Dr Rajiv Desai
An Educational Blog
Monkeypox (Tinypox)
Monkeypox (Tinypox):
_
_
Section-1
Prologue:
On May 7, 2022 the world was alerted to a confirmed case of monkeypox in the United Kingdom. Cases have since cropped up across the globe, from Germany and Spain, to the U.S. and Canada. The global monkeypox outbreak, the largest in history, is highly unusual because the virus is circulating widely in countries where it is not normally found. Historically, monkeypox has circulated in remote parts of West and Central Africa. In that context, people normally caught the virus from animals. There was little spread between people. Monkeypox is now spreading widely between people, mostly through close contact during sex among gay and bisexual men.
_
Monkeypox is an illness caused by monkeypox virus, which belongs to the Orthopoxvirus genus of the Poxviridae family; other members of Orthopoxvirus genus include variola virus (which causes smallpox), vaccinia virus (used in smallpox vaccines), cowpox virus, and various other animal poxviruses. It is a viral zoonotic infection, meaning that it can spread from animals to humans. It can also spread from humans to other humans and from the environment to humans. Monkeypox is not related to chickenpox. Chickenpox is a herpes virus in a separate family from monkeypox and smallpox. Monkeypox was first identified in 1958 at a laboratory in Copenhagen, Demark when it was discovered in macaque monkeys kept for research, hence the name ‘monkeypox.’ It does infect monkeys, and has been isolated from monkeys, but they’re not the primary reservoir for the disease. The first case in humans was not reported until 1970 when a nine-month-old boy admitted to a hospital in the Democratic Republic of Congo was found to have been infected with the virus.
_
Since then, cases have appeared throughout Africa and beyond, including Singapore, the U.K, Israel and the U.S. Most infections occur in people who live, have travelled to, or have been in contact with individuals or animals from endemic regions of Central and West Africa. For example, in 2003 over 70 people in the U.S. fell ill with monkeypox after handling prairie dogs that were co-housed with infected Gambian pouched rats and dormice imported from Ghana. However, infections don’t always follow this transmission pattern, as evidenced by the current spread of monkeypox among people who have not travelled to endemic countries or been in contact with those known to be infected with monkeypox. The recent multinational outbreak of monkeypox cases since early May 2022 has revealed a changing epidemiological trend, those confirmed cases had no sojourn history in endemic areas and with a high proportion of cases involving men who have sex with men (MSM). Among the MSM cases, many of them presented with atypical clinical manifestations of monkeypox and with other sexually transmitted co-infections. Combined with the high social interactivity in this community, there is likely a higher risk of monkeypox transmission in this population.
_
The World Health Organisation (WHO) has declared the global monkeypox outbreak a ‘public health emergency of international concern’ (PHEIC) in July 2022, one step below that of a ‘pandemic.’ A PHEIC, according to the WHO, constitutes “…an extraordinary event, which constitutes a public health risk to other States through the international spread, and which potentially requires a coordinated international response.” More than 72,000 monkeypox cases and 28 deaths have been confirmed in 109 countries as of 14th October 2022 since the outbreak began earlier this year and the vast majority of monkeypox cases – more than 95 per cent, are among men who have sex with men, with a median age of 36. And among cases where Human Immuno-deficiency Virus (HIV) status is known, about 40 per cent of reported monkeypox cases are among people who are also living with HIV. When the first few dozen cases of monkeypox emerged in Europe, spreading of the virus through sexual contact and genital lesions came as a surprise to many scientists. But it shouldn’t have. Nigerian researchers reported a similar pattern in 2017, when they documented 228 cases, many of them young men with genital ulcers. The virus spread primarily among young men who had genital ulcers.
_
Few people outside Africa and the public health community had even heard of monkeypox. In just a few months, it has become a household word. As the fear of the coronavirus disease 2019 (COVID-19) pandemic subsides, countries around the globe are now dealing with a fear of the epidemic surrounding the prevalence of monkeypox cases in various regions. Previously endemic to regions of Africa, the majority of monkeypox cases associated with the 2022 outbreak are being noted in the western hemisphere. While contact-tracing projects are being conducted by various organizations, it is unknown how this outbreak began. Monkeypox cases received attention during the 1970s, after the global eradication of smallpox. The smallpox vaccine provided cross-immunity to the monkeypox virus. Upon the cessation of smallpox vaccine administration, monkeypox cases became more prevalent. It was not until the 2003 US outbreak that monkeypox truly gained global attention. Despite the virus being named monkeypox, monkeys are not the origin of the virus. Monkeypox name is something of a misnomer: Monkeys (and humans) are just incidental hosts of the disease, which is thought to be found primarily in rodents. Monkeypox classically presents with rash, fever, lymphadenopathy, and a clinical course similar to that of smallpox but milder. But in the current 2022 outbreak, many patients presented atypically with genital or peri-anal rash prior to or without prodromal symptoms which may not spread to other parts of the body, and having lesions at different stages of development. Although mortality is low in the current 2022 outbreak, many of those infected report severe pain that sometimes requires hospitalization to manage.
_
Let’s be clear and state facts. First, anyone can get monkeypox. Second, the current 2022 outbreak is overwhelmingly concentrated among men who have sex with men. And third, a growing body of evidence and data suggests that sex among these men is the primary means through which monkeypox is presently spreading. While it’s true that there are other ways the virus can be transmitted, recognizing and reporting these facts is not anti-gay. Yes, monkeypox can spread from close physical contact regardless of any sexual orientation or race but vast majority of monkeypox cases are among men who have sex with men, therefore targeting advice to members of this community is not anti-gay.
While monkeypox is endemic in central and western Africa, it’s misleading to describe the virus as “being African,” wrote more than 20 scientists in a recent paper detailing the need for a non-discriminatory, non-stigmatizing name for the disease. Critics say the name “monkeypox” plays into racist stereotypes about Black people, Africa and LGBT people — and, they note, it falsely suggests monkeys are the main source of the virus.
We have to follow science to curtail outbreak and not appease or displease any community or race. Today I am renaming ‘Monkeypox’ as Tinypox. I do not want to stigmatize & discriminate against either African people or LGBT community. Let’s call it Tinypox. It is POX because it resembles smallpox and it is TINY because it is less transmissible & lethal than smallpox. I hope WHO accepts new name ‘Tinypox’ to combat racism and homophobia generated by the name ‘Monkeypox’.
_
Since cases of monkeypox began to emerge in Europe, beliefs about the virus have been shared widely on social media that appear to be recycled from the COVID-19 pandemic. There are important differences that make monkeypox a much less serious threat than COVID-19. These include:
- Monkeypox does not spread easily.
- Infected people are easier to identify.
- Outbreaks are easier to contain.
- There are two vaccines that are effective against monkeypox.
Early on in the Covid-19 pandemic, SARS-CoV-2 was assumed to spread through droplets from symptomatic patients, whereas actually aerosol (long distance) transmission was overwhelming, with asymptomatic or pre-symptomatic patients dominating transmission chains. While fears about the monkeypox outbreak are understandable, this virus is not like Covid, it is not airborne, asymptomatic transmission rare and its spread will be limited. Since May 2022, clusters of monkeypox infections have caused global concern but the number of infections is growing slowly. Monkeypox is considerably less transmittable than COVID-19, with the average infected individual spreading the disease to only one or two other people, compared to as many as 15 with recent strains of COVID-19. It is much harder to pass on than Covid, we already have available vaccines and treatments, and people appear to be infectious only once symptoms appear – making it easier to spot and isolate. So, restrictions such as lockdowns or mass vaccinations are really not going to be the way to respond to this. Instead, isolation measures and vaccines are currently being targeted at infected people or their close contacts. The monkeypox virus is not airborne like COVID-19, highly transmissible like smallpox or long-lasting in the body like HIV.
Social media accounts and news outlets in Ukraine, Russia, China and the US have all made accusations that the monkeypox outbreak was the result of a laboratory leak, or the use of monkeypox as a biological weapon. The genetic sequences of DNA we have so far for the virus all trace it back to the strain of monkeypox which commonly circulates in West Africa. That tells us this is not something manufactured. Monkeypox virus is not a novel virus unlike novel coronavirus of COVID-19. The risks posed by monkeypox have been well documented for years and cases have been on the increase, and outbreaks of infection are a fact of life. While monkeypox outbreak is attracting headlines, there is little threat of a massive global pandemic – and no comparison to COVID-19. While monkeypox poses little threat to the general public, public should be aware of its spread through direct physical contact, whether sexual or non-sexual.
______
______
Abbreviations and synonyms:
MPX = monkeypox
MPXV = monkeypox virus = MPV
ORXP = orthopoxvirus
VARV = variola virus = VAR = smallpox virus
VACV = vaccinia virus = VV = VAC
CPXV = cowpox virus = CPV
NHP = non-human primate
MSM = men who have sex with men
GBMSM = gay, bisexual and other men who have sex with men
LGBT = lesbian, gay, bisexual, and transgender
PFU = plaque-forming units
STI = sexually transmitted infection
STD = sexually transmitted disease
Cq = quantification cycle
Ct = cycle threshold
qPCR = quantitative polymerase chain reaction
MVA = modified vaccinia Ankara
VZV = varicella zoster virus = virus causing chickenpox & herpes zoster
ST-246 = Tecovirimat = TPOXX
VIG = Vaccinia Immune Globulin
CMX001 = Brincidofovir
CFR = case fatality rate
PEP = post-exposure prophylaxis
PrEP = pre-exposure prophylaxis
______
______
Section-2
Pox virus family:
Monkeypox is one of the many zoonotic viruses that belong to the Orthopoxvirus genus of the Poxviridae family, as presented in figure below. Poxviridae viruses are large, enveloped, double-stranded DNA viruses. The major hosts of Poxviruses are rodents, rabbits, and non-human primates, which can occasionally be transmitted to humans facilitating the occurrence of human-to-human transmission.
Taxonomically, the Poxviridae family is further categorized into two families: Entomopoxvirinae and Chorodopoxvirinae. The subfamily classification is based on whether the virus will infect insects, such as Entemopovirinae, or infect vertebrates, as is the case with Chorodopoxvirinae. The Chorodopoxvirinae family is further classified into 18 genera, as depicted in figure below. Each of the 18 genera within the Chorodopoxvirinae subfamily list several viruses, the majority of which are of zoonotic origin.
Figure above shows Taxonomy and Classification of Monkeypox virus within Poxviridae Lineage.
_
The last endemic case of smallpox occurred in 1977, total eradication was confirmed in 1980, and the official account of the disease and its eradication has appeared. Consequently, smallpox is not discussed in detail. However, its importance should not be forgotten. It helped to shape history, and it made history by being the first disease to be controlled by immunization and the first to be eradicated. Table below lists the features that made smallpox an ideal candidate for eradication.
_
Figure below shows genera and species of family Poxviridae, subfamily Chorodopoxvirinae, that affect humans:
The poxviruses are highly relevant to human beings, among which the orthopoxviruses are the best-known. These include the smallpox virus (variola virus, VARV), the monkeypox virus (MPXV), the cowpox virus (CPXV), and the vaccinia virus (VACV or VV). Human monkeypox virus (MPXV) is a double-stranded DNA virus of the Orthopoxvirus genus of the family Poxviridae. Two genetic clades of the monkeypox virus have been characterized: West African and Central African. There is a range of animal poxviruses, several of which have zoonotic potential. Infections in humans have been described for vaccinia virus, cowpox virus, buffalopox virus, and sporadic cases of camelpox. Monkeypox infects a wide range of mammalian species, but its natural host reservoir remains unknown.
_
Orthopoxviruses are large, complex DNA viruses within the family Poxviridae. Four orthopoxvirus species are known to cause human disease: variola virus (smallpox), vaccinia virus (smallpox vaccine), cowpox virus, and monkeypox virus. Variola virus is likely the best known member of the orthopoxvirus genus. As the causative agent of smallpox, this virus caused untold human suffering and loss of life until its eradication in 1980 following the successful completion of a global eradication campaign. Ordinary smallpox presented with fever and flu-like symptoms after an incubation period of 10–14 days. Rash generally followed within 2–3 days and was characterized by a centrifugal distribution and stepwise progression through macular, papular, vesicular, and pustular stages. Mortality rates were estimated as high as 30 %. Edward Jenner was the first person to recognize the ability of orthopoxviruses to induce cross-reactive antibodies that protect against infection from other orthopoxvirus species and pioneer the use of vaccination to prevent disease. Vaccinia virus is still in use today as a vaccine as well as a subject and tool for biomedical research. Human vaccinia infections generally cause self-limited, localized lesions though severe and life-threatening complications can occur, particularly in high-risk populations such as immunocompromised individuals and those with atopic dermatitis. In addition, vaccinia infections present a risk of inadvertent inoculation from infectious virus present in vaccinial lesions. Most vaccinia infections are related to vaccination. However, both vaccinia virus and cowpox virus cause sporadic zoonotic infections as well. Cowpox virus is classically associated with occupational exposure to cattle though other sources include rats, cats, and zoo and circus elephants. In contrast, vaccinia virus is only known to occur naturally in cattle and buffalo in Brazil and select regions of the Middle East and Southeast Asia. Monkeypox virus is also transmitted zoonotically; one or more species of squirrels or other rodents is believed to be the natural reservoir of monkeypox virus. The incidence of monkeypox appears to be increasing since the cessation of routine smallpox vaccination following eradication. The presentation of monkeypox is similar to that of ordinary smallpox with lymphadenopathy being the distinguishing clinical features of monkeypox. Overall, monkeypox is less severe compared to smallpox with an estimated mortality rate of < 10 %. An outbreak of human monkeypox occurred in the United States in 2003 demonstrating the capacity for spread of the disease outside of the previously observed geographic boundaries. Prevention of human orthopoxvirus infections is largely accomplished through vaccination. Few treatment options are available for orthopoxvirus infections after the onset of symptoms. Vaccinia immune globulin (VIG) has been used successfully in treating certain severe adverse events from smallpox vaccine. Other drugs with antiviral activity against orthopoxviruses are currently licensed for this indication. Orthopoxviruses pose a threat to public health based on their ability to cause zoonotic outbreaks and potential to be used as a biological weapon or agent of bioterrorism. These concerns continue to drive poxvirus research and efforts to develop preparedness and response plans, improved vaccines, antivirals, and other medical countermeasures.
_
The epidemiology of smallpox, caused by orthopoxvirus variola, is understood through detailed studies conducted during the end of the eradication campaign. Interhuman transmission of variola virus generally occurred through the inhalation of large airborne respiratory droplets of infectious virus. Transmission usually required prolonged face-to-face or other close contact, although airborne transmission over longer distances had been reported. Transmission by fomites or contact with infectious material from the rash also occurred. Aggregate data, collected during the smallpox eradication campaign, suggest a secondary attack rate of 58.4% in unvaccinated close or household contacts and a secondary attack rate of 3.8% in previously vaccinated close or household contacts. Case-fatality rates for variola major varied with the type of disease manifested, but aggregate rates of 10 to 30% in various outbreaks have been recorded. Severity of disease correlated with rash burden and was also more severe in children and pregnant women. Variola alastrim minor, a variant of variola with a case-fatality rate of less than 1%, had similar human-to-human disease transmission characteristics.
Monkeypox has a more complex epidemiology. The virus is zoonotic, and two genetically discrete virus clades have been described, each with apparent distinct clinical and epidemiologic parameters. Human infections in western and central Africa were first identified in 1970. Investigations in the Congo basin country Zaire, now the Democratic Republic of Congo, demonstrated that human-to-human transmission of monkeypox was less prevalent than that of smallpox. The secondary attack rate in unvaccinated contacts of monkeypox cases was calculated to be 9.3% versus 37 to 88% for smallpox. Previous smallpox vaccination (administered 3 to 19 years prior) appeared to be 85% protective in preventing disease acquisition in contacts and also ameliorated the severity of disease. Overall, most identified cases acquired disease from presumed animal exposure; only 28% of cases were ascribed to person-to-person transmission. A case-fatality rate of approximately 10% was observed in unvaccinated persons, and the majority of fatalities and the most severe disease manifestations were observed in children younger than 5 years. In a more recent series of 122 confirmed cases in Nigeria, the case-fatality rate was 6%. Serosurveys suggested that subclinical infection may have occurred in up to 28% of close contacts of monkeypox patients in some communities; this relatively low rate may contribute to the rarity of sustained generations of human-to-human transmission in household and other close-contact situations. However, more recent studies of household attack rates in an outbreak setting suggest that up to 50% of infections may be transmitted from human to human, and the seroprevalence of anti-orthopoxvirus antibodies in unvaccinated individuals is about 20 to 25% in central and western Africa.
______
Orthopoxvirus:
Virion:
Enveloped, brick-shaped virion, on average 300nm long and 240nm wide. The surface membrane displays surface tubules or surface filaments. Two distinct infectious virus particles exist: the intracellular mature virus (IMV) and the extracellular enveloped virus (EEV).
Genome:
Linear, dsDNA genome of 170-250kb. The linear genome is flanked by inverted terminal repeat (ITR) sequences which form covalently closed hairpin termini at each extremity.
Cytoplasmic replication:
-1. Attachment of the viral proteins to host glycosaminoglycans (GAGs) mediates endocytosis of the virus into the host cell. The virus can be uptaked also by apoptotic mimicry
-2. Fusion with the plasma membrane to release the core into the host cytoplasm.
-3. Early phase: early genes are transcribed in the cytoplasm by viral RNA polymerase. Early expression begins at 30 minutes post-infection.
-4. Core is completely uncoated as early expression ends, viral genome is now free in the cytoplasm.
-5. Intermediate phase: Intermediate genes are expressed, triggering genomic DNA replication at approximately 100 minutes post-infection.
-6. Late phase: Late genes are expressed from 140 min to 48 hours post-infection, producing all structural proteins.
-7. Assembly of progeny virions starts in cytoplasmic viral factories, producing an spherical immature particle. This virus particle matures into brick-shaped intracellular mature virion (IMV).
-8. IMV virion can be released upon cell lysis, or can acquire a second double membrane from trans-Golgi and bud as external enveloped virion (EEV).
_
Figure above shows Electron micrographs of negatively stained, naturally released virions of (A) vaccinia virus and (B) parapoxvirus. The outer envelope is particularly obvious in (A). (bar = 100 nm)
_
Viruses in the family Poxviridae, genus Orthopoxvirus, consist of numerous pathogens known to infect humans, including variola virus (VARV), monkeypox virus (MPXV), cowpox virus (CPXV), and vaccinia virus (VACV). Genomes of these viruses are ≈200 kb long, have highly conserved central regions coding for replication and assembly machinery, and have more variable terminal ends that contain genes involved in host range determination and pathogenesis. Although orthopoxviruses are antigenically and genetically similar, they have diverse host range and virulence properties. Comparative genomics studies have shown that the evolution of orthopoxviruses is ongoing and can be driven by selective pressure from a host species. It has been postulated that progressive gene loss, primarily at the terminal ends of the genome, has been a driving force behind the evolution of these viruses. CPXV, which causes only mild infection in humans, contains the largest genome of all sequenced orthopoxviruses (≈220 kb), encodes 223 open reading frames (ORFs), and has a broad host range that includes rodents, humans, felids, bovids, and voles. Conversely, VARV, the causative agent of smallpox, is highly pathogenic (case-fatality rate ≈30%), has the smallest genome of all naturally occurring orthopoxviruses (≈186 kb), is predicted to encode 20% fewer functional proteins than CPXV, and has a host range restricted to humans.
_______
General Concepts of pox viruses:
Clinical Manifestations:
Smallpox has been eradicated. Poxvirus infections are characterized by the production of skin lesions. With most poxviruses there is typically just a primary lesion, but generalized lesions develop with monkeypox and molluscum. In human cowpox and parapox infections the lesion develops at the site of inoculation (usually the hand), and infection may be spread to other sites such as the face and/or genitals by scratching. When seen by the physician, cowpox and parapox lesions are usually hemorrhagic crusting ulcers, but early in infection the former are usually vesicular and the latter nodular. The lesions of molluscum, usually multiple, are firm, pearly, flesh-colored nodules.
Figure above shows localized and generalized poxvirus infections. Numbers indicate progression of infection.
Parapox and molluscum infections are relatively painless and cause very little constitutional disturbance. Human cowpox is very painful, particularly in young children, usually causes pyrexia and marked lymphadenopathy; patients often require hospitalization. Rare encephalitic complications of cowpox have been reported, and erythema multiforme is a complication of parapox infections. Infection in immunocompromised or eczematous individuals is more severe and usually results in generalized illness, and in cowpox has caused deaths.
Smallpox vaccination has been associated with serious complications. However, routine use of smallpox vaccine has been discontinued, and any future use of recombinant vaccinia virus vaccines will involve attenuated strains, thus reducing the chances of complications.
Although human monkeypox is rare and geographically localized, it is a serious generalized infection, which clinically resembles mild smallpox. A febrile prodrome precedes the development of a vesicular or pustular rash, typically centrifugal in distribution. Detailed examination of more than 300 cases in Zaire showed an overall mortality of 10 percent, reaching 15 to 20 percent in unvaccinated children. Respiratory complications were seen in about 12 percent of unvaccinated patients.
_
Pathogenesis:
The pathogenesis of localized poxvirus infections is simple. Virus invades through broken skin, replicates at the site of inoculation, and causes dermal hyperplasia and leukocyte infiltration. With cowpox, and to a lesser extent with parapox, there is limited lymphatic spread; this causes lymphadenopathy and elicits an immune response. The lesion of molluscum is circumscribed by a connective tissue capsule, and the dermis, although distorted, is not usually broken. Some poxviruses express an epidermal growth factor and host range genes which play a role in pathogenesis and cell tropism.
Human monkeypox is can be acquired via direct contact or respiratory tract, and during a 12-day incubation period viremia distributes infection to internal organs, which are damaged by virus infection. Spread to the skin initiates the clinical phase, and the lesions progress through the classic stages of macule to papule to vesicle to pustule to crust. Lymphadenopathy, usually involving the cervical and inguinal areas, is often marked.
_
Host Defenses:
With the exception of human monkeypox, which is can be acquired via the respiratory route, human poxvirus infections are acquired by inoculation into the skin or contact with broken skin. Consequently, unbroken skin presents the first line of defense. Interferon, nonspecific inflammation, and probably pyrexia play a role in limiting infection during the early stages. Infection induces humoral and cellular immune responses to naturally released virions and to viral antigens on the surface of virus-infected cells. Responses to the extra antigens on the envelope of naturally released virions are particularly important, determining the speed and extent of recovery and the prevention or attenuation of future infection. In general, the immune response is related to the severity of infection; the immunity elicited by a mild infection may be insufficient to prevent reinfection, as is often the case with human parapox infections.
_______
_______
Is monkeypox taking over where smallpox left?
Ten thousand years ago, when smallpox first emerged, humankind could do little more than pray to the gods for succor. Later known as variola, the virus that caused the disease first attacked the linings of the nose or throat, spreading throughout the body until a characteristic rash followed by virus-filled blisters developed on the skin. Over the course of recorded history, the “speckled monster” killed up to a third of the people it infected. During the 20th century alone, it felled more than 300 million men, women and children.
By the late 1970s, however, the deadly scourge had been eliminated from the face of the earth thanks to mass vaccination campaigns that protected millions and left them with a small scar on their upper arm. With nowhere to hide in the natural world—humans are the virus’s only host—variola was beaten into extinction. Today the only known viral samples are locked in two specialized government laboratories, one in the U.S. and the other in Russia. Barring a catastrophic lab accident, deliberate release or the genetic reengineering of the virus, smallpox will never again spread death and misery across the globe.
The World Health Organization, which had organized the eradication campaign, sounded the official all clear in 1979, two years after the last sporadic case was recorded, in a Somali hospital worker. Since then, no country has routinely vaccinated its citizens against smallpox, although the U.S. began inoculating certain health personnel and selected members of its armed forces after the terror attacks on September 11, 2001. Thus, an entire generation has reached adulthood without any exposure to either the disease or the vaccine, which sometimes caused serious side effects.
And therein lies the rub. The smallpox vaccine did not protect just against the variola virus. Anyone who was vaccinated against smallpox also developed immunity to infection with variola’s viral cousins—including monkeypox and cowpox. Given the much larger scale of smallpox infections at the time, this secondary protection was seen as a minor benefit.
Now that the smallpox vaccine is no longer widely given, the question becomes: Could these obscure pathogens, which, like smallpox, belong to the Orthopoxvirus genus, pose a new danger to humans? There are reasons to worry. Unlike smallpox, cowpox and monkeypox naturally lurk in rodents and other creatures, so they can never be fully eliminated. The number of cases of monkeypox and cowpox in humans has steadily risen in recent years. And both viruses have begun to infect different creatures beyond their normal hosts, raising the possibility that they might spread through new paths around the planet.
No one knows how monkeypox and cowpox will change over time, but virologists worry that if they mutate to jump more easily from one person to the next, they could devastate large parts of the globe. That grim possibility drives a small band of virologists to learn more about these—or any other—potential pox plagues in the making, so as to sound the alarm if they show signs of developing into more threatening forms.
Scientists still cannot explain why poxviruses vary so greatly in their severity. In most people, cowpox, camelpox and raccoonpox infections trigger little more than a skin rash, with virus-filled pustules that harmlessly clear up on their own. Monkeypox infections, on the other hand, can be quite deadly in humans. Even at that, not all monkeypox viruses are equally dangerous. The worst subtype, found in the Congo Basin, kills about 10 percent of people who are infected, whereas another version, from West Africa, rarely if ever ends in death. As it happens, the West African strain in 2003 caused the first-ever recorded cases of monkeypox in the Western Hemisphere. The outbreak, which occurred in six states in the U.S., led to the hospitalization of 19 people, including a child who suffered encephalitis and a woman who was blinded, necessitating a corneal transplant. Investigators traced the infection to rodents imported from Ghana that passed the virus to pet prairie dogs, which in turn infected their owners. Such intermediary animals allow a virus that normally lives in animals with little human contact to reach potentially large numbers of people.
Subtle genetic differences may help explain the shifting severity of pox infections. For example, some poxviruses possess genes for proteins that interfere with the ability of the immune system to respond effectively to the infection. When researchers compared the genes from different poxviruses, they zeroed in on one that was found in several different kinds of poxviruses. In the most deadly strains of variola, this gene triggered the production of a protein that evidence suggests prevents some immune cells from efficiently coordinating their counterattack against the virus. But the equivalent gene in the Congo Basin strains of monkeypox (which are less deadly than smallpox) provided the hereditary instructions for a much shorter protein. When researchers looked at the milder West African version of monkeypox, the gene was missing altogether and the protein in question could not be manufactured. Thus, the evidence suggested that the shorter protein in the Congo Basin strains of monkeypox somehow made them less deadly than smallpox.
Speculation among researchers about how different species of poxvirus acquired this and other genes indicates why monkeypox and its cousins could potentially become more dangerous threats than they are now. The genes, which are not essential for poxvirus replication, appear to be faithful copies of genes the viruses acquired at some point in the evolutionary past from organisms they infected. Yet, curiously, the viruses do not in the normal course of an infective cycle come anywhere near the genetic material stored in the nucleus of the host cells.
One possible explanation, popular among pox virologists, posits the simultaneous infection of a human or other vertebrate host with a poxvirus and a retrovirus. Such co-infections are probably pretty common, researchers say. Retroviruses are known for incorporating their own genes into their host’s DNA. (About 8 percent of the human genome consists of DNA that originated in retroviruses.) It is possible that the unusual biochemical activity of the retrovirus inside the cell could allow the poxvirus to capture its host’s genes.
If true, this hypothesis could prove ominous. Poxviruses are genetically stable and do not usually mutate quickly. If they can steal genes from their hosts that make them more virulent, then there is no predicting what a relatively harmless, not to mention an already deadly, poxvirus might do under the right circumstances. The change from mild to dangerous threat could occur more quickly and unpredictably than anyone might have previously suspected.
______
______
Section-3
Monkeypox virus (MPXV), its genome and mutations:
Monkeypox is a rare zoonotic disease that is caused by the MPXV from the Orthopoxvirus genus, which includes the variola virus, the causative agent of smallpox. With an incubation period of 5–21 days making its movement hard to track; human disease typically begins with fever, myalgia, fatigue and headache, often followed by maculopapular rash. Although the natural reservoir of MPXV remains unknown, animals such as rodents and non-human primates may harbor the virus, leading to occasional spill-over events to humans. MPXV is endemic in West and Central African countries, and the rare reports outside these regions have been associated with imports from those endemic countries. We are now facing the first multi-country outbreak without known epidemiological links to West or Central Africa, with more than 70,000 confirmed cases reported worldwide since the first confirmed case on 7 May 2022 in the United Kingdom. Several measures are being recommended by international health authorities to contain MPXV transmission, including the use of vaccines for selected close contacts of patients with monkeypox (post-exposure) and for groups at risk of occupational exposure to monkeypox (pre-exposure). The virus can be transmitted from human to human by close contact with lesions, body fluids, respiratory droplets and contaminated materials but the current epidemiological context poses some degree of uncertainty about the viral transmission dynamics and outbreak magnitude.
_
Figure below shows comparison of monkeypox, HIV, SARS CoV-2, and polio viruses’ size. Membranes and membrane-bound proteins are in purple, capsids are in dark blue, and genomes and nucleoid-associated proteins are in turquoise.
The virus is mainly found in the tropical forests of Central Africa and West Africa. It was first discovered in monkeys in 1958, and in humans in 1970. Between 1970 and 1986, over 400 cases in humans were reported. Small viral outbreaks with a death rate in the range of 10% and a secondary human-to-human infection rate of about the same amount occur routinely in equatorial Central and West Africa. The primary route of infection is thought to be contact with the infected animals or their bodily fluids. The first reported outbreak outside Africa occurred in 2003 in the Midwestern United States in Illinois, Indiana, and Wisconsin, with one occurrence in New Jersey. No deaths occurred. A significant outbreak in Nigeria occurred in 2017. After a few years of sporadic cases outside of Africa, the 2022 monkeypox outbreak infected tens of thousands of people worldwide.
_
Poxviruses are large viruses with a deoxyribonucleic acid (DNA) genome. The orthopoxvirus genus is especially interesting because its members elicit cross-reactive and cross-protective immunity to each other. Despite the eradication of smallpox, poxviruses can cause emerging endemic diseases. Several outbreaks have been caused by MPXV, which produces smallpox-like lesions. In 2022, a massive outbreak occurred involving over 100 countries. Not only was this spread by community transmission, but the route was by sexual contact. This was quite unlike earlier, much smaller epidemics confined to endemic regions in western and central Africa, following contact with potentially infected animal hosts. In July 2022, the MPX outbreak was declared a global health emergency by the World Health Organization (WHO). Orthopoxviruses are a possible bioweapon, with their ability to spread rapidly and their potential mortality risk in the absence of poxvirus immunity ever since smallpox vaccination was stopped almost 50 years ago.
_
Poxviruses are large, linear, double-stranded DNA viruses with a genome size ranging from 130 to 360 kbp that replicate in the cytoplasm of vertebrate or invertebrate cells. DNA viruses typically replicate and express their genomes in the nucleus, making extensive use of cellular proteins, however, this is not the case for poxviruses. Poxviruses are different in the sense that they rely heavily on virus-encoded proteins that enable them to replicate in the cytoplasm. The central part of the genome contains genes involved in key functions, such as transcription and virus assembly, whereas those located at the termini are involved in virus-host interactions. Of more than 150 genes encoded by poxviruses, 49 are common to all sequenced members of this family and 90 are common within the subfamily of chordopoxviruses. The majority of these conserved genes among the viruses are related to viral function and form the central part of the genome.
_
Monkeypox is from the family: Poxviridae, subfamily: chordopoxvirinae, genus: orthopoxvirus, and species: Monkeypox virus.
On electron microscopy, the monkeypox virus is relatively large (200-250 nanometers). Poxviruses are brick-shaped, surrounded by a lipoprotein envelope with a linear double-stranded DNA genome. Aside from their reliance on host ribosomes for mRNA translation, poxviruses include all necessary replication, transcription, assembly, and egress proteins in their genome.
Figure above shows electron microscope image of monkeypox virions from a human skin sample. On the left are mature, oval-shaped virus particles, and on the right are the crescents, and spherical particles of immature virions.
_
Like all poxviruses, monkeypox virions are large, enveloped and “brick-shaped.” Encapsulated within each virion is a core containing a linear, double-stranded DNA genome and enzymes required for virus uncoating and replication. At the onset of infection, poxvirus particles attach to the host cell membrane through various viral-host protein interactions. Notably, while other mammalian DNA viruses replicate in the nucleus, poxviruses replicate in the cytoplasm in small compartments known as ‘factories’, formed from the host rough endoplasmic reticulum (ER). Though they come wielding their own transcriptional machinery, poxviruses rely on host ribosomes to translate mRNAs into the structural components of the virion, as well as proteins that dismantle the factory’s ER membrane. Such dismantling gives rise to small, membranous crescents that grow to encapsulate the genome of assembling virions. Progeny poxviruses are decorated with additional membranes from the trans-Golgi network before exiting the cell via plasma membrane fusion.
_
The MPXV infects both animal reservoir hosts and other incidental hosts, including humans, tree squirrels, dormice, and Gambian pouched rats. While first identified in Asian monkeys in a polio research laboratory in Denmark, it was again reported in a captive monkey colony.
The first human infection was documented in 1970 in the Democratic Republic of Congo. The original orthopoxvirus lineage diverged about 3500 years ago into a common ancestor that gave rise to MPXV and VARV. MPXV itself first appeared in West Africa 600 years ago.
Orthopoxviruses develop between 10-5 and 10-6 mutations per replication site. The large genome, containing almost 200,000 base pairs, changes by 1 or 2 nucleotides a year.
However, the currently circulating strain has changed by about 50 nucleotides over the last 4 years – a 12-fold increase in the mutation rate. This may indicate that it has adapted excellently to its human host, thus permitting the spread of this virus within this species.
Phylogenetically, two clades are distinguished, the West and Central African clades, which are supposed to have diverged between 560-860 years ago. Significant differences in their terminal regions encode proteins responsible for modulating the host immune response distinguish them.
While the West Africa clade (Clade 2) is less virulent and shows a less extensive drift, the Central Africa clade (Clade 1) is more virulent and spreads more rapidly because it prevents T-cell-receptor-mediated T cell activation and resulting cytokine production. This clade has probably expanded by migration, founder effect, or bottleneck events.
Recently a third clade was identified, Clade 3. Both clade 2 and clade 3 belong to the older Western Africa clade. Clade 3 was responsible for the outbreak of 2017/2019, with multiple mutations that seem to indicate adaptation to humans. In fact, there are 47 nucleotides that differ between the currently circulating strain and the 2017/2019 clade 3 strain.
This number is much larger when compared to the expected number of mutations in just 3 years. Some researchers suggest calling the current strain hMPXV1, to emphasize its adaptation to the human host.
Even so, this subclade shows significant variation in genomes. Using the Pango system to name MPXV, it has been suggested that the ancestor of the human strain be called lineage A, with its descendants A.1, A.2, and A.1.1. The current strain would then be called B.1.
_
Due to the larger size of poxviruses, it makes it harder for viruses such as monkeypox to breach host defenses by passing through gap junctions. The larger size of the virus also makes it difficult for the virus to replicate rapidly and orthopoxviruses need a more comprehensive strategy to survive within the host. The larger size of the orthopoxviruses alerts the immune system of the individual very early on and thus, generates an immune response very easily. To be able to evade the host immune system, orthopoxviruses are equipped with a set of molecules encoded by virulence genes that will act as modulators by being directed against components of the host’s immune system. These proteins that are responsible for modulatory actions against the host’s immune response can be categorized into two groups according to whether they worked intracellularly or extracellularly.
These proteins that are responsible for modulatory actions against the host’s immune response can be categorized into two groups, as highlighted in Figure below. Intracellular proteins are virotransducer proteins and virostealth proteins. The virotransducer proteins act by playing a role in interfering with the cell’s ability to respond to the infection, including the oxidative burst and apoptotic pathways. The virostealth proteins, which also act intracellularly, reduce the likelihood of detection of the virus by the host’s immune system via the downregulation of immune recognition molecules such as the major histocompatibility complex class 1 (MHC 1) and CD+4. While there are two different types of intracellular modulatory proteins that aid monkeypox in evading the host’s immune response, there is only one type of extracellular protein, viromimic proteins. Figure below shows that there are two different classifications of viromimic proteins, and both function to modulate the immune system’s response. The viroreceptors are secreted or are present as cell surface glycoproteins that bind host cytokines and chemokines competitively and, thus, interfere with their actions. Consequently, virokines form viral mimics of host cytokines, chemokines, and growth factors that are effective in both subverting host responses that are detrimental to virus survival and in promoting responses appropriate for viral replication and spread. These modulatory proteins work simultaneously to evade the host’s immune system to allow for viral replication. Without the presence of these proteins, Orthopoxviruses such as monkeypox would be unable to evade the immune system.
Figure above shows Intracellular and Extracellular Modulatory Proteins of Monkeypox.
_
Poxviruses’ replication cycle provides an insight into how the replication cycle of the monkeypox virus functions. As with other viruses, poxviruses also have proteins that enable and aid the binding of the virus to a cell, membrane fusion, and entry into the host cell. In the case of poxvirus, the mature virion (MV), which has a single membrane, and the extracellular enveloped virion (EV), which has an additional outer membrane, are disrupted before the fusion. There are four viral proteins that are associated with the MV and all these collectively will facilitate the attachment of MV to a host cell by binding glycosaminoglycans or laminin on the cell surface. Regardless of whether the MV or EV mediate infection, the fusion of the virus to the host cell is dependent on 11 to 12 non-glycosylated, transmembrane proteins that range in size from 4 to 43 kDa. MVs are very stable and are thought to mediate transmission between host animals, whereas EVs have a fragile outer membrane and are specifically specialized for exiting the intact cell and spreading within the host.
_
Poxvirus DNA replication progresses within cytoplasmic structures that originally were called Guarnieri bodies and are now commonly called factories. Each factory derives from a single infecting particle and in the early stages of infection, they are compact DNA-containing structures that are surrounded by membranes that seemed to drive from the cell’s rough endoplasmic reticulum (RER). These factories will enlarge with continuing DNA synthesis and will gradually adopt a more irregular appearance as cavities form containing viral mRNA and host translation factors. In the later stages of the replication cycle, a complex of late gene products and a collective of viral membrane assembly proteins will act to dismantle the surrounding endoplasmic reticulum membranes and produce crescent-shaped structures as substrates for the assembly of the immature virions (IV). The IV is then processed into MV, which are the most abundant infectious species. These MV will exit the cell via fusion with the cytoplasmic membrane.
_______
_______
Animal models of MPXV:
MPXV is highly pathogenic for a variety of laboratory animals, and so far, many animal models have been developed by using different species and different routes of exposure (Table below). Because of the unavailability of variola virus to develop animal models and resulting disease manifestations in humans that are similar, MPXV is one of the pox viruses that are used very heavily to develop a number of small animal models via different routes of exposure. Wild-derived inbred mouse, STAT1-deficient C57BL/6 mouse, prairie dogs, African dormice, ground squirrels are highly susceptible to MPXV by different exposure routes.
MPXV Animal Models:
|
Virus species |
Route of exposure |
Characteristics of human disease |
Animal model |
Route of exposure |
Clinical outcome in animals |
|
MPXV |
Direct contact with body fluids or lesions of infected person or animal and/or aerosol |
Fever, malaise, lymphadenopathy, rash |
Mice |
Intranasal |
Weight loss, viremia, mortality |
|
Intraperitoneal |
Weight loss, viremia, mortality |
||||
|
Prairie dogs |
Intraperitoneal |
Rash, viremia, mortality |
|||
|
Intranasal |
Rash, viremia |
||||
|
Intradermal |
Rash, viremia |
||||
|
Ground squirrels |
Intraperitoneal |
Anorexia, lethargy, viremia, mortality |
|||
|
Intranasal |
Anorexia, lethargy, viremia, mortality |
||||
|
Subcutaneous |
Anorexia, lethargy, viremia, mortality |
||||
|
Dormice |
Intranasal |
Weight loss, viremia, hemorrhage in internal organs |
|||
|
NHP |
Aerosol |
Fever, lymphadenopathy, rash (−/+), bronchopneumonia, viremia |
|||
|
IV |
Fever, lymphadenopathy, vesiculopustular rash, viremia, |
||||
|
Intranasal |
Fever, weight loss, rash, viremia |
||||
|
Intratracheal |
Fever, weight loss, lymphadenopathy, rash, viremia |
||||
|
Intrabronchial |
Fever, rash, viremia |
Small animal models, other than non-human primates (NHP) models, have the advantage that large numbers of animals are available. Furthermore, maintenance costs are lower compared to monkeys. However, small animal models have limitations: Disease pathology, a shortened time course of disease, pharmacokinetic behavior of compounds, and tissue distribution can vary from human conditions. Therefore, animals, which are closely related to humans, and whose physiological and pathological reactions are, therefore, more comparable to humans, are more suitable. NHP are the next relatives to humans, recapitulate human condition as closely as possible, and are, therefore, very appropriate to evaluate new vaccines, treatments, or pathogenesis. Thus, NHP are the gold-standard for MPXV models, as their resemblance to humans allows the best predicative value for effects or side effects of new therapeutics or vaccines in humans.
_
NHP can be infected with MPXV experimentally via different techniques described in the table above.
Figure below shows Monkeypox virus infected Macaca mulatta (M. mulatta) with multifocal severe papular dermatitis.
Figure below shows skin of Monkeypox virus infected M. mulatta with multiple vesicular and erosive to ulcerative skin lesions.
______
______
Analysis of the Monkeypox Virus Genome, 2002 study:
The similar clinical manifestation of monkeypox and smallpox led to a hypothesis that MPV is the evolutionary ancestor of VAR (Fenner, 1977; Marennikova et al., 1972; Noble, 1970). Comparisons of VAR and MPV, based on genomic restriction endonuclease maps (Esposito and Knight, 1985; Mackett and Archard, 1979) or nucleotide sequences of individual viral genes (Douglass and Dumbell, 1992; Esposito and Knight, 1984; Hutin et al., 2001; Mukinda et al., 1997), were interpreted by some as indicating that MPV and VAR evolved independently (Douglass and Dumbell, 1992) and by others that VAR is ancestral to MPV (Bugert and Darai, 2000). Because a reliable answer to this question could be obtained only through comparisons of complete genomes, authors sequenced the DNA of a recent human MPV isolate, strain ZAI-96-I-16 (MPV-ZAI), and concluded that MPV was not the immediate ancestor or descendent of VAR (Shchelkunov et al., 2001). Authors carried out a detailed analysis of the 196,858-bp MPV-ZAI DNA sequence, comprising the entire genome with the exception of part of the covalently closed terminal hairpin loops, and compared this with the corresponding complete genome sequences of VAC (Antoine et al., 1998; Goebel et al., 1990), VAR (Massung et al., 1994; Shchelkunov et al., 1993d, 1995, 2000), and the partial sequence of CPV (Shchelkunov et al., 1998).
Figure above shows comparison of the terminal region of MPV and other orthopoxviruses. Upper line shows the sequenced part of the MPV-ZAI terminal loop. Sequences, which are necessary for telomere resolution, are boxed. Nucleotides, which differ from VAC-WR sequence, are printed in bold font. ZAI, BSH, COP and WR represent the virus strains Zaire-96-I-16, Bangladesh, Copenhagen and Western Reserve, respectively.
The 196,858-bp MPV genome was analyzed with regard to structural features and open reading frames. Each end of the genome contains an identical but oppositely oriented 6379-bp terminal inverted repetition, which similar to that of other orthopoxviruses, includes a putative telomere resolution sequence and short tandem repeats. Computer-assisted analysis was used to identify 190 open reading frames containing 60 amino acid residues. Of these, four were present within the inverted terminal repetition. MPV contained the known essential orthopoxvirus genes but only a subset of the putative immunomodulatory and host range genes. Sequence comparisons confirmed the assignment of MPV as a distinct species of orthopoxvirus that is not a direct ancestor or a direct descendent of variola.
_______
Monkeypox virus genome:
MPXV belongs to the genus Orthopoxvirus with a double-stranded DNA genome, whose clinical manifestations are similar to the smallpox virus. The orthopoxvirus encodes ~200 genes, whereas MPXV encodes about 190 genes which could be assigned to the genome as three parts, a core region, a left arm, and a right arm. Viral replication and assembly genes are encoded by the core region, which is relatively conserved in the genome. The MPXV’s left and right variable regions were known to be more involved in the host range and the pathogenicity of the orthopoxvirus. These regions contain an identical but opposite sequence called inverted terminal repeats (ITR), which is prone to forming hair-pin loop-outs. Although a similar genome composition was observed in other viruses, the variation across different poxviruses is apparent.
MPXV is a linear DNA genome of ≈197 kb and contains ≈190 nonoverlapping ORFs >180 nt long. Like all orthopoxviruses, the central coding region sequence (CRS) at MPXV nucleotide positions ≈56000–120000 is highly conserved and flanked by variable ends that contain inverted terminal repeats (ITRs). (See figure below) VACV homologs to genes found in the terminal ends of the MPXV genome are predominantly involved in immunomodulation, and most are either predicted or known to influence host range determination and pathogenicity. Unlike VARV, which lacks ORFs in the ITR region, MPXV contains at least 4 ORFs in the ITR region.
Poxvirus was considered to undergo high-frequency recombination and a gradual process that starts with nonsense mutations and small indels, which leads to gene gain or loss. Inter-species recombination between cowpox virus and ectromelia virus and intra-species recombination of vaccinia viruses were documented. Recombination within the variola viruses might cause gene loss resulting in a lower virulence. The gain or loss of genetic material had also been reported in the MPXV. The West Africa (WA) lineage and the Congo Basin (CB) lineage differed by about 900 bp in genome length. The WA lineage has a case fatality rate of 3.6%, whereas the CB lineage has a case fatality rate of 10.6%. The MPXV should, in principle, have a low mutation rate based on the stability of the double-strand DNA genome. However, 46 single nucleotide polymorphisms (SNPs) have been observed in the MPXV-2022 strains compared to the NCBI Monkeypox reference sequence NC_063383.
Poxvirus genomes usually consist of a central region with about 100 genes that are mostly involved in creating new copies of the virus, and terminal regions with another 100 or so genes that interact with the host, for instance to counteract immune defenses. Those terminal genes appear to be a key site of evolution. Generalist poxviruses that infect many different hosts, including monkeypox and cowpox, tend to have more genes in the terminal regions, whereas smallpox, which specializes in infecting humans, has many fewer.
Researchers sequenced the 197-kb genome of MPV isolated from a patient during a large human monkeypox outbreak in Zaire in 1996. The nucleotide sequence within the central region of the MPV genome, which encodes essential enzymes and structural proteins, was 96.3% identical with that of variola (smallpox) virus (VAR). In contrast, there were considerable differences between MPV and VAR in the regions encoding virulence and host-range factors near the ends of the genome.
_
Genome evolution:
-The mutation rate of poxviruses is estimated to be between 10-5 and 10-6 mutations per replication site.
– Because these viruses infect immune cells, they are subject to cytoplasmic DNA editing by cellular enzymes such as APOBEC3, which can significantly increase the mutagenicity induced by viral DNA polymerase errors.
-Poxviruses are able to evolve by homologous recombination between coinfecting strains. They can also acquire foreign genes by non-homologous recombination.
_
Genome comparisons of the west and central African strains yielded a set of candidate genes that may be involved in the differentiating clade virulence. The open reading frames in the west African clade contained deletions and fragmentations that contribute to its reduced virulence. Central African monkeypox prevents T-cell receptor-mediated T-cell activation, prohibiting inflammatory cytokine production in human cells derived from previously infected monkeypox patients. Hammarlund et al. observed that T-cell mediated cytokine responses were decreased by 80% in the presence of a low viral load of monkeypox, suggesting that monkeypox may produce a modulator that suppresses host T-cell responses. The monkeypox virus inhibitor of complement enzymes (a gene that inhibits complement enzymes) is absent in west African strains, and it has been implicated as an important immune-modulating factor contributing to the increased virulence of central African strains. Moreover, the central African strain selectively downregulates the host responses, specifically, apoptosis in the host. Transcriptional studies have shown that central African monkeypox appears to selectively silence the transcription of genes involved in host immunity during infection.
_
Clades:
MPXV is a brick-shaped, enveloped, 200-250 nm sized, double-stranded DNA, zoonotic virus of the Orthopoxvirus genus in the Poxviridae family, which includes smallpox (variola), cowpox, and vaccinia viruses. A clade is defined as a group of organisms that can be traced back to common ancestors or a common genetic lineage. Consensus is reached to now refer to the former Congo Basin (Central African) clade as Clade one (I) and the former West African clade as Clade two (II). Additionally, it was agreed that the Clade II consists of two subclades. The proper naming structure will be represented by a Roman numeral for the clade and a lower-case alphanumeric character for the subclades. Thus, the new naming convention comprises Clade I, Clade IIa and Clade IIb, with the latter referring primarily to the group of variants largely circulating in the 2022 global outbreak.
Clades and subclades of Monkeypox virus:
|
Name |
Former names |
Nations |
Case fatality rate (CFR) |
|
|
Clade I |
Congo Basin Central African |
|
~10.6% |
|
|
Clade II |
Clade IIa Now called clade 2 |
West African |
|
~3.6% for clade 2 0.03 % for clade 3 (MPXV 2022) |
|
Clade IIb Now called clade 3 |
2022 monkeypox outbreak worldwide |
|||
______
Relationship between the genome of the virus currently circulating in Europe and the West African strain:
Monkeypox is a DNA virus, which means that it is less likely to mutate than an RNA virus like SARS-CoV-2.
It is actually quite simple to determine whether we are dealing with a West African or Congo Basin strain. We just need to sequence out short sections of its DNA. But given the large size of the viral genome, it takes time and effort to obtain a complete sequence. We need this complete sequence in order to detect differences in sequences more precisely, which would allow us to identify chains of transmission and find out how cases are linked. However, if our experience with SARS-CoV-2 has taught us anything, it’s that a large-scale global effort can be of great help in moving things along.
_
Figure above shows Phylogenetic tree depicting the ‘family’ relationships between the different strains of monkeypox virus responsible for outbreaks.
_
Initial sequencing carried out on samples from a Portuguese and a Belgian patient have shown the genetic proximity of the virus to strains isolated in Nigeria and during the previous out-of-African spread of the virus in 2018, with genomes of the ongoing outbreak being highly similar. This is in favour of a single introduction followed by community spread in Western countries after superspreading events.
More detailed genomic analyses comparing 2022 strains to those of 2018 identified around 50 mutations (tenfold the expected rate of mutations) with a pattern specific of the action of an antiviral enzyme called APOBEC which may reveal the sustained circulation of the virus in a new animal intermediate host, or in humans. This observation, possibly indicating a recent increase in viral circulation in Nigeria, matches the documentation of cases in peri-urban areas of Nigeria like Abuja, together with increased frequencies of overseas exportation of cases.
_______
Genomic analysis of the recent monkeypox outbreak, June 2022:
The Monkeypox virus is the etiological cause of a recent multi-country outbreak, with nearly one hundred distinct cases detected outside the endemic areas of Africa in May 2022. In this article, authors analyze the sequences of two full genomes of Monkeypox virus from Portugal and Belgium, and compare them with all available Monkeypox sequences, annotated by year and geographic origin, as well as related Cowpox and Variola (smallpox) virus sequences. Their results show that the recent outbreak is most likely originating from the West African clade of Monkeypox, with >99% sequence identity with sequences derived from historical and recent cases, dating from 1971 to 2017. Authors analyze specific mutations occurring in viral proteins A42R (for which a crystal structure is available) and H3L (an important epitope in host immune recognition), highlighting specific amino acids varying between the current outbreak, previous Monkeypox and Cowpox sequences and the historical Variola virus. Genome-wide sequence analysis of the recent outbreak and other monkeypox/cowpox/variola viruses shows a very high conservation, with 97.9% (protein-based) and 97.8% (nucleotide-based) sequence identity.
_______
_______
Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus, June 2022, nature medicine:
The largest monkeypox virus (MPXV) outbreak described so far in non-endemic countries was identified in May 2022. In this study, shotgun metagenomics allowed the rapid reconstruction and phylogenomic characterization of the first MPXV outbreak genome sequences, showing that this MPXV belongs to clade 3 and that the outbreak most likely has a single origin. Although 2022 MPXV (lineage B.1) clustered with 2018–2019 cases linked to an endemic country, it segregates in a divergent phylogenetic branch, likely reflecting continuous accelerated evolution. An in-depth mutational analysis suggests the action of host APOBEC3 in viral evolution as well as signs of potential MPXV human adaptation in ongoing microevolution. Authors findings also indicate that genome sequencing may provide resolution to track the spread and transmission of this presumably slow-evolving double-stranded DNA virus.
_
To rapidly get the first insights on phylogenetic placement and evolutionary trends of the 2022 outbreak-causing MPXV, authors focused analysis on a first outbreak-related MPXV genome sequence, publicly released on 20 May 2022 by Portugal, as well as on additional sequences released in the National Center for Biotechnology Information (NCBI) before 27 May 2022, with 15 sequences in total (most of them from Portugal). The rapid integration of the first sequence into the global MPXV genetic diversity (Figure below) confirmed that the 2022 outbreak virus belongs to the MPXV clade 3 (within the formerly designated ‘West African’ clade, which also includes clade 2). MPXV from clades 2 and 3 are most commonly reported from western Cameroon to Sierra Leone and usually carries a <1% case–fatality ratio (CFR), in contrast with viruses from the clade 1 (formerly designated as ‘Central African’ or ‘Congo Basin’ clade), which are considered more virulent with a >10% CFR. All outbreak MPXV strains sequenced so far tightly cluster together (Figure below), suggesting that the ongoing outbreak has a single origin. The 2022 outbreak cluster (lineage B.1) forms a divergent branch descendant from a branch with viruses (lineage A.1) associated with the exportation of MPXV in 2018 and 2019 from an endemic country (Nigeria) to the United Kingdom, Israel and Singapore, with genetic linkage to a large outbreak occurring in Nigeria in 2017–2018 (Figure below). Given these findings and the MPXV historical epidemiology (rare cases in non-endemic countries), it is likely that the emergence of the 2022 outbreak resulted from importation(s) of this MPXV from an endemic country, with the MPXV detected in 2022 potentially representing the continuous circulation and evolution of the virus that caused the 2017–2018 Nigeria outbreak. The recent release of an MPXV sequence from a 2021 travel-associated case from Nigeria to the United States (USA_2021_MD; accession no. ON676708) phylogenetically placed between 2018–2019 and 2022 sequences (Figure below) is aligned with such hypothesis.
_
Figure below shows Phylogenetic analysis of MPXV viral sequences associated with the 2022 worldwide outbreak.
-a, MPXV global phylogeny showing that the 2022 outbreak cluster (lineage B.1) belongs to clade 3. Clade and lineage are designated according to the nomenclature proposed by Happi et al.
-b, Genetic diversity within the outbreak cluster, including the 15 sequences analyzed in this study (released in the NCBI before 27 May 2022). The deletion symbol (Δ) denotes a large deletion (11,335–12,247 in the MPXV-UK_P2-010 gene) shared by sequences segregating in a small subcluster.
-c, Outbreak phylogenetic tree updated with sequences available in the NCBI as of 15 June 2022 (provided during revision for more updated contextualization).
_
Notably, the 2022 MPXV diverges from the related 2018–2019 viruses by a mean of 50 single-nucleotide polymorphisms (SNPs), which is far more (roughly 6–12-fold more) than one would expect considering previous estimates of the substitution rate for Orthopoxviruses (1–2 substitutions per genome per year). Such a divergent branch might represent accelerated evolution. Of note, among the 46 SNPs (24 non-synonymous, 18 synonymous and four intergenic) separating the 2022 MPXV outbreak virus from the reference sequence (MPXV-UK_P2, 2018; GenBank accession no. MT903344.1), three amino acid changes (D209N, P722S and M1741I) occurred in the immunogenic surface glycoprotein B21 (MPXV-UK_P2-182). Serological studies have previously indicated that the monkeypox B21 protein might be an important antibody target with several key immunodominant epitopes. Fine inspection of the mutation profile of those 46 SNPs further revealed a strong mutational bias, with 26 (14 non-synonymous, ten synonymous and two intergenic) and 15 (nine non-synonymous and 16 synonymous) being GA > AA and TC > TT nucleotide replacements, respectively. A tool was built to rapidly screen these and other mutation profiles. The observed (hyper)mutation signature might suggest the potential action of apolipoprotein B mRNA-editing catalytic polypeptide-like 3 (APOBEC3) enzymes in the viral genome editing. Also, MPXV is A:T rich, so a mutation bias leading to further incorporation of A/T suggests the action of a non-random mutational driver, such as APOBEC3. In fact, APOBEC3 enzymes can be upregulated in response to viral infection, being capable of inhibiting a wide range of viruses by introducing mutations through deaminase and deaminase-independent mechanisms. In some circumstances (for example, lower levels of deamination), APOBEC3-mediating mutations might not completely disrupt the virus, thus increasing the likelihood of producing hyper-mutated (but viable) variants with altered characteristics (for example, HIV immune escape variants). The repertoire and level of APOBEC3 enzymes depend on the host species/tissue, and different enzymes display different preferences for the nucleotide or motif (such as dinucleotides or tetranucleotides) to be mutated. For instance, the GA > AA and TC > TT nucleotide replacements observed in the 2022 outbreak MPXV were also found to be the preferred mutational pattern of human APOBEC3A enzymes (expressed in keratinocytes and skin) during genetic editing of human papillomavirus (HPV) in HPV1a plantar warts and HPV16 pre-cancerous cervical biopsies. Whether the excess of mutations seen in the 2022 MPXV is a direct consequence of APOBEC3-mediated genome editing in the human host cannot be discerned at this stage. Also, the putative APOBEC3 effect on MPXV evolution augments the uncertainty regarding the 2022 outbreak origins and introductions, in addition to the complexity of the epidemiological context. This raises the need for future studies focusing on the weight of APOBEC3 in MPXV diversification. In particular, functional studies assessing whether this mutational driver triggers MPXV adaptive evolution toward altered phenotypic features, such as enhanced transmissibility, are warranted.
_
In summary, authors genomic and phylogenomic data provide insights into the evolutionary trajectory of the 2022 MPXV outbreak strain and shed light on potential mechanisms and targets of human adaptation. The observed accelerated evolution of this human MPXV, potentially driven by the APOBEC3 action, suggests that viral genome sequencing might provide sufficient resolution to track the transmission dynamics and outbreak spread, which seemed to be challenging for a presumably slow-evolving double-stranded DNA virus. Together with the adopted strategy of real-time data sharing, this study may help guide novel outbreak control measures and subsequent research directions.
______
______
MPXV mutations:
RNA viruses mutate faster than DNA viruses, single-stranded viruses mutate faster than double-strand virus, and genome size appears to correlate negatively with mutation rate. Viral mutation rates are modulated at different levels, including polymerase fidelity, sequence context, template secondary structure, cellular microenvironment, replication mechanisms, proofreading, and access to post-replicative repair. Additionally, massive numbers of mutations can be introduced by some virus-encoded diversity-generating elements, as well as by host-encoded cytidine/adenine deaminases.
_
The monkeypox virus spreading across the US, Europe and the UK; is mutating surprisingly fast, according to a study conducted by Portuguese researchers and published in the journal Nature Medicine [vide supra]. Researchers found the current strain diverges from the original strain by 50 single nucleotide polymorphisms (SNPs), and several mutations made the virus more transmissible. The strain belongs to clade 3 of the West African strain of the virus, which is less fatal than the Congo Basin clade. Monkeypox outbreaks from clade 3 are typically reported from western Cameroon to Sierra Leone and usually carry a less than 1% case-fatality rate. The authors said the outbreak was likely not caused by undetected silent spread, or from an animal-to-human crossover event. Instead, current data points for a scenario of more than one introduction from a single origin, with superspreader event(s) (e.g., saunas used for sexual encounters) and travel abroad likely triggering the rapid worldwide dissemination. The authors also said the 50 SNPs that diverge from the original strain are far more (roughly sixfold to 12-fold more) than one would expect considering previous estimates of the substitution rate for orthopoxviruses, which typically have 1 to 2 substitutions per genome per year.
_
Monkeypox is a brick-shaped virus carrying a double-strand of DNA. This is good news because it means the virus is relatively stable and less likely to mutate into more lethal or more transmissible variants. The Sars-CoV-2 virus that causes Covid-19 contains genetic material made from a single stranded RNA. RNA viruses mutate very effectively – they’re diabolical. Monkeypox is a double-stranded DNA zoonotic virus. DNA viruses mutate slower than RNA viruses. The monkeypox virus is made of DNA, which tends to mutate less often than RNA in viruses such as SARS-CoV-2, which causes COVID. Unlike SARS-CoV-2, a rapidly evolving RNA virus whose variants have regularly eluded immunity from vaccines and prior infection, monkeypox is caused by a relatively large DNA virus. DNA viruses are better at detecting and repairing mutations than RNA viruses, which means it’s unlikely that the monkeypox virus has suddenly mutated to become adept at human-to-human transmission. Viral mutation rates are not merely caused by polymerase errors, but also by the ability of a virus to correct DNA mismatches by proofreading and/or post-replicative repair. The replicative life cycles of many DNA viruses have been shown to engage components of the host DNA damage and repair machinery. On average, poxviruses—a family that includes orthopoxviruses such as monkeypox and smallpox—tend to mutate once per year. DNA viruses typically don’t mutate that quickly, even with the 50 mutations we’ve seen in monkeypox, we don’t see mutations having an impact on the severity of the disease. Part of the reason for this may be because more than half of the mutations seen in the virus over the past four years are considered to be “silent” – meaning they don’t change any of the viral proteins it needs to infect cells and evade the immune system. Even so, some researchers have expressed surprise at how many mutations the virus has accumulated in the past three or four years. The tests showed that the virus had mutated 50 times — up to 12 times more than they would have expected — since that previous outbreak in 2018. This data completely challenges what is known about the mutation rate of monkeypox, said study author Joao Paulo Gomes, a researcher at Portugal’s National Health Institute.
_
The World Health Organisation (WHO) said that studies are underway to see if mutations are the cause behind rapid spread of Monkeypox.
João Paulo Gomes, head of the Genomics & Bioinformatics Unit at the National Institute of Health in Portugal who co-authored the nature medicine study, said it is not known whether the mutations have contributed to increased transmissibility between people. The researchers say their work shows that viral genome sequencing of monkeypox might be precise enough to track the spread of the current outbreak and see how transmission might be changing. This, in turn, would allow decision makers to introduce measures to curb monkeypox spread. Vaccines are already available.
Grant McFadden, PhD, a monkeypox expert at Arizona State University, who was not involved with the nature medicine paper, noted that it’s “hard to say” whether any of the mutations have changed viral behavior. “I don’t know how quickly we will know whether or not the fundamentals of the disease have changed, but the disease characteristics look a little milder than what we saw in West Africa, in terms of the number of lesions and the way they’re distributed,” McFadden said. “The changes in nucleotides may just be a collection of mutations that have arisen over a longer time than we would have predicted,” he added. “And now that it’s in a new host, the selection pressures are a bit different on the virus than they were in rodents, and that may be exerting a selective pressure to acquire more alleles. But we don’t know what the functional significance of any of the changes are.” Nonetheless, he said, it will be important to do that work.
Stephen Goldstein, PhD, an evolutionary virologist at the University of Utah in Salt Lake City, who wasn’t involved with the paper said that the continued adaptive changes are still speculative. “Without understanding the functional relevance of any of these mutations, it’s pretty difficult to make that argument,” Goldstein said. “We know so little about the transmission of these viruses,” he continued. “We have not observed smallpox transmission in the era of modern genomics, and we don’t have much more with respect to monkeypox. I just don’t think we understand enough about the transmission of these viruses to link any kind of genetic changes to different transmission dynamics.”
