Dr Rajiv Desai
An Educational Blog
MUCORMYCOSIS
Mucormycosis:
(Previously called zygomycosis: misnomer black fungus)
_
_____
Section-1
Prologue:
Fungi are important to everyday human life. Fungi are important decomposers in most ecosystems. Mycorrhizal fungi are essential for the growth of most plants. Fungi, as food, play a role in human nutrition in the form of mushrooms, and also as agents of fermentation in the production of bread, cheeses, alcoholic beverages, and numerous other food preparations. Secondary metabolites of fungi are used as medicines, such as antibiotics and anticoagulants. Fungi are model organisms for the study of eukaryotic genetics and metabolism.
Fungi are relatively uncommon causes of disease in healthy and immunocompetent human hosts, even though hosts are constantly exposed to infectious propagules. However fungal diseases have occurred originating from opportunistic and pathogenic fungi. Opportunistic fungi have a preferred habitat independent from the living host and cause infection after accidentally penetration of intact skin barriers, or when immunologic defects or other debilitating conditions exist in the host. There was an outbreak of mucormycosis during the 2011 tornado in Joplin, Missouri. After the tornado, dirt and soil were all turned upside down, and people had cuts and bruises on them through which spores entered. In contrast, pathogens are defined as having advantage of the host; in obligatory pathogens the host is indispensable to complete their life-cycle and for nutrient acquisition, growth, niche establishment, and reproduction.
Human fungal diseases pose a significant, but often overlooked, burden on public health, affecting over 1 billion people worldwide. Fungal infections may be broadly divided into superficial and systemic mycoses, which are caused by different species of fungi. Superficial mycoses affect the skin, keratinous tissues, and mucosal surfaces, whereas systemic mycoses manifest in the form of bloodstream infections and major organ involvement, for example, fungal infections such as candida, aspergillosis, cryptococcus, histoplasmosis, coccidioidomycosis and mucormycosis. Mucormycosis, candida and aspergillosis are the ones observed more in those with low immunity. Life-threatening fungal infections that invade the blood, lungs and other organs pose a serious risk to millions of immunocompromised people. Despite available antifungal drugs, invasive fungal infections are associated with high mortality rates worldwide, causing an estimated 1.6 million deaths each year, a number comparable to tuberculosis.
Mucormycosis is a serious but rare fungal infection caused by a group of molds called mucormycetes. These fungi live throughout the environment, particularly in soil and in decaying organic matter, such as leaves, compost piles, or rotten wood. The term black fungus is actually a misnomer. It is not a true black fungus as it does not produce melanin pigment unlike other black fungus like dematiaceous fungi. In common man’s language it is called black fungus as it produces tissue necrosis which is black in color. The term also got associated with mucormycosis due to the presence of black dots among the culture of white fungal colonies.
The epidemiology of mucormycosis seems to be different between developed and developing countries. In developed countries, the disease is still a rarity, and at present is mostly seen in patients with hematological malignancies, those undergoing chemotherapy, in bone marrow transplant recipients, and as an emerging infection in patients receiving voriconazole therapy or prophylaxis. However, in developing countries, especially in India, the number of mucormycosis cases seems to be on the rise, occurring commonly in patients with uncontrolled diabetes.
Mucormycosis has exploded across India on the coattails of the coronavirus pandemic. During the second wave, which struck India in April 2021, its creaky, underfunded medical system lacked beds, oxygen and other necessities as infections and deaths soared. The viral pandemic has precipitated an epidemic of fungus. The Indian Health Ministry said the country reported at least 40,845 cases of mucormycosis and 3,129 fatalities from mucormycosis during the second wave of the pandemic at the end of June 2021. Of the total number of mucormycosis patients, 34,940 had Covid, 26,187 had the co-morbidity of diabetes, and 21,523 were on steroids. By July 15, total of 45,432 cases of Mucormycosis have been reported in India and 4,252 have died. Exponential rise in mucormycosis in India is alarming; not seen anywhere in the world. Brazil, Chile, Mexico, Uruguay, Honduras, Paraguay, United States of America, Italy, Russia, United Kingdom, Pakistan, Nepal, Iran and Bangladesh have also detected isolated cases of mucormycosis in patients recovering from Covid-19.
The spores of mucormycetes are flying all around—they land on plants, the ground, anything. Our immune systems are usually able to combat and filter out fungi spores as they enter our bodies. When immune defense is compromised, the fungus starts to grow and spread. In the most common form, it colonizes the nose, the sinuses and the eyes, and from there it makes its way to the brain. It can also affect the lungs and intestinal tract. If you’ve just recovered from Covid-19 and find your nose is stuffy, teeth are mobile or eyes red, it will be useful to get checked for mucormycosis. Treatment for the disease involves complex, often disfiguring surgery and an uncommon & expensive drug, contributing to a mortality rate above 50 percent. Improving development of, and access to, new and affordable rapid diagnostics and antifungal therapies, and strengthened public health and research capabilities are needed to combat epidemic of mucormycosis.
The exact incidence and prevalence of mucormycosis in India is unknown due to the lack of population-based studies. Whatever numbers I have quoted for mucormycosis incidence/prevalence before covid and during covid are from studies done in Indian hospitals. No doubt, there is ongoing epidemic of mucormycosis during coronavirus pandemic in India. Doctors say, “it is something like earlier there were five cases in 25 years, and now we are seeing 25 in five days…Such is the situation”. This epidemic inspired me to write on fungi in general and Mucorales in particular.
_____
_____
Abbreviations and synonyms:
Mold = Mould = fungus that grows in the form of multicellular filaments called hyphae
Corticosteroids = steroids = glucocorticosteroids
HM = hematological malignancy
HSCT = hematopoietic stem-cell transplantations
C-AMB = conventional amphotericin = amphotericin B deoxycholate = AMB
ABCD = amphotericin B colloid dispersion
L-AMB = liposome amphotericin B
ABLC = amphotericin B lipid complex
LFAB = Lipid formulations of amphotericin B (ABLC & L-AMB)
BAL = bronchoalveolar lavage fluid
GM-CSF = granulocyte macrophage colony stimulating factor
CAM = COVID-19 associated mucormycosis
ROCM = Rhino-orbital-cerebral mucormycosis
PM = pulmonary mucormycosis
HM = hematological malignancy
CRS = Chronic Rhinosinusitis
AFRS = Allergic fungal rhinosinusitis
DM = diabetes mellitus = diabetes
DKA = diabetic ketoacidosis
GRP78 = Glucose Regulated Protein 78
CotH = spore coat protein homologs of Mucorales
DFO = deferoxamine
DFP = deferiprone
DFX = deferasirox
SOM = Solid organ malignancy,
SOT = Solid organ transplantation
IPA = invasive pulmonary aspergillosis
CAPA = COVID-19-associated pulmonary aspergillosis
IM = invasive mucormycosis
IPM = invasive pulmonary mucormycosis;
CBCT = Cone beam CT
G-CSF = granulocyte colony-stimulating factor
IFN-γ = interferon-γ
_____
_____
Terminology:
Mycorrhiza: a symbiotic association between a fungus and the roots of a vascular plant
Spore: a reproductive particle, usually a single cell, released by a fungus that may germinate into another. Fungi reproduce by spores, which are produced by either sexual or asexual methods, and the majority of fungal spores are adapted for airborne dispersal. Asexually produced spores of primitive fungi are called sporangiospore and of advanced fungi are called conidia.
Sporangium: a case, capsule, or container in which spores are produced by an organism
Hyphae: branching filaments of a fungus.
Mycelium: a network of hyphae.
Lichen: any of many symbiotic organisms, being associations of fungi and algae; often found as white or yellow patches on old walls, etc.
Glucan: any polysaccharide that is a polymer of glucose
Thallus: vegetative body of a fungus
Saprophyte: any organism that lives on dead organic matter, as certain fungi and bacteria
Chitin: a complex polysaccharide, a polymer of N-acetylglucosamine, found in the exoskeletons of arthropods and in the cell walls of fungi; thought to be responsible for some forms of asthma in humans
Homothallic: male and female reproductive structures are present in the same plant or fungal mycelium
Gametangium: an organ or cell in which gametes are produced that is found in many multicellular protists, algae, fungi, and the gametophytes of plants
Karyogamy: the fusion of two nuclei within a cell
Plasmogamy: stage of sexual reproduction joining the cytoplasm of two parent mycelia without the fusion of nuclei
Heterotroph: An organism that cannot make its own food and must obtain nutrients from other organic sources.
Yeast: Single-celled fungi.
_____
_____
Section-2
Introduction to fungi:
Fungi are eukaryotic organisms and include yeasts, moulds and mushrooms. Some fungi are multicellular, while others, such as yeasts, are unicellular. Most fungi are microscopic, but many produce the visible fruitbodies we call mushrooms. The word fungus comes from the Latin word for mushrooms. Indeed, the familiar mushroom is a reproductive structure used by many types of fungi. However, there are also many fungi species that don’t produce mushrooms at all. Being eukaryotes, a typical fungal cell contains a true nucleus and many membrane-bound organelles. Fungi are eukaryotes with an enormous variety of body plans and, along with land plants and animals, are one of the major evolutionary lineages to occupy land. Edible mushrooms, yeasts, black mold, and the producer of the antibiotic penicillin, Penicillium notatum, are all fungi. “Fungi” is plural for “fungus”. A fungus is any member of the group of eukaryotic organisms that includes unicellular microorganisms such as yeasts and multicellular organisms such as molds. Biologists classify these organisms as a kingdom Fungi, separate from the other life-kingdoms of plants, animals, protists, and bacteria. One difference that places fungi in a different kingdom is that their cell walls contain chitin, unlike the cell walls of plants, bacteria and some protists. Similar to animals, fungi are heterotrophs, that is, they acquire their food by absorbing dissolved molecules, typically by secreting digestive enzymes into their environment. Growth is their means of mobility, except for spores (a few of which are flagellated), which may travel through air or water. Fungi function as the principal decomposers in ecological systems. Like bacteria, fungi play an essential role in ecosystems because they are decomposers and participate in the cycling of nutrients by breaking down organic materials to simple molecules.
_
These are organisms separate from the plants and animal kingdoms. They are ubiquitous in nature and are found in the soil, plants, decaying organic matter, water, air, damp places, and also in humans and animals. There are many thousands of different fungi that share our environment, and we are constantly being exposed to fungi in the air we breathe, the food we eat, and the water we drink. They play a very important role in our ecosystem along with bacteria, by degrading organic matter into simpler forms for the consumption of plants. They include the household yeast, molds, mushrooms, and several others. There are about 1.5 million species of fungi, out of which some of them are pathogenic to humans. The most common being Candida, Aspergillus, Cryptococcus, Histoplasma, Pneumocystis, and Mucormycetes.
_
Fungi comprise one of the seven kingdoms of living organisms, more closely related to the animal kingdom than to the plant kingdom. Fungi are eukaryotic organisms with chromosomes within membrane-bound nuclei, dividing through mitosis. Fungi have chitin-containing cell walls, a polysaccharide found also in insect exoskeletons. Fungi may be unicellular, syncytial (many nuclei not separated into different cells) and multicellular (nuclei separated by septa). Complex life cycles have multiple life stages, with both sexual and asexual reproduction. ‘Holomorph’ refers to the fungus throughout its entire life cycle, with ‘anamorph’ referring to the asexual reproductive stage and ‘teleomorph’ to the sexual reproductive stage. Sometimes the alternate life stage is not known, with only the anamorph or the teleomorph identified. Anamorphs without a known teleomorph stage are frequently classified as Deuteromycota, or Fungi Imperfecta: an artificial taxon, a paraphyletic group united only by asexual propagation.
_
Fungi are a group of spores producing eukaryotic organisms that lack chlorophyll. They may reproduce asexually or sexually. Some fungal organisms multiply only asexually, whereas others undergo both asexual reproduction and sexual reproduction with alternation of generations. Asexual reproduction occurs by budding, fragmentation, or the production of spores. Most fungi produce a large number of spores, which are haploid cells that can undergo mitosis to form multicellular, haploid individuals. Most but not all fungi can also reproduce sexually through meiosis and fusion to give rise to diploid nuclei, also producing spores. This introduces genetic variation into the general fungi population, distinct from asexual reproductive methods where the spores are genetically identical to the parent cell.
_
Fungi exist in two main forms, yeasts and molds. Yeasts are solitary cells that reproduce by budding. Examples of yeasts include Candida spp. and Cryptococcus spp. Molds, such as aspergillus, form multicellular hyphae and can grow by apical extension (McGinnis and Tyring, 1996). A number of fungi may exist phenotypically in both morphologies depending on the temperature and environment. These fungi are called dimorphic fungi, of which Histoplasma spp., Coccidioides spp., Blastomyces dermatitidis, Paracoccidioides spp., and Sporothrix spp. are some examples.
_
Fungi often interact with other organisms, forming beneficial or mutualistic associations. For example, most terrestrial plants form symbiotic relationships with fungi. The roots of the plant connect with the underground parts of the fungus forming mycorrhizae. Through mycorrhizae, the fungus and plant exchange nutrients and water, greatly aiding the survival of both species Alternatively, lichens are an association between a fungus and its photosynthetic partner (usually an alga). Fungi also cause serious infections in plants and animals. In humans these include skin diseases such as athletes’ foot, ringworm and thrush. Fungal infections may be broadly divided into superficial and systemic mycoses, which are caused by different species of fungi. Superficial and subcutaneous mycoses affect the skin, keratinous tissues, and mucosal surfaces, whereas systemic mycoses manifest in the form of bloodstream infections and major organ involvement. The term “endemic fungi” refers to fungi that occupy a specific ecological niche in the environment. In the United States, the three main endemic fungi are histoplasmosis, coccidioidomycosis, and blastomycosis, while histoplasmosis, penicilliosis, and sporotrichosis are the most common in the Asia-Pacific region (Chakrabarti and Slavin, 2011). In humans, fungal infections are generally considered challenging to treat. Unlike bacteria, fungi do not respond to traditional antibiotic therapy because they are eukaryotes. Fungal infections may prove deadly for individuals with compromised immune systems.
_
Abundant worldwide, most fungi are inconspicuous because of the small size of their structures, and their cryptic lifestyles in soil or on dead matter. Fungi include symbionts of plants, animals, or other fungi and also parasites. They may become noticeable when fruiting, either as mushrooms or as molds. Fungi perform an essential role in the decomposition of organic matter and have fundamental roles in nutrient cycling and exchange in the environment. They have long been used as a direct source of human food, in the form of mushrooms and truffles; as a leavening agent for bread; and in the fermentation of various food products, such as wine, beer, and soy sauce. Since the 1940s, fungi have been used for the production of antibiotics, and, more recently, various enzymes produced by fungi are used industrially and in detergents. Fungi are also used as biological pesticides to control weeds, plant diseases and insect pests. Many species produce bioactive compounds called mycotoxins, such as alkaloids and polyketides, that are toxic to animals including humans. The fruiting structures of a few species contain psychotropic compounds and are consumed recreationally or in traditional spiritual ceremonies. Fungi can break down manufactured materials and buildings, and become significant pathogens of humans and other animals. Losses of crops due to fungal diseases (e.g., rice blast disease) or food spoilage can have a large impact on human food supplies and local economies.
_
The fungus kingdom encompasses an enormous diversity of taxa with varied ecologies, life cycle strategies, and morphologies ranging from unicellular aquatic chytrids to large mushrooms. However, little is known of the true biodiversity of Kingdom Fungi, which has been estimated at 2.2 million to 3.8 million species. Of these, only about 148,000 have been described, with over 8,000 species known to be detrimental to plants and at least 300 that can be pathogenic to humans. Ever since the pioneering 18th and 19th century taxonomical works of Carl Linnaeus, Christiaan Hendrik Persoon, and Elias Magnus Fries, fungi have been classified according to their morphology (e.g., characteristics such as spore color or microscopic features) or physiology. Advances in molecular genetics have opened the way for DNA analysis to be incorporated into taxonomy, which has sometimes challenged the historical groupings based on morphology and other traits. Phylogenetic studies published in the first decade of the 21st century have helped reshape the classification within Kingdom Fungi, which is divided into one subkingdom, seven phyla, and ten subphyla.
______
Types of fungi:
Fungi are subdivided on the basis of their life cycles, the presence or structure of their fruiting body and the arrangement of and type of spores (reproductive or distributional cells) they produce.
The three major groups of fungi are:
-1. Multicellular filamentous moulds.
-2. Macroscopic filamentous fungi that form large fruiting bodies. Sometimes the group is referred to as ‘mushrooms’, but the mushroom is just the part of the fungus we see above ground which is also known as the fruiting body.
-3. Single celled microscopic yeasts.
_
-1. Multicellular filamentous moulds
Moulds are made up of very fine threads (hyphae). Hyphae grow at the tip and divide repeatedly along their length creating long and branching chains. The hyphae keep growing and intertwining until they form a network of threads called a mycelium. Digestive enzymes are secreted from the hyphal tip. These enzymes break down the organic matter found in the soil into smaller molecules which are used by the fungus as food. Some of the hyphal branches grow into the air and spores form on these aerial branches. Spores are specialised structures with a protective coat that shields them from harsh environmental conditions such as drying out and high temperatures. They are so small that between 500 – 1000 could fit on a pin head. Spores are similar to seeds as they enable the fungus to reproduce. Wind, rain or insects spread spores. They eventually land in new habitats and if conditions are right, they start to grow and produce new hyphae. As fungi can’t move, they use spores to find a new environment where there are fewer competing organisms.
-2. Macroscopic filamentous fungi
Macroscopic filamentous fungi also grow by producing a mycelium below ground. They differ from moulds because they produce visible fruiting bodies (commonly known as mushrooms or toadstools) that hold the spores. The fruiting body is made up of tightly packed hyphae which divide to produce the different parts of the fungal structure, for example the cap and the stem. Gills underneath the cap are covered with spores and a 10 cm diameter cap can produce up to 100 million spores per hour.
-3. Yeasts
Yeasts are small, lemon-shaped single cells that are about the same size as red blood cells. They multiply by budding a daughter cell off from the original parent cell. Scars can be seen on the surface of the yeast cell where buds have broken off. Yeasts such as Saccharomyces play an important role in the production of bread and in brewing. Yeasts are also one of the most widely used model organisms for genetic studies, for example in cancer research. Other species of yeast such as Candida are opportunistic pathogens and cause infections in individuals who do not have a healthy immune system.
_
Examples of fungi:
Many species of fungus produce the familiar mushroom (a) which is a reproductive structure. This (b) coral fungus displays brightly-colored fruiting bodies. This electron micrograph shows (c) the spore-bearing structures of Aspergillus, a type of toxic fungi found mostly in soil and plants.
______
A typical fungus is built up of long, thin cells, the hyphae. As the hyphae are so thin, often smaller than 1/100 mm, a microscope is needed to study them. They have apical growth and branch into a dense network, called mycelium – the vegetative part of the fungus. The hyphae are protected by a cell wall of chitin and glucan. The mycelium can be septated (Ascomycota and Basidiomycota), or unseptated, (other fungal phyla). The content of the cytoplasm can move through pores in the septa from one hyphal compartment to another. Dense mycelia can be seen by the naked eye, e.g., mould on bread, cheese or jam. Most often, however, the mycelia are hidden in soil or wood. However, difficult to see, it is supposed that a lump of soil of the size of a piece of sugar can contain as much as 10 km. The thin and branched hyphae have a large surface and are extremely efficient at absorbing nutrients. Some fungi lack mycelium, e.g., the budding yeast fungi and many chytrids, which consist of single, rounded cells.
Unlike bacteria which have simple prokaryotic cells, fungi have complex eukaryotic cells like animals and plants. Fungi are heterotrophic. This means that fungi cannot produce their own food by photosynthesis or chemosynthesis, but rely on organic compounds from plants, animals or other fungi for nutrition. They are unable to fix atmospheric nitrogen. In contrast to animals, fungi utilise extracellular digestion. A fungus is a eukaryote that digests food externally and absorbs nutrients directly through its cell walls. Various enzymes are produced in the cytosol of the hyphae. They are transported with organic acids through the membrane of the hyphae and secreted into surrounding substrates. Depending on the enzyme, they can break down cellulose, hemicellulose, lignin, proteins or other complicated organic compounds to simpler soluble substances like sugars (mono- or disaccharides), amino acids or oligopeptides. These substances can be transported through the hyphal membrane into the fungal cell (hyphal compartment). Some fungi obtain their nutrients from a living host (plant or animal) and are called biotrophs; others obtain their nutrients from dead plants or animals and are called saprotrophs (saprophytes, saprobes). Some fungi infect a living host, but kill host cells in order to obtain their nutrients; these are called necrotrophs.
Fungi vary drastically in size. Some parasitic fungi live inside of plant cells or on pollen grains. On the other hand, in North America, there are giant fungi whose mycelia can cover several square kilometres and weigh up to 600 tons (more than three blue whales!). In the Arctic, Agaricus aristocratus produce fairy rings up to 50 m in diameter. The ring widens every year as the mycelium grows radially from the primary germination point.
______
It is well known that fungi require certain optimum conditions for each phase of their growth. In this regard, associations with temperature and moisture have been well documented in the mycological literature. It has been also established that spore concentrations in the atmosphere fluctuate with weather. Temperature, humidity, and rainfall also play important roles. However, air spore levels also vary for biological reasons, such as growth and differentiation of spores- or pollen producing organs (Gregory, 1973). Fungi are able to grow at a relative humidity (RH) below that of bacteria and algae. The competitive ability of fungi is facilitated by its ability to respond by sporulating when the RH decreases. The minimum RH permitting growth varies between 75 and 95% for different species of mould (Gravesen, 1979). The fungi are mainly mesophilic and optimal temperature for growth is 20–40˚C, Some fungi (e.g., certain Cladosporium species) are psychrophilic (optimum below 20˚C), and cause serious problems in refrigerated food storage (Gravesen, 1979). In contrast to bacteria and algae, fungi are entirely heterotrophic. Their food demands range over a broad spectrum of organic material (Gravesen, 1979).
______
The word fungus usually evokes images of athlete’s foot, unseemly looking nails, or scrumptious cheese and mouth-watering mushrooms. However, few realize that over 300 million people suffer from serious fungal-related diseases, or that fungi collectively kill over 1.6 million people annually, which is more than malaria and similar to the tuberculosis death toll. Fungi and oomycetes destroy a third of all food crops each year, which would be sufficient to feed 600 million people. Furthermore, fungal infestation of amphibians has led to the largest disease-caused loss of biodiversity ever recorded, while fungi also cause mass mortality of bats, bees and other animals, and decimate fruit orchards, pine, elm and chestnut forests. Headline-grabbing statistics, one would imagine. There are an estimated 1.5 million fungal species, of which over 8,000 are known to cause disease in plants and 300 to be pathogenic to humans. Candida, Aspergillus, Pneumocystis and Cryptococcus spp. are the most common cause of serious disease in humans, and five diseases — wheat stem rust, rice blast, corn smut, soybean rust and potato late blight — are the most devastating for crop production. Infections primarily occur in immunocompromised patients, such as those undergoing chemotherapy or infected with HIV, and many are acquired in hospitals. However, infections of otherwise healthy people are on the rise.
______
How old are fungi?
Fungi are an ancient group—not as old as bacteria, which fossil evidence suggests may be 3. 5 billion years old—but the earliest fungal fossils are from the Ordovician, 460 to 455 million years old (Redecker et al. 2000). Based on fossil evidence, the earliest vascular land plants didn’t appear until approximately 425 million years ago, and some scientists believe that fungi may have played an essential role in the colonization of land by these early plants (Redeker et al. 2000). Mushrooms exquisitely preserved in amber from the Late Cretaceous (94 million years ago) tell us that there were mushroom-forming fungi remarkably similar to those that exist today when dinosaurs were roaming the planet (Hibbett et al. 2003). However, the fungal fossil record is incomplete and provides only a minimum time estimate for when different groups of fungi evolved. Molecular data suggest that fungi are much older than indicated by the fossil record, and may have arisen more than one billion years ago (Parfrey et al. 2011).
______
______
Taxonomy of fungi:
Before the introduction of molecular methods for phylogenetic analysis, taxonomists considered fungi to be members of the plant kingdom because of similarities in lifestyle: both fungi and plants are mainly immobile, and have similarities in general morphology and growth habitat. Like plants, fungi often grow in soil and, in the case of mushrooms, form conspicuous fruit bodies, which sometimes resemble plants such as mosses. The fungi are now considered a separate kingdom, distinct from both plants and animals, from which they appear to have diverged around one billion years ago (around the start of the Neoproterozoic Era). Some morphological, biochemical, and genetic features are shared with other organisms, while others are unique to the fungi, clearly separating them from the other kingdoms:
-1. Shared features:
-With other eukaryotes: Fungal cells contain membrane-bound nuclei with chromosomes that contain DNA with noncoding regions called introns and coding regions called exons. Fungi have membrane-bound cytoplasmic organelles such as mitochondria, sterol-containing membranes, and ribosomes of the 80S type. They have a characteristic range of soluble carbohydrates and storage compounds, including sugar alcohols (e.g., mannitol), disaccharides, (e.g., trehalose), and polysaccharides (e.g., glycogen, which is also found in animals).
-With animals: Fungi lack chloroplasts and are heterotrophic organisms and so require preformed organic compounds as energy sources.
-With plants: Fungi have a cell wall and vacuoles. They reproduce by both sexual and asexual means, and like basal plant groups (such as ferns and mosses) produce spores. Similar to mosses and algae, fungi typically have haploid nuclei.
-With euglenoids and bacteria: Higher fungi, euglenoids, and some bacteria produce the amino acid L-lysine in specific biosynthesis steps, called the α-aminoadipate pathway.
-The cells of most fungi grow as tubular, elongated, and thread-like (filamentous) structures called hyphae, which may contain multiple nuclei and extend by growing at their tips. Each tip contains a set of aggregated vesicles—cellular structures consisting of proteins, lipids, and other organic molecules—called the Spitzenkörper. Both fungi and oomycetes grow as filamentous hyphal cells. In contrast, similar-looking organisms, such as filamentous green algae, grow by repeated cell division within a chain of cells. There are also single-celled fungi (yeasts) that do not form hyphae, and some fungi have both hyphal and yeast forms.
-In common with some plant and animal species, more than 70 fungal species display bioluminescence
-2. Unique features:
-Some species grow as unicellular yeasts that reproduce by budding or fission. Dimorphic fungi can switch between a yeast phase and a hyphal phase in response to environmental conditions.
-The fungal cell wall is composed of glucans and chitin; while glucans are also found in plants and chitin in the exoskeleton of arthropods, fungi are the only organisms that combine these two structural molecules in their cell wall. Unlike those of plants and oomycetes, fungal cell walls do not contain cellulose.
Most fungi lack an efficient system for the long-distance transport of water and nutrients, such as the xylem and phloem in many plants. To overcome this limitation, some fungi, such as Armillaria, form rhizomorphs, which resemble and perform functions similar to the roots of plants. As eukaryotes, fungi possess a biosynthetic pathway for producing terpenes that uses mevalonic acid and pyrophosphate as chemical building blocks. Plants and some other organisms have an additional terpene biosynthesis pathway in their chloroplasts, a structure that fungi and animals do not have. Fungi produce several secondary metabolites that are similar or identical in structure to those made by plants. Many of the plant and fungal enzymes that make these compounds differ from each other in sequence and other characteristics, which indicates separate origins and convergent evolution of these enzymes in the fungi and plants.
_
Fungi are eukaryotes, having a nucleus with an RNA-rich nucleolus and cytoplasmic organelles including mitochondria, vacuoles, endoplasmic reticulum, ribosomes, Golgi apparatus, and other cytoplasmic inclusions. Fungi do not have chloroplasts and do not produce chlorophyll. These organisms are delineated within the eukaryotes by their lack of flagella (nonmotile), the development of spores during asexual reproduction, and their predominantly aerobic growth requirements. The fungi produce an ergosterol-rich cell membrane and a cell wall composed of a mixture of polysaccharides including chitin, glucan, and glycoproteins. The cell wall is similar but not identical for each fungus, allowing variations in the cell wall composition to be used to differentiate one fungus form another.
_
The kingdom Fungi is further divided into three phyla on the basis of differences in the mode of sexual reproduction of the organisms and on the basis of morphologic features (see figure below). The phylum Basidiomycota is delineated by the formation of sexual basidiospores on the surface of a club-shaped basidium. These spores are formed by either sexual (meiosis) or asexual (mitosis) mechanisms. This phylum contains mushrooms, toad stools, puffballs, rusts, smuts, and other related organisms. It also includes human pathogens including the sexual stage of Cryptococcus neoformans, Filobasidiella neoformans. The Ascomycota includes the higher fungi that reproduce sexually by the production of ascospores. This phylum contains several pathogens important to humans, including the teleomorphs of the dermatophytes and Histoplasma capsulatum and Blastomyces dermatitides. A variety of other yeast and filamentous human pathogens and nonpathogens also fall into this category. The third phylum, Zygomycota, is composed of fungi that form coenocytic hyphae and reproduce sexually by the production of zygospores. A catch-all category of mitosporic fungi (formerly the form phylum Deuteromyces) represents the “holding cell” for fungi whose sexual (teleomorph) phase has not yet been identified. Since these fungi were identified only by their asexual phase (“mitosporic” indicating reproduction by mitosis only), they have been designated Fungi Imperfecti, or imperfect fungi. A large number of fungi, and most of the human fungal pathogens, fall into this category. Included in this group are the yeast-like fungi including the human pathogens Candida spp. and other related yeasts. Many filamentous fungi with septate mycelium which reproduce by formation of conidia are also thrown into this group. Included in this former “form class Coelomycetes” are both hyaline and dematiaceous fungi. Important members of this group include the agents of aspergillosis, penicillin producers, the agents of subcutaneous mycosis and chromoblastomycosis, and other fungi. It is believed that with the use of molecular techniques, these organisms will eventually be linked with their sexual phase and reassigned into their correct phyla of the kingdom Fungi. Neither the form phylum Deuteromyces nor its form classes are recognized taxonomic designations any longer.
Note: “-mycota” is used to designate a phylum while “-mycetes” formally denotes a class or is used informally to refer to all members of the phylum.
Zygomycetes – These are formed by the fusion of two different cells. The sexual spores are known as zygospores while the asexual spores are known as sporangiospores. The hyphae are without the septa.
Ascomycetes – They are also called as sac fungi. They can be coprophilous, decomposers, parasitic or saprophytic. The sexual spores are called ascospores. Asexual reproduction occurs by conidiospores. E.g., Saccharomyces
Basidiomycetes – Mushrooms are the most commonly found basidiomycetes and mostly live as parasites. Sexual reproduction occurs by basidiospores. Asexual reproduction occurs by conidia, budding or fragmentation. E.g., Agaricus
Deuteromycetes – They are otherwise called imperfect fungi as they do not follow the regular reproduction cycle as the other fungi. They do not reproduce sexually. Asexual reproduction occurs by conidia. E.g., Trichoderma.
_
Taxonomic organization of the zygomycetes:
The zygomycetes fall into a distinctive phylum, the phylum Zygomycota. It is composed of the organisms that are characterized by the formation of wide ribbon-like aseptate hyaline hyphae (coenocytic hyphae) and sexual reproduction with the formation of zygospores. This phylum is divided into two classes, the Trichomycetes, which are obligate symbionts of arthropods and contain no human pathogens and the Zygomycetes, the class containing the human pathogens. This class is subdivided into two orders, which contain the agents of human zygomycosis, the Mucorales and Entomophthorales.
_
Traditionally, the Mucorales are divided into six families of significance in causing human or animal disease: Mucoraceae, Cunninghamellaceae, Saksenaea, Thamnidiaceae, Syncephalastraceae, and Mortierellaceae. Under this classification system, the vast majority of human zygomycotic disease is caused by the members of the family Mucoraceae. Members of this family include zygomycetes that produce asexual sporangiospores in a sack-like structure called sporangia. A more recently proposed reclassification of the Mucorales by von Arx places the mucoraceous zygomycetes into seven families containing human pathogens. In addition to the six families mentioned in the traditional classification system, the family Absidiaceae is added based upon the presence of an apophysis, the widening of the terminal portion of the sporangiophore during sporangium formation. von Arx defined the family Mucoraceae as nonapophysate sporangium producers that may or may not produce stolons and rhizoids and includes in this family members of the genera Mucor and Rhizomucor. The proposed family Absidiaceae contains the zygomycetes that produce apophysate sporangia with deliquescent (dissolving) or persistent sporangial walls, produce both stolons and rhizoids, and produce zygospores with opposed suspensors. The most common pathogens in this family are in the genera Rhizopus and Absidia. Most of the recent texts on mycology adhere to the traditional taxonomic scheme, with only a very few authors supporting the suggested reclassification scheme. The reader is alerted to the possibility that this reclassification may become the accepted nomenclature in time, particularly with better dissemination of its proposal.
_
The order Entomophthorales has two families that contain human pathogens, Ancylistaceae and Basidiobolaceae. Similar to all zygomycetes, the Entomophthorales are characterized by the production of coenocytic hyphae and by their sexual reproduction by production of zygospores. The Entomophthorales are distinguished from the Mucorales by their production of actively expelled asexual sporangioles and by their markedly compact and glabrous mycelial morphology. Both of these features define this order within the class Zygomycetes. Although several species of Basidiobolus exist in nature, all cases of human disease are now known to be caused by Basidiobolus ranarum. Conidiobolus contains several species that are pathogenic to mammals. Conidiobolus coronatus is the major human pathogen. C. incongruus has also been implicated in several relatively invasive infections in humans. C. lamprauges is pathogenic only to horses. A single human case of a Conidiobolus infection by another member of the species has also been described.
_
The term zygomycosis encompasses agents that cause mucormycosis and entomophthoramycosis, but the term has been discarded in the modern taxonomy literature. The previous taxonomy had been based on similarities in the structure, life cycle, and ecology of the fungi. However, more recent analysis of the molecular biology has separated the two into different subphyla. In the revised classification, all agents of mucormycosis have been placed under the subphylum Mucormycotina, whereas agents of entomophthoramycosis are now classified under the subphylum Entomophthoramycotina. Because most fungal pathogens associated with mucormycosis are also in the order Mucorales, the disease name of mucormycosis is now considered more taxonomically and clinically accurate than the previously used (and now obsolete) term zygomycosis.
In addition, entomophthoramycosis is clinically different from mucormycosis. Unlike mucormycosis, entomophthoramycosis is most commonly found in tropical climates, affects immunocompetent patients, and causes a chronic infection. For these reasons, the more specific terms mucormycosis, Mucor infection, or simply Mucor are preferred over zygomycosis. Most cases of mucormycosis are caused by members of the genus Rhizopus or Mucor. However, there are many other genera in the order Mucorales that can cause infections in humans as seen in the figure below.
Figure above delineates taxonomic hierarchy of the genera that most commonly cause mucormycosis.
__
The taxonomy of fungi is in a state of constant flux, especially due to recent research based on DNA comparisons. These current phylogenetic analyses often overturn classifications based on older and sometimes less discriminative methods based on morphological features and biological species concepts obtained from experimental mating. There is no unique generally accepted system at the higher taxonomic levels and there are frequent name changes at every level, from species upwards. Efforts among researchers are now underway to establish and encourage usage of a unified and more consistent nomenclature. Fungal species can also have multiple scientific names depending on their life cycle and mode (sexual or asexual) of reproduction. The 2007 classification of Kingdom Fungi is the result of a large-scale collaborative research effort involving dozens of mycologists and other scientists working on fungal taxonomy. It recognizes seven phyla, two of which—the Ascomycota and the Basidiomycota—are contained within a branch representing subkingdom Dikarya, the most species rich and familiar group, including all the mushrooms, most food-spoilage molds, most plant pathogenic fungi, and the beer, wine, and bread yeasts. The true fungi, which make up the monophyletic clade called kingdom Fungi, comprise seven phyla: Chytridiomycota, Blastocladiomycota, Neocallimastigomycota, Microsporidia, Glomeromycota, Ascomycota, and Basidiomycota (the latter two being combined in the subkingdom Dikarya). Subphylum mucoromycotina belong to phylum Glomeromycota and order Mucorales belong to Subphylum mucoromycotina.
_
On the basis of nutrition, kingdom fungi can be classified into 3 groups.
Saprophytic – The fungi obtain their nutrition by feeding on dead organic substances. Examples: Rhizopus, Penicillium and Aspergillus.
Parasitic – The fungi obtain their nutrition by living on other living organisms (plants or animals) and absorb nutrients from their host. Examples: Taphrina and Puccinia.
Symbiotic – These fungi live by having an interdependent relationship association with other species in which both are mutually benefited. Examples: Lichens and mycorrhiza. Lichens are the symbiotic association between algae and fungi. Here both algae and fungi are mutually benefited as fungi provide shelter for algae and in reverse algae synthesis carbohydrates for fungi.
_
Fungus-like organisms:
Because of similarities in morphology and lifestyle, the slime molds (mycetozoans, plasmodiophorids, acrasids, Fonticula and labyrinthulids, now in Amoebozoa, Rhizaria, Excavata, Opisthokonta and Stramenopiles, respectively), water molds (oomycetes) and hyphochytrids (both Stramenopiles) were formerly classified in the kingdom Fungi, in groups like Mastigomycotina, Gymnomycota and Phycomycetes. The slime molds were studied also as protozoans, leading to an ambiregnal, duplicated taxonomy. Unlike true fungi, the cell walls of oomycetes contain cellulose and lack chitin. Hyphochytrids have both chitin and cellulose. Slime molds lack a cell wall during the assimilative phase (except labyrinthulids, which have a wall of scales), and ingest nutrients by ingestion (phagocytosis, except labyrinthulids) rather than absorption (osmotrophy, as fungi, labyrinthulids, oomycetes and hyphochytrids). Neither water molds nor slime molds are closely related to the true fungi, and, therefore, taxonomists no longer group them in the kingdom Fungi. Nonetheless, studies of the oomycetes and myxomycetes are still often included in mycology textbooks and primary research literature.
The Eccrinales and Amoebidiales are opisthokont protists, previously thought to be zygomycete fungi. Other groups now in Opisthokonta (e.g., Corallochytrium, Ichthyosporea) were also at given time classified as fungi. The genus Blastocystis, now in Stramenopiles, was originally classified as a yeast. Ellobiopsis, now in Alveolata, was considered a chytrid. The bacteria were also included in fungi in some classifications, as the group Schizomycetes.
The Rozellida clade, including the “ex-chytrid” Rozella, is a genetically disparate group known mostly from environmental DNA sequences that is a sister group to fungi. Members of the group that have been isolated lack the chitinous cell wall that is characteristic of fungi.
The nucleariids may be the next sister group to the eumycete clade, and as such could be included in an expanded fungal kingdom. Many Actinomycetales (Actinobacteria), a group with many filamentous bacteria, were also long believed to be fungi.
______
______
Morphology, characteristics and reproduction of fungi:
_
Structure of Fungi:
The structure of fungi can be explained in the following points:
-1. Almost all the fungi have a filamentous structure except the yeast cells.
-2. They can be either single-celled or multicellular organism.
-3. Fungi consist of long thread-like structures known as hyphae. These hyphae together form a mesh-like structure called mycelium.
-4. Fungi possess a cell wall which is made up of chitin and polysaccharides.
-5. The nucleus is dense, clear, with chromatin threads. The nucleus is surrounded by a nuclear membrane.
_
Morphology:
Microscopic structures:
Most fungi are multicellular organisms. They display two distinct morphological stages: the vegetative and reproductive. The vegetative stage consists of a tangle of slender thread-like structures called hyphae (singular, hypha), whereas the reproductive stage can be more conspicuous. Most fungi grow as hyphae, which are cylindrical, thread-like structures 2–10 µm in diameter and up to several centimeters in length. Hyphae grow at their tips (apices); new hyphae are typically formed by emergence of new tips along existing hyphae by a process called branching, or occasionally growing hyphal tips fork, giving rise to two parallel-growing hyphae. Hyphae also sometimes fuse when they come into contact, a process called hyphal fusion (or anastomosis). These growth processes lead to the development of a mycelium, an interconnected network of hyphae. Hyphae can be either septate or coenocytic (aseptate). Septate hyphae are divided into compartments separated by cross walls (internal cell walls, called septa, that are formed at right angles to the cell wall giving the hypha its shape), with each compartment containing one or more nuclei; coenocytic hyphae are not compartmentalized. Septa have pores that allow cytoplasm, organelles, and sometimes nuclei to pass through; an example is the dolipore septum in fungi of the phylum Basidiomycota. Coenocytic hyphae are in essence multinucleate supercells.
Many species have developed specialized hyphal structures for nutrient uptake from living hosts; examples include haustoria in plant-parasitic species of most fungal phyla, and arbuscules of several mycorrhizal fungi, which penetrate into the host cells to consume nutrients.
Although fungi are opisthokonts—a grouping of evolutionarily related organisms broadly characterized by a single posterior flagellum—all phyla except for the chytrids have lost their posterior flagella. Fungi are unusual among the eukaryotes in having a cell wall that, in addition to glucans (e.g., β-1,3-glucan) and other typical components, also contains the biopolymer chitin.
_
Monochrome micrograph below showing Penicillium hyphae as long, transparent, tube-like structures a few micrometers across. Conidiophores branch out laterally from the hyphae, terminating in bundles of phialides on which spherical condidiophores are arranged like beads on a string. Septa are faintly visible as dark lines crossing the hyphae.
An environmental isolate of Penicillium shows following stricture under microscope:
-1. hypha
-2. conidiophore
-3. phialide
-4. conidia
-5. septa
_
The vegetative body of a fungus is a unicellular or multicellular thallus. Unicellular fungi are generally referred to as yeasts. Saccharomyces cerevisiae (baker’s yeast) and Candida species (the agents of thrush, a common fungal infection) are examples of unicellular fungi. Some fungi grow exclusively or mostly as yeasts, defined as single-celled fungi that reproduce by budding or fission. In contrast to apical growth that is characteristic of hyphae, yeasts exhibit wall growth over the entire cell surface, often resulting in a nearly spherical cell. There are also fungi that can switch between mycelial growth and yeast-like growth, dependent upon the environmental conditions. The ability to grow in different forms is called dimorphism, and is exhibited by some members of phyla Ascomycota, Basidiomycota and Zygomycota. Dimorphic fungi can change from the unicellular to multicellular state depending on environmental conditions.
Figure below is example of a unicellular fungus: Candida albicans is a yeast cell and the agent of candidiasis and thrush. This organism has a similar morphology to coccus bacteria; however, yeast is a eukaryotic organism (note the nucleus).
C. albicans is commonly used as a model organism for fungal pathogens. It is generally referred to as a dimorphic fungus since it grows both as yeast (figure above) and filamentous cells (figure below). The ability of pathogenic fungi to switch between a multicellular hyphal and unicellular yeast growth form is a tightly regulated process known as dimorphic switching. Dimorphic switching requires the fungus to sense and respond to the host environment and is essential for pathogenicity. Figure below shows gram stain of Candida albicans from a vaginal swab from a woman with candidiasis, showing hyphae, and chlamydospores, which are 2–4 µm in diameter.
_
Inside the fungal cell:
Most of the organelles present in fungal cells are similar to those of other eukaryotes. Fungal nuclei are usually small (< 2 µm diameter), and can compress and/or stretch to move through septal pores and into developing spores. Fungi have been found to possess between 6 and 21 chromosomes coding for 6,000 to nearly 18,000 genes. The average genome size of Ascomycota group of fungi is 36.91 Mb. The average genome size of Basidiomycota group is 46.48 Mb. The average genome size of Oomycota group of fungi is 74.85 Mb which is the highest among all groups. If we consider about the coding gene sequence in fungi, in average the Acomycota, Basidiomycota, Oomycota and Mucoromycotina groups encodes for 11129.45, 15431.51, 24173.33, 13306 no. of genes respectively in their genomes. Genome sizes range from 8. 5 megabase pairs (Mb) to just over 400 Mb in filamentous fungi (Zolan 1995; Spanu et al. 2010; Duplessis et al. 2011), making fungal genomes among the smallest of eukaryotic organisms on average—approximately 1% the size of mammalian genomes and only 1. 3 times the size of the largest known bacterial genome (Stover et al. 2000). Many fungi (Ascomycota) have a life cycle that is predominantly haploid, while others (Basidiomycota) have a long dikaryotic phase.
_
Macroscopic structures:
Many fungi produce spores inside or upon a fruiting body. The apothecium—a specialized structure important in sexual reproduction in the ascomycetes—is a cup-shaped fruit body that is often macroscopic and holds the hymenium, a layer of tissue containing the spore-bearing cells. Many people are familiar with the mushroom, a type of fruiting body produced by some Basidiomycota. You may recognize other fungal fruiting bodies such as puffballs, or shelf fungi. These are examples of large, conspicuous fruiting bodies, but there is an even greater diversity of microscopic fruiting bodies produced by various fungi. What all fruiting bodies have in common is that they produce spores and provide a mechanism for dispersing those spores.
_
Figure below shows a cluster of large, thick-stem, light-brown gilled mushrooms growing at the base of a tree:
_
The mass of hyphae is a mycelium. It can grow on a surface, in soil or decaying material, in a liquid, or even on living tissue. Although individual hyphae must be observed under a microscope, the mycelium of a fungus can be very large. Fungal mycelia can become visible to the naked eye, for example, on various surfaces and substrates, such as damp walls and spoiled food, where they are commonly called molds. Mycelia grown on solid agar media in laboratory petri dishes are usually referred to as colonies. These colonies can exhibit growth shapes and colors (due to spores or pigmentation) that can be used as diagnostic features in the identification of species or groups. Some individual fungal colonies can reach extraordinary dimensions and ages as in the case of a clonal colony of Armillaria solidipes, which extends over an area of more than 900 ha (3.5 square miles), with an estimated age of nearly 9,000 years.
_______
Growth Characteristics:
Fungi require free oxygen for growth. Most fungi can grow over a wide range of pH (2–8.5) but some are favoured by an acid pH. Fungi in general can utilize foods ranging from simple to complex substrates. Most fungi possess hydrolytic enzymes such as amylases, pectinase, proteinase and lipase. The growth of fungi is slow compared to that of bacteria and yeast. Most fungi are mesophilic, growing between 25 and 31°C. In general, most fungi require less available moisture than yeasts and bacteria. Water activity or aw is a measure of available water. Water activity ranges from 1.00 for pure water to 0.00 for a bone-dry material. The minimum water activity ((aw) for spore germination has been found to be as low as 0.62 for some fungi and as high as 0.93 for others, e.g., Mucor, Rhizopus and Botrytis. Sorbic acid, propionates and acetates specifically act as fungicides in nature.
General characteristics:
Fungi are classified into a separate group of organisms differing from both plants and animals, primarily by the type of nutrition. Fungi are not autotrophs, they have no chloroplasts, they can only use the energy stored in organic compounds. This distinguishes fungi from plants. As against animals, fungi are osmotrophic: they obtain food by absorbing nutrients from the environment. These feeding features correlate with fungal morphology and physiology.
-1. The body of most fungi is made of mycelium consisting of very branched hyphae. Such a structure allows a maximum occupation of the substrate, whether it is soil or plant, to extract nutrients. Fungi absorb nutrients by the entire body.
-2. The osmotrophic type of feeding makes a vegetative body plunge fully into the substrate, which impedes its propagation and occupation of new substrates. Therefore, in most fungi, spores are brought out above the substrate in special structures, which in many cases have a complex arrangement (sporangiophores, conidiophores, and fruit bodies). Sporiferous structures of endophytic fungi (those developing inside plants) are released through stomata or breaches in epidermis.
-3. Fungi need to use, as energy sources, complex organic compounds that cannot pass to the cell through cellular covers because of large molecular weight. Therefore, fungi release the enzymes depolymerases to the environment that cause degradation of polymers. Degradation products enter cells in a dissolved form. Fungi are sources of highly active depolymerases.
-4. Fungi need to develop high turgor pressure in the cells to provide entrainment of nutrient solutions from the substrate to the mycelium.
Both saprotrophic and parasitic fungi feed mostly on plant tissues. Apparently, the association of fungi and plants developed at the very early stages of their evolution. The most primitive fungi Chytridiomycetes and Oomycetes parasitize on the most primitive plants, algae. Some mycologists believe that fungi came to live on the land under the cover of plants that had come to live on the land, as their parasites and symbiotes. Symbiotic fungi are also believed to have provided adaptation of green plants to life on land. There are almost no fungi living in symbiosis with animals, while a huge number of fungi live in continuous symbiotic relations with plants. The enzymatic system of fungi is designed to decompose carbohydrates – structural materials and reserve nutrients of plants. It is not only parasitic fungi that mainly attack plants, but also saprotrophic fungi feeding on dead plants, leaving dead animals to bacteria. Dead wood is almost entirely decomposed by fungi.
_______
Fungal reproduction:
Most fungi can reproduce through both sexual and asexual reproduction. Asexual reproduction occurs through the release of spores or through mycelial fragmentation when the mycelium separates into multiple pieces that grow separately or budding/fission (yeast). In sexual reproduction, separate individuals fuse their hyphae together. The exact life cycle depends on the species, but generally multicellular fungi have a haploid stage (where they have one set of chromosomes), a diploid stage, and a dikaryotic stage where they have two sets of chromosomes but the sets remain separate. In some fungi, the fusion of two haploid hyphae does not result in the formation of a diploid cell. In such cases, there appears an intermediate stage called the dikaryophase. This stage is followed by the formation of diploid cells.
Fungi frequently reproduce by the formation of spores. A spore is a survival, reproductive and dispersal unit, consisting of one or a few cells, that is capable of germinating to produce a new hypha. Unlike plant seeds, fungal spores lack an embryo, but contain food reserves needed for germination. Spores can become dormant for a long time until conditions are favorable for growth. This is an adaptation for opportunism; with a sometimes unpredictable food source availability, spores can be dormant until they are able to colonize a new food source. Most of fungal spores are transported by wind. Such species often produce dry or hydrophobic spores which do not absorb water and are readily scattered by raindrops, for example.
Fungi produce spores through sexual and asexual reproduction. Many fungi produce more than one type of spore as part of their life cycles. Fungal spores may be formed via an asexual process involving only mitosis (mitospores), or via a sexual process involving meiosis (meiospores). The manner in which meiospores are formed reflects the evolutionary history and thus the classification for the major groups (phyla) of fungi.
Examples of meiospores—spores that are the products of meiosis—include ascospores (phylum Ascomycota) and basidiospores (phylum Basidiomycota). Ascospores are formed inside a sac-like structure called an ascus. An ascus starts out as a sac of cytoplasm and nuclei, and by a process called “free cell formation” (Kirk et al. 2008) a cell wall forms de novo around each nucleus and surrounding cytoplasm to form ascospores (typically eight per ascus). Ascospores vary in size, shape, color, septation, and ornamentation among taxa. Basidiospores are formed on a basidium and are typically one-celled with one or two haploid nuclei. Basidiospores vary in size, color and ornamentation depending upon the taxonomic group.
_
Sporangiospores (spores) are produced in a sporangium. Release of spores from a sporangium is depicted in the figure below:
This bright field light micrograph shows the release of spores from a sporangium at the end of a hypha called a sporangiophore. The organism depicted is a Mucor sp. fungus: a mold often found indoors.
_
_
The characteristic features and size of the spores determine how deep they may penetrate into respiratory tract, whereby the exact site of allergic response can be determined. Spores larger than 10 µm diameter are deposited in the nasopharynx causing rhinitis; spores smaller than 5 µm penetrate to the alevoli causing alevolitis. Spores <10 µm size mostly deposit in the bronchi and bronchioles causing asthma (Lacey, 1996).
_
The spores of VA mycorrhizae are highly resistant and can live for many years in the absence of plant roots. When roots come near, they germinate and colonize the roots. Thus the shelf life of Agbio-Endos/Ectos can be years in some cases, but always at least two years. The fungal spores of ringworm can stay alive on clothing, bedding, and elsewhere as long as their food supply (dead skin cells) is present, and they have a moist and warm environment. These spores can live for as long as 12 to 20 months in the right environment. Mushrooms must shed their spores fast as both mushrooms and spores often live for only a few days. Fungal Spores can survive in environment for few days to few years depending on type of fungus and type of environment.
_
Teleomorph and anamorph:
Many fungi are able to reproduce by both sexual and asexual processes. Sexual and asexual reproduction may require different sets of conditions (e. g., nutrients, temperature, light, moisture). In some fungi, two sexually compatible strains must conjugate (mate) in order for sexual reproduction to occur. The terms ‘anamorph’ and ‘teleomorph’ are used to convey the asexual and sexual reproduction morphological types, respectively, in a particular fungus. The concept of anamorph and teleomorph is a confusing one for many students, as we are not accustomed to thinking about organisms with such reproductive flexibility.
_
Sexual Reproduction:
Sexual reproduction introduces genetic variation into a population of fungi. In fungi, sexual reproduction often occurs in response to adverse environmental conditions. Two mating types are produced. When both mating types are present in the same mycelium, it is called homothallic, or self-fertile. Heterothallic mycelia require two different, but compatible, mycelia to reproduce sexually. Although there are many variations in fungal sexual reproduction, all include the following three stages. First, during plasmogamy (literally, “marriage or union of cytoplasm”), two haploid cells fuse, leading to a dikaryotic stage where two haploid nuclei coexist in a single cell. During karyogamy (“nuclear marriage”), the haploid nuclei fuse to form a diploid zygote nucleus. Finally, meiosis takes place in the gametangia (singular, gametangium) organs, in which gametes of different mating types are generated. At this stage, spores are disseminated into the environment.
_
Some fungi exist primarily as filamentous dikaryotic organisms:
The sexual phase is begun when haploid hyphae from two different fungal organisms meet and fuse. When this occurs, the cytoplasm from the two cells fuses, but the nuclei remain separate and distinct. The single hypha produced by fusion typically has two nuclei per “cell”, and is known as a dikaryon, meaning “two nuclei”. The dikaryon may live and grow for years, and some are thought to be many centuries old. Eventually, the dikaryon forms sexual sporangia in which the nuclei fuse into one, which then undergoes meiosis to form haploid spores, and the cycle is repeated.
_
Some fungi, especially the chytrids and zygomycetes, have a life cycle more like that found in many protists. The organism is haploid, and has no diploid phase, except for the sexual sporangium. A number of fungi have lost the capacity for sexual reproduction, and reproduce by asexual spores or by vegetative growth only. These fungi are referred to as Fungi Imperfecti, and include, among other members, the athlete’s foot and the fungus in bleu cheese. Other fungi, such as the yeasts, primarily reproduce through asexual fission, or by fragmentation — breaking apart, with each of the pieces growing into a new organism.
_____
Fungi may utilize both asexual and sexual stages of reproduction. Types of fungal reproduction is depicted in figure below:
______
______
Section-3
Ecology of fungi:
Wherever there is moisture, moderate temperatures, and a supply of organic food there are fungi. Since they digest their food outside of their bodies, they literally live within their food supplies. When the area around them is depleted, they grow into a new supply. They occur worldwide, although there are an estimated 1.5 million species of fungi, less than 10 percent of them have been described. About 500 species are marine; the rest are terrestrial with several thousand described symbionts and plant and animal pathogens. Fungi usually are the primary decomposers in their natural habitats and are capable of digesting a wide array of organic materials—including, unfortunately, some substances of economic importance to humans. Most are saprobes, but some, like their animal relatives, attack living prey, a notorious example being the fungus that sets hyphal traps, ensnares and then digests nematodes. Many fungi are parasitic and the major pathogens of many crop plants such as corn and wheat.
_
Fungi are involved in a wide range of activities—some fungi are decomposers, parasites or pathogens of other organisms, and others are beneficial partners in symbiosis with animals, plants or algae. Although often inconspicuous, fungi occur in every environment on Earth and play very important roles in most ecosystems. Along with bacteria, fungi are the major decomposers in most terrestrial (and some aquatic) ecosystems, and therefore play a critical role in biogeochemical cycles and in many food webs. As decomposers, they play an essential role in nutrient cycling, especially as saprotrophs and symbionts, degrading organic matter to inorganic molecules, which can then re-enter anabolic metabolic pathways in plants or other organisms. With their versatile metabolism, fungi can break down organic matter which would not otherwise be recycled in the ecosystem. Some elements, such as nitrogen and phosphorus, are required in large quantities by biological systems, and yet are not abundant in the environment unless this breakdown takes place. Even trace elements present in low amounts in many habitats are essential for growth would remain tied up in rotting organic matter if fungi and bacteria did not return them to the environment via their metabolic activity. Thus, fungi make it possible for other living things to be supplied with the nutrients they need to live.
_
Because of their varied metabolic pathways, fungi can fulfil many important roles. Not only do they help to stabilize ecosystems and supply us with food, but they are also directly used in the production of beer, cheese, and bread, as well as various medicines. Some fungi are also extremely sensitive to air pollution, especially to abnormal levels of nitrogen and sulphur. The U.S. Forest Service and National Park Service can monitor air quality by measuring their relative abundance and health in an area. Currently, fungi are being investigated as potential tools in bioremediation; for example, some species of fungi can be used to break down diesel oil, polycyclic aromatic hydrocarbons (PAHs), and even heavy metals, such as cadmium and lead.
_
Relatively little is known of the effects of the environment on the distribution of fungi that utilize dead organic material as food (i.e., saprobic fungi). The availability of organic food is certainly one of the factors controlling such distribution. A great number of fungi appear able to utilize most types of organic materials, such as lignin, cellulose, or other polysaccharides, which have been added to soils or waters by dead vegetation. Most saprotrophic fungi are widely distributed throughout the world, only requiring that their habitats have sufficient organic content to support their growth. However, some saprotrophs are strictly tropical and others are strictly temperate-zone forms; fungi with specific nutritional requirements are even further localized. Fungi are found in areas that have sufficient organic material and moisture to support their growth. For example, members of the genus Armillaria are often found in forests living on trees such as hardwoods or conifers.
_
Moisture and temperature are two important ecological factors (besides organic material) that are important in determining the distribution of fungi. Laboratory studies have shown that many, perhaps the majority, of fungi are mesophilic, meaning they have an optimum growth temperature of 20–30 °C (68–86 °F). Thermophilic species are able to grow at 50 °C (122 °F) or higher but are unable to grow below 30 °C. Although the optimum temperature for growth of most fungi lies at or above 20 °C, a large number of species are able to grow close to or below 0 °C (32 °F). The so-called snow molds and the fungi that cause spoilage of refrigerated foods are examples of this group. Obviously, temperature relationships influence the distribution of various species. Certain other effects of temperature are also important factors in determining the habitats of fungi. Many coprophilous (dung-inhabiting) fungi, such as Pilobolus, although able to grow at a temperature of 20–30 °C, require a short period at 60 °C (140 °F) for their spores to germinate.
______
Fungal symbioses:
Symbiosis is any type of a close and long-term biological interaction between two different biological organisms, be it mutualistic, commensalistic, or parasitic. Many fungi have important symbiotic relationships with organisms from most if not all kingdoms. These interactions can be mutualistic or antagonistic in nature, or in the case of commensal fungi are of no apparent benefit or detriment to the host.
When both members of the association benefit, the symbiotic relationship is called mutualistic. Fungi form mutualistic associations with many types of organisms, including cyanobacteria, algae, plants, and animals.
Among the examples of fungal-plant mutualism are the endophytes: fungi that live inside tissue without damaging the host plant. Endophytes release toxins that repel herbivores, or confer resistance to environmental stress factors, such as infection by microorganisms, drought, or heavy metals in soil.
For the most common example, most terrestrial plants form symbiotic relationships with fungi via their roots. The roots of the plant connect with the underground parts of the fungus forming mycorrhizae (from the Greek words myco meaning fungus and rhizo meaning root). In a mycorrhizal association, the fungal mycelia use their extensive network of hyphae and large surface area in contact with the soil to channel water and minerals from the soil into the plant. In exchange, the plant supplies the products of photosynthesis to fuel the metabolism of the fungus. Even some plants, such as orchids, have developed so strong an association with fungi that their seeds generally cannot germinate and grow without a fungal mycorrhiza partner!
Mutualistic relationships between fungi and animals involves numerous insects; Arthropods depend on fungi for protection, while fungi receive nutrients in return and ensure a way to disseminate the spores into new environments.
As pathogens and parasites:
Many fungi are parasites on plants, animals (including humans), and other fungi. Serious pathogens of many cultivated plants causing extensive damage and losses to agriculture and forestry. Some fungi can cause serious diseases in humans, several of which may be fatal if untreated. These include aspergillosis, candidiasis, coccidioidomycosis, cryptococcosis, histoplasmosis, mycetomas, and paracoccidioidomycosis. Furthermore, persons with immuno-deficiencies are particularly susceptible to disease by genera such as Aspergillus, Candida, Cryptoccocus, Histoplasma, and Pneumocystis. Other fungi can attack eyes, nails, hair, and especially skin, the so-called dermatophytic and keratinophilic fungi, and cause local infections such as ringworm and athlete’s foot. Fungal spores are also a cause of allergies, and fungi from different taxonomic groups can evoke allergic reactions.
The organisms which parasitize fungi are known as mycoparasitic organisms. Certain species of the genus Pythium, which are oomycetes, have potential as biocontrol agents against certain fungi. Fungi can also act as mycoparasites or antagonists of other fungi, such as Hypomyces chrysospermus, which grows on bolete mushrooms. Fungi can also become the target of infection by mycoviruses.
______
______
Human use of fungi:
Fungi have played important roles as foods and medicines in both ancient and modern biotechnological processes. Fungi range from microscopic yeasts and molds to macroscopic mushrooms. Their applications include production of antibiotics, alcohols, enzymes, organic acids, and numerous pharmaceuticals. The advent of recombinant DNA technology enables fungi to utilize novel carbon sources and to be hosts for the production of heterogonous proteins.
The human use of fungi for food preparation or preservation and other purposes is extensive and has a long history. Mushroom farming and mushroom gathering are large industries in many countries. The study of the historical uses and sociological impact of fungi is known as ethnomycology. Because of the capacity of this group to produce an enormous range of natural products with antimicrobial or other biological activities, many species have long been used or are being developed for industrial production of antibiotics, vitamins, and anti-cancer and cholesterol-lowering drugs. More recently, methods have been developed for genetic engineering of fungi, enabling metabolic engineering of fungal species. For example, genetic modification of yeast species—which are easy to grow at fast rates in large fermentation vessels—has opened up ways of pharmaceutical production that are potentially more efficient than production by the original source organisms.
-1. Therapeutic uses:
Many species produce metabolites that are major sources of pharmacologically active drugs. Particularly important are the antibiotics, including the penicillins, a structurally related group of β-lactam antibiotics that are synthesized from small peptides. Although naturally occurring penicillins such as penicillin G (produced by Penicillium chrysogenum) have a relatively narrow spectrum of biological activity, a wide range of other penicillins can be produced by chemical modification of the natural penicillins. Modern penicillins are semisynthetic compounds, obtained initially from fermentation cultures, but then structurally altered for specific desirable properties. Other antibiotics produced by fungi include: ciclosporin, commonly used as an immunosuppressant during transplant surgery; and fusidic acid, used to help control infection from methicillin-resistant Staphylococcus aureus bacteria. Other drugs produced by fungi include griseofulvin isolated from Penicillium griseofulvum, used to treat fungal infections, and statins (HMG-CoA reductase inhibitors), used to inhibit cholesterol synthesis. Examples of statins found in fungi include mevastatin from Penicillium citrinum and lovastatin from Aspergillus terreus and the oyster mushroom. Fungi produce compounds that inhibit viruses and cancer cells. The shiitake mushroom is a source of lentinan, a clinical drug approved for use in cancer treatments in several countries, including Japan. In Europe and Japan, polysaccharide-K (brand name Krestin), a chemical derived from Trametes versicolor, is an approved adjuvant for cancer therapy. Recently, new drug candidates from fungi have been found with anti-tumor, antihypertensive, immunosuppressant, anti-diarrheal, or anti-mutagenic properties. Increasing scientific evidence from animal tests and clinical studies has supported the idea that some fungi could be used as adjuvant cancer treatments.
Traditional and folk medicine:
Certain mushrooms enjoy usage as therapeutics in folk medicines, such as Traditional Chinese medicine. Notable medicinal mushrooms with a well-documented history of use include Agaricus subrufescens, Ganoderma lucidum, and Ophiocordyceps sinensis.
-2. Foods:
Baker’s yeast or Saccharomyces cerevisiae, a unicellular fungus, is used to make bread and other wheat-based products, such as pizza dough and dumplings. Yeast species of the genus Saccharomyces are also used to produce alcoholic beverages through fermentation. Shoyu koji mold (Aspergillus oryzae) is an essential ingredient in brewing Shoyu (soy sauce) and sake, and the preparation of miso, while Rhizopus species are used for making tempeh. Several of these fungi are domesticated species that were bred or selected according to their capacity to ferment food without producing harmful mycotoxins, which are produced by very closely related Aspergilli. Quorn, a meat substitute, is made from Fusarium venenatum.
Edible mushrooms include commercially raised and wild-harvested fungi. Agaricus bisporus, sold as button mushrooms when small or Portobello mushrooms when larger, is the most widely cultivated species in the West, used in salads, soups, and many other dishes. Many Asian fungi are commercially grown and have increased in popularity in the West. They are often available fresh in grocery stores and markets, including straw mushrooms (Volvariella volvacea), oyster mushrooms (Pleurotus ostreatus), shiitakes (Lentinula edodes), and enokitake (Flammulina spp.).
Many other mushroom species are harvested from the wild for personal consumption or commercial sale. Milk mushrooms, morels, chanterelles, truffles, black trumpets, and porcini mushrooms (Boletus edulis) (also known as king boletes) demand a high price on the market. They are often used in gourmet dishes.
Certain types of cheeses require inoculation of milk curds with fungal species that impart a unique flavor and texture to the cheese. Examples include the blue color in cheeses such as Stilton or Roquefort, which are made by inoculation with Penicillium roqueforti. Molds used in cheese production are non-toxic and are thus safe for human consumption; however, mycotoxins (e.g., aflatoxins, roquefortine C, patulin, or others) may accumulate because of growth of other fungi during cheese ripening or storage.
Poisonous fungi:
Many mushroom species are poisonous to humans and cause a range of reactions including slight digestive problems, allergic reactions, hallucinations, severe organ failure, and death. Genera with mushrooms containing deadly toxins include Conocybe, Galerina, Lepiota, and, the most infamous, Amanita. The latter genus includes the destroying angel (A. virosa) and the death cap (A. phalloides), the most common cause of deadly mushroom poisoning. The false morel (Gyromitra esculenta) is occasionally considered a delicacy when cooked, yet can be highly toxic when eaten raw. Tricholoma equestre was considered edible until it was implicated in serious poisonings causing rhabdomyolysis. Fly agaric mushrooms (Amanita muscaria) also cause occasional non-fatal poisonings, mostly as a result of ingestion for its hallucinogenic properties. Historically, fly agaric was used by different peoples in Europe and Asia and its present usage for religious or shamanic purposes is reported from some ethnic groups such as the Koryak people of northeastern Siberia.
As it is difficult to accurately identify a safe mushroom without proper training and knowledge, it is often advised to assume that a wild mushroom is poisonous and not to consume it.
