Dr Rajiv Desai
An Educational Blog
Desalination
Desalination:
_
The history of water is equivalent to the history of the world and the history of water quality is equivalent to the history of life.
—Andreas N. Angelakis
_
Section-1
Prologue:
All early civilizations were built on the banks of rivers. Whether it was the Euphrates in the Fertile Crescent or the Tiber in Rome, rivers gave early settlements easy access to abundant streams of fresh water, essential not only for drinking but also for irrigating crops. The availability of water was one of the biggest constraints on the growth of settlement and population size. The layout of Ancient Egypt reflected this. It stretched out like a long snake, hugging the banks of the Nile River. The invention of aqueducts by the Romans first enabled water to be carried long distances, providing the crucial utility to remote stretches of its sprawling empire and allowing populations to settle far from fixed sources of water. The Roman method of diverting freshwater to new habitations is still the essential means modern cities use to provide water to their residents. Water is life, and without water, there would be no civilizations and a vacant Earth. Water is considered an abundant natural resource on the earth. Water covers about 70% of the planet, but only 2.5% of that water is fresh, and only about half of that fresh water is accessible. This small portion of the available water supplies the needs of humans and animals. However, freshwater that exists in underground, rivers, and lakes is insufficient to cover all the world’s water demands. Few, if any, countries have the luxury of unlimited water resources. For many countries, water resources are becoming increasingly limited in both quantity and quality. Human water consumption has increased beyond sustainable levels in many regions, resulting in depletion of natural water sources, and lower quality water in drinking water reservoirs, including groundwater systems. Thus, water saving, water reuse, rainwater harvesting, stormwater utilization, and desalination are critical for maintaining water supplies for the future of humanity.
_
I was brought up and educated in Mumbai. Mumbai water supply solely depends on rainfall. Every year we used to look up in the sky for adequate rainfall to prevent water shortage. We were at the mercy of nature. Nobody thought of desalination plant for Mumbai in those days although Mumbai is a coastal city. I was serving as medical specialist in the Red Sea coastal town of Umm Laaj in the northern Tabuk region of Saudi Arabia between 2001 to 2006. The entire town was supplied with desalinated seawater. I used desalinated water daily from 2001 to 2006. I was wondering why my hometown Mumbai has no desalination plant. The Brihanmumbai Municipal Corporation (BMC) supplies 3,900 million litres of water daily (MLD) to the city. However, the city’s reliance on its seven lakes for potable water causes anxiety every year as lake levels recede. So, to augment water supply, the BMC has initiated the ambitious project of a desalination plant. The plant will be of a capacity of 200 MLD that can be expanded to 400 MLD at a later stage and will be set up in Manori, Malad. It is expected to be completed in the next four years.
_
Oceans cover 70 per cent of the planet. They provide nourishment for over three billion people and absorb 30 per cent of carbon dioxide released into the atmosphere and 90 per cent of the heat from climate change. Increasingly, they are also providing freshwater for a burgeoning population. Did you know that several countries, such as the Maldives, Malta and the Bahamas, meet all their water needs through the desalination process—that is, they convert seawater to freshwater? Scientists have known that the Earth’s natural hydrologic cycle continuously desalinates water, using solar energy as the water evaporates from the oceans and lakes, leaving behind the salt and mineral content. The resulting freshwater vapors form clouds which produce rain and snow. This hydrological cycle continuously moves salt from land to the oceans. The rain that falls on the land contains some dissolved carbon dioxide from the surrounding air. This causes the rainwater to be slightly acidic due to carbonic acid. The rain physically erodes the rock and the acids chemically break down the rocks and carries salts and minerals along in a dissolved state as ions. The ions in the runoff are carried to the streams and rivers and then to the ocean. Oceans are salty because when water evaporates, the salts remain and accumulate.
_
Desalination is simply the process of removing salt from water. Or, sometimes, removing the water from the salt. While this may sound simple, it is a problem that scientists have been trying to efficiently solve for decades. Considering that 25% of the world’s population does not have access to properly sanitized water, there is a major incentive to develop cheap, sustainable desalination technologies. Moreover, 40% of the world’s population lives within 60 miles of the coast. This means that there is a ready supply of water for much of the human population if only the salt can be removed. Presently about 500 million people depend on desalination (desal) as their primary source of water every day. Large number of countries around the world, and especially in the MENA (the Middle East and North Africa) region, suffer from extreme levels of water scarcity throughout the year. MENA region embraces around 47.5 % of the world’s desalination capacity.
_
In its most recent annual risk-assessment report, the World Economic Forum lists water crisis as the most significant potential global risk driven by water stress, increasing world population, improving living standards, changing consumption patterns, and expansion of irrigated agriculture because of rainfall shortage and increasing food consumption. Currently, 4 billion people are under water stress at least one month per year, and 33 countries are expected to experience extremely high water stress by 2040. Water is critical for sustainable development, environmental integrity and the eradication of poverty and hunger. Inaccessibility of clean water sources negatively impacts health, ability to work and the economy. Water facilities must be available for use at a price that is affordable to economically challenged people. Many regions of the world are facing formidable freshwater scarcity. Although there is substantial scope for economizing on the consumption of water without affecting its service level, the main response to water scarcity has been to increase the supply. To a large extent, this is done by transporting water from places where it is abundant to places where it is scarce. At a smaller scale and without a lot of public and political attention, people have started to tap into the sheer limitless resource of desalinated water. As demand for water is going to rise exponentially and water supply will continue to be scarce and even more erratic, desalination of sea water appears to be only mode for ensuring water security of the nation.
_
Most of the desalination plants in the Middle East were based on the evaporation and condensation technology. But in addition to evaporation, Americans were working on another type of technology that would turn out to have the most promise for the future: membranes. Essentially, the water was pushed through a series of barriers with openings of a size and shape that only water molecules could pass through, leaving behind most salt and other impurities. By the 1980s membranes had become the standard technology used in many of the world’s desalination plants. Today further advancements in nanotechnology are making the membranes smaller and more effective at filtering out unwanted particles, and distillation plants are now in the minority. Israel, Saudi Arabia, the United Arab Emirates, and Australia now obtain a significant percentage of their water from ocean-fed plants that push water through membranes. Kuwait and Qatar are entirely dependent on desalinated water for both domestic and industrial uses and spend billions of dollars each year to keep it flowing.
_
Currently, desalinated water only provides about 1% of the world’s drinking water, but the International Water Association expects production capacity to double by 2030. It may need to grow even faster to keep up with climate change. A paper published by the American Meteorological Society predicted that the total percentage of Earth’s land in extreme drought at any given time will have grown from 1% in 2006 to 30% by the end of the 21st century. As climate change redistributes water around the planet, causing wet places to become wetter and dry places to become drier, more and more people are going to be dependent on alternate water sources. Even the United States, where droughts have again increased during the past two decades, has begun reinvesting in desalination. A number of membrane plants have come online in Texas and California since 2005, and others are planned for Los Angeles and Corpus Christi.
_
Beyond the energy consumption and expense, desalination has another major downside: it produces extra-salty brine that sinks to the sea floor where it kills marine life. Additionally, such plants would have enormous carbon footprints. Critics of desalination argue that it’s wasteful to spend money and energy on desalinating seawater (even if the plants are powered by solar panels) when humans dump billions of gallons of wastewater into lakes and rivers every day. Why not recycle the leftover water from showers and toilets and send it back into the system instead of wasting energy desalting ocean water? Part of the reason some governments have been resistant to recycling water is because the concept sounds a little gross; detractors call it “toilet to tap.” On the other hand, around the world there are at least 1.2 billion people living in areas that don’t have water to recycle to begin with. For those people the only options appear to be desalination. With climate change causing more frequent droughts, desalination offers a potential solution to sustain growing populations in the most arid regions of the planet. Given that the raw water source in desalination is virtually inexhaustible, it offers a drought-free solution for municipalities and industries, ensuring water security. One more factor that works out in favour of the proposal is that the cost of desalinated water has been decreasing over the years, thanks to technological advancements. My endeavour is to study whether desalination is sustainable and affordable alternative water source or not.
______
______
Abbreviations and synonyms:
Desalination = Desal = Desalinization = Desalinisation
CAPEX = Capital Expenditures
CSP = Concentrated Solar Power
GCC = Gulf Cooperation Council
GHG = Greenhouse Gas
MD = Membrane Desalination
MED = Multi-Effect Distillation = Multi-Effect Evaporation = MEE
MENA = Middle East and North Africa
MSF = Multi-Stage Flash
MVC = Mechanical Vapour Compression
OPEX = Operating Expenditures
PV = Photovoltaic
SW = seawater
BW = blackish water
WW = waste water
RW = river water
RO = Reverse Osmosis
TDS = Total Dissolved Salts
VCD = Vapour Compression Distillation
VC = vapor-compression
ED = electro-dialysis
NF = nanofiltration
MF = microfiltration
UF = ultrafiltration
FO = forward osmosis
EDR = electro-dialysis reversal
TVC = thermal vapor-compression
π = osmotic pressure
c = molar solute concentration
R = gas constant
T = absolute temperature
VMD = vacuum MD
SWRO = seawater reverse osmosis
SEC = specific energy consumption
GO = graphene oxide
GOR = gained output ratio
RR = recovery ratio
ERD = energy recovery devices
BWRO = brackish water reverse osmosis
CDI = capacitive deionization
CNT = carbon nanotube
ZLD = zero liquid discharge
TBT = top brine temperature
LCA = life-cycle assessment
HPRO = high-pressure reverse osmosis
MLD = million liters per day
MGD = million gallons per day
MCM = million cubic meters
One cubic meter (m3) = 1000 liters ≈ 264 gallons
One acre foot = 1233.48 cubic meters (m3)
______
______
Section-2
Water characteristics and standards:
By definition, drinking water should have a salt content of no more than 0.01 per cent. The term ‘fresh water’ is used for a salt content of up to 0.05 per cent, while most crops can tolerate a salt content of up to 0.2 per cent. Water covers about 71% of the Earth’s surface. Over 96.5% of the Earth’s water supply remains in seas and oceans but, because of its saltiness, is not suitable for drinking. Just 2.5% of all the water on the planet is fresh, which means it could be directly used by humans and animals. Out of total fresh water, 70 per cent isn’t accessible – trapped, for instance, in polar ice. What remains is less than one per cent. Half of the world’s groundwater is either too contaminated with other substances or too saline to be suitable for drinking without treatment. This so-called brackish water has a salt content of between 0.05 and three per cent. For all of these different types of water, the same basic formula applies: the higher the percentage of salt, the more difficult, damaging and energy consuming becomes the process of desalination – at least for those technologies currently in use in significant enough numbers.
_
Salinity:
Salinity is the saltiness or amount of salt dissolved in a body of water. Salinity is expressed in the unit g/kg, which is often written as ppt (part per thousand) or ‰. Note that % means grams per deciliter and ‰ means grams per kilogram.
|
Fresh water |
Brackish water |
Saline water |
Brine |
|
< 0.05% |
0.05 – 3% |
3 – 5% |
> 5% |
|
< 0.5 ‰ |
0.5 – 30 ‰ |
30 – 50 ‰ |
> 50 ‰ |
Sodium is a mineral and it is one of the chemical elements that are found in salt. Our bodies need a certain amount of sodium to function. However, too much sodium can be harmful. People cannot drink saline water, but saline water can be turned into freshwater in a process called desalination.
_
Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sodium bicarbonate which dissolve into ions. The concentration of dissolved chloride ions is sometimes referred to as chlorinity. Operationally, dissolved matter is defined as that which can pass through a very fine filter (historically a filter with a pore size of 0.45 μm, but nowadays usually 0.2 μm). Salinity can be expressed in the form of a mass fraction, i.e. the mass of the dissolved material in a unit mass of solution.
Seawater typically has a mass salinity of around 35 g/kg, although lower values are typical near coasts where rivers enter the ocean. Rivers and lakes can have a wide range of salinities, from less than 0.01 g/kg to a few g/kg, although there are many places where higher salinities are found. The Dead Sea has a salinity of more than 200 g/kg. Precipitation typically has a TDS of 20 mg/kg or less.
_
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world’s oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately 35 grams (1.2 oz) of dissolved salts (predominantly sodium (Na+) and chloride (Cl−) ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at 4 °C (39 °F)) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about −2 °C (28 °F). The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was −2.6 °C (27.3 °F). Seawater pH is typically limited to a range between 7.5 and 8.4. Most natural freshwaters have pH values in the range from 6.5 to 8.0. Most waters have some capacity to resist pH change through the effects of the carbonate-buffer system.
_
TDS vs salinity:
The key difference between TDS and salinity is that TDS is the measurement of all types of solid compounds in a given liquid sample whereas salinity is the measurement of the amount of salt that is dissolved in a given liquid sample. Often, people use the terms TDS and salinity interchangeably though they are two different terms. The term TDS stands for total dissolved solids while salinity refers to the dissolved salt amount in water.
______
______
TDS:
TDS is total dissolved solids. It is a measure of the dissolved combined content of all inorganic and organic substances in a liquid. Common inorganic salts that can be found in water include calcium, magnesium, potassium and sodium, which are all cations, and carbonates, nitrates, bicarbonates, chlorides and sulfates, which are all anions. Cations are positively charged ions and anions are negatively charged ions. The unit of measurement of this TDS parameter is usually “part per million (ppm)”. For water, 1 ppm = approximately 1 mg/L of contaminant in water. We can easily determine the TDS level of water using a digital meter. The most important application of TDS parameter is the study of water quality for streams, rivers, and lakes. There are several different primary sources of TDS including,
-1. Agricultural runoff
-2. Residential runoff
-3. Clay-rich mountain waters
-4. Leaching of soil contamination
-5. Point source water pollution from industrial sites
-6. Sewage treatment plants
The form of dissolved chemical substances in liquids with a high TDS level can be cations, anions, molecules or agglomerates. The toxic chemical components that can cause harmful health effects due to high TDS levels in water are pesticides that arise from surface runoff. Some naturally occurring total dissolved solids come from the weathering and dissolution of rocks and soils.
_
Why should you measure total dissolved solids?
Total dissolved solids can affect your water quality, your health, your home plumbing system, and even daily tasks, such as cooking and cleaning. By measuring your water for TDS, you can better understand your water quality and how it affects your everyday life, allowing you to make an informed decision to solve your water quality problem and install the most effective filtration system for your home.
-1. Taste and smell
Tap water with a high concentration of total dissolved solids (TDS) can have a bitter taste and unpleasant smell. The higher the concentration of total dissolved solids, the more bitter your water will be.
-2. Health
High TDS water is not necessarily unhealthy to drink, but certain substances, such as lead and copper, are health hazards. For example, lead exposure can cause brain and nervous system damage and high levels of copper exposure can cause nausea.
-3. Filter maintenance
Water filtration systems are a great solution to reduce total dissolved solids but are subject to normal wear and tear. Routine testing for TDS can provide assurance that your filter system is working properly and can alert you when maintenance is required.
-4. Plumbing and appliances
Water that contains high levels of dissolved calcium and magnesium is hard water and can result in high TDS levels. When calcium and magnesium salts dissolve, they collect in pipes and form scale buildup, which results in costly pipe replacements and shortens the lives of your appliances.
-5. Cooking
Though not detrimental to your health at levels below 1000 ppm, cooking with elevated TDS water can change the taste of food. For example, if your water has high levels of chlorine, you may find that your pasta absorbs an unpleasant taste from the boiling water.
-6. Cleaning
If your dishes have water spots no matter how well you clean them, your clothes fade in the wash, and you have buildup in your sinks, your cleaning woes may be caused by high levels of total dissolved solids.
_
Taste of Water with Different TDS:
In a study by the World Health Organization, a panel of tasters came to the following conclusions about the preferable level of TDS in water:
TDS Level in parts per million(ppm) Palatability Quotient:
|
TDS in Water (measured in PPM) |
Suitability for Drinking Water |
|
Between 50-150 |
Excellent for drinking |
|
150-250 |
Good |
|
250-300 |
Fair |
|
300-500 |
Poor, not good for drinking |
|
Above 1200 |
Unacceptable |
A very low concentration of TDS has been found to give water a flat taste, which is undesirable to many people. Generally, the TDS level between 50-150 is considered as the most suitable and acceptable. If the TDS level is about 1000 PPM, it is unsafe and unfit for human consumption.
_
According to the Bureau of Indian Standards (BIS), the upper limit of TDS levels in water is 500 ppm. The TDS level recommended by WHO, however, is 300 ppm. The World Health Organization (WHO) sets out guidelines for drinking water quality that include the recommendation that water with TDS below 300 PPM is considered safe for drinking. However, most authorities accept 500 PPM. In the United States, the average is 350 PPM of TDS in standard drinking water from the tap. It’s important to note that with measurements being predicted in parts per million, it’s going to take some sophisticated equipment to catch every substance. Usually, total dissolved solids are tested using a TDS meter which uses electricity to determine the conductivity of the water and uses that data to determine TDS level results.
_
Three of the most common ways to reduce TDS in water are:
-1. Reverse Osmosis (RO): TDS is lowered through Reverse Osmosis by pushing the water using pressure through an artificial membrane. The membrane has tiny holes that allow only particles smaller than 0.0001 microns to go through. Since the particles of dissolved metals and salts are bigger than water molecules, only water goes through the membrane, leaving the metals and salts behind.
-2. Distillation: This method involves boiling water to create water vapor. The vapor goes up to a cool surface and changes back into liquid. The dissolved salts can’t turn into vapor and stay in the boiling water.
-3. Deionization (DI): Deionization is a chemical process for removing minerals from water by extracting ions, or electrically charged particles, from the water supply.
All three methods are advanced and unless you have an RO water filter at home, there is not much you can do to reduce the TDS of the water. It is recommended that you at least boil the water properly before consuming it, as it kills most of the bacteria and viruses present in the water. Boiling water removes hard water causing bicarbonates of magnesium and calcium, as well as nasty contaminants in the main supply, such as chlorine and lead. Once boiled and cooled, you can pour the remaining water into another container, ensuring it retains its purity and softness.
Does boiling salt water get rid of the salt?
No. Boiling it will make it saltier as the water will start to evaporate leaving the salt behind in less water. You can boil fresh water for drinking purpose and not salty water.
_
TDS versus hardness:
Hardness measures the presence of calcium and magnesium salts in forms like bicarbonates, chlorides, and sulfates, while TDS includes all minerals, not just calcium and magnesium. When measuring water treated with water softeners, high levels of total dissolved solids do not correlate to hard water, as water softeners do not reduce TDS; rather, they replace magnesium and calcium ions, which cause hard water, with an equal charge of sodium or potassium ions, e.g. Ca2+ ⇌ 2 Na+, leaving overall TDS unchanged or even increased.
Hard water leaves deposits and films on fixtures, and on the insides of hot water pipes and boilers. Soaps and detergents do not produce as much lather with hard water as with soft water. Hard water can cause scale buildup in pipes, valves, and filters, reducing performance and adding to system maintenance costs. These effects can be seen in aquariums, spas, swimming pools, and reverse osmosis water treatment systems.
_
Desalination and TDS:
Desalination is a general term for the process of removing salt from water to produce fresh water. Fresh water is defined as containing less than 1000 mg/L of salts or total dissolved solids (TDS) (Sandia, 2003). Above 1000 mg/L, properties such as taste, color, corrosion propensity, and odor can be adversely affected. Many countries have adopted national drinking water standards for specific contaminants, as well as for TDS, but the standard limits vary from country to country or from region to region within the same country. Most desalination facilities are designed to achieve a TDS of 500 mg/L or less. Desalinated water used for other purposes, such as crop irrigation, may have a higher TDS concentration; irrigation water standards often include concentration limits for TDS, chloride, sodium, and boron. Depending on the type of crop, the chloride standard can range from 350 mg/L to more than 2000 mg/L.
The feed water salinity for desalination facilities ranges from approximately 1000 mg/L TDS to 60,000 mg/L TDS, although feed waters are typically labeled as one of two types: seawater or brackish water. Although most seawater sources contain 30,000–45,000 mg/L TDS, seawater reverse osmosis membranes are used to treat waters within the TDS range 10,000 – 60,000 mg/L. Brackish water reverse osmosis membranes are used to treat water sources (often groundwater sources) within a range of 1000–10,000 mg/L TDS. The feed water type can dictate several design choices for a treatment plant, including desalination method, pretreatment steps, waste disposal method, and product recovery (the fraction of influent water that becomes product).
_____
_____
Causes of saltiness of water:
-1. From precipitation to the land to the rivers to the sea….
The rain that falls on the land contains some dissolved carbon dioxide from the surrounding air. This causes the rainwater to be slightly acidic due to carbonic acid. The rain physically erodes the rock and the acids chemically break down the rocks and carries salts and minerals along in a dissolved state as ions. The source of the salts in water is the surrounding rocks, e.g. the mineralogy of the aquifer. Therefore, surface water is usually less salty than groundwater, which is surrounded by rocks. The ions in the runoff are carried to the streams and rivers and then to the ocean. Many of the dissolved ions are used by organisms in the ocean and are removed from the water. Others are not used up and are left for long periods of time where their concentrations increase over time.
-2. The second source is actually from the ocean itself. Gashes in the seafloor, called hydrothermal vents, produce large amounts of steam and heat. This causes a variety of chemical reactions. Among these reactions is the release of metal ions into the water, like sodium, chloride, and magnesium.
-3. Oceans are salty because when water evaporates, the salts remain and accumulate.
_
The two ions that are present most often in seawater are chloride and sodium. These two make up over 90% of all dissolved ions in seawater. The concentration of salt in seawater (its salinity) is about 35 parts per thousand; in other words, about 3.5% of the weight of seawater comes from the dissolved salts. In a cubic mile of seawater, the weight of the salt (as sodium chloride) would be about 120 million tons. A cubic mile of seawater can also contain up to 25 pounds of gold and up to 45 pounds of silver! But before you go out and try alchemy on seawater, just think about how big a cubic mile is: 1 cubic mile contains 1,101,117,147,000 gallons of water!
_
Near the equator, the tropics receive the most rain on a consistent basis. As a result, the fresh rain water falling into the ocean decreases the salinity of the surface water in that region. Rain decreases further from the equator, and with less rain and more sunshine, evaporation increases. Evaporation of water vapor from the ocean to the atmosphere leaves behind the salt, resulting in higher salinity. Toward the poles, fresh water from melting ice decreases the surface salinity once again. The saltiest locations in the ocean are the regions where evaporation is highest or in large bodies of water where there is no outlet into the ocean. The saltiest ocean water is in the Red Sea and in the Persian Gulf region (around 40ppt) due to very high evaporation and little fresh water inflow.
_____
Sea water intrusion:
Seawater intrusion is among the primary groundwater contamination processes in coastal areas, where saline water is moving moves towards or mixing with freshwater aquifers. When the extreme freshwater abstraction from a coastal aquifer surpasses the natural recharge of freshwater from surface water, salty water is drawn into the aquifer. Thus, seawater intrusion is a major issue in many continents, such as European, north, and south Mediterranean coastal areas. Seawater intrusion has also become an essential issue in North Africa and Middle East, the USA, and Turkey. Several research studies on seawater intrusion in coastal areas have been conducted. These studies showed that the mismanagement of the coastal aquifers resulted in an uncontrolled saltwater intrusion. All these results indicate that many coastal areas have been affected by seawater intrusion processes.
_____
_____
If saltwater is still water, why can’t we drink it?
Accidentally consuming small quantities of clean seawater is not harmful, especially if the seawater is taken along with a larger quantity of fresh water. However, drinking seawater to maintain hydration is counterproductive; more water must be excreted to eliminate the salt (via urine) than the amount of water obtained from the seawater itself. In normal circumstances, it would be considered ill-advised to consume large amounts of unfiltered seawater.
Seawater contains salt. When humans drink seawater, their cells are thus taking in water and salt. While humans can safely ingest small amounts of salt, the salt content in seawater is much higher than what can be processed by the human body. Additionally, when we consume salt as part of our daily diets, we also drink liquids, which help to dilute the salt and keep it at a healthy level. Living cells do depend on sodium chloride (salt) to maintain the body’s chemical balances and reactions; however, too much sodium can be deadly.
Human kidneys can only make urine that is less salty than salt water. Therefore, to get rid of all the excess salt taken in by drinking seawater, you have to urinate more water than you drank. Eventually, you die of dehydration even as you become thirstier.
Survival manuals consistently advise against drinking seawater. A summary of 163 life raft voyages estimated the risk of death at 39% for those who drank seawater, compared to 3% for those who did not. The effect of seawater intake on rats confirmed the negative effects of drinking seawater when dehydrated.
_
Some animals can drink saltwater, so why can’t we?
Some animals, in ocean ecosystems have adaptations that allow them to safely drink saltwater. Seabirds such as albatrosses, gulls and penguins, which might spend weeks on the open ocean with no freshwater in sight, have specialized salt glands and grooves in their bills for filtering and purging excess salt from ingested water before it hits their stomachs and is absorbed into their blood. Marine mammals such as whales, dolphins and seals have also evolved adaptations to life in an environment where freshwater is scarce or absent. Marine mammals have adapted special enzymes and cellular structures that allow them to purge excess salt from their systems. It’s like they have super kidneys.
_
Since humans and a fair portion of the planet’s plant and animal population can’t subsist on saltwater, people have long looked enviously at the sea to provide the water they require, whether it’s for drinking, hygiene, agriculture or more recently, industrial purposes. Historically, desalination was deemed too expensive to be considered a viable large-scale option; it simply required too much energy. But newer technologies, such as reverse osmosis and multistage flash distillation, have, since the 1950s, slowly started to change that opinion, especially in places where sources of freshwater are scarce, and people are plentiful.
______
______
Difference between desalination and demineralization:
Desalination and demineralization are both processes used to purify water, but they differ in their source, purpose, process, technology, and applications. Desalination is used to remove salt and other minerals from seawater to make it drinkable, while demineralization is used to remove minerals and impurities from freshwater for various purposes. Desalination is used to purify seawater, while demineralization is used to purify freshwater. Desalination often uses reverse osmosis or distillation, while demineralization often uses ion exchange or other chemical treatments.
The demineralisation process produces high-quality water – without removing the minerals, the water could interfere with and contaminate the various industrial processes that demineralised water is used for, including:
Laboratory testing
Analytical chemistry
Cleaning lab equipment
Coolant systems
______
Differences between desalination and water treatment systems:
-1. Different purposes:
The purpose of a seawater desalination system is to convert saltwater (mainly seawater) into fresh water. This is a widely used technology in areas with relatively scarce freshwater resources, especially near the ocean. The main principle is to remove salt from seawater through methods such as reverse osmosis and distillation, and produce fresh water suitable for human consumption and industrial use.
The purpose of a water treatment system is to improve the quality of existing water sources. This may include removing pollutants, heavy metals, bacteria, etc. from natural water sources to ensure that the water meets specific water quality standards. Water treatment systems are mainly used in water treatment plants, industrial wastewater treatment plants, etc., to provide clean water sources for various purposes.
-2. Source of raw water:
The raw water of seawater desalination systems mainly comes from high salinity water bodies such as oceans and saltwater lakes. These water sources themselves cannot be directly used by humans and require desalination treatment.
The raw water of the water treatment system can include tap water, river water, lake water, etc., with relatively low salinity. The water treatment system mainly deals with various pollutants and impurities in natural water sources.
-3. Process principle:
The core processes of seawater desalination systems include technologies such as reverse osmosis and multi-stage distillation. Reverse osmosis removes salt through a semi-permeable membrane, while distillation evaporates and re-condenses water through heating and cooling, achieving salt separation.
The process of water treatment system involves various technologies, such as filtration, sedimentation, disinfection, etc., to remove pollutants from water. These processes vary depending on the water source and are typically purified through various methods such as physical, chemical, and biological methods.
______
Differences between RO water and distilled water:
- Reverse osmosis water and distilled water are very similar because they’re both pure water sources.
- The main difference between reverse osmosis water and distilled water is the method of purification: RO water is produced by membrane separation, while distilled water is made by evaporation and condensation.
- RO water may contain some volatile organic compounds (VOCs) and chemicals, and distilled water may contain some chemicals and heavy metals.
_______
_______
Section-3
Water scarcity:
_
World water:
About 71 percent of the Earth’s surface is water-covered, and the oceans hold about 96.5 percent of all Earth’s water. Water also exists in the air as water vapor, in rivers and lakes, in icecaps and glaciers, in the ground as soil moisture and in aquifers, and even in humans and animals. Figure below shows how almost all of Earth’s water is saline and is found in the oceans. Of the small amount that is actually freshwater, only a relatively small portion is available to sustain human, plant, and animal life.
_
Major Stocks of Water on Earth
|
Location |
Amount (106 km3) |
Percentage of World Water |
|
Ocean |
1338.0 |
96.5 |
|
Glaciers and permanent snow |
24.1 |
1.74 |
|
Groundwater (brackish or saline) |
12.9 |
0.94 |
|
Groundwater (fresh) |
10.5 |
0.76 |
|
Ground ice/permafrost |
0.30 |
0.022 |
|
Freshwater lakes |
0.091 |
0.007 |
|
Freshwater stream channels |
0.002 |
0.0002 |
The average annual rainfall over land amounts to 119000 km3, of which some 74000 km3 evaporate back into the atmosphere. The remaining 45000 km3 flow into lakes, reservoirs and streams or infiltrate into the ground to replenish the aquifers. This represents what is conventionally called “water resources”. Not all of these 45000 km3 are accessible for use because part of the water flows into remote rivers and during seasonal floods. An estimated 9000 – 14000 km3 are all that are economically available for human use, a teaspoon in a full bathtub compared to the total amount of water on earth.
_
As noted in Table above, nearly 1 percent of the world’s water exists as brackish or saline groundwater. In most inland cases, groundwater salinity results from the dissolution of minerals present in the subsurface, possibly concentrated further by evapotranspiration. Coastal aquifers form another class of brackish water, which is created from the natural mixing of seawater with groundwater that is discharging to the ocean. The thickness of this brackish mixing zone is sometimes increased by coastal groundwater pumping. Brackish groundwater exists at elevations less than 305 m (1,000 feet) across much of the conterminous United States (Feth, 1965) and almost certainly at comparable depths in Hawaii and Alaska. Both coastal and inland communities are increasingly considering brackish groundwater as a possible water supply resource.
_
The distribution of freshwater around the world is not uniform. In this case, unbalanced distribution caused that some parts of the groundwater resources have become greatly available to several specific areas with low population and convenient access to freshwater such as the northern parts of Russia, Scandinavia, Canada, Alaska and southern parts of South America. Additionally, areas with a high population or areas with industrial growth are more vulnerable to water stress and areas that are in arid regions also have a degree of water stress based on the ratio of water consumption to the amount of available water. Obviously considering the significance of upstream water use on downstream stress has a direct effect on water distribution (Munia et al. 2016). The index of water stress is essentially linked to per capita water use.
______
______
Daily water consumption per capita:
Demands for freshwater in developed countries are increasing. For example, the daily consumption per person in the USA is 400 L. Some developed countries with the help of restrictions and regulations have reduced the consumption of water up to 150 L. However, the studies indicated that with the difficulty of access to freshwater resources in some areas around the world, a low amount of water would be consumed. For example, the consumption per person in Africa is 20 L per day. The World Health Organization (WHO) considers that consuming 15–20 L per day is necessary for human survival. On the other hand, for some uses, such as hospitals and schools, consumption of 50 L per person per day is essential.
_
The average water consumption per capita in Saudi Arabia is 270 l/day for municipal purposes according to the Water Statistics Report in GCC Countries of 2018. It means that for 1 million citizens we need to build water desalination plant of capacity 270000 m3/day. We considered only water demand for municipal purposes of one million people, but what if we include also the industrial and agricultural water demand. Obviously, the water consumption would be much more than in a previous case. It comes to 2460 l/day. Knowing the total amount of water that is required for one person per capita for agricultural, industrial and municipal purposes, we could calculate the capacity of desalination plant.
_____
_____
Water scarcity in the world:
Water is very essential for life. It is one of the most abundant resources of the earth, covering about 3/4th of earth’s surface. Though it covers earth’s major portion yet there is severe shortage of potable water in many countries around the world mainly in developing countries and middle east region countries. The reason for this situation is that nearly 97.5% of earth’s water is salt water and remaining 2.5% is fresh water which is in the form of ground water, ice-mountains, lakes and rivers, which serves most human and animal needs. For every 20 years, the consumption rate of water is doubling exceeding by two times the rate of population growth. The potable water resources are on the decline and water demand is high. In recent times various industrial and developmental activities have resulted in increasing pollution and deteriorating the quality of water. Thus, water shortages and unreliable quality of water are considered to be major hindrances for sustainable development of society.
_
Water is a vital source of life on earth, particularly for Human Life. Around 785 million people have no access to clean water, and 144 million people fulfil their needs from surface water. Water vulnerability is affecting 1.42 billion people in the world. Only 2.5% of water is fresh in the World, and this water is becoming scarce due to mismanagement, misuse, contamination, and over-extraction. Urbanization, population growth, and industrialization have increased the water demands in the world. Climate change also contributes to water scarcity as extreme weather conditions like floods, droughts, change in precipitations, and sea-level rise contaminate freshwater resources, destroy water and sewage infrastructure, and reduce available water. Water-related disasters occurred about 74% from 2001 to 2018, destroying water and sanitation infrastructure. These disasters’ frequency will increase due to climate change. On the other hand, global water demand will increase about 20−30% by 2050 because of population growth, high living standards, and food and energy demand.
_
According to UNEP (United Nations Environment Programme) 1/3rd of the world’s population lives in countries with insufficient freshwater resources. Hence enormous efforts are required to make new water resources available and minimize water deficiency in countries with shortage of fresh water. World Health Organization guidelines state that the permissible limits of salinity in drinking water are 500 ppm and in few cases, it may extend up to 1000 ppm. Most of the water on earth has salinity ranging up to 10,000 ppm and for sea water it may be in the range of 35,000–45,000 ppm due to its dissolved salts.
_
The existing water resources are decreasing
- Due to unbalanced distribution of rain water and drought
- Extreme exploitation of ground water resources and its un sufficient recharge
- Degradation of water quality due to the discharge of domestic and industrial wastes without sufficient treatment
_
Figure below shows the global distributions of rainfall across the globe highlighting the regions that have water access and the regions that lack it.
Since the fresh water resources are very limited to serve the major population needs and salt water is unsuitable for many applications, desalination of salt water (sea water) emerges as a boon to most of the population to serve their needs.
_
Water can be scarce for many reasons: demand for water may be exceeding supply, water infrastructure may be inadequate, or institutions may be failing to balance everyone’s needs. Water scarcity is an increasing problem on every continent, with poorer communities most badly affected. To build resilience against climate change and to serve a growing population, an integrated and inclusive approach must be taken to managing this finite resource. Population growth, increasing consumption per capita and climate changes are three main stressors in the water resources. The World Water Program (WWP) estimates that by 2030 only 60% of the water needed will be available, and the Organization for Economic Co-operation and Development (OECD) has predicted that by 2050 this amount will reach 55%. By the end of the century, 40% of the world’s population will live in areas with water stress (Caldera et al. 2016). Overall, water demands will be doubled every 20 years (Eltawil et al. 2009; Kalogirou 2005). About 70% of the freshwater needed is for the agriculture section, 20% for the industry sector and only 10% for houses uses (El-Dessouky and Ettouney 2002). High rates of population growth and climate change have highlighted the need for new freshwater resources. Shortages of water resources are leading to a decline in the standard of living and economic growth.
_
The term ‘water shortage’ is defined by the UN as a situation in which there isn’t sufficient access to drinking water to fulfil human needs. The World Health Organisation (WHO) states that in times of crisis, every person needs guaranteed access to at least 15 litres of water per day – preferably all drinking water; at least fresh water. According to the World Water Institute, almost two billion people in 17 countries are heading straight for an acute water crisis in the coming years. This will lead to between 24 and 700 million people being displaced by as early as 2040, the UN has warned. ‘Water is among the top five global risks in terms of impacts, reaching far beyond socio-economic and environmental challenges and impacting livelihoods and well-being of the people,’ says Manzoor Qadir, an environmental scientist who focuses on water recycling and safe reuse at the United Nations University in Hamilton, Canada. In a recent study, Qadir and his colleagues concluded that ‘statistics demonstrate that “conventional” sources of water such as rainfall, snow-melt and river runoff captured in lakes, rivers and aquifers are no longer sufficient to meet human demands in water-scarce areas’. Qadir is calling for more desalination plants. ‘Desalination can extend water supplies beyond what is available from the hydrological cycle, providing an “unlimited”, climate-independent and steady supply of potable water,’ he says.
_____
Facts and Figures of water scarcity:
- 2.3 billion people live in water-stressed countries, of which 733 million live in high and critically water-stressed countries. (UN-Water, 2021)
- 3.2 billion people live in agricultural areas with high to very high water shortages or scarcity, of whom 1.2 billion people – roughly one-sixth of the world’s population – live in severely water-constrained agricultural areas. (FAO, 2020)
- Today, 1.42 billion people – including 450 million children – live in areas of high or extremely high water vulnerability. (UNICEF, 2021)
- About 4 billion people, representing nearly two-thirds of the global population, experience severe water scarcity during at least one month of the year. (Mekonnen and Hoekstra, 2016)
- 72% of all water withdrawals are used by agriculture, 16% by municipalities for households and services, and 12% by industries. (UN-Water, 2021)
- When a territory withdraws 25% or more of its renewable freshwater resources it is said to be ‘water-stressed’. Five out of 11 regions have water stress values above 25%, including two regions with high water stress and one with extreme water stress. (UN-Water, 2021)
____
Forecast of water scarcity in 2025 is depicted in figure below:
The figure above shows how water related issue were in 1995 and the forecast of how worse could it be in 2025. According to this source, the world will face severe water shortages. The countries that have water withdrawal as a percentage of total available water from 10% to 20% would become even more water stressed reaching to numbers from 20% to 40%. The situation would be even more rough with the countries that already were water stressed. As climate change makes rainfall less predictable and droughts more common, a growing number of countries are turning to desalination. The term is used to refer to removing salt from both seawater and subterranean “brackish” water, as well as the treatment of waste water (aka sewerage) to make it drinkable.
_
Water scarcity is described as a condition where water demand exceeds over available water supply. A country or a region faces “water scarcity” when the availability of natural hygienic water falls below 1000 m3 per person per year (Pereira et al., 2002; Dehghani et al., 2019). Water scarcity is something that is not a concern with only a single living being at a particular location and time, but it affects a larger population within a certain geographic region (e.g., country) and pertains to larger timescales (years or months). It can occur because of natural low water availability as well as human-imposed activities to degrade the available natural water. The natural water body has the potential to renew or restore its originality. However, water pollution is the dominant factor to aggravates water scarcity by degrading the quality of water resources. The detrimental effect caused by nature and human intervention has led to severe water challenges and water scarcity, which is elaborated in the upcoming section.
_
Water scarcity arises in situations where there is insufficient water to simultaneously support both human and ecosystem water needs (White, 2014). Most often this arises as a result of a basic lack of water (i.e., physical water scarcity), but it may also result from a lack of suitable infrastructure to provide access to what might otherwise be considered ample available water resources, which is referred to as economic water scarcity. Physical water scarcity may occur as a result of both natural phenomena (e.g., aridity, drought) as well as from human influences (e.g., desertification, water storage; Pereira et al., 2009; White, 2014), although these influences are often coupled. For example, the process of desertification often commences as a result of water overuse during periods of temporary drought; droughts are more common in arid regions (McMahon et al., 1992). A key distinction between these various processes is in degree of permanency and reversibility. In the case of drought and water overuse, for example, the impacts may be temporary; however, those arising from aridity and desertification are more likely to be irreversible (Water, 2006). As Pereira et al. (2009) point out, this distinction is often confused when discussing water scarcity and its impacts, but it may be important in understanding both impacts and mitigation options.
_
Map below shows the global distribution of regions affected by water scarcity.
_
Regardless of the cause, water scarcity impacts both human populations and natural ecosystems on all continents (Figure above). For example, recent estimates suggest approximately 4 billion people live under conditions of water scarcity for at least one month each year, with roughly 0.5 billion people exposed to severe water scarcity all year round (Mekonnen and Hoekstra, 2016). These figures nearly double previous estimates, in part by considering the flows required to remain in rivers to sustain flow-dependent ecosystems, as well as the goods and services they provide for people. In most, though not all regions, climate change is forecast to exacerbate water scarcity even further (Gosling and Arnell, 2016). These assessments highlight the massive global impacts of water scarcity on human livelihoods and on natural systems, and many global programs such as those of the United Nations focus on improved human access to water within a more sustainable ecosystem footprint.
_
Drought:
A drought is a period of dry weather that can extend for weeks, months, or years. It results in a shortage of water sources, including ground and rainwater. During a drought, plants begin to die from the lack of rain. If it lasts for several months, a drought can cause food shortages due to a decrease in crops. Droughts also negatively impact land and wildlife. At least 23 countries, including India, declared drought emergencies at a national or sub-national level during 2022-23, showed new data from a global drought map compiled by the United Nations, pointing to unprecedented urgency on a planetary scale. And the U.N. has warned that 130 more countries could face droughts by 2100 if we do nothing to curb climate change. But as soon as 2025, two-thirds of the global population could face water shortages, according to the World Wildlife Fund. This could result in conflicts, political instability, and the displacement of millions of people.
__
There are several different methods to measure water scarcity. One of these methods is the Falkenmark Indication, which looks at the number of people in an area and the volume of available water. According to this method, a volume of 1700m3/cap/year of available renewable freshwater per person means that the given area is experiencing water scarcity. A value of 1000m3/cap/year demonstrates high water scarcity, and 500m3/cap/year demonstrates absolute scarcity. The water use to availability ratio can also be used, where the available renewable water resources and amount of water used are looked at. The measure of water stress is the ratio of total water use (domestic, industrial or agricultural) to produced renewable water, including runoff in rivers and underground sources with little depth. With these different standards to determine water scarcity, there are also two different types of water scarcity, physical and economic. Physical water scarcity is associated with limited access to water resources, with the Middle East and North Africa (MENA) region, southeast Europe, Western Australia, and India being areas experiencing physical water scarcity. Economic water scarcity is the inability to have access to available water resources due to institutional, political influences, or other contributing factors. Central and Latin America, South and Central Africa and Asia regions demonstrate economic water scarcity.
__
The scarcity of fresh water may also make it harder to decarbonize society—something we must do to prevent catastrophic climate change—because some strategies to do this could further stress water resources. Green hydrogen, seen as key to eliminating emissions from aviation, shipping, trucking, and heavy industry, is produced by electrolysis, which splits water into hydrogen and oxygen. However, the process requires large amounts of purified water. One estimate is that nine tons of it are needed to produce one ton of hydrogen, but actually the treatment process used to purify the water requires twice as much impure water. In other words, 18 tons of water are really needed to produce one ton of green hydrogen. Nuclear energy, seen by the IPCC as an important tool for achieving our climate goals, also depends on fresh water for cooling, but as water shortages increase, nuclear plants may be forced to reduce their capacity or shut down.
_
As a result of water scarcity, some parts of the world have turned to desalination for drinking water. Desalination (desal) involves removing salt and minerals from salty water, usually seawater. This process occurs naturally as the sun heats the ocean—fresh water evaporates off the surface and then falls as rain. Arid regions like the Middle East and North Africa have long depended on desal technology for their fresh water. Today over 120 countries have desal plants with Saudi Arabia producing more fresh water through desal than any other nation. The United States also has a number of desal plants with the largest in the western hemisphere located in Carlsbad, CA. A new $1.4 billion desal plant in Huntington Beach, CA is likely to be approved soon.
_____
_____
Desalination as a solution to water scarcity:
Rapid population growth and urbanization are two main drivers for the over-abstraction of conventional freshwater resources in various parts of the world, which leads to the situation of water scarcity (per capita availability <1000 m3/year). The general trend showed that most of the water-scarce countries withdraw freshwater mainly for agricultural activities, municipal use, and industrial use. The highest use was in agriculture, which ranged from 0.02 to 9.13 m3/cap/day, with an average of 1.19 m3/cap/day. The average water withdrawal for municipal purposes was 0.140 m3/cap/day, which is eight times lower than for the agricultural sector. Predictions based on the World Bank projected population data and the FAO AQUASTAT database for freshwater availability show that by 2050, 2 billion people living in 44 countries will likely suffer from water scarcity, of which 95% may live in developing countries. Among these, the countries that will likely be most strongly hit by water scarcity by 2050 are Uganda, Burundi, Nigeria, Somalia, Malawi, Eritrea, Ethiopia, Haiti, Tanzania, Niger, Zimbabwe, Afghanistan, Sudan, and Pakistan. Currently, these countries have not yet established desalination to meet their freshwater demand. However, the current global trend shows that membrane-based desalination technology is finding new outlets for supplying water to meet growing water demand in most of the water-scarce countries. These 14 water-scarce countries will demand an additional desalination capacity of 54 Mm3/day by 2050 in order to meet the standard of current municipal water demand and to compensate for the withdrawal of renewable resources. Case studies from India, China, and South Africa have highlighted that other countries may apply the strategy of using desalinated water for industrial users.
_
The potential technical solutions to solve water scarcity are:
|
Saving water |
Increasing productivity in agriculture and industry |
|
Reducing leakages in public water supply |
|
|
Imposing progressive tariffs |
|
|
Increasing rainwater harvesting |
|
|
Water transport |
Transporting from long distances |
|
Aquifer storage |
Storing river water during high flow |
|
Water reuse |
Increasing reuse/recycling in industry |
|
and domestic wastewater in agriculture |
|
|
Desalination |
Using brackish water, wastewater, seawater |
_
Alternative water sources:
Traditionally, our water supply comes from surface water (rivers and lakes) and from groundwater. But increasingly, we are looking to other sources for our water – referred to collectively as ‘alternative water sources’.
These include:
- Recycled Water: Water from a municipal wastewater plant that has been treated to the point that it can be safely used again.
- Desalinated Water: Water from the ground or surface (e.g., brackish water or seawater) that has had the excess salts removed from it for use.
- Stormwater/rainwater: Water from runoff from precipitation events that is captured and sometimes treated for use.
- Greywater: Wastewater from households or office buildings that does not contain human waste and that is diverted and sometimes treated for reuse for landscapes and for flushing toilets.
Diversification of water supply portfolio – using non-traditional water sources – improves our water supply reliability and our ability to withstand drought conditions. Alternative water sources are crucial to that effort.
_
Among the different alternative solutions for solving the issues of water scarcity, desalination is only implemented as a last resort when conventional freshwater resources have been stretched to the limit. Desalination is considered as a drought-proof water source, since it does not depend on river flows, reservoir levels, or climate change. Desalination may be an option to alleviate scarcity in industry and for coastal cities. The report published by the United Nations showed that approximately 44% of the global population, and 8 out of the 10 largest metropolitan areas in the world, are located within a distance of 150 km from the coastline. The rate of population growth in the coastal regions is accelerating, and increasing tourism adds to the pressure on the environment (UN Atlas of the Ocean, 2017). Therefore, the possibility for widespread application of seawater desalination in the future is very likely. Although the most well-known application of desalination (and related membrane technology) is to produce freshwater from seawater, it can also be used to treat slightly saline (brackish) water, low-grade surface and groundwater, and treated effluent resources. The current global trend shows that desalination technology is finding new outlets as an alternative source for supplying water to meet growing water demands in most of the water-scarce countries. However, there have been barriers to the widespread adoption of this technology, mainly due to its cost, energy requirements, a lack of expertise, and its carbon footprint.
_
Why desalinate?
Three principal reasons for desalination:
-1. Creating a new standalone water source – Sweet-water shortages existing presently and expected in the future can be filled by the production of high-quality water, at reasonable cost, from the unlimited water source that is the sea.
-2. Water quality – The quality of water produced by desalination plants stands up to the most rigid quality control demands required of drinking water by WHO standards. The water is significantly softer, so that it precipitates significantly less scale and reduces the energy spent on water heating by the industrial and private sectors. Another advantage is receiving waste water with much less salinity than currently, making improved agricultural crops possible increasing the possibilities for use of wastewater, no less significantly, improving the groundwater.
-3. Economic benefit – Thanks to technological improvements and marketplace competition, desalination costs are dropping and production is becoming better and more efficient. Although desalinated water is more expensive than natural sweet water, the extra cost is insignificant when compared against the economic damage from dried-out agricultural land and parks. The drop in desalination cost is reflected in the tenders which were published over the years. In the Sorek B tender, the water price was set at approximately 1.5 NIS (0.4 $) per cubic meter.
_
How does desalination work?
These are the two chief methods:
-1. Evaporation processes (the older technology) – The water gradually evaporates as it passes through a series of chambers that vary in temperature and pressure. In each chamber a certain quantity of water evaporates, and the salts remain in the water that has not evaporated. The salty water (about half the incoming water) is dumped back into the sea as brine. The water vapor passes through a condensation stage in which the drops of water are collected, and the product is desalinated water.
-2. Membrane processes (the more modern technology) – The best-known and most widespread of these processes is reverse osmosis. In this process, the salt water is squeezed through membranes that permit the passage of water only and prevent salts from passing through. The water that does pass through the membranes is desalinated water, and the water that remains as a solution is a concentrate that is dumped back into the sea.
Because of their high energy consumption, the evaporation processes are suitable only for countries where electricity is very inexpensive, whereas reverse osmosis is much more energy-efficient and therefore is increasingly used.
______
Desalination refers to production of drinkable water from seawater (typical salinity ranging from 30,000 to 44,000 milligrams per liter) and from brackish water (salt content below 10,000 milligrams per liter). While desalination of brackish water is less expensive, but the source itself remains limited and already almost fully used in arid regions (World Bank, 2019). Oceans, however, seem like an unlimited desalination source as they contain almost 97% of the global water endowment.
Most of the world uses groundwater as the main source of water for domestic, agriculture, and industrial use. Increasing droughts indicate that regional inland aquifers and rivers will not be able to continue to meet these water demands in the coming decades. With 67% of the global population located away from coastal regions, there is a growing need for inland desalination plants, which treat brackish or wastewater (industrial or municipal) to fill this deficit.
_
Desalination at sea and inland:
Removing salt and other impurities from sea-, ground- and wastewater could solve the world’s looming freshwater crisis. And yet, while industrial-scale seawater desalination plants do exist in coastal areas where the freshwater challenge is most acute, the process of making undrinkable water drinkable is largely out of reach for inland water sources due to the high cost of concentrate disposal. When we desalinate water, we are left with a pure water stream and a concentrated waste stream. Inland brackish water and wastewater desalination plants are costly to build and to operate because we don’t have easy disposal options for the concentrate stream. Compounding this problem is that some inland wastewaters from industrial sources can have up to 10 times higher concentration of dissolved solids than seawater. Concentrating and disposing of concentrated brine could unlock vast new water resources, but it’s just too expensive at this time.
_______
Desalination is an effective technique for removing salts and undesirable quantities of minerals from water. It produces freshwater, which is potable and also useable for sanitary purposes. Desalination of seawater and brackish water is the best alternative technique to cope with the rising issue of scarcity of potable water. This can potentially make the saline water usable on a commercial scale, but energy- and cost-effective solutions are needed along with environmental friendliness and social acceptability. The costs of desalination plants are high because a huge amount of power is required for plant operation. Reverse Osmosis (RO) is currently applied in 85% of operational desalination plants and about 91% of under-construction plants. The Middle East region accounts for 39% of global desalination capacity, and most operational desalination plants are present here. Fossil fuel (thermal) based desalination plants account for 2/3 of total desalination plants in the Middle East and North Africa (MENA) region for freshwater production. The remaining desalination plants rely on Natural gas to operate RO in the region. The excess amount of Solar and Wind energy potential is present in MENA region compared to Europe, which is abundant with geothermal energy. Solar leads the way in terms of easy access and availability in MENA region. Water desalination is a better option for people of MENA region as 50% population lives nearby coast that makes them accessible of sea water. Combining sea water desalination with solar energy technologies can make best outcome for sustainable water production. Solar assisted desalination remains a viable solution for meeting the growing water demands, yet some technical hurdles needs to be addressed for the commercialization.
_
Driven by rising demand and commercial innovation, the cost of desalination has decreased significantly over the years, and it is becoming an increasingly feasible option. In 2018, 18,426 desalination plants were reported to be in operation in over 150 countries, producing 87 million cubic meters of clean water each day and supplying over 300 million people. Almost half this capacity (44 percent) is in the still-growing Middle East market, but other regions are growing even faster, notably Asia (in particular China), the United States, and Latin America. Figure below shows how the desalination industry has evolved over the sixty years. All in all, it is fair to say that desalination has solidified its position as a formidable complementary source in today’s water resources landscape.
_
Figure above shows the evolution of the desalination industry across the globe over the years.
_
Globally, nearly 2 billion people – about half of them in sub-Saharan Africa – lack access to safe drinking water. Water demand has increased by more than double the rate of population growth in the past century. The United Nations Environment Program estimates that by 2030, we could face a 40% shortfall in water supply if no drastic changes are implemented in water resources management. In fact, one-third of the world’s aquifers are in distress, mainly due to excessive withdrawals and changing rainfall patterns due to climate change. Averting major water shortages in vulnerable areas will require developing sustainable and energy-efficient ways of producing and managing water resources. Desalinated seawater is an unconventional water resource that can play a key role in achieving SDG6 and alleviating water poverty in coastal countries. However, desalination technologies are energy-intensive, so as the uptake of desalination expands globally, so will energy demand. This could be a major barrier in energy-constrained countries.
_
Desalination as risk management:
Desalination is also a good tool of risk management. Its raw material (the ocean) is practically limitless. Desalination is thus drought proof, and it is a good way to deal with climate change risks. Desalination is also a good response to exogenous risks such as dependency. Singapore, for example, opted for large-scale desalination to reduce its dependence on increasingly expensive imported water. The stable, efficient supplies of urban and industrial water that desalination provides can help governments manage a range of economic, social, and political risks.
_
Desalination as a strategic option:
Despite significant reduction in cost, desalination remains largely more expensive and needs to be used strategically to address a limited range of problems. However, today the instances of these problems are fast expanding. Desalination is proving appropriate for certain markets that require high quality and complete reliability of service and in which customers or governments can afford to pay the higher cost. For example, desalination can produce high-quality potable water that suits the needs of large cities in which there are high concentrations of people who demand a quality 24 hours per day, seven days per week water service and who are prepared to pay for that service. Desalination can also provide a reliable supply of large volumes of water to high-value industry, commerce, and tourism. In these uses, demand is going up with incomes, demographics, and urbanization; it is also in these uses that the value of water is typically the highest.
_
Desalination is of specific interest in certain locations in which the alternatives are high cost or the risk of supply failure is high. Desalination is, however, demanding in terms of location. Water has a very high ratio of bulk to value and is very expensive to lift or transport. This drives the location of a desalination plant: it should be near its raw material, the sea; it should be close to its market or point of use; and geographically it should not be too far below its market because pumping up elevation is very expensive. Hence, the typical location of a desalination plant is along a coastal city or coastal industrial zone, supplying a relatively well-off industrial, commercial, or domestic demand. Fortunately, already over one-third of the world’s population lives in urban centers bordering the ocean and in many arid parts of the world (such as the Middle East, Australia, Northern Africa, and Southern California) the population concentration along the coast exceeds 75 percent. Where the physical and socioeconomic conditions are right, seawater desalination provides a strategic solution for the sustainable, long-term satisfaction of part of this growing water demand.
_____
_____
Water transport to alleviate water scarcity:
Water transmission from rich resources to arid areas is not an innovative idea. For example, in the southwestern areas of the USA, water is transmitted for household, industrial and agricultural uses. Los Angeles now supplies 85% of its water requirements from outside of the region. Of course, water transfers from other areas are not always favorable, and protests against the transmission have always been a problem. In the USA, for instance, the diversion of the Colombia River for water supply in the western states has been a hot topic in Oregon for 35 years. Furthermore, in Spain, representatives of various parties are vigorously competing for water in the southeast (Kucera 2019). Additionally in the transmission systems, water quality is getting worse. Preventive potential for biological regrowth can be achieved by chlorinating the water. Even more disturbing, scheduling the control systems in water transmission lines is another concern for this strategy (Jung et al. 2015; Al-Jasser 2007). In addition to social and political pressures, technical problems such as long distances for water transmission, especially in high-altitude areas, makes it impossible to apply this method to all areas of the earth.
_
While noting costs are falling, and generally positive about the technology for affluent areas in proximity to oceans, a 2005 study argued, Desalinated water may be a solution for some water-stress regions, but not for places that are poor, deep in the interior of a continent, or at high elevation. Unfortunately, that includes some of the places with the biggest water problems, and, indeed, one needs to lift the water by 2000 m, or transport it over more than 1600 km to get transport costs equal to the desalination costs. Thus, it may be more economical to transport fresh water from somewhere else than to desalinate it. In places far from the sea, like New Delhi, or in high places, like Mexico City, transport costs could match desalination costs. Desalinated water is also expensive in places that are both somewhat far from the sea and somewhat high, such as Riyadh and Harare. By contrast in other locations transport costs are much less, such as Beijing, Bangkok, Zaragoza, Phoenix, and, of course, coastal cities like Tripoli. After desalination at Jubail, Saudi Arabia, water is pumped 320 km inland to Riyadh. For coastal cities, desalination is increasingly viewed as a competitive choice.
_
The costs of water produced by desalination have dropped considerably over the years as a result of reductions in price of equipment, reductions in power consumption and advances in system design and operating experiences. As the conventional water supply tends to be more expensive due to overexploitation of aquifers and increasing contaminated water resources, desalted water becomes a viable alternative water source. Desalination costs are competitive with the operation and maintenance costs of long-distance water transport system. As Saudi Arabia has already demonstrated, water can be pipped inland to landlocked cities which means desalination is not limited to coastal cities and can ensure the prosperity of sizeable regions.
_____
_____
Section-4
Introduction to desalination:
The desalting of seawater is an ancient notion. Aristotle described an evaporation method used by Greek sailors of the 4th century BCE. An Arab writer of the 8th century CE produced a treatise on distillation. In the 19th century the development of steam navigation created a demand for noncorroding water for boilers, and the first patent for a desalination process was granted in England in 1869. The same year, the first water-distillation plant was built by the British government at Aden, to supply ships stopping at the Red Sea port. The first large still to provide water for commercial purposes was built in 1930 in Aruba, near Venezuela. By 2019 about 18,000 desalination plants producing a total of more than 95 million cubic metres (in excess of 3.4 billion cubic feet) of potable water per day were in operation throughout the world.
_
In order to increase the fresh-water quantity to meet their basic needs, humans have practiced various forms of purification technologies on sea and brackish waters since long. This process, which is called as desalination, is an attractive way to tackle the water shortage problem, as it is the only inexhaustible source of water available in the globe. The UN World Water Development Report states unequivocally that water stress and scarcity is endemic in many regions and is increasing globally. Furthermore, it describes how we are very far from on-track to meet the goal of safe access to water for all by 2030. Desalination is often cited as a potential solution. Desalination is the broad term referring to the removal of salts from saline, brackish, contaminated, or otherwise highly mineralized sources of water. This technology holds obvious promise given the climate predictions for arid regions of the world. Currently, more than half a billion people are estimated to receive water by this means globally and the desalination industry is expanding, with market size approaching US$30 billion. Over the past decade in particular, the desalination industry has proliferated beyond its traditional hubs in the Middle East, the USA, Europe, Australia and China, and new mega-projects are being rolled out in diverse countries including Morocco, Chile, Ghana and India. Many commentators argue that the turn to desalination will be one of the most essential transitions in the water sector over the coming decades. In mid-October 2023, the world’s foremost authority on desalination, the International Desalination Association (IDA), held their Summit on Water and Climate Change in Seville, Spain, marking the association’s 50th anniversary.
_
The increase in world population, accompaniment with increase in industrial and agricultural activities in the recent decade, has led to excessive exploitation of available water resources and freshwater resources pollution. Therefore, adopting various methods for converting polluted water or salty water into potable water is necessary. In general, water is divided into five main categories:
Freshwater (0.5 g/L and less salinity)
Brackish water (0.5–30 g/L salinity)
Saline water (30–50 g/L salinity)
Sea water (35 g/L salinity)
Brine water (50 g/L and more salinity)
One of the most popular methods to produce potable water is “Desalination”, in which the salty water is
converted into potable water by the removal of salt content.
_
Globally, over 40 percent of the population is grappling with water shortages, while over 700 million lack clean, drinkable water. Close to two billion people live in river basins that require supplementary sources of clean water, the UN posits. In Africa, the UN further says that up to 250 million people will be living in areas with high water scarcity, which will lead to the displacement of between 24 and 700 million people triggered by unbearable living conditions. And as the global population burgeons and is expected to reach 9.7 billion by 2050, there has been an urgent need to embrace water technologies to address the worrying pressure this will place on resources. The need for water is always rising to keep up with the demands of population growth, improving living conditions, expanding green space, rising per capita consumption, urban development, and industrial growth. Over-pumping groundwater to meet the rising water demand has resulted in the depletion of significant aquifers and the worsening of groundwater quality.
_
Among the innovations being touted is water desalination (desal for short), which has received a warm welcome from both government and industry players. Desalination is the process of purifying saltwater by removing extra salt and other dissolved compounds. The World Health Organization’s 500 ppm drinking water limit is reached or exceeded by this method, which lowers salt content. The process of removing salt, impurities and other minerals on a massive scale from saltwater can quench the thirst of millions and be used in other activities, like farming, largely involves two techniques. The less technical one is the heating of sea or salty water to draw pure vapour, which is then cooled into liquid that is safe for drinking; the more complicated process, dubbed reverse osmosis, uses membranes that push water through filters at a high pressure in order to remove salt and other impurities.
_
The over 20,000 desalination plants available globally embrace these processes, producing approximately 25 billion gallons of desalinated water every day and supply over 300 million people. Up to 40 percent of the global water desalination initiatives are happening in the Middle East and North Africa, with the global market set to reach $32.1 billion by 2027. Due to its energy consumption, desalinating sea water is generally more costly than fresh water from surface water or groundwater, water recycling and water conservation. However, these alternatives are not always available and depletion of reserves is a critical problem worldwide. The energy intensity has improved: It is now about 3 kWh/m3 (in 2018), down by a factor of 10 from 20-30 kWh/m3 in 1970: Nevertheless, desalination represented about 25% of the energy consumed by the water sector in 2016.
_
Seawater provides an unlimited source of water for desalination processes. The second potential source is brackish water, which is sourced mainly from underground sources in many regions. The average salt content of seawater is 35,000 mg/L (range: 24,000 to 42,000 mg/L depending on the location). Brackish waters are less salty (ranging: 2000 to 10,000 mg/L). In desalination processes, membrane technologies play a dominant role nowadays – 69–73% of all installed systems globally, while thermal techniques account for ca. 27%. Among membrane techniques, reverse osmosis (RO) dominates the global market. RO is currently the most economical process for a wide range of salinity (seawater and brackish water). For low salinity feeds, mature processes such as electrodialysis (ED) and electrodialysis reversal desalination (EDR) are considered. Other emerging processes, such as forward osmosis (FO), adsorption desalination (AD), capacitive deionization (CDI), and membrane distillation (MD) are under development and may have a great potential in the future. Hybrid systems, which combine different desalination techniques and energy sources, appear to offer the most promising solutions. In addition, research is being conducted worldwide to improve the efficiency of already commonly used desalination processes (e.g. RO) and to find new solutions. Despite the progress in desalination technologies which has been observed over the last decades, they are still widely regarded as energy- intensive, and as a result solutions are required to reduce unit cost and thus improve the economic viability of such projects. One direction is the use of renewable energy sources (RESs), which, given important trends in the fight against climate change, play a particularly important role. However, there is still a need to identify efficient and economical RES-based solutions that can support desalination processes for long term operation.
______
______
High energy cost:
What makes desalination so expensive is the energy required to power the different technologies. Currently, most of the world’s desalination plants use one of two methods: thermal desalination (distillation) or reverse osmosis. The former, which has been in use for longer, involves evaporating saltwater and then condensing the water vapour, leaving the salt behind. Most thermal desalination plants utilise waste heat from power plants to heat up seawater or brackish water. The heated water then evaporates in a vacuum and the steam condenses on pipes that contain a cooling liquid. This method is becoming less popular, but remains significant across the Arab world. These types of plants require an enormous amount of energy to take the salt out of the seawater. That’s why energy-rich countries have the advantage. The energy cost represents between 40 and 50 per cent of the entire production cost of these plants, depending on which technology is being used and the age of the plant. Therefore, reducing energy consumption is also the most sensible way to make desalination more cost efficient.
_
Experts and activists point to a vicious cycle: droughts and water shortage increase the need for desalination, but if desalination uses fossil fuels, the burning of these fuels increases the emissions responsible for climate change. This is one of the reasons why most of the world prefers reverse osmosis for desalination nowadays –about 80 per cent of all plants. But it will still take some time until the remaining 20 per cent of thermal plants disappear. Reverse osmosis operates by pushing saltwater under high pressure through a semi-permeable membrane whose pores are too small for the salt molecules to pass through. From the point when its advantages were scientifically and practically established, it still took reverse osmosis about three decades to overtake thermal desalination as the market-leading technology. Back then, the energy demand for reverse osmosis was 15–20 kWh/m³. Today, we’re down to 3.5–4.5 kWh/m³. This energy comes from electricity, so a plant’s sustainability depends on how that electricity is being generated. Generally speaking, osmosis has a lower energy demand, but the higher the salinity of the original water, the higher the cost. Higher salinity causes a higher osmotic pressure in the saltwater, which means more pressure is needed to push the water through the membrane. Engineers have managed to reduce these costs by about two-thirds by developing more sophisticated membranes. And yet, despite improvements, reverse osmosis has a physical limitation that puts the method at a disadvantage compared with some of the even newer technologies now being developed. The theoretical minimum of energy required for desalination is 0.7 kWh/m³. That is the physical limit – it will never get any better than that.
_______
Brine:
The other big problem with desalination lies in its waste product. Worldwide, plants generate 160 million cubic metres of a hypersaline concentrate per day. This brine is one of the main problems for desalination and a puzzle that scientists have been trying to solve for decades. Producing a litre of drinking water creates 1.6 litres of salty brine and activists are concerned about the byproduct’s effect on the environment, especially marine ecosystems – a concern that was one of the reasons why the plant in Huntington Beach wasn’t approved.
_
Waste brine is commonly mixed with seawater to a lower salt concentration and then pumped back into the ocean. This kind of waste management is affordable. All that’s required is a pumping system that’s capable of sending the brine back into the ocean. The amount of energy required to pump the brine back depends on its salinity and the plant’s distance from the ocean – which is why there aren’t many desalination plants designed to cleanse brackish water inland.
But environmental activists are concerned about the effects on fragile maritime ecosystems. The high level of salinity alone can damage seagrass beds and fish larvae. The brine can also cause a lack of oxygen in certain layers of the ocean, which could be dangerous to larger marine fauna. In addition, the pumps can hurt or even kill fish and mammals when they suck in water. ‘These plants contribute to climate change, destroy fragile coastal ecosystems, and salt being introduced into the environment poses a risk as well,’ says Fabienne McLellan of the international environmental group Ocean Care.
_
According to plant operators, if the process is correctly conducted, after the concentrated brine has been mixed down, the same quantity of salt that has been taken out of the ocean is pumped back. But even proponents of desalination have to admit that there are even more problematic pollutants in that brine. In order to operate these plants, and to keep their different mechanical parts functional for as long as possible, chemical additives are being used. These can include antifouling agents, descalers or anti-foaming agents. Today, these high-energy systems with a worrying waste product may work for water-stressed but wealthy states, but they don’t offer a solution for poorer nations facing water crises, such as Yemen or Somalia. There, NGOs and others are working on smaller, less cost-intensive desalination projects, often replacing fossil fuels with solar power to keep the plants running.
______
Meanwhile, scientists are working on technological solutions that could solve both the environmental issues and the energy-efficiency problems. Researchers are currently working on more than 50 desalination technologies that are, in parts, so fundamentally diverse, that they follow different disciplines of the natural sciences. One day, some of these technologies will replace reverse osmosis, although it will take a long time for new plants to come online. In the past, we were too focused on attempting to squeeze fresh water out of the salt water. Now, new technologies aim to directly remove salt and solids, which amount to about three per cent of the volume of seawater.
A technology called capacitive deionisation appears to be particularly promising. Here an electrostatically charged surface is submerged in saltwater. The static causes the salt ions to attach themselves to porous electrodes, often made from carbon. When the surface is lifted from the water, the salt can be released by changing the polarity of the electrodes. It’s just as if you had some kind of electrostatic shovel. Currently, the main issue is that the ‘shovel’ needs to unload the salt ions regularly, so the process has to be repeatedly interrupted. To improve on that, scientists at RWTH Aachen University have developed a variation of the process – called flow-electrode capacitive deionisation – that uses a liquid suspension rather than a solid surface, so the metaphorical shovel doesn’t need to be unloaded.
Other methods that promise to offer stand-alone desalination in the future are currently being trialled as complementary processes in reverse osmosis plants. At Columbia University in New York, scientists have developed a solvent-based extraction method called TSSE (temperature swing solvent extraction). Saltwater is combined with a liquid solvent and vigorously shaken. The salt separates from the mixture, which, when heated, separates further into solvent, salt and drinking water. The method ‘uses a solvent that acts like a “sponge”, soaking up just the water but not the dissolved salt. Warming up the solvent is analogous to squeezing the sponge to release the water. The method doesn’t require membranes or the high temperatures needed for distillation, can be powered by renewable energies and is generally more cost-efficient. Using reverse osmosis, when there is too much salt, the pressure needed is too high for the membrane to sustain and therefore reverse osmosis is restricted to seawater salt concentrations and below. TSSE doesn’t have such a limitation and can desalinate more salty water. At this stage, TSSE can help to reduce the environmental burden of reverse osmosis plants by cleaning up the high-saline-brine waste product. TSSE has made tremendous progress in the past two years. The technology can extract all of the water from the brine and achieve what the industry calls ‘zero liquid discharge (ZLD)’, where all of the water in the brine is converted to desalinated water and only solid minerals of the salt are left behind. Current methods to obtain ZLD are all very energy intensive and costly. TSSE will be the answer to many of the environmental questions that are still posed by desalination naysayers.
_____
_____
Commercial desalination technologies are grouped into two categories; membrane desalination, mainly RO, and thermal desalination, mainly MED and MSF. Emerging desalination technologies, that are still in the research and development stages, to a large extent, include membrane distillation (MD), forward osmosis (FO), capacitive deionization (CDI), freezing, humidification dehumidification (HDH) and gas hydrate-based (GH) desalination. In addition, there are a number of supporting or pretreatment technologies to increase desalination plant efficiency; these include ultrafiltration (UF), nanofiltration (NF) and ionic filtration (IF). Apart from the major standalone desalination technologies, blends of technologies including MSF-MED, MED-adsorption (MED-AD) and RO-MSF are currently being considered to increase desalination plant efficiency, by combining the strengths in each technology such that the blend could overcome deficiencies in each technology.
_
Salt removal from seawater consumes a relatively high amount of energy; in terms of the electric energy required in membrane desalination to overcome the natural osmotic pressure of seawater, or in terms of both electric and thermal energy required in thermal desalination to vaporize or flash a portion of seawater into distillate. RO desalination technology, for example, is nearly 5 times more energy-intensive than traditional surface-water (such as lakes and rivers) treatment technology. The energy required to desalt seawater in thermal desalination is even higher than that required in RO, for plants that are not combined heat and power plants. The main reason for this disparity in energy consumption in these two dominant technologies is the rapid advancement in membrane technology. RO accounts for about 69% share of the installed desalination capacity and most of the new contracts are awarded with membrane desalination technologies in mind. Meanwhile, despite (i) huge resources expended on, (ii) research activities carried out on, and (iii) fame associated with membrane technologies for water desalination, the current specific energy required to desalt seawater via these techniques (especially RO) still hovers around 2–4 kWh/m3 (against 0.7 kWh/m3 thermodynamic minimum requirement), depending on the salinity of the feed water and other external factors. This range is still significantly higher than the energy consumption of non-saline surface water treatment technologies.
_
Compared to thermal desalination methods, the Achilles heel of RO is membrane fouling. The most common fouling mechanisms are colloidal and particulate, organic, inorganic and biofouling mechanisms. Hence, pretreatment steps are required to maximize the efficiency of RO as well as elongate the life span of RO membranes. Most times, based on the quality of feed water, multiple pretreatment steps may be required. MSF and MED are the two well-established or mature large-scale thermal desalination technologies in the world. They are both assessed based on widely accepted thermal energy performance factors such as (1) gain output ratio (GOR) and/or (2) performance ratio (PR). GOR measures the amount of distilled water produced per kilogram of steam consumed in a desalination process. PR represents the produced quantity of the distilled water per amount of input energy. These factors influence the water production cost. The maximum operational efficiency, in terms of PR, recorded for an MSF plant is 8.6 kg/2326 kJ, having 24 stages with top brine temperature (TBT) of 112 °C, whereas the highest recorded PR for an MED plant is 14.6 kg/2326 kJ with TBT of 70 °C and 16 effects.
_
MSF as a desalination technology has been around for more than 60 years. Research activities on this technology have always been focused on the control of two major factors: corrosion and scale formation. Buildup of scales on the process equipment limits heat transfer, which leads to high energy consumption. Although a number of research activities has been reported on antiscalants to improve MSF operational efficiency, each antiscalant comes with additional challenges that have effects on the overall MSF plant efficiency. The second challenge associated with MSF technology is corrosion buildup inside the stages. The most important corrosion control strategy is right material selection for the heat exchange surfaces, especially the condenser tubes. For example, the first plants constructed in the GCC used carbon steel as the material of construction for evaporators but the experience garnered over the years, coupled with astute research and development in material science, has led to new plants being built with corrosion-resistant alloys.
_
The major technology challenge in MED also involves scale buildup. As a result, the current MED plants are built with a maximum of 12 effects, operating at TBT of 65 °C. The low value of the temperature limits the number of effects that can be incorporated to 12. To address these challenges in thermal desalination technologies, most MSF and MED plants employ pretreatment options. The salinity of seawater can be reduced and hardness (Ca2+ and Mg2+) can be removed by pretreatment prior to application of either MSF or MED. This allows TBT to be increased without much penalty of scale buildup. Cost of desalinated water production is also strongly related to capital cost. Capital cost contributes more to the specific cost of production than operating cost.
_
It is observed that the global installed desalination capacity has been increasing steadily at the rate of about 7% per annum since year 2010 to the end of 2019. Extra-large plants are few but they supply most of the global desalination capacity. There is a sharp rise in the desalination capacities of regions that did not really embrace desalination in the past, including Europe and Africa. The power industry remains the largest owner of installed capacity for industrial purposes. Filtration and dissolved air flotation remains the most prominent pretreatment methods. Seawater and Engineering-Procurement-Construction (EPC) model are the most frequently used feed water and plant delivery method, accounting for 57% and 71.7% of global installed capacity, respectively.
_
Desalination as a Municipal Water Supply in the United States:
Existing desalination capacity and the prospects for future adoption varies across US states and differs for brackish water and seawater. Brackish water desalination—treatment of waters with dissolved solids of 1000 and 10,000 milligrams per liter (mg/L)—in states like Florida, California, and Texas has made the United States a global leader in this type of desalination. In contrast, the United States has only a limited number of municipal seawater desalination facilities. As of 2015, treated brackish water and seawater were not significant municipal water sources at the national level; water from desalination represented less than 1% of US municipal water supplies. This situation, however, does not reflect the future role that desalination may play in addressing regional and local shortages of developed freshwater supplies.
______
______
Desalination in nature:
Evaporation of water over the oceans in the water cycle is a natural desalination process. The formation of sea ice produces ice with little salt, much lower than in seawater.
Seabirds distil seawater using counter current exchange in a gland with a rete mirabile. The gland secretes highly concentrated brine stored near the nostrils above the beak. The bird then “sneezes” the brine out. As freshwater is not usually available in their environments, some seabirds, such as pelicans, petrels, albatrosses, gulls and terns, possess this gland, which allows them to drink the salty water from their environments while they are far from land.
Mangrove trees grow in seawater; they secrete salt by trapping it in parts of the root, which are then eaten by animals (usually crabs). Additional salt is removed by storing it in leaves that fall off. Some types of mangroves have glands on their leaves, which work in a similar way to the seabird desalination gland. Salt is extracted to the leaf exterior as small crystals, which then fall off the leaf.
Willow trees and reeds absorb salt and other contaminants, effectively desalinating the water. This is used in artificial constructed wetlands, for treating sewage.
_____
_____
Basics of Desalination:
Desalination is a term used to describe the process of producing freshwater out of saline water (brackish water, seawater or brine). In more detail, the desalination is a procedure that is performed on an aqueous solution to separate the salts from the solution or to separate the water from the salts; however, the exact procedure depends on the type of technology used (Panagopoulos et al., 2019). The freshwater produced should contain a content of total dissolved solids (TDS) that is appropriate for domestic or industrial use. Specifically, although there is no worldwide regulation on the freshwater purity for human consumption, it is suggested that drinking water should contain <500 mg/L TDS (Rosborg, 2019; European Community, 1998). On the other hand, the purity of the water has to be much higher (10–20 mg/L TDS) in many industrial applications such as pharmaceuticals, semiconductors, etc. (Agalloco and Carleton, 2007; Reinhardt and Reidy, 2011). Besides freshwater, a by-product called ‘brine’, ‘reject’ or ‘concentrate’ is produced. The by-product is at least 1.6 times more saline than seawater and its management is a crucial issue as brine has adverse effects on the environment (Heck et al., 2016; Missimer and Maliva, 2018; Frank et al., 2017). Overall, input streams include feed water, energy and chemicals; while output streams include freshwater produced, brine and GHGs emissions.
_
With drought and population growth increasing demand, desalination has become essential to securing adequate fresh water supply in many regions. However, removing salts and minerals from seawater or brackish sources poses complex challenges. Desalination is an alternative solution to increase available water resources. In some regions of the world, it is the only source of water available. Desalination allows converting seawater into a life sustaining and usable resource. Seawater covers 71% of the planet’s surface and represents 97% of the world’s water. Processes to desalinate seawater and render it potable have been around for many decades but recent years have brought innovations that have greatly improved their energy consumption and environmental footprint. The oceans represent a fantastic resource for humanity, a vital one in water-scarce regions, and we are now able to capitalize on it in a more sustainable way. Seawater desalination is an increasingly key solution for scarcity of water guaranteeing water supply even in periods or areas of drought.
_
Currently, the total desalination capacity installed worldwide stands at around 21,123 plants, producing approximately 142,000,000 m3/day of freshwater (IDA and GWI DesalData, 2019). It is interesting to mention that the aforementioned amount of freshwater produced per day is significant as it is equivalent to the water volume of 56,800 Olympic-sized swimming pools. Most countries systematically use desalination, with most desalination plants in Saudi Arabia, the United States of America, the United Arab Emirates, Kuwait, France, Japan, etc. (Eslamian, 2016; IDA and GWI DesalData, 2019). Currently, the largest desalination plant is the Jubail Plant (Saudi Arabia) which produces 1,401,000 m3/day of freshwater (Guinness World Records, 2019). RO technology is the most prevalent, with 74% of the world’s installed capacity using this technology in 2019, while another 21% and 3% remained in the use of thermal technologies (namely, MED and MSF) and ED, respectively; the remaining 2% refers to technologies that they were unable to dominate the market due to their costs and/or existing technical constraints (IDA and GWI DesalData, 2019).
_
Desalination technologies and their application have evolved substantially over the past 50 years. The key elements of a desalination system (see figure above), for either brackish water or seawater desalination, are as follows:
-1. Intakes—the structures used to extract source water and convey it to the process system;
-2. Pretreatment—removal of suspended solids and control of biological growth, to prepare the source water for further processing;
-3. Desalination—the process that removes dissolved solids, primarily salts and other inorganic constituents, from a water source;
-4. Post-treatment—the addition of chemicals to the product water to prevent corrosion of downstream infrastructure piping; and
-5. Concentrate management—the handling and disposal or reuse of waste residuals from the desalination system.
Depending on the source water and the desalination technology used, specific elements may vary in their importance in the overall system. For example, inland brackish groundwater desalination facilities will use wells and pumps to bring the source water to the facility, and these systems may need little or no pretreatment. In contrast, seawater reverse osmosis (RO) desalination may use more elaborate intake structures, depending on the specific site conditions, and may require extensive pre- treatment.
_
Three streams are involved in the process:
-1. Source water (or feedwater stream) permits address the location and means of obtaining the source water used by the desalination facility.
-2. Potable water (or finished water stream) permits address the use of the finished water produced by the desalination facility.
-3. Waste (concentrate and other associated waste stream/ Brine) permits address the treatment or discharge of the waste streams, including concentrate, chemical wastes from cleaning processes, and any other waste associated with the operation of the facility.
_
Source Water Consideration:
Several factors influence how desalination plants determine which source waters to treat, such as:
- The plant’s location relative to available water sources
- The source water quality
- The treated water’s delivery destination
- Available pretreatment options
- The environmental impacts of treatment
_
The two most common sources of water desalination plants evaluate are seawater and brackish water. As illustrated in figure below, the majority of the desalination plants (>80%) desalinates brackish water or seawater.
_
Seawater Desalination:
Seawater has an exceptionally high concentration of live salts, making it more difficult to treat than brackish waters. To remove the salts from the salt water (also referred to as saline water), water quality professionals first measure the concentration of dissolved salt by weight in parts per million (ppm). Thus, if water has a 10,000ppm concentration, 1% of the water’s weight comes from dissolved salt. By identifying how much salt is present, desalination plants can treat seawater and all saline waters appropriately.
For reference, here are the ppm concentrations of each water source:
- Freshwater – less than 1,000ppm
- Slightly saline (brackish) water – between 1,000ppm and 3,000ppm
- Moderately saline water – between 3,000ppm and 10,000ppm
- Highly saline (ocean) water – between 10,000ppm and 35,000ppm
While desalination can effectively treat seawater and create safe drinking water, it also produces an environmentally harmful by product called brine — a highly concentrated solution of salts and contaminants.