Another notable finding from the study is that most of the mutations are of a particular type that could have been introduced by a human defence mechanism called APOBEC3, which works by introducing mutations to viruses in order to stop them from working properly, said Pam Vallely, professor of medical virology at the University of Manchester. “However, in this case the mutations are apparently not making the virus non-viable and may be helping it to adapt to human-human transmission,” Vallely, who was not involved in the study said. “This is just a theory that fits the current evidence, and lots more work is going to be needed to see if this is what is really happening. I don’t think we can say the mutations have made it more contagious, but maybe they have made it better adapted to humans.”
_
The current monkeypox outbreak is moving faster than any in recent history, but Washington State University virologist Heather Koehler does not see a reason for the rapid spread in the virus itself. An expert in virus-host interactions, Koehler is researching the monkeypox strain that is currently circulating, working to understand the virus’ genetics by studying its DNA sequence and protein structures. “There are no new, large mutations that could account for the change in transmission,” said Koehler. “I’m not an epidemiologist; I don’t want to predict how or where we should be vaccinating, but there’s something that’s not lining up.” Koehler said it could be containment strategies are not being implemented appropriately or vigorously enough, or that infected people are not recognizing the symptoms and seeking treatment in time. Past outbreaks have been relatively small and quickly contained. This time, however, the virus has spread to more than 16,500 cases as of late July, mostly in countries that historically have not had many reported cases of the virus. Yet, little has changed in the monkeypox genome, said Koehler. While it is possible that a small change could be driving the spread, researchers have found no obvious changes that would seem to have this large of an impact. Analysis has shown there are less than 100 out of over 197,000 nucleotides, a type of DNA base pair, that are different in the current virus than the one from a smaller 2017 outbreak in the U.K. “It’s pretty much the same virus,” she said. “This is something that’s spreading faster than any virologist would have predicted based on prior outbreaks that were rapidly contained,” she said. “The widespread nature of this outbreak is unprecedented.” There have been many situations in the past where people traveling to endemic regions come back with the disease, but through isolation and vaccination of people’s contacts it was contained. One problem could be that people are not identifying as having been exposed to monkeypox, Koehler said. The disease has an incubation period, but people are not usually contagious until symptoms appear. Symptoms include, fever, muscle fatigue and severe headache, but skin lesions are a tell-tale sign of the virus. “The lesions are pretty characteristic or distinct. It’s not like there’s much room for interpretation,” Koehler said. For instance, monkeypox lesions can appear anywhere on the body including the palms of the hands and the bottoms of the feet—unlike lesions from other diseases such as herpes or chicken pox.
Koehler also fears that misrepresentation of the disease as spreading primarily by men having sex with men is making the problem worse.
_______
Family tree of viral genomes:
Figure above shows MPXV family tree with sequenced genomes. Getting high-quality sequences is harder and more expensive than it is for SARS-CoV-2, not just because the monkeypox genome is so vast but also because crucial regions near its ends can be full of repetitions or deletions that can trip up researchers when they assemble sequences.
What mutations can do is give researchers a clock to determine how long ago monkeypox began to circulate in humans. Comparing genomes from different time points suggests the virus is currently adding about six APOBEC3-related changes per year. A family tree of virus genomes from the current outbreak suggests viruses circulating in Nigeria in late 2017 already carried nine APOBEC3-type mutations, which would mean the virus jumped into humans sometime in early 2016, about a year and a half before the outbreak was recognized in Nigeria (see figure above). The analysis also suggests the virus has been continuously circulating in humans since then.
Mutations seen in monkeypox samples allow researchers to trace the roots of the current outbreak, which began in Nigeria. The virus was exported to other countries several times and eventually spread around the world. Because a human protein, APOBEC3, likely introduced many of the mutations, they can also show how long the virus has been spreading in people.
Evolutionary virologists have concentrated on the influenza virus, HIV, and other small viruses whose genomes consist of RNA. Poxviruses, by contrast, are made of DNA, and are much larger and more complex. With roughly 200,000 nucleotides and 200 genes, the monkeypox genome is more than 20 times the size of HIV’s. It’s not clear what many of those genes do, let alone how they interact with each other or how changes in any of them might affect their impact on humans.
One thing is clear, however: Poxviruses mutate slowly compared with RNA viruses. Their genomes are pretty stable and don’t change quickly. And although poxviruses have ways of tricking the immune system, they don’t change their surface proteins to escape immunity, as SARS-CoV-2 does. An infection with smallpox, if you survived it, provided immunity for life, and the vaccines remained very effective right until the end of the eradication campaign. That, too, offers some hope that monkeypox won’t transform into a bigger threat.
_______
_______
Genomic annotation and molecular evolution of monkeypox virus outbreak in 2022, July 2022 study:
Monkeypox virus (MPXV) has generally circulated in West and Central Africa since its emergence. Recently, sporadic MPXV infections in several nonendemic countries have attracted widespread attention. Here, authors conducted a systematic analysis of the recent outbreak of MPXV-2022, including its genomic annotation and molecular evolution. The phylogenetic analysis indicated that the MPXV-2022 strains belong to the same lineage of the MPXV strain isolated in 2018. However, compared with the MPXV strain in 2018, in total 46 new consensus mutations were observed in the MPXV-2022 strains, including 24 nonsynonymous mutations. By assigning mutations to 187 proteins encoded by the MPXV genome, authors found that 10 proteins in the MPXV are more prone to mutation, including D2L-like, OPG023, OPG047, OPG071, OPG105, OPG109, A27L-like, OPG153, OPG188, and OPG210 proteins. In the MPXV-2022 strains, four and three nucleotide substitutions are observed in OPG105 and OPG210, respectively.
_
There are increasing concerns about monkeypox’s global distribution among human populations. Although the human-to-human transmissions of the WA lineage have been observed previously, the recent outbreak of novel MPXV sporadically across multiple nonendemic countries has raised significant concerns. Authors data suggest that 2022 outbreak strains have most likely been derived from WA-clade 3. However, it is still unclear if the MPXV-2022 outbreak strain originated from humans or other hosts.
_
MPXV is a double-stranded DNA virus with a low mutation frequency. However, 46 common mutations among the MPXV-2022 outbreak strains were identified. Although the mechanisms responsible for generating these mutations are unclear, recent studies pointed out the possible contribution of the host APOBEC3-like deaminase to many of these SNPs. Although authors showed that 41/46 mutations could be classified as APOBEC3-like driving mutations, how a host antiviral gene could lead to mutations accumulated in the outbreak of MPXV remains an open question. Therefore, the continued evolution of the transmission ability of the MPXV requires further attention.
_
The present mutations in the MPXV-2022 strains affected at least 20 proteins. It is crucial to determine whether these mutations contribute to the transmission or pathogenesis of the virus or help the virus evade host immunity. The results showed that OPG105 and OPG210 have nucleotide substitutions in the 2022 outbreak strain. Both proteins have been mutated in multiple lineages of the MPXV. Previous experimental studies have shown that the homologous protein L6R of OPG105 has the epitopes conserved among vaccinia and variola viruses. And the homologous protein of OPG210 was also shown to be associated with monkeypox-specific antibody epitopes. More investigation is needed to further evaluate the impact of these two protein mutations on viral function.
_
The smallpox vaccine was considered the most effective way to fight against MPXV. The US CDC has suggested the FDA-approved MVA-BN as a potential vaccine strain for MPXV. Authors found that the vaccine strain differed by 10 proteins by comparing the protein composition and identifying sequence identities between the vaccine strain (MVA-BN) and the MPXV-2022 consensus sequence. Together, this study provided an initial assessment of the genetic composition of the 2022 outbreak and showed comprehensive mutation profiling that may serve as a reference for future studies on the transmission and pathogenesis of the current strains.
_____
_____
UAMS Researchers find Changes in Monkeypox Genome that may explain its Recent Rapid Spread:
Comparison of Monkeypox virus genomes from the 2017 Nigeria outbreak and the 2022 outbreak, September 2022
Aims:
The current Monkeypox virus outbreak is not only the largest known outbreak to date caused by a strain belonging of the West-African clade, but also results in remarkably different clinical and epidemiological features compared to previous outbreaks of this virus. Here, authors consider the possibility that mutations in the viral genome may be responsible for its changed characteristics.
Methods and Results:
Six genome sequences of isolates from the current outbreak were compared to five genomes of isolates from the 2017 outbreak in Nigeria and to two historic genomes, all belonging to the West-African clade. Authors report differences that are consistently present in the 2022 isolates but not in the others. Although some variation in repeat units was observed, only two were consistently found in the 2022 genomes only, and these were located in intergenic regions. A total of 55 single nucleotide polymorphisms (SNPs) were consistently present in the 2022 isolates compared to the 2017 isolates. Of these, 25 caused an amino acid substitution in a predicted protein.
Conclusions:
The nature of the substitution and the annotation of the affected protein identified potential candidates that might affect the virulence of the virus. These included the viral DNA helicase and transcription factors.
Discussion:
The rapid spread of monkeypox is unlike the virus’ past outbreaks and may be a result of genetic mutations identified by University of Arkansas for Medical Sciences (UAMS) researchers.
The team compared the genomes of the 2022 virus to monkeypox genomes from a 2017 outbreak in Nigeria, plus sequenced genomes from localized outbreaks in 1965 and 1970. None of the previous monkeypox variants spread beyond their place of origin in Africa.
The UAMS team’s bioninformatics analysis using advanced genomic sequencing methods revealed 25 mutations, 14 of which appear to change protein function and bear further research, said Ussery, a professor in the College of Medicine Department of Biomedical Informatics and director of the Arkansas Center for Genomic and Epidemiology Medicine at UAMS. “At least one of the differences we found could be responsible for why the current virus is causing a pandemic while past strains of monkeypox viruses did not,” he said.
The team’s article notes that the current monkeypox virus outbreak is not only the largest known outbreak to date, the infections result in much different clinical and epidemiological features compared to previous outbreaks.
______
______
DNA levels of monkeypox correlate with virus infectivity:
MPXV is a double-stranded deoxyribonucleic acid (DNA) zoonotic virus that belongs to the family Poxviridae of the genus Orthopoxvirus. MPXV human infection was reported in central Africa for the first time in 1970.
Subsequently, several sporadic outbreaks were reported in Central and West Africa. In 2003, MPXV outbreaks outside Africa were recorded for the first time and were due to the import of exotic animals from Africa.
During the current outbreak, individuals have reported skin/mucosal lesions of the genital, perianal, and/or oropharyngeal regions. Polymerase chain reaction (PCR) analysis has been used to detect MPXV infection.
Since the presence of viral DNA in a clinical specimen does not imply infectivity, scientists have tested the clinical samples of MPXV patients to determine the relationship between the MPXV DNA copies and infectious status in a recent Euro Surveillance study. The number of MPXV DNA copies was measured through PCR (quantitation cycle (Cq) value), whereas infectious status was measured in plaque formation unit (pfu)/mL using a plaque assay. The threshold PCR value that indicated MPXV infection was determined in this study.
A strong and negative correlation between PCR results related to MPXV oropharyngeal, rectal swabs, dermal lesions, and virus infectivity on cultured cells indicated higher infectivity in specimens with low Cq values.
In the evaluation of paired oropharynx and dermal lesion samples of the same patients, most dermal lesion samples were associated with a higher viral load and low Cq values as compared to oropharynx lesion specimens. This suggests that dermal lesions present a higher infectivity risk. A Cq value greater than or equal to 35 indicated negative or minor MPXV infection. This finding is consistent with a previous study that reported a Cq value of 29 in semen samples that indicated the person had successfully recovered from MPXV.
Although the present study estimated a ratio of 172 DNA copies/pfu, a previous study contradicted this finding and reported a ratio of 10-100 DNA copies/pfu. This contradictory finding was attributed to the fact that the current study estimated the presence of viral DNA rather than infectious particles. Additionally, as compared to viruses originating from culture, the viruses isolated from clinical samples had decreased infectivity towards cell lines.
Storing the samples for up to 48 hours prior to analysis might influence the result.
Taken together, the study findings indicate a strong correlation between MPXV Cq values and virus infectivity. It further suggested that a Cq value greater than or equal to 35 corresponded to minor or non-infectivity.
The researchers of the current study recommend that infectiousness must be evaluated based on overall clinical manifestation, such as lesion location and stage. These findings may be used to develop guidelines and protective measures for MPXV-infected patients and close contacts.
_____
_____
Modeling study shows potential growth of outbreak:
In another study, this one published in The Lancet Microbe, scientists use modeling to predict what will happen in nonendemic countries if public health measures to curb ongoing outbreaks are not taken. They predict that, without interventions, the introduction of 3 cases in a country could cause 18 secondary cases, 30 could cause 118 secondary cases, and 300 cases could cause 402 secondary cases. Contact tracing and surveillance, isolation of symptomatic cases, and ring vaccination would reduce the number of secondary cases by up to 86.1% and the duration of the outbreak by up to 75.7%, the authors conclude. The authors also said the outbreak is a moderate international concern.
_______
_______
Section-4
Introduction to human monkeypox disease:
Orthopoxviruses, including smallpox, cowpox, and monkeypox, cause disease in both humans and other animals. Many people are familiar with smallpox, a disease caused by the variola virus that killed approximately 400 million people in the 20th century. The name “smallpox” originated as a description of the disease’s hallmark skin lesions, which were smaller than those of “great-pox”, or syphilis. The discovery of a smallpox vaccine eventually led to global eradication of the disease. In the United States, routine vaccination against smallpox stopped in 1972, and the World Health Organization officially declared that smallpox was eradicated worldwide in May 1980. Currently, both the United States and Russia possess legal sources of the variola virus, and United States government has a stockpile of smallpox vaccination available in the event that the virus is ever used as a bioterrorism agent.
_
Smallpox eradication, coordinated by the WHO and certified 40 years ago, led to the cessation of routine smallpox vaccination in most countries. It is estimated that over 70% of the world’s population is no longer protected against smallpox, and through cross-immunity, to closely related orthopox viruses such as monkeypox. Monkeypox is now a re-emerging disease. Monkeypox is endemic in as yet unconfirmed animal reservoirs in sub-Saharan Africa, while its human epidemiology appears to be changing. Monkeypox in small animals imported from Ghana as exotic pets was at the origin of an outbreak of human monkeypox in the USA in 2003. Travellers infected in Nigeria were at the origin of monkeypox cases in the UK in 2018 and 2019, Israel in 2018 and Singapore in 2019. Together with sporadic reports of human infections with other orthopox viruses, these facts invite speculation that emergent or re-emergent human monkeypox might fill the epidemiological niche vacated by smallpox.
_
Monkeypox, was first discovered in 1958. Despite its name, monkeypox can also be carried by rats, squirrels, and shrews, and these rodents may play a significant role in transmitting the disease to humans. Although monkeypox was originally described in monkeys in 1958, rodents are likely to be the natural reservoir of this virus with primates—including humans—being incidental hosts. The first documented case of monkeypox in humans occurred in the Democratic Republic of the Congo in September 1970, when a 9-month-old child suspected of having smallpox was actually discovered to be infected with monkeypox instead. Monkeypox is endemic to Central Africa and West Africa, where cases likely spread from local wildlife species to humans. There are two distinct lineages of monkeypox virus, with the western Africa strain generally causing less severe disease than the central African—also known as the Congo Basin—strain (i.e., 1–5% vs 10% case fatality).
_
Monkeypox was first recognized by Von Magnus in Copenhagen in 1958 as an exanthem of primates in captivity. Two outbreaks of a nonfatal pox-like disease were initially observed in two shipments of cynomolgus monkeys arriving in Copenhagen in 1958. An orthopoxvirus was subsequently isolated on the chorioallantoic membrane, producing grayish pocks with a hemorrhagic center after three days of incubation at 35 ºC (easily distinguishable from the larger hemorrhagic pocks of cowpox virus and opaque white pocks of variola virus). After the virus has been given recognition as a standalone species of the genus Orthopoxvirus, it was named monkey poxvirus; in addition, the Copenhagen strain is still regarded as a reference strain. After this discovery, in the next ten years, a total of nine monkeypox outbreaks were seen in captive monkey colonies throughout Europe and the US.
In most of these outbreaks, no clinical signs were typically detected until the rash appeared and developed into papules on the face, trunk, palms, and soles. The papules first become vesicular, then pustular, before scabs form 10 days after the onset of rash. The severity of clinical presentation varied among different species, with orangutans being particularly susceptible.
_
The disease was also seen in other captive animals including primates in zoos and animal import centers. Particular attention was focused on it in 1970 when smallpox surveillance activities in Africa revealed cases of human monkeypox, clinically indistinguishable from smallpox, particularly in Zaire (now the Democratic Republic of the Congo [DRC]). Serosurveys and virologic investigations in the 1980s in the DRC by the WHO indicated that monkeys are sporadically infected, as are humans; three-fourths of cases, mainly in children younger than 15 years, resulted from animal contact; vaccinia vaccination has about 85% protective efficacy; monkeypox virus probably has a broad host range including squirrels (Funisciurus spp. andHeliosciurus spp.); and human monkeypox has a secondary attack rate of 9% among unvaccinated contacts within households (i.e., it is much less transmissible than smallpox). Since 1970 the disease has been seen in the DRC, Liberia, Ivory Coast, Sierra Leone, Nigeria, Benin, Cameroon, Gabon, Central African Republic, and South Sudan; most cases have been in the DRC, which in 1980 had a population of about 30 million (338 cases were discovered prospectively during WHO-intensified monkeypox surveillance in Zaire in 1981–86). Human monkeypox has continued to be reported from the DRC, mainly in children younger than 15 years. On the basis of reported monkeypox onset dates in a largely retrospective study complicated by a concurrent outbreak of chickenpox, about 250 sero-substantiated cases of monkeypox occurred among 0.5 million people in 78 villages, from February 1996 to October 1997. About three-fourths of the cases appeared to result from human-to-human transmission; however, the secondary attack rate of 8% among unvaccinated contacts within households appeared to be about the same as in the 1981–86 surveillance.
_
Sporadic outbreaks continue to occur and cause concern, but the most detailed clinical, epidemiologic, and ecologic information about virology laboratory–confirmed disease in Africa was obtained before 1988. Initial animal surveys in Zaire detected monkeypox-specific antibodies in 85 of 347 squirrels (25%) sampled but from none of 233 terrestrial rodents. Monkeypox-specific antibody has been detected in very few monkeys, which, similar to humans, are probably only occasional hosts. Subsequent work in the DRC found evidence of orthopoxvirus seroreactivity in some small terrestrial mammals tested including Gambian rats(Cricetomys emini) and elephant shrews(Petrodromus tetradactylus). Studies in the 1980s that used direct virus sampling of trapped animals revealed virus in only one Funisciurus species.
_
Human infections originate from contact with an infected animal or human. Subsequent human-to-human transmission can occur through large respiratory droplets or contact with a skin lesion (including through fomites). The incubation period ranges from 5 days to 21 days with shorter incubation periods occurring with more invasive exposure (e.g., bite vs scratch vs light touch). Symptomatic cases are usually self-limited (i.e., resolved by themselves without treatment) and the main symptoms are fever, chills, and malaise that precede the development of a centrifugal rash involving the palms of the hands and soles of the feet. Although monkeypox has a distinctive rash that appears on the palms of the hands and soles of the feet, it is frequently confused with chickenpox. The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Although fever can last for up to a week, the rash evolves from maculopapular to vesicular to pustular to crusting over a period of 2–4 weeks. Unlike smallpox, typical monkeypox infections are usually characterised by lymphadenopathy. Notably, it has become clear in the current outbreak that monkeypox can also present atypically without fever or rash, and with only one to a few skin lesions that can be asynchronous in appearance. Often these lesions are only present on the genitalia, oral mucosa, or rectal mucosa consistent with the points of skin contact in sexual settings. This association with sexual contact has led to misdiagnosed cases of monkeypox, and wrong or delayed treatment, and new clinical syndromes associated with monkeypox such as urethritis, rectal pain, and urinary retention. Many men in the current outbreak have lesions on their genitalia, but those can be mistaken for sexually transmitted infections such as syphilis, gonorrhea and chlamydia. To untrained eyes, monkeypox could easily be mistaken for other dermatological diagnoses within sexual health clinics or primary care (e.g., chickenpox, herpes zoster, herpes simplex, syphilitic chancre, gonorrhea, or molluscum contagiosum). An emergency drill in the USA showed that six of 13 patients with simulated smallpox were discharged with diagnoses that included West Nile virus and upper respiratory infections. The consequences of monkeypox infection in pregnant women are unclear, though monkeypox virus can cross the placenta. Additionally, initial reports from Germany and Italy of monkeypox-positive PCR assays of semen, followed by a report from August, 2022 of infectious virus isolated from semen have surfaced raising concerns that monkeypox virus could also be sexually transmitted.
_
Monkeypox outbreaks have occurred episodically in parts of Africa where the virus has become endemic, most notably in the Democratic Republic of the Congo, Nigeria in 2017, and in other parts of central and western Africa over the past 5 years. However, the number of infections occurring in endemic parts of Africa as well as outbreaks occurring in non-endemic parts of the world have been increasing. This rise could be related to a combination of factors, including the increasing number of people with no orthopoxvirus cross-protection due to the cessation of smallpox vaccination after eradication in 1980, and the growing ease and rapidity of global travel that allows previously isolated clusters to quickly become global epidemics. For example, in the 2003 US outbreak there were 71 monkeypox cases stemming from the sale of pet prairie dogs that became infected through contact with illegally imported and infected rodents from Africa in a shared distribution center. Lastly, the effect of genetic changes in the virus on transmissibility needs to be evaluated.
_
Monkeypox has been reported as endemic in several other central and western African countries such as: Cameroon, Central African Republic, Cote d’Ivoire, Democratic Republic of the Congo, Gabon, Liberia, Nigeria, Republic of the Congo, and Sierra Leone. This has been also reported in certain non-endemic countries e.g., USA, UK Belgium, France, Germany, Italy, Netherlands, Portugal, Spain, Sweden, Australia, Canada, Austria, Canary Islands, Israel and Switzerland. According to World Health Organization (WHO), in the present series of outbreaks being reported, this is the first time that chains of transmission are reported in Europe without known epidemiological links to West or Central Africa. Beginning in May, 2022, a large, multinational outbreak was identified, which at the time of writing this article includes more than 70,000 PCR-confirmed cases across 105 countries, predominantly in networks of men who have sex with men (MSM). Criteria such as little to no population-level immunity and evidence of infections across WHO regions, technically fit the definition of a pandemic. Cases of monkeypox (MPX) acquired in the EU have recently been reported in nine EU Member States (Austria, Belgium, France, Germany, Italy, Portugal, Spain, Sweden, and the Netherlands). Monkeypox (MPX) does not spread easily between people. Human-to-human transmission occurs through close contact with infectious material from skin lesions of an infected person, through respiratory droplets in prolonged face-to-face contact, and through fomites. The predominance, in the current outbreak, of diagnosed human MPX cases among men having sex with men (MSM), and the nature of the presenting lesions in some cases, suggest transmission occurred during sexual intercourse. Although monkeypox isn’t known to be sexually transmitted, sexual activity certainly constitutes close contact. The most likely explanation for this unexpected pattern of transmission is that the virus was coincidentally introduced into an MSM community, and has continued circulating there. It is possible the number of onward transmissions per case is artificially elevated because of the settings in which the virus is currently being transmitted (high rates of close contact).
_______
Since it was first identified in a colony of monkeys in Copenhagen in 1958, monkeypox has been largely overlooked by the Western world. An infectious poxvirus that causes fever, chills and rashes, the disease is endemic, or consistently regionally present, in ten African countries. Until recently, however, it was rarely found in Europe and the Americas—a trend that has, historically, led Western public health officials to disregard its spread elsewhere. “It’s a phenomenon of ‘not in my backyard,’” says Martin Hirsch, editor-in-chief of the Journal of Infectious Diseases and an immunologist at Harvard University. “There’s not much interest in Western health groups about something that’s only circulating in Africa.”
The scientific community is hesitant to the importance of MPX which can be demonstrated by a limited number of research articles in the biomedical literature. The biggest number of articles was published in a year when MPX was reported outside of the African continent for the first time in history. In the past decades, we have seen a similar reluctance to research and control of other pathogens with epidemic potential (Zika and Ebola as recent examples) which were geographically limited to the Southern Hemisphere. Monkeypox outbreaks are rarely reported, badly managed and little described leading to an incomplete picture of the disease’s importance. MPX is the next most pathogenic poxvirus disease after smallpox but never received appropriate attention to prevent it to become an epidemic.
Literature on monkeypox transmission is severely lacking. In a PubMed search for “monkeypox AND transmission” that yielded only 224 manuscripts, published from 1962 to 2022, and more reviews were published on the subject than original research studies in humans. 15 of the studies involving humans investigated transmission, the largest study being that of 2510 contacts of 214 patients with monkeypox in Zaire from 1980 to 1984; here the investigators reported infected cases without exanthema. In 2005, smallpox-vaccinated individuals with monkeypox breakthrough infection were reported to have few or no skin lesions. Prolonged upper respiratory tract viral DNA shedding after skin lesion resolution was also seen in patients with monkeypox admitted to dedicated high consequence infectious diseases centers between 2018 and 2021.
Early on in the COVID-19 pandemic, SARS-CoV-2 was assumed to spread through droplets from symptomatic patients, whereas actually aerosol (long distance) transmission was overwhelming, with asymptomatic or pre-symptomatic patients dominating transmission chains. Although assumptions of asymptomatic and airborne transmission of monkeypox might be premature, in the context of yet another outbreak rapidly spreading across the world, the possibility of such transmission modalities must be considered. We must take a precautionary infection control approach to control the spread of the virus while completing urgent research to understand better the human-to-human monkeypox transmission process.
_______
_______
Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo, a 2010 study:
Studies on the burden of human monkeypox in the Democratic Republic of the Congo (DRC) were last conducted from 1981 to 1986. Since then, the population that is immunologically naïve to orthopoxviruses has increased significantly due to cessation of mass smallpox vaccination campaigns. To assess the current risk of infection, authors analyzed human monkeypox incidence trends in a monkeypox-enzootic region. Active, population-based surveillance was conducted in nine health zones in central DRC. Epidemiologic data and biological samples were obtained from suspected cases. Cumulative incidence (per 10,000 population) and major determinants of infection were compared with data from active surveillance in similar regions from 1981 to 1986. Between November 2005 and November 2007, 760 laboratory-confirmed human monkeypox cases were identified in participating health zones. The average annual cumulative incidence across zones was 5.53 per 10,000 (2.18–14.42). Factors associated with increased risk of infection included: living in forested areas, male gender, age < 15, and no prior smallpox vaccination. Vaccinated persons had a 5.2-fold lower risk of monkeypox than unvaccinated persons (0.78 vs. 4.05 per 10,000). Comparison of active surveillance data in the same health zone from the 1980s (0.72 per 10,000) and 2006–07 (14.42 per 10,000) suggests a 20-fold increase in human monkeypox incidence. Thirty years after mass smallpox vaccination campaigns ceased, human monkeypox incidence has dramatically increased in rural DRC. Improved surveillance and epidemiological analysis is needed to better assess the public health burden and develop strategies for reducing the risk of wider spread of infection.
_______
_______
Disease characteristics:
Monkeypox (MPX) is a zoonotic disease and is currently the most prevalent orthopoxvirus infection in humans after the eradication of smallpox and the cessation of universal smallpox vaccination. Human MPX cases are increasingly reported in several African countries after its first identification as a human pathogen in the DRC in 1970, due to a combination of factors including both increased exposure (deforestation, conflict and displacement), as well as improved surveillance and laboratory capacity in the African region. Many cases in Africa have been traced back to contact with wild animals or the use of animal products for medicinal or cultural practices. As deforestation and urbanization drive people and animals into closer quarters, more viruses may make the jump to human hosts. Monkeypox is most likely to leap to people from rodents. There are some 2,000 species of rodents worldwide, composing 40 percent of all mammalian species. The African rope squirrel is a leading candidate as the primary reservoir for monkeypox, but there are other contenders, including striped mice and dormice, giant pouched rats, rusty-nosed rats and brush-tailed porcupines. In endemic areas, MPXV is probably maintained in nature through circulation among a number of mammals, including squirrels, Gambian pouched rats (Cricetomys gambianus), striped mice, dormice and primates, with occasional spill-over events to humans. In endemic areas, MPXV is transmitted to humans through a bite or direct contact with an infected animal’s blood, meat, bodily fluids or cutaneous/mucosal lesions.
Sequencing has identified two distinct clades of MPXV. The West African clade is known to occur from western Cameroon to Sierra Leone and carries a <1% CFR, whereas the Congo Basin clade has been detected from central and southern Cameroon to the DRC and is considered more virulent with a CFR >10%.
The largest West African clade MPX outbreak identified to date was in Nigeria in 2017, with 146 suspected and 42 confirmed cases. In 2018, three unlinked travel-related MPX cases were identified in Israel, the UK, and Singapore. These exportations represent the first time that a human host was documented to transfer MPXV from the African continent. However, MPX outbreaks in animals in laboratories and zoos, with no clearly identified source of infection, have been reported outside the African continent.
In 2003, the US Centers for Disease Control (CDC) reported a total of 71 human MPX cases after close contact with pets. All people infected with monkeypox became ill after having contact with infected pet prairie dogs. No human-to-human transmission was identified, and none resulted in death. The cases were connected to the importation of small mammals from Ghana to Texas as the probable source of introduction of the virus into the US. The spread of the virus between federal states was connected to infected pet prairie dogs that were-housed with rodents of African origin.
Monkeypox does not spread easily between people. Between humans, the virus can be transmitted by respiratory droplets during direct and prolonged face-to-face contact. In addition, monkeypox virus can be transmitted by direct contact with body fluids of an infected person, contact of mucosa or non-intact skin with open rash lesions or with virus-contaminated objects, such as bedding or clothing. Sexual transmission of monkeypox has been described, but infrequently, in the literature. Ogoima et al., in reporting the 2017 human MPX outbreak in Nigeria, hypothesized that sexual transmission was a plausible route of infection as it involved close skin-to-skin contact during sexual intercourse or transmission via genital secretions.
Infection of sexual partners, both female and male, has been previously reported for vaccinia virus, another virus of the Orthopoxvirus genus, post smallpox vaccination. Vaginal lesions occurred in the female partner of a recently vaccinated military man who removed bandages covering his vaccination site, four days after unprotected sexual intercourse, preceded by digital vaginal contact. A painful perianal rash and a lesion on the upper lip were reported by a male patient ten days after sexual intercourse with a recently vaccinated man who did not cover his vaccination site. Further transmission from this patient, while he was experiencing perianal rash, occurred in a male sexual contact who experienced general symptoms and papular lesions on his penis two days after sexual intercourse.
The incubation period for MPX is usually 6 to 13 days but can range from 5 to 21 days. The illness typically lasts for two to four weeks. Disease usually begins with fever, myalgia, fatigue and headache. Within three days from the onset of the prodrome symptoms, a centrifugal maculopapular rash starts from the site of primary infection and rapidly spreads to other parts of the body. Palms and soles are involved in cases of the disseminated rash, which is a characteristic of the disease. The lesions progress, usually within 12 days, simultaneously from the stage of macules to papules, vesicles, pustules, crusts and scabs before falling off. The lesions may be centrally depressed and can be painful and secondary bacterial infection may occur if scratching occurs. Lesions on oral or ophthalmic mucosa (enanthem) may also be present. Prior to and concomitant with the rash, lymphadenopathy is observed in many patients, which is usually not observed in smallpox or varicella. It should be noted that the clinical manifestations in travel-related cases detected in western countries were usually mild, sometimes with very few lesions. The onset of the rash is considered the start of the infectious period; however, it is believed that persons with prodrome symptoms can also transmit MPXV.
In severe forms, monkeypox can trigger sepsis, encephalitis, or secondary infections, These can be fatal, especially in people with preexisting conditions. Some people more likely to develop severe cases of monkeypox include:
- Very young children
- Immunocompromised groups
- People with preexisting skin conditions like eczema
Monkeypox can spread through both direct and indirect contact:
- Direct contact could include touching an infected person’s scabs or bodily fluids, or prolonged exposure to respiratory secretions.
- Indirect contact involves touching items an infected person has left secretions on, like blankets or clothes.
Monkeypox is considered less transmittable than COVID-19, with the average infected individual spreading the disease to only one or two other people, compared to as many as 15 with recent strains of COVID-19. It’s not entirely clear how common asymptomatic transmission of monkeypox may be, but it’s likely less common than in COVID-19. That reduces the risk of spread, since it’s easier to identify infected individuals and ask them to quarantine.
The majority of human MPX cases experience mild to moderate symptoms. Complications in endemic countries include encephalitis, secondary skin bacterial infections, dehydration, conjunctivitis, keratitis, and pneumonia. The case-fatality rate of MPX ranges from 0% to 11% in outbreaks in endemic areas with mortality mostly affecting young children. Little information is available on MPX in immunocompromised patients. In the 2017 Nigeria outbreak, patients with concurrent HIV-infection had more severe morbidity with more skin lesions and associated genital ulcers as compared with HIV-negative individuals. No deaths were reported among HIV-positive patients. Major disease sequelae are usually disfiguring scars and permanent corneal lesions.
The route of infection (invasive, such as animal bite, vs. exposure to fomites) plays a role, with invasive modes of exposure causing more severe disease and shorter incubation period.
Considering varicella as the most relevant differential diagnosis, electron microscopy was traditionally used in the past to distinguish herpesviruses from orthopoxviruses. Currently, MPXV real-time polymerase chain reaction (Real Time-PCR) on suspected skin lesions is used. Scabs, swabs and aspirated lesion fluid should be preferably used for PCR over blood due to limited duration of viremia. These samples can be transported at room temperature and without transport media; blood and serum for serological tests can be transported at room temperature, however, tissue biopsies should be shipped frozen on dry ice. Formalin-fixed samples can be sent at room temperature. Results from scabs, swabs and aspirated lesion fluid specimens show the best correlation with both infectivity and the clinical course of infection. Recent Real Time-PCR approaches can also discriminate the two MPXV clades described above. Serology has limited value due to the immunological cross-reactivity between human-pathogenic orthopoxviruses, but it is used to monitor antibody response in vaccinated individuals. However, for contact investigations, IgM and IgG detection is available in some laboratories. Immunohistochemistry can potentially be used to identify antigens in biopsy samples.
Treatment is primarily symptomatic and supportive (alleviation of fever, pain and pruritus, hydration), including prevention and treatment of secondary bacterial infections. Antivirals tecovirimat, brincidofovir and cidofovir are potential options for severe cases. Only Tecovirimat has market authorisation in the EU for the treatment of orthopoxvirus infection, including MPX. Limited data on efficacy and safety exist currently, while clinical studies are ongoing in Africa.
Various orthopoxviruses share similar genetic and antigenic features and, as such, infection by any of these viruses may provide substantial protection against infection by the other viruses from the orthopoxvirus genus. Due to cross-immunity, an immune response against any illness caused by any orthopoxvirus will decrease the likelihood of an infection by a different orthopoxvirus. Previous vaccination against smallpox can confer cross-protection against monkeypox, which was estimated from older studies to be as high as 85%. The protective effect of smallpox vaccination wanes with time, although serosurveys indicate that it can last more than 20 years. However, it is believed that despite the waning effect smallpox vaccine confers, lifelong protection against severe disease can occur due to memory B and T cells therefore some degree of protection should be expected in the population of adults in the EU/EEA currently over 50 years of age. Because of the inferior safety profile of the smallpox vaccine in immune compromised persons, pregnant women, or persons with atopic dermatitis, routine smallpox vaccinations were stopped in the 1980s following a recommendation from WHO. Only military personnel, selected healthcare, and laboratory workers still get the vaccine. Consequently, the number of people with lacking immunity, not only against smallpox, but also against other zoonotic OPXV infections is increasing, in humans, as well as in animals. In the Democratic Republic of Congo there is a massive (20-fold) increase in human monkeypox incidence.
Monkeypox virus is not considered a biological agent of concern for biosecurity according to the U.S. CDC list of bioterrorism agents, while it is considered an ‘agent with high threat for deliberate release’ using the matrix developed by the EU task force on Bioterrorism (BICHAT). Although the case-fatality rate of the pathogen is low, its relative environmental stability and persistence and transmission pathways, in addition to the lack of immunity in the population, the limited availability of effective treatments and vaccination, make it an agent which could represent a biological threat in case of accidental spill or intentional release.
_______
_______
Cases should remain isolated until their rash heals completely, avoiding contact with children, immunosuppressed persons and pets. Abstaining from sexual activity and close physical contact is also advised until the rash heals. Most cases can remain at home with supportive care.
Close contacts of MPX cases should self-monitor for the development of symptoms up to 21 days from the last exposure to a case.
Healthcare workers should wear appropriate PPE (gloves, water-resistant gown, FFP2 respirator) when screening suspected cases or caring for a MPX case. Laboratory personnel should also take precautions to avoid occupational exposure.
Close contacts of a MPX case should be deferred from blood, organ or bone marrow donations for a minimum of 21 days from the last day of exposure.
_____
When the first monkeypox cases were identified in early May 2022, European health officials were stumped. The virus was not known to spread easily among people, let alone infect dozens — and soon hundreds — of young men. The origins of the outbreak are now becoming clearer. Genetic analysis suggests that although the monkeypox virus is rapidly spreading in the open, it has been silently circulating in people for years. For monkeypox to be detected in people with no apparent connection to one another suggests that the virus might have been spreading silently — a fact that Andrea McCollum, an epidemiologist who heads the poxvirus team at the US Centers for Disease Control and Prevention in Atlanta, Georgia, calls “deeply concerning”. Unlike SARS-CoV-2, which can spread without causing symptoms, monkeypox does not usually go unnoticed when it infects a person, in part because of the skin lesions it causes. If monkeypox could spread asymptomatically, it would be especially troubling, because it would make the virus harder to track, McCollum says. Health officials have already identified two versions of monkeypox among American patients, suggesting at least two separate chains of transmission. Researchers in several countries have found cases with no known source of infection, indicating undetected community spread. And one research team argued that monkeypox had already crossed a threshold into sustainable person-to-person transmission. The genetic information available so far indicated that, at some point in the last few years, the virus became better at spreading between people, said Trevor Bedford, an evolutionary biologist at the Fred Hutchinson Cancer Research Center in Seattle. “Genomic patterns would suggest this occurred around 2018,” Dr. Bedford said. If the virus has adapted to include people as hosts, monkeypox outbreaks could become more frequent and more difficult to contain. That carries the risk that monkeypox could spill over from infected people into animals — most likely rodents — in countries outside Africa, which has struggled with that problem for decades. The virus may persist in infected animals, sporadically triggering new infections in people. “We can also transmit this back to animals that can spread the disease within wildlife and back to humans,” said Sagan Friant, an anthropologist at Pennsylvania State University who has studied human-animal interactions in Nigeria for about 15 years. The longer it takes to contain the virus, the higher the odds that it will find a permanent new home in people or animals, Dr. Friant said.
______
Reported cases are going down in many Western countries—most likely as a result of behavioral changes and vaccination—and public health officials in Europe are already talking about eliminating the virus in the region. But infections are still on the rise elsewhere in the world. In many places vaccines are unavailable, or people at risk either lack information about how to avoid infection or fear asking for it, because gay sex is criminalized. “I don’t think [monkeypox] will cause massive numbers of infections, but it will stay there and it will be difficult to eradicate,” poxvirologist Antonio Alcamí says. “Decision-makers have to realize that this is not going away anytime soon,” adds Christian Drosten, a virologist at Charité. Science can’t do more than hint at how the virus might evolve as it continues to circulate. One reason is that research interest in poxviruses has dwindled after the worldwide smallpox eradication campaign ended in triumph in 1980. “I always had to start my talks by almost apologizing for working on poxviruses,” Alcamí says.
_______
_______
Section-5
Past outbreaks of monkeypox before May 2022 outbreak:
Monkeypox has a relatively recent history. People first discovered it in monkeys in 1958, although a “vesicular disease in monkeys” was described in the 1860s. The disease, and eventually the causative virus, was named monkeypox because the lesions (pox) seen in monkeys developed like other known pox-forming diseases (pustules that eventually break open, ulcerate, crust over, and some pox form scars in the skin). Later studies showed the “monkeypox” virus was actually sustained endemically in African rodents. It was not until 1970 in Africa (Zaire, now the Democratic Republic of Congo also termed Republic of the Congo, DRC, and Congo) when a 9-year-old boy (who developed smallpox-like lesions) was the first person to eventually be diagnosed with monkeypox. This situation initially caused concern that smallpox may also have an animal reservoir or endemic population that would make eradication of smallpox impossible. Fortunately, this was not the case because monkeypox was found to be a different species of poxvirus, and smallpox was eradicated from the human population by vaccinations in 1979 (currently, only a few research labs have access to smallpox viruses). Monkeypox is now the major Orthopoxvirus (also termed orthopox) that infects humans and fortunately, not frequently. However, vigilance is warranted, as there have been several outbreaks of monkeypox since the 1970s. Although most have occurred in Africa (mainly western and central Africa), there was an outbreak in the U.S. in 2003. This apparently happened when an animal distributor either housed or transported monkeypox-infected African rodents (Gambian rats) with prairie dogs that people later purchased as pets, became “sick,” and transmitted the disease to their owners. Other animals like the rope squirrel (Funisciurus anerythrus) and the sun squirrel (Heliosciurus rufobrachium) may transmit the virus to humans in Africa.
_
In 2017, an outbreak of monkeypox began in Nigeria. This large outbreak is thought to be triggered by river flooding that has caused infected wild animals (especially rodents and monkeys) to more closely associate with humans, thus spreading this zoonotic (transmitted to humans from animals) disease. From 2017 to the present, Nigeria has recorded 446 cases. In September 2018, Dr. Beadsworth in England reported treating three people with monkeypox who had visited Nigeria. The three patients likely were exposed to the virus while visiting Nigeria. On July 15, 2021, a person was diagnosed with monkeypox in Dallas, Texas, and the CDC confirmed this. He traveled by air from Nigeria to Atlanta, Georgia, and then flew on to Dallas, Texas. Another case was diagnosed in Maryland in 2021. The current outbreak (May 2022) has spread to non-endemic countries according to the World Health Organization (WHO). The countries include Australia, Belgium, Canada, France, Germany, Italy, Netherlands, Portugal, Spain, Sweden, the United Kingdom (Britain), and the USA.
_
Outbreak overview of monkeypox in humans:
The outbreak overview below covers all cases published via official channels (research articles and reviews, books, WHO reports). All reported outbreaks are summarized in Table below:
Cases of human monkeypox reported in the World from 1970 till 2018:
|
1970-1990 |
1991-1999 |
2000-2009 |
2010-2018 |
|
|
Democratic Republic of Congo (former Zaire) |
386 (confirmed) + 2–5 |
511 |
Not fully enumerable |
Not fully enumerable |
|
Central African Republic |
6 (confirmed) |
N/A |
4 |
At least 68 (at least 29 confirmed) |
|
Cameroon |
2 (confirmed) |
4 (1 confirmed) |
N/A |
16 (1 confirmed) |
|
Nigeria |
10 (3 confirmed) |
N/A |
N/A |
244 (101 confirmed) |
|
Ivory Coast |
2 (confirmed) |
N/A |
N/A |
N/A |
|
Liberia |
4 (confirmed) |
N/A |
N/A |
2 (confirmed) |
|
Sierra Leone |
1 (confirmed) |
N/A |
N/A |
At least 2 (2 confirmed) |
|
Gabon |
1–10 (one confirmed) |
N/A |
N/A |
N/A |
|
USA |
N/A |
N/A |
47 (37 confirmed, 10 probable) |
N/A |
|
Republic of Congo |
N/A |
N/A |
12 (3 confirmed, 8 probable) |
98 (9 confirmed) |
|
South Sudan |
N/A |
N/A |
49 (10 confirmed, 9 probable) |
N/A |
_
Monkeypox has already occurred in 10 African countries and is known to be endemic in the DRC. It has once crossed the borders of the African continent when it was imported into the USA in 2003, before which it was assumed that the disease was geographically limited. The summary of recorded cases above demonstrates an increase in the incidence of monkeypox cases in recent years, together with a broader geographical occurrence. However, the data collected is often incomplete and unconfirmed which hampers realistic estimations of prevalence and incidence of monkeypox over time. Nevertheless, the active surveillance conducted by Rimoin et al. between 2005 and 2007 concluded that there was a 20-fold increase in monkeypox incidence compared to the historic data between 1981 and 1986 (WHO active surveillance). Also other official reports (e.g., DRC’s passive surveillance program 2010-2016 data) and unofficial source data underline the emerging character of the disease. Four explanations, possibly occurring simultaneously, were raised for the monkeypox incidence increase:
-1. The cessation of the smallpox vaccination in 1980 and the consequent drop of immunity against orthopoxviruses. Eradication of smallpox and waning immunity against after vaccine cessation created an immunologic niche for monkeypox. In 2010, only 24.5% of the DRC population had evidence of a smallpox vaccination scar. The influence of vaccination cessation is reflected in the age pattern of incidence. After 1980, more individuals are susceptible to MPXV infection every year. Similar causality was shown for measles and yellow fever when a decreased vaccination coverage led to increased incidence.
-2. Higher or more frequent exposure to animal reservoir species. Significant anthropogenic and demographic changes have occurred in the DRC since 1980, and may have increased the exposure of the local population to reservoir species of MPXV. Logging of the rain forest increases the opportunities of human exposure to displaced animals, thus for zoonotic transmission of MPXV. Additionally, recurrent war, civil unrest and poverty force the affected population to flee and eventually look for shelter deeper in the rain forest, and to rely more extensively on bush meat (monkeys, small rodents, etc). Similar conclusions were drawn from studies on Nipah virus and rabies virus where environmental and anthropogenic changes led to a higher chance of exposure to (infected) animals, leading to increased incidence.
-3. Increased human-to-human transmission rate. Increased prevalence in humans, particularly immunocompromised hosts, may provide more opportunity for MPXV to acquire mutations that increase its fitness in human hosts, possibly leading to increased transmissibility, virulence, and pathogenic potential. Also probabilistic arguments suggest that a zoonotic pathogen with an R0 near to one (such as monkeypox) retains a greater potential to evolve to a state of higher transmissibility as transmission chains lengthen and as the number of primary introductions increases. This assumes that the population lacks the vaccine-derived immunity, as it is the case nowadays because the smallpox vaccination was ceased. It needs to be studied whether the rising monkeypox incidence is a result of increased human-to-human transmission because greater circulation among humans opens the possibility of geographic spread by travelers. To demonstrate, a novel H1N1 influenza virus crossed the species barrier from swine into humans in 2009 and multiple mutations during human-to-human transmissions allowed incremental changes in viral fitness, which may have contributed to the apparent increase in disease severity.
-4. Advancement in diagnostic capacity and health education. Development of molecular techniques and point-of-care systems in recent years made the diagnosis of monkeypox more accurate and rapid than before. Also, more attention is paid to health education of medical workers and the general public. Despite these improvements which could cause higher reporting and confirming of cases, modern molecular techniques are more expensive, while today’s surveillance programs have less funding and manpower resources than the WHO active surveillance program between 1981 and 1986. That is why the few active surveillance programs conducted nowadays are likely to be conservative estimates of a true monkeypox incidence increase.
_
The majority of data we currently have come from passive surveillance which often miss a proportion of cases, leading to an underestimation of the burden of MPXV infections in humans. Despite this, recent research papers/reports based on passive surveillance (even though limited in number) provide evidence of the frequent occurrence of human MPXV infections. The incidence of monkeypox clearly increased but no detailed data is available to evaluate whether other aspects, like human-to-human transmission rate, morbidity and mortality rates or patterns of transmission changed.
The emergence of monkeypox as a significant human pathogen is indisputably a realistic scenario. Firstly, poxviruses were shown to be capable to rapidly adapt against host defenses despite their low mutation rates. Secondly, multiple countries were projected to have a suitable environment for MPXV by ecological niche modeling where MPXV might be circulating undetected in animal hosts. In these countries, evolutionary, ecological, or epidemiologic changes could tip the balance in favor of emergence and possibly sustained transmission. A good example is Ghana where MPXV circulates in animals but the country never reported human cases of MPX. The importation of MPXV-infected rodents to the USA, however, did lead to a human outbreak of MPX.
It is clear that surveillance but also research on the ecology, epidemiology, natural history and pathogenesis of the infection has to be addressed in order to design and implement needed prevention and control measures. Additionally, specific improvements in laboratory diagnostics and infection control measures are needed to detect cases, treat patients, and prevent further spread of the virus.
_______
_______
Section-6
Current 2022 outbreak of human monkeypox:
A multi-country outbreak of monkeypox is currently underway in places where the virus has not been typically found before, in Europe, the Americas, Africa, the Western Pacific, countries of the Eastern Mediterranean and in South East Asia. More cases than normal have been reported in 2022 in parts of Africa that have previously reported cases, such as Nigeria, the Democratic Republic of the Congo, and the Central African Republic.
Monkeypox has been reported in some African countries in the years before this outbreak began. These include Cameroon, the Central African Republic, the Republic of the Congo, Côte d’Ivoire, the Democratic Republic of the Congo, Gabon, Liberia, Nigeria, and Sierra Leone. Some of these countries only had a few cases and others have had persistent or recurrent outbreaks. Occasional cases in other countries have been linked to travel from Nigeria. The current outbreak affecting many countries at once is not typical of previous outbreaks.
_
An ongoing outbreak of monkeypox, a viral disease, was confirmed in May 2022. The initial cluster of cases was found in the United Kingdom, where the first case was detected on 6 May 2022 in an individual with travel links to Nigeria (where the disease is endemic). The outbreak marked the first time monkeypox has spread widely outside Central and West Africa. From 18 May onwards, cases were reported from an increasing number of countries and regions, predominantly in Europe but also in North and South America, in Asia, in Africa, and in Oceania. On 23 July, the Director-General of the World Health Organization (WHO), Tedros Adhanom Ghebreyesus, declared the outbreak a public health emergency of international concern (PHEIC). As of October 2022, there had been more than 70,000 confirmed cases in over 100 countries, most of them seeing their first monkeypox cases. The United States has the highest number of monkeypox cases in the world.
_
How is the current outbreak different?
This is the first time that chains of transmission were reported in Europe without links to West or Central Africa, according to the European Center for Disease Prevention and Control. The agency also said this year’s cases included the first that have been reported among men who have sex with men.
_
The virus has been circulating for decades in some places, including parts of West and Central Africa. In early research posted, scientists at the Institute of Evolutionary Biology at the University of Edinburgh described how the genetic pattern they’re seeing suggests that “there has been sustained human to human transmission since at least 2017.” In that research, genetic sequences showed that the first monkeypox cases in 2022 appear to have descended from an outbreak that resulted in cases in Singapore, Israel, Nigeria and the United Kingdom from 2017 to 2019. Michael Worobey, an evolutionary biologist and professor at the University of Arizona who was not involved in the research, said it suggests that “this outbreak has been going on for a long time, locally,” as in where the virus is endemic. And it means the world has failed to protect those in resource-limited areas where it has been endemic and to control it at its source before it spread globally, he added.
_
As of July 1, 2022, the Center for Disease Control and Prevention (CDC) confirms 5783 cases of monkeypox, distributed in 52 different countries around the globe. Figure below is a visual depiction of the regional distribution of the cases globally.
Many of the cases of monkeypox, currently, are concentrated within regions of Europe and within the western hemisphere. Within Europe, current reports show that the largest number of cases are present in the United Kingdom. Currently, many of the confirmed cases of monkeypox are prevalent amongst individuals under the age of 40 years with a median age of 31 years. This is a population born only after the discontinuation of the smallpox vaccination campaign, therefore further reflecting the lack of cross-protective immunity. There is also a higher prevalence of monkeypox cases among males as infections in the global outbreak have occurred so far in men who have sex with men (MSM).
_
_
Spread of monkeypox as of 31 August 2022 is depicted in the figure below:
______
______
Monkeypox declared a global emergency:
The World Health Organization’s director-general, Tedros Adhanom Ghebreyesus, rang the agency’s highest alarm on 23 July 2022 to signal the seriousness of the global monkeypox outbreaks. Over the weekend, the World Health Organization (WHO) declared that the monkeypox outbreak spreading globally is a ‘public health emergency of international concern’ (PHEIC). Researchers hope that the declaration — the agency’s highest alarm — might serve as a wake-up call for countries as they struggle to contain the spread of the virus that causes monkeypox. Since the first cases were detected outside Africa in May, as of September more than 62,000 people have been confirmed infected in nearly 105 countries that don’t typically see cases. Monkeypox has been circulating in parts of Africa for decades.
This is the seventh time since the alarm system originated in 2005 that the WHO has declared a PHEIC — a step it reserves for events that pose a risk to multiple countries, and that require a coordinated international response. Two of those warnings, for COVID-19 and polio, are still in place.
In an unprecedented move, WHO director-general Tedros Adhanom Ghebreyesus declared the PHEIC on 23 July, after a panel of advisers failed to come to a consensus. Although the panel doesn’t formally vote, six members were in favour of declaring a PHEIC, while nine were against, Tedros said at a press conference announcing his decision. The panel had previously met in late June, but at that time only three members were for declaring a PHEIC and 11 were opposed, so Tedros decided against sounding the alarm at the time. Opponents of the declaration have pointed out that cases of monkeypox are concentrated in a dozen countries in Europe and the Americas, and show no sign of exponential increase. The vast majority of people recover without requiring hospitalization, and death remains an extremely rare occurrence. There has been speculation that a PHEIC declaration could stigmatize MSM communities, as well as spark demand for the monkeypox vaccine among people who are at very low risk of infection, which could further reduce the already constricted supplies.
_
The highest alarm:
The WHO has declared a ‘Public Health Emergency of International Concern,’ or PHEIC, seven times since the alarm system originated in 2005.
2009: H1N1 (swine flu) originates in Mexico and spreads to the United States.
2014: Polio resurges in Afghanistan, Pakistan and Nigeria.
2014: Ebola virus infections spread throughout Guinea, Sierra Leone and Liberia.
2016: A Zika virus epidemic causes microcephaly and other neurological disorders in the Americas.
2019: An Ebola outbreak spreads in a conflict zone in the Democratic Republic of the Congo.
2020: The first cases of COVID-19 appear in China in late 2019, and the SARS-CoV-2 coronavirus spreads to many other countries, becoming a pandemic.
2022: Monkeypox infections pop up and spread widely in countries outside Central and West Africa, where they had been slowly circulating for 50 years.
Declaring the monkeypox outbreak a global emergency is a good decision, says Anne Rimoin, an epidemiologist at the University of California, Los Angeles, who is a member of the panel and has studied monkeypox in the Democratic Republic of the Congo for more than a decade. “It sends the right message, and it will hopefully mobilize attention and resources to prevent this virus from gaining a foothold globally.”
_
A window closing:
Researchers have been warning that the window of opportunity for containing the global monkeypox outbreak is rapidly closing. The virus is already established in an animal reservoir in some parts of Africa, making its eradication a difficult task. One fear is that the virus could spread from humans to animals elsewhere in the world, establishing further reservoirs from which humans could be infected repeatedly. Even as cases are soaring in Germany, Spain and the United States, some think that containment is still possible, however. The PHEIC signals loudly to public-health officials that the time to act is now, says Caitlin Rivers, an epidemiologist at Johns Hopkins University in Baltimore, Maryland. “We can’t accept this as an endemic virus,” she says. Countries should work to increase the number of people tested, boost contact-tracing efforts and isolate people as early as possible after symptoms are detected, Rivers adds. The PHEIC sends a clear message to countries that their participation in the global response — which might include sharing vaccines and treatments — is necessary for containment. The WHO issued guidelines for countries when announcing the alarm, offering recommendations on testing, public-health measures and messaging, travel advisories, infection prevention and control, and global coordination.
_
Containment concerns:
Wealthy countries have raced to deploy smallpox vaccines, thought to be highly effective against monkeypox because the viruses that cause these diseases are related. Although some children and women have been infected with monkeypox, almost all the infections in the global outbreak have occurred so far in men who have sex with men (MSM), especially those with multiple sexual partners. This has led public-health officials to focus their messaging and vaccine stockpiles on this population. At first, some countries, such as Canada and the United Kingdom, used a ‘ring vaccination’ approach, which inoculates the close contacts of people infected with monkeypox to cut off routes of transmission. They then moved quickly to offer the vaccine more widely to high-risk communities, including MSM and health-care workers. Researchers know that monkeypox spreads mainly through close contact. They are still investigating whether the virus can be sexually transmitted, but it has clearly taken hold in the MSM community. Some members of the WHO panel were reluctant to support a PHEIC declaration because they worried that it would stigmatize that community and hinder efforts to contain the virus. “People are not always willing to disclose sexual history”, especially in countries where homosexuality is criminalized, says Boghuma Titanji, an infectious-disease physician at Emory University in Atlanta, Georgia.
While wealthy countries use vaccines for monkeypox, Titanji warns that the ongoing outbreaks in Africa, where there have been more than 70 suspected deaths from the virus this year, “cannot be relegated to a footnote”. (So far, very few deaths have occurred in people infected outside Africa.) She hopes that the WHO will promote equity in the global monkeypox response by helping to scale-up surveillance and testing for the virus in Africa, and by allocating money for research and vaccines there. “We got into this problem by allowing monkeypox to rage on for 50 years in Africa,” she says.
_
About 97% of the 17,422 cases of monkeypox for which there are data on sexual orientation have been in MSM. Viral DNA has been detected in seminal fluid, although it remains unclear as to whether this translates to an infection risk. “Monkeypox is turning into another sexually transmitted disease; that is not entirely unexpected, from what we know about the virus, but it is still a new development in terms of the history of the disease”, David Evans, a virologist in the Department of Medical Microbiology and Immunology at the University of Alberta (Edmonton, AB, Canada), told The Lancet Infectious Diseases.
Notably, MSM do not seem to be driving new infections in the endemic regions of west and central Africa. Around 17,000 case reports from around the world include information on sex. They indicate that 99% of infections in the ongoing monkeypox outbreak are in males. Lack of testing capacity means that data from the endemic countries of Africa are scarce, but the available figures show that women comprise more than a third of infections. Researchers at the Pasteur Institute have calculated that in a completely susceptible population, the monkeypox virus would have a basic reproduction number (R0) of 1·46–2·67. However, many people older than 50 years are likely to have cross-protection conferred by the now-discontinued smallpox vaccination campaigns. In a population with 10–25% immunity, the R0 for monkeypox is likely to be somewhere in the region of 1·1–2·4.
_
On Aug 5, the USA declared a public health emergency for monkeypox. At the time, the country had registered over 6600 cases of the disease. In the days preceding the declaration, New York state, Illinois, and California, which together accounted for roughly half the American cases of monkeypox, had all declared emergencies. New York City and San Francisco have taken similar measures. The mayor of New York City and the health commissioner issued a joint statement asserting that the “outbreak must be met with urgency, action, and resources, both nationally and globally, and this declaration of a public health emergency reflects the seriousness of the moment”. They went on to suggest that 150,000 city residents could be at risk of contracting monkeypox. A national public health emergency declaration in the USA releases funding and opens a path for the Secretary of Health and Human Services to take action to address the crisis.
_
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention (CDC). Men aged 26-40 make up the highest proportion of cases. The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the Division of HIV, Infectious Diseases, and Global Medicine at the University of California San Francisco. It is most common in younger to middle-aged aged groups, and less common in children and older individuals. As of August 21, only 17 children younger than 15 have been diagnosed with monkeypox in the US, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Gandhi says. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, she said, “and that isn’t something we worry about with school-spread illness.”
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from August 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Gandhi says, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
_
Is this 2022 monkeypox outbreak a pandemic?
No.
Monkeypox, while it is spreading around the world, is still considered to be an outbreak. There are not really any official criteria for a pandemic, but the World Health Organization ultimately makes the call as to whether a disease outbreak qualifies, and that hasn’t happened yet. The last pandemic to be declared was COVID-19 in March 2020. Monkeypox is less contagious and less dangerous than COVID-19. Monkeypox is not as contagious as some other infections because it requires close contact with someone who has monkeypox (e.g., face-to-face, skin to skin, mouth-to-skin, mouth-to-mouth or sexual), with a contaminated environment or with an infected animal, to spread. With R0 between 1 and 2, it is unlikely to spread like Covid and now there are reports of its decline in the U.S. and Europe. We can control this outbreak by working closely with communities and groups at higher risk to stop transmission. While there is risk of infection for everyone, men who have sex with men are most affected. The MVA vaccine for monkeypox is safe and effective when used before exposure and even after exposure up to 4 days.
______
______
Section-7
Epidemiology of monkey pox:
The epidemiology of smallpox, caused by orthopoxvirus variola, is understood through detailed studies conducted during the end of the eradication campaign. Interhuman transmission of variola virus generally occurred through the inhalation of large respiratory droplets of infectious virus. Transmission usually required prolonged face-to-face or other close contact, although airborne transmission over longer distances had been reported. Transmission by fomites or contact with infectious material from the rash also occurred. Aggregate data, collected during the smallpox eradication campaign, suggest a secondary attack rate of 58.4% in unvaccinated close or household contacts and a secondary attack rate of 3.8% in previously vaccinated close or household contacts. Case-fatality rates for variola major varied with the type of disease manifested, but aggregate rates of 10 to 30% in various outbreaks have been recorded. Severity of disease correlated with rash burden and was also more severe in children and pregnant women. Variola alastrim minor, a variant of variola with a case-fatality rate of less than 1%, had similar human-to-human disease transmission characteristics.
_
Monkeypox was first isolated in Denmark in the late 1950s from a colony of laboratory monkeys from Singapore that were going to be used for polio virus research. During the following decade, additional outbreaks of monkeypox were seen in laboratory animals in the United States as well as zoo animals in Rotterdam. Monkeypox was first identified as a cause of disease in humans in the 1970s in the Democratic Republic of the Congo. Monkeypox has a more complex epidemiology. The virus is zoonotic, and two genetically discrete virus clades have been described, each with apparent distinct clinical and epidemiologic parameters. Human infections in western and central Africa were first identified in 1970. Investigations in the Congo basin country Zaire, now the Democratic Republic of Congo, demonstrated that human-to-human transmission of monkeypox was less prevalent than that of smallpox. The secondary attack rate in unvaccinated contacts of monkeypox cases was calculated to be 9.3% versus 37 to 88% for smallpox. Previous smallpox vaccination (administered 3 to 19 years prior) appeared to be 85% protective in preventing disease acquisition in contacts and also ameliorated the severity of disease. Overall, most identified cases acquired disease from presumed animal exposure; only 28% of cases were ascribed to person-to-person transmission. A case-fatality rate of approximately 10% was observed in unvaccinated persons, and the majority of fatalities and the most severe disease manifestations were observed in children younger than 5 years. In a more recent series of 122 confirmed cases in Nigeria, the case-fatality rate was 6%. Serosurveys suggested that subclinical infection may have occurred in up to 28% of close contacts of monkeypox patients in some communities; this relatively low rate may contribute to the rarity of sustained generations of human-to-human transmission in household and other close-contact situations. However, more recent studies of household attack rates in an outbreak setting suggest that up to 50% of infections may be transmitted from human to human, and the seroprevalence of anti-orthopoxvirus antibodies in unvaccinated individuals is about 20 to 25% in central and western Africa.
_
Geographic distribution:
Since discontinuation of smallpox immunization, which also protects against monkeypox, most cases of monkeypox have occurred in Central and West Africa. Thought to be a rare and self-limiting disease, monkeypox has not attracted much attention since its discovery 70 years ago. The frequency and geographic distribution of human monkeypox cases have increased in recent years in a specific region of Africa (figure below), and monkeypox has been recognized as an increasing public health threat, particularly in regions in West Africa where there is close interaction between humans and wild animal reservoirs and in particular where there is evidence that the infection attack rate is increasing. The clinical presentation of monkeypox is similar to that of smallpox in terms of symptom onset, timing of rash occurrence, and rash distribution, but generally less severe than smallpox in terms of complication rate, case fatality rate, and levels of scarification.
Figure above shows Map of Africa showing countries reporting human Monkeypox cases (1971–2019).
