Dr Rajiv Desai
An Educational Blog
HYDROGEN
Hydrogen:
_
_
Section-1
Prologue:
Primary energy sources include fossil fuels (petroleum, natural gas, and coal), nuclear energy, and renewable sources of energy. Energy carriers include electricity and heat as well as solid, liquid and gaseous fuels. They occupy intermediate steps in the energy-supply chain between primary sources and end-use applications. Energy carriers allow the transport of energy in a usable form from one place to another. Hydrogen is an energy carrier, not an energy source and can deliver or store a tremendous amount of energy. Hydrogen is the chemical element with the symbol H and atomic number 1. H is in the upper left corner of the Periodic Table. Hydrogen is the most basic chemical element — just one proton and one electron — and makes up nearly three-quarters of the mass in the universe. Stars such as the Sun are mainly composed of hydrogen in the plasma state. A molecule of hydrogen consists of two protons and two electrons held together by electrostatic forces with the molecular formula H2. Most of the hydrogen on Earth exists in forms such as water and organic compounds.
When Ahmed Sheikh Yamani, Saudi oil minister during the oil shocks of the 1970s and 80s, famously said that “the Stone Age did not end for lack of stone, and the oil age will end long before the world runs out of oil”, he was not thinking of renewable energy and electric vehicles, he was thinking of hydrogen. Hydrogen is a fascinating energy carrier. It can be produced from electricity and water. Its conversion to heat or power is simple and clean. When combusted with oxygen, hydrogen forms water. No pollutants are generated or emitted. The water is returned to nature where it originally came from. But hydrogen, the most common chemical element on the planet, does not exist in nature in its pure form. It has to be separated from chemical compounds, by electrolysis from water or by chemical processes from hydrocarbons or other hydrogen carriers. The electricity for the electrolysis may eventually come from clean renewable sources such as solar radiation, kinetic energy of wind and water or geothermal heat. Therefore, green hydrogen may become an important link between renewable energy and chemical energy carriers. Jules Verne told us about green hydrogen more than 125 years ago in his novel The Mysterious Island: “What are they going to burn instead of coal?… Water. Water broken down into its elements by electricity will one day be used as fuel.” In electrolysis, we put in electricity and water and we get out hydrogen and oxygen. In a fuel cell, we put in hydrogen and air (oxygen), and we get out electricity and water.
Despite a 5.8% drop in global carbon emissions in 2020 due to the COVID-19 pandemic, emissions are still at critical levels. Global CO2 emissions were 31.5Gt – an increase of 54% since 1990. With that in mind, the quest to find a viable alternative to carbon-based fuel and energy production processes is continually accelerating. We must remember that doing nothing is not an option. We cannot continue to burn fossil fuels and meet climate change obligations, it’s impossible. From passenger vehicles to domestic energy, those at the forefront of alternative fuel and energy storage technology are exploring options. Today, the world produces 75 million tons of hydrogen each year. Nearly all of the hydrogen is used by industry for refining petroleum, treating metals, producing fertilizer, and processing foods. Most of it is generated from fossil fuels, mainly natural gas and coal. This accounts for 6% of the global natural gas consumption, 2% of the global coal consumption, and results in 830 million tons of carbon dioxide being released every year – almost as much as Germany’s annual CO2 emissions. There are three main types of hydrogen discussed today. First, ‘grey’ hydrogen. The vast majority of hydrogen in use — and there is plenty of it, mainly in industry — is made from natural gas and coal. The process emits CO2. ‘Grey’ hydrogen becomes ‘blue’ hydrogen when the CO2 given out during its production is locked up through carbon capture and storage (CCS) processes. But while the CO2 output is lowered, this process is quite expensive. ‘Grey’ and ‘blue’ hydrogen, thus, are both produced by the same processes, the only difference for ‘blue’ hydrogen being that the CO2 produced is sequestered. Finally, ‘green’ or ‘renewable’ hydrogen — which every hydrogen advocate says is the ultimate goal — is made from the electrolysis of water powered by renewables and it will help to substantially reduce emissions. Production cost of grey hydrogen is $1 per kilogram and blue is $2 per kilogram at the cheapest. Green hydrogen costs upwards of $4 per kilogram.
Legend has it that when Henry Ford was asked if he developed the Model T in response to customer demands, he famously responded: “If I had asked people what they wanted, they would have said faster horses.” This quote has been used for 100 years to describe the paradox between what customers perceive they want based on their own life experiences versus what could be possible based on current science and technology. Today’s energy transformation is caught in a similar paradox. The “faster horses” offered by cheap natural gas and oil need to be replaced with technology that can minimize CO2 emissions and mitigate climate change. Hydrogen technologies offer the potential to change the way we produce, store, and use energy by enabling more widespread use of carbon free energy sources.
A new star has exploded back onto the climate scene: hydrogen. It offers possibilities to move away from fossil fuels, but it brings its own challenges. For climate experts, green or renewable hydrogen — made from the electrolysis of water powered by solar or wind — is indispensable to climate neutrality. It features in all eight of the European Commission’s net zero emissions scenarios for 2050. In theory, it can do three things: store surplus renewables power when the grid cannot absorb it, help decarbonize hard-to-electrify sectors such as long-distance transport and heavy industry, and replace fossil fuels as a zero-carbon feedstock in chemicals and fuel production. The International Energy Agency lauded its “vast potential” in a first ever report on hydrogen in June 2019. Bloomberg New Energy Finance said clean hydrogen “can help address the toughest third of global greenhouse gas emissions by 2050” in March 2020. Net-zero requires a full fossil fuel phase-out. It puts the spotlight on gas for the first time. And the gas industry is turning to hydrogen for a new lease of life. Yet the climate community is cautious. The risk is that the hydrogen hype triggers a reversal of priorities. Energy efficiency, renewables, nuclear, switch from coal to natural gas and direct electrification are the bulk solutions to climate change. Moreover, the climate impact of hydrogen depends entirely on how it is made. Hydrogen is not a technology; it is an energy carrier that can be produced clean or dirty.
With global warming and climate change becoming an ever-increasing issue, sustainable power is growing in demand. In the last decade, electric vehicles have had a surge in popularity, but could hydrogen be a better option? Elon Musk, the CEO of electric car company Tesla stated that hydrogen cars are ‘mind-bogglingly stupid’. Hydrogen’s impact in the car market may indeed remain modest due to efficiency and economic problems. However, ongoing improvements along with advantages of abundance, low emissions and high energy density mean that in the new market of sustainable larger vehicles hydrogen power will soon gain significant market share. The hydrogen economy could be all-encompassing, or it could fill a series of niches, depending on hydrogen availability, cost and performance relative to alternatives, for each potential application. Are you for or against hydrogen? That seems to be the wrong question. The correct question is: where do you really need to use it?
_______
Abbreviations and synonyms:
°C = degree Celsius
K = kelvin, the SI base unit of temperature
EJ = exajoule
Gt/yr = gigatons per year
GW = gigawatt
h = hour
kg = kilogram
kW = kilowatt
kWh = kilowatt hour = unit of electricity
One tonne of oil equivalent (toe) = 41.868 GJ
MJ = megajoule = 106 Joule
GJ = gigajoule= 109 Joule = 0.95 million Btu
1 kWh = 3.6 megajoules = 3412.14 Btu
Btu = British thermal unit = the amount of energy required to heat one pound of water by one degree Fahrenheit
1 Btu = 1055.055853 Joule
MPa = megapascal
MW = megawatt
Nm3 = normal cubic meter (under normal or standard conditions of temperature & pressure)
TWh = terawatt hour
ALK = alkaline
BEV = battery electric vehicle
CAPEX = capital expenditure
CCS = carbon capture and storage
CCU = carbon capture and utilisation
COP21 = 21st Conference of the Parties to UN Framework Convention on Climate Change
CO₂ = CO2 = carbon dioxide
CSP = concentrated solar power
DRI-H = direct reduction via hydrogen
e-fuel = electrofuel
FCEV = fuel cell electric vehicle
HHV = high heating value
HRS = hydrogen refuelling station
H₂ = H2 = hydrogen
LH2 = liquid hydrogen
LDV = light-duty vehicle
LCOH = levelised cost of hydrogen
LHV = lower heating value
LOHC = liquid organic hydrogen carrier
OPEX = operating expenditure
PEM = proton exchange membrane
PV = photovoltaic
P2G = power-to-gas
P2X = power-to-x
P2P = power-to-power
SMR = steam-methane reforming
SOEC = solid oxide electrolyser cell
VRE – variable renewable energy
FC = fuel cell
gal = gallon
ICE = internal combustion engine
IHIG = International Hydrogen Infrastructure Group
Psi = pounds per square inch
CNG = Compressed Natural Gas
HCNG = Hydrogen and CNG blend
CO = Carbon monoxide
H2O = Water
IRENA = International Renewable Energy Agency
LCA = Life cycle assessment
LPG = Liquefied petroleum gas
MCFC = Molten carbonate fuel cell
PEMEL = Proton exchange membrane electrolyser
PEMFC = Proton exchange membrane fuel cell
AEL = alkaline electrolyzer
TOC = total cost of ownership
One bar = 0.987 atm = 14.50 psi = 100,000 pascals (N/m2) = 0.1 MPa = 750.06 mmHg pressure
CHP = Combined heat and power
CHP is concurrent production of electricity or mechanical power and useful thermal energy (heating and/or cooling) from a single source of energy. It is type of distributed generation, which, unlike central station generation, is located at or near the point of consumption. CHP technology is often referred to as cogeneration, but there are important differences. Cogeneration is the process where a simple cycle gas turbine produces electricity and steam—as well as the steam that is used in other processes, such as drying. However, the steam is not used to drive a steam turbine. CHP combined-cycle power plants can deliver concurrent production of electricity and useful thermal energy from a common fuel. The captured thermal energy (steam or hot water) can be used for processes like heating and cooling, and to generate power for other industrial purposes. CHP applications can operate at about 75% efficiency, a significant improvement over the national average of about 50% for these services when provided separately.
Feedstock:
Feedstock is raw material used for processing or manufacturing another product. As feedstock, the term connotes it is a bottleneck asset critical to the production of other products. A feedstock is also defined as any renewable, biological material that can be used directly as a fuel, or converted to another form of fuel or energy product. Hydrogen is a feedstock for the production of ammonia, various plastics, refining, synthetic fuels, stationary power and mobile power.
______
Heat Values of Various Fuels:
The heat value of a fuel is the amount of heat released during its combustion. Also referred to as energy or calorific value, heat value is a measure of a fuel’s energy density, and is expressed in energy (joules) per specified amount (e.g., kilograms).
HHV and LHV:
HHV (High Heating Value) and LHV (Low Heating Value) are engineering terms used since the 19th century.
All biomass fuels, such as wood, straw, charcoal and others, contain carbon, hydrogen and oxygen, which are described in this chemical formula CxHyOz. In a complete combustion, organic fuels react with oxygen molecules in the atmosphere to form two products: carbon dioxide CO2and water H2O and to release heat. This heat released is called heat of combustion. Some of the heat released are used to vaporize the existing moisture in the fuel and the water product. Higher heating value (HHV) is calculated with the product of water being in liquid form while lower heating value (LHV) is calculated with the product of water being in vapor form. LHV number usually is more realistic. The chart below compares HHV and LHV values for several fuels. Notice that the difference is greater for hydrogen. Any device that makes hydrogen will look worse if LHV numbers are used to calculate efficiency. The error lies in mixing LHV and HHV numbers inconsistently. In this article, LHV of hydrogen 120MJ/kg is used frequently.
|
|
HHV |
LHV |
Difference |
|
Hydrogen |
142 MJ/kg |
120 MJ/kg |
18% |
|
Natural Gas |
38 MJ/kg |
35 MJ/kg |
8% |
|
Gasoline |
35 MJ/kg |
32 MJ/kg |
11% |
_______
Units for Hydrogen Production and Use:
Hydrogen production capacity is usually given in units of standard cubic feet (scf) produced per day, normal cubic meters (Nm3) per day, gigajoules per day, or kilowatts of hydrogen output (on a continuous basis). Specific capital costs for production plants are expressed as dollars ($) per kilowatt of hydrogen output capacity. All energy and power units are usually based on the higher heating value (HHV) of hydrogen. Hydrogen storage capacity is given in volume units (scf or Nm3), in tons, or in energy stored (gigajoules). Capital costs for storage are given in $ per ton of hydrogen stored or $ per gigajoule stored.
Because hydrogen is delivered in a compressed gaseous form, volume measurement would not be suitable due to the dependency on pressure and temperature of the gas. So hydrogen is measured in kilograms. A 100% efficient electrolyser requires 39 kWh of electricity to produce 1 kg of hydrogen. The devices today require as much as 50 kWh/kg. So, if electricity costs are 0.05 US$/kWh, the power cost for the electrolysis process alone is 2.50 US$/kg of hydrogen.
A kilogram of hydrogen contains 141.8 megajoules of energy. In electrical terms, the energy density of hydrogen is equal to 33.6 kWh of usable energy per kg, versus diesel which only holds about 12–14 kWh per kg. What this really means is that 1 kg of hydrogen, used in a fuel cell to power an electric motor, contains approximately the same energy as a gallon of diesel.
_____
Electricity units generated by power plants:
When you see a 60 watt light bulb in your house, it means that the light bulb draws electricity from the grid at a rate of 60 Watts, or 60 Joules per second.
One electricity unit = 1 kilowatts generated for 1 hour = 1 kwh = 3.6 megajoules
A 60 watt bulb will draw 1 unit electricity in 16.6 hour.
300 MW power station will produce 300 million watts per second; that is 300,000 kilowatts per second; that is 300,000 kilowatt hour every hour; that is 300,000 units of electricity generated every hour; that is 7.2 million units of electricity per day. So a 300 MW power station will generate 7.2 million units of electricity in 24 hours provided it is 100 % efficient. In other words, 300 MW power station will produce 300 MWh electricity every hour.
_______
_______
Section-2
Hydrogen basics:
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula H2. Hydrogen is found naturally in the molecular H2 form. At room temperature and under normal pressure, hydrogen is a colorless, odourless and non-poisonous gas which is lighter than air and helium. In the environment H2 can be freely found in volcanic gasses, but its lightness allows it to escape from the earth’s atmosphere. Hydrogen burns with a pale blue, almost invisible flame. At temperatures under –253 ºC hydrogen is in a liquid state. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H, named Protium) each atom has one proton, one electron, and no neutrons.
In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 years later during the recombination epoch, when the plasma had cooled enough for electrons to remain bound to protons.
_
Hydrogen is an essential for life, the universe and just about everything. Life, in fact, is multiply dependent on it. Without hydrogen we wouldn’t have the Sun to give us heat and light. There would be no useful organic compounds to form the building blocks of life. And that most essential substance for life’s existence, water, would not exist. It’s only thanks to a special trick of hydrogen’s that we can use water at all. Hydrogen forms weak bonds between molecules, latching onto adjacent oxygen, nitrogen or fluorine atoms. It’s these hydrogen bonds that give water many of its properties. If they didn’t exist, the boiling point of water would be below -70 degrees Celsius. Liquid water would not feature on the Earth.
_
Hydrogen is nonmetallic, except at extremely high pressures, and readily forms a single covalent bond with most nonmetallic elements, forming compounds such as water and nearly all organic compounds. Hydrogen plays a particularly important role in acid–base reactions because these reactions usually involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) where it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H+. The H+ cation is simply a proton (symbol p) but its behavior in aqueous solutions and in ionic compounds involves screening of its electric charge by nearby polar molecules or anions. Because hydrogen is the only neutral atom for which the Schrödinger equation can be solved analytically, the study of its energetics and chemical bonding has played a key role in the development of quantum mechanics.
_
Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. In 1766–81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means “water-former”.
Industrial production is mainly from steam reforming natural gas, and less often from more energy-intensive methods such as the electrolysis of water. Most hydrogen is used near the site of its production, the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market. Hydrogen is problematic in metallurgy because it can embrittle many metals, complicating the design of pipelines and storage tanks.
Hydrogen is the only element that can exist without neutrons. Hydrogen’s most abundant isotope has no neutrons. Hydrogen forms both positive and negative ions. It does this more readily than any other element. Hydrogen is the only atom for which the Schrödinger equation has an exact solution. Liquid hydrogen has the lowest density of any liquid. Solid, crystalline hydrogen has the lowest density of any crystalline solid. Because hydrogen is so light, the pure element isn’t commonly found on the Earth. It would just float away. The prime components of air, nitrogen and oxygen, are fourteen and sixteen times heavier, giving hydrogen dramatic buoyancy. This lightness of hydrogen made it a natural for one of its first practical uses – filling balloons. No balloon soars as well as a hydrogen balloon.
The first such aerial vessel was the creation of French scientist Jacques Charles in 1783, who was inspired by the Montgolfier brothers’ hot air success a couple of months before to use hydrogen in a balloon of silk impregnated with rubber. Hydrogen seemed to have a guaranteed future in flying machines, reinforced by the invention of airships built on a rigid frame, called dirigibles in the UK but better known by their German nickname of Zeppelins, after their enthusiastic promoter Graf Ferdinand von Zeppelin.
These airships were soon the liners of the sky, carrying passengers safely and smoothly across the Atlantic. But despite the ultimate lightness of hydrogen it has another property that killed off airships – hydrogen is highly flammable. The destruction of the vast zeppelin the Hindenburg, probably by fire caused by static electricity, was seen on film by shocked audiences around the world. The hydrogen airship was doomed.
Yet hydrogen has remained a player in the field of transport because of the raw efficiency of its combustion. Many of NASA’s rockets, including the second and third stages of the Apollo Program’s Saturn V and the Space Shuttle main engines, are powered by burning liquid hydrogen with pure oxygen.
_
Basics of Hydrogen element:
|
Discovery date |
1766 |
|
Discovered by |
Henry Cavendish |
|
Origin of the name |
The name is derived from the Greek ‘hydro’ and ‘genes’ meaning water forming. |
|
Allotropes |
H2 |
|
Atomic number |
1 |
|
State at 20°C |
Gas |
|
Melting point |
−259.16°C, −434.49°F, 13.99 K |
|
Boiling point |
−252.879°C, −423.182°F, 20.271 K |
|
Density (g cm−3) |
0.000082 |
|
Relative atomic mass |
1.008 |
|
Key isotopes |
1H, 2H |
_
History of hydrogen:
Hydrogen has received increased attention as an environmentally friendly option to help meet today’s energy needs. The road leading to an understanding of hydrogen’s energy potential presents a fascinating tour through scientific discovery and industrial ingenuity.
1766 – Hydrogen was first identified as a distinct element by British scientist Henry Cavendish after he separated hydrogen gas by reacting zinc metal with hydrochloric acid. In a demonstration to the Royal Society of London, Cavendish applied a spark to hydrogen gas yielding water. This discovery led to his later finding that water (H2O) is made of hydrogen and oxygen.
1783 – Jacques Alexander Cesar Charles, a French physicist, launched the first hydrogen balloon flight. Known as “Charliere,” the unmanned balloon flew to an altitude of three kilometers. Only three months later, Charles himself flew the first manned hydrogen balloon.
1788 – Building on the discoveries of Cavendish, French chemist Antoine Lavoisier gave hydrogen its name, which was derived from the Greek words – “hydro” and “genes,” meaning “water” and “born of.”
1800 –English scientists William Nicholson and Sir Anthony Carlisle discovered that applying electric current to water produced hydrogen and oxygen gases. This process was later termed “electrolysis.”
1839 – The fuel cell effect, combining hydrogen and oxygen gases to produce water and an electric current, was discovered by Swiss chemist Christian Friedrich Schoenbein.
1845 – English scientist and judge Sir William Grove demonstrated Schoenbein’s discovery on a practical scale by creating a “gas battery.” For his achievement he earned the title “Father of the Fuel Cell.”
1905 – Germans developed the Haber process to make ammonia from nitrogen in the air and hydrogen. Ammonia is the starting point for making fertilizer and explosives.
1920s – German engineer Rudolf Erren converted the internal combustion engines of trucks, buses and submarines to use hydrogen or hydrogen mixtures. British scientist and Marxist writer J.B.S. Haldane introduced the concept of renewable hydrogen in his paper, Science and the Future, by proposing that “there will be great power stations where during windy weather the surplus power will be used for the electrolytic decomposition of water into oxygen and hydrogen.”
1931 – Harold Urey and his colleagues at Columbia University in the US detected a second, rarer, form of hydrogen. This has twice the mass of normal hydrogen, and they named it deuterium.
1937 – After ten successful trans-Atlantic flights from Germany to the United States, the Hindenburg, a dirigible inflated with hydrogen gas, erupted into flames while landing in Lakewood, New Jersey.
1958– The United States formed the National Aeronautics and Space Administration (NASA). NASA’s space program currently uses the most liquid hydrogen worldwide, primarily for rocket propulsion and as a fuel for fuel cells. NASA uses hydrogen both as a fuel for its rockets and for fuel cells to generate electricity.
1959– Francis T. Bacon of Cambridge University in England built the first practical hydrogen-air fuel cell. The 5-kilowatt (kW) system powered a welding machine. He named his fuel cell design the “Bacon Cell.” Later that year, Harry Karl Ihrig, an engineer for the Allis – Chalmers Manufacturing Company, demonstrated the first fuel cell vehicle: a 20–horsepower tractor. Hydrogen fuel cells, based upon Bacon’s design, have been used to generate on-board electricity, heat and water for astronauts aboard the famous Apollo spacecraft and all subsequent space shuttle missions.
1970 – Electrochemist John O’M. Bockris coined the term “hydrogen economy.” He later published Energy: The Solar-Hydrogen Alternative, describing his envisioned hydrogen economy where cities in the United States could be supplied with solar energy.
1972– A 1972 Gremlin, modified by The University of California at Los Angeles, entered the 1972 Urban Vehicle Design Competition and won first prize for the lowest tailpipe emissions. Students converted the Gremlin’s internal combustion engine to run on hydrogen supplied from an onboard tank.
1973–The OPEC oil embargo and the resulting supply shock suggested that the era of cheap petroleum had ended and that the world needed alternative fuels. The development of hydrogen fuel cells for conventional commercial applications began.
1974– Professor T. Nejat Veziroglu of the University of Miami, FL, organized The Hydrogen Economy Miami Energy Conference (THEME), the first international conference held to discuss hydrogen energy. Following the conference, the scientists and engineers who attended the THEME conference formed the International Association for Hydrogen Energy (IAHE).
1977– International Energy Agency (IEA) was established in response to global oil market disruptions. IEA activities included the research and development of hydrogen energy technologies. The U.S. Department of Energy (DOE) was also created.
1978– National Science Foundation transferred the Federal Hydrogen R&D Program to the U.S. DOE.
1988– The Soviet Union Tupolev Design Bureau successfully converted a 164-passenger TU-154 commercial jet to operate one of the jet’s three engines on liquid hydrogen. The maiden flight lasted 21 minutes.
1989– The National Hydrogen Association (NHA) formed in the United States with ten members. Today, the NHA has nearly 100 members, including representatives from the automobile and aerospace industries, federal, state and local governments, universities, researchers, utilities and energy providers. The International Organization for Standardization’s Technical Committee for Hydrogen Technologies was also created.
1990– The world’s first solar powered hydrogen production plant at Solar-Wasserstoff-Bayern, a research and testing facility in southern Germany, became operational. The U.S. Congress passed the Spark M. Matsunaga Hydrogen, Research, Development and Demonstration Act (PL 101-566), which prescribed the formulation of a 5-year management and implementation plan for hydrogen research and development in the United States. The Hydrogen Technical Advisory Panel (HTAP) was mandated by the Matsunaga Act to ensure consultation on and coordination of hydrogen research.
1991– Georgetown University in Washington, D.C. begins development of three 30-foot Fuel Cell Test Bed Buses (TBB) as part of their Generation I Bus Program. In 2001, Georgetown finished their second Generation II bus, which uses hydrogen from methanol to power a 100kW fuel cell “engine.”
1992– The Partnership for a New Generation of Vehicles (PNGV), a cooperative R&D program, was established by the Clinton Administration as a joint effort between the government and automobile manufactures for the research and development of new vehicles technologies and alternative fuels, including hydrogen.
1994– Daimler Benz demonstrated the NECAR I (New Electric CAR), its first hydrogen fuel cell vehicle, at a press conference in Ulm, Germany.
1995– The Chicago Transit Authority unveiled the first of their three hydrogen fuel cell buses. The small pilot fleet began operation the following year.
1997– Retired NASA engineer Addison Bain challenged the belief that hydrogen caused the Hindenburg accident. The hydrogen, Bain demonstrated, did not cause the catastrophic fire but rather it was the combination of static electricity and highly flammable material on the skin of the airship.
1998– Iceland unveiled a plan to create the first hydrogen economy by 2030.
1999– Europe’s first hydrogen fueling stations were opened in the German cities of Hamburg and Munich. The Royal Dutch/Shell Company committed to a hydrogen future by forming a hydrogen division. Also, a consortium of Icelandic institutions, headed by the financial group New Business Venture Fund, partnered with Royal Dutch/Shell Group, DaimlerChrysler (a merger of Daimler Benz and Chrysler) Norsk Hydro to form the Icelandic Hydrogen and Fuel Cell Company, Ltd. to further the hydrogen economy in Iceland.
2001– Ballard Power Systems launched the world’s first volume-produced proton exchange membrane (PEM) fuel cell system designed for integration into a wide variety of industrial and consumer end-product applications.
2002– Executives from DaimlerChrysler Corporation, Ford Motor Company and General Motors Corporation, along with Secretary of Energy Spencer Abraham, announced a new cooperative automotive research (CAR) partnership between the U.S. Department of Energy and the U.S. Council for Automotive Research (USCAR). The program, FreedomCAR, focuses on developing enabling technologies, such as hydrogen fuel cells, for petroleum-free cars and light trucks.
2003– President George W. Bush announced in his 2003 State of the Union Address a $1.2 billion hydrogen fuel initiative to develop the technology for commercially viable hydrogen powered fuel cells, such that “the first car driven by a child born today could be powered by hydrogen and pollution free.” U.S. Secretary of Energy Spencer Abraham launched the International Partnership for the Hydrogen Economy (IPHE) to foster global cooperation in the development of hydrogen technology.
2004– U.S. Energy Secretary Spencer Abraham announced over $350-million devoted to hydrogen research and vehicle demonstration projects, nearly one-third of President Bush’s commitment. The funding encompasses over 30 lead organizations and more than 100 partners selected through a competitive review process.
______
______
Properties of hydrogen:
Common hydrogen has a molecular weight of 2.016 g/mole. As a gas it has a density of 0.071 g/l at 0ºC and 1 atm. Its relative density, compared with that of the air, is 0.0695. Hydrogen is the most flammable of all the known substances. Hydrogen is slightly more soluble in organic solvents than in water. Many metals absorb hydrogen. Hydrogen absorption by steel can result in brittle steel, which leads to fails in the chemical process equipment.
At normal temperature hydrogen is a not very reactive substance, unless it has been activated somehow; for instance, by an appropriate catalyser. At high temperatures it’s highly reactive.
Although in general its diatomic, molecular hydrogen dissociates into free atoms at high temperatures. Atomic hydrogen is a powerful reductive agent, even at ambient temperature. It reacts with the oxides and chlorides of many metals, like silver, copper, lead, bismuth and mercury, to produce free metals. It reduces some salts to their metallic state, like nitrates, nitrites and sodium and potassium cyanide. It reacts with a number of elements, metals and non-metals, to produce hydrides, like NAH, KH, H2S and PH3. Atomic hydrogen produces hydrogen peroxide, H2O2, with oxygen.
Atomic hydrogen reacts with organic compounds to form a complex mixture of products; with ethylene, C2H4, for instance, the products are ethane, C2H6, and butane, C4H10. The heat released when the hydrogen atoms recombine to form the hydrogen molecules is used to obtain high temperatures in atomic hydrogen welding.
Molecular H2 is unreactive compared to diatomic elements such as halogens or oxygen. The thermodynamic basis of this low reactivity is the very strong H-H bond, with a bond dissociation energy of 435.7 kJ/mol. The kinetic basis of the low reactivity is the nonpolar nature of H2 and its weak polarizability. It spontaneously reacts with chlorine and fluorine to form hydrogen chloride and hydrogen fluoride, respectively. The reactivity of H2 is strongly affected by the presence of metal catalysts. Thus, while mixtures of H2 with O2 or air combust readily when heated to at least 500 C by a spark or flame, they do not react at room temperature in the absence of a catalyst.
Hydrogen has one of the highest energy densities per unit mass (between 120 and 142 MJ/kg), and as it has a higher combustion energy per unit mass than other fuels, it has become of interest to the renewable energy community.
An understanding of the properties of hydrogen is critical for the proper design of a facility or workspace. A workspace can be configured to mitigate hazards by understanding and taking advantage of some of the characteristics of hydrogen.
_
-1. Gaseous Hydrogen
Gaseous hydrogen has some outstanding specifications compared to other fuel types, as can be seen in table below:
|
|
Petrol |
Methane |
Propane |
Hydrogen |
|
Lower explosion limit (%, air) |
15 |
5 |
2,1 |
4 |
|
Upper explosion limit (%, air) |
8 |
15 |
9.5 |
75.6 |
|
Flash point ͦC |
-20 |
-188 |
-104 |
-270.8 |
|
Lowest ignition energy mJ |
0.8 |
0.3 |
0.25 |
0.017 |
|
Density (20 ͦC, 1 bar) |
0.7-0.78 kg/l |
0.718 kg/m3 |
2.01 kg/m3 |
0.089 kg/m3 |
|
Boiling point ͦC |
30-215 |
-161.5 |
-42 |
-252.7 |
|
Critical temperature ͦC |
|
-82.5 |
96.6 |
-239.3 |
|
Critical pressure bar |
|
45 |
42.2 |
13 |
|
Diffusion coefficient cm2/s |
|
0.16 |
0.12 |
0.61 |
Hydrogen is colorless, odorless, tasteless, non-toxic, and non-poisonous. It’s also non-corrosive, but it can embrittle some metals. Hydrogen is the lightest and smallest element and is a gas under atmospheric conditions. Natural gas and propane are also odorless, but industry adds a sulfur-containing odorant so people can detect them. Currently, odorants are not used with hydrogen because there are no known odorants light enough to “travel with” hydrogen at the same dispersion rate. Current odorants also contaminate fuel cells, which are an important application for hydrogen.
Hydrogen is about 57 times lighter than gasoline vapor and 14 times lighter than air. This means that if it is released in an open environment, it will typically rise and disperse rapidly. This is a safety advantage in an outside environment. For indoor applications, it means that hydrogen will concentrate at the ceiling.
Hydrogen is a very small molecule with low viscosity, and therefore prone to leakage. In a confined space, leaking hydrogen can accumulate and reach a flammable concentration. Any gas other than oxygen is an asphyxiant in sufficient concentrations. In a closed environment, leaks of any size are a concern, since hydrogen is impossible for human senses to detect and can ignite over a wide range of concentrations in air. Proper ventilation and the use of detection sensors can mitigate these hazards.
Hydrogen has a high energy content by weight, but not by volume, which is a particular challenge for storage. In order to store sufficient quantities of hydrogen gas, it’s compressed and stored at high pressures. The easiest way to decrease the volume of a gas, at constant temperatures, is to increase its pressure. Thus, at 700 bar, hydrogen has a density of 42 kg/m3, compared to 0.089 kg/m3 under normal pressure and temperature conditions. At this pressure, 5 kg of hydrogen can be stored in a 125 liter tank. For safety, hydrogen tanks are equipped with pressure relief devices that will prevent the pressures in the tanks from becoming too high.
_
-2. Hydrogen Combustion
Hydrogen gas (dihydrogen or molecular hydrogen) is highly flammable:
2 H2(g) + O2(g) → 2 H2O(l) + 572 kJ (286 kJ/mol)
The enthalpy of combustion is −286 kJ/mol.
Hydrogen gas forms explosive mixtures with air in concentrations from 4–74% and with chlorine at 5–95%. The explosive reactions may be triggered by spark, heat, or sunlight. The hydrogen autoignition temperature, the temperature of spontaneous ignition in air, is 500 °C (932 °F). While the flammable limits for a fuel such as natural gas is in the range of 5.0%–15% (gas to air volume ratio), hydrogen has a range from 4.0% to 74.0%. It has a relatively low ignition energy (0.02 milli-Joule). Hydrogen has the smallest ignition energy, much lower than that required for other common fuels. This means that small sparks can easily ignite it.
Hydrogen burns with a pale blue flame that is nearly invisible in daylight, so it is almost impossible to detect by the human senses. Impurities such as sodium from ocean air or other burning materials will introduce color to the hydrogen flame. Both hydrogen and flame detectors are almost always installed with hydrogen systems to quickly identify any leak and minimize the potential for undetected flames. In addition, hydrogen flames radiate little infrared (IR) heat, but substantial ultraviolet (UV) radiation. This means that when someone is very close to a hydrogen flame, there is little sensation of heat, making inadvertent contact with the flame a significant concern. UV overexposure is also a concern, since it can result in sunburn-like effects.
_
If a large hydrogen cloud comes into contact with an ignition source, ignition will likely result in the flame flashing back to the source of the hydrogen. In open spaces with no confinement, flames will propagate through a flammable hydrogen-air cloud at several meters per second, and even more rapidly if the cloud is above ambient temperature. The result is a rapid release of heat, but little overpressure, and the combustion product is steam. It should be noted that hydrogen combustion is more rapid than combustion of other fuels. A hydrogen cloud will burn within seconds, and all of the energy of the cloud will be released. However, a hydrogen gas mixtures ignited in a confined space can generate pressures high enough to rupture equipment, exploding buildings and throw shrapnel. So, keeping hydrogen equipment and piping outdoors is an inherent safety advantage.
A large leak in a pressurized [>1400 kPa (>200 psi)] hydrogen system will result in a jet that may extend for some meters. If ignited, the jet flame can cause serious damage to anything it encounters. This is the reason for establishing separation distances between hydrogen systems and objects that can be harmed by a jet flame.
_
-3. Liquid Hydrogen
Liquid hydrogen has different characteristics and additional potential hazards than gaseous hydrogen, so additional control measures are used to ensure safety. Liquid hydrogen (LH2) is the liquid state of the element hydrogen. To exist as a liquid, H2 must be cooled below its critical point of 33 K. However, for it to be in a fully liquid state at atmospheric pressure, H2 needs to be cooled to 20.28 K (−252.87 °C; −423.17 °F). One common method of obtaining liquid hydrogen involves a compressor resembling a jet engine in both appearance and principle. Liquid hydrogen is typically used as a concentrated form of hydrogen storage. As for any gas, storing it as liquid takes less space than storing it as a gas at normal temperature and pressure. However, the liquid density is very low compared to other common fuels. Once liquefied, it can be maintained as a liquid in pressurized and thermally insulated containers. As a liquid, hydrogen is stored under pressure at around -245°C (-410°F), a temperature that can cause cryogenic burns or lung damage. Detection sensors and personal protective equipment are critical when dealing with a potential liquid hydrogen leak or spill.
The volume ratio of liquid to gas is approximately 1:848. So, if you picture a gallon of liquid hydrogen, that same amount of hydrogen, existing as a gas, would occupy about 848 gallon containers without compression. Hydrogen undergoes a rapid phase change from liquid to gas, so ventilation and pressure relief devices are built into hydrogen systems to ensure safety.
Liquid hydrogen is also colorless. It is extremely cold and only persists if maintained in a cryogenic storage vessel. Storage is usually under pressures up to 1000 kPa (150 psi). If spilled on ambient-temperature surfaces, liquid hydrogen will rapidly boil and its vapors will expand rapidly, increasing 848 times in volume as it warms to room temperatures. If the liquid hydrogen is confined (such as between valves closing off a length of pipe) and left to warm without pressure relief, pressures approaching 170 MPa (25,000 psi) are possible. Confinements will likely rupture under such pressures, producing high-pressure jets of gas and high-speed shrapnel. Ignition is extremely likely under such circumstances.
The density of liquid hydrogen is only 70.85 g/L (at 20 K), a relative density of just 0.07. Although the specific energy is more than twice that of other fuels, this gives it a remarkably low volumetric energy density, many fold lower.
Due to its cold temperatures, liquid hydrogen is a hazard for cold burns. Elemental hydrogen as a liquid is biologically inert and its only human health hazard as a vapor is displacement of oxygen, resulting in asphyxiation. Because of its flammability, liquid hydrogen should be kept away from heat or flame unless ignition is intended.
_
-4. Volumetric density of hydrogen:
-The volumetric density of gaseous hydrogen at atmospheric pressure is 0.09 kg/m³. Consequently, under normal conditions, much space is required to store gaseous hydrogen. Therefore, hydrogen is virtually not stored or transported in gaseous form at atmospheric pressure because it is simply not efficient.
-At a pressure of 350 bar, the volumetric density of gaseous hydrogen is 21 kg/m³. This increased pressure makes it possible to store considerably more gaseous hydrogen in the same space. The pressure of 350 bar is used in the tanks of gaseous hydrogen trucks, for example, the ones from Hyzon. A loaded 55-ton truck needs about 50-70 kg of hydrogen to travel 500 to 600 km.
-At a pressure of 700 bar, the volumetric density of gaseous hydrogen is 42 kg/m³. This relatively high pressure is used, among others, for gaseous hydrogen passenger cars such as the Hyundai NEXO. With a 125 liter tank containing 5 kg of hydrogen, a car can drive about 600 km.
-In liquid form and at a temperature of -252.9 centigrade, hydrogen has a volumetric density of 71 kg/m³. Liquid hydrogen is also used as an energy carrier for sustainable trucks and aircraft, which are currently under development. To drive about 1000 km, a truck needs about 80 kg of liquid hydrogen. This applies, for example, to the liquid hydrogen-powered Daimler GenH2. Liquid hydrogen also offers excellent potential for aircraft because the energy in liquid hydrogen is so high and hydrogen as a fuel is a lot lighter than kerosene. This is a vast advantage for aircraft. However, the volume of liquid hydrogen is a lot more than the volume of kerosene. To carry the same amount of total energy on board, you need four times the volume of liquid hydrogen compared to kerosene. Fortunately, there are ways to use fuel efficiently. For example, fuel cells are more efficient than fuel engines, and superconductivity makes them even more economical. By applying these techniques, it is not always necessary to take a massive amount of hydrogen on board.
______
Isotopes of hydrogen:
Hydrogen has three naturally occurring isotopes, denoted 1H, 2H and 3H. Other, highly unstable nuclei (4H to 7H) have been synthesized in the laboratory but not observed in nature.
Deuterium and tritium are isotopes of hydrogen, the most abundant element in the universe. Whereas all isotopes of hydrogen have one proton, deuterium also has one neutron and tritium has two neutrons, so their ion masses are heavier than protium, the isotope of hydrogen with no neutrons.
1H is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the nucleus of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name protium. It is unique among all stable isotopes in having no neutrons.
2H, the other stable hydrogen isotope, is known as deuterium and contains one proton and one neutron in the nucleus. All deuterium in the universe is thought to have been produced at the time of the Big Bang, and has endured since that time. Deuterium is not radioactive, and does not represent a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called heavy water. Water made from deuterium is about 10 percent heavier than ordinary water. It will actually sink to the bottom of a glass of ordinary water. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for 1H-NMR spectroscopy. Heavy water is used as a neutron moderator and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial nuclear fusion.
3H is known as tritium and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into helium-3 through beta decay with a half-life of 12.32 years. Tritium is rare in nature. Exposing the more abundant element of lithium to energetic neutrons can generate tritium. It is so radioactive that it can be used in luminous paint, making it useful in such things as watches. The glass prevents the small amount of radiation from getting out. Small amounts of tritium are produced naturally by the interaction of cosmic rays with atmospheric gases; tritium has also been released during nuclear weapons tests. It is used in nuclear fusion reactions, as a tracer in isotope geochemistry, and in specialized self-powered lighting devices. Tritium has also been used in chemical and biological labeling experiments as a radiolabel.
_
When deuterium and tritium fuse, they create a helium nucleus, which has two protons and two neutrons. The reaction releases an energetic neutron. Fusion power plants would convert energy released from fusion reactions into electricity to power our homes, businesses, and other needs. Fortunately, deuterium is common. About 1 out of every 5,000 hydrogen atoms in seawater is in the form of deuterium. This means our oceans contain many tons of deuterium. When fusion power becomes a reality, just one gallon of seawater could produce as much energy as 300 gallons of gasoline. A working fusion power plant would need enriched lithium to breed the tritium it needs to close the deuterium-tritium fuel cycle. Current R&D efforts are focused on advanced designs of tritium breeding blankets using lithium originally obtained from Earth based sources. To avoid certain R&D challenges including structural material damage from energetic neutrons, fusion scientists are interested also in aneutronic fusion reactions (such as deuterium-helium-3 and proton-boron fusion) even though these fusion reactions occur at higher ion temperatures than for deuterium and tritium. Hydrogen would become energy source when commercial nuclear fusion power generation becomes reality.
______
Spin isomers of hydrogen:
A molecule of dihydrogen contains two atoms, in which the nuclei of both the atoms are spinning. Depending upon the direction of the spin of the nuclei, the hydrogens are of two types:
Ortho hydrogen molecules are those in which the spins of both the nuclei are in the same direction. Molecules of hydrogen in which the spins of both the nuclei are in the opposite direction are called para hydrogen. Ordinary dihydrogen is an equilibrium mixture of ortho and para hydrogen.
The amount of ortho and para hydrogen varies with temperature as:
-At 0°K, hydrogen contains mainly para hydrogen which is more stable.
-Liquid hydrogen consists of 99.79% parahydrogen and 0.21% orthohydrogen.
-At the room temperature, the ratio of ortho to para hydrogen is 3:1.
-Even at very high temperatures, the ratio of ortho to para hydrogen can never be more than 3:1.
Thus, it has been possible to get pure para hydrogen by cooling ordinary hydrogen to a very low temperature (close to 20 K) in liquid form but it is never possible to get a sample of hydrogen containing more than 75% of ortho hydrogen.
At room temperature, gaseous hydrogen is mostly in the ortho isomeric form due to thermal energy, but an ortho-enriched mixture is only metastable when liquified at low temperature. It slowly undergoes an exothermic reaction to become the para isomer, with enough energy released as heat to cause some of the liquid to boil. To prevent loss of the liquid during long-term storage, it is therefore intentionally converted to the para isomer as part of the production process, typically using a catalyst such as iron(III) oxide, activated carbon, platinized asbestos, rare earth metals, uranium compounds, chromium(III) oxide, or some nickel compounds. If orthohydrogen is not removed from rapidly liquified hydrogen, without a catalyst, the heat released during its decay can boil off as much as 50% of the original liquid.
_______
Cosmic prevalence and distribution:
Hydrogen, as atomic H, is the most abundant chemical element in the universe, making up 75 percent of normal matter by mass and more than 90 percent by number of atoms. (Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as dark matter and dark energy.) This element is found in great abundance in stars and gas giant planets. Molecular clouds of H2 are associated with star formation. Hydrogen plays a vital role in powering stars through the proton-proton reaction in case of stars with very low to approximately 1 mass of the Sun and the CNO cycle of nuclear fusion in case of stars more massive than our Sun.
States of hydrogen:
Throughout the universe, hydrogen is mostly found in the atomic and plasma states, with properties quite distinct from those of molecular hydrogen. As a plasma, hydrogen’s electron and proton are not bound together, resulting in very high electrical conductivity and high emissivity (producing the light from the Sun and other stars). The charged particles are highly influenced by magnetic and electric fields. For example, in the solar wind they interact with the Earth’s magnetosphere giving rise to Birkeland currents and the aurora. Hydrogen is found in the neutral atomic state in the interstellar medium because the atoms seldom collide and combine. They are the source of the 21-cm hydrogen line at 1420 MHz that is detected in order to probe primordial hydrogen.
Under ordinary conditions on Earth, elemental hydrogen exists as the diatomic gas, H2. Hydrogen gas is very rare in the Earth’s atmosphere (1 ppm by volume) because of its light weight, which enables it to escape from the atmosphere more rapidly than heavier gases. However, hydrogen is the third most abundant element on the Earth’s surface, mostly in the form of chemical compounds such as hydrocarbons and water. The discoveries of hundreds of natural H2 seepages, generally connected with circulation of hydrothermal fluids through ultramafic rocks, both under the seafloors and on the continents, raise important questions regarding the energy potential that these sources can represent. Much has been learned about natural hydrogen (H2) seepages and accumulation, but present knowledge of hydrogen behavior in the crust is so limited that it is not yet possible to consider exploitation of this resources.
A molecular form called protonated molecular hydrogen ((H+3) is found in the interstellar medium, where it is generated by ionization of molecular hydrogen from cosmic rays. This ion has also been observed in the upper atmosphere of the planet Jupiter. The ion is relatively stable in the environment of outer space due to the low temperature and density. H+3 is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium. Neutral triatomic hydrogen H3 can exist only in an excited form and is unstable. By contrast, the positive hydrogen molecular ion (H+2) is a rare molecule in the universe.
_______
_______
The challenge of cutting carbon:
The atmosphere contains greenhouse gases, of which CO2, CH4, N2O and H2O are the primary ones. These gases permit the radiation of the sun to reach the surface of the earth while simultaneously preserving part of the heat that radiates from the earth within the atmosphere. This is called the greenhouse effect. In the same way that the glass in a greenhouse prevents the loss of heat, the greenhouse gases prevent the total escape of heat from the earth’s surface to outer space. Without these gases the average temperature on the earth would be about 35 degrees lower than it is today (in other words, a temperature of about –20 degrees Celsius).
_
The releases of fossil CO2 disturb a naturally renewable balance, already unbalanced by the activities of mankind through the cutting and burning of forest and vegetation at a more rapid pace than it is replanted and regrown. In other words, more CO2 is released (through the burning of wood and so on) than the plants are able to take up through photosynthesis. Furthermore, dead biological material (paper, food leftovers, textiles) are left to rot without the presence of oxygen, thereby resulting in a conversion to the climate gas methane (CH4) rather than CO2. Methane’s greenhouse effect is a factor of 21 times higher than that of CO2. In addition, new chemicals (for example, chlorofluorocarbon gases) have been introduced that also remain in the atmosphere and contribute towards intensifying the greenhouse effect.
_
The amount of the various gases in the atmosphere have varied throughout the earth’s history due to volcano eruptions and other natural phenomena. However, over the course of the last two hundred years, mankind has made an ever-increasing impact on the makeup of these atmospheric gases. We are disturbing the natural CO2 cycle to a steadily increasing degree. In the course of a few short decades, we have extracted, refined and consumed fossil material that the earth has used millions of years to produce, releasing large amounts of carbon dioxide. Nature partly manages to absorb the ”fossil” CO2 gas by binding it in the sea and in plants through the process of photosynthesis, but large amounts nonetheless end up in the atmosphere.
_
As the chart above shows, the current greenhouse gas trajectory will far exceed the global warming limits set by the Paris Agreement. Countries across the globe adopted an historic international climate agreement at the UN Framework Convention on Climate Change (UNFCCC) Conference of the Parties (COP21) in Paris in December 2015. In anticipation of this moment, countries publicly outlined what post-2020 climate actions they intended to take under the new international agreement, known as their Intended Nationally Determined Contributions (INDCs). The climate actions communicated in these INDCs largely determine whether the world achieves the long-term goals of the Paris Agreement: to hold the increase in global average temperature to well below 2°C, to pursue efforts to limit the increase to 1.5°C, and to achieve net zero emissions in the second half of this century. The transition to renewables depends on new energy technologies being scaled-up to provide workable substitutes for our dependence on fossil fuels.
_
All nations wish to tackle climate change. Many have very strong policies focused on green electricity, at both the national and regional level. California just announced that it is aiming to achieve 100% green electricity on its network by 2045. Europe will reach 30% by 2020 and is aiming for 50% by 2030. Electricity, however, represents less than a quarter of energy use in Europe. Around half of total energy use is for heating, which is mainly covered by natural gas. The other big part is transport, which represents a third of the total, and is mainly covered by liquid fuels.
One can argue that biomass and biogas help decarbonise heating in Europe (covering 19% of total heating needs) and biofuels help decarbonise transport in Europe (with 7% of the total). But we still face three major facts about Europe, which are true of other regions too.
-1. Greening electricity networks is great and should be further encouraged but even if half our electricity was produced by renewables, we would still only decarbonise about 10% of the energy we use. As 80% of worldwide CO2 emissions are related to energy, it is key that we understand where to put the emphasis in order to reduce them faster.
-2. Heating and transport, which represent 75% of our energy needs, are mainly covered by solid, gas and liquid fuels, which are emitting CO2 (even biomass, biogas and biofuels). And we know that if we do not want to warm up the planet by more than 2°C, we cannot release more CO2 than we did between 1985 and today. So, it is only if we have major CO2 sinks (such as large forests and carbon capture and storage sites) that we can expect to cope with this reality. If not, we need to look for a substitute to these fuels which does not emit CO2.
-3. Increasing green electricity levels on the grid leads to major instabilities due to the intermittent nature of solar and wind resources. We therefore need to find ways to store these electrons on a massive scale for later use. Battery-based large storage exists, but it fills up very quickly and is best for storing and releasing electricity within a day, not for long-term storage.
_
Green hydrogen offers a solution:
It is the only gas that does not contain any carbon, and so using it for heating and transport does not generate any CO2 – only water. Generating it from green power helps store it and balance the grid.
On the heating side, green hydrogen can be mixed up to 20% with natural gas in pipelines, or dedicated pure hydrogen pipelines can be laid (there are several thousands of kilometres of them around the world already). It can then be used in existing gas appliances or dedicated fuel cells to generate heat and power.
On the transport side, hydrogen can power fuel cell-based vehicles, such as electric vehicles carrying a hydrogen tank and a fuel cell that transforms on-demand hydrogen into electrons to power the car.
Green hydrogen is set to enter industrial processes, mobility, homes, buildings and cities. As such, we must ask ourselves how it will impact our activities as it develops into a new energy source, and much sooner and faster than initially thought.
_
Green Goals:
Many more countries have announced full hydrogen strategies in 2020
Hydrogen could be used to meet 27% of Canada’s primary energy needs
The Nord Stream 2 gas pipeline could deliver hydrogen from Russia to Europe
EU aims to install 40GW of renewable hydrogen electrolyzers by 2030
Japan was the first to adopt a comprehensive hydrogen strategy
Germany’s $10B strategy outspends the rest
South Korea wants to lead in the production and use of hydrogen vehicles
China is already the world’s largest producer, but mostly from coal
Australia aims to be among the top three exporters of hydrogen to Asian markets by 2030
Chile aims to produce the world’s cheapest green hydrogen by 2030.
_______
_______
Section-3
Energy and fuel:
_
The energy challenge:
Energy is the most important need for the human life and development worldwide. Energy is a topical subject in our daily life. The rapid growth of population and increase of personal income are the key drivers behind growing energy demand. It is projected that by 2035, an additional 1.6 billion people will demand energy when the global population reaches 8.7 billion. The major problem faced is the conflict between increasing energy demand and the scarceness of existing fossil fuel supply, together with concerns associated with the utilisation of conventional fossil fuels, such as greenhouse gas emissions leading to climate change, as well as the negative impact on human health from other associated pollutants.