Brackish Water Desalination:
Though brackish water is significantly less salty than seawater, desalination plants still need to treat it to make it safe for human consumption. Commonly found in inland seas, brackish water occurs when groundwater and seawater mix in deep fossil aquifers. As precipitation seeps in over time, the salt from mineral deposits dissolves and produces salty water. Traditionally, communities have undervalued and underutilized brackish water. However, with modern desalination processes and the depletion of freshwater sources, water quality professionals are looking to it more and more as a source for generating drinking water. Because it’s less salty than seawater, brackish water is easier and cheaper to desalinate.
Disposal of Brine:
Many desalination plants dispose of brine by dumping it back into the sea, a process that requires costly pumping systems and can damage marine ecosystems. A more environmentally friendly alternative is to turn brine into valuable chemicals like sodium hydroxide. Water desalination facilities can use this chemical to pretreat saline water, resulting in a process that’s not only safer for the environment but also more efficient and cost-effective.
Technologies have been developed exclusively for the treatment of desalination brine, such as brine concentrator (BC), brine crystallizer (BCr), spray dryer (SD) (GEA Process Engineering, 2019; Kerone, 2018; Veolia Water Technologies, 2018). The reason behind this is that brine treatment is an upcoming sector of the water treatment industry since it is possible to recover higher volumes of freshwater and resources (e.g., salts) (Panagopoulos et al., 2019).
_
Figure below shows global online desalination capacity by feed water types, categorised by plant size as of 2021.
Note: S = under 1000 m3/day; M = 1000 to 10,000 m3/day; L = 10,000 to 100,000 m3/day; XL = over 100,000 m3/day.
_
Nowadays, desalination plants operate in more than 120 countries in the world. Figure below shows the global desalination capacities (m3/d) and the source water types.
_
Figure below shows principal methods for desalinating water; reverse osmosis dominates in terms of installed capacity, followed by multi-stage flash and multi-effect distillation.
_
There are two main types of seawater desalination technologies: membrane-based (RO) and thermal (MED, MSF). Reverse Osmosis (RO) desalination uses the principle of osmosis to remove salt and other impurities by transferring water through a series of semi-permeable membranes. Thermal desalination uses heat, from power plants or refineries, to evaporate and condense water to purify it. Desalination is an artificial process by which saline water (generally sea water) is converted to fresh water. The most common desalination processes are distillation and reverse osmosis.
The advent of the modern desalination era started with thermal desalination technology. The core idea behind thermal desalination is relatively simple; introducing thermal energy to the saline water would eventually turn it from a liquid to a gas phase (e.g., the vapor or steam) which would be considered free of salt, minerals, or other contaminants initially dissolved or suspended in the saline water. The vapor or steam would then be condensed back to the liquid form passed by desalination units. From a practical standpoint, there are different ways to implement the idea of thermal desalination, the most notable of which are multi-effect desalination (MED), multistage flash (MSF) desalination, and vapor compression (VC) (e.g., mechanical vapor compression (MVC) and thermal vapor compression (TVC)). A simplified process diagram of thermal or membrane desalination is depicted in figure below.
_
Figure above shows simplified process diagram of thermal or membrane desalination units.
_
Multistage flash distillation is a thermal process for desalting relatively large quantities of seawater. Based on the fact that the boiling temperature of water is lowered as air pressure drops, this process is carried out in a series of closed tanks (stages) set at progressively lower pressures. When preheated seawater enters the first stage, some of it rapidly boils (flashes), forming vapour that is condensed into fresh water on heat-exchange tubes. Fresh water is collected in trays as the remaining seawater flows into the next stage, where it also flashes, and the process is continued. One of the largest of these systems, located in Al-Jubayl, Saudi Arabia, can produce more than 750 million litres (200 million gallons) of desalted water per day.
_
In small communities where salt water and intense sunlight are both abundant, a simple thermal process called solar still can be used. The heat of the Sun partially vaporizes salt water under a transparent cover. On the underside of the cover, the vapour condenses and flows into a collecting trough. The principal difficulty in this process is that large land areas are required, and energy is needed for pumping the water. Another thermal process makes use of the fact that, when salt water is frozen, the ice crystals contain no salt. In practice, however, objectionable amounts of salt water remain trapped between the crystals, and the amount of fresh water needed to wash the salt water away is comparable to the amount of fresh water produced by melting the crystals.
_
Another practical approach to desalination is through a phenomenon technically referred to as the membrane process. The central principle behind the membrane process is to enforce the transport of a substance, say water, through semi-permeable membranes against the chemical potential gradient. This process would allow the selective passage of particles through the membrane. In the context of desalination, to this day, there are three known practical ways to enforce the membrane process, namely, (I) pressure-driven, (II) using an electric potential gradient, and (III) using temperature gradient. One of the most prevalent membrane process-based desalination units is reverse osmosis (RO). Other notable membrane process-based desalination are electrodialysis (ED), membrane distillation (MD), and freeze desalination (FD). It should be noted that forward osmosis (FO), hydrate-based desalination (HyDesal), and pervaporation desalination (PVD) are some of the other promising cutting-edge technologies that are for the most still in the research/development phase (Wang et al. 2016; Babu et al. 2018; Suwaileh et al. 2020).
_
Reverse osmosis (RO), nanofiltration (NF), and electrodialysis (ED) are the three membrane processes available for desalination. ED membranes operate under an electric current that causes ions to move through parallel membranes and are typically only used for brackish water desalination (Reahl, 2004). NF membranes are a newer technology developed in the mid-1980s (Singh, 1997) and have been tested on a range of salt concentrations (Hilal et al., 2005, Tanninen et al., 2006, Wang et al., 2005, Wang et al., 2006). Research has shown that NF, as a singular process, cannot reduce seawater salinity to drinking water standards, but NF has been used successfully to treat mildly brackish feed water (Bohdziewicz et al., 1999, Lhassani et al., 2001, M’nif et al., 2007). Coupled with RO, NF can be used to treat seawater (Hamed, 2005, Hassan et al., 1998, Hilal et al., 2005). In particular, NF membranes are used to remove divalent ions, such as calcium and magnesium that contribute to water hardness, as well as dissolved organic material (Choi et al., 2001, Gorenflo et al., 2002, Wilf, 2003).
RO membranes, however, are able to reject monovalent ions, such as sodium and chloride. Today, seawater RO membranes have salt rejections greater than 99% (Bates and Cuozzo, 2000, Brehant et al., 2003, Reverter et al., 2001); some membranes, when operated under standard test conditions (32,000 mg/L NaCl, 5.5 MPa, 25 °C, pH 8, 8% recovery), can achieve as high as 99.7–99.8% salt rejection (Reverberi and Gorenflo, 2007, Hydranautics, 2007). RO membrane technology has developed for both brackish and seawater applications. Brackish water RO membranes typically have higher product water (permeate) flux, lower salt rejection, and require lower operating pressures (due to the lower osmotic pressures of less saline waters), while seawater RO membranes require maximum salt rejection. Membranes designed for higher salt rejection, have lower permeate fluxes, due to the trade-off between membrane selectivity (salt rejection) and membrane permeability (permeate flux). In addition, seawater RO membranes must operate at higher pressures to compensate for the higher osmotic pressure of seawater.
_
RO:
Reverse osmosis (RO) is defined by Mindler and Epstein (1986) as ‘a pressure driven separation of water from a saline solution across a membrane, the pressure being adequate to overcome osmotic pressure of the saline solution and to provide an economically acceptable flux’. In reverse osmosis, water is pushed through semi-permeable membranes, using pressure. The salts do not pass through the membrane, while water molecules do.
Based on the quality of the input processed, RO processes can be grouped into brackish water RO plants (BWRO) where the salinity is in the range of 500 mg/L to 10,000 mg/L and seawater RO plants (SWRO) where the salinity is around 30,000 mg/L. BWRO is further sub-grouped into low salinity BRWO that process feed water with salinity between 500 and 2500 mg/L and high salinity BRWO plants that process water with salinity between 2500 and 10,000 mg/L. The efficiency of RO depends on a number of factors including the operational parameters, the employed membrane, and the feed water characteristics. One of the largest reverse-osmosis desalination plants now in operation is located in Sorek, Israel, and can produce some 627,000 cubic metres (22 million cubic feet) of desalted water per day.
Although there is some selectivity to specific ions, desalination with reverse osmosis removes most salts from the water and the amount of salts removed depends mostly on the pressure and on the ratio between the amount of desalinated water produced and the amount of brine water rejected and discharged as waste. The higher the salt concentration of the intake water is, the higher the pressure that is required. RO requires pressure between 2–17 bar (30–250 psi) for fresh and brackish water, and 40–82 bar (600–1200 psi) for seawater.
Since dissolved salts, which are removed by reverse osmosis, are much smaller than viruses, reverse osmosis also removes viruses and bacteria. However, presence of certain bacteria prior to the RO plant often results in membrane clogging and operation problems.
______
Desalination Technologies Classification:
- By Technology:
-Reverse Osmosis (RO)
-Multi-Stage Flash (MSF)
-Multi-Effect Distillation (MED)
- By Application:
-Municipal
-Industrial
-Agricultural
- By Region:
-North America
-Latin America
-Europe
-East Asia
-South Asia & Oceania
-MENA
______
Figure below shows desalination technologies used at plants worldwide in 2019. The technologies include reverse osmosis (red pie slice), thermal-based technologies (green pie slice), electrodialysis (light blue pie slice) and other technologies (light yellow pie slice).
RO’s dominance over the last decade can be attributed to its efficiency, scalability and modularity as there have been many advancements over the last decade (Kucera, 2015; Trishitman et al., 2020). Typically, the mature desalination technologies are primarily used in the desalination of brackish water and seawater.
______
Key issues in Seawater Desalination:
The most widely applied and commercially available technologies for sea water desalination can be divided in two types: membrane processes and thermal processes. Reverse osmosis (RO) is the leading sea water desalination solutions. The advances in key equipment (membranes, pumps, energy recovery device), turned the process energy efficient, resulting in a low investment cost (CAPEX) and low operational cost (OPEX). Nowadays, desalination has become a very affordable solution to cope with fresh water shortage typically in tropical as well as of off-shore areas.
_
The desalination core process is based on Reverse Osmosis Membrane technology, but stand alone, it doesn’t provide safe drinking water, nor does it guarantee an efficient plant. The pretreatment includes all the necessary treatment step ahead of the reverse osmosis plant. It is determining for plant life time and to minimise chemical cleaning and membrane replacement. It has a direct impact on the plant performance. There are as many membrane types as applications. They range from “high rejection” to “ultra low energy” or ” high boron rejection”. The reverse osmosis process can also be built with one or two passes, depending on the product water requirements and the seawater salinity and temperature. In most cases, 1 pass is sufficient to rich the EU drinking water standards, specially regarding the boron content (1 mg/L). To rich WHO boron guideline (0.5mg/L), a second pass might be necessary (Boron removal process)
_
- The energy recovery device is the key factor that determines the plant electrical costs. It must be chosen carefully based on the local energy costs and environment policies.
- Post-treatment and/or polishing steps are required to condition the water after the reverse osmosis membrane process to make it suitable to your application.
- Brine disposal can be an environmental and economical issue in some areas where the fauna and flora are sensitive to local seawater salinity increase. Brine disposal should be studied and engineered case by case.
- The art of desalination is to determine and combine available technologies to optimize water production costs and quality.
_
Qualities of a Good Seawater Desalination System:
A good desalination system is one that effectively removes salt and other minerals from seawater to produce high-quality fresh water. And a good desalination system produces fresh water that can be used directly for industrial applications or municipal water. A good system should have the following important characteristics:
Durability:
The desalination system uses high-quality equipment materials, and its internal structure is strong enough to withstand the harsh marine environment. The system should be durable and not easily damaged to affect its use.
Reliability:
The machinery can be capable of performing consistently and dependably, especially under difficult circumstances like excessive salinity or erratic water flows.
Efficiency:
With little energy input, the system should be able to generate significant amounts of fresh water. This is crucial for the system’s cost-effectiveness as well as for lowering its environmental impact.
Environmental impact:
The system’s operation should have a negligibly small impact on the environment, both in terms of the energy needed to run it and the waste products it generates.
Flexibility:
This desalination system should be able to adjust to various water sources and environmental factors, such as shifting water currents or variable salinity levels.
_
Key performance criteria for desalination:
- energy consumption: the desalination technology must have a low energy consumption to be environmentally and economically feasible;
- renewable energy: it must be easy to integrate renewable energy to power the desalination process in order to reduce the associated GHG emissions;
- pre-treatment: this stage must not be energy and chemically intensive to reduce water treatment costs and environmental pollution;
- water recovery: this parameter must be maximised to increase process efficiency and reduce the volume of brine;
- water cost: the water production costs must be low to ensure the process is economically feasible and cost-competitive with current technologies.
_
All type of water can be produced from a desalination plant:
- WHO or EU drinking water
- Irrigation water
- Process water: boiler feed water, cooling water
- Demi or Ultrapure water
All type of natural seawater source can be treated:
- Shallow Surface seawater
- Deep seawater
- Brackish river water
- Beach well seawater
_____
_____
Desalination statistics, growth and progress:
According to the new International Desalination Association (IDA) Water Security Handbook, the total global installed desalination capacity stands at 97.4 million cubic meters per day (m3/day). While, the total global cumulative contracted capacity is 104.7 million m3/d in 2022. Water is a national resource that requires a national strategy for integrated water-resources management and to address the difficulties brought on by the increased water demand. Desalination has solidified its place in recent years as a means of reducing the world’s water shortage. Desalination uses a lot of energy, though, and historically has relied on fossil fuel-based processes. Desalination of brackish water and seawater has spread fast throughout the world. More than 17,000 desalination units were operational in 2013, delivering around 80×10^6 m3/d to 300 million people across 150 nations. By 2015, the production capacity had nearly reached 97.5×10^6 m3/d, and by 2050, it is anticipated that there will be 192×10^6 m3/d of desalinated water available. The top 10 countries using desalination are listed in Table below:
|
No. |
Country |
Total capacity (million M3/d) |
Market share (%) |
|
1 |
Saudi Arabia |
9.9 |
16.5 |
|
2 |
USA |
8.4 |
14.0 |
|
3 |
UAE |
7.5 |
12.5 |
|
4 |
Spain |
5.3 |
8.9 |
|
5 |
Kuwait |
2.5 |
4.2 |
|
6 |
China |
2.4 |
4.0 |
|
7 |
Japan |
1.6 |
2.6 |
|
8 |
Qatar |
1.4 |
2.4 |
|
9 |
Algeria |
1.4 |
2.3 |
|
10 |
Australia |
1.2 |
2.0 |
_
Figure below shows global distribution of desalination plants disaggregated by feedwater quality, plant capacity and desalination technology:
The large desalination systems in many countries, such as China or those that in Middle East which can reach a daily production more than 500000 m3, use mainly thermal desalination methods of large scales, sometimes combining additionally with the membrane ones. For medium size systems from 5000 to 90000 m3/day both thermal and membrane methods are possible. However, for small desalination units, with daily capacity of 1000–5000 m3 membrane technologies are the dominant way.
_
Desalination plays an integral role in the supply of freshwater to the Middle East, where nearly half of global desalination capacity is located, and the biggest markets in the Middle East are Saudi Arabia, the United Arab Emirates, Kuwait, Qatar, and Israel. Desalination is largely deployed in wealthy, energy rich areas of the world, like the United States, Australia, China, Europe, and Japan. But the next growth centers for the technology will be in rapidly growing countries in Africa and Asia such as Senegal, the Philippines, India, Morocco, and South Africa.
Figure below shows global desalination capacity (% by region):
_____
_____
Desal growth:
Desalination is more energy intensive than traditional water treatment methods and depending on the technology, both electricity and heat are required. Total online desalination capacity has been increasing globally, both in the Gulf Cooperation Council (GCC) countries and elsewhere. Seawater reverse osmosis (SWRO) has rapidly increased, and thermal desalination technologies have plateaued as seen in the figure below.
SWRO is projected to remain the dominant desalination technology owing to the lower costs and energy consumption and technological improvements. Nevertheless, while the energy consumption for surface freshwater treatment is about 0.6 kWh/m3, the least energy intensive SWRO desalination plants today still consume around 3 kWh/m3. Consequently, conventional SWRO plants are heavily dependent on diminishing and costly fossil fuel resources. In addition, the burning of the fossil fuels results in greenhouse gas emissions, only further contributing to one of the causes of water scarcity – climate change.
_
Current Trends in a Global Desalination Industry:
Large- scale seawater desalination began in the 1960s, using thermal distillation processes such as multi-stage flash (MSF) and multi-effect distillation (MED), which dominated the market until 2000 (Figure below). The membrane-based technology reverse osmosis (RO) was introduced into the market in the 1970s, mainly to treat brackish water. Since the 1980s, advances in membrane technology and materials have made it possible to use RO technology for seawater applications. As a result of this advancement, since 1999, membrane-based technologies, including RO, electrodialysis (ED), and nanofiltration (NF), have become the most dominant technologies for water desalination (Figure below). Since that time, the average growth in desalination capacity throughout the world is about 7.5% per year, of which membrane desalination makes up about two-thirds of the total installed capacity. The total desalination capacity (installed and projected, 2021) is about 115 Mm3/d, of which 77% (~88 Mm3/ d) uses RO technology. In fact, the ratio is likely to change, since most new contracted desalination plants are founded on membrane-based technologies.
_
Figure above shows global desalination capacity with regards to desalination technology and RO source water.
_
Based on the available data, RO is currently the most commonly applied technology. Almost half (53%) of the RO desalinated water is from seawater, and the rest is mainly from brackish, freshwater, and treated wastewater (Figure above). Extra-large seawater RO (SWRO) plants (>50,000 m3/ d) are already in service, comprising approximately 40% of the total installed capacity. The remaining plants are categorized as follows: 24% as large plants (10,000–50,000 m3/d), 15% as medium plants (1000–10,000 m3/d), and 3% as small plants (<1000 m3/d). Most of the extra-large plants are located in the Middle East and East Asia/Pacific.
_
Considering the commercial technologies for desalination, the trends of the installed capacity and number of plants are reported in Figure below. Thermally driven desalination plants (MSF and MED) dominated this sector until the 1990s. As the freshwater demand is still growing, new plants are being installed. MSF continues to grow linearly, while the installed capacity of RO is increasing very quickly.
Figure above shows trend of installed capacity and operative desalination plants and upward trending graph showing the growth of the number of desalination plants as well as increasing desalination.
_
Increasing water scarcity is the major driver. At the same time, if you look at the countries where desalination has tremendously increased, those are the countries that can afford it. Desal, for all its faults, isn’t going anywhere. As it gets cheaper, adoption will continue to grow. Middle Eastern countries full-tilt rely on it, while other regions, like Southern California, use it to supplement traditional—and increasingly unpredictable—sources of water. A plant run by Poseidon Water, for instance, produces 10 percent of San Diego County’s water supply. That is enough water to serve 400,000 residents. This is the only new water supply in the county that is not dependent on snowpack in the Sierras or local rainfall—truly climate-resilient.
Except, that is, for the fact that sea levels are rising due to climate change, which threatens seaside desalination plants the world over. And ironically enough, these facilities are sucking up massive amounts of energy, thus contributing to the emissions problem. From an impact perspective, the energy intensity is huge. Even if powered by renewable energy sources such as solar or wind, you’re still using a tremendous amount of energy, which in principle could go elsewhere to displace fossil fuel consumption. Desalination is not a panacea but it can be a complement to more traditional sources of water like snowpack. And while the efficiency of these plants will improve, this is still a fundamentally energy-ravenous technology. There are theoretical limits to the energy intensity reductions that are possible for seawater desalination. It will never be cheap.
_____
_____
Applications of desalination:
Figure above shows that major applications of desalination is municipal and industrial use worldwide.
_
Desalination of industrial water has increased by around 20% in recent years thanks to its use in the following industries:
- Petroleum
- Gas
- Mining
- Electronics
In fact, there are studies that corroborate that approximately half of all desalination plants produce water for the industrial sectors. Although it should be noted that the municipal sector is undoubtedly the largest user of this service.
Therefore, it is considered that the main uses of desalinated water are the following groups:
-1. Municipal services (human consumption)
-2. Industrial services (In the sectors mentioned above)
-3. Irrigation services
-4. Energy services
Figure below is a map of desalination plants around the world, by size and customer type:
Figure above illustrates the currently installed desalination plants worldwide. It can be clearly seen that a high number of desalination plants have been installed in the Middle East, USA, Australia, China, Central Europe, the Mediterranean Region, and Japan. As indicated on the map, most of the desalination plants are located along the coastline.
_
Applications of RO:
Reverse osmosis is very effective in treating brackish (surface and ground), tap and sea water for both small and large applications.
Some examples of applications:
- Municipal drinking water
- Food and beverage industry
- Agricultural irrigation
- Industrial ultrapure water
- Industrial process water
- Waste water reuse
- Power industry (boiler feed water, cooling towers)
- Municipal/industrial water reuse
- Households
_____
_____
Desalination Technologies Market:
The desalination technologies market size in terms of revenue is valued at US$ 16.87 billion in 2023, and is projected to reach US$ 47.9 billion by 2033-end, growing at double digit CAGR (Compound Annual Growth Rate) of 11% during the forecast period from 2023 to 2033. Desalination technologies encompass a range of processes designed to extract salt, contaminants, and other minerals from water sources. Feedwater origins include seawater, brackish water, as well as groundwater and surface water. Desalination technology plays a crucial role in addressing water scarcity issues in regions where access to freshwater is limited.
_
Growth Drivers of desalination technologies:
-1. Increasing Water Scarcity:
One of the primary drivers for the desalination technologies market is the escalating global water scarcity crisis. Growing populations, urbanization, and industrialization have heightened demand for fresh water, and in many regions, traditional water sources are becoming insufficient. Desalination provides an alternative source of freshwater, especially in arid and water-stressed areas.
-2. Technological Advancements:
Ongoing advancements in desalination technologies have played a crucial role in driving market growth. Research and development efforts have led to improvements in efficiency, cost-effectiveness, and environmental sustainability of desalination processes. Innovations in membrane technology, energy recovery systems, and other components contribute to making desalination more accessible and economically viable.
-3. Government Initiatives and Investments:
Governments around the world are increasingly recognizing the importance of desalination as a strategic solution to water scarcity issues. Many countries have implemented or are planning desalination projects, often backed by substantial investments. Government initiatives, subsidies, and regulatory support contribute to the expansion of desalination infrastructure. For example, in water-stressed regions like the Middle East, governments have invested significantly in desalination plants to secure a stable water supply.
_
Key Trends in Desalination Technologies Industry:
- Innovations in membrane technology are leading to more efficient and selective salt removal, further improving water recovery rates and energy efficiency.
- Water desalination equipment is increasingly being coupled with renewable energy sources such as solar power to reduce environmental impact and operational costs.
- Adoption of IoT (Internet of Things) and AI (Artificial Intelligence) technologies for real-time monitoring and predictive maintenance is enhancing the operational reliability and performance of desalination solutions.
- Demand for reverse osmosis (RO) desalination technologies is estimated to rise at a robust CAGR of 12% over the next 10 years.
_____
_____
Answers to Frequently Asked Questions on desalination:
_
How can water users not located on the coast benefit from desalinated seawater?
Development of seawater desalination along sea coast will help relieve stress on existing conventional surface water and groundwater supply sources in coastal areas which in turn could make these resources available to water users located away from the coast. In this context, seawater desalination can indirectly benefit people living hundreds of miles away from the coastline.
_
What is the average unit cost of desalinated water?
Unit cost of desalinated water is a function of capital cost, debt service, and operating cost. Advancements in reverse osmosis technology have brought desalination costs closer to other alternatives. Ten years ago, desalinated water cost more than $9 per 1000 gallons, but today, the range is $2 to $5 per 1000 gallons. Israel’s world-largest desal water costs about $2 per 1000 gallons and the recently completed 25 MGD Tampa Bay plant produces water at about $3 per 1000 gallons. The cost depends on whether the source water is brackish groundwater or seawater. Brackish water desalination costs less than seawater desalination because it contains less dissolved salts. While there are many variables related to the cost of desalinated water, Texas Water Development Board states a good rule of thumb is $1.10-2.40 per 1,000 gallons for brackish water and $2.46-4.30 per 1,000 gallons for seawater desalination. The total costs also depend on the amount of pre-treatment and post-treatment needed. Because of available grants, subsidies and innovative financing, the costs are not entirely passed to the end user.
_
How long does it take to build a plant, from the permit phase to the final construction phase?
The time required for full implementation of a desalination plant varies from project to project. Obviously, it depends on the size and complexity of the plant, and whether it has to be built from scratch or can use existing water intake structures. Texas does not yet have a seawater desalination plant, but using an example of a large brackish groundwater desalination plant (the El Paso-Fort Bliss plant) that is presently under construction it may take at least 5 years.
_
Which country desalinates the most water?
There are approximately 16,000 operational desalination plants, located across 177 countries, which generate an estimated 95 million m3/day of fresh water as of 2018. Saudi Arabia is the country that relies most on desalination – mostly of seawater. The US is in second place. Per capita SA desalinates more, but the USA desalinates more water overall.
There are about 325 brackish groundwater desalination plants in the United States. Almost half of them (45 percent) are in Florida, 14 percent in California, and 9 percent in Texas (Mickley and others, 2011 and Nicot and others, 2005). The 50-MGD desalination plant at Carlsbad, California is the largest seawater desalination plant in the US and the 25-MGD desalination plant at Tampa Bay, Florida, is the second largest. California has a total of 10 operating seawater desalination facilities, where 6 plants are active and four are not (Cooley, 2016). Of the six active seawater desalination plants, three are used for municipal purposes. California is also proposing about nine desalination plants along the Pacific Coast.
_
Why isn’t desalination the solution to our water problems?
Desalination’s energy-intensive process is expensive and environmentally harmful, making it a costly strategy to bolster regional water supplies. The average price per acre foot of desalinated water is often 2-4 times more expensive than other water sources. Ocean desalination is not efficient. It requires roughly two gallons of ocean water for every one gallon of freshwater produced. This means one large desalination facility is not going to solve regional water supply problems.
_
Are there alternatives to desalination?
Water conservation, water use efficiency, storm water capture and reuse, and recycled water expansion are proven effective strategies to increase regional water supplies and often cost less than desalination. In addition, these alternatives provide pollution abatement, habitat restoration, and flood control benefits, which are commonly overlooked during cost/benefit assessments.
_
What are the advantages and disadvantages of desalination?
Advantages include a reliable and abundant water supply, a solution to freshwater scarcity, diverse application possibilities, and increased independence. Disadvantages involve high energy consumption, negative environmental impacts, high costs, and limited geographical availability.
_
Is desalinated water OK to drink?
Yes, desalinated water is generally safe to drink. It undergoes rigorous purification and is often re-mineralized to ensure it meets drinking water standards. Desalinated water, when properly treated and mineralized, is safe for long-term consumption. However, the absence of natural minerals in desalinated water can be a concern if not properly addressed.
_______
_______
Section-5
History of desalination:
Desalination has been an ongoing process on earth—the natural process of water evaporation from the sea and the condensation in the atmosphere to form rain. The freezing of available seawater near the Polar and Arctic Regions, where different ice crystals are shaped, originates from pure water as salt is excluded from crystal growth. One cannot tell when humanity first became frustrated at its inability to drink salt water and began to consider the matter of salt water purification. However, some of the earliest writings suggest that even in earliest times, it was a matter of some interest.
___
Desalination in Antiquity:
In China, according to the books Huai Nan Wan Bi Shn (c. 200 BC) and Qi Min Shn by Jia Si-Xie (c. AD 540), people would concentrate their wine by immersing the leaves of Guan-Pu grass in it. The leaves were more permeable to and adsorptive of water than alcohol and, therefore, the wine was strengthened (S. Wang, personal communication). In addition, during the same period in China, Guan-Pu leaves were used to concentrate brines to make pickling solution. Similarly, in China, approximately 2000 years ago there were reports of desalting using specially woven bamboo sheeting and earthenware filters. Their method of employment is unclear, but it might have involved the formation of a dynamic gel layer on the “sieving surface” (S. Wang, personal communication). However, other regions were also suggesting desalination by filtration through earthen vessels. Unfortunately, there is no first-hand substantiation of these legends.
_
In the Bible, there is the observation that “for He maketh small the drops of water: they pour down rain according to the vapor thereof; which the clouds do drop and distil upon man abundantly” (Job xx: xx).
In the Bible it is also written that:
and they went three days in the wilderness, and found no water. And when they came to Marah, they could not drink the waters of Marah for they were bitter. … And the people murmured against Moses, saying, What shall we drink? And he cried unto the Lord; and the Lord shewed him a tree, which when he had cast into the waters, the waters were made sweet (Exod. Xx: xx). (This reference to water treatment with vegetable products is similar to but different from that of the Guan-Pu leaves in China. In the biblical instance, the plant material took up the salt rather than the water.)
_
In his Meteorologica, Aristotle (384-322 BC) wrote that “Salt water when it turns into vapour becomes sweet and the vapour does not form salt water again when it condenses” (Forbes 1948). He further speculated that
“Some again believe that the sea is, as it were, the sweat of the earth which it sweats out when the sun heats it: which is the reason why it is salt because sweat is salt. Others suppose that the earth is the cause of its saltiness: just as water strained through ashes becomes salt, so the sea is salt because the earth with this property is mixed with it”- (Nebbia and Menozzi 1966).
And so we see in the above an early consideration of a hydrological cycle.
Aristotle was also among the first of many to report that “If one plunges a water-tight vessel of wax into the ocean, it will hold, after 24 hours, a certain quantity of water, that filtered into it through the waxen walls, and this water will be found to be potable, because the earthy and salty components have been sieved off”. Such a claim was to be reported repeatedly through the ages and was also published by Democritus (c. 500 BC) (Underwood 1935; Nebbia and Menozzi 1966). Although this may suggest a reverse osmosis type of mechanism at work, it is more likely just an attractive myth. Firstly, to overcome the natural osmotic pressure of seawater, such a “waxen vessel” would need to withstand great pressures. Neither a waxen vessel nor an unglazed earthenware vessel, perhaps coated with wax, would be likely to do so. Secondly, such a vessel would need to be lowered to and retrieved reliably from a depth of approximately 500 m. It is doubtful that the technology existed at the time for such an undertaking. Yet the concept was too attractive to be dropped and persisted for centuries.
_______
Dawn of Science:
During the first and second centuries AD, Alexandria was the scene of experimentation on distillation by Maria the Jewess, Cleopatra, and others (Badger 1926). While seawater was not the specific subject of their investigations, the skills and concepts were passed on to subsequent generations. Particularly important was the design of equipment for evaporation and condensation, including the alembic vessel which has come to be one of the more popular “logos” of chemistry. Alexandria was also the home of Hero whose steam-powered “aeropile”, while only a toy, demonstrated the potential of steam as a useful tool.
_
Alexander of Aphrodisias commented c. AD 200 that “sailors at sea boil sea water and suspend large sponges from the mouth of a bronze vessel to imbibe what is evaporated. In drawing this off the sponge, they find it to be sweet water” (Forbes 1948).
Figure above shows Condensing Steam in Sponges as sailors producing freshwater with seawater distillation. This method of collection of steam also appears in the “steaming cotton-padded jacket” approach reportedly (S. Wang, personal communication) used hundreds of years ago in China.
Alexander further built upon the hydrological cycle suggested by Aristotle by stating in his Natural Problems that “Some say that the origins of the saltiness of the sea is the earth itself. The water, in fact, running through the ground, takes its same properties” (Nebbia and Menozzi 1966). St Basil, writing in the fourth century, commented upon having been shipwrecked on a desert island and being saved by the seamen placing sponges over pots of boiling seawater to condense and collect the steam (Huber 1898). (This is the first time that the method of the fleeces or sponges is supported by an eyewitness account.)
_
Before the Industrial Revolution, desalination was primarily of concern to oceangoing ships, which otherwise needed to keep on board supplies of fresh water. Sir Richard Hawkins (1562-1622), who made extensive travels in the South Seas, reported in his return that he had been able to supply his men with fresh water by means of shipboard distillation. Additionally, during the early 1600s, several prominent figures of the era such as Francis Bacon or Walter Raleigh published reports on water desalination. These reports and others, set the climate for the first patent dispute concerning desalination apparatus. The two first patents regarding water desalination date back to 1675 and 1683 (patents No.184 and No. 226, published by Mr. William Walcot and Mr. Robert Fitzgerald (and others), respectively). Nevertheless, neither of the two inventions was really put into service as a consequence of technical problems derived from scale-up difficulties. No significant improvements to the basic seawater distillation process were made for some time during the 150 years from the mid-1600s until 1800.
_
When the frigate Protector was sold to Denmark in the 1780s (as the ship Hussaren) the desalination plant was studied and recorded in great detail. In the newly formed United States, Thomas Jefferson catalogued heat-based methods going back to the 1500s, and formulated practical advice that was publicized to all U.S. ships on the backs of sailing clearance permits. Beginning about 1800, things started changing very rapidly as consequence of the appearance of the steam engine and the so-called age of steam. The development of a knowledge of the thermodynamics of steam processes and the need for a pure water source for its use in boilers, generated a positive effect regarding distilling systems. Additionally, the spread of European colonialism induced a need for freshwater in remote parts of the world, thus creating the appropriate climate for water desalination. In parallel with the development and improvement of systems using steam (multiple-effect evaporators), this type of devices quickly demonstrated their potential in the field of desalination.
_
In 1852, Alphonse René le Mire de Normandy, was issued a British patent for a vertical tube seawater distilling unit which thanks to its simplicity of design and ease of construction, very quickly gained popularity for shipboard use. Land-based desalting units did not significantly appear until the later half of the nineteenth century. In the 1860s, the US Army purchased three Normandy evaporators, each rated at 7000 gallons/day and installed them on the islands of Key West and Dry Tortugas. Another important land-based desalter plant was installed at Suakin during the 1880s which was able to provide freshwater to the British troops placed there. It consisted of six-effect distillers with a capacity of 350 tons/day.
_
Significant research into improved desalination methods occurred in the United States after World War II. The Office of Saline Water was created in the United States Department of the Interior in 1955 in accordance with the Saline Water Conversion Act of 1952. This act was motivated by a water shortage in California and inland western United States. The Department of the Interior allocated resources including research grants, expert personnel, patent data, and land for experiments in order to further advancements in desalination. The results of these efforts were manifold, including the construction of over 200 electrodialysis and distillation plants globally, promising reverse osmosis research, and international cooperation on the cause (for example, the First International Water Desalination Symposium and Exposition in 1965). The Office of Saline Water was eventually merged into the Office of Water Resources Research in 1974. The first industrial desalination plant in the United States opened in Freeport, Texas in 1961 with the hope of bringing water security to the region after a decade of drought. Vice-president Lyndon B. Johnson attended the plant’s opening on June 21, 1961. President John F. Kennedy recorded a speech from the White House, describing desalination as “a work that in many ways is more important than any other scientific enterprise in which this country is now engaged.”
______
The current situation of seawater desalination:
At present, the desalination industry and devices have covered more than 150 countries and regions in the world, such as Saudi Arabia, Oman, UAE, Spain, Cyprus, Malta, Gibraltar, Cape Verde, Portugal, Greece, Italy, India, China, Japan and Australia.
The Middle East is one of the regions in the world with severe water scarcity, where the climate is hot, precipitation is scarce and the land is arid, but the local oil resources are abundant and the economy is strong, so there is an urgent need for desalination technology and devices. It has the economic basis for the development of desalination industrialization. Only this region of Saudi Arabia, the United Arab Emirates, Kuwait, Qatar, and Bahrain five countries desalination device total water production is accounting for 44.3% of the global total; and in the coastal areas of the Persian Gulf, some countries have desalinated seawater volume accounted for 80-90% of the country’s freshwater use.
In contrast, in the birthplace of the world’s first modern desalination plant in the United States, the total amount of desalinated water accounts for only 18% of the global total, and 70% of Israel’s water comes from desalination.
Saudi Arabia’s annual rainfall is only 100mm, half of its land area is desert, and the per capita possession of water resources is only 1.2% of the world average. The Saudi government, in order to solve the serious water shortage problem, has invested heavily to build 25 large desalination plants.
_
Renewable energy has become a viable option from the practical side to replace conventional energy resources. As such, over the years, it has secured a more significant portion of the energy market. In fact, from 2007 onward, renewable energy resources showed a steady monotonic upward trend, to the point that in 2019, they accounted for 26.49% of the world’s total electricity generation (IEA 2021). This ongoing development in the energy market landscape could profoundly impact the desalination industry, as more countries could turn to this more environmentally friendly resource to fuel the desalination process. Saudi Arabia, for instance, made a conscious attempt to move toward powering desalination plants with renewable energy resources. In that spirit, they commissioned the construction of the world’s first full-size solar-powered seawater desalination plant in 2015. Australia is another notable example that has been working on a pilot case in Garden Island, south of Perth in Western Australia, to use a wave energy system for powering a desalination plant (Palmer 2015). From the environmental perspective, this switch could have huge implications as it can indicate a potential reduction in this practice’s greenhouse gas (GHG) emissions.
_
The advancement of desalination technology is another important factor that could ultimately shape the future of this industry. For the most part, however, this has not been a drastic and fast change, as the core technology in most modern desalination plants has stayed relatively similar since the late 1960s. In fact, most progress in the desalination industry can be described as a steady shift toward upgrading outdated desalination plants with state-of-the-art technologies such as RO desalination units. As of now, thermal desalination is the most common approach in the Middle East region, where energy prices are relatively reasonable (Kucera 2019). RO units, however, are expected to become the standard desalination practice in the future (Tan et al. 2022), which, as a more energy-efficient technology, can be seen as a making a positive contribution to global energy consumption. In the meantime, energy recovery devices (e.g., isobaric chambers and positive-displace pumps) and regular maintenance can be viable, practical options to maintain or even decrease the energy consumption and, in turn, offset the overall cost of desalination (Leon et al. 2021). Incorporating stem technologies, such as nanotechnology and nanocomposite membrane, is also a promising future direction, though thus far, scaling up these technologies to generate a commercially viable flux still pose some practical challenges (Palmer 2015; Kucera 2019).
_______
_______
Section-6
Technology of desalination:
In general, a desalination plant includes different processes to obtain freshwater, among which the desalination unit is the most energy expensive component. A desalination plant normally includes:
- Intake, composed by pumps and pipes to take water from the source (sea or brackish water)
- Pre- treatment, consisting of the filtration of raw water to remove solid components and the addition of chemical substances to reduce the salt’s precipitation and the corrosion inside the desalination unit
- Desalination, where freshwater is extracted from saltwater
- Post-treatment, to correct pH by adding selected salts to meet the requirements of the final uses.
Before analyzing the specific solutions, a classification is required. Alkaisi suggested three main categories: Evaporation and Condensation, Filtration and Crystallization.
______
Pretreatment:
Pretreatment is generally required for all desalination processes. Pretreatment ensures that constituents in the source water do not reduce the performance of the desalination facility. Thermal processes require pretreatment to avoid scaling and to control corrosive constituents of the source water. Some removal of sand or gritlike suspended solids may also be necessary to avoid pipe erosion. In membrane desalination, pretreatment involves these considerations as well as further pretreatment to remove suspended solids of both biological and mineral origin to avoid membrane fouling. Biological growth may need to be inhibited by a disinfectant or biocide.
_
Thermal systems like MSF and MED tend to need much less pre-treatment than other systems, often only screening and chemical treatment (adding chlorine, antiscalant and antifoam) is required. Chlorination is typically used to avoid biological fouling of the desalination step. Where chlorine intolerant membranes are used, dechlorination may be essential in order to avoid oxidation of the membrane. Dechlorination is not normally required with thermal systems.
RO pre-treatment technologies include conventional (e.g., coagulation-flocculation, media filtration, disinfection, scale inhibition) and non-conventional (e.g., MF, UF, and NF). As per the available literature, UF, MF and coagulation-flocculation are considered the most widely used pre-treatment technologies.
_
The major cause of performance deterioration in distillation and RO processes is the deposition of materials on heat exchanger (Hx) and RO membrane surfaces. The fouling of Hx and RO membrane is a complex phenomenon involving the deposition of several different but related types of foulants on the surfaces. The fouling problem in these processes is becoming more important as the use of lower quality feed water increases. Membrane and Hx fouling via deposits results in decreased production, unscheduled shutdowns, poor product water quality, and premature equipment failure. The fouling of Hx and RO surfaces has a pronounced effect on the cost of the produced water. If the fouling of an RO membrane is allowed to go unchecked, the membrane may become irreversibly damaged which will necessitate replacement. According to Graham membranes can account for approximately 20 % of the installed costs of a typical brackish water plant and 30 % of a sea water plant. This is significant enough to compel the operator to maintain the membranes in their best condition. The heat exchangers in a distillation system can usually be cleaned more rigorously when they foul, but the economic penalty for operating a fouled heat exchanger may be greater. This again suggests that vigilance is always warranted.
_
Fouling:
Membrane fouling represents a serious challenge in RO processes due to its significant contribution to energy requirements and process economy (e.g., flux decline, permeate quality, membrane lifespan, increased feed pressure, increased pre-treatment and membrane maintenance cost). Membrane fouling types include colloidal, organic, inorganic, and biological fouling.
Seawater contains suspended particles, natural organic matter, mono- and multivalent ions, microorganisms, and organic and inorganic colloids. Some of these constituents block the pores of the RO membranes, also known as fouling, rendering them inefficient after short operation times. Colloidal, particulate, organic or biological fouling (biofouling) as well as scaling occurs very easily during desalination using RO membranes. It is essential to remove the foulants to prevent the failure of the RO processes.
Bio-fouling:
Biofouling —the process by which organisms colonize all forms of submerged substrata—negatively affects materials and structures and can even destroy man-made installations. Such microorganisms, mainly bacteria, fungi, and diatoms, rapidly foul the RO membranes and create a sudden increase of differential pressure by restricting water flux, which ultimately impairs the salt rejection process, an issue which costs billions of US dollars to the desalination industry to address and prevent. The purpose of disinfection in RO desalination is to prevent the colonization of microbes at the surface of membranes.
Membrane biofouling occurs when bacteria from seawater sticks to the RO membrane and forms a layer of biofilm, reducing water flow. As biofilm clogs the pores on the membrane, more pressure is required for water to pass through, reducing production efficiency and raising operating costs. Once the membrane is severely clogged, it needs to be cleaned or replaced—both time-consuming and costly operations. It’s not only about the cost of the membrane or the cost of operation. There’s also an environmental impact. To clean the biofilm, you need to use cleaning chemicals, which enter the membrane system and are discharged back into the sea, potentially harming the marine environment. The best way to minimize biofilm from forming in the first place is to reduce the amount of biomass and nutrients in seawater entering the RO filter. This can be done by improving pretreatment procedures.
_
Pretreatment is a critical step in seawater and brackish water membrane desalination systems that utilize feedwater from surface water sources, because the suspended and colloidal particles, organisms, and natural organic matter need to be removed before the feedwater reaches the membranes. Indeed, proper pretreatment of feedwater is the most important factor in the successful operation of an RO plant, and pilot testing of the pretreatment process is a critical part of plant design.
Brackish water desalination systems that treat groundwater require very minimal, if any, pretreatment to remove particulates because the water typically contains very low concentrations of suspended solids and organic matter. Nevertheless, brackish groundwater may require pretreatment to remove selected constituents such as dissolved iron, manganese, and sulfides, which, if oxidized, create particulates that can foul RO membranes (USBR, 2003).
_
The quality of source water available at a particular site will also affect the extent of pretreatment needed for membrane desalination. Source water quality will depend on local site factors such as source water depth, turbidity, boat traffic, oil contamination, nearby outfalls, wind conditions, tides, and the influence of runoff. Subsurface seawater intakes, aquatic filter barriers, and deep ocean water intakes can greatly reduce the need for pretreatment. Due to permitting regulations and available land, however, desalination plants cannot always be sited where they will have the lowest pretreatment costs. The most common pretreatment processes are discussed below.
_
Scaling and Corrosion Control:
Scaling is caused by the precipitation of minerals, such as calcium carbonate, from solution. Calcium sulfate scaling can be controlled via temperature control or through pretreatment by nanofiltration to remove the calcium ions. Acidification of the feedwater can prevent calcium carbonate or magnesium hydroxide formation and scaling. Finally, the use of chemical antiscalants such as sodium hexametaphosphate or polymeric acids can sequester the cations that can lead to scaling problems.
Corrosion can be reduced by removing corrosive gases in pretreatment. Carbon dioxide can be controlled through acidification, and oxygen can be controlled with an oxygen scavenger such as sodium bisulfate or ferrous sulfate. Alternatively, corrosion can be controlled in some systems through the formation of a protective film within the system by adding zinc orthophosphate.
_
Conventional Solids Removal Methods:
Conventional solids removal methods such as coagulation and sedimentation followed by media filtration are still the predominant pretreatment processes for seawater RO. Chemicals such as ferric chloride or polyelectrolytes are added to enhance the coagulation of suspended solids prior to settling and filtration. Traditional gravity flow filtration has been successfully used at many seawater RO plants around the world. At Point Lisas, Trinidad, gravity filters with greater-than normal depth proved to be successful in pretreating seawater that encounters severe spikes in turbidity due to the intake location in a ship turning basin (Jacangelo and Grounds, 2004). These are mature technologies, although novel approaches to conventional filtration continue to be examined. For example, at Tampa Bay, an upflow dual sand process was installed that had previously only been used for industrial and wastewater applications. Nevertheless, there is a need to improve the quality and stability of influent to RO membranes; thus, other pretreatment options continue to emerge.
_
Microfiltration and Ultrafiltration:
Microfiltration (MF) and ultrafiltration (UF) membranes are increasingly being used in the pretreatment processes for membrane desalination. Water molecules and salts are free to pass through, and water is pushed (or pulled) through the membrane at very low pressures. Particles larger than the membrane pore size (0.03-10 µm for MF and 0.002-0.1 µm for UF) are removed. Membranes are commercially available in flatsheet, tubular, hollow-fiber, and spirally wound configurations. Among the benefits of MF/UF pretreatment compared to conventional pretreatment technologies are (1) production of feedwater to the RO system of constant and high quality regardless of source water fluctuations; (2) reduced RO fouling, which results in less cleaning and longer membrane life; (3) smaller footprint; and (4) lower consumption of chemicals. Potential disadvantages include higher costs and negative environmental impacts of concentrate from these membranes. MF and UF membrane processes have been developed and piloted for application in seawater pretreatment.
_
Biofouling Control:
Chlorine or hypochlorite have been the standard oxidants used for biofouling control, but thin-film composite polyamide membranes commonly used in RO desalination cannot tolerate oxidants like chlorine, and the chlorine needs to be removed in pretreatment by addition of a reducing agent such as sodium bisulfite. Sodium bisulfite and copper sulfate can also be used as biocides in membrane systems.
Ultraviolet (UV) and ozone treatment are being considered potential replacements for chlorine-based biological growth control of RO feedwater. Both UV and ozone have merit and have been successfully used in small to midsized drinking water and water reuse applications globally. UV will not cause problems with oxidant-sensitive membranes. Ozone is a much more effective disinfectant, but it poses a problem to the oxidant sensitive RO membranes.
_
Post-treatment:
Distillation results in a product water with very low mineral content. This water can be aggressive (causing corrosion to metal pipes) and would not be considered potable. Remineralisation is therefore conducted to stabilise the water. Even though the pore size may be much smaller than the membranes typically used for disinfection, Reverse Osmosis (RO) is not generally considered to be a disinfection step, as significant quantities of water can pass the membrane. It is therefore normal to have a separate step of primary disinfection following reverse osmosis. Even where the desalination step results in completely sterile water, it would also be normal to add a residual disinfectant in order to avoid recontamination in distribution.
_
Role of Bacteria Filters:
If the desalination system is equipped with a chemical treatment system for the water produced, then bacteria filtration may not be needed as part of the system. If the system has no chlorination, ozonation or similar process then the water is stored and distributed with no chemical protection against bacteria. Any organisms that enter the system will be viable and may be distributed downstream to all users. Many waterborne organisms form biofilms which, once established, are extremely difficult to remove. Some systems may be designed and operated with periodic chemical or heat sanitization processes, which can inhibit the formation of biofilms. However, bacteria can still enter through open tank vents, open distribution lines or ‘dead legs’ and move downstream, possibly interfering with the intended use of the water. In such situations ‘bacteria removal’ filter can be used. Removing bacteria before the water is moved very far downstream reduces the opportunity for biofilm formation.
______
______
Classification of desalination technologies:
Different desalination processes have been developed, some of them are at present under research and development. The two major technologies that are mainly used for desalination are
- Thermal desalination technology
- Membrane desalination technology
Both the technologies include a number of different processes, apart from these there are alternative technologies like freezing and ion exchange which are not generally used. All these technologies need energy to operate. Conventional energy or renewable energy is generally used in these methods.
_
Thermal desalination processes:
It is generally known as distillation. It is one of the most ancient ways of desalinating sea water and converting them to drinking water. This technology is rarely used for desalinating brackish water since it is expensive. This technology is based on principles of boiling the saline water and evaporating it and then collecting the condensed vapour to obtain pure water. The salt is left behind and the distillate is collected.
The thermal desalination processes are subdivided into the following types
- Multi-stage flash distillation (MSF)
- Multi-effect distillation (MED)
- Vapour compression evaporation (VC)
- Cogeneration
- Solar water desalination
_
Membrane processes:
Initially membrane applications were confined to municipal water treatment such as microfiltration and desalination but due to advancements in technology and development of new membranes, it is used not only for water purification but also in chemical separations, concentration of enzymes and purification of beverages.
Membrane processes uses a relatively permeable membrane to move either water or salt to produce two zones of differing concentrations to produce fresh water. These processes are also useful in municipal water treatment. Reverse osmosis and electro dialysis (ED) are replacing other phase change desalting technologies for supplying water to coastal and island communities all over the world. RO is emerging as an economical alternative to the traditional water softening processes.
Membrane technology consists of several processes, but the major difference between them lies in the size of the ions, molecules and suspended particles that are retained or allowed to pass via the membranes. Major separation processes include nano-filtration, ultra-filtration, microfiltration and filtration used in the pre-treatment stages of desalination that are used to remove large particles, bacteria, ions and for water softening.
The membrane processes are further categorized into
- Reverse osmosis
- Electro dialysis
- Membrane distillation
____
Reverse osmosis (RO), Forward osmosis (FO), multi-effect distillation (MED), multi-stage flash distillation (MSF), Vapor-compression (VC), Ion exchange, Membrane processes, Electro-dialysis (ED), Capacitive Deionization (CDI), Nano-filtration (NF), Membrane distillation (MD), Hydration (HY), Secondary Refrigerant Freezing (SRF), Solar Still Distillation (SSD), and Solar Chimney (SC) are all processes used in water desalination plants. Many cogeneration facilities where the thermal energy needed to desalinate water are also used to generate electricity. The most common method for pumping brackish water through membranes while utilizing electrical energy is reverse osmosis.
_
Figure below shows an upgrade of the classification proposed by Alkaisi, integrating the new technologies currently under investigation.
Figure above shows classification of desalination technologies by working principle.
_
Evaporation and Condensation technologies are the first desalination techniques to be historically introduced and used for civil freshwater production. The idea is to supply thermal energy to seawater, producing a vapor, and then condensate it. This energy can be generated by using the heat from a thermal process (for example, waste heat or fuel combustion), or through a mechanical process. In the first case, the most common technologies are MED, MSF and Thermal Vapor Compression (TVC). Currently other approaches are under investigation, and among these we can find few new solutions supplied by solar radiation: Solar Still Distillation (SSD), Solar Chimney (SC) and Humidification-Dehumidification (HDH) desalination.
Regarding the mechanical processes used to produce freshwater through the evaporation and condensation of seawater, the main technique is Mechanical Vapor Compression (MVC).
In case of filtration technologies, all solutions are essentially based on a semipermeable membrane, i.e., a layer that shows a different mode of crossing behavior according to the sizes or nature of molecules. The only exception is Ion-exchange resins (IXR), where natural or artificial materials are used to capture the dissolved ions in a chemical way.
In this context RO is the most used technology for desalination. The Electrodialysis (ED) and Ion Exchange Resin (IXR) are used to produce water with a very limited concentration of salts. Other techniques, as Forward Osmosis (FO), Nano Filtration (NF) and Capacitive Deionization (CDI) are in the development stage.
Finally, the Crystallization category comprises techniques that extract freshwater producing ice as intermediate product. As example, the main techniques are Secondary Refrigerant Freezing (SRF), Hydration (HY) and Vacuum Freezing (VF) desalination. All these approaches are under investigation.
_
Thermal- based procedures like MED, multi-stage flash distillation (MSF), and MVC need a lot more energy than membrane methods like forward osmosis (FO), RO, and electrodialysis (ED). While salt content affects the energy requirements for membrane processes, salt concentration has no impact on the energy needs for thermal desalination systems. The substantially lower energy requirements of membrane technologies versus thermal ones are probably their most well-known benefit. While MSF uses between 10 and 16 (kWh/m3) and MED uses between 5.5 and 9 (kWh/m3), RO energy requirements remain at 3 to 4 (kWh/m3) for seawater or decrease to 0.5 to 2.5 (kWh/m3) for brackish water.
When pressure is applied to the saltwater feed solution during the desalination process that is larger than just its osmotic pressure or the minimal pressure that prohibits the intake of pure water by osmosis, water is ejected through a semipermeable membrane. RO is not advised for desalinating highly concentrated salt solutions because increasing osmotic pressure necessitates very high pump pressure. An alternative desalination method called membrane distillation, which combines heat and membrane processes, is more suited for working with extremely salty solutions, especially for solutions having salinity between 70 and 300 g of salt per kilogram of solution.
_______
_______
Thermal based distillation techniques for desalination:
Distillation is a thermal process, in which the sea water is converted to pure water by evaporation and condensation. Distillation is one of mankind’s earliest forms of separating fresh water from a salt-water solution. When salt water is boiled, the dissolved salt remains behind as the fresh water vapor is boiled away. In a distillation process, water is first boiled and then the steam, or water vapor, is cooled. This cooling condenses the steam into water again. Thus, distillation involves adding heat energy to salt water in order to vaporize the water and then removing the heat energy from the steam to condense it into fresh water. In nature, this basic process is responsible for the hydrologic cycle. The sun causes water to evaporate from surface sources such as lakes, oceans, and streams. The water vapor eventually comes in contact with cooler air, where it re-condenses to form dew or rain. This process can be imitated artificially, and more rapidly than in nature, using alternative sources of heating and cooling.
_
Distillation is a phase separation method. Heat is added to seawater, so the water vaporizes; but the salt, which has a different boiling point, doesn’t. When this water in its gas phase returns to its liquid phase through condensation, all that’s left is pure H2O. Generating the heat to boil seawater requires much more energy than drawing water from a well or stream or collecting rainwater. That’s why for most of human history, thermal distillation of seawater was only a last resort that took place at a small scale, as when ancient mariners boiled pots of seawater and used sponges to collect the condensed steam. However, after the industrial revolution, when oceangoing ships began to use steam for power, things started to change. Throughout the 1800s marine distillation became increasingly widespread and more energy-efficient through a series of inventions, including multiple-effect evaporators, that are still in use today. Large-scale, land-based thermal distillation began in 1928 when a 60 m3/ day plant using multiple-effect distillation (MED) was installed on Curaçao. Throughout the rest of the 1900s, hundreds of other thermal distillation desalination plants were built around the world. Despite their relative energy intensity, many of these first-generation desalination plants are still in use today. Engineers made great strides in improving the energy efficiency of thermal distillation throughout the 20th century.
_
When water is heated, its temperature increases until the boiling point is reached. While water is boiling, the steam and the boiling water are at the same temperature. However, raising water to its boiling point is not enough to cause it to boil. More heat must be added to change the water into steam. The amount of heat required to change water at its boiling point into steam at the same temperature is called heat of vaporization of water. The heat of vaporization is of major importance in distillation. The amount of heat required to vaporize water into steam is approximately five times greater than the heat needed to raise water from its freezing point to its boiling point (at ordinary sea-level atmospheric pressure (14.7 psi) water boils at 100 deg C). The heat of vaporization of water is about 2,260 kJ/kg.
_
Distillation under reduced pressure is used to purify a liquid that has a tendency to decompose when heated to a high temperature. Under the conditions of reduced pressure, the liquid will boil at a temperature lower than its boiling point, and as a result, the liquids will not degrade as they would otherwise. The various distillation processes used to produce potable water, including MSF, MED, VC, and waste-heat evaporators, all generally operate on the principle of reducing the atmospheric pressure within the unit to permit boiling to occur at lower temperatures, without the use of additional heat.
_
Distillation is a two-step process involving both evaporation and condensation, heat must be added in one step and removed in the other. If these two steps were accomplished independently, the process would be inefficient and costly. In all the distillation processes, the steam is condensed by transferring heat from the steam to salt water as part of the heat source required to convert more water into steam. In this way some of the heat energy used in one step is recovered and used in the other step.
_
Three types of distillation used for desalination are as follows.
In multi-stage flash distillation, there are three sections namely heating (brine heater), heat rejection and heat recovery (flash chambers). The seawater gets pre-heated in the condensing coils of the flash chambers before entering into the brine heater where it is actually heated. Simultaneously, the condensing coils preheat the seawater and condense the flashed steam to produce pure water. After reaching the brine heater, the water is boiled at 70-110°C and enters the flash chambers where the flashing (sudden evaporation) takes place. While the hot brine entering the flash chambers, the vapour pressure of each flash chamber must be controlled in order to avoid violent evaporation. The vapour rises to the upper part and condenses to form pure water. The brine gets deposited in the bottom of the chamber and the remaining brine enters the next chamber where the process repeats again.
Multiple effect distillation (MED) consists of multiple chambers (Effect). The seawater feed is preheated before sending into the first effect. In the first effect, the feed is sprayed on the evaporator tubes and where a part it gets converted into hot vapour. This hot vapour is fed into the second effect which acts as a heating medium for the evaporation process. The remaining feed that is not vapourised gets settled at the bottom of the effect and transferred to next effect for the process to repeat again.
Vapour compression distillation consists of an evaporator, a condenser and a compressor (Mechanical or Thermal). Seawater is sprayed on the evaporator tube, which gets evaporated. The water vapour after evaporation gets compressed by the compressor, mechanical or steam jet. This compressed air acts as a heating source for the incoming seawater to vaporize and condenses inside the tubes to get pure water (distillate). The seawater that is not evaporated gets collected at the bottom of the vessel and recirculated.
_
Distillation becomes a key source of freshwater for most of the world’s regions. Because of the ample water, the distillation procedure is mostly considered in coastal places. The most significant feature of this procedure is that it is completely safe for everyone involved—in other words, it has no negative consequences for the environment. According to a survey conducted in the preceding decade, roughly 75 million people around the world rely on distillation for their everyday requirements. Distillation is the only source of freshwater for many countries.
_
Distillation has various advantages such as (i) the capacity to take care of a wide range of feed flow rate range, meaning they can handle high and low flow rates contrary to some alternative techniques. For example, facultative, stabilization, oxidation, and maturation ponds all require a high flow rate of feed; (ii) it can remove various and lots of substances from feed concentrations. Numerous alternative treatments have different stages or include varied chemicals for a particular impurity removal. For example, alum is used mainly to reduce solids through coagulation and chlorine is used only for the elimination of pathogens; so, it cannot remove suspended solids or other impurities; (iii) it can produce water of very high quality (pure); this is contrary to other techniques that partially treat or only reduce the impurity level of the feed. Distillation is a very well-known technique for purification because of its robustness and versatility. One of the major issues with distillation in desalination is the high energy demand for the process.
_
Advantages:
-1. Distillation offers significant savings in operational and maintenance costs compared with other desalination technologies.
-2. In most cases, distillation does not require the addition of chemicals or water softening agents to pretreat feedwater.
-3. Low temperature distillation plants are energy-efficient and cost-effective to operate.
-4. Many plants are fully automated and require a limited number of personnel to operate.
-5. Distillation has minimal environmental impacts, although brine disposal must be considered in the plant design.
-6. The technology produces high-quality water, in some cases having less than 10 mg/1 of total dissolved solids.
-7. Distillation can be combined with other processes, such as using heat energy from an electric-power generation plant.
Disadvantages:
-1. Some distillation processes are energy-intensive, particularly the large-capacity plants.
-2. Disposal of the brine is a problem in many regions.
-3. The distillation process, particularly MSF distillation, is very costly.
-4. Distillation requires a high level of technical knowledge to design and operate.
-5. The technology requires the use of chemical products, such as acids, that need special handling.
_____
_____
Membrane based techniques for desalination:
Membrane desalination is the process by which salt and minerals are removed from water solution when it passes through a semipermeable membrane. They can be divided further into the following major subcategories:
-1. Reverse osmosis (RO) and
-2. Electrodialysis (ED)
Reverse osmosis:
Osmosis is the process by which water flows through a semipermeable membrane from a dilute solution to a more concentrated solution. The flow continues until the resulting osmotic head is equal to the osmotic pressure of the solution. RO happens when a pressure (typically 55–82 bar for seawater) higher than the natural osmotic pressure of the solution is applied to it. The direction of the water flow is then reversed and the solution becomes more concentrated. Purified water is obtained on the other side of the membrane. RO accounts for the other half of the desalination market and consumes about 3-5 kWh/m3 of electrical energy. Typically the feed seawater is pretreated to sterilize, filter, prevent scaling and biofouling, and heated to increase the efficacy of production. Two types of RO membranes commonly used commercially are spiral wound (SW) membranes and hollow-fiber (HF) membranes. The distillate is also posttreated to sterilize, stabilize, and mineral-enrich. The efficiency of the RO can be greatly enhanced if the reject brine pressure is recovered by a turbine, which may save of up to 40% of the consumed energy. RO units are considered more complex to operate but with lower capital cost than thermal units and sensitive pretreatment requirements.
Electrodialysis:
ED is similar to ion exchange by which ions present in water would be attracted to electrodes with an opposite charge. However, ED is different in using selective membranes that allow either anions or cations (but not both) to pass when placed between a pair of electrodes. Like RO, ED requires a pump to push water through membranes but is generally more expensive. However, since ED is more resistant to membrane fouling, cost associated with their replacement, and cleaning can reduce overall cost.
______
Membrane distillation:
Membrane distillation is a thermal based membrane-separation process. In this process, the membrane is porous and hydrophobic in nature, which allows the vapour to pass through the membrane. The temperature difference induces a vapour pressure gradient across the membrane that acts as a driving force for the membrane distillation process. It is a cost-effective process. The mechanism of membrane distillation was carried out by Knudsen diffusion and molecular diffusion principles. The Membrane Distillation process is formation of vapour gap (Hot-side) at the feed membrane interface, flow of vapours through pores and at the membrane-permeate interface (Cold side) condensation of vapour.
Pervaporation:
Pervaporation is a membrane separation process in which the feed solution is in direct contact with one side of the membrane and the vapour permeate is collected from another side of the membrane and then condensed. The chemical potential gradient acts as a driving force for the transfer of permeate from the feed stream to permeate stream of the membrane. The membranes act as a selective barrier for separation, which must be dense and hydrophilic in case of polymeric material and molecularly porous for inorganic material. The mechanism of pervaporation is based on solubility and diffusivity of membrane material.
______
______
The major membrane types that can be used for desalination and/or pretreatment are depicted below:
Microfiltration (MF)
The pore size on microfiltration membranes ranges from 0.1 – 5 um, and has the largest pore size of the four main membrane types. Its pores are large enough to filter out such things as bacteria, blood cells, flour, talc and many other kinds of fine dust in solution. Because its pores are relatively large compared to other membranes, it can be operated under low pressures and therefore low energy.
Ultrafiltration (UF)
Ultrafiltration has a pore size range of 0.1um to 0.01um. UF membranes reject particles such as silica, viruses, endotoxins, proteins, plastics and smog/fumes such as ZnO. Due to the decrease in pore size, the osmotic pressure required is higher than that of MF.
Nanofiltration (NF)
Nanofiltration has a pore size range of 0.001-0.01um. NF membranes can filter particles up to and including some salts, synthetic dies and sugars, however it is unable to remove most aqueous salts and metallic ions, as such, NF is generally confined to specialist uses.
Reverse Osmosis (RO)
Reverse Osmosis has a pore size range of 0.0001 – 0.001 um. It is by far the finest separation material available to industry. It is used on a large scale for the desalination and purification of water as it filters out everything but water molecules, with pore sizes approaching the radius of some atoms in many cases. This pore size means it is the only membrane that can reliably filter out salt and metallic ions from water. The small pore size of RO membranes means that a significant amount of osmotic pressure is required to force filtration.
_
As shown in Figure below, filtration technologies are classified according to the size of the particles and molecules that are stopped by the membrane.
Figure above shows Filtration technologies by required gradient pressure and porous size.
_
Various membrane techniques and their general characteristics in their applications for water treatment and desalination are shown in Table below:
|
Membrane Type |
Particle Capture Size |
Typical Contaminants Removed |
Typical Operation Pressure Ranges |
Key Applications |
|
Microfiltration |
0.1–10 µm |
Suspended solids, bacteria, and protozoa |
0.1–2 bar |
Water treatment plants, pretreatment in desalination plants, the preparation of sterile water for industries, such as pharmaceuticals, etc. |
|
Ultrafiltration |
0.003–0.1 µm |
Colloids, proteins, polysaccharides, most bacteria, viruses (partially) |
1–5 bar (cross-flow) 0.2–0.3 bar (dead-end |
Drinking water treatment, the pretreatment process in desalination, and membrane bioreactors |
|
Nanofiltration |
<0.001 µm |
Viruses, natural organic matter, multivalent ions (including hardness in water) |
5–20 bar |
Treatment of fresh, process and wastewaters |
|
Reverse osmosis |
<0.0001 µm |
Almost all impurities, including monovalent ions |
10–100 bar |
Treatment of fresh, process and wastewaters, desalination of seawater and blackish water |
Note:
1 micrometer (μm) = 1000 nanometers.
A bar is a pressure unit defined as 100 kilopascals or 0.1 MPa. This makes one atmosphere nearly equal to one bar, specifically: 1 atm = 1.01325 bar.
_
Figure below shows substances and contaminants nominally removed by pressure driven membrane processes:
_____
_____
Emergence of Membrane Technology:
Membrane technologies arose as a result of a breakthrough in the use of polymer films for separating salt from water in the late 1950s/early 1960s. A brief history of the development of RO membranes is shown in Figure below.
_
Reid and Breton (1959) first demonstrated the possibility of desalination using polymeric cellulose films, and thus the first polymeric RO membranes were created. Loeb and Sourirajan (1963) then showed that an asymmetric cellulose acetate membrane can be used for desalination. The permeabilities of these early membranes were low, and RO membranes were considered a novelty separation technique rather than a solution to desalination.
_
An innovation in the packaging of large membrane areas into small volumes was the development of the spiral wound module (Figure below) by General Atomics in 1963. The spiral wound configuration is now common in RO applications. In this module, “leaves” of membranes, with feed and permeate spacers, are connected to a perforated permeate tube and rolled up in a “jelly roll” configuration. Hollow-fiber modules containing thin fibers were developed a few years later by DuPont, but this configuration is less commonly used for RO.
_
A major advance in membrane chemistry that has made possible the application of RO membranes is the development of the thin film composite (TFC) architecture. Previously, membranes were either several-micron-thick polymer layers with a uniform architecture or similar-size polymer layers with an “asymmetric” structure with a nonporous salt-rejecting top surface opening up to a more porous support. Cadotte (1981) patented the design for the three-layer TFC membrane that is now the industry standard. It provides high permeability while maintaining selectivity for water (vs. salt or other solutes). His major innovation was to make the crosslinked “active layer” of the membrane of nanoscale thickness and support it on a microporous membrane (Figure below). A 20–200 nm thin crosslinked polyamide layer is supported on (or indeed grown from) a microporous polysulfone layer that is in turn supported on a polyester fabric.
_
The most common chemistry for modern RO membranes is interfacial polymerization, another major advance in RO membrane manufacturing. The procedure, described in Figure below, has been the standard for making RO membranes for the past 5 decades.
______
Membranes used for desalination:
The membrane functions as a selective barrier, transporting the desired substances while other substances are rejected (Qiu et al., 2019). In recent years, membrane separation technology (MST) has gained widespread attention due to its potential application in energy demand and sustainable green development (Zeng et al., 2022). MST offers significant advantages like high separation accuracy, high operability, low energy requirements, low cost, and low carbon footprint (Ali et al., 2020; Ang et al., 2020; Zuo et al., 2021). Advances in membrane engineering have promoted desalination and gas separation. Reverse osmosis (RO) membrane desalination technology is crucial to solving human survival challenges.
Current commercial RO membranes used in the desalination industry are mainly thin film composite (TFC) membranes, containing a relatively dense selective layer and a porous support layer. The selective layer is typically composed of polyamides formed via interfacial polymerization (IP) reaction. Moreover, the microstructure of the polyamide layer can be adjusted to apply in different membrane separation processes, for instance, nanofiltration (NF), forward osmosis (FO). In contrast, current microfiltration (MF) and ultrafiltration (UF) membranes are generally prepared by phase inversion methods. RO membranes are typically either cellulose acetate or polysulfone coated with aromatic polyamides. NF membranes are made from cellulose acetate blends or polyamide composites like the RO membranes, or they could be modified forms of UF membranes such as sulfonated polysulfone.
Membrane-based technologies for applications in separations are to be reliable and game-changing in terms of energy efficiency when compared to thermal processes. The current prevalent polymeric membranes experience poor selectivity, low fouling resistance, and low stability to abide heat or chemical lured decadence. Therefore, searching and exploring a novel material, with mended selectivity, permeability, resistance to fouling, and chemical stability concurrently, for membrane fabrication has been a consistent endeavor.
In the past decade, Graphene oxide membranes (GOms) constituting tunable microstructures and multifunctional reactive groups have attained enormous potential to demonstrate high water permeability, thus captivating significant scientific and technological interest. The water permeability in GO membrane is governed either via the oxidized zones that serve as spacers to administer sufficient interlayer distance to accept water molecules, or via pure graphitic zones by enabling practically unhindered flow.
While graphene has gained attention as a promising material for membranes, Singaporean researcher Darren Sun has spent years looking into another candidate: titanium dioxide. The Nanyang Technological University professor developed nanofibers made from titanium dioxide crystals. He then formed them into membranes to filter the water. The membranes could have multiple applications, including in a method of desalination known as forward osmosis. Sun believes they would outperform today’s polymer-based membranes because they can kill bacteria and reduce the clogging that prompts their replacement.
_______
Channel-Based membranes as an alternative to conventional RO Membranes:
Research reveals that water transport is driven by a pressure gradient within the RO membranes and solvent permeance depends on the membrane pore size, kinetic diameter of solvent molecules, and solvent viscosity. In contrast, biological membranes conduct efficient and selective channel-based transport, in which water or selected solutes are transported “straight through” protein channels (membrane proteins, MPs). MP channels are approximately 4 nm in length in comparison to the tortuous unconnected pores in the 20–200 nm thick RO membrane active layers.
_
Attention has recently been focused on water channel proteins called aquaporins (AQPs) and their synthetic analogs, carbon nanotubes (CNTs). AQPs selectively transport water across cell membranes in many forms of life (including in humans) (Agre 2004). Both AQPs and CNTs efficiently transport water at the rate of several billions of molecules per second. They consist of narrow pores lined with hydrophobic surfaces, resulting in single-file water transport (de Groot and Grubmuller 2001; Hinds 2007). While CNTs cannot be made at dimensions that are substantially less than 10 Å in diameter and thus cannot reject salt (hydrated sodium and chloride ions are about 7.2 and 6.6 Å in diameter respectively; Israelachvili 2011), AQPs are highly water selective due to their small pore size (~3 Å) and the presence of amino acid residues that reject charged ions (Agre et al. 2002). The exceptional permeability and selectivity of AQPs has led to research on their incorporation in water purification membranes (Shen et al. 2014), and AQP-based biomimetic membranes were proposed in the mid- to late 2000s in several patents and papers.
_
Graphene-based membranes can also be considered as an example of channel-based membranes and may be promising as next-generation RO membranes (Cohen-Tanugi and Grossman 2012; Mi 2014; Werber et al. 2016b). Graphene is a single thin layer of sp2 hybridized carbon that has unusual mechanical, thermal, and electrical properties and may lend itself to a variety of applications. Pores drilled into graphene may be an option for filtration membranes but currently the pores cannot be made small enough to reject salt (Wang and Karnik 2012). More practical for desalination is the use of oxidized graphene or graphene oxide sheets stacked together so that the distance between the layers can be small enough to reject solutes (Mi 2014). This work is rapidly progressing and could be a new material for sustainable desalination.
_
Fouling-Resistant Membranes:
A major challenge during operation of RO membranes is the deposition of colloidal materials and organic macromolecules on the membrane surface and the growth of microbes. This deposition leads to cake formation, irreversible adsorption, and growth of persistent biofilms, collectively referred to as fouling. Fouling can cause a substantial increase in power consumption due to additional resistance to flow. In addition, salt accumulates in fouling cake layers. The cake-enhanced concentration polarization and, for biofilms, biofilm-enhanced osmotic pressure (Herzberg and Elimelech 2007; Hoek and Elimelech 2003) increase the effective osmotic pressure to be overcome, thus decreasing the driving force for membrane filtration and increasing power consumption. Several membrane modification strategies are under consideration to reduce membrane fouling in RO systems. These include the grafting of superhydrophilic or amphiphilic molecules that can prevent adsorption of macromolecules and biological cells; use of nanoparticles, carbon-based materials such as CNTs, and graphene oxide flakes to impart biocidal properties to the RO membrane surface; and use of electroactive or magnetically actuated surfaces to prevent deposition or cause cell death. Methods that interrupt or manipulate cell-to-cell communication are also being explored for biofouling control.
______
______
Reverse osmosis process:
RO now accounts for 65% of installed capacity for desalination and well more than 50% of the new capacity contracted over the past several years. (Panel a of the figure below shows an RO plant in Barcelona, Spain.) The process is conceptually simple: A pressurized saline stream—typically at 60–70 bar for seawater systems—passes over a membrane that admits water molecules but rejects dissolved salts. As long as the hydraulic pressure of the saline stream exceeds its osmotic pressure (explained in panel b of the figure below), fresh water passes across the membrane and leaves the brine behind. We still don’t perfectly understand the rejection and transport mechanisms, but a theory called solution–diffusion, posits that water on the saline side dissolves into the membrane material and diffuses across to the pure side much faster than the salt does. Latest research reveals that water transport is driven by a pressure gradient within the membranes, not by a water concentration gradient, in marked contrast to the classic solution-diffusion model. It is further shown that water molecules travel as clusters through a network of pores that are transiently connected. Permeation experiments with water and organic solvents using polyamide and cellulose triacetate RO membranes showed that solvent permeance depends on the membrane pore size, kinetic diameter of solvent molecules, and solvent viscosity. This observation is not consistent with the solution-diffusion model, where permeance depends on the solvent solubility.
_
Figure above shows Reverse Osmosis: Plant, principle, prospects. (a) The Barcelona, Spain, seawater desalination plant was inaugurated in 2009. (b) When salt water (green) and fresh water (blue) are separated by a semipermeable membrane that lets only fresh water through, water moves from the fresh volume to the salty one in a process called osmosis (left). Once the pressure P in the salty volume reaches the so-called osmotic pressure π (center), the system achieves equilibrium. If pressure is applied to the salty region so that P > π (right), fresh water will be forced out of the salty volume; that process is desalination by reverse osmosis. (c) Water permeability A and salt permeability B characterize membrane performance. Here the permeabilities are given relative to those for a typical commercial seawater (sw) membrane. Membranes in current use tend to fall along the red line. According to simulations, nanoporous graphene membranes and other ultrapermeable materials may achieve water permeabilities up to 1000 times greater than that of commercial seawater membranes. (Salt permeabilities for such membranes have not yet been simulated.)
_____
In the reverse osmosis (RO) process, the osmotic pressure is overcome by applying external pressure higher than the osmotic pressure on the seawater (Figure below). Thus, water flows in the reverse direction to the natural flow across the membrane, leaving the dissolved salts behind with an increase in salt concentration. The amount of fresh water that could be obtained from a seawater range between 30 and 75% of the volume of the feed water, depending on the initial water quality, the quality of the product needed, and the technology and membranes that is applied (Cipollina et al., 2014). There is no need for heating or phase separation change for this process. This is the main difference from the old thermal techniques, where desalination process is based on water heating. It means that RO process does not require thermal energy and all processes could be done using only electricity.
_
Figure above shows schematic diagram of RO system.
_
Thus, in RO desalination technique the major energy is required for pressurizing the seawater feed for desalting it. Two developments have helped to reduce the energy demand of RO plants during the past decade: the development of membranes that can operate efficiently with longer duration, and the use of energy recover devices (ERD) (Voutchkov, 2017). ERD transfers pressure energy from a high pressure fluid stream to a low pressure fluid stream. Energy consumption of RO plant is approximately 6 – 8 kW h/m3 without energy recovery. Installing an ERD reduces the energy demand quite significantly to 4–5 kW h/m3.
_
RO is a desalination technology based on semipermeable membranes, which are specific layers allowing the passage only to selected molecules. In nature, if two solutions with different concentrations of solutes are separated by a semipermeable membrane, the solvent flows spontaneously from the more diluted solution to the more concentrated one, in order to balance the energy potential of both solutions, as shown in Figure below (see case a). This flow can be progressively reduced if an increasing external pressure gradient Δ𝑝 is applied to the concentrated solution (see case b).
Figure above depicts phenomenon of osmosis, according to the external pressure applied to the two sides of the membrane. Case (a). Forward osmosis. Case (b) Retarded osmosis. Case (c) Zero flow. Case (d) Reverse osmosis.
The exact value able to stop the solvent flow is defined as Osmotic Pressure Δ𝑝𝑜𝑠𝑚 (see case c). If the external pressure gradient is greater than the osmotic pressure, the solvent flow is inverted, so the solvent can be extracted from the concentrated solution (see case d).
_
For each solution the absolute osmotic pressure 𝑝𝑜𝑠𝑚 can be defined according to van’t Hoff’s equation:
𝑝𝑜𝑠𝑚=𝜄[𝑐]𝑅𝜏
assuming the following notation:
- 𝜄 is the dimensionless van’t Hoff index (also called the number of osmotically active particles), given by the relation 𝜄=1+𝜖(𝜈−1), where 𝜖 is the degree of dissociation representing the ratio of how many original solute molecules are dissociated, and 𝜈 is the number of ions formed by the molecule dissociation (the stoichiometric coefficient of dissociation reaction). As an example, in the case of sodium chloride (NaCl), 𝜖≈1, 𝜈=2, consequently 𝜄=2.
- [𝑐] is the molar concentration of the solute.
- 𝑅 is the ideal gas constant equal to 8.31441 J/mol K [0.0821 L atm/mol K]
- 𝜏 is the absolute temperature of the solution.
As the salt concentration is negligible in freshwater and consequently its osmotic pressure (the minimal pressure required to stop the solvent flow) is equal to the osmotic pressure of saline water. For seawater, the salt concentration ranges between 0.51 and 0.68 mol / L. Thus, considering an environmental temperature equal to 25 °C, the osmotic pressure according to van’t Hoff’s equation ranges between 25 and 33 bar. Greater values can be measured, as in the extreme case of Dead Sea, where the osmotic pressure is equal to 290 bar.
_
By applying an external pressure gradient greater than the osmotic pressure, freshwater is extracted from saltwater. For desalination purposes, an external pressure between 15 and 25 bar is normally applied for brackish water, and between 54 and 80 bar for seawater. Thus, according to the values reported above, the RO essentially requires electrical (or mechanical) energy to run the pumps to significantly increase the seawater pressure before the semipermeable membrane. Seawater, after a pre-treatment to remove solid particles, is pressurized by a High Pressure Pump (HPP) in order to supply the RO desalination unit. About 3.5 kilowatt hours (kWh) of electricity are needed to desalinate 1 cubic metre of seawater – 1.3kWh to pump seawater to the plant and 2.2kWh for the reverse osmosis process. Pumping a cubic metre of fresh water distances of more than 200km requires more energy than desalinating the same amount of seawater.
_
In desalination applications, the Recovery Ratio (RR) is defined as the ratio between the freshwater flow and the saline feedwater flow.
𝑅𝑅=𝑄f/𝑄s
Considering the working conditions, RR assumes values between 35% and 50%, so practically only half (or less) of the seawater flow becomes freshwater and the remaining part is expelled as brine. To increase the freshwater extraction, the pressure before the semipermeable membrane should be increased, but there are several technical constraints, essentially related to the mechanical resistance of the membrane. As semipermeable membranes are not perfect, a limited amount of salts can be found in the freshwater output.
_
The brine flow has a high energy potential, as its pressure is practically the same as the saline input water. In fact, the pressure drop inside the brine circuit is about 2–3 bar. To reduce the total energy consumption for desalination, many studies have been realized since the 1970s. In addition to the improvement of membrane properties, the main goal was energy recovery from the brine flow by the introduction of an Energy Recovering Device (ERD). The solutions can be classified as:
- Centrifugal device
- Isobaric device
_____
The maximum operating pressure for a standard sea water RO membrane is typically around 80 bar in order to overcome the retentate osmotic pressure of seawater treated to 50% recovery (∼70,000 mg/L; π ≈ 59 bar). Because of hydraulic-pressure limitations of RO, hypersaline brines (≥70000 mg /L), could be further treated via thermal processes.