Food Safety:
Many fungi produce biologically active compounds, several of which are toxic to animals or plants and are therefore called mycotoxins. Of particular relevance to humans are mycotoxins produced by molds causing food spoilage, and poisonous mushrooms. Particularly infamous are the lethal amatoxins in some Amanita mushrooms, and ergot alkaloids, which have a long history of causing serious epidemics of ergotism (St Anthony’s Fire) in people consuming rye or related cereals contaminated with sclerotia of the ergot fungus, Claviceps purpurea. Other notable mycotoxins include the aflatoxins, which are insidious liver toxins and highly carcinogenic metabolites produced by certain Aspergillus species often growing in or on grains and nuts consumed by humans, ochratoxins, patulin, and trichothecenes (e.g., T-2 mycotoxin) and fumonisins, which have significant impact on human food supplies or animal livestock.
Mycotoxigenic fungi, such as Aspergillus, Fusarium, and Penicillium, pose serious problems and toxicological risks at preharvest and postharvest stage as well as in processed food products. Biological control using beneficial fungi (filamentous and yeasts) allows managing contamination by mycotoxins at both pre- and postharvest levels and can contribute to decontamination and/or detoxification of these dangerous compounds from food and feeds.
For instance, atoxigenic isolates of Aspergillus are widely used to prevent aflatoxin contamination of crops, such as nuts and maize, in several parts of the world. Field experiments have demonstrated that Trichoderma gamsii and Fusarium equiseti can reduce FHB incidence and DON contamination on wheat. The yeastlike Aureobasidium pullulans or the yeast Metschnikowia fructicola are able to reduce OTA contamination by Aspergillus sp. on grape and can degrade OTA to a less toxic compound in vitro.
Finally, patulin- and OTA-producing fungi on apples and pears can be controlled by the application of Aureobasidium pullulans or Candida sake, resulting also in a reduction of mycotoxin contamination.
Food spoilage:
Fungi play a major role in recycling organic material and are also responsible for major spoilage and economic losses of stored food.
-3. Pest control:
In agriculture, fungi may be useful if they actively compete for nutrients and space with pathogenic microorganisms such as bacteria or other fungi via the competitive exclusion principle, or if they are parasites of these pathogens. For example, certain species may be used to eliminate or suppress the growth of harmful plant pathogens, such as insects, mites, weeds, nematodes, and other fungi that cause diseases of important crop plants. This has generated strong interest in practical applications that use these fungi in the biological control of these agricultural pests. Entomopathogenic fungi can be used as biopesticides, as they actively kill insects. Examples that have been used as biological insecticides are Beauveria bassiana, Metarhizium spp., Hirsutella spp., Paecilomyces (Isaria) spp., and Lecanicillium lecanii. Endophytic fungi of grasses of the genus Neotyphodium, such as N. coenophialum, produce alkaloids that are toxic to a range of invertebrate and vertebrate herbivores. These alkaloids protect grass plants from herbivory, but several endophyte alkaloids can poison grazing animals, such as cattle and sheep. Infecting cultivars of pasture or forage grasses with Neotyphodium endophytes is one approach being used in grass breeding programs; the fungal strains are selected for producing only alkaloids that increase resistance to herbivores such as insects, while being non-toxic to livestock.
-4. Bioremediation:
Bioremediation is a process where biological organisms are used to remove or neutralize an environmental pollutant by metabolic process. Certain fungi, in particular white-rot fungi, can degrade insecticides, herbicides, pentachlorophenol, creosote, coal tars, and heavy fuels and turn them into carbon dioxide, water, and basic elements. Fungi have been shown to biomineralize uranium oxides, suggesting they may have application in the bioremediation of radioactively polluted sites.
-5. Model organisms:
Several pivotal discoveries in biology were made by researchers using fungi as model organisms, that is, fungi that grow and sexually reproduce rapidly in the laboratory. For example, the one gene-one enzyme hypothesis was formulated by scientists using the bread mold Neurospora crassa to test their biochemical theories. Other important model fungi are Aspergillus nidulans and the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe, each of which with a long history of use to investigate issues in eukaryotic cell biology and genetics, such as cell cycle regulation, chromatin structure, and gene regulation. Other fungal models have more recently emerged that address specific biological questions relevant to medicine, plant pathology, and industrial uses; examples include Candida albicans, a dimorphic, opportunistic human pathogen, Magnaporthe grisea, a plant pathogen, and Pichia pastoris, a yeast widely used for eukaryotic protein production.
-6. Others:
Fungi are used extensively to produce industrial chemicals like citric, gluconic, lactic, and malic acids, and industrial enzymes, such as lipases used in biological detergents, cellulases used in making cellulosic ethanol and stonewashed jeans, and amylases, invertases, proteases and xylanases. The fungus can even make diesel compounds from cellulose, which would make it a better source of biofuel than anything we use at the moment. The fungus, which has been named Gliocladium roseum, produces a number of different molecules made of hydrogen and carbon that are found in diesel.
_______
_______
Section-4
Pathogenic fungi:
Pathogenic fungi are fungi that cause disease in humans or other organisms. Approximately 300 fungi are known to be pathogenic to humans. Markedly more fungi are known to be pathogenic to plant life than those of the animal kingdom. The study of fungi pathogenic to humans is called “medical mycology”. Although fungi are eukaryotic, many pathogenic fungi are microorganisms. The study of fungi and other organisms pathogenic to plants is called plant pathology. Humans are exposed to hundreds of fungal spores daily, usually not producing any harmful effect on their health. This protection is by various defense mechanisms that effectively eliminate the fungal spores.
Pathogenic fungi represent an enormous, but under-appreciated burden on human health, with invasive mycoses killing approximately one and a half million people each year. The most common invasive fungal diseases of humans, and the dominant aetiological agents are: aspergillosis (Aspergillus fumigatus), candidiasis (Candida albicans), cryptococcosis (Cryptococcus neoformans), mucormycosis (Rhizopus oryzae/delemar), pneumocystis (Pneumocystis jirovecii), and the endemic mycoses histoplasmosis (Histoplasma capsulatum), coccidioidomycosis (Coccidioides immitis), blastomycosis (Blastomyces dermatitidis), paracoccidioidomycosis (Paracoccidioides brasiliensis) and penicilliosis (Penicillium marneffei). These major fungal pathogens are highly diverse from both evolutionary and ecological perspectives. Pathogenic species are distributed throughout the Fungal Kingdom and found in three of the major fungal phyla: the Mucormycotina (Rhizopus), the Basidiomycota (Cryptococcus) and the Ascomycota (all other major invasive pathogenic species).
Coccidioides immitis, Histoplasma capsulatum, Blastomyces dermatitidis, Paracoccidioides brasiliensis and dermatophyte fungi can infect healthy, immunologically competent individuals. By contrast, species such as Candida, Aspergillus, Rhizopus and Fusarium are normally avirulent in healthy people, but can cause disseminated fatal infections in patients with suppressed immunity. These are called opportunistic pathogenic fungi. The fungus Cryptococcus neoformans can be considered both as a true and opportunistic pathogen since it can cause infections in immunologically competent as well as immunocompromised hosts.
The occurrence of superficial as well as invasive opportunistic fungal infections has increased significantly over the past two decades. This increase can be attributed to the growing number of immunocompromised patients- including those with AIDS, neoplastic disease, advanced age, long-standing diabetes mellitus, undergoing blood and marrow transplantation, solid-organ transplantation, major surgery, receiving immunosuppressive therapy and premature infants. Genetic predisposition to invasive fungal infection has been reported recently owing to defective NADPH oxidase activity, abnormal production of tumor necrosis factor- α, interleukin 10 and other cytokines. Of course, trauma, burns, and malnutrition also increase susceptibility to fungal infection.
The spectrum of opportunistic fungal infections is changing. The majority of invasive fungal infections are still due to Aspergillus and Candida species; but infections due to mycelial fungi other than Aspergillus and non-albicans species of Candida are becoming increasingly common. Any fungus present in the environment can be potentially pathogenic in immunocompromised patients.
_
Pathogenic mechanisms:
Ustilago maydis is a pathogenic plant fungus that causes smut disease in maize and teosinte. Plants have evolved efficient defense systems against pathogenic microbes such as U. maydis. A rapid defense reaction after pathogen attack is the oxidative burst where the plant produces reactive oxygen species at the site of the attempted invasion. U. maydis can respond to the oxidative burst with an oxidative stress response, regulated by the gene YAP1. The response protects U. maydis from the host defense, and is necessary for the pathogen’s virulence. Furthermore, U. maydis has a well-established recombinational DNA repair system which acts during mitosis and meiosis. The system may assist the pathogen in surviving DNA damage arising from the host plant’s oxidative defensive response to infection.
Cryptococcus neoformans is an encapsulated yeast that can live in both plants and animals. C. neoformans usually infects the lungs, where it is phagocytosed by alveolar macrophages. Some C. neoformans can survive inside macrophages, which appears to be the basis for latency, disseminated disease, and resistance to antifungal agents. One mechanism by which C. neoformans survives the hostile macrophage environment is by up-regulating the expression of genes involved in the oxidative stress response. Another mechanism involves meiosis. The majority of C. neoformans are mating “type a”. Filaments of mating “type a” ordinarily have haploid nuclei, but they can become diploid (perhaps by endoduplication or by stimulated nuclear fusion) to form blastospores. The diploid nuclei of blastospores can undergo meiosis, including recombination, to form haploid basidiospores that can be dispersed. This process is referred to as monokaryotic fruiting. This process requires a gene called DMC1, which is a conserved homologue of genes recA in bacteria and RAD51 in eukaryotes, that mediates homologous chromosome pairing during meiosis and repair of DNA double-strand breaks. Thus, C. neoformans can undergo a meiosis, monokaryotic fruiting, that promotes recombinational repair in the oxidative, DNA damaging environment of the host macrophage, and the repair capability may contribute to its virulence.
_
Fungal pathogens afflicting humans are subdivided into those that remain superficial (i.e., restricted to the epithelial surface) and those that invade deep organs and tissues (deep fungi). Some species are considered opportunistic (infecting only immunocompromised hosts) and others truly pathogenic (i.e., capable of infecting normal persons). Mucormycosis (formerly zygomycosis or phycomycosis) is the name most widely familiar for any infection caused by a fungus that is a member of the class Zygomycetes (formerly Phycomycetes), order Mucorales and genera Absidia, Rhizopus, and Mucor. They are found in soil, dung and dust throughout the world and are common causes of food spoilage. Overall, mucormycosis is the third leading cause of invasive opportunistic fungal infection after Aspergillus and Candida spp.
____
____
Innate Defense against Fungal Pathogens:
Human fungal infections have been on the rise in recent years and proved increasingly difficult to treat as a result of the lack of diagnostics, effective antifungal therapies, and vaccines. Most pathogenic fungi do not cause disease unless there is a disturbance in immune homeostasis, which can be caused by modern medical interventions, disease-induced immunosuppression, and naturally occurring human mutations. The innate immune system is well equipped to recognize and destroy pathogenic fungi through specialized cells expressing a broad range of pattern recognition receptors (PRRs).
Human fungal pathogens are responsible for more than a million life-threatening infections annually, which can be associated with mortality rates reaching 95% (Brown et al. 2012). Fungal infections are difficult to treat and control because of rising problems of antifungal drug resistance and the lack of diagnostics, novel antifungal drugs, and vaccines. Fortunately, the majority of pathogenic fungi are opportunistic pathogens (Table below) and, as such, do not normally cause disease unless there are alternations in immune defense. The use of immunosuppressive drugs, the human immunodeficiency virus (HIV) epidemic, and modern clinical interventions result in such alternations and have contributed substantially to the recent increases of systemically infected patients. Fungi also cause nonlethal skin and mucosal infections that are equally difficult to treat and often recurring.
_
Species of pathogenic fungi that cause disease in humans, the barrier tissue breached and organs infected, and the PRRs needed for protective immunity:
|
Selected disease(s) |
Barriers breached/site of infection |
Selected PRRs involved in protective immunity |
|
|
Ascomycota |
|||
|
Candida albicans |
Disseminated candidiasis |
Blood, kidneys, brain, heart |
TLR-2/TLR-4/TLR-6 |
|
Aspergillus fumigatus |
Invasive pulmonary aspergillosis |
Lung, blood |
TLR-9/TLR-3 |
|
Pneumocystis carinii |
Pneumonia |
Lung |
Dectin-1 |
|
Blastomyces dermatitidis |
Blastomycosis (pneumonia) |
Lung |
?? |
|
Histoplasma capsulatum |
Histoplasmosis (pneumonia) |
Lung |
?? |
|
Paracoccidioides brasiliensis |
Paracoccidioidomycosis |
Lung |
TLR-2/TLR-4 |
|
Coccidioides immitus |
Coccidioidomycosis (Valley fever) |
Lung, blood |
Dectin-1 |
|
Fonsecaea pedrosi |
Chromoblastomycosis |
Skin |
TLRs |
|
Basidiomycota |
|||
|
Cryptococcus neoformans |
Cryptococcosis/cryptococcal meningitis |
Lung, brain/CNS |
MR |
|
Trichosporon rubrum |
Onychomycosis |
Skin/nails |
?? |
|
Malassezia sympodialis |
Atopic dermatitis |
Skin |
Mincle |
CNS, central nervous system; CR3, complement receptor 3; GI, gastrointestinal; MR, mannose receptor; PRRs, pattern recognition receptors; TLR, Toll-like receptor.
_
The innate immune system is the first line of defense against pathogens and broadly protects against invading microorganisms. Genetically inherited receptors, called pattern recognition receptors (PRRs), are used by innate cells for recognition of conserved pathogen-associated molecular patterns. Signaling downstream from PRRs activates cellular responses and killing mechanisms, and also helps initiate and shape adaptive immune responses. Adaptive immunity, unlike innate, is activated by specific antigens recognized by noninherited T-cell/B-cell receptors and has a faster response time with each subsequent challenge (immune memory) (Wüthrich et al. 2012).
The vast predominance of fungi in the environment results in continual human exposure. It is estimated that we inhale several hundred Aspergillus fumigatus spores a day (Rivera et al. 2011), and many humans are also colonized with commensal fungi (e.g., Candida albicans) (Iliev et al. 2012). For this reason, our innate system is equipped to recognize fungal particles and maintain commensal relationships, but also destroy the pathogenic fungi that we are exposed to.
_____
_____
Mycosis:
Mycoses are infectious diseases caused by pathogenic fungi. They include fungal infections of the skin, just under the skin and ones that are more deep or widespread such as histoplasmosis and blastomycosis. They include yeast infections such as candidiasis and pityriasis versicolor, and include several other opportunistic fungal infections such as aspergillosis and mucormycosis. Several, including sporotrichosis, chromoblastomycosis and mycetoma are neglected. Mycoses are common and a variety of environmental and physiological conditions can contribute to the development of fungal diseases. Inhalation of fungal spores or localized colonization of the skin may initiate persistent infections; therefore, mycoses often start in the respiratory track or on the skin. Fungal infections of the skin was the 4th most common skin disease in 2010 affecting 984 million people. An estimated 1.6 million people die each year of invasive fungal infections. In ICD-10CM, mycoses have 14 entries coding from B35 to B49.
______
Household molds:
Molds are a group of fungi, which are specifically multi-cellular microscopic organisms characterised by the presence of multi-cellular filaments, the hyphae. Whether it is a cold, damp winter or a warm, humid summer, activities at home can result in moisture indoors and the appearance of mold. Mold can grow on walls, clothes, books, toys, and even CDs. It can turn prized possessions into musty relics that only look fit for the garbage. Molds are a form of fungus. There are many different types, and they can occur both indoors and outdoors. Molds produce spores, which spread by floating around in the air. Mold spores are present in all indoor environments. There is no way to prevent spores, and they can persist in conditions where mold itself cannot grow. Mold spores thrive in environments that are moist and warm, so when they land on a damp spot, they begin to grow. Molds can grow on a variety of different surfaces, including fabric, paper, wood, glass, and plastic. As they grow, they may digest the material they are growing on. While mold cannot get nutrients from inorganic material such as concrete, glass and metal, it can grow on the dirt present on these surfaces,
Common indoor molds include:
Alternaria: This occurs in damp places indoors, such as showers or under leaky sinks.
Aspergillus: This often grows indoors, on dust, powdery food items, and building materials, such as drywall.
Cladosporium: This can grow in either cool or warm areas. It tends to appear on fabrics and wood surfaces.
Penicillium: This tends to grow on materials with water damage. It often has a blue or green appearance.
Molds take a variety of forms and textures. They can be white, black, yellow, blue, or green and often look like discoloration or stain to a surface. They can also have a velvety, fuzzy, or rough appearance, depending on the type of mold and where it is growing. Mold can pose a health problem, especially for people with an allergy, an existing respiratory problem, or a weakened immune system.
______
Fungi inside refrigerators and on onions are not the ones causing mucormycosis:
Inside a refrigerator and on damp walls, some bacteria (Bacillus and Acinetobacter species), yeast (Saccharomyces and Candida) and different molds such as stachybotrys chartarum can grow. The black mold found inside refrigerators is stachybotrys chartarum. According to the United States Centers for Disease Control and Prevention, stachybotrys chartarum and other similar molds may cause health symptoms that are non-specific. However, if stachybotrys chartarum or other molds are found, they should be removed.
According to the US department of agriculture, the black mold on onions is caused by aspergillus niger, a common fungus found in soil. This kind of fungus causes infection in rare cases. However, onions should always be thoroughly washed before consuming.
Mucormycosis (earlier known as zygomycosis) is a serious but rare fungal infection caused by a group of molds called mucormycetes. These molds live throughout the environment. Mucormycosis mainly affects people who have health issues or those who consume medicines that lower the body’s ability to fight germs and sickness. The fungus that causes mucormycosis cannot survive in the refrigerator. Also, it is incorrect to distinguish fungus on the basis of colour. Thus, it is clear that the fungus which forms a black layer in refrigerators and the one that creates a black covering on onions are completely different from the fungus that causes mucormycosis.
______
______
Section-5
Introduction to mucormycosis:
Many people recovering from Covid-19 have of late been afflicted by black fungus – or mucormycosis – disease. The fungus invades the sinus and makes its way into the intraorbital and intracranial regions. If its progression is not checked early, 50-80% of patients could die.
People experience fungi most often in their kitchens, when fruits rot or the bread turns moldy. Fungi play an important role on Earth. They have helped plants move from their aquatic habitats to land, and still help them obtain minerals from the soil. Fungi decompose organic litter and recycle the nutrients locked up in the leaves and wood. Some of them have also evolved to become plant pathogens: they infect plants, multiply and disperse to other plants, leaving destruction in their wake. The great Irish famine of 1845 that left a million people dead was the work of the fungus Phytophthora infestans, which wiped out the country’s staple potato crop. While fungal diseases are common among plants, only a very small fraction of them assail humans. One reason is that animals, including humans, have evolved intricate immune systems. However, when the immune system has been breached by another illness, fungi that are otherwise harmless take advantage and invade human tissues. These are called opportunistic infections. Even so, unlike their pathogenic bacterial counterparts, fungi rarely cause life-threatening diseases. A few fungi, like the Candida yeast, can sometimes kick off a serious infection. Candida lives on the skin and inside the mouth, throat and vagina of healthy persons without causing any problems. But if the host’s body has been weakened by another disease or drugs, it can cause oral thrush, diaper rash and vaginal infections.
The Mucoralean fungi are even less problematic. They include the genera of Mucor and Rhizopus. These are ubiquitous molds occurring in the soil, compost, animal dung, rotting wood and plant material. You may have seen them as the black growth on old fruits and bread. Mucoralean fungi are generally the first colonisers of dead or decaying plant material. They rapidly utilise the limited amount of simple carbohydrates available before other fungi show up for the more complex carbohydrates, such as cellulose.
Like most fungi, Mucor produces millions of microscopic spherical, dark-hued structures called spores, which are dispersed in air. When the spores land on moist surfaces, like soil or plant material, they begin to germinate and produce thread like structures called mycelia. The mycelia branch out and feed on sugars in their surroundings and grow.
Fungal spores measure one thousandth to one hundredth of a millimeter. The density of the spores – the number of spores per cubic metre of atmosphere – varies depending on the fungus, the location (vegetation and exposed earth) and season. In tropical areas like in India, spore counts are generally higher during the summer than during the monsoons. But compared to the 1,000-5,000 spores per cubic meter outdoors, the count inside homes is typically 100-250 only. Five to 10 species account for more than 90% of the total spore density in the air. Most people come in contact with microscopic fungal spores every day, so it’s probably impossible to completely avoid coming in contact with mucormycetes. These fungi aren’t harmful to most people. However, for people who have weakened immune systems, breathing in mucormycetes spores can cause an infection in the lungs or sinuses which can spread to other parts of the body.
As it happens, hospitals are not free from these spores. A study in Tehran in 2014 suggested that hospital air could carry many opportunistic pathogenic fungi like Candida, Aspergillus, Penicillium and Rhizopus. In recent years, health-care-associated mucormycosis is increasingly documented. Mucormycosis has been reported following the use of elastoplast and the use of tongue depressors. Outbreaks have also been linked to hospital bed sheets, negative-pressure rooms, water leaks, poor ventilation, contaminated medical equipment, and building works. When a patient whose immune system has been compromised inhales Mucor spores, they may develop mucormycosis. This is a rare, non-contagious disease – but it can be debilitating or fatal if not treated quickly. The frequency of mucormycosis infections has increased in the last decade, principally because of the greater number of organ transplants. People who have received transplanted organs depend on immunosuppressant drugs to keep their bodies from rejecting the new organs, but in this state they are also predisposed to infection.
People suffering from Covid-19 and other viral diseases, congenital bone marrow disease, severe burns, cancers and untreated or irregularly treated diabetes have reduced immunity and are prone to developing mucormycosis. Covid-19 patients who have received steroids are particularly at risk because steroids suppress the immune system. This is why steroids should not be used unless absolutely necessary.
Experiments with rats and rabbits have found that the inhaled spores in healthy animals are quickly killed by white blood cells. But if the host’s immune response has been suppressed, the body produces fewer white blood cells. In this condition, the spores germinate and grow rapidly as thin, wire-like tubes that branch out and enter the blood vessels and kill them.
_____
Mucormycosis is caused by the fungi belonging to the order Mucorales. In most cases it is due to an invasion of the genera Rhizopus and Mucor, common bread molds. Humans acquire the infection predominantly by inhalation of sporangiospores, occasionally by ingestion of contaminated food or traumatic inoculation. The fungi under Mucorales are ubiquitous, and morphologically appear as broad, aseptate or sparsely septate ribbon-like hyphae. Eleven genera and ~27 species under Mucorales are associated with human infections. Mucormycosis is an angioinvasive disease that is characterised by tissue infarction and necrosis. The clinical presentation of mucormycosis is broad, depending on the underlying immunosuppression of the host. Although some overlap exists, the clinical presentation can be broadly grouped on the basis of anatomic localisation, such as rhino-orbital-cerebral (ROCM), pulmonary, gastrointestinal, cutaneous, disseminated mucormycosis and uncommon presentations of mucormycosis (e.g., renal). Sites involved in invasive mucormycosis are the sinuses (39%), lungs (24%) and skin (19%). Dissemination into the CNS occurs in approximately 23%. The overall mortality rate of patients suffering from mucormycosis was estimated as follows: 66% in patients with malignancies, 44% in diabetics and 35% in patients without underlying conditions. Not only does the patients’ immune status impact on the mortality rate, but also the site of infection. Patients suffering from disseminated infections have a mortality of up to 96%, patients with gastrointestinal infections up to 85% and patients suffering from pulmonary infections 76%. Gastrointestinal mucormycosis represents a rare disease entity and is frequently associated with patients’ exposure to high spore loads in combination with severe malnutrition. Isolated renal mucormycosis is a sporadic entity with a higher overall success rate (~65%) compared with all other invasive forms of invasive mucormycosis and may affect immunocompetent hosts.
_
Patients with diabetes mellitus, haematological malignancy and chemotherapy, haematopoietic stem cells, and solid-organ transplant recipients on immunosuppressive therapy, with iron overload, on peritoneal dialysis, extensive skin injury, and voriconazole therapy are at increased risk of acquiring mucormycosis. A considerable number of mucormycosis cases are reported in immunocompetent hosts. Though mucormycosis is globally distributed, certain risk factors, clinical forms, and causative agents of the disease are prevalent in India.
Uncontrolled diabetes mellitus is the most common underlying disease associated with mucormycosis in India, in contrast to haematological-malignancy patients and solid-organ transplant recipients in developed countries. Nevertheless, recent reports from India identified haematological malignancy and solid-organ transplant recipients as important risk factors, but the overwhelming number of patients with uncontrolled diabetes overshadows the picture. The ROCM type is the most common form of the disease in India, followed by the pulmonary and cutaneous types; however, the pulmonary form is the most common clinical presentation in developed countries. The cutaneous type is commonly seen in patients with trauma or burns. Isolated renal mucormycosis in a healthy host is a unique clinical presentation in India.
Seasonal fluctuations may exist, as studies show a higher occurrence of infections during autumn, as well as differences in epidemiology between developed and developing countries. In the latter, especially in India, mucormycosis, although sporadic, occur mainly in patients with uncontrolled diabetes or trauma. In tropical and subtropical countries such as India, Apophysomyces elegans is an emerging agent of mucormycosis. This species, unlike other Mucorales, is capable of infecting apparently immunocompetent hosts with severe cutaneous, rhino–orbito–cerebral, and renal forms of the disease. In developing countries R. homotallicus and M. irregularis are frequently found. In developed countries disease remains mostly seen in patients with hematological malignancies, undergoing chemotherapy and allogeneic stem cell transplants. Overall, different genera of Mucorales display a different pathogenicity or disease profile. Some of the Mucorales behave like Entomophthorales (Mucor irregularis) and Entomophthorales behave like Mucorales (Conidiobolus).
Mucormycosis is rare, affecting fewer than 1.7 people per million population each year in the US. However, it is around 80 times more prevalent in India. People of any age may be affected, including premature infants. The first case of mucormycosis was possibly one described by Friedrich Küchenmeister in 1855. The disease has been reported in natural disasters; 2004 Indian Ocean tsunami and the 2011 Missouri tornado. During the Covid-19 pandemic, a number of cases linked to immunosuppressive treatment for Covid-19 were reported in India in 2020 and 2021.
_
Mucormycosis is a rare, emerging fungal infection, with high morbidity and mortality. Due to the rarity of the disease, it is almost impossible to conduct large, randomized clinical trials, and most of the available data regarding epidemiology, diagnosis, and treatment, originate from case reports and case series. The first effort to analyze all the available literature was made by Roden et al. in 2005. Relatively large epidemiological studies were performed either on a national level or in patients with selected underlying diseases, for example, hematopoietic stem cell transplantation (HSCT). Registries are another source of valuable information, despite their inherent limitations.
_
Mucorales are ubiquitous throughout the environment and commonly found in decaying organic matter, soil, compost, and animal excreta. Mucorales characteristically produce large, ribbon-like hyphae with irregular diameter and only occasional septae, hence, their frequent characterization in tissue as aseptate fungi. Identification of the fungi is confirmed during culture when characteristic, saclike fruiting structures (sporangia) are observed that produce internally yellow or brown spores (sporangiospores). These sporangiospores range from 3 to 11 micrometers in diameter and are easily aerosolized and cause infections in susceptible hosts when inhaled or introduced through the cutaneous or percutaneous route. Mucorales fungi are ubiquitous, saprophytic and not fastidious fungi located in soil or decaying organic matter, with three genera that are known to be human pathogens, namely, Rhizopus, Absidia and Mucor. The optimal temperature for growth is 28 to 30°C under aerobic conditions, with an incubation period of 2 to 5 days. Incubation begins with inhalation of the spores or their direct inoculation into abraded skin. Whatever the route of infection (inhalation of airborne spores, ingestion, or direct skin inoculation), the hyphae invade blood vessels, causing tissue infarction and necrosis.
_
Given the ubiquitous nature of these fungi, most humans are exposed to these organisms. Sporangiospores, 3–11 μm in diameter according to the species involved, are easily aerosolized. A mature sporangium may contain up to 100,000 sporangiospores, which then become dispersed into the environment; in general, it is estimated that humans may inhale more than 170.000 airborne fungal spores per day. When person breathes, every breath contains between 1 and 10 spores. Even though high numbers of spores enter the respiratory tract, these fungi infrequently cause infections in immunocompetent humans. At risk are mainly patients with a hampered immune system, among those are patients with poorly controlled diabetes mellitus, those receiving glucocorticosteroids, those suffering from neutropenia, who have undergone transplantation, those with iron overloads, or those with burns. As mucormycetes mainly cause infections in immunocompromised hosts, they are regarded as pathogens with low virulence. In addition, infections have been described in patients requiring dialysis, intravenous drug abusers, and individuals suffering from severe malnutrition, persistent diarrhea, gastric or intestinal ulcers and colitis. So far, the relative contribution of each condition to mucormycosis risk is unknown. The route of infection is thought to be via inhalation, traumatic inoculation or ingestion. Ingestion is regarded as the route of infection for the rare but frequently lethal gastrointestinal mucormycosis. Since the first description of mucormycosis in 1855, numerous clinical cases have been reported from all over the world. Post-mortem surveys reveal that mucormycosis is 10–50-times less frequent than candidiasis and aspergillosis, representing 1–5 cases of 10,000 autopsies. The true incidence of mucormycosis is not known and is probably underestimated owing to difficulties in antemortem diagnosis and the low autopsy rates. Nevertheless, a rise of infections has been observed over the last years, which is attributed to rising numbers of immunocompromised hosts. Furthermore, there are increasing reports of breakthrough mucormycosis in antifungal prophylaxis or treatment (e.g., voriconazole, echinocandins) that is effective against Aspergillus, but not mucormycosis.
_
In a review of more than 900 reported human cases of mucormycosis, Roden and colleagues found the majority of human mucormycosis cases were caused by fungi classified under the following genera:
Rhizopus (47%)
Mucor (18%)
Cunninghamella (7%)
Apophysomyces (5%)
Absidia species (5%)
Saksenaea species (5%)
Rhizomucor pusillus (4%)
Other genera belonging to Mucorales represented less than 3% of culture confirmed cases.
_
Types of mucormycosis:
- Rhinocerebral (sinus and brain) mucormycosis is an infection in the sinuses that can spread to the brain. Rhino-orbital-cerebral mucormycosis (ROCM) is an acute, often fatal, fungal infection caused by members of the order Mucorales. The genus Rhizopus accounts for most cases of ROCM. The disease is characterized by fungal hyphal invasion of blood vessels resulting in thrombosis and infarction of the nasal, paranasal sinus, orbital, and cerebral tissues. The most commonly associated condition is diabetes mellitus; other associated conditions include immunocompromised states, renal disease, deferoxamine use, and acidotic states.
- Pulmonary (lung) mucormycosis is the most common type of mucormycosis in people with cancer and in people who have had an organ transplant or a stem cell transplant.
- Gastrointestinal mucormycosis is more common among young children than adults, especially premature and low birth weight infants less than 1 month of age, who have had antibiotics, surgery, or medications that lower the body’s ability to fight germs and sickness.
- Cutaneous (skin) mucormycosis: occurs after the fungi enter the body through a break in the skin (for example, after surgery, a burn, or other type of skin trauma). This is the most common form of mucormycosis among people who do not have weakened immune systems.
- Disseminated mucormycosis occurs when the infection spreads through the bloodstream to affect another part of the body. The infection most commonly affects the brain, but also can affect other organs such as the spleen, heart, and skin.
_____
The class Zygomycetes contains hyaline fungi that produce wide ribbon-like, coenocytic hyphae in human tissues. Their asexual reproductive phase is characterized by the production of sporangiospores in sack-like structures. Sporangiospore formation occurs as a result of cleavage of the protoplasm inside the sporangium (free cell formation). This is distinguished from the process of conidiation, where hyphal elements are converted into conidial spores. Their sexual reproductive phase is marked by the production of zygospores. Sexual reproduction may occur within a single isolate (homothallic) or may require mating between oppositely oriented mating strains (heterothallic). Heterothallic sexual reproduction predominates among the members of the order Mucorales. Zygospore formation occurs when specialized (sexually oriented) hyphal branches called zygophores are attracted to one another. Zygophores develop near the ends of rapidly growing mycelia which secrete chemical attractants. These two elements contact one another and swell, forming the progametangia. These fuse to produce the gametangium, which undergoes plasmogamy (the mixing of cytoplasm) and karyogamy (the fusion of nuclei). The wall becomes thickened, with multiple layers, and forms the zygosporangium. It is the zygosporangium that accounts for the coloration and surface ornamentation that is characteristic for each isolate.
Like all fungi, the zygomycetes are eukaryotes. They lack flagella and are thus nonmotile, and they are predominantly aerobic. While most of these organisms are considered to be saprophytic, the Mucorales may also be parasitic and predaceous, especially in regard to causing disease in humans and animals. In all cases, the fungi absorb their nutrients rather than synthesizing them. They require no light for growth. Dimorphic conversion has been identified in some species.
___
The hyphae of the Mucorales are distinct and allow for a presumptive identification from clinical specimens. The hyphae are broad (5-to-25-micron diameter), irregularly branched, and have rare septations. This is in contrast with the hyphae of ascomycetous molds, such as Aspergillus, which are narrower (2-to-5-micron diameter), exhibit regular branching, and have many septations. The lack of regular septations may contribute to the fragile nature of the hyphae and the difficulty of growing the agents of mucormycosis from clinical specimens. Grinding clinical specimens can cause excessive damage to the hyphae. Thus, finely mincing tissues is preferred for culturing tissue samples that may contain molds.
____
The fungi that cause mucormycosis are commonly found on decaying food, soil, and animal excrement.
Macroscopic photograph below shows Mucor sporangia growing on old bread.
_
A spore from the environment divides and forms ribbonlike hyphae on the substrate. In asexual reproduction, an upright stalk called a sporangiophore grows from the hyphae. The tip of the sporangiophore differentiates into a rounded sac called a sporangium from which new spores develop. The wall of the sporangium dissolves on maturity, exposing the spores. Sinus and lung infection occur by inhalation and deposition of the spores in these tissues. Cutaneous infection can occur when there is disruption of the skin in the case of burns or trauma.
_
Drawing of a sporangium at the tip of a hyphal stalk releasing spores.
____
Genome Architecture and Structure:
Sequencing of the R. delemar stain 99–880 genome revealed a highly repetitive genome indicative of an ancestral whole-genome duplication (WGD) event, which resulted in the replication of gene families related to cell growth, signal transduction, and cell-wall synthesis (Ma et al., 2009). Similar patterns were seen in the genomes of M. circinelloides and Phycomyces blakesleeanus, a non-pathogenic member of the Mucorales order known for its phototrophic growth (Corrochano et al., 2016). Both fungi showed evidence of widespread genome duplication that was concurrent with R. delemar, suggesting that the WGD event occurred early in the Mucoromycotina subdivision lineage. Fungi in the mucoromycotina subdivision appear to have more duplicated regions than other fungal genomes; however, evidence of a WGD event is not found in all of them. While the whole genome sequence of Lichtheimia corymbifera indicates a high occurrence of gene duplication and expansion, there appears to be no evidence of WGD. Rather, the duplicative nature of the L. corymbifera genome appears to be mediated by the high occurrence of tandem duplications (Schwartze et al., 2014). This pattern was also observed in whole genome sequencing and comparison of Apophysomyces species, which demonstrated extensive gene duplication and expansion across its genome but no evidence of a WGD (Prakash et al., 2017).
Other relevant features of Mucorales genomes have been elucidated through genome sequencing. Rhizopus species demonstrate remarkable variety in genome length, notably within Rhizopus microsporus (Gryganskyi et al., 2018). Additionally, there is wide variety in the structure of the mating type locus within Rhizopus genomes that vary from typical arrangements seen in Mucoralean fungi (Gryganskyi et al., 2018). Similar to L. corymbifera (Schwartze et al., 2014), Apophysomyces species had a lower number of transposable elements (TEs) in their genome when compared to other Mucorales species (Prakash et al., 2017). Both cases were associated with multiple copies of heterokaryon incompatibility (HET) genes and genes associated with RNA interference (RNAi) pathway (Schwartze et al., 2014; Prakash et al., 2017).
Chromatin immunoprecipitation sequencing (ChIP-seq) of M. circinelloides showed that it has a unique “mosaic” centromere structure in Mucoromycotina, with characteristics from point centromeres (seen in Saccharomyces cerevisiae) and regional centromeres (seen in C. albicans, Candida tropicalis, Magnaporthe oryzae, Schizosaccharomyces pombe, and C. neoformans) (Navarro-Mendoza et al., 2019).
_____
There are three ways humans can contract Mucormycosis:
-By inhaling spores, or
-By swallowing spores in food or medicines, or
-When spores contaminate wounds
Out of the three ways, inhalation of spores is the most common way to get infected with this fungal disease. Fungal spores, when gets deposited in the nasal cavity, lead to rhinocerebral disease. Similarly, spores attacking the lungs lead to pulmonary disease, or entering the intestine leads to GI disease. Finally, if they enter through cuts in the skin, they lead to cutaneous disease.
_____
_____
Fungus, nose and sinus:
An estimated 1.5 million fungal species inhabit Earth, with the vast majority poorly described or undiscovered. Because fungi are present throughout the environment, human exposure is inevitable and normal respiration will routinely deposit fungal elements within the nose and paranasal sinuses. In most instances, the presence of fungal elements in the nose is of no consequence and will remain unknown to the individual unless elaborate culture techniques are used. In select instances, fungal species can cause sinonasal disease, with clinical outcomes ranging from mild symptoms to intracranial invasion and death. Fungal rhinosinusitis has been categorized primarily based on whether the fungus invades local tissues or not, a characteristic intimately associated with the status of the host’s immune system. Non-invasive fungal rhinosinusitis includes fungal colonization, fungal ball, and allergic fungal rhinosinusitis (AFRS). Spread of fungus into local tissues characterizes acute invasive, chronic invasive, and chronic granulomatous forms of fungal rhinosinusitis.
_
Fungal ubiquity on sinonasal mucosa:
Considering how common fungal species are in the environment, it should not be surprising to also find them in the human upper respiratory tract. However, early culture techniques and staining methods were relatively insensitive and failed to identify fungal species in most cases. It was not until the development of enhanced culture techniques and speciation via polymerase chain reaction that the true prevalence of sinonasal fungal elements was appreciated. Ponikau et al. in 1999 examined 210 consecutive patients with chronic rhinosinusitis (CRS) and were able to culture fungal species in 96%. Kim et al. identified fungal elements in 76/82 (92.5%) of CRS patients compared with only 23.2% using standard cultures. Fungal elements are not unique to patients with CRS but are also seen in most healthy controls. The Ponikau and Kim studies cultured fungi in 100 and 97.5% of normal controls, respectively. The ubiquitous presence of fungi in the sinonasal tract suggests that fungal-related sinonasal disease has less to do with the presence or absence of fungi. Instead, the presence of fungal rhinosinusitis and its various forms relates more to the status of the host’s immune system and subsequent host–microbe interaction.
_
Immune status and fungal rhinosinusitis:
Assessing the viability of the host’s immune system is central to correctly differentiating and managing fungal rhinosinusitis. Immune dysfunction, whether overt or subtle, is the key factor predisposing to fungal invasion of sinonasal tissues and must be considered in all patients with Chronic Rhinosinusitis (CRS). Presumably, fungi are unable to penetrate the epithelial layer when the immune system is functioning normally. Paranasal mycoses occur in two distinct forms. A benign or non-invasive infection which is seen in immunocompetent individuals and presents as fractious sinusitis and nasal polyposis that fails to respond to repeated courses of antibiotics. It has a slow mild course with no tissue invasion, expansion or erosion of the sinus walls. Another is more serious invasive form is usually associated with immunocompromised patient such as poorly controlled diabetic and is usually characterized by its rapid onset and its ability to invade tissues and cause destruction. Suppression of the immune system, such as from diabetes mellitus, chemotherapy, or corticosteroids, creates a condition in which fungi are able to penetrate normal mucosal barriers and invade host tissues. On the other end of the spectrum, AFRS likely represents a hypersensitive response of a competent host immune system to fungal elements. In AFRS, chronic mucosal inflammation may be mediated in part through IgE-mediated (type 1) reactions to fungal species trapped in sinonasal mucous. An appreciation of the host’s immune status, together with other key clinical characteristics, thus directs the proper diagnosis and classification of fungal sinonasal disorders.
Fungal species are ubiquitously present in the environment, including the human sinonasal tract. Fungal rhinosinusitis encompasses a wide spectrum of disease, ranging from simple colonization to acute invasion. Each disease entity has a characteristic presentation and clinical course, with the immune status of the host playing a critical pathophysiological role. Accurate diagnosis and targeted treatment strategies are necessary to achieve optimal outcomes.
_
Orbits and sinuses:
The orbits are pear-shaped bony cavities that contain the globes, extraocular muscles, nerves, fat and blood vessels. The walls of the orbit are comprised of seven bones. The periosteal covering of the orbital bony cavity fuses anteriorly with the orbital septum and posteriorly with the dura mater. Abscesses can localize in the subperiosteal space. The roof, medial wall, and floor of the orbit separate it from adjacent paranasal sinuses, including the maxillary, frontal, ethmoid, and sphenoid sinuses. The paranasal sinuses arise from and drain into the nasal cavity. Thus, an intimate anatomical relationship exists between the orbit and the adjacent paranasal sinuses, and the latter may be the source of an orbital infection as seen in the figure below.
(Left) The human eye in situ with the tunics peeled back, exposing a portion of the vasculature of the retina, lens, and anterior chamber as seen from the side.
(Right) The relationship of the paranasal sinuses to the eye, three views.
-1. The top figure shows a side view.
-2. (Bottom left) Relationship of the sinuses with the orbit as seen by coronal section just posterior to the bridge of the nose.
-3. (Bottom right) Relationship of the sinuses with the orbits with the head tilted backwards.
_
_
Invasive Aspergillus and mucormycetes infections have a marked predilection for the orbit and sinonasal infection can spread to orbit. Because mucormycosis may involve the orbit and other ocular structures, the ophthalmologist may be the first physician to see a patient with this highly morbid condition. Thus, it is important to have this disease in the differential diagnosis, as a delay in diagnosis may be fatal. Aggressive medical and surgical management is critical, and patients will likely require co-management with multiple services. Excellent communication among these services and an in-depth discussion with the family regarding prognosis are fundamental in caring for these patients.
_
Rhinocerebral mucormycosis:
The most common presentation is rhino-orbital-cerebral mucormycosis (ROCM). Inoculation is presumed to start with inhalation of spores in a susceptible host. The presentation typically is a rapidly progressive infection but cases of indolent disease have been reported. Common symptoms include sinusitis, nasal stuffiness and purulent discharge, headache, and fever. Pain may be present or absent at the time of presentation. The infection can spread to adjacent structures and cause more widespread and devastating disease. Signs of disease include the following:
-Soft tissue: edema, necrosis, eschars, destruction of turbinates, cyanosis or blanching of overlying skin or mucosa due to vaso-occlusion of the surrounding vessels by the hyphae.
-Orbit: periorbital edema, proptosis, motility deficits
-Cavernous sinus: multiple cranial nerve palsies, thrombosis, invasion of the carotid artery
-Frontal lobe: mental status changes, obtundation, and death
-Sensory branch of CN V: facial numbness due to the invasion of nerves by the hyphae
_______
_______
Common symptoms of mucormycosis in initial stages:
Sinus headache
Facial pain
Stuffy nose
Blood nasal discharge
Blurring of vision, double vision
Dental pain or loosening of teeth, in the initial stages.
Common symptoms of mucormycosis in later stages:
Facial swelling
Facial skin colouration
Ptosis (closure of eyelids)
Proptosis (swelling of the eyeballs)
Restricted eye movements
Palatal blackish discolouration
Brownish discharge from the nose
Recognising the symptoms early is the surest way to diagnose and treat the disease!
Investigations needed:
Complete blood count
Blood sugar test
Renal function test
Deep nasal swab – KOH staining to detect the fungus
Nasal endoscopy, biopsy and culture
CT scan of the nose and PNS helps in further assessment of the disease
MRI of the brain
_____
Although rare, Mucorales is the third most common cause of invasive opportunistic fungal infection after Candida and Aspergillus species. Mucormycosis is an acute opportunistic infection which is caused by a broad, aseptate, saprophytic fungus which is found in soil, air, bread mould, rotten fruit and vegetables. It can be cultured from the mouth, nasal tract, throat and the faeces of healthy persons. These molds live throughout the environment and their spores are present in the air. They get lodged in the nasal cavity and adjoining sinuses. On reaching a favourable milieu they ensconce themselves within the tissue. The spores germinate, hyphae (filamentous processes) outgrow and release destructive juices which digest the host tissue and provide nutrition to the rapidly growing fungi. As they grow in the nasal cavity, they relentlessly destroy the surrounding host tissue. Mucormycosis typically originates in the nasal or oral mucosa, spreads to the paranasal sinuses, and enters the orbit via the ethmoid and maxillary sinuses or via the nasolacrimal duct. Intracerebral extension may occur from the orbit via orbital apex, orbital vessels, or via cribriform plate The bones in the nasal cavity and sinuses are destroyed. These include the hard palate, the orbital bones, and the skull base bones. Black masses may be seen in the nasal cavity and oral cavity. If it destroys the orbit and enters the eye socket it may cause bulging of the eyes, pain, frozen eye movements, and blindness. Once it enters the cranial cavity by breaching the skull base it blocks major arteries and venous lakes resulting in major life-threatening brain strokes and bleeds. The hallmark of mucormycosis is angioinvasion, causing arteritis, vessel thrombosis, tissue ischaemia, and necrosis with bony destruction. Angioinvasion also allows the organism to disseminate to other organs, and the ischaemic necrosis impedes the delivery of antifungal agents to target sites. Spores can sometimes travel into the depths of the respiratory system and get comfortably lodged in the lung parenchyma (alveoli and bronchioles). Here the fungi grow rapidly, destroying the lung tissue and compromising blood oxygenation. From there it can spread into the circulatory system resulting in an existential crisis. Mucormycosis can also develop on the skin after the fungus enters the skin through a cut, scrape, burn, or other type of skin trauma. The disease is not contagious and doesn’t spread from one person to another. Successful treatment of mucormycosis requires early diagnosis, reversal of underlying risk factors, prompt administration of antifungal therapy and surgical debridement when applicable. Antifungal treatment options consist of lipid formulations of amphotericin B, amphotericin B deoxycholate or posaconazole. First-line treatment is with amphotericin B, preferably with liposomal amphotericin B. Debridement of necrotic tissue in combination with medical therapy is mandatory for patient survival. Repeated surgery may be required, especially for rhinocerebral mucormycosis. Rapid and reliable diagnostic methods are lacking and current diagnosis is dependent on culture and histopathological examination.
_____
_____
History of mucormycosis:
The first case of mucormycosis was possibly one described by Friedrich Küchenmeister in 1855. Fürbringer first described the disease in the lungs in 1876. In 1884, Lichtheim established the development of the disease in rabbits and described two species; Mucor corymbifera and Mucor rhizopodiformis, later known as Lichtheimia and Rhizopus, respectively. In 1943, its association with poorly controlled diabetes was reported in three cases with severe sinus, brain and eye involvement. In 1953, Saksenaea vasiformis, found to cause several cases, was isolated from Indian forest soil, and in 1979, P. C. Misra examined soil from an Indian mango orchard, from where they isolated Apophysomyces, later found to be a major cause of mucormycosis. Several species of Mucorales have since been described. When cases were reported in the United States in 1955, the author thought it to be a new disease resulting from the use of antibiotics, ACTH and steroids. Until the latter half of the 20th century, the only available treatment was potassium iodide. In a review of cases involving the lungs diagnosed following flexible bronchoscopy between 1970 and 2000, survival was found to be better in those who received combined surgery and medical treatment, mostly with amphotericin B.
The disease has been reported in natural disasters and catastrophes; 2004 Indian Ocean tsunami and the 2011 Missouri tornado. The first international congress on mucormycosis was held in Chicago in 2010, set up by the Hank Schueuler Foundation, which was established in 2008 for the research of children with leukaemia and fungal infections. A cluster of infections occurred in the wake of the 2011 Joplin tornado. By July 19, 2011 a total of 18 suspected cases of mucormycosis of the skin had been identified, of which 13 were confirmed. Ten people required admission to an intensive-care unit, and five died. A 2018 study found many freshly laundered hospital linens delivered to U.S. transplant hospitals were contaminated with Mucorales.
Naming:
Arnold Paltauf coined the term ‘Mycosis Mucorina’ in 1885, after describing a case with systemic symptoms involving the sinus, brain and gastrointestinal tract, following which the term ‘mucormycosis’ became popular. The disease named “mucormycosis” was subsequently used by an American pathologist R. D. Baker to denote a mycosis caused by some members of Mucorales in 1957. ‘Mucormycosis’ is often used interchangeably with ‘zygomycosis’, a term made obsolete following changes in classification of the kingdom Fungi. The former phylum Zygomycota, included Mucorales, Entomophthorales, and others. Mucormycosis describes infections caused by fungi of the order Mucorales.
Other animals:
Mucormycosis in other animals is similar, in terms of frequency and types, as in people. Cases have been described in cats, dogs, cows, horses, dolphin, bison, and seals.
Dentists and mucormycosis:
Dentists are seeing a growing number of medically compromised patients in their practices. Invasive fungal infections (mycoses) are uncommon, but when they occur, they are devastating to patients. These infections are opportunistic — they occur when organisms to which we are frequently exposed gain entry to the body due to a decrease in host defenses or through an invasive portal, such as a dental extraction. This organism is frequently found to colonize the oral mucosa, nasal mucosa, paranasal sinuses and pharyngeal mucosa of asymptomatic patients. These fungi do not usually cause disease in healthy people with intact immune systems, but patients with a number of conditions can be predisposed to the development of invasive fungal disease. These conditions include diabetes mellitus, renal failure, malignancies, intravenous drug abuse, malnutrition states, as well as immunosuppression and corticosteroid therapy.
Mucormycosis of the oral cavity can be of two different origins. One is from disseminated infection where the gateway of entry is inhalation (through the nose) and the other is through direct wound contamination with dissemination to other viscera as a common complication. When it arises from nose and PNS, the infection may cause palatal ulceration leading to necrosis and the affected area appears black in preponderance of the cases. When the infection spreads from direct wound contamination (e.g., tooth extraction), the clinical findings may appear anywhere in the oral cavity, including the mandible. A significant difference between infection involving the maxilla and mandible is cavernous sinus thrombosis, a serious complication of maxillary infections.
Following some easy dental hygiene tips might lower the odds of contracting this rare but deadly disease. After a person recovers from COVID-19, continuous use of steroids can allow bacteria or fungus to thrive in the oral cavity. Brushing your teeth is the first step in your dental hygiene regimen. The two-minute period can be divided into 30 second intervals between each quadrant (upper and lower right and left sides). Brushing twice a day should be done in the morning and at night. Flossing will be a regular part of your oral hygiene routine at night. Plaque and food particles can be removed from between your teeth by flossing at night. Rinsing is the next step in your dental hygiene programme. Antiseptic mouthwash not only promotes fresh breath, but it also aids in the prevention of hazardous microorganisms. Mouthwash is a simple technique to maintain proper dental hygiene because the results are instant. Your breath will be notably fresh and your mouth will feel visibly clean.
______
______
Section-6
Epidemiology of mucormycosis:
Most commonly, mucormycosis develops as a nosocomial infection affecting a wide range of susceptible hosts. Predisposing conditions include in metabolic disorders such as diabetes mellitus with ketoacidosis, chronic renal insufficiency, hematologic malignancy, organ transplant, malnutrition, increased serum iron, prolonged neutropenia, immunosuppressive drugs such as steroids, cytotoxics, broad-spectrum antibiotics, deferoxamine therapy, etc. In approx. 50 % of mucormycosis patients, diabetes mellitus serves as a predisposing factor due to the pronounced availability of glucose, lower pH and impaired host defense mechanism. Rhizopus species have an active ketone reductase system that enables them to thrive in an acidic pH and glucose-rich medium. Hyperglycemia enhances fungal growth and impairs neutrophil chemotaxis; therefore, individuals with diabetic ketoacidosis are commonly affected. Rhizopus species also favor an iron-rich environment and are frequently isolated in patients receiving deferoxamine therapy (an iron-chelating agent). Breakthrough infection in neutropenic patients receiving voriconazole is an increasingly recognized risk factor for mucormycosis. The major mode of disease transmission is through inhalation of spores from environmental sources. Cutaneous routes of infection are particularly important in surgical, trauma, and burn patients. The development of mucormycosis in areas of skin breakdown has been associated with a variety of contaminated adhesive products, elastic bandages, and tongue depressors used in the hospital setting. The ingestion of contaminated milk, vegetables, bread, may play a role in promoting gastrointestinal mucormycosis.
_
The fungal spores enter the human organism by inhalation, ingestion or direct inoculation. The most common species all over the world is Rhizopus arrhizus (formerly Rhizopus oryzae). Other isolated fungi belong to the genera Lichtheimia, Mucor, Rhizomucor, Cunninghamella, Saksenaea, Apophysomyces, Cokeromyces, Actinomucor and Syncephalastrum. In the global review by Jeong et al. Rhizopus spp., Lichtheimia spp. and Mucor spp. accounted for 75% of all cases. The agents of mucormycosis vary depending on the geographical area. In the ECMM study from Europe, Rhizopus spp. were isolated in 34% of cases, Lichtheimia spp. in 19% and Mucor spp. in 19%. In the RetroZygo study from France, Rhizopus spp. were the causative agents in 52%, while the second most common genus was Lichtheimia (29%). In India, although Rhizopus species are the most common cause of the disease, Apophysomyces elegans, A.variabilis and Rhizopus homothallicus are emerging species and uncommon agents such as Mucor irregularis and Thamnostylum lucknowense are also being reported. Another new species of Apophysomyces, namely, A. mexicanus, has been reported from Mexico. In a recent study, presenting the epidemiology of mucormycosis in Australia, trauma patients were more often infected with uncommon, non-Rhizopus spp.; the patients infected with Apophysomyces spp. or Saksenaea spp. were all immunocompetent, had predominantly acquired infection through trauma, and had infection frequently localized to the skin, soft tissues, and bones. Necrotizing fasciitis due to Apophysomyces variabilis or A.elegans and Saksenaea erythrospora, after intramuscular injections, have also been reported from India. Cunninghamella infection has been associated with poorer out-come. New species are emerging, including Rhizopus homothallicus, Thamnostylum lucknowense, Mucor irregularis and Saksenaea erythrospora. Seasonal variations affect the incidence of mucormycosis, with most infections occurring from August to November.
_
The incidence of mucormycosis has been increasing in recent decades, mainly due to the growth of the number of severely immunocompromised patients. Now mucormycosis cases are being reported from all over the world, but differences in the epidemiology seem to exist between developed and developing countries. In developed countries, the disease remains uncommon and is mostly seen in patients with hematological malignancies (HM). In contrast, in developing countries, especially in India, mucormycosis is more common and cases occur mainly in patients with uncontrolled diabetes mellitus (DM) or trauma. Accordingly, the prevalence of mucormycosis varies from 0.01 to 0.2 per 100,000 population in Europe and the United States of America and is much higher in India (14 per 100,000 population).
_
The most common clinical presentations of mucormycosis are rhino-orbito-cerebral, pulmonary, cutaneous, and disseminated. The percentages reported in the review by Jeong et al. were 34%, 21%, 20%, and 14%, respectively, while in the European study of the Working Group on Zygomycosis the corresponding numbers were 27%, 30%, 26%, and 15%. In patients with HM, the main clinical form of the disease is pulmonary. In India rhino-orbito-cerebral presentation associated with uncontrolled DM was the predominant characteristic, and isolated renal mucormycosis has emerged as a new clinical entity. In a large study from Mexico, reviewing 418 cases, diabetes was the underlying disease in 72% of patients, and it was associated with sinusitis. In the group of patients with underlying malignancies, pulmonary and sinus presentations were similar.
_
Infections by Mucorales are typically rapidly progressive. However, an emerging opportunistic fungus, Mucor irregularis (formerly Rhizomucor variabilis var. variabilis) initially reported in farmers from China, is the cause of an infection with a completely different epidemiology and clinical presentation. The infection is chronic, persisting for years, it occurs inimmunocompetent patients, without any apparent risk factors and it affects the skin and subcutaneous tissues, leading finally to severe disfigurement.
_
Mucormycosis in children was recently analyzed in cases extracted from two global registries. Fungal isolates included Rhizopus spp. (39.7%), Lichtheimia spp. (17.5%), Mucor spp. (12.7%), Cunninghamella bertholletiae (6.3%), and unspecified species (23.8%). Underlying conditions were HM (46%), other malignancies (6.3%), HSCT (15.9%), solid organ transplantation, trauma/surgery and DM (4.8% each) and a variety of other diseases (7.9%); in 9.5%, no underlying medical condition was found. Neutropenia was recorded in 46% of patients. The main sites of infection were lungs (19%), skin and soft tissues (19%), paranasal sinus/sino-orbital region (15.8%), and rhino-cerebral region (7.9%). Disseminated infection was present in 38.1%. Mortality, in the same study, was 33.3%. In adults, the reported mortality ranges from 20% to 100%, depending on the underlying risk factors, site of infection and treatment.
______
- Disease Burden:
Mucormycosis is prevalent worldwide, but the exact global burden of disease is not clear. A study conducted in San Francisco indicated that the incidence is around 1.7 per million population in the US annually. Moreover, it has been suggested by experts that the incidence of mucormycosis is increasing.
- Mortality Rate:
A study by the Centers for Disease Control and Prevention (CDC), Atlanta, has indicated a mortality rate of 54 percent. In this context, it should be noted that the mortality rate can vary depending on the health of the patient, type of fungus, and site infected. For example, the mortality rate for disseminated mucormycosis is 96 percent, while for pulmonary and sinus mucormycosis, it is 76 percent and 46 percent, respectively.
- Reservoir:
Mucormycetes are found everywhere in the environment, but most prominently in the soil. The specific environmental niches that are inhabited by these fungi vary with their genera and species. Mucorales are thermo-tolerant fungi with a ubiquitous distribution. They are found on organic substrates such as decaying fruit and vegetable matter, crop debris, bread, compost piles, animal excreta and soil within indoor and outdoor environments. An ecological study conducted in India revealed presence of many species of Mucorales in soil. Mucorales are also present in indoor environments such as air conditioning filters. A recent study from North India reported large numbers of Mucorales spores in both hospitals and outdoor air. The mean spore count of Mucormycetes in outdoor samples ranged between 0.73 to 8.60 cfu/m3 across different seasons. Within the hospital, the mean spore count was slightly higher in an airconditioned area (0.88 – 1.72 cfu/m3) compared to a non-airconditioned area (0.68 – 1.12 cfu/m ) (21). R. arrhizus was the predominant Mucorales isolated in both indoor and outdoor air.
Note: Two different methods are generally used to quantify spores in fungal preparations. One technique is based on colony forming units (cfu) resulting from the plating of diluted spore suspensions on some agar medium. The second method uses direct counts of conidia in a diluted spore suspension with a hemocytometer. The counts are then adjusted by conidial viability determined by the percent spore germination after a standard incubation time on an agar medium.
- Incubation period:
Mucormycosis has an incubation period of 2 to 5 days from deposition of spores in nasal mucosa till development of hyphae. From the sinus, it takes around 2 to 4 days to reach to the eyes. And to more concern, it takes merely a day to reach from eyes to the human brain. In such cases, to prevent the fungus from further being spread, eyes are evacuated.
- Transmission:
These fungi can infect the body through various routes, but most commonly through inhalation of the spores from the environment. The spores can also be ingested. Hospital outbreaks have been reported that originated from contaminated bandages, tongue depressors, linen, and non-sterile medical devices, among others. Community outbreaks have been reported, which started from traumatic injuries sustained during natural disasters.
The major mode of disease transmission for the mucormycetes is presumed to be via inhalation of spores from environmental sources. Experimentally, when rabbits were infected by nasal instillation of a spore solution, they developed upper and lower respiratory disease with subsequent spread to the central nervous system. Inhalation of spores in dust likewise provides the exposure seen in the allergic interstitial pneumonitis or alveolitis syndrome seen in employees of both the malt and lumber industries and in outbreaks of rhinocerebral or pulmonary mucormycosis linked to excavation, construction, or contaminated air conditioning filters.
Percutaneous routes of exposure are also very important in causing infection by the mucormycetes. Traumatic implantation of spores in dirt has been seen in a number of patients. Needle-stick exposures have been implicated in mucormycotic infections occurring at the site of medicine injection, catheter insertion sites, injection sites for illicit drug use, and tattooing. Insect bites or stings have also been implicated in disease transmission in cases of cutaneous and subcutaneous mucormycosis. The development of wound mucormycosis has been seen with a variety of adhesive products used in the hospital setting.
The ingestion of fermented milk with dried bread products or fermented porridges and alcoholic drinks derived from corn may play a role in promoting gastric mucormycosis. Spore-contaminated herbal or homeopathic remedies have likewise been linked to gastrointestinal disease. A series of cases were presumably transmitted orally by spore contaminated tongue depressors used for oropharyngeal examinations in a hematology/oncology clinic. Consumption of moldy hay or grains is the most likely way in which infection is acquired in animals.
- Is mucormycosis contagious?
No. Mucormycosis can’t spread between people or between people and animals.
______
Fungi growing in water of oxygen humidifier:
Fungal colonization of oxygen humidifier and nebulizer chambers, a 2011 study:
Humidified oxygen and nebulizers are routinely used in hospitalized patients suffering from respiratory ailments. These can however be potential source of allergens or infection if colonized by fungi. Authors undertook a study to determine if the oxygen humidifier chambers of portable cylinders and central lines at our hospital were colonized by fungi. The Hudson’s chambers of nebulizers were also studied as they remain wet after use. Samples of these were obtained using sterile swabs on Tuesday as these chambers are usually cleaned on every Saturday. Spot samples were taken from ICUs, wards, casualty and OPD on a single day. Air samples were also obtained on the same day to determine if the fungal spore load in the inhaled room air was normal or high. 46/53 (86.79%) swabs form oxygen humidifiers and 7/17 (41.17%) swabs from Hudson’s chambers grew fungi. There were a total of 14 species of fungi identified altogether. 4 of them are virulent strains and 6 are known allergens for asthmatics. The colonization was less in shallow Hudson’s chambers (35.71%) as compared to the reusable long ones (66.66%). The air samples showed insignificant growth. The study indicates a potential in-hospital source of allergens and infection. The oxygen and nebulizer chambers need to be cleaned more frequently with disinfectants.
So there is some truth that the fungi must be growing in dirty water of oxygen humidifiers. However, the pure oxygen stored in cylinders is likely to be detrimental to the growth of microorganisms of all kinds.
______
Myths about transmission:
Several theories about the source of mucormycosis infections are circulating on social media, many of them unfounded.
-1. Person-to-person transmission
Crucially, mucormycosis cannot be transmitted from person to person, so there is no need for people to isolate — unless, of course, they have an ongoing SARS-CoV-2 infection. Rather, the source of infection is environmental, from airborne spores produced by the fungi. Fungi are common in the environment, and people breathe in or come in contact with fungal spores every day without getting sick. However, in people with weak immune systems, these fungi are more likely to cause an infection.
-2. Face masks harbor mucormycosis fungus
This is a myth. There is no evidence that face masks can harbor Mucorales fungi. Someone who already has a fungal infection in their lungs/sinus could possibly contaminate their own mask, but not the other way around.
Yeast infection can occur anywhere there is warm, moist, creased skin. Candida albicans is the most common culprit that takes advantage of this environment, which can be created by masks to create a superficial fungal infection. Yeast is present on the skin and usually causes no ill effects. However, the right combination of factors can allow it to develop into an infection. It’s not the mask itself that causes the problem. But wearing a mask for extended periods of time, particularly in these hot, sticky days of summer, can encourage the factors that lead to infection.
-3. Onions are to blame
Another popular theory is that the black mold sometimes seen on onions is Mucorales fungus and, therefore, a potential source of infection. In fact, the black mold found on onions and garlic is usually the fungus Aspergillus niger. In a 2019 paper, Prof. Richardson and his co-author explain that Mucorales fungi grow on moldy bread, decaying fruit and vegetables, crop debris, soil, compost, and animal excreta. He points out that they have a high moisture requirement and are unlikely to survive on common building materials, such as wood, painted surfaces, and ceramic tiles. He concludes: “All of these observations suggest that house residents are not generally exposed to mucormycetes in their home environment, apart from mould-contaminated food items, such as bread and fruit.” However, they are commonly found in homes and one study indicated that Mucorales species were present in 98% of samples taken from home dust (Gravesen, 1978; Quandahl and Cooper, 2018).
_______
_______
Statistics of mucormycosis:
Mucormycosis (previously called zygomycosis), an aggressive infection caused by mucoralean fungi, is the third most prevalent fungal disease after candidiasis and aspergillosis, among populations at high risk, including those with uncontrolled diabetes, solid organ or allogeneic stem cell transplant recipients, and patients undergoing immunosuppressive therapies (Petrikkos et al., 2012; Danion et al., 2015; Farmakiotis and Kontoyiannis, 2016; Hoenigl et al., 2018; Cornely et al., 2019). The reported annual incidence of mucormycosis is for Europe (from 0.2 cases in Denmark to 95 cases in Portugal), USA (3.0 cases), Canada (1.2 cases) and Australia (0.6 cases) per 1,000,000 individuals (Skiada et al., 2012). A computational-based approach estimated the prevalence of mucormycosis at 140 cases per million populations in India, with the prevalence ranging between 137,807 cases to 208,177 with the mean of 171,504 (SD: 12,365.6; 95% CI: 195,777–147,688) and a mean attributable mortality at 65,500 (38.2%) per year. Although few studies in Asia have reported the prevalence of Mucorales infections (Yamazaki et al., 1999; Chakrabarti and Singh, 2014; Vaezi et al., 2016), a national investigation of medical autopsies revealed that the incidence of these infections in Japan increased by 16% in a 20-year span (Prakash and Chakrabarti, 2019).
_
Mucormycosis is rare, but the exact number of cases is difficult to determine because no national surveillance exists in the United States. Population-based incidence estimates for mucormycosis were obtained from laboratory surveillance in the San Francisco Bay Area during 1992–1993 and suggested a yearly rate of 1.7 cases per 1 million population. The number of mucormycosis incidences is increasing and is estimated to be 500 cases per year in the United States. Data from the Center for Disease Control and Prevention (CDC) Transplant Associated Infection Surveillance Network (TRANSNET), which surveyed 25 U.S. Transplantation Centers from 2001-2006, noted a 1-year cumulative incidence rate of 3.8 per 1,000 HSCT and 0.6 per 1,000 solid organ transplants. The overall incidence increased from 1.7 per 1,000 in 2001 to more than 6.2 per 1000 in 2004, with Rhizopus spp. as the most common pathogen; however, the rate decreased in the subsequent 2 years of the study. The prevalence of mucormycosis in autopsy series has ranged from 1 to 5 cases per 10,000 autopsies, approximately10- to 50- fold less common than Candida or Aspergillus infection, respectively. Finally, in patients at higher risk, such as those undergoing allogeneic bone marrow transplantation, the prevalence of mucormycosis has been described to be as high as 2 to 3%. It is around 80 times more prevalent in India, where it is estimated that there are around 0.14 cases per 1000 population, and where its incidence has been rising. Diabetes is the main underlying disease in low and middle income countries, whereas, blood cancers and organ transplantation are the more common underlying problems in developed countries. As new immunomodulating drugs and diagnostic tests are developed, the statistics for mucormycosis have been changing. In addition, the figures change as new genera and species are identified, and new risk factors reported such as tuberculosis and kidney problems.
