Dr Rajiv Desai
An Educational Blog
OXYGEN THERAPY
Oxygen (O2) Therapy:
_____
While we inhale 21% of oxygen and 0.04% of carbon dioxide, we exhale 17% of oxygen and 4% of carbon dioxide.
_____
Prologue:
Oxygen (O2) is a vital element in human survival and plays a major role in a diverse range of biological and physiological processes. Oxygen therapy means using an oxygen cylinder or a machine to breathe in air that contains more oxygen than normal. Oxygen is widely available and commonly prescribed by medical and paramedical staff. In medical practice, it is among the most universally used agents for the treatment of critical illness and part of the routine treatment in acute shock and emergency medicine. Proper application of oxygen therapy and airway management is lifesaving. In the absence of O2 (hypoxia), cellular respiration ceases and irreversible cellular injury and death occur within minutes. Administered correctly it may be lifesaving. However, renaissance physician Paracelsus noted: “nothing is without poison—the poison is in the dose”. This accounts for many aspects in medicine but may also be applicable to the oxygen molecule. Oxygen is often given without careful evaluation of its potential benefits and side effects. Like any drug there are clear indications for treatment with oxygen and appropriate methods of delivery. Inappropriate dose and failure to monitor treatment can have serious consequences. In a recent hospital survey 21% of oxygen prescriptions were inappropriate. 85% of patients were inadequately supervised. While there are many benefits to oxygen by inhalation, it is not without hazards and toxic effects. There is another aspect of oxygen therapy. Over 60 children have died at Baba Raghav Das (BRD) Medical College hospital in Gorakhpur, India between August 7 and 12, 2017, reportedly due to lack of oxygen. The local government denied that the deaths of children were due to shortage of oxygen blaming viral encephalitis for the tragedy. How far oxygen really helps in saving life? I have seen many critical patients receiving oxygen failed to get any benefit of oxygen but still I tell all juniors that you must give oxygen in medical emergency. The reason is that patient’s relatives are satisfied when they see oxygen given to their loved one irrespective of outcome. Failure to give oxygen amounts to medical negligence irrespective of benefit. Is oxygen therapy a ‘hype’ created by media and society? Is room air containing 21% oxygen sufficient in many serious illnesses? Is overzealous oxygen therapy harmful to patient? Let me discuss science of oxygen therapy.
______
Note:
Please read my article ‘Pulse Oximetry’ posted on August 24, 2015 in this website as it is complementary to ‘Oxygen Therapy’ posted here.
______
______
Abbreviations, synonyms and terminology:
CO2 = Carbon dioxide
BTS = British Thoracic Society
EWS = Early Warning Score
O2 = Oxygen
ABG= arterial blood gases
CaO2 = oxygen content of blood
COPD = Chronic Obstructive Pulmonary Disease
PCO2 = carbon dioxide tension = partial pressure of CO2
PO2 = oxygen tension = partial pressure of O2
FiO2: Fraction of inspired oxygen (%). Room air FiO2 21% (0.21)
PiO2 = partial pressure of oxygen in inspired air
PaCO2: The partial pressure of CO2 in arterial blood. It is used to assess the adequacy of ventilation.
PaO2: The partial pressure of oxygen in arterial blood. It is used to assess the adequacy of oxygenation. Normal value 80 to 100mm Hg.
PvO2: Partial pressure of oxygen in venous blood, normal about 40 mmHg
PAO2: The partial pressure of oxygen in alveolus = alveolar oxygen tension
SaO2: Arterial oxygen saturation measured from blood specimen.
SpO2: Arterial oxygen saturation measured via pulse oximetry. Normal value 95 to 100 %.
SvO2: oxygen saturation of hemoglobin in venous Blood, normal about 75%.
NBO = normobaric oxygen
HBO = hyperbaric oxygen
ROS = reactive oxygen species = reactive oxygen intermediates (ROI) = free radicals
HIF = hypoxia inducible factor
PHD = prolyl hydroxylases
ROP = Retinopathy of Prematurity
STOT = Short-term oxygen therapy
LTOT = Long term oxygen therapy
NOT = Nocturnal oxygen therapy
AOT = Ambulatory oxygen therapy
POT = Palliative oxygen therapy
SBOT = short-burst oxygen therapy
HFO = high flow oxygen
HFNC = high flow nasal cannula
OCD = Oxygen Conserving Device
ACS = Acute coronary syndrome,
ADHF = Acute decompensated heart failure
AMI = Acute myocardial infarction
ARDS = Acute respiratory distress syndrome
TBI = Traumatic brain injury
High flow: High flow systems are specific devices that deliver the patient’s entire ventilatory demand, meeting, or exceeding the patients Peak Inspiratory Flow Rate (PIFR), thereby providing an accurate FiO2.
Low flow: Low flow systems are specific devices that do not provide the patient’s entire ventilatory requirements; room air is entrained with the oxygen, diluting the FiO2.
l/m = l/min = LTM =liter/minute oxygen flow rate
Humidification is the addition of heat and moisture to a gas. The amount of water vapour that a gas can carry increases with temperature.
Hypercapnia: Increased amounts of carbon dioxide in the blood.
Hypoxemia: Low arterial oxygen tension (in the blood)
Hypoxia: Low oxygen level at the tissues.
Hyperoxia occurs when cells, tissues and organs are exposed to higher than normal partial pressure of oxygen.
Minute ventilation: The total amount of gas moving into and out of the lungs per minute. The minute ventilation (volume) is calculated by multiplying the tidal volume by the respiration rate, measured in litres per minute.
Peak Inspiratory Flow Rate (PIFR): The fastest flow rate of air during inspiration, measured in litres per second.
Tidal Volume: The amount of gas that moves in, and out, of the lungs with each breath, measured in millilitres (6-10 ml/kg).
Ventilation – Perfusion (V/Q) mismatch: An imbalance between alveolar ventilation and pulmonary capillary blood flow.
Extracorporeal membrane oxygenation (ECMO) is an extracorporeal technique of providing prolonged cardiac and respiratory support to persons whose heart and lungs are unable to provide an adequate amount of gas exchange or perfusion to sustain life.
Medical Air is a colorless, odorless, and tasteless gas that is similar in composition to the air that we breathe. It is made up of approximately 78% Nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, hydrogen, argon, and other various components. Medical air USP is used either by itself (i.e. to transport inhaled medications) or mixed with other gases to support patient respiration. The main use of medical air is to aid in long-term life support as in intensive care units, critical care units, and neonatal intensive care units.
Oxygen Therapy = Oxygen = Supplemental oxygen = Oxygen treatment
_______
_______
The Earth was probably formed about 4,600 million years ago by the gravitational coalescence of cold material. Initially there was a tenuous atmosphere of hydrogen and helium which was lost because of a weak gravitational field. The secondary atmosphere was created by the thermal and radioactive decay of various Earth’s constituents. Ammonia dissociated into nitrogen and hydrogen, and water vapour into hydrogen and oxygen. However, by far the greatest source of oxygen was, and still is, from photosynthesis. The atmosphere of planet earth was anaerobic until the advent of water-splitting, O2-evolving photosynthesis. The accumulation of O2 changed the environment for, and therefore changed the selection pressures on, all living organisms. It also increased the mutation rate and therefore hastened subsequent evolution. There is some evidence to suggest that the atmospheric concentration of oxygen cannot have changed for the past 345 million years. Advantages could be gained by using the O2 to increase the useful energy derivable from foodstuffs, to carry out novel metabolic transformations, to solubilize and detoxify numerous compounds and even to generate heat and light. But there was a price to pay for these benefits and that was to provide defenses against the considerable toxicity of this paramagnetic gas. Those organisms that succeeded in developing the requisite defenses could reap the benefits, and they gave rise to the enormous variety of aerobic life forms that are now so evident on earth. Those that could not accommodate to the challenge of O2 toxicity evolved into the sensitive microscopic anaerobes now restricted to those anaerobic niches that remain even on a thoroughly aerobic planet.
_
_
Oxygen is a chemical element, atomic number 8, atomic weight 15.999. It is a colorless and odorless gas that makes up about 21 per cent of the atmosphere. Oxygen makes up 20.9% of air by volume and 23% air by weight. It constitutes 50% of Earth’s crust by weight (in air water and combined with other elements). It can combine with all other elements except other inert gases to form oxides. Oxygen is therefore characterized as an oxidizer. It is a non-flammable gas but accelerates combustion. At -182.9 deg C (-300 deg F) oxygen is a pale blue liquid. Its critical temperature is -118.4 deg C (above this critical temperature oxygen can only exist as a gas regardless of the pressure). In combination with hydrogen, it forms water; by weight, 90 per cent of water is oxygen. It is the third most abundant of all the elements of nature. Large quantities of it are distributed throughout the solid matter of the earth because it combines readily with many other elements. With carbon and hydrogen, oxygen forms the chemical basis of much organic material. Oxygen is essential in sustaining all kinds of life. Among the land animals, it is obtained from the air and drawn into the lungs by the process of respiration.
_
Oxygen is required in aerobic metabolism for:
- Production of high energy phosphate compounds (ATP)
- Dehydrogenation of flavo proteins
- Biotransformation of drugs
- Oxidation of certain other substrates.
_
Oxygen Balance and Oxygen Debt:
The need of every cell for oxygen requires a balance in supply and demand. But this balance need not be exact at all times. In fact, in strenuous exercise the oxygen needs of muscle cells are greater than the amount the body can absorb even by the most intense breathing. Thus, during athletic competition, the participants make use of the capacity of muscles to function even though their needs for oxygen are not fully met. When the competition is over, however, the athletes will continue to breathe heavily until the muscles have been supplied with sufficient oxygen. This temporary deficiency is called oxygen debt. Severe curtailment of oxygen, as during ascent to high altitudes or in certain illnesses, may bring on a variety of symptoms of hypoxia, or oxygen lack. A number of poisons, such as cyanide and carbon monoxide as well as large overdoses of sedatives, disrupt the oxygen distribution system of the body. Such disruption occurs also in various illnesses, such as anemia and diseases of lungs, heart, kidneys, and liver.
_______
_______
History of oxygen:
_
_
Oxygen is an atmospheric gas essential for survival of all living things. The presence of an “air” vital for survival of humans was recognised in the ancient Greek as well as in Vedic Hindu literature more than 2000 years ago. But it was only in the eighteenth century that the gas was isolated by Joseph Priestley and its importance in respiratory physiology was described by Antoine Lavoisier. Antoine Lavoisier in 1777 coined the term ‘oxygen’. The first recorded case of inhaled oxygen being used in medicine was by the French physician Caillens in 1783. Over the next hundred years, the popularity and credibility of the therapeutic use of oxygen fluctuated. One early reference to specially designed masks for the administration of oxygen is found in the work of Hill published in the British Medical Journal in 1912. Prior to this, descriptions of a multitude of methods of application of oxygen can be found including via the stomach for resuscitation, and per rectum and per vaginum for conditions such as cholera and inflammatory diseases. In 1914, Howitt wrote: ‘In my experience the old method of inhalation was a failure’. He went on to extol the virtues of subcutaneous injection of oxygen for a variety of diseases including eclampsia, pertussis, diabetes, emphysema and even asystole! He declared the method of raising a subcutaneous lump as large as the closed fist as the formation of an artificial lung. The works of Haldane (1917) united the physiological and theoretical basis for inhalational oxygen therapy. Furthermore, it dispelled many of the myths and prejudices of the day and, at last, it became a fully accepted therapy. In support of Haldane’s work, Meltzer, writing in the Journal of the American Medical Association, also went on to say that the failure of some practitioners to see any favourable effect ‘is probably due essentially to the inefficient method of administration’. Meltzer’s statement still holds true today where the effective, accurate and efficient administration of oxygen is not seen universally, often due to confusion about the various devices and methods for its delivery. It was around second decade of the twentieth century and later that the oxygen therapy was adopted for indications based on firm scientific foundations.
______
______
Physiology of oxygen metabolism in humans:
_
We have always emphasized two things: airway and oxygenation in emergency situation. In reality, we should be emphasizing ventilation. Without an airway, your patient cannot ventilate. Without ventilation, you cannot assess the airway. They’re inseparably linked. Likewise, without ventilation, oxygenation is impossible. But ventilation involves much more than oxygenation. It involves the elimination of carbon dioxide and toxins and plays a role in other important biological processes. While the exchange of oxygen and carbon dioxide between the bloodstream and the pulmonary airspace works by diffusion and requires no external work, air must be moved into and out of the lungs to make it available to the gas exchange process. In spontaneous breathing, a negative pressure is created in the pleural cavity by the muscles of respiration, and the resulting gradient between the atmospheric pressure and the pressure inside the thorax generates a flow of air.
_
The process of taking oxygen from the inspired air and using it to sustain aerobic cellular metabolism throughout the body can be conceptualized as having three steps:
–Oxygenation
–Oxygen delivery
–Oxygen consumption
Oxygenation is the process of oxygen diffusing passively from the alveolus to the pulmonary capillary, where it binds to hemoglobin in red blood cells or dissolves into the plasma. Insufficient oxygenation is termed hypoxemia. Oxygenation is necessary to maintain life and health. Clients with compromised oxygenation status need careful assessment and thoughtful nursing care to achieve an adequate and comfortable level of oxygenation function.
Oxygen delivery is the rate of oxygen transport from the lungs to the peripheral tissues
Oxygen consumption is the rate at which oxygen is removed from the blood for use by the tissues.
_
_
Unit of pressure measurement:
One pascal is the pressure exerted by a force of magnitude one newton perpendicularly upon an area of one square metre. Kilopascal is a metric unit and equals to 1000 force of newton per square meter. The abbreviation is “kPa”. It’s used widely globally although it’s replaced by psi (pound force per square inch) in many countries. Torr is a pressure of fluid from 1 millimeter of mercury.
1 kPa (Kilopascal) = 7.50061683 Torr (mmHg)
1 kPa = 0.145038 psi
One atmospheric pressure (atm) at sea level = 101 kPa = 14.7 psi = 760 mm Hg = 1 ATA (atmospheres absolute)
Bar is the atmospheric pressure at the sea level, which is around 100 kilopascals. Since the difference between atm and bar is so small, in some applications bar unit is used.
1 megapascal (Mpa) = 1,000,000 pascals = 10 Bar = 1000 kPa
_
Concept of partial pressure:
It is the individual pressure exerted independently by a particular gas within a mixture of gases. Partial pressure is the pressure exerted on a surface by the molecules of individual gases. The air we breathe is a mixture of gasses: primarily nitrogen, oxygen, & carbon dioxide. So, the air you blow into a balloon creates pressure that causes the balloon to expand (& this pressure is generated as all the molecules of nitrogen, oxygen, & carbon dioxide move about & collide with the walls of the balloon). However, the total pressure generated by the air is due in part to nitrogen, in part to oxygen, & in part to carbon dioxide. That part of the total pressure generated by oxygen is the ‘partial pressure’ of oxygen, while that generated by carbon dioxide is the ‘partial pressure’ of carbon dioxide. A gas’s partial pressure, therefore, is a measure of how much of that gas is present (e.g., in the air, blood or alveoli). The partial pressure exerted by each gas in a mixture equals the total pressure times the fractional composition of the gas in the mixture. So, given that total atmospheric pressure (at sea level) is about 760 mm Hg and, further, that air is about 21% oxygen, then the partial pressure of oxygen in the air is 0.21 times 760 mm Hg or 160 mm Hg.
The partial pressure of any component gas in a mixture is calculated as:
Partial pressure = total absolute pressure × volume fraction of gas component
For the oxygen component,
PO2 = P × FO2
Where PO2 = partial pressure of oxygen, P = total pressure, FO2 = volume fraction of oxygen content
_
Partial Pressures of O2 and CO2 in the body (normal, resting conditions):
- Alveoli
PO2 = 110 mm Hg
PCO2 = 40 mm Hg
- Alveolar capillaries
Entering the alveolar capillaries
PO2 = 40 mm Hg (relatively low because this blood has just returned from the systemic circulation & has lost much of its oxygen)
PCO2 = 45 mm Hg (relatively high because the blood returning from the systemic circulation has picked up carbon dioxide)
In alveolar capillaries, oxygen will enter blood from alveolus and carbon dioxide will leave blood into alveolus, through the process of passive diffusion created by partial pressure difference and hence blood leaving alveolus will have PO2 100mm and PCO2 40mm.
_
PO2 in air, alveolus, blood and tissues:
The oxygen tension drops from 160mmHg just outside the mouth to 100mmHg in the alveolus air at sea level. This decrease is due to several factors: (1) the addition of water vapor; (2) the addition of a volume of carbon dioxide as well as the removal of a volume of oxygen from the alveolus; and (3) the incomplete gas exchange with every breath.
_
Partial pressure of inspired oxygen (PiO2) in airways:
We’re getting the air from the atmosphere at atmospheric pressure (which is 760 mm Hg at sea level). However, as we inhale the air, water is added to the air prior to it reaching the alveoli – so we need to account for the water vapor that has been added by the airways. We account for this by subtracting the water vapor pressure (the partial pressure of the water that has been added) from the total atmospheric pressure: at body temperature, the water vapor pressure is 47 mm Hg:
P(atm) – PH20 = 760 mm Hg – 47 mmHg = 713 mm Hg
Now – oxygen does not make up all the air other than the water vapor pressure – it is only 21% of the air we breathe in, so we have more step to do at this point – we have to figure out how much oxygen is there:
PiO2= (Patm – 47 mm Hg) X FiO2
PiO2= (Patm – 47 mm Hg) X 0.21 = 713 x 0.21 = 149.7 mm Hg
_
Oxygen cascade:
Oxygen is delivered via the respiratory tract to the alveoli and then diffuses across the alveolar-capillary membrane into the blood. Oxygen cascade refers to the progressive decrease in the partial pressure of oxygen from the ambient air to the cellular level. Oxygen moves down the pressure or concentration gradient from a relatively high level in air, to the levels in the respiratory tract and then alveolar gas, the arterial blood, capillaries and finally the cell as seen in the figure below. The PO2 reaches the lowest level (1-1.5kPa) in the mitochondria, the structures in cells responsible for energy production. This decrease in PO2 from air to the mitochondrion is known as the oxygen cascade. The movement of oxygen at the level of the microcirculation occurs mainly by passive diffusion. The successive steps down in PO2 occur for physiological reasons shown above, but they can be influenced by pathological states, for instance hypoventilation, ventilation/ perfusion inequality, or diffusion abnormality, that will result in tissue hypoxia.
_
Fraction of inspired oxygen:
Fraction of inspired oxygen (FiO2) is the fraction or percentage of oxygen in the air that is inspired in lungs. Medical patients experiencing difficulty breathing are provided with oxygen-enriched air, which means a higher-than-atmospheric FiO2. Natural air includes 21% oxygen, which is equivalent to FiO2 of 0.21. Oxygen-enriched air has a higher FiO2 than 0.21; up to 1.00 which means 100% oxygen. FiO2 is typically maintained below 0.5 even with mechanical ventilation, to avoid oxygen toxicity. If a patient is wearing a nasal cannula or a simple face mask, each additional liter/min of oxygen adds about 4 percentage points for each of the first 3 liters and only 3 Percentage point for every liter thereafter to their FiO2 (for example, a patient with a nasal cannula with 4L/min of oxygen flow would have an FIO2 of 21% + (3 x 4%) + (1 x 3%) =36%). There are also other formulae of calculating FiO2 from oxygen flow.
_
FiO2 is the same at all altitude but PiO2 falls at high altitude:
Often used in medicine, the FiO2 is used to represent the percentage of oxygen participating in gas-exchange. If the barometric pressure changes, the FiO2 may remain constant while the partial pressure of oxygen changes with the change in barometric pressure. The percentage of individual gases in air (oxygen, nitrogen, etc.) doesn’t change with altitude, but the atmospheric (or barometric) pressure does. FIO2, the fraction of inspired oxygen in the air, is thus 21% (or 0.21) throughout the breathable atmosphere. PaO2 declines with altitude because the inspired oxygen pressure declines with altitude (inspired oxygen pressure is fraction of oxygen times the atmospheric pressure). Average barometric pressure at sea level is 760 mm Hg; it has been measured at 253 mm Hg on the top of Mt. Everest.
__
PaO2/FiO2 ratio:
The ratio of partial pressure arterial oxygen and fraction of inspired oxygen, sometimes called the Carrico index, is a comparison between the oxygen level in the blood and the oxygen concentration that is breathed. This helps to determine the degree of any problems with how the lungs transfer oxygen to the blood. A sample of arterial blood is collected for this test. Normal PaO2/FiO2 = 100 mmHg/0.21 ≈ 500. The lower the ratio, the worse the disease process. PaO2/FiO2 < 300 is consistent with ALI (acute lung injury). PaO2/FiO2 < 200 is consistent with ARDS (acute respiratory distress syndrome). A high FiO2 has been shown to alter the ratio of PaO2/FiO2.
___
Increasing the FiO2 will obviously cause an increase alveolar oxygen-tension which may be calculated as follows:
PAO2 = (PB– PH2O) X FiO2 – PaCO2/RQ
PAO2 = Alveolar O2 tension
PB = Barometric pressure (760mmHg at sea level)
PH2O = Water vapor tension (47mmHg)
FiO2 = fraction of inspired oxygen
PaCO2 = arterial carbon dioxide tension
The respiratory quotient or respiratory coefficient (RQ) is the ratio of CO2 produced divided by the O2 consumed, and its value is typically 0.8 (RQ = CO2 eliminated / O2 consumed).
_
Alveolar ventilation is another factor that affects PAO2 besides FiO2 as seen in the figure below:
Alveolar oxygen tension is directly proportional to FiO2 and alveolar ventilation.
Other factors that affect PAO2 are distribution of ventilation to perfusion, mixed venous oxygen content and alveolar gas exchange.
_
The alveolar gas equation illustrates how increasing the inspired O2 fraction (FiO2) increases the alveolar PO2 (PAO2) and subsequently the arterial PO2 (PaO2):
PaO2 is determined by the alveolar PO2 (PAO2), which is determined by the fraction of inspired oxygen, the barometric pressure and the PaCO2 (i.e., the alveolar gas equation); and the architecture of the lungs. In cases of shunt (V/Q=0), supplemental O2 therapy has little effect on PaO2. If the cause of hypoxemia is low V/Q or diffusion defect, supplemental O2 therapy will effectively increase the PaO2.
_
Supplemental O2 is FiO2 > 21%.
Supplemental oxygen means FiO2 greater than the 21% oxygen in room (ambient) air. When you give supplemental oxygen you are raising the patient’s inhaled FiO2 to something over 21%; the highest FiO2 possible is 100%. To give even more oxygen requires a hyperbaric chamber.
_
Air transit:
Room air consists of about 21% oxygen, 78% nitrogen, and 1% other gases, so the fractional concentration of oxygen in inspired air (FiO2) is 21%. By giving supplemental oxygen, you can raise the patient’s FiO2 to as much as 100% oxygen. Oxygen is transported to the tissues in two ways: About 98% of oxygen is bound to hemoglobin, and the other 2% is dissolved in plasma. The arterial blood gas (ABG) analysis measures PaO2—the pressure of oxygen dissolved in plasma. A value of 80 to 100 mm Hg is considered normal, but will increase if the patient receives a higher oxygen concentration. The saturation of hemoglobin with oxygen can be measured via pulse oximetry (SpO2) or ABG analysis (SaO2). A normal SpO2 or SaO2 value is above 94%. An SaO2 or SpO2 value below 90% means the PaO2 is below 60 mm Hg, indicating that the patient isn’t adequately oxygenated.
_
O2 Content of Blood:
_
Above figures show that about 5 ml of oxygen is extracted from blood by human tissues per 100 ml of blood per minute. Average adult consumes about 225-250 ml of oxygen per min. It increases 10 fold during exercise. Oxygen reserve is very small in the body; hence human can stand for only 4-6 min after cessation of spontaneous ventilation.
_
Oxygen delivery:
Oxygen delivery to the periphery is determined by oxygen content of the arterial blood (CaO2) and cardiac output( CO):
DO2 = (CO) X (CaO2) X 10
DO2 = 5 X 19 X 10
DO2 is O2 delivery in liters /min = 0.95 liter/minute
About 250 ml oxygen extracted by tissues and remaining 700 ml returned back through venous blood so that oxygen saturation of venous blood is about 75 %.
__
Dissolved oxygen in oxygen therapy:
Dissolved O2 in plasma = 0.003ml / mm PO2 / 100ml of blood:
Breathing Air (PaO2 100mm Hg) 0.3ml / 100ml of blood
O2 therapy:
- Normobaric Breathing (1 Atm.) 100% O2 (PaO2 > 600mmHg) = >1.8ml / 100ml of blood
- Hyperbaric Breathing 100% O2 at 3 Atm. Pressure (PaO2> 2000mmHg) = 6 ml /100ml of blood
Remember 98% oxygen is carried by hemoglobin and hemoglobin cannot carry more oxygen if PaO2 becomes more than 100mm, so SpO2/SaO2 cannot rise above 100 % no matter how high PaO2 is. Yes, oxygen saturation will fall if PaO2 becomes less than 80 mm.
_
Oxygen enters blood from lungs as dissolved oxygen (PaO2) and then binds with hemoglobin (SaO2). After oxygen has entered and dissolved within the blood, then and only then, oxygen can bind to the hemoglobin in the blood. Oxygen leaves blood to be utilized by tissues as dissolved oxygen first and then dissociates from hemoglobin. So dissolved oxygen is available to tissues first and then oxygen bound to hemoglobin. Supplemental oxygen therapy increases dissolved oxygen resulting in increased oxygen saturation; and even if oxygen saturation is 100% and cannot rise beyond it, oxygen therapy increases oxygen content and delivery by increasing dissolved oxygen in blood although the amount of dissolved oxygen is much lesser than bound oxygen.
_______
_______
Physiology of oxygenation:
The delivery of oxygen to the body’s cells is a process that depends upon the interplay of the pulmonary, hematologic, and cardiovascular systems. Specifically, the processes involved include ventilation, alveolar gas exchange, oxygen transport and delivery, and cellular respiration.
_______
- Ventilation:
The first step in the process of oxygenation is ventilation, which is the movement of air into and out of the lungs for the purpose of delivering fresh air into the lung’s alveoli. Ventilation is regulated by respiratory control centers in the pons and medulla oblongata, which are located in the brain stem. The rate and depth of ventilation are constantly adjusted in response to changes in the concentrations of hydrogen ion (pH) and carbon dioxide (CO2) in the body’s fluids. For instance, an increase in carbon dioxide in the blood or a decrease in pH in the body’s fluids will stimulate faster and deeper ventilation. A decrease in blood oxygen concentration (hypoxemia) will also stimulate ventilation, but to a lesser degree. Inhalation of air is initiated when the diaphragm contracts, pulling it downward and thus increasing the size of the intrathoracic space. This space is also increased by contraction of the external intercostal muscles, which elevate and separate the ribs and move the sternum forward. The effect of increasing the space inside the thorax is to decrease the intrathoracic pressure, so that air will be drawn in from the atmosphere. Stretch receptors in the lung tissue send signals back to the brain to cause cessation of inhalation, preventing overdistension of the lungs. Exhalation occurs when the respiratory muscles relax, thus reducing the size of the intrathoracic space, increasing the intrathoracic pressure, and forcing air to exit the lungs. Under normal conditions, exhalation is a passive process. When the movement of air is impeded, additional muscles may be used to increase the ventilatory ability. These accessory muscles of ventilation include the sternocleidomastoid muscle, the abdominal muscles, and the internal intercostal muscles. In some disease states, exhalation is impaired, requiring that the individual actively force air out of the lungs rather than passively exhaling. Forced expiration is aided by the intercostal muscles and the abdominal recti. When additional muscular force is required for breathing, the work of breathing is said to be increased.
_
Hypoventilation:
The hallmark of hypoventilation is a high PaCO2 level as adequate ventilation is necessary for the removal of CO2. Ventilation is also required for oxygenation, and hypoventilation leads to low PAO2 and subsequent low PaO2. Another unique feature of hypoventilation is normal P(A-a)O2 gradient as the alveolar – capillary membrane is intact in this condition. Prolonged hypoventilation, however, may lead to atelectasis of some parts of the lungs and widening of P(A-a)O2 gradient. Hypoventilation does not produce significant hypoxemia in healthy lung, but in the presence of lung diseases, hypoxemia can be severe. One characteristic feature of hypoventilation induced hypoxemia is that it is easily correctible by supplemental oxygen. Oxygen therapy corrects hypoxemia even when hypoventilation and hypercapnia persists. Normal pulse oximetry in a patient breathing room air indicates adequacy of ventilation (normal PaCO2). However, it cannot be used to judge the adequacy of ventilation in patients on supplemental oxygen if hypoventilation persists. Patients of COPD, asthma, ILD, and other lung diseases initially cause Type-1 respiratory failure but after certain period of time may develop Type-2 respiratory failure due to alveolar hypoventilation.
Various causes of hypoventilations are given below:
- Impaired central drive
- Drug overdose: Opioids, benzodiazepines, alcohol
- Brainstem hemorrhage, infarction
- Primary alveolar hypoventilation
- Spinal cord level: Amyotrophic lateral sclerosis, cervical spinal cord injury
- Nerve supplying respiratory muscle: Guillain–Barre syndrome
- Neuromuscular junction: Myasthenia gravis, Lambert–Eaton syndrome
- Respiratory muscles: Myopathy
- Defects in chest wall: Kyphoscoliosis, thoracoplasty, fibrothorax.
Characteristics
- Hypoxemia shows good response to oxygen therapy
- P(A-a)O2 is usually normal
- PaCO2 is high
- PaO2 and PaCO2 move in opposite direction to the same extent.
_
Several mechanisms exist to keep the airways clear of microorganisms and debris. As air is inhaled through the nose, the larger particles are filtered out through hairs lining the nasal passages. The mucous membranes of the nasopharynx and sinuses warm and humidify the inspired air, and the film of mucus lining these membranes traps smaller particles. Closure of the glottis protects the airway from aspiration of food and fluids during swallowing. In the trachea and larger bronchi, tiny hair-like cilia continually produce wavelike movements to propel mucus and particles upward, where they can be coughed out. If any invaders manage to reach the alveoli, specialized alveolar macrophages will engulf and destroy the offending organism. Disease processes can interfere with any of these protective mechanisms, increasing the individual’s vulnerability to infection and injury.
_
Ventilation Perfusion Mismatch:
_
Alveolar gas exchange depends not only on ventilation of the alveoli but also on circulation of blood through the alveolar capillaries. This makes sense. You need both oxygen in the alveoli, and adequate blood flow past alveoli to pick up oxygen, otherwise oxygen cannot be delivered. When the proper balance is lost between ventilated alveoli and good blood flow through the lungs, ventilation/perfusion mismatch is said to exist. The ventilation/perfusion ratio is often abbreviated V/Q. V/Q mismatch is common and often effects patient’s ventilation and oxygenation. There are 2 types of mismatch: dead space and shunt. Shunt is perfusion of poorly ventilated alveoli. Dead space is ventilation of poor perfused alveoli.
_
Dead space:
Dead space is the volume of a breath that does not participate in gas exchange. It is ventilation without perfusion. In physiology, dead space is the volume of air which is inhaled that does not take part in the gas exchange, either because it (1) remains in the conducting airways, or (2) reaches alveoli that are not perfused or poorly perfused. In other words, not all the air in each breath is available for the exchange of oxygen and carbon dioxide. Mammals breathe in and out of their lungs, wasting that part of the inspiration which remains in the conducting airways where no gas exchange can occur. In humans, about a third of every resting breath has no change in O2 and CO2 levels. In adults, it is usually in the range of 150 ml.
_
Dead space is the portion of each tidal volume that does not take part in gas exchange. There are two different ways to define dead space– anatomic and physiologic. Anatomic dead space is the total volume of the conducting airways from the nose or mouth down to the level of the terminal bronchioles, and is about 150 ml on the average in humans. The anatomic dead space fills with inspired air at the end of each inspiration, but this air is exhaled unchanged. Thus, assuming a normal tidal volume of 500 ml, about 30% of this air is “wasted” in the sense that it does not participate in gas exchange. Physiologic dead space includes all the non-respiratory parts of the bronchial tree included in anatomic dead space, but also factors in alveoli which are well-ventilated but poorly perfused and are therefore less efficient at exchanging gas with the blood. Because atmospheric PCO2 is practically zero, all the CO2 expired in a breath can be assumed to come from the communicating alveoli and none from the dead space. By measuring the PCO2 in the communicating alveoli (which is the same as that in the arterial blood) and the PCO2 in the expired air, one can use the Bohr Equation to compute the “diluting,” non-CO2 containing volume, the physiologic dead space. In healthy individuals, the anatomic and physiologic dead spaces are roughly equivalent, since all areas of the lung are well perfused. However, in disease states where portions of the lung are poorly perfused, the physiologic dead space may be considerably larger than the anatomic dead space. Hence, physiologic dead space is a more clinically useful concept than is anatomic dead space. Since perfusion is less; removal of CO2 by high V/Q unit is low. Although the impact of high V/Q unit on blood oxygenation is minimal, it can cause hypoxemia if the compensatory rise in total ventilation is absent. Since the high V/Q unit receiving less perfusion, blood from this area is diverted to other areas leading to the development of low V/Q in other areas of the lungs. It results in the development of hypoxemia unless the compensatory rise in total ventilation is impaired. The compensatory rise in ventilation can lead to normalization of V/Q ratio of the low V/Q areas.
_
Factors that increase dead space:
- General anaesthesia – multifactorial, including loss of skeletal muscle tone and bronchoconstrictor tone
- Anaesthesia apparatus/circuit
- Artificial airway
- Neck extension and jaw protrusion (can increase it twofold)
- Positive pressure ventilation (i.e. increased airway pressure)
- Upright posture as opposed to supine (because of decreased perfusion to the uppermost alveoli)
- Pulmonary embolus, PA thrombosis, hemorrhage, hypotension, surgical manipulation of pulmonary artery tree – anything that decreases perfusion to well-ventilated alveoli
- Emphysema (blebs, loss of alveolar septa and vasculature)
- Age
- Anticholinergic drugs
_
Pulmonary shunt:
A pulmonary shunt is a pathological condition which results when the alveoli of the lungs are perfused with blood as normal, but ventilation (the supply of air) fails to supply the perfused region. In other words, the ventilation / perfusion ratio (the ratio of air reaching the alveoli to blood perfusing them) is zero. A pulmonary shunt often occurs when the alveoli fill with fluid, causing parts of the lung to be unventilated although they are still perfused. Intrapulmonary shunting is the main cause of hypoxemia (inadequate blood oxygen) in pulmonary edema and conditions such as pneumonia in which the lungs become consolidated. The shunt fraction is the percentage of blood put out by the heart that is not completely oxygenated. In pathological conditions such as pulmonary contusion, the shunt fraction is significantly greater and even breathing 100% oxygen does not fully oxygenate the blood. Poor response to oxygen therapy is the feature that differentiates shunt from other mechanisms of hypoxemia. Failure to improve PaO2 by oxygen therapy is due to the inability of oxygen to improve PAO2 in unventilated lung units. Hypercapnia is uncommon in shunt until the shunt fraction reaches 50%. Lack of hypercapnia is due to stimulation of the respiratory center by chemoreceptor as the PCO2 in the arterial blood leaving the shunt unit is high. PaO2/FiO2 is a rough estimate of shunt fraction. If PaO2/FiO2 is <200, shunt fraction is more than 20%, whereas a PaO2/FiO2 of more than 200 indicates a shunt fraction of <20%.
_
The Ventilation-Perfusion (V/Q) ratio:
The ventilation-perfusion ratio is exactly what you think it should be – the ratio between the amount of air getting to the alveoli (the alveolar ventilation, V, in ml/min) and the amount of blood being sent to the lungs (the cardiac output or Q – also in ml/min). Calculating the V/Q ratio is quite easy:
V/Q = alveolar ventilation/cardiac output
V/Q = (4 l/min) / (5 l/min)
V/Q = 0.8
When the V/Q is higher than 0.8, it means ventilation exceeds perfusion.
When the V/Q is < 0.8, there is a VQ mismatch caused by poor ventilation.
Because the lung is centered vertically around the heart, part of the lung is superior to the heart, and part is inferior. This has a major impact on the V/Q ratio:
- apex of lung – higher
- base of lung – lower
These two variables, V & Q, constitute the main determinants of the blood oxygen (O2) and carbon dioxide (CO2) concentration. The V/Q ratio can be measured with a ventilation/perfusion scan. A V/Q mismatch can cause a type 1 respiratory failure. V/Q mismatch can be caused by anything which increases or decreases ventilation of the lungs or increases or decreases perfusion of the lungs. In other words, anything that interferes with the ability of fresh air to get to the alveoli, or anything that prevents blood flow to the capillaries. Both of these can disrupt the balance between ventilation and perfusion.
_
Understanding Ventilation vs. Oxygenation:
Ventilation and oxygenation are separate physiological processes. Ventilation is the act or process of inhaling and exhaling. To evaluate the adequacy of ventilation, a provider must exercise eternal vigilance. Chest rise, compliance (as assessed by the feel of the bag-valve mask), and respiratory rate are qualitative clinical signs that should be used to evaluate the adequacy of ventilation. Capnography, long the standard of care in the operating room and intensive care unit, can also be used to assess ventilation. Also, continuous quantitative waveform capnography has become the standard of care for monitoring endotracheal tube placement. Capnography can be used to assess end-tidal carbon dioxide (EtCO2) concentration or tension. Normal values of EtCO2 are 35–37 mmHg, and in normal lungs, the EtCO2 approximates the arterial CO2 concentration in the blood with a value that is usually lower 2–5 mmHg. Use of capnography is not limited to intubated patients; nasal cannulas and face masks can be modified to detect EtCO2. Oxygenation refers to the process of adding oxygen to the body system. There is no way to reliably measure arterial oxygenation via clinical signs alone. Cyanosis, pallor and other physical findings are not reliable. The pulse oximeter, which relies on a spectral analysis of oxygenated and reduced hemoglobin as governed by the Beer-Lambert law, represents the principle means of assuring adequate oxygenation in a patient. Saturation of peripheral oxygen (SpO2) levels measured with a pulse oximeter correlate highly with arterial oxygenation concentrations. Despite years of use in a wide variety of settings, even experienced physicians and nurses have significant knowledge deficits regarding the limitations and interpretation of pulse oximetry. Pulse oximetry has several limitations. Hypoxia follows hypoventilation, and it may take 30 seconds or more for the pulse oximeter to reflect conditions of life-threatening hypoxia. Relying on the pulse oximeter alone can decrease the margin of safety because corrective actions taken after the pulse oximeter falls may be too late. Hypovolemia, vasoconstriction, peripheral vascular disease or nail polish may cause false readings. Ideally, when monitoring ventilation and oxygenation in the prehospital environment, capnography should be combined with pulse oximetry. With capnography, providers are able detect respiratory insufficiency early and are able to institute early interventions, thereby preventing arterial oxygen desaturation. However, as with any monitoring technology, the best “monitor” is the provider. Pulse oximeters and capnometers do not treat patients. Integrating the information from your monitors and clinical assessment to make sound clinical decisions is the key to successful airway management. As evidenced by the astute assessment and action of a paramedic, knowing the difference between ventilation and oxygenation is a critical concept that must be understood.
_
Remember, oxygenation and ventilation are different. Ventilation exchanges air between the lungs and the atmosphere so that oxygen can be absorbed and carbon dioxide can be eliminated. Oxygenation is simply the addition of oxygen to the body. If you hyperventilate with room air, you will lower your arterial carbon dioxide content (PaCO2) significantly, but your oxygen levels won’t change much at all. On the other hand, if you breathe a high concentration of oxygen, but don’t increase or decrease your respiratory rate, your arterial oxygen content (PaO2) will greatly increase, but your PaCO2 won’t change. Oxygenation mostly changes PaO2. Ventilation mostly changes PaCO2. If you’re providing baby with extra oxygen, she may not become hypoxic right away because enough oxygen will still reach her alveoli to maintain her oxygen saturation for a while. However, she is barely moving her dead space gas back and forth so her ventilation is poor. As a result, her carbon dioxide starts to rise. Hypoventilation leads to increased PaCO2. Acute values above 50 mmHg are significant and require treatment; values above 70 mmHg can be life-threatening because of respiratory acidosis among other things. Each 10 mmHg change in PCO2 roughly changes your pH by 0.1. So all other things being equal, a PCO2 of 70 is associated with a pH of 7.1. If carbon dioxide rises into the 70–80mmHg range it will also profoundly sedate the patient. This worsens hypoventilation, and increases carbon dioxide even more. Respiratory rate eventually slows and the patient can stop breathing. It’s important to realize that by providing extra oxygen, a good practice, you delay the onset of hypoxia, but you may also delay the diagnosis of dangerous hypoventilation if you’re not looking for it.
_
Another mechanism of hypoxemia caused by Hypoventilation:
Hypoventilation is a common cause of too little oxygen in the blood. When breathing room air, CO2 takes up space in the alveoli, leaving less room for oxygen. Let’s see how big an effect this is. The concentration of oxygen in the alveoli can be calculated using the Alveolar Gas Equation discussed earlier:
PAO2 = FiO2 (PB – PH2O) – PACO2/ RQ
Let’s say that emergency room patient with a narcotic overdose, at sea level and breathing room air, has an alveolar PACO2 of 80 mmHg, or twice normal. That carbon dioxide takes up space and leaves less room for oxygen.
Using the Alveolar Gas Equation, that PAO2 calculation is:
PAO2 = 0.21 (760 – 47) – 80/0.8 = 49 mmHg
Normal PAO2 is about 100 mmHg, so this is quite hypoxic, especially since the alveolar PAO2 is always a little higher than the arterial PaO2. If it weren’t, oxygen would not flow out of the alveoli into the blood — it would stay in the alveoli.
Now let’s treat this patient with 50% oxygen and see what happens:
PAO2 = 0.5 (760 – 47) – 80/0.8 = 256 mmHg
That’s a five-fold increase in alveolar oxygen without changing ventilation at all. Putting the patient on oxygen will buy you time for treatment. If this is a quickly reversible process, such as a narcotic overdose, you may not need to intubate. However, if this is not quickly reversible, then oxygen protects brain and heart while you manually ventilate or intubate. This is also a good time to point out that a patient can have a normal oxygen saturation and even a normal arterial oxygen concentration and still be in respiratory distress or failure because ventilation and CO2 elimination is failing. In the above example our treated patient’s O2 saturation would be 100%, but with a PaCO2 of 80 mmHg, the pH would be about 7, a dangerous and potentially life-threatening respiratory acidosis. Don’t be lulled into missing a patient’s tenuous status just because the oxygen saturation looks good.
_
Now let’s look at a CO2 retaining emphysema patient relying on hypoxic drive — which by the way, is only a very small minority of patients with end stage pulmonary disease. This patient was in respiratory distress from pneumonia with an arterial PaO2 of 65 mmHg upon arrival to the hospital. The nurse placed her on 50% oxygen. After oxygen therapy, her blood gas shows her PaO2 is now 256 (good) and her PaCO2 is now 80 (bad) and she’s getting sleepy, probably from the high CO2. The high oxygen levels have decreased this particular patient’s drive to breathe.
Seeing CO2 retention, the nurse might be tempted to take all the oxygen off this patient in order to stimulate her breathing and get her CO2 down — but that would be the wrong thing to do. Why?
As we saw in the calculation above, we’d expect the alveolar PAO2 to abruptly drop to 49 with this change. A better way to deal with this situation would be to wean the oxygen back slowly, maintaining a good oxygen level while allowing the respiratory drive to improve. Keep reminding the patient to take deep breaths. Intubation might still be needed so watch the patient carefully. Never let the fear of CO2 retention stop you from treating a COPD patient with oxygen in an emergency. The vast majority of patients with COPD do not retain CO2. And even if the patient you happen to be treating does retain CO2, the worst-case scenario is that you relieve their hypoxia and protect their brain and heart (good) but might have to temporarily assist ventilation. Also, the reason a high FiO2 may raise PaCO2 in a patient with COPD is not only because the extra oxygen cuts off the hypoxic drive but modest rise in PaCO2 occurs mainly because the extra oxygen alters V/Q relationships within the lungs, creating more physiologic dead space.
_
The physiological response of an increase in PaCO2 due to high concentration oxygen therapy has been demonstrated not only in stable and acute exacerbations of COPD, but also in severe asthma, community-acquired pneumonia and obesity hypoventilation syndrome. Proposed mechanisms for oxygen-induced hypercapnia include increased ventilation perfusion mismatch due to reduced hypoxic pulmonary vasoconstriction, reduced ventilatory drive, atelectasis and the Haldane effect, with the contribution of each likely to depend on the clinical situation.
______
- Alveolar Gas Exchange:
Once fresh air reaches the lung’s alveoli, the next step in the process of oxygenation begins. The exchange of oxygen from the alveolar space into the pulmonary capillary blood is referred to as oxygen uptake; it may also be called external respiration. Oxygen diffuses across the alveolar membrane in response to a concentration gradient; that is, it moves from an area of higher concentration (the alveoli) to an area of lower concentration (the pulmonary capillary blood), seeking equilibrium. At the same time, carbon dioxide diffuses from the blood to the alveolar space, also in response to a concentration gradient. The exchange of gases (O2 & CO2) between the alveoli & the blood occurs by simple diffusion: O2 diffusing from the alveoli into the blood & CO2 from the blood into the alveoli. Diffusion requires a concentration gradient. So, the concentration (or pressure) of O2 in the alveoli must be kept at a higher level than in the blood & the concentration (or pressure) of CO2 in the alveoli must be kept at a lower level than in the blood. We do this, of course, by breathing – continuously bringing fresh air (with lots of O2 & little CO2) into the lungs & the alveoli.
_
The walls of alveoli are coated with a thin film of water & this creates a potential problem. Water molecules, including those on the alveolar walls, are more attracted to each other than to air, and this attraction creates a force called surface tension. This surface tension increases as water molecules come closer together, which is what happens when we exhale & our alveoli become smaller (like air leaving a balloon). Potentially, surface tension could cause alveoli to collapse and, in addition, would make it more difficult to re-expand the alveoli (when you inhaled). Both of these would represent serious problems: if alveoli collapsed they would contain no air & no oxygen to diffuse into the blood &, if re-expansion was more difficult, inhalation would be very, very difficult if not impossible. Fortunately, our alveoli do not collapse & inhalation is relatively easy because the lungs produce a substance called surfactant that reduces surface tension.
_
Diffusion limitation:
It occurs when the oxygen transport across the alveolar-capillary membrane is impaired. Diffusion limitation may be due to decrease in lung surface area for diffusion, inflammation, and fibrosis of the alveolar-capillary membrane, low alveolar oxygen, and extremely short capillary transit time. Since both oxygen and carbon dioxide transport occur through the alveolar-capillary membrane, theoretically it should cause both hypoxemia and hypercapnia. However, hypercapnia is uncommon due to diffusion limitation. Since CO2 is 20 times more soluble in water than O2, it is less likely to be affected by diffusion limitation. Another reason could be hypoxemia-mediated stimulation of ventilation, leading to CO2 washout. Normal pulmonary capillary transit time is 0.75 s, and the time required to complete gas exchange is 0.25 s. One important characteristics of diffusion limitation is the development or worsening of hypoxemia during exercise. During exercise, the capillary transit time is shortened due to rise in cardiac output. Moreover, mixed venous oxygen level also falls due to increase oxygen extraction by the tissues. However, hypoxemia usually does not develop due to the following reasons: Recruitment of capillaries, distension of capillaries, and rise in alveolar oxygen. Patients with pulmonary fibrosis fail to recruit additional capillaries and develop exercise-induced/exaggerated hypoxemia. Important causes of diffusion limitation are emphysema and ILDs.
Characteristics of diffusion limitation:
- Hypoxemia shows good response to oxygen therapy
- P (A-a) O2 is elevated
- PaCO2 is usually normal.
_____
- Oxygen Transport in the Blood:
Once the diffusion of oxygen across the alveolar-capillary membrane occurs, the oxygen molecules are dissolved in the blood plasma. Three factors influence the capacity of the blood to carry oxygen: the amount of dissolved oxygen in the plasma, the amount of hemoglobin, and the tendency of the hemoglobin to bind with oxygen. However, the plasma is not able to carry nearly enough dissolved oxygen to meet the metabolic needs of the body. The oxygen-carrying capacity of the blood is greatly enhanced by the presence of hemoglobin in the erythrocytes. The amount of oxygen carried in a sample of blood is measured in two ways. Oxygen dissolved in plasma is expressed as the partial pressure of oxygen (PaO2). The normal PaO2 in arterial blood is about 80 to 100 mm Hg. The oxygen dissolved in plasma, however, represents only about 1% to 2% of the total oxygen content of the blood. The vast majority of oxygen in the blood is carried bound to the hemoglobin molecule. The amount of oxygen bound to hemoglobin is expressed as the percentage of hemoglobin that is saturated with oxygen (SaO2), with 100% being fully saturated. Since the SaO2 is a percentage indicating the relationship between oxygen and hemoglobin, we should interpret the client’s SaO2 measurement with the hemoglobin level. Normal saturation of arterial blood (SaO2) is about 96% to 98%.
_
Hemoglobin molecules have the ability to form a reversible bond with oxygen molecules, so that the hemoglobin readily takes up oxygen in the lungs, while it also readily releases oxygen to the body’s cells in the systemic capillary beds. This seemingly paradoxical shift in hemoglobin’s affinity for oxygen is represented by the oxyhemoglobin dissociation curve, which is a graphic representation of the relationship between the partial pressure of oxygen and oxygen saturation.
_
The affinity of hemoglobin for oxygen is highest when the PaO2 (the measure of oxygen dissolved in the arterial blood plasma) is 70 mm Hg or higher; in this portion of the curve, further increases in PaO2 result in very little change in SaO2. This characteristic of the oxyhemoglobin dissociation curve accounts for the rapid uptake of oxygen by hemoglobin in the pulmonary circulation and allows for some decrease in PaO2 (such as might occur with disease or in high altitudes) without significantly sacrificing SaO2. As the oxygen-saturated blood is circulated to the peripheral capillary beds, dissolved oxygen diffuses out of blood. This decrease in dissolved oxygen causes hemoglobin to lose its affinity for oxygen, so the oxygen is then released to the body’s cells. Once the partial pressure of oxygen in the blood drops below 60 mm Hg, hemoglobin releases oxygen very easily. This release is represented in the lower left portion of the curve, also known as the venous portion, and permits rapid unloading of oxygen to the cells.
_
You can see that at high partial pressures of O2 (above about 40 mm Hg), hemoglobin saturation remains rather high (typically about 75 – 80%). This rather flat section of the oxygen-hemoglobin dissociation curve is called the ‘plateau.’ Recall that 40 mm Hg is the typical partial pressure of oxygen in the cells of the body. Examination of the oxygen-hemoglobin dissociation curve reveals that, under resting conditions, only about 20 – 25% of hemoglobin molecules give up oxygen in the systemic capillaries. This is significant (in other words, the ‘plateau’ is significant) because it means that you have a substantial reserve of oxygen. In other words, if you become more active, & your cells need more oxygen, the blood (hemoglobin molecules) has lots of oxygen to provide. When you do become more active, partial pressures of oxygen in your (active) cells may drop well below 40 mm Hg. A look at the oxygen-hemoglobin dissociation curve reveals that as oxygen levels decline, hemoglobin saturation also declines – and declines precipitously. This means that the blood (hemoglobin) ‘unloads’ lots of oxygen to active cells – cells that, of course, need more oxygen.
_
Several physiological factors may alter the affinity of hemoglobin for oxygen, and these shifts can be represented on the oxyhemoglobin dissociation curve. A shift to the left occurs when affinity is increased so that, for a given PaO2, the associated SaO2 will be higher. This means that, although the arterial blood may be carrying adequate oxygen, little of it is being released to the tissues. A shift to the left may be caused by increased pH (alkalosis), hypothermia, or a decrease in the red blood cell enzyme 2,3-diphosphoglycerate (2,3-DPG), which may occur after massive transfusions of banked blood. A shift to the right of the oxyhemoglobin dissociation curve means that, for a given PaO2, the SaO2 will be lower. This phenomenon represents a decreased affinity of hemoglobin for oxygen so that oxygen is more readily released to the tissues. This shift occurs in response to acidosis, hyperthermia, and hypoxia (which induces increased production of 2,3-DPG) and results in improved delivery of oxygen to the tissues.
_
Oxygen carrying capacity of the blood:
Most oxygen is carried in the blood attached to haemoglobin with only a small amount (typically less than 2% if PaO2<14 kPa) dissolved in the plasma. Despite this, the optimum haemoglobin concentration in critically ill patients is 100-110 g/l, which represents the balance between maximising oxygen content and the adverse microcirculatory effects associated with the marked rise in viscosity that occurs at higher packed cell volumes.
_
Effect of increasing levels of supplemental oxygen and transfusion in an anaemic hypoxemic patient showing importance of saturation and haemoglobin concentration:
| FiO2 | PaO2 (kPa) | SaO2 (%) | Hb (g/l) | Dissolved O2 (ml/l) | CaO2 (ml/l) | CaO2 (% exchange) | |
| Air | 0.21 | 6 | 75 | 80 | 1.4 | 83 | — |
| 35% O2 | 0.35 | 9.5 | 93 | 80 | 2.2 | 103 | 24 |
| 60% O2 | 0.6 | 16.5 | 98 | 80 | 3.8 | 110 | 7 |
| Transfusion | 0.6 | 16.5 | 98 | 120 | 3.8 | 164 | 48 |
As saturation and hemoglobin increases, more oxygen is carried by blood.
_
Circulation:
Once oxygen is bound to hemoglobin, the oxygen is delivered to the cells of the body by the process of circulation. Circulation of the blood is the function of the heart and blood vessels.
_____
- Internal Respiration:
During pulmonary gas exchange (external respiration) oxygen from inhaled air is diffused into the alveoli in the lungs and the waste product carbon dioxide from the body diffuses out through the alveoli to be exhaled back into the air. With the help of the cardiovascular system the freshly inhaled O2 rich blood is transported to the tissues of the body. At this point the final stage of respiration occurs as the much needed O2 is absorbed by the tissues and the waste CO2 that the tissues have created is diffuses back into the blood and is transported back to the lungs to be exhaled. This exchange of gases at tissue level is called peripheral gas exchange. Peripheral gas exchange is also known as ‘internal respiration’, as it involves the respiratory processes that occur within the tissues of the body rather than the lungs. This can be seen in the image below.
_____
Oxygen consumption:
Approximately 250ml of oxygen are used every minute by a conscious resting person (resting oxygen consumption) and therefore about 25% of the arterial oxygen content is used every minute. The haemoglobin in mixed venous blood is about 73% saturated (98% minus 25%). At rest, oxygen delivery to the cells of the body exceeds oxygen consumption. During exercise, oxygen consumption increases. The increased oxygen requirement is usually provided by an increased cardiac output and increased oxygen extraction. A low cardiac output, low haemoglobin concentration (anaemia) or low oxygen saturation will result in reduced tissue oxygen delivery, unless there is a compensatory change in one of the other factors.
_____
Factors affecting delivery of oxygen to tissues
- Inspired oxygen concentration FiO2
- Alveolar ventilation
- Ventilation-perfusion distribution within lungs
- Haemoglobin Hb and concentrations of agents which may bind to Hb as carbon monoxide
- Influences on the oxygen Hb dissociation curve
- Cardiac output and atherosclerosis of blood vessels
- Distribution of capillary blood flow within tissues
_____
Oxygen stores:
In spite of our reliance on oxygen, the stores of oxygen in the body are small and would be unable to sustain life for more than a few minutes. If breathing ceases, oxygen stores are limited to the oxygen in the lung and oxygen in the blood. The amount of oxygen in the blood depends on the blood volume and haemoglobin concentration. The amount of oxygen in the lung is dependent on the lung volume at functional residual capacity (FRC) and the alveolar concentration of oxygen. The FRC is the volume of air (about 3 litres in an adult) that is present in the lungs at the end of a normal expiration; at this volume the elastic recoil of the lung (its tendency to collapse) is balanced by the tendency of the chest wall and diaphragm to resist lung collapse. When breathing air, the total oxygen stores (in blood and lung) are small. The major component of this store is the oxygen bound to haemoglobin; only a small part of these stores can be released without an unacceptable reduction in PaO2 (when haemoglobin is 50% saturated, the PaO2 will have fallen to 3.5kPa). Breathing 100% oxygen causes a large increase in the total oxygen stores as the FRC fills with oxygen. The major component of the store is now in the lung and 80% of this oxygen can be used without any reduction in haemoglobin saturation (PaO2 is still about 14kPa). This is the reason why pre-oxygenation is so effective [vide infra].
_____
_____
Measures of Oxygenation:
- arterial oxygen saturation (SaO2)/ SpO2
- arterial oxygen tension (PaO2)
- A-a (alveolar-arterial) oxygen gradient
- PaO2/FiO2 ratio
- A-a oxygen ratio
- Oxygenation index (OI)
- Oxygen Extraction Ratio (O2ER)
__
PaO2, SaO2, CaO2 are all related but different.
PaO2, the partial pressure of oxygen in the arterial blood, is determined solely by the pressure of inhaled oxygen (the PiO2), the PaCO2, and the architecture of the lungs. The most common physiologic disturbance of lung architecture is ventilation-perfusion (V-Q) abnormality; less commonly, there can be diffusion block or anatomic right to left shunts. If the lungs are normal, then PaO2 is affected only by the alveolar PO2 (PAO2), which is determined by the fraction of inspired oxygen, the barometric pressure and the PaCO2 (i.e., the alveolar gas equation). PaO2 is a major determinant of SaO2, and the relationship is the familiar sigmoid-shaped oxygen dissociation curve. SaO2 is the percentage of available binding sites on hemoglobin that are bound with oxygen in arterial blood. The O2 dissociation curve (and hence the SaO2 for a given PaO2) is affected by PaCO2, body temperature, pH and other factors. However, SaO2 is unaffected by the content of hemoglobin, so anemia does not affect SaO2. CaO2 is arterial oxygen content. Unlike either PaO2 or SaO2, the value of CaO2 directly reflects the total number of oxygen molecules in arterial blood, both bound and unbound to hemoglobin. CaO2 depends on the hemoglobin content, SaO2, and the amount of dissolved oxygen. Units for CaO2 are ml oxygen/100 ml blood.