There is an urgent need to source clean alternative and sustainable fuel to replace existing non-renewable fossil fuels. The world has seen a rapid growth in the development of renewable power generation. However, there are numerous disadvantages inherent in renewable power plants. Typically, renewable power plants are far away from the demand site, as a result, the transport of renewable energy presents a difficulty. The inherent intermittent nature and fluctuation of renewable power sources indicate that the power generation is unpredictable and there is an inevitable mismatch between the renewable power generation and the load demand. Hence, with existing centralised power generation and distribution networks, increasing decentralised renewable power plants, like PV arrays and wind farms, will have significant impact on the stability of the grid. As a result, curtailment is a mechanism currently used to solve those problems which is expensive and prevents the further penetration of renewable power. Curtailment is the reduction of output of a renewable resource below what it could have otherwise produced. Curtailment is permitted on grounds of maintaining grid stability and system safety. Energy storage is a solution to address the aforementioned problems. Among various energy storage mechanisms, such as pumped hydro, battery, compressed air, flywheels, capacitor, and others, hydrogen is a promising candidate to help construct our future energy system.
Energy security is a major issue. Fossil fuel, particularly crude oil, is confined to a few areas of the world and continuity of supply is governed by political, economic and ecological factors. These factors conspire to force volatile, often high fuel prices while, at the same time, environmental policy is demanding a reduction in greenhouse gases and toxic emissions.
A coherent energy strategy is required, addressing both energy supply and demand, taking account of the whole energy lifecycle including fuel production, transmission and distribution, and energy conversion, and the impact on energy equipment manufacturers and the end-users of energy systems. In the short term, the aim should be to achieve higher energy efficiency and increased supply from renewables. In view of technological developments, vehicle and component manufacturers, transport providers, the energy industry, and even householders are seriously looking at alternative energy sources and fuels and more efficient and cleaner technologies – especially hydrogen and hydrogen-powered fuel cells. In the long term, a hydrogen-based economy will have an impact on all these sectors.
_
Fuel:
A fuel is any material that can be made to react with other substances so that it releases energy as heat energy or to be used for work. The main purpose of fuel is to store energy, which should be in a stable form and can be easily transported to the place of use. Almost all fuels are chemical fuels. The user employs this fuel to generate heat or perform mechanical work, such as powering an engine. It may also be used to generate electricity, which is then used for heating, lighting, or other purposes.
Alternative fuel, known as non-conventional and advanced fuels, are any materials or substances that can be used as fuels, other than conventional fuels like; fossil fuels (petroleum oil, coal, and natural gas), as well as nuclear materials such as uranium and thorium, as well as artificial radioisotope fuels that are made in nuclear reactors.
Some well-known alternative fuels include bio-diesel, bio-alcohol (methanol, ethanol, butane), refuse-derived fuel, chemically stored electricity (batteries and fuel cells), hydrogen, non-fossil methane, non-fossil natural gas, vegetable oil, propane and other biomass sources.
_
Hydrogen as an Alternative Fuel:
Hydrogen is considered an alternative fuel under the Energy Policy Act of 1992 of the US. The interest in hydrogen as an alternative transportation fuel stems from its ability to power fuel cells in zero-emission vehicles, its potential for domestic production, and the fuel cell’s fast filling time and high efficiency. In fact, a fuel cell coupled with an electric motor is two to three times more efficient than an internal combustion engine running on gasoline. Hydrogen can also serve as fuel for internal combustion engines. However, unlike FCEVs, these produce tailpipe emissions and are less efficient.
It takes more energy to produce hydrogen (by separating it from other elements in molecules) than hydrogen provides when it is converted to useful energy. However, hydrogen is useful as an energy source/fuel because it has a high energy content per unit of weight, which is why it is used as a rocket fuel and in fuel cells to produce electricity on some spacecraft. Hydrogen is not widely used as a fuel now, but it has the potential for greater use in the future.
_
The hydrogen atom is the lightest, simplest and most common element in the universe. However, it occurs only in combination with other elements, primarily with oxygen in water and with carbon, nitrogen and oxygen in living materials and fossil fuels. Hydrogen is not a primary source of energy. However, it becomes an attractive energy carrier when split from these other elements by using a source of energy. Hydrogen, as clean energy carrier, is considered to be the clean fuel of future particularly for energy storage and transport. The energy storage capacity of hydrogen is excellent because calculations show that one kilogram of hydrogen contains approximately 33 kWh of usable energy.
The advantages of hydrogen are: (i) energy security by reducing oil imports, (ii) sustainability by taking advantage of the RE sources, (iii) less pollution and better urban air quality by producing near-zero carbon, hydrocarbon, GHG and NOx emissions at the point of use, and (iv) economic viability by potentially shaping the future global energy markets. Therefore, Hydrogen is a worldwide-accepted clean energy carrier as it is source-independent and has a high energy content per mass compared to petroleum as listed in Table below. Although there are some nitrogen oxides produced during high temperature combustion, environmental pollutant can be fully removed during low temperature utilization such as by fuel cells.
Comparison between Hydrogen and different fuel as source of energy:
|
Fuel |
Energy content (MJ/kg) |
|
Hydrogen |
120 |
|
Liquefied natural gas |
54.4 |
|
Propane |
49.6 |
|
Aviation gasoline |
46.8 |
|
Automotive gasoline |
46.4 |
|
Automotive diesel |
45.6 |
|
Ethanol |
29.6 |
|
Methanol |
19.7 |
|
Coke |
27 |
|
Wood(dry) |
16.2 |
|
Begasse |
9.6 |
_
What makes hydrogen more powerful than gasoline?
The simplicity of hydrogen (two hydrogen atoms held together in a single H-H bond) makes for very fast rates of energy release, or rapid kinetics. Compare this to octane, a primary constituent of gasoline, which has 25 chemical bonds per molecule (7 carbon-carbon and 18 carbon-hydrogen bonds). This not only bodes well for hydrogen’s use in conventional combustion, but it also opens its use in high-efficiency electrochemical energy transformers, such as fuel cells. The electrochemical option is estimated to offer more than twice the fuel economy of an internal combustion engine-based powertrain while producing no polluting emissions.
_
Gasoline, which is derived from refining crude oil, contains much more energy than coal (twice the lower grade bituminous) or wood (three times). Liquid natural gas (LNG) is almost entirely composed of methane, while natural gas has about 85% of its mass accounted by methane. Jet A-1 is the standard fuel used by commercial jet planes and is mostly composed of kerosene and several additives (antifreeze, antioxidant and antistatic) since the fuel must meet rigorous specifications as it will be exposed to high altitudes and low temperatures. Conversely, Bunker C fuel, which is the primary fuel used for maritime shipping, can be considered one of the lowest quality fuels in liquid form but suitable for the vast ship engines.
The energy in 2.2 pounds (1 kilogram) of hydrogen gas is about the same as the energy in 1 gallon (6.2 pounds, 2.8 kilograms) of gasoline. No matter how it is used, the by-product the burning of hydrogen is water. The World Energy Council (WEC) says that “combusting one kilo of hydrogen releases three times more energy than a kilo of gasoline and produces only water”. Then, there are hydrogen fuel cells, which is “an electrochemical cell that converts the chemical energy of hydrogen and oxygen into electricity”, whose waste product, again, is water. “Fuel cells can produce electricity continuously for as long as hydrogen and oxygen are supplied,” WEC says. Because hydrogen has a low volumetric energy density, it is stored onboard a vehicle as a compressed gas to achieve the driving range of conventional vehicles. Most current applications use high-pressure tanks capable of storing hydrogen at either 5,000 or 10,000 pounds per square inch (psi), that is 350 to 700 bar. For example, the FCEVs in production by automotive manufacturers and available at dealerships have 10,000 psi tanks. Retail dispensers, which are mostly co-located at gasoline stations, can fill these tanks in about 5 minutes. Fuel cell electric buses currently use 5,000 psi tanks that take 10–15 minutes to fill. Other ways of storing hydrogen are under development, including bonding hydrogen chemically with a material such as metal hydride or low-temperature sorbent materials.
_
Hydrogen is often advocated as an energy carrier. Here are some relevant facts.
Energy carriers allow the transport of energy in a usable form from one place to another. Hydrogen, like electricity, is an energy carrier that must be produced from another substance. Hydrogen can be produced—separated—from a variety of sources including water, fossil fuels, or biomass and used as a source of energy or fuel.
-1. Hydrogen is the lightest of the elements with an atomic weight of 1.0. Liquid hydrogen has a density of 0.07 grams per cubic centimeter, whereas water has a density of 1.0 g/cc and gasoline about 0.75 g/cc. These facts give hydrogen both advantages and disadvantages. The advantage is that it stores approximately 2.6 times the energy per unit mass as gasoline, and the disadvantage is that it needs about 4 times the volume for a given amount of energy. A 15 gallon automobile gasoline tank contains 90 pounds of gasoline. The corresponding liquid hydrogen tank would be 60 gallons, but the hydrogen would weigh only 34 pounds.
-2. When hydrogen is burned in air the main product is water. Some nitrogen compounds may also be produced and may have to be controlled. Should greenhouse warming turn out to be an important problem, the key advantage of hydrogen is that carbon dioxide (CO2) is not produced when hydrogen is burned.
-3. Since hydrogen is not available in significant quantities in nature in pure form, the main present way of getting hydrogen is steam methane reforming, and this will probably remain the most economical way as long as methane (natural gas) is available cheaply and in large quantities, and hydrogen is required only in small quantities. When the price of methane goes up to more than three times its present price because of scarcity, hydrogen will be obtained by splitting water H2O into hydrogen H2 and oxygen O2.
The chemical reaction is written 2H2O + energy => 2H2 + O2.
The well developed way of splitting water is by electrolysis. If fossil fuels, e.g., coal, oil or natural gas, are used to generate the electricity, there is no advantage over using the fossil fuels directly. Indeed you still get all the CO2, and there is a considerable loss of energy. Therefore, the large scale use of hydrogen depends on using either nuclear or solar electricity. The law of conservation of energy tells us that all the energy to be obtained by burning the hydrogen must be supplied by the primary source, e.g. nuclear or solar. Of course, since these processes aren’t 100 percent efficient, there is some loss of energy. Therefore, the use of hydrogen as an intermediate is justified only when there is some reason not to use the primary source directly. For vehicles the reason is that both nuclear nor solar power plants are too big to carry around, except that nuclear power is suitable for large ships.
-4. If there is large scale use of solar energy, the energy is likely to be generated far from where it is used and at a different time. Hydrogen has been proposed as both a storage and transmission medium. It should work for these purposes. Hydrogen can be transported by pipelines similar to those used to transport natural gas. There are some additional problems, because hydrogen tends to leak more and can embrittle some metals used for pipelines. The existence of a 240 km hydrogen pipeline in Germany operated by the company Air Liquide provides evidence that these difficulties can be overcome. There is also 879 km hydrogen pipeline network in Belgium, France, and the Netherlands operated by Air Liquide.
______
______
Hydrogen energy density:
Different fuels have different energy density levels (see figure below), which can be measured in terms of equivalent energy released through combustion. Energy density is the amount of energy that can be released by a given mass or volume of fuel. It can be measured in terms of gravimetric energy density (per unit of mass) or volumetric energy density (per unit of volume). Gravimetric energy density is relevant when comparing the energy efficiency of fuels. At the same time, volumetric energy density is relevant when comparing transportation modes as storage space must be present to carry the fuel propelling a vehicle. The higher the energy density, the higher the fuel quality, which is inversely proportional to its chemical complexity. High-quality fuels are gases, while low-quality fuels are solids, with liquids in between. The highest energy density fuel is hydrogen, which is also the simplest chemical component in existence.
_
The gas weighs almost nothing but has an extremely high gravimetric energy density. One kilogram of hydrogen contains a vast amount of energy, making it an efficient and lightweight energy carrier. In turn, the volumetric energy density of hydrogen is particularly low. Per volume, the energy content of hydrogen is even a lot lower than that of most other fuels and energy carriers. Consequently, storing or using hydrogen at atmospheric pressure and temperature requires a substantial amount of space. Fortunately, there is a solution to this. By compressing or liquefying hydrogen, it is possible to raise the low volumetric energy density. This makes the storage, transportation, and application of hydrogen considerably easier.
|
Results from various sources |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
Fuel and storage method energy densities |
||||||||||||||||||||
_
At any pressure, hydrogen gas clearly carries less energy per volume than methane (representing natural gas), methanol, propane or octane (representing gasoline). At 800 bar pressure gaseous hydrogen reaches the volumetric energy density of liquid hydrogen. But at any pressure, the volumetric energy density of methane gas exceeds that of hydrogen gas by a factor of 3.2. The common liquid energy carriers like methanol, propane and octane (gasoline) surpass liquid hydrogen by factors 1.8 to 3.4, respectively. But at 800 bar or in the liquid state hydrogen must be contained in hi-tech pressure tanks or in cryogenic containers, while the liquid fuels are kept under atmospheric conditions in unsophisticated containers.
Although methane and hydrogen both have higher energy density than gasoline, their gaseous form creates storage difficulties. One of the most efficient energy storage devices, the lithium battery, can only hold about the equivalent of 0.5 MJ per kilogram, underlining the challenge of developing electric vehicles.
____
Hydrogen is locked up in enormous quantities in water, hydrocarbons, and other organic matter. One of the challenges of using hydrogen as a fuel comes from being able to extract hydrogen efficiently from these compounds. Now, steam reforming, which combines high-temperature steam with natural gas, accounts for the majority of the hydrogen produced. This method of hydrogen production occurs at temperatures between 700-1100 °C, and has a resultant efficiency of between 60-75%. Hydrogen can also be produced from water through electrolysis, which is less carbon-intensive if the electricity used to drive the reaction does not come from fossil-fuel power plants but rather renewable or nuclear energy instead. The efficiency of water electrolysis is between about 70-80%, with a goal set to reach 82-86% efficiency by 2030 using proton exchange membrane (PEM) electrolyzers. Once produced, hydrogen can be used in much the same way as natural gas – it can be delivered to fuel cells to generate electricity and heat, used in a combined cycle gas turbine to produce larger quantities of centrally produced electricity or burned to run a combustion engine; all methods producing no carbon or methane emissions. In each case hydrogen is combined with oxygen to form water. This is also one of its most important advantages as hydrogen fuel is environmentally friendly. The heat in a hydrogen flame is a radiant emission from the newly formed water molecules. The water molecules are in an excited state on the initial formation and then transition to a ground state; the transition releasing thermal radiation. When burning in air, the temperature is roughly 2000 °C (the same as natural gas). Historically, carbon has been the most practical carrier of energy, as hydrogen and carbon combined are more volumetrically dense, although hydrogen itself has three times the energy density per mass as methane or gasoline. Although hydrogen is the smallest element and thus has a slightly higher propensity to leak from venerable natural gas pipes such as those made from iron, leakage from plastic (polyethylene PE100) pipes is expected to be very low at about 0.001%.
The reason steam methane reforming has traditionally been favoured over electrolysis is that whereas methane reforming directly uses natural gas, electrolysis requires electricity. As the cost of producing electricity (via wind turbines and solar PV) falls below the cost of natural gas, electrolysis becomes cheaper than SMR.
______
______
Section-4
Introduction to Hydrogen as chemical, feedstock and fuel:
_
Hydrogen is not found in free form (H2) but must be liberated from molecules such as water or methane. It is therefore not an energy source and must be made, using energy. It is already a significant chemical product, about half of annual pure hydrogen production being used in making nitrogen fertilisers via the Haber process and about one-quarter to convert low-grade crude oils (especially those from tar sands) into liquid transport fuels. There is a lot of experience handling hydrogen on a large scale, though it is not as straightforward as natural gas.
_
Most hydrogen today is made by steam reforming of natural gas or coal gasification, both with carbon dioxide (CO2) emissions. Future demand will be mainly for zero-carbon hydrogen. Plans for increased hydrogen production are essentially based on electrolysis using electricity from intermittent renewable sources. Off-peak capacity of conventional nuclear reactors or other power plants can also be used. In future, a major possibility for zero-carbon hydrogen production is decomposition of water by direct use of heat from nuclear energy, using a thermochemical process enabled by high-temperature reactors. The rapidly-growing demand for hydrogen by oil refineries and chemical plants favours technologies with low costs. Limited hydrogen pipeline networks already exist, allowing production facilities to be some way from users.
_
According to the International Energy Agency (IEA), in 2018 demand for pure hydrogen was about 74 million tonnes (Mt), of which 38.2 Mt was used in oil refining and 31.5 Mt in ammonia production. There was a further 42 Mt of demand for hydrogen mixed with other gases such as carbon monoxide i.e., synthesis gas. Of this, 12 Mt was used in methanol production and 4 Mt in direct-reduced iron (DRI) for steel. Like electricity, hydrogen is an energy carrier (but not a primary energy source). Hydrogen has some potential to replace oil as a transport fuel and in other applications. It is the preferred fuel for fuel cell electric vehicles (FCEVs), though portable storage at vehicle scale is a challenge. Hydrogen can also be used in internal combustion engines.
_
While hydrogen can replace liquid hydrocarbons, it is never as energy-dense by volume or convenient to store and transport. However, it does compare well with batteries, which is why it and hydrogen-based liquid fuels have a lot of potential. Electricity and hydrogen are convertible one to the other as energy carriers. However, the overall efficiency of electricity-hydrogen-fuel cell-electricity is no more than 40%. One approach to mitigate intermittency of wind and solar electricity is to make hydrogen by electrolysis and feed this into the gas grid, the power-to-gas strategy. It has been suggested that most electricity from wind might be used thus, greatly simplifying electrical grid management.
_
In future some hydrogen produced for fuel may be converted into ammonia as a more energy-dense carrier medium volumetrically for trade or long-term energy storage. The mass energy density of hydrogen is LHV 120 or HHV 142 MJ/kg, compared with methane 50 MJ/kg, propane 46 MJ/kg and ammonia 19 MJ/kg. Volumetrically, the energy density of hydrogen is low – LHV 10.8 or HHV 12.75 MJ/Nm3 (or as liquid: LHV 8.5 or HHV 10.0 MJ/L). Ammonia is the main hydrogen derivative in consideration for transport.
All this points to the fact that while a growing hydrogen economy already exists, linked to the worldwide chemical and refining industry, and potentially for steelmaking, a much greater one is in sight. With new uses for hydrogen as a fuel, the primary energy demand for its production may exceed that for electricity production.
Transport is expected to constitute the largest demand for hydrogen in both the EU and South Korea by 2050, reflecting the conversion of the heavy vehicle and large passenger car fleet from diesel to hydrogen FCEVs. Heating for buildings is expected to be the next largest demand, replacing natural gas. Railways can replace diesel fuel with hydrogen. If hydrogen is to fuel shipping or aviation, refuelling needs to be available worldwide, so Europe’s ambitious plans will need much wider take-up.
A September 2020 report by LucidCatalyst said that in addition to deployment of renewables for power generation: “The only known way to address the ‘difficult-to-decarbonize’ economic sectors is with the large-scale use of hydrogen as a clean energy carrier and as a feedstock for synthetic fuels such as ammonia.” But clean hydrogen needs to come from non-fossil sources, at a lower price – under $1/kg – than is prospective today. An abundant supply of low-cost hydrogen would greatly boost world agricultural productivity through increased availability of nitrogen fertilizers, as well as fuelling transport.
_
Salient points:
- Hydrogen is increasingly seen as a key component of future energy systems if it can be made without carbon dioxide emissions.
- It is starting to be used as a transport fuel, despite the need for high-pressure containment.
- The use of hydrogen in the production of liquid transport fuels from crude oil is increasing rapidly, and is vital where tar sands are the oil source.
- Hydrogen can be combined with carbon dioxide to make methanol or dimethyl ether (DME) which are important transport fuels.
- Hydrogen also has future application as industrial-scale replacement for coke in steelmaking and other metallurgical processes.
- Nuclear energy can be used to make hydrogen electrolytically, and in the future high-temperature reactors are likely to be used to make it thermochemically.
- The energy demand for hydrogen production could exceed that for electricity production today.
_
Although a considerable part of the global energy demand is currently served by fossil fuels, the harmful impacts of the combustion of fossil fuels are unignorable: greenhouse gas, acid rain, etc., which are devastating to the environment and human beings. To this end, global energy transformation is gaining momentum, which is accelerated by the rapid development of using renewable energy. To enhance this momentum and to mitigate emissions, hydrogen has been explored as a substitute energy carrier, while generating electricity from hydrogen using a fuel cell causes no local pollution because the only byproduct is pure water. Another advantage of hydrogen lies in its high specific energy density. It can provide three times more energy than gasoline combustion per unit mass. Also, hydrogen can be locally produced, which reduces countries’ dependence on external energy suppliers. Besides, hydrogen can be extracted from an extensive range of substances, such as water, oil, gas, biofuels, sewage sludge, etc. In particular, the abundance of water on earth assures the production of hydrogen in a rather sustainable way. Splitting water by electrolysis offers promising opportunities for synergy with the renewable energy. The hydrogen can be produced before it is used due to the intermittent nature of some renewable energy resources so that it is suitable for distributed production and centralised production connected directly to the remote renewable resources. The hydrogen produced from an electrolyser is perfect for use with fuel cells. Stationary fuel cell technologies also facilitate the development of distributed power backup, stand-alone power plants and co-generation. It provides a substituted option of the traditional power grid because combined with a fuel cell, the electricity can be produced when and where it is needed so that the hydrogen does not necessarily to be stored.
_
Hydrogen does not generally exist in the free-state rather it occurs in compounds which means other energy sources have to be used to separate it. Broadly speaking, the compounds are water, natural gas and coal or biomass. Selection of the method of separation determines the overall environmental impact of hydrogen. Once produced, hydrogen can be in the form of a compressed gas, cryogenic liquid or chemical, each requiring specific methods of storage essential to successful distribution. As an energy carrier, hydrogen has many applications which can be categorised as stationary, mobile, backup or speciality. For hydrogen to make it into general use, production, distribution and application must be moderated by safety, reliability, the market place and education. Technical difficulties still present hurdles to the everyday use of hydrogen. These are being confronted and dealt with by researchers and developers around the world.
_
The reduction in the cost of renewable electricity has fueled the ‘green hydrogen hope.’ Interestingly, this is not the first time hydrogen is being projected to solve contemporary energy issues. Historically, hydrogen was first included as a part of energy policy in the 1970s after the oil embargo, but new discoveries of oil reserves damped out the efforts in subsequent decades. Although it was perceived as a solution to climate change on two separate occasions; once in the 1990s and again in the 2000s, the low oil prices, economic and financial crisis, and popularity of alternative renewable technologies like solar PV and wind affected the concrete financial support required for its development. Today, high technology costs, lack of adequate international supply chain, and lack of awareness impact the commercialization, infrastructure development, and demand creation of hydrogen-based technology.
______
IEA 2018 report:
The time is right to tap into hydrogen’s potential to play a key role in a clean, secure and affordable energy future. At the request of the government of Japan under its G20 presidency, the International Energy Agency (IEA) has produced a landmark report to analyse the current state of play for hydrogen and to offer guidance on its future development. The report finds that clean hydrogen is currently enjoying unprecedented political and business momentum, with the number of policies and projects around the world expanding rapidly. It concludes that now is the time to scale up technologies and bring down costs to allow hydrogen to become widely used. The pragmatic and actionable recommendations to governments and industry that are provided will make it possible to take full advantage of this increasing momentum.
-1. Hydrogen can help tackle various critical energy challenges.
It offers ways to decarbonise a range of sectors – including long-haul transport, chemicals, and iron and steel – where it is proving difficult to meaningfully reduce emissions. It can also help improve air quality and strengthen energy security. Despite very ambitious international climate goals, global energy-related CO2 emissions reached an all time high in 2018. Outdoor air pollution also remains a pressing problem, with around 3 million people dying prematurely each year.
-2. Hydrogen is versatile.
Technologies already available today enable hydrogen to produce, store, move and use energy in different ways. A wide variety of fuels are able to produce hydrogen, including renewables, nuclear, natural gas, coal and oil. It can be transported as a gas by pipelines or in liquid form by ships, much like liquefied natural gas (LNG). It can be transformed into electricity and methane to power homes and feed industry, and into fuels for cars, trucks, ships and planes.
-3. Hydrogen can enable renewables to provide an even greater contribution.
It has the potential to help with variable output from renewables, like solar photovoltaics (PV) and wind, whose availability is not always well matched with demand. Hydrogen is one of the leading options for storing energy from renewables and looks promising to be a lowest-cost option for storing electricity over days, weeks or even months. Hydrogen and hydrogen-based fuels can transport energy from renewables over long distances – from regions with abundant solar and wind resources, such as Australia or Latin America, to energy-hungry cities thousands of kilometers away.
-4. There have been false starts for hydrogen in the past; this time could be different.
The recent successes of solar PV, wind, batteries and electric vehicles have shown that policy and technology innovation have the power to build global clean energy industries. With a global energy sector in flux, the versatility of hydrogen is attracting stronger interest from a diverse group of governments and companies. Support is coming from governments that both import and export energy as well as renewable electricity suppliers, industrial gas producers, electricity and gas utilities, automakers, oil and gas companies, major engineering firms, and cities. Investments in hydrogen can help foster new technological and industrial development in economies around the world, creating skilled jobs.
Hydrogen can be used much more widely. Today, hydrogen is used mostly in oil refining and for the production of fertilisers. For it to make a significant contribution to clean energy transitions, it also needs to be adopted in sectors where it is almost completely absent at the moment, such as transport, buildings and power generation.
_______
David Yellen, program assistant for the Global Energy Center at the Atlantic Council, called green hydrogen the “Swiss Army knife” of the transition to clean energy. It is a single product that can service multiple markets and, if produced using low- or zero-emissions energy sources, it can help us significantly cut greenhouse emissions. The last decade has seen “amazing cost reductions” in solar and wind power and especially in batteries. But those energy sources won’t do well for heavy manufacturing, long-distance transport such as trucking, cargo ships and aircraft, so green hydrogen, with its high-energy density, is expected to fill the gaps. For this big investment for hydrogen to pay off, it’s always going to have to compete with batteries. The weight of a battery is an issue for air travel. Jet fuel burns as it goes, but batteries stay the same weight throughout the trip. Also, the space batteries take up make them problematic for trucks, while hydrogen can be used in steel mills due to its ability to produce high-quality heat and in the form of ammonia on ships (which serves as fuel). Hydrogen also beats batteries for long-term storage. Compared with batteries, hydrogen can release more energy per unit of mass. This means that in contrast to electric battery-powered cars, it can allow passenger vehicles to cover longer distances without refuelling. Refuelling is quicker too, and is likely to stay that way. The benefits are potentially even greater for heavy vehicles such as buses and trucks which already carry heavy payloads, and where lengthy battery recharge times can affect business models.
Hydrogen can also play an important role in energy storage, which will be increasingly necessary both in remote operations such as mine sites, and as part of the electricity grid to help smooth out the contribution of renewables such as wind and solar. This could work by using the excess renewable energy (when generation is high and/or demand is low) to drive hydrogen production via electrolysis of water. The hydrogen can then be stored as compressed gas and put into a fuel cell to generate electricity when needed.
____
____
Types of hydrogen:
Hydrogen gas is colourless and completely invisible. Hydrogen is produced via different methods and the ascribed colour merely serves to indicate the way in which it has been produced. You might encounter the terms ‘grey’, ‘blue’, ‘green’ being associated when describing hydrogen technologies. It all comes down to the way it is produced. Hydrogen emits only water when burned but creating it can be carbon intensive. Depending on production methods, hydrogen can be grey, blue or green – and sometimes even pink, yellow or turquoise. However, green hydrogen is the only type produced in a climate-neutral manner making it critical to reach net zero by 2050.
_
Grey Hydrogen:
The most common form of hydrogen, it’s created from fossil fuels and the process releases carbon dioxide which is not captured. The process used to create hydrogen from natural gas is called steam methane reforming (SMR), where high-temperature steam (700°C–1,000°C) is used to produce hydrogen from a methane source, such as natural gas. In steam methane reforming, methane reacts with steam under 3–25 bar pressure (1 bar = 14.5 pounds per square inch) in the presence of a catalyst to produce hydrogen, carbon monoxide, and a relatively small amount of carbon dioxide. Steam reforming is endothermic — that is, heat must be supplied to the process for the reaction to proceed.
There is also a gasification process which uses coal as a feedstock, creating brown hydrogen, which also releases carbon dioxide and can be put in the same category as grey.
According to studies, steam reforming produces about 9.3 kg of CO2 for every kilogram of hydrogen produced. The head of business development at the renewable energy giant Enel has described hydrogen as a “climate killer” as it stands right now due to almost all of it being grey: “98% of it is produced from steam reforming and gasification, which equates to yearly carbon emissions comparable to that of Indonesia and the UK combined,” he said. “Just 2% is produced from electrolysis.”
Clearly then, grey hydrogen is not a long-term solution.
_
Blue Hydrogen:
Blue hydrogen uses the same process as grey, except this time the carbon is captured and stored. This makes it much more environmentally friendly, but comes with added technical challenges and a big increase in cost.
Carbon capture and storage (CCS) has been around a while, with the technology being used by heavy industry and power generation companies burning fossil fuels. The technology can capture up to 90% of the CO2 produced, so it isn’t perfect but clearly a massive improvement. Most of the time, this CO2 is then transported by a pipeline and stored deep underground, often in salt caverns or depleted oil and gas reservoirs.
Countries which do not have access to such underground options will find it very challenging to establish a blue hydrogen industry, and it may be more cost-effective to develop green hydrogen as their primary solution.
Some forward thinking organisations like Drax in the UK have been combining CCS with biomass fuels, aiming to become carbon negative — removing more carbon dioxide from the atmosphere than it produces.
When it comes to hydrogen production, blue hydrogen is often seen as a stepping stone from grey to green, and it’s proven to be divisive among industry professionals. Its proponents see it as a necessary step until technologies allow a full transition to green hydrogen. However, at least 10-20% of the CO2 generated always escapes capture systems, so blue hydrogen cannot be considered a clean, low-carbon form of energy.
_
Green hydrogen:
Rather than using fossil fuels, green hydrogen is made by using a process called electrolysis to split water into hydrogen and oxygen. If that process is powered by a renewable energy source, such as wind or solar power, then the hydrogen is referred to as being green. It has the potential to be a major part in solving the intermittent generating capacity of most renewable energy sources. Excess electricity can be used to create hydrogen, which is then stored as a gas or liquid until needed. It faces many challenges, but the momentum behind it is growing with governments around the world recognising the potential benefits and developing policies to help drive development and adoption.
Creating green hydrogen needs a huge amount of electricity, which means a mind-blowing increase in the amount of wind and solar power to meet global targets. Some current estimates are that that we need to install more offshore wind capacity than in the previous 20 years, every year for the next 30 years.
To become competitive, the price per kilogram of green hydrogen has to reduce to a benchmark of $2/kg, with Bloomberg New Energy Finance reporting that $1/kg is achievable by 2050. At these prices, green hydrogen can compete with natural gas. Costs for producing green hydrogen have fallen 50% since 2015 and could be reduced by an additional 30% by 2025 due to the benefits of increased scale and more standardized manufacturing, among other factors.
_
Other hydrogen colours:
Turquoise – This hydrogen color refers to thermal splitting of methane using methane pyrolysis. This method removes carbon in the form of a solid instead of a gas and remains an experimental method of H2 production.
Black or Brown – This H2 is made using methods powered by bituminous (black) or lignite (brown) coal. The technique used for H2 production is called coal gasification, which is highly polluting and releases copious carbon dioxide and carbon monoxide into the atmosphere, among other biproducts.
Purple, Pink, and Red – These hydrogen colors refer to H2 produced using nuclear power plants. The purple form uses nuclear power and heat to split water via combined chemo thermal electrolysis. Pink uses the electricity produced by a nuclear plant to power water electrolysis. Red uses nuclear power thermal energy to power high-temperature catalytic water splitting.
Cyan Hydrogen -This is hydrogen produced on site (de-centralized production) by steam methanation of Renewable Natural Gas with carbon capture. Renewable Natural Gas is obtained from organic waste sites (farms, landfill gas, municipal waste, and food spoilage).
_
Hydrogen is the simplest and smallest element in the periodic table. No matter how it is produced, it ends up with the same carbon-free molecule. However, the pathways to produce it are very diverse, and so are the emissions of greenhouse gases like carbon dioxide (CO2).
Graphic below showing the green, grey and blue hydrogen.
Many other colours have been added to the palette, but the focus on colour is a distraction. What really matters is the carbon intensity of the production process — that is, the tonnes of carbon produced for each tonne of hydrogen.
_
Low-carbon production of hydrogen:
There are several ways to produce hydrogen, with varying carbon footprints. Figure below depicts the lower-carbon options of hydrogen production.
To date, neither renewable hydrogen (green) nor hydrogen produced from fossil fuels with CO2 capture systems (blue) can compete with the production costs of hydrogen generated from fossil fuels (grey). The estimated costs of green hydrogen range between €2.5 and €5.5 per kilogram, compared to €2/kg for blue hydrogen and €1.5/kg for grey hydrogen in Europe. The costs of green hydrogen are falling rapidly. Over the past 10 years, electrolyser costs have fallen by 60% and are expected to halve by 2030. Renewables solar/wind costs are also falling. The sector surely promises interesting developments.
____
____
Countries exploring the use of hydrogen:
Influential organisations, including the IEA, Hydrogen Council and BP, have all revealed their visions for its future significance and others have heralded the 2020s as the “decade of hydrogen”. The pipeline for “green” hydrogen – produced using renewable electricity – is expanding rapidly. However, these projects still make a marginal contribution to the global energy system.
Only six of the 197 parties to the 2015 Paris Agreement mentioned “hydrogen” in their first nationally determined contributions to the deal, but interest is growing as a wave of countries, encouraged by net-zero targets, set out national hydrogen strategies. A recent review by the World Energy Council’s German chapter found that 20 countries have introduced such strategies or are “on the verge of doing so”. The map below is based on updated analysis by the council shared with Carbon Brief. It shows another 33 countries moving in this direction. This led the group to conclude that by 2025 these strategies will likely cover countries representing over 80% of global GDP.
Map above shows extent of hydrogen support in various countries, including national strategies, pilot and demonstration projects and policy discussions.
One of the most significant announcements is the European Commission’s “hydrogen strategy for a climate-neutral Europe”, released in July 2020, which includes an ambitious target of 40 gigawatts (GW) of European electrolyser capacity to produce “green” hydrogen by 2030.
_____
_____
Circulative Hydrogen Energy that does not rely on Fossil Fuel:
Hydrogen is not naturally occurring, but can be found in various other substances, and can be extracted. Hydrogen can be generated from natural gas and biomass, and electrolysis using renewable energies such as solar, wind or hydro-power generation. As hydrogen can be generated using methods suited to regional characteristics and climate, an ideal hydrogen cycle that does not rely on fossil fuel can be established in the future. Electricity can be stored in batteries, but large capacities cause problems. Electricity is normally generated at power plants, and is constantly supplied based on usage forecasts. Hydrogen can be made, transported and stored, and used to generate electricity when required.
_______
_______
Hydrogen production paths:
Hydrogen is not an energy source, but is an energy vector or carrier. This means that it has to be produced from one of the primary energy sources: fossil fuels, nuclear, solar, wind, biomass, hydro, geothermal and urban waste resources. All the energy we use, including hydrogen, must be produced from one of these primary energy resources. On earth, hydrogen is found combined with other elements. For example, in water, hydrogen is combined with oxygen. In fossil fuels, it is combined with carbon as in petroleum, natural gas or coal. The challenge is to separate hydrogen from other naturally occurring compounds in an efficient and economic manner.
See the “Hydrogen Production Paths” chart below for unique ways to produce hydrogen from these sources.
Chart above displays possible hydrogen production paths.
Most hydrogen is produced through steam methane reforming, a high-temperature process in which steam reacts with a hydrocarbon fuel to produce hydrogen.
Another common hydrogen production method takes water, and separates the molecule H2O into oxygen and hydrogen through a process called electrolysis. Electrolysis takes place in an electrolyzer, which functions much like a fuel cell in reverse—instead of using the energy of a hydrogen molecule, like a fuel cell does, an electrolyzer produces hydrogen from water molecules using electricity energy.
Biological processes can also produce hydrogen through biological reactions using microbes such as bacteria and microalgae. In these processes, microbes consume plant material and produce hydrogen gas.
There are many ways to produce hydrogen using sunlight, including photobiological, photoelectrochemical, photovoltaic-driven electrolysis, and solar thermochemical processes.
_____
_____
Hydrogen uses:
Most of this hydrogen is used as a chemical, rather than a fuel, in a variety of commercial applications:
-Commercial fixation of nitrogen from the air to produce ammonia for fertilizer (about two-thirds of commercial hydrogen is used for this)
-Hydrogenation of fats and oils, in which vegetable oils are changed from liquids to solid
-Methanol production, in hydrodealkylation, hydrocracking, and hydrodesulphurization
-Welding
-Hydrochloric acid production
-Metallic ore reduction
-Cryogenics and the study of superconductivity (liquid hydrogen)
-Hydrogen’s main use as a fuel is in the space program. Today hydrogen fuels both the main engine of the Space Shuttle and the onboard fuel cells that provide the Shuttle’s electric power.
-As of 2021, there are two models of hydrogen cars publicly available in select markets: the Toyota Mirai, which is the world’s first mass-produced dedicated fuel cell electric vehicle (FCEV), and the Hyundai Nexo
-In Germany, the world’s first hydrogen train has been running in regular service since 2018
-Several manufacturers are working on the development of hydrogen-powered trucks
-Public transport company De Lijn has commissioned five hydrogen buses in Antwerp
-Retail group Colruyt has 13 hydrogen-powered cars and 75 hydrogen-powered forklifts
-Hydrogen is used as cooking and heating fuel in homes and buildings blended with natural gas.
_
One of the most potentially useful ways to use hydrogen is in electric cars or buses in conjunction with a fuel cell which converts the hydrogen into electricity. Fuel cells are attractive because they’re far more efficient than the internal combustion engines they can replace – though the latter can still be used with hydrogen fuels if desired. At the moment, hydrogen is most commonly produced from natural gas. In this situation, a typical fuel cell car generates 70–80g CO2 for each kilometer driven – similar to a modern gasoline hybrid or to a battery electric vehicle charged with today’s UK grid electricity. These emissions can be reduced towards zero if the hydrogen is produced using low-carbon electricity sources such as renewables, nuclear or CCS to electrolyse water. The downside is that in this situation only around half as much electricity comes out of the fuel cell as was put in to produce the hydrogen in the first place. The rest is lost as heat. Partly for this reason, and partly due to concerns over the commercial readiness of hydrogen fuel cell cars, battery-based electric cars have received more attention in recent years than hydrogen cars. However, hydrogen vehicles retain a number of important advantages: they can be rapidly refuelled in just a couple of minutes and have a range of many hundreds of kilometers. So the best technology depends on the final cost, carbon mitigation potential, and consumer needs in each case.
___
Potential Applications:
-1. Hydrogen can be used in fuel cells to power a wide variety of applications, both mobile and stationary, small- and large-scale. Fuel cells can be used to provide clean energy for transportation. And because they are modular, fuel cells can provide heat and electricity for single homes or to supply the energy to run an entire large commercial building, to provide a small amount of electricity to a community grid, or a large amount of electricity to a large grid network.
-2. Hydrogen can be used to generate electricity for our homes and office buildings, through the use of gas turbines and microturbines (small gas turbines). Conventional gas turbines can be modified to run efficiently on hydrogen or hydrogen/natural gas blends. Microturbines can provide high-efficiency reliable power for smaller-scale applications. Hydrogen and ammonia can be used in gas turbines to increase power system flexibility. Ammonia could also be used in coal-fired power plants to reduce emissions.
-3. Hydrogen can also be used in internal combustion engines for both stationary and mobile applications, powering industrial processes, ocean fleets, cars, and buses. As with gas turbines, conventional combustion engines can be modified to run efficiently on hydrogen or hydrogen/natural gas mixtures for these applications.
-4. Hydrogen may also be useful as a way to store renewable energy from intermittent sources – for example, when the wind is blowing but there is not high demand for electricity. In this context, it’s an alternative to large-scale batteries or other storage systems.
-5. In transport, the competitiveness of hydrogen fuel cell cars depends on fuel cell costs and refuelling stations while for trucks the priority is to reduce the delivered price of hydrogen. Shipping and aviation have limited low-carbon fuel options available and represent an opportunity for hydrogen-based fuels.
It is the flexibility that hydrogen offers that makes it so potentially useful within future low-carbon energy systems. It can be produced from a wide variety of resources and can be used in a wide range of applications, such as power generation, as a transport fuel for low carbon vehicles, for the chemical industry, and for low carbon heating. Moreover, hydrogen is already used extensively in the chemical industry so industry is familiar with its production, handling and distribution on a large scale. For all these reasons, many experts see hydrogen as a key enabler of the lowest-cost low-carbon energy system.
_______
_______
Challenges and problems with using hydrogen as energy carrier:
-1. It takes energy to produce hydrogen:
Most hydrogen in the world is made by applying heat to natural gas in a process known as “reforming” hydrogen. Making hydrogen from natural gas is 72% efficient, which means you lose 28% of the energy contained in the natural gas to make hydrogen. It also takes energy to extract and deliver the natural gas to the hydrogen plant. Hydrogen generation via electrolysis requires a greater energy input than directly using renewable energy.
-2. Infrastructure:
The first hydrogen-powered cars will probably be sold as fleet vehicles to companies covering a limited geographical area, for example taxis, busses, local council vehicles, and courier cars. The absence of “hydrogen filling stations” on every corner would not present a problem for these types of vehicles. The introduction of hydrogen as a propellant fuel on a larger scale and for the use in private automobiles would however require an established infrastructure. The development of hydrogen infrastructure is slow and holding back widespread adoption. Hydrogen prices for consumers are highly dependent on how many refuelling stations there are, how often they are used and how much hydrogen is delivered per day. Tackling this is likely to require planning and coordination that brings together national and local governments, industry and investors.
The German auto maker BMW has equipped their first hydrogen cars with tanks for both hydrogen and gasoline such that the engine automatically changes over from hydrogen to gasoline in the event that the hydrogen tank should run dry. This is technically possible because the cars utilise an ordinary combustion engine. However, BMW assumes that there will be a sufficient number of German hydrogen stations in the course of the next few years.
-3. It all comes down to cost:
Cost of hydrogen:
The various obstacles green hydrogen faces can actually be reduced to just one: cost. Julio Friedmann, senior research scholar at Columbia University’s Center on Global Energy Policy, believes the only real challenge of green hydrogen is its price. The fact that 75 million tons of hydrogen are produced every year and that it is shipped in pipelines around the U.S. shows that the technical issues of distributing and using hydrogen are “straightforward, and reasonably well understood,” he said.
The problem is that green hydrogen currently costs three times as much as natural gas in the U.S. And producing green hydrogen is much more expensive than producing grey or blue hydrogen because electrolysis is expensive, although prices of electrolyzers are coming down as manufacturing scales up. Currently, grey hydrogen costs about €1.50 euros per kilogram, blue costs €2 to €3 per kilogram, and green costs €3.50 to €6 per kilogram, according to a recent study.
Producing hydrogen from low-carbon energy is costly at the moment. IEA analysis finds that the cost of producing hydrogen from renewable electricity could fall 30% by 2030 as a result of declining costs of renewables and the scaling up of hydrogen production. Fuel cells, refuelling equipment and electrolysers (which produce hydrogen from electricity and water) can all benefit from mass manufacturing.
Cost of hydrogen car:
Hydrogen fuel cell cars are relatively expensive to buy. The 2022 Toyota Mirai cost $49,500, far more costly than fully electric 2022 Nissan Leaf S 40 kW: $28,375. There are a range of reasons why hydrogen fuel cell cars are still expensive. In addition to small volumes, which means that production is still to be industrialized, there’s also the question of the need for the precious metal platinum, which acts as a catalyst during power generation. The amount of platinum needed for vehicle fuel cells has already been greatly reduced. Another reason for the high purchase price is that hydrogen fuel cell cars tend to be quite large because the hydrogen tank(s) take up a lot of space.
-4. Storage & transport:
Hydrogen contains a lot of energy per unit of weight while the content of energy per unit of volume is quite low. This poses a potential problem in terms of storing large amounts of hydrogen in a small space. Hydrogen has the lowest volumetric energy density of any fuel. At room temperature and pressure, hydrogen takes up 3,000 times the volume of gasoline containing an equivalent amount of energy. To be useable, hydrogen needs to be compressed or liquefied and more energy is lost:
-Compressing hydrogen to 10,000 psi is a multi-stage process that loses 15% of the energy contained in the hydrogen. At 10,000 psi pressure, compressed hydrogen is dangerous and must be stored in heavy tanks.
-Liquefying hydrogen allows more hydrogen energy to fit into a smaller container, but a further 30-40% of the hydrogen’s energy is lost in the process. Handling liquid hydrogen requires extreme precautions because it’s so cold (– 253ºC) and volatile. Fueling is typically done mechanically with a robot arm. A vehicle using liquid hydrogen needs a cryogenic support system, which is heavy, and insulation adds more weight. Also, the cold temperatures cause plugged valves and other problems.
It is difficult and expensive to transport:
Currently, hydrogen is transported through dedicated pipelines, in low-temperature liquid tanker trucks, in tube trailers that carry gaseous hydrogen, or by rail or barge.
Canister trucks can carry enough fuel for 60 cars. These trucks weigh 40,000 kg but deliver only 400 kg of hydrogen. For a delivery distance of 150 miles, the delivery energy used is nearly 20% of the usable energy in the hydrogen delivered; at 300 miles it is 40%. The same size truck carrying gasoline delivers 10,000 gallons of fuel, enough to fill about 800 cars.
Today 1,600 miles of hydrogen pipelines deliver gaseous hydrogen around the U.S., mainly in areas where hydrogen is used in chemical plants and refineries, but that is not enough infrastructure to accommodate widespread use of hydrogen.
Natural gas pipelines are sometimes used to transport only a limited amount of hydrogen because hydrogen can make steel pipes and welds brittle, causing cracks. When less than 5 to 10 percent of it is blended with the natural gas, hydrogen can be safely distributed via the natural gas infrastructure. To distribute pure hydrogen, natural gas pipelines would require major alterations to avoid potential embrittlement of the metal pipes, or completely separate hydrogen pipelines would need to be constructed. The average cost of a natural gas pipeline is about $5 million per mile. The US has about 300,000 miles of natural gas pipeline. Hydrogen-specific steel pipelines can cost as much as 68 percent more than natural gas pipelines, depending on pipe diameter and operating pressure. The major operating cost of hydrogen pipelines is compressor power and maintenance. Compressors in the pipeline keep the gas moving, using hydrogen energy to push the gas forward. After 620 miles, 8% of the hydrogen will have been used to move it through the pipeline.
-5. Standards and legal framework:
Regulations currently limit the development of a clean hydrogen industry. Government and industry must work together to ensure existing regulations are not an unnecessary barrier to investment. Trade will benefit from common international standards for the safety of transporting and storing large volumes of hydrogen and for tracing the environmental impacts of different hydrogen supplies. The early introduction of international standards for all countries is important in avoiding unnecessary extra costs such as redesign as a consequence of diverging standards and safety requirements. Standardisation would also simplify the international trading of hydrogen technology. In this vein, the International Organisation for Standardisation (ISO) has established a technical committee for hydrogen technology, the ”ISO/TC 197 – Hydrogen technology”.
In March 1999 the first hydrogen standard was published: – ISO 13984 “Liquid Hydrogen – Land vehicle fuelling system interface”.
In addition, the following seven standards are presently under development:
-ISO 13985 ”Liquid Hydrogen – Land vehicle fuel tanks”- ISO 13986 ”Tank containers for multimodal transportation of liquid hydrogen”
-ISO 15594 ”Airport hydrogen fuelling facility”
-ISO 15866 ”Gaseous hydrogen and hydrogen blends – Service stations”
-ISO 15869 ”Gaseous hydrogen and hydrogen blends – Land vehicle fuel tanks”
-ISO 15916 ”Basic requirements for the safety of hydrogen systems”
-ISO 17268 ”Gaseous hydrogen – Land vehicle fuelling connectors”
Another co-operative effort is the European Integrated Hydrogen Project (EIHP) to develop regulations for hydrogen-powered vehicles so as to co-ordinate development of the technology in Europe. EIHP is a co-operation between European car manufacturers and government agencies.
-6. Safety issues:
Its flammability and its lightness mean that hydrogen, like other fuels, needs to be properly handled. Many fuels are flammable. Compared to gasoline, natural gas, and propane, hydrogen is more flammable in the air. However, low concentrations of hydrogen have similar flammability potential as other fuels. Since hydrogen is so light—about 57 times lighter than gasoline fumes—it can quickly disperse into the atmosphere, which is a positive safety feature. Hydrogen has one of the widest explosive/ignition mix ranges with air. This means that any leak of hydrogen from a hydrogen: air mixture will most likely lead to an explosion if it comes into contact with a spark or flame. This limits the use of hydrogen as a fuel, especially in enclosed areas such as tunnels or underground parking. Pure hydrogen-oxygen flames burn in the UV range and are invisible, so a flame detector is needed to detect if a hydrogen leak is burning. Hydrogen is also odorless, so leaks cannot be detected by smell.
-7. Carbon footprint:
Hydrogen is almost entirely supplied from natural gas and coal today. Hydrogen is already with us at industrial scale all around the world, but its production is responsible for annual CO2 emissions equivalent to those of Indonesia and the United Kingdom combined. Harnessing this existing scale on the way to a clean energy future requires both the capture of CO2 from hydrogen production from fossil fuels and greater supplies of hydrogen from clean electricity.
-8. Fuel Cell Problems:
Fuel cell technology has been constrained by the high cost of fuel cells because platinum, which is expensive, is used at the anode as a catalyst to split hydrogen. The average amount of a platinum in a hydrogen fuel cell is between 30 and 60 grams. Research is ongoing to improve the performance of fuel cells and to find more efficient and less costly materials. Many of the component pieces of a fuel cell are costly. For PEMFC systems, proton exchange membranes, precious metal catalysts (usually platinum), gas diffusion layers, and bipolar plates make up 70 percent of a system’s cost. In order to be competitively priced (compared to gasoline-powered vehicles), fuel cell systems must cost $35 per kilowatt. Currently, the projected high-volume production price is $73 per kilowatt. In particular, researchers must either decrease the amount of platinum needed to act as a catalyst or find an alternative. Researchers must also develop PEMFC membranes that are durable and can operate at temperatures greater than 100 degrees Celsius and still function at sub-zero ambient temperatures. A 100 degrees Celsius temperature target is required in order for a fuel cell to have a higher tolerance to impurities in fuel. Because you start and stop a car relatively frequently, it is important for the membrane to remain stable under cycling conditions. Currently membranes tend to degrade while fuel cells cycle on and off, particularly as operating temperatures rise. Because PEMFC membranes must by hydrated in order to transfer hydrogen protons, researches must find a way to develop fuel cell systems that can continue to operate in sub-zero temperatures, low humidity environments and high operating temperatures. At around 80 degrees Celsius, hydration is lost without a high-pressure hydration system. The SOFC has a related problem with durability. Solid oxide systems have issues with material corrosion. Seal integrity is also a major concern. The cost goal for SOFC is less restrictive than for PEMFC systems at $400 per kilowatt, but there are no obvious means of achieving that goal due to high material costs. SOFC durability suffers after the cell repeatedly heats up to operating temperature and then cools down to room temperature.
______
______
Hydrogen council:
Hydrogen Council is the largest industry-led effort to develop the hydrogen economy. Launched in January 2017 at the World Economic Forum, its members include leading companies that invest along the hydrogen value chain, including transportation, industry, and energy exploration, production, and distribution. The 13 inaugural members included Air Liquide, Alstom, Anglo American plc, BMW, Daimler AG, ENGIE, Honda, Hyundai Motor Company, Kawasaki Heavy Industries, Royal Dutch Shell, The Linde Group, Total S.A., and Toyota Motor Corporation. In a formal statement made at the Forum, Air Liquide Chairman and CEO Benoît Potier stated that the aim of the initiative is “to explain why hydrogen emerges among the key solutions for the energy transition, in mobility as well as in the power, industrial and residential sectors”. The key ambitions of the Hydrogen Council are to 1) accelerate significant investment in the development and commercialization of the hydrogen and fuel cell sectors and 2) encourage key stakeholders to increase their backing of hydrogen as part of the future energy mix. The Council now counts 109 companies from 20+ countries around the world, bringing together an even wider range of sectors along the entire hydrogen value chain.