In 2022, the WHO reported that monkeypox was endemic in several African countries, including Benin, Cameroon, the Central African Republic, the Democratic Republic of the Congo, Gabon, Ghana (identified in animals only), Ivory Coast, Liberia, Nigeria, the Republic of the Congo, Sierra Leone, and South Sudan. From January to May 2022, most suspected cases of monkeypox occurred in the Democratic Republic of the Congo, with 1284 cases and 58 deaths reported.
Nonendemic countries:
The first outbreak of monkeypox in the Western hemisphere occurred in the United States in 2003. Since then, sporadic cases have been reported in several nonendemic countries, mostly related to travel. Several sporadic cases of monkeypox have been reported after travel to endemic areas. In one study evaluating the 2017 outbreak in Nigeria, a small pool of related isolates was the likely source for the exported infections.
Between 2018 and 2021, seven cases of monkeypox were diagnosed in the United Kingdom; four cases were related to travel from endemic countries, two cases resulted from household transmission from one of the index cases, and one case occurred in a health care worker who acquired infection nosocomially.
In July 2021, a patient was diagnosed with monkeypox in Dallas, Texas. This patient developed symptoms during his return trip from Nigeria. In November 2021, another travel-related case was reported in a United States resident in Maryland who had recently returned from Nigeria. No additional cases were linked to these two patients.
An ongoing outbreak associated with person-to-person transmission was reported in May 2022 and has involved thousands of individuals in dozens of countries outside Africa and not related to travel to endemic areas.
_______
_______
Agent:
Monkeypox virus (MPXV) is an enveloped double-stranded DNA virus that belongs to the Orthopoxvirus genus of the Poxviridae family. There are two distinct genetic clades of the monkeypox virus – the Central African (Congo Basin) clade and the West African clade. The Congo Basin clade has historically caused more severe disease and was thought to be more transmissible. The geographical division between the two clades has so far been in Cameroon – the only country where both virus clades have been found.
Host:
Natural reservoir is yet unknown. However, certain rodents (including rope squirrels, tree squirrels, Gambian pouched rats, dormice) and non-human primates are known to be naturally susceptible to monkeypox virus. The virus is naturally occurring in animals only on the continent of Africa where infection has been documented in at least 10 nonhuman primate species and four squirrel species. Squirrels are believed to be the major disease reservoir in Africa (Reid and Dagleish, 2011). The virus has a broad host range of Asian, African, and South American nonhuman primates including select apes, and New and Old World monkeys (Wachtman and Mansfield, 2012). Most of the infections of captive nonhuman primates have involved Asian macaques (Fenner, 1990). Human is incidental host.
Incubation period:
The incubation period (interval from infection to onset of symptoms) of monkeypox is usually from 6 to 13 days but can range from 5 to 21 days.
Period of communicability:
1-2 days before the rash to until all the scabs fall off/gets subsided.
Infectious dose:
There are no definite data on the required infectious dose with monkeypox virus in humans. However, in contrast to variola virus, a significantly higher dose is assumed to be required to trigger infection. In non-human primates, infection could be initiated by intrabronchial application of 5×104 plaque-forming units (PFU). Orthopoxviruses are reported to remain infectious under dry conditions and different temperatures. Dried vaccinia virus is stable up to 35 weeks (at 4 °C) without loss of infectivity.
Mode of transmission [vide infra]:
- Human-to-human transmission is known to occur primarily through large respiratory droplets generally requiring a prolonged close contact. It can also be transmitted through direct contact with body fluids or lesion material, and indirect contact with lesion material, such as through contaminated clothing or linens of an infected person.
- Animal-to-human transmission: may occur by bite or scratch of infected animals like small mammals including rodents (rats, squirrels) and non-human primates (monkeys, apes) or through bush meat preparation.
Reproduction number (R0) of monkeypox outbreak 2022:
Monkeypox, a fast-spreading viral zoonosis outside of Africa in May 2022, has scientists on alert. Researchers estimated the reproduction number to be 1.29 (95% CrI: 1.26, 1.33) by aggregating all cases in 70 countries as of July 22, 2022; according to Journal of Travel Medicine July 2022.
Race, sex, and age:
Poxvirus infections have no racial predilection, and the incidence is equal in males and females, except in the 2022 epidemic, where patients are overwhelmingly male. A survey of 528 infections confirmed from April 27, 2022 to June 24, 2022, in 16 countries found that 98% of patients were men who have sex with men, 75% were White, and 41% were HIV positive. The median patient age was 38 years.
In the African epidemics, 90% of the patients were children younger than 15 years. In the 2003 US outbreak, of the confirmed cases (n = 35), 11 patients were younger than 18 years and 24 were older. Although the highest age-specific incidences and the greatest number of cases occur among persons younger than 15 years, a trend toward increasing incidence among persons aged 15-30 years has been seen in recent years.
Cross-Immunity and Protection:
Various orthopoxvirus species share genetic and antigenic features, and an infection by any of these species may confer substantial protection against infection by the others. Vaccination with vaccinia virus protects against disease caused by VARV, MPXV, or CPXV. The immunologic mechanisms underlying cross-protection by immunization with vaccinia virus seem to be diverse, with neutralizing antibodies among the principal components. Consistent with the ability of smallpox vaccine to provide cross-protection for humans against monkeypox, monkeys can be protected against monkeypox by immunization with the human smallpox vaccine.
Ever since smallpox vaccinations were discontinued in 1978, cross-protective immunity to various orthopoxviruses has waned, particularly in younger individuals lacking vaccinia-induced immunity, and the number of unvaccinated, susceptible individuals has grown worldwide. Indeed, these changes have been accompanied by an increased frequency and geographic distribution of human monkeypox cases in recent years.
______
______
The paradigm shift in monkeypox epidemiology:
The current global monkeypox outbreak has thrown doctors, epidemiologists, and infectious disease experts off-balance. Unlike previous outbreaks, the world is seeing hundreds of infections outside of Africa. Cases have been found in over 105 countries in Europe, the United States, Australia, and Asia. These cases raise a number of concerns.
Why do we have outbreaks in multiple countries?
Why do these cases occur, mainly outside endemic West and Central Africa?
Was the monkeypox disease spreading undetected in the west, and could this explain why, so far, none of the current cases has been traced to an endemic country?
Why is there a higher incidence in gay and bisexual populations?
Answers to these questions and many more will help epidemiologists, infectious disease experts and clinicians find a solution to a suspected shift in the epidemiology of the disease.
For many years, monkeypox was endemic only in Africa, until the first case outside of Africa was reported in 2003. Since then, there have been pockets of intermittent outbreaks in America, Europe, and Asia, most of which can be traced back to African origins, either through travel or importing animals. The latest outbreak, however, is not following the same pattern – with epidemiologists suspecting transfer to people who have not been to Africa.
A large number of these infections have occurred in bisexuals and men who have sex with men, suggesting the possibility of sexual transmission. This suggestion cannot be dismissed, even if it does not support the already known epidemiology of the disease. This may involve new knowledge and previously unknown facts. Examples of emerging and re-emerging infectious diseases that were later found to be potentially sexually transmitted were the Zika and Ebola virus diseases. These viruses were later isolated from semen, vaginal fluids, and breast milk to confirm the possibility of their sexual and postnatal mother-to-child transmission.
According to the World Health Organisation (WHO), there are currently outbreaks of monkeypox in three West African (Sierra Leone, Liberia and Nigeria) and four Central African countries (Congo, Cameroon, the Central African Republic and the Democratic Republic of the Congo). About 1,440 cases have been reported since early 2022 but only 44 of them have been laboratory confirmed. Cases have not been reported in any of the non-endemic African countries. However, it is not out of place to reflect on the evolution of epidemiological changes based on further insight into the reported cases.
In recent years, outbreaks have been identified in new areas and locations in endemic countries. Data reported by the Nigeria Center for Diseases Control has shown varying levels of surveillance between states and ease of movement within the country. In the past , monkeypox was mainly reported in the south of the country, but since 2020, the virus has spread to the central, eastern, and northern parts of the country.
With the ongoing outbreaks in non-endemic countries, evidence suggesting sexual transmission, and incidents outside of endemic geographic areas, such as those seen recently in Nigeria, there may be an ongoing change in monkeypox epidemiology. We have to be vigilant of a global monkeypox outbreak, with 105 non-endemic countries, more than 72,000 laboratory-confirmed cases in October, 2022.
______
Epidemiological trends and clinical features of the ongoing monkeypox epidemic: A preliminary pooled data analysis and literature review: 12 June 2022:
An emerging outbreak of monkeypox infection is quickly spreading worldwide, being currently reported in more than 30 countries, with slightly less than 1000 cases. In the present preliminary report, authors collected and synthesized early data concerning epidemiological trends and clinical features of the ongoing outbreak and compared them with those of previous outbreaks. Data were pooled from six clusters in Italy, Australia, the Czech Republic, Portugal, and the United Kingdom, totaling 124 cases (for 35 of which it was possible to retrieve detailed information). The ongoing epidemic differs from previous outbreaks in terms of age (54.29% of individuals in their thirties), sex/gender (most cases being males), risk factors, and transmission route, with sexual transmission being highly likely. Also, the clinical presentation is atypical and unusual, being characterized by anogenital lesions and rashes that relatively spare the face and extremities. The most prevalent sign/symptom reported was fever (in 54.29% of cases) followed by inguinal lymphadenopathy (45.71%) and exanthema (40.00%). Asthenia, fatigue, and headache were described in 22.86% and 25.71% of the subjects, respectively. Myalgia was present in 17.14% of the cases. Both genital and anal lesions (ulcers and vesicles) were reported in 31.43% of the cases. Finally, cervical lymphadenopathy was described in 11.43% of the sample, while the least commonly reported symptoms were diarrhea and axillary lymphadenopathy (5.71% of the case series for both symptoms). Some preliminary risk factors can be identified (being a young male, having sex with other men, engaging in risky behaviors and activities, including condomless sex, human immunodeficiency virus positivity (54.29% of the sample analyzed), and a story of previous sexually transmitted infections, including syphilis).
______
Epidemiologic and Clinical Characteristics of Monkeypox Cases — United States, May 17–July 22, 2022:
Monkeypox, a zoonotic infection caused by an orthopoxvirus, is endemic in parts of Africa. On August 4, 2022, the U.S. Department of Health and Human Services declared the U.S. monkeypox outbreak, which began on May 17, to be a public health emergency. After detection of the first U.S. monkeypox case), CDC and health departments implemented enhanced monkeypox case detection and reporting. Among 2,891 cases reported in the United States through July 22 by 43 states, Puerto Rico, and the District of Columbia (DC), CDC received case report forms for 1,195 (41%) cases by July 27. Among these, 99% of cases were among men; among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before symptom onset. Among the 88% of cases with available data, 41% were among non-Hispanic White (White) persons, 28% among Hispanic or Latino (Hispanic) persons, and 26% among non-Hispanic Black or African American (Black) persons. Forty-two percent of persons with monkeypox with available data did not report the typical prodrome as their first symptom, and 46% reported one or more genital lesions during their illness; 41% had HIV infection. Data suggest that widespread community transmission of monkeypox has disproportionately affected gay, bisexual, and other men who have sex with men and racial and ethnic minority groups. Compared with historical reports of monkeypox in areas with endemic disease, currently reported outbreak-associated cases are less likely to have a prodrome and more likely to have genital involvement. CDC and other federal, state, and local agencies have implemented response efforts to expand testing, treatment, and vaccination. Public health efforts should prioritize gay, bisexual, and other men who have sex with men, who are currently disproportionately affected, for prevention and testing, while addressing equity, minimizing stigma, and maintaining vigilance for transmission in other populations. Clinicians should test patients with rash consistent with monkeypox, regardless of whether the rash is disseminated or was preceded by prodrome. Likewise, although most cases to date have occurred among gay, bisexual, and other men who have sex with men, any patient with rash consistent with monkeypox should be considered for testing. CDC is continually evaluating new evidence and tailoring response strategies as information on changing case demographics, clinical characteristics, transmission, and vaccine effectiveness become available.
What is added by this report?
Among U.S. monkeypox cases with available data, 99% occurred in men, 94% of whom reported recent male-to-male sexual or close intimate contact; racial and ethnic minority groups appear to be disproportionately affected. Clinical presentations differed from typical monkeypox, with fewer persons experiencing prodrome and more experiencing genital rashes.
What are the implications for public health practice?
Public health efforts should prioritize gay, bisexual, and other men who have sex with men, who are currently disproportionately affected, for prevention and testing, address equity, and minimize stigma, while maintaining vigilance for transmission in other populations. Clinicians should test persons with rash consistent with monkeypox, regardless of whether the rash is disseminated or was preceded by prodrome.
_______
_______
Case definition:
WHO suggested 2022 outbreak case definition:
-1. Suspected case:
(1) A person who is a contact of a probable or confirmed monkeypox case in the 21 days before the onset of signs or symptoms, and who presents with any of the following: acute onset of fever (>38.5°C), headache, myalgia (muscle pain/body aches), back pain, profound weakness or fatigue.
OR
(2) A person presenting since 01 January 2022 with an unexplained acute skin rash, mucosal lesions or lymphadenopathy (swollen lymph nodes). The skin rash may include single or multiple lesions in the ano-genital region or elsewhere on the body. Mucosal lesions may include single or multiple oral, conjunctival, urethral, penile, vaginal, or ano-rectal lesions. Ano-rectal lesions can also manifest as ano-rectal inflammation (proctitis), pain and/or bleeding.
AND
for which the following common causes of acute rash or skin lesions do not fully explain the clinical picture: varicella zoster, herpes zoster, measles, herpes simplex, bacterial skin infections, disseminated gonococcus infection, primary or secondary syphilis, chancroid, lymphogranuloma venereum, granuloma inguinale, molluscum contagiosum, allergic reaction (e.g., to plants); and any other locally relevant common causes of papular or vesicular rash.
N.B. It is not necessary to obtain negative laboratory results for listed common causes of rash illness in order to classify a case as suspected. Further, if suspicion of monkeypox infection is high due to either history and/or clinical presentation or possible exposure to a case, the identification of an alternate pathogen which causes rash illness should not preclude testing for MPXV, as co-infections have been identified.
_
-2. Probable case:
A person presenting with an unexplained acute skin rash, mucosal lesions or lymphadenopathy (swollen lymph nodes). The skin rash may include single or multiple lesions in the ano-genital region or elsewhere on the body. Mucosal lesions may include single or multiple oral, conjunctival, urethral, penile, vaginal, or ano-rectal lesions. Anorectal lesions can also manifest as ano-rectal inflammation (proctitis), pain and/or bleeding.
AND
One or more of the following:
- has an epidemiological link to a probable or confirmed case of monkeypox in the 21 days before symptom onset
- Identifies as gay, bisexual or other man who has sex with men
- has had multiple and/or casual sexual partners in the 21 days before symptom onset
- has detectable levels of anti-orthopoxvirus (OPXV) IgM antibody (during the period of 4 to 56 days after rash onset); or a four-fold rise in IgG antibody titer based on acute (up to day 5-7) and convalescent (day 21 onwards) samples; in the absence of a recent smallpox/monkeypox vaccination or other known exposure to OPXV
- has a positive test result for orthopoxviral infection (e.g., OPXV-specific PCR without MPXV-specific PCR or sequencing)
_
-3. Confirmed case:
A person with laboratory confirmed monkeypox virus infection by detection of unique sequences of viral DNA by real-time polymerase chain reaction (PCR) and/or sequencing.
_
Discarded case:
A suspected or probable case for which laboratory testing of lesion fluid, skin specimens or crusts by PCR and/or sequencing is negative for MPXV.
Conversely, a retrospectively detected probable case for which lesion testing can no longer be adequately performed (i.e., after the crusts fall off) and no other specimen is found PCR-positive, would remain classified as a probable case. A suspected or probable case should not be discarded based on a negative result from an oropharyngeal, anal or rectal swab.
_
Definition of a contact:
A person who has been exposed to an infected person during the infection period i.e., the period beginning with the onset of the index case’s first symptoms and ending when all scabs have fallen off, and who has one or more of the following exposures with a probable or confirmed case of monkeypox:
- direct skin-to-skin and skin-to-mucosal physical contact (such as touching, hugging, kissing, intimate or sexual contact)
- contact with contaminated materials such as clothing or bedding, including material dislodged from bedding or surfaces during handling of laundry or cleaning of contaminated rooms
- prolonged face-to-face respiratory exposure in close proximity
- respiratory exposure (i.e., possible inhalation of) or eye mucosal exposure to lesion material (e.g., scabs/crusts) from an infected person
- the above also apply for health workers potentially exposed in the absence of proper use of appropriate personal protective equipment (PPE)
______
______
Who is at risk:
People who live with or have close contact (including sexual contact) with someone who has monkeypox are most at risk. Anyone living with someone who has monkeypox should take steps to reduce the risk of becoming infected. A person who has a monkeypox infection should be assessed by a health care provider to determine if they are well enough to be cared for at home and if isolation can be safely managed at home. Health workers should follow infection prevention and control measures to protect themselves while caring for patients with monkeypox. Newborn infants, young children and people with underlying immune deficiencies may be at higher risk of more serious symptoms, and in rare cases, death from monkeypox.
People who were vaccinated against smallpox may have some protection against monkeypox. However, younger people are unlikely to have been vaccinated against smallpox because smallpox vaccination stopped in most settings worldwide after the disease was eradicated in 1980. People who have been vaccinated against smallpox should continue to take precautions to protect themselves and others.
_
What is the risk of further spread of current monkeypox outbreak?
Human-to-human transmission of MPX occurs through close contact with infectious material from skin lesions of an infected person, and also through respiratory droplets in prolonged face-to-face contact and through fomites. The predominance, in the current outbreak, of diagnosed human MPX cases among MSM, and the nature of the presenting lesions in some cases, suggest that transmission occurred during sexual intercourse. Transmission through intact skin contact is less likely but cannot be excluded. Although sequencing data are not yet available to indicate that the outbreak is the result of one introduction, the cases of MPX within parts of the MSM community whose sexual networks are inter-connected could be considered a possible source of introduction.
_
Particular sexual practices (e.g. having multiple casual sexual contacts and/or multiple sexual partners, attending chemsex parties) that may be present within some parts of the MSM community could further facilitate the transmission of monkeypox. Outbreaks of other sexually transmitted infections among MSM can be linked to travel abroad and to social and mass gathering events (e.g., pride events). Several such events are taking place in Europe over the spring and summer months, which can contribute to further accelerate the transmission of MPXV. In addition, smallpox vaccination, which confers cross-protection, has been discontinued since the 1980s and only a small percentage of military and frontline health professionals have been vaccinated in recent years. Therefore, a large part of the population is vulnerable to MPXV. The probability of further spread of MPXV among persons with multiple sexual partners in interconnected sexual networks (including some groups of MSM) in EU/EEA countries and globally, in the coming months, is therefore assessed as high.
_
While most MPX cases reported thus far in this outbreak have been described as mild, the number of reported cases is too low to reliably estimate rates of severe morbidity and mortality, and a clear overview of the clinical presentations in the reported cases is currently lacking. Severity estimates in the literature exist from endemic countries and the 2003 USA outbreak. In Nigeria, the CFR is estimated at 3.3% for cases diagnosed between 2017-2022, however, it is a different health care and population setting, where the disease is endemic and is probably transmitted through different routes (e.g., more frequent contact with animals). In the 2003 outbreak in the USA, which was exclusively driven by contact with infected pets (rodents), five out of 34 confirmed cases (15%) were defined as severely ill, and no deaths were reported. Patients under 18 years of age did not develop severe illness more frequently, compared to older patients. These severity estimates are probably biased upwards. Immunocompromised patients are believed to be more at risk for severe disease and the prevalence of HIV among MSM is higher than in the broader population. However, most people living with human immunodeficiency virus (PLWHIV) in EU/EEA (range 67–87%) are receiving antiretroviral treatment, and are not severely immunocompromised. Moreover, some treatment options are available for severe MPX cases. Therefore, the impact of MPX is assessed as low, which combined with the high probability of infection leads to an overall moderate risk for persons with multiple sexual partners.
It should be noted that the above-mentioned moderate risk may be higher for older people who have multiple sexual partners or people with untreated HIV infection.
_
Risk for the broader population:
Based on the evidence from the cases in this outbreak detected to date, overall, the probability of further spread of MPXV among the broader population in EU/EEA countries and globally in the coming months, is assessed as very low leading to an overall low risk for the general population. However, the individual risk for very young children, pregnant women, elderly or immunocompromised individuals among close contacts of MPX cases may be high due to the higher impact of the disease in these groups.
_
Risk for health professionals:
Healthcare workers:
Transmission to HCWs exposed to patients with MPX is possible, given the risk of transmission of other orthopoxviruses, such as smallpox, and has been reported in outbreaks in endemic countries. In a study of 57 HCWs exposed to patients with MPX, including nursing staff, radiology technicians, emergency department staff and physicians, no case of infection was documented. One HCW in this study had evidence of recent orthopoxvirus seroconversion but had also received smallpox vaccination four months before being exposed. In another outbreak report, monkeypox was transmitted to a HCW, whose only identified exposure was the changing of potentially contaminated bedding of a hospitalised patient with MPX.
The probability of MPX transmission to HCWs wearing appropriate personal protective equipment (a disposable gown, disposable gloves, disposable shoe or boots covers, respiratory protection (Filtering Face Piece (FFP) 2 respirator), and eye splash protection (goggles or visor) is very low, with the disease having an estimated low impact, leading to an overall low risk.
The risk to HCWs with unprotected close contact with MPX cases (e.g. contact face-to-face for prolonged time, contact with open lesions without gloves, intubation or other invasive medical procedure) is assessed as moderate, equivalent to that of a close contact.
Laboratory personnel:
Occupational exposure and infection from orthopoxviruses have been occasionally reported among laboratory personnel handling virus-containing specimens.
The risk of occupational exposure is estimated to be low for trained laboratory personnel following appropriate biosafety procedures.
Unprotected occupational exposure in a laboratory, particularly involving spillage or aerosolisation with exposure of mucosa, carries high probability of infection and moderate risk of the disease (due to the direct exposure of mucosae to potentially significant quantity of virus). The risk for unprotected laboratory personnel is assessed as high.
Due to an expected higher impact, the risk may be higher for exposed HCWs and laboratory personnel who are older or immunocompromised.
_
Table shows summary of risk assessed for the different population categories:
|
|
Persons with multiple sexual partners, including some MSM |
Broader population |
|
Health professionals |
||
|
|
|
HCWs |
Laboratory personnel |
|||
|
|
Proper PPE |
Unprotected exposure |
Proper procedure and PPE |
Unprotected exposure |
||
|
Probability |
High |
Very low |
Very low |
High |
Very low |
High |
|
Impact |
Low |
Low |
Low |
Low |
Low |
Moderate |
|
|
|
|
|
|
||
|
Overall risk |
Moderate |
Low |
Low |
Moderate |
Low |
High |
The risk may be higher for certain people in some of the above categories, particularly very young children, pregnant women, elderly, or immunocompromised persons.
_
Risk of transmission through substances of human origin:
No cases of monkeypox virus transmission through substances of human origin have ever been documented. However, there are reported cases of virus transmission from mother to child during pregnancy, and animal studies show the presence of virus in blood, tissues and organs of infected animals. Existence of viremia (i.e. blood specimens positive for viral DNA) has been shown. The duration of viremia is unclear, and there are no data on viraemia in asymptomatic patients (including during the incubation period). Even though information is limited, it is likely that monkeypox virus is transmissible through substances of human origin, but the overall risk for recipients in the EU/EEA is low.
_
Risk of spill-over event to animal species in Europe:
Currently, little is known about the suitability of European peri-domestic (mammalian) animal species to serve as a host for monkeypox virus. However, rodents, and particularly species of the family of Sciuridae (squirrels) are likely to be suitable hosts, more so than humans, and transmission from humans to (pet) animals is theoretically possible. Such a spill-over event could potentially lead to the virus establishing in European wildlife and the disease becoming an endemic zoonosis. In the US, there is no evidence that the virus became enzootic in wildlife, however, animal health authorities carried out systematic surveillance and an aggressive campaign for exposed animals during the 2003 outbreak. The probability of this spill-over event is very low.
______
______
Section-8
Monkeypox Transmission:
Figure above shows transmission of human monkeypox. In endemic countries, spillover events occur from zoonotic animal reservoirs into humans, potentially leading to limited outbreaks usually facilitated by close human contact. Outbreaks can also occur in nonendemic regions through introduction of the virus via human travel or importation of animals harboring the virus. Subsequent human-to-human transmission can then occur via household contacts and via other close contacts.
_
Animal-to-human and human-to-human transmission can occur. Human to animal transmission can also occur. Monkeypox is a zoonotic disease, meaning that it can spread between animals and people, and is caused by Monkeypox virus, an Orthopoxvirus. Monkeypox virus is believed to have several modes of transmission, all of which are associated with direct contact with infected animals or the with infected humans, as depicted in Figure below. Human infections have been linked to contact with animals, but the precise exposure of a human case can be difficult to pinpoint in areas where contact with animals via household rodent infestations and the hunting or preparation of bushmeat from a variety of species is common. The exact mode of transmission of monkeypox is still under investigation, however, the suspected modes of the transmission shown in Figure below are those that were listed as risk factors for monkeypox contraction by Bunge et al. Animal to human transmission is direct contact or exposure with infected animals and most commonly, due to bodily fluids such as saliva, respiratory excretions, or could be the exudate from cutaneous or mucosal lesions. Viral shedding via feces may represent another exposure source. Exposure to feces of infected animals can be an important risk factor in endemic regions of Africa where resources and infrastructure are scarce, causing individuals to sleep outside, on the ground, or live near or visit the forest where infected animals are much more prevalent. In areas of scarce resources, such as food, households are left with no choice but to hunt and cook small mammals, increasing their risk of exposure to monkeypox. Although human-to-human transmission is less common than animal-to-human, it usually involves respiratory droplets with prolonged face-to-face contact or contact with lesions of an infected individual. Contaminated objects/surfaces, such as sleeping on the same bedding, living in the same household, or eating or drinking from the same dishes as an infected individual, are deemed a risk factor for viral transmission among individuals of the same household. Amid the current, ongoing monkeypox epidemic, it has also been observed that the disease is more common in males who have sex with males. According to the World Health Organization (WHO), it is not yet known whether monkeypox is sexually transmitted or not, however, the transmission can be attributed to close contact. The pathogenesis and pathophysiology of monkeypox begin from the transmission of the virus, whether it be human-to-human transmission or animal-to-human transmission.
Figure above shows Suspected Modes of Transmission of Monkeypox to Humans.
_____
_____
Animals to humans:
Animal-to-human (zoonotic) transmission can occur from direct contact with the blood, bodily fluids, or cutaneous or mucosal lesions of infected animals. In Africa, evidence of monkeypox virus infection has been found in many animals including rope squirrels, tree squirrels, Gambian pouched rats, dormice, different species of monkeys and others. The natural reservoir of monkeypox has not yet been identified, though rodents are the most likely. Eating inadequately cooked meat and other animal products of infected animals is a possible risk factor. People living in or near forested areas may have indirect or low-level exposure to infected animals. Monkeypox can spread to people when they come into physical contact with an infected animal such as a non-human primate, terrestrial rodent, antelope, gazelle, or tree squirrel – for example through bites or scratches, or during activities such as hunting, skinning, trapping, cooking, playing with carcasses. The risk of catching monkeypox from animals can be reduced by avoiding unprotected contact with wild animals, especially those that are sick or dead (including their meat and blood). In countries where animals carry monkeypox, any foods containing animal parts or meat should be cooked thoroughly before eating.
The 2003 outbreak isn’t the only time rodents—specifically, pets—have been incriminated in virus transmission. Over the past 20 years, European researchers have repeatedly identified cowpox, a related virus in the orthopox genus, passing to kids from pet rats that may have come from dodgy breeders, or from pet cats who similarly might have been infected at a breeding facility—or might have caught infected rodents and then become infected themselves. There is one documented case of a wild rodent passing that disease to a human, a 14-year-old girl who found an injured rat outdoors and tried to nurse it back to health. (The rat died. The girl survived.)
_____
_____
Human to human:
Human-to-human transmission can result from close contact with respiratory secretions, skin lesions of an infected person or recently contaminated objects. Transmission via droplet respiratory particles usually requires prolonged face-to-face contact, which puts health workers, household members and other close contacts of active cases at greater risk. However, the longest documented chain of transmission in a community has risen in recent years from 6 to 9 successive person-to-person infections. This may reflect declining immunity in all communities due to cessation of smallpox vaccination. Transmission can also occur via the placenta from mother to fetus (which can lead to congenital monkeypox) or during close contact during and after birth. While close physical contact is a well-known risk factor for transmission, monkeypox can be transmitted through sexual transmission routes.
While the virus itself is not a sexually transmitted infection, which are generally spread through semen and vaginal fluids, the most recent surge in cases appears to have been spread among men who have sex with other men but anyone can contract monkeypox. Many diseases can be spread through sexual contact. You could get a cough or a cold through sexual contact, but it doesn’t mean that it’s a sexually transmitted disease. The virus is spread through close contact with people, animals or material infected with the virus. It enters the body through broken skin, the respiratory tract, the eyes, nose and mouth. Though human-to-human transmission is believed to occur through respiratory droplets as well, that method requires prolonged face-to-face contact because the droplets cannot travel more than a few feet.
Route of person-to-person transmission — Human-to-human transmission of monkeypox virus can occur in several ways:
- Direct contact – Spread is thought to occur primarily through direct contact with infectious sores, scabs, or body fluids. As such, monkeypox can spread during activities that include close, personal contact with an infected individual. Mucous membrane microabrasions are a potential portal of entry.
During the ongoing worldwide outbreak of monkeypox reported beginning in May 2022 in nonendemic countries, close contact with infectious material from skin lesions (e.g., occurring during sexual and/or close intimate contact) is considered the main risk factor for acquisition. Although most cases during this outbreak have been seen in men who have sex with men, anyone who has direct skin-to-skin contact with someone who has monkeypox is at risk.
- Indirect contact through fomites – Transmission can occur through contact with materials or fomites that have become contaminated with infected material, such as clothing or linens contaminated with infectious material from body fluids or sores.
- Respiratory secretions – Monkeypox virus is also thought to be spread through respiratory secretions, although prolonged face-to-face contact may be required for transmission to occur via this route.
Activities resulting in resuspension of dried material from lesions (e.g., shaking contaminated linens) may also present a risk and should be avoided.
- Vertical transmission – The virus can cross the placenta from the mother to her fetus, which can lead to congenital monkeypox, although the rate of transmission or risk by trimester is not known. In a report of four pregnant women with monkeypox from the Democratic Republic of the Congo, one gave birth to a healthy infant, two had miscarriages in the first trimester, and one had fetal death with the stillborn showing diffuse cutaneous maculopapillary skin lesions consistent with vertical transmission.
_
Viral shedding and period of infectiousness:
Typically, a person with MPX is infectious and can pass the virus on to others from when they first develop symptoms until their lesions or scabs crust, dry or fall off. A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelialization has occurred. The infectious period will normally last for around two to four weeks.
A report of seven cases in the United Kingdom between 2018 and 2021 demonstrated polymerase chain reaction (PCR) positivity in blood and upper respiratory tract samples for at least three weeks in three of the patients. However, it is not clear what this means with respect to infectivity, and the correlation of PCR and culture positivity was not described in the report. Thus, PCR testing of throat or blood samples is generally not done for clinical decision making, and the duration of isolation is determined by clinical evaluation. The infectiousness of individuals who have no symptoms is uncertain.
_
Person-to-person transmission of MPXV classically occurs through large respiratory droplets, close contact with skin lesion exudates, and contaminated fomites. Pooled estimates suggest a secondary attack rate of approximately 8% (range 0–11%) among household contacts who are unvaccinated against smallpox. Sexual transmission might be possible given the detection of MPXV DNA in seminal fluid and the high rate of primary genital and anal mucosal lesions following condomless sexual activity in the 2022 outbreak. The caveat, however, is that isolating MPXV in seminal fluid is not necessarily evidence of infectivity because viremia is known to seed the reproductive tract. The basic reproduction number, R0, for monkeypox is estimated to be 0.8 but >1 among men who have sex with men. For context, SARS-CoV-2 has a strain-dependent R0 of 2.5 (original strain), 7 (delta variant B.1.617.2) and 10 (omicron variant), respectively, while smallpox had an R0 between 3.5 – 6.21
Because of their large DNA (~197 kb), orthopoxviruses are better at detecting and repairing mutations than RNA viruses (e.g., SARS-CoV-2). Consequently, this had previously resulted in only 1-2 substitutions per genome per year, which made MPXV a virus with presumably low epidemic potential. However, genomic sequencing studies have revealed that the 2022 MPXV strain contains 6-12 times the expected number of single-nucleotide polymorphisms, suggesting accelerated evolution and increased human adaptation. These might have contributed to cryptic human transmission of monkeypox for years before the global outbreak was amplified by super-spreading events in 2022.
_
While anyone can get MPX, the current global outbreak has disproportionately impacted:
- men who have sex with men (MSM)
- people who have sex with MSM. This may include people of any gender or sexual identity, whether they are transgender or cisgender, and non-binary people.
There is higher risk for these communities, particularly if there are multiple or anonymous sexual partners. Anyone who will have close physical or sexual contact while overseas, or with people who’ve recently been overseas, is advised to be mindful of MPX symptoms. This is particularly important for MSM, their sexual partners and anyone who has multiple or anonymous sexual partners.
_____
_____
Human to animals:
Recent reports of monkeypox transmission from humans to pets (dogs) are currently being investigated. Since many species of animals are known to be susceptible to the monkeypox virus, there is the potential for spillback of the virus from humans to susceptible animal species in different settings, which could lead to the formation of novel animal reservoirs. People who have confirmed or suspected monkeypox should avoid close physical contact with animals, including pets (such as cats, dogs, hamsters, gerbils etc.), livestock and wildlife.
According to a study, doctors from Pitié-Salpêtrière Hospital reported two cohabitating male patients showing symptoms of monkeypox, including fever, headaches, and rash. Twelve days following the onset of symptoms, the patient’s male Italian greyhound tested positive for the monkeypox virus. The dog also had lesions associated with the disease. The dog owners told doctors they slept with their dog in the same bed. Doctors reportedly compared skin lesion samples from both the dog and its owners and found it was the same virus strain. Based on the first suspected case of human-to-dog monkeypox transmission, the CDC has a message for pet parents — if you contract monkeypox, stay away from your pets for 21 days to avoid transmission:
- Infected people should not take care of exposed pets.
- The person with monkeypox should avoid close contact with the exposed pet, and when possible, ask another household member to care for the animal until the person with monkeypox is fully recovered.
- Cats and dogs should be kept under household isolation with regular checkups to ensure no signs of monkeypox have appeared.
- Pocket pets (like hamsters, gerbils, etc.) should also be removed for a quarantine period of three weeks to test and remove the infection, as they’re actually much more likely to carry the virus and pass it to humans (per the UK Health Security Agency).
Symptoms of monkeypox to look for in your pets:
- Lesions/rash
- Cough and other respiratory signs
- Fever
- Conjunctivitis (aka pink eye)
- Decreased appetite
Reach out to your veterinarian with your concerns if any of these signs appear in your pet.
_
The risk of reverse zoonotic transmission to pet animals during the current global monkeypox outbreak, United Kingdom, June to mid-September 2022:
Authors report results of surveillance between June and mid-September 2022 of pet animals living in households of confirmed human monkeypox (MPX) cases. Since surveillance commenced, 154 animals from 40 households with a confirmed human MPX case were reported to the United Kingdom Animal and Plant Health Agency. No animals with clinical signs of MPX were identified. While a risk of transmission exists to pets from owners with a confirmed MPX virus infection, authors assess this risk to be low. This British study found no human-to-pet transmissions, even after reports of two such cases in Brazil and France primed fears that the virus could spill into a new wild-animal reservoir. However, authors highlighted the possibility of monkeypox spilling over to rodents, which, they wrote, could then infect domesticated animals that could in turn transmit the virus to humans.
______
______
Airborne or not:
According to CDC, monkeypox primarily spreads through close contact with an infected person or items that they may have touched, such as bedsheets. “All the cases we’ve seen to date in this outbreak are related to direct contact, either through skin-to-skin contact or through bed sheets,” said CDC Director Rochelle Walensky.
In addition, the agency acknowledged that monkeypox may sometimes be spread through “droplet transmission” by saliva or large respiratory secretions, but said there is no evidence to suggest the virus is airborne. “When we consider airborne transmission at the CDC, we’re talking about small viral particles that become suspended in the air and can stay there for long periods of time,” Walensky said. “We have not seen documentation of that through our experience with this virus or with prior similar viruses.”
However, the World Health Organization and other health experts say airborne transmission, particularly over small distances, continues to be a possibility, and precautions, including masking for those in close contact with infected patients, may be necessary. “Airborne transmission may not be the dominant route of transmission nor very efficient, but it could still occur,” said Linsey Marr, an expert on airborne viruses at Virginia Tech.
Clinical features and management of human monkeypox: a retrospective observational study in the UK in august 2022 found that prolonged upper respiratory tract viral DNA shedding after skin lesion resolution challenged current infection prevention and control guidance. All lesions had crusted by day 12 of illness; however, despite clearance of viraemia, monkeypox DNA remained detectable by PCR in upper respiratory tract swabs until day 20. The limitations of this study are its observational nature, the small number of cases, and author’s inability to confirm positive PCR results with viral culture assays to demonstrate ongoing shedding of viable virus. So the infectivity of patients with positive upper respiratory tract swabs and crusted skin lesions remains undetermined.
So far, no cases of long-range airborne transmission have been reported. Some research from the United Kingdom suggests it may be possible for monkeypox to become aerosolized, particularly during certain activities like changing contaminated bedding. According to the Public Health Agency of Canada (PHAC), evidence of airborne transmission of smallpox also raises a concern that monkeypox may spread in a similar way. Even so, airborne transmission “does not appear to be the primary mode of transmission,” PHAC says.
_
Air and surface sampling for monkeypox virus in UK hospitals, July 2022:
Background:
An unprecedented outbreak of monkeypox virus (MPXV) infections in non-endemic countries has been recognised since 12 May 2022. More than 6000 cases have been identified globally with more than 1500 in the UK by July 2022. Transmission of MPXV is believed to be predominantly through direct contact with lesions or infected body fluids, with possible involvement of fomites and large respiratory droplets. Importantly, a case of monkeypox in a UK healthcare worker in 2018 was suspected to be due to virus exposure while changing bedding.
Methods:
Authors investigated environmental contamination with MPXV from infected patients admitted to isolation rooms in the UK, to inform infection prevention and control measures. Surface swabs of high-touch areas in isolation rooms, of healthcare worker personal protective equipment (PPE) in doffing areas, and from air samples collected before and during bedding change were analysed using MPXV qPCR to assess contamination levels. Virus isolation was performed to confirm presence of infectious virus in key positive samples.
Findings:
Authors identified widespread surface contamination (66 positive out of 73 samples) in occupied patient rooms (MPXV DNA Ct values 24·7-38·6), on healthcare worker personal protective equipment after use, and in doffing areas (Ct 26·3-34·3). Five out of fifteen air samples taken were positive. Significantly, three of four air samples collected during a bed linen change in one patient’s room were positive (Ct 32·7-35·8). Replication-competent virus was identified in two of four samples selected for viral isolation, including from air samples collected during the bed linen change.
Interpretation:
These data demonstrate significant contamination in isolation facilities and potential for aerosolisation of MPXV during specific activities. PPE contamination was observed after clinical contact and changing of bed linen. Additionally, contamination of hard surfaces in doffing areas supports the importance of cleaning protocols, PPE use and doffing procedures.
_
There is some evidence to support that the virus can be suspended in the air, according to a July 2022 study narrated above. The U.K. researchers conducted the study to inform infection prevention control measures after a U.K. healthcare worker was suspected to have contracted the virus while changing bedding. The study took place in U.K. healthcare facilities, and the researchers found the virus in air samples collected after bedding changes in isolation rooms used for infected patients, supporting the theory the virus may be present in suspended skin particles or dust.
Eric Feigl-Ding, PhD, an epidemiologist and former faculty member at Boston-based Harvard Medical School, has also raised concern about the potential for respiratory transmission. “It’s not really true that it’s ‘not known to linger in the air,'” he wrote in a July 28 tweet, referencing the U.K. study and a CDC tweet that said monkeypox is not known to linger in the air. But even with early evidence suggesting the virus can become airborne in some capacity, it’s not yet fully clear whether it can infect someone.
_
My view:
As DNA is an environmentally stable molecule, detection of viral DNA by PCR cannot be equated with infectious virus. Unless you are able to grow virus in cultures from environmental samples, mere detection of DNA by PCR in environmental samples do not prove that infection can occur from contact with these surfaces or air. Infection-competent virus means viral particles capable of infecting a cell and resulting in the production of new viral particles. Detection of virus, even replication-competent virus, in environmental samples does not mean that transmission leading to infection would necessarily occur if someone were exposed to that virus, as there are many factors that can influence successful infection of a human. These factors include routes of transmission, host susceptibility, environmental factors that could weaken the virus’ ability to infect cells and replicate, and the amount of virus to which one is exposed.
One study did find monkeypox virus (DNA and virus by isolation) in environmental air samples from health-care settings where air sample was collected during a bedding change; and detection of monkeypox virus DNA in air samples collected at distances of greater than 1·5 m from the patient’s bed and at a height of about 2 m supports the theory that monkeypox virus can be present in either aerosols or suspended skin particles or dust containing virus, and not only in large respiratory droplets that fall to the ground within 1–1·5 m of an infected individual.
The epidemiologic data we have so far in this 2022 outbreak support that people are not contracting monkeypox through touching contaminated surfaces or breathing aerosols. The preponderance of data indicates it’s being transmitted through direct physical contact, whether sexual or non-sexual.
In the current 2022 outbreak, the vast majority of monkeypox cases – more than 95 per cent, are among men who have sex with men, with a median age of 36. If monkeypox was airborne, these infected MSMs would have transmitted it to their non-sexual contacts but it hasn’t happened. Every infected MSM has many non-sexual contacts including family members and co-workers but they haven’t got it. So airborne transmission of monkeypox is unlikely. Even previous outbreaks in Africa haven’t shown airborne spread. The inoculum dose and host susceptibility for a particular transmission mode affect the onward transmission of all viruses. Previous studies suggested that pathogens capable of aerosol transmission should be associated with a high reproductive number (R0) except pertussis that transmits via droplets has a much higher R0 than tuberculosis pathogen transmitted via aerosols. Despite vast susceptible population available unvaccinated by smallpox vaccines, the R0 of monkeypox is between 1 to 2 and therefore highly unlikely to be transmitted by air. In fact, higher R0 is seen due to sexual transmission in MSM community while R0 was less than 1 in endemic Africa due to droplet/contact transmission. There are no definite data on the required infectious dose with monkeypox virus in humans. However, in contrast to variola virus, a significantly higher dose is assumed to be required to trigger infection. The infectious dose of variola (small pox) is 10 to 100 virions and smallpox was airborne in addition to droplet/contact transmission. In non-human primates, monkeypox infection could be initiated by intrabronchial application of 5×104 plaque-forming units (PFU). Despite similarities between smallpox and monkeypox transmission, such high dose inoculum compared to variola makes monkeypox unsuitable for airborne transmission. Remember, infectious dose of tuberculosis bacteria is reported to be between 1 and 200 bacilli and a single aerosol can contain anywhere from 1 to 400 bacilli.
All in all, in my view, monkeypox is not airborne.
______
______
Fomite transmission or not:
There is past evidence from previous monkeypox outbreaks of someone catching monkeypox after touching contaminated objects. Objects, surfaces and fabrics can become contaminated with the monkeypox virus if they are touched by someone with monkeypox. The virus has been found to survive on some surfaces for some time in certain conditions. However, in this current outbreak, studies are still underway on whether people can catch monkeypox by touching surfaces and objects. As of now, nearly all cases are linked to close contact like touching or sex. Objects and surfaces can be cleaned with soap and water and common household disinfectants or a bleach product to kill the monkeypox virus.
_
A study in Morbidity and Mortality Weekly Report shows multiple surface sites testing positive for monkeypox virus genetic material in a household of two people infected with monkeypox in Utah. The two case-patients, who acquired the disease while traveling internationally, had isolated at home for 20 days before their home was entered for sampling by officials from the Utah Department of Health and Human Services (UDHHS). Agents collected samples from 30 objects in nine areas of the home. Of the 30 specimens, 21 (70%) yielded positive real-time polymerase chain reaction (PCR) results, indicating the presence of monkeypox virus DNA. The swabbed areas included those from all three porous items (cloth, furniture and blankets), 17 of 25 (68%) nonporous surfaces (handles and switches), and one of two mixed-surface types (chairs). The investigators attempted to grow live virus in the lab from PCR-positive samples but noted, “No specimen yielded a positive viral culture result.”
The contamination occurred despite the patients reporting showering once or twice each day, performing hand hygiene approximately 10 times daily, laundering bedding and clothing weekly, and performing routine household cleaning, such as mopping and daily use of a multi-surface spray on most high-contact surfaces, the authors said.
“Persons living in or visiting the home of someone with monkeypox should follow appropriate precautions against indirect exposure and transmission by wearing a well-fitting mask, avoiding touching possibly contaminated surfaces, maintaining appropriate hand hygiene, avoiding sharing eating utensils, clothing, bedding, or towels, and following home disinfection recommendations,” the authors concluded.
Infectious disease expert Michael Osterholm, PhD, MPH, however, put the findings in perspective. “The epidemiologic data we have so far in this 2022 outbreak support that people are not contracting monkeypox through touching contaminated surfaces. The preponderance of data indicates it’s being transmitted through direct physical contact, whether sexual or non-sexual.”
_
Evidence of surface contamination in hospital rooms occupied by patients infected with monkeypox, Germany, June 2022 study:
Since May 2022, the largest west-African-clade-monkeypox outbreak to date in countries with non-endemic occurrences has been described. The outbreak involves transmission among people in close physical contact with symptomatic cases, in contrast to previous outbreaks, where zoonotic transmission was reported as the main mechanism of spread. Nevertheless, events of person-to-person transmission have been previously described. Additionally, transmission to personnel taking care of patients was reported on rare occasions. Indirect transmission via contaminated objects is also discussed in the literature. However, there are insufficient data on the environmental contamination of surfaces with monkeypox virus. Authors systematically examined surfaces of two hospital rooms occupied by monkeypox patients and the adjacent anterooms, which are used for donning and doffing personal protective equipment (PPE), for monkeypox virus contamination using PCR. In addition, authors assessed the infectivity on cell culture of the collected samples by virus isolation. ‘Potentially’ infectious monkeypox viral loads was detected on high-touch hospital room surfaces, study finds. While researchers were unable to say whether viral loads detected in patient rooms and adjoining anterooms could lead to infection, they said new findings underscore the importance for hospital staff to follow recommended infection control and monkeypox protection measures.
In this study monkeypox virus was successfully isolated from three different samples, each with a total of at least 106 virus copies. Thus, contaminated surfaces with such viral loads or higher, could potentially be infectious and it cannot be ruled out that their contact with especially damaged skin or mucous membranes, could result in transmission. Detection of up to 1.1×viral 106 copies on gloves is consistent with the detection of viral DNA on surfaces typically handled only by medical staff such as the door handles of the anteroom. The detection of the virus at very low concentrations even outside the isolation unit indicates that containment protocols may not have been fully adhered to.
The findings in this report are subject to some limitations. As DNA is an environmentally stable molecule, detection of viral DNA by PCR cannot be equated with infectious virus. Despite high contamination with up to 105 cp/cm2 as well as the successful recovery of monkeypox virus from samples with a total of > 106 copies, these findings do not prove that infection can occur from contact with these surfaces. No secondary case in the context of clinical care of the two patients in this study has been observed so far. The study was performed only for two cases and might not be generalized to other cases. In particular, in certain cases, depending on the skin regions mainly affected and the number of lesions, the levels of contamination of different surfaces may vary.
_
Viable Monkeypox virus in the environment of a patient room, September 2022 study:
Authors conducted a prospective environmental surveillance study to investigate the air, surface, dust and water contamination of a room occupied by a patient infected with Monkeypox virus (MPXV) at various stages of his illness. The patient tested positive for MPXV from a throat swab and skin lesions. Environmental sampling was conducted in a negative pressure room with 12 unidirectional HEPA air changes per hour and daily cleaning of the surfaces. A total of 179 environmental samples were collected on days 7, 8, 13, and 21 of his illness. Air, surface, and dust contamination was highest during the first eight days of the illness, with a gradual decline to the lowest contamination level by day 21. Viable MPXV was isolated from surfaces and dust samples and no viable virus was isolated from the air and water samples.
The researchers recovered viable MPXV in nearly all air samples of the patient’s room, though not culturable, and extensive surface contamination of the patient’s chair, toilet seat, and dust from bed linen in the first week of infection, with gradual decline later. While the detection of MPXV DNA across sampling days showed continued viral shedding throughout the disease course, the recovery of viable virus from the chair and toilet seat correlated with the location of skin lesions. Likewise, viable MPXV in surface swabs and dust indicated a possibility of fomite-based transmission, especially in home settings.
The environmental contamination reduced from the second week of infection when the patient stopped developing new skin lesions. This finding highlighted the importance of disinfection of the surfaces of chairs, toilets, and floors and taking precautions when handling linens. The researchers found MPXV material only in particles of >4 μm sizes, which nullified the possibility that MPXV was transmitted via breathing or talking in this case. It could be due to 12 HEPA-filtered uni-directional air changes per hour or high ventilation rates. Therefore, future studies should examine direct breath samples in the environment with typical air conditions for a better understanding of the respiratory source of MPXV transmission.
Nevertheless, the presence of live MPXV in dust samples suggested lesion shedding as the potential source of air contamination. Likely, the inoculum dose and host susceptibility for a particular transmission mode affect the onward transmission of all viruses. Therefore, future studies should assess the MPXV transmission dynamics, including the infectious dose required to cause the disease.
In a nutshell, viable monkeypox virus found in nearly all environmental samples from patient’s room in first week of infection.
_
High attack rate in tattoo-linked outbreak:
Published in the Lancet Infectious Diseases is a report on several monkeypox cases linked to a piercing and tattoo parlor in Cadiz, Spain. Of 54 exposed clients, 20 (37%) contracted the disease from Jul 19 to Aug 3. All piercings and tattoos were performed by one female employee who did not have monkeypox, but was in close contact with a possible index case on Jul 6. That person got a piercing while experiencing a rash and generalized skin inflammation. Among the 20 exposed clients, 8 were under the age of 18 and 13 were female. In 90% of the cases, patients developed a rash at the piercing or tattoo site. Subsequent surface sampling showed extensively contaminated and unhygienic conditions, with detectable monkeypox viral DNA on work tables and chairs, and sharps and other work instruments. “Together, these findings suggest that monkeypox virus can be transmitted through exposure to contaminated piercing or tattoo material and, potentially through contaminated hands, due to poor aseptic measures and handling of materials,” the authors concluded.
_
Nurses infected after collecting samples:
In another study highlighting transmission routes, practitioners writing in Emerging Infectious Diseases describe the infections of two Brazilian nurses 5 days after collecting samples from a monkeypox patient. The authors say the virus was most likely transmitted via contact with contaminated objects. The nurses visited with the patient in his home and had no skin-to-skin contact with the patient, and they reported no sharps injuries. They wore N95 respirators and gloves, but did not sanitize clipboards and specimen-collection boxes. “Our report provides evidence supporting the hypothesis that both [healthcare workers] infections observed in this study were transmitted through fomite exposure with surfaces in the patient’s home, their own [personal protective equipment], or outer surfaces of the specimen transport box,” the authors wrote.
______
______
Section-9
Sexual transmission of monkeypox in current outbreak:
Ever since monkeypox started to sicken thousands of people worldwide this spring, two big questions have loomed:
Why is a virus that has never managed to spread beyond a few cases outside Africa suddenly causing such a big, global outbreak?
And why are the overwhelming majority of those affected men who have sex with men (MSM)?
A long history of work on sexually transmitted infections (STIs) and early studies of the current outbreak suggest the answers may be linked: The virus may have made its way into highly interconnected sexual networks within the MSM community, where it can spread in ways that it cannot in the general population. The virus did not spread well between people in the past but may have found a new niche in tightly connected sexual networks. An epidemiological modeling study by researchers at the London School of Hygiene & Tropical Medicine (LSHTM) supports that idea. It suggests the outbreak will keep growing rapidly if the spread isn’t curtailed. It also has implications for how to protect those most at risk and limit spread, while suggesting the risk for the wider population remains low.
Before this year’s global outbreak, scientists typically thought the monkeypox virus (MPXV) primarily reached humans through contact with infected animals, leading to household transmission and limited outbreaks in regions of West and Central Africa where this pathogen is endemic. But there were hints MPXV was spreading through sexual networks as well, with Nigerian scientists ringing alarms during a countrywide outbreak in 2017 that largely impacted sexually active young men, often causing genital lesions. So it is possible that monkeypox has also been spreading through sexual contact and has kind of flown under the radar, and found its way into very densely connected sexual networks that allowed it to be amplified.
In Canada, the disease is mostly reported among men who have sex with men, and 99 per cent of infected individuals identify as male, with a median age of 36.
Among U.S. monkeypox cases with available data, 99 per cent also occurred in men, including 94 per cent who reported recent male-to-male sexual or close intimate contact.
Broad European data also suggests cases remain primarily among men who have sex with men between the ages of 18 to 50. According to the latest European Centre for Disease Prevention and Control risk assessment, the likelihood of MPXV spreading further in networks of people with multiple sexual partners is “considered high,” while the chance of it spreading into the broader population is “assessed as very low.”
But there are still many uncertainties, and communication is fraught because of the risk of stigmatizing MSM—and because communicating frankly about sexual behavior is hard. “I think we have to talk more about sex,” says Yale School of Public Health epidemiologist and former HIV activist Gregg Gonsalves. “Everybody has been very clear about stigma, and saying it over and over again. The point is that you still have to address the risk of infection in our community.” It’s important to alert that community and do it the right way, Gonsalves says. “We should say: It’s not about who you are. It’s about what you’re doing. And we’re not going to stigmatize it. But just know that you’re at greater risk if you fit this profile.”
_______
Sex between men, not skin contact, is fueling monkeypox:
The claim that skin-to-skin contact during sex between men, not intercourse itself, drives most monkeypox transmission is likely backward, a growing group of experts say.
Since the outset of the global monkeypox outbreak in May 2022, public health and infectious disease experts have told the public that the virus is largely transmitting through skin-to-skin contact, in particular during sex between men.
Now, however, an expanding cadre of experts has come to believe that sex between men itself — both anal as well as oral intercourse — is likely the main driver of global monkeypox transmission. The skin contact that comes with sex, these experts say, is probably much less of a risk factor.
In recent weeks, a growing body of scientific evidence — including various studies published in peer-reviewed journals, as well as reports from national, regional and global health authorities — has suggested that experts may have framed monkeypox’s typical transmission route precisely backward.
_
Reconceiving the primary risk factors for transmission is crucial because of how it may affect guidance on reducing the risk of infection, including the question of whether demanding that people with the virus self-isolate has any substantial impact on transmission.
“A growing body of evidence supports that sexual transmission, particularly through seminal fluids, is occurring with the current MPX outbreak,” said Dr. Aniruddha Hazra, medical director of the University of Chicago Sexual Wellness Clinic, referring to monkeypox and to recent studies that found the virus in semen. Consequently, scientists said that the Centers for Disease Control and Prevention and other public health authorities should update their monkeypox communication strategies to more strongly emphasize the centrality of intercourse among gay and bisexual men, who comprise nearly all U.S. cases, to the virus’ spread.
On Aug. 14, Dr. Jeffrey Klausner, an infectious disease physician at the University of Southern California, and Dr. Lao-Tzu Allan-Blitz, a resident physician in global health at Brigham and Women’s Hospital in Boston, published an essay on Medium in which they reviewed the science supporting the argument that during the current outbreak, monkeypox is largely transmitting through anal and oral intercourse between men. “It looks very clear to us that this is an infection that is transmitting sexually the vast majority of the time,” Allan-Blitz said.
This debate, however, is far from settled.
Dr. Rosamund Lewis, technical lead for monkeypox at the World Health Organization said that it was “unfortunate but true” that “we don’t know yet” whether the virus is predominantly transmitted through intercourse. “Completely reading the situation as uniquely due to anal or oral sex is highly likely to be overreach,” she said. “The correlation may appear to be strong, but that does not explain the whole picture of disease caused by this virus. So we need to keep an open mind.”
Some experts in infectious disease see evidence supporting the argument that monkeypox at least transmits more readily through intercourse. “At this point,” said Dr. Paul Adamson, an infectious disease specialist at the UCLA School of Medicine, “I’m not sure we can say it is primarily the sexual transmission and not the skin-to-skin contact that also occurs during sex that is contributing to the most transmission during this current outbreak. However, emerging data seem to suggest that monkeypox might be more efficiently transmitted sexually.”
_
In an interview, Klausner, who has submitted a version of his and Allan-Blitz’s essay to a scientific journal for publication, distilled the evidence that he said supports the hypothesis that sex itself fuels the global outbreak into four major points.
-1. First, he noted that, according to the WHO, more than three quarters of global monkeypox cases are among men 18 to 44 years old. This is a typical age breakdown for diagnoses of sexually transmitted infections among gay and bisexual men, he said. What’s more, in recent studies of pooled monkeypox cases among this demographic, 17% to 32% of those diagnosed with the virus received a sexually transmitted infection (STI) diagnosis at the same time.
-2. Second, during the global outbreak, atypical to what has historically been seen in the 11 African nations where the virus has become endemic since first being identified in humans in 1970, monkeypox lesions have in the majority of cases occurred in men’s genital and anorectal areas. This suggests that these were the sites where the virus first passed into the body.
In a study of 197 monkeypox cases in London men published July 28 in The BMJ, the British Medical Association’s journal, researchers found that 56% had lesions in the genital area and 42% had them in their anorectal regions. And in a study published July 21 in The New England Journal of Medicine, a global team of researchers pooled 538 monkeypox cases — also all in men — from around the world and found that 73% had lesions in the genital or anorectal areas.
-3. Third, researchers have found monkeypox in semen and have been able to culture that virus, which suggests it could transmit through ejaculation. Also, the authors of two recent studies have detected the virus after taking anal swabs among men who had monkeypox but were asymptomatic, which indicates that the virus might transmit from the anorectal area during anal intercourse before people develop symptoms. Experts say more research is needed on both these fronts.
Referring to bodily fluids such as semen, vaginal fluids and blood, the WHO’s Lewis said, “Research is underway to find out more about whether people can spread monkeypox through the exchange of these fluids during and after symptomatic infection.”
-4. Finally, Klausner noted that scientists have identified an association between specific sexual acts and the location of monkeypox lesions.
The authors of a paper published Aug. 8 in The Lancet documenting 181 cases of the virus in Spain found that 38% of the men who reported having receptive anal intercourse, called “bottoming,” developed proctitis, or inflammation of the rectum. Just 7% of the men who reported sex with men without bottoming developed this potentially excruciating symptom. Additionally, 95% of the men with tonsillitis reported performing oral sex on a man.
_
Dr. Oriol Mitjà, an associate professor in infectious disease at the University Hospital Germans Trias i Pujol in Spain and the joint senior co-author of the study in The Lancet, said monkeypox transmits most efficiently when lesions come into contact with mucus membranes in the anorectal area, genitals, mouth and throat. Monkeypox is more likely to transmit through oral or anal sex than through contact with external skin, which would need some sort of defect, such as a wound, to allow entry of the virus, Mitjà said.
Dr. Dimie Ogoina, a professor of medicine and infectious diseases at Niger Delta University in Nigeria, acknowledged Mitjà’s research supporting the connection between types of sex between men and monkeypox outcomes. “This is not to say that females or heterosexuals are not at risk of monkeypox or that the female genital mucosa is not prone to abrasions during sexual activity,” Ogoina said.
_
Some experts, like the WHO’s Lewis, maintain that the main mode of monkeypox transmission remains skin-to-skin contact — including during sex. Others, like Klausner and Adamson, say a number of infectious disease experts may resist believing intercourse is a predominant driver of the current outbreak because that is not how monkeypox has tended to spread in past decades. “Historically, the primary mode of transmission of monkeypox was through skin-skin contact, though there might have been some suggestion of sexual transmission in prior outbreaks. It takes some time and additional data to overturn our understanding of transmission,” Adamson said.
Monkeypox has been diagnosed in 62,000 people in 105 countries during this current global outbreak, and the WHO reports that among cases with proper data, 97% have been diagnosed in gay, bisexual and other men who have sex with men. The consistency with which cases have remained so overwhelmingly in this demographic, some experts argue, is further evidence that the virus transmits among them through a behavior that is exclusive to the group — anal intercourse and oral sex between men.
_
Meanwhile, across the global outbreak, the virus is also apparently following the same transmission patterns traditionally seen in Africa. But experts assert that just as in those African nations, when the virus transmits through nonsexual means, it does so with dramatically lower efficiency — and thus at a rate similar to the relatively slow spread seen in Africa.
Specifically, the authors of The New England Journal of Medicine paper estimated that just 0.8% of the cases they analyzed were due to nonsexual close contact and 0.6% were due to household contact. By contrast, 95% of these cases were likely acquired during sex between men. The authors of the Lancet paper estimated that 3% of the cases they analyzed transmitted through nonsexual household contact.
Dr. Monica Gandhi, an infectious disease physician at University of California, San Francisco, said the small number of global monkeypox cases in children have likely been transmitted through cuddling or hugging. She pointed to various STIs, including herpes, that in rare cases can also transmit nonsexually.
“STIs such as syphilis or chancroid are commonly found in children in the tropics, where abrasions on the arms and legs are common,” Mitjà said.
Referring to the recent rapid expansion of the global outbreak, Ogoina said, “It is all about numbers — the more sexual partners, the greater the likelihood for many to become exposed.”
If monkeypox is indeed overwhelmingly being transmitted through intercourse and rarely through more casual means, this challenges burdensome public health guidelines recommending that people with the virus isolate for the course of their illness, which can last for weeks, Mitjà and his coauthors argued in their paper.
Klausner called for updated communications from the CDC and other health authorities to emphasize the importance of sexual intercourse to monkeypox’s transmission.
“If we accept that this is how it’s spread, we know how to reduce the spread: by awareness and education and encouraging people for the time being to reduce sex with multiple partners until they get vaccinated,” Klausner said “And if they can’t reduce the behavior, to try to use a condom.”
CDC spokesperson Kristen Nordlund said the agency’s recent analyses “show most diagnosed cases of monkeypox in the United States are associated with sexual and intimate contact, which can involve a range of behaviors. Additional analyses are needed to understand if specific sexual and intimate behaviors that occur during sex are disproportionately contributing to spread.”
Harvard’s Lao-Tzu Allan-Blitz acknowledged the pervasive concern that telling the public that monkeypox transmits sexually among gay men will fuel homophobia. He said there is, however, also a cost to keeping quiet about how the virus apparently transmits: This keeps people at risk from best understanding how to protect themselves. “In our silence, we can also do harm,” he said.
_______
_______
Monkeypox Virus Infection in Humans across 16 Countries — April–June 2022, August 25, 2022 NEJM:
Authors report 528 infections diagnosed between April 27 and June 24, 2022, at 43 sites in 16 countries. Overall, 98% of the persons with infection were gay or bisexual men, 75% were White, and 41% had human immunodeficiency virus infection; the median age was 38 years. Transmission was suspected to have occurred through sexual activity in 95% of the persons with infection. In this case series, 95% of the persons presented with a rash (with 64% having ≤10 lesions), 73% had anogenital lesions, and 41% had mucosal lesions (with 54 having a single genital lesion). Common systemic features preceding the rash included fever (62%), lethargy (41%), myalgia (31%), and headache (27%); lymphadenopathy was also common (reported in 56%). Concomitant sexually transmitted infections were reported in 109 of 377 persons (29%) who were tested. Among the 23 persons with a clear exposure history, the median incubation period was 7 days (range, 3 to 20). Monkeypox virus DNA was detected in 29 of the 32 persons in whom seminal fluid was analyzed. Antiviral treatment was given to 5% of the persons overall, and 70 (13%) were hospitalized; the reasons for hospitalization were pain management, mostly for severe anorectal pain (21 persons); soft-tissue superinfection (18); pharyngitis limiting oral intake (5); eye lesions (2); acute kidney injury (2); myocarditis (2); and infection-control purposes (13). No deaths were reported.