_
The incidence of mucormycosis is rising globally, but the rise is very high in India and China among patients with uncontrolled diabetes mellitus. However, a recent review of 851 cases over the period January 2000 through January 2017, provides a different indication that the disease burden is higher in Europe than in Asia, as they reported 34% in Europe, followed by Asia (31%) and North or South America (28%), Africa (3%), Australia and New Zealand (3%). The contrary data may be due to under-reporting during this period from Asian countries. The Leading International Fungal Education (LIFE) portal has estimated the burden of serious fungal infections globally. According to their estimate, the annual prevalence of mucormycosis might be around 10,000 cases in the world barring India. After the inclusion of Indian data, the estimate of mucormycosis rose to 910,000 cases globally.
_
It is likely that mucormycosis will continue to increase in incidence because the number of organ transplantations, cancer patients, and diabetic patients is on the rise. Just in the United States in 2015, the number of transplant patients exceeded 30,000, with an increase of nearly 5% over 2014 (Organ Procurement and Transplantation Network [OPTN]). According to WHO, the number of people affected by diabetes quadrupled in the past 4 decades, reaching >420 million in 2014. Additionally, due to global warming, natural disasters with outbreaks of mucormycosis are likely to occur with higher frequency, similar to what happened with the 2004 Southeast Asia tsunami and the 2011 Joplin tornado.
Recent data have demonstrated a striking increase in the number of reported cases of mucormycosis, possibly due to the rising prevalence of risk factors including diabetes, cancer, and organ transplantation in the ageing population of developed countries. For example, there has been an alarming rise in the incidence of mucormycosis at major transplant centers and the number of cases over a 15 year period has more than doubled. In fact, the prevalence of mucormycosis is up to 8% in autopsied patients with leukemia. Additionally, a recently published population based study demonstrated a 70% increase in mucormycosis cases between 1997 and 2006. Further, data from a tertiary care center demonstrated ≥ 400% increase in the incidence of mucormycosis, mainly among DKA patients between 1991 and 2007. These studies suggest that the incidence of mucormycosis is increasing in both immunocompromised and DKA patients alike.
______
______
A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment, a 2018 study:
Mucormycosis due to Mucorales is reported at large numbers in uncontrolled diabetics across India, but systematic multicenter epidemiological study has not been published yet. The present prospective study was conducted at four major tertiary care centers of India (two in north and two in south India) during 2013–2015 to compare the epidemiology, treatment strategies and outcome of mucormycosis between the two regions. Molecular techniques were employed to confirm the identity of the isolates or to identify the agent in biopsy samples. A total of 388 proven/probable mucormycosis cases were reported during the study period with overall mortality at 46.7%. Uncontrolled diabetes (n = 172, 56.8%) and trauma (n = 31, 10.2%) were the common risk factors. Overall, Rhizopus arrhizus (n = 124, 51.9%) was the predominant agent identified, followed by Rhizopus microsporus (n = 30, 12.6%), Apophysomyces variabilis (n = 22, 9.2%) and Rhizopus homothallicus (n = 6, 2.5%). On multivariate analysis, the mortality was significantly associated with gastrointestinal (OR: 18.70, P = .005) and pulmonary infections (OR: 3.03, P = .015). While comparing the two regions, majority (82.7%) cases were recorded from north India; uncontrolled diabetes (n = 157, P = .0001) and post-tubercular mucormycosis (n = 21, P = .006) were significantly associated with north Indian cases. No significant difference was noted among the species of Mucorales identified and treatment strategies between the two regions. The mortality rate was significantly higher in north Indian patients (50.5%) compared to 32.1% in south India (P = .016). The study highlights higher number of mucormycosis cases in uncontrolled diabetics of north India and emergence of R. microsporus and R. homothallicus across India causing the disease.
_
This prospective multicenter study was conducted during January 2013 through December 2015 at four major tertiary care centres in India: from north India, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh (inpatient beds: 1948 beds with ∼85000 admissions per year) and All India Institute of Medical Sciences (AIIMS), New Delhi (2362 beds with ∼230000 admissions per year); from south India, St. John’s Medical College (SJMC), Bengaluru (1400 beds with ∼50000 admissions per year) and Nizam’s Institute of Medical Sciences (NIMS), Hyderabad (1300 beds with ∼42000 admissions per year). A total of 388 proven/probable mucormycosis cases were reported during the study period with overall mortality at 46.7%.
Total admissions in these 4 centers 407,000 per year and 1,221,000 in 3 years. Out of them, only 388 mucormycosis cases were reported in these 3 years which comes to 0.0317 % of all hospital admissions.
_____
Epidemiology and Diagnosis of Mucormycosis: An Update, a 2020 study:
Mucormycosis is an angioinvasive fungal infection, due to fungi of the order Mucorales. Its incidence cannot be measured exactly, since there are few population-based studies, but multiple studies have shown that it is increasing. The prevalence of mucormycosis in India is about 80 times the prevalence in developed countries, being approximately 0.14 cases per 1000 population. Diabetes mellitus is the main underlying disease globally, especially in low and middle-income countries. In developed countries the most common underlying diseases are hematological malignancies and transplantation. The epidemiology of mucormycosis is evolving as new immunomodulating agents are used in the treatment of cancer and autoimmune diseases, and as the modern diagnostic tools lead to the identification of previously uncommon genera/species such as Apophysomyces or Saksenaea complex. In addition, new risk factors are reported from Asia, including post-pulmonary tuberculosis and chronic kidney disease. New emerging species include Rhizopus homothallicus, Thamnostylum lucknowense, Mucor irregularis and Saksenaea erythrospora. Diagnosis of mucormycosis remains challenging. Clinical approach to diagnosis has a low sensitivity and specificity, it helps however in raising suspicion and prompting the initiation of laboratory testing. Histopathology, direct examination and culture remain essential tools, although the molecular methods are improving. The internal transcribed spacer (ITS) region is the most widely sequenced DNA region for fungi and it is recommended as a first-line method for species identification of Mucorales. New molecular platforms are being investigated and new fungal genetic targets are being explored. Molecular-based methods have gained acceptance for confirmation of the infection when applied on tissues. Methods on the detection of Mucorales DNA in blood have shown promising results for earlier and rapid diagnosis and could be used as screening tests in high-risk patients, but have to be validated in clinical studies. More, much needed, rapid methods that do not require invasive procedures, such as serology-based point-of-care, or metabolomics-based breath tests, are being developed and hopefully will be evaluated in the near future.
_____
_____
Mucormycosis and poultry:
Chickens (or poultry) can develop mucormycosis. The infection usually happens in chickens with immune problems because of stress, malnutrition, and environmental factors. However, mucormycosis does not spread between humans and animals. There is no evidence to suggest that people become infected with mucormycosis by eating cooked chicken. Raw chicken should never be eaten since there is a high risk of foodborne illness from bacteria like camphylobacer and salmonella.
_____
_____
Infection control issues:
-1. Patient-to-patient transmission of mucormycosis is unlikely. However, outbreaks or pseudo-outbreaks of healthcare-associated mucormycosis have been reported with contaminated bandages, tongue depressors, and other medical solutions or devices as previously described. Construction, excavation, or cleaning of airducts may aerosolize large inocula of Mucorales that, when inhaled, have been associated with slowly progressing pulmonary mucormycosis even in immunocompetent hosts.
-2. Patients with soft tissue mucormycosis often have a history of preceding trauma that resulted in subcutaneous inoculation of fungal spores. Cutaneous mucormycosis has been reported even with minor trauma, including insect bites and tattooing.
-3. Gloves, gown, and masking are unnecessary to prevent transmission of mucormycosis to healthy people except visiting construction or excavation sites.
-4. There is no vaccine for mucormycosis.
-5. Given the uncommon nature of infection, primary antifungal prophylaxis for mucormycosis is not recommended. However, secondary antifungal prophylaxis or chronic suppressive therapy should be considered for persistently immunosuppressed patients with intensification of therapy during periods of more severe immunosuppression. Anecdotal cases of mucormycosis reactivation in patients with acute leukemias and/or hematopoetic stem cell transplantation have been reported following more than 2 years of continuous treatment and prior good clinical response to antifungal therapy and tapering immunosuppression.
______
______
Section-7
Pathophysiology and pathogenesis of mucormycosis:
Mucorales are ubiquitous fungi that are commonly found in soil and in decaying matter. Rhizopus can be found in moldy bread. Given the ubiquitous nature of these fungi, most humans are exposed to these organisms on a daily or weekly basis. Nonetheless, they rarely cause disease because of the low virulence of the organisms; instead, they mainly affect individuals with immunocompromising conditions. Immunocompromised hosts with poorly controlled diabetes mellitus (especially with ketoacidosis), who are receiving glucocorticosteroids, who have neutropenia in the setting of hematologic or solid malignancy, who have undergone transplantation, who have iron overload, and who have burns are at risk for disease.
The major route of infection is via inhalation of spores; other routes include ingestion and traumatic inoculation. Ingestion leads to GI disease and occurs primarily among malnourished patients but can also occur after ingesting non-nutritional substances (pica). Regarding cutaneous disease, nonsterile tape and contaminated wooden splints have caused wound infections. Such cases are associated with trauma/surgery, the presence of a pre-existing wound or line, or both. In addition, mucormycosis should be considered in the differential diagnoses of a necrotic-appearing wound or one with a poor response to antibiotic treatment following natural disasters (e.g., hurricanes, tsunamis, tornadoes).
When spores are deposited in the nasal turbinates, rhinocerebral disease develops; when spores are inhaled into the lungs, pulmonary disease develops; when ingested, GI disease ensues; and when the agents are introduced through interrupted skin, cutaneous disease develops.
In humans, the infection is thought to be caused by asexual spore formation. The tiny spores then become airborne and land on the oral and nasal mucosa of humans. In immunocompetent hosts, these spores will be contained by a phagocytic response. If this fails, germination will occur and hyphae will develop. The fungus shows a remarkable affinity for arteries, veins and lymphatics. It invades the vessel wall producing extensive endothelial damage, thrombosis, and infarction resulting in progressive tissue ischemia and necrosis of deep tissues, including muscle, fat and bone. It further progresses as hyphae propagate within the vessel walls and lumens causing thrombosis, ischemia, and infarction with dry gangrene of the affected tissues. Hematogenous spread to other organs can occur (lung, brain, and so on) and results in sepsis and multiorgan failure. Neutrophils are the key host defense against these fungi; thus, individuals with neutropenia or neutrophil dysfunction (e.g., diabetes, steroid use) are at highest risk. Few cases of mucormycosis have been reported in patients with acquired immunodeficiency syndrome (AIDS), suggesting that the host defense against this infection is not primarily mediated by cellular immunity.
Figure above shows pathophysiology of invasive mucormycosis.
_
Pathophysiology involves:
-Angioinvasion
-Vessel thrombosis
-Tissue necrosis
_
Mucorales have many common characteristics with other moulds, including portals of entry (airways as well as disrupted mucosal and skin barriers), innate host defenses (polymorphonuclear neutrophil and mononuclear phagocytes, specific ligands in fungal spores such as pathogen-associated molecular patterns, and immune cells such as Toll-like receptors) as well as histopathological and clinical features. However, R. oryzae and certain other Mucorales, including Lichtheimia, Rhizomucor, and Mortierella spp, are characterized by distinctive virulence factors that enable them to infect patients with diabetic ketoacidosis or other forms of acidosis, and exert unique host-pathogen interactions compared to other fungi, thus facilitating host evasion and disease progression despite treatment.
In addition, mucormycosis is characterized by extensive angioinvasion that leads to vessel thrombosis and tissue necrosis. Angioinvasion results in hematogenous dissemination of the organism, whereas necrosis of the affected tissues prevents penetration of immune cells and antifungal agents to the infection focus. Certain Mucorales, such as R. oryzae, have reduced susceptibility to innate host defense as compared to other fungi, such as Aspergillus or Candida, making them more difficult to treat and, therefore associated with increased mortality.
_
Host defenses include monocyte/macrophages and neutrophils. In fact, an increased risk for developing mucormycosis appears to involve functional and/or quantitative deficiencies of these cells. The paucity of reports of mucormycosis in HIV-infected patients who do not inject intravenous illicit drugs indicates that T-cell dysfunction alone is not a major determinant in the development of the disease.
Increased iron availability in tissue and serum is a unique risk factor for mucormycosis as demonstrated in patients with diabetic ketoacidosis, those receiving iron chelation therapy, and those with iron overload due to frequent blood transfusions in the setting of underlying hematologic malignancy. Fungi secure iron from the host by using high-affinity iron permeases or low-molecular-weight iron chelators.
Metabolic acidosis is also a key factor in predisposing patients to mucormycosis. It has been demonstrated that low serum pH diminishes the phagocytic and chemotactic ability of neutrophils. Furthermore lower blood pH disrupts transferrin binding of iron, leading to increased iron availability.
________
Pathogenesis:
Regardless of the manifestation of the disease, a hallmark of mucormycosis is the ability of the causative organism to aggressively and rapidly invade blood vessels, which results in hematogenous dissemination, vessel thrombosis, and subsequent tissue necrosis. Therefore, interactions between invading fungi and endothelial cells lining blood vessels represent a major step in the pathogenesis of mucormycosis. Similarly, the unique predisposition of DKA patients and deferoxamine-treated patients to mucormycosis points to the importance of hyperglycemia, iron, and acidifying ketone bodies in the virulence of Mucorales.
Mucorales infect the host either through inhalation, ingestion and/or through direct inoculation of fungal spores through an abraded skin due to trauma. Therefore, during early steps of the infection Mucorales interact directly with epithelial cells and basement membranes which separate the host cells from the underlying stroma. Further, clinical hallmarks of R. oryzae infection include its remarkable angiotropism and the susceptibility of patients with increased available serum iron and/or altered phagocytic function. The angioinvasion and subsequent hematogenous dissemination during mucormycosis indicate that the organism interacts in vivo with: 1) endothelial cells lining the vasculature; 2) the subendothelial membrane which is made accessible to the fungus upon damaging endothelial cells. The hyper-susceptibility of patients with increased available serum iron to infection by Mucorales, but not other pathogenic fungi, highlights the central role of iron metabolism in the organisms’ virulence strategy. For example, patients in DKA are uniquely susceptible to mucormycosis. These patients are known to have elevated free iron generated by proton-mediated liberation from transferrin due to the acidic pH of their blood. Finally, the susceptibility of patients lacking functional phagocytes underscores the vital role of these cells in the host defense against Mucorales. Consequently, for Mucorales to be successful pathogens, they must adhere to and invade host tissues while evading host defense mechanisms. Therefore, understanding the mechanisms by which these processes occur may lead to new approaches to prevent and/or treat mucormycosis.
_
-1. Interactions between R. oryzae and extracellular matrix components
Basement membranes are extracellular protein matrices that separate epithelial and endothelial cells from underlying stroma. They provide structural support for these cells and serve as barriers to the passage of macromolecules and invading pathogens. The majority of basement membrane proteins consist of laminin and collagen IV. Epithelial cell damage has been reported in patients who are susceptible to mucormycosis, such as diabetics or patients receiving chemotherapy. This damage in turn exposes the extracellular matrix proteins so that they can directly interact with the pathogen. In this respect, an early study showed that R. oryzae can adhere to laminin and type IV collagen, but not to fibronectin. This attachment occurs with spores prior to germination and decreases dramatically when the spores germinate. Furthermore, adherence of R. oryzae to laminin and collagen is specific as determined by anti-laminin and anti-collagen antibodies blocking studies as well as receptor competition experiments.
R. oryzae is known to harbor an expanded family of genes encoding for the proteolytic enzymes, including secreted aspartic proteinase (SAP) and subtilases gene families and multiple studies demonstrate the production of these enzymes by R. oryzae. Genes specifying these lytic enzymes, which have been shown to contribute to the virulence of other organisms, are present in larger numbers in R. oryzae compared to other fungi (28 SAP genes and 23 subtilases genes). These genes are expressed in patients with mucormycosis and their products likely facilitate the penetration of the organism through extracellular matrix proteins and invasion of host cells.
_
-2. Host Defense against mucormycosis
Studies have shown that hosts with higher levels of immune response cells such as monocytes/macrophages, dendritic cells, and invariant natural killer (iNK) T-cells exhibited greater control of fungal growth and protection against systemic infection. Pattern recognition receptors (PRRs) play an important role in inducing an immune response by recognizing specific fungal pathogens and initiating an immune response. In the case of mucosal candidiasis, the cells that produce cytokine IL-17 are extremely important in maintaining innate immunity.
_
Phagocytes are the major line of defense against Mucorales. Inhalation of Mucorales spores by immunocompetent animals does not result in the development of mucormycosis. Innate immune responses in healthy hosts typically clear sporangiospores before infection can be established. In contrast, neutropenic hosts are at increased risk for developing mucormycosis. Furthermore, corticosteroids and diabetes, both of which are known to suppress phagocyte functions, cause animals inhaling R. oryzae spores to die from progressive pulmonary and hematogenously disseminated infection.
Both mononuclear and polymorphonuclear phagocytes of normal hosts kill Mucorales by the generation of oxidative metabolites and the cationic peptides, defensins. To establish infection, spores must overcome killing by mononuclear and polymorphonuclear phagocytes to germinate into hypal forms, the angioinvasive form of the infection. Most inhaled spores can avoid upper host defenses and reach distal alveolar spaces. However, larger spores (>10 micrometers) may lodge in nasal turbinates, predisposing patients to sinusitis. A recent study showed that exposure of neutrophils to R. oryzae hyphae results in up-regulation in Toll-like receptor 2 expression and in a robust proinflammatory gene expression with rapid induction of NF-κB pathway–related genes. However, during DKA where there is hyperglycemia and acidosis, phagocytes display dysfunctional chemotaxis and intracellular killing of R. oryzae by both oxidative and non-oxidative mechanisms. The dysfunction in phagocyte anti-Rhizopus activities during DKA is likely due to the direct effects of hyperglycemia and acidosis. Also the elevated free iron found in DKA patients might be toxic to phagocytes. Additionally, spleen cells of mice fed excess levels of iron secrete less IFN-γ, a cytokine that upregulates killing of several Mucorales family members (including R. oryzae) by human polymorphonuclear leukocytes (PMNLs). Therefore, it is likely that during DKA excess levels of iron lead to impaired phagocytic function. This hypothesis is supported by impaired chemotaxis of neutrophils in response to R. oryzae infection in mice given excessive amount of iron compared to normal mice. This impaired chemotaxis is reversed upon treating mice with the Mucorales cidal iron chelator, deferasirox.
Concordant with these clinical observation, inhalation of Mucorales sporangiospores by immunocompetent animals does not result in the development of mucormycosis. In contrast, corticosteroid-immunosuppressed or animals with DKA die of progressive pulmonary and hematogenously disseminated infection. Moreover, the ability of inhaled sporangiospores to germinate and form hyphae in the host is critical for establishing infection. Although pulmonary alveolar macrophages harvested from lungs of immunocompetent mice are able to ingest and inhibit germination of R. oryzae sporangiospores, these bronchoalveolar macrophages have limited capacity to kill the organism in vitro. In contrast, corticosteroid treatment affects the ability of mouse bronchoalveolar macrophages to prevent germination of the spores in vitro or after in vivo infection induced by intranasal inoculation.
It is also likely that members of the Mucorales order suppress the immune recognition during infection. In this respect, a study using whole genome expression profiling in Drosophila melanogaster after infection with R. oryzae identified host genes that was selectively down-regulated, act in pathogen recognition, innate immune defense mechanisms and tissue repair mechanisms. These findings might further explain the success of Mucorales in evading host defenses and in causing extensive tissue necrosis.
Mucorales-specific T cells emerge in the course of invasive mucormycosis, according to a 2011 study. Such T cells predominantly produced IL-4, IFN-γ, IL-10, and to a lesser extent IL-17 and belonged to either CD4(+) or CD8(+) subsets. The specific T cells that produced IFN-γ were able to directly induce damage to Mucorales hyphae.
_
Figure below shows pathogenetic mechanisms of and host defense mechanisms against mucormycosis.
To cause disease, the agents of mucormycosis must scavenge from the host sufficient iron for growth, must evade host phagocytic defense mechanisms, and must access vasculature to disseminate. A) In a normal host, primary defense mechanisms against mucormycosis include sequestration of iron in serum by specialized iron-binding proteins (1), phagocytes including circulating neutrophils (2a) and tissue macrophages (2b), and endothelial cells (3), which regulate vascular tone and permeability. Acting in concert, these mechanisms prevent establishment of infection in tissue and subsequent endovascular invasion. B) In susceptible hosts, normal defense mechanisms break down. For example, in diabetic ketoacidosis (DKA), the acidic pH of the serum causes dissociation of free iron from sequestering proteins (1). This release of free iron allows rapid fungal growth. Defects in phagocytic defense mechanisms (2), for example, deficiency in cell number (neutropenia) or functional defects caused by corticosteroids or the hyperglycemia and acidosis of diabetic ketoacidosis, allow proliferation of the fungus. Finally, adherence to and damage of endothelial cells by the fungus (3) allows fungal angioinvasion and vessel thrombosis and subsequent tissue necrosis and dissemination of the fungal infection.
_
The exact mechanisms by which phagocytes are impaired by ketoacidosis, diabetes mellitus, and corticosteroids are yet to be determined. Furthermore, phagocyte dysfunction alone cannot explain the high incidence of mucormycosis among patients with DKA, because the incidence of mucormycosis among these patients is increased more than the incidence of infections caused by other pathogens. Therefore, Mucorales must possess unique virulence traits that enable the organism to exploit the unique state of immunosuppression and physiologic impairment seen in this subset of patients.
_
The recent completion of Rhizopus delemar 99-880 (aka R. oryzae) genome sequence revealed the existence of putative virulence factors that are likely to be critical for invasion and survival of the fungus in the host during infection. Additionally, the epidemiology, risk factors and clinical hallmarks of the disease point to the critical role of quantitative and/or qualitative phagocytic defects, as well as the high iron and glucose concentrations, in mediating angioinvasion during mucormycosis.
These virulence traits are summarized in Table below:
|
Virulence traits |
Function |
Role in virulence/immunopathogenesis |
|
Iron uptake |
||
|
Reductase/permease |
Iron uptake in iron depleted environments |
Proven |
|
Permease (FTR1) |
Proven |
|
|
Reductases |
putative |
|
|
Cu-oxidases |
putative |
|
|
Siderophore |
Siderophore-mediated iron uptake |
Proven |
|
Rhizoferrin |
putative |
|
|
Deferoxamine |
Proven |
|
|
Heme oxygenase |
Iron-uptake from heme |
Putative |
|
CotH |
Host cell invasion |
Proven |
|
GRP78 |
Host cell receptor |
Proven |
|
Proteinases |
Protein lysis |
Putative |
|
Chitin/chitosan |
Cell and structure assembly |
Putative |
|
Ergosterol biosynthesis |
Cell membrane fluidity and azole resistance |
Putative |
|
Cell size |
Proven |
|
|
Sex loci |
Mating |
Putative |
|
NK cells |
Host defense |
Proven |
|
Mucorales specific T cells |
Host defense |
Proven |
_
The skin barrier represents a host defense against cutaneous mucormycosis, as evidenced by the increased risk for developing mucormycosis in persons with disruption of this barrier. The agents of mucormycosis are typically incapable of penetrating intact skin. However, burns, traumatic disruption of the skin, and persistent maceration of skin enables the organism to penetrate into deeper tissues. These organisms could originate from traumatic implantation of contaminated soil or water (e.g., the outbreaks after natural disasters, as was seen after the tsunami in Indonesia in 2004 and after the destructive tornadoes that occurred in Joplin, Missouri, in June 2011). Contaminated surgical dressings and nonsterile adhesive tape have been shown to be the source of primary cutaneous mucormycosis. Furthermore, mucormycosis can even be introduced through direct access, as was seen with the use of contaminated tongue depressors in neonates or the use of contaminated wooden applicators used to mix drugs given to immunocompromised patients. These cases illustrate an alarming shift in mucormycosis cases from mainly community-acquired infections to nosocomial infections in susceptible hosts.
_
-3. Endothelial cell-R. oryzae interactions
Damage of and penetration through the endothelial cell lining of the blood vessels is likely a critical step in the pathogenetic strategy of Mucorales because angioinvasion is a hallmark of mucormycosis. This angioinvasion often results in vessel thrombosis and subsequent tissue necrosis which can prevent delivery of leukocytes and antifungal agents to the foci of infection, thereby further exacerbating the disease.
How Mucorales adhere to and invade the endothelium?
Studies mainly utilize germinated R. oryzae (germlings) since this is the form that is likely to interact with endothelial cells during tissue invasion.
-1. Adherence and invasion of human umbilical vein endothelial cells by R. oryzae.
R. oryzae germlings adhere avidly to endothelial cells but not to bare plastic. Furthermore, R. oryzae germlings are able to cause endothelial cell injury in vitro independent of any serum factors. This process requires direct contact between the organism and endothelial cells because membrane inserts separating R. oryzae from endothelial cells completely abrogates injury. Endothelial cell injury requires internalization of R. oryzae (i.e., invasion of the endothelium) because the use of the endothelial cell microfilament inhibitor, cytochalasin D, blocks internalization of and R. oryzae-induced endothelial cell injury. In addition, chelation of endothelial cell iron prevents R. oryzae from invading and damaging endothelial cells, which suggests that host iron can modulate the ability of Mucorales to cause disease. Of note, mice treated with the deferiprone and deferasirox (two iron chelators that deprive R. oryzae from acquiring external iron) are protected from hematogenously disseminated mucormycosis.
Unlike other fungi (e.g., C. albicans, Cryptococcus neoformans, etc.), fungal-induced endothelial cell injury does not require fungal viability since dead germlings (heat-, ethanol-, or glutaraldehyde-killed) are able to cause a similar degree of injury to endothelial cells as do live organisms. This ability of dead germlings to cause injury is also dependent on their internalization by endothelial cells. Injury mediated by dead germlings is induced by cell-associated rather than soluble factors since cell debris, but not the supernatant, from broken germlings as well as cell wall material from regenerating protoplasts of R. oryzae germlings cause equivalent injury to endothelial cells as live organisms do. Similar results are obtained when cell wall materials are collected from other members of the Mucorales order such as R. microsporus, Mucor, Cunninghamella, and Absidia. These results indicate that endothelial cell injury caused by Mucorales is dependent, at least in part, on a toxin like substance(s) that is associated with the cell wall. Researchers have recently demonstrated that Mucorales fungi produce a toxin, which plays a central role in virulence. Polyclonal antibodies against this toxin inhibit its ability to damage human cells in vitro and prevent hypovolemic shock, organ necrosis and death in mice with mucormycosis. Inhibition of the toxin in Rhizopus delemar through RNA interference compromises the ability of the fungus to damage host cells and attenuates virulence in mice. This 17 kDa toxin has structural and functional features of the plant toxin ricin, including the ability to inhibit protein synthesis through its N-glycosylase activity, the existence of a motif that mediates vascular leak and a lectin sequence.
-2. GRP78 is a novel endothelial cell receptor for Mucorales
To identify the host receptor(s) that are utilized by Mucorales to invade endothelial cells, researchers used the affinity purification process developed by Isberg and Leong, in which extracts of endothelial cell membrane proteins were incubated with intact R. oryzae germlings. A 78 kDa endothelial cell protein found to bind to R. oryzae but not S. cerevisiae (which does not adhere to or invade endothelial cells). The major band at 78 kDa was identified as Glucose Regulated Protein 78 (GRP78). This protein is a novel host receptor which mediates invasion and subsequent injury of endothelial cells by Mucorales, but not C. albicans or A. fumigatus. Additionally, GRP78 is a specific and universal receptor for germlings and not spores of several Mucorales members. Although GRP78 is utilized by R. oryzae to invade endothelial cells, it does not play a role in initial fungal adherence to host cells. These results provide support to a model by which the fungus invades endothelial cells through a two step approach that initially involves adherence of the fungus to a receptor followed by binding to GRP78, which triggers invasion.
GRP78 (also known as BiP/HSPA5) was discovered as a cellular protein induced by glucose starvation. It is a member of the HSP70 protein family that is mainly present in the endoplasmic reticulum. It functions as a major chaperone that is involved in many cellular processes, including protein folding and assembly, marking misfolded proteins for proteosome degradation, regulating Ca2+ homeostasis, and serving as a sensor for endoplasmic reticulum stress. Despite its main function as a cellular chaperone protein, recent studies reported the translocation of a fraction of GRP78 to the cell surface in a variety of cells.
Elevated concentrations of glucose and iron, consistent with those seen during DKA, enhance GRP78 surface expression and resulting invasion and injury of endothelial cells in a receptor-dependent manner. These results are concordant with finding that chelation of endothelial cell iron protects these cells from R. oryzae-induced injury in vitro. Researchers also found that mice in DKA, which have enhanced susceptibility to mucormycosis, have increased expression of GRP78 in their sinus, lungs, and brain versus normal mice and that anti-Grp78 immune serum protects these mice from mucormycosis. Collectively, these data offer an explanation for the longstanding mystery as to why hosts in DKA are uniquely predisposed to mucormycosis infection and provide a foundation for novel therapeutic interventions against this deadly infection.
_
Although not much is known about how Mucorales interact with epithelial cells, Rhizopus adheres to and invades endothelial cells by specific recognition of the host receptor glucose-regulator protein 78 (GRP78). This recognition causes host cellular death by induction of the endothelial cell–mediated fungus endocytosis. GRP78, which was first discovered as a heat shock protein involved in stress-related responses, binds to R. delemar as well as other Mucorales germlings but not spores. Binding to germlings is consistent with the hypothesis that hyphae are the invading form of Mucorales. Similarly, induced endocytosis and active penetration of epithelial cells by Candida albicans is linked to the formation of hyphal structures, and mutants impaired in hyphal formation are defective in host cell invasion. Aspergillus fumigatus, instead, invades lung epithelial or endothelial cells by expressing the thaumatin-like protein CalA on both germlings and conidia. Although an earlier study showed that CalA is required for adherence of A. fumigatus to laminin, hyphal adhesion to host cells and macromolecules was shown to be predominantly mediated by the polysaccharide galactosaminogalactan. Suppression of GRP78 expression by short hairpin RNA (shRNA) or blocking its function by antibodies suppresses fungal invasion of host cells and drastically decreases endothelial cell injury caused by R. delemar but not that of other fungal pathogens like C. albicans or A. fumigatus. Importantly, anti-GRP78 antibodies protect DKA mice from mucormycosis.
_
The fact that GRP78 does not bind to C. albicans or A. fumigatus and anti-GRP78 antibodies do not affect endothelial cell invasion of these 2 fungi clearly shows that a unique mechanism of Mucorales-mediated endothelial cell invasion and injury exists. Also, the lack of complete abrogation of Rhizopus-mediated endothelial cell invasion and injury when GRP78 is blocked or suppressed indicates that other factors are involved in Rhizopus interacting with endothelial cells. In this respect, in preliminary results of the transcriptome of endothelial cells interacting with R. delemar, R. oryzae, or M. circinelloides, it was shown that the platelet derived growth factor (PDGF) pathway is activated, similarly to C. albicans. Indeed, the use of 2 small molecules that inhibit the phosphorylation of PDGF receptor partially reduces Rhizopus-mediated endothelial cell injury in vitro. Future investigations are required to delineate whether GRP78 and PDGF receptor act as coreceptors or independently in facilitating Mucorales invasion of endothelial cells.
_
The fungal ligand that binds to GRP78 during invasion of the endothelium belongs to the spore coating (CotH) protein family. Similar to the previous discoveries for GRP78, blocking the function of CotH proteins either biochemically by using anti-CotH antibodies or genetically by attenuating CotH expression reduces the ability of R. delemar to invade and injure endothelial cells in vitro and reduces disease severity in mice. CotH proteins are universally present in Mucorales and absent from any other forms of life for which the genome has been sequenced. In other pathogenic fungi, invasion of host cells is mediated by other cell surface proteins. For example, agglutinin-like sequence (Als) proteins and thaumatin-like protein (CalA) have been reported to act as invasins for C. albicans and A. fumigatus, respectively. All 3 classes of protein families are characterized by the presence of secretion-signal and glycophosphatidylinositol (GPI)-anchored sequences. However, they all bind to different host receptors (e.g., Als proteins bind to cadherins while CalA binds to integrins).
_
The most commonly isolated Mucorales from patients (Rhizopus, Mucor, and Lichtheimia) contain 3–7 copies of CotH, while those that are only occasionally the cause of the disease (Apophysomyces, Cunninghamella, Saksenaea, and Syncephalastrum) only contain 1–2 copies. Interestingly, isolates of Entomopthorales, which were previously taxonomically considered close to Mucorales but do not cause invasive disease, lack the presence of CotH. Collectively, these data point to the unique interaction between Mucorales CotH and endothelial cell GRP78 receptor and to the ability of CotH to mediate invasive disease. Moreover, it appears that Mucorales fungi harboring more copies of CotH can cause more frequent disease. Alternatively, it is possible that GRP78 among individuals harbors SNPs that make the attachment to certain sequences of CotH more avid compared to others. This assumption is supported by the fact that CotH2 and CotH3, which have 77% sequence identity, can avidly bind GRP78 while CotH1, which has about 20%–24% amino acid identity to CotH2 and CotH3, does not. Future studies are necessary to verify these hypotheses and their effect on the interaction between GRP78/CotH and the frequency and severity of the disease.
_
Glucose, iron, and acidosis by β-hydroxy butyrate modulate GRP78/CotH interactions:
DKA and deferoxamine-treated patients are uniquely predisposed to mucormycosis. Clearly, diabetic patients suffer from an elevated concentration of glucose. Hyperglycemia can induce excessive glycosylation of proteins such as transferrin and ferritin, diminishing their iron affinity. Moreover, in the presence of an acidotic condition due to accumulation of ketone bodies (e.g., β-hydroxy butyrate [BHB]), the low pH in the blood vessels strongly impairs the ability of transferrin to chelate iron. Glucose, iron, and BHB enhance the growth of the fungus (see figure below). They also induce the expression of GRP78 and CotH, and this enhanced expression results in augmented fungal invasion and subsequent injury of the endothelium in vitro (see figure below) It appears that the BHB-related acidosis exerts a direct effect on both GRP78 and CotH expression (an effect not seen with lactic acid) and an indirect effect by compromising the ability of transferrin to chelate iron, because iron chelation combined with reversal of pH by sodium bicarbonate completely protects endothelial cells from Rhizopus-mediated invasion and injury. Importantly, DKA mice, or those treated with BHB, suffer from lower blood pH, have elevated available serum iron, express more GRP78 in their target organs (e.g., lungs and sinuses), and are extremely susceptible to mucormycosis. Consistent with the clinical observation, ketoacidosis does not predispose mice to aspergillosis. It is also worth noting that physiological concentrations of glucose, iron, and BHB augment the fungal growth and have detrimental effect on the host immune response via suppression of T-lymphocyte induction, interferon-Ɣ production, and phagocyte-mediated killing (see figure below). Thus, the unique interactions of GRP78 and CotH proteins and their enhanced expression under hyperglycemia and ketoacidosis explain the specific susceptibility of DKA patients to mucormycosis.
_
Figure above shows the interactions of Mucorales with endothelial cells during hematogenous dissemination/organ seeding and the effect of host factors on these interactions and on the immune response.
(A) Hyperglycemia and ketoacidosis result in liberation of iron from serum-sequestering proteins (e.g., transferrin) via glycosylation and protonation, respectively. (B) Ketone bodies (e.g., β-hydroxy butyrate [BHB]) and free iron negatively affect the immune response to the infection, while sodium bicarbonate (NaHCO3) reverses this negative effect by preventing iron release from transferrin and neutralizing acidity. (C) Surface expression of glucose-regulator protein 78 (GRP78) on endothelial cells is enhanced to cope with the stress elicited by hyperglycemia, free iron, and ketone bodies. (D) Glucose, free iron (transported by the high affinity iron permease [Ftr1p]), and BHB also enhance the expression of fungal cell surface CotH, which results in invasion of the endothelium and augmentation of fungal growth. (E) In deferoxamine-treated hosts, the iron-rich ferrioxamine binds to its fungal receptor (ferrioxamine binding proteins [Fob1/Fob2]) then releases iron via a reductive step prior to feeding invading Mucorales via Ftr1p transportation.
_
To emphasize the importance of GRP78/CotH protein interactions in the pathogenesis of mucormycosis, therapeutic treatment with either anti-GRP78 or anti-CotH antibodies protect DKA and neutropenic mice from mucormycosis. Also and potentially of clinical relevance is the finding that reversal of ketoacidosis in Rhizopus-infected mice by administration of sodium bicarbonate (in lieu of insulin) improves survival. This protection is believed to be caused by reversal of the enhanced fungal growth, restoration of the immune function, and halting of host tissues fungal invasion. It is currently unknown what role the GRP78/CotH interactions play in the neutropenic host, the other major patient population susceptible to mucormycosis. However, the fact that anti-CotH antibodies protect cyclophosphamide/cortisone acetate–treated mice from Mucorales infections argues that at least CotH proteins play a major role in the virulence of Mucorales in this host. Also, it has been known that GRP78 expression induces resistance of cancer cells to chemotherapy. Therefore, it is possible that cyclophosphamide treatment results in induction of GRP78 expression in mice.
_
Other factors contributing to endothelium injury:
The recognition of CotH by GRP78 does not appear to be the only mechanism through which Mucorales damage endothelial cells. It was observed that nonviable Rhizopus, killed by heat or chemicals such as glutaraldehyde or ethanol, was able to cause a comparable amount of damage to endothelial cells as viable cells. These results suggest the contribution of toxin-like substances in mucormycosis pathogenesis. However, rhizotoxin, which is produced by the Rhizopus symbiont bacterium Burkholderia, does not contribute to the virulence of Rhizopus in humans. Therefore, it is reasonable to speculate the presence of toxin-like secondary metabolites produced directly from Mucorales, which mediate the interaction between the pathogen and the host, especially with the recent report showing M. circinelloides to be the cause of food poisoning illness. Genomic evidence also supports the notion that Mucorales possess pathways for secondary metabolites including polyketide synthases (PKSs), nonribosomal peptide synthetases (NRPs), and L-tryptophan dimethylallyl transferases (DMATs).
_______
_______
Role of zinc and iron in pathogenesis of mucormycosis:
_
Zinc uptake by human fungal pathogens:
The ability of pathogenic microorganisms to assimilate nutrients from their host environment is one of the most fundamental aspects of infection. To counteract this, hosts attempt to withhold essential micro-nutrients from potentially harmful microbes to limit, or even prevent, their growth. This process is called nutritional immunity. For example, vertebrates, such as humans, express several iron-binding molecules to maintain extremely low free levels of this metal in the body. To overcome this restriction, successful pathogens have evolved sophisticated mechanisms to assimilate iron. These include high affinity transporters, siderophores, and transferrin-, ferritin-, and haem-binding proteins. Indeed, iron acquisition is considered a vital virulence factor for many pathogens. However, nutritional immunity does not begin and end with iron. Vertebrates have also developed mechanisms to sequester other essential metals, such as zinc. The importance of zinc sequestration and the strategies that successful pathogens employ to overcome this has only recently been realized.
_
Zinc is essential for life, with an astonishing 9% of eukaryotic proteins predicted to be zinc metalloproteins. The role of this metal in human health is well-documented and zinc is known to play key roles in both adaptive and innate immunity. However, host zinc sequestration from pathogens, as a means to control microbial growth, is an emerging field. Accompanying this, a growing body of literature is illuminating the role of bacterial zinc acquisition systems in virulence. But what about fungal pathogens? Fungi also rely on zinc for growth, as this metal serves as a cofactor for several enzymes, including superoxide dismutase and alcohol dehydrogenase, along with numerous other proteins, such as transcription factors. Therefore, in order to cause infections, pathogenic fungi must assimilate zinc from their host environment. In fungi, zinc binding to transcription factors and other proteins ranges from approximately 9% of the proteome in Aspergillus fumigatus and 7.5% in Saccharomyces cerevisiae. Zinc relevance is further stressed by our immune system that, when confronted by a fungal pathogen, often deploys an array of mechanisms in order to manipulate microbial access to the metal. Neutrophils release extracellular traps to restrict extracellular zinc availability via calprotectin, a zinc binding protein, released at inflammatory sites. Zinc limitation is also observed intracellularly when infected macrophages restrict fungal growth by pumping the metal out of the phagosome. To ensure survival, pathogenic fungi express an array of zinc transporters with variable degrees of affinity to zinc and, thus, maintain growth in harsh conditions.
_
Progressive studies have been published in order to understand how those transporters work in pathogenic fungi, unveiling two major groups of zinc transporters: the Zrt- and Irt-like Protein (ZIP) and the Cation Diffusion Facilitator (CDF) families of transporters. The ZIP family consists of membrane proteins responsible for the transport of zinc into the cytosol, either from the extracellular environment or from intracellular membrane compartments, such as the vacuole or endoplasmic reticulum. The CDF family transports zinc from the cytosol to organelles, maintaining essential zinc-depending metabolic processes as well as quickly reducing zinc cytosolic levels in case of “zinc shock”. All the main proteins of both families are tightly regulated by a single transcription factor, capable of sensing minor alterations in cytosolic zinc levels.
_
Despite evolutionary and ecological differences, various fungal species are devastating human pathogens and must therefore be able to effectively utilise zinc from their host environment during infection. Based on the observations made in C. albicans and A. fumigatus, it is evident that our immune system has learned to recognise this fungal zinc uptake system. This seems to be a recurrent immunological theme, as several bacterial zinc uptake systems are effectively recognised by our immune systems and are now being considered as vaccine targets. From the perspective of nutritional immunity, this is perhaps not surprising: during infection, zinc is a generally limiting factor, and thus, in this setting, pathogens must express high affinity uptake systems in order to proliferate. Indeed, it is possible that our immune system has developed this positive feedback loop for the recognition and killing of microbial invaders. By creating a circuit whereby zinc deficiency is enforced via nutritional immunity and microbial high-affinity zinc uptake systems are targeted, our immune systems force the invader to reveal itself.
_
Rhizopus delemar (previously R oryzae), one of the main species causing Mucormycosis, encodes 3 cell surface zinc importers. Zinc actually acts as growth factor of Mucormycosis. In vitro study it has been seen that zinc chelator like clioquinol or phenanthroline or other zinc chelator inhibit the growth of this fungus. That means zinc deprivement neutralise the growth. Not only this, difficult to grow this fungus in zinc deficient tissue.
______
______
Iron uptake by human fungal pathogens:
Iron is an essential element for cell growth and development, contributing to DNA synthesis and regulating the G1-phase to S-phase transition. Moreover, iron is important for the virulence of the majority of microorganisms, and the function of the genes regulating iron uptake is coupled with the manifestations of the virulence phenotype. Iron is required by virtually all microbial pathogens for growth and virulence.
Iron is present in two readily available ionization states, Fe2+ (ferrous) and Fe3+ (ferric). Because of its ability to exist in either of these two states, iron has the ability to donate and accept electrons, and therefore can participate in a wide variety of cellular oxidation-reduction reactions. However, the chemical properties of iron place two limitations on its cellular accumulation and utilization by microorganisms.
First, the metal is mainly found in nature in an insoluble state, typically comprised of Fe3+ hydroxides. The insolubility of Fe3+ hydroxides limit the ability of microorganisms to transport the iron intracellularly. Therefore, fungi have devised a variety of strategies to overcome this problem.
The second problem limiting iron utilization by fungi is that iron is potentially toxic because of its ability to catalyze the production of oxygen free radicals via the Fenton reaction or the Haber-Weiss reaction. Iron catalyzed production of oxygen free radicals lead to cellular injury by causing oxidative damage to a wide variety of cellular substrates. Therefore, proper storage of excess iron is essential to prevent toxicity. For instance, soon after uptake, iron can be found in the ferrous form bound to polyphosphates in vacuoles of S. cerevisiae. Alternatively, iron can be stored as part of iron-rich proteins (ferritins). To date, the only fungi identified that store iron in ferritins are members of the class Zygomycetes. Three types of iron-rich proteins have been identified in Zygomycetes: 1) mycoferritin, which is closely related to the mammalians ferritins; 2) bacterioferritin; and 3) zygoferritin, which is unique to Zygomycetes. Also, fungi can store iron as part of small proteins called siderophores, which specialize in obtaining iron from the environment. This mechanism of storage is common among fungi belonging to the ascomycetes and basidiomycetes classes.
_
In mammalian hosts, very little serum iron is available to microorganisms because it is highly bound to carrier proteins such as transferrin. Sequestration of iron by serum is a major host defense mechanism against R. oryzae in particular. The organism grows poorly in serum and this growth inhibition is reversed when exogenous iron is added.
Importantly, patients with elevated levels of available serum iron are uniquely susceptible to infection by R. oryzae and other Zygomycetes, but not to other pathogenic fungi, such as Candida or Aspergillus. For example, patients treated with the iron chelator, deferoxamine, have a markedly increased incidence of invasive mucormycosis, which is associated with a mortality of >80% in these patients. While deferoxamine acts as an iron chelator with respect to the human host, its effect on R. oryzae is just the opposite. Deferoxamine predisposes patients to Rhizopus infection by acting as a siderophore, which supplies previously unavailable iron to the fungus. Rhizopus obtains iron from the iron-deferoxamine complex by intracellular transport of the reduced iron without deferoxamine internalization. This transport is likely mediated by high-affinity iron permeases.
Patients with diabetic ketoacidosis have elevated levels of available serum iron, likely due to release of iron from binding proteins in the presence of acidosis. Artis et al. showed that sera collected from patients with diabetic ketoacidosis supported growth of R. oryzae in the presence of acidic pH (7.3-6.88) but not in the presence of alkaline pH (7.78-8.38). Furthermore, adding exogenous iron to serum allowed R. oryzae to grow profusely at acidic conditions but not at pH ≥7.4. Finally, simulated acidic conditions decreased the iron-binding capacity of serum samples collected from healthy volunteers, suggesting that acidosis per se disrupts the capacity of transferrin to bind iron, probably by proton-mediated displacement of ferric iron from transferrin. As proof of principle, animal data showed that mice with DKA were protected from R. oryzae infection by administration of iron chelators, such as deferiprone and deferasirox, which are not used by Mucorales as xenosiderophores. However, not all Mucorales have the same susceptibility to effective iron chelators. For example, a study showed that Cunninghamella bertholletiae and Mucor species display higher deferasirox minimal inhibitory and fungicidal concentrations than do Rhizopus species.
_
Another clinical observation highlights the central role of host iron availability in predisposing patients to mucormycosis. Patients receiving dialysis who are treated with the iron chelator deferoxamine are also uniquely susceptible to a deadly form of mucormycosis. The bacterial siderophore, deferoxamine, predisposes patients to Rhizopus infection by acting as a xenosiderophore. Deferoxamine strips ferric iron from transferrin and attaches itself on the mold through an inducible receptor, and the iron is transported intracellularly by an active reduction of the ferric form into the more soluble ferrous form. Concordant with these results, administration of deferoxamine worsens survival among guinea pigs infected with Rhizopus but not among those infected with Candida albicans. In addition, in vitro studies of radiolabeled iron uptake from deferoxamine in serum show that Rhizopus is able to incorporate 8-fold and 40-fold more iron than can Aspergillus fumigatus and C. albicans, respectively. Finally, a major risk factor for mucormycosis in transplantation includes underlying myelodysplastic syndrome, which probably predisposes patients to the disease because of iron overload resulting from repeated blood transfusions.
_
Fungi can obtain iron from the host by using high-affinity iron permeases or low-molecular-weight iron chelators (siderophores). The high-affinity iron permeases are present in fungi as part of a reductive system containing redundant surface reductases that reduce ferric into the more soluble ferrous form. The reduced ferrous iron generated by the surface reductase is, in turn, captured by a protein complex consisting of a multicopper oxidase and a ferrous permease. The genome sequencing project identified 3 ferric reductases, 6 copper oxidases, and 1 high-affinity iron permease. Indeed, recent data show that the gene encoding high-affinity iron permease (FTR1) is expressed by R. oryzae during murine infection and inhibition of FTR1 gene expression by RNA-I, or reduction of FTR1 copy number by gene disruption reduces the virulence of the fungus in animal models of mucormycosis. Of importance, passive immunization with anti-Ftr1p immune serum protected mice with DKA from infection with R. oryzae . Thus, FTR1 is a crucial virulence factor for R. oryzae, and anti-Ftr1p passive immunotherapy represents a promising strategy to improve outcomes of deadly mucormycosis.
_
Rhizopus is known to secrete rhizoferrin, a siderophore that belongs to the polycarboxylate family. This siderophore supplies Rhizopus with iron through a receptor-mediated, energy-dependent process. In this regard, the genome-sequencing project of R. oryzae identified 13 possible siderophore permeases that might act as receptors for siderophores, including rhizoferrin or deferoxamine. However, it is not currently known whether rhizoferrin transports iron by release of iron extracellularly or whether the siderophore is internalized before releasing iron in the cytoplasm. What is known is that rhizoferrin is inefficient in obtaining iron from serum; therefore, the contribution of the organism’s endogenous siderophores to its virulence in a mammalian host is likely to be minimal. The lack of rhizoferrin ability to take iron from serum is also highlighted by the adaptation of the organism to use xenosiderophores, such as deferoxamine, which are more efficient in obtaining iron from the host.
_
A third mechanism by which fungi can obtain iron from the host is through use of heme. The Rhizopus genome project revealed 2 homologues of the heme oxygenase. These 2 R. oryzae homologues may enable R. oryzae to obtain iron from host hemoglobin and might explain the angioinvasive nature of R. oryzae. Of interest, researchers found that R. oryzae that had reduced copy numbers of FTR1 also had lagging growth on media supplemented with heme. Therefore, FTR1 in R. oryzae may act as a cytoplasmic membrane permease that facilitates intracellular heme uptake, which is followed by release of ferric iron through degradation with heme oxygenases intracellularly. Other genes likely to be involved in the ability of R. oryzae to take up iron include SreA, a transcriptional regulator that has been described in A. fumigatus to be required for adaptation to the ambient iron availability, and 2 orthologes probably encoding ferritin required for intracellular storage of iron.
_
Figure below shows the 3 mechanisms of iron uptake that are likely to be operative during mucormycosis.
Proposed mechanisms of iron uptake by Mucorales during mucormycosis. A, Because of the angioinvasive nature of the disease, heme (H) is likely to represent a source of iron to the invading fungus, which either takes up heme intracellularly or strips ferric iron from heme by the action of the reductase-permease system. If heme is transported intracellulary, ferric iron is obtained by the action of heme oxygenase in the cytoplasm. B, In patients with DKA, proton (H+)–mediated displacement of ferric iron (Fe3+) from transferrin (T) increases the availability of iron, which is transported intracellularly by the reductase-permease system. C, Deferoxamine (D) directly chelates iron from transferrin, resulting in ferrioxamine (iron-deferoxamine complex). The fungus then liberates ferrous iron from ferrioxamine by reduction at the cell surface. In all cases, iron is transported across the cell membrane by the copper oxidase–iron permease (FTR1) complex.
_
The Role of Deferoxamine (DFO):
The well-documented and repeatedly reported increased susceptibility to zygomycosis of haemodialysis patients during treatment with DFO, an iron chelator that is capable of removing tissue iron, initially appeared to be a paradox. It became clear, however, that although DFO chelates iron, from the perspective of Zygomycetes it is a xenosiderophore, as fungal siderophores have higher affinity for iron than DFO and therefore are capable of easily and effectively detaching iron from it and providing it to the fungi. This ability is particularly prominent in Zygomycetes, and these species can remove eight and 40 times greater amounts of iron from DFO than A. fumigatus and C. albicans, respectively.
Figure above shows effect of human serum and of ferrioxamine on the growth of Rhizopus microsporus. Spores of Rhizopus were cultivated for 24 h at 37C in standard BDM culture medium alone (a), in BDM with 40% human serum (b), or in BDM with 40% serum + 1 lM Fe deferoxamine (Fe.DFO) (c).
The rapid and effective iron uptake by Zygomycetes results in rapid growth in serum. The growth of Rhizopus rhizopodiformis spores, isolated from a dialysis patient with zygomycosis while on DFO therapy, was studied in an iron-deficient medium containing human serum at increasing concentrations and with human serum enriched with different concentrations of ferrioxamine (DFO–iron complex). A concentration of 40% human serum inhibited fungal growth by >50%. However, in the presence of serum, ferrioxamine produced significant growth stimulation at 24 h that persisted at 48 h. The effect of human serum and of ferrioxamine on the growth of Rhizopus found in this study is demonstrated in figure above. Data from animal models emphasize the exceptional requirement of iron for Rhizopus pathogenicity, as administration of DFO or free iron worsens the survival of animals infected with Rhizopus, but not with Candida.
Patients with hemochromatosis (iron overload) are predisposed to mucormycosis because of the essential role free iron plays in the growth of Mucorales in vivo. Historically, patients with severe hemochromatosis received treatment with the chelation agent deferoxamine. However, deferoxamine can be utilized by some Mucorales (Rhizopus) as a xenosiderophore (foreign iron carrier protein) to form a ferrioxamine complex, which makes iron, which was previously unavailable, available to the fungus. Hence, deferoxamine therapy is associated with increased risk for fulminant mucormycosis. A similar phenomenon does not take place with deferiprone.
____
Potential novel therapies:
The two newer iron chelators deferiprone (DFP) and deferasirox (DFX) do not act as xenosiderophores, apparently because the fungal iron uptake systems are incapable of detaching iron from them. This could be due either to inadequate molecular access, as they are smaller molecules than DFO, or to their higher affinity for iron, which means that DFP and DFX might form more stable chemical structures with iron that are not destabilized in the presence of fungal enzymes or siderophores. Moreover, the demonstration of clear inhibitory activity of the two newer chelators on fungal growth suggests that these molecules are probably capable of detaching iron from the fungal iron uptake molecules and holding it more strongly. This has been proven in vivo, using animal models of zygomycosis, in which treatment of Rhizopus-infected mice or guinea pigs with DFP markedly improved survival. In cultures of Rhizopus oryzae, DFP has fungistatic activity at 24 h, confirmed at 48 h.
The introduction of DFX and the recognition of the safety and efficacy profile of the drug encouraged its use in sporadic cases of systemic zygomycosis and in experimental animal studies. DFX induces an iron-starvation response in R. oryzae and activates RFTR1 expression. Addition of DFX to cultures of different members of the Mucorales produced a fungicidal effect, which was reversed by the addition of iron. The MIC90s of DFX against various Mucor spp. were much lower than the levels achieved by the administration of the usual daily dose of 20 mg/kg. Treatment with routine doses of DFX of diabetic ketoacidotic mice infected with spores of R. oryzae led to significantly improved survival as compared with controls, and resulted in a more than ten-fold reduction of brain and kidney fungal burden as compared with placebo-treated animals. The kidneys of DFX-treated mice had no visible hyphae and there was an effective neutrophil inflammatory reaction, whereas kidneys of placebo-treated mice had extensive filamentous fungi and manifested a poor or complete absence of a neutrophil inflammatory response.
In another experiment, mice infected intranasally with 107 spores of R. oryzae were treated for 7 days, starting 24 h post-infection, with either DFX 10 mg/kg twice daily or placebo. As controls, infected or uninfected mice were treated with DFO 50 mg/kg. DFX was significantly more protective than placebo or DFO. As expected, DFO worsened the survival of infected mice, although it had no effect on uninfected mice. Treatment with DFX resulted in significantly increased Th1 and Th2 splenocyte subpopulations, and in significantly higher splenic and kidney levels of the proinflammatory cytokines tumour necrosis factor-a and interferon-c, than those in mice treated with saturating iron or placebo.
As mentioned above, patients with elevated available serum iron, be it free iron or ferrioxamine iron, are at high risk of acquiring mucormycosis. Experimental data strongly indicated that the use of iron chelators that are not utilised as xenosiderophores by Mucorales can be of benefit in treating the disease alone or as an adjunctive therapy. In 2005, deferasirox became the first orally bioavailable iron chelator approved for use in the US by the FDA to treat iron overload in transfusion-dependent anaemia. This led to the off-label use of deferasirox in treating advanced cases of mucormycosis with reported success as an adjunctive therapy mainly in diabetic patients with ketoacidosis. However, a subsequent phase II, double-blind, randomised, placebo-controlled trial of adjunctive deferasirox therapy that enrolled a total of twenty patients failed to demonstrate a benefit of the combination regimen in patients with mucormycosis. In fact significantly higher mortality rates were found in patients randomised to receive deferasirox at 30 (45% vs. 11%) and 90 days (82% vs. 22%, P = 0.01). It is imperative to note that although this study represents the first completed clinical trial of evaluating a novel treatment option for mucormycosis, it suffered from major imbalances between the two study arms with patients receiving deferasirox were more likely than placebo patients to have active malignancy, neutropenia, corticosteroid therapy and less likely to have received additional antifungal, making the results of this pilot trial hard to interpret. Thus, conclusions regarding the use of deferasirox cannot be drawn from this small study. Indeed subsequent studies to the Phase II clinical trial continue to suggest the successful use of deferasirox as an adjunctive therapy against mucormycosis especially in DKA patients. Therefore, only a large, Phase III trial, potentially enrolling only diabetic or corticosteroid-treated patients (as suggested by the animal studies and anecdotal studies), and excluding cancer/neutropenia patients, could further elucidate the safety and efficacy of initial, adjunctive deferasirox (and other iron chelators) for the treatment of mucormycosis.
______
______
Endothermy:
Endothermy can be defined as any mechanism of heat generation without shivering that increases body temperature and resting metabolic rate. Homeothermy is thermoregulation that maintains a stable internal body temperature regardless of external influence. Mammalian endothermy and homeothermy are potent nonspecific defenses against most fungi. A comparative genomic study found that in opportunistic fungi there are few if any specialised virulence traits consistently linked to opportunistic pathogenicity of fungi in humans apart from the ability to grow at 37 °C.
Of the 1.5 million fungal species, only a few hundred are pathogenic to mammals. Fungal diseases in mammals often reflect impaired immune function, and fungi did not emerge as major pathogens for humans until the late 20th century. For example, candidiasis was uncommon until the 1950s, when thrush was associated with the introduction of antibiotics that disrupted bacterial flora. Similarly, diseases such as cryptococcosis, aspergillosis, and histoplasmosis were rare until recently, when their prevalence increased with the human immunodeficiency virus epidemic and the development of immunosuppressive therapies. In contrast, the number of fungal species pathogenic to plants and insects is estimated to be 270,000 and 50,000, respectively. Amphibians are particularly vulnerable to certain fungal infections, as evidenced by the current catastrophic epidemic of chytridiomycosis in frogs
The resistance of mammals with intact immune systems to systemic fungal diseases, coupled with their endothermic and homeothermic lifestyles, suggested that these costly physiological adaptations were evolutionarily selected because they conferred a survival advantage by protecting against environmental pathogens. The paucity of fungal diseases in mammals relative to insects, amphibians, and plants is due to mammalian endothermy and homeothermy that are potent nonspecific defenses against most fungi and have provided a strong evolutionary survival advantage against fungal diseases. A study analyzed the thermal tolerance of 4802 fungal strains from 144 genera and found that most cannot grow at mammalian temperatures. Fungi from insects and mammals had greater thermal tolerances than did isolates from soils and plants. Every 1°C increase in the 30°C–40°C range excluded an additional 6% of fungal isolates, implying that fever could significantly increase the thermal exclusion zone.
_______
_______
Opportunistic vs non-opportunistic fungi:
For fungi to successfully infect a human body, they have to overcome several obstacles, such as high temperature (Robert and Casadevall 2009), low water activity and low pH in case of skin penetration (Elias 2007), oxidative bursts of human phagocytes and severe iron limitation (Hamad 2008; Kumamoto 2008). In the case of the few true fungal pathogens (also named primary pathogens), which can infect healthy individuals, specialised mechanisms to counter the above-described immune defences possibly evolved as a response to selection pressures during an infection. The infection potential of these species enhances their fitness and is therefore considered as an essential part of their natural lifestyle. However, such adaptive evolution seems improbable in the case of opportunists, a much longer list of species limited to sporadic infections of often immunocompromised hosts. Compared to the large populations of opportunistic pathogens outside the host the infection events are extremely rare and it is unlikely that they would noticeably contribute to the biological success of the species. Therefore, traits promoting virulence in opportunists have likely evolved for purposes other than survival within the host (Song et al. 2017). Published literature suggests various selection pressures driving the emergence of such pre-existing adaptations (exaptations), among them adaptations to stress encountered outside the mammalian host. These traits would primarily promote the survival of the fungus in the environment, but (as an unintentional side-effect) also allow its establishment in the host (van Burik and Magee 2001; Casadevall 2007). For example, in the prominent pathogen Cryptococcus neoformans the mechanisms that enable its survival during infection are thought to have evolved in response to stress in its primary ecological niche, bird manure (Brown et al. 2007). Several examples of polyextremotolerant fungi that are also opportunistic pathogens are found among dothideomycetous and eurotiomycetous black yeasts, a group of melanised ascomycetous species. If opportunistic infections are indeed accidental colonisations enabled by adaptability and stress tolerance, opportunistic potential should be phylogenetically linked to polyextremotolerance. Opportunists should contain few if any traits important in virulence compared to their non-opportunistic relatives.
______
______
Section-8
Predisposing factors for mucormycosis:
The most important conditions that predispose to mucormycosis, according to various studies (see table below), include diabetes mellitus (DM), with or without ketoacidosis, hematological malignancies (HM), other malignancies, transplantation, prolonged neutropenia, corticosteroids, trauma, iron overload, illicit intravenous drug use, neonatal prematurity and malnourishment. Immunocompetent patients can also be affected, when the spores of the fungus are directly inoculated in the skin, as a result of trauma or burns.
Risk factors for mucormycosis:
|
Reference |
Characteristics of Studies |
Risk Factors/Underlying Diseases (%) |
|||||||||||
|
Countries of Origin of Cases |
Prospective Study |
Multicenter Study |
Time Period |
Total no. of pts |
DM |
HM |
HSCT |
SOM/ SOT |
AI/CO |
Trauma # |
HIV |
None |
|
|
Roden et al. 2005 |
Global |
No |
Yes |
1940–2003 |
929 |
36 |
15.8 |
5 |
1/7 |
1 |
8 # |
2 |
19 |
|
Jeong et al. 2019 |
Global |
No |
Yes |
2000–2017 |
851 |
40 |
32 |
1/14 |
3/33 |
20 |
18 |
||
|
Skiada et al. 2011 |
Europe |
Yes |
Yes |
2005–2007 |
230 |
17 |
44 |
5/4 |
44 |
17 |
2 |
8 |
|
|
Lanternier et al. 2012 |
France |
No |
Yes |
2005–2007 |
101 |
23 |
50 |
12 |
2/3 |
13 |
18 |
1 |
1 |
|
Pagano et al. 2009 |
Italy |
Yes |
Yes |
2004–2007 |
60 |
18 |
62 |
3 |
8/ |
3/50 |
2 |
17 |
3 |
|
Kontoyiannis et al. 2016 |
USA |
No |
Yes |
2005–2014 |
555 |
52 |
40 |
11 |
6/15 |
NA |
4 |
2 |
NA |
|
Nucci et al. 2019 |
South America |
No |
Yes |
1960–2018 |
143 |
42 |
11 |
2 |
/13 |
NA |
20 |
2 |
7.7 |
|
Corzo-Leon et al. 2017 |
Mexico |
No |
Yes |
1982–2016 |
418 |
72 |
17 |
1/ |
1 |
2.3 |
0.7 |
4 |
|
|
Chakrabarti et al. 2006 |
India |
No |
No |
2000–2004 |
178 |
73.6 |
1.1 |
/0.6 |
1.7 |
7.3 |
0.6 |
11.8 |
|
|
Chakrabarti et al. 2009 |
India |
Yes |
No |
2006–2007 |
75 |
44 |
9 |
/5 |
29 |
11 |
1 |
3 |
|
|
Prakash et al. 2019 |
India |
Yes |
Yes |
2013–2015 |
303 |
56.8 |
6 |
/6 |
9.9 |
10 |
– |
10.5 |
|
|
Patel et al. 2020 [11] |
India |
Yes |
Yes |
2016–2017 |
465 |
74 |
8 |
1 |
1.5/6.5 |
/3.7 |
6.9 |
– |
11.8 |
|
Dolatabadi et al. 2018 |
Iran |
No |
Yes |
2008–2014 |
208 |
75 |
3 |
2 |
3/3 |
NA |
4 |
– |
2 |
|
Vaezi et al. 2016 |
Iran |
No |
Yes |
1990–2015 |
98 |
48 |
6 |
1 |
/23 |
NA |
1 |
– |
10 |
|
El Zein et al. 2018 |
Lebanon |
No |
No |
2008–2018 |
20 |
35 |
65 |
/5 |
70 |
– |
– |
– |
|
|
Kennedy et al. 2016 |
Australia |
No |
Yes |
2004–2012 |
74 |
27 |
48.6 |
18 |
3/11 |
12/ 53 |
23 |
11 |
|
|
Stemler et al. 2020 |
Middle East and North Africa |
No |
Yes |
1968–2019 |
310 |
49.7 |
16.5 |
2/17 |
21.6 |
12 |
0.3 |
5.8 |
|
# Penetrating trauma and surgery. SOM = Solid organ malignancy, SOT = Solid organ transplantation, AI = Autoimmune disease, CO = corticosteroids.
In several studies there are multiple risk factors so the total is more than 100% in the table above.
Risk factors for mucormycosis vary considerably by geographical area. In studies from Europe the most common underlying disease was a hematological malignancy, while in India, Iran and Mexico it was diabetes mellitus. Diabetes was also the leading underlying condition in the study by Stemler et al. in countries of the Middle East and North Africa.
_
Relationship between predisposing condition and site of infection:
Based on clinical presentation and the involvement of a particular anatomic site, mucormycosis can be divided into at least six clinical categories: (i) rhinocerebral, (ii) pulmonary, (iii) cutaneous, (iv) gastrointestinal, (v) disseminated, and (iv) miscellaneous. Of note, these categories of invasive mucormycosis tend to occur in patients with specific defects in host defense (see table below). Several studies have shown that the underlying disease is correlated to the site of infection. Hematological malignancies and neutropenia are associated with pulmonary mucormycosis and diabetes mellitus with sinusitis and rhinocerebral disease, while trauma usually leads to cutaneous mucormycosis.
|
Predisposing Condition |
Predominant Site of Infection |
|
Diabetes mellitus |
Rhinocerebral, sino-orbital, cutaneous |
|
Haematological malignancy |
Pulmonary, sinus, cutaneous, sino-orbital |
|
Hematopoetic stem cell transplantation |
Pulmonary, disseminated, rhinocerebral |
|
Solid organ transplantation |
Sinus, cutaneous, pulmonary, rhinocerebral, disseminated |
|
Intravenous drug use |
Cerebral, endocarditis, cutaneous, disseminated |
|
Deferoxamine therapy |
Disseminated, pulmonary, rhinocerebral, cutaneous, gastrointestinal |
|
Trauma |
Cutaneous, ocular |
|
Malnutrition |
Gastrointestinal |
|
Corticosteroids |
Pulmonary, disseminated, or rhinocerebral |
|
Neutropenia |
Pulmonary and disseminated |
______
Diabetes Mellitus and mucormycosis:
Studies have reported diabetes mellitus as a predisposing factor to mucormycosis in 36%–88% of cases. DM is a clinical syndrome associated with deficiency of insulin secretion and/or function. It is considered as one of the largest emerging threats to health in the 21st century. It is estimated that there will be 380 million cases of DM in 2025. Diabetes mellitus is the leading underlying disease in patients with mucormycosis globally. According to the World Health Organization (WHO) “the global prevalence (age-standardized) of diabetes has nearly doubled since 1980, rising from 4.7% to 8.5% in the adult population. Globally, an estimated 422 million adults were living with diabetes in 2014, compared to 108 million in 1980”. Diabetes prevalence has risen faster in low- and middle-income countries than in high-income countries. The number of people aged 20–79 years with diabetes in 2011 was 61.3 million in India, and it is estimated to rise to 101.2 million in 2030. A great rise in the diabetic population is also predicted for China, Brazil, Japan, Mexico, Egypt and Indonesia. Accordingly, the cases of mucormycosis are expected to increase.