_
Hydrogen council members are depicted in the figure below:
The 109 members of the Hydrogen Council represent over USD 6.8 trillion in market capitalization and more than 6.5 million employees.
_
Hydrogen council vision: The hydrogen economy in 2050:
Hydrogen could become a major asset in the fight against climate change, cutting CO2 emissions by 20% between now and 2050, according to a McKinsey study for the Hydrogen Council. The prospects opened up by “Hydrogen scaling up” (the title of the study) make the energy vector a highly credible alternative to fossil fuels and a substantial addition to existing renewables: in less than 25 years, hydrogen could account for 18% of global energy consumption and reduce CO2 emissions from current levels by some 6 gigatonnes. The impact would also be economic, with hydrogen generating $2,500 billion in revenue and creating more than 30 million jobs. Hydrogen can play seven major roles in this transformation:
-Enabling large-scale renewable energy integration and power generation
-Distributing energy across sectors and regions
-Acting as a buffer to increase energy system resilience
-Decarbonizing transportation
-Decarbonizing industrial energy use
-Helping to decarbonize building heat and power
-Providing clean feedstock for industry.
In all seven application areas, hydrogen can offer economically viable and socially beneficial solutions.
_
Figure below shows global hydrogen projects across the value chain:
Globally, there are 228 hydrogen projects across the value chain. Of these, 17 are already-announced giga-scale production projects (i.e., more than 1 GW for renewable and over 200 thousand tons a year for low-carbon hydrogen), with the biggest in Europe, Australia, the Middle East and Chile.
______
______
Section-5
Hydrogen in Energy Transition:
The Paris Agreement aims to limit the rise in average global temperature to “well below 2 °C” in this century as compared to pre-industrial levels. Achieving this will require substantial GHG emissions reductions across all sectors. To achieve the targets in the Paris Agreement, the global energy system must undergo a profound transformation from one largely based on fossil fuels to an efficient and renewable low-carbon energy system According to analysis by the International Renewable Energy Agency (IRENA, 2018), over 90 % of the necessary global CO₂ emission reductions could come from these measures; renewable energy is expected to contribute 41 % of the required emission reductions directly and an additional 13 % through electrification. To meet this objective, renewable energy’s share of global final energy consumption needs to increase from 18 % today to 65 % in 2050. Variable renewable energy in the power system, in particular wind and solar, will make up the vast majority of generation capacity and 60 % of all electricity generation The power system needs to become more flexible to economically integrate such large shares of variable generation.
Today, one-third of global energy-related emissions come from economic sectors for which there is presently no economic alternative to fossil fuels (IRENA, 2017a) These emissions originate mostly from the energy intensive industry sectors and freight transport.
Hydrogen could be the “missing link” in the energy transition from a technical perspective: hydrogen from renewable electricity allows large amounts of renewable energy to be channeled from the power sector into sectors for which electrification (and hence decarbonisation) is otherwise difficult, such as transport, buildings and industry. The iron and steel industry globally is currently responsible for around 7 per cent of global CO2 emissions with the dominance of coal-based blast furnace technology. Switching to a direct-reduction process using low-carbon hydrogen could eliminate around 95 per cent of these emissions.
Hydrogen could thus play a key role in facilitating three positive outcomes: the decarbonisation of these sectors; the integration of large amounts of variable renewable energy (VRE); and the decoupling of VRE generation and consumption through the production of transportable hydrogen. However, hydrogen is not economically competitive at present, and therefore significant reductions in the cost of production and distribution need to take place for the decarbonisation of such sectors to take place.
This notion is becoming well recognised around the globe as a result of various developments, which include the need for deep decarbonisation (COP21), the increasing share and decreasing cost of renewable energy sources, wind and solar in particular, and the associated need for additional flexibility in power systems. In parallel, technological advancements and cost reductions in hydrogen-related technologies are increasing the competitiveness of hydrogen from renewable electricity.
_
The last two years have seen growing momentum behind the global recognition of the urgency of the ‘climate emergency’, with more and more countries committing to achieve net zero emissions, typically by 2050 (e.g., UK, European Union, Japan, and South Korea) and by 2060 in the hugely significant case of China. The same two years have also seen a growing conviction that hydrogen will play a significant role in the decarbonisation of the energy system. Electrification will certainly play a much-enlarged role in future, with many commentators suggesting that the share of electricity in final consumption is likely to rise from typically around 20 per cent today to around 50 per cent by 2050. Even if that proves to be an underestimate, it will still leave considerable demand for low-carbon molecules, and, with current technologies, the most likely low-carbon (or preferably zero-carbon) molecule is hydrogen. A growing number of countries have now published national hydrogen strategies, and more such strategies are under development. These strategies set bold ambitions for development of hydrogen but are relatively unclear on the pathways and steps to reach those ambitions.
The scale of transformation of the energy system from one based largely on fossil fuels to one where fossil fuels play a very minor role is enormous, and to complete such a transformation within 30 years requires unprecedented speed. Low-carbon hydrogen is starting from a small base, and current costs do not support a commercial business case. For hydrogen to achieve the ambitious targets which have been set for it in various strategies will require many players across the energy industry (private sector, government, regulators, and consumer groups) to work together to drive the required policies and behaviours. The structures to enable that collaboration will need to be developed as a matter of urgency in the next year or two.
_
Hydrogen status today:
The hydrogen industry is well established and has decades of experience in industry sectors using hydrogen as a feedstock. The hydrogen feedstock market has a total estimated value of USD 115 billion and is expected to grow significantly in the coming years, reaching USD 155 billion by 2022. In 2015 total global hydrogen demand was estimated to be 8 exajoules (EJ) (Hydrogen Council, 2017). The largest share of hydrogen demand is from the chemicals sector for the production of ammonia and in refining for hydrocracking and desulphurisation of fuels. Other industry sectors also use hydrogen, such as producers of iron and steel, glass, electronics, specialty chemicals and bulk chemicals, but their combined share of total global demand is small. Over 95 % of current hydrogen production is fossil-fuel based Steam-methane reforming (SMR). Oil and coal gasification are also widely used, particularly in China and Australia, albeit to a lesser extent than SMR. Only around 4 % of global hydrogen supply is produced via electrolysis, mainly with chlor-alkali processes.
_
Today’s society depends crucially on the uninterrupted availability of affordable fossil fuels which, in future, will be increasingly concentrated in a smaller number of countries – creating the potential for geopolitical and price instability. Hydrogen opens access to a broad range of primary energy sources, including fossil fuels, nuclear energy and, increasingly, renewable energy sources (e.g., wind, solar, ocean, and biomass), as they become more widely available. Thus, the availability and price of hydrogen as a carrier should be more stable than any single energy source. The introduction of hydrogen as an energy carrier, alongside electricity, would enable world to exploit resources that are best adapted to regional circumstances.
Hydrogen and electricity also allow flexibility in balancing centralised and decentralised power, based on managed, intelligent grids, and power for remote locations (e.g., island, and mountain sites). Decentralised power is attractive both to ensure power quality to meet specific customer needs, as well as reducing exposure to terrorist attack. The ability to store hydrogen more easily than electricity can help with load levelling and in balancing the intermittent nature of renewable energy sources. Hydrogen is also one of the few energy carriers that enables renewable energy sources to be introduced into transport systems.
_
Technical opportunities and challenges:
Hydrogen has long been touted as an important piece of the clean energy puzzle. It is the lightest and most abundant element in the universe, but hydrogen on Earth only rarely exists in its pure form. It is almost always chemically combined with other elements, most notably as water molecules (H2O). Once you free the element from its compound, hydrogen can be converted into electricity through fuel cells, it can be combusted to produce heat or power, or it can be used as a feedstock. When burned in an engine or when combined with oxygen in a fuel cell, hydrogen produces heat or electricity with only water vapor as a by-product, and no other pollutants or emissions.
Hydrogen can be employed across a wide range of applications, across virtually all sectors, from transport to industry to buildings. The IEA sees significant opportunity for hydrogen-based fuels in high-temperature heat production and industries, space heating, powering high-mileage vehicles as well as planes and ships, and seasonal storage for the grid.
It is important to note that hydrogen is not an energy source but an energy carrier. Just like electricity, it needs to be produced using other sources of energy. Today, hydrogen is mainly produced from natural gas (“grey” hydrogen) and coal (“black” hydrogen). Only a negligible part of current production is from fossil fuels equipped with carbon capture technologies (“blue” hydrogen) or from electrolysis powered by renewables (“green” hydrogen). Converting renewable electricity via hydrogen into other energy carriers – gases, liquids, and heat – and chemical feedstocks is a process known as “Power-to-X” (PtX or P2X). Each of the “downstream derivates” of hydrogen (e.g., synthetic methane, synthetic diesel, methanol, ammonia) comes with its own value-chains. By enabling these conversions, hydrogen has the potential to connect different parts of the energy system, al so known as “sector coupling”.
Several technical and economic limitations have held back hydrogen, including its explosiveness, low energy density per volume, ability to cause embrittlement in metals and, accordingly, costly infrastructure for production, storage, and distribution. As a consequence, past waves of enthusiasm have not translated into sustained investments or policy support. Between 2008 and 2018, worldwide government spending on hydrogen declined by about 35%.
Without some form of “climate neutral molecules” (biogas, hydrogen, synthetic fuels, etc.), however, it will be very hard to achieve deep decarbonization. For sectors such as long-haul transport, chemicals, and metallurgy, it is difficult to curb emissions through electrification alone. Efficiency, new materials, the circular economy, and behavioural changes could help to lower overall energy demand in those hard-to-abate sectors. For instance, substituting short-distance air travel with high-speed rail could dent overall demand for jet fuel. Yet, modelling shows the need for some form of green gases or fuels to successfully transition to a zero-carbon energy system. Hydrogen and derived fuels such as methanol, ethanol, and ammonia may thus be the “missing link” in the energy transition. Moreover, the rapid expansion of cheap renewable power can simultaneously bring down hydrogen’s cost and carbon emissions.
_______
Utility of hydrogen and fuel cells:
Hydrogen is used to power hydrogen fuel cell vehicles. Because of its energy efficiency, a hydrogen fuel cell is two to three times more efficient than an internal combustion engine fueled by gas. And a fuel cell electric vehicle’s refueling time averages less than four minutes. Because they can function independently from the grid, fuel cells can be used in the military field or in disaster zones and work as independent generators of electricity or heat. When fixed in place they can be connected to the grid to generate consistent reliable power.
_
A sustainable high quality of life is the basic driver for providing a clean, safe, reliable and secure energy supply in the world. To ensure a competitive economic environment, energy systems must meet the following societal needs at affordable prices:
–Mitigate the effects of climate change;
–Reduce toxic pollutants; and
–Plan for diminishing reserves of oil.
Failure to meet these needs will have significant negative impacts on:
–the economy;
–the environment; and
–public health.
Measures should therefore be introduced which promote:
–more efficient use of energy; and
–energy supply from a growing proportion of carbon-free sources.
The potential effects of climate change are very serious and most important of all, irreversible. World cannot afford to wait before taking remedial action, and it must aim for the ideal – an emissions-free future based on sustainable energy. Electricity and hydrogen together represent one of the most promising ways to achieve this, complemented by fuel cells which provide very efficient energy conversion.
Hydrogen is not a primary energy source like coal and gas. It is an energy carrier. Initially, it will be produced using existing energy systems based on different conventional primary energy carriers and sources. In the longer term, renewable energy sources will become the most important source for the production of hydrogen. Regenerative hydrogen, and hydrogen produced from nuclear sources and fossil-based energy conversion systems with capture, and safe storage (sequestration) of CO2 emissions, are almost completely carbon-free energy pathways.
_
Producing hydrogen in the large quantities necessary for the transport and stationary power markets could become a barrier to progress beyond the initial demonstration phase. If cost and security of supply are dominant considerations, then coal gasification with CO2 sequestration may be of interest for large parts of world. If the political will is to move to renewable energies, then biomass, solar, wind and ocean energy will be more or less viable according to regional geographic and climatic conditions. For example, concentrated solar thermal energy is a potentially affordable and secure option for large-scale hydrogen production, especially for Southern Europe. The wide range of options for sources, converters and applications, shown in Figures 1 and 2 below, although not exhaustive, illustrates the flexibility of hydrogen and fuel cell energy systems.
_
Fuel cells will be used in a wide range of products, ranging from very small fuel cells in portable devices such as mobile phones and laptops, through mobile applications like cars, delivery vehicles, buses and ships, to heat and power generators in stationary applications in the domestic and industrial sector. Future energy systems will also include improved conventional energy converters running on hydrogen (e.g., internal combustion engines, Stirling engines, and turbines) as well as other energy carriers (e.g., direct heat and electricity from renewable energy, and bio-fuels for transport).
_
Figure 1 above shows primary energy sources, energy converters and applications of hydrogen.
Note: Size of “sectors” has no connection with current or expected markets.
_
The benefits of hydrogen and fuel cells are wide ranging, but will not be fully apparent until they are in widespread use. With the use of hydrogen in fuel-cell systems there are very low to zero carbon emissions and no emissions of harmful ambient air substances like nitrogen dioxide, Sulphur dioxide or carbon monoxide. Because of their low noise and high power quality, fuel cell systems are ideal for use in hospitals or IT centers, or for mobile applications. They offer high efficiencies which are independent of size. Fuel-cell electric-drive trains can provide a significant reduction in energy consumption and regulated emissions. Fuel cells can also be used as Auxiliary Power Units (APU) in combination with internal combustion engines, or in stationary back-up systems when operated with reformers for on-board conversion of other fuels – saving energy and reducing air pollution, especially in congested urban traffic.
_
In brief, hydrogen and electricity together represent one of the most promising ways to realise sustainable energy, whilst fuel cells provide the most efficient conversion device for converting hydrogen, and possibly other fuels, into electricity. Hydrogen and fuel cells open the way to integrated “open energy systems” that simultaneously address all of the major energy and environmental challenges, and have the flexibility to adapt to the diverse and intermittent renewable energy sources.
_
Figure 2 above shows fuel cell technologies, possible fuels and applications.
Note: Size of “sectors” has no connection with current or expected markets.
PEM = Proton Exchange Membrane Fuel Cell; AFC = Alkaline Fuel Cells; DMFC = Direct Methanol Fuel Cell; PAFC = Phosphoric Acid Fuel Cell; MCFC = Molten Carbonate Fuel Cell; SOFC = Solid Oxide Fuel Cell
______
Greenhouse gas reduction:
Hydrogen can be produced from carbon-free or carbon-neutral energy sources or from fossil fuels with CO2 capture and storage (sequestration). Thus, the use of hydrogen could eventually eliminate greenhouse gas emissions from the energy sector. Fuel cells provide efficient and clean electricity generation from a range of fuels. They can also be sited close to the point of end-use, allowing exploitation of the heat generated in the process.
The table below illustrates how, in a mature hydrogen oriented economy, the introduction of zero carbon hydrogen-fuelled vehicles could reduce the average greenhouse gas emissions from the European passenger car fleet, compared to the average level of 140g/km CO2 projected for 2008.
The European Automobile Manufacturers’ Association (ACEA) has made a voluntary commitment to reduce the average level of CO2 emissions to 140 g/km for new vehicles sold on the European market in 2008. The average level today is around 165-170 g/km.
|
YEAR |
% of new cars(1) fuelled by zero carbon hydrogen |
% of fleet fuelled by zero-carbon hydrogen |
Average CO2 reduction (all cars)(2) |
CO2 avoided per year (MtCO2) |
|
2020 |
5 |
2 |
2.8 g/km |
15 |
|
2030 |
25 |
15 |
21.0 g/km |
112 |
|
2040 |
35 |
32 |
44.8 g/km |
240 |
|
(1) Figures based on an assumed European fleet of 175m vehicles. The fleet size will increase significantly by 2040, with correspondingly larger benefits. (2) Calculation is independent of total number of cars. |
||||
The last column shows the corresponding amounts of CO2 emissions that could be avoided. This may be compared to a projected total level of 750-800 MtCO2 emissions for road transport in 2010. The numbers for H2-fuelled cars are an assumption based on a survey of experts for conventional and alternative automotive drive trains, but not a prediction of future production or sales.
Greenhouse gas savings of about 140 MtCO2 per year (14% of today’s levels of CO2 emissions from electricity generation) could be achieved if about 17% of the total electricity demand, currently being supplied from centralised power stations, is replaced by more efficient decentralised power stations, incorporating stationary high-temperature fuel-cell systems fuelled by natural gas. Fuel-cell systems will be used as base load in the future decentralised energy systems.
These examples are not proposed as targets, but merely to serve as illustrations of the CO2 savings that could be achieved with quite modest penetrations of hydrogen vehicles and fuel cell-based stationary power generation. Together, 15% regenerative hydrogen vehicles and the above distributed fuel cell/gas turbine hybrid systems could deliver about 250 MtCO2 savings per year. This is approximately 6% of the energy-related CO2 emissions forecast in 2030, and progress such as this would allow Europe to move beyond the Kyoto Protocol.
______
New dependencies between states:
Today, hydrogen is still a very localized industry. Some 85% of hydrogen is produced and consumed on-site, mostly at refineries. To scale up production, industrialized countries may set up hydrogen plants at home or import hydrogen from states rich in renewable (or fossil) energy resources. For major economies like the EU or Japan, importing green hydrogen from regions with comparatively cheap, abundant renewables may help to reduce the cost of the energy transition as well as pressures on domestic resources (space on sea and land) linked to large-scale deployment of renewables. However, such cross-border maritime trade in hydrogen could produce new dependencies between states and give rise to new maritime shipping risks.
Hydrogen thus has the potential to reshape the global map of energy trade and create a new class of exporters (see Figure below). Countries such as Japan and South Korea are anticipating large-scale imports of hydrogen. By contrast, the hydrogen strategies of countries like Australia, Chile, and New Zealand focus on the potential for exports. New trade links may thus emerge and, to the extent that hydrogen displaces fossil fuels, it could potentially reduce the pressure on key maritime chokepoints for oil (e.g., Strait of Hormuz) or pivotal transit countries for natural gas (e.g., Ukraine until recently). At the same time, new shipping lanes may gain importance on the map of global energy trade.
_
Figure above shows costs of different hydrogen types by location, USD per kg of hydrogen.
For countries with close geographic proximity, hydrogen may be shipped through pipelines. In Northwestern Europe, for instance, a 900 km hydrogen-pipeline network connects Rotterdam (the Netherlands), Antwerp (Belgium), and Dunkirk (France). Worldwide, there exist already more than 4,500 km of hydrogen pipelines. German gas pipeline operators have recently unveiled plans to build a hydrogen grid of around 5,900 km, which would be by far the world’s largest. While these regional and local networks could be combined into transregional networks, there is as of yet no experience with long-distance hydrogen pipeline transportation.
Several countries are already engaging in what could be called “hydrogen diplomacy.” The Dutch government has even appointed a special “hydrogen envoy.” Japan’s diplomats and industrial stakeholders are engaging Australia, Brunei, Norway, and Saudi Arabia on hydrogen fuel procurement. Germany has signed a cooperation agreement with Morocco on methanol production from hydrogen, South Korea has its eyes on Norway, the Netherlands is targeting Portugal as a potential supplier of hydrogen, and industrial players in Belgium are looking towards Oman and Chile for large-scale hydrogen imports.
If the current trend toward bilateral partnerships continues, the market could start from a highly fragmented base, mimicking the experience with the initial phases of the LNG market. The first LNG projects were subject to inflexible, bilateral, long-term contracts with oil-indexed prices—and were therefore sometimes referred to as “floating pipelines.” Japan spearheaded the development of the LNG market by emerging as the first big buyer. Its commitment to large-scale hydrogen imports could make it, once again, global gas market pioneer, this time in hydrogen.
One of the key differences with trade in crude oil or natural gas is that hydrogen trade will be less asymmetric. It is technically possible to produce hydrogen almost everywhere in the world. The fact that many countries could become prosumers (both producers and consumer of hydrogen) and that hydrogen can be stored makes it almost impossible for exporters to weaponize hydrogen trade or for importers to be trapped by a small cartel of suppliers. Yet, hydrogen trade will not be as reciprocal as cross-border trade in electricity, where electrons actually travel both ways depending on supply and demand conditions on both sides of the border. Still, international trade in hydrogen will boost the energy security of importers as it will provide a back-up to the electricity system.
_______
Hydrogen’s roles in decarbonizing major sectors of the economy:
Hydrogen’s unique properties make it a powerful enabler for the energy transition, with benefits for both the energy system and end-use applications.
-1. Enable large-scale, efficient renewable energy integration
In the power sector, the timing of variable electricity supply and demand is not well matched (neither over the day nor between seasons). Integration of an increasing share of intermittent sources up to targeted levels (above 40% of the electricity mix) will enhance the need for operational flexibility. Increased electrification and limited storability of electricity will require adequate storage solutions. Various options exist to resolve the various issues, such as grid infrastructure upgrades or technologies for short- or longer-term balancing of supply and demand, e.g., flexible back-up generation, demand-side management, or energy storage technologies.
Hydrogen offers valuable advantages in this context, as it avoids CO2 and particles emission, can be deployed at large scale, and can be made available everywhere. There are two ways in which hydrogen improves the efficiency and flexibility of the energy system:
-a-Electrolysis can convert excess electricity into hydrogen during times of oversupply. The produced hydrogen can then be used to provide back-up power during power deficits or can be used in other sectors such as transport, industry or residential. It thus valorizes excess electricity.
The potential of valorization of otherwise curtailed renewable energy is considerable. For instance, in Germany alone, in a scenario with 90% renewables, curtailment of more than 170 TWh/year is projected for 2050, equivalent to about half the energy needed to fuel the German passenger car fleet with hydrogen. This would create an opportunity for around 60 GW of electrolysis capacity to operate economically (depending on improvements in grid interconnectivity).
Hydrogen offers a centralized or decentralized source of primary or backup power. Like gas, power from hydrogen (or one of its compounds) is switched on and off quickly. Thus, hydrogen helps deal with sudden drops in renewable energy supply, e.g., during adverse weather events. In addition, electrolysers may provide ancillary services to the grid, such as frequency regulation.
Hydrogen can also be used in specific fuel cell CHPs in industry and buildings, linking heat and power generation. This enhances the efficiency of generated electricity and heat for these sectors and improves flexibility of the energy system as a whole.
-b-Hydrogen can serve as long-term carbon-free seasonal storage medium.
Hydrogen represents the optimal overall solution for long-term, carbon-free seasonal storage. While batteries, super-capacitors, and compressed air can also support balancing, they lack either the power capacity or the storage timespan needed to address seasonal imbalances (see Figure below).
Figure above proves that Hydrogen is most promising for long-term carbon-free seasonal storage.
Pumped hydro offers an alternative to hydrogen for large-scale, long-term energy storage; it currently accounts for more than 95% of global power storage (162 GW worldwide). However, its remaining untapped potential is subject to local geographic conditions and limited to about 1% of annual global energy demand (0.3 EJ). This is not enough to handle seasonal demand differences. For instance, in Germany energy demand is about 30% higher in winter than in summer, while renewable generation is typically 50% lower in winter than in summer.
At this point in time, hydrogen remains a novel way to store energy, but more and more large, hydrogen- based storage demonstration projects are being planned, announced, or launched around the world – e.g., in Denmark, Canada, Japan, and the Asia-Pacific region. In addition, underground storage of large volumes of hydrogen is a well-established industry practice and does not present a major technological barrier. With an increasing share of renewable energy sources, the deployment of hydrogen as a long-term storage solution is expected to accelerate. As that happens, the cost of hydrogen storage is projected to decrease to €140/MWh (power to power) in 2030 for hydrogen stored in salt caverns. This is even less than the projected cost for pumped hydro storage (about €400/ MWh in 2030). In Germany the constrained potential for storage in caverns is about 37 billion cubic meters. This would be sufficient to store 110 TWhth hydrogen, covering the projected full seasonal storage need.
All in all, hydrogen permits to integrate more economically large amounts of intermittent energy sources in the system and provides the much needed flexibility to maintain the resilience of the system.
_
-2. Distribute energy across sectors and regions
The power system will require distribution of renewable energy for several reasons. Some countries, such as Japan, are not well positioned to generate energy with wind or solar power alone. Other countries may need time to raise the necessary investments. In some cases, importing renewable energy might be more economical, e.g., bringing low-cost solar energy from sun-belt countries to less sunny regions. As hydrogen and its compounds have a high energy density and are easily transported, they will help to (re)distribute energy effectively and flexibly.
While transporting electricity over long distances can cause energy losses, pipeline transportation of hydrogen reaches almost 100% efficiency. This benefit makes hydrogen an economically attractive option when transporting renewable energy at scale and over large distances, e.g., from areas with a high potential for renewable power generation, such as the Middle East, to areas with high energy demand like Europe. Import of hydrogen might serve as a long-term strategy, aimed at handling the ramp-up period for renewables or ensuring adequate energy supply during the winter, when renewable energy sources produce less electricity. Japan is planning to launch the first technical demonstration of a liquefied hydrogen carrier ship to enter international trade. Today, hydrogen pipelines and gaseous or liquefied tube trailers are the most common modes of transport. As the flow of hydrogen increases, the costs for liquefaction and transport are expected to drop by 30 to 40% in the next 15 years. Use of existing gas grids to transport hydrogen has been tested but not applied at large scale.
_
-3. Act as a buffer to increase system resilience
Hydrogen can help align global energy storage with changing energy demand. Its high energy density, long storage capacity, and variable uses make hydrogen well suited to serve as an energy buffer and strategic reserve. Today, the energy system has backup capacity of about 90 EJ (24% of final annual energy consumption), held almost exclusively by fossil energy carriers. There is no indication that the amount of buffering need could decrease significantly in the future. But, as consumers and the power sector switch to alternative energy carriers, the use of fossil fuels as backup might shrink, since this buffer serves only applications that consume fossil fuels. The most efficient buffer would mix energy carriers that reflect (or could transform into) end-use applications. This mix would include fossil fuels, biofuels/biomass/synthetic fuels, and hydrogen.
_
-4. Decarbonize transport
Fuel cell electric vehicles (FCEVs) have an important role to play in decarbonizing transport. Today oil dominates the fuel mix that meets the world’s transport needs. Gasoline and diesel account for 96% of total fuel consumption and 21% of global carbon emissions.
Efficient hybrid vehicles like hybrid electric vehicles (HEVs) and plug-in hybrid electric vehicles (PHEVs) are already reducing vehicle emissions. However, fully decarbonizing transport will require deployment of zero-emission vehicles like hydrogen-powered FCEVs and battery electric vehicles (BEVs), or hybrid combinations thereof. Advances in technology and new trends in mobility (e.g., connected cars, autonomous driving technology, and shared mobility) will influence relative levels of deployment and the transition speed. Both electric vehicle types make use of similar and complementary technologies and are specifically suited to serve different segments and customers. Besides lowering CO2 emissions, both also support local air quality improvements and noise reductions.
FCEVs offer several significant benefits. Firstly, they can drive long distances without needing to refuel (already more than 500 km), a feature highly valued by consumers. Secondly, they refuel quickly (3 to 5 minutes), similar to current gasoline/diesel cars, which adds to consumer convenience. Thirdly, thanks to a much higher energy density of the hydrogen storage system (compared to batteries), the sensitivity of the FCEV powertrain cost and weight to the amount of energy stored (kWh) is low. This increases its attractiveness and likelihood of adoption of vehicles that require significant energy storage (e.g., heavy load capacity and/or long range/heavy use). Lastly, FCEV infrastructure can build on existing gasoline distribution and retail infrastructure, creating cost advantages and preserving local jobs and capital assets.
FCEVs will emerge in all segments. Considering the above indicated benefits, they will be especially important in decarbonizing passenger cars (e.g., medium to large cars, fleets, and taxis), heavy-duty transportation, buses, and nonelectrified trains. Application of synthetic fuels made out of hydrogen to shipping and aviation is also being explored.
For passenger cars, total cost of ownership (TCO) for FCEVs is currently higher than for internal combustion engine (ICE) vehicles, while travel cost (fuel cost per kilometer traveled) is already similar to the cost of HEVs in Japan. When FCEVs reach at-scale commercialization, cost parity (from a TCO perspective) can be reached by 2025 for medium to large passenger cars. Selected car fleets and buses will reach cost parity even sooner, as their infrastructure rollout tends to be simpler and thus cheaper.
Major automotive players are pursuing a dual solution for zero-emission products. Three leading manufacturers already offer commercially available FCEVs, while many others have announced the intention to launch their own FCEVs soon. FCEVs are starting to become commercially available, with more than a thousand vehicles already on the road in Japan and the US, and a few hundred in Europe. China has set the goal of having 50,000 FCEVs on the road by 2025 and 1 million by 2030. Japan plans to deploy 200,000 FCEVs by 2025 and 0.8 million by 2030.
While the current market share of FCEV buses is still small (~ 500 on roads around the world), recent investments show increasing momentum to shift mass transit to FCEV solutions. For example, Lianyungang Haitong Public Transport (China) plans for 1,500 FCEV buses, Europe has announced to deploy in total 600 to 1,000 FCEV buses by 2020 and South Korea plans to replace 27,000 CNG buses with FCEVs by 2030.
Leading Western and Asian countries are planning to roll out significant hydrogen infrastructure over the coming decade. In Europe the number of stations is expected to double biannually, with up to 400 stations in Germany alone by 2023, and California has set the goal of having 100 stations by 2020. Japan already has more than 80 stations operating, and South Korea and China are planning to setup a hydrogen network, together aiming for 830 stations by 2025. The total targeted number of more than 3,000 stations in 2025 will be sufficient to provide hydrogen for about 2 million FCEVs. After this initial development phase, refueling infrastructure will be self-sustained.
_
-5. Decarbonize industry energy use
Today, natural gas, coal, and oil provide energy for industrial processes and thus generate about 20% of global emissions. Industry needs to improve energy efficiency (including waste heat recovery), thus reducing the need for energy. Steam electrolysis technologies can help valorize waste heat into hydrogen. Industry also needs to decarbonize the sources of process heat, for both low- and high-grade heat.
Industry has many options for decarbonizing low-grade heat. While heat pumps and electric resistance heating offer advantages in certain geographic locations, hydrogen is clearly advantageous when it is available as a by-product of the chemical industry or when a specific industry needs an uninterruptable power supply (as provided by a fuel cell), along with heat. As hydrogen can be combusted in hydrogen burners or be used in fuel cells, it offers a zero-emission alternative for heating.
High-grade heat – above 400°C – is harder to decarbonize. Hydrogen burners can complement electric heating to generate high-grade heat, depending on local conditions: some regions might favor industrial use of hydrogen technologies instead of electricity, given the constraints they have in the design of their energy system.
Today, industry uses hydrogen in low-grade heat applications, such as process heating and drying. In the future, industry might also use a mix of hydrogen burners and fuel cells to meet their low- and high-grade heat needs. Fuel cells have a higher efficiency than burners and simultaneously provide heat and power, but their deployment still requires significant investment. Burners, on their side, require only adjustments of existing equipment.
_
-6. Serve as feedstock using captured carbon
Hydrogen-based chemistry could serve as a carbon sink and complement or decarbonize parts of the petrochemical value chain. Today, crude oil (derivatives) are used as feedstock in the production of industrial chemicals, fuels, plastics, and pharmaceutical goods. Almost all of these products contain both carbon and hydrogen. If the application of carbon capture and utilization (CCU) technology takes off (as part of a circular economy or an alternative to carbon storage), the technology will need (green) hydrogen to convert the captured carbon into usable chemicals like methanol, methane, formic acid, or urea. This use of hydrogen would make CCU a viable alternative for other hard-to-decarbonize sectors like cement and steel production, and would contribute to the decarbonization of part of the petrochemical value chain.
The use of hydrogen and captured carbon to produce chemical feedstocks is in the research and development phase, with initial pilot projects being launched. Iceland has an operational geothermal plant that uses geothermal CO2 and generated electricity to produce hydrogen and then methanol. This methanol production is stated to be cost-competitive with an electricity price of EUR 30/MWh; other local conditions might produce different results. Sweden has planned a similar project that will use carbon captured from iron ore processing. Germany is combining carbon from steel production emissions with hydrogen from excess electricity to produce chemicals. The project is still in the concept phase and is expected to reach scale in 15 years.
_
-7. Help decarbonize building heating
Heating and warm water supply account for about 80% of residential energy consumption. About 50 EJ of energy is used for residential heating, responsible for 12% of global emissions. Hydrogen will be part of a portfolio of solutions for decarbonizing building heating. Local conditions will dictate the choice of options.
Building heating can use hydrogen as a fuel or leverage hydrogen technologies, or ideally a combination of both: hydrogen technologies such as fuel cell micro CHPs serve as energy converters. They offer high efficiency for heat and power generation (> 90%). Hydrogen itself can serve as a fuel either pure or blended with gas, partially decarbonizing the gas grid. For houses connected to a natural gas grid, switching to hydrogen-combustion based heating offers an opportunity to keep using the existing gas grid. With relatively small adjustments and investments, the grid can safely transport a mixture of hydrogen and natural gas. Full decarbonization requires a total switch to hydrogen, as contemplated by UK gas grid operators in Leeds.
On a global scale, about 190,000 buildings are already heated with hydrogen-based fuel cell micro CHPs. Most micro-CHPs (> 95%) are located in Japan, where about half run on methane combined with a reformer to produce hydrogen. The project has shown the ability of micro CHPs to meet heating requirements and supplement the electricity balance. By 2030, some 5.3 million Japanese households will use micro CHPs. Economies of scale have already cut prices more than 50%, from 2.4 USD/W installed in 2009 to 1 USD/W installed in 2014.
______
______
Section-6
Green hydrogen:
Green hydrogen is hydrogen that is generated entirely by renewable energy. Green hydrogen has significantly lower carbon emissions than grey hydrogen, which is produced by steam reforming of natural gas and represents 95% of the market. Certified green hydrogen requires an emission reduction of >60-70% (depending on the certification body) below the benchmark emissions intensity threshold (= GHG emissions of grey hydrogen, for example benchmark values according to the renewable energy directive RED II).
There are several ways to produce green hydrogen:
-1. Electrolysis of water with electricity generated by low-carbon power sources
-2. Steam reforming of biomethane
-3. Pyro-reforming of glycerine of renewable origin
-4. Electrolysis of salt solution using electricity from renewable sources
Green hydrogen is predominantly produced by splitting water using electricity generated from low-carbon sources. The high cost of production is the main factor behind the low use of green hydrogen. To date, most of the hydrogen produced is grey hydrogen and is thus not carbon friendly. Green hydrogen currently accounts for less than 1% of all hydrogen production. The biggest hurdle to scalability of clean hydrogen production has been its cost. Clean gas is not cost competitive when compared to fossil-based hydrogen: the production, transportation, and storage costs are substantial (e.g., the estimated costs for fossil-based production of hydrogen are 1.5 €/kg, compared to 2.5 to 5.5 €/kg for hydrogen produced using renewable energy). The current push for green hydrogen comes as production costs of renewable power are rapidly falling, making the cost savings resulting from the use of fossil-based hydrogen less significant. In particular, according to the EU Hydrogen Strategy, the cost of electrolysers used to produce clean hydrogen have already fallen, having been reduced by 60% in the last 10 years, and thanks to economies of scale, are expected to be half of their current costs by 2030. The wide-scale deployment of green hydrogen therefore requires a combination of factors, including critical mass in investment, an attractive regulatory framework, sustained research and innovation, and a large-scale infrastructure network, all of which make the EU well placed to lead the hydrogen revolution.
The United States Department of Energy forecasts that the hydrogen market is expected to grow, with the cost of hydrogen production falling from $6/kg in 2015 to as low as $2/kg by 2025. The price of $2/kg is considered a potential tipping point that will make green hydrogen competitive against other fuel sources. Siemens has already developed offshore wind turbines which are equipped for a hydrogen blend and, consequently help increase production of green hydrogen. The number of investments in green hydrogen has risen from almost none in 2020 to 121 gigawatts across 136 projects in planning and development phases totalling over $500 billion in 2021. Companies across countries have formed alliances to increase production of the fuel fifty fold in the next six years.
_____
Figure below shows how green hydrogen can be produced, converted and used across the energy system.
_____
Why green hydrogen is needed:
Most experts agree that green hydrogen will be essential to meeting the goals of the Paris Agreement, since there are certain portions of the economy whose emissions are difficult to eliminate. In the U.S., the top three sources of climate-warming emissions come from transportation, electricity generation and industry.
Energy efficiency, renewable power, and direct electrification can reduce emissions from electricity production and a portion of transportation; but the last 15 percent or so of the economy, comprising aviation, shipping, long-distance trucking and concrete and steel manufacturing, is difficult to decarbonize because these sectors require high energy density fuel or intense heat. Green hydrogen could meet these needs. While wind and solar energy can provide the electricity to power homes and electric cars, green hydrogen could be an ideal power source for energy-intensive industries like concrete and steel manufacturing, as well as parts of the transportation sector that are more difficult to electrify. Operating a plane or a large ship, for instance, requires so much energy that any battery used to store electricity from solar or wind would likely be too large and heavy for the vessel. Green hydrogen, on the other hand, can come in liquid form and is lighter.
_____
Like any other form of energy, green hydrogen has advantages and disadvantages of which it is important to be aware.
The positive aspects are:
100% sustainable: it does not emit any form of polluting gas;
storable: it is relatively easy to store and can be used either immediately or at a later date;
versatile: it can be converted into electricity or synthetic gas, used for commercial, industrial, domestic or mobility purposes;
transportable: the same pipelines as natural gas can be used for transport, because up to a certain percentage (about 20%) can be mixed with methane without consequences.
The negative aspects are:
high costs: electrolysis generated from renewable sources is even more expensive than electrolysis produced by other methods;
high energy consumption: with the current level of technology development, hydrogen production generally requires more energy than other fuels;
safety: hydrogen is a very flammable and volatile material, so safety measures must be taken to avoid leaks and explosions.
______
Green hydrogen energy solutions:
Green hydrogen is an important piece of the energy transition. It is not the next immediate step, as we first need to further accelerate the deployment of renewable electricity to decarbonize existing power systems, accelerate electrification of the energy sector to leverage low-cost renewable electricity, before finally decarbonize sectors that are difficult to electrify – like heavy industry, shipping and aviation – through green hydrogen.
For green hydrogen, we might witness a similar story to that of solar PV. It is capital intensive, therefore we need to reduce investment cost through scaling up manufacturing of renewable technologies and electrolysers. This will lead to a stable, decreasing cost of green hydrogen. Renewable energy technologies reached a level of maturity already today that allows competitive renewable electricity generation all around the world, a prerequisite for competitive green hydrogen production. Electrolysers though are still deployed at very small scale, needing a scale up of three orders of magnitude in the next three decades to reduce their cost threefold.
Today the pipeline for green hydrogen projects is on track and this combined with large projects located where the best renewable resources are, can lead to competitive green hydrogen to be available at scale in the next 5-10 years. This does not leave much time for blue hydrogen – still at pilot stage today – to scale up from pilot to commercial scale, deploy complex projects (e.g., the long term geological CO2 storage) at commercial scale and competitive cost, and recover the investments made in the next 10-15 years. Several governments have now included hydrogen fuel technologies in their national strategies. We will need green hydrogen to reach net zero emissions, in particular for industry, shipping and aviation.
The main actions to accelerate decarbonisation are:
-1) energy efficiency
-2) electrification
-3) rapid acceleration of renewable power generation
Once this is achieved, we are left with 40% of demand to be decarbonised, and this is where we need green hydrogen, modern bioenergy and direct use of renewables. Once we further scale up renewable power to decarbonise electricity, we will be in a position to further expand renewable power capacity to produce competitive green hydrogen and decarbonise hard-to-abate sectors at minimal extra cost.
We see the opportunity for rapid uptake of green hydrogen in the next decade where hydrogen demand already exists: decarbonising ammonia, iron and other existing commodities. Many industrial processes that use hydrogen can replace grey with green or blue, provided CO2 is adequately priced or other mechanisms for the decarbonisation of those sectors are put in place.
For shipping and aviation, the situation is slightly different. Drop-in fuels, based on green hydrogen but essentially identical to jet fuel and methanol produced from oil, can be used in existing planes and ships, with minimal to no adjustments. However, those fuels contain CO2, which has to be captured from somewhere and added to the hydrogen, to be released again during combustion: this reduces but does not solve the problem of CO2 emissions. Synthetic fuels can be deployed before 2030, if the right incentives are in place to justify the extra cost of reduced (not eliminated) emissions.
In the coming years, ships can switch to green ammonia, a fuel produced from green hydrogen and nitrogen from the air, which does not contain CO2, but investments will be needed to replace engines and tanks, and green ammonia is currently much more expensive than fuel oil.
Hydrogen (or ammonia) planes are further away, and these will be essentially new planes that have to be designed, built and sold to airlines to replace existing jet-fuel-powered planes – clearly not feasible by 2030: in this sense, green jet fuel – produced with a combination of green hydrogen and sustainable bioenergy – is a solutions that can be deployed in the near term.
______
Utility of green hydrogen is depicted in the figure below:
_______
Green Hydrogen can fill gaps in renewable energy:
When hydrogen burns, the only by-product is water—which is why hydrogen has been an alluring zero-carbon energy source for decades. Yet the traditional process for producing hydrogen, in which fossil fuels are exposed to steam, is not even remotely zero-carbon. Hydrogen produced this way is called grey hydrogen; if the CO2 is captured and sequestered, it is called blue hydrogen.
Green hydrogen is different. It is produced through electrolysis, in which machines split water into hydrogen and oxygen, with no other by-products. Historically, electrolysis required so much electricity that it made little sense to produce hydrogen that way. The situation is changing for two reasons. First, significant amounts of excess renewable electricity have become available at grid scale; rather than storing excess electricity in arrays of batteries, the extra electricity can be used to drive the electrolysis of water, “storing” the electricity in the form of hydrogen. Second, electrolyzers are getting more efficient.
Companies are working to develop electrolyzers that can produce green hydrogen as cheaply as grey or blue hydrogen, and analysts expect them to reach that goal in the next decade. Meanwhile energy companies are starting to integrate electrolyzers directly into renewable power projects. For example, a consortium of companies behind a project called Gigastack plan to equip Ørsted’s Hornsea Two offshore wind farm with 100 megawatts of electrolyzers to generate green hydrogen at an industrial scale.
Current renewable technologies such as solar and wind can decarbonize the energy sector by as much as 85 percent by replacing gas and coal with clean electricity. Other parts of the economy, such as shipping and manufacturing, are harder to electrify because they often require fuel that is high in energy density or heat at high temperatures. Green hydrogen has potential in these sectors. The Energy Transitions Commission, an industry group, says green hydrogen is one of four technologies necessary for meeting the Paris Agreement goal of abating more than 10 gigatons of carbon dioxide a year from the most challenging industrial sectors, among them mining, construction and chemicals.
Although green hydrogen is still in its infancy, countries—especially those with cheap renewable energy—are investing in the technology. Australia wants to export hydrogen that it would produce using its plentiful solar and wind power. Chile has plans for hydrogen in the country’s arid north, where solar electricity is abundant. China aims to put one million hydrogen fuel–cell vehicles on the road by 2030. Similar projects are underway in South Korea, Malaysia, Norway and the U.S., where the state of California is working to phase out fossil-fuel buses by 2040. And the European Commission’s recently published 2030 hydrogen strategy calls for increasing hydrogen capacity from 0.1 gigawatt today to 500 gigawatts by 2050. All of which is why Goldman Sachs predicted that green hydrogen will become a $12-trillion market by 2050.
______
______
Green hydrogen plan:
Europe:
Brussels distinguished three phases for the development of the clean hydrogen economy.
First phase
In the first phase, from 2020 to 2024, the aim is to decarbonise all existing hydrogen production and promote new applications. In this phase, at least 6 Gigawatts of green hydrogen electrolysers will have to be installed in Europe. Currently, production stands at 1 Gigawatt.
Second phase
In the second phase (2024-2030), hydrogen will become part of an integrated energy system. The aim will be to install at least 40 Gigawatts of green hydrogen electrolysers by 2030 and to increase production to 10 million tonnes. In this phase, the use of green hydrogen will be extended to sectors such as steel production, heavy transport, railways and some segments of maritime transport.
Third phase
From 2030 to 2050, renewable hydrogen technologies are expected to reach maturity, with large-scale deployment to decarbonise all sectors where the use of other alternative energies is unsuitable or more expensive.
Goldman Sachs estimates hydrogen will be 15% of the EU energy mix by 2050. Six European Union member states: Germany, Austria, France, the Netherlands, Belgium and Luxembourg, requested hydrogen funding be backed by legislation. Germany has already invested €9 billion to construct 5 GW of hydrogen capacity by 2030. Many member countries have created plans to import hydrogen from other nations, especially from North Africa.
Saudi Arabia:
Saudi Arabia is constructing a futuristic city in the desert on the Red Sea called Neom. The $500 billion city — complete with flying taxis and robotic domestic help — is being built from scratch and will be home to a million people. And what energy product will be used both to power this city and sell to the world? Not oil. The Saudis are going big on green hydrogen — a carbon-free fuel made from water by using renewably produced electricity to split hydrogen molecules from oxygen molecules. U.S. gas company, Air Products & Chemicals, announced that as part of Neom it has been building a green hydrogen plant in Saudi Arabia for the last four years. The plant is powered by 4 gigawatts from wind and solar projects that sprawl across the desert. It claims to be the world’s largest green hydrogen project — and more Saudi plants are on the drawing board.
Australia:
In Australia, green hydrogen has cost twice as much as conventional hydrogen and blue hydrogen, but a 2020 Australian National University report estimated that Australia could be producing it for much cheaper, even currently, and it could equal the price of conventional and blue hydrogen (at about A$2 per kilogram) by 2030, which would be cost-competitive with fossil fuels. An energy market analyst suggested in early 2021 that the price of green hydrogen would drop 70% over the coming 10 years in countries which have cheap renewable energy. In 2020, the government fast tracked approval for the world’s largest planned renewable energy export facility in the Pilbara region. The following year, energy companies announced plans to construct a “hydrogen valley” in New South Wales at a cost of $2 billion which would replace the region’s coal industry.
China:
China is the leader of the global hydrogen market with an output of 20 million tons, accounting for ⅓ of global production. Sinopec aims to generate 500,000 tonnes of green hydrogen by 2025. Researchers from the Harvard China Project have indicated that hydrogen generated from wind energy could provide a cost effective alternative for coal-dependent regions like Inner Mongolia.
Japan:
In order to become carbon neutral, the Japanese government intends to transform the nation into a “hydrogen society”. The energy demand in Japan would require the government to import 36 million tons of liquefied hydrogen. The nation’s commercial imports are projected to be 100 times less than this amount by 2030, when the use of the fuel is expected to commence, which represents a serious challenge. Japan has published a preliminary road map that called for hydrogen and related fuels to supply 10% of the power for electricity generation as well as a significant portion of the energy for other uses like shipping and steel manufacture by 2050. The country has created a hydrogen highway consisting of 135 subsidized hydrogen fuels stations and plans to construct 1,000 by the end of the decade.
______
The potential of hydrogen:
Hydrogen, specifically green hydrogen, has been a topic of interest for the renewable energy economy for a very long time. Hydrogen remains one of the most abundantly available and commonly known elements in the world, and it will become a gamechanger with its noteworthy contribution to clean energy transitions. Hydrogen is light, storable, energy-heavy and does not produce direct carbon emissions or greenhouse gases (GHG). Sectors such as oil refining and ammonia, methanol, and steel production have been using hydrogen extensively. Hydrogen will play a critical role in the transition to clean energy with the advancement of its applications in sectors such as transportation (fuel cell vehicles), buildings (hydrogen blending) and power generation.
_______
A net-zero world would require 306 million tonnes of green hydrogen derived from renewable energy per year by 2050, IEA study:
Achieving global net-zero emissions by 2050 will require about 306 million tonnes of green hydrogen derived from renewable energy each year, according to the International Energy Agency (IEA) report, Net Zero by 2050 – A Roadmap for the Global Energy Sector. The landmark IEA study, which sets out the steps needed to get the world to net-zero emissions by mid-century, also says that 197.6 million of blue hydrogen would be required annually, derived from natural gas or coal with carbon capture and storage (CCS). A further 16 million tonnes of low-carbon electrolytic hydrogen would be also produced annually from electrolysis powered by nuclear power and fossil-fuel power plants with CCS. In total, the report says, 520 million of renewable and low-carbon hydrogen would be used across a wide range of industries. By comparison, 87 million tonnes of largely grey hydrogen were produced from unabated natural gas and coal in 2020, mainly for use in the chemicals and oil refining sectors. This would require a compound average annual growth rate (CAAGR) in clean hydrogen production of 66% between now and 2030, and 23% between 2030 and 2050, the IEA says.
The 322 million tonnes of green and electrolytic hydrogen in 2050 would require a global electrolyser capacity of 3,585GW, up from about 300MW today, and roughly 14,500TWh of electricity — about 20% of the world’s electricity supply (71,164TWh). Vast amount of clean hydrogen would consume about 20% of the world’s electricity.
According to the IEA, reaching net-zero emissions would require the following power capacities to be installed in 2050 (with 2020 installed capacities in brackets):
Solar PV: 14,458GW (737GW)
Wind: 8,265GW (737GW)
Hydro: 2,599GW (1,327GW)
Hydrogen power plants: 1,867GW (zero)
Nuclear: 812GW (415GW)
Bioenergy: 640GW (171GW)
Coal-fired with CCS: 222GW (1GW)
Gas-fired with CCS: 171GW (zero)
Concentrating solar power (CSP): 426GW (6GW)
Geothermal: 126GW (15GW)
Marine (wave and tidal): 55GW (1GW)
The IEA says that blue hydrogen from natural gas will cost around $1-2 per kg by 2050, with green hydrogen at $1-2.50/kg.
______
______
Section-7
Hydrogen production:
Hydrogen production technologies are technologies that relate to the production and use of hydrogen. Hydrogen technologies are applicable for many uses. Some hydrogen technologies are carbon neutral and could have a role in preventing climate change and a possible future hydrogen economy. Hydrogen is a chemical widely used in various applications including ammonia production, oil refining and energy. The most common methods for producing hydrogen on an industrial scale are: Steam reforming, oil reforming, coal gasification and water electrolysis. These four main sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis account for 48%, 30%, 18% and 4% of the world’s hydrogen production respectively. Hydrogen is not a primary energy source, because it is not naturally occurring as a fuel. It is, however, widely regarded as an ideal energy storage medium, due to the ease with which electric power can convert water into its hydrogen and oxygen components through electrolysis and can be converted back to electrical power using a fuel cell. There are a wide number of different types of fuel and electrolysis cells.
_
There are two general types of hydrogen generation technologies: reforming and water splitting.
Reforming technologies use fossil fuels or biomass and steam to produce hydrogen at the lowest cost—but they also produce carbon dioxide.
Water splitting technologies, meanwhile, fall into three general categories: thermo‐chemical cycles, electrolysis, and direct photoelectrochemical (PEC).
Thermo‐chemical cycles utilize heat—sourced from a nuclear plant or from concentrated solar plants, for example—and chemical reactions to produce hydrogen and oxygen, but they also involve corrosive acids or volatile chemicals.
The PEC pathway directly uses solar radiation to split water using semiconductor‐based devices, but it currently has a fairly low technology readiness level. For now, nearer-term electrolysis is often elected as the better option for “green” hydrogen production.