Thermal technologies are used to concentrate brine streams to approximately 250,000 mg/L (π≈290 bar). Thermal-based brine crystallizers then concentrate the waste stream above its solubility limit (e.g., 357,000 mg L/1 for NaCl) to extract solid salts for disposal. These technologies typically present high CAPEX and specially high OPEX (high energy required for the process).
With recent developments on RO membrane market, ultra-high-pressure reverse osmosis (UHPRO) spiral wound membranes have been developed capable of withstanding 1800 psi (120 bar). UHPRO membranes enable the concentration of saline streams up to 130,000 mg/L total dissolved solids (TDS) for NaCl and 150,000 mg/L for Na2SO4 which means a 50% decrease in brine volume comparing to the standard types of membranes. This results in more fresh water produced and less brine to be disposed or further treated by thermal technologies
_____
Energy and capital costs:
Salt water and fresh water spontaneously mix. When freshwater and saltwater come into contact, the ions of the saltwater will move towards the freshwater. The instigator of this mixing process is the increase in entropy, in popular terms: the disorder of the system. The saltwater will gradually become more fresh and the freshwater more saline. When this mixing process is occurring in an uncontrolled way – like in nature, or at a discharge – it is irreversible. By controlling the mixing process in a reversible way, a part of the energy that is otherwise lost, can be converted into electricity. In the Netherlands this has become known as Blue Energy. Therefore, according to the laws of thermodynamics, a minimum energy is required to unmix them—that is, to desalinate. For a typical seawater system producing a half kilogram of fresh water per kilogram of seawater, that minimum is about 1 kWhe (kilowatt hour of electrical work) per cubic meter of fresh water, roughly the energy needed to run a window-unit air conditioner for an hour on a hot summer day. New, large-scale seawater RO plants generally use about 3–4 kWhe/m3; by way of contrast, the best large-scale evaporative desalination systems require a heat input equivalent to about 20 kWhe/m3. The takeaway here is that with modern RO, we cannot expect order-of-magnitude improvements to energy consumption—we’re already pretty good.
_
Two developments have also helped to reduce the operating cost of RO plants during the past decade.
-1. The development of operational membranes with high durability and lower prices.
-2. The use of energy recovery devices that are connected to the concentrated stream as it leaves the pressure vessel. The concentrated brine loses only ∼1–4 bar relative to the applied pressure from the high-pressure pump. The devices are mechanical and generally consist of turbines or pumps that can convert a pressure difference into rotating energy that can be used to reduce energy costs.
_
Further improvements are possible. To drive pure water out of a saline feed, the pressure of the feed has to exceed its osmotic pressure. As the saline stream concentrates, its osmotic pressure increases. So the highest pressure in the system must exceed the highest osmotic pressure—that of the concentrated salt water—by enough to yield a reasonable water flux.
The RO process need not always run at its maximum pressure. In a staged system, the applied pressure is stepped up in accordance with increases in osmotic pressure. Much of the purifying flow occurs at relatively low pressure, which reduces overall energy consumption. The drawback is the extra cost of the additional pumps and membranes needed to produce the same amount of fresh water. One innovation that provides similar benefits to staging without the additional cost is called closed-circuit RO, which recirculates the salty stream and slowly pressurizes it as it concentrates.
The average electricity bill for industrial users in the US in 2014 was about 7 cents/kWhe, so the 3–4 kWhe/m3 energy budget of a large-scale RO plant corresponds to an outlay of 21–28 cents/m3. The total cost to operate such plants is in the range of 60–80 cents/m3, roughly half that of a typical residential water bill.
_____
_____
RO system:
_
A typical RO system consists of four major subsystems
- pretreatment system,
- high-pressure pump,
- membrane modules, and
- posttreatment system.
Feed water pretreatment is a critical factor in the operation of an RO system due to membrane sensitivity to fouling. Pretreatment commonly includes feed water sterilization, filtration, and addition of chemicals in order to prevent scaling and biofouling. In most cases, an RO plant must be preceded by a proper pre-treatment to avoid membrane fouling/scaling by sediments, hardness, organic matter, bacteria, silica, metal oxides or even chlorine.
The posttreatment system consists of sterilization, stabilization, and mineral enrichment of the produced freshwater. RO permeate water is often more acidic than the feed water due to dissolved carbon dioxide. Common post-treatment are pH neuralisation and remineralisation. When an additional polishing step is required after the RO treatment to reach extra pure water quality Electrodeionization (EDI) and Ion exchange (IX) can be added as treatment steps.
_
The pretreated feed water is forced by a high-pressure pump to flow across the membrane surface. RO operating pressure varies from 17 to 27 bar for brackish water and from 55 to 82 bar for seawater. Part of the feed water passes through the membranes, removing from it the majority of the dissolved solids resulting in the so-called product or permeate water. The remaining water together with the rejected salts emerges from the membrane modules at high pressure as a concentrated reject stream (brine).
In large plants, the reject brine pressure energy is recovered by a turbine, recovering from 20% up to 40% of the consumed energy. In fact this is one of the most significant issues in RO technological development and innovation. The energy saving, that is, the percentage of the mechanical energy that can be recovered pressurizing the feed water, and the water recovery ratio – the ratio of the desalinated water output volume to the seawater input volume used to produce it – are the critical parameters in the RO process. RO processes have been characterized by a significant reduction in energy consumption.
__
Apart from its need for an elaborate pretreatment plant, the RO process has many advantages such as the following:
- The modular structure of the process makes it flexible enough to handle different plant capacities.
- The process is conducted at ambient temperature, which minimizes corrosion hazard.
- There is an embedded potential of water–power cogeneration and coupling with energy recovery systems.
- The rate of development in RO technology is high compared with other desalination processes and this fact promises for more cost reduction of desalted water produced by RO in the near future.
- Desalination by RO results in high salt rejection (up to 99%) and high recovery ratios (up to 40%).
- Seawater RO (SWRO) can produce potable water with salt content of about 500 ppm.
______
All essential process steps in RO desalination plants are depicted in the figure below:
_
A Typical SWRO Plant:
Most SWRO plants follow the same type of layout. They can be divided into four sections.
The first is the low-pressure seawater intake, where the maximum pressure is 1 MPa and the seawater is filtered and treated. This is necessary because the membrane cells are the single highest cost item in a reverse osmosis plant, and it is important to prevent degradation of the membranes to extend their life. The seawater may have chlorine and/or hypochlorite (ClO-) injected near the seawater intake to prevent fouling. It then passes through coarse filters to remove plastic bags, fish, and other large items. The seawater is then usually dosed with a chemical to aid coagulation as it passes through the dual media filters. These are large vessels with layers of various materials to remove organic matter and other material from the seawater. Finally, the water is fine filtered through cartridge filters to ~1 μm.
Before the high-pressure pumps, depending on the pH of the feedwater, it would be normal to add an antiscalant to limit precipitation of calcium and magnesium salts. Chlorine and/or hypochlorite is a powerful oxidizing agent and it will cause damage to the membranes, unless it is removed. This is usually done by monitoring the redox potential and dosing with sodium metabisulfite (Na2S2O5) to bring the redox potential down to an acceptable value. It is also common to add a nonoxidizing biocide to limit organic fouling of the membranes.
The second or high-pressure section can operate from 6 to 10 MPa, with 7 MPa being a typical value, while high-recovery systems may operate closer to 10 MPa. In the membrane cells chloride is removed in two or more passes, depending on how pure the final water must be.
After chloride removal, the third section handles the permeate (pure water) and is ~0.1 MPa pressure and it is then pumped to storage or a distribution system. Although this is low pressure and low chloride, it is quite corrosive because the water has little tendency to form protective scales of calcium carbonate (CaCO3).
The fourth section is the reject brine section, where the chloride concentration may be twice that in natural seawater. The pressure is still high (~6.9 MPa) and this energy is not wasted, but is hydraulic energy recovery, which transfers hydraulic energy from the brine directly to the feedwater either just prior to or just after the high-pressure pumps. There are two types of energy recovery device, the centrifugal type and the isobaric type. The energy recovered reduces the power of pump required and is much more efficient than electricity generation.
_
General design data for Reverse Osmosis Plants:
|
|
TWRO Tap water |
BWRO Brackish water |
SWRO Sea water |
|
Salinity |
< 1500 ppm |
< 8000 ppm |
< 35000-45000 ppm |
|
Recovery |
80% |
65-80% |
35-45% |
|
Working pressure |
< 15 bar |
< 15-25 bar |
50-75 bar |
|
Membrane types |
Tap / Brackish 4″ or 8″ |
Seawater 4″ or 8″ |
|
|
Flux |
30-35 l/h.m2 |
25-30 l/h.m2 |
15-20 l/h.m2 |
|
Specific energy at 25°C |
< 0.75 kWh / m3 |
< 1.5 kWh / m3 |
< 5 kWh / m3 with energy recovery |
|
Configuration |
Skid mounted or containerized 10 to 40 ft |
||
As with any industrial facility, seawater reverse osmosis (SWRO) and brackish water reverse osmosis (BWRO) desalination plants are prone to wear and tear. If not appropriately managed, scaling and fouling are very common issues, leading to membrane clogging and even mechanical failure, affecting the plant’s performance, productivity, and profitability.
_
Note:
As seen in the table above, higher salinity of feedwater needs higher pressure, higher energy use and result in lower recovery and lower flux. Lower salinity of feedwater needs lower pressure, lower energy use and results in higher recovery and higher flux. Flux is used to express the rate at which water permeates a reverse osmosis membrane. Typical units of measurement are gallons per square foot per day (i.e. GFD or GSFD) or litres per square meter per hour (l/m2/hr).
_____
High pressure pump:
The high pressure pump pushes water through the membrane. Typical pressures for brackish water range from 1.6 to 2.6 MPa (225 to 376 psi). In the case of seawater, they range from 5.5 to 8 MPa (800 to 1,180 psi). This requires substantial energy. Where energy recovery is used, part of the high pressure pump’s work is done by the energy recovery device, reducing energy inputs.
There are two types of high pressure pumping units on seawater RO systems: centrifugal and positive displacement (PD) plunger pumps. Because plunger pumps operate at much higher efficiencies, these most often are the pumps of choice for plants less than 150,000 gpd and where high-energy costs exist. In larger plants, the centrifugal pumps are used most often because these pumps may approach 80 percent efficiency, are less costly and require less maintenance. Plunger pumps produce large output pressure variance (pulsation) due to their reciprocating action, which translates to vibration. This vibration not only is potentially damaging to the pump but to all other system components as well especially plumbing, instrumentation and the systems framework. In order to minimize vibration damage to system components, the pump requires a discharge pulsation dampener and, in some cases, a suction stabilizer (depending on the acceleration head attributed to systems feed plumbing). Another important factor is pump speed in RPM. The slower the pump speed, the less vibration transfers. Mechanical design for vibration isolation also is key to minimizing vibration damage from the pumping system.
Because seawater RO pumps can generate pressures in excess of 1,000 psig, it is recommended that a safety switch, in combination with a pressure relief valve, be incorporated in the design. Severe damage or injury could occur if the pump pressure exceeds material strengths of the RO design.
_____
Membrane assembly (vide supra):
The membrane assembly consists of a pressure vessel with a membrane that allows feedwater to be pushed against it. The membrane must be strong enough to withstand the pressure. RO membranes are made in a variety of configurations. The two most common are spiral-wound and hollow-fiber.
Only part of the water pumped onto the membrane passes through. The left-behind “concentrate” passes along the saline side of the membrane and flushes away the salt and other remnants. The percentage of desalinated water is the “recovery ratio”. This varies with salinity and system design parameters: typically, 20% for small seawater systems, 40% – 50% for larger seawater systems, and 80% – 85% for brackish water. The concentrate flow is typically 3 bar/50 psi less than the feed pressure, and thus retains much of the input energy.
The desalinated water purity is a function of the feed water salinity, membrane selection and recovery ratio. To achieve higher purity a second pass can be added which generally requires another pumping cycle. Purity expressed as total dissolved solids typically varies from 100 to 400 parts per million (ppm or mg/litre) on a seawater feed. A level of 500 ppm is generally the upper limit for drinking water, while the US Food and Drug Administration classifies mineral water as water containing at least 250 ppm.
______
Energy recovery:
Energy recovery can reduce energy consumption by 50% or more. Much of the input energy can be recovered from the concentrate flow, and the increasing efficiency of energy recovery devices greatly reduces energy requirements. Devices used, in order of invention, are:
- Turbine or Pelton wheel: a water turbine driven by the concentrate flow, connected to the pump drive shaft provides part of the input power. Positive displacement axial piston motors have been used in place of turbines on smaller systems.
- Turbocharger: a water turbine driven by concentrate flow, directly connected to a centrifugal pump that boosts the output pressure, reducing the pressure needed from the pump and thereby its energy input, similar in construction principle to car engine turbochargers.
- Pressure exchanger: using the pressurized concentrate flow, via direct contact or a piston, to pressurize part of the membrane feed flow to near concentrate flow pressure. A boost pump then raises this pressure by typically 3 bar / 50 psi to the membrane feed pressure. This reduces flow needed from the high-pressure pump by an amount equal to the concentrate flow, typically 60%, and thereby its energy input. These are widely used on larger low-energy systems. They are capable of 3 kWh/m3 or less energy consumption.
- Energy-recovery pump: a reciprocating piston pump. The pressurized concentrate flow is applied to one side of each piston to help drive the membrane feed flow from the opposite side. These are the simplest energy recovery devices to apply, combining the high pressure pump and energy recovery in a single self-regulating unit. These are widely used on smaller low-energy systems. They are capable of 3 kWh/m3 or less energy consumption.
- Batch operation: RO systems run with a fixed volume of fluid (thermodynamically a closed system) do not suffer from wasted energy in the brine stream, as the energy to pressurize a virtually incompressible fluid (water) is negligible. Such systems have the potential to reach second-law efficiencies of 60%.
_____
Remineralization and pH adjustment:
The desalinated water is stabilized to protect downstream pipelines and storage, usually by adding lime or caustic soda to prevent corrosion of concrete-lined surfaces. Liming material is used to adjust pH between 6.8 and 8.1 to meet the potable water specifications, primarily for effective disinfection and for corrosion control. Remineralization may be needed to replace minerals removed from the water by desalination, although this process has proved to be costly and inconvenient in order to meet mineral demand by humans and plants as found in typical freshwater. For instance water from Israel’s national water carrier typically contains dissolved magnesium levels of 20 to 25 mg/liter, while water from the Ashkelon plant has no magnesium. Ashkelon water created magnesium-deficiency symptoms in crops, including tomatoes, basil, and flowers, and had to be remedied by fertilization. Israeli drinking water standards require a minimum calcium level of 20 mg/liter. Askelon’s post-desalination treatment uses sulfuric acid to dissolve calcite (limestone), resulting in calcium concentrations of 40 to 46 mg/liter, lower than the 45 to 60 mg/liter found in typical Israeli fresh water.
_____
Disinfection:
Post-treatment disinfection provides secondary protection against compromised membranes and downstream problems. Disinfection by means of ultraviolet (UV) lamps (sometimes called germicidal or bactericidal) may be employed to sterilize pathogens that evade the RO process. Chlorination or chloramination (chlorine and ammonia) protects against pathogens that may have lodged in the distribution system downstream.
_____
_____
Benefits and drawbacks of Reverse Osmosis Desalination:
Reverse osmosis (RO) desalination is a widely used method for producing potable water from seawater or brackish water. It has several benefits and drawbacks compared to other methods of producing potable water. Here are some key points to consider:
Benefits of Reverse Osmosis Desalination:
High Water Quality:
Reverse osmosis is effective in removing a wide range of contaminants, including dissolved salts, minerals, bacteria, viruses, and other impurities. It produces high-quality drinking water that meets stringent quality standards. After reverse osmosis, the water is so pure we actually have to put minerals back into it. The process removes the minerals of water that humans need as well as the tastes we are familiar with.
Widely Applicable:
RO desalination can be used in various settings, including coastal areas, islands, arid regions, and remote locations where freshwater sources are limited. It provides a viable solution for water scarcity issues in these areas.
Scalability:
Reverse osmosis systems can be scaled up or down to meet different water demands. From small-scale household systems to large-scale industrial plants, RO desalination offers flexibility in capacity and application.
Energy Efficiency:
Compared to thermal desalination methods like multi-stage flash (MSF) and multi-effect distillation (MED), reverse osmosis is relatively energy-efficient. Advancements in RO technology have led to reduced energy consumption, making it more sustainable.
Corrosion:
Material corrosion problems are significantly less compared with MSF and MED processes due to the ambient temperature conditions.
Modular systems:
Modular systems are designed to be compact and easy to move and install in order to reduce capital costs. They are great for municipal or commercial drinking water applications (such as hotels) where space may be limited, but they need to provide for a large number of people.
Higher yield:
The only other currently used desalination treatment is of the thermal variety. It works the same way as the water cycle, evaporating water into steam and when it is condensed it provides clean water. This approach is very effective at removing unwanted particles, but collecting and condensing steam is inefficient and produces much lower yields of pure water than RO. For the same output volume of water, thermal processes would require nearly three times as much seawater.
_
Drawbacks of Reverse Osmosis Desalination:
Pretreatment needed:
Reverse osmosis membranes are very sensitive. So, unless some more resistant membrane material is developed, pretreatment is an important requirement. Without it, the membrane can become practically useless, decreasing yield or producing impure water. Improperly pretreated seawater can deposit particulate matter on the membrane. These contaminants affect proper membrane flow and pressure which increases operating cost.
High Energy Consumption:
Although RO is more energy-efficient than thermal methods, it still requires a significant amount of energy, primarily for pumping water at high pressure through the membranes. The energy demand can be a limitation, especially in regions where electricity is scarce or expensive.
Environmental Impact:
The brine concentrate, a byproduct of the desalination process, is typically discharged back into the ocean or other water bodies. The high salinity and other chemical constituents in the brine can harm marine ecosystems if not properly managed.
Cost:
Reverse osmosis desalination systems often require substantial upfront investment and ongoing maintenance costs. The expenses include equipment installation, membrane replacement, energy costs, and brine disposal. These costs can make RO desalination less economically feasible in some regions. Many nations of the world don’t have the capability or resources to construct and operate desalination projects. The drinking water produced from the seawater desalination process is typically more expensive than treated ground water, brackish water or surface water sources.
Membrane Fouling:
RO membranes are prone to fouling due to the accumulation of impurities, scaling, and biofouling. Fouling reduces system efficiency and necessitates regular cleaning and membrane replacement, which adds to the operational costs.
Water Waste:
The reverse osmosis process generates a significant amount of reject water or brine, typically about 30-50% of the feedwater. Disposing of the brine can be challenging, and improper discharge may harm the environment.
______
______
Brief review of other desalination technologies:
In thermal distillation atmospheric pressure is reduced, thus lowering the temperature required to evaporate the water. Liquids boil when the vapor pressure equals the ambient pressure and vapor pressure increases with temperature. Effectively, liquids boil at a lower temperature, when the ambient atmospheric pressure is less than usual atmospheric pressure. Thus, because of the reduced pressure, low-temperature “waste” heat from electrical power generation or industrial processes can be employed.
_
Multiple-Effect Distillation (MED):
The multiple-effect distillation (MED) process is the oldest desalination method and is very efficient thermodynamically. The MED process takes place in a series of evaporators called effects, and uses the principle of reducing the ambient pressure in the various effects (Figure below). This process permits the seawater feed to undergo multiple boiling without supplying additional heat after the first effect. The seawater enters the first effect and is raised to the boiling point after being preheated in tubes. The seawater is sprayed onto the surface of evaporator tubes to promote rapid evaporation. The tubes are heated by externally supplied steam from a normally dual-purpose power plant.
_
Figure above shows schematic diagram of MED unit.
_
Multiple-effect distillation or multi-effect distillation (MED) is a distillation process often used for sea water desalination. It consists of multiple stages or “effects”. In each stage the feed water is heated by steam in tubes, usually by spraying saline water onto them. Some of the water evaporates, and this steam flows into the tubes of the next stage (effect), heating and evaporating more water. Each stage essentially reuses the energy from the previous stage, with successively lower temperatures and pressures after each one. Multiple-effect distillation (MED) is the low temperature thermal process of obtaining fresh water by recovering the vapour of boiling sea water in a sequence of vessels, (called effects) each maintained at a lower temperature than the last. Because the boiling point of water decreases as pressure decreases, the vapour boiled off in one vessel can be used to heat the next one, and only the first one (at the highest pressure) requires an external source of heat.
|
PRESSURE |
1 BAR |
0.47 BAR |
0.32 BAR |
0.25 BAR |
0.1 BAR |
|
BOILING POINT |
100°C |
80°C |
70°C |
65°C |
45°C |
By maintaining the effects at low pressure the sea water remains at temperatures below 65°C, thus avoiding unnecessary heating and allowing a good control of scaling. More energy-efficient than MSF, MED plants operate at approximately 14 – 21 kWh/m3 of distilled water.
_
The advantages:
- Very low electrical consumption compared to other thermal processes such as Multi Stage Flash (MSF). Hence MED technology can be considered to be cost effective and more efficient than MSF technology in terms of potable water production.
- Operate at low temperature (< 70°C) and at low concentration (<1.5) to avoid corrosion and scaling
- Produce steadily high purity distillate
- Do not need complex pre-treatment of sea water and are tolerant to variations of sea water conditions
- Are highly reliable and simple to operate
- Reduce civil works cost thanks to reduced footprint
- Are simple to install with packaged units mounted on skids and delivered ready for use, after easy installation
- Have a low maintenance cost (no rotating parts except low pressure pumps)
- Operate 24 hours a day with minimum supervision
- Ideal for coupling with power plants, steam can be used efficiently at pressure as low as 0.35 bar abs or less
- Can be adapted to any heat source including hot water
- Allow very high thermal efficiencies and savings in fuel costs
- Range up to 15 MIGD (68000 m3/day) per unit
Disadvantages:
- Incompatible with higher temperature heat sources due to scaling issues during spray evaporation.
- Difficult to scale down to small sizes due to complexity and large numbers of parts required.
_____
______
Multi-Stage Flash (MSF):
The multi-stage flash (MSF) distillation process is based on the principle of flash evaporation. The MSF includes the use of distillation through multiple chambers called stages (Figure below). In this process, each following stage of the plant operates at relatively lower pressures. The process starts with heating of feed water under high pressure. The heated water enters into first stage, at lower pressure triggering the sudden evaporation called flashing. The flashing of a percentage of the feed water proceeds in each consecutive stage, because of the reduction of pressure in each successive stage. The heat exchanger tube bank that runs through each stage condenses the vapor and converts it into potable water (Khan et al., 2018).
_
Figure above shows schematic diagram of MSF unit. MSF plants operate at approximately 20 – 27 kWh/m3.
_
The MSF process shows some similitudes with MED, previously described. In fact, an initial heat supply is also required, using steam spilled from a power plant, and the decreasing pressure is used to force the vapor production. Electricity is required to run the several pumps distributed along the desalination plant.
Advantages and disadvantages of MSF:
- MSF plants are relatively simple to construct and easy to operate.
- They have no moving parts, other than conventional pumps, and contains only some amount of connection tubing.
- The quality of effluent water contains 2–10 ppm of dissolved solids which means a high level of purification. So it is re-mineralized in the post-treatment process to make it palatable and fit for consumption.
- Though operating plants at higher temperatures (over 115°C) improves their efficiency but causes scaling problems because the salts such as calcium sulphate precipitate on the tubes surfaces and cause thermal and mechanical problems like tube clogging.
- It is considered as an energy intensive process, which requires both thermal and mechanical energy, but it can be overcome by the cogeneration system.
- Adding more stages in MSF improves its efficiency and increases water production, but it increases the capital cost and causes operational complexity.
____
____
Vapor Compression (VC):
Vapor compression (VC) is a technique that is used for small scale plants. The technique is comparable to MED, but it is based on compression of the vapor generated by evaporating water instead of condensation, so that the latent heat of the vapor can be efficiently reused in the evaporation process (Figure below). Vapor compression can be seen as a variation of MED, but technically somewhat more complex, so that application is limited to smaller plants. VC is used mainly for small systems with production around 1000 m3/day.
_
Figure above shows schematic diagram of VC unit.
Mechanical vapor compression (MVC) plants use pressure turbines to compress water vapor to create additional heat and vapor. Employed primarily in thermoelectric and medium-sized plants, MVC is the most energy-efficient form of thermal distillation, requiring between 7 – 12 kWh/m3 of distilled water.
_
Advantages and disadvantages of vapour compression evaporation:
- This method is simple and reliable and hence it can be considered as a better option for small-scale desalination units. They usually have a capacity of 3000 m3/day and are generally used for resorts, industries and drilling sites where fresh water is in shortage.
- The operating temperature of VC distillation or evaporation is low which makes it a simple and efficient process in terms of power consumption.
- Since the operating temperatures are low (below 70°C), the potential for scale formation and tube corrosion is reduced.
_____
_____
Electrodialysis (ED):
Electrodialysis (ED) is an electrochemical desalination process. This technology uses a combination of semipermeable membranes and the generation of an electric field to remove the dissolved ions from the solution. The electric field is generated by two electrodes, supplied in direct current voltage. Each ion has an electric charge (positive or negative). Due to the electric field, each ion is affected by an electric force directly proportional to the amplitude of the electric field and the value of the ion charge. The cations (positive ions, as Na+, Ca2+) are attracted by the anode, while the anions (negative ions, as Cl−, HCO3−, CO32−) are attracted by the cathode. Anionic and cationic semipermeable membranes are alternatively installed in the region between the two electrodes. The first one allows the flow to the anions, the latter to the cations. In this way, the migration of ions generated by the electric field is selectively stopped by the semipermeable membranes.
Electrodialysis (ED) is a mass separation technique utilizing ion exchange membranes and an electrical potential difference for separation of ionic species from an aqueous solution and other uncharged components (Figure below). ED is mostly applied for desalination of brackish water representing the main process for the production of potable water in some parts of the world (Ali et al., 2017). When a feed solution is pumped through these compartments and an electrical potential is maintained between the electrodes, the cations pass through the CEMs and migrate towards the cathode whereas the anions pass through the AEMs and migrate towards the anode. Consequently, the salts are depleted from the feed solutions to form a freshwater in dilute compartments and concentrated effluent in concentrate compartments. The cost for desalination largely depends on the concentration of salts to be removed. The process becomes uneconomical for large salt fractions, i.e. seawater, but is competitive for brackish water desalination. For water with low salt concentrations, ED is considered to be the most advantageous technique (Bruggen et al., 2002). This technology is currently used to produce freshwater from brackish water (salinity up to 2000 ppm).
_
Figure above shows schematic diagram of ED unit.
_
Electrodialysis processes are different from distillation techniques and other membrane based processes (such as reverse osmosis (RO)) in that dissolved species are moved away from the feed stream, whereas other processes move away the water from the remaining substances. Because the quantity of dissolved species in the feed stream is far less than that of the fluid, electrodialysis offers the practical advantage of much higher feed recovery in many applications.
_
Advantages and disadvantages of ED:
- It has the capability of high recovery in terms of more fresh water product and less brine.
- ED is feasible for brackish water with a salinity of <6 g/l of dissolved solids, but not suitable for water with dissolved solids of <0.4 g/l.
- The desalination of water with concentrations of dissolved solids higher than 30 g/l, like seawater, is possible, but it is not economically viable.
- The major energy requirement is the direct current to separate the ionic substances in the membrane.
- Energy usage is proportional to the salts removed.
- It can treat feed water with a higher level of suspended solids than RO.
- Chemical usage for pre-treatment is low.
_____
_____
Membrane distillation (MD):
Membrane distillation (MD) is considered one of the effective and alternative techniques for the treatment of water and wastewater containing high amount of dissolved salt. Traditional water desalination methods like reverse osmosis are too expensive and considerable membrane fouling can happen as it is a pressure driven system. Driving force of MD separation process is vapour pressure differential between cold and hot sides and it is created due to the temperature difference involved in the process. Membrane distillation uses a temperature difference across a membrane to evaporate vapor from a brine solution and condense pure water on the colder side. The design of the membrane can have a significant effect on efficiency and durability. A study found that a membrane created via co-axial electrospinning of PVDF-HFP and silica aerogel was able to filter 99.99% of salt after continuous 30 day usage.
MD has been divided into different types based on the creation of pressure vapour difference of two side of the contactor. The configuration of direct contact membrane distillation (DCMD) is called when a hot liquid on the feed side has direct contact with a cold solution on the permeate side of the contactor. There is a microporous hydrophobic membrane between feed and cold sides. Transmembrane flux is carried out because of the difference in temperature between the hot and cold streams which results in a vapour pressure difference between two sides of the microporous membrane. When the vapour phase passed through membrane is vacuumed on the permeate side, it is called vacuum membrane distillation (VDM). In air/water gap MD (AGMD or WGMD), There are a microporous membrane as well as a condensation surface which air or water is confined between the membrane and condensation plate.
Six extremely important critical influencing factors (CIFs) in the desalination MD are feed temperature; feed concentration or feed salinity; feed flow rate; membrane hydrophobicity; pore size; and membrane material. Moderately important CIFs are feed solution properties, membrane thickness, feed channel geometry, and pressure difference along the feed channel. Finally, the least preferred CIFs are MD configuration, duration of test, specific heat of feed solution, and viscosity.
_
Advantages and disadvantages of MD:
- The main advantages are its simplicity and the low operating temperature rise it requires to operate. These facilitate and make utilizing the waste heat as a preferable energy source possible, such as by coupling the MD units with solar energy sources, which is attractive.
- It requires a lower operating pressure than pressure-driven membrane processes, and reduced vapour space compared with conventional distillation.
- MD requires more space than other membrane processes; however, the recent R&D will reduce the size.
- Energy consumption is approximately the same as that of MSF and MED plants.
- The MD process requires that the feed water should be free of organic pollutants; this explains the limited use of this method.
______
______
Forward Osmosis (FO):
Forward osmosis (FO) is an osmotic process that, like reverse osmosis (RO), uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a “draw” solution of high concentration (relative to that of the feed solution), is used to induce a net flow of water through the membrane into the draw solution, thus effectively separating the feed water from its solutes. In contrast, the reverse osmosis process uses hydraulic pressure as the driving force for separation, which serves to counteract the osmotic pressure gradient that would otherwise favor water flux from the permeate to the feed. Hence significantly more energy is required for reverse osmosis compared to forward osmosis.
An additional distinction between the reverse osmosis (RO) and forward osmosis (FO) processes is that the permeate water resulting from an RO process is in most cases fresh water ready for use. In FO, an additional process is required to separate fresh water from a diluted draw solution. Types of processes used are reverse osmosis, solvent extraction, magnetic and thermolytic. Depending on the concentration of solutes in the feed (which dictates the necessary concentration of solutes in the draw) and the intended use of the product of the FO process, the addition of a separation step may not be required. The membrane separation of the FO process in effect results in a “trade” between the solutes of the feed solution and the draw solution. The forward osmosis process is also known as osmosis or in the case of a number of companies who have coined their own terminology ‘engineered osmosis’ and ‘manipulated osmosis’.
_
Forward osmosis is quite a recent commercial process used for water desalting using a gradient of salt concentration (osmotic pressure) as the driving force through the membrane. If or in case the feed (e.g., seawater) is at one side of the membrane, on the other side of the membrane, there is the higher osmotic pressure “draw” (reusable) solution. As a natural migration process, the water from the feed solution will migrate to the draw solution deprived of employing an external pressure through the membrane. Then, the diluted solution is treated to separate the draw solution from the product as shown in Figure below.
_
Whilst FO hold great potentials that might be harnessed as an alternative seawater desalination technology, its efficacy is constraint by some key drivers impacting its applicability including membrane developments, membrane fouling, concentration polarization, draw solutes development and draw solution recovery, and reverse salt flux. These challenges remained to be overcome.
_
Advantages:
Forward osmosis (FO) has many positive aspects in the treating of industrial effluents containing many different kinds of contaminants and also in the treating of salty waters. When these draw effluents have moderate to low concentrations of removable agents, the FO membranes are really efficient and have the flexibility of adapting the membrane depending on the quality desired for the product water. FO systems are also really useful when using them combined with other kinds of treatment systems as they compensate the deficiencies that the other systems may have. This is also helpful in processes where the recovery of a certain product is essential to minimize costs or to improve efficiency such as biogas production processes.
Disadvantages:
The main disadvantage of the FO processes is the high fouling factor that they may experience. This occurs when treating a high saturated draw effluent, resulting in the membrane getting obtruded and no longer making its function. This implies that the process has to be stopped and the membrane cleaned.
______
______
Nanofiltration (NF)
Nanofiltration is a membrane filtration process used to remove dissolved ions or organic matter to produce soft water, i.e., water with a limited number of ions that are responsible for scaling (Ca2+, Mg2+…). This technique is conceptually similar to RO. The main difference is the action used to remove the ions from the saltwater, as shown in Figure below.
Figure above shows the working principles and schema of a nanofiltration unit.
The prefix “Nano” is related to the pore sizes, ranging from 1 to 10 nanometers, so smaller than other filtration techniques (microfiltration and ultrafiltration) but larger than in the RO. As a consequence, this technology removes mostly divalent ions (e.g., Ca2+ and Mg2+), with an efficiency of between 90% and 98%. The removal of monovalent ions is limited (between 60% and 85%).
As the soft water produced by the NF process has a greater ion concentration than RO, a lower pressure gradient must be applied to the semipermeable membrane (between 34 and 48 bar). As NF requires a lower energy demand than RO, this solution is under investigation for seawater desalination, introducing a dual-stage unit.
NF is used in several applications such as water and wastewater, pharmaceutical and food processing. The applications for the desalination of seawater are limited, since these semipermeable membranes are more porous, allowing the passage of some dissolved solids.
______
______
Solar distillation:
Solar distillation mimics the natural water cycle, in which the sun heats sea water enough for evaporation to occur. After evaporation, the water vapor is condensed onto a cool surface. There are two types of solar desalination. The first type uses photovoltaic cells to convert solar energy to electrical energy to power desalination. The second type converts solar energy to heat, and is known as solar thermal powered desalination.
Wave-powered desalination:
Wave powered desalination systems generally convert mechanical wave motion directly to hydraulic power for reverse osmosis. Such systems aim to maximize efficiency and reduce costs by avoiding conversion to electricity, minimizing excess pressurization above the osmotic pressure, and innovating on hydraulic and wave power components. One such example is CETO, a wave power technology that desalinates seawater using submerged buoys. Wave-powered desalination plants began operating on Garden Island in Western Australia in 2013 and in Perth in 2015.
Freeze–thaw:
Freeze–thaw desalination (or freezing desalination) uses freezing to remove fresh water from salt water. Salt water is sprayed during freezing conditions into a pad where an ice-pile builds up. When seasonal conditions warm, naturally desalinated melt water is recovered. This technique relies on extended periods of natural sub-freezing conditions.
A different freeze–thaw method, not weather dependent and invented by Alexander Zarchin, freezes seawater in a vacuum. Under vacuum conditions the ice, desalinated, is melted and diverted for collection and the salt is collected.
Microbial desalination:
Microbial desalination cells are biological electrochemical systems that implements the use of electro-active bacteria to power desalination of water in situ, resourcing the natural anode and cathode gradient of the electro-active bacteria and thus creating an internal supercapacitor.
Microbial Desalination Cell (MDC) is a novel technology able to produce sustainable drinking water by using the energy provided from the metabolism of electroactive bacteria when organic matter is degraded, allowing simultaneous desalination of water, treatment of waste water and production of electricity. MDC consists of an electrochemical device with three compartments. The anodic compartment comprises an electrode covered by a biofilm that oxidizes the organic matter contained in the wastewater, transferring electrons from the substrate (i.e., organic matter) to the electrode. Then, the electrons use an external circuit to reach the cathodic compartment, where the reduction reaction takes place. The electric potential forces the migration of ions. Therefore, desalination takes place when positive ions move through the cation exchange membrane (CEM) from the saline compartment to the cathode and negative ions move through the anion exchange membrane (AEM) from saline to the anodic compartment.
The first concept of MDC was proposed by Cao et al. in a cell of 9 cm2 (cross section) with a saline volume chamber of 11 mL, achieving 90% of salt removal batchwise, at initial salt concentrations ranging from 5 to 35 NaCl g·L−1 (Cao et al., 2009). Different MDC configurations have been reported in the literature, including cubic and tubular reactors (Mehanna et al., 2010; Jacobson et al., 2011a,b; Ping and He, 2013), stacked cells (Chen et al., 2011; Kim and Logan, 2011), using batch recirculation (Chen et al., 2012; Qu et al., 2012), biocathodes (Wen et al., 2012), increasing water production by applying external voltage (Ge et al., 2014), or integrating innovative membranes (forward osmosis) (Zhang and He, 2012; Yuan et al., 2015), ion exchange resins in the compartments (Zhang et al., 2012), or microfiltration processes (Zuo et al., 2017, 2018). Up to date, the biggest MDC ever built (100 L) was reported to achieve partial desalination of sea water with a nominal desalination rate of 0.077 L·m−2·h−1 (Zhang and He, 2015).
Since its introduction in 2009, the MDC technology has evolved quickly in designs and in uses but is faced with a number of problems too.
_____
_____
Hybrid systems:
Generally, the majority of desalination facilities have been designed to support only one desalination method, such as RO, MED, or MSF. However, there is now more interest in designing facilities that support hybrid systems and have several desalination techniques operating at the same location. The aims of integrating two or more techniques are to boost effective recovery throughout, lessen the release of brine, and reduce the cost and energy requirements.
Hybrid plants use a combination of treatment technologies, which enables the plant to reuse energy and optimize plant performance. As an example, the Cape Hatteras desalination plant in North Carolina operates a hybrid RO/ion exchange plant using brackish water. The plant withdraws water from two separate wells; the high salinity water from Well 1 is processed by the RO process, and the high organic content water from Well 2 is processed by the ion exchange process. The treated water from the RO and ion exchange processes are blended as the final product water. This plant has also incorporated an energy recovery turbine into the RO treatment process.
Basically, there are two main types of hybrid desalination plants: homogeneous and heterogeneous. Homogeneous hybrid plants, as the name implies, utilize a single desalination technique, either membrane- or thermal-based desalination. In contrast, heterogeneous hybrid plants utilize more than one desalination technique. For instance, a thermal-based method may precede a membrane-based method or vice versa.
_
Various homogeneous hybrid systems have been used for desalination, and one of the most prominent is the membrane hybrid system that couples NF and RO and is referred to as the NF-RO system. In particular, NF-RO systems have proven to be effective for the treatment of seawater; utilizing NF during the pretreatment stage results in improved efficiency via an increase in the permeation current and reliable seawater reverse osmosis (SWRO). It was reported that the recovery performance can be increased from 1 L/min and 16.7% to 4.8 L/min and 48%. The primary contributing factors to this performance boost include (1) reduced scaling and membrane fouling, (2) increased feedwater quality, and (3) reduced polarization during the SWRO step. Including an NF step results in the elimination of a substantial amount of organics and scale, which leads to the formation of divalent ions. Hence, there is a reduction or elimination of SWRO membrane fouling and scaling. The performance of homogeneous NF-RO systems is also higher than that of RO systems due to the NF step effectively increasing the feedwater quality; NF removes monovalent ions from the seawater. In homogeneous NF-RO desalination systems, polarization is reduced during the SWRO step, which means that a steady flux can be maintained throughout the process.
_
Furthermore, a brackish water reverse osmosis (BWRO) system connected to an electrodialysis reversal (EDR) system has been shown to be effective in brackish water recovery, recording up to 97% recovery. Taking into account how well EDR works with selective ion membranes, connecting EDR to conventional BWRO resulted in enhanced brine recapture from a maximum of 75% up to 97%, with a final brine salinity of 100 g/L and around a 17% increase in total product water. Achieving greater recovery is essential since it lessens the need to dispose of brine from BWRO inland desalination systems, which can be challenging.
_
Altaee and Hilal, reported an overall improvement in recovery (to > 90%) when high-salinity brackish water was treated using a tri-hybrid NF-forward osmosis (FO)-BWRO system. Similarly, Tang and Ng also explored using an FO technique to concentrate brine at salinities of 0.6–1 M NaCl. For various feed-draw solution combinations, they found that the water recovery from brine streams ranged from 30% to 75%. Their system also generated reduced volumes of brine for disposal.
_
The potential benefits associated with combining two methods into one system have also been examined for thermal-based hybrid desalination systems consisting of MSF and MED steps. Dahdah and Mitsos went a step further and studied superstructures consisting of MED-MSF and MED-MSF coupled with TVC; they compared the energy usage and total productivity optimization relative to stand-alone MSF, MED, and TVC systems. They found that two novel tri-hybrid systems consisting of MED-TVC + MED + MSF and of MED-TVC + MSF were capable of satisfying higher GOR, and reducing heat transport surfaces, the size of the equipment, and cooling water requirements. Furthermore, Mabrouk and Fath, compared the techno-economic features of a novel MSF-MED device to those of various independent systems, such as MSF and MED systems. They found that the cost and pumping power requirements for the integrated system were 16% and 21% less than those of the MSF and MED systems, respectively.
_
Heterogeneous hybrid desalination systems include a variety of distinct membrane-based desalination methodologies that are typically coupled with thermal-based desalination methodologies. Such systems are commonly denoted as bi- or tri-hybrid desalination systems. NF has been extensively studied, primarily as a pretreatment step before MSF to eliminate divalent ions. Significant reductions in scaling issues caused by some salts have been reported. For example, Zhao et al. reported up to 97.7, 96.98, 94.86, and 99.8% reductions in BaSO4, CaSO4, CaF, and SrSO4, respectively. Al-Shammiri et al. reported that in a hybrid system that included MSF, when the top temperature was above 120 °C, the system used less energy.
_
Hybrid thermal-membrane plants have a more flexible power-to-water ratio, efficient operation even with significant seasonal and daily fluctuations of the electricity and water demand, less primary energy consumption and an increase of plant efficiency, thus improving economics and reducing environmental impacts. MSF+RO or MED-TVC+RO hybrid plants exploit the best features of each technology for different quality products or a blended product. RO/MSF desalination systems are one of the most well-known types of heterogeneous hybrid systems, and Helal et al. have shown the efficacy of such systems. They established that it was possible to lower the price of water produced using MSF systems by at least 17–24% by implementing heterogeneous hybrid systems. El-Sayed et al. also studied a hybrid MSF/RO system and demonstrated that the electrical energy consumption was reduced by 25% compared to an SWRO system. Similarly, a pilot plant-scale NF-SWRO-MSF tri-hybrid system was studied by Hamed et al. and showed an increase in the permeate and distillate recovery of the MSF step and plant-scale operational consistency.
_
With thousands of RO desalination plants currently operating and with no current plans for deployment of large-scale plants utilising alternative methods, it could be a long time before the desalination industry replaces RO. Therefore, hybrid systems could be an excellent opportunity to test, further develop and allow the sector to grow confidence in the new systems.
The major hybrid configurations reported in the literature over the past ten years are summarised in Table below. These were selected for consideration here if studies used seawater as the initial feed water and they reported key information on energy consumption, water recovery and salt rejection. The period is limited to the past decade to reflect the rapid improvements in the efficiencies of the technologies.
|
Hybrid type |
Energy consumption, kWh/m3 |
Water recovery, % |
Salt rejection, % |
Remarks |
|
FO-ROb FO-RO FO-Crystallisation- RO RO-MD RO-CD-UF-MD RO-NF-MD RO-MD RO-MD-PRO |
1.37–1.82 1.5 17.4 4.8d 4.8d 4.8d 2.81 2.68 |
~35–55 2 68 84.6 66.9 73.4 30% of RO brine 30% of RO brine |
99 98 – – – – – – |
Wastewater and seawater used Water desalination cost: $0.91/m3 Water desalination cost: $0.64–0.70/m3 Water desalination cost: $0.63/m3d Water desalination cost: $1.05/m3d Water desalination cost: $0.70/m3d Operational cost: $1.04/m3 Operational cost: $1.07/m3 |
|
RO-MD-RED RO-MD-MDC RO-MD-MDC RO-MD-MDCb RO-CDI |
6.5 – 3 in addition to RO alone – 3.17 |
90 90 99.8 – – |
100 – – – >99.9 |
Energy recovered from saline brine Production of 21 kg/m3 of NaCl crystals Equipment and energy costs: €1.09/m3; recovery of CaCO3, NaCl and KCl Addition of MDC contributes >0.5% of the energy consumption Ultrapure water production (TDSc< 2 ppm) |
|
RO-MCDI RO-MCDI |
0.15–0.21 in addition to RO alone 4.24–11 |
50% of RO permeate 14.6 |
>99.9 – |
Bromide removal efficiency: 68.7–70.3% Small-scale plant, 2 m3/d |
|
RO-FCDI |
1.3 in addition to RO alone |
45 |
95% |
Used after one-pass RO module |
CD: Chemical deposition; CDI: Capacitive deionisation; FCDI: flow-electrode capacitive deionisation; FO: Forward osmosis; MCDI: Membrane capacitive deionisation; MD: Membrane distillation; MDC: Membrane crystallisation; NF: nanofiltration; OARO: Osmotically assisted reverse osmosis; PRO: Pressure retarded osmosis; RED: Reverse electrodialysis; RO: Reverse osmosis; UF: ultrafiltration.
b Theoretical study. c Total dissolved solids. d Assumes 100% of the thermal energy is provided as free waste heat.
_
Hybrid desalination facilities may also integrate multiple processes in series to increase the separation or concentration capabilities of the facility. These series hybrids are typically smaller in capacity. For example, zero liquid discharge (ZLD) systems (i.e., facilities with no offsite liquid-waste discharges) often concentrate the desalination waste stream by separating the process into logical steps and optimizing the entire system, using RO systems followed by distillation concentrators and crystallizers. Another hybrid example is the combination of ED and RO proposed by Davis (2006). The process uses ED to reduce the salinity of the reject stream from the RO so that the salt-depleted reject stream can be recycled to the RO to increase recovery. Hybrid configurations in series can also be used to create ultrapure water required by some industrial processes. The multitude of possible combinations of desalination processes in hybrid configurations is limited only by ingenuity and the identification of economically viable applications.
______
______
Key Benefits of Commonly Used Desalination Methods:
Each desalination method has its advantages and disadvantages. Table below provides a summary of those key benefits. For decision making in terms of choice of desalination method, it is important to assess the specific circumstances of a given situation from different angles before making a decision.
Key Advantages and Disadvantages of Different Desalination Methods:
|
Desalination method |
Key advantages |
Key disadvantages |
|
MSF |
Easiest to operate Generally, requires less land Lowest O&M costs More cost-effective than RO for seawater with TDS > 46 ppt Low TDS and boron product water quality Source water quality has limited impact on performance |
Highest energy use Highest thermal discharge footprint Low recovery ratio |
|
MED-TVC |
Lower energy demand than MSF Uses less chemicals than MSF and RO Cost of water comparable to RO for large plants Low TDS and boron product water |
More complex to operate than MSF Higher energy use than RO Low recovery ratio |
|
SWRO |
No need for steam Lowest total energy use Lowest capital and water production costs Discharge does not create thermal pollution Higher recovery ratio |
Highest O&M costs Most complex operation Reliability is sensitive to source water quality |
|
Hybrid |
Lower capital costs Lowest RO energy use Lowest RO production cost Second-pass RO system not needed |
Most complex desalination plant configuration |
_____
_____
Main parameters for performance and efficiency of desalination:
Capacity = Production of water (usually in m3/d)
Quality = Water quality expressed by amount of total dissolved solids (TDS) in the product (in ppm)
Recovery ratio (RR) = The ratio of the desalinated water volume to the feed water volume used to produce it is called the recovery ratio.
Most useful criterion to measure the performance of a given combination of an energy source and desalination plant is the lifetime levelized unit cost of the water produced ($/m3) or ($/kWh)
Levelized cost = total water production cost/total amount of water produced
Specific for thermal:
Gain Output Ratio (GOR) = It measures the amount of distilled water produced per kilogram of steam consumed in a desalination process. It is a measure of how efficiently thermal energy is used in a desalination process.
Performance ratio (PR) = PR represents the produced quantity of the distilled water per amount of input energy.
Top Brine Temperature (TBT) = The maximum temperature of the brine in the first stage/effect. Defines the quality of heat needed and affects GOR.
Specific for membrane:
Pressure: The feedwater pressure used to pump the feedwater through the membrane. Usually related with the membrane type and mechanical properties.
_
Several parameters can be used to evaluate the performance and efficiency of desalination technologies. In each case, context-specific factors, such as temperature and salinity of feed water or the stringency of health regulations for drinkable water in given jurisdiction, will have considerable influence upon the final value of these parameters. For this reason, it is advisable to benchmark parameters to local and regional peers rather than to global average, if data availability permits.
_
Recovery ratio is a simple measure of efficiency of desalination processes which can be used for comparison of different technology choices. Nevertheless, there are several factors which make comparison difficult. Recovery ratio (RR) is the “volumetric processing efficiency of the purification process which indicates the proportion of intake water that is converted into high quality (low salinity) water for sectoral use” (Jones et al., 2019). As table below shows, amongst the commercially available technologies, RO has higher recovery ratios than thermal technologies (MSF, MED). Several technologies in the right part of table below have far higher recovery ratio but are currently not commercially viable options – these are nanofiltration, electrodialysis, electrodialysis-reversal and electrodeionization. Furthermore, RR also depends on the quality of feed water which serves as the input for desalination: the difficulty and expensiveness of desalinating with high RR increase with the salinity and temperature of feed water. On the other hand, specific energy consumption and the operating pressure decrease as the temperature of the feed water increased from 23 to 35 °C for RO desalination due to apparent increase in the membrane permeability that is caused by lower feed viscosity as a function of temperature.
_
Estimation of recovery ratios for different combinations of desalination technologies and feed water types are depicted in table below:
_
The efficiency and economic viability of different desalination technologies depend on what kind of water is used as an input. Consequently, few technology-feed water combinations are far more widely used than others: just eight combinations of technology-feed water account together for more than 90% of the global production of desalinated water (Jones et al., 2019). Those combinations are SW-RO, BW-RO, SW-MSF, SW-MED, RW-RO, WW-RO, BW-ED, RW-ED.
_
The advantage of RO is that the technology is economically viable with several types of feed water. In their recent survey of current desalination assets worldwide, Jones et al. (2019) found that 50% of desalinated water produced by RO desalination technologies originates from SW and another 27% from BW, accounting respectively for 34% and 19% of the global desalination capacity. Conversely, thermal technologies are used almost exclusively to desalinate highly saline types of feed water: SW constitutes 99.9% and 92% of feed water used for MSF and MED desalination, respectively.
_
Energy consumption of desalination is another important aspect to consider when assessing the performance of different desalination technologies. Table below presents the energy consumed by different desalination methods with seawater as an input. When benchmarking different desalination technologies, it is crucial to keep in mind that RO uses only electrical energy; while both MSF and MED desalination plants require electrical as well as thermal energy. Therefore, the assessment of energy consumption of both technologies requires a common unit of comparison: total equivalent electrical energy (in kWh) per cubic meter of water produced.
Estimation of energy consumption of the leading commercially available desalination methods with seawater as an input is depicted in table below:
_
The performance of desalination technologies is highly dependent upon local context due to the strong influence of factors such as water quality, temperature and salinity. Keeping this variability in mind, the following table presents a global overview of the usual range of the main operational and performance parameters of different desalination technologies, based on the data collected by Shahzad et al. (2017). The range of parameter values in some cases reflects the high context-dependency. One should keep in mind that even two identical desalination plants located in very different environments would perform differently. Moreover, the two last columns in red emphasize that these technologies are not commercially available.
Summary of operational and performance parameters of different desalination processes is depicted in table below:
_
Several important points can be made based on the information contained in table above. Both MD and hybrid MED+AD desalination technologies show significantly higher recovery ratios than commercially available technologies, but they remain in the R&D phase. Among all the commercially available technologies, SWRO has the lowest carbon footprint and the highest electrical energy consumption yet it does not require any thermal energy input, unlike thermal based competitors, which require both energies as an input. This point will be crucial for proper comparison of the performance of different technologies. One can also note the differences in plant sizes and in capital costs.
_____
_____
Thermal vs membrane desalination:
Thermal desalination using multistage flash distillation (MSF) and multi-effect distillation (MED) has been the primary desalination process over the 1950s–1970s era. Thermal desalination is preferred for power and water production, i.e., co-generation, and where low energy cost, high salinity, and temperature seawater. Thermal desalination currently accounts for about 25% of the global desalination capacity and mainly practiced in the gulf cooperation council GCC countries (Jones et al., 2019). Membrane desalination using reverse osmosis (RO) and nanofiltration (NF) membranes has been evolved in the 1970s–1980s. RO and NF currently dominate the desalination market due to its lower energy consumption and modular nature, with a 70% share of the global desalination capacity (Jones et al., 2019; Malaeb and Ayoub, 2011).
_
Two main advantages of thermal over membrane:
-1. Thermal desalination processes such as MSF (multi-stage flash) and MED (multiple effect distillation) have in the past provided the majority of potable water in regions such as the Middle East, where excess heat from power plants is used to heat and desalinate seawater. This excess heat otherwise is wasted. So, we are increasing energy efficiency plus desalinating water.
-2. Thermal desal is good for high salinity, high temperature and poor quality feed water as RO will need pretreatment raising cost.
_
Thermal desalination has the advantage of being suitable for high-salinity, high-temperature, low-quality feedwaters, with less feedwater pretreatment requirements, recovery is independent of feedwater salinity, and more economically and operationally attractive when coupled with power plants (Son et al., 2020). However, the main disadvantages are the high energy consumption, and hence higher cost, and higher environmental impacts (EIs) due to greenhouse gases (GHGs) emissions and discharge of hot brine (Park et al., 2020). On the other hand, membrane desalination has the advantages of lower energy consumption, and hence cost, suitability to a broad range of feedwater salinity (such as wastewater, both domestic and industrial, brackish groundwater, and seawater), plant size scalability (from few m3/d to hundreds of thousands m3/d) (Swaminathan et al., 2019). In membrane desalination, the two main distinct technologies of RO and NF give broader options for feedwater/product water salinity, this is mainly due to the difference in salt rejection with about 99.5% for RO, while it varies for NF from 50 to 90% for mono-valent ions such as Na+ and Cl− and up to 99.5% for divalent ions such as Ca2+ and SO42−, and accordingly RO operates at high pressure up to 70 bars, while NF operates up to 20 bars (Yusuf et al., 2020). However, RO has higher pretreatment requirements, not suitable for high feedwater salinities as the maximum attainable recovery decrease as the feedwater salinity increase, and more prone to scaling and fouling being a pressure filtration-based process (Roy et al., 2020).
_
Although thermal and membrane desalination technologies have a proven reliability and technology maturity, it has some challenges such as the high energy consumption in case of thermal desalination, and high pretreatment requirements in case of membrane desalination, in addition to the different EIs (Elimelech and Phillip, 2011; Kress and Galil, 2018). In addition to thermal and membrane desalination processes, there are a set of new and emerging desalination processes that are currently under development. The main drivers for developing such emerging desalination processes are lower energy consumption, lower desalination cost, environmentally friendly, and many others (Lu et al., 2019; Miller et al., 2015; Yusuf et al., 2020).
______
Will Reverse Osmosis replace Thermal Desalination in GCC Region?
As to why GCC nations seemingly favor MSF over RO technology, one must first note that, during the desalination boom in the said region, thermal technology was the only option with a proven track record, especially for large-scale plants (Baten and Stummeyer 2013). Easy access to cheap fossil fuels was another incentive that would justify resorting to thermal technologies (Baten and Stummeyer 2013; Kucera 2019). Finally, one should also take into consideration that the poor seawater quality of the region (relatively high salinity and temperature) for long was not compatible with membrane processing desalination units because it would cause biofouling of the membranes (Kucera 2019). It is worth noting that while the increase in salinity and temperature in feedwater could potentially decrease the efficiency of membrane desalination, they do not affect or favor thermal evaporation desalination (Altmann et al. 2022). However, modern membrane materials with higher permeability and salt-rejecting membranes that operate at lower pressure, as well as options for energy recovery and hybrid MSF-RO technology, have allowed RO to become a more viable, cost-effective option in the GCC region. Recent data on the use of RO in Saudi Arabia and UAE reflects this (Kucera 2019). As a result, currently, thermal desalination is almost exclusively used in installations that intend to use a waste heat source, such as thermal and nuclear power plants.
_
The advantages of RO over thermal technologies are well known in terms of lower energy consumption and the cost of produced water; but are not yet taken advantage of in the GCC zone. One of the reasons is blamed on high feed water salinity and bad water quality; other reasons such as lack of experience, red tides and reliability are contributed to the dominance of thermal plants. However, field experience showed that good pretreatment and optimized RO design may overcome the problems of high feed salinity and bad water quality. Several RO plants, such as Fujairah in UAE, are good examples of a working RO technology in the harsh water environment. Good RO design includes design and optimization of both pretreatment and post-treatment. Field experience showed that most of RO plants failure was due to inefficient pretreatment which resulted in providing low quality water to the RO membrane that caused fouling. Fouling, including biological and scaling, can be handled once an efficient pretreatment process is available. Recent advances in pre-treatment techniques include the combination of Forward Osmosis (FO) with RO among other methods. Recent studies by the authors including commercial implantations have shown that the combination of FO with RO addresses the most technical challenge of RO process and that is fouling, which results in lower energy consumption and less chemical additives. Experience showed fouling in FO process in reversible, i.e. can be removed by backlashing while fouling in conventional RO process is irreversible.
_
The current trends in the global desalination market show that RO is the most popular available technology. In fact, countries such as Spain, Australia, and Algeria are investing heavily in RO for their national desalination programs (Kucera 2019). Various reasons can explain the popularity of this technology over traditional thermal desalination units, one being the lower capital costs required due to less-expensive construction materials and smaller infrastructure (Kucera 2019). In addition, while the operational costs of RO desalination units vary from case-to-case, they are often far less than MSF units and marginally better than MED units (Baten and Stummeyer 2013). In terms of environmental impacts and carbon footprint of these technologies, while it is important to note that this should ideally be determined on a case-by-case base, considering global averages, RO technology [2.562 (kgCO2eq/m3)] is a better choice than MSF [2.988 kgCO2eq/m3] and often competitive with MED units [1.280 kgCO2eq/m3] (Saleh et al. 2019). What is important to note here, however, is that the efficiency of RO desalination units can be improved through using energy-recovery systems and regular maintenance of the system (e.g., changing the RO membranes) (Dolnicar and Schäfer 2009; Cornejo et al. 2014; Leon et al. 2021). As a rule of thumb, the larger the desalination units, the more efficient the process becomes (Martínez-Alvarez et al. 2019). Using this fact within a water resources planning perspective could in some cases help offset the capital and investment costs.
_
Thermal to membrane in Middle East:
Membrane technologies that use electricity, such as reverse osmosis, are the most common desalination technology installed worldwide. But the Middle East is an exception. The low cost of oil and gas and the prevalence of co-generation facilities for power and water means the region relies heavily on fossil fuel-based thermal desalination (such as multi-stage flash or multiple-effect desalination). Two-thirds of the water produced from seawater desalination in the region today is from fossil fuel-based thermal desalination, while the rest is from membrane-based desalination that relies heavily on electricity produced using natural gas. Overall, the Middle East accounts for roughly 90% of the thermal energy used for desalination worldwide, led by the United Arab Emirates and Saudi Arabia. But the use of membrane technologies is growing in the region. Reverse osmosis technologies accounts for 60% of capacity in Oman and roughly half of the capacity in Saudi Arabia. All of the contracted plants currently under construction in Saudi Arabia and a majority of planned capacity are reverse osmosis desalination plants.
_
By 2040, the production of desalinated seawater in the Middle East is projected to increase almost fourteen-fold, and there is a concerted shift towards membrane-based desalination. Why the shift in approach? There are a few main reasons:
- The cost of membrane-based technologies for desalination continues to decline, making them the technologies of choice for new capacity.
- The disadvantage of using domestic hydrocarbons for thermal desalination is underlined by anticipated reforms to energy pricing, which reduce fossil-fuel consumption subsidies. The use of domestic oil and gas resources for thermal desalination also cuts into potential export revenues.
- The electricity mix is changing, with many countries in the region looking to exploit their (highly under-utilised) potential for renewables. The region has some of the highest solar irradiation rates in the world and some countries have received some of the lowest bids seen so far for solar projects, but there is only around 1 GW of solar capacity in the Middle East today, compared with some 90 GW of oil-fired generation capacity.
- Even more importantly, pairing more co-generation plants with reverse osmosis technologies instead of thermal technologies would allow for greater operational flexibility and for the system to be used as a demand response facility: it could help ensure an outlet during periods of excess electricity production from solar, with water storage tanks effectively serving as energy storage.
- In addition, relying more on renewables, depending on the technologies, can reduce the water intensity of electricity generation and thus water demand from the power sector, as the water needs for solar photovoltaic and wind compared to other technologies or fuels is low.
By 2040, over three-quarters of the water produced in the Middle East will be from membrane-based desalination. However, because the power sector remains heavily reliant on natural gas and oil for power generation in 2040, most desalination still depends on fossil fuel-based electricity.
_____
_____
Section-7
Energy requirement of desalination:
The amount of energy required for a desalination process is dependent on the quality of feed water, level of water treatment, treatment technology used by the facility, and plant capacity. Compared to the other water resources, desalination of seawater (SW) is the most energy intensive. Even though energy costs are lower for groundwater and surface water treatment, the supply from these sources is not enough to meet the increasing demand for fresh water. Therefore, desalination of SW seems to be the world’s most suitable solution for water scarcity regardless of the energy costs associated with it. For typical seawater at ambient temperature and 3.5% concentration by weight of dissolved salts, the universal thermodynamic limit (TL) to separate water from the solution (but at zero recovery) is 0.78 kWh per cubic meter. However, the practical specific energy consumption for seawater desalination plants available hitherto may vary from 5- to 8-folds higher than the ideal limit.
_
Desalination is energy-intensive. Reverse Osmosis needs up to 6 kWh of electricity per cubic metre of water (depending on its original salt content), hence 4 MWe will produce about 16,000 to 24,000 m3 per day from seawater. MSF and MED require heat at 70-130°C and use 25-200 kWh/m³, though a newer version of MED (MED-MVC) is reported at 10 kWh/m3 and competitive with RO. A variety of low-temperature and waste heat sources may be used, including solar energy, so the above kilowatt-hour figures are not properly comparable. For brackish water and reclamation of municipal wastewater RO requires only about 1 kWh/m3. The choice of process generally depends on the relative economic values of fresh water and particular fuels, and whether cogeneration is a possibility.
_
The desalination stage of early SWRO systems installed in the 1970s consumed as much as 20 kWh per cubic meter of produced freshwater (kWh/m3). As reverse osmosis technology continued to improve, the power consumption dropped to 8 kWh/m3 in the 1980s and then to 5 kWh/m3 in the 1990s. By 2000, newly built SWRO plants were consuming 3.5 kWh/m3. Shortly after the turn of the century, isobaric pressure exchangers with an energy recovery efficiency greater than 96% were introduced, enabling a further drop in the power consumption of SWRO desalination at 50% recovery to nearly 2 kWh/m3, where it remains today. In terms of specific energy consumption, the current “state of the art” for SWRO plants using isobaric ERDs, axial piston high-pressure pumps, and the latest membrane technology can now be lower than 2 kWh/m3, or less than half of what it was at the turn of the 21st century.
_
Let’s put seawater RO desalination power requirements in perspective!
- Based on nationwide data from the Energy Information Administration, a typical refrigerator average annual energy usage is 1,400-1,500 kwh. Using the average US water use per house- hold of 100,000 gallons per year, the energy requirement for supplying desalinated water to a house in the US will be less than an old refrigerator, but the same as a newer, more efficient refrigerator power use.
- Based on the data from the Office of the Energy Markets and End Use, the average annual household energy power consumption is 11.0 MW. If the entire community is served by seawater desalinated water, the annual power required for the desalination plant to serve a house is 1.0 MW, or an increase of less than 10%. In most cases, however, the existing traditional supply sources are augmented with seawater and/ or brackish water desalinated waters, which further reduces the percent increase to typically less than 5%.
_
The specific energy consumption (SEC), in kWh per m3 of product water, is the single most important parameter characterizing the performance of the desalination process, particularly from the standpoint of overall process sustainability. SEC is comprised of contributions from the operation of the various sections of an entire membrane desalination plant; i.e. (1) the feed-water intake facility, (2) the pre-treatment section, (3) the main desalination section (that includes high-pressure pumps, RO membrane trains and energy recovery devices [ERD]), the product post-treatment section and the brine treatment/disposal facility. The largest contribution to SEC, usually varying between 60% and 80% (depending on feed-water type, local conditions, technology employed) is due to the main section where the membrane desalination process is carried out.
_
Thermal and membrane processes are the two primary technologies that drive the desalination sector. In the thermal desalination process, energy is utilized in the form of heat to vaporize pure water from its salt mixtures, and in commercial membrane desalination processes, electric energy is used to run high pressure feed pumps to filter out the dissolved solids. In both processes, the stream of water containing less dissolved solid is the main end product and a concentrate water stream is the reject. Due to the progress in membrane technologies, desalination industries have gradually shifted towards it. By 2006, 96% of US based desalination industries and 56% of global industries had shifted from thermal-based to membrane-based processes.
Table below shows the energy consumption of different technologies used for desalination. The membrane technologies, specifically the RO, are preferred over the other technologies, mainly due to lower energy requirements as can be seen from Table below. The specific energy consumption (SEC) of different technologies varies widely, and depending on the process control and operation as well as the quality of the produced water, this value further differs significantly for a particular technology. The energy consumption in a process typically contributes a significant amount to the total cost of desalination, hence, understanding various factors affecting the SEC of the process becomes highly important for the development of sustainable energy-water sources.
Specific energy consumption (SEC) by different desalination techniques:
|
|
Electric |
Thermal |
Total Electric Equivalent |
|
BWRO |
0.5–3 |
– |
0.5–3 |
|
SWRO |
3–6 |
– |
3–6 |
|
ED |
1–3.5 |
– |
1–3.5 |
|
EDR |
1–2 |
– |
1–2 |
|
MVC |
7–15 |
– |
7–15 |
|
FO |
0.2–0.5 |
20–150 |
10–68 |
|
MD |
1.5–4 |
4–40 |
3–22 |
BWRO = brackish water reverse osmosis; SWRO = seawater reverse osmosis; ED = electrodialysis; EDR = electrodialysis reversal; MVC = mechanical vapor compression; FO = forward osmosis; MD = membrane distillation.
______
______
Theoretical Minimum Energy of Desalination:
Calculating the minimum amount of energy required to separate pure water from seawater allows us to understand how effectively energy inputs are being used by a desalination process. This theoretical minimum energy, which is independent of the desalination method chosen, is realized when the separation occurs as a reversible thermodynamic process. Thus, the energy for the separation will be equal in magnitude but opposite in sign to the free energy of mixing.
Thermodynamic principles state that any method of water desalination will be most efficient, if it involves a reversible thermodynamic process. The same energy is invested in any reversible desalination process, and it is independent of the detailed technology employed, exact mechanism, or number of process stages. Calculation of the energy for a particular reversible system is good for all, and it serves as the lower energy limit for any other process.
_
Osmosis is, in principle, a reversible process, though, its application deviates from reversibility. The energy of seawater desalination by osmosis is calculated here. Osmosis is the phenomenon of water flow through a semi permeable membrane that blocks the transport of salts through it. The external pressure on the salt solution determines the speed and direction of water flow through the membrane.
Figure above shows a vessel divided by a semi permeable membrane. The left side contains seawater, and a moving partition pushes the water through the membrane. The membrane blocks salt transport and only pure water flows through it. Therefore, the right side of the vessel will contain only pure water. The work W done by the partition is equal to the force F acting on it, multiplied by the distance x that it travels. The process is reversible since the direction of the partition movement, and therefore, also of the water flow through the membrane, can be reversed at any given moment.
_
The force acting on the partition is equal to the osmotic pressure multiplied by the partition area. The osmotic pressure π is given by van’t Hoff formula:
π = cRT
where c is the molar concentration of the salt ions, R = 0.082 (liter∙bar) / (deg∙mol), is the gas constant, and T = 300 K is the ambient temperature on the absolute temperature scale (Kelvin).
The amount of salt in seawater is about 33 gram / liter. Seawater contains a variety of salts, but the calculation will be simplified by assuming that all the salt is sodium chloride (NaCl). The atomic weight of sodium is 23 gram, and of chlorine is 35.5 gram, so the molecular weight of NaCl is 58.5 gram. The number of NaCl moles in seawater is, therefore, 33 / 58.5 = 0.564 mol / liter.
When NaCl salt dissolves in water it dissociates into Na+ and Cl- ions. There are two ions per salt molecule, so the ions’ concentration is twice the molecules’ concentration. c = 2 x 0.564 = 1.128 mol / liter. Inserting the values into the van’t Hoff formula yields the osmotic pressure:
π = 1.128 x 0.082 x 300 = 27.8 bar or, 27.8 kilogram per square centimeter.
Assume now that the partition area is one square centimeter. It then has to travel a distance of 1000 cm, or, 10 meters, in order to push one liter of solution through the membrane. The work of this travel is:
W = F x d = 27.8 x 1 x10 = 278 kg meter / liter or, 2780 Joules / liter, since 10 Joules are equal to 1 kg∙meter. (Or, 2780 / 3600 = 0.77 kWatt hour / cubic meter). One kilocalorie (kcal) is equal to about 4200 Joules, therefore, the work is 2780 / 4200 = 0.66 kcal / liter.
So 0.66 kcal / liter is the minimum energy required to desalination of one liter of seawater, regardless of the technology applied to the process. It comes to 0.77 kWatt hour /m3 of sea water.
_
Note:
The work W of desalination is:
W = F x d = π x A x d = π x V
Where π is the osmotic pressure, A is the partition’s area, and A x d = V is the volume of pumped water.
In different units:
W = π x V x100
for W in Joules (Watt seconds), π in bars, and V in Liters. Or:
W = π x V x (100 / 3600) = πV / 36
for W in kWatt hours, π in bars, and V in cubic meters.
_
It is interesting to compare this energy to the heat required to boil one liter of water and condense its vapors. About 70 kcal are required to heat one liter to the boiling temperature, then more 540 kcal are required to boil it. Most of the invested heat comes back during condensation and a lot of it is recoverable by use of heat exchangers. Yet it seems difficult to compete with the energy efficiency of desalination by reverse osmosis.
Producing a volume of desalinated water requires the pumping of a higher volume of seawater. Part of the input seawater is transformed into output desalinated water, and part of it goes back to the sea as a high-salinity water. The ratio of the desalinated water output-volume to the seawater input-volume used to produce it is called the water recovery ratio. Practical desalination systems are never fully reversible and there are energy losses that are due unavoidable irreversible contributions. These losses, that depend on the water recovery ratio, increase the energy of desalination above the reversible thermodynamic limit.
__
In efforts to reduce the energy demands, it is important to realize the theoretical minimum energy required for desalination processes. High water costs remain as one of the major barriers in extending the desalination technology, which in turn is influenced by the energy consumed by the conventional and membrane desalination processes, accounting almost 50-60% of the total costs. So we need to realize the theoretical minimum energy required for separating fresh water from SW, in order to push our efforts for reducing the desalination energy demands. Thermodynamics places a lower bound on the energy required for desalination processes. At present, we are operating close to the thermodynamic limit for membrane desalination processes. Hence, reducing the energy for such processes is becoming more challenging.
_
Minimum theoretical energy required for separating the salts from seawater to produce freshwater is 0.7 kWh/m3. In practice, much higher energy is required by currently available desalination technologies. Energy demands for water desalination range from 650 kWh/m3 for energy-intensive single-stage evaporation of seawater to 27 kWh/m3 for multistage flash evaporation and 3.7 kWh/m3 for RO.
_
Today, RO desalination plants have been built and are in operation in some regions of the world, especially for water supply in areas under water scarcity. These plants require minimal energy consumption, part of which can be covered by renewable energy sources, such as air turbines and solar panels. These plants minimize the burden to the local energy networks and the environment. More efficient process designs also help enhance energy efficiency as seen in table below.
The primary descriptor of importance for desalination processes is the amount of dissolved solids (primarily inorganic salts) represented by the total dissolved solids (TDS; the solids left over after water is evaporated from particle-free water). Table below shows Typical Water Sources for Desalination and their Total Dissolved Solids (TDS) Ranges as well as the Calculated Minimum Energy for Separation per Unit Volume (specific energy consumption). 2008 data:
|
Water Source |
Total Dissolved Solids (mg/L) |
Minimum Energy for Separation (kwh/m3) in RO |
|
Seawater |
15,000–50,000 |
0.67 |
|
Brackish water |
1,500–15,000 |
0.17 |
|
River water |
500–3,000 |
0.04 |
|
Pure water |
< 500 |
< 0.01 |
|
Wastewater (untreated domestic) |
250–1,000 |
0.01 |
|
Wastewater (treated domestic) |
500–700 |
0.01 |
In addition to being a measure of usability (such as for consumption), TDS levels determine the bounds for the minimum energy needed to remove these solutes from water (or to move water away from these solutes). Energy is needed to separate the solute from the solvent and is dependent on the concentration of the solute. Table above shows that higher-salinity water (such as seawater) requires larger amounts of energy for desalination, whereas water from low-salinity streams (such as those from wastewater reuse) could be much lower.
The growing pressure on limited freshwater sources has focused the world’s attention on seawater and the recovery of water from marginal sources such as brackish ground- and surface water as well as recycled wastewater. It has also raised awareness and catalyzed the implementation of wastewater reuse, where wastewater is treated to a high quality and in some cases used for direct or indirect potable reuse. Desalination is thus a critical technology for humanity to allow for sustainable development.
__
Table above showed minimal energy for separation for different types of water.
What about actual energy for separation?
Table below shows the energy required to produce 1 m3 of fresh water from distinct types of water sources using RO.
|
Water Source |
Energy (kWh/m3) |
|
Seawater |
2.58–8.5 |
|
Wastewater reuse |
1.0–2.5 |
|
Wastewater treatment |
0.62–0.87 |
|
Groundwater |
0.48 |
_
Indeed, removing the salt from seawater is an energy-intensive process. These energy requirements have declined dramatically over the past 40 years due to a variety of technological advances, and desalination designers and researchers are constantly seeking ways to further reduce energy consumption. Despite these improvements, desalination consumes more energy per gallon than most other water supply and treatment options as seen in the figure below:
Figure above shows the energy intensity, in kilowatt-hours (kWh) per million gallons, of various water supply options in California. Local sources of groundwater and surface water are among the least energy-intensive options available.
_
To produce fresh water, conventional desalination systems demand high energy requirements, which are usually obtained from fossil fuels. Table below shows the specific energy requirements to produce one cubic meter of fresh water from currently available technologies for commercial seawater desalination.
Typical total electrical energy consumptions in different desalination technologies.
|
Desalination Technology |
Specific Energy Requirements |
|
Multi-Effect Distillation |
14–21 kWh/m3 |
|
Multi-Stage Flash |
20–27 kWh/m3 |
|
Mechanical Vapor Compression |
7–12 kWh/m3 |
|
Thermal Vapor Compression |
16.26 kWh/m3 |
|
Seawater Reverse Osmosis |
4–6 kWh/m3 |
_
Table below shows range of operational energy requirement for seawater main desalination systems.
Note that MED is also called multi-effect evaporation (MEE).
_
Several options have been developed to improve the energy footprint of desalination technologies, among which is energy recycling and recovery, hybrid processes, process modifications, use of waste heat and integration with renewable energies. Broadly, the efficiency of the low-temperature heat is quantified by the gain output ratio (GOR), which measures the thermal energy consumed in the desalination process, and is defined as the ratio between the mass of distillate and the mass of steam input (kgdistillate/kgsteam). For MED, commercial manufacturers provide a GOR between 10 to 16; for MSF between 8 and 12 and for MVC, a GOR of around 12 kgdistillate/kgsteam. This explains why the high technological growth trend of MED over MSF, in particular, when coupled with other desalination systems to form more efficient hybrid systems, with less environmental impact and higher quality of the water produced.
Generally, thermal-based desalination techniques significantly consume more energy compared to membrane techniques. Only 131 desalination plants in the world, representing approximately 1% of the world’s water desalination capacity work with energy from renewable sources. However, use of renewable energy for seawater desalination has increased from 2% in 1998 to 19.3% in 2015.
_____
_____
Factors determining the energy consumption:
Different factors have been searched through the existing literature that affect energy footprint of water desalination. Some of them have greater influence on energy footprint than others. These factors are: capacity of desalination plant (small, medium, large), type of required energy (electrical or thermal energy), type of feed water (brackish or seawater), desalination method (thermal or membrane), use of renewable energy sources (RES) (solar, wind, geothermal), necessity of feed pretreatment (mechanical and/or chemical). All these factors determine the total energy consumption of desalination plant. They are categorized in the figure below.
Figure above shows factors determining energy consumption for desalination process.
Each of these factors affect the total energy consumption of the desalination plant. This means that for the main target of energy footprint reduction all these factors need to be considered. Environmental features, local area, design of desalination plant needs to be taken into account for the possibility to implement different types of technologies in order to create the most economically beneficial combination of these factors. Some combinations are widely applied, for example seawater reverse osmosis of large scale using electrical energy with pretreatment. Others are not, for example, seawater vapor compression of small scale using thermal energy without pretreatment.
__
Type of required energy:
Desalination processes require different types of energy, either thermal or electrical. Membrane technologies do not need thermal energy, all process steps are going using electricity. For RO plants, energy is needed to generate high pressure to force water to pass through the membrane. For ED plants, electrical energy is required to create electrical potential difference. Thermal technologies need both electrical and thermal type of energy to vaporize seawater in order to separate salts in them. Desalination techniques, such as MSF and MED, use thermal energy as a primary source and electricity to drive associated pumps as a secondary source. Thermal methods are more expensive than membrane ones because of the large quantities of fuel required to vaporize salt water. However, they are more efficient in terms of desalination of very salty waters than the membrane technologies. Electricity could be generated from fossil fuel like coal, oil, diesel, gas, renewable energy, and nuclear sources. Thermal energy could be produced from fossil-fuel-fired boilers, power-plant waste heat, renewable energy sources, and industrial-waste heat sources. Basically, high energy consumption is a critical factor that affects the economics of desalination. There is a need to make desalination processes as much energy efficient as possible by improving technology and equipment.
__
Type of feed water:
Energy demand of water desalination strongly depend on the type of water, whether it is seawater or brackish water, especially for membrane technologies. The salinity content of brackish water is up to 10000 parts per million (ppm) or 10 g/l and seawater normally has salinity in the range of 35000 – 45000 ppm or 35-45 g/l in the form of total dissolved salts making these waters unsuitable for drinking and most domestic uses. According to World Health Organization, drinking water quality permits a salinity of 500 ppm and 1000 ppm in certain cases.
Feed water has great influence on energy requirements of desalination plants. The energy that is required for desalination of seawater is higher than for the brackish water one, because of higher concentration rate of salt in a seawater. Brackish water desalination requires less energy because of the lower salinity, which enable to apply lower pressure and to obtain much higher water recovery. Thus, energy for desalination of seawater and brackish are different. Compared to seawater desalination, brackish water desalination requires around 0.5–2.5 kWh/m3 less energy. Thermal methods are more energy consumptive because of the large quantities of fuel required to vaporize salt water, however they seem to be more effective than membrane methods in terms of efficiency in the desalination of very salty seawaters.
_
Pretreatment:
Feed pretreatment is another factor determining the total energy consumption of desalination installation. This is particularly imperative for RO, but for distillation processes it is also highly important. Traditional pretreatment is based on mechanical treatment for removing from the feed debris and other unnecessary substances using screens and filters. Mechanical pretreatment supported by an extensive chemical treatment, including chlorination, flocculant dosing, dosing for scaling prevention. This process require energy, basically electrical for the pumps and mixing devices. Conventional intake system where the supply source is nearby the SWRO facility, power consumption is in range from 15% to 20% of the total power consumed by the water desalination process.
_____
_____
Comparison of energy consumptions:
Figure below shows energy consumption of different type of desalination technologies:
To understand how efficient certain method, it is good to compare desalination techniques with each other. Figure above depicts comparison of energy consumption of most applied desalination methods ranging from lowest to highest energy demand in kWh per m3. Membrane technologies are shown with blue color, thermal ones –with orange color and desalination methods that use renewable sources as an energy supply are shown with green color. Membrane technologies powered by renewable energy sources are marked with light green and thermal technologies powered by renewable energy sources marked with dark green.
_
Membrane-based desalination methods due to their less energy-intensive nature and small footprint became more popular than the thermal ones. Substantial efforts have been observed in integrating membrane technologies, mainly reverse osmosis (RO) and electrodialysis (ED), and relatively green sources of energy (wind, solar). It could be observed that brackish water ED powered by solar energy and brackish water RO powered by wind energy have the lowest energy demand among the others renewably powered desalination plants. However, they application is limited due to several factors, like availability at the same location both the brackish water and enough solar radiance or wind capacity. So, practically their application is restricted up to 200 m3/day for BW ED and up to 2000 m3/day for BW RO. This capacity is not enough for overcome the existing severe water shortages in many regions.
_
Next come membrane technologies powered by conventional energy sources. First come brackish water RO of medium and large scale and brackish water ED of medium and large scale with 1.9 and 2.9 kW h/m3 energy demand respectively. But availability of brackish water is somewhat limited, thus, although these technologies have relatively small energy demand per cubic meter of water, however they are not applied in large scales globally. The picture is quite different with seawater, which availability is practically unlimited because of seas and oceans. Seawater RO of medium size has energy consumption of 4.3 kW h/m3 and seawater RO of large scale has 4.4 kW h/m3 energy footprint. Due to selective nature of RO membrane, ions and other solute particles are rejected under the high pressure. Compared to its thermal counterparts, RO possesses the advantages of significantly less energy consumption, non-corrosive equipment, small footprints and relatively safer operation. Thus, seawater RO of medium and large scales are the most popular and economically efficient type of desalination method, making the installed share of SW RO of total globally installed desalination capacity more than 65%.