_
Sexual activity, largely among gay or bisexual men, was by far the most frequently suspected route of transmission. The strong likelihood of sexual transmission was supported by the findings of primary genital, anal, and oral mucosal lesions, which may represent the inoculation site. Monkeypox virus DNA that was detectable by PCR in seminal fluid in 29 of the 32 cases in which seminal fluid was tested further supports this hypothesis. However, whether semen is capable of transmitting infection remains to be investigated, since it is unknown whether the viral DNA detected in these specimens was replication competent. Reports of clusters associated with sex parties or saunas further underscore the potential role of sexual contact as a promoter of transmission. International travel and attendance at large gatherings linked to sex-on-site activities may explain the global spread of monkeypox infections amplified through sexual networks.
_
“It is important to stress that monkeypox is not a sexually transmitted infection in the traditional sense; it can be acquired through any kind of close physical contact,” said first author John Thornhill, in a statement. “However, our work suggests that most transmissions so far have been related to sexual activity — mainly, but not exclusively, amongst men who have sex with men,” he added. “This research study increases our understanding of the ways it is spread and the groups in which it is spreading which will aid rapid identification of new cases and allow us to offer prevention strategies.” Overall, 98 percent of infected people were gay or bisexual men, 41 percent had HIV and the median age was 38. Their median number of sex partners in the prior three months was five, and around a third were known to have visited sex-on-site venues such as sex parties or saunas within the previous month. Although sexual activity was behind most cases, the researchers stressed in a statement that the virus can be spread via any close physical contact, such as respiratory droplets and potentially through clothing and other surfaces.
______
______
Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain. June 2022:
Up to 22 June 2022, 508 confirmed cases of monkeypox (MPX) have been reported in the Madrid region of Spain, 99% are men (n = 503) with a median age of 35 years (range: 18–67). In this ongoing outbreak, 427 cases (84.1%) reported condomless sex or sex with multiple partners within the 21 days before onset of symptoms, who were predominantly men who have sex with men (MSM) (n = 397; 93%). Both the location of the rash, mainly in the anogenital and perineal area, as well as the presence of inguinal lymphadenopathy suggest that close physical contact during sexual activity played a key role in transmission. Several cases reported being at a sauna in the city of Madrid (n = 34) or a mass event held on the Spanish island of Gran Canaria (n = 27), activities which may represent a conducive environment for MPX virus spread, with many private parties also playing an important role. Because of the rapid implementation of MPX surveillance in Madrid, one of the largest outbreaks reported outside Africa was identified.
HIV infection and its level of control may modify the severity and duration of the clinical signs. Although 44.3% of cases reported being HIV-positive, there has been no observed increase in severity. This is likely because of the adequate immune control with undetectable viral load in almost all cases. A MPX outbreak studied in Nigeria in 2017–18 resulted in seven deaths among 118 confirmed cases (6%), of whom four were HIV-positive with poor disease control. The high percentage of HIV-positive cases in this report could be because of the ease of patient access to the Spanish healthcare system, and therefore a high testing rate.
There is some debate about the protection provided by the smallpox vaccine against disease occurrence and severity. In Spain, smallpox vaccination was discontinued in the early 1980s, so people under 40 years of age would not have been vaccinated. In this outbreak, the median age of cases was 35 years, and 65% were aged under 40 years, so they would be less protected than other age groups against MPX, but vaccination status was unknown for most cases. Thus far, however, this has not translated into a higher severity of cases.
_______
_______
Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022:
Authors aimed to characterize viral shedding to better understand the possible role of different bodily fluids in disease transmission and investigated the presence of MPX virus DNA in saliva, rectal swab, nasopharyngeal swab, semen, urine and faecal samples, from 12 MPX patients in Barcelona, Spain.
At the time of diagnosis, MPX DNA was detected in swabs of skin lesions in all 12 cases. In most cases (9/12), high viral loads (quantification cycle (Cq) value range: 16–21) were observed in skin pustules and some patients presented with additional oral, pharyngeal and rectal lesions.
In all cases, MPX DNA was detected in several follow-up samples taken between 4 and 16 days post-symptom onset, and in one third (Patients 06, 09, 10, and 12), DNA was detected at some point during the follow-up period in all types of clinical samples analysed. High viral loads (Cq values ≤ 21) were observed in some saliva, rectal swab, semen, urine and faecal samples. Intermittent shedding (negative PCR results that became positive in the following time point collected) was also observed in samples such as urine or saliva (Patients 03 and 05). Importantly, MPX virus was detected in saliva from all 12 patients studied, and in some cases, at low Cq values indicative of high viral loads. In addition, the other clinical samples tested were frequently positive for MPX virus: rectal swab (11/12 cases), nasopharyngeal swab (10/12 cases), semen (7/9 cases), urine (9/12) and faeces (8/12).
Authors did not perform cell culture, and a clear correlation between real-time PCR and virus isolation has not been reported in existing literature. However, results from studies in animal samples that quantified MPX virus and performed cell culture indicate that virus isolation can be successful with viral loads in the range of 105 – 106 copies/ml. Furthermore, during the present outbreak, MPX virus has been isolated from skin lesion samples with a Cq of 20 in one case. With the low Cq values observed in this study in a variety of samples such as saliva, rectal swab, semen, urine and faecal samples, further research on the infectious potential of these bodily fluids and their potential role in disease transmission by close physical contact during sexual activity is warranted.
_______
_______
Viral loads in clinical samples of men with monkeypox virus infection: a French case series, September 29, 2022
The new analysis of 356 samples from 50 men in France infected with monkeypox shows that viral DNA detection via polymerase chain reaction (PCR) was more frequent from skin (88% of men), throat (77%), and anus (71%) swabs, than from semen (54%), blood (29%), or urine (22%).
The highest viral DNA loads were consistently found in skin and anus swabs, and lesions were common on the anus and genitals, which continues to suggest sexual contact as the main route of transmission. The study, published in The Lancet Infectious Diseases is one of several new studies that aim to describe real-world transmission risks of the poxvirus.
The researchers assessed viral load using cycle threshold (Ct) values, which are lower when viral loads are high. Viral loads were significantly higher from skin lesions (Ct, 19.8) and anal samples (Ct, 20.9) than from throat (27.2), semen (27.8), urine (31.1), or blood (32.8) samples.
The median age in the study was 34, with 44% of the men HIV-positive. By day 14 of swabbing, the proportion of positive samples dramatically decreased on the skin, and in anus, throat, blood, urine, and semen samples.
“High MPXV viral loads from skin and mucosa, including genital and anal sites, suggest that transmission most likely occurs through direct body contact rather than through the respiratory route or contact with body fluids, which should help to refine the prevention messages delivered to individuals most exposed to the virus,” the authors wrote.
They added, “The detection of MPXV at high concentrations in the anal region, in the mouth, and in semen is consistent with the sexual practices potentially involved in the spread of the virus among men who have sex with men, in addition to skin contact related to sexual or non-sexual proximity.”
_______
_______
Monkeypox study spotlights role of sexual transmission, October 2022:
A new study of GeoSentinel Network data involving 226 monkeypox cases from 15 countries shows that, of 219 patients for whom data were available, 216 (99%) reported sexual or close intimate contact in the 21 days before symptom onset. The study is published in The Lancet Infectious Diseases and adds to a growing body of literature that shows the monkeypox outbreak is primarily fueled by sexual contact. Other important findings show that men who have sex with men (99% of all male patients) reported a median of three recent sexual partners, and clinical manifestations of the virus differed by HIV status, but HIV status did not correlate with more severe disease outcomes. Monkeypox patients who were HIV-positive were more likely to report diarrhea, anal rash and lesions, and a higher rash burden. A significant proportion of patients reported attending large mass gatherings before developing monkeypox symptoms. Of 161 patients with available information, 37 (23%) met their sexual partners at such gatherings, including the Maspalomas Festival on Spain’s Gran Canaria island, and various other Pride-related festivities in Europe and the United States, the authors said.
_______
_______
Italian researchers reported in The Lancet in august 2022 that they had found monkeypox DNA in the semen of a 39-year-old patient living with HIV who self-identified as a man who has sex with men, and a sex worker. He had reported condomless sex with several male partners in the month before infection. “Overall, our findings support that prolonged shedding of monkeypox virus DNA can occur in the semen of infected patients for weeks after symptoms onset, and show that semen collected in the acute phase of infection (day six after symptom onset) might contain a replication-competent virus and represent a potential source of infection,” according to the researchers, from the National Institute for Infectious Diseases in Italy. “Whether infectious monkeypox virus found in semen could be associated with seminal cells or if viral replication occurs in the genital tract remains to be established,” they note. However, they add that “the isolation of live replication-competent monkeypox virus from semen, and prolonged viral DNA shedding, even at low viral copies, might hint at a possible genital reservoir”. No monkeypox virus DNA was detected in urine and blood samples, suggesting absence of semen cross-contamination from other potential sources. The team also detected monkeypox virus DNA in semen samples from 11 (79 per cent) of 14 patients, and isolated live and replication-competent viruses from two patients with HIV.
In another study, researchers have detected monkeypox virus in the testes of macaques during the acute phase of infection for up to 37 days during convalescence although virus was cleared from most organs—and from healed skin lesions.
_
The vast majority of cases in the growing monkeypox outbreak are among men who have sex with men, according to the World Health Organization. WHO Director-General Tedros Adhanom Ghebreyesus advised members of this community to limit their exposure to the virus by reducing their number of sex partners and reconsidering sex with new partners.
Remember that close physical contact, including sex, may increase your risk of exposure. Having multiple and frequent sexual contacts, including with anonymous partners, may put you more at risk of infection of monkeypox. To protect yourself practice safer sex,
WHO recommends the use of condoms as a precautionary measure because we don’t know how much of the infection is transmitted through semen, but it is also because it reduces skin-to-skin contact. It’s preferable to avoid skin-to-skin contact altogether if someone has monkeypox, but at the very least, using a condom may reduce that risk while we do more studies to learn more. This applies to bisexual and gay men who have sex with men and anyone who has multiple sexual partners. The CDC says that wearing a condom may help, but alone, it probably will not protect against the spread of monkeypox. However, the agency still emphasizes that condoms can prevent other sexually transmitted infections.
______
______
Is monkeypox sexually transmitted infection/disease (STI/STD):
STIs, sometimes called sexually transmitted diseases (STDs), are infections or diseases that primarily spread through sexual contact. A sexually transmitted disease is commonly defined as one that mainly spreads through sexual contact. But some STDs can be spread in other ways, too. HIV can spread through shared needles. Syphilis can spread through kissing. A common, parasite-caused sexual infection called trichomoniasis has been found to spread through the sharing of damp, moist objects like sponges or towels.
_
Monkeypox has not usually spread easily among people, and experts are still trying to understand exactly how it moves from person to person. In Africa, where small outbreaks have been common for years, people have been infected through bites from rodents or small animals. But in May 2022, cases began emerging in Europe, the United States and elsewhere that showed a clear pattern of infection through intimate contact with an infected person, like many other sexually transmitted diseases.
Experts have reservations about classifying monkeypox strictly as an STI albeit there is data supporting the fact that this current monkeypox outbreak is sexually transmitted. We’re seeing levels of monkeypox DNA in people’s semen, in their rectum. We’re seeing it in sexual sites. But experts say that monkeypox can be spread in many different ways, and sexual transmission is one of them. MPX is not a ‘gay disease’. There’s nothing inherent in the biology of the virus that limits it to men who have sex with men. The virus spreads through tight-knit social networks; it does not discriminate. Person-to-person transmission is possible through “close physical contact with monkeypox sores, items that have been contaminated with fluids or sores (clothing, bedding, etc.), or through respiratory droplets following prolonged face-to-face contact. Although the overwhelming majority of infections in this current outbreak are among men who have sex with men, according to the WHO, anyone can get monkeypox. In the U.S., there have been five cases of monkeypox in kids and one case in a pregnant woman. Monkeypox may spread sexually, but it is clearly spreading through non-sexual close contact as well. This is not a sexually transmitted disease per se, but it is certainly an ‘intimacy-communicable’ disease. Monkeypox can be described more accurately as “sexually transmissible,” as sex is one of the ways the virus can spread — but not the only way.
_
WHO officials said that 99% of all the monkeypox cases beyond Africa were in men and that of those, 98% involved men who have sex with men. Experts suspect that monkeypox outbreaks in Europe and North America were ignited by sex at two raves in Belgium and Spain. The statistics are the same for cases reported in the United States, according to the Centers for Disease Control and Prevention. As in Europe, cases have emerged in other groups too, including at least 13 people who were women and at least two children. New England Journal of Medicine published a study of hundreds of monkeypox infections in 16 countries. It found that the suspected means of transmission in 95% of the cases was sexual close contact, as reported by doctors. That idea seemed to be further supported by the finding that most of the men had lesions in the genital or anal areas or in the mouth — areas of sexual contact, the researchers said. Scientists from Italy and Spain have found monkeypox virus in the semen of infected people. And in another study, published in The Lancet Infectious Diseases, scientists demonstrated that monkeypox virus from semen can infect human cells. But – and this is a key point – monkeypox doesn’t spread only during sexual contact. It can also spread through a few other routes. Sexual transmission is almost certainly contributing to this outbreak. Misinformation is saying transmission is exclusively sexual.
_
While there is broad agreement among health officials that monkeypox is being transmitted during sexual encounters, some experts debate whether it should be called an STD. They worry that the term unfairly stigmatizes and that it could undermine efforts to identify infections and tame the outbreak. Considering monkeypox as an STI seems logical in order to face the current outbreak, but the stigma and discrimination this could cause is a major problem. An infection acquired through sex is still something that causes guilt and fear of rejection by society. STIs are still viewed by many as a punishment for certain behaviours. Additionally, classifying monkeypox as an STI may create a false sense of security for people who may think they’re not at risk. When a disease is defined as a sexually transmitted infection that mainly affects men who have sex with men, many people may begin to think of it as “a gay disease” that poses no risk to them. That’s what happened in the early days of the AIDS epidemic in the 1980s, which contributed to the spread of HIV to other groups. If we look at how the AIDS response unfolded, for example, it took almost a decade to get the heterosexual community to pay attention and realize that HIV was not a gay disease. We cannot allow the same form of inaccurate information to guide our public health practice today.
Both stigma and a low perception of risk can hinder efforts for early identification of cases, rapid isolation and limitation of the outbreak. Worst of all, stigma related to this outbreak would perpetuate harms to the LGTBQ2SA+ community. There are consequences to calling monkeypox an STI based on a demographic it is prevalent in, especially because of bias toward the LGBTQ+ community. Monkeypox is behaving like an STI during this global outbreak, so including this diagnosis as part of sexual health management may be beneficial to stop transmission. However, bigger efforts addressing stigma and discrimination are necessary.
_______
_______
Asymptomatic sexual transmission?
Most people infected with monkeypox virus are symptomatic, but subclinical infection can occur. Sero-epidemiological studies in Africa suggest some patients may have subclinical or asymptomatic monkeypox infection. Serologic studies of household contacts of acutely infected cases in the Democratic Republic of Congo suggest that approximately 28% of all monkeypox infections are subclinical. More recently, immunologic evidence of exposure to monkeypox virus was identified in several asymptomatic contacts of infected people in the USA. In the 2022 outbreak, some studies found that asymptomatic infections are rare. As an example, in an update from the European Centre for Disease Prevention and Control (ECDC) on June 30, 2022, only one of 1435 patients notified with monkeypox was listed as “asymptomatic”. The potential for transmission from an individual with asymptomatic infection is uncertain. In a study performed at the beginning of the 2022 outbreak in Belgium, stored anogenital and oropharyngeal specimens from 224 men who had been tested for gonorrhea and chlamydia were PCR tested for monkeypox; three men had anorectal specimens positive for monkeypox DNA despite reported absence of symptoms or exposure to a person with monkeypox. However, because of limited records, it is uncertain whether those men were completely free of symptoms or signs of infection. Although the finding raises concern that people with mild disease could contribute to ongoing transmission, none of the contacts of the three men developed clinical monkeypox, and follow-up monkeypox testing performed 21 to 37 days after the initial positive sample was negative. In a study published in August in the Annals of Internal Medicine, among 200 anal swabs tested for monkeypox at a routine sexual screening clinic in France, 13 were positive (6.5 percent). All of the subjects in these cases were initially asymptomatic, and two subsequently developed symptoms of monkeypox infection. This study determines that positive MPVX infection results can occur in asymptomatic patients. However, whether this is suggestive of viral shedding leading to transmission is still unclear. Recently, the French authorities have recommended vaccination for all MSM with multiple partners to prevent the spread of MPVX infection.
These studies suggest that not all the patients present with symptoms. So monkeypox infections can occur without symptoms but subtle, unrecognized symptoms could not be ruled out completely. We know that asymptomatic monkeypox infections occur infrequently but we need more studies to determine whether asymptomatic transmission, including sexual transmission from asymptomatic carriers occurs or not.
______
______
Section-10
Monkeypox Pathogenesis, Immunity, Reinfection and Relapse:
_
Infection biology:
MPXV is known to have a wide-reaching host tropism and can infect many different species. This generality also translates into its cell and tissue tropisms; the virus has been found to infect tissues ranging from the heart and brain to the ovaries and lymphoid tissue.
Once inside the body, MPXV infects cells through a series of interactions between viral and cellular proteins, for instance the viral D8L protein, which binds to the cell surface receptor chondroitin sulfate. Once bound, the virus enters the cell by fusing with the cell membrane or by endocytosis. In this manner, the virus can enter cells, replicate and then infiltrate the bloodstream, after which it can spread through the bloodstream to any of the many tissue types that it is capable of infecting.
_
Pathophysiology and pathogenesis:
The phases of MPXV viremia help correlate the signs and symptoms of monkeypox infection. Following exposure to MPXV from any route (e.g., oropharynx, nasopharynx, intradermal and possibly anogenital [as seen in the 2022 outbreak]), the virus replicates at the site of inoculation before spreading to locoregional lymph nodes. From there, MPXV enters the bloodstream, producing a primary viremia that seeds the hematopoietic system. The duration of primary viremia corresponds with the incubation period of monkeypox infection (7 – 14 days with an upper limit of 21 days). Further replication of MPXV produces a secondary viremia, which results in a prodrome lasting approximately two days, characterized by fever, headache, myalgia, and tender lymphadenopathy. The latter may be cervical or inguinal (1 – 4 cm in diameter) and is typical of monkeypox infection. Approximately 1 – 3 days following the onset of fever, MPXV seeds the skin and mucous membranes with virions at various stages of assembly within the cytoplasm of keratinocytes. This causes an enanthem (oral cavity lesions) and a skin exanthem due to ballooning degeneration of basal keratinocytes and full thickness necrosis of the epidermis. Monkeypox virus, like other poxviruses, has evolved mechanisms to evade host immune responses. Monkeypox virus is likely to be stable on fomites and the number of virions required for infection is thought to be high compared to variola virus. Strain differences may exist—monkeypox strains circulating in western Africa appear to be more attenuated and less transmissible than those in the Congo basin. HIV and other conditions that suppress cell mediated immunity may alter the natural history of disease. Other poxvirus infections, specifically vaccinia and molluscum contagiosum viruses, are more severe in those that are infected with HIV.
_
Monkeypox virus follows a similar infectious pathway to smallpox, beginning with the exposure of the oropharyngeal or respiratory mucosa of the host. Following the viral entry, the monkeypox virus replicates at the site of inoculation; in human-to-human transmission, the site of inoculation is the respiratory and oropharyngeal mucosa in outbreaks of Africa. Following viral replication, in primary viremia, the viral load spreads to the local lymph nodes. In secondary viremia, the viral load will reach the distant lymph nodes and organs through circulation. The entire process represents the incubation period, typically lasting seven to 14 days with an upper limit of 21 days. Clinical manifestation of monkeypox is not visible during the incubation stage and the incubation period is not contagious. The symptoms and clinical manifestation of monkeypox can be correlated to the prodromal stage. During the prodromal stage, secondary viremia occurs from the lymphoid organs to the skin and tertiary organs such as the lungs, eyes, gastrointestinal tract, etc. It is during the prodromal state that an individual is deemed to be the most infectious.
Figure above shows Pathogenesis of Monkeypox.
_
MPXV infection has an incubation period of 5–21 days, and common symptoms include fever (between 38.5 °C and 40.5 °C), headache and myalgia. A distinguishing feature of MPXV infection is the presence of swelling at the maxillary, cervical or inguinal lymph nodes (lymphadenopathy). In the recent outbreak, a report from Portugal suggested that inguinal lymphadenopathy was more common than cervical and axillary lymphadenopathy. Rashes appear following the onset of fever, beginning on the face, tongue and oral cavity (enanthem) before spreading across the body. In the later stages of infection, lesions in the oral cavity can cause difficulties in drinking and eating. However, in the recent outbreaks, several atypical clinical observations have been reported. In patients who are MSM, these include the presence of genital lesions that subsequently spread to other sites in the body, as well as anal ulcers, and it appears that skin lesions may be more limited in distribution than reported in previous outbreaks.
Disease severity can be classified using the lesion count, as higher lesion counts correlate with increased risk of severe complications. Patients with severe complications may experience respiratory and gastrointestinal issues, encephalitis, septicaemia and ocular infections leading to permanent vision loss. Skin lesions also increase the likelihood of dermal bacterial infections, especially in patients who are not vaccinated against smallpox.
Lesions typically progress through four stages — macular, papular, vesicular and pustular — before falling off as scabs. Patients are typically considered non-contagious once lesions have crusted over. However, scabs have been reported to contain significant quantities of MPXV DNA even after falling off, which may indicate the presence of infectious viral material. Of note, viable VARV has been isolated from scabs of patients with smallpox.
During pregnancy, MPXV can be vertically transmitted from the mother to the fetus. In one study involving four pregnant women who were infected with MPXV in the DRC, only one gave birth to a healthy infant. Two women experienced miscarriage in the first trimester and one had a stillbirth. In the stillborn, skin lesions were observed across the body. In another study, four out of five women who were infected with MPXV in the DRC had fetal demise and lesions were observed on the maternal surface of the placenta. The studies did not report which clade of MPXV these patients were infected with, although given the location of the studies it is very likely the Central African clade.
A study conducted in Zaire during 1980–1985 reported a higher incidence of fatal disease in young children infected with MPXV, with a case fatality rate of 14.9% in children aged between 0 and 4 years compared with a rate of 0% in individuals aged 10 years or older. This disparity could potentially be due to differences in their immune responses. Currently, data on severity of infection in children infected with the West African clade are lacking. Nevertheless, the severe outcomes of monkeypox in pregnant women and in young children highlight the importance of future public health efforts to limit the spread of MPXV and minimize the risk of adverse events.
______
MPXV immunopathogenesis:
The clinical outcome of orthopoxviral infection in a vertebrate host is strongly dependent on the entry route used by the virus to establish the primary infection (Figure below). For several orthopoxviruses, such as the highly contagious VARV and MPXV, the respiratory/oral cavity is a possible common entry route following the inhalation of aerosolized respiratory secretions or the ingestion of bodily fluids from individuals who are infected. The virus then infects the oral and respiratory tract mucosae, with the upper, middle and lower airway epithelium as main targets for primary infection. This phase of infection is asymptomatic, with no signs of oropharyngeal lesions. Virus spread progresses with the infection of nearby tissue-resident immune cells, potentially including antigen-presenting cells such as monocytes, macrophages, B cells and dendritic cells. The mechanisms whereby orthopoxviruses relocate from the initial site of infection to nearby draining lymph nodes are a matter of debate. It has been observed, for example, that VACV-infected murine dendritic cells migrate from the lung epithelium to draining lymph nodes, likely contributing to virus dissemination. Conversely, VACV infection of human monocyte-derived dendritic cells has been shown to be abortive, affecting dendritic cell maturation and their migratory potential, and arguing against a role of dendritic cells supporting the initial lymphatic spread of VACV. Importantly, the rapid relocation of VACV to draining lymph nodes within hours of inoculation indicates direct viral access to lymphatic vessels as a dissemination mechanism.
_
Immunopathogenesis of human monkeypox is depicted in the figure below:
Monkeypox virus (MPXV) might enter the body via the respiratory (panel a) or skin (panel b) route. In the respiratory tract, the virus can infect airway epithelial cells such as ciliated cells. Antigen-presenting cells such as dendritic cells and macrophages (MΦ) are also susceptible to MPXV infection. Upon inoculation in the skin, the virus infects keratinocytes and fibroblasts. Skin-resident immune cells such as Langerhans cells, dendritic cells and macrophages are also targeted. In both scenarios (panels a and b), it is hypothesized that infected antigen-presenting cells travel to nearby draining lymph nodes and facilitate its spread through the lymphatic system (panel c). Direct viral access to the lymphatics has been also speculated. A common feature of human monkeypox is swelling of lymph nodes (lymphadenopathy). The abnormal proliferation and retention of natural killer cells might be one of the causes. Following its spread through lymphoid tissue, MPXV may target other large organs such as the spleen and liver (panel d). Of note, MPXV antigens have been previously been detected in both hepatocytes and Kupffer cells in non-human primate (NHP) models. The viraemia wave could then allow the virus to further spread to distant organs such as the skin and gonads. Recently, MPXV was isolated from semen of infected individuals, highlighting the possibility of sexual transmission (panel e). The infection of skin and mucosae leads to the appearance of infective pustules (panel f) and ulcers (panel g). The latter release high quantities of virus into the saliva, which potentially leads to aerosolized transmission of MPXV (panel h).
_
Following the infection of nearby draining lymph nodes, orthopoxviruses replicate extensively in lymphoid tissues. Clinical studies of human monkeypox suggest that lymphoid tissues in the neck and throat are areas of primary MPXV replication. This was supported by studies in a cynomolgus macaque model of aerosolized MPXV infection, where the tonsils and the mandibular and mediastinal lymph nodes were active areas of early virus replication. Poxvirus tropism in lymphoid tissue has been associated with infection of monocyte/macrophages, dendritic cells, B cells and activated T cells, which could also be targets for MPXV. The processes leading to abnormal lymph node swelling upon natural MPXV infection have not been elucidated; however, during experimental infection of non-human primates (NHPs) with MPXV, massive proliferation and accumulation of natural killer cells was observed in the lymph nodes surrounding the site of inoculation.
After the development of low-grade primary viraemia resulting from the infection of the lymphoid tissues, orthopoxviruses can disseminate to distant organs via the lymphohaematogenous route. In experimental mousepox models, the spleen and liver are the main targets for infection after primary lymphatic spread. Virus infection in these organs releases a second major viraemia wave (believed to be through infected cells) that results in viral dissemination to the lungs, kidneys, intestines, skin and other organs. Similarly, in an NHP model of aerosolized MPXV infection, viral antigen was observed in the liver, particularly in highly specialized macrophages such as Kupffer cells. Antigen was also detected in hepatocytes, although to a lower extent. Enlarged spleen and liver have also been reported during MPXV infection in humans. In VARV infection, it is believed that lymphoid organs such as the spleen and bone marrow support virus replication, but there is less evidence for hepatic involvement.
The presence of orthopoxviruses in the small dermal blood vessels marks the start of skin infection and development of skin lesions. However, how the virus reaches the upper skin layers, which lack blood and lymphatic vessels, is not well understood. It is possible that infected migratory skin dendritic cells such as Langerhans cells might be responsible, as they are known to be susceptible to VACV infection. Infiltration of macrophages, dendritic cells and CD3+ T cells has been observed around the infective pustule. The role of skin-infiltrating CD3+ T cells in the context of MPXV infection requires characterization; this is particularly true for cytotoxic T lymphocyte responses, which have been associated with better virus control in vaccinated rhesus macaques. Importantly, epithelial lesions (enanthema) also appear during MPXV infection in the oropharyngeal mucosa, tongue, pharynx, larynx, trachea and oesophagus, eventually evolving into ulcers which release infectious viral particles into the saliva.
Infection can also occur via the skin. It has been postulated that infection of the dermal keratinocytes, fibroblasts and tissue-resident antigen-presenting cells such as monocytes, macrophages, Langerhans cells and dendritic cells might occur and migratory antigen-presenting cells could contribute to virus dissemination through the lymphatics. Nonetheless, recent evidence from a mouse model of orthopoxvirus skin infection suggested that dendritic cell migration from the skin to draining lymph nodes is impaired on VACV. The relocation of the virus from the skin to the lymphatics might also be supported by other mechanisms such as direct lymphatic vessel access, as observed in skin infection models of Zika virus.
Sexual transmission of monkeypox has been speculated, and MPXV was recently identified in the semen of three male patients in Italy. Cases of monkeypox with exclusive genital lesions have also been reported, which might indicate preferential MPXV tropism into the testes. Being an immune-privileged tissue, the testes could act as a site for latent MPXV infection, but this requires further investigation. Nonetheless, recent animal studies showed that the related VACV exhibits tropism for testicular and ovarian tissues. Viral shedding was also reported in faeces and contact with the rectal mucosa might increase the likelihood of MPXV transmission. It was previously noted in patients with human immunodeficiency virus 1 (HIV-1) that these tissues could act as a reservoir for the virus. A recent study determined that the rectal mucosa immune environment in MSM was significantly different compared with individuals who are heterosexual, with a higher presence of immune activities indicative of mucosal injuries. This condition could lead to the recruitment of immune cells, which could then be easily targeted by infectious agents, such as observed with HIV-1. This could similarly apply to MPXV transmission and infection in MSM. However, this does not indicate that monkeypox has become a sexually transmitted disease, as MPXV can spread through any form of close contact with the infectious pustules that are symptomatic of monkeypox.
_______
_______
Immunity to MPXV:
Even though the virus was identified decades ago, human immunity to MPXV infection has not been extensively characterized. As such, inferences on MPXV interaction with the host immune system are often drawn from studies performed with VACV and related orthopoxviruses. In the following paragraphs, the potential mechanisms of host immunity against MPXV are highlighted and the immune evasion strategies utilized by MPXV during active infection is discussed.
_
Innate immune responses to MPXV:
Innate immune cells typically act as the first line of defence following active viral infection, but these cells also serve as targets for some viruses. Numerous in vitro and in vivo studies have demonstrated that monocytes are the initial targets of poxviruses. Early detection of poxvirus antigen in both monocytes and neutrophils has been suggested to be a strong predictor of MPXV lethality. Susceptible monocytes are actively recruited to sites of infection, with marked expansion of CD14+ monocytes in the lungs of cynomolgus macaques experiencing viral pneumonia following MPXV infection. Mouse CD45+CD11b+GR-1int inflammatory monocytes have also been shown to be permissive to VACV replication and may be plausible vehicles for virus dissemination. It was also reported that human primary M2-like macrophages allowed VACV replication and dissemination. Following VACV infection, these primary macrophages formed actin tails, cell linkages, lamellipodia and branching structures associated with the VACV virions, indicating that these cells might participate in virus spread. However, it was also reported that depletion of phagocytic cells did not abolish spread of VACV in infected mice, suggesting that monocytes and macrophages are not the only immune cells that are capable of facilitating virus dissemination. Nevertheless, Ly6G+ innate immune cells (both neutrophils and Ly6G+ monocytes) were responsible for infiltrating and controlling virus-infected cells, thus limiting viral tissue damage. These results were indirectly confirmed by a study that found an association between low numbers of blood neutrophils with moribundity in MPXV-infected animals. It is also worth noting that immune cells recruited to the site of infection control only local pathogenesis and tissue pathology, but not virus dissemination, and a systemic immune response is required to prevent widespread infection.
Natural killer cells are an important component of innate immunity and, similar to monocytes, are capable of shaping the adaptive immune response. In MPXV-infected rhesus macaques, numbers of natural killer cells expand significantly in both peripheral blood (a mean 23-fold increase by day 7 post infection) and lymph nodes (a mean 46-fold increase by days 8–9 post infection). Prior to this rapid proliferation, the migratory capacity of the various natural killer cell subsets was significantly impaired by MPXV infection, which severely affected their recruitment into the lymphoid and/or inflamed tissues. A downregulation of chemokine receptors such as CXCR3, CCR5, CCR6 and CCR7 on these cells was also reported. Moreover, natural killer cells isolated from both lymph nodes and blood were reported to lose their ability to degranulate and to secrete IFNγ and TNF. Although no correlation between viral clearance and natural killer cell numbers and activities was reported in this NHP model, the importance of natural killer cells in controlling MPXV viral load was demonstrated in CAST/EiJ mice. This strain is exceptionally susceptible to orthopoxvirus infection owing to low numbers of natural killer cells. IL-15 treatment protected CAST/EiJ mice from lethal MPXV infection even when both CD4+ and CD8+ T cells were depleted. This implies that the expanded natural killer cells were responsible for the protective effect as treatment with IL-15 is known to transiently increase the numbers of IFNγ-secreting natural killer cells and CD8+ T cells.
Similarly, natural killer cells are also needed to control both Ectromelia virus (ECTV) and VACV infection in C57BL/6 mice. ECTV is a natural orthopoxviral pathogen of mice, and is often used to induce experimental mousepox as a model for other clinically important orthopoxviruses. Interestingly, it was suggested that the expression of CD94 on natural killer cells in C57BL/6 mice is absolutely essential for conferring resistance to ECTV infection. This is mediated by the NKG2E and CD94 receptors on natural killer cells, which bind complexes of MHC class I with the peptide Qa-1b, which are expressed by infected cells. NKG2D has also been reported to participate in natural killer cell-mediated protection against ECTV infection and it was postulated that CD94-NKG2E and NKG2D may have synergistic activity in inducing optimally protective natural killer cells. However, the exact mechanisms behind this apparent synergism remain to be elucidated, and further studies will be required to better understand the roles of natural killer cells in mousepox infections. Given that CD94 and NKG2 are highly conserved between humans and rodents, these receptors may also play a protective role against MPXV infection in humans.
In humans infected with MPXV, the roles of many innate immune cells — including monocytes/macrophages, neutrophils, natural killer cells, conventional dendritic cells, plasmacytoid dendritic cells and innate lymphoid cells — are currently unknown. The characterization and profiling of these immune cells during MPXV infection will be essential for understanding their functions and identifying important biomarkers for disease prognosis.
VARV infection in animal models triggers systemic cytokine responses that correlate with disease outcomes. In unvaccinated cynomolgus macaques, significant changes in host gene expression were detected following infection with VARV. In particular, transcription of a cluster of interferon-associated genes was upregulated; this cluster was enriched for genes regulated by both type I and type II interferons, including PKR, STAT1, STAT2, MX1, MX2, IP10, OAS1, OAS2 and OAS3. The animals that succumbed to the infection (two out of seven) had minimal interferon responses, indicating that this early interferon response protects against fatality. Human IFNβ has been demonstrated to inhibit MPXV replication and spread. However, MPXV does not robustly activate TNF-regulated and NF-κB-regulated genes, especially in animals that succumb to infection. This is not surprising, given that VARV and other orthopoxviruses harbour genes that can modulate TNF and NF-κB pathways.
Although host immunity is required to combat infections, aberrant immune signalling can adversely affect infection outcomes. In another study of VARV-infected cynomolgus macaques, the levels of IL-8, CCL2 (also known as MCP1), CCL4 (also known as MIP1β), IL-6 and IFNγ were significantly increased during the first 4 days post infection. These cytokines drive monocytosis, which might facilitate enhanced virus dissemination through monocytic cell-associated viraemia. Importantly, the macaques eventually succumbed to VARV infection, where high levels of these cytokines might have contributed to a ‘cytokine storm’ leading to their demise. Likewise, the levels of IL-1RA, IL-2, IL-6, IL-8, IFNγ, CCL2, CCL5 (also known as RANTES), G-CSF, GM-CSF and sCD40L were found to be elevated in MPXV-infected cynomolgus macaques. Furthermore, a relative expansion (0.97-fold to 16.3-fold) of CD14+ monocytes was reported during acute infection, suggesting that the general immune milieu promoted the development and recruitment of monocytes following MPXV infection.
In reported cases of human MPXV infection, numerous cytokines are elevated following infection (regardless of disease severity) — these include IL-1β, IL-1RA, IL-2R, IL-4, IL-5, IL-6, IL-8, IL-13, IL-15, IL-17, CCL2 and CCL5. However, in patients with serious disease (defined as having >250 lesions), concentrations of IL-2R, IL-10, GM-CSF and CCL5 were higher compared with those in patients with less severe disease, whereas the concentration of pro-inflammatory IL-6 was lower. This cytokine profile suggests a dominant T helper 2 cell response characterized by higher levels of IL-4, IL-13 and IL-10. Likewise, the reduced levels of IL-2, TNF, IFNα and IFNγ also suggest an anti-inflammatory microenvironment involving regulatory T cells.
VACV can evade immune responses by downregulating inflammatory and antiviral immune responses, and MPXV may use a similar strategy to subvert host immunity. Given that immune mediators often facilitate crosstalk between immune cells, future studies should identify the functional relationships between cytokine profiles and immune cells in order to clarify the mechanisms of pathogenesis and determine immune correlates of protection during MPXV infection.
_
B cell and antibody protection:
The importance of B cells and immunoglobulins against poxviruses was first demonstrated with the successful global vaccination campaign that eradicated smallpox, which used a live VACV vaccine. It was further demonstrated that treatment with vaccinia immune globulin (VIG) isolated from the serum of vaccinees successfully protected close contacts of patients with smallpox from infection. In rhesus macaques, VACV-specific B cell responses were instrumental in protecting against a lethal MPXV infection. Importantly, epidemiological studies have further demonstrated that the VACV vaccine confers protection against other poxviruses, including MPXV. The VACV-specific memory B cells and antibody levels induced by vaccination were exceptional, in some cases lasting longer than 50 years. However, only ~50% of vaccinated individuals at >20 years after vaccination had neutralizing antibody titers greater than 1:32, a correlate of protection suggested to confer protective immunity against smallpox. It is likely that cross-protective immunity against monkeypox may similarly wane over time.
Fourteen MPXV proteins have been shown to be recognized by cross-reactive VACV-induced immunoglobulins from human vaccinees. Three proteins in particular — MPXV (Zaire-1979_005) proteins D8, H3 and A26 — were targeted by neutralizing antibodies against MPXV in infected macaques. In VACV, orthologue D8 and H3 proteins are involved in the attachment of mature virions, whereas A26 associates with A27 to bind surface laminin. MPXV (Zaire-1979_005) proteins C19, A33 and A44 were also prominent antigens for IgM isolated from MPXV-infected macaques during the acute phase of infection — these proteins could thus be further developed as antigen-based serological diagnostic tools. In another study, prophylactic treatment with a cocktail of two mAbs — c7D11 and c8A — successfully protected marmosets against lethal MPXV infection. C7D11 and c8A target the VACV proteins L1 and B5, respectively, and have been recently formulated as a potential mRNA vaccine encapsulated in lipid nanoparticles. Gilchuk et al. showed that a mixture of human-derived mAbs targeting the VACV proteins D8, H3, A33, A27, B5 and L1 effectively cross-neutralized four clinically relevant orthopoxviruses, including MPXV and live VARV. However, despite knowledge of the MPXV proteins that are recognized by neutralizing antibodies, MPXV-specific epitopes (both conformational and linear) have not been extensively characterized.
The isotype composition of the anti-MPXV response may provide an important clue to pre-existing immunity and protection, as IgM antibodies typically dominate in primary immune responses, whereas IgG antibodies dominate in secondary immune responses. In a cohort of 200 patients infected with MPXV who were recruited between March 2007 and August 2011 in the DRC, individuals with both IgM and IgG responses were 5.09 times more likely to develop severe lesions compared with individuals who had IgG-only responses. Similarly, in a cohort of infected individuals from the 2003 MPXV outbreak in the United States, patients with moderate/severe disease had an overall higher titer of anti-orthopoxvirus IgM compared with those with mild disease, and anti-orthopoxvirus IgG responses were much reduced and less frequent in patients with moderate/severe disease. It is plausible that an IgG-only response reflects robust levels of cross-protective IgG+ memory B cells that produce a dominant secondary antibody response, whereas the lack of such memory necessitates an IgM-dominated primary response that is less effective at preventing disease. Thus, IgM responses may be a biomarker for disease severity. This also emphasizes the critical and immediate need to extensively characterize the antibody profile of patients with MPXV in different cohorts. Likewise, VACV vaccine correlates of protection must be determined to explain why certain vaccinated patients experience breakthrough infections.
_
T cell immunity:
CD4+ T cells, particularly T follicular helper cells, play a role in enhancing recall and differentiation of memory B cells into antibody-secreting cells. Memory CD4+ T cells were found to persist for up to 50 years or longer following VACV vaccine, with an estimated half-life of 8–15 years. These VACV-specific CD4+ T cells were capable of producing IFNγ and TNF following stimulation. However, no direct correlation was reported between numbers of virus-specific CD4+ T cells and anti-VACV antibody titers. By contrast, the number of CD4+ T cells was shown to be critically important in inducing a protective antibody response against lethal MPXV infection in VACV-vaccinated rhesus macaques. Simian immunodeficiency virus (SIV)-infected macaques with CD4+ T cell counts <300 cells mm^3 were not able to produce VACV-specific IgG following vaccination and died when challenged with MPXV. This observation is of high concern to both VACV-vaccinated and unvaccinated individuals with uncontrolled HIV-1 infection, as their CD4+ T cell counts are typically very low. This group of individuals is therefore at high risk of developing severe MPXV infection if exposed. They might also experience a more complicated pathology and provide the virus with an opportunity to acquire mutations that result in higher virulence or transmission potential. By contrast, a recent patient infected with MPXV who was on antiretroviral treatment for HIV-1 had a CD4+ T cell count >700 cells mm^3 and did not experience a severe disease outcome. This may suggest an important role for CD4+ T cells in regulating monkeypox severity. Nevertheless, more studies will need to be carried out to fully understand the role of CD4+ T cells during MPXV infection.
In addition to supporting antibody development, T cells can play direct antiviral roles. Given that orthopoxviruses, including MPXV, infect and disseminate in macrophages, cytolytic T cells can be instrumental in killing infected macrophages to prevent viral spread. CD8+ T cells have been shown to eradicate virus-infected monocytes and minimize virus dissemination in a mouse model of VACV infection. In fact, activation of CD8+ T cells in response to VACV infection has been shown to be dependent on γδT cells, which present VACV peptides via MHC class I molecules. Moreover, γδT cells also upregulate co-stimulatory molecules CD80 and CD86 and secrete IL-1 and IFNα for activation of CD8+ T cells. In a mouse model of VACV respiratory infection, IFNγ secretion by primary activated effector CD8+ T cells was shown to protect against lethality. Indeed, CD8+ T cell-derived IFNγ was sufficient for protection even in the absence of CD4+ T cells and B cells, highlighting the possibility that CD8+ T cells also confer protection against infection with other orthopoxviruses. Likewise, memory CD8+ T cells, which are induced following VACV immunization, were also demonstrated to protect against lethal ECTV infection in mice. These memory CD8+ T cells execute their protective effects via a combination of IFNγ and perforin secretion, and work concomitantly with primary effector CD8+ T cells to achieve optimal protection. In humans, standard smallpox vaccine administered by scarification was also able to induce primary cytotoxic CD8+ T cells and IFNγ-producing T cells. This observation was supported by another study, in which participants received live vaccinia smallpox vaccine (Dryvax). High levels of IFNγ-producing CD8+ and CD4+ T cells were detected following immunization. In humans infected with VACV, activated CD4+ T cells were shown to upregulate genes related to cytolytic activities. Interestingly, MHC class II-restricted cytolytic CD4+ T cells have also been reported in individuals immunized with VACV. These cells could be responsible for virus clearance in vaccinees with reduced or missing memory CD8+ T cell responses. In the experimental mousepox model, perforin-dependent cytolytic CD4+ T cells have been reported. Taken together, these observations highlight the importance of T lymphocytes in controlling orthopoxviral infections.
Across the orthopoxvirus proteome, numerous CD4+ and CD8+ T cell epitopes have been identified in humans, mice and NHPs. Many are conserved among the major orthopoxviruses and bind to human MHC class I and class II molecules. In particular, CD8+ T cells specific for two identified epitopes (MHC class II-restricted GRVFDKADGKSKRDA and MHC class I-restricted NPVTVINEY) in the immediate-early E3 protein of VACV were capable of killing infected cells and halting the spread of VACV. Both epitopes are conserved in the MPXV homologue, which is encoded by the MPXV F3L gene. An earlier study showed that VACV missing the E3 protein did not protect cynomolgus macaques from subsequent MPXV infection. As E3 protein is detected within 30 min of VACV infection, it should be readily processed and presented by infected cells, allowing T cell-mediated lysis at an early infection stage before virion production and release. These properties make E3 an excellent possible candidate for future vaccine designs targeting all major orthopoxviruses.
Despite the potential importance of T cells in disease protection, smallpox vaccination does not necessarily provide robust T cell-mediated immunity against MPXV. In two out of five vaccinated individuals who subsequently contracted MPXV, orthopoxvirus-specific CD4+ and CD8+ T cells were not detectable throughout convalescence. Furthermore, similar levels of orthopoxvirus-specific CD4+ T cell reactivity were observed in vaccinated and unvaccinated patients, and orthopoxvirus-specific CD8+ T cell responses were in fact higher in unvaccinated patients. The association of CD4+ and CD8+ T cell responses with the severity of MPXV infection remains inconclusive in human studies.
_______
_______
MPXV immune evasion:
Orthopoxviruses have accrued an arsenal of genes that encode proteins which interfere with host cell signalling pathways that are involved in virus recognition, apoptosis and immune regulation (Figure below).
Monkeypox virus (MPXV) is known to encode numerous viral proteins that are involved in evading the host immunity. These can be involved in interfering with the signalling cascade of pathogen recognition receptors, disrupting key transcription factors for the expression of inflammatory genes, such as interferon regulatory factor 3 (IRF3) and NF-κB. MPXV can also interfere with interferon signalling by blocking IFNα/β binding or suppressing IFNα/β production and by blocking protein kinase R (PKR)-mediated pathways. In addition, MPXV secretes proteins that can target key inflammatory molecules such as TNF, IFNγ, IL-1β, IL-18 and IL-6. Moreover, MPXV can prevent apoptosis in infected cells by expressing numerous viral proteins that target the apoptotic pathways. The Central African MPXV Zaire strain also expresses D14 which blocks the activation of the complement cascade. However, this viral protein is not expressed in the West African MPXV strain. Lastly, MPXV can also downregulate the activities of natural killer cells and T cells by interfering with their activation processes.
PRR, pattern recognition receptor.
_
Preventing cellular signalling:
Mammalian cells can detect the presence of microbial infection through pattern recognition receptors (PRRs). These can trigger intracellular signalling cascades involving numerous host cofactors such as MYD, TRAM, TIRAP and TRIF, eventually activating important immune transcription factors such as NF-κB and interferon regulatory factors (IRFs). Numerous orthopoxviral proteins can antagonize these signalling processes. For example, the VACV genome encodes numerous B cell lymphoma 2 (BCL-2)-like proteins that inhibit NF-κB and IRF3 activation by interacting with cofactors that are recruited following PRR binding. BCL-2-like proteins are generally conserved across orthopoxviruses — A47, B13, P1, C6 and D11 are orthologues of BCL-2-like proteins in MPXV. VACV also encodes the E3 protein, which binds to double-stranded RNA (dsRNA) that is produced late in its replication cycle, and prevents the dsRNA from being detected by host intracellular PRRs. This leads to complete inhibition of the protein kinase R (PKR)-mediated pathway, which can otherwise block protein synthesis by phosphorylating the eukaryotic translation initiation factor subunit 2α (eIF2α). The F3 protein of MPXV is a homologue of the VACV E3 protein with a 37 amino acid truncation at the amino terminus. Unlike a similarly truncated recombinant VACV (VACVΔ37N), MPXV can still inhibit host immune responses. Interestingly, another recombinant VACV expressing the MPVX F3L gene (VACV-F3L) did not inhibit host PKR activation, suggesting that MPXV has evolved to encode for yet undiscovered proteins that compensate for the missing N-terminal amino acids of F3 in limiting host antiviral activities.
One of the most important transcription factors downstream of PRR binding is IRF3, which controls the expression of the crucial antiviral molecules IFNα and IFNβ. The VACV B19 protein directly interacts with soluble interferons and inhibits their binding to receptors. Although deletion of the B19R gene in VACV did not affect its virulence, deletion of B19 in ECTV did severely attenuate its ability to establish infection. The MPXV orthologue B16 can inhibit IFNβ signalling. Interestingly, the interferon response is known to be stronger in children and has been shown to protect against severe SARS-CoV-2 and RSV infections, but the reverse was observed in children infected with MPXV. This emphasizes the need to better understand host–pathogen interactions and further characterize the mechanisms of MPXV pathogenesis.
The activation of NF-κB is controlled by proteins of the IκB family, which contain ankyrin repeats. NF-κB consists of two subunits (p65/p50), and in its inactive form the p65 subunit is bound by the inhibitory protein IκBα (which contains six ankyrin repeats). During NF-κB activation, IκBα is phosphorylated by IκBα kinase (IKK), followed by ubiquitination and subsequent degradation. To prevent NF-κB activation, orthopoxviruses express ankyrin-like proteins that compete with IκBα for phosphorylation by IKK. Eight ankyrin-like genes (J3L, D1L, D7L, D9L, O1L, C1L, B5R, B17R, N4R and J1R) are encoded by the MPXV genome — J3L and J1R as well as D1L and N4R are duplicated ORFs in left and right inverted terminal repeats within the viral genome.
_
Regulation of apoptosis:
Another mechanism of orthopoxvirus immune evasion involves regulating apoptosis. The BCL-2-like proteins encoded by orthopoxviruses may interfere with the BCL-2-mediated regulation of the intrinsic apoptotic pathway. Numerous orthopoxvirus-encoded serine protease inhibitors (SPIs; serpins) have also been reported, such as CrmA in cowpox virus (CPXV); its homologue SPI-2 (B13) in VACV is considered the virus’s most potent inhibitor of apoptosis. CrmA interferes with granzyme B, which is secreted by cytotoxic T cells to initiate cell death in the virus-infected target cells. It also inhibits caspase 1 and caspase 8, thereby interfering with their respective pyroptotic or apoptotic pathways. In MPXV, an SPI-2 orthologue is encoded by the B12R gene.
TNFR orthologues are also commonly used by orthopoxviruses to interfere with host inflammation and apoptotic events Decoy viral TNFRs, which lack signalling domains, are secreted and compete for the binding of TNF. Five orthopoxviral viral TNFRs — CrmB, CrmC, CrmD, CrmE and vCD30 — have been identified. The MPXV genome encodes only CrmB, which is reported to bind to both TNF and TNFβ based on investigations performed with the CPXV CrmB. Interestingly, CrmB from VARV is extremely potent and exhibits an affinity for TNF that is stronger than etanercept, a commercially available competitive inhibitor of TNF.
_
Antagonism of immune mediators:
Orthopoxviruses also evade host immune responses by secreting proteins that antagonize the functions of host IFNγ, CC and CXC chemokines, IL-1β and the complement system. Interestingly, the West African MPXV clade does not express any complement-modulating proteins, whereas the Central African strains encode the monkeypox inhibitor of complement enzyme (MOPICE) from the D14L gene. Despite truncation in one of its short consensus repeat (SCR) domains, MOPICE inhibits complement activation by binding to C3 and C5 convertases. However, deletion of MOPICE did not affect MPXV virulence in rhesus macaques infected with a Central African isolate, although it did dampen the adaptive immune response.
_
Reduction of cellular activation:
Orthopoxviruses also evade T cell-mediated and natural killer cell-mediated cytotoxicity. T cells identify virus-infected cells by detecting foreign peptides loaded on surface-expressed MHC class I. Meanwhile, natural killer cells continually survey cells via NKG2D for the absence of MHC class I, thereby ensuring that the MHC system is not compromised. MPXV overcomes this system first by secreting the orthopoxvirus MHC class I-like protein (OMCP) encoded by the N3R gene, which resembles the MHC class I molecule and binds to NKG2D. This suppresses the typical NKG2D-dependent natural killer cell lysis of infected cells that do not express MHC class. Evasion of natural killer cell surveillance allows the virus to reduce MHC class I expression, thus reducing T cell recognition. In addition, CPXV also expresses proteins D10 and B8 that impair peptide loading and MHC class I trafficking within the endoplasmic reticulum. In MPXV, the B10R gene encodes for an orthologue of the CPXV B8 protein. Orthopoxviruses also directly modulate natural killer cells and T cells in a paracrine fashion. For instance, orthopoxviruses produce an IL-18-binding protein that further blocks the cytotoxic activities of natural killer cells. MPXV also suppresses T cell-mediated immunity by triggering a state of T cell unresponsiveness via an MHC-independent mechanism. Subversion of T cell responses may explain why orthopoxvirus-specific memory T cells in vaccinated NHPs failed to protect against lethal MPXV infection in the absence of neutralizing antibodies.
_______
_______
Immunological Memory after Exposure to Variola Virus, Monkeypox Virus, and Vaccinia Virus; 2007 study:
Authors compared cellular and humoral immunity to vaccinia virus (VV) in individuals exposed to 3 different orthopoxviruses: 154 individuals previously vaccinated with VV, 7 individuals with a history of monkeypox virus infection, and 8 individuals with a history of variola virus infection. Among individuals vaccinated >20 years prior, 9 (14%) of 66 individuals demonstrated VV-specific interferon (IFN)—γ enzyme-linked immunospot (ELISPOT) assay responses; 21 (50%) of 42 had lymphoproliferative (LP) responses, and 29 (97%) of 30 had VV-specific neutralizing antibodies. One year after monkeypox virus infection, 6 of 7 individuals had IFN-γ ELISPOT responses, all had VV-specific LP responses, and 3 of 7 had VV-specific neutralizing antibodies. Of 8 individuals with a history of variola virus infection, 1 had a VV-specific IFN-γ ELISPOT response, 4 had LP responses against whole VV, 7 had LP responses against heat-denatured vaccinia antigen, and 7 had VVspecific neutralizing antibodies. Survivors of variola virus infection demonstrated VV-specific CD4 memory cell responses and neutralizing antibodies >40 years after infection.
In summary, survivors of variola virus infection display sustained vaccinia-specific neutralizing-antibody responses; in this study, 7 of 8 individuals had VV-specific proliferative responses to heat-denatured VacAg, indicating the presence of low-level circulating VV-specific CD4 memory cells >40 years after infection. Survivors of monkeypox virus infection had strong cell mediated responses 1 year after infection, although the cytolytic T cell response was more limited. Authors also found that only 14% of individuals had IFN-γ—producing lymphocytes 20 years after the last VV vaccination, although 50% had detectable VV-specific proliferative responses; this contrast suggests the longer persistence of CD4 memory cells after natural infection or vaccination with orthopoxviruses.
______
Monkeypox-Induced Immunity and Failure of Childhood Smallpox Vaccination To Provide Complete Protection, 2007 study:
Following the U.S. monkeypox outbreak of 2003, blood specimens and clinical and epidemiologic data were collected from cases, defined by standard definition, and household contacts of cases to evaluate the role of preexisting (smallpox vaccine-derived) and acquired immunity in susceptibility to monkeypox disease and clinical outcomes. Orthopoxvirus-specific immunoglobulin G (IgG), IgM, CD4, CD8, and B-cell responses were measured at ∼7 to 14 weeks and 1-year postexposure. Associations between immune responses, smallpox vaccination, and epidemiologic and clinical data were assessed. Participants were categorized into four groups: (i) vaccinated cases, (ii) unvaccinated cases, (iii) vaccinated contacts, and (iv) unvaccinated contacts. Cases, regardless of vaccination status, were positive for orthopoxvirus-specific IgM, IgG, CD4, CD8, and B-cell responses. Antiorthopoxvirus immune responses consistent with infection were observed in some contacts who did not develop monkeypox. Vaccinated contacts maintained low levels of antiorthopoxvirus IgG, CD4, and B-cell responses, with most lacking IgM or CD8 responses. Preexisting immunity, assessed by high antiorthopoxvirus IgG levels and childhood smallpox vaccination, was associated (in a nonsignificant manner) with mild disease. Vaccination failed to provide complete protection against human monkeypox. Previously vaccinated monkeypox cases manifested antiorthopoxvirus IgM and changes in antiorthopoxvirus IgG, CD4, CD8, or B-cell responses as markers of recent infection. Antiorthopoxvirus IgM and CD8 responses occurred most frequently in monkeypox cases (vaccinated and unvaccinated), with IgG, CD4, and memory B-cell responses indicative of vaccine-derived immunity. Immune markers provided evidence of asymptomatic infections in some vaccinated, as well as unvaccinated, individuals.
The findings from this study suggest that remote (30 years prior) vaccinia (smallpox) vaccination does not provide complete protection against systemic OPX infection (even against a relatively mild disease variant); in some cases, it may prevent systemic disease, but the relative contributions of infectious inoculum and route of exposure, in addition to remote vaccination, may significantly impact whether systemic illness, asymptomatic infection, or atypical illness manifests.
______
______
Reinfection with monkeypox:
Experts believe you are not likely to become reinfected with monkeypox after you’ve been infected or vaccinated. Based on what scientists know of other orthopox infections — such as smallpox, monkeypox’s close cousin — immunity to the disease should be lifelong. In studies of smallpox survivors, researchers have noted that immune cells that help coordinate the body’s ability to fight off the virus and kill infected cells can still be found in people’s blood up to 83 years after their original smallpox infection. Similarly, antibodies that are able to neutralize the smallpox virus seem to stick around for decades after an infection. Viruses that tend to mutate slowly — like measles, mumps or the Epstein-Barr virus, which causes mononucleosis — also prompt a strong immune response. As a result, immunity after infection tends to be lifelong. Your body learns to recognize the virus and remembers how to fight it in case of future encounters.
Upper respiratory illnesses such as the flu, the common cold and Covid are notable exceptions. The viruses that cause these illnesses adapt quickly, with mutations that make them significantly different from what the body may have dealt with in the past — as we all learned with the Omicron variant of Covid.
________
Monkeypox relapse:
Adler et al. conducted a retrospective observational study of monkeypox cases in the UK from 2018 to 2021 and identified seven patients with monkeypox infection. Four cases acquired their infection abroad, while one was a health-care worker exposed nosocomially and two were infected in the household. All patients recovered but they were hospitalized for up to 39 days and one patient developed an abscess. Of note, one patient experienced a short relapse 6 weeks after hospital release with PCR-positive lesions and upper-respiratory tract swabs. Monkeypox virus may persist in the body for 10 weeks, even after the tell-tale rash has disappeared. So relapse is possible albeit rarely.
______
Can monkeypox, like Ebola, pose a post-infection risk of transmission?
Monkeypox is a poxvirus, as its name implies. Ebola is a filovirus. Filoviruses can squirrel themselves away in parts of the body where immune system weaponry cannot reach them. These are known as “privileged sites” — the eyeball, synovial fluid (the fluid in joints), spinal fluid, and most importantly for this discussion, the testicles. From the very earliest days of recorded outbreaks of filoviruses, there was a suspicion that survivors could harbor viruses and pass them to others, primarily through sex. Later, it became clear that viral persistence, as it is called, is a feature of these infections. A portion of people who survive filovirus infections will suffer a relapse later; a Scottish nurse infected when she worked on the West African Ebola outbreak in 2014 had two subsequent resurgences of illness. In other cases, male survivors have infected sex partners months, even years after recovering.
It is not known if monkeypox virus can similarly lodge in testicles and pose a post-infection transmission risk. But the fact that scientists are discovering monkeypox viral DNA in semen, and in one case even managing to grow live virus from semen, is raising the question.
It is thought that monkeypox is a one-and-done infection, that people who survive — as most people do — have life-long immunity. They cannot be reinfected and pose no transmission risk after they recover. But that calculus changes if survivors have monkeypox virus hiding in their testicles or other parts of their bodies.
Because of the unanswered questions, the Health Security Agency in the United Kingdom is recommending men who have had monkeypox wear condoms during sex for at least 12 weeks after recovering. The CDC says wearing condoms is advisable, but at present there is little data on which to make such a recommendation.
______
______
Section-11
Clinical manifestation:
MPXV has an incubation period ranging from five days to three weeks, and the symptoms can last for nearly 2–5 weeks. Persons with a history of an animal bite or scratch may have a shorter incubation period than those with only tactile exposures (9 versus 13 days, respectively). The findings of 2006 study indicate that route of infection can influence monkeypox illness manifestations. A report from the 2022 outbreak found the mean incubation period was 8.5 days (5th to 95th percentiles: 4.2 to 17.3). Early symptoms include shivers, headaches, fainting, backaches, and myalgia, but they are not specific. During the 2022 global outbreak most patients with monkeypox have been symptomatic. Asymptomatic infections appear to be rare.
The most common symptoms observed before the rash development are fever, restlessness and lymphadenopathy. Human MPX has similar clinical features to ordinary and modified smallpox, but generally more moderate. As swollen lymph nodes are not a common sign of smallpox and are seen in 90% of MPX patients, they are considered a distinctive hallmark of MPX. These enlargements can be observed in the neck, the groin and submandibular areas. Since lymphatic adenopathy is one of the most frequently observed sign, which is a differential sign from other diseases, it is imperative to emphasize its importance in the initial examination of a suspected patient.
During the five days following the fever, various sizes of rashes develop, initially on the face and then spreading across the trunk area and extremities. The rashes often appear on the palms and soles of the feet. These lesions measure approximately 0.5 cm in diameter, and some can reach up to 1 cm. The rash evolves sequentially from macules (lesions with a flat base) to papules (slightly raised firm lesions), vesicles (lesions filled with clear fluid), pustules (lesions filled with yellowish fluid), and crusts which dry up and fall off. The number of lesions varies from a few to several thousand. In severe cases, lesions can coalesce until large sections of skin slough off. Exanthems progress through different developmental phases, resolving into crusts that fall off during the healing phase. Lesion co-infection is recurrent and plays a major role in future skin marking.
While MPX symptoms are less severe than those of smallpox, it is still considered a fatal disease, that causes death at a fluctuating rate of up to 10%. Death usually occurs within the second week of the infection. The risk is higher in children and young adults, and the disease can take a severe course in immunocompromised patients. The disease can present with numerous complications, such as co-infections, respiratory disorders, encephalitis, blindness-related keratitis, and gastrointestinal symptoms like vomiting and diarrhea.
Smallpox vaccination can offer some protection against MPX and can alter the course of the disease. Studies between 1980 and 1990 indicated a change in the pathogenesis of human MPX, because more people without immunity to smallpox developed the disease. Moreover, the pathological picture was less severe in the vaccination group, and the skin infection was milder. This was in contrast to the unvaccinated, whose skin infection was more severe, with multiple forms and a higher probability of death.
Since the symptoms of MPX are varied and non-specific, many diseases can be included in a differential diagnosis. The primary differential diagnosis is severe chickenpox with lesions in palms and soles. The lesions in chickenpox are more superficial and occur in clusters of the same stage, with denser manifestations on the trunk than on the face and extremities. Because of the nonspecific nature of the symptoms and signs of monkeypox, a wide variety of differential diagnoses should be considered, ranging from chickenpox, molluscum contagiosum, measles, rickettsial infections, bacterial skin infections (such as those caused by Staphylococcus aureus), anthrax, scabies, syphilis, and drug reactions to other noninfectious causes of rash. A clinical sign differentiating monkeypox from smallpox and chickenpox is the presence of enlarged lymph nodes, particularly submental, submandibular, cervical, and inguinal nodes.