_
In the latest review by Jeong et al. diabetes mellitus was the most common underlying condition in 40% of cases and 20% had documented ketoacidosis. Uncontrolled, type II, diabetes is the most common type in diabetic patients with mucormycosis. In a recent study comparing North and South India, diabetic ketoacidosis was found in 90% of cases from North India and 10% of cases from South India. Diabetes has been reported as a risk factor for mucormycosis in 73.5% of cases in India, 75% in Iran and 72% in Mexico. In contrast, the percentages from the European ECMM study were 17%, from Italy 18%, from France 23% and from Lebanon 35%. In the Indian publications, mucormycosis was the unmasking disease for diabetes mellitus in 12–31% of patients.
When diabetes is poorly controlled, blood sugar is high and the tissues become relatively acidic – a good environment for Mucorales fungi to grow. This was identified as a risk for mucormycosis in India (where diabetes is increasingly prevalent and often uncontrolled) and worldwide well before the Covid-19 pandemic. Of all mucormycosis cases published in scientific journals globally between 2000-2017, diabetes was seen in 40% of cases. A recent summary of Covid-19-associated mucormycosis showed 94% of patients had diabetes, and it was poorly controlled in 67% of cases. A multicenter study of 187 cases of CAM after the first COVID wave, noted a 2.1-fold increase in the cases of mucormycosis during the peak COVID-19 period as compared to pre-COVID-19 time. Uncontrolled diabetes was noted in 62.7% of cases. COVID-19 was the only risk factor in 32.6% CAM patients among whom 78.7% received glucocorticoid treatment for COVID-19 management. Inappropriate glucocorticoid use was independently associated with late CAM. The US has a very high prevalence of diabetes – 9.3% of the population is estimated to have the condition. It also has the highest number of Covid cases globally. But mucormycosis is very rare – diabetes cases there are largely managed with only 3% going undiagnosed, according to the US Centers for Disease Control.
In a meta-analysis of 175 ROCM cases published between 1994 and 2005, diabetes mellitus was the predominant underlying condition (64% of the cases), followed by hematological malignancy (15%) and renal diseases (13%). In two different studies, Rhizopus was predominantly associated with rhino-cerebral forms. This finding might be explained by differences in virulence between genera in the order of Mucorales. In experimental studies, ketoacidosis has been found to predispose mice to Rhizopus spp. but not to Lichtheimia spp. infection. This could be linked with the fact that the glucose-regulated protein 78 (GRP78), an endoplasmic reticulum chaperone protein of the HSP70 family, induced notably by elevated concentrations of glucose has been identified as the host receptor for R. arrhizus in endothelial cells in mice.
_
Beside the classical complications of the disease, DM has been associated with reduced response in T cells, neutrophil function, and responsible for disorders of humeral immunity. Consequently, DM increases the susceptibility to infections by most common microbial pathogens as well as fungal agents that cause mucormycosis. Diabetes mellitus tends to change the normal immunological response of body to any infection in several ways. Hyperglycemia stimulates fungal proliferation and also causes decrease in chemotaxis and phagocytic efficiency which permits the otherwise innocuous organisms to thrive in acid-rich environment. In the diabetic ketoacidosis patient, there is an increased risk of mucormycosis caused by Rhizopus oryzae as these organisms produce the enzyme ketoreductase, which allows them to utilize the patient’s ketone bodies. It has been established that diabetic ketoacidosis temporarily disrupts the ability of transferrin to bind iron, and this alteration eliminates a significant host defense mechanism and permits the growth of Rhizopus oryzae. The mechanism for ketoacidosis preferentially causing susceptibility to the rhinocerebral form of the disease remains unclear. As mentioned earlier, patients in ketoacidosis, or indeed any systemic acidosis, have increased available iron in serum due to dissociation of iron from sequestering proteins in acidic conditions. However, the predominant presentation of mucormycosis in the setting of deferoxamine therapy is disseminated disease, indicating that increased available iron cannot, by itself, explain the preferential occurrence of rhinocerebral disease in ketoacidosis. Furthermore, while it is known that hyperglycemia and acidosis negatively impact neutrophil chemotaxis and phagocytic activity, these observations cannot explain the preferential occurrence of rhinocerebral disease in diabetic ketoacidosis because neutropenic patients more commonly develop pulmonary mucormycosis than rhinocerebral disease.
_
When Uncontrolled Diabetes Mellitus and Severe COVID-19 Converge: The Perfect Storm for Mucormycosis, a 2021 study:
Mucormycosis has been increasingly described in patients with coronavirus disease 2019 (COVID-19) but the epidemiological factors, presentation, diagnostic certainty, and outcome of such patients are not well described. Authors review the published COVID-19-associated mucormycosis (CAM) cases (total 41) to identify risk factors, clinical features, and outcomes. CAM was typically seen in patients with diabetes mellitus (DM) (94%) especially the ones with poorly controlled DM (67%) and severe or critical COVID-19 (95%). Its presentation was typical of mucormycosis seen in diabetic patients (mostly rhino-orbital and rhino-orbital-cerebral presentation). In sharp contrast to reported COVID-associated aspergillosis (CAPA) cases, nearly all CAM infections were proven (93%). Treating physicians should have a high suspicion for CAM in patients with uncontrolled diabetes mellitus and severe COVID-19 presenting with rhino-orbital or rhino-cerebral syndromes. CAM is the convergence of two storms, one of DM and the other of COVID-19.
_______
Hematological Malignancy and Hematopoietic Stem Cell Transplantation:
Hematological malignancies (HMs) and hematopoietic stem cell transplantation (HSCT) are the most common underlying diseases in mucormycosis in Europe, USA and Australia. In India HM was a risk factor in 1–9% and in Iran in 3%. The majority of patients with HM and mucormycosis in various studies had acute myeloid leukemia (48% in the ECMM study, 46% in Italy, 34% in France, 38% in Lebanon and 42% in the global review by Jeong et al.). The rest of the patients with HM had acute lymphoblastic leukemia, non-Hodgkin’s lymphoma, myelodysplastic syndrome and other, more rare, malignancies. The risk is higher when the patient has prolonged neutropenia. HSCT is also an important risk factor. In the global analysis of mucormycosis in France from 2005 to 2007, HSCT was the underlying condition in 12% of cases. Conversely, in a French nationwide retrospective study from 2003 to 2008, the prevalence of mucormycosis among HSCT recipients was found to be 0.4%. In order to investigate the epidemiology of infections due to Mucorales and other molds in transplant recipients, Park et al. analyzed the data from the Transplant-Associated Infection Surveillance Network (TRANSNET), where 23 transplant centers in USA conducted a prospective surveillance for invasive fungal infections during 2001–2006. The 12-month mucormycosis cumulative incidence was 0.29% for HSCT. Mucormycosis incidence among HSCT recipients varied widely, from 0.08% to 0.69%. The incidence of HSCT as a risk factor for mucormycosis in developing countries was reported as 1% for India, 2% for Iran and 2% for South America countries.
_
Predisposing risk factors for mucormycosis in patients with hematologic malignancies and/or stem cell transplantation:
|
Prolonged (> 3 wk) and severe (ANC < 200) neutropenia |
|
Monocytopenia (< 100 mm3) |
|
Prolonged (> 3 wk) high-dose systemic corticosteroids (e.g., prednisone or equivalent of > 1 mg/kg/d) |
|
Iron overload (assessed by high iron indices, high iron storage by MRI, or high iron staining in bone marrow biopsy) |
|
High-risk SCT (e.g., matched-unrelated donor SCT, haploidentical donor SCT, cord blood SCT, T cell-depleted SCT) |
|
Severe GVHD and its treatment (especially corticosteroids) |
|
Prolonged hyperglycemia (fasting serum glucose > 200 mg/dL), corticosteroid-associated hyperglycemia, diabetes mellitus |
|
Colonization by mucormycetes or heavy environmental exposure? |
|
Previous exposure to Aspergillus-active antifungal agents, especially voriconazole? |
|
Relapsed leukemia |
ANC indicates absolute neutrophil count.
_
Solid Organ Malignancies and Solid Organ Transplantation:
Solid organ malignancies (SOMs) and solid organ transplantation (SOT), although not as common as HM and HSCT, are also important risk factors for mucormycosis. The reported prevalence varies, ranging from 0.6% to 23%, but the studies cannot be compared because the methods of collecting data are different. The numbers are higher in reviews of the literature, where there is publication bias. In the RetroZygo study from France SOT was the underlying disease in 3% of cases. In the TRANSNET analysis the 12-month mucormycosis cumulative incidence for SOT was 0.07% and mucormycosis accounted for 2% of all invasive fungal infections in these patients. The results were similar in the Prospective Antifungal Therapy Alliance (PATH Alliance) study, where 7526 invasive fungal infections in a total of 6918 adults SOT recipients were included, Mucormycosis accounted for 1.6% of these infections. The incidence also depends on the organ being transplanted. In the review by Almyroudis et al. the incidence in renal transplant recipients was 0.4–0.5, in liver recipients 4–16, in heart recipients 8 and in lung recipients 13.7–14, all per 1000 patients. In a prospective, matched case-control study, renal failure, diabetes mellitus and prior voriconazole and/or caspofungin use were associated with a higher risk of mucormycosis, whereas tacrolimus, a calcineurin-inhibitor, was associated with a lower risk. Calcineurin plays a vital role in the virulence and pathogenicity of several opportunistic fungi and its involvement in the virulence of Mucorales is currently a hot topic of research.
_
At transplant centers there has also been an alarming rise in the incidence of mucormycosis. For example, at the Fred Hutchinson Cancer Center, Marr et al. have described a doubling in the number of cases from 1985 to 1989 to 1995 to 1999. Similarly, Kontoyianis et al. have described a greater than doubling in the incidence of mucormycosis in transplant patients over a similar time span. In patients undergoing hematological stem cell transplantation, mucormycosis develops at least as commonly in nonneutropenic periods as in neutropenic periods. For example, two major transplant centers have recently reported that more than half the cases of mucormycosis occurred more than 90 days after transplantation. These cases illustrate an alarming trend in the epidemiology of mucormycosis. Mucormycosis, formerly virtually always community acquired and often in the setting of diabetic ketoacidosis, is rapidly becoming a nosocomial infection in patients with malignancy or undergoing organ or hematopoietic cell transplantation.
_
Major risk factors for mucormycosis in the transplant setting include underlying myelodysplastic syndrome (possibly due to iron overload from repeated blood transfusions) and graft-versus-host disease treated with steroids. Administration of antithymocyte globulin may also pose a risk for mucormycosis. Although less than half of these patients are neutropenic at the time of disease onset, prolonged neutropenia is a risk factor for mucormycosis in this setting, as are diabetes mellitus and steroid use. The role of antifungal prophylaxis in predisposing patients to developing mucormycosis is increasingly being described. Prophylaxis with either itraconazole or voriconazole has been implicated in predisposing to mucormycosis.
_______
Corticosteroids and Other Immunosuppressive Agents:
Chronic administration of corticosteroids and other immunosuppressive agents is an important risk factor for mucormycosis. They are used in the treatment of malignancies, transplantation and autoimmune diseases. Corticosteroids impair migration, ingestion and phagolysosome fusion in macrophages. In addition they may lead to drug-induced diabetes. Prolonged (>3 weeks) high-dose systemic corticosteroids are risk factors for mucormycosis. However, there have been reports of mucormycosis associated with short courses of corticosteroids. In the ECMM European study 46% of patients had received corticosteroids in the month prior to diagnosis of mucormycosis and 44% had received other immunosuppressive agents. In the global review by Jeong et al. 3% of patients had an autoimmune disease, while in Australia, Kennedy et al. reported that 12% had an autoimmune disease. There are few cases of systemic lupus erythematosus associated mucormycosis in the literature, but the mortality of these infections is exceedingly high (80%) There have also been reports of mucormycosis in other autoimmune diseases, where the infection mimicked the relapse of Wegener’s granulomatosis or, in another case, was confused with giant cell arteritis. Although these cases are extremely rare, they may be underdiagnosed and mucormycosis should be included in the differential diagnosis.
_
Risks of invasive fungal infection with corticosteroids:
Invasive aspergillosis:
Among 228 renal transplant recipients, a case control study revealed the impact of higher doses of prednisolone on acquisition of invasive aspergillosis.
|
Prednisolone dose |
Invasive aspergillosis (%) |
No invasive aspergillosis (%) |
|
>1.25mg/Kg |
8 (89) |
8 (33) |
|
<1.25mg/Kg |
1 (11) |
16 (67) |
P = 0.0057
In 331 recipients of allogeneic stem cell (bone marrow) transplants (HSCT), use of high-dose prednisone (0·5–1·0 mg/kg per day) increased invasive aspergillosis and other fungal infections six-fold compared with regimens with low dose prednisone (0·25 mg/kg per day). Prednisone doses of <1·9 mg/kg, 1·9–3·0 mg/kg and >3 mg/kg daily were associated with a risk of aspergillosis of 5%, 10%, and 14%, respectively in the late post-bone-marrow-transplant period. Cumulative prednisone receipt of >700 mg in the 3 months prior to admission was associated with the occurrence of invasive aspergillosis in COPD patients. There are many other examples of increased risk of invasive aspergillosis after corticosteroids.
Candidaemia and invasive candidiasis:
Corticosteroids are a risk factor for candidaemia and invasive candidiasis. Premature neonates treated for hypotension with corticosteroids developed invasive candidiasis 7.5-fold more often than those not receiving corticosteroids. Breakthrough candidaemia was associated with corticosteroid therapy (53%) compared with non-breakthrough candidaemia (23%). Corticosteroids predispose patients undergoing surgery, including transplant surgery, those on haemodialysis for acute renal failure, HSCT and those with SLE to candidaemia. Some species differences are apparent for risk of infection.
Pneumocystis pneumonia (PCP):
Corticosteroids are a key risk factor in ~90% of patients with cancer who develop PCP, if not given co-trimoxazole (trimethoprim + sulphamethoxazole) prophylaxis. In particular, PCP often follows high dose dexamethasone given for brain tumours. Likewise corticosteroids with or without chemotherapy are a risk factor for PCP in patients with chronic lymphocytic leukaemia, with chronic graft-versus-host disease following HSCT, and nonmalignant conditions such as autoimmune disorders, especially SLE and Wegener’s granulomatosis. A median daily dose of 40 mg prednisone in patients with SLE is associated with development of PCP.
Cryptococcal meningitis and infection:
Cortisone acetate reduces the ability of alveolar macrophages to attach to and ingest C. neoformans, the chemotactic activity of cerebrospinal fluid toward PMNs and monocytes and impairs microglial (brain macrophage) function. Corticosteroids predispose non-AIDS patients with cancer or sarcoidosis and HSCT and solid-organ transplant recipients to cryptococcosis. Corticosteroids are not helpful for raised intracranial pressure in patients with cryptococcal meningitis.
Histoplasmosis:
Disseminated histoplasmosis is associated with corticosteroid treatment, notably in SLE and cancer. Prednisone treatment >20 mg/day or prior therapy with corticosteroids are risk factors.
Mucormycosis:
Cumulative prednisone dose of >600 mg prior to infection predisposes cancer patients to zygomycosis and methylprednisone dose of 2–7 g was associated with mucormycosis after liver or pancreas-kidney transplantation. Patients with lupus erythematosus, auto-immune disease, Wegener granulomatosis, when treated with high-dose of steroid for a long time, are at increased risk of mucormycosis especially disseminated form and the mortality rate is very high (88%). Corticosteroids make these people susceptible by reducing the function of macrophages and neutrophils, and/or steroid induced diabetes.
Worse outcomes in patients with invasive fungal infection continuing on corticosteroids:
The risk of dying with or of invasive aspergillosis was higher (80%) in HSCT recipients who received a cumulative prednisolone dose of >7 mg/kg in the week before infection, compared with similar patients who received a cumulative prednisolone dose of <7 mg/kg (12%). Among 94 patients with multiple underlying diseases with invasive aspergillosis, use of corticosteroid therapy increased the risk of dying 10.6-fold. A daily prednisone-equivalent dose of 30 mg administered for 12 weeks (median) was clearly associated with a worse outcome from Pneumocystis pneumonia. Relapse of cryptococcal meningitis was intimately associated with continuing corticosteroid therapy (at least 20mg prednisone-equivalent daily) after antifungal therapy has stopped. Corticosteroids are associated with a worse outcome from disseminate Fusarium infection; 70% versus 33% mortality in haematological cancer patients receiving and not receiving glucocorticoids respectively. Dexamethasone in acute cryptococcal meningitis in AIDS produces slower sterilisation of the CSF and overall a worse outcome.
Better outcomes with adjunctive corticosteroids in treatment with antifungal therapy:
Several randomised studies have shown that adjunctive corticosteroids improve survival of patients with PCP in AIDS. The overall reduction in mortality was 46% at 1 month and 33% at 3-4 months of follow-up. The impact was greatest if antiretroviral therapy cannot be given. The need for mechanical ventilation is also reduced by adjunctive corticosteroids. This effect is not clearly seen in non-HIV patients who develop PCP, possibly because corticosteroids are the major risk factor for disease.
Corticosteroids are also important in the management of several other fungal infections:
-Exacerbations of allergic bronchopulmonary aspergillosis (ABPA) are typically treated with oral steroids.
-Allergic fungal rhinosinusitis patients are treated post-surgery with oral and/or local steroid, probably with benefit.
-Topical corticosteroids combined with topical antifungal therapy are valuable in reducing the inflammatory component of seborrheic dermatitis and kerion (tinea capitis).
-Mulch pneumonitis in chronic granulomatous disease appears to respond better to a combination of antifungal therapy (such as voriconazole) and adjunctive corticosteroids.
________
Iron Overload:
Increased serum iron is a risk factor for mucormycosis, as iron plays a crucial role in the pathogenesis of this infection. Iron is normally attached to transferrin and ferritin, and is not available to the Mucorales fungi. In patients with diabetic ketoacidosis or other forms of acidosis there is decreased affinity of these proteins to bind iron. Serum iron may also be increased in patients undergoing dialysis or multiple transfusions. In the past, the iron chelator deferoxamine was used in these cases. This is a bacterial siderophore and is actually utilized by Mucorales as a xenosiderophore for acquiring iron from the host. It soon became apparent that deferoxamine was a risk factor for mucormycosis, most often disseminated. The newer chelators, deferasirox and deferiprone, do not have xenosiderophore activity. Iron overload has also been implicated in the increased susceptibility of liver transplant recipients to disseminated mucormycosis.
Deferoxamine and iron overload:
Deferoxamine, which chelates both iron and aluminum, increases the risk of mucormycosis by enhancing growth and pathogenicity. The deferoxamine-iron chelate, called feroxamine, is a siderophore for the species Rhizopus, increasing iron uptake by the fungus, which stimulates fungal growth and leads to tissue invasion.
Iron overload itself may predispose to mucormycosis in the absence of deferoxamine therapy. In addition, individuals with diabetic ketoacidosis have elevated concentrations of free iron in their serum, which supports the growth of Rhizopus oryzae at an acidic, but not at an alkaline pH.
Deferoxamine was once used commonly as an aluminum chelator in patients with renal failure; however, aluminum excess is rarely seen today. Currently, patients at risk for deferoxamine-associated mucormycosis are those who have received multiple blood transfusions and are treated with this chelating agent for iron overload. The majority of patients with deferoxamine-associated infection present with disseminated disease that is rapidly fatal, with a mortality rate that approaches 90 percent.
In contrast with deferoxamine, other iron chelating agents, such as deferasirox and deferiprone, do not act as siderophores and therefore do not increase the risk of mucormycosis. Limited studies of their adjunctive use in mice with mucormycosis have suggested benefit, but results in humans have been mixed.
Deferoxamine may be associated with a tense form of mucormycosis as it can cause inhibition of Iron-catalyzed peroxidase production of free radicals (that is important for killing fungi). Increased serum iron intensifies mucormycosis as it holds a siderophoric affinity to the fungus. The iron bound to siderophores (an iron-binding compound secreted by microorganisms) can be used by the fungi, whereas that bound to transferrin cannot be used.
______
Breakthrough Mucormycosis:
Another factor that may predispose to mucormycosis is the use of antifungal prophylaxis or treatment, which is effective against Aspergillus but not Mucorales (voriconazole and echinocandins). The first reports of possible association of prophylaxis with voriconazole and mucormycosis were published in 2004. Marty et al. hypothesized that a possible explanation for this was prolonged use of voriconazole or increased survival among profoundly immunosuppressed patients, i.e., patients who had received HSCT. Studies in animal models suggested that voriconazole might increase the virulence of certain Mucorales. Breakthrough invasive fungal infections continued to emerge when the newer azoles, posaconazole and isavuconazole were introduced, despite their activity against Mucorales. In a single-center study from Austria 13% of HSCT patients receiving prophylaxis with posaconazole developed breakthrough invasive fungal infections and 55% of them were due to mucormycetes. A recent review by Lionakis et al. showed that the incidence and spectrum of breakthrough invasive mold infections vary significantly depending on the specific mold-active antifungal used for prophylaxis, local epidemiology and patient characteristics.
______
Other factors:
Other diseases associated with mucormycosis are intravenous drug use, AIDS, renal failure, liver diseases, chronic alcoholism, malnutrition and low birth weight infants. Mucormycosis in patients who are HIV positive is extremely rare. In an old retrospective study of 1630 autopsies of patients who died of AIDS from 1984 to 2002, only 2 patients had mucormycosis. In the recent review of the literature by Moreira et al. only 4 out of 67 cases (5.9%) had no other risk factors. The commonest comorbidities were a history of intravenous drug use (IVDU; 50%), neutropenia (29.7%) and corticosteroid use (25%).
Patients with a history of IVDU who develop mucormycosis, most often present with isolated cerebral infection. Conversely, in a review of 68 patients with isolated cerebral mucormycosis, 82% had a history of IVDU, and the authors concluded that the presence of lesions in the basal ganglia, rapidly progressive symptoms and a history of IVDU should raise suspicion for mucormycosis and for initiation of amphotericin B and stereotactic aspiration. In a study from India, published in 2019, post-pulmonary tuberculosis (6.9%) and chronic kidney disease (8.9%) were emerging risk factors.
_____
No Underlying Disease:
In a significant proportion of cases mucormycosis develops in immunocompetent patients. In the two large reviews by Roden et al. and Jeong et al., 19% and 18% of patients respectively, had no underlying disease. In such cases trauma or burns is the usual predisposing factor, resulting in cutaneous disease. The trauma can be minor (injection sites, animal bites, gardening, etc.) or major, including motor vehicle accident, natural disasters and surgery. In cases associated with natural disasters uncommon species have been isolated: wound infections due to Apophysomyces elegans were reported after the tsunami in Sri-Lanka and Syncephalastrum racemosum was isolated from respiratory samples following hurricane Katrina. An outbreak of necrotizing soft tissue infections causes by Apophysomyces trapeziformis occurred in patients with traumatic injuries resulting from a tornado in Joplin, Missouri, in 2011. Invasive skin and soft tissue infections occur in patients with disrupted cutaneous barriers, as a result of either traumatic implantation of soil, maceration of skin by a moist surface, or even via direct access through intravenous catheters or subcutaneous injections.
Mucormycosis has also been reported following combat-related injury. Invasive fungal infections, including mucormycosis, have been reported in United States military personnel who sustained blast injuries during combat in Afghanistan. Between June 2009 and through December 2010, a total of 37 cases were identified, including 20 proven cases (with histopathologic evidence of angioinvasion), 4 probable cases (with histopathologic evidence of nonvascular tissue invasion), and 13 possible cases (with a positive fungal culture, but without histopathologic evidence).
Another clinical entity, reported mainly from India and China, is isolated renal mucormycosis in immunocompetent patients. Finally, there have been several reports from Asia, about an infection in immunocompetent patients by Mucor irregularis (formerly Rhizomucor variabilis), leading to primary cutaneous mucormycosis, which progresses very slowly, in contrast to the classical presentation of mucormycosis.
_______
Healthcare Associated Mucormycosis:
Though mucormycosis was considered a community-acquired disease, nosocomial mucormycosis has been increasingly reported from many hospitals. Documented cases of mucormycosis have been noted after use of the contaminated umbilical catheter and elastoplast adhesive dressings. Outbreaks associated with contaminated wooden tongue depressors, wooden sticks, karaya ostomy bags and bandages have been described. The underlying diseases of those patients included diabetes mellitus (22%), solid organ transplantation (24%), steroid therapy (37%), and malignancy (12%). The skin was the most common site involved (57%), followed by gastrointestinal tract (15%), lungs (8%), sinuses and brain (4%). Disseminated infection was reported in 2% of patients. An outbreak of intestinal mucormycosis had been reported in a haematology ward of a hospital over six months period in China possibly after ingestion of allopurinol tablets and ready-to-eat food. Corn-starch was possibly the source of contamination, as it was used to prepare both preparations. Duffy et al. reported outbreak associated with contaminated linens in the United States hospital and Rhizopus species was isolated from 42% of the samples collected from clean linens. Since invasive mucormycosis is found to be an important cause of mortality in debilitated patients, a high index of suspicion should exist among the clinicians to predict the outbreaks in the hospital environment.
As mentioned, there has also been a shift from community onset to nosocomial onset of disease. Nosocomial mucormycosis has been associated with iatrogenic immunosuppression and a variety of procedures or devices used in hospitals, including antifungal prophylaxis, bandages or medication patches, intravenous catheters, and even tongue depressors. There have been multiple reports of healthcare-associated mucormycosis, either as isolated cases or as outbreaks. Two studies — published in 2014 and 2016, respectively — implicate hospital linens from poorly managed laundries as a source. A 2009 review of research into hospital outbreaks identifies ventilation systems, wooden tongue depressors, adhesive bandages, and ostomy bags as other possible sources of infection. Pathologists at the University of Kentucky in Lexington report that another possible transmission route is the inhalation of spores in dust from nearby building works, or contaminated air-conditioning filters. They also highlight the importance of infection through the skin, for example via burns, catheter insertion sites, needlestick injuries, insect bites, and stings.
In a publication from India, 75 cases of mucormycosis were reported during an eighteen-month period, of which 9% were nosocomial. Healthcare-associated mucormycosis has been attributed to various exposures in the hospital environment:
-(a) The use of non-sterile products is the most commonly suspected cause of infection. Bandages, adhesives, nitroglycerin patches, contaminated linen, wooden tongue depressors, ostomy bags and probiotics have all been implicated.
-(b) Various procedures and medical devices, such as catheters, insulin pumps and finger sticks, and insertion of tubes, tooth extractions and surgery.
-(c) Environmental factors may also be a source of infection. Molds may be found in the air, dust or any surfaces in the hospital. Construction works increase the risk of invasive fungal infections. Outbreaks have been linked to defective ventilation systems and water leakage.
The clinical presentation varies, depending on the source of infection. Infections due to bandages, adhesives or contaminated wound dressings are mostly cutaneous. Percutaneous exposure in immunocompromised patients has led to disseminated disease. Inhalation leads to pulmonary and rhino-cerebral infection, while ingestion of tablets or food, and the use of tongue depressors, are responsible for gastrointestinal mucormycosis. Dialysis catheters have been linked to peritonitis.
______
______
Hypoxia and mucormycosis:
At the peak of the second wave in India, when oxygen supplies were in short, oxygen was being rationed, hospitals were trying to maintain patients at low oxygen levels between 90 and 94; some experts hypothesized that low oxygen concentration becomes an ideal environment to nurture fungus growth. Patients who are hypoxic offer a conducive environment for the fungus to grow. In fact, it is one of the leading causes of mucormycosis. I disagree.
_
Molecular oxygen functions as an electron acceptor for aerobic respiration and a substrate for key metabolisms and cellular processes. Microbes that have diverse habitats, especially pathogenic microbes, are exposed to varying oxygen levels in both terrestrial and host environments having different oxygen states. Hypoxia also occurs at most infection sites and generates significant environmental stress on hosts and pathogens. Therefore, out of necessity, eukaryotes evolved sophisticated mechanisms for sensing and adapting to altered oxygen levels. Most eukaryotes develop direct or indirect oxygen sensors and reprogram transcriptional and translational metabolisms to adapt to altered oxygen availability under varying oxygen concentrations.
_
Molecular oxygen plays an essential role as an electron acceptor in the generation of chemical energy via mitochondrial respiration but is also critical for the biosynthesis of sterols, mono- and polyunsaturated fatty acids, NAD, and porphyrin and in other metabolic and biosynthetic pathways. Thus, the amount of available oxygen to eukaryotic cells is a critical factor in determining overall cellular metabolism. As most eukaryotic human fungal pathogens are generally considered obligate aerobes, oxygen availability during fungal pathogenesis may play a critical role in the outcome of infection from the perspective of both the host and the fungus. Pathogenic fungi like Candida albicans, Cryptococcus neoformans, Mucorales and Aspergillus fumigatus are exposed to oxygen-limited or hypoxic microenvironments during fungal pathogenesis. Human fungal pathogens manipulate transcriptional levels of genes related to virulence as well as oxygen-dependent metabolisms such as ergosterol homeostasis when they are confronted with oxygen limitation (hypoxia) during infection.
_
In a given environment, oxygen availability is usually described as anaerobic or anoxic (complete absence of oxygen), hypoxic (reduction in available oxygen compared to atmospheric levels), or normoxic (atmospheric levels of generally 21% O2 or an O2 partial pressure [pO2] of 159 mm Hg at sea level). In the context of microbial pathogenesis, it is generally accepted that hypoxia occurs at sites of infection, thus generating significant environmental stress on most host and microbial pathogen cells. An exact oxygen level that defines localized hypoxia in vivo is difficult to pinpoint and will likely vary with anatomical location and distinct pathologies. However, the mammalian hypoxic response starts (as monitored through induction of the mammalian hypoxia transcription factor hypoxia-inducible factor 1 [HIF-1]) in most cells at an oxygen level of ∼6% (pO2, 40 mm Hg). In healthy tissues in the human body, oxygen levels of 2.5% to 9% are considered normal, while oxygen levels of ≤1% that have been described in tumors and wounds are typically considered hypoxic. In the healthy human lung, the initial deposition site of many human fungal infections, alveolar pO2 is around 100 to 110 mm Hg (∼14% O2).
_
When an invading microbe interacts with host cells, tissue damage due to inflammation, thrombosis, and necrosis is thought to decrease available oxygen concentrations due to decreased tissue perfusion at the site of infection. For example, it has recently been observed that production of the nonribosomal peptide gliotoxin and other potential secondary metabolites by the mold Aspergillus fumigatus contributes to the inhibition of angiogenesis in the lung. Inhibition of neovascularization at the site of infection by the fungus is likely to cause significant tissue necrosis, prevent tissue repair, and thus contribute to the development of localized and systemic hypoxia. Moreover, it is likely that oxygen-dependent metabolism of both pathogen and host cells also contributes to the rapid utilization of available oxygen, though this remains to be definitively confirmed. Importantly, at least within the lung, the co-occurrence of microbial infection and hypoxia is often associated with poor clinical outcomes.
_
Oxygen concentrations at sites of human fungal infection have not been measured directly in vivo, though hypoxemia is often described as part of the clinical picture associated with these infections, even in the lung, that may require invasive or noninvasive oxygen therapy. In addition, CO2 production is directly coupled to oxygen consumption of eukaryotic cells and sites of hypoxia in vivo often contain increased levels of this gas, whose sensing has been linked to fungal virulence.
_
With regard to Candida albicans, one of the most frequently occurring human fungal pathogens, its normal anatomical location is the human gastrointestinal tract, which contains significant regions of hypoxia. For cryptococcal meningitis caused by Cryptococcus neoformans, oxygen concentrations in the human brain are also significantly lower than in the atmosphere, indicating that C. neoformans is also faced with reduced oxygen levels during infection. In further support of the idea that fungal pathogens face significant oxygen depletion during pathogenesis, hypoxia at the site of infection has recently been confirmed in murine models of invasive pulmonary aspergillosis (IPA). In two related studies of murine IPA models, a luciferase-producing A. fumigatus strain showed decreased luminescence in vivo after reaching a maximum at day 1 postinfection (despite an increase in fungal burden). The authors hypothesized that this observation was due to severe tissue damage caused during infection, which may decrease oxygen availability. Thus, the lack of luminescence may be attributable to hypoxia at the site of infection, as oxygen is essential for the light-producing luciferase reaction.
Additional indirect evidence that hypoxia is an important component of the in vivo microenvironment during a pulmonary fungal infection is the recent detection of ethanol production in bronchoalveolar lavage fluid from a chemotherapeutic murine model of IPA. It was further found that, under normoxic conditions, A. fumigatus did not produce detectable ethanol levels in culture supernatants but, upon exposure to hypoxia, in vitro culture supernatants from shake flask cultures contained significant amounts of ethanol. These data suggest that in response to hypoxia A. fumigatus can ferment glucose or other fermentable carbon sources into ethanol. In addition to in vivo ethanol detection, hypoxia was directly monitored in the lung in three immunologically distinct murine models of IPA using the hypoxia detection agent pimonidazole hydrochloride (Hypoxyprobe-1). The results of this study suggest that the influx and activity of host immune cells are strong contributors to the development of hypoxia during an invasive pulmonary fungal infection, as more-extensive hypoxia was detected in murine models characterized by strong inflammatory responses than those characterized by fungal proliferation and tissue invasion.
In summary, these observations suggest that human fungal pathogens are faced with rapidly changing oxygen availability during fungal pathogenesis, which may suggest that the ability to adapt to low-oxygen environments is critical for fungal virulence.
_
Fungal adaptation to hypoxia:
As the majority of human fungal pathogens do not normally inhabit the human body, at least as far as we currently understand, and are often associated with infection only in immunocompromised patients, a key question is how these fungi evolved and maintained their ability to adapt to hypoxia. The mold A. fumigatus is typically found in soil and decaying organic material such as compost heaps. These environments are relatively oxygen poor, as oxygen concentrations in compost piles rapidly change with the metabolic activity of the microflora and range from atmospheric (21%) to hypoxic (1.5% and lower). This indicates that organisms that thrive in such environments have evolved hypoxia adaptation mechanisms. Moreover, the soil itself can become hypoxic after heavy rains or due to increased CO2 levels, and thus soilborne organisms have evolved mechanisms to tolerate low and rapidly changing oxygen levels. Although most molds are traditionally considered obligate aerobes, A. fumigatus has been observed to tolerate oxygen levels as low as 0.1%, and several older studies even suggest that, under the right nutrient conditions, A. fumigatus can survive and grow anaerobically. In addition, Fusarium species seem particularly adept at tolerating hypoxic and even anoxic conditions, which is consistent with their resident ecological niche, soil. Thus, these studies strongly suggest that molds like A. fumigatus and F. oxysporum, which cause human disease, may not be typical obligate aerobes but rather behave like facultative anaerobes. In my view, they are aerobes but they adapt to hypoxia. They are obligate aerobic organisms and generally do not grow under anaerobic conditions, although enhanced germination of conidia may occur at lower O2 levels. Aspergillus grows well in aerobic culture media at 30–35°C. No growth at oxygen concentrations of 0 or 0.025% but growth occurs at 0.5 and 2.5%. So pathogenic Aspergillus spp. are capable of growth at low oxygen tensions but it is adaptation.
With regard to human-pathogenic yeasts, C. albicans is capable of low levels of growth under anaerobic conditions and can also ferment glucose to ethanol predominantly under low-oxygen conditions, suggesting a Pasteur effect typically associated with other facultative fermentative yeasts. In contrast, C. neoformans seems to be a true obligate aerobe, though the precise effects of low-oxygen conditions on C. neoformans or Cryptococcus gattii growth and metabolism have not been elucidated to the degree that they have in C. albicans. In RPMI 1640, a medium commonly used for Clinical and Laboratory Standards Institute (CLSI) antifungal drug susceptibility screening, oxygen is a limiting factor for C. neoformans growth and cell cycle progression also seems dependent on oxygen availability. Taken together, these data suggest that human-pathogenic fungi have evolved mechanisms to adapt to low-oxygen environments that occur in their natural environments as well as during fungal pathogenesis. It is unclear whether hypoxia adaptation mechanisms directly contribute to the distinction between pathogenic and nonpathogenic fungi, but data suggest that responses are similar in nonpathogens such as Schizosaccharomyces pombe.
_
Mucor irregularis:
Mucor irregularis, a causal agent of cutaneous mucormycosis, thrives in a skin where the oxygen pressure is only 41 mmHg. (~6% oxygen concentration). Therefore, M. irregularis must overcome hypoxia in the human body to survive. M. irregularis showed growth retardation in hypoxia compared to normoxia, suggesting that M. irregularis may slowly grow during the invasion of human skin to adapt to the hypoxic skin microenvironment. According to the transcriptome data, M. irregularis may use the intralipid pool and the extracellular lipid absorbed through endocytosis instead of the carbohydrates as energy source during infection. RNA-seq under 6% O2 concentration revealed that genes involved in carbohydrate metabolism such as glycolysis, pentose phosphate pathway, oxidative phosphorylation, and GABA shunt were downregulated, while genes involved in gluconeogenesis, lipid/fatty acid metabolism, beta-oxidation, and endocytosis were upregulated. The ergosterol biosynthesis-related genes were not significantly affected by hypoxia, except for genes encoding sterol reductase and a cytochrome P450.
_
My view:
Human fungal pathogens including Morales are generally considered obligate aerobes and therefore hypoxia cannot be conducive environment for the fungus to grow. When an invading microbe interacts with host cells, tissue damage due to inflammation, thrombosis, and necrosis is thought to decrease available oxygen concentrations due to decreased tissue perfusion at the site of infection. Moreover, it is likely that oxygen-dependent metabolism of both pathogen and host cells also contributes to the rapid utilization of available oxygen. Human fungal pathogens are faced with rapidly changing oxygen availability during fungal pathogenesis, therefore their ability to adapt to low-oxygen environments is critical for fungal virulence. The mold Mucorales is typically found in soil and decaying organic material. These environments are relatively oxygen poor and organisms that thrive in such environments have evolved hypoxia adaptation mechanisms. These fungi reprogram transcriptional and translational metabolisms to adapt to altered oxygen availability under varying oxygen concentrations. Experiments with M. irregularis the causative agent of cutaneous mucormycosis revealed genes involved in lipid metabolism and endocytosis activation were upregulated in response to hypoxic conditions. This result was of significant interest since M. irregularis infections are often found in facial skin lesions where sebaceous glands are abundant (Sebum of sebaceous glands have high fatty acid concentrations). Furthermore, genes involved in carbon metabolism were downregulated, leading the authors to hypothesize that M. irregularis uses intracellular lipid pools rather than carbohydrates as an energy source (Xu W. et al., 2018). In other words, hypoxia is not an ideal environment to nurture fungi growth but fungi adapt to it for survival. So hypoxemia in covid-19 did not encourage mucormycosis but treatment with steroids did encourage mucormycosis. We have to treat hypoxaemia to save human life no matter the cause of it but hypoxaemia certainly does not encourage fungi to grow in humans.
_______
_______
Section-9
Covid-19 Associated Mucormycosis (CAM):
COVID-19 has already claimed more than 4 million lives worldwide. In the absence of an effective vaccine or antiviral therapy, supportive care plays a vital role in the management of COVID-19. Glucocorticoids, monoclonal antibodies, anticoagulants and probably remdesivir are the only drugs proven to be beneficial in COVID-19. Glucocorticoids are inexpensive, widely available, and have been shown to reduce mortality in hypoxemic patients with COVID-19. Nevertheless, glucocorticoids can increase the risk of secondary infections. Moreover, the immune dysregulation caused by the virus and the use of concurrent immunomodulatory drugs such as tocilizumab could further increase the risk of infections in COVID-19 patients.
A complex interplay of factors, including pre-existing diseases, such as diabetes mellitus, previous respiratory pathology, use of immunosuppressive therapy, the risk of hospital-acquired infections, and systemic immune alterations of COVID-19 infection itself may lead to secondary infections, which are increasingly being recognized in view of their impact on morbidity and mortality. Secondary infections are known to complicate the clinical course of coronavirus disease (COVID-19). Bacterial infections are the most common secondary infections, but increasing reports of systemic fungal infections are causing concern. In the early part of the COVID-19 pandemic, <1% of secondary infections reported in COVID-19 patients were fungal. Pre-existing conditions, indiscriminate use of antimicrobial and glucocorticoid drugs, and lapses in infection control practices are putative factors contributing to the emergence of systemic fungal infections in severe COVID-19 cases. In a recent review, 62/806 (8%) patients had secondary bacterial or fungal infections during hospital admission. White et al. screened 135 adults with COVID-19 infection and reported an incidence of invasive fungal infections of 26.7% (commonly aspergillosis (14.1%), or yeast, usually candida (12·6%)), nonetheless, no case of mucormycosis in their subjects was detected. Patients with invasive fungal diseases had higher mortality (53% with vs 31% without), which was significantly reduced by appropriate therapy. Corticosteroid therapy and a past history of chronic pulmonary disease were associated with a higher risk of invasive fungal disease. Similarly, high incidences have been seen in Pakistan (23/147, 15.6%) and Italy (30/108, 27.7%), with the authors suggesting that the development of invasive fungal infections alters the natural history of the disease. Song et al. have suggested an algorithm for the early diagnosis and management of common invasive fungal infections (aspergillus, candidiasis, cryptococcosis, and mucormycosis).
There have been case reports of mucormycosis in patients diagnosed with coronavirus disease 2019 (COVID-19), but the relationship of these two infections is unclear. Some of the infections of mucormycosis were diagnosed several days to a couple weeks after being admitted for COVID-19, and it seems reasonable to assume that the mucormycosis (rhinocerebral and pulmonary in these cases) was a secondary infection arising in a critically-ill patient on steroids. The other case reports describe patients who were diagnosed with rhinocerebral mucormycosis and COVID-19 simultaneously.
_
Viral pneumonia increases patients’ susceptibility to bacterial and fungal superinfections, including invasive pulmonary aspergillosis (IPA). Influenza-associated pulmonary aspergillosis (IAPA) has complicated the clinical course of many critically ill patients with acute respiratory distress syndrome (ARDS). In December, 2019, COVID-19 emerged from Wuhan, China, and has become pandemic. There have been several reports of COVID-19-associated pulmonary aspergillosis (CAPA), raising concerns about this superinfection as an additional contributing factor to mortality. Indeed, in a prospective cohort of 108 critically ill patients with ARDS, a higher 30-day mortality was observed in patients with CAPA than in patients without aspergillosis (44% vs 19%), and the association of COVID-19-associated fungal disease with mortality was also supported by other studies. The population of patients with CAPA harbours many baseline prognostic factors with negative effects on survival, which might be further compromised by azole-resistant CAPA, with an increasing number of patients reported in the literature.
_
Respiratory viruses cause direct damage to the airway epithelium, enabling aspergillus to invade tissue. Furthermore, viral infection hampers ciliary clearance and leads to immune dysfunction or dysregulation, or both, locally or systemically. The extent of dysregulation that is associated with ARDS is not yet fully understood, however, some patients develop pronounced immunosuppression, facilitating bacterial and fungal superinfection. Moreover, a distinctive immune-cell event that is observed in patients with COVID-19 is the decrease of T-cell populations, especially in patients with severe disease. Decline of lymphocyte counts can be accompanied by defective function. Severe lymphopenia has been established as a factor predicting the risk of invasive mould disease in patients with haematological malignancies although mucormycosis in patients who are HIV positive (having T-cell dysfunction) is extremely rare.
_
While COVID-19 associated pulmonary aspergillosis (CAPA) has received much attention, mucormycosis, another devastating disease, remains unrecognized. The diagnosis of CAPA relies on the presence of risk factors, consistent radiology, and demonstration of Aspergillus in tissue culture or microscopy. While galactomannan in bronchoalveolar lavage is a useful marker of invasive pulmonary aspergillosis, its role in CAPA has not been confirmed. Invasive mold infections (invasive pulmonary aspergillosis and pulmonary mucormycosis) share similar risk factors, clinical presentation, and radiology. The diagnosis of CAM is thus even more challenging. A lack of clinical suspicion and difficulty isolating the causative fungi might contribute to the underdiagnosis of mucormycosis. In recent months, an increase in reports of cases of COVID-19 associated Mucormycosis (CAM) has been observed mainly in people with underlying diseases, such as diabetes mellitus (DM), diabetic ketoacidosis, or on steroids. In these patients, the most frequent clinical manifestation is rhino-orbital mucormycosis, followed by rhino-orbital-cerebral mucormycosis, which present as secondary infections and occur after SARS CoV-2 infection. Globally, the highest number of cases has been reported in India, where it is estimated that there are more than 40,000 people with CAM.
_____
Pathogenesis of CAM:
As the novel coronavirus disease (COVID-19) continues to rampage, an abrupt increase in the number of opportunistic fungal infections has been observed. Globally, several cases of mucormycosis have been described in patients with COVID-19, an entity being described as COVID-19-associated mucormycosis (CAM). Although a causal link between COVID-19 and mucormycosis remains unearthed, multiple factors including glucocorticoids and worsening of blood glucose have been implicated in the development of mucormycosis in patients with COVID-19.
Figure above shows association between the novel coronavirus disease (COVID-19) and mucormycosis.
_
The sporangiospores of Mucorales vary in size depending on species (range 3-11 µm). These spores are released from the sporangium and dispersed into the air, where a vulnerable host population may acquire an infection. Inhalation is the major route of acquiring mucormycosis. The relatively larger spores of R. arrhizus get trapped in the nasal epithelium and sinuses and thus may lead to ROCM, whereas the relatively smaller spores of Cunninghamella spp. can reach the lower respiratory tract leading to pulmonary mucormycosis. However, available evidence suggests that any pathogenic Mucorales species can produce any type of clinical presentation.
_
Post COVID-19 sepsis is what occurs after SARS- CoV-2 has had a rampage in the human body and we are literally left picking up the pieces. It leads to a dysregulated innate immune response, ciliary dysfunction, cytokine storm, thrombo-inflammation, microvascular coagulation and eventual immune exhaustion. This cascade of events facilitates secondary bacterial and fungal infections especially in critically ill patients subjected to emergency invasive procedures, mechanical ventilation, CRRT, ECMO, poor nursing ratios, prolonged hospital stays and breaches in asepsis. Further, the use of corticosteroid treatment and anti-IL-6-directed strategies in these highly susceptible hosts along with high fungal spore counts in the environment creates the perfect setting for mould infections.
_
Uncontrolled diabetes in CAM:
The major underlying disease noted in mucormycosis cases in LMICs such as India, Iran or Mexico is uncontrolled diabetes with or without ketoacidosis. Among COVID-19 patients, uncontrolled diabetes had been reported at 7-21%. However, the prevalence in India is above 30%, with 2% of patients developing diabetic ketoacidosis. Acute or stress-induced hyperglycaemia has been noted in 50% of hospitalised COVID-19 patients. SARS-CoV-2 itself can induce acute diabetes and diabetic ketoacidosis by damaging pancreatic islets cells, which have a high expression of angiotensin converting enzyme-2 receptors, as has been noted with SARS-CoV-1, and indirectly by damaging small blood vessels supplying pancreatic beta cells. Increased resistance to insulin due to the profound inflammatory reaction, may also play some role in the induction of hyperglycaemia. In addition, a few cases of euglycemic diabetic ketoacidosis have been reported in COVID-19 cases, especially those receiving sodium-glucose co-transporter-2 inhibitors (SGLT2).
Type 2 diabetes mellitus itself is an immunocompromised state which leads to dysregulated, dysfunctional innate and adaptive immune cells making the host susceptible to infections by Mucorales. Due to increased glycosylation, IL-10 production by lymphocytes and macrophages is significantly reduced. Diabetes mellitus also reduces polymorphonuclear leukocyte mobilization and chemotaxis. Hyperglycaemia and acidosis can induce phagocytic cell dysfunction leading to increased risk of Mucorales infections.
_
Role of corticosteroids in CAM:
In a multicentre study from India, inappropriate corticosteroid use was noted in 63.3% patients. The situation was aggravated further during the second wave of COVID-19, when steroids were used indiscriminately and inappropriately to overcome the challenge of the oxygen acquisition crisis and resulting oxygen desaturation in patients. The over-the-counter availability and practice of self-medication with corticosteroids complicates the situation even further. The use of corticosteroids in the treatment of COVID-19 may act as a double-edged sword. Firstly, corticosteroids can increase blood glucose levels by acting as a substrate for oxidative stress metabolism with lipolysis, proteolysis, and hepatic glucose production. They also increase insulin resistance in up to 60%-80% of patients depending on the dose and type used. Secondly, they affect virtually all immune cells. Some major immune suppressive actions of corticosteroids are: i) antagonism of macrophage maturation and differentiation, ii) decrease of interleukin-1, interleukin-6, tumour necrosis factor, proinflammatory prostaglandins and leukotrienes production by macrophages, iii) suppression of microbicidal activity of activated macrophages. They also suppress neutrophil adhesion to endothelial cells and impair lysosomal enzyme release, respiratory burst, and chemotaxis to the site of infection. Increased risk of infection following glucocorticoid therapy is well established, but the dose and the duration of steroid therapy required to increase the risk of different infections is not as well defined. One Indian study reveals that steroids were not used in the treatment of only 14 per cent of covid-19 patients.
Thousands of Covid-19 patients in India took high doses of steroids for an extended period of time against medical guidelines. Steroids are prescribed to help ward off the “cytokine storm” — an excessive inflammatory response that hurts the body without stopping the infection — caused by coronavirus. But they also reduce immunity and raise sugar levels, creating fertile ground for the fungus to grow. India, home to the largest number of diabetics in the world after China, has thousands of patients at risk. The World Health Organization advises coronavirus patients receive a daily dose of 6mg of dexamethasone, a corticosteroid, or its equivalent for seven to 10 days to reduce mortality in patients that are critically or severely ill. But Indian patients have taken up to 500mg a day and often for as long as a month. Taking steroids too early in the course of a Covid-19 infection can also affect the immune system and is suspected of leading to unnecessary hospitalisations of young adults who could otherwise fight off the disease. Under the intense pressure of the second wave, doctors have prescribed a laundry list of medications including steroids and antibiotics for people with even mild symptoms. The indiscriminate use of steroids has led to mucormycosis epidemic in India.
Instructions for steroid use in mild COVID cases:
Apart from frequently monitoring the oxygen levels and blood sugar levels, inhaled steroids such as budesonide can be given to mild COVID cases which have also have high risk factors such as being above 65 years of age, having comorbidities and manifest an oxygen level above 92 per cent.
Consider inhaled budesonide two puffs, twice daily (1600 mcg/day), until symptoms improve. After using the inhaler, instruct the patient to rinse the mouth with water and spit out safely.
Instructions for steroid use in moderate/severe COVID cases:
The instructions issued by the coalition of experts identified moderate to severe COVID19 patients as those that have oxygen levels below 92 per cent. For such patients, it stated that following oral steroids can be given: Dexamethasone 8mg (6mg salt), or Methylprednisolone 32 mg or Prednisone 40 mg.
These oral steroids are to be given once a day for a standard duration of five days and a maximum duration of 10 days.
The blood sugar levels of COVID19 patients have to be strictly monitored and should be maintained at below 180 mg/dL (milligram per decilitre). Also suggested is the use of insulin in ensuring the healthy sugar levels of the COVID19 patients on oral steroids.
_
Iron and hyperferritinaemia:
Iron overload and deferoxamine therapy are well known risk factors for mucormycosis. The free iron captured by siderophores of Rhizopus species helps in its growth. Severe COVID-19 is a hyperferritinaemic state due to hyperinflammation. In severe COVID-19 patients, ferritin level rises 1.5 to 5 times higher than in non-severe cases (average ferritin concentration of >800 μg/L). High IL-6 concentrations in COVID-19 patients have been correlated to disease severity. IL-6 directly stimulates ferritin production and increases the synthesis of hepcidin which in turn sequesters iron in enterocytes and macrophages thus preventing them to efflux from these cells leading to increased intracellular iron load. This excess intracellular iron generates reactive oxygen species (ROS) causing damage to the tissue and free iron is released in the circulation and available to Mucorales.
_
Endothelial damage:
Comparison of autopsy lung specimens obtained from expired patients due to COVID-19 and those with acute respiratory distress syndrome (ARDS) secondary to influenza A (H1N1) infection, has revealed severe vascular endothelial injury along with the presence of intracellular virus and disrupted cell membranes in COVID-19 deceased patients. Pulmonary vessels show widespread thrombosis along with significantly higher (9 times, P<0.001) alveolar capillary microthrombi in COVID-19 patients compared to patients with influenza.
Thrombosis can also occur in small veins supplying the pancreas, thereby damaging it and causing insulin deficiency leading to diabetes mellitus. As endothelial adhesion and penetration is an early step in establishing mucormycosis, endothelial damage observed in severe COVID-19 disease may play an important pathogenic role.
_
Overexpression of GRP78:
Hyperglycaemia is a stress condition that induces the overexpression of the glucose regulated protein (GRP78) which is present in the lumen of the endoplasmic reticulum and expressed in mammalian cells. The CotH protein kinase belonging to the spore coating protein family in Rhizopus acts as the ligand for GRP78, which helps the fungus to adhere and invade endothelial and nasal epithelial cells. Sabrili et al. demonstrated significantly higher serum GRP78 levels in COVID 19 patients compared to a COVID 19 negative control group. These findings suggest amplified pathogenetic role of GRP78 in CAM.
_
Other factors in covid-19 associated mucormycosis:
-1. Zinc supplements:
To enhance immunity, people have been consuming multivitamins and supplements since the outbreak of the coronavirus infection. The sale of these has increased drastically since the beginning of the Covid-19 pandemic. These supplements are over-the-counter products or non-prescription medicine. They fall under the food supplements category and not drugs, so purchasing these tablets are easy. The increase in the sale of such supplements has been seen as panic buying. Most of the time when they are not prescribed, it is driven by unproven social media messages saying that the supplements boost immunity and helps in the fight against Covid-19.
Inadvertent pumping of too much of zinc could also be contributing to this epidemic of mucormycosis along with other risk factors. Fungi thrive in zinc-rich environment, and mammalian cells try to keep zinc away from fungus to avoid infection. Fungi feed on zinc and mammalian cells try to escape fungal invasion by ‘starving’ the fungus by hiding zinc. These self-defence processes against fungi are known as nutritional immunity. People have taken zinc supplements indiscriminately & this has allowed fungus to grow as zinc is prerequisite for its growth. The US government agency National Institute of Health (NIH) in its Covid-19 treatment guidelines advised against zinc supplementation above the usual dietary allowance, except in a clinical trial. It said that there are insufficient data for the COVID-19 Treatment Guidelines Panel to recommend either for or against the use of zinc for the treatment of Covid-19. In addition, a study published in JAMA Network says that treatment with zinc, ascorbic acid, or both does not affect SARS-CoV-2 symptoms.
-2. Oxygen delivery:
Some experts claimed unhygienic delivery of oxygen to patients was the root cause. They said medical oxygen as opposed to industrial oxygen was highly purified. It undergoes a range of processes such as compression, filtration and purification. Even its cylinders undergo disinfection and cleanliness processes. They point out possible role of unclean delivery of industrial oxygen for Covid treatment as a probable reason for the mucormycosis epidemic. Also, the water used in humidifier bottles through which oxygen is given needs to be sterile and clean. If not, the risk of contamination increases by breathing in fungal spores.
Other experts said that there was no direct link between industrial oxygen and sanitation of cylinders to the sudden surge in mucormycosis cases. When it comes to oxygen — whether industrial or not — it passes through water (humidifier) and mycologists have pointed out that fungi cannot produce spores in water. Also, use of industrial oxygen per se cannot be a reason because that is almost 100 per cent pure oxygen and would kill off all microorganisms — it could only be more sterilising.
-3. Antibiotics misuse:
A study on black fungus involving 210 patients found that antibiotics were used to treat 100 per cent of these Covid-19 patients, who were later diagnosed with mucormycosis. Antibiotics – Azithromycin, Doxycycline and Carbapenems – being prescribed for Covid patients in India, are known to increase the risk of fungal infections. Misuse of antibiotics could wipe out commensal bacteria lining nasal mucosa and leads to fungal invasion.
-4. Excessive steam inhalation:
Excessive steam inhalation is widespread practice in India during covid-19 pandemic. Steam in excess can damage the delicate mucus layer and even cause burns along the mucosa, making it easy for fungus to breach our natural defence. Excessive use of steam inhalation has led to scalding of the nasal passages in patients, which on exposure to fungal spores can serve as a site for infection. The normal flora has also been destroyed by the indiscriminate steaming which patients & people have done, thinking that will kill the virus.
_
The pathogenesis of CAM is a complex issue, in which environment, vulnerable patients, corticosteroid use, and COVID-19 play synergistic roles in the disease pathogenesis.
Figure below shows schematic representation of pathogenesis of COVID-19 associated mucormycosis.
_____
_____
Studies on CAM:
-1. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India, 2021study:
Overall, 101 cases of mucormycosis in people with COVID-19 have been included in the study, of which 82 cases were from India and 19 from the rest of the world. Mucormycosis was predominantly seen in males (78.9%), both in people who were active (59.4%) or recovered (40.6%) from COVID-19. Pre-existing diabetes mellitus (DM) was present in 80% of cases, while concomitant diabetic ketoacidosis (DKA) was present in 14.9%. Corticosteroid intake for the treatment of COVID-19 was recorded in 76.3% of cases. Mucormycosis involving nose and sinuses (88.9%) was most common followed by rhino-orbital (56.7%). Mortality was noted in 30.7% of the cases. An unholy trinity of diabetes, rampant use of corticosteroid in a background of COVID-19 appears to increase mucormycosis. All efforts should be made to maintain optimal glucose and only judicious use of corticosteroids in patients with COVID-19.
_
-2. COVID-19-associated mucormycosis: An updated systematic review of literature, 2021 study:
In its wake, the COVID-19 pandemic has ushered in a surge in the number of cases of mucormycosis. Most cases are temporally linked to COVID-19; hence, the entity is described as COVID-19-associated mucormycosis (CAM). The present systematic review was undertaken to provide an up-to-date summary of the hitherto available literature on CAM. PubMed, Scopus, and Google Scholar databases were systematically searched using appropriate keywords till May 14, 2021, to identify case reports/case series pertaining to mucormycosis in patients with COVID-19. Relevant data extracted included demographic characteristics, comorbidity profile, clinical category of mucormycosis, glucocorticoid use, treatment offered and patient outcome. Authors identified 30 case reports/case series, pooling data retrieved from 99 patients with CAM. Most cases were reported from India (72%). The majority of the patients was male (78%) and had diabetes mellitus (85%). A prior history of COVID-19 was present in 37% patients with mucormycosis developing after an initial recovery. The median time interval between COVID-19 diagnosis and the first evidence of mucormycosis infection or CAM diagnosis was 15 days. Glucocorticoid use was reported in 85% of cases. Rhino-orbital mucormycosis was most common (42%), followed by rhino-orbito-cerebral mucormycosis (24%). Pulmonary mucormycosis was observed in 10 patients (10%). The mortality rate was 34%; the use of adjunct surgery, which was undertaken in 81% of patients, was associated with better clinical outcomes (p<0.001). In conclusion, CAM is an emerging problem necessitating increased vigilance in COVID-19 patients, even those who have recovered. CAM portends a poor prognosis and warrants early diagnosis and treatment.
_
-3. ECMM/ISHAM recommendations for clinical management of COVID -19 associated mucormycosis in low- and middle-income countries, 2021 study:
Reports are increasing on the emergence of COVID-19 associated mucormycosis (CAM) globally, driven particularly by low- and middle-income countries. The recent unprecedented surge of CAM in India has drawn worldwide attention. More than 28,252 mucormycosis cases are counted and India is the first country where mucormycosis has been declared a notifiable disease. However, misconception of management, diagnosing and treating this infection continue to occur. Thus, European Confederation of Medical Mycology (ECMM) and the International Society for Human and Animal Mycology (ISHAM) felt the need to address clinical management of CAM in low- and middle-income countries. This article provides a comprehensive document to help clinicians in managing this infection. Uncontrolled diabetes mellitus and inappropriate (high dose or not indicated) cortico-steroid use are the major predisposing factors for this surge. High counts of Mucorales spores in both the indoor and outdoor environments, and the immunosuppressive impact of COVID-19 patients as well as immunotherapy are possible additional factors. Furthermore, a hyperglycaemic state leads to hyper-ferritinaemia and increased expression of glucose regulated protein (GRP- 78) in endothelial cells that may help the entry of Mucorales into tissues. Rhino-orbito-cerebral (ROC) mucormycosis is the most common presentation followed by pulmonary mucormycosis. Recommendations are focused on the early suspicion of the disease and confirmation of diagnosis. Regarding management, glycaemic control, elimination of corticosteroid therapy, extensive surgical debridement and antifungal therapy are the standards for proper care.
My view:
Hyperglycemia itself cannot lead to hyper-ferritinaemia as described above, but elevated glucose and iron levels upregulate GRP78 expression and promote endothelial cell invasion and damage by R. oryzae in a receptor-dependent manner.
_
-4. Multicenter Epidemiologic Study of Coronavirus Disease–Associated Mucormycosis, India, 2021 study:
During September–December 2020, authors conducted a multicenter retrospective study across India to compare epidemiology and outcomes among cases of coronavirus disease (COVID-19)–associated mucormycosis (CAM). Among 287 mucormycosis patients, 187 (65.2%) had CAM; CAM prevalence was 0.27% among hospitalized COVID-19 patients. Authors noted a 2.1-fold rise in mucormycosis during the study period compared with September–December 2019. Uncontrolled diabetes mellitus was the most common underlying disease among CAM and non-CAM patients. COVID-19 was the only underlying disease in 32.6% of CAM patients. COVID-19–related hypoxemia and improper glucocorticoid use independently were associated with CAM. The mucormycosis case-fatality rate at 12 weeks was 45.7% but was similar for CAM and non-CAM patients. Age, rhino-orbital-cerebral involvement, and intensive care unit admission were associated with increased mortality rates; sequential antifungal drug treatment improved mucormycosis survival. The COVID-19 pandemic has led to increases in mucormycosis in India, partly from inappropriate glucocorticoid use.
_
-5. Mucormycosis and COVID-19: An Epidemic within a Pandemic in India, 2021 study:
This prospective, observational, multi-centre study included 47 consecutive patients with mucormycosis, diagnosed during their course of Covid-19 illness, between January 3 to March 27, 2021. Of the 2567 COVID-19 patients admitted to 3 tertiary centers, 47 (1.8%) were diagnosed with mucormycosis. Mean age was 55±12.8years and majority suffered from diabetes mellitus (n=36, 76.6%). Most were not COVID-19 vaccinated (n=31, 66.0%) and majority (n=43, 91.5%) had developed moderate-to-severe pneumonia, while 20 (42.6%) required invasive ventilation. All patients had received corticosteroids and broad-spectrum antibiotics while most (n=37, 78.7%) received at least one anti-viral medication. Mean time elapsed from COVID-19 diagnosis to mucormycosis was 12.1±4.6days. Eleven (23.4%) subjects succumbed to their disease, mostly (n=8, 72.7%) within 7-days of diagnosis. Among the patients who died, 10 (90.9%) had pre-existing diabetes mellitus, only 2 (18.2%) had received just one vaccine dose and all developed moderate-to-severe pneumonia, requiring oxygen supplementation and mechanical ventilation.
Mucormycosis can occur among COVID-19 patients, especially with poor glycemic control, widespread and injudicious use of corticosteroids and broad-spectrum antibiotics, and invasive ventilation. Owing to the high mortality, high index of suspicion is required to ensure timely diagnosis and appropriate treatment in high-risk populations.
_
-6. The Emergence of COVID-19 Associated Mucormycosis: Analysis of Cases From 18 Countries, 2021 study:
Coronavirus disease 2019 (COVID-19)–associated mucormycosis (CAM) has recently been increasingly reported, particularly among patients with uncontrolled diabetes. Patients with diabetes and hyperglycemia often display an inflammatory state that may be potentiated by the activation of antiviral immunity to SARS-CoV-2, and thus may favor secondary infections. Authors analyze 80 published and unpublished cases of CAM, with a predominance (42/80) of cases from India. Uncontrolled diabetes mellitus as well as systemic corticosteroid treatment represented major comorbid predisposing factors and rhino-orbital cerebral mucormycosis was the most frequent presentation of disease. Mortality was high at 49%, driven particularly by those with pulmonary or disseminated mucormycosis and those with cerebral involvement. Furthermore, a significant proportion of surviving patients suffered life-changing morbidities (loss of vision in 46% of survivors). This review indicates that CAM may be a relevant complication of severe COVID-19, particularly in those with uncontrolled diabetes.
_______
_______
Characteristics of the CAM cases.
|
Author |
Singh et al. |
John el al. |
Ravani et al. |
Sharma et al. |
Pakdel et al. |
|
Type of study |
Systematic review |
Systematic review |
Retrospective institutional cohort |
Prospective observational study |
Prospective descriptive study |
|
Number of cases |
101 cases |
41 cases |
31 cases |
23 cases |
15 cases |
|
Country |
82 cases – India |
USA |
India |
India |
Iran |
|
COVID positivity |
Active – 59.5% |
Mild – 2.4% |
19 cases – 61.2% |
Active – 17.4% |
Mild – 13.3% |
|
Age/Age range |
27–78 years |
46–61 years |
20–80 years |
Not available |
14–71 years |
|
Gender |
78.9% – Male |
82.9% – Male |
64.5% – Male |
65.2% – Male |
66% – Male |
|
Comorbidity |
83.3% – DM |
80.4% – DM |
96.7% – DM |
52.17% – DM (Uncontrolled) |
86% – DM |
|
Corticosteroids |
76.3% – Yes |
87.8% – Yes |
61.2% – Yes |
100% – Yes |
46.6% – Yes |
|
Location |
88.9% – Nasal/Sinus |
7.3% – Sinusitis alone |
77.41% – Pansinusitis |
100% – Sinuses |
47% – Rhino Orbital |
|
Mortality |
30.7% |
48.7% |
9.78% |
Not available |
46.6% |
CAM: COVID-19 associated mucormycosis, NR: Not reported, DM: Diabetes mellitus, DKA: Diabetes ketoacidosis
_______
_______
Section-10
Clinical manifestations of mucormycosis:
Based on anatomic localization, mucormycosis can be classified in various forms: (1) rhinocerebral, (2) pulmonary, (3) cutaneous, (4) gastrointestinal, (5) disseminated, and (6) uncommon presentations.