_
It is clear from figure above that membrane desalination plants powered by renewable energy sources are in between of conventional membrane and conventional thermal methods. They lose in energy demand to membrane technologies, but win the thermal ones. This could give a good incentive to continue improvements of implementation of renewable energy sources in desalination processes in the future.
_
The energy demand of thermal methods is quite large comparing with membrane technologies. They are more expensive because of the large quantities of fuel required to vaporize salt water. Thermal desalination now exists mainly in the regions enriched in petroleum resources such as Middle East. The power consumption of an MED plant is significantly lower than that of an MSF plant, and the performance ratio of the MED plant is higher than that of the MSF plant. MED technology has 11.9 kW h/m3 energy requirement and MSF has 17.1 kW h/m3. Therefore, MED is more efficient than MSF. However, there is a serious problem with corrosion in both thermal methods. Still the problem with corrosion is easier to solve with MSF compared to MED, because the design is less complex. This is the main reason that MSF has received wider global application than MED for desalination of very salty waters, despite the fact that energy demand for MSF higher than for MED. Thermal technologies powered by renewable energy sources have even more energy footprint. Wind VC has 13.5 kW h/m3 and solar PV MED has 21.1 kW h/m3, which make them extremely inefficient regarding with small plant sizes.
______
______
ENERGY FOR DESALINATION: A STATE-OF-THE-ART REVIEW 2020:
The utilization of seawater for drinking purposes is limited by the high specific energy consumption (SEC) (kW-h/m3) of present desalination technologies; both thermal and membrane based. Many technologies are already working near their thermodynamic limit, whilst posing challenges in further SEC reductions.
Highlights:
- Improvements in desalination SEC are necessary for efficient system performances.
- RO is operating very near to its thermodynamic limit.
- Thermal technologies operate far from their current thermodynamic limit.
- Desalination hybrids are attractive solutions for reduced energy consumptions.
- RE sources can be used for supplying energy to various desalination processes
_____
_____
Reduction in energy consumption for desalination:
To reduce energy consumption in desalination, seawater reverse osmosis (SWRO) has been intensively investigated. Recently, alternative desalination technologies, including forward osmosis, pressure-retarded osmosis, membrane distillation, capacitive deionization, renewable-energy-powered desalination, and desalination batteries have also been actively studied. Related major consortium-based desalination research projects and their pilot plants suggest insights into lowering the energy consumption of desalination and mitigation of the environmental impact of SWRO brine as well.
_
The energy consumption of RO technology has dramatically declined thanks to improvements in formulation, manufacturing procedures, and processes, such as energy recovery from pressurized brine. These advances rapidly enhanced sustainability and exponentially increased the implementation of these membranes for seawater and brackish water desalination as well as wastewater reuse.
Figure above shows decline in specific energy consumption of reverse osmosis membranes, 1978–2008.
_
For some cases, such as seawater reverse osmosis, it is argued that current membranes have reached very close to the thermodynamic limit of ~1 kWh/m3 and that further improvement in materials may not yield additional energy sustainability (Elimelech and Phillip 2011). On the other hand, advances in permeability and selectivity can still yield major gains in brackish water treatment and wastewater reuse.
Ultrapermeable membranes with very high salt rejection appropriate for reverse osmosis may substantially reduce the necessary energy (~45 percent) or plant infrastructure (pressure vessels, up to 65 percent) in low-salinity sources (Cohen-Tanugi et al. 2014) such as brackish water desalination and water reuse. The energy advantage is significantly lower for high-salinity seawater applications (15 percent less energy) but the plant size can be reduced by 44 percent (Cohen-Tanugi et al. 2014).
A focus on increasing selectivity rather than simply increasing membrane permeability has been proposed in recent work as a sustainable approach to improve membrane materials (Werber et al. 2016a).
_
Reverse osmosis (RO) desalination is reported to reach 3.0 kWh/m3 by a certain specific case, and an average product water cost close to 0.75 US$/m3 was achieved. After the introduction of an energy recovery device, RO desalination has now reached a technical barrier in energy consumption. To allow for further improvement, two major research trends have recently been highlighted: (1) development of high flux RO membranes, and (2) development of new technologies such as membrane distillation (MD) and forward osmosis (FO). The performance of RO membranes has been improved from 6000 gallons per day (GPD) to 12,000 GPD, due to novel membrane fabrication technologies. Also, membrane modifications are being spotlighted utilizing aquaporin, carbon nanotube, graphene, and other materials. The world’s first standalone FO desalination process, by Modern Water (Al Khaluf, Oman), with a capacity of 100 m3/d, has demonstrated its potential for commercial application. Nonetheless, FO still lacks economic feasibility due to the inefficiency in draw solute recovery. MD has a high potential to replace prevailing thermal desalination technologies, but in order for it to be economically feasible, a reliable (low grade) heat source is required. Such alternative desalination technologies currently have drawbacks in replacing RO desalination, thus hybridization with RO desalination is now consistently addressed for practical application: FO-RO, utilizing FO as pretreatment for RO or for reducing energy consumption in RO, RO-MD hybrid for improving RO recovery, or RO-PRO (pressure retarded osmosis) hybrid for converting chemical potential to electrical energy. Such hybridizations have just started to be considered in research laboratories and pilot plants, but guidelines for design, operation, and maintenance of such processes are yet to be established.
_
Energy Recovery Devices:
The high pressure pumping required to overcome the osmotic pressure in saline feedwater results in a saline concentrate stream which is highly pressurized. ERDs are commonly used to recover this hydraulic energy and transfer it to the feed stream, reducing both the amount of energy otherwise required by the HPPs and the size of HPP required (Guirguis, 2011). The earliest ERDs used in SWRO plants were centrifugal-type devices such as the Francis Turbine, Pelton Wheel and Turbocharger (Urrea et al., 2019). These devices convert the hydraulic energy of the concentrate into mechanical energy to drive a piston or pump, which transfers hydraulic energy back into the feed. Since around 2000, isobaric chamber ERDs have replaced centrifugal devices in most new SWRO plants. Isobaric ERDs transfer hydraulic energy from the concentrate directly into the feed, as the two streams come into direct contact (with minimal mixing). As a result of the single energy conversion, efficiency loss is reduced when compared with centrifugal ERDs.
__
What’s coming next?
Will the next generation of desalination technology also reduce energy consumption by half – or more? When will incremental SEC improvements in high-efficiency SWRO technologies reach the point of diminishing returns, and open the door for the next game-changing breakthroughs? It’s too early to tell, but many interesting technologies will most likely reduce the energy consumption and the carbon footprint of desalinated water even further. Here are just a few innovations we might be seeing more of soon:
- Improved membranes: Although already close to what is possible thermodynamically, membrane technology will almost certainly improve as they become even more permeable and fouling resistant.
- Better ERDs: Next-generation ERDs will likely reduce the impacts of brine-feedwater mixing and lubrication limitations. We can also expect less complexity in terms of the number of units that can be connected – as well as CAPEX and OPEX reductions over time.
- Renewable-energy driven desalination: Wind, solar, wave, and geothermal energy can all be harnessed to power desalination – and they will do so increasingly to reduce desalination’s carbon footprint. Many small-scale SWRO systems powered by renewable energy already exist, and larger plants that serve municipalities are also coming online, including the world’s biggest solar-powered SWRO plant in Al Khafji, Saudi Arabia, which produces 60,000 m3/d. The World Bank estimates that the cost of solar-powered thermal desalination will drop to as low as USD 0.90/m3 by 2050.
- Alternatives to SWRO:
Although replacing high-efficiency SWRO is unlikely for years to come, several technologies, some more viable and scalable than others, may compete for a share of the growing desalination market:
-Humidification-dehumidification desalination (HDH)
-Semi-batch/Batch RO – CCRO/Batch+ brine concentration
-Forward osmosis (FO) brine concentration and FO-RO
-FO-RO hybrid
-Radial deionization (RDI)
-Desalination by freeze crystallization
The combined effects of demographic growth, climate change, and water scarcity will almost certainly increase future demand for desalinated water. However, for desalination to be part of the solution – instead of adding to the problem by increasing CO2 emissions as it increases water output – more innovation will be needed.
______
______
Cogeneration system for power and water desalination:
It is possible to use energy in a dual use or cogeneration systems in which the energy sources can perform several different functions such as electric power generation and water desalination. Most of the desalinated potable water and electricity in the Arabian Gulf countries, and North Africa are produced by cogeneration plants associated with multi-stage flash desalination units operating on seawater. Although other distillation processes such as thermal vapour compression and MED are starting to find their way into the market, the MSF process is still considered as the workhorse of the desalination industry. This process has proven its reliability and flexibility over almost 50 years of plant design and operation. For large desalination capacity, the MSF process can be considered as the only candidate commercially. However, on the cogeneration plant side, the situation is different in that several alternatives are commercially available to provide the required electrical power and steam for desalination. In cogeneration plants, the electricity is produced with high-pressure steam to operate the turbines; the steam produced by boilers at temperatures up to 540°C. As this steam expands in turbines, its temperature and energy level are reduced. Distillation plants need steam with temperatures lower than 120°C and this can be obtained easily at the end of the turbine after much of its energy has been utilized in electric power generation. This steam is used in the desalination process and the condensate from the steam is then returned to the boiler to be reheated again for use in the turbine. The main advantage of this system is that it uses much less fuel than each plant operating separately and energy costs are a crucial factor in any desalination process. In contrast, one of the disadvantages is the permanent coupling between the desalination plant and the power plant which can create a problem in water production when the demand for electricity is reduced or when the turbine or generator is down for repair. The size of the desalination plant can be efficiently integrated with a power cogeneration plant, so that the ratio of desalted water to power production is consistent with water and power requirements of the community it serves. Cogeneration plants have also reduced power costs. Meanwhile, other types of cogeneration plants have achieved lower costs by benefiting from heat recovery systems on gas turbines, heat pumps and other industrial processes such as burning solid wastes in an incinerator.
__
A combined-cycle gas turbine (CCGT) power plant is essentially an electrical power plant in which a gas turbine and a steam turbine are used in combination to achieve greater efficiency than would be possible independently. The association of Power Generation and Desalination can save a lot of energy as seen in the figure below:
In this approach, a typical power plant produces high pressure/temperature steam, which is normally expelled from the power plant as waste. A cogeneration plant uses this steam as an additional energy source during the desalination process in order to reduce fossil fuel use.
The power plant gains extra revenue by selling the waste steam to the desalination plant, while the desalination plant avoids having to pay for the construction and operation of its own energy source.
__
Cogeneration is generating excess heat and electricity generation from a single process. Cogeneration can provide usable heat for desalination in an integrated, or “dual-purpose”, facility where a power plant provides the energy for desalination. Alternatively, the facility’s energy production may be dedicated to the production of potable water (a stand-alone facility), or excess energy may be produced and incorporated into the energy grid. Cogeneration takes various forms, and theoretically any form of energy production could be used. However, the majority of current and planned cogeneration desalination plants use either fossil fuels or nuclear power as their source of energy. Most plants are located in the Middle East or North Africa, which use their petroleum resources to offset limited water resources. The advantage of dual-purpose facilities is they can be more efficient in energy consumption, thus making desalination more viable.
The current trend in dual-purpose facilities is hybrid configurations, in which the permeate from reverse osmosis desalination is mixed with distillate from thermal desalination. Basically, two or more desalination processes are combined along with power production. Such facilities have been implemented in Saudi Arabia at Jeddah and Yanbu.
A typical supercarrier in the US military is capable of using nuclear power to desalinate 1,500,000 L of water per day.
__
Advantages and disadvantages:
- The major advantage of cogeneration system is that it uses very less fuel than other plants operating separately and the energy costs are less for desalination process.
- In contrary, one of the disadvantages is that, problems can occur due to permanent coupling between the desalination plant and the power plant which can create a problem in water production when the need for electricity is reduced or when the turbine or generator has a problem.
_____
_____
Section-8
Cost of desalination:
There are two ways to think about the cost of desalination: the cost of a desalination plant, and the cost of water.
A typical large scale desalination plant produces 100,000 cubic meters of water per day. Assuming a per capita consumption of 300 liters per day, this equates to 300,000 people. The installed cost of desalination plants is approximately $1m for every 1,000 cubic meters per day of installed capacity. Therefore, a large scale desalination plant serving 300,000 people typically costs in the region of $100 million. The costs of infrastructure to distribute water must be added to this.
The cost of desalinated seawater, the majority of which is accounted for by plant capital costs and energy costs, is typically in the range of $0.5 to $3 per cubic meter of water. The lower end of the scale corresponds to regions where electricity costs are low (e.g. Middle East) and the higher end to regions where electricity costs are high (e.g. Australia, where electricity is sometimes mandated to be from renewable energy).
_
Factors that determine the costs for desalination include capacity and type of facility, location, feed water, labor, energy, plant technology, specific conditions of the project, financing and concentrate disposal. Therefore, it is challenging to provide an exact cost for a liter of desalinated water from the ocean as it can vary significantly. However, as a general estimate, the cost of desalinated water typically ranges from $0.50 to $3 per cubic meter ($0.0005 to $0.003 per liter). These figures can vary depending on the factors mentioned earlier, as well as local factors such as labor costs, infrastructure requirements, and the availability and quality of feedwater (ocean water) for desalination.
Costs of desalinating sea water (infrastructure, energy, and maintenance) are generally higher than fresh water from rivers or groundwater, water recycling, and water conservation; primarily due to the high energy requirements and capital investments associated with the desalination process, but alternatives are not always available.
It’s important to consider that advancements in desalination technology, economies of scale, and improved energy efficiency may contribute to reducing the cost of desalinated water in the future. Additionally, regional factors and government subsidies or incentives can also influence the cost of desalinated water projects.
In the future, costs are likely to fall with greater adoption of technologies like vacuum boiling, which can cut energy use by over 60%. Improved membranes and renewable energy integration can also reduce costs. However, seawater desalination is likely to remain relatively more expensive than freshwater sources like lakes and aquifers. Government subsidies and private-public partnerships can help improve affordability.
_____
Economic cost of Desalination:
The economic cost of desalination plants is divided into two main parts. The capital cost is the construction cost of all the operational units and supporting units in the desalination plant. Operational cost, which includes all operations, maintenance, replacement, etc.
Capital Cost:
Capital cost, the acronym CAPEX is often used to express the Capital Expenditures of desalination plants. The capital expenditures include construction the infrastructure and operational units. The capital cost also includes the inputs to the feed water and the outputs of the saline wastes and the treated fresh water. The main operational units and the supporting units as well as the pre-treatment units are included in the capital cost which include pumps, membranes, filters and all accessories.
Operating Cost:
Operating costs OPEX, is the cost that relates to operating, maintaining and replacing parts in desalination plants. Also, the operational cost includes energy cost, annual cost of labor, maintenance of units and replacement of damaged parts such as filter membranes, chemicals in pretreatment units. In addition to the periodic cost of monitoring and analysis units.
Total Cost to Desalinate Water:
The total cost includes all operational and capital expenditures. Thus, the total cost is on an annual basis for each cubic meter or gallon of water produced from the desalination plant.
____
Figure below shows a typical breakdown of the seawater desalination costs.
_____
Although desalination has been considered a critical source for fresh water worldwide, one of the main challenges to extend it is its high cost. According to the Global Water Intelligence (GWI) DesalData, a total of $93,700 million is expected to be spent on desalination projects in the coming four years. Approximately, $51,600 million is dedicated only for operating expenditures. As shown in figure below, operating costs are divided into four main services which are energy, labor, replacements, and chemicals. Almost 50% of the operating expenditures will be spent on thermal and electrical energies. This high cost of energy for desalination is not surprising given that in 2014, desalination was classified as the most energy-intensive water treatment process which consumes 75.2 TWh of energy per year.
Figure above shows Desalination operating expenditure by service.
______
The cost of desalination can be broken down into the following elements:
- Capital cost (CAPEX) (40 percent)
- Labor (3 percent)
- Membrane replacement (3 percent)
- Maintenance and parts (7 percent)
- Consumables (3 percent)
- Electrical energy (44 percent)
Seawater Desalination has historically been perceived as a more expensive option compared with the traditional treatment of surface or groundwater. However, there an improvement in the overall cost, including operational costs (OPEX) and the initial capital expenditure (CAPEX). Very recently, project tenders in Abu Dhabi, Saudi Arabia, and Israel have seen the price fall to the range of $0.50/m3 for the first time.
_____
Determinants of costs of desalination:
Theoretically, all desalination processes, including those yet to be invented, have certain minimum requirements for energy. However, inefficiencies arise in all desalination processes due to the transport of energy in the process, or transport of matter at phase boundaries (National Academy of Sciences, 1962; Water Corporation, 2000). These inefficiencies increase the energy requirements of desalination methods, thus raising unit water costs.
Since all desalination processes can use some combination of energy sources and can be designed for different levels of energy efficiency, simple economic comparisons are difficult to make. However, it is clear that the cost of desalting is determined by a number of technical and economic factors. The major categories are capital costs, and operating and maintenance costs. These two categories are interdependent; that is, if one component is increased the other component usually decreases. Many factors play a significant role in determining the cost of desalinated water. These factors include the type of technology used, plant size, geographical location, plant capacity, pretreatment requirements, quality of feed water and power cost. Out of these, three factors have a dominant effect on the cost of desalination per unit of fresh water produced: the feedwater salinity level, energy costs, and economies of size.
Feedwater salinity level:
By definition, the feedwater is divided into two saline categories: brackish water and seawater. The general trend is increasing the salt content of the feedwater increases the operating costs, as more apparatus (such as membrane area or the number of stages of distillation) is needed (Popkin, 1968; Khan, 1986; Buros, 1999). Typically, the cost of desalting seawater is three to five times the cost of desalting brackish water from the same size plant for both membrane and thermal methods (Buros, 1999; Water Corporation, 2000). Seawater with high levels of salt or other impurities may require more extensive pre-treatment, increasing costs. Membrane processes most economically achieve brackish water desalting with reverse osmosis.
Energy costs:
Desalination is an energy-intensive process, and the cost of energy can significantly impact the overall cost per gallon. Countries with low energy costs or access to renewable energy sources can produce desalinated water at a lower cost. A major characteristic of all desalination processes is their requirement for thermal or electric energy input, which can represent 50 to 75 percent of operating costs. The future of desalination technology will depend largely on reducing the energy cost by optimising power and water generation. The form of energy available and environmental constraints related to the energy source contribute to the cost of energy for desalination. Reverse osmosis has the lowest energy demand and this consequently makes it more attractive in many instances, compared to the well-tried multistage flash distillation.
Economies of size:
Economies of size arise when increases in the plant size (kilolitres of water produced per day) bring decreases in the unit fresh water cost (i.e. lower average total costs). As shown in figure below, there is an economy of scale associated with increasing plant capacity to effectively lessen membrane desalination plant unit construction costs.
Economies of size are evident in all desalination processes, but to different extents. Reverse osmosis exhibits some scope for economies of size, while distillation processes show the greatest potential. Larger plants can achieve economies of scale, resulting in lower costs per gallon. Smaller plants, however, may have higher costs due to lower production volumes and less efficient operations. The operating and maintenance costs are not subject to economies of size, but are directly affected by the water quality to be treated. Exploiting economies of size for distillation methods has been proven an efficient means of reducing the cost of desalted water.
Desalination technology:
Different plants may use different desalination technologies, such as reverse osmosis, multi-stage flash, or electrodialysis, each with varying levels of cost efficiency and energy consumption.
Geographic location:
The location of the desalination plant can affect costs in several ways. Factors such as access to seawater, proximity to energy sources, and local regulations can all influence the overall cost of desalination.
Environmental considerations:
Desalination plants must comply with environmental regulations, which can impact the cost of operations. For example, plants may be required to invest in technologies to mitigate their environmental impact, such as brine disposal or greenhouse gas emissions reduction.
Maintenance and operational costs:
The ongoing maintenance and operation of a desalination plant can contribute to the overall cost per gallon. Factors such as labor costs, membrane replacement, and chemical usage can all influence the operational costs of a desalination facility.
Labor costs:
Labor costs vary across the world, with some regions having higher wages and associated costs than others. This can influence the overall cost of desalination.
Local infrastructure and logistics:
The availability of existing infrastructure, such as pipelines, storage facilities, and transportation networks, can impact the cost per gallon. Inadequate or costly infrastructure can lead to higher desalination costs.
_
Figure below shows site-specific cost-determining factors for desalination:
_______
_______
Overall costs have been rapidly decreasing. Recent typical costs of water production show considerable reductions for both thermal technologies (MSF and MED), but particularly for SWRO, which is now registering costs as low as US$ 0.64 per cubic meter (2016) in favorable physical and business environments. A database was built containing over 50 desalination projects from around the world constructed over the last two decades. Table below draws on this database, shows the actual costs of desalination by technology and feedwater source for SWRO plants.
Summary of Worldwide Seawater Desalination Costs in 2016:
|
Desalination method |
Capital costs (million US$/MLD) |
O&M costs (US$/m3) |
Cost of water production (US$m3) |
|||
|
Range |
Average |
Range |
Average |
Range |
Average |
|
|
MSF |
1.7–3.1 |
2.1 |
0.22–0.30 |
0.26 |
1.02–1.74 |
1.44 |
|
MED-TVC |
1.2–2.3 |
1.4 |
0.11–0.25 |
0.14 |
1.12–1.50 |
1.39 |
|
SWRO Mediterranean Sea |
0.8–2.2 |
1.2 |
0.25–0.74 |
0.35 |
0.64–1.62 |
0.98 |
|
SWRO Arabian Gulf |
1.2–1.8 |
1.5 |
0.36–1.01 |
0.64 |
0.96–1.92 |
1.35 |
|
SWRO Red Sea |
1.2–2.3 |
1.5 |
0.41–0.96 |
0.51 |
1.14–1.70 |
1.38 |
|
SWRO Atlantic and Pacific oceans |
1.3–7.6 |
4.1 |
0.17–0.41 |
0.21 |
0.88–2.86 |
1.82 |
|
MSF/MED Hybrid |
1.5–2.2 |
1.8 |
0.14–0.25 |
0.23 |
0.95–1.37 |
1.15 |
|
SWRO |
1.2–2.4 |
1.3 |
0.29–0.44 |
0.35 |
0.85–1.12 |
1.03 |
MED-TVC = multiple effect distillation with thermal vapor compression; MLD = million liters per day; MSF = multistage flash distillation; O&M = operation and maintenance; SWRO = seawater reverse osmosis.
Key criteria for choice include the following:
- RO desalination is the most cost-competitive technology for less saline environments, but thermal technology is more competitive for higher salinity environments.
- MSF is the most expensive desalination technology in terms of CAPEX, but it is easier to operate and yields higher economy of scale benefits for megasize projects than RO.
- MED-TVC technology is more competitive than MSF for small- and medium-size desalination projects.
- Source water conditions make a big difference to costs for SWRO, but not for thermal technology.
- Hybrid thermal/RO projects can be the most competitive when there is access to cheap energy and there is a large unmet demand for water.
- Regulatory regimes also affect costs.
- Energy use and GHG emissions can be factors, going forward under the Paris Climate Agreement and the 2030 Sustainable Development Agenda.
_____
_____
Economics of water desalination using membrane processes:
The cost of membrane desalination technology has steadily decreased from its commercial introduction in 1970s until today, despite rising energy prices. Recent developments in membrane materials, pumping and energy recovery systems have dramatically reduced the energy consumption in RO desalination processes. The cost of water desalination in membrane processes varies according to the type and composition of the feed water. Large-scale RO plants can use brackish water containing total dissolved solids (TDS) of from 2000 to 10 000 ppm, but, as TDS concentrations increase, the unit cost of the desalinated water also increases. The cost of brackish water desalination in the Middle East with a TDS concentration of 2300 ppm is 0.26 $/m3, while in Florida, for brackish water with a TDS of 5000 ppm, it is 0.27 $/m3. In small-scale RO units, the costs are greater. For example, the cost of a desalination unit of 1000 m3/day ranges from 0.78 to 1.23 $/m3 or even 1.33 $/m3. For the desalination of seawater, the RO method has been used more and more in recent years, as the cost of membranes has fallen, but it is still slightly costly. For instance, the estimated water production cost for the world’s largest RO seawater desalination plant at Ashkelon in Israel with a capacity of 320000 m3/day is 0.52US$/m3 and costs at an RO plant with a capacity of 94 600 m3/day in Tampa Bay in USA was reported to be at $0.56/m3. Table below shows typical costs for RO desalination of brackish water and seawater.
Cost of desalinated water in membrane (RO) plants:
|
Type of feed water |
Capacity of desalination plant (m3/day) |
Desalination cost per m3 (US$) |
|
Brackish water |
<20 |
5.63–12.9 |
|
20–1200 |
0.78–1.33 |
|
|
40 000–46 000 |
0.26–0.54 |
|
|
Seawater |
<100 |
1.5–18.75 |
|
250–1000 |
1.25–3.93 |
|
|
15 000–60 000 |
0.48–1.62 |
|
|
100 000–320 000 |
0.45–0.66 |
_____
_____
Cost Reduction Strategies:
Operation costs account for two-thirds of water production costs, while one-third is based on capital cost depreciation. Energy accounts for 50% of the operation cost when membranes, pumps and energy recovery devices with standard efficiency are used. Basic thermodynamics explains the inherent energy cost of producing potable water from seawater. These analyses use second-law considerations to estimate inefficiencies from concentration differences and from converting one stream into two streams at different thermodynamic states. Calculations show that the minimum energy needed for 38,000 mg/L of seawater at 45% recovery with a product water of 300 ppm is 2.1 kJ/kg of permeate, which corresponds to a usage of 0.6 kWh/m3. However, the historical energy consumption of seawater plants has been in the range of 2.5 kWh/m3 to 4 kWh/m3, or four to six times higher than the thermodynamic requirement.
This energy loss is due to different factors:
- Inefficiency of feed pump and motor;
- Water permeating through the membrane;
- Energy lost with brine.
Even if the system used 100% efficient pumps, motors and energy recovery, the energy required to permeate water through conventional membranes adds 1.1 kWh/m3 to the thermodynamic requirement of 0.6 kWh/m3, which triples the energy requirement! With pressurised brine, the system can lose between 0.1 and 1.9 kWh/m3, depending on the use of energy recovery devices and their efficiency (e ER). Depending on pump efficiency, the system can lose an additional 0.4 to 1.6 kWh/m3. Consequently, efficiency increases in pump, motor and energy recovery devices lead to considerable energy savings. The largest fraction of energy use is the permeation of water through the membrane. To minimise energy consumption and reach levels as low as 2.0 kWh/m3, elements with higher productivity, which can also reduce other costs, such as the pretreatment or capital cost, are needed. A new generation of SWRO membranes enables further cost reduction through high fouling resistance, increased productivity and rejection, and improved cleanability. The savings depends on site conditions and permeate quality requirements.
Designing systems around high-flow, low-energy elements can enable significant reductions in capital expense (CAPEX), operational expense (OPEX) or both.
To reduce capital expense, designers can use the membranes’ higher productivity to increase capacity using the same number of pressure vessels and membrane elements, or use fewer pressure vessels and membrane elements for the same capacity.
To reduce operating expense, the feed pressure can be reduced while using the same number of elements to achieve the same flow rate, resulting in lower energy costs at lower flux.
To reduce both capital and operating expense, water production and recovery can be increased using the same number of membranes, resulting in lower pump and pre-treatment capital and operating costs.
_
There are several strategies that can be employed to reduce the cost per gallon of desalinated water:
- Energy efficiency improvements: By investing in more energy-efficient technologies and processes, desalination plants can reduce their energy consumption and, consequently, their costs. For example, using waste heat from other industrial processes or integrating renewable energy sources can significantly lower energy costs.
- Pre-treatment optimization: Improving pre-treatment processes can reduce the need for chemicals, maintenance, and energy, resulting in cost savings. Advanced monitoring and control systems can help optimize pre-treatment and reduce fouling, scaling, and corrosion.
- Recovery of valuable by-products: Some desalination processes produce by-products, such as salt and minerals, which can be sold or used in other applications. This can help offset the costs of desalination and reduce the overall cost per gallon. Brine can be recycled within the desalination process. The use of waste brine in the manufacture of sodium hypochlorite, a chemical disinfectant that can substitute chlorine, is a promising development. Research has shown that on-site sodium hypochlorite production can save Caribbean desalination plants more than £300,000 per year.
- Public-private partnerships and financing: Collaborating with private entities can help secure financing for desalination projects and reduce costs. Governments can provide incentives, such as tax breaks or low-interest loans, to encourage private investment in desalination infrastructure.
- Research and development of novel technologies: Investing in the research and development of new desalination technologies can lead to more efficient and cost-effective solutions. For example, capacitive deionization is an emerging technology that have the potential to significantly reduce the cost of desalination.
_
Technology advances are expected to reduce the cost of desalinated water by 20% in the next five years, and by up to 60% in the next 20 years (see Table below), making it a viable and cost-effective competitor for potable water production.
Forecast of Desalination Costs for Medium and Large Size Projects in 2016:
|
Parameter for Best-in Class Desalination Plants |
Year 2016 |
Within 5 Years |
Within 20 Years
|
|
Cost of Water (US$/m3) |
0.8 – 1.2 |
0.6 – 1.0 |
0.3 – 0.5 |
|
Construction Cost (US$/MLD) |
1.2 – 2.2 |
1.0 – 1.8 |
0.5 – 0.9 |
|
Electrical Energy Use (kWh/m3) |
3.5 – 4.0 |
2.8 – 3.2 |
2.1 – 2.4 |
|
Membrane Productivity (m3/membrane) |
28-47 |
35-55 |
95-120 |
RO desalination plants have limitations in spatial requirements but could be easily adapted to the variations in productivity. The manufacturing process is relatively simple. It necessitates the flexibility of RO installations being adapted to the water demand variability with small footprints. Also, the cost of water production needs to be kept as low as possible, even though it remains higher than the other processes. However, it is less expensive than the cost needed for drinking water transportation. Technological progress in desalination is expected to cut down the cost of water production by 20% within 5 years and by ~60% in the next 20 years (see Table above), making the RO more viable and cost-effective for potable water production.
_
Desalinated water has become cheaper:
Energy Monitor notes that “globally, around 1% of the world’s drinking water is desalinated, but in Israel, that figure is around 25%.” Israel’s desalinated water comes from five desalination plants. The Sorek B plant has a capacity to desalinate 52.8 billion gallons a year and is contracted to produce water for $0.41 per cubic meter. There are around 264 gallons per cubic meter, so this puts the cost at about a penny per 6.4 gallons.
_
Water from desalination is inexpensive, and could be even cheaper if cheaper electricity is supplied. For instance, the cost of production of water from the 100 MLD Nemmeli plant in Chennai, run by VA Tech Wabag, works out to ₹38 for 1,000 litres (around ₹55 including finance costs, depreciation and transportation); cost of electricity alone accounts for 70 per cent of it. The plant buys electricity at ₹6.35 a kWhr. Compare this with the national average power purchase cost, which is ₹3.60/kWhr or the Tamil Nadu’s utility’s average power purchase cost of ₹4.29, you will see how much cheaper electricity a desalination plant could buy power. Improvements in technology have made desal (desalination) water cheaper. In the 1980s, a desalination plant used to consume 9 kWhr of electricity to produce thousand litres of water. Now it has come down to 3.8 kWhr, because of systems that recover energy from the pressure of the discharge water. Power costs — and consequently cost of water — could further come down if electricity is made available to these plants cheaper. If a solar power plant is linked to a desalination unit and wheeling charges are waived, the cost of water would drastically come down.
_____
_____
Economic sense of desalination:
Desalination is still the most energy-intensive technology for producing drinking water, and it is usually only implemented as a last resort when conventional freshwater resources have been stretched to the limit. The global concerns over climate change, water scarcity, rapid urbanization, and industrialization are some factors that directed many scientists and engineers to think of desalination to meet the demand of freshwater supply worldwide. However, the costs required to produce and distribute freshwater from seawater through desalination is still a matter of debate when compared with the costs associated with conventional water supply systems (coagulation, flocculation, sedimentation, and filtration scheme). As illustrated in Table below, the cost of desalinated water was approximately two times higher compared to the cost of the conventional water supply. Likewise, the cost related to energy consumption was also approximately 5–25 times higher for desalinated water compared to conventionally treated water.
Comparison of water costs for conventional and desalination water supply options (Voutchkov, 2011, 2014; Plappally, 2012).
|
Range |
Energy Requirements (kWh/m3) |
Water Production Costs ($/m3) |
||
|
Conventional Water Supplies |
Seawater Reverse Osmosis (SWRO) |
Conventional Water Supplies |
Seawater Reverse Osmosis (SWRO) |
|
|
Low |
0.1–0.5 |
2.5–2.8 |
0.25–0.75 |
0.5–0.8 |
|
Medium |
1.0–2.5 |
3.0–3.5 |
0.75–2.50 |
1.0–1.5 |
|
High |
2.5–4.5 |
4.0–4.5 |
2.50–5.00 |
2.0–4.0 |
_
In most of the urban cities, the available freshwater water resources have reached the capacity limit mainly because of population growth and urbanization. This circumstance has forced many cities to treat their brackish water, seawater, and wastewater via desalination or to transport their freshwater from long distances. The choice depends upon the cost requirements or the decisions of the governments of the respective countries. Gude (2016) compared the relative average cost for providing drinking water in various countries using conventional treatment. For instance, people in Beijing, China, pay the average cost of about USD 1.13/m3 for desalinated municipal water. The desalinated seawater in Beijing is collected from a distance of 135 km, with an elevation difference of 100 m from the source to the distribution site. Likewise, in Delhi, India, the cost for the desalinated water is about USD 1.9/m3. In this city, the desalinated water is transported from a distance of 1050 km, with an elevation difference of 500 m. On the other hand, the costs paid for municipal water by most European citizens are much higher compared to the costs paid by citizens from developing countries. The difference in the cost for water could be due either to government policy or due to strict environmental and economic standards of these countries. For instance, the lower water prices in India and China compared to European countries could be related to the fact that, in these countries, the water prices are highly subsidized by the government.
_
These considerations are central to decision making around desalination.
-1. Portfolio effects – understanding the sum of the parts
The cost of desalination is often compared to the cost of new dams. While understanding the cost per unit of water produced has some value, it does not tell the full story. It is important to account for portfolio effects which consider how any new source works together with existing sources to meet the desired objective. For example, investing in a new dam might be the cheapest option on an average dollar per kilolitre basis, but if the dam is located in a catchment where rainfall is highly correlated with rainfall to existing dams, it might contribute little towards meeting the appropriate level of water supply security. Desalination might be more expensive on a dollar per kilolitre basis, but it can supply water when it is most needed. These portfolio effects can be readily assessed to determine whether desalination fits the optimal mix for any city or town.
-2. Customer preferences – how much does the community value water security?
The appropriate level of water supply security depends on the costs and benefits of increasing (or decreasing) water supply security. Understanding the costs of increasing water supply security is mostly a technical engineering problem. However, understanding the benefits is an economic problem requiring an understanding of customer preferences. This matters for desalination because the higher the appropriate level of water supply security, the greater the benefits from desalination will tend to be.
-3. Climate resilience – is the investment robust over different climate sequences?
The benefits of desalination also depend on future rainfall and temperature. If the construction of a desalination plant is followed by a sequence of relatively wet years, the benefits are likely to be far lower than under a sequence of extremely dry years.
-4. Construction and operation – optimising desalination
Because we simply do not know exactly when it will rain and when desalination plants will be required, there is a risk of getting investment decisions wrong with the benefit of hindsight. Indeed, many decision makers have been harshly criticised for just that. While judgement should be based on the quality of the risk-based decisions made at a point in time, not with the benefit of hindsight, there are embedded options that need to be considered based on their ability to reduce the cost of uncertainty. These include readiness options (i.e. splitting the build decision into component parts and completing various stages that reduce the lead time for implementation such as environmental and planning approvals) and staging (i.e. the idea of building with the option for modular additional components of supply to come online flexibly over time). In terms of operation, desalination plants are expensive to run, including as a result of significant energy costs. However, their effectiveness is limited if storages are left to decline too far before being turned on. To maximise the benefits from desalination, it is important to consider the full range of options for constructing and operating desalination plants.
_____
_____
Costs of Water Transport:
Seawater desalination plants are typically located in the coastal area. However, not all the water scarce regions are close to the coast, which generates a need to transport water from desalination plants to where water is needed. Transport costs range from a few cents per cubic meter to over a dollar for 100 kilometers. A 100 m vertical lift is about as costly as a 100 km horizontal transport (0.05-0.06$/m3). Transport makes desalinated water prohibitively expensive in highlands and continental interiors, but not elsewhere. Kally’s [1993] cost estimates make clear that horizontal distance is not the main driver of water transport costs, but the vertical distance is.
______
______
Section-9
Challenges and problems of desalination:
Globally, more than five hundred million people depend on desalinated water for day-to-day water needs. Presently more than twenty-one thousand desalination plants function in 150 countries. Desalination tech works with both seawater water & brackish water. However, there are some issues faced during the desalination process. As policymakers increasingly fear water shortages, private companies are marketing desalination as a solution. While they offer to take the salt out of seawater for two or more times the cost of other water sources, they fail to advertise the toxic chemicals, marine life damage, carbon emissions and other social and environmental ills that come along with the process.
_
-1. Cost:
Ocean desalination is expensive. Although the price tag varies and the true price is often hidden by corporate underestimates and government subsidies, it consistently costs at least twice as much as other options. The cost of desalinization is about $2000 per acre-foot, which is roughly the amount of water a family of five uses in a given year. This is equivalent to a cost of about $0.61 for every 100 gallons. Piping and pumping the water will increase these costs. Such an expense means that desalinization is usually a cost-effective option for wealthy, coastal cities. However, for elevated or inland areas, it is often cheaper to simply transport freshwater or to focus on water-conservation strategies.
_
-2 Environmental effects:
The first is the environmental impact of the intake system. Desalinization plants require the intake of massive volumes of water, usually from a nearby ocean. These inevitably run the risk of sucking in fish, fish eggs, shellfish, and other organisms. Smaller organisms are usually killed by heat or exposure to chemicals. Meanwhile, larger organisms can be trapped inside intake pipes or pressed against screens by the pressure of the flowing water.
The second issue is the environmental impact of the plant’s outflowing water. By definition, desalinization plants extract fresh water and leave behind a salty brine. Desalination typically reclaims only 60 to 85 percent of brackish water that enters a plant, and only 35 to 60 percent of ocean water. The remaining water ends up as a brine two to 10 times more concentrated than the source water. The salt content of the brine, as well as its temperature and the existence of pretreatment chemicals, makes this output an environmental hazard. But its effect can be minimized by diluting the brine. This can be done by mixing it with other outflowing water (i.e. cooling waters from a nearby power plant) or by releasing it gradually (i.e. splitting the outflow pipes into branches). Brine disposal can be troublesome for domestic desalination, where it can’t be discharged into surface water bodies or municipal sewers, requiring the necessity of reinjection wells or evaporation ponds.
_
-3. Human health:
The portion of the water that reaches the customer can contain unregulated chemicals not present in normal drinking water, which may endanger the public health. These contaminants include chemicals such as endocrine disruptors, pharmaceuticals, personal care products and toxins from marine algae. Desalinized water is inevitably low in iodine, as that element is filtered out by the reverse-osmosis membranes or left behind during the evaporation process. This can lead to iodine deficiencies (which, for example, can cause goiter) in a population that is habitually drinking desalinized water.
_
-4. Membrane Performance:
If possible, engineers want to attain the highest salt removal at a very low operating pressure. Typically, SWRO operates with pressures in the range of 60-70 bar. Generally, if the pressure is low, less salt is eliminated from the finished water. To save on energy & reduce costs, membranes that should maintain salt removal at pressures of 50-55 bar are required. Therefore, researchers are attempting to create membrane materials that can be functioned at high pressures & salinities. Moreover, this not only includes new membrane materials but also how the membranes are assembled & operated.
_
-5. Altering Seawater Conditions:
Sudden & variable seawater conditions—particularly harmful phytoplankton blooms—might subdue the intake pre-treatment system for desalination plants. In certain cases, stand-by DAF (dissolved-air-flotation) systems might be installed for such an occasion, but it’s a large capital outlay for a subsystem that might not run very often.
_
-6. Membrane Fouling:
Material gradually accumulates on membranes in the course of operation, like organic material contaminating the feed water, inorganic solutes, & biofilms. The overall effect is a depletion of permeability, which is generally offset by slightly increasing pressure to keep up the water production. Moreover, this adds to the power consumption & cost of water over the lifetime of the plant. The membrane surface fouling throughout operation reduces the membrane productivity, and if the fouling conditions continue, the salt rejection will suffer. Even though membrane cleaning is regular, it takes the plant offline & generates liquid waste to be discarded.
There are three sources for membrane fouling: particles entrained in feedwater, build-up of sparingly soluble minerals, and by-products as a result of growth of microorganisms.
A frequent cleaning is required to handle these conditions, which is costly and leads to a shorter service life of the membrane elements. In general, the suspended solids should be eliminated from the feed to the membranes, and for the membrane plant to function well, a suitable pretreatment for the feed is needed. Sparsely soluble minerals are mainly barium and silica, and these contribute to hardness. Microorganism’s growth is most pronounced within the temperature range of 30–45°C.
Membrane fouling — organic, inorganic, and biological — is a challenge to pressure-driven membrane desalination. During pretreatment, chlorine is added to prevent biofouling; however, chlorine can cause oxidative damage to the membrane. This requires additional steps of de-chlorination and re-chlorination. To simplify treatment processes, chlorine-tolerant membranes need to be developed. Another limitation of reverse osmosis is insufficient rejection of small neutral solutes, such as boron. This can be overcome with additional treatment steps.
_
-7. Feedwater limitations prior to the RO permeates:
The following are the feedwater limitations prior to the RO membranes set by the permeate manufacturers. The pretreatment process should take into consideration the following guidelines that are shown in Table below.
Feedwater limitations prior to RO permeate:
|
Feed iron, aluminium, and manganese |
Not more than 0.05 mg/l |
|
Feed bacteriological content |
0 |
|
Feed chlorine or other oxidants |
0 |
|
Feed SDI after filtration |
<3 |
|
Feed organic content (TOC, BOD5, COD) |
0 |
|
Feed oil, hydrocarbons, grease content |
0 |
|
Feed H2S |
0 |
|
Suspended solids |
<1.0 mg/l |
|
Feed barium, strontium, fluoride |
Traces |
_
-8. Scaling:
At higher concentrations of RO brine, salts tend to silt on equipment surfaces, stopping or slackening the desalination process. Another way to address this issue is to deliberately precipitate & take out these salts before desalination or as a temporary measure, thereby enabling additional desalination. However, there is a lot of work going on in the inclusion of precipitation into both membrane & thermal desalination methods, & everyone hopes to see continued innovation in this field.
__
-9. Energy Consumption:
Both reverse osmosis (RO) and distillation methods face the challenge of energy consumption. The need for energy-intensive processes, whether in pressurizing water for RO or heating water for distillation, poses economic and environmental challenges. Most plants in the world are still powered by fossil fuels. This isn’t good for energy sustainability. The use of solar and offshore wind could help.
_
-10. Concentration Polarization:
Concentration polarization refers to the concentration gradient of salts on the high pressure side of the reverse osmosis membrane surface created by the less than immediate redilution of salts left behind as water permeates through the membrane itself. The salt concentration in this boundary layer exceeds the concentration of the bulk water. This phenomenon impacts the performance of the RO process by increasing the osmotic pressure at the membrane’s surface leading to the following:
- Reduced flux
- Increased salt leakage
- Increased probability of scale development
Increasing the velocity (turbulence) of the brine stream helps to reduce the concentration polarization.
_
-11. Pre-Treatment of Sea Water before Desalination is essential:
(1. Remove Suspended Solids, Bacteria, SDI, Colour & Turbidity:
When sea water is extracted for desalination it brings along a lot of organic waste, plastic waste, bacteria, and silt. All this has to be removed before the seawater is fit for desalination. Sea water with all these impurities leads to a shorter RO membrane life, short operation period and high maintenance. Pre-filtering protects the RO membrane and keeps it working efficiently for a longer time.
(2. Remove Oil from Sea Water:
Pollution in the sea and its impact on marine life is increasing at an alarming rate. Contamination by hydrocarbons, due to oil spills, heavy naval traffic, and increased production of petrochemicals is highly acute. If this contaminated water goes directly into the desalination plant, it causes caking and clogging, and impedes the efficiency of the desalination equipment. Oil removal equipment like Gopani’s Clarysorb Cartridge Filter is perfect for a preventive pretreatment process so that the desalination plants perform consistently for a long time.
(3. Bring Down the Operating Cost of Desalination Plants:
If membrane fouling reduces considerably, then operational costs drop significantly. Also the desalination plants are expensive and a prefiltration device can effective protect this expensive equipment from damage and increase its service life.
______
Challenges to the widespread adoption of desalination exist such as expense, significant energy use, the need for specialized staff training, the large carbon footprint of facilities, environmental issues such as greenhouse gas emission (GHGs), chemical discharge, and operational problems such as membrane fouling. The price of desalinated water has trended downward in recent years, driven by requirements from desalination plant developers and owners for ever-lower costs per m3/gallons of desalinated water. How desalination plant operators can continue to meet these expectations will be determined by how they respond to the following six critical challenges:
-1. Maximizing equipment efficiency to further improve power consumption
-2. Optimizing plant designs and controls
-3. Financing creative projects
-4. Ensuring the reliability and availability of clean water
-5. Producing consistently high-quality water
-6. Partnering with suppliers who can provide the right equipment, services and technical long-term support
______
______
Drivers and restraints of the desalination market:
Desalination is “often chemically, energetically and operationally intensive, focused on large systems, and thus requires a considerable infusion of capital, engineering expertise, and infrastructure”. The main “Achilles heel” for the efficient operation of a membrane-based desalination systems is membrane fouling. The problems associated with membrane fouling are decreased membrane permeability, increased operating pressure, increased frequency of chemical cleaning, and membrane deterioration. Despite all these facts, desalination is gaining a market to meet the demand of freshwater shortage worldwide. However, several factors that drive the feasibility of desalination plants need to be considered. Figure below indicates the drivers and restraints of the desalination market. As illustrated, the main drivers for desalination markets are saltwater intrusion, the willingness of private investors to invest, water shortages, reduced plant prices, etc. On the other hand, the environmental impact, high capital cost, and political instability are main restraints on the desalination market. As illustrated in the Figure below, the significance of each factor (for both drivers and restraints) is indicated by the length of the arrow. The longer the arrow in the figure, the higher the importance of the factor. Contrastingly, the dotted arrows highlight the forces whose importance is gradually decreasing, for instance, factors such as high capital investment are decreasing, as the operational cost and energy consumption of membrane-based desalination have been reduced dramatically over time due to the improvements in membrane technologies, energy recovery systems, and the use of renewable energy sources. This drastic decline in operational costs encourages investors to invest more in desalination projects. In summary, the most important driver for desalination projects is mainly water shortages due to the depletion of freshwater because of high water demand (population growth, industrial expansion) and increasing saltwater intrusion.
Figure above shows Key desalination market forces.
It is therefore recommended to perform the PESTLE Risk Analysis, which includes analysis of various factors (political, economic, social, technological, legal, and environmental) that have a direct or long-lasting impact on processes and technologies. This analysis identifies opportunities and external risks that must be considered and not ignored.
_______
_______
Barriers to Seawater Desalination:
- Effects of the concentrated waste stream on ecosystems and the impact of seawater intakes on aquatic life;
- Disposal of the waste stream produced from desalination can have negative effects on the environment due to its high concentration of salts and traces of chemicals, though this is improving with recent technological advances
- Desalination techniques are relatively expensive and require a lot of energy, though there are increasing possibilities for using renewable energy, such as solar or wind-driven desalination coupling
- Developing countries, which often have the greatest freshwater needs, may not be able to use desalination, as the best opportunities for its implementation are in well-managed water sectors with clear water policies
- Optimal utilization requires training, regular maintenance and access to spare parts, which could be a limiting factor in remote and smaller communities
The environmental impacts of desalination must be weighed against those of expanding use of freshwater sources (e.g. groundwater depletion, diverting surface water flows) (Gassan, 2007). Although RO product water is almost totally pure, it is possible that some compounds of possible concern could get into product water; pre-treatment or post-treatment processes can be used to address the few compounds that are not removed well by RO (e.g., boron).
_
Opportunities for Seawater Desalination:
By securing new water resources, these technologies can make it possible to provide a stable supply of water for households and industry. Desalination enables utilities in many water poor areas to access a nearly unlimited water resource. However, desalination can sometimes exacerbate the problems of a poorly functioning water sector (WHO, 2007; World Bank, 2005). Therefore, the best opportunities for implementation are in water sectors that are functioning well, with well-defined water policy, well-characterized water resource availability and demand, technical expertise, and relatively little waste and inefficiency. Opportunities for desalination are greatest when:
- Freshwater resources are inadequate to meet demand (water stress or water scarcity)
- It provides climate change adaptation benefits in water scarce areas through water source diversification and reduced pressure on freshwater sources
- For membrane systems, an abundant source of brackish water with low salt/TDS concentration is available; or, for thermal systems, the population is located on a coastline with an adjacent facility (e.g., a power plant) that yields abundant waste heat
- Consumers are opposed to the reuse of treated wastewater
- Technological advances are continuously reducing the economic and environmental impacts of desalination
- It has the potential to provide a near unlimited supply of water if sustainable energy utilization methods and safe discharge are deployed
_____
_____
Limitations of RO:
RO offers significant advantages over previous desalination technologies, including lower energy consumption, lower operating costs and smaller internals, resulting in more compact equipment. It is providing reliably clean, potable water for millions of people whose lives depend on it. However, it is a very energy-demanding process, leading to high GHG emissions. The use of electrical energy also means that RO is inaccessible or too expensive in many regions with high water demand. Other key limitations include membrane fouling and limited water recovery, leading to large amounts of waste brine.
Desalination limitations inspire Innovations:
Renewable energy sources may be the key to getting around desalination’s high energy cost. Solar panels are the perfect candidate for powering desalination facilities. They are increasingly inexpensive, and areas with little rainfall (in other words, areas that benefit most from additional water sources) have abundant sunlight. Some small facilities have already successfully implemented solar panels. Saudi Arabia will open the first municipal-scale solar-powered desalination plant.
States such as California have created disposal regulations to minimize brine’s environmental impact. But brine may actually be a useful resource. Farid Benyahia, a chemist from Qatar University, figured out how to use brine for making baking soda and calcium chloride. Calcium chloride is used for preserving canned vegetables and tanning leather. Once refined, Benyahia’s method could be more efficient than current industrial processes.
_
Limitations of FO:
One of the most substantial FO limitations is the energy consumed during the recovery of the draw solutes (DS). A study by McGovern et al. compared FO with RO based on theoretical energy requirements. They found that even with an optimal DS recovery, the overall desalination energy would not be significantly lower than for RO, despite the much lower energy requirements for the desalination step. These findings are in agreement with Awad et al., who investigated 15 pilot FO studies and found that a 40–50% decrease in energy consumption for recovering the DS is required before it can compete with RO. A way to minimise the energy consumption of DS regeneration is to use a solution that does not need to be recovered, effectively eliminating the recovery process. However, this would lead to generation of additional waste through discarded DS. Other approaches include investigating new materials, such as magnetic nanoparticles (MNPs) and ionic liquids (ILs).
_
Limitations of MD:
Despite having advantages over RO, MD is still not a widely commercialised technology. It is generally agreed that the two largest issues for industrial-scale MD systems are pore wetting and low thermal efficiency. Fouling and low water flux also have a considerable effect on the MD performance.
Pore wetting can occur when amphiphilic molecules (surfactants) attach to the surface of the membrane pore. Liquids and substances within the water can also act as pore wetting agents if they have low surface tension (alcohols). Contact of the pore-wetting agent on the membrane surface results in a reduction of the pore liquid entry pressure. Once the liquid entry pressure is below the transmembrane hydraulic pressure difference, a channel is created whereby the feed water is able to pass through the membrane. The effects are usually shown by a sharp increase in permeate conductivity, as the salt rejection capability is reduced.
______
______
Why is it so hard to make salt water drinkable?
First let me discuss, how does water dissolve salts?
At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges on opposite sides in the molecule. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. Likewise, a water molecule is ionic in nature, but the bond is called covalent, with two hydrogen atoms both situating themselves with their positive charge on one side of the oxygen atom, which has a negative charge. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules.
The positively-charged side of the water molecules are attracted to the negatively-charged chloride ions and the negatively-charged side of the water molecules are attracted to the positively-charged sodium ions. Essentially, a tug-of-war ensues with the water molecules winning the match. Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules. Once this happens, the salt is dissolved, resulting in a homogeneous solution. One oxygen from the water cannot stabilize the Na+ alone, but several oxygens from different waters can surround the Na + and their combined partial negative charges can stabilize the Na+. The chloride is stabilized in the same way by the partially charges hydrogen side of the waters. Figure below illustrates above phenomenon.
_
Why is desalination so expensive/hard?
Because salt really likes to dissolved in water as they’re both polar molecules. Therefore, simpler, cheaper separation techniques don’t work.
Also, gravity separation won’t work for salt, that would work fine for sand or pine needles.
Also, liquid-liquid extraction or any other absorption into some other solvent (which would be viable for getting a hydrocarbon out of water into an immiscible hydrocarbon) or adsorption on a surface like activated carbon or a zeolite to remove carcinogens like benzene from water and onto activated carbon, won’t work for salt.
Ion-exchange only swaps one ion like sodium for another like calcium which can “soften” your water (make it less “hard” to soap up and rinse off) but doesn’t reduce the salts in your water, only changes them from one metal to another.
And water has a very high heat of vaporization – it takes a lot more energy to boil water than most other liquids. That’s why sweat cools us so effectively, but it increases the energy requirements of distillation-style desalination. The energy required for this distillation process today makes it prohibitively expensive on a large scale. A lot of the current market for so-called “thermal desalination” has therefore been in oil-rich, water-poor countries in the Middle East. Thermal desalination requires roughly 6 times as much energy as reverse osmosis.
Most desalination plants today use reverse osmosis. In this process, seawater is pushed through a membrane to remove the salts. The membranes are so fine that water can get through, but salts really can’t, and the clean water that comes through is taken for drinking water. Although this method requires less energy than thermal desalination, it still uses quite a bit of electricity, about four times what we would typically spend on our municipal water use, including wastewater treatment. But for every gallon of seawater that plants process through reverse osmosis, only half is recovered as fresh water. That’s because the process has a built-in trade-off: As water is pushed through the membrane, the seawater on the starting side gets more concentrated, which makes it even harder to pass through the membrane. This, in turn, increases the energy needed to push the water through.
The super-concentrated seawater that’s left over, called brine, is a common byproduct of reverse osmosis plants. Once the filtration is done, plants typically pump the brine back into the ocean, far away from shore, while mixing it with seawater so it doesn’t create extra salty zones. Some researchers have pointed out that having multiple plants in one area operating for an extended period could lead to an increase in ocean salinity and negatively affect the surrounding ecosystem. But with proper design and monitoring the effect should be minimal.
Although seawater is plentiful, removing the salt is an energy-intensive process that may only make sense in specific scenarios. Our need for water as humans is never-ending, but our supply of fossil fuels is limited. One way to bring down desalination energy costs is by coupling plants with renewable energy sources.
______
______
Section-10
Improving and emerging desalination technologies:
_
Improving desalination:
By 2030, 47% of the global population will face water scarcity. With global demand for drinking water growing exponentially, it’s now critical more than ever that we come up with a way to reduce the projected increases in CO2 emissions from desalination. And it’s equally essential that we make the desalination process cleaner and more affordable.
_
The Middle East is home to nearly half the world’s desalination capacity and demand for desalination across the region continues to grow. Burgeoning population multiplied by high water consumption per capita are important drivers behind this upward trend. Growth in capacity has also been helped by the steady decline in costs. Desalinated water costs of 0.5-1 USD/m3 are readily achieved, on par with many conventional water sources. Several Middle Eastern countries, including Bahrain, Kuwait and Qatar, are entirely reliant on desalination today. The continued desire to reduce costs has caused a technology shift in desalination. The shift has been away from the traditional thermally-driven technologies of multistage flash (MSF) and multi-effect distillation (MED) towards electrically-driven process of reverse osmosis (RO). RO uses several times less energy than MSF and MED, which means lower costs and lower emissions. Nevertheless, the environmental impacts of RO remain significant – not only because of CO2 emitted to the atmosphere, but also because of the massive quantities of brine discharged to the sea. Even state-of-the-art RO desalination plants give rise to >1 kg of CO2 and >1 m3 of brine for each cubic meter of freshwater produced.
______
Technology improvement:
-1. Batch RO
Batch RO may offer increased energy efficiency, more durable equipment and higher salinity limits. Rather than keeping a constant flow of seawater at those high pressure levels, a batch process takes in a set quantity of water at one time; processes it; discharges it; and then repeats the process with the next batch. Each batch runs for about one to two minutes. Engineers ramp up the pressure over time, reduce the volume over time, and they end up using much less energy to produce the same amount of fresh water. Though some desalination plants have attempted to use semibatch techniques, none has ever implemented a full batch system – partly because of the time breaks between batches. It takes time and energy to pump each batch of water out, and then pump the next batch of water in for processing. Expending that time and energy generally cancels out the efficiency gains you would get from using the batch process. That’s why engineers developed a solution called ‘double-acting batch reverse osmosis. This new process uses a piston tank — a high-pressure vessel with a piston in the middle. While one side of the piston sends seawater forward into the processing loop, the other side of the piston simultaneously fills up with the next batch of seawater in the queue. When one batch process ends, the piston seamlessly injects the next batch of seawater into the system while simultaneously filling its other side with the next batch of seawater in the queue, and the process repeats continuously. Instead of fully emptying the piston each time or using some other liquid or gas to pressurize the piston, they are filling it with the next batch of seawater. So rather than one side of the piston being essentially dead space, they are using the seawater itself to get double-duty out of this piston, so there’s almost no downtime. This proposed system offers the lowest energy consumption ever for seawater desalination. It’s a best-in-class milestone.
_
-2. Desal Without Membranes
Columbia University engineers led by Yip, developed a method called temperature swing solvent extraction (TSSE) that doesn’t use membranes at all to desalinate. The efficient, scalable, and low-cost technique uses a solvent whose water solubility—the amount of a chemical substance that can dissolve in water—changes according to temperature. At low temperatures, the solvent mixed with salt water draws in water molecules but not salt. After all the water is sucked into the solvent, the salts form crystals that can easily be removed. The solvent and its absorbed water are then heated to a moderate temperature, enabling the solvent to release the water, which forms a separate layer below. The water can then be collected. Yip explained that the process is designed to deal with very salty water, which reverse osmosis cannot handle. For example, the water that comes up during oil and gas extraction can be five to seven times saltier than regular seawater. The textile industry also produces very salty water because of the solutions it uses to dye cloth. According to Yip, TSSE is not the best way to obtain drinking water, but it could help replenish our water resources for other needs.
_
-3. Innovative approach of combining different techniques with one goal – better outcomes across efficiency levels, water quality, and environmental impact reduction.
Today, with Zero Liquid Discharge (ZLD) and Minimal Liquid Discharge (MLD) frameworks, various desalination technologies can be combined to recover as much freshwater as possible. For example, conventional desalination technologies, such as reverse osmosis (RO), multi-stage flash distillation (MSF), multi-effect distillation (MED), electrodialysis (ED), electrodialysis reversal (EDR), nanofiltration (NF), etc. can be combined with emerging desalination technologies such as forward osmosis (FO), membrane distillation (MD), electrodialysis metathesis (EDM), etc. to achieve a very high freshwater recovery (up to 99%)
-Fusion strategies combining thermal distillation processes with membrane-based techniques offer potential advantages over conventional standalone operations.
-New-age nanofiltration membranes paired with electrodialysis can reduce energy requirements.
-Coupling capacitive deionization technology alongside existing RO practices promises potentially enhanced salt removal capabilities from respective water source.
_
-4. Improving Current Membranes
Current RO membranes, thin-film composite (TFC) polyamide membranes, are being studied to find ways of improving their permeability. Through new imaging methods, researchers were able to make 3D models of membranes and examine how water flowed through them. They found that TFC membranes with areas of low flow significantly decreased water permeability. By ensuring uniformity of the membranes and allowing water to flow continuously without slowing down, membrane permeability could be improved by 30%-40%.
_
-5. Low-pressure High-recovery (LPHR)
Another approach is low-pressure high-recovery multistage RO (LPHR). It produces concentrated brine and freshwater by cycling the output repeatedly through a relatively porous membrane at relatively low pressure. Each cycle removes additional impurities. Once the output is relatively pure, it is sent through a conventional RO membrane at conventional pressure to complete the filtration step. LPHR was found to be economically feasible, recovering more than 70% with an OPD between 58 and 65 bar and leaving no more than 350 ppm TDS from a seawater feed with 35,000 ppm TDS.
_
-6. Carbon Nanotubes (CNTs)
Carbon nanotubes are meant to potentially solve the typical trade-off between the permeability and the selectivity of RO membranes. CNTs present many ideal characteristics including: mechanical strength, electron affinity, and also exhibiting flexibility during modification. By restructuring carbon nanotubes and coating or impregnating them with other chemical compounds, scientists can manufacture these membranes to have all of the most desirable traits. The hope with CNT membranes is to find a combination of high water permeability while also decreasing the amount of neutral solutes taken out of the water. This would help decrease energy costs and the cost of remineralization after purification through the membrane.
_
-7. Graphene
Graphene membranes are meant to take advantage of their thinness to increase efficiency. Graphene is a singular layer of carbon atoms, so it is about 1000 times thinner than existing membranes. Graphene membranes are around 100 nm thick while current membranes are about 100 µm. Many researchers were concerned with the durability of graphene and if it would be able to handle RO pressures. New research finds that depending on the substrate (a supporting layer that does no filtration and only provides structural support), graphene membranes can withstand 57MPa of pressure which is about 10 times the typical pressures for seawater RO.
_
-8. Improved Water Pumps
The desalination industry is heavily reliant on water pumps. There are tons of water to be moved around. Water has to be pumped in from the sea and freshwater has to be transferred to the storage tanks before being transported to the city. Leading industrial pump manufacturers offer a range of pumps for desalination plants. These pumps are specifically designed to meet the challenges of desalination plants and deliver high performance at low energy consumption.
_
-9. Smarter Digital Technology
Today’s desalination plants are fitted with high-range sensors that better detect and anticipate problems, thereby reducing plant downtime. This, in turn, cuts down costs and enhances the flow of day-to-day operations.
_______
Renewable Energy Integration:
With the increasing emphasis on environmental protection, the deepening of sustainable development strategies, and the reduction of dependence on traditional energy sources, more and more new energy sources are available on the market for use in the desalination process. For example, wind energy, solar energy, wave energy, tidal energy, and so on. The challenges and ongoing research in both reverse osmosis and distillation underscore the dynamic nature of desalination technologies. As advancements continue, the future outlook includes more sustainable, cost-effective, and energy-efficient desalination solutions to address global water challenges.
_
Desalination by solar evaporation array (SEA) panels is one of the latest developments in solar technology. SEA panels are self-contained water purification devices that can be set up in minutes and are cheaper than traditional desalination methods for small-scale applications. In 2020, researchers at MIT and Shanghai Jiao Tong University showcased a method for passive solar-powered desalination. This system could provide more than 1.5 gallons of fresh drinking water per hour for every square meter of solar collecting area and guarantee water security in water-scarce areas. It involves a multilayer solar still device that relies on a set of evaporating and condensing components. It uses flat panels to absorb heat and then transfer that heat to a layer of water so that it begins to evaporate. The vapor then condenses on the next panel. That water gets collected, while the heat from the vapor condensation gets passed to the next layer. The multilayer evaporator makes sure that released heat flows to the next evaporating layer which recycles the solar heat and boosts overall efficiency of the device.
Other forms of renewable energy, like wind, are also possible solutions. In Australia, a wind-powered desalination plant has been providing the town of Perth with nearly 40 million gallons of drinking water every day for twelve years. This plant and others are connected via a grid to local wind farms, lowering overall energy costs while reducing carbon emissions.
European companies are developing the Floating WINDdesal in the Middle East, a seawater desal plant powered almost entirely by wind energy. The floating semi-submersible plant is being built in three sizes, with the largest expected to be able to produce enough water for 500,000 people. The plants can be moved by sea, making them easy to mobilize for emergencies and can be deployed in deeper water where brine disposal would have less impact on marine life. Because they float, they will not be affected by rising sea levels.
______
Brine management improvement:
Diluting brine can lessen its impacts. You take more seawater, and you premix it [with the brine] in an engineered reactor. Now the salinity of that mix is not two times saltier than seawater. It’s still saltier than seawater, but it’s lower. And instead of discharging it at one point, you discharge it at several points with diffusers. These are engineering approaches to try to minimize the impacts of brine. Brine impacts can be lessened by how much brine is discharged and how the desal process is carried out.
_
Seawater and brine can be concentrated by solar evaporation as is the case in traditional salt works – the main downside being large land utilisation and maintenance costs of the salt works. But wind-driven evaporation can also be used with considerable land and cost saving. In fact, a wind-driven evaporator can provide benefits of cooling as demonstrated by Seawater Greenhouse technology in the Horn of Africa, thus reducing the cost below zero. An optimised system combining seawater desalination, brine concentration, greenhouse cultivation and mineral recovery, could provide a winning solution at the water-energy-food nexus.
_
The desalination industry has made fantastic progress in reducing energy consumption, but has paid relatively less attention to brine discharge so far. Management of brines not only reduces the impact on marine ecosystems, but also creates the chance to build a whole new industry around ‘mining’ the numerous elements contained in seawater. This opportunity has not escaped the attention of research funding agencies around the world. For example, the EU-funded sea4value project aims to recover nine elements from seawater, including magnesium, lithium and rubidium. Other recent initiatives include the Sandooq Al Watan ‘Think Brine Challenge’ and the King Adbullah University of Science and Technology ‘Brains for Brine’ competition.
Stanford University researchers have developed a device that can turn brine into useful chemicals. Through an electrochemical process, it splits the brine into positively charged sodium and negatively charged chlorine ions. These can then be combined with other elements to form sodium hydroxide, hydrogen, and hydrochloric acid. Sodium hydroxide can be used to pretreat seawater going into the desal plant to minimize fouling of the membranes. It is also involved in the manufacture of soap, paper, detergents, explosives and aluminum. Hydrochloric acid is useful for cleaning desal plants, producing batteries, and processing leather; it is also used as a food additive and is a source of hydrogen. Turning brine components into chemicals that have other purposes would decrease brine waste and its environmental damage, as well as improve the economic viability of desalination.
_
The final stages of brine concentration and mineral recovery typically remain the territory of thermal technologies, because RO is unable to handle the extreme salinities encountered. However, the thermal technologies have less work to do (and spend less energy) if supplied with a feed that is already preconcentrated somewhat. This idea is currently driving several developments in high-recovery RO that range from stages of technological readiness, from the purely conceptual to operational industrial plant. For example, Yale University has proposed ‘Low Salt Rejection Reverse Osmosis’ (LSRRO) whereby RO membranes should be tuned to have imperfect salt rejection. A modest amount of salt leakage lowers the differential osmotic pressure across the membrane, which might otherwise exceed 200 atmospheres for brine desalination. Use of several such membranes in series is theoretically predicted to provide near 100% rejection with an energy consumption just a fraction of traditional brine concentration methods. A more mature technology is that of counter flow RO, in which the feed is diluted by water transferred from brine exiting the system, to the point where it can be handled by a conventional RO device. As in LSRRO, the trick is to avoid a large concentration difference across the RO membrane while the absolute concentrations remain high, thus avoiding the destructive pressures otherwise associated with high salinity RO. Counter flow RO is currently being implemented in western Saudi Arabia.
_____
_____
Emerging desalination technologies:
As the demand for desalination continues to grow, a few factors have forced researchers to explore alternative technologies. First, the overall energy consumption of RO plants remains high. Although development of energy-efficient pumps, energy recovery devices and high flux membrane materials have significantly reduced energy consumption of seawater RO (SWRO) from 20 kWh/m3 in the 1970s to 2-5 kWh/m3 today, the increasing energy requirement with increasing feed salinity still makes it challenging to treat feed solutions with high salinity from an energy standpoint. This includes hypersaline reject brine from desalination, the safe disposal of which is a budding environmental concern. Treatment of brine has received significant interest with increasing volume of desalination byproducts and stringent regulations on their disposal. Hypersaline feed conditions are also present in regions with high evaporation rates, such as the Arabian Gulf. In fact, the high salinity and harsh feed conditions that necessitate rigorous pretreatment methods are both contributing factors in the slow transition from thermal technologies to reverse osmosis in the region. Another incentive to diversify desalination technologies is the presence of other applications where mature technologies would make little economic or practical sense. Examples include zero liquid discharge (ZLD), brine management, saline wastewater treatment, produced water treatment, food and dairy industry, pharmaceuticals and electronics. In recent years, emerging desalination technologies have shown remarkable promise in these areas. Researchers have turned to developing processes with low energy requirements to overcome existing challenges in reverse osmosis, MED and MSF technologies. Emerging desalination technologies have the potential to compete with conventional technologies for seawater desalination, or to outperform these technologies in niche areas; however, their transition to full-scale employment depends on further scientific advances to achieve threshold performance and energy efficiency.
_
The increase in the desalination market size reflects growing demand for water. In 2017, the global water desalination market was valued at US $15.43 billion; by 2025, this is expected to increase to US $27 billion. Owing to the relatively high cost of desalination, 67% of plants are located in high-income countries, such as Saudi Arabia (15.5%), USA (11.2%) and UAE (10.1%). Over the last few decades, there has been a significant market change from thermal- to membrane- based desalination methods, most notably to seawater reverse osmosis (RO). The latter now accounts for 84% of the total number of operating desalination plants, contributing 69% to the desalinated water produced globally. The main driver for this change has been the need to reduce the operating costs associated with high energy consumption of thermal desalination. The specific energy consumption (SEC) for medium to large seawater RO plants has reduced significantly over the last few decades and is currently between 2.5 and 7 kWh/m3. However, RO requires high-grade energy (electricity), meaning that even low SEC values lead to high energy destruction. Despite the reduced SEC, RO desalination still has high greenhouse gas (GHG) emissions related to the energy use, which range from 0.835 to 6.1 kg CO2 eq. per m3 of potable water. This is due to the vast majority of desalination plants still relying on energy from fossil fuels. For example, the Middle East, the largest producer of water from seawater desalination, produced only 0.7% of the total water using renewable energy.
_
The global production of 141.5 million m3/day of brine is another environmental concern. Marine ecosystems with sandy seafloors, high wave action and those already impacted by human activity may not be affected greatly by the release of RO brine. However, areas with low ocean currents and sensitive marine life are at high risk. Changes in the salinity of seawater can affect the development and growth rate of larvae and the saline brine reduces the amount of dissolved oxygen in seawater, which can cause hypoxia in marine organisms. Chemicals within the brine, such as iron chloride, sodium hypochlorite and sodium bisulphite, can also have lethal toxic effects on marine life through acidification and anoxia. The brine itself also contains valuable resources, including magnesium sulphate, calcium carbonate and lithium, which cannot be recovered using current RO technology.
_
Reverse osmosis (RO), currently the most widely adopted technique, has caused environmental concerns over the high associated greenhouse gas emissions and generation of large amounts of chemicals-containing brine. Significant consumption of electricity for RO desalination is an additional challenge, particularly in remote locations. Emerging technologies, such as forward osmosis (FO), membrane distillation (MD) and membrane capacitive deionisation (MCDI) are being explored to reduce the energy consumption, fouling and brine issues associated with desalination. These emerging technologies offer significant advantages over RO, such as higher salt rejection (CDI, MD), higher recovery of water (MD), fewer pre-treatment stages (MD, FO) and the ability to use low-grade energy (MD, FO). In their current state, stand-alone technologies cannot compete with RO until certain challenges are addressed, including pore-wetting (MD) and high energy consumption (MD, CDI, FO). Hybrid systems that combine RO and emerging technologies may be useful for feed waters that cannot be treated by RO alone and their benefits may be able to offset the increase in capital costs.
_
Summary comparison of emerging technologies and reverse osmosis:
|
Parameter |
Reverse osmosis |
Forward osmosis |
Membrane distillation |
Membrane capacitive deionisation |
|
Thermal energy consumption, kWh/ m3 potable water |
– |
– |
49–350 |
– |
|
Electrical energy consumption, kWh/ m3 potable water |
2.5–7 |
3–68 The forward osmosis process has low energy consumption but regeneration of draw solution can be energy intensive. Additional ~0.25 kWh/m3 required for circulation pumps. |
0.6–1.8 required for circulation pumps. |
83.2 |
|
Type of energy required when integrated with renewable energy |
Reliable electricity. |
Energy for regeneration dependent on draw solution; can potentially use low-grade energy. |
Low-grade energy/waste heat. |
Electricity |
|
Ease of pre-treatment |
Extensive pre-treatment steps are required to mitigate membrane fouling; must be chemically cleaned. |
Fouling is reversible and can be mechanically removed. |
Fewer pre-treatment chemicals required. Antiscalants required to reduce calcium scaling. |
Absence of hydraulic pressure reduces fouling. |
|
Operating pressure and temperatures |
50–70 bar, ambient temperature. |
Atmospheric pressure but high pressures/ temperatures may be required during draw solution regeneration. |
1 bar; 30–90 ◦C; higher temperatures maximise flux |
1 bar, ambient temperature. |
|
Water recovery |
35–50% |
Up to 50%, although rarely used as stand- alone technology. |
Usually ~5–40%, although >90% is possible. |
Uncertain, potentially significantly higher than 50% |
|
Water desalination cost, $/m3 |
0.5–3 |
0.8–2 |
0.64–5.2 |
Unknown, however capital costs and membrane costs are higher than RO |
|
Current market share in desalination |
Most widely used (~65 % share) |
Emerging (<2%) |
Emerging (<2%) |
Not commercialised for seawater desalination. |
_
Table above summarises the major performance characteristics of the evaluated emerging technologies against RO. As can be seen, FO and MD experience lower fouling rates and need fewer pre-treatment stages, which could reduce the environmental impacts from the release of chemicals into the brine. FO and MCDI operate at ambient pressure and temperature, avoiding the need for high-tolerance construction materials. Nevertheless, the advantages of FO are extremely dependent on the DS recovery stage, which needs to be improved. However, all of the emerging technologies have higher energy consumptions than RO at this stage and require further developments to improve their economic feasibility. It is worth highlighting that MD and FO can operate using low-grade energy, which could enable the development of desalination systems powered by waste heat, thus reducing consumption of fossil- fuel-derived energy and related environmental impacts.
In addition, there are indications that coupling the emerging technologies with RO to form hybrid systems could help overcome some of the limitations of the stand-alone technologies.
_
Figure below shows summary comparison of stand-alone and hybrid systems:
Figure above provides a qualitative comparison of RO, FO, MD, MCDI and their hybrid counter-parts. It is interesting to note that the hybrid systems tend to perform overall better than the single technologies.
However, these comparisons should be treated with caution due to a lack of data related to commercial applications of the emerging technologies. Also, some criteria may be considered more important than others, particularly the energy consumption and costs. Finally, it should be borne in mind that RO is the only option available at scale and hence optimised – if deployed commercially, the emerging technologies would also benefit from the economies of scale and their performance would improve.
______
______
Section-11
Worldwide desalination experience:
The majority of Gulf countries now largely depend on desalinated water for their inhabitants’ consumption: in the United Arab Emirates (UAE), 42% of drinking water comes from desalination plants producing more than 7 million cubic meters (m3) per day, in Kuwait it is 90%, in Oman 86%, and in Saudi Arabia 70%. In 2022, there were more than 21,000 seawater desalination plants in operation worldwide, almost twice as many as a decade ago, and the sector’s capacity is growing at between +6% and +12% per year.
_
By 2030, desalination capacity in Middle Eastern countries is expected to almost double, as part of plans announced in the region to prepare these economies for their transition to “post-oil” and to foster resilience. Saudi Arabia’s desalination capacity is set to increase from 5.6 million cubic meter (m3) per day in 2022 to 8.5 million m3 per day in 2025, and it will have to cover more than 90% of the country’s water consumption. The same holds for the UAE, Kuwait, Bahrain and Israel, where the production of desalinated water will more than double by 2030.
_
With the rise of available solutions to meet all such needs, these technologies are now in demand on virtually every continent, while the Middle East today represents only 50% of installed capacity worldwide. In Africa, large-scale projects have recently been announced in Algeria and Morocco, countries that until now have had sufficient resources. Other countries such as Ghana, Senegal and Kenya supply many cities with desalinated seawater. This is also the case for Cairo. In the Indo-pacific region, particularly in China and India, the needs for desalinated water are increasing, driven by growing industries and decreasing available water. In 2020 alone, the construction of more than 35 desalination plants was announced in China, as well as six in the Philippines, and six in Taiwan. In the Americas, the west coast of the United States stands out with important projects in California, and Texas is not far behind. In Latin America, new projects are emerging in Peru and Chile, driven mainly by the needs of the mining industry, while in Mexico the demand for desalinated water notably comes from the population. Finally, island areas stand out for their strong needs for desalinated water: Cebu in the Philippines, Cape Verde, the Canary Islands and the Maldives are increasingly using desalination capabilities.
_
Seawater desalination had been used for years in arid areas across the world such as the Middle East, the Mediterranean, the Caribbean Islands and Australia. Scarcity of alternative, cheaper sources of water make desalination a viable option for these areas. The lower cost of fossil fuels in Middle Eastern countries significantly brings down the cost of desalinating seawater. Seawater desalination will make sense when production plants are situated near coastal areas. Equally important factors are the site’s elevation (higher elevation will translate to higher cost of pumping), feedwater characteristics (higher salinity has higher costs), and the consumer’s capacity and willingness to pay for water.
_
Desalination in Saudi Arabia:
Saudi Arabia is the largest country in the world without running surface water and has one of the highest rates of water consumption in the world. Providing new sources of potable water for the Kingdom’s growing population and expanding industry has long been a matter of national importance to the desert country. With daily water consumption at 263 liters per capita (in 2019), total water consumption has exceeded 8 million cubic meters per day (m3/d) and is forecast to reach 12.3 million m3/d by 2040. The country has relied on desalinated water since the 1950s and has since come to be the leading desalinated water producer in the world, with 7.6 million m3 produced daily accounting for 22 percent of global production. As of 2019, 60 percent of the country’s water comes from desalination, with nonrenewable groundwater (less than 40 percent) taking most of the remaining share and reclaimed wastewater surface water and surface water supplies supplying the rest. The Kingdom’s overall water demand stands at an estimated 25.29 billion m3 annually but is projected to grow slightly to 25.79 billion m3 by 2025. As of October 2020, the Kingdom had a total of 33 desalination plants in 17 locations run by the Saline Water Conversion Corporation (SWCC), a government-run organization responsible for approximately 69 percent of desalination in the Kingdom (5.6 million m3/d) and 20 percent of worldwide desalination.
_
Desalination in China:
The total amount of freshwater resource in China is 2.7 × 10^12 m3. The annual water supply capacity has been stabilized around 6.0 × 10^11 m3/year, while the national population and economy is growing in a linear trend over the last decade and expanding at an annual rate of 4.8–5.9‰ and 6.7–9.0%, respectively. Thus, it is a great challenge to supply the entire country with enough water. The water resource per capita decreased from 4225 m3 in 1962 to about 2008 m3 in 2018. The freshwater demand in China is even more urgent in consideration of its ever-growing population, accelerated industrialization and urbanization. However, China has a fairly long coastline of ~32,000 km and a vast ocean area of ~3000,000 km2, providing a great geographical advantage for the large-scale promotion of seawater desalination. Therefore, seawater desalination technologies have attracted great attention and developed rapidly in China for the last six decades. By the end of 2018, 142 seawater desalination plants have been installed with a total production of 1.202 million m3/d in China. China is going to be one of the world’s most promising market for seawater desalination.
_
Desalination in America:
In the U.S., over 400 municipal desalination plants have been opened since 1971 and an estimated 200 or more are currently in operation, though the precise number is not known for certain. Most are in California, Florida and Texas. The biggest seawater desalter in the western hemisphere is the Claude “Bud” Lewis Carlsbad Desalination Plant in San Diego County, California. Completed in 2015, the plant can produce up to 60 million gallons of desalted water in one day. In a region plagued with heat and drought, it’s the only water supply in the county that is not dependent on snow or rainfall.
Brackish Water Desalination in America:
Desalination doesn’t just refer to de-salting sea water—it can also be used to transform water that’s not as salty as the ocean, but still too salty to drink, into fresh water. Known as “brackish water,” this water contains less salt in parts per million (or ppm) compared to sea water, which contains about 35,000 ppm of salt. Fresh water has less than 1,000 ppm of salt, and brackish water falls somewhere in between the two. Brackish water appears in sources on the surface, like lakes and rivers, as well as in underground aquifers. According to research studies done in 2010, over 95% of the desalination facilities in the U.S. are located inland, away from the ocean, and most are designed to treat brackish groundwater. In Texas, 27 of the state’s 31 aquifers contain brackish groundwater. The state is also home to the largest inland desalination plant in the country. Located in El Paso, far from any ocean, the Kay Bailey Hutchison Desalination Plant transforms previously unusable groundwater into fresh water, and it’s capable of making up to 27.5 million gallons of fresh water a day. To meet the growing demand for water, the plant plans to expand so it can produce up to 42 million gallons in a day.
Brackish water desalination poses a different set of challenges compared to seawater desalination. While there are fewer dissolved particles to remove from brackish water, it can be harder to dispose of the leftover waste. And though less energy is required to pump the brackish water through filters than sea water, more energy is sometimes required to pump it from its source.