__
Since a normal PaO2 is between 80-100 mmHg, some people may think that an O2 saturation of 90 is normal as well — after all 90 is easy to remember. However, this interpretation is very wrong. An O2 sat of 90% corresponds to a PaO2 of 60 mmHg. This is the minimum oxygen concentration providing enough oxygen to prevent ischemia in tissues. Once the O2 sat falls below 90%, the PaO2 drops quickly into the dangerously hypoxic range as fewer and fewer oxygen molecules are bound to Hb. We want to try to keep O2 saturation above 90%.
_
Is Oxygen Saturation of 100% always Normal?
No, it’s not.
Let’s take an example of a patient breathing 50% FiO2 who has a PaO2 of 100. A simple formula to estimate what the arterial oxygen concentration should be is to multiply the inspired oxygen concentration by 5 to 6. Someone breathing room air at 21% oxygen should have a PaO2 of about 100. So if the patient is breathing 50%, we know that his PaO2 should be about 250 and if it is not, then something is very wrong. But if you look at the Oxygen-Hemoglobin Dissociation Curve, a PaO2 of 100 and 250 both have an O2 sat of 100% because both provide enough oxygen molecules to fill all of the Hb binding sites. So in this case O2 saturation doesn’t help us very much.
_
Generally speaking, your SpO2 matches up with your PaO2 as follows:
- 90 percent SpO2 = 60 PaO2 (this is your normal range, and usually no oxygen therapy is needed)
- 80 percent SpO2 = 50 PaO2 (this is your hypoxemic range, meaning oxygen therapy is needed)
- 70 percent SpO2 = 40 PaO2 (this is severe hypoxemia, immediate treatment is needed to prevent tissue damage and death)
_
PaO2 is a sensitive and non-specific indicator of the lungs’ ability to exchange gases with the atmosphere. In patients breathing ambient or “room” air (FiO2 = 0.21), a decreased PaO2 indicates impairment in the gas exchange properties of the lungs, usually signifying V-Q imbalance. PaO2 is a very sensitive indicator of gas exchange impairment; it can be reduced from virtually any lung problem, including asthma, chronic obstructive pulmonary disease, pneumonia, ARDS and atelectasis that doesn’t show up on a chest x-ray.
Normal PaO2 decreases with age:
A patient over age 70 may have a normal PaO2 around 70-80 mm Hg, at sea level. A useful rule of thumb is normal PaO2 at sea level (in mm Hg) = 100 minus the number of years over age 40.
_
Target oxygen saturation range: 92-96% Versus 94-98: a 2017 study:
This scientific letter considers the rationale for the target oxygen saturation measured by pulse oximetry (SpO2 ) range of 92-96% for oxygen therapy in adult patients without COPD or other conditions associated with chronic respiratory failure, recommended by the Thoracic Society of Australia and New Zealand, in contrast to the 94-98% target range recommended by the British Thoracic Society. Authors conclude from the available evidence that the SpO2 target of 92-96% may be preferable to 94-98%.
_
Alveolar to arterial (A-a) oxygen gradient:
Alveolar to arterial (A-a) oxygen gradient = PAO2 – PaO2. The A-a oxygen gradient indicates the integrity of the alveolar-capillary membrane and effectiveness of gas exchange. Pathology of the alveolar-capillary unit widens the gradient. Therefore, hypoxemia due to V/Q mismatch, diffusion limitation, and shunt will have widened gradient, whereas hypoxemia due to hypoventilation would have normal gradient. Unlike PaO2, PAO2 is not measured but calculated by using the alveolar gas equation. In young person, the A-a oxygen difference is <10 mmHg. The A-a oxygen difference increases with age. It is primarily due to age-induced decrease in the PaO2 level because of the rise in V/Q mismatch. The drop in PaO2 after 70 years is about 0.43 mmHg per year. High FiO2 by increasing both the alveolar and arterial oxygen level widens the gradient. The rise in gradient is due to disproportionate increase in alveolar oxygen level. The arterial blood oxygen level does not rise to the same proportion as the alveolar oxygen level due to its admixing with unoxygenated blood coming from bronchial veins, mediastinal veins, and thebesian veins.
_
Oxygenation Index (OI):
The usual way to describe the severity of pulmonary dysfunction in ventilated ICU patients is by using the PaO2/FiO2 ratio (PF). The PF may be adjusted by the ventilator pressure settings in order to reduce inspiratory oxygen fraction but the PF does not take the mean airway pressure (MAP) into account. In contrast, the Oxygenation Index (OI) is defined as the reciprocal of PF times MAP: OI = (FiO2×mean airway pressure)/PaO2. As such, the OI is a better representative of oxygenation dysfunction. The oxygenation index is used to assess the intensity of ventilatory support required to maintain oxygenation. It is used in neonatology and pediatrics to assess the need for potential ECMO therapy. A lower oxygenation index is better. As the oxygenation of a person improves, they will be able to achieve a higher PaO2 at a lower FiO2.
__
Oxygen Extraction Ratio (O2ER):
Oxygen extraction ratio (O2ER) is the ratio of oxygen consumption (VO2) to oxygen delivery (DO2). Global oxygen delivery (DO2) is the total amount of oxygen delivered to the tissues per minute, irrespective of the distribution of blood flow. Oxygen consumption (VO2) is the total amount of oxygen removed from the blood due to tissue oxidative metabolism per minute. Under resting conditions with normal distribution of cardiac output, DO2 is more than adequate to meet VO2 and ensure that aerobic metabolism is maintained. Oxygen that is not extracted returns to the mixed venous circulation. SvO2 of 70% indicates oxygen delivery is adequate (assuming normal microcirculatory function).
O2ER = VO2 / DO2 = (SaO2-SvO2) / SaO2
In a normal 75 kg adult undertaking routine activities:
- VO2 is approximately 250 ml/min
- DO2 is approx. 1 L/min
- O2ER is 25% (increases to ~70% during maximal exercise in an athlete)
- SvO2 70%
_
High O2ER suggests inadequate oxygen delivery (reduced DO2):
- Hypoxic hypoxia: low FiO2 gas or high altitude; lung disease)
- reduced hemoglobin (anemia)
- Heart failure
- Shock/ hypoperfusion due to other causes
Or increased oxygen consumption (increased VO2):
- fever and inflammatory states, e.g. sepsis, burns, trauma, surgery
- increased metabolic rate, e.g. hyperthyroidism, adrenergic drugs, hyperthermia, burns
- increased muscular activity, e.g. exercise, shivering, seizures, agitation/anxiety/pain, weaning from ventilation/ increased respiratory effort
_
Low O2ER suggests increased oxygen delivery:
-hyperoxia, e.g. high FiO2 gas, hyperbaric oxygen or ECMO
Or decreased oxygen consumption:
- decreased metabolic rate, e.g. hypothyroidism, sedatives/ hypnotics, hypothermia
- decreased muscular activity e.g. sedation/analgesics, muscle paralysis, ventilatory support
- antipyretics
- Starvation/hyponutrition
- Sepsis due to shunting
- Histotoxic hypoxia, e.g. cyanide poisoning
_____
Synopsis of measures of oxygenation:
______
______
Carbon Dioxide Transport and Excretion:
Carbon dioxide – transported from the body cells back to the lungs as:
1 – Bicarbonate (HCO3) – 60% -formed when CO2 released by cells making ATP combines with H2O due to the enzyme in red blood cells called carbonic anhydrase
2 – Carbaminohemoglobin – 30% -formed when CO2 combines with hemoglobin (hemoglobin molecules that have given up their oxygen)
3 – Dissolved in the plasma – 10%
Carbon dioxide is a natural byproduct of glucose metabolism. Like oxygen, it exists normally as a gas and can be dissolved in the plasma as well as loosely bound to the hemoglobin molecule (although carbon dioxide attaches to a different binding site on the hemoglobin molecule than does oxygen). In the lungs, carbon dioxide is released into the alveoli by diffusion, and when the individual exhales, the carbon dioxide exits to the atmosphere. In the body fluids, carbon dioxide functions as an acid because, combined with water, it produces carbonic acid. The hydrogen ions that are liberated in this process stimulate the respiratory control centers in the pons and medulla to increase the rate and depth of breathing; more carbon dioxide is then released by the lungs and the pH of the body is brought back to normal. Likewise, increased production of carbon dioxide, as may be associated with fever or exercise, is often a cause of increased ventilatory rate (tachypnea) and depth. Elevated blood levels of carbon dioxide (hypercapnia) indicate inadequate alveolar ventilation.
______
______
Hypoxia and hypoxemia:
Hypoxia literally means “deficient in oxygen”, that is an abnormally low oxygen availability to the body or an individual tissue or organ. Hypoxia is deficiency of O2 at tissue levels. Hypoxemia refers to reduced O2 tension in arterial blood, that is decreased partial pressure of oxygen in blood. Hypoxemia leads to low levels of oxygen in the blood (low blood oxygen saturation or content). Hypoxia is inadequate oxygen in tissues for normal cell and organ function, and hypoxia results from hypoxemia. Hypoxemia occurs frequently in diseases like lower respiratory tract infection (severe pneumonia or bronchiolitis), upper airway obstruction, severe asthma, common neonatal conditions like birth asphyxia and in respiratory distress syndrome, severe sepsis, heart failure, cardiac arrest, trauma, carbon monoxide poisoning, and obstetric and perioperative emergencies. Anoxia is when there is no oxygen available at all.
_
As all the functions of the human body require oxygen, oxygen deprivation can have severe adverse effects on the cells that perform important biological processes. Lack of oxygen leads very quickly to dysfunction of the organ systems, and death. Therefore, hypoxemia is a life-threatening condition that requires early detection and treatment.
Arterial oxygen saturation is referred to as SaO2 when measured by gas analysis and as SpO2 when measured by pulse oximetry. The normal range of SpO2 at sea level is 97–99%, with a lower limit (mean minus 2 standard deviations) of 94%. Therefore, the percentage is lower in children living at high altitude because of a lower partial oxygen pressure (PaO2) at higher altitude. The amount of oxygen used varies with the threshold at which hypoxemia is defined and oxygen is given. In one hospital, it was found that 13% of children with pneumonia were hypoxemic at SpO2 < 85%, 26% at SpO2 < 90% and 44% at SpO2 < 93%. In practice, the threshold at which oxygen is given is often SpO2 < 90%, which corresponds to the flat part of the haemoglobin–oxygen dissociation curve and represents a safe margin of error where there are sufficient oxygen supplies. Small reductions in SpO2 below 90% may represent a dangerous fall in PaO2 (steep part of the curve). Oxygen therapy at higher thresholds than 90% SpO2 are required in some conditions, such as serious impairment of oxygen delivery from the lungs to body tissues and when the vital organs are particularly susceptible to low oxygen levels. Examples include severe anaemia (in which haemoglobin may be normally saturated but provides too little oxygen because of too little haemoglobin), severe heart failure, severe sepsis or brain injury or in critically ill children with emergency signs. In these conditions, especially during the resuscitation phase, give oxygen if the SpO2 is < 94%.
_
John Scott Haldane, who formulated much of our understanding of gas physiology, said in 1917, “Hypoxia not only stops the motor, it wrecks the engine.” Patients begin to suffer impaired mental function at oxygen saturations below 64 percent. People typically lose consciousness at saturations less than 56 percent, giving airplane passengers no more than 60 seconds to breathe supplemental oxygen when an airplane flying at 30,000 feet suddenly depressurizes.
__
_
Mechanisms of Hypoxemia:
- Inadequate PAO2
–Alveolar Hypoventilation
–Decreased PiO2/Increased Altitude
- V/Q Mismatch
- Shunt (Will not improve with O2)
–Intrapulmonary (Pneumonia, ARDS)
–Intracardiac
–Intravascular
- Diffusion Abnormality
- Low SvO2
_
Note:
Hypoxemia due to ventilation perfusion mismatch, hypoventilation, high altitude (low PiO2) or diffusion impairment is reversed by giving oxygen.
__
There are four types of hypoxia:
- Hypoxemic hypoxia (hypoxic hypoxia) – occurs when there is poor gas exchange between the alveoli and the capillary
- Ischaemic (Stagnant) hypoxia – Occurs when there is obstruction to blood flow servicing areas of the body
- Histotoxic hypoxia- the body’s cells are unable to use the oxygen even if there is sufficient oxygen circulating. This is likely to occur in some cases of poisoning
- Anaemic hypoxia – occurs when the ability to transport oxygen is impaired due to lack of haemoglobin or inefficient haemoglobin.
Indications for oxygen therapy include a blood oxygen saturation (SaO2) of less than 90%, partial oxygen pressure (PaO2) of less than 60 mmHg on room air, cardiac instability, or hypoventilation. Hypoventilation also needs assisted ventilation to remove excess CO2 from blood.
__
Respiratory failure results from inadequate gas exchange by the respiratory system, meaning that the arterial oxygen, carbon dioxide or both cannot be kept at normal levels. The normal partial pressure reference values are: oxygen PaO2 more than 80 mmHg (11 kPa), and carbon dioxide PaCO2 lesser than 45 mmHg (6.0 kPa). Type 1 respiratory failure is defined as a low level of oxygen in the blood (hypoxemia) without an increased level of carbon dioxide in the blood (hypercapnia), and indeed the PaCO2 may be normal or low. It is typically caused by a ventilation/perfusion (V/Q) mismatch. Type 2 respiratory failure is hypoxemia (PaO2 <60 mm) with hypercapnia (PaCO2 >50mm). Type 2 respiratory failure is caused by inadequate alveolar ventilation; both oxygen and carbon dioxide are affected. It is defined as the build-up of carbon dioxide levels (PaCO2) that has been generated by the body but cannot be eliminated. Occasionally type 2 respiratory failure has near normal oxygen level but high carbon dioxide in blood.
_
Figure below depicts causes of hypoxemia:
There are five cause of hypoxemia. Decreased PaO2 can be caused by hypoventilation, low PiO2, diffusion limitation, low V/Q regions or shunt. In contrast with other causes, hypoxemia due to shunt characteristically responds poorly to increased FiO2. Low V/Q regions and shunt are by far the most common causes of clinically encountered hypoxemia. Hypoventilation and low V/Q regions also impair CO2 removal, but the magnitude of the effect on PaCO2 is less and modified by the ventilatory response to hypercapnia. High V/Q regions and alveolar and apparatus dead space cause increased wasted ventilation and, thus, impaired CO2 elimination. The primary response to increased wasted ventilation is, in most situations, increased minute ventilation and work of breathing, not increased PaCO2. The effect of V/Q mismatch on gas exchange efficiency can be quantified using calculations of PA–aO2, venous admixture and wasted ventilation. Low and high V/Q regions cause hypoxemia, impaired CO2 elimination and increased work of breathing in COPD patients. Shunt is the most important cause of hypoxemia in patients with ARDS and pneumonia.
_
On a cellular level, mitochondrial function declines, anaerobic glycolysis occurs, and lactate/pyruvate ratio increases. Patients may have impaired judgment at low levels of hypoxemia, with progressive loss of cognitive and motor functions, and eventually loss of consciousness as severe hypoxemia ensues. Other nonspecific symptoms of hypoxemia include headache, breathlessness, palpitations, angina, restlessness, and tremor.
_
Obvious breathing is a sign of hypoxia:
Breathing in normal healthy person is subtle. If a patient’s breathing is obvious on initial contact (for example, when you first see the patient on walking into the room) it is abnormal. Normal breathing at rest is simply not obvious; one has to look very closely for chest movement to appreciate breathing. Six signs that may make someone’s breathing obvious to the observer – all abnormal – are:
- flaring of nostrils with breathing
- tachypnea (generally, to be obvious, respiratory rate is > 24 breaths/min)
- noisy breathing (wheezing, stridor, moaning, etc.)
- use of accessory breathing muscles (neck muscles, intercostal muscles, etc.)
- pursed lip breathing (often seen in severe COPD)
- Cheyne-Stokes breathing (alternating periods of apnea with tachypnea; apnea periods may last up to 30 seconds).
__
The Cellular Response to Hypoxia:
The bulk of cellular oxygen is used by mitochondria as an electron acceptor that allows adenosine triphophate to be generated from the energy released during the oxidation of carbon in dietary sugars and fats (oxidative phosphorylation). Oxygen delivery to the mitochondria depends on the arterial oxygen content (primarily determined by the hemoglobin concentration and the oxyhemoglobin saturation), the cardiac output, the vascular supply to the tissue, and the metabolic rate of the tissue. The last is responsible for the observation that the PO2 of the tissues is substantially lower than the arterial blood even under normal conditions. For example, in metabolically active tissues including the liver and the kidney, diffusion gradients between the vasculature and individual cells result in a normal tissue PO2 as low as 30 torr. The ability of cells to tolerate these levels of oxygen is explained by the finding that isolated mitochondria do not become substrate limited by oxygen until the PO2 falls to <1 torr. This complex interrelationship between oxygen delivery and metabolism explains how some patients with remarkably low arterial oxygen concentrations can remain clinically “stable” for prolonged periods prior to the initiation of oxygen therapy. Unlike exposure to hypoxia, ischemia resulting from vascular occlusion interrupts both oxygen and glucose delivery and prevents the clearance of CO2 and lactate, resulting in tissue hypoxia, hypercapnia, and acidosis.
_
In response to a reduced tissue PO2, cells initiate an adaptive program mediated by the transcription factors hypoxia inducible factor (HIF)-1 and HIF-2. The HIFs are heterodimers composed of HIF-1α or HIF-2α and HIF-1β (also known as the aryl hydrocarbon receptor nuclear translocator). Under normoxic conditions, HIF-1β can be found in the cytoplasm; however, HIF-1α is nearly undetectable because it is rapidly targeted for ubiquitin-mediated degradation by its E3 ligase, the von Hippel Lindau protein. For the von Hippel Lindau protein to target HIF-1α, it must be hydroxylated at specific proline residues by prolyl hydroxylases (PHDs). As the intracellular PO2 falls below about 30 torr, the activity of the PHDs is inhibited, preventing the hydroxylation and subsequent ubiquitin-mediated degradation of HIF-1. Once stabilized, HIFs bind to hypoxic-responsive elements in the genome to induce the transcription of hundreds of genes. Although this HIF-induced program of gene expression varies as a function of cell type, in most cells, HIFs increase the expression of glycolytic genes and angiogenic factors, including vascular endothelial growth factor (VEGF). This adaptive program promotes cellular survival and revascularization but often comes at the cost of reducing the function of the cell with respect to the tissue in which it resides (e.g., reducing contractile force generation in a cardiac myocyte). Prolonged expression of this transcriptional program can even disrupt tissue architecture. For example, the HIF-mediated release of VEGF from hypoxic cells in the retina is implicated in both diabetic retinopathy and the retinopathy of prematurity.
_
In addition to the HIF-mediated alterations in gene expression, arterial hypoxia causes physiologically important changes in the pulmonary vasculature. In most vascular beds, hypoxia-induced vasodilation increases blood flow to the hypoxic tissue. In contrast, the pulmonary vasculature constricts in response to hypoxia, which allows pulmonary blood flow to be distributed to the better-ventilated regions of the lung and, under normal conditions, ensures a ratio of ventilation to perfusion near unity. In patients with lung disease, large regions of the lung can become hypoxic, causing a significant increase in pulmonary vascular resistance, which can acutely contribute to the development of right-sided heart failure, limiting global perfusion. In these patients, physicians might use supplemental oxygen therapy as a pulmonary vasodilator. Because the pulmonary circulation is sensitive to changes in PO2 as high as 70 to 80 torr, the use of oxygen to vasodilate the pulmonary circulation might require the administration of a higher FiO2 than that required to fully saturate hemoglobin.
_____
Figure below shows effects of chronic hypoxemia on tissues:
_____
Physiological Responses to Reduced Oxygenation:
When oxygen delivery is inadequate to meet the metabolic needs of the body, various responses to this deficit can be expected, including changes in metabolic pathways and efforts to increase the extraction of available oxygen. If these efforts fail, cells will be damaged and ultimately die.
- Increased Oxygen Extraction:
Under normal conditions, the cells of the body do not extract all of the oxygen carried in the arterial blood. In fact, blood returning to the heart via the venous circulation is typically about 75% saturated with oxygen. In response to poor oxygen delivery or increased oxygen need, the cells can extract more oxygen from the arterial blood.
- Anaerobic Metabolism:
The utilization of food (glucose) for cellular energy occurs via metabolic pathways that use oxygen; this is known as aerobic metabolism. Many cells are also capable of utilizing alternate metabolic pathways in the absence of oxygen for short periods of time; this is referred to as anaerobic metabolism. Anaerobic metabolism is limited by several factors:
-a. Not all cells are capable of significant anaerobic metabolism (most notably brain cells).
-b. Anaerobic metabolism yields less energy per unit of fuel than does aerobic metabolism.
Glycolysis produces only 2 ATP molecules, but somewhere between 30 and 36 ATPs are produced by the oxidative phosphorylation of the 10 NADH and 2 succinate molecules made by converting one molecule of glucose to carbon dioxide and water, while each cycle of beta oxidation of a fatty acid yields about 14 ATPs.
-c. Anaerobic metabolism results in the accumulation of acid byproducts, such as lactate, which upset the chemical environment of the cell and induce the release of cell-damaging (lysosomal) enzymes.
- Tissue Ischemia and Cell Death:
Prolonged oxygen deprivation (hypoxia) will lead to a syndrome ending in cellular death. The decreased production of adenosine triphosphate (ATP) resulting from anaerobic metabolism reduces the amount of energy available for cellular metabolic functions and results in a breakdown in all cellular functions. The integrity of the cell membrane becomes impaired, and the cell begins to swell. Cellular organelles may become damaged and lysosomal enzymes released, killing the cell. The destruction of tissues or organs as a result of oxygen deprivation is known as an infarction. Widespread cellular death resulting from oxygenation disturbances is the underlying characteristic of a devastating syndrome known as multiple-organ-system failure.
____
Benefits of oxygen therapy:
_
Assessing the need for oxygen therapy 3 basic ways:
- Laboratory measures: PAO2, PaO2, SaO2, SpO2 monitoring
- Clinical Problem or condition: postoperative patients, pneumonia, atelectasis, pulmonary edema, etc…
- Symptoms of hypoxemia: tachycardia, tachypnoea, hypertension, cyanosis, dyspnoea, disorientation, clubbing, etc.
_____
_____
Introduction to oxygen therapy:
Oxygen (O2) must be present in every breathing gas. This is because it is essential to the human body’s metabolic process, which sustains life. The human body cannot store oxygen for later use as it does with food. If the body is deprived of oxygen for more than a few minutes, unconsciousness and death result. The tissues and organs within the body (notably the heart and brain) are damaged if deprived of oxygen for much longer than four minutes.
_
Oxygen therapy may be defined as the administration of oxygen to a patient at an inspired concentration greater than that of oxygen concentration in ambient air. Oxygen therapy is one of the important parts of therapy for treating hypoxemia and widely used across a whole range of specialties. Oxygen is a drug. It should not be given, it should be prescribed. So, it mandates to be used cautiously but also no patient should be devoid of it when required and indicated. It is on the World Health Organization’s List of Essential Medicines, the most effective and safe medicines needed in a health system. Oxygen therapy is widely used in the management of a number of chronic and acute health conditions. The therapy may be used in a hospital setting or pre-hospital setting (e.g. in the ambulance) to manage emergency situations or in the home setting to manage long-term health conditions. The mode of delivery and device used for oxygen therapy depends upon several factors including the patient’s specific needs and the opinion of the medical professionals involved. The use of oxygen in medicine became common around 1917. The cost of home oxygen is about 150 USD a month in Brazil and 400 USD a month in the United States. Home oxygen can be provided either by oxygen tanks or an oxygen concentrator. Oxygen is believed to be the most common treatment given in hospitals in the developed world.
_
Oxygen therapy, also known as supplemental oxygen, is the use of oxygen as a medical treatment. This can include for low blood oxygen, carbon monoxide toxicity, cluster headaches, and to maintain enough oxygen while inhaled anesthetics are given. Long term oxygen is often useful in people with chronically low oxygen such as from severe COPD or cystic fibrosis. Oxygen can be given in a number of ways including nasal cannula, face mask, and inside a hyperbaric chamber. Oxygen is required for normal cell metabolism. Excessively high concentrations can cause oxygen toxicity such as lung damage or result in respiratory failure in those who are predisposed. Higher oxygen concentrations also increase the risk of fires, particularly while smoking, and without humidification can also dry out the nose. The target oxygen saturation recommended depends on the condition being treated. In most conditions a saturation of 94-98% is recommended, while in those at risk of carbon dioxide retention saturations of 88-92% are preferred, and in those with carbon monoxide toxicity or cardiac arrest they should be as high as possible. Air is typically 21% oxygen by volume while oxygen therapy can increase this up to 100%.
_
To deliver emergency oxygen, you need:
- An oxygen cylinder.
- A regulator with pressure gauge and flowmeter.
- A delivery device, such as a nasal cannula, resuscitation mask, non-rebreather mask or a BVM (bag valve mask or AMBU bag).
_
Normobaric hyperoxia (normobaric oxygen, NBO) is applied via a wide variety of masks that allow delivery of inspired oxygen of 24% to 90%. Higher concentrations can be delivered via masks with reservoirs, tightly fitting continuous positive airway pressure-type masks, or during mechanical ventilation. There are two methods of administering oxygen at pressures higher than 0.1 MPa (1 atmosphere absolute, 1 ATA) (hyperbaric oxygen, HBO). In the first, a small hyperbaric chamber, usually designed for a single occupant, is used. The chamber is filled with 100% oxygen, which is compressed to the pressure required for treatment. With the second method, the treatment is given in a large multiplace hyperbaric chamber. The chamber is filled with compressed air while the patients breathe 100% oxygen at the same ambient pressure via a mask or hood.
_
Tissue oxygenation is dependent on optimal or adequate delivery of oxygen to the tissues. Increasing the concentration of inhaled oxygen is an effective method of increasing the partial pressure of oxygen in the blood and correcting hypoxemia. Simply stated, oxygen therapy is a means to provide oxygen according to target saturation rates (as per physician orders or hospital protocol) to achieve normal or near normal oxygen saturation levels for acute and chronically ill patients (British Thoracic Society, 2008). Those administering oxygen must monitor the patient to keep the saturation levels within the required target range. Oxygen should be reduced or discontinued in stable patients with satisfactory oxygen saturation levels (Perry et al., 2014). Hypoxemia or hypoxia is a medical emergency and should be treated promptly. Failure to initiate oxygen therapy can result in serious harm to the patient. The essence of oxygen therapy is to provide oxygen according to target saturation rates, and to monitor the saturation rate to keep it within target range. The target range (SaO2) for a normal adult is 94% to 98%. For patients with COPD, the target SaO2 range is 88% to 92% (Alberta Health Services, 2015; British Thoracic Society, 2008; Kane, et al., 2013).
_
Although all medications given in the hospital require a prescription, oxygen therapy may be initiated without a physician order in emergency situations. Most hospitals will have a protocol in place to allow health care providers to apply oxygen in emergency situations. The health care provider administering oxygen is responsible for monitoring the patient response and keeping the oxygen saturation levels within the target range. The most common reasons for initiating oxygen therapy include acute hypoxemia related to pneumonia, shock, asthma, heart failure, pulmonary embolus, myocardial infarction resulting in hypoxemia, post-operative states, pneumothorax, and abnormalities in the quality and quantity of hemoglobin. There are no contraindications to oxygen therapy if indications for therapy are present (Kane et al., 2013).
_
Oxygen is probably the most common drug to be used in the care of patients who present with medical emergencies; approximately 34% of ambulance journeys involve oxygen use at some stage and national audit data suggest that 18% of hospital inpatients in the UK are being treated with oxygen at any given time. At present, oxygen is administered for three main indications:
- to correct hypoxemia, as there is good evidence that severe hypoxemia is harmful
- to prevent hypoxemia in unwell patients
- to alleviate breathlessness.
Only the first of these indications is evidence based. Recent evidence suggests that the administration of oxygen to prevent hypoxemia in ill patients may actually place them at increased risk if severe hypoxemia does actually develop. Additionally, there is no evidence that oxygen relieves breathlessness in non-hypoxemic patients and there is evidence for lack of effectiveness in non-hypoxemic breathless patients with chronic obstructive pulmonary disease (COPD) or advanced cancer. However, patients at risk of hypoxemia should be adequately monitored in an appropriate clinical setting.
_
Oxygen is essential for cell metabolism, and in turn, tissue oxygenation is essential for all normal physiologic functions. Prompt recognition of life threatening situations and immediate administration of oxygen appropriately will benefit the patient in reducing morbidity and mortality. Oxygen is a lifesaving treatment. It should be treated like any other drug; it should be prescribed in writing, with the required flow rate and the method of delivery clearly specified. Failure to correct hypoxemia (PaO2 > 8 kPa ) for fear of causing hypoventilation and carbon dioxide retention is unacceptable clinical practice. Careful monitoring of treatment is essential and will detect those patients at risk of carbon dioxide retention.
_
Oxygen prescription:
Oxygen therapy is one of the most critical considerations in the management of diseases crossing different medical and surgical specialities. But this is one subject which remains poorly understood and inadequately practiced. Invariably, enormous errors are committed in the use of oxygen. Oxygen prescription often comprises of a single written word — oxygen. Frequently, it is administered merely on verbal orders. Reports on assessment of uses and misuses of oxygen are almost universal including those from the developed countries of the West. Several authors have found gross inadequacies in oxygen-prescription on analysis of over thousands of indoor files and treatment charts. Oxygen is a medicine and it must be prescribed for you to get it.
_
A detailed oxygen prescription indicates:
The oxygen dose (liters per minute, or l/m), the number of hours per day that oxygen therapy is required, the dose required during exercise, the oxygen supply system: concentrator, compressed gas cylinder, or liquid oxygen reservoir, and the delivery device: nasal cannula, demand-flow device, reservoir cannula, or transtracheal oxygen catheter etc.
Best practice is to prescribe a target range for all hospital patients at the time of admission so that appropriate oxygen therapy can be started in the event of unexpected clinical deterioration with hypoxemia and also to ensure that the oximetry section of the early warning score (EWS) can be scored appropriately. The target saturation should be written (or ringed) on the drug chart or entered in an electronic prescribing system.
_
The challenge:
Oxygen is included on the World Health Organization (WHO) Model List of Essential Medicines (EML), but listed only under “anaesthetics” with no specification for use as a treatment for hypoxemia. Lack of such global endorsement and normative guidance serves to limit the use of oxygen therapy, which remains inaccessible to a large proportion of patients admitted to health facilities in low and middle-income countries (LMICs). Furthermore, when oxygen technologies are available, their use is often complicated by inadequate maintenance, missing equipment, or lack of proper staff training on safe use and/or treatment guidelines. As a result, newborns, children, and pregnant women in need of oxygen often do not receive it or receive it unsafely, creating a serious barrier to improving health outcomes, and putting particularly vulnerable patients at risk.
_____
_____
Rationale of oxygen therapy:
Oxygen is one of the most commonly used therapeutic agents. Injudicious use of oxygen at high partial pressures (hyperoxia) for unproven indications, its known toxic potential, and the acknowledged roles of reactive oxygen species in tissue injury led to scepticism regarding its use. A large body of data indicates that hyperoxia exerts an extensive profile of physiologic and pharmacologic effects that improve tissue oxygenation, exert anti-inflammatory and antibacterial effects, and augment tissue repair mechanisms. These data set the rationale for the use of hyperoxia in a list of clinical conditions characterized by tissue hypoxia, infection, and consequential impaired tissue repair. Data on regional hemodynamic effects of hyperoxia and recent compelling evidence on its anti-inflammatory actions incited a surge of interest in the potential therapeutic effects of hyperoxia in myocardial revascularization and protection, in traumatic and nontraumatic ischemic-anoxic brain insults, and in prevention of surgical site infections and in alleviation of septic and nonseptic local and systemic inflammatory responses. Although the margin of safety between effective and potentially toxic doses of oxygen is relatively narrow, the ability to carefully control its dose, meticulous adherence to currently accepted therapeutic protocols, and individually tailored treatment regimens make it a cost-effective safe drug.
_
Oxygen is one of the most widely used therapeutic agents. It is a drug in the true sense of the word, with specific biochemical and physiologic actions, a distinct range of effective doses, and well-defined adverse effects at high doses. Oxygen is widely available and commonly prescribed by medical staff in a broad range of conditions to relieve or prevent tissue hypoxia. Although oxygen therapy remains a cornerstone of modern medical practice and although many aspects of its physiologic actions have already been elucidated, evidence-based data on its effects in many potentially relevant clinical conditions are lagging behind. The cost of a single use of oxygen is low. Yet in many hospitals, the annual expenditure on oxygen therapy exceeds those of most other high-profile therapeutic agents. The easy availability of oxygen lies beneath a lack of commercial interest in it and the paucity of funding of large-scale clinical studies on oxygen as a drug. Furthermore, the commonly accepted paradigm that links hyperoxia to enhanced oxidative stress and the relatively narrow margin of safety between its effective and toxic doses are additional barriers accounting for the disproportionately small number of high-quality studies on the clinical use of oxygen at higher-than-normal partial pressures (hyperoxia). Yet it is easy to meticulously control the dose of oxygen (the combination of its partial pressure and duration of exposure), in contrast to many other drugs, and therefore clinically significant manifestations of oxygen toxicity are uncommon.
_
Tissue oxygenation:
Delivery of oxygen to tissues depends on adequate ventilation, gas exchange, and circulatory distribution. When air is breathed at normal atmospheric pressure, most of the oxygen is bound to hemoglobin while only very little is transported dissolved in the plasma. On exposure to hyperoxia, hemoglobin is completely saturated with oxygen. This accounts for only a small increase in arterial blood oxygen content. In addition, the amount of physically dissolved oxygen in the blood also increases in direct proportion to the ambient oxygen partial pressure. Due to the low solubility of oxygen in blood, the amount of dissolved oxygen in arterial blood attainable during normobaric exposures to 100% oxygen (about 2 vol%) can provide only one third of resting tissue oxygen requirements. However, on exposure to oxygen at a pressure of three atmospheres (in a hyperbaric chamber), there is sufficient oxygen dissolved in the plasma (about 6 vol%) to meet the average requirements of resting tissues by means of dissolved oxygen alone without contribution from oxygen bound to hemoglobin. This is part of the rationale behind the use of hyperoxia in situations in which the hemoglobin’s oxygen-carrying capacity has been impaired (for example, in carbon monoxide poisoning and in severe anemia when transfusion of blood is not possible).
Deliberations on the effect of hyperoxia on the availability of molecular oxygen to tissues which are based on changes in arterial blood oxygen content undervalue the main effect of hyperoxia that is related to changes in its partial pressure in the blood (see table below). The flow of oxygen into tissues occurs by diffusion. The driving force for diffusion of oxygen is determined by its partial pressure gradient between capillary blood and tissue cells and much less so by increased oxygen content. Inhalation of 100% oxygen yields a 5- to 7-fold increase in arterial blood oxygen tension at normal atmospheric pressure and may reach values close to 2,000 mm Hg during hyperbaric exposure to oxygen at 0.3 MPa (3 ATA). The marked increase in oxygen tension gradient from the blood to metabolizing cells is a key mechanism by which hyperoxygenation of arterial blood can improve effective cellular oxygenation even at low rates of tissue blood flow.
_
Alveolar oxygen partial pressure while breathing room air or 100% oxygen at different ambient pressures from 1 to 3 ATA
| Total pressure mm Hg | PAO2 on air
(FiO2 21%) |
PAO2 on 100% O2
(FiO2 100%) |
|
| ATA | |||
| 1 | 760 | 102 | 663 |
| 2.5 | 1,900 | 342 | 1,803 |
| 3 | 2,280 | 422 | 2,183 |
ATA, atmosphere absolute; PAO2, alveolar oxygen partial pressure.
At high altitude atmospheric pressure falls, so PiO2 falls, so PAO2 falls despite 21% FiO2.
_
According to textbook physiology, increasing the FiO2 from 0.21 (i.e., air) to 1.0 (i.e., 100 % O2) will moderately affect total blood O2 content under conditions of normal cardiopulmonary function: at normal pH and temperature, arterial PO2 levels of 90–100 mmHg lead to hemoglobin O2 saturations close to 100 % due to the sigmoid shape of the hemoglobin-O2-dissociation curve. Therefore, pure O2 breathing will only raise the amount of physically dissolved O2, the maximum effect being a five-fold increase, while hardly modifying the amount of O2 bound to hemoglobin. It is self-evident from the afore-mentioned estimate that the effect of pure O2 breathing on total blood O2 content will be the more important the lower the hemoglobin concentration. Therefore, ventilation with 100 % O2 was particularly protective in various models comprising critical hemodilution (reviewed in Calzia et al.): the most impressive evidence in this context are the data reported in the “Live without blood” experiment as early as in 1960: in pigs subjected to hemodilution to a hematocrit <1–2 %, mechanical ventilation with pure O2 allowed preventing the otherwise marked ECG signs of myocardial ischemia, and no sequelae were observed after blood re-transfusion and return to air breathing. Strikingly, however, despite its frequent routine use, so far there are no clinical data on the role of mechanical ventilation with FiO2 = 1.0 during the management hemorrhagic shock, most likely due to ethical constraints. The available pre-clinical data are equivocal: deleterious and beneficial effects as well as no therapeutic efficacy at all were reported, depending on the species used, the severity of shock, and the concomitant use of therapeutic hypothermia.
_
A recent surge of interest in the value of increasing the availability of oxygen to tissues in critical conditions yielded important studies like the one on early goal-directed therapy in sepsis that assessed a resuscitation protocol aimed at increasing tissue oxygenation. Regrettably, the specific value of oxygen therapy was not assessed in this study. Yet a recent study that compared the influence of allogeneic red blood cell transfusion with 100% oxygen ventilation in volume-resuscitated anemic patients after cardiac surgery demonstrated a superior effect of normobaric hyperoxia (NBO) on tissue (skeletal muscle) oxygen tension .
_
Hemodynamic effects:
The availability of oxygen to tissues is also determined by its effects on hemodynamic variables. In healthy animals and humans, oxygen causes a temporary increase in blood pressure by increasing total peripheral vascular resistance secondary to systemic peripheral vasoconstriction. This transient change is rapidly counterbalanced by a decrease in heart rate and cardiac output that prevents a sustained effect on arterial blood pressure. The unique combination of hyperoxia-induced vasoconstriction and high blood oxygen tension affords an advantage by decreasing a vasogenic component of increased tissue hydrostatic pressure while preserving a high blood-to-tissue oxygen partial pressure gradient and is therefore considered beneficial in crush injury and compartment syndrome as well as brain edema, particularly when the latter develops in situations in which additional indications for HBO therapy exist, such as carbon monoxide poisoning and air embolism. Recent experimental evidence supports the role of hyperoxia in cerebral ischemic-anoxic insults such as stroke, head injury, near drowning, asphyxia, and cardiac arrest. In the specific case of traumatic brain injury, it has repeatedly been shown that, although HBO causes cerebral vasoconstriction, it increases brain tissue pO2 (partial pressure of oxygen) and restores mitochondrial redox potential. NBO has also been shown to decrease intracranial pressure and improve indices of brain oxidative metabolism in patients with severe head injury. A significant body of experimental data that suggested beneficial effects of hyperoxia in ischemic stroke was followed by clinical trials that failed to demonstrate clear-cut benefits. Yet significant shortcomings of the available clinical data call for re-evaluation of the effect of hyperoxia on the outcome of stroke and on the possibility to use it to extend the narrow therapeutic time window for stroke thrombolysis.
Another area of controversy is the use of NBO in asphyxiated newborn infants. Initial laboratory and clinical studies suggested an inferior effect of resuscitation with 100% oxygen compared with room air. Later cumulative clinical experience and systematic review of the literature have not indicated a significant difference in the effectiveness of either gas source or in the final outcome in this specific group of patients. Yet a recent systematic review and meta-analysis of the few available randomized or quasirandomized studies of depressed newborn infants have shown a significant reduction in the risk of mortality and a trend toward a reduction in the risk of severe hypoxic ischemic encephalopathy in newborns resuscitated with 21% oxygen. Taken together, the available data definitely do not support an overall beneficial effect of hyperoxia in this condition, although the superiority of room air in neonatal resuscitation may still be regarded as controversial.
In contrast to the knowledge on the effects of hyperoxia on central hemodynamics, much less is known about its effects on regional hemodynamics and microhemodynamics. Studies that looked at hyperoxia-induced changes in regional hemodynamics in healthy animals both in normal atmospheric pressure and in hyperbaric conditions yielded conflicting results, indicating an increase, a decrease, or no change in regional blood flows to specific vascular beds. Only limited and scattered information on regional hemodynamic effects of hyperoxia in relevant models of disease is available. In this regard, a study in an acute canine model of ischemia and reperfusion (IR) of the external iliac artery showed that HBO did not induce vasoconstriction in the affected regional vascular bed until oxygen deficit was corrected. Such findings support suggestions that a dynamic situation may exist in which vasoconstriction is not always effective in severely hypoxic tissues and therefore may not limit the availability of oxygen during hyperoxic exposures and that hyperoxic vaso-constriction may resume after correction of the regional hypoxia. Furthermore, in a severe rat model of hemorrhagic shock, authors have shown that normobaric hyperoxia increased vascular resistance in skeletal muscle and did not change splanchnic and renal regional resistances. This yielded redistribution of blood flow to the small intestine and kidneys ‘at the expense’ of skeletal muscle. A similar divergent effect of normobaric hyperoxia that augmented hind-quarter vascular resistance without a significant effect on the superior mesenteric bed was also found in a rat model of splanchnic IR. In this regard, NBO-induced redistribution of cardiac output to the hepatosplanchnic regions was recently reported in a pig model of severe sepsis. NBO was also shown to redistribute blood flow to ischemic myocardium and improve contractile function during low-flow myocardial ischemia. So the claim that hyperoxia is a universal vasoconstrictor in all vascular beds is an oversimplification both in normal and pathologic states. Furthermore, understanding of the effects of hyperoxia on regional hemodynamics cannot be based on simple extrapolations from healthy humans and animals and warrants careful evaluation in selected clinical states and their animal models.
_
Effects on inflammation:
Tissue hypoxia activates a large variety of vascular and inflammatory mediators that trigger local inflammation and may lead to a systemic inflammatory response (SIR) that in many cases culminates in multiple organ dysfunction and multiple organ failure (MOF). The wish to prevent or treat hypoxia-induced inflammatory responses yielded studies that evaluated the effects of hyperoxia on the microvascular-inflammatory response. Most of the attention focused on models of IR (immune response) which frequently provoke local inflammatory response, SIR, and MOF. The potential beneficial effects of hyperoxia are confronted by the understanding of the central role of reactive oxygen species (ROS) in IR injury. The demonstration of increased production of ROS during exposure of normal tissues to hyperoxia evoked concerns that oxygen therapy could exacerbate IR injury. The seemingly rational unease related to the use of hyperoxia in IR must be weighed against a gradually growing body of evidence on beneficial effects of hyperoxia in diverse IR models. Hyperoxia appears to exert a simultaneous effect on a number of steps in the proinflammatory cascades after IR, including interference with polymorphonuclear leukocyte (PMNL) adhesion and production of ROS. In this regard, HBO has been shown to decrease rolling and adhesion of PMNL in the microcirculation following IR of skeletal muscle, small bowel, skin flaps, heart, and liver as well as after carbon monoxide poisoning. All in all, the ameliorating effects of hyperoxia on the acute net proinflammatory response after IR and other conditions may be related to direct inhibitory effects of oxygen on mechanisms that enhance PMNL rolling, adhesion, activation, and transmigration to tissues. Hyperoxia may also exert indirect effects on the inflammatory response simply by ameliorating tissue hypoxia – a key trigger of inflammation.
Sepsis is one of the most common clinical causes of SIR. In a study of early hyperdynamic porcine septic shock, Barth and colleagues demonstrated beneficial effects of NBO on apoptosis in the liver and the lungs, on metabolic acidosis, and on renal function. Researchers found a dose-related beneficial effect of NBO (100% oxygen for 6 hours per day) on the pulmonary inflammatory response in sepsis induced by cecal ligation and puncture (CLP) in rats. Buras and colleagues studied the effects of hyperoxia at 1, 2.5, and 3 ATA applied for 1.5 hours twice a day on survival in a mouse CLP model of sepsis and reported that HBO at 2.5 ATA improved survival. They also presented data suggesting that augmented production of the anti-inflammatory cytokine interleukin-10 may be an important mechanism of the salutary effects of HBO in this model. The steadily growing body of data on beneficial effects of hyperoxia in severe local and systemic inflammation warrants appropriate clinical studies to define its role as a clinically relevant modifier of hyperinflammation.
_
Effects on microorganisms and tissue repair mechanisms:
HBO has been studied and used in a large variety of infections for over 40 years. Early demonstrations of its beneficial effects in clostridial myonecrosis (gas gangrene) and in chronic refractory osteomyelitis were followed by a large body of experimental data on in vitro effects of increased ambient oxygen partial pressures on microorganisms and reports on in vivo effects of HBO in infection. HBO exerts direct bacteriostatic and bactericidal effects mostly on anaerobic microorganisms. These effects have been attributed to deficient defense mechanisms of anaerobic microorganisms against increased production of ROS in hyperoxic environments. Beyond a direct activity against microorganisms, HBO has been shown to re-establish defense mechanisms that are critically impaired by the typically hypoxic microenvironment in infectious sites. Both phagocytosis and microbial killing by PMNLs are severely impaired in hypoxic environments. By increasing tissue oxygen tensions, HBO therapy restores phagocytosis and augments the oxidative burst that is needed for leukocyte microbial killing. Furthermore, the activity of a number of antibiotics is impaired in hypoxic environments and is restored and even augmented during exposure to HBO. Other important beneficial effects of hyperoxia in infection are attributed to enhancement of key components of tissue repair such as necrotic tissue proteolysis, fibroblast proliferation, collagen deposition and angiogenesis, migration of epithelial cells, and bone remodeling by osteoblastic/osteoclastic activity, which are all severely impaired in hypoxic tissues. Altogether, direct activity on bacteria (for example, pseudomonas, some strains of Escherichia, and Clostridium perfringens), improvement of cellular defense mechanisms, synergistic effects on antibiotic activity, modulation of the immune response, and augmentation of mechanisms of tissue repair form the basis for the use of HBO as adjunctive therapy in combination with antibiotics and surgery for treating tissue infections involving both anaerobic and aerobic microorganisms in hypoxic wounds and tissues and in sepsis-induced SIR.
As for normobaric hyperoxia, two recent prospective randomized clinical studies reported significant beneficial effects of perioperative administration of supplemental oxygen (80% oxygen at normal atmospheric pressure) on surgical site infection (SSI) after elective colorectal surgery. A third study on patients undergoing various open abdominal procedures reported a higher incidence of SSI in the higher oxygen group and ignited a yet unsettled debate on the routine use of normobaric hyperoxia to prevent SSI.
Hyperoxia has also been shown to inhibit the growth of some fungi and to potentiate the antifungal effect of amphthericin B. Data from case reports, small groups of patients, and compilations of previous reports support the use of adjunctive HBO treatment together with amphotericin B and surgery in invasive rhinocerebral mucormycosis. The level of evidence on the effects of HBO in other fungal infections is less compelling.
_
The proven pathophysiologic profile of actions of hyperoxia set the basis for its use in selected clinical conditions. Sufficient clinical evidence is available for the use of HBO in carbon monoxide poisoning, decompression sickness, arterial gas embolism, radiation-induced tissue injury, clostridial myo-necrosis, problem wounds, crush injury, and refractory osteomyelitis. Effects of NBO in these and in other potentially relevant clinical states are much less studied. Studies that evaluate a range of oxygen doses in both the normobaric and hyperbaric pressure range are largely unavailable and should be encouraged by appropriate allocation of research funding.
______
______
Objectives of Oxygen therapy:
- To overcome the reduced partial pressure and oxygen quantity in blood
- To increase the quantity of oxygen carried in solution in the plasma, even when the Hemoglobin is fully saturated.
The primary goal of oxygen therapy is to correct hypoxemia and/or tissue hypoxia. Therefore, any disorder causing hypoxia is a potential indication for oxygen administration. But the tissue oxygen delivery depends upon an adequate function of cardiovascular (cardiac output and flow), haematological (Hb and its affinity for oxygen) and the respiratory (arterial oxygen pressure) systems. Therefore, tissue hypoxia is not relieved by oxygen therapy alone – functioning of all the three organ systems also needs to be improved. Oxygen therapy should be administered according to guidelines. Proper monitoring of oxygen therapy is recommended to ensure adequate oxygenation and to save precious oxygen from wastage. The use of pulse oximeter is a simple, quick, non-invasive, and reliable method to assess it.
_
Goals of oxygen therapy:
- Correcting Hypoxemia
-By raising Alveolar & Blood levels of Oxygen
-Easiest objective to attain & measure
-Give oxygen therapy in a way which prevents excessive CO2 accumulation i.e. selection of the appropriate flow rate and delivery device.
- Decreasing symptoms of Hypoxemia
-Supplemental O2 can help relieve symptoms of hypoxia
-Lessen dyspnoea/work of breathing
-Improve mental function
- Minimizing Cardiopulmonary workload
-Cardiopulmonary system will compensate for Hypoxemia by: Increasing ventilation to get more O2 in the lungs & to the Blood, Increased work of breathing, Increasing Cardiac Output get more oxygenated blood to tissues but it is hard on the heart, especially if diseased.
-Hypoxia causes Pulmonary vasoconstriction & Pulmonary Hypertension, These cause an increased workload on the right side of heart, Over time the right heart will become more muscular & then eventually fail (Cor Pulmonale and heart failure). Supplemental O2 can relieve hypoxemia & relieve pulmonary vasoconstriction & hypertension, reducing right ventricular workload.
- Ensure adequate clearance of secretions and limit the adverse events of hypothermia (infants) and insensible water loss by use of optimal humidification (dependent on mode of oxygen delivery).
_
Benefits of Oxygen Therapy:
Besides saving life in medical emergencies, oxygen has many benefits including:
- Improves mood and sleep.
- Increases mental alertness and stamina.
- Allows for normal body functioning.
- Prevents heart failure in people with severe lung disease.
- Improves ability to exercise for longer periods of time at higher intensities.
In addition, oxygen therapy prolongs survival for some patients who use is at least 15 hours a day.
______
Other methods to increase Oxygen saturation in blood:
The use of oxygen delivery systems is only one component to increasing oxygen to the alveolar capillary bed to allow for optimal oxygenation to the tissues. Additional methods to increase oxygen saturation levels in the body include (Perry et al., 2014):
- Maintaining satisfactory airway
- Optimizing oxygen-carrying capacities (hemoglobin levels)
- Reversing any respiratory depressants
- Using invasive or non-invasive ventilation when necessary
- Treating airflow obstruction with bronchodilators and sputum-clearing techniques
- Treating pulmonary edema as required
_______
_______
Different types of oxygen therapy:
_
There are two types of oxygen administration techniques:
- Normobaric oxygen therapy
- Hyperbaric oxygen therapy
_
There are several different kinds of oxygen therapy:
- Short-term oxygen therapy (STOT) for medical emergency and acute care
- Long term oxygen therapy (LTOT) – used to stabilise oxygen levels for at least 15 hours per day in chronic lung diseases.
- Nocturnal oxygen therapy (NOT) – used to improve oxygen levels during sleep
- Ambulatory oxygen therapy (AOT) – used to improve oxygen levels during activity
- Palliative oxygen therapy (POT) – used to manage severe intractable breathlessness
- short-burst oxygen therapy (SBOT) – used to improve oxygen levels following a flare-up or exacerbation
- Intermittent oxygen therapy is often indicated for people diagnosed with a restrictive lung condition.
_
Oxygen should be considered a drug with clear guidelines and indications for its use. Oxygen is used for short-term hospitalized patients and as long-term therapy in patients with chronic lung disease. Oxygen is costly therapy and hence a rational understanding regarding its use is required. In the United States, home oxygen therapy is provided to approximately 1 million Medicare recipients costing nearly $2 billion dollars per year.
_
Short-term Oxygen Therapy in the Acute Care Setting:
The most common indication for oxygen therapy in the acute setting is arterial hypoxemia with a PaO2 of less than 60 mm Hg. Ventilation perfusion mismatch is the most common cause of arterial hypoxemia. Ventilation and blood flow are mismatched in various regions of the lung and all gas transfer becomes inefficient. Alveolar hypoventilation occurs when the volume of fresh gas going to alveoli (alveolar ventilation) is reduced. This is commonly caused by diseases outside the lung such as CNS insults, drug overdose, thoracic cage abnormalities, and upper airway obstruction and is often easily corrected with supplemental oxygen. Shunts, either in the form of extreme ventilation perfusion mismatch or anatomic right-to-left shunts, are often less responsive to administration of supplemental oxygen. A shunt occurs when blood reaches the arterial system without passing through ventilated regions of the lung. The role of diffusion impairment as a cause of hypoxemia is controversial, although it is thought to play a role in exercise oxygen desaturation seen in advanced interstitial lung disease. A PaO2 of 60 mm Hg is often set as a reasonable goal in the initial treatment of arterial hypoxemia. Accepted indications for short-term oxygen therapy include acute hypoxemia, cardiac and respiratory arrest and low cardiac output with metabolic acidosis. Questionable indications for which supplemental oxygen is used clinically, but for which there is little supporting data, include uncomplicated acute myocardial infarction, dyspnea without hypoxemia, sickle cell crisis and angina.
_
Long-term Oxygen Therapy:
Long term oxygen therapy (LTOT) is delivered to reduce long-term complications of chronic hypoxemia, particularly cor pulmonale. Hypoxemia induces physiologic responses that aim to maintain adequate oxygen delivery to tissues. These include increased heart rate and stroke volume, pulmonary vasoconstriction and increases in erythropoietin and hemoglobin concentration. Oxygen supplementation in patients with chronic hypoxemia has been shown to improve survival, pulmonary hemodynamics, exercise capacity and neuropsychological performance.
_
There were two landmark trials of LTOT in the 1980s- the British Medical Research Council (MRC) Working Party Trial and the American Nocturnal Oxygen Therapy Trial (NOTT). The MRC trial compared COPD patients receiving oxygen for 15 hours/day with controls receiving no oxygen. The NOTT trial compared continuous daily oxygen (17.7 hours/day) with overnight oxygen use (average 12 hours/day). The main outcome in both trials was improved survival in those patients receiving oxygen for at least 15 hours/day, although this improved survival was not seen in the MRC trial until after a year of oxygen therapy. The NOTT trial showed a reduced exercise Pulmonary Arterial Pressure (PAP) after 6 months of continuous or nocturnal oxygen therapy. The MRC trial failed to show a fall in mean PAP with LTOT. The reason for the improved survival with LTOT is not clear. Although LTOT is often prescribed in patients with pulmonary infiltrative or vascular disease, COPD is the disease for which LTOT is most commonly prescribed and the disease in which the original studies were completed.
_
Indications for LTOT in non COPD patients:
Studies similar to the NOTT and MRC are not available in non COPD patients with chronic hypoxemia. But extending the use of LTOT for patients with resting hypoxemia from other cardiopulmonary conditions include conditions such as interstitial lung diseases, cystic fibrosis, pulmonary arterial hypertension, chronic cardiac disease and neuromuscular diseases causing ventilatory failure. Long-term oxygen therapy is also sometimes prescribed for the dyspnea of lung cancer or other causes of disabling dyspnea due to terminal disease.
_
Before LTOT is considered, the patient must be clinically stable and on appropriate optimum therapy such as antibiotics, bronchodilators, physiotherapy and having stopped smoking tobacco. Many patients first present for LTOT with profound hypoxemia and hypercapnia during an infective, often oedematous exacerbation of their lung disease. Assessments should occur during convalescence when the patient is clinically stable. They should be shown to have a PaO2 less than 7.3 kPa and/or a PaCO2 greater than 6 kPa on two occasions at least 3 weeks apart. FEV1 should be less than 1.5 litres, and there should be a less than 15% improvement in FEV1 after bronchodilators. All patients should be assessed by an experienced chest physician. Patients with a PaO2 between 7.3 and 8 kPa who have polycythaemia, right heart failure or pulmonary hypertension may gain benefit from LTOT but this is still to be clearly proven.
_
The American Thoracic Society recommends long-term oxygen therapy (LTOT) for the following people:
- Patients with a resting partial pressure of oxygen (PaO2) (as measured by an arterial blood gas study) of < 55 mmHg, with an oxygen saturation level of < 88%.
- Patients whose PaO2 is 55-59 mmHg, with a corresponding oxygen saturation level of 89%, who exhibit signs of tissue hypoxia (lack of oxygen to the body tissues), including those with pulmonary hypertension, cor pulmonale, polycythemia, fluid retention from right heart failure, or impaired mental status.
____
Benefits of Long Term Oxygen Therapy:
Significant improvements are shown in several parameters of chronic respiratory insufficiency of COPD.
- Duration of survival. An increase in the duration of survival was the most significant benefit seen in both the studies mentioned earlier. The same observations have been made in most of the subsequent studies and clinical experience.