______
The common, nonspecific symptoms, as depicted in Figure below, begin to develop one to two weeks after an individual has been infected with the monkeypox virus. During the prodromal stage, nonspecific symptoms triggering the immune system emerge such as fever, lymphadenopathy, myalgias, etc. Due to the nonspecific nature of these initial symptoms, an infected individual may attribute these symptoms to seasonal flu or the common cold. Initial activation of the immune system will always cause enlargement of lymph nodes, including maxillary, cervical and inguinal, occurring in synchrony with the onset of fever. In cases prior to the 2022 outbreak, rashes would be observed appearing one to three days after the onset of fever and lymphadenopathy. In the emergence of new cases in 2022 outbreak, for some patients, those prodromal symptoms might be mild, or not even noticed at all, suggesting that some individuals may not be aware of any symptomology at all, until the appearance of the rash. In typical cases, the fever often declines on the day of, or up to three days after, the onset of the rash. The rash will first appear on the face and will quickly appear in a centrifugal distribution across the body. A centrifugal distribution means that there will be more lesions on the extremities and the face rather than on the abdomen and trunk. Figure below mentions lesions that are often noted in the oral cavity, and these lesions cause difficulties with eating and drinking and thus, hinder the nutritional intake of an infected individual. The skin lesions cause extensive perturbation of the skin, and this raises concerns about secondary bacterial infection of the skin, and this has been a complication that has been noticed in 19% of unvaccinated monkeypox patients. The rash observed in infected individuals follows a very distinct presentation.
Figure above shows Categorization of Nonspecific Symptoms and Complications of Monkeypox.
_
The hallmark feature of monkeypox is a disseminated vesiculopustular rash. The rash itself has been noted as going through several stages before they enter the desquamation phase in which the scabs begin peeling off. It has been noted that these distinctive lesions often present first as enanthem, macular, papular, then vesicular, and pustular, as is depicted in Figure below. These lesions will ultimately become crusted within two to three weeks. Prior to the rash appearing on the skin, an individual will see lesions developing on the tongue and mouth; these lesions are called enanthem. Enanthem or enanthema, is a rash inside the body i.e. rash on mucous membrane, by contrast, a rash on the outside of the body is called an exanthem or exanthema i.e. rash on skin. Once the crusted lesions peel off and reveal new skin underneath, an individual is no longer considered infectious. This is referred to as the desquamation phase. In some cases, individuals may be left with scars once scabs peel off. Some individuals may even present with regions of hyperpigmentation and hypopigmentation where the rash was more concentrated. The lesions are painful in all stages listed in Figure below until the desquamation phase, at which point, the crusting causes individuals extreme itchiness. Histopathologic analysis of the earliest stage of lesion development in humans reveals epidermal necrosis at the center of individual lesions concurrent with nascent extension into the superficial layers of the dermis. It has also been observed in monkeypox-infected non-human primates that lesion pathology intensifies as pustules form, with progressive ulceration, necrosis, and interstitial hyperplasia. Furthermore, edema is prominent at the margins of necrotic areas and clefts develop in interstitial spaces between cells where the fluid and cellular debris accumulates. Eventually, inflammation and necrosis of the superficial dermis predominate, and destruction of sebaceous glands and follicles is evident. Together, these attributes lead to the characterization of the affected areas as ‘partial-thickness wounds,’ and an injury of this extent points to the need for active prevention of complications such as secondary bacterial infections and possible cellulitis. Interventional studies demonstrated that the use of moist occlusive therapies successfully promoted re-epithelialization and healing at herpes lesions sites and as such, the use of moist occlusive dressings could be contemplated for patients with extensive facial coverage of rash lesions.
Figure above shows stages of the vesiculo-pustular rash in Monkeypox Patients.
_
Figure below shows stages of monkeypox lesion development.
Figure above shows stages of skin presentation and progression of monkeypox rash.
_
The incubation period has been estimated at 5 to 21 days, and duration of symptoms and signs at 2 to 5 weeks. The illness begins with nonspecific symptoms and signs that include fever, chills, headaches, lethargy, asthenia, lymph node swellings, back pain, and myalgia (muscle ache) and begins with a fever before rashes appear. Within 1 to 5 days after the onset of fever, rashes of varying sizes appear, first on the face (Fig. X below), then across the body, hands, and legs and feet (Fig. Y below). The rash undergoes several stages of evolution from macules, papules, vesicles (fluid-filled blisters) (see Fig. X below), and pustules, followed by resolution over time with crusts and scabs (Fig. Z below), which drop off on recovery. Various stages of the rash may show at the same time. Areas of erythema and/or skin hyperpigmentation are often seen around discrete lesions. Detached scabs may be considerably smaller than the original lesion. Inflammation of the pharyngeal, conjunctival, and genital mucosae may also be seen.
_
Figure X. Maculo-papular-vesicular-pustular monkeypox skin lesions of varying sizes on the face.
_
Figure Y. Papular-pustular monkeypox skin lesions on the hands, legs, and feet.
_
Figure Z. Extensive papulo-pustular monkeypox rashes with crust and scar formation.
_
Note:
Vaccinated individuals were noted to have fewer lesions, smaller lesions, and better presentation of regional monomorphism and centrifugal distribution of rash.
_
Gastrointestinal symptoms that arise by the second week of illness such as vomiting and diarrhea can contribute to severe dehydration in an infected individual. The mouth and throat ulcers cause difficulties with maintaining nutrition, furthering chances of dehydration amongst patients. Of the other complications, the most serious complication of monkeypox is the corneal infection. Ocular infections may result in corneal scarring and permanent vision loss. The majority of the complications associated with a monkeypox infection are detected in unvaccinated individuals (74%) than vaccinated patients (39.5%). Since the eradication of smallpox, the routine vaccine is no longer provided to today’s population. Due to cross-immunity, individuals that had been vaccinated against smallpox prior to the 1970s are much less likely to be developing the complications associated with a monkeypox virus infection. Sepsis and septic shock may also occur, and this is largely due to overly exaggerated immune responses. Monkeypox is a self-limiting viral illness and due to the nature of the illness, lifelong complications are very rarely noted.
Although bronchopneumonia is a complication of monkeypox infection, it is a complication that is more commonly noted in individuals that are co-infected with the influenza virus. The respiratory challenge of non-human primates across a range of infectious doses has been reproducibly shown to result in the development of focal necrosis of lung tissues, diffuse pulmonary consolidation, and fulminant bronchopneumonia. Serious inflammation and bronchopneumonia can restrict air intake and diminish a patient’s willingness and/or ability to ingest food and fluids.
_
The most reliable clinical sign differentiating monkeypox from smallpox and chickenpox is enlarged lymph nodes, especially the submental, submandibular, cervical, and inguinal nodes. Note the image below.
Figure above shows lymphadenopathy in monkeypox. Large nodes in the mandibular, cervical, or inguinal region are commonly seen in monkeypox. The presence of significant lymphadenopathy helps differentiate monkeypox from smallpox and chickenpox.
_
Is itching a symptom of monkeypox?
Monkeypox lesions are typically described as more painful than itchy in the beginning, adding that patients have reported itching towards the end when the sores are crusting and scabbing over. The characteristic monkeypox rash is a painful lesion, and that pain can become very intense. Some patients have actually had to be admitted to hospital for pain management. This may include topical treatments such as lidocaine or oral pain medication.
_____
_____
The May 2022 outbreak has produced atypical symptoms when compared to previous monkeypox outbreaks. These symptoms may include:
- Scant or even single lesions or even the complete absence of skin lesions
- Lesions mostly confined to the genital and perianal areas, presenting with anal pain and bleeding
- Lack of prodromal symptoms, such as fever, myalgias, fatigue, and headache before the appearance of a rash
- Some patients experience cutaneous lesions at the point of sexual contact prior to further symptoms.
Many cases in the 2022 monkeypox outbreak presented with genital and peri-anal lesions, fever, swollen lymph nodes, and pain when swallowing, with some patients manifesting only single sores from the disease.
Genital and anal lesions are of relevance in this outbreak because of their frequency. (see figure below).
Images represent both a range of presentations and a series of progression, giving an insight into the clinical course of the disease in an outbreak largely centered on men who have sex with men and bisexual men.
_
Figure above shows progression of penile lesions and penile oedema.
_
Principal findings different from previous outbreaks:
The characteristics of the cohort described in May 2022 outbreak differ from those of populations affected in previous outbreaks in endemic regions. In previous outbreaks where a higher proportion of the population had been vaccinated against smallpox, most infections occurred in young children. More recently, outbreaks of the West African and Congo Basin clades have affected both adults and children, with male patients being disproportionately represented in some reports in West and Central Africa. In contrast with previous reports, the current cohort comprised men only, and most (99.5%) identified as gay, bisexual, or other men who have sex with men.
At presentation, almost half (47.2%) of this cohort had exclusively mucocutaneous manifestations or developed systemic symptoms after rather than preceding the onset of lesions. The predilection of lesions to genital, perianal, and perioral or tonsillar areas, and the history of recent sexual contact in 96% of our cohort suggests lesions may initially form at the site of inoculation, followed by the development of systemic symptoms and subsequent dissemination of lesions. However, some of the participants, such as those with solitary lesions, did not develop further dissemination. More than a third (35.5%) of cohort described a polymorphic rash, a finding that has been recognised in other emerging evidence from this outbreak. Lesions appearing at different stages and timepoints could be a consequence of autoinoculation. Widespread maculopapular rashes were also observed that did not become pustular or ulcerated. These patterns represent a change in the clinical presentation of the disease.
Solitary lesions and tonsillar signs were not previously known to be typical features of monkeypox infection. On initial presentation, single lesions could be mistaken for other conditions such as syphilis, lymphogranuloma venereum, and ingrown hair follicles. Throat features included ulcers, pain, secondary bacterial superinfection, and quinsy, which could all be mistaken for bacterial tonsillitis. Infection in patients presenting in such ways may have gone undiagnosed in the community for some time. This could help to explain why the outbreak had become so widespread at the point of detection.
Just under a third (31.5%) of the cohort screened for a sexually transmitted infection had a co-infection. The most common co-infections were N gonorrhoeae and C trachomatis on rectal sampling, which might have increased the severity of rectal symptoms at presentation. In those who tested negative for monkeypox virus, the most common alternative diagnoses were syphilis, herpes simplex virus, varicella zoster virus, N gonorrhoeae, and C trachomatis. It is imperative to screen all people for sexually transmitted infections who present to healthcare settings with suspected monkeypox infection to ensure prompt diagnosis and treatment of co-infections.
_
Figure below shows Lesions in persons with Confirmed Human Monkeypox Virus Infection in May 2022 outbreak:
Panel A shows the evolution of cutaneous lesions in a person with monkeypox; images a1 and a2 show facial lesions, images b1 through b3 show a penile lesion, and images c1 and c2 show a lesion on the forehead. The polymerase-chain-reaction (PCR) status is indicated if available. IM denotes intramuscular, and MSM man who has sex with men.
Panel B shows oral and perioral lesions (image a, perioral umbilicated lesions; image b, perioral vesicular lesion on day 8, PCR positive; image c, ulcer on the left corner of the mouth on day 7, PCR positive; image d, tongue ulcer; image e, tongue lesion on day 5, PCR positive; and images f, g, and h, pharyngeal lesions on day 0, 3, and 21, respectively, PCR positive on day 0 and 3 and negative on day 21).
Panel C shows perianal, anal, and rectal lesions (image a, anal and perianal lesions on day 6, PCR positive; images b and c, rectal and anal lesions in a single person, PCR positive; image d, perianal ulcers, PCR positive; image e, anal lesions; image f, umbilicated perianal lesion on day 3, PCR positive; image g, umbilicated perianal lesions on day 3, PCR positive; and image h, perianal ulcer on day 2, PCR positive).
______
Monkeypox characteristics in MSMs:
Interestingly, many confirmed monkeypox cases in the MSM population do not exhibit the classic clinical manifestations. The most notable features are genital and perianal rashes, which often appear as the first symptoms. Furthermore, the morphology of the rash does not progress from maculopapular rash to blisters and pustules as in typical cases, instead, pustules have appeared before systemic symptoms (e.g., fever) in some cases. The initial appearance of genital or perianal rash may imply that close physical contact during sexual intercourse acts as a possible transmission route. All reported cases of monkeypox in MSM had sexual exposure with or without using condoms prior to symptom onset. It is unclear whether condom use has a protective effect on monkeypox transmission. However, condom use is still strongly recommended to prevent other sexually transmitted diseases (STDs). Reported monkeypox cases in MSM include both HIV-positive cases with viral suppression and HIV-negative cases receiving pre-exposure prophylaxis, and many are diagnosed alongside other STDs. Coinfection with hepatitis A, B, or C is also common.
______
Clinical features and novel presentations of human monkeypox in a central London Centre during the 2022 outbreak: 22 July 2022:
Participants were 197 patients with polymerase chain reaction confirmed monkeypox infection.
The median age of participants was 38 years. All 197 participants were men, and 196 identified as gay, bisexual, or other men who have sex with men. All presented with mucocutaneous lesions, most commonly on the genitals (n=111 participants, 56.3%) or in the perianal area (n=82, 41.6%). 170 (86.3%) participants reported systemic illness. The most common systemic symptoms were fever (n=122, 61.9%), lymphadenopathy (114, 57.9%), and myalgia (n=62, 31.5%). 102/166 (61.5%) developed systemic features before the onset of mucocutaneous manifestations and 64 (38.5%) after (n=4 unknown). 27 (13.7%) presented exclusively with mucocutaneous manifestations without systemic features. 71 (36.0%) reported rectal pain, 33 (16.8%) sore throat, and 31 (15.7%) penile oedema. 27 (13.7%) had oral lesions and 9 (4.6%) had tonsillar signs. 70/195 (35.9%) participants had concomitant HIV infection. 56 (31.5%) of those screened for sexually transmitted infections had a concomitant sexually transmitted infection. Overall, 20 (10.2%) participants were admitted to hospital for the management of symptoms, most commonly rectal pain and penile swelling.
These findings confirm the ongoing unprecedented community transmission of monkeypox virus among gay, bisexual, and other men who have sex with men seen in the UK and many other non-endemic countries. A variable temporal association was observed between mucocutaneous and systemic features, suggesting a new clinical course to the disease. New clinical presentations of monkeypox infection were identified, including rectal pain and penile oedema. These presentations should be included in public health messaging to aid early diagnosis and reduce onward transmission.
______
______
Section-12
Laboratory diagnosis of monkeypox:
_
Evaluation for a person with suspected monkeypox infection:
For any patient with a rash or anogenital lesion, clinicians should collect a detailed travel and sexual history and perform a physical examination that includes an evaluation of lymph nodes and oral, genital, and rectal mucosa.
Clinicians should isolate their patient in a single-person room if available. Clinicians should consult their infection and control practice in their hospital system, their state health department (State Contacts), or CDC as soon as monkeypox is suspected to ensure proper testing and reporting. Two-step diagnostic testing includes obtaining multiple samples from different lesions and sending the sample for an initial orthopoxvirus polymerase chain reaction test. If orthopoxvirus is confirmed, the specimens are sent for monkeypox virus-specific testing. Co-infections with monkeypox virus and sexually transmitted infections (STIs) have been reported; therefore, a broad testing approach for STIs should be considered.
Additionally, clinicians should use appropriate infection prevention measures when collecting specimens for monkeypox evaluation. These measures include the use of personal protective equipment such as eye protection, gown, gloves, and a particulate respirator approved by the National Institute for Occupational Safety and Health (NIOSH), e.g., N95.
_
The diagnosis of monkeypox should be suspected in patients who present with a rash or other symptoms that could be consistent with monkeypox and epidemiologic risk factors for infection (e.g., close or intimate in-person contact with individuals who have suspected or confirmed monkeypox or are part of a social network experiencing monkeypox activity; recent travel to Central or West Africa or other areas where large outbreaks of monkeypox have been reported). The diagnosis should also be suspected in patients who do not fall in groups described above but present with genital ulcer disease or proctitis that does not respond to empiric treatment for typical sexually transmitted infections. If the diagnosis of monkeypox is being considered, infection prevention and control measures should be implemented to reduce the risk of transmission.
_
Any individual meeting the definition for a suspected case should be offered testing. The decision to test should be based on both clinical and epidemiological factors, linked to an assessment of the likelihood of infection. Due to the range of conditions that cause skin rashes and because clinical presentation may more often be atypical in this outbreak, it can be challenging to differentiate monkeypox solely based on the clinical presentation, particularly for cases with an atypical presentation. It is therefore important to consider other potential causes of discrete skin lesions or a disseminated rash; Examples of other aetiologies for similar-appearing skin lesions at the different stages of development include herpes simplex virus, varicella zoster virus, molluscum contagiosum virus, enterovirus, measles, scabies, Treponema pallidum (syphilis), bacterial skin infections, medication allergies, parapoxviruses (causing orf and related conditions) and chancroid. Lymphadenopathy during the prodromal stage of illness can be a clinical feature to distinguish monkeypox from chickenpox or smallpox. If monkeypox is suspected, health workers should collect an appropriate sample and have it transported safely to a laboratory with appropriate capability. Confirmation of monkeypox depends on the type and quality of the specimen and the type of laboratory test. Polymerase chain reaction (PCR) is the preferred laboratory test given its accuracy and sensitivity.
_
The recommended specimen type for diagnostic confirmation of monkeypox in suspected cases is skin lesion material, including swabs of lesion exudate, roofs from more than one lesion, or lesion crusts. Where feasible, biopsy is an option. Laboratory confirmation of specimens from a suspected case is done using nucleic acid amplification testing (NAAT), such as real-time or conventional polymerase chain reaction (PCR). NAAT can be generic to orthopoxvirus (OPXV) or specific to monkeypoxvirus (MPXV, preferable). In addition to NAAT, sequencing is useful to determine virus clade and to understand epidemiology. Affected countries are strongly encouraged to share MPXV genetic sequence data in available and publicly accessible databases.
Lesion samples must be stored in a dry, sterile tube (no viral transport media) and kept cold. Viral DNA present in lesion material is stable for a long period of time if kept in a relatively dark, cool environment, an important factor to consider when cold chain is not readily available. PCR blood tests are usually inconclusive because of the short duration of viremia relative to the timing of specimen collection after symptoms begin and should not be routinely collected from patients. Rapid diagnosis is made via a positive real-time polymerase chain reaction (rt-PCR) on swabs from skin lesions.
Virus isolation and culture, immunochemistry, and immunofluorescence imaging are also potentially useful to confirm the diagnosis. A viral culture should be obtained by an oropharyngeal or nasopharyngeal swab. Skin biopsies of vesiculopustular rash or a sample of the roof of an intact skin vesicular lesion are valuable for analyses. Reference laboratories with high containment facilities are required to make a definitive diagnosis using electron microscopy, culture and molecular analysis identification by polymerase chain reaction, and sequencing.
Serologic testing requires paired acute and convalescent sera for MPXV-specific immunoglobulin M detection within 5 days of presentation, or immunoglobulin G detection after 8 days. As orthopoxviruses are serologically cross-reactive, antigen and antibody detection methods do not provide monkeypox-specific confirmation. Serology and antigen detection methods are therefore not recommended for diagnosis or case investigation where resources are limited. Additionally, recent or remote vaccination with a vaccinia-based vaccine (e.g. anyone vaccinated before smallpox eradication, or more recently vaccinated due to higher risk such as orthopoxvirus laboratory personnel) might lead to false positive results.
Histology and immunohistochemistry of papular lesions may show acanthosis, individual keratinocyte necrosis, and basal vacuolization, along with a superficial and deep perivascular lymphohistiocytic infiltrate in the dermis. Vesicular lesions show spongiosis with reticular and ballooning degeneration, multinucleated epithelial giant cells with epidermal necrosis with numerous eosinophils and neutrophils, and features of vasculitis and viral inclusions in keratinocytes. Intracytoplasmic, round-to-oval inclusions with sausage-shaped structures centrally, measuring 200 to 300 nanometers, may be seen on electron microscopic observation.
_
The NICD currently offers PCR testing and electron microscopy for the investigation of acute suspected monkeypox cases. Monkeypox has two disease phases and different specimens can be collected in each phase. During the prodromal phase specimens to be collected include tonsillar tissue swab, nasopharyngeal swab, acute serum and whole blood. Specimens to be collected during the rash/lesion phase includes lesion biopsy, fluid, scab or crust, acute serum and whole blood. More than one lesion should be sampled, preferably from different locations on the body and/or from different looking lesions
_____
In order to diagnose monkeypox, health providers should collect a proper specimen and send it carefully to a capable lab. Verifying human MPX virus relies on the sample type and the available laboratory tests. The confirming techniques that are used for analyzing specimens and determine MPX include genetic, phenotypic and immunological methods. Table below lists the diagnostic methods that can be used to identify human MPX. These approaches work better when are combined with the medical and epidemiological information including the patient’s immunization history.
The diagnostic methods that can be used to identify human MPX:
|
Genetic Methods |
Phenotypic Methods |
Immunological Methods |
Electron Microscopy |
|
|
Based on |
PCR or qPCR |
Clinical diagnosis |
Sensitive detection of IgG or IgM antibodies against MPX using Elisa test. Immunohistochemical (IHC) to spot virus antigens |
Electron microscopy (EM). |
|
Pros |
PCR is the standard test for detecting MPX-specific DNA sequences due to its high accuracy and sensitivity For genetic testing, the recommended diagnostic samples are from cutaneous lesions (a smear from the surface of the lesion and/or exudate, or crusts of the lesion) or from a biopsy when possible |
possible diagnosing based on clinical signs is essential in order to expose suspected cases during examination |
Increased antiviral antibodies and T-cell activation against MPX have been documented with disease onset When a rash develops, IgM and IgG can be detected in serum about 5 days and more than 8 days in a row If both IgM and IgG are present in unvaccinated persons with a history of rash and symptoms of severe disease, then an indirect diagnosis can be founded |
Can distinguish Orthopoxvirus from herpes simplex virus. It gives evidence that virus may belong to the Poxviridae family |
|
Cons |
Highly sensitive examinations where there are justified concerns about sample contamination These tests demand high-cost tools, reagents, and expert techniques |
According to a study conducted on a group of 645 individuals whose clinical diagnosis of MPX was not accompanied by a laboratory confirmation, it had a high sensitivity (93–98%) but low specificity (9%–26%) |
The above methods are not considered qualitative for human MPX |
Orthopoxviruses are indistinguishable from each other. Hence, it requires more specific testing diagnose |
_
A detailed medical history with focus on specific information, such as recent traveling to an endemic area, vaccinating with the smallpox vaccine, along with linking clinical information to the existing symptoms, can be extremely directing to the disease diagnosis, but it is not sufficient to establish a definitive one. The golden test to establish the diagnose is the polymerase chain reaction. Beside its high accuracy and sensitivity, the viral DNA within the lesion persists constant for a long time if kept in a dark and comparatively cool atmosphere. Polymerase chain reaction (PCR) testing should be performed on lesion samples. Lesions should be vigorously swabbed to collect skin cells that come off the lesion. Because the lesion is deep seeded, the lesion does not need to be unroofed for specimen collection. If there are multiple lesions, a few of them can be sampled (using separate swabs). It is recommended to take two swabs from each specimen.
The health setting of initial presentation reflected referral patterns and included sexual health or HIV clinics, emergency departments, and dermatology clinics and, less commonly, primary care. A positive PCR result was most commonly obtained from skin or anogenital lesions (97%) in a study; other sites were less frequently sampled. The reported percentages of positive PCR results were 26% for nasopharyngeal specimens, 3% for urine specimens, and 7% for blood specimens. Semen was tested in 32 persons from five clinical sites and was PCR positive in 29 persons.
Immunological tests against orthopoxviruses have a cross-reactivity with other Orthopoxviruses. Still, these tests may be valuable when there is previous indication to explain the disease cause.
Although IgG alone cannot provide a definitive diagnosis to a patient who has been exposed to orthopoxvirus during his life through vaccination, IgM is considered more effective in diagnosing newly infections patients retrospectively.
______
The U.S. Food and Drug Administration (FDA) is advising people to use swab samples taken directly from a lesion (rash or growth) when testing for the monkeypox virus. The FDA is not aware of clinical data supporting the use of other sample types, such as blood or saliva, for monkeypox virus testing. Testing samples not taken from a lesion may lead to false test results.
Potential Risk of False Monkeypox Test Results:
- False-negative monkeypox test results mean the test says the person does not have monkeypox, but they do have monkeypox. A false-negative result may lead to delayed diagnosis or inappropriate treatment. False-negative results can also lead to the infected person not taking action to limit their exposure to others, and further spread of the monkeypox virus.
- False-positive monkeypox test results mean the test says the person has monkeypox, but they do not have monkeypox. A false-positive result may lead to a delay in a correct diagnosis or the right treatment for the actual cause of the person’s illness, which could be a disease other than monkeypox.
______
Roche rolls out trio of PCR research tests for monkeypox:
If there’s one positive outcome of the COVID-19 pandemic, it’s the speed at which diagnostic test makers learned to develop and begin churning out kits to test for a virus. Roche, for one, is already applying what it learned from COVID to the next infectious disease outbreak. Only a few weeks after a spate of monkeypox cases first cropped up in Europe, the company has completed development of three separate tests for the virus. Developed in conjunction with its subsidiary TIB Molbiol, Roche’s tests are already available to scientists for research use in the majority of countries worldwide.
All three of Roche’s LightMix Modular Virus test kits analyze samples using quantitative PCR technology, requiring the tests to be run on one of the company’s PCR analyzers.
The first test looks for signs of any orthopoxvirus, a strain of viruses that includes smallpox, cowpox and horsepox as well as monkeypox.
Roche’s second test looks for monkeypox specifically and is able to detect both the West African and Central African clades of the virus.
The third test combines the two. Alongside identifying the presence of orthopoxviruses, it also returns results clarifying whether either form of monkeypox is the orthopoxvirus in question.
______
GeneXpert MPX/OPX test for monkeypox:
Molecular diagnostics for human MPX are currently limited to real-time quantitative polymerase chain reaction (qPCR) assays in specialized laboratory settings. How long does it take to get results from monkeypox testing? Typically, you should receive your test result within 48 hours. Delayed clinical diagnosis and lack of etiologic confirmation (via laboratory testing) are impediments to controlling the spread of MPX. Increased diagnostic capabilities in areas at risk for MPX would allow for an efficient and timely public health response in the case of an outbreak. Therefore, a relatively simple system designed to perform well in less sophisticated laboratories and field conditions would aid in surveillance and disease control activities.
The Cepheid GeneXpert system is a backpack-sized, consolidated analytic workstation that combines sample preparation with real-time PCR amplification and detection. The system uses a self-contained cartridge to minimize contamination risks, and results are obtained from minimally processed samples in less than 90 minutes. This technology has been used for the detection of a number of infectious diseases, most notably Mycobacterium tuberculosis with rifampicin resistance, methicillin-resistant Staphylococcus aureus, and, most recently, Ebola virus disease (EVD). The accuracy and utility of a multiplex MPXV and OPX assay using the GeneXpert platform provided an alternative to traditional PCR detection methods. The GeneXpert MPX/OPX assay performed has a sensitivity of 98.8% and specificity of 100%. The GeneXpert MPX/OPX assay displayed high sensitivity, specificity in specimens from suspect cases. The assay was highly accurate regardless of the type of specimen collected (crust versus vesicular swab), and demonstrated the ability to identify MPX infection at any stage of the rash. Blood viremia after MPXV infection is often time dependent and unreliable for acute diagnosis, so crust and vesicular swab specimens were preferable, and also reduced the risks associated with needle use for the patients. Further, crusts and vesicular swabs were used for their reliability and stability with the GeneXpert cartridge. GeneXpert MPX/OPX test is specific and accurate test for detecting human MPX infection. Compared with traditional real-time PCR diagnostic methods, it is not only easier and much faster to use, but also offers lower risk of contamination and increases user safety through its all-in-one cartridge system.
____
There is a growing possibility that monkeypox will become an endemic sexually transmitted infection that will need to be considered in patients with a rash-like illness following intimate exposure with a new partner. As the case count increases, new tests will likely be developed, including those that allow for an individual to collect their own specimen from home (but send that sample to a lab for testing), multiplex tests that analyze a specimen for several diseases (such as monkeypox, herpes, syphilis) in the same test, and potentially in the future, those that do not require samples to be submitted to a clinical laboratory. Hopefully, the lessons learned from Covid-19 will result in innovative testing options to curb the spread of monkeypox.
_____
Biological risk management:
It is recommended that all manipulations of specimens originating from suspected, probable or confirmed cases of monkeypox in the laboratory be conducted according to a risk-based approach. Each laboratory should conduct a local (that is, institutional) risk assessment. When manipulating biological specimens, core biosafety requirements, similar to those previously referred to as biosafety level 2, must be met and heightened control measures should be applied based on local risk assessment.
MPXV may be contracted during the specimen processing stage from contaminated material or faulty processes. Therefore, heightened biosafety measures are recommended in addition to the core requirements, including the following for the purpose of clinical testing without virus propagation.
- Specimens from patients with suspected MPXV infection must be handled in a functioning Class I or II biosafety cabinet, prior to sample inactivation. Properly inactivated specimens do not require a biosafety cabinet.
- Laboratory personnel should wear appropriate PPE, especially for handling specimens before inactivation.
- Where use of a centrifuge is required for a procedure, safety cups or sealed rotors should be used.
Additional control measures should be considered for specific procedures, including aerosol-forming procedures, according to the local risk assessment.
_
Occupational health:
Various smallpox vaccines, containing vaccinia virus, provide cross-protection against other OPXV, including monkeypox, therefore national health authorities should conduct a risk assessment and consider whether arranging immunization for health care workers, including laboratory personnel, and other staff that are at risk of exposure to individuals or specimens with MPXV is required. A non-replicating vaccine consisting of the modified vaccinia Ankara strain known as MVA-BN was approved for prevention of smallpox (which was declared eradicated in 1980) in 2013. In 2019, it was also approved for the prevention of monkeypox by two stringent regulatory authorities. This vaccine can also be considered for prevention of monkeypox in the occupational setting.
______
______
Section-13
Differential diagnosis of monkeypox:
To begin with, let’s split the word ‘monkeypox’ in half to understand its etymology. While monkeys aren’t exactly the culprits to blame, the virus was first identified in a monkey colony used for research in 1958. And pox refers to any disease that produces a rash of pimples that become pus-filled and leave pockmarks on healing. Hence the name ‘monkey-pox’.
Similarly, chickenpox has nothing to do with chickens. There are two theories behind the naming of the disease: the first suggests that when the disease was discovered, the blisters on the diseased person looked similar to chickpeas; the other indicates that the rashes looked like peck marks caused by a chicken.
As for smallpox, the ‘small’ was used to distinguish it from syphilis (also recognised by rashes), which was called ‘greatpox’ in the 16th Century.
_
Various infections and skin diseases should be considered in differential diagnosis. Both clinical and epidemiological data and specific laboratory studies can lead to different diagnoses. In the current outbreak, cases of both monkeypox and other sexually transmitted infections have been reported. therefore, diagnosis of an infection such as syphilis or lymphogranuloma venereum should not rule out infection with the monkeypox virus.
Figure below shows lesions of the various differential diagnoses of monkeypox:
_____
Attending to the characteristics of its lesions, monkeypox will have to be differentiated from other diseases that cause a vesicular rash and a fever, including chickenpox, smallpox [eradicated], herpes zoster and erythema multiforme.
Different rash-like conditions can be confused with monkeypox and are thus included in its differential diagnosis. The various conditions that should be differentiated from monkeypox include:
Impetigo:
It commonly presents with pimple-like lesions surrounded by erythematous skin. Lesions are pustules, filled with pus, which then break down over 4-6 days and form a thick crust. It’s often associated with insect bites, cuts, and other forms of trauma to the skin.
Insect bites:
The insect injects formic acid, which can cause an immediate skin reaction often resulting in a rash and swelling in the injured area, often with formation of vesicles.
Kawasaki disease:
Commonly presents with high and persistent fever, red mucous membranes in mouth, “strawberry tongue”, swollen lymph nodes and skin rash in early disease, with peeling off of the skin of the hands, feet and genital area.
Measles:
Commonly presents with high fever, coryza and conjunctivitis, with observation of oral mucosal lesions (Koplik’s spots), followed by widespread skin rash.
Rubella:
Commonly presents with a facial rash which then spreads to the trunk and limbs, fading after 3 days, low grade fever, swollen glands, joint pains, headache and conjunctivitis. The rash disappears after a few days with no staining or peeling of the skin. Forchheimer’s sign occurs in 20% of cases, and is characterized by small, red papules on the area of the soft palate.
Atypical measles:
The symptoms commonly begin about 7-14 days after infection and present as fever, cough, coryza and conjunctivitis. Observation of Koplik’s spots is also a characteristic finding in measles.
Coxsackievirus:
The most commonly caused disease is the Coxsackie A disease, presenting as hand, foot and mouth disease. It may be asymptomatic or cause mild symptoms, or it may produce fever and painful blisters in the mouth (herpangina), on the palms and fingers of the hand, or on the soles of the feet. There can also be blisters in the throat or above the tonsils. Adults can also be affected. The rash, which can appear several days after high temperature and painful sore throat, can be itchy and painful, especially on the hands/fingers and bottom of feet.
Acne:
It is typical of teenagers, usually appears on the face and upper neck, but the chest, back and shoulders may have acne as well. The upper arms can also have acne, but lesions found there are often keratosis pilaris, not acne. The typical acne lesions are comedones and inflammatory papules, pustules, and nodules. Some of the large nodules were previously called “cysts”
Syphilis:
It commonly presents with generalized systemic symptoms such as malaise, fatigue, headache and fever. Skin eruptions may be subtle and asymptomatic It is classically described as:
-Non-pruritic bilateral symmetrical mucocutaneous rash
-Non-tender regional lymphadenopathy
-Condylomata lata and
-Patchy alopecia.
Molluscum contagiosum:
The lesions are commonly flesh-colored, dome-shaped, and pearly in appearance. They are often 1-5 millimeters in diameter, with a dimpled center. Generally not painful, but they may itch or become irritated. Picking or scratching the lesions may lead to further infection or scarring. In about 10% of the cases, eczema develops around the lesions. They may occasionally be complicated by secondary bacterial infections.
Infectious Mononucleosis:
Common symptoms include low-grade fever without chills, sore throat, white patches on tonsils and back of the throat, muscle weakness and sometime extreme fatigue, tender lymphadenopathy, petechial hemorrhage and skin rash.
Toxic erythema:
It is a common rash in infants, with clustered and vesicular appearance.
Rat-bite fever:
It commonly presents with fever, chills, open sore at the site of the bite and rash, which may show red or purple plaques.
Parvovirus B19:
The rash of fifth disease is typically described as “slapped cheeks,” with erythema across the cheeks and sparing the nasolabial folds, forehead, and mouth.
Cytomegalovirus:
The common symptoms include sore throat, swollen lymph nodes, fever, headache, fatigue, weakness, muscle pain and loss of appetite.
Scarlet fever:
It commonly includes fever, punctate red macules on the hard and soft palate and uvula (Forchheimer’s spots), bright red tongue with a “strawberry” appearance, sore throat and headache and lymphadenopathy.
Rocky Mountain spotted fever:
The symptoms may include maculopapular rash, petechial rash, abdominal pain and joint pain.
Stevens-Johnson syndrome:
The symptoms may include fever, sore throat and fatigue. Commonly presents ulcers and other lesions in the mucous membranes, almost always in the mouth and lips but also in the genital and anal regions. Those in the mouth are usually extremely painful and reduce the patient’s ability to eat or drink. Conjunctivitis of the eyes occurs in about 30% of children. A rash of round lesions about an inch across, may arise on the face, trunk, arms and legs, and soles of the feet, but usually not on the scalp.
Herpes zoster:
It commonly starts as a painful rash on one side of the face or body. The rash forms blisters that typically scab over in 7-10 days and clears up within 2-4 weeks.
Chickenpox:
It commonly starts with conjunctival and catarrhal symptoms and then characteristic spots appearing in two or three waves, mainly on the body and head, rather than the hands, becoming itchy raw pox (small open sores which heal mostly without scarring). Touching the fluid from a chickenpox blister can also spread the disease.
Meningococcemia:
It commonly presents with rash, petechiae, headache, confusion, and stiff neck, high fever, mental status changes, nausea and vomiting.
Rickettsial pox:
The first symptom is commonly a bump formed by a mite-bite, eventually resulting in a black, crusty scab. Many of the symptoms are flu-like including fever, chills, weakness and muscle pain but the most distinctive symptom is the rash that breaks out, spanning the person’s entire body.
Meningitis:
It commonly presents with headache, nuchal rigidity, fever, petechiae and altered mental status.
______
______
Differential diagnosis of genital and perianal lesions that mimic monkeypox in MSM:
-1. Genital herpes
-Caused by HSV (herpes simplex virus), mostly HSV-2
-Painful thin-walled vesicular lesions on erythematous base; often bilateral on labia, vulva, perineum, perianal areas, or shaft or glans of penis
-Vesicles rupture to form small painful ulcers
-Diagnosis confirmed by polymerase chain reaction, antigen assays, and microscopic examination
-2. Syphilis
-Caused by Treponema pallidum spirochete
-Firm, generally single, painless genital lesion with clean base, indurated border, and associated regional painless adenopathy; frequently found in perineum, cervix, anogenital area, lips, oropharynx, and hands
-Diagnosis confirmed with VDRL testing (Venereal Disease Research Laboratory) or RPR testing (rapid plasma reagin) followed by confirmatory treponemal antigen testing, demonstration of Treponema pallidum on darkfield microscopy, or detection with polymerase chain reaction
-3. Chancroid
-Caused by Haemophilus ducreyi
-Nonindurated, painful, exudative genital ulcer with necrotic base that bleeds when scraped
-Tender and suppurative inguinal adenopathy
-Definitive diagnosis is determined by culture of exudate from lesion and exclusion of genital herpes and syphilis
-4. Lymphogranuloma venereum
-Caused by Chlamydia trachomatis
-Unilateral small papules or pustules that are painless and may erode, resulting in formation of small ulcers
-Tender inguinal lymphadenopathy, usually unilateral, is common
-Diagnosis confirmed via culture or nucleic acid amplification testing, performed on swab of skin lesion or lymph node aspirate
-5. Genital warts
-Caused by HPV (human papillomavirus)
-Flat, papular, or pedunculated growths in or around the anogenital area; warts are highly variable in size and appearance
-Diagnosis is made by visual inspection of external genital lesions with ascertainment of typical genital wart morphology; biopsy can be diagnostic if lesions are atypical
-6. Granuloma inguinale
-Caused by Klebsiella granulomatis
-Painless, beefy red, foul-smelling ulcer and absence of regional lymphadenopathy, although pseudobuboes may form
-Diagnosis is usually clinical, but Giemsa- or Wright-stained smears may be confirmatory
______
Differential diagnosis of diffuse rash that mimic monkeypox:
-1. Syphilis
-Secondary syphilis can result in a maculopapular erythematous rash that involves the trunk and extremities, including palms and soles
-Diagnosis confirmed with VDRL testing (Venereal Disease Research Laboratory) or RPR testing (rapid plasma reagin) followed by confirmatory treponemal antigen testing, demonstration of Treponema pallidum on darkfield microscopy, or detection with polymerase chain reaction
-2. Varicella (chickenpox)
-Caused by varicella-zoster virus
-Lesions evolve from macules to papules to thin-walled vesicles, which become cloudy or pustular in appearance and may umbilicate before crusting; presence of lesions in various stages of healing is characteristic
-Lesions are superficial and typically denser on trunk than on face and extremities
-Diagnosis is usually clinical, but it may be confirmed by polymerase chain reaction assay, culture, direct antigen testing, or serology
-3. Disseminated herpes zoster (shingles) infection
-Caused by reactivation of varicella-zoster virus acquired from previous varicella infection or vaccination
-Usually has limited dermatomal distribution, but disseminated disease may occur, especially in immunocompromised patients
-Erythematous maculopapular lesions evolve over several days into clusters of vesicles, which may coalesce to form bullae
-Diagnosis is usually clinical, but it may be confirmed by viral culture, polymerase chain reaction, Tzanck test, or direct fluorescent antibody test
-4. Disseminated herpes simplex infection
-Immunocompromised patients may develop severe disseminated infection
-Superficial painful vesicles that can rupture to form small ulcers
-Diagnosis is confirmed by polymerase chain reaction, antigen assays, and microscopic examination
-5. Molluscum contagiosum
-Caused by molluscum contagiosum virus
-In children, trunk and proximal extremities are commonly affected, although lesions can occur on any part of body, including face and genitals, via autoinoculation. Sexually transmitted infection in adults primarily affects the perineum and thighs
-Skin-colored, dome-shaped, smooth, pearly papules with central umbilication
-Diagnosis is usually clinical, but histopathology can be diagnostic
-6. Other poxvirus infections
-Other orthopoxviruses that can cause disease in humans include the causative agents of smallpox, vaccinia, and cowpox
-Overall clinical course and features of monkeypox are quite similar to those of smallpox, which has been eradicated globally and is no longer seen
-Lymphadenopathy is frequently present in monkeypox but absent in smallpox
-Vaccinia occurs in the context of smallpox vaccination or direct contact with the unhealed inoculation site of a person who has recently been vaccinated
-Cowpox occurs after exposure to infected animals (cows and others)
-CDC and certain authorized commercial laboratories can perform monkeypox-specific polymerase chain reaction testing to differentiate monkeypox from other orthopoxviruses; however, CDC states there are no other clinically significant orthopoxviruses in circulation in the United States at this time
_______
_______
Monkeypox vs. chickenpox:
Given the worldwide eradication of smallpox, the most likely diagnostic consideration in a patient presenting with a vesicular rash is varicella (chickenpox). Although they both cause skin rashes, different viruses cause monkeypox and chickenpox. Monkeypox is an orthopoxvirus, while chickenpox is a herpes virus. Both viruses can be spread through skin-to-skin or face-to-face contact, but chickenpox is very contagious and spreads more easily than monkeypox. Chickenpox is airborne while monkeypox isn’t. In several outbreaks, it has been difficult to distinguish the two. One feature that may help distinguish these infections is lymphadenopathy, which is often a distinctive feature of monkeypox compared with varicella. In addition, unlike varicella where vesicular lesions are characteristically in different stages of development and healing, monkeypox lesions are generally at the same stage. However, during the global monkeypox outbreak that started in May 2022, some reports describe lesions that were in different stages of development. The presence of lymphadenopathy, pre-eruptive fever and slower maturation of skin lesions are the most important clinical signs supporting correct diagnosis of monkeypox.
_
It’s actually not easy for most people to tell the difference, says Thomas Russo, M.D., professor and chief of infectious disease at the University at Buffalo in New York. “They’re both viral infections that cause pox-like lesions,” he says. “Rarely in our lifetimes have we had we had two pox diseases co-circulating at the same time.” In general, monkeypox and chickenpox tend to appear in “very different demographic groups,” Dr. Russo says. (Monkeypox has largely shown up in men who have sex with men; chickenpox typically is more common in kids). However, he points out, “adults can get chickenpox if they’re not vaccinated” and there have been at least two cases of monkeypox in children.
There are some potential cues with the rashes, too, which “are both similar and different,” says William Schaffner, M.D., an infectious disease specialist and professor at the Vanderbilt University School of Medicine. Chickenpox, for example, creates a “very thin-walled, fragile blister” that’s usually filled with clear fluid, Dr. Schaffner says. But monkeypox causes a “deep-seated, firm, or rubbery kind of lesion—it’s much more firm and stable than the chickenpox lesion,” he explains. Chickenpox lesions can “break easily, whereas monkeypox lesions do not,” Dr. Schaffner says. Monkeypox lesions also tend to change over time and may “umbilicate,” which means they form an indentation or little crater in the middle, Dr. Schaffner says. How long you’re sick for factors in, too. “It will take two to four weeks for those monkeypox lesions to resolve completely, whereas chickenpox lesions resolve much more quickly,” Dr. Schaffner says. (The CDC notes that chickenpox usually lasts about four to seven days.)
______
______
Monkeypox vs. tomato flu:
A new illness known as tomato flu, or tomato fever, has emerged in India in the state of Kerala in children younger than 5 years. The rare viral infection is in an endemic state and is considered non-life-threatening; however, because of the dreadful experience of the COVID-19 pandemic, the vigilant management is desirable to prevent further outbreaks. Although the tomato flu virus shows symptoms similar to those of COVID-19 (both are associated with fever, fatigue, and bodyaches initially, and some patients with COVID-19 also report rashes on the skin), the virus is not related to SARS-CoV-2. Tomato flu could be an after-effect of chikungunya or dengue fever in children rather than a viral infection. The virus could also be a new variant of the viral hand, foot, and mouth disease, a common infectious disease targeting mostly children aged 1–5 years and immunocompromised adults, and some case studies have even shown hand, foot, and mouth disease in immunocompetent adults. Tomato flu is a self-limiting illness and no specific drug exists to treat it.
The primary symptoms observed in children with tomato flu are similar to those of chikungunya, which include high fever, rashes, and intense pain in joints. Tomato flu gained its name on the basis of the eruption of red and painful blisters throughout the body that gradually enlarge to the size of a tomato. These blisters resemble those seen with the monkeypox virus in young individuals. Rashes also appear on the skin with tomato flu that lead to skin irritation. As with other viral infections, further symptoms include, fatigue, nausea, vomiting, diarrhoea, fever, dehydration, swelling of joints, body aches, and common influenza-like symptoms, which are similar to those manifested in dengue. In children with these symptoms, molecular and serological tests are done for the diagnosis of dengue, chikungunya, zika virus, varicella-zoster virus, and herpes; once these viral infections are ruled out, contraction of tomato virus is confirmed. Because tomato flu is similar to chikungunya and dengue as well as hand, foot, and mouth disease, treatment is also similar—i.e., isolation, rest, plenty of fluids, and hot water sponge for the relief of irritation and rashes. Supportive therapy of paracetamol for fever and bodyache and other symptomatic treatments are required.
Monkeypox rash is a painful red, flat bump, and these bumps covert into blisters that are filled with pus. These are commonly seen on the face, inside the mouth, or hands accompanied by lymphadenopathy. The rashes develop after 2-3 days of fever and these lasts usually from 2-4 weeks.
_
Monkeypox vs. Hand, foot and mouth disease (HFMD):
HFMD, which is typically caused by coxsackieviruses in the U.S., is quite contagious. The disease spreads easily from person to person through close contact with body fluids — saliva, nasal secretions, stool, fluid from blisters — and through respiratory droplets. For example, touching toys that have the virus on them and then touching your eyes, nose and mouth can spread the disease, along with close contact, such as hugging, kissing or sharing utensils and cups, with an infected person.
HFMD usually starts with a sore throat or sore mouth. In younger, nonverbal children, it often manifests as a refusal to eat or drink. Low-grade fevers that last a day or two sometimes occur. The classic sign is the development of mouth sores, most commonly on the tongue and on the inside lining of the cheek. These start out as small, flat red spots which progress to blisters and then ulcers. About 75% of those infected with HFMD develop a rash on the palms of their hands and soles of their feet and occasionally on the buttocks, upper thighs and arms. The rash may appear as red spots that are flat or slightly raised or blisters, similar to chickenpox, and may or may not be painful. It is usually not itchy.
_
Figure below shows Hand, foot, and mouth disease in adult.
Hand, foot, and mouth disease can be confused for monkeypox because both illnesses can cause fevers as well as lesions on the hands, feet and mouth. HFMD lesions usually get better without treatment within ten days. The best way to tell between monkeypox and hand, foot, and mouth is with a lab test.
_
Table below compare monkeypox, chickenpox, and hand-foot-and-mouth disease:
|
Category |
Monkeypox |
Chickenpox |
Hand-Foot-and-Mouth Disease |
|
Virus |
Monkeypox virus |
Varicella zoster virus (VZV) |
Enteroviruses (e.g. Coxsackievirus A16, Enterovirus 71) |
|
Incubation period |
5-21 days |
10-21 days, but can be up to 28 days in breakthrough infections |
3-7 days |
|
Fever |
May occur, most commonly 1-3 days before rash onset |
Unvaccinated: Mild if present, 1-2 days before rash2 #Breakthrough: Less common |
1-2 days before oral vesicles |
|
Lymphadenopathy |
May occur |
Less common |
Less common |
|
Rash appearance |
*Appears at the site of inoculation, then may appear on other parts of the body, including: •oral mucosa, •genital area, •conjunctiva, •palms of the hands, and •soles of the feet
|
Usually appears on the chest, back, and face then spreads to other parts of the body Rarely, lesions may appear on palms of the hands and soles of the feet in immunocompromised individuals |
Usually, vesicles appear in or on the mouth, then may appear on other parts of the body, including •palms of the hands, •soles of the feet, •knees, •elbows, •buttocks, or •genital area
|
|
Rash progression |
Slowly progresses through macules, papules, vesicles, pustules, and crusting/scab. May have central umbilication Lesions may develop simultaneously and evolve together on any given part of the body (i.e., monomorphic) |
Unvaccinated: Pleomorphic rash; Rapidly progresses through macules, papules, vesicles, and crusting/scab #Breakthrough: Usually, maculo-papular lesions that do not progress to vesicles Lesions occur in “crops” with old and new lesions present at the same time |
Macules, sometimes with vesicles Vesicles may break open and progress to crusting/scab |
|
Rash duration |
14-28 days |
Unvaccinated: 4-7 days Breakthrough: Shorter duration of illness compared to unvaccinated |
7-10 days |
#Breakthrough varicella is an infection that occurs in a varicella vaccinated person more than 42 days after varicella vaccination.
*Atypical presentations of monkeypox include initial signs of a genital or peri-anal rash prior to or without prodromal symptoms which may not spread to other parts of the body, and having lesions at different stages of development.
____
____
Monkeypox vs. Molluscum contagiosum:
Molluscum contagiosum is probably one of the things that can look most similar to monkeypox. Both cause indented lesions and, like monkeypox, the molluscum contagiosum virus spreads through direct skin contact. This means it can be seen in similar areas of the body. Molluscum contagiosum can be seen it in the groin and the anus, but we can also see it anywhere on the body such as the knees, hands, arms. Molluscum contagiosum generally causes “hard bumps,” whereas monkeypox lesions were pus-filled. Molluscum contagiosum lesions are skin-colored, dome-shaped, smooth, pearly papules with central umbilication. Most healthy people with molluscum contagiosum don’t tend to get sick and the rash gets better without treatment within a year.
_____
_____
What is monkey fever?
The virus that causes monkey fever (also known as Kyasanur forest illness) is a member of the Flaviviridae family. It is a disease spread by a vector. Both humans and monkeys are typically affected by it. Monkey Fever is transmitted by ticks. The tick’s bite causes humans to become infected. The first instance of it was in Karnataka’s Kyasanur Forest in March 1957, where many monkeys died and later people became sick.
______
______
Monkeypox and varicella-zoster co-infection:
A Tale of Two Viruses: Coinfections of Monkeypox and Varicella Zoster Virus in the Democratic Republic of Congo, 2020 study:
Recent enhanced monkeypox (MPX) surveillance in the Democratic Republic of Congo, where MPX is endemic, has uncovered multiple cases of MPX and varicella zoster virus (VZV) coinfections. The purpose of this study was to verify if coinfections occur and to characterize the clinical nature of these cases. Clinical, epidemiological, and laboratory results were used to investigate MPX/VZV coinfections. A coinfection was defined as a patient with at least one Orthopoxvirus/MPX-positive sample and at least one VZV-positive sample within the same disease event. Between September 2009 and April 2014, 134 of the 1,107 (12.1%) suspected MPX cases were confirmed as MPX/VZV coinfections. Coinfections were more likely to report symptoms than VZV-alone cases and less likely than MPX-alone cases. Significantly higher lesion counts were observed for coinfection cases than for VZV-alone but less than MPX-alone cases. Discernible differences in symptom and rash severity were detected for coinfection cases compared with those with MPX or VZV alone. Findings indicate infection with both MPX and VZV could modulate infection severity. Collection of multiple lesion samples allows for the opportunity to detect coinfections. As this program continues, it will be important to continue these procedures to assess variations in the proportion of coinfected cases over time.
The possibility of monkeypox and varicella-zoster virus (VZV) co-infection, seen in 12% of individuals in the DRC, is an epidemiologic observation worth highlighting because women from tropical and subtropical regions are more likely to be non-immune to VZV. For example, only 80.9% of pregnant women in Tunisia have VZV IgG antibodies compared to 96.1% and 98.8% of pregnant women in Spain and France. Co-infection carries important implications for similarly susceptible groups because both viruses carry a risk of vertical transmission. Given that co-infection also modifies the severity of the skin rash, delayed diagnoses and treatment could result in worse perinatal outcomes, particularly in resource-limited settings.
______
______
Section-14
Treatment of monkeypox:
The disease is usually self-limited; resolution occurs in 2-4 weeks. In the African cases, the mortality rate was 1-10%, and death was related to the patients’ health status and other comorbidities. Most patients died of secondary infections. No fatalities were reported in the 2003 US outbreak. Many people infected with monkeypox virus have a mild, self-limiting disease course in the absence of specific therapy. However, the prognosis for monkeypox depends on multiple factors, such as previous vaccination status, initial health status, age, immune status, concurrent illnesses, and comorbidities among others. Patients who should be considered for treatment might include:
-1. People with severe disease (e.g., hemorrhagic disease, confluent lesions, sepsis, encephalitis, or other conditions requiring hospitalization)
-2. People who may be at high risk of severe disease
-People with immunocompromise (e.g., human immunodeficiency virus/acquired immune deficiency syndrome infection, leukemia, lymphoma, generalized malignancy, solid organ transplantation, therapy with alkylating agents, antimetabolites, radiation, tumor necrosis factor inhibitors, high-dose corticosteroids, being a recipient with hematopoietic stem cell transplant <24 months post-transplant or ≥24 months but with graft-versus-host disease or disease relapse, or having autoimmune disease with immunodeficiency as a clinical component)
-Pediatric populations, particularly patients younger than 8 years of age
-People with a history or presence of atopic dermatitis, persons with other active exfoliative skin conditions (e.g., eczema, burns, impetigo, varicella zoster virus infection, herpes simplex virus infection, severe acne, severe diaper dermatitis with extensive areas of denuded skin, psoriasis, or Darier disease [keratosis follicularis])
-Pregnant or breastfeeding women
-People with one or more complications (e.g., secondary bacterial skin infection; gastroenteritis with severe nausea/vomiting, diarrhea, or dehydration; bronchopneumonia; concurrent disease or other comorbidities)
-3. People with monkeypox virus aberrant infections that include accidental implantation in eyes, mouth, or other anatomical areas where monkeypox virus infection might constitute a special hazard (e.g., the genitals or anus)
_
Patients often feel poorly during the febrile stage of the illness; therefore, bedrest along with supportive care may be necessary. Newly identified cases of MPX should undergo a medical assessment for severity and risk factors (e.g. underlying conditions or medications affecting immune competence, untreated HIV infection etc.). Those at increased risk of severe disease from MPX may require hospitalisation and/or treatment with antivirals. People at increased risk for severe disease include infants and young children, pregnant women, elderly and severely immunocompromised persons. There is no specific treatment for MPX as to date. CDC recommends administering the smallpox vaccine within 4 days of exposure which may prevent the disease from happening, and within 2 weeks to reduce symptoms severity. In immunocompromised patients, first and second generations smallpox vaccines are contradictive, and are replaced with vaccinia immune globulin. FDA approved Smallpox antivirals tecovirimat and brincidofovir can be used to treat MPX but there are no studies that prove their efficacy. Tecovirimat has the potential to cause resistance to pox viruses, therefore, careful monitoring of treated patients should be undertaken, particularly the immunosuppressed. Potential combination with brincidofovir can also be explored. A study found that total 5% of the 528 persons received monkeypox-specific treatment. The drugs administered included intravenous or topical cidofovir (in 2% of persons), tecovirimat (2%), and vaccinia immune globulin (<1%). The majority of MPX cases reported so far in this outbreak have been mild with localised disease and self-limiting symptoms. Therefore, hospitalisation is not necessary, unless the patient’s clinical condition requires it. Patients can remain isolated at home with supportive care (analgesia, hydration). If isolation is not possible at home, then hospitalisation or other arrangement can be considered. Hospitalization may be necessary in more severe cases; a negative pressure room is preferable. To avoid infection of health care workers and close contacts, airborne and contact precautions should be applied. Isolation must be continued until the last crust is shed.
MPXV can be transmitted to anyone, regardless of sexual orientation or gender identity, through contact with body fluids, monkeypox sores, or shared items. Therefore, cases should be instructed to isolate until the rash scabs fall off, which indicates the end of infectiousness. Cases should remain in their own room, when at home, and use designated household items (clothes, bed linen, towels, eating utensils, plates, glasses), which should not be shared with other members of the household. Cases should also avoid contact with immunocompromised persons until their rash heals. A MPX case should be monitored daily by public health authorities (e.g. via telephone calls) and can temporarily leave their home (e.g. for medical appointments and necessary exercise for their mental health stability), provided they wear a medical face mask and their rash is covered (e.g. long sleeves and pants). They should also be instructed to avoid close or intimate contact (hugging, kissing, prolonged face-to-face contact in closed spaces) with other people until their rash heals completely.
Careful hand and respiratory hygiene are recommended for the case and everyone in the household; a medical face mask should be used when in contact with other people. Cases should abstain from sexual activity until scabs fall off. While the use of condoms is consistently encouraged during sex for prevention of HIV and other STIs, cases should be made aware that the use of condoms alone cannot provide full protection against MPXV infection, as contact with skin lesions is involved for its transmission. Because transmission through droplets is possible, avoidance of close, physical contact is recommended until the scabs fall off.
Health authorities and policy makers should consider that sex workers may be disproportionately affected by this outbreak and may need incentives to be able to comply with the full recommendation of isolation until the rash heals completely which may last up to four weeks.
Finally, instructions should be given to MPX cases to avoid contact with any mammal pets, and in particular pet rodents (mice, rats, hamsters, gerbils, guinea pigs, squirrels etc), due to the possibility of human-to-pet transmission. Any recent contact with such pets should be noted and animal health services should be contacted for advice.
_
For individuals exposed to the virus, temperature and symptoms should be monitored twice per day for 21 days because that is the accepted upper limit of the monkeypox incubation period. Infectiousness aligns with symptom onset; therefore, close contacts need not isolate while asymptomatic. In some cases, post-exposure vaccination with modified vaccinia Ankara vaccine (smallpox and monkeypox vaccine, live, non-replicating) is recommended. Contact between broken skin or mucous membranes and an infected patient’s body fluids, respiratory droplets, or scabs is considered a “high risk” exposure that warrants post-exposure vaccination as soon as possible.
The replication-defective modified vaccinia Ankara vaccine is a two-shot series, four weeks apart, with a superior safety profile compared to first and second-generation smallpox vaccines. Unlike live vaccinia virus preparations, administering modified vaccinia, Ankara does not create a skin lesion or pose a risk of local or disseminated spread. In addition, clinical trials have shown that modified vaccinia Ankara is safe and stimulates antibody production in patients with atopy and compromised immune systems, which are known contraindications to live vaccinia administration.
_
Despite the suggested treatment interventions, supportive and symptomatic therapy has been deemed the basis of managing a monkeypox viral infection. Tables below allows for insight into potential supportive treatment options that can be utilized to aid symptomatic individuals. It is important to understand that besides symptomatic management and preventing complications, there is no clear treatment for monkeypox. With the 2003 US outbreak of monkeypox and the current presentation of monkeypox cases, internationally, more research must be conducted before any treatment or vaccine can be commissioned.
General supportive management of Monkeypox:
|
Component of management |
Symptoms/Signs |
|
|
Management |
|
Protection of compromised skin and mucous membranes
|
Skin rash |
|
|
• Clean with simple antiseptic • Mupironic Acid/Fucidin • Cover with light dressing if extensive lesion present • Do not touch/ scratch the lesions • In case of secondary infection relevant systematic antibiotics may be considered |
|
Genital ulcers |
|
|
Sitz bath |
|
|
Oral ulcers |
|
|
Warm saline gargles/ oral topical anti-inflammatory gel |
|
|
Conjunctivitis |
|
|
• Usually, self-limiting • Consult Ophthalmologist if symptoms persist or there are pain/ visual disturbances |
|
|
Rehydration therapy and nutritional support |
Dehydration can occur in association with poor appetite, nausea, vomiting and diarrhea |
• Encourage ORS or oral fluids • Intravenous fluids if indicated • Encourage nutritious and adequate diet |
||
|
Symptom alleviation |
Fever |
• Tepid sponging • Paracetamol as required |
||
|
Itching/Pruritus |
• Topical Calamine lotion • Antihistaminic |
|||
|
Nausea and vomiting |
Consider anti-emetics |
|||
|
Headache/ malaise |
Paracetamol and adequate hydration |
|||
_
Complications and Potential Supportive Treatment:
|
Complication |
Supportive Treatment |
|
Respiratory distress/Bronchopneumonia |
Oral/intravenous antibiotics for prophylaxis, nebulizer treatments, non-invasive ventilation (e.g., CPAP) |
|
Sepsis |
Oral/intravenous antibiotics, supplemental oxygen, corticosteroids, |
|
Gastrointestinal/vomiting & diarrhea |
Oral/intravenous antiemetic and antidiarrheal medications, oral/intravenous rehydration |
|
Superinfection skin |
Oral/intravenous antibiotics, incision, and drainage, advanced wound management e.g., negative pressure wound therapy |
|
Lymphadenopathy |
Oral/intravenous anti-inflammatory/analgesic medications |
|
Corneal infection |
Ophthalmic antibiotics/antivirals and corticosteroids |
|
Skin scarring/Cellulitis/Skin lesions |
Application of moist occlusive dressings to promote re-epithelization |
______
______
Doctors may struggle with Incomplete Treatment Guidance, Study Suggests:
A lack of high quality and comprehensive information on how to treat and care for monkeypox may be hindering clinicians’ ability to respond to the current global outbreak, according to a new study published in British Medical Journal Global Health, which found existing guidelines are contradictory and lack key information.
Key points:
-In a search of six major databases, researchers identified 14 relevant clinical management guidelines—a tool used by front-line physicians to respond to outbreaks—on monkeypox treatment and care, but most were of low quality, according to a framework used to evaluate health guidance.
-Most guidelines were missing critical information for several groups at risk of becoming infected: Only five gave advice for children and only three offered guidance for pregnant women or people living with HIV.
-Information on monkeypox treatments were particularly inconsistent, researchers found, with none offering details on best dosage, timing and length of treatment.
-This lack of robust guidance may create “uncertainty” for clinicians treating monkeypox, especially those with no previous experience, and may negatively impact patient care, researchers wrote in the study.
______
______
Promising therapeutics:
Several compounds have shown promise as antiviral therapeutics against Orthopoxvirus species; 3 of the most promising compounds are summarized in Table below. Cidofovir has antiviral activity against a variety of viruses by inhibiting viral DNA polymerase. CMX-001 is a modified cidofovir compound that lacks the extent of nephrotoxicity seen with cidofovir. Antiviral activity of CMX-001 has been demonstrated with a variety of Orthopoxvirus species. The drug ST-246 blocks the release of the intracellular virus from the cell, and has shown promising results against a variety of Orthopoxvirus species, including variola virus. These compounds have been used in varying combinations, also with vaccinia immune globulin, investigationally, to treat severe vaccine-associated adverse events. Development of strategies to use these drugs in endemic areas to treat disease will need to be considered.
Promising Therapeutics for the Treatment of Orthopoxvirus Infections:
|
Antiviral Therapeutic |
Mechanism of Action |
Clinical Considerations |
Stage of Development or Use |
|
Cidofovir |
Inhibits DNA polymerase |
Intravenous administration with hydration and probenecid; nephrotoxicity has been seen |
Licensed for the use of cytomegalovirus retinitis in AIDS patients. Has been used to treat other poxvirus infections (molluscum contagiosum and orf virus). |
|
CMX-001 |
Modified cidofovir compound; inhibits DNA polymerase |
Lacks nephrotoxicity seen with cidofovir; oral administration |
In development. |
|
ST-246 |
Inhibits release of intracellular virus |
Oral administration |
Is maintained in the United States in the Strategic National Stockpile. Available for other Orthopoxvirus infections under an investigational protocol. |
_____
Medical Treatment of Monkeypox:
Currently there is no treatment approved specifically for monkeypox virus infections. However, antivirals developed for use in patients with smallpox may prove beneficial against monkeypox.