Figure below shows cutaneous and rhino-orbito-cerebral mucormycosis:
(A) Extensive primary cutaneous mucormycosis of the left leg due to Apophysomyces variabilis, after a car accident. (B) Erythematous skin, ptosis, palpebral oedema, limited ocular motility, and right maxillary pain, 6 days after symptom onset in uncontrolled diabetes. (C) Proptosis, palpebral erythema, and cavernous sinus syndrome, 7 days after symptom onset in uncontrolled diabetes. (D) Necrotic, purulent palatal ulcer and cavernous sinus syndrome, 8 days after symptom onset in uncontrolled diabetes. (E) Rhinocerebral mucormycosis in a female child, 2 years old with acute lymphoblastic leukaemia and lethal outcome. (F) 52-year-old man with persistent neutropenia post chemotherapy, sinusitis, and skin necrosis. (G) Black eschar as typical skin lesion in mucormycosis; one of several lesions on the right forehead, ear and cheek in a non-diabetic, haematopoietic stem cell transplant recipient with pansinusitis due to Lichtheimia corymbifera.
_
Manifestations of mucormycosis depend on the location of involvement, rhinocerebral and pulmonary infections are the most common. A classic clinical sign of mucormycosis is the rapid onset of tissue necrosis with or without fever. Necrosis is the result of invasion of blood vessels and subsequent thrombosis.
- Rhinocerebral mucormycosis is the most common form in patients with diabetes and with renal transplants. It also occurs in neutropenic cancer patients and hematopoietic stem cell transplant or solid organ transplant recipients. Symptoms may include unilateral facial swelling, headaches, nasal or sinus congestion or pain, serosanguinous nasal discharge, and fever. As the infection spreads, ptosis, proptosis, loss of extraocular muscle function, and vision disturbance may occur. Necrotic black lesions on the hard palate or nasal turbinate and drainage of black pus from eyes are useful diagnostic signs.
- Pulmonary mucormycosis generally occurs in patients with hematologic malignancy or profound neutropenia. The symptoms are non-specific and include fever, cough, chest pain, and dyspnea. Angioinvasion results in tissue necrosis, which may ultimately lead to cavitation and/or hemoptysis.
- Cutaneous mucormycosis may be primary or secondary. Primary infection is usually caused by direct inoculation of the fungus into disrupted skin and is most often seen in patients with burns or other forms of local skin trauma, and can occur in patients who are not immunosuppressed. Primary infection produces an acute inflammatory response with pus, abscess formation, tissue swelling, and necrosis. The lesions may appear red and indurated and often progress to black eschars. Secondary cutaneous infection is generally seen when the pathogen spreads hematogenously; lesions typically begin as an erythematous, indurated, and painful cellulitis and then progress to an ulcer covered with a black eschar.
- Gastrointestinal mucormycosis is less common than the other clinical forms and is believed to result from ingestion of the organism. It typically occurs in malnourished patients or premature infants. The stomach, colon, and ileum are most commonly affected. Non-specific abdominal pain and distension, nausea, and vomiting are the most common symptoms, and gastrointestinal bleeding can occur. It is the most common form of mucormycosis among neonates and is challenging to diagnose partly because of its clinical resemblance to necrotizing enterocolitis, a far more common disease.
- Disseminated mucormycosis may follow any of the forms of mucormycosis described above but is usually seen in neutropenic patients with a pulmonary infection. The most common site of spread is the brain, but the spleen, heart, skin, and other organs can also be affected.
_____
_____
Sinus Mucormycosis:
The clinical manifestations of mucormycosis may be classified as sinus (localized or extended to the orbit and/or brain), pulmonary, cutaneous, gastrointestinal, miscellaneous, and disseminated infection. Sinus infection is the most common presentation. Patients with diabetes mellitus most commonly present with sinus disease but seldom with pulmonary infection; whereas, neutropenic patients frequently develop pulmonary infection, as well as sinus disease. As the clinical manifestations of invasive mucormycosis may be nonspecific, an otherwise unexplained infection in the appropriate setting should alert physicians to the possibility of this infection.
_
Mucormycosis frequently presents as invasive sinusitis. Approximately two-thirds of cases occur in diabetics, often with ketoacidosis; however, sinus infection may also occur in association with other forms of immunosuppression, including neutropenia, HSCT, and solid organ transplantation. The process can remain within the paranasal sinuses or progress to the orbit (sino-orbital) and/or brain parenchyma (rhinocerebral). Progression of infection with extension to neighboring tissues from the sinuses may be rapid and constitutes a medical and surgical emergency in compromised hosts. The ethmoid sinus is a critical site from which infection may extend through the lamina papyracea into the orbit, extraocular muscles, eye, and optic nerve. The brain may be seeded by invasion of the ethmoidal and orbital veins, which drain into the cavernous sinuses.
_
The initial clinical features of sinus mucormycosis include nasal congestion, dark blood-tinged rhinorrhea or epistaxis, sinus tenderness, retro-orbital headache, fever, and malaise. More advanced sinus infection may present as facial or periorbital swelling and numbness, blurred vision, lacrimation, chemosis, diplopia, proptosis, and loss of vision in the affected eye. A painful black necrotic ulceration may develop on the hard palate, indicating extension from the maxillary sinus into the oral cavity. Facial lesions and exophytic or necrotic lesions of the hard palate are often signs of rapidly progressing infection, as illustrated in figure below:
The absence of lesions does not rule out sinus mucormycosis, as necrotic or hard palate lesions may only be present in 50% of patients.
_
Patients with suspected mucormycosis should undergo nasal endoscopy and oral examination with biopsy from a suspicious area. Rhinoscopy or nasal endoscopy should be performed as soon as possible to look for areas of tissue ischemia or necrosis. Biopsies and/or surgical exploration of suspicious lesions should be performed to establish an early definitive diagnosis.
Endoscopic evaluation of the maxillary sinus and middle turbinate revealed necrotic blackened tissue as seen in the figure below:
Endoscopic image showing necrosis of the middle turbinate. The necrotic tissue of the middle turbinate was removed and sent to the pathology department for evaluation. Fluid was drained out of the maxillary sinus, and the necrotic tissues were debrided.
Nasal endoscopy may reveal necrotic ulcers along the nasal mucosa or turbinates. Infection can extend to adjacent bone and ultimately to the skull base. Progression to the central nervous system occurs via the optic nerve or from the ethmoid sinuses by way of the cavernous sinus. Abnormal mentation often signifies cerebral involvement. Vision loss, ophthalmoplegia, corneal anesthesia and facial anhidrosis may indicate cavernous sinus thrombosis, which may be further complicated by internal carotid artery thrombosis with contralateral hemiplegia.
Sinusitis with acute onset of blurred vision or diplopia in a diabetic or otherwise immunocompromised patient should prompt careful clinical and radiological evaluation for mucormycosis, as well as rapid therapeutic intervention. One should note that the clinical manifestations of cavernous sinus thrombosis may precede radiological findings in the central nervous system. Thus, medical interventions and surgical consultation should not be delayed when cavernous sinus thrombosis is clinically evident but not yet apparent in diagnostic imaging studies.
Computed tomography (CT) and magnetic resonance imaging (MRI) of the sinuses are superior to plain films for delineating the extent of infection and can guide surgical debridement. The principal radiographic findings of mucormycosis of the sinuses are opacification of the paranasal sinuses, fluid levels, bone destruction, and osteomyelitis.
_____
Rhinocerebral disease:
Rhinocerebral mucormycosis continues to be the most common form of the disease, accounting for between one-third and one-half of all cases of mucormycosis. About 70% of rhinocerebral cases (occasionally referred to as craniofacial) are found in diabetic patients in ketoacidosis. More rarely, rhinocerebral mucormycosis has also occurred in patients who received a solid organ transplant or those with prolonged neutropenia. Recently, rhinocerebral disease has been an increasing problem in patients undergoing hematopoietic stem cell transplantation. These cases have largely been associated with steroid use for graft-versus-host disease.
Rhinocerebral disease may manifest as unilateral, retro-orbital headache, facial pain, numbness, fever, hyposmia, and nasal congestion, which progresses to black discharge. Initially, mucormycosis may mimic bacterial sinusitis. Late symptoms that indicate invasion of the orbital nerves and vessels include diplopia and visual loss (see the following image). These late symptoms indicate a poor prognosis and are usually followed by a reduced level of consciousness. Most patients with rhinocerebral disease have diabetes (especially with ketoacidosis) or have malignancies with associated neutropenia and may be receiving broad-spectrum antibiotics.
Orbital swelling and facial cellulitis are progressive. Necrotic eschars with black purulent discharge can be noted in the nasal cavity, on the hard palate, or on the face. Although these lesions suggest mucormycosis, their absence does not exclude the possibility of this disease. Proptosis, ptosis, chemosis, and ophthalmoplegias indicate retro-orbital extension. Cranial nerves V and VII are the most commonly affected. Loss of vision can occur with retinal artery thrombosis. A reduced level of consciousness state denotes brain involvement.
The incubation period is measured in days. The clinical course can progress from normal to symptomatic in a week and from sinus opacification to uncal herniation and death in just a few days.
Cranial nerve findings represent extensive infection and signal a grave prognosis. Progressive vision loss and ultimately blindness may result either from involvement of the optic nerve or from arteriolar invasion resulting in infarction or from cavernous sinus thrombosis. Cranial nerves third and fifth may also be affected, resulting in ipsilateral loss of facial sensation and ptosis and pupillary dilation. Infection can also spread posteriorly from either the orbit or sinuses to the central nervous system. A bloody nasal discharge may be the first sign that infection has invaded through the terbinates and into the brain. When there is extensive central nervous system involvement, the angioinvasive nature of the fungus may result in cavernous sinus thrombosis and internal carotid artery encasement and thrombosis with extensive resulting cerebral infarctions. Occasionally cerebral vascular invasion may lead to hematogenous dissemination of the infection, with or without development of mycotic aneurysms.
_
A high index of suspicion is required to make the diagnosis of rhinocerebral mucormycosis, as evidenced by the fact that autopsy series have found up to half of cases are diagnosed post-mortem. Imaging techniques may be suggestive of mucormycosis but are rarely diagnostic. Indeed, the initial imaging study is frequently negative or has only subtle findings. The most common finding on computerized tomography (CT) scanning of the head or sinuses is subtle sinus mucosal thickening or thickening of the extraocular muscles. It is also common to detect no abnormalities in the bones of the sinuses despite clinical evidence of progressive disease. However, when present, the finding of bony erosion of the sinuses is strongly suggestive of the diagnosis in the appropriate clinical context (e.g., patient in diabetic ketoacidosis with proptosis). It should be emphasized that it is very uncommon to visualize an organized retroorbital mass.
Although evidence of infection of the soft tissues of the orbit may sometimes be seen by CT scan, magnetic resonance imaging is more sensitive. Still, as with CT scans, patients with early rhinocerebral mucormycosis may have a normal magnetic resonance imaging, and surgical exploration with biopsy of the areas of suspected infection should always be performed in high-risk patients. It is critically important to emphasize that if mucormycosis is suspected, initial empirical therapy with a polyene antifungal should begin while the diagnosis is being confirmed, rather than waiting while a protracted series of diagnostic tests are completed.
Given the limitations of imaging studies, diagnosing mucormycosis almost always requires histopathologic evidence of fungal invasion of the tissues. Culturing organisms from a potentially infected site is rarely sufficient to establish the diagnosis of mucormycosis because the causative agent is ubiquitous, may colonize normal persons, and is a relatively frequent laboratory contaminant. Additionally, the organism may be killed during tissue grinding, which is routinely used to process tissue specimens for culture. Thus, a sterile culture does not rule out the infection. Furthermore, waiting for the results of the fungal culture may delay the institution of appropriate therapy.
_
There are no reliable serologic, PCR-based, or skin tests for mucormycosis. Therefore, the diagnosis should be made by biopsy of infected tissues. The biopsy should demonstrate the characteristic wide, ribbon-like, aseptate hyphal elements that branch at right angles. The organisms are often surrounded by extensive necrotic debris. Other fungi, including Aspergillus, Fusarium, or Scedosporium spp, may look similar to the Mucorales on biopsy. However, these molds have septae, are usually thinner, and branch at acute angles. The genus and species of the infecting organism may be determined by culture of the infected tissue. However, the organism is rarely isolated from cultures of blood, cerebrospinal fluid, sputum, urine, feces or swabs of infected areas.
_______
Pulmonary Mucormycosis:
Lower respiratory tract infection develops most frequently in severely immunocompromised patients, particularly those with hematological malignancies and allogeneic HSCT. Key factors associated with development of pulmonary mucormycosis include prolonged and profound neutropenia, corticosteroid use, and GVHD. Pulmonary disease also develops in solid organ transplant recipients.
Pulmonary mucormycosis may appear radiologically as solitary lung nodules, segmental or lobar consolidation, halo signs, and cavitary or bronchopneumonic lesions. Persistent fevers and pulmonary infiltrates refractory to broad-spectrum antibacterial agents in a compromised patient should suggest fungal pneumonia. As the presentation of pulmonary mucormycosis is similar to that of invasive pulmonary aspergillosis, a diagnostic procedure such as bronchoalveolar lavage (BAL) is important.
The pulmonary lesions of mucormycosis typically are rapidly progressive in compromised hosts. Invasion of pulmonary vessels may lead to thrombosis and pulmonary infarcts. Infection may extend across tissue planes to involve the chest wall, pericardium, myocardium, superior vena cava, and diaphragm. Invasion of the great vessels may lead to aneurysm formation with fatal hemoptysis, as well as endobronchial disease with airway obstruction. High-resolution CT is superior to plain chest radiographs for early diagnosis and for delineating the extent of pulmonary and mediastinal involvement. The air crescent and halo signs, which are typically associated with invasive pulmonary aspergillosis, may also be observed in pulmonary mucormycosis. As lower respiratory tract infection may occur alone, concomitant with sinus disease, or as part of a disseminated process, radiological assessment of extrapulmonary sites should be considered in the initial evaluation.
There are some features in pulmonary mucormycosis that may be helpful in distinguishing this infection from pulmonary aspergillosis. The reversed halo sign, which was seen in approximately 4% of patients with pulmonary mycoses, is most frequently observed in patients with mucormycosis. A review of clinical features and CT findings observed that patients with cancer and pulmonary mucormycosis (n = 16) in comparison to contemporaneous patients with cancer and pulmonary aspergillosis (n = 29) have a higher frequency of concomitant sinusitis (odds ratio (OR), 25.7; 95% confidence interval (CI), 1.47–448.15; P = 0.026), history of voriconazole prophylaxis (OR, 7.76; 95% CI, 1.32–45.53; P = 0.023), presence of multiple (≥10) nodules (OR, 19.8; 95% CI, 1.94–202.29; P = .012) and pleural effusions (OR, 5.07; 95% CI, 1.06–24.23; P = 0.042).
Unfortunately, sputum culture is highly unreliable. In two case series, sputum and bronchiolar alveolar lavage cultures were negative in 18 of 19 cases of biopsy-proven pulmonary mucormycosis. Therefore, biopsy with histopathological assessment remains the best modality to diagnose pulmonary mucormycosis.
If pulmonary infection is not treated, hematogenous dissemination to the contralateral lung and other organs frequently occurs. Patients with untreated pulmonary mucormycosis usually die from disseminated disease before respiratory failure occurs. The notable exception is the rare patient with massive hemoptysis. The overall mortality of pulmonary mucormycosis is approximately 50 to 70% but is >95% if the pulmonary mucormycosis is part of a disseminated process.
Pulmonary infection may occur in conjunction with sinus infection.
_______
Cutaneous Mucormycosis:
Cutaneous mucormycosis may be confined to the skin and subcutaneous tissues, extend into adjacent soft tissue and even bony structures, or serve as a source for disseminated infection. Unlike cutaneous fusariosis, cutaneous mucormycosis seldom disseminates to the skin from a distant site with multiple lesions. Cutaneous mucormycosis most commonly occurs after primary inoculation of the skin. With direct inoculation of the organism in a compromised host, infection usually presents with signs of acute inflammation with edema, induration, and necrosis. The lesions are initially erythematous and indurated, but often progress to form necrotic eschars. The eschars are typically dark and firm with a woody consistency in more advanced stages. Necrotic tissue may also ulcerate. While primary cutaneous infections disrupt the epidermis and may invade adipose tissue, muscle, fascia, and bone, cutaneous lesions developing from disseminated mucormycosis tend to be nodular with only minimal destruction of the epidermis.
There are important prognostic implications to the staging of lesions of cutaneous mucormycosis. Among 176 patients with cutaneous mucormycosis, patients with localized infection had an overall mortality of 10% and deep extension in 26%, while those with cutaneous lesions as part of a disseminated process had a 94% mortality. Recognizing that most cases of cutaneous mucormycosis are the result of direct inoculation provides a rationale for urgent local surgical control in order to prevent dissemination to other sites in immunocompromised hosts.
Cutaneous disease manifests as cellulitis, which progresses to dermal necrosis and black eschar formation. The progressive black necrotic lesion of cutaneous mucormycosis reflects the vascular invasion characteristic of all forms of the disease.
As mentioned, patients who are at high risk of developing cutaneous mucormycosis are those with disruption of the normal protective cutaneous barrier. The agents of mucormycosis are typically incapable of penetrating intact skin. However, burns, traumatic disruption of skin, and persistent maceration of skin enables the organisms to penetrate into deeper tissues. A typical case results from traumatic implantation of soil, for example, as a result of a motor vehicle accident or penetrating injury with plant material (e.g., a thorn). In diabetic and immunocompromised patients, cutaneous lesions may also arise at insulin injection or catheter insertion sites. Contaminated surgical dressings have also been implicated as a source of cutaneous mucormycosis. Cutaneous mucormycosis has also occurred in the context of contaminated tape used to secure an endotracheal tube in a ventilated patient.
______
Gastrointestinal Mucormycosis:
Gastrointestinal infection is an uncommon form of mucormycosis. Typically occurring after ingestion of sporangiospores by malnourished patients, premature neonates, or immunocompromised hosts. Gastrointestinal mucormycosis carries a reported mortality of 85%. While the stomach, ileum, and large intestine are the most commonly infected sites, virtually any part of the gastrointestinal tract may be affected. As the organisms may invade the mucosa, submucosa, and vascular structures, bowel wall perforation and peritonitis caused by mixed flora of Mucorales and bacteria may ensue. Early recognition of the clinical manifestations of gastrointestinal mucormycosis, including fever, nausea, vomiting, hematemesis, abdominal pain and distension, and hematochezia, may allow for prompt surgical and medical interventions.
_____
Other Forms of Mucormycosis:
Endocarditis and isolated cerebral, renal or peritoneal infection are uncommon but potentially lethal manifestations of mucormycosis. Endocardial mucormycosis occurs typically in the setting of prosthetic valves or other cardiac surgery, but may also develop in immunosuppressed patients, hemodialysis recipients, and intravenous drug users. Infected emboli may disseminate from large, valvular vegetations and cause distal infarctions. Endocardial infection also may extend transmurally to involve the myocardial wall and pericardium with substernal chest pain and tamponade.
Isolated mucormycosis of the central nervous system (CNS) may develop in immunocompromised patients and in users of illicit intravenous drugs. Focal neurological deficits, including hemiparesis, loss of visual fields, seizures and cranial nerve deficits, may be the earliest clinical manifestations of isolated CNS mucormycosis. Cerebral mucormycosis is clinically indistinguishable from CNS aspergillosis. Patients sustaining open head trauma may suffer direct inoculation of fungi into CNS tissue.
Solitary renal mucormycosis occurs in the setting of illicit intravenous drug use or contamination of a central venous catheter. The lesions of solitary renal zygomycosis may expand to occupy a large portion of the renal parenchyma before they elicit symptoms. Fever, flank pain, and hematuria may be present. Cytological analysis of the spun sediment of a 24-hour urine collection may establish an early diagnosis, especially in resource-limited settings where transcutaneous needle aspirate technology may not be readily available.
Various studies from India documented the rise in patients with isolated renal mucormycosis from 5.4% to 14% among all mucormycosis cases. In China and India, 33–100% of isolated renal mucormycosis cases had no underlying illness. Patients usually present with fever, flank pain, haematuria or anuria. Computer tomography and ultrasound are helpful in the early diagnosis of renal mucormycosis. CT of the abdomen shows bilaterally enlarged kidneys with thickening of the renal pelvis, infarction in the parenchyma.
_____
Disseminated Mucormycosis:
Disseminated mucormycosis is defined by involvement of at least two non-contiguous sites. Roden et al described the crude mortality of disseminated mucormycosis as 94% to 100%. Disseminated mucormycosis typically occurs in severely immunocompromised patients and in those receiving deferoxamine. Although hematogenous dissemination may originate from any site, the lungs are the most common primary source of infection. That pulmonary mucormycosis may be complicated by fatal hematogenous dissemination underscores the importance of early diagnosis and therapeutic intervention.
This form of mucormycosis is the second most common clinical presentation in patients with hematological malignancies. The symptoms and evolution of disseminated mucormycosis vary widely, reflecting the host as well as the location and degree of vascular invasion and tissue infarction in the affected organs. Immunosuppression and deferoxamine therapy appear to be the most significant risk factor for disseminated mucormycosis. Other risk factors for dissemination include organ transplantation, chemotherapy, and corticosteroids therapy. Recent case series have described frequent dissemination in the context of voriconazole prophylaxis of transplant patients. In five recent case series, a total of 18 cases of disseminated mucormycosis have occurred in patients post-allogeneic hematopoietic stem cell transplantation who were receiving voriconazole either prophylactically or therapeutically for other infections. It has been pointed out that the increase in frequency of mucormycosis in transplant patients preceded the availability of voriconazole and that therefore the precise role of voriconazole in predisposing patients to mucormycosis is unclear. For example, increasingly intensive immunosuppressive regimens and broader availability of allogeneic transplantation (e.g., to patients of increasing age) may be playing a role. Furthermore, the increasing use of peripheral stem cell transplants (in lieu of bone marrow-derived cells), non-myeloablative conditioning regimens, and unrelated donor and/or HLA-mismatched transplants have increased the incidence of graft-versus-host disease. As mentioned, graft-versus-host disease and its treatment with corticosteroids are strongly linked with the risk of mucormycosis in the transplant setting. Indeed, most of the reported cases of mucormycosis occurring during voriconazole therapy have occurred in patients receiving corticosteroids for graft-versus-host disease. Nevertheless, the uniformity of the reports of mucormycosis in patients receiving voriconazole infections implicates a link between the drug and the disease. Voriconazole has broad activity against Aspergillus, Candida, and Scedosporium spp. and the dematiaceous fungi, but has no clinically relevant activity against the agents of mucormycosis. Therefore, it is possible that the predisposition to mucormycosis is due to selective inhibition of other fungi, which allows the agents of mucormycosis to colonize the patient. As well, it is also possible that voriconazole is preventing early-onset deadly infections caused by other species of fungi (i.e., Candida and Aspergillus), thereby allowing highly immunocompromised patients, who in the past would have died earlier posttransplant, to live long enough to become infected with the agents of mucormycosis.
______
______
Section-11
Diagnosis of mucormycosis:
Early diagnosis of mucormycosis is of utmost importance, since it may improve outcome. Studies have shown that it increases survival, and it may also reduce the need for or extent of surgical resection, disfigurement and suffering. Since the disease is rare, a high index of suspicion is very important. Diagnosis consists of recognition of risk factors, assessment of clinical manifestations, early use of imaging modalities and prompt initiation of diagnostic methods based on histopathology, cultures and advanced molecular techniques.
_
Fungal infections caused by members of the Mucorales order are rapidly progressing and fatal. The importance of mucormycosis has grown in recent years as the number of patients with predisposing factors has increased dramatically. Clinical symptoms are elusive and conventional techniques are often insensitive and unspecific; in particular, cultures are often negative even though direct microscopy is positive.
The symptoms, signs and radiographic manifestations of mucormycosis are nonspecific, and in order to obtain a conclusive diagnosis, direct identification of the characteristic hyphae in biopsy specimens obtained from the site of infection are required. Direct examination of sputum, paranasal sinus secretions or bronchoalveolar lavage fluid is the most rapid approach for a first orientation of diagnosis and has to be considered as evidence of infection in blood cultures are rarely positive. Diagnosis requires identifying the mold in the affected tissue by biopsy and confirming it with a fungal culture. Because the causative fungi occur all around, a culture alone is not decisive. Blood tests include a complete blood count to look specifically for neutropenia. Other blood tests include iron levels, blood glucose, bicarbonate, and electrolytes.
_
Timely diagnosis is paramount in cases of mucormycosis. Persons with suspected rhinocerebral disease should undergo emergent computed tomography (CT) imaging of the paranasal sinuses and an endoscopic examination of their nasal passages with biopsies of any suggestive lesions. The diagnosis of mucormycosis is established by obtaining a biopsy specimen of the involved tissue, and frozen tissue samples should be immediately evaluated for signs of infection. Tissue should also be sent for routine pathology examination and cultures. Swabs of tissue or discharge are unreliable. For pulmonary disease, a bronchoalveolar lavage (BAL), biopsy, or both may assist in the diagnosis. For cutaneous disease, a skin biopsy for pathology and culture should be obtained. In cases of central nervous system (CNS) involvement, cerebrospinal fluid (CSF) findings may include elevated protein levels and a modest mononuclear pleocytosis. CSF cultures are typically sterile. A CT scan should precede a lumbar puncture to assess for evidence of elevated intracranial pressure, which could lead to herniation.
Blood cultures can be obtained; however, they are usually negative despite the angioinvasive nature of the organism. Blood cultures may be useful to detect bacteremia in addition to Mucorales infection. One study of pulmonary mucormycosis identified concurrent bacteremia as an independent predictor of 28-day mortality. There are no specific biomarkers to identify mucormycosis. Bronchoalveolar lavage (BAL) of fluid culture has a low yield, with a sensitivity of 20%-50%. Antigen tests (beta-D-glucan or galactomannan) are not useful for detecting this infection.
The use of quantitative polymerase chain reaction (qPCR) for detection of circulating DNA from common Mucorales species (Lichtheimia species, Rhizomucor species, and Mucor/Rhizopus species), while not yet commercially available, has been described and appears promising for the early diagnosis of mucormycosis in high-risk patients.
_
Problems in the diagnosis of mucormycosis:
-The clinical signs and symptoms are non-specific
-Imaging signs are non-specific
-Various non-invasive tests (PCR, antigens etc.) are not yet standardized
-Biopsy cannot always be performed due to severe thrombocytopenia in many cases
-Definite diagnosis is usually made in an advanced stage of the disease
____
____
Clinical diagnosis:
The prerequisites for the diagnosis of mucormycosis are a high index of suspicion, recognition of host factors, and prompt assessment of clinical manifestations. Diplopia in a patient with diabetes or pleuritic pain in a neutropenic host may be a sign of this infection and should lead to the prompt use of imaging modalities and subsequent acquisition of samples for testing by histology, microbiology, and advanced molecular methods. As already mentioned, the most common clinical presentations of Mucorales infection are rhinocerebral, pulmonary, soft tissue, and disseminated disease; however, virtually any organ can be affected. Tissue necrosis is the hallmark of mucormycosis, but presentation and syndrome-oriented approach to diagnosis lacks sensitivity and specificity. Other fungi, such as Aspergillus or Fusarium, may produce the same clinical signs. Furthermore, in countries where tuberculosis is endemic, the two infections may coexist, for example, as reported in a diabetic patient. Nevertheless, there are some features which should lead to a higher index of suspicion for invasive pulmonary mucormycosis. These include a history of prior prophylaxis with voriconazole or the emergence of breakthrough fungal infection in an immunocompromised patient receiving agents active against Aspergillus but not Mucorales. Corzo-Leon et al. proposed an algorithm for the diagnosis of rhinocerebral mucormycosis in diabetic patients. The list of signs and symptoms that should be considered to be “red flags” includes a cranial nerve palsy, diplopia, sinus pain, proptosis, periorbital swelling, orbital apex syndrome, and ulcers of the palate.
______
The 1950 Smith and Krichner criteria for the clinical diagnosis of mucormycosis are still considered to be gold standard and include:
(i) Black, necrotic turbinate’s easily mistaken for dried, crusted blood,
(ii) Blood-tinged nasal discharge and facial pain, both on the same side,
(iii) Soft peri-orbital or peri-nasal swelling with discoloration and induration,
(iv) Ptosis of the eyelid, proptosis of the eyeball and complete ophthalmoplegia and,
(v) Multiple cranial nerve palsies unrelated to documented lesions.
______
When and how to clinically suspect CAM:
During the present mucormycosis epidemic in India, nearly 90% of cases presented as ROCM and less than 10% as pulmonary or disseminated disease. Cutaneous mucormycosis is rarely reported in SARSCoV-2 infected patients. Early diagnosis of CAM cases helps in prompt treatment which, in turn, reduces the mortality or morbidity of this infection. CAM can occur while the patient is actively suffering from the disease, during the in-hospital recovery stage or post-discharge after recovering from SARS-CoV-2 infection. Some important points to be considered while clinically diagnosing these conditions are described below. One should suspect CAM in any SARS -CoV-2 infected (present or past) uncontrolled diabetic patient who received corticosteroid therapy and develop the following features.
Rhino-orbito-cerebral mucormycosis (ROCM):
Early warning signs of ROCM include facial pain, nasal blockade or congestion, bloody/brown/black discharge with or without local tenderness or pain. This can be associated with fever, nausea, and headache. Nasal ulcers or crusts turning black later in the course of disease are often noted. Furthermore, patients may develop facial numbness or oedema during involvement of the maxillary, frontal, or ethmoidal paranasal sinuses. Palatal involvement may be noted in the form of an ulcer over the upper palate leading to a dark necrotic area. It may also present as toothache and/or loosening of maxillary teeth and restriction of jaw movement which was not noted during pre-COVID-19 times. With invasion into the orbit the patient may initially have blurring of vision or diplopia, orbital pain, paraesthesia, or proptosis. The orbital apex syndrome without pain has also been well-described. An eschar may be seen in the nasal septum, palate, eyelid, face, or orbital areas. Cranial nerve palsies are also common. With invasion of the brain the patient may present with features of cerebral oedema such as coma, and vascular invasion may lead to cerebral infarcts. Another common presentation includes sinus cavernous thrombosis following loss of vision.
Pulmonary mucormycosis:
Solitary pulmonary CAM is difficult to diagnose and a radiological imaging is needed as several symptoms may overlap with pulmonary features of COVID-19 and of invasive aspergillosis. Fever, cough, haemoptysis, chest pain and pleural effusions are generally present. However, features such as haemoptysis, and tissue infarction are particularly characteristic of pulmonary mucormycosis. Pulmonary mucormycosis is usually the form observed in neutropenic patients.
Basic investigations such as regular determination of blood glucose levels, ferritin levels, arterial blood gas measurements to rule out acidosis, serum electrolyte imbalance, along with screening for serum or urine ketone bodies are recommended for severe COVID-19 patients.
For diagnosis of CAM, COVID-19 should be confirmed by a single RT PCR test detecting the RNA of SARS-CoV-2, or SARS-CoV-2 antigen testing along with clinical, radiological, histopathological, or microbiological evidence suggestive of mucormycosis.
_____
_____
Early diagnosis of mucormycosis:
Invasive mucormycosis causes severe morbidity and mortality in hematopoietic stem cell transplant and solid organ transplant recipients and in patients with hematological malignancies, diabetes mellitus, burns, trauma, and low birth weight. Timely initiation of treatment improves the outcome of invasive mycoses. Similarly, early treatment is predicated on early diagnosis. Early diagnosis of mucormycosis is important, and prompt therapeutic intervention may prevent progressive tissue invasion and its sequelae. These sequelae include (1) angioinvasion and direct tissue injury of the respiratory tract, (2) direct extension from lungs into the great vessels, (3) invasion from the paranasal sinuses into the orbit and brain, and (4) hematogenous dissemination to central nervous system tissues. Early diagnosis may also reduce the need for or extent of surgical resection, disfigurement, and suffering. Finally, early diagnosis may improve outcome and survival. Supporting the importance of early diagnosis of invasive mucormycosis, Chamilos and colleagues demonstrated the impact of delayed amphotericin B–based therapy on outcome among 70 consecutive patients with hematological malignancy and mucormycosis. Delayed therapy resulted in a 2-fold increase in mortality at 12 weeks, compared with early treatment (82.9% vs 48.9%). Moreover, delayed treatment of invasive mucormycosis was an independent predictor of poor outcome in multivariate analysis (odds ratio, 8.1 [95% confidence interval, 1.7–38.2]; P = .008).
_
The symptoms, signs, and radiographic manifestations of mucormycosis are nonspecific, and a definitive diagnosis requires direct identification of the characteristic hyphae or recovery of the organism in culture from specimens obtained from the site of infection. Direct examination of sputum, paranasal sinus secretions, or bronchoalveolar lavage (BAL) fluid is frequently nondiagnostic, but isolation of Mucorales organisms from such specimens in a susceptible host with corresponding clinical manifestations should be considered a priori as compelling evidence for infection. Establishing a diagnosis of pulmonary mucormycosis may also reveal concomitant infections caused by other organisms, including Aspergillus spp. and other fungal pathogens.
Laboratory Methods for Detection and Diagnosis of Mucormycosis:
-1. Direct examination
-Wet mount
-Calcofluor
-2. Cytopathological examination
-Periodic acid–Schiff stain
-Gomori methenamine silver stain
-3. Histopathological examination
-Periodic acid–Schiff stain
-Gomori methenamine silver stain
-4. Immunohistochemistry analysis
-Culture
-Antigen detection
-5. Molecular methods
-Direct sequencing of cultured organism or formalin-fixed tissue
-Fluorescent in situ hybridization
-Quantitative PCR of blood, BAL fluid, or tissue
Samples for direct microscopy by wet mount, cytopathological, or histopathological examination may be collected by radiographically guided percutaneous needle aspirate and by transbronchial or direct biopsies of lesions. Although histopathological examination is specific and reliably establishes the diagnosis of mucormycosis in most cases in which characteristic hyphae are observed, obtaining biopsy material from deep tissue sites is frequently difficult in patients with thrombocytopenia or coagulopathy.
_
Benefits of Early Diagnosis of Mucormycosis:
Prevention of angioinvasion
Prevention of direct tissue injury of lung, brain, and sinuses
Prevention of extension into critical sites: eyes, brain, great vessels
Prevention of progression to dissemination
Reduced need for or extent of surgical resection
Reduced need for disfiguring surgery
Reduced suffering (invasion of sensory nerve fibers)
Improved outcome and survival
______
______
Diagnostic pathway for mucormycosis:
The capability of diagnosing mucormycosis depends on the availability of imaging techniques, trained personnel, and mycological and histological investigations. Patients with suspected mucormycosis should be referred immediately to a facility with the highest care level. If all diagnostic options are available, one should follow the management pathway depicted in figure below.
Depending on the geographical location not all recommended tests might have regulatory approval for use in clinical settings. HSCT=haematopoietic stem cell transplantation. SOT=solid organ transplantation. PAS=periodic acid Schiff. GMS=Grocott-Gomori’s methenamine-silver strain. qPCR=quantitative PCR. HRM=high resolution melting. ITS=internal transcribed spacer. rDNA=ribosomal DNA.
_____
_____
Microbiological and Histopathological diagnosis:
In clinical practice, laboratory diagnosis of mucormycosis includes histopathology, direct examination of wet mounts and culture.
-1. Histopathology:
A definitive diagnosis is based on the demonstration of fungal hyphae typical for mucormycetes in biopsies of affected tissues, or bronchoalveolar lavage (BAL) in patients with pulmonary mucormycosis. Histopathology is a very important diagnostic tool since it distinguishes the presence of the fungus as a pathogen in the specimen from a culture contaminant and is indispensable to define whether there is blood vessel invasion. It can furthermore reveal coinfections with other molds. Mucorales genera produce typically non-pigmented, wide (5–25 μm), thin-walled, ribbon-like hyphae with no or few septations (pauciseptate) and right-angle branching, in contrast to those of the Aspergillus species or other hyaline molds, which are typically 3–5 μm wide, septate and form acute-angle branching. Routine hematoxylin and eosin (H&E) stains may show only the cell wall with no structures inside, or occasionally, very degenerate hyphae. Stains that can help highlight the fungal wall include Grocott methenamine-silver (GMS) and periodic acid-Schiff PAS stains, although PAS gives a better visualization of the surrounding tissue compared to GMS.
Hyphae may be observed within necrotic tissue with signs of angioinvasion and infarction; neutrophilic infiltrates or granuloma formation may be present in patients who are not granulocytopenic or with more chronic infection, respectively. Occasionally, immunohistochemistry with commercially available antizygomycete antibodies may help in the diagnosis.
In tissue, Mucorales hypahe can be differentiated from other more common opportunistic molds, such as Aspergillus and Fusarium, by their broad (5-25 micrometer), empty, thin-walled, mostly aseptate hyphae.
Tissue sections may show mixed hyphal forms that include folded, twisted, or compressed hyphae that can be mistaken for septae. Mistaken histologic identification is common, especially in laboratories not attuned to diagnosis of mucormycosis, and can lead to inappropriate therapy. A variety of stains, including hemtoxylin and eosin, Grocott-Gomori methenamine silver, and periodic acid-schiff stains, reveal characteristic hyphal elements in tissue.
_
-2. Direct Microscopy:
Demonstration of hyphae in clinical samples by direct microscopy is important because it is rapid and highly suggestive of disease. Specimens can be observed after treatment with potassium hydroxide, staining with an optical brightener (calcofluor white), or with Gomori methamine-silver. Hyphae are hyaline, non- or pauciseptate, ribbon-like with a large diameter (5-25 μm). Width is irregular with branching angles of 90°. When hyphae are fragmented, a definitive diagnosis of mucormycosis can be difficult by direct examination and culture is required to confirm the diagnosis.
For a rapid presumptive diagnosis of mucormycosis direct microscopy of KOH wet mounts can be used. It can be applied to all materials sent to the clinical laboratory, preferably using fluorescent brighteners such as Blankophor and Calcofluor White together with KOH, which enhance the visualization of the characteristic fungal hyphae, in this case requiring a fluorescent microscope. Treatment with fluorescent stains, such as Calcofluour white, Blankofluor, or Uvitex, may enhance detection of hyphae during microscopic examination and improve the discrimination between septate and aseptate molds in biopsy specimens. Direct microscopy of fresh material is an inexpensive, yet invaluable method to rapidly give a presumptive diagnosis and to define clear surgical margins for invasive fungal infection intraoperatively, and it is strongly recommended, along with histopathology, by a panel of experts of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium (ECMM/MSG ERC). These methods, however, are not able to identify a fungus to the genus or species level. Another method, immunohistochemistry using monoclonal antibodies against R. arrhizus (recently commercially available) can aid in the diagnosis when cultures are negative and has been proven useful for differentiating aspergillosis from mucormycosis (sensitivity 100%, specificity 100% for mucormycosis) and has gained a moderate recommendation of B IIu in the recent ECMM/MSG ERC guidelines.
_
-3. Culture:
Culture of specimens is essential for the diagnosis of mucormycosis since it allows identification to the genus and species level, and eventually antifungal susceptibility testing. Most medically important Mucorales are thermotolerant and are able to grow rapidly at temperatures of 37 °C. They grow on virtually any carbohydrate substrate, colonies appearing usually within 24–48 h and identification is based on colonial and microscopic morphology and growth temperature. The media most commonly used are potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA). Matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) identification of cultured Mucorales is a promising method for those laboratories that are accordingly equipped, but the commercially available databases should be expanded further, and more validation data are needed. Until then, molecular identification remains the gold standard.
The spores from asexual reproduction are easily airborne and may be demonstrated during sampling of both outdoor and indoor air. The small sporangiospore size (mean size, 6.6 μm) allows easy dissemination by the airborne route. Particles of this size have a settling rate that is very low and, as a result, may remain airborne even with very slight movements in air. The Mucorales may be seen in the laboratory as clinical contaminants, presumably as a result of airborne contamination of the culture medium, or they may be seen in clinical specimens as a result of oral or nasal ingestion in food or air prior to sample collection. Growth of mucormycetes in culture may therefore not represent clinically significant invasive disease. Demonstration of invasive disease by these organisms generally requires the identification of fungal elements directly in the clinical specimen or organism growth from more than one specimen obtained from a normally sterile site. When isolates are obtained from nonsterile sites such as sputa, culturing the same organism from multiple specimens or culturing large numbers of colonies from these specimens might suggest the diagnosis; however, these results might also reflect superficial transient colonization. A positive culture linked to a hyphal identification in cytologic specimens or tissue sections, however, is considered diagnostic.
A positive culture from a sterile site confirms the diagnosis, while a positive culture from a non-sterile site could be due to a contaminant and must be combined with clinical and radiological data to establish a probable diagnosis. Hence, there is a caveat for falsely positive results, especially when histopathology is not available.
The major concern about culture, however, is its low sensitivity, as it can be falsely negative in up to 50% of mucormycosis cases. This can be attributed to a number of reasons, such as grinding or homogenization of tissue specimens, which may destroy the delicate hyphae of mucormycetes, the presence of genera that require special culture conditions, recent or ongoing therapy with antifungals effective on Mucorales, or even a lack of expertise. Proper sampling and handling of the specimens before examination are a prerequisite for an optimal yield. Therefore, upon suspicion of a case, good communication and close collaboration between clinicians and the microbiology laboratory is essential to ensure that all steps of the diagnostic procedure will be taken properly.
_____
-Because of the ubiquitous nature of the fungus in the environment, positive cultures occasionally reflect culture contamination rather than true disease. However, discovery of Mucorales in a specimen from the “right host” (i.e., hematopoietic cell transplant recipient) is an important diagnostic clue that should be confirmed with tissue biopsy/histopathologic confirmation.
-Skin and sinus infection generally can be definitively diagnosed through biopsy, even in severely thrombocytopenic patients, given the accessibility of these infection sites.
-Tissue swabs and cultures of sputum, sinus secretions, nasal mucosa, as well as bronchoalveolar lavage fluid are typically nondiagnostic but occasionally grow the fungus.
-Blood cultures rarely grow Mucorales, despite the angioinvasive nature of the infection.
-Identification of Mucorales to the genus and species level requires growth of the fungus in culture to identify reproductive fruiting structures of the fungus. Most species grow rapidly on rich media, such as sabouraund dextrose agar when incubated at 25-30°C. Unfortunately, culture recovery of Mucorales from tissue is inherently poor because of the friable nature of the non-septate hyphae in tissue. Therefore, tissue should be minced (not homogenized) during preparation. The material taken from biopsies should be carefully managed so as not to be crushed because zygomycetes are fragile, and culture may thus remain negative. Recovery from tissue may also be aided by growing the cultures in a reduced oxygen environment at 37°C. Growth is rapid and usually occurs during incubation for few days at 25-37°C. Culture of a sterile site confirms mucormycosis infection and allows precise genus and species identification. Blood cultures are almost always negative and their positivity should evoke the suspicion of contamination. Similarly, agents of mucormycosis are rarely present in the cerebrospinal fluid even during central nervous system infections.
______
A definitive diagnosis can be made by microbiological and/or histopathological examination of tissues obtained from different lesions. Nasal samples from ROCM, broncho alveolar lavage (BAL) samples, mini-BAL, non-bronchoscopic lavage, transbronchial biopsy from pulmonary mucormycosis may provide an early clue of infection. The samples are first examined with KOH or KOH calcofluor-white, which demonstrate characteristic broad aseptate or pauci-septate hyphae (measuring 6-16 or even up to 25 µm) with folding of the hyphae giving a ribbon-like appearance (Figure below 4A). Right-angle branching can be often noted especially on histopathological examination. Demonstration of this kind of hyphae and isolation of Mucorales from endoscopically collected debrided tissue/biopsy or a CT guided biopsy from a lung lesion confirms the diagnosis of mucormycosis. For successful isolation of Mucorales, tissue or biopsy samples should not be homogenized or grinded but instead it should be cut into small bits using scissors before inoculating culture media. Homogenization damages the fragile cell wall of Mucorales making the fungi non-viable resulting in no growth on the media. In culture, Mucorales can easily be identified as they typically produce cottony growth (Figure below 4B), and with histopathology stains, angioinvasion and occlusion of the vessels with thrombi, necrosis, and haemorrhagic infarction can be noted. PCR based molecular techniques have been shown to have high sensitivity especially from fresh tissue sections. A real time PCR-based kit is commercially available for the detection of Mucorales DNA in various clinical specimens. Identification of Mucorales to species level is based on microscopic features (Figure below 4C), PCR followed by sequencing and Matrix-Assisted Laser Desorption/Ionization-Time of Flight (MALDI-TOF). Though no biomarker is available for the diagnosis of mucormycosis, repeated negative galactomannan antigen, 1,3-ß-D-glucan, and Aspergillus specific PCR results in a patient with strong suspicion of invasive mould infections of the lungs may suggest pulmonary mucormycosis
Figure above shows broad aseptate hyphae on potassium hydroxide wet mount (4A), classical cottony appearance of Rhizopus arrhizus colony (4B) Microscopic picture of Rhizopus arrhizus (4C).
_
The wet mounts can also be examined with Calcofluor/blankophor white under Nikon 90i fluorescent microscope with excitation filter (300–412 nm). The size, morphology, and quantity of any fungal elements are noted.
Calcofluor white staining of tissue sample showing fluorescent ribbon-shaped broad sparsely septate hyphae (1000 × magnification).
______
In one patient, sputum culture on Sabouraud dextrose agar (SDA) at 25 °C and 37 °C grew a pure culture of cottony grayish white colony 6 days after incubation. The microbiology culture report revealed cotton wooly appearance colonies which was suggestive of fungal infection as seen in the figure below.
Lactophenol cotton blue (LCB) mount from the growth revealed aseptate hyphae with nodal rhizoids and short sporangiophores with terminal spherical sporangia filled with brownish sporangiospores (figure below), suggestive of Rhizopus microsporus.
The identification was confirmed by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF; Bruker Daltonics, Billerica, MA, USA), which gave a good discriminatory score of 2.1. The in vitro antifungal susceptibility testing (AFST) of the isolate was performed by the microbroth dilution method as per the Clinical Laboratory Standards Institute (CLSI)-M38A2 guidelines. The minimum inhibitory concentrations (MICs) of the isolate were as follows: amphotericin B, 0.5 µg/mL; itraconazole, 0.03 µg/mL; posaconazole, 2.0 µg/mL
A specific mouse monoclonal anti-Rhizomucor-antibody has been employed for immunohistochemical analysis; however, this test was previously shown to react with other Mucorales and Entomophthorales. The use of in situ hybridization targeting 5S and 18S ribosomal RNA sequences remains investigational.
_____
Mucormycosis is usually suspected based on results of direct microscopy of clinical specimens, preferably stained with fluorescent brighteners calcofluor white (Sigma Aldrich, St Louis, MO, USA) or blankophor (Tanatax Chemicals, Ede, The Netherlands). To confirm an infection, non-pigmented hyphae showing tissue invasion must be shown in tissue sections stained with haematoxylin-eosin (HE), periodic acid-Schiff stain (PAS), or Grocott-Gomori’s methenamine-silver stain (GMS), or both. Histopathologically, Mucorales hyphae have a variable width of 6–16 μm, but may be up to 25 μm, and are non-septate or pauci-septate. In tissue, the hyphae appear ribbon-like with an irregular pattern of branching (figure below A–C). Hyphae can artefactually seem to have septae because tissue can fold over itself during processing, which can create artificial lines that can be confused with septations. Similarly, the historically described 90° branching angle of Mucorales in tissue, versus 45° branching angle of septate moulds, can be difficult to identify in tissue due to interstitial pressures exerted on the fungi by the tissue and alterations in tissue architecture during processing. Thus the wider and irregular (ribbon-like) nature of the hyphae are more reliable distinguishing characteristics than septations and angle of branching.
Figure above shows Hyphal morphology in mucormycosis and aspergillosis:
(A) Typical hyphal morphology in mucormycosis lesions (GMS, × 200). Mucorales hyphae are at least 6–16 µm wide, ribbon-like, pauci-septate, and branch irregularly. (B) Hyphal structure covered with Splendore-Hoeppli phenomenon (HE, × 1000). The eosinophilic material likely represents antigen-antibody complexes. First described by Splendore in 1908, and by Hoeppli in 1932. (C) Typical hyphal morphology in aspergillosis lesions (PAS, x 200). Aspergillus hyphae are 3–5 µm wide, regularly septated, with dichotomous branching. (D–F) Sizes and branching angles for Mucorales and aspergillus stained by calcofluor-white. D and F correspond to Rhizopus arrhizus and E to Aspergillus fumigatus. Measurements correspond to the size of the white lines; hyphal diameter were performed with the Leica software LAS-AF and are expressed in µm. Diagnosis needs to be confirmed by culture, molecular techniques, or both.
The lesions of mucormycosis are characteristic but non-specific. In acute lesions, haemorrhagic infarction, coagulation necrosis, angioinvasion, infiltration by neutrophils (in non-neutropenic hosts), and perineural invasion are characteristic features; whereas, in chronic lesions, a pyogranulomatous inflammation with presence of giant cells, and sometimes hyphae covered by the Splendore-Hoeppli phenomenon, which describes deeply eosinophilic material surrounding the pathogen are seen.
Obtaining a diagnosis of mucormycosis on histomorphological basis is challenging, and the most common cause for incorrect morphological diagnosis is the misidentification of Mucorales as Aspergillus spp. The application of immunohistochemistry with commercially available monoclonal antibodies or PCR techniques on either fresh or formalin-fixed paraffin-embedded tissue, have been shown to be highly specific, although a variation in sensitivity has been reported, in addition, these tests might not be widely available.
Hyphae of Mucorales can be distinguished from septate hyaline moulds due to their greater width and irregular pattern of branching. However, there are no data available to describe the accuracy of distinguishing Mucorales from other moulds based on these characteristics. Therefore, it is strongly recommended to confirm the diagnosis of mucormycosis in tissue by culture or by application of molecular or in-situ identification techniques, at centers where such assays are available
Culture of specimens is strongly recommended for genus and species identification, and for antifungal susceptibility testing. Homogenisation of tissue should be avoided before culturing. Incubation at 30°C and 37°C separately is strongly recommended. Direct microscopy with fluorescent brighteners from clinical specimens is strongly recommended mainly focusing on septation, branching angle, and hyphal width.
_____
_____
Species Identification and antifungal susceptibility:
Species identification is of interest because Mucorales may have different susceptibility to azoles. Newer azoles (posaconazole, isavuconazole but not voriconazole) may be active on Mucorales and can be given for long-term oral maintenance therapy or in the case of intolerance to liposomal amphotericin B therapy. The most common genera in invasive mucormycosis are Rhizopus, Rhizomucor, Lichtheimia and Mucor, accounting for 90% of all cases. Other genera (Cunninghamella, Apophysomyces, Saksenaea, Cokeromyces, Actinomucor and Syncephalastrum) species are individually responsible for <1% to 5% of reported cases of mucormycosis.
Species identification is of interest for a better epidemiological understanding of mucormycosis and may be of value for outbreak investigations. Mucorales fungi can easily be differentiated from Aspergillus fungi on culture. The study by Alvarez et al. demonstrated that morphological features alone, when assessed by individuals with expertise in fungal identification, can provide a high level of accuracy. However, morphological species identification is difficult and may be associated with failures in speciation.
Biochemical tests display standard procedures for species identification of bacteria and yeasts, with carbon assimilation profiles (Analytical Profile Index [API] ID32C and API 20C; Bio Mérieux SA, Marcy l’Etoile, France) being widely used for yeasts. In principle, these methods work excellently and misidentification is rare. Such biochemical tests were evaluated for 57 mucormycetes (15 different species) using ID32C kit (bio Merieux, Marcy l`Étoile, France) and API 50CH (bio Merieux). API 50CH worked best for Mucor spp., while ID32C was most reliable for L. corymbifera and R. pusillus. Mucor circinelloidides and M. rouxii were not distinguished by either test. ID32C combined with positivity for melezitose assimilation is associated with L. ramosa.
MALDI-TOF mass spectrometry for species identification of mucormycetes was found to be promising, but is not yet validated for all Mucorales. Lichtheimia sp. was successfully discriminated from other Mucorales sp. as only two out of 34 Lichtheimia were not accurately identified. In another analysis of 103 filamentous fungi, eight tested Mucorales were correctly identified to species level.
Another reliable approach is the application of molecular based assays focusing on the internal transcribed spacer region.
Although some genera, such as Cunninghamella, can be associated with an increased mortality rate in patients and have been shown to be more virulent in experimental models, there is currently sparse evidence that identification of the causative Mucorales to the genus or species level, or both, could guide the choice of the antifungal treatment. M. circinelloides shows high minimum inhibitory concentrations (MIC) against posaconazole, and Rhizopus and Cunninghamella against amphotericin B. Some Apophysomyces isolates have also increased MIC against amphotericin B. The role of such data is unclear for patient treatment but needs to be further analyzed.
By contrast, identification to the species level is of importance for improved epidemiological knowledge of the disease. In particular, the clinical picture can be different depending on the species. Moreover, species identification is valuable for investigation of healthcare associated mucormycosis and outbreaks.
_____
_____
Microculture:
The current gold standard for diagnosing mucormycosis is based on histopathological and mycological findings, followed by unspecific radiological criteria. However, these procedures require specialized expertise and the results are often not available in a timely fashion (Hammond et al., 2011; Cornely et al., 2019). To enhance outcomes, patients suspected to have mucormycosis should be immediately treated. Despite our improved understanding of the disease and the availability of various medico-surgical treatments, the survival rate in mucormycosis patients remains poor (Kontoyiannis and Lewis, 2011; Katragkou et al., 2014; Pilmis et al., 2018; Skiada et al., 2018). Therefore, there is a need for novel diagnostic assays. Several new molecular methods for the diagnosis of mucormycosis have been reported (Hammond et al., 2011; Dadwal and Kontoyiannis, 2018); however, these techniques may lack sensitivity, can be time-consuming and expensive to perform, and are not universally available (Katragkou et al., 2014).
The choice of an effective treatment regimen against mucormycosis requires early diagnosis and identification of the causative pathogen and its antifungal susceptibility profile, for which a positive culture is needed (Walsh et al., 2014; Hoenigl et al., 2018; Cornely et al., 2019). However, due to the unique physiology of these etiological agents (e.g., fragile and non-septate hyphae), cultures are frequently negative, and the processing of clinical specimens requires a suspicion of Mucorales as the causative agent and experienced laboratory personnel than may be required for fungi with septate hyphae (Ben-Ami et al., 2009; Hammond et al., 2011; Lewis et al., 2013; Cornely et al., 2019).
So, there is a role of microculture assay as a putative, rapid, and low-cost culture-based method for the early diagnosis of mucormycosis.
Rapid mycological diagnostic methods may assist with timely initiation of appropriate antifungal therapy, which may prevent progressive tissue invasion, lead to decreased mortality, and overall improvement in healthcare utilization (i.e., shorter hospitalization duration and reduced costs). Histological analysis is an important diagnostic tool in the early management of this devastating disease (Skiada et al., 2018); however, the 24-h turnaround time of the microculture approach is considerably shorter than that of histological analysis (48–72 h) (Dekio et al., 2015) or conventional culturing (3–7 days) (Skiada et al., 2018).
Conventional tissue fungal cultures are typically positive in only 50% of mucormycosis cases (Skiada et al., 2018). Positive cultures and fungal identification, even at the genus level only, allow for the appropriate choice of antifungal regimens and further assessment of antifungal resistance patterns and emerging resistance (Cornely et al., 2014; Beardsley et al., 2018). Although some molecular identification methods may be able to provide results within a few hours, the microculture assay does not require specialized training or equipment. Surprisingly, in one study, 26 of 90 blood samples were positive by microculture, while all blood samples were negative with traditional culture. Increased CO2 levels during incubation, leading to a lower pH, may facilitate the growth of R. arrhizus in microculture tubes. Microculture tubes are microcapillary tubes. Culture-based methods for fungal identification are generally practical, economical, and accessible. The microculture method is relatively rapid and easy to perform. Hence, this approach could also be considered for the diagnosis of fungal infections, which may be challenging using traditional culture methods.
_____
_____
Serological diagnosis:
The panfungal β-D-glucan test and Aspergillus galactomannan tests do not detect antigen components of the Mucorales cell wall, and a positive test is assumed to provide strong evidence for excluding mucormycetes as causative agents of infection, even though comprehensive evidence-based data supporting this statement are lacking. In the past, various techniques, such as ELISA, immunoblots and immunodiffusion tests, have been designed for the diagnosis of mucormycosis, yet with variable success. Some assays applied showed either poor specificity and sensitivity, yielded cross reactivity with Candida and Aspergillus species, or showed a lack of clinical validation. Recently, Mucorales-specific T cells were detected by an enzyme-linked immunospot (ELISpot) assay in three hematological patients developing invasive mucormycosis. None of the controls had Mucorales-specific T cells. Whether such specific T cells will act as surrogate diagnostic markers will be the subject of further studies.
_____
_____
Immunological diagnostic tests for mucormycosis:
|
Method |
Target structure |
Clinical specimens |
Clinical studies |
Sensitivity/specificity |
Crossreactivity |
Comments |
|
ELISA |
Rhizopus arrhizus and Rhizopuspusillus antibody |
Sera from patients infected with Mucorales spp. (n = 43) |
None |
81%/94% |
Candida spp., Aspergillus spp. |
Minimal positivity titer dilutionof 1:400 |
|
Western immunoblotting |
R. arrhizus antigen homogenates |
Sera from patients infected with R. arrhizus (n = 5) |
None |
NA/NA |
NA |
NA |
|
ELISA, Western blot, immunohistochemistry with anti-R. arrhizus monoclonal mouse antibody |
Cytoplasm, hyphae, walls and septae, of R. arrhizus WSSAs |
Tissue samples from patients with systemic mycoses (n = 40) |
Limited data available |
NA/NA |
NA |
14–110 kDa R. arrhizus 1–14 to 52 kDa Lichtheimia corymbifera 20–28 kDa R. pusillus. |
|
ELISA (ELISpot) or immunocytofluorimetric assays |
Mucorales specific IFN-γ-producing T cells |
Peripheral blood samples from patients with proven IM (n = 80) |
None |
R. pusillis (6/4) |
None |
Positivity for 2–10 SFCs |
______
______
Metabolomics-Breath Test:
In an experimental murine model of invasive mucormycosis (IM), Koshy et al. examined breath volatile metabolite profiles, using the three Mucorales species that most commonly cause human IM—Rhizopus oryzae, R. delemar and R. microsporus, by thermal desorption gas chromatography/tandem mass spectrometry (GC–MS). Mice infected with Aspergillus fumigatus were used as controls. They also analyzed breath volatile metabolites from five patients eventually diagnosed with proven IM caused by R. microsporus, sampled prospectively. The findings showed that the three Mucorales species had distinct breath profiles of the volatile metabolite sesquiterpene, which could be used to identify these infections in vivo. These profiles distinguished the infections from each other and from aspergillosis, therefore this method has the potential to diagnose fungal infection non-invasively, and perhaps monitor response to therapy. Additionally, it could be used in a high risk population such as patients with neutropenia due to treatment for leukemia or those undergoing hematopoietic cell transplantation, to screen for mold infections additionally to Aspergillus galactomannan. This method appears to be very appealing and promising, but needs further evaluation.
______
______
Molecular diagnosis:
When cultures are negative, molecular identification from tissue samples can confirm the histological diagnosis. However, at present, there is no standardized method available. Fresh or frozen samples are preferred; however, based on recent inter-laboratory experimental and clinical data, formalin-fixed paraffin-embedded tissues may also be used. Molecular identification of agents of mucormycosis can help to confirm diagnosis and identify the fungus to the genus and species level. Different techniques have been reported: DNA probes targeting 18S subunit, ITS1 sequencing after polymerase chain reaction (PCR) with pan-fungal primers, 18S-targeted semi-nested PCR and real-time PCR targeting cytochrome b gene.
Currently, in the absence of a standardised test, the use of molecular methods on both fresh clinical material and paraffin sections for the diagnosis of mucormycosis is moderately supported. Fresh material is preferred over paraffinembedded tissue because formalin damages DNA.
Detection of DNA in serum as well as in other body fluids is very promising but because of lack of standardisation supported with moderate strength only.
______
Molecular Assays for Mucormycosis Diagnosis:
Recent advances in molecular biology have contributed to the development of rapid, accurate, and sensitive methods for pathogen detection. Most commonly used are conventional PCRs, RFLP, DNA sequencing of defined gene regions, and melt curve analysis of PCR products. All assays described above can be used either for detection or identification of mucormycetes. The efficiency of these in-house assays has not been widely studied, lacks thorough clinical evaluation and therefore cannot be recommended as stand-alone, single approach in clinical routine diagnostics. However, they can be recommended as valuable add-on tools that complement conventional diagnostic procedures. Such combined strategies may result in a species-specific diagnosis of the causative agent. A detailed overview on so far published molecular tools is given in Table below. The majority of the molecular assays are based on the internal transcribed spacer (ITS) region that was chosen as the pan-fungal barcode by the International Subcommission on Fungal Barcoding. By contrast, only a few assays are based on RNA detection. From the latter, the most promising is a 18S rRNA-based FISH procedure that is combined with PCR. The fluorescence probes were described to differentiate Candida spp., Aspergillus spp. and mucormycetes.
It needs to be highlighted that the performance and sensitivity of all nucleic acid-based methods is highly dependent on the DNA/RNA extraction method used. Purity and amount in DNA/RNA extracts is not only dependent on the clinical material, but also on the extraction method. Depending on the clinical material and its specific composition, the extraction protocol or extraction kit used needs to be modified to minimize the risk of amplification inhibition (e.g., proteins, high amount of human DNA) and to guarantee a sufficient amount of intact nucleic acids from the causative pathogen.
_
Molecular tools for detecting and/or identifying causative agent of invasive mucormycosis:
|
Method |
Target structure |
Clinical samples |
Species to be targeted |
Sensitivity/specificity |
Cross-reactivity |
LOD |
|
FISH |
18S rRNA |
FFEs (n = 61) |
Mucorales, Aspergillus spp. and Candida spp. |
95%/NA |
NA |
1:40 titer dilution |
|
PCR |
ITS |
Paraffin tissues (n = 3), Serum (n = 1) |
Rhizopus spp. |
100%/NA |
NA |
NA |
|
|
ITS |
Cultures (n = 57) |
Mucorales spp. |
98%/98% |
Yes |
10 pg |
|
RFLP |
18S rRNA |
Biopsy (n = 1) |
Rhizopus microsporus |
100% (f.t)/100% |
NA |
NA |
|
|
18S rRNA |
Clinical samples (n = 2) |
Lichtheimia corymbifera, R. microsporus spp., Rhizopus arrhizus, Rhisopus azygosporus, Mucor circinelloides, Mucor hiemalis, Mucor indicus, Rhizomucor miehei, Rhizomucor pusillus |
100%/100% |
NA |
100 pg/µl |
|
|
FTR1 |
None |
Rhizopus spp., Rhizomucor spp. and Syncephalastrum spp. |
100% (c)/100% (c) |
None |
NA |
|
|
ITS |
Biopsy (n = 8) |
Apophysomyces elegans |
100%/100% |
NA |
NA |
|
Sequencing |
ITS |
Mouse tissue |
R. arrhizus, L. corymbifera, R. pusillus, R. microsporus, M. circinelloides, M. indicus |
70%/NA |
NA |
NA |
|
|
ITS |
Mouse tissue |
R. arrhizus, R. microsporus, L. corymbifera, R. pusillus, M. circinelloides |
93%/100% |
NA |
NA |
|
RT-PCR |
Cytochrome B |
Clinical samples (n = 12), FFPE (n = 9) |
Lichtheimia spp., Apophysomyces spp., Conidiobolus spp., Cunninghamella spp., Mucor spp., Rhizomucor spp., R. microsporus, R. arrhizus, Saksenae spp., Syncephalastrum spp. |
100% (f.t)/92% (c) |
None |
10 targets/µl |
|
|
28S rRNA |
BAL, lung tissue homogenates, rabbit, plasma |
20 species from five genera |
NA/NA |
NA |
10 fg R. arrhizus, R. microsporus and R. circinelloides, 10 pg R. pusillus |
|
|
18S rRNA |
Paraffin wax embedded tissue |
Rhizomucor spp., M. hiemalis, Lichtheimia corymbifera, R. arrhizus and Aspergillus fumigatus |
100%/100% |
NA |
0.1 fg plasmid DNA |
|
|
18S rRNA |
F.t and FFPE (n = 12) |
Rhizopus spp., R. microsporus, R. pusillis, Mucor racemosus, M. circinelloides, L. corymbifera and other fungi |
2 × 107–2 × 1010 copies/5 µl/NA |
NA |
0.1 fg plasmid DNA |
|
|
18S rRNA |
Serum (n = 51) from patients (n = 10) |
Mucor spp., Rhizopus spp., Lichtheimia spp. and Rhizomucor spp. |
90%/NA |
None |
3.7–15 fg/10µl |
|
|
ITS |
Biopsies (n = 12) |
R. arrhizus, M. circinelloides and R. microsporus |
100%/100% |
None |
1 fg/µl |
BAL: Bronchoalveolar lavage; c: Cultures; FFPE: Formalin-fixed, paraffin-embedded tissue; F.t: Fresh tissue; FTR1: High-affinity iron permease; ITS: Internal transcribed spacer; LOD: Limit of detection; RT-PCR: Real-time PCR.
_______
PCR-Based Approach Targeting Mucorales-Specific Gene Family for Diagnosis of Mucormycosis, 2018 study:
Mucormycosis is an aggressive, life-threatening infection caused by fungi in the order Mucorales. The current diagnosis of mucormycosis relies on mycological cultures, radiology and histopathology. These methods lack sensitivity and are most definitive later in the course of infection, resulting in the prevention of timely intervention. PCR-based approaches have shown promising potential in rapidly diagnosing mucormycosis. The spore coating protein homolog encoding CotH genes are uniquely and universally present among Mucorales. Thus, CotH genes are potential targets for the rapid diagnosis of mucormycosis. Authors infected mice with different Mucorales known to cause human mucormycosis and investigated whether CotH could be PCR amplified from biological fluids. Uninfected mice and those with aspergillosis were used to determine the specificity of the assay. CotH was detected as early as 24 h postinfection in plasma, urine, and bronchoalveolar lavage (BAL) samples from mice infected intratracheally with Rhizopus delemar, Rhizopus oryzae, Mucor circinelloides, Lichtheimia corymbifera, or Cunninghamella bertholletiae but not from samples taken from uninfected mice or mice infected with Aspergillus fumigatus. Detection of CotH from urine samples was more reliable than from plasma or BAL fluid. Using the receiver operating characteristic method, the sensitivity and the specificity of the assay were found to be 90 and 100%, respectively. Finally, CotH was PCR amplified from urine samples of patients with proven mucormycosis. Thus, PCR amplification of CotH is a promising target for the development of a reliable, sensitive, and simple method of early diagnosis of mucormycosis.
______
Circulating DNA:
Circulating Mucorales DNA detection is a recent promising diagnostic tool that may lead to improving the diagnosis and prompting therapeutic initiation that should include antifungal treatment, correction of the underlying disease and surgery when feasible.