- Intellectual functions. There is significant improvement in memory, motor coordination, mood and other hypochondrial symptoms on long term oxygen therapy.
- Pulmonary hypertension. There is a decrease in pulmonary vasoconstriction and vascular resistance. This improves the severity of right heart failure.
- Red cell mass. Long term oxygen decreases red cell mass and haematocrit levels. Therefore, complications of polycythaemia are diminished.
_
Potential Benefits.
There are reports on improvement of several other parameters. Although some of these benefits are not necessarily proved with placebo-controlled studies. These include the following:
(i) increased exercise ability;
(ii) improved quality of life, patients resuming gainful employment and participating in their own care, more actively;
(iii) decrease in dyspnoea;
(iv) decrease in hospitalisation and exacerbations of respiratory failure, and
(v) delayed development of cor-pulmonale.
There is little evidence to support oxygen supplementation during exercise training in COPD patients during pulmonary rehabilitation. But evidence does support the use of oxygen supplementation for patients with severe hypoxemia at rest or with exercise.
_
Long-term oxygen therapy: Are we prescribing appropriately?
Long-term oxygen therapy (LTOT) is the treatment proven to improve survival in chronic obstructive pulmonary disease (COPD) patients with chronic respiratory failure. It also appears to reduce the number of hospitalizations, increase effort capacity, and improve health-related quality of life. Standard LTOT criteria are related to COPD patients who have PaO2 <60 mmHg, are in a clinical stable situation, and are receiving optimal pharmacological treatment. According to LTOT guidelines, oxygen should be prescribed for at least 18 hours per day although some authors consider 24 hours would be more beneficial. The benefits of LTOT depend on correction of hypoxemia. Arterial blood gases should be measured at rest. During exercise, an effort test should be done to assure adequate SaO2. During sleep, continuous monitoring of SaO2 and PaCO2 should be performed to confirm correction of SaO2 overnight. An arterial blood gas sample should be taken at awakening to assess PaCO2 in order to prevent hypoventilation from the oxygen therapy. Several issues that need to be addressed are the use of LTOT in COPD patients with moderate hypoxemia, the efficacy of LTOT in patients who desaturate during exercise or during sleep, the optimal dosage of oxygen supplementation, LTOT compliance, and the LTOT prescription in diseases other than COPD
_
During exercise:
There are no specific recommendations about oxygen prescription during exercise. Some guidelines advise using an effort test to assess the adequate oxygen flow to correct oxygen desaturation. To establish the appropriate oxygen flow it is recommended to test patients under increasing oxygen flow until SaO2 ≥90%. The most frequently used effort test is the 6-minute walking test (6Wt), which some authors have shown to be a reliable measurement. Comparing SaO2 during the 6WT with that of ambulatory pulse oximetry, Morante and colleagues (2005) showed that the 6WT is a good reflection of desaturation during daily activities measured by ambulatory pulse oximetry. These authors also demonstrated a good correlation between the two measurements when patients are breathing oxygen (p < 0.01). Wijkstra and colleagues’ (2001) international survey showed that most countries assess patients during exercise to achieve SaO2 ≥90%. However, ATS guidelines and some European countries recommend the resting flow rate should be increased by 1 l/minute or that the same flow rate as at rest should be used without testing the oxygen flow rate needed during exercise.
During sleep:
There are three ways to adjust the oxygen flow during sleep: maintaining the resting flow rate, increasing the resting flow rate by 1 or 2 l/minute, or setting the flow rate according to continuous monitoring of SaO2 to maintain SaO2 ≥90%.
_
British Thoracic Society (BTS) guideline:
LTOT is indicated for the following conditions having chronic hypoxemia:
COPD
Interstitial lung disease
Cystic fibrosis
Bronchiectasis
Pulmonary arterial disease
Pulmonary hypertension
Severe heart failure
In patients with chronic hypoxemia, LTOT should usually be prescribed after appropriate assessment, when the PaO2 is consistently at or below 7.3 kPa (55 mm Hg) when breathing air during a period of clinical stability. Clinical stability is defined as the absence of exacerbation of chronic lung disease for the previous five weeks. The level of PaCO2 (which may be normal or elevated) does not influence the need for LTOT prescription.
In addition, LTOT can be prescribed in chronic hypoxemia patients when the clinically stable PaO2 is between 7.3kPa and 8kPa, together with the presence of one of the following:
Secondary polycythaemia.
Clinical and or echocardiographic evidence of pulmonary hypertension.
LTOT should not be prescribed in patients with chronic hypoxemia with a PaO2 value above 8kPa.
Assessment for LTOT requires referral to a physician with a specialist interest in these disorders. LTOT will normally be used as an adjunct to non-invasive ventilation (NIV) or continuous positive airway pressure (CPAP).
_____
Nocturnal oxygen therapy (NOT):
NOT is oxygen administered overnight alone without additional oxygen therapy during awake or daytime hours. Before daytime resting hypoxemia develops, many patients develop nocturnal or sleep time oxygen desaturation due to a combination of worsening V/Q mismatch in a supine posture and lack of drive to ventilator muscles during sleep. Patients who develop significant decreases in arterial oxygen during sleep may also benefit from chronic oxygen administration. These include patients with primary sleep disordered breathing (obstructive sleep apnea or obesity hypoventilation syndrome) and patients with chronic lung disease with nocturnal desaturation. In patients with primary sleep disorders, oxygen therapy may need to be given in conjunction with continuous positive airway pressure (CPAP) or other invasive or noninvasive ventilator support for the treatment of hypercarbia. Nocturnal oxygen therapy (NOT) is not recommended in patients with COPD who have nocturnal hypoxemia but who fail to meet the criteria for LTOT. Other causes of nocturnal desaturation in COPD should be considered, such as obesity hypoventilation, respiratory muscle weakness or obstructive sleep apnoea (OSA). Patients with OSA, obesity hypoventilation syndrome or overlap syndrome should not have NOT alone ordered. It can be considered in patients with evidence of established ventilatory failure, where it should be given with NIV support. Nocturnal oxygen therapy may be prescribed for occasional individuals with lung disease who desaturate to SpO2 ≤ 88% for more than one third of the night, particularly if they suffer sequelae such as pulmonary hypertension or polycythaemia.
_____
Ambulatory Oxygen Therapy (AOT):
AOT is defined as the use of supplemental oxygen during exercise and activities of daily living. Most patients needing this will also be using LTOT. Patients should be assessed for AOT taking into account daily activities, treatment regimens and active lifestyles. If a patient’s SpO2 drops >=4% to <90% during a baseline endurance shuttle walk test (ESWT) or 6 minute walk test (6MWT) they meet the criteria for AOT.
The indications for ambulatory oxygen are poorly researched. Ambulatory oxygen may be suitable for:
- Patients on LTOT who are mobile.
- Those with exercise desaturation and who show an improvement in exercise capacity or dyspnea with oxygen. Oxygen should be titrated to limit exercise desaturation, aiming for an oxygen saturation of >90%.
_____
Palliative Oxygen Therapy:
Palliative Oxygen Therapy (POT) is defined as the use of oxygen to relieve the sensation of refractory persistent breathlessness in advanced disease or life-limiting illness irrespective of underlying pathology. Refractory breathlessness is defined as breathlessness at rest or on limited exertion that persists despite optimal treatment of the underlying conditions, in advanced chronic disease, or towards the end of life. This stage of disease can be clinically challenging and from the evidence presented in the new BTS guidelines, POT should not be routinely prescribed in patients who are not hypoxic. However several recommendations are made for patients with intractable breathlessness covering non-pharmacological methods, opioids and oxygen. Supplemental oxygen may provide symptomatic relief for patients with intractable dyspnoea and significant hypoxemia (despite maximal treatment) due to terminal illnesses, including late‐stage lung disease. However, hypoxemia may not necessarily be associated with the subjective experience of dyspnoea and reversal of hypoxemia with oxygen therapy may not necessarily relieve dyspnoea. The two are known to be poorly correlated.
______
Short Burst Oxygen Therapy (SBOT):
Short-burst therapy (e.g., for 10-20 minutes) is indicated to relieve dyspnoea in palliative care or episodic breathlessness, not relieved by other treatments in severe COPD, interstitial lung disease or heart failure. SBOT does not improve exercise tolerance or reduce breathlessness when administered either before or following exercise to hypoxemic or non-hypoxemic patients with moderate to severe COPD. SBOT does not improve health-related quality of life or reduce healthcare utilisation when ordered for patients following an acute exacerbation of COPD. SBOT delivering high flow oxygen (12 L/min via a non-re-breather mask) is a first line effective treatment for acute cluster headache attacks.
______
______
Oxygen and nebulisation:
In medicine, a nebulizer is a drug delivery device used to administer medication in the form of a mist inhaled into the lungs. Nebulizers are commonly used for the treatment of cystic fibrosis, asthma, COPD and other respiratory diseases or disorders. Nebulizers use oxygen, compressed air or ultrasonic power to break up solutions and suspensions into small aerosol droplets that can be directly inhaled from the mouthpiece of the device. An aerosol is a mixture of gas and solid or liquid particles. The most commonly used nebulizers are jet nebulizers, which are also called “atomizers”. Jet nebulizers are connected by tubing to a compressor, that causes compressed air or oxygen to flow at high velocity through a liquid medicine to turn it into an aerosol, which is then inhaled by the patient. A nebulizer treatment delivers liquid, prescription medicine in a fine mist. Inhaling the mist, through a mouth piece or mask, takes the medicine right into your lungs. The nebulizer therapy is complete after the medicine is used up, usually in 10 to 20 minutes. Most patients use nebulizer therapy two to four times a day. Asthma is a condition often treated with nebulizer therapy.
_
Nebulizers use compressed gas to propel liquid medication into an aerosol, with specific therapeutically sized droplets, for deposition in the appropriate, desired portion of the airway. A typical compressed gas flow rate of 8-10 L/min is used to nebulize medications, saline, sterile water, or a mixture of the preceding into a therapeutic aerosol for inhalation. In the clinical setting room air (ambient mix of several gasses), molecular oxygen, and Heliox are the most common gases used to nebulize a bolus or a continuous volume of therapeutic aerosols.
_
Useful overlap:
Powering a nebulizer with oxygen is a convenient way to cover two needs with one treatment, for continuous oxygen users. But after your nebulizer runs dry you’ll need to disconnect the oxygen from your nebulizer and reconnect your cannula right away. In patients with COPD, titration of oxygen therapy should continue during bronchodilator administration, if required, to achieve the 88–92% target oxygen saturation range. This can be done by giving titrated oxygen through nasal cannula and giving the bronchodilator through an air-driven nebuliser. There is evidence from a randomised controlled trial for this approach. An alternative to nebulisation that allows for the ongoing titration of oxygen therapy is to give the bronchodilator from a metered dose inhaler via a spacer. When nebulised therapy is administered to patients at risk of hypercapnic respiratory failure, it should be driven by compressed air. If necessary as decided by the doctor, supplementary oxygen should be given concurrently by nasal prongs at 1-4 litres per minute to maintain an oxygen saturation of 88-92% or other specified target range documented on oxygen/nebuliser prescription chart. All patients requiring 35% or greater oxygen therapy should have their nebulised therapy driven by oxygen at a flow rate of greater than 6 litres/minute as prescribed by the doctor.
_
Asthma:
Guideline does recommend that the nebulised route (oxygen-driven) be used for the delivery of high-dose beta agonists in acute asthma with life-threatening features. If a nebuliser is used in the emergency situation, there are theoretical risks of oxygen desaturation whilst using air-driven compressors. Therefore, nebulisers should be oxygen-driven with a ‘high flow regulator’ fitted to the cylinder in order to provide the necessary flow rate of 6 L/minute. Hypoxemia during nebulization with air-driven nebulizers can easily be prevented by simple addition of oxygen source to the air inlet of available nebulizers, since oxygen has to be given to children in severe attacks of asthma not only before and after but also during treatment with ß2-agonist. This is important in preventing continued deaths occurring from asthma.
_
COPD:
Although patients with COPD are considered to have irreversible bronchoconstriction, most show some reversibility with high-dose bronchodilators. The nebulised aqueous vapour is also believed to alter viscosity of mucus and assist expectoration. Therefore, air-driven nebulisers are used frequently in the treatment of acute exacerbations and maintenance of COPD. If a patient is hypercapnic or acidotic the nebuliser should be driven by compressed air, not oxygen (to avoid worsening hypercapnia). If oxygen therapy is needed it should be administered simultaneously by nasal cannula. The driving gas for nebulised therapy should always be specified in the prescription.
_
It is important to take care when selecting which gas to use. The use of oxygen as the driving gas can be harmful for patients who are at risk of hypercapnia (e.g. patient with severe COPD) and these patients normally need nebulisers driven by air (with nasal oxygen if necessary). But for patients with other conditions or who are dependent on higher levels of oxygen, the use of medical air as the driving gas exposes them to the risk of hypoxemia; where patients are dependent on very high levels of oxygen, discontinuing it even for a few minutes taken to give a nebuliser driven by air can prove fatal.
_____
_____
Indications for oxygen therapy:
_
Management of the acutely hypoxemic patient requires evaluation and treatment of the underlying cause of the hypoxemia. Oxygen therapy relieves hypoxemia, but not the underlying cause. Oxygen is a drug and it should be prescribed for specific indications. This prescription should include the target range for oxygen saturation. The response to oxygen administration requires regular monitoring. Oxygen is used as a medical treatment in both chronic and acute cases, and can be used in hospital, pre-hospital or entirely out of hospital, dependent on the needs of the patient and their medical professionals’ opinions. In the first assessment of an unwell patient, oxygen saturations can be determined by pulse oximetry. However, clinicians need to be aware that the accuracy of pulse oximetry is variable in clinical practice. Arterial blood gases should be measured in patients who are critically unwell, when an oximetry reading cannot be obtained or when hypercapnia is suspected. In view of the widespread use of venous blood gas measurements, clinicians need to be aware that this method cannot accurately determine arterial carbon dioxide. Oxygen therapy is indicated in patients with oxygen saturations below the target saturation range. It is not indicated for the treatment of breathlessness in patients with adequate oxygen saturations, apart from certain patients with carbon monoxide poisoning and with pneumothorax. There is no evidence to support the use of supplemental oxygen to reduce dyspnea in non-hypoxemic patients with advanced COPD” (Mariciniuk et.al, 2011). The BTS Guideline for Oxygen Use in Adult Patients (2009) is based on the premise that oxygen is a treatment for “hypoxemia, not breathlessness”. Further, the guideline states that “oxygen has not been shown to have any effect on the sensation of breathlessness in non-hypoxemic patients”. Breathlessness does not always indicate hypoxemia and Oxygen is not a treatment for breathlessness.
_
Indications of STOT:
Accepted Indications:
-Acute hypoxemia (PaO2 < 60 mmHg; SaO2 < 90%)
-Cardiac and respiratory arrest
-Hypotension (systolic blood pressure < 100 mmHg)
-Low cardiac output and metabolic acidosis (bicarbonate < 18 mmol /L)
-Respiratory distress (respiratory rate > 24/min)
Questionable Indications:
-Uncomplicated myocardial infarction
-Sickle cell crisis
-Angina
Others:
-Carbon monoxide poisoning if hyperbaric oxygen not available. High concentration oxygen treatment is essential despite a normal PaO2 because oxygen competes with carbon monoxide for haemoglobin binding sites and reduces the half-life of carboxyhaemoglobin from about 320 to 80 minutes.
-Dyspnea without hypoxemia
-Accelerating resorption of pneumothorax
_
Oxygen therapy in Acute conditions:
Oxygen is widely used in emergency medicine, both in hospital and by emergency medical services or those giving advanced first aid. In the pre-hospital environment, high flow oxygen is definitively indicated for use in resuscitation, major trauma, anaphylaxis, major haemorrhage, shock, active convulsions and hypothermia. It may also be indicated for any other patient where their injury or illness has caused hypoxemia, although in this case oxygen flow should be moderated to achieve target oxygen saturation levels, based on pulse oximetry (with a target level of 94–98% in most patients, or 88–92% in COPD patients). For personal use, high concentration oxygen is used as home therapy to abort cluster headache attacks, due to its vaso-constrictive effects. Patients who are receiving oxygen therapy for hypoxemia following an acute illness or hospitalization should not routinely have a prescription renewal for continued oxygen therapy without a physician’s re-assessment of the patient’s condition.
_
In acutely ill patients oxygen delivery relies on maintaining a patent airway. This should always be checked first. Give oxygen empirically in patients with cardiac or respiratory arrest or when there is respiratory distress or hypotension. Arterial blood gases should be analysed as soon as possible to assess the degree of hypoxemia, partial pressure of carbon dioxide (PCO2), and acid-base state.
_
Guidelines for initial oxygen dose:
| Condition | Fraction of oxygen in inspired air (%)
FiO2 |
| Cardiac or respiratory arrest | 100 |
| Hypoxemia with PCO2<5.3 kPa | 40-60 |
| Hypoxemia with PCO2>5.3 kPa | 24 initially |
_
In the acute situation the dose of oxygen administered may be critical. Inadequate oxygen accounts for more deaths and permanent disability than can be justified by the relatively small risks associated with high dose oxygen. In many acute conditions (for example, asthma, pulmonary embolus), inspired oxygen concentrations of 60-100% for short periods may preserve life until more specific treatment can be instituted. Thereafter oxygen should be given at a dose that will correct hypoxemia and minimise side effects (increase the PaO2 to 8.0-10.6 kPa). When necessary, oxygen must be given continuously.
_
Increasing the fraction of inspired oxygen (FiO2) increases oxygen transport by ensuring that blood haemoglobin is fully saturated and by raising the quantity of oxygen normally carried in solution in the plasma. However, the solubility of oxygen in blood is low. Even when the inspired oxygen concentration is 100%, dissolved oxygen provides only one third of resting tissue oxygen requirements. Therefore, oxygen treatment must be aimed at correcting arterial hypoxemia; when tissue hypoxia occurs in the absence of arterial hypoxemia, treatment should always be directed at correcting the underlying cause (that is, heart failure, anaemia).
_
High dose oxygen given to patients with chronic obstructive pulmonary disease who have type II respiratory failure can reduce the hypoxic drive to breathe and increase ventilation-perfusion mismatching. This causes carbon dioxide retention and a respiratory acidosis that may be lethal. In these patients initial treatment with low oxygen concentrations (24-28%) should be progressively increased on the basis of repeated blood gas analysis with the aim of correcting hypoxemia to a PaO2>6.65 kPa without decreasing arterial pH below 7.26. Non-invasive positive pressure ventilation may help achieve adequate oxygenation and prevent carbon dioxide retention by raising minute ventilation in patients with type II respiratory failure. Type II respiratory failure occurs in 10-15% of patients with chronic obstructive pulmonary disease. In patients without type II respiratory failure, the risk of hypercapnia is often overstressed, and under-treatment of serious hypoxemia can result in unnecessary death.
_
Acute use of oxygen therapy: a 2015 study:
A major change is needed in the entrenched culture of routinely administering high-concentration oxygen to acutely ill patients regardless of need. Oxygen is a drug that should be prescribed for specific indications. There should be a documented target range for oxygen saturation, and regular monitoring of the patient’s response. There are risks from unrelieved hypoxemia due to insufficient oxygen therapy, and from provoked hyperoxemia due to excessive oxygen therapy. Oxygen therapy should therefore be titrated so that the saturation is within a range that avoids these risks. If oxygen requirements are increasing, the clinician should review the patient and consider transfer to a higher level of care. If oxygen requirements are decreasing, consider reducing or discontinuing oxygen therapy.
_
Target oxygen saturation ranges:
The recommended target saturation range should be included as part of the patient’s oxygen prescription on the drug chart.
COPD and conditions associated with chronic respiratory failure:
In the treatment of exacerbations of chronic obstructive pulmonary disease (COPD), oxygen should be titrated to achieve a target oxygen saturation range of 88–92%. This results in a greater than twofold reduction in mortality, compared with the routine administration of high-concentration oxygen therapy. In a randomised controlled trial, ambulances were allocated to treat patients having an acute exacerbation of chronic obstructive pulmonary disease with either:
- titrated oxygen therapy – oxygen delivered by nasal cannulae as required to achieve target pulse oximetry saturations of 88–92% and bronchodilators delivered by an air-driven nebuliser
or
- high-concentration oxygen therapy – 8 L/min via a non-rebreather mask, regardless of oxygen saturation, and bronchodilators given by an oxygen-driven nebuliser.
Key findings were:
- mortality was over two times higher in patients who received routine high-concentration oxygen compared with those who received titrated oxygen therapy
- the number needed to harm (death) with the routine use of high-concentration oxygen was 14 (one additional person died for every 14 treated).
Uncontrolled oxygen therapy for patients with COPD can cause hypercapnia. Due to concerns that the risks of high-concentration oxygen therapy may also apply in other conditions that place patients at risk of hypercapnic respiratory failure (cystic fibrosis, neuromuscular disorders, chest wall disorders, morbid obesity), the saturation target of 88–92% has also been recommended for these patients.
Other acute medical conditions:
Due to limited evidence from randomised controlled trials to guide clinical practice, it has been difficult to set a target saturation range for other acute medical conditions, such as asthma, pneumonia and acute coronary syndrome. A pragmatic guide is to only give oxygen if saturations are under 92%, with a target saturation range of 92–96%.
Selecting the appropriate delivery method:
Oxygen can be delivered through a number of devices. For most patients, standard nasal cannulae are the preferred method of delivery. The flow rate is varied to achieve the target oxygen saturation.
Patients who improve:
If the patient’s clinical condition improves to the extent that their oxygen saturation exceeds the target oxygen saturation range, this is an indication to reduce the concentration of inspired oxygen. Monitoring of oxygen saturations should be continued to detect subsequent deterioration of the underlying condition and the requirement to increase or resume oxygen therapy.
Patients who deteriorate:
If oxygen saturations fall or increasing oxygen concentrations are required to maintain oxygen saturation within the target range, review the patient and consider measurement of their arterial blood gases.
In hospital a need for a fraction of inspired oxygen (FiO2) greater than 40% should trigger a review by a senior clinician. If the patient requires FiO2 greater than 50%, consultation with intensive care is recommended. Increased monitoring and non-invasive or invasive ventilation should be considered.
If oxygen-induced hypercapnia develops, oxygen therapy should not be abruptly stopped. This may lead to rebound hypoxemia (with a fall in oxygen saturation to below the level seen before oxygen was given). Oxygen should be gradually down-titrated and non-invasive ventilation considered.
Prophylactic oxygen therapy:
There are risks in the practice of administering prophylactic oxygen to a breathless patient who is not currently hypoxemic, in the belief that it may prevent hypoxemia if the underlying condition deteriorates. This practice has the potential to cause delay in recognising clinical deterioration and reduce the time available to start additional treatment.
______
Oxygen therapy for chronic conditions:
The therapy may be used to deliver supplemental oxygen to patients with chronic obstructive pulmonary disease (COPD) on a long-term basis. COPD is one of the long-term effects of smoking and patients may need additional oxygen, either through periods where their condition has worsened or as a permanent support throughout the day and night. The use of supplemental oxygen is indicated when a patient with COPD has a partial pressure of oxygen (PaO2) level of 55 mmHg or less or an oxygen saturation (SaO2) level of 88% or less. The therapy has been shown to significantly increase patients’ lifespan.
Other examples of chronic conditions that may benefit from oxygen therapy include:
- Chronic asthma
- Cystic fibrosis
- Pulmonary hypertension
- Obstructive sleep apnea
- Heart failure
_____
_____
Practical use of oxygen in anaesthesia:
_
Inspired oxygen concentration:
The efficiency of oxygenation during anaesthesia is reduced due to hypoventilation and venous admixture. Inspired oxygen in the range of 25% to 30% is usually effective in restoring the PaO2 to normal when hypoxemia is due to hypoventilation. When hypoxemia is due to venous admixture it is possible to restore the PaO2 by increasing the inspired oxygen concentration if the venous admixture does not exceed the equivalent of a 30% shunt. The inspired oxygen concentration during maintenance of anaesthesia should routinely be increased to 30% whenever possible to compensate for hypoventilation and shunt which normally accompany anaesthesia. Additional oxygen may need to be administered to patients at risk of decreased oxygen delivery (anaemia or decreased cardiac output) or increased oxygen consumption (fever).
_
Pre-oxygenation:
The small volume of the oxygen stores of a patient breathing air means that there will be a rapid fall in oxygen saturation during apnoea (e.g. following induction of anaesthesia, during laryngospasm or during upper airway obstruction). Pre-oxygenation involves breathing 100% oxygen for three minutes through an anaesthetic circuit with a face mask firmly applied to the face. This is the time taken to replace the nitrogen in the FRC with oxygen using normal tidal ventilation (‘denitrogenation’). Although FRC falls on induction of anaesthesia the extra oxygen contained within the FRC provides an essential store of oxygen for periods of apnoea, particularly during rapid sequence induction or difficult intubation. Patients with a small FRC (infants, pregnancy, obese) or a low haemoglobin concentration, and therefore smaller oxygen stores, desaturate more rapidly and pre-oxygenation is especially indicated in these patients.
_
Postoperative oxygen:
The causes of increased venous admixture (V/Q mismatch – shunt and airway closure) and the abnormal response to hypoxia continue into the postoperative period for up to three days following major surgery. Postoperative hypoventilation is common and may be due to the residual effect of anaesthesia, the use of opioid analgesia, pain or airway obstruction. Shivering in the immediate postoperative period causes an increase in oxygen consumption. Additional oxygen should therefore be given to all unconscious patients in recovery and to those awake patients who either shiver, hypoventilate, are desaturated or who are considered to be at increased risk (e.g. ischaemic heart disease). On the ward during the postoperative period, episodes of airway obstruction during sleep are common and may aggravate borderline oxygenation due to the above factors. This is usually due to the use of opioid analgesia and a change in sleep pattern that occurs on the second and third postoperative nights. After major surgery, the risk of hypoxemia extends well into the postoperative period. Small degrees of cyanosis are not easy to detect clinically, especially in anaemic patients, and therefore oxygen should be given to these patients wherever possible, especially overnight. Postoperative pain should be effectively treated, particularly following abdominal or thoracic surgery. If opioid analgesics are indicated, hypoventilation should be anticipated, and oxygen saturation monitored as a routine.
______
______
Contraindications to oxygen therapy:
Many EMS protocols indicate that oxygen should not be withheld from any patient, while other protocols are more specific or circumspect. However, there are certain situations in which oxygen therapy is known to have a negative impact on a patient’s condition. Oxygen should never be given to a patient who is suffering from paraquat poisoning unless they are suffering from severe respiratory distress or respiratory arrest, as this can increase the toxicity. (Paraquat poisoning is rare — for example 200 deaths globally from 1958 to 1978). Mild to moderate hypoxia should not be routinely treated with oxygen in paraquat poisoning as it will worsen oxidative stress and it greatly increases lethality in animal models. Oxygen therapy is not recommended for patients who have suffered pulmonary fibrosis or other lung damage resulting from bleomycin treatment. Supplemental oxygen therapy is considered to be a synergistic toxin with bleomycin in bleomycin induced lung injury. Exposure to bleomycin appears to sensitise the lungs and potentially fatal acute lung damage can occur at an inspired oxygen concentration which is otherwise considered normal for a healthy individual. The use of some O2 delivery devices e.g., nasal cannulas and nasopharyngeal catheters is disallowed in neonates and pediatric patients that have nasal obstructions. Oxygen is not indicated for patients with severe cardiopulmonary disease whose main complaint is dyspnoea, but who maintain a PaO2 greater than 60 mm Hg (8 kPa) and who show no secondary effects of chronic hypoxia. Oxygen therapy is not indicated in patients who continue to smoke cigarettes because of the increased fire risk and the probability that the poorer prognosis conferred by smoking will offset treatment benefit. Oxygen therapy is not indicated for patients who have not received adequate therapy for the underlying medical condition(s) responsible for causing hypoxemia, or who are not sufficiently motivated to undertake the discipline required in using oxygen therapy for the prescribed number of hours per day. Remember ‘contraindicated’ and ‘not indicted’ are not same.
______
______
Oxygen sources:
Oxygen can be separated by a number of methods, including chemical reaction and fractional distillation, and then either used immediately or stored for future use. Oxygen has historically been obtained by fractional distillation of liquid air, but is increasingly obtained by non-cryogenic technologies such as pressure swing adsorption (PSA) and vacuum swing adsorption (VSA) technologies. It is stored as a liquid to reduce the size of the storage container. 1 L of liquid O2 produces 860 L of gaseous O2.
_____
There are several ways supplemental oxygen is delivered:
- High-Pressure Oxygen System:
High-pressure oxygen systems are usually found in hospitals and other medical facilities. Medical oxygen provided from wall source provides 50 psi (pounds per square inch) of pressure. The figure below shows wall outlet with pressure valve & flow meter.
While this is the most versatile system for supplemental oxygen, it’s rare to find this type of system outside of licensed facilities.
______
- Liquid oxygen:
In extremely cold temperatures (-300°F) oxygen changes from a gas state to a liquid state. Liquid oxygen is stored in chilled tanks until required, and then allowed to boil at a temperature of −182.96 °C to release oxygen as a gas. This is widely used at hospitals due to their high usage requirements, but can also be used in other settings. The primary benefit of liquid oxygen is that a large amount of oxygen can be supplied in a relatively small container because one liter of liquid oxygen offers approximately 860 liters of gaseous oxygen. This occurs because liquid O2 is stored at about -300°F and when heated transforms to gaseous oxygen. The systems designed to store home liquid oxygen are Thermos-like containers with capacities of 20-40 liters for home storage units to 0.5-1.0 liter for portable units. Liquid oxygen is kept under far less pressure than compressed oxygen, making it safer and less volatile. The down-side is that the ports are exceptionally cold, and can burn the skin quite badly if touched. The advantage of liquid oxygen is availability of compact portable units that offer a wide range of liter flow and duration. A disadvantage is that while the storage containers are efficient they are not perfect and heat does cause wasteful dissipation of oxygen. The units are also quite costly and require regular home deliveries.
_____
- Compressed oxygen cylinder:
_
Compressed oxygen cylinders are perhaps the most well-known, and possibly the most iconic type of oxygen delivery system. They are the most basic and simple form of oxygen therapy, and also one of the most reliable. The cylinders consist of a tank filled with compressed oxygen, a valve that is manipulated to deliver a specific amount of oxygen and a plastic delivery tube. Tanks for home use are generally very large and bulky, rendering their mobility challenging. The tanks are supplied and home-delivered by a medical supply company. The tanks then need to be secured in a sturdy manner, in order to ensure the tanks’ safety. Smaller portable tanks are available for mobile use.
_
Compressed oxygen is placed inside of steel tanks which are built to withstand the internal pressure of the compressed gas. A flow regulator allows oxygen to be delivered to a person, at a lower pressure, while still maintaining the pressure inside the tank. Oxygen is a volatile gas and under pressure, it can become dangerous. The tanks are constructed to keep the oxygen from combusting, but additional care should be taken to secure the tanks in an upright position, at relatively low temperatures, and away from any potential falling objects. While the bulky size of the larger tanks renders them impractical for portable use, it makes them highly effective for home-use. A key is used to open and close the tank’s valve. The tanks carry enough oxygen to last a considerable amount of time, depending on the relative flow rate being utilized. Once the tank approaches empty they must be replaced immediately, as to prevent a life-threatening situation for someone that is oxygen dependent. It is wise to keep a small replacement cylinder on hand just in case the large oxygen cylinder runs low without being noticed. Because these tanks operate on their own, they do not need an outside source of power, such as a home’s electrical outlet or a battery.
_
Oxygen cylinders are the primary source for home oxygen portable systems. The advantages of oxygen cylinders include the fact that they come in a variety of sizes, do not waste oxygen, they can have their duration increased with the use of a conserving device and can provide high liter flows. The disadvantages include often cumbersome size, limited duration, need for frequent refills and the high storage pressure of the gas (often up to 2000 psi).
_
The oxygen gas is compressed in a gas cylinder, which provides a convenient storage, without the requirement for refrigeration found with liquid storage. Large oxygen cylinders hold 6,500 litres (230 cu ft) and can last about two days at a flow rate of 2 litres per minute. A small portable M6 (B) cylinder holds 164 or 170 litres (5.8 or 6.0 cu ft) and weighs about 1.3 to 1.6 kilograms (2.9 to 3.5 lb). These tanks can last 4–6 hours when used with a conserving regulator, which senses the patient’s breathing rate and sends pulses of oxygen. Conserving regulators may not be usable by patients who breathe through their mouths.
_
Oxygen cylinder math:
D cylinder contains 350 liters of oxygen
E cylinder contains 625 liters of oxygen
M cylinder contains 3,000 liters of oxygen
Safe residual pressure is 200 psi.
To use the math formula each cylinder size has its own CONSTANT.
D cylinder is 0.16
E cylinder is 0.28
M cylinder is 1.56
Formula of how long cylinder last in minutes:
(PSI in tank – safe residual pressure) X CONSTANT
___________________________________________
Flow rate in LPM
_
Example A)
You are to run a non-rebreather mask at 10 LPM. Your D cylinder has 1800 psi remaining. How long will the tank last? So take 1800 minus 200 multiply by 0.16 then divide number by 10. The answer is in minutes. 25.6 minutes is the answer.
Example B)
You are to run a non-rebreather mask at 12 LPM. Your M cylinder has 1500 psi remaining. How long will the tank last? So take 1500 minus 200 multiply by 1.56 then divide number by 12. The answer is 169 minutes.
____
- Oxygen Concentrator:
_
Oxygen concentrators take in the ambient air from the environment, isolate the oxygen, and deliver a concentrated dose of oxygen. They consist of an air compressor and zeolite-filled container. The compressor brings the ambient air into the machine. Ambient air is made up of roughly 78% nitrogen and 21% oxygen. The zeolite minerals absorb the nitrogen to separate it from the oxygen, resulting in a concentrated dose of up to 95% pure oxygen.
_
_
Oxygen concentrators come in two types: stationary and portable. Both types vary regarding functionality, but they both work on the same principle of concentrating oxygen from the ambient air and outputting enough flow to reach therapeutic levels. Stationary concentrators are a great option if you’re only prescribed oxygen at night. However, if you need oxygen throughout the day, portable oxygen concentrators are an excellent choice because they can be taken with you on-the-go. Home oxygen concentrators feature oxygen outputs ranging anywhere from 2 Liters per Minute (LPM) up to 10 LPM. Regular oxygen concentrators deliver oxygen at around 7 psi. High Pressure oxygen concentrator delivers an output of up to 10 LPM at 20 psi. When deciding what kind of oxygen concentrator is best for you, it is important to determine what kind of oxygen delivery you would prefer: Pulse Dose or Continuous Flow. Pulse Dose (PD) is based on breathing and inhaling, whereas Continuous Flow (CF), as its name implies, is delivered at a constant rate indiscriminate of the user’s breathing. Pulse Dose mechanisms are more sensitive, utilizing an oxygen conserver and other technology to deliver oxygen based on breathing rates and other factors. When an oxygen concentrator is said to be a “single-solution” for on-the-go, at home, and sleep, it usually employs this type of technology so that it can deliver the proper amount of oxygen during all phases of daily activity and during rest.
_____
Figure below shows oxygen delivery from source to patient:
Oxygen delivery system consists of
- Oxygen source
- Pressure regulator and flow meter
- Oxygen delivery device
- Patient
Oxygen cylinder operates at 1800-2400 psi which is very high pressure and cannot be directly delivered to patient or run the ventilator. It needs pressure regulator with flow meter. The pressure regulator controls the pressure coming out of the cylinder by reducing high pressure into low pressure which is indicated on the gauge in psi. The flow meter controls how rapidly the oxygen flows from the cylinder/wall source to the victim. The flow rate can be set from 1 to 25 L/min. Oxygen flow rate determines the amount of oxygen delivered to the patient and measured in liters per minute. The rate varies according to patient’s conditions and type of delivery device. COPD patient on LTOT may need oxygen flow of 2 L/min while a patient in acute respiratory failure may need more than 25 L/min.
_____
_____
Oxygen delivery devices:
_
Oxygen delivery systems:
From a practical point of view, there are two types of delivery systems: those for patients who are breathing on their own and can protect their airways, and those for intubated or tracheostomized patients.
For patients who aren’t intubated and don’t require airway protection, you can choose from a variety of high or low-flow options or consider an enclosure device.
_
Currently, there is a wide array of oxygen delivery devices available to the respiratory therapist (RT) to utilize for administration. The choice of oxygen delivery devices depends on the patient’s oxygen requirement, efficacy of the device, reliability, ease of therapeutic application and patient acceptance. Although design plays an important role in selection of these devices, clinical assessment and performance ultimately determines how and which device should be selected. Oxygen delivery devices range from very simple and inexpensive designs to more complex and costly. Oxygen percentage delivery can be inconsistent or precise depending on the type of administration device selected. Oxygen administration can be delivered via low-flow or high-flow systems, with humidity or not, and with a reservoir or not. Monitoring of oxygen delivery effectiveness includes arterial blood gas analyses, oxygen saturation monitoring, and clinical assessment. Oxygen can be considered toxic if percentages are delivered in percentages greater than 60% and in the chronic carbon dioxide retention patient population it may diminished ventilator drive and produce life threatening hypercarbia. It can also cause absorption atelectasis by washing out nitrogen gas when delivered in high concentrations.
_
Oxygen delivery devices have historically been categorized into three basic types based on their design: low-flow, reservoir, and high-flow. Regarding the FiO2 range, oxygen systems can be divided into those indicated for low oxygen (<35%), moderate delivery (35%-60%) or high delivery (>60%). Some devices can deliver a wide range of oxygen percentages. When selecting an oxygen delivery device the respiratory therapist must address two key questions. First, how much oxygen can the device deliver? Second, is the FiO2 delivered consistent, or can it vary with changing respiratory patterns?
_
There is a wide range of oxygen delivery devices broadly divided into the low flow oxygen devices and the high flow oxygen devices.
The low flow devices consist of the nasal cannula, simple face mask, partial rebreathing mask and the non-rebreathing mask. The high flow oxygen delivery devices consist of venturi masks, oxygen tent and oxygen hood. Many people only require a slight increase in oxygen in the air they breathe, rather than pure or near-pure oxygen. This can be delivered through a number of devices dependent on the situation, the flow required and in some instances patient preference. Oxygen is most commonly delivered to the patient via a nasal cannula or mask attached to the tubing.
_
High-Flow and Low-Flow Oxygen Delivery Systems:
An oxygen delivery system is a device used to administer, regulate, and supplement oxygen to a subject to increase the arterial oxygenation. In general, the system entrains oxygen and air to prepare a fixed concentration required for administration. Oxygen delivery systems are generally classified as low-flow or variable-performance devices and high-flow or fixed-performance devices. Low-flow systems provide oxygen at flow rates that are lower than patients’ inspiratory demands; thus, when the total ventilation exceeds the capacity of the oxygen reservoir, room air is entrained. Typical low-flow oxygen systems provide supplemental oxygen often less than the patient’s total minute ventilation. Because the patient minute ventilation exceeds flow, the oxygen delivered by the device will be diluted with ambient air and thus the inspired oxygen delivery is less than anticipated. The final concentration of oxygen delivered depends on the ventilatory demands of the patient, the size of the oxygen reservoir, and the rate at which the reservoir is filled. At a constant flow, the larger the tidal volume, the lower the FiO2 and vice versa. In contrast, the high-flow systems provide a constant FiO2 by delivering the gas at flow rates that exceed the patient’s peak inspiratory flow rate and by using devices that entrain a fixed proportion of room air. Also, there is a tendency to confuse flow systems with oxygen concentrations. However, both are mutually exclusive in that a high-flow system, viz. Venturi mask, can deliver FiO2 as low as 0.24, whereas a low-flow system like a nonrebreather mask can deliver FiO2 as high as 0.8. Thus, if the ventilatory demand of the patient is met completely by the system, then it is a high-flow system. In contrast, if the system fails to meet the ventilatory demand of the patient, then it is classified as a low-flow system.
_
There are a number of methods of classifying devices that deliver oxygen to the patient’s upper airway. These include: (i) fixed versus variable performance; (ii) ‘patient-dependent’ versus ‘patient-independent’; and (iii) low versus high gas flows. The fixed performance systems are usually considered as ‘patient-independent’ because the patient receives a constant, predetermined inspired oxygen concentration (FiO2) regardless of changes in respiratory parameters. The variable performance systems are ‘patient-dependent’ because the patient receives a variable FiO2 as their respiratory parameters change. Table below classifies oxygen therapy devices by considering the total gas flow delivered to the patient. While there is no defined cut-off point between low and high flow, it is reasonable to place it at about 10–15 litre/min. Low flow systems can deliver a known FiO2 when there is a tight fitting mask and a reservoir, such as anaesthetic breathing systems. High flow devices become patient-independent when the flow exceeds the patient’s peak inspiratory flow. Conversely, when the flow is less than the peak inspiratory flow, even the high flow devices become patient-dependent.
_
Nasal cannula:
A nasal cannula (NC) is a thin tube with two small nozzles that protrude into the patient’s nostrils. It can only comfortably provide oxygen at low flow rates, 2–6 litres per minute (LPM), delivering a concentration of 24–40%. The nasal cannula is usually the delivery device of choice since it is well tolerated and doesn’t interfere with the patient’s ability to communicate, eat, or drink. The concentration of oxygen inhaled depends upon the prescribed flow rate and the ventilatory minute volume (MV). Nasal cannulas are most commonly used in our day to day practice. Usually, patients are more comfortable with the nasal cannula but we should keep in mind whether the fraction of inspired oxygen (FiO2) delivered by the nasal cannula is sufficient for the patient. The maximum fraction of inspired oxygen (FiO2) provided by the nasal cannula is 0.44 at flow of 6 L/min. With further increase in flow, there is no further increase in FiO2.Appropriate devices and flow rates should be used in order to achieve target range of oxygen saturation for the patient. Prolonged use of nasal cannula may lead to drying of mucosa and crusting of secretions and may lead to epistaxis. Nasal cannulas are used to deliver oxygen when a low flow, low or medium concentration is required, and the patient is in a stable state. They deliver oxygen in a variable manner; this means the amount of oxygen inspired depends on the patient’s breathing rate and pattern. Since the delivered oxygen percentage is very inconsistent during respiratory distress, a nasal cannula is not recommended for acute severe hypoxemia or patients that breathe on a hypoxic drive where to high of an oxygen concretion may led to respiratory depression. This includes some patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) having hypercapnia; these patients retain carbon dioxide and require a Venturi oxygen mask. In other acute situations patients may need a higher concentration of oxygen and a non-rebreathe mask or simple oxygen mask is often used. A nasal cannula utilizes no external reservoir of oxygen and relies on the patient’s upper airway as an oxygen reservoir. A humidification device is recommended for flows greater than four liters to insure humidification of the dry inspired gas. Even with humidity added flows 6-8 liters per minute can cause nasal dryness and bleeding. The best clinical indications for the nasal cannula is for patients who have a relative stable respiratory pattern, required low oxygen percentage, need supplement oxygen during an operative or diagnostic procedure or for chronic home care.
_
Transtracheal catheter:
Transtracheal catheters deliver oxygen directly into the trachea. There are washout and storage effects that promote gas exchange, as well as provide high-flow oxygen. High-flow transtracheal catheters may reduce the work of breathing and augment CO2 removal in the chronic oxygen user. Transtracheal oxygen therapy improves the efficiency of oxygen delivery by creating an oxygen reservoir in the trachea and larynx. Consequently, mean oxygen savings amount to 50% at rest and 30% during exercise. Transtracheal oxygen reduces dead space ventilation and inspired minute ventilation, while increasing alveolar ventilation slightly, which may result in a reduction of the oxygen cost of breathing. As a result, patients using this device may experience improved exercise tolerance and reduced dyspnea. This delivery device is best used for home care and ambulatory patients who required long periods of mobility and do not feel comfortable wearing a nasal cannula.
_
Face mask (oxygen face mask):
Simple face mask provides an additional reservoir of 100-200mL of oxygen. It can provide a FiO2of up to 0.6 to the patient. The flow rate must be a minimum of 5 L/min to prevent rebreathing and prevent carbon dioxide (CO2) accumulation. It has an advantage of being light weight and simple to use but it needs to be removed while eating and speaking. It is difficult to get a proper seal in patients with Ryle’s tube. It cannot be used in patients with facial injury and burns. If the face mask moves up accidentally towards the eyes, then it may lead to irritation and dryness of eyes. Simple face mask is often used between 5 and 8 LPM, with a concentration of oxygen to the patient of between 28% and 50%.
_
Types of masks:
_
Partial rebreathing and non-rebreathing masks have reservoir bag attached to the mask which provides additional reservoir volume. In partial rebreathing masks, as the name suggests some amount of rebreathing is allowed. Exhaled oxygen from the anatomic dead space which does not take part in gas exchange gets collected in the reservoir bag. This is especially helpful in situations where oxygen supply is less and conservation of oxygen would be helpful. Partial rebreathing mask delivers a FiO2 between 0.6 and 0.8. Reservoir bag must remain inflated throughout to ensure the delivery of highest FiO2 with adequate CO2 evacuation. For this, fresh gas flows of more than 8 L/min is required. Non-rebreathing masks have additional valves to the partial rebreathing masks. The valves allow unidirectional flow of gases. The flutter valves on the side ports prevent room air entrainment. The exhaled gases are prevented from entering the reservoir bag by incorporating a valve between the mask and the reservoir bag. It can provide the highest FiO2of 0.9 to 1.0 without intubation at fresh gas flow of 12 – 15 L/min. Prolonged use can be uncomfortable for the patient as it requires a tight seal and the entire equipment is heavy. Malfunction of the equipment may lead to CO2 build up and suffocation.
_
Venturi Mask:
The mechanism of action is usually incorrectly quoted as depending on the venturi effect. Despite there being no evidence for this, many textbooks and journal articles cite this as the mechanism. This fixed performance oxygen delivery system, despite often being called a venturi mask, works on the principle of jet mixing. As the oxygen enters the mask through a narrow jet, it entrains a constant flow of air, which enters via surrounding holes.
Example: with oxygen flow of 4 liters/min, total flow (oxygen + air) of approx, 40 liters/min is delivered to the patient. At such high flow rates, there is negligible rebreathing of expired gas and, therefore, no CO2 accumulation. This type of oxygen delivery is advocated for patients with chronic hypercarbia for the in management of moderate to severe hypoxemia. Venturi mask can delivers O2 concentrations varying from 24% to 40% or 50% –at flow rates of 4L to 10L/m.
_
Color coding of venture masks:
_
Exhalation filters for oxygen masks:
Filtered oxygen masks have the ability to prevent exhaled, potentially infectious particles from being released into the surrounding environment. These masks are normally of a closed design such that leaks are minimized and breathing of room air is controlled through a series of one-way valves. Filtration of exhaled breaths is accomplished either by placing a filter on the exhalation port, or through an integral filter that is part of the mask itself. These masks first became popular in the Toronto (Canada) healthcare community during the 2003 SARS Crisis. SARS was identified as being respiratory based and it was determined that conventional oxygen therapy devices were not designed for the containment of exhaled particles. Common practice of having suspected patients wear a surgical mask was confounded by the use of standard oxygen therapy equipment. In 2003, the HiOx80 oxygen mask was released for sale. The HiOx80 mask is a closed design mask that allows a filter to be placed on the exhalation port. Several new designs have emerged in the global healthcare community for the containment and filtration of potentially infectious particles. Other designs include the ISO-O2 oxygen mask, the Flo2Max oxygen mask, and the O-Mask. The use of oxygen masks that are capable of filtering exhaled particles is gradually becoming a recommended practice for pandemic preparation in many jurisdictions. Typical oxygen masks allow the patient to breathe in room air in addition to their therapeutic oxygen, but because filtered oxygen masks use a closed design that minimizes or eliminates the patient’s contact with and ability to inhale room air, delivered oxygen concentrations to the patient have been found to be higher, approaching 99% using adequate oxygen flows. Because all exhaled particles are contained within the mask, nebulized medications are also prevented from being released into the surrounding atmosphere, decreasing the occupational exposure to healthcare staff and other patients.
_
Oxygen hood in infants:
An oxygen hood is used for babies who can breathe on their own but still need extra oxygen. Oxygen hood is a high flow device, used to deliver oxygen in infants. A hood is a plastic dome or box with warm, moist oxygen inside, that surrounds the baby’s head. This device is useful in infants requiring more than 40% oxygen. Oxygen delivered by this method should be warmed and humidified. The total flow must be more than 10 L/min to prevent CO2 accumulation. Desired oxygen concentration can be achieved by combining the flow of oxygen and air. The oxygen delivered needs to be humidified and warmed. The gas should not be allowed to blow directly into infants face. And hood should not rub against infant’s neck, chin or shoulder. Probable complications of oxygen hood include hypoxemia, hyperoxemia, hyperthermia, hypothermia, irritation and pain in the neck.
_
Oxygen tent:
An oxygen tent consists of a canopy placed over the head and shoulders, or over the entire body of a patient to provide oxygen at a higher level than normal. Some devices cover only a part of the face. O2 flow should be 15L/Mt for 5 mts initially and then adjust the flow meter according to the orders. Delivers approximately 30% of O2
_
Special considerations:
- Review the protocol at your health authority prior to initiating any high-flow oxygen systems, and consult your respiratory therapist.
- In general, nasal prongs and a simple face mask (low-flow oxygen equipment) may be applied by a health care provider. All other oxygen equipment (high-flow systems) must be set up and applied by a respiratory therapist.
- For patients with asthma, nebulizer treatments should use oxygen at a rate greater than 6 L/min. The patient should be changed back to previous oxygen equipment when treatment is complete.
- Oxygenation is reduced in the supine position. Hypoxic patients should be placed in an upright position unless contraindicated (e.g., if they have spinal injuries or loss of consciousness).
- In general, for most patients with COPD, target saturation is 88% to 92%. It is important to recognize COPD patients are at risk for hypercapnic respiratory failure.
- Check the function of the equipment and complete a respiratory assessment at least once each shift for low-flow oxygen and more often for high-flow oxygen.
- In acutely ill patients, oxygen saturation levels may require additional ABGs to regulate and manage oxygen therapy.
- Oxygen saturation levels and delivery equipment should be documented on the patient’s chart.
__
Selection of appropriate oxygen delver devices:
In order to make an optimal choice of oxygen delivery, few basic principles need to be understood and then oxygen is prescribed using an appropriate delivery device. The very first thing to consider is the patient’s status. In patients who are relatively stable but at risk of type 2 respiratory failure, due to underlying lung or systemic pathology, then the target oxygen saturation should be 88-92% and titrate the oxygen dosage appropriately. Inappropriate high flow and high saturation in these patients can be deleterious, increasing both mortality and morbidity, whereas titrated oxygen delivery is proven to have significantly reduced mortality, hypercapnia, and respiratory acidosis. These patients are ideally treated by starting off with a fixed flow oxygen delivery device which can provide reliable and predictable FiO2, thus ideally started off with blue or white venturi while maintaining patient arterial oxygen saturation (SpO2) of 88-92%. And then titrating the oxygen to lowest dose of FiO2while maintaining the target SpO2. For patients who are not at risk of type 2 respiratory failure and requiring oxygen therapy because of hypoxemia, the target should be to maintain a SpO2 of 94-98%. Such patients who present initial severe hypoxemia with SpO2 less than 85%, they should be started with high flow oxygen of 10-15 L/min through reservoir mask. Others who are is relatively better with SpO2 85-94% can be started with low flow device such as nasal cannula at 2-6 L/min or simple face mask at 5-10 L/min and titrated ±2L/min thereafter to maintain the target SpO2. Other than the target SpO2 described in various conditions, permissive hypoxemia is a relatively new strategy for maintaining SpO2 of 82-88% in critically ill patients to improve outcomes.
_
The atmospheric content of oxygen within room air is only 21%. Although this amount is adequate for healthy individuals, those with certain diseases can benefit from an increased oxygen fraction in the gas they breathe, which will increase the oxygen content of their blood. For most of these diseases, increasing the oxygen fraction to around 30% to 35% is enough to make a significant difference to the blood oxygen level. This supplementary level of oxygen can be achieved using a nasal cannula, a thin tube with an individual nozzle for each nostril. This can provide oxygen at a low flow rate of 1 to 6.00 l/m, to achieve an oxygen level of 24% to 40%. For oxygen at greater concentrations, various face masks can be used. This includes the simple face mask, which can deliver oxygen at 5 to 15 l/m flow, to achieve an oxygen level between 28% and 50%; the Venturi mask which can provide oxygen to the trachea at concentrations of up to 40%; and a partial re-breathing mask which is similar to a simple mask but includes a reservoir bag and can deliver 40% to 70% oxygen at 5 to 15 l/m. For patients who require 100% oxygen, several devices are available, the most common of which is the non-breather mask or reservoir mask. This is based on a similar design to the re-breathing mask but a number of valves line the device to stop air that has been exhaled returning to the bag. This device achieves a minimum flow of 10 l/m and the fraction of inspired oxygen achieved is between 60% and 80%. Air or oxygen that has been warmed or humidified can also be administered via nasal cannula so that the patient can still talk, eat and drink while they undergo therapy. For patients who are unable to breathe independently, positive pressure may be needed to force air into their lungs. Systems used to deliver this therapy vary in complexity but essentially, they provide artificial respiration.
____
Oxygen Delivery Devices and Flow Rates Chart:
Given below is the respiration management table which shows the oxygen percentage exhaled, measured against a number of respiratory measurement tools such as Nasal Cannula, simple mask, venturi mask, partial re-breathing mask, non-rebreathing mask. This oxygen delivery devices and flow rates chart shows the O2% delivered measured for each tool. This table helps doctors choose the right type of mask for the patients depending upon the type of respiratory ailment of the patients.
| Device | Flow/Rate(L/min) | % O2 Delivered |
| Nasal Cannula | 1 | 24 |
| 2 | 28 | |
| 3 | 32 | |
| 4 | 36 | |
| 5 | 40 | |
| 6 | 44 | |
| Simple Mask | 5-6 | 40 |
| 7-8 | 50 | |
| 10 | 60 | |
| Venturi Mask(with Appropriate Entrainment Port) | 4 | 24 |
| 4 | 28 | |
| 6 | 31 | |
| 8 | 35 | |
| 8 | 40 | |
| 10 | 50 | |
| Partial Rebreathing Mask | 6-10 | Up to 80% |
| Non Rebreathing Mask | 10 | 80-100% |
This oxygen delivery devices and flow rates chart shows how many oxygen percentages delivered according to the different flow rates. The percentage of oxygen inspired depends on the flow rate and the delivery device. However FiO2 also depends on patient factors (vide infra).
__
__
Synopsis of oxygen delivery devices:
_____
Performance of oxygen delivery devices:
The administration of a known concentration of oxygen is an important part of routine care of the sick patient. Many devices are currently available. The actual concentration of oxygen that can be delivered by these devices can be affected by several factors, both from the patient as well as the device itself. Measuring the Fio2 delivered to the lungs in vivo can be both difficult and potentially uncomfortable for the subjects. Modern management of the sick patient involves the administration of oxygen in supposedly known concentrations. There are a many delivery systems commercially available whose performances are documented in the literature. This is usually reported as the actual delivery from the system, the resultant inspired concentration of oxygen, or the arterial oxygenation achieved.
_
From a physics perspective, the actual concentration of oxygen delivered is determined by the interaction between the delivery system and the patient’s breathing pattern. In contrast, most of the in vivo measurements that have been reported were carried out in normal subjects breathing at rest or trained to vary their tidal volumes. Several of these studies do point out that there are multiple factors which may compromise the performance of these devices and result in an effective FiO2 that is less, or occasionally more, than that expected. The factors that influence the concentration actually inspired by the patient are summarised in Table below.
_
Factors that influence the FiO2 delivered to a patient by oxygen delivery devices:
| Patient factors | Device factors |
| Inspiratory flow rate | Oxygen flow rate |
| Presence of a respiratory pause | Volume of mask |
| Vent resistance | |
| Tightness of fit |
_
Few studies have tried to evaluate delivery system performance in the adverse circumstances in which the systems are commonly used. Practical difficulties include the instantaneous and simultaneous measurement of FiO2 and gas flow. This is compounded by the problems of identifying and accessing a suitable sampling site. The predominant problem is the relationship of the oxygen flow into the mask, the size of the reservoir effect of the mask and the peak inspiratory flow rate, the combination of which will determine air entrainment and therefore oxygen dilution.
_
A given liter flow rate of nasal O2 does not = any specific FiO2.
The oft-quoted rule that 2 l/min = FiO2 of 24%, 3 l/min = 28%, etc., is an illusion, based on nothing experimental or scientific.
The actual FiO2 with nasal oxygen depends on the patient’s breathing rate and tidal volume, i.e., the amount of room air inhaled through the mouth and nose that mixes with the supplemental oxygen. FiO2 depends on ventilatory minute volume and flow rate of oxygen. This is illustrated by calculating the FiO2 at a fixed oxygen flow rate of 2 l/min in a patient with an exacerbation of chronic obstructive pulmonary disease before and after treatment.
As you can see, at constant oxygen flow rate of 2 l/m, FiO2 delivered during respiratory distress was only 26 % while FiO2 delivered after relief of respiratory distress was 53%.
_
Face masks cannot deliver 100% oxygen unless there is a tight seal. So-called non-rebreather face masks can deliver FiO2 up to around 80%. It is a mistake to label a patient with any loose-fitting face mask as receiving “FiO2 of 100%.” (Again, 100% oxygen can only be delivered with a ventilator or tight-fitting face mask.)