-1. Tecovirimat (also known as TPOXX, ST-246)
Tecovirimat is an antiviral medication that is approved by the United States Food and Drug Administration (FDA) for the treatment of smallpox in adults and children. Data are not available on the effectiveness of tecovirimat in treating monkeypox infections in people, but studies using a variety of animal species have shown that tecovirimat is effective in treating disease caused by orthopoxviruses. Clinical trials in people showed the drug was safe and had only minor side effects. CDC holds an expanded access protocol (sometimes called “compassionate use”) that allows for the use of stockpiled tecovirimat to treat monkeypox during an outbreak. Tecovirimat is available as a pill or an injection. For children who weigh less than 28.6 pounds, the capsule can be opened, and medicine mixed with semi-solid food.
_
The antiviral works by preventing orthopoxviruses, such as smallpox, from creating a certain protein. Once this type of virus hijacks a host cell and replicates, the newly formed viruses use this protein to escape from the infected cell and spread to other cells. By blocking the virus from making the protein, tecovirimat “essentially traps the virus inside infected cells”, leaving the immune system’s antibodies and T cells to take care of the situation.
Is there any evidence that tecovirimat works against monkeypox?
A 2018 trial in about 450 people, 90 of whom received a placebo, showed that the drug is safe and has few side effects. But demonstrating its efficacy against orthopoxviruses in humans has been less explored.
Of the 369 patients in 2022 study who were administered Tpoxx orally, adverse events were reported in 3.5% of patient, with only one adverse event classified as serious. Roughly half of those administered the drug had HIV, and the median interval from initiation of tecovirimat to subjective improvement was 3 days and did not differ by HIV infection status, the authors said. Tecovirimat was taken for a prescribed 14-day regimen.
_
Tecovirimat is an antiviral drug that was approved for the treatment of smallpox disease under a regulation known as the “Animal Rule.” This pathway allows for approval of drugs for serious or life-threatening conditions when it is not ethical to conduct efficacy studies in humans and not feasible to conduct field trials to study the effectiveness of a drug or biologic product. Under the Animal Rule, efficacy is established on the basis of adequate and well-controlled studies in animal models of the human disease or condition of interest; safety must be adequately evaluated in people.
Because smallpox, monkeypox and cowpox are either eradicated (smallpox) or occur sporadically in the West, studies to assess the effectiveness of Tecovirimat in infected people could not be carried out. The effectiveness of Tecovirimat was therefore evaluated based on studies in animals infected with lethal doses of orthopoxviruses, on studies on the medicine’s effects in the human body, and on the way the medicine is absorbed, modified and removed from the body in humans and animals (pharmacodynamics and pharmacokinetics studies).
Studies in animals who had received lethal doses of either monkeypox or rabbitpox viruses showed that treatment with Tecovirimat for 14 days significantly increased survival rates: when treatment started either 4 or 5 days after infection, between 80 and 100% of the animals that were treated with Tecovirimat survived. No animals in the placebo groups survived. The survival rate was 50% when treatment started 6 days after the infection.
The dose that is needed in humans to ensure that Tecovirimat will work as expected was determined based on comparative pharmacokinetics and pharmacodynamics studies carried out in animals and in humans.
_
Dose of tecovirimat:
Oral:
- 40 to <120 kg: 600 mg PO q12hr
- ≥120 kg: 600 mg PO q8hr
- Treatment duration: 14 days, but may be longer (not to exceed 90 days) or shorter depending on disease progression and patient’s clinical condition
IV:
- 35 to <120 kg: 200 mg IV q12 hr
- ≥120 kg: 300 mg IV q12 hr
- Switch to oral capsules to complete treatment course as soon as oral therapy tolerated
Dosage Modifications:
Renal impairment
- Oral
Mild, moderate, severe, or patients with ESRD requiring hemodialysis: No dosage adjustment required
- IV
Mild-to-moderate (CrCl 30-89 mL/min): No dosage adjustment necessary
Severe (CrCl <30 mL/min): Contraindicated
Hepatic impairment
- Mild, moderate, or severe (Child-Pugh class A, B, or C): No dosage adjustment required.
_
Compassionate Use of Tecovirimat for the Treatment of Monkeypox Infection, JAMA. Published August 22, 2022:
Monkeypox is a zoonotic orthopoxvirus in the same genus as variola (the causative agent of smallpox). A recent global outbreak has led to more than more than 39 000 cases reported as of August 18, 2022. Monkeypox is typically self-limited with symptoms generally lasting between 2 and 4 weeks in prior outbreaks. Hospitalization was required in 13% of patients in a recent study, suggesting the need for effective therapy.
Tecovirimat is an antiviral that inhibits p37, a protein involved in release of enveloped virus, dissemination, and viral virulence. In vitro testing has shown activity against both smallpox and monkeypox, and tecovirimat appears to have a favorable clinical safety profile based on the experience of healthy volunteers. Authors assessed adverse events and clinical resolution of systemic symptoms and lesions in an uncontrolled cohort study of patients with monkeypox who were treated with tecovirimat on a compassionate use basis.
As of August 13, 2022, 25 patients with confirmed monkeypox infection had completed a course of tecovirimat therapy. All patients were self-reported male and the median age was 40.7 years (range, 26-76). Nine patients had HIV, 1 patient had received the smallpox vaccine more than 25 years prior, and 4 received 1 dose of JYNNEOS vaccination after symptom onset. At the time of treatment, systemic symptoms, lesions, or both were present for a mean of 12 days (range, 6-24). Systemic symptoms included fever in 19 patients (76%), headache in 8 (32%), fatigue in 7 (28%), sore throat in 5 (20%), chills in 5 (20%), backache in 3 (12%), myalgia in 2 (8%), nausea in 1 (4%), and diarrhea in 1 (4%). Almost all patients (23 [92%]) had genital and/or perianal lesions, and 13 (52%) had fewer than 10 lesions over their entire body. All patients had pain associated with lesions.
One patient received 21 days of therapy while the remainder were treated for 14 days. Complete resolution of lesions was reported in 10 patients (40%) on day 7 of therapy, while 23 (92%) had resolution of lesions and pain by day 21. Treatment with tecovirimat was generally well tolerated with no patient discontinuing therapy. The most frequently reported adverse events on day 7 of therapy included the following: fatigue in 7 patients (28%), headache in 5 (20%), nausea in 4 (16%), itching in 2 (8%), and diarrhea in 2 (8%).
In this preliminary study, oral tecovirimat was well tolerated by all patients with monkeypox infection, with minimal adverse effects. However, adverse effects could not always be differentiated from symptoms related to the infection. No control group was included, limiting conclusions of antiviral efficacy pertaining to duration of symptoms or severity. Time from symptom onset to presentation was variable among patients, and conclusions related to antiviral use vs natural evolution of disease should be made with caution.
Limitations of the study include the small number of patients, lack of a control group, and selection bias. Additional large-scale studies are needed to elucidate antiviral efficacy, dosing, and adverse events.
____
-2. Vaccinia Immune Globulin Intravenous (VIGIV):
VIGIV is licensed by FDA for the treatment of complications due to vaccinia vaccination including eczema vaccinatum, progressive vaccinia, severe generalized vaccinia, vaccinia infections in individuals who have skin conditions, and aberrant infections induced by vaccinia virus (except in cases of isolated keratitis). CDC holds an expanded access protocol that allows the use of VIGIV for the treatment of orthopoxviruses (including monkeypox) in an outbreak.
Data are not available on the effectiveness of VIG in treatment of monkeypox virus infection. Use of VIG has no proven benefit in the treatment of monkeypox and it is unknown whether a person with severe monkeypox infection will benefit from treatment with VIG. However, healthcare providers may consider its use in severe cases.
VIG can be considered for prophylactic use in an exposed person with severe immunodeficiency in T-cell function for which smallpox vaccination following exposure to monkeypox virus is contraindicated.
___
-3. Cidofovir (also known as Vistide)
Cidofovir is an antiviral medication that is approved by the FDA for the treatment of cytomegalovirus (CMV) retinitis in patients with Acquired Immunodeficiency Syndrome (AIDS). Data is not available on the effectiveness of Cidofovir in treating human cases of monkeypox. However, it has shown to be effective against orthopoxviruses in in vitro and animal studies. CDC holds an expanded access protocol that allows for the use of stockpiled Cidofovir for the treatment of orthopoxviruses (including monkeypox) in an outbreak. It is unknown whether or not a person with severe monkeypox infection will benefit from treatment with Cidofovir, although its use may be considered in such instances. Brincidofovir may have an improved safety profile over Cidofovir. Serious renal toxicity or other adverse events have not been observed during treatment of cytomegalovirus infections with Brincidofovir as compared to treatment using Cidofovir.
____
-4. Brincidofovir (also known as CMX001 or Tembexa)
Brincidofovir is a prodrug of cidofovir diphosphate. Cidofovir diphosphate selectively inhibits orthopoxvirus DNA polymerase-mediated viral DNA synthesis. Brincidofovir is an antiviral medication that was approved by the FDA on June 4, 2021 for the treatment of human smallpox disease in adult and pediatric patients, including neonates. Data is not available on the effectiveness of Brincidofovir in treating cases of monkeypox in people. However, it has shown to be effective against orthopoxviruses in in vitro and animal studies. CDC is currently developing an expanded access investigational new drug (EA-IND), also called compassionate use, to help facilitate use of Brincidofovir as a treatment for monkeypox.
_____
_____
Recommendations for the management of monkeypox in MSMs:
A well-developed infectious disease surveillance system facilitates early detection of diseases and contact tracing. Some countries, such as United Kingdom and the Republic of Korea, have listed monkeypox as a statutory notifiable disease. Healthcare workers should be highly vigilant about monkeypox transmission in MSM. It is necessary to consider the diagnosis of monkeypox in MSM patients with a typical rash and risky sexual behaviour, especially in those with a history of sexual contact at the site of the disease outbreak. Patients having a sexual history in MSM should be actively screened for HIV and other STD infections. For those with HIV co-infection, antiretroviral therapy and viral load monitoring should be provided as an urgent public health priority. Finally, although current monkeypox outbreaks are mainly in the MSM populations, it is crucial to avoid stigmatisation. Effective communication and community engagement are paramount to ending the monkeypox outbreak.
_
Health threat of monkeypox in MSMs:
Monkeypox is ordinarily a self-limiting disease. Clinical outcomes are related to the degree of viral exposure, patient’s health status, and the nature of complications. Immunodeficiency, such as advanced or uncontrolled HIV infection may lead to more severe clinical manifestations. Since HIV patients usually have multiple comorbidities, such as hepatitis, tuberculosis, and/or other STDs, this can cause additional complications and complicate treatment. In addition, due to similarities in the presentations of the monkeypox rash and some STDs, misdiagnosis may be more common in the MSM population than in the general population. It was reported that some cases were misdiagnosed as herpes simplex virus (HSV) or varicella-zoster virus (VZV) infection in this 2022 outbreak, resulting in late detection and management, thus increasing the risk of community transmission. Furthermore, the high social interactivity in MSM contributes to a high risk of monkeypox transmission in this population.
_
Persons with HIV Infection:
The 2022 outbreak has disproportionately affected gay, bisexual, and other men who have sex with men. During the global monkeypox outbreak that started in May 2022, 30 to 50 percent of patients have had concomitant HIV. About 40% of people monkeypox also had HIV, according to a CDC study that included almost 2,000 monkeypox cases in the U.S. that occurred during the first two months of the outbreak. It is not known if HIV infection increases a person’s risk of acquiring monkeypox disease after exposure. However, people with advanced HIV or those who are not virologically suppressed with antiretroviral therapy (ART) may be at increased risk of severe disease related to monkeypox virus infection.
For persons with HIV who have monkeypox, anti-monkeypox virus therapy should be considered for those who are felt to be immunocompromised and at risk for severe disease (e.g., CD4 count <500 cells/microL). In addition, ART should be continued. For persons with newly diagnosed HIV and those who are not taking ART, ART should be started as soon as possible. Potential drug interactions with tecovirimat and ART can be found on the CDC website; these guidelines discuss considerations for increasing the dose of doravirine, rilpivirine, and maraviroc during the two weeks of treatment with tecovirimat and for two weeks after the end of therapy.
Post-exposure prophylaxis with the MVA vaccine should be administered to those who warrant prophylaxis. Patients with CD4 counts >350 cells/microL had antibody responses after MVA vaccination similar to non-HIV-infected patients. In another study that included HIV-infected patients with CD4 counts between 200 and 350 cells/microL, antibody responses were present, but lower than non-HIV-infected patients.
The ACAM2000 vaccine is contraindicated in immunocompromised persons since it is a live, replication-competent vaccine. However, if only ACAM2000 is available for post-exposure prophylaxis, it can be considered on a case-by-case basis for those with a CD4 count of >500 copies/microL and no other contraindications.
______
______
Section-15
Monkeypox complications and mortality:
Most cases are usually mild and self-limiting, and most patients will recover within 2 to 4 weeks without treatment.
- More than 90% of survivors have no complications, regardless of smallpox vaccination status. In survivors who do develop long-term complications, the most common sequelae are disfiguring scarring of the skin (including pitted scars) and blindness.
- The acute infectious illness results in immunity following recovery. Relapse of disease is rare, but is possible. One UK patient experienced a mild relapse 6 weeks after hospital discharge in 2019. The relapse was short, and was not associated with detectable viraemia.
_
Complications of monkeypox:
- Pitted scarring (pockmarks) is the most common long-term sequela for survivors
- Oral lesions can lead to difficulties with swallowing and thus with eating and drinking
- Perianal lesions can lead to severe pain, tenesmus, and bleeding
- Secondary bacterial infections of skin
- Secondary infections of lungs, resulting in bronchopneumonia and respiratory distress
- Vomiting and diarrhea, resulting in severe dehydration
- Encephalitis
- Myocarditis
- Septicemia
- Ocular infections, which can result in corneal scarring and permanent vision loss
_
Hospitalization rate:
During the 2022 global outbreak, few hospitalizations have been reported and most were for the purpose of isolating the patient. Other reasons for hospitalization included provision of adequate pain management and the need to treat secondary infections.
In a recent study, total of 70 persons (13%) were admitted to a hospital. The most common reasons for admission were pain management (21 persons), mostly for severe anorectal pain, and treatment of soft-tissue superinfection. Other reasons included severe pharyngitis limiting oral intake (5 persons), treatment of eye lesions (2), acute kidney injury (2), myocarditis (2), and infection-control purposes (13). There was no difference in the frequency of admission according to HIV status. Three new cases of HIV infection were identified.
Two types of serious complications were reported: one case of epiglottitis and two cases of myocarditis. The epiglottitis occurred in a person with HIV infection who had a CD4 cell count of less than 200 per cubic millimeter; the person was treated with tecovirimat and recovered completely. The myocarditis cases were self-limiting (<7 days) and resolved without antiviral therapy. One occurred in a person with HIV infection who had a CD4 cell count of 780 per cubic millimeter, and one occurred in a person without HIV infection. No deaths were reported.
During the 2003 outbreak in the United States, 9 of the 34 patients had been hospitalized for a variety of reasons, including nausea, vomiting, and dysphagia. The discharge diagnoses of two of the most seriously ill patients were encephalopathy and a retropharyngeal abscess. All of the patients in this case series survived with supportive therapy; no antiviral therapy was administered.
_
Ocular complications associated with acute monkeypox virus infection, DRC 2014:
A considerable number of MPX cases (23.1%) had “conjunctivitis” as a symptom of their illness. The majority of these were young children (<10 yrs.) who also had a higher frequency of other symptoms. These individuals were also more likely to be “bed-ridden”. MPX cases with “conjunctivitis” are at risk for corneal scarring, which can cause blindness. Understanding the underlying cause of “conjunctivitis” in monkeypox patients will be important, as some may be amenable to treatment (e.g., Triflourodine has been used to treat Orthopoxvirus-associated corneal lesions). Improving the availability of ophthalmologic resources in areas endemic for monkeypox may diminish risks for significant visual sequelae among patients.
_
Monkeypox tied to neurologic complications:
New research from the University College London (UCL) shows some monkeypox patients develop significant neurological complications, including encephalitis (brain inflammation), seizures, and confusion. The study was published in eClinicalMedicine recently. The meta-analysis combed 19 cohort studies that included a total of 1,512 monkeypox patients. The investigators found that 2.7% patients experienced seizures (95% confidence interval [CI], 0.7% to 10.2%), confusion 2.4% (95% CI, 1.1% to 5.2%), and encephalitis 2.0% (95% CI, 0.5% to 8.2%). The patients were seen in outbreaks in the United States, Nigeria, Democratic Republic of the Congo, Republic of Congo, and the United Kingdom. The study findings include patient data from previous outbreaks and a mix of virus clades, the authors warned. Senior author Jonathan Rogers, MB BChir, of UCL, said, “We found that severe neurological complications such as encephalitis and seizures, while rare, have been seen in enough monkeypox cases to warrant concern, so our study highlights a need for further investigation.”
______
______
Mortality:
In 1987, researchers from the WHO’s long-defunct smallpox eradication unit and the Democratic Republic of the Congo’s monkeypox surveillance team published a major piece of work that charted the disease course of 282 people who contracted the virus between 1980 and 1985. There were no deaths among the monkeypox patients who had a smallpox vaccination scar. But among the 250 who did not, there were 27 fatal cases. All the deaths occurred in children under the age of 8, and the case fatality rate was more than twice as high among those 4 years of age and younger than among kids 5 to 9. Nineteen of the children who died developed bronchopneumonia and pulmonary distress. One developed septicemia, an infection on the blood stream. One developed encephalitis — inflammation of the brain.
A paper on Nigeria’s 2017-2018 monkeypox, published in the journal The Lancet Infectious Diseases in 2019, reported that among 122 cases there, seven had been fatal. Four of those were people who were living with HIV but in whom the disease was untreated at the time of their monkeypox infection. They died rapidly, the authors reported, though they noted a precise cause of death was not available in these cases. Two other deaths were attributed to secondary bacterial infections of monkeypox lesions, with apparent sepsis — a dangerous condition where the body’s attempt to curb an infection backfires and leads to organ damage. The seventh death was in a one-month-old infant.
A systematic review published in February in PLOS Neglected Tropical Diseases — presciently titled “The changing epidemiology of human monkeypox — A potential threat?” — noted that from the 1970s through the 1990s, 100% of recorded fatal monkeypox cases were in children younger than age 10. But in the first two decades of this century, pediatric deaths declined to 37.5% of monkeypox cases.
One thing that is becoming clear in this multi-country outbreak is that the monkeypox case fatality rate is not as high as was previously estimated, at least for clade II viruses (the former West African clade) and when infections are primarily in adults. Figures ranging from 1% to 3% have historically been cited. But in the current 2022 outbreak, out of more than 70,000 cases that have been detected, there have been roughly 26 deaths reported.
_
Severe or complicated disease and death occurs more commonly in younger children and immunocompromised people.
- Most reported deaths have occurred in younger children and immunocompromised people (e.g., poorly controlled HIV infection).
- In the early years of human infection, 100% of deaths were in children <10 years of age. However, between 2000 and 2019, children <10 years of age accounted for only 37.5% of deaths.
- Patients with fatal disease had higher viral loads of the virus in their blood, maximum skin lesion count, and elevated transaminases.
- Severe complications and sequelae have been found to be more common among unvaccinated patients (74%) compared with vaccinated patients (40%).
_
Case fatality rates vary according to virus clade, geographical location, and availability of medical facilities, and are vulnerable to case ascertainment bias during outbreaks.
- Historically, the case fatality rate (CFR) of the clade I virus has been estimated to be 1% to 10%, while the CFR of the clade II virus has been estimated to be <3%. More recent data for the clade II virus report a CFR of 1.4%.
- The estimated pooled CFR was 8.7% for both clades in one systematic review (10.6% for clade I and 3.6% for clade II).
- The overall CFR was 0% in an outbreak in the US in 2003.
- The overall CFR in the current ongoing 2022 multi-country outbreak is approximately 0.03% as of 18 August 2022 (0.013% in countries that have not reported monkeypox previously, and 2% in Africa).
______
May 2022 outbreak has case fatality rate of roughly 0.03%. Yet, the World Health Organization reports monkeypox death rates around 3-6% in recent years, and rates as high as 11% historically.
So why aren’t we seeing death tolls in the hundreds now?
-1. Mild cases previously under-represented
One factor is huge selection biases in the data on monkeypox prior to 2022. Until recently, monkeypox was rarely seen outside of Central and West Africa where access to care can be very limited. And most monkeypox infections are mild and resolve without treatment, so many people may not seek care unless they are very ill. As such, only the sickest people may be represented in case counts, meanwhile they miss the mild cases that never make it to healthcare.
-2. Less deadly strain currently circulating
The monkeypox virus driving the current global outbreak likely diverged from the strain that caused an ongoing outbreak in Nigeria, according to research published in Nature Medicine. Both belong to clade 3 (aka IIb), a group of strains dominant in West Africa that are considered less deadly than those in Central Africa’s clade 1. Monkeypox outbreaks caused by clade 3 viruses typically have case fatality rates below 1%, while the fatality rates of clade 1 outbreaks may top 10%, according to the authors of the Nature Medicine study.
-3. Different populations affected
Another factor to consider is the different demographics of those infected in the global outbreak versus those in endemic areas. Outside of Africa, monkeypox virus is disproportionately affecting generally young and healthy men who have sex with men from wealthy nations who may be less likely to suffer complications than pregnant women, children, or immunocompromised people, for example, from poorer endemic regions. Age is a key risk factor – children under age 10 accounted for all monkeypox deaths in the 1970s to 1990s, and more than a third of such deaths since then. Access to care is also important as bacterial superinfection is a leading cause of morbidity and death related to monkeypox.
______
______
Section-16
Monkeypox in children and adolescents:
Historically, monkeypox has been documented in children and adolescents living in endemic regions. Once illness occurs, the clinical presentation is expected to be similar to that in adults. Monkeypox can spread through contact with the fluids (e.g., lesion exudates and respiratory secretions) of people or animals with monkeypox or through contact with fomites (e.g., shared clothing, towels, toiletries, and bedding). Monkeypox also can be transmitted to the fetus during pregnancy or to the newborn by close contact during and after birth.
While data about monkeypox in children are limited, there is evidence from patients infected with Clade 1 of Monkeypox virus that the disease is more likely to be severe in children under 8 years of age. Additionally, anyone with immunocompromising conditions or certain skin conditions, such as eczema, is at risk of severe monkeypox disease. The 2022 Multinational Monkeypox Outbreak is caused by the Clade 3 virus, which typically causes less severe disease than Clade 1. Data on potential complications of infections with Clade 3 in children are lacking. Compared with healthy adults, complications associated with Monkeypox are more frequent in children and people who are immunocompromised, as noted an article in the medical journal The Lancet.
_
Monkeypox has been reported in 17 children and adolescents up to 15 years old by the CDC, as of Aug. 21, 2022 in current outbreak but their risk of monkeypox is described by the CDC as low at this time. Schools are being advised to keep up with “everyday operational guidance that reduces the transmission of infectious diseases,” including disinfecting. The risk of children getting infected with monkeypox virus is low. Children and adolescents are more likely to be exposed to monkeypox if they live in or have recently traveled to a community with higher rates of infection. Infants, young children (under 8 years of age), children with eczema and other skin conditions and children with immunocompromising conditions may be at increased risk of severe disease when they contract monkeypox.
Rash is the most common monkeypox symptom, and it can look similar to rashes seen more commonly in children, including rashes caused by chickenpox, herpes, allergic skin rashes and hand, foot, and mouth disease. The rash typically begins as maculopapular lesions and then progresses to vesicles, pustules and scabs. Other common symptoms include fever, lymphadenopathy, fatigue and headache, although these symptoms are not always present. Data on children with monkeypox are limited. Although there is evidence that the disease is more likely to be severe in children younger than eight years infected with the Congo Basin clade of monkeypox virus, there is less experience with the West African clade.
_
Monkeypox remains contagious until the rash is fully resolved (scabs fall off and new skin has formed), which can take up to 2-4 weeks. While contagious, the following precautions should be taken:
- Children with monkeypox should cover their skin lesions.
- Parents/caregivers should encourage children not to scratch skin lesions or touch their eyes.
- Children with monkeypox should avoid contact with other people and pets. If possible, one person should be the caregiver of a child with monkeypox and should avoid skin-to-skin contact with the rash.
- Children who are at least 2 years of age who have monkeypox should wear a well-fitting mask when interacting with a caregiver, and the caregiver should wear a respirator or well-fitting mask and gloves when skin contact with the child may occur, and when handling bandages or clothing.
- Children should not return to school or childcare while contagious. The decision to end isolation and return to school or childcare should be made in collaboration with local or state public health authorities.
_
While most cases of monkeypox resolve without treatment, treatment should be considered for the following groups:
- Children and adolescents with severe disease (e.g., hemorrhagic disease, confluent lesions, encephalitis, airway obstruction due to lymphadenopathy, or other conditions requiring hospitalization)
- Children and adolescents with complications from monkeypox (g., pneumonia, sepsis, ocular lesions, cellulitis, or abscess)
- Children and adolescents at risk of severe disease including:
-Children under 8 years of age
-Children and adolescents with immunocompromising conditions
-Children and adolescents with a history or presence of atopic dermatitis, or with other active exfoliative skin conditions (e.g., eczema, burns, impetigo, varicella zoster, herpes simplex, severe acne, severe diaper dermatitis with extensive areas of denuded skin, psoriasis, or Darier disease [keratosis follicularis])
-Children and adolescents with aberrant infections, such as those involving the eyes, face, or genitals
_
Treatment:
Pending additional information, treatment with tecovirimat may be warranted in certain children with severe disease (airway obstruction, confluent lesions, encephalitis), those with complications (cellulitis/abscess, ocular lesions, pneumonia, or sepsis), and those felt to be at increased risk for severe disease (e.g., children <8 years of age, children with eczema and other skin conditions, immunocompromised children, children with infections involving the eyes, face, or genitals).
Prevention:
Post-exposure vaccination with the MVA vaccine should be considered for those >6 months of age after a high-risk exposure. Although the MVA vaccine is not approved for those younger than 18, there are no known contraindications.
If the MVA vaccine is not available, the decision to use the replication-competent smallpox vaccine (ACAM2000) in healthy children older than 12 months must be individualized as it is associated with more severe adverse reactions and complications.
For infants <6 months, post-exposure vaccination may not be effective, and other prophylactic measures (e.g., VIG, antiviral therapy) can be considered on a case-by-case basis.
______
______
Section-17
Monkeypox in pregnancy:
It is unknown if pregnant people are more susceptible to monkeypox virus acquisition or if the disease is more severe during pregnancy. However, an increased risk of maternal mortality and morbidity has been documented with other poxvirus infections. Monkeypox virus can be transmitted to the fetus during pregnancy or to the newborn by close contact during and after birth.
_
Limited information on monkeypox in pregnancy:
Data on monkeypox infection in pregnancy are restricted to very few case reports and small case series in endemic regions. A non-laboratory-confirmed case of monkeypox in a pregnant woman (~24 weeks’ gestation) in the Democratic Republic of Congo (DRC), was associated with premature birth, with the infant exhibiting a skin rash consistent with monkeypox and dying of malnutrition 6 weeks later. An observational study of 222 symptomatic infected hospitalized individuals, also in the DRC, included 4 pregnant women, 3 of whom experienced fetal demise. Two had first trimester miscarriages while the third patient had moderately severe disease at 18 weeks’ gestation and suffered an intrauterine fetal demise. The fourth woman delivered a healthy baby at term. There was no testing of pregnancy tissue for the miscarriages. In the case of the intrauterine fetal death, virological, histological, and serological evidence were consistent with vertical transmission, with the fetus demonstrating features of monkeypox infection, including the characteristic rash.
_
How did smallpox compare?
The overall maternal CFR from smallpox infection in pregnancy was 34.3%, and the crude proportion of miscarriage and preterm birth was 39.9%. In the largest series of 389 pregnant women with smallpox, 75% miscarried before 24 weeks’ gestation, 55% delivered preterm, and 10% suffered stillbirths at term. Congenital smallpox occurred in 9% of fetuses and resulted in a neonatal mortality rate of 100%. Maternal mortality from smallpox was the highest in the third trimester of pregnancy; expectant mothers were 2 – 4 times more likely than nonpregnant women to die from the infection and vaccinated pregnant women were about three times less likely to succumb than those who were unvaccinated. Hemorrhagic smallpox – characterized by petechiae, ecchymoses, profound thrombocytopenia and multi-organ failure – occurred seven times more frequently during pregnancy than in men and non-pregnant women regardless of vaccination status and carried a CFR of 100%. It is likely that smallpox represented the extreme end of the disease severity spectrum from orthopox infection during pregnancy.
Importantly, however, monkeypox and smallpox differ in the regions encoding virulence factors (e.g., IFN resistance genes and interleukin-1 inhibitors) at the terminal ends of the viral genome which might explain the variation in clinical presentation and disease severity between the two infections. Additionally, no hemorrhagic form of monkeypox has been described in humans, although MPXV clade 1 has demonstrated the potential for pulmonary hemorrhage, epistaxis, and impaired coagulation parameters in animal studies.
_
The monkeypox case count in the current global outbreak surpassed 52,000 on Sept 1, 2022. Community transmission is affecting people considered to be at high risk of severe disease, including pregnant women and neonates, albeit in small numbers so far. As of Sept 2, 2022, ten cases of monkeypox in pregnant women have been reported worldwide, mostly via local news media rather than medical or public health publications, with the first case reported in the USA on July 23, 2022. Based on available information, vertical transmission did not occur; the neonate received prophylactic vaccinia immunoglobulin and did not develop monkeypox disease.
On Aug 4, 2022, the Government of São Paulo, Brazil, announced that two pregnant women had been diagnosed with monkeypox and were being monitored by health-care professionals. By Aug 26, the Brazilian health authorities had reported a total of nine cases in pregnancy (four in São Paulo, three in Rio de Janeiro, one in Minas Gerais, and one in Ceará). Eight had monkeypox PCR-confirmed by Sept 1, whereas the woman in Ceará tested negative. On Aug 5, a local newspaper in São Paulo reported that one of the infected pregnant women had passed the transmission phase, with both mother and baby in a stable condition, but there was no information on vertical transmission. In Minas Gerais, the 26-year-old pregnant woman with monkeypox presented to hospital with skin lesions on Aug 4 and gave birth to a healthy infant on Aug 14. She was isolated from her baby after birth and discharged healthy on Aug 17. There was no vertical transmission; the neonate was asymptomatic but remained in hospital when the mother was discharged.
Reassuringly, it appears that, so far, none of the monkeypox infections reported in pregnant women have been severe, and there has been no evidence that pregnant women have more severe disease or worse outcomes than non-pregnant people. There is, however, an urgent need for an international registry or reporting system to better understand the course, management, treatment, and outcomes of monkeypox, as well as the safety and effectiveness of vaccination, in populations at high risk, including the mother–fetus dyad, so that patients worldwide can be provided with accurate advice and evidence-based care. Unfortunately, we are currently having to rely on news outlets providing sparse information that is not externally verifiable.
_
Reasons for and underlying mechanisms of maternal-fetal susceptibility to MPX virus:
Pregnant women are highly prone to vertical MPX virus (MPXV) transmission due to immunological vulnerability, waning of anti-smallpox immunity among women of reproductive age (15 to 49 years), and since orthopoxviruses can overcome the syncytiotrophoblast placental barrier.
A gestational bias towards a helper T cell type 2 (Th2)-dominant environment (from a Th1-dominant environment) enhances maternal vulnerability to viral diseases. Th1 cytokines such as type 1 interferon (IFN) inhibit viral replication through direct antiviral and indirect immunoregulatory mechanisms. MPXV expresses IFNa/b-binding proteins (IFNa/bBP) that evade IFN-induced antiviral host immune responses.
Further, smallpox eradication and cessation of smallpox vaccinations created a niche for monkeypox (genetically similar to smallpox) due to waning natural immunity against smallpox. Reproductive age unimmunized women are prone to MPX due to lacking cross-protective antiviral immunity. Cross-border MPX transmission in populations lacking prior humoral immunity and in immunosuppressed persons could allow MPXV to evolve with mutations increasing MPXV virulence genetically.
Several mechanisms may be involved in the vertical transmission of MPXV since MPXV does not express cell-specific receptors that facilitate cellular tropism. MPXV may reach the fetus via the hematogenous spread arriving at the intervillous space from maternal uterine spiral arteries and binding to trophoblast cells, subsequently infecting cytotrophoblasts syncytiotrophoblasts, and cells of the fetal endothelium within the anchored or floating villi to eventually invade blood cells of the fetus.
MPXV could also directly ascend from the genital lesions via uterine and cervical tissue and colonize the decidua and chorionic membranes or could breach the placental barrier by fusing with trophoblasts facilitating internalization of viral DNA and viral replication in the host.
_______
Diagnosis and management of monkeypox infection in pregnancy is summarized in figure below:
Pregnant women may present with an unexplained centrifugal rash (head, hands and feet) or a rash on any part of the body, and report one or more classical symptom(s) of monkeypox infection, including intense headache, lymphadenopathy, arthralgia and/or backache and fever (>38.5 C). They may have an epidemiological link to a confirmed or probable case of monkeypox, or travel history to West or Central Africa in the 21 days before symptom onset. Staff wearing personal protective equipment (PPE) should take a sample for polymerase chain reaction (PCR) (throat, pustules, scabs or urine) for monkeypox virus. The patient should be masked, lesions covered, and isolated; monkeypox treatment options should be discussed, and the consultant virologist contacted. The obstetrician should assess the fetus (ultrasound/fetal heart rate monitoring) and mother and consider cesarean section (CS) if delivery is indicated or the woman is in labour. Staff examining patients should wear PPE at all times; contact with vulnerable staff (pregnant/ immunosuppressed) should be minimized. If patients are discharged, they should wear a mask and cover all body lesions, self-isolate, refrain from intimate contact, and their contacts should be followed up. After delivery, breastfeeding should be considered when benefits may outweigh the potentially increased risk of neonatal monkeypox infection (for example in low- and middle-income countries, breastfeeding may carry greater benefit to the baby than the potential risk of neonatal monkeypox infection). VIG: vaccinia immune globulin.
_______
Treatment during pregnancy and lactation:
Pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment if needed. Most infected people will have a mild, self-limiting illness. However, CDC guidance indicates that patients who should be considered for treatment following infection include:
-1. People with severe disease and one or more complications requiring hospitalization (ie, hemorrhagic disease, encephalitis, secondary bacterial skin infection, poor oral intake, severe pain)
-2. People at high risk of severe disease, including pregnant or breastfeeding people, people with immunocompromise, people with a history of active exfoliative skin conditions and pediatric patients, particularly those younger than age 8.
There are no specific treatments for monkeypox virus infection. Two antivirals and vaccinia immune globulin are available from the Strategic National Stockpile under expanded access investigational new drug protocols held by the Centers for Disease Control and Prevention. The risks and benefits of treatment should be discussed with the patient using shared decision making.
Most people with infection have a mild, self-limiting illness. Severe disease includes hemorrhagic disease, sepsis, encephalitis, or other conditions requiring hospitalization.
Persons with monkeypox infection should also be counseled to isolate for the duration of the illness. The decision to treat and monitor a pregnant person as an outpatient or inpatient should be individualized. As CDC recommends, if treatment is indicated, tecovirimat should be considered the first-line antiviral drug for pregnant, recently pregnant, and breastfeeding people (see Table below).
_
Drugs Used for Treatment of Monkeypox:
|
Drug Availability Administration
|
Pregnancy data |
Breastfeeding data |
||
|
Tecovirimat (TPOXX, ST-246) |
Limited to health department/CDC expanded access protocol |
Weight-based
Intravenous and oral
|
Pregnant patients not included in pharmacokinetic studies
Adverse events not observed in animal reproduction studies |
Breastfeeding patients not included in pharmacokinetic studies
Not known if present in breastmilk
|
|
Cidofovir (Vistide) |
Off-label
Available for use in an outbreak setting |
Intravenous
Contraindicated in patients with CrCl ≤55mL/min, serum Cr > 1.5 mg/dL; use with or within 7 days of nephrotoxic agents |
No human data
Animal data suggest embryolethality and teratogenicity |
No human data
Breastfeeding not recommended during period of exposed lesions
|
|
Brincidofovir (CMX001, Tembexa)
|
Availability limited to Strategic National Stockpile distribution |
Oral
Weight-based
Dose adjustment for hepatic impairment
No contraindications in manufacturer labeling |
Animal data suggest embryolethality and teratogenicity |
No human data
Breastfeeding not recommended during period of exposed lesions |
|
Vaccinia intravenous immune globulin (VIGIV) |
Limited to health department/CDC expanded access protocol |
Intravenous |
No human or animal data
Immune globulins known to cross the placenta without severe adverse effects |
No human or animal data
Immune globulins known to cross the placenta without severe adverse effects |
CDC, Centers for Disease Control and Prevention; Cr, creatinine; CrCl, creatine clearance
_
Vaccines against monkeypox and administration during pregnancy:
Two vaccines directed against monkeypox are currently available. ACAM2000 is a replicating viral vaccine licensed for the prevention of smallpox. It is contraindicated in pregnant or breastfeeding people due to the risk of pregnancy loss, congenital defects, and vaccinia virus infection.
JYNNEOS, the Modified Vaccinia Ankara (MVA) vaccine is a live, nonreplicating viral vaccine licensed for the prevention of both smallpox and monkeypox disease. Post-vaccination follow-up of fewer than 300 pregnant women did not identify an increase in adverse outcomes, but MVA vaccine is not yet approved for use against monkeypox in pregnancy. This vaccine, however, contains non-replicating virus and animal studies have not identified any adverse fetal effects, but the general advice remains that MVA-BN vaccine should be used during pregnancy only if the benefits, in terms of protection of both mother and baby, of preventing monkeypox likely outweigh any potential unknown risks associated with the vaccine. On the other hand, MVA-BN is considered safe during breastfeeding and should be offered to breastfeeding women with significant exposure to the virus after discussing the risks of monkeypox to them and their infants. The JYNNEOS vaccine requires two doses administered 28 days apart for maximum effectiveness.
_
Antenatal fetal surveillance for patients with suspected or confirmed monkeypox virus:
Currently, the data are insufficient to guide recommendations for antepartum fetal surveillance. It is unknown when or how often vertical transmission occurs during pregnancy, nor how infection during pregnancy contributes to stillbirth risk. Fetal surveillance during acute illness should be guided by maternal disease severity, gestational age, and comorbid conditions. Clinicians can consider a follow-up growth ultrasound once the illness has resolved and following completion of the isolation period.
_
Delivery in patients with monkeypox:
The relationship between the timing of infection in pregnancy, risk for congenital infection, and transplacental vs. intrapartum transmission is unknown. It is unclear whether preterm or early term delivery protects against adverse neonatal outcomes, nor is it clear whether cesarean delivery mediates the risk of perinatal infection. There has been no clear evidence of transmission through vaginal fluids; however, these data are limited. Further, nothing is known regarding genital tract shedding.
Currently, in the absence of obstetric indications, preterm or early term delivery is not recommended. Decisions regarding the mode of delivery should be individualized. Cesarean delivery can be considered if lesions are present and cannot be covered in or near the vaginal, anal, or perineal regions to reduce the risk of neonatal contact during delivery. However, the guidance will continue to evolve as pregnancy-specific data emerge.
_
Mother-Infant Contact:
While the benefits of skin-to-skin contact and rooming-in on breastfeeding are well-known, given the risk of neonatal transmission of monkeypox virus with close contact and the potential for severe disease in newborns, direct contact between a patient in isolation for monkeypox and their newborn is not advised.
Infant Feeding with Breast Milk:
Breast milk is the best source of nutrition for most newborns, and it provides protection against many illnesses. However, given that the monkeypox virus is spread by close contact and neonatal monkeypox infection may be severe, breastfeeding should be delayed until criteria for discontinuing isolation have been met (i.e., all lesions have resolved, the scabs have fallen off, and a fresh layer of intact skin has formed). Breast pumping will allow patients to maintain supply during a breastfeeding pause. However, until there are data evaluating the risk of transmission through breastmilk, current recommendations are to discard breastmilk pumped until the person is considered no longer infectious.
Breastfeeding should be considered when benefits may outweigh the potentially increased risk of neonatal monkeypox infection, for example in low- and middle-income countries, breastfeeding may carry greater benefit to the baby than the potential risk of neonatal monkeypox infection.
_
Infection Control:
Infection control practices for the care of patients who are pregnant with monkeypox infection are the same as those for patients who are not pregnant with monkeypox infection – including appropriate isolation of patients with monkeypox; training for healthcare personnel on maternity and newborn care units on correct adherence to infection control practices and PPE (gown, gloves, eye protection, and NIOSH-approved particulate respirator equipped with an N95 filter or higher) use and handling; and ensuring sufficient and appropriate PPE supplies are positioned at all points of care.
Further, visitors to pregnant or postpartum patients with monkeypox should be limited to those essential to the patient’s care and wellbeing. The use of alternative mechanisms for patient and visitor interactions, such as video-call applications, should be encouraged for any additional support. Visitors should have no direct contact with the patient.
_______
_______
Section-18
Prevention and control of monkeypox:
Raising awareness of risk factors and educating people about the measures they can take to reduce exposure to the virus is the main prevention strategy for monkeypox. Scientific studies are now underway to assess the feasibility and appropriateness of vaccination for the prevention and control of monkeypox. Some countries have, or are developing, policies to offer vaccine to persons who may be at risk such as laboratory personnel, rapid response teams and health workers.
Prevention of transmission of infection by respiratory and contact routes is required. Appropriate respiratory isolation is essential for suspected and confirmed cases. Scabs are also infectious and care must be taken to avoid infection through handling bedding, clothing, and so on.
Monkeypox virus is classified as an ACDP Hazard Group 3 pathogen and all laboratory work with live virus must be conducted at full Containment level 3 (CL3), in accordance with the Control of Substances Hazardous to Health Regulations 2002 (as amended).
Clinical laboratories should be informed in advance of samples submitted from suspected or confirmed diagnosis of monkeypox. Laboratories must ensure that appropriate controls commensurate to CL3 are in place to minimize risk to laboratory workers so that they can safely perform laboratory tests that are essential to clinical care.
______
The risk of monkeypox transmission in community and health care settings can be mitigated through implementation of infection prevention and control measures.
The following recommendations are based on CDC, World Health Organization (WHO) and other institutions:
-1. Home isolation:
Patients with suspected or confirmed infection and with mild or uncomplicated disease who are not at high risk for severe or complicated disease may be isolated at home for the duration of the infectious period, provided that a home assessment determines that infection prevention and control conditions can be met in the home setting. Consider admission to a health facility for patients who are at higher risk of severe disease (e.g., children, pregnant women, immunocompromised people, people with skin conditions) for closer monitoring. Also consider admitting patients who live with vulnerable populations where adequate infection prevention and control precautions cannot be met. Hospital admission may be required for a small proportion of patients with painful or infected skin or mucosal lesions for pain management and/or antibiotic therapy.
-2. Patient placement (room selection):
A patient with suspected or confirmed monkeypox infection should be placed in a single-person (private) room with dedicated toileting facilities. If a patient is in a semi-private room at the time that monkeypox is suspected, the patient with suspected monkeypox should be moved to a private room as soon as possible. Pending that transfer, both patients should be masked, the curtain between the beds should be closed, and both patients should be provided with a commode to use. Patients should not share the bathroom. If the patient is eventually diagnosed with monkeypox, any patients who shared the room should be evaluated to determine the exposure risk.
Special air handling is generally not required. However, an airborne infection isolation room (AIIR; “negative pressure”) should be used for any procedures that are likely to aerosolize oral secretions. An AIIR should also be used pending the initial diagnostic work-up when diseases that require an AIIR, such as varicella (chickenpox), are being considered. If an AIIR is not available in these circumstances, the patient should be placed in a private, standard room, with the door closed when it is safe to do so. Patients should don a facemask when any individual enters the room or when the patient needs to travel outside the room.
-3. Personal protective equipment
Personal protection equipment (PPE) is required for all health care personnel (HCP) interacting with a patient with suspected or confirmed monkeypox or interacting with the patient’s environment (e.g., environmental services HCP).
All HCP should use a gown, gloves, eye protection (goggles or face shield), and a National Institute for Occupational Safety and Health (NIOSH)-approved N95 filtering facepiece or equivalent or higher-level respirator. This combination of PPE reflects contact precautions (gown and gloves), droplet precautions (eye protection), and respiratory protection from airborne precautions. While there is no epidemiologic evidence to date that monkeypox is spread by the airborne route, at this time the CDC recommends respiratory protection be used. Careful attention must be paid to doffing (removal) of PPE in the correct order and in a manner that reduces the risk of self- and cross-contamination.
All HCP determined to have had an exposure to monkeypox should be monitored for symptoms for 21 days from the day of last interaction. The approach to monitoring (active or passive) and the need for post-exposure prophylaxis depend upon the type of exposure, use of PPE, and local regulations.
-4. Patient transport
Patient transport outside the room should be limited to those essential for the care of the patient and for procedures or interventions that cannot be performed in the patient room. If transport is necessary, the patient should wear a medical mask during transport and any exposed skin lesions should be covered with a clean sheet or gown. During transport, there must be an HCP with clean, nongloved hands who is able to open doors and push elevator buttons, as needed. The receiving department should be notified in advance of the required precautions.
-5. Care of the environment
Standard cleaning and disinfection procedures should be performed using an Environmental Protection Agency (EPA)-registered hospital-grade disinfectant with an emerging viral pathogen claim.
When handing soiled laundry (e.g., bedding, towels, personal clothing), contact with lesion material that may be present on the laundry should be avoided. In addition, soiled laundry should be gently and promptly contained in an appropriate laundry bag and should never be shaken or handled in a manner that may disperse infectious material.
Activities such as dry dusting, sweeping, or vacuuming should be avoided. Wet cleaning methods are preferred.
-6. Waste management
If the patient is suspected or confirmed to be infected with the West African clade of monkeypox virus (clade IIb), waste (i.e., bodily fluids such as urine, stool, blood, sharps, used PPE, and other waste generated in the course of care) is managed as routine hospital medical waste. By contrast, waste generated from a patient suspected or confirmed to be infected with the Central African clade (clade I) is considered Category A waste and additional precautions should be taken.
In nonendemic countries, it is reasonable to assume that patients with suspected or confirmed monkeypox during the 2022 outbreak have infection with the West African clade (clade IIb). However, individual facilities should review this approach with their local and state public health officials as local considerations may apply (e.g., if there are epidemiologic data that could implicate infection with the Central African clade [clade I] of monkeypox virus).
-7. Community settings
The majority of patients with monkeypox will have mild disease and can be cared for at home in the community. Such patients should not leave the home except for follow-up medical care. Unexposed persons who do not have an essential need to be in the home should not visit while the individual remains infectious.
Patients with monkeypox virus should be isolated in a room or area separate from other family members and pets. This is particularly important for persons with extensive lesions that cannot be easily covered (excluding facial lesions) and those with respiratory symptoms. If around others, skin lesions should be covered (e.g., long sleeves, long pants) to minimize risk of contact with monkeypox lesions. Individuals with monkeypox should also wear a facemask; ideally, household members should wear a facemask when in the presence of the person with monkeypox as well.
Household members providing care to patients with monkeypox should use disposable gloves for direct contact with lesions. The gloves should be disposed of after use, followed by hand hygiene with an alcohol-based hand rub or if visibly soiled, with soap and water. Disposal of contaminated waste (such as dressings and bandages) should be determined in consultation with state or local health officials.
Hand hygiene should also be performed regularly by infected individuals and by household contacts after any unprotected contact with lesions or potentially contaminated surfaces.
Similar to health care settings, care should be used when handling soiled laundry to avoid direct contact with contaminated material. Soiled laundry should not be shaken or otherwise handled in a manner that may disperse infectious particles. Laundry may be washed in a standard washing machine with water and detergent.
-8. When to discontinue isolation
Persons with monkeypox should be considered infectious until all lesion scabs have fallen off and re-epithelialization has occurred, which typically lasts two to four weeks. Decisions regarding discontinuation of isolation precautions in both the health care facility and the community should be made in consultation with the local or state health department.
-9. Precautions after recovery
Condom use is recommended during any sexual activity for 12 weeks after recovery as culturable virus is found in semen of infected individuals.
_______
_______
Three key groups should be considered as priority groups for community engagement strategies during the current MPX outbreak.
-1. Since the current event has seen several cases in MSM, this group must be made aware of the risk of infection as well as prevention measures they may take. Applications used by MSM for meeting partners can be explored to reach those most at risk and provide health promotion information. Various organisations exist at the regional, national, sub-national and local level working on health for LGBTQIA+, including activist groups and community testing organisations (i.e., checkpoints). These should be contacted, informed and asked to engage with their members, users and networks about the situation and hear their perceptions and concerns. Other organisations working on sexual health may also be mapped and contacted for similar purposes. Key messages should focus on the fact that MPXV is spread through close contact with infectious individuals, may possibly be sexually transmitted and that condoms can mitigate the risk of many sexually-transmitted infections, but cannot offer full protection against transmission of MPXV, since contact with lesions may be sufficient for transmission to occur.
-2. As people who are immunocompromised have been shown to be more vulnerable to severe disease, support organisations for immunocompromised people should also be identified, kept informed, and supported in conducting outreach to their members. Particular focus should be given to their heightened risk of severe disease, and the importance of seeking treatment should they develop symptoms of MPX infection. However, it is important to clarify that people living with HIV under appropriate treatment are not considered immunocompromised, and those with untreated HIV should be referred to HIV treatment.
-3. Healthcare workers’ unions and associated professional networks should be engaged with so they can be equipped to detect and treat cases early as well as provide health advice and disseminate messages about case definitions and strategies for contact tracing. They should also be informed about the particular susceptibility to severe disease of people with untreated HIV and those who are otherwise immunocompromised so that they can provide appropriate treatment and support for such patients. Health workers should also be made aware that their own close contact with patients may put them at increased risk of infection and therefore they should protect themselves accordingly.
______
______
Management of monkeypox contacts:
Close contacts of the currently reported MPX cases include mainly sexual partners and people living in the same household or anyone sharing the same bedding or clothing with an MPX case. Sharing the same workspace for several hours seated within one to two meters or being a co-passenger in longer flights, train or bus rides may also qualify as a close contact in certain situations, but this would require a case-by-case assessment. From outbreaks in Africa, the secondary attack rate is estimated at 9-12% among unvaccinated contacts within households, however other estimates are as high as 50%, while in the 2003 US outbreak it was 0%. Although some of the reported cases are epidemiologically linked, no further onward transmission to close contacts that are not sexual partners has been documented yet in this outbreak.
_
Contact tracing of newly identified MPX cases should be performed carefully and exhaustively, building on longstanding good practices implemented for the management of STIs and the HIV epidemic and the ongoing COVID19 pandemic. Partner notification should be rapidly initiated; however, this might be challenging in the case of anonymous sexual partners. Involvement of sexual health services, who are experienced in partner notification for sexually-transmitted diseases is recommended to ensure the best possible outcome.
Awareness raising in MSM communities about the ongoing MPX outbreak is extremely important and should happen with the engagement of the community. Contact tracing should pay particular attention to identifying MPX contacts who are immunocompromised. Working with associations supporting people living with human immunodeficiency virus (PLWHIV) and immunocompromised patients would be valuable.
_
All identified close contacts (see Table below) of a MPX case should be instructed to self-monitor for fever and MPX symptoms daily for 21 days after their last exposure. Instructions should be provided, if they develop any symptoms during this period, to self-isolate except for attending medical assessments or testing. In general, symptomatic contacts should be isolated during their investigation until MPX is excluded. Close contacts should be advised to avoid close physical contact with young children, pregnant women and immunocompromised persons until MPX is excluded.
_
Sexual contacts of MPX cases should abstain from sex for a duration of at least 21 days or until the infection is excluded. While all persons are encouraged to use condoms consistently during sexual activity for prevention of HIV and other STIs, they should be made aware that the use of condoms alone cannot offer full protection against transmission of MPXV. Because transmission through droplets in prolonged face-to-face contact is also possible, avoidance of close, physical contact is recommended for the duration of 21 days following exposure. Sex workers may be a group potentially exposed to MPX and consideration should be given to the fact that they would need financial support to comply with the recommended duration of self-monitoring.
_
Close contacts of MPX cases would benefit from an exposure assessment, including history of past smallpox vaccination, and should be carefully evaluated for the potential need of post-exposure prophylactic (PEP) smallpox vaccination. Use of PEP smallpox vaccination should only be offered after a careful risk/benefit ratio assessment for the individual person, including the type and timing of last exposure, their age group, their medical history particularly as regards their immune status and other underlying conditions that would indicate that they are at increased risk for severe MPX disease. The time from vaccination until developing the expected antibody protection also plays a role. In addition, the profile, indication and availability of the nationally available smallpox vaccine should be considered (which generation of vaccine is available, number of doses etc.). Use of the smallpox vaccine up to four days after exposure to MPX can prevent the onset of symptoms, while after that and until 14 days postexposure prophylactic vaccination may modify the disease course. If the currently authorised 3rd generation MVA vaccine for smallpox would be used, then two doses would be needed 28 days apart.
For passengers in an aircraft, bus or train sitting within a radius of two meters of a symptomatic case (i.e. seated one-two seats around the case), an exposure assessment by health authorities should be carried out and monitoring implemented accordingly. Exposure on longer flights or rides (more than eight hours) can be considered as riskier. From when smallpox was circulating, no transmission on aircraft was documented.
_
Table below presents the overview of the advice for the management of contacts of a MPX case:
|
Type of contact Description |
Management guidance |
|
|
Close contact |
• Sexual partner • Person(s) living in same household, or similar setting (e.g camping, overnight sleeping etc) • Person(s) sharing clothing, bedding, utensils etc, while the patient had a rash • Person(s) sharing the same closed workspace/office for long periods of time • Caregivers of MPX case, while symptomatic • HCW who had contact with MPX case (lesions or prolonged face-to-face contact) without appropriate PPE • HCW or other person who suffered a sharps injury or was exposed to MPX case body fluids or aerosol generating procedure without PPE • Laboratory staff suffering exposure to occupational accident with virus-containing sample (splash, sharp or aerosol exposure etc) • Co-passenger seated one -two seats distance around case while they were symptomatic, in airplane, bus or train ≥ 8 hours duration |
• Careful benefit/risk assessment for the need for PEP smallpox vaccination • Self-monitor for fever or other MPX symptoms (headache, back ache etc) or new unexplained rash for 21 days from last exposure. In that case self-isolate and abstain from sexual activity until MPX is excluded. • Careful hand hygiene and respiratory etiquette. • Abstain from sexual activity and avoid close physical contact for 21 days or until MPX is excluded. • Avoid contact with mammal pets for 21 days or until MPX is excluded |
|
All other contacts |
• Brief social interactions • Work colleagues not sharing same office • Persons sharing fitness equipment or sharing the same sauna or bath, without sexual contact • Social encounters/ acquaintances • HCW contact with appropriate PPE
|
• Depending on the certainty of contact, some of these contacts may be asked to self-monitor for fever or other MPX symptoms (headache, back ache etc.) or new unexplained rash for 21 days from last exposure. |
_______
_______
Reducing the risk of human-to-human transmission:
Surveillance and rapid identification of new cases is critical for outbreak containment. During human monkeypox outbreaks, close contact with infected persons is the most significant risk factor for monkeypox virus infection. Health workers and household members are at a greater risk of infection. Health workers caring for patients with suspected or confirmed monkeypox virus infection, or handling specimens from them, should implement standard infection control precautions. If possible, persons previously vaccinated against smallpox should be selected to care for the patient.
Samples taken from people and animals with suspected monkeypox virus infection should be handled by trained staff working in suitably equipped laboratories. Patient specimens must be safely prepared for transport with triple packaging in accordance with WHO guidance for transport of infectious substances.
_
Global outbreaks of monkeypox infections in 2022 have indicated that sexual contact is the primary route of monkeypox transmission among men with multiple male sex partners. Sexual contact increases the spread of monkeypox. Concerns concerning sexual behaviour have arisen since the World Health Organization proclaimed monkeypox a worldwide health emergency. This is because the virus can transmit by any type of close contact, including kissing, caressing, oral, penetrative vaginal, or anal intercourse with a person who has the infection. According to the WHO, rashes, body fluids (pus, or blood from skin lesions), and scabs are extremely contagious. Because the virus can be transmitted by saliva, ulcers, lesions, or sores may also be contagious. Contact with items like eating utensils or items like clothing, bedding, or towels that have been in contact with the infected individual could also be a cause of infection.
_
There are several different ways people can prevent monkeypox infection, including avoiding close, skin-to-skin contact with a monkeypox rash; not handling or touching bedding, clothing, or towels of a person who has monkeypox; and washing hands often with soap and water or using an alcohol-based hand sanitizer, especially after contact with people sick with the virus. The best way to protect yourself from monkeypox is to avoid sex and other intimate contact with multiple or anonymous partners. A study conducted by scientists from the US Centers for Disease Control and Prevention (CDC) indicates that reducing one-time sexual partnerships among gay, bisexual, and other men who have sex with men can significantly reduce the transmission of the monkeypox virus. Reducing the number of one-time or casual sex partners can substantially control monkeypox outbreaks in non-endemic countries.
_
Guideline on how to avoid monkeypox transmission during sex:
- Have “virtual sex with no in-person contact”
- Masturbate together “at a distance of at least six feet, without touching each other and without touching any rash or sores”
- Have sex “with your clothes on or covering areas where rash or sores are present, reducing as much skin-to-skin contact as possible”
- Avoid kissing
- Limit sexual partners. Reduce your number of partners, especially those you do not know or whose recent sexual history you do not know.
- Ask your partners if they have monkeypox symptoms or feel sick. If you or your partners are sick, especially if you or they have a new or unexpected rash or sore, do not have sex or close physical contact.
- Avoid sex parties, circuit parties and other spaces where people are having sex and other intimate contact with multiple people.
- Since it may be possible the virus can be transmitted through semen, use latex condoms during sex.
- Do not share towels, clothing, fetish gear, sex toys or toothbrushes.
- Wash your hands, fetish gear and bedding. Sex toys should be washed after each use or sex act.
_
Would using a condom help?
Monkeypox can spread by hugging, kissing or coming in contact with bedding, clothes or objects used by an infected person, so barrier methods like using a condom may not be effective. Close physical non-sexual contact can transmit monkeypox, so use of condom may not prevent transmission. However, condom can prevent transmission of other STDs including HIV.
_
WHO recommends gay and bisexual men limit sexual partners to reduce the spread of monkeypox:
Key points:
- Men who have sex with men are at the highest risk of infection right now from monkeypox, according to the WHO.
- About 99% of cases are among men, and at least 95% of those patients are men who have sex with other men, according to WHO.
- WHO said men who have sex with men should consider limiting their sexual partners to lower their risk of infection and reduce the spread.
- WHO called on media, public health authorities and government to fight stigma and discrimination, which will only fuel the outbreak.
_
ECDC is working with multiple civil society organisations to reach out to MSM groups by disseminating the following message to all European countries:
“Monkeypox virus is spreading in Europe, in particular among men who have sex with men. It is transmitted through close contact, like during sexual intercourse or through contaminated bedding, sex toys. If you or any recent (last 21 days) partner have unusual sores or rash, contact your sexual health provider”.
_____
_____
Reducing the risk of zoonotic transmission:
Over time, most human infections have resulted from a primary, animal-to-human transmission. Unprotected contact with wild animals, especially those that are sick or dead, including their meat, blood and other parts must be avoided. Additionally, all foods containing animal meat or parts must be thoroughly cooked before eating.
Preventing monkeypox through restrictions on animal trade:
Some countries have put in place regulations restricting importation of rodents and non-human primates. Captive animals that are potentially infected with monkeypox should be isolated from other animals and placed into immediate quarantine. Any animals that might have come into contact with an infected animal should be quarantined, handled with standard precautions and observed for monkeypox symptoms for 30 days.
Examining Animals with Suspected Monkeypox:
Veterinarians who decide to treat animals with suspected monkeypox should use infection control precautions to protect themselves, staff, clients, as well as other animal patients in the clinic.
Illness presentation may vary amongst animal species. Symptoms that were observed in prairie dogs during the 2003 U.S. outbreak included cough, history of fever, conjunctivitis, lack of appetite, respiratory signs, and rash. Similar symptoms have also been observed in non-human primates that have been challenged with monkeypox virus in research studies. Some animals may experience a milder form of illness with fewer symptoms.
Animals suspected of being infected should not be allowed to enter through the waiting area of a veterinarian clinic nor should they be taken to a common treatment room. All treatment and diagnostics should be performed in an examination room. The number of staff allowed in the exam room and that come in contact with the animal should be limited to as few persons as possible.
_____
_____
Environmental persistence and disinfection:
Poxviruses show extraordinary resistance to drying, and increased temperature and pH tolerance when compared with other enveloped viruses. These characteristics strongly impact their environmental persistence: materials from infected patients (e.g., dermal crusts) or fomites (e.g., bed linens) remain infective for months to years.
The monkeypox virus can survive on surfaces for 15 days or more, particularly in a dark, cool, and dry environment, according to a study by the Centers for Disease Control and Prevention (CDC). Porous materials, such as bedding and clothing, can harbor the virus for longer periods of time than non-porous surfaces, such as plastic, metal, and glass. The persistence of the virus demonstrates the importance of properly disinfecting any item or surface that may have come into contact with it.
Monkeypox can survive on surfaces for a long time because it has a protective outer layer known as the envelope. Despite having an outer envelope, the virus is sensitive to common household disinfectants like Lysol and Clorox.
Cleaning of the room where a MPX case stayed should be done without stirring a lot of dust or causing the formation of aerosols and should use regular cleaning products followed by disinfection using a 0.1 % sodium hypochlorite (NaOCl) (dilution 1:50, if household bleach is used, usually at an initial concentration of 5%). Particular attention should be paid to toilets and frequently touched surfaces. Contaminated clothing and linens should be collected and washed at 60°C cycles. Carpets, curtains and other soft furnishings can be steam cleaned.
Single-use disposable cleaning equipment (e.g. disposable towels) is recommended. If disposable cleaning equipment is not available, the cleaning material (cloth, sponge etc.) should be placed in a disinfectant solution effective against viruses, or 0.1% sodium hypochlorite. If neither solution is available, the material should be discarded.
Gauzes or other material soaked with lesion fluid or containing scabs from the MPX case should be preferably handled in a healthcare facility as infectious waste, or according to instructions from the local public health authority.
Washing your hands with soap and water should be enough to kill the monkeypox virus, but sanitizers that contain at least 60% alcohol are the most ideal for proper hand hygiene. Using hot water and regular detergent is enough to kill the virus on linens and clothing. Always wear gloves and wash your hands properly before and after handling contaminated items.
______
______
Prevention with vaccines:
The smallpox vaccine is the first vaccine to be developed against a contagious disease. In 1796, the British doctor Edward Jenner demonstrated that an infection with the relatively mild cowpox virus conferred immunity against the deadly smallpox virus. Cowpox served as a natural vaccine until the modern smallpox vaccine emerged in the 20th century. From 1958 to 1977, the World Health Organization (WHO) conducted a global vaccination campaign that eradicated smallpox, making it the only human disease to be eradicated. Although routine smallpox vaccination is no longer performed on the general public, the vaccine is still being produced to guard against bioterrorism, biological warfare, and monkeypox.
The term vaccine derives from the Latin word for cow, reflecting the origins of smallpox vaccination. Edward Jenner referred to cowpox as variolae vaccinae (smallpox of the cow). Allan Watt Downie demonstrated in 1939 that the modern smallpox vaccine was serologically distinct from cowpox, and vaccinia was subsequently recognized as a separate viral species. Whole-genome sequencing has revealed that vaccinia is most closely related to horsepox, and the cowpox strains found in Great Britain are the least closely related to vaccinia.