A multicentre study shows that Mucorales DNA can be detected in at least 36/44 (81%) patients with probable or proven mucormycosis. Due to the retrospective design of the study, serum sampling and volume were not optimal, and the 81% sensitivity rate is probably underestimated. When excluding cases with insufficient serum sample volume and the case due to a species not targeted by the qPCR, sensitivity reaches 92% (34/37). More importantly, the first positive qPCR sample observed with a median of 9 days before diagnosis was supported by histological or mycological evidence. If positive qPCR had been available, specific treatment could have been initiated 7 days earlier on average for 81% of the patients in this study. With the pulmonary or sinus imaging available, often performed before the mycological investigations, it is observed that qPCR results could be positive at least 2 days (range 0–24) before. This suggests that qPCR results can trigger earlier treatment, which is crucial for improving prognosis. This study showed that Mucorales quantitative PCR could not only confirm the mucormycosis diagnosis when other mycological arguments were present but could also anticipate this diagnosis. Development of qPCR negativity after treatment was associated with higher survival rates (48% vs 4%), suggesting that this modality could eventually be used for treatment monitoring.
_____
_____
Radiological diagnosis:
Imaging is often performed, such as CT scan of lungs and sinuses. Imaging should be used to investigate areas of suspected mucormycosis. Because subclinical disease may be present, a thorough history and physical examination are recommended in addition to imaging (CT) of the brain, sinuses, chest, and abdomen. Signs on chest CT scans, such as nodules, cavities, halo signs, pleural effusion and wedge-shaped shadows, showing invasion of blood vessels may suggest a fungal infection, but does not confirm mucormycosis. A reverse halo sign in a person with a blood cancer and low neutrophil count, is highly suggestive of mucormycosis. CT scan images of mucormycosis can be useful to distinguish mucormycosis of the orbit and cellulitis of the orbit, but imaging may look identical to those of aspergillosis.
______
Rhinocerebral mucormycosis:
Radiological features of ROCM are mucosal thickening and opacification of the sinuses, oedema, inflammation, or infarction of the brain.
Plain orbit or sinus radiography:
Not reliable. Plain films may show sinus involvement with mucosal thickening, air-fluid levels, and/or bony erosions.
Computed Tomography (CT):
A sensitive indicator of the extent of orbital and cranial involvement. Shows opacification of the paranasal sinuses, thickening of the sinus mucosa, bone destruction and air-fluid level. Soft tissue swelling, proptosis, and swelling of the extra ocular muscles can also be seen. Head and facial CT imaging should be used as the initial investigation in rhinocerebral infections. CT scans may show sinusitis of the ethmoid and sphenoid sinuses, as well as orbital and intracranial extension. As the disease progresses, bony erosion may occur and the infection may spread into the brain or orbits.
Magnetic resonance imaging (MRI):
Imaging characteristics of mucormycosis infection generally demonstrate a rim of soft-tissue thickness along the paranasal sinuses. Sinus opacification, air-fluid concentration, and obliteration of the nasopharyngeal tissue planes are features of sinonasal mucormycosis infection. On MR imaging, variable intensity within the sinuses on T1- and T2-weighted images is usually seen. Fungal elements themselves may cause a low signal intensity on T2 sequences. Furthermore, slightly decreased T2 signal intensity with rhinocerebral mucormycosis can be due to the involved mucosa itself. DWI sequences may aid in the diagnosis by showing an increased signal intensity of the affected sinus. The infarcted mucosa may lead to a restriction of diffusion.
T2-weighted MR images may demonstrate intracerebral extension while contrast-enhanced MRI scans may demonstrate the perineural spread of disease. Magnetic resonance (MR) imaging is quite useful in identifying the intradural and intracranial extent of ROCM, cavernous sinus thrombosis, and thrombosis of cavernous portions of the internal carotid artery. Contrast-enhanced MR imaging can also demonstrate perineural spread of the infection. Although evidence of infection of orbital soft tissues may be seen on CT scans, MR imaging is more sensitive for this. Magnetic resonance imaging (MRI) of the facial sinuses and brain is superior to a CT scan in assessing the degree of tissue invasion and need for ongoing surgery.
Still, as with CT scans, patients with early ROCM may have normal MR images, and high-risk patients should always undergo surgical exploration with biopsy analysis of the suspected areas of infection. Imaging studies are nonspecific for ROCM, and diagnosing ROCM almost always requires histopathological evidence of fungal tissue invasion. Given the rapidly progressive nature of ROCM and marked increase in the mortality rate when the fungus penetrates the cranium, any diabetic patient with a headache and visual changes is a candidate for prompt evaluation using imaging studies and nasal endoscopy to rule out mucormycosis.
Initial CT and MRI scans are sometimes unremarkable in patients during the initial stages of rhinocerebral mucormycosis but show evidence of rapid infection progression 48-72 hours later. Therefore, serial radiographic imaging is important in patients with suspected mucormycosis. Due to the aggressive nature of mucormycosis CT or MRI scans should be obtained at frequent intervals to monitor disease extension and response to therapy.
Angiography Or Surgical Exploration:
Used in areas of anatomic complexity, like orbit, where reactive inflammation may be difficult to distinguish from true invasion with computed tomography or MRI.
_
Radiographic imaging often suggests severe sinusitis but is not specific for mucormycosis. Patients may also have superimposed bacterial sinusitis or bacterial meningitis following invasion of the dura mater. Computed tomography of the sinuses typically reveals mucosal thickening, air-fluid levels, and bony erosion.
CT radiograph demonstrating right-sided sinusitis (arrows) in patients with rhinocerebral mucormycosis.
_
Coronal view of magnetic resonance imaging of paranasal sinuses showing bilateral maxillary and ethmoidal sinusitis (2C), more involvement on right side, contrast study showed post contrast enhancement (2D).
_
Figure above shows three-dimensional computed tomographic scan of the facial skeleton of a patient with rhinocerebral mucormycosis showing extensive skeletal defects after orbitomaxillary resection.
CT scans produce 2-dimensional images of a “slice” or section of the body, but the data can also be used to construct 3-dimensional images.
_
Fungus ball in a 56-year-old man presenting with left nasal obstruction and chronic sinus pain. On noncontrast coronal CT image (below) of the paranasal sinuses, there is complete opacification of the atelectatic left maxillary sinus, with associated thickened walls, which is consistent with chronic sinusitis (arrowhead). There is a coarse nodular calcification replacing the left inferior turbinate, representing a fungus ball (arrow).
A fungus ball is an extramucosal fungal proliferation that completely fills one or more paranasal sinuses and usually occurs as a unilateral infection. It is mainly caused by Aspergillus spp in an immunocompetent host, but some cases of paranasal fungal balls reportedly have been caused by Mucor spp. A Mucor fungus ball is usually found in the maxillary sinus and/or the sphenoid sinus and may be black in color. Patients with mucormycosis, or a Mucor fungal ball infection, usually present with facial pain or headache.
___
MRI brain of a 45-year-old diabetic patient presented with orbital symptoms and fifth cranial nerve palsy.
(A) Magnetic resonance imaging (MRI) fat-saturated T1 post gadolinium coronal image shows lack of enhancement of the left cavernous sinus (arrow), corresponding to mucormycosis invasion. (B) MRI post gadolinium T1-weighted axial image shows perineural spread through the left fifth cranial nerve with brainstem and middle cerebellar peduncle invasion (arrows). (C) MR diffusion-weighted image shows restricted diffusion (arrow). (D) MR fat-saturated T1 post gadolinium coronal image shows interval progression in invasion of the cavernous sinus and carotid artery (arrow). Perineural (V3) spread through the foramen ovale is also noted (arrowhead). (E) MR angiography time of flight shows thinning of the left cavernous internal carotid artery and MCA branches (arrows). (F) MR flair axial image shows left hemispheric infarcts (arrows).
_____
The “Black Turbinate” Sign: An early MR Imaging finding of nasal mucormycosis:
Postcontrast T1-weighted coronal MR image shows non-enhancing soft tissue, “black turbinate sign,” within the left maxillary sinus (asterisk). The mucosa of the inferior turbinate and a portion of the left middle turbinate demonstrate focal lack of the enhancement (arrowheads).
Mucormycosis affects patients who are immunocompromised and is caused by fungi in the order Mucorales, including Mucor, Rhizopus, and Absidia species. The spores invade the nasal mucosa and are not phagocytized as in an immunocompetent individual which then germinate, forming angioinvasive hyphae that cause infarction of the involved tissue, resulting in a “dry” gangrene appearance. This devitalized mucosa appears on contrast-enhanced MR imaging as contiguous foci of non-enhancing tissue.
______
Imaging features of rhinocerebral mucormycosis: A study of 43 patients in 2018:
CT and MR imaging of 43 patients showed predominant involvement of the ethmoid (37, 86%) and maxillary (34, 79%) sinuses. Extension to the orbit (32, 76%) and face (24, 57%) preceded involvement of the deep skull base (5, 12%) and brain (13, 31%). CT showed minimally enhancing hypodense soft tissue thickening as the predominant finding in involved areas, while MRI showed T2 isointense to mildly hypointense soft tissue thickening and heterogeneous post contrast enhancement as the main finding. Bone erosion was seen less often (17, 40%), with rest (26, 60%) of the patients showing extrasinus extension across grossly intact appearing bones on imaging.
Conclusion:
CT and MRI show a spectrum of findings in rhinocerebral mucormycosis. Imaging plays a major role in assessing the extent of involvement and complications.
______
______
CBCT:
In most of the case of mucormycosis computed tomography (CT) is the cornerstone of modern medical radiology to diagnose the extension of lesion in rhinomaxillary region. Cone beam CT (CBCT), which is a comparatively recent scanning technology in dentistry, provides images equivalent to medical CT at reduced costs and radiation doses. The radiation dose to the patient with CBCT is 40 % lesser than that of multi-slice CT dose but is 3-7 times higher than conventional panoramic radiograph exposure dose. CBCT has been considered the examination of choice in various instances, since it gives high resolution imaging, diagnostic consistency and risk benefit assessment. However, usefulness of CBCT in diagnosis of mucormycosis is may be limited, especially if there are intracranial extensions.
CBCT shows bony erosion, involvement of sinus, and nasal cavity mucosal thickening in mucormycosis cases. These features may be seen in other diseases, but if CBCT shows bony erosion and sinus involvement in an immunocompromised patient, invasive fungal sinusitis should be one of the differential diagnosis.
_
Sagittal plane of CBCT image shows perforation of the palate with involvement of the maxillary sinus present.
_
CBCT revealed erosion of the maxillary bone with moth eaten appearance in three-dimensional view.
_
Invasive fungal infections with intracranial and orbital extensions are incompletely evaluated in CBCT. Besides, soft tissue infiltration with fat stranding are unlikely to be recognized in CBCT. In such cases, magnetic resonance imaging (MRI) or Multidetector computed tomography (MDCT) are better imaging modalities. Therefore, CBCT may have a role in the early stages of mucormycosis, providing detailed information about extensions into all the sinuses; but if there orbital and intracranial extensions, MDCT or MRI would be the imaging of choice.
_____
_____
Pulmonary mucormycosis:
The radiological manifestations of pulmonary mucormycosis are mostly non-specific. An abnormal chest roentgenogram result is present in >80% of patients. The reported findings include consolidation, cavitation, the air-crescent sign, the halo sign, the reversed halo sign, solitary or multiple pulmonary nodules or masses, bronchopleural fistulae, pulmonary artery pseudoaneurysms, lymphadenopathy and pleural effusion. Cavitation is observed in as many as 40% of cases, but the air-crescent sign is uncommon. CT can show findings that alter the management or diagnostic approach in as many as 26% of patients. The presence of the air-crescent sign often portends a poor prognosis if surgical therapy is delayed. Similar to invasive pulmonary aspergillosis, pulmonary mucormycosis is detected with the highest sensitivity when using high-resolution chest CT to determine the extent of the disease. This technique also usually finds evidence of the infection earlier than standard chest radiographs. The right lung is more commonly involved than the left, and there is a predilection for the involvement of the upper lobes, although the reason for this remains unknown.
_
Chest x-ray shows opacification of the left upper lobe, multiple air/fluid levels, and a 4 cm round mass-like lesion projecting over one of the air/fluid levels.
_
Serial chest radiographs of the subject in different stages of resolution. A: On admission, showing a large cavity in the right parahilar lung field with normal-looking surrounding parenchyma. B: Ten weeks after the initial presentation, showing unresolved consolidation in the right mid and upper lung fields. C: Following completion of antifungal therapy, demonstrating near-complete resolution of the radiopacity.
_
While chest radiography is often the initial test performed, its sensitivity and specificity for mucormycosis are low. Nonenhanced high-resolution CT scanning is the imaging modality of choice. The most common findings include pleural effusion, nodules, consolidation, and ground-glass opacities. With disease progression, consolidation can become multilobar. The reverse halo sign (i.e., a nodule with central ground-glass opacity and a ring of peripheral consolidation) strongly suggests pulmonary mucormycosis and is rarely seen in invasive aspergillosis. The halo sign (i.e., a nodule surrounded by ground-glass opacity) represents a lung infarct surrounded by alveolar hemorrhage; it is associated with invasive mold infections but can be present in bacterial or viral infections and noninfectious lung disease (e.g., Wegener granulomatosis, sarcoidosis, malignancy). Other findings such as the air crescent sign and hypodense sign are less specific and may occur in later stages.
_
Chest computed tomography (CT) scan above showing pulmonary mucormycosis with left basal consolidation and widespread nodules due to Rhizopus oryzae infection. The patient was receiving cytotoxic chemotherapy for myelodysplastic syndrome and had iron overload from numerous blood transfusions.
The CT scan of the same patient below shows resolution of pulmonary mucormycosis after 5 months of antifungal treatment.
_
Thrombosis of pulmonary vessels with fungal invasion often results in wedge-shaped infarcts, as illustrated in figure below.
Large wedge-shaped infarct (arrow) in patient with pulmonary mucormycosis.
_
One of the diagnostic challenges in patients suspected of having invasive fungal pneumonia is differentiating aspergillosis from mucormycosis. Chamilos et al studied 45 patients with IPA or PM. According to their findings, clinical clues that suggest PM rather than IPA include concurrent sinus infection and previous voriconazole therapy. Imaging findings that are more suggestive of PM than IPA include the presence of more than 10 lesions and the presence of pleural effusion.
_
Radiologically, multiple (≥10) nodules, and pleural effusion are reportedly more common in mucormycosis. Another finding on computerized tomography (CT) scan, which seems to indicate the presence of mucormycosis, is the reverse halo sign (RHS).
Reverse Halo Sign:
The reverse halo sign is defined as a ground-glass lesion with a peripheral rim of consolidation. While most fungal pneumonia shows nonspecific signs at imaging, the reverse halo sign has been shown to be a specific sign of mucormycosis, occurring in 19%–94% of patients with PM.
Coronal CT image above shows a lesion with a reverse halo sign.
The reverse halo sign can help distinguish between other fungal pneumonias, particularly IPA. In a study of 189 patients with fungal pneumonia and 32 with PM, Wahba H et al found eight patients in whom CT depicted a reverse halo sign. Seven of the eight patients had PM, and one patient had IPA. Nam et al showed a characteristic progression of CT findings in 15 patients with PM. In that imaging series, nodules, masses, or consolidation appearing with a CT halo sign progressed to a reverse halo sign, followed by central necrosis, and finally, an air crescent sign. This progression was associated with a tendency toward a gradual increase in the neutrophil count, although this association was not statistically significant. In another study, the CT scans of 24 patients with lung mucormycosis were compared to those of 96 patients with invasive lung aspergillosis. The RHS was more common in patients with mucormycosis (54%) than in those with aspergillosis (6%, P < .001), whereas some airway-invasive features, such as clusters of centrilobular nodules, peribronchial consolidations, and bronchial wall thickening, were more common in patients with aspergillosis. While these findings are not conclusive, they may be used as indicators to start aggressive diagnostic laboratory tests.
The reverse halo sign can represent other processes in different contexts. The differential diagnosis includes organizing pneumonia, bland pulmonary infarct, and lung cancer. The clinical context should help differentiate these entities. Most patients with PM have some form of immunodeficiency. In addition, mucormycosis shows relatively rapid progression at serial chest radiography compared with these other entities.
Another emerging imaging technique, which may eventually aid in the diagnosis and management of mucormycosis is the positron emission tomography-computed tomography (PET/CT) with [18F]-fluorodeoxyglucose (FDG). When feasible, endobronchial ultrasound-guided fine needle aspiration is also a useful diagnostic tool.
_
Radiographical signs suggestive of pulmonary mucormycosis are shown in figure below.
Four imaging signs can suggest pulmonary mucormycosis in an appropriate clinical setting. (A) Halo sign on CT, a ring of ground glass opacity surrounding a nodular infiltrate, which pathophysiologically represents a region of ischaemia, and which is also typical of invasive pulmonary aspergillosis (arrow). (D & B) Reversed halo sign on CT, also known as inversed halo or atoll sign, an area of ground glass opacity surrounded by a ring of consolidation (arrow). (E) Hypodense sign on MRI, T1 weighted, a central hypodensity in a lung consolidation or nodule, corresponding to a central area of necrosis caused by vascular obstruction with secondary lung infarction and sequestration. Magnetic resonance imaging shows pulmonary nodule with central hypodensity in right upper lobe (arrow), corresponding to a central area of necrosis caused by vascular obstruction with secondary lung infarct and sequestration. (C) Vascular occlusion sign on CT angiography, defined as interrupted vessel at the border of a focal lesion without depiction of the vessel inside the lesion or peripheral to the lesion (arrow). Particularly aggressive forms of mucormycosis are F. Contiguous spread on CT, presence of a mass or consolidation exhibiting invasion of adjacent organs by traversing tissue planes, including the diaphragm, chest wall, pleura, and spleen. (G) Typical rapidly progressive pulmonary mucormycosis on CT, associated with clinical deterioration. Day 8 and Day 15 CT scans showing a reversed halo sign.
______
In patients with haematological malignancy and suspected pulmonary mucormycosis, pulmonary CT scan is recommended for the detection of the reversed halo sign, an area of ground glass opacity surrounded by a ring of consolidation on thoracic CT, or vessel occlusion on CT pulmonary angiography. In diabetic patients with facial pain, sinusitis, proptosis, ophthalmoplegia, or newly diagnosed amaurosis, or both, cranial CT or MRI is strongly recommended to determine if sinusitis is present. If sinusitis is diagnosed, endoscopy is strongly recommended to diagnose mucormycosis. If disease of the eye or brain is suspected, MRI should be conducted in lieu of a CT scan due to substantially greater sensitivity. If mucormycosis is a potential diagnosis, biopsy is strongly recommended. Once mucormycosis has been proven in a patient with underlying malignancy, cranial, thoracic and abdominal imaging studies to determine the extent of disease are recommended with moderate strength. In view of the rapid progress of mucormycosis, weekly CT scans are strongly recommended, particularly in unstable patients.
______
______
Section-12
Differential diagnosis of mucormycosis:
Clinicians must have a high index of suspicion to differentiate rhinocerebral mucormycosis from other diseases having similar overlapping symptoms and involving similar sites. When evaluating a patient with suspected rhinocerebral mucormycosis, also consider the following:
-Bacterial orbital cellulitis
-Cavernous sinus thrombosis
-Rapidly growing orbital tumor
-Aspergillosis
-Pseudallescheria boydii infection (pseudallescheriasis)
-Fusarium infection (fusariosis)
Aspergillosis, pseudallescheriasis, fusariosis, nocardiosis, Wegener granulomatosis, pulmonary embolism, pulmonary tuberculosis and malignancy are also considered when evaluating a patient with suspected pulmonary mucormycosis.
Cutaneous disease considerations include ecthyma gangrenosum associated with pseudomonal infection and anthrax. Gastrointestinal disease considerations include bowel obstruction and ileocecal tuberculosis.
_
Selected Differential Diagnosis of Proptosis and Palatal Necrotic Ulcers:
|
Condition |
Characteristics |
|
Bacterial orbital cellulitis |
Proptosis, extraocular muscle impairment, diplopia, vision loss |
|
Cavernous sinus thrombosis |
Headache, loss of vision or visual acuity, ophthalmoplegia |
|
CNS aspergillosis |
More common in patients with neutropenia; blurred vision, proptosis, chemosis; vascular invasion with subsequent infarction and tissue necrosis is a hallmark |
|
Ecthyma gangrenosum |
Occurs as a result of sepsis from perivascular bacterial invasion; painless, nodular lesions affecting the skin or mucous membranes form into a central hemorrhage, ulcerate, and necrose |
|
Rhinocerebral mucormycosis |
More common in patients with hyperglycemia and associated metabolic acidosis; sinus involvement with spread to the CNS; ocular involvement (proptosis, periorbital edema, ophthalmoplegia, or vision loss); nasal and palatal necrosis |
_____
_____
Mucormycosis versus aspergillosis:
Mucormycosis must be differentiated from other conditions with similar presentation. Invasive fungal disease should be considered in any immunocompromised patient presenting with a new cranial neuropathy or ocular motility abnormality. The mucormycetes may be easily differentiated from other fungal agents of infection on examination of cytologic specimens or tissue sections. Mucormycetes in respiratory specimens are distinguished from the dimorphic fungal pathogens and yeasts since they do not produce a yeast phase in this site. They may be differentiated from dematiaceous fungi in clinical specimens by their lack of both darkly pigmented vegetative mycelium and septate hyphae. The major differentiation must be made between the mucormycetes, the other hyaline filamentous fungi, and members of the genus Candida. The morphology of the hyphae is important in making this distinction. Mucormycetes produce wide, coenocytic, ribbon-like hyphae with wide-angle branching, while the other filamentous fungi (often times Aspergillus spp.) present as septate hyphae. Candida spp. produce pseudohyphae and blastoconidia in clinical specimens. Features that help to differentiate these fungal pathogens microscopically are summarized in figure below:
Microscopic morphology of Rhizopus spp., Aspergillus spp., and Candida spp. in tissue. (A) Rhizopus spp. in tissue section stained with GMS. The mucoraceous zygomycetes produce wide ribbon-like aseptate hyphae in tissues. There is a great deal of variation of hyphal width. Branching occurs at wide angles nearing 90° (arrowheads). A frothy or bubbly tissue appearance may be seen in areas of tissue where the hyphae are cross-sectioned (upper left hand corner of the frame). (B) Aspergillus spp. in tissue section stained with GMS. Aspergillus spp. produce thin hyphae with relatively consistent diameters. Hyphae are septate, with no constriction of the fungus seen at the point of septation (arrowheads). Blastoconidia are not produced, although areas where hyphae are cross-sectioned may be confused with yeast cells (asterisk). Hyphae branch at acute angles of about 45° (arrow). (C) Candida spp. in tissue section stained with GMS. Fungal elements in tissue appear as pseudohyphae with blastoconidia. Fungal elements constrict or “bud” at sites of septation (arrowheads). Branching occurs at acute angles (arrow). Pseudohyphae are thin, and their diameter is very similar to that seen for the true hyphae of the Aspergillus spp.
_
Another figure showing difference in microscopic morphology:
(A) Septate fungal hyphae branching at a 45° angle (arrowhead), which is characteristic of Aspergillus spp. (magnification: 400×); (B) broad-based, aseptate hyphae, which are characteristic of Mucoromycete (white star), and the other septate fungal hyphae are Aspergillus spp. (black star) (magnification: 400×); (C) Mucoromycete characterized with broad-based, aseptate hyphae (arrowhead) (magnification: 400×).
_
Differentiating features of the Mucormycetes, Aspergillus spp., and Candida spp. in tissue sections:
|
Morphologic characteristic |
Mucormycetes |
Aspergillus spp. |
Candida spp. |
|
Hyphal type |
Aseptate or nearly aseptate hyphae; may also present as gnarled or “crinkled cellophane” balls in specimens |
Septate hyphae |
Pseudohyphae |
|
Hyphal width |
Variable and wide (5–25 μm wide) |
Consistently thin (2–3 μm wide) |
Consistently thin (2–3 μm wide) |
|
Blastoconidia |
Absent |
Absent |
Present |
|
Sporulation or conidiation |
Absent in tissue |
May be present if infected space communicates with air |
Not applicable |
|
Angioinvasion |
Present |
Present |
May be present |
_______
Mucormycosis and Aspergillosis are rare, life-threatening fungal infections with mortality rates reported to be over 50% despite surgical debridement and antifungal therapy. Rhizopus arrhizus, which is responsible for 70% of all cases of mucormycosis, is associated with a number of clinical diseases (rhino-orbital-cerebral, cutaneous, gastric, and pulmonary mucormycosis) in adults. This fungal infection is particularly recognized worldwide by the healthcare community due to its highly pathogenic nature, which is characterized by rapid tissue destruction and invasion across tissue planes. The major risk factors for mucormycosis include uncontrolled diabetes mellitus with ketoacidosis, hematological malignancy, stem cell and solid organ transplantations, iron chelation therapy with deferoxamine, and corticosteroid usage. The diagnosis of mucormycosis is usually made by the identification of causative fungal organisms by histopathological analysis of tissue specimens from patients with suggestive signs and symptoms. Cultures are only occasionally positive. Initial treatment of mucormycosis typically requires early aggressive surgical debridement of infected tissues, combined with administration of amphotericin B deoxycholate (Amb) or liposomal amphotericin B (L-AmB).
_
Aspergillosis is an infection caused by Aspergillus, allergic reaction, or fungal growth of a common mold that lives indoors and outdoors. The fungus which grows on dead leaves and decaying vegetation is commonly found fungus in our environment and most of us breathe in Apergillus spores every day without getting sick. Still, contracting the illness is very rare. However, those with weakened immune systems or lung diseases, both common in COVID-19 patients, are at high risk of infection.
More than 300 different types of Aspergillus have been identified and more are continuing to be identified. Most of these molds are harmless, however, some types can cause a variety of diseases in humans ranging from simple allergic reactions to life-threatening invasive disease. Collectively, this group of diseases is referred to as aspergillosis and is broadly broken down into three categories – allergic, chronic and invasive. Four main clinical types of aspergilloses are usually identified – allergic bronchopulmonary aspergillosis, aspergilloma, invasive aspergillosis, and chronic necrotizing aspergillosis. Aspergillosis rarely develops in healthy individuals; it is much more likely to develop in individuals with asthma, cystic fibrosis, diabetes mellitus, and lung disease or in individuals who have a weakened immune system, who take corticosteroid drugs or who have had a bone marrow or organ transplant. In most cases, aspergillosis develops when susceptible individuals breathe in (inhale) Aspergillus spores.
Aspergillus fumigatus, Aspergillus flavus, and Aspergillus niger account for most cases of human Aspergillus infection. A. fumigatus and A. flavus are the most common fungal contaminants of the sinuses. A. fumigatus is the most common species affecting immunocompromised patients while A. flavus is common in immunocompetent. Noninvasive aspergillosis (allergic fungal sinusitis and sinus mycetoma) rarely involves the orbit but they can be precursors of invasive fungal infection of the orbit.
_
According to CDC, there are different types of aspergillosis. Some types are mild, but some of them are very serious. However, Aspergillosis can’t spread between people or between people and animals from the lungs.
Allergic bronchopulmonary aspergillosis (ABPA): Occurs when Aspergillus causes inflammation in the lungs and allergy symptoms such as coughing and wheezing, but doesn’t cause an infection.
Allergic Aspergillus sinusitis: Occurs when Aspergillus causes inflammation in the sinuses and symptoms of a sinus infection (drainage, stuffiness, headache) but doesn’t cause an infection.
Azole-Resistant Aspergillus fumigatus: Occurs when one species of Aspergillus, A. fumigatus, becomes resistant to certain medicines used to treat it. Patients with resistant infections might not get better with treatment.
Aspergilloma: Occurs when a ball of Aspergillus grows in the lungs or sinuses, but usually does not spread to other parts of the body. Aspergilloma is also called a “fungus ball.”
Chronic pulmonary aspergillosis: Occurs when Aspergillus infection causes cavities in the lungs, and can be a long-term (3 months or more) condition. One or more fungal balls (aspergillomas) may also be present in the lungs.
Invasive aspergillosis: Occurs when Aspergillus causes a serious infection, and usually affects people who have weakened immune systems, such as people who have had an organ transplant or a stem cell transplant. Invasive aspergillosis most commonly affects the lungs, but it can also spread to other parts of the body.
Cutaneous (skin) aspergillosis: Occurs when Aspergillus enters the body through a break in the skin (for example, after surgery or a burn wound) and causes infection, usually in people who have weakened immune systems. Cutaneous aspergillosis can also occur if invasive aspergillosis spreads to the skin from somewhere else in the body, such as the lungs.
_
When to suspect mucormycosis over aspergillosis?
As the pathophysiology, mode of acquisition and underlying patient risk factors for mucormycosis are similar to aspergillosis, clinical distinction between the two entities is difficult. Both infections are acquired primarily through inhalation of spores, which are ubiquitous in the environment leading to sinopulmonary disease. Nevertheless, some scenarios and underlying risk factors and elements of the clinical and radiographic presentation should prompt a high index of suspicion for incipient mucormycosis (Table below). Other clinical forms of mucormycosis, including primary cutaneous, gastrointestinal, rhino-orbital, or single-organ involvement are less common in hematology patients compared with other classic risk groups, such as the diabetic patients (rhino-orbital or rhinocerebral form), trauma victims, or premature neonates (primary cutaneous form). Mucormycosis is also more likely to initially present as a disseminated infection in severely immunosuppressed patients, even though the extent of dissemination is rarely appreciated antemortem.
_
Factors favouring mucormycosis over aspergillosis:
|
Clues |
Comments |
|
Epidemiologic and host clues |
|
|
Institution with high background rates of mucormycosis |
Unique geographic exposures vs institution-specific differences in immunosuppression and anti-infective practices |
|
Iron overload |
The most reliable method of diagnosis is unclear |
|
Hyperglycemia with or without DM |
Degree and duration are undefined |
|
Prior voriconazole or echinocandin use |
The magnitude and specificity of such association are debatable |
|
Clinical, radiologic, and laboratory clues |
|
|
Community-acquired sinusitis |
Pansinusitis or ethmoid involvement are important clinical clues of mucormycosis |
|
Oral necrotic lesions in hard palate or nasal turbinates |
|
|
Chest wall cellulitis adjacent to a lung infarct |
Mucormycosis can spread across tissue planes |
|
Acute vascular event (e.g., MI, GI bleeding) |
Resulting from the acute haemorrhagic infarct caused by Mucorales |
|
Multiple (n > 10) nodules in CT and pleural effusion |
|
|
Reverse halo sign in CXR or CT |
Halo sign is as common in IPM as in IPA |
|
Presumed (by CT findings) fungal pneumonia with adequate (e.g., > 2 μg/mL) voriconazole levels |
|
|
Presumed (by CT findings) fungal pneumonia with repetitively negative GM and G-glucan serum levels |
|
DM indicates diabetes mellitus; MI, myocardial infarction; GI, gastrointestinal; CXR, chest x-ray; GM, galactomannan; IPM, invasive pulmonary mucormycosis; and IPA, invasive pulmonary aspergillosis.
______
______
Section-13
Treatment of mucormycosis:
_
Consultations:
Patient survival from mucormycosis requires rapid diagnosis and aggressive coordinated medical and surgical therapy. To that effect, consultations with various specialists are critical. Infectious disease consultation is warranted for management of antifungal therapy and coordination of medical care.
Surgical specialty consultations depend on the location of disease, as follows:
- Otolaryngology (ENT) consultation and neurosurgery consultation for rhinocerebral mucormycosis
- Thoracic surgery consultation for pulmonary involvement
- Gastroenterology (GI) surgery consultation for gastrointestinal involvement
- Plastic surgery consultation for cutaneous involvement
In addition, endocrinology consultation may be necessary for the management of unstable diabetes, hematology/oncology consultation may be needed for the management of issues related to underlying malignancy, and surgical intensive care unit (SICU) consultation is important for perioperative care.
___
Suspected mucormycosis requires urgent intervention, because of the often rapidly progressive and destructive nature of the infection. Delayed initiation of therapy is associated with increased mortality. Maximizing survival rates requires rapid diagnostic and therapeutic intervention, including immediate involvement of a multidisciplinary medical, surgical, radiological, and laboratory based team. Readily available guidance is important to ensure efficient diagnosis and treatment, and to optimize patient prognosis. Optimal management depends on recognising disease patterns and the available diagnostic and therapeutic options, which differ between the regions of the world. Patients with mucormycosis should be treated in a tertiary care center with subspecialty units experienced in the management of this condition and its underlying risk factors. Correction of the underlying abnormality, prompt initiation of liposomal amphotericin B therapy, and surgical resection are critical. Mucormycosis infection requires team of doctors such as an Infection disease expert, ENT Surgeon, Maxillofacial surgeon, ophthalmologist, and Neurologist.
____
Effective treatment of mucormycosis includes (1) early and rapid diagnosis; (2) reversal of underlying risk factors; (3) surgical debridement where applicable; and (4) prompt antifungal therapy.
-1. First, early diagnosis depends on a high index of clinical suspicion. Onset of mucormycosis can be nonspecific with malaise and fever. With orbital involvement, there can be orbital cellulitis, orbital apex syndrome, and cavernous sinus thrombosis. Occurrence of mental state changes, hemiparesis, or seizures suggest intracranial extension. Endoscopy and radiography appearance lag behind clinical progression, so in suspicious cases, blind biopsies of sinus mucosa or thickened extraocular muscles are warranted. Computed tomography findings are nonspecific, with sinusitis or thickening of extraocular muscles, but may be useful in delineating the extent of the infection and in guiding surgical debridement. Magnetic resonance imaging is more sensitive than computed tomography for detecting orbital and cerebral involvement, and may demonstrate focal lack of enhancement with devitalised sinus mucosa. Definitive diagnosis is made by demonstration of fungal hyphae in tissue specimens. Fungal invasion may be patchy, so multiple biopsies may be required for definitive diagnosis.
Early diagnosis is important because small, focal lesions can often be surgically excised before they progress to involve critical structures or disseminate. Unfortunately, there are no serologic or PCR-based tests to allow rapid diagnosis. As mentioned, autopsy series have reported that up to half the cases of mucormycosis are diagnosed postmortem, underscoring the critical need to maintain a high index of clinical suspicion and to aggressively pursue diagnostic biopsy. The initial imaging study is frequently negative or has subtle findings. Radiographic findings lag behind clinical progression in this disease, and a negative imaging study does not provide a rationale to delay more aggressive diagnostic maneuvers (e.g., endoscopy with biopsy) if clinical suspicion is high. The appearance of tissue at endoscopy may also lag behind invasion, as the mucosa can appear pink and viable during the initial phase of fungal invasion. Therefore, if the suspicion for disease is high, blind biopsies of sinus mucosa and/or thickened extraocular muscles are warranted to make the diagnosis.
Finally, time is of the essence in the management of mucormycosis. Because patients with rhinocerebral disease may initially present with normal mental status and appear clinically stable, the urgency for establishing the diagnosis is frequently underappreciated. The key concept is that initial spread of the fungus to the brain may be relatively asymptomatic. Once the fungus has penetrated the cranium or entered the major intracranial vasculature, mortality increases substantially. Additionally, starting the patient on an antifungal is not definitive therapy, since surgery may be a key addition to the treatment strategy. The sensitivity of the organisms varies considerably, so that a patient on amphotericin B alone may be receiving completely ineffective therapy during the diagnostic period. Minutes and hours count, and if the clinical suspicion is high, the workup should proceed on an emergent basis even if the patient currently appears clinically stable. Indeed, delayed diagnosis has been associated with a dramatically worse outcome. One strategy to expedite the workup is to rely upon frozen sections to guide further diagnostic and therapeutic decisions rather than waiting for fixed and stained histopathology from a biopsy. Use of frozen sections in this setting has been shown to shorten the time to diagnosis and has been associated with improved outcomes in two recent case series.
Initiation of polyene therapy within 5 days after diagnosis of mucormycosis was associated with improvement in survival, compared with initiation of polyene therapy at ≥6 days after diagnosis (83% vs. 49% survival). Therefore, establishing an early diagnosis of mucormycosis is critical to enable early initiation of active antifungal therapy.
-2. Second, it is critical to reverse any underlying immunocompromised state. This includes aggressive management to restore euglycemia and normal acid base in diabetic ketoacidosis patients, stopping immunosuppressive agents, and avoiding iron and blood transfusions. Interrupt deferoxamine therapy; hydroxypyridine chelating agents may be substituted for deferoxamine It is critical to reverse or prevent underlying defects in host defense when treating patients with mucormycosis. Immunosuppressive medications, particularly corticosteroids, should be administered at reduced dosages or stopped if at all possible.
-3. Third, surgical debridement is critical, as blood vessel thrombosis and resulting tissue necrosis impedes delivery of necessary antifungal agents to the site of infection. Involved tissues rarely bleed, so surgeons should debride until well-perfused bleeding tissue is encountered. Daily repeated debridement may be needed, and subsequent surgeries may be needed for reconstruction when the infection subsides.
Surgery when needed and possible must be very aggressive. Not only necrotic tissues but also surrounding infected healthy-looking tissues should be removed, as the speed of the extension of the infection by the Mucorales hyphae is enormous. Surgery is particularly useful in rhino-orbito-cerebral infection and in soft tissue infection. In cases of a single localized pulmonary lesion, it may be helpful. It is obviously impossible in cases of disseminated mucormycosis or when infection of difficult-to-reach organs (i.e., certain parts of brain or lung parenchyma close to great vessels) exists. In cases with a successful outcome, plastic surgery will be used to correct disfigured body areas.
-4. Fourth, first-line antifungal treatment includes lipid-based amphotericin B, which destroys the cell wall of the fungus. This is given systemically, locally irrigated, and packed in affected areas. Early treatment after an accurate diagnosis is crucial for achieving a better prognosis in treating mucormycosis. Treatment consists of systemic antifungal treatment, causative therapy for the underlying disease, and radical surgical debridement. Mucormycosis is a serious infection and needs to be treated with prescription antifungal medicine, usually amphotericin B, posaconazole, or isavuconazole. These medicines are given through a vein (amphotericin B, posaconazole, isavuconazole) or by mouth (posaconazole, isavuconazole). Other medicines, including fluconazole, voriconazole, and echinocandins, do not work against fungi that cause mucormycosis. Conventional antifungal treatment involves injection amphotericin B (dose 1–1.5 mg/kg/day) and its lipid formulation (5 mg/kg/day). Lipid formulations of amphotericin B are considered superior over amphotericin B deoxycholate in the treatment of mucormycosis, as the former has less nephrotoxic effects, better penetration to the brain, and has superior immunomodulatory effects.
The European Society of Clinical Microbiology and Infectious Diseases and European Confederation of Medical Mycology recommended immediate first-line antifungal therapy followed by radical surgical debridement. Since thrombosis and tissue necrosis associated with mucormycosis lead to poor penetration of antifungal agents at the sites of infection and compromise their efficacy, surgical debridement must be performed very aggressively. Most debridement may cause large defects which may require rehabilitation by hard- and soft-tissue reconstruction. Antifungal therapy alone and surgical therapy by itself are ineffective.
Apart from treating the underlying causes such as metabolic diseases, other options include leukocyte transfusions, treatment with interferon-gamma, hyperbaric oxygenation, iron-chelating agents, and colistin. Correcting acidosis, hyperglycemia, and electrolyte abnormalities is critical to the successful management of this condition. Poor survival is associated with delayed diagnosis and treatment (61% if commenced within first 12 days of presentation compared to 33% if after 13 days), cerebral involvement (hemiparesis or hemiplegia), bilateral sinus involvement, renal disease, and possibly facial necrosis.
Owing to the aggressive nature of mucormycosis, the mortality rate is high. Although an infrequent diagnosis, there should be a high index of suspicion for mucormycosis in patients with predisposing factors and orbital symptoms, in order to prevent treatment delay.
______
Current targets of therapy for mucormycosis:
As a result of recent translational research, funded by the US National Institutes of Health and industry, agents are now available to attack the Mucorales at multiple biochemical targets as seen in the figure below:
As a result of recent translational research, strategies are available to attack 4 biochemical targets in Mucorales. These targets include (1) polyene binding to ergosterol in the cell membrane, resulting in creation of pores in the membrane; (2) posaconazole inhibition of cytochrome p450 14-α-demethylase, blocking synthesis of cell membrane-stabilizing ergosterol; (3) echinocandin inhibition of cross-linking of β-glucan in the fungal cell wall; and (4) deferasirox iron chelation therapy, blocking uptake of iron, which is essential for fungal growth. In addition, adjunctive therapy with host immune enhancing strategies, such as (5) granulocyte transfusions and (6) cytokine therapy, are possible. Granulocytes can damage the fungal cell and can be activated by recombinant cytokines, including granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), and interferon-γ (IFN-γ). Polymorphonuclear leukocytes also can be delivered to the site of infection in neutropenic hosts by granulocyte transfusions. Polymorphonuclear leukocytes and lipid formulations of amphotericin B act synergistically to damage hyphae of Rhizopus species.
_____
Treatment approaches to mucormycosis:
The ability to treat mucormycosis effectively depends on the availability of the surgical techniques and antifungal drugs. If all treatment options are available, one should follow the management pathways detailed in figure below:
Figure above shows optimal treatment pathways for mucormycosis in adults.
_____
Treatment approaches for CAM:
Standard approaches for the management of CAM are similar to the management of mucormycosis in non-COVID-19 patients, which are outlined above. For instance, early diagnosis of mucormycosis based on clinical, microbiological, histopathological, or radiological features is key for successful management. Controlling or eliminating the underlying predisposing factors such as diabetes, ketoacidosis, corticosteroids intake, immunomodulators, and any immunosuppressant agents are crucial and help in preventing worsening of the disease. Invasive mucormycosis is a medical emergency condition and frontline antifungal therapy with amphotericin B (optimally lipid-based formulations) should be initiated at the earliest, as a delay of ≥6 days in certain patients is associated with 2-fold increase in mortality rate at 12 weeks. Management of mucormycosis is a team effort depending on presentation and extent of involvement. The team generally constitutes an infectious diseases specialist, microbiologist, ENT surgeon, general surgeon, maxillofacial surgeon, intensivist, ophthalmologist, neurologist, histopathologist, radiologist, and pharmacologist.
Surgical management:
Early and aggressive surgical resection and debridement of affected tissues is necessary for local control of mucormycosis. In ROCM infection complete debridement of the external infected tissues including bones as well as internal tissues by endoscopic debridement or orbital exenteration results in higher survival rates. In cases of recurrence, repeated resection and debridement is necessary. In pulmonary mucormycosis, resection of the affected lung (if localized or single lobe is involved) may be beneficial to the patient by preventing him/her from undergoing emergency surgery for controlling bleeding in a later course of disease further increasing the chance of survival.
Antifungal therapy:
Amphotericin B (if available as lipid-based formulations) is the drug of choice for first line therapy of mucormycosis. Among azoles, posaconazole and isavuconazole are effective, whereas itraconazole has shown in-vitro activity against Mucorales. Of all the different injectable amphotericin B formulations (liposomal amphotericin B, amphotericin B deoxycholate, amphotericin B lipid complex, amphotericin B colloidal dispersion) available, liposomal amphotericin B is strongly recommended at a dose of 5mg/kg per day in 200ml of 5% dextrose over 2-3 h for 3-6 weeks.
The current epidemic of mucormycosis in India has led to limited availability or even nonavailability (in certain regions) of amphotericin B, posaconazole or isavuconazole, making the situation imperative to use alternative antifungals. In the current Indian scenario where, antifungal drugs are not easily available, the recommendation of Fungal Infection Study Forum is depicted in figure below.
Treatment algorithm for CAM prepared by the Fungal Infections Study Forum (modified)
[CVC- central venous catheter; PICC – peripherally inserted central catheter; TDM – therapeutic drug monitoring]
Itraconazole is generally not recommended for the management of mucormycosis. In fact, there are only few case reports available using itraconazole either alone or in combination with amphotericin B for mucormycosis. However, in vitro antifungal susceptibility testing has consistently shown itraconazole is active against Mucorales but trial has not been done to evaluate its effect in vivo. In a recent multicentric study from India, it has been shown that the minimum inhibitory concentration values of itraconazole against all species of Mucorales are less than the epidemiological cut-off value (2 µg/ml) defined for R. arrhizus by Espinel Ingroff et al. Hence when amphotericin B, isavuconazole and posaconazole are not available, itraconazole therapy, 200 mg thrice a day for 3-6 weeks may be considered. Intravenous therapy followed by itraconazole suspension are the preferred formulations. If only itraconazole capsules are available, they should be taken with acidic beverages such as cola. Concomitant use of proton-pump inhibitors decreases the absorption of this drug. Therapeutic drug monitoring should be done after 5 days of treatment with itraconazole.
Although a few reports on the use of iron chelators, especially deferasirox have shown improvement along with antifungal therapy, the evidence is not robust. Deferasirox may be considered in those patients who have diabetes as a risk factor but should probably be avoided in patients with a haematological malignancy.
______
_____
Antifungal Agents for Mucormycosis:
The two main classes of antifungal medications used to treat mucormycosis are the polyenes (amphotericin formulations) and triazoles (isavuconazole and posaconazole). Amphotericin B and isavuconazole are the two agents currently FDA approved for the primary therapy of mucormycosis. Posaconazole can be used off-label for salvage treatment in patients intolerant to amphotericin B. It has also been used as step-down therapy after initial control of the disease with amphotericin.
Surgical debridement should be performed whenever feasible in parallel to antifungal treatment. The drug of choice is liposomal amphotericin B. In case of renal failure, posaconazole or isavuconazole were shown to be effective. If a patient is intolerant to liposomal amphotericin B, its dose can be reduced, but should stay ≥5 mg/kg bodyweight. In case of extensive disease, rapid progression, or poor general condition, the addition of isavuconazole or posaconazole can be considered. Treatment should be continued until resolution of initially indicative findings on imaging and reconstitution of host immune system. Isavuconazole or posaconazole may be administered as maintenance therapy.
Amphotericin B binds with ergosterol, a component of fungal cell membranes, forming pores that cause rapid leakage of monovalent ions (K+, Na+, H+ and Cl−) and subsequent fungal cell death. This is amphotericin B’s primary effect as an antifungal agent. Four formulations of Amphotericin B are available: conventional amphotericin B (C-AMB, also known as amphotericin B deoxycholate), amphotericin B colloid dispersion (ABCD), liposome amphotericin B (L-AMB), and amphotericin B lipid complex (ABLC). Lipid formulations of amphotericin B (amphotericin B lipid complex and liposomal amphotericin B) are newer preparations of amphotericin B with fewer side effects and are reportedly more effective against ROCM. Isavuconazole and posaconazole inhibits the synthesis of ergosterol, a key component of the fungal cell membrane, through the inhibition of cytochrome P-450 dependent enzyme lanosterol 14-alpha-demethylase. This enzyme is responsible for the conversion of lanosterol to ergosterol.
In patients with hematologic malignancy, posaconazole is superior to other triazoles as prophylaxis for invasive mold infection. Isavuconazole is a novel triazole antifungal agent with many advantages, including excellent oral bioavailability, linear and predictable pharmacokinetics, and minimal CYP450 interactions. Owing to its safety, tolerability, and comparable efficacy to amphotericin B, isavuconazole is promising as a primary and salvage therapy. Further study is needed prior to its use as primary mold prophylaxis in the setting of prolonged neutropenia.
Echinocandin agents, including anidulafungin, caspofungin, and micafungin, competitively inhibit the beta-1,3-D-glucan synthase enzyme complex. In Candida species, beta-glucan depletion causes cell lysis via loss of resistance to osmotic force; in Aspergillus, it is fungistatic owing to impaired growth at hyphal branching points. Echinocandins have minimal activity against the Mucorales, which contain little or no beta-1,3-D-glucan. Some studies have suggested combination therapy with amphotericin and an echinocandin may improve survival; however, a more recent retrospective study showed no such mortality benefit. There is no current evidence-based recommendation for the addition of an echinocandin to amphotericin B or isavuconazole for the treatment of mucormycosis.
The benefit of combination therapy with different classes of antifungals (including echinocandins) is uncertain. Despite advances in medical management, surgical evaluation is essential in the management of mucormycosis, and overall mortality rates remain high.
__
A major obstacle for clinicians to choose among the current available antifungal agents in treating mucormycosis is the lack of available clinical trials. Prospective interventional study of mucormycosis has been impractical for several reasons. First, although the disease is unusually deadly, it occurs at a lower frequency relative to other opportunistic infections. By extrapolation from studies of Aspergillus infection, which is more common, dozens to possibly even hundreds of trial sites and multiple years would be required to accrue sufficient patients to adequately power a standard phase III superiority study. Such a study would undoubtedly cost tens of millions of dollars, and mucormycosis cases represent an insufficient potential market to spur any pharmaceutical company to sponsor such a study.
An additional barrier to clinical trials of mucormycosis is the abysmal rate of success of monotherapy. Because of this low success rate, it might be considered unethical to randomize patients in a clinical trial to any “less intensive” regimen (i.e., standard-dose versus high-dose monotherapy, monotherapy versus combination therapy, etc.). For these reasons, prospective interventional trials have not been performed. Lacking any significant clinical trial data, physicians have been forced to rely upon anecdotal case reports, limited retrospective reviews, and unpublished observations in determining the first-line therapy for mucormycosis. Such reports are intrinsically subject to publication and observer bias and allow no comparison of the relative efficacies of various treatment strategies. For these reasons, animal models of mucormycosis are essential to provide well-controlled comparative analyses of antifungal therapies.
_
Several murine models have been developed to study mucormycosis in vivo, including intravenous, intranasal, and intrasinus diabetic mice models. Additionally, neutropenic, corticosteroid, and deferoxamine-treated mouse models and a deferoxamine-treated guinea pig model have been reported. More rarely, immunocompetent mice have been studied. A variety of species have been utilized in these models, including R. oryzae, R. microsporus, and Mucor and Absidia spp. There is no clear advantage to any one of these models in evaluating the efficacy of different antifungal regimens, and none of the models completely accurately recapitulates the normal route of infection (inhalation) of the majority of mucormycosis infections. Nevertheless, given the lack of controlled clinical trials for mucormycosis, these models are essential to evaluating the relative merits of different antifungal strategies.
_
From January, 2018, authors from 33 countries in all United Nations regions analysed the published evidence on mucormycosis management and provided consensus recommendations addressing differences between the regions of the world as part of the “One World One Guideline” initiative of the European Confederation of Medical Mycology (ECMM). First-line treatment with high-dose liposomal amphotericin B is strongly recommended, while intravenous isavuconazole and intravenous or delayed release tablet posaconazole are recommended with moderate strength. Both triazoles are strongly recommended salvage treatments. Amphotericin B deoxycholate is recommended against, because of substantial toxicity, but may be the only option in resource limited settings.
First-line antifungal monotherapy:
First-line treatment with liposomal amphotericin B 5–10 mg/kg per day is strongly supported across all patterns of organ involvement. If substantial renal toxicity develops, the dose can be reduced as necessary, but doses below 5 mg/kg per day are recommended with marginal strength only. Doses should not be slowly increased over several days; rather, the full daily dose should be given from the first treatment day. Amphotericin B lipid complex 5 mg/kg per day is recommended with moderate strength for patients without CNS involvement. Use of amphotericin B deoxycholate is discouraged whenever alternatives are available. Isavuconazole is recommended with moderate strength for the first-line treatment of mucormycosis. Marginal recommendation includes use of posaconazole oral suspension, and posaconazole delayed release tablets and infusion for first-line treatment.
First-line antifungal combination therapy:
In animal models, some antifungal combinations have shown the potential to improve cure and survival rates with no antagonism noted. Results from some patient series are promising. However, a historical control study and a propensity score analysis failed to show benefits of double and triple antifungal combinations in patients with haematological malignancy. In trauma patients, specifically in blast injury, more than one mould species can cause mixed infection warranting empirical combination therapy with liposomal amphotericin B and either posaconazole or voriconazole. The downsides of combination therapy are unclear aside from potential added toxicity, drug interactions, and cost.
There are no definitive data to guide the use of antifungal combination therapy. Limited data support combinations of polyenes and azoles or polyenes plus echinocandins. Combination therapy can be rationally given due to lack of enhanced toxicity with possible but unproven benefit; however, data are too limited to support this beyond a marginal recommendation.
Prophylaxis:
In neutropenic patients or those with graft versus host disease, primary prophylaxis with posaconazole delayed release tablets is recommended with moderate strength, and prophylaxis with oral suspension is recommended with marginal strength to prevent mucormycosis.
Secondary prophylaxis:
In immunosuppressed patients with previous diagnosis of mucormycosis, surgical resection and continuation or restart of the last drug effective in that patient is strongly recommended.
_
Other azoles:
Fluconazole and voriconazole have no meaningful activity against agents of mucormycosis in vitro and in experimental models. Clinical data have suggested that use of voriconazole for prophylaxis or empirical therapy may explain an increase in incidence of mucormycosis. Whether voriconazole really impacts on incidence or just allows for longer survival and, therefore, exposure to other opportunistic pathogens of high-risk patients successfully treated for voriconazole-susceptible fungal infection remains a matter of debate.
Itraconazole has variable in vitro activity with differences between and within genera, best activity being reported in Lichtheimia spp. In an experimental model, itraconazole reduced mortality of immunocompetent mice infected with Lichtheimia corymbifera and Apophysomyces elegans but not in animals infected with Rhizopus microspores. Despite rare case reports, data are insufficient to support its use as monotherapy for mucormycosis in clinical practice, although Indian studies show that when amphotericin B, isavuconazole and posaconazole are not available, itraconazole therapy, 200 mg thrice a day for 3-6 weeks may be considered.
_
Echinocandins:
Caspofungin, anidulafungin and micafungin have no efficacy against agents of mucormycosis as single agents when tested by standard techniques in vitro. However, Rhizopus oryzae expresses the target enzyme of echinocandins, 1,3-D-glucan synthase, and caspofungin has shown some efficacy in an animal model of infection but with an unexplained inverse-dose response relationship: low doses were more effective in reducing mortality than high doses. This inverse dose-response relationship may be similar to the paradoxical effect previously described with caspofungin against Candida albicans. No clinical data are available with echinocandin monotherapy in mucormycosis and occurrence of mucormycosis has been documented in HM currently receiving or recently exposed to caspofungin. However, efficacy of combination therapy including an echinocandin has been reported.
_
Flucytosine:
Flucytosine lacks activity against agents of mucormycosis.
Terbinafine:
Despite some in vitro activity, oral terbinafine failed to show efficacy in a murine model of mucormycosis, although absorption was demonstrated. No clinical data are available for terbinafine monotherapy in mucormycosis.
______
______
Antifungal Resistance in Mucorales:
Mucor Mucoraceous fungi are resistant to most antifungals in vitro, including voriconazole. Amphotericin B is the most active drug, except for some Cunninghamella and Apophysomyces isolates. Posaconazole and isavuconazole are also active, while itraconazole and terbinafine show some activity against certain strains. Mucorales species are intrinsically resistant to many antifungal agents, including fluconazole, voriconazole, flucytosine, and echinocandins. There seems to be some correlation between the degree of susceptibility of Mucorales isolates to amphotericin B and outcomes. In a small study by Lamoth et al. MIC ≤0.5 μg/ml was significantly associated with better 6-week outcome. A similar correlation was reported in mice, where the efficacy of posaconazole was higher in animals infected with strains of Rhizopus oryzae that had lower MICs. There are still not enough data to make a strong recommendation, but the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) / European Confederation of Medical Mycology (ECMM) guidelines recommend susceptibility testing to guide treatment of mucormycosis and to establish epidemiological knowledge.
Recent sequencing of the Rhizopus oryzae suggests that the fungus has a 2-10-fold enrichment in gene families involved in ergosterol biosynthesis, cell wall biogenesis, cell growth, iron uptake, and production of known virulence factors compared to the more common Aspergillus fumigatus. This genetic arsenal may explain the remarkable capacity of this pathogen for rapid growth in hostile environments (i.e., host inflammatory milieu), as well as its resistance to multiple antifungal classes.
Data on the susceptibility of Mucorales are limited, and MIC testing is rarely performed outside of research laboratories or regional mycology reference testing centers. Standardized methods for MIC testing have been developed for filamentous fungi, but the sometimes rapid growth of Mucorales often makes test results inconsistent or difficult to interpret. Moreover, interpretative resistance breakpoints have not been defined for Mucorales. Therefore, MIC testing does not play a routine role in detecting resistance of managing patients with mucormycosis.
_
Mechanisms of antifungal resistance:
Resistance to antifungal drugs can occur through various mechanisms. These can include: (1) nonsynonymous point mutations within the gene encoding the target enzyme leading to alterations in the amino acid sequence, (2) increased expression of the target enzyme through increased transcription of the gene encoding it, (3) decreased concentrations of the drug within the fungal cells due to drug efflux, (4) changes in the biosynthetic pathway resulting in reduced production of the target of the antifungal drugs. For the azoles, each of these mechanisms have been associated with reduced susceptibility in Candida albicans, and several are associated with resistance in other Candida species. Alterations in the target enzyme (lanosterol 14-α-demethylase) due to point mutations in the encoding gene ERG11 leads to decreased susceptibilities to the azoles. Overexpression of the CDR1, CDR2, and MDR1 genes that encode for efflux pumps leads to azole resistance. Azole resistance has also been documented in A. fumigatus and is due to point mutations within the CYP51A gene that encodes the enzyme responsible for converting lanosterol to ergosterol. In isolates with environmental exposure to the azoles tandem repeats in the promoter region along with along with point mutations in the gene (e.g., TR34/L98H and TR46/Y121F/T289A) have been found and cause increased expression of CYP51A.
_____
_____
Role of Surgery:
Mucormycosis is frequently rapidly progressive, and antifungal therapy alone is often inadequate to control the infection. The numerous agents of mucormycosis have a broad range of susceptibilities to antifungal agents; some strains may be highly resistant to amphotericin B. Furthermore, the hallmark angioinvasion, thrombosis, and tissue necrosis of this disease result in poor penetration of anti-infective agents to the site of infection. Therefore, even if the causative organism is susceptible to the treating antifungal agent in vitro, the antifungal may be ineffective in vivo. Finally, surgery is necessary due to the massive amount of tissue necrosis occurring during mucormycosis, which may not be prevented by killing the organism and even dead hyphae can cause ischemia and necrosis. Surgical debridement of infected and necrotic tissue should be performed on an urgent basis. The mainstay of therapy is extensive debridement of all infected and necrotic tissue, with drainage of all sinus and abscess fluid collections. An endoscopic approach is preferred over the open surgery in patients with early, limited disease, or with significant medical comorbidities. Open surgeries are preferred for extensive disease, and include maxillectomy, orbital exenteration and/or craniofacial resection. The extent and timing of surgical debridement necessary to maximize outcomes of mucormycosis has never been defined.
_
The most comprehensive review of mucormycosis so far, that included 929 cases published between 1885 and 2005, found higher survival rates for patients treated with antifungal therapy and surgery (328 of 470, 70%) compared with patients treated with d-AmB alone (51 of 90, 57%) or surgery alone (324 of 532, 61%). Similarly, a review of 106 cases of solid organ transplant recipients with mucormycosis reported from 1970 to 2002 found a reduced mortality rate (34.3%) among patients receiving surgery in combination with antifungal treatment compared to those with antifungal therapy alone (62.5%). A favorable outcome was associated with limited disease accessible to surgical intervention and early surgery together with antifungal therapy.
_
In rhinocerebral mucormycosis, early surgical excision of the infected sinuses and appropriate debridement of the retro-orbital space can often prevent the infection from extending into the eye, thereby obviating the need for enucleation and resulting in extremely high cure rates (>85%). Repeated surgical exploration of the sinuses and orbit may be necessary to ensure that all necrotic tissue has been debrided and the infection has not progressed. Published case series continue to support the need for surgical debridement to optimize outcomes. For example, in a case series totaling 49 patients with rhinocerebral mucormycosis, the mortality was 70% in cases treated with antifungal agents alone versus 14% in cases treated with antifungal agents plus surgery. Similarly, in a combined series of rhinocerebral, cutaneous, and pulmonary mucormycosis, 11 of 17 (65%) patients treated with surgery plus antifungal agents survived the infection, compared to zero of seven (0%) patients treated with antifungal agents alone. Clearly there is the potential for selection bias in these case series, as patients who do not undergo surgery may have fundamental differences in severity of illness or comorbidities. Nevertheless, the observational clinical data support the concept that surgical debridement is necessary to optimize cure rates.
In patients with pulmonary mucormycosis, surgical treatment plus antifungal therapy also greatly improves outcome compared to the use of antifungal therapy alone. In one series, the mortality of patients treated with antifungal agents alone was 68%, versus 11% in patients treated with antifungal agents plus surgery.
Finally, localized (nondisseminated) cutaneous mucormycosis treated with aggressive surgical debridement and adjunctive antifungal therapy has a mortality of <10%. A similar experience has been described with isolated renal mucormycosis. However, because surgical debridement of necrotic tissue is frequently highly disfiguring, if the patient survives the acute phase of the disease, major reconstructive surgery may be necessary.
_
Mucormycosis in intensive care unit: surgery is a major prognostic factor in patients with hematological malignancy, 2020 study:
Mucormycosis was diagnosed in 74 patients during the study period. Among them, 60 patients (81%) were immunocompromised: 41 had hematological malignancies, 9 were solid organ transplant recipients, 31 received long-term steroids, 11 had diabetes, 24 had malnutrition. Only 21 patients survived to ICU stay (28.4%) with a median survival of 22 days (Q1–Q3 = 9–106) and a survival rate at day 28 and day 90, respectively, of 35.1% and 26.4%. Survivors were significantly younger (p = 0.001), with less frequently hematological malignancies (p = 0.02), and less malnutrition (p = 0.05). Median survival in patients with hematological malignancies (n = 41) was 15 days (Q1–Q3 = 5–23.5 days). In this subgroup, curative surgery was a major factor associated with survival in multivariate analysis (odds ratio = 0.71, [0.45–0.97], p < 0.001).
Conclusion:
Overall prognosis of mucormycosis in ICU remains poor, especially in patients with hematological malignancies. In this subgroup of patients, a therapeutic strategy including curative surgery was the main factor associated with survival.
_____
Life after surgery for mucormycosis:
Mucormycosis can lead to loss of the upper jaw and sometimes even the eye. Patients would need to come to terms with loss of function due to a missing jaw — difficulty with chewing, swallowing, facial aesthetics and loss of self-esteem. Be it the eye or upper jaw, these can be replaced with appropriate artificial substitutes or prostheses. While prosthetic replacement of the missing facial structures can commence once the patient stabilises after surgery, it is important to reassure patient about the availability of such interventions instead of leaving him to panic with the sudden unforeseen loss, augmenting a post-Covid stress disorder which is already a reality. Prosthetic reconstruction can be effected after surgery, but interim solutions should be planned even before surgery of the jaws for better long-term outcomes. Prosthetic reconstruction can ensure that the cure is not more dreadful than the disease itself.
_____
_____
Adjunctive Therapy:
Reversal of immunosuppression is an important pillar of therapy for mucormycosis, along with surgery and appropriate early antifungal agents. Most patients who die of this disease have poor recovery of bone marrow function or require prolonged immunosuppressive therapy (such as those with GVHD). Therefore, any effort to reverse neutropenia in hematology patients should be made, by using hematopoietic growth factors, or in selected cases, by white cell transfusions. Patients with immunosuppression from corticosteroids, such as patients with autoimmune diseases should be tapered or transitioned to alternative non-steroidal therapy, if possible. Patients with HIV/AIDS should be started on anti-retroviral therapy, in order to restore their immunity. Aggressive glycemic control is paramount for patients with uncontrolled diabetes and/or ketoacidosis. Reversal of acidemia by administration of sodium bicarbonate is able to partially block the ability of Rhizopus oryzae to invade endothelial cells, and to restore host iron chelation and neutrophil function.
-1. Hyperbaric oxygen
It is hypothesized that hyperbaric oxygen might be useful for treating mucormycosis in conjunction with standard therapy because higher oxygen pressure improves the ability of neutrophils to kill the organism. High oxygen concentrations may improve wound healing through release of tissue growth factors, and putative oxidative killing mechanisms of amphotericin B. Additionally, high oxygen pressure inhibits the germination of fungal spores and growth of mycelia in vitro. Whether hyperbaric oxygen actually improves the outcome of patients with mucormycosis remains to be established through appropriately controlled prospective clinical trials. In a review of 28 cases adjunctive HBO was beneficial in diabetic patients (94% survival), but not in patients with haematological malignancies or bone marrow transplants (33% survival; p 0.02). Prolonged courses of HBO were associated with a higher survival (100% survival; p 0.003), although this can be explained by “survival bias”. Yet, the lack of prospective studies and controls make the efficacy of the method debatable. A recent report summarizing the experience with hyperbaric oxygen as adjunct treatment gathered over the past 40 years concludes that there is not sufficient evidence to define the efficacy of this expensive intervention.
-2. Adjunctive cytokines
It is well known that PMLs and macrophages constitute an important defense mechanism against the agents of mucormycosis, providing a rationale for the use of granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and interferon-γ (IFN-γ) as adjunctive treatment beyond the setting of granulocytopenia. GCSF and GM-CSF have been shown to increase phagocytosis, oxidative burst and fungicidal activity of PMLs, and IFN-γ to induce a T-helper cell type 1 (Th1) immunological response that favors resistance to invasive fungal infections and enhances PML’s antifungal activities. G-CSF and GMCSF are routinely given to neutropenic patients with invasive fungal diseases including mucormycosis. G-CSF and GMCSF have also been used in a limited number of cases of mucormycosis in non-neutropenic patients as adjunctive treatment with favorable outcomes. While individual non-neutropenic patients with extensive or refractory disease may benefit from the use of adjunctive cytokine treatment, further studies are needed to assess the general utility of IFN-γ, G-CSF or GM-CSF as adjuncts to antifungal chemotherapy.
Granulocyte transfusions have also been tested with unclear success but with some risk for inflammatory lung injury. Statins have shown in vitro and in vivo activity against Rhizopus spp., but reliable clinical data are lacking. In a recent case report, an immunosuppressed, trauma patient with intractable mucormycosis was successfully treated with combination of interferon-γ and nivolumab, a monoclonal antibody that decreases programmed death-1 (PD-1) expression on T-cells. Interferon-γ restores monocyte function and has been used as rescue therapy for life-threatening fungal infections while nivolumab binds to PD-1, blocks interaction with its ligands, and enhances PD-1 pathway-mediated inhibition of T-cell proliferation and cytokine production. Anti-PD-1 has shown activity in animal models of fungal sepsis. Given the limited evidence, the relative benefit of adjunctive strategies must be balanced against the cost and potential for harm, on an individual patient basis.
-3. Topical amphotericin B
Irrigation and packing of the involved orbit and paranasal sinuses with amphotericin B (1 mg/ml) can achieve excellent results by increasing the delivery of the drug to the infected site. Twice daily, intraconal injection of amphotericin B (1 mg/ml) for 9 days was successfully used along with intravenous amphotericin B in one patient of ROCM and diabetic ketoacidosis and exenteration was avoided. Many authors have described methods of intraorbital infusion of amphotericin B (concentration 0.25–1.25 mg/ml; volume 1–15 ml; frequency 1–4 times daily; duration 5 days to 4 weeks) along with intravenous amphotericin B, and exenteration was avoided in most of these cases. In an attempt to increase the effectiveness of local drug delivery, Kahana and Lucarelli described the use of radiopaque silicone catheter that allows the surgeon to radiologically confirm ideal catheter placement for intraorbital drug delivery.