_
Desalination in Australia:
Australia is the driest habitable continent on Earth and its installed desalination capacity has been increasing. Until a few decades ago, Australia met its demands for water by drawing freshwater from dams and water catchments. As a result of the water supply crisis during the severe 1997–2009 drought, state governments began building desalination plants that purify seawater using reverse osmosis technology. The first modern large-scale desalination plant was the Perth Seawater Desalination Plant, completed in November 2006 and over 30 plants are currently operating across the country. Many plants are utilizing nearby wind or wave farms to use renewable energy and reduce operating costs, and solar powered desalination units are used for remote communities. Achieving water security using desalination is now a priority for the majority of Australia’s capital cities, all but one of which are on the coast. Using the abundance of sea water as a source, this approach seeks to “climate proof” their cities’ water supplies.
_
Desalination in India:
India has an extensive network of rivers and the annual rainfall measured across India amounted to 1,257 millimeters in 2022. Despite this abundance, India regularly experiences a water crisis, which is affecting the economy, people’s wellbeing, and the ecological state of the country. High population density and increased water stress indices in cases like Kolkata and Chennai justify the need for seawater desalination. Chennai, in fact, hosts the largest desalination plant in the country, the Minjur Desalination Plant. The plant generates 36.5 million cubic meters of water per year, servicing 2.5 million residents in Chennai. Another desalination plant located in Nemmeli, Chennai, supplies fresh water at the same capacity. Being able to ease a quarter of the city’s water requirement with just the two plants, the state government plans to replicate more desalination plants in the city. Another desalination plant situated in Jamnagar of Gujarat produces approximately 96,000m3/day.
Compared with freshwater-starved countries whose only viable choice is desalinated water, India has several available freshwater resources that are not being utilized to the fullest. Technology, policy control, and most importantly, an open mindset can lead to a lot of opportunities for efficiently harnessing what’s already available.
_____
_____
World’s Largest Desalination Plants:
Ras Al Khair, Saudi Arabia: 1,036,000 m3/day:
Commonly regarded as the desalination heavyweight of the world, the massive Ras Al-Khair is a hybrid project that uses both thermal multistage flash (MSF) and reverse osmosis (RO) technologies. Located 75km north-west of Jubail and serving Riyadh, the site also has a substantial power generation component, with a capacity of 2,400MW. The main contractor for plant construction was Doosan and its consortium partner Saudi Archirodon, with Poyry acting as the consultant for the project.
Taweelah, UAE – 909,200 m3/day:
The Emirates Water and Electricity Company and ACWA Power, have signed the water purchase agreement, for the world’s largest sea water reverse osmosis desalination plant to be constructed at Taweelah Power and Water Complex, 50 km north of Abu Dhabi. ACWA Power, with the lead developer of the project and a 40 per cent shareholder, confirmed the successful financial closing of the world’s largest SWRO plant, at a cost US$847m, has the tariff of desalinated water 49.05 cents/m3. Construction of the project commenced in May 2019 and the plant is expected to deliver 909,200 cubic meter of water a day. Once complete, the Taweelah power and water development is expected to raise the emirate’s proportion of desalinated produced water by RO from 13 per cent today to 30 per cent.
Shuaiba 3, Saudi Arabia – 880,000 m3/day:
A consortium involving Siemens of Germany for the power plant and Doosan for the thermal desalination plant were selecting by ACWA Power to provide project engineering, procurement and construction of the plant. One expansion to the plant has completed and one expansion is in the final construction stage with a total additional 400,000 m3/day of RO capacity added, according to ACWA Power. When complete, Shuaiba will eventually overtake Ras Al Khair as the largest operating desalination plant with total capacity of 1,282,000 m3/day.
Sorek, Israel – 624,000 m3/day:
Sorek could be the heavyweight membrane plant of the world in operation with an enormous 624,000 m3/day capacity. Located 15km south of Tel Aviv in Israel and developed by IDE Technologies, the project was and continues to be unique in the use of 16-inch seawater reverse osmosis membranes but in a vertical formation. A further development – Sorek 2 – IDE Technologies and Bank Leumi have won the Israeli government’s PPP tender to build and operate the Sorek 2 water desalination plant. IDE has now won four of the five tenders to operate desalination plants in Israel. The bid, with an unprecedented price of USD 0.41 per cubic meter of water, calls for the annual production of 200 million cubic meters of water (nominal capacity of 548,000 m3/day). Once complete, Sorek 2 will be the sixth desalination plant to operate in Israel alongside Hadera, Ashkelon, the first Sorek, Palmachim and Ashdod.
JUBAIL 3A IWP – 600,000 m3/day:
The ACWA consortium submitted the lowest levelized water cost of USD 0.41 per m3. With an investment value of USD $650 million, the Jubail 3A Independent Water Plant (IWP) will generate 600,000 m3 of potable water/day. The greenfield seawater reverse osmosis desalination project will be in Jubail, Kingdom of Saudi Arabia. The Engineering Procurement Construction contract got award to a consortium consisting of Power China, SEPCO-III and Abengoa.
_
Advantages of Large-Scale Water Desalination Systems:
Large-scale water desalination systems offer several benefits in addressing water scarcity and ensuring a sustainable water supply. These advantages include:
-1. Reliable Source of Clean Water
Large-scale water desalination systems provide a reliable source of clean, potable water, regardless of the availability of freshwater sources. This is especially crucial in arid regions where traditional water sources are limited or contaminated.
-2. Independence from Rainfall Patterns
Unlike traditional water sources, which rely on rainfall patterns, large-scale water desalination systems are not dependent on weather conditions. They can operate consistently, ensuring a stable water supply even during droughts or dry seasons.
-3. Mitigation of Water Stress
By tapping into seawater or brackish water sources, large-scale water desalination systems alleviate the pressure on freshwater sources, reducing water stress in regions facing water scarcity issues. This helps preserve existing freshwater resources for other essential purposes.
-4. Reduction of Environmental Impact
Large-scale water desalination systems, particularly those utilizing reverse osmosis technology, have a lower environmental impact compared to other water purification methods. They consume less energy and produce less brine, minimizing their carbon footprint and potential harm to marine ecosystems.
_____
Small scale desalination technologies: A comprehensive review of 2023:
In recent decades, problems related to fresh water has become a very important issue for humans. Small-scale desalination (SSD) systems, besides large-scale desalination (LSD) systems, fulfil an important role in meeting freshwater demand by eliminating the cost of transmission and have the advantage of treating water on-site. In this study, for the first time, a comprehensive review of previous studies has been carried out on SSD systems (less than 25 m3/d water production). These systems are powered using renewable, non-renewable or hybrid sources of energy, incorporating different treatment technologies such as: reverse osmosis (RO); electro dialysis (ED); capacitive deionization (CDI); membrane desalination (MD); humidification–dehumidification processes (HDH); multi-effect desalination (MED); and hybrid technologies, including a combination of RO-UF, RO-ED and RO-MED. The advantages and drawbacks of the systems that operate using fossil fuels and renewable energy (RE) systems have been studied, considering membrane, evaporation and salinity features. Among these, solar-based desalination systems are the most popular. Accordingly, numerous studies on RO, ED, MD, HDH and MED technologies for solar-SSD systems have been compared in terms of their freshwater productivity, energy consumption and cost of produced water. Attention has also been paid to SSD systems powered via wind, geothermal, tidal and hybrid energies. It has been determined that the RO system holds the largest market share in both non-renewable (25 %) and renewable energy (40 %) systems. In addition, a comparison of low-cost SSD and LSD systems shows that SSD systems are economically competitive with LSD systems. The outlook for the future shows that the use of SSD systems powered using non-renewable energy is likely to decrease, except in areas where energy costs are very low. In addition, the use of solar-SSD systems is likely to increase, where systems that operate solely on wind or geothermal energy will be replaced by hybrid renewable energy systems.
_____
Floating desalination plant:
While desalination facilities are usually built onshore, floating desalination barges provide the same benefits but stand several advantages. The cost of transferring seawater to the plant offshore (feed-water intake) and the price of the area holding the plant are significantly lower while there is the added advantage of having them easily moved (towed) to other locations – as needed. Floating barges also carry lower environmental footprints with waste liquid being further diluted onsite using existing seawater, thus offering additional protection to marine life.
Furthermore, the high degree of the modularized design and delivery based on the pre-assembled plant modules minimizes the workforce required at the shipyard. It also reduces the installation and commissioning time leading to commercial operation. Due to the plant being mobile, the floating barges don’t face typical marine and soil project risks usually resulting from brownfield activities.
This innovative solution allows governments to meet surging water demand due to pressing environmental challenges, and local requirements in regions where economic growth is rapid and/or where demand varies significantly with seasons. Ultimately, water can be deployed to any location on the coastline when needed and backup supplies for contingency planning and emergency salutations secured in a timely manner.
Integrating the barge desalination units with power generators results in quick mobilization, expedited construction timelines, fewer marine works, and reduced adverse impacts of climate change.
_____
_____
Wealthy nations built desal plants but poorer countries can’t afford desalination:
Big desalination plants do not come cheap. The capital cost of large-capacity plants typically runs into the hundreds of millions of dollars: not surprisingly, most plants built in recent years have been in wealthy countries like the UAE and Israel, or to supply big cities in Australia or the US. The latest desalination market report from Global Industry Analysts, a market research company, anticipates the global desalination market to grow by 9.8% annually from $15.2bn in 2022 to $22.5bn in 2026. However, this growth will largely be driven by China and the US, say the authors, rather than the many poorer countries of the world struggling to adapt to climate change.
_
The operating cost of desalination is also a major burden for countries producing desalinated water. This varies around the world, depending on conditions and technology used: US Department of Energy guidelines suggest that operators should target $1.50 per cubic metre (/m3) for high-salinity water, such as brine from oil and gas operations, and $0.50/m3 for lower-salinity water such as sea water. In Israel, the Sorek B desalination plant currently under construction is contracted to produce water for $0.41/m3, which the Israeli government suggests offers a “new benchmark for seawater desalination water prices on a global scale”.
Analysis from Christopher Gasson, publisher of the industry magazine Global Water Intelligence, points out that the low cost achieved in Israel may not be replicable in other countries. This is because the cost of project finance is typically much lower in wealthy countries, while Israel also has much lower labour costs than other wealthy countries like Australia and the US. Gasson adds that for much of the world outside of the Middle East, desalination will be needed as a “temporary solution to getting through droughts”, in which case cheaper and more inefficient plants will be built, which will produce more expensive water.
_
While the costs of producing desalinated water have decreased significantly — to around $0. 50 per cubic meter today — it is still a business of rich countries. The key issue is that it is still not affordable for low-income countries. Over 90% of desalination happens in upper middle- and high-income countries around the world, even though poorer countries such as those in sub-Saharan Africa, are predicted to become water scarcity “hotspots” by 2050. While smaller solar or wind powered off-grid desalination plants are being developed, these products are not reaching the marginalized communities that need them most.
______
______
Desalination politics:
As other states across the Arab world and further afield look to desalination as a solution to their water needs, there are signs that desalination diplomacy may become an important political tool, with GCC countries able to take advantage of their position as first movers in the sector, exporting technology, know-how, and even water to other states in the region. Desalination negates some of the key drivers of water conflict. Most obviously, when relying on sea water rather than river water, it reduces the tensions that can develop between upstream and downstream riparian states, such as have occurred between Egypt and Ethiopia over the latter’s construction of a new dam.
That being said, desalination may also become the locus of political conflict and produce its own geopolitical dynamics. For example, Gulf states must contend with their reliance on the same body of water for their potable water needs. As has been noted, the Gulf’s waters have become a water security concern. Dotted with offshore oil rigs and plied by the world’s largest oil tankers, an oil spill there would have the potential to disrupt the water supply of multiple Gulf countries. Moreover, security analysts have pointed to the potential threat of an attack targeting a country’s desalination infrastructure, noting that their coastal locations make them particularly vulnerable. The First Gulf War offers a clue as to how desalination infrastructure might be targeted during a conflict. In 1991, as the Iraqi Army retreated from Kuwait, former Iraqi dictator Saddam Hussein destroyed the country’s desalination plant and then released Kuwaiti oil into the Gulf, creating a large oil slick and disrupting the wider region’s desalination plants. There are concerns that a potential cross-Gulf conflict with Iran could again see water infrastructure targeted during wartime.
______
______
Portable desalination:
A portable desalination device is an innovative solution tailored to turning saline water into potable water, especially crucial in isolated or crisis-hit environments. In 2022, researchers from MIT developed an efficient model, compactly sized like a suitcase. Its energy efficiency is so optimized that a budget-friendly solar panel can drive it. This device is different from traditional models because it bypasses the need for filters. Instead, it harnesses electrical power for water purification, drastically cutting down on upkeep. Such advancements make it invaluable for regions like remote islands, ships on extended voyages, refugees impacted by natural calamities or soldiers in remote military outposts. “This is really the culmination of a 10-year journey that I and my group have been on. We worked for years on the physics behind individual desalination processes, but pushing all those advances into a box, building a system, and demonstrating it in the ocean, that was a really meaningful and rewarding experience for me,” says senior author Jongyoon Han, a professor of electrical engineering and computer science and of biological engineering, and a member of the Research Laboratory of Electronics (RLE).
Commercially available portable desalination units typically require high-pressure pumps to push water through filters, which are very difficult to miniaturize without compromising the energy-efficiency of the device. Instead, their unit relies on a technique called ion concentration polarization (ICP), which was pioneered by Han’s group more than 10 years ago. Rather than filtering water, the ICP process applies an electrical field to membranes placed above and below a channel of water. The membranes repel positively or negatively charged particles — including salt molecules, bacteria, and viruses — as they flow past. The charged particles are funneled into a second stream of water that is eventually discharged. The process removes both dissolved and suspended solids, allowing clean water to pass through the channel. Since it only requires a low-pressure pump, ICP uses less energy than other techniques. But ICP does not always remove all the salts floating in the middle of the channel. So the researchers incorporated a second process, known as electrodialysis, to remove remaining salt ions.
The portable system desalinates brackish water and seawater (2.5–45 g/L) into drinkable water (defined by WHO guideline), with the energy consumptions of 0.4–4 (brackish water) and 15.6–26.6 Wh/L (seawater), respectively. In addition, the process can also reduce suspended solids by at least a factor of 10 from the source water, resulting in crystal clear water (<1 NTU) even from the source water with turbidity higher than 30 NTU (i.e., cloudy seawater by the tide). Authors built a fully integrated prototype (controller, pumps, and battery) packaged into a portable unit (42 × 33.5 × 19 cm3, 9.25 kg, and 0.33 L/h production rate) controlled by a smartphone, tested for battery-powered field operation. The demonstrated portable desalination system is unprecedented in size, efficiency, and operational flexibility. Therefore, it could address unique water challenges in remote, resource-limited regions of the world.
____
QuenchSea, a portable, low cost device that turns seawater into drinking water:
QuenchSea is a portable, low cost desalination device that turns saltwater into fresh water. to do so, the apparatus combines a hydraulic system, a triple pre-filtration process, and a small reverse osmosis membrane to desalinate seawater into freshwater using human power only, at sea or on the coast, convert seawater into clean fresh drinking water instantly. In ideal conditions, QuenchSea can produce three liters of water within one hour.
The device features a hydraulic system that is able to build pressure up to 60 bars, removing salts from seawater through a reverse osmosis membrane. The in-built ultrafiltration and microfiltration system removes suspended solids, pathogens, parasites and microplastics. Then, through the process of adsorption, an advanced activated carbon filter ensures that the taste of water is palatable and the odor is pleasant. The reserve osmosis process is the one in charge of demineralizing the water where the osmosis membrane rejects larger molecules such as dissolved salts (ions) and other impurities such as pathogens to produce highly purified water for drinking.
_____
_____
Desalination on ships:
The challenge of maintaining an adequate water supply at sea had bedevilled mariners for centuries. In primitive times, a captain would carry as much water as possible because, once out of sight of land, their lives depended on it. Why didn’t they just boil sea water to drink? The answer is simple, boiling seawater does not remove salt, too much of which is deadly for humans. Fortunately, today’s mariners have the technology to convert enormous amounts of saltwater into drinking water through a process known as distillation. Distillation is accomplished by heating seawater to the boiling point to vaporize it. The vapors condense into a liquid which removes impurities and contaminants. Potable water purity from the distillation process results in clean drinking water, which is treated with chlorination solutions to ensure purity and safety for consumption. The chemical additive process varies from ship to ship, but the Navy Bureau of Medicine and Surgery provides very clear water-processing guidelines to ensure personnel safety and health. Treated water is stored in large tanks designated solely for potable water and is supplied to the ship’s services via pumps operated by highly trained sailors.
The current design for Navy ships requires standardized water purification plants of two capacities: 12,000-gallons per day reverse osmosis plants and 100,000 gallons distilling plants. A modern aircraft carrier produces up to 200,000 gallons of drinkable water daily. But not all water meant for drinking. Ships use this fresh water for cooking, washing, bathing, and even to cool important machinery onboard.
Before the advent of modern desalinization plants, mariners relied on the fresh water they collected from rain and stowed while at sea. Today, Sailors and Marines benefit from high-tech, reverse osmosis (RO) desalinization plants aboard most Navy ships. It takes energy to make water, and that energy comes from burning fuel to spin turbine generators that produce electricity necessary for ship systems, including RO plants. A more efficient desalinization plant translates into a more efficient ship, which uses less fuel, extends combat capability and reduces its carbon footprint.
The third process that can be used to create desalinated water on cruise ships involves water bunkering. Fresh water bunkering involves providing ships with clean, potable water while they are in port or at anchor. This essential service ensures that vessels have an adequate supply of drinking and operational water, ensuring the well-being of crew and compliance with regulations. The reason for this particular issue is that cruise ships spend a considerable period of time at a port, which means that these ships don’t have as much time at sea to produce potable water. High amounts of shore-based impurities could also lead to a lower production of potable water.
Keep in mind that cruise ships are also required to reach a certain distance from shore before being able to evaporate the water. As for RO units, these are meant to be switched on once the ship reaches a minimum depth of around 50 meters, which limits the risk associated with fouling and damage to RO membranes. Cruise ships prepare for these issues by bunkering potable water on a weekly basis. This process usually occurs at the initial port or the destination port.
_____
_____
Section-12
Pros and Cons of desalination:
_
Benefits of installing desalination plants:
-1. Respite in times of distress and droughts:
Droughts are becoming more frequent and severe due to climate change, posing a significant threat to agriculture, ecosystems, and communities. Desalination plays a pivotal role in mitigating the impacts of drought by providing a stable water supply during times of scarcity.
In California, the Carlsbad Desalination Plant has been instrumental in tackling the state’s recurring drought challenges. Since its inception, the plant has produced over 56 billion liters (15 billion gallons) of freshwater, meeting approximately 10% of the region’s water needs. By diversifying the water portfolio and reducing dependence on traditional sources, seawater desalination plants offer a lifeline during drought periods, safeguarding communities and ecosystems.
With drought comes several challenging situations such as hunger, landscape degradation, higher fire risk, famine, and whatnot. Since all the nearby water bodies dry up, surviving becomes very difficult for plants, animals, and humans. All of us know that the ocean is an inexhaustible source, and it can deliver a continuous supply of water during such hard times. This is where desalination systems step in and deliver access to water that is fit for human use. Having a desalination system might even prevent such a disaster from happening in the first place. Also, this will allow farmers to continue with their agricultural production even when the temperature hits the peak.
-2. Conserving Freshwater Resources:
Desalination has the ability to alleviate pressure on traditional freshwater sources such as rainfall and underground reservoirs. By tapping into the vast reservoirs of seawater, desalination plants provide a sustainable alternative to dwindling freshwater supplies. This is particularly vital in regions with limited access to freshwater, such as arid coastal areas.
Take the example of Israel, a country that has embraced desalination technology on a large scale. With nearly 85% of its domestic water coming from desalination plants, Israel has significantly reduced its reliance on freshwater sources. This shift has not only preserved precious freshwater reserves but has also increased the overall resilience of the country’s water supply.
-3. Diversifying and expanding water Sources:
Relying solely on freshwater sources is increasingly risky in the face of climate change. By incorporating desalination plants into the water supply infrastructure, a nation can diversify its water sources and reduce dependency on vulnerable supplies, such as snowpacks and groundwater. This diversification ensures a more resilient and adaptable water management system. Given the current global situation on the availability of freshwater sources, it’s essential to look for alternative ways that could expand our sources of water supply. One of the very obvious alternatives is the sea. Holding around 71% of the surface on earth, the ocean proves to be a feasible alternative. It can efficiently expand the most precious resource: Water. Expansion of water sources will eliminate water scarcity and will preserve the available freshwater sources across the world.
-4. Promoting Public Health:
Access to clean and safe drinking water is essential for public health. With desalination, seawater is purified, eliminating harmful contaminants and ensuring a constant supply of potable water. Desalination plants can significantly improve public health outcomes by reducing the risk of waterborne diseases and contamination.
-5. Protecting Agriculture:
Agriculture is a significant consumer of water resources, and droughts can severely impact crop yields and food production. Farmers can ensure consistent water availability, bolster crop resilience, and sustain food production even during prolonged dry spells by integrating desalinated water into agricultural irrigation systems.
It is also worth noting that seawater is not only undrinkable but also unsuitable for irrigation due to the high concentration of salt that would render a land unable to support plant life. Note that it is also impossible to feed livestock with saline water. Hence, as the human population increases, water consumption also increases not only for drinking but also for supporting agricultural activities and food production. Desalination provides a solution for addressing both water and food requirements of a growing population. A study by D. Zarzo, E. Campos, and P. Terrero explored the experience of Spain in water desalination for agriculture. They noted that the country provides a prime example of success in using desalinated water for agricultural purposes. Currently, Spain has large capacity plants and farmer-owned facilities for producing irrigation water from saline water sources.
-6. Supporting Industrial Growth:
Industries heavily reliant on water, such as energy production and manufacturing, face operational challenges during water scarcity. Desalination plants can provide these industries with a dependable water source, safeguarding their productivity and supporting economic growth.
-7. Preserving Ecosystems:
Traditional freshwater sources like rivers and aquifers are essential for maintaining healthy ecosystems. By reducing freshwater extraction for human consumption, desalination plants can help preserve natural habitats and protect aquatic life that rely on these ecosystems. Pressure on sources of freshwater leaves the aquatic animals with a poor habitat. Not only do they harm the aquatic animals, but aquatic plants also cease to exist. Desalination systems facilitate the growth of aquatic life by reducing the burden on sources of freshwater.
-8. Conservation of Land Resources:
When it comes to preserving our land resources, we need to think about the way we get our fresh water. Traditional sources like reservoirs and dams require a huge chunk of land to set up and it is a massive undertaking. Saltwater desalination machines offer a nifty alternative. They can be built on smaller plots of land, which means we can manage our land resources much better. This is super helpful in crowded areas where land is already in short supply. By using desalination, we can optimize our land use and protect the precious natural habitats, agricultural land, and ecosystems that would otherwise suffer from those big water infrastructure projects. It’s a win-win for both people and the environment.
-9. Enables Co-generation Applications:
Another benefit of water desalination is that it allows for the application of co-generation. Countries rich in fossil fuel resources benefit from water desalination through co-generation. They burn fossil fuels not only for electricity generation but also for producing desalinated water, thus maximizing the utility and capacity of their power plants. The International Atomic Energy Agency also noted that water desalination using nuclear energy or utilizing dual-purpose nuclear power plants has benefited countries such as Japan, India, and Kazakhstan. Again, co-generation maximizes the utility not only of power plants but also of energy resources or inputs.
-10. Modular system:
If you believe seawater desalination systems consume a wide area and money, you’re wrong! With constant experiments, experts have managed to design modular systems, which are compact and can be easily moved and installed. Not only this saves you space, but it also saves installation expenses. Such systems are the perfect fit for commercial or municipal drinking applications with limited space and a huge water requirement.
-11. It could create a freshwater reserve:
There are times when freshwater resources are plentiful. During those times, the desalination plant could continue to operate, producing a healthy reserve of water that could be used at a later time. That would allow habitats and agricultural efforts to be maintained, potentially over a period of several years, which could reduce or eliminate the changing economic and agricultural conditions that drought could provide.
______
______
Disadvantages of desalination:
-1. High Costs:
The financial cost of desalination is substantial. Building and maintaining a desalination plant requires significant investment. For example, the construction of the Carlsbad plant cost about $1 billion. The operational costs, primarily due to high energy consumption, make desalinated water more expensive than traditional freshwater sources. With the addition of costs from infrastructure construction, operation, and maintenance, producing desalinated water is generally more expensive than tapping water sources from lakes and rivers, groundwater, or through water recycling and water conservation. In some regions, desalinated water can cost twice as much as water from conventional sources. However, in other regions (especially in remote areas), desalinated water is actually cheaper than conventional water sources.
Desalination is inherently expensive for countries with no abundant supply of natural energy resources. They need to import these resources or build more capacities for alternative energy production. The costs would make desalinated water expensive for end-use consumption.
Although advances in technology have driven down the cost of water desalination, a study by Y. Zhou and R. S. J. Tol argued that it would still be costly for developing countries, those that are placed on the interior of a landmass, or those situated at high evaluation. Interestingly, these countries usually have substantial water problems. Zhou and Tol further explained that aside from the costs associated with constructing and maintaining a plant, including the cost involved in energy input, transporting the desalinated water inland or in elevated regions provide additional costs that can equal the costs of water desalination.
-2. High Energy Requirements:
Desalination is an energy-intensive process. It requires approximately 5 times more energy than conventional water treatment of freshwater. For instance, the Carlsbad Desalination Plant in California consumes around 38 megawatt-hours of electricity per day. This high energy demand often leads to increased operational costs and carbon footprint, particularly when the energy sourced is from fossil fuels.
-3. Negative Impacts on the Environment:
Another drawback of desalination is its various negative impacts on the environment. Take note that aside from having high energy requirements, which translates to carbon emission in the case facilities powered by hydrocarbons, the construction, operation, and maintenance of these facilities produce a number of negative environmental externalities.
The following are the specific adverse environmental impacts of desalination:
- Affects Marine Species: The U.S. Environmental Protection Agency explained that the intake of water from seas could suck fish and shellfish, as well as their eggs. Once sucked, these marine species may be killed due to physical stress, exposure to chemical, or heat. Some larger organisms are instantly killed when trapped at the front of intake structures.
- Produces Brine: A byproduct of desalination is wastewater with a high-concentration solution of salt called brine. Brine is strong enough to kill wildlife and vegetation by coming into contact with it. Also, there are usually chlorine-removing chemicals and anti-scaling agents in it. It poses an environmental risk because of its corrosive effects and the toxicity of other chemicals diluted in it. The organisms most commonly affected by brine and chemical discharge from desalination plants are plankton and phytoplankton, which form the base of all marine life by forming the base of the food chain. Desalination plants therefore have the ability to negatively affect the population of animals in the ocean. Nonetheless, there are different ways to properly dispose of brine such as through dilution or wastewater treatment.
- Waste Disposal: As with any process, desalination has by-products that must be taken care of. The process of desalination requires pre-treatment and cleaning chemicals, which are added to water before desalination to make the treatment more efficient and successful. These chemicals include chlorine, hydrochloric acid and hydrogen peroxide, and they can be used for only a limited amount of time. Once they’ve lost their ability to clean the water, these chemicals are dumped, which becomes a major environmental concern. These chemicals often find their way back into the ocean, where they poison plant and animal life.
- Other Impacts: Facilities need to be built on about 25 acres of land on or near a shoreline. Hence, there is an issue of land use. Building them far from a water source or on inland also produces environmental risks due to energy inefficiency and possibilities of leaks from the piping system that could contaminate freshwater sources such as aquifers.
-4. Pretreatment needed:
Reverse osmosis membranes are very sensitive. So, unless some more resistant membrane material is developed, pretreatment is an important requirement. Without it, the membrane can become practically useless, decreasing yield or producing impure water. Improperly pretreated seawater can deposit particulate matter on the membrane. These contaminants affect proper membrane flow and pressure which increases operating cost.
-5. Limited Availability:
Desalination’s feasibility is heavily dependent on geographical location. It’s most viable in coastal areas with access to seawater. Landlocked regions or areas far from the sea face logistical challenges in adopting this technology, as it requires proximity to large water bodies like oceans, seas, or in some cases, brackish water sources. This limitation restricts the widespread adoption of desalination, making it a less practical solution for many inland areas.
-6. Water Quality Issues:
Water quality is part of both the pros and cons of desalination. While desalinated water has the potential to be of high quality, surpassing conventional sources, its quality is not uniformly guaranteed. It depends on the methods used, the original water quality, and how the desalination process is managed. Well-maintained plants with stringent quality control measures can produce high-quality water consistently, whereas plants with less rigorous standards may produce water of variable quality.
-7. Health Hazards:
RO removes both harmful contaminants and desirable minerals. Some studies report some relation between long-term health effects and consumption of water low on calcium and magnesium.
-8. It might be used to earn dirty money:
Experts, including the consultant for WWF and author of a 2007 report on desalination, Phil Dickie, argue that process plants are almost never built where they are needed the most, such as in the sub-Saharan Africa, to solve chronic water shortages. Instead, some desalination plants are built in places where they generate healthy financial returns. These experts also maintain that this technology is quickly emerging as a distraction to policies for better use of water.
-9. Disadvantages of household RO:
Household RO units use a lot of water because they have low back pressure. Household RO water purifiers typically produce one liter of usable water and 3-25 liters of wastewater. The remainder is discharged, usually into the drain. Because wastewater carries the rejected contaminants, recovering this water is not practical for household systems. Wastewater is typically delivered to house drains. A RO unit delivering 20 liters (5.3 U.S. gal) of treated water per day also discharge between 50 and 80 liters (13 and 21 U.S. gal). This led India’s National Green Tribunal to propose a ban on RO water purification systems in areas where the total dissolved solids (TDS) measure in water is less than 500 mg/liter. In Delhi, large-scale use of household RO devices has increased the total water demand of the already water-parched National Capital Territory of India.
_____
_____
Section-13
Desalination in Agriculture:
Agriculture represents nearly 70% of global water withdrawals and also contributes to considerable water pollution mainly caused by excess utilization of fertilizers, pesticides, and other pollutants. Urbanization and increased living standards along with contamination of water resources and climate change have all resulted in the current world’s water scarcity. Given such circumstances, seeking noncompeting water resources for agriculture has increasingly become a focus of global attention. In 2021, about 20% of total irrigated lands, contributing to one-third of global food production, are salt-affected while almost 4.4% of the world’s agricultural lands are annually degraded due to salt accumulation mainly because of irrigation with low-quality water. In many regions around the world, saltwater is the only available water resource for irrigation which can adversely affect crop growth and yield.
_
Effects of water salinity on crops:
Water salinity is a major problem for agriculture. Saline water contains high concentrations of salts, which crops might not tolerate.
Saline water affects crops in two ways:
-1. It increases the osmotic pressure and reduces the ability of the plant to absorb water, even up to a point in which the plant wilts.
-2. High concentrations of specific ions in the saline water may be toxic to the plant.
Groundwater may contain high concentrations of ions such as sodium, chlorides, sulphate, bicarbonates, calcium, magnesium. It may also contain trace elements such as boron, iron, manganese and fluoride, which may be present at relatively low concentrations, but might become toxic to the plant if their concentrations exceed certain thresholds (such thresholds are usually crop-specific). Water‐scarce countries especially the Middle East countries located in the arid and semi‐arid zones will have to rely more on the use of non‐conventional irrigation water resources such as saline aquifers to partly alleviate water scarcity and most of these water resources contain dissolved solids and chemicals such as salts. The application of these water resources for irrigation purposes often result to the detrimental effect of salinization of soils, environmental degradation and low crop yield.
_
Beyond 1,000 ppm freshwater begins to become saline, with the water in the ocean having a TDS content of ~35,000 ppm. The agricultural need for fresh water is imperative in order to maintain crop yields and preserve soil quality. Many vegetable crops begin to experience diminished yields as TDS levels begin to exceed ~300 ppm. Hence, desalination, which is any process that removes salt from water to produce desalinized water, is increasingly considered a source of water for agriculture. Even though soil salinity has been affecting agriculture for thousands of years, significant research has been conducted only in the past 100 years.
_
Retention of salts in soils diminishes crop performance by creating ion toxicities, nutrient lockup, and desiccating osmotic effects. As modern farming practices are additionally dependent upon chemical fertilizers in order to maintain large crop yields, copious amounts of freshwater are required in order to leech these excess salts from the soil to prevent toxicities. Salts in soil and irrigation water may be either naturally present as products of geochemical weathering of rocks and parent materials or derived directly from sea water flooding, spray or intrusion into groundwater sources and/or caused by irrigation mismanagement, particularly when internal soil drainage is impeded. Due to the presence of salts, most saline lands are virtually uncultivated in the dry season because of strong salinity and lack of water in good quality and quantity. Salinization is one of the land degradation processes rendering millions of hectares of land unproductive for crop cultivation. Salinity is when an ‘excessive’ amount or concentration of soluble salts occurs in the soil, either naturally or as a result of mismanaged irrigation water. Although, salt‐affected soils are most abundant in arid regions worldwide, the extent of saline soils is variable.
_
Not every crop is sensitive to salt:
While many crops are highly salt sensitive, there are some which are not that sensitive to salt, which means that farmers are able to mix the desalinated water with regular water in order to reduce costs. This gives the water an acceptable level of salinity as not all crops need completely desalinated water. By mixing the two types of water, farmers do not have to produce as high a volume of desalinated water.
_
Water resources for agricultural production have been declining globally. This is due to an increase in water demand over limited resources and poor quality water, which has a negative impact on crop quality and yield and deteriorates soil properties. Desalination, the process of reducing the salt content in water to an acceptable level, could be an option for improving water quality, increasing water sources, and decreasing competition among various water users. As a result, desalination could improve crop quality, yield, and all year crop production, making it an important tool for effective agricultural water management. Water desalination is a well-established technology mainly for drinking-water supply in water scarce regions such as the Middle East. However, with agriculture accounting for 69 percent of all water withdrawals compared to domestic use of about 10 percent and industry 21 percent, it is the main source of potable water in the Persian Gulf countries and in many islands around the world and it is also being used in certain countries to irrigate high-value crops. However, it has proven much less economic for agricultural application than the reuse of treated wastewater, even where the capital costs of the desalination plants are subsidized.
_
Desalination for agriculture is expensive:
Desalinated water is more expensive than conventional water resources and it is not affordable for most crops. However, desalinated water might be affordable for high value crops, especially where subsidies on capital costs are provided. Desalinated water is of high quality and can have less negative impact on soils and crops in comparison with direct use of brackish water. For cost considerations, brackish water desalination is more suitable for agricultural production than is seawater desalination. Moreover, desalination facilities near the point of use are preferred in order to minimize transfer costs.
Waste water treatment vs desalination in agriculture:
Wastewater and water desalination constitute potential sources of water for agriculture and other uses. Technologies for tertiary wastewater treatment and desalination have very much in common. However, the cost of treatment varies depending on the type of treatment and the intended final use of product water. Treated wastewater reuse in agriculture is less expensive than is desalinated water. With its associated benefits, treated wastewater reuse also has problems in terms of public acceptance, and potential health and environmental risks. Although the World Health Organization (WHO) and FAO have specified guidelines for wastewater reuse, no common standards have been set owing to difficulties in systematic implementation in countries around the world.
_
Desalination technologies may provide one opportunity for generating cost-effective and potentially climate-independent water resources of controlled quality for agriculture applications. It is estimated that about 69% of available water resources around the world are used for irrigation and as water demands increase the number of desalination plants for irrigation for agriculture has also increased. Consequently there is increased emphasis on enabling cost effective desalination technologies to provide water of suitable quantity and quality for agricultural applications. Drier countries such as Australia and Spain have a long history with desalination technologies. In the past, the high capital and operating costs of desalination and the energy required have been major constraints to large-scale production of freshwater from brackish waters and seawater. However, desalinated water is becoming more competitive for urban use because desalinating costs are declining associated with increasing demand from population growth and reduced security of supply from surface water and usable groundwater and it is expected that these increases in efficiency will flow through to the agricultural sector. However, in spite of these developments, currently the cost of desalinated water is still too high for the use of this resource in broad-scale irrigated agriculture. An exception appears to be intensive horticulture for high-value cash crops, such as vegetables and flowers (mainly in greenhouses) grown in coastal areas where safe disposal of brines is easier than in inland areas. For example, Sundrop farms (Sundrop-Farms, Personal communication), uses 860,000 m3 of fresh water yearly to irrigate 2000 m2 of greenhouses. If the costs for providing desalinated water continue to reduce, its use is expected to become more viable because desalination for agricultural purposes has a number of significant advantages including:
- Tailored conductivity for irrigation water
- Assured supply
- Enables agricultural products of consistent quality
- Production may be increased compared to other water sources.
- The water may attain a higher resale price due to quality and supply assurance.
- It allows recovery of saline soils by irrigation with high quality water.
_
According to Desaldata many countries are beginning to use desalinated water in agriculture, albeit at varying rates. The highest proportion of desalinated water use in agriculture occurs in Spain, where the current installed capacity is 1.4 million m3/day and 22% is used in agriculture for high value crops, such as vegetables, fruits including tomatoes and peppers, and vineyards for table grape production. In Kuwait, where the current installed capacity is in excess of 1 million m3/day, 13% is used for agriculture and in Saudi Arabia, the world’s largest single producer of desalinated water; only 0.5% of its desalination capacity is used for agricultural purposes. Other countries which use desalinated water for food production are Italy (desalination capacity 64,700 m3/day — 1.5% for agriculture), Bahrain (620,000 m3/day — 0.4%), Qatar (0.1%), USA (1.3%) and Israel. The wider application of desalination technologies for agriculture is limited by its relatively higher cost, as well as by the need for agriculture to be close to saline and brackish feedwater resources as well as a safe and cost effective disposal option for brines. National assessments of the applicability of desalination technologies to support agricultural water supply are currently under way in Chile, China and Australia.
______
______
Benefits of desalination in agriculture:
- Water security: The major benefit of desalination technologies to agriculture is water security. While extremely site specific, desalination technologies can support agricultural resilience to drought and climate variability, de‑risking agricultural businesses.
- Water quality improvement: Where water in the upper range of a crop’s salt tolerance is used for irrigation, reduction in water salinity (via desalination) improves crop productivity. For example, a 25% reduction in water salinity leads to a 100% increase in grape vine crop productivity.
- Water conditioning: Permeate can be further conditioned by adding fertilisers and/or minerals (‘fertigation’) directly to the irrigation stream, customised to individual crops.
- Water use efficiency: The expanding use of desalinated water for agriculture is closely correlated with developing water‑saving irrigation techniques and high‑value crops.
- Increasing agricultural productivity: Desalination and permeate post‑treatment allows delivery of ‘fit‑for‑purpose’ water, which supports advanced agricultural productivity. This would lead to an increase in farming profitability, helping to offset the cost of desalinated water.
_
Desalination is a water saving alternative to brackish water irrigation. By implication, it could increase the possible sources of water for irrigation, and as such enhance sustainable all‐year round crop production. The low level of salinity of desalinized water is an extra benefit, because the salts [especially Sodium (Na+) and Chlorine (Cl−)] damage soils, stunt plant growth and harm the environment. Hence, desalinized water could improve the quality of irrigation water thereby reducing the possibilities of the incidence of soil salinity with its consequent adverse effect on crop growth and yield via its deteriorating effects on soil properties. Furthermore, desalination could increase the size of land area for cultivation, the number of crops (including salt sensitive crops) cultivated, improve crop quality, increase crop productivity and increase the broad band of water use for other purposes. Desalination solutions offer a promising and innovative approach to address water scarcity in agriculture. Desalinated water can increase crop yield, reduce reliance on rainfall, and minimize soil salinization. Desalination has been reported to improve farmers’ income.
_
Hidden benefit:
The concept here is centered around the idea that this newly introduced resource would add water to the regional hydrological cycle (Pistocchi et al. 2020). Studies show that, on average, each cubic meter of plant transpiration yields 570 L of precipitation (Van der Ent et al. 2010). Implementing desalinated water for irrigation follows the same basic principle, although quantifying its local significance would require reconstruction of the relevant climate variables at high resolutions (Van der Ent et al. 2010). It should be noted that, however, given the general time and space scales of recycling of soil moisture in arid regions, in most cases, this seems likely to be an insignificant effect. The importance of this phenomenon notwithstanding, a more apparent positive effect of introducing desalinated water to meet agricultural demands is that it reduces the pressure on other water resources. Problems that would otherwise result from over-abstraction from freshwater resources could be mitigated to some extent (Calatrava et al. 2022).
_____
Challenges to desalination in agriculture:
- Seasonality of water demands: The wide variability of irrigation water demand (daily, monthly and annually) can lead to a high cost of desalination if it is used as an ‘emergency supply’. Desalination plants operate at the lowest unit water cost when water is produced at a constant rate (100% capacity, i.e. ‘base load’). This issue can be resolved by deploying desalination in conjunction with water storage. MAR (managed aquifer recharge or water storage in an aquifer) may be cost‑effective where suitable aquifers are available.
- Current water price: Within Australia, the unit water cost (from sources other than desalination) is highest in South Australia ($0.383/kL to $2.307/kL) and lowest in Queensland ($0.133/kL to $0.405/kL). The unit cost of desalinated water is commonly greater than $1/kL (could be much higher) and is highly dependent upon factors such as feedwater quality and salinity, and brine disposal options.
- Perception: In addition to cost, lack of expertise and low implementation confidence can be factors limiting the wider use of desalinated water in agriculture.
- The potential for gradual degradation of soil structure: To understand the root of this problem, one should first note that the chemical composition of desalinated water often varies from other conventional water resources. Though the specifics may vary from one case to another, desalinated water for agriculture is often expected to contain a higher concentration of Sodium and Boron ions (Silber et al. 2015; Martínez-Alvarez et al. 2019). Besides the well-documented phytotoxicity effect of the said element (Monterrey-Viña et al. 2020), prolonged exposure to excessive amounts of sodium could degrade the physical properties of the soil; specifically, it can cause clay dispersion (Martínez-Alvarez et al. 2016). The problem could manifest itself as deterioration of aggregate stability, increased susceptibility to surface sealing and soil erosion problems, soil compaction, and decreased soil aeration and hydraulic conductivity (Martínez-Alvarez et al. 2016, 2018). Concerning boron, although it is known to be an essential nutrient for fruits and vegetables implied in physiological processes, plant growth, and development (Hilal et al. 2011), its excess may represent a real boron-toxicity damage risk for sensible crops, and hence, different management options such as water blending or boron reduction strategies must be considered for the sake of sustainability (Imbernón-Mulero et al. 2022). Additionally, calcium and magnesium ions are almost completely removed in the desalination process; hence, reintroducing these elements through the post-treatment process may be a practical solution to mitigate the said effects (Martínez-Alvarez et al. 2018). Alternatively, this issue can be controlled through proper planning and management, such as adopting a suitable irrigation treatment to help leach the accumulated excess minerals (Martínez-Alvarez et al. 2018). However, improper execution of the latter plan could lead to washing the said elements into aquifers (Silber et al. 2015).
______
______
Desalination for greenhouse agriculture:
By 2050, based on current consumption patterns and farming practices, projected water availability will be insufficient to feed the world’s population from current croplands. Part of the solution may rely on shifting from conventional open-air soil-based farming to more water efficient and higher yielding greenhouse based hydroponic agriculture (see figure below), where plants are grown in a growing media with nutrients delivered using nutrient solutions and the climate around the plants controlled.
Figure above shows Tomatoes growing in a hydroponic greenhouse owned by CEICKOR in Queretaro, Mexico. As an example, for growing the same amount of lettuce, hydroponic greenhouse agriculture consumed 12.5 times less water and used 10.5 times less area than conventional farming. The high yields and low water usage has resulted in a high adoption rate of greenhouse based agriculture, with the $22.9 billion dollar greenhouse industry growing annually at a rate of 8.9 %.
_
Desalination is very important for the greenhouse industry. Since greenhouses replace soil with nutrient solutions, the quality of water is of central importance to greenhouse producers. Greenhouses regularly monitor the pH and the salinity or total dissolved solids (TDS) of their waters to ensure the water is of the desired quality. Many greenhouses use municipal water to meet their water needs; however, municipal water is expensive in several parts of the world and may not be readily available. These reasons have driven a number of greenhouses to use groundwater to meet their water needs. About half the groundwater resources in the world are too brackish (0.5 ≤ S ≤ 5 g/kg) for food production, requiring desalination before use. These reasons have led the greenhouse industry to be early adopters of desalination within the agriculture industry. New desalination technologies designed to serve the needs of 21st century agriculture are likely to first serve the greenhouse industry sub-sector before being adopted for conventional field farming.
_____
Integrated Aqua-Agriculture (IAA):
Integrated Aqua-Agriculture (IAA) is a technology that utilizes an unconventional approach to fish and crop production. Brackish water desalination is performed using reverse osmosis (RO), the permeate is directed to an aquaculture unit, and the fish effluent is used as irrigation water for crops. Wastewater from the fish production unit is utilized as both irrigation water and a fertilizer solution to provide plants with nutrients, thus enhancing plants’ vegetative growth and fruit production. IAA system involves irrigating plants with fish effluents to maximize crop and fish production while promoting limited water use and fertilizer applications. Previous studies have shown a significant increase in water use efficiency and crop yield when plants are irrigated with fish effluents (Schneider et al. 2005; Dey et al. 2010; Mariscal-Lagarda et al. 2012). The IAA system’s advantage is that it can utilize the linkages and the synergies between the different agro-production activities such as fish, livestock, vegetables, and other high-value crops (Saha et al. 2016; Kimera et al. 2021a). The system demonstrates promising semi-commercial food production results and could provide new agricultural entrepreneurship and investment opportunities (Prein 2002; Abdul-Rahman et al. 2011; Murshed-E-Jahan and Pemsl 2011; Kimera et al. 2021b).
______
______
Section-14
Environmental Impacts of Desalination:
Although desalination plays an indisputable role in providing a steady supply of water in regions where freshwater resources are limited, it has diverse effects on the environment. Depending on the type of feed-water used, the desalination method employed, and how waste brine is managed, the desalination process has distinct and variable environmental consequences.
Although the constraints on global freshwater consumption can be alleviated by desalinating SW, especially by membrane desalination, the process itself contributes to energy-consumption-related carbon emissions and high-recovery-related highly concentrated brine discharge, which significantly impact the environment and local marine biota. It has been shown that local marine biota mortality increased, and fertility, embryo-survival rate, hatching rate, and embryo larval length decreased with increasing salinity. Increases in mortality became statistically significant at salinities ≥40,000 ppm, and 50% of marine plants died within the first 15 days at salinities near 45,000 ppm.
According to Lienhard et al., desalination constitutes ~0.2% of total global energy consumption and is projected to increase with increasing freshwater demand. Recently, it has been reported that desalination processes emit between 2.1 and 3.6 kg of carbon dioxide per cubic meter of freshwater produced. Elimelech et al. also reported that current state-of-the-art SW reverse osmosis (SWRO) plants consume between 3 and 4 kWh of electricity and emit between 1.4 and 1.8 kg of carbon dioxide per cubic meter of freshwater produced. It remains difficult for current state-of-the-art RO systems to further reduce specific energy consumption and brine discharge owing to obvious thermodynamic limitations.
More concerning and potentially the most dangerous environmental threat from desalination is brine discharge. SWRO returns 50–80% more total dissolved solid (TDS) to the sea. In addition to concentrated brine discharge, chemicals added to water during desalination pretreatment stages may harm marine life in vicinities of outlet pipes, which can significantly affect regional grids and ecosystems, thereby further increasing climate variability and disrupting historical patterns of salt precipitation and water storage.
_
The effects of desalination on the environment can be classified according to the process’s inputs from/outputs to nature and other processes (Source). Inputs from nature include feedwater, fuel, and air, while inputs from other processes include electrical and thermal energy, construction material, and chemicals. Outputs to nature include brine, chemicals, and gas emissions, while outputs to other processes include produced water and solid waste. Therefore, desalination affects the environment in many ways as depicted in the figure below:
-Energy consumption and GHG emissions:
Desalination requires a significant amount of energy, usually from fossil fuel-based power plants or electricity grids. This leads to greenhouse gas emissions and contributes to climate change.
-Marine ecosystem disruption
Intake and discharge of seawater during the desalination process can harm marine life. The intake of water can trap or kill marine organisms, while the discharge of highly concentrated brine back into the ocean can create localized areas of high salinity, affecting marine ecosystems.
-Chemical use and pollution
Desalination plants often use chemicals such as chlorine to disinfect water and prevent biological fouling. If not properly managed, the release of these chemicals into the environment can harm aquatic life. Increases air pollution occurs through COx, NOx, SOx, and particulate matter (PM) emissions from fossil fuels due to its high energy requirements.
-Brine disposal
The brine concentrate produced as a byproduct of desalination is typically discharged back into the ocean. If not properly diluted and dispersed, discharges brine can harm the marine environment and aquatic life due to its salinity, pH, temperature, residual chemicals, and heavy metal content.
-Habitat destruction
The construction of desalination plants may require clearing land, altering coastal ecosystems, and disrupting natural habitats.
-Contamination of Groundwater
A potential environmental issue is the potential contamination of groundwater aquifers near desalination plants. When constructing feedwater pumps, there is a chance that the drilling operation will contaminate the groundwater. Groundwater aquifers may be harmed by leaks from pipes that transport feedwater into desalination plants and highly concentrated brine out of them.
_____
Desalination facilities typically fall into one of two categories: thermal and membrane. With thermal, you suck in seawater, heat it up to get the pure vapor, and pump the remaining brine back out to sea. With membranes, you push seawater at great pressures through a series of filters, which pull out all the salt and other contaminants. Thermal is the more old-school method—prior to the 1980s, 84 percent of desalinated water went through this process. Since the beginning of the new millennium, though, a particular kind of membrane technology, reverse osmosis (RO for short), has proliferated exponentially. RO facilities now produce 69 percent of desalinated water worldwide. Why? Because RO is cheaper and more efficient. Advances in membrane technology mean facilities require less and less pressure, and therefore energy, to filter seawater. As an added benefit, RO produces less brine. With thermal, 75 percent of the water you bring in might leave as brine. With RO, it’s more 50-50 freshwater to wastewater. Scientists haven’t had a good idea of just how much brine the 16,000 operating desal facilities worldwide have been producing. Until now. Researchers report recently that global desal brine production is 50 percent higher than previous estimates, totaling 141.5 million cubic meters a day, compared to 95 million cubic meters of actual freshwater output from the facilities.
_
Irrespective of the technique(s) used, each desalination plant requires an intake and an outfall, the size and type of which are dependent on the desalination technique(s) used. Intakes can generally be divided into two types: open sea intakes, which consist of open surface intakes and submerged intakes that are typically positioned above the seafloor; and subsurface intakes, which consist of infiltration galleries and wells. Similarly, according to, outfalls can also be divided into two main types: open outfalls and submerged outfalls. The following factors are considered when choosing an outfall and intake type, together with their associated costs: the plant size and desalination technology, the local plant site’s hydro-geomorphological characteristics, and other environmental considerations. Thus, thermal- and membrane-based desalination plants near power stations may have similar intakes or outfalls. It is also worth noting that the selection, development, and utilization of the intake significantly impacts the seawater pretreatment process and the efficacy of the entire desalination process. Intakes and outfalls both have direct effects on the marine environment, for example, via the introduction of new structures. Therefore, careful consideration must be given to reducing the effects that intakes and outfalls have on the environment.
_____
Environmental concerns about desalination:
The intake of large volumes of seawater, discharge of brine, and release of emissions from desalination plants are major areas of environmental concern. Water intake may change the sediment transport pattern, increase soil salinity, and result in seawater intrusion into ground water. Also, the force of the operation removes organisms from their natural habitats, and tiny organisms like phytoplankton, fish eggs, and larvae may pass through the finest screen size of intake systems and die, while slightly bigger animals become trapped between intake screens with no way to escape. Even large animals can be injured or killed when the high flow velocity of feedwater pins them to external intake screens.
_
These organisms experience further damage higher mortality when desalination plants release brine as a waste product. Because desalination plants typically dispose of brine in the ocean, it influences marine environment health through its salinity, temperature, and residual chemicals. Brine from desalination plants has 1.5 to 2 times the salinity of seawater and contains chemicals that were added during desalination. These high levels of salinity, along with the chemical additives such as antiscalants and coagulants, and toxic compounds formed from disinfection byproducts, cause marine organisms with low tolerance for such environmental contamination to die. If their tolerance is high enough to escape death, organisms still face possible changes in their metabolic and growth rates and must contend with other effects of brine on their environment, such as the decrease in light penetration depth caused by heavy metals and the increase in sediment salinity and reduction of oxygen solubility brought about by increased seawater salinity. Altogether, these effects reshape benthic communities and may even introduce non-indigenous species to the area.
_
Both membrane and thermal desalination methods result in the effects described above, but thermal desalination has a unique impact due to the temperature of its discharge, which is 5-15 ºC higher than ambient seawater temperature. This increases the overall seawater temperature in the area, which can cause thermal shock, increasing organisms’ mortality rate. Combined with the increase in salinity, the higher water temperature also contributes to the reduction of its concentration of dissolved oxygen, putting marine organisms under greater stress.
_
Desalination plants also damage marine environments and organisms through their emissions. The processes undertaken by plants are energy-intensive. Seawater membrane desalination consumes 2-7 kWh of electrical energy per 1 m3 of product water, and thermal plants consume a combination of electrical and thermal energy, anywhere from 1.5 kWh electrical and 6 kWh thermal to 3.5 kWh electrical and 12 kWh thermal per 1 m3 of product water, depending on the temperature being used. Fossil fuels are still the primary source used to create this energy, which means that coal, oil, and natural gas power the majority of desalination plants around the world. The use of fossil fuels to meet these high energy demands makes desalination plants significant emitters of greenhouse gases, such as CO2, NOx, and SOx, as well as particulate matter. Greenhouse gas (GHG) emissions contributes to climate change. Desalination is an energy-intensive technology, which implies that relying on this resource rather than conventional water resources would emit more GHG into the atmosphere (Martin-Gorriz et al. 2021). As such, it is believed that desalination can exacerbate climate change. It is also important to note that climate change adversely impacts marine ecosystems (Manes et al. 2021), making them more susceptible to potential risks associated with desalination technologies. Incorporating renewable energy resources as a substitute for conventional fossil fuels is a logical solution to the GHG emissions associated with desalination (Kucera 2019). Greenhouse gases hurt the Earth in a variety of ways, but the harm to marine life specifically comes when these emissions make their way into ocean water.
_
Anthropogenic CO2 emissions, such as those from burning fossil fuels, are changing the chemistry of seawater in a process called ocean acidification. This process has been intensifying as oceans absorb increasing quantities of CO2, and around the world, marine animals that cannot adjust to the rapidly changing conditions are dying. For example, shellfish such as mussels, oysters, and abalone are having trouble building their shells and reproducing, and other fish are finding it more difficult to detect predators or locate suitable habitats. The destruction of these species puts many food webs at risk, impacting organisms of all shapes and sizes. Significant environmental impacts like these raise concerns about the establishment and operation of desalination plants and push us to examine our relationship with the environment.
_
Some of the most pressing environmental issues are associated with water intake and discharge sites. The problem here is that in both situations, the desalination problem is innately interfering with the environment and ecosystem and, as such, can potentially disturb the established natural order. For seawater desalination, the intake problem, for instance, usually manifests itself as impingement and entrainment. The former refers to the losses of aquatic organisms as they collide with intake screens, while the latter describes the situation in which these organisms are drawn into the plant with the feedwater (Lattemann et al. 2010). It should be noted that the mere construction of intake infrastructures can cause disturbance in aquatic ecosystems and potentially endanger marine life. Infiltration galleries and beach-well intakes have been proposed as alternatives to open seawater intakes to mitigate such impacts (Lattemann et al. 2010).
_
Another inherent challenge in desalination is the effective disposal of liquid waste via discharge points. Based on the quality of intake water and the technology used in the desalination unit, the said by-product can be in the form of brine water or sludge. Brine water, which is concentrated seawater, can be seen in thermal and membrane-based desalination units. The second form of waste flow, however, has much lesser volume and importance due to the washing of filters (e.g., organic matter and suspended solids), and the chemical cleaning of the installations (e.g., detergents, acids, alkalis, antiscalant, antifouling agents) is primarily seen in RO units. Traditionally, the produced sludge would have been mixed with brine. Reportedly, for seawater desalination, the sludge problem in the medium to large RO units (> 100,000 m3/day) that are prone to this specific challenge can even sum up to a few dozen tons per day (Baten and Stummeyer 2013). The current trend is, however, to incorporate installations in the desalination plants for the treatment and purification of these flows, and often thanks to proper environmental regulations, sludge disposal is no unmanageable issue in most cases. Apart from sheer volume, which can be problematic in and of itself, the chemical composition of the sludge can cause issues as well, most notably when the treated sludge is released directly back into the environment. In such cases, ferric salt may be employed in pretreatment (e.g., the process of removing particles, debris, microorganisms, suspended solids, and silt from the intake water prior to the desalination process) to remove the suspended materials. Though in and of itself, the presence of ferric salt may not cause a notable environmental impact, the discoloration caused by ferric salt can have an adverse aesthetic effect on the receiving water. This was, for instance, the case in Ashkelon, Israel (Baten and Stummeyer 2013). Alternatively, the pretreatment process can use centrifuge filtration that creates a high-solid waste product that can be disposed of in designated landfills. This, however, can be logistically challenging and introduce additional costs to the desalination process. As such, there is no universally accepted sustainable sludge disposal procedure, and for the most part, such strategies need to be determined on a case-by-case basis.
_
Disposing of brine is another common issue in the desalination industry in general. For instance, studies suggest that taking a global average, using RO technology, 1.5 m3 of brine is produced per every 1 m3 of desalinated water (Jones et al. 2019). Accordingly, improper brine disposal can lead to several environmental problems, ultimately endangering marine ecosystems like seagrass meadows or benthic populations (Pistocchi et al. 2020). However, the severity of these issues varies from case to case, to the point that under certain circumstances, it can even be considered negligible (Lattemann et al. 2010; McEvoy and Wilder 2012). This highlights the sheer importance of conducting an environmental impact analysis to get a better and more realistic sense of the potential impact of desalination on a local scale, as well as to develop environmental monitoring programs for the seafloor in the raw water intake and the brine discharge areas.
_
Like the sludge problem, brine’s chemical content, which depends on the desalination process and the feedwater quality, can also cause environmental concerns. Depending on these factors, brine composition could contain residual chlorine and chlorination by-products, antiscalant and antifoaming agents, and even certain heavy metals such as copper or nickel (Lattemann et al. 2010). The concentration of these chemical compounds is diluted following discharge, markedly if the discharge velocity is high. This, however, can result in the formation of high-salinity density currents that propagate hundreds to thousands of meters along the seafloor with limited dilution (Pistocchi et al. 2020).
_
Elevating seawater temperature near the discharging zones is another issue especially for thermal desalination (Kucera 2019). In MED, MSF, and VC desalination units, for instance, while there are certain preventive measures to tamper with the discharged brines, it often has higher temperatures than the seawater. The difference between the temperature of the discharge and the ambient water dictates how these interact and mix. The chemical composition, due to its effect on buoyancy, also has an effect. Discharged water with negatively buoyant properties enhances the risk of plume sinking and seafloor spreading, endangering benthic ecosystems. Neutrally or positively buoyant, on the other hand, could cause plumes to spread in the water column and affect nektonic species (Lattemann et al. 2010; Kucera 2019). Though much remains unclear about the local environmental impacts of the discharge, to the point that such effects need to be determined on a case-by-case basis, often it is assumed that the design of outfalls, particularly outlet water velocity or mixing with cooling water from power plants, could mitigate or even negate such potential problems (Pistocchi et al. 2020).
______
______
The marine environmental impacts of desalination:
Lettemann and Hopner (2008) provide an extensive list of the potential impacts of desalination on the marine environment; however, the main impacts broadly grouped into 4 categories:
-1. Impingement and entrainment in seawater intakes: large organisms are drawn into intakes and caught on screens and filters (impingement) as well as smaller and unicellular organisms which pass through the initial screening and enter the water stream within the plant (entrainment),
-2. Thermal discharges: the discharge of heated water either directly from thermal desalination plants, or indirectly as cooling water from power plants that generate power for non-thermal desalination processes such as reverse osmosis,
-3. Brine discharges: the discharge of concentrated brines in the residual outflow water following the removal of purified water,
-4. Chemical discharges: various chemical treatments are used to condition water to control biofouling, remove suspended solids, antiscalants, foam control additives (not required for RO), and cleaning. Additional chemical contamination of discharges can be due to corrosion of metal parts within the system. Chemical contamination of discharges varies between plants due to plant- specific process controls and different chemical composition of commercial treatment products (Pervov and Andrianov, 2017).
___
Counterpoint:
It is a myth that putting the highly salty water (brine) from the RO plants back into the sea is harmful to marine life — only, it has to be done right. Pre-dilute it, use proper diffusers. West Asia, for decades, been getting almost all of its water needs from desalination of sea water, no environmental issue is seen in the Gulf. On the contrary, in December 2019, research conducted by Professor Brendan Kelaher of the National Marine Science Center of Australia’s Southern Cross University, found that the hypersaline water pumped into the sea by Sydney’s desalination plant had actually increased fish life by 279 per cent.
A white paper produced by the IDA on the effects of brine discharge concludes that the discharge is fully safe and does not result in negative impacts on the marine habitat.
______
______
Pollutants Produced by Desalination:
One of the biggest environmental concerns associated with desalination is the pollution that results from the process. One of the main sources of pollution from desalination is the brine waste generated during the process. Brine is the highly concentrated, salt-rich water that is produced as a by-product of desalination. The brine is often discharged back into the ocean, where it can have negative impacts on the marine environment. The high salt content of brine can cause changes in the water chemistry and harm aquatic life, including fish, crustaceans, and other marine organisms. The brine can also create “dead zones” in the ocean, where the increased salinity makes it difficult for other species to survive.
_
Another source of pollution from desalination is the chemicals used in the process. Desalination requires the use of various chemicals, including antifoulants, biocides, and disinfectants, to keep the equipment clean and free of biofouling. These chemicals can be harmful to the environment if they are not properly managed and disposed of. In addition, some desalination processes also require the use of energy-intensive pumps and membranes, which can consume significant amounts of electricity and contribute to greenhouse gas emissions. The disposal of desalination residuals, such as filter backwash and concentrate, is another source of pollution associated with the process. These residuals can contain high levels of dissolved solids and other pollutants, including heavy metals, organic matter, and pathogens, which can pose risks to human health and the environment if they are not properly managed.
_
In addition to the pollution generated during the desalination process itself, the construction and operation of desalination plants can also have negative impacts on the environment. For example, the construction of new desalination plants can cause damage to sensitive habitats, such as wetlands and intertidal zones, and may lead to the displacement of wildlife and other species. The large scale infrastructure required for desalination can also lead to the degradation of natural resources, such as sand, gravel, and minerals, which can cause long-term impacts on the environment. Furthermore, the transport and distribution of desalinated water can also contribute to pollution and environmental degradation. This can occur through the release of pollutants during transportation, such as spills and leaks from pipelines, as well as the release of chemicals and other pollutants from treatment facilities.
_
A closer look at what kinds of chemicals and pollutants are used during desalination is provided in table below. The table outlines the main types of pollutants resulting from desalination and provides their typical concentrations for both reverse osmosis and thermal (MED and MSF) desalination technologies. At each stage of the desalination process, chemicals are added or further concentrated. Most of them are simply discharged at the very end of the desalination process. Some chemicals are discharged continuously as part of the brine discharge while others in periodic intervals during clearing and maintenance of the facility (Miller et al., 2015).
Key characteristics of main pollutants used in RO, MED and MSF desalination are depicted in table below:
______
______
Carbon Footprint of Desalination Plants:
GHG emissions from water reuse and desalination systems come from: (1) direct emissions from on-site sources, (2) indirect emissions associated with off-site energy production, and (3) other indirect emissions (i.e. production of chemicals, materials, fuels, etc.). The carbon footprint is defined as the sum of individual GHG emissions, in which carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) are expressed in carbon dioxide equivalents (CO2eq) by converting CH4 and N2O emissions using their global warming potential. Reverse osmosis (RO) technologies were found to have lower CO2 emissions than thermal desalination technologies and the estimated carbon footprint of seawater RO desalination (0.4–6.7 kg CO2eq/m3) is generally larger than brackish water RO desalination (0.4–2.5 kg CO2eq/m3) and water reuse systems (0.1–2.4 kg CO2eq/m3).
_
Figure above shows GHGs emissions per m3 of freshwater produced by the major desalination technologies when fossil fuels are used (red bars) and GHGs emissions per m3 of freshwater produced by the major desalination technologies when renewable energy sources or waste heat are used (green bars). The use of fossil fuels to generate the necessary energy is associated with emissions of GHGs. The thermal-based technologies, namely MSF and MED, have at least ten times higher GHGs emissions than RO, as can be seen from figure above. Consequently, desalination has significant environmental impacts on air quality.
_____
_____
Solutions of environmental impacts of desalination:
The desalination plants have a significant environmental impact. Despite many efforts, there are still some environmental concerns such as:
-disposal of material use
-land use
-energy use to desalinate water and greenhouse gas (GHGs) emissions
-brine discharge
-high volume of chemical use
-loss of aquatic organisms from marine pollution and open seawater intake
The use of fossil fuels to desalinate water emits greenhouse gases, including carbon monoxide (CO), nitric oxide (NO), nitrogen dioxide (NO2), and sulfur dioxide (SO2). Likewise, the use of a high volume of chemicals during the pre- and post-treatment of seawater is another environmental concern. The main concern is the discharge of chemicals into the natural water, which affects the ecological imbalance. Furthermore, the design of open seawater intake has a potential role in the loss of aquatic organisms, as these organisms sometimes collide with the intake screen or can be drawn into the plant. Some of the possibilities for sustainable solutions to prevent/minimize the issue listed above are:
-implement low or no chemical technologies
-treat the chemicals before discharging into the natural water bodies
-disperse the concentrate through a multiport diffuser in a suitable marine site
-use subsurface or submerged intakes with low intake velocities
-reuse of material
-recover salts from the brine (resource recovery)
-use renewable energy sources to partially/completely fulfil the energy requirements
-use energy recovery devices to recover hydraulic energy
_
Figure above shows Environmental concerns and sustainable solutions for the desalination plant to minimize the environmental impact.
_
Overall, the measures for addressing desalination’s environmental impacts are illustrated in Table below.
Summary of mitigation measures and future prospects for reducing the environmental impacts of desalination and brine treatment:
|
Impacts |
Mitigation measures and future prospects |
|
Brine discharge |
Near-field and far-field modeling approaches Environmental monitoring plans (EMPs) Dilution with cooling water from power plants Zero Liquid Discharge (ZLD) Minimal Liquid Discharge (MLD) Resource recovery (Brine mining) |
|
Energy consumption |
Co-generation power-desalination plants Efficient energy usage plan Energy recovery devices |
|
GHGs emissions |
Renewable Energy Sources Waste heat from industrial processes |
|
Chemicals |
Novel green antiscalants Novel green corrosion inhibitors |
|
Feed water intake |
Multiport diffusers More corrosion-resistant metallic materials Locating intake areas with minimal impact Low speeds in the intake channels |
______
Desalination and environment: A critical analysis of impacts, mitigation strategies, and greener desalination technologies, a 2021 review:
The desalination of seawater is perceived as one of the most viable processes to fulfill the mounting demand for freshwater. Despite enormous economic, social, and health benefits offered by desalination, there are several concerns regarding its prospective environmental impacts (EIs). The objective of this work is to critically evaluate the potential EIs of seawater desalination, and assess the prospects of greener desalination. The EIs of desalination on marine environment, land, groundwater, and air quality was systematically reviewed. An attempt has been made to analyze the actuality of these so-called impacts with reference to evidence from real desalination plants. The mitigative measures to counterbalance these unfavorable impacts are critically appraised. Furthermore, the brine management technologies for the disposal of reject stream, the recovery of precious materials and water, and the production of useful chemicals are also reviewed. Current challenges to minimize the adverse impacts of desalination and prospects of sustainable greener desalination to overwhelm global water scarcities are also discussed. The current desalination approaches have moderate and minor negative EIs. However, with proper mitigation and utilization of modern technologies, these impacts can be lessened. Furthermore, by employing various modern techniques, reject brine can be utilized for several useful applications while reducing its adverse impacts simultaneously. Recent advancements in desalination technologies have also offered many alternative approaches that provide a roadmap towards greener desalination. This review article will be beneficial for all the stakeholders in the desalination industry.
______
LCA:
To evaluate the potential environmental impact of the water desalination plants, a life cycle assessment (LCA) tool can be a useful tool for application across the whole life cycle. The LCA takes into account all the phases of a product’s life cycle, starting from the acquisition of raw materials to the end-of-life phase (collection/sorting, reuse, recycling, waste disposal). The LCA technique has four phases: goal and scope definition, inventory analysis, impact assessment, and interpretation. There is various software available that can be applied to the LCA, such as EIME V5, Cycle IT System V1.1, e-LICCO, Open LCA 1.2, GaBi, SimaPro Analyst 7.3.3, Umberto 5.6, and others. LCA is a useful method for decision-makers to detect environmental hotspots and develop strategies to reduce negative environmental externalities.
_____
_____
Is the development of desalination compatible with sustainable development of the Arabian Gulf? A 2021 study:
The development of desalination has been essential to the rapid economic development of the countries bordering the Arabian Gulf. The current production capacity of sea water desalination plants drawing water from Gulf is over 20 million m3 day^−1, which may rise to 80 million m3 day^−1 by 2050. Whilst supporting aspects of sustainable development related to water and sanitation, desalination impacts the marine environment through impingement and entrainment of organisms in intakes, and through thermal, brine and chemical discharges. This may compromise other objectives for sustainable development related to sustainable use of the oceans. Under business as usual scenarios, by 2050, the impact of individual desalination plants will combine causing a regional scale impact. Without mitigating actions to avoid the business as usual scenario, by 2050, desalination in combination with climate change, will elevate coastal water temperatures across more than 50% of the Gulf by at least 3 °C, and a volume of water equivalent to more than a third of the total volume of water between 0 and 10 m deep will pass through desalination plants each year. This will adversely impact the coastal ecosystem of the Gulf, with impacts on biodiversity, fisheries and coastal communities and may cause potential loss of species and habitats from the Gulf. Given the significant implications of these preliminary findings, and in light of the precautionary approach to management, it is recommended that mitigating options addressing behavioural, regulatory and technological change are rapidly evaluated and implemented to avoid the development of desalination in the region along a business as usual pathway, and multidisciplinary research studies should be conducted to reduce uncertainty in predictions of future impacts.
______
______
Section-15
Brine and brine management:
Brine refers to a hot, salty concentrate containing chemicals. It is an unwanted by-product of the desalination procedure. As the recent study by Jones et al. (2019) showed, the volume of brine is superior by up to 50% to the volume of water produced by desalination facilities. Previous estimates placed the produced volumes of brine at roughly the same levels as volumes of produced water. This recent study shows that the situation of brine is an even more concerning issue than previously thought. Brine resulting from the RO desalination is more saline than brine resulting from the use of thermal technologies, reaching up to 80000 mg of salt per liter of discharged water. The exact composition of brine is highly dependent on the type of desalination technology and on the choice of chemicals used during the desalination procedure.
_
The environmental impacts of brine are felt locally in areas making heavy use of desalination. Brine produced just by four countries – Saudi Arabia, UAE, Kuwait and Qatar – accounts for 55% of the global brine output (Jones et al., 2019). The main reason for this unwanted leading position is the combination of relatively challenging environmental conditions for desalination (hot and saline water of the Gulf) and significant presence of thermal desalination facilities in the region which produce higher volumes of less concentrated brine. The Middle East and Northern Africa region leads the table terms of produced brine, accounting cumulatively for 70.3% of global brine production, which implies that these zones would be particularly interesting target for impact-based financing of brine mitigation strategies. The municipal sector is an end user responsible for 75.2% of brine production according to the data provided by Jones et al. (2019), which once again suggests a potential trade-off between the socio-economic need for drinkable water and the environmental concerns related to brine.
_
For every liter of freshwater output, desalination plants produce about 1.5 liters of brine (depending on the feedwater salinity and desalination technology). A recent UN-backed study has determined that nearly 16,000 desalination plants worldwide pump out 142 million cubic meters (5 billion cubic feet) of salty brine every day, 50% more than previous estimates, to produce 95 million cubic meters of fresh water. Brine is comprised of about 5% salt. Seawater is typically only about 3.5% salt. Brine often includes toxins and heavy metals which can accumulate in the environment and have negative effects on plant and animal species. According to marine scientists, brine is denser than seawater and often sinks to the ocean floor, where it can lower oxygen levels in seawater with devastating impacts on aquatic ecology. The problem with all this hyper salty water is that it often contains other contaminants and can pose a significant threat to marine life. There is an increase in the temperature of this zone of the sea, together they decrease the dissolved oxygen level, which is called hypoxia and that impacts the aquatic life in that zone. Hypoxia often leads to what are called dead zones in the oceans. Scientists say these zones have quadrupled since 1950, mainly as a result of climate change. Now the salt is adding to these problems. High salinity and reduced dissolved oxygen levels can have profound impacts on benthic organisms, which can translate into ecological effects observable throughout the food chain. To mitigate the effects, facilities can dilute brine with seawater before releasing it and take care to distribute it where ocean currents are strongest to encourage dissipation. In other cases, they can also evaporate brine into crystalized form.
_
The exact scope and nature of damage caused by brine to the environment depends on the methods chosen for brine discharge from desalination plant. The choice of these methods in turn depends on the location of the plant itself. For desalination plants located in proximity to saline water surfaces, brine is discharged directly, often without any treatment. Inland desalination plants usually do not have saline water bodies nearby and therefore have to adopt alternative methods for brine disposal such as injection of brine into wells. This is a cheaper method for landlocked desalination plants relative to the transport of brine towards the sea. However, it can result in unwanted pollution and contamination of underground waters.
_
The environmental impacts caused by brine are strongest around the discharge point. Since brine tends to have elevated temperature and salinity and also contains several chemicals (antiscalants, coagulants, surfactants, acids and bases for pH control), its discharge is hazardous for marine ecosystems. For illustration, coagulants increase water turbidity and discharge of antiscalants adds organic phosphorus into ambient waters, hereby causing eutrophication (Petersen et al., 2018), which alters the levels of dissolved oxygen and leads oxygen depletion. Depending on the volumes of discharged brine, oxygen depletion can create so called “dead zones” where aquatic life cannot survive due to insufficient quantities of available oxygen dissolved in the water.
Other consequences of the discharge of brine into seawaters are the increased salinity of surrounding water and alteration of the levels of pH, nutrients and alkalinity of the water. Combined, these impacts can threaten marine life or change the composition and functioning of marine ecosystems (Roberts et al., 2010).
_
Besides freshwater, a by-product called ‘brine’, ‘reject’ or ‘concentrate’ is produced during desalination. The by-product is at least 1.6 times ore saline than seawater (Table below) and its management is a crucial issue as brine has adverse effects on the environment (Heck et al., 2016; Missimer and Maliva, 2018; Frank et al., 2017).
Table below shows composition of brackish water, seawater and brine:
|
Saline solution |
Total dissolved solids (TDS) (mg/L) |
Ca2+ (mg/L) |
Mg2+ (mg/L) |
Na+ (mg/L) |
K+ (mg/L) |
Cl− (mg/ L) |
SO24− (mg/L) |
HCO3− (mg/L) |
PO34− (mg/L) |
|
Brackish water |
2480 |
230 |
66.8 |
142 |
– |
382 |
72.4 |
– |
– |
|
Brackish water |
1691.4 |
102 |
78.5 |
340 |
6 |
645 |
80 |
350 |
– |
|
Seawater |
34,483 |
400 |
1262 |
10,556 |
380 |
18,980 |
2649 |
140 |
– |
|
Seawater |
39,017 |
474 |
1356 |
12,245 |
434 |
21,535 |
2772 |
146 |
– |
|
Brine |
57,400 |
521 |
1738 |
18,434 |
491 |
32,127 |
4025 |
– |
2.5 |
|
Brine |
70,488 |
790 |
2479 |
21,921 |
743 |
38,886 |
5316 |
173 |
– |
Note: Different studies have given differing figures.
_
Brine salinity produced by membrane-based technologies, mainly seawater reverse osmosis (SWRO), ranges from 60 g/L TDS to 85 g/L TDS, while brine salinity produced by thermal technologies (i.e., MSF and MED) ranges from 55 g/L TDS to 65 g/L TDS, respectively. Compared to thermal-based plants, this differentiation can be attributed to the higher recovery rates (40–45%) present in commercial and well-established RO plants. As for the temperature of the brine, membrane-based plants produce brine at ambient temperature, much like the temperature of the feed water. In particular, a maximum difference of 1–2.5 ◦C has been reported, which could be from heat dissipation in the pumps and/or friction in the channels of the RO elements (Nagy, 2019; Spellman, 2015). MD and MCr are the only exceptions in membrane-based technologies, as these technologies are thermal-driven and produce brine at significantly higher temperatures (>30 ◦C). On the other hand, thermal-based plants produce brine of higher temperature (25–40 ◦C) than the ambient temperature as evaporation takes place (Cambridge et al., 2017; Missimer et al., 2015).
_
The quality and amount of the brine are governed by the quality of the feed water, pre-treatment process, desalination process, water recovery rate, and disposal technique (Panagopoulos et al., 2019) (Table above). The amount of brine produced is determined by the capacity of the desalination plant and the water recovery rate, which refers to the percentage of freshwater produced from the total volume of feed water used. As a result, feed water with higher salinity levels will produce more concentrated brine if the water recovery rate remains constant, and thus, better feed water quality will lead to a higher recovery rate (Jones et al., 2019). The brine generated will become more concentrated and lesser as the water recovery rate increases. Brine effluents released from MSF and RO seawater desalination facilities have physical properties (such as salinity and temperature); cleaning, bio-fouling, scale, and foam control chemicals; coagulants added to remove suspended solids; and pollutants resulting from corrosion, such as heavy metals (Lin et al., 2013). Although RO relies on hydraulic pressure and does not change the temperature of the seawater used, it does have highly complex pre-treatment steps that include antiscalants and coagulants that may change the pH of the water, resulting in brine that is the same temperature as ambient water but with a much higher salinity (Missimer and Maliva, 2018; Frank et al., 2019; Matin et al., 2019). MSF desalination, on the other hand, relies on evaporating and condensing the input saltwater, resulting in brine effluent with higher temperature and salinity levels, although the salinity is generally significantly lower than with RO (Hashim and Hajjaj, 2005; Bandi et al., 2016).
_
Desalination brine effluents include significant levels of Cl- and Na+, as well as other ions such as Ca2+, Mg2+, and SO2−4 (Panagopoulos et al., 2019). Several studies have been published on the presence of heavy metals such as copper (Cu) in desalination brine effluents and seawater. MSF, on the other hand, relies on the use of stainless steel with strong corrosion resistance and non-metal equipment. Brine discharges from RO often include metals at trace quantities, such as iron (Fe), nickel (Ni), chromium (Cr), and molybdenum (Mo). Copper contamination is more frequent in MSF effluents and is derived from Cu-based alloys used as components in the distillation process (Lin et al., 2013). Furthermore, chemicals such as biocides, surface−active agents, anti-scale additives, and solid residues from filter backflushing may be present in the effluent discharge on a continuous or periodic basis, posing a risk to the environment (Frank et al., 2019). Brine effluents from RO desalination plants not only have a high salt content but typically also contain compounds from the desalination process, such as phosphonate-based antiscalants and ferric (or alum) sulphate-based coagulants (Frank et al., 2019).
_
Factors influencing the physicochemical composition of brine are summarized in the figure below:
_
Discharge Approach:
Risks associated with the construction of desalination plants include construction of water intake infrastructure and the network of pipes transporting the feedwater to the plant, which may impact environmentally sensitive areas. However, concentrate (reject salt) still remains the most critical environmental problem, which also affects the cost-effectiveness of desalination. RO plants produce high TDS concentrates (>65,000 mg/L) that may also contain some toxic chemicals used during feedwater pretreatment and post-treatment (cleaning) processes. Selection of a concentrate management option depends on several factors: concentrate volume and quality, the location of the desalination plant, and the pertinent environmental regulations. Examples of concentrate management practices include:
- Surface water disposal. Disposing of concentrate in tidal rivers and streams, coastal waters such as oceans, estuaries, and bays adjacent to or near the plant is the most common method of concentrate disposal. Environmental concerns include adverse impact on the receiving waters’ ecosystems, and the long-term effect on the water quality of coastal aquifers.
- Submerged disposal. Concentrate is transported away from the desalination plant via underwater pipes to an estuarine and/or ocean location. Environmental concerns include potential impact of sinking concentrate on benthic marine organisms living on the sea bottom.
- Deep well injection. Concentrate is directly injected into deep groundwater aquifers. This practice is site specific due to geologic condition and potential impact on drinking water sources.
- Evaporation ponds. Constructed ponds with liners are used to allow water evaporation from the concentrate while the remaining salts accumulate in the pond bottom. Evaporation ponds are a cost-effective option in areas of warm climates and high evaporation rates. However, they are moderately land-intensive and also cause significant loss of the basic water resource through evaporation.
- Land application. This method uses spray irrigation, infiltration trenches and percolation ponds. Land application provides an opportunity for a beneficial use of concentrate. For example, concentrate can be used to irrigate salt-tolerant crops and grasses such as those used on golf courses. The feasibility of land application depends on the local climate, vegetation tolerance to salinity, the availability of land, and the location of the groundwater table.