_
For over a 100 years since Jenner, smallpox vaccination went from ‘arm to arm’ in humans. In 1860 scientists in France and Italy started using animals – initially cows but later sheep, horses, and donkeys – to amplify the smallpox vaccine. We now know that maintaining viruses in non-natural hosts over multiple generations changes them in unpredictable ways. By the 1930s, the smallpox vaccine cocktail largely included a virus called ‘vaccinia’, whose origin and animal reservoir remain unknown to this day. Due to its cross-protective immunity, this live vaccinia virus was used to eradicate smallpox and it saved millions of lives.
The first-generation smallpox vaccine was made by growing vaccinia virus in the skin of animals – mostly cows, but also sheep. The second-generation vaccine used live vaccinia virus grown in eggs or in cell culture, being introduced in the late 1950s and early 1970s, respectively. A freeze-drying method developed in the early 1950s allowed the vaccine to be stored and transported at room temperature. Between 1958 and 1977 it was mainly the second-generation vaccine that was used in smallpox eradication programs. Third-generation vaccines introduced in the late 1970s are based on a Turkish strain – Modified Vaccinia Ankara (MVA) – which had lost the ability to multiply in humans; it could still be grown well in chicken embryos.
Two smallpox vaccines are currently approved for use against monkeypox.
ACAM2000 is a second-generation vaccine that includes live vaccinia virus, which replicates in the recipient and has unpleasant side effects.
MVA-BN (Modified Vaccinia Ankara – Bavarian Nordic) is a third-generation vaccine, which uses MVA and has a better safety profile. It is licensed under three brand names – Jynneos (US), Imvanex (EU) and Imvamune (Canada).
_
Note:
Chickenpox is a herpes virus in a separate family from monkeypox and smallpox, so vaccination against chickenpox would not be expected to provide any protection from monkeypox.
_
Immune responses to one orthopoxvirus can recognise other orthopoxviruses and result in varying levels of protection depending on how closely related the different orthopoxviruses are. It has been hypothesized that the increase in monkeypox incidence since the cessation of smallpox vaccination is due to an increasingly immunologically naive population. This immunological cross-reactivity has enabled researchers to develop various animal models of smallpox infection that were used to test vaccines and antivirals. This cross-reactivity is primarily due to two factors. First, the high degree of sequence similarity between orthopoxviruses, especially among immunologically relevant proteins, leading to a large number of shared immune epitopes. Second, the wide breadth of the response, with antibodies targeting at least 24 membrane and structural proteins. Similarly, T-cell responses recognise epitopes within a wide diversity of viral proteins, with CD4 T cells preferentially recognising structural proteins, whereas CD8 T cells target proteins produced early (e.g., virulence factors) in the viral lifecycle. Neutralising antibody was established as a correlate of protection against smallpox (caused by the variola virus) in humans and against other orthopoxviruses in animal models. Although T cells are not necessary for protection, they do contribute to viral clearance.
Some of the earliest evidence that vaccinia-specific immune responses can protect against monkeypox comes from studies done in the 1960s. In three separate studies involving chimpanzees, rhesus macaques, and cynomolgus macaques, respectively, vaccination with Dryvax (Wyeth Laboratories, PA, USA) or other first-generation smallpox vaccines provided complete protection against disease in almost all vaccinated animals. The single exception was an animal that did not develop a take (i.e., a characteristic blister at vaccination site) after vaccination. These early studies involved small numbers of animals but the results suggested that a large degree of cross-protective immunity was conferred by smallpox vaccination.
Data showing clinical effectiveness (~85%) of smallpox vaccines against monkeypox come from surveillance studies conducted in central Africa in the 1980s and later during outbreaks in the same area. These data are supported by a large number of animal studies (primarily in non-human primates) with live virus challenge by various inoculation routes. The benefit of smallpox vaccination with vaccinia virus to prevent monkeypox was demonstrated in a study of human-to-human transmission of monkeypox in Africa. Secondary attack rates varied greatly among 2278 household contacts depending on their prior smallpox vaccination status (7.5 compared with 1.3 percent in vaccinated and unvaccinated subjects). In another study that showed an increasing incidence of human monkeypox cases in Africa, vaccinated people had a fivefold lower risk of monkeypox as compared with unvaccinated persons (0.78 versus 4.05 per 10,000); vaccine efficacy was estimated to be approximately 81 percent in those with a distant history of smallpox vaccination.
In the 2003 United States outbreak, an investigation using experimental techniques identified three asymptomatic monkeypox infections in individuals who had received smallpox vaccination 13, 29, and 48 years prior to their exposure to monkeypox. These individuals were unaware that they had been infected because they did not have any recognizable disease symptoms, and no transmission was documented. These findings suggested that distant vaccination prevented symptomatic monkeypox disease.
These studies uniformly showed a high degree of protection and immunity against monkeypox virus following vaccination with various smallpox vaccines. Smallpox vaccines represent an effective countermeasure that can be used to control monkeypox outbreaks. Both the CDC and the WHO estimate that the available smallpox vaccines are about 85% effective against monkeypox. But experts caution that the highly cited figure should not be taken at face value, particularly in the context of the current outbreak. The historic smallpox vaccines administered in above mentioned studies are no longer in production. But newer ones, like MVA-BN, a third-generation smallpox vaccine manufactured by Bavarian Nordic, are expected to have similar efficacy against monkeypox, based on studies in people that showed comparable antibody responses. However, they haven’t been directly tested against the disease in clinical trials. MBA-BN, known in the U.S. as Jynneos, is currently the only vaccine licensed by the Food and Drug Administration to prevent monkeypox infection. Its initial approval for smallpox was based on favorable data from nearly two dozen clinical trials with more than 7,500 participants. But when the agency later approved the vaccine for monkeypox, its decision was based on data from animal experiments, including non-human primate studies. Eighty to 100% of the monkeys who got the jab later survived a lethal dose of the virus, compared to zero to 40% of the placebo group.
Surveillance studies hints that first-generation shots had been effective; 30 years after mass smallpox vaccination campaigns ceased in central Africa, incidence of monkeypox cases there increased 20-fold. But such data cannot be extrapolated to the current outbreak — which is primarily spreading through sexual contact. Mucosal surfaces are easier for the virus to infect, and the prolonged contact that happens during sex is likely exposing people to much larger doses of the virus than the groups of people who were studied in western and central Africa in previous decades. We are expecting more from these vaccines than they were designed for. This kind of intense, often repeat, mucosal exposures are very different from animal exposure, household, fomite, or respiratory droplet transmission.
These perhaps outdated estimates of vaccine effectiveness are based on studies of pre-exposure vaccination. There’s even less data supporting the ring vaccination strategy being deployed by countries including the U.S., the U.K., and Canada. That strategy hinges on having rigorous contact tracing and a long incubation period in which to operate. And while the approach has proven very effective at curbing smallpox it’s not obvious yet that it will work as well for monkeypox.
_
More research is needed before relying on vaccination alone to curb the monkeypox outbreak:
A study published in the CDC’s Morbidity and Mortality Weekly Report, was based on 5402 monkeypox cases that occurred between 31 July and 3 September, a period when the vaccine was widely available in the US but most people had only received their first dose. Preliminary results suggest that men who received a single vaccine dose at least two weeks prior were 14 times less likely to contract monkeypox than eligible men who were not vaccinated. But this analysis was not able to control for confounding factors. For example, men who were early vaccine adopters might have also changed their sexual behaviour. “This early finding suggests that a single dose of Jynneos vaccine provides some protection against monkeypox infection,” the study authors wrote. “The degree and durability of such protection is unknown, and it is recommended that people who are eligible for monkeypox vaccination receive the complete two-dose series.”
A smaller Israeli study, published as a pre-print, looked at outcomes among 1970 vaccine-eligible men who either were on HIV PrEP or were HIV-positive and had a recent STI. Of these, 873 received one dose of the MVA-BN vaccine between 31 July and 18 August and had at least 25 days of follow-up. Three vaccinated and 15 unvaccinated men contracted monkeypox. This works out to a vaccine effectiveness estimate of 79%, though the number of cases was small and the confidence interval is wide (ranging from 24% to 94%). “Our results suggest that a single dose of MVA is associated with a significantly lower risk for monkeypox virus infection in high-risk individuals,” the researchers concluded.
But some experts think it’s premature to say the vaccine provides such a high level of protection – especially with only one dose — and other recent reports suggest caution is warranted.
A French study, published as a pre-print, looked at post-exposure vaccination of people who had high-risk contact with a person known to have monkeypox. The analysis included 276 individuals, mostly men who have sex with men, who received a single dose of MVA-BN between 27 May and 13 July with a median delay of 11 days after exposure. Of these, 12 (4%) contracted monkeypox, but none of the cases was severe. Ten of the 12 developed monkeypox within five days after vaccination, but two had breakthrough infections at 22 and 25 days post-vaccination. The vaccination strategy “was well tolerated and effective against monkeypox but did not completely prevent breakthrough infections,” Dr Michael Thy of Hôpital Bichat in Paris and colleagues concluded.
Dr Aniruddha Hazra and colleagues from Howard Brown Health in Chicago — the largest monkeypox vaccination site in the US Midwest — analysed infections after a single dose of MVA-BN. The study, published in JAMA, included 7339 people who received their first vaccine dose between 28 June and 9 September. Of this group, 90 contracted monkeypox. More than three quarters of these cases (77%) occurred during the first week (37 cases) or second week (32 cases) after vaccination. But eight people tested positive more than a month after their first dose, including two who had had their second dose more than three weeks earlier. All but one had mild illness with fewer than 10 lesions.
In another study published as a pre-print, Dr Luca Zaeck of Erasmus University Medical Centre in Rotterdam and colleagues compared antibody responses in people who received the MVA-BN vaccine, those who received an older smallpox vaccine and those who tested positive for monkeypox. They found that MVA-BN recipients had low levels of neutralising antibodies compared with the other two groups. People who received just one dose “hardly developed antibody responses” at four and eight weeks post-vaccination. Antibodies remained relatively low even after the second dose, but a third dose boosted their levels. “As the role of monkeypox virus neutralising antibodies for protection against disease and transmissibility is currently unclear and no correlate of protection against monkeypox virus infection has been identified yet, this raises the question how well vaccinated individuals are protected,” the researchers concluded.
In contrast, researchers at Bavarian Nordic reported in another pre-print that single and double subcutaneous doses of MVA-BN induced durable neutralising antibody responses comparable to those seen with older smallpox vaccines. Antibody levels remained high for at least six months but then returned to pre-vaccine levels by two years. However, this does not mean immunity was lost, as B-cells and T-cells provide longer-lasting protection. This study also found that a MVA-BN booster jab given to people who previously received an older smallpox vaccine induced a rapid increase in antibodies, reaching higher levels than one or two doses of MVA-BN without prior vaccination. A rapid antibody response was also seen when people who received one or two initial doses of MVA-BN were given a booster two years later, suggesting the booster activates existing memory B-cells.
Given the mixed evidence to date, experts caution that vaccinated people may still be at risk, and more research is needed before relying on vaccination alone to curb the monkeypox outbreak. While the MVA-BN vaccine certainly provides some immunity, it does not prevent infection entirely, especially after just one dose. Indeed, some evidence suggests a third booster dose may be needed for optimal protection.
Until there’s more data, the most important thing that patients should know is that the vaccine, even two doses, does not provide 100% protection. It’s an important part of a combined strategy for lowering the risk of infection, but it’s not the only thing people should be doing.
_
How long does protection from prior smallpox vaccination last?
Researchers have estimated that protection against severe disease erodes after about 32 years and protection against fatal disease lasts nearly 52 years. But those estimates are based 1) on protection against smallpox and 2) calculated using data from a 1903 smallpox outbreak in Australia. Smallpox Vaccination 40 years ago, even if not currently protective against monkeypox disease, may offer some protection against a fatal outcome. Should you be exposed to monkeypox in the current outbreak, you should definitely be revaccinated because vaccination after exposure to an infected monkeypox patient, even four days later, can prevent monkeypox disease.
______
Orthopoxvirus vaccines:
Types of vaccines — There are two available vaccines that can reduce the risk of developing monkeypox. The modified vaccinia Ankara (MVA) vaccine (JYNNEOS in the United States, IMVANEX in the European Union, and IMVAMUNE in Canada) and ACAM2000 vaccine.
- MVA vaccine – The MVA vaccine is made from a highly attenuated, nonreplicating vaccinia virus and has an excellent safety profile, even in immunocompromised people and those with skin disorders. In the United States, JYNNEOS is approved for the prevention of smallpox and monkeypox. JYNNEOS is safer and can be used in immunocompromised individuals and potentially in pregnant women. Two doses are required 28 days apart, with full protection being present only 14 days after the second dose.
- ACAM2000 – ACAM2000 is a replication-competent smallpox vaccine that can only be used in select patients and is associated with more adverse events that the MVA vaccine. In the United States, ACAM2000 is approved for the prevention of smallpox. It can be used for monkeypox under an expanded access investigational new drug (IND) application through the CDC. ACAM2000 is capable of replication and can cause serious complications, such as progressive vaccinia infection, eczema, myocarditis and pericarditis, and encephalitis. ACAM2000 requires a single dose, with peak protection at 28 days.
Post-exposure prophylaxis with these vaccines has been reported, and ring vaccination is being practiced at present in the USA, UK, and Canada to arrest the MPX epidemic. CDC and WHO suggest vaccination with the MVA vaccine given its safety profile. However, if the MVA vaccine is not available, and vaccination is indicated, ACAM2000 may be used in certain clinical settings (e.g., healthy, nonpregnant, immunocompetent person with a high-risk exposure). This vaccine is administered through a process called scarification, in which an infectious dose is placed on a bifurcated sterile needle and gently penetrated 15 times into the epidermis of the deltoid region of the arm.
______
While it’s best to get the vaccine before you’re exposed to monkeypox, getting it afterward may still help prevent the disease or make the symptoms less serious. Experts call this prophylaxis.
Vaccine strategies for monkeypox include:
-1. Post-exposure prophylaxis (PEP):
Post-Exposure Prophylaxis (PEP) is for known close contacts of monkeypox cases who are identified by public health via case investigation, contact tracing, and risk exposure assessments. CDC recommends that the monkeypox vaccine be given within 4 days from the date of exposure in order to prevent onset of the disease. If given between 4-14 days after the date of exposure, vaccination may reduce the symptoms of disease, but may not prevent the disease.
Persons who are eligible for PEP include:
- Those who had a sexual partner in the past 14 days who was subsequently diagnosed with monkeypox; or
- Gay, bisexual, or other men who have sex with men, or transgender people who, in the past 14 days, have had any of the following: sex with multiple partners (or group sex); sex at a commercial sex venue; or sex in association with an event, venue, or defined geographic area where monkeypox transmission is occurring.
The goal of this strategy is to help slow the spread of the current outbreak by providing vaccination to people in the community who are most likely to have a recent exposure to monkeypox. As supplies of vaccine become more available, vaccines may be more widely distributed as pre-exposure prophylaxis. Peak immunity is expected 14 days after the second dose of vaccine.
-2. Post-Exposure Prophylaxis (PEP)++
It is for individuals with certain risk factors who are more likely to have been recently exposed to monkeypox even if they have not had documented exposure to someone with confirmed monkeypox, such as people who attended an event or venue where there was known monkeypox exposure.
-3. Pre-exposure prophylaxis (PrEP):
Pre-Exposure Prophylaxis (PrEP) is for individuals at occupational risk of monkeypox according to Advisory Committee on Immunization Practices (ACIP) guidance, including: laboratory workers who perform monkeypox testing, and clinical and public health workers who collect monkeypox specimens. This vaccine strategy is used for those who are at high risk for exposure.
In 2021, the Advisory Committee on Immunization Practices (ACIP) voted to recommend the use of the MVA vaccine for certain workers at high risk for occupational exposure to orthopoxvirus infection, such as research laboratory personnel and specialized clinical laboratory personnel performing diagnostic testing for orthopoxviruses (e.g., labs that are part of the Laboratory Response Network [LRN]), as well as designated response team members who are at risk for occupational exposure to orthopoxviruses, in consultation with local public health authorities. The ACIP also recommended offering vaccination to those who administer ACAM2000 (or care for patients infected with replication-competent orthopoxviruses, based on shared clinical decision-making). In 2022, the United States Centers for Disease Control and Prevention (CDC) approved the updated ACIP smallpox vaccine recommendations related to PrEP, including the use of booster vaccination as immunity can wane.
______
______
ACAM2000™ vaccine:
ACAM2000™ is a live-attenuated smallpox vaccine that is also effective against MPX. ACAM2000™ is manufactured by Sanofi. Administration of ACAM2000™ requires specialised training and facilities.
ACAM2000™ is not suitable for:
- severely immunocompromised people
- people who are pregnant or breastfeeding
- people with cardiac disease or cardiac risk factors
- people with active eczema
- infants below 12 months of age.
_
JYNNEOS® vaccine:
JYNNEOS® is a modified vaccinia Ankara strain vaccine (MVA-BN) that contains a virus that has been altered so it cannot multiply in the human body. JYNNEOS® is manufactured by Bavarian Nordic. It is given as 2 doses, at least 28 days apart for people 18 years and over. Vaccination with JYNNEOS® in children can be considered, especially for people in high-risk groups aged 16 years and older, after discussing the risks and benefits with their vaccine provider.
JYNNEOS® can be injected subcutaneously (under the skin, preferably into the upper arm) or intradermally (into the outer layers of skin). Data from a 2015 clinical study of the MVA vaccine evaluated a two-dose series given intradermally compared to subcutaneously. Individuals who received the vaccine intradermally received a lower volume (one fifth) than individuals who received the vaccine subcutaneously. The results of this study demonstrated that intradermal administration produced a similar immune response to subcutaneous administration, meaning individuals in both groups responded to vaccination in a similar way. Administration by the intradermal route resulted in more redness, firmness, itchiness and swelling at the injection site, but less pain, and these side effects were manageable. Intradermal administration of this vaccine is not recommended for children and anyone with a weakened immune system or a history of keloid scarring. Most people living with HIV with undetectable viral load on ART can have the vaccine this way. You also need to have a CD4 count above 200.
_
Adverse effects of monkeypox vaccines:
- Redness, soreness, swelling, and itching where the shot is given are the most common things that happen after vaccination with JYNNEOS™.
- Fatigue (tiredness), headache, and muscle pain can also sometimes happen after vaccination with JYNNEOS™.
- In the ACAM2000 clinical trial experience, seven individuals of the 2,983 ACAM2000 first-time recipients and three individuals of the 868 Dryvax first-time recipients were suspected to have myocarditis/pericarditis. There were no cases in those subjects who had been vaccinated previously. More commonly observed side effects include: itching, sore arm, fever, headache, body ache, mild rash and fatigue.
______
Coadministration with other vaccines:
JYNNEOS typically may be given at the same time as other vaccines. However, because of the observed risk for myocarditis after receipt of ACAM2000 vaccine and mRNA and Novavax COVID-19 vaccines and the unknown risk for myocarditis after JYNNEOS, experts recommend waiting 4 weeks after JYNNEOS or ACAM2000 before receiving a Moderna, Novavax, or Pfizer-BioNTech COVID-19 vaccine. If an orthopoxvirus vaccine is recommended for prophylaxis in the setting of an outbreak, orthopoxvirus vaccination should not be delayed because of recent receipt of a Moderna, Novavax, or Pfizer-BioNTech COVID-19 vaccine; no minimum interval between COVID-19 vaccination with these vaccines and orthopoxvirus vaccination is necessary. If you get ACAM2000 vaccine then you should not get a live injectable vaccine (such as measles, mumps, and rubella vaccine (MMR) or varicella vaccine (chickenpox)) on the same day. They should be separated by at least 28 days.
______
______
Impact of vaccinia virus-based vaccines on the 2022 monkeypox virus outbreak:
There has been a noticeable difference between the outbreak of monkeypox in 2022 (MPXV-2022) and previous outbreaks. The previous outbreaks led to a small number of infections and were mostly localized, whereas the current outbreak has led to more than 70,000 confirmed cases in more than 100 countries within a few months of the first report on 7th May 2022. The monkeypox outbreak of 2022 was declared a global health emergency of international concern by the World Health Organization (WHO) on 23rd July 2022. Phylogenetic analyses of genomic sequences obtained from samples that were collected from at least 15 countries reported the West African clade of MPXV (MPXV-WA) to be involved in the 2022 outbreak. However, this is unusual since this clade has a historically low outbreak-causing potential.
Vaccines based on the vaccinia virus (VACV), which were initially developed against smallpox, can be used to prevent and control monkeypox. Three prominent VACV-based vaccines are available, including first, second, and third-generation vaccines. The use of first-generation vaccines such as Dryvax is not recommended against MPXV due to safety concerns. However, second-generation vaccines such as ACAM2000 are relatively safer compared to first-generation vaccines and can be used against monkeypox in the US. Currently, one third-generation vaccine, Bavarian Nordic’s modified vaccinia virus Ankara (MVA-BN), is recommended by the US Centers for Disease Control and Prevention (CDC) as well as WHO, primarily for high-risk groups. MVA-BN is also a VACV-based vaccine but cannot replicate in humans and therefore is safer than the previous generation vaccines. However, the availability of MVA-BN is currently limited.
Although these VACV-based vaccines have been observed to show differences in safety profiles and replication, they have been reported to produce strong T-cell responses and high neutralizing antibody titers. Some previous studies also indicated their cross-reactive and protective immune responses against different MPXV. Although MVA-BN and ACAM2000 have been found to prevent MPXV infections, those studies were conducted using the Congo Basin clade of MPXV (MPXV-CB). There is limited data to support the efficacy and cross-reactivity of these vaccines against the MPXV-WA clade responsible for the current outbreak.
_
A new study “Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus, 2022” aimed to investigate the cross-reactivity of VACV-based vaccines against the MPXV viruses responsible for the 2022 outbreak. The study involved downloading complete genome sequences of MPXV-2022, genome reference sequences of MPXV-CB and VACV, and reference sequences for MVA-BN, Dryvax, and ACAM2000 vaccines from several databases for multiple sequence alignment. Assessment of the cross-reactivity of the vaccines was carried out using the VACV reference sequence since it served as a representative of the VACV-based vaccines. In addition, pairwise sequence alignments were carried out to detect genetic similarities.
The data on VACV-derived T cell and B cell epitopes were obtained from the Immune Epitope Database (IEDB). Identification of eight VACV proteins was carried out that served as targets for neutralizing antibodies in humans, while 121 VACV proteins were identified as targets of T cells. Finally, the visualization of VACV protein crystal structures was carried out.
_
Figure above maps mutations observed in MPXV-2022 and MPXV-CB on the structure available for VACV (A) H3L [PDB ID: 5EJ0] and (B) D8L [PDB ID: 4E9O] surface proteins. The core structure of each protein is shown in gray, while mutations and their labels are colored according to the scheme in the legend.
_
The study results reported approximately 84% genetic similarity between MPXV-2022 sequences and VACV reference sequences. The sequences were found to contain about 13% insertion/deletion (indel) and 3% single nucleotide polymorphisms (SNPs), which is equal to 27.5 k indels and 6.5 k SNPs. A 94 to 98% genetic similarity was observed for the eight identified immunogenic proteins between VACV as well as the MPXV-CB reference sequence and MPXV-2022 consensus sequence.
The same site of mutations was observed in 4 of the 8 proteins between VACV and the two MPXV sequences. D8L and H3L were found to be the two proteins with the highest number of mutations that were similar to VACV. Moreover, all the common and unique mutations were observed to be exposed and, therefore, can be targeted by neutralizing antibodies.
Furthermore, a high degree of genetic similarity was also observed between T cell epitope associated 121 proteins of VACV with both MPXV-CB and MPXV-2022. 71.6% of VACV-derived T cell epitopes were found to have exact similarities with both MPXV-CB and MPXV-2022. However, genetic variation was observed in over one-quarter of the T cell epitopes between VACV and both MPXV orthologs. Additionally, 89.2% of the T cell epitopes were observed to be identical between MPXV-CB and MPXV-2022.
Therefore, this study demonstrated a high degree of genetic similarity between the current MPXV-2022 and MPXV-CB. Furthermore, the genetic similarity was also observed between these two MPXV orthologs and the VACV reference sequence. This suggests that the currently available VACV-based vaccines can protect against MPXV-2022. However, further studies are required to determine the efficacy of these vaccines against MPXV-2022.
_____
_____
Decline in monkeypox cases in the US:
The seven-day average has declined by 50% in September 2022. After weeks of rising cases, the monkeypox outbreak appears to be significantly slowing down in the United States.
There are two reasons why monkeypox infections are trending downward.
-1. First reason is that at-risk people have changed their behaviors.
The outbreak has primarily been concentrated in men who have sex with men, a group that includes people who identify as gay, bisexual, transgender and nonbinary, although health officials have said anyone — regardless of sexual orientation — is at risk if they have direct contact with an infected patient. Those at risk have been inundated with information about how to reduce their risk and have followed doctors’ advice. There’s been a terrific amount of public health education that’s gone out and it’s gone out particularly to the MSM community and the LBGTQ community that’s been primarily affected. These communities been literally flooded with information about monkeypox, and what you as an individual can do to protect yourself against becoming infected. A joint survey from the CDC, Emory University and Johns Hopkins University found about one-half of gay, bisexual, and other men who have sex with men reduced their number of sexual partners, one-time anonymous partners, and reduced use of dating apps.
-2. Second reason is vaccinations.
As of Sept. 20, more than 684,000 JYNNEOS vaccine doses have been distributed in the U.S. To increase the number of doses available, the U.S. Food and Drug Administration authorized a new strategy to inject the vaccine intradermally, just below the first layer of skin, rather than subcutaneously, or under all the layers of skin — allowing one vial of vaccine to be administered as five separate doses rather than a single dose.
The battle is not over yet and there should be a sustained effort on the part of public health officials, clinicians and community leaders to keep spreading information on the seriousness of monkeypox and how to reduce risk as well as how to get vaccinated.
_______
_______
Is this monkeypox outbreak potentially a major global health threat, like COVID?
Given the rapid rise of cases and the potential to contribute to health inequities, monkeypox needs to be taken seriously. However, we have a much greater chance of controlling monkeypox than COVID. For one, monkeypox is not as transmissible, unless the biology has changed drastically, and that seems unlikely. Secondly, it takes much longer for a monkeypox infection to develop within an individual and to become transmissible. Therefore, there is a greater opportunity to protect contacts. Third, monkeypox may not be very transmissible before its visible rash highlights the need for quarantine, while COVID can be transmitted before symptoms emerge and even in asymptomatic cases.
______
______
Will the virus continue to primarily infect men who have sex with men?
In the U.S. and Europe, monkeypox has so far primarily affected men who have sex with men. But historically, outbreaks that begin in one community do not stay there. HIV famously spread far beyond gay and bisexual men in the ’80s, fueled in part by officials who ignored early warnings that there was no such thing as a gay disease. In the 2000s, an outbreak of drug-resistant bacteria was first spotted in gay men but ultimately spread to athletes and took its greatest toll on people in prison.
For now, monkeypox’s next move remains unclear. Much of the news is positive: The virus appears to be more difficult to transmit through casual contact than initially feared, narrowing its potential paths. Cases have also been declining in New York, San Francisco, and much of Europe, as more vaccines become available and people minimize their risk of exposure through sex. If we can contain this outbreak in men who have sex with men, that is our best shot.
And yet the news is not good everywhere. The monkeypox response has so far been marked by inequities. In North Carolina, 72% of people diagnosed with monkeypox are Black, but only 24% of vaccines have gone to Black residents — a glaring example of a racial disparity seen in much of the country. The longer these disparities go unaddressed, the more likely the virus is to stick around and spread broadly, including into prisons and other overcrowded settings, or to even become endemic among animals in North America. We need equity in vaccine distribution and change in behaviour of MSM community to control the outbreak. Wealthy countries have been criticized for not doing more to help the African nations that have animal reservoirs of the virus fight the pathogen, even as there were signs of more human-to-human transmission in recent years. Even now, as countries in Europe and North America have scooped up global vaccine supplies, those African countries don’t have access to vaccines.
Even if individual cities, or countries, can eliminate monkeypox, they won’t be able to move on entirely. They’ll have to continue surveillance to ensure the virus didn’t get established in animals locally — a source for potential future outbreaks.
______
______
Section-19
Monkeypox stigma:
MSMs suffering from monkeypox are looking terrified, grappling with the stigma and shame that is traveling along with the monkeypox outbreak in big cities from Washington D.C. to San Francisco and Minneapolis to Houston, and in small towns from Appleton, Wisconsin to Waco, Texas. The monkeypox virus arrived in the U.S. at a terrible time: right before Gay Pride events across the country, when LGBTQ+ people celebrate the freedom of being whoever they want to be and loving whoever they want to love. This virus spreads mainly through skin-to-skin contact, and can affect anyone. But in this 2022 outbreak, it has spread like wildfire through sexual networks, as HIV did — and continues to do — in the LGBTQ+ community. Data has shown how the monkeypox outbreak has been disproportionately impacting black and brown folks in the LGBTQ+ community in the U.S. We’ve seen these communities bear the brunt of illness and governmental failures to control viruses in the past, perhaps most notably with COVID-19 and with the HIV pandemic. And with the monkeypox virus — which is related to the smallpox virus but causes milder illness — cases have come hand and hand with stigma, with intersectional minorities being hurt the most. And the messaging around the issue is fraught, with LGBTQ+ and black and brown people seemingly most at risk for infection right now, but also being stigmatized for the spread of a virus that impacts us all.
_
When news of the multi-country monkeypox outbreak began May 2022, some patients pointed that many of the photos in news stories about the virus featured mostly black people. This could be stigmatizing, they worried, even if journalists were just using the stock photos that were available based on previous cases, as the infection is endemic to parts of Africa. Now we have more equitable representation but initially there was a concern that this would be further stigmatizing to people of color based on the representations of the disease.
Concerns around monkeypox virus stigma also extend to the LGBTQ+ community. So far, cases have been spreading primarily among men who have sex with men. The current outbreak is thought to be related to two raves that occurred this spring in Spain and Belgium that were primarily attended by men who have sex with men. Centers for Disease Control and Prevention (CDC) has said that this cohort is at “high risk” of being infected. A New England Journal of Medicine article looking at infections diagnosed between April 27 and June 24 of this year came to the same conclusion, noting that 98% of those infected identified as gay or bisexual men. So some labelled it as STD among MSMs although monkeypox is transmitted by non-sexual routes as well.
Stigma against the illness has prompted concern from activists, who say monkeypox has become a tool for anti-LGBTQ haters against the community. LGBTQ activists say they have noted a rise in homophobic or transphobic messages about monkeypox online, that may make those diagnosed with monkeypox — LGBTQ or not — reluctant to come forward about their illness. Many health experts have raised concerns about stigma toward the MSM community, which is currently the most at risk of the current outbreak of monkeypox infection. Men who have sex with men in many settings are often stigmatized and discriminated against, and then that’s kind of going to stand in the way of us being able to control this outbreak. And this could have ripple effects to the broader population as well. If you stigmatize a group of individuals, what’s going to happen is that they’re not going to come forth to be diagnosed if they suspect that they may have this infection. They are not going to come in and get vaccinated. And that jeopardizes control of the outbreak.
_
The WHO guidance targeted to MSM put the agency at odds with health officials and activists who have insisted on a sex-positive approach—or one that avoids asking people to change their sexual behavior—to combat the virus. There is concern that monkeypox messaging centered on the risks of sex between men could fuel the blaming of the outbreak on the sexual behavior of gay men, rather than the testing, vaccine procurement, and treatment failures allowing the virus to go unchecked. In addition to concerns of anti-LGBTQ+ discrimination, a large reluctance to label monkeypox an STD stems from the worry that doing so could lead people outside of MSM sexual networks to think that the disease poses no risk to them. Such was the case in the early days of the AIDS epidemic, when popular misconceptions of HIV as a “gay disease” allowed the virus to spread beyond MSM.
_
Meanwhile, the stigmatization has already led to discrimination. It’s led to people worrying about touching certain groups of people, based on a misunderstanding of the most common ways it spreads; and to making assumptions about people who get it. There’s been demonizing and slut-shaming and homophobia that’s come along with this virus, often due to misinformation and misunderstandings. Many doctors agree that stigmatizing monkeypox as a virus that only impacts LGBTQ+ folks, especially people of color, could lead to many of the same mistakes we made during the early days of HIV, when homophobia in government impacted research, treatments, and the way information was disseminated. And drawing comparisons between HIV and monkeypox, as has been done, must be much more nuanced. It’s important to learn from HIV to inform our responses to everything as we go forward, but to compare the diseases is an act of futility. Very few people have died from monkeypox, and it’s not the same… However, we see some similarities and it’d be irresponsible of us not to honor and note them. It’s impacting a similar community, and based on the ways that the community was alienated and stigmatized and oppressed, we can learn from that. If we didn’t, we’d be shooting ourselves in the foot. Getting treatment to those most in need is proving difficult, both due to a lack of vaccine supply as well as a warranted lack of trust in the medical establishment due to racism and homophobia. In order to avoid stigmatizing monkeypox while also making sure that those who are most at risk get the care they need, we need to acknowledge that anyone can get monkeypox, and this is something we should all be concerned about treating and getting under control epidemiologically.
______
Media must separate monkeypox risk from stigma:
Media coverage of monkeypox paints it as an African virus. The world is in the midst of a monkeypox outbreak. The World Health Organization has recorded more than 70,000 cases in 100 countries this year – including the United Kingdom, the United States and a number of European nations. And how do Western media outlets illustrate the story? The BBC, the Independent, CNBC and ABC News are among those that have used a stock photo of a Black person with monkeypox blisters. Africans and health equity advocates have been swift in reacting to the Western media’s use of Black arms and faces with monkeypox. Nigerian journalist Mercy Abang tweeted, “Here is an example of media bias at its finest; monkeypox has been reported in nations worldwide, but a search shows only [photos of] blacks. Tragic.” Dr. Madhu Pai, professor of epidemiology and global health at McGill University, tweeted, “Journalists and editors of global North media outlets badly need training on how to not be racist & stigmatizing in their reporting Ebola, Covid, monkeypox.”
_
The U.S. National health headline is that gay and bisexual men are spreading the rare virus akin to smallpox, monkeypox. For those who survived the AIDS pandemic, it’s an uncomfortable reminder of how stigmatization starts. CNBC, a major news outlet, presented the disease as a sexually transmitted gay illness although anyone can contract monkeypox through close personal contact regardless of sexual orientation. Many of the people affected globally so far are men who identify as gay or bisexual but the risk isn’t limited only to the gay and bisexual community. Monkeypox can thus infect anyone, and is not limited to the LGBTQ+ community – it is very important to separate risk of exposure from stigma.
_
The U.N. has denounced what it terms “homophobic and racist” reporting on monkeypox spread, noting that media portrayals of cases among African and LGBT people are fueling blame, as infections are reported in Europe, US and Australia. UNAIDS, the United Nations’ AIDS agency, warned of “exacerbating stigma and undermining the response to the growing outbreak.” UNAIDS said “a significant proportion” of recent monkeypox cases have been identified among gay, bisexual and other men who have sex with men, but also said “transmission was most likely via close physical contact with a monkeypox sufferer and could affect anyone.” UNAIDS deputy executive director, Matthew Kavanagh said, “Stigma and blame undermine trust and capacity to respond effectively during outbreaks like this one.” Kavanagh added, “Experience shows that stigmatising rhetoric can quickly disable evidence-based response by stoking cycles of fear, driving people away from health services, impeding efforts to identify cases and encouraging ineffective, punitive measures.” UNAIDS has expressed concern that some public reporting and commentary on Monkeypox has used language and imagery, particularly portrayals of LGBT and African people, that reinforce homophobic and racist stereotypes and exacerbate stigma. Lessons from the AIDS response show that stigma and blame directed at certain groups of people can rapidly undermine outbreak response.
______
Misinformation:
Misinformation and conspiracy theories spread online in various social media networks. In an article published by the BBC in May 2022 countering misinformation about the monkeypox outbreak, journalist Rachel Schraer noted that social media accounts and news outlets from different countries including China, Russia, Ukraine and the United States have been promoting the idea that the outbreak was caused by a lab leak or that monkeypox is being used as a bioweapon. The Institute for Strategic Dialogue described this as “Reviving the spread of a set of cut-and-paste… conspiracies”, referring to conspiracy theories used during the COVID-19 pandemic. The BBC also made it clear that the genetic sequences of the virus, as far as is known, date back to a West African strain.
_______
Combatting Racism and Stigma:
More than 72,000 cases of the monkeypox virus have now been identified across nearly 100 countries. Vaccines and treatments are being rolled out by health agencies around the world, most of which are responding to the first significant monkeypox outbreak within their country. Early outbreak response bears a striking resemblance to the early HIV/AIDS response; we have seen the use of harmful and stigmatizing language, widespread circulation of images of African bodies with rashes, and the conflation of sexuality and monkeypox infection.
Let’s be clear and state three facts:
First, anyone can get monkeypox.
Second, the current outbreak is overwhelmingly concentrated among men who have sex with men.
And third, a growing body of evidence and data suggests that sex among these men is the primary means through which monkeypox is presently spreading.
While it’s true that there are other ways the virus can be transmitted, recognizing and reporting these facts is not anti-gay or anti-science, and neither is targeting advice to members of this community given they are the ones who are presently most at risk.
And yet, whether through fears of perpetuating stigma or just general squeamishness about using the words “anal sex” in headlines, health officials and the news media have appeared extremely hesitant in speaking frankly about sex to who is most at risk. In Washington, DC, officials even broadened vaccine eligibility to include people of all genders in part due to a desire to “[destigmatize] the individuals who may need a vaccine.”
Given this current outbreak is spreading so predominantly among these networks of men who sleep with each other, experts say that prolonged skin-to-skin contact during sex is what is driving most of these cases. “Based on the data we have, it looks pretty convincing to me that sex is playing a dominant role in the spread of monkeypox, together with the fact that perhaps these patients had sex with multiple partners,” Gerardo Chowell, an epidemiologist at Georgia State University School of Public Health in Atlanta said. “And that’s probably why we haven’t seen as many cases among heterosexuals.” If fomites and aerosols were huge drivers of monkeypox, the data would show a lot of cases occurring outside of gay men, bisexual men, and other men who have sex with men (GBMSM). Not all transmission is associated with sex, but most is. If this were transmitted more commonly by aerosols, fomites, or incidental contact, we’d see way more household transmission and spread into the larger community. GBMSM don’t live in isolation: they have kids, families, coworkers. We’d see more cases in those people if the spread weren’t driven primarily by sex. Though again, there is some nonsexual transmission occurring, just not that much.
Various findings together suggest that close skin-to-skin contact during sex is probably the dominant transmission route in the current outbreak. There is some evidence that monkeypox might be spreading via semen. As Jeffrey Klausner, a professor at the University of Southern California’s Keck School of Medicine, and Lao-Tzu Allan-Blitz, a physician at Brigham and Women’s Hospital and Boston Children’s Hospital, summarized in a Medium post, “Taken in context, the temporal and anatomic association with various sex practices, the high prevalence of sexual risk behavior among patients with human monkeypox, and the in vitro infectivity of human monkeypox DNA isolated from semen strongly suggest that human monkeypox is transmitted through sexual activity.”
Even though data and studies suggest monkeypox is overwhelmingly spreading via sex in this outbreak, researchers aren’t yet comfortable classifying it as a sexually transmitted infection, or STI, which would mean it’s being transmitted via the close contact or exchange of fluids that only really happens in sex. This was because of what scientists know about the ways the virus has previously spread. There is also presently a minority of patients, including a few children, who have become infected without having sex.
Wary of perpetuating anti-gay stigma, health officials have struggled to communicate the twin ideas that while anyone can get monkeypox, it’s GBMSM who are currently most at risk. It’s important to tailor public health messaging to those most at risk, experts believe, but that will inherently cause some level of public stigma.
“The labeling of the outbreak as a disease among gay, bisexual, and other men who have sex with men is certainly both necessary from a harm reduction perspective, but is also a stigmatizing message at the same time,” said Jason Farley, director of Johns Hopkins’ Center for Infectious Disease and Nursing Innovation. Rasmussen, the virologist in Canada, said public health authorities need to give frank and practical advice focused on harm reduction, such as information on safer sex, that targets queer men. At the same time, experts need to leave open the possibility that their recommendations might need to be tweaked again if the virus spreads into other sexual or social networks. “What’s challenging is communicating that while this not a ‘gay disease,’ our limited resources need to go to the people at the highest risk, currently GBMSM,” Rasmussen said. “And it’s also important to communicate the risk is highest with sexual activity without shaming the queer community that has already been profoundly harmed by medical stigmatization.”
And while well-meaning people may want the media or public health authorities not to focus on the LGBTQ community for fear of spreading stigma, allowing the virus to spread may cause much greater harm.
Gettysburg College history professor Jim Downs argued in a piece published in the Atlantic, titled “Asking Gay Men to Be Careful Isn’t Homophobia,” that officials should not “tiptoe” around how the virus is spreading and instead state plainly that queer men need to refrain from high-risk sex until they are fully vaccinated. “As a gay man and a historian of infectious disease, I know about the harm that comes when public policy becomes infused with homophobia,” he wrote. “Yet protecting gay men from discrimination and stigmatization today does not require public-health officials to tiptoe around how monkeypox is currently being transmitted.”
Linking monkeypox to sexual transmission would equip gay and bisexual men with the health information they need to protect themselves. Frank and positive messaging on sex is the first step to curbing spread and protecting the most vulnerable.
_______
_______
We need new name for monkeypox:
Critics say ‘monkeypox’ is a racist name. But it’s not going away anytime soon. Nearly weeks after the World Health Organization said it will change the name of the monkeypox disease, agreeing with scientists who called it “discriminatory and stigmatizing,” the controversial label doesn’t seem to be going anywhere. Critics say the name “monkeypox” plays into racist stereotypes about Black people, Africa and LGBTQ people — and, they note, it falsely suggests monkeys are the main source of the virus. “Monkeypox should be renamed for two major reasons,” said Dr. Ifeanyi Nsofor, a global health equity advocate and senior New Voices fellow at the Aspen Institute. “First, there is a long history of referring to Blacks as monkeys. Therefore, ‘monkeypox’ is racist and stigmatizes Blacks.” “Second, ‘monkeypox’ gives a wrong impression that the disease is only transmitted by monkeys. This is wrong,” he adds. Yet despite growing criticism of the name, the International Committee on Taxonomy of Viruses said that even if the name is changed in the next year or two, the term “monkey” will likely still be part of any revamped name.
So, today I am renaming ‘Monkeypox’ as Tinypox. We do not want to stigmatize & discriminate against either African people or LGBT community. Let’s call it Tinypox. It is POX because it resembles smallpox and it is TINY because it is less transmissible & lethal than smallpox. I hope WHO accepts new name as ‘Tinypox’ to combat racism and homophobia generated by the name ‘Monkeypox’.
________
________
Moral of the story:
_
-1. Monkeypox was first identified in 1958 at a laboratory in Copenhagen, Demark when it was discovered in macaque monkeys kept for research and the disease caused lesions that were similar to those seen in smallpox — so scientists called it monkeypox. It does infect monkeys, and has been isolated from monkeys, but they’re not the primary reservoir for the disease.
Are monkeys involved in human monkeypox? No. Monkeys likely have nothing to do with the disease humans are getting. Several rodents and small mammals have been attributed as the source of the virus. Monkeypox is a viral zoonotic disease, which means that humans catch the virus from animals and then human-to-human transmission occurred.
_
-2. Monkeypox is an illness caused by monkeypox virus, which belongs to the Orthopoxvirus genus of the Poxviridae family; other members of Orthopoxvirus genus include variola virus (which causes smallpox), vaccinia virus (used in smallpox vaccines), cowpox virus, and various other animal poxviruses. It is a viral zoonotic infection, meaning that it can spread from animals to humans. It can also spread from humans to other humans; from the environment to humans; and from humans to animals. Poxvirus infections are characterized by the production of skin lesions. With most poxviruses there is typically just a primary lesion, but generalized lesions develop with smallpox [eradicated], monkeypox and molluscum. Monkeypox is not related to chickenpox. Chickenpox is a herpes virus in a separate family from monkeypox and smallpox. Few people outside Africa and the public health community had even heard of monkeypox. In last few months, it has become a household name.
_
-3. Smallpox killed an estimated 300–500 million people in the 20th century before a vaccine campaign eradicated the virus by 1979. Since then, no country has routinely vaccinated its citizens against smallpox. And therein lies the rub. The smallpox vaccine did not protect just against the variola virus. Anyone who was vaccinated against smallpox also developed immunity to infection with variola’s viral cousins—including monkeypox and cowpox. The orthopoxvirus genus is especially interesting because its members elicit cross-reactive and cross-protective immunity to each other. Now that the smallpox vaccine is no longer widely given, these obscure pathogens, which, like smallpox, belong to the Orthopoxvirus genus, pose a new danger to humans. There are reasons to worry. Unlike smallpox, cowpox and monkeypox naturally lurk in rodents and other creatures, so it would be difficult to eliminate them fully. Poxviruses are genetically stable and do not usually mutate quickly but they can mutate to become more transmissible or more lethal.
_
-4. The MPXV infects both animal reservoir hosts and other incidental hosts, including humans, tree squirrels, dormice, and Gambian pouched rats. Though the exact host reservoir for monkeypox is still unknown, there are data that suggest that monkeys are, like humans, incidental hosts and that the reservoir is likely to be one or numerous species of rodents or squirrels that inhabit the secondary forest of central Africa. Rodents are likely to be the natural reservoir of this virus with primates—including humans—being incidental hosts. If the virus has adapted to include humans as hosts, monkeypox outbreaks could become more frequent and more difficult to contain.
_
-5. Monkeypox is one of the many zoonotic viruses that belong to the Orthopoxvirus genus of the Poxviridae family. Poxviridae viruses are large, enveloped, double-stranded DNA viruses. The major hosts of Poxviruses are rodents, rabbits, and non-human primates, which can occasionally be transmitted to humans facilitating the occurrence of human-to-human transmission. The poxviruses are highly relevant to human beings, among which the orthopoxviruses are the best-known. These include the smallpox virus (variola virus, VARV), the monkeypox virus (MPXV), the cowpox virus (CPXV), and the vaccinia virus (VACV or VV).
MPXV belongs to the genus Orthopoxvirus with a double-stranded DNA genome of ≈197 kb whose clinical manifestations are similar to the smallpox virus. The orthopoxvirus encodes ~200 genes, whereas MPXV encodes about 190 genes which could be assigned to the genome as three parts, a core region, a left arm, and a right arm. Viral replication and assembly genes are encoded by the core region, which is relatively conserved in the genome. The MPXV’s left and right variable regions are known to be more involved in the host range and the pathogenicity of the orthopoxvirus.
The nucleotide sequence within the central region of the MPXV genome, which encodes essential enzymes and structural proteins, is 96.3% identical with that of variola (smallpox) virus (VARV). In contrast, there were considerable differences between MPXV and VARV in the regions encoding virulence and host-range factors near the ends of the genome. Monkeypox and smallpox viruses differ in the regions encoding virulence factors (e.g., IFN resistance genes and interleukin-1 inhibitors) at the terminal ends of the viral genome which might explain the variation in clinical presentation and disease severity between the two infections.
_
-6. A clade is defined as a group of organisms that can be traced back to common ancestors or a common genetic lineage. There are two distinct genetic clades of the monkeypox virus: the central African (Congo Basin) clade and the west African clade. The Congo Basin clade has historically caused more severe disease and was thought to be more transmissible. The West Africa (WA) lineage and the Congo Basin (CB) lineage differed by about 900 bp in genome length. The WA lineage has a case fatality rate of 3.6%, whereas the CB lineage has a case fatality rate of 10.6%.
Consensus is reached to now refer to the former Congo Basin (Central African) clade as Clade one (I) and the former West African clade as Clade two (II). Additionally, it was agreed that the Clade II consists of two subclades. The new naming convention comprises Clade I, Clade IIa and Clade IIb, with the latter referring primarily to the group of variants largely circulating in the 2022 global monkeypox outbreak. Clade I, IIa and IIb are also known as clade1, 2 and 3. Both clade 2 and clade 3 belong to the older Western Africa clade. Clade 3 was responsible for the outbreak of 2017/2019 and there are about 50 nucleotides that differ between the currently circulating 2022 strain and the 2017/2019 clade 3 strain.
The World Health Organization’s official death toll for the 2022 outbreak as of 14 October stands at 28, but this excludes large numbers of suspected deaths in countries where there is little laboratory testing. There have been 120 suspected deaths from monkeypox in the Democratic Republic of the Congo this year, and the virulent Clade I virus may be to blame. There are fears it could follow the milder Clade IIb and go global.
_
-7. Genome comparisons of the west and central African strains yielded a set of candidate genes that may be involved in the differentiating clade virulence. The open reading frames in the west African clade contained deletions and fragmentations that contribute to its reduced virulence. Central African monkeypox prevents T-cell receptor-mediated T-cell activation, prohibiting inflammatory cytokine production in human cells derived from previously infected monkeypox patients. The monkeypox virus inhibitor of complement enzymes (a gene that inhibits complement enzymes) is absent in west African strains, and it has been implicated as an important immune-modulating factor contributing to the increased virulence of central African strains. Moreover, the central African strain selectively downregulates the host responses, specifically, apoptosis in the host. Transcriptional studies have shown that central African monkeypox appears to selectively silence the transcription of genes involved in host immunity during infection.
_
-8. Despite the eradication of smallpox, poxviruses can cause emerging endemic diseases. Several outbreaks have been caused by MPXV, which produces smallpox-like lesions. Historically, monkeypox has circulated in remote parts of West and Central Africa. In that context, people normally caught the virus from animals. There was little spread between people. The recent multinational outbreak of human monkeypox cases beginning in early May 2022 with more than 72,000 confirmed cases reported worldwide has revealed a changing epidemiological trend, those confirmed cases had no sojourn history in endemic areas and with a high proportion of cases involving men who have sex with men (MSM). Not only was this spread by community transmission, but the route was by sexual contact. This was quite unlike earlier, much smaller epidemics confined to endemic regions in western and central Africa, following contact with potentially infected animal hosts. In the current 2022 outbreak, the vast majority of monkeypox cases – more than 95 per cent, are among men who have sex with men, with a median age of 36. And about 40 per cent of reported monkeypox cases are among people who are also living with HIV. Among the MSM cases, many of them presented with atypical clinical manifestations of monkeypox and with other sexually transmitted co-infections. Clinical presentations differed from typical monkeypox, with fewer persons experiencing prodrome and more experiencing genital rashes.
_
-9. RNA viruses mutate very effectively – they’re diabolical. Monkeypox virus is a double-stranded DNA virus. DNA viruses mutate slower than RNA viruses such as SARS-CoV-2 which causes COVID. Unlike SARS-CoV-2, a rapidly evolving RNA virus whose variants have regularly eluded immunity from vaccines and prior infection, monkeypox is caused by a relatively large DNA virus. DNA viruses are better at detecting and repairing mutations than RNA viruses, which means it’s unlikely that the monkeypox virus has suddenly mutated to become adept at human-to-human transmission. Viral mutation rates are not merely caused by polymerase errors, but also by the ability of a virus to correct DNA mismatches by proofreading and/or post-replicative repair. The replicative life cycles of many DNA viruses have been shown to engage components of the host DNA damage and repair machinery. On average, poxviruses—a family that includes orthopoxviruses such as monkeypox and smallpox—tend to mutate once per year.
_
-10. Genome evolution of MPXV is by various mechanisms:
-1. Orthopoxviruses develop between 10-5 and 10-6 mutations per replication site per year. The large genome, containing almost 200,000 base pairs, changes by 1 or 2 nucleotides a year.
-2. Because these viruses infect immune cells, they are subject to cytoplasmic DNA editing by cellular enzymes such as APOBEC3, which can significantly increase the mutagenicity induced by viral DNA polymerase errors.
-3. Poxviruses are able to evolve by homologous recombination between coinfecting strains. They can also acquire foreign genes by non-homologous recombination. If they can steal genes from their hosts that make them more virulent, then there is no predicting what a relatively harmless, not to mention an already deadly, poxvirus might do under the right circumstances.
The 50 single nucleotide polymorphisms (SNPs) of 2022 MPXV that diverge from the original strain of 2018, are far more (roughly sixfold to 12-fold more) than one would expect considering previous estimates of the substitution rate for orthopoxviruses, which typically have 1 to 2 substitutions per genome per year. Whether the excess of mutations seen in the 2022 MPXV is a direct consequence of APOBEC3-mediated genome editing in the human host cannot be discerned at this stage. APOBEC3 works by introducing mutations to viruses in order to stop them from working properly; but in this case the mutations are apparently not making the virus non-viable and may be helping it to adapt to human-human transmission.
Mutations seen in monkeypox samples allow researchers to trace the roots of the current outbreak, which began in Nigeria in 2018. The phylogenetic analysis indicated that the MPXV-2022 strains belong to the same lineage of the MPXV strain isolated in 2018 in Nigeria. The virus was exported to other countries several times and eventually spread around the world. Travelers infected in Nigeria were at the origin of monkeypox cases in the UK in 2018 and 2019, Israel in 2018 and Singapore in 2019. However, compared with the MPXV strain in 2018, in total about 50 mutations (SNPs) are observed in the MPXV-2022 strains. You may say that only about 50 SNPs change out of over 197,000 nucleotides means it is almost the same virus in 2022 as it was in 2018 outbreak.
_
-11. The R0 of monkeypox in previous outbreaks in Africa was between 0.8 to 1 and the R0 of current 2022 outbreak is between 1 to 2. Previous outbreaks in Africa were localized and affected few people but current outbreak is spread in 100 countries with more than 72,000 cases in few months. What explains rapid and massive spread?
-1. The rapid spread of monkeypox is unlike the virus’ past outbreaks and may be a result of genetic mutations. The genomic sequencing studies have revealed that the 2022 MPXV strain contains 6-12 times the expected number of single-nucleotide polymorphisms, suggesting accelerated evolution and increased human adaptation. The present mutations in the MPXV-2022 strains affected at least 20 proteins. It is crucial to determine whether these mutations contribute to the transmission or pathogenesis of the virus or help the virus evade host immunity.
-2. It could be containment strategies are not being implemented appropriately or vigorously enough, or that infected people are not recognizing the symptoms and seeking treatment in time. One problem could be that people are not identifying as having been exposed to monkeypox.
-3. The long incubation period of 5–21 days is making its movement hard to track.
-4. There is vast susceptible population with no orthopoxvirus cross-protection due to the cessation of smallpox vaccination after eradication in 1979.
-5. The growing ease and rapidity of global travel allows previously isolated clusters to quickly become global epidemics.
-6. Increased prevalence of immunocompromised people, may provide more opportunity for MPXV to acquire mutations that increase its fitness in human hosts, possibly leading to increased transmissibility, virulence, and pathogenic potential.
-7. Advancement in diagnostic capacity and health education. Development of molecular techniques and point-of-care systems in recent years made the diagnosis of monkeypox more accurate and rapid than before. Also, more attention is paid to health education of medical workers and the general public resulting in greater awareness and diagnosis.
-8. Most cases of current outbreak are in MSMs and prolonged physical contact that happens during sex is likely exposing people to much larger doses of the virus than animal exposure, household, fomite, or respiratory droplet transmission seen in western and central Africa in previous decades. Having multiple and frequent sexual contacts, including with anonymous partners, may allow transmission of virus efficiently in MSM community. Furthermore, the high social interactivity in MSM contributes to a high risk of monkeypox transmission in this population. Also, there is stigma of monkeypox linked to sexual behavior, so people might not come forward for diagnosis & isolation and thereby spread it, might not want to comply with contact-tracing efforts making ring vaccination much harder.
_
-12. Can remote smallpox vaccination prevent monkeypox?
Immune responses to one orthopoxvirus can recognise other orthopoxviruses and result in varying levels of protection depending on how closely related the different orthopoxviruses are. This immunological cross-reactivity is primarily due to two factors. First, the high degree of sequence similarity between orthopoxviruses, especially among immunologically relevant proteins, leading to a large number of shared immune epitopes. Second, the wide breadth of the response, with antibodies targeting at least 24 membrane and structural proteins. Similarly, T-cell responses recognise epitopes within a wide diversity of viral proteins, with CD4 T cells preferentially recognising structural proteins, whereas CD8 T cells target proteins produced early (e.g., virulence factors) in the viral lifecycle. Due to cross-immunity, an immune response against any illness caused by any orthopoxvirus will decrease the likelihood of an infection by a different orthopoxvirus.
Previous vaccination against smallpox can confer cross-protection against monkeypox, which was estimated from older studies to be as high as 85%. Data showing clinical effectiveness (~85%) of smallpox vaccines against monkeypox come from surveillance studies conducted in central Africa in the 1980s and later during outbreaks in the same area. These data are supported by a large number of animal studies (primarily in non-human primates) with live virus challenge by various inoculation routes. Various studies uniformly showed a high degree of protection and immunity against monkeypox virus following vaccination with various smallpox vaccines. Smallpox vaccines represent an effective countermeasure that can be used to control monkeypox outbreaks. The protective effect of smallpox vaccination wanes with time, although serosurveys indicate that it can last more than 20 years. However, it is believed that despite the waning effect, smallpox vaccine confers lifelong protection against severe disease due to memory B and T cells; therefore, some degree of protection should be expected in the population of adults over 50 years of age who had taken smallpox vaccine before 1980.
On the other hand, past data cannot be extrapolated to the current 2022 outbreak — which is primarily spreading through sexual contact. Mucosal surfaces are easier for the virus to infect, and the prolonged contact that happens during sex is likely exposing people to much larger doses of the virus than the groups of people who were studied in western and central Africa in previous decades. This kind of intense, often repeat, mucosal exposures are very different from animal exposure, household, fomite, or respiratory droplet transmission. We are expecting more from smallpox vaccines than they were designed for. The remote (30 years prior) vaccinia (smallpox) vaccination does not provide complete protection against monkeypox infection (even against a relatively mild disease variant in Africa); in some cases, it may prevent systemic disease, but the relative contributions of infectious inoculum and route of exposure, in addition to remote vaccination, may significantly impact whether systemic illness, asymptomatic infection, or atypical illness manifests.
_
-13. Due to the larger size of poxviruses, it makes it harder for viruses such as monkeypox to breach host defenses by passing through gap junctions. The larger size of the virus also makes it difficult for the virus to replicate rapidly and orthopoxviruses need a more comprehensive strategy to survive within the host. The larger size of the orthopoxviruses alerts the immune system of the individual very early on and thus, generates an immune response very easily. To be able to evade the host immune system, orthopoxviruses are equipped with a set of molecules (proteins) encoded by virulence genes that will act as modulators by being directed against components of the host’s immune system. Without the presence of these proteins, Orthopoxviruses such as monkeypox would be unable to evade the immune system.
Although poxviruses have ways of tricking the immune system, they don’t change their surface proteins to escape immunity, as SARS-CoV-2 does. An infection with smallpox, if you survived it, provided immunity for life, and the vaccines remained very effective right until the end of the eradication campaign. That offers some hope that monkeypox won’t transform into a bigger threat.
_
-14. IgM antibodies typically dominate in primary immune responses, whereas IgG antibodies dominate in secondary immune responses. In a cohort of 200 patients infected with MPXV who were recruited between March 2007 and August 2011 in the DRC, individuals with both IgM and IgG responses were 5.09 times more likely to develop severe lesions compared with individuals who had IgG-only responses. Similarly, in a cohort of infected individuals from the 2003 MPXV outbreak in the United States, patients with moderate/severe disease had an overall higher titer of anti-orthopoxvirus IgM compared with those with mild disease, and anti-orthopoxvirus IgG responses were much reduced and less frequent in patients with moderate/severe disease. It is plausible that an IgG-only response reflects robust levels of cross-protective IgG+ memory B cells that produce a dominant secondary antibody response, whereas the lack of such memory necessitates an IgM-dominated primary response that is less effective at preventing disease. Thus, IgM responses may be a biomarker for disease severity.
_
-15. Simian immunodeficiency virus (SIV)-infected macaques with CD4+ T cell counts <300 cells mm^3 were not able to produce VACV-specific IgG following vaccination and died when challenged with MPXV. This observation is of high concern to both VACV-vaccinated and unvaccinated individuals with uncontrolled HIV-1 infection, as their CD4+ T cell counts are typically very low. This group of individuals is therefore at high risk of developing severe MPXV infection if exposed. They might also experience a more complicated pathology and provide the virus with an opportunity to acquire mutations that result in higher virulence or transmission potential.
_
-16. The interferon response is known to be stronger in children and has been shown to protect against severe SARS-CoV-2 and RSV infections, but the reverse was observed in children infected with MPXV. A study conducted in Zaire during 1980–1985 reported a higher incidence of fatal disease in young children infected with MPXV, with a case fatality rate of 14.9% in children aged between 0 and 4 years compared with a rate of 0% in individuals aged 10 years or older.
_
-17. MPXV is known to have a wide-reaching host tropism and can infect many different species. This generality also translates into its cell and tissue tropisms; the virus has been found to infect tissues ranging from the heart and brain to the ovaries and lymphoid tissue. Tropism in which the virus replicates in one cell type but not another is known as cellular tropism. Poxvirus tropism at the cellular level seems to be regulated by intracellular events downstream of virus binding and entry, rather than at the level of specific host receptors as is the case for many other viruses. Several routes are involved in the transmission of MPXV since MPXV does not express cell-specific receptors that facilitate cellular tropism. The cellular tropism of HIV is largely determined by the cell surface receptors it uses for binding and entry. HIV infects and eventually destroys T-helper lymphocytes but not T-killer lymphocytes, because the T-helper cells express CD4 whereas the cytotoxic T-cells express CD8.
_
-18. Monkeypox (MPX) does not spread easily between people. Human monkeypox has a secondary attack rate of 8 to 9% among unvaccinated contacts within households while smallpox has a secondary attack rate of 58.4% in unvaccinated close or household contacts, so monkeypox is much less transmissible than smallpox. Researchers have estimated the reproduction number (R0) of the current monkeypox outbreak to be 1.29 by aggregating all cases in 70 countries as of July 22, 2022.
_
-19. Since May 2022, the largest west-African-clade-monkeypox outbreak to date in countries with non-endemic occurrences has been described. The outbreak involves transmission among people in close physical contact with symptomatic cases, in contrast to previous outbreaks, where zoonotic transmission was reported as the main mechanism of spread.
Monkeypox virus is believed to have several modes of transmission, all of which are associated with direct contact with infected animals or the with infected humans, Animal to human transmission is direct contact or exposure with infected animals and most commonly, due to bodily fluids such as saliva, respiratory excretions, or could be the exudate from cutaneous or mucosal lesions. Viral shedding via feces may represent another exposure source. In areas of scarce resources, such as food, households are left with no choice but to hunt and cook small mammals, increasing their risk of exposure to monkeypox.
Although human-to-human transmission is less common than animal-to-human, it usually involves respiratory droplets with prolonged face-to-face contact or contact with lesions of an infected individual. Contaminated objects/surfaces, such as sleeping on the same bedding, living in the same household, or eating or drinking from the same dishes as an infected individual, are deemed a risk factor for viral transmission among individuals of the same household.
Amid the current, ongoing monkeypox epidemic, it has also been observed that the disease is most common in men who have sex with men (MSM). The virus may have made its way into highly interconnected sexual networks within the MSM community, where it can spread in ways that it cannot in the general population. The virus did not spread well between people in the past but may have found a new niche in tightly connected sexual networks.
WHO reports that among cases with proper data, 97% have been diagnosed in gay, bisexual and other men who have sex with men. Sex between men, not mere skin contact, is fueling monkeypox. A growing body of evidence supports that sexual transmission, particularly through seminal fluids, is occurring with the current MPX outbreak. Researchers have found monkeypox virus in semen and have been able to culture that virus, which suggests it could transmit through ejaculation. The isolation of live replication-competent monkeypox virus from semen, and prolonged viral DNA shedding, even at low viral copies, might hint at a possible genital reservoir. Researchers have detected monkeypox virus in the testes of macaques during the acute phase of infection for up to 37 days during convalescence although virus was cleared from most organs—and from healed skin lesions.
The detection of MPXV at high concentrations in the anal region, in the mouth, and in semen is consistent with the sexual practices potentially involved in the spread of the virus among men who have sex with men, in addition to skin contact related to sexual or non-sexual proximity.