________
Infection control:
Under normal circumstances, low-flow oxygen systems (including cannulas and simple masks) do not present clinically important risk of infection and need not be routinely replaced. High-flow systems that employ heated humidifiers and aerosol generators, particularly when applied to patients with artificial airways, can pose important risk of infection. In the absence of definitive studies to support change-out intervals, results of institution-specific and patient-specific surveillance measures should dictate the frequency with which such equipment is replaced.
____
____
Oxygen Conserving Device (OCD) and Demand Oxygen Delivery System (DODS):
The oxygen conserving device (OCD) is the device on your small compressed gas tank that makes the oxygen supply last longer. It causes the oxygen to be delivered only when you take a breath. Not all OCDs deliver the same amount of oxygen as a continuous flow would, so it is important that your oxygen saturation be tested at rest and with activity while you are using the OCD, to make sure you are getting enough oxygen. An oxygen conserving device (OCD) controls the flow of oxygen from the oxygen source to the patient. The OCD releases oxygen only when the patient inhales, thus dramatically increasing the amount of time a patient can use the oxygen supply. This offers patients increased mobility and comfort from avoiding a continuous flow of oxygen into the nostrils. Ultimately, OCDs look to make oxygen therapy more efficient, more portable and less intrusive. Oxygen conserving devices allow all types of oxygen systems to last longer increasing patient independence. Oxygen conservation can be accomplished through the interface or through the delivering apparatus. The advantage of these delivery systems is that they increase the durations of tanks and portable oxygen concentrators by 30% to 60%. The noise created by the pulse of oxygen, limited liter flow (up to 6 l/m) and inability to maintain adequate oxygen saturation in some patients are all considered disadvantages of oxygen conserving devices.
_
Recently demand oxygen delivery system (DODS) has been preferred to continuous flow oxygen (CFO) method for long term oxygen therapy in patients with chronic obstructive pulmonary disease, since DODS supplies oxygen during only inhalation and saves oxygen consumption. Demand oxygen delivery systems (DODS) or oxygen resuscitators deliver oxygen only when the person inhales, or, in the case of a non-breathing person, the caregiver presses a button on the mask. Demand oxygen delivery systems (DODS) allot oxygen by interrupting the oxygen flow during exhalation, when it would mostly be wasted. Pulse-type DODS deliver oxygen only early in inhalation. Demand-type DODS provide oxygen flow throughout inhalation. Pulse-type DODS generally deliver a fixed volume of gas, at a relatively high flow that does not vary with changes in respiratory frequency. Demand-type DODS generally deliver a smaller bolus of gas at the onset of inhalation and then maintain a flow at or below the implied continuous-flow setting for the remainder of inhalation. These systems greatly conserve oxygen compared to steady-flow masks, which is useful in emergency situations when a limited supply of oxygen is available and there is a delay in transporting the patient to higher care. They are very useful in performing CPR, as the caregiver can deliver rescue breaths composed of 100% oxygen with the press of a button. Care must be taken not to over-inflate the patient’s lungs, and some systems employ safety valves to help prevent this. These systems may not be appropriate for unconscious patients or those in respiratory distress, because of the effort required to make them breathe.
_____
_____
High flow oxygen (HFO) therapy via high flow nasal cannula (HFNC):
Traditional oxygen therapy is up to 16 L/min and high flow oxygen therapy is up to 60 L/min. A relatively new oxygen delivery device is high-flow nasal cannula (HFNC) delivery system. Nasal oxygen has been administered at flows ranging from 10-60 liters. When this oxygen is warmed to body temperature and saturated to full humidity via molecular humidification, despite its high flows, it is deemed comfortable. High-flow oxygen (HFO) consists of a heated, humidified high-flow nasal cannula that can deliver up to 100% heated and humidified oxygen at a maximum flow of 60 LPM via nasal prongs or cannula. Humidified high flow nasal cannula enables flows exceeding a patient’s peak inspiratory flow demand to be delivered via nasal cannula, thus providing FiO2 of up to 100% because there is no entrainment of room air, even with the mouth open. This also allows the patient to continue to talk, eat and drink while still receiving the therapy. This type of delivery method is associated with greater overall comfort, and improved oxygenation and respiratory rates than with face mask oxygen.
_
The device consists of an air/oxygen blender connected via an active heated humidifier to a nasal cannula, through a single-limb heated inspiratory circuit. It delivers a fraction of inspired oxygen (FiO2) from 21% to 100%, with a flow rate up to 60 l/m. FiO2 adjustments are independent of the setting flow rate, so that the patient is administered heated humidified high-flow oxygen, with a flow that can be set above the patient’s maximum inspiratory flow rate, thus increasing confidence about the FiO2 being delivered to the patient. These device characteristics make it more promising in comparison with conventional low- and high-flow oxygen devices (e.g. nasal cannula, non-rebreathing masks and Venturi masks), especially in patients with high inspiratory flow rates, such as patients with acute respiratory failure.
_
Nasal high flow is a promising novel oxygen delivery device, whose mechanisms of action offer some beneficial effects over conventional oxygen systems. The administration of a high flow of heated and humidified gas mixture promotes higher and more stable inspiratory oxygen fraction values, decreases anatomical dead space and generates a positive airway pressure that can reduce the work of breathing and enhance patient comfort and tolerance. Nasal high flow has been used as a prophylactic tool or as a treatment device mostly in patients with acute hypoxaemic respiratory failure, with the majority of studies showing positive results.
_
In a 2013 study of high flow oxygen via HFNC devices for adult and perinatal patients, Ward concluded that high flow oxygen had the following therapeutic effects:
- Increased fraction of inspired oxygen (FiO2)
-Gas inlet flow prevents secondary room-air entrainment;
-Provides anatomic oxygen reservoirs using nasopharynx and oropharynx;
-Washing out of airway dead space;
- Development of a CPAP effect
-Decreases atelectasis;
-Improvement in pulmonary ventilation-perfusion;
-Decreases work of breathing: counteracts intrinsic PEEP;
- Greater patient comfort
-Warmed and humidified nasal oxygen can be better tolerated, especially with flows >6 LPM.
_
Physiological effects of HFNC oxygen therapy:
_
Roca et al compared HFNC with conventional oxygen therapy via a mask in 20 critical care patients and demonstrated that HFNC via Optiflow was better tolerated and more comfortable than an oxygen mask. HFNC also was associated with an improvement in oxygenation and a reduction in respiratory rate. Kernick and Magarey compiled a systematic review that examined eight studies comparing oxygen therapy with HFNC in adult ICU patients. The study demonstrated that HFNC may be used to improve oxygenation in adult critical care patients. In a randomized crossover of 50 mechanically ventilated patients deemed suitable for ventilator liberation, Tiruvoipati et al demonstrated that HFNC was as effective in providing oxygenation as a conventional oxygen system and was better tolerated by patients. In addition, six case studies on adult critical care patients demonstrated improved oxygenation and a reduction of respiratory rate or dyspnea. Lenglet et al examined the utilization of HFO in the emergency department for patients with acute respiratory insufficiency who may benefit from noninvasive ventilation. In addition to the ability to generate high flow and concentration of supplemental oxygen, HFNC generates a low level of positive airway pressure, especially with the mouth closed. In hypoxemic patients, HFNC may provide effective support with greater ease of use and patient comfort than techniques requiring a tight face mask. High flow oxygen administration also has been utilized in the end-of-life clinical arena. In a recent study conducted by Peters et al, it was demonstrated that patients who would have been treated via NIV were successfully maintained with HFO. NIV is typically initiated in the ICU setting, so that it is common for a patient with do-not-resuscitate (DNR) or do-not-intubate (DNI) status to be admitted or transferred to the ICU specifically for consideration of NIV. The outcome of this study showed that humidified HFNC oxygen therapy can provide adequate oxygenation for many patients with hypoxemic respiratory failure, and may be an alternative to NIV for patients who decline intubation.
_
Based on clinical evidence, the utilization of high flow oxygen (HFO) therapy via high flow nasal cannula (HFNC) in appropriate patients can improve oxygenation, decrease the patient’s work of breathing, and serve as an alternative to more invasive forms of treatment, such as mechanical ventilation. Improving gas exchange and decreasing work of breathing are clinical end-points when managing patients with respiratory compromise. Oxygen administration has been one of the most common interventions used over the past several decades. Almost every patient admitted to an emergency department or intensive care unit is placed on some form of oxygen administration. Often the choices are to provide oxygen via a mask, or a more aggressive form of clinical management, such as noninvasive ventilation (NIV) or invasive mechanical ventilation. However, a high flow oxygen (HFO) therapy system can deliver a high flow air/oxygen blend through a nasal cannula or tracheal adapter, providing an alternative to other forms of ventilation. By providing flow rates of up to 60 LPM, high molecular humidity, and precise oxygen delivery, HFO may reduce the need for noninvasive ventilation and intubation in selected patient populations.
_
Historically, oxygen delivery devices have been labelled as either low flow or high flow, based on design application. Each of these systems has inherent advantages and liabilities when compared to each other. Low flow systems often are more comfortable, but the ability to deliver a precise oxygen concentration in various respiratory breathing patterns is limited. A high flow system can deliver very accurate oxygen concentrations, but is often uncomfortable and obtrusive. Recently HFNC delivery device has been developed that can deliver precise oxygen concentrations at various flow rates and provide a level of comfort to the patient. It also can deliver positive end-expiratory pressure (PEEP) that may splint conducting airways or recruit collapsed alveoli. In these models, for approximately every 10 liters of flow delivered, about 1 cm/H2O of positive pressure is obtained. Since it utilizes a molecular humidification system, it can increase humidity delivery and promote mucokinesis, thus helping to prevent mucous plugging. HFO increases patient comfort during oxygen administration and may allow some patients who are NIV dependent to eat or receive nursing care without potential life-threatening desaturation.
_
High flow oxygen therapy in chronic conditions:
Humidified high flow oxygen therapy has been used successfully in COPD, bronchiectasis, end-stage cancer and do-not-intubate patients.
High flow oxygen therapy is intended to:
- Eliminate most of the anatomic dead space
- Create a reservoir with high FiO2 in the nasal cavity
- Improve gas exchange
- Significantly reduce the work of breathing
Other positive effects of high flow oxygen therapy (with humidification):
- Using humidified (warmed) gas keeps mucus more fluid and aids airway recovery (e.g., after surgery)
- Reduced respiratory rate
- Less dyspnoea (laboured breathing or shortness of breath / breathlessness) and mouth dryness, and greater overall comfort
- Ease of set-up – easier to fit a nasal cannula than an oxygen mask
- Low level of patient compliance needed (sedation possible but not required)
- Patients say it is very comfortable and allows them to communicate
___
Stable Hypercapnic COPD may benefit from At-Home High-Flow Nasal Cannula Oxygen Therapy: 2018 study:
High-flow nasal cannula oxygen therapy for COPD has been shown to be effective in the acute setting. At-home treatment with high-flow nasal cannula (HFNC) oxygen therapy for 6 weeks in patients with stable hypercapnic chronic obstructive pulmonary disease (COPD) improved health-related quality of life (HRQOL) and reduced hypercapnia according to a study published in the Annals of the American Thoracic Society. However, HFNC/LTOT did not improve the arterial partial pressure of oxygen, dyspnea, spirometry measures, lung volumes, the 6-minute walk test, or physical activity. The most frequently encountered adverse effect of HFNC therapy was nocturnal sweating, which occurred in 20.7% of participants. During the trial, 4 severe adverse events occurred (2 in each group), but these were deemed not to be the result of therapy. Growing evidence indicates that HFNC oxygen therapy is beneficial in the acute setting. These findings suggest that the benefits of HFNC may apply to at-home therapy as well.
______
______
So far I discussed oxygen therapy devices for patients who can breathe well on their own without any assistance. Now I discuss oxygen therapy devices for patients who cannot breathe well without any assistance. These patients are provided positive pressure ventilation through machine.
_
Positive pressure delivery:
Patients who are unable to breathe on their own will require positive pressure to move oxygen into their lungs for gaseous exchange to take place. Systems for delivering this vary in complexity (and cost), starting with a basic pocket mask adjunct which can be used by a basically trained first aider to manually deliver artificial respiration with supplemental oxygen delivered through a port in the mask. Many emergency medical service and first aid personnel, as well as hospitals, will use a bag-valve-mask (BVM), which is a malleable bag attached to a face mask (or invasive airway such as an endotracheal tube or laryngeal mask airway), usually with a reservoir bag attached, which is manually manipulated by the healthcare professional to push oxygen (or air) into the lungs. Automated versions of the BVM system, known as a resuscitator or pneupac can also deliver measured and timed doses of oxygen direct to patient through a facemask or airway. These systems are related to the anaesthetic machines used in operations under general anaesthesia that allows a variable amount of oxygen to be delivered, along with other gases including air, nitrous oxide and inhalational anaesthetics. For patients who are intubated or have a tracheostomy, additional care must be directed toward temperature control, humidification, and infection control. Remember that in these patients you’ve bypassed the upper airway. The function of the nose is to warm, filter, and humidify air.
_
Mechanical ventilation:
Mechanical ventilation is the medical term for artificial ventilation where mechanical means is used to assist or replace spontaneous breathing. Mechanical ventilation is indicated when the patient’s spontaneous ventilation is inadequate to maintain life. It is also indicated as prophylaxis for imminent collapse of other physiologic functions, or ineffective gas exchange in the lungs. Because mechanical ventilation serves only to provide assistance for breathing and does not cure a disease, the patient’s underlying condition should be correctable and should resolve over time. In addition, other factors must be taken into consideration because mechanical ventilation is not without its complications. In general, mechanical ventilation is instituted to correct blood gases and reduce the work of breathing.
_
Ventilatory support can be achieved through a variety of interfaces (mouth piece or nasal, face, or helmet mask), using a variety of ventilatory modes (e.g., volume ventilation, pressure support, bilevel positive airway pressure [BiPAP], proportional-assist ventilation [PAV], continuous positive airway pressure [CPAP]) with either ventilators dedicated to noninvasive ventilation (NIV) or those capable of providing support through an endotracheal tube or mask. Older models of noninvasive ventilators required oxygen to be bled into the system, but current models incorporate oxygen blenders for precise delivery of the fraction of inspired oxygen (FIO2). The ventilator blows gas (air plus oxygen as needed) into a person’s lungs. It can help a person by doing all of the breathing or just assisting the person’s breathing. The ventilator can deliver higher levels of oxygen than delivered by a mask or other devices. The ventilator can also provide what is called positive end expiratory pressure (PEEP). This helps to hold the lungs open so that the air sacs do not collapse. The tube in the windpipe also makes it easier to remove mucus if someone has a weak cough. Noninvasive ventilation (NIV) refers to the administration of ventilatory support without using an invasive artificial airway (endotracheal tube or tracheostomy tube).
_
Positive-pressure ventilation means that airway pressure is applied at the patient’s airway through an endotracheal / tracheostomy tube, or without intubation in NIV. The positive nature of the pressure causes the gas to flow into the lungs until the ventilator breath is terminated. As the airway pressure drops to zero, elastic recoil of the chest accomplishes passive exhalation by pushing the tidal volume out Positive-pressure ventilators work by increasing the patient’s airway pressure through an endotracheal or tracheostomy tube. The positive pressure allows air to flow into the airway until the ventilator breath is terminated. Then, the airway pressure drops to zero, and the elastic recoil of the chest wall and lungs push the tidal volume — the breath-out through passive exhalation.
_
CPAP machine is different than mechanical ventilator:
A continuous positive airway pressure (CPAP) machine was initially used mainly by patients for the treatment of sleep apnea at home, but now is in widespread use across intensive care units as a form of ventilation. Obstructive sleep apnea occurs when the upper airway becomes narrow as the muscles relax naturally during sleep. This reduces oxygen in the blood and causes arousal from sleep. The CPAP machine stops this phenomenon by delivering a stream of compressed air via a hose to a nasal pillow, nose mask or full-face mask, splinting the airway (keeping it open under air pressure) so that unobstructed breathing becomes possible, reducing and/or preventing apneas and hypopneas. It is important to understand, however, that it is the air pressure, and not the movement of the air, that prevents the apneas. The CPAP machine blows air at a prescribed pressure (also called the titrated pressure). The necessary pressure is usually determined by a sleep physician after review of a study supervised by a sleep technician during an overnight study (polysomnography) in a sleep laboratory. The titrated pressure is the pressure of air at which most (if not all) apneas and hypopneas have been prevented, and it is usually measured in centimetres of water (cm H2O). The pressure required by most patients with sleep apnea ranges between 6 and 14 cm H2O. A typical CPAP machine can deliver pressures between 4 and 20 cm H2O. More specialized units can deliver pressures up to 25 or 30 cm H2O. CPAP treatment can be highly effective in treatment of obstructive sleep apnea.
___
Devices for Patients Requiring Ventilation Assistance:
| Name | Invasive? | Indications and Management |
| CPAP | no | CPAP (Continuous Positive Pressure Ventilation) pushes air continuously into the airway to keep the airways expanded during inhalation and exhalation, enhancing the quality of the patient’s breaths. Often used to treat sleep apnea (the pressure prevents the airway obstruction responsible for most sleep apnea). It’s also used in emergencies when a patient needs assisted ventilation but an invasive airway hasn’t been established yet. |
| BiPAP | no | Similar to CPAP, BiPAP (Bilevel Positive Airway Pressure) pushes air into the airway but allows for a lower pressure during exhalation. |
| Tracheostomy (with mask or collar) | yes | Fits loosely over a tracheostomy to deliver oxygen; Tracheostomies are surgically established artificial airways involving a hole cut into the trachea through the neck to deliver oxygen. These devices will require humidification since mouth and nose are bypassed. |
| t-tube/Briggs adaptor | yes | Attaches directly to endotracheal tube (ET tube) or tracheostomy to deliver oxygen & ‘breathe for the patient’; An ET tube is a non-surgical airway involving a tube is inserted through the mouth (or nose, rarely) to establish an artificial airway. These will require humidification since mouth and nose are bypassed |
_
Ventilatory support indicated
- In patients with hypercapnic respiratory failure, in whom an ABG measurement shows a pH of <7.35 and PaCO2 of >45 mm Hg, non-invasive ventilation (NIV) or invasive ventilation should be considered. COPD patients with a pH of <7.26 managed with NIV require more intensive monitoring with a low threshold for intubation.
- In patients in whom oxygen-induced hypercapnia is suspected, oxygen therapy should be titrated to maintain the 88–92% target oxygen saturation range and not be abruptly stopped due to the risk of profound rebound hypoxemia.
- In patients with severe cardiogenic pulmonary oedema continuous positive airway pressure should be considered.
- NIV is not routinely recommended in acute hypoxemic respiratory failure, as results from clinical trials and observational studies have provided mixed results for various patient groups; however, there is some evidence of benefit in certain patients with immunosuppression.
- It is recommended that patients receiving ventilatory support are located in a ward area such as ICU, a close observation unit or monitored bed unit, where there are adequate numbers of staff experienced in ventilatory support to provide an appropriate level of monitoring and titration of therapy.
_____
Oxygen provided by nasal cannula forms a reservoir in the nasopharynx that is entrained during inspiration. The concentration of oxygen in the trachea can be increased progressively by creating a larger reservoir for oxygen outside the nasopharynx. Examples include venturi masks and “nonbreather” masks, which are equipped with an expiratory valve to prevent CO2 rebreathing. With any reservoir system, the tracheal FiO2 decreases with higher minute ventilations and higher inspiratory flow rates. This can be overcome partially by increasing the flow rate of the inspired oxygen. For example, tight-fitting external masks with high flow rates can deliver an FiO2 near 1.0 to the trachea and can be combined with PEEP valves to provide continuous positive airway pressure. Some newer systems for nasal cannula oxygen delivery use very high flow rates, which actively replenish the nasopharyngeal reservoir during inspiration and generate modest continuous positive airway pressure. Conventional mechanical ventilators use centralized oxygen supplies, which have no flow rate limitation, allowing for precise control of the inspired FiO2. In contrast, many systems for noninvasive ventilation rely on the entrainment of oxygen into the ventilator tubing and oropharyngeal mask, resulting in limitations to oxygen delivery similar to those of the nasal cannula or simple mask delivery systems. This is typically not a problem because noninvasive ventilation has a high failure rate in patients with acute hypoxemic respiratory failure due to noncardiogenic causes, and is used infrequently. However, in selected patients, higher oxygen concentrations can be achieved during noninvasive ventilation using specifically designed noninvasive ventilators or standard mechanical ventilators with tubing adapted to fit noninvasive masks.
_____
Is positive pressure ventilation (PPV) similar to hyperbaric oxygen?
No.
Normal atmospheric pressure is 760 mm Hg and hyperbaric oxygen is at three times atmospheric pressure i.e. at 2280 mm Hg.
Positive pressure ventilation require insufflations pressures of 15, 20, 25, and 30 cm H2O (depending on underlying conditions) to produce normal tidal volumes. Maximum being 30 cm H2O.
1 cm H2O = 0.73 mm Hg
30 cm H2O = 22 mm Hg
So total pressure exerted on airways and alveoli is 760 + 22 = 782mm Hg nowhere near 2280 mm Hg. Even if you give 100 % oxygen through positive pressure ventilation, maximum PAO2 will be 663 +22 = 685 mm Hg while 100 % oxygen in hyperbaric chamber at 3 Atm pressure will generate PAO2 of 2183 mm Hg. PPV can never be hyperbaric. Use alveolar gas equation for these calculations.
Controlled trials were made of different methods of resuscitation in young rabbits, subjected to asphyxia or anoxia to beyond the last gasp. Hyperbaric O2 was significantly less effective than positive pressure ventilation with O2. Positive pressure ventilation with air was as effective as with O2.
_____
_____
Synopsis of oxygen therapy is depicted in the figure below:
____
____
Treatment algorithm for oxygen therapy
____
____
Humidification of oxygen:
Oxygen therapy can be delivered using a low flow or high flow system. When oxygen is delivered at a flow rate of 14 l/min by mask or nasal prongs the oropharynx or nasopharynx provides adequate humidification. All high flow systems require humidification. At higher flow rates or when oxygen is delivered directly to the trachea humidification is necessary. The type of humidification device selected will depend on the oxygen delivery system in use, and the patient’s requirements. The humidifier should always be placed at a level below the patient’s head.
Rationale:
-Cold, dry air increases heat and fluid loss
-Medical gases, including air and oxygen, have a drying effect on mucous membranes resulting in airway damage.
-Secretions can become thick & difficult to clear or cause airway obstruction
-In some conditions e.g. asthma, the hyperventilation of dry gases can compound bronchoconstriction.
Oxygen can be drying to the mucous membranes of the upper airway (Pilkington 2004). The National heart, lung and blood institute (2012) reports how oxygen therapy can cause dryness, bloody nose, skin irritation and mucus dryness. Dryness of nostrils and mouth can be prevented through good oral hygiene, application of E45 cream and adequate fluid intake. Never use petroleum jelly near oxygen, however, because of its potentially flammable nature (NHS 2011). Oxygen administered for more than a short period can be humidified, particularly if the concentration administered is high, for example, over 35 per cent, or at a rate of 4 L per minute or above (O’Driscoll et al, 2008).
___
Patient Monitoring:
Things to monitor in patients receiving oxygen therapy include:
- make sure oxygen delivery and monitoring equipment are intact and working properly
- monitoring SpO2 (make sure alarms are turned on if available to notify of change in patient condition) and performing blood gases as ordered
- make sure mucous membranes are moist and intact (supplemental oxygen can dry membranes out; humidification can be added if needed)
- checking all vital signs; changes in SpO2 and respiratory rate as well as in heart rate and blood pressure can indicate decline in respiratory status
- physical assessment: look especially for changes in skin color, swelling in legs or abdomen, abnormal lung or heart sounds
- patient subjective assessment of their own breathing
- In patient with acute respiratory failure, close monitoring is essential and arterial blood gases taken on presentation should be repeated within 20 min to establish that treatment has achieved acceptable PAO2 levels of 7kPa(55mmHg) or O2 saturation of 88%.
___
Weaning of oxygen:
There is no purpose of continuing oxygen after its requirement has ceased. The thought of weaning should start the moment the patient is comfortable. There are several clinical and laboratory parameters which need to be continuously assessed. Symptoms and signs of hypoxemia and tissue hypoxia should be carefully monitored. Weaning should be initiated once the patient’s underlying disease process is stabilised and bedside evaluation of respiratory rate, heart rate, blood pressure, skin colour and pulse oximetry are normal. Weaning can be gradually attempted by either discontinuing oxygen altogether or lowering its concentration for a fixed period and re-evaluating the clinical parameters and SpO2. An initial attempt of withdrawal for about 30 minutes is followed by longer periods. If there is no deterioration, oxygen may be completely withdrawn. In patients with underlying chronic respiratory diseases, oxygen may be required at lower concentrations for longer periods. Prospects of long term domiciliary oxygen need to be discussed with the patient after the acute need is over.
Warning:
Never try to get off of oxygen-assisted breathing without asking a doctor beforehand. Some people are not physically able to breathe on their own and removing the oxygen can be fatal.
___
___
Oxygen administration guidelines:
Why guidelines?
Oxygen is the most commonly used drug in emergency medicine. 34% of ambulance patients receive oxygen during transit and 15–17% of hospital inpatients will be receiving oxygen at any given time. Yet prior to 2008, there was no national or international guidance available for the safe use of oxygen. There are common misconceptions regarding the safe use of oxygen and many people are unaware of the dangers of hyperoxemia. It is widely believed that that supplemental oxygen relieves dyspnoea in the absence of hypoxemia (low arterial oxygen levels). No evidence of benefit exists for administering oxygen in patients who are normoxemic (normal arterial oxygen levels) or very mildly hypoxemic. Dyspnoea can occur for many reasons other than cardiorespiratory disease, including metabolic acidosis, anxiety and pain, and treatment with oxygen is not indicated in these cases. Another common misconception is that one “can’t give too much oxygen” and there is general lack of appreciation for the dangers of “hyperoxemia” (higher than normal arterial oxygen levels). Historically, high levels of oxygen were given to all patients with dyspnoea and critical illness. It is well established that severe hypoxemia results in rapid organ failure and death. Oxygen saves lives when used appropriately to correct hypoxemia and is an essential component in resuscitation of the critically ill; however, there is little evidence that supra-physiological levels of oxygen have a clinical benefit in most instances. Evidence does exist, however, that inappropriate use of oxygen can be detrimental.
_
Hyperoxemia can cause coronary vasoconstriction. Paradoxically, therefore, giving too much oxygen at the time of an acute infarction may worsen oxygen delivery to the cardiac muscle. Use of high-flow oxygen has been associated with increased reperfusion injury, infarct size and mortality in myocardial infarction. Theoretically, hyperoxemia may have similar effects on cerebral blood flow. One randomised controlled trial found that in minor or moderate stroke, oxygen administration was linked to increased mortality when compared with air. High-flow oxygen is commonly used in intensive therapy units (ITU) and hyperoxemia is common in these wards. Studies in critical care have shown that in cardiac arrest survivors and in patients receiving ITU care, hyperoxemia is linked to worse outcomes than normoxemia.
_
In the ward setting, patients using high-flow oxygen without a target saturation range may have high oxygen saturation (>98%), which can be falsely reassuring to staff. The ability of pulse oximetry to detect clinical deterioration is masked by the high oxygen saturation and patients may become severely hypoxic before the staff are alerted to the deterioration in gas exchange. By contrast, if oxygen administration was titrated against patient need, aiming to achieve a normal oxygen saturation target range, pulse oximetry should allow early detection of increasing oxygen requirements.
_
Inappropriate oxygen use in patients at risk of type 2 respiratory failure (T2RF) can result in life-threatening hypercapnia (higher than normal levels of carbon dioxide in arterial blood), respiratory acidosis, organ dysfunction, coma and death. Vulnerable groups include not only chronic obstructive pulmonary disease (COPD), where high concentrations of inspired oxygen are linked with increased mortality during acute exacerbation, but also severe asthma, cystic fibrosis, bronchiectasis, chest wall disorders, neuromuscular disease and obesity hypoventilation . All at-risk patients need to be identified when prescribing and administering oxygen.
_
In 2010, the UK National Patient Safety Agency (NPSA) reported nine deaths directly attributable to oxygen therapy over a 5-year period. The four reported deaths due to over-oxygenation are thought to be a gross underestimation as previous work in COPD has estimated that, in the UK, a few thousand deaths could be avoided each year by controlled oxygen use. The NPSA relies on clinicians reporting adverse events.
_
Oxygen prescribing is poor:
Despite being a drug, oxygen is often not prescribed appropriately, signed for on drug charts or regularly reviewed. The 2008 national oxygen audit (carried out prior to the publication of the emergency oxygen guideline) showed that, in UK hospitals, less than one-third of patients receiving oxygen had any written prescription, in only 10% of cases was consideration given to a target saturation range and in only 5% of cases was oxygen signed for to indicate it had been administered.
_______
The British Thoracic Society (BTS) Emergency Oxygen Guideline, published in 2008, addresses many of the issues surrounding the use and prescribing of oxygen, specifically regarding target saturation ranges. The recommendations aim to guide clinicians, encouraging levels of oxygenation that are appropriate for each patient, based on a combination of what is believed to be safe and normal or near-normal.
_
BTS guideline for oxygen use in adults in healthcare and emergency settings:
Philosophy of the guideline
- Oxygen is a treatment for hypoxemia, not breathlessness. Oxygen has not been proven to have any consistent effect on the sensation of breathlessness in non-hypoxemic patients.
- The essence of this guideline can be summarised simply as a requirement for oxygen to be prescribed according to a target saturation range and for those who administer oxygen therapy to monitor the patient and keep within the target saturation range.
- The guideline recommends aiming to achieve normal or near-normal oxygen saturation for all acutely ill patients apart from those at risk of hypercapnic respiratory failure or those receiving terminal palliative care.
_
- Assessing patients
-For critically ill patients, high-concentration oxygen should be administered immediately and this should be recorded afterwards in the patient’s health record.
-Clinicians must bear in mind that supplemental oxygen is given to improve oxygenation but it does not treat the underlying causes of hypoxemia which must be diagnosed and treated as a matter of urgency.
-The oxygen saturation should be checked by pulse oximetry in all breathless and acutely ill patients, ‘the fifth vital sign’ (supplemented by blood gases when necessary) and the inspired oxygen concentration should be recorded on the observation chart with the oximetry result. (The other vital signs are pulse rate, blood pressure, temperature and respiratory rate).
-Pulse oximetry must be available in all locations where emergency oxygen is used. Clinical assessment is recommended if the saturation falls by ≥3% or below the target range for the patient.
-All critically ill patients outside of a critical care area should be assessed and monitored using a recognised physiological track and trigger system such as the National Early Warning Score (NEWS).
_
- Target oxygen prescription
-Oxygen should be prescribed to achieve a target saturation of 94–98% for most acutely ill patients or 88–92% or patient-specific target range for those at risk of hypercapnic respiratory failure.
-Best practice is to prescribe a target range for all hospital patients at the time of admission so that appropriate oxygen therapy can be started in the event of unexpected clinical deterioration with hypoxemia and also to ensure that the oximetry section of the early warning score (EWS) can be scored appropriately.
-The target saturation should be written (or ringed) on the drug chart or entered in an electronic prescribing system.
_
3 Oxygen administration
-Oxygen should be administered by staff who are trained in oxygen administration.
-These staff should use appropriate devices and flow rates in order to achieve the target saturation range.
-Staff should be trained in the use of a range of different oxygen delivery devices to ensure oxygen is delivered safely.
_
4 Monitoring and maintenance of target saturation
-Oxygen saturation and delivery system (including flow rate) should be recorded on the patient’s monitoring chart.
-Oxygen delivery devices and flow rates should be adjusted to keep the oxygen saturation in the target range.
-Prompt clinical assessment is required if oxygen therapy needs to be initiated or increased due to a falling saturation level.
-Oxygen should be prescribed and a signature should be entered on the drug chart on each drug round.
_
5 Weaning and discontinuation of oxygen therapy
-Oxygen should be reduced in stable patients with satisfactory oxygen saturation.
-Oxygen should be discontinued once the patient can maintain saturation within or above the target range breathing air but the prescription for a target range should be left in place in case of future deterioration and to guide EWS.
_______
Clear your concepts about oxygen therapy:
- Oxygen should be considered as a drug that is prescribed and administered for specific indications, with a documented target oxygen saturation range and with regular monitoring of the patient’s response.
- Oxygen is prescribed for the relief of hypoxemia, not breathlessness.
- Hypoxemia is both a marker of risk of a poor outcome due to the severity of the underlying disease(s) that has caused hypoxemia, and an independent risk factor of poor outcome.
- There are risks associated with both hypoxemia and hyperoxemia, which underlie the importance of prescribing oxygen only if required, and to within a target oxygen saturation range.
- The ‘swimming between the flags’ concept of titrating oxygen therapy, to within a specific target oxygen saturation range applies to a wide range of clinical situations, in addition to exacerbations of chronic obstructive pulmonary disease (COPD).
- The variable accuracy of pulse oximetry in the estimation of arterial oxygen saturation (SaO2) represents the major limitation in its use to guide the titration of oxygen therapy.
- The use of high concentration oxygen in a breathless patient in an attempt to protect against hypoxemia in the event of a subsequent deterioration has the potential to cause delay in recognizing clinical deterioration and reduce the time available to initiate additional treatment.
- If a patient who requires a high fraction of inspired oxygen (FiO2) to maintain adequate oxygen saturations deteriorates, there is limited opportunity to increase FiO2 to avoid life-threatening hypoxemia. For this reason, patients who need high FiO2 should receive senior clinician review and transfer to an area where there are appropriate numbers of competent staff able to provide more intensive monitoring and therapy.
- Supplemental oxygen relieves hypoxemia but does not improve ventilation or treat the underlying cause of the hypoxemia. Monitoring of SpO2 indicates oxygenation not ventilation. Therefore, beware the use of high FiO2 in the presence of reduced minute ventilation.
- Many children in the recovery phase of acute respiratory illnesses are characterised by ventilation/perfusion mismatch (e.g. asthma, bronchiolitis, and pneumonia) and can be managed with SpO2 in the low 90’s as long as they are clinically improving, feeding well and don’t have obvious respiratory distress.
- Normal SpO2 values may be found despite rising blood carbon dioxide levels (hypercapnia). High oxygen concentrations have the potential to mask signs and symptoms of hypercapnia.
- Children with cyanotic congenital heart disease normally have SpO2 between 60%- 90% in room air. Increasing SpO2 > 90% with supplemental oxygen is not recommended due to risk of over circulation to the pulmonary system while adversely decreasing systemic circulation. However, in emergency situations with increasing cyanosis supplemental oxygen should be administered to maintain their normal level of SpO2.
____
____
Home oxygen therapy:
Do you qualify for home oxygen therapy?
The qualifications for home oxygen are set by the Centers for Medicare and Medicaid Services (CMS), and they prefer numbers over subjective signs and symptoms. So qualifying is determined by the following test results:
- Arterial Blood Gas: Your PaO2 is at or below 55 mmHg
- Pulse Oximetry: Your SpO2 is at or below 88 percent
The basic premise goes like this: you qualify for oxygen therapy if your:
- PaO2 is 55 or less or your SpO2 is 88 percent or less at rest while breathing room air.
- PaO2 is 55 or less or your SpO2 is 88 percent or less while you are sleeping; or, if your PO2 drops 10 percent or more, or your SpO2 drops 5 percent or more, while you are sleeping, and you are also displaying symptoms of hypoxemia.
- PaO2 is 55 or less or your SpO2 is 88 percent or less while you are exercising.
How much oxygen do you need?
The goal of oxygen therapy is to provide you with the least amount of supplemental oxygen to maintain an SpO2 at 90 percent or greater. Usually, a low flow of 2-3 l/m using a nasal cannula works great. And, considering the benefits, most people tolerate it very well.
_
The types of home oxygen therapy (vide supra) are:
▸ long-term oxygen therapy (LTOT)
▸ nocturnal oxygen therapy (NOT)
▸ ambulatory oxygen therapy (AOT)
▸ palliative oxygen therapy (POT)
▸ short burst oxygen therapy (SBOT).
LTOT is provided in the home via oxygen concentrator, liquid oxygen or oxygen cylinders. Current technology and reimbursement practices have changed the availability and provision of home LTOT. The choice of which system is best for each patient is determined by the oxygen requirements, liter flow and duration, the available systems and insurance reimbursement.
_
Patient education:
Education should cover diagnosis, use of ambulatory oxygen therapy, principles of treatment, maintenance of portable equipment, servicing arrangements and electricity reimbursement, use of nasal cannulae or masks, requirement for humidifier, contact telephone number and advice on travel. Further education is provided by the engineer at the time of delivery. A family member or carer should attend the education sessions. The patient should be made aware of the dangers of smoking and fire risk. People should know that there are a few side-effects of oxygen therapy. These include a dry or bloody nose, skin irritation from the face mask or nasal prongs, tiredness and morning headaches. If these happen, they should be encouraged to let their clinician know, as they may be able to change the prescription to ease the patient’s problems.
____
____
Oxygen safety:
Oxygen is not a flammable gas but it does support combustion (rapid burning). Due to this the following rules should be followed:
- Do not smoke in the vicinity of oxygen equipment.
- Do not use aerosol sprays in the same room as the oxygen equipment.
- Turn off oxygen immediately when not in use. Oxygen is heavier than air and will pool in fabric making the material more flammable. Therefore, never leave the nasal prongs or mask under or on bed coverings or cushions whilst the oxygen is being supplied.
- Oxygen cylinders should be secured safely to avoid injury.
- Do not store oxygen cylinders in hot places.
- Keep the oxygen equipment out of reach of children.
- Do not use any petroleum products or petroleum by-products e.g. petroleum jelly/Vaseline whilst using oxygen.
- When using oxygen cylinders, store them upright, chained, or in appropriate holders so that they will not fall over.
- Keep oxygen delivery systems at least 2 metres from any heat source.
- Determine that electrical equipment in the room or home is in safe working condition. A small electrical spark in the presence of oxygen will result in a serious fire. The use of a gas stove, kerosene space heater, or smoker is unsafe in the presence of oxygen. Avoid items that may create a spark (e.g., electrical razor, hair dryer, synthetic fabrics that cause static electricity, or mechanical toys) with nasal cannula in use.
- Do not defibrillate someone when oxygen is free-flowing.
- Flash fires ignited by electrocautery and oxygen flow from nasal cannula during facial surgery under local anaesthesia have been described, and Reyes et al. used a facial flash fire model to show how nasal cannula tubing can be ignited by an electrocautery spark at an oxygen flow rate of 2 litres per min and at a linear distance of 5 cm from the oxygen source.
_
Fire risk:
Highly concentrated sources of oxygen promote rapid combustion. Oxygen itself is not flammable, but the addition of concentrated oxygen to a fire greatly increases its intensity, and can aid the combustion of materials (such as metals) which are relatively inert under normal conditions. Fire and explosion hazards exist when concentrated oxidants and fuels are brought into close proximity; however, an ignition event, such as heat or a spark, is needed to trigger combustion. A well-known example of an accidental fire accelerated by pure oxygen occurred in the Apollo 1 spacecraft in January 1967 during a ground test; it killed all three astronauts. Concentrated O2 will allow combustion to proceed rapidly and energetically. Steel pipes and storage vessels used to store and transmit both gaseous and liquid oxygen will act as a fuel; and therefore the design and manufacture of O2 systems requires special training to ensure that ignition sources are minimized. Highly concentrated oxygen in a high-pressure environment can spontaneously ignite hydrocarbons such as oil and grease, resulting in fire or explosion. The heat caused by rapid pressurization serves as the ignition source. For this reason, storage vessels, regulators, piping and any other equipment used with highly concentrated oxygen must be “oxygen-clean” prior to use, to ensure the absence of potential fuels. This does not apply only to pure oxygen; any concentration significantly higher than atmospheric (approximately 21%) carries a potential risk. Hospitals in some jurisdictions, such as the UK, now operate “no-smoking” policies, which although introduced for other reasons, support the aim of keeping ignition sources away from medical piped oxygen. Recorded sources of ignition of medically prescribed oxygen include candles, aromatherapy, medical equipment, cooking, and unfortunately, deliberate vandalism. Smoking of pipes, cigars and cigarettes is of special concern. These policies do not entirely eliminate the risk of injury with portable oxygen systems, especially if compliance is poor.
_
In 2003-2006, hospital emergency rooms saw an estimated average of 1,190 thermal burns per year caused by ignitions associated with home medical oxygen.
-Eighty-nine percent of the victims suffered facial burns.
-In most cases, the fire department was not involved.
_
During 2002-2005, oxygen administration equipment was involved in an estimated average of 182 home fires reported to local fire departments per year.
-These fires caused an average of 46 civilian deaths and 60 civilian injuries per year.
-One of every four such fires resulted in death.
-Smoking is by far the leading cause of burns, reported fires, deaths, and injuries involving home medical oxygen.
-Cooking and candles were other common factors.
_
Fire Safety Tips for Home Medical Oxygen Users:
The use of home oxygen systems has increased over the past decade. It’s important for people to practice fire safe behaviors when oxygen is in use. Oxygen itself does not burn but a fire needs oxygen to start and to keep burning. When more oxygen is in the air, the fire will burn hotter and faster. Smoking should not be allowed in a home where oxygen is used. Even if oxygen is not being used, it may have saturated the home including clothing, curtains, furniture, bedding, hair, and anything in the area.
Safety Tips
- Never smoke in a home where oxygen is used.
- Post “no smoking” signs in and outside of the home to remind residents and guests not to smoke.
- If oxygen is used in the home, the amount of oxygen in the air, furniture, clothing, hair, and bedding goes up, making it easier for a fire to start and spread. This means that there is a higher risk of both fires and burns.
- Never use an open flame, such as candles, matches, wood stoves, and sparking toys, when oxygen is in use.
- People who may have difficulty escaping a fire should have a phone near their bed or chair.
- Make sure that the home has smoke alarms. Test them at least monthly.
- Have a home fire escape plan with two ways out of every room and an outside meeting place.
- Practice the plan at least twice a year.
_
Most patients on home oxygen use nasal cannulae. Nasal cannula tubing is a polyvinyl chloride product which, when ignited, emits an intense flame, possibly owing to the release of highly flammable vinyl chloride gas. The prongs of a cannula are intended to direct oxygen into the nose. Greco et al. showed, however, that a significant amount of oxygen exits the nose and constantly leaks out and bathes the lower face. An oxygen-enriched environment facilitates ignition and combustion of any material. The use of a less combustible material for cannula tubing and a more efficient oxygen delivery system may reduce the incidence of such burns.
____
____
Hyperbaric oxygen (HBO) therapy:
Hyperbaric oxygen therapy involves breathing pure oxygen in a pressurized room or tube. Hyperbaric oxygen therapy is inhalation of oxygen at higher than normal atmospheric pressure with or without increased oxygen concentrations. Usually 100 percent oxygen is given at two to three times the atmospheric pressure resulting in arterial oxygen tension in excess of 2000 mm Hg and oxygen tension in tissue of almost 400 mm Hg. Such doses of oxygen have a number of beneficial biochemical, cellular, and physiologic effects. Since dissolved oxygen in blood is 0.003 ml per mm of oxygen tension per 100 ml of blood, >2000 mm PaO2 will result in dissolved oxygen of 6 ml per 100 ml blood i.e. 60 ml/liter of blood. Since cardiac output is 5 liter/min, HBO will provide 60 X 5 = 300 ml/min of dissolved oxygen to whole body which is more than total resting tissue requirement of oxygen 250 ml/min. So it meets the average requirements of resting tissues by means of dissolved oxygen alone without contribution from oxygen bound to hemoglobin. Remember dissolved oxygen is available to tissues first and then oxygen bound to hemoglobin. Remember when 100 % normobaric oxygen is given, PaO2 becomes 600 mm and dissolved oxygen 18 ml per liter of blood, it will provide only 90 ml dissolved oxygen per min to whole body, quite insufficient to provide resting tissue oxygen requirement. The ability of HBO to augment oxygen content and independently meet resting tissue oxygen requirements has led to its use in conditions of compromised oxygen delivery, such as profound anemia, carbon monoxide (CO) poisoning, and both acute and chronic ischemia. The value of hyperbaric oxygen therapy in decompression illness and arterial gas embolism depends on the physical properties of gases. The volume of a gas in an enclosed space is inversely proportional to the pressure exerted on it (Boyle’s law). At 300 kPa gas bubble volume is reduced by about two thirds. Dissolution of the gas bubble is enhanced by replacing the inert gas nitrogen in the bubble with oxygen, which is then rapidly metabolised by the tissues.
_
A typical treatment protocol for patients in the hyperbaric chamber involves the patient diving to three atmospheres, once or twice a day for approximately 11/2 to 2 hours. To benefit from hyperbaric oxygen therapy, you’ll likely need more than one session. The number of sessions depends on your medical condition. Some conditions, such as carbon monoxide poisoning, might be treated in three visits. Others, such as nonhealing wounds, may require 20 to 40 treatments. There are two types of hyperbaric oxygen chambers. Multiplace chambers are walk-in rooms capable of diving three to six atmospheres above sea level with many people inside. The monoplace chamber is more common, is capable of holding one patient. The monoplace chamber can dive to approximately 3 atmospheres above sea level, it must use 100% oxygen to be therapeutic. A monoplace hyperbaric oxygen treatment that begins under ambient air conditions requires a high oxygen flow in order to eliminate the presence of nitrogen within the chamber. Oxygen flows through the chamber at a rate of up to 500 liters/min. Once the nitrogen has been purged from the chamber and the internal oxygen concentration has exceeded 95%, high flows are no longer needed to maintain the patient’s hyperoxygenation level. Once you are oriented and your vitals are taken, the hyperbaric technician will position you on the gurney and place you inside the chamber. The pressure will then be gradually increased, and you may feel fullness inside your ears. The technician will instruct you about how to clear any discomfort during this 10-15 minute phase. Once the chamber reaches the desired treatment pressure, you may watch movies or relax and take a nap. When your treatment ends, the pressure inside the chamber will be gradually decreased to normal atmospheric pressure. During this time, the chamber may get a little cooler than normal. You may feel fullness in your ears again from the reduction of pressure in the chamber – this is similar to what you feel when you are driving over the mountains or flying. The technician will ensure that you are comfortable at all times by providing you with instructions during the entire treatment.
_
Cellular and biochemical benefits of hyperbaric oxygen:
- Promotes angiogenesis and wound healing
- Kills certain anaerobes
- Prevents growth of species such as Pseudomonas
- Prevents production of clostridial alpha toxin
- Restores neutrophil mediated bacterial killing in previously hypoxic tissues
- Reduces leucocyte adhesion in reperfusion injury, preventing release of proteases and free radicals which cause vasoconstriction and cellular damage
_
Under normal circumstances, oxygen is transported throughout the body only by red blood cells. With HBOT, oxygen is dissolved into all of the body’s fluids, the plasma, the central nervous system fluids, the lymph, and the bone and can be carried to areas where circulation is diminished or blocked. In this way, extra oxygen can reach all of the damaged tissues and the body can support its own healing process. The increased oxygen greatly enhances the ability of white blood cells to kill bacteria, reduces swelling and allows new blood vessels to grow more rapidly into the affected areas. It is a simple, non-invasive and painless treatment.
_
Therapeutic uses of hyperbaric oxygen:
Strong scientific evidence:
Main treatment
- Decompression sickness
- Arterial gas embolism
- Severe carbon monoxide poisoning and smoke inhalation
Adjunctive treatment
- Prevention and treatment of osteoradionecrosis
- Improved skin graft and flap healing
- Clostridial myonecrosis (gas gangrene)
Suggestive scientific evidence:
Adjunctive treatment
- Refractory osteomyelitis
- Radiation induced injury
- Acute traumatic ischaemic injury
- Prolonged failure of wound healing
- Exceptional anaemia from blood loss
_
Therapeutic uses in detail:
- Decompression sickness (DCS) and arterial gas embolism (AGE):
One atmosphere (101 kPa or 14.7 psi) is also the pressure caused by the weight of a column of fresh water of approximately 10.3 m (33.8 ft). Thus, a diver 10.3 m underwater experiences a pressure of about 2 atmospheres (1 atm of air plus 1 atm of water). When scuba diving, additional oxygen and nitrogen dissolve in body tissues. The additional oxygen is consumed by the tissues, but the excess nitrogen must be washed out by the blood during decompression. During or after ascent this excess nitrogen gas can form bubbles in the tissues, analogous to the carbon dioxide bubbles that form when a carbonated beverage container is opened. These bubbles may then cause symptoms that are referred to as decompression sickness. Trapping of gas within the lungs during ascent, either because the lung is diseased or because of breath-holding, can cause bubbles to be forced into the bloodstream i.e. arterial gas embolism or AGE, where they can block the flow of blood or damage the lining of blood vessels supplying critical organs such as the brain. AGE can also occur in non-divers, due to entry of air into the body, such as during medical diagnostic or therapeutic procedures. Symptoms of DCS or AGE can include joint pain, numbness, tingling, skin rash, extreme fatigue, weakness of arms or legs, dizziness, loss of hearing, and in serious cases, complete paralysis or unconsciousness. Emergency treatment of DCS or AGE includes administration of oxygen and measures to maintain adequate blood pressure, such as lying the patient down and fluid (either oral or intravenous, depending upon availability and severity of the illness). Definitive treatment for DCS or AGE is administration of 100% oxygen at increased atmospheric pressure in a hyperbaric chamber (typically at a pressure 2-3 times greater than normal atmospheric pressure). While some delay in transporting a patient to a hyperbaric chamber is usually unavoidable, the success in relieving symptoms is greater if the treatment is administered within a few hours after the onset of symptoms. Some improvement might be expected, particularly in mild cases, even after a day or more of delay. The vast majority of cases respond satisfactorily to a single hyperbaric oxygen treatment. Sometimes, repetitive treatments are recommended until no further improvement can be observed. A small minority of divers with severe neurological injury may require 15-20 repetitive treatments. The success of hyperbaric oxygen treatment for DCS or AGE has borne the test of time, and continues to be the standard of care for the treatment of these disorders. Divers who surface too quickly are at risk of decompression sickness (DCS), sometimes called “the bends,” or of an air gas embolism (AGE). Jointly, these are known as decompression illness (DCI), and they both relate to problems with air in the body. Consequences can be severe. HBOT is the primary treatment for both. When divers surface too rapidly the partial pressure of nitrogen dissolved in the tissues may exceed the ambient atmospheric pressure sufficiently to form gas bubbles in the blood and the tissues. Although less common, rapid ascent to over 5500 m can result in high altitude decompression sickness.
- Carbon monoxide poisoning:
Carbon monoxide poisoning is an important cause of death from poisoning, particularly in the United States. Carbon monoxide binds to haemoglobin with an affinity 240 times that of oxygen. This reduces the oxygen carrying capacity of the blood. In addition, carbon monoxide binds to the large pool of myoglobin increasing tissue hypoxia. Hyperbaric oxygen provides an alternative source of tissue oxygenation through oxygen dissolved in the plasma. It also facilitates dissociation of carbon monoxide from the haemoglobin and myoglobin; the carboxyhaemoglobin half-life is 240-320 min breathing air, 80-100 min breathing 100% oxygen, and about 20 min with hyperbaric oxygen. In addition, hyperbaric oxygen dissociates carbon monoxide from cytochrome oxidase, improving electron transport and cellular energy state. If carbon monoxide poisoning results in unconsciousness, convulsions, neurological impairment (including abnormal gait or mental state test results) or severe metabolic acidosis the case should be discussed with the nearest regional centre. A single session of hyperbaric oxygen therapy will usually reverse the acute, potentially life threatening effects of carbon monoxide poisoning, but additional treatments may be needed to reduce the delayed neuropsychological sequelae. Patients with less severe poisoning should be treated with 100% oxygen.
- Necrotising infections and osteomyelitis:
The primary treatment of myonecrosis and gas gangrene of soft tissues resulting from clostridial infection and alpha toxin production is surgical debridement and antibiotics. However, experimental evidence and clinical experience suggest that adjunctive treatment with hyperbaric oxygen improves systemic illness and decreases tissue loss by demarcating the border between devitalised and healthy tissue. This reduces the extent of surgical amputation or debridement. Controlled trials of hyperbaric oxygen and normobaric 100% oxygen are not available. In necrotising fasciitis (rapidly progressive skin infection without muscle disease) retrospective studies suggest that hyperbaric oxygen is beneficial in combination with surgical debridement but prospective controlled trials are lacking. Hyperbaric oxygen is also claimed to be helpful in refractory osteomyelitis. Animal experiments show improved healing of osteomyelitis compared with no treatment, but the effect is no better than that with antibiotics alone and the two treatments have no synergistic effect. Uncontrolled trials of surgery and antibiotics combined with hyperbaric oxygen in refractory osteomyelitis have reported success rates of as high as 85%, but controlled trials are needed.
- Post radiation damage:
Soft tissue radionecrosis and osteonecrosis after surgery on irradiated mandibles are reduced by hyperbaric oxygen. In a controlled study comparing osteoradionecrosis at six months postoperatively, the incidence was 5% in patients receiving 30 preoperative hyperbaric oxygen treatments compared with 30% in patients who received only preoperative antibiotics. A similar improvement in wound healing after surgery has been shown in patients with irradiated tissue who receive preoperative hyperbaric oxygen therapy. Normobaric 100% oxygen does not seem to confer the same benefits. The higher partial pressures achieved with hyperbaric oxygen may stimulate new vessel growth and healing in damaged irradiated tissue which has lost the capacity for restorative cellular proliferation. To prevent mandibular osteonecrosis after surgery on irradiated facial and neck tissue 30 preoperative 90 minute sessions and 10 postoperative sessions are recommended
- Skin grafts, flaps, and wound healing:
In poorly vascularised tissue hyperbaric oxygen improves both graft and flap survival compared with routine postoperative surgical care alone. The effect of normobaric 100% oxygen was not examined in these studies. In the United States problem wounds are the commonest indication for a trial of adjunctive hyperbaric oxygen therapy and include diabetic and other small vessel ischaemic foot ulcers. Several studies have shown improved healing and a lower incidence of amputation with 4-30 sessions. Hyperbaric oxygen should be considered for problem wounds if the facility is readily available.
Other indications:
Hyperbaric oxygen has been used successfully to treat haemorrhagic shock in patients who refuse blood on religious grounds or for whom suitable blood was not available. Similarly, there is evidence for benefit in acute traumatic ischaemic injuries including compartmental syndromes and crush injuries.
_
Conditions which do not benefit:
Hyperbaric oxygen has been tried in numerous conditions and is often reported to be beneficial. However, in many of these situations the scientific evidence is flimsy and use should be restricted to randomised controlled trials. Hyperbaric oxygen has been clearly shown not to be beneficial in several diseases including multiple sclerosis and senility.
- Lack of randomised controlled trials makes it difficult to assess the efficacy of hyperbaric oxygen in many diseases
- Side effects are usually mild but can be life threatening
- Clear evidence of benefit has been found in decompression sickness and a few other conditions
- Much work remains to be done to establish the timing, indications, and therapeutic regimens required to obtain the best clinical and cost effective results
- The cellular, biochemical, and physiological mechanisms by which hyperbaric oxygen achieves beneficial results are not fully established
_
The evidence is insufficient to support claims that hyperbaric oxygen therapy can effectively treat the following conditions:
- AIDS/HIV
- Allergies
- Alzheimer’s disease
- Arthritis
- Asthma
- Autism
- Bell’s palsy
- Brain injury
- Cancer
- Cerebral palsy
- Chronic fatigue syndrome
- Cirrhosis
- Depression
- Fibromyalgia
- Gastrointestinal ulcers
- Heart disease
- Heatstroke
- Hepatitis
- Migraine
- Multiple sclerosis
- Parkinson’s disease
- Spinal cord injury
- Sports injury
- Stroke
______
Cancer, hypoxia and hyperoxia:
The most common myth associated with cancer is the claim that cancer cannot survive in a high oxygen atmosphere. The origin of the claim stem from a statement made by 1931 Nobel Peace Prize winner Otto Warburg. What Otto Warburg stated is that there was a respiratory defect in cancer cells causing them to ferment regardless of how much oxygen was present. Somehow this statement got twisted in to the claim that Warburg stated the cause of cancer was a lack of oxygen. Warburg won the Nobel Prize for discovering an enzyme he called “iron oxidase”. Warburg’s hypothesis about there being a respiratory defect in cancer cells has since been disproven. The fact is that all cells, healthy and cancerous rely on both anaerobic and aerobic metabolism for energy production. And just like healthy cells, cancer cells will die if deprived of oxygen. This is actually the basis for the formation of blood vessels to a tumor through growth factors such as vascular endothelial growth factor. Once the tumor reaches a certain size, it can no longer be maintained though oxygen diffusion and the tumor starts to die. In response the tumor releases vascular growth factors that stimulate blood vessel formation to the tumor. This creates a more effective glucose and oxygen source for the tumor to help it survive and proliferate. Bottom line is that cancer cells are highly dependent on oxygen for survival. This is why hyperbaric oxygen therapy (HBOT) does not cure cancer despite supersaturating the tissues with pure oxygen. This concept confuses some people because some oxygen therapies such as ozone therapy will readily destroy cancer cells. Ozone though is not the same as the oxygen we routinely breathe and has numerous properties and benefits that plain oxygen does not provide.
_
Studies on HBO and cancer have recently focused on whether enhanced oxygen acts as a cancer promoter or not. As oxygen is believed to be required for all the major processes of wound healing, one feared that the effects of HBO would be applicable to cancer tissue as well and promote cancer growth. Furthermore, one also feared that exposing patients who had been treated for cancer, to HBO, would lead to recurrence. Nevertheless, two systematic reviews on HBO and cancer have concluded that the use of HBO in patients with malignancies is considered safe. Based on the present as well as previous reviews, there is no evidence indicating that HBO neither acts as a stimulator of tumor growth nor as an enhancer of recurrence. On the other hand, there is evidence that implies that HBO might have tumor-inhibitory effects in certain cancer subtypes. This is so because hypoxia is a critical hallmark of solid tumors and involves enhanced cell survival, angiogenesis, glycolytic metabolism, and metastasis.