- Integration with a wastewater treatment plant. Options here include concentrate discharge to the front of the wastewater treatment plant or to the end of the wastewater treatment plant. However, discharge to the front of a wastewater treatment plant is not recommended because conventional wastewater treatment plants do not remove TDS and concentrate TDS level can have a significant impact on the biological treatment process. Concentrate disposal to the end of a wastewater treatment plant results in diluted concentrate due to it mixing with treated wastewater. It’s important to note that, despite dilution, this practice may adversely impact receiving water. Also, there will be additional cost for constructing a separate pipeline (and perhaps using a pump) to carry the concentrate to the wastewater treatment plant.
- Brine concentrators. The brine concentrator process uses heat exchangers, deaerators, and vapor compression to convert liquid concentrate into slurry form. Using a brine concentrator, 95 percent of the water can be recovered as a high-purity distillate with less than 10 mg/L of TDS concentration. The remaining 5 percent of concentrated slurry can be reduced to dry solids in a crystallizer to create dry, solid cake, which is easy to handle for land disposal. As a cautionary note, some chemicals may be present in the dry solid.
- Zero liquid discharge (ZLD). The ZLD technique originally developed for solid waste management is promoted as a new technology for concentrate management. The ZLD technique uses an evaporation process to convert a liquid concentrate (brine) into a dry solid that can then be utilized for beneficial purposes. As a cautionary note, some chemicals may be present in the dry solid. Furthermore, the ZLD process is a high-energy-cost technique. Using thermal technologies may facilitate more cost-effective ZLD application for concentrate management.
_
The quality, content, and quantity of brine, as well as the geographical location at the point of discharge, the validity of the choice, and the availability of an acquisition site, all influence the optimal brine disposal strategy (Mansour et al., 2017; Mavukkandy et al., 2019). More than 90% of seawater desalination facilities use the surface water approach to discharge brine into open water bodies (Panagopoulos et al., 2019). This method is commonest owing to the short distance between the desalination plant and the sea. Accordingly, the inland disposal option has become undesirable (Ahmad and Baddour, 2014).
Surface discharge and sub-surface discharge are the two types of brine discharge to open water bodies. The first type discharges brine via an outfall structure very near to the coast, resulting in a build-up of highly concentrated saltwater in that area (Ahmad and Baddour, 2014). In contrast, submerged brine discharge, which is used by the majority of large desalination plants, allows brine to be discharged deeper and further into the mixing zone of the receiving water body via pipes with diffusers or vertical risers embedded at the discharging end. This method allows for better dilution of brine with ambient seawater to reduce salinity, thus minimizing the impact on the marine environment (Ahmad and Baddour, 2014; Mansour et al., 2017; Missimer and Maliva, 2018).
_
The vertical riser can be used in deep-sea brine discharge. It is positioned vertically and equipped with a nozzle that increases the momentum of the discharged brine, resulting in dilution of the brine with seawater. The brine is discharged vertically with great momentum, causing it to ascend to a certain height then descend due to its negative buoyancy, eventually dispersing around the discharge point on the sea floor (Ahmad and Baddour, 2014; Missimer and Maliva, 2018). Diffusers are commonly used in sub-surface brine discharges, which can include rosette or multi-port diffusers, which are essentially multiple nozzles on the end of the discharge pipe that promote mixing of brine with ambient saltwater in the water column to prevent brine accumulation on the sea bottom (Missimer and Maliva, 2018).
_
The salinity of brine is reduced by diluting it with regular seawater, municipal wastewater, and cooling water from a neighboring power station prior to disposal (Ahmad and Baddour, 2014; Mansour et al., 2017; Missimer and Maliva, 2018). However, because the temperature and salinity of water discharged from cooling and desalination plants are both higher than that of the receiving seawater, the cooling power plant discharge can float on the surface, although because the desalination discharge is more salinic and heavier, it drags the cooling power plant water with it as it descends. Hence, the entire water column is involved in the salinity and heat dissipation process, which speeds up the dilution of discharged water.
_
Brine disposal costs can account for up to a third of total desalination expenses, and avoid damaging environmental impacts. There is no single disposal method that has only advantages. For example, deep-well injection is unsuitable for countries with high seismic activity (e.g., Greece); evaporation pond is the costliest method since it requires high footprint area; sewage discharge and land application can only be used for small amounts of brine; and surface water discharges have a direct impact on the marine environment (Panagopoulos et al., 2019). Therefore, even with the adopted disposal methods, brine may have an adverse impact on the environment.
_____
_____
Solutions for mitigating environmental impacts of brine:
While brine is by its very nature a harmful by-product of the desalination procedure, several mitigation measures can be put in place in order to reduce the impact of related negative externalities. The exact impact of brine upon marine ecosystems depends not only on the chemical composition of brine but also upon the proprieties of receiving water bodies.
-1. Mixing of brine with cooling water before discharge:
One way to make brine less harmful is to make it less concentrated when discharged. Brine can be diluted by mixing with cooling water from power plants adjacent to the desalination plant. Since the water used for mixing is much less saline, the mixing reduces brine salinity via dilution (Giwa et al., 2017). Desalination plants often have adjacent power generation facilities. The cooling water from such power plants can be mixed with brine, diluting it before the discharge into ambient waters. This can partially mitigate the issues related to the high salinity, elevated temperature and chemical content of brine.
Depending on the volume of cooling water used for mixing, brine can be denser than the ambient seawater and sinks to the seabed where it flows as a concentrated stream. The location of where brine plunges to the seafloor can vary in locations with changing water currents (Petersen et al., 2018). This “bottom ponding” can, however, be restricted by mixing brine waters with receiving waters at the point of discharge, for instance by using pressurized dispersion nozzles (Roberts, 2015).
-2. Optimal brine discharge infrastructure:
Computer modelling can be used to determine the optimal length of brine discharge pipelines, the number of openings discharging brine into ambient water or the number and type of diffusers facilitating the mixing of brine with water. The exact impact of brine discharge upon the marine environment is context-specific, depending on a variety of factors related to the composition of water, strength and direction of underwater currents as well as behavior and migratory patterns of local organisms. Computer models can be used to account for the influence of a set of factors such as the chemical properties of receiving water or the strength and direction of underwater currents to determine the optimal structure of brine discharge infrastructure given the local conditions.
-3. Neutralizing chemicals used during desalination:
Brine can be made less environmentally harmful before it is discharged. Various chemicals used during the successive stages of the desalination process can be neutralized before they are added into the brine. The exact modalities and related costs will depend on the type of chemicals used, but the point is generally applicable.
-4. Brine minimization technology:
Several technologies can be used at desalination facility to reduce the volume of produced brine. The exact choice of technology depends on the salinity of brine. Brine with relatively low salinity is best suited for membrane-based brine minimization technologies such as forward osmosis, vibratory shear enhanced processing (VSEP) and electrodialysis metathesis (EDM). For brines of higher salinity, thermal-based technologies can be used.
-5. Recovery of metals and salt from brine:
While usually considered as an unwanted byproduct of desalination, brine itself can also be used for other purposes. In this perspective, it is no longer an unsolicited environmental headache but a valuable resource for potential commercial exploitation. Several possibilities exist but are not yet used on an industrial scale. Investments in this direction would have the double benefit of turning “wasted” brine into an exploitable resource while avoiding the environmental impact of conventional ways of obtaining elements extracted from brine.
Adsorption is a process enabling the extraction of metals from brine. Depending on the exact composition of brine, recovered metals can include uranium, lithium, rubidium and cesium (Loganathan et al. 2017). While the recovered metals could provide a relatively high return on investment, the technology has not yet been demonstrated at a large scale. A co-benefit of metal recovery from brine would be a potential reduction of negative environmental impacts of mining.
Except from metals, brine can also provide another valuable resource: salt. Chemical precipitation, crystallization and evaporation all enable salt recovery from brine. For illustration, sodium phosphate and sodium carbonate are used around the Mediterranean and the Red Sea for this purpose.
-6. Use of brine for aquaculture:
Finally, brine can also be used as a resource for aquaculture since some organisms thrive in saline water. Two documented cases are “brine shrimp” used as a food for fish and shellfish, and microalgae “Dunaliella salina” used in pharmaceutical and food industries. Brine can be used as a feedstock for such aquaculture activities, hereby also providing opportunities for local employment and economic activity.
_____
_____
Brine disposal to brine treatment:
Brine, also known as concentrate, is the by-product of the desalination process that has an adverse impact on the environment due to its high salinity and chemicals. Hence, viable and cost-effective brine management systems are needed to reduce environmental pollution. Brine is commonly disposed of in the environment with various methods, such as surface water discharge, sewer discharge, deep-well injection, evaporation ponds and land application (Mickley, 2018). Brine, in addition to its high salinity, may contain dangerous pretreatment chemicals, organics and heavy metals. Numerous researchers investigated the negative environmental impact of brine disposal on the marine environment, groundwater and soil quality. Thus, potential environmental damage includes eutrophication, pH fluctuations, increase of heavy metals in marine environments, etc. (Heck et al., 2016; Petersen et al., 2018; Heck et al., 2018). Despite the fact that disposal methods have been widely used so far, the concern about a long-term impact on the environment and human health leads to the need for a different approach.
Nowadays, brine treatment is considered one of the most promising alternatives to brine disposal, since treatment results in the reduction of environmental pollution, minimization of waste volume and production of freshwater with high recovery. To eliminate the demand for brine disposal, desalination brine can be treated using the Zero Liquid Discharge (ZLD) approach. ZLD effectively minimizes wastewater discharge and enables freshwater and salt to be recovered (Wenten et al., 2017). ZLD can be achieved through various membrane-based and thermal-based technologies. Two technologies specifically developed for brine treatment, the brine concentrator and crystallizer, are currently being applied in full scale. To date, these technologies have very high capital and operating costs, so their adoption is limited (Mansour et al., 2018). Consequently, with the development of new emerging technologies and the enhancement of the existing commercial technologies, more effective ZLD systems can be available.
_
Disposal of desalination brine and reduction of its associated environmental effects are two of the focal issues of new technologies (Mansour et al., 2017). As a result, appropriate and efficient actions must be taken to ensure the safe disposal of desalination brine and its related toxic substances that occur in the brine from desalination processes (Loutatidou et al., 2017). In modern-day brine management, two basic techniques are used: The first is volume reduction, which aims to retrieve additional amounts of water equal to or beyond the saturation concentration, and the second is zero liquid discharge or crystallization approaches, which aims to retrieve water and various salts from the highly concentrated brine (Mavukkandy et al., 2019).
Zero liquid discharge is defined as a synthesis of desalination technologies that aims to generate high-quality water without producing any liquid waste at all. These processes achieve a water recovery rate of 95–99%, and the water produced is very pure and can be used for a variety of purposes including drinking and irrigation. In addition to the production of water, the solutes are compressed into their solid state, which can be processed further to produce a useful substance (Muhammad and Lee, 2019).
There is no universal zero liquid discharge system to be adopted by all desalination plants because zero liquid discharge systems differ in their design, arrangement, and operation, making each system unique. Beyond mitigating environmental impacts, ZLD also presents an economic advantage through resource recovery. By extracting valuable salts from the leftover brine, plants can generate additional revenue which helps offset operation costs. In essence: embracing technologies like ZLD isn’t just good practice – it can be a smart business strategy too.
_____
_____
Drivers, challenges, and emerging technologies for desalination of high-salinity brines: a 2022 review:
Hypersaline brines are of growing environmental concern. While high-salinity desalination and zero liquid discharge (ZLD) are increasingly attractive treatment options, the high salt and scalant contents pose considerable technical difficulties to existing desalination techniques. In this review, authors introduce sources of hypersaline brines, examine factors driving high-salinity desalination, and present the thermodynamic minimum energy of hypersaline desalination and ZLD, highlighting effects of mineral precipitation and imperfect salt rejection. Authors then critically examine prospects and challenges of 10 alternative technologies for hypersaline desalination: electrodialysis, osmotically-mediated reverse osmosis, forward osmosis, membrane distillation, humidification-dehumidification, solvent extraction desalination, supercritical water desalination, freeze desalination, clathrate hydrate desalination, and solar thermal desalination. Although electrodialysis and osmotically-mediated reverse osmosis show promise of having competitive energy efficiencies, these membrane-based techniques are still constrained by concentrate salinity limits. Recovery and reuse of heat will be vital for competitiveness of thermally-driven approaches. Technologies that intrinsically precipitate salts in bulk solution, namely solvent extraction desalination, supercritical water desalination, and humidification-dehumidification, can advantageously avoid mineral scaling. Due to the highly heterogeneous nature of hypersaline streams and the wide array of end-use goals, the high-salinity desalination market will ultimately be best served by a range of different technologies with distinctive capabilities.
_____
_____
Can brine from a desalination plant be made into consumable salt?
The problem is that this hypersaline brine contains not just sodium chloride, but also heavy metals and other contaminants and chemicals. You are dealing with a mountain of salt… inland desalination plants that convert brackish water into potable water, and then they evaporate the brine and waste and cart it away. This isn’t table salt; we make table and sea salt from places where the seawater is relatively clean. The brine that comes off desalination is extremely toxic stuff. Also, a study was conducted to evaluate the potential of transferring desalination brine to solar saltworks, so that its disposal to the sea is avoided. The analysis showed that brine transfer by trucks is prohibitively expensive.
A better alternative would be to extract metals such as gold and uranium from the brine to offset the costs of production. One alternative that is in use is the evaporation of the brine for the production of road salt for deicing roads, but so far, this has only been used with inland desalination plants for fear of introduction of toxic chemicals back into already destabilized aquafers.
_______
_______
Creating useful products from desalination waste:
Researchers at MIT proposes a direct electrosynthesis (DE) process to produce sodium hydroxide (NaOH) and hydrochloric acid (HCl) from desalination brine as an alternative to current technologies. In the proposed DE process, the water is split to produce hydrogen (H+) and hydroxide (OH-) ions, which are combined with the brine stream to produce NaOH and HCl. NaOH can be used to pretreat water going into the desalination plant. It changes the pH of the water, which helps to prevent fouling of reverse osmosis (RO) membranes. Fouling is a major cause of interruptions and failures in typical RO desalination plants. NaOH pretreatment also helps to prevent scaling, in which salts such as calcium carbonate become supersaturated and precipitate onto the membrane. Industry uses and spends a lot of money on NaOH, and the ability to make it on site would offer a “big advantage”.
Using established processing methods, HCl could be produced on site, from desalination waste. HCl is also used by desalination plants, as well as in many other industrial processes. In desalination plants HCl can be used for cleaning and it is widely used in chemical production and as a hydrogen source.
John Lienhard, Professor of Mechanical Engineering at MIT, said: “Environmentally safe discharge of brine is manageable with current technology, but it’s much better to recover resources from the brine and reduce the amount of brine released.”
This research, published in ACS Sustainable Chemistry & Engineering, outlines an efficient method for transforming water with very high concentrations of salt and chemicals, known as brine, into commercially valuable chemicals as part of the desalination process. The approach avoids the need for disposing potentially hazardous chemicals in local ecosystems.
_
In recent years, the hunt for a sustainable and “inexhaustible” supply of raw minerals and metals has emerged as a crucial challenge. With the global population expected to grow, the demand for raw materials is projected to double by 2060, leading to a 70 % surge in waste production by 2050. Reliance on land-based mining can no longer be taken into consideration. The main reasons for this are: (i) the gradual depletion of high-grade ores, (ii) the increase of water and energy requirements for the extraction of minerals from low-grade ores and (iii) the reduced purity of the final products recovered from hydro metallurgical facilities, leading to amplified costs and environmental concerns. Among the different initiatives to recover critical raw materials from secondary sources, seawater mining has emerged as an attractive option due to the comprehensive presence of elements in the ocean, spanning the majority of the periodic table. The abundance of certain elements Na+ > Mg2+ > Ca2+, K+ (for cations) and Cl− > SO42− > HCO3− > Br− (for anions), allows for their economically feasible extraction. For example, due to the abundance of NaCl, its extraction from seawater has been conducted since ancient times. However, other elements, referred to as “trace elements”, hold higher economic value but are found in such low concentrations in seawater (below 1 mg/L) that their retrieval is currently not economically viable. Many studies have shifted their interest to valorising brine originated from seawater desalination (i.e. seawater reverse osmosis brine (SWRO brine)) due to a concentration almost the double of that of seawater and the fact that pumping cost is covered by the water production stage. As technologies for seawater brine mining develop, desalination concentrate as a source of minerals becomes more economically and environmentally viable. The economic gain obtained by extracting minerals is proportional to the increase in the concentration of minerals in the concentrate as well as the market price of these minerals. In this respect, mining of compounds of elements including Mg, Na, Ca, K, Sr, Li, Br, B, and Rb could potentially be economically attractive for harvesting from concentrate, if suitable methods of brine concentration and extraction are developed. Economically, the cost of extraction needs to be weighed against the revenue achievable, which relies on market fluctuations of commodity prices. Environmentally, extraction from brine is less intrusive than conventional mining with the added benefit of reduction in brine volumes.
______
______
Section-16
Health risks related to desalinated water:
Saline sources are different from freshwater sources in that they always require a substantive treatment step. However, while the desalination process usually provides a significant barrier to both pathogens and chemical contaminants, this barrier is not necessarily absolute, and a number of issues could potentially have an impact on public health. Some of these are similar to the challenges encountered in most piped water systems, but others, such as those related to stabilizing and remineralizing the water to prevent it from being excessively aggressive, are different and therefore must be addressed within the context of a site-specific health risk management plan.
_
Source water and potential hazards:
Source water for desalination can be marine or brackish surface water or highly mineralized groundwater. By definition, this water has a significant content of naturally occurring inorganic ions, and the objective of treatment is to reduce the concentration of, or remove, these substances. These naturally occurring substances include some that would be of potential concern if present in sufficient concentrations after treatment. Like all surface water sources and some groundwater sources, there can be contamination by pathogenic viruses, bacteria and parasites and by a variety of chemical contaminants from human activities.
There are notable differences between freshwater sources and brackish or saline sources. In particular, the survival of many microbial pathogens is significantly reduced in saline waters, especially in combination with a high level of solar radiation. However, some pathogens, such as Vibrio cholerae, do survive well in saline waters. There are also many marine algae that can produce toxins of concern to human health.
Chemical constituents of interest include boron (borate), bromide, iodide, sodium and potassium; they may require additional actions for removal (boron) or are present in such concentrations as to leave significant residues. While natural organic matter (NOM) varies significantly, there are a number of organic substances, coming from both natural and anthropogenic sources, that are of particular interest.
_
Desalination processes:
The desalination process is primarily intended to remove natural ionic contaminants, but some substances are not as well removed as others. For example, boron, which can be present in significant concentrations in saline waters, is not well removed by reverse osmosis (RO). While most systems remove a significant proportion of microbial pathogens, in some circumstances, there is a significant potential for some pathogen transfer. Electrodialysis reversal systems do not provide any barrier against pathogens, and electrodialysis reversal is, therefore, rarely considered to serve for drinking-water production.
In addition, a number of chemical treatments are used to prepare and maintain the desalination systems, and procedures must be established to ensure that the associated compounds do not reach final water in unacceptable concentrations. Cleaning of membranes is fundamental for optimal treatment performance and the quality of water coming from the membranes. When cleaning agents are applied, either online
or offline, these chemicals can be present in the system at high concentrations that could negatively affect treated water quality. Therefore, the membranes should be properly flushed before installation and before the system goes back online, and the flushing solution should be disposed of suitably as waste. Pretreatment of the waste will be necessary, and it is important that this waste stream be disposed of properly so that it does not contaminate either source waters or waters that might be used for blending with desalinated water.
Materials such as piping and contact surfaces in treatment systems and processes that come into contact with drinking-water need to be assessed to ensure that no harmful chemicals or substances in these materials are introduced that could cause WHO guideline values to be exceeded, pose a hazard to health or have an impact on the acceptability of the final water.
_
Disinfection:
Desalinated waters constitute a relatively easy disinfection challenge because of their low total organic carbon and particle content, low microbial loads and minimal oxidant demand after desalination treatments. Turbidity is not likely to affect chemical disinfectant performance, as turbidity values of desalinated water are low. Post-treatment (e.g. with lime) can cause an increase of inorganic turbidity that would not interfere with disinfection by chlorine. The target levels of inactivation for pathogens remaining in desalinated waters can readily be achieved by appropriate disinfection processes, discussed in the WHO Guidelines for drinking-water quality (WHO, 2011). Once the target levels of disinfection have been achieved, it is good practice to maintain an appropriate level of residual disinfectant in the product water during distribution.
Issues to be considered as specific to the disinfection of desalinated water are:
- the potential passage of viruses through some RO membranes, which may require adequate virus inactivation downstream of RO.
- the potential loss of integrity of membranes, which could lead to the passage of pathogens into the process water.
These issues can be addressed in most cases by applying effective post-desalination disinfection using chlorine-based or alternative disinfection processes (ultraviolet light, ozone, etc.) as an additional barrier to reduce the possible risks.
_
Blending and remineralization:
Desalinated water is low in minerals and is poorly buffered. It is usually aggressive to cementitious and metallic materials used in storage, distribution and plumbing and requires conditioning to address this problem. Blending desalinated water with source water or partially treated water is a common practice, and the addition of minerals to achieve a balanced mineral content in desalinated water is increasingly being adopted. This latter approach may also be used to make a contribution to the mineral intake of consumers in regions where traditional sources of water have contained significant levels of minerals.
_
Storage and distribution of processed water:
Desalinated water usually undergoes storage prior to or during distribution, and the problems encountered during storage and distribution are similar to those encountered for other supplies derived from fresh water. These relate to the potential introduction of contaminants, both microbial and chemical, the problem of corrosion of materials and the potential for materials and corrosion products to affect water quality, and the growth of pathogenic and potential nuisance organisms. Similar to most drinking-water supplies, there is often a requirement for blending with existing drinking-water streams.
______
______
Chemicals of concern for desalination processes:
-1. Boron and borate
Most of the inorganic components will be significantly removed in the desalination process, either thermal desalination or RO desalination, although some sodium chloride and bromide may be present in the treated water from membrane plants and possibly from some older distillation plants. In terms of key contaminants of direct interest for health and the environment, the most important is probably boron, which can be of significance in RO plants, as the rejection ratio of boron-containing anions (probably mostly as borate) is less than that for most other inorganics. In the fourth edition of the WHO Guidelines for drinking-water quality, the health-based guideline value for boron (borate) in drinking-water is 2.4 mg/l (WHO, 2011). This value represents a revision from earlier values and is based upon a review of the toxicological data and studies in areas with high background exposures. Although boron is an essential element for plant growth, it is herbicidal at higher levels, and some plants are sensitive at 0.5 mg/l. The latter is the principal issue for residual boron—that is, its effect as a herbicide if present in sufficient amounts in irrigation water, particularly in areas where rainfall is so low as to not cause sufficient leaching of salts from soils. Acceptable boron concentrations in desalinated water in areas where desalinated water has significant applications for irrigation may best be determined by authorities on a case-by-case basis, reflecting costs, end uses, climate and agricultural activity in the area.
_
-2. Bromide and bromate
Bromide is initially present in seawater in relatively large amounts (~80 mg/l in some regions), so even high (e.g. >95%) percentage removals will allow some bromide, on the order of 1 mg/l to several milligrams per litre, to be present in the finished water. The concentrations of bromide in desalinated water will be approximately proportional to the chloride concentration because of similar removal mechanisms for these analogous anions. Inorganic bromide is also present in many fresh waters, especially groundwaters and coastal aquifers affected by seawater intrusion, at up to milligram per litre levels. FAO/WHO (1988) developed an acceptable daily intake for bromide of 1 mg/kg body weight; assuming a 60 kg adult drinking 2 litres of water per day with a 20% allocation of the acceptable daily intake to drinking-water could give a health based reference value in the range of 6 mg/l. A similar conclusion is recommended in the fourth edition of the WHO Guidelines for drinking-water quality (WHO, 2011).
If ozonation or other similar oxidation processes are applied to waters with sufficient residual bromide under appropriate conditions, bromate will be formed at concentrations that will likely exceed the current WHO guideline value of 10 µg/l (WHO, 2011). Packaged waters produced by bottling distributed desalinated waters derived from high-bromide source water are often treated by ozonation prior to bottling. This would increase the bromate levels in the bottled water beyond the concentrations in the original distributed water if residual bromide is present. Production of chlorine by electrolysis of seawater will also produce large amounts of bromate. Bromate is carcinogenic in rats and mice in lifetime tests under high-dose conditions, with cancers in the kidney, thyroid and testes being observed, although there are no data available for humans (WHO, 2005a). However, there are strong indications that small amounts of bromate are metabolized and detoxified following ingestion before they can reach the target cells.
_
-3. Sodium and potassium
Sodium concentrations in seawater are in the range of 10000–15000 mg/l, depending upon the location. Sodium is an essential nutrient, and there is no health-based WHO guideline value for sodium, which is normally present in relatively low concentrations in drinking-waters derived from freshwater sources. The taste threshold is in the region of 200–250 mg/l, depending upon the associated anions. Daily dietary intake may approach 10000 mg/day for some individuals, which is well above the required daily intake. Sodium is essential for adequate functioning of human physiology, although the requirement of infants for sodium is lower than that for children and adults, and high sodium intake may lead to hypernatremia. This is a problem for bottle-fed infants and is the reason why sodium levels in infant formulas have been reduced significantly over time. There have been concerns expressed about the contribution of sodium intake to increasing hypertension across populations. A number of WHO Member States are concerned about the overall intake of salt from all sources, but particularly food, which is the major source of sodium intake, and are seeking to persuade their populations to decrease salt intake. In contrast, hyponatraemia can be a serious, including fatal, acute risk if significant perspiration causes high loss of sodium and there is inadequate sodium intake from the total diet. It is probable that the presence of some sodium in drinking-water in very warm climates might be beneficial for persons engaging in heavy physical activity.
Usually, seawater, brackish water and many fresh waters also contain potassium. Potassium concentrations in seawater are in the region of 450 mg/l, but about 98% of the potassium is removed in the desalination process. Potassium is also an essential nutrient, and the recommended daily dietary requirement is more than 3000 mg/day. There is currently no specific WHO guideline value for potassium; residual concentrations in desalinated water are expected to be small and well below any significant contribution to recommended daily dietary intakes.
_
-4. Magnesium and calcium
Magnesium and calcium are essential nutrients and are present in seawaters at concentrations of about 1200–1700 mg/l and 400–500 mg/l, respectively. They are the principal defining components of “hard water”. They are very efficiently removed by desalination, including NF, but may be added back to finished water by some processes used to stabilize the water and reduce corrosive potential. Desalinated bottled water contained significantly lower levels of Ca, Mg and F– than other water types, according to 2014 study in Qatar. The WHO recommends 10 mg/L of Magnesium and 30 mg/L of Calcium for drinking water.
_
-5. Organic chemicals found naturally in source waters
Naturally occurring chemicals include natural organic matter (NOM), such as humic and fulvic acids, and the by-products of algal and seaweed growth, where this growth occurs to a significant extent. Such chemicals can include substances that can cause taste and odour in the final water, such as geosmin from cyanobacteria, particularly in brackish water; and a range of toxins from a variety of different organisms, including cyanobacteria and dinoflagellates, that can form significant blooms, although these are usually intermittent in nature. Only one of these potential contaminants, the cyanotoxin microcystin-LR, which arises from freshwater cyanobacterial blooms, has a WHO guideline value (provisional) of 1 µg/l (WHO, 2011). Desalination processes will significantly control algal toxins.
The nature of the natural organic molecules is such that most of them have sufficiently high molecular weights or low volatilities that they would not be expected to carry over in thermal desalination processes, although the potential for some carryover by steam distillation remains a possibility. Volatile organics are usually vented as part of the distillation process. The carryover would be expected to be small, but for substances such as geosmin, which has an odour threshold measured in nanograms per litre, this could still be of concern for the potential acceptability of the final product. Most of the organic molecules are relatively large (e.g. greater than ~200 daltons) and would be expected to be excluded by membranes used in desalination; for example, two of the main marine toxins, saxitoxin and domoic acid, have been shown to be rejected by membranes used in desalination (N. Voutchkov, personal communication, 2006). However, low molecular weight polar compounds might require further study in that regard. Solvent-type low molecular weight neutral organics can pass through membranes to a significant degree.
_
-6. Disinfection byproducts (DBPs)
Although chlorine is the most commonly used disinfectant in desalination plants, its reaction with organic matter produces various disinfection by-products (DBPs) (e.g., trihalomethanes [THMs], haloacetic acids [HAAs], and haloacetonitriles [HANs]), and some DBPs are regulated in many countries due to their potential risks to public health. To reduce the formation of chlorinated DBPs, alternative oxidants (disinfectants) such as chloramines, chlorine dioxide, and ozone can be considered, but they also produce other types of DBPs. In addition, due to high levels of bromide and iodide concentrations in seawater, highly cytotoxic and genotoxic DBP species (i.e., brominated and iodinated DBPs) may form in distribution systems, especially when desalinated water is blended with other source waters having higher levels of organic matter. Even at low concentrations and long-term exposure, these DBPs can induce tumors/cancers and even cause death. Nevertheless, the genotoxicity of the desalination processes is an understudied issue, and there is a lack of information on drinking water from desalination. A 2023 study from China demonstrate that the genotoxicity of the desalinated seawater is much lower than the conventional drinking water with fresh source water.
_______
_______
Efficiency of desalination processes for removing pathogens:
Reverse osmosis:
RO has been shown to remove bacteria and larger pathogens and all or a large fraction of viruses (Gagliardo et al., 1997; Adham et al., 1998a; van der Hoek et al., 2000). High-quality RO processes are good treatment barriers to pathogens if properly selected and maintained. The pore size of the reverse osmosis membrane is very small, usually between 0.5 and 10 nanometers, which is the accuracy that even nanofiltration and ultrafiltration cannot be achieved. Usually, the size of bacteria in the water is between 0.5 and 5 microns, and the conversion to nanometers is 500-5000 nanometers. Viruses are much smaller than bacteria, usually between 50 and 100 nanometers in diameter. The volume of viruses and bacteria is much larger than the pore size of the reverse osmosis membrane. There is no doubt that the reverse osmosis membrane is able to filter bacteria and viruses, and the filtration effect is excellent.
WHO provides guidance on target removals for bacteria, viruses and protozoa, removals that are achieved by typical and enhanced water treatment processes (WHO, 2011). Removal of viruses by RO membranes may vary significantly and is a function of the membrane itself as well as its condition and the integrity of the entire system, including seals. Removals ranging from 2.7 to more than 6.8 logs, depending on the type of RO membrane, have been reported at bench scale using MS2 bacteriophage as the model virus, and Adham et al. (1998b) suggested that the selection of membranes is an important factor in determining virus removal. Kitis et al. (2002, 2003) reported removals of MS2 ranging from 5 logs for a dual element unit to more than 6.8 logs for a multistage unit. In pilot-scale studies conducted to investigate the potential of integrated ultrafiltration and NF membrane systems for the removal of various microorganisms, including viruses, protozoa (Cryptosporidium oocysts and Giardia cysts), bacterial spores (Clostridium perfringens) and bacteriophage (MS2 and PRD-1), Lovins et al. (1999) observed removals, including those resulting from pretreatment, ranging from 6.1 to 10.1 logs. This shows that membrane treatment exceeds the microbial removal attained by other combinations of process units, such as coagulation, filtration and disinfection of surface water. Although RO constitutes an excellent barrier to microorganisms, the maintenance of that barrier depends on the integrity of the system. Breaches of integrity in the membranes or the O-rings could lead to the passage of pathogens into the process water and must be monitored by integrity testing.
Bacteria have been found in permeate samples of NF and RO effluent, and they can proliferate in discharge lines. This does not mean that pathogens are not rejected, but rather that sterile conditions cannot be maintained (Taylor & Jacobs, 1996). As bacteria have been shown to traverse through membrane defects, membranes cannot be considered as completely effective for disinfection and are commonly succeeded by a disinfection step.
_
Thermal processes:
When thermal processes are used for desalination, microbial inactivation will be controlled by the temperature attained and the time the water remains at that temperature. Typical temperatures to ensure the inactivation of vegetative cells by humid heat vary from 50 °C to 60 °C when maintained for 5–30 minutes to achieve pretreatment. Spores, endospores and other resistant forms are more resistant to heat and require higher temperatures (70–100 °C) held for longer periods of time. Most vegetative pathogens are inactivated under flash pretreatment conditions (temperature of 72 °C for 15 seconds). The condensate is unlikely to contain pathogens after the distillation process because of the killing impact of heat and because pathogens are unlikely to be entrained. However, reduced pressures are used in some desalination processes to reduce the boiling point and reduce energy demand. Temperatures as low as 50 °C may be utilized (USBR, 2003) and might not achieve the required inactivation targets.
_
Counterview:
By specification commercial RO membranes state that they remove 99.9% of bacteria. This in no way indicates that RO removes all bacteria. If the inquiry is to determine if RO water is sterile and can be counted on to be bacteria free, the answer is an absolute no. Here are the reasons why.
-1. Bacteria are living organisms that multiply. Even if only 0.01% of the bacteria in the feed water pass through the RO membrane, that minute amount of bacteria, over time will multiply and can increase exponentially in number. As a result, the water in a storage tank filled with a properly functioning RO system can have profuse amounts of bacteria in it.
-2. The rating of 99.9% rejection of bacteria is for a new RO membrane only. Once a membrane has been placed into service it will begin to degrade under normal wear and tear, pressure and friction. With time, the bacterial rejection rate of an RO system will decrease and more bacteria will pass.
______
______
Remineralization:
Minerals contained in “normal” water are depicted in table below:
|
|
Total dissolved solids |
Calcium |
Magnesium |
Sodium |
Chlorine |
Sulphate |
|
mg/L |
400 |
30-150 |
0-100 |
30-150 |
0-200 |
0-200 |
_
The lack of dissolved minerals in the high-purity water produced by desalination processes raises some problems. High-purity water tends to be highly reactive and, unless treated, it can create severe corrosion difficulties during its transport in conventional pipelines. Also, untreated desalinated water cannot be used directly as a source of drinking water. A certain degree of remineralisation is necessary in order to make the water palatable and for re-introducing some essential ions required for health considerations. Desalinated water is slightly acidic, lacks minerals and cannot be used un-buffered, thus making remineralization an important component downstream of desalination.
Post-treatment involves preparing the water from the desalination process for its end use. It may include disinfection, corrosion control as well as degasification, depending on the gases present in the desalination product. Re-mineralizing desalinated water to control its pH, alkalinity and hardness is considered an important step in post-treatment. In today’s times, most RO plants put the water through a ‘post-treatment’ process whereby salts are added to make TDS around 300 mg/l.
_
Although the composition of desalinated water varies depending on plant design and technique used, removal of salts in desalinated water results in a product that is very low, often too low, in minerals, has a slightly acidic pH, and very low buffering capacity. Before delivery to the water network, the water needs to go through a series of post-treatment steps to prevent and/or control various problems such as:
-1. Corrosion, which has been known since the development of water networks. Various empirical parameters have been defined to characterize corrosion potential, for example Chloride to Sulfate Mass Ratio (CSMR) or Langelier Saturation Index (LSI).
-2. Negative effects on human health potentially leading to diseases. The main minerals considered are calcium and magnesium; however fluoride, iodide and trace elements also impact human health.
-3. Irrigation with lack of essential nutrients for crops or soil compatibility such as sodium, potassium, calcium, magnesium and sulfate, or presence of toxic trace elements such as boron.
_
The most common processes for the remineralisation of desalinated water are as follows:
- Dosage of chemical solutions based on calcium chloride and sodium bicarbonate
- Lime dissolution by carbon dioxide
- Limestone dissolution by carbon dioxide. This is the simplest and most widely used process.
Two solutions are widely used to remineralize/neutralize water:
|
Description |
Minerals |
|
CO2 addition + Calcite Limestone (CaCO3, MgO) percolation + Na2CO3 |
80 mg/L CaCO3 |
|
Addition of CaCl2 + NaHCO3 |
100 mg/L CaCO3 |
While diet remains the principal source of nutrients and minerals, drinking-water may provide supplemental amounts that could be important for some people.
_
WHO expert consultations on calcium and magnesium in drinking-water (WHO, 2005b, 2006; Cotruvo, 2006) concluded that there was evidence of dietary deficiency of both calcium and magnesium in many parts of the world. This would be particularly acute in developing countries and in women, as well as in some sectors of the population, such as the elderly, who are also at highest risk of mortality from ischaemic heart disease. Hard water and particularly magnesium, a component of hardness, have been negatively (i.e. beneficially) associated with these conditions in a number of epidemiological studies. Although uncertainties about this association remain, in circumstances where a supply is moving from a source that has significant levels of calcium and magnesium to low-mineral desalinated water, it would be appropriate to consider remineralizing with calcium and magnesium salts. Additionally, calcium intake may reduce osteoporosis risk, and magnesium deficiency may also be associated with metabolic syndrome, indicating a prediabetic condition. However, any decision should be taken in conjunction with health and nutrition authorities in the light of total dietary intakes of nutrient minerals. Blending with 1% seawater provides about 15 mg of magnesium per litre and about 5 mg of calcium per litre to the finished water.
The overall conclusion based on identified case–control and cohort studies was that there is no evidence of an association between water hardness or calcium and acute myocardial infarction or deaths from CVD (acute myocardial infarction, stroke and hypertension). There does not appear to be an association between drinking-water magnesium and myocardial infarction. However, the studies do show a negative association (i.e. protective effect) between CVD mortality and drinking-water magnesium. Although this association does not necessarily demonstrate causality, it is consistent with the well-known effects of magnesium on cardiovascular function.
_
A recommendation of a WHO working group was for a minimum fluoride concentration of 0.2 mg/l, but this recommendation may require examination and confirmatory studies (WHO, 2005b). The recommended WHO guideline value for fluoride is 1.5 mg/l, but the optimal value is usually in the range of 0.5–1 mg/l, based upon average ambient temperatures and water consumption patterns. The appropriate value provides a balance between the benefits of fluoridation of drinking-water and minimizing the occurrence of dental fluorosis.
WHO states that there is clear evidence that long-term exposure to an optimal level of fluoride results in diminishing levels of caries in both child and adult populations and that fluoride is being widely used on a global scale, with much benefit (WHO, 2006). However, good dental care, use of fluoride toothpaste and low sugar consumption are also important dental health factors. Water fluoridation is controversial in some quarters but generally believed by the dental community and many public health officials to be beneficial and without demonstrable risk. Water fluoridation is a matter of national policy. Seawater is naturally low in fluoride, and the fluoride is further depleted by the desalination process. Optimal fluoridation of the desalinated water can be a significant contributor to daily intake and can reduce the incidence of dental caries in some populations, just as it does with fluoridated fresh waters.
_____
_____
Safe Drinking-water from Desalination: Israeli experience:
For Israel, seawater desalination has become an indispensable technology in the fight against the region’s deteriorating water situation. In 2019, the country produces around 600 million cubic meters of desalinated seawater per year, which constitutes 60% of the water provided to Israeli households and 50% of the nation’s entire water supply (including for agriculture). Despite its paramount importance to water security in the drying Middle East, desalination turned out to cause adverse human health effects – which are the concern of scientists as well as government officials in Israel. Desalinated seawater is lacking four essential minerals that are vital to human health – calcium, magnesium, fluoride, and iodine. The minerals are removed during the desalination process, along with the salts. The potential risk for public health due to the consumption of low–mineral water hasn’t escaped the eyes of the authorities.
_
Israel, like many other countries, has a long experience in reintroducing minerals to drinking water – especially fluoride, which has been added to the water since the 1980s. Adding fluoride to tap water is known to prevent dental caries, and is, therefore, a convenient method to increase public dental health. Moreover, the costs of adding fluoride are marginal at about $8 – $10 million per year. The Ministry of Health (MoH) assures that fluoride-enriched water at the levels added in Israel (0.7 mg/L) is safe to drink and that no studies exist linking water fluoridation to cancer or other any diseases, contrary to those who oppose the practice. Consequently, a lack of fluoride likely does not entail severe health consequences and is being addressed. But this isn’t the case with other minerals.
_
Low iodine intake potentially leads to iodine-deficiency disorders such as thyroid dysfunction, which particularly in young children might result in poor intellectual and cognitive abilities. In pregnant women, low iodine intake can cause severe impairments of the fetus like mental retardation, goiter (enlargement of the thyroid gland), and physical deformation. In 2016, Israeli researchers for the first time examined the possibility of desalinated water being the cause of iodine deficiency and thyroid diseases. The study, conducted at the Barzilai University Medical Center in Ashkelon – a city located on Israel’s Mediterranean coast, getting most of its municipal water from the nearby desalination facility – showed that 70% of the 74 participants had iodine intake below the Estimated Average Requirements (EAR). Twenty-nine participants were diagnosed with non-autoimmune thyroid disease (NATD). It was concluded that the iodine intake of those patients living in areas depending on desalinated seawater is too low, which might be directly connected to iodine deficiency and thyroid health. The Ministry of Health (MoH) acknowledges the problem of iodine deficiency in Israel but dismisses the notion of adding iodine to desalinated seawater due to the risks involved in such an application. The ministry thus recommends the public to switch from regular salt to iodized salt, and keep a balanced diet rich in iodine.
Unlike iodine, calcium seems to be less of a problem in Israel, as it is already being added to desalinated seawater in order to avert pipe corrosion. Still, the MoH does not publish any figures regarding the cost of adding calcium to the water.
_
Magnesium is critical for energy production, the synthesis of proteins and nucleic acids, the regulation of vascular tone, and for insulin sensitivity. A deficiency in magnesium means a high risk for hypertension, cardiac arrhythmias, atherosclerosis, diabetes, and colon cancer. While the optimal daily Mg intake is 300 (woman) – 410 mg (man), Israelis, on the average, only ingest between 228-270 mg per day. WHO Guidelines for Drinking-Water Quality (2011) states that the contents of magnesium in drinking water may contribute to the daily intake of Mg, and is mostly important for those who have marginal Mg intake. The WHO recommendation to consider adding Mg to the desalinated seawater has tremendous implications for Israel and Israeli Ministry of Health has recommended adding Mg to desalinated seawater at 20-30 mg/L. The Health Ministry estimated in 2012 that approximately 250 deaths each year could be attributed to low magnesium levels.
_____
_____
Occurrence and Health Risk Assessment of Trace Metals in Desalinated Seawater Using Two Desalination Technologies, a 2020 study:
Desalination of seawater is an effective way to alleviate the world’s water shortage. However, the transmission and health risk of trace metals in the different processes of seawater plants are highly uncertain. Here, authors investigated the concentrations of 29 ions and trace elements in desalinated water based on two desalination techniques – multiple effect distillation (MED) and reverse osmosis (RO) – and evaluated the potential health risks (carcinogenic risk (CR) and non-carcinogenic risk (n-CR)) of trace metals in these processes. The results showed that most ions and trace elements were efficiently removed using both the RO and MED methods. After desalination, the trace metal concentrations in the desalinated water treated with the MED method were higher than those in the desalinated water treated with the RO method. The n-CR values for the trace metals in the desalinated water using the MED and RO methods were within the neglectable level. The CR values based on the two methods were lower than the maximal acceptable risk levels designated by the US Environmental Protection Agency. The total health risks using the MED method were higher than those using the RO method. The results indicated that seawater desalination using both treatment methods is safe.
_____
_____
Section-17
Desalination based on Renewable Energy:
Desalination is an energy-intensive process. Over the long term, desalination with fossil energy sources would not be compatible with sustainable development; fossil fuel reserves are finite and must be conserved for other essential uses, whereas demands for desalted water would continue to increase. A sustainable, non-polluting solution to water shortages is essential. Renewable energy sources, such as wind, solar, and wave power, may be used in conjunction to generate electricity and to carry out desalination, which could have a significant impact on reducing potential increased greenhouse gas emissions. Nuclear energy seawater desalination also has a tremendous potential for the production of freshwater.
_
Desalination is an energy-intensive process that uses fossil fuels for power generation, a non-renewable energy source. Thus, the potential impact on climate change is a major issue for desalination plants, and two specific aspects of energy use by desalination plants must be addressed. First, there is a need to reduce the overall amount of energy consumed. Second, there is a need to shift to renewable energy sources to reduce GHG emissions. Currently, due to their high energy consumption and use of non-renewable fossil fuels, desalination plants contribute to air pollution by emitting COx, NOx, SOx, and particulate matter. According to Mezher et al., the typical amount of CO2 emitted during SWRO desalination powered by natural gas is 2.79, 2.13, and 1.75 kg-CO2/m3 produced water for the steam cycle, internal combustion engine, and combined cycle, respectively. Similarly, Tarnacki et al. calculated the global warming potential of SWRO desalination plants and found that they produce 1.77 kg-CO2/m3 produced water. They also calculated the acidification potential in terms of SO2 and reported a value of 25 g-SO2/m3 produced water. Additionally, Ameen et al. reported that an SWRO desalination plant has a carbon footprint of 2.3–2.5 kg-CO2/m3 for 0.63 MCM/day. In a study of 20 desalination plants with mixed SWRO + BWRO systems with a total capacity of 1.736 MCM/day, it was found that the carbon footprint was equivalent to 1,193 Kt CO2eq annually, which equates to 1.9 kg-CO2/m3. Global CO2 emissions from carbon-powered desalination plants could reach 218 million tons in 2020. An expensive solution that accelerates climate change is far from ideal, particularly for the many low and middle-income countries most impacted by water scarcity.
_
One of the desired methods for overcoming freshwater scarcity is developing new desalination technologies. However, these methods demand high amounts of heat and energy to drive desalination units. Hence, renewable energy sources like geothermal and solar energies play an important role in achieving a desalination system. Not only does renewable energy help producers bypass the cost of extracting and purifying fossil fuels, but it is also environment-friendly and guarantees sustainability. Unlike the availability of fossil fuels, the need for water is relatively time-infinite. Some of these renewable energy technologies are the use of wind energy, direct and indirect solar energy, geothermal energy, and wave energy to desalinate seawater. The Global Clean Water Desalination Alliance has set a goal for 20% of new desalination plants to be powered by renewables between 2020-2025. Founded by the International Desalination Association, the alliance includes energy and desalination industries, water utilities, governments, financing institutions, academia, and R&D with the goal to reduce CO2 emissions from existing water desalination plants and to scale up the use of clean desalination technologies through coordinated actions. Globally, the current share of renewable energy used in desalination is around 1%. Saudi Arabia is using solar power to make water desalination, a highly energy-intensive process, more environmentally friendly. The Jazlah plant in Jubail city is the first in the country to integrate desalination with solar power on a large scale, saving around 60,000 tons of carbon emissions annually. In Western Australia, all new desalination plants must use renewable energy. From Europe to India, to China, many others are following their lead. These nations may be obliged to reduce emissions under the Paris Agreement on Climate Change. They are certainly driven by the humanitarian and economic crisis of water scarcity.
_
Desalination is already responsible for 0.4% of the world’s electricity consumption, and around 10% of the electricity consumed in Israel. If the process is to become compatible with a net-zero future, it must be powered by renewables. Unfortunately, this is far from the case in Israel today: more than 90% of the country’s electricity comes from fossil fuels. Other countries in the Middle East that use desalination to produce a majority of their drinking water do so with high carbon intensity. The UAE, which produces all of its municipal water from desalinated water, does so by producing 15kg of CO2 equivalent per cubic metre of water treated as seen in the figure below.
Note:
CO2e is a measurement of the total greenhouse gases emitted, expressed in terms of the equivalent measurement of carbon dioxide. On the other hand, CO2 only measures carbon emissions and does not account for any other greenhouse gases.
All the countries in the Sahara and Arabian Deserts – which typically have both the wealth and dry conditions to make desalination viable – have a renewable electricity share of less than 20%. If desalination is to offer a viable climate adaptation method for these water-scarce countries, then it must be developed alongside an effective carbon mitigation strategy that involves a rapid scale-up of renewable energy supplies.
Increasing the share of renewables in the electricity supply that powers desalination will also help lower the price of the technology since renewable electricity is now much cheaper than fossil fuel power in much of the world. A 2020 study led by professor of solar economy Christian Breyer found that the global average levelised cost of drinking water from desalination plants could decline from €2.40/m3 ($2.60/m3) in 2015 to €1.05/m3 by 2050 if solar power and storage systems are used to decarbonise the sector.
Other tactics such as public campaigns to encourage lower water use – which could reduce existing demand by 30–50% in many urban areas – as well as improving water recycling processes and introducing high-tech farming methods like drip irrigation will also decrease the need for desalinated water and the overall cost of maintaining a water supply.
_
In order to supply the desalination process with renewable energy sources, it is convenient to distinguish the energy sources that can be used to produce electricity (or mechanical energy) from those producing thermal energy. With this goal, the renewable energy sources can be sorted in the following categories, according to the usual energy output that can be produced:
- Electricity producers, such as wind, hydro, tidal and wave.
- Thermal and electrical energy producers, such as solar, geothermal and biomass.
The energy output is usually selected according to the features of the local energy resource.
Combining the technologies for renewable energy sources utilization and the desalination solutions, figure below is obtained.
Figure above shows possible coupling between desalination technologies and renewable energy sources (RES).
_
Desalination based on the use of renewable energy sources can provide a sustainable way to produce fresh water. It is expected to become economically attractive as the costs of renewable technologies continue to decline and the prices of fossil fuels continue to increase. Using locally available renewable energy resources for desalination is likely to be a cost-effective solution particularly in remote regions, with low population density and poor infrastructure for fresh water and electricity transmission and distribution. The present deployment of renewable-based desalination – i.e. less than 1% of desalination capacity based on conventional fossil fuels (EU, 2008) – does not reflect the advantages of this technology option. Renewable desalination is mostly based on the RO process (62%), followed by thermal processes such as MSF and MED. The dominant energy source is solar photovoltaics (PV), which is used in some 43% of the existing applications, followed by solar thermal and wind energy (EU, 2008). The right combination of a renewable energy source with a desalination technology can be the key to match both power and water demand economically, efficiently and in an environmentally friendly way. Assessing the technical feasibility and cost effectiveness of renewable desalination plants requires a detailed analysis, including a variety of factors, such as location, quality (salinity) of feed-water input and fresh-water output, the available renewable energy source, plant capacity and size, and the availability of grid electricity. Operation and maintenance requirements, feed-water transportation and pre-treatment needs are also part of the decision-making process. Some technology solutions are better suited to large size plants, while others are better for small-scale applications (EU, 2008). Most common renewable options are shown in Table below.
Table above shows possible combinations of renewable energy and desalination technologies.
_
Potable water is considered to be a scarce commodity especially in arid and remote regions. While conventional desalination technologies offer an excellent solution to meet water demand, they are considered to be energy intensive processes. Conventional desalination technologies are well suited for large scale applications but they are not-efficient and not suited for small scale water demand. Conventional desalination processes are expensive to operate and require continues maintenance which prevent their utilization in remote areas. Renewable desalination is growing especially in arid regions with huge solar energy potentials such as the MENA region. Many of the existing renewable desalination systems are implemented in small capacities from a few m3 up to 100 m3/d. Only a few medium-size applications exist in the MENA region. The world’s largest solar PV desalination plant using novel nano-membrane technology is under construction in the city of Al Khafji, in Saudi Arabia. It is part of the project launched by KACST (King Adbulaziz City for Science and Technology) in cooperation with IBM. It will be implemented in three stages over nine years. In the first phase, a desalination plant with a production capacity of 30,000 m3/d will meet the needs of some 100,000 people. Saudi Arabia presently uses1.5 million barrels of oil per day at its desalination plants, which provide between 50% and 70% of the country’s drinking water. Other desalination plants powered by renewable energy can be seen in Cyprus, Egypt, Jordan, Morocco, Turkey, Abu Dhabi and the Canary Islands.
_
The status of renewable-powered desalination technologies is depicted in figure below:
__
Coupling desalination facilities with carbon-free or low-carbon power sources such as solar, wind, or nuclear power plants could make it possible to gain the benefits of clean water without the climate impact. But some of these renewable energy sources do not deliver power continuously, and some types of desalination technology encounter difficulties when their operation is not constant. For example, variations in the operation of the plants can lead to increased fouling of the membranes that separate the salt from the water. Wind and solar installations produce variable power, so to avoid the ramping up and down of the desalination plants, these power sources might need to be coupled with storage systems, raising the cost. And nuclear plants tend to be larger than needed for desalination, so such facilities might have to be coupled with power production for other uses. Boris Liberman, vice president and chief technology officer of IDE Technologies, the Israel-based company responsible for the design and construction of that county’s giant new seawater desalination plants, including the largest such plant in the world, said that those plants have now demonstrated that with proper design and operation it is possible to operate efficiently even with power supplies that ebb and flow. The key, he said, is to maintain constant pressure inside the system while allowing the flow rate and freshwater output to rise and fall. The company’s largest plant, called Sorek, which produces 150 million cubic meters of water per year, “has worked for two years with no fouling,” he said.
______
______
Solar Energy and Desalination Process:
One can achieve desalination using solar energy either directly or indirectly, depending on the technique adopted for solar energy harnessing. Direct solar desalination does not require a large step up of auxiliaries to convert solar thermal energy or electricity from solar panels into other forms of energy. Indirect solar desalination first collects the solar energy and then converts it into another energy form used in desalination. In the current world scenario of climate change and global warming concerns, Photovoltaics (PV) and concentrated solar power (CSP) are the attractive energy source for the desalination of water. Usually, desalination processes are selected based on feed water quality, product purity, climatic conditions, environmental impacts, and, most importantly, site specifications.
Figure below shows the link between solar energy type and different desalination processes.
_
Photovoltaic (PV) technology can be connected directly to RO or ED desalination processes, which are based on electricity as the input energy. Many small PV-based desalination systems have been demonstrated throughout the world, especially in remote areas and islands, including Gran Canaria, Canary Islands (PV-RO, seawater, 1–5 m3/d), Riyadh, Saudi Arabia (PV-RO, brackish water, 5 m3/d), and Ohshima Island, Japan (PV-ED, seawater, 10 m3/d) (Kalogirou, 2005). The main issue of PV desalination is the (still) high cost of PV cells and batteries for electricity storage. Careful maintenance and operation of battery systems are also necessary. Further technology advances in electricity storage associated to PV could lead to wider use of PV desalination. PV-solar-based desalination also provides electricity for the membrane desalination (MD) process and auxiliary units. MD systems are suitable for brackish water treatment. Solar configuration coupled with the MD process is used in most areas, but it has a small capacity for water production.
CSP plants collect solar radiation and provide high-temperature heat for electricity generation. Therefore, they can be associated with either membrane desalination units (e.g. reverse osmosis, RO) or thermal desalination units. CSP plants are often equipped with thermal storage systems to extend operation when solar radiation is not available, and/or combined with conventional power plants for hybrid operation. This paves the way to a number of design solutions which combine electricity and heat generation with water desalination via either thermal or membrane separation processes. CSP plants are also large enough to provide core energy for medium- to large-scale seawater desalination. In desert regions (e.g. MENA) with high direct solar irradiance, CSP is considered a promising multi-purpose technology for electricity, heat and district cooling production, and water desalination. An analysis by the German Aerospace Centre (DLR, 2007) for the MENA region shows that the choice between the CSP-MED process and the CSP-RO may depend on feed-water quality. The CSP-MED process is more energy efficient than the CSP-RO process in the Arabian Gulf where seawater has a high salinity level.
The most appropriate solar energy choice for a solar desalination system is CSP because of backup energy and working hours after sunset. The CSP produces electricity and connects with RO System. It also has some limitations, such as in cloudy weather the direct irradiance reduces, and it only uses direct irradiance from the sun. Due to relatively high humidity in coastal areas, direct irradiance is disturbed. On the other hand, PV has no limitations and can be used on a large-scale system. PV systems are suitable for highly densely populated areas where desalination can use solar energy.
_
Existing PV-RO desalination plants in the MENA region
|
Location |
Year |
Additional Power Supply |
Production [m3/day] |
Operator |
|
Abu Dhabi, UAE |
2008 |
Diesel |
20 |
NEWRC |
|
GECOL, at Ras Ejder, Libya |
2005 |
Wind & grid |
300 |
GECOL |
|
El Hamrawein, Egypt |
1986 |
– |
240 |
– |
|
Hassi Khebi, Algeria |
1988 |
– |
22.8 |
– |
|
Sadous Riyadh Region, KSA |
2001 |
– |
14.4 |
– |
|
Maagan Michael, Israel |
1997 |
Diesel |
9.6 |
– |
|
Aqaba, Jordan |
2004 |
– |
81.6 |
NERC |
|
Ksar Ghilane, Tunisia |
2006 |
– |
50.4 |
ITC |
|
Benhsaine, Morocco |
2007 |
– |
24 |
ITC |
|
Msaim, Morocco |
2007 |
– |
24 |
ITC |
|
Jordan Valley, Jordan |
2010 |
– |
30 |
– |
|
Tasekra, Morocco |
2008 |
– |
24 |
– |
_
Existing solar membrane distillation desalination plants in the MENA
|
Location |
Year |
Additional Power Supply |
Production [L/day] |
Operator |
|
Morocco |
2005 |
– |
150 |
Fraunhofer ISE |
|
Aqaba, Jordan |
2005 |
– |
1000 |
Fraunhofer ISE |
_
Existing solar CSP multi-effect distillation desalination plants in the MENA
|
Location |
Year |
Additional Power Supply |
Production [m3/day] |
Operator |
|
Qatar |
2012 |
– |
150 |
Fischer |
|
Alexandria, Egypt |
2018 |
– |
250 |
Fraunhofer ISE |
_____
Light can vaporize water without Heat: Photomolecular Effect:
Researchers at MIT have discovered a new phenomenon: that light can cause evaporation of water from its surface without the need for heat. They have named this the photomolecular effect, by analogy with the photoelectric effect that was discovered by Heinrich Hertz in 1887 and finally explained by Albert Einstein in 1905. That effect was one of the first demonstrations that light also has particle characteristics, which had major implications in physics and led to a wide variety of applications, including LEDs. Just as the photoelectric effect liberates electrons from atoms in a material in response to being hit by a photon of light, the photomolecular effect shows that photons can liberate entire molecules from a liquid surface, the researchers say. The finding of evaporation caused by light instead of heat provides new disruptive knowledge of light-water interaction. It could help us gain new understanding of how sunlight interacts with cloud, fog, oceans, and other natural water bodies to affect weather and climate. It has significant potential practical applications such as high-performance water desalination driven by solar energy. Solar desalination research also shows that there are happenings beyond what conventional physics would predict based on current knowledge of how water acts. Scientists say that, in principle, they think it may be possible to increase the limit of water produced by solar desalination, which is currently 1.5 kilograms per square meter, by as much as three- or fourfold using this light-based approach. This could potentially really lead to cheap desalination.
_____
_____
Wind Power Desalination:
The electrical and mechanical power generated by a wind turbine can be used to power desalination plants, notably RO and ED desalination units, and vapor compression (VC) distillation process (in particular, Mechanical Vapor Compression, MVC). In the MVC, the mechanical energy of the wind turbine is used directly for VC without further conversion into electricity. In general, wind power based desalination can be one of the most promising options for seawater desalination, especially in coastal areas with high wind potential. Various wind-based desalination plants have been installed around the world, including Gran Canaria, Canary Islands (Wind-RO, seawater, 5–50 m3/d), Fuerteventura Island, Spain (Wind-diesel hybrid system, seawater, 56 m3/d), and the Centre for Renewable Energy Systems Technology in the United Kingdom (Wind-RO, seawater, 12 m3/d). Same as for PV and CSP, a drawback of wind desalination is the intermittence of the energy source. Possible combinations with other renewable energy sources, batteries or other energy storage systems can provide smoother operating conditions. Water desalination itself can provide an excellent storage opportunity in the case of electricity generation exceeding the demand (Gude et al., 2010).
The West Australian Government has announced the northern suburb of Alkimos as its preferred location for its third desalination plant and it will be powered by renewable wind energy and provide safe, secure drinking water to millions of Western Australians.
____
____
Geothermal Desalination:
As geothermal energy can produce electricity and heat, it can be combined with both thermal and membrane desalination technologies. Low-temperature geothermal energy, typically in the range of 70–90°C, is ideal for MED desalination. A project on Milos Island, Greece, has proposed a geothermal desalination system to produce 1,920 m3/d of water. The plant consists of a dual system with hot water from geothermal wells being employed to run either an organic Rankine cycle (ORC) with a 470-kWe turbine for electricity generation or a MED desalination unit. The system can benefit the local community by producing desalinated water at a very low cost, i.e. USD 2/m3 (Constantine, 2004). However, the exploitation of geothermal energy very much depends on the specific local conditions, with upfront investment costs that are usually high.
_____
_____
Wave-Powered Desalination:
Using the power of ocean waves, innovators from Boston, U.S., have developed a technology that can produce fresh water off-grid and without the costly infrastructure of desalination plants. The technology, Wave2OTM, was developed by start-up company Resolute Marine Energy. Chief Operating Officer Olivier Ceberio says it “targets ‘off-grid’ coastal communities in developing nations where a solution to persistent water shortages is urgently needed”. Importantly, it fills a gaping hole between industrial-scale utilities that are costly and time-consuming to build, and micro-scale solutions for individual households. The only technology currently offered in between involves diesel-powered desalination systems. And Wave2O can be delivered competitively because it uses “free energy from a consistent and inexhaustible renewable energy resource: ocean waves,” says Ceberio.
To generate power, a flap moves with the waves and sends seawater – sourced away from contamination off-shore – and the power generated to a standard reverse osmosis unit. The energy can also be diverted for other purposes. About 35% of the water is filtered and the rest is released back into the ocean using a manifold that maximizes the dispersion. This relatively low recovery rate produces low brine salinity with several advantages including lower maintenance, extended membrane life and minimal impact on marine flora and fauna when back in the sea. The smaller module can produce 500 cubic meters of water per day, depending on wave energy – that’s half a million liters, enough to supply five to 10 thousand people’s personal and domestic needs. The only limitations include wave energy availability, the topology of the ocean floor, land use and local regulations.
Oneka, a wave-powered desalination technology uses floating buoys tethered to the ocean floor use wave power to drive a pump that forces seawater through filters and reverse osmosis membranes. The fresh water is then piped ashore again powered solely by the natural motion of waves.
Australian wave energy developer Carnegie Wave Energy has announced the world’s first wave-powered seawater reverse osmosis desalination plant that is now fully integrated and operational in 2015. The wave-powered desalination pilot plant is co-located with its Perth Wave Energy Project, on Garden island. While the desalination plant was originally running off the conventional electricity grid, the integration with the CETO wave energy power plant means the desalination plant is now able to run both off the grid and directly off hydraulic power provided by the wave energy power plant, or a combination of both. The CETO system is a wave energy solution which operates underwater, converting ocean wave energy into zero-emission electricity and desalinated water. Unlike conventional surface-type wave energy generators, submersion protects it from large storms. It is also invisible from the shore, and located in areas away from breaking waves and beach goers.
____
____
Desalination water costs by renewable energy sources:
In general, desalination based on renewable energy sources is still expensive if compared with conventional desalination, as both investment and generation costs of renewable energy are higher. However, under certain circumstances – e.g. installations in remote areas where distributed energy generation (heat and power) is more convenient than centralized energy generation, transmission and distribution – renewable desalination could compete with conventional systems. The desalination systems can be operated by the use of conventional and renewable energy sources and the vast majority of desalination plants over the world are currently operated by fossil fuel instead of renewable energy due to technical and economical barriers. From the literature, it has been concluded that the cost of water produced from desalination systems using a conventional source of energy is much lower than those powered by renewable energy sources. Generally, water desalination prices have been decreased over the recent years due to technical improvements and research advancements in technologies. In conventional systems, the cost for seawater desalination ranges from 0.35 Euro/m3 to more than 2.7 Euro/m3, while for brackish water desalination, the cost is almost half. When renewable energy sources are used, the cost is much higher, and in some cases can reach even 10.32 Euro/m3, due to most expensive energy supply systems. However, this cost is counter balanced by the environmental benefits.
Table below summarizes the cost of fresh water when the desalination system is powered by conventional and renewable energy sources.
|
Type of feed water |
Type of energy |
Water cost (Euro/m3) |
|
Brackish water |
Conventional fuel |
0.21–1.06 |
|
Photovoltaic cells |
4.5–10.32 |
|
|
Geothermal |
2 |
|
|
Sea water |
Conventional fuel |
0.35–2.70 |
|
Wind energy |
1.0–5.0 |
|
|
Photovoltaic cells |
3.14–9.0 |
|
|
Solar collectors |
3.5–8.0 |
__
Fossil fuels are still the primary global energy source and the overall water production costs of integrated solar energy systems and water desalination plants are higher than those of conventional water desalination plants due to the high cost of solar energy systems.
Table below shows summary of economic aspects of thermal and membrane seawater desalination methods.
|
Water Desalination Method |
Total Capacity (m3/day) |
Specific Energy Consumption (kWh/m3) |
Water Production Cost ($/m3) |
|
MSF |
50,000–70,000 |
13.5–25.5 |
0.84–1.6 |
|
MED |
5000–35,000 |
6.5–28 |
1.21–1.59 |
|
CSP + MED |
>5000 |
|
2.5–3 |
|
RO |
15,000–320,000 |
3–8 |
0.7–0.66 |
|
PV + RO |
<100 |
4–5 |
11.7–15.6 |
|
PV + RO |
1000 |
2.4 |
1.74–2.59 |
|
PV + RO |
50,000–190,000 |
2.5–6.6 |
0.89–1.8 |
Table above demonstrates that the total water production cost of thermal and membrane technologies of water desalination has increased as a result of integration into CSP and PV systems. It is also important to mention that the water production cost is substantially related to daily solar irradiance and seawater salinity. In this regard, Bilton and Dubowsky has constructed three low-capacity RO systems (10 m3/day) powered by PV panels in three locations to investigate the influence of water salinity and daily solar irradiance on the water production cost. They confirmed that water production costs are 7.01, 5.64 and 4.96 $/m3 for Boston, Los Angeles, and Saudi Arabia, respectively. They attributed these values to the solar intensity of 4.4, 5.6 and 6.6 kWh/m2/day and feed salinity of 32,664 ppm, 33,505 ppm, and 38,340 ppm for Boston, Los Angeles, and Saudi Arabia, respectively. The corresponding specific energy consumption was estimated to be 2.92 kWh/m3, 3 kWh/m3, and 3.3 kWh/m3, respectively.
______
______
Section-18
Nuclear desalination:
Beginning in the 1960s, the prospect of using nuclear energy to desalinate water started to gain traction. The process of nuclear desalination involves converting seawater to potable water using a facility in which a nuclear reactor operates as the power source. A variety of methods have been developed for the desalination process, which mostly involve reverse osmosis, distillation, or a hybrid of reverse osmosis and distillation. During the 1960s, the International Atomic Energy Agency (IAEA) implemented studies to explore the economic and technical feasibility of implementing nuclear desalination programs. The results of these studies indicated that desalinating seawater using nuclear power could be feasible technically and could also compete economically with renewable and fossil energy sources. Throughout the next several decades, use of nuclear energy to desalinate water gained traction. Use of nuclear power has become increasingly attractive as conversation over limiting fossil fuels has increased. Desalination in general has become a common practice worldwide over the past few decades, with desalination plants worldwide at a capacity of 100 million cubic meters of water desalinated per day compared to only 5 million cubic meters/day in 1980. Current technologies can reject 99.70-99.75% of salt from saltwater, meaning that the average ocean water, with a salinity of 35 g/kg, can be reduced to a salinity of 87.5 mg/kg. More countries are seriously considering desalination powered by nuclear energy to address their water needs, while avoiding carbon emissions. As desalination is a very energy intensive technology, it is imperative to power it with large-scale, zero-carbon sources, such as nuclear energy, in order to continue providing essential access to clean water to an increasing number of people worldwide, while simultaneously addressing climate change and commitments to net zero.
_
Nuclear desalination has been defined as the use of both electricity and heat generated by nuclear power plant to remove salt and minerals from seawater. It has accumulated a couple of hundred of reactor-years of successful operations around the globe. A combination of a variety of desalination techniques (thermal or membrane in single or hybrid mode) have been shown to be successfully coupled with different types of nuclear power plants to produce water and electricity at different scales. The economics of nuclear desalination has been found to be competitive with other desalination techniques driven by other sources of energy. Nuclear desalination doesn’t require additional safety measures than those already existing for the nuclear power plant. Special consideration for potential water radiation contamination is achieved through insertion of additional physical barrier between the nuclear island and pathways of final water product. Marine, coastal, atmospheric, siting, and socioeconomic impacts of nuclear desalination have been shown to be either equivalent or (in some cases) better than those when other energy sources are used. Finally, efforts are under way to improve existing desalination techniques and invent new ones to increase the efficiency of nuclear desalination. Integrated solutions and systems have also been proposed to use multiple energy sources, including nuclear and renewable energies to meet multiple needs, including water desalination, industrial process steam, hydrogen production, electricity generation, and district heating. This will allow for resource optimization while minimizing the overall environmental impact of the proposed integrated solution.
_
Thermal desalination is an energy intensive process that satisfies its requirement from conventional fossil fuel sources. Current research efforts aim at finding alternatives for fossil fuels to power thermal desalination. Nuclear energy offers a feasible option for power cogeneration and production of fresh water due to the significant amount of recovered useful heat. The heat is exploited to produce steam and generate electricity on-site to power thermal and membrane desalination facilities. Large or small/medium nuclear reactors (SMR) can be used. Small modular reactors (SMRs) are advanced nuclear reactors that have a power capacity of up to 300 MW(e) per unit, which is about one-third of the generating capacity of traditional nuclear power reactors.
SMRs, which can produce a large amount of low-carbon electricity, are:
Small – physically a fraction of the size of a conventional nuclear power reactor.
Modular – making it possible for systems and components to be factory-assembled and transported as a unit to a location for installation.
Reactors – harnessing nuclear fission to generate heat to produce energy.
_
Both small modular and advanced nuclear power reactors could be attractive energy sources for future large-scale seawater desalination and other cogeneration applications, the Technical Working Group on Nuclear Desalination (TWG-ND) acknowledged at its 2016 meeting at the IAEA.
Small modular reactors are especially suitable for remote areas with limited infrastructure. The members of the TWG-ND agreed that it would be useful to examine the techno-economics and prospects for non-electrical applications using micro- and small modular reactors. Advanced nuclear power reactors provide unique features for electricity generation, such as higher temperatures and thermodynamic efficiencies, which could also enable their larger deployment in nuclear desalination.
Cogeneration involves the integration of nuclear power plants with other applications. Aside from its use for desalination and hydrogen production, the heat generated by nuclear power plants can be used to produce a vast range of other products.
_
Most desalination plants in the world use fossil fuels to power them, but it’s even better to power them with nuclear energy. The new fleet of Small Modular Nuclear Reactors (SMRs) are ideal as they produce both thermal energy and electrical energy without producing greenhouse gases. But only 15 out of the thousands of desalination plants operating today worldwide are powered by nuclear energy. In contrast, all nuclear-powered naval vessels routinely use nuclear energy to desalinate seawater.
SMRs allow places with smaller electrical grids and limited infrastructure to add new electrical and water capacity in small increments and allow countries to site them as needed at many distributed locations. Refueling of any SMR does not require the nuclear plant to shut down. The small size and large surface area-to-volume ratio of the reactor core, that sits below ground in a super seismic-resistant heat sink, allows natural processes to cool it indefinitely in the case of complete power blackout, with no humans needed to intervene, no AC or DC power, no pumps, and no additional water for cooling. This reactor cannot melt down.
_
In 2022, through its technical cooperation programme, the IAEA hosted a national training course in Amman, Jordan, to build capacity in the use of small modular reactors (SMRs) to desalinate water. Through the IAEA Platform on Small Modular Reactors and their Applications, the Jordan Atomic Energy Commission (JAEC) requested a review by IAEA nuclear power experts of a nuclear desalination study that employs SMRs. The study found that using nuclear energy for desalination is feasible in Jordan, and it offers competitive prices for fresh water to end consumers, in comparison with imported energy sources.
_
A key strength of nuclear power plants (NPPs), aside from technical maturity, is their capacity to supply either thermal or electrical energy, or both, to respective desalination processes, at varying scales. In Abu Dhabi, UAE, four nuclear power units of 1400 MW are being constructed at Al Barakah, which could advance the Abu Dhabi power and desalination program. “We can think of nuclear-powered desalination in terms of two main applications. One is to serve make-up water resources for the plant; the other is to produce potable water. Both applications have already been demonstrated,” said Ibrahim Khamis from the International Atomic Energy Agency (IAEA) department of nuclear energy. Precedent notwithstanding, Khamis explained that “nuclear-powered desalination of potable water isn’t currently widespread or deployed at large scale.”
_
One of the primary benefits of nuclear desalination is that it can be much more energy-efficient than traditional desalination methods. While traditional desalination methods typically rely on fossil fuels, nuclear desalination does not produce any greenhouse gases. Additionally, nuclear power plants have a high capacity factor, meaning that they can continually produce energy, making it a reliable source of power for desalination plants.
Another advantage of nuclear desalination is the ability to produce freshwater at a much lower cost than traditional desalination methods. While the initial capital costs of constructing a nuclear power plant are high, the long-term operating costs are much lower than those of other desalination methods. This is because nuclear power plants have a lifespan of up to 60 years and require minimal maintenance compared to other power plants.
With advances in technology, nuclear desalination is becoming more efficient and safe. Modern nuclear power plants have numerous safety features, including automatic shutdown systems, multiple backup generators, and containment buildings, which make them much safer than older nuclear reactors. Additionally, nuclear waste can be recycled and reused in nuclear reactors.
_
The development of a floating nuclear plant is one of the more surprising solutions to the desalination problem. S.S. Verma of the Department of Physics at SLIET in Punjab, points out that small floating nuclear power plants represent a way to produce electrical energy with minimal environmental pollution and greenhouse gas emissions. Such plants could be sited offshore anywhere there is dense coastal population and not only provide cheap electricity but be used to power a desalination plant with their excess heat.
Raha and colleagues at the Desalination Division of the Bhabha Atomic Research Centre, in Trombay, point out that Low-Temperature Evaporation (LTE) desalination technology utilizing low-quality waste heat in the form of hot water (as low as 50 Celsius) or low-pressure steam from a nuclear power plant has been developed to produce high-purity water directly from seawater. Safety, reliability, viable economics, have already been demonstrated. BARC itself has recently commissioned a 50 tons per day low-temperature desalination plant.
B.M. Misra, formerly head of BARC, suggests that solar, wind, and wave power, while seemingly cost effective approaches to desalination, are not viable for the kind of large-scale fresh water production that an increasingly industrial and growing population needs. India already has plans for the rapid expansion of its nuclear power industry. Misra suggests that large-scale desalination plants could readily be incorporated into those plans. “The development of advanced reactors providing heat for hydrogen production and large amount of waste heat will catalyze the large-scale seawater desalination for economic production of fresh water,” he says.
_____
_____
Nuclear reactors as heat sources:
The heat produced by nuclear reactors can be used either for the production of electricity or it can be applied directly in industrial processes. Depending on the type of reactor, heat can be extracted at various temperatures in the form of hot gas, steam or hot water. The application of low pressure and low temperature steam in industrial processes is particularly promising because commercially available nuclear reactors can produce large quantities of such steam at relatively low cost. For the distillation process, the heat requirements in the form of low pressure and low temperature steam are 45 to 65 kWh thermal per cubic metre (160 to 240 kJ per kilogram) of product water.
There are two approaches to the use of nuclear reactors in desalination: they can be used for a single purpose (i.e. heat production) or for dual purpose (generating electricity and heat production). For either application, the nuclear reactor must meet several requirements: (1) it should be available in sizes of practical interest for dual power/ desalination or for desalination only; (2) the technology of the particular reactor type should be well developed to guarantee safe and reliable operation; (3) the economics of heat production should be attractive and competitive with other available energy sources.
_
The size of the nuclear power plants to be used for desalination is of particular importance.
The smallest nuclear power plant commercially available at present is 600 MWe, or 1900 MWth. If used for the single purpose of desalination, this plant size could satisfy the heat: requirements of a desalination plant with a capacity of 700,000 to 800,000 cubic metres per day of desalted water, sufficient for a city of 1.5 million inhabitants. However. the desalination plants that are presently available are not large enough to handle all of the heat output from a 1900 MWth reactor. Therefore, nuclear reactors with 135 MWth, 260 MWth or 1100 MWth outputs are being considered as potentially more suitable for desalination plants with production capacities of, respectively, 50,000 100,000 or 400,000 cubic metres of desalted water per day.
_
Another solution to the problem of plant size mismatch is to use commercially available nuclear power plants for dual purpose operation. In a dual purpose power plant, the steam is normally extracted from the turbine after being expanded to some degree. In principle, one can select the ratio of water/electricity production by deciding at what temperature and pressure the process steam should be extracted. Typical production ratio may vary from 120 litres/kWh down to 20 litres/kWh. In all cases, a reduction in the amount of electricity that is produced cannot be avoided. This reduction varies from 56% for the 120 1/kWh ratio to 10—20% for the 20 1/kWh ratio
_
Dual purpose nuclear power plants might be an adequate solution for the water supply problems of some rapidly expanding metropolitan or arid industrial areas. The size of the desalination plant can be tailored to the existing demand and the excess capacity of the nuclear plant can be used to generate electricity.
For other metropolitan areas, however, it may be preferable that the first desalination plants use smaller power plant units that can satisfy the short and medium term needs for water and provide at the same time sufficient experience for future, larger desalination operations.
_
Small nuclear reactors in the size range 300 to 1000 MWth might be best suited to situations where water production capabilities have to be added to the existing water supply system at regular intervals. Construction of desalination plants in smaller sizes may be justified because the scaling factor in desalination installations does not play a significant role in the capital cost of water production. Water production is proportional to the heat transfer area of the evaporator installation, and operation of the small power plants may have advantages with respect to safety and availability.
_
Aside from the aspect of size, safety and high availability are the next most important factors in considering nuclear reactors for desalination. Higher than usual safety is required so that the plant can be located close to a metropolitan area in order to keep water transport to a minimum. High availability means that the reactor should be capable of operating continuously for long periods without forced outages.
______
______
Nuclear Desalination Experience:
Nuclear desalination as a concept has been around for almost 50 years as an economically reasonable option, though it has never achieved wider application. Still, sufficient experience was accumulated (~ 200 reactor years) in the use of nuclear power as the energy source for seawater desalination plants in various countries.
Nuclear desalination has proven to be a viable solution in countries like Kazakhstan, India, and Japan. While concerns have been raised about the environmental effects of nuclear power, the technology is becoming safer and more efficient. Large-scale deployment of nuclear desalination on a commercial basis will depend primarily on economic factors. Indicative costs are US$ 70-90 cents per cubic metre, much the same as fossil-fuelled plants in the same areas. One obvious strategy is to use power reactors which run at full capacity, but with all the electricity applied to meeting grid load when that is high and part of it to drive pumps for RO desalination when the grid demand is low.
_
The BN-350 fast reactor at Aktau, in Kazakhstan, successfully supplied up to 135 MWe of electric power while producing 80,000 m³/day of potable water over some 27 years, about 60% of its power being used for heat and desalination. The plant was designed as 1000 MWt but never operated at more than 750 MWt, but it established the feasibility and reliability of such cogeneration plants. (In fact, oil/gas boilers were used in conjunction with it, and total desalination capacity through ten MED units was 120,000 m³/day.)
In Japan, some ten desalination facilities linked to pressurised water reactors operating for electricity production yield some 14,000 m³/day of potable water, and over 100 reactor-years of experience have accrued. MSF was initially employed, but MED and RO have been found more efficient there. The water is used for the reactors’ own cooling systems.
India has been engaged in desalination research since the 1970s. In 2002 a demonstration plant coupled to twin 170 MWe nuclear power reactors (PHWR) was set up at the Madras Atomic Power Station, Kalpakkam, in southeast India. This hybrid Nuclear Desalination Demonstration Project (NDDP) comprises a reverse osmosis (RO) unit with 1800 m3/day capacity and a multi-stage flash (MSF) plant unit of 4500 m³/day costing about 25% more, plus a recently-added barge-mounted RO unit. This is the largest nuclear desalination plant based on hybrid MSF-RO technology using low-pressure steam and seawater from a nuclear power station. They incur a 4 MWe loss in power from the plant. In 2009 a 10,200 m3/day MVC plant was set up at Kudankulam to supply fresh water for the new plant. It has four stages in each of four streams. An RO plant there supplies the plant’s township. A low temperature (LTE) nuclear desalination plant uses waste heat from the nuclear research reactor at Trombay has operated since about 2004 to supply make-up water in the reactor.
_____
_____
Environmental impact of nuclear desalination:
There is no dismissing the environmental impact of nuclear desalination but some studies have reported various impacts due to many factors, such as technology, operation, hydrology, geographical, desalination capacity, and meteorological conditions. Therefore, it is important to consider environmental assessments to investigate possible impacts and propose optimal solutions. Table below presents some of the major impacts associated with nuclear desalination.
Summary of the main environmental impacts of nuclear desalination:
|
Impact |
Reason |
|
Coastal |
Noise, construction, visual impacts, and land requirements. |
|
Marine |
Intake of seawater, brine discharge, operations, characteristics, etc. |
|
Siting and co-location |
Water transport, environmental concerns, and overall impact. |
|
Sustainability |
Energy and water availability, relocation of the population, economic competitiveness, sustainability, etc. |
|
Public health |
Quality, reliability, radiation, safety, and water produced. |
|
Public perception |
Plant safety and fresh water production without radiations. |
_____
Potential radioactive contamination of the potable water produced:
One of the main concerns in nuclear desalination is the risk of transporting radioactive material into the desalination process stream, which must be prevented by all means and for all circumstances. Several radioactive isotopes could be released because of a leakage or an accident; among them, tritium is the primary concern that could contaminate the potable water produced. Its gaseous form has high permeability, and it could get into the desalination loop where it can react with oxygen and form tritiated water (HTO). In comparison, gaseous tritium is much less toxic than tritiated water, the ratio is 1:25,000 times, and therefore it is crucial to maintain constant monitoring of the desalination system for any leakage.