Electrolysis generally falls into two categories. Low‐temperature electrolysis (LTE), which is already commercially available and often the choice for emerging power-to-gas projects, involves placing electrodes in an electrolytic solution or using membranes to separate the hydrogen from the oxygen. High‐temperature steam electrolysis (HTSE) utilizes heat and electricity to split water into hydrogen and oxygen, so, compared to LTE, the additional heat reduces the amount of work needed. In HTSE, solid oxide electrolysis cells are used to electrochemically separate the hydrogen and oxygen from steam at temperatures around 800 deg C.
_
Hydrogen can be produced from diverse, domestic resources, including fossil fuels, biomass, and water electrolysis with electricity. The environmental impact and energy efficiency of hydrogen depends on how it is produced. Several projects are underway to decrease costs associated with hydrogen production.
There are several ways to produce hydrogen:
-1. Natural Gas Reforming/Gasification: Synthesis gas—a mixture of hydrogen, carbon monoxide, and a small amount of carbon dioxide—is created by reacting natural gas with high-temperature steam. The carbon monoxide is reacted with water to produce additional hydrogen. This method is the cheapest, most efficient, and most common. Natural gas reforming using steam accounts for the majority of hydrogen produced in the United States annually. A synthesis gas can also be created by reacting coal or biomass with high-temperature steam and oxygen in a pressurized gasifier. This converts the coal or biomass into gaseous components—a process called gasification. The resulting synthesis gas contains hydrogen and carbon monoxide, which is reacted with steam to separate the hydrogen.
-2. Electrolysis: An electric current splits water into hydrogen and oxygen. If the electricity is produced by renewable sources, such as solar or wind, the resulting hydrogen will be considered renewable as well, and has numerous emissions benefits. Power-to-hydrogen projects are taking off, using excess renewable electricity, when available, to make hydrogen through electrolysis.
-3. Renewable Liquid Reforming: Renewable liquid fuels, such as ethanol, are reacted with high-temperature steam to produce hydrogen near the point of end use.
-4. Fermentation: Biomass is converted into sugar-rich feedstocks that can be fermented to produce hydrogen.
_
Several hydrogen production methods are in development:
-1. High-Temperature Water Splitting: High temperatures generated by solar concentrators or nuclear reactors drive chemical reactions that split water to produce hydrogen.
-2. Photobiological Water Splitting: Microbes, such as green algae, consume water in the presence of sunlight and produce hydrogen as a by-product.
-3. Photoelectrochemical Water Splitting: Photoelectrochemical systems produce hydrogen from water using special semiconductors and energy from sunlight.
The primary challenge for hydrogen production is reducing the cost of production technologies to make the resulting hydrogen cost competitive with conventional transportation fuels. Government and industry research and development projects are reducing the cost as well as the environmental impacts of hydrogen production technologies.
_____
Hydrogen can be produced from a large number of different feedstocks such as water, coal, natural gas, biomass, hydrogen sulfide, boron hydrides, and others, through thermal, electrolytic or photolytic processes. A brief summary of the methods, along with their concise descriptions, materials and required energy supply is provided in Table below:
General hydrogen production methods:
|
Method |
Description |
Material |
Energy |
|
Water electrolysis |
Water decomposition into oxygen and hydrogen by passing a direct current that drives electrochemical reactions |
Water |
Electrical |
|
High temperature steam electrolysis |
Steam decomposition by using direct current assisted by thermal energy to drive electrochemical reactions to split water molecule |
Steam |
Electrical, Thermal |
|
Photoelectrochemical water splitting |
Uses electric and photonic energy to electrolyse water and generate H2 and O2 |
Water |
Photonic, Electric |
|
Photocatalysis |
Uses photonic energy and catalysts to decompose water molecule |
Water |
Photonic |
|
Biophotolysis |
Uses a reversible reducible cofactor and photometabolically active microbes to generate hydrogen from water |
Water |
Photonic, Biochemical |
|
Anaerobic digestion |
Uses biological energy manipulated by microbes to extract hydrogen from biodegradable materials in the absence of oxygen |
Biomass |
Biochemical |
|
Thermolysis |
Uses thermal energy to decompose water molecule at very high temperature (2500◦C) |
Water |
Thermal |
|
Thermochemical water splitting |
Thermally driven chemical reactions performed in a loop with the overall result of water splitting |
Water |
Thermal |
|
Thermocatalytic cracking |
Uses thermal energy to break the carbon-hydrogen bonds of hydrocarbons and eventually generate hydrogen |
Fossil fuels |
Thermal |
|
Gasification |
Converts solid carbonaceous materials into carbon monoxide and hydrogen by reacting them with O2 and/or steam |
Water, fossil fuels, biomass |
Thermal |
|
Reforming |
Reacts carbon-based liquid or gaseous fuels with steam at high temperature to produce carbon dioxide and hydrogen |
Water, fossil fuel or biofuels |
Thermal |
_____
_____
The production of hydrogen can be catalogued as four main routes; renewable, fossil fuel, nuclear and biomass as demonstrated in figure below:
Figure above shows the route of hydrogen production.
Currently, over 75 million tons of hydrogen is produced worldwide annually. However, an estimated 95% or more is from fossil fuels. Meanwhile, a large amount of the hydrogen produced is used for industrial applications, such as metal refining, chemical production as well as fats and oil production.
______
______
Some hydrogen production methods in detail:
-1. Electrolysis of water using electricity
Figure above illustrates inputs and outputs of simple electrolysis of water production of hydrogen.
The electrolysis of water is a simple method of producing hydrogen. Electrolysis requires high-purity water. Therefore, the water is first treated to remove the minerals and ions prior to the electrolysis process. A low voltage current is run through the water, and gaseous oxygen forms at the anode while gaseous hydrogen forms at the cathode. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals. (Iron, for instance, would oxidize, and thus decrease the amount of oxygen given off.) The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is in the range 88–94%.
2 H2O(l) + energy → 2 H2(g) + O2(g)
This is the opposite reaction of what happens in a fuel cell.
_
Electrolyzers can range in size from small, appliance-size equipment that is well-suited for small-scale distributed hydrogen production to large-scale, central production facilities that could be tied directly to renewable or other non-greenhouse-gas-emitting forms of electricity production.
Polymer Electrolyte Membrane Electrolyzers:
In a polymer electrolyte membrane (PEM) electrolyzer, the electrolyte is a solid specialty plastic material. Water reacts at the anode to form oxygen and positively charged hydrogen ions (protons). The electrons flow through an external circuit and the hydrogen ions selectively move across the PEM to the cathode. At the cathode, hydrogen ions combine with electrons from the external circuit to form hydrogen gas.
Anode Reaction: 2H2O → O2 + 4H+ + 4e-
Cathode Reaction: 4H+ + 4e- → 2H2
Alkaline Electrolyzers:
Alkaline electrolyzers operate via transport of hydroxide ions (OH-) through the electrolyte from the cathode to the anode with hydrogen being generated on the cathode side. Electrolyzers using a liquid alkaline solution of sodium or potassium hydroxide as the electrolyte have been commercially available for many years. Newer approaches using solid alkaline exchange membranes (AEM) as the electrolyte are showing promise on the lab scale.
Solid Oxide Electrolyzers:
Solid oxide electrolyzers, which use a solid ceramic material as the electrolyte that selectively conducts negatively charged oxygen ions (O2-) at elevated temperatures, generate hydrogen in a slightly different way. Steam at the cathode combines with electrons from the external circuit to form hydrogen gas and negatively charged oxygen ions. The oxygen ions pass through the solid ceramic membrane and react at the anode to form oxygen gas and generate electrons for the external circuit.
Solid oxide electrolyzers must operate at temperatures high enough for the solid oxide membranes to function properly (about 700°–800°C, compared to PEM electrolyzers, which operate at 70°–90°C, and commercial alkaline electrolyzers, which typically operate at less than 100°C). Advanced lab-scale solid oxide electrolyzers based on proton-conducting ceramic electrolytes are showing promise for lowering the operating temperature to 500°–600°C. The solid oxide electrolyzers can effectively use heat available at these elevated temperatures (from various sources, including nuclear energy) to decrease the amount of electrical energy needed to produce hydrogen from water.
_
Electrolysis is an energy intensive process. The power consumption at 100% efficiency is about 40 kWh/kg hydrogen; however, in practice it is closer to 50 kWh/kg. Since electrolysis units operate at relatively low pressures (10 atmospheres), higher compression is needed to distribute the hydrogen by pipelines or tube trailers compared to other hydrogen production technologies. This process offers the potential to produce hydrogen with almost no pollution or greenhouse gas production. The environmental effects of renewable electrolysis depend on the technique that is used to produce electricity. Nuclear energy can also produce carbon free electricity that can be used to split water into hydrogen and oxygen.
The source of the required electricity—including its cost and efficiency, as well as emissions resulting from electricity generation—must be considered when evaluating the benefits and economic viability of hydrogen production via electrolysis. In many regions, today’s power grid is not ideal for providing the electricity required for electrolysis because of the greenhouse gases released and the amount of fuel required due to the low efficiency of the electricity generation process. Hydrogen production via electrolysis is being pursued for renewable (wind, solar, hydro, geothermal) and nuclear energy options. These hydrogen production pathways result in virtually zero greenhouse gas and criteria pollutant emissions; however, the production cost needs to be decreased significantly to be competitive with more mature carbon-based pathways such as natural gas reforming.
Potential for synergy with renewable energy power generation:
Hydrogen production via electrolysis may offer opportunities for synergy with dynamic and intermittent power generation, which is characteristic of some renewable energy technologies. For example, though the cost of wind power has continued to drop, the inherent variability of wind is an impediment to the effective use of wind power. Hydrogen fuel and electric power generation could be integrated at a wind farm, allowing flexibility to shift production to best match resource availability with system operational needs and market factors. Also, in times of excess electricity production from wind farms, instead of curtailing the electricity as is commonly done, it is possible to use this excess electricity to produce hydrogen through electrolysis.
In Germany they have wind power at a time they can’t use. Big utilities operating these wind plants lose billions of euros every year, so they need to store that fluctuating energy. Even if you have a less than efficient electrolyzer, and you can keep half of the electricity stored in your hydrogen to reuse for power when it is necessary, that is still cheaper than wasting it.
_
-2. Methane pyrolysis
Figure above illustrates inputs and outputs of methane pyrolysis, a process to produce hydrogen.
Hydrogen production using natural gas methane pyrolysis is a recent “no greenhouse gas” one-step process. Developing volume production using this method is the key to enabling faster carbon reduction by using hydrogen in industrial processes, fuel cell electric heavy truck transportation, and in gas turbine electric power generation. Methane pyrolysis uses methane CH4 bubbled up through the molten metal catalyst at high temperatures (1340 K, 1065 °C or 1950 °F) to produce non-polluting hydrogen H2 gas in high volume, at low cost and produces non-polluting solid carbon C with no emission of greenhouse gas.
CH4(g) → C(s) + 2 H2(g) ΔH° = 74 kJ/mol
The industrial quality solid carbon may be sold as manufacturing feedstock or permanently landfilled, it is not released into the atmosphere and no ground water pollution in landfill. Methane pyrolysis is in development and considered suitable for commercial bulk hydrogen production. Volume production is being evaluated in the BASF “methane pyrolysis at scale” pilot plant. Further research continues in several laboratories, including at Karlsruhe Liquid-metal Laboratory (KALLA) and the chemical engineering laboratory at University of California – Santa Barbara.
_
-3. Steam reforming of natural gas
Figure above illustrates inputs and outputs of steam reforming of natural gas, a process to produce hydrogen.
Hydrogen is produced presently from natural gas in a chemical process at about 800°C to 900°C. You burn natural gas for heat for a steam reforming reaction, to react natural gas with water and get a syngas. This gets reacted a second time and produces hydrogen and carbon dioxide. Commercial bulk hydrogen is usually produced by the steam reforming of natural gas with release of atmospheric greenhouse gas or with capture using CCS and climate change mitigation. Steam reforming is also known as the Bosch process and is widely used for the industrial preparation of hydrogen.
At high temperatures (1000–1400 K, 700–1100 °C or 1300–2000 °F), steam (water vapor) reacts with methane to yield carbon monoxide and H2.
CH4 + H2O → CO + 3 H2
This reaction is favored at low pressures but is nonetheless conducted at high pressures (2.0 MPa, 20 atm or 600 inHg). This is because high-pressure H2 is the most marketable product, and pressure swing adsorption (PSA) purification systems work better at higher pressures. The product mixture is known as “synthesis gas” because it is often used directly for the production of methanol and related compounds. Hydrocarbons other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon:
CH4 → C + 2 H2
Consequently, steam reforming typically employs an excess of H2O. Additional hydrogen can be recovered from the steam by use of carbon monoxide through the water gas shift reaction, especially with an iron oxide catalyst. This reaction is also a common industrial source of carbon dioxide:
CO + H2O → CO2 + H2
This is known as water-gas shift reaction (WGSR) of carbon monoxide and water vapor to form carbon dioxide and hydrogen.
Other important methods for CO and H2 production include partial oxidation of hydrocarbons:
2 CH4 + O2 → 2 CO + 4 H2
and the coal reaction, which can serve as a prelude to the shift reaction above.
C + H2O → CO + H2
Hydrogen is sometimes produced and consumed in the same industrial process, without being separated. In the Haber process for the production of ammonia, hydrogen is generated from natural gas. Electrolysis of brine to yield chlorine also produces hydrogen as a co-product.
_
-4. Metal-acid
Many metals react with water to produce H2, but the rate of hydrogen evolution depends on the metal, the pH, and the presence alloying agents. Most commonly, hydrogen evolution is induced by acids. The alkali and alkaline earth metals, aluminium, zinc, manganese, and iron react readily with aqueous acids. This reaction is the basis of the Kipp’s apparatus, which once was used as a laboratory gas source:
Zn + 2 H+→ Zn2++ H2
In the absence of acid, the evolution of H2 is slower. Because iron is widely used structural material, its anaerobic corrosion is of technological significance:
Fe + 2 H2O → Fe(OH)2 + H2
Many metals, such as aluminium, are slow to react with water because they form passivated coatings of oxides. An alloy of aluminium and gallium, however, does react with water. At high pH, aluminium can produce H2:
2 Al + 6 H2O + 2 OH−→ 2 Al(OH)−4 + 3 H2
Some metal-containing compounds react with acids to evolve H2. Under anaerobic conditions, ferrous hydroxide (Fe(OH)2) can be oxidized by the protons of water to form magnetite and H2. This process is described by the Schikorr reaction:
3 Fe(OH)2 → Fe3O4 + 2 H2O + H2
This process occurs during the anaerobic corrosion of iron and steel in oxygen-free groundwater and in reducing soils below the water table.
_
-5. Thermochemical decomposition of water
More than 200 thermochemical cycles can be used for water splitting. Many of these cycles such as the iron oxide cycle, cerium(IV) oxide–cerium(III) oxide cycle, zinc zinc-oxide cycle, sulfur-iodine cycle, copper-chlorine cycle and hybrid sulfur cycle have been evaluated for their commercial potential to produce hydrogen and oxygen from water and heat without using electricity. A number of laboratories (including in France, Germany, Greece, Japan, and the USA) are developing thermochemical methods to produce hydrogen from solar energy and water.
Thermochemical water splitting uses high temperatures—from concentrated solar power or from the waste heat of nuclear power reactions—and chemical reactions to produce hydrogen and oxygen from water. This is a long-term technology pathway, with potentially low or no greenhouse gas emissions. The most efficient way to produce solar hydrogen leverages solar heat for a thermochemical reaction. There are overall efficiency advantages. When electrolysis is powered by solar photovoltaic an efficiency of only 12 percent to 14 percent is reported. For the thermochemical water-splitting reaction, some solar reactor systems have efficiencies of over 60 percent. The two-step solar thermochemical conversion is still subject to research, but shows a long-term efficiency potential of up to 25 percent. In solar thermal (heat) energy, thousands of “suns” can be concentrated by mirrors and focused into a solar reactor to produce temperatures up to 1,500°C. There are no carbon emissions when hydrogen is made by a thermochemical reaction splitting water using the heat of a solar reactor.
_
-6. Serpentinization reaction
In deep geological conditions prevailing far away from the Earth’s atmosphere, hydrogen (H2) is produced during the process of serpentinization. In this process, water protons (H+) are reduced by ferrous (Fe2+) ions provided by fayalite (Fe2SiO4). The reaction forms magnetite (Fe3O4), quartz (SiO2), and hydrogen (H2):
3Fe2SiO4 + 2 H2O → 2 Fe3O4 + 3 SiO2 + 3 H2
fayalite + water → magnetite + quartz + hydrogen
This reaction closely resembles the Schikorr reaction observed in anaerobic oxidation of ferrous hydroxide in contact with water.
_
-7. Biomass Gasification
The process of biomass gasification starts by heating the biomass to produce a syn gas consisting mostly of hydrogen, carbon monoxide, carbon dioxide, and water. The gas is cleaned and steam is introduced to cause the water gas shift reaction to convert energy in carbon monoxide into hydrogen. Pressure swing absorption separates the hydrogen from carbon dioxide. This process is similar to coal gasification in many ways. Biomass has several advantages over coal as a hydrogen feedstock. The feedstock is relatively inexpensive. However, it is uneconomical to build biomass plant as big as coal plants since biomass has less energy density by volume and therefore is more expensive to transport. The cost and availability of feedstock is probably the most important consideration for the future of biomass gasification.
______
Hydrogen is omnipresent but not readily available: energy is required to extract it from chemical compounds. If hydrogen is to play a major role in a future energy economy, the whole spectrum of primary energies (fossil, nuclear, renewable) for its production must be considered. Different hydrogen production methods are given in Table below which lists the benefits and barriers of the different technologies.
|
Technology |
Benefits |
Barriers |
|
Steam reforming: Splitting of hydrocarbons with heat and steam. |
Well understood at large scale; commercially available with proven technology; widely available feedstock; highly economic at present; CO2 sequestration at large scale; ideal for centralized production. |
Small scale units not commercial; CO2 emissions; H2 contains some impurities; primary fuel may be used directly; subject to natural gas price fluctuations; in distributed form not yet verified. |
|
Gasification: Splitting of heavy hydrocarbons and biomass into hydrogen and other gases for reforming. |
Well understood at large scale; can be used for solids and liquids; abundance of (coal) resources. |
Less hydrogen-rich than methane; lower efficiency; High levels of CO2 emissions from coal; feedstock requires pretreatment; H2 requires cleaning prior to use; biomass gasification still at pilot plant scale; low energy density of biomass. |
|
Electrolysis: Splitting of water using electricity. |
Well understood; commercially available with proven technology; high purity hydrogen; modular; convenient for renewable electricity; ideal for distributed production. |
Electricity price strongly impacts cost of H2; efficiency of whole chain is low; need for development of durable HTSE cells; competition with direct use of renewable electricity. |
|
Thermochemical cycles: Splitting of water using inexpensive high temperature heat from nuclear or solar. |
Potentially massive production at low cost; no GHG emissions; high efficiency (~50% expected); International collaboration on R&D and deployment. |
Not commercial; aggressive chemistry; much R&D work still needed on process and materials technology; high capital cost; high temperature nuclear reactor deployment needed. |
|
Biological production: Algae and bacteria produce hydrogen directly under certain conditions. |
Potentially large resource; no feedstock required. |
Slow hydrogen production rates; large area needed; low efficiency; appropriate organisms not yet found; still at R&D level. |
The first step toward a hydrogen economy will always be based on existing technologies and established processes. In the near and medium terms, fossil fuels are expected to remain the principal source of hydrogen. Natural gas, the ‘cleanest’ fuel among the hydrocarbons, is expected to have advantages as a starting point for the initial hydrogen market (in the transition phase) as a source of hydrogen in terms of environmental impact (highest H:C ratio), availability and economy. In the long term, hydrogen production technologies will be strongly focused on CO2 neutral or CO2 free methods.
______
______
Hydrogen production energy efficiency:
Table below summarises the nominal efficiency and energy requirements for different hydrogen production methods. Early demonstrations have seen much lower efficiencies in practice though. For example, hydrogen production efficiency at dozens of filling stations in California and Japan averaged 55.8 ± 8.4% efficiency from natural gas, while electrolysers averaged 55.9 ± 3.5% LHV (for those with >4 operating hours per day).
Efficiency and energy consumption of hydrogen production pathways:
|
Efficiency (LHV) |
Energy requirement (kWh per kgH2) |
|
|
Methane reforming |
72% (65–75%) |
46 (44–51) |
|
Alkaline Electrolysis |
61% (51–67%) |
55 (50–65) |
|
Coal gasification |
56% (45–65%) |
59 (51–74) |
|
Biomass gasification |
46% (44–48%) |
72 (69–76) |
Tradeoffs:
Of the various production routes, there are multiple trade-offs between scale of production, cost and GHG emissions. Natural gas, oil, and coal-derived hydrogen is lowest cost at large scales, but has poor environmental credentials. If CCS can be deployed, hydrogen from natural gas exhibits relatively low emissions with costs that will depend on the available CCS infrastructure. However, GHG emissions may still be significant and will be governed by the carbon capture rate, and the embodied upstream supply chain emissions. Methane emissions from shale gas productions vary widely between sites and are a key contributor to total global warming potential. Biomass gasification may also offer large scale centralised hydrogen production, but at a higher capital cost. Emissions are significantly lower than hydrogen from natural gas, but are again non-negligible and are largely dependent on the biomass feedstock. Hydrogen production from electrolysis is highest cost but is more suitable for small-scale generation given the modular nature of electrolysers. Total greenhouse gas emissions may be very low if supplied with low-carbon electricity (e.g., combined with offshore wind), and so electrolysis represents a key technology if cost profiles improve.
______
______
Section-8
Hydrogen storage, distribution and infrastructure:
_
Hydrogen Storage:
Hydrogen storage is an important component in hydrogen economy, and one of the most urgent and challenging applications is to develop safe, reliable, efficient and effective storage mechanisms. There are several key parameters in the selection of hydrogen storage methods and materials, including: (a) gravimetric and volumetric hydrogen densities, (b) energy efficiency, (c) refueling time, (d) durability, (e) cost, (f) standards, (g) technology maturity and (h) life-cycle and efficiency analysis. Energy efficiency deals with the energy consumed during both the storage and release of hydrogen to and from its storage states or hydrogen storage materials. Furthermore, durability is correlated to its lifetime, especially in case of reversible hydrogen storage materials. Standards for the storage systems and interface are required in order to facilitate the implementation of the storage technology, as well as safety and public acceptance. The successful development of hydrogen storage is crucial for the future of hydrogen economy. In order to store hydrogen effectively, different hydrogen storage technologies have been studied and developed. These include compressed and liquefied hydrogen, liquid organic carriers, metal hydrides, methanol (CH3OH) and ammonia (NH3). Hydrogen storage covers both mobile and stationary systems.
_
There are three typical approaches to store hydrogen:
- Physical storage as compressed gas
- Physical storage as cryogenic liquid hydrogen
- Materials-based storage or solid state storage
Among them, the first two methods, i.e., store hydrogen as compressed gas and in its liquid form, are the most mature and widely used methods. Hydrogen can be stored physically as either a gas or a liquid. Storage of hydrogen as a gas typically requires high-pressure tanks (350–700 bar [5,000–10,000 psi] tank pressure). Storage of hydrogen as a liquid requires cryogenic temperatures because the boiling point of hydrogen at one atmosphere pressure is −252.8°C. On a mass basis, hydrogen has nearly three times the energy content of gasoline—120 MJ/kg for hydrogen versus 44 MJ/kg for gasoline. On a volume basis, however, the situation is reversed; liquid hydrogen has a density of 8 MJ/L whereas gasoline has a density of 32 MJ/L Onboard hydrogen storage capacities of 5–13 kg hydrogen will be required to meet the driving range for the full range of light-duty vehicle platforms.
_
Ideal Storage Method:
Before the evaluation of hydrogen storage techniques, an ideal storage medium for mobility can be defined by qualifying or quantifying the characteristics of each system. High volumetric and gravimetric energy densities are clearly desirable for mobile applications. Gasoline and diesel are currently the ubiquitous fuels for surface transportation, and they can be used as a benchmark. The energy densities of these fuels vary because complex mixtures and different blends are available on the market. However, values close to 38 wt % and 35 MJ/L are typical. Pure hydrogen at ambient temperature and pressure offers excellent gravimetric but poor volumetric energy densities of 120 MJ/kg (100 wt %) and 0.01 MJ/L, respectively. Another important performance metric is the speed of kinetics. This term designates the rate at which the system can release hydrogen upon demand and stop this release when required. The rates should match transportation applications, e.g., acceleration and braking of an automobile. Temperature-dependent hydrogen storage techniques imply the addition of a heat management system, which adds costs, complexity, and possibly mass. Ideally, this technique should be avoided, and operation near ambient temperature throughout refueling, standby, and discharge is desirable. Another important aspect to consider is the operating pressure. Pressure vessels must be reinforced with high strength materials that are subject to strict regulation and testing, which negatively impacts gravimetric density and costs. The final important thermodynamic property is efficiency. If hydrogen is used in an effort to capture renewable energy and displace hydrocarbons, then efficiency should be as high as possible to make optimal use of available renewable energy. For an ideal storage method, safety is essential, especially for general public use. Toxicity, flammability, danger of explosion or projections, etc., are not desirable, but they are difficult to quantify. The use of materials that require resource intensive extraction or designs that make recycling difficult or impossible should also be avoided. All the aforementioned characteristics should be affordable to ensure market penetration.
_
The high mass-based energy density of hydrogen makes it one of the most promising future fuels. Hydrogen contains 33.33 kWh usable energy per kilo, compared to 12 kWh of petrol and diesel. However, storing the same amount of hydrogen requires a larger volume. The development of hydrogen storage technologies is, therefore, a fundamental premise for hydrogen powered energy systems. Conventional technologies store the hydrogen as compressed gas and cryogenic liquid, while for large-scale applications, underground storage turns out to be a preferable method. In recent years, solid-state hydrogen storage has seen rapid development and is believed to be the safest hydrogen storage mode.
Different technologies of hydrogen storage have been summarized in figure below:
_____
Compressed gas:
Compressed hydrogen is the simplest way to store hydrogen, although its hydrogen density is low (42.2 kg-H2/m3 at 69 MPa). Compressed hydrogen requires high pressure to effectively store the gaseous hydrogen. In the case of a hydrogen vehicle, a high pressure tank of about 70 MPa is currently required in order to store the hydrogen to achieve a similar driving range to conventional vehicles. Moreover, as hydrogen is a very light and small element, leakage from high pressure can easily occur, in addition to the problem related to hydrogen embrittlement. Storing hydrogen under pressure has been done successfully for many years.
The three main types of tanks are:
Steel
Aluminium core encased with fiberglass (composite)
Plastic core encased with fiberglass (composite)
To store more hydrogen a smaller volume, being compressed to high pressure is one of the options. The most common way of storing hydrogen is to compress it into steel gas cylinders under a pressure of up to 700 bar. In stationary systems where weight and size are not decisive factors, steel tanks are a good solution, but for vehicles, traditional pressure tanks are problematic regarding both weight and volume. There has been considerable breakthrough the last few years in the development of a new type of composite tank which can store hydrogen at 350 bar pressure and at the same time meet the current safety standards. This type of tank has a storage capacity of 10-12 weight percent hydrogen, whereby the weight of the tank no longer is a problem. Progress is also being made on tanks which can store hydrogen at 700 bar pressure. This will reduce the tank volume, which is necessary to achieve the desirable driving distance. Light weight composite tanks which utilise space better than the usual cylindrical tanks have also been designed. Special H2 compressors are normally used to pressurise the hydrogen. If pressure electrolysers are used to supply compressed hydrogen, the process of compressing could be reduced or eliminated all together depending on what pressure level is needed. This would be a more efficient system, and a simpler and less expensive solution.
_
Pressure vessel materials according to their type:
|
Type |
Materials |
Typical Pressure (bar) |
Cost ($/kg) |
Gravimetric Density (wt %) |
|
I |
All-metal construction |
300 |
83 |
1.7 |
|
II |
Mostly metal, composite overwrap in the hoop direction |
200 |
86 |
2.1 |
|
III |
Metal liner, full composite overwrap |
700 |
700 |
4.2 |
|
IV |
All-composite construction |
700 |
633 |
5.7 (Toyota Mirai) |
_
Figure below shows Type-IV composite overwrapped hydrogen pressure vessel:
Compressed hydrogen is a storage form whereby hydrogen gas is kept under pressures to increase the storage density. Compressed hydrogen in hydrogen tanks at 350 bar (5,000 psi) and 700 bar (10,000 psi) is used for hydrogen tank systems in vehicles, based on type IV carbon-composite technology. By compressing the hydrogen gas to 700 bar, it reaches a volumetric density of 42 kg/m3 compared with 0.090 kg/m3 under normal pressure and temperature conditions.
_
Table below shows volume of 1kg of hydrogen depending on the storage pressure:
_
According to Züttel, the gravimetric density of high-pressure gas cylinders is 13% at pressure of 800 bar. In contrast, according to the Toyota Motor Corporation, the gravimetric density of the 2017 Mirai tank is 5.7 wt % at 700 bar. The Mirai tank has an internal volume of 122.4 L, with volumetric energy density up to 4.90 MJ/L. This significant difference shows that even for established commercial technologies, performance may vary widely depending on the application. Energy is required to compress hydrogen, and it takes a minimum of 4.1 wt % to compress hydrogen from 20 to 700 bar. All gases, hydrogen included, release heat when compressed. A common strategy to avoid overheating the tank during refill by compression is to cool the gas beforehand. This requires an additional 1.8–3.6 wt % for hydrogen pre-cooling. However, there is no need for a thermal management system onboard the vehicle. The pressure is extremely high and demands an extremely robust tank. This limits the shape of the tank to a cylinder and makes its integration into the vehicle architecture more difficult. The kinetics of compressed gas are ideal, and the fuel flow can increase or decrease in a virtually limitless manner. From a safety point of view, the typical materials involved, such as carbon fiber and nylon-6, are not toxic or environmentally harmful. High pressure, however, always represents a risk.
_____
Liquid Hydrogen (LH2):
Liquid hydrogen is also considered promising and efficient as a hydrogen storage option, because it has higher hydrogen density (70.8 kg-H2/m3), which is about 800 times that of uncompressed hydrogen (0.090 kg/m3 at standard temperature and pressure (STP)), as well as high purity. However, in order to bring hydrogen into the liquid phase, refrigeration to a very low temperature (−253 °C) is required, leading to high energy consumption. Moreover, due to this cooling requirement, liquid hydrogen is not preferred for long term storage or long distance of transportation, because the energy input needed to keep the temperature very low is also intensive. Hydrogen is liquefied by reducing its temperature to −253 °C, similar to liquefied natural gas (LNG) which is stored at −162 °C. At -252.87°C and 1.013 bar, liquid hydrogen has a density of close to 71 kg/m3. At this pressure, 5 kg of hydrogen can be stored in a 75-liter tank. Liquid hydrogen tanks do not have to withstand high pressure, but they must be heavily insulated, which results in reservoirs with thick walls. The vessel must be properly insulated to reduce heat transfer to a minimum. Heat transfer from the environment to the liquid increases the pressure inside the tank. Since the tank is not designed to hold high pressure, hydrogen is allowed to escape through a relief valve, which is sometimes referred to as “boil-off”. This causes a daily loss of approximately 3% of the stored volume. Because thermal insulation is never perfect, an unused hydrogen reservoir stored in a warm environment will eventually deplete itself. Liquid hydrogen storage is a mature technology and is the basis of the existing industrial infrastructure network for storage and delivery. It is important to note that the cost of hydrogen liquefaction is significant, both in terms of energy and equipment.
_
LH2 is particularly interesting for long distance transportation purposes and as fuel in spacecraft and airplanes. However, the volumetric energy density of liquid hydrogen is almost 4 times lower than that of kerosene, even excluding the volume required for insulation. Therefore, liquid hydrogen has an unacceptably short range for a commercial airliner. A great deal of experience has been accumulated over the years when it comes to the usage and handling of LH2. In order to cool the hydrogen down, energy equalling 30-40% of that in the fuel is needed. Development of a new cooling process that would cut the energy use in half is considered feasible. LH2 is especially well suited for use in air and space travel, where its characteristics rate is higher than any other fuel. Today, LH2 is the most frequently used fuel within space travel.
BMW has studied use of liquid hydrogen in combustion engines in cars for over 20 years and says that using liquid hydrogen in automobiles is a good alternative. The German company Linde has developed a tank for liquid hydrogen where the cold from some of the liquid hydrogen is used to cool down the insulation surrounding the tank; this is done with cooling elements. This way the tank keeps the hydrogen in a liquid state for up to 12 days. This type of tank is now being tested and will probably be installed in BMW’s hydrogen cars among others. Japan has a liquid hydrogen (LH2) storage site in Kobe port.
______
Solid storage:
In this method, hydrogen atoms or molecules are tightly bound with other elements. It is perhaps the most promising hydrogen storage method as it is possible to store large amount of hydrogen within a relatively small volume. There are two basic bonding mechanisms for such material-based solid state storage:
- Chemisorption (i.e., absorption): hydrogen molecules are dissociated into hydrogen atoms and integrated in the lattice of the materials. This method makes it possible to storage large quantities of hydrogen in small volume under low pressure and ambient temperature.
- Physisorption (i.e., adsorption): hydrogen atoms or molecules are attached to the surface of the materials
It is preferable that the storage material will have high gravimetric and volumetric capacity, reversibility of hydriding and dehydriding steps, favourable equilibrium temperature-pressure characteristics, adequate stability of the hydride formed and low sensitivity to impurities present in feed gas. However, after years of research, a single material that possess all those features remains elusive.
_
The hydrogen molecule is split into hydrogen atoms which are absorbed by the metal, whereby hydrogen is stored in the metallic matrix. Certain metals and metal alloys have the ability to absorb hydrogen under moderate pressure and temperature, creating hydrides. Hydrogen can react with metals like Li, Na, Mg, Ti, alloy or intermetallic compound (IMC) to form metal hydride. Hydride is a compound which contains hydrogen and one or more other elements. Metal hydrides, such as MgH2, NaAlH4, LiAlH4, LiH, LaNi5H6, TiFeH2, ammonia borane, and palladium hydride represent sources of stored hydrogen. A metal hydride tank contains, in addition to a heat manipulation system, granular metal which absorbs the hydrogen like a sponge absorbs water. The heat system draws heat away when hydrogen is filled into the tank, and applies heat when the hydrogen is taken out of the tank. The hydrogen is released from the metal hydride when heat is applied. This heat may, for example, be excess heat from the fuel cells. A metal hydride tank is considered to be a very safe fuel system in the event of a collision because the loss of pressure in a punctured tank will cool down the metal hydride, which will then cease to release hydrogen. Several metal hydrides are available commercially, representing a good solution for hydrogen storage where the weight factor is not a problem. For vehicles, the problem with metal hydride is the high weight compared to the amount of hydrogen stored. The problem of weight has still not been solved in spite of extensive research. Researchers are therefore trying to think in new directions, by trying to lighten the alloys for one, and finding methods of packing the hydrogen in higher concentrations.
_
Another choice of storing hydrogen is by adsorption, which physically adsorbs hydrogen using porous materials, such as metal–organic frameworks and carbon materials. Compared with metal hydrides which offer high volumetric capacities through dissociative absorption of hydrogen, high surface area absorbent materials offer the advantages of fast hydrogen kinetics and low hydrogen binding energies. In this physisorption process, the hydrogen gas molecule interacts with atoms at the surface of the material and the distance between the gas molecule and the surface diminished. Hence, it potentially reduces the thermal management issues during charging and discharging of hydrogen. Due to such weak interaction, the adsorption is only observed at low temperature. For adsorption, the major problem is to provide light carrier materials with sufficient amount of bonding sites. A number of materials can be used for adsorption, such as graphite, carbon nanotubes, C60 buckyballs, zeolites, metal organic frameworks, intercalation compounds and so on. The big advantages of adsorption are the low operating pressure, the inexpensive material used, and the simple design of the storage system. However, major drawbacks including the requirement of low temperature and high pressure to storage hydrogen, together with low gravimetric and volumetric hydrogen density. The physical adsorption hydrogen storage is still far away from large commercialization as the filling time is still under satisfaction when considering the storage capacity.
______
Liquid organic hydrogen carriers:
Liquid organic hydrogen carriers (LOHC) are organic compounds that can absorb and release hydrogen through chemical reactions. LOHCs can therefore be used as storage media for hydrogen. In principle, every unsaturated compound (organic molecules with C-C double or triple bonds) can take up hydrogen during hydrogenation. The sequence of endothermal dehydrogenation followed by hydrogen purification is considered as the main drawback which limits the overall efficiency of the storage cycle.
______
Summary of weight and volume of different tank types. All examples have the same driving distance when using the same type of vehicle.
|
Vehicles with equal driving distances per fill-up |
Mass (kg) |
Volume (liter) |
|
Gasoline/combustion engine |
50 |
70 |
|
Compressed hydrogen (350 bar) / fuel cells |
90 |
320 |
|
Compressed hydrogen (700 bar) / fuel cells |
~ 100 |
180 |
|
Liquid hydrogen / fuel cells |
45 |
190 |
|
Hydrogen in metal hydride / fuel cells |
200-600 |
180 |
________
________
Hydrogen stored as another chemical fuel:
It is evident that pure hydrogen is difficult to store and transport due to its physicochemical properties, such as low volumetric energy density. Therefore, it is worth investigating pathways to chemically store hydrogen. The term chemical hydrogen is used to describe the strategy of storing hydrogen by synthesizing molecules that contain hydrogen. Chemical storage could offer high storage performance due to the high storage densities. For example, supercritical hydrogen at 30 °C and 500 bar only has a density of 15.0 mol/L while methanol has a density of 49.5 mol H2/L methanol and saturated dimethyl ether at 30 °C and 7 bar has a density of 42.1 mol H2/L dimethyl ether.
_
Methane (CH4):
Methane, the simplest hydrocarbon, can be synthesized by a process known as methanation. The global decarbonization trend spurred renewed interest in this process. Methanation can be carried out biologically or catalytically, from carbon monoxide or carbon dioxide. It produces hydrogen from electrolysis of water. After that, catalytic hydrogen–carbon dioxide methanation effectively converts electricity to methane. While an industrial infrastructure for natural gas exists, the same cannot be said for methane. However, because natural gas is composed mostly of methane, the current natural gas technologies provide an acceptable estimation of the methane potential. The volumetric energy densities of hydrogen compressed at 700 bar and natural gas compressed at 250 bar are roughly the same. The volumetric energy density of liquefied natural gas is twice as high as that of hydrogen compressed at 700 bar. However, similar to liquid hydrogen tanks, liquid natural gas tanks are subject to boil-off, and methane is a potent greenhouse gas. The chemical bonds between the carbon and hydrogen atoms are very stable, thus hydrogen is not readily extracted from methane. Therefore, methane must be utilized differently to pure hydrogen. A common way to produce hydrogen from methane is steam reforming, but this reaction is highly endothermic, i.e., it requires a lot of energy. Consequently, it is not suitable for mobile applications. Methane cracking is possible, but its energy balance is unclear. Joglekar et al. developed a direct methane fuel cell to produce electricity directly from methane. However, it uses a platinum catalyst and is far from commercial application. Solid oxide fuel cells (SOFCs) are perhaps the most interesting candidate for methane conversion. One of the promising characteristics of SOFCs is their fuel flexibility. Given the modest energy density of methane, other candidates should be considered for chemical hydrogen storage.
_____
Methanol (CH3OH):
Most methanol production today is from synthesis gas (carbon monoxide and hydrogen) derived from biomass or fossil fuels, or from hydrogen by steam reforming of natural gas. Most methanol use is for making plastics. About 14% of it is used as a petrol additive and 7% to make DME. Methanol production accounts for about 13% of the word’s hydrogen demand. Methanol is also a promising candidate for hydrogen storage, as well the utilization of CO2 via hydrogenation. The adoption of methanol is strongly correlated with the idea of power-to-product (P2X), which utilizes surplus electricity to produce chemical fuels. Hydrogen can be released from methanol through thermolysis, steam reforming and partial oxidation. However, the adoption of methanol to store hydrogen leads to environmental problems in the utilization site because of the release of CO2 when methanol is directly utilized or decomposed. This leads to a non CO2-free energy system. In addition, the separation of CO2 is also energy intensive. The established CO2 separation based on absorption using amine solution consumes approximately 1.1 kWh/kg-CO2.
Any future liquid fuel for cars needs to compete with petrol at 32 MJ/L or diesel fuel at 39 MJ/L and be no more difficult to store and refuel than LPG. Methanol has 16 MJ/L. Methanol has a high content of hydrogen which can relatively easily be extracted by reforming. There are people who say methanol would be a good transitional fuel solution for smaller cars. The advantage of methanol is that it is liquid under normal air pressure and room temperature, and has a high content of hydrogen compared to other fossil fuels. In a methanol car with a reformer, the methanol will be reformed into hydrogen which is then used in the fuel cell. The energy loss in these two processes is high and the system efficiency therefore low. For diesel engines, dimethyl ether (CH3-O-CH3, DME) is better, and this is made by dehydrating a couple of methanol molecules. It is a gas but can be stored under low pressure as a liquid, like LPG. DME has an energy density of 18-19 MJ/L, so less than oil-based fuels, but usable and easily stored.
At present, the methanol route has a low energy efficiency due to the energy consuming intermediate step of synthesis gas. If a catalyst were discovered which is capable of converting methane directly and easily to methanol, then this would also be a very attractive fuel for use in Direct Methanol Fuel Cells (DMFC). However, as things stand at present, hydrogen can be used as a secondary energy carrier in fuel cells without emitting any CO2, so it does not make sense to convert hydrogen to methanol (another secondary energy carrier), which does emit CO2 when used in a fuel cell.
_____
Ammonia (NH3):
Organic nitrogen in the world’s soils is only sufficient to feed one-third of today’s population. The rest must come from inorganic additions. Most of the world’s nitrogen fertilizers are made combining hydrogen with abundant atmospheric nitrogen. The resulting ammonia is then oxidised to nitrates. The largest single use of hydrogen in the world is in ammonia manufacture, which consumes about two-thirds of the world’s hydrogen production. Ammonia is manufactured by the so-called Haber-Bosch process, in which hydrogen and nitrogen react in the presence of a catalyst at pressures around 1,000 atmospheres and temperatures around 500° C. The Haber Process (Haber-Bosch Process) combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The reaction is reversible and the production of ammonia is exothermic. German scientist Fritz Haber invented the process to combine atmospheric nitrogen with hydrogen in 1909 and received the Nobel Prize for chemistry in 1918 for creating “an exceedingly important means of improving the standards of agriculture and the well-being of mankind,” which now looks like a considerable understatement. It was scaled up by the chemical engineer Karl Bosch, so is often known as the Haber-Bosch process. Bosch received a Nobel Prize in 1931.
N2 + 3H2 ⇌ 2NH3
∆H -92 kJ/mol (exothermic, using a metal catalyst at high temperature and pressure)
The Haber process produces about 180 million tonnes of anhydrous ammonia per year for nitrogen fertilizers and consumes about 3-5% of the world’s natural gas production to make the hydrogen for it. The nitrogen is obtained cryogenically from air. Ammonia is currently adopted as an agricultural fertilizer, refrigerant gas and in the manufacture of explosives, pesticides and other chemicals.
Looking to the future, ammonia could have a key role in the storage and transport of hydrogen. It may also be used as a fuel. In Japan projects are exploring co-firing ammonia with coal in boilers, and ammonia with natural gas in combustion turbines. Ammonia also has potential use as a maritime fuel, since it can be used in ship engines with only minor modification. Also it can be used in some fuel cells.
_
Facts about ammonia:
-Ammonia is a colorless gas with a very distinct odor. Ammonia gas can be dissolved in water. This kind of ammonia is called liquor ammonia or aqueous ammonia at room temperature. There is another liquid ammonia when it is cooled down to minus 33°C. Both liquid and liquor ammonia are in the liquid state. Liquid ammonia has only ammonia molecules, but liquor ammonia has both ammonia and water. The key difference between liquid ammonia and liquor ammonia is that liquid ammonia contains NH3 molecules while liquor ammonia contains NH4OH.
-Ammonia (NH3) is used today primarily for fertilizer.
-The high content of hydrogen makes ammonia suitable as a fuel and energy carrier.
-Liquor ammonia is able to store hydrogen in volumes much higher (121 kg-H2/m3) than liquid hydrogen (70.8 kg-H2/m3), which is about 1.7 times as high. Liquor ammonia can be stored at relatively low pressure (0.99 MPa at a temperature of 25 °C), which is significantly lower than that of compressed hydrogen. Therefore, liquor ammonia is easier to store and transport.
-Green ammonia is considered the best carbon-free fuel alternative for long-haul shipping.
-Green ammonia is made from green hydrogen which is produced using renewable energy.
_
Characteristic data for comparable energy carriers:
|
Liquid |
Volumetric Energy density |
Gravimetric energy density |
Boiling point |
|
Ammonia |
12.7 MJ/litre |
18.6 MJ/kg |
-33.4 °C |
|
Diesel oil |
36.2 MJ/litre |
43.1 MJ/kg |
180–380 °C |
|
Hydrogen |
8.5 MJ/litre |
119.9 MJ/kg |
-252.87 °C |
|
Propane |
26 MJ/litre |
46 MJ/kg |
-42 °C |
__
Ammonia (NH3) releases H2 in an appropriate catalytic reformer. Ammonia provides high hydrogen storage densities as a liquid with mild pressurization and cryogenic constraints: It can also be stored as a liquid at room temperature and pressure when mixed with water. Ammonia is the second most commonly produced chemical in the world and a large infrastructure for making, transporting, and distributing ammonia exists. Ammonia can be reformed to produce hydrogen with no harmful waste, or can mix with existing fuels and under the right conditions burn efficiently. Since there is no carbon in ammonia, no carbon by-products are produced; thereby making this possibility a “carbon neutral” option for the future. Pure ammonia burns poorly at the atmospheric pressures found in natural gas fired water heaters and stoves. Under compression in an automobile engine it is a suitable fuel for slightly modified gasoline engines. Ammonia is a suitable alternative fuel because it has 18.6 MJ/kg energy density at NTP and carbon-free combustion byproducts.
_
Ammonia (NH3) makes a good carrier of hydrogen, or means of long-term storage. It is a key part of the hydrogen picture, for storing it or moving it over long distance at much lower cost than for hydrogen itself.
Ammonia will be made by some refinement of the Haber Bosch process, requiring 2-3 kWh/kgH2 or 14 kWh/kg ammonia, then dissociated to release the hydrogen, requiring about 8 kWh/kgH2. Conversion to and from ammonia is likely to add about $1/kg to the hydrogen production cost.
Ammonia has thermal properties similar to propane, is easy to cool and compress into liquid form for transport and storage (boiling point minus 33°C), and has an energy density that is competitive with carbon-based fossil fuels. It contains 17.7% hydrogen by weight. Ammonia’s energy density of 18.6 MJ/kg (4.32 kWh/L or 5.18 kWh/kg) is comparable to that of compressed natural gas and methanol, but about half that of petrol/gasoline, diesel fuel and liquefied petroleum gas (LPG).
Ammonia is seen as a large-scale solution to the problems in storage and movement of hydrogen beyond pipelines. According to the source of hydrogen, the ammonia may be ‘green’, ‘blue’ or ‘grey’. Already, over 100 million tonnes of ammonia is transported each year, so handling is well-established. It can be stored and moved at pressures of 800-1000 kPa, similar to propane, or in refrigerated tanks.
After storage or transport, the ammonia can be dissociated or ‘cracked’ back into hydrogen and nitrogen by thermal catalytic decomposition or electro-oxidation, though recovery of high-purity hydrogen is unproven at scale. Alkaline fuel cells use impure hydrogen (c. 90%) cracked from ammonia, but PEM fuel cells require high-purity product (99.9%). Ammonia may also be used directly in fuel cells or marine internal combustion engines, or blended with petrol/gasoline.
Of the 46 Mt of clean hydrogen expected to be required for shipping in 2050, 74% of this would be for ammonia, with 16% for methanol and 10% directly as fuel, according to the International Renewable Energy Agency (IRENA). That ammonia – 183 Mt – is expected to comprise 43% of the shipping sector fuel needs.
Ammonia, which consists of three hydrogen atoms and one nitrogen atom, can, like pure hydrogen, be used both for energy storage and as fuel in fuel cells. As a fuel, ammonia is particularly suitable for long-haul shipping. Shipping accounts for about two per cent of the world’s greenhouse gas emissions, and 80 per cent of these emissions come from long-haul ships. It has been proposed to use pure hydrogen as fuel in ships, but the challenge is that hydrogen has a relatively low energy density in gaseous form. If you want enough fuel for long-distance transport on ships, the hydrogen must be kept liquid, which requires cooling to minus 253 degrees Celsius. This is why ammonia is a better option. Ammonia becomes liquid already at minus 33 degrees Celsius, which makes it significantly easier to produce and transport as fuel. Ammonia also has a higher energy density in liquid form than hydrogen. In addition, the infrastructure for ammonia is already in place, and ammonia is cheaper to produce, store and transport. So it’s difficult to see how liquid hydrogen could compete with ammonia for long-haul ships.
_
Advantages and disadvantages of ammonia vis-à-vis hydrogen:
Ammonia has several challenges to widespread adaption as a hydrogen storage material. Ammonia is a toxic gas with a potent odor at standard temperature and pressure. The odour on the other hand has the advantage that a leak will be detected long before the concentration in the air is dangerous. Additionally, advances in the efficiency and scalability of ammonia decomposition are needed for commercial viability, as fuel cell membranes are highly sensitive to residual ammonia and current decomposition techniques have low yield rates.
Hydrogen is not toxic, but it is explosive. In compressed form, the substance is a light gas that rises rapidly into the air, but in liquid form it is heavier and thus poses additional challenges related to safety. Combustion of hydrogen only results in water vapour, while ammonia emits nitrous gases (NOx). NOx is of course a challenge, but there are technical solutions that reduce the problem, including a catalyst made of ammonia. The most important advantage of using ammonia is that it’s a practical and feasible opportunity to reduce greenhouse gas emissions from shipping.
_
Four major types of energy preservation technologies are currently available: electrical, mechanical, electrochemical, and chemical. Of these four categories, the future of long-term energy storage is more often associated with the electrochemical (batteries) and chemical (e.g., natural gas, hydrogen, and ammonia) options. Unlike other options, these can store large volumes of energy for a long time in a transportable form, so that power can be transferred across both time and space. Of these, only hydrogen and ammonia—two substances that can be generated carbon-free—are able to preserve the same amounts of energy as fossil fuels, potentially cost-efficiently, while not emitting any CO2 when combusted. Of these two, ammonia can deliver more energy within the same volume, and it has an established infrastructure and lower handling costs. Ammonia is highly valued as a potential hydrogen storage option. It has high hydrogen density (17.7 wt%), as well as high flexibility in its utilization, including mobile and stationary applications. Due to its stability for long-term storage and transportation, ammonia can fulfill the demand to store the energy in time (stationary energy storage) and in space (energy export and import). Ammonia can be utilized by extracting its stored hydrogen or directly utilized as fuel.
_
The combined application of water electrolysis and Haber-Bosch process is called power-to-ammonia (P2A) technology. The advantage of nitrogen-based fuels is that nitrogen, abundant in the atmosphere, can be used as feedstock, whereas methanation or methanol production require CO2. Power-to-ammonia could provide grid services such as seasonal storage, emergency backup, and energy transmission. This could reduce the need for significant excess capacity in the electricity system and minimize the need to expand electricity grid capacity. A number of challenges which need to be overcome, in addition to the usual suspects like increasing scale, decreasing cost and obtaining required government support. Significantly, the ammonia synthesis process generally requires continuous operation to avoid damaging the catalysts, which limits its ability to provide higher-value grid-balancing services with intermittent renewable power generation. In addition, the toxicity of ammonia leads to limited social acceptability and stringent storage and handling requirements.