The rectal mucosa immune environment in MSM is significantly different compared with individuals who are heterosexual, with a higher presence of immune activities indicative of mucosal injuries. This condition could lead to the recruitment of immune cells, which could then be easily targeted by infectious agents, such as MPXV. This could lead to MPXV infection and transmission in MSM.
Monkeypox lesions have in the majority of cases occurred in men’s genital, oral and anorectal areas. This suggests that these were the sites where the virus first passed into the body. Monkeypox is more likely to transmit through oral or anal sex than through contact with external skin, which would need some sort of defect, such as a wound, to allow entry of the virus. Reports of clusters associated with sex parties or saunas further underscore the potential role of sexual contact as a promoter of transmission. The prolonged contact that happens during sex is likely exposing people to much larger doses of the virus than animal exposure, household, fomite, or respiratory droplet transmission seen in western and central Africa in previous decades. International travel and attendance at large gatherings linked to sex-on-site activities may explain the global spread of monkeypox infections amplified through sexual networks.
The virus is also apparently following the same transmission patterns traditionally seen in Africa. But just as in those African nations, where the virus transmits through nonsexual means, it does so with dramatically lower efficiency — and thus at a rate similar to the relatively slow spread seen in Africa. Thus in the current 2022 outbreak, only 0.8% to 3 % of the cases are due to nonsexual close contact and by contrast, more than 95% of cases are likely acquired during sex between men. Close contacts of the currently reported MPX cases include mainly sexual partners and people living in the same household or anyone sharing the same bedding or clothing with an MPX case. Sharing the same workspace for several hours seated within one to two meters or being a co-passenger in longer flights, train or bus rides may also qualify as a close contact in certain situations. Although some of the reported cases are epidemiologically linked, no further onward transmission to close contacts that are not sexual partners has been documented yet in this outbreak. So monkeypox is indeed overwhelmingly transmitted through anal and oral intercourse between men in the current 2022 outbreak.
Although predominant transmission is in MSMs in America and Europe, the same is not seen in Africa in this 2022 outbreak. MSMs do not seem to be driving new infections in the endemic regions of west and central Africa. Why? May be because homosexual relations are criminalized in 32 countries in Africa, so people are not coming forward. If you have better explanation, please send it to me. I will publish it with your name below this article.
_
-20. Recent reports of monkeypox transmission from humans to pets (dogs) are currently being investigated. Since many species of animals are known to be susceptible to the monkeypox virus, there is the potential for spillback of the virus from humans to susceptible animal species which could lead to the formation of novel animal reservoirs. Such a spill-over event could potentially lead to the virus establishing in wildlife and the disease becoming an endemic zoonosis. The virus is already established in an animal reservoir in some parts of Africa, making its eradication a difficult task. One fear is that the virus could spread from humans to animals elsewhere in the world, establishing further reservoirs from which humans could be infected repeatedly. The longer it takes to contain the virus, the higher the odds that it will find a permanent new home in animals. However, the probability of this spill-over event is very low. People who have confirmed or suspected monkeypox should avoid close physical contact with animals, including pets (such as cats, dogs, hamsters, gerbils etc.), livestock and wildlife.
_
-21. As DNA is an environmentally stable molecule, detection of viral DNA by PCR cannot be equated with infectious virus. Unless you are able to grow virus in cultures from environmental samples, mere detection of DNA by PCR in environmental samples do not prove that infection can occur from contact with these surfaces or air. Infection-competent virus means viral particles capable of infecting a cell and resulting in the production of new viral particles. Detection of virus, even replication-competent virus, in environmental samples does not mean that transmission leading to infection would necessarily occur if someone were exposed to that virus, as there are many factors that can influence successful infection of a human. These factors include routes of transmission, host susceptibility, environmental factors that could weaken the virus’ ability to infect cells and replicate, and the amount of virus to which one is exposed.
One study did find monkeypox virus (DNA and virus by isolation) in environmental air samples from health-care settings where air sample was collected during a bedding change; and detection of monkeypox virus DNA in air samples collected at distances of greater than 1·5 m from the patient’s bed and at a height of about 2 m supports the theory that monkeypox virus can be present in either aerosols or suspended skin particles or dust containing virus, and not only in large respiratory droplets that fall to the ground within 1–1·5 m of an infected individual.
The epidemiologic data we have so far in this 2022 outbreak support that people are not contracting monkeypox through touching contaminated surfaces or breathing aerosols. The preponderance of data indicates it’s being transmitted through direct physical contact, whether sexual or non-sexual.
In the current 2022 outbreak, the vast majority of monkeypox cases – more than 95 per cent, are among men who have sex with men, with a median age of 36. If monkeypox was airborne, these infected MSMs would have transmitted it to their non-sexual contacts but it hasn’t happened. Every infected MSM has many non-sexual contacts including family members and co-workers but they haven’t got it. So airborne transmission of monkeypox is unlikely. Even previous outbreaks in Africa haven’t shown airborne spread. The inoculum dose and host susceptibility for a particular transmission mode affect the onward transmission of all viruses. Previous studies suggested that pathogens capable of aerosol transmission should be associated with a high reproductive number (R0) except pertussis that transmits via droplets has a much higher R0 than tuberculosis pathogen transmitted via aerosols. Despite vast susceptible population available unvaccinated by smallpox vaccines, the R0 of monkeypox is between 1 to 2 and therefore highly unlikely to be transmitted by air. In fact, higher R0 is seen due to sexual transmission in MSM community while R0 was less than 1 in endemic Africa due to droplet/contact transmission. There are no definite data on the required infectious dose with monkeypox virus in humans. However, in contrast to variola virus, a significantly higher dose is assumed to be required to trigger infection. The infectious dose of variola (small pox) is 10 to 100 virions and smallpox was airborne in addition to droplet/contact transmission. In non-human primates, monkeypox infection could be initiated by intrabronchial application of 5×104 plaque-forming units (PFU). Despite similarities between smallpox and monkeypox transmission, such high dose inoculum compared to variola makes monkeypox unsuitable for airborne transmission. Remember, infectious dose of tuberculosis bacteria is reported to be between 1 and 200 bacilli and a single aerosol can contain anywhere from 1 to 400 bacilli.
All in all, in my view, monkeypox is not airborne.
_
-22. Monkeypox virus can be transmitted to the fetus during pregnancy or to the newborn by close contact during and after birth. Monkeypox in pregnancy can also increase one’s risk of adverse events such as miscarriage or stillbirth. Pregnant women are highly prone to vertical MPXV transmission due to immunological vulnerability, waning of anti-smallpox immunity among women of reproductive age (15 to 49 years), and since orthopoxviruses can overcome the syncytiotrophoblast placental barrier. As of Sept 2, 2022, ten cases of monkeypox in pregnant women have been reported worldwide, but vertical transmission did not occur; the neonate received prophylactic vaccinia immunoglobulin and did not develop monkeypox disease. So far in the current outbreak of 2022, none of the monkeypox infections reported in pregnant women have been severe, and there has been no evidence that pregnant women have more severe disease or worse outcomes than non-pregnant people.
_
-23. Based on the evidence from the cases in the current 2022 outbreak detected to date, overall, the probability of further spread of MPXV among the broader population is very low leading to an overall low risk for the general population. While anyone can get monkeypox, the men who have sex with men (MSM) community and people who have sex with MSM are at higher risk. Having multiple and frequent sexual contacts, including with anonymous partners, may put MSMs more at risk of infection of monkeypox. The risk to HCWs with unprotected close contact with MPX cases (e.g. contact face-to-face for prolonged time, contact with open lesions without gloves, intubation or other invasive medical procedure) is assessed as moderate, equivalent to that of a close contact. The risk for unprotected laboratory personnel is assessed as high. Newborn infants, young children and people with underlying immune deficiencies may be at higher risk of more serious symptoms, and in rare cases, death from monkeypox. People who were vaccinated against smallpox may have some protection against monkeypox.
_
-24. The route of infection can influence monkeypox illness manifestations. The route of infection (invasive, such as animal bite) plays a role, with invasive modes of exposure causing more severe disease and shorter incubation period.
_
-25. The clinical presentation of monkeypox is similar to that of smallpox in terms of symptom onset, timing of rash occurrence, and rash distribution, but generally less severe than smallpox in terms of complication rate, case fatality rate, and levels of scarification. The incubation period (interval from infection to onset of symptoms) of monkeypox is usually from 6 to 13 days but can range from 5 to 21 days. A report from the 2022 outbreak found the mean incubation period was 8.5 days. The “classic presentation” of monkeypox involves prodromal symptoms — fever, malaise, headache, and swollen lymph nodes — which precede the emergence of lesions but in the current 2022 outbreak patients are seeing lesions without any prodromal symptoms, and in some cases these symptoms develop after the rash develops.
Some people who contract monkeypox develop lesions over a variety of body parts — the torso, the face, the soles of feet, the palms of hands, and especially in this 2022 outbreak, which is occurring mostly in gay and bisexual men who have sex with men, in the anogenital area. Some of the men infected in this outbreak have had their lesions situated almost exclusively on their penises or in or around their anuses. The predilection of lesions to genital, perianal, and perioral or tonsillar areas, and the history of recent sexual contact in 96% of cohort suggests lesions may initially form at the site of inoculation, followed by the development of systemic symptoms and subsequent dissemination of lesions. Some, perplexingly, have had only a few lesions. Some diagnosed cases have had a single lesion. Some of the participants, such as those with solitary lesions, did not develop further dissemination. A single lesion or sore in their mouth or on their genitals could lead to clinicians to misdiagnose monkeypox as another sexually transmitted infection (STI). such as syphilis, lymphogranuloma venereum, and ingrown hair follicles. Throat features included ulcers, pain, secondary bacterial superinfection, and quinsy, which could all be mistaken for bacterial tonsillitis.
Vaccinated individuals were noted to have fewer lesions, smaller lesions, and better presentation of regional monomorphism and centrifugal distribution of rash.
_
-26. A monkeypox patient is infectious from the onset of prodromal symptoms and throughout rashes period till all skin lesions have scabbed over and re-epithelialization has occurred. The dermal lesions present a higher infectivity risk compared to oropharyngeal and rectal lesions. The infectious period normally last for around two to four weeks. Cases should remain isolated until their rash heals completely, avoiding contact with children, immunosuppressed persons and pets. Abstaining from sexual activity and close physical contact is also advised until the rash heals. Most cases can remain at home with supportive care.
_
-27. Close contacts of MPX cases should self-monitor for the development of symptoms up to 21 days from the last exposure to a case. Close contacts of a MPX case should be deferred from blood, organ or bone marrow donations for a minimum of 21 days from the last day of exposure.
_
-28. It is thought that monkeypox is a one-and-done infection, that people who survive — as most people do — have life-long immunity. They cannot be reinfected and pose no transmission risk after they recover. But that calculus changes if survivors have monkeypox virus hiding in parts of their bodies. Occasionally, monkeypox virus may persist in the body for 10 weeks, even after the tell-tale rash has disappeared. So rarely, relapse is possible and whether persistent virus is transmissible or not is unclear at present.
_
-29. Many people infected with monkeypox virus have a mild, self-limiting disease course with resolution occurs in 2-4 weeks in the absence of specific therapy. However, the prognosis for monkeypox depends on multiple factors, such as previous vaccination status, age, immune status, route of infection, dose of inoculum, concurrent illnesses, and comorbidities among others. During the 2022 global outbreak, few hospitalizations have been reported and most were for the purpose of isolating the patient. Other reasons for hospitalization included provision of adequate pain management and the need to treat secondary infections.
_
-30. Most people infected with monkeypox virus are symptomatic, but subclinical infection can occur. Sero-epidemiological studies in Africa suggest some patients may have subclinical or asymptomatic monkeypox infection. Serologic studies of household contacts of acutely infected cases in the Democratic Republic of Congo suggest that approximately 28% of all monkeypox infections are subclinical. More recently, immunologic evidence of exposure to monkeypox virus was identified in several asymptomatic contacts of infected people in the USA. Some studies found that not all the patients present with symptoms in the current 2022 outbreak. We know that asymptomatic monkeypox infections occur infrequently but we need more studies to determine whether asymptomatic transmission, including sexual transmission from asymptomatic carriers occurs or not. If asymptomatic transmission can occur, then post-exposure ring vaccination strategies might not be sufficient to contain further spread of the virus. (Ring vaccination involves immunizing individuals who have been exposed to monkeypox through close contact with an infected person. The idea is to catch people early enough that their immune systems can nip a potential infection in the bud before they become contagious.)
_
-31. The overall case fatality rate (CFR) in the current ongoing 2022 multi-country outbreak is approximately 0.03% as of 18 August 2022 (0.013% in countries that have not reported monkeypox previously, and 2% in Africa). The children and people with immunodeficiency problems are at risk for more severe cases. In previous outbreaks in Africa, most reported deaths have occurred in younger children and immunocompromised people (e.g., poorly controlled HIV infection) and the case fatality ratio was around 3–6%. Patients with fatal disease had higher viral loads in their blood, maximum skin lesion count, and elevated transaminases.
CFR is low in current 2022 outbreak because 95% patients are MSMs with median age of 36 years and although 40% of them have HIV, it is well treated resulting in high CD4 counts. A monkeypox outbreak studied in Nigeria in 2017–18 resulted in seven deaths among 118 confirmed cases (6%), of whom four were HIV-positive with poor disease control.
Although the case-fatality rate of the pathogen is low, its relative environmental stability & persistence and transmission pathways, in addition to the lack of immunity in the population, the limited availability of effective treatments and vaccination, make it an agent which could represent a biological threat in case of accidental spill or intentional release.
_
-32. More than 90% of monkeypox survivors have no complications. Disease severity can be classified using the lesion count, as higher lesion counts correlate with increased risk of severe complications. Patients with severe complications may experience respiratory and gastrointestinal issues, encephalitis, septicemia and ocular infections leading to permanent vision loss. Skin lesions also increase the likelihood of dermal bacterial infections, especially in patients who are not vaccinated against smallpox. Major disease sequelae are usually disfiguring scars and permanent corneal lesions.
_
-33. Differential diagnosis of monkeypox range from chickenpox, molluscum contagiosum, measles, rickettsial infections, herpes simplex virus, enterovirus, parapoxviruses (causing orf and related conditions), chancroid, herpes zoster, lymphogranuloma venereum, granuloma inguinale, bacterial skin infections (such as those caused by Staphylococcus aureus), anthrax, scabies, syphilis, and drug reactions to other noninfectious causes of rash.
Due to similarities in the presentations of the monkeypox rash and some STDs, misdiagnosis may be more common in the MSM population than in the general population. It was reported that some cases were misdiagnosed as herpes simplex virus (HSV) or varicella-zoster virus (VZV) infection in this 2022 outbreak, resulting in late detection and management, thus increasing the risk of community transmission.
_
-34. In several outbreaks, it has been difficult to distinguish monkeypox from chickenpox. As enlarged lymph nodes are not a common sign of chickenpox and are seen in 90% of monkeypox patients, they are considered a distinctive hallmark of monkeypox, especially the submental, submandibular, cervical, and inguinal nodes. In addition, unlike varicella where vesicular lesions are characteristically in different stages of development and healing, monkeypox lesions are generally at the same stage. However, during the global monkeypox outbreak that started in May 2022, some reports describe lesions that were in different stages of development. It will take two to four weeks for monkeypox lesions to resolve completely, whereas chickenpox lesions resolve much more quickly. Chickenpox creates superficial, irregular border, very thin-walled, fragile blister that’s usually filled with clear fluid but monkeypox causes deep-seated, well-circumscribed, umbilicated, firm, or rubbery kind of lesion—it’s much more firm and stable than the chickenpox lesion. Chickenpox lesions can break easily, whereas monkeypox lesions do not. The presence of lymphadenopathy, pre-eruptive fever and slower maturation of skin lesions are the most important clinical signs supporting diagnosis of monkeypox.
_
-35. The monkeypox and chickenpox co-infection was seen in 12% of individuals in a study in DRC, and that is an epidemiologic observation worth highlighting. Coinfections were more likely to report symptoms than VZV-alone cases and less likely than MPX-alone cases. Significantly higher lesion counts were observed for coinfection cases than for VZV-alone but less than MPX-alone cases. Co-infection carries important implications for susceptible groups because both viruses carry a risk of vertical transmission. Given that co-infection also modifies the severity of the skin rash, delayed diagnoses and treatment could result in worse perinatal outcomes, particularly in resource-limited settings.
_
-36. Does past vaccination against chickenpox or history of chickenpox illness provide any protection against monkeypox?
No.
Chickenpox is caused by a different virus, the varicella zoster virus from Herpesviridae family. Past vaccination against chickenpox or having suffered from chickenpox illness does not provide any protection against monkeypox as monkeypox virus is an orthopoxvirus from Poxviridae family. The only thing common between chickenpox and monkeypox is the term POX meaning contagious disease causing vesicular/pustular skin rash.
_
-37. Containment strategy for current 2022 monkeypox outbreak include contact tracing and surveillance, isolation of symptomatic cases, ring vaccination, vaccination of MSM community, testing and educational messaging to MSMs and change in behaviour of MSM community. Men who have sex with men are at the highest risk of infection right now from monkeypox. Reducing the number of one-time or casual sex partners among gay, bisexual, and other men who have sex with men can significantly reduce the transmission of the monkeypox virus and control monkeypox outbreaks in non-endemic countries. Condoms can mitigate the risk of many sexually-transmitted infections, but cannot offer full protection against transmission of MPXV, since any physical contact with lesions may be sufficient for transmission to occur.
_
-38. Can current smallpox vaccine prevent monkeypox?
Vaccines based on the vaccinia virus (VACV), which were initially developed against smallpox, can be used to prevent and control monkeypox. Three prominent VACV-based vaccines are available, including first, second, and third-generation vaccines. The use of first-generation vaccines such as Dryvax is not recommended against MPXV due to safety concerns. However, second-generation vaccines such as ACAM2000 are relatively safer compared to first-generation vaccines and can be used against monkeypox. In immunocompromised patients, first and second generations smallpox vaccines are contraindicated. Currently, one third-generation vaccine, Bavarian Nordic’s modified vaccinia virus Ankara (MVA-BN), is recommended by the US Centers for Disease Control and Prevention (CDC) as well as WHO, primarily for high-risk groups. MVA-BN is also a live VACV-based vaccine but cannot replicate in humans and therefore is safer than the previous generation vaccines. Data on effectiveness in clinical use are limited. Given the mixed evidence to date, experts caution that vaccinated people may still be at risk, and more research is needed before relying on vaccination alone to curb the monkeypox outbreak. While the MVA-BN vaccine certainly provides some immunity, it does not prevent infection entirely, especially after just one dose. Because local health departments are challenged with limited MVA-BN availability, many have implemented a single-dose strategy to maximize current supply; further strategies to deliver lower-volume doses intradermally have also been adopted. The MVA vaccine is used before exposure and even after exposure up to 4 days. Between 4 to 14 days postexposure, it cannot prevent disease but it can reduce severity. Until there’s more data, the most important thing that people should know is that the vaccine, even two doses, does not provide 100% protection. It’s an important part of a combined strategy for lowering the risk of infection, but it’s not the only thing people should be doing. Since the probability of further spread of MPXV among the broader population is very low and since availability of MVA vaccine is limited, vaccines are not recommended for general public at present.
_
-39. Optimal clinical specimens for laboratory analyses include specimens from skin lesions such as swabs of vesicular lesions, exudate, or crusts stored in a dry, sterile tube (no viral transport media) and kept cold. Viral DNA present in lesion material is stable for a long period of time if kept in a relatively dark, cool environment, an important factor to consider when cold chain is not readily available. Rapid diagnosis is made via a positive real-time polymerase chain reaction (RTPCR) on swabs from skin lesions. Viremia is transient, limiting the utility of blood tests. Virus isolation and culture, immunochemistry, and immunofluorescence imaging are also potentially useful to confirm the diagnosis. Specific MPXV antibodies may be detected by enzyme-linked immunosorbent assays (ELISA) after 5 (IgM) and 8 (IgG) days of infection onset, respectively. However, cross-reactivity makes this test less useful as a specific tool for MPX diagnosis. As orthopoxviruses are serologically cross-reactive, antigen and antibody detection methods do not provide monkeypox-specific confirmation. Additionally, recent or remote vaccination with a vaccinia-based vaccine (e.g. anyone vaccinated before smallpox eradication, or more recently vaccinated due to higher risk such as orthopoxvirus laboratory personnel) might lead to false positive results.
The golden test to establish the diagnose is the polymerase chain reaction due its high accuracy and sensitivity, Molecular diagnostics for human MPX are currently limited to real-time quantitative polymerase chain reaction (qPCR) assays in specialized laboratory settings. Typically, you should receive your test result within 48 hours. Blood viremia after MPXV infection is often time dependent and unreliable for acute diagnosis, so crust and vesicular swab specimens from skin lesions are preferable. You may also take swab from oropharyngeal and rectal lesions if no skin lesion available. Remember, skin lesion samples have a higher viral load as compared to oropharynx and rectal lesions.
_
-40. A study found that total 5% of the 528 persons received monkeypox-specific treatment. The drugs administered included intravenous or topical cidofovir (in 2% of persons), tecovirimat (2%), and vaccinia immune globulin (<1%). The majority of MPX cases reported so far in this 2022 outbreak have been mild with localised disease and self-limiting symptoms, requiring no antiviral therapy.
_
-41. Tecovirimat is an antiviral that inhibits p37, a protein involved in release of enveloped virus, dissemination, and viral virulence. In vitro testing has shown activity against both smallpox and monkeypox, and tecovirimat appears to have a favorable clinical safety profile based on the experience of healthy volunteers. Because smallpox, monkeypox and cowpox are either eradicated (smallpox) or occur sporadically, studies to assess the effectiveness of tecovirimat in infected people could not be carried out. The effectiveness of Tecovirimat was therefore evaluated based on studies in animals infected with lethal doses of orthopoxviruses, on studies on the medicine’s effects in the human body, and on the way the medicine is absorbed, modified and removed from the body in humans and animals (pharmacodynamics and pharmacokinetics studies). There is no RCT done on efficacy of tecovirimat in humans infected with monkeypox. Compassionate use without control found complete resolution of lesions in 40% on day 7 of therapy, while 92% had resolution of lesions and pain by day 21. RCTs are currently underway in the United States, United Kingdom and Democratic Republic of Congo to assess use of tecovirimat for treatment of human Monkeypox. Although the early data on tecovirimat are promising for severe disease, only a randomized controlled trial will provide the level of evidence we need to treat patients with confidence.
_
-42. Poxviruses show extraordinary resistance to drying, and increased temperature and pH tolerance when compared with other enveloped viruses. The monkeypox virus can survive on surfaces for 15 days or more, particularly in a dark, cool, and dry environment. MPXV can survive on surfaces for a long time because it has a protective outer layer known as the envelope. Despite having an outer envelope, the virus is sensitive to common household disinfectants like Lysol and Clorox. Washing your hands with soap and water should be enough to kill the monkeypox virus, but sanitizers that contain at least 60% alcohol are the most ideal for proper hand hygiene. Using hot water and regular detergent is enough to kill the virus on linens and clothing. Always wear gloves and wash your hands properly before and after handling contaminated items.
_
-43. Considering monkeypox as sexually transmitted disease (STD) seems logical in order to face the current 2022 outbreak, but the stigma and discrimination this could cause is a major problem. An infection acquired through sex is still something that causes guilt and fear of rejection by society. STDs are still viewed by many as a punishment for certain behaviours. So infected people may not come forward for diagnosis, treatment, isolation and contact tracing. Additionally, classifying monkeypox as an STD may create a false sense of security for people who may think they’re not at risk. When a disease is defined as a sexually transmitted infection that mainly affects men who have sex with men, many people may begin to think of it as “a gay disease” that poses no risk to them. That’s what happened in the early days of the AIDS epidemic in the 1980s, which contributed to the spread of HIV to other groups. Both stigma and a low perception of risk can hinder efforts for early identification of cases, rapid isolation and limitation of the outbreak. Worst of all, stigma related to this outbreak would perpetuate harms to the LGBT community. Instead of sexually transmitted disease, Monkeypox can be described more accurately as “sexually transmissible,” as sex is one of the ways the virus can spread — but not the only way. Monkeypox can be spread in many different ways, and sexual transmission is one of them. However, one cannot overlook the fact that 95% cases in current 2022 monkeypox outbreak are among MSMs.
_
-44. Media reporting and commentary on Monkeypox has used language and imagery, particularly portrayals of LGBT and African people, that reinforce homophobic and racist stereotypes and exacerbate stigma & discrimination. Lessons from the AIDS response show that stigma and blame directed at certain groups of people can rapidly undermine outbreak response.
_
-45. The current 2022 monkeypox outbreak is overwhelmingly concentrated among men who have sex with men. And a growing body of evidence and data suggests that sex among these men is the primary means through which monkeypox is presently spreading. While it’s true that there are other ways the virus can be transmitted, recognizing and reporting these facts is not anti-gay or anti-science, and neither is targeting advice to members of this community given they are the ones who are presently most at risk. While well-meaning people may want the media or public health authorities not to focus on the LGBT community for fear of spreading stigma, allowing the virus to spread may cause much greater harm. Linking monkeypox to sexual transmission would equip gay and bisexual men with the health information they need to protect themselves. Frank and positive messaging on sex is the first step to curbing spread and protecting the most vulnerable. We have to choose between truth, control of disease and little stigma on one hand; and lies, spread of disease and appeasement on the other hand. I would choose the former.
_
-46. After weeks of rising cases, the monkeypox outbreak appears to be significantly slowing down in the United States and Europe. There are two reasons why monkeypox infections are trending downward.
-1. First reason is that at-risk people (MSMs) have changed their behaviors. The outbreak has primarily been concentrated in men who have sex with men, a group that includes people who identify as gay, bisexual, transgender and nonbinary. A joint survey from the CDC, Emory University and Johns Hopkins University found about one-half of gay, bisexual, and other men who have sex with men reduced their number of sexual partners, one-time anonymous partners, and reduced use of dating apps.
-2. Second reason is vaccinations. As of Sept. 20, more than 684,000 JYNNEOS vaccine doses have been distributed in the U.S. To increase the number of doses available, the U.S. Food and Drug Administration authorized a new strategy to inject the vaccine intradermally, just below the first layer of skin, rather than subcutaneously, or under all the layers of skin — allowing one vial of vaccine to be administered as five separate doses rather than a single dose.
Almost six months after the virus started to spread, vaccination efforts and behavioural changes seem to be containing the current strain—at least in the United States and Europe. But the situation could still play out in several ways. At best, the outbreak might fizzle out over the next few months or years. At worst, the virus could become endemic outside Africa by reaching new animal reservoirs, making it nearly impossible to eradicate.
What would it take to eliminate monkeypox altogether?
In areas where animal-to-human transmission occurs, it will be impossible to eliminate the virus completely without a vaccine for people and (eventually) animals. Yet despite the risk that the virus might spread out of Africa again, African countries have not yet received any vaccines. That’s because wealthy nations have not yet donated any doses to countries that cannot afford them. Even if vaccines do arrive, behavioural changes will be needed to curb monkeypox. If we do not control monkeypox in endemic areas, then no matter the efforts put into non-endemic countries, we’re not going to achieve control.
_
-47. The scientific community is hesitant to the importance of monkeypox which can be demonstrated by a limited number of research articles in the biomedical literature. Monkeypox outbreaks are rarely reported, badly managed and little described leading to an incomplete picture of the disease’s importance. Literature on monkeypox transmission is severely lacking. PubMed search for “monkeypox AND transmission” yielded only 224 manuscripts, published from 1962 to 2022, and more reviews were published on the subject than original research studies in humans. A lack of high quality and comprehensive information on how to treat and care for monkeypox may be hindering clinicians’ ability to respond to the current global outbreak, according to a new study published in British Medical Journal Global Health, which found that existing guidelines are contradictory and lack key information. Until recently, monkeypox was rarely found in Europe and the Americas—a trend that has, historically, led Western public health officials to disregard its spread elsewhere. There’s not much interest in Western health groups about something that’s only circulating in Africa. Western countries have been criticized for not doing more to help the African nations that have animal reservoirs of the virus fight the pathogen, even as there were signs of more human-to-human transmission in recent years. Monkeypox is the next most pathogenic poxvirus disease after smallpox but never received appropriate attention to prevent it resulting in the current 2022 outbreak.
______
Dr. Rajiv Desai. MD.
October 18, 2022
_______
Postscript:
When I said Covid is airborne in June 2020, nobody believed me and the rest is history. Today I say Monkeypox is not airborne and let me see the response.
______

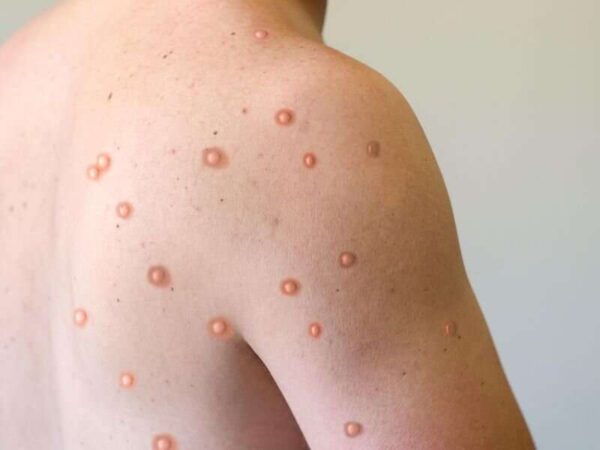
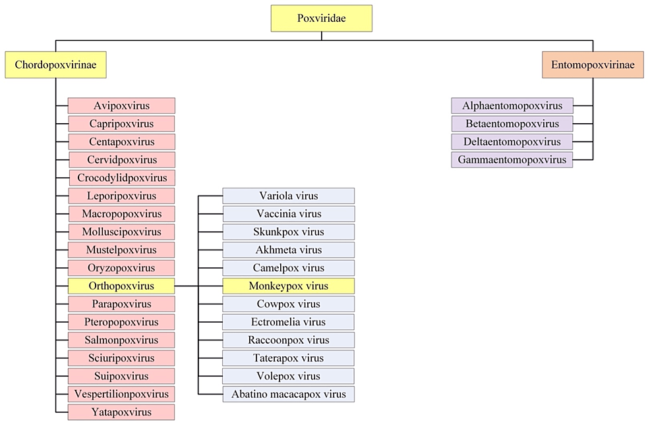

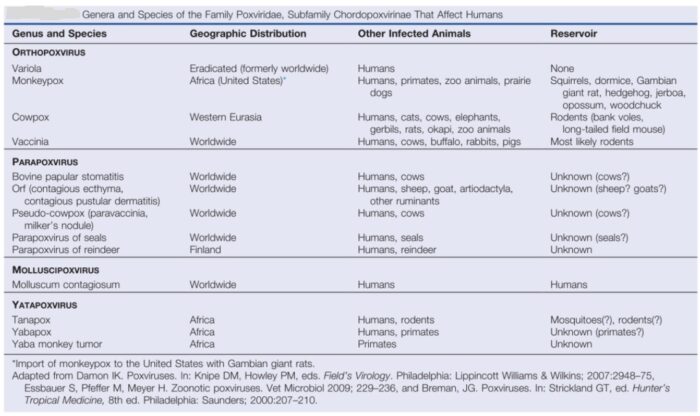
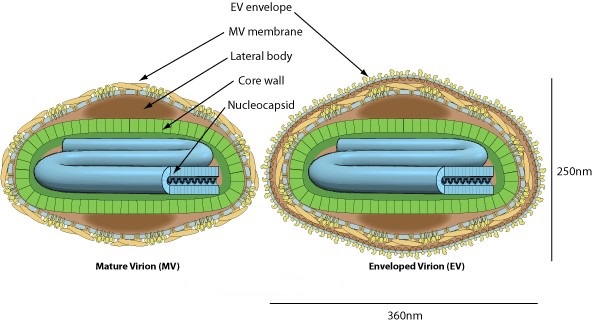
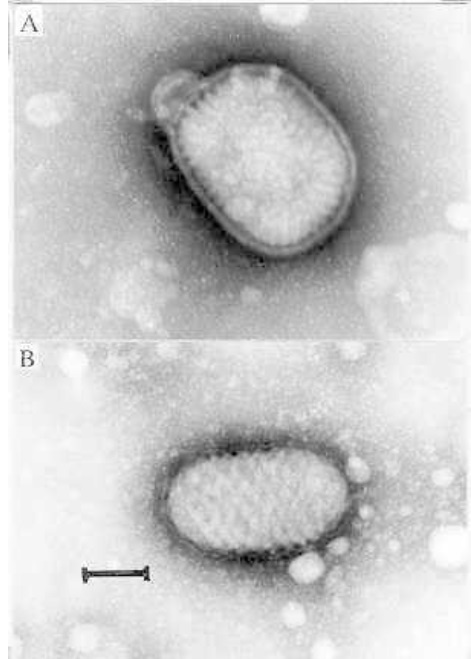
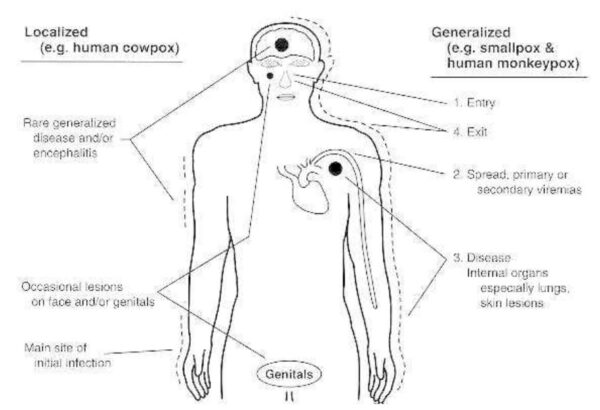
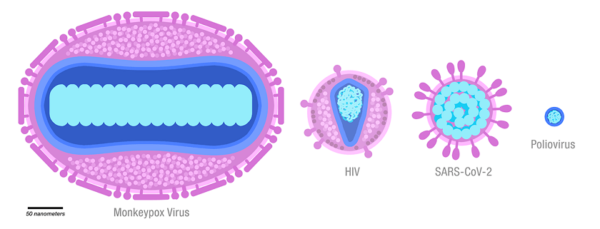
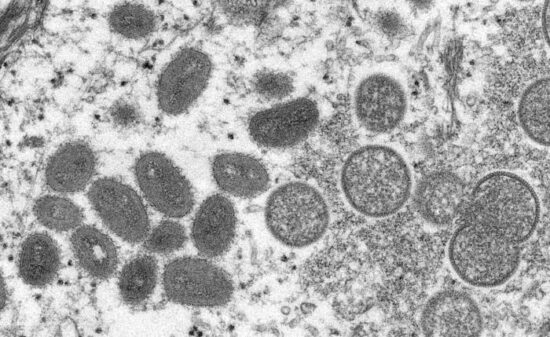
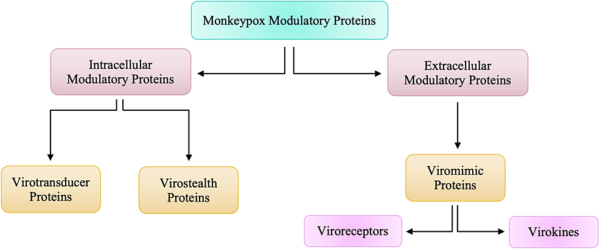
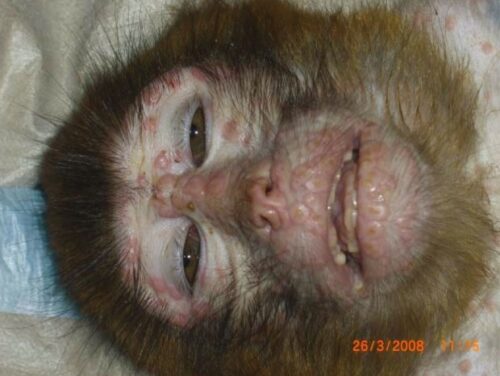
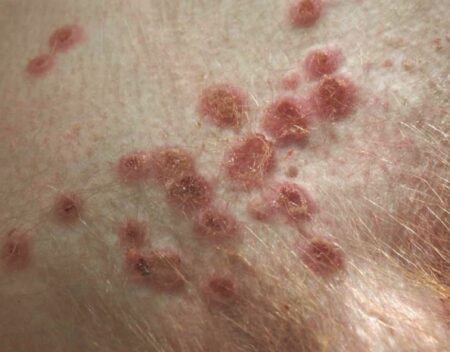

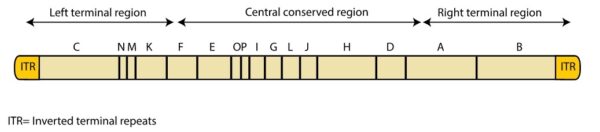
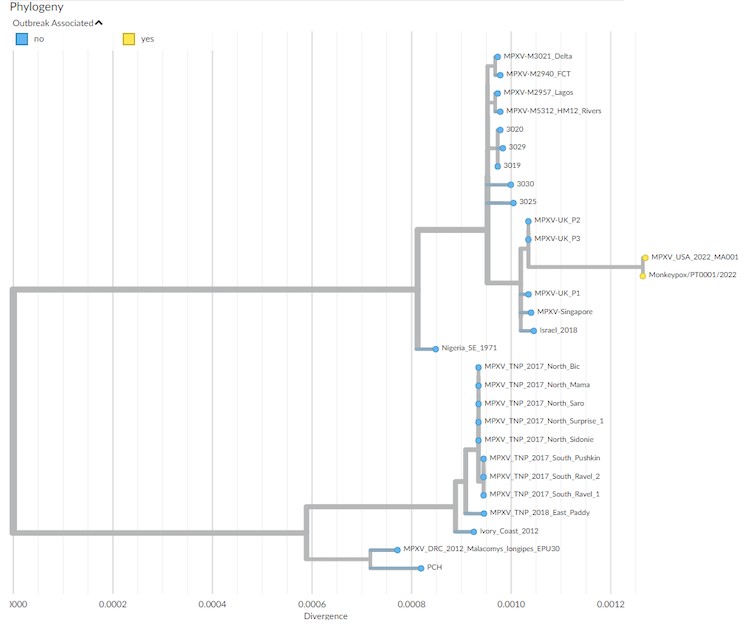
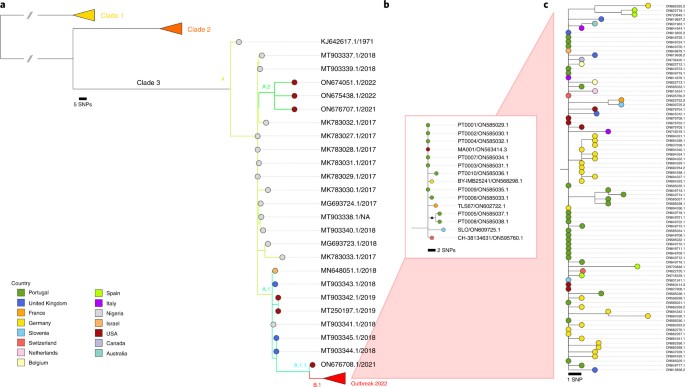
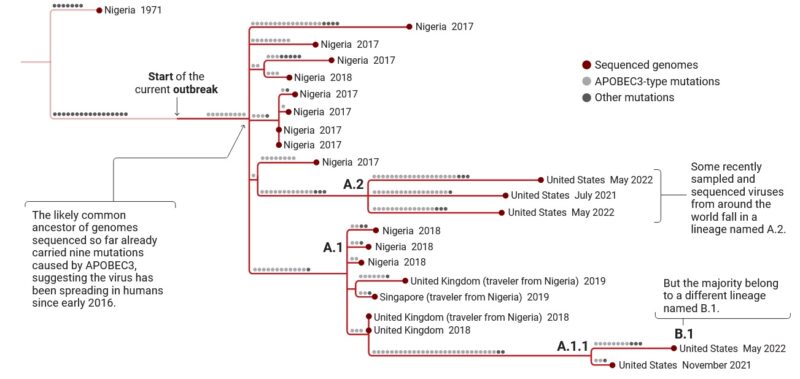
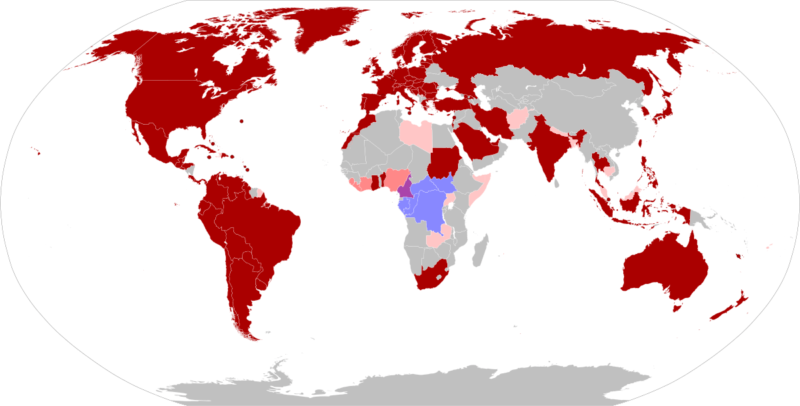

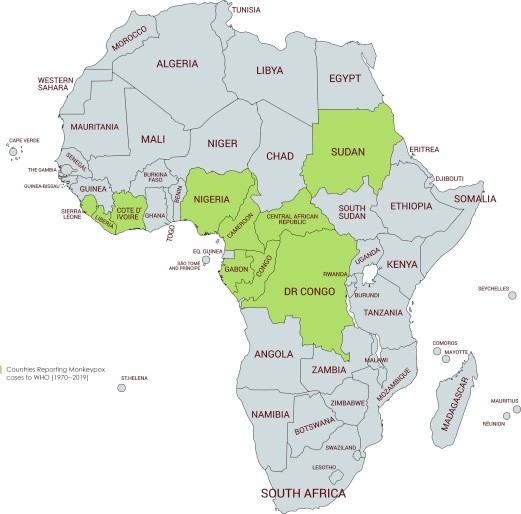
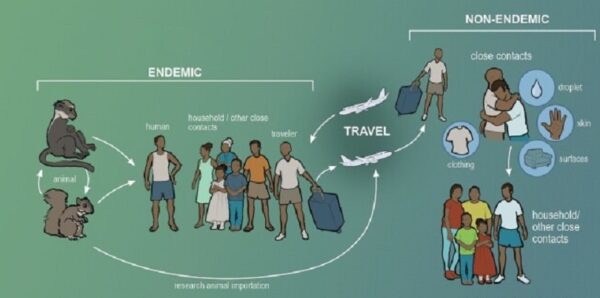
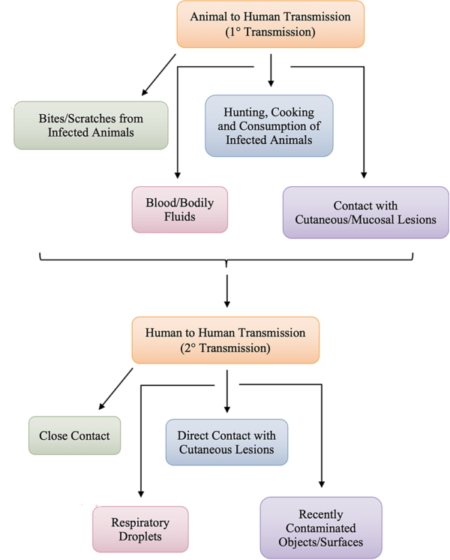
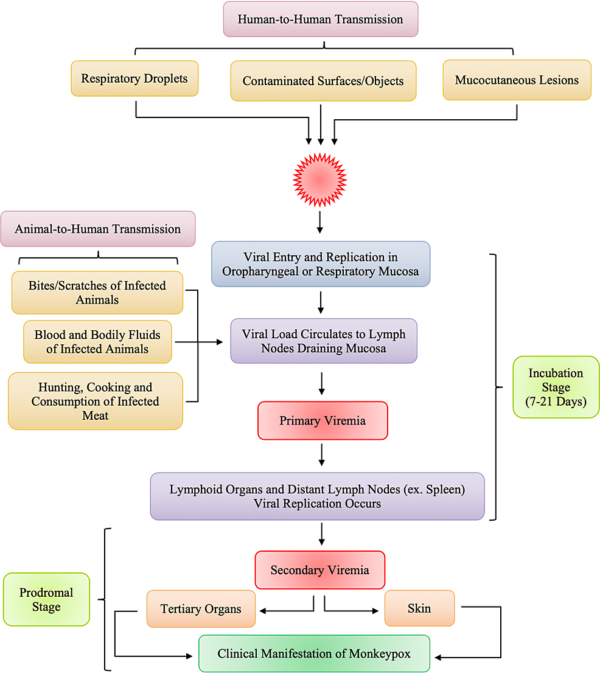
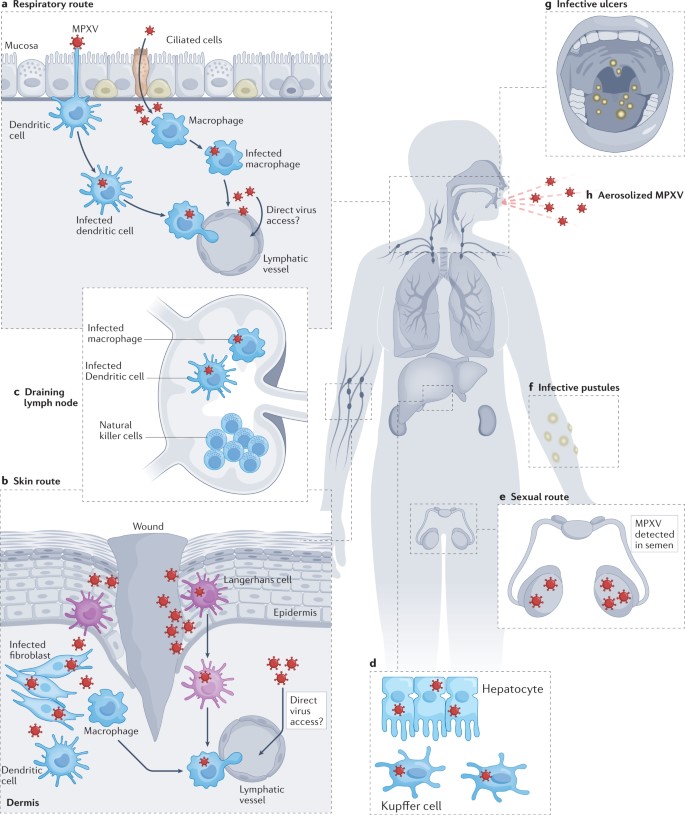
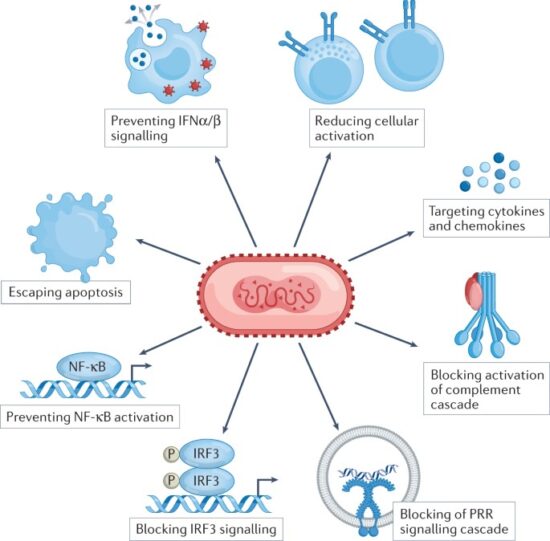
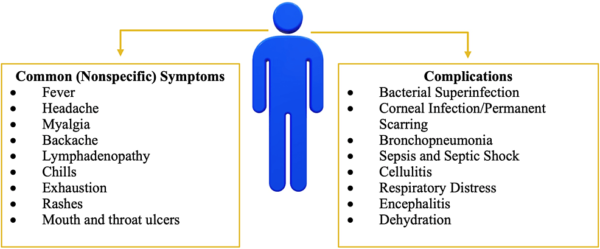
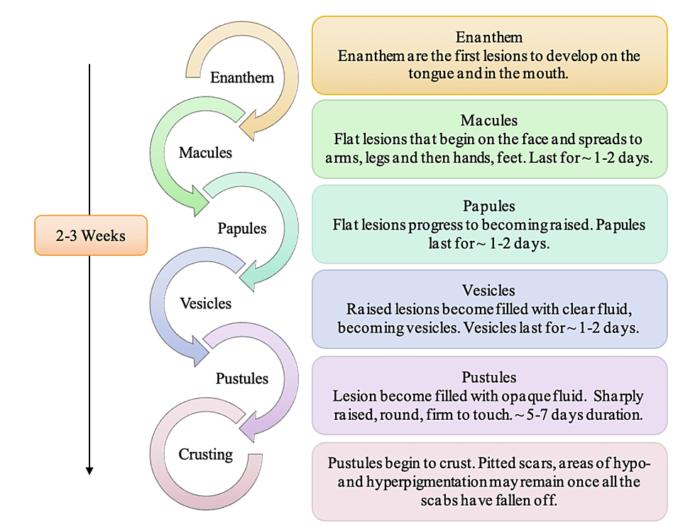
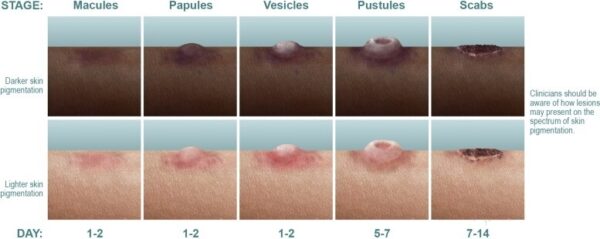
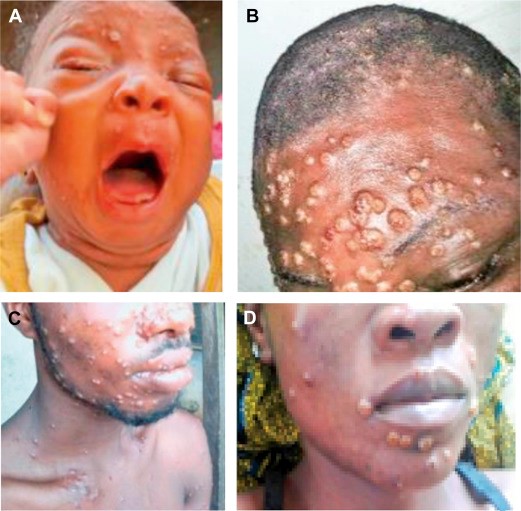
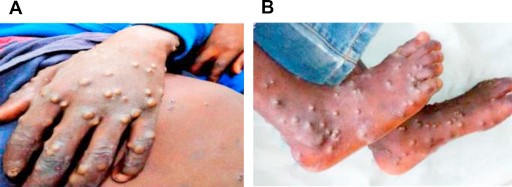
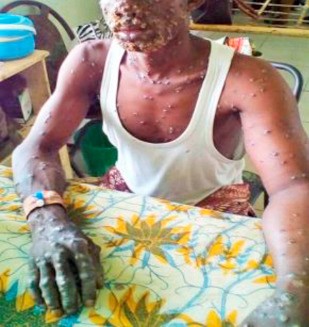
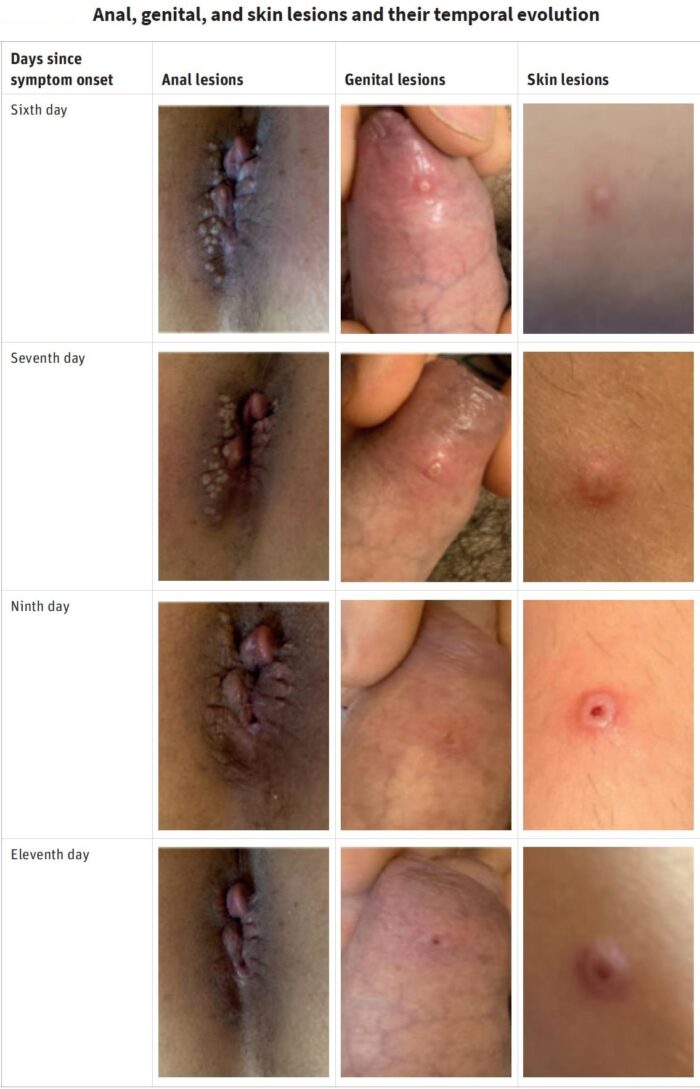
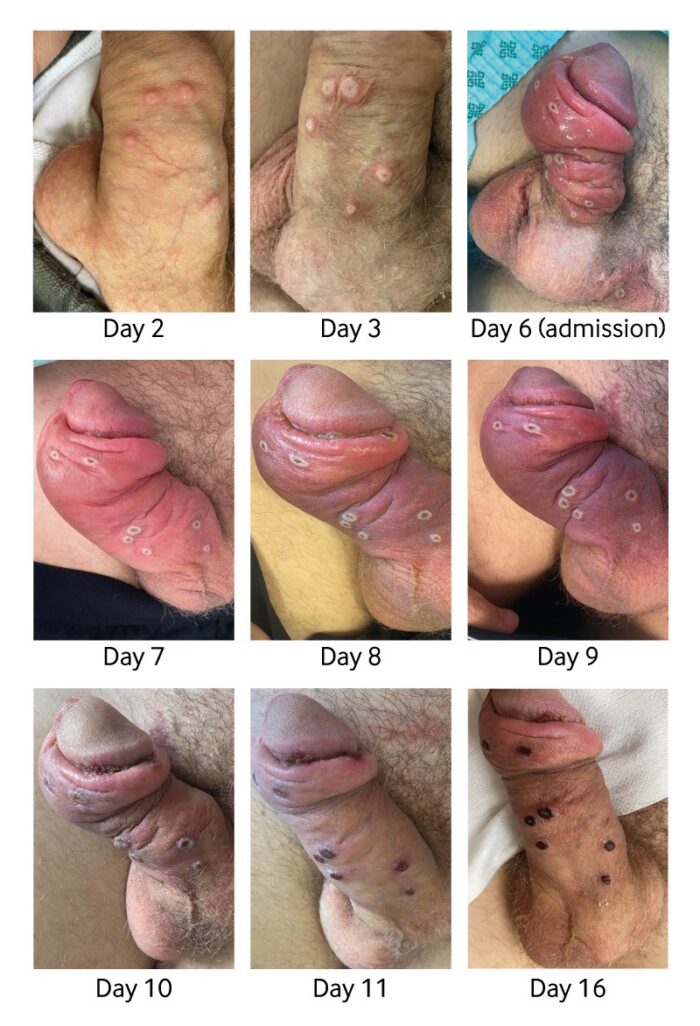
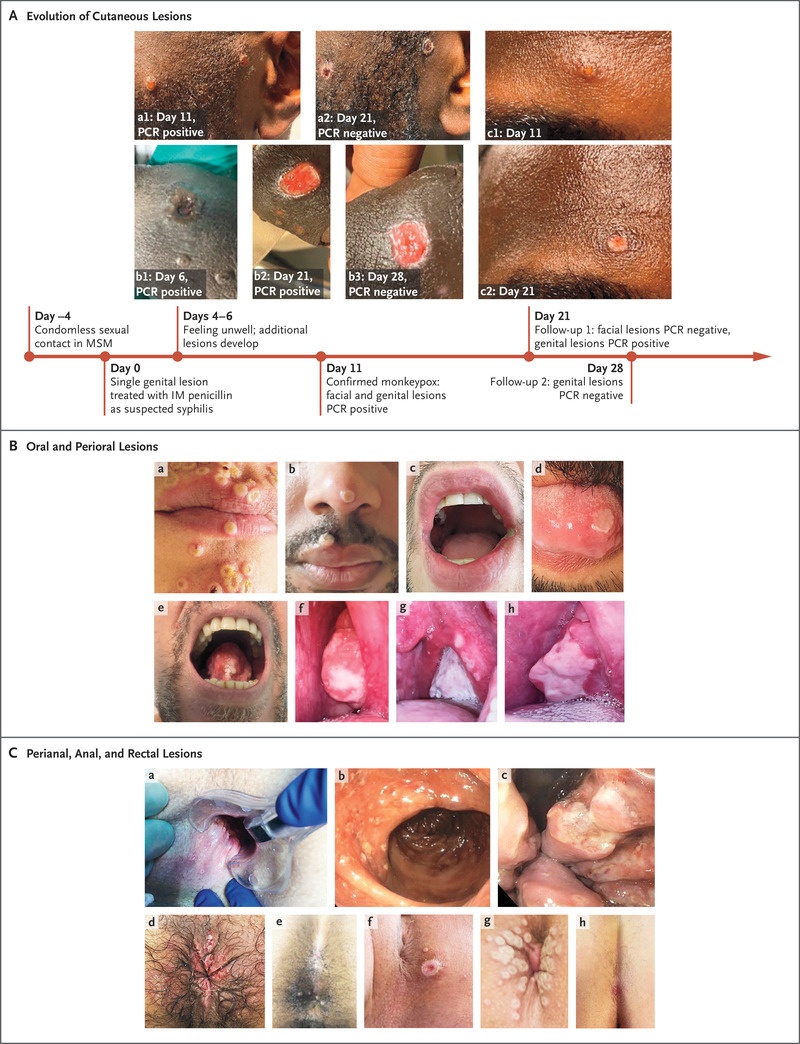
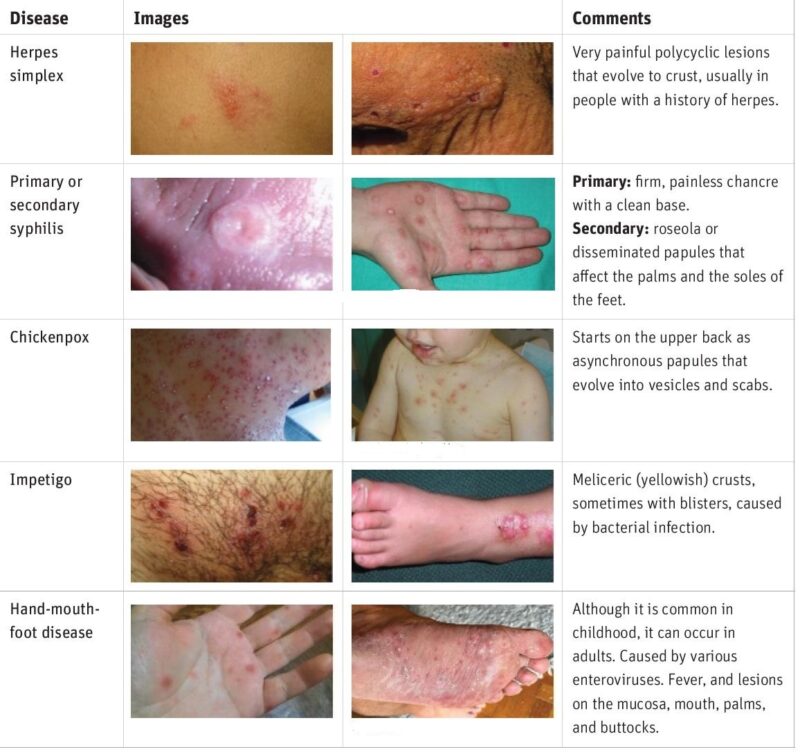
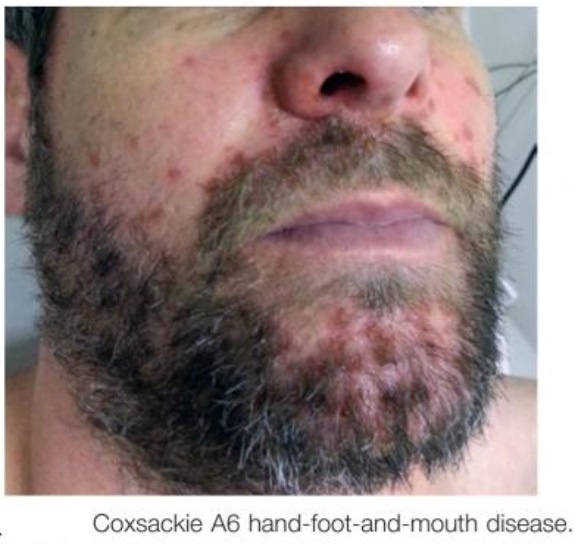
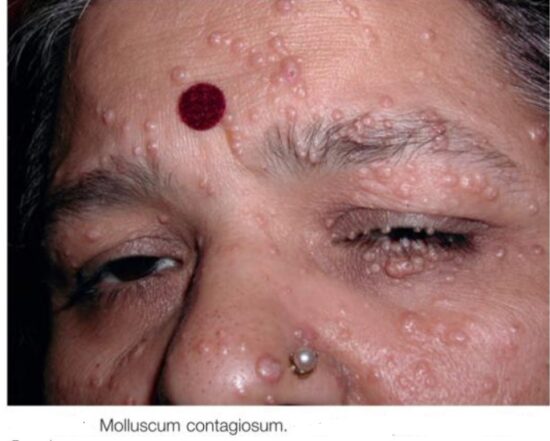
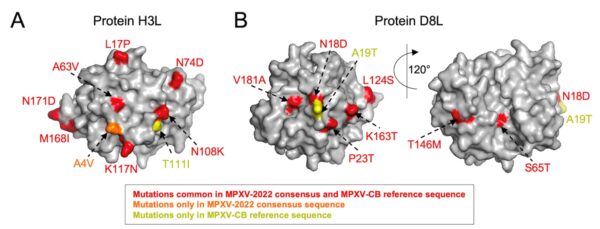
Wonderful work! This is the kind of information that are supposed to be shared around the net. Disgrace on Google for not positioning this put up higher! Come on over and talk over with my website . Thanks =)
Very nice info and straight to the point. I don’t know if this is really the best place to ask but do you people have any ideea where to employ some professional writers? Thx 🙂
Great blog! Do you have any helpful hints for aspiring writers? I’m planning to start my own site soon but I’m a little lost on everything. Would you suggest starting with a free platform like WordPress or go for a paid option? There are so many choices out there that I’m totally overwhelmed .. Any suggestions? Thanks!
Great web site. A lot of helpful information here. I am sending it to a few pals ans additionally sharing in delicious. And obviously, thanks to your sweat!
Yeah bookmaking this wasn’t a bad decision great post! .
I would like to thnkx for the efforts you have put in writing this blog. I am hoping the same high-grade blog post from you in the upcoming as well. In fact your creative writing abilities has inspired me to get my own blog now. Really the blogging is spreading its wings quickly. Your write up is a good example of it.
I genuinely enjoy examining on this web site, it has got great blog posts. “Never fight an inanimate object.” by P. J. O’Rourke.
You made some first rate factors there. I appeared on the web for the issue and located most people will go along with with your website.
Great info and straight to the point. I am not sure if this is truly the best place to ask but do you folks have any thoughts on where to employ some professional writers? Thanks 🙂