_
Cancerous tumors are heterogeneous tissues with a dynamic microenvironment. They exhibit an oxygen gradient with outer regions of well-oxygenated (normoxic) tissue alongside poorly-oxygenated regions experiencing hypoxia.
_
Hypoxia is essential for tumor development and many studies have shown that tumor cells in hypoxic regions distant from blood vessels show resistance to chemotherapy or radiation therapy.
Solid tumors often contain areas subjected to acute or chronic hypoxia, though with variable severity in patients both within and among different tumor types. Although severe or prolonged hypoxia is deleterious, adaptation to the hypoxic microenvironment has allowed cancer cells to survive and proliferate in this hostile milieu. Tumor hypoxia develops due to the structural and functional abnormalities of the tumor vasculature since cancer growth often overrides the ability of the cancer vasculature to adapt to the increasing oxygen demand. Traditionally, hypoxia was thought of as a factor limiting cancer growth by reducing the ability of cells to divide. However, more recently, hypoxia has proven to be a causative factor in many pathophysiological events, including cancer progression. Multiple reports have demonstrated that decreased oxygen tension selects for more malignant cells and induces multiple cellular adaptations, which again sustains and fosters cancer progression and thereby induces cancer growth. Hypoxia is reported to result in cellular responses which improve oxygenation and viability through induction of angiogenesis, an alteration in metabolism by increased glycolysis and upregulation of genes involved in cell survival/apoptosis. Hypoxia has also been shown to increase genetic instability, activate invasive growth, and preserve the undifferentiated cell state. Studies have demonstrated that hypoxia is implicated in the resistance to conventional therapy. Oxygen concentration has an especially crucial role in radiation oncology and radiation resistance. The epithelial-to-mesenchymal transition in cancer has been shown to be induced by hypoxic conditions, leading to cancers with an invasive or metastatic phenotype. Given its important role as a negative prognostics and predictive factor, hypoxia may be considered as one of the targets in cancer treatment. There are several physicians who advocate the use of Hyperbaric Oxygen Treatment alongside other conventional cancer treatments.
_____
Contraindication to HBOT:
The only absolute contraindication to hyperbaric oxygen therapy is untreated pneumothorax. The reason is concern that it can progress to tension pneumothorax.
The following are relative contraindications — meaning that special consideration must be made by specialist physicians before HBO treatments begin:
- Cardiac disease
- COPD with air trapping – can lead to pneumothorax during treatment.
- Upper respiratory infections – These conditions can make it difficult for the patient to equalise their ears or sinuses, which can result in what is termed ear or sinus squeeze.
- High fevers – In most cases the fever should be lowered before HBO treatment begins. Fevers may predispose to convulsions.
- Emphysema with CO2 retention – This condition can lead to pneumothorax during HBO treatment due to rupture of an emphysematous bulla.
- History of thoracic (chest) surgery – This is rarely a problem and usually not considered a contraindication. However, there is concern that air may be trapped in lesions that were created by surgical scarring. These conditions need to be evaluated prior to considering HBO therapy.
- Malignant disease: Cancers thrive in blood-rich environments but may be suppressed by high oxygen levels. HBO treatment of individuals who have cancer presents a problem, since HBO both increases blood flow via angiogenesis and also raises oxygen levels. Taking an anti-angiogenic supplement may provide a solution. A study by Feldemier, et al. and recent NIH funded study on Stem Cells by Thom, et al., indicate that HBO is actually beneficial in producing stem/progenitor cells and the malignant process is not accelerated.
- Middle ear barotrauma is always a consideration in treating both children and adults in a hyperbaric environment because of the necessity to equalise pressure in the ears.
Pregnancy is not a relative contraindication to hyperbaric oxygen treatments, although it may be for underwater diving. In cases where a pregnant woman has carbon monoxide poisoning there is evidence that lower pressure (2.0 ATA) HBOT treatments are not harmful to the fetus, and that the risk involved is outweighed by the greater risk of the untreated effects of CO on the fetus (neurologic abnormalities or death.) In pregnant patients, HBO therapy has been shown to be safe for the fetus when given at appropriate levels and “doses” (durations). In fact, pregnancy lowers the threshold for HBO treatment of carbon monoxide-exposed patients. This is due to the high affinity of fetal hemoglobin for CO.
____
Complications of Hyperbaric Oxygen Therapy:
While it’s generally very safe, as with all medical treatments, Hyperbaric Oxygen Therapy carries with it the risk of complications that in rare instances can be life threatening and/or result in permanent or long-term disability.
- Barotrauma of the Ear
Barotrauma is a term that refers to injury due to increased pressure. Barotrauma of the ear is the most frequent complication of HBO. The middle-ear is an air-filled cavity behind the ear drum that connects to the throat through a slit-like passage called the eustachian tube. During compression, if the air pressure in the middle-ear cannot be equalized with the external pressure, the eardrum will bow inward, leading to pain and possibly rupture, leading to hearing loss.
- Round or Oval Window Rupture
Round and/or oval window rupture is a phenomenon related to ear barotruama. The round and oval windows are membranes separating the air-filled middle ear from the fluid-filled inner ear. Rarely, over-vigorous attempts to equalize the pressure in the middle ear can lead to increased pressure in the inner-ear and can rupture these membranes. Deafness is the result. So, while rupture of these windows is not related to pressure change per-se, it is related to maneuverers used to prevent another complication.
- Sinus Squeeze
Similarl to the middle-ear, the sinuses are air-filled spaces in the skull. Failure to equalize the pressure in the sinuses and the external environment leads to severe pain and possibly bleeding into the sinuses.
- Tooth Squeeze
Recent dental work can leave air-filled voids in teeth. The inability to equalize the pressure in these pockets can lead to pain and even cracking of the teeth.
- Pneumothorax or Pulmonary Barotrauma
Pulmonary barotruama refers to damage to the lung tissue as a result of pressure change, resulting in air leaking from the lungs into the chest cavity causing a dropped lung, or pneumothroax. This generally occurs in patients with air trapping lesions in the lungs, such as can occur in emphysema or asthma. During decompression, these air-filled pockets will begin to expand, and, if the pressure is not relieved by the airways in the lungs, these pockets can rupture. This released air can cause excess pressure in the chest cavity leading to difficulty breathing and decreased blood pressure that can result in death if untreated. Treatment consists of emergency evacuation of air from the chest cavity by inserting a needle through the chest wall, and, subsequently, placing a chest tube to re-expand the lung.
- Oxygen Toxicity Seizures
The high level of oxygen in the blood that occurs during HBO treatments can be toxic to the central nervous system and can result in seizure activity. While this is rare during clinical hyperbaric treatments, it does occur and may be more likely in those with pre-existing seizure disorders or hypoglycemia (low blood sugar). Treatment consists of simply removing the supplemental oxygen from the patient, which will terminate the seizure.
- Pulmonary Oxygen Toxicity
Elevated oxygen concentrations can be detrimental to the lungs. Prolonged exposure to high levels of oxygen can eventually lead to chest pain, difficulty breathing, and eventually, respiratory failure. In the early stages of the condition, the lungs rapidly return to baseline once the oxygen concentration is decreased. Thus, due to the intermittent nature of HBO treatments, pulmonary oxygen toxicity is rarely seen in clinical practice. On the other hand, this can become a concern in critically ill patients who must be maintained on supplemental oxygen between treatments or those patients who require unusually frequent or prolonged treatment courses.
- Decompression Sickness
Decompression sickness, or the bends, is a result of the uptake of nitrogen into the blood when air (which is about 80 percent nitrogen) is breathed at increased ambient pressure. This is generally more of a concern for the inside attendants, who breathe air during a treatment, rather than patients, who are breathing 100% oxygen. This can become a concern if a patient must be removed from oxygen for prolonged periods of time during the dive. Decompression sickness can result in pain, neurological injury, cardiopulmonary collapse, and possibly death.
- Claustrophobia, which appears to be present in about 2% of the general patient population, may cause some degree of confinement anxiety, even in a multiplace chamber. Occasionally, mild sedation is required for such individuals to continue to receive daily HBO2 therapy.
- Progressive myopia has been observed in some patients undergoing prolonged periods of daily HBO2 therapy. Although the exact mechanism remains obscure, it is apparently lenticular in origin and usually reverses completely within a few days to several weeks after the last therapy.
- Hypoglycemia
Some patients with diabetes experience a drop in blood sugar during hyperbaric treatments. In order to prevent this, patients are encouraged to eat before coming for treatments and blood glucose is monitored during the dive at appropriate intervals.
- Cardiovascular responses to hyperbaric hyperoxia include a rate-dependent reduction in cardiac output and systemic vasoconstriction with an increase in peripheral vascular resistance. Although these effects are well tolerated by normal individuals, the occurrence of acute pulmonary edema in three patients during hyperbaric oxygen therapy, with one related fatality, has been reported. All three patients had cardiac disease with reduced left ventricular ejection fractions. The risk of pulmonary edema may have been increased by the fact that they remained supine in a monoplace chamber during treatment. The present inability to identify patients who are unusually susceptible to this rare complication requires a comprehensive risk-benefit assessment when evaluating patients with compromised cardiac function.
In general, if pressures do not exceed 300 kPa and the length of treatment is less than 120 minutes, hyperbaric oxygen therapy is safe. Overall, severe central nervous system symptoms occur in 1-2% of treated patients, symptomatic reversible barotrauma in 15-20%, pulmonary symptoms in 15-20%, and reversible optic symptoms in up to 20% of patients.
_____
_____
Oxygen therapy in COPD:
Oxygen therapy offers significant short- and long-term benefits in those with COPD. Immediate benefits include alleviation of hypoxemia and its sequelae, improvement in exercise capacity, reduction of dyspnea, and possibly sleep consolidation. Long-term use has been shown to improve survival in severely hypoxemic patients with COPD and cause a slight reduction in pulmonary artery pressure. On the basis of various studies, patients with a resting PaO2 of less than 55 mm Hg (SaO2 < 88%), those with a PaO2 of 56–59 mm Hg with signs of tissue hypoxemia (e.g., cor pulmonale, polycythemia, impaired cognition), and those who experience desaturation during sleep or exercise should be considered for LTOT. The benefits of oxygen therapy in those with mild to moderate hypoxemia, although they may not include a reduction in mortality, may include improvements in mood, neurocognitive function, and quality of life. Adherence to LTOT ranges from 45% to 70% and utilization for more than 15 hours per day is widely accepted as efficacious.
_
Benefits of Oxygen Therapy in COPD:
- Increased Survival
By far, the most important benefit of LTOT for people with COPD is that, when used a minimum of 15 hours per day, it prolongs life. In fact, the average survival for those using LTOT for at least 18 hours a day is approximately double that of those who don’t use supplemental oxygen. Despite this evidence, studies show that the average number of hours that a patient uses daily supplemental oxygen is typically less than what the doctor has prescribed. Voluntary under-use of oxygen therapy can limit its effectiveness.
- Reduced COPD Complications
COPD is associated with a number of complications that can significantly impact your quality of life, including pulmonary hypertension, secondary polycythemia and cor pulmonale. Supplemental oxygen helps reduce COPD complications by stabilizing pulmonary hypertension, reducing secondary polycythemia, and decreasing arrhythmias (irregular heart rhythms) and ECG findings suggestive of myocardial ischemia (a lack of oxygen to the heart). Additionally, according to the American Lung Association, oxygen therapy helps prevent heart failure in people who have severe lung diseases like COPD.
- Lessened COPD Symptoms
Dyspnea, or shortness of breath, is not only the hallmark symptom of COPD; it’s also the most disabling and difficult to control. In patients who need it, supplemental oxygen can help relieve dyspnea and other symptoms related to COPD, including fatigue, dizziness, and depression.
- Improved Health-Related Quality of Life
When you don’t get an adequate supply of oxygen, every organ in your body is affected, which eventually takes a toll on your health and well-being. Using supplemental oxygen has a positive impact on health-related quality of life. Not only does it improve sleep and mood, it also increases mental alertness and stamina, allowing you to get more done during the day. Moreover, it may reduce the number of exacerbations and hospitalizations associated with COPD.
- Increased Exercise Tolerance
Exercise is one of the most important aspects of COPD management; in fact, a regular physical training program can increase survival and improve the quality of life in COPD. Many patients with COPD, however, have poor exercise tolerance that dramatically limits their ability to exercise. Studies suggest that using oxygen during exercise improves exercise endurance, heightens exercise performance and ultimately decreases the sensation of breathlessness.
- Improved Sex Life
Impotence is a common occurrence among men who have COPD. Supplemental oxygen can help. One study showed that 42 percent of sexually impotent men with COPD experienced a reversal of sexual impotence when they used LTOT for at least one month. Additionally, using supplemental oxygen during sex can help you prolong intimacy, an added benefit for both partners.
- Safer Air Travel
It’s not uncommon for patients with COPD to experience severe hypoxemia when they travel by airplane. Supplemental oxygen during air travel helps patients prevent severe hypoxemia and can benefit many patients, even those who don’t normally use oxygen. If you experience severe COPD symptoms when you travel, talk to your healthcare provider about using supplemental oxygen when you fly. Commercial passenger aircraft operate at cabin pressures similar to ambient pressures experienced at up to 2500 m above sea level. This is analogous to breathing 15% oxygen at sea level. At this ‘altitude’, the PaO2 for healthy people falls to around 53–64 mm Hg (7.1–8.5 kPa), with corresponding oxygen saturations of 85–91%. As a general rule, supplemental oxygen is unlikely to be required if the resting oxygen saturation is ≥95% but is recommended for patients who qualify for continuous oxygen therapy at home or who have a demonstrated fall in SpO2 to <85% during an altitude simulation test. In the United States, most airlines restrict the devices allowed on board aircraft. As a result, passengers are restricted in what devices they can use. Some airlines will provide cylinders for passengers with an associated fee. Other airlines allow passengers to carry on approved portable concentrators.
- Improved Social Life
How many times has COPD interfered with your social life? If breathlessness prevents you from enjoying a movie or dinner invitation, maybe it’s time you look into using supplemental oxygen by way of a portable oxygen concentrator. Extremely lightweight and compact, portable oxygen concentrators are much more versatile than their home-based counterparts, allowing you freedom and independence to go about your business in the usual fashion. If COPD often puts a damper on your plans, talk to your health care provider about going portable.
_______
COPD, oxygen therapy and hypercapnia:
Care needs to be exercised in patients with chronic obstructive pulmonary disease, such as emphysema, especially in those known to retain carbon dioxide (type II respiratory failure). Such patients may further accumulate carbon dioxide and decreased pH (hypercapnation) if administered supplemental oxygen, possibly endangering their lives. This is primarily as a result of ventilation–perfusion imbalance. In the worst case, administration of high levels of oxygen in patients with severe emphysema and high blood carbon dioxide may reduce respiratory drive to the point of precipitating respiratory failure, with an observed increase in mortality compared with those receiving titrated oxygen treatment. However, the risk of the loss of respiratory drive is far outweighed by the risks of withholding emergency oxygen, and therefore emergency administration of oxygen is never contraindicated. Transfer from field care to definitive care, where oxygen use can be carefully calibrated, typically occurs long before significant reductions to the respiratory drive. A 2010 study has shown that titrated oxygen therapy (controlled administration of oxygen) is less of a danger to COPD patients and that other, non-COPD patients, may also, in some cases, benefit more from titrated therapy.
_
What is carbon dioxide?
Carbon dioxide (CO2) is a gas in the air that makes up about 0.04% of the earth’s atmosphere. CO2 is also a “waste gas” and a by-product of the body’s metabolism (biochemical processes occurring in cells and which are required to sustain life). During metabolism, oxygen is used and CO2 is produced. CO2 is chiefly removed from the body through the lungs when you breathe out. A high CO2 level is usually an indication that the lungs are not able to keep up with the body’s needs. In a healthy person, a high CO2 level can occur suddenly during an acute illness. Some people adapt over time to a new “baseline” where the CO2 level in the body is higher than in healthy lungs. Some people with stable Chronic Obstructive Pulmonary Disease (COPD) can manage with a higher CO2 level than people with healthy lungs.
Effects of high CO2 levels (hypercapnia):
The presence of a high CO2 level in the blood is known as hypercapnia and can cause headaches, lethargy, drowsiness, confusion and, if severe, can lead to coma and death. People with hypercapnia may be flushed and warm to touch, and they may also show a classic “flapping tremor” of the hands. When asked to hold their arms out in front and bend their wrists back, they are unable to maintain the position of the hands, and as a result, the hands will “flap”.
_
How does oxygen cause hypercapnia?
Too much supplemental oxygen can cause or worsen hypercapnia by a number of different mechanisms. In individuals with chronic obstructive pulmonary disease who receive supplemental oxygen, carbon dioxide accumulation may occur through three main mechanisms:
- Ventilation/perfusion mismatching:
The pulmonary vasculature can dilate and constrict to alter blood flow and match ventilation to perfusion, and the primary driver of vascular dilation and increased perfusion is alveolar O2. Under-ventilated lung usually has a low oxygen content which leads to localised vasoconstriction limiting blood flow to that lung tissue. In COPD, patients optimise their gas exchange by hypoxic vasoconstriction leading to altered alveolar ventilation-perfusion (V/Q) ratios. Supplemental oxygen abolishes this constriction, leading to poor ventilation/perfusion matching. This redistribution of blood to areas of the lung with poor ventilation reduces the amount of carbon dioxide eliminated from the system. Diseased sections of lung see increased PaO2 and steal perfusion away from better functioning areas. This results in shunting, dead space ventilation, and eventually hypercarbia.
- The Haldane effect:
Most carbon dioxide is carried by the blood as bicarbonate, and deoxygenated hemoglobin promotes the production of bicarbonate. Increasing the amount of oxygen in the blood by administering supplemental oxygen reduces the amount of deoxygenated hemoglobin, and thus reduces the capacity of blood to carry carbon dioxide. Deoxygenated hemoglobin (Hb) binds CO2 with greater affinity than oxygenated hemoglobin (HbO2). Oxygen therefore induces a right shift of the CO2 dissociation curve, which is called the Haldane effect. In patients with severe COPD who cannot increase minute ventilation, the Haldane effect accounts for about 25% of the total PaCO2 increase due to O2 administration.
- Respiratory homeostasis:
In healthy individuals, a rise in carbon dioxide causes an increase in the drive to breathe. However, in some patients with chronic obstructive pulmonary disease, this response has been blunted, leaving low oxygen levels as the main stimulus of respiration (hypoxic drive). Hence, giving supplemental oxygen reduces their stimulus to breathe, causing respiration to slow (hypoventilation), and allowing carbon dioxide to accumulate in the body. In hypoxic drive, the peripheral chemoreceptors located at the bifurcations of the aortic arteries and the aortic arch monitor partial pressure of arterial oxygen (PaO2). This drive only becomes active when the PaO2 is less than 60 mmHg. This hypoxic response is far slower than signals sent by central chemoreceptors, and therefore the hypoxic drive has only a minor role in breathing. Modern evidence suggests that the hypercapnic drive is never completely blunted, and therefore even COPD patients with chronically elevated PaCO2 will not stop breathing in the presence of higher oxygen levels. Many studies suggested that hypoventilation due to loss of hypoxic respiratory drive is not the cause of hypercarbia after O2 administration, and that other factors, like the Haldane effect and V/Q mismatching are more likely to blame.
Uncontrolled oxygen therapy, or receiving too much oxygen, can make people who usually have higher CO2 levels retain more until it reaches dangerous levels.
_
Prevention:
In people with chronic obstructive pulmonary disease, carbon dioxide toxicity can be prevented by careful control of the supplemental oxygen. Just enough oxygen is given to maintain an oxygen saturation of 88 – 92%. The critical oxygen level is an oxygen saturation of approximately 90% (this is measured by a finger pulse oximeter), equivalent to a blood oxygen level of 55-60 mmHg (this is measured from a blood sample taken from an artery, commonly in the wrist). This blood test is known as an arterial blood gas or ABG. Therefore, controlled oxygen therapy, to maintain oxygen saturation at around 90% (88-92% is an acceptable range) will minimise the risk of hypercapnia. It is important to avoid too much oxygen and minimise the risk of worsening CO2 levels in this situation. Some patients who are very sensitive to the adverse effects of too much oxygen may choose to wear a medical alert bracelet to alert paramedics about their lung condition in the event of an emergency.
_
Some people with low oxygen levels are prescribed supplemental oxygen at home as a long-term treatment for their lung condition. In this instance, the treating physician usually determines the oxygen flow within an acceptable range. It is important that oxygen therapy is used to maintain blood levels within this acceptable range and not in an effort to reduce perceived breathlessness. Breathlessness in COPD is rarely due to low oxygen levels alone. If someone experiences worsening breathlessness they need to see their doctor and address the possible causes. Patients should not increase the oxygen flow simply to treat the symptom of breathlessness.
__
Oxygen therapy management in COPD:
- Target SaO2 88-92% in these patients
- the targeted approach is associated with decreased mortality in COPD patients and less respiratory acidosis
- The oxygen flow rate administered is not important, the (alveolar) PAO2 (and, indirectly, the SaO2) achieved is
- Never withhold oxygen from a seriously ill hypoxic patient due to fear of cause hypercapnic respiratory failure.
_______
The Role of LTOT in Patients with COPD and Severe Hypoxemia at Rest:
Supplemental oxygen is a well-established therapy with clear evidence for benefit in patients with COPD and severe resting hypoxemia, which is defined as a room air PaO2 ≤ 55 mm Hg or ≤ 59 mm Hg with signs of right-sided heart strain or polycythemia. Oxygen was the first treatment shown to prolong life in people with COPD. Current recommendations for prescribing LTOT are based on results from two randomized trials in patients with COPD published almost 30 years ago: the Nocturnal Oxygen Therapy Trial (NOTT) and the Medical Research Council (MRC) study.
_
The Role of LTOT in Patients with COPD and Moderate Hypoxemia:
In contrast with the results of the MRC study and NOTT, supplemental oxygen has not been shown to improve survival in patients with COPD and moderate hypoxemia. Górecka et al randomly assigned 135 patients with a resting room air PaO2 of 56 to 65 mm Hg to receive supplemental oxygen for > 17 h/d (to raise PaO2 to ≥ 65 mm Hg) or no supplemental oxygen. Over a mean observation of 40.9 months, cumulative survival in the treatment and control groups did not differ significantly. Furthermore, no survival difference was observed for patients using supplemental oxygen for more than 15 h/day vs. those using it for shorter periods. A large Randomized Trial of Long-Term Oxygen for COPD with moderate desaturation in 2016 found that in patients with stable COPD and resting or exercise-induced moderate desaturation, the prescription of long-term supplemental oxygen did not result in a longer time to death or first hospitalization than no long-term supplemental oxygen, nor did it provide sustained benefit with regard to any of the other measured outcomes. A systematic review and meta-analysis suggested that oxygen therapy may reduce dyspnea in patients with COPD and mild or no hypoxemia but this trial found no consistent benefit of long-term supplemental oxygen with regard to measures of quality of life, depression, anxiety, or functional status. This Long-Term Oxygen Treatment Trial randomly assigned 738 participants with COPD (73% of whom were men) and mild-to-moderate hypoxemia at rest or during a 6-minute walk test to receive either long-term supplemental oxygen or no long-term supplemental oxygen. The supplemental oxygen was prescribed as 2 liters of oxygen per minute continuously in participants with resting hypoxemia (57% of the participants) and as an adjusted oxygen dose during exercise and 2 liters of oxygen per minute during sleep in participants with exertional hypoxemia only (43%). During a median follow-up of 18.4 months, there was no significant between-group difference in the rate of death or first hospitalization in the time-to-event analysis (primary outcome) or in mortality and the rate of hospitalizations separately, COPD exacerbations, quality of life, anxiety, depression, or functional status. This landmark study is the largest to date with regard to long-term oxygen therapy. On the basis of all available current data, long-term oxygen therapy should be prescribed to prolong survival among patients with COPD who have chronic (>3 weeks) severe resting hypoxemia (PaO2 of ≤55 mm Hg or SpO2 of <88%) while they are breathing ambient air. Since a lack of evidence of effect is not evidence of a lack of any clinical effectiveness, a trial of oxygen use might still be appropriate in selected patients with moderate exertional hypoxemia and intractable breathlessness despite appropriate evidence-based treatment. Which patients with moderate hypoxemia benefit from long-term oxygen therapy? A 2017 study found that LTOT is not indicated in moderate hypoxemia except in the few patients with polycythemia or signs of right-sided heart failure, which may reflect more chronic and severe hypoxemia.
_
The Role of Supplemental Oxygen in Patients with Hypoxemia during Activity:
Important challenges in ascertaining the effectiveness of supplemental oxygen during activity in patients with COPD are the lack of uniform criteria for defining exertional desaturation and standardized exercise protocols. Threshold values for oxygen desaturation range from 88% to 90%, and relative declines vary from 2% to 5% in published investigations. Some studies require maintenance of the oxygen saturation by pulse oximetry (SpO2) below a threshold value for a specified interval (usually between 0.5 min and 5 min). The techniques for inducing exertion vary from activities of daily living to incremental maximal cycle ergometry. Several studies suggested that exertional desaturation may portend a poor prognosis for patients with COPD. In a retrospective review of 144 patients, Takigawa and coworkers showed that a fall in SpO2 ≥ 6% during a 6-min walk predicted mortality. Similarly, in a prospective study of 576 patients with stable COPD, Casanova and colleagues demonstrated that desaturation (a decrease in the SpO2 ≥ 4% or SpO2 < 90% on 6-min walk) predicted mortality with a relative risk of 2. The PaO2 slope (rate of change of PaO2 and oxygen consumption) during incremental cardiopulmonary exercise testing and age were the most significant independent prognostic factors associated with survival in 120 patients with COPD. In a cohort of 64 patients with hypercapnia followed for ≤ 15 years, the decline in arterial oxygen saturation (SaO2) and increase in PaCO2 during exercise were significantly greater in those who died. A retrospective review of 471 subjects with emphysema, resting normoxemia, and exertional desaturation randomized to medical treatment in the National Emphysema Treatment Trial demonstrated no differences in survival among subjects treated with continuous oxygen, intermittent oxygen, or no supplemental oxygen. Although exertional desaturation in patients with COPD and resting normoxemia appears to predict a poor prognosis, the effect of continuous supplemental oxygen on survival in this group has not been prospectively assessed in a large population.
_
As-Needed or Short-Burst Oxygen Therapy:
Short duration, intermittent supplemental oxygen has been used to relieve breathlessness with exercise. There is no uniform definition of the amount or duration of oxygen therapy used for short periods of time. Published reports have used oxygen to relieve breathlessness as needed, before exercise, during exercise, or after exercise. Several early studies suggested that short-burst oxygen immediately before or just after exertion reduces dyspnea and increases 6-min walk distance. Subsequent studies failed to demonstrate such benefits. It is to be noted that short-burst oxygen i.e. oxygen inhaled immediately prior and/or following exertion with the aim of relieving breathlessness or improving exercise tolerance is not effective (O’Neill 2006, O’Driscoll 2008) [evidence level I]. Short-burst oxygen either before or after a 6-min walk does not improve the distance walked or the Borg dyspnea scale in patients with COPD and normoxemia at rest and desaturation with exertion. Compared with air, supplemental oxygen after the completion of exercise decreases the time to recovery from dynamic hyperinflation but does not affect the time to return to baseline breathlessness or maximal perception of dyspnea during recovery. Thus, there are few randomized controlled studies of short-term supplemental oxygen use. A meta-analysis of short-burst oxygen therapy concluded that there is no reduction in breathlessness and inconsistent effects on exercise capacity.
_
Ambulatory oxygen therapy:
In patients who qualify for long-term oxygen therapy (LTOT), ambulatory oxygen therapy can be used in order to maximize usage achieve an average usage of 18 hours day. In patients who do NOT quality for LTOT, available evidence does not allow any firm conclusions to be made about the use of long-term intermittent ambulatory domiciliary oxygen therapy in patients with COPD who do not meet the criteria for LTOT. This conclusion is based on a Cochrane Review comprising four studies (total of 331 patients) (Ameer 2014) who received oxygen or air (blinded) for between two and 12 weeks in the home setting. This review found no significant difference in exercise tolerance or mortality in those receiving supplemental oxygen compared to breathing air supplied by a cylinder.
_
The Role of LTOT in Patients with COPD and Nocturnal Desaturation:
Nocturnal oxygen desaturation (NOD) has been reported in patients with COPD with an awake PaO2 > 60 mm Hg. The most significant episodes of NOD occur during rapid eye movement sleep, with a reported prevalence of 27%. However, there are no accepted standards for the level or duration of desaturation that define NOD in patients with COPD. It is not clear whether patients with COPD who desaturate during the day with exercise also desaturate at night. Because the mechanisms of desaturation during exercise and during sleep differ, patients who desaturate with activity may not desaturate at night.
Mortally:
Although retrospective data suggest a decreased survival in patients with NOD, only a few studies examined the impact of nocturnal supplemental oxygen therapy on mortality in patients with COPD and NOD. In patients with mild to moderate daytime hypoxemia (PaO2, 56-69 mm Hg) and associated NOD, no improvement in survival was noted with nocturnal supplemental oxygen therapy at the end of 2 years. A similar lack of improvement in survival was seen in patients with COPD and isolated NOD who were randomized to nocturnal oxygen therapy for 3 years. Therefore, based on limited available data in small numbers of subjects, it is unknown whether continuous supplemental oxygen therapy affects survival in patients with COPD and isolated NOD.
Sleep Quality:
Sleep quality is poor in patients with COPD. Subjective complaints include difficulty falling and staying asleep, morning tiredness, early awakenings, and excessive daytime sleepiness. Objective assessment of sleep quality demonstrates increased sleep latency, decreased total sleep time, increased number of arousals, and a decrease in rapid eye movement sleep. The results of studies investigating the effects of oxygen therapy on sleep quality are limited and conflicting, with one study demonstrating improved sleep quality and another noting no change. Therefore, although sleep quality is known to be poor in patients with COPD, the effects of nocturnal supplemental oxygen therapy are unknown.
______
Does oxygen help people Quit Smoking?
There are no studies looking at the role of oxygen therapy in smoking cessation. Logically, people who smoke and need oxygen might consider quitting smoking to preserve their lung function. (Experience tells us that this is not always the case.) Equally, oxygen is flammable, and one could assume that people who use oxygen would stop smoking. (Again, experience tells us that this is not the case. It is not unusual to see people smoking while attached to an oxygen cylinder. If in doubt, stand outside any hospital for a few minutes.)
Bottom Line:
There is no connection between oxygen therapy and smoking cessation.
______
______
Oxygen therapy in acute coronary syndrome:
Most patients with acute coronary syndromes (ACS) receive oxygen therapy as part of their emergency treatment, initiated by paramedics during transfer and before their first contact with a physician. A survey among physicians involved with acute myocardial infarction cases found that 96% of their patients with ACS received oxygen therapy. About 50% of all responders believed that oxygen decreases mortality, 25% thought it helps to relieve pain and 25% thought it has no effect. Many therapies and interventions are not based on proven benefit, but on anecdotal evidence, expert opinion and tradition. This is especially true for oxygen therapy, which is usually not questioned and has been used for over 100 years. Oxygen supplementation is a well-accepted therapy for hypoxemic patients, because it increases the delivery of oxygen to cells and is thus believed to reverse the effects of hypoxia. However, most patients who present with acute coronary syndrome (ACS) are not hypoxemic, and the value of oxygen therapy in these patients remains unknown. We could argue that as long as it does no harm, it does not really matter whether we continue to provide oxygen in these situations. However, is it really harmless?
_
From a physiological perspective, treating ACS patients with oxygen may seem reasonable. In ACS there is a lack of myocardial perfusion and consequently a lack of oxygenation of the myocardium. Therefore, it seems logical to increase the oxygenation of the blood reaching the jeopardised myocardium by administering oxygen therapy. Oxygen, via face mask or nasal cannula, is often administered to patients with suspected acute myocardial infarction (AMI) in an attempt to increase myocardial oxygenation and decrease infarct size. While treating AMI with oxygen makes sense from a physiologic standpoint, no studies have convincingly demonstrated that oxygen therapy improves outcomes in AMI. In addition, recent data suggest that this practice may even be harmful.
_
Mechanisms of oxygen harms in ACS/AMI:
- Oxygen may increase microvascular resistance, leading to reduced coronary blood flow thus reducing cardiac output and increasing radical oxygen species, which can have multiple negative effects including increased risk for arrhythmias and cell damage leading to heart failure. Arterial oxygen tension is a major determinant of coronary artery tone, with any increment in arterial oxygen content reducing coronary flow regardless of initial saturation. In human studies of subjects with cardiac disease, hyperoxia from high concentration oxygen therapy causes a marked reduction in coronary blood flow. In patients with myocardial infarction, high concentration oxygen therapy also causes a reduction in cardiac output and stroke volume, and increases systemic vascular resistance and blood pressure.
- Primary percutaneous coronary intervention (PCI) is now the gold standard treatment for AMI, with thrombolysis very rarely used in Western countries. While reperfusion with PCI is successful to achieve revascularisation in nearly all cases, thrombolysis achieves sufficient revascularisation in only 50% of cases. How, and if, a patients get re-perfused is potentially very important, and the effect of delivering oxygen may differ depending on the method used. While reperfusion is vital, it can also lead to myocardial damage, the so-called ‘reperfusion injury’. This reperfusion damage can lead to 25% of the overall myocardial damage. Reperfusion injury may be influenced by oxygen therapy, for example by increasing the concentration of radical oxygen species.
_
The use of oxygen to relieve angina pectoris was first described by Steele over 100 years ago. Russek and colleagues, in 1950, cautioned that the administration of 100% oxygen may actually be contraindicated in patients with myocardial infarction or angina pectoris in whom the arterial oxygen saturation is normal. Since this warning, there have been numerous reports of potentially harmful effects of high flow oxygen in the treatment of myocardial infarction, yet it is routine clinical practice to administer oxygen to virtually all patients in this situation. Russek and colleagues observed that 100% oxygen via a face mask led to more pronounced and longer duration of the electrocardiograph (ECG) manifestations of myocardial ischemia and failed to prevent the onset or influence the duration of anginal pain. These observations led the authors to suggest that the administration of 100% oxygen may actually be contraindicated in patients in whom arterial oxygen saturation is normal, and to hypothesize that hyperoxygenated blood may interfere with the reactive hyperaemia which accompanies an ischaemic myocardium. They stressed that oxygen should be freely administered if indicated (i.e. for relief of hypoxia with pulmonary oedema and Cheyne-Stokes respiration) but that ‘its indiscriminate employment may result in more harm than good.’ These observations contrasted with the pragmatic but unproven medical view of the period that the administration of high flow oxygen was an important therapeutic measure, regardless of arterial hypoxia, on the basis that it increased the oxygen supply to the myocardium and reduced the size of the infarct. There is little evidence by which to determine the efficacy and safety of high concentration oxygen therapy in myocardial infarction. The evidence that does exist indicates that the use of high concentration oxygen therapy in uncomplicated myocardial infarction results in a significant increase in infarct size (as determined by cardiac enzyme levels) and possibly mortality with an odds ratio of death of 3.03 (95% CI 0.93 to 9.83). In terms of resuscitation following cardiac arrest, oxygen therapy resulting in hyperoxia is independently associated with increased mortality, compared with either hypoxia or normoxia. However, considering the related treatment of hyperbaric oxygen therapy (which is administration of 100% oxygen at pressures greater than one atmosphere absolute) in patients with AMI, evidence from small randomised trials suggests a reduction in myocardial damage and mortality. So if hyperbaric oxygen does not cause harm, and is even potentially beneficial, might it be rather unlikely that oxygen therapy at 4-6 L/min would increase mortality?
_
Oxygen therapy for acute myocardial infarction: Cochrane review 2013:
Many people who are having a heart attack are routinely given oxygen to breathe. Authors looked for the evidence to support this practice by searching for randomised controlled trials that compared the outcomes for people given oxygen to the outcomes for those given normal air to breathe. Authors were primarily interested in seeing whether there was a difference in the number of people who died, but authors also looked at whether administering oxygen reduced pain. Authors found four randomised controlled trials that compared one group given oxygen to another group given air. These trials involved a total of 430 participants of whom 17 died. In that group, more than twice as many people known to have been given oxygen died compared to those known to have been given air. However, because the trials had few participants and few deaths, this result does not necessarily mean that giving oxygen increases the risk of death. The difference in numbers may have occurred simply by chance. Nonetheless, since the evidence suggests that oxygen may in fact be harmful, authors think it is important to evaluate this widely-used treatment in a large trial as soon as possible, to make sure that current practice is not causing harm to people who have had a heart attack.
_
AVOID trial: 2015:
This prospective multicenter randomized controlled trial of air versus oxygen in STEMI diagnosed by paramedics found that mean peak troponin was similar between the groups, but the mean peak in creatine kinase (CK) was higher in the oxygen group, with higher rates of recurrent MI, arrhythmia, and increased infarct size on cardiac MR scanning at six months, suggesting harm associated with oxygen use. Further analysis of the AVOID data by Nehme, et al. published in 2016 specifically looked at the effects of supplementary oxygen on biochemical and cardiac MRI measures of myocardial injury. It proposed that every 100L of oxygen exposure was associated with a 1.4% (95% CI 0.6% to 2.2%, p<0.001) and 1.2% (95% CI 0.7% to 1.8%, p<0.001) increase in the mean troponin and CK, respectively. A 1.2% (95% CI 0.1% to 2.3%, p=0.03) increase in the cardiac MR infarct mass and a 0.9% (95% CI 0.01% to 1.9%, p=0.06) increase in the infarct size as a proportion of left ventricular mass was observed in a subgroup of 139 patients undergoing a six-month cardiac MR scan.
_
Determination of the Role of Oxygen in Suspected Acute Myocardial Infarction (DETO2X-AMI) trial: 2017:
This prospective, randomised, open-label trial enrolled 6229 patients with suspected heart attack from 35 hospitals across Sweden. Half of the patients were assigned to oxygen given through an open face mask and the other half to room air without a mask. The primary outcome, the mortality rate one year after randomisation, was not statistically different between the two groups (5.0% in the oxygen group versus 5.1% in the air group). Similarly, there was no significant difference between the two groups for secondary endpoints, including the risk of a new heart attack or heart muscle injury measured by markers in the blood. Even in patients at high risk, such as smokers, older patients, patients with diabetes, or patients with previous heart disease, the results were similar concerning mortality within one year. Routine use of supplemental oxygen in patients with suspected myocardial infarction who did not have hypoxemia was not found to reduce 1-year all-cause mortality.
_
The 2004 American (AHA/ACC) ST-elevation myocardial infarction (STEMI) guidelines recommend that oxygen be administered to hypoxemic STEMI patients (SaO2 <90%, level of evidence B) and state that ‘‘it is reasonable to administer supplemental oxygen to all patients during the first 6 hours” (level of evidence C). More recently published updates do not address the administration of oxygen. The current European non-ST-elevation myocardial infarction (NSTEMI)-ACS guidelines recommend oxygen supplementation if oxygen saturation is <90%. The recently published European STEMI guidelines suggest a different cut-off that defines hypoxia and advocate oxygen therapy only if oxygen saturation levels are <95%. The recommendations in both guidelines are supported with a low level of evidence (C). Recently, several scientific societies have reviewed and modified their guidelines on ACS management regarding oxygen supplementation—the new European guidelines now recommend oxygen therapy only in hypoxemic patients. Also, the Scottish Intercollegiate Guidelines Network and the British National Clinical Guidelines Centre for acute and chronic conditions advocate oxygen therapy only in hypoxic patients (SaO2 <94%). These new revisions, however, are based on expert opinion (level C), not solid clinical data.
_
My view:
There is insufficient evidence to support its routine use in uncomplicated ACS. There is good quality evidence to nudge us beyond “no benefit” in giving supplemental oxygen to patients with ACS and SpO2 ≥ 94% to “potential for harm.” If the patient is dyspnoeic, hypoxemic, or has obvious signs of heart failure, providers should titrate therapy, based on monitoring of oxygen saturation to 94%.
______
______
Oxygen therapy in stroke:
Despite its relatively small size (2% of total body weight), mammal brain ranks second after the heart as the organ with the highest O2 consumption. As its function strictly depends on continuous oxygenation, any decrease in the O2 supply results into potentially lethal cerebral hypoxia. Brain hypoxia is a dangerous feature in hemorrhage, anemia, trauma, stroke, perinatal encephalopathy, cardiopulmonary failure and high altitude exposure. Hyperoxic oxygenation is therefore a mandatory therapy for brain survival. Although necessary to guarantee life, however, excess O2 may become dangerous when the body antioxidant properties become inadequate to deal with higher than physiological levels of O2, a potentially toxic element. Brain cells, especially neurons, are known to be highly vulnerable to the deleterious effects of the reactive O2 species (ROS) produced during oxidative stress. Because of its high O2 consumption and relatively low antioxidant defense, brain is thus particularly sensitive to ROS. Therefore, although hyperoxia is often used therapeutically in traumatic brain injury and ischemic stroke, it may imbalance the redox status thereby inferring cerebral damage.
_
Brain edema is a life-threatening complication of cerebral infarction, which aggravates ischemic brain injury by compression of the cerebral vasculature and reduction of blood flow to the penumbral region. Vasogenic edema is characterized by increased extracellular fluid volume due to destruction of the blood-brain barrier (BBB). This type of edema develops within the first few hours to days after a stroke. Transient brain ischemia and subsequent reperfusion enhances the production of oxygen free radicals and lipid peroxidation as one of the main causes of BBB destruction and subsequent vasogenic cerebral edema. Tissue hypoxia is the main cause of cell death following cerebral ischemia. Hence, it is believed that brain tissue oxygenation might be a logical and effective strategy for ischemic stroke treatment. Previous studies have suggested a neuroprotective effect of normobaric oxygen therapy (NBO) when used for a short time and early after the occurrence of ischemic stroke. However, there are also controversial reports that NBO does not produce neuroprotective effects or that exacerbates brain injury. It was reported that oxygen therapy in stroke patients may increase mortality rates due to excessive production of reactive oxygen species (ROS) and subsequent lipid peroxidation. Early hyperoxia after cerebral ischemia could increase the generation of ROS due to mitochondrial respiration impairment. Thus, NBO may cause severe oxidative damage of the brain and BBB disruption by lipid peroxidation, especially during reperfusion. Dubinsky et al. also suggested that neuronal cell death caused by glutamate excitotoxicity requires the presence of oxygen. In addition, it was suggested that oxygen therapy may impair cerebral blood flow by vasoconstriction and worsen stroke outcome. Oxygen has vasoconstrictive effects on the circulatory system, reducing peripheral circulation and hyperoxia may compromise cerebral blood flow via vasoconstriction and thereby worsen the functional outcome of ischemic stroke. However, when additional oxygen is given to the patient, additional oxygen is dissolved in the plasma. This allows a compensating change to occur and the dissolved oxygen in plasma may support embarrassed (oxygen-starved) neurons, reduces inflammation and post-stroke cerebral edema. Since 1990, hyperbaric oxygen therapy has been used in the treatments of stroke in some centers. In rare instances, hyperbaric oxygen therapy patients have had seizures. However, because of extra available dissolved oxygen to neurons, there is usually no negative sequel to the event. Such seizures are generally a result of oxygen toxicity, although hypoglycemia may be a contributing factor, but the latter risk can be eradicated or reduced by carefully monitoring the patient’s nutritional intake prior to oxygen treatment. It appears that treatment of acute ischemic stroke might depend on the severity of the brain injury, timing and duration of oxygen therapy, and the age of the patient. Oxygen therapy may exacerbate stroke outcome in aged patients with severe brain damage.
_
A Pilot Study of Normobaric Oxygen Therapy in Acute Ischemic Stroke: 2005:
Therapies that transiently prevent ischemic neuronal death can potentially extend therapeutic time windows for stroke thrombolysis. Authors conducted a pilot study to investigate the effects of high-flow oxygen in acute ischemic stroke. They found that High-flow oxygen therapy is associated with a transient improvement of clinical deficits and MRI abnormalities in select patients with acute ischemic stroke. Further studies are warranted to investigate the safety and efficacy of hyperoxia as a stroke therapy.
_
Early oxygen therapy does not protect the brain from vasogenic edema following acute ischemic stroke in adult male rats: 2017:
The findings showed that the antioxidant power of brain tissue was significantly reduced in NBO-treated ischemic rats. The results of the present study show that early oxygen therapy following acute ischemic stroke does not reduce vasogenic brain edema, nor does it protect against oxidative stress-induced BBB destruction in adult male Sprague-Dawley rats. Additionally, cerebral edema formation occurs in conjunction with an increased mortality rate, serious brain injury, and impairment of brain antioxidant power. Reduction of brain tissue antioxidant power and increases in lipid peroxidation may be a possible mechanism for early oxygen therapy failure. Further experimental studies are needed to clarify the beneficial effects and potential side effects of early oxygen therapy before clinical use.
__
No benefit seen for routine low-dose oxygen after stroke: 2017:
The Stroke Oxygen Study (SO2S) was a single-blinded, randomized, controlled trial that recruited 8,003 adults with a diagnosis of acute stroke within 24 hours of hospital admission, drawing from 136 centers in the United Kingdom. A total of 7,677 participants (96%) had data available for analysis of the primary outcome measure, a composite of death and disability 90 days post stroke. Participants, who were not hypoxic at enrolment, were randomized 1:1:1 to receive continuous oxygen supplementation for the first 72 hours after stroke, to receive supplementation only at night, or to receive oxygen when indicated by usual care protocols. The average participant age was 72 years and 55% were men in all study arms, and all stroke severity levels were included in the study. Routine use of low-dose oxygen supplementation in the first days after stroke doesn’t improve overall survival or reduce disability, according to a large new study. The poststroke death and disability odds ratio was 0.97 for those receiving one of two continuous low-dose oxygen protocols, compared with the control group (95% confidence interval, 0.89-1.05; P = .47).
_
My view:
Early hyperoxia after cerebral ischemia could increase the generation of ROS due to mitochondrial respiration impairment. The human brain is at particularly high risk for damage by free radicals because of its high degree of metabolism compared to other tissues, while lacking the levels of antioxidant protection found elsewhere in the body. Thus, oxygen therapy may cause severe oxidative damage of the brain and BBB disruption by lipid peroxidation, especially during reperfusion. In addition, hyperoxia may compromise cerebral blood flow via vasoconstriction and thereby worsen the functional outcome of ischemic stroke. Clinical trials have failed to demonstrate clear-cut benefits of hyperoxia in ischemic stroke. One randomised controlled trial found that in minor or moderate stroke, oxygen administration was linked to increased mortality when compared with air. So oxygen therapy in ischemic stroke is to be given only if patient presents with hypoxemia.
_______
_______
Oxygen therapy in dyspnoea:
Dyspnea has been defined as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations varying in intensity. The experience derives from interactions among multiple physiological, psychological, social, and environmental factors.” Prevalence of severe dyspnea among terminally ill patients has been reported as 65%, 70%, and 90% for heart failure, lung cancer, and chronic obstructive pulmonary disease (COPD) patients, respectively. Dyspnea often presents as a chronic condition that intensifies during the dying process; it can erode quality of life (QOL), psychological well-being, and social functioning. The exact nature and cause, and therefore appropriate treatment, of dyspnea remain elusive. Objective measures, such as desaturation with exercise, may point toward underlying pathology, but do not reliably indicate subjective experience. Current pharmacologic treatments include opioids, psychotropic drugs, inhaled frusemide, Heliox, and oxygen; opioids remain the mainstay of treatment. Palliative interventions seek primarily to alleviate the sensation of breathlessness; they are generally applied in palliative care irrespective of underlying pathology and respiratory functioning.
_
Long-term oxygen therapy (LTOT) is indicated for COPD patients with severe hypoxemia (PaO2≤55 mm Hg at rest); treatment improves survival, dyspnea, and functional status. Palliative oxygen is frequently prescribed to manage dyspnea in people with advanced life-limiting illness, irrespective of PaO2, and is generally considered standard of care. Over 70% of physicians caring for dyspneic palliative care patients report prescribing palliative oxygen, usually for refractory symptoms (65%) or at patient request (30%). There is not, however, clear evidence demonstrating symptomatic benefit of palliative oxygen, though the intervention entails cost and logistical burden. Across the world, hospices commonly prescribe oxygen based on symptomatic, rather than pulse oximetry, criteria. In Canada, compassionate-use oxygen not meeting LTOT criteria represents 30% of the oxygen therapy budget. Lack of evidence to support palliative oxygen use and lack of available clinical practice guidelines have led to inconsistent access and variable utilization.
_
Effect of palliative oxygen versus medical (room) air in relieving breathlessness in patients with refractory dyspnea: a double-blind randomized controlled trial: 2010:
This international double-blind randomized controlled trial evaluated effectiveness of oxygen vs. medical (room) air for relieving breathlessness in patients with life-limiting illness, refractory dyspnea, and PaO2>55 mm Hg. Participants were recruited from outpatient clinics at 9 sites (Australia, United States, England). Participants received oxygen or medical air via concentrator through nasal cannulae at 2 liters/minute for 7 days. The primary outcome measure was breathlessness (0-10 numerical rating scale [NRS]), measured twice daily. This adequately powered study demonstrated no additional symptomatic benefit of oxygen over room air delivered by nasal cannulae for relieving refractory breathlessness related to life-limiting illness in patients with PaO2>55 mm Hg. Dyspnea intensity decreased across the study period in both arms, temporally related to the provision of the gas; improvement in QOL scores and exertional capacity mirrored changes in breathlessness. Breathlessness scores of patients with moderate to severe dyspnea improved most, irrespective of medical gas administered. The temporal relationship between gas delivery and breathlessness reduction suggests that medical air is an intervention, not a placebo. Prior small studies of palliative oxygen vs. medical air have also demonstrated improvements with both gases. Possible reasons are that: the movement of any gas across the nasal passages influences the sensation of dyspnea; the obvious presence of an intervention alleviates the patient’s anxiety and related breathlessness; the concentrator itself may function as a placebo, inducing expectation of benefit; or, the extra attention that the patient receives during study participation improves psychological status, thereby reducing breathlessness. In a similar longitudinal study, dyspnea gradually worsened over an 8-day period, suggesting that study participation does not, in itself, lessen dyspnea.
_
Implications for clinical practice:
Palliative oxygen is widely prescribed in palliative care. These results should therefore be placed in clinical context, providing practical guidance to inform care of patients with refractory breathlessness and advanced life-limiting illness. Interpreted cautiously, these results suggest that moving gas near the nasal passages, and specifically delivered via nasal cannulae, may lead to improved symptoms. The gas, however, need not be oxygen. Effect can be achieved in the setting of other palliative interventions, such as opioids (the option best supported by evidence). Currently, it is difficult to prescribe medical air; prescription of oxygen may be substituted but with important caveats. Oxygen is flammable; smoking patients, and those with smoking caregivers, should not be prescribed oxygen. Oxygen is expensive and may be difficult to obtain. Potentially hypercarbic patients, and especially people with central hypoventilation syndromes, should have close supervision when prescribed oxygen. Given that air motion seems to be an operative factor in relieving breathlessness, a simple hand-held or table-top fan may be a helpful, inexpensive, first step. Treatment of breathlessness with a medical gas – whether oxygen or moving air – may be advisable to alleviate other related symptoms in addition to dyspnea, such as fatigue. Additionally, and especially for patients with less severe dyspnea, nonpharmacological options such as pulmonary rehabilitation should be considered.
________
________
Does oxygen therapy increase the resolution rate of primary spontaneous pneumothorax? 2017 study:
Background:
Patients with small pneumothoraces are usually treated with oxygen therapy. However, evidence that oxygen therapy increases resolution rate is based on small populations with secondary spontaneous pneumothorax. Therefore, this study aimed to confirm whether oxygen therapy increases the resolution rate of primary spontaneous pneumothorax (PSP).
Methods:
Authors retrospectively reviewed records of patients with PSP who had undergone outpatient observation (room air group) and those who were admitted for oxygen therapy (O2 group) between March 2005 and February 2016. The initial chest posteroanterior (PA) radiograph was compared with the last chest PA radiograph before the pneumothorax disappeared. The size of the pneumothorax was measured using the Collins’ method.
Results:
A total of 175 episodes were identified in 160 patients. Of these, 128 episodes (73.1%) occurred in patients in the O2 group. The mean age was 19.24±4.74 years. The mean initial size of the pneumothorax was smaller in the room air group (23.32%±7.00% vs. 20.26%±6.78%, P=0.011). The resolution rate was higher in the O2 group [(4.27%±1.97%) vs. (2.06%±0.97%)/day, P<0.001]. The initial size of the pneumothorax, time interval between radiographs, and use of oxygen therapy were significantly associated with the resolution rate in multivariate analysis.
Conclusions:
Oxygen therapy increases the resolution rate of PSP. However, routine use of oxygen therapy in patients with small pneumothoraces should be considered more carefully. Well-controlled prospective studies are required to confirm the indication of oxygen therapy.
My view:
The only absolute contraindication to hyperbaric oxygen therapy is untreated pneumothorax. Don’t mix up hyperbaric oxygen therapy with normobaric oxygen therapy.
______
______
Paediatric oxygen therapy:
There remains a lack of consensus regarding fundamental issues in paediatric oxygen therapy, but principle differences from adult care must be taken into account in the care of children:
- assessment process – difficulty to obtain arterial blood samples
- clinical conditions in infancy are exclusive although overlaps exist in adolescents
- prognosis in infancy usually positive – children often require oxygen therapy for limited periods
- many children require long term oxygen therapy overnight only – this differs from the 15 hours forming the adult long term therapy definition
- low flow equipment is sometimes required
- supervision from parent/carer essential
- oxygen provision may be necessary within school
_
Indications for oxygen therapy in children:
Where considering the application of oxygen therapy it is essential to perform a thorough clinical assessment of the child
- Transient, self-correcting desaturations that have no other physiological correlates (e.g. Tachycardia, cyanosis) may not routinely require oxygen therapy in most cases.
- The threshold for oxygen therapy can vary with the child’s general state and point in the illness.
- There is no physiological basis for the application of low flow oxygen therapy to a child with normal SpO2 and increased work of breathing.
- The treatment of documented hypoxia/hypoxemia as determined by SpO2 or inadequate blood oxygen tensions (PaO2).
- Achieving targeted percentage of oxygen saturation (as per normal values unless a different target range is specified on the observation chart.)
- The treatment of an acute or emergency situation where hypoxemia or hypoxia is suspected, and if the child is in respiratory distress manifested by:
-dyspnoea, tachypnoea, bradypnoea, apnoea
-pallor, cyanosis
-lethargy or restlessness
-use of accessory muscles: nasal flaring, intercostal or sternal recession, tracheal tug
_
Oxygen flow rate in children:
Normal flow requirement: 3-4 time the minute ventilation (MV = TV X RR)
For example, 5 kgs child breathing at rates of 60/min:
Flow rates needed: 3-4 X (60 X 6 X 5) = 5400-7200 ml/min
_
Oxygen during Neonatal Care in the Neonatal Intensive Care Unit:
The establishment of optimal oxygen saturation targets for preterm infants needing oxygen supplementation in the NICU remains elusive. Preterm infants are very sensitive to hyperoxia, which may lead to lung and retinal damage and also to hypoxia, which may cause increased mortality, NEC (Necrotizing Enterocolitis), or white matter injury. The lung of the extremely preterm infant has a tendency to suffer oxidative stress and inflammation because it lacks an adequately developed antioxidant defense system, is structurally and functionally immature, and frequently requires mechanical ventilation and supplemental oxygen. Additionally, the lungs are prone to infection and are exposed to increased circulating free iron. A connection between oxygen, oxidative stress, mechanical ventilation, and genetic factors and later appearance of BPD has been substantiated in various studies. Thus, preterm neonates who later developed BPD (bronchopulmonary dysplasia) exhibited elevated concentrations in blood and tracheal aspirates of carbonyl adducts, which represent by-products of the attack of oxygen free radicals upon structural and functional proteins of the lung. Similarly, elevated plasma isofurans immediately after birth with higher oxygen load and F2α-isoprostanes in the first week after birth have also been associated with later development of BPD and periventricular leukomalacia, indicating an important role for oxidative injury. In addition to the acute and direct effects of free radicals upon the lung tissue, evidence reveals that specific oxygen species such as hydroperoxides may act as signaling molecules inducing the expression of transcription factors that may alter cell growth, differentiation, chemotaxis, inflammatory response, and/or apoptosis. Exposure to elevated oxygen concentration leads to the release of specific mediators such as VEGF and angiopoietin capable of disrupting the alveolar capillary membrane and thus causing pulmonary edema and subsequent lung injury. Other cytokines are also released from lung cells and attract inflammatory cells to the lung. These inflammatory cells, as well as hyperoxia per se, release ROS, which can initiate the mitochondrial-dependent cell death pathway.
_
Air versus oxygen for resuscitation of infants at birth:
About 5 to 10% of infants need resuscitation at birth. Many experts recommend that these babies be resuscitated with 100% oxygen, but other experts think that normal room air is as good as or better than 100% oxygen. Too much oxygen can make breathing difficult for babies and can cause other problems such as problems with brain development, an eye condition (retinopathy of prematurity), and a lung condition (bronchopulmonary dysplasia). The authors of 2005 Cochrane review questioned whether resuscitation with room air resulted in fewer deaths or disabilities than 100% oxygen. After searching the literature, they found five studies. There were a total of 1302 infants in these studies; 24% of them were premature. In the studies, fewer babies died when resuscitated with room air than with 100 % oxygen. The Cochrane review published in 2005 concluded that a reduction in mortality was seen in infants resuscitated with room air compared with 100% oxygen, and no evidence of harm was demonstrated. Updating the Cochrane review in 2017 found no change in recommendation from the initial review to start in 21% oxygen and have 100% oxygen available as backup.