______
______
Underground hydrogen storage:
Salt caverns, exhausted oil and gas fields or aquifers can all provide underground hydrogen storage on an industrial scale. Such underground storage sites have been used for natural gas and crude oil for years, where they were held to balance supply or demand fluctuations or in preparation for a crisis. For storage of very large amounts of hydrogen, the most economical method is underground storage under pressure. Various solutions have been proposed for large-scale hydrogen storage. Except for the buried tanks compressing hydrogen in gas and liquid, hydrogen underground storage solutions, such as aquifers, depleted deposits of natural gas and oil and salt caverns are the principal choices for large-scale hydrogen storage in medium and long term. The first two types are of porous structure and their capacity may be influenced by the geological conditions. Most underground hydrogen storage of the world is in depleted deposits, approximately 75%. In recent years, salt caverns have seen great interests in storing hydrogen gas owing to their stability and imperviousness of their walls of salt caverns. The volume of a salt cavern can range from 100,000 to 1000,000 m3 working at a maximum pressure of 200 bar. The expenses of storing hydrogen in caverns will vary according to the geological formations, but this could be an inexpensive option. The German town of Kiel is supposed to have stored city gas with a hydrogen content of 60-65% in a gas storage hall with a volume of 32,000 m3 under 80-100 bar pressure since 1971. Usable as such underground storage areas may be empty reservoirs, aquifers, caverns or empty cavities in salt formations. However, the development of salt cavern hydrogen storage is limited by some technical aspects where the tightness of the boreholes and the transfer capacity of the surface installation are of significant importance. Besides, environmental limitations and sustainable development should also be taken into consideration when making location plans.
______
Storage in Pipelines:
Piping systems are usually several miles long, and in some cases may be hundreds of miles long. Because of great length, and therefore great volume, of these piping systems, a slight change in the operating pressure of a pipeline system can result in a large change in the amount of gas contained within the piping network. By making small changes in operating pressure, the pipeline can be used to handle fluctuations in supply and demand, avoiding the cost of onsite storage.
A natural gas network may be used for the storage of hydrogen. Before switching to natural gas, the UK and German gas networks were operated using town gas that contained about 50% hydrogen. The town gas was used for cooking, heating and lighting. The storage capacity of the German natural gas network is more than 200,000 GWh which is enough for several months of energy requirement. By comparison, the capacity of all German pumped storage power plants amounts to only about 40 GWh. Similarly UK pumped storage is far less than the gas network. The transport of energy through a gas network is done with much less loss (<0.1%) than in a power network (8%). While it is generally accepted that gas with 10-20% hydrogen content could be introduced into the existing natural gas system without causing a negative impact on end users or pipeline infrastructure, a number of critical components have been deemed unsuitable for use at these levels of hydrogen concentration.
______
______
Distribution of hydrogen:
Compressed gas can be transported using high-pressure cylinders, tube trailers or pipelines. If hydrogen is to be transported as a gas, it should be compressed to a very high pressure to maximize tank capacities. High pressure gas cylinders, for example, are rated as 40 Mpa and hold about 1.8 kg of hydrogen, but are very expensive to handle and transport. Tube trailers, consists of several steel cylinders mounted to a protective framework, can be configured to hold 63-460 kg of hydrogen depending on the number of tubes. Operating pressures are 2060 Mpa. Metal hydrides can be used for transport by absorbing hydrogen with a metal hydride, then loading the entire container onto a truck or railcar for transport to the customer’s site where it can be exchanged for an empty hydride container, or used as a conventional tanker. Most hydrogen used in the United States is produced at or close to where it is used—typically at large industrial sites. The infrastructure needed for distributing hydrogen to the nationwide network of fueling stations required for the widespread use of fuel cell electric vehicles still needs to be developed. The initial rollout for vehicles and stations focuses on building out these distribution networks, primarily in southern and northern California.
_
Hydrogen is distributed through various methods:
-1. Pipeline:
Gaseous hydrogen can be transported through pipelines much the way natural gas is today. Approximately 1,600 miles of hydrogen pipelines are currently operating in the United States. Owned by merchant hydrogen producers, these pipelines are located where large hydrogen users, such as petroleum refineries and chemical plants, are concentrated such as the Gulf Coast region. Transporting gaseous hydrogen via existing pipelines is a low-cost option for delivering large volumes of hydrogen. The high initial capital costs of new pipeline construction constitute a major barrier to expanding hydrogen pipeline delivery infrastructure. Natural gas is compressed in transmission pipelines to pressures typically ranging from 500 to 1400 psi. Today’s hydrogen pipelines, which are associated with industrial facilities such as oil refineries and chemical plants, operate at pressures around 500–1200 psi. While transmission pipelines may operate at pressures over 1000 psi, distribution systems operate at much lower pressures. High-pressure natural gas (and hydrogen pipelines) today use steel alloys, while natural gas “distribution” pipes can be made of a variety of materials such as cast iron, copper, steel or plastic (PVC or PE). In the natural gas distribution network, pressure is low, around 4 bar, and so cheaper plastic pipe is usually used. PVC (Poly Vinyl Chloride) and the newer HDPE (High Density Poly Ethylene) are too porous and not usable for transporting high pressure hydrogen. With little or no changes, the majority of existing steel natural gas lines can be used to transport mixtures of natural gas and hydrogen. It is also possible, with certain modifications, to use pure hydrogen in certain existing natural gas lines. This depends on the carbon levels in the pipe metal. Newer gas pipelines such as those in the North Sea, have low carbon content and are therefore suitable for transporting hydrogen. If the speed is increased by a factor of 3.2 to compensate for hydrogen having 3.2 times lower energy density per volume than natural gas, the same amount of energy can be moved. The fact is that by using efficient hydrogen technology such as fuel cells, etc., the same amount of transported energy will yield increased output at final consumption.
_
Hydrogen embrittlement is not a problem for hydrogen gas pipelines. Hydrogen embrittlement only happens with ‘diffusible’ hydrogen, i.e., atoms or ions. Hydrogen gas, however, is molecular (H2), and there is a very significant energy barrier to splitting it into atoms. Carrying hydrogen in steel pipelines (grades: API5L-X42 and X52; up to 1,000psi/7,000kPa, constant pressure/low pressure cycling) does not lead to hydrogen embrittlement. Hydrogen is typically stored in steel cylinders without problems. Coal gas (also known as town gas) is 50% hydrogen and was carried in cast-iron pipes for half a century without any embrittlement issues. Over great distances, pipeline transport of hydrogen could be an effective way of transporting energy. The energy loss in an electric power grid can be up to 7.5-8% of the energy it is transferring. This is about double of what is needed to feed gas through a pipeline of the same length. Hydrogen pipes that are in use today are constructed of regular pipe steel, and operate under pressure at 10-20 bar, with a diameter of 25-30 cm.
_
Research today focuses on overcoming technical concerns related to pipeline transmission, including:
-The potential for hydrogen to embrittle the steel and welds used to fabricate the pipelines
-The need to control hydrogen permeation and leaks
-The need for lower cost, more reliable, and more durable hydrogen compression technology.
Potential solutions include using fiber reinforced polymer (FRP) pipelines for hydrogen distribution. The installation costs for FRP pipelines are about 20% less than that of steel pipelines because the FRP can be obtained in sections that are much longer than steel, minimizing welding requirements.
_
-2. High-Pressure Tube Trailers:
Transporting compressed hydrogen gas by truck, railcar, ship, or barge in high-pressure tube trailers is expensive and used primarily for distances of 200 miles or less.
_
-3. Liquefied Hydrogen Tankers:
Cryogenic liquefaction is a process that cools hydrogen to a temperature where it becomes a liquid. Although the liquefaction process is expensive, it enables hydrogen to be transported more efficiently (compared with high-pressure tube trailers) over longer distances by truck, railcar, ship, or barge. If the liquefied hydrogen is not used at a sufficiently high rate at the point of consumption, it boils off (or evaporates) from its containment vessels. As a result, hydrogen delivery and consumption rates must be carefully matched.
Liquid hydrogen is transported using special double walled insulated tanks to prevent boil off of the liquid hydrogen. Some tankers also use liquid nitrogen heat shields to cool the outer wall of the liquid hydrogen vessel to further minimize heat transfer. Tank trucks can carry 360-4,300 kg of liquid hydrogen where as rail cars have capacities ranging from 2,300 to 9,100 kg of hydrogen. Boil off rates for trucks and rail cars are 0.3%-0.6%/day.
_
-4. Ocean transportation
Hydrogen can be transported as a liquid in tank ships. These are not too different from LNG tankers, aside from the fact that better insulation is required to keep the hydrogen cooled down over long distances. The Japanese WE-NET and the German-Canadian Euro Quebec have reported on the use of such tanks. The evaporated hydrogen may be used as fuel onboard. In 1990, the German institute for materials research declared that LH2 could be given the same safety rating as LPG and LNG, and transport of LH2 into German harbours was approved. Barges or sea-going vessels have been considered for long distance transport of hydrogen. Each barge would carry 21,000 kg of hydrogen with no venting during a 50-day trip.
_
-5. Air transportation
There are several advantages in transporting LH2 by air rather than by ship. LH2 is lightweight and the delivery time is much shorter, and evaporation is therefore not a big problem. Studies on this have been done by CDS Research Ltd. in Canada, with support from the WE-NET program.
_
Insulated pipeline (which includes a super conducting wire) can also be considered. The liquid hydrogen acts as a refrigerant for superconductor and would allow long distance transport of electricity without the high current losses of conventional power lines. The main problem with this would be the specialized insulating requirement and losses from pumping and re-cooling the liquid hydrogen along the way.
______
Hydrogen is not always an ideal vector to carry energy from its place of production to the end user, because a fairly high amount of energy is lost during handling, storage and transportation as seen in the table below.
|
Hydrogen stages of application |
Energy cost in % of HHV |
|
Production: electrolysis on-site production |
43 65 |
|
Packaging: compression 20 MPa compression 80 MPa liquefaction chemical hydrides |
8 13 40 60 |
|
Distribution: road, 20 MPa, 100 km road, liquid, 100 km pipeline, 1000 km |
6 1 10 |
|
Storage: liquid, 10 d |
5 |
|
Transfer: 20 MPa to 20 MPa |
1 |
If the hydrogen is packaged in liquid synthetic fossil fuels, the overall energy consumption can be considerably lower. In the evaluation of the cost of green hydrogen i.e., that produced by electrolysis of water using low carbon power (the likely alternative, production from methane with steam reforming and carbon capture is referred to as ‘blue’ hydrogen) the analysis must account for the cost and efficiency of an electrolyser, and replacement of its stacks, the compression and storage of hydrogen, the cost of transporting hydrogen and, finally, the efficiency of dispensing hydrogen.
______
High energy and economic cost of hydrogen transport:
A 40 ton truck can deliver 26 tons of gasoline to a conventional gasoline filling station. One daily delivery is sufficient for busy station. A 40 ton truck carrying compressed hydrogen can deliver only 400 kilograms. That is because of the weight of the tank capable of holding 200 atmospheres of pressure. An empty truck will weigh almost as much as a full one. The compressed hydrogen tank must be robust. The energy used to compress the hydrogen to 200 atmospheres would be released instantly if a tank ruptured. The fireball would cover a football field. Hydrogen is more energy dense than gasoline (by weight) and hydrogen powered transportation is more energy efficient. Yet the hydrogen filling station will require 15 deliveries every day, everything else being equal. The energy cost of truck transport becomes unacceptable unless the source of hydrogen is very close to the point of use. A cryogenic truck could carry more hydrogen but the energy cost to liquefy hydrogen makes this infeasible in most cases.
Creating an infrastructure for hydrogen distribution and delivery to thousands of future individual fueling stations presents many challenges. Because hydrogen contains less energy per unit volume than all other fuels, transporting, storing, and delivering it to the point of end-use is more expensive on a per gasoline gallon equivalent basis. Building a new hydrogen pipeline network involves high initial capital costs, and hydrogen’s properties present unique challenges to pipeline materials and compressor design. However, because hydrogen can be produced from a wide variety of resources, regional or even local hydrogen production can maximize use of local resources and minimize distribution challenges.
There are trade-offs between centralized and distributed production to consider. Producing hydrogen centrally in large plants cuts production costs but boosts distribution costs. Producing hydrogen at the point of end-use—at fueling stations, for example—cuts distribution costs but increases production costs because of the cost to construct on-site production capabilities.
______
______
Hydrogen Infrastructure:
A hydrogen infrastructure is the infrastructure of hydrogen pipeline transport, points of hydrogen production and hydrogen stations (sometimes clustered as a hydrogen highway) for distribution as well as the sale of hydrogen fuel, and thus a crucial prerequisite before a successful commercialization of automotive fuel cell technology.
Hydrogen production plants:
The most common method for hydrogen production is steam reforming, accounting for near 95% of the world’s hydrogen production. Methods such as electrolysis of water are also used. The world’s largest facility for producing electrolytic hydrogen fuel is claimed to be the Fukushima Hydrogen Energy Research Field (FH2R), a 10MW-class hydrogen production unit, inaugurated on 7 March 2020, in Namie, Fukushima Prefecture. The site occupies 180,000 square meters of land, much of which is occupied by a solar array; but power from the grid is also used to conduct electrolysis of water to produce hydrogen fuel.
Hydrogen pipeline transport:
Hydrogen pipeline transport is a transportation of hydrogen through a pipe as part of the hydrogen infrastructure. Hydrogen pipeline transport is used to connect the point of hydrogen production or delivery of hydrogen with the point of demand, pipeline transport costs are similar to CNG, the technology is proven, however most hydrogen is produced on the place of demand with every 50 to 100 miles (80 to 161 km) an industrial production facility.
Hydrogen highways:
A hydrogen highway is a chain of hydrogen-equipped filling stations and other infrastructure along a road or highway which allow hydrogen vehicles to travel.
Hydrogen refuelling stations:
A hydrogen station is a storage or filling station for hydrogen. Hydrogen stations which are not situated near a hydrogen pipeline get supply via hydrogen tanks, compressed hydrogen tube trailers, liquid hydrogen trailers, liquid hydrogen tank trucks or dedicated onsite production. Some firms as ITM Power are also providing solutions to make your own hydrogen (for use in the car) at home. According to FuelCellsWorks, an industry group, at the end of 2019, 330 hydrogen refueling stations were open to the public worldwide. As of June 2020, there were 178 publicly available hydrogen stations in operation in Asia. 114 of these were in Japan. There were at least 177 stations in Europe, and about half of these were in Germany. There were 44 publicly accessible stations in the US, 42 of which were located in California. The UK has rapidly developed 5000 electric charging locations to rival its 8500 petrol stations, compared to just 15 hydrogen stations. While 15 hydrogen dispensers could deliver comparable throughput to 900 BEV fast-chargers, they do not offer the same geographic coverage and convenience. A hydrogen fueling station costs between $1 million and $4 million to build. The hydrogen is dispensed by weight. There are two filling pressures in common use. H70 or 700 bar, and the older standard H35 or 350 bar.
“We have a chicken and egg problem with hydrogen fuel cell technology,” explains BMW expert Rücker. “As long as the network of refueling stations for hydrogen-powered cars is so thin, the low demand from customers will not allow for profitable mass production of fuel cell vehicles. And as long as there are hardly any hydrogen cars on the roads, the operators will only hesitantly expand their refueling station network.”
To make up for the lack of conveniently available hydrogen refueling stations, several FCEV manufacturers currently include three years of free hydrogen fuel with a vehicle – valued at around $15,000. Much like refueling with gas, it takes only about three to five minutes to refill a FCEV tank.
_
Numerous production and distribution pathways exist, as summarised in figure below, and include several incremental steps which do not require a wholesale infrastructure transformation. Developing a cost-efficient infrastructure from these options that may evolve over time with developing demand is a significant challenge.
The upper half of figure depicts centralised production methods that rely on new distribution networks, synonymous with the ‘hydrogen economy’ vision. Incremental and less infrastructurally-intensive routes also exist (the lower half of the figure), which utilise existing gas or electricity networks and reduce large up-front costs, albeit at the expense of lower efficiency. Indeed, only 60 small refuelling stations with onsite hydrogen production would be sufficient to supply most of the UK population in the early stages of a transition to fuel cell vehicles, with additional infrastructure deployed as demand increased. This suggests that infrastructure development might not be as challenging as some have suggested.
_
Aside from the energy generation, hydrogen production could be centralized, distributed or a mixture of both. While generating hydrogen at centralized primary energy plants promises higher hydrogen production efficiency, difficulties in high-volume, long range hydrogen transportation (due to factors such as hydrogen damage and the ease of hydrogen diffusion through solid materials) makes electrical energy distribution attractive within a hydrogen economy. In such a scenario, small regional plants or even local filling stations could generate hydrogen using energy provided through the electrical distribution grid or methane pyrolysis of natural gas. While hydrogen generation efficiency is likely to be lower than for centralized hydrogen generation, losses in hydrogen transport could make such a scheme more efficient in terms of the primary energy used per kilogram of hydrogen delivered to the end user.
______
______
Section-9
Hydrogen fuel cell:
A hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. In many ways fuel cells are similar to batteries, such as those you might find in a car or in a portable electronic device like an MP3 player. However, there are some important differences between batteries and fuel cells. Similar to a battery, a fuel cell with a supply of hydrogen and oxygen can be used to power devices that use electricity. While both batteries and fuel cells convert chemical energy into electrical energy, batteries store this chemical energy inside the battery itself. This means that a battery will run down, or need recharging, when there is no longer enough stored chemical energy available to produce sufficient electricity to power the device connected to the battery. Rather than storing chemical energy inside itself, a hydrogen fuel cell receives a supply of chemical energy from the outside. This chemical energy is stored in the hydrogen that is supplied to the anode of the fuel cell. A hydrogen fuel cell essentially consumes hydrogen and oxygen. When a fuel cell is continuously supplied with hydrogen and oxygen, and the product water is removed, the fuel cell can generate electricity.
_
Hydrogen fuel cells and batteries are both electrochemical cells. They each have two electrodes in contact with a material that can conduct ions, called an electrolyte. One electrode is the anode and the other is the cathode. In a hydrogen fuel cell electrons are released from the hydrogen that is supplied to the anode whereas in a battery the electrons are released from the material in the anode itself. Because battery electrodes actively participate in the conversion of chemical energy to electrical energy, over time this can have a damaging effect on the electrodes and therefore on the effectiveness of the battery. Unlike batteries, the electrodes in hydrogen fuel cells are relatively stable since they act as catalysts in the release or acceptance of electrons and are not chemically changed during this process.
_
Fuel cells can offer many potential benefits over batteries. Batteries can be recharged just as a canister of hydrogen can be refilled, but the capacity of batteries decreases over many recharge cycles. A container for hydrogen and a fuel cell, on the other hand, do not degrade over time. Furthermore, once batteries are fully charged, they continuously “leak” energy when not in use, while a container of hydrogen does not leak and has a longer shelf-life. Additionally, a container for hydrogen can typically be refilled with hydrogen from an external source much faster than a battery can be fully recharged. When batteries are used in an electric vehicle, the performance of the vehicle degrades as the battery discharges. In contrast, fuel cell performance remains the same as long as there is hydrogen fuel in the tank.
_
Basics of fuel cell:
A fuel cell is a device that generates electricity by a chemical reaction. There are several kinds of fuel cells, and each operates a bit differently. But in general terms, hydrogen is fed to the anode, oxygen is fed to the cathode; they are separated by a catalyst and an electrolyte membrane that only allows positively charged protons through to the cathode. The catalyst splits off the hydrogen’s negatively charged electrons, allowing the positively charged protons to pass through the electrolyte to the cathode. The electrons, meanwhile, travel via an external circuit—creating electricity that can be put to work—to meet the protons at the cathode, where they react with the oxygen to form water. In other cell types the oxygen picks up electrons and then travels through the electrolyte to the anode, where it combines with hydrogen ions. The electrolyte plays a key role. It must permit only the appropriate ions to pass between the anode and cathode. If free electrons or other substances could travel through the electrolyte, they would disrupt the chemical reaction. Whether they combine at anode or cathode, together hydrogen and oxygen form water, which drains from the cell. As long as a fuel cell is supplied with hydrogen and oxygen, it will generate electricity. Even better, since fuel cells create electricity chemically, rather than by combustion, they are not subject to the thermodynamic laws that limit a conventional power plant (Carnot Limit). Therefore, fuel cells are more efficient in extracting energy from a fuel. Waste heat from some cells can also be harnessed, boosting system efficiency still further. One great appeal of fuel cells is that they generate electricity with very little pollution. A single fuel cell generates a tiny amount of direct current (DC) electricity. In practice, many fuel cells are usually assembled into a stack. The purpose of a fuel cell is to produce an electrical current that can be directed outside the cell to do work, such as powering an electric motor or illuminating a light bulb or a city. Because of the way electricity behaves, this current returns to the fuel cell, completing an electrical circuit. The chemical reactions that produce this current are the key to how a fuel cell works.
__
Design features in a fuel cell include:
-The electrolyte substance, which usually defines the type of fuel cell, and can be made from a number of substances like potassium hydroxide, salt carbonates, and phosphoric acid.
-The fuel that is used. The most common fuel is hydrogen.
-The anode catalyst, usually fine platinum powder, breaks down the fuel into electrons and ions.
-The cathode catalyst, often nickel, converts ions into waste chemicals, with water being the most common type of waste.
-Gas diffusion layers that are designed to resist oxidization.
_
A hydrogen fuel cell is an electrochemical cell that uses a spontaneous redox reaction to produce current that can do work. The net reaction is exothermic.
|
H2 + 1/2 O2 = H2O |
∆Gf = -229 kJ/mol |
|
H2 = 2 H+ + 2 e– |
E0= 0.00 V |
|
O2 + 4 e– + 4 H+ = 2 H2O |
E0= 1.23 V |
The hydrogen flows to a platinum catalyst connected to the anode. Molecular hydrogen is dissociated to atomic hydrogen on the metal surface and the atomic hydrogen is oxidized. The protons travel through electrolyte and the electrons travel through the external circuit. Molecular oxygen is reduced at the cathode and combined with protons to form water.
The theoretical voltage for a hydrogen fuel cell should be 1.23 V, however a typical fuel cell produces a voltage from 0.6 to 0.7 V at full rated load. Voltage decreases as current increases, due to several factors:
-Activation loss
-Ohmic loss (voltage drop due to resistance of the cell components and interconnections)
-Mass transport loss (depletion of reactants at catalyst sites under high loads, causing rapid loss of voltage).
To deliver the desired amount of energy, the fuel cells can be combined in series to yield higher voltage, and in parallel to allow a higher current to be supplied. Such a design is called a fuel cell stack. The cell surface area can also be increased, to allow higher current from each cell.
Electricity generated from other means (nuclear power, hydro power, or solar energy) can produce hydrogen through electrolysis. This is the reverse of the hydrogen fuel cell reaction.
_
Scientists and inventors have designed many different types and sizes of fuel cells in the search for greater efficiency, and the technical details of each kind vary. Many of the choices facing fuel cell developers are constrained by the choice of electrolyte. The design of electrodes, for example, and the materials used to make them depend on the electrolyte. Today, the main electrolyte types are alkali, molten carbonate, phosphoric acid, proton exchange membrane (PEM) and solid oxide. The first three are liquid electrolytes; the last two are solids. Some cells need pure hydrogen, and therefore demand extra equipment such as a “reformer” to purify the fuel. Other cells can tolerate some impurities, but might need higher temperatures to run efficiently. Liquid electrolytes circulate in some cells, which requires pumps. The type of electrolyte also dictates a cell’s operating temperature–”molten” carbonate cells run hot, just as the name implies.
The cleanest fuel type is hydrogen, but there are many fuel cell designs that can utilize other fuels. Common fuels include hydrogen, methanol, ethanol, and ammonia. Each type of fuel cell has advantages and drawbacks compared to the others, and none is yet cheap and efficient enough to widely replace traditional ways of generating power, such coal-fired, hydroelectric, or even nuclear power plants.
_
There are several types of fuel cells with different characteristics and uses. In general, all fuel cells have the same basic configuration — an electrolyte and two electrodes. But there are different types of fuel cells, classified primarily by the kind of electrolyte used. The electrolyte determines the kind of chemical reactions that take place in the fuel cell, the temperature range of operation, and other factors that determine its most suitable applications. Fuel cells are classified in the same way as electrolysers, usually according to the electrolyte that is used as seen in the table below.
|
Fuel Cell Type |
Operating Temperature |
System Output |
Efficiency |
Applications |
|
Alkaline (AFC) |
90–100ºC 194–212ºF |
10kW–100kW |
60–70% electric |
• Military • Space |
|
Phosphoric Acid (PAFC) |
150–200ºC 302–392ºF |
50kW–1MW (250kW module typical) |
80–85% overall with combined heat and power (CHP) (36–42% electric) |
• Distributed generation |
|
Polymer Electrolyte Membrane or Proton Exchange Membrane (PEM) |
50–100ºC 122–212ºF |
<250kW |
50–60% electric |
• Back-up power • Portable power • Small distributed generation • Transportation |
|
Molten Carbonate (MCFC) |
600–700ºC 1112–1292ºF |
<1MW (250kW module typical) |
85% overall with CHP (60% electric) |
• Electric utility • Large distributed generation |
|
Solid Oxide (SOFC) |
650–1000ºC 1202–1832ºF |
5kW–3 MW |
85% overall with CHP (60% electric) |
• Auxiliary power • Electric utility • Large distributed generation |
_____
Regenerative fuel cells:
Simply put, this is a fuel cell which produces electricity and heat, and which can reverse the process. When supplied with electricity, it can be used in electrolysis of water to produce hydrogen and oxygen. In other words the same unit is used for two functions, possibly saving on weight and costs compared to a system with separate fuel cells and electrolyser. The efficiency rating for the one function in a regenerative fuel cell is not necessarily any less than for dedicated fuel cells or electrolysers. But the catalyst in the system cannot be optimised for both. In other words, efficiency is not at its height in both processes. Therefore, a system that will primarily produce hydrogen, for example, should be at peak performance for electrolysis. Regenerative fuel cell systems are most often based on PEM technology.
_____
Role of catalyst:
The two main difficulties preventing us from having hydrogen power everything we have are storage and production. At the moment, hydrogen production is energy-intensive and expensive. Normally, industrial production of hydrogen requires high temperatures, large facilities and an enormous amount of energy. In fact, it usually comes from fossil fuels like natural gas – and therefore isn’t actually a zero-emission fuel source. Making the process cheaper, efficient and sustainable would go a long way toward making hydrogen a more commonly used fuel.
An excellent – and abundant – source of hydrogen is water. But chemically, that requires reversing the reaction in which hydrogen releases energy when combining with other chemicals. That means we have to put energy into a compound, to get the hydrogen out. Maximizing the efficiency of this process would be significant progress toward a clean-energy future. One method involves mixing water with a helpful chemical, a catalyst, to reduce the amount of energy needed to break the connections between hydrogen and oxygen atoms. Chemically speaking, a catalyst lowers the amount of energy needed for two compounds to react. Catalysts are used in reactions that create the hydrogen gas that serves as fuel for the fuel cell. In the most desirable, fossil-fuel independent case, renewable electrical energy can be used to split water molecules (two hydrogen atoms and one oxygen) in the presence of a catalyst. The reaction splits the water into oxygen and hydrogen gases. The more efficient the catalyst, the less energy is needed to split the water.
At the most basic level, fuel cells split hydrogen into its two components, a proton and an electron. This splitting requires a material such as platinum to catalyze the reaction. The negatively charged electrons flow toward a positively charged pole in the fuel cell. This flow of electrons is the current that the fuel cell generates, which can power engines or other electrical devices. Some advanced fuel cells, called regenerative fuel cells, combine both reactions. But most current fuel cells rely on hydrogen created by separate systems and sold as fuel.
Right now, the best catalysts for both reactions (in electrolyser and fuel cell) are platinum group metals. The researchers don’t think that will change because platinum is almost perfect. With platinum group metals, the electrochemical reactions to draw out the hydrogen are quick and efficient, plus the metals can stand up to the harsh acidic conditions currently required for such reactions. The problem, though, is that the platinum is rare and costly. There are several promising catalysts for hydrogen generation, including molybdenum sulfide, graphene and cadmium sulfate.
______
______
Why Fuel Cells?
-1. Fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts.
-2. Hydrogen-powered fuel cells are not only pollution free, but also can have two to three times the efficiency of traditional combustion technologies.
- A conventional combustion-based power plant typically generates electricity at efficiencies of 33 to 35 percent, while fuel cell systems can generate electricity at efficiencies up to 60 percent (and even higher with cogeneration).
- The gasoline engine in a conventional car is less than 20% efficient in converting the chemical energy in gasoline into power that moves the vehicle, under normal driving conditions. Hydrogen fuel cell vehicles, which use electric motors, are much more energy efficient and use 40-60 percent of H2 the fuel’s energy — corresponding to more than a 50% reduction in fuel consumption, compared to a conventional vehicle with a gasoline internal combustion engine.
-3. Fuel cells operate quietly, have fewer moving parts, and are well suited to a variety of applications.
-4. Scalability: The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure at which gases are supplied. A single fuel cell produces approximately less than 1 volt — barely enough electricity for even the smallest applications. To increase the amount of electricity generated, individual fuel cells are combined in series to form a stack. (The term “fuel cell” is often used to refer to the entire stack, as well as to the individual cell.) Depending on the application, a fuel cell stack may contain only a few or as many as hundreds of individual cells layered together. This “scalability” makes fuel cells ideal for a wide variety of applications, from laptop computers (50-100 Watts) to homes (1-5kW), vehicles (50-125 kW), and central power generation (1-200 MW or more).
______
Capabilities of Hydrogen Fuel Cells:
Fuel cells have three main applications: transportation, portable uses, and stationary installations.
-1. Stationary fuel cells can be utilized as a backup source of power, power for remote locations, distributed power generation and co-generation. Stationary fuel cells are the largest, most powerful fuel cells. They are designed to provide a clean, reliable source of on-site power to hospitals, banks, airports, military bases, schools, and homes. Fuel cells are very useful as power sources in remote locations, such as spacecraft, remote weather stations, large parks, communications centers, rural locations including research stations, and in certain military applications. A fuel cell system running on hydrogen can be compact and lightweight, and have no major moving parts. Because fuel cells have no moving parts and do not involve combustion, in ideal conditions they can achieve up to 99.9999% reliability. This equates to less than one minute of downtime in a six-year period. There are many different types of stationary fuel cells so efficiencies vary, but most are between 40% and 60% energy efficient. However, when the fuel cell’s waste heat is used to heat a building in a cogeneration system this efficiency can increase to 85%. This is significantly more efficient than traditional coal power plants, which are only about one third energy efficient.
Cogeneration:
Combined heat and power (CHP) fuel cell systems, including micro combined heat and power (MicroCHP) systems are used to generate both electricity and heat for homes, office building and factories. The system generates constant electric power (selling excess power back to the grid when it is not consumed), and at the same time produces hot air and water from the waste heat. As the result CHP systems have the potential to save primary energy as they can make use of waste heat which is generally rejected by thermal energy conversion systems. A typical capacity range of home fuel cell is 1–3 kW electrical, 4–8 kW thermal. CHP systems linked to absorption chillers use their waste heat for refrigeration. The waste heat from fuel cells can be diverted during the summer directly into the ground providing further cooling while the waste heat during winter can be pumped directly into the building. Co-generation systems can reach 85% efficiency (40–60% electric and the remainder as thermal).
In Hwasung City, South Korea, the Gyeonggi Green Energy fuel cell park is currently the largest of its kind. With a 59 megawatt (MW) capacity, the facility delivers renewable energy to the South Korean power grid and high-quality heat to the district’s heating system. The United States is not far behind, with 56 large-scale fuel cell generating units with capacities greater than one MW. Recognising the vulnerabilities of grid dependency, a number of organisations are looking at fuel cells to help supply a reliable source of backup power. After Hurricane Sandy wreaked havoc across the Caribbean and the US East Coast in 2012, fuel cells provided emergency backup power for telecommunications towers in both the Bahamas and the United States, allowing communication to remain open. Fuel cells can be monitored and controlled remotely, making them an ideal back up source for a range of power applications.
_
-2. Fuel cells have the capacity to power any portable application that uses batteries – from hand-held devices to portable generators. Fuel cells can power almost any portable device or machine that uses batteries. Unlike a typical battery, which eventually goes dead, a fuel cell continues to produce energy as long as fuel and oxidant are supplied. Laptop computers, cellular phones, video recorders, and hearing aids could be powered by portable fuel cells.
Portable power systems:
Portable fuel cell systems are generally classified as weighing under 10 kg and providing power of less than 5 kW. The potential market size for smaller fuel cells is quite large with an up to 40% per annum potential growth rate and a market size of around $10 billion, leading a great deal of research to be devoted to the development of portable power cells. Within this market two groups have been identified. The first is the microfuel cell market, in the 1-50 W range for power smaller electronic devices. The second is the 1-5 kW range of generators for larger scale power generation (e.g., military outposts, remote oil fields).
Microfuel cells are primarily aimed at penetrating the market for phones and laptops. This can be primarily attributed to the advantageous energy density provided by fuel cells over a lithium-ion battery, for the entire system. For a battery, this system includes the charger as well as the battery itself. For the fuel cell this system would include the cell, the necessary fuel and peripheral attachments. Taking the full system into consideration, fuel cells have been shown to provide 530Wh/kg compared to 44 Wh/kg for lithium ion batteries. However, while the weight of fuel cell systems offer a distinct advantage the current costs are not in their favor. while a battery system will generally cost around $1.20 per Wh, fuel cell systems cost around $5 per Wh, putting them at a significant disadvantage.
As power demands for cell phones increase, fuel cells could become much more attractive options for larger power generation. The demand for longer on time on phones and computers is something often demanded by consumers so fuel cells could start to make strides into laptop and cell phone markets. The price will continue to go down as developments in fuel cells continues to accelerate. Current strategies for improving micro fuel cells is through the use of carbon nanotubes. It was shown by Girishkumar et al. that depositing nanotubes on electrode surfaces allows for substantially greater surface area increasing the oxygen reduction rate.
Fuel cells for use in larger scale operations also show much promise. Portable power systems that use fuel cells can be used in the leisure sector (i.e., RVs, cabins, marine), the industrial sector (i.e., power for remote locations including gas/oil well sites, communication towers, security, weather stations), and in the military sector. The key advantage of fuel cells in this market is the great power generation per weight. While fuel cells can be expensive, for remote locations that require dependable energy fuel cells hold great power. For a 72-h excursion the comparison in weight is substantial, with a fuel cell only weighing 15 pounds compared to 29 pounds of batteries needed for the same energy.
_
-3. Fuel cells power transportation such as personal vehicles, trucks, buses and marine vessels; it can also provide auxiliary power to traditional transportation technologies. Hydrogen-powered fuel cells are also far more energy efficient than traditional combustion technologies.
Automobiles:
By year-end 2019, about 18,000 FCEVs had been leased or sold worldwide. Three fuel cell electric vehicles have been introduced for commercial lease and sale: the Honda Clarity, Toyota Mirai and the Hyundai Nexo. Fuel cell electric vehicles feature an average range of 314 miles between refuelings. They can be refueled in less than 5 minutes. The U.S. Department of Energy’s Fuel Cell Technology Program states that, as of 2011, fuel cells achieved 53–59% efficiency at one-quarter power and 42–53% vehicle efficiency at full power, and a durability of over 120,000 km (75,000 mi) with less than 10% degradation. In a 2017 Well-to-Wheels simulation analysis that “did not address the economics and market constraints”, General Motors and its partners estimated that per mile traveled, a fuel cell electric vehicle running on compressed gaseous hydrogen produced from natural gas could use about 40% less energy and emit 45% less greenhouse gasses than an internal combustion vehicle.
Hydrogen fuel cells have found their way into a number of marine applications. Some boats like the Energy Observer even use onboard solar panels and wind turbines to generate their own hydrogen for a fuel cell system. For military stealth submarines like the German Type 212, hydrogen fuel cells offer an alternative to nuclear power with long range, silent cruising, and low exhaust heat.
From package delivery to search and rescue operations, many new applications of UAVs (i.e., drones) are significantly limited by the power and range provided by traditional batteries. Both military and private industry plan to overcome these challenges with hydrogen fuel cells that boast up to three times the range of battery-based systems. Fuel cells also have a higher energy to mass ratio and can be refueled in a few minutes.
Several experimental projects like the Pathfinder and Helios prototypes have explored application of hydrogen fuel cells in aerospace. These long-range unmanned vehicles utilized a hybrid system with hydrogen fuel cells which were replenished by electrical power from solar arrays, allowing for theoretically indefinite day and night continuous flight.
______
______
Advantages of hydrogen fuel cell:
-1. Low emissions and higher efficiency:
Hydrogen fuel cells work by combining hydrogen and oxygen to produce electrical energy. The only emissions that result from this process are water (H20) and hot air – meaning that no harmful gases are released into the atmosphere, in contrast to the 4.6 metric tons of carbon dioxide a typical passenger vehicle emits each year. In addition, hydrogen fuel is more efficient – internal combustion engine (ICE) vehicles convert fuel into kinetic energy at 20 % efficiency and a traditional combustion-based power plant produces electricity at 33-35% efficiency; Hydrogen fuel cells do so at 60% efficiency. Hydrogen fuel cells are a very effective way of energy production compared to the other sources of energy. This fuel efficiency provides a higher production rate per kg of fuel.
-2. Relatively low barrier to entry:
Fuel cell electric vehicles (FCEVs) have low barriers to entry in terms of societal change. They operate and perform very similarly to the conventional ICE vehicles we are used to, allowing you to refuel at a station within minutes, as opposed to having to wait for an electric vehicle (EV) to charge. An electric vehicle typically has a range of around 230 miles, whereas FCEVs can reach 310-370 miles range without having to be refuelled. In addition, an EV can take up to 8 hours to charge from empty to full – it takes roughly 5 minutes to refuel a hydrogen tank.
-3. Effective in heavy-duty applications
While this is a challenge for “light-duty” FCEVs, it is less so for larger, heavy-duty vehicles where the footprint of the gas tank is less of an issue. Bulkier vehicles that need to travel long distances, carry heavy loads and refuel with minimal downtime are good candidates. For that reason, hydrogen fuel has been tested in vehicles such as trucks, boats, trains and planes.
-4. Renewable and Promptly Available:
Hydrogen is the most abundant ingredient in the Universe and, despite the challenges related to its removal from water, is a uniquely renewable and plentiful source of energy, ideal for our future zero-carbon requirements for combined power and heat supplies.
-5. Very small Noise Pollution:
Hydrogen fuel cells do not create noise pollution like other renewable energy sources, such as wind power. This also indicates that, much like electric cars, hydrogen-powered vehicles are much quieter than those that use traditional internal combustion engines.
-6. Perfect for application in Remote Areas
Hydrogen availability through local production and storage is an alternative to diesel-based power and heating in distant areas. This will diminish the necessity to transport fuels and improve the life quality of those living in remote areas by offering non-polluting energy obtained from a readily available natural resource.
-7. The Versatility of Adoption
As the technology progress, hydrogen fuel cells will be able to afford energy for a range of stationery and portable applications.
-8. The democratization of Power Supply
Hydrogen fuel cells can diminish the dependency of a nation on fossil fuels, which will help democratize energy and power supplies worldwide. This increased independence will establish an interest for various countries that are currently reliant on fossil fuel stocks.
______
Disadvantages of hydrogen fuel cell:
-1. Extraction of Hydrogen
Despite being the most plentiful ingredient in the Universe, hydrogen does not exist on its own, so it needs to be extracted from the water via electrolysis or insulated from carbon fossil fuels. Both of these methods need a notable amount of energy to accomplish. This energy can be more than that obtained from the hydrogen itself as well as being costly. Besides, this extraction typically requires the use of fossil fuels, which in the absence of CCS undermines hydrogen’s green credentials.
-2. Considerable Investment for Development
Hydrogen fuel cells require finance to be developed to the point where they become a genuinely viable power source. This will also need the political inclination to spend the money and time on developing to enhance and develop the technology. The global objection for developing sustainable and widespread hydrogen energy is how best to build the supply and demand chain most cost-effectively.
-3. Raw Materials Cost
Precious metals such as iridium and platinum are typically needed as catalysts in fuel cells and some water electrolyzer types, suggesting that the initial cost of fuel cells (and electrolyzers) can be expensive. This high cost has prevented some from investing in hydrogen fuel cell technology. Such costs require to be reduced to make hydrogen fuel cells a viable fuel source for all.
-4. Overall Expense
The price for a unit of power from hydrogen fuel cells is now higher than other energy sources, such as solar panels. This may switch as technology advances, but nowadays, this cost is a barrier to hydrogen’s general use even though it is more effective once created. This expense also affects costs further down the line, such as with the price of hydrogen-operated vehicles, causing widespread adoption questionable at the moment.
-5. Hydrogen Accommodation
Transportation and storage and of hydrogen are more complicated than those required for fossil fuels. This means extra costs to consider for hydrogen fuel cells as an energy source. High-density hydrogen storage is a challenge for both portable and stationary applications. The storage solutions we have available currently typically require the storage of large volumes of hydrogen in gaseous form. To reach the performance and efficiency goals for light-duty FCEVs, large-volume, high-pressure compressed gas tanks would need to be used, which can have a significant footprint.
-6. Infrastructure
Since fossil fuels have been employed for decades, the infrastructure for this power supply previously exists. Gasoline is still being widely used to this day. And as of the moment, there just isn’t any infrastructure that can support hydrogen as fuel. This is why it becomes highly expensive to just think about replacing gasoline. Also, cars need to be refitted in order to accommodate hydrogen as fuel. Large-scale adoption of hydrogen fuel cell technology for automotive applications will need a new refueling foundation to support it. However, for long-range applications such as those for delivery trucks and HGVs, start-to-end refueling will plausibly be utilized.
-7. Less efficient than batteries
When comparing hydrogen fuel cells to other potential alternatives to hydrocarbon power, the picture becomes slightly less positive. The viability of FCEVs is being threatened by the continued development of more cost-effective battery technology and lowering costs of electricity-based transport systems. BEV and hybrid vehicles overall offer better efficiency than FCEVs. Electric batteries lose only 17% of their initial input of energy through inefficiencies when charging and discharging. The cycle used to create electrical energy within a hydrogen fuel cell wastes more than 40% of its energy.
-8. It is highly flammable.
Since it is a very powerful source of fuel, hydrogen can be very flammable. In fact, it is on the news frequently for its many risks. Hydrogen gas burns in air at very wide concentrations – between 4 and 75 percent.
-9. Fossil fuels are often used to produce it.
Although hydrogen energy is renewable and has a minimal environmental impact, other non-renewable sources such as coal, oil and natural gas are often used to separate it from oxygen or carbon. While the point of switching to hydrogen is to get rid of using fossil fuels, fossil fuels are often still used to produce hydrogen fuel. Renewable energy like solar and wind can be used to generate hydrogen energy and is a greener choice.
______
______
Battery, fuel cell and conventional generator:
Many people get confused by the difference between a battery and a fuel cell. Both can be used as sources of power – but in different ways. The biggest difference between the two is that a battery stores energy, while a fuel cell generates energy by converting available fuel. A fuel cell can have a battery as a system component to store the electricity it’s generating. The electrical energy contained within a battery is either from the factory where it was made, or from charging the battery via an outlet. If your battery dies, you are dependent on either being near a source of electricity to re-charge, or near a store to buy a new one.
A fuel cell is different. It takes an energy source, such as hydrogen, propane or natural gas, and converts it into electrical energy. As long as you have access to your energy source, you have access to electricity any time you need it – wherever you may be. Whether you are at sea, out camping, in an emergency situation or when the neighborhood power goes out, you can use a fuel cell to create your own electricity.
Some people have back-up generators for emergency situations. A typical generator that you might buy combusts the fuel source to create electricity. Essentially, there is a small explosion as the fuel is combusted. That explosion moves a piston, converting chemical energy to mechanical, and then through a series of mechanical steps electricity is produced. Combustion engines have changed little since they were invented over a hundred years ago. By combusting the fuel to make electricity, generators create a lot of noise, smoke, exhaust and toxic fumes. They also tend to be inefficient, large, heavy and unwieldy.
Unlike a generator, a fuel cell directly “converts” an energy source into electricity through a chemical reaction – one step rather than multiple steps. This allows a fuel cell to remain efficient, quiet and clean. Since a fuel cell “converts” a fuel’s chemical energy rather than “combusts” the fuel the way a generator does, the result is a fuel cell that can create clean electricity, efficiently and effectively.
When it comes to power, portability is a big factor. A 20 lb. propane tank can be converted into nearly 3400-amp hours of power. In comparison, a standard lead acid battery, weighing about 60 lbs., has only 80-amp hours. A boater would need approximately 80 plus batteries to match the amp hour rates – a very expensive boat anchor at $190 for each battery. The portable fuel cell system weighs less than 30 lbs.
With a portable fuel cell, you can hand-carry the fuel cell with you. From camping, to sailing, to storms, to remote locations and power outages, you can create your own electricity, anywhere, any time.
With a portable fuel cell, creating electricity is easy. You attach a fuel source such as a propane tank or canister, press a switch and start charging your batteries quietly and efficiently. No more dead batteries. Electronic devices and electrical systems are up and running.
______
______
Section-10
Burning hydrogen:
Hydrogen can be burned in the normal way in air (oxygen), and the heat resulting from the combustion can be used for either heating, cooking, turbines, boilers or in combustion engines. Because of hydrogen’s high burning temperature, large amounts of NOx will be released under standard combustion methods. It is therefore better to turn to other processes which have lower NOx emissions. Catalytic burners use a catalyst to reduce the burning temperature, thereby reducing the creation of NOx. There are several burners which use diffusion (i.e., primus stove principle) for low NOx burning of hydrogen. Up to 15% H2 may be added to regular natural gas, without any need to adjust conventional burner.
_
Hydrogen internal combustion engine (HICE):
Hydrogen internal combustion engine cars are different from hydrogen fuel cell cars. The hydrogen internal combustion car is a slightly modified version of the traditional gasoline internal combustion engine car. These hydrogen engines burn fuel in the same manner that gasoline engines do; the main difference is the exhaust product. Gasoline combustion results in emissions of mostly carbon dioxide and water, plus trace amounts of carbon monoxide, NO x , particulates and unburned hydrocarbons, while the main exhaust product of hydrogen combustion is water vapor plus NOx.
In 1807 Francois Isaac de Rivaz designed the first hydrogen-fueled internal combustion engine. In 1965, Roger Billings, then a high school student, converted a Model A to run on hydrogen. In 1970 Paul Dieges patented a modification to internal combustion engines which allowed a gasoline-powered engine to run on hydrogen. Mazda has developed Wankel engines burning hydrogen, which are used in the Mazda RX-8 Hydrogen RE. The advantage of using an internal combustion engine, like Wankel and piston engines, is the lower cost of retooling for production. HICE forklift trucks have been demonstrated based on converted diesel internal combustion engines with direct injection.
_
For a long time, hydrogen combustion engines were disregarded, as the very high costs of hydrogen made the powertrain uneconomical. Today, however, some automotive OEMs, component suppliers, and start-ups are reconsidering hydrogen combustion as an additional component of their future powertrain portfolios, alongside batteries and fuel cells. Despite impressive developments, batteries and fuel-cell technology are not yet ready to meet the very high-power requirements needed for the harsh conditions to which many heavy-duty vehicles (especially in the off-highway segment) are exposed. Mining trucks, for instance, require several megawatts worth of power, run around the clock, and are exposed to extreme vibrations and heat development, as well as dirt in the air. Internal combustion engines have met these requirements for decades, and a switch from diesel to hydrogen could be a straightforward way to decarbonize these engines, with a relatively minor requirement for further technical innovation.
_
Even where batteries and fuel cells are technically feasible, hydrogen combustion could carve out niches. Low capex requirements for combustion engines, decreasing hydrogen prices, and the relatively high efficiencies achieved by HICEs at high loads create conditions in which hydrogen combustion can be a TCO-competitive solution. Moreover, since bi-fuel combustion engines can run on hydrogen, liquefied natural gas (LNG), or diesel (or hydrogen–gas blends), depending on availability, they can help decarbonize vehicle segments where hydrogen supply and infrastructure have not yet achieved full coverage.
Beyond these considerations, HICEs offer other advantages for automotive OEMs and component suppliers: they make use of current engineering know-how and jobs, draw on existing supply chains and production capacities in the automotive industry, and do not create sustainability and integrity concerns around the supply and recycling of precious metals or rare earths.
_
Hydrogen combustion and hydrogen fuel cells are complementary, as they thrive in the same ecosystem
One concern about HICEs is their perceived competition with hydrogen fuel cells. However, while there are some applications in which the two technologies could compete, it is more likely that both can help grow hydrogen’s share in the future powertrain mix and propel each other’s success.
For both powertrains, the availability of hydrogen refueling stations and the cost of hydrogen at the pump are the key factors that will determine success and are of greatest concern today. However, both powertrains require (largely) the same infrastructure; thus, each HICE vehicle will help to bring down the costs for hydrogen fuel cells, and vice versa. Similarly, both powertrains share the same hydrogen-tank technology—a significant share of overall powertrain costs. Allowing OEMs and tank suppliers to amortize R&D and capex over a larger number of vehicles will help bring down the cost curve for all hydrogen vehicles and support the competitiveness of both solutions. Finally, hybrid solutions with hydrogen combustion engines, fuel cells, and batteries are being actively developed by some players to maximize efficiencies for variable load profiles.
_
Achieving zero emissions across transportation segments globally is a tremendous challenge; nevertheless, HICEs can play their part with multiple applications to provide complementary solutions to FCEVs and BEVs.
HICE’s advantages include lower payload penalties and space requirements, faster refueling times compared to BEV trucks, lower costs, and higher tolerances for heat and vibrations. Various vehicle segments could benefit from these advantages, including these:
-light-duty vehicles, such as tow trucks
-medium-duty vehicles, such as medium-haul and fire trucks
-heavy-duty vehicles, such as concrete trucks
-mining and construction vehicles, such as crawler dozers, excavators, and dump trucks
-agricultural vehicles, such as harvesting machinery and tractors
_
The impact of using hydrogen as a supplementary fuel for spark ignition (SI) and compression ignition (CI) engines on engine performance and gas emissions were investigated in various studies. By adding hydrogen as a fuel in internal combustion engines, the torque, power, and brake thermal efficiency of the engines decrease, while their brake-specific fuel consumption increase. Hydrogen reduces the emissions of CO, UHC, CO2, and soot; however, NOx emission is expected to increase.
_
Hydrogen is environmentally friendly when it is produced from renewable sources in a sustainable and efficient process. In comparison to hydrocarbon fuels such as natural gas, gasoline, and diesel, utilizing hydrogen as a fuel in internal combustion engines can improve thermal efficiency while decreasing carbon emissions. A key benefit of using hydrogen for transportation is that it increases our reliance on renewable sources while reducing our usage of non-renewable fossil fuels. Hydrogen has a heating value 4, 2.8, and 2.4 times higher than those of coal, gasoline, and methane, respectively. When compared to fossil fuels, hydrogen, the most abundant element, has the highest specific energy content.