_
Hydrogen in its natural form is composed of 99.98% of 1H, 0.02% of deuterium, and traces of tritium; therefore potable water will contain tritium, and international standards accept it up to a specific limit. The maximum average annual tritium levels, as measured in the municipal drinking water of Canadian communities neighbouring nuclear facilities, are about 18 Bq/l. In comparison the Aktau desalination plant reported no more than 6 Bq/L. One Becquerel (Bq) is one radioactive decay per second. The experience from Kalpakkam, India is similar. No specific value for the tritium activity in the drinking water was reported since it was below the measurable limit. As can be seen, nuclear power plants coupled with heat driven systems, such as desalination, are well capable of controlling the tritium activity level in the product water through system design and operation practices.
______
______
Section-19
Alternatives to desalination:
Desalination is an expensive method to increase local water supply because it uses a lot of energy and has negative environmental effects. Desalinated water is frequently 2–4 times more expensive per acre-foot than other water sources. Desalination may seem like a panacea, but from a cost and energy standpoint it’s the worst deal out there. Desalination by the ocean is ineffective. For every gallon of freshwater generated, approximately two gallons of ocean water are needed. This means that a single, massive desalination plant cannot address the issues with the local water supply. Increased regional water supplies can be achieved by water conservation, water use efficiency, storm water capture, reuse, and recycling, which are frequently more affordable than desalination. These alternatives also offer benefits that are frequently disregarded in cost–benefit analyses, such as flood control, habitat restoration, and pollution abatement. Increased water conservation and efficiency remain the most cost-effective approaches in areas with a large potential to improve the efficiency of water use practices. Wastewater reclamation provides multiple benefits over desalination of saline water, although it typically uses desalination membranes. Urban runoff and storm water capture also provide benefits in treating, restoring and recharging groundwater.
___
Wastewater and its types:
Wastewater is used water. Wastewater is used water from any combination of domestic, industrial, commercial or agricultural activities, surface runoff / storm water, and any sewer inflow or sewer infiltration. In everyday usage, wastewater also called domestic wastewater or municipal wastewater, is wastewater that is produced by a community of people. Wastewater has TDS of around 1000 mg/l while Seawater has 35,000 mg/l.
Wastewater are of two types, blackwater and greywater.
-1. Sewage, commonly known as blackwater, is wastewater from toilets and bathrooms that contains faeces and urine. Because of pathogens and grease contamination, kitchen and dishwasher water is also considered black water. It is also known as sewage or brown water, and it can spread diseases and bacteria that are harmful. To destroy bacteria when recycling and treating blackwater for use as fertilizer, it must be properly processed and decomposed. Composting generates heat, which can kill bacteria in blackwater.
-2. Greywater is wastewater from plumbing systems other than toilets, such as hand basins, washing machines, showers, and baths. Greywater can be safely reused in the garden if handled properly. Recycled grey water is commonly used in irrigation and built-in wetlands – as long as no harmful chemicals are present. Grey water containing food particles can be used to irrigate plants; it can also be used to wash clothes and clean bathrooms. Grey water is valuable in areas where water is scarce.
_____
The water crisis is a global problem that is expected to worsen in the coming years due to climate change, population growth, and increasing demand for water. There are a number of solutions that can be implemented to address the water crisis besides desalination include:
- Improving water efficiency in agriculture.
Agriculture is the largest consumer of water worldwide, accounting for about 70% of global water use. Improving irrigation efficiency can help to reduce water demand in agriculture. This can be done by using more efficient irrigation methods, such as drip irrigation, and by planting crops that are more water-efficient.
- Reducing water losses in urban areas.
Water losses in urban areas can be significant, due to leaks in pipes and other infrastructure. Reducing these losses can help to conserve water. This can be done by investing in better infrastructure and by educating the public about water conservation. Every year in the U.S., approximately 9 billion tons of drinking water are lost due to leaking faucets, pipes and water mains, and defective meters. President Biden’s $1.2 billion infrastructure plan includes substantial sums for upgrading clean drinking water and wastewater infrastructure.
- Recycling and reusing wastewater.
Wastewater can be treated and reused for a variety of purposes, such as irrigation, industrial use, and toilet flushing. This can help to reduce the demand for freshwater. Recycling or reusing treated wastewater is often less expensive than desalination. Technology and regulations in this area are advancing, and this is already making large investments in recycling possible in many arid regions. Water reuse definitely has to be an important part of the solution. Our wastewater can get treated, either to potable standards, like it’s been done in other parts of the world, or to a different standard that can be used for agriculture or other things. Recycling the approximately 50 million tons of municipal wastewater that is discharged daily around the U.S. into the ocean or an estuary could supply 6 percent of the nation’s total water use. Recycled water can be used for irrigation, watering lawns, parks and golf courses, for industrial use and for replenishing aquifers. The House of Representatives is considering a bill that would direct the Secretary of the Interior to establish a program to fund water recycling projects and build water recycling facilities in 17 western states through 2027.
The technology to recycle water has been around for 50 years. Wastewater treatment facilities add microbes to wastewater to consume the organic matter. Membranes then are used to filter out bacteria and viruses, and the filtered water is treated with ultraviolet light to kill any remaining microbes. The water can be used for agriculture or industry, or it can be pumped into an aquifer for storage. When it is needed for drinking water, it can be pumped out and repurified. If the water is for human consumption, some minerals are added back in to make it more drinkable.
- Water conservation
Water conservation includes all the policies, strategies and activities to sustainably manage the natural resource of fresh water, to protect the hydrosphere, and to meet the current and future human demand (thus avoiding water scarcity). One of the strategies in water conservation is rain water harvesting. Rainwater harvesting is the collection and storage of rainwater for use later. This can be a way to collect water during wet seasons for use during dry seasons. In the U.S., 42 billion tons of untreated stormwater enter the sewage system and waterways and ultimately the ocean each year. This means that the rainwater that could soak into the ground to replenish groundwater supplies is lost. Green infrastructure, such as green roofs, rain gardens, trees, and rain barrels, would reduce some of this water waste.
Storage capacity for enhanced capture of stormwater, even in areas where it rains infrequently, can be doubled or quadrupled in regions like Los Angeles and parts of Australia, at one-third to one-half of the cost per unit of desalinated water.
An additional strategy to water conservation is practicing sustainable methods of utilizing groundwater resources.
- Water pricing.
Water pricing can be used to encourage water conservation. By making water more expensive, people are more likely to use it wisely.
- Education and awareness.
Raising awareness of the water crisis and the importance of water conservation is essential to addressing the problem. This can be done through public education campaigns, school programs, and other initiatives. Determining the most reasonable and economical uses for water would help everyone understand and appreciate its true value.
- Water transport
A proposed alternative to desalination in the American Southwest is the commercial importation of bulk water from water-rich areas either by oil tankers converted to water carriers, or pipelines.
These are just some of the solutions that can be implemented to address the water crisis. The best solution for a particular area will depend on the specific circumstances. However, it is clear that a combination of approaches will be needed to solve this global problem.
_
In addition to the solutions mentioned above, there are a number of other emerging technologies that have the potential to help address the water crisis. These include:
- Drought-resistant crops. Scientists are developing drought-resistant crops that can thrive in dry conditions. This could help to reduce the demand for water in agriculture.
- Smart irrigation systems. Smart irrigation systems use sensors to monitor soil moisture and adjust irrigation accordingly. This can help to improve irrigation efficiency and reduce water waste.
- Water purification technologies. New water purification technologies are being developed that can remove pollutants from water more effectively. This could make it possible to use wastewater and other sources of polluted water for human consumption.
The water crisis is a complex problem, but there are a number of solutions that can be implemented to address it. By taking action now, we can help to ensure a sustainable future for water resources.
_____
Atmospheric Water Gen Technology:
Water-Gen represents more than a mere technological advancement; it signifies a fundamental shift in how we source water. Utilizing Atmospheric Water Generation (AWG), this method condenses atmospheric moisture into liquid form. Although AWG is an established concept, Israeli technologists have propelled it forward, devising a unique method that curtails energy use, rendering it suitable for widespread industrial application. Contrary to expectations of high energy demands, Water-Gen distinguishes itself with its astonishing efficiency. Data reveals that Water-Gen apparatus can produce one liter of water using merely 250 Wh of energy—significantly less than the energy a standard bulb consumes over the same period. This efficiency represents a pivotal advantage, especially for industries where energy conservation is crucial.
Water-Gen’s ability to source water from air has profound ecological benefits. It mitigates the reliance on groundwater, thus conserving natural aquifers, and diminishes the need for water transport, cutting down on associated carbon emissions and energy consumption.
______
Water 4.0 to Hitler’s sweater!
Desalination, wastewater recycling, and capturing rainwater are three pillars of future water systems. With world water demands rising and extreme droughts expected to grow more frequent and widespread as the climate warms, drawing fresh water from oceans and other salty sources will be increasingly important. “Eventually, we’ll have to develop new sources of water,” said David Sedlak, a University of California-Berkeley professor of civil and environmental engineering and author of Water 4.0: The Past, Present and Future of the World’s Most Vital Resource. Desalination, along with wastewater recycling and capturing and storing rainwater, will be “three main pillars,” he said, to replace “water supplies that are going to become less reliable and less available in the future.” However, desalination is expensive, energy-intensive, and can damage marine ecosystems. Moreover, while seawater accounts for 60 percent of desalinated water today, Sedlak and others say it’s much more practical and sustainable to desalinate less-salty brackish water and use the technology to recycle wastewater.
“Accepting recycled wastewater is kind of like being asked to wear Hitler’s sweater,” says Paul Rozin, a social psychologist at the University of Pennsylvania who has consulted water utilities on marketing toilet-to-tap programs to residents. “No matter how many times you clean the sweater, you just can’t take the Hitler out of it.” But the purity you get from the RO process is quantifiably better than the water you get from conventional treatments — better even than some bottled water. Whereas tap water is often treated with chemical coagulants and chlorine, RO filtration is a mechanical filtration of water contaminants that cuts the need for those chemicals.
______
______
Water recycling (wastewater reuse):
Also known as reclamation or reuse, water recycling is an umbrella term encompassing the process of treating wastewater and storing, distributing, and using recycled water. According to (Water Code § 13050) “’Recycled water’ means water which, as a result of treatment of waste, is suitable for a direct beneficial use or a controlled use that would not otherwise occur and is therefore considered a valuable resource.” Water can be recycled or reused on farms, within industrial complexes, and at home. However, municipal recycled water is what most people refer to as recycled water. Municipal recycled water originates at a municipal wastewater treatment plant and is sewer water that has been collected, treated, and reused so that it can be beneficially reused again.
_
Sewage treatment to water recycling:
In every location that has a centralized sewage system, the sewage is pumped through pipes to a sewage treatment facility. The treatment process occurs in a few steps. The first step is Preliminary Screening which removes large debris such as wood, dead animals, or clothing. Once sewage has passed through screens, it is then mixed vigorously and pumped with air induce the decaying process of organic waste. After aeration, the sewage enters settling tanks where the heavy sludge sinks to the bottom and the lighter materials float to the top; both of which are removed from the water. After the majority of the macro waste is removed, the water is then chlorinated to kill any remaining microbes that could cause harm. The last step of the process is to neutralized chlorine in the water and stabilizing the pH. Once, the sewage has been processed, it can now be safely return to the river or ocean.
The only thing difference between the sewage treatment process and water recycling is what happens to the water when it leaves the plant. Water that leaves a sewage plant can be used for any number of non potable purposes instead of disposing it.
_
Direct and indirect potable reuse:
Potable reuse can be “direct”: water produced in an advanced wastewater treatment facility is introduced into the raw water supply immediately upstream of a drinking water treatment facility, or directly into a drinking water supply distribution system. The latter has successfully been in place for decades in Windhoek, Namibia.
Direct recycling of wastewater into drinking water by purifying the output from wastewater treatment plants is called Direct Potable Reuse (DPR), also known colloquially as “toilet to tap,” is legal in Texas, and Arizona allows it on a case by case basis. Science supports adopting this method, but political acceptance requires getting the public to focus on the safety of the water coming from the tap more than whence it comes.
Potable reuse can also be “indirect”: highly purified water is introduced into an environmental buffer before being withdrawn for potable purposes. The buffer provides an additional barrier for the protection of public health, storage and means of transport. It may be a groundwater aquifer, like in Orange County, California, or a surface water reservoir, like in Singapore.
_
As an alternative to RO seawater desalination, water reuse through advanced wastewater treatment plants can also technically provide water of drinking water quality, but the main challenge in (potable) water reuse so far has been to set best practices, policies and high control standards to increase public acceptance. Moreover, the removal of (organic) micropollutants (also called trace organic contaminants, TrOCs) that are not fully removed by conventional (biological) wastewater treatment plants requires specific attention. As such, planned indirect potable reuse (IPR), which consists of blending an extensively treated wastewater with another source of fresh water, for example through recharging the treated wastewater into a subsurface ground water or into an above-ground surface water reservoir before drinking water treatment, is currently the most used in water reuse schemes. In this case, the reservoir acts as environmental buffer and the drinking water purification step provides an additional barrier to potential pollution. Planned IPR schemes are already in use in few places of the world such as Singapore, Belgium, California and Australia. However, implementation of these schemes can require extensive pumping costs related to transport of the treated effluent back to upstream reservoirs, which is affecting their economic viability. Alternatively, direct potable reuse (DPR) implies the injection of extensively treated wastewater within the local drinking water supply. Such scheme requires even more stricter control than IPR of wastewater treatment but may avoid extensive piping and pumping costs.
_
For both IPR and DPR, to assure drinking water quality and to avoid health risk of such scheme especially with regards to organic contaminants, pathogens and TrOCs, the multiple barrier approach has been developed. Specific treatment towards TrOCs removal or degradation were assessed and implemented: dense membrane technologies such as nanofiltration (NF) or RO, advanced oxidation or adsorption on active carbon proved to be efficient treatments. Water reuse treatment consists of pursuing the purification of a secondary treated wastewater through an advanced wastewater treatment plant. Typically, such plant consists in passing through two sets of membrane processes (for example ultrafiltration (UF) and RO) and a disinfection step (ultraviolet, ozonation) as described in Figure below. As a result of such an extensive treatment train, direct potable water reuse remains as costly as desalination, with main case studies and practical examples providing numbers in the range of 0.69–1.23 $·m−3 of water produced.
_
Figure above shows examples of typical potable water reuse and desalination treatment trains.
_
In practice, desalinated seawater remains the main alternative source for drinking water, while water reuse is mainly dedicated to irrigation or industrial purposes and as such, both streams are very distinct. Seawater desalination is therefore the first option for safe drinking water production but its energy consumption remains the main obstacle. Ultimately, both seawater desalination and water reuse schemes require further improvement and more attractive economics to allow for broader development.
_
Current uses of Water Recycling:
For as simple as water recycling is, only a few nations actually implement water recycling on a noticeable scale.
- Currently, Israel leads the way with water recycling because it recycles 100 percent of its sewage water. The result of this is that Israel now has 70 percent more water to use for agriculture (the other 30 percent is used as grey water or water used by industry). Considering that the 70 percent of the world’s water is already used for agriculture, water recycling, on a global scale, could reduce the amount of water pulled from sources by 25 percent.
- NEwater:
NEWater is Singapore’s brand of high-grade reclaimed water. NEWater is recycled from treated sewage (‘used water’) and produced using a rigorous 3-step purification process involving ultrafiltration/microfiltration, reverse osmosis (RO) and ultraviolet (UV) disinfection. As compared to desalination, NEWater is more energy-efficient and cost-efficient to produce because of the lower salt content in treated used water, as opposed to seawater. NEWater is used for both direct non-potable use (DNPU) and indirect potable use (IPU). DNPU is in the form of NEWater that is supplied to water-intensive industries such as wafer fabrication plants (fabs), power generation and petrochemical industries, commercial and public buildings for air-con cooling towers. The quality of NEWater surpasses WHO’s as well as the US Environmental Protection Agency’s water standards, thereby making it safe for potable use. However, it is not used directly. Instead, NEWater is injected into reservoirs to allow it to mix with rainwater before being collectively treated at the water treatment plants for potable use. This is done to be mindful of public attitudes and acceptance of reused water, as well as to provide an environmental buffer and allow for trace minerals to be reintroduced by blending with reservoir water. Over the years, PUB, Singapore’s National Water Agency has expanded NEWater supply capacity to meet up to about 40% of Singapore’s total water demand. Future plans aim to increase NEWater capacity to meet up to 55% of total water demand by 2060. The technical rigour embodied in the development of NEWater, was complemented with extensive public and community engagement to increase its acceptability and convince Singaporeans it was safe to use. Members of Parliament and grassroots leaders were briefed to engage with the community about NEWater using exhibitions, posters, brochures and advertisements. Bottled NEWater was provided to the public to taste and distributed at community events and at the National Day Parades.
The public communications message that followed these activities was consistent and stressed the following – i) that potable reuse of water has been practised successfully around the world and is not something new; ii) the treatment process employed in its production is reliable and safe; iii) indirect potable use provides an additional environmental buffer; and iv) NEWater provides a sustainable water source for Singapore.
NEWater improves Singapore’s water security, increases its resilience to climate change, and also reduces the need for large water storage capacity as water is constantly recycled, thereby freeing up limited land for other uses. While technology makes the production of NEWater viable, strong political will, effective engagement (with the public, media, industry, experts), positive messaging and organisational changes have been instrumental in its successful implementation.
- Australia has several highly successful water recycling projects. Sydney introduced the Rouse Hill recycled water scheme in 2001. Highly treated wastewater is piped into 32,000 suburban properties in distinct purple pipes. Each property also has the normal “potable” drinking water supply. Recycled water can play an important role in agricultural schemes. There are successful examples in South Australia (Virginia Irrigation Scheme), Victoria (Werribee) and New South Wales (Picton). Perth has gone further by embracing water recycling for urban use with plans to treat it to a drinking water standard. Part of the extensive treatment process involves reverse osmosis, which is also used in desalination. The treated water is then pumped into groundwater aquifers and stored. This “groundwater replenishment” adds to the groundwater that contributes about half of the city’s water supply. The Water Corporation of Perth has a long-term aim to recycle 30% of its wastewater. Southeast Queensland, too, has developed an extensive recycled water system. The Western Corridor Recycled Water Scheme also uses reverse osmosis and can supplement drinking water supplies during droughts.
- The American Southwest is another location that implements water recycling as that portion of the country is a desert and is constantly under water stress. Irvine California uses recycled water for toilet flushing which only adds 9 percent to plumbing costs. However, by using recycled water for the number one source of domestic water use (26.7 percent of domestic water is used for toilet flushing), the city requires almost a quarter less water per year. The other major use of recycled water is industry. For example, recycled water is used to cool the Palo Verde Nuclear Reactor in Phoenix Arizona. As can be seen by the purple arrow in the image below, water recycling can supplement many different needs in society.
In figure above, the purple arrows represent uses of recycled water (also known as grey water) and the blue arrows represent “new” water.
Because water recycling simply reuses treated water that would have otherwise been disposed of, it is both a cheap and highly effective method to reduce net water usage.
__
Taking into account that about 66% of the global urban population lives in urban centers bordering the ocean, the potential implementation of seawater desalination plants is therefore especially significant. However, it may not be a viable solution for water-stressed regions that are located a long distance from the coast or at a high altitude. In fact, when water demand sites are located far away to the coast and/or at high altitudes, exploiting other conventional or non-conventional water resources may be more economical than obtaining water from the sea. Therefore, water transportation costs can significantly contribute to total water production costs, affecting the economic viability of the seawater desalination process. The reuse of municipal wastewater could be a viable alternative to address water scarcity for these cases. In this context, there are already European countries that reuse treated wastewater for non-potable and potable uses. The conventional treatment of municipal wastewater is usually based on primary treatment followed by secondary treatment, which usually involves a biological process to remove organic matter from wastewater, in order to meet the standards needed for its discharge. In order to reuse treated municipal wastewater, a tertiary treatment (e.g., membrane-based separation processes) is needed to remove the remaining pollutants from secondary treated effluent, such as inorganic and organic compounds, pathogens, or nutrients, in order to meet water standards. The reuse of municipal wastewater allows for an increase in the water supply flow rate, but its additional costs, consisting of both the extra treatment needed to reach the water quality requirement and the transportation of the produced water to the reuse site, should be considered.
_____
_____
Desalination vs Water Recycling (Reuse):
The main differences between desalination and water recycling might be:
-Type of feed water
Desalination mainly uses saltwater and water with a high salt content as a water input. It can also use brackish water. Water recycling on the other hand mainly uses different types of waste water as an input, which come from a range of different parts of society and all contain less salt.
-Types of processes
Desalination uses the desalination process, which removes salt and other unwanted impurities from the water using RO or thermal methods. Water can be recycled using the membrane to filter out the contaminants. Methods like microfiltration, ultrafiltration, RO or nanofiltration are effective. With this filtration plant, you can carry out different water treatment functions like deionization or pre-treatment.
-Energy
Reverse osmosis (RO) is a technology commonly used for both wastewater treatment and desalination. Depending on the nature of the water to be treated, energy requirements differ. For example, the amount of total dissolved solids (TDS) to be removed from seawater is considerably higher than that of municipal wastewater – generally above 35 g/L compared with 0.1-1 g/L in municipal wastewater. The higher TDS, the more energy is required to supply the necessary pressure to remove these.
-Cost of water
The price to produce water from seawater desalination is more expensive than both indirect potable reuse and non potable reuse, with brackish desalination the cheapest of the four options. The energy component is considered to be between 50% (Dawoud, 2005) and 44% (Hinkebein and Price, 2005) of the cost of desalinating water. Seawater desalination requires four times higher feed pressure, and higher feed flow compared to reuse. Dreizin (2006) describes greywater reclamation and brackish water desalination as incurring very similar costs. Beyond that, there’s variables that can make both water sources more expensive, such as dedicated pipes for recycled water, and long transport distances can increase the price to produce water for both water recycling and desalination.
-Sustainability & Environmental Impact
Desalination can be energy intensive, and can produce a brine by-product from the desalination process, which is essentially a form of highly concentrated salt. Brine affects marine environment and desalination plants contribute to GHG emissions and air pollution. Water recycling also relies on energy to operate, and can also sometimes discharge treated wastewater with residual pollutants/contaminants (that can’t be removed from the wastewater) into surface water sources, which essentially is a form of water pollution but recycling wastewater requires less energy than the treatment of seawater with desalination. Additionally, wastewater recycling may have sustainable benefits to consider when recovering or reusing nutrients found in the water as a fertilizer.
There are cities around the world that use both desalination and water recycling as part of their overall water supply strategy. Perth in Western Australia might be an example of this.
In summary, reuse is more energy-efficient than seawater desalination but poses other treatment challenges. It requires more complex – i.e., more expensive – technologies that have greater potential for scaling and biofouling than seawater and have higher maintenance costs.
___
In many cities, desalination is chosen over potable reuse.
Is this because of cost? Of energy efficiency? Or because of public perception?
How do potable reuse costs compare to seawater desalination? The cost of treatment depends on many localized factors. The costs discussed here include both annualized capital costs and operation and maintenance costs, including energy. Costs for advanced water treatment, including Reverse Osmosis, which is needed for direct potable reuse, range between $0.45 and $0.75 /m3. Costs are lower for advance water treatment without reverse Osmosis, ranging from $0.32/m3 to $0.55/m3. Costs for desalination of seawater for large facilities varies from $0.50/m3 to $1.80/m3, dependent upon energy cost and location. So the cost analysis would often weigh towards potable reuse, though handling of concentrates or managing the buffer could tip the balance the other way.
Let’s take a look at energy consumption. An advanced water treatment facility requires 0.95 kWh/m3, whereas the state of the art desalination plants require about 3.3 kWh/m3. The energy required for potable reuse is significantly lower than for desalination, and the carbon footprint of desalination is three times higher.
So why don’t we have more potable reuse projects? It’s often because the public acceptance towards reuse is harder to obtain for city or government officials. Would you drink recycled toilet water? How do you get the citizens and the regulators to embrace that?
We need to build water wise communities – the inhabitants, the professionals, the policy makers and the leaders, who can make the right decisions for a water wise world. Desalination is still needed as a source of raw water in many places, but given its cost and its energy consumption, we should consider a combination of desalination and potable reuse.
____
Desalinated versus recycled water — public perceptions:
The Australian population once perceived desalinated water as environmentally unfriendly, and recycled water as a public health hazard. The general level of knowledge about these two concepts as potential water sources has historically been low. After nearly five years of serious drought, accompanied by severe water restrictions across most of the country, and subsequent media attention on solutions to water scarcity, Australians now show more acceptance of desalinated water for close-to-body uses, and less resistance to recycled water for garden watering and cleaning uses. Australians currently have fewer reservations about desalinated water than recycled water, despite the fact that identical water quality is assumed. Australians are mainly concerned about health issues that may be related to using water from alternative sources in their households while at the same time having only a low level of factual knowledge about the true health risks associated with desalinated and recycled water.
_
The primary source of recycled water is municipal wastewater, and this has prompted community concerns about water quality. Seawater is seen as a more pristine source. Wastewater carries what humans excrete and discharge to the drain from sources such as toilet, bathroom, kitchen, and laundry, or miscellaneous dumps of household or garden toxins or pharmaceuticals. Toze (2006) summarizes the primary concerns as being microorganisms including bacteria, viruses, protozoa, and helminthes, which are excreted from ill persons and carry infectious disease. Such organisms are eradicated by several “barriers” during water recycling, although the risk of treatment failure exists. However, this risk is relatively small and requires the combination of multiple, simultaneous systems failures. A technology used for both water recycling and desalination is reverse osmosis. This technology is used commonly for both application and hence lends itself for direct comparison. Reverse osmosis can treat both seawater and wastewater to a quality higher than required for most water applications. Reverse osmosis usually achieves a water quality better than most tap or bottled waters.
_
Seawater desalination and water reuse schemes have already been implemented worldwide, but their broader development remains limited due to both public perception and overall treatment costs/energy usage. In fact, it has been shown that public acceptance of alternative water scenarios is mainly driven by the lack of conventional water sources, i.e., only if there is real water shortage, acceptance is increased. It is clear that better education of the public on alternative water sources, and increased awareness of water scarcity are of utmost importance. However, broader implementation of alternative water schemes also requires technical progress to ensure safe drinking water (high and constant level of pollutant rejection) at lower treatment costs.
______
______
Desalination and water reuse together:
There is merit in discussing desalination and treated wastewater reuse together for the following reasons.
-1. First, they are the two most rapidly expanding sources of ‘new’ or unconventional water today. According to the Global Water Intelligence database, cumulative online desalination capacity went from virtually zero in 1966 to around 21 million cubic metres per day (m3/day) in 2000, to over 91 million m3/day in 2021. By then, 21,055 desalination plants were operational (GWI, 2022). Meanwhile, direct use of treated wastewater has also increased significantly worldwide, although, overwhelmingly, water reuse continues to concern untreated wastewater and/or treated wastewater that is released into existing water bodies. Thebo et al. (2017) thus calculated that around 35.9 million hectares (ha) of irrigated croplands have high levels of dependence on urban wastewater flows and that 82% of this cropland is located in catchments with low levels of wastewater treatment. Global cumulative installed capacity in municipal treated wastewater reuse rose slowly up until the mid-2000s, however, increasing from about 57 million m3/day in 1991 to 67 million m3/day in 2005. It then accelerated sharply to 169.1 million m3/day in 2020 (IDA, 2022: 6-14). The area covered by some planned reuse of treated wastewater is estimated to be around 1.35 million ha, and is mainly in the Middle East, North Africa and Western Europe (Drechsel et al., 2022). Underlying this progression are increasingly massive investments from both public and private sources, with large projects typically costing hundreds of millions or even billions of US dollars. Both desalination and treated wastewater reuse are thus at the centre of extraordinary metabolic transformations of the hydrosocial cycle in diverse contexts and at different scales and magnitudes. Both are likely to continue to shape water governance and politics in many places throughout the 21st century.
-2. The second reason to discuss desalination and treated wastewater together is that, although most critical research has so far considered them separately, industry and governing actors are increasingly considering them together under the heading of unconventional water. Critical social research should do the same if we are to engage with, and critique, this emerging narrative.
-3. Third, although wastewater reuse is materially and infrastructurally much more heterogeneous that desalination, both processes often use similar membrane technologies, which are often delivered by the same global water companies. Unlike dams, both are also usually located in coastal or low-lying periurban areas and thus face specific constraints in terms of access to land and energy costs.
-4. High operating costs are related, in particular, to high energy consumption, both to power production processes, especially in the case of desalination (i.e. reverse osmosis) and to pump water upstream, as both desalination plants and wastewater treatment plants are located in low-lying areas. High costs may also be related to high land prices in metropolitan areas. This encourages the adoption of more intensive, denser and more expansive technologies such as (in the case of Beirut) sophisticated processes of biofiltration in wastewater treatment plants. Finally, in the case of treated wastewater, it is also due to high monitoring costs, as wastewater carries particular health risks that require more stringent regulations, and more thorough enforcement, than is the case for conventional water.
_____
Opportunities and Challenges of Combining Desalination and Water Reuse Schemes:
Forty percent of the world’s population lives in urban coastal areas, which are typically faced with the joint presence of multiple water sources of different qualities and salinity levels (e.g., river water, wastewater, seawater…). In several densely populated (dry) coastal regions, water is not reused, but drinking water is produced from seawater desalination. In these cases, typically wastewater treatment plant effluents and seawater intake points are in a relatively close geographic area (as illustrated in Figure below). In other examples, water reuse and seawater desalination are both implemented, such as in California or several regions in Australia. In Singapore, since the implementation of the NEWater program, both water reuse and desalination participate to the overall potable water supply but through distinct water purification scheme. So far, water reuse and desalination have always been considered as separate and independent streams to solving water shortage.
_
Figure above shows potential combination of wastewater reuse and seawater desalination to support potable water needs.
_
Implementation of a single scheme combining both concepts requires some prerequisites such as proximity of both streams, and ideally the location of water reuse and desalination facilities in one place, which require long term planning in water management, along with technical and economic justification. However, combining a desalination facility with another plant, also called co-sitting scheme, have already been proposed by implementing desalination close to a power plant to lower water intake costs, optimise energy efficiency and eventually combine water streams. This concept has been extended to hybrid systems, such as combined RO-multi-stage flash (MSF) distillation systems, or membrane distillation (MD). As already demonstrated in other co-sitting plants, integrating wastewater treatment and desalination in one plant can also result in potential economic benefits. As such, combining water reuse and desalination schemes with the FO-RO hybrid process could present major advantages in water management, as combining these schemes could synergistically lower water intake costs and could optimise energy efficiency of water treatment.
______
Seawater desalination plus water reuse can be a win-win fix to our water cycle:
While we increasingly turn to desalination as a secure water supply, it is still perceived as an expensive and environmentally damaging solution, affordable only for affluent societies. We need to counter this narrative.
Formula: desalinate once, reuse again and again.
-1. The benefits of desalination go beyond the single-use value of the water produced. Desalination can be a sustainable way to replenish our water cycle: after primary uses (industrial or domestic), reuse of desalinated water for irrigation enables agriculture in otherwise unproductive regions, and/or forest growth. The ensuing evapotranspiration feeds the water cycle, further enhancing precipitation, while enabling carbon sequestration by plants. In this way, desalination may not only reduce freshwater abstraction, but also provide a net water surplus, and thus help preserve and restore freshwater-dependent ecosystems.
-2. The negative effects of desalination can be effectively controlled. Energy efficient desalination is now ready to harness renewable energy sources (RE), particularly photovoltaics (PV) combined with battery storage, in increasingly competitive ways, so as to become carbon-neutral. Brine disposal affects marine ecosystems only at local scale, and appropriate design of outfalls can minimize impacts through dilution.
-3. Resource recovery from brine is of marginal help with current technologies, in spite of the appreciable content of valuable minerals and energy, because their concentration is so low that revenues from their recovery cannot offset the costs of water production. Yet, when operated with cheap energy (e.g. using passive solar evaporation or waste heat) concentration of brine may yield additional water resources while reducing the costs of sea disposal.
-4. When fresh water becomes scarce, its cost tends to go up, making desalination increasingly economic. Moreover, desalination can have little environmental costs. Considering the environmental costs of over-abstraction of freshwater, desalination tilts the balance in its favour. While carbon-neutral desalination can already be affordable, it may become mainstream as societies understand its broader benefits. With market expansion, costs will decrease further, triggering a virtuous cycle of water resources replenishment. For this scenario to come true, desalination cannot work in isolation, but through integrated water management including reuse for irrigation, maximizing the social and environmental return.
Closing the water cycle by desalination and wastewater purification promises to provide virtually unlimited volumes of freshwater.
______
______
Desalination versus water treatment for municipal use in Chennai:
Third desalination plant, with a capacity of 150 million litres a day (MLD), have raised hopes among Chennaites. Convinced that their water woes can be successfully addressed with advanced technology, citizens welcome the state government’s proposal of the plant that would make sea water potable. But, are desalination plants really the solution we need? Environmentalists are often deeply critical of pollution caused by desalination plants. But, even if we put aside the concerns around environmental damage for the present discussion, desalination plants themselves do not appear to be economically viable for the city.
The government spends mammoth Rs 1.36 crore every day in Operational and Maintenance (O&M) costs to source 200 MLD water from the existing desalination plants at Minjur and Nemmeli. This hefty drain on the exchequer could be reduced by more than four times if we can rely on surface water resources — lakes, ponds and quarries. In simpler terms, the government would be spending only Rs 29 lakh to acquire the same amount of water from the water bodies of Chennai. That should not be a great challenge given that the proposed Chennai Metropolitan Area is blessed with 4100 water bodies. This expenditure of Rs 29 lakh is on account of operations and maintenance of plants that treat water from the water bodies to make it potable.
Data shared by Chennai Metrowater Supply and Sewerage Department (CMWSSD) specifies the breakup of the expenditure on various modes of procuring water as in the table below:
|
Source |
Capital Cost per MLD (in Rs) |
O & M Cost per MLD (in Rs) |
|
Desalination plants |
15.30 crores |
68,000 |
|
Surface Water resources |
7.70 crores |
6000-23,000 (average of 14,500) |
Minjur and Nemmeli plants yield 100 MLD water each, amounting to 200 MLD. The cost of converting the saline water to potable drinking water (of 200 MLD) can then be estimated at Rs 1.36 crore (68,000 X 200). Thus, the above table indicates that the state government spends close to Rs 500 crore per annum to get just about a fourth of Chennai’s water requirement (of more than 830 MLD). The current cost of procurement of 200 MLD from desalination plants is higher than the 450 MLD sourced from the four reservoirs, agricultural wells and quarries. The capital cost per MLD of a desalination plant is again double that of water treatment plants. The capital cost to tap water from surface water resources includes laying pipelines and setting up treatment plants. But it is not necessary to set up a treatment plant at every lake at that cost. We could divert water from the lakes to the existing plants for treatment.
Chennai water problems are essentially due to mismanagement of water bodies, and Chennai is not a rain-starved city. Chennai’s average annual rainfall of 139 cm is sufficient to recharge its aquifers. They don’t have perennial rivers, but they have abundant surface water resources. The city of London, with just 60 cm of annual average rainfall, relies on surface water resources. “It is shameful that we have opted for the extravagant choice of desalination plants instead,” said Sai Praneeth, Director, Hydro-Meteorological Innovative and Explorative Solutions (HYMIES).
______
Desalination versus surface water for agriculture use in America:
The world doesn’t have a shortage of water; it has a shortage of cheap water. And the cost of desalination has a physics limit: it will always take 1 kWh or more of energy to desalinate a cubic meter of seawater no matter what method used.
- 1 kilowatt-hour to desalinate 1 cubic meter of seawater
- 1.2 Megawatt-hour to desalinate 1 acre-foot of seawater
(The numbers are rounded to make them easier to remember.)
In the California, the cost of electricity is about 30¢ per kWh retail. Farmers in California can currently buy fresh water at less than $200 per acre foot. Putting this into a list, we get the costs of fresh water today for an acre-foot of fresh water in California:
- less than $200 (river water)
- $360 (desalinated water, at physics limit, retail electricity, not yet achieved)
- $2700 (desalinated water at California, using best desalination technology available)
Desalinated water is cheaper than bottled water, but far more expensive than currently available farm water in the California. It is affordable if you need water to drink, but not if you are using it for agriculture in a world market. Santa Barbara installed their desalination plant during a severe drought. The drought ended in the late 1980s, so they turned it off (the water was too expensive). On July 21, 2015, in response to exceptional drought conditions, the Santa Barbara City Council voted unanimously to reactivate Desalination Plant. The high costs and the physics limit make it look as if desalination will never be cheap enough for agriculture.
_______
_______
Can desalination solve the water crisis?
Alone, no. But it might help as part of a broader range of efforts to cut water use and increase water supplies. Its technologies are growing more energy-efficient, and there are new ways to reduce the environmental harm of the salty wastewater. And it could be used in especially parched parts of the world where water is desperately needed and where there are few alternatives. “The benefits of desalination go beyond the single-use value of the water produced,” the authors of the European Commission study argued, advocating for wider use of desalination in more-vulnerable and poorer regions of the globe. The technology can provide “plentiful water for human use, with all the benefits that entails, while helping preserve and restore ecosystems.”
But in the United States, even proponents of the technology say desalination is likely to supply only a sliver of the American West’s water needs in the coming years, leaving some of the biggest water users — notably the agriculture industry — to look for water elsewhere.
Los Angeles recently unveiled a $3.4 billion proposal to recycle and reuse its wastewater, for example, instead of treating the waste and pumping it into the ocean, as is currently done. Advocates say the change would significantly ease the pressure on the city’s water sources farther north in California and the Colorado River — all without the need to lean more heavily on desalination.
Conservation, recycling, all of those things are important first and if you can’t solve your water supply problem, then do desal, but do it right.
_
Why is desalination not the answer to all the word’s water problems, considering that two-thirds of the earth’s surface is ocean?
Not all of world’s population live next to the oceans. Let’s assume interior Karnataka has a water shortage [they often do]. From which ocean would you pump the water to that region hundreds of kilometers away from any coast? This is a key difference between India and Israel – Israel is a narrow strip of land next to a calm sea. Indian ocean is more violent bringing terrific cyclones and thus majority of our population live interior.
Water itself is seldom the problem. Even a dry region like Rajasthan has had two floods in 2 years. The collection, storage and distribution of water is the key problem. The same problem would remain even if we desalinate everything. Not to mention, the cost of desalination is still very high. It might be affordable for drinking water, but not really for agriculture and some industrial use.
______
______
Section-20
Experimental technologies for desalination:
_
Freezing:
The principle involved in freeze desalination (FD) is that, in the process of freezing, the dissolved salts present in the feed water are separated during the formation of ice crystals. Seawater can be desalinated by cooling the water to form crystals under controlled conditions. Before the total amount of feed water has been frozen, the mixture is washed and rinsed to remove the salts present in the remaining water or that is sticking to the ice crystals. The ice is then melted to produce fresh water. Since the main heat transfer processes involved are freezing and melting which are regenerative, this method is said to have very high energy efficiency.
Thermodynamically, the latent heat of freezing and vaporization of water is 330 kJ/kg and 2256 kJ/kg, respectively. The FD process needs approximately 1/7th of the energy required by the vaporization-based desalination processes. The insensitiveness to the fouling is another important benefit of FD compared to the membrane-based desalination processes, which typically are prone to fouling and require frequent maintenance. In the case of excessive fouling, the cleaning of membranes is very difficult. There is no need for intensive pretreatment of the saltwater for FD. The involvement of sub-zero temperature in FD reduces the risk of corrosion and scaling. Furthermore, both vaporization and membrane-based desalination methods produce concentrated brine, which causes environmental damage. FD can treat concentrated brine close to zero liquid discharge either standalone or integrating with membrane distillation and crystallization. The disadvantage in FD process is handling ice and water mixtures which are mechanically complicated to move and to be processed.
_
Ion exchange:
Ion exchangers are generally organic or inorganic solids which are capable of exchanging one type of cation (or anion) immobilized on the solid surface for another type of cation (or anion) present in solution. For example, Na+ ions in solution can be replaced with H+ by a cation exchanger and Cl− ions can be similarly replaced with OH− by an anion exchanger, resulting in the complete ‘demineralization’ of a NaCl solution. This process can be reversed by regenerating the cation exchanger with an acid, and the anion exchanger with a base.
_
Directional solvent extraction process:
Directional Solvent Extraction process is an alternative desalination technology to the distillation and membrane based processes. For directional solvent extraction process, the solvent must possess four conditions as follows (1. Water must be soluble in the solvent; (2. Increase in temperature increases the solubility of water in a solvent; (3. The solvent must be insoluble in water; (4. Salt does not dissolve in the solvent. The solvents used for directional solvent extraction process are hexanoic acid, soyabean oil, decanoic acid and octanoic acid. The solvents extract the water leaving behind the salts and other contaminants. The advantages of directional solvent extraction process are membrane free, low thermal and electrical energy consumption compared to reverse osmosis and distillation process.
_
Aquaporins:
Aquaporin proteins (AQP) are present in living organism for the selective water transport across the membrane. These aquaporin proteins are used in the biomimetic membrane for desalination because of their high permeability and salt rejection. Aquaporin is incorporated into the biomimetic membrane and can be classified into two types (1. AQP incorporated supported membrane layer where lipids or polymers act as supported layers (SMLs) and (2. AQP incorporated vesicle encapsulated membranes where proteoliposomes or proteo-polymersomes acts as a vesicle (VEMs). Aquaporin incorporated biomimetic membrane is used for nanofiltration, reverse osmosis and forward osmosis. The AQP incorporated proteoliposome was deposited on the hollow fiber polyethersulfone membrane which was prepared by the dry-jet wet spinning method and it was coated with polyamide layer by interfacial polymerization. This biomimetic membrane exhibited a permeate flux of 40 L m^−2 h^−1 and salt rejection (NaCl solution 500ppm) of 97.5% in reverse osmosis process. Moreover, it has also been used for forward osmosis process which exhibited a superior water flux compared to other forward osmosis thin film composite membrane. The surface modification of cellulose acetate membrane was done by (trimethoxy-silyl) propyl methacrylate which acts as a substrate. The selective layer was deposited on the surface modified cellulose acetate substrate by vesicle rupture and UV polymerization of triblock polymer (ABA) vesicles. The different ratio of Aquaporin (AQPZ): ABA was prepared 1:50 1:100 and 1:200. Finally, a planar biomimetic aquaporin membrane was prepared and exposed to UV radiation. The biomimetic membrane performance for nanofiltration was elucidated. The membrane with the 1:50 of AQPZ: ABA ratio showed a water permeability of 34 LMH bar^−1and a salt rejection more than 30%. The quantity of aquaporins plays a major role in water permeability and salt rejection. This increase in water flux was observed when there is an increase in the concentration of AQP.
_
Capacitive Deionization (CDI):
Capacitive Deionization (CDI) is a process system that removes charged species from water using an electrical potential difference (electrical driving force on the ions) between a pair of electrodes made often of porous carbon. One electrode which is positively charged adsorbs anions (negatively charged ions) and the other electrode which is negatively charged adsorbs cations (positively charged ions). The absence of hydraulic pressure means that OPEX can be reduced and fouling can be controlled in contrast to pressure-driven membrane processes. What’s more, a relatively low voltage is required (< 1.8 V) which means significant advantages in terms of low energy requirements with substantial water recovery.
CDI has already been put into practical use for industrial desalination by commercial companies such as EST Water and Technologies (Changzhou, China) and Atlantis Technologies (Dana Point, CA, USA). Numerous studies about CDI have been carried out to upgrade the desalination efficiency of CDI.
Standard CDI techniques desalinate water by separating the water’s ions. A typical CDI cell consists of two electrodes attached on opposite sides of a flow channel. The electrodes capture the salt ions through electrical exchanges that occur when an electrical current is applied to the cell. The cell is then regenerated by releasing the salt ions in a second cycle by alternating the direction of the applied electrical current. Since CDI does not require membranes and has lower energy requirements than other popular methods, it is becoming a competitive technology for removing salt from water. The problem with CDI systems is that they are limited by low salt adsorption when using the typically applied 1.2 volts. Increasing the applied voltage does improve the salt adsorption, but it also increases the potential for unintended side reactions that waste energy and can create permanent electrode corrosion.
To combat this problem, researchers have come up with a desalination method called battery electrode deionization (BDI). BDI improves upon standard capacitive deionization (CDI) techniques by eliminating the regeneration stage and lowering the voltage required to complete the process.
_
Membrane capacitive deionisation (MCDI):
Instead of removing the bulk water from the salt (as in RO, FO and MD), MCDI removes the salt from the water. Research on capacitive deionisation (CDI) began without using the membrane. In both CDI and MCDI systems, the feed-water flows between a porous carbon anode and cathode and relies on an electric field between them. The salt ions form electrical double layers at the surface of the electrodes’ pores and are held in place by electrostatic attraction. Once the pore surface is saturated with electrosorbed ions, the salt ions are discharged from the electrodes by reducing or reversing the cell voltage. This process regenerates the electrodes while forming a highly saline brine solution. The regeneration step also recovers a portion of the initial charging energy; Długołęcki et al. found that 83% of the initial charging energy can be recovered this way. The addition of an ion-exchange membrane to the CDI system prevents the adsorption of ions onto the electrode during regeneration, leading to lower energy requirements and higher salt removal capacities than conventional CDI. A more recent development is flow-electrode capacitive deionisation (FCDI), whereby the electrodes are formed of a non-static carbon slurry. These systems can operate continuously as the carbon slurry can be regenerated downstream, while new slurry is added back upstream.
Advantages over reverse osmosis:
The recent rise in MCDI research can be attributed to the low-energy and cost-efficient brackish water desalination, which have led to investigations into using MCDI for seawater applications. MCDI has the potential for a much higher rejection of solutes than RO because it removes the salt from the water rather than the other way round. This is intuitively a more energy efficient approach, given that there is much less salt than the water in both brackish and seawater. MCDI can be highly selective such that it can be used for the separation of monovalent and divalent ions, which is an important issue in the controlling of hardness in water. Selectively removing monovalent cations is useful within seawater resource recovery and positively impacts the remineralisation process. A further advantage of MCDI over RO is that it operates under ambient pressures and temperatures and, as such, has a lower propensity for fouling.
_
Redox Flow Desalination (RFD):
Electrochemical processes are emerging as promising technologies for water desalination owing to their energy efficiency and eco-friendliness. Two classes of electrochemical water desalination processes have received significant attention: capacitive deionization (CDI) and electrodialysis (ED). CDI involves the desalination of water by removing ionic substances, driven by the electrochemical adsorption/desorption of these ions in an electrical double layer formed on a pair of porous carbonaceous electrodes. Sequential charging and discharging of CDI electrodes enable adsorption and regeneration over multiple cycles. ED utilizes alternatively arranged ion-exchange (cationic and anionic) membranes, in which the charged species migrate through the membranes and produce dilute and concentrated water streams. In contrast with the discontinuous operation of CDI, which requires subsequent charging and discharging steps, ED systems rely on faradaic reactions to drive continuous desalination. Furthermore, through combination with ion-exchange techniques, ED has overcome the operational drawbacks of concentration polarization. This class of processes is referred to as Electrodeionization (EDI). ED has been scaled-up and implemented to various industrial applications, such as water desalination, salt pre-concentration, water softening, production of electrodialytic energy, and acid-base recovery.
Redox-mediated electrodialysis, also known as redox flow desalination (RFD), has recently emerged as a promising technology for electrochemically driven desalination, consisting of a hybrid system of CDI and ED processes. RFD improves upon traditional ED technologies by employing a reversible redox reaction to drive deionization, rather than the water-splitting reaction in ED, thus leveraging low-energy-driven faradaic reactions, analogous to battery systems. Thus, RFD presents several advantages that combine faradaic and non-faradaic characteristics. One advantage is the low operating cell voltage resulting from the use of reversible redox couples and the possibility of energy recovery from these redox couples, as opposed to the large cell voltage required in conventional ED. Another advantage is the utilization of commercially available electrodes (e.g., activated carbon), in contrast with the use of expensive metal oxide electrodes (e.g., dimensionally stable anodes) in the ED system to withstand extreme reaction conditions.
_
Waste heat use:
Thermally-driven desalination technologies are frequently suggested for use with low-temperature waste heat sources, as the low temperatures are not useful for process heat needed in many industrial processes, but ideal for the lower temperatures needed for desalination. In fact, such pairing with waste heat can even improve electrical process: Diesel generators commonly provide electricity in remote areas. About 40–50% of the energy output is low-grade heat that leaves the engine via the exhaust. Connecting a thermal desalination technology such as membrane distillation system to the diesel engine exhaust repurposes this low-grade heat for desalination. The system actively cools the diesel generator, improving its efficiency and increasing its electricity output. This results in an energy-neutral desalination solution. An example plant was commissioned by Dutch company Aquaver in March 2014 for Gulhi, Maldives.
_
Low-temperature thermal:
Low Temperature Thermal Desalination (LTTD) process utilizes the temperature gradient between two water bodies to evaporate the warmer water at low pressures and condense the resultant fresh using the colder water to obtain high quality fresh water. Low-temperature thermal desalination (LTTD) takes advantage of water boiling at low pressure, even at ambient temperature. The system uses pumps to create a low-pressure, low-temperature environment in which water boils at a temperature gradient of 8–10 °C (14–18 °F) between two volumes of water. Cool ocean water is supplied from depths of up to 600 m (2,000 ft). This water is pumped through coils to condense the water vapor. The resulting condensate is purified water. LTTD may take advantage of the temperature gradient available at power plants, where large quantities of warm wastewater are discharged from the plant, reducing the energy input needed to create a temperature gradient.
Experiments were conducted in the US and Japan to test the approach. In Japan, a spray-flash evaporation system was tested by Saga University. In Hawaii, the National Energy Laboratory tested an open-cycle OTEC plant with fresh water and power production using a temperature difference of 20 °C (36 °F) between surface water and water at a depth of around 500 m (1,600 ft). LTTD was studied by India’s National Institute of Ocean Technology (NIOT) in 2004. Their first LTTD plant opened in 2005 at Kavaratti in the Lakshadweep islands. The plant’s capacity is 100,000 L /day, at a capital cost of INR 50 million (€922,000). The plant uses deep water at a temperature of 10 to 12 °C (50 to 54 °F). In 2007, NIOT opened an experimental, floating LTTD plant off the coast of Chennai, with a capacity of 1,000,000 L/day. A smaller plant was established in 2009 at the North Chennai Thermal Power Station to prove the LTTD application where power plant cooling water is available.
_
Hydrogel based desalination:
Hydrogels are three-dimensional networks composed of hydrophobic polymers synthesized by crosslinking water-soluble polymers. Hydrogels can retain a large quantity of water within their network without disturbing their original structure. This imparts flexibility and swelling properties to the hydrogel structures.
The idea of the method is in the fact that when the hydrogel is put into contact with aqueous salt solution, it swells absorbing a solution with the ion composition different from the original one. This solution can be easily squeezed out from the gel by means of sieve or microfiltration membrane. The compression of the gel in closed system lead to change in salt concentration, whereas the compression in open system, while the gel is exchanging ions with bulk, lead to the change in the number of ions. The consequence of the compression and swelling in open and closed system conditions mimics the reverse Carnot Cycle of refrigerator machine. The only difference is that instead of heat this cycle transfers salt ions from the bulk of low salinity to a bulk of high salinity. Similarly to the Carnot cycle this cycle is fully reversible, so can in principle work with an ideal thermodynamic efficiency. Because the method is free from the use of osmotic membranes it can compete with reverse osmosis method. In addition, unlike the reverse osmosis, the approach is not sensitive to the quality of feed water and its seasonal changes, and allows the production of water of any desired concentration.
Researchers at Nankai University in Tianjin, China, developed the concept of a solar-powered desalination system that produces fresh water by using smart DNA hydrogels that does not consume additional energy. The same process can be used simultaneously to extract uranium from seawater or treat uranyl containing nuclear wastewater. The new solar-powered concept, which fabricates a DNA hydrogel matrix, incorporates the ability to absorb sunlight and reduce the amount of energy required to evaporate water. In addition, the smart DNA hydrogels used in the new concept incorporate functional DNA molecules that can respond to various stimuli, such as changes in pH or metal ions, and therefore can be used to extract uranyl ions found in low concentrations in seawater.
_
Small-scale solar:
The United States, France and the United Arab Emirates are working to develop practical solar desalination. AquaDania’s WaterStillar has been installed at Dahab, Egypt, and in Playa del Carmen, Mexico. In this approach, a solar thermal collector measuring two square metres can distil from 40 to 60 litres per day from any local water source – five times more than conventional stills. It eliminates the need for plastic PET bottles or energy-consuming water transport. In Central California, a startup company WaterFX is developing a solar-powered method of desalination that can enable the use of local water, including runoff water that can be treated and used again. Salty groundwater in the region would be treated to become freshwater, and in areas near the ocean, seawater could be treated.
_
Metal-organic framework (MOF):
There are many ways to desalinate water, but one of the most effective is membrane desalination. In this method, water is pushed through a thin membrane with tiny holes. The water flows through the pores, but the salt ions can’t, leaving only fresh water on the other side. A conductive metal-organic framework (MOF) is a new type of membrane for water desalination. These membranes consist of both the metal center and organic compound. The organic compound and metal connect in a pentagonal pattern, leaving a hole in the center that serves as a pore. If you look at them, they are like a honeycomb.
There are a couple of reasons why the framework is more effective. First, it’s incredibly thin. It’s a few atoms thick, which means there’s very little friction as the water molecules pass through the pores. Additionally, the placement of the pores helps with permeation. When you don’t have adjacent pores, there’s a huge pressure from the wall on the molecules. This makes the desalination process less efficient. To understand why, just imagine pouring water into a funnel. The water moves more slowly through the hole at the end because it’s pushed against the walls and forced through a small space.
The MOF, on the other hand, has multiple adjacent pores. There’s no pressure from the wall side. And that gives them this opportunity to pass more easily through the pore. Imagine pouring water through a strainer this time—it moves much more quickly, because it has multiple exit points it can escape through.
Finally, the MOF has more structural integrity than other materials. In most materials, scientists have to drill tiny holes in order to create the needed pores, which limits the amount that can be created per surface area. If you want to make a lot of pores, graphene or MoS2 can’t do that. Structurally they can’t hold the pressure. But thanks to its honeycomb structure, MOF is intrinsically porous. This allows a higher ratio of pores to surface area. It also saves on time and energy, since the pores don’t need to be drilled, or even adjusted in size.
The differences between the MOF and other typical membranes are notable, both in terms of how quickly water passes through and how many ions are rejected. And that’s just looking at a simulation of a few pores. A desalination plant can have billions of pores, raising its efficiency exponentially. In the scale of a large operation, it would be huge. Even a slight increase in efficiency would mean a huge leap.
_
Silica Gel Adsorption:
This approach uses low-grade heat such as waste heat from a process or solar energy to generate potable water and, depending on the cycle details, cooling as well. The low-grade heat is used to form water vapour from the saline or brackish source. The vapour is then passed through a bed of silica into which it adsorbs until the silica is saturated. Once saturated, the bed of silica is heated using further low-grade heat to drive off the now desalinated water before being re-condensed in a receiving vessel.
Adsorption-based desalination has a range of advantages, including: (1) it is driven by low-grade heat that is free and/or would otherwise go to waste; (2) it has few moving parts, leading to reduced maintenance costs; (3) fouling and corrosion is reduced due to the low operating temperatures and confinement of the saline/brackish water to a fraction of the total system; and (4) it offers the ability to treat/desalinate saline and brackish waters containing organic compounds. Besides these advantages, which may be shared in part with some conventional desalination methods, it also offers two unique benefits: (1) it has the ability to co-generate cooling along with the potable water; and (2) it yields double distillate, minimizing the possibility of so-called ‘(bio) gen-contamination’.
_
Thermally enhanced reverse osmosis:
The concept of thermally-enhanced RO is largely motivated by the fact that warmer feed water permeates more quickly across a RO membrane, making it possible to produce more water with a given unit of mechanical work. For example, some studies have shown mechanical energy savings of up to 12 % when seawater feed is heated from 15 to 45 °C, and up to 45 % when brackish water feed is heated from 15 to 45 °C. This is mostly the result of an apparent increase in the membrane permeability that is caused by lower feed viscosity as a function of temperature. The other advantage of warm feed is that high permeability facilitates operation at lower applied pressures, which is helpful for mitigating the risks of membrane mechanical failure and membrane fouling. For these reasons, it is generally agreed that RO operation in warm climates is favorable. However, it is less clear whether it is advantageous to use an external source of thermal energy to pre-heat RO feed water. In such a case, the energy savings from reduced pumping work (and associated cost savings) would need to outweigh the thermal energy input (and associated costs). A study found that overall energy savings of up to 12 % are observed for seawater desalination when feed is heated from 20 to 41 °C, and up to 18 % for brackish water with feed heated to 46 °C.
_
Dewvaporation:
Dewvaporation is a specific process of humidification-dehumidification desalination, which uses air as a carrier-gas to evaporate water from saline feeds and form pure condensate at constant atmospheric pressure.
The heat needed for evaporation is supplied by the heat released by dew condensation on opposite sides of a heat transfer wall. Since external heat is needed to establish a temperature difference across the wall, and since the temperature of the external heat is versatile, the external heat source can be from waste heat, from solar collectors, or from fuel combustion. The unit is constructed out of thin water wettable plastics and operated at atmospheric pressure. Dewvaporation is a novel desalination technology developed at Arizona State University as an energy efficient tool for freshwater procurement and saline waste stream management. The system has relatively low installation costs and low operation and maintenance requirements.
_
Biodesalination:
Biodesalination has emerged as a compelling study area, presenting an alternative approach to reducing salt concentration in seawater using microorganisms like algae and cyanobacteria. These microorganisms can remove salt via biosorption (a rapid, metabolism-independent process) and bioaccumulation (a slower, metabolism-dependent process). Biodesalination offers the potential to be a low-energy-demanding technology and could serve as an alternative to develop hybrid desalination plants, ultimately reducing the cost of seawater treatment. The reason for selecting biodesalination units as a pre-treatment approach is that they tend to reduce the salt content in the seawater, which requires further RO process treatment. It reduces the energy requirement of RO membrane processes to eliminate salt content from seawater.
_
Satellites-Based Monitoring of Harmful Algal Blooms for Sustainable Desalination:
The presence of harmful algal blooms (HABs) in the areas surrounding the intakes of desalination plants makes the seawater desalination process quite challenging. HABs, also known as red tide, are caused by the presence of large concentrations of harmful aquatic microorganisms where the bloom takes on a red or brown color. HABs are generally associated with the production of natural toxins that lead to the depletion of dissolved oxygen and cause wildlife mortalities of marine and coastal species. The occurrence frequency of HAB outbreaks has increased significantly in the past few decades. The effects of HABs on water desalination include (but not limited to) affecting the quality of the produced water, obstruction to coagulation, clogging of filters, and bio-filming. In many cases, HAB outbreak events have caused the shutdown of desalination plant operation for extended periods of time. Removal of HAB species from the desalination process seems to be an obvious solution to this problem, but their sudden eruption and a lack of preparedness represent the major challenges facing desalination plant operators. Consequently, an early algal bloom detection tool is required in order to perform operational changes and maintain the desired production capacity. Remote sensing, combined with hydrodynamic modeling systems, can be an alternative solution, which can detect the outbreak of HABs from space and monitor and forecast their evolution in space and time.
_
Forward Osmosis—Reverse Osmosis (FO-RO) Hybrids:
Forward osmosis (FO) is a promising membrane technology to combine seawater desalination and water reuse. More specifically, in a FO-reverse osmosis (RO) hybrid process, high quality water recovered from the wastewater stream is used to dilute seawater before RO treatment. As such, lower desalination energy needs and/or water augmentation can be obtained while delivering safe water for direct potable reuse thanks to the double dense membrane barrier protection. Typically, FO-RO hybrid can be a credible alternative to new desalination facilities or to implementation of stand-alone water reuse schemes. However, apart from the societal (public perception of water reuse for potable application) and water management challenges (proximity of wastewater and desalination plants), FO-RO hybrid has to overcome technical limitation such as low FO permeation flux to become economically attractive. Recent developments (i.e., improved FO membranes, use of pressure assisted osmosis, PAO) demonstrated significant improvement in water flux. However, flux improvement is associated with drawbacks, such as increased fouling behaviour, lower rejection of trace organic compounds (TrOCs) in PAO operation, and limitation in FO membrane mechanical resistance, which need to be better considered.
_
Co-axially electrospun superhydrophobic nanofiber membranes with 3D-hierarchically structured surface for desalination by long-term membrane distillation:
A research team in KICT, led by Dr. Yunchul Woo, has developed co-axial electrospun nanofiber membranes fabricated by an alternative nano-technology, which is electrospinning. The co-axial electrospinning technique is one of the most favorable and simple options to fabricate membranes with three-dimensional hierarchical structures. Dr. Woo’s research team used poly(vinylidene fluoride-co-hexafluoropropylene) as the core and silica aerogel mixed with a low concentration of the polymer as the sheath to produce a co-axial composite membrane and obtain a superhydrophobic membrane surface. In fact, silica aerogel exhibited a much lower thermal conductivity compared with that of conventional polymers, which led to increased water vapor flux during the membrane distillation process due to a reduction of conductive heat losses.
The co-axial electrospun nanofiber membrane performed a 99.99% salt rejection for 1 month. Based on the results, the membrane operated well without wetting and fouling issues, due to its low sliding angle and thermal conductivity properties. Temperature polarization is one of the significant drawbacks in membrane distillation. It can decrease water vapor flux performance during membrane distillation operation due to conductive heat losses. The membrane is suitable for long-term membrane distillation applications as it possesses several important characteristics such as, low sliding angle, low thermal conductivity, avoiding temperature polarization, and reduced wetting and fouling problems whilst maintaining super-saturated high water vapor flux performance. Dr. Woo’s research team noted that it is more important to have a stable process than a high water vapor flux performance in a commercially available membrane distillation process. Dr. Woo said that “the co-axial electrospun nanofiber membrane has strong potential for the treatment of seawater solutions without suffering from wetting issues and may be the appropriate membrane for pilot-scale and real-scale membrane distillation applications.”
_
Nanotechnology for Seawater Desalination:
Nanotechnology is a rapidly growing field that has numerous applications, including seawater desalination. In desalination, nanotechnology is used to create membranes with nanopores, which are capable of filtering out even smaller impurities than traditional RO and NF membranes. These membranes are also more durable and resistant to fouling. Hermetic, sulphonated nano- composite membranes have shown to be capable of removing various contaminants to the parts per billion level, and have little or no susceptibility to high salt concentration levels. Nanotechnology can increase the efficiency of the desalination process by reducing energy consumption and increasing water recovery rates.
Fluorine nanotubes:
You’ve probably seen how effortlessly wet ingredients slide across a nonstick Teflon-coated frying pan if you’ve ever used one. Fluorine, a lightweight ingredient that is inherently water-repellent, or hydrophobic, is a crucial component of Teflon. Teflon can also be used to enhance the flow of water by lining pipes with it. Associate Professor Yoshimitsu Itoh of the University of Tokyo’s Department of Chemistry and Biotechnology, as well as his colleagues, were intrigued by this behavior. Thus, they were inspired to investigate how fluorine pipelines or channels may work on a different scale, the nanoscale.
The researchers developed test filtration membranes by chemically manufacturing nanoscopic fluorine rings that were stacked and implanted in an otherwise impenetrable lipid layer, similar to the organic molecules found in cell walls as seen in the figure below. They developed multiple test samples with nanorings ranging in size from 1 to 2 nanometers. A human hair is almost 100,000 nanometers wide for comparison. Itoh and his colleagues evaluated the presence of chlorine ions, one of the major components of salt (the other being sodium), on either side of the test membrane to determine the effectiveness of their membranes. They found that it works 2400 times faster than even experimental carbon nanotube-based desalination devices.
As fluorine is electrically negative, it repels negative ions such as the chlorine found in salt. But an added bonus of this negativity is that it also breaks down what is known as water clusters, essentially loosely bound groups of water molecules, so that they pass through the channels quicker. The team’s fluorine-based water desalination membranes are more effective, faster, require less energy to operate, and are made to be very simple to use as well. Reducing the energy and thus financial cost, as well as improving the simplicity of water desalination, could help communities around the world with poor access to safe drinking water.
______
______
Moral of the story:
_
-1. The rain that falls on the land contains some dissolved carbon dioxide from the surrounding air. This causes the rainwater to be slightly acidic due to carbonic acid. The rain physically erodes the rock and the acids chemically break down the rocks and carries salts and minerals along in a dissolved state as ions. The ions in the runoff are carried to the streams and rivers and then to the ocean. Oceans are salty because when water evaporates, the salts remain and accumulate. Another source of salts in the ocean is hydrothermal fluids, which come from vents in the seafloor.
_
-2. Salinity is the saltiness or amount of salt dissolved in a body of water. Salt is mainly sodium chloride. Seawater typically has a salinity of around 35 g/kg, although lower values are typical near coasts where rivers enter the ocean. Rivers and lakes can have a wide range of salinities, from less than 0.01 g/kg to a few g/kg, although there are many places where higher salinities are found. Surface water is usually less salty than groundwater as groundwater is surrounded by rocks. Precipitation (rain water) typically has a 20 mg of salt per kg or less. The term ‘fresh water’ is used for a salt content of up to 0.5 gms per kg water but some authors have classified freshwater as containing less than 1 gms salt per kg of water. Out of total Earth’s water supply, less than 1% is available as freshwater to humans and animals for consumption. We use freshwater for drinking, agriculture, recreation, and industrial processes. 72% of all freshwater withdrawals are used by agriculture, 16% by municipalities for households and services, and 12% by industries.
Note:
Fresh water is not always potable water, that is, water safe to drink by humans. Much of the earth’s fresh water (on the surface and groundwater) is to a substantial degree unsuitable for human consumption without some treatment.
_
-3. The key difference between TDS (total dissolved solids) and salinity is that TDS is the measurement of all types of solid compounds in a given liquid sample whereas salinity is the measurement of the amount of salt that is dissolved in a given liquid sample, although people use the terms TDS and salinity interchangeably. The unit of measurement of TDS is usually “part per million (ppm)”. For water, 1 ppm = approximately 1 mg/L of dissolved solids in water. A very low concentration of TDS has been found to give water a flat taste, which is undesirable to many people. Generally, the TDS level between 50-150 is considered as the most suitable and acceptable. If the TDS level is about 1000 ppm, it is unsafe and unfit for human consumption. Most common methods to reduce high TDS are Evaporation and Condensation, Filtration and Crystallization.
_
-4. Do not confuse water hardness with TDS. Hardness measures the presence of calcium and magnesium salts, while TDS includes all minerals, not just calcium and magnesium. When measuring water treated with water softeners, high levels of TDS do not correlate to hard water, as water softeners do not reduce TDS; rather, they replace magnesium and calcium ions, which cause hard water, with sodium or potassium ions, leaving overall TDS unchanged or even increased. Soaps and detergents do not produce as much lather with hard water as with soft water. Hard water can cause scale buildup in pipes, valves, and filters, reducing performance and adding to system maintenance costs.
_
-5. Why can’t we drink seawater?
While humans can safely ingest small amounts of salt, the salt content in seawater is much higher than what can be processed by the human body. Additionally, when we consume salt as part of our daily diets, we also drink liquids, which help to dilute the salt and keep it at a healthy level. Living cells do depend on sodium chloride (salt) to maintain the body’s chemical balances and reactions; however, too much sodium can be deadly. Human kidneys can only make urine that is less salty than seawater. Therefore, to get rid of all the excess salt taken in by drinking seawater, you have to urinate more water than you drank. Eventually, you die of dehydration even as you become thirstier. A summary of 163 life raft voyages estimated the risk of death at 39% for those who drank seawater, compared to 3% for those who did not. People cannot drink saline water, but saline water can be turned into freshwater in a process called desalination.
_
-6. If you don’t have RO water purifier at home, it is recommended that you at least boil the water properly before consuming it, as it kills most of the bacteria and viruses present in the water. Boiling water also removes hard water causing bicarbonates of magnesium and calcium, as well as nasty contaminants in the main supply, such as chlorine and lead. Once boiled and cooled, you can pour the remaining water into another container, ensuring it retains its purity and softness.
Does boiling water get rid of the salt?
No. Boiling it will make it saltier as the water will start to evaporate leaving the salt behind in less water. You can boil fresh water for drinking purpose and not salty water.
RO purifiers at home are ideal for treating water with a TDS level of 500 – 2000 mg/l while seawater has TDS 35000 ppm. RO water purifier at home cannot clean seawater. Do not confuse between RO purifier at home and RO desalination. You should not use household RO water purifier in areas where TDS in water is less than 500 mg/liter as household RO water purifiers typically produce one liter of usable water and 3-25 liters of wastewater so you will be wasting lot of water; instead of RO boil such water.
_
-7. Water is becoming scarce due to mismanagement, misuse, contamination, and over-extraction. Urbanization, population growth, and industrialization have increased the water demands in the world. Climate change also contributes to water scarcity as extreme weather conditions like floods, droughts, unpredictable change in precipitations, and sea-level rise contaminate freshwater resources, destroy water and sewage infrastructure, and reduce available water. One-third of the world’s aquifers are in distress, mainly due to excessive withdrawals and changing rainfall patterns due to climate change. Excessive withdrawals from coastal aquifers resulted in an uncontrolled saltwater intrusion in many countries. Over-pumping groundwater to meet the rising water demand has resulted in the depletion of significant aquifers and the worsening of groundwater quality. A country or a region faces “water scarcity” when the availability of freshwater falls below 1000 m3 per person per year. Conventional sources of water such as rainfall, snow-melt and river runoff captured in lakes, rivers and aquifers are no longer sufficient to meet human demands in water-scarce areas. Water vulnerability is affecting 1.42 billion people in the world and World Water program (WWP) estimates that by 2030 only 60% of the water needed will be available. Water scarcity could result in conflicts, political instability, and the displacement of millions of people. The scarcity of fresh water may also make it harder to decarbonize society.
_
-8. Among the different alternative solutions for solving the issues of water scarcity, desalination is only implemented as a last resort when conventional freshwater resources have been stretched to the limit. Desalination is considered as a drought-proof water source, since it does not depend on river flows, reservoir levels, or climate change. Although the most well-known application of desalination is to produce freshwater from seawater, it can also be used to treat slightly saline (brackish) water, low-grade surface and groundwater, and treated effluent resources. Desalination can extend water supplies beyond what is available from the hydrological cycle, providing an unlimited, climate-independent and steady supply of potable water.
_
-9. Desalination is a procedure that is performed on an aqueous solution to separate the salts from the solution or to separate the water from the salts. Desalination is a general term for the process of separating salt from saltwater to produce fresh water. The freshwater produced should contain a content of total dissolved solids (TDS) that is appropriate for domestic, agriculture or industrial use. Current technologies can reject 99.75% of salt from saltwater, meaning that the average ocean water, with a salinity of 35 g/kg, can be reduced to a salinity of 87.5 mg/kg. Most desalination facilities are designed to achieve a TDS of 500 mg/L or less. Desalinated water used for other purposes, such as crop irrigation, may have a higher TDS concentration. The feed water salinity for desalination facilities ranges from approximately 1000 mg/L TDS to 60,000 mg/L TDS. Although feed waters are typically labeled as seawater or brackish water; low-grade surface & groundwater and treated wastewater can also be used as feed water in desalination plant. The feed water type can dictate several design choices for a treatment plant, including desalination method, pretreatment steps, waste disposal method, and product recovery (the fraction of influent water that becomes product).
_
-10. Currently total desalination capacity installed worldwide stands at around 21,123 plants, producing approximately 142,000,000 m3/day of freshwater supplying over 500 million people. RO technology is the most prevalent, with 74% of the world’s installed capacity using this technology, while another 21% use of thermal technologies (namely, MED and MSF). Many countries simply could not function without it. The Middle East accounts for just under half of total capacity, while Asia, China, the United States, and South America are scaling up their desalination capacity fast. The two most common sources of water desalination plants evaluate are seawater and brackish water.
_
-11. Desalination plants at sea versus inland:
Water has a very high ratio of bulk to value and is very expensive to lift or transport. This drives the location of a desalination plant: it should be near its raw material, the sea; it should be close to its market or point of use; and geographically it should not be too far below its market because pumping up elevation is very expensive. Hence, the typical location of a desalination plant is along a coastal city or coastal industrial zone, supplying a relatively well-off industrial, commercial, or domestic demand. Fortunately, already over one-third of the world’s population lives in urban centers bordering the ocean.
Removing salt and other impurities from sea-, ground- and wastewater could solve the world’s looming freshwater crisis. And yet, while industrial-scale seawater desalination plants do exist in coastal areas, the process of making undrinkable water drinkable for inland water sources is problematic due to the high cost of concentrate (brine) disposal. There are many desalination facilities in the U.S. located inland, away from the ocean, and most are designed to treat brackish groundwater. The reject brine from these plants cannot be economically discharged to the ocean, as is done with most coastal desalination plants. In such instances, evaporation ponds may be useful. In other cases, alternatives such as waste minimisation, discharge to surface water, discharge to wastewater treatment plants, deep well injection, land application, and wastewater evaporators may be appropriate. The reject brine from desalination plants can alter the physical and chemical properties of the soil. The brine may also find its way to groundwater and can alter its properties. Landlocked regions or areas far from the sea face logistical challenges in adopting this technology, as it requires proximity to large water bodies like oceans, seas, or in some cases, brackish water sources. This limitation restricts the widespread adoption of desalination, making it a less practical solution for many inland areas. In southern California, a ‘Brine Line’ allows inland plants to discharge their brine to the ocean rather than to sewer or surface waters. Due to the ocean’s naturally high salinity, the environmental risks of brine discharge are lower. Concentrating or crystallizing and disposing of concentrated/crystallized brine could unlock vast new water resources, but it’s just too expensive at this time.
_
-12. Cost estimates make clear that horizontal distance is not the main driver of water transport costs, but the vertical distance is. Consequently, it costs 5 to 6 cents/m3 per 100 km to transport water and a 100 m vertical lift is about as costly as a 100 km horizontal transport (0.05-0.06$/m3). When one needs to lift the water by 1600 m, or transport it over more than 1600 km, then transport costs equal to the desalination costs. Transporting the desalinated water inland or in elevated regions provide additional costs that can equal the costs of water desalination. Pumping a cubic metre of water to distances of more than 200km requires more energy than desalinating the same amount of seawater. In fact, when water demand sites are located far away to the coast and/or at high altitudes, exploiting other conventional or non-conventional water resources may be more economical than obtaining water from the sea.
However, the costs of water produced by desalination have dropped considerably over the years as a result of reductions in price of equipment, reductions in power consumption and advances in system design and operating experiences. As the conventional water supply tends to be more expensive due to overexploitation of aquifers and increasing contaminated water resources, desalinated water becomes a viable alternative water source. Desalination costs are competitive with the operation and maintenance costs of long-distance water transport system. Even though desalination water cost remains higher than the other processes, it is less expensive than the cost needed for drinking water transportation. Desalination has also become cheaper than building new infrastructure to transport water over long distances: the cut-off is roughly 500km according to Acciona, a major operator. As Saudi Arabia has already demonstrated, water can be pipped inland to landlocked cities which means desalination is not limited to coastal cities and can ensure the prosperity of sizeable regions.
_
-13. In RO desalination, we take in saltwater, a highly concentrated solution, and force it through the membrane by adding pressure. On the other side, we obtain salt-free water, while back on the first side the remaining water still holds the salt the membrane prevented from passing through. Current commercial RO membranes used in the desalination industry are mainly thin film composite (TFC) membranes, containing a relatively dense selective layer and a porous support layer. The selective layer is typically composed of polyamides formed via interfacial polymerization reaction. By applying an external pressure greater than the osmotic pressure of feed water, freshwater is extracted from saltwater. Thus, water flows in the reverse direction to the natural flow across the membrane, leaving the dissolved salts behind with an increase in salt concentration. For desalination purposes, an external pressure between 15 and 25 bar is normally applied for brackish water, and between 54 and 80 bar for seawater. About 3.5 kilowatt hours (kWh) of electricity are needed to desalinate 1 cubic metre of seawater – 1.3kWh to pump seawater to the plant and 2.2kWh for the reverse osmosis process. Desalination by RO results in high salt rejection (up to 99%) and high recovery ratios (up to 40%). Seawater RO (SWRO) can produce potable water with salt content of less than 500 ppm from seawater of 35000 ppm. Reverse osmosis is effective in removing a wide range of contaminants, including dissolved salts, minerals, bacteria, viruses, and other impurities. It produces high-quality drinking water that meets stringent quality standards. After reverse osmosis, the water is so pure we actually have to put minerals back into it.
In RO desalination plant, higher salinity of feedwater (seawater) needs higher pressure, higher energy use and result in lower recovery and lower flux. Lower salinity of feedwater (blackish water) needs lower pressure, lower energy use and results in higher recovery and higher flux.
_
-14. In thermal distillation atmospheric pressure is reduced, thus lowering the temperature required to evaporate the water. Liquids boil when the vapor pressure equals the ambient pressure and vapor pressure increases with temperature. Effectively, liquids boil at a lower temperature, when the ambient atmospheric pressure is less than usual atmospheric pressure. Distillation under reduced pressure is used to purify a liquid that has a tendency to decompose when heated to a high temperature. Under the conditions of reduced pressure, the liquid will boil at a temperature lower than its boiling point, and as a result, the liquids will not degrade as they would otherwise. The various distillation processes used to produce potable water, including MSF, MED, VC, and waste-heat evaporators, all generally operate on this principle. This allows water to evaporate even at 40°C leaving the dissolved solids behind, which require about 300°C to volatilize. Also, because of the reduced pressure, low-temperature “waste” heat from electrical power generation or industrial processes can be employed.
Thermal desalination has the advantage of being suitable for high-salinity, high-temperature, low-quality feedwaters, with less feedwater pretreatment requirements, recovery is independent of feedwater salinity, and more economically and operationally attractive when coupled with power plants i.e., co-generation of power and water. Thermal desalination processes such as MSF (multi-stage flash) and MED (multiple effect distillation) have in the past provided the majority of potable water in regions such as the Middle East, where excess heat from power plants is used to heat and desalinate seawater. However, the main disadvantages are the high energy consumption, and hence higher cost, and higher environmental impacts (EIs) due to greenhouse gases (GHGs) emissions and discharge of hot brine.
_
-15. Pretreatment is generally required for all desalination processes. Pretreatment ensures that constituents in the source water do not reduce the performance of the desalination facility. Thermal processes require pretreatment to avoid scaling and to control corrosive constituents of the source water. Pretreatment is a critical step in seawater and brackish water membrane desalination systems that utilize feedwater from surface water sources, because the suspended and colloidal particles, organisms, and natural organic matter need to be removed before the feedwater reaches the membranes. Indeed, proper pretreatment of feedwater is the most important factor in the successful operation of an RO plant.
RO pre-treatment technologies include conventional (e.g., coagulation-flocculation, media filtration, disinfection, scale inhibition) and non-conventional (e.g., MF, UF, and NF). As per the available literature, UF, MF and coagulation-flocculation are considered the most widely used pre-treatment technologies. Experience showed that most of RO plants failure was due to inefficient pretreatment which resulted in providing low quality water to the RO membrane that caused fouling. Several membrane modification strategies are under consideration to reduce membrane fouling in RO systems.
_
-16. Compared to its thermal counterparts, RO possesses the advantages of significantly less energy consumption, lower cost, non-corrosive equipment, small carbon footprints and relatively safer operation. Thus, seawater RO of medium and large scales are the most popular and economically efficient type of desalination method, making the installed share of SWRO of total globally installed desalination capacity more than 65%. The advantage of RO is that the technology is economically viable with several types of feed water salinity (such as wastewater, both domestic and industrial, brackish groundwater, and seawater), modular nature and plant size scalability (from few m3/d to hundreds of thousands m3/d). However, RO has higher pretreatment requirements, not suitable for high feedwater salinities as the maximum attainable recovery decrease as the feedwater salinity increase, and more prone to scaling and fouling being a pressure filtration-based process.
In most cases, an RO plant must be preceded by a proper pre-treatment to avoid membrane fouling/scaling by sediments, hardness, organic matter, bacteria, silica, metal oxides or even chlorine.
_
-17. Why GCC nations seemingly favor MSF over RO technology:
(1. Easy access to cheap fossil fuels and waste heat source such as thermal power plants result in co-generation facilities for power and water.
(2. The poor seawater quality of the region (relatively high salinity and temperature) for long was not compatible with membrane processing desalination units because it would cause fouling of the membranes.
(3. Lack of experience, red tides and reliability.
_
-18. Electrodialysis processes are different from distillation techniques and reverse osmosis in that dissolved species are moved away from the feed stream, whereas other processes move away the water from the remaining substances. Because the quantity of dissolved species in the feed stream is far less than that of the fluid, electrodialysis offers the practical advantage of much higher feed recovery in many applications. The desalination of water with concentrations of dissolved solids higher than 30 g/l, like seawater, is possible, but it is not economically viable.
_
-19. Membrane distillation (MD) is considered one of the effective and alternative techniques for the treatment of water and wastewater containing high amount of dissolved salt. RO is not advised for desalinating highly concentrated salt solutions because of need of very high pressure (high energy-cost) and membrane fouling. Membrane distillation combines heat and membrane processes and is more suited for working with extremely salty solutions, especially for solutions having salinity between 70 and 300 g of salt per kilogram of solution.
_
-20. Whilst forward osmosis (FO) holds great potentials that might be harnessed as an alternative seawater desalination technology, its efficacy is constraint by some key drivers impacting its applicability including membrane developments, membrane fouling, concentration polarization, draw solutes development and draw solution recovery, and reverse salt flux. These challenges remained to be overcome.