The dangers from giving oxygen to neonates have also been long appreciated. The most compelling outcome studies of neonates published in 2004 and repeated in 2007 showed a significant increase in mortality of depressed newborns resuscitated with oxygen (13 percent) versus room air (8 percent). This led to the current neonatal resuscitation recommendations for use of room air positive pressure ventilation. A large number of both experimental and clinical studies have primed pediatricians with great awareness of the risks of hyperoxia. For neonatal resuscitation, the routine use of 100 % oxygen has been abandoned after numerous associations with myocardial, neurological, and kidney injury and retinopathy, inflammation, and increased mortality. However, strict adherence to lower target ranges of oxygen saturation among preterm infants did not significantly reduce disability or deaths. Results from a prospective large-scale meta-analysis investigating the most appropriate level of oxygenation for extremely preterm neonates suggested that functional oxyhemoglobin saturation be targeted at 90–95 % in the post-natal period.
_
A series of randomized controlled trials has tried to assess the optimal SpO2 range for preterm infants needing oxygen supplementation after postnatal stabilization. In the BOOST I trial, the effect of higher (95% to 98%) versus lower (91% to 94%) targeted saturations for babies of <30 weeks gestation was compared. The use of higher SpO2 limits was associated with an increased length of oxygen therapy, increased incidence of chronic lung disease, and increased frequency of babies discharged on home oxygen therapy, whereas it did not improve neurodevelopment or somatic growth. In another randomized controlled trial (STOP-ROP, or Supplemental Therapeutic Oxygen for Prethreshold `Retinopathy of Prematurity), a group of preterm infants with prethreshold ROP was randomized to SpO2 limits set between 89% and 94% or between 95% and 99% for a minimum of 2 weeks. The beneficial effect of higher SpO2 on the evolution of eye disease was minimal, whereas the negative effects such as prolonged hospitalization, respiratory morbidity, and prolonged need for oxygen supplementation were significantly higher.
_
Five large multicenter, international, masked, randomized controlled trials, the NeOProM studies, have been conducted. These trials enrolled nearly 5000 extremely low birth-weight (ELBW) infants of <28 weeks gestation and sought to identify the optimal saturation target while following almost identical designs to allow for subsequent individual-level patient meta-analysis. All of these studies compared low-target SpO2 (85% to 89%) with high-target (91% to 95%). The SUPPORT trial confirmed that surviving ELBW infants randomly assigned to lower SpO2 target ranges (85% to 89%) had a lower risk of ROP (8.6% vs 17.9%; p < 0.01) than those in the higher target group (91% to 95%). However, unexpectedly, a significantly increased mortality was present in the low saturation group (19.9% vs 16.2%; p< 0.04). In addition, the BOOST II trial performed in the United Kingdom, Australia, and New Zealand showed similar results, with higher ROP in the babies maintained in the high saturation range and higher mortality in babies kept in the low saturation range. In contrast, the Canadian Oxygen Trial, using a primary outcome that was a composite of death, gross motor disability, cognitive or language delay, severe hearing loss, or bilateral blindness at a corrected age of 18 months, with secondary outcomes of ROP and brain injury, did not find significant differences in death or disability in babies in the lower compared to the higher saturation range. In a meta-analysis that included all the NeOProM studies, with a total of approximately 5000 babies, it was concluded that when targeting SpO2 in the lower range there was an increased risk of mortality (risk ratio [RR], 1.41 [95% CI, 1.14 to 1.74]) and NEC (RR, 1.25 [95% CI, 1.05 to 1.49]). However, in the lower saturation range there was a significantly decreased risk of ROP (RR, 0.74 [95% CI, 0.59 to 0.92]). The authors of this meta-analysis concluded that SpO2 targets of 90% to 95% for babies born at <28 weeks gestation needing supplemental oxygen were recommended until 36 weeks postmenstrual age.
_
Although all of these trials had an impeccable design, some concerns especially from a technical perspective have raised doubts about the practical applicability of their conclusions. It is relevant to know that the accuracy of the reading of the pulse oximeters employed was 2.9%. Thus, if the displayed SpO2 is 88%, true saturation may be in the 85% to 91% range in 68% of observations and in the 82% to 94% range in 95% of observations. In addition, the algorithm used in these trials resulted from the fusion of a high- and a low-range algorithm. The effect of this dual curve was that the SpO2 values in the region of 87% to 90% (at the junction of the lower and higher algorithm curves) were shifted upward. Readings were therefore factitiously elevated around 2% in the proximity of saturations in the 90% range. Given the relatively small separation in the mean SpO2 between the high and the low target groups, these technical considerations make interpretation of the data challenging, and the optimal saturation range for extremely preterm infants needing oxygen supplementation remains elusive. It is likely that there is no fixed SpO2 range or oxygen supply that safely satisfies metabolic demands of all infants born at different gestational ages. Moreover, even for a given gestational age, postnatal age is also a relevant factor to be taken into consideration when establishing oxygen saturation limits. While SpO2 is relatively easily measured, it is not a direct reflection of tissue oxygen delivery. In addition to oxyhemoglobin saturation, oxygen delivery at the tissue level is affected by oxygen carrying capacity (hemoglobin level) and circulation and may more directly reflect the organism’s oxygen sufficiency or excess. Finally, it is important to note that the findings of these trials may be largely related to our limited ability to maintain the SpO2 within the target ranges during routine care. The exposure to extreme levels of SpO2 may be more strongly related to the observed outcomes than the target ranges. In these trials the actual SpO2 levels did not exactly match the target ranges, and the exposure to extremely high or low SpO2 ranges may have differed between the target ranges. In daily practice, during routine care SpO2 levels above the target range are frequently tolerated to reduce hypoxemia, but this practice increases the exposure to high SpO2. Conversely, targeting lower SpO2 ranges to avoid hyperoxemia can increase exposure to very low SpO2 levels. Tolerance of high SpO2 to avert hypoxemia spells or targeting low SpO2 ranges to avoid hyperoxemia may not be necessary if the maintenance of the intended range of SpO2 could be improved and exposure to extreme high or low SpO2 minimized. Until further evidence is available, keeping preterm babies within a range from 90% to 95% seems a reasonable approach. Minimizing fluctuation in SpO2 would be desirable, because alternating hypoxia and hyperoxia is known to be a proinflammatory stimulus. To that effect, there is a great deal of interest in improving oxygen saturation targeting by automatic control of FiO2.
_
Patent ductus arteriosus (PDA) and oxygen therapy in neonate:
In the developing fetus, the DA (ductus arteriosus) is the vascular connection between the pulmonary artery and the aortic arch that allows most of the blood from the right ventricle to bypass the fetus’ lungs, which are fluid-filled and compressed. During fetal development, this shunt protects the right ventricle from pumping against the high resistance in the lungs, which can lead to right ventricular failure if the DA closes in utero. When the newborn takes his or her first breath, the lungs open and pulmonary vascular resistance decreases. After birth, the lungs release bradykinin to constrict the smooth muscle wall of the DA and reduce blood flow as it narrows and then completely closes. In most newborns with a patent ductus arteriosus (PDA), the blood flow is reversed from that of in utero flow; i.e., the blood flow is from the higher-pressure aorta to the now lower-pressure pulmonary arteries. In normal newborns, the DA is substantially narrowed within 12–24 hours after birth, and seals completely after three weeks. The primary stimulus for closure of the DA is an increase in neonatal blood oxygen content. Withdrawal from maternal circulating prostaglandins also contributes to ductal closure. So neonatal hypoxia may prevent closure of DA. Clinically significant PDA is quite frequent in very sick preterm infants with RDS, prolonged hypoxia and acidosis. Noori et al.’s study explores these by comparing the incidence of what they term as haemodynamically significant patent ductus arteriosus (PDA) in the two epochs either side of the change in oxygen policy. Noori et al. showed only small increases in PDA as defined in this study with lower oxygen saturation targeting. Although this is reassuring, the trend is there and, with greater statistical power, this might become more significant.
______
______
Ozone therapy:
Ozone (O3), a gas discovered in the mid-nineteenth century, is a molecule consisting of three atoms of oxygen in a dynamically unstable structure due to the presence of mesomeric states. The gas is colorless, acrid in odour and explosive in liquid or solid form. High in the atmosphere, some oxygen (O2) molecules absorbed energy from the Sun’s ultraviolet (UV) rays and split to form single oxygen atoms. These atoms combined with remaining oxygen (O2) to form ozone (O3) molecules, which are very effective at absorbing UV rays. High up in the atmosphere, ozone forms a layer that shields the Earth from ultraviolet rays. However, at ground level, ozone is considered a major air pollutant. Ozone is diamagnetic, which means that its electrons are all paired. In contrast, O2 is paramagnetic, containing two unpaired electrons. It has a half-life of 3 days at 20°C in air. Although O3 has dangerous effects, yet researchers believe it has many therapeutic effects. The beginning of precise medical O3 generators has only recently allowed the mechanisms, action and possible toxicity of O3 to be evaluated by clinical trials. Ozone has a capacity to oxidize organic compounds, and has well-known toxic effects on the respiratory tract when present in smog. In medical use the gas produced from medical grade oxygen is administered in precise therapeutic doses, and never via inhalation, and advocates that it has excellent health benefits in dental caries, decrease blood cholesterol and stimulation of antioxidative responses, modifies oxygenation in resting muscle and is used in complementary treatment of hypoxic and ischemic syndromes.
_
Mechanism of action:
- Inactivation of bacteria, viruses, fungi, yeast and protozoa:
Ozone therapy disrupts the integrity of the bacterial cell envelope through oxidation of the phospholipids and lipoproteins. In fungi, O3 inhibits cell growth at certain stages. With viruses, the O3 damages the viral capsid and upsets the reproductive cycle by disrupting the virus-to-cell contact with peroxidation. The weak enzyme coatings on cells which make them vulnerable to invasion by viruses make them susceptible to oxidation and elimination from the body, which then replaces them with healthy cells.
- Stimulation of oxygen metabolism:
Ozone therapy causes an increase in the red blood cell glycolysis rate. This leads to the stimulation of 2,3 diphosphoglycerate which leads to an increase in the amount of oxygen released to the tissues. Ozone activates the Krebs cycle by enhancing oxidative carboxylation of pyruvate, stimulating production of ATP. It also causes a significant reduction in NADH and helps to oxidize cytochrome C. There is a stimulation of production of enzymes which act as free radical scavengers and cell-wall protectors: glutathione peroxidase, catalase and superoxide dismutase. Production of prostacyline, a vasodilator, is also induced by O3.
- Activation of the immune system:
Ozone administered at a concentration of between 30 and 55 μg/cc causes the greatest increase in the production of interferon and the greatest output of tumor necrosis factor and interleukin-2. The production of interleukin-2 launches an entire cascade of subsequent immunological reactions.
- Mechanism of action of O3 on the human lung:
Ozone exposure induces a significant mean decrement in vital capacity. It significantly increases mean airway resistance and specific airway resistance but does not change dynamic or static pulmonary compliance or viscous or elastic work. It also significantly reduces maximal transpulmonary pressure. And further more significantly increases respiratory rate and decreased tidal volume.
_
Medication forms in a gaseous state are somewhat unusual, and it is for this reason that special application techniques have had to be developed for the safe use of O3. In local applications as in the treatment of external wounds, its application in the form of a transcutaneous O3 gas bath has established itself as being the most practical and useful method, for example at low (sub-atmospheric) pressure in a closed system guaranteeing no escape of O3 into the surrounding air. Ozonized water, whose use is particularly known in dental medicine, is optimally applied as a spray or compress. Diseases treated are infected wounds, circulatory disorders, geriatric conditions, macular degeneration, viral diseases, rheumatism/arthritis, cancer, SARS and AIDS.
_
Intraarticular O3 therapy for pain control in osteoarthritis of the knee:
Ozone is being currently tested for its effectiveness in relieving the pain in patients suffering from osteoarthritis of the knee. The current status of the study is phase 2 which is sponsored by Ben-Gurion University of the Negev and the study being conducted by NCT00832312.
The Effect of Ozone Therapy for Lumbar-Herniated Disc:
Ozone is also being evaluated for its efficacy infiltration and its effectiveness in comparison with microdiscectomy in the treatment of lumbar-herniated disc with criteria for surgery. The study is currently in its phase 2 studies, which is sponsored by Kovacs Foundation and trials being carried out by NCT00566007. The study also evaluates the efficacy of infiltration in presence of corticoids, anaesthetics, which is being compared by replacing O3 by oxygen.
_______
_______
Heliox therapy:
The atmosphere comprises various distinct gases, the most abundant being nitrogen (~78%), followed by oxygen (~21%). Helium is only present in five parts per million (0.0005%) in the lower atmosphere. Nitrogen and helium have comparable viscosity, but helium has higher thermal conductivity compared to nitrogen. As a result, when a heliox gas mixture (79% helium and 21% oxygen) is produced, it has a viscosity similar to, but a density nearly six times lowers than atmospheric air. Due to these properties, heliox has potential applications in respiratory medicine. Heliox is a low density gas mixture of helium and oxygen commonly used in deep diving (> 6 ATM). This mixture has been also used for clinical purposes, particularly in the critical care setting. Heliox gas mixtures are known to be nontoxic, noncarcinogenic, and have no lasting effects on any human organs. Due to its lower density, inhalation of heliox results in significantly lower turbulence, particularly in the more distal portions of the lung. This effect translates to a greater proportion of laminar flow and lower overall airway resistance. The decreased turbulence effect results in increased flow rates by up to 50% during heliox inhalation. This decreased turbulence remained evident even when airflow was restricted, as in the case of obstructive lung disease. Combining helium and oxygen gas (heliox) results in a gas with a viscosity similar to air but with a density substantially lower. Because low density gas has the potential to decrease resistance to gas flow, heliox has been advocated for treating obstructive lesions of the larynx, trachea, and airways since 1935.
_
The clinical benefits ascribed to helium-oxygen mixtures include:
- improved ventilation and reduced barotrauma;
- reduced peak airway and plateau pressures;
- increased tidal volumes;
- reduced inspiratory-expiratory ratios;
- improved homogeneity of gas distribution;
- improved elimination of carbon dioxide; and
- movement of the equal-pressure point of the airways upstream.
_
Care must be taken to administer heliox in a safe and effective manner. To avoid administration of a hypoxic gas mixture, it is recommended that 20% oxygen/80% helium is mixed with oxygen to provide the desired helium concentration and FiO2. If FiO2 requirement >40%, the limited concentration of helium is unlikely to produce clinical benefit. When using an oxygen-calibrated flow meter for heliox therapy, it must be remembered that the flow of heliox (80% helium and 20% oxygen) will be 1.8 times greater than the indicated flow. For spontaneously breathing patients, heliox is administered by face mask with a reservoir bag. Y-piece attached to the mask allows concurrent delivery of aerosolized medications. Sufficient flow is required to minimize contamination of the heliox with ambient air i.e.12 to 15 L/min. Administration during mechanical ventilation can be problematic as density, viscosity, and thermal conductivity of helium affect the delivered tidal volume and the measurement of exhaled tidal volume
_
The physical properties of helium make it an ideal agent for use as a carrier gas for oxygen in place of nitrogen for various conditions affecting the respiratory tract. The low density and viscosity of Heliox helps to promote greater and smoother gas flow, decrease airway resistance, and decrease the work of breathing in selected patients. There is a body of evidence to support its use in parallel with conventional forms of treatment in asthma, COPD, croup, and a variety of other respiratory conditions, although further work will be required to define its precise role. Helium-oxygen use can reduce work of breathing in severe airway obstruction in patients with asthma and fixed airway obstruction. Heliox rapidly improves ventilation in patients presenting to an emergency department with acute severe asthma with respiratory acidosis and a short duration of symptoms. In spontaneously breathing patients with asthma, heliox decreases PaCO2, increases peak flow, and decreases pulsus paradoxus. Heliox reduces resistance with upper airway obstruction (post extubation stridor).
______
______
Why oxygen in airplane?
At sea level, our bodies are subjected to about 14.7 pounds of pressure per square inch from column of air. As we climb in altitude, the amount of air pressure acting on us decreases rapidly. You notice the decrease when your ears pop while driving up a mountain or riding a fast elevator. Although the atmosphere is 300 miles thick, most of the air molecules are squashed down to within a few thousand feet of the earth’s surface. As we climb higher, air molecules are spread farther apart. When we breathe, our lungs take in less air, and less oxygen. At 18,000 feet, the atmospheric pressure is down to 7.3 psi, about half the sea-level pressure. There just isn’t enough oxygen in a breath of air to adequately supply the brain. At this pressure, a healthy adult has only 20-30 minutes of useful consciousness. Airliners fly between 30,000 and 43,000 feet. At those altitudes the atmosphere provides less than 4 psi of pressure. If you tried breathing at that altitude, your useful consciousness would be less than a minute (followed soon after by death).
Table below shows oxygen in your blood at high altitudes.
| Altitude (feet): | PAO2: | PaO2: | SaO2: |
| 0 | 101.8 | 82.8 | 96.0% |
| 2000 | 90.7 | 72.7 | 94.6% |
| 4000 | 80.0 | 63.0 | 89.7% |
| 6000 | 70.1 | 54.1 | 87.8% |
| 8000 | 60.7 | 45.7 | 81.5% |
| 10000 | 52.1 | 38.1 | 72.0% |
Airplanes that travel at 30,000 to 40,000 feet are usually pressurized to an equivalent altitude of 6000 to 8000 feet and the lower limit of normal for the SaO2 of airplane travellers is usually considered to be between 89% and 91%. However if a plane suffers decompression at 30,000 feet, the first priority is to get down to a safe altitude, and give everybody oxygen to keep them alive.
______
______
Oxygen bar:
An oxygen bar is an establishment, or part of one, that sells oxygen for recreational use. Individual flavored scents may be added to enhance the experience. The flavors in an oxygen bar come from bubbling oxygen through bottles containing aromatic solutions before it reaches the nostrils: most bars use food-grade particles to produce the scent, but some bars use aroma oils. Modelled after the “air stations” in polluted downtown Tokyo and Beijing, the first official oxygen bar (the O2 Spa Bar) opened in Toronto, Canada, in 1996. The trend continued in North America and by the late 1990s bars were in use in New York, California, Florida, Las Vegas and the Rocky Mountain region. Customers in these bars breathe oxygen through a plastic hose inserted into their nostrils. Oxygen bars can now be found in many venues such as nightclubs, salons, spas, health clubs, resorts, tanning salons, restaurants, coffee houses, bars, airports, ski chalets, yoga studios, chiropractors, and casinos. They can also be found at trade shows, conventions and corporate meetings, as well as at private parties and promotional events.
_
Oxygen bar guests pay about one U.S. dollar per minute to inhale a percentage of oxygen greater than the normal atmospheric content of 21 % oxygen. This oxygen is produced from the ambient air by an industrial (non-medical) oxygen concentrator and inhaled through a nasal cannula for up to about 20 minutes. The machines used by oxygen bars or oxygen vendors differ from the typical medical-issue machine, although customers use the cannula, the rubber tube apparatus that fits around the ears and inserts in the nostrils, to breathe in the oxygen. Customers can enhance their experience by using aromatherapy scents to be added to the oxygen, such as lavender or mint.
_
Proponents claim this practice is not only safe, but enhances health and well-being, including strengthening the immune system, enhancing concentration, reducing stress, increasing energy and alertness, lessening the effects of hangovers, headaches, and sinus problems, and generally relaxing the body. It has also been alleged to help with altitude sickness. However, no long-term, well-controlled scientific studies have confirmed any of the proponents’ claims. Furthermore, the human body is adapted to 21 percent oxygen, and the blood exiting the lungs already has about 98 percent of the oxygen that it could carry bound to hemoglobin. Having a higher oxygen fraction in the lungs serves no purpose, and may actually be detrimental. Higher than normal oxygen partial pressure can also indirectly cause carbon dioxide narcosis in patients with severe chronic obstructive pulmonary disease (COPD). The FDA warns that in some situations, droplets of flavoring oil can be inhaled, which may contribute to an inflammation of the lungs. Oxygen may also cause serious side effects at excessive doses. Although the effects of oxygen toxicity at atmospheric pressure can cause lung damage, the low fraction of oxygen (30–40%) and relatively brief exposures make pulmonary toxicity unlikely. Nevertheless, due caution should be exercised when consuming oxygen. Another concern is the improper maintenance of oxygen equipment. Some oxygen concentrators use clay filters which cause micro-organisms to grow creating an additional danger that can cause lung infections. Also, concentrated oxygen is a flame accelerant which should be kept away from cigarettes and other sources of ignition.
____
____
Oxygen therapy for animal patients:
Figure below shows a Labrador retriever with a nasal catheter placed for oxygen supplementation.
_
Oxygen therapy is indicated for any animal patient presented in respiratory distress. Even animal patient with mild-to-moderate respiratory distress should receive supplemental oxygen. Animal patients in severe distress require oxygen supplementation immediately, before attempts to identify the cause. If quantitative measurements are available, oxygen supplementation should be provided to any animal patient with oxygen saturation (SaO2) or pulse oximetry reading of (SpO2) of <93% or with an arterial partial pressure of oxygen (PaO2) of <80 mm Hg. Minimal risk is associated with short-term oxygen supplementation, which rapidly benefits most hypoxic animal patients. Oxygen supplementation can be provided while a quick animal patient assessment is performed. This includes evaluation for upper airway obstruction that may require immediate treatment with endotracheal intubation or tracheostomy. Oxygen supplementation should also be provided for animal patients with respiratory distress during catheter placement, thoracocentesis, radiography, or sedation for any other procedures. Oxygen can be supplied from various sources (e.g., centralized in-house oxygen, portable oxygen tanks, anaesthetic machines) and in many different ways, depending on the severity of respiratory distress, the need to handle the animal patient while providing oxygen, the duration supplementation is needed, the available equipment, and the clinical experience and skills of the veterinary clinician.
______
______
Side-effects, complications and toxicity of normobaric oxygen therapy:
I have already discussed complications of hyperbaric oxygen (HBO) therapy. Oxygen safety issues have also been discussed earlier. Contraindication of oxygen therapy is also discussed earlier. Now I discuss side-effects, complications and toxicity of normobaric oxygen (NBO) therapy i.e. routine oxygen therapy in hospitals and homes.
_
Side-effects of oxygen therapy:
Some patients who use oxygen therapy may have side effects, including:
- Dry or bloody nose
- Irritated skin around the cannula or mask
- Tiredness or drowsiness
- Morning headaches
These side effects can be relieved by:
- Changing the oxygen delivery equipment
- Adjusting the amount of oxygen
- Changing how often oxygen is used
- Using a humidifier or nasal spray
The side effects may include a dry or bloody nose, skin irritation from the nasal cannula or face mask, fatigue, tiredness and morning headaches. Some people only suffer side effects initially upon first use and then they disappear, however if these problems persist then all you need to do is to inform your doctor and provider. Depending upon the problems all your doctor may need to do is to alter the oxygen flow rate or length of time you’re using the equipment. If nose dryness is a problem then you may just require an additional nasal spray or to have a humidifier attached to your equipment to reduce the dryness effect of the oxygen. If you experience irritation from the mask or cannula then your provider can try other devices that may fit you better and can recommend over-the-counter gels and devices designed to help lessen skin irritation. Appropriate flow rates are imperative for effective and safe oxygen delivery. Drying and dehydration of the nasal mucosa, respiratory epithelial degeneration, and impaired mucosal ciliary clearance increase the risk of infection in patients receiving supplemental oxygen. Humidification decreases the risk of mucosal damage exponentially. If you use transtracheal oxygen therapy then complications can potentially be a bit more serious due to the more invasive way that the oxygen is delivered via a tube inserted into your trachea. You may develop mucus balls which can cause coughing and clog the trachea, plus infection and injury to trachea.
_
CO2 narcosis:
Increase in PaCO2 due to high concentration oxygen therapy in severe COPD and other respiratory illnesses has been discussed earlier.
_
Absorption atelectasis:
Atelectasis refers to the partial or complete collapse of the lungs. About 80% of the gas in the alveoli is nitrogen. Nitrogen helps keep the alveoli open and prevents the collapse of the alveoli. If high concentrations of oxygen are provided, the nitrogen is displaced. When the oxygen diffuses across the alveolar-capillary membrane into the bloodstream, the nitrogen is no longer present to distend the alveoli (called a nitrogen washout). This reduction in alveolar volume results in a form of collapse called absorption atelectasis. This situation also causes an increase in the physiologic shunt and resulting hypoxemia.
_____
_____
Oxygen toxicity:
High fractions of inspired oxygen (FiO2) have been associated with several effects on lung tissue/gas exchange including diminished lung volumes and hypoxemia due to absorptive atelectasis, accentuation/production of hypercapnia, and damage to airways and pulmonary parenchyma. The term “oxygen toxicity” is usually reserved for the last of these consequences, i.e., tracheobronchial and alveolar damage.
_
Oxygen is prescribed in many medical emergencies in which tissue oxygenation is threatened because of respiratory failure, (defined as PaO2 < 8.0 kPa) and/or reduced tissue perfusion. Depending on the mode of delivery, the fraction of inspired oxygen (FiO2) associated with oxygen therapy can range from 25 % to 100 %, compared with normal FiO2 of 21 % when breathing ambient air at sea level. Since FiO2 determines PaO2, high-dose oxygen therapy (FiO2 > 50 %) can cause PaO2 to rise well in excess of the upper limit of the reference range, a condition called hyperoxemia that potentially results in hyperoxia (increased oxygen in tissues). Notwithstanding the general appreciation that oxygen in excess is potentially toxic to tissue cells, it has been assumed that, with the notable exception of neonates who are particularly vulnerable, transient hyperoxia is a side effect of high-dose oxygen therapy that is essentially harmless if not unduly prolonged, and well worth the cost of avoiding tissue hypoxia. This assumption is now being challenged, and there is a growing body of clinical study directed at establishing the real safety profile of hyperoxia during oxygen therapy.
_
My definition of hyperoxia:
Hyperoxia occurs when cells, tissues, blood and organs are exposed to higher than normal partial pressure of oxygen. Hyperoxia definition need not entail harm just as definitions of Hypertension and Diabetes entail no harm in it.
Hyperoxia means PAO2 > 110mm and/or PaO2 > 100mm and/or tissue PO2 > 50 mm.
For example, FiO2 is increased to 60 % from normal 21% to increase PAO2 to 380mm but due to severity of illness e.g. massive pneumonia or pulmonary edema, PaO2 is still 60mm, it is hyperoxia for lungs although rest of the body is hypoxic. If such situation continues for several days, patient may develop lung injury due to oxygen toxicity although rest of the body is hypoxic.
_
Hyperoxia is a state of excess supply of O2 in tissues and organs. Oxygen toxicity occurs when the partial pressure of alveolar O2 (PAO2) exceeds that which is breathed under normal conditions. With continuous exposure to supraphysiologic concentrations of O2, a state of hyperoxia develops. Under hyperoxic pathological conditions, a large influx of reactive O2 species (ROS) are produced. In intracellular and extracellular biological systems, the mass effect of ROS elevation, caused by O2 overexposure, disrupts the balance between oxidants and antioxidants, and this disruption of homeostasis can result in damage to cells and tissues. Oxygen toxicity, caused by excessive or inappropriate supplemental oxygen, can cause severe damage to the lungs and other organ systems. High concentrations of oxygen, over a long period of time, can increase free radical formation, leading to damaged membranes, proteins, and cell structures in the lungs. It can cause a spectrum of lung injuries ranging from mild tracheobronchitis to diffuse alveolar damage. The latter is histologically indistinguishable from that observed in the acute respiratory distress syndrome (ARDS). For this reason, oxygen should be administered so that appropriate target saturation levels are maintained.
_
Oxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen (O2) at increased partial pressures. It is also known as oxygen toxicity syndrome, oxygen intoxication, and oxygen poisoning. Oxygen toxicity is a concern for underwater divers, those on high concentrations of supplemental oxygen (particularly premature babies), and those undergoing hyperbaric oxygen therapy. The result of breathing increased partial pressures of oxygen is hyperoxia, an excess of oxygen in body tissues. The body is affected in different ways depending on the type of exposure. Central nervous system toxicity is caused by short exposure to high partial pressures of oxygen at greater than atmospheric pressure. Pulmonary and ocular toxicity result from longer exposure to increased oxygen levels at normal pressure. Symptoms may include disorientation, breathing problems, and vision changes such as myopia. Prolonged exposure to above-normal oxygen partial pressures, or shorter exposures to very high partial pressures, can cause oxidative damage to cell membranes, collapse of the alveoli in the lungs, retinal detachment, and seizures. Oxygen toxicity is managed by reducing the exposure to increased oxygen levels. Studies show that, in the long term, a robust recovery from most types of oxygen toxicity is possible.
_
History of oxygen toxicity:
In 1774, Joseph Priestley published his discovery of the substance that Antoine Lavoisier subsequently called “oxygen.” In his original report, Priestley recognized intuitively the double-edged nature of oxygen therapy, writing that “it might be peculiarly salutary to the lungs in certain morbid cases … but…it might not be so proper for us in the usual healthy state of the body.” Lavoisier confirmed these suspicions when he noted that all guinea pigs placed in “pure” oxygen died with “lungs that were very flaccid but very red” after several days of exposure. By 1939, an already impressive body of literature suggested that rodents and dogs died with injury to the lungs after several days of exposure to hyperoxia. Becker-Freyseng and Clamann, who were skeptical that these findings were applicable to humans, subjected themselves to 65 h of continuous hyperoxia (90% oxygen). After 1 day of exposure, both men complained of progressively worsening paresthesias and dyspnea and developed leukocytoses. By the end of the exposure, one of the men was hospitalized with fever, tachycardia, and a progressive drop in his vital capacity. His examination revealed “fluid in the right thorax and signs of bronchopneumonia” and his vital capacity did not recover until 6 weeks after the exposure. More rigorous confirmation of these findings in subsequent studies has left modern physicians to grapple with concern about oxygen’s toxicity in the face of its obvious clinical benefit. Detailed guidelines have been published for the monitoring of arterial oxygen saturations and the provision of oxygen therapy in acutely ill adults.
_
Oxygen toxicity affecting various organs and tissues:
Oxidative damage may occur in any cell in the body but the effects on the three most susceptible organs (brain, eyes and lungs) will be the primary concern. It may also be implicated in damage to red blood cells (haemolysis), the liver, heart, endocrine glands (adrenal glands, gonads, and thyroid), or kidneys, and general damage to cells.
_
The major limitation confronting a much more liberal clinical use of hyperoxia is its potential toxicity and the relatively narrow margin of safety that exists between its effective and toxic doses. However, an awareness of the toxic effects of oxygen and an acquaintance with safe pressure and duration limits of its application, combined with the ability to carefully manage its dose, provide an acceptable basis for expanding the current list of clinical indications for its use. The most obvious toxic manifestations of oxygen are those exerted on the respiratory system and central nervous system (CNS).
_
The lungs are exposed to higher oxygen tensions than any other organ. At exposures to ambient oxygen pressures of up to 0.1 MPa (1 ATA), the lungs are the first organ to respond adversely to the toxic effects of oxygen. The response involves the entire respiratory tract, including the airway epithelium, microcirculation, alveolar septa, and pleural space. Pulmonary oxygen toxicity is characterized by an initial period in which no overt clinical manifestations of toxicity can be detected – termed the ‘latent period’. The duration of this ‘silent’ clinical interval is inversely proportional to the level of inspired oxygen. Pulmonary oxygen toxicity occurs when 100% O2 given for 12 hours or more; 80% O2 for more than 24hrs; and 60% O2 more than 36hrs.
- Acute tracheobronchitis is the earliest clinical syndrome that results from the toxic effects of oxygen on the respiratory system. It does not develop in humans breathing oxygen at partial pressures of below 0.05 MPa (0.5 ATA or 50% oxygen at normal atmospheric pressure i.e. FiO2 50%). In healthy humans breathing more than 95% oxygen at normal atmospheric pressure (0.1 MPa), tracheobronchitis develops after a latent period of 4 to 22 hours and may occur as early as 3 hours while breathing oxygen at 0.3 MPa (3 ATA). It can start as a mild tickling sensation, later followed by substernal distress and inspiratory pain, which may be accompanied by cough and, when more severe, by a constant retrosternal burning sensation. Tenacious tracheal secretions may accumulate. Upon termination of hyperoxic exposure, the symptoms subside within a few hours, with complete resolution within a few days.
- Longer exposures to oxygen (usually more than 48 hours at 0.1 MPa) may induce diffuse alveolar damage (DAD). The clinical symptoms as well as the laboratory, imaging, and pathologic findings of oxygen-induced DAD are not significantly different from those of ARDS from other causes.
- Resolution of the acute phase of pulmonary oxygen toxicity or prolonged exposures to oxygen at sublethal concentrations such as during prolonged hyperoxic mechanical ventilation may result in a chronic pulmonary disease characterized by marked residual pulmonary fibrosis and emphysema with tachypnea and progressive hypoxemia.
The relative contributions of hyperoxia, the underlying clinical condition, and mechanical ventilation to the occurrence of chronic pulmonary fibrosis and emphysema in human adults have yet to be clarified.
_
CNS oxygen toxicity occurs in humans at much higher oxygen pressures, above 0.18 MPa (1.8 ATA) in water and above 0.28 MPa (2.8 ATA) in dry exposures in a hyperbaric chamber. Hence, CNS toxicity does not occur during normobaric exposures but is the main limitation for the use of HBO in diving and hyperbaric treatments. The ‘latent’ duration until the appearance of symptoms of CNS oxygen toxicity is inversely related to the oxygen pressure. It may last for more than 4 hours at 0.17 to 0.18 MPa and may be as short as 10 minutes at 0.4 to 0.5 MPa.
- The most dramatic manifestation of CNS oxygen toxicity is a generalized tonic-clonic (grand mal) seizure. Hyperoxia-induced seizures are believed to be reversible, causing no residual neurologic damage and disappearing upon reduction of the inspired oxygen partial pressure. Early abnormal changes in cortical electrical activity were reportedly seen on exposure to HBO a few minutes prior to the full development of the electrical discharges. Unfortunately, no real-time on-line definition of the preseizure electroencephalogram (EEG) activity which could serve as an early EEG indicator of CNS oxygen toxicity is available.
- Other symptoms of CNS toxicity include nausea, dizziness, sensation of abnormality, headache, disorientation, light-headedness, and apprehension as well as blurred vision, tunnel vision, tinnitus, respiratory disturbances, eye twitching, and twitching of lips, mouth, and forehead. CNS toxicity does not appear to have warning signs as there is no consistency in the pattern of appearance of symptoms and no typical gradual sequence of minor signs appearing prior to the full development of the seizures.
The most dramatic personal factor that may modify the sensitivity to CNS oxygen toxicity is an increase in blood pCO2 (partial pressure of carbon dioxide). Hypercapnia occurs in patients due to hypoventilation, chronic lung diseases, effects of analgesics, narcotics, other drugs, and anaesthesia, and should be taken into consideration in designing individual hyperoxic treatment protocols. Various pharmacologic strategies were tested in animal models for postponing hyperoxic-induced seizures. However, none of them has shown clinically relevant efficacy.
Due to its potential toxic effects, HBO is currently restricted to short sessions (less than 2 hours), at pressures below the threshold of CNS toxicity (0.28 MPa), with ‘recovery’ breaks of few minutes during which the patient is switched to air breathing at the treatment pressure. As for NBO, whenever possible, it should be restricted to periods shorter than the latent period for development of pulmonary toxicity. When used according to currently employed standard protocols, oxygen therapy is extremely safe.
_
Premature infants ventilated with hyperoxic gas mixtures are at high risk of the development of retinopathy of prematurity and bronchopulmonary dysplasia. The retinopathy of prematurity has been hypothesized as resulting from an initial hyperoxia-induced stimulation of the PHDs, which prevents the stabilization of HIF. The resulting loss of VEGF and other HIF target genes impairs development of the retinal vasculature. As the nonvascular tissue in the retina develops and becomes increasingly metabolically active, the imbalance between oxygen demand and supply causes local tissue hypoxia and excessive HIF-mediated release of VEGF, which induces the neovascularization characteristic of the retinopathy of prematurity. In support of this hypothesis, the risk of retinopathy is increased both by exposure to hyperoxic gas mixtures and by large fluctuations in arterial oxygen saturation, and both restrictive and liberal oxygen saturation targets in neonates have been associated with adverse long-term outcomes. Determining optimal strategies for oxygen therapy in these patients remains an active area of investigation. Retinopathy of prematurity occurs when PaO2 more than 80mmhg for more than 3 hrs in new born and very premature babies are more susceptible.
Pulmonary and ocular damage are most likely to occur when supplemental oxygen is administered as part of a treatment, particularly to newborn infants, but are also a concern during hyperbaric oxygen therapy.
_
Hyperventilation of atmospheric air at atmospheric pressures does not cause oxygen toxicity, because sea-level air has a partial pressure of oxygen of 0.21 bar (21 kPa) whereas toxicity does not occur below 0.3 bar (30 kPa). Contrary to popular myth, hyperventilating air at ordinary pressures never causes oxygen toxicity and the dizziness is due to CO2 levels dropping too low.
_
The diagram above shows that oxygen toxicity depends upon both the oxygen pressure and the duration of exposure. The safe duration of exposure becomes shorter as the partial pressure of oxygen increases.
Below 0.5 bar, indefinite exposure appears to be safe
Between 0.5 and 1.6 bar, pulmonary toxicity occurs after prolonged exposures but CNS effects are not detectable
Above 2.5 bar (HBO), CNS toxicity appears before pulmonary effects are detectable.
_
Exposure time, atmospheric pressure, and fraction of inspired O2 (FIO2) determine the cumulative O2 dose leading to toxicity. Exposure of most mammalian species to an FiO2 >0.9 for more than 72 to 96 h is required to induce lung injury, whereas exposure to a lower level of hyperoxia (FiO2 between 0.8 and 0.85) for more than a week causes lung injury with fibroproliferation. Based on these data, most investigators speculate that an FIO2 <0.7 is nontoxic, and these recommendations form the basis for most protocols of ventilator management. Similarly, the lowest acceptable level of arterial oxygen saturation is difficult to define because tissue oxygen delivery depends on the arterial oxygen content, the cardiac output, and the health of the vasculature. In the absence of definitive data, most guidelines define an arterial oxygen saturation of 88% as the lowest acceptable level. In individual patients, these targets might be adjusted based on global measures of perfusion including the mixed venous oxygen saturation and arterial lactate levels. As an example, the current ARDS Network guidelines for the management of oxygen therapy in patients with ARDS recommend the use of an FIO2 >0.7 only in patients who require >12 cm H2O of positive end-expiratory pressure (PEEP) to maintain an arterial oxygen saturation >88% or a PaO2 >55 torr.
__
Diagnosis of oxygen toxicity:
Diagnosis of central nervous system oxygen toxicity in divers prior to seizure is difficult as the symptoms of visual disturbance, ear problems, dizziness, confusion and nausea can be due to many factors common to the underwater environment such as narcosis, congestion and coldness. However, these symptoms may be helpful in diagnosing the first stages of oxygen toxicity in patients undergoing hyperbaric oxygen therapy. In either case, unless there is a prior history of epilepsy or tests indicate hypoglycaemia, a seizure occurring in the setting of breathing oxygen at partial pressures greater than 1.4 bar (140 kPa) suggests a diagnosis of oxygen toxicity. Diagnosis of bronchopulmonary dysplasia in newborn infants with breathing difficulties is difficult in the first few weeks. However, if the infant’s breathing does not improve during this time, blood tests and x-rays may be used to confirm bronchopulmonary dysplasia. In addition, an echocardiogram can help to eliminate other possible causes such as congenital heart defects or pulmonary arterial hypertension. The diagnosis of retinopathy of prematurity in infants is typically suggested by the clinical setting. Prematurity, low birth weight and a history of oxygen exposure are the principal indicators, while no hereditary factors have been shown to yield a pattern.
_
Clinical Implications:
In patients with acute hypoxemic respiratory failure, targeting a “normal” arterial oxygen concentration may require the administration of a high FiO2. This strategy has several advantages, including the maintenance of tissue oxygen delivery, the prevention of organ dysfunction resulting from anoxic injury to vascular watershed areas, and an improvement in right-sided heart function as a result of pulmonary arterial vasodilation. These benefits come at the cost of an increased risk of oxidant-mediated lung injury. In addition, increased oxygen concentrations in the lung and intentional or unintentional increases in arterial and tissue oxygen concentrations above normal inhibit the activation of HIFs and consequently the transcription of genes including VEGF that promote adaptation to hypoxia as well as lung and tissue repair.
______
______
Mechanism of cellular injury in hyperoxia:
Oxygen is a requirement for cellular respiration in the metabolism of glucose and the majority of O2 consumed by the mitochondria is utilized for adenosine triphosphate (ATP) generation. The mitochondrial electron transport chain reduces the elemental molecular O2 to ionic O2 by the relay of electrons making O2 usable for ATP generation, and during this process, oxidizing free radicals are generated. In a biological context, ROS (reactive oxygen species) are formed as a natural byproduct of the normal metabolism of oxygen and have important roles in cell signaling and homeostasis. Adequate oxygen tension at the wound increases superoxide production by neutrophils, which leads to greater bacterial destruction. Examples include peroxides, superoxide, hydroxyl radical, and singlet oxygen. Toxic levels of O2 lead to the formation of additional ROS, which can impose damage to lipid membranes, proteins, and nucleic acids. Reactive O2 species mediate physiological and pathophysiological roles within the body. Free radicals are a type of unstable, reactive, short-lived chemical species that have one or more unpaired electrons and may possess a net charge or be neutral. The species is termed free because the unpaired electron in the outer orbit is free to interact with surrounding molecules. Cells generate free radicals, or ROS, by the reduction of molecular O2 to water (H2O) as seen in the figure below:
Figure above shows reduction of oxygen in which a single-electron transfer which converts molecular oxygen to the superoxide anion, creating an unstable molecule. The decomposition of hydrogen peroxide can be a source of the hydroxyl radical; this reaction requires both superoxide and hydrogen peroxide as precursors. These steps reduce oxygen to water by the addition of four electrons, yielding three reactive oxygen species: superoxide anion, hydrogen peroxide, and hydroxyl radical. These ROS-producing reactions occur endogenously involving enzymes and organelles such as the mitochondria, and exogenously induced by radiation, pollutants, xenobiotics, and toxins. Cellular survival and adaptation in an oxidative atmosphere are dependent upon sufficient antioxidant defenses to counteract the effects of ROS on cells and tissues.
_
The NADH and FADH2 formed in glycolysis, fatty acid oxidation, and the citric acid cycle are energy-rich molecules because each contains a pair of electrons having a high transfer potential. When these electrons are used to reduce molecular oxygen to water, a large amount of free energy is liberated, which can be used to generate ATP. Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers. This process, which takes place in mitochondria, is the major source of ATP in aerobic organisms. For example, oxidative phosphorylation generates 30 to 36 molecules of ATP that are formed when glucose is completely oxidized to CO2 and H2O. ROS are produced in the mitochondria as by-products of oxidative phosphorylation for ATP synthesis. Cytosol can produce ROS from many endogenous (growth factors, cytokines, and metabolisms) or exogenous sources (nutrients, radiation, microbiome, and xenobiotics). On the other hand, cytosol can accumulate ROS produced by mitochondria and redoxosomes, especially superoxide and hydroperoxides. ROS and RNI, accumulating into the cytosol, can diffuse easily (depending on half-life) into the nucleoplasm, interacting with nucleic acids and other nuclear components.
_
Paramagnetism and the univalent pathway:
Rotating electrical charges generate magnetic fields. This applies to electrical current in a coil of wire or to a single spinning electron. The pairing of electrons with opposite spin states neutralizes this effect. Most substances are not influenced by imposed magnetic fields because the electrons they contain are all in spin-opposed pairs. Such substances are diamagnetic. O2 is unusual in being paramagnetic, and that implies unpaired electronic spins. Indeed, O2 contains two unpaired electrons and they have the same spin state. This electronic structure constitutes a barrier to the insertion of a pair of electrons. Thus, the electrons of the incoming spin-opposed pair would be trying to join the parallel-spinning unpaired electrons of O2, and one of them would have the same spin state as its partner to be. This situation is energetically very unfavorable; as stated by the Pauly exclusion principle. There is a way around this barrier, and that way is to add the electrons to O2 one at a time. This works because electronic spins can be inverted by interaction with nuclear spins. However, this is a slow process relative to the lifetime of collisional complexes and is not likely while the reacting partners are in contact. But when the electrons are added one at a time, during separate collisional events, there is time between collisions for the inversion of electronic spin. As a result, the facile route of O2 reduction is by a series of univalent electron transfers. The reduction of O2 to 2H2O requires four electrons. Hence, intermediates will be encountered on this univalent pathway and these are superoxide (O2-), hydrogen peroxide (H2O2) and hydroxyl radical (HO-). It is these intermediates that are responsible for the toxicity of O2, and defenses against that toxicity must include minimizing their production to the maximum extent possible and eliminating those whose production cannot be avoided. Most of the O2 consumed by respiring cells is reduced by cytochrome c oxidase which, by virtue of two ferrihemes and two Cu(II) prosthetic groups, manages the four-electron reduction of O2 to 2H2O without releasing intermediates. But there are other enzymes that reduce O2 to H2O2, and there are both enzymic and spontaneous processes within cells that produce O2-.
_
Classes of ROS and their properties:
| Radical | Structure | Reactivity | Half-life | Production/localization | Diffusion | Targets | Biological effect | Pathological effect |
| Hydroxyl radical | High | 10−9 sec | Mitochondria Phagosome Endoplasmic reticulum (ER) |
Highly localized where is produced | Any cell component | Unknown | Toxicity | |
| Superoxide | Low | 1–15 minutes | Mitochondria cytosol ER Peroxisome |
Localized, it can diffuse through an anion channel | Fe-S centers Nitric oxide |
Protein modification (activation or inhibition) | Protein damage | |
| Hydrogen peroxide | H2O2 | Moderate Reversible |
Hours to days | Mitochondria cytosol ER Peroxisome |
Diffuse, it can travel through aquaporins | Iron-sulphur Cysteine residues |
Activation of signalling | Mutation, accumulation, and genomic instability |
_
At present, the acronym ROS may also include also several nitrogen-containing compounds or RNS (Reactive Nitrogen Species), such as nitric oxide (NO), nitroxyl anion (NO−), and peroxynitrite (ONOO−). NO is produced by the activity of inducible nitric oxide synthase (iNOS) and reacts with superoxide to give rise to the other RNS. ROI (Reactive Oxygen Intermediates) and RNI (Reactive Nitrous Intermediate) are additional acronyms used to indicate ROS.
_
Hyperoxia is not precisely defined but significant elevations of the partial arterial pressure of oxygen (PaO2) are found only when the fraction of inspired oxygen (FiO2) is greater than 21 percent of atmospheric pressure. Adverse events may result from increased oxygen tension in the alveoli, blood, or at the cellular level. Hyperoxia appears to produce cellular injury through increased production of reactive oxygen intermediates (ROIs), such as the superoxide anion, the hydroxyl radical, and hydrogen peroxide. When the production of these (ROIs) increases and/or the cell’s antioxidant defenses are depleted, they can react with and impair the function of essential intracellular macromolecules, resulting in cell death.
_
Oxygen toxicity is believed to result from the formation of ROS in excess of the quantity that can be detoxified by the available antioxidant systems in the tissues. Although mechanisms of free radical damage to a substantial array of cellular systems (proteins, enzymes, membrane lipids, and nucleic acids) have already been characterized, large gaps exist in our understanding of the intermediate stages in the pathophysiologic cascades that follow such reactions and result in functional deficits and clinical phenomena. Oxygen free radicals may also promote a deleterious inflammatory response, leading to secondary tissue damage and/or apoptosis. Much of the evidence supporting direct cellular injury due to ROIs comes from studies in transgenic mice with altered superoxide dismutase activity. Mice with augmented antioxidant mechanisms are relatively tolerant to hyperoxia, while manganese superoxide dismutase knockout mice die shortly after birth with extensive mitochondrial injury within degenerating neurons and cardiac myocytes. Data from animal models suggest possible roles for insulin growth factor and angiopoietin in the pathogenesis of hyperoxia-induced lung injury.
_
Oxygen (O2) is life essential but as a drug has a maximum positive biological benefit and accompanying toxicity effects. Oxygen is therapeutic for treatment of hypoxemia and hypoxia associated with many pathological processes. Pathophysiological processes are associated with increased levels of hyperoxia-induced reactive O2 species (ROS) which may readily react with surrounding biological tissues, damaging lipids, proteins, and nucleic acids. Protective antioxidant defenses can become overwhelmed with ROS leading to oxidative stress. Activated alveolar capillary endothelium is characterized by increased adhesiveness causing accumulation of cell populations such as neutrophils, which are a source of ROS. Increased levels of ROS cause hyperpermeability, coagulopathy, and collagen deposition as well as other irreversible changes occurring within the alveolar space. In hyperoxia, multiple signaling pathways determine the pulmonary cellular response: apoptosis, necrosis, or repair. Understanding the effects of O2 administration is important to prevent inadvertent alveolar damage caused by hyperoxia in patients requiring supplemental oxygenation.
_
Can Hypoxia generate ROS?
Yes.
Physiological hypoxia results in a host of responses which include increased ventilation, constriction of the pulmonary artery, and a cellular transcriptional program which promotes glycolysis, angiogenesis, and erythropoiesis. Mitochondria are the primary consumers of cellular oxygen and have thus been speculated for years to be the site of cellular oxygen sensing. Many of the cellular responses to hypoxia are now known to be mediated by the production of reactive oxygen species at mitochondrial complex III. To survive, respiring organisms must sense and respond to changes in environmental oxygen levels. Complex III of the mitochondrial electron transport chain (ETC) has been implicated in the O2 sensing pathway in mammals through its ability to increase production of reactive oxygen species (ROS) during hypoxia. Hypoxia induced ROS activates HIF1α by inactivating its inhibitor, PHD (Prolyl Hydroxylase Domain). HIF induces genes for transcriptional activation of erythropoietin (EPO), vascular endothelial growth factor (VEGF), and glycolytic enzymes. Systemically, these responses enhance the delivery of O2 to cells and facilitate the production of glycolytic ATP. However Mitochondrial ROS accumulation following hypoxia can, in turn, activate NADPH-oxidases through a mechanism requiring protein kinase Cε and leading to further ROS increase and cellular damage.
_
Reactive oxygen species (ROS) are involved in the cell growth, differentiation, progression, and death. Low concentrations of ROS may be beneficial or even indispensable in processes such as intracellular signaling and defense against micro-organisms. Nevertheless, higher amounts of ROS play a role in the aging process as well as in a number of human disease states, including cancer, ischemia, and failures in immunity and endocrine functions. Although a small amount of ROS are produced through cellular respiration, a large amount of free radicals are created after any ischemic insult. With an overwhelming increase in reactive ions, systemic anti-oxidant defenses rapidly become depleted. Oxygen-derived free radicals subsequently cause direct damage to epithelial cells, and result in cell death. Effects of membrane damage are seen in all organ systems, but are especially prominent in the brain, lungs, GI tract, and vascular endothelium. The CNS is especially sensitive to oxidative damage because of its high lipid content and low antioxidant levels.
A study found that hyperoxia with 100% oxygen after hypoxia-ischemia can cause more damage in the cerebral cortex than room air in newborn rats. Excessive oxygen therapy can exacerbate primary brain injury and result in severe secondary damage, especially in cases of trauma. On a pulmonary level, oxidative destruction of the epithelial lung lining leads to airway inflammation, increased tissue permeability, pulmonary edema, and ultimately fibrosis. In the GI tract, ROS driven compromise predisposes to ulceration and bacterial translocation, increasing the risk of sepsis. Finally, vasoplegia can result secondary to capillary leak and microvascular sludging. This can lead to refractory hypotension in extreme cases. Ischemic conditions carry additional risk for the development of oxygen’s adverse effects, as their pathology involves an element of oxidative damage prior to oxygen therapy. Patients suffering from traumatic brain injury, post arrest syndrome, compartment syndrome, or prolonged hypotension often have a buildup of reactive intermediates which only need additional oxygen to incite a cascade of oxidative injury. Reperfusion injury occurs when oxygen is reintroduced to ischemic tissues. Stated another way, the injury does not occur during periods of hypoxia. It occurs after oxygen is restored to the affected tissues. The primary mechanism is thought to be release of additional “reactive oxygen species” or “free radicals.” In these cases, supplemental oxygen should only be provided if there is evidence of hypoxia. The severity and time to develop oxidative injury are dependent on the fraction of oxygen provided (FiO2) and duration of therapy. As a general guideline, FiO2 of over 60% should not be administered for longer than 24-48 hours. Used judiciously, oxygen therapy is one of the most important tools available for critical patients, but it is not an innocuous therapy. It is imperative to be conscious of oxygen “as a drug” and use it accordingly.
_
Antioxidants:
Oxidant antioxidant homeostasis is highly regulated and essential for maintaining cellular and biochemical functions. A change in the balance toward an increase in the oxidant over the capacity of the antioxidant defines oxidative stress and can lead to oxidative damage. Changing the balance toward an increase in the reducing power of the antioxidant can also cause damage and is defined as reductive stress. Reduction, antioxidant and oxidation, or pro-oxidant reactions result from a gain or a loss of electrons and a loss or a gain in O2. Antioxidant defenses may be classified as nonenzymatic and enzymatic; or endogenous and dietary. Examples of nonenzymatic antioxidants are glutathione (GSH), ascorbic acid, vitamin E, beta-carotene, and uric acid. Major enzymatic antioxidants are superoxide dismutase (SOD), catalase, and glutathione peroxidase which divert or dismutate ROS into harmless products. Endogenous or dietary antioxidants are based on the ability of the antioxidant to be synthesized by humans. Endogenous antioxidants are SOD, catalase, GSH peroxidase, uric acid, and bilirubin. Dietary antioxidants are ascorbic acid, vitamin E, and beta-carotene. Ascorbic acid, vitamin E, uric acid, bilirubin, and GSH scavenge ROS by expendable, replaceable, or recyclable substrates. Vitamin E and beta-carotene quench ROS by absorption of electrons and/or energy. Antioxidants can also be classified into four categories based on function. (1) Preventive antioxidants which suppress formation of ROS, (2) radical scavenging antioxidants which suppress chain initiation and/or break chain propagation reactions, (3) the repair and de novo antioxidants such as proteolytic enzymes and the repair enzymes of DNA, and (4) antioxidants which allow for adaptation that occurs when the signal for the production and reactions of ROS induces oxidant formation and transport.
_
_
Antioxidant tasks are accomplished by enzymes as catalases, glutathione peroxidases, thioredoxins, and peroxyredoxins. These enzymes use electron donors in order to avoid the intermediate formation of the hydroxyl radical (OH-), which is a strongly reactive oxidant. In this process, superoxide dismutase is an important antioxidant enzyme as it efficiently reduces the concentration of the superoxide anion (O2–) by facilitating its rapid conversion in hydrogen peroxide (H2O2) or oxygen (O2). In general, ROS generation from mitochondria increases with oxygen tension and is dependent on the clinical balance between the underlying condition and oxygen supply. In response to bacterial invasion, neutrophils can also produce large amounts of ROS that may initially be beneficial in the host defense against several pathogens. Fortunately, the lungs are principally well protected against oxygen toxicity by adequate intra—and extracellular antioxidant activity. Besides this physiological activity, additional antioxidants can be recruited in the epithelial lining fluid. However, when the production of ROS exceeds the limits of counteraction by antioxidant responses, ROS concentrations reach toxic levels and a cellular state of oxidative stress manifests. Oxidative stress refers to the imbalance caused by increased ROS formation or deficient oxidant suppressors. When antioxidant systems are insufficient during critical illness and mechanical ventilation, supplemental oxygen can cause accumulation of oxygen radicals and may initiate or perpetuate oxygen toxicity. Moreover, ROS control can be markedly influenced by aging, genetic factors, and pharmacochemical agents.
_
Cell death:
When the delicate homeostatic balance is disturbed, oxidative stress leads to damage of nucleic acids, proteins, and lipids, resulting in cell death by both apoptotic and necrotic pathways. Necrosis is characterized by incomplete apoptosis and supported by integrity loss of the cell membrane and cytoplasmic swelling. Programmed cell death by apoptosis can be achieved through extrinsic or intrinsic pathways, concomitantly. The extrinsic pathway is triggered by extracellular signals that stimulate intracellular apoptotic cascades after binding the cell membrane. The intrinsic apoptotic pathway is initiated by increased mitochondrial ROS formation. Subsequently, the opening of transition pores is facilitated, making the outer mitochondrial membrane more permeable for pro-apoptotic components. These components can then pass to the cytoplasm and induce a state of intracellular stress. When this occurs in both endothelial and epithelial cells, lytic damage and cell death contribute to interstitial pulmonary edema and impaired gas exchange by means of alveolar collapse and disintegration of the alveolar-capillary barrier.