Compression ignition (CI) diesel engines are widely used in heavy transport, power generation, and agricultural applications. Some of their benefits include increased torque, increased power, increased thermal efficiency, and lower fuel consumption. Unfortunately, diesel engines create more nitrogen oxides (NOx), unburned hydrocarbons (UHC), carbon monoxide (CO), and soot. Despite the fact that heavy-duty engines emit pollutants into the environment, they are unlikely to be phased out anytime soon, as alternatives such as large-scale fuel cell manufacturing remain difficult and expensive. Hydrogen fuel in CI engines can minimize CO2 emissions while preserving or even enhancing engine performance.
Because of its unique properties, hydrogen is more suited for use in spark ignition (SI) engines than in compression combustion (CI) engines. The adiabatic flame rate of hydrogen, for example, is much higher than that of gasoline, which enhances the combustion stability. Furthermore, hydrogen has a significantly greater diffusion coefficient than gasoline, resulting in a more homogeneous combination of air and fuel. When added, hydrogen’s broad flammability makes it easier to run engines in lean circumstances. In addition, because hydrogen has a higher combustion temperature (about 858 K), it is better suited to SI engines than to compression combustion (CI) engines.
_
Adaptation of existing engines:
The differences between a hydrogen ICE and a traditional gasoline engine include hardened valves and valve seats, stronger connecting rods, non-platinum tipped spark plugs, a higher voltage ignition coil, fuel injectors designed for a gas instead of a liquid, larger crankshaft damper, stronger head gasket material, modified (for supercharger) intake manifold, positive pressure supercharger, and high temperature engine oil. All modifications would amount to about one point five times (1.5) the current cost of a gasoline engine. These hydrogen engines burn fuel in the same manner that gasoline engines do.
The theoretical maximum power output from a hydrogen engine depends on the air/fuel ratio and fuel injection method used. The stoichiometric air/fuel ratio for hydrogen is 34:1. At this air/fuel ratio, hydrogen will displace 29% of the combustion chamber leaving only 71% for the air. As a result, the energy content of this mixture will be less than it would be if the fuel were gasoline. Since both the carbureted and port injection methods mix the fuel and air prior to it entering the combustion chamber, these systems limit the maximum theoretical power obtainable to approximately 85% of that of gasoline engines. For direct injection systems, which mix the fuel with the air after the intake valve has closed (and thus the combustion chamber has 100% air), the maximum output of the engine can be approximately 15% higher than that for gasoline engines. Therefore, depending on how the fuel is metered, the maximum output for a hydrogen engine can be either 15% higher or 15% less than that of gasoline if a stoichiometric air/fuel ratio is used. However, at a stoichiometric air/fuel ratio, the combustion temperature is very high and as a result it will form a large amount of nitrogen oxides (NOx), which is a criteria pollutant. Since one of the reasons for using hydrogen is low exhaust emissions, hydrogen engines are not normally designed to run at a stoichiometric air/fuel ratio.
Typically hydrogen engines are designed to use about twice as much air as theoretically required for complete combustion. At this air/fuel ratio, the formation of NO x is reduced to near zero. Unfortunately, this also reduces the power output to about half that of a similarly sized gasoline engine. To make up for the power loss, hydrogen engines are usually larger than gasoline engines, and/or are equipped with turbochargers or superchargers. A small amount of hydrogen can be burned outside the combustion chamber and reach into the air/fuel mixture in the chamber to ignite the main combustion.
_
Pitfalls of HICE:
They create nitrogen oxide, which isn’t good for people or the environment. Even though carbon isn’t part of the hydrogen combustion process, NOx isn’t a compromise as automakers look to zero-emission vehicles.
Second, hydrogen-combustion engines aren’t as efficient as a hydrogen fuel cell in numerous ways. By the time hydrogen makes its way to the engine, through a transmission, and into a differential to power a car’s wheels, only 25 percent of the hydrogen’s potential energy is transferred. In a hydrogen fuel cell, the hydrogen works its way to the fuel cell where electrons are sent to a converter, then to a power control unit, and to an electric motor. The motor then powers a gear reduction to power the car’s wheels. Despite numerous transfers, the hydrogen’s fuel energy is more effectively transmitted to the wheels, up to 50 percent. Since hydrogen internal combustion engines are heat engines, their maximum efficiency is limited by the Carnot efficiency. In comparison, the efficiency of a fuel cell is limited by the Gibbs free energy, which is typically higher than that of Carnot.
In a basic sense, fuel cell-powered cars are electric cars powered with hydrogen. The efficiencies have a cascading effect, too. Since hydrogen takes up a lot of space when in storage, hydrogen fuel cell-powered cars can have smaller fuel tanks compared to hydrogen-combustion cars. And since hydrogen isn’t exactly cheap, the fuel cell is far more efficient to operate and uses 25 percent less energy to do the same work as a hydrogen-combustion engine. The fuel cell solves dual purpose as it can run the vehicle and can generate standby power in case of emergencies as well.
_____
Ford Motor Company recently introduced the P2000, a new car with a hydrogen internal combustion engine (ICE) that “could help bridge the gap between gasoline vehicles and the fuel cell vehicles of the future.” The engine is not much different from an ordinary gasoline engine. The use of hydrogen greatly reduces emissions although nitrous oxides are still a problem. Engine efficiency about equals a diesel, about 35%. The hydrogen is stored in a tank that is rated at 240 atmospheres (240 bars). The range is only 62 miles. Ford does not give the price of the P2000, but it should be inexpensive given that all of the components are rather ordinary.
______
______
Hydrogen gas power plant:
The hydrogen power plant includes an H2-fired gas turbine (e.g., SGT5-9000HL, SGT-800, or SGT-400), electrolyzers with H2 compression, storage and management system to integrate all components including renewable energy sources feeding electricity into the electrolyzer.
_
Converting a gas turbine to a hydrogen turbine:
The use of hydrogen as a gas turbine fuel has been demonstrated commercially, but there are differences between natural gas and hydrogen that must be taken into account to properly and safely use hydrogen in a gas turbine. The usability of hydrogen in turbines has been verified by several turbine manufacturers, notably GE. Currently cheaper than fuel cells, turbines may be considered a transitional technology. Gas turbines can run on varying levels of hydrogen. In addition to differences in the combustion properties of hydrogen and natural gas, it’s also important to consider the impact to all gas turbine systems, as well as the overall balance of plant. In a power plant with one or more hydrogen-fueled turbines, changes may be needed to the fuel accessories, bottoming cycle components, and plant safety systems. As gas turbines are inherently fuel-flexible, they can be configured to operate on green hydrogen or similar fuels as a new unit, or be upgraded even after extended service on traditional fuels, i.e., natural gas. The scope of the required modifications to configure a gas turbine to operate on hydrogen depends on the initial configuration of the gas turbine and the overall balance of plant, as well as the desired hydrogen concentration in the fuel. GE has more experience running gas turbines on hydrogen than any other OEM. In total, GE has 100+ gas turbines supporting power generation with hydrogen and associated fuels around the world. GE has combustion technologies that are capable of operating on a wide range of hydrogen concentrations up to ~100% (by volume).
A fuel mix of 20% hydrogen can be used without any technological improvements, and if you use a gas turbine with an output capacity of 500MW, and a turbine efficiency rating of 60%, it requires 1.4 tons of hydrogen per hour. This equals the volume of hydrogen used by around 100,000 to 130,000 fuel-cell vehicles. If you are going to proceed in earnest with hydrogen use, it’s imperative that you quickly move to upgrade the hydrogen infrastructure, through measures such as proactively increasing the number of turbines using hydrogen.
_
In 2018, Mitsubishi Power, a member of Mitsubishi Heavy Industries Group, with the support of the Japan’s New Energy and Industrial Technology Development Organization (NEDO), developed a gas turbine that runs on 30% hydrogen and 70% natural gas — a major step towards a carbon-free society. Their mixed-fuel turbine produces around 10% less CO2 than those powered by natural gas alone. Hydrogen–gas turbines have many environmental and economic benefits, and Mitsubishi Power is committed to facilitating the transition. Their turbines can be fitted into existing power plants and can run on less pure forms of hydrogen, which can be carried in any form, from liquid hydrogen to ammonia. They can also operate in combined-cycle power plants, which are more efficient because they use surplus heat to generate steam that powers a second turbine. They have already achieved 64% power-generation efficiency in their natural-gas, combined-cycle plant, and they can theoretically improve on this by raising the combustion temperature of the gases. Fully hydrogen powered turbines are being developed at Mitsubishi Power, helping Japan’s push for a carbon-free economy.
_
Hybrids:
By integrating solid oxide fuel cell technology with turbines, the electrical efficiency of a gas power plant can reach up to 80% under optimum conditions. Fuel cells alone have the potential to utilise 60% of the energy in the fuel. The rest is lost in the form of low quality heat, but also because the fuel cells are not capable of utilising all the fuel. The excess fuel in the exhaust gas can be used however with the help of gas turbines. Such a plant would still produce NOx unless pure O2 is used in the afterburner, but to a lesser degree than in a conventional power plant. Siemens Westinghouse has started a 220 kW SOFC micro turbine “hybrid” system at the University of California in Irvine. This is the first of its kind and the efficiency is 52-53%. A 550 kW system is under development.
_
A consortium of European companies, research institutes, and universities have launched the world’s first demonstration of a fully integrated power-to-hydrogen-to-power project, at industrial scale and in a real-world power plant application. The four-year project to demonstrate HYFLEXPOWER, which has achieved a technology readiness level of 7, will convert a 12-MWe combined heat and power (CHP) plant at Engie Solutions’ Smurfit Kappa pulp-and-paper industrial site in Saillat-sur-Vienne, France, to demonstrate the entire power-to-hydrogen-to-power cycle.
The 2007-installed CHP facility currently uses natural gas from the French grid via a Siemens Gas and Power SGT-400 gas turbine and a recovery boiler to produce power, which is sold to French utility EDF under a regulated feed-in-tariff. It also produces steam, which is used by the Smurfit Kappa papermill to dry pulp from recycling of waste paper to make new paper for cardboard.
Siemens Gas and Power, which will play the crucial role of project coordinator for the HYFLEXPOWER pilot, will provide an electrolyzer system to produce hydrogen from surplus renewable power in the region. While part of that hydrogen may be used for storage, Siemens will also upgrade the existing SGT-400 industrial gas turbine at the CHP plant to burn a variety of natural gas and hydrogen mixes for power generation, working to gradually step up hydrogen’s volume of the fuel to at least 80%, and eventually, to 100%. Engie, meanwhile, will build the hydrogen production and storage facility, including the natural gas and hydrogen mixing station. HYFLEXPOWER seeks to prove that hydrogen can be produced and stored from renewable electricity and then added with up to 100% to the natural gas currently used with combined heat and power plants.
A Leap for Power-to-X-to-Power:
If successful, the demonstration’s biggest takeaway may be that it will show that hydrogen has a role in long-term energy storage on a renewables-heavy grid. Chemical storage appears to be the most promising long-term energy storage technology. Among chemical storage technologies, hydrogen is expected to dominate as it can be produced by electrolysis of water using excess energy from [renewable energy sources], easily compressed and stored, and finally re-electrified using gas turbines.
Power-to-gas (P2G) describes the process of converting renewable energy to gaseous energy carriers such as hydrogen or methane via water electrolysis—mainly alkaline electrolysis, proton exchange membrane (PEM) electrolysis, and solid oxide electrolysis cells. The produced green hydrogen can be used in a range of pathways that promise to decouple renewable generation from electricity demand—helping to avoid surplus curtailment and potentially providing the sector with an assortment of new revenue streams.
Over the past two decades, more than 200 projects have started operation to convert electricity and water into hydrogen via electrolysis to reduce emissions—from transport, natural gas use, and industrial sectors—or to support the integration of renewables into the energy system, but most have been pilots or demonstration projects under 1 MW. More recently, owing to increased discussions about P2G’s place in future energy systems and technology advancements, several much larger and more ambitious projects are in planning.
______
______
Section-11
Energy efficiency vis-à-vis hydrogen:
_
Hydrogen fuel cell energy efficiency:
The energy efficiency of a system or device that converts energy is measured by the ratio of the amount of useful energy put out by the system (“output energy”) to the total amount of energy that is put in (“input energy”) or by useful output energy as a percentage of the total input energy. In the case of fuel cells, useful output energy is measured in electrical energy produced by the system. Input energy is the energy stored in the fuel.
According to the U.S. Department of Energy, fuel cells are generally between 40 and 60% energy efficient. This is higher than some other systems for energy generation. For example, the typical internal combustion engine of a car is about 25% energy efficient. In combined heat and power (CHP) systems, the heat produced by the fuel cell is captured and put to use, increasing the efficiency of the system to up to 85–90%.
In a fuel cell vehicle the tank-to-wheel efficiency is greater than 45% at low loads and shows average values of about 36% when a driving cycle like the NEDC (New European Driving Cycle) is used as test procedure. The comparable NEDC value for a Diesel vehicle is 22%. In 2008 Honda released a demonstration fuel cell electric vehicle (the Honda FCX Clarity) with fuel stack claiming a 60% tank-to-wheel efficiency.
_
It is also important to take losses due to fuel production, transportation, and storage into account. Fuel cell vehicles running on compressed hydrogen may have a power-plant-to-wheel efficiency of 22% if the hydrogen is stored as high-pressure gas, and 17% if it is stored as liquid hydrogen. Fuel cells cannot store energy like a battery, except as hydrogen, but in some applications, such as stand-alone power plants based on discontinuous sources such as solar or wind power, they are combined with electrolyzers and storage systems to form an energy storage system. As of 2019, 90% of hydrogen was used for oil refining, chemicals and fertilizer production, and 98% of hydrogen is produced by steam methane reforming, which emits carbon dioxide.
The overall efficiency (electricity to hydrogen and back to electricity) of such plants (known as round-trip efficiency), using pure hydrogen and pure oxygen can be “from 35 up to 50 percent”, depending on gas density and other conditions. The electrolyzer/fuel cell system can store indefinite quantities of hydrogen, and is therefore suited for long-term storage.
_
Solid-oxide fuel cells produce heat from the recombination of the oxygen and hydrogen. The ceramic can run as hot as 800 degrees Celsius. This heat can be captured and used to heat water in a micro combined heat and power (m-CHP) application. When the heat is captured, total efficiency can reach 80–90% at the unit, but does not consider production and distribution losses. CHP units are being developed today for the European home market.
_
Hydrogen fuel cells are more energy-efficient than internal combustion engines. However, lithium-ion batteries are still the most energy efficient and the highest performing energy source for vehicles. Coulombic efficiency (CE), also called faradaic efficiency or current efficiency, describes the charge efficiency by which electrons are transferred in batteries. CE is the ratio of the total charge extracted from the battery to the total charge put into the battery over a full cycle. Li-ion has one of the highest CE ratings in rechargeable batteries. It offers an efficiency that exceeds 99 percent. This, however, is only possible when charged at a moderate current and at cool temperatures. While the coulombic efficiency of lithium-ion is normally better than 99 percent, the energy efficiency of the same battery has a lower number and relates to the charge and discharge C-rate. In the real world, the Tesla Roadster is said to have an energy efficiency of 86 percent. At its highest, fuel cell energy efficiency is around 60%. Typical internal combustion engines have 20-30% efficiency. This comparison is in terms of the “tank-to-wheels” efficiency; however when you factor in the losses from production to utilization, hydrogen fuel cells have significantly higher energy losses than batteries. Hydrogen can be stored as either gas in high-pressure tanks or as a liquid in cryogenic temperatures, but it must be in the gas form to be used for lift truck fuel cells. Both methods of hydrogen storage involve an inherent loss of energy. Compressing the hydrogen requires about 13% of the total energy content of the hydrogen itself, and if it is liquefied it loses about 40%.
_______
_______
Comparison of hydrogen fuel cell and battery electric vehicle efficiency:
Gravimetric energy density of compressed hydrogen is 40,000 Wh/Kg while Lithium-ion batteries are able of achieving of 260 Wh/Kg. Because of its energy density and its lightweight, hydrogen is being able to provide extended range without adding significant weight, which is a significant barrier of incorporating into aviation industry. Each kilogram of battery weight to increase range requires extra structural weight, higher torque motor, heavier brakes, and in turn more batteries to carry the extra mass. The weight compounding limits the vehicle range until a new improvement in the battery development improves the energy density per Kg. For hydrogen fuel cell vehicles, the weight compounding in not an issue. In addition, refuelling of the vehicle takes much less time with hydrogen, compared with recharging.
_
Fuel Cell Vehicle (FCV) Efficiency:
Hydrogen requires more energy to produce and it usually found in water, hydrocarbons (such as methane) and other organic material. The biggest challenge which prevents from being used as an energy storage mechanism comes from being able to efficiently extracted from the previous mentioned compounds. One process to extract the hydrogen comes from a method called “steam methane reforming reaction”. Despite it is the most common method for industrial production of hydrogen, it requires a lot of energy for heat, which results to high inefficiency.
Another method to produce hydrogen is “electrolysis”. While the energy that requires for that process can be generated by renewables sources, it requires more energy input than steam reforming and it ends up of losing 30% of energy from the original energy input from the renewables (Arnold, 2017). A slightly more efficient method of producing hydrogen is “Proton Exchange Membrane” (PEM) electrolysis with a loss of only 18%.
Additionally, there is more energy loss from the transport and storage of the produced hydrogen. Hydrogen has low density in gas and liquid format, so to achieve sufficient energy density we have to increase its actual density. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen itself. Alternatively, the hydrogen can be liquefied cryogenically but with an efficient loss of 40%.
After the production and storage of hydrogen, a viable hydrogen infrastructure requires that hydrogen can be delivered from the origin of production to the end point of use. The production site of the hydrogen can have a significant impact on the cost and the delivery. A centrally located facility, capable of producing large amounts of hydrogen, can produce in lower prices but it cost more to deliver the hydrogen because the end point of use is far away. A distributed production facility can produce hydrogen on the place of demand with low delivery cost. However, the cost to produce is higher because the production volume is less. Due to the current and tested infrastructure of delivering energy through pipes, we have to assume that the hydrogen would be transferred by truck and pipelines where the energy losses can range from 10% to 40% (Interstate Natural Gas Association of America, 2010).
Another reason why efficiency is reduced by using hydrogen is the tank-to-wheel conversation efficiency. For hydrogen fuel vehicles, the hydrogen in the tank must be reconverted into electric power, which is done through fuel cell. According to the U.S. Department of Energy, the fuel cell technology has the potential of achieving 60% of efficiency, with most of the rest of the energy lost as heat (U.S. Department of Energy, 2011). Another study found 47% efficiency of the PEM fuel cell (Pellow, Emmott, Barnhart, & Benson, 2015).
_
Battery Efficiency:
Lithium Ion batteries have seen extensive development for the last 20 years in response for the increase in electric vehicle sales. The energy density of Lithium Ion batteries has nearly doubled between the periods of the mid-1990s to the mid-2000s. In the case that the energy used to recharge batteries comes from renewable sources, we have to consider the transmission losses to the grid. Using the EU for transmission and distribution losses, the average value is 6%. In addition, the charging infrastructure has an efficiency loss of only 1%. Like hydrogen fuel cell, batteries have inefficiencies and losses. The grid provides AC power while the batteries store the power in DC. For the conversion, there is a need of a charger with a peak efficiency of 95%. In addition, due to the fact that most of the electric vehicles are using AC motors, an inverter is needed. The peak efficiency of a high quality inverter can be close to 95%. Finally, Lithium Ion batteries can lose energy due to leakages. An estimate for the charging efficiency can be close to 90%.
_
Comparison of the efficiency of two types of power-trains is depicted in the figure below:
The “power-plant-to-wheel” efficiency of a fuel cell vehicle operated on compressed gaseous hydrogen is about 23 to 30 % while the same for BEV is 70 to 80% according to various studies. In other words, the hydrogen fuel cell requires double the amount of energy. To quote BMW: “The overall efficiency in the power-to-vehicle-drive energy chain is only half the level of an electric vehicle.”
_
According to the best-case scenario of having high efficiency rates across the whole procedure, battery electric vehicles provide the most efficient solution to power a vehicle. So, despite the fact that the fuel cell vehicle might be able to go further with full tank of hydrogen compared to a battery powered, the cost that is needed to fully charge the tank is higher due to energy losses and the inefficiencies. The cost per kilometre is a little more than 3 times greater for hydrogen.
|
|
Tesla Model 3 (75kWh) |
Toyota Mirai |
|
Price to fully charge or fill |
15 € |
47.5 € |
|
Range (km) |
499 |
502 |
|
Price/km |
0.030 € |
0.095 € |
Note: At Average Electricity Price of 0.20 € per kWh
Additional costs will affect further the price per kilometre like the cost of the construction of the facility and the profit of the hydrogen station. At the moment, the above mentioned energy losses and inefficiencies are driving the market where the majority of investment and research is forward to battery electric vehicles.
Passenger cars driven by electric motors (EVs) have higher well-to-wheel efficiency than cars with an internal combustion engine (ICE) powered by fossil fuels. Concerns about battery capacity and range provide a technological incentive to further increase energy efficiency. The same is true for electric buses and trucks. However, EV motor efficiency depends on the load profile and system boundaries, which complicates how this is evaluated.
There is a potential for hydrogen fuel cells to successfully implemented to long-haul lorries, train, and ambulances that would benefit from longer driving ranges and the development of infrastructure could be easily deployed in order to fuel up to their bases. Nevertheless, based on the current methods of producing, storing and converting hydrogen to electricity, the inefficiencies would limit the increase of share to the vehicle market.
The electric vehicle sales growth will continue with a greater pace as long as the battery cost is declining and the energy density is being improving. Significant innovations in battery chemistry will be required to maintain the growth and supply challenges with cobalt must be solved.
______
______
Energy inefficiency of using hydrogen for heating buildings:
Heat pumps require some electricity to run, but it’s a relatively small amount. Modern heat pump systems can transfer three or four times more thermal energy in the form of heat than they consume in electrical energy to do this work – and that the homeowner pays for. A report from the International Council on Clean Transportation found that in homes, heat pumps are up to six times more efficient than hydrogen boilers as they transfer rather than generate heat, and because green hydrogen is very energy intensive to produce.
Graphic below shows why electrification is more efficient than hydrogen in heating.
Using electricity to run a heat pump is a far more efficient way of heating a home than using green hydrogen. Contrary to industry marketing, it makes no sense to burn hydrogen in our homes. The gas distribution system can’t deliver significant volumes of hydrogen to homes and businesses without creating safety hazards in pipelines and household appliances. Instead, the best way forward for homes is electrification.
______
______
Economics and security may outdo efficiency:
All energy carriers, including fossil fuels, encounter efficiency losses each time they are produced, converted or used. In the case of hydrogen, these losses can accumulate across different steps in the value chain. After converting electricity to hydrogen, shipping it and storing it, then converting it back to electricity in a fuel cell, the delivered energy can be below 30% of what was in the initial electricity input.
This makes hydrogen more ‘expensive’ than electricity or the natural gas used to produce it. It also makes a case for minimising the number of conversions between energy carriers in any value chain. That said, in the absence of constraints to energy supply, and as long as CO2 emissions are valued, efficiency can be largely a matter of economics, to be considered at the level of the whole value chain.
Indeed, conventional energy systems based on fossil fuels are already highly inefficient, with combustion engine cars returning as little as 20% of the energy in petrol as useful forward motion. Similarly, the average efficiency of coal-fired power plants is just 33%.
This suggests low efficiency is not a fundamental barrier to the use of hydrogen. Instead, low efficiency may hold back hydrogen via higher costs and the need for a larger energy supply.
Although cost, efficiency and technical performance are all important factors for hydrogen to address, there are some really critical drivers beyond techno-economics. Ultimately for the EU, it’s not necessarily driven by cost, it’s driven by security of energy supply, reduction of Russian oil and gas imports, and job creation. Those aspects of the hydrogen opportunity are underplayed at the moment and they are what is going to drive things forward.
For some such as Samuele Furfari, professor in energy geopolitics at the Université Libre de Bruxelles in Belgium, hydrogen of any colour makes little sense. It makes much more sense to use fossil fuels or electricity directly. “Each [conversion] step is a waste of energy,” he says. “The processes are technically feasible but they are nonsense from an energy and economic point of view. Hydrogen has re-emerged because we need a solution to the intermittency of renewables.”
Ad van Wijk, professor for future energy systems at Delft University of Technology in the Netherlands and a founding father of the hydrogen economy concept, counters that efficiency is no longer the benchmark: “a solar panel in the Sahara generates 2–3 times as much power as one in the Netherlands. If you convert that power to hydrogen, transport it here and turn it back into power via a fuel cell, you are left with more energy than if you install that solar panel on a Dutch roof. In a sustainable energy system, you calculate in terms of system costs, not efficiency.”
van Wijk sums up: “even if all production and consumption was electric, more than half of that power would have to be converted to hydrogen for [cost-effective] transport and storage.” Electricity cables can transport up to 1–2 GW, but the average gas pipeline can carry 20 GW (and is 10–20 times cheaper to build). The challenge is converting existing gas pipelines from natural gas to hydrogen, says van Wijk.
_____
_____
Section-12
Cost of hydrogen:
_
Cost to consumers:
Hydrogen cars are expensive to buy and there are only two currently on sale. The Hyundai Nexo is around £70,000 and the Toyota Mirai costs £50,000. That, however, is £15,000 cheaper than the first generation Mirai and hints that costs could come down. It’s also comparable to EVs from brands such as Audi, Tesla and BMW. Once you’ve bought the car, the running costs are currently more than for a conventional or BEV car.
In the UK, hydrogen fuel costs between £10 and £15 per kg (it’s measured in kilogrammes rather than litres). That means filling a Hyundai Nexo’s 6.33kg tank, which offers a claimed 414 miles of range will cost anywhere between £63 and £95 pounds. With consumption of 0.95kg per 100km (62 miles) that means it will cost around £11.40 to cover 100km (at a cost of £12 per kg). An equivalent diesel with economy of 55mpg (5.1l/100km) will cost around £6.76 to cover the same distance. A petrol with economy of 49mpg (the UK new car average in 2019) will cost £7.48. To charge a BEV such as the Hyundai Kona, which requires 19.4kWh per 100km, will cost around £2.79 per 100km.
In other words, fuel cost of running BEV is cheapest and fuel cost of running FCEV is most expensive while petrol/diesel would come in between.
_
The average price of hydrogen for a light-duty fuel cell electric vehicle (passenger car) in California is $16.51 per kilogram, according to the 2019 Joint Agency Report. As more retail stations open and have higher utilization, the price per kilogram of hydrogen is projected to drop to ranges more competitive with the prices of gasoline. For example, in late 2019, the True Zero Oakland hydrogen station opened with three times the capacity of previous stations. It offers hydrogen at $13.11 per kilogram (tax included) due, in part, to the larger volume and other factors. In addition, drivers of fuel cell electric cars are offered free fuel by automakers for three years, to bridge the time it takes the market to become more competitive with other fuel options. Reports, studies, and white papers from the Hydrogen Council, NREL and Shell, among others, all point to reductions in the price of fuel and fueling infrastructure for various reasons (scaling up, standardization, etc.).
_____
Capital cost:
Capital cost is about capital investments at the beginning of the system installation. Current capital costs of electrolysers, compressed tanks and fuel cells have been summarised in and several cost effectiveness analysis has been conducted in recent projects and researches.
The capital cost of the current hydrogen production system using alkaline electrolyzers (AELs) ranges from 1000 to 1500 EUR/kW including installation and that of PEMELs is twice these numbers, i.e., 2000 to 3000 EUR/kW. Although alkaline water electrolysis has been well developed, the production volume is rather low. This is because the electrolyser providers tend to fabricate small-volume electrolysers for niche market, which increases at the same time the cost of BoPs. Therefore, potential cost reduction for AELs depends on more cost-efficient production, while for further reduction in the cost of PEMEL, breakthroughs in technological developments are required.
Different dimensions of stationary fuel cell systems are deployed to meet various demands from serving residential buildings to industrial applications. Fuel cell micro-CHPs for family homes and small buildings with an installed capacity of 0.3–5 kW now cost around 10,000 EUR/kW. Some mid-sized installation for larger buildings of 5–400 kW now cost 4500 EUR/kW to 7500 EUR/kW and large scale installations of 0.4–30 MW costs 2000 to 3000 EUR/kW for specific industrial applications. The capital costs have the potential to be largely reduced in the coming future owing to more mature installation technologies and economies of scale. Target capital expense (CAPEX) values for 2030 are expected to reach 3500 EUR/kW, 1500–4000 EUR/kW and 1200–1750 EUR/kW for micro-CHP, mid-size and large scale applications, respectively.
With the gradual maturity of technologies, the capital costs of both electrolyser systems and fuel cell systems are expected to decrease significantly by 2030, especially the stack cost. Technological advances in increasing the active area of the stack are required, which can reduce the number of cells for producing a certain amount of hydrogen, and therefore, decrease the cost.
______
Hydrogen production cost:
The cost of hydrogen production is an important issue. Hydrogen produced by steam reformation costs approximately three times the cost of natural gas per unit of energy produced. This means that if natural gas costs $6/million BTU, then hydrogen will be $18/million BTU. Also, producing hydrogen from electrolysis with electricity at 5 cents/kWh will cost $28/million BTU — slightly less than two times the cost of hydrogen from natural gas. Note that the cost of hydrogen production from electricity is a linear function of electricity costs, so electricity at 10 cents/kWh means that hydrogen will cost $56/million BTU.
_
The cost of steam reforming hydrogen is mainly shaped by the gas prices, which currently ranges from 1.4 to 1.8 EUR/kg with CO2 capture. However, driven by the exhaustion of fossil fuels and the decreasing cost of renewable electricity, electrolytic hydrogen becomes competitive and is about to see continuous increasing deployments shortly. The current hydrogen production cost of AEL is 3.2–5.2 EUR/kg, while using PEMEL, the cost is 4.1–6 EUR/kg. Regarding hydrogen’s compressing, storing and dispensing, the pipeline scenario cost is 1.8–2.6 EUR/kg and the distributed scenario cost is 2.1–3 EUR/kg. This cost is expected to be reduced to 1.6 EUR/kg as the ultimate goal.
Although producing hydrogen through water electrolysis is a promising solution, the consumption of electricity should be considered. If the hydrogen is produced through water electrolysis with an assumed efficiency of 60%, all today’s dedicated hydrogen demand requires 3600 TWh of electricity consumption, which exceeds the total annual electricity generation in Europe. One promising solution for lowering down the electricity price is to generate electricity from renewables or nuclear power. As renewable energy sources, e.g., solar and wind, have been explored with declining costs, renewable electricity becomes less expensive. Although the hydrogen produced using renewable energy may suffer from high transmission and distribution cost as the locations could be remote, the final profit is considerable. A cost–benefit analysis of an integrated wind–hydrogen system in Corvo island has been conducted in, which found that the local renewable energy can cover 80% of the electricity demand of the island. Projects for installing electrolysers at the locations with excellent solar and wind resources have been launched all over the world and future large-scale industrial deployments are about to be undertaken.
_
At present, the most widely used and cheapest method for hydrogen production is the steam reforming of methane (natural gas). This method includes majority of the world hydrogen production, and hydrogen price is about 7 USD/GJ. A comparable price for hydrogen is provided by partial oxidation of hydrocarbons. However, greenhouse gases generated by thermochemical processes must be captured and stored, and thus, an increase in the hydrogen price by 25–30% must be considered. The further used thermochemical processes include gasification and pyrolysis of biomass. The price of hydrogen thus obtained is about three times greater than the price of hydrogen obtained by the SR process. Therefore, these processes are generally not considered as cost competitive of steam reforming. The price of hydrogen from gasification of biomass ranges from 10–14 USD/GJ and that from pyrolysis 8.9–15.5 USD/GJ. It depends on the equipment, availability, and cost of feedstock.
_
Green hydrogen is expensive to produce today. In a report published in 2019, the International Energy Agency put the cost of green hydrogen at $3 to $7.50 per kilo, compared to $0.90 to $3.20 for production using steam methane reformation. Cutting the cost of electrolyzers will be critical to reducing the price of green hydrogen, but that will take time and scale. Electrolyzer costs could fall by half by 2040, from around $840 per kilowatt of capacity today, the IEA said.
As of 2020 green hydrogen costs between $2.50-6.80 per kilogram and turquoise hydrogen $1.40-2.40/kg or blue hydrogen $1.40-2.40/kg compared with high-carbon grey hydrogen at $1–1.80/kg. Deployment of hydrogen can provide a cost-effective option to displace carbon polluting fossil fuels in applications where emissions reductions would otherwise be impractical and/or expensive. These may include heat for buildings and industry, conversion of natural gas-fired power stations, and fuel for aviation and importantly heavy trucks.
___
To qualitatively assess the costs derived from each hydrogen production (renewable and fossil fuel based) method, variables such as energy source, feed stock and capital investment cost, and hydrogen production cost (per kg of hydrogen) have been shown in Table below.
Comparison of different hydrogen production methods in 2019:
|
Process |
Energy source |
Feedstock |
Capital cost (M$) |
Hydrogen production cost ($/kg) |
|
SMR with CCS |
Standard fossil fuels |
Natural gas |
226.4 |
2.27 |
|
SMR without CCS |
Standard fossil fuels |
Natural gas |
180.7 |
2.08 |
|
CC with CCS |
Standard fossil fuels |
Coal |
545.6 |
1.63 |
|
CG without CCS |
Standard fossil fuels |
Coal |
435.9 |
1.34 |
|
ATR of methane with CCS |
Standard fossil fuels |
Natural gas |
183.8 |
1.48 |
|
Methane pyrolysis |
Internally generated steam |
Natural gas |
– |
1.59–1.70 |
|
Biomass pyrolysis |
Internally generated steam |
Woody biomass |
53.4–3.1 |
1.25–2.20 |
|
Biomass gasification |
Internally generated steam |
Woody biomass |
149.3–6.4 |
1.77–2.05 |
|
Direct bio-photolysis |
Solar |
Water + algae |
50 $/m2 |
2.13 |
|
Indirect bio-photolysis |
Solar |
Water + algae |
135 $/m2 |
1.42 |
|
Dark fermentation |
– |
Organic biomass |
– |
2.57 |
|
Photo-fermentation |
Solar |
Organic biomass |
– |
2.83 |
|
Solar PV electrolysis |
Solar |
Water |
12–54.5 |
5.78–23.27 |
|
Solar thermal electrolysis |
Solar |
Water |
421–22.1 |
5.10–10.49 |
|
Wind electrolysis |
Wind |
Water |
504.8–499.6 |
5.89–6.03 |
|
Nuclear electrolysis |
Nuclear |
Water |
– |
4.15–7.00 |
|
Nuclear thermolysis |
Nuclear |
Water |
39.6–2107.6 |
2.17–2.63 |
|
Solar thermolysis |
Solar |
Water |
5.7–16 |
7.98–8.40 |
|
Photo-electrolysis |
Solar |
Water |
– |
10.36 |
There are some uncertainties regarding the cost of hydrogen production. This cost is strongly affected by the production technology’s advancement level, availability of existing infrastructure, and the feedstock price. According to the table above, the most financially advantageous methods for hydrogen production are steam methane reforming, coal, and biomass gasification. Nuclear thermochemical cycles (CueCl and SeI) also seem to be competitive to fossil fuel and biomass prices. Wind and solar electrolysis give the highest production cost per kg of hydrogen. Since one of the major advantages of electrolysis is its local applications, distributed, small-scale production assumption is made when calculating the cost of electrolysis.
______
Hydrogen Cost estimated by Hydrogen Council:
Costs associated with 40 hydrogen technologies used in 35 applications, including for heat and power, could tumble dramatically over the next decade as the scale-up of hydrogen production, distribution, and equipment and component manufacturing continues. For some applications, hydrogen could become competitive with other low-carbon alternatives, and even conventional options. Those are key findings from an in-depth assessment of industry-provided hydrogen application costs that the Hydrogen Council—an international CEO-level advisory body—underscored in a recent report. For example, “scaling fuel cell production from 10,000 to 200,000 units can reduce unit costs by as much as 45%, irrespective of any major technological breakthroughs, and can impact multiple end-use cases. Scaling up to 70 GW of electrolysis will lead to electrolyser costs of less than $400 per kW,” it claims.
The report “Path to Hydrogen Competitiveness: A Cost Perspective” broke down hydrogen value chain to 35 applications, which span from transport—to include fuel cell trains and forklifts, aviation, and ships—to industry feedstock, and heat and power. It further breaks down hydrogen heat and power by applications for buildings, and for industry and the grid. For each application, it assessed the total cost of ownership (TOC) for a “low-carbon hydrogen solution” from 2020 to 2050, and then compared the costs with other low-carbon solutions, such as batteries, vehicles, and heat pumps, as well as conventional technologies, such as diesel-powered vehicles and gas boilers. Among its major findings is that, while it depends greatly on the region, for power applications, the eventual costs of renewable hydrogen matter. Today, renewable hydrogen from electrolysis costs about $6/kilogram (kg). Generally, however, it concludes that in the short-term (through 2025), hydrogen could become competitive in transportation, mostly for large vehicles with long ranges—trains and coaches, for example—and forklifts. By 2030, if costs of hydrogen production and distribution continue to fall, hydrogen solutions could compete with other low-carbon alternatives in simple cycle hydrogen turbines for peak power, hydrogen boilers, and industry heating.
______
Julio Friedmann, senior research scholar at Columbia University’s Center on Global Energy Policy detailed three strategies that are key to bringing down the price of green hydrogen so that more people will buy it:
-1. Support for innovation into novel hydrogen production and use. He noted that the stimulus bill Congress just passed providing this support will help cut the cost of fuel cells and green hydrogen production in years to come.
-2. Price supports for hydrogen, such as an investment tax credit or production tax credit similar to those established for wind and solar that helped drive their prices down.
-3. A regulatory standard to limit emissions. For example, half the ammonia used today goes into fertilizer production. “If we said, ‘we have an emission standard for low carbon ammonia,’ then people would start using low carbon hydrogen to make ammonia, which they’re not today, because it costs more,” said Friedmann. “But if you have a regulation that says you have to, then it makes it easier to do.” Another regulatory option is that the government could decide to procure green hydrogen and require all military fuels to be made with a certain percentage of green hydrogen.
______
______
Section-13
Hydrogen and natural gas mixture:
_
Hydrogen Gas Mixtures in combustion engine:
Hydrogen can be used advantageously in internal combustion engines as an additive to a hydrocarbon fuel.
Hydrogen is most commonly mixed with high pressure natural gas for this purpose since both gases can be stored in the same tank. If hydrogen is blended with other fuels, it usually has to be stored separately and mixed in the gaseous state immediately before ignition. In general, it is impractical to use hydrogen in conjunction with other fuels that also require bulky storage systems, such as propane.
Gaseous hydrogen cannot be stored in the same vessel as a liquid fuel. Hydrogen’s low density will cause it to remain on top of the liquid and not mix. Furthermore, liquid fuels are stored at relatively low pressures so that very little hydrogen could be added to the vessel.
Liquid hydrogen cannot be stored in the same vessel as other fuels. Hydrogen’s low boiling point will freeze other fuels resulting in fuel “ice”!
Hydrogen can be used in conjunction with compact liquid fuels such as gasoline, alcohol or diesel provided each are stored separately. In these applications, the fuel tanks can be formed to fit into unused spaces on the vehicle. Existing vehicles of this type tend to operate using one fuel or the other but not both at the same time. One advantage of this strategy is that the vehicle can continue to operate if hydrogen is unavailable.
Hydrogen cannot be used directly in a diesel (or “compression ignition”) engine since hydrogen’s autoignition temperature is too high (this is also true of natural gas). Thus, diesel engines must be outfitted with spark plugs or use a small amount of diesel fuel to ignite the gas (known as pilot ignition). Although pilot ignition techniques have been developed for use with natural gas, no one is currently doing this with hydrogen.
One commercially available gas mixture known as Hythane contains 20% hydrogen and 80% natural gas. At this ratio, no modifications are required to a natural gas engine, and studies have shown that emissions are reduced by more than 20%. Mixtures of more than 20% hydrogen with natural gas can reduce emissions further but some engine modifications are required.
_
Hydrogen and natural gas blending:
HCNG or H2CNG (hydrogen compressed natural gas) is a mixture of compressed natural gas and 4–9 percent hydrogen by energy. It may be used as a fuel gas for internal combustion engines and home appliances. In the town of Nes on the island of Ameland in the Netherlands, a four-year (2008-2011) field test was carried out where 20% hydrogen was added to the local distribution net supplying a complex of 14 apartments. The appliances involved were kitchen stoves, condensing boilers, and micro-CHP boilers. The use of existing natural gas pipelines for HCNG was studied by NaturalHy. To get the most out of an internal combustion engine in transportation if higher levels of hydrogen are added, modifications have to be made to the engine and the control strategy. The hydrogen in the blend leads to lower CO2 emissions. Hydrogen produced through clean pathways can be injected into natural gas pipelines, and the resulting blends can be used to generate heat and power with lower emissions than using natural gas alone.
_
Basic physicochemical properties of hydrogen and natural gas:
|
Property |
Hydrogen |
Natural Gas |
|
Density at 273 K (kg/m3) |
0.09 |
0.65 |
|
Boiling point at atmospheric pressure(K) |
20.3 |
111.2 |
|
Liquid density (kg/m3) |
70.8 |
450.0 |
|
Flammability concentration limits in air (vol %) |
4–75 |
5–15 |
|
Diffusion coefficient in air (cm2/s) |
0.61 |
0.16 |
|
Gravimetric HHV in MJ/kg |
142 |
55.6 |
|
Volumetric HHV in MJ/m3 |
12.7 |
40 |
At any pressure, the volumetric energy density of methane gas exceeds that of hydrogen gas by a factor of 3.2 so at any pressure, hydrogen gas clearly carries less energy per volume than natural gas.
_
The industry is currently very interested in expanding the hydrogen energy market as a result of diminishing fossil fuel resources and the requirements to reduce (if possible, eliminate) GHG emissions. A very possible method to incorporate hydrogen into the current energy market could be achieved via mixing it into the existing natural gas delivery systems and distributing it using the existing natural gas grid. The International Gas Union (IGU) has indicated that substituting 10 vol% of a natural gas supply with hydrogen cuts CO2 emissions by about 3%. Another study conducted by NATURALHY has presented that around 15% CO2 emissions cutbacks can be attained with mixing up to 50 vol% hydrogen gas into the existing natural gas grid. It is essential to note that many governments do not permit 50% hydrogen and 50% natural gas mixture by volume. This is limited to 25 vol% on a Wobbe number basis. The Wobbe is a measure of the interchangeability of fuel gases when introduced into a heater via a burner with a fixed differential pressure. Two gases with the same Wobbe Index will deliver the same amount of heat into a combustion process per unit of time regardless of the composition.
Blends in excess of 20% hydrogen require end-user appliances to be converted because of the effects of hydrogen on the Wobbe index.
_
Wobbe number is calculated as follows:
_
When deciding on whether or not it is beneficial to add increasing amounts of hydrogen into the existing natural gas grid, all potential effects should be considered thoroughly, including the effects on:
- Net and overall energy densities: increasing the amount of hydrogen in the mixture reduces its volumetric energy density.
- Wobbe number: increasing the amount of hydrogen in the mixture (up to around 70 vol%) reduces its Wobbe number slightly (it should be noted that this might not be regulated in some countries).
- Ignition characteristics: increasing the amount of hydrogen in the mixture reduces its “knock tendency.”
- Burning velocity: increasing the amount of hydrogen in the mixture (up to around 30 vol%) increases its burning velocity.
_
It should be noted that there are possible risks related to mixing hydrogen and natural gas supplies. For example, there are reliability risks of the grid network and fuel processing sites that might seriously affect the operators. Another impact might be the performance decrease of household appliances. Frequency of explosions and the risk of fire hazard might increase as well. Increasing hydrogen amounts in the existing natural gas pipelines might increase NOx emissions. These are some of the risks associated with introducing (or increasing the amounts of) hydrogen in the existing natural gas grid.
And, all the risks mentioned earlier should be comparatively and thoroughly assessed by considering the reductions in carbon emissions that can be achieved by mixing hydrogen and natural gas instead of using natural gas alone. In addition, natural gas can be utilized for distributed heat and power supply systems via stationary CHP systems together with hydrogen. The United Kingdom Hydrogen and Fuel Cell Industry (UKHFCA) has shown that by using fuel cell micro-CHP technologies to replace today’s traditional boilers, about 2.5 t equivalent of CO2 emissions can be reduced. This amount is equal to about 40%–50% of a typical European household’s annual carbon footprint.
_____
HyDeploy project:
Hydrogen heating at Keele University successfully showed it is possible to mix 20% hydrogen into the gas supply and have everything work as normal. Adding the hydrogen will reduce the amount of CO2 that’s being produced through heating and cooking. Critics fear hydrogen will prove too expensive for mass usage, but supporters of the technology have high hopes.
As a fuel, hydrogen functions in much the same way as natural gas. So staff in the university canteen say cooking on the 20% hydrogen blend has made no difference to their cooking regime. The project – known as HyDeploy – is the UK’s first live trial of hydrogen in a modern gas network. Keele was chosen because it has a private gas system. Its hydrogen is produced in an electrolyser – a device that splits water (H2O) into its constituents: hydrogen and oxygen. The machine is located in a glossy green shipping container in the corner of the university’s sports field. The gas distribution firm Cadent, which is leading the project, says that if a 20% blend were to be rolled out across Britain, it would reduce emissions of CO2 by six million tonnes – equivalent to taking 2.5 million cars off the road. The hydrogen could be generated pollution-free by using surplus wind power at night to split water molecules using electrolysis.
Why not add more than 20% hydrogen?
The 20% proportion was chosen because it’s an optimal blend that won’t affect gas pipes and appliances.
_
Unfortunately, it’s not quite that simple:
Natural gas is a fossil fuel. It’s mostly methane, and when burned it produces carbon dioxide. Natural gas produces 19% of all global greenhouse gas emissions, very similar to oil which accounts for 21% or coal which accounts for 25%. In the UK, using natural gas for heating generates about a third of our greenhouse gas emissions. So, boilers that run on natural gas will have to go. Given the need to ditch gas and move to renewable and zero-carbon fuel sources, the discussion has turned to whether hydrogen could replace natural gas as the fuel that powers boilers and cooks meals.
The recent test at Keele University showed it was possible to continue using appliances built to run on natural gas by using a mix of 20% hydrogen, 80% natural gas. Unlike natural gas, which releases carbon dioxide when it’s burned, burning hydrogen only releases water. That means it has the potential to be a zero-carbon fuel. The test only used 20% hydrogen because that’s the maximum amount that can be mixed with natural gas without needing to change pipes and appliances to be able to handle pure hydrogen. To be able to use 100% hydrogen new pipe infrastructure, new boilers and cooking appliances would have to be installed. So, the switch to a hydrogen grid will not be easy or cheap.
_
Hydrogen doesn’t emit carbon dioxide when it is burnt, but it can still be carbon-intensive depending on how it is made. At the moment, most hydrogen is made from natural gas via a process called steam reforming. This is energy-intensive and it takes more natural gas to produce the hydrogen using this process than would be required if we just kept using natural gas. Emissions would actually go up by switching to hydrogen. No one is advocating just switching to hydrogen that’s made using steam reforming and letting the CO2 just go into the atmosphere. But the gas industry is advocating for using steam reforming with carbon capture and storage. This means the natural gas is converted to hydrogen, but the carbon dioxide is captured and then pumped back underground.
There are three major issues with this approach.
-Firstly, carbon capture and storage is not yet a proven technology at scale. There have been tests, but we don’t have a good idea how much it will cost to capture the carbon and pump it into safe storage sites under the North Sea. Betting on carbon capture is a major risk.
-Second, it still means we’ll need a lot of gas. Because of the conversion losses of steam reforming, it’s estimated running the gas grid on hydrogen would require 47% more natural gas then continuing to run on natural gas as it does now. That’s good news for the gas industry, but really terrible news for the rest of us because it means higher heating bills and the need to import huge amounts of gas from produces like Russia and Qatar. Rather than freeing ourselves from the need to import fuels by switching to renewables, hydrogen may make us more reliant on energy imports than ever. The H21 report by Northern Gas Networks, which looks at switching Leeds to a hydrogen gas grid, estimated that hydrogen gas produced via steam reforming would cost almost double the current price of gas.
-Finally, there’s the cost of the switch over to pure-hydrogen infrastructure, which would entail a huge upgrade of the gas network to handle hydrogen, the new hydrogen production facilities, and the need for consumers to all switch to new cookers and boilers that can operate on hydrogen. With these major disadvantages, converting to a hydrogen grid doesn’t seem like a magic bullet for decarbonising heating.
_
There is another way of creating hydrogen that is currently done only on a small scale but could theoretically be expanded to create the hydrogen that the UK would need – electrolysis. This uses electricity to create hydrogen from water. It’s clean and doesn’t produce any CO2, so there’d be no need to rely on unproven carbon capture and storage technology. But making hydrogen from electricity (electrolysis) has one major drawback, which is why it currently accounts for only a very small fraction of hydrogen production – it’s really expensive. Electricity is already much more expensive per kWh than gas, then you factor in the significant conversion losses in electrolysis (about half the energy is lost) and you find that hydrogen produced via electrolysis is extremely costly. Electricity already costs over four times the price of gas per kwh, but with the added conversion losses from making the hydrogen, you’re potentially looking at hydrogen being six to eight times as expensive as natural gas. Bill payers are going to be very upset when the fuel supply switches over and they see their heating bill has octupled.
_
Is there a better way?
Switching from natural gas to hydrogen will either lock us into using even more natural gas than we are now (if the hydrogen is made via steam reforming) or will be horrendously expensive for consumers (the electrolysis option). But we need to move away from natural gas rapidly if we are to meet our climate objectives, so is there any other way to achieve this?
The answer is simple: reduce demand and electrify the residual heating need.
Reducing demand means making buildings more efficient so they require less heating. That means saving both energy and money in the long run. Citu Homes require ten to twenty times less heating than an average UK home. That means the heating demand is so low you don’t need a gas-powered central heating system (or a hydrogen one for that matter). Little electric radiators are sufficient to provide the small amount of heating needed. An MVHR system recycles heat from exiting stale air and transfers it onto incoming fresh air, reducing heating requirements by 90% when combined with the homes air-tight membrane and excellent insulation. All new homes should therefore be required to be built to these high standards of energy efficiency, where so little heating is needed that gas boilers are not required. That works for new buildings, but older buildings that aren’t as efficient will still need central heating systems. Heating demand can be reduced considerably by incentivising better home insulation via low-cost loans for home efficiency improvements, subsidies for insulation and by linking the stamp duty system to energy performance to encourage households to improve the efficiency of their homes.
Then heating can be switched from carbon-intensive natural gas to clean electric heat pumps, which can take advantage of the electricity grid’s rapid decarbonisation. In future, it will also be able to benefit from the rapidly falling cost of renewable power, and it’ll mean we’ll no longer rely on gas imports. Air source heat pumps are easy to install and don’t require digging up gardens like ground source heat pumps do. Their costs are falling rapidly, and they are getting more efficient. A typical air-source heat pump will deliver about 3 kWh of heat for every 1 kWh of electricity used to power it. This is six times as efficient as using hydrogen created via electrolysis for heating. Every kWh of electricity could be used either to make 0.5 kWh worth heat by producing hydrogen, or 3 kWh of heat via an air source heat pump.
This doesn’t mean hydrogen doesn’t have a part to play in transitioning to a zero-carbon economy. Hydrogen will have an important role to play in decarbonising industrial processes that can’t be electrified because they require extremely high temperatures. But it simply isn’t a good solution to decarbonising home heating: it’ll be good for the gas industry because it’ll take large amounts of natural gas to produce it, but it’ll be terrible for consumers who’d have to pay double for their energy costs. Far better to invest in drastically increasing efficiency so gas is no longer required and use highly efficient heat pumps for central heating in older properties.