_
-21. Nanofiltration (NF) technology removes mostly divalent ions (e.g., Ca2+ and Mg2+), with an efficiency of between 90% and 98%. The removal of monovalent ions is limited (between 60% and 85%). As the soft water produced by the NF process has a greater ion concentration than RO, a lower pressure gradient must be applied to the semipermeable membrane (between 34 and 48 bar). As NF requires a lower energy demand than RO, this solution is under investigation for seawater desalination, introducing a dual-stage unit.
_
-22. Hybrid systems have several desalination techniques operating at the same location. The aims of integrating two or more techniques are to boost effective recovery throughout, lessen the release of brine, reduce the cost, reuse energy, reduce energy requirements and optimize plant performance. In particular, NF-RO systems have proven to be effective for the treatment of seawater; utilizing NF during the pretreatment stage results in improved efficiency via an increase in the permeation current and reliable seawater reverse osmosis (SWRO). Hybrid thermal-membrane plants have a more flexible power-to-water ratio, efficient operation even with significant seasonal and daily fluctuations of the electricity and water demand, less primary energy consumption and an increase of plant efficiency, thus improving economics and reducing environmental impacts.
_
-23. Different factors affect energy footprint of water desalination. These factors are: capacity of desalination plant (small, medium, large), type of required energy (electrical or thermal energy), type of feed water (brackish or seawater), desalination method (thermal or membrane), necessity of feed pretreatment (mechanical and/or chemical), product post-treatment and the brine treatment/disposal facility. All these factors determine the total energy consumption of desalination plant. As a rule of thumb, the larger the desalination units, the more efficient the process becomes. Generally speaking, RO has a lower energy demand, but higher the salinity of the original water, higher the energy needed. Higher salinity causes a higher osmotic pressure in the saltwater, which means more pressure is needed to push the water through the membrane. While salt content affects the energy requirements for membrane processes, salt concentration has no impact on the energy needs for thermal desalination systems. Thermal distillation technology is rarely used for desalinating brackish water since it is expensive.
_
-24. Energy conundrum:
Salt water and fresh water spontaneously mix. When freshwater and saltwater come into contact, the ions of the saltwater will move towards the freshwater. The instigator of this mixing process is the increase in entropy, in popular terms: the disorder of the system. The saltwater will gradually become more fresh and the freshwater more saline. When this mixing process is occurring in an uncontrolled way – like in nature, or at a discharge – it is irreversible. By controlling the mixing process in a reversible way, a part of the energy that is otherwise lost, can be converted into electricity. In the Netherlands this has become known as Blue Energy. Therefore, according to the laws of thermodynamics, a minimum energy is required to unmix them—that is, to desalinate.
For typical seawater at ambient temperature and 35000 ppm (35 gms/Kg), the universal theoretical thermodynamic limit to separate water from the solution (but at zero recovery) is 0.78 kWh per cubic meter.
For a typical seawater system producing a half kilogram of fresh water per kilogram of seawater, that minimum is about 1 kWhe (kilowatt hour of electrical work) per cubic meter of fresh water.
Practical desalination systems are never fully reversible and there are energy losses that are due unavoidable irreversible contributions. These losses, that depend on the water recovery ratio, increase the energy of desalination above the reversible thermodynamic limit and these loses are far greater in thermal desalination as compared to membrane desalination.
TDS levels determine the bounds for the minimum energy needed to generate freshwater from saltwater. Higher TDS water (such as seawater) requires larger amounts of energy for desalination, whereas water from low TDS streams (such as blackish water and wastewater reuse) could be much lower. While salt content affects the energy requirements for membrane processes, salt concentration has no impact on the energy needs for thermal desalination systems. Thermal methods are more energy intensive than membrane ones because of the large quantities of fuel required to vaporize salt water. However, they are more efficient in terms of desalination of very salty waters than the membrane technologies.
New, large-scale seawater RO plants generally use about 3–4 kWhe/m3, and decreases to 0.5 to 2.5 (kWhe/m3) for brackish water; by way of contrast, the best large-scale evaporative desalination systems require a heat input equivalent to about 20 kWhe/m3. The takeaway here is that with modern RO, we cannot expect order-of-magnitude improvements to energy consumption—we’re already pretty good. The current “state of the art” SWRO plants using isobaric ERDs, axial piston high-pressure pumps, and the latest membrane technology can be at 2 kWhe/m3. The substantially lower energy requirements of membrane technologies versus thermal ones are probably their most well-known benefit. RO uses several times less energy than MSF and MED, which means lower costs and lower emissions. Today RO is operating very near to its thermodynamic limit. Thermal technologies operate far from thermodynamic limit due to the irreversible losses in real processes.
_
-25. Several options have been developed to improve the energy footprint of desalination technologies, among which is energy recycling and recovery, hybrid processes, process modifications, use of waste heat and integration with renewable energies. Recent developments in membrane materials, pumping and energy recovery systems have dramatically reduced the energy consumption in RO desalination processes.
_
-26. Brackish water desalination poses a different set of challenges compared to seawater desalination. While there are fewer dissolved particles to remove from brackish water, it can be harder to dispose of the leftover waste. And though less energy is required to pump the brackish water through filters than sea water, more energy is sometimes required to pump it from its source.
_
-27. The energy consumption for surface freshwater treatment is about 0.6 kWh/m3, and brackish water and reclamation of municipal wastewater RO requires about 1 kWh/m3, while the least energy intensive SWRO desalination plants today still consume around 2-3 kWh/m3.
_
-28. It is possible to use energy in a dual use or cogeneration systems in which the energy sources can perform several different functions such as electric power generation and water desalination. The major advantage of cogeneration system is that it uses very less fuel than other plants operating separately and the energy costs are less for desalination process. However, problems can occur due to permanent coupling between the desalination plant and the power plant which can create a problem in water production when the need for electricity is reduced or when the turbine or generator has a problem.
_
-29. Factors that determine the costs for desalination include capacity and type of facility, location, feed water salinity, labor, energy, plant technology, pretreatment requirement, specific conditions of the project, financing and concentrate disposal. Therefore, it is challenging to provide an exact cost of desalinated water from the ocean as it can vary significantly. Operation costs account for two-thirds of water production costs, while one-third is based on capital cost depreciation. High water costs remain as one of the major barriers in extending the desalination technology, which in turn is influenced by the energy consumed by desalination processes, accounting almost 50% of the total costs. The size of desalination installation has great impact on the energy consumption and cost of desalination process. With increase of desalination plant’s capacity, it becomes more efficient in term of cost.
_
-30. The cost of desalinated seawater, the majority of which is accounted for by plant capital costs and energy costs, is typically in the range of $0.5 to $3 per cubic meter of water. The lower end of the scale corresponds to regions where electricity costs are low (e.g. Middle East) and the higher end to regions where electricity costs are high. The cost of brackish water desalination in the Middle East with a TDS concentration of 2300 ppm is 0.26 $/m3, while in Florida, for brackish water with a TDS of 5000 ppm, it is 0.27 $/m3. RO desalination is the most cost-competitive technology for less saline environments, but thermal technology is more competitive for higher salinity environments. Source water conditions make a big difference to costs for RO, but not for thermal technology.
_
-31. Producing desalinated water is generally more expensive than tapping water sources from lakes and rivers, groundwater, or through water recycling and water conservation; primarily due to the high energy requirements and capital investments associated with the desalination process, but alternatives are not always available.
The cost of desalinated water is approximately two to four times higher compared to the cost of the conventional water supply. Desalination might be more expensive but it can supply water when it is most needed as it is rainfall independent. Desalination has the ability to alleviate pressure on traditional freshwater sources such as rainfall and underground reservoirs. By incorporating desalination plants into the water supply infrastructure, a nation can diversify its water sources and reduce dependency on vulnerable supplies, such as snowpacks and groundwater.
_
-32. The Middle East is one of the regions in the world with severe water scarcity, where the climate is hot, precipitation is scarce and the land is arid, but the local oil resources are abundant and the economy is strong, so there is an urgent need for desalination technology and devices. Saudi Arabia’s annual rainfall is only 100mm, half of its land area is desert, and the per capita possession of water resources is only 1.2% of the world average. The Saudi government, in order to solve the serious water shortage problem, has invested heavily to build 25 large desalination plants. There are theoretical limits to the energy reductions that are possible for seawater desalination. It will never be cheap. Increasing water scarcity is the major driver. If you look at the countries where desalination has tremendously increased, those are the countries that can afford it. Poorer countries can’t afford desalination. Over 90% of desalination happens in upper middle- and high-income countries around the world, even though poorer countries such as those in sub-Saharan Africa, are predicted to become water scarcity “hotspots” by 2050.
_
-33. Challenges to the widespread adoption of desalination are expense, significant energy use, need for specialized staff training, large carbon footprint of facilities, chemical discharge, large amount of brine discharge and operational problems such as membrane fouling in RO plants, and corrosion & scale formation in MSF and MED plants. The problem with corrosion is easier to solve with MSF compared to MED, because the design is less complex. This is the main reason that MSF has received wider global application than MED for desalination of very salty waters, despite the fact that energy demand for MSF is higher than for MED.
_
-34. Recovery Ratio (RR) is defined as the ratio between the freshwater flow and the saline feedwater flow. In comparison to membrane desalination, the recovery ratio in thermal desalination is generally lower but recovery is independent of feedwater salinity. With thermal, 75 percent of the seawater you bring in might leave as brine. With RO, it’s more 50-50 freshwater to seawater. RR is higher for blackish water RO as compared to seawater RO. Because desalination plants typically dispose of brine in the ocean, it influences marine environment through its salinity, temperature, and residual chemicals.
_
-35. The main “Achilles heel” for the efficient operation of a membrane-based desalination systems is membrane fouling. Some of the constituents of feed water may block the pores of the RO membranes, also known as fouling, rendering them inefficient after short operation times. The problems associated with membrane fouling are decreased membrane permeability, increased operating pressure, increased frequency of chemical cleaning, and membrane deterioration. Membrane fouling represents a serious challenge in RO processes due to its significant contribution to energy requirements and process economy (e.g., flux decline, permeate quality, membrane lifespan, increased feed pressure, increased pre-treatment and membrane maintenance cost). Hence, pretreatment steps are required to maximize the efficiency of RO as well as elongate the life span of RO membranes.
_
-36. Advantages of desalination include a reliable and abundant water supply, a solution to freshwater scarcity, diverse application possibilities, and increased independence. Disadvantages involve high energy consumption, negative environmental impacts, high costs, and limited geographical availability.
_
-37. Desalination negates some of the key drivers of water conflict. Most obviously, when relying on sea water rather than river water, it reduces the tensions that can develop between upstream and downstream riparian states.
_
-38. Agriculture is a significant consumer of water resources, and droughts can severely impact crop yields and food production. Farmers can ensure consistent water availability, bolster crop resilience, and sustain food production even during prolonged dry spells by integrating desalinated water into agricultural irrigation systems.
The agricultural need for fresh water is imperative in order to maintain crop yields and preserve soil quality. Many vegetable crops begin to experience diminished yields as TDS levels begin to exceed ~300 ppm. In many regions around the world, saltwater is the only available water resource for irrigation which can adversely affect crop growth and yield. In 2021, about 20% of total irrigated lands, contributing to one-third of global food production, are salt-affected. The application of saline water resources for irrigation purposes often result to the detrimental effect of salinization of soils, environmental degradation and low crop yield. Retention of salts in soils diminishes crop performance by creating ion toxicities, nutrient lockup, and desiccating osmotic effects.
Desalination solutions offer a promising and innovative approach to address water scarcity in agriculture. Desalinated water can increase crop yield & crop quality, reduce reliance on rainfall, and minimize soil salinization. However, desalination is expensive for agriculture, and currently the cost of desalinated water is still too high for the use of this resource in broad-scale irrigated agriculture. An exception appears to be intensive horticulture for high-value cash crops, such as vegetables and flowers (mainly in greenhouses) grown in coastal areas where safe disposal of brines is easier than in inland areas. Desalination has proven much less economic for agricultural application than the reuse of treated wastewater, even where the capital costs of the desalination plants are subsidized.
The wider application of desalination technologies for agriculture is limited by its relatively higher cost, as well as by the need for agriculture to be close to saline and brackish feedwater resources as well as a safe and cost-effective disposal option for brines.
_
-39. Over the long term, desalination with fossil energy sources would not be compatible with sustainable development; fossil fuel reserves are finite and must be conserved for other essential uses, whereas demands for desalted water would continue to increase. Also, global CO2 emission from fossil fuel powered desalination plants is about 218 million tons in 2020. The typical amount of CO2 emitted during SWRO desalination powered by natural gas is 2 to 3 kg CO2/m3 produced water. Renewable energy is environment-friendly and guarantees sustainability. Currently about 131 desalination plants in the world, representing approximately 1% of the world’s water desalination capacity work with energy from renewable sources. However, desalination based on renewable energy sources is expensive as compared to conventional desalination. On the other hand, water desalination itself can provide an excellent storage opportunity in the case of electricity generation exceeding the demand during renewable energy capture.
Only under certain circumstances – e.g. installations in remote areas where distributed energy generation (heat and power) is more convenient than centralized energy generation, transmission and distribution – renewable desalination could compete with conventional systems. Using locally available renewable energy resources for desalination is likely to be a cost-effective solution particularly in remote regions, with low population density and poor infrastructure for fresh water and electricity transmission and distribution.
_
-40. Nuclear desalination has been defined as the use of both electricity and heat generated by nuclear power plant to remove salt and minerals from seawater. Nuclear desalination is generally very cost-competitive with using fossil fuels. More countries are seriously considering desalination powered by nuclear energy to address their water needs, while avoiding carbon emissions. As desalination is a very energy intensive technology, it is imperative to power it with large-scale, zero-carbon sources, such as nuclear energy, in order to continue providing essential access to clean water to an increasing number of people worldwide, while simultaneously addressing climate change and commitments to net zero.
_
-41. Depending on the type of feed-water used, the desalination method employed, and how waste brine is managed, the desalination process has distinct and variable environmental consequences. The environmental impacts of desalination must be weighed against those of expanding use of freshwater sources (e.g. groundwater depletion, diverting surface water flows). Environmental impact of desalination includes air pollution, marine pollution, noise pollution, carbon footprint, and altering quality of land/soil and ground water.
The estimated carbon footprint of seawater RO desalination (0.4–6.7 kg CO2eq/m3) is generally larger than brackish water RO desalination (0.4–2.5 kg CO2eq/m3) and water reuse systems (0.1–2.4 kg CO2eq/m3). The thermal-based technologies, namely MSF and MED, have at least ten times higher GHGs emissions than RO. The use of a high volume of chemicals during the pre- and post-treatment of seawater is another environmental concern. The main concern is the discharge of chemicals into the natural water, which affects the ecological imbalance.
Furthermore, the design of open seawater intake has a potential role in the loss of aquatic organisms, as these organisms sometimes collide with the intake screen or can be drawn into the plant.
Besides freshwater, a by-product called ‘brine’, ‘reject’ or ‘concentrate’ is produced during desalination. Producing a litre of drinking water creates 1.6 litres of salty brine and this by-product is at least 1.6 times more saline than seawater. Brine refers to a hot, salty concentrate containing chemicals. It is an unwanted by-product of the desalination procedure. The global production of 141.5 million m3/day of brine is environmental concern. Thermal desalination produces higher volumes of less concentrated brine while RO desalination produces lower volumes of high concentrated brine due to higher recovery rates of RO compared to thermal. Since brine tends to have elevated temperature and salinity and also contains several chemicals (antiscalants, coagulants, surfactants, acids and bases for pH control), its discharge is hazardous for marine ecosystems. The quality and amount of the brine are governed by the quality of the feed water, pre-treatment process, desalination process, water recovery rate, and disposal technique.
_
-42. Diluting brine can lessen its impacts. You take more seawater, and you premix it [with the brine] in an engineered reactor. Now the salinity of that mix is not two times saltier than seawater. It’s still saltier than seawater, but it’s lower. And instead of discharging it at one point, you discharge it at several points with diffusers. These are engineering approaches to try to minimize the impacts of brine. A white paper produced by the IDA on the effects of brine discharge concludes that the discharge is fully safe and does not result in negative impacts on the marine habitat. Many desal plants have been constructed on the North Africa coast, where no significant impact of brine has been reported. This could be due to the relatively large sea area and abundant rain; otherwise, constant brine discharge will make seawater source too salty to accommodate the desalination cost while the salinity level keeps rising to a peak. As understood from the peak oil theory, the point after which oil extraction declines, peak salt may make desalination unfeasible for the economic and environmental aspects.
_
-43. Brine is commonly disposed of in the environment with various methods, such as surface water discharge, sewer discharge, deep-well injection, evaporation ponds and land application. Nowadays, brine treatment is considered one of the most promising alternatives to brine disposal, since treatment results in the reduction of environmental pollution, minimization of waste volume and production of freshwater with high recovery. To eliminate the demand for brine disposal, desalination brine can be treated using the Zero Liquid Discharge (ZLD) approach. Zero liquid discharge is defined as a synthesis of desalination technologies that aims to generate high-quality water without producing any liquid waste at all. ZLD effectively minimizes wastewater discharge and enables freshwater and salt to be recovered. ZLD can be achieved through various membrane-based and thermal-based technologies.
_
-44. Desalinated water is low in minerals and is poorly buffered. It is usually aggressive to cementitious and metallic materials used in storage, distribution and plumbing and requires conditioning to address this problem. Blending desalinated water with source water or partially treated water is a common practice, and the addition of minerals to achieve a balanced mineral content in desalinated water is increasingly being adopted. Untreated desalinated water cannot be used directly as a source of drinking water. Desalinated seawater is lacking four essential minerals that are vital to human health – calcium, magnesium, fluoride, and iodine. The minerals are removed during the desalination process, along with the salts. The potential risk for public health due to the consumption of low–mineral water hasn’t escaped the eyes of the authorities. A certain degree of remineralisation is necessary in order to make the water palatable and for re-introducing some essential ions required for health considerations. In today’s times, most RO plants put the water through a ‘post-treatment’ process whereby salts are added to make TDS around 300 mg/l. Common post-treatment are pH neuralisation and remineralisation. Desalinated water, when properly treated and mineralized, is safe for long-term consumption. However, the absence of natural minerals in desalinated water can be a concern if not properly addressed.
_
-45. Desalination is an expensive method to increase local water supply because it uses a lot of energy and has negative environmental effects. Thermal desalination requires roughly 6 times as much energy as SWRO and SWRO requires about five times as much energy as fresh water treatment and three times as much for recycled wastewater treatment. Desalinated water is frequently 2–4 times more expensive per acre-foot than other water sources. Increased regional water supplies can be achieved by water conservation, water use efficiency, storm/rain water capture, and recycling (reuse of treated wastewater), which are frequently more affordable than desalination. In addition, these alternatives provide pollution abatement, habitat restoration, and flood control benefits, which are commonly overlooked during cost/benefit assessments. Because we simply do not know exactly when it will rain and when desalination plants will be required, there is a risk of getting investment decisions wrong with the benefit of hindsight. For much of the world outside of the Middle East, desalination will be needed as a “temporary solution to getting through droughts”, in which case cheaper and more inefficient plants will be built, which will produce more expensive water.
_
-46. Desalination alone cannot solve the water crisis. But it might help as part of a broader range of efforts to cut water use and increase water supplies. Water itself is seldom the problem. The collection, storage and distribution of water is the key problem. The same problem would remain even if we desalinate everything not to mention high cost of desalination. Desalination might be affordable for drinking water, but not really for agriculture and some industrial use. Even if powered by renewable energy sources such as solar or wind, you’re still using a tremendous amount of energy, which in principle could go elsewhere to displace fossil fuel consumption. Desalination is not a panacea but it can be a complement to more traditional sources of water.
_
-47. Droughts and water shortage increase the need for desalination, but if desalination uses fossil fuels, the burning of these fuels increases GHG emissions responsible for climate change. And climate change leads to water scarcity. So, we have a vicious cycle of water scarcity to desalination to climate change to water scarcity.
Seawater desalination uses huge amounts of electricity, and the cost of this power generation is an increase in GHG emissions, which increases sea levels due to global warming and the rising sea levels will actually threaten the plants before their use-by date. Sea level rise is caused primarily by two factors related to global warming: the added water from melting ice sheets and glaciers, and the expansion of seawater as it warms.
_
-48. Recycled water means water which, as a result of extensive treatment of wastewater, is suitable for a direct beneficial use or a controlled use. It is better to recycle and reuse wastewater instead of dumping it into the ocean or other water bodies. The conventional treatment of municipal wastewater is usually based on primary treatment followed by secondary treatment, which usually involves a biological process to remove organic matter from wastewater, in order to meet the standards needed for its disposal. In order to reuse treated municipal wastewater, a tertiary treatment (e.g., membrane-based separation processes like RO) is needed to remove the remaining pollutants from secondary treated effluent, such as inorganic and organic compounds, pathogens, or nutrients, in order to meet water standards. Technologies for tertiary wastewater treatment and desalination have very much in common. However, the cost of treatment varies depending on the type of treatment and the intended final use of product water. Treated recycled wastewater is cheaper than desalinated water. Remember, wastewater has TDS of around 1000 mg/l while seawater has 35,000 mg/l, so wastewater treatment would consume less energy for RO and thereby cheaper than SWRO.
Why don’t we have more wastewater reuse projects?
It’s often because the public acceptance towards reuse is harder to obtain for city or government officials. The primary source of recycled water is municipal wastewater, and this has prompted community concerns about water quality while seawater is seen as a more pristine source. Israel leads the way with water recycling because it recycles 100 percent of its sewage water. The result of this is that Israel now has 70 percent more water to use for agriculture.
How can we drink recycled toilet water?
People do not know that Reverse Osmosis can treat wastewater to a quality higher than required for most water applications. Reverse osmosis usually achieves a water quality better than most tap or bottled waters. Indirect potable reuse (IPR) consists of blending an extensively treated wastewater with another source of fresh water, and it is already in use in few places of the world such as Singapore, Belgium, California and Australia.
_
-49. Desalination and water reuse (recycle) together:
So far, water reuse and desalination have always been considered as separate and independent streams to solving water shortage. However, wastewater reuse and seawater/blackish water desalination can be combined to support potable water needs.
Seawater/blackish water desalination plus water reuse can be a win-win fix to our water cycle:
Formula: Desalinate once, reuse treated wastewater, plus use treated wastewater as feedwater for desalination plant.
Desalination can be a sustainable way to replenish our water cycle: after primary uses (industrial or domestic), reuse of desalinated water for irrigation enables agriculture in otherwise unproductive regions, and/or forest growth. The ensuing evapotranspiration feeds the water cycle, further enhancing precipitation, while enabling carbon sequestration by plants. In this way, desalination may not only reduce freshwater abstraction, but also provide a net water surplus, and thus help preserve and restore freshwater-dependent ecosystems.
Also, treated wastewater can be taken as feed water for desalination plant instead of seawater.
The negative effects of desalination can be effectively controlled by using renewable energy so as to become carbon-neutral. Brine disposal affects marine ecosystems only at local scale, and appropriate design of outfalls can minimize impacts through dilution. Considering the environmental costs of over-abstraction of freshwater, desalination tilts the balance in its favour.
Closing the water cycle by desalination and wastewater reuse promises to provide virtually unlimited volumes of freshwater.
Although wastewater reuse is materially and infrastructurally much more heterogeneous that desalination, both processes often use similar membrane technologies, which are often delivered by the same global water companies. Also, it’s much more practical and sustainable to desalinate less-salty brackish water and use the technology to recycle wastewater.
______
Dr. Rajiv Desai. MD.
May 7, 2024
______
Postscript:
There is delay in publishing this article due to mental torture caused by daily sting operations conducted against me by Indian regime and media. Their priorities are misplaced as rapidly rising water scarcity is a reality in India and is expected to worsen in the coming years.
______
______


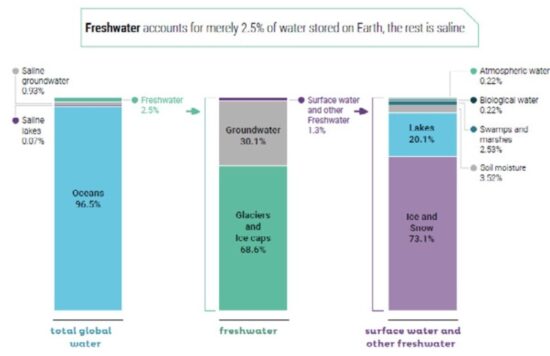
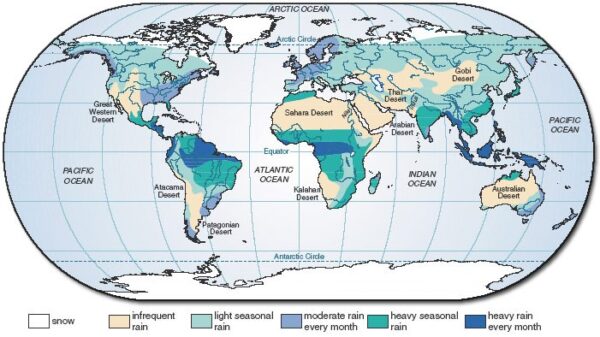
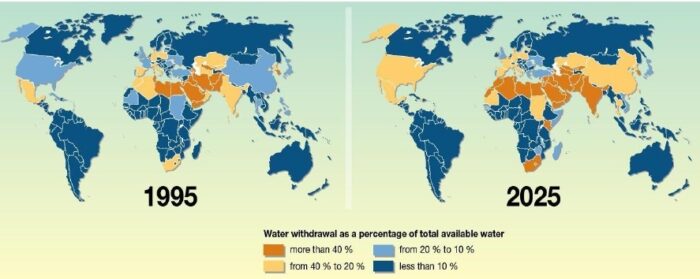
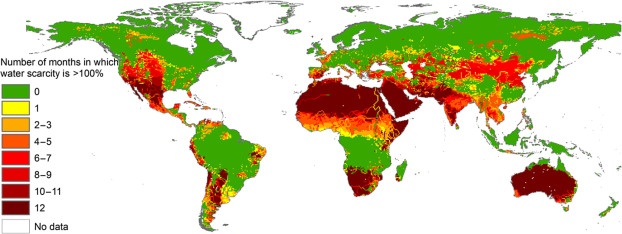
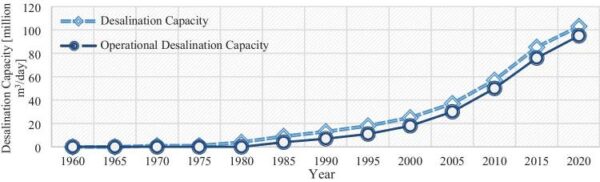
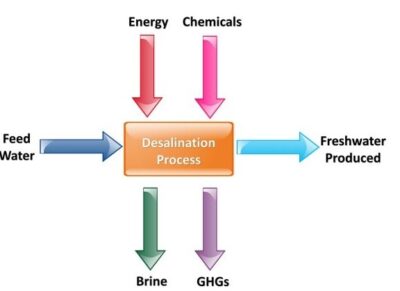
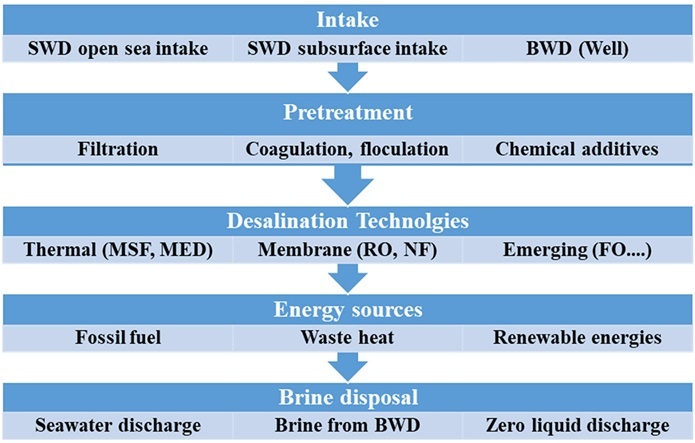
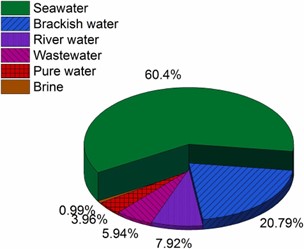
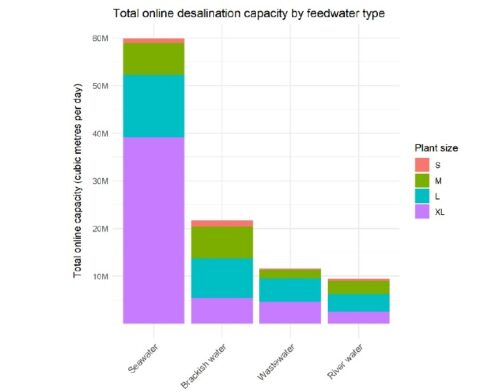
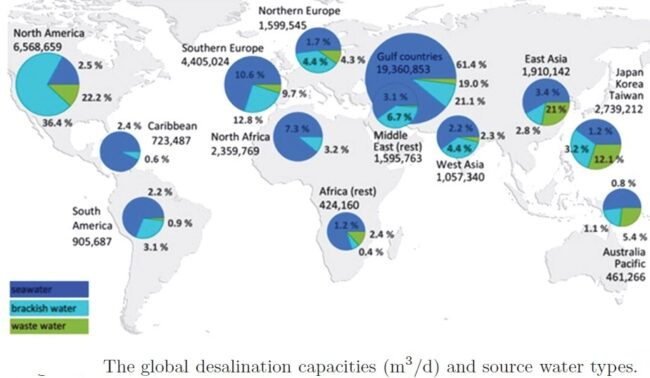
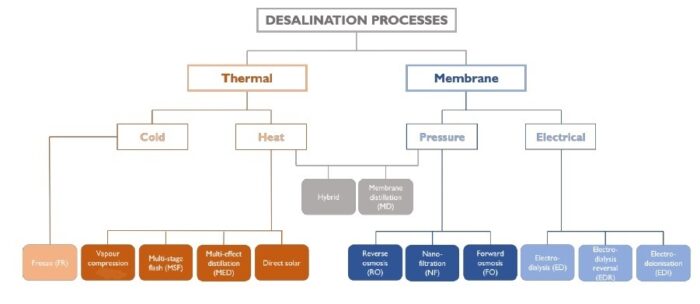
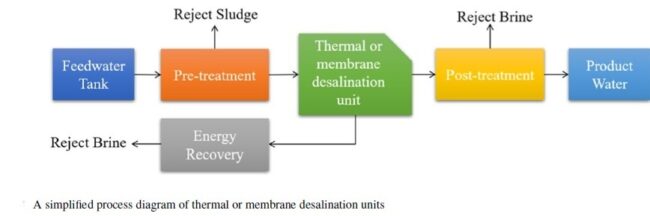
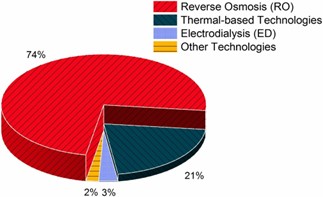
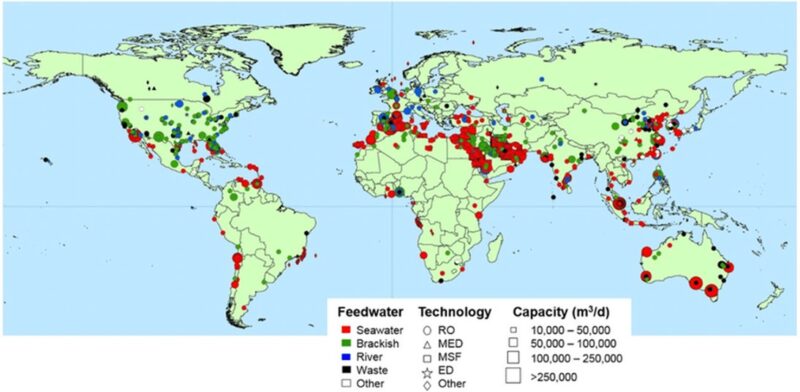
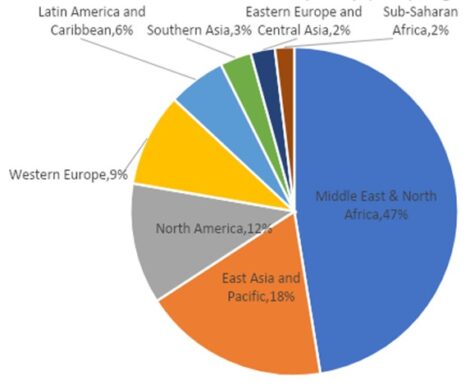
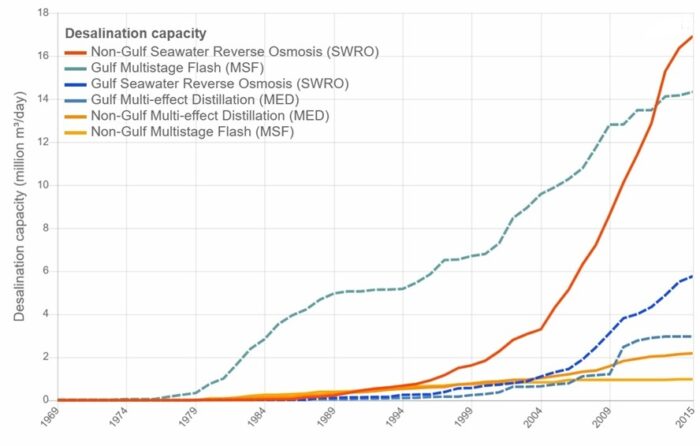
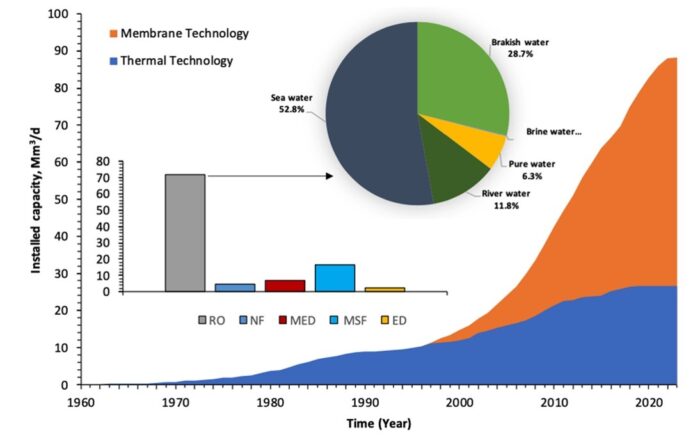
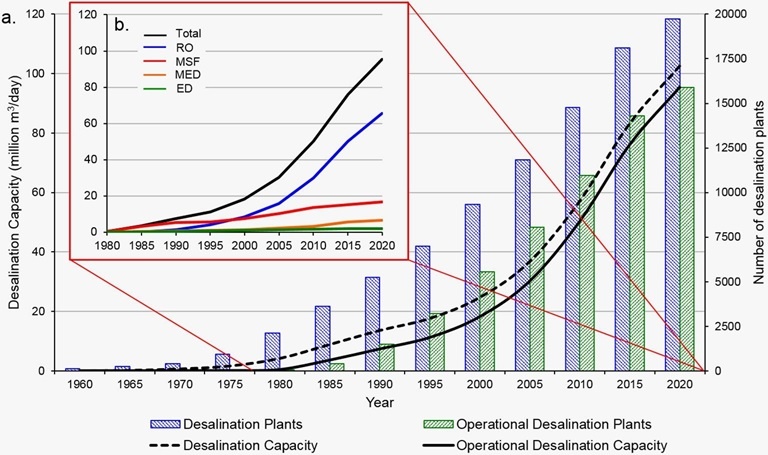
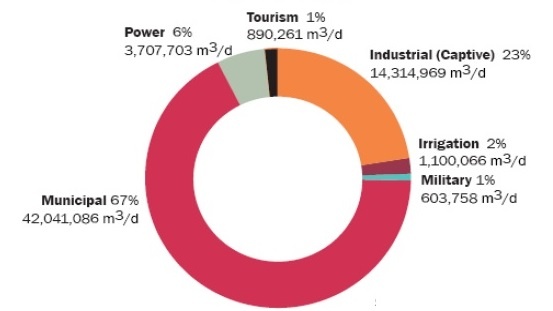
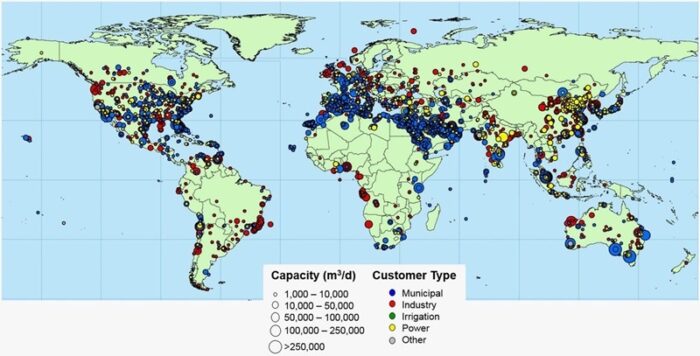
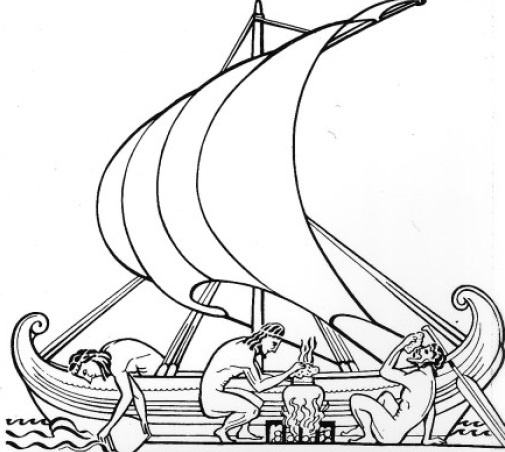
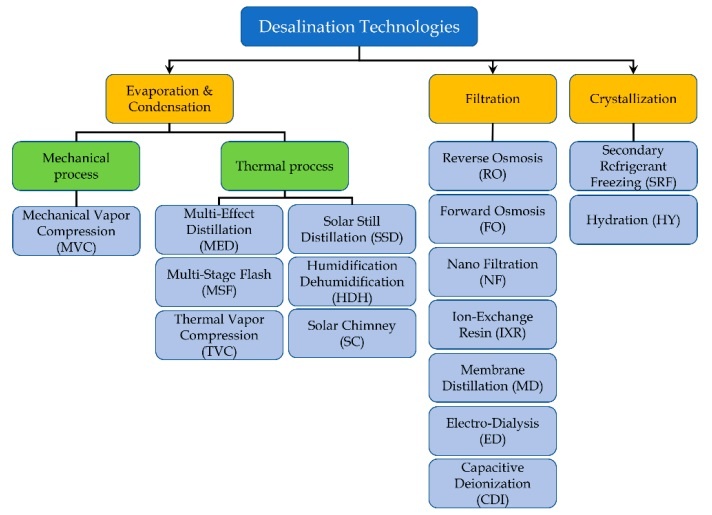
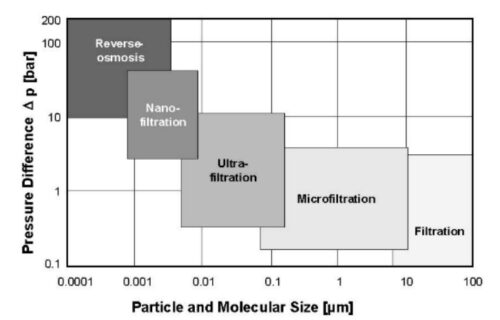
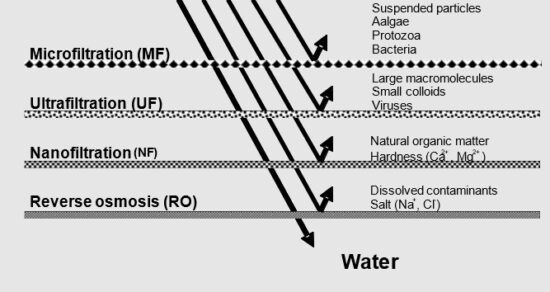
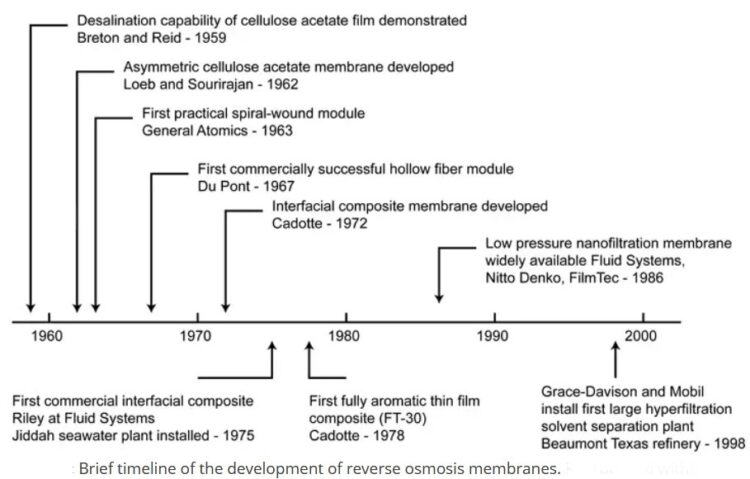
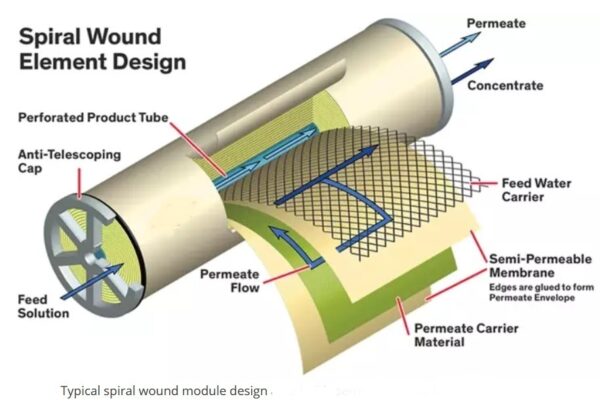
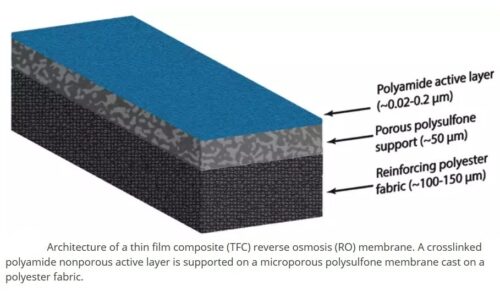
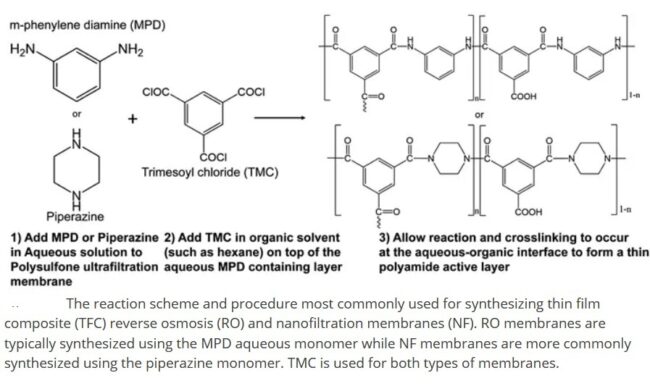
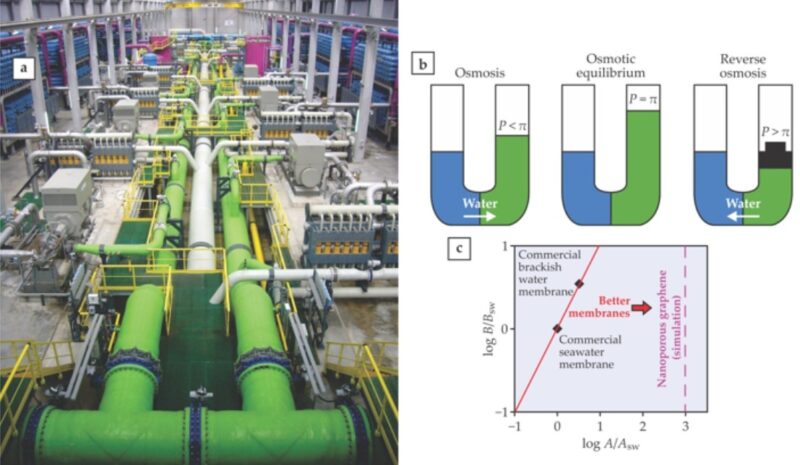
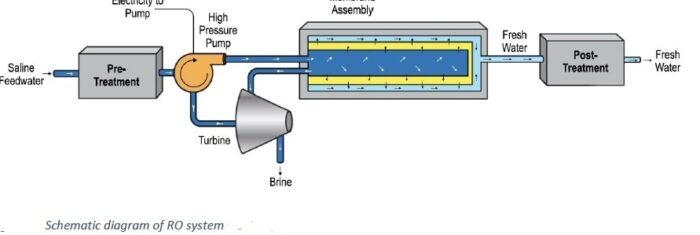
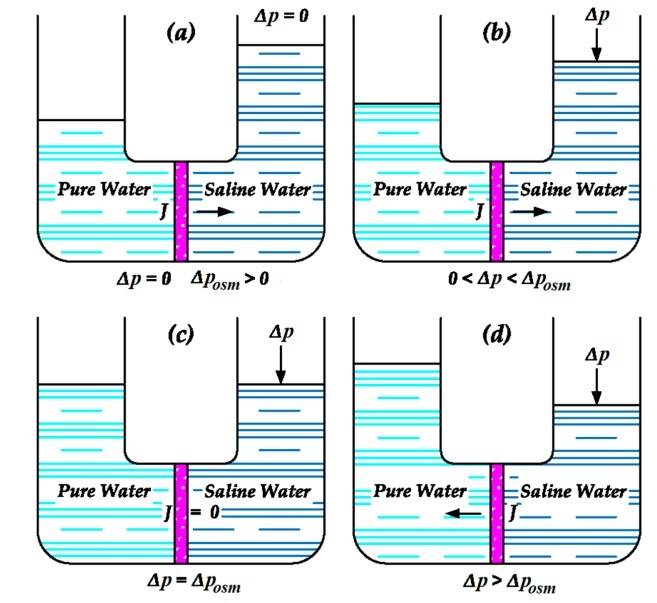
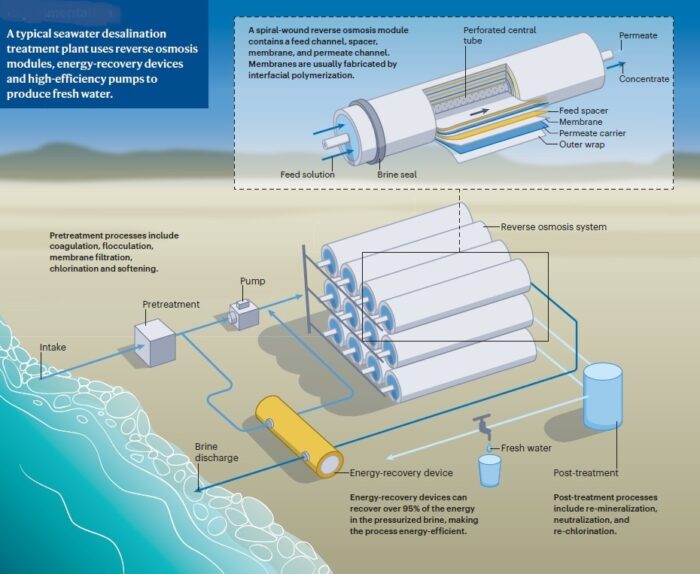
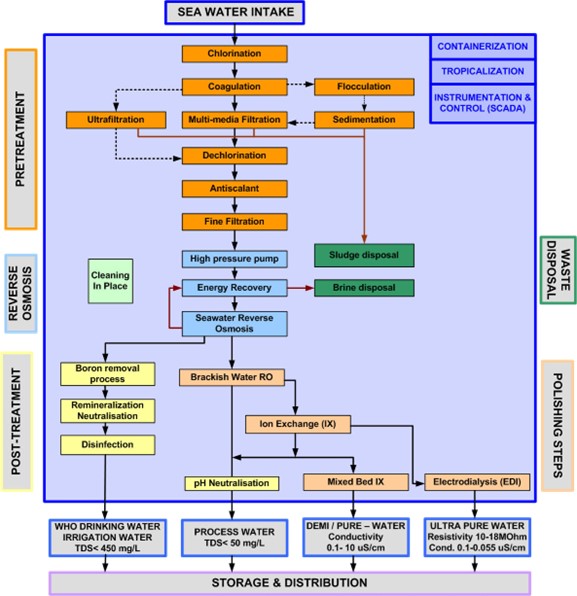
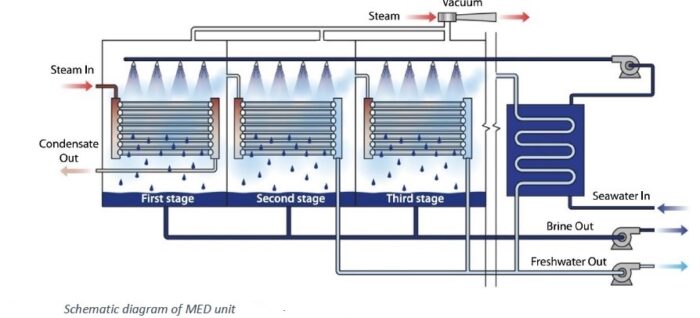
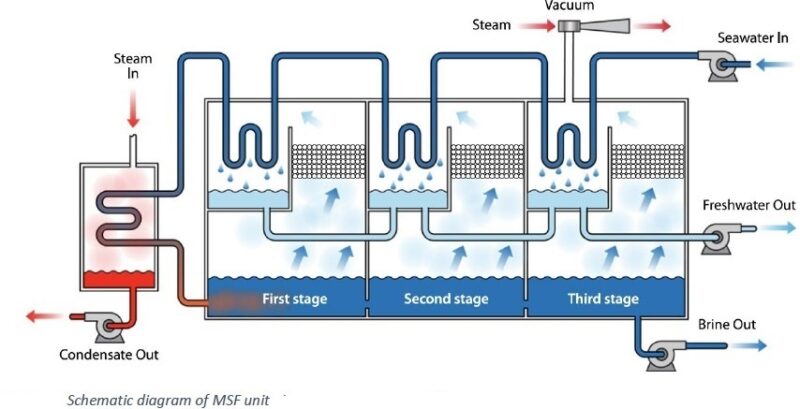
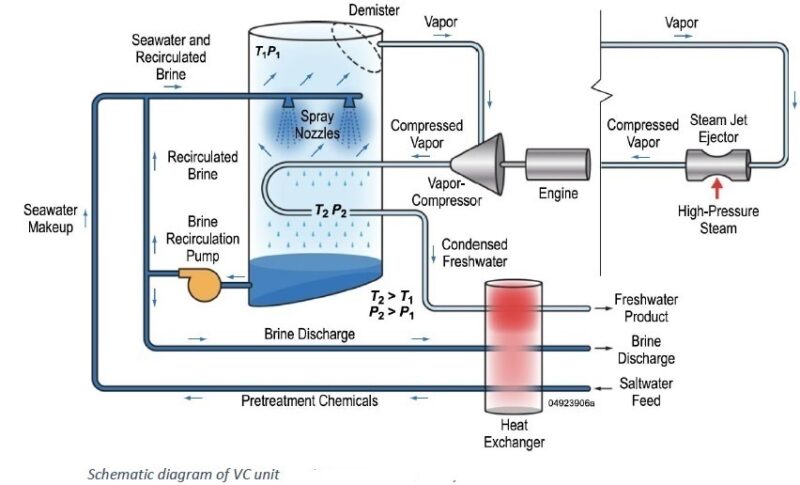
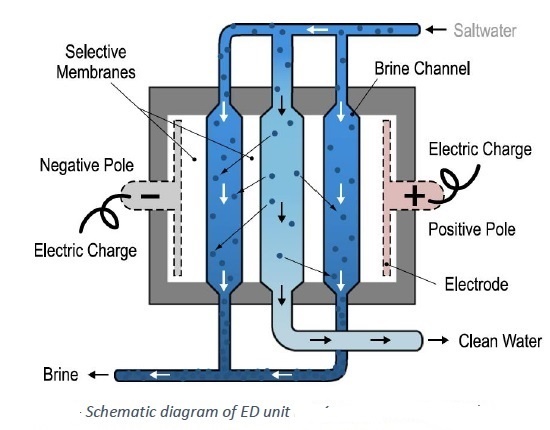
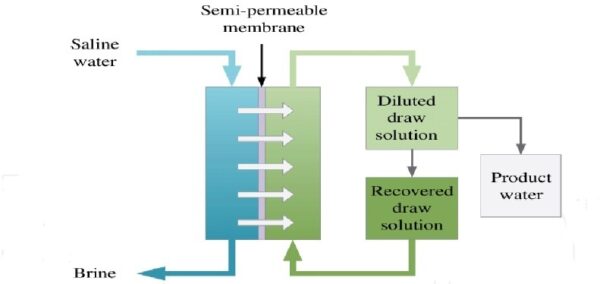
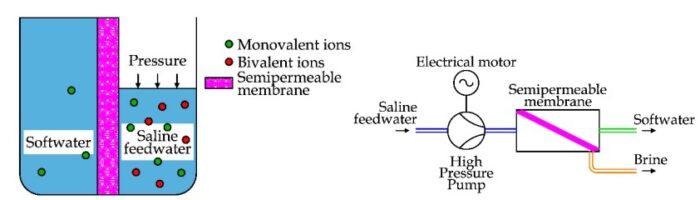
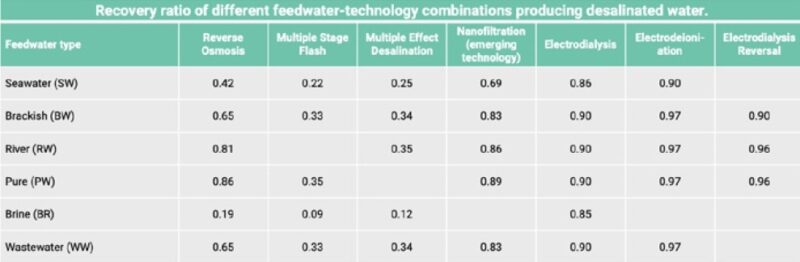
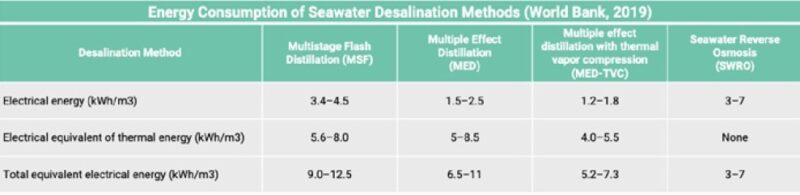
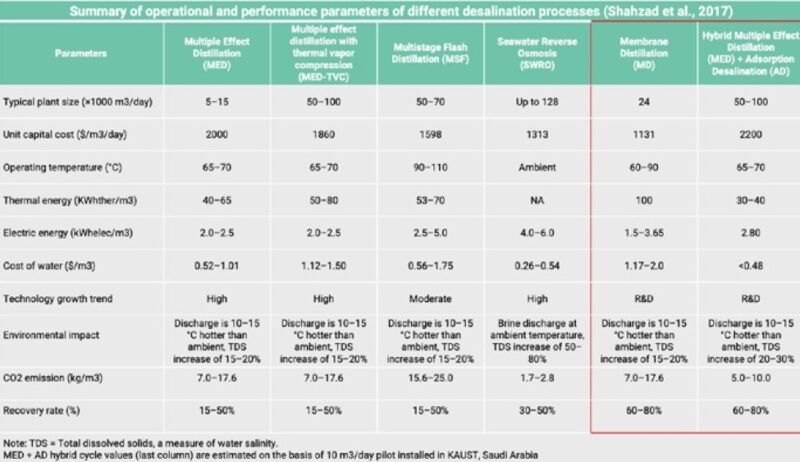
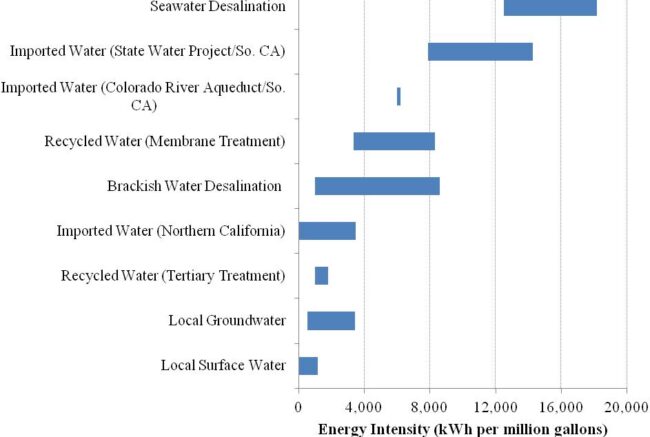
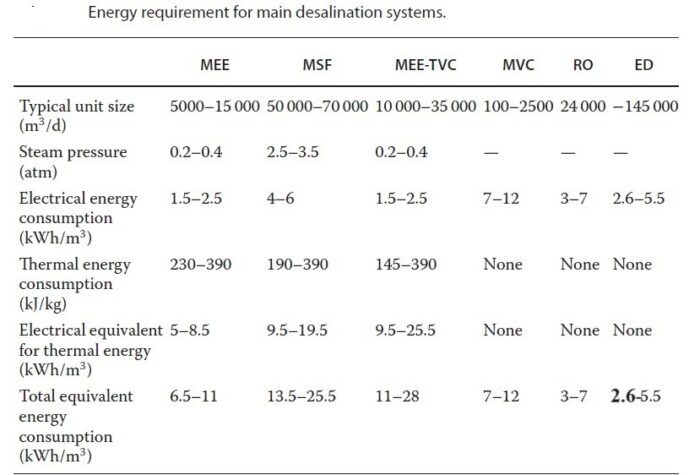
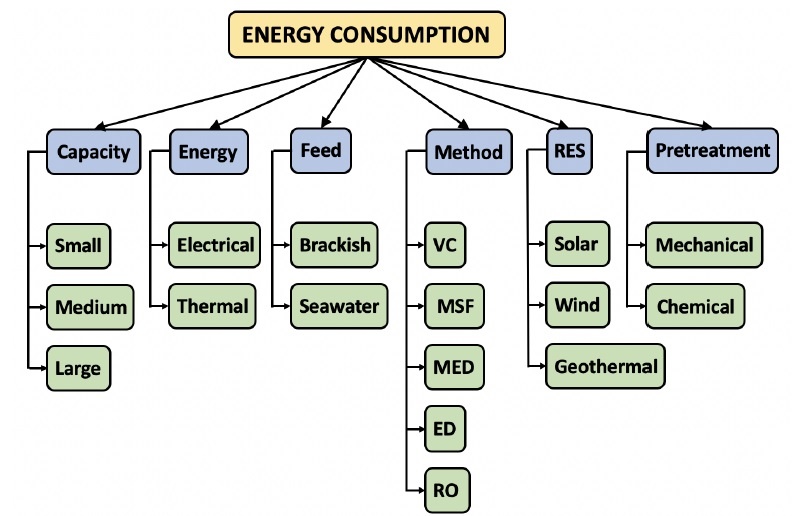
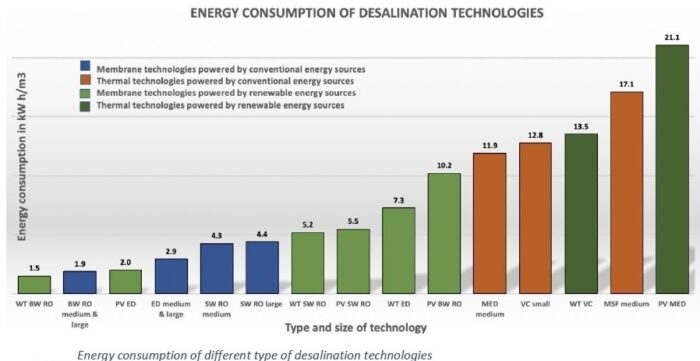
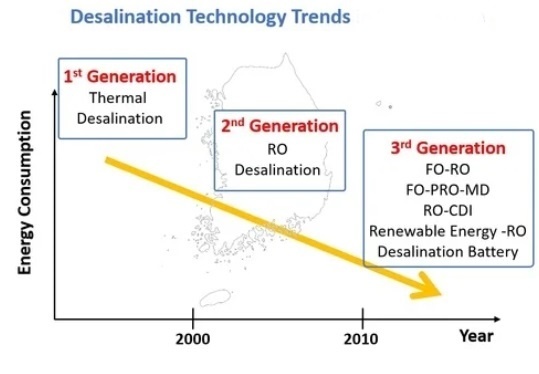
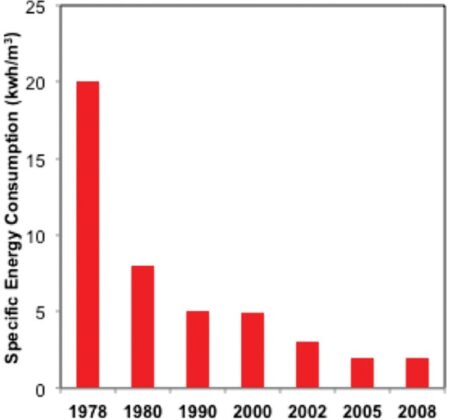

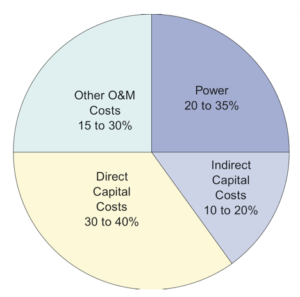
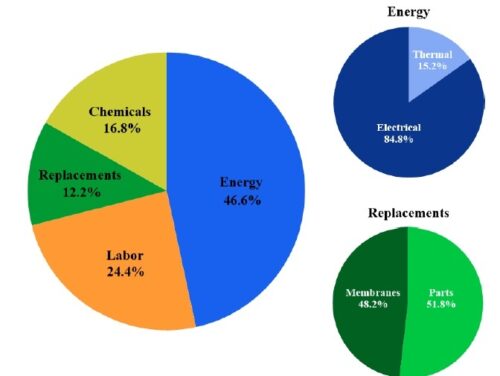
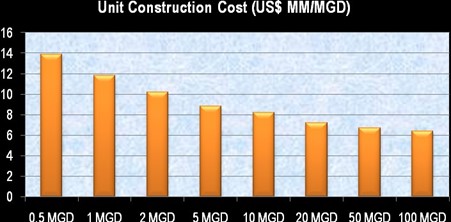
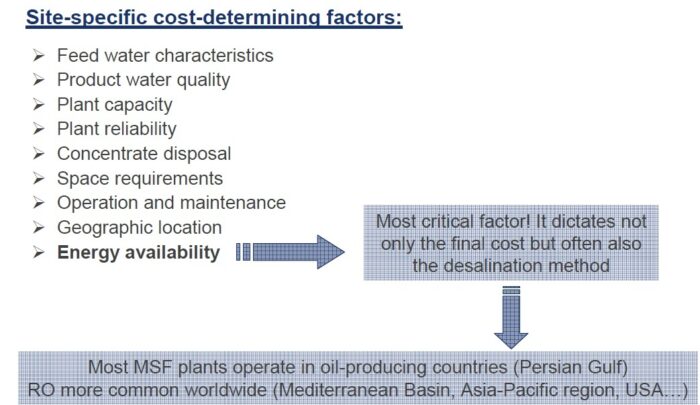
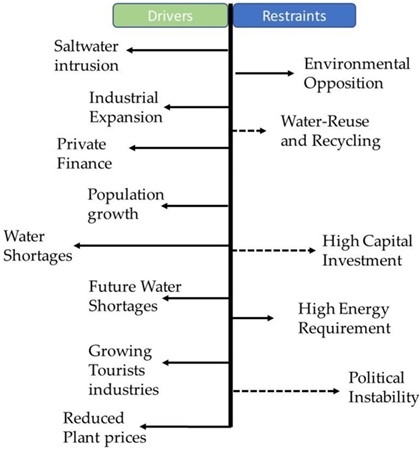
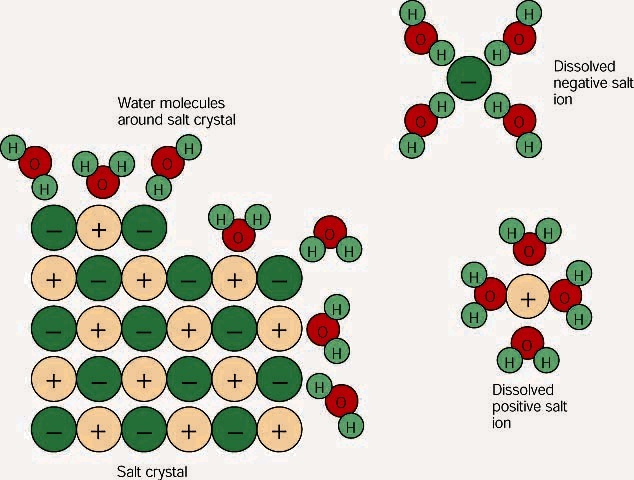
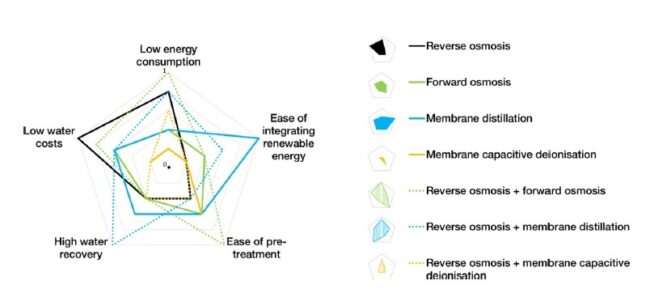
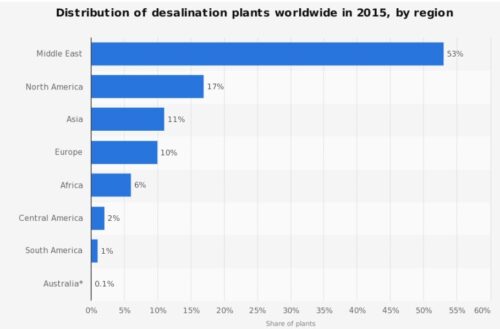

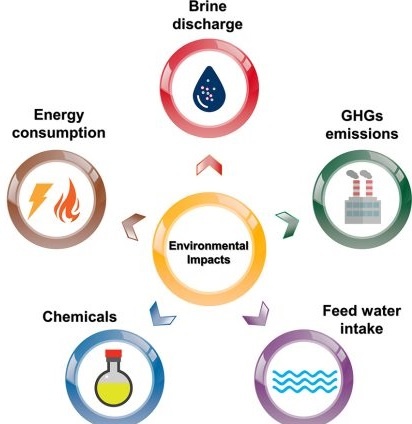
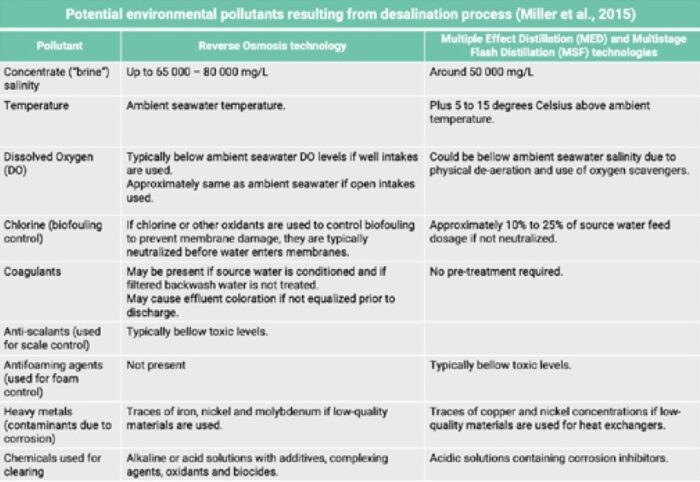
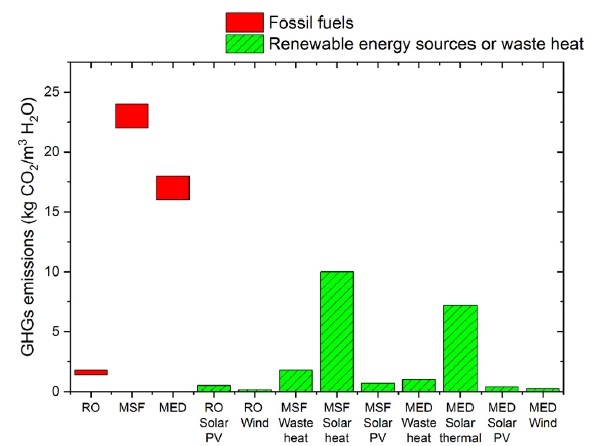
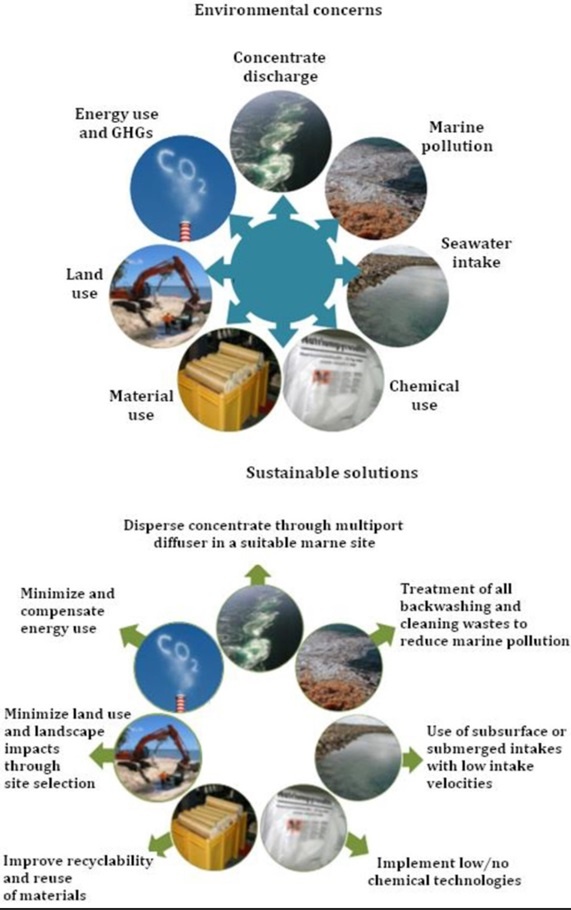
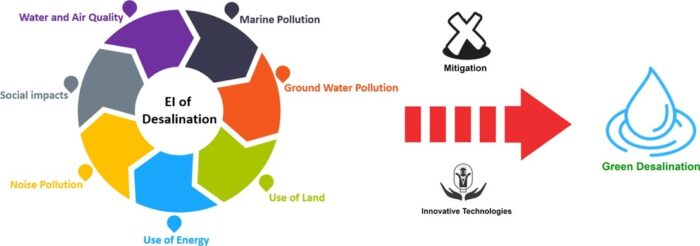
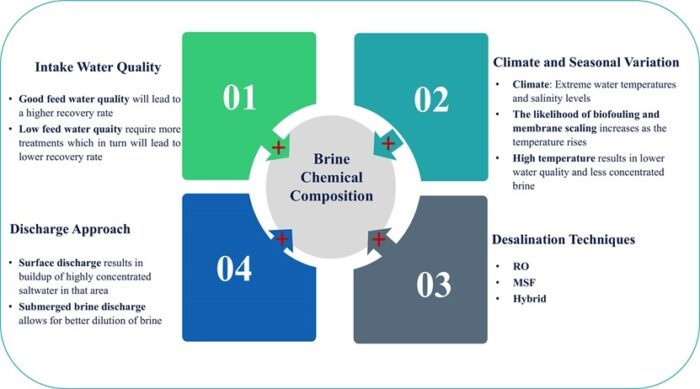
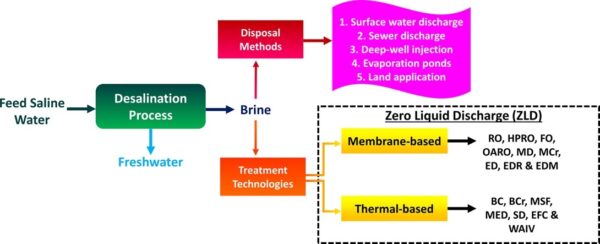
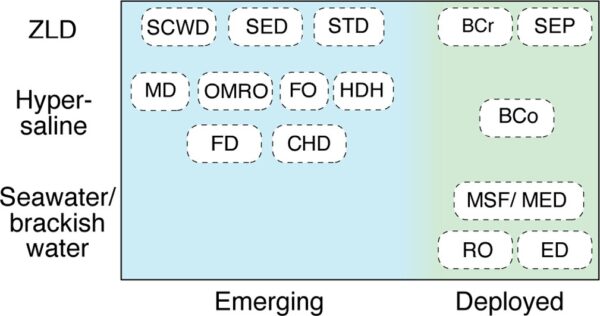
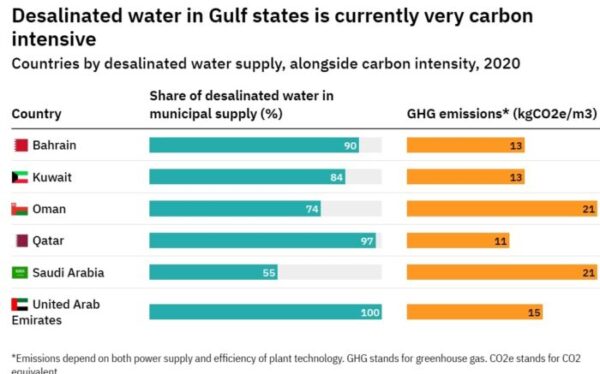
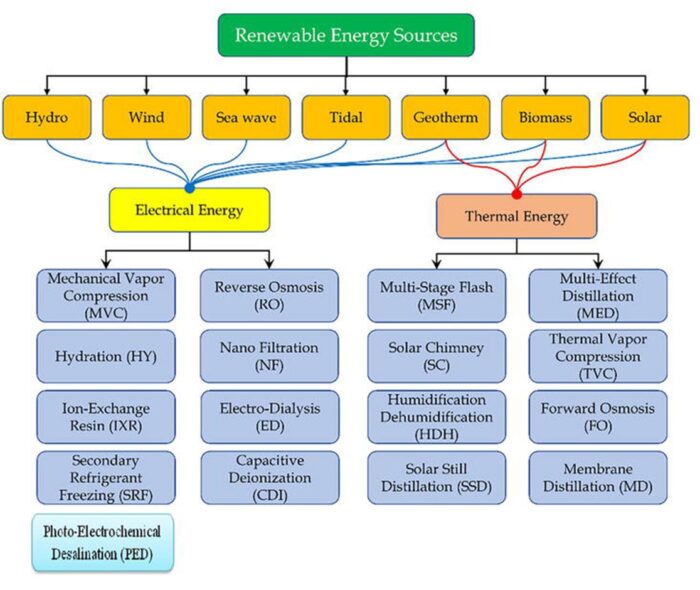
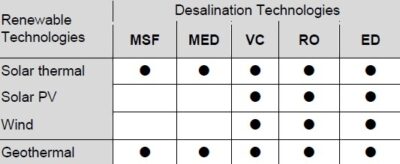
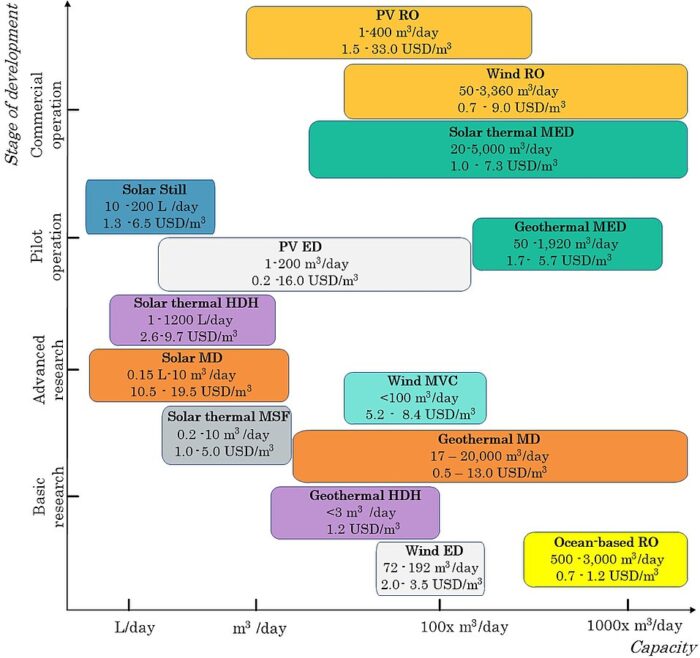
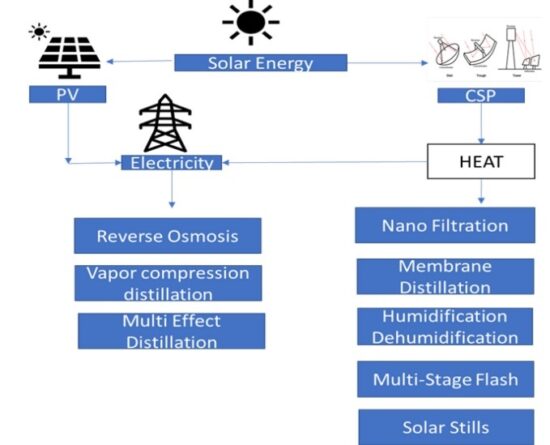
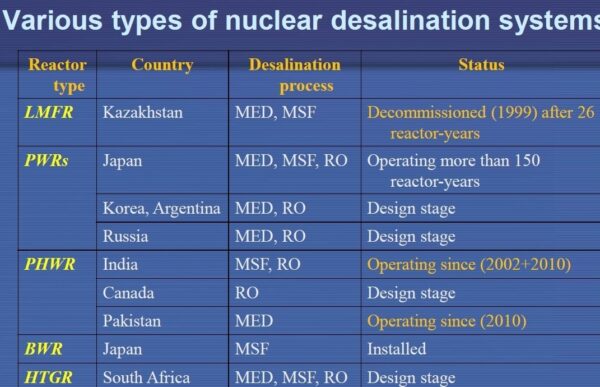
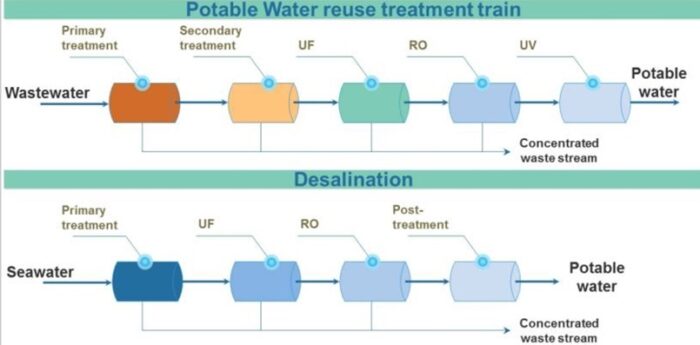
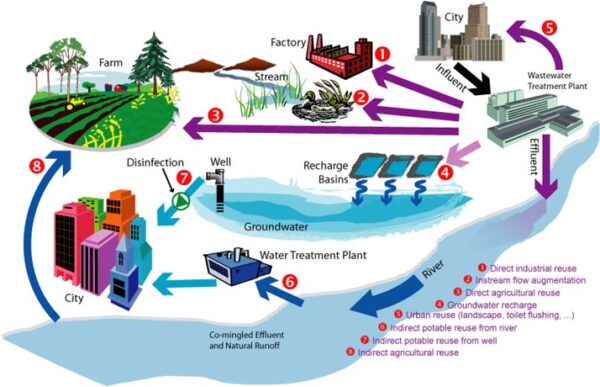
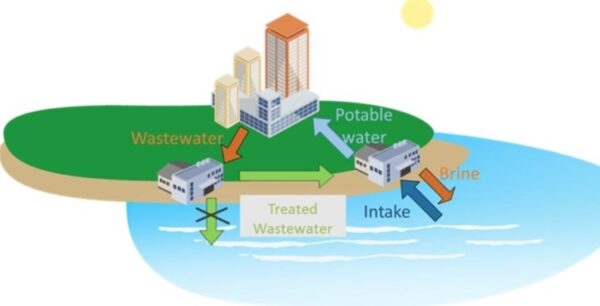
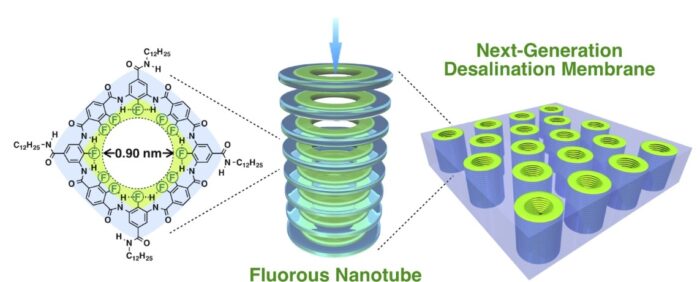
I am curious to find out what blog platform you have been using? I’m experiencing some small security problems with my latest blog and I’d like to find something more secure. Do you have any recommendations?
You can definitely see your expertise within the paintings you write. The arena hopes for even more passionate writers such as you who are not afraid to mention how they believe. All the time go after your heart.
Nice i really enjoyed reading your blogs. Keep on posting. Thanks
Стильные советы по созданию стильных луков на любой день.
Мнения стилистов, новости, все коллекции и шоу.
https://pitersk.ru/articles/2024-09-10-7-veshchey-v-kotoryh-demna-gvasaliya-ne-imeet-ravnyh/