_
Cell damage and inflammatory pathways:
In addition to direct cell death by necrosis or apoptosis, cellular disruption caused by hyperoxia and ROS has been shown to release endogenous damage-associated molecular pattern molecules (DAMPs) that alert the innate immune system. DAMPs, or alarmins, are cell fragments released during cellular dysfunction and sterile injury and act as pleiotropic modulators of inflammation. During oxidative stress, mitochondrial damage is a pivotal cause of extracellular hazardous content including both free radicals and DAMPs. Because they resemble bacterial DNA, circulating mitochondrial DAMPs are efficiently recognized by pattern recognition receptors and activate polymorphonuclear neutrophils (PMNs). Subsequently, PMNs release interleukins and contribute to a sterile inflammatory reaction and, ultimately, neutrophil-mediated organ injury. In response to hyperoxia-mediated ROS production, resident lung cells initiate the release of various cytokines. Chemotactic factors orchestrate the inflammatory response by attracting inflammatory cells to the pulmonary compartment. Recruited neutrophils and monocytes, in turn, are significant sources of additional ROS, conserving a vicious cycle leading to further tissue damage. Under enduring conditions of injury to pulmonary epithelium and increasing alveolar permeability, cytokines can translocate from the alveolar space to the systemic circulation, creating a systemic inflammatory response, in which cytokines are efficiently activated and phagocytosis by alveolar macrophages is hampered. Cytokine concentrations decrease after long-term exposure, suggesting that a fast upregulation of inflammatory action is followed by a gradual impairment of the innate immune system. Besides mitochondrial damage, the inflammatory actions of oxygen are importantly modulated by hypoxia-inducible factor (HIF). HIF-1α is thought to be upregulated during relative changes in oxygenation and accordingly responds to normoxia as a relative hypoxic state directly after hyperoxia. Through this mechanism, intermittent hyperoxia may trigger a paradoxical phenomenon in which the genetic expression of inflammatory mediators and erythropoietin is stimulated in the absence of true tissue hypoxia.
_
Oxidation is loss of electrons and Reduction is gain of electrons. Oxidising agents get reduced and reducing agents get oxidised. Oxygen, a strong electron acceptor, enables organisms to extract more energy from organic molecules via oxidative phosphorylation than can be extracted using other terminal electron acceptors. The oxidizing properties of molecular oxygen, however, come at a cost. During the reduction of O2 during respiration, oxygen can be converted to various intermediates, like superoxide, hydroxyl radical, hydrogen peroxide, and singlet oxygen. Singlet oxygen is a high-energy form of oxygen with physical properties differing only subtly from those of the more prevalent triplet ground state of O2. In terms of its chemical reactivity, however, singlet oxygen is far more reactive toward organic compounds. These highly reactive bi-products of O2 reduction are quite toxic, causing damage to cellular molecules. To grow in an oxygen- rich environment, then, organisms must have defenses against the damage caused by these reactive oxygen species. These defences include enzymatic antioxidants like superoxide dismutase (SOD), catalase, and glutathione peroxidase which are evolutionary biologically evolved and genetically controlled. There is limit to what extent they can work. When their limits are crossed oxygen starts harming us.
_
Figure below shows vicious cycle of hyperoxia-induced cell injury:
AP activator protein, DAMP damage-associated molecular pattern molecules, H2O2 hydrogen peroxide, IFN interferon gamma, IL interleukin, MAPK mitogen-activated protein kinase, NADPH nicotinamide adenine …
_
_
The “double-edged sword” character of molecular oxygen (O2) is well established and has been a matter of debate since its discovery at the end of the eighteenth century. On the one hand, O2 plays a crucial role during adenosine triphosphate (ATP) synthesis. On the other hand, its chemical characteristics lead to strong oxidizing properties, capable of damaging any biological molecule, and thereby defining the paradigm of oxygen toxicity. This phenomenon is due to the formation of reactive oxygen species (ROS), its magnitude being directly correlated to the level of the O2 partial pressure either low or high. During mitochondrial respiration, 1–3 % of O2 consumption leads to ROS formation. Like O2, ROS also exert Janus-headed properties: while being of importance for host defense and signaling cascades, their toxic effects are well known. Certain structures should be protected from reactive oxygen species. Namely cell wall structures, mitochondria and DNA. On the other hand, white blood cells depend upon highly reactive oxidative zones to kill bacteria or to have anticancer properties. Then there are physicians who treat cancer and other infectious processes with hydrogen peroxide and/or ozone (triple oxygen) therapies; although FDA warned consumers not to buy or use high-strength hydrogen peroxide products, including one sold as “35 Percent Food Grade Hydrogen Peroxide,” for medicinal purposes because “they can cause serious harm or death when ingested.” On the top of it, ROS production has been strictly associated with cancer, ageing, diabetes, obesity, neurodegeneration, and other age-related diseases such as age-related retinopathy, cochlear degeneration, and chronic inflammatory diseases. The production of reactive oxygen species (ROS) from the inner mitochondrial membrane is one of many fundamental processes governing the balance between health and disease. It is well known that ROS are necessary signalling molecules in gene expression, yet when expressed at high levels, ROS may cause oxidative stress and cell damage. Both hypoxia and hyperoxia may alter ROS production by changing mitochondrial PO2 (PmO2).
_
Figure below summarizes the key pro and con arguments concerning the use of O2 therapy during shock states.
Figure above shows beneficial (green arrows) and deleterious (red arrows) effects of hyperoxia, i.e., breathing pure oxygen, during circulatory shock and/or in medical emergencies.
FiO2 fraction of inspired oxygen, PO2 oxygen partial pressure, µ micro, Hb haemoglobin
_____
Brain adaptation to hypoxia and hyperoxia in mice: 2016 study:
Mice were exposed to mild hypoxia (10%O2), normoxia (21%O2) or mild hyperoxia (30%O2) for 28 days, sacrificed and brain tissue excised and analyzed. Although one might expect linear responses to %O2, only few of the examined variables exhibited this pattern, including neuroprotective phospho- protein kinase B and the erythropoietin receptor. The major reactive oxygen species (ROS) source in brain, NADPH oxidase subunit 4 increased in hypoxia but not in hyperoxia, whereas neither affected nuclear factor (erythroid-derived 2)-like 2, a transcription factor that regulates the expression of antioxidant proteins. As a result of the delicate equilibrium between ROS generation and antioxidant defense, neuron apoptosis and cerebral tissue hydroperoxides increased in both 10%O2 and 30%O2, as compared with 21%O2. Remarkably, the expression level of hypoxia-inducible factor (HIF)−2α (but HIF-1α) was higher in both 10%O2 and 30%O2 with respect to 21%O2. Prolonged mild hyperoxia leads to persistent cerebral damage, comparable to that inferred by prolonged mild hypoxia. The underlying mechanism appears related to a model whereby the imbalance between ROS generation and anti-ROS defense is similar, but occurs at higher levels in hypoxia than in hyperoxia. Mild hyperoxia represents a condition as challenging as mild hypoxia with respect to redox imbalance and brain damage. This suggests that, despite profound fluctuations in Earth atmosphere %O2 in the past geological eras, terrestrial mammals are now adapted to live in an atmosphere containing 20.96%O2, and that any deviation from this value, irrespectively if higher or lower, might trigger the occurrence of a potentially lethal situation. This may have important clinical implications for the 800,000 individuals that need supplemental oxygen at a cost of 1.8 billion dollars/year in the US.
_____
_____
Oxygen administration is uniformly used in emergency and intensive care medicine and has life-saving potential in critical conditions. However, excessive oxygenation also has deleterious properties in various pathophysiological processes and consequently both clinical and translational studies investigating hyperoxia during critical illness have gained increasing interest. Reactive oxygen species are notorious by-products of hyperoxia and play a pivotal role in cell signaling pathways. The effects are diverse, but when the homeostatic balance is disturbed, reactive oxygen species typically conserve a vicious cycle of tissue injury, characterized by cell damage, cell death, and inflammation. The most prominent symptoms in the abundantly exposed lungs include tracheobronchitis, pulmonary edema, and respiratory failure. In addition, absorptive atelectasis results as a physiological phenomenon with increasing levels of inspiratory oxygen. Hyperoxia-induced vasoconstriction can be beneficial during vasodilatory shock, but hemodynamic changes may also impose risk when organ perfusion is impaired. In this context, oxygen may be recognized as a multifaceted agent, a modifiable risk factor, and a feasible target for intervention. Although most clinical outcomes are still under extensive investigation, careful titration of oxygen supply is warranted in order to secure adequate tissue oxygenation while preventing hyperoxic harm.
_______
_______
Clinical studies on role of oxygen therapy in Critical care:
Recent studies assessing the clinical effects of arterial hyperoxia or normobaric supplemental oxygen in critical care are listed in Table below. As highlighted in recent meta-analyses, the effects on major clinical endpoints are conflicting and may be partially explained by heterogeneous methodology and subgroup differences in critically ill patients. Pooled effect estimates favoring normoxia are quite consistent, but the harmful effects were previously shown to be impacted by the definition of hyperoxia and may be more pertinent to specific subgroups and at specific moments of admission.
_
Studies assessing the clinical effects of arterial hyperoxia or supplemental oxygen in subgroups of critically ill patients:
| Author | Country | Study type | Inclusion period | Subgroup | Sample size | Harm | Conclusions |
| Eastwood et al. (2012) | Australia and New Zealand | Cohort | 2000–2009 | MV | 152,680 | – | Hypoxia in first 24 h of admission was associated with increased in-hospital mortality, but hyperoxia was not. |
| de Jonge et al.(2008) | The Netherlands | Cohort | 1999–2006 | MV | 36,307 | + | High FiO2 and both low PaO2 and high PaO2 in first 24 h of admission were associated with in-hospital mortality |
| Suzuki et al. (2014) | Australia | Before-after pilot | 2012 | MV | 105 | +/– | Conservative oxygen therapy in mechanically ventilated ICU patients was feasible and free of adverse biochemical, physiological, or clinical outcomes while allowing a marked decrease in excess oxygen exposure |
| Aboab et al. (2006) | France | Experimental | NA | ARDS | 14 | +/– | In mechanically ventilated patients with ARDS, the breathing of pure oxygen leads to alveolar derecruitment, which is prevented by high PEEP |
| Austin et al. (2010) | Australia | RCT | 2006–2007 | COPD | 405 | + | Titrated oxygen treatment significantly reduced mortality, hypercapnia, and respiratory acidosis compared with high-flow oxygen in acute exacerbations of COPD |
| Cameron et al. (2012) | New Zealand | Cohort | 2005–2008 | COPD | 180 | + | Serious adverse clinical outcomes are associated with both hypoxaemia and hyperoxaemia during acute exacerbations |
| Perrin et al. (2011) | New Zealand | RCT | 2007–2009 | Asthma | 106 | + | High-concentration oxygen therapy causes a clinically significant increase in transcutaneous CO2 during severe exacerbations |
| Bellomo et al. (2011) | Australia and New Zealand | Cohort | 2000–2009 | CA | 12,108 | – | Hyperoxia did not have a robust or consistently reproducible association with mortality |
| Elmer et al. (2014) | USA | Cohort | 2008–2010 | CA | 184 | + | Severe hyperoxia was independently associated with decreased survival to hospital discharge |
| Ihle et al. (2013) | Australia | Cohort | 2007–2011 | CA | 584 | – | Hyperoxia within the first 24 h was not associated with increased hospital mortality |
| Janz et al. (2012) | USA | Cohort | 2007–2012 | CA | 170 | + | Higher levels of the maximum measured PaO2 were associated with increased in-hospital mortality and poor neurological status on hospital discharge |
| Kilgannon et al. (2010) | USA | Cohort | 2001–2005 | CA | 6326 | + | Arterial hyperoxia was independently associated with increased in-hospital mortality compared with either hypoxia or normoxia |
| Kilgannon et al. (2011) | USA | Cohort substudy | 2001–2005 | CA | 4459 | + | Supranormal oxygen tension was dose-dependently associated with the risk of in-hospital death |
| Kuisma et al. (2006) | Finland | RCT pilot | NA | CA | 28 | – | No indication that 30 % oxygen with SpO2 monitoring did worse than the group receiving 100 % oxygen |
| Lee et al. (2014) | Korea | Cohort | 2008–2012 | CA | 213 | – | Mean PaO2 was not independently associated with in-hospital mortality |
| Nelskyla et al. (2013) | Australia | Cohort | 2008–2010 | CA | 122 | – | No statistically significant differences in numbers of patients discharged from the hospital and 30-day survival between patients with hyperoxia exposure and no exposure |
| Spindelboeck et al.(2013) | Austria | Cohort | 2003–2010 | CA | 145 | – | Increasing PaO2 was associated with a significantly increased rate of hospital admission and not with harmful effects |
| Vaahersalo et al. (2014) | Finland | Cohort | 2010–2011 | CA | 409 | – | Hypercapnia was associated with good 12-month outcome, but harm from hyperoxia exposure was not verified |
| Minana et al. (2011) | Spain | Cohort | 2003–2009 | ADHF | 588 | – | Admission PaO2 was not associated with all-cause long-term mortality |
| Ranchord et al. (2012) | New Zealand | RCT pilot | 2007–2009 | STEMI | 136 | – | No evidence of benefit or harm from high-concentration compared with titrated oxygen |
| Stub et al. (2012) | Australia | RCT | 2011–2014 | STEMI | 441 | + | Supplemental oxygen therapy in patients with STEMI but without hypoxia increased myocardial injury, recurrent myocardial infarction, and cardiac arrhythmia and was associated with larger myocardial infarct size at 6 months. Further results anticipated. |
| Sutton et al. (2014) | Australia and New Zealand | Cohort | 2003–2012 | Post cardiac surgery | 83,060 | – | No association between mortality and hyperoxia in the first 24 h in ICU after cardiac surgery |
| Ukholkina et al. (2005) | Russia | RCT | NA | AMI | 137 | – | Inhalation of 30–40 % oxygen within 30 min prior to endovascular myocardial reperfusion and within 4 h thereafter reduced the area of necrosis and peri-infarction area, improved central hemodynamics, and decreased the rate of post-operative rhythm disorders as compared with patients breathing ambient air |
| Zughaft et al. (2013) | Sweden | RCT | NA | ACS | 300 | – | The use of oxygen during PCI did not demonstrate any analgesic effect and no difference in myocardial injury measured with troponin- t or in the morphine dose |
| Asher et al. (2013) | USA | Cohort | NA | TBI | 193 | – | PaO2 threshold between 250 and 486 mm Hg during the first 72 h after injury was associated with improved all-cause survival independently of hypocarbia or hypercarbia |
| Brenner et al. (2012) | USA | Cohort | 2002–2007 | TBI | 1547 | + | Hyperoxia within the first 24 h of hospitalization was associated with worse short-term functional outcomes and higher mortality |
| Davis et al. (2009) | USA | Cohort | 1987–2003 | TBI | 3420 | + | Both hypoxemia and extreme hyperoxemia were associated with increased mortality and a decrease in good outcomes |
| Quintard et al. (2014) | Switzerland | Cohort | 2009–2013 | TBI | 36 | + | Incremental normobaric FiO2 levels were associated with increased cerebral excitotoxicity independently from brain tissue oxygen and other important cerebral and systemic determinants |
| Raj et al. (2013) | Finland | Cohort | 2003–2012 | TBI | 1116 | – | Hyperoxemia in the first 24 h of admission was not predictive of 6-month mortality |
| Rincon et al. (2013) | USA | Cohort | 2003–2008 | TBI | 1212 | + | Arterial hyperoxia was independently associated with higher in-hospital case fatality |
| Jeon et al. (2014) | USA | Cohort | 1996–2011 | Stroke | 252 | + | Exposure to hyperoxia was associated with delayed cerebral ischemia |
| Rincon et al. (2014) | USA | Cohort | 2003–2008 | Stroke | 2894 | + | Arterial hyperoxia was independently associated with in-hospital death as compared with either normoxia or hypoxia |
| Ali et al. (2014) and Roffe et al. (2011) | UK | RCT pilot | 2004–2008 | Stroke | 289 | – | Routine oxygen supplementation started within 24 h of hospital admission with acute stroke led to a small improvement in neurological recovery at 1 week, but no outcome differences were observed at 6 months |
| Ronning et al. (1999) | Norway | Quasi-RCT | 1994–1995 | Stroke | 310 | + | Supplemental oxygen should not routinely be given to non-hypoxic patients with minor or moderate strokes |
| Singhal et al. (2005) | USA | RCT pilot | NA | Stroke | 16 | – | High-flow oxygen therapy is associated with a transient improvement of clinical deficits and MRI abnormalities |
| Young et al. (2012) | Australia and New Zealand | Cohort | 2000–2009 | Stroke | 2643 | – | Worst arterial oxygen tension in the first 24 h was not associated with outcome |
| Stolmeijer et al. (2014) | The Netherlands | Cohort | NA | Sepsis | 83 | – | No association between mortality and hyperoxia, nor between lower FiO2 and other detrimental effects |
NA, not available; +, study found harm from supplemental oxygen or arterial hyperoxia; –, no harm found from supplemental oxygen or arterial hyperoxia
_
When pure O2 is introduced to the lungs, autonomic reflex increases respiration. The increased rate of breathing means that a much larger load of carbon dioxide is released from the body, which causes the blood vessels to constrict. Despite the increased amount of available oxygen in the lungs, the circulatory system is hampered, and cannot deliver precious O2 as well as it could when breathing normal atmosphere. Ronald Harper, a neurobiology professor at UCLA, conducted observations on a group of healthy teenagers breathing various gas mixes using functional magnetic resonance imaging (fMRI). His findings showed that in some subjects the pure O2 caused the brain to go clinically bonkers. Brain structures such as the hippocampus, the insula, and the cingulate cortex all displayed an adverse reaction; they in turn spurred the hypothalamus, the body’s main regulatory gland, into a fervour. The hypothalamus regulates a myriad of things, including heart rate, body temperature, and is the master of a variety of other glands. The introduction of pure oxygen prompts the hypothalamus to flood the body with a cocktail of hormones and neurotransmitters which serve to hamper heart rate, and further reduce the circulatory system’s effectiveness. But Harper also found that by adding a mere 5% CO2, all the detrimental effects found in pure oxygen are negated. There are circumstances, however, where even the proper mix of gases would prove inadequate. Modern medicine has long taught that after respiration stops, the brain can only survive for six minutes without oxygen before its cells begin to die in droves. In order to combat this, standard procedure has been to aggressively attempt to restore breathing and heartbeat immediately upon cessation. The base premise on which this protocol is designed may be in error. Upon examining heart cells and neurons deprived of oxygen under a microscope, Dr Lance Becker of the University of Pennsylvania found there was no indication that the cells were dying after five or six minutes. In fact, they seemed to endure the state for up to an hour without adverse effect. Given this unexpected observation, the researchers were forced to investigate why human resuscitation becomes impossible after only a few minutes of clinical death. The answer they uncovered was that the body’s cells were not dying of oxygen starvation; they were expiring due to reperfusion—the sudden reintroduction of oxygen to a dormant cell. Inside the cells, the culprit seems to be in the mitochondria, which is the cell’s power plant where sugar and oxygen are converted to usable energy. Mitochondria are also responsible for apoptosis—the organized, controlled self-destruction of a cell. Normally apoptosis occurs in situations such as the cell being damaged beyond repair, infected by a virus, an attempt to prevent cancer, or aiding in initial tissue development. The process effectively kills and dismantles the cell allowing the body’s usual waste management functions to carry the cell’s remains away. For reasons not entirely clear, reperfusion triggers apoptosis—the oxygen intended to save the cell actually causes cellular suicide. Armed with this new information about how cells react to oxygen, it is clear that current emergency care is not altogether ideal, and new protocols are under investigation. Dr Becker proposes that induced hypothermia may slow cell degradation, and if a means can be found to safely reintroduce oxygen to tissues, a clinically dead person—who still has trillions of living cells—could be resuscitated after being an hour dead. This glorious future is still on the horizon.
But the contrary view emerges from a recently published study from the Australian and New Zealand Intensive Care Research Centre based at Monash University in Melbourne. Investigators here sought to examine the notion, suggested by previous studies, that hyperoxia occurring during resuscitation from cardiac arrest is an independent risk factor for death. Clearly if this were indeed the case, more conservative use of supplemental oxygen during cardiac resuscitation would be warranted. The data suggests that patients with hyperoxia during resuscitation from cardiac arrest are less likely to survive, not because of the hyperoxia per se, but because they are sicker, i.e. already less likely to survive before oxygen administration. Rather than being a contributory cause of death, hyperoxia is more likely just an incidental (innocent) consequence of higher oxygen dose delivered in response to poorer clinical condition. In view of all recent data, supplemental oxygen administration during resuscitation still appears desirable, but hyperoxia should be avoided in the post-resuscitation phase and saturation should be targeted at 94–96 %.
_
Research on patient outcomes after hyperoxia:
What is new are prehospital research studies comparing outcomes of patients treated without oxygen or with oxygen titrated to saturations versus patients routinely given high flow oxygen. These data are frightening; they invariably show impressive patient harm from even short periods of hyperoxia. In 2002, a study of 5,549 trauma patients in Texas showed prehospital supplemental oxygen administration nearly doubled mortality. A Tasmanian study of prehospital difficulty breathing patients published in 2010 compared patients treated with oxygen titrated to saturations of 88 to 92 percent to patients treated with non-rebreather oxygen masks. It showed a reduction in deaths during subsequent hospitalization of 78 percent in COPD patients and 58 percent in all patients. We’ve known since 1999 that oxygen worsened survival in patients with minor to moderate strokes and made no difference for patients with severe stroke. In fact, the American Heart Association recommended in 1994 against supplemental oxygen for non-hypoxemic stroke patients.
_
Perioperative care:
Liberal oxygen supply is usually accepted in perioperative care in order to avoid potentially life-threatening consequences of hypoxia during surgery. Further effects of perioperative hyperoxia have been comprehensively summarized in meta-analyses enrolling over 7000 patients and generally showed a reduced risk of surgical site infections and postoperative nausea without luxation of postoperative atelectasis. However, risks may outweigh benefits in specific age groups and different subsets. This was recently highlighted in patients undergoing cancer surgery in whom 80 % oxygen supply in the perioperative setting showed a significantly increased long-term all-cause mortality compared with those randomly assigned to 30 %.
_
Although oxygen remains of life-saving importance in critical care, accumulating evidence has demonstrated the prominent role of hyperoxia and the consequent formation of ROS in the pathogenesis of several life-threatening diseases. The toxic effects of supraphysiological oxygen concentrations are driven by cell damage, cell death, and inflammation. These aspects are of special concern in the pulmonary compartment, where absorptive atelectasis impairs respiratory function at high inspiratory oxygen levels. The cerebral and coronary circulations are at specific risk when vascular alterations manifest. Long-term exposure to hyperoxia impairs the innate immune response and increases susceptibility to infectious complications and tissue injury. Given that critically ill patients are prone to inflammation, cardiovascular instability, and depleted antioxidant mechanisms, the most rational practice may be to supply oxygen conservatively and titrate the therapy carefully to the patient’s needs. However, our understanding of oxygen toxicity is limited in humans, and conflicting findings hamper the constitution of compelling guidelines. Further research is warranted to study hyperoxia-induced effects in clinical practice, to elucidate time—and dose-response relationships, and to provide evidence-based oxygenation targets and interventions through robust clinical trials.
_____
_____
Supplementary oxygen in healthy subjects and those with COPD increases oxidative stress and airway inflammation: 2004:
There is accumulating evidence that oxidative stress may play an important pathophysiological role in chronic obstructive pulmonary disease (COPD) in which increased oxidative stress has been demonstrated, especially in severe disease and during exacerbations. Supplementary oxygen therapy which is used in patients with severe COPD may further increase oxidative stress, theoretically resulting in enhancement of inflammation and worsening of the disease. There is an increase in markers of oxidative stress in plasma and airways after hyperbaric oxygen therapy in humans, indicating that hyperoxia may worsen pulmonary disease toxicity. Patients with severe COPD who develop cor pulmonale are commonly treated with long term oxygen therapy. To investigate whether short term supplementary oxygen (28%) increases oxidative stress and inflammation in the airways authors measured 8-isoprostane and IL-6 concentrations in EBC of healthy subjects and COPD patients after breathing room air and then after exposure to 28% oxygen given continuously through a face mask for 1 hour. The findings suggest that short term supplementary oxygen may enhance oxidative stress and inflammation in the airways. Whether this happens with long term oxygen therapy needs to be determined.
______
Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update:
Hyperoxia (i.e., ventilation with a FiO2 = 1.0) is a cornerstone of the acute management of circulatory shock, a concept which is based on compelling experimental evidence that compensating the imbalance between O2 supply and requirements (i.e., the oxygen debt) is crucial for survival, at least after trauma. On the other hand oxygen toxicity due to the increased formation of ROS limits its use, because it may cause serious deleterious side effects, especially in conditions of ischemia/reperfusion. While these effects are particularly pronounced during long-term administration, i.e., beyond 12–24 h, several retrospective studies suggest that even hyperoxemia of shorter duration is also associated with increased mortality and morbidity. In fact, albeit the clinical evidence from prospective studies is surprisingly scarce, a recent meta-analysis suggests that hyperoxia is associated with increased mortality at least in patients after cardiac arrest, stroke and TBI. Most of these data, however, originate from heterogenous, observational studies with inconsistent results, and therefore, there is a need for the results from the large scale, randomized, controlled clinical trials on the use of hyperoxia, which can be anticipated within the next 2–3 years. Consequently, until then, “conservative” O2 therapy, i.e., targeting an arterial hemoglobin O2 saturation of 88–95 % as suggested by the guidelines of the ARDS Network and the Surviving Sepsis Campaign, represents the treatment of choice to avoid exposure to both hypoxemia and excess hyperoxemia.
_____
Why are we still using oxygen to resuscitate term infants? 2010 study:
This article summarizes the historical background for the use of oxygen during newborn resuscitation and describes some of the research and the process of changing the previous practice from a high- to a low-oxygen approach. Findings of a recent Cochrane review suggest that more than 100,000 newborn lives might be saved globally each year by changing from 100 to 21% oxygen for newborn resuscitation. This estimate represents one of the largest yields for a simple therapeutic approach to decrease neonatal mortality in the history of pediatric research. Available data also suggest that, for the very low birth weight infant, use of the low-oxygen approach should be considered with the understanding that some of the smallest and sickest preterm neonates will need some level of oxygen supplementation during the first minutes of postnatal life. As more data are needed for the very preterm population, creation of strict guidelines for these infants would be premature at present. However, it can be stated that term and late preterm infants in need of resuscitation should, in general, be started on 21% oxygen, and if resuscitation is not started with 21% oxygen, a blender should be available, enabling the administration of the lowest FiO2 possible to keep heart rate and SaO2 within the target range. For extremely low birth weight infants, initial FiO2 could be between 0.21 and 0.30 and adjusted according to the response in SaO2 and heart rate.
_____
Oxygen and ARDS: 2015 study:
A recent study suggested that targeting a partial pressure of oxygen (PaO2) between 85 and 110 mm Hg might improve neurocognitive outcomes for patients with acute respiratory distress syndrome (ARDS). A subsequent review in the same journal questioned this practice and critically appraised the existing data on oxygen targets and safety. The review points out that there is no established “threshold” value below which supplemental oxygen is definitively safe, and physicians tend to underestimate the harmful effects. The authors conclude with a review of all of the instances in which the urge to “normalize” physiology has done more harm than good. In the ICU alone, we’ve seen this phenomenon play out with intensive insulin therapy, blood transfusions, and erythropoietin administration. Attempts at normalization did not translate to improvements in clinical outcomes. The authors aren’t the first to call attention to the harmful effects of hyperoxia. Another recent review from a separate group of researchers called for a more individualized approach to oxygen levels, along with the acceptance of permissive hypoxia or at least targeting a lower oxygen level than saturation [SpO2] of 95%.
______
There are over 70,000 published articles on oxygen, including 6,186 clinical trials. To put this into context, there are 12,000 studies and less than 300 clinical trials on helium gas.
_____
_____
Where do we go from here?
Knowing that both hypoxia and hyperoxia are bad, EMS providers must stop giving oxygen routinely. Oxygen saturations should be measured on every patient. Protocols need to be aligned to reflect the current ACLS and BLS ECC guidelines: administer oxygen to keep saturations between 94 and 96 percent. No patient needs oxygen saturations above 97 percent and in truth, there is little to no evidence suggesting any clinical benefit of oxygen saturations above 90 percent in any patient. Modifications in prehospital equipment will be inherent in controlling oxygen doses administered to patients. In all likelihood, the venturi mask will make a comeback, allowing EMS providers to deliver varied concentrations of oxygen as needed to keep oxygen saturations between 94 and 96 percent. Few patients will require non-rebreather masks which are prone to deliver too much oxygen (hyperoxia). CPAP (Continuous Positive Airway Pressure) devices will also need redesign as most conventional EMS CPAP delivers 100 percent oxygen. A study conducted by Bledsoe, et al in Las Vegas found that prehospital CPAP using low oxygen levels (28 to 30 percent) was highly effective and safe.
Bottom line:
The drug oxygen we use most often can cause harm if we give it without good reason. In the absence of low saturations, oxygen will not help patients with shortness of breath and it may actually hurt them. The same holds true for neonates and virtually any patient with ongoing tissue injury from stroke, heart attack or trauma. Indeed, oxygen can be bad.
____
____
FAQ on oxygen (oxygen therapy):
_
I am so short of breath. Why can’t I have oxygen?
Many things contribute to shortness of breath, and though it is commonly believed by the lay person that shortness of breath means you need oxygen, that is not always the case. The converse is also true. Patients with significant lung disease who have chronically low blood oxygen levels sometimes refuse to use oxygen because they “don’t feel short of breath.” For an accurate assessment of who needs oxygen and who doesn’t we must rely on measured blood levels. This is done directly on blood drawn through a needle from an artery or indirectly by a simple device called pulse oximeter, which can accurately measure your blood oxygen level through your finger. There are criteria that doctors use to determine who does and does not need oxygen, whether that will be part time or 24-hours daily. These criteria are well established in the medical literature. You can be quite short of breath and yet have an entirely normal blood oxygen level. In that case all of the oxygen we could possibly give is not going to affect your shortness of breath. There are other factors involved in that situation which must be treated in order to improve your shortness of breath.
_
I am not short of breath. Why must I use the oxygen?
Even patients who are not short of breath but yet have low blood oxygen levels can benefit from oxygen therapy in terms of quality of life and better functioning of important organs such as the brain, the heart, and the kidneys.
_
If I start on oxygen, won’t I get addicted to it?
No, this is a myth. We have all essentially been “addicted” to oxygen since birth. We can’t live without it. The air we breathe is approximately 21% oxygen. When a patient is started on supplemental oxygen they are often put on something in the range of 24 to 30% oxygen, so it is just a little more than is in the usual air you breathe. Using supplemental oxygen does not make you dependent on it any more than you have already been dependent on it since your first breath. It is just that with lung disease you may need a little bit more oxygen going into your lungs in order to get an adequate amount through your diseased lungs into your blood stream. Addiction to alcohol or addictive drugs, like narcotics, is characterized by compulsive use, craving, loss of control and continued use. Supplemental oxygen is not like a narcotic; using it does not create an increased need for it. Although oxygen saturation may drop when supplemental oxygen is discontinued, it doesn’t mean you’re hooked on it; it only means that you’re unable to get enough oxygen from the air you breathe and you need a higher concentration of oxygen to maintain health and quality of life, and to prevent further complications.
Using supplemental oxygen does not result in increasing demand for oxygen. The increased need is simply related to the progression of your underlying lung disease. Often patients who need oxygen find that as years go by and their lung disease gradually worsens that it takes more oxygen to get the same blood level. This is not because they were started on supplemental oxygen. It is merely because the disease has progressed (as is the natural course of many lung diseases). Often times patients feel that oxygen may be addictive because once they are started on supplemental oxygen they “can never get off it.” That is not because they have used the oxygen. It is just, again, related to their underlying disease. If the underlying lung disease is significant enough that oxygen is required in the first place, oxygen is likely going to always be required.
Sometimes, when people are right on the verge of needing oxygen, but not quite needing it on an everyday basis, they may come to need oxygen temporarily during an acute illness. This is something that can happen when a person is near the need for continuous oxygen therapy and with an acute illness their body will be stressed enough to require oxygen temporarily. Then after recovery their oxygen level may be back up and they can come off oxygen again. Sometimes an acute illness will be the last straw that causes you to need supplemental oxygen from that point on.
If your doctor feels you need to use oxygen, you should use it without fear of it causing dependence. Studies show that if you meet the criteria for needing oxygen and use it according to your doctor’s prescription that you will survive better and longer than a similar patient with a similar disease who chooses not to use oxygen.
_
Can oxygen cure me?
No. Your oxygen does not treat your underlying lung disease. It just helps to fulfil needs that your diseased lungs cannot manage on their own. It is helping you to do a little better with what you have, but it can’t improve your lung function. It is kind of like having to wear glasses because your eyes aren’t entirely normal. Wearing glasses doesn’t fix your eye problem. They just help your eyes to see better. If you take your glasses off, your eye problem is still there and you can’t see very well. The same is true for your oxygen. It just helps you do a little better with the lungs you have. Your lungs are still the way they were. Your body cannot store oxygen, so if you take your oxygen off, your blood oxygen level will go back down again within minutes.
Yes. In emergency with low blood oxygen level, oxygen can save life provided underlying illness improves. In decompression illness, air embolism and carbon monoxide poisoning, hyperbaric oxygen can save life and cure illness.
_
I am on oxygen now. Why can’t I breathe any better?
It might make sense that if your O2 (oxygen) levels are fine, all is right with the world, but that is not always the case. Dyspnea, or the sensation of difficult breathing does not always correlate well with the amount of oxygen (O2) in the blood – so oxygen levels may be fine, but breathing is hard. When O2 levels are okay and you may feel like you “can’t breathe,” your dyspnea may be caused by anxiety (often caused by the feeling of not being able to breathe). It’s a vicious cycle. This is where pursed lip breathing is most useful, because is slows down breathing, relaxes you and often makes breathing easier. Another hint to decrease the sensation of difficult breathing is to sit in front of a fan – cool air facial stimulation decreases the sensation of dyspnea. Pulmonary rehabilitation patients say that the most important thing they learn from rehab is how to breathe correctly.
It is also possible that your underlying illness is so severe that oxygen given to you is unable to reach blood or tissues. In many instances, supplemental oxygen does relieve shortness of breath; in many cases it doesn’t. This is particularly true of COPD patients who lead a sedentary lifestyle. Persistent inactivity leads to muscle weakness and fatigue and impairs the body’s ability to utilize oxygen. When weak, tired muscles are called upon to perform any type of physical activity, shortness of breath worsens, sometimes to such a degree that supplemental oxygen can’t relieve it.
_
Do all people with COPD eventually need oxygen?
No. COPD is a progressive disease in and of itself; smoking makes the progression that much faster. As smoking continues, more lung tissue is destroyed, lung function worsens and the risk of needing supplemental oxygen increases. Smoking cessation prevents excessive lung function decline in COPD and in some cases, even normalizes it. If smoking cessation occurs before lung damage is too extensive, supplemental oxygen may not be necessary, even in the presence of COPD.
_
Does supplemental oxygen cause side effects?
It is important to wear your oxygen as your provider ordered it. If you start to experience headaches, confusion or increased sleepiness after you start using supplemental oxygen, you might be getting too much. Oxygen settings of 4 liters per minute or above can cause dryness and bleeding of the lining of the nose. A humidifier attached to your oxygen equipment or certain ointments can help prevent or treat the dryness.
_
Can too much oxygen hurt me?
You should consider oxygen as a medication. Accordingly it should be treated like any other drug prescribed, that is, it should be used in appropriate doses. Just because some is good, it doesn’t mean more oxygen is necessarily better. You should rely on the advice of your doctor who knows your particular case. Some lung diseases require a certain level of supplemental oxygen when the patient is at rest, but more oxygen is needed during times of exertion. Other lung diseases require a set amount of supplemental oxygen on a continuous basis, and increasing the dosage beyond the prescribed amount could be detrimental. You will have to discuss your own individual situation with your doctor to determine what is best for you. Under some circumstances it can be detrimental to increase your own oxygen without consulting your doctor, just as if you increased your own heart medicine, blood pressure medicine, or diabetes medicine without consulting your doctor. Again, the best way to go about it is to think of your oxygen like another drug prescription and to follow the specific instructions very carefully just as you would with any of your other medicines. There are some very specific situations in which it can be harmful to be on too much oxygen. People with severe COPD who are retaining carbon dioxide may worsen with too much oxygen. Oxygen toxicity due to hyperoxia harms eyes, brain and lungs, especially in neonates.
_
What about oxygen at night?
Nocturnal oxygen therapy (NOT) is not recommended in patients with COPD who have nocturnal hypoxemia but who fail to meet the criteria for LTOT. Other causes of nocturnal desaturation in COPD should be considered, such as obesity hypoventilation, respiratory muscle weakness or obstructive sleep apnoea (OSA). The oxygen that gets into your blood by using supplemental oxygen leaves your system within several minutes after removing your cannula. Therefore, although the oxygen you use during the night can have many long-term beneficial effects, the oxygen itself is gone from your system fairly soon after you turn off the oxygen tank or concentrator.
_
Can oxygen into my nose get in even when I have clogged sinuses?
That depends on the degree of obstruction/sinus congestion. If the nasal passages are completely swollen/blocked, a cannula might not be as effective but if your sinuses are congested a little, you are likely breathing more through your mouth, then the oxygen going into the nasal passages will be pulled into the lungs by the air coming in through the mouth. Patients who put their cannula in their mouth, does not usually make the delivery of oxygen to the lungs any more effective.
_
Can anyone use a portable oxygen concentrator?
Patients who need a high flow of oxygen might not be able to use a portable oxygen concentrator, since there are currently no regular portable oxygen concentrators that provide more than 3 LPM of continuous flow. If you need 4 LPM or more of continuous flow, you can use liquid oxygen tanks, which will provide you this volume of oxygen, for longer than a compressed oxygen tank. High Pressure oxygen concentrator can deliver an output of up to 10 LPM at 20 psi.
_
What do I need to do when I travel?
Talk with your healthcare provider to see if you will need oxygen on an airplane. Contact the airline well in advance to make sure it can be available. The airline will require a prescription from your health care provider. The airline may have limited choices for oxygen flows and the cost varies between airlines. If you use oxygen continuously, you must arrange for oxygen to use while waiting at the airport, at stopovers, or at your final destination. You also need to work with your healthcare provider to set up oxygen at your travel destination.
_
Is it true that Medical, Welders and Aviation Oxygen are different?
No. There is practically no difference between industrial and medical oxygen. The two come from the same source and are produced the same way but to sell oxygen as medical gas, as with any prescription drug, regulations must be complied with to ensure that it is being properly dispensed and that it is traceable, with a lot number in the event of a recall. Industrial oxygen contains no harmful contaminants. If you go to a local oxygen supplier and ask, (and they are being honest) they will tell you that they fill the welding oxygen, the aviators oxygen and the medical grade oxygen tanks from the exact same bulk tank, which is to say, they are all medical grade. Furthermore, because of the chemical nature of oxygen it must be as pure and dry from water as possible if stored under pressure. Oxygen is produced to be better than 99.9% pure, if not damage or contamination will result to equipment. Oxygen even holding the slightest amount of water moisture~ which is added during delivery for medical and industrial purposes may have helped to cause confusion in the industry. As far as the FDA is concerned any oxygen cylinder marked as USP or medical is a drug, and has to be held~ dispensed~ and used under strict medical protocols outlined by the FDA and cannot be lawfully used for aviation purposes. Oxygen cylinders labelled as AVO, which is aviator’s oxygen, or otherwise is not under the auspices of the FDA and are lawfully used for aviation purposes.
_
Do I have to worry about oxygen exploding or burning?
This is probably one of the most common misconceptions about supplemental oxygen. In truth, oxygen is combustible; not flammable. This means that materials burn more readily in its presence. Oxygen alone will not “explode” and does not burn. Oxygen will make a flame burn hotter and brighter. Oxygen itself will not explode, but it does feed a flame, which will cause it to grow rapidly and make it appear to “blow up”. That is why it’s dangerous for you to get close to an open flame while using your oxygen concentrator or other means of oxygen therapy. The oxygen could catch the flame and cause the fire to spread to your clothing, or surrounding materials such as carpet or upholstery. Because oxygen supports combustion, precautionary measures must be taken anytime it’s in use. You should never smoke while using oxygen. Your nose, hair, and clothing can catch fire very quickly and cause life-threatening burns. Do not use any petroleum products or petroleum by-products e.g. petroleum jelly/Vaseline whilst using oxygen. Keep oxygen at least 6 feet (2 meters) away from any heat source. If a cylinder falls and cracks, it propels like a torpedo. Stabilize all cylinders by placing in a safe area or by securing them to a wall.
____
____
Moral of the story:
_
- Oxidation is loss of electrons and Reduction is gain of electrons. Oxidising agents get reduced and reducing agents get oxidised. Oxygen (O2), a strong electron acceptor, enables organisms to extract more energy from organic molecules via oxidative phosphorylation in the mitochondria than can be extracted using other terminal electron acceptors. The oxidizing properties of molecular oxygen, however, come at a cost. The usual route of O2 metabolism is through its complete reduction to H2O by accepting four electrons. O2 contains two unpaired electrons and they have the same spin state. This electronic structure constitutes a barrier to the insertion of a pair of electrons. There is a way around this barrier, and that way is to add the electrons to O2 one at a time. Hence, intermediates will be encountered on this univalent pathway and these are free radicals like superoxide anion, hydroxyl radical, hydrogen peroxide, and singlet oxygen, best known as ROS (reactive oxygen species) or ROI (reactive oxygen intermediates). Free radicals are named so because the unpaired electron in the outer orbit is free to interact with surrounding molecules.
_
- Partial pressure is the individual pressure exerted independently by a particular gas within a mixture of gases. The total pressure generated by the air is due in part to nitrogen, in part to oxygen, & in part to argon, carbon dioxide and water vapor. That part of the total pressure generated by oxygen is the ‘partial pressure’ of oxygen, while that generated by carbon dioxide is the ‘partial pressure’ of carbon dioxide. Given that total atmospheric pressure (at sea level) is about 760 mm Hg and air is about 21% oxygen, then the partial pressure of oxygen in the air is 21% of 760 mm Hg or 160 mm Hg. A gas’s partial pressure, therefore, is a measure of how much of that gas is present (e.g., in air, blood or alveoli).
_
- The oxygen tension (partial pressure of oxygen) drops from 160 mmHg just outside the mouth to110 mmHg in the alveolar air at sea level. This decrease is due to several factors: (1) the addition of water vapor; (2) the addition of a volume of carbon dioxide as well as the removal of a volume of oxygen from the alveolus; and (3) the incomplete gas exchange with every breath.
_
- Fraction of inspired oxygen (FiO2) is the fraction or percentage or concentration of oxygen in the air that is inspired in lungs. Room air contains 21% oxygen, which is equivalent to FiO2 of 21 % or 0.21. Medical patients experiencing difficulty in breathing are provided with oxygen-enriched air, which means a higher-than-room air (atmospheric) FiO2. PaO2 is determined by PAO2 [which in turn determined by the fraction of inspired oxygen, the barometric pressure and the PaCO2 i.e. the alveolar gas equation] and the architecture of the lungs. Increasing FiO2 increases PAO2 and consequently PaO2 as oxygen passively diffuses along pressure gradient across alveolar-capillary membrane, provided alveolar ventilation is normal.
_
- Oxygenation is the process of oxygen diffusing passively from the alveolus to the pulmonary capillary, where it binds to hemoglobin in red blood cells or dissolves into the plasma. Insufficient oxygenation is termed hypoxemia. SaO2 or SpO2 value below 90% means PaO2 is below 60 mm Hg, indicating that the patient isn’t adequately oxygenated. Oxygenation is necessary to maintain life and health.
_
- Oxygenation and ventilation are different. Ventilation exchanges air between the lungs and the atmosphere so that oxygen can be absorbed and carbon dioxide can be eliminated. Oxygenation is simply the addition of oxygen to the body. Oxygenation mostly changes PaO2. Ventilation mostly changes PaCO2 although alveolar hypoventilation does reduce PaO2 when person is breathing room air. Hypercapnia occurs mainly due to alveolar hypoventilation but can also occur due to high V/Q (increased dead space). A patient can have normal oxygen saturation and even normal arterial oxygen tension and still be in respiratory distress or failure because ventilation and CO2 elimination is failing.
_
- Although alveolar hypoventilation causes hypercapnia, it also causes hypoxemia due to two mechanisms:
-1. Ventilation is requires not only to remove carbon dioxide but also for oxygenation and hypoventilation leads to low PAO2 and subsequent low PaO2.
-2. Hypoventilation causes hypercapnia and excess carbon dioxide readily diffuses out of alveolar-capillary membrane into alveoli and this excess carbon dioxide takes up alveolar space and leaves less room for oxygen.
_
- Hypoxemia refers to reduced O2 tension in arterial blood i.e. decreased partial pressure of oxygen in arterial blood. Hypoxia is inadequate oxygen in tissues for normal cell and organ function, and hypoxia results not only from hypoxemia but also from reduced tissue perfusion, reduced or dysfunctional hemoglobin and inability of cells to use the oxygen even if there is sufficient oxygen circulating. The human body cannot store oxygen for later use as it does with food. If the body is deprived of oxygen for more than a few minutes, unconsciousness and death result.
_
- Oxygen therapy, also known as supplemental oxygen, is the use of oxygen as medical treatment by inhalation. Oxygen therapy is defined as the administration of oxygen to a patient at an inspired concentration greater than that of oxygen concentration in ambient air. Supplemental oxygen means FiO2 greater than the 21% oxygen in room (ambient) air. When you give supplemental oxygen you are raising the patient’s inhaled FiO2 to something over 21%; the highest FiO2 possible is 100%. To give even more oxygen requires a hyperbaric chamber. In other words, oxygen therapy means FiO2 > 21% at normal atmospheric pressure (760mmHg) i.e. normobaric oxygen (NBO) therapy or FiO2 > 21% at more than normal atmospheric pressure i.e., hyperbaric oxygen (HBO) therapy. In airplanes, pressurization systems are designed to keep the interior cabin pressure between 11 and 12 psi at cruise altitude (36000 feet) where outside atmospheric pressure is only 3.3 psi, and atmospheric pressure at sea level is 14.7 psi. So anybody receiving oxygen therapy in airplane due to any reason will be hypobaric oxygen therapy.
_
- The ability of cells to tolerate low levels of oxygen is explained by the finding that isolated mitochondria do not become substrate limited by oxygen until the PO2 falls to <1 mm Hg (normal PO2 in mitochondria 7-10 mmHg). This explains how some patients with remarkably low arterial oxygen concentrations can remain clinically “stable” for prolonged periods prior to the initiation of oxygen therapy.
_
- Increasing the inspired O2 fraction (FiO2) increases the alveolar PO2 (PAO2) and consequently the arterial PO2 (PaO2): In cases of shunt (V/Q = 0), supplemental O2 therapy has little effect on PaO2. If the cause of hypoxemia is low V/Q, high V/Q (increased dead space), diffusion defect, alveolar hypoventilation or high altitude (low PiO2), supplemental O2 therapy will effectively increase the PaO2. Oxygen therapy is most helpful in hypoxemic hypoxia (except shunt) and somewhat helpful in anemic and stagnant hypoxia. When tissue hypoxia occurs in the absence of arterial hypoxemia, treatment should always be directed at correcting the underlying cause (i.e. heart failure, anaemia).
_
- If oxygen flow rate is set at 1 l/min, patient will receive 1 l/min of 100% oxygen mixed with room air having 21% oxygen. From physics perspective, the actual concentration of oxygen delivered is determined by the interaction between the delivery system and the patient’s breathing pattern. FiO2 depends on ventilatory minute volume and flow rate of oxygen, meaning the patient’s breathing rate and tidal volume, i.e., the amount of room air inhaled through the mouth and nose that mixes with the supplemental oxygen. For example, ventilatory minute volume is 20 liters/minute in a patient with respiratory distress and oxygen given 2 liter/minute, patient will inhale 18 liter air (FiO2 21%) and 2 liter oxygen (FiO2 100%), so actual FiO2 received by patient is (1 X 2) + (0.21 X 18) / 20 = 0.29 (29%).
_
- Oxygen is one of the most widely used therapeutic agents. It is a drug in the true sense of the word, with specific biochemical and physiologic actions, a distinct range of effective doses, and well-defined adverse effects at high doses. The dose of oxygen is the combination of its partial pressure and duration of exposure. Oxygen prescription often comprises of a single written word — oxygen. Frequently, it is administered merely on verbal orders. To ensure safe and effective treatment, prescriptions should cover the flow rate, delivery system, duration, the target range for oxygen saturation and monitoring of treatment.
_
- Oxygen is probably the most common drug used in the care of patients who present with medical emergencies; approximately 34% of ambulance patients receive oxygen during transit and 15–17% of hospital inpatients will be receiving oxygen at any given time.
_
- Oxygen therapy is useful in anaesthesia as pre-oxygenation before anaesthesia, FiO2 25 to 30 % during anaesthesia and postoperative oxygen.
_
- The most common indication for oxygen therapy in the acute setting is arterial hypoxemia with a PaO2 of less than 60 mm Hg. Ventilation perfusion mismatch is the most common cause of arterial hypoxemia. A PaO2 of 60 mm Hg is often set as a reasonable goal in the initial treatment of arterial hypoxemia. Accepted indications for short-term oxygen therapy include acute hypoxemia, cardiac and respiratory arrest and low cardiac output with metabolic acidosis.
_
- It is well established that severe hypoxemia results in rapid organ failure and death. Increasing the concentration of inhaled oxygen is an effective method of increasing the partial pressure of oxygen in the blood and correcting hypoxemia. Oxygen saves lives when used appropriately to correct hypoxemia and is an essential component in resuscitation of the critically ill. Oxygen supplementation is a well-accepted therapy for hypoxemic patients, because it increases the delivery of oxygen to cells and is thus believed to reverse the effects of hypoxia. Hypoxemia is both a marker of risk of a poor outcome due to the severity of the underlying disease(s) that has caused hypoxemia, and an independent risk factor of poor outcome. Supplemental oxygen relieves hypoxemia but does not improve ventilation or treat the underlying cause of the hypoxemia. Monitoring of SpO2 indicates oxygenation not ventilation. Therefore, beware the use of high FiO2 in the presence of reduced minute ventilation. Also high FiO2 is linked to oxygen toxicity.
_
- Titrated oxygen therapy (controlled administration of oxygen) means maintaining oxygen saturation between 94 to 98% in most cases (92-96% preferred by other authors), while in those at risk of carbon dioxide retention maintain saturation between 88 to 92%, and in those with carbon monoxide toxicity or cardiac arrest it should be as high as possible. The oxygen flow rate administered is not important; the PAO2, the PaO2 and the SaO2 (or SpO2) achieved are most important. It is an increase in FiO2 that increases the oxygen saturations of a patient, not the oxygen flow rate although oxygen flow rate contributes to FiO2. Effective oxygen therapy is about finding a balance between delivering the lowest FiO2 in order to achieve prescribed oxygen saturations for the patient. Also when lowest FiO2 achieve oxygen saturation in target range, continuous real-time pulse oximetry allows early detection of deterioration in patient’s illness.
_
- Lowest FiO2 to achieve target oxygen saturation means:
-avoiding hypercapnia
-avoiding oxygen toxicity especially in conditions of ischemia/reperfusion
-early detection of deterioration
_
- More than 98% oxygen in blood is carried by hemoglobin and hemoglobin is fully saturated with oxygen (SaO2/SpO2 100%) at PaO2 80-100 mmHg. Hemoglobin cannot carry more oxygen even if PaO2 becomes more than 100mm; therefore SpO2 cannot rise above 100 % no matter how high PaO2 is. Therefore hyperoxemia cannot be diagnosed with SpO2. Therefore no oxygen therapy guideline recommends 100 % oxygen saturation except carbon monoxide poisoning, cardiorespiratory arrest, air embolism and decompression illness.
_
21.There are no contraindications to oxygen therapy if indications for therapy are present. Although oxygen should not be withheld from any critical patient, oxygen is contraindicated in paraquat poisoning and bleomycin induced lung injury.
_
- Oxygen therapy is indicated in patients with oxygen saturations below the target saturation range. It is not indicated for the treatment of breathlessness in patients with adequate oxygen saturations, apart from certain patients with carbon monoxide poisoning and with pneumothorax. Dyspnoea can occur for many reasons other than cardiorespiratory disease, including metabolic acidosis, anxiety and pain, and treatment with oxygen is not indicated in these cases. There are risks in the practice of administering prophylactic oxygen to a breathless patient who is not currently hypoxemic, in the belief that it may prevent hypoxemia if the underlying condition deteriorates. This practice has the potential to cause delay in recognising clinical deterioration and reduce the time available to start additional treatment.
_
- Treatment of breathlessness in patients with refractory breathlessness and advanced life-limiting illness by medical air specifically delivered via nasal cannula masquerading as oxygen therapy does improve symptoms due to presence of an intervention that alleviates patient’s anxiety and related breathlessness, intervention inducing expectation of benefit, gaseous flow across nasal passages influences the sensation of dyspnea and the extra attention that the patient receives.
_
- Oxygen cylinder operates at 1800-2400 psi which is very high pressure and cannot be directly delivered to patient or run the ventilator. It needs pressure regulator with flow meter. The pressure regulator controls the pressure coming out of the cylinder by reducing high pressure into low pressure which is indicated on the gauge in psi. The flow meter controls how rapidly the oxygen flows from the cylinder/wall source to the patient. Regular home oxygen concentrators deliver oxygen at around 7 psi and does not need pressure valve.
_
- While there is no defined cut-off point between low and high flow, it is reasonable to place it at about 10–15 litre/min. If the ventilatory demand of the patient is met completely by the system, then it is a high-flow system. In contrast, if the system fails to meet the ventilatory demand of the patient, then it is classified as a low-flow system. Low flow systems often are more comfortable, but the ability to deliver a precise oxygen concentration in various respiratory breathing patterns is limited. A high flow system can deliver very accurate oxygen concentrations, but is often uncomfortable and obtrusive.
_
- 100% oxygen administered means no admixture of room air. 100% oxygen can only be delivered with a ventilator, a tight-fitting non-rebreather face mask and high flow oxygen (HFO) therapy via high flow nasal cannula (HFNC).
_
- Traditional oxygen therapy is up to 16 l/min and high flow oxygen therapy is up to 60 l/min. Humidified high flow nasal cannula (HFNC) enables flows exceeding a patient’s peak inspiratory flow demand to be delivered via nasal cannula, thus providing FiO2 of up to 100% because there is no entrainment of room air, even with the mouth open. This also allows the patient to continue to talk, eat and drink while still receiving the therapy. This type of delivery method is associated with greater overall comfort, and improved oxygenation and respiratory rates than with face mask oxygen. The administration of a high flow of heated and humidified gas mixture promotes higher and more stable inspiratory oxygen fraction values, decreases anatomical dead space and generates a positive airway pressure that can reduce the work of breathing and enhance patient comfort and tolerance. Based on clinical evidence, the utilization of high flow oxygen (HFO) therapy via high flow nasal cannula (HFNC) in appropriate patients can improve oxygenation, decrease the patient’s work of breathing, and serve as an alternative to non-invasive ventilation and intubation in selected patient populations.
_
- The physiological response of an increase in PaCO2 due to high concentration oxygen therapy has been demonstrated not only in stable and acute exacerbations of COPD, but also in severe asthma, community-acquired pneumonia and obesity hypoventilation syndrome. Proposed mechanisms for oxygen-induced hypercapnia include increased ventilation perfusion mismatch due to reduced hypoxic pulmonary vasoconstriction, reduced ventilatory drive, atelectasis and the Haldane effect, with the contribution of each likely to depend on the clinical situation. FiO2 of 40-60% is adequate in most situations, 100% needed during resuscitation. Increasing requirement of FiO2 to maintain same SpO2 is an ominous sign. If oxygen-induced hypercapnia develops, oxygen therapy should not be abruptly stopped. This may lead to rebound hypoxemia (with a fall in oxygen saturation to below the level seen before oxygen was given). Oxygen should be gradually down-titrated and non-invasive ventilation considered. Never withhold oxygen from a seriously ill hypoxic patient due to fear of causing hypercapnic respiratory failure. Never let the fear of CO2 retention stop you from treating a COPD patient with oxygen in an emergency. The vast majority of patients with COPD do not retain CO2. And even if the patient you happen to be treating does retain CO2, the worst-case scenario is that you relieve their hypoxia and protect their brain and heart (good) but might have to temporarily assist ventilation.
_
- On the basis of all available current data, long-term oxygen therapy (LTOT) should be prescribed to prolong survival among patients with COPD who have chronic (>3 weeks) severe resting hypoxemia (PaO2 of ≤55 mm Hg or SpO2 of <88%) while they are breathing ambient air, and they have receiving optimal pharmacological treatment. Patients with moderate hypoxemia having polycythemia or signs of right-sided heart failure may also benefit from long-term oxygen therapy. Oxygen supplementation in patients with chronic hypoxemia has been shown to improve survival, pulmonary hemodynamics, exercise capacity and neuropsychological performance. According to LTOT guidelines, oxygen should be prescribed for at least 18 hours per day. Oxygen should be titrated to achieve a target oxygen saturation range of 88–92%. This results in a greater than twofold reduction in mortality, compared with the routine administration of high-concentration oxygen therapy. Work in COPD has estimated that in the UK, a few thousand deaths could be avoided each year by controlled oxygen use. Only in patients who qualify for long-term oxygen therapy (LTOT), ambulatory and nocturnal oxygen therapy can be used. Short-burst oxygen i.e. oxygen inhaled immediately prior and/or following exertion with the aim of relieving breathlessness or improving exercise tolerance is not effective. It is unknown whether continuous supplemental oxygen therapy affects survival in patients with COPD and isolated nocturnal oxygen desaturation. Although exertional desaturation in patients with COPD and resting normoxemia appears to predict a poor prognosis, the effect of continuous supplemental oxygen on survival in this group has not been prospectively assessed in a large population.