_____
_____
Salvage treatment:
In general, there are two drug-related reasons for treatment failures, refractory mucormycosis or toxicity of first-line regimens—i.e., intolerance to a drug. For amphotericin B formulations, particularly renal toxicity can be a limiting factor, while for the azole class hepatic toxicity has the highest prevalence. Toxicity can be caused by previous antifungals, or expected due to pre-existing organ damage. Only two drug classes have proven efficacy in mucormycosis, thus salvage treatment mostly means switching to the other class. Isavuconazole salvage treatment was successful in both clinical scenarios, refractory disease, and intolerance or toxicity. In Europe, isavuconazole is licenced for salvage treatment of mucormycosis only. Posaconazole treatment with oral suspension achieved cure in two non-randomised clinical trials and in case series. Liposomal amphotericin B was effective as salvage treatment, as was amphotericin B lipid complex, and amphotericin B colloidal dispersion.
Deferasirox or posaconazole are reasonable salvage options for patients with mucormycosis refractory to or intolerant of polyene therapy (table below). Substantially more clinical data are available for posaconazole in this setting. If deferasirox is used, it should be administered for 2–4 weeks during salvage therapy, because in preclinical studies of non–iron-overloaded primates, deferasirox toxicity increased beyond 4 weeks of therapy. In contrast, posaconazole appears to be quite safe, despite dosing for months to years.
_
Salvage therapy for mucormycosis:
|
Drug |
Recommended dosage |
Advantages and supporting studies |
Disadvantages |
|
Posaconazole with or without lipid polyenes |
200 mg po qid or 400 mg po bid |
Convenient oral dosing of posaconazole; retrospective case series demonstrated 60%–70% “success” rates (complete plus partial response) |
Monotherapy posaconazole efficacy less than polyenes in murine studies; combination posaconazole plus LFAB no better than LFAB alone in murine studies |
|
Deferasirox plus lipid polyenes |
20 mg/kg po qd for 2–4 weeks |
Convenient oral dosing of deferasirox; success in case report |
Limited published data |
|
Granulocyte transfusions (for persistently neutropenic patients) |
∼109 cells/kg |
Neutrophils and ABLC interact synergistically against Mucorales in vitro; case reports of patients supported with granulocyte transfusions |
Limited clinical data; infusion related toxicity and alloimmunization |
|
Recombinant cytokines G-CSF, GM-CSF, or IFN-γ |
Dose G-CSF at 5 μg/kg/day; GM-CSF at 100–250 μg/m2; IFN-γ at 50 μg/m2 for those with body surface area ≥0.5 m2 and 1.5 μg/kg for those with body surface area <0.5 m2 |
In vitro studies demonstrate augmented host response of PMNs to hyphal elements of Rhizopus species; individual case reports |
Limited clinical data |
ABLC, amphotericin B lipid complex; bid, twice per day; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; PMN, polymorphonuclear leukocyte; po, oral; qd, once per day.
Granulocyte colony-stimulating factor and granulocyte transfusions may provide additional support for persistently neutropenic patients until recovery from neutropenia. Administration of granulocyte macrophage colony-stimulating factor or interferon-γ may further augment host response and antifungal effect in nonneutropenic patients with refractory infection.
______
______
Total duration of therapy:
The duration of therapy necessary to treat mucormycosis is unknown. In general, weeks to months of therapy are given. Successful courses of amphotericin B typically last 4-6 weeks. Primary or salvage isavuconazole therapy may be continued for several months given its higher tolerability compared with amphotericin. If immune defect is resolved—e.g., diabetes is controlled, neutropenia definitively resolved, immunosuppression can be tapered or stopped, therapy can be continued until resolution of signs and symptoms of infection, and substantial radiographical improvement. Median duration of isavuconazole first-line or salvage treatment was 84 days intravenous or oral route or both. Across several posaconazole oral suspension studies, treatment duration ranged from 1 week to almost 3 years, mean duration was approximately 6 months. The wide range reflects the pattern of organs involved, with competing risks from underlying conditions. Late relapse in long-term survivors have been documented. Repeated surgical debridement of necrotic tissue identified by follow-up head computed tomography (CT) scan or magnetic resonance imaging (MRI) is often indicated.
In the absence of comparative data, the total duration of therapy for mucormycosis should be individualized for each patient. In general, antifungal therapy for mucormycosis should be continued until all of the following objectives are attained: (1) there is resolution of clinical signs and symptoms of infection, (2) there is resolution or stabilization of residual radiographic signs of disease on serial imaging, and (3) there is resolution of underlying immunosuppression. Such a case is illustrated in a patient with lymphoma and renal mucormycosis.
For patients with mucormycosis who are receiving immunosuppressive medications, secondary antifungal prophylaxis is typically continued for as long as the immunosuppressive regimen is continued. Posaconazole may be an option if polyenes cannot be used for prolonged periods. For patients with intermittent immunosuppression, such as those receiving intermittent cycles of chemotherapy who have adequate leukocyte counts between cycles, secondary prophylaxis should be reinitiated during neutropenia and should continue until the recovery from neutropenia.
Educate patients about the signs of disease, such as facial swelling and black nasal discharge, and instruct patients to present promptly for evaluation if these signs occur.
______
______
Novel therapy of mucormycosis:
Iron chelation therapy (vide supra):
Deferoxamine iron chelation therapy predisposes to mucormycosis, because deferoxamine actually enhances delivery of iron to Mucorales. Indeed, animals infected with R. oryzae that are treated with iron or deferoxamine have markedly worse survival than do animals treated with placebo. However, other iron chelators cannot be used by Mucorales to acquire iron. In 2005, a new orally available iron chelator, deferasirox, was approved by the US Food and Drug Administration for the treatment of iron overload among patients with transfusion-dependent anemia. Deferasirox was fungicidal for clinical isolates of Mucorales in vitro, with an MIC90 of 6.25 μg/mL The drug exhibited time-dependent killing, with cidality occurring at 12–24 h of drug exposure. Based on trough serum levels >15 μg/mL in patients who are treated with deferasirox at 20 mg/kg/day, it should be feasible to maintain deferasirox serum levels in excess of the MICs of Mucorales.
The toxicities of deferasirox therapy in nonhuman primates and in clinical trials have been extensively reviewed. Gastrointestinal symptoms (e.g., nausea and diarrhea) are the most common adverse effects of deferasirox therapy. However, the primary toxicity of concern is renal. Elevations in creatinine occurred in up to one-third of patients in deferasirox clinical trials, but they were usually mild and reversible upon cessation of drug use. There have been rare post-marketing reports of severe acute renal failure resulting in hemodialysis for or death of iron-overloaded patients receiving deferasirox. However, these patients typically had other underlying risk factors for renal failure. Therefore, the contribution of deferasirox to the renal failure in these cases is unclear.
These iron chelators appear to be a rational adjunct to antifungal treatment. However, the limited evidence currently available is insufficient to estimate the role of deferasirox or deferiprone as adjunctive treatment for mucormycosis in combination with surgery and antifungal treatment. Routine use of adjunctive iron chelator therapy is not recommended. Deferasirox is a reasonable salvage options for patients with mucormycosis refractory to or intolerant of polyene therapy.
_____
Zinc chelation therapy:
It is long known that zinc (Zn) starvation inhibits microbial growth in tissues. Zn deficiency induces stress in fungal cells and hampers fungal development by restricting the activity of Zn-binding proteins, which are mainly transcription factors involved in many biological processes. Published data about Zn homeostasis in fungi have inferred that compounds that interfere with this metabolic process would inhibit fungal growth.
It is known that fungi are susceptible to zinc deprivation, so researchers tested the in vitro effect of the zinc chelators clioquinol, phenanthroline, and N,N,N′,N′-tetrakis (2-pyridylmethyl) ethane-1,2-diamine combined with amphotericin B or posaconazole against 25 strains of Mucorales. Clioquinol-posaconazole was the most active combination, although results were strain dependent.
It is known that metal-chelating agents are able to inhibit biological processes that are essential in every cellular system. Zn-chelator concentration-related toxicity has been described and should be taken into consideration. It is thus clear that a Zn-depletion-based strategy for mucormycosis therapy would be plausible only if undesired effects of ion sequestration could be avoided with the development of fungal-specific ion chelators. This concept should be added to the drug development pipeline.
_____
Newer antifungal agents:
The number of therapeutic options for the treatment of mucormycosis is quite limited when compared with those available to treat bacterial or other fungal infections. Indeed, only two classes of molecules, polyenes and azoles, are currently used in clinical practice. Unlike invasive candidiasis and aspergillosis, the majority of hematology patients who develop mucormycosis still die from their infection despite the administration of systemic antifungal therapy, emphasizing the need for new antifungal drugs with activity against Mucorales. Yet, it is difficult to find unique targets for Mucorales. Recent genome sequencing of Rhizopus oryzae revealed evidence of a whole-genome duplication event during its evolution, offering a remarkable genetic plasticity to this pathogen and explaining its relative resistance to multiple antifungal classes.
The investigational drug VT-1161, an inhibitor with selective activity against the fungal CYP51, has in vitro activity against Mucorales including R. oryzae, Lichtheimia and Cunninghamella. VT-1161 was shown to prolong survival of neutropenic mice with mucormycosis due to R. oryzae when given therapeutically or prophylactically. Although additional studies are required to establish the efficacy of VT-1161 against other Mucorales (higher MIC values were noticed versus R. delemar), this ergosterol synthesis inhibitor might prove to be an additional asset in our armamentarium against mucormycosis.
APX001A (formerly E1210) is an antifungal agent that targets Gwt1, an early step in the conserved glycosylphosphotidyl inositol (GPI) post-translational modification pathway of surface proteins in eukaryotic cells. Although it has modest in vitro activity against other Mucorales, in a recent study, it protected immunosuppressed mice from Rhizopus delemar infection. APX001A has entered phase I clinical trials. Finally, another novel agent, haemofungin inhibits in vitro the growth of several fungi including Rhizopus.
______
Oral Amphotericin B:
A technology developed by researchers at the Indian Institute of Technology (IIT) Hyderabad to treat ‘kala-azar’ has been repurposed to treat mucormycosis patients in a cost-effective and convenient manner. An innovation reported two years ago by researchers at IIT-Hyderabad in the formulation of oral tablets used in the treatment of kala-azar or black fever has come to the aid of black fungus patients. In 2019, researchers from IIT- H’s Creative and Advanced Research Based on Nanomaterials (CARBON) lab reported using gelatin nanofibers to manufacture Amphotericin B in a sustained-release tablet form. The research was intended to deliver Amphotericin B (AmB) orally at an extremely slow rate within the therapeutic window. The purpose was to increase the drug absorption and reduce aggregation, to lower the drug toxicity. For this, the team has selected gelatin an FDA-approved polymer as an excipient for drug molecules. Explaining the reason for oral administration not preferred despite the comfortable and effective route, the researchers said that due to its amphiphilic nature, the AmB has poor aqueous solubility and forms aggregates in the system, which stresses renal filtration and thus causing nephrotoxicity. No pharmaceutical company in the country has advanced this innovation in the last two years by conducting clinical trials, obtaining necessary regulatory approvals, and manufacturing tablets. Amphotericin-B is currently manufactured in the form of an injection by a few pharmaceutical companies, and its use in the treatment of mucormycosis costs lot of rupees — a 50 mg vial costs close to Rs 4,000, and up to 60-100 vials are required for one patient. Amphotericin B tablets would be significantly less expensive than injections, costing around Rs 200 per tablet of 60 mg dose. Manufacturing the drug in tablet form would also make it easier to scale up production to meet the high demand.
_______
Antifungal Peptides and Other Immuno-therapeutics:
Compounds with direct antifungal activity derived from, or directly influenced by, the immune system includes monoclonal antibodies (mAb), immunomodulatory AMP (antimicrobial peptides), vaccines, checkpoint inhibitors, interferon and colony stimulating factors as well as immune cell therapies. Antimicrobial peptides are promising candidates as therapeutics for the treatment of fungal infection and are much needed in clinical practice due to the limited array of treatment options and increasing resistance to existing antifungals. Unfortunately, we are not seeing enough drug candidates making it through the drug development pipeline, as in vitro and in vivo testing approaches are not always appropriate and/or optimised for AMP. The same is true in part for clinical efficacy trials which must be appropriate for AMP (end points in particular). These factors are undoubtedly part of the reason behind there not being more AMP progressing through the drug development cycle and/or AMP candidates are confined to topical therapy status as delivery systems, formulation, routes of administration and duration of therapy for AMP have not been adequately optimised. The time is now coming for greater exploitation of AMP and other immune-therapeutics as antifungal drug candidates as we gain a greater understanding of how best to test these drug candidates in vitro and how regulatory pathways and clinical studies can be more accommodating for peptides (Table below).
_
Selected immunology-based approaches for the treatment of fungal infection.
|
Antifungal therapy |
Target fungal infection |
Developmental therapeutic |
Target pathogen/s |
Development stage as antifungal |
|
AMP |
||||
|
Antifungal |
Onychomycosis |
NP213 |
Dermatophytes |
Phase IIb |
|
Onychomycosis |
HXP124 |
Dermatophytes |
Phase I/IIa |
|
|
VVC |
CZEN-002 |
Candida spp. |
Phase I/IIa |
|
|
Oral candidiasis |
P113 |
Candida spp. |
Phase IIb |
|
|
Dermal infection |
Omiganan |
Candida spp. |
In vivo (porcine) |
|
|
Prophylaxis in HSCT patients |
hLF1-11 |
Not Specified |
Phase I |
|
|
Oral mucositis |
Iseganan |
Yeasts |
Phase III |
|
|
Not specified |
LTX-109 |
S. cerevisiae |
In vitro |
|
|
Aspergillosis & Candidiasis |
NP339 |
Aspergillus spp., Candida spp., mucorales |
In vitro |
|
|
Fungal infection |
D2A21 |
Mucor spp., T. mentagrophytes |
In vitro |
|
|
Systemin infection |
ETD151 |
C. albicans, A. fumigatus |
In vivo (murine) |
|
|
Anti-biofilm |
Not specified |
Histatin-5 |
C. albicans |
In vitro |
|
Not specified |
LL-37 |
C. albicans |
In vitro |
|
|
Not specified |
hLF1-11 |
C. albicans |
In vitro |
|
|
Not specified |
LTX-109 |
S. cerevisiae |
In vitro |
|
|
Immunostimulatory molecules |
Clinical |
|||
|
Interferon-γ |
Systemic infection |
IFN- γ |
Aspergillus spp., Candida spp. |
|
|
Colony stimulating factors |
Prophylaxis |
G-CSF |
Fungal |
Clinical |
|
Prophylaxis |
GM-CSF |
Fungal |
Clinical |
|
|
Prophylaxis |
M-CSF5 |
Candida spp. |
Phase I/II |
|
|
Antibodies |
||||
|
Prophylaxis |
Cryptococcosis |
18B7 |
Cryptococcus neoformans |
Phase I |
|
Candidiasis |
mAb 3D9.3 |
C. albicans |
In vitro |
|
|
Fungal |
mAb C7 |
Candida spp., Cryptococcus spp., A. fumigatus, Scedosporium prolificans |
In vitro |
|
|
Therapeutic |
Disseminated candidiasis |
Ab119 & Ab120 |
Candida spp. |
In vivo (murine) |
|
Vaccines |
VVC |
NDV-3A |
Candida spp. |
Phase II |
|
VVC |
PEV7 |
Candida spp. |
Phase I |
|
|
VVC |
D.651 |
Candida spp. |
Phase II |
|
|
Immune checkpoint inhibitors |
Mucormycosis |
Nivolumab |
Mucorales |
Case study (1 patient) |
|
Cell-based therapies |
||||
|
Antifungal-loaded leukocytes |
Pulmonary aspergillosis |
Posaconazole-loaded leukocytes |
Aspergillus spp. |
In vivo (murine) |
|
CAR-T |
Murine lung infection |
D-CAR+ T cells6 |
Aspergillus spp. |
In vivo (murine) |
_____
Fungal viruses:
Certain fungal viruses, when properly understood and harnessed, can become helpful and useful. Some, for instance, make the fungi they infect less aggressive, a phenomenon called hypovirulence. One example is the hypovirus CHV1, which reduces virulence in the tree pathogen Cryphonectria parasitica, one of the most devastating of all plant-killing fungi. It decimated natural populations of the American chestnut tree, beginning in the early 1900s. CHV1 was first discovered in Europe in the 1960s after people noticed that European chestnut trees affected by C. parasitica had begun to recover. They did not suffer the same devastation that befell the American species. Hypovirulent viruses have been of great interest to researchers because of their potential as biocontrol agents of fungi that cause serious plant diseases.
Double-stranded RNA viral elements have been found in Mucorales species (Vágvölgyi et al., 1993, 1998; Papp et al., 2001); however, there have been little to no published attempts to further characterize these mycoviruses or identify novel Mucorales infecting mycoviruses. NGS-based approaches have already been utilized to discover and characterize mycoviruses in pathogenic fungi, including Aspergillus (Vainio et al., 2015; Zoll et al., 2018). This approach has already shown potential in the Mucorales field. Whole genome sequencing and phylogenomic comparison of Rhizopus species showed the presence of pol fragments from Caulimovirus (plant virus) in one-third of the analyzed genomes (Gryganskyi et al., 2018). Recently, a transcriptomic analysis of M. irregularis demonstrated the presence of a gene for a predicted RNA polymerase domain specific to a negative strand RNA virus (Barata et al., 2019). RNA sequencing has also led to the discovery of two Narnavirus members in R. microsporus in a novel fungal-bacterial-viral holobiont system (Espino-Vazquez et al., 2020). The role of these viruses in Rhizopus biology are still questioned but it is clear that they play a role of in Rhizopus biology. Infection of the viruses alone decreased asexual reproduction by reducing the number of R. microsporus sporangiospores. We have to research and harness a fungal virus that can infect Mucorales fungi and make them less virulent.
______
______
Complications of mucormycosis:
The complications of mucormycosis are serious and are related to the body area initially infected but also can occur in other body regions because the fungi often spread to the organs or tissues that physically contact or are near the originally infected area or by haematological dissemination. In addition, because surgical debridement is almost uniformly needed, some normal tissue may be destroyed because the surgeon must remove all tissue that is dead or dying. Unfortunately, that means the surgeon may have to remove some normal tissue to insure all of the fungi are removed. An example is infection of the eye orbit; often the whole eye must be removed.
Complications of mucormycosis include:
blindness,
meningitis,
brain abscesses,
cerebral infarction,
intracranial hemorrhage,
cavernous sinus thrombosis,
osteomyelitis,
pulmonary hemorrhages,
gastrointestinal hemorrhages,
amphotericin B-related renal failure,
cavitary lesions in organs and eventually secondary bacterial infections, sepsis, and death.
_____
_____
Prognosis of mucormycosis:
-1. The site of infection and underlying host factors (immunosuppression) are key prognostic determinants of mucormycosis outcome.
-2. Patients with active hematologic malignancy, allogeneic hematopoietic cell transplantation, and disseminated infection have the poorest outcome with most patients dying within 12 weeks of diagnosis.
-3. Early diagnosis, correction of underlying immunosuppression combined with aggressive multi-faceted treatment (i.e., systemic antifungal therapy, surgery) offers the best opportunity for patient survival.
_
Mucormycosis is frequently a life-threatening infection. A review of published mucormycosis cases found an overall all-cause mortality rate of 54%. The mortality rate varied depending on the rapidity of diagnosis and treatment, the site of infection, the patient’s underlying conditions and degree of immunosuppression, and the type of fungus (for example, the mortality rate was 46% among people with sinus infections, 76% for pulmonary infections, and 96% for disseminated mucormycosis).
_
Previously, cases of rhinocerebral mucormycosis were almost consistently fatal. Although the mortality rate of rhinocerebral disease remains high, the infection can be cured when diagnosed early and treated with aggressive surgery and antifungal agents. Recent series have described a mortality of approximately 40% in diabetics with rhinocerebral mucormycosis and a similar survival rate for rhinocerebral disease in patients with hematological malignancies. Of note, the prognosis is much better if the disease has not penetrated beyond the sinus prior to surgical debridement; in local sinonasal disease, the mortality has been reported to be <10%. The nature of the underlying disease and the reversibility of the immune dysfunction are also important determinants of survival. One study showed that 75% of patients with rhinocerebral disease who had no underlying immune compromise survived, while 60% of those with diabetes and only 20% of patients with other immunocompromised states were cured.
The overall survival rate of patients with mucormycosis is approximately 50%, although survival rates of up to 85% have been reported more recently. Much of the variability in outcome is due to the various forms of the disease. Rhinocerebral mucormycosis has a higher survival rate than does pulmonary or disseminated mucormycosis because the rhinocerebral disease can frequently be diagnosed earlier and the most common underlying cause, diabetic ketoacidosis, can be treated readily. In contrast, pulmonary mucormycosis has a high mortality (≈65% at 1 year) because it is difficult to diagnose and it frequently occurs in neutropenic patients. For example, in one large study, only 44% with pulmonary mucormycosis were diagnosed premortem, and the overall survival rate was only 20%. In a separate study in which 93% of the infections were diagnosed premortem, the survival rate was 73%. Mortality in patients with disseminated disease approaches 100%, in large part because surgical removal of infected tissues is not feasible and in part because these patients tend to be the most highly immunocompromised (e.g., allogeneic stem cell transplantation).
_
All-cause mortality rates for mucormycosis range from 40% to 80% with varying rates depending on underlying conditions and sites of infection. The highest survival rates are reported in patients with a healthy immune status and those without comorbidities. The poorest prognosis is observed in patients with haematological malignancies and HSCT recipients and in patients with extensive burns. Disseminated disease, especially to the CNS is often associated with mortality rates higher than 80%. Conversely, lower mortality is seen with localised sinus or skin infection, where earlier tissue-based diagnosis is often feasible and surgical debridement may result in cure. Mortality is also high in neonates and other immunocompromised patients with gastrointestinal mucormycosis, possibly related to delay in diagnosis and polymicrobial sepsis. Generally, improved survival is related to earlier diagnosis and application of early, multidisciplinary treatment approaches involving aggressive surgical debridement. Despite improved understanding of the disease and the availability of more therapeutic options, survival rates in mucormycosis remain poor.
_____
_____
Section-14
Prevention of mucormycosis:
Mucormycosis is commonly found in dirt (or soil), animal manure (or dung), decaying plant materials (e.g., compost, fruits, vegetables, leaves), and in moist or wet areas. According to CDC, many people come into contact with fungal spores every day. They also suggest that it is impossible to avoid coming into contact with mucormycetes (the types of fungi that cause mucormycosis). The spores are not harmful for most people. Common risk factors for mucormycosis infection include immune system problems, diabetes, past or current COVID-19 infection, and steroid treatments. To avoid infection, the India Council of Medical Research (ICMR) suggests that people “use masks if you are visiting dusty construction sites; wear shoes, long trousers, long sleeve shirts and gloves while handling soil (gardening), moss or manure; and maintain personal hygiene including thorough scrub bath.” The CDC suggests similar actions and include avoiding water damaged buildings after floods or heavy rains. They note that these preventive measures “haven’t been proven to prevent mucormycosis.”
_
Measures to decrease the incidence of mucormycosis in patients at risk are difficult at best. There is no routine antifungal prophylaxis available, and with the low prevalence of mucormycosis, there is no real indication to provide it. The most common preventive interventions attempted regard modifications and controls in the environment that reduce the risk of exposure to airborne spores. Most of these control measures are focused on easily identified patients at risk, i.e., those expected to be profoundly neutropenic for prolonged periods. Transplantation and chemotherapeutic wards are often isolated with Hepafilter treatment of the air supply and positive pressure to exclude the recruitment of dust into the ward. Dust should be kept to a minimum in the environment that houses these neutropenic patients. Additionally, flower arrangements and live plants are often excluded from such wards since they may harbor a variety of fungal agents. Patients when neutropenia below 1,000/microliter are asked to wear masks when leaving the cancer or transplant wards, particularly when going outside. The monitoring of air quality, particularly during times of building renovation and excavation in the vicinity of transplant centers, is also important on infection control measure.
Preventive measures for patients other than the transplant and chemotherapy population require addressing the underlying risk factors for developing mucormycosis. Adequate control of diabetes, the use of iron chelators other than deferoxamine, limiting the use of aluminum-containing buffers in dialysis, and aggressive direct and culture-based detection of mucormycosis are among the best preventive measures that may be taken. Keeping a high level of suspicion for mucormycosis in patients who are at risk can aid an early diagnosis and implementation of appropriate therapy.
_____
It’s difficult to avoid breathing in fungal spores because the fungi that cause mucormycosis are common in the environment. There is no vaccine to prevent mucormycosis. For people who have weakened immune systems, there may be some ways to lower the chances of developing mucormycosis.
-1. Protect yourself from the environment.
It’s important to note that although these actions are recommended, they haven’t been proven to prevent mucormycosis.
-Try to avoid areas with a lot of dust like construction or excavation sites. If you can’t avoid these areas, wear an N95 respirator (a type of face mask) while you’re there.
-Avoid direct contact with water-damaged buildings and flood water after hurricanes and natural disasters.
-Avoid activities that involve close contact to soil or dust, such as yard work or gardening.
If this isn’t possible,
-Wear shoes, long pants, and a long-sleeved shirt when doing outdoor activities such as gardening, yard work, or visiting wooded areas.
-Wear gloves when handling materials such as soil, moss, or manure.
-To reduce the chances of developing a skin infection, clean skin injuries well with soap and water, especially if they have been exposed to soil or dust.
-2. Antifungal medication.
If you are at high risk for developing mucormycosis (for example, if you’ve had an organ transplant or a stem cell transplant), your healthcare provider may prescribe medication to prevent mucormycosis and other mold infections. Doctors and scientists are still learning about which transplant patients are at highest risk and how to best prevent fungal infections.
_____
ICMR’s things to-do:
-Control hyperglycemia
-Monitor blood glucose level post- COVID-19 discharge
-Use steroids judiciously – correct timing, dose and duration
-Use clean, sterile water for humidifiers during oxygen therapy
-Use antibiotics/antifungals judiciously
ICMR’s things not-to-do:
-Do not miss warning signs and symptoms
-Do not consider all cases with blocked nose to be bacterial sinusitis, especially in the context of immunosuppressors and/or COVID-19 patients on immunomodulators
-Do not hesitate to seek aggressive interventions
-Tests like KOH staining, microscopy, culture, MALDI-TOF can be done to detect fungal aetiology
-Don’t lose crucial time by hesitating to initiate treatment
_____
Preventive measures suggested by Indian medical association (IMA) are depicted in figure below:
______
Mucormycosis can be avoided with a strict ICU vigil, shows study by Mumbai hospital:
The mucormycosis crisis sweeping across India’s post-Covid patients could have been avoided with strict bedside monitoring, shows a new study by a Mumbai hospital. None of the 1,000-plus patients admitted to Fortis Hospital’s ICU since March 2020 developed the deadly fungal infection that can disfigure or kill a patient. Of the 1,027 patients admitted to ICU between March 2020 and May 2021, 915 received steroids and 417 had diabetes. Yet, on follow-up it was found that none developed mucormycosis. The results underline that “if you manage patients well”, the fungal infection can be avoided. Even if some patients develop mucormycosis, doctors catch it so early that the patient does not need surgery.
_____
According to ECMM/ISHAM recommendations for clinical management of COVID -19 associated mucormycosis in low- and middle-income countries in 2021, steps to prevent or reduce the occurrence of covid associated mucormycosis and misconception on the terminology and acquisition of infection are depicted in figure below.
Figure above depicts prevention and misconception regarding COVID-19 associated mucormycosis.
An urgent attention is essential to curb the epidemic of CAM in low- and middle-income countries, especially in India. Recent multi-centre study in India has confirmed that proper glycaemic control and appropriate steroid therapy behaviour are essential while managing COVID-19 patients. Early diagnosis is important for better outcome of CAM patients. Clinicians while discharging from COVID wards, should advice the diabetic patients on the early symptoms and signs of CAM. Mucormycosis management requires comprehensive facility of accurate diagnosis, competent surgical and medical team. The epidemic has taught us that fungal diseases should not be neglected; training on managing fungal infections among laboratory personnel and clinicians are essential in each country especially in countries having high rate of fungal infection. Research is essential in fungal diseases especially those that disproportionately affect the large population of low- and middle income countries.
______
______
Section-15
Why does a rare condition end up becoming an epidemic in India?
[Also read section 9]
Millions of patients suffering from diabetes or on steroid or cancer patients are admitted to hospital in India every year but occasionally gets this infection. Millions all over the world are getting infected with COVID, are diabetic and put on steroids but get mucormycosis occasionally. Then why is there epidemic of Mucormycosis during 2nd wave of covid in India?
_
While COVID-19-associated pulmonary aspergillosis (CAPA) has received much international attention, the Indian epidemiology of invasive mould infections in the ICU reveals a significant burden of invasive mucormycosis. This has recently emerged as a life-threatening complication of COVID-19 in India. Although the predisposing factors and pathogenesis are somewhat similar to that of other mould infections, certain unique characteristics and key distinguishing factors must be kept in mind in order to promptly suspect the infection, confirm the diagnosis and offer timely therapeutic intervention. Mucorales are ubiquitous moulds, abundantly found in the environment on decaying organic matter. Various studies from hospitals across India have revealed heavy mould spore counts even in hospital air due to predominantly hot, humid conditions in India’s tropical climate. Unlike CAPA, invasive mucormycosis has been observed even in patients with mild to moderate SARS- CoV-2 infections. The strongest predisposing factor appears to be hyperglycemia in undiagnosed or uncontrolled diabetics.
_
The hospital of the Mahatma Gandhi Institute of Medical Sciences, a medical school in the town of Sevagram in the Indian state of Maharashtra, has been taking in patients afflicted with COVID since May 2020. But in the middle of May 2021, something changed. Patients arrived with problems the physicians there had not yet seen in the pandemic: people were not only breathless and feverish yet had pain and pressure behind their cheekbones and around their eyes. Their cases were some of the earliest indications of a wave of illness that swamped India, an epidemic within the pandemic: infections with a rare group of fungi called mucormycetes. The infection they cause, mucormycosis— “black fungus,” colloquially—can infest the sinuses and bones of the face and invade the brain or cause patients to lose an eye. When not diagnosed early— treatment is prolonged and difficult—mucormycosis can kill up to half of those who contract it.
_
Public health challenges are those that have solutions beyond the perimeters of narrow medical interventions. Regarding a potential public health menace as merely a medical challenge until numbers rise to hideous levels is bad practice. The mucormycosis (commonly and mistakenly called ‘black fungus’) epidemic superimposed on the second wave of the Covid-19 pandemic is a classic case in point. Within months, it has gone from a rare condition caused by ubiquitous and largely innocuous fungi to being declared an epidemic by a number of Indian states. Unfortunately, it continues to garner scarce recognition as a problem that warrants steadfast public health measures.
_
A study conducted by the Indian Council of Medical Research (ICMR) between June and September 2020, with data from 17,534 Covid patients in 10 hospitals across eight cities, reported that nearly 3.6% (in the range of 1.7% to 28% between hospitals) of total patients developed a secondary infection, with around 78% having been reported two days after hospital admission and an average mortality of 56.7%. However, none of these infections were mucormycosis. Notwithstanding the possibility of missed mucormycosis cases during the first wave, it raises the question as to why it has transformed into a veritable onslaught during the second wave. The ICMR study also noted that nearly three-fourth of patients with secondary infections were given antibiotics from the ‘Watch’ and ‘Reserve’ lists of the WHO, which includes drugs having a greater propensity to bacterial resistance and must be reserved for certain special situations. Another study on Covid patients at the trauma centre of AIIMS, Delhi, noted multi-drug resistance in 60% of studied isolates with overall resistance up to 84%. One can expect the situation to have only worsened in the second wave. These forebode the horror of widespread antimicrobial resistance soon after the pandemic due to their indiscriminate use, which could be a much bigger and more enduring concern than mucormycosis.
_
Why should we take mucormycosis seriously?
Firstly, all individuals affected with COVID-19 are at risk of this complication, irrespective of severity of the infection. Secondly, its diagnosis is often delayed leading to incorrect or neglected care. Most importantly, the mortality rate of Mucormycosis is 50-100 percent depending on the severity, which implies that more than half of the people affected by it won’t survive.
_
India was capital of mucormycosis even before Covid-19 pandemic:
The Leading International Fungal Education (LIFE) portal has estimated the burden of serious fungal infections globally. According to their estimate, the annual prevalence of mucormycosis might be around 10,000 cases in the world barring India. After the inclusion of Indian data, the estimate of mucormycosis rose to 910,000 cases globally. So mucormycosis is already far more common in India compared to other countries. The prevalence of mucormycosis in India was about 80 times the prevalence in developed countries even before the pandemic. This tells us that India was already a hotbed for fungal infections. Mucormycosis was already more common in India than in any other country even before the pandemic and India contributed to approximately 71% of the global cases of mucormycosis in patients with COVID-19 from December, 2019, to the start of April, 2021. Indian environment apparently promote growth of molds and Indian air contains high spore count. A study published in the journal Mycoses in 2014 explains that certain species of fungi like Apophysomyces elegans and Rhizopus homothallicus are emerging in the Indian subcontinent, leading to an alarming frequency of mucormycosis in India in the last decade. The study also notes that Indian mucormycosis has certain unique features which is why it’s often categorised as rhino-orbital-cerebral mucormycosis (ROCM). Given the new association with COVID-19, it’s more important than ever to control and prevent mucormycosis in India.
_
Another factor could be due to the fact that India is the diabetes capital of the world. Of all mucormycosis cases published in scientific journals globally between 2000 and 2017, diabetes was seen in 40 per cent of the cases. An estimated 77 million people are affected with diabetes in India, representing the largest number of any country in the world. Unfortunately, more than half the cases of diabetes in India are undiagnosed. In the recent study, 94 per cent of the people who developed mucormycosis after Covid had diabetes and 67 per cent of them had uncontrolled sugar levels. The incidence of mucormycosis has been increasing in the last couple of decades as indicated by some single- and multi-centre studies, possibly attributable among other factors to the rising prevalence of diabetes and suitable climatic conditions in India. A study across 16 institutions between September and December 2020 noted 2.5 times increase in mucormycosis infections. Since December 2020, a palpable rise in cases can be made out from clinician testimonies. Technically, therefore, mucormycosis becoming an epidemic is not a recent phenomenon, but one that is many months old and is only bursting at the seams now.
_
A study from 2013-2015 at four major tertiary care hospitals in India reported 388 mucormycosis cases, nearly 56% of whom were reported as having “uncontrolled diabetes”. A multicentre observational study on the epidemiology, risk factors, management and outcomes of 465 mucormycosis cases in India in 2019 found diabetes mellitus as a predisposing factor in 73.5% cases. Recently the Indian Health Ministry said the country reported at least 40,845 cases of mucormycosis and 3,129 fatalities from mucormycosis during the second wave of the pandemic at the end of June 2021. Of the total number of mucormycosis patients, 34,940 had Covid, 26,187 had the co-morbidity of diabetes, and 21,523 were on steroids.
India is not the only country with an increase in mucormycosis cases. Pakistan, Nepal and Bangladesh have been reporting COVID-19-associated mucormycosis; however, these countries have not been affected by a wave of COVID-19 cases in the same way. About 15% of people in these countries have received their first dose of vaccine, meaning that there is potential for similar mucormycosis epidemics to occur across other low- and middle-income countries. This would put a high burden on healthcare services which are already struggling from the pandemic. In fact one Indian survey found that 86% mucormycosis cases are among covid unvaccinated. Therefore, it is vital to ensure vaccine equity across the world to protect healthcare systems and patients from COVID-19, as well as other associated, deadly, infections.
_
A perfect storm in India:
People with diabetes and obesity tend to develop more severe Covid-19 infections. This means they’re more likely to receive corticosteroids, which are frequently used to treat Covid-19. But the corticosteroids – along with diabetes – increase the risk of mucormycosis.
-1. Poorly controlled diabetes has an established and strong correlation with invasive mucormycosis. Diabetes mellitus was identified in 40% of patients and was the most common predisposing condition in one of the largest epidemiological studies conducted thus far. Hyperglycemia impairs chemotaxis and the oxidative and non-oxidative fungicidal mechanisms used by phagocytic cells – the main defensive mechanism against mucormycosis. Hyperglycemia also increases the expression of GRP78 (a 78 kDa glucose-regulated protein) which acts as the endothelial receptor for the ligand (spore-coating protein homolog) used by the agents that cause mucormycosis. In states of acidosis related to hyperglycemia, free iron becomes readily available in the serum. Mucorales can acquire this excess endogenous iron through siderophores (low-molecular-weight chelators) or iron permeases enhancing their virulence.
There are many ways by which diabetes occurs in covid-19 patients besides pre-existing diabetes. SARS-CoV-2 itself can induce acute diabetes directly by damaging pancreatic islets cells which have a high expression of angiotensin converting enzyme-2 receptors, and indirectly by damaging small blood vessels supplying pancreatic beta cells. Increased resistance to insulin due to the profound inflammatory reaction, may also play some role in the induction of hyperglycaemia. Corticosteroids can increase blood glucose levels by acting as a substrate for oxidative stress metabolism with lipolysis, proteolysis, and hepatic glucose production. They also increase insulin resistance in up to 60%-80% of patients depending on the dose and type used.
-2. Besides hyperglycemia, corticosteroids also cause impairment in the migration, ingestion, and phagolysosome fusion of macrophages. Steroids also suppress neutrophil adhesion to endothelial cells and impair lysosomal enzyme release, respiratory burst, and chemotaxis to the site of infection. Coupled with the potential adverse effect of steroid-induced hyperglycemia, a diabetic patient receiving corticosteroids is exceptionally vulnerable to the development of mucormycosis. There are few case reports of pulmonary mucormycosis resulting from short courses (5–14 days) of steroids for exacerbations of chronic obstructive disease in the setting of well-controlled diabetes mellitus.
While long term use of corticosteroids has often been associated with several opportunistic fungal infection including aspergillosis and mucormycosis, even a short course of corticosteroids has recently been reported to link with mucormycosis especially in people with DM. A cumulative prednisone dose of greater than 600 mg or a total methyl prednisone dose of 2–7 g given during the month before, predisposes immunocompromised people to mucormycosis. There are few case reports of mucormycosis resulting from even a short course (5–14 days) of steroid therapy, especially in people with DM. Surprisingly, 46% of the patients had received corticosteroids within the month before the diagnosis of mucormycosis in the European Confederation of Medical Mycology study. These findings need a relook in the context of COVID-19 pandemic where corticosteroids are often being used.
The landmark RECOVERY trial published in June 2020 has served as a ‘license’ to use steroids in patients with COVID-19. However, the fine print clearly revealed some important messages that Indians seem to have overlooked. Benefit was specifically shown with low dose, short duration dexamethasone in moderate to severe illness. In a multi-center study from India, inappropriate corticosteroid use was noted in 63.3% patients. While the standard prescribed dose of steroid in Covid-19 is 6 mg dexamethasone per day, many doctors in every corner of India have misused these guidelines, gave overdose of steroids for long time, and prescribed steroids to those who were not seriously ill. On top of it, patients started self-medicating with steroids owing to their over-the-counter availability and low price in the country. Consequently, a large proportion of cases took steroids even when they didn’t need it and the unsupervised prescriptions led to irrational doses and duration of steroid intake. Some doctors say they have encountered patients who have received 30mg dexamethasone in a day. In certain cases, doctors appear to have overprescribed steroids in efforts to compensate for the shortage of oxygen — even though standard clinical treatment protocols do not recommend this. But the worst is giving steroids in high doses to people without covid. I have seen many patients taking high dose of steroids merely on high CRP level without any virological or radiological evidence. I am sure that many non-covid mucormycosis were due to abuse of steroids in non-covid patients presumed to be covid due to fever and/or high CRP.
-3. While oxygen has to be given to patients who need it, care has to be taken regarding its source and humidification. Owing to the acute shortage of medical grade oxygen across the country, the Empowered Group II under the Government of India directed all major oxygen manufacturers to convert industrial grade oxygen cylinders for medical use. They have also been directed by state governments to convert nitrogen/argon cylinders into oxygen cylinders. Industrial oxygen production and storage is not done under strict asepsis as required for medical grade oxygen, thus, carries a significant risk of contamination with microbes including bacteria and fungi. Although there are SOPs for these procedures, whether the manufacturers, cylinder handlers, transport personnel and distributors followed the precautions rigorously or not is a highly debatable topic. Inside the hospitals, the water used to humidify the oxygen is another potential source of contamination. In an environment where molds are already present, any laxity in the manufacturing or humidification, under work-stress or timeline-pressure, can have serious consequences. A 2011 study found that 86.79% swabs form oxygen humidifiers grew fungi. Oxygen humidifiers must be cleaned and disinfected, and use sterile water for humidification. However, nearly 100 % oxygen stored in cylinders (medical or industrial) is likely to be detrimental to the growth of microorganisms of all kinds including fungi. Even humans get oxygen toxicity caused by exposure to oxygen at partial pressures greater than those to which the body is normally exposed. So inhalation of humidified oxygen is unlikely to cause mucormycosis.
-4. Respiratory viruses cause direct damage to the airway epithelium, enabling fungi to invade tissue. Furthermore, viral infection hampers ciliary clearance and leads to immune dysfunction or dysregulation. The dysregulated innate immune response, ciliary dysfunction, cytokine storm, thrombo-inflammation, microvascular coagulation and eventual immune exhaustion facilitates secondary bacterial and fungal infections especially in critically ill patients subjected to emergency invasive procedures. Fungal spores are everywhere, but we are pretty efficient at clearing them from our respiratory tract. But COVID damages the respiratory tract, so then you have a double whammy: reduced capacity to naturally clear the spores and reduced immune response as a result of steroids. As endothelial adhesion and penetration is an early step in establishing mucormycosis, endothelial damage observed in severe COVID-19 disease may play an important pathogenic role. So damage to tissue and blood vessels from Covid-19 infection, treatment with corticosteroids, high background rates of diabetes in the population most severely affected by the coronavirus, and, importantly, more widespread exposure to the fungus in the environment are all likely to be playing a part in the situation we’re seeing with mucormycosis in India.
In severe COVID-19 patients, ferritin level rises 1.5 to 5 times higher than in non-severe cases. High IL-6 concentrations in COVID-19 patients have been correlated to disease severity. IL-6 directly stimulates ferritin production and increases the synthesis of hepcidin which in turn sequesters iron in enterocytes and macrophages thus preventing them to efflux from these cells leading to increased intracellular iron load. This excess intracellular iron generates reactive oxygen species (ROS) causing damage to the tissue and free iron is released in the circulation and available to Mucorales.
CAM prevalence was 0.27% among hospitalized COVID-19 patients during first wave and rose to more than 1.8 % during second wave; while mucormycosis prevalence in Indian hospitals before covid was only 0.0317 %. A retrospective study among 560 American hospitals with 104 million patients, estimated the prevalence of mucormycosis related hospitalizations at only 0.12 per 10,000 discharges before covid. One study noted 2.1-fold rise in mucormycosis cases during last quarter of 2020 compared to last quarter of 2019 in India. No doubt covid-19 is a predisposing factor for mucormycosis and covid-19 associated mucormycosis is a reality.
-5. Misuse of zinc supplements and steam inhalations also contributes to mucormycosis epidemic. Fungi thrive in zinc-rich environments… steam in excess can damage the delicate mucus layer and even cause burns along the mucosa, making it easy for fungus to breach our natural defence. Rampant distribution of zinc, iron and vitamin C supplements plus azithromycin and doxycycline to all suspected or confirmed cases of covid-19 was visible in India. Misuse of antibiotics like azithromycin and doxycycline harmed commensal bacteria lining nasal mucosa and led to fungal invasion. Even iron supplements cause increase in serum iron and help fungus acquire iron for its growth.
-6. Malnourishment can affect how well the immune system responds to infections. In fact, malnourishment is a growing problem in poorer sectors of India and could be one of the many contributing factors to rising Mucorales infections.
-7. The fact that a high proportion of mucormycosis cases are hospital-acquired points towards poor infection prevention and control (IPC) practice in the hospitals of India.
-8. India’s health system approach to mucormycosis fails to move beyond arranging doses of liposomal Amphotericin B and loosely pronounced advisories on rational use of drugs, with insufficient attention to epidemiology. A secondary epidemic superimposed on a pre-existing pandemic must not be accorded secondary priority. In fact, there is a range of distinctive reasons that make a public health response extremely crucial for combating the fungal threat: high fatality rate; high burden of comorbid risks like uncontrolled diabetes in the population; considerable toxicity profile of the drug used for the treatment; shortage of the drug and its prohibitive price; and so on.
All above points plus high counts of Mucorales spores in both the indoor and outdoor environments in India caused mucormycosis epidemic during 2nd wave of covid-19 pandemic in India.
In a nutshell, factors responsible for mucormycosis epidemic during 2nd wave of covid-19 pandemic in India can be summarized in the figure below:
_____
_____
Moral of the story:
_
-1. A fungus is any member of the group of eukaryotic organisms that includes unicellular microorganisms such as yeasts and multicellular organisms such as molds. Biologists classify these organisms as a kingdom Fungi, separate from the other life-kingdoms of plants, animals, protists, and prokaryotes. Most fungi are multicellular organisms. They display two distinct morphological stages: vegetative and reproductive. The vegetative stage consists of a tangle of slender thread-like structures called hyphae, whereas the reproductive stage can be more conspicuous. Most fungi grow as hyphae, which are cylindrical, thread-like structures 2–10 µm in diameter and up to several centimeters in length. A mycelium is a network of fungal threads or hyphae. Although individual hyphae must be observed under a microscope, the mycelium of a fungus can be very large. Fungal mycelia can become visible to the naked eye, for example, on various surfaces and substrates, such as damp walls and spoiled food, where they are commonly called molds. Some fungi produce visible fruit bodies called as mushrooms that sprout from a mycelium but there is an even greater diversity of microscopic fruiting bodies produced by various fungi. What all fruiting bodies have in common is that they produce spores and provide a mechanism for dispersing those spores.
_
-2. Fungi play an essential role in ecosystems because they are decomposers and participate in the cycling of nutrients by breaking down organic materials to simple molecules. With their versatile metabolism, fungi can break down organic matter which would not otherwise be recycled in the ecosystem. Some elements, such as nitrogen and phosphorus, are required in large quantities by biological systems, and yet are not abundant in the environment unless this breakdown takes place. Even trace elements present in low amounts in many habitats are essential for growth would remain tied up in rotting organic matter if fungi did not return them to the environment via their metabolic activity. Thus, fungi make it possible for other living things to be supplied with the nutrients they need to live.
Not only do they help to stabilize ecosystems and supply us with food, but they are also directly used in the production of beer, cheese, and bread, as well as various medicines. The human use of fungi for food preparation or preservation and other purposes is extensive and has a long history. They have long been used as a direct source of human food, in the form of mushrooms and truffles; as a leavening agent for bread; and in the fermentation of various food products, such as wine, beer, and soy sauce. Because of the capacity of this group to produce an enormous range of products with biological activities, many species have long been used or are being developed for industrial production of antibiotics, vitamins, enzymes, and anti-cancer & cholesterol-lowering drugs. Fungi are also used as biological pesticides to control weeds, plant diseases and insect pests. More recently, methods have been developed for genetic engineering of fungi, enabling metabolic engineering of fungal species.
_
-3. The word fungus usually evokes images of athlete’s foot, unseemly looking nails, or scrumptious cheese and mouth-watering mushrooms. However, few realize that fungi collectively kill over 1.6 million people annually, similar to the tuberculosis death toll. Fungi and oomycetes destroy a third of all food crops each year, which would be sufficient to feed 600 million people. Furthermore, fungal infestation of amphibians has led to the largest disease-caused loss of biodiversity ever recorded, while fungi also cause mass mortality of bats, bees and other animals, and decimate fruit orchards, pine, elm and chestnut forests. There are an estimated 1.5 million fungal species, of these only about 148,000 have been described, of these over 8,000 are known to cause disease in plants and 300 to be pathogenic to humans.
Fungi did not emerge as major pathogens for humans until the late 20th century. For example, candidiasis was uncommon until the 1950s, when thrush was associated with the introduction of antibiotics that disrupted bacterial flora. Similarly, diseases such as cryptococcosis, aspergillosis, and histoplasmosis were rare until recently, when their prevalence increased with the human immunodeficiency virus epidemic and the development of immunosuppressive therapies.
_
-4. Fungi have been found to possess between 6 and 21 chromosomes coding for 6,000 to nearly 18,000 genes. The average genome size of Ascomycota group of fungi is 36.91 Mb. The average genome size of Basidiomycota group is 46.48 Mb. The average genome size of Oomycota group of fungi is 74.85 Mb which is the highest among all groups. Genome sizes of fungi make fungal genomes among the smallest of eukaryotic organisms on average—approximately 1% the size of mammalian genomes.
_
-5. Fungi are classified into a separate group of organisms differing from both plants and animals, primarily by the type of nutrition. Fungi are not autotrophs, they have no chloroplasts, they can only use the energy stored in organic compounds. This distinguishes fungi from plants. As against animals, fungi are osmotrophic: they obtain food by absorbing nutrients from the environment. These feeding features correlate with fungal morphology and physiology. Wherever there is moisture, moderate temperatures, and a supply of organic food there are fungi. Since they digest their food outside of their bodies, they literally live within their food supplies. When the area around them is depleted, they grow into a new supply.
On the basis of nutrition, fungi can be classified into 3 groups, saprophytic, parasitic and symbiotic.
Saprophytic fungi obtain their nutrition by feeding on dead organic substances. Examples: Rhizopus, Penicillium and Aspergillus.
Parasitic fungi obtain their nutrition by living on other living organisms (plants or animals) and absorb nutrients from their host. Examples: Taphrina and Puccinia.
Symbiotic fungi live by having an interdependent relationship association with other species in which both are mutually benefited. Examples: Lichens (with alga) and mycorrhiza (with plants).
Mucormycosis is caused by saprophytic fungi.
_
-6. Fungi can reproduce asexually, sexually or both. Some fungal organisms multiply only asexually, whereas others undergo both asexual reproduction and sexual reproduction with alternation of generations. Asexual reproduction occurs by budding, fragmentation, or the production of spores. Most fungi produce a large number of spores, which are haploid cells that can undergo mitosis to form multicellular, haploid individuals. Most but not all fungi can also reproduce sexually through fusion to give rise to diploid nuclei which undergo meiosis producing spores. This introduces genetic variation into the general fungi population, distinct from asexual reproductive methods where the spores are genetically identical to the parent cell. Spores are always haploid cells no matter produced sexually or asexually. Many fungi produce more than one type of spore as part of their life cycles. A spore is a survival, reproductive and dispersal unit, consisting of one or a few cells, that is capable of germinating to produce a new hypha. Unlike plant seeds, fungal spores lack an embryo, but contain food reserves needed for germination. Majority of fungal spores are adapted for airborne dispersal but rain and insects can also spread spores. Most of fungal spores are transported by wind. Such species often produce dry or hydrophobic spores which do not absorb water and are readily scattered by raindrops. They eventually land in new habitats and if conditions are right, they start to grow and produce new hyphae. The exact life cycle depends on the species, but generally multicellular fungi have a haploid stage (where they have one set of chromosomes), a diploid stage, and a dikaryotic stage where they have two sets of chromosomes but the sets remain separate.
_
-7. Fungi are evolutionary link between prokaryotes & protists on one side and plants & animals on the other side. Fungi can switch between a multicellular hyphal and unicellular yeast form. Fungi can also switch between asexual and sexual reproduction. Fungi can also switch between a haploid stage, a diploid stage, and a dikaryotic stage.
_
-8. The paucity of fungal diseases in mammals relative to insects, amphibians, and plants is due to mammalian endothermy and homeothermy that are potent nonspecific defenses against most fungi and have provided a strong evolutionary survival advantage against fungal diseases. Most fungi cannot grow at mammalian temperatures. Most fungi are mesophilic, growing between 25 and 31°C. In opportunistic fungi there are few if any specialised virulence traits consistently linked to opportunistic pathogenicity of fungi in humans apart from the ability to grow at 37 °C. Rhizopus are able to grow at between 20 and 40°C and therefore most common genera of mucormycosis in humans.
_
-9. Fungal spores can become dormant for a long time until conditions are favorable for growth. Fungal spores can survive in environment for few days to few years depending on type of fungus and type of environment. A single spore can develop into a mycelium.
_
-10. Fungal spores measure one thousandth to one hundredth of a millimeter. The density of the spores – the number of spores per cubic meter of atmosphere – varies depending on the fungus, the location (vegetation and exposed earth) and season. In tropical areas like in India, spore counts are generally higher during the summer than during the monsoons. But compared to the 1,000-5,000 spores per cubic meter outdoors, the count inside homes is typically 100-250 only.
The characteristic features and size of the spores determine how deep they may penetrate into respiratory tract. Spores larger than 10 µm diameter are deposited in the nasopharynx; spores smaller than 5 µm penetrate to the alveoli; and spores <10 µm size mostly deposit in the bronchi and bronchioles.
When person breathes, every breath contains between 1 and 10 spores. It is estimated that humans may inhale more than 170,000 airborne fungal spores per day. Even though high numbers of spores enter the respiratory tract, these fungi infrequently cause infections in immunocompetent humans.
_
-11. Like most fungi, Mucorales produces millions of microscopic spherical, dark-hued structures called spores, which are dispersed in air. When the spores land on moist surfaces, like soil or plant material, they begin to germinate and produce thread like structures called mycelia. The mycelia branch out and feed on sugars in their surroundings and grow. Spores produced by asexual reproduction of Mucorales are called sporangiospores and by sexual reproduction are called zygospores. These sporangiospores range from 3 to 11 micrometers in diameter and are easily aerosolized and cause infections in susceptible hosts when inhaled, ingested or introduced through the cutaneous route. Incubation begins with inhalation of the spores or their direct inoculation into abraded skin. Whatever the route of infection (inhalation of airborne spores, ingestion, or direct skin inoculation), the hyphae invade blood vessels, causing tissue infarction and necrosis. Inhalation is the major route of acquiring mucormycosis. The relatively larger spores of R. arrhizus get trapped in the nasal epithelium & sinuses and thus may lead to ROCM, whereas the relatively smaller spores of Cunninghamella spp. can reach the lower respiratory tract leading to pulmonary mucormycosis. However, available evidence suggests that any pathogenic Mucorales species can produce any type of clinical presentation. The term mucormycosis is now preferred for infections caused by molds belonging to the order Mucorales.
_
-12. Fungal species are ubiquitously present in the environment, including the human sinonasal tract. The ubiquitous presence of fungal spores in the sinonasal tract suggests that fungal-related sinonasal disease has less to do with the presence or absence of fungi. Instead, the presence of fungal rhinosinusitis and its various forms relates more to the status of the host’s immune system and subsequent host–microbe interaction.
_
-13. Fungal pathogens afflicting humans are subdivided into those that remain superficial (i.e., restricted to the epithelial surface) and those that invade deep organs and tissues (deep fungi). Some species are considered opportunistic (infecting only immunocompromised hosts) and others truly pathogenic (i.e., capable of infecting normal persons). Overall, mucormycosis is the third leading cause of invasive opportunistic fungal infection after Aspergillus and Candida spp.
The occurrence of superficial as well as invasive opportunistic fungal infections has increased significantly over the past two decades. This increase can be attributed to the growing number of immunocompromised patients- including those with AIDS, neoplastic disease, advanced age, long-standing diabetes mellitus, undergoing HSCT and marrow transplantation, solid-organ transplantation, major surgery, receiving immunosuppressive therapy and premature infants. Of course, trauma, burns, and malnutrition also increase susceptibility to fungal infection. Genetic predisposition to invasive fungal infection has been reported recently.
_
-14. Mucormycosis is an uncommon, life-threatening infection caused by fungi belonging to the subphylum Mucormycotina, order Mucorales. Eleven genera and ~27 species under Mucorales are associated with human infections. Mucormycosis is an acute opportunistic invasive fungal infection caused by a broad, non-septate, saprophytic fungus which is found in soil, compost, animal dung, rotting wood and plant material. You may have seen them as the black growth on old fruits and bread. Humans acquire the infection predominantly by inhalation of sporangiospores, occasionally by ingestion of contaminated food or traumatic inoculation. Its spores can be cultured from the mouth, nasal tract, throat and the faeces of healthy persons but it is kept in check by the body’s defense mechanisms. Our immune systems are usually able to combat and filter out fungal spores as they enter our bodies. When immune defense is compromised, the fungal spore germinates into hyphae which grows and spreads. In most cases it is due to an invasion of the genera Rhizopus and Mucor.
Mucormycosis has an incubation period of 2 to 5 days from deposition of spores in nasal mucosa till development of hyphae. From the sinus, it takes around 2 to 4 days to reach to the eyes. And to more concern, it takes merely a day to reach from eyes to the human brain. Infections by Mucorales are typically rapidly progressive.
_
-15. The epidemiology of mucormycosis is evolving as new immunomodulating agents are used in the treatment of cancer and autoimmune diseases, as the modern diagnostic tools lead to the identification of previously uncommon genera/species, and as we are facing the triad of covid-19 pandemic, uncontrolled diabetes and overuse of corticosteroids.
_
-16. Mucormycosis is not contagious; can’t spread between people or between people and animals. Since mucormycosis cannot be transmitted from person to person, there is no need for people to isolate — unless, of course, they have an ongoing SARS-CoV-2 infection. Rather, the source of infection is environmental, from airborne spores produced by the fungi. Mucormycosis, formerly virtually always community acquired and often in the setting of diabetic ketoacidosis, is rapidly becoming a nosocomial infection in patients with malignancy or undergoing organ or hematopoietic stem cell transplantation, and now covid-19 with diabetes & steroids. Nosocomial mucormycosis has been associated with iatrogenic immunosuppression and a variety of procedures or devices used in hospitals, including antifungal prophylaxis, bandages or medication patches, intravenous catheters, and even tongue depressors. Since invasive mucormycosis is found to be an important cause of mortality in debilitated patients, a high index of suspicion should exist among the clinicians to predict the outbreaks in the hospital environment.
_
-17. In a 2019 paper, Prof. Richardson concludes: “All of these observations suggest that house residents are not generally exposed to mucormycetes in their home environment, apart from mould-contaminated food items, such as bread and fruit.” However, other studies indicated that Mucorales species were present in 98% of samples taken from home dust (Gravesen, 1978; Quandahl and Cooper, 2018).
_
-18. Mucorales spores are routinely found in upper respiratory tract of healthy individuals. It is impossible to avoid coming into contact with Mucorales spores as they are ubiquitous. Therefore gloves, gown, and masking are unnecessary to prevent transmission of mucormycosis to healthy individuals except visit to construction or excavation sites where large inocula of Mucorales can be inhaled resulting in slowly progressing pulmonary mucormycosis even in immunocompetent hosts. There is no evidence that face masks can harbour Mucorales fungi. Someone who already has a fungal infection in their lungs/sinus could possibly contaminate their own mask, but not the other way around. The spores are not harmful for most people except those with compromised immunity or breach in skin/mucous membrane. Although various preventive measures have been advocated for high-risk individuals, these preventive measures haven’t been proven to prevent mucormycosis.
Transplantation and chemotherapeutic wards are often isolated with Hepa-filter treatment of the air supply and positive pressure to exclude the recruitment of dust into the ward. Additionally, flower arrangements and live plants are often excluded from such wards since they may harbor a variety of fungal agents. Patients when neutropenia below 1,000/microliter are asked to wear masks when leaving the cancer or transplant wards, particularly when going outside. Preventive measures for patients other than the transplant and chemotherapy population require addressing the underlying risk factors for developing mucormycosis i.e., proper glycaemic control, appropriate steroid therapy, use of iron chelators other than deferoxamine, limiting the use of aluminum-containing buffers in dialysis etc. Keeping a high level of suspicion for mucormycosis in patients who are at risk can aid an early diagnosis and implementation of appropriate therapy.
_
-19. The hallmark of mucormycosis is angioinvasion, causing arteritis, vessel thrombosis, tissue ischaemia, and necrosis with bony destruction. Angioinvasion results in hematogenous dissemination of the organism, whereas ischemia and necrosis of the affected tissues prevents penetration of immune cells and antifungal agents to the infection focus. Invasive aspergillosis is also characterized by angioinvasion.
_
-20. The innate immune system is well equipped to recognize and destroy pathogenic fungi through specialized cells expressing a broad range of pattern recognition receptors (PRRs). It is the innate immunity expressed by mononuclear (macrophage) and polymorphonuclear (neutrophil) phagocytes of normal hosts that kill Mucorales by generation of oxidative metabolites and cationic peptides defensins. To establish infection, spores must overcome killing by mononuclear and polymorphonuclear phagocytes to germinate into hyphal forms, the angioinvasive form of the infection. A recent study showed that exposure of neutrophils to R. oryzae hyphae results in up-regulation in Toll-like receptor 2 expression and in a robust proinflammatory gene expression with rapid induction of NF-κB pathway–related genes. In a normal host, primary defense mechanisms against mucormycosis include sequestration of iron in serum by specialized iron-binding proteins; phagocytes including circulating neutrophils and tissue macrophages; and endothelial cells, which regulate vascular tone and permeability. Acting in concert, these mechanisms prevent establishment of infection in tissue and subsequent endovascular invasion. To cause disease, the agents of mucormycosis must scavenge from the host sufficient iron for growth, must evade host phagocytic defense mechanisms, and must access vasculature to disseminate.
_
-21. Neutrophils are the key host defense against spores, germlings and hyphae of Mucorales fungi; thus, individuals with neutropenia or neutrophil dysfunction (e.g., diabetes, steroid use, acidosis, high serum iron) are at highest risk.
_
-22. Only few cases of mucormycosis have been reported in patients with acquired immunodeficiency syndrome (AIDS), suggesting that the host defense against this infection is not primarily mediated by cellular immunity although Mucorales-specific T cells emerged in the course of invasive mucormycosis in one study. The paucity of reports of mucormycosis in HIV-infected patients who do not inject intravenous illicit drugs indicates that T-cell dysfunction alone is not a major determinant in the development of the disease. Mucormycosis in patients who are HIV positive is extremely rare. In retrospective study of 1630 autopsies of patients who died of AIDS from 1984 to 2002, only 2 patients had mucormycosis.
_
-23. Glucose Regulated Protein 78 (GRP78) is host receptor which mediates invasion and subsequent injury of endothelial cells by Mucorales, but not C. albicans or A. fumigatus. GRP78 is a specific and universal receptor for germlings and not spores of several Mucorales members. Binding to germlings is consistent with the hypothesis that hyphae are the invading form of Mucorales. This recognition causes host cellular death by induction of the endothelial cell–mediated fungus endocytosis. The CotH protein kinase belonging to the spore coating protein family in Rhizopus acts as the ligand for GRP78, which helps the fungus to adhere and invade endothelial and nasal epithelial cells. Blocking the function of CotH proteins either biochemically by using anti-CotH antibodies or genetically by attenuating CotH expression reduces the ability of R. delemar to invade and injure endothelial cells in vitro and reduces disease severity in mice. CotH proteins are universally present in Mucorales and absent from any other forms of life for which the genome has been sequenced.
_
-24. Mucormycosis was already more common in India than in any other country even before the covid-19 pandemic. The reported annual incidence of mucormycosis is for Europe (from 0.2 cases in Denmark to 95 cases in Portugal), USA (3.0 cases), Canada (1.2 cases) and Australia (0.6 cases) per 1,000,000 individuals. However, this is likely to be an underestimate because of the low rate (23–50%) of antemortem diagnosis and also because a large number of cases remain undiagnosed due to the decline in autopsies in the United States and Europe from around 60% in the 1960s to around 10% at present. According to WHO, the incidence rate of mucormycosis globally varies from 0.005 to 1.7 per million population. The exact incidence of mucormycosis in India is unknown due to the lack of population-based studies. A computational-based approach estimated the prevalence of mucormycosis at 140 cases per million populations in India which is 80 times the prevalence in developed countries. Indian environment apparently promote growth of molds and Indian air contains high spore count. Various studies from hospitals across India have revealed heavy mold spore counts even in hospital air due to predominantly hot, humid conditions in India’s tropical climate. In my view, unclean environment due to untreated animal dung and decaying plant materials contribute more than climate as many nations have climate similar to India without epidemic of mucormycosis. There is widespread exposure to the fungus from the environment in India as compared to other nations.
_
-25. The most important conditions that predispose to mucormycosis according to various studies include diabetes mellitus (DM) with or without ketoacidosis, hematological malignancies (HM), other malignancies, transplantation, prolonged neutropenia, corticosteroids use, trauma, iron overload, illicit intravenous drug use, neonatal prematurity and malnourishment. Immunocompetent patients can also be affected, when the spores of the fungus are directly inoculated in the skin as a result of trauma or burns. Several studies have shown that predisposing conditions are correlated to the site of infection. Hematological malignancies and neutropenia are associated with pulmonary mucormycosis and diabetes mellitus with sinusitis and rhinocerebral disease, while trauma usually leads to cutaneous mucormycosis.
_
-26. Of all mucormycosis cases published in scientific journals globally between 2000-2017, diabetes was seen in 40% of cases. A multicenter study of 187 cases of CAM after the first COVID wave found uncontrolled diabetes in 62.7% of cases. The US has a very high prevalence of diabetes – 9.3% of the population and also has the highest number of Covid cases globally. But mucormycosis is very rare because diabetes cases there are largely managed with only 3% going undiagnosed. In India, 50 % diabetes cases are undiagnosed.
Hyperglycemia stimulates fungal proliferation as fungi utilize glucose of growth. Type 2 diabetes mellitus itself is an immunocompromised state which leads to dysregulated, dysfunctional innate and adaptive immune cells making the host susceptible to infections by Mucorales. Due to increased glycosylation, IL-10 production by lymphocytes and macrophages is significantly reduced. Hyperglycemia impairs chemotaxis and the oxidative and non-oxidative fungicidal mechanisms used by phagocytic cells – the main defensive mechanism against mucormycosis. Hyperglycemia is a stress condition that induces the overexpression of the glucose regulated protein (GRP78) resulting in endothelial cell invasion by Mucorales. In the diabetic ketoacidosis patient, there is an increased risk of mucormycosis caused by Rhizopus oryzae as these organisms produce the enzyme ketoreductase, which allows them to utilize the patient’s ketone bodies. In states of acidosis related to hyperglycemia, free iron becomes readily available in the serum as acidosis temporarily disrupts the ability of transferrin to bind iron. Mucorales can acquire this excess endogenous iron through siderophores (low-molecular-weight chelators) or iron permeases enhancing their virulence. Fungal growth is enhanced in high-glucose, high-iron, and acidic environments. Glucose, iron, and acidosis by β-hydroxy butyrate modulate GRP78/CotH interactions. It is also worth noting that physiological concentrations of glucose, iron, and β-hydroxybutyrate (BHB) also have detrimental effect on the host immune response via suppression of T-lymphocyte induction, interferon-Ɣ production, and phagocyte-mediated killing.
_
-27. Metabolic acidosis due to any cause is a key factor in predisposing patients to mucormycosis. It has been demonstrated that low serum pH diminishes the phagocytic and chemotactic ability of neutrophils. Furthermore, lower blood pH disrupts transferrin binding of iron, leading to increased iron availability. Animal Studies showed with reversal of pH by sodium bicarbonate completely protects endothelial cells from Rhizopus-mediated invasion and injury. Reversal of ketoacidosis in Rhizopus-infected mice by administration of sodium bicarbonate (in lieu of insulin) improves survival. I recommend short course of sodium bicarbonate tablets to all patients with mucormycosis (if not contraindicated) to improve survival. People take sodium bicarbonate tablets as antacids, as urine alkalizer and also in CKD. This is a novel use.