_____
Benefits of hydrogen blending in gas networks highly variable, 2022 study finds:
Efforts to lower carbon emissions from gas networks by replacing some of the gas with hydrogen could see higher bills for consumers, a study has found. Fraunhofer IEE has assessed the technical feasibility, emission savings and cost impacts from plans to add hydrogen to the existing gas transport network. The process, known as ‘hydrogen blending’, is currently being discussed by the EU as a way to lower carbon emissions from gas networks across its member states. But the study found that the carbon benefits of such a practice are highly variable depending on the type of hydrogen used and believes that other industries may be more suited to using the fuel. Furthermore, the measures for hydrogen blending are currently estimated to increase costs for end users by up to 43 per cent for industry and up to 16 per cent for households at a blending level of 20 per cent of the total gas volume. Instead the study recommends using hydrogen in sectors where there are currently few alternatives for decarbonisation such as steel production or other industrial applications, and shipping and aviation fuels.
_____
_____
Section-14
Nuclear hydrogen production:
Energy has been universally recognized as one of the most important inputs for economic growth and human development. Economic growth implies the availability of cost effective and environmentally benign energy sources. A future energy economy will need to replace oil and reduce greenhouse gas emissions (GHGs) for climate protection. The worldwide interest in hydrogen as a clean fuel has led to comprehensive research, development and demonstration activities whose main objective is the transition from a fossil based to a “CO2 lean” energy structure.
_
The vast majority of the world’s commercial hydrogen — over 95% by most estimates — is produced using the steam methane reforming process (SMR). In this process, natural gas is reacted with steam at an elevated temperature to produce carbon monoxide and hydrogen (which is synthesis gas, or simply syngas). A subsequent reaction — the water gas shift reaction — then reacts additional steam with the carbon monoxide to produce additional hydrogen and carbon dioxide. The carbon footprint of hydrogen production via SMR is high. In fact, more carbon is generated in the production of hydrogen via SMR than if you simply burned the methane used to make the hydrogen. So, why do we make hydrogen using this method? It has historically been the cheapest method of large scale hydrogen production. Grey hydrogen denotes hydrogen produced from fossil fuels, such as via the SMR process. Thus, most of the world’s hydrogen production is grey. However, it is possible to capture the carbon dioxide produced in this process. The carbon can then be sequestered or otherwise used for other purposes. This lowers the carbon footprint, and can result in the subsequent hydrogen being classified as “blue hydrogen.” Blue hydrogen is produced using non-renewable resources, but it meets the threshold of a low carbon footprint. Depending on the process, blue hydrogen can be produced from fossil fuels, but it can also be produced from nuclear power. Green hydrogen meets the low-carbon threshold, but it is produced using renewable resources. For example, electricity from solar power can be used to electrolyze water into its constituents, hydrogen and water. Renewable production of hydrogen is the idealized vision of the hydrogen economy, but there are some obstacles that have thus far kept this vision from being realized. The biggest issue with green hydrogen is the cost. It simply isn’t yet cost effective enough to produce hydrogen using intermittent renewables. It could become cost effective if the renewable supply is overbuilt, and hydrogen production only takes place when there is excess electricity being produced. However, that means that all of the associated hydrogen production equipment is only being utilized a small fraction of the time. Because of the low capacity factor of renewables, the subsequent capital costs of the hydrogen equipment drive the price quite high per unit of mass of hydrogen produced. Current estimates put green hydrogen production at roughly twice the cost of hydrogen production via SMR, but with a carbon footprint that is about 80% lower. Costs are expected to come down, but it will be challenging because of the intermittency.
_
As hydrogen production has grown in popularity, so has the term “green hydrogen,” which refers to using renewable sources to produce hydrogen. This mirrors mandates and pledges we see to drive down emissions in our energy sector through wind, solar and other carbon-free technologies. But what is discounted most often is the role that nuclear energy should have in this exciting new market. According to EON’s study, nuclear can also serve as the cheapest carbon-free source for hydrogen fuel and be economically competitive with natural gas—which is the leading method for making hydrogen currently—if used on a large scale. An inherent advantage over technologies that only produce electricity (like wind and photovoltaic solar) is nuclear’s capacity to produce both electricity and heat, affording it the ability to take advantage of all hydrogen production technology options. This is where nuclear power can make a huge impact. A hydrogen economy will require a massive increase in hydrogen production. That means scalable options. Hydrogen can be produced from nuclear power in a scalable fashion.
The simplest way is using nuclear power to produce electricity, which is then used to electrolyze water. This would be the same process as that used to produce green hydrogen, except in this case, it would utilize nuclear power at a capacity factor of 90% instead of renewables at 20% to 40% capacity factor. That may drive down the cost of hydrogen production. A 2020 paper in Applied Energy estimated the carbon footprint of hydrogen production via a number of different methods, and concluded that hydrogen production via nuclear electricity has a comparable carbon footprint to hydrogen produced by renewables. However, the cost via this route is still high. It has been estimated to be comparable in cost to the renewables route. The primary reason is that electrolysis isn’t especially efficient. Generally about 20% of the power used to produce hydrogen from electrolysis is utilized in the process. Or, to put it another way, for a given input of electricity you only get 0.8 equivalent units of hydrogen back out.
_
Hydrogen could provide a link between the electricity and liquid fuels markets. It can be generated from excess electricity produced by means of technologies with low operating costs and stored until needed. Hydrogen produced from off-peak electricity can be used for premium markets where the unique characteristics of hydrogen add value. Peak electricity production from stored hydrogen can be provided today from gas turbines, which may be replaced in the future with high temperature fuel cell plants and/or liquid hydrogen (LH2)–LOX steam generator cycles. Similar to electricity, hydrogen decouples the energy demand from the energy resources. It can enhance energy supply security in that individual countries can choose their own sources of energy.
Centralized energy production in large quantities favours the use of nuclear plants, which should operate as the baseload power source, whereas conventional plants would cover peak load. A principal advantage of a nuclear based energy supply is eliminating supply uncertainty and the sensitivity of energy prices to volatility of natural gas and other fuel prices. Nuclear, with virtually no emissions of airborne pollutants, appears to be an ideal option for large scale centralized H2 production as seen in the figure below.
Figure above shows routes for nuclear assisted hydrogen production.
Nuclear hydrogen production technologies have great potential and advantages over other sources that might be considered for a growing the hydrogen share in a future world energy economy. The selection of hydrogen technologies (to be coupled to nuclear power reactors) greatly depends on the type of the nuclear power plant itself. Some hydrogen production technologies, such as conventional electrolysis, require only electric power. Whereas others, such as thermochemical cycles, may require only process heat (which may be delivered at elevated temperature values) or hybrid technologies such as the high temperature steam electrolysis (HTSE) and hybrid thermochemical cycles, which require both heat and electricity.
The plant’s relative proximity to key markets is ideal for reducing transport distances. The nuclear plant should be within 150 miles of major existing hydrogen consumers, such as oil refineries, steel manufacturers, syngas, and chemical plants. The location also has the right inputs for the necessary electricity and water.
According to EON’s study, meeting the energy demands of the maritime transportation industry by 2050 from nuclear power alone “would require as much as 650 gigawatts of advanced nuclear reactors” for hydrogen production. The 650 gigawatts needed for this portion of the transportation sector alone is more than six times the capacity of all nuclear plants in the United States, so in other words it’s big. Wind and solar will play a major role in providing hydrogen too, but that’s still a huge opportunity for the nuclear industry.
_
Nuclear power already produces electricity as a major energy carrier with well-known applications. Operating at very high capacity factors, nuclear energy is well placed to produce zero-carbon hydrogen as an emerging energy carrier with a wide range of applications. The evolution of nuclear energy’s role in hydrogen production over perhaps two decades is seen to be:
-1. Conventional electrolysis of water, using off-peak capacity (needs 50-55 kWh/kg).
-2. Low-temperature steam electrolysis, using heat and electricity from nuclear reactors.
-3. High-temperature steam electrolysis, using heat and electricity from nuclear reactors.
-4. High-temperature thermochemical production using nuclear heat.
In addition, nuclear heat can assist the process which provides most of the world’s hydrogen today:
-5. Use of nuclear heat to assist steam reforming of natural gas (methane).
Steam reforming of methane (SMR) requires temperatures of over 700 °C to combine methane and steam to produce hydrogen and carbon monoxide. A nuclear heat source would reduce natural gas consumption by about 30% (i.e., that portion of feed which would simply be for heat), and eliminate flue gas CO2 emissions.
Starting with electrolysis, the efficiency of the whole process (primary heat to hydrogen) moves from about 25% with today’s reactors driving electrolysis (33% for reactor x 75% for cell) to 36% with more efficient reactors doing so, to 45% for high-temperature electrolysis of steam, to about 50% or more with direct thermochemical production.
_
Nuclear hybrid energy systems for hydrogen production:
|
|
Alkaline electrolysis |
PEM electrolysis |
Solid oxide electrolysis |
Steam methane reforming |
Thermochemical S-I |
|
Technology readiness |
9 |
6-8 |
5 |
9 |
4 |
|
Temperature (°C) |
60 |
60 |
800 |
870 |
910 |
|
Pressure (atm) |
1 |
1 |
1.57 |
4.1 |
3.85 |
|
Efficiency (HHV, %) |
30 |
27 |
36 |
79 |
25 |
|
Electricity (MJ) |
180 |
200 |
146 |
1.4 |
75 |
|
Heat (MJ) |
26 |
26 |
30 |
0 |
375 |
|
Water (kg) |
11.5 |
11.5 |
83 |
10.3 |
9 |
|
Natural gas (kg) |
0 |
0 |
0 |
2.9 |
0 |
|
CO2 out (kg) |
0 |
0 |
0 |
5-11 |
0 |
|
Production cost |
$5.92 |
$3.56-5.46 |
$2.24-3.73 |
$1.54-2.30 |
$2.18-5.65 |
Note: Efficiency assumes 40% heat to electricity conversion.
_
Nuclear energy is poised to be the future alternative for a hydrogen economy. It can produce hydrogen not only in large quantities but also at high quality at a relatively low cost without any GHG emissions. All types of nuclear reactors can be used for the production of hydrogen as they can provide electricity and process heat. An important factor to be considered when selecting a reactor for hydrogen production is the power size. Large reactors are more suitable for cogeneration of electricity and hydrogen production. Whereas small sized plants are more suitable for hydrogen production only as a single purpose plant. Current light water reactors can be used for hydrogen production, especially using off-peak power or cogeneration for better economics. Small and medium power reactors based on high temperature gas cooled reactors are an attracting option for hydrogen production.
_
Nuclear power plants can produce hydrogen in a variety of methods that would greatly reduce air emissions while taking advantage of the constant thermal energy and electricity it reliably provides. Existing nuclear plants could produce high quality steam at lower costs than natural gas boilers and could be used in many industrial processes, including steam-methane reforming. However, the case for nuclear becomes even more compelling when this high-quality steam is electrolyzed and split into pure hydrogen and oxygen.
A single 1,000 megawatt nuclear reactor could produce more than 150,000 tonnes of hydrogen each year. Ten nuclear reactors could provide about 1.5 million tonnes annually or 15% of current hydrogen produced in the United States. This process would allow utilities to produce and sell hydrogen regionally as a commodity in addition to providing clean and reliable electricity to the grid. For instance, reactors in Ohio could sell hydrogen to iron and steel manufacturing plants. The Midwest could target fertilizer producers and California could market hydrogen stations for fuel cell electric vehicles. This new revenue stream could also help build an economic case to keep the nation’s at-risk reactors up and running—possibly providing higher market value for hydrogen commodities in states and countries that are looking to reduce emissions.
Advanced reactors are expected to operate at considerably higher temperatures and would allow nuclear plants to more efficiently produce hydrogen to dramatically scale-up the industry. High temperature reactors could even be used to significantly reduce the emissions produced by conventional steam-methane reforming processes by replacing the natural gas that is burned to produce steam and to provide the essential heat to reform the natural gas/steam mixtures. New electrochemical processes are also being developed to directly convert natural gas into hydrogen and plastics using nuclear, which would completely avoid air emissions and achieve significantly higher efficiencies. Ultimately, nuclear energy could support the nation’s manufacturing industries across multiple sectors by providing clean energy to produce hydrogen, fuels, fertilizers, steel, plastics, and other chemicals.
_
Electrolysis at ambient temperature is being undertaken in at least four US projects at nuclear power plants and is planned for the Kola plant in Russia from 2023. Alkaline and proton exchange membrane (PEM) technology is employed. In August 2021 Nel Hydrogen was contracted to build a 1.25 MW PEM electrolyser at Exelon’s Nine Mile Point nuclear power plant to show integrated production, storage and normal use at the plant.
Low-temperature steam electrolysis improves the efficiency of electrolysis at ambient temperatures and utilizes waste heat at up to 200 °C from a conventional reactor. The US Department of Energy in October 2020 selected two projects to advance flexible operation of light water reactors with integrated hydrogen production systems to receive cost-shared funding. Two other projects are already under way. The IEA’s Global Hydrogen Review 2021 described about a dozen projects that are intended to use electricity from nuclear power plants to produce hydrogen using electrolysis. Most of these projects are based in Canada, China, Russia, the USA and the UK. However, only a few of these were actually launched.
_
The International Atomic Energy Agency (IAEA) has developed the Hydrogen Economic Evaluation Program (HEEP) to assess the economics of large-scale hydrogen production using nuclear energy. The IAEA HEEP was developed and released as a free tool which can be used to assess the economics of large scale hydrogen production using nuclear energy. The software can be used to evaluate the economics of the four most promising processes for hydrogen production: high temperature electrolysis, thermochemical processes including S-I process, low temperature conventional electrolysis and steam reforming. The IAEA HEEP software is suitable for comparative studies not only between nuclear and fossil energy sources for hydrogen production but also for solely hydrogen production or cogeneration with electricity. The HEEP models are based on some economic and technical data, and on cost modelling which include various aspects of hydrogen economy including storage, transport, and distribution with options to eliminate or include specific details as required by the users.
_
Hydrogen Calculator (HydCalc)
One of the most important factors to be considered when deciding on nuclear hydrogen production is the size of the nuclear power reactor. Larger reactors are more suitable for cogeneration while smaller and modular reactors are more appropriate for hydrogen generation as a single commodity. Economics of hydrogen production is another deciding factor that becomes more effective with the Carbon tax in effect. HydCalc was developed as a single window calculator to make rough estimate of the hydrogen production cost utilizing different technologies. It uses current price estimate from publications and articles in open literature, and provides cost value of hydrogen production based on average estimated CO2 release. It also considers the effect of CO2 tax on the production cost.
_____
Advantages of nuclear power and hydrogen cogeneration:
Every year, about 75Mt of hydrogen are consumed worldwide. Nuclear power plants and hydrogen production systems are well aligned to give nuclear an economical advantage over traditional hydrogen production energy sources. Nuclear power plants can supply the required heat and electricity without generating any carbon emissions. Producing hydrogen will serve as energy storage and decouple power production from the consumption of electricity. The hydrogen stored can either be used as fuel for generators based on combustion or sold for other industrial purposes.
If nuclear is considered as the primary energy source for hydrogen production, it should generate minimal emissions and have minimal impact on the environment. Today, we are in search of solutions to drive down carbon emissions. Already, nuclear energy comprises nearly 55 percent of the carbon-free energy in the United States and is viewed as a key part of any viable climate solution. The potential for a nuclear and hydrogen partnership is a natural fit and worthy of future investments. Using all available carbon-free sources, including nuclear, for hydrogen production will be game-changing. Carbon-free hydrogen powered by nuclear energy can reduce carbon emissions even more, protecting our climate while fueling the future transportation and industrial sectors.
MIT Energy Initiative (MITEI) researcher Jesse Jenkins and his colleagues at Argonne National Laboratory considered pairing renewable resources with flexible nuclear power plants. In a paper for Applied Energy Jenkins claims it makes more sense to operate a nuclear power plant at lower performance and to absorb as much free wind and sun as possible. That way nuclear operates flexibly to integrate renewable energy and reduce carbon dioxide emissions. Flexible operations improve reactor ownership revenue by reducing the amount of waste fuel, improving system quality, and reducing customer energy costs.
_
Challenges of nuclear power and hydrogen cogeneration:
Nuclear cogeneration faces major challenges, including the disparities between nuclear and heat markets. There are also specific issues and concerns to be addressed around nuclear plant whose design has been altered so it is better suited to produce hydrogen (settlement, the time needed to plan, construction and financial risk), the demonstration of industry-specific nuclear plant and licensing of custom nuclear units.
Nuclear power generation is feasible and economically viable; however, any nuclear reactor is subject to a set of operational limitations arising from nuclear reactor physics and these are different from the technological restrictions of traditional coal or gas power plants. For example if, over the fuel irradiation cycle, the minimally stable performance of a nuclear reactor changes, production cannot ramp up or down too rapidly without loading the nuclear fuel rods and the reactor itself.
At high power levels there is surplus energy available and curtailing it is considered to be largely unfavourable to the plant. The surplus energy available would suffer if the plant were operated flexibly to accommodate demand management.
The potential benefits of nuclear hydrogen over other sources are significant and could result in a growing share of hydrogen production in a future global energy economy. However, nuclear hydrogen processes are technically uncertain and require comprehensive research and a strong development effort. Safety issues and the storage and delivery of hydrogen are critical areas for development to promote a prosperous hydrogen economy.
______
______
Section-15
Hydrogen safety:
Hydrogen poses a number of hazards to human safety, from potential detonations and fires when mixed with air to being an asphyxiant in its pure, oxygen-free form. In addition, liquid hydrogen is a cryogen and presents dangers (such as frostbite) associated with very cold liquids. Hydrogen dissolves in many metals and in addition to leaking out, may have adverse effects on them, such as hydrogen embrittlement, leading to cracks and explosions. Hydrogen gas leaking into external air may spontaneously ignite. Moreover, hydrogen fire, while being extremely hot, is almost invisible, and thus can lead to accidental burns.
Even interpreting the hydrogen data (including safety data) is confounded by a number of phenomena. Many physical and chemical properties of hydrogen depend on the parahydrogen/orthohydrogen ratio (it often takes days or weeks at a given temperature to reach the equilibrium ratio, for which the data is usually given). Hydrogen detonation parameters, such as critical detonation pressure and temperature, strongly depend on the container geometry.
______
All fuels come with some degree of risks associated with them. EVs running on lithium battery are prone to fire hazard if not handled carefully. Gasoline and diesel are highly combustible and poses a risk. Natural gas is stored at high pressure, and damage to the container can cause catastrophic accidents. These all accidents and incidents have already occurred, and researchers and design engineers are working tirelessly to ensure more safety to these systems. Here are some incidents with hydrogen:
In June 2019, an incident occurred at the Air Products and Chemicals, Inc. facility in Santa Clara, California. The hydrogen transfill facility had an explosion during the loading of a tanker truck that was being fueled. This resulted in the temporary shutdown of multiple hydrogen fueling stations in the San Francisco area.
In June 2019 Uno-X fueling station in Norway experienced an explosion, resulting in the shutdown of all Uno-X hydrogen fueling stations and a temporary halt in sales of fuel cell vehicles in Norway. Based on preliminary investigation findings neither the electrolyzer or the dispenser used by customers had anything to do with this incident. Therefore, the electrolyzer division will now return to business as usual. June 27, 2019 Nel ASA announces the root cause of the incident has been identified as an assembly error of a specific plug in a hydrogen tank in the high-pressure storage unit.
In December 2019, a gas explosion at an Airgas facility in Waukesha, Wisconsin injured one worker and caused 2 hydrogen storage tanks to leak.
On 7 April 2020, an explosion occurred at the OneH2 Hydrogen Fuel plant in Long View, North Carolina, causing significant damage to surrounding buildings. The blast was felt several miles away, damaging about 60 homes. No injuries from the explosion were reported. The incident remains under investigation.
On 11 June 2020, an explosion occurred at the Praxair Inc., 703 6th St. Texas City, Texas, a hydrogen production plant.
On 30 September 2020, a hydrogen tanker crashed and exploded in Changhua City, Taiwan, killing the driver.
On 9 August 2021, the Medupi Power Station in South Africa was severely damaged after an explosion in Unit 4 caused by an improper procedure while the generator was being purged of hydrogen.
_____
Hydrogen properties vis-à-vis safety issues:
At standard temperature and pressure conditions, hydrogen is a colourless, odourless, tasteless, nontoxic, non-corrosive, non-metallic diatomic gas, which is in principle physiologically not dangerous. One of its most important characteristics is its low density. It is positively buoyant above a temperature of 22 K that is over (almost) the whole temperature range of its gaseous state. The positive buoyancy of hydrogen is a favourable safety effect in unconfined areas, but can cause a hazardous situation in (partially) confined spaces, where the hydrogen can accumulate, for example underneath a roof. Hydrogen gas exhibits a high diffusivity and a high buoyant velocity thus it rapidly mixes with the ambient air upon release.
Hydrogen forms molecules of small size with small molecular weight and has low viscosity. As a result, hydrogen can leak at a larger molecular flow rate, permeates through materials and passes through smaller leak paths than other gases. Diffusion in small amount is possible even through intact materials, in particular organic materials, which may lead to gas accumulation in confined spaces. Hydrogen leaks can support combustion at very low flow rates, as low as 4 micrograms/s.
Hydrogen also exhibits a positive Thompson-Joule effect at temperatures above 193 K, the inversion temperature. This means that the temperature of hydrogen gas increases upon depressurization, which in turn may lead to ignition. For example, if a sudden pressure drop from 20 MPa to ambient pressure takes place the temperature changes by six degrees. This makes hydrogen more susceptible to ignition after sudden release from high pressure containment.
_
Compressed hydrogen fuel tanks will operate under high (5000–10,000 psi) pressure and will be subject to potential failures associated with pressure vessels. In the event of a failure, hydrogen will leak through the cracks developed in these vessels, triggering an increase in the temperature, due to the negative value of the Joule–Thompson coefficient of hydrogen. The net effect of a leakage in a confined space, such as that from a vehicle parked in a garage, will be the creation of a hazardous situation, which may cause severe damage to property and human health. The potential for such hazards (from stationary vehicles) must be considered and quantified to the greatest possible extent when evaluating the various on-board hydrogen storage alternatives.
_
Energy carriers used as fuel are by definition explosive and fire hazards. Different types of fuel have quite varying physical properties, and must therefore be handled differently. No fuel system can be described as totally safe, but the risk of accidents can be reduced significantly by appropriate storage, handling and transportation methods. Safety is important in a large-scale energy infrastructure. Hydrogen is no more or less dangerous than any other energy carrier and furthermore that hydrogen has properties that in certain areas make it safer than other energy carriers: it is not poisonous, and has the ability to dissipate quickly into the atmosphere because of its light weight compared to air. There is very little likelihood that hydrogen will explode in open air, since it will quickly rise upwards due to its lightness. This is the opposite of what we find for heavier gases such as propane or gasoline fumes, which, hovering near the ground, constitute great danger for explosion.
A hydrogen flame burns quickly and emits very little heat (hydrogen radiates only 10% of the heat from hydrocarbons per comparable unit of energy). This means that a hydrogen fire will do much less damage to the immediate surroundings than a gasoline fire, while consequently creating less damaging gases caused by the burning of “secondary” materials.
Other properties of hydrogen necessitate special considerations when handling. Hydrogen consists of small molecules, which require special qualities in materials used in storage and transportation means. Hydrogen creates flammable and explosive mixtures of air over a broad spectre (see Table below). These mixtures need very little energy to ignite. Ventilation is therefore an important factor in areas where hydrogen is used.
|
Characteristic |
Hydrogen |
Natural gas |
Gasoline |
|
Lower heating value (KJ/g) |
120 |
50 |
44,5 |
|
Self-ignition temperature (ºC) |
585 |
540 |
228-501 |
|
Flame temperature (ºC) |
2045 |
1875 |
2200 |
|
Flammability limits in air (vol %) |
4-75 |
5.3-15 |
1.0-7.6 |
|
Minimum ignition energy in air (microjoule) |
20 |
290 |
240 |
|
Detonability limits in air (vol %) |
18-59 |
6.3-13.5 |
1.1-3.3 |
|
Theoretical explosive energy (kg TNT/m3 gas) |
2.02 |
7.03 |
44.22 |
|
Diffusion coefficient in air (cm2/s) |
0.61 |
0.16 |
0.05 |
Hydrogen is extremely flammable. However this is mitigated by the fact that hydrogen rapidly rises and often disperses before ignition, unless the escape is in an enclosed, unventilated area. It is very difficult to detonate hydrogen in open air. This has to do with the fact that hydrogen is much lighter than air (14.4 times) and rises at a speed of 20 m/s. Hydrogen has many characteristics that warrant its being handled with great care. Hydrogen-air mixtures can ignite or explode at both lower and higher concentrations of the gas in the air than CNG or methane. Hydrogen is more easily ignited than other fuels. The impact of this is negligible, however, as most other fuels can already be ignited by small amounts of static electricity. Hydrogen has a lower radiant heat than conventional gasoline, meaning the air around the flame of hydrogen is not as hot as around a gasoline flame. Therefore, the risk of hydrogen secondary fires is lower. Demonstrations have shown that a fuel fire in a hydrogen-powered vehicle can burn out completely with little damage to the vehicle, in contrast to the expected result in a gasoline-fueled vehicle. Gasoline has been the preferred fuel for cars for over a hundred years, despite the considerable number of accidents where gasoline fires take the lives of people. We are accustomed to the risks involved in gasoline, while hydrogen has undeservedly been given a bad name.
______
Hydrogen combustion:
Hydrogen burns in a non-luminous, almost invisible pale blue, hot flame to water vapour liberating the chemically bound energy as heat (gross heat of combustion). The flammability range of hydrogen (at room temperature) is between 4 and 75 % vol. in air, whereas the maximum flame temperature of a burning (premixed stoichiometric) hydrogen-air mixture is 2403 K.
The auto-ignition temperature for hydrogen, which is the minimum temperature of a hot surface that can ignite a flammable mixture, is 858 K. It is relatively high, but can be lowered by catalytic surfaces. Hydrogen gas does not have a flash point as it is already a gas at ambient conditions. Therefore, cryogenic hydrogen will flash at all temperatures above its boiling point of 20 K.
For a given combustible mixture and ignition type, there is a minimum energy below which ignition does not occur (minimum ignition energy). The minimum ignition energy varies with composition and has a minimum value where the mixture is nearer to stoichiometry. Over the flammable range of hydrogen-air mixtures the minimum ignition energy varies by almost three orders of magnitude and can be as low as 0.017 mJ, a value much lower than that of hydrocarbon-air mixtures.
The burning velocity of hydrogen in air at stoichiometric ambient conditions is 2.55 m/s reaching a maximum of 3.2 m/s at a concentration of 40.1%, which would even increase to 11.75 m/s in pure oxygen. These values are higher than the ones of hydrocarbon fuel-air mixtures due to the fast chemical kinetics and high diffusivity of hydrogen. The detonability is usually in the range of 18% to 59% of hydrogen concentration in air by volume.
_____
Hydrogen safety covers the safe production, handling and use of hydrogen – particularly hydrogen gas fuel and liquid hydrogen. The main concern in working with hydrogen is flammability.
Figure above shows fire diamond hazard sign for both elemental hydrogen gas and its isotope deuterium.
Hydrogen possesses the NFPA 704’s highest rating of 4 on the flammability scale because it is flammable when mixed even in small amounts with ordinary air; ignition can occur at a volumetric ratio of hydrogen to air as low as 4% due to the oxygen in the air and the simplicity and chemical properties of the reaction. However, hydrogen has no rating for innate hazard for reactivity or toxicity. The storage and use of hydrogen poses unique challenges due to its ease of leaking as a gaseous fuel, low-energy ignition, wide range of combustible fuel-air mixtures, buoyancy, and its ability to embrittle metals that must be accounted for to ensure safe operation. Liquid hydrogen poses additional challenges due to its increased density and the extremely low temperatures needed to keep it in liquid form.
_
Leaks:
Hydrogen is odorless, colorless and tasteless, so most human senses won’t help to detect a leak. By comparison, natural gas is also odorless, colorless and tasteless, but industry adds a sulfur-containing odorant called a mercaptan to make it detectable by people. Currently, all known odorants contaminate fuel cells (a popular application for hydrogen). However, given hydrogen’s tendency to rise quickly, a hydrogen leak indoors would briefly collect on the ceiling and eventually move towards the corners and away from places where people might be exposed to it. For that and other reasons, industry often uses hydrogen sensors to help detect hydrogen leaks and has maintained a high safety record using them for decades. Researchers are investigating other methods that might be used for hydrogen detection: tracers, new odorant technology, advanced sensors and others.
_
Flame:
Hydrogen burns with a pale blue flame that is nearly invisible in daylight. The flame may appear yellow if there are impurities in the air like dust or sodium. A pure hydrogen flame will not produce smoke. A hydrogen flame can be most easily identified by the mirage-like effect on the air over and around the flame, as it otherwise does not produce significant heat radiation. While hydrogen flames can be hard to see with the naked eye, they show up readily on UV/IR flame detectors. More recently Multi IR detectors have been developed, which have even faster detection on hydrogen-flames.
_
Liquid hydrogen:
Condensed and solidified atmospheric air, or trace air accumulated in manufacturing, contaminates liquid hydrogen, thereby forming an unstable mixture. This mixture may detonate with effects similar to those produced by trinitrotoluene (TNT) and other highly explosive materials. Liquid hydrogen requires complex storage technology such as the special thermally insulated containers and requires special handling common to all cryogenic substances. This is similar to, but more severe than liquid oxygen. Even with thermally insulated containers it is difficult to keep such a low temperature, and the hydrogen will gradually leak away. (Typically it will evaporate at a rate of 1% per day)
_______
Hydrogen gas has nothing to do with hydrogen bomb:
A fusion is an amalgamation of two light atom nuclei to a heavier atom nucleus. The source of energy on the sun is the continual fusion of deuterium (hydrogen atom with one neutron and one proton in the nucleus) into helium. The reaction requires high pressure and temperatures of several million degrees. In a hydrogen bomb, a nuclear fusion takes place. In a hydrogen bomb lithium-6-deutride [6Li2H] is packed into a conventional atom bomb to reach a high enough temperature and pressure level. It is under no circumstance possible to come anywhere close to a fusion reaction with regular hydrogen, in a car accident or plane crash. Hydrogen bombs has very little to no relation with FCEV technology. The only real connection is the usage of the word ‘hydrogen.’ Hydrogen is a gas 14 times lighter than the air, rises at a speed of 20m/s, 6 times faster than natural gas which means that when leaked, it rises and disperses quickly. It means that hydrogen concentrations under normal pressure dissolve to incombustible levels very quickly. This also means that under ambient air pressure hydrogen has very little energy density per unit of volume compared to other vehicle fuels. In the case of the hydrogen bomb, materials are not pressurized hydrogen gas, but deuterium and tritium. Even if deuterium and tritium were present, getting the explosive power of a hydrogen bomb will require extreme heat and pressure, which is over a hundred million degrees at thousands of bars of pressure.
During the 2011 Fukushima nuclear accident, three reactor buildings were damaged by hydrogen explosions. Exposed Zircaloy cladded fuel rods became very hot and reacted with steam, releasing hydrogen. The containments were filled with inert nitrogen, which prevented hydrogen from burning in the containment. However, the hydrogen leaked from the containment into the reactor building, where it mixed with air and exploded. To prevent further explosions, vent holes were opened in the top of the remaining reactor buildings.
_____
Hindenburg disaster. May 6, 1937:
The Hindenburg airship was 245 meters long and was driven by four 1,100 horsepower Daimler-Benz diesel engines, reaching a speed of 135 km/h and ranging 14,000 km. It flew a regular route between Germany and USA for DELAG and transported over 1,000 passengers in 1936 in 10 round-trip tours over the Atlantic Ocean; it took 65 hours going east and 52 hours going west.
On the evening of May 6, 1937, the Hindenburg crashed in Lakehurst, New York. Of the 97 passengers onboard, 35 lost their lives. One person in the ground crew of 200 was killed when one of the motors fell out. Of those that died, 27 had jumped out in panic while still in the air and the other 8 died due to burn injuries from burning diesel. An investigative commission engaged by the Zeppelin company concluded that some hydrogen had leaked out from the internal tanks and was ignited by a spark.
For several years Addison Bain has carried out extensive investigations to try to find out exactly what the cause of the accident was. The conclusion, after having analysed bits of the materials used in the canvassing around the blimp, was that the Hindenburg burned because this material was extremely flammable. The fire was sparked by static electricity as the result of an error in design. The hydrogen gas used for buoyancy had no direct influence on the accident.
______
Many people fear the risk of explosion when they think of hydrogen fuel vehicles:
Though many of us think of this type of fuel as highly explosive, this is based on a common misconception. The Hindenburg is a typical example of what people consider to be the dangers associated with hydrogen, however, that disaster was caused by the fabric of the blimp and not nearly as much by the gas.
In fact, many feel that H2 can be considered to be considerably safer than cars powered by gasoline. Primarily, if there is a gasoline leak, there is a mess and a significant fire risk. In the event of a hydrogen leak, the gas simply dissipates harmlessly.
Fire-and-Impact-Proof Hydrogen Tanks:
Hydrogen fuel tanks require the highest level of safety and have to survive a strict regulatory certification, such as the UN’s global integrated standard, one of the harshest regulations in the world; the tanks have withstood permeability tests for gas leakage, fire-resistant tests in case of a vehicle fire, and impact tests for traffic accidents. The tanks that contain H2 are thick walled and carefully designed to prevent leaking, even after a substantial crash. The tanks in the Mirai, for example, are carbon fiber wrapped and can withstand a .50 caliber bullet without suffering a leak. The Nexo are made differently but can withstand the pressure of the gas up to 10,000 psi. The tanks in hydrogen fuel vehicles also feature relief devices that cause the gas to be vented in certain circumstances in order to avoid heat-induced explosions. For example, should the tanks ever be punctured, the device allows for a managed venting of the gas.
There are multiple real-time sensors that detect any leakage on the fuel tank. If a hydrogen leak is detected, either during standard operation or in an external impact that damages the feed system, the driver’s dashboard display puts up a warning. The safety system may even choke the hydrogen fuel tank valve, preventing mass ejection of hydrogen from the tank, which may lead to other hazards. Sensors throughout the vehicle also work to detect unexpected gas presence to shut everything down and bring the vehicle to a stop before anything can be permitted to ignite.
______
Hydrogen safety standards have come a long way:
Industry has been using hydrogen in rocket fuel, oil refineries, and fertilizer production for the past 40 years—more than enough time for scientists and engineers to develop and adopt robust safety protocols. Today, the Hydrogen Industry Panel on Codes, International Code Council, and National Fire Protection Association work together to develop stringent standards for hydrogen systems and fuel cells. Years of R&D and experience have made it possible to develop the appropriate engineering controls and guidelines to mitigate the risks of hydrogen’s high flammability and low ignition energy (the energy required to ignite something).
For example, because hydrogen is colorless and odorless, sensors are a requirement for hydrogen fueling stations, equipment, and facilities. Today’s technology enables remote hydrogen sensing to ensure robust detection of any hydrogen leak. Hydrogen storage tanks in fuel cell cars are also subject to rigid testing standards, such as exposure to extreme temperatures and pressures, before they can be deployed. These are just a few examples of the standards and codes that have supported a safe hydrogen industry for the last four decades.
Hydrogen collects under roofs and overhangs, where it forms an explosion hazard; any building that contains a potential source of hydrogen should have good ventilation, strong ignition suppression systems for all electric devices, and preferably be designed to have a roof that can be safely blown away from the rest of the structure in an explosion. It also enters pipes and can follow them to their destinations. Hydrogen pipes should be located above other pipes to prevent this occurrence. Hydrogen sensors allow for rapid detection of hydrogen leaks to ensure that the hydrogen can be vented and the source of the leak tracked down. As in natural gas, an odorant can be added to hydrogen sources to enable leaks to be detected by smell but odorants are not used with hydrogen because there are no known odorants light enough to “travel with” hydrogen at the same dispersion rate. Current odorants and potential odorants have negative impacts on fuel cell performance. Current hydrogen detectors are more reliable than the odorant–human detection system and should provide increased safety. However most durable hydrogen sensors are far too costly and cumbersome for automotive use. Existing sensors are too easily jostled, and their reactive metals, which include palladium and tin oxide, are ruined by contact with gases and particles that are common on the road. Research is continuing on odorant for hydrogen gas containing at least one acrylic acid C1-C6-alkyl ester and acetophenone.
In event of fire, shut off supply; if not possible and no risk to surroundings, let the fire burn itself out; in other cases, extinguish with water spray, powder, carbon dioxide.
_
To ensure that hydrogen is handled properly, the International Organization for Standardization (ISO) is developing international safety standards; the Canadian Hydrogen Installation Code (CHIC), for instance, defines the requirements applicable to the installation of hydrogen equipment while the Society of Automotive Engineers (SAE) defines standards whereby the principal emphasis is placed on the transportation industry. Several standards for hydrogen applications have also been published during the last few years. The ISO is currently developing new standards relating to hydrogen applications. Furthermore, companies that manufacture hydrogen and fuel cell products and build hydrogen fueling stations use many features that continue to be validated through safety tests. Hydrogen has been safely produced, stored, transported and used in large amounts in industrial applications.
_____
_____
Section-16
Hydrogen applications:
The world produces about 75 million tonnes of hydrogen a year. Until recently (for the past 100 years), the primary uses for hydrogen were for fertilizer production and for hydrocracking. This has changed with the advent of the hydrogen economy. People are now viewing hydrogen as a replacement for fossil fuels.
The uses of hydrogen fuel in general can be divided into categories: stationary (power plants to fuel cells), mobile (land, air, water, and space transport), backup (portable systems for any practice), and specialty (sometimes the most interesting), depending on the selected platform. Note that the categories will often cross lines because, for example, a hydrogen fuel cell can be placed in a production facility (stationary), an automobile (mobile), a backup generator (backup), or a laptop computer (specialty).
The distinction must be made between the direct use of hydrogen and the use of hydrogen in fuel cells. For example, hydrogen can be used directly as in combustion in automobiles or power plants, but it can also be used secondarily, as in fuel cells. The categories discussed above include both of these applications, sometimes in combination.
With regard to transportation, light duty fuel cell vehicles (FCEVs) are already on the market, including personal vehicles, taxis, and fleet vehicles. They compete well with conventional vehicles in mileage (250–350 miles per tank) and refueling experience. With the additional advantage of being quiet and having no emissions at the tailpipe, they are prime additions to the hydrogen economy. Captive fleet vehicles have been predicted to be “more competitive in 2030 than diesel, compressed natural gas, and biomethane-powered vehicles by a significant margin”.
Larger means of transport such as buses are being demonstrated all over Europe and in the United States, which should lead to greater numbers over the next decade. The use of hydrogen in other vehicles such as forklifts and heavy-duty trucks is increasing and the capital expenditure for hydrogen-powered forklifts is now competitive with battery-powered forklifts. Trains and light rail are becoming another use for hydrogen. Quebec actually demonstrated the first one in 2002and Japan in 2006. Hydrogen-powered boats were of interest at least fifty decades ago and are now receiving renewed interest with the US Navy in submarines, ferries, and other boats. This arena is trailing the others but is picking up momentum.
Although hydrogen has only minimally been considered with regard to flight, it is currently making inroads in the arena of drones and for use (fuel cells), while the airplane is on the ground as a means to reduce fuel consumption.
With regard to the use of hydrogen for power and fuel, it is seeing more applications in the energy sector, in particular as a storage medium after water has been electrolyzed using renewable electricity. Some organizations and nations are also combining hydrogen with other fuels to produce both power and heat.
____
When transport can be fully electrified, why do we need hydrogen?
Some forms of transport can’t be fully electrified. Airliners need to carry enough energy to take them thousands of miles, which is impossible using batteries for the foreseeable future. Liquid green or blue hydrogen, or fuels based on hydrogen, can be a solution to clean air travel. Cargo ships will struggle to cross great oceans on battery power, and again hydrogen can be the answer, in the form of ammonia, which is easy to store as a liquid, and can be used directly as fuel in slightly modified diesel engines.
On the ground, vehicles powered by hydrogen fuel cells have some advantages over battery EVs. Hydrogen is very quick to refill – in minutes, rather than hours for a battery charge. It is also far lighter than batteries, an important asset when it comes to a vehicle’s range and decreasing road damage. This makes it appealing to replace todays diesel heavy transport such as trucks and some trains, with Daimler, Volvo and Scania already looking at the technology.
____
Figure below is synopsis of current hydrogen market:
_____
Applications of hydrogen in brief are as follows:
-1. Petrochemical industry
Large quantities of H2 are used in the “upgrading” of fossil fuels. Key consumers of H2 include hydrodealkylation, hydrodesulfurization, and hydrocracking. Many of these reactions can be classified as hydrogenolysis, i.e., the cleavage of bonds to carbon. Illustrative is the separation of sulfur from liquid fossil fuels:
R-S-R + 2 H2 → H2S + 2 RH
-2. Hydrogenation
Hydrogenation, the addition of H2 to various substrates is conducted on a large scale. The hydrogenation of N2 to produce ammonia by the Haber-Bosch Process consumes a few percent of the energy budget in the entire industry. The resulting ammonia is used to supply the majority of the protein consumed by humans. Hydrogenation is used to convert unsaturated fats and oils to saturated fats and oils. The major application is the production of margarine. Methanol is produced by hydrogenation of carbon dioxide. It is similarly the source of hydrogen in the manufacture of hydrochloric acid. H2 is also used as a reducing agent for the conversion of some ores to the metals.
-3. Coolant
Hydrogen is commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules. These include low density, low viscosity, and the highest specific heat and thermal conductivity of all gases.
-4. Energy carrier
Hydrogen is not an energy resource as a combustion fuel because there is no naturally occurring source of hydrogen in useful quantities. The Sun’s energy comes from nuclear fusion of hydrogen, but this process is difficult to achieve controllably on Earth. Elemental hydrogen from solar, biological, or electrical sources requires more energy to make than is obtained by burning it, so in these cases hydrogen functions as an energy carrier, like a battery. Hydrogen may be obtained from fossil sources (such as methane), but these sources are unsustainable.
The energy density per unit volume of both liquid hydrogen and compressed hydrogen gas at any practicable pressure is significantly less than that of traditional fuel sources, although the energy density per unit fuel mass is higher. Nevertheless, elemental hydrogen has been widely discussed in the context of energy, as a possible future carrier of energy on an economy-wide scale. For example, CO2 sequestration followed by carbon capture and storage could be conducted at the point of H2 production from fossil fuels. Hydrogen used in transportation would burn relatively cleanly, with some NOx emissions, but without carbon emissions. However, the infrastructure costs associated with full conversion to a hydrogen economy would be substantial. Fuel cells can convert hydrogen and oxygen directly to electricity more efficiently than internal combustion engines.
-5. Semiconductor industry
Hydrogen is employed to saturate broken (“dangling”) bonds of amorphous silicon and amorphous carbon that helps stabilizing material properties. It is also a potential electron donor in various oxide materials, including ZnO, SnO2, CdO, MgO, ZrO2, HfO2, La2O3, Y2O3, TiO2, SrTiO3, LaAlO3, SiO2, Al2O3, ZrSiO4, HfSiO4, and SrZrO3.
-6. Rocket propellant
Liquid hydrogen and liquid oxygen together serve as cryogenic fuel in liquid-propellant rockets, as in the Space Shuttle main engines.
-7. Niche and evolving uses
Shielding gas: Hydrogen is used as a shielding gas in welding methods such as atomic hydrogen welding.
Cryogenic research: Liquid H2 is used in cryogenic research, including superconductivity studies.
Buoyant lifting: Because H2 is lighter than air, having only 7% of the density of air, it was once widely used as a lifting gas in balloons and airships.
Leak detection: Pure or mixed with nitrogen (sometimes called forming gas), hydrogen is a tracer gas for detection of minute leaks. Applications can be found in the automotive, chemical, power generation, aerospace, and telecommunications industries. Hydrogen is an authorized food additive (E 949) that allows food package leak testing, as well as having anti-oxidizing properties.
Neutron moderation: Deuterium is used in nuclear fission applications as a moderator to slow neutrons.
Nuclear fusion fuel: Deuterium is used in nuclear fusion reactions.
Isotopic labeling: Deuterium compounds have applications in chemistry and biology in studies of isotope effects on reaction rates.
Rocket propellant: NASA has investigated the use of rocket propellant made from atomic hydrogen, boron or carbon that is frozen into solid molecular hydrogen particles that are suspended in liquid helium. Upon warming, the mixture vaporizes to allow the atomic species to recombine, heating the mixture to high temperature.
Tritium uses: Tritium, produced in nuclear reactors, is used in the production of hydrogen bombs, as an isotopic label in the biosciences, and as a source of beta radiation in radioluminescent paint for instrument dials and emergency signage.
-8. Biological reactions
H2 is a product of some types of anaerobic metabolism and is produced by several microorganisms, usually via reactions catalyzed by iron- or nickel-containing enzymes called hydrogenases. These enzymes catalyze the reversible redox reaction between H2 and its component two protons and two electrons. Creation of hydrogen gas occurs in the transfer of reducing equivalents produced during pyruvate fermentation to water. The natural cycle of hydrogen production and consumption by organisms is called the hydrogen cycle.
Hydrogen is the most abundant element in the human body in terms of numbers of atoms of the element but, it is the 3rd most abundant element by mass, because hydrogen is so light. H2 occurs in the breath of humans due to the metabolic activity of hydrogenase-containing microorganisms in the large intestine. The concentration in fasted people at rest is typically less than 5 parts per million (ppm) but can be 50 ppm when people with intestinal disorders consume molecules they cannot absorb during diagnostic hydrogen breath tests. Hydrogen gas is produced by some bacteria and algae and is a natural component of flatus, as is methane, itself a hydrogen source of increasing importance.
Water splitting, in which water is decomposed into its component protons, electrons, and oxygen, occurs in the light reactions in all photosynthetic organisms. Some such organisms, including the alga Chlamydomonas reinhardtii and cyanobacteria, have evolved a second step in the dark reactions in which protons and electrons are reduced to form H2 gas by specialized hydrogenases in the chloroplast. Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to efficiently synthesize H2 gas even in the presence of oxygen. Efforts have also been undertaken with genetically modified alga in a bioreactor.
-9. Home Heating with Hydrogen
As many sectors turn to low or zero-carbon sources of fuel, home heating is one area that has lagged behind. Many countries around the world are finding a sizable portion of their carbon dioxide emissions come from residential heating, which still largely relies on fossil fuels, mostly natural gas and coal. Among many technologies addressing carbon emission, interest has turned to hydrogen as a possible method to decarbonize heating.
In the United States, residential heating and cooling contribute heavily to the country’s overall emissions. Direct CO2 emissions from commercial and residential buildings made up 12.6% of the country’s carbon emissions, amounting to over 2 billion tons of carbon dioxide released into the atmosphere each year, a 9% increase since 1990. As for the United Kingdom, about 25% or more of their emissions come from residential use. Clearly, this will be a major hurdle in the race towards net-zero emissions.
There are many advantages to hydrogen fuel that makes it an ideal substitute for fossil fuels in heating buildings. Hydrogen used for power can be created through electrolysis or steam reformation. Hydrogen produced by both electrolysis and steam reformation can be carbon-free when using renewable or zero-emission feedstocks such as wind or solar electricity or renewable natural gas.
Using electricity to run a heat pump is a far more efficient way of heating a home than using green hydrogen.
Hydrogen can also be blended with the current source of heating power, natural gas, to reduce the amount of fossil fuel burned and cut emissions. While testing and demonstration of hydrogen blending is currently underway, it is expected that hydrogen concentrations up to 20% of heating fuel supply would not require replacement or alteration of existing infrastructure or appliances. This can provide a great starting point for conversion while a state or country increases its hydrogen-producing capacity and updates its pipelines and in-home systems
_
Note:
Some applications of hydrogen are already discussed in earlier sections of Hydrogen fuel cell and Burning hydrogen.
_____
_____
Hydrogen ladder:
Michael Liebreich, a clean-energy guru has developed a “hydrogen ladder” which ranks uses from indispensable to unaffordable (see diagram above). An intriguing borderline case is afforded by domestic heating. On an efficiency basis, electrically powered heat pumps beat domestic boilers fired by hydrogen quite handily. But retrofitting urban housing already equipped with boilers to burn hydrogen may be more attractive in some places than trying to fit heat pumps on to every building. Britain is likely to be a test case for this trade-off. Its government unveiled plans for 5GW of low-carbon hydrogen production capacity by 2030 to replace natural gas in domestic and industrial applications.
Near the bottom of Mr Liebreich’s ladder are fuel-cell electric vehicles (FCEVs) used as cars. Toyota, a Japanese automobile giant, has longed to build them since the early 1990s, investing billions in the technology. Official visitors were ferried around Tokyo in such vehicles during the recent Olympic games, and the Japanese government has plans to expand the country’s fleet of FCEVs, which numbered just 3,600 in 2019, to 200,000 by 2025. The Chinese government says it wants 1m of the things by 2030.
But as Mr Liebreich and many others point out, this does not seem sensible if the competition is a battery-powered electric car. Fuel cells add to an electric car’s price and complexity while offering no benefit in performance. They are also inefficient. About four-fifths of the power fed into a battery-powered electric vehicle gets used; conversion losses mean that an FCEV is likely to manage only half that level of efficiency. A veteran Japanese utility executive whispers that Toyota’s stance makes no sense: “Millions of fuel-cell cars won’t happen. Even Honda gave up. Pride is why Toyota is sticking with it.”
That does not rule out other forms of road transport. Many of the world’s big lorry-makers, including Europe’s Volvo and Daimler, are racing against startups like Hyzon to bring hydrogen-fuelled heavy lorries to market on the basis that the weight and recharging time of batteries means they are not able to be used. According to DHL, a logistics company, when lorries with heavy loads need to travel farther than 200km (120 miles) batteries become unattractive.
______
______
Applications of hydrogen in power systems:
As hydrogen plays an important role in various applications to store and transfer energy, there are four typical applications of integrating hydrogen into power systems: energy storage, power-to-X system, fuel cell co- and tri-generation and vehicular applications.
______
-1. Energy Storage Systems (ESS):
In the literature, various types of energy storage can be used such as: compressed air energy storage (CAES), flywheel energy storage (FES), pumped hydro energy storage (PHES), battery energy storage (BES), flow battery energy storage (FBES), superconducting magnetic energy storage (SMES), super capacitor energy storage (SCES), hydrogen energy storage, synthetic fuels, and thermal energy storage (TES). ESS technologies can be used for different application depending on various characteristics such as: specific parameters such as: energy and power density, response time, cost and economies scale, lifetime, monitoring and control equipment, efficiency and operating constraint.
A suitable energy storage system should have a number of properties: (a) high gravimetric and volumetric energy and power densities, (b) easy deployment and integration with RE sources and the existing energy network, (c) high energy efficiency, (d) economic viability in storing large amount of energy, (e) extended life span and reliability of the systems and components, and (f) safe in operation. Table below presents a brief comparison between the different types of ESSs.