_
- Oxygen-driven nebulizer must be used for delivery of high-dose beta agonists in acute bronchial asthma having life-threatening features with oxygen at a rate greater than 6 l/min. However, when nebulised therapy is administered to patients at risk of hypercapnic respiratory failure (e.g. severe COPD), it should be driven by compressed air, and if necessary supplementary oxygen should be given concurrently by nasal prongs at 1-4 litres per minute to maintain an oxygen saturation of 88-92%.
_
- My definition of hyperoxia:
Hyperoxia occurs when cells, tissues, blood and organs are exposed to higher than normal partial pressure of oxygen. Hyperoxia definition need not entail harm just as definitions of Hypertension and Diabetes entail no harm in it.
Hyperoxia means PAO2 > 110mm and/or PaO2 > 100mm and/or tissue PO2 > 50 mm Hg.
For example, FiO2 is increased to 60 % from normal 21% to increase PAO2 to 380mm but due to severity of illness e.g. massive pneumonia or pulmonary edema, PaO2 is still 60mm, it is hyperoxia for lungs although rest of the body is hypoxic. If such situation continues for several days, patient may develop lung injury due to oxygen toxicity although rest of the body is hypoxic. Hyperoxia can be caused by breathing air with FiO2 more than 21 % at normal atmospheric pressure (normobaric hyperoxia) or breathing air or oxygen at pressures greater than normal atmospheric pressure (hyperbaric hyperoxia). PaO2 more than 100 mm Hg is hyperoxemia which will causes hyperoxia in various tissues.
_
- In hyperbaric oxygen (HBO) therapy, 100 percent oxygen is given at two to three times the atmospheric pressure resulting in arterial oxygen tension in excess of 2000 mm Hg and oxygen tension in tissue of almost 400 mm Hg. Such doses of oxygen have a number of beneficial biochemical, cellular, and physiologic effects. Since dissolved oxygen in blood is 0.003 ml per mm of oxygen tension per 100 ml of blood, >2000 mm PaO2 will result in dissolved oxygen of 6 ml per 100 ml blood i.e. 60 ml/liter of blood. Since cardiac output is 5 liter/min, HBO will provide 60 X 5 = 300 ml/min of dissolved oxygen to whole body which is more than total resting tissue requirement of oxygen 250 ml/min. So it meets the average requirements of resting tissues by means of dissolved oxygen alone without contribution from oxygen bound to hemoglobin. Remember dissolved oxygen is available to tissues first and then oxygen bound to hemoglobin. Remember when 100 % normobaric oxygen is given, PaO2 becomes 600 mm and dissolved oxygen 18 ml per liter of blood, but it will provide only 90 ml dissolved oxygen per min to whole body, quite insufficient to provide resting tissue oxygen requirement. Also, marked increase in oxygen tension gradient from the blood to metabolizing cells in HBO therapy is a key mechanism by which hyperoxygenation of arterial blood can improve effective cellular oxygenation even at low rates of tissue blood flow.
_
- Sufficient clinical evidence is available for the use of HBO in carbon monoxide poisoning & smoke inhalation, decompression sickness, arterial gas embolism, radiation-induced tissue injury, clostridial myo-necrosis (gas gangrene), problem wounds, crush injury, and refractory osteomyelitis. Due to its potential toxic effects, HBO is currently restricted to short sessions (less than 2 hours), at pressures below the threshold of CNS toxicity (< 3 Atm), with ‘recovery’ breaks of few minutes during which the patient is switched to air breathing at the treatment pressure.
_
- All cells, healthy and cancerous rely on both aerobic and anaerobic metabolism for energy production. And just like healthy cells, cancer cells will die if deprived of oxygen. However hypoxia is a critical hallmark of solid tumors and involves enhanced cell survival in cancer due increased genetic instability, upregulation of genes, angiogenesis, activation of invasive growth, and preservation of undifferentiated cell state. Studies have demonstrated that hypoxia is implicated in the resistance to conventional cancer therapy. Given its important role as a negative prognostics and predictive factor, hypoxia may be considered as one of the targets in certain cancer subtypes. So hyperbaric oxygen therapy may be used as adjuvant to conventional treatment in certain cancer subtypes.
_
- The only absolute contraindication to hyperbaric oxygen therapy is untreated pneumothorax. Normobaric oxygen therapy accelerates resorption of pneumothorax. So don’t mix up hyperbaric oxygen therapy with normobaric oxygen therapy.
_
- Maximum PAO2 achieved though PPV (positive pressure ventilation) is 685 mmHg and maximum PAO2 achieved through HBO is 2183 mmHg. So PPV and HBO are different although the word ‘Pressure’ is used in positive pressure ventilation and oxygen delivered at 2 to 3 times of normal atmospheric pressure in HBO therapy.
_
- A large body of data indicates that hyperoxia exerts an extensive profile of physiologic and pharmacologic effects that improve tissue oxygenation, exert anti-inflammatory and antibacterial effects, and augment tissue repair mechanisms. These data set the rationale for the use of hyperoxia in a list of clinical conditions characterized by tissue hypoxia, infection, and consequential impaired tissue repair. On the other hand, the potential risks of hyperoxia due to high concentration oxygen therapy include respiratory (increased PaCO2, absorption atelectasis and direct pulmonary toxicity), cardiovascular (increased systemic vascular resistance and blood pressure, reduced coronary artery blood flow, reduced cardiac output), cerebrovascular (reduced cerebral blood flow) effects, ocular effects and increased reperfusion injury.
_
- Adverse effects due to hyperoxia are divided in to two groups:
-1. Adverse effects on normal organs and tissues (oxygen toxicity):
Oxidative damage may occur in any cell in the body but the effects on the three most susceptible organs (brain, eyes and lungs) will be the primary concern, more so in neonates. It may also be implicated in damage to red blood cells (haemolysis), the liver, heart, endocrine glands (adrenal glands, gonads, and thyroid), or kidneys, and general damage to cells. Exposure time, atmospheric pressure, and fraction of inspired O2 (FiO2) determine the cumulative O2 dose leading to oxygen toxicity. Pulmonary oxygen toxicity occurs when 100% O2 given for 12 hours or more; 80% O2 for more than 24hrs; and 60% O2 more than 36hrs. As for NBO, whenever possible, it should be restricted to periods shorter than the latent period for development of pulmonary toxicity. FiO2 is typically maintained below 0.5 even with mechanical ventilation, to avoid oxygen toxicity. When used according to currently employed standard protocols, oxygen therapy is extremely safe. CNS toxicity does not occur during normobaric exposures but is the main limitation for the use of HBO in diving and hyperbaric treatments. Pulmonary and ocular damage are most likely to occur when supplemental oxygen is administered as part of a treatment, particularly to newborn infants, but are also a concern during hyperbaric oxygen therapy.
-2. Adverse effects on diseased organs and tissues when oxygen is given as part of treatment for these diseases:
For example, harms produced by oxygen to lungs and heart when given in COPD and AMI respectively.
_
- There are risks from unrelieved hypoxemia due to insufficient oxygen therapy, and from provoked hyperoxemia due to excessive oxygen therapy. Oxygen therapy should therefore be titrated so that the saturation is within a range that avoids these risks. The ‘swimming between the flags’ concept of titrating oxygen therapy, to within a specific target oxygen saturation range applies to a wide range of clinical situations as there are risks associated with both hypoxemia and hyperoxemia. Although the margin of safety between effective and potentially toxic doses of oxygen is relatively narrow, the ability to carefully control its dose, meticulous adherence to currently accepted therapeutic protocols, and individually tailored treatment regimens make it a cost-effective safe drug. In the acute situation the dose of oxygen administered may be critical. Inadequate oxygen accounts for more deaths and permanent disability than can be justified by the relatively small risks associated with high dose oxygen.
_
- There is a common misconception is that one “can’t give too much oxygen” and there is general lack of appreciation for the dangers of hyperoxemia and hyperoxia.
_
- Although oxygen remains of life-saving importance in critical care, accumulating evidence has demonstrated the prominent role of hyperoxia and the consequent formation of ROS in the pathogenesis of several life-threatening diseases. The data from pre-hospital research studies comparing outcomes of patients treated without oxygen or with oxygen titrated to saturations versus patients routinely given high flow oxygen are frightening; they invariably show impressive patient harm from even short periods of hyperoxia. In 2002, a study of 5,549 trauma patients in Texas showed pre-hospital supplemental oxygen administration nearly doubled mortality. Study of patients undergoing cancer surgery in whom 80 % oxygen supply in the perioperative setting showed a significantly increased long-term all-cause mortality compared with those randomly assigned to 30 %.
_
- Hyperoxemia can cause coronary vasoconstriction. Paradoxically, therefore, giving too much oxygen at the time of an acute myocardial infarction may worsen oxygen delivery to the cardiac muscle. Use of high-flow oxygen has been associated with increased reperfusion injury, infarct size and mortality in myocardial infarction. If the patient is dyspnoeic, hypoxemic, or has obvious signs of heart failure, oxygen therapy should be given in acute coronary syndrome based on monitoring of oxygen saturation to 94%.
_
- The human brain is at particularly high risk for damage by free radicals because of its high degree of metabolism compared to other tissues & its high lipid content while lacking the levels of antioxidant protection found elsewhere in the body. Thus, oxygen therapy may cause severe oxidative damage to the brain and blood-brain-barrier (BBB) disruption by lipid peroxidation, especially during reperfusion. In addition, hyperoxia may compromise cerebral blood flow via vasoconstriction and thereby worsen the functional outcome of ischemic stroke. Clinical trials have failed to demonstrate clear-cut benefits of hyperoxia in ischemic stroke. One randomised controlled trial found that in minor or moderate stroke, oxygen administration was linked to increased mortality when compared with air. So oxygen therapy in ischemic stroke is to be given only if patient presents with hypoxemia.
_
- For neonatal resuscitation of term infant, the routine use of 100 % oxygen has been abandoned after numerous associations with myocardial, neurological, and kidney injury; and retinopathy, inflammation, and increased mortality. The current neonatal resuscitation recommendation of term infant is use of room air positive pressure ventilation. Cochrane review suggested that more than 100,000 newborn lives might be saved globally each year by changing from 100 to 21% oxygen for newborn resuscitation. Preterm infants are very sensitive to hyperoxia, which may lead to lung and retinal damage and also to hypoxia, which may cause increased mortality, NEC (Necrotizing Enterocolitis), or white matter injury. Keeping preterm babies within a range from 90% to 95% SpO2 seems a reasonable approach limiting exposure to extreme high or low SpO2. For extremely low birth weight infants, initial FiO2 could be between 0.21 and 0.30 and adjusted according to the response in SaO2 and heart rate.
_
- Hyperoxia appears to produce cellular injury through increased production of reactive oxygen species (ROS), such as the superoxide anion, the hydroxyl radical, and hydrogen peroxide. When production of these ROS increases and/or the cell’s antioxidant defenses are depleted, they can react with and impair the function of essential intracellular macromolecules. Oxidative stress refers to the imbalance caused by increased ROS formation or deficient oxidant suppressors. Oxidative stress leads to damage of nucleic acids, proteins, and lipids, resulting in cell death by both apoptotic and necrotic pathways. Oxygen toxicity is believed to result from the formation of ROS in excess of the quantity that can be detoxified by the available antioxidant systems in the tissues. The enzymatic antioxidants like superoxide dismutase (SOD), catalase, and glutathione peroxidase are evolutionary biologically evolved and genetically controlled. There is a limit to their work. When that limit is crossed oxygen starts harming us. And that limit may be different in different individuals, so conflicting results of oxygen therapy trials.
_
- Hypoxia also generates ROS and hyperoxia worsen it. Patients suffering from traumatic brain injury, post arrest syndrome, compartment syndrome, or prolonged hypotension often have a build-up of ROS which only need additional oxygen to incite a cascade of oxidative injury. In these cases, supplemental oxygen should only be provided if there is evidence of hypoxia.
_
- Both oxygen and ROS are Janus-headed. On the one hand, O2 plays a crucial role during adenosine triphosphate (ATP) synthesis. On the other hand, its chemical characteristics lead to strong oxidizing properties, capable of damaging any biological molecule, and thereby defining the paradigm of oxygen toxicity. This phenomenon is due to the formation of reactive oxygen species (ROS), its magnitude being directly correlated to the level of the O2 partial pressure either low or high. ROS also exert Janus-headed properties: on the one hand, ROS is useful for host defense (white blood cells generate excessive ROS to kill bacteria or to have anticancer properties), signalling cascades and homeostasis. On the other hand, ROS production has been associated with cancer, ageing, diabetes, obesity, neurodegeneration, and other age-related diseases such as age-related retinopathy, cochlear degeneration, and chronic inflammatory diseases. Why blame O2 and ROS for being Janus-headed? My theory of duality of existence shows that there is a dual existence of everything in universe and therefore a single frame of logic/rationale/laws cannot explain all phenomena associated with it. Randomness and certainty coexist simultaneously depending on the knowledge of all variables. So O2 and ROS show dual behaviour depending on their concentration (existence) and since we do not know all variables in a biological system, we are getting conflicting results in their clinical studies.
_
- Review of all of the instances in which the urge to “normalize” physiology has done more harm than good. In the ICU alone, this phenomenon is played out with intensive insulin therapy, blood transfusions, and erythropoietin administration. Attempts at normalization did not translate to improvements in clinical outcomes. And oxygen therapy is no exception. Hypoxia does harm but hyperoxia also harms. A more individualized approach to target oxygen levels is desirable. The goal of oxygen therapy is to avoid hypoxia and hyperoxia. If the patient’s oxygen saturation and ventilation are adequate, supplemental oxygen is probably not required. If the patient is hypoxic and/or hypercapnic, then you must determine whether the problem can be remedied through increased ventilation, increased oxygenation, or both. Thus, you have to assess the problem, recognize and understand the pathophysiological processes involved, plan an appropriate therapy (within the scope of your protocols), and provide the needed therapy. There is no ‘one size fits all’ oxygen therapy.
_
- Low concentrations of ROS may be beneficial or even indispensable in processes such as intracellular signalling molecules in gene expression and defense against micro-organisms or cancer. High concentrations of ROS either due to hypoxia or hyperoxia or hyperoxia following hypoxia is harmful. Normoxia generates a small amount of ROS produced through cellular respiration that is helpful to us and therefore the goal of oxygen therapy is to treat hypoxia to make normoxia and not hyperoxia barring exception of hyperbaric oxygen therapy which uses increased dissolved oxygen in blood and physical properties of gases to benefit us in few specific conditions overriding harms of increased ROS. Barring few exceptions, no patient needs oxygen saturations above 97 percent and in truth, there is little to no evidence suggesting any clinical benefit of oxygen saturations above 90 percent in any patient.
_
- A 2016 study on brain adaptation to hypoxia and hyperoxia in mice found that mild hyperoxia represents a condition as challenging as mild hypoxia with respect to redox imbalance and brain damage. This suggests that, despite profound fluctuations in Earth atmosphere %O2 in the past geological eras, terrestrial mammals are now adapted to live in an atmosphere containing 21%O2, and that any deviation from this value, irrespectively if higher or lower, might trigger the occurrence of a potentially lethal situation. Both hypoxia and hyperoxia alter ROS production by changing mitochondrial PO2 (PmO2) and high or low PmO2 provoke excessive ROS. Both hypoxia and hyperoxia generate moderate amount of ROS but hyperoxia following hypoxia generate maximum ROS. In other words, be very cautious treating severe hypoxemia (critical illness) by oxygen therapy, not to overshoot and cause hyperoxia. On the other hand, dangers of severe hypoxemia are far greater than those of hyperoxia following hypoxia, and just like fluid management; the majority of patients get too little rather than too much.
_______
Dr. Rajiv Desai. MD.
February 12, 2018
_____
Postscript:
Oxygen therapy is not a ‘hype’ but room air containing 21% oxygen is sufficient in many serious illnesses including neonatal resuscitation.
______

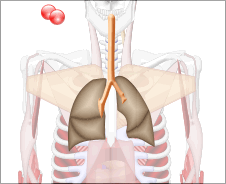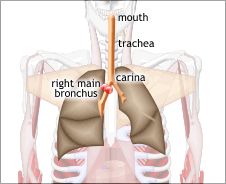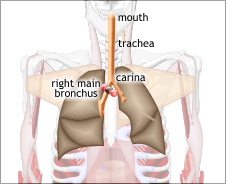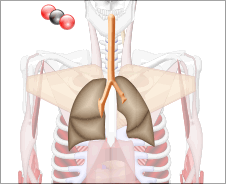


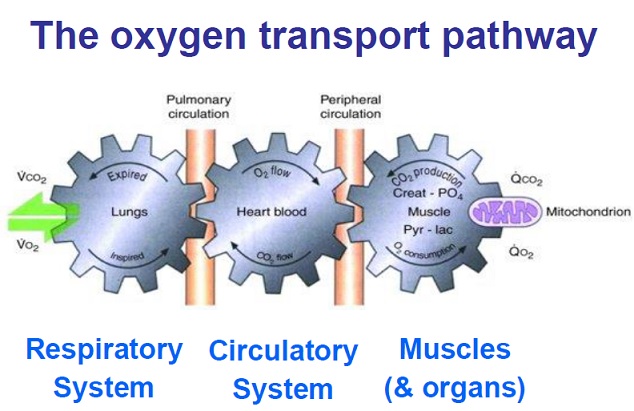
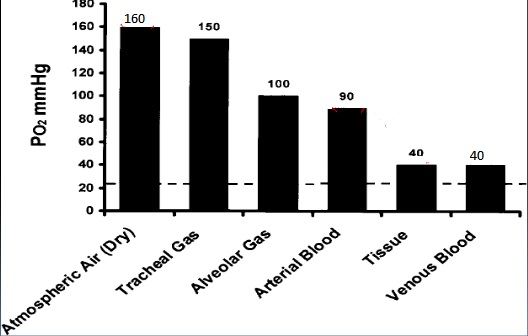
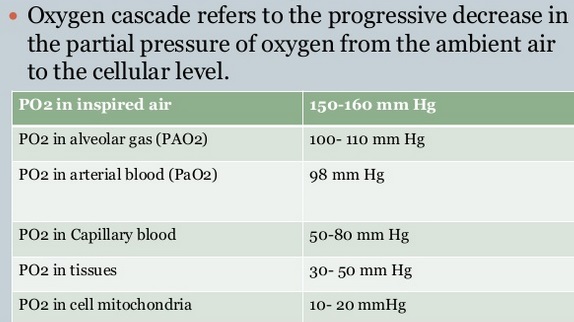
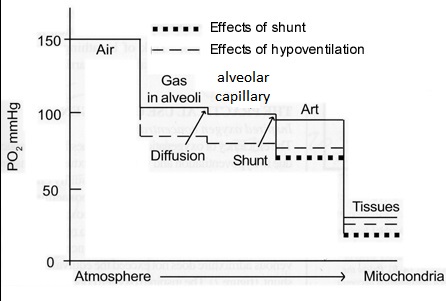
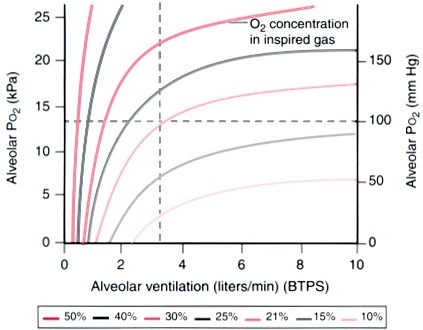
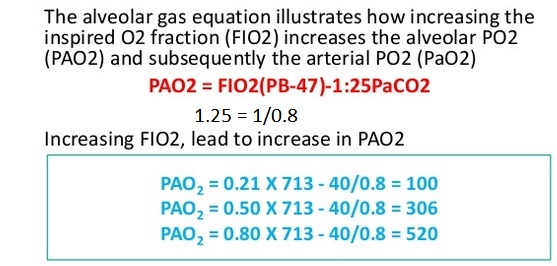


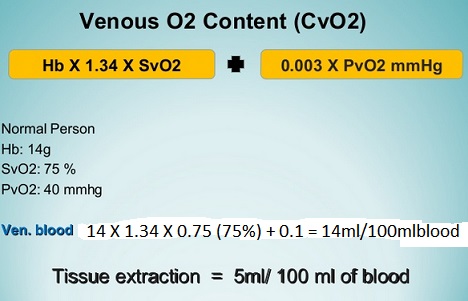
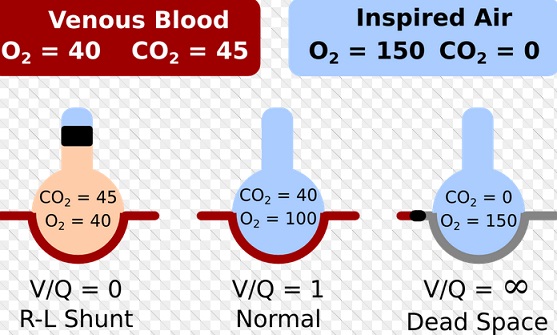
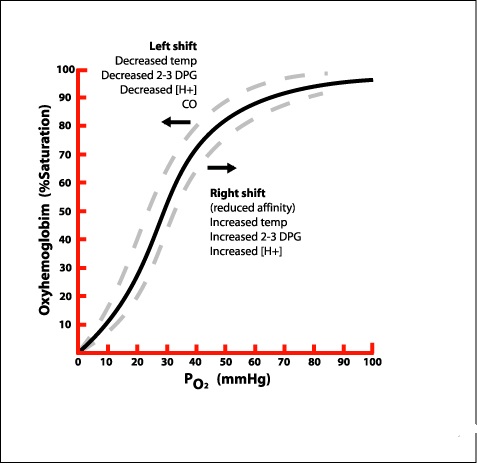
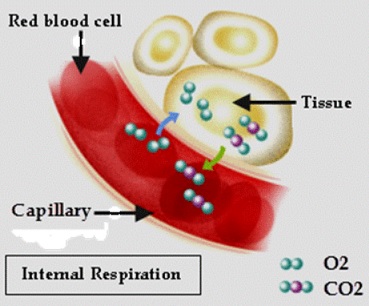

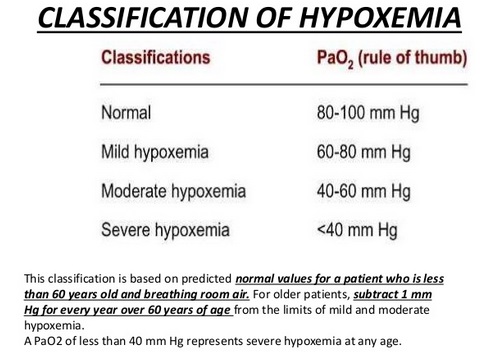
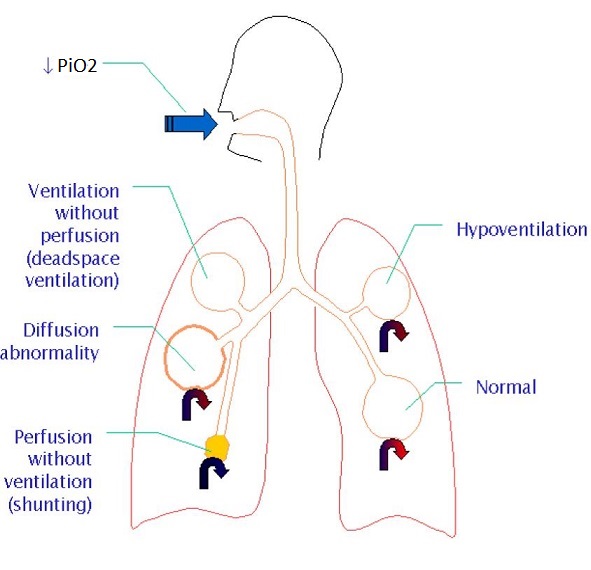
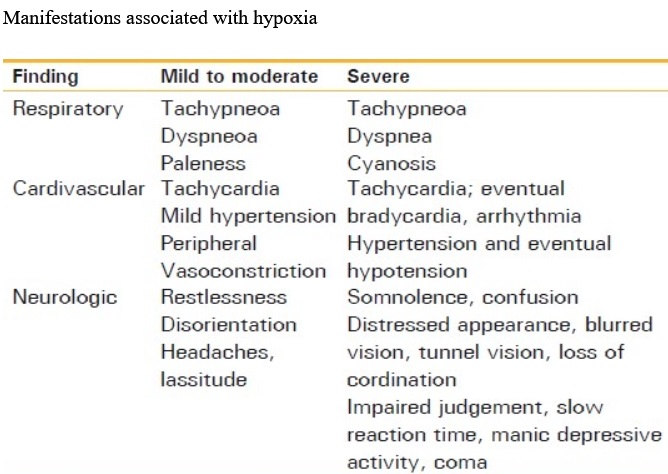
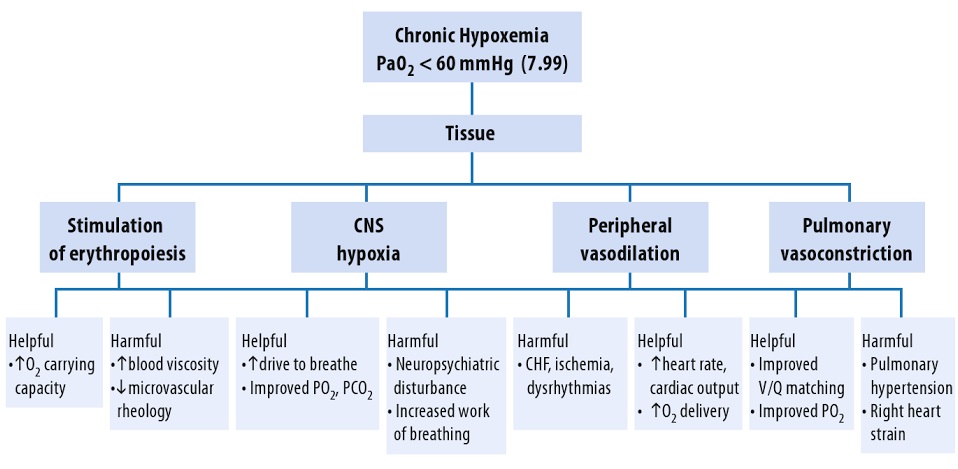
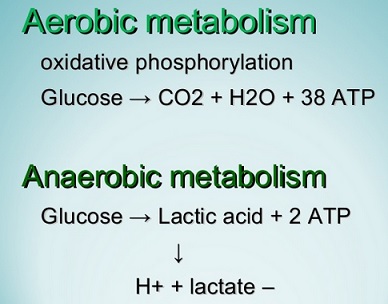
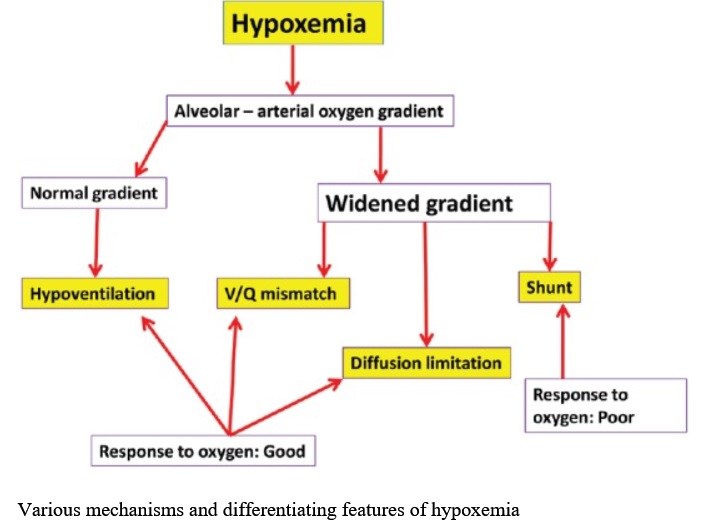

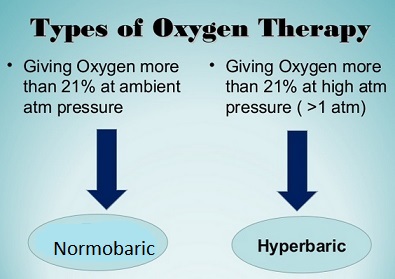



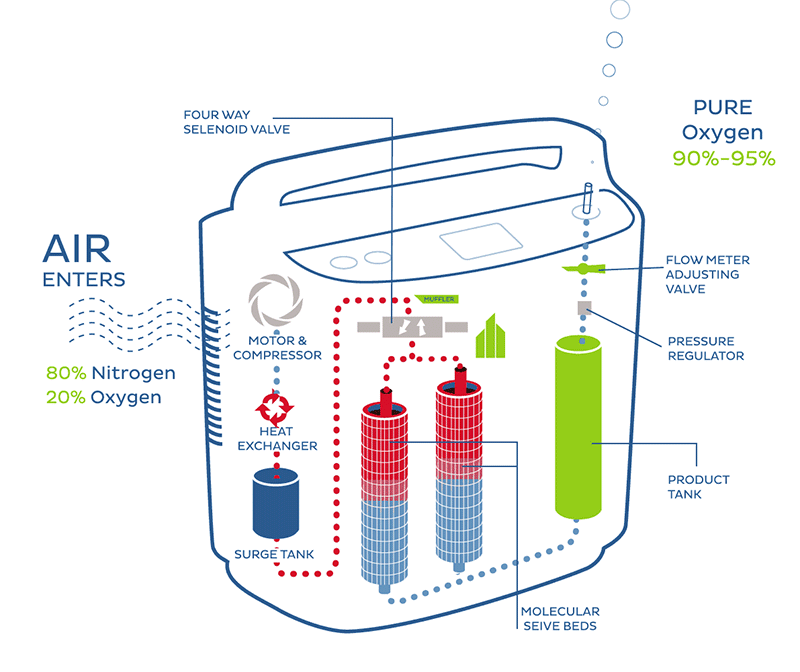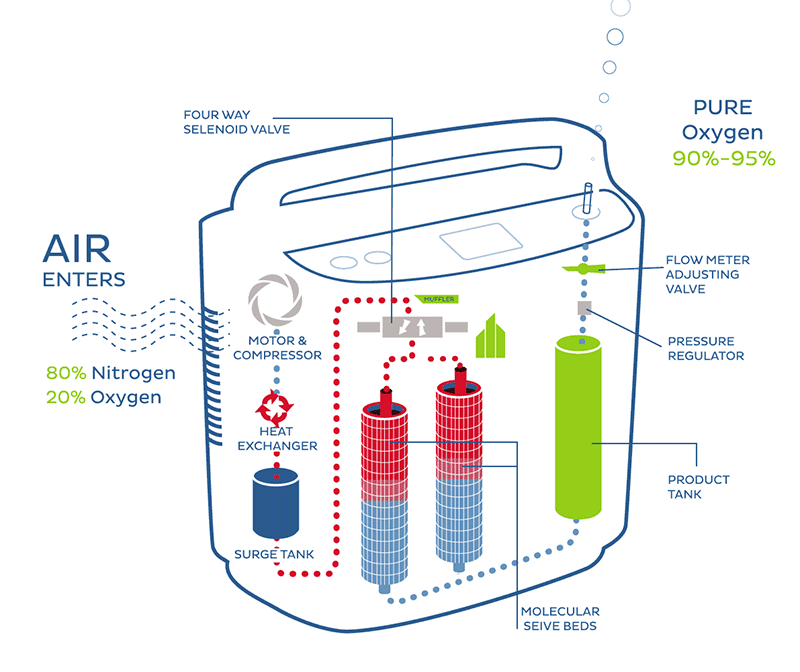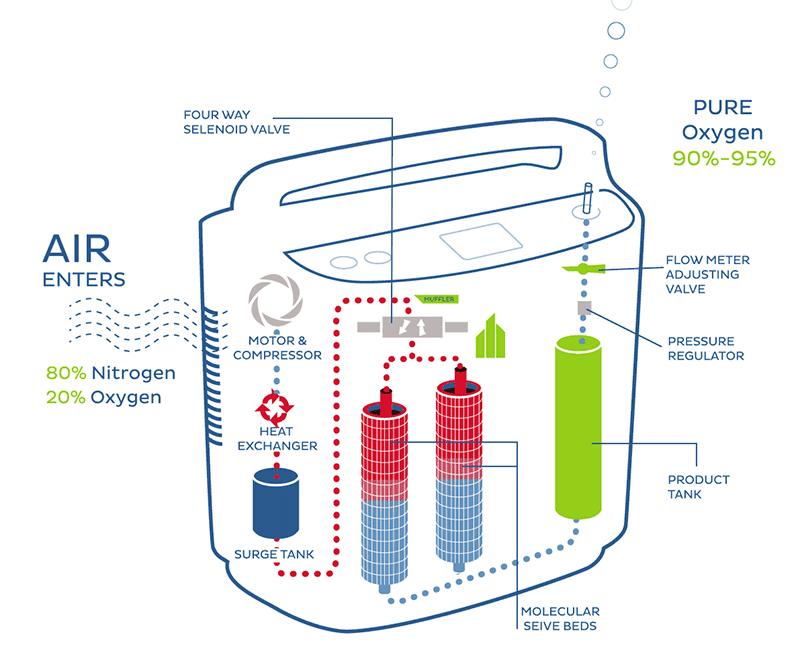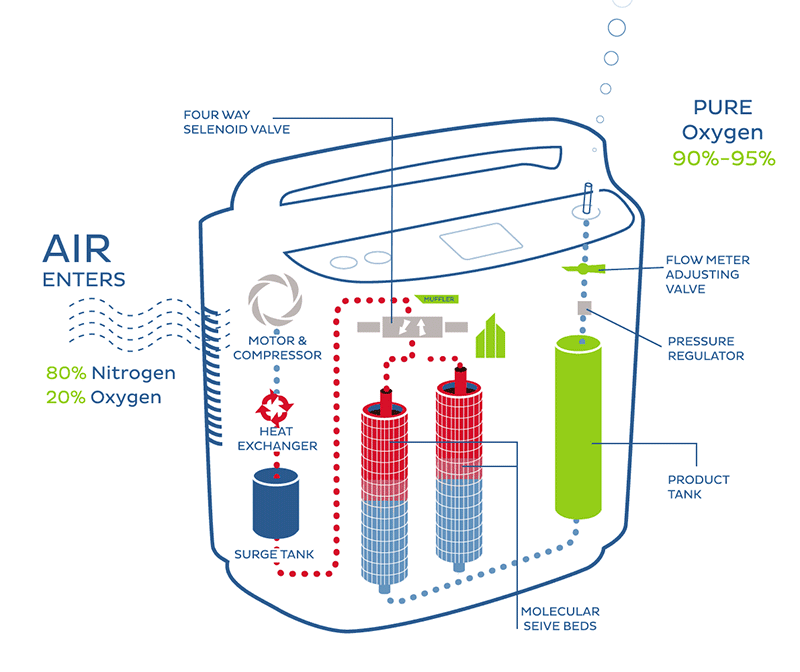
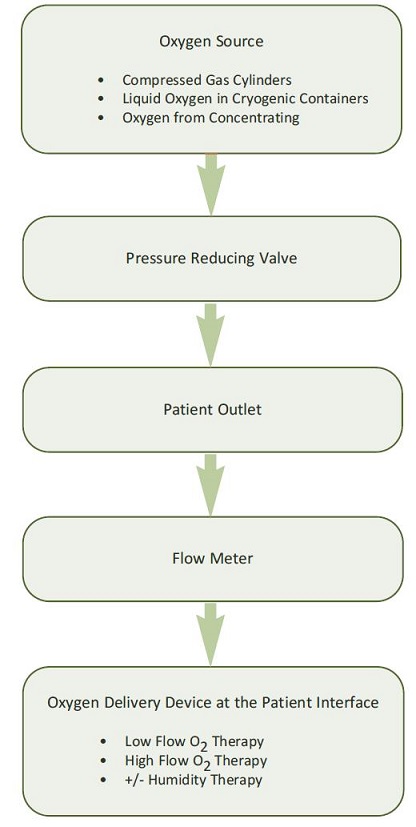

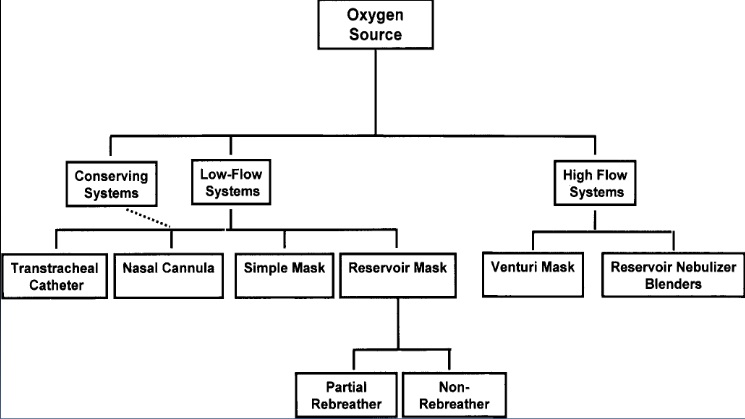
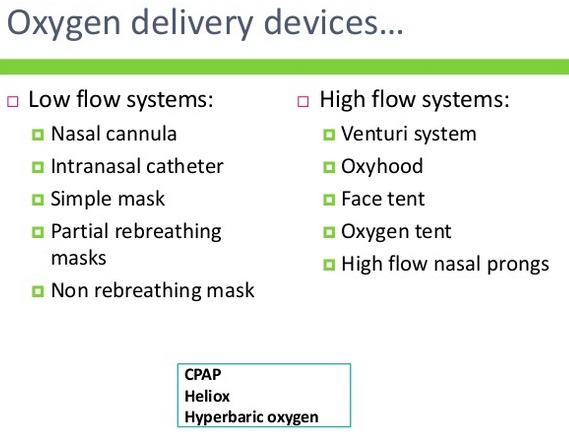

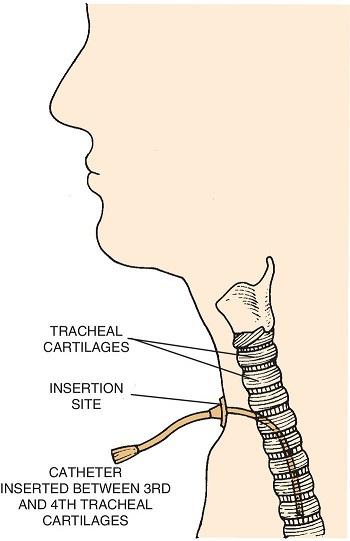
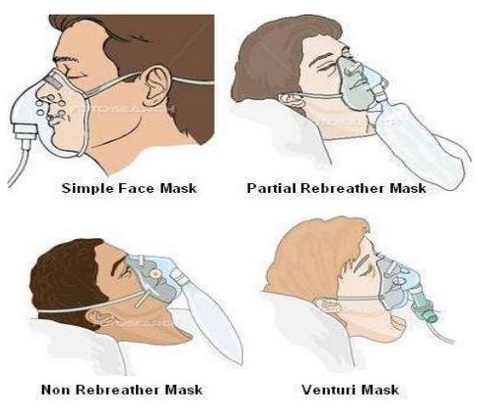
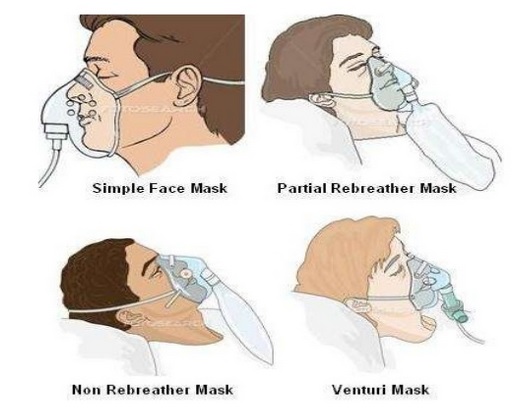
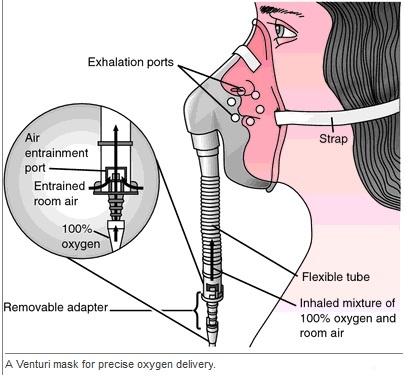

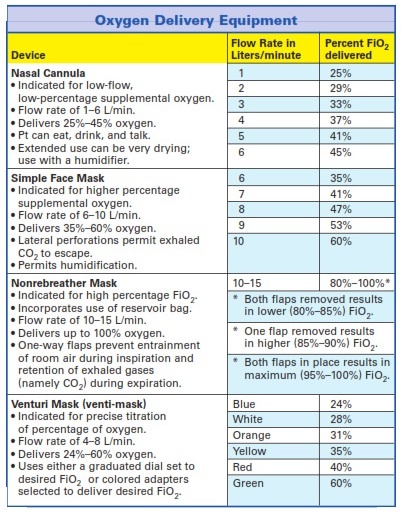

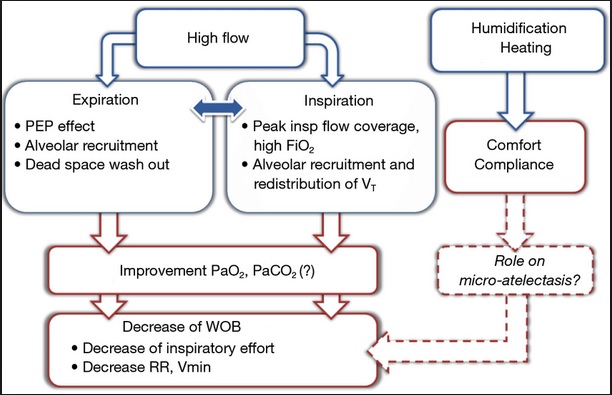
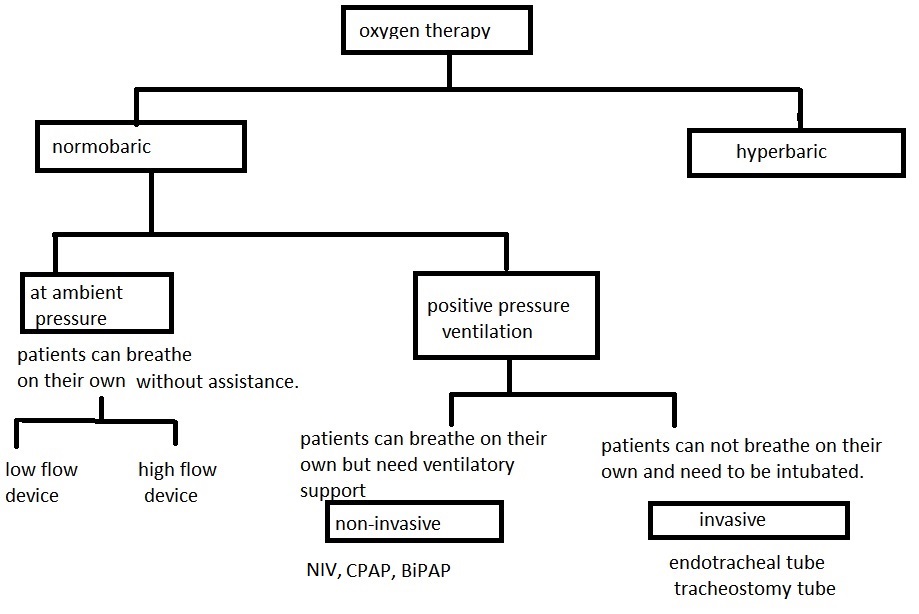
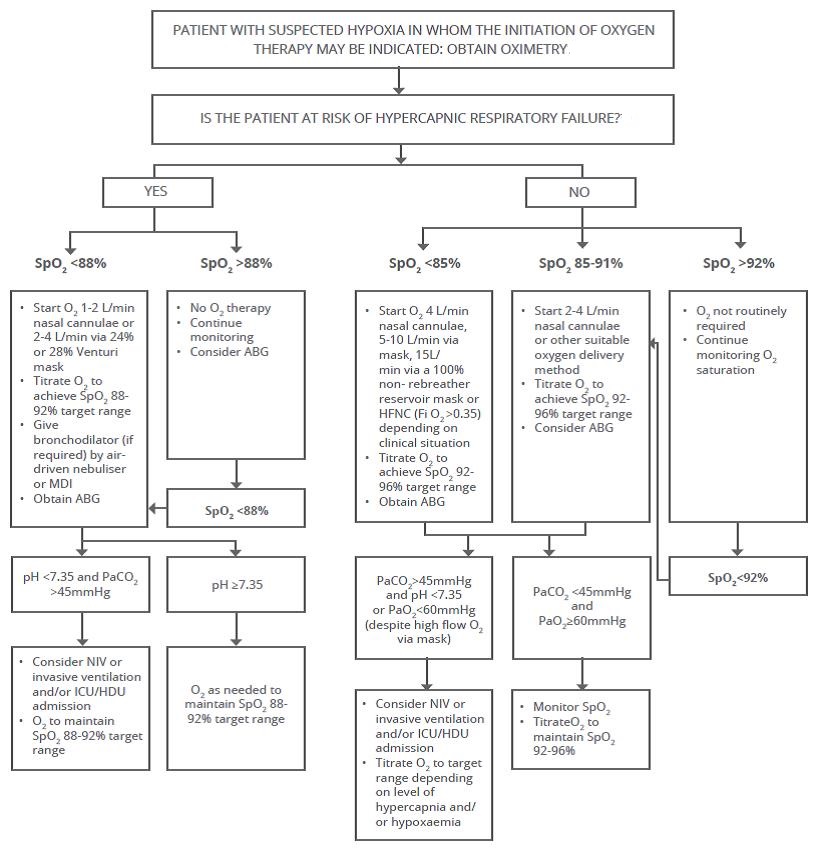
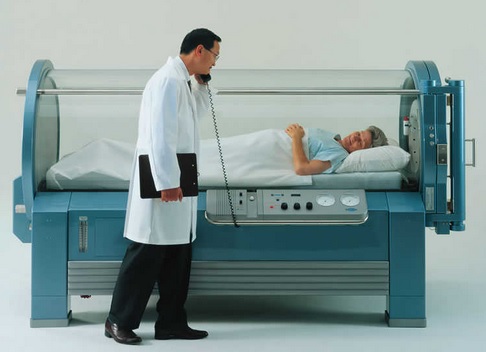
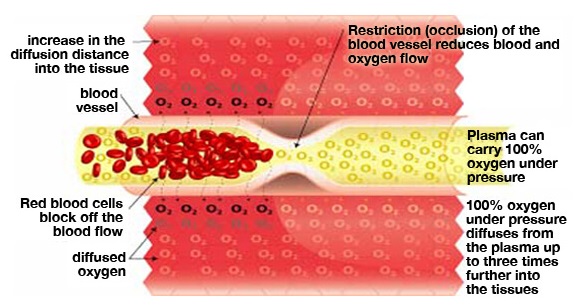
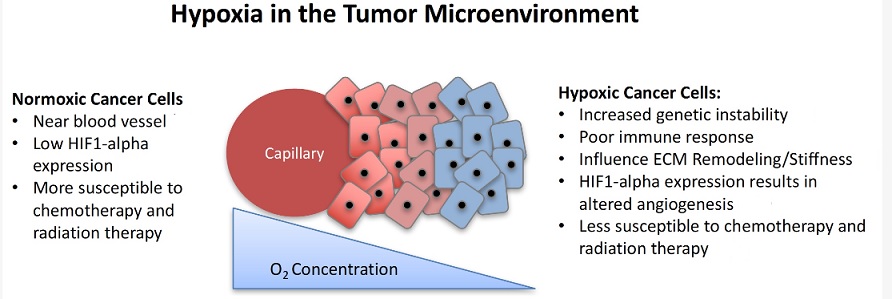
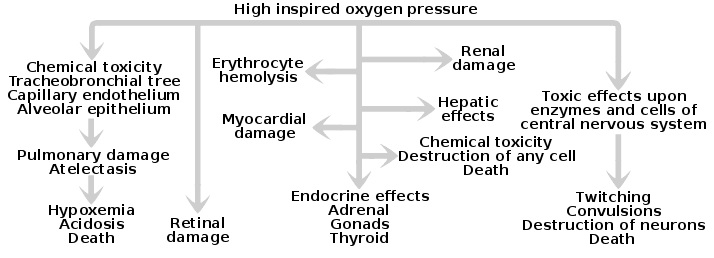
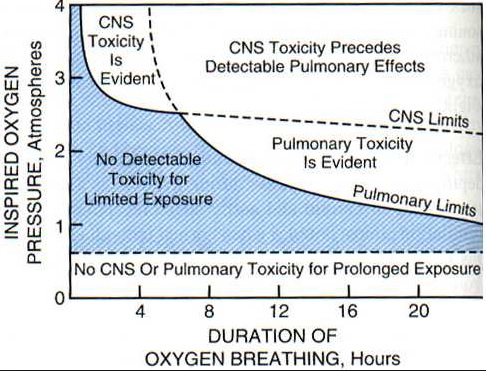
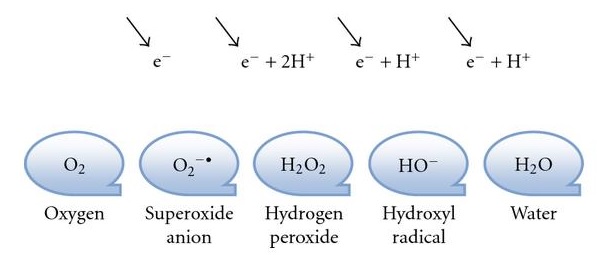
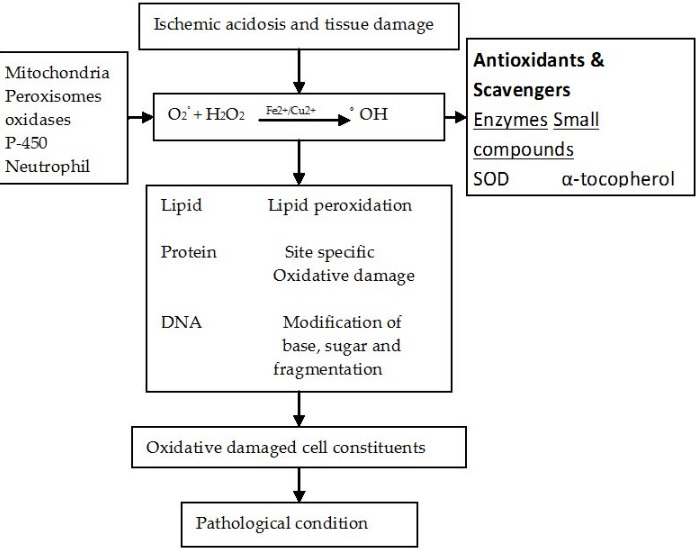
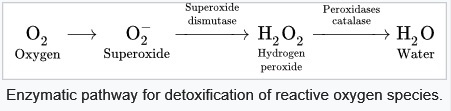
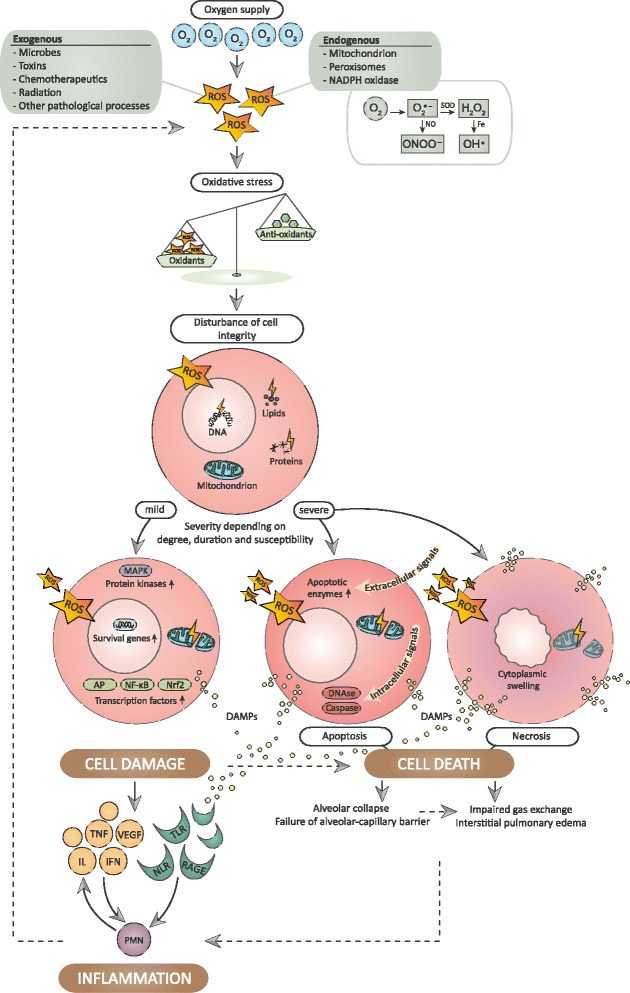
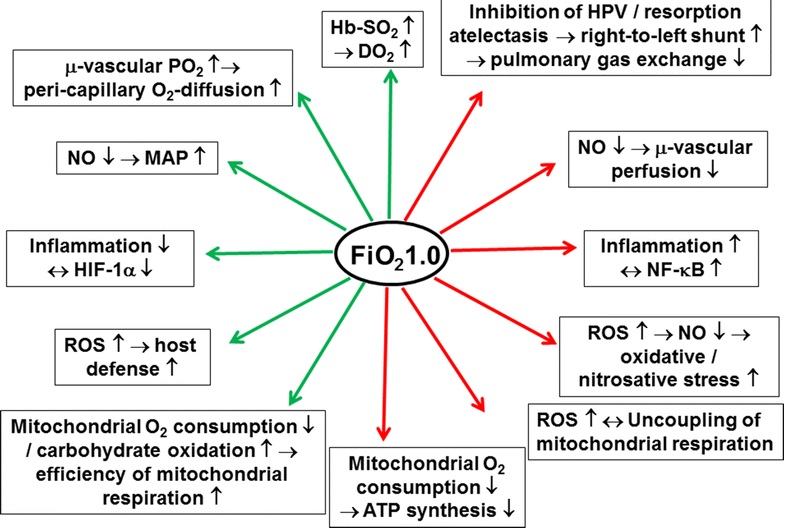
This is a topic that’s close to my heart… Cheers! Where can I find the contact details for questions?
Spot on with this write-up, I actually believe that this web site needs far more attention. I’ll probably be back again to read more, thanks for the advice!
There is certainly a great deal to know about this topic. I really like all the points you’ve made.
Everything is very open with a clear clarification of the issues. It was truly informative. Your website is useful. Thanks for sharing!
There is definately a great deal to learn about this topic. I really like all of the points you have made.
Great article! We will be linking to this great content on our site. Keep up the good writing.
Very good post! We will be linking to this great content on our site. Keep up the good writing.
Your style is so unique compared to other folks I have read stuff from. Thanks for posting when you have the opportunity, Guess I’ll just bookmark this blog.
Excellent post. I absolutely love this website. Stick with it!
Iím impressed, I have to admit. Seldom do I come across a blog thatís both educative and engaging, and let me tell you, you have hit the nail on the head. The problem is an issue that not enough folks are speaking intelligently about. Now i’m very happy that I found this in my search for something regarding this.
I really love your site.. Great colors & theme. Did you make this site yourself? Please reply back as Iím hoping to create my own personal website and want to learn where you got this from or just what the theme is named. Thanks!
This is a topic that’s close to my heart… Take care! Exactly where are your contact details though?
You need to take part in a contest for one of the best websites online. I’m going to recommend this website!
This is a very good tip especially to those new to the blogosphere. Brief but very precise informationÖ Thank you for sharing this one. A must read post!
This is a topic that is close to my heart… Cheers! Exactly where are your contact details though?
Iím amazed, I must say. Seldom do I come across a blog thatís both educative and interesting, and let me tell you, you have hit the nail on the head. The problem is something that not enough folks are speaking intelligently about. Now i’m very happy that I found this during my search for something regarding this.
Good blog you’ve got here.. Itís hard to find high-quality writing like yours these days. I truly appreciate people like you! Take care!!
It’s a jealous article. It’s very awesome and fresh. Who happen to be you to write this kind of special article?
You have actually covered this subject professionally.
You designed such an interesting article to read, giving just about every subject enlightenment for us to achieve knowledge. Thanks for sharing this such details with us to see this specific.
Aw, this was an exceptionally good post. Spending some time and actual effort to create a top notch articleÖ but what can I sayÖ I procrastinate a whole lot and never seem to get nearly anything done.
Thanks for Usefull information
Whoa. Guru. You’re a good professional. Say thanks to you.
Hi, i could not resist commenting. Your blog post is very well written. I would suggest you to write more articles like this. if you are looking fot the best pulse oximeter for home use that is pretty cheap with all features check out my website.
world is changing
Simply desire to say your article is as surprising.
The clarity on your publish is simply nice and i can assume
you’re a professional in this subject. Fine with your permission let me
to snatch your feed to stay up to date with coming near near
post. Thank you a million and please carry on the rewarding
work.
There is definately a lot to learn about this issue.
I love all the points you’ve made.
Hi mates, how is the whole thing, and what you want to say on the
topic of this article, in my view its truly amazing in support of me.
Hi there! Quick question that’s completely off topic. Do you know how to make your
site mobile friendly? My website looks weird
when browsing from my iphone. I’m trying to find a theme or plugin that might be
able to fix this issue. If you have any suggestions, please share.
Thank you!
This post is worth everyone’s attention. How can I find out more?
It’s really very difficult in this active life to listen news on Television, so I simply use the web for that reason, and take the newest information.
Hi! I just want to give you a huge thumbs up for your excellent information you have got right here on this
post. I am returning to your blog for more soon.