_
-28. Corticosteroids affect virtually all immune cells. Some major immune suppressive actions of corticosteroids are: i) antagonism of macrophage maturation and differentiation, ii) decrease of interleukin-1, interleukin-6, tumour necrosis factor, proinflammatory prostaglandins and leukotrienes production by macrophages, iii) suppression of microbicidal activity of activated macrophages. Corticosteroids impair migration, ingestion and phagolysosome fusion in macrophages. They also suppress neutrophil adhesion to endothelial cells and impair lysosomal enzyme release, respiratory burst, and chemotaxis to the site of infection. In addition, they may lead to hyperglycemia and worsen pre-existing diabetes. Hyperglycemia itself is a risk factor for mucormycosis (vide supra). Corticosteroids can increase blood glucose levels by acting as a substrate for oxidative stress metabolism with lipolysis, proteolysis, and hepatic glucose production. They also increase insulin resistance in up to 60%-80% of patients depending on the dose and type used. Prolonged (>3 weeks) high-dose systemic corticosteroids are risk factors for mucormycosis. However, there have been reports of mucormycosis associated with short courses of corticosteroids especially in covid-19 patients.
_
-29. Iron is required by virtually all microbial pathogens for growth and virulence. In mammalian hosts, very little serum iron is available to microorganisms because it is highly bound to carrier proteins such as transferrin and also stored as ferritin. Sequestration of iron by serum is a major host defense mechanism against R. oryzae in particular. The organism grows poorly in serum and this growth inhibition is reversed when exogenous iron is added. Fungi secure iron from the host by using high-affinity iron permeases or low-molecular-weight iron chelators (siderophores). R. oryzae additionally obtain iron from host hemoglobin and might explain the angioinvasive nature of R. oryzae. In fact, the angioinvasion may be due to the need of iron which get fulfilled from hemoglobin.
Serum iron may be increased in patients undergoing dialysis, multiple transfusions and liver transplant recipients; and they are susceptible to mucormycosis. Data from animal models emphasize the exceptional requirement of iron for Rhizopus pathogenicity, as administration of deferoxamine or free iron worsens the survival of animals infected with Rhizopus, but not with Candida. Patients with elevated available serum iron, be it free iron or ferrioxamine iron, are at high risk of acquiring mucormycosis. Increased iron levels upregulate GRP78 expression and promote endothelial cell invasion and damage by R. oryzae in a receptor-dependent manner. There is also impaired chemotaxis of neutrophils in response to R. oryzae infection in mice given excessive amount of iron compared to normal mice.
Increased serum iron is a risk factor for mucormycosis, as iron plays a crucial role in the pathogenesis of this infection. Indiscriminate use of iron supplements to covid-19 patients with diabetes and steroids may have contributed to mucormycosis epidemic in India as iron supplements can cause increase in serum iron and help fungus acquire iron for its growth.
We need further studies to know how common mucormycosis is among iron deficient people as significant population of India is iron deficient. Logic suggests that untreated iron deficient population should have lower incidence of mucormycosis due to lower serum iron, all other factors being equal.
_
-30. The well-documented and repeatedly reported increased susceptibility to mucormycosis during treatment with deferoxamine (DFO), an iron chelator that is capable of removing tissue iron initially appeared to be a paradox. It became clear, however, that although DFO chelates iron, from the perspective of mucormycetes it is a xenosiderophore, as fungal siderophores have higher affinity for iron than DFO and therefore are capable of easily and effectively detaching iron from it and providing it to the fungi. Deferoxamine predisposes patients to Rhizopus infection by acting as a siderophore, which supplies previously unavailable iron to the fungus. Rhizopus obtains iron from the iron-deferoxamine complex by intracellular transport of the reduced iron without deferoxamine internalization. This transport is likely mediated by high-affinity iron permeases. A similar phenomenon does not take place with deferiprone and deferasirox. These two newer iron chelators deferiprone (DFP) and deferasirox (DFX) do not act as xenosiderophores, apparently because the fungal iron uptake systems are incapable of detaching iron from them. This could be due either to inadequate molecular access, as they are smaller molecules than DFO, or to their higher affinity for iron, which means that DFP and DFX might form more stable chemical structures with iron that are not destabilized in the presence of fungal enzymes or siderophores. Mice treated with the deferiprone and deferasirox (two iron chelators that deprive R. oryzae from acquiring external iron) are protected from hematogenous disseminated mucormycosis. Deferasirox is a reasonable salvage options for patients with mucormycosis refractory to or intolerant of polyene therapy.
_
-31. It is becoming increasingly apparent that efficient zinc acquisition represents a critical component of microbial pathogenicity. Rhizopus delemar (previously R oryzae), one of the main species causing Mucormycosis, encodes 3 cell surface zinc importers. Zinc actually acts as growth factor of Mucormycosis. Our immune system has learned to recognize this fungal zinc uptake system and evolved sophisticated mechanisms to withhold this essential micronutrient from invading pathogens. Zn deficiency induces stress in fungal cells and hampers fungal development by restricting the activity of Zn-binding proteins, which are mainly transcription factors involved in many biological processes. Indiscriminate use of zinc supplements during 2nd covid wave may have contributed to epidemic of mucormycosis in India.
_
-32. A 2011 study found that 86.79% swabs form oxygen humidifiers grew fungi. Oxygen humidifiers must be cleaned and disinfected, and use sterile water for humidification. However, nearly 100 % oxygen stored in cylinders (medical or industrial) is likely to be detrimental to the growth of microorganisms of all kinds including fungi. Even humans get oxygen toxicity caused by exposure to oxygen at partial pressures greater than those to which the body is normally exposed. So inhalation of humidified oxygen is unlikely to cause mucormycosis.
_
-33. Unlike other fungi (e.g., C. albicans, Cryptococcus neoformans, etc.), fungal-induced endothelial cell injury in Mucorales does not require fungal viability since dead germlings (heat, ethanol, or glutaraldehyde-killed) are able to cause a similar degree of injury to endothelial cells as do live organisms. So antifungal agents may kill fungal hyphae and prevent extension of disease but cannot obliterate pathogenicity because even dead hyphae can cause ischemia and necrosis. So physical removal of hyphae (live or dead) by surgical debridement is the best way to treat mucormycosis (albeit causing disfigurement) along with antifungals. Surgical resection of necrotic tissues is the core of mucormycosis therapy. Surgical debridement is critical, as blood vessel thrombosis and resulting tissue necrosis impedes delivery of necessary antifungal agents to the site of infection as well as prevent phagocytes to reach site of infection.
_
-34. Based on anatomic localization, mucormycosis can be classified in different forms: (1) rhinocerebral, (2) pulmonary, (3) cutaneous, (4) gastrointestinal, (5) disseminated, and (6) uncommon presentations. Tissue necrosis is the hallmark of mucormycosis, but presentation and syndrome-oriented approach to diagnosis lacks sensitivity and specificity. As the pathophysiology, mode of acquisition and underlying patient risk factors for mucormycosis are similar to aspergillosis, clinical distinction between the two entities is difficult. Both infections are acquired primarily through inhalation of spores, which are ubiquitous in the environment leading to sinopulmonary disease. Furthermore, in countries where tuberculosis is endemic, the two infections may coexist especially among diabetics.
_
-35. The symptoms, signs, and radiographic manifestations of mucormycosis are nonspecific, and a definitive diagnosis requires direct identification of the characteristic hyphae or recovery of the organism in culture from specimens obtained from the site of infection. A high index of suspicion is required to make the diagnosis of rhinocerebral mucormycosis, as evidenced by the fact that autopsy series have found up to half of cases are diagnosed post-mortem. Mucormycosis diagnosis consists of recognition of risk factors, assessment of clinical manifestations, early use of imaging modalities and prompt initiation of diagnostic methods based on direct microscopy of wet mounts, histopathology, cultures and advanced molecular techniques.
_
-36. Minutes and hours count, and if the clinical suspicion is high, the workup should proceed on an emergent basis even if the patient currently appears clinically stable. Indeed, delayed diagnosis has been associated with a dramatically worse outcome. Early diagnosis of mucormycosis is important, and prompt therapeutic intervention may prevent progressive tissue invasion and its sequelae. These sequelae include (1) angioinvasion and direct tissue injury of the respiratory tract, (2) direct extension from lungs into the great vessels, (3) invasion from the paranasal sinuses into the orbit and brain, and (4) hematogenous dissemination to central nervous system tissues. Early diagnosis may also reduce the need for or extent of surgical resection, disfigurement, and suffering. Finally, early diagnosis may improve outcome and survival.
_
-37. For a rapid presumptive diagnosis of mucormycosis direct microscopy of KOH wet mounts can be used. It can be applied to all materials sent to the clinical laboratory, preferably using fluorescent brighteners such as Blankophor and Calcofluor White together with KOH, which enhance the visualization of the characteristic fungal hyphae, in this case requiring a fluorescent microscope. Direct microscopy with fluorescent brighteners from clinical specimens is strongly recommended mainly focusing on septation, branching angle, and hyphal width. Direct microscopy of fresh material is an inexpensive, yet invaluable method to rapidly give a presumptive diagnosis and to define clear surgical margins for invasive fungal infection intraoperatively, and it is strongly recommended, along with histopathology.
_
-38. A definitive diagnosis is based on the demonstration of fungal hyphae typical for mucormycetes in biopsies of affected tissues, or bronchoalveolar lavage (BAL) in patients with pulmonary mucormycosis. Histopathology is a very important diagnostic tool since it distinguishes the presence of the fungus as a pathogen in the specimen from a culture contaminant and is indispensable to define whether there is blood vessel invasion. It can furthermore reveal coinfections with other molds. Mucorales genera produce typically non-pigmented, wide (5–25 μm), thin-walled, ribbon-like hyphae with no or few septations (pauciseptate) and right-angle branching, in contrast to those of the Aspergillus species or other hyaline molds, which are typically 3–5 μm wide, septate and form acute-angle branching.
The wider and irregular (ribbon-like) nature of the Mucorales hyphae are more reliable distinguishing characteristics than septations and angle of branching.
Hyphae may be observed within necrotic tissue with signs of angioinvasion and infarction; neutrophilic infiltrates or granuloma formation may be present in patients who are not granulocytopenic or with more chronic infection, respectively.
Obtaining a diagnosis of mucormycosis on histomorphological basis is challenging, and the most common cause for incorrect morphological diagnosis is the misidentification of Mucorales as Aspergillus spp. The application of immunohistochemistry with commercially available monoclonal antibodies or PCR techniques on either fresh or formalin-fixed paraffin-embedded tissue, have been shown to be highly specific, although a variation in sensitivity has been reported, in addition, these tests might not be widely available.
_
-39. Culture of specimens is essential for the diagnosis of mucormycosis since it allows identification to the genus and species level, and eventually antifungal susceptibility testing. Identification of Mucorales to the genus and species level requires growth of the fungus in culture to identify reproductive fruiting structures of the fungus. Fungal colonies can exhibit growth shapes and colors (due to spores or pigmentation) that can be used as diagnostic features in the identification of species or groups. Mucorales can easily be identified as they typically produce cottony growth, a macro-morphological feature. Identification of Mucorales to species level is based on micro-morphological features.
A positive culture from a sterile site confirms the diagnosis, while a positive culture from a non-sterile site could be due to a contaminant and must be combined with clinical and radiological data to establish a probable diagnosis. The Mucorales may be seen in the laboratory as clinical contaminants, presumably as a result of airborne contamination of the culture medium, or they may be seen in clinical specimens as a result of oral or nasal ingestion in food or air prior to sample collection. Hence, there is a caveat for falsely positive results, especially when histopathology is not available. The major concern about culture, however, is its low sensitivity, as it can be falsely negative in up to 50% of mucormycosis cases. This can be attributed to a number of reasons, such as grinding or homogenization of tissue specimens, which may destroy the delicate hyphae of mucormycetes, the presence of genera that require special culture conditions, recent or ongoing therapy with antifungals effective on Mucorales, or even a lack of expertise. Furthermore, waiting for the results of the fungal culture may delay the institution of appropriate therapy.
_
-40. Identification of Mucorales to species level is based on microscopic features, PCR followed by sequencing and Matrix-Assisted Laser Desorption/Ionization-Time of Flight (MALDI-TOF) mass spectrometry. Identification to the species level is of importance for improved epidemiological knowledge of the disease. There is currently sparse evidence that identification of the causative Mucorales to the genus or species level, or both, could guide the choice of the antifungal treatment. However European Society of Clinical Microbiology and Infectious Diseases (ESCMID) / European Confederation of Medical Mycology (ECMM) guidelines recommend susceptibility testing to guide treatment of mucormycosis.
_
-41. Several new molecular methods for the diagnosis of mucormycosis have been reported; however, these techniques may lack sensitivity, can be time-consuming and expensive to perform, and are not universally available. Fresh or frozen biopsy samples are preferred for molecular diagnosis. The efficiency of these assays has not been widely studied, lacks thorough clinical evaluation and therefore cannot be recommended as stand-alone, single approach in clinical routine diagnostics.
The use of quantitative polymerase chain reaction (qPCR) for detection of circulating DNA from common Mucorales species (Lichtheimia species, Rhizomucor species, and Mucor/Rhizopus species), while not yet commercially available, has been described and appears promising for the early diagnosis of mucormycosis in high-risk patients. Assay positivity was observed at an average of 9 days before diagnosis was supported by histological or mycological evidence and 2 days prior to positive imaging findings often performed before the mycological investigations. Development of PCR negativity after treatment was associated with higher survival rates (48% vs 4%), suggesting that this modality could eventually be used for treatment monitoring.
_
-42. Histological analysis is an important diagnostic tool in the early management of this devastating disease, however, the 24-h turnaround time of the microculture approach is considerably shorter than that of histopathological analysis (48–72 h) or conventional culturing (3–7 days). So, there is a role of microculture assay as a putative, rapid, and low-cost culture-based method for the early diagnosis of mucormycosis.
_
-43. The initial imaging study is frequently negative or has subtle findings. Radiographic findings lag behind clinical progression in this disease, and a negative imaging study does not provide a rationale to delay more aggressive diagnostic manoeuvres (e.g., endoscopy with biopsy) if clinical suspicion is high. The appearance of tissue at endoscopy may also lag behind invasion, as the mucosa can appear pink and viable during the initial phase of fungal invasion. Endoscopy and radiography appearance lag behind clinical progression, so in suspicious cases, blind biopsies of sinus mucosa or thickened extraocular muscles are warranted to make the diagnosis. Patients with suspected mucormycosis should undergo nasal endoscopy and oral examination with biopsy from a suspicious area. Fungal invasion may be patchy, so multiple biopsies may be required for definitive diagnosis.
_
-44. Head and facial CT imaging should be used as the initial investigation in rhinocerebral infections. CT scans may show sinusitis of the ethmoid and sphenoid sinuses, as well as orbital and intracranial extension. Magnetic resonance (MR) imaging is quite useful in identifying the intradural and intracranial extent of ROCM, cavernous sinus thrombosis, and thrombosis of cavernous portions of the internal carotid artery. Contrast-enhanced MR imaging can also demonstrate perineural spread of the infection. Although evidence of infection of orbital soft tissues may be seen on CT scans, MR imaging is more sensitive for this. MRI of the facial sinuses and brain is superior to a CT scan in assessing the degree of tissue invasion and need for ongoing surgery. Imaging studies are nonspecific for ROCM, and diagnosing ROCM almost always requires histopathological evidence of fungal tissue invasion.
Initial CT and MRI scans are sometimes unremarkable in patients during the initial stages of rhinocerebral mucormycosis but show evidence of rapid infection progression 48-72 hours later. Therefore, serial radiographic imaging is important in patients with suspected mucormycosis. Due to the aggressive nature of mucormycosis CT or MRI scans should also be obtained at frequent intervals to monitor disease extension and response to therapy.
_
-45. Concomitant sinusitis; history of voriconazole prophylaxis; multiple (≥10) nodules, pleural effusion and reverse halo sign (RHS) on CT scan are more likely in pulmonary mucormycosis than aspergillosis. Though no biomarker is available for the diagnosis of mucormycosis, repeated negative galactomannan antigen, 1,3-ß-D-glucan, and Aspergillus specific PCR results in a patient with strong suspicion of invasive mold infections of the lungs may suggest pulmonary mucormycosis. Unfortunately, sputum culture is highly unreliable. In two case series, sputum and bronchiolar alveolar lavage cultures were negative in 18 of 19 cases of biopsy-proven pulmonary mucormycosis. Therefore, biopsy with histopathological assessment remains the best modality to diagnose pulmonary mucormycosis.
_
-46. Mucormycosis is a medical and surgical emergency. No treatment is not an option, as untreated mucormycosis is universally fatal. Suspected mucormycosis requires urgent intervention, because of the often rapidly progressive and destructive nature of the infection. Delayed initiation of therapy is associated with increased mortality. Effective treatment of mucormycosis includes (1) early and rapid diagnosis; (2) reversal of underlying risk factors; (3) surgical debridement where applicable; and (4) prompt antifungal therapy.
Patients with suspected mucormycosis should be referred immediately to a facility with the highest care level. It is critically important to emphasize that if mucormycosis is suspected, initial empirical therapy with a polyene antifungal should begin while the diagnosis is being confirmed, rather than waiting while a protracted series of diagnostic tests are completed. Immediate first-line antifungal therapy must be followed by radical surgical debridement where applicable. Unlike bacteria, fungi do not respond to traditional antibiotic therapy because they are eukaryotes.
_
-47. Lipid formulations of amphotericin B are considered superior over amphotericin B deoxycholate in the treatment of mucormycosis, as the former has less nephrotoxic effects, better penetration to the brain, and has superior immunomodulatory effects. Liposomal amphotericin B is strongly recommended at a dose of 5 to 10 mg/kg per day for 4-6 weeks. Primary or salvage isavuconazole therapy may be continued for several months given its higher tolerability compared with amphotericin. Repeated surgical debridement of necrotic tissue identified by follow-up head computed tomography (CT) scan or magnetic resonance imaging (MRI) is often indicated in rhinocerebral mucormycosis.
The current epidemic of mucormycosis in India has led to limited availability or even nonavailability (in certain regions) of amphotericin B, posaconazole or isavuconazole, making the situation imperative to use alternative antifungals. When amphotericin B, isavuconazole and posaconazole are not available, itraconazole therapy, 200 mg thrice a day for 3-6 weeks may be considered as in vitro antifungal susceptibility testing has consistently shown itraconazole active against Mucorales.
_
-48. There is no vaccine for mucormycosis. Given the uncommon nature of infection, primary antifungal prophylaxis for mucormycosis is not recommended. In neutropenic patients or those with graft versus host disease, primary prophylaxis with posaconazole delayed release tablets is recommended. The spectrum of posaconazole includes covers both Aspergillus and Mucorales, which should theoretically reduce the risk of mucormycosis cases in high-risk patients when administered as prophylaxis. However, posaconazole is not a panacea. Compliance issues, suboptimal absorption, and drug-drug interactions frequently result in low posaconazole serum levels and occasional breakthrough mucormycosis infections.
_
-49. Mucormycosis is frequently a life-threatening infection. All-cause mortality rates for mucormycosis vary from 40% to 80% depending on the rapidity of diagnosis and treatment, the site of infection, the patient’s underlying conditions, the degree of immunosuppression, and the type of fungus.
_
-50. Why does a rare condition like mucormycosis end up becoming an epidemic during 2nd wave of covid-19 pandemic in India?
-1. There is high counts of Mucorales spores in both the indoor and outdoor environments in India. The prevalence of mucormycosis in India was about 80 times the prevalence in developed countries even before the pandemic.
-2. There was explosion of covid-19 cases during 2nd wave in 2nd quarter of 2021 in India. The dysregulated innate immune response, ciliary dysfunction, cytokine storm, thrombo-inflammation, microvascular coagulation and eventual immune exhaustion during covid-19 facilitates secondary bacterial and fungal infections especially in critically ill patients subjected to emergency invasive procedures. CAM prevalence was 0.27% among hospitalized COVID-19 patients during first wave and rose to more than 1.8 % during second wave; while mucormycosis prevalence in Indian hospitals before covid was only 0.0317 %. A retrospective study among 560 American hospitals with 104 million patients, estimated the prevalence of mucormycosis related hospitalizations at only 0.12 per 10,000 discharges before covid. One study noted 2.1-fold rise in mucormycosis cases during last quarter of 2020 compared to last quarter of 2019 in India. No doubt covid-19 is a predisposing factor for mucormycosis and covid-19 associated mucormycosis is a reality.
-3. India is the diabetes capital of the world. An estimated 77 million people are affected with diabetes in India, representing the largest number of any country in the world. Unfortunately, more than half the cases of diabetes in India are undiagnosed.
-4. There was overuse and misuse of corticosteroids by people as well as doctors during 2nd wave of covid-19 in India.
-5. There was rampant distribution of zinc & iron supplements plus azithromycin & doxycycline to all suspected or confirmed cases of covid-19 in India.
-6. There was excessive steam inhalation by normal as well as covid-19 patients during 2nd wave of covid-19
-7. Malnourishment is a growing problem in poorer sectors of India and could be one of the many contributing factors to rising Mucorales infections.
-8. There is poor infection prevention and control (IPC) practice in many hospitals of India.
-9. India’s health system approach to mucormycosis fails to move beyond arranging doses of liposomal Amphotericin B with insufficient attention to epidemiology. A secondary epidemic superimposed on a pre-existing pandemic must not be accorded secondary priority.
However, millions all over the world are getting infected with COVID, are diabetic and put on steroids and reside in countries where malnutrition and poor infection control practices exists but gets mucormycosis occasionally.
Then why is it that only India gets epidemic of Mucormycosis during covid-19 pandemic?
Well, it boils down to high counts of Mucorales spores in both the indoor and outdoor environments in India; very large number of undiagnosed and uncontrolled diabetes; overuse and misuse of corticosteroids, antibiotics, zinc & iron supplements by people themselves and by doctors as well.
However, high counts of Mucorales spores in both the indoor and outdoor environments and very large number of undiagnosed and uncontrolled diabetes existed in India during first wave of covid-19. CAM prevalence was 0.27% among hospitalized COVID-19 patients during first wave and rose to more than 1.8 % during second wave; i.e., more than six times. Mucormycosis epidemic happened predominantly in India during 2nd wave as compared to first wave and rest of the world. So, there is something in India during 2nd wave which did not happen in first wave and did not happen in rest of the world. It boils down to overuse and misuse of corticosteroids, antibiotics, zinc & iron supplements by people themselves and by doctors as well, as the root cause of mucormycosis epidemic. People’s awareness increased due to experience of the first wave and doctors got panicked due to non-availability of oxygen, hospital beds and remdesivir. So, everybody took lots of corticosteroids, zinc, iron and antibiotics to save lives no matter whether they are covid-19 patients, suspects, doubtful or even healthy. Inappropriate glucocorticoid use was seen even during first wave but it worsened exponentially during second wave precipitating mucormycosis epidemic. This is the truth.
_____
Dr. Rajiv Desai. MD.
July 21, 2021
_____
Postscript:
The events unfolded during coronavirus pandemic in India are unparalleled in the world. Be it migrant crisis; be it loss of millions of jobs; be it unprecedented decline in GDP resulting in millions pushed into poverty; be it death of hundreds of people due to non-availability of oxygen; be it death of thousands of people due to mucormycosis (black fungus); be it hundreds of corpses floating in the river; be it vaccine politics, shortage, slackening and hesitancy; be it covid inappropriate behavior by majority of people resulting in explosive 2nd wave; be it overuse & misuse of corticosteroids, zinc, iron and antibiotics by people and doctors; be it hoarding and black-marketing of remdesivir, amphotericin B, oximeters and oxygen cylinders; be it availability of spurious remdesivir and posaconazole; be it fake vaccine; be it high Covid-19 bills pushing families into poverty; be it massive discrepancy between the official Covid-19 death figures and the actual numbers on the ground, be it high court and supreme court monitoring covid management; and so on and so forth.
_____

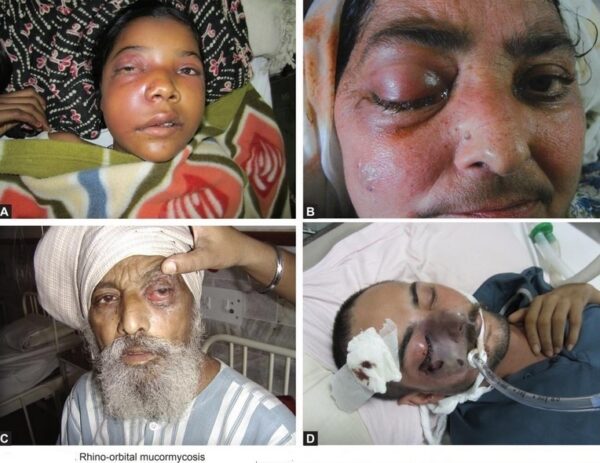

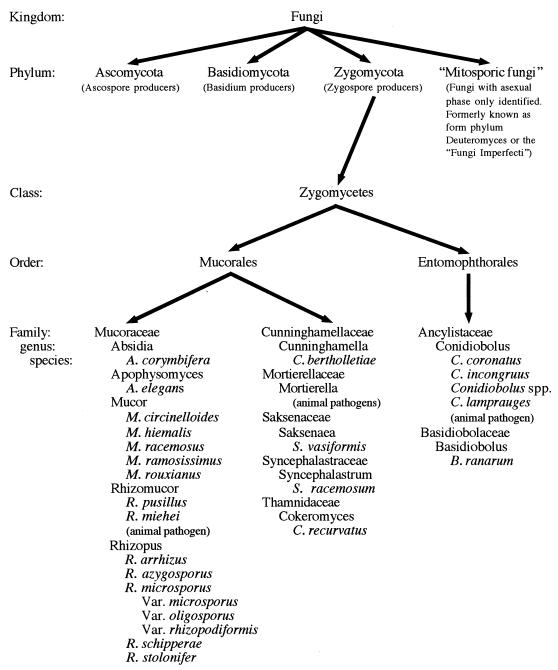
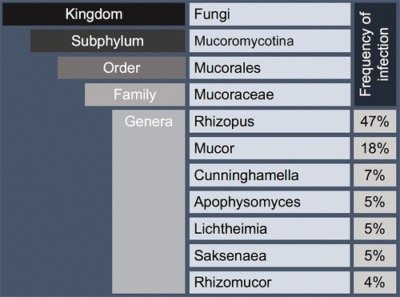
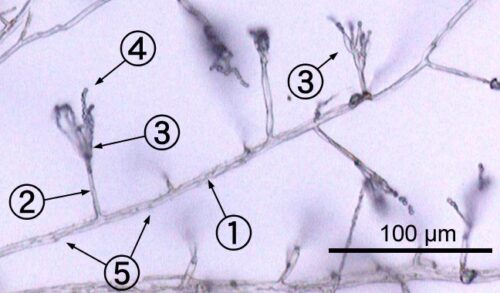
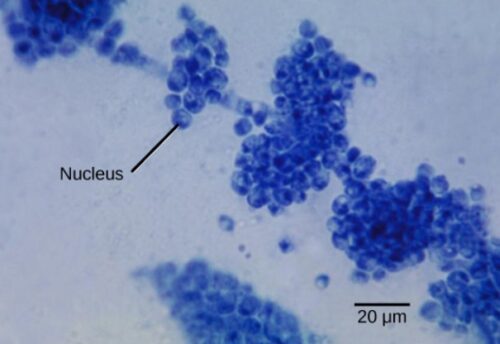

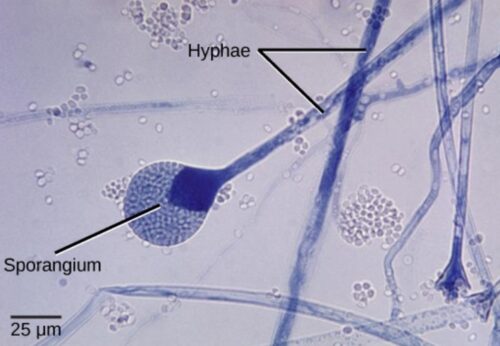
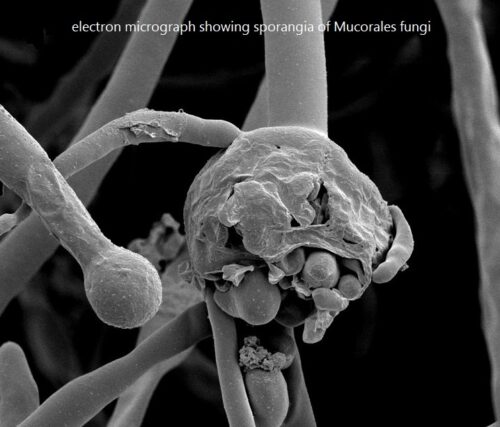
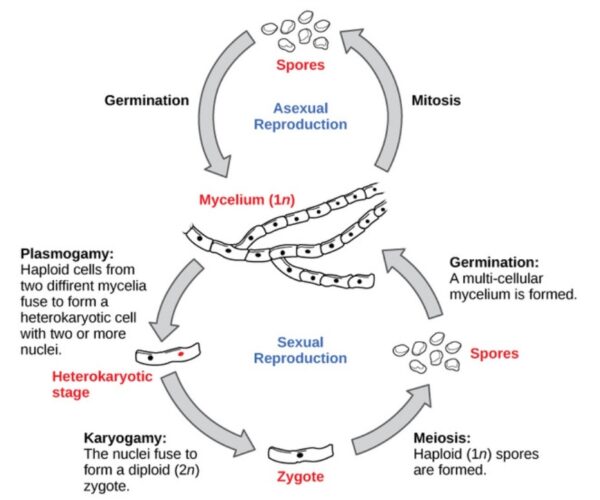

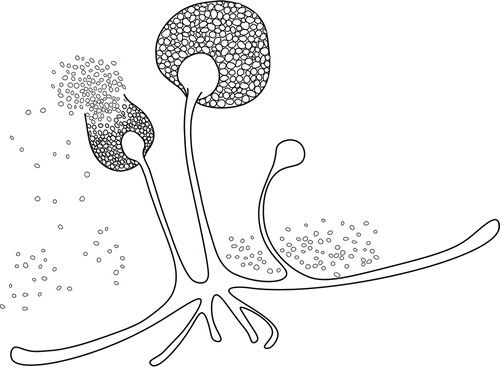
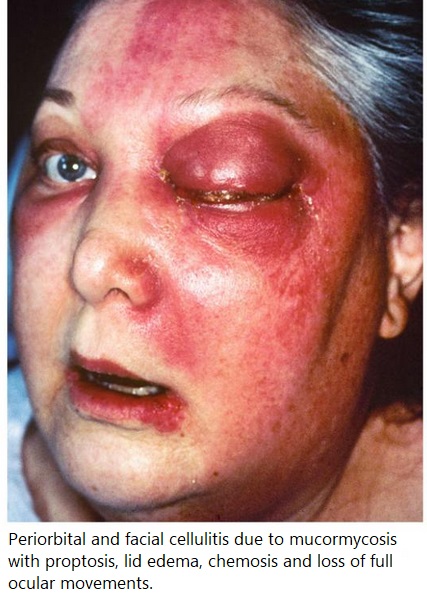
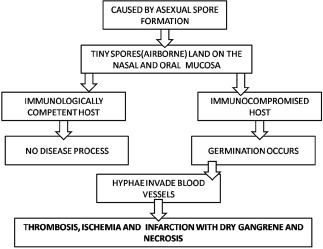
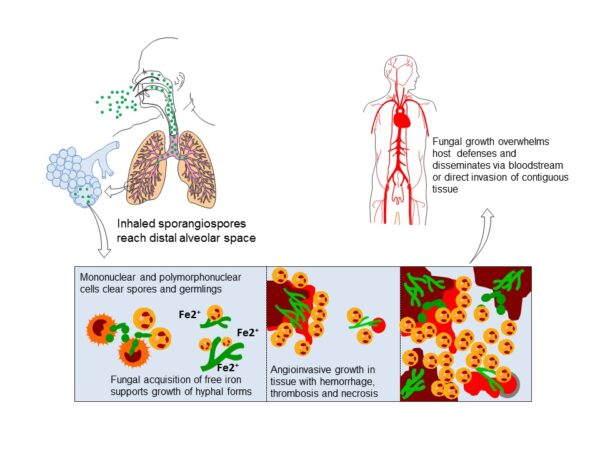
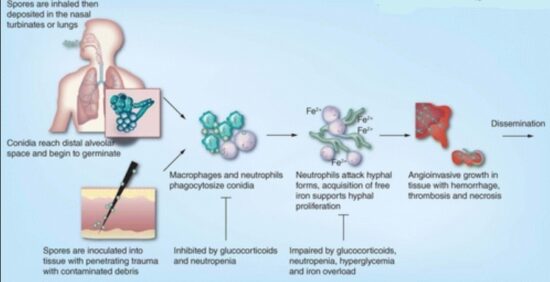
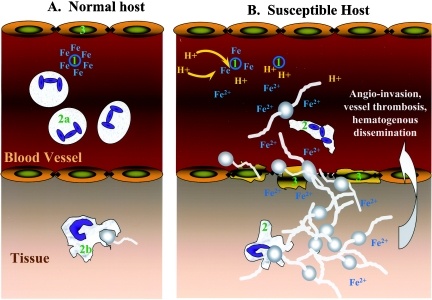
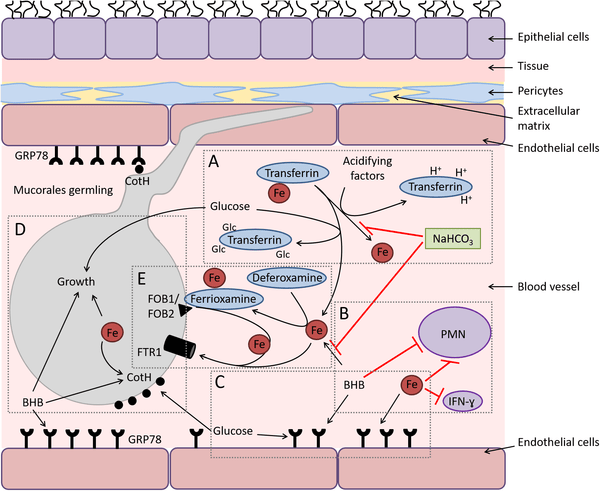
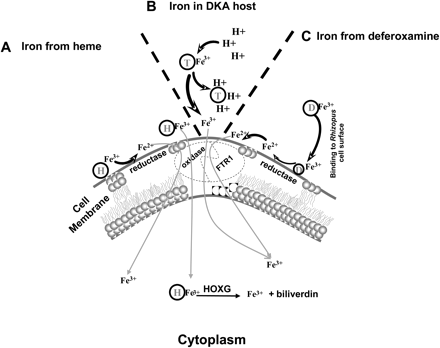
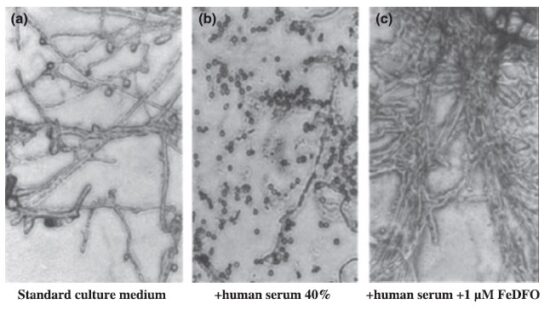
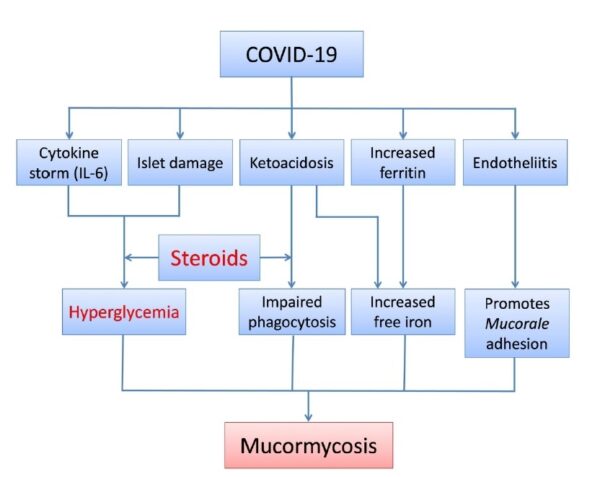
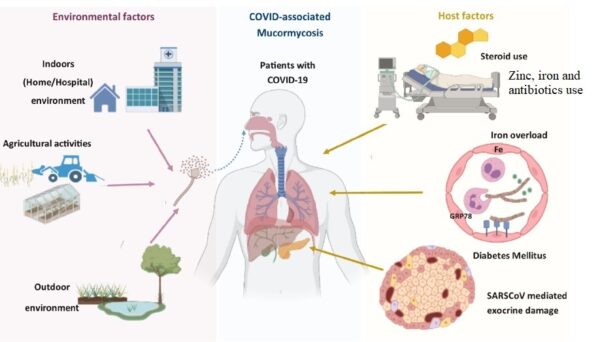
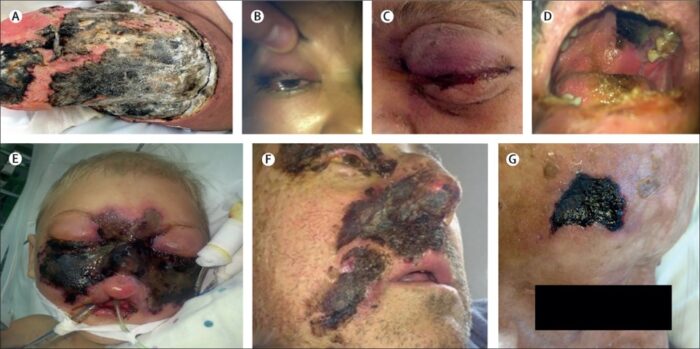
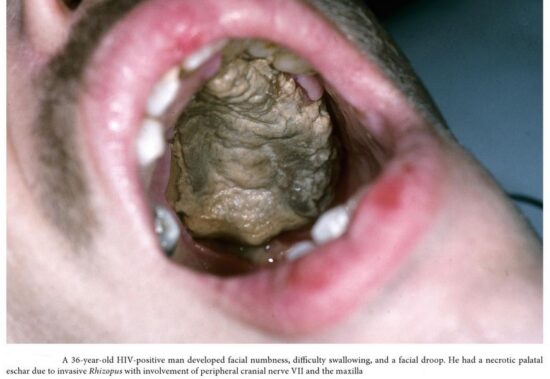
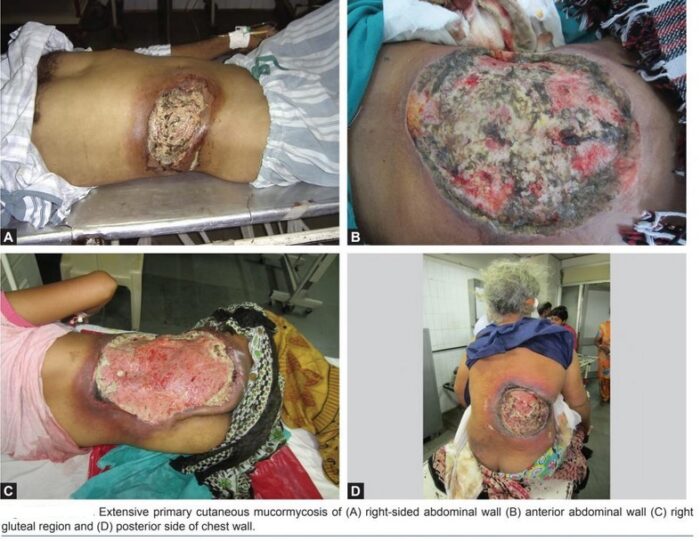
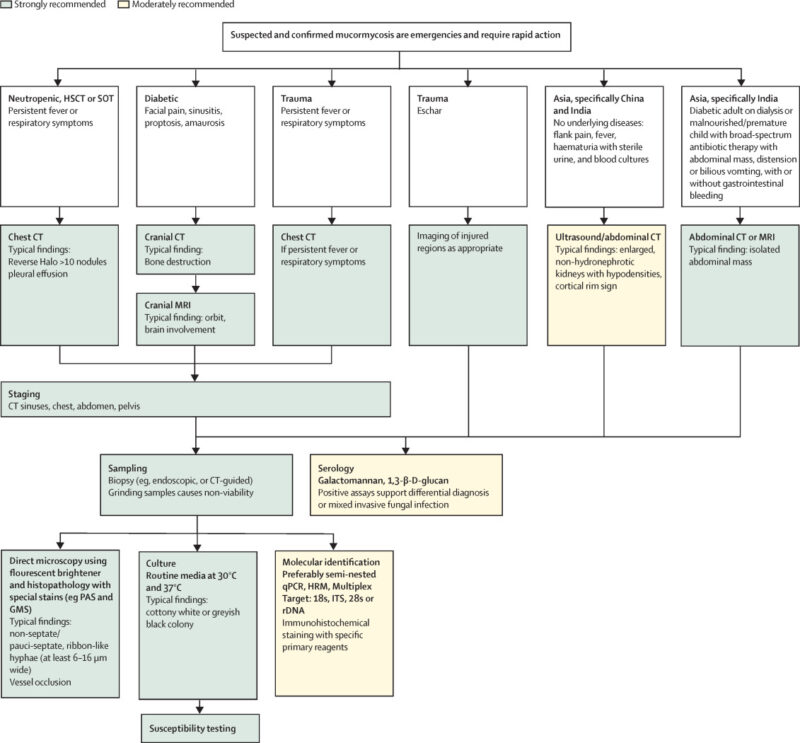
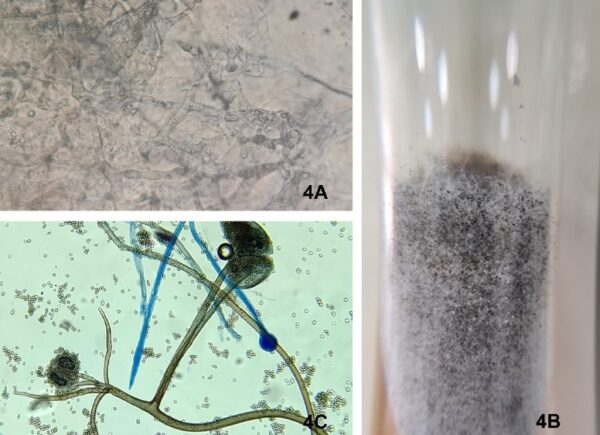
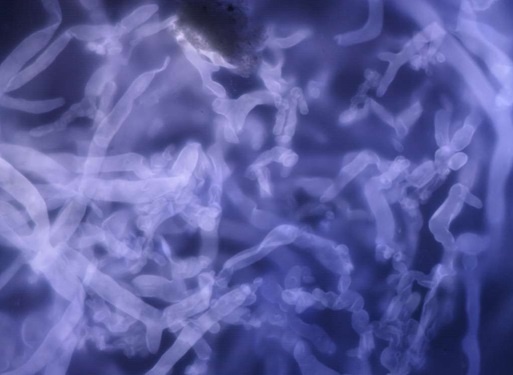

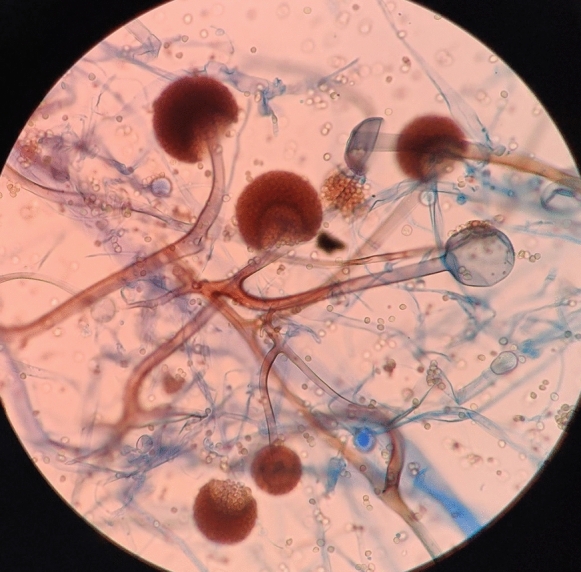
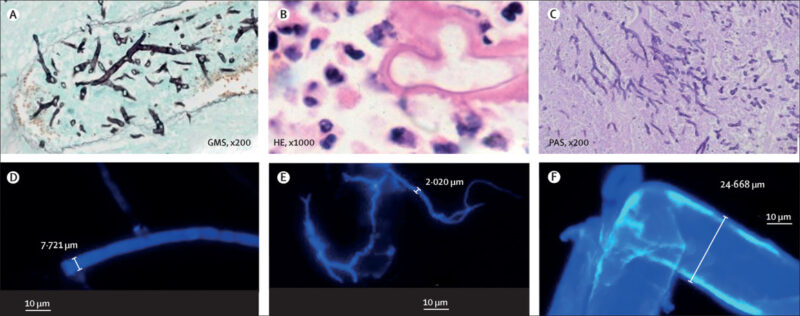
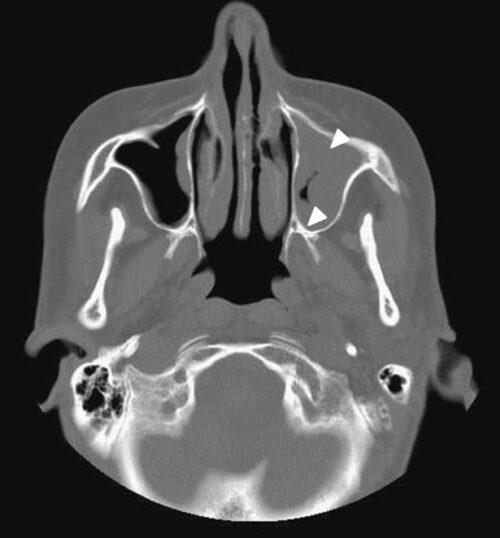
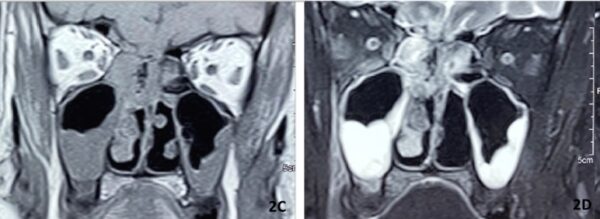
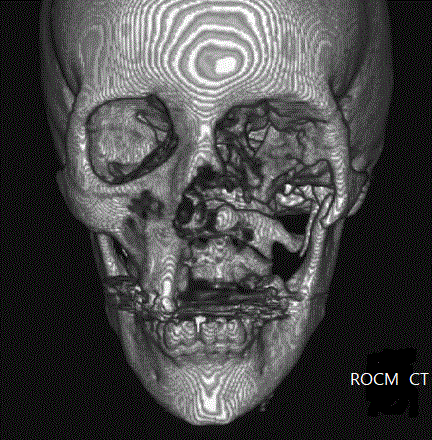
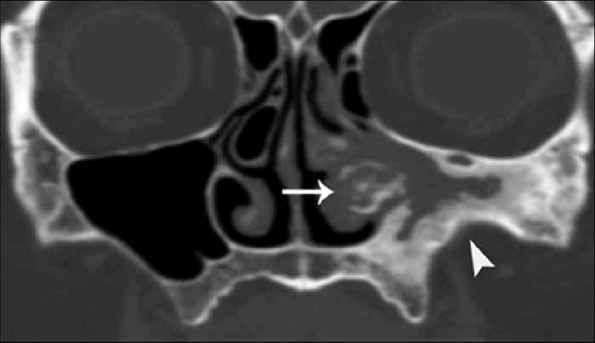
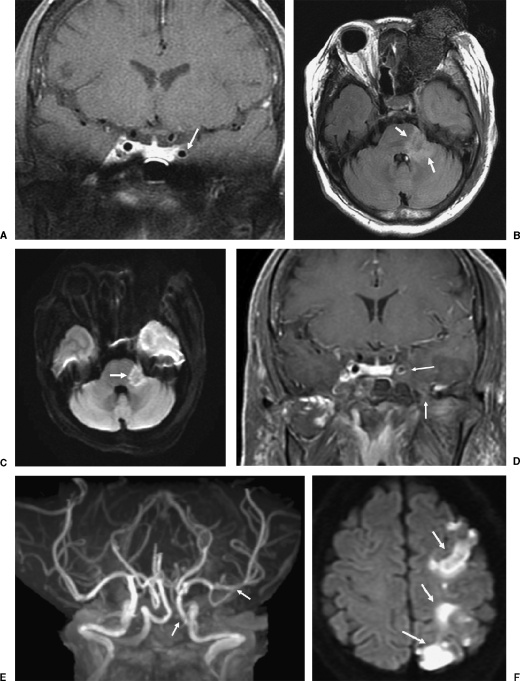
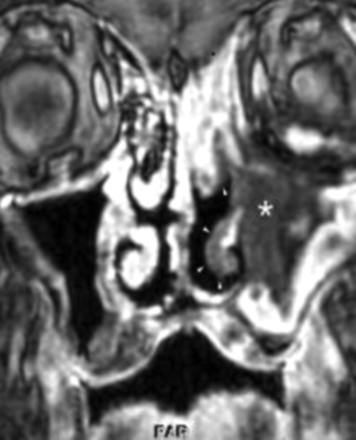
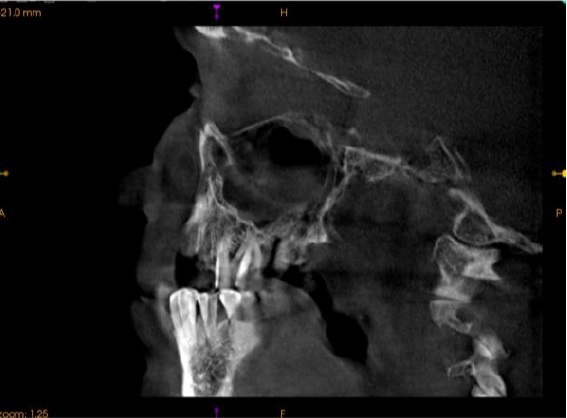
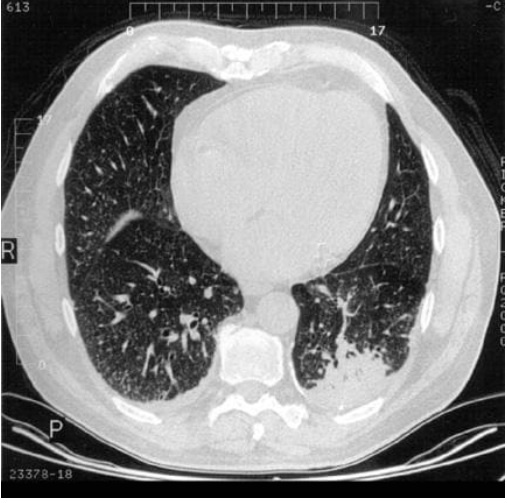
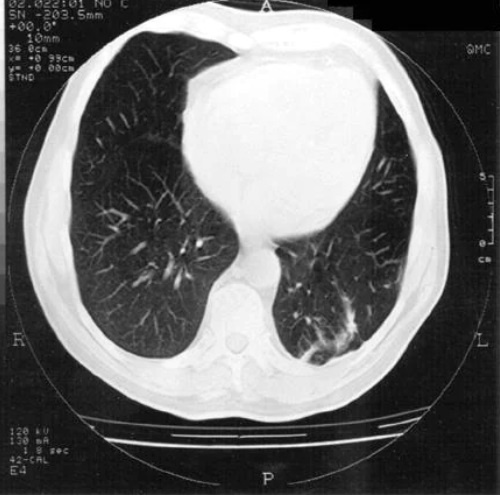
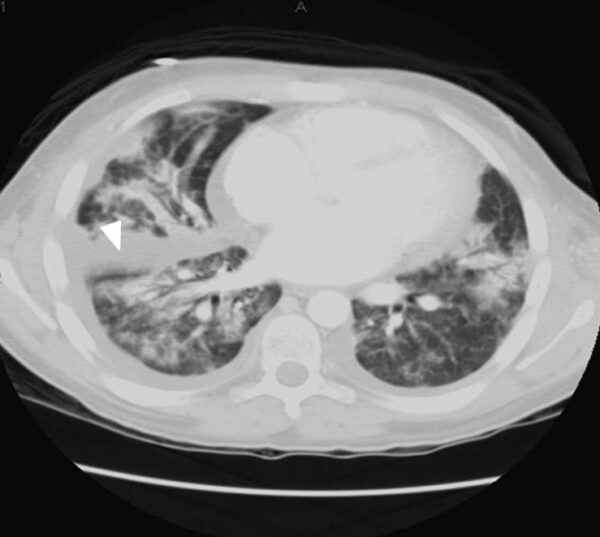
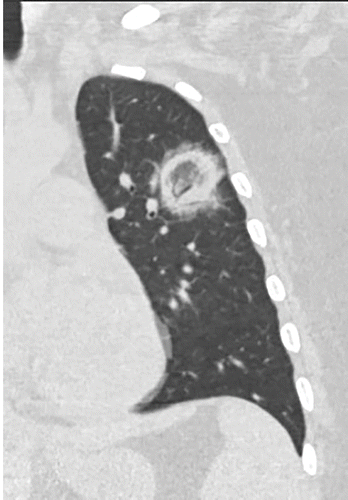
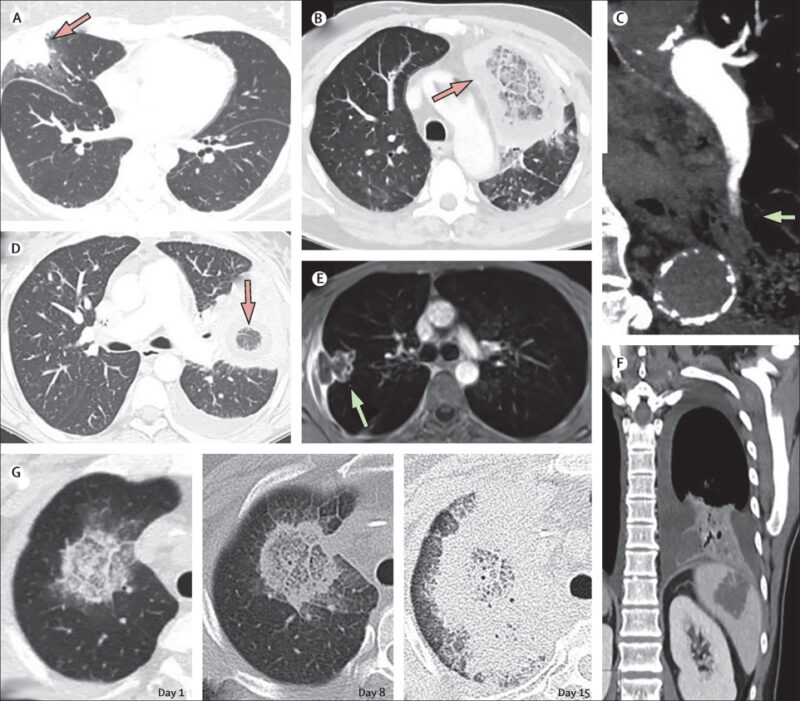
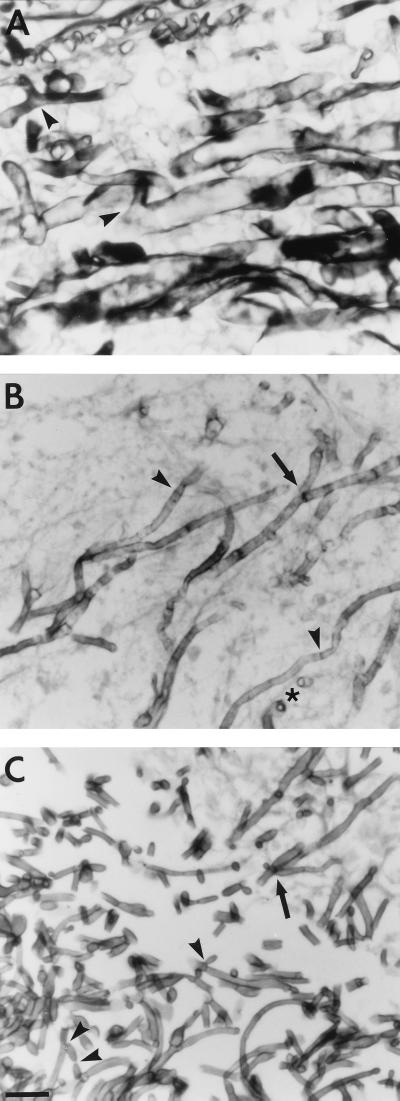
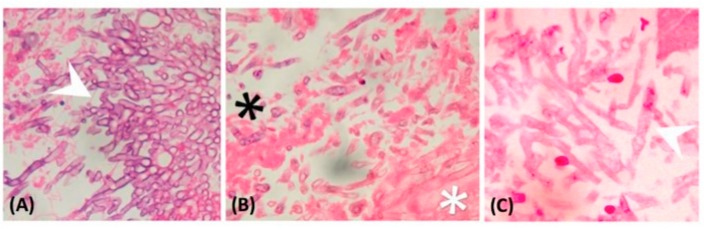
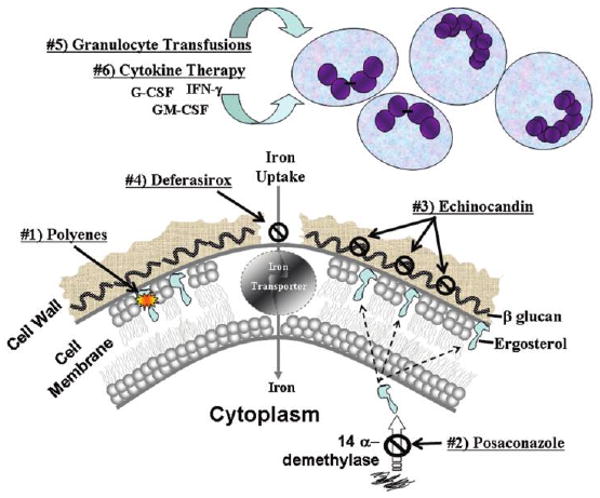
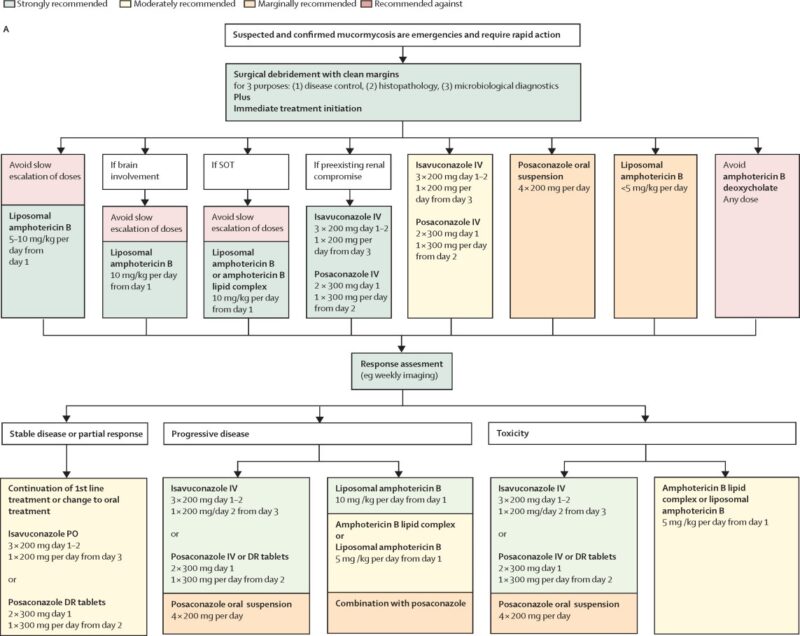
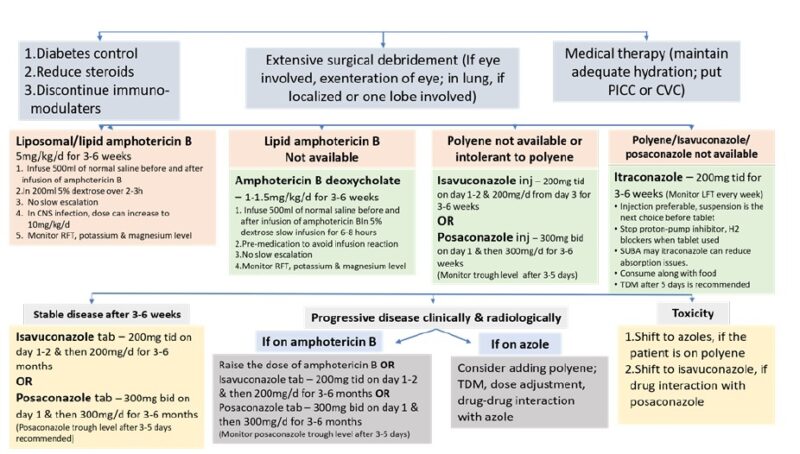
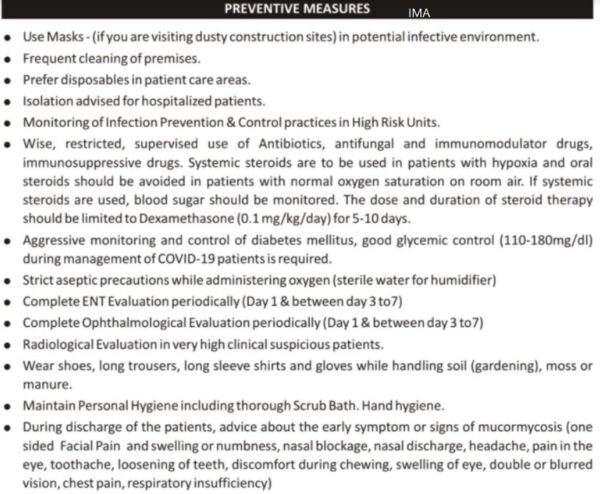

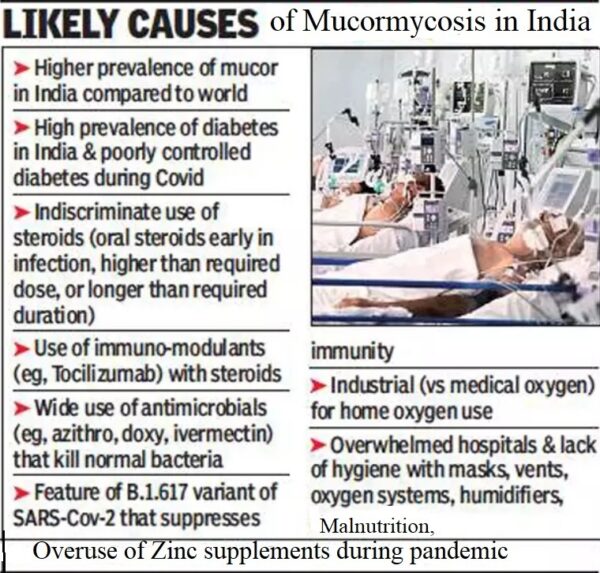
Thank you for every other great article. The place else may just anybody get that type of information in such a perfect way of writing? I’ve a presentation next week, and I’m at the search for such information.|
You always recognize just how to make me really feel much better. I appreciate your guts and resolution.
I cannot thank you enough for the blog.Really thank you! Cool.
Great blog.Really thank you! Keep writing.
It is a one of its kind. Like no other.
I think this is a real great article post.Really thank you! Really Great.
There’s certainly a lot to know about this topic.I like all of the points you’ve made.
A big thank you for your blog article.Thanks Again. Much obliged.
Im thankful for the article.Thanks Again. Awesome.
I loved your blog. Fantastic.
This is a topic that’s near to my heart…Many thanks! Where are your contact details though?
I like it when people come together and share thoughts. Great site, continue the good work!
So pleased to possess found this publish.. Respect the admission you presented.. Undoubtedly handy perception, thanks for sharing with us.. So content to have identified this publish..
Appreciate you sharing, great blog article.Really thank you! Great.
Thank you for your article.Much thanks again. Really Cool.
I really liked your blog. Really Great.
Looking forward to reading more. Great article.Much thanks again. Great.
I couldnÃt resist commenting. Perfectly written!
Im obliged for the blog.Much thanks again. Great.
I cannot thank you enough for the blog article.Much thanks again. Really Cool.
I am so grateful for your post.Really looking forward to read more. Great.
Sometimes, the sheer magnitude of the information seems overwhelming.
Great article.Much thanks again. Great.
I savour, lead to I found exactly what I used to be taking a look for. You have ended my four day lengthy hunt! God Bless you man. Have a nice day. Bye
This is one awesome article post.Really looking forward to read more. Cool.
Wow, great blog post.Thanks Again.
Very informative blog. Much obliged.
Apg Hydraulic Process Clamping Machine
Adjustable Passive RFID Wristband Silicone RFID Wristband NFC TAG Waterproof Smart RFID Bracelet
Borussia
China SmallOrders G0151 birthday present Special and practical Christmas suppliers
Backlit Panel Light Suppliers
Cationic Red XGRL Free Sample
persianas dúo
Basalt Fiber Cloth
Keren abiss
Canapé en cuir
Newest HPMC powder paint grade
ブランドコピー代引き
1-Isopropyl-4-methylcyclohexa-1 3-diene
Hard Plastic Case
スーパーコピーバッグ
Hi there! I’m at work browsing your blog from my new iphone 4! Just wanted to say I love reading through your blog and look forward to all your posts! Keep up the fantastic work!
I journal frequently and also I really cherish your web content. This wonderful blog post has absolutely peaked my curiosity. I am going to book mark your website and also keep looking for brand-new info regarding once a week. I opted in for your RSS feed in addition.
Analytical tools for trading binary options – https://crypto-vfxalert-signals.xyz
How to earn and multiply with your mind – link to the video -https://youtu.be/AXlXFhKCQn8
Visit the blog about binary options trading – https://binaryvfx123.blogspot.com/2020/09/binary-signal-from-vfxalert-how-to-work.html
[url=https://crypto-vfxalert-signals.xyz]binary signals[/url]
do catholic hospitals cover viagra the best viagra in india
スーパーコピーバッグ
Dial Indicator Magnetic Stand
Chanelシャネルブレスレットスーパーコピー
Gate
MUCORMYCOSIS – Dr Rajiv Desai
avswxqpoq
[url=http://www.ghccq7159c0944q41htr73zgsz8988nws.org/]uvswxqpoq[/url]
vswxqpoq http://www.ghccq7159c0944q41htr73zgsz8988nws.org/
Super Site
[url=https://nike.com]nike.com[/url]
[url=https://creativecommons.org]creativecommons.org[/url]
[url=http://www.fruityloopsstudio.ru]fruityloopsstudio.ru[/url]
[url=https://transparencyreport.google.com]transparencyreport.google.com[/url]
[url=https://nomadcapitalist.com]nomadcapitalist.com[/url]
[url=https://unsplash.com]unsplash.com[/url]
[url=https://cse.google.com]cse.google.com[/url]
[url=https://gstatic.com]gstatic.com[/url]
[url=https://discord.com]discord.com[/url]
[url=https://scholar.google.com]scholar.google.com[/url]
[url=https://developers.google.com]developers.google.com[/url]
Hello. And Bye.
I simply couldn’t go away your site prior to suggesting that I actually loved the standard information an individual provide on your guests? Is going to be again incessantly in order to investigate cross-check new posts.