Comparison between the different types of ESSs in 2015:
|
Technology |
Capital cost ($/kWh) |
Durability (Year) |
Power density (W kg-1) |
Gravimetric energy density (Wh kg-1) |
Energy Efficiency (%) |
|
Li-ion battery |
600-2500 |
5-20 |
100-5000 |
75-250 |
85-90 |
|
Super-capacitor |
300-2000 |
20+ |
500-5000 |
0.05-30 |
97 |
|
Pumped-hydro |
5-100 |
40-100 |
– |
0.5-1.5 |
70-87 |
|
Hydrogen |
2-20 |
30 |
– |
400-1000 |
– |
|
Flywheel |
1000-5000 |
15-20 |
400-1600 |
5-130 |
80-99 |
|
Pressurized air |
2-50 |
20-100 |
– |
30-60 |
40-80 |
Obviously, ESSs can be employed for either power-intensive (power for relatively short periods of time) or energy-intensive (energy for relatively long periods of time) applications. Among energy storage systems, batteries are the most common choice for short-term storage. However for longer-term energy storage, their application might be inappropriate owing to their low energy storage density and unavoidable self-discharge. In this respect, it is clear that Energy storage systems based on hydrogen technologies are one of the most interesting options. In hydrogen storage systems, excess electricity can be converted to hydrogen through an electrolyser (EL) and stored in pressurized tanks. The stored hydrogen can later be used to produce electricity.
_
Renewable energy sources are experiencing a period of rapid growth, with the U.S. Energy Information Agency forecasting that they will be the fastest growing source of electricity generation in the near future. However, renewable energy sources such as solar and wind suffer from supply and demand imbalances, because their most productive periods are when electricity demand is lowest, leading to a surplus of unused energy, and they are least productive when electricity demand peaks, leading to energy shortages that must be filled by other means. To address this issue, renewables must be supplemented with other dispatchable energy sources, which can instantaneously adjust output to match shifts in energy demand. One promising option to fulfill this dispatchable energy role is hydrogen energy storage.
_
Hydrogen energy storage is a process wherein the surplus of energy created by renewables during low energy demand periods is used to power electrolysis, a process in which an electrical current is passed through a chemical solution in order to separate hydrogen. Once hydrogen is created through electrolysis it can be used in stationary fuel cells, for power generation, to provide fuel for fuel cell vehicles, injected into natural gas pipelines to reduce their carbon intensity, or even stored as a compressed gas, cryogenic liquid or wide variety of loosely-bonded hydride compounds for later use. Hydrogen created through electrolysis is showing great promise as an economic fuel choice, with data from the International Energy Agency predicting that hydrogen generated from wind will be cheaper than natural gas by 2030.
_
While other forms of energy storage such as batteries and pumped water storage facilities can fulfill the same dispatchable energy needs, both have limitations that hydrogen energy storage can overcome. Batteries suffer from storage degradation, and can only store a limited amount of energy, whereas hydrogen fuel can be stored for long periods of time, and in quantities only limited by the size of storage facilities. According to Steve Szymanski, Director of Business Development at FCHEA member Nel Hydrogen, “batteries are best suited to discharge times that are 4 hours or less… [Hydrogen energy storage] can address longer duration needs (say days or even weeks).” Although pumped water storage does not suffer from the same duration and capacity limitations of chemical batteries, it can only be used in limited geographic areas where hills or mountains are present, requires vast areas of land, and can be prohibitively expensive to build.
_
Hydrogen offers several advantages over other grid-scale storage options. Firstly, the use of hydrogen in the power or gas grid offers the opportunity to decarbonize all economic sectors. Secondly, hydrogen can store larger amounts of energy per unit volume than other large-scale energy storage options being considered: it has over 200 times the volumetric energy storage density of pumped hydro storage and 50 times that of compressed air; see figure below for a comparison of storage densities against discharge time. Thirdly, hydrogen can be used for both intra-day and inter-seasonal storage, enabling a greater degree of flexibility with day/night and seasonal variations.
Figure below shows storage technologies and power/energy characteristics:
_
Hydrogen energy storage has proven its merit beyond the lab through real-world projects. For example, in 2018 Enbridge Gas Distribution and FCHEA member Hydrogenics opened North America’s first multi-megawatt power-to-gas facility using renewably-sourced hydrogen, the 2.5 MW Markham Energy Storage Facility in Ontario, Canada. The facility is currently providing grid regulation services under contract to the Independent Electricity System Operator of Ontario.
In Europe many hydrogen energy storage projects have been created, such as the Energiepark Mainz in Germany, a project involving FCHEA member Linde, in partnership with Siemens, the Rhein Main University of Applied Sciences and the Mainzer Stadtwerke. The Energiepark uses excess wind energy to create hydrogen fuel, which is later used to generate energy when wind power cannot match demand.
Orsted, Denmark’s largest energy firm, is planning to use excess energy from its proposed North Sea wind farms to power electrolysis and create renewable hydrogen energy. The proposed wind farms would have a nameplate capacity of 700 MW and be linked directly to the grid. During periods of time where the wind farms oversupplied energy, this excess power would be used to generate hydrogen through electrolysis which would later be sold to large industrial customers.
In the United States, hydrogen energy storage has begun to show promise through ongoing tests, and promising projects. For example, SoCalGas, a natural gas provider based in Southern California, has partnered in hydrogen energy storage projects. With the National Fuel Cell Research Center at the University of California at Irvine, SoCalGas installed an electrolyzer powered by the on-campus solar electric system, which generates renewable hydrogen to be fed into the campus power plant. With the National Renewable Energy Laboratory, SoCalGas constructed a biomethanation reactor system, which uses a water electrolyzer to produce hydrogen from renewable power, through a bioreactor that converts hydrogen and carbon dioxide into methane and water.
Beyond tests, promising full-scale hydrogen energy projects have also been constructed. Mitsubishi Hitachi Power Systems and Magnum Developer are planning to develop a 1,000 MW power facility in Millard County, Utah, which will be used to store renewable hydrogen, while also deploying flow batteries and solid oxide fuel cells at the site. Xcel Energy, a large utility provider, is partnering with the National Renewable Energy Laboratory to create an 110 kW wind-to-hydrogen project which would use excess wind energy to create hydrogen to be stored for later use at the site’s hydrogen fueling station or converted back to electricity and fed to the utility grid during peak-demand hours.
As the American energy grid is becoming increasingly fueled by renewable energy sources, it should continue to embrace hydrogen energy storage as a dispatchable energy source to manage the supply and demand imbalances which will come with a renewable energy powered grid.
_
Considering the high storage capacity of hydrogen, hydrogen-based energy storage has been gaining momentum in recent years. It can satisfy energy storage needs in a large time-scale range varying from short term system frequency control to medium and long-term (seasonal) energy supply and demand balance.
Medium to long-term energy storage:
The recent years have seen rapid growth in renewable energy generation. However, the intermittent nature of some renewable energy resources makes them time and season-dependent. Therefore, the generated renewable energy needs to be stored in a reliable form, which should be tolerant to the fluctuation and randomness of those renewable energy sources. There are several existing energy storage options, e.g., pumped hydro energy storage, compressed air energy storage, batteries, etc. Compared with them, hydrogen has its advantages of high energy storage capacity, long storing period and flexibility. It can smooth out the energy volatility and uncertainty and absorb, especially, the excess renewable energy generation. It can be applied to deal with:
- Energy time shift: Hydrogen is used to equilibrate the demand and supply by storing the excess of the energy generated by renewables when the supply is larger than demand and when it is needed, the hydrogen can be used for power generation or grid injection through, for example, stationary fuel cells. In particular, the energy generated during low demand and low electricity price period tends to be stored in hydrogen to lower the energy cost and in contrary, the hydrogen is used to produce electricity during high demand and high electricity price period, gaining the most benefit. Besides, the storage duration of hydrogen is much longer than batteries, up to weeks or months, compared to hourly or weekly storage of batteries.
- Seasonal variation: Hydrogen can also be used to shift the renewable resources across the seasons due to the seasonal difference in energy production. Moreover, hydrogen storage capacity can reach up to MWh, even TWh, owing to its high energy density, while batteries tend to be used in kWh to MWh applications, i.e., one needs to expand the size of the instrument to reach a greater storage capacity.
Numerous hydrogen energy storage projects have been launched all around the world demonstrating the potential of its large industrial use. For example, DATAZERO is a project aiming at integrating renewable energy in data centers, solving the sizing, optimisation and control problems on both software and hardware levels. It has proposed an all green solution to supply the electrical loads by photovoltaics (PVs) and wind turbines and using hydrogen storage, batteries and supercapacitors to handle the limited flexibility and controllability of the IT infrastructure. Underground Sun Storage is an Austrian project that stores the energy from wind and underground solar power below ground.
Ancillary services:
Fuel cells and electrolysers can also play a role in providing ancillary services to the grid. These services mainly come in the form of flexibility, which is the main requirement to integrate renewable energy sources. Examples of ancillary services include congestion mitigation, reducing negative price occurrences, frequency regulation, voltage support and black start.
_
The scope of hydrogen energy storage (HES):
The core process is electrolysis, the conversion of electrical energy into hydrogen energy by splitting water into its constituent parts, hydrogen and oxygen. Conceptually, the electrolysis process can be thought of as establishing a new intertie with the electricity network to allow wholesale energy deliveries to other energy networks. In effect, this becomes a new electricity export option that can simultaneously support high-value electricity grid and ancillary services. The resulting hydrogen may be consumed in one of three ways:
-1. Directly as a fuel—Hydrogen can be used in near-term markets, such as for Material Handling Equipment (MHE) such as fork-lift trucks, or backup power systems (e.g., telecom towers), or in emerging markets, such as FCEVs. Hydrogen can be converted to electricity through a fuel cell or combustion engine (e.g., turbine or internal combustion engine). The oxygen byproduct of electrolysis can be used to improve conversion efficiency, such as in high efficiency hydrogen-oxygen turbines.
-2. As a feedstock—Conventional feedstock uses include hydrogen in refineries, for hydrocracking or sulfur removal, and in ammonia production. An important energy storage pathway is the biological or chemical combination of hydrogen and carbon dioxide to produce synthetic natural gas (2H2 + CO2 = CH4 +O2), which can then be injected into natural gas pipelines. When the hydrogen is produced from renewable sources, the resulting synthetic gas is referred to as a renewable gas. Hydrogen is also an important feedstock for some advanced biofuel production processes.
-3. Blended with natural gas—At relatively low concentrations, such as 2% to 10%, hydrogen may be injected into some natural gas pipeline systems with only minor modifications to supply infrastructure or end-use devices. Acceptable concentrations and required modifications are very pipeline and utility dependent.
These three general uses are indicated with reference to natural gas and electrical supply pathways in figure below.
Figure above shows processes and pathways for HES systems.
The figure generally flows from energy production on the left to end-use markets on the right. Across the top of the figure is the conventional electrical grid (solid black lines with grey shadows) with natural gas plants producing electricity, which is delivered via transmission lines to substations and then to end users through the distribution grid. Across the bottom of the figure is the conventional natural gas pipeline grid (double-lined pathways with red shadows), with natural gas production in the bottom left and gas transmission pipelines and large-scale storage (i.e., caverns) upstream of the pressure letdown stations, where the gas pressure is reduced before entering the distribution pipeline system. Interspersed between these two conventional grid systems are various hydrogen production and conversion processes and pathways (shown with blue shadows) that are proposed as a means of adding value to overall grid sustainability and resiliency. These include examples of each of the three general uses listed above, including blending hydrogen into natural gas pipelines (which results in the pathways shown with purple shadows). The figure does not include all possible processes and pathways, but it does serve as an introduction to the scope of HES systems. Additionally, a single system may be able to pursue multiple processes and pathways.
__
Lithium-Ion’s Limits:
In order for wind and solar power to fully replace fossil fuel and nuclear plants, they would need to be constructed with several times the capacity of the more dependable fossil fuel and nuclear power sources. Considerable energy storage capacity would also be required to store surplus power generated during periods of high wind and solar availability, then release the power to the grid during times when renewables are less productive.
Energy storage can be accomplished through multiple technologies, including chemical storage in fuels and batteries; thermal storage in heated or cooled materials; mechanical storage in springs, flywheels, etc.; electrical storage in capacitors; and hydrologic storage in reservoirs. Hydrologic storage is currently the most prevalent source of energy storage in the U.S,. with 1,460 conventional reservoirs where water is pumped uphill into a reservoir, followed by energy recovery, when the water is allowed to flow downhill. However, lithium-ion batteries are rapidly becoming the innovation of choice for both grid storage and electric vehicles.
Battery technology has been successfully applied to limited grid applications such as short-term (a few hours) substitution for peaking power plants (usually powered by natural gas) that are used sporadically to address unusually high power demand. However, implementation of batteries at the scale required for grid stability is not close to being economically feasible. MIT estimates $2.5 trillion in investment would be required to provide adequate battery storage to ensure U.S. grid stability.
Battery technology is also the predominant source of energy storage in electric vehicles (BEVs). BEV sales currently comprise about 2.6% of global car sales but are increasing rapidly, registering a 40% increase in 2019. However, BEV performance continues to lag conventional vehicles powered by fossil fuels in a number of aspects: cost, weight, range, charging time and performance in cold weather. Additionally, spent batteries often generate considerable waste because of limited recycling capacity. The supply chain associated with lithium ion battery production is also a concern.
Better battery technologies will certainly emerge in the years to come. However, improvements will likely be incremental because the required advances in chemistry, physics, and material science do not usually occur at the same rates as say, advances in information technology. As a recent analysis by MIT points out: “Countless breakthroughs have been announced over the last decade. Time and time again these advances have failed to translate into commercial batteries with anything like the promised improvements in cost and energy storage.”
For grid-level power, lithium-ion batteries present a long list of shortcomings:
-1. Short-term storage: Most battery-based solutions store energy from one to four hours. Longer-lasting solutions (12+ hours) are not cost-effective. Hydrogen can be held indefinitely in storage until needed. Natural gas storage can be repurposed to store hydrogen after evaluation for porosity.
-2. Limited battery life: Batteries last for a finite number of recharge cycles, and their life is shorter in cold weather use.
-3. Overheating risk: The risks are seen when operating temperatures are high, and when batteries are charged in freezing temperatures.
-4. Environmental concerns of lithium: Production of lithium batteries is energy intense with a high carbon footprint, and the disposal of such batteries is an environmental concern. Lithium at a large scale is associated with resource depletion, global warming, ecological toxicity, and human health impacts.
__
Hydrogen technology faces efficiency disadvantage in power storage race:
Converting power to hydrogen and then using the fuel to generate power has a relatively low round-trip efficiency. Round-trip efficiency is the percentage of electricity retrieved after being stored.
The technology to convert power to hydrogen and back to power has a round-trip efficiency of 18%-46%, according to data from the Massachusetts Institute of Technology and scientific journal Nature Energy. In comparison, two mature long-duration technologies, pumped-storage hydropower and compressed air energy storage, boast round-trip efficiencies of 70%-85% and 42%-67%, respectively. Flow batteries, a rechargeable fuel cell technology that is less mature, have a round-trip efficiency of 60%-80%. A typical lithium ion battery will lose only 5% of energy round-trip (95% efficiency), compared to 20-25% losses for lead-acid systems. Both lead-acid and lithium-ion technologies perform well with regards to self-discharge, with losses of around 5% of capacity per month. And pumped hydro and compressed air energy come with geographic and environmental constraints, pumped hydro requires a water reservoir, while conventional compressed air energy requires burning fossil fuels.
Achieving the economics that will drive the adoption of storage technologies at scale will require low up-front capital expenditures and ongoing operating expenses that can be recouped quickly through future revenues.
______
______
-2. Power to X:
Power-to-X describes methods for converting electrical energy into liquid or gaseous chemical energy sources through electrolysis and further synthesis processes. Using electrical current, water is split into oxygen and hydrogen – a 100% CO₂ emission-free process. Being a key technology for the energy transition, Hydrogen can be easily stored and further used or processed in many ways. Sector coupling via power-to-X has the potential to reduce primary fossil energy consumption by 50% even while power demand grows by 25%
Application of Power-to-X
Mobility:
Power-to-X produces synthetic fuels for immediate application: e-Methane, e-Methanol, e-Diesel, e-Gasoline or e-Jet fuel – ready for instant use. They can be blended gradually with fossil fuels until they fully replace fossil fuels as a primary energy source. Existing infrastructure such as gas pipelines, gas stations, or storage facilities can be used as well as existing and low-cost consumer applications, powered by e-Fuels.
Power generation:
Modern gas turbines can be operated with a mix of hydrogen and natural gas, with a hydrogen share of 5 to 100%. Hydrogen can be cached, transported in gas grids and re-electrified in gas turbines, combined cycles or fuel cell power plants.
Industry:
Large heat demand; H2 enables CO2-free metal production; Green hydrogen as feedstock for production of ammonia and other products.
_
Power-to-power (P2P):
Electricity is used to generate hydrogen via electrolysis. The hydrogen generated by this process is then stored in a pressurized tank (for small-scale applications) or an underground cavern (for grid-scale applications) or re-electrified when needed using a fuel cell (kW to MW scale) or a hydrogen gas turbine (multi-MW scale). The hydrogen produced in this case can also be used as a fuel for FCEVs in the transport sector, which is referred to as power-to-fuel.
_
Power-to-gas (P2G):
Power-to-gas is an application which usually uses electric power to produce a combustible gas. Electricity is used to generate hydrogen via electrolysis. The hydrogen generated by this process is then either injected into the gas distribution grid (mixed with natural gas or used on its own) or transformed to synthetic CH4 in a subsequent methanation step. The methanation step combines hydrogen with captured CO2 in a methanation reactor (either thermochemical or biological). The hydrogen gas produced can be stored in both natural gas pipelines and storage sites. This option is gaining growing interest, especially because it can be combined with biogas plants being used for synthetic CH4 production, which enables direct use of the CO2 from the biogas for conversion into CH4 with hydrogen from water electrolysis. This combination, with CCS added, is also interesting for the concept of negative CO2 emissions.
_
The concept of power-to-gas (P2G) is to feed electrolysers with cheap surplus renewable electricity to produce hydrogen and injecting this into the gas grid. There are two major routes, direct injection and methanation, as shown in figure below.
Figure above shows principle of power-to-gas technology.
The simplest way is to inject hydrogen directly into the natural gas grid. It possesses some advantages such as one step process, no further investment and energy lost, and no additional hydrogen storage required. However, there is research suggesting that the direct injection of significant quantities of hydrogen may have an influence on the thermodynamic and transportation properties of the natural gas as well as the natural gas pipeline and end use applications such as gas turbines and gas burners. Some potential problems include hydrogen embrittlement for high pressure steel pipelines, reduction in gas grid capacity and efficiency due to high volume of hydrogen injection, and potential safety risk for end use applications such as flashback. Although there are suggestions that at Standard Temperature and Pressure the gas grid can cope with up to 20 % of hydrogen by volume without any difficulties, there is also legislation in various countries that constrains the amount of hydrogen content in the natural gas grid.
For countries such as Italy, UK, US and Japan, indications are that by injecting an amount of 1 vol% of hydrogen into the distributed natural gas on an average annual basis, the corresponding energy consumption will exceed 10% of total wind + solar production, which demonstrates a high possibility to mitigate the production instability of large wind farms. Recent research into the investigation of direct injection of renewable hydrogen gas to Great Britain’s gas grid also confirms that significant reduction in both wind power curtailment and operating cost of the combined gas and electricity network can be achieved.
Alternatively, the hydrogen can react with CO2 to generate other useful products. One well-known methanation process, also called the Sabatier reaction, involves reaction of CO2 with H2 over a metal catalyst to produce methane. The same process can also be used to convert CO to methane.
The reactions are expressed as:
CO2 + 4H2 → CH4 + 2H2O
CO + 3H2 → CH4 + H2O
The greatest advantage of methanation of hydrogen is that the synthesized methane can be fed directly into the gas distribution network without any limitations. Although the second reactant in the methanation process CO2 can be separated from air, the related energy and economic effort is very high. Hence, the methanation plants should be located close to a CO2 source, such as fossil fuel power stations, industrial or biomass plants in order to get access to abundant of CO2 with low economic and energy effort. So such systems can also recycle CO2 emissions from existing CO2 sources.
However, the disadvantages are also obvious. Due to the requirement of the additional step in the P2G chain, further investment on methanation plant and hydrogen storage is required, and such an additional step also causes further losses in energy and efficiency. After evaluating 41 realised and 7 planned P2G plants, it was concluded that the design and sizing, control strategy and system integration of the P2G plants have great influence on their overall efficiency, reliability and economics.
The view of P2G technology is still controversial. There are supporters believe that P2G technology can solve the energy storage and grid congestion problems at the same time, but there are still researchers questioning that P2G does not seem to be an optimal storage system from economic and environmental perspective.
According to the European Power to Gas Platform, there are approximately 40 P2G demonstration projects in Europe. Germany is currently leading the way in terms of demonstrating the P2G and P2P concept at grid scale: 20 plants were reported to be in operation with 10 facilities being planned or under construction in August 2015 with a power range of 100 kWel to 6 MWel.
P2P & P2G group of technologies suffers from high cost and low efficiency. An analysis of the total energy efficiency over the whole energy conversion chain is a good starting point for comparing the different pathways. Owing to the number of transformation steps the final efficiencies are rather low for P2P and P2G, in the range 20–30%.
_____
_____
-3. Fuel cell Co- and tri-generation
To improve the efficiency and to reduce the cost, fuel cells can be used as prime movers for combined heat and power (CHP) generation or combined cold and power (CCP) generation, known as co-generation, or to be used for combined cold heat and power (CCHP) generation, known as tri-generation. The mechanism of running a tri-generation system to produce electricity and heat from renewable energy sources through electrolysers and PEMFCs.
Co-generation:
The process of using fuel cells as prime movers to produce both electricity and heat concurrently is called co-generation, in which the electricity is used to provide the electrical needs, while the released heat is used for heating applications so that the total efficiency can reach up to 95%. A typical fuel cell co-generation system is made up of a stack, a fuel processor (a reformer or an electrolyser), power electronics, heat recovery systems, thermal energy storage systems (typically a hot water storage system), electrochemical energy storage systems (accumulators or supercapacitors), control equipment and additional equipment (fans, pumps, communication devices, etc.).
Nowadays, a great number of commercial projects are launched to develop fuel cell co-generation applications. Japan is a leader country on small-scale co-generation installations driven by the ENE-FARM project (about 300,000 units in 2018), which provides electricity and heat for home use by deploying PEMFCs from 0.3 to 1 kW. As homes are supplied with liquefied petroleum gas (LPG), a reformer is used to convert the LPG into hydrogen and the residual heat can be used to heat up water. Then the PEMFC stack combine hydrogen with ambient oxygen into water and at the same time, produce electricity and heat to meet the electrical needs and to heat water for kitchen, bathroom, room heating, etc.
In Europe, micro-co-generation for residential applications is currently in commercial development. The first European project for micro-co-generation using fuel cells is the ene.field project. From 2012 to 2017, over 1000 residential micro-CHP fuel cells were installed across 10 European countries. Project PACE follows up the work of ene.field project. This project started in 2016 and ends in 2021, in which 2800 microCHP fuel cells over 10 European countries are being installed. In the ene.field project, an environmental life cycle assessment of micro-CHP fuel cell has been carried out. It concludes that in all the scenarios investigated, fuel cell co-generation produced less greenhouse gas compared to gas boilers and heat pumps. For these two projects, they have reached an electrical efficiency and overall efficiency of 60% and 95%, respectively.
Tri-generation:
Tri-generation is an extended application of co-generation, which couples a prime mover to thermally driven equipment to produce cooling. Typically, a heat pump is used to produce cold from a thermal sink, which contains two reactors, a condenser and an evaporator. The two reactors consist of an absorption/adsorption reactor and a desorption reactor. The vapour or gas extracted from the absorbent passes through the condenser where it transforms into a liquid by rejecting heat, then the refrigerant liquid passes through the evaporator at low pressure, where it absorbs heat to evaporate. Compared to the traditional distributed cold, heat and electricity, fuel cell tri-generation can lower the carbon emissions and increase energy efficiency.
_____
_____
-4. Application of hydrogen in transportation
Hydrogen-fuelled electric powertrains provide a solution for long distance driving with clean energy, while battery-powered vehicles suffer from range limitations. 3% of global vehicle sales in 2030 are expected to be hydrogen-fuelled, and this percentage could reach 36% in 2050. Several companies are developing fuel cell powertrains in terms of their quality, reliability and dependableness to accelerate their commercialisation in the vehicle market.
Other than fuel cell vehicles, fuel cell ships have been in development in recent years. The high pollution caused by ships, counting for around 2.5% of total global greenhouse gas emissions makes the shipping sector to shift to more sustainable sources of energy, i.e., hydrogen. Fuel cells are capable of powering ships sailing relatively long distances compared with those powered by batteries and meeting the auxiliary energy needs of larger ships. The same is true for fuel cell trains. Hydrogen-fuelled regional multi-unit trains have been put into operation in Europe and are expected to have even higher market share in the future, which may take place of 30% of the currently used diesel fleets.
______
______
Portable hydrogen-powered generator goes to market in Japan:
Japanese startup Scitem will begin marketing this spring a portable emergency power generation system fueled by replaceable hydrogen cartridges. The power generators are about the size of a briefcase. The hydrogen from the cartridges — which look like gas canisters — reacts with oxygen in the air to produce electricity through an internal hydrogen fuel cell. The electricity can be used to recharge smartphones and other devices through plugs or USB cables. Power generation can be maintained by replacing the hydrogen cartridges. Unlike batteries, the cartridges do not discharge electricity, making them resistant to degradation. Scitem has developed a prototype system with a 30-watt output, which is compatible with notebook computers. Scitem is still exploring pricing, although it expects to sell the system for about 500,000 yen ($4,400) upon mass production. The company plans to market generators with varying electrical outputs based on the orders. There is no engine, meaning vibration is reduced and no carbon dioxide is emitted. The technology can be used for a wide range of applications such as automated guided systems and kick scooters. The hydrogen cartridges “can be replaced like dry-cell batteries so continuous operation is possible,” said Scitem President Kan Tanaka. “There’s no need to build hydrogen stations.”
_____
Hydrogen power plant for home in Australia:
Many of us dream of living off-grid, powering our homes with solar power. In order to make this a reality, a home solar system needs a way to store the energy generated when the sun is not out. At the moment, this can be done using lithium battery systems, such as Tesla’s Powerwall. Now, Australian energy company Lavo has built an integrated hybrid hydrogen battery that combines with rooftop solar panels to keep the home lights burning.
The Lavo Green Energy Storage System is a 324 kg (714 lb) box that connects to the homes’ solar inverter and mains water, through a water purifier. The Lavo uses solar energy to electrolyse the water, splitting the oxygen and hydrogen. The oxygen is released and the hydrogen is stored in the LAVO’s patented metal hydride “sponge”.
The hydrogen gas is then converted back into electricity when it is needed, using a fuel cell. There is also a DC converter and a small, 5 kW lithium buffer battery to deliver a regulated voltage. The system includes Wi-Fi connectivity and an app that allows direct monitoring and control – it is essentially a small power plant. The system can store around 40 kilowatt-hours of electricity – enough to power the average home for two days. Anyone needing more power can run several LAVO’s in parallel.
The LAVO is not cheap – at A$34,750 it is around three times the cost of a Powerwall, but also holds around three times the energy. The company also expects each one to last around 30 years – roughly double the life expectancy of a lithium battery setup. It could also work as a solution for rural villages, to replace diesel generators, or for those cut off from the main grid by natural disasters. Lavo Chief Executive Officer Alan Yu has said that the companies’ mission is, “to try and change the way people live with energy.”
Sunny Australia uses the most rooftop-solar panels in the world, with around 29 per cent of households equipped. However, although this makes the LAVO seem like a natural fit, there are a few hurdles yet. In the event of a fire, the hydrogen gas may become combustible, and generating hydrogen by electrolysis is not as efficient as other methods. On top of this, the system’s maximum continuous power output of 5 kW could limit its use. Air conditioning is also common in Australia, and many systems draw more than 7 kW – which could be a problem when the grid connection is not active. LAVO began installing the systems in 2021 and hopes to sell 10,000 units a year by 2022.
_____
GM announces plans to make mobile power generators using hydrogen fuel cells:
General Motors announced plans to manufacture mobile power generators using its Hydrotec-branded hydrogen fuel cells. The aim is to replace polluting gas- and diesel-powered generators with zero-emission hydrogen-powered ones. GM’s Mobile Power Generator can fast-charge EVs without having to expand the grid or install permanent charge points in places where there is only a temporary need for power. GM has condensed its Hydrotec system into a “power cube” encompassing 300 individual hydrogen fuel cells. The cubes can then be deployed in a variety of applications, including mobile generators and temporary EV chargers. The company is working Renewable Innovations, a Utah-based company that will manufacture the generators.
The hydrogen-powered generators will only be sold to commercial and military customers to start out, but the automaker said it plans on offering versions for residential use in the future. GM said the ideal application would be at an outdoor concert venue, thanks to the hydrogen generator’s much lower noise profile as compared to gas-powered power sources. Another use case would involve temporary electric vehicle chargers installed at locations where demand for charging hasn’t yet resulted in a permanent charging station.
GM is planning on offering these generators in multiple sizes for a variety of uses. Each unit will put out power ranging from 60kW to 600kW, depending on size and use case.
One of the biggest challenges, though, is the dearth of hydrogen charging and refueling infrastructure. Despite the technology having been in development for decades, there are only a little more than two dozen fueling stations in California, mostly clustered around Los Angeles and the Bay Area. The East Coast is trying to get in on the action. A handful of stations are up and running, and more are in the works in New York, New Jersey, Massachusetts, Connecticut, and Rhode Island.
_____
_____
Section-17
Hydrogen fuel cell versus battery electrical versus gasoline vehicle:
An overview of the differences between gasoline/petrol cars, BEVs and hydrogen FCEVs can be seen in the table below.
|
Car type |
Gasoline/petrol |
BEV |
FCEV |
|
Charging time (full charge) |
≈ 5 min. |
Several hours |
≈ 5 min. |
|
Efficiency (well-to-wheel) |
≈ 13 % |
≈ 73 % |
≈ 22 % |
|
Range (km) |
Up to ≈ 1200 |
≈ 200-500 |
≈ 600 |
|
Pollution |
Tailpipe emissions from tailpipe. |
No tailpipe emissions and the environmental effects depend on power production in the energy system. |
No tailpipe emissions. |
|
Gravimetric energy density (MJ/kg) of fuel |
≈ 46 |
≈ 0.46 to 0.72 |
≈ 120 |
|
Consumption per 100 km |
7.04 l/100 km |
17-20 kWh/100 km |
0.84 kg/100 km |
|
Cost per km (EUR/100 km) |
9.152 |
0.238-6.02 |
6.65 |
_____
_____
FCEV and BEV comparison:
A hydrogen-powered vehicle is classed as a Hydrogen Fuel Cell Electric Vehicle (HFCEV or FCEV). Hydrogen gas is fed at high pressure to a fuel cell where electricity is produced to power the motor, with water and heat by-products. This compares to a Battery Electric Vehicle (BEV or EV) which is powered by electric motors that pull current from a rechargeable battery. The power to recharge these batteries is normally drawn from the main electricity grid and may come from a wide range of sources. There are hundreds of types of batteries and at least 16 ways to produce hydrogen. Some of the batteries and the methods of making hydrogen are greener than others. All of the methods and batteries differ in detail, so the comparison has been generalized here.
_
As of 2021, there are two models of hydrogen cars publicly available in select markets: the Toyota Mirai (2014–), which is the world’s first mass-produced dedicated fuel cell electric vehicle (FCEV), and the Hyundai Nexo (2018–). The Honda Clarity was produced from 2016 to 2021. Most companies that had been testing hydrogen cars have switched to battery electric cars; Volkswagen has expressed that the technology has no future in the automotive space, mainly because a fuel cell electric vehicle consumes about three times more energy than a battery electric car for each mile driven. As of December 2020, there were 31,225 passenger FCEVs powered with hydrogen on the world’s roads.
_
The benefits of hydrogen technology are fast refueling time (comparable to gasoline) and long driving range on a single tank. The drawbacks of hydrogen use are high carbon emissions when hydrogen is produced from natural gas, capital cost burden, low energy content per unit volume at ambient conditions, production and compression of hydrogen, the investment required in filling stations to dispense hydrogen, transportation of hydrogen to filling stations, and lack of ability to produce or dispense hydrogen at home.
_
Basics of hydrogen and lithium:
Hydrogen:
Hydrogen is the simplest and lightest element and is the most common element in the universe. Hydrogen is non-toxic, and when it reacts with oxygen releases significant amounts of energy and produces pure water: 2 H2 + O2 ⇌ 2 H2O + energy
When one mole of hydrogen molecules (two grams) combines with half a mole of oxygen molecules (16 grams) to form one mole of water molecules (18 grams), the energy given off turns out to be 286 kilo-joules, assuming that the water comes out as a gas rather than as a liquid. The energy released from this reaction can be in the form of heat (which can run a steelmaking furnace or power an engine), or electricity from a fuel cell.
Both fuel cells and battery cells exploit similar physics to convert between chemical and electrical energy, except in fuel cells energy is stored externally in a tank, whereas in battery cells energy is stored inside the battery itself.
Since oxygen is plentiful in the air around us and also free, we need only store and transport hydrogen to store and transport energy.
Hydrogen has both advantages and drawbacks. Because of hydrogen’s low boiling point at normal pressure (20 degrees Kelvin which is super cold), it is harder to store and transport than room temperature liquid fuels like gasoline. Small molecules like hydrogen tend to leak from pipe fittings and can also penetrate the surface of inexpensive metals, making them brittle. Luckily, hydrogen is light and dissipates very rapidly and alternative materials are impervious to hydrogen.
Lithium:
Lithium sits directly underneath hydrogen on the periodic table and is the lightest solid element at room temperature and pressure. Lithium is around 500 times rarer on earth than hydrogen, but around 20 times more plentiful than other elements like nickel and cobalt that are also used in some lithium-ion batteries. Large reserves of lithium exist in South America, Australia, and other parts of the world. Like hydrogen, lithium can be oxidized to release heat, but this is a disadvantage because when lithium is mixed with water, it produces hydrogen gas, which is flammable. This is why trying to put out a lithium-based battery fire with water makes things worse.
The real advantage of lithium is when it is used in battery cells. They work like fuel cells, but with internal instead of external storage of energy,
Energy per Mass:
Hydrogen stores very high amounts of chemical energy per mass — more than 100 times the electrical energy in the active parts of lithium-ion battery cells. This is why lithium-ion batteries are not practical for long-range aircraft — they simply weigh too much — and why hydrogen is a common rocket fuel. As vehicle size scales up, the 100X higher energy per mass of hydrogen gives hydrogen a much greater mass advantage in trucks, trains, ships, long-range aircraft, and spacecraft.
Adding more battery capacity to a vehicle yield diminishing marginal range. With an internal combustion engine or fuel cell, you get essentially a 1:1 increase in driving range for each additional measure of energy storage capacity. Said another way, if the car in your garage has a 15-gallon fuel tank with 400 miles of range, doubling the size of the tank would yield slightly less than 2X that range. The additional mass of the fuel and bigger tank means you can’t get an exact 100 percent increase. This nearly 1:1 increase of range with added energy storage is also true with hydrogen fuel cells. The reason battery packs don’t enjoy this same kind of scale with relation to driving range is that battery packs, compared with both fuel cells and gas/diesel engines, hold comparatively little energy. Despite the enormous efficiency advantage BEVs have over conventional vehicles lithium-ion batteries – the best batteries in high-volume production today – only store 1/100th, or 1 percent, the energy density of gasoline. Hydrogen also has very high energy storage density than lithium-ion batteries in terms of energy stored per unit weight. So lighter, smaller vehicles are better candidates for battery electric powertrains, while heavier, larger vehicles are better suited for fuel cells. But not only are BEVs best suited for lighter vehicles that require much less energy than larger/heavier vehicles, but BEVs are best suited for travel within cities, rather than between them, in part because of recharging times and in part because of this disadvantage relative to fuel cells on energy storage.
Because of hydrogen’s very high energy per mass, the fuel tank of the Toyota Mirai carries only a tiny mass — 5 kg (11 lbs) — of hydrogen fuel, yet can power the Mirai over 400 miles between refueling.
Of course, to do a fair comparison, we must also take into account the additional mass beyond the active energy storage elements of each system. For example, the 2021 Toyota Mirai FCEV weighs a few hundred pounds less than the similar size and range Tesla Model S long-range Battery Electric Vehicle (BEV). And a gasoline-powered Internal Combustion Engine (ICE) Camry weighs a few hundred pounds less than the Mirai.
Beyond hydrogen’s lower mass, the much larger mass of lithium-ion batteries needed by a long-range passenger car BEV consumes more natural resources, emits more carbon during manufacture, and is likely more costly to recycle than the equivalent equipment in an FCEV or Plug-In Hybrid Electric Vehicle (PHEV). And, because lithium-ion batteries are still improving and will be supply-limited for some time, placing many lithium-ion batteries into fewer long-range BEVs rather than many more smaller-battery FCEVs and PHEVs can result in less total carbon emissions reduction.
Hydrogen doesn’t degrade like a battery. It stays hydrogen and does not change its energy content. Batteries have a limited lifetime: they degrade and lose their capacities over time. Lead acid is the oldest and most commonly used battery technology, but it has a much shorter service life and degrades in performance quickly throughout the discharge cycle. Lithium-ion batteries keep a constant voltage level during the entire discharge cycle, so they are able to maintain consistent performance until they need recharging. Hydrogen fuel cells deliver better performance than lead acid batteries, but they do not beat lithium-ion batteries when it comes to efficiency, costs, and safety. When considering fuel cells to power your forklifts, make sure you understand all of their advantages and disadvantages.
_____
Recharge Time;
It is the refueling time where FCEVs edge ahead. Filling up a tank with hydrogen takes as much time as filling it up with petrol, thereby saving precious minutes, which can be subtracted from the overall duration of your journey. While fast charging a Tesla Model S can give you 80% power in half an hour, a regular AC charger takes up to 5 hours to fully charge an EV. Take into account that a li-ion battery can only take a limited number of fast charging cycles, hydrogen clearly comes out as the winner in terms of sheer practicality. Long-haul transport trucks cannot have heavy batteries as it will force them to reduce their cargo weight. A smaller battery would reduce the range considerably and add to the overall time required to deliver cargo.
A hydrogen tank can be recharged 10–100 times faster than lithium-ion batteries without the lifetime degradation suffered by rapidly charged lithium-ion batteries. This advantage becomes critical in larger vehicles like trucks, trains, planes, and ships, which must quickly replenish much larger reserves of energy. Because the energy content of chemical fuels is so high, the rate of energy transfer when a car’s fuel tanks are being filled with gasoline or hydrogen is 1–10 MegaWatts (MW). That’s 10–100 times the power of a 100kW level 3 DC BEV Supercharger. That is why it takes so much less time to fill up a car’s fuel tank (gasoline or hydrogen) than to fully recharge a BEV’s empty batteries.
_____
Cost:
Hydrogen fuel cells and lithium-ion batteries have similar disadvantages, namely the higher costs compared with diesel engines. The cost of lithium ion batteries however is coming down swiftly as energy density continues to improve. Bloomberg predicts that the crossover point — when electric vehicles become cheaper than their combustion engine equivalents — could be as soon as 2022. Because the development and roll-out of hydrogen vehicles has been limited it has been difficult to measure similar gains.
_
Chart above shows progress in reducing fuel cell system cost from $124/kW in 2006 to $55/kW in 2014. It also shows the $40/kW target for 2020 and an ultimate target of $30/kW.
FCVs are currently more expensive than conventional vehicles and hybrids. Nexo, for instance, is the most expensive Hyundai on sale in the U.S., with a starting price of $59,345 (starting prices for the brand’s comparably-sized Santa Fe start at $24,250). The Toyota Mirai and Honda Clarity fuel cell models have a similar MSRP in the $59,000 range. These car purchases are eligible for government rebates — in California there is a $5,000 tax rebate available.
In the US hydrogen prices are roughly at $13.99 per kg. For context the Honda Clarity on a full 5.46 kg tank, would cost $57.36 to fill, roughly $0.21 a mile. In comparison at $0.20/kWh for electricity, a Tesla Model 3 can achieve $0.05 per mile. The annual fuel costs for the Toyota Mirai, Honda Clarity Fuel Cell and Hyundai Nexo is at $4,495, which is three to four times the cost of gas-powered alternatives.
Car makers must continue to lower costs, especially for the fuel cell stack and hydrogen storage, for FCEVs to compete with conventional vehicles. Hydrogen prices also have to come down to compete with BEV or gasoline cars.
_
In January 2022, the City of Montpellier, France made the decision to cancel an order for 51 hydrogen fuel cell buses instead opting for electric ones, scrapping the 29-million-euro ($33 million USD) Montpellier Horizon Hydrogène project. In collaboration with EDF subsidiary Hynamics, the intention had been to construct a 2MW electrolyzer with hydrogen storage, powered by 2.8MW of photovoltaics, to generate 800 kg of green hydrogen per day for the bus fleet. However, reviewing the finances for the project the conclusion was reached that the operating cost of the fuel cell buses would have been 0.95 euros ($1.08) per km in comparison to 0.15 euros ($0.17) per km for battery electric. The hydrogen buses would have been six times more expensive to operate, costing 3 million euros per year compared to 500k euros per year for the battery buses.
_______
Emissions and noise:
Both technologies are exceptionally clean when it comes to tank-to-wheel emissions. Neither emit CO2 or NOx, and in the case of fuel cells, the only by-products are water vapor and warm air. When it comes to well-to-wheel emissions, the climate impact varies greatly depending on how the energy is sourced. If the electricity stored in a battery or the hydrogen used in the fuel cell, is generated from renewable sources, then the overall climate impact will be very low. This however is still a challenge today as most electricity grids around the world are still powered by coal, oil or gas despite the push for more renewables. Hydrogen today is either extracted from fossil fuels or made using electrolytic processes powered by fossil fuels which increases hydrogen’s overall climate impact. There are also many more steps in the energy life cycle process of hydrogen vehicles, compared with the direct use of electricity in battery electric vehicles.
Both technologies are very quiet compared to combustion engines and can offer the same advantages when it comes to providing low-noise transport.
_____
Range:
Hydrogen fuel cells offer greater energy density than batteries, and therefore can provide greater range to a vehicle. In fact the energy to weight ratio of a fuel cell is ten times higher than a battery. Hydrogen provides hundreds of times as much energy per kilogram, which gives a vehicle a much longer range without making it considerably heavier – a crucial impediment for BEVs which cannot extend their range without adding to the vehicle’s weight.
Simply put, li-ion batteries simply aren’t as power dense as a tank full of hydrogen. An incremental change in the size of a hydrogen tank can add to the range considerably. In comparison, any increase in the size of a li-ion battery proves to be a self-defeating concept as the extended range must also cater to the added weight, reducing overall efficiency.
The general consensus is that FCEVs are better for long-distance journeys, while BEVs are preferable for shorter runs. At present the average FCEV can outrun the average BEV by about 160km before running out of juice.
The hydrogen-powered 2020 Honda Clarity has a range of 366 miles on a full tank whereas, the electric Tesla Model 3 has a range of 322 miles for the long-range edition. When comparing other transportation methods, the gap widens significantly, for example, semi-trucks. Tesla recently announced the Tesla-Semi truck boasting a range of 300-500 miles, with a potentially substantial charge time. The hydrogen Nikola One Semi-Truck has a range of 1200 miles, and a fuelling time of 10-15 minutes, like a standard diesel. Overall in both classes of larger vehicle, hydrogen power prevails in terms of range and refuelling being competitive with diesel.
______
Energy Efficiency:
Hydrogen-based fuel cell technology has one crucial disadvantage: it is very inefficient – both in terms of efficiency and operating costs. This is also confirmed in detail by a Horváth & Partners study, comparing both types of drive for e-cars from the customer’s point of view. In its study “Automotive Industry 2035 – Forecasts for the Future”, the management consultancy recently had a detailed investigation carried out into whether battery- or hydrogen-powered e-cars will become established in the future. The study was prepared over six months, accompanied by 80 people/interview partners and financed by the management consultancy itself. So which energy storage system has the best efficiency and is the most cost-effective for powering electric cars?
Figure above shows efficiencies in comparison.
With battery-powered e-cars, only eight percent of the energy is lost during transport before the electricity is stored in the vehicle’s batteries. When the electrical energy is converted to drive the electric motor, another 18 percent is lost. Depending on the model, the battery-powered e-car thus achieves an efficiency of between 70 to 80 percent Well-to-Wheel.
In the case of the hydrogen-powered e-car, the losses are much greater: 45 percent of the energy is already lost during the production of hydrogen through electrolysis. Of this remaining 55 percent of the original energy, another 55 percent is lost when converting hydrogen into electricity within the vehicle. This means that the hydrogen-powered e-car only achieves an efficiency of between 25 to 35 percent Well-to-Wheel, depending on the model. For the sake of completeness: the efficiency is even worse with alternative fuels. The overall efficiency here is only 10 to 20 percent.
In concrete terms this means that a hydrogen car consumes two to three times more electricity for the same distance than a battery car. But we cannot afford this kind of energy waste. The scarce green electricity must be used as efficiently as possible in the future. Hydrogen would therefore be a serious mistake for passenger cars.
However, hydrogen offers very promising prospects – although not for cars. The authors of the study conclude that investments should rather focus on other areas where they make ecological and economic sense. “We believe that there is great potential if green hydrogen is pushed into applications where it can really establish itself in the long term. Above all in industry, but also in heavy-duty transport, aviation and shipping,” says Frank Klose, co-author of the study.
The conclusion is clear: in the case of the passenger car, everything speaks in favor of the battery and practically nothing speaks in favor of hydrogen. “No sustainable economy can afford to use twice the amount of renewable energy to drive with fuel cell passenger cars rather than battery-powered vehicles,” says study leader Dietmar Voggenreiter. This is also the view of customers: In Germany there are already more than 130,000 battery cars on the road – but only 507 hydrogen cars.
_____
Durability
In terms of durability, BEVs are at a disadvantage. While most BEV manufacturers offer up to 8 years or 160000km of warranty on their lithium-ion batteries, the batteries themselves can only take a limited amount of charging cycles before they start to lose their ability to retain electric charge despite being protected by thermal management systems and battery buffers (which prevent the battery from being fully charged or depleted, thereby extending its lifespan). A lithium-ion battery at the end of its life cycle offers considerably less range, and while it is replaceable, it is always an expensive proposition, far more expensive than replacing a fuel cell. Battery lifetimes are affected by local climate, overcharging, deep discharge and high charging/discharging rates; Tesla expect batteries to last 10–15 years, yet most BEVs are <5 years old so such lifetimes are unproven. In contrast to batteries, hydrogen tanks can undergo fast refilling and frequent, deep discharging without compromising lifetime, and fuel cell stacks are expected to outlive other drivetrain components. A fuel cell has an estimated life span of 5000 hours, or 240000km, giving it the upper hand. However, research has proven that short-distance driving puts severe stress on a fuel cell’s membrane and that is what reduces its lifespan. Continuous driving, wherein a fuel cell isn’t wetted and dried constantly, would allow a fuel cell to last almost 8 times as long as it does on average. Therefore it’s far more suited to long-distance journeys where it isn’t required to make frequent pit stops.
_____
Safety
The dangers of hydrogen-powered cars remain largely theoretical. Hydrogen has been transported for industrial use for decades, and there have been no notable incidents with the major FCEVs on road. The storage and transportation of hydrogen, along with the refuelling process does pose certain risks. Given that compressed hydrogen poses a greater risk than a lithium-ion battery, a BEV is a comparatively safer option.
______
Infrastructure:
The development of lithium-ion batteries has progressed far further than hydrogen fuel-cells. The technology is already being used and has proven to be commercially viable, particularly in urban transport operations. There are large-scale manufacturers, like Volvo Trucks, and suppliers of all the necessary components, and scaling up production would be relatively simple compared to hydrogen vehicles. The infrastructure for electric vehicles is also growing steadily. In 2021/22, there are about 25,000 hydrogen fuel-cell cars on the road, two FCEV models available to purchase (Toyota Mirai and Hyundai Nexo), and about 540 hydrogen filling station in operation around the world. In contrast, there are likely to be about 15 million battery electric and plug-in hybrid vehicles on the road across the world. Almost all manufacturers now sell such vehicles, with more than 350 models available globally. And while most battery electric vehicle (BEV) drivers currently charge at home, there are about 1.3 million public charging points in operation — a quarter of which are fast-chargers (at least 22kW) — and that more than 1,000 public chargers of up to 300kW are now available in Europe. In fact, with the exception of Japan and Germany, most countries are yet to build a proper network of hydrogen stations. A direct consequence of this is that there are very few passenger car FCEVs being manufactured (Toyota and Hyundai are the only key players) and even fewer infrastructure companies across the world willing to invest in the transport and the setting-up of hydrogen refuelling stations. It’s a chicken-and-egg problem that can be solved partially through government policy.
______
FCEV vs. BEV Final Conclusion:
With a BEV, once the electricity is generated – hopefully from a renewable source – the supply of this to your vehicle charging location loses about 5%. The charging and discharging of the battery then lose another 10%. Finally, the motor wastes another 5% driving the vehicle. That makes for a total loss of 20%. With a hydrogen fuel cell, however, you first have to convert the electricity to hydrogen via electrolysis, which is only 75% efficient. Then the gas has to be compressed, chilled and transported, which loses another 10%. The fuel cell process of converting hydrogen back to electricity is only 60% efficient, after which you have the same 5% loss from driving the vehicle motor as for a BEV. The grand total is a 62% loss – more than three times as much. Or, to put it another way, for every kW of electricity supply, you get 800W for a BEV, but only 300W for an FCV – less than half as much. That’s a huge inefficiency if you’re hoping for a greener future, and doesn’t even take into account the fact that 95% of hydrogen is currently generated from fossil fuel sources.
Nevertheless, hydrogen still has niches where its main strengths – lightness and quick refuelling – give it a clear advantage. While you can fit your personal driving lifestyle around strategic battery charging stops, this is not ideal for a commercial vehicle that needs to run for very long periods and distances with only short waits to refuel. The weight of batteries for eight hours of continual usage would also be prohibitive in a train, for example. So, for industrial vehicles, hydrogen seems like a viable option, despite the inefficiency.
But for personal car users, it’s no contest. Hydrogen evangelists are still arguing that FCEVs are the future of personal transport and the technology will take off in near future. It’s likely that FCEV energy supply-chain efficiency will be improved over time and more renewable energy sources used in hydrogen production. However, considering the number of BEVs already on the road, FCEVs have lost this battle already and will never catch up. A BEV is a viable form of personal transportation right now in most developed Western nations. There are lots of options with over 200 miles of range, and Tesla has even hit 400 miles. There are charging points springing up all the time, with more than twice as many EV charging points in the UK as petrol stations. The battle for the future of green personal transportation is over, and battery electric vehicles have already won.
_
The conclusion is clear: fuel cell e-cars have many advantages (range, fast refueling, no heavy battery on board), but one decisive disadvantage: it is comparatively inefficient – both in terms of efficiency and cost. No sustainable economy can afford to use twice as much renewable energy to drive fuel cell cars instead of battery-powered vehicles. Hydrogen could only be used in niches, in trucks and buses, and over long distances. Battery weight, range and fueling time play a decisive role here.
Lithium, when used in lithium-ion batteries, has high energy efficiency and uses existing charging infrastructure, but has low energy per mass and limited charging rate, making it impractical for large vehicles. By contrast, Hydrogen, as used in hydrogen fuel cells and engines, has high energy per mass and a high charging rate, but lower energy efficiency and needs new charging infrastructure. In contrast to lithium-ion batteries, hydrogen particularly excels in large vehicles. Each approach has its benefits and its drawbacks, and each is strong where the other is weak:
_
What is clear is that hydrogen-powered e-cars will increasingly become more expensive to drive than battery-powered vehicles, not only in terms of purchase, but above also in terms of operation. The double primary energy requirement of hydrogen-powered vehicles compared to battery-powered vehicles will be reflected in consumer prices. Drivers are already paying around nine to twelve euros per 100 kilometers for hydrogen-powered cars, but only two to seven euros per 100 kilometers (depending on the electricity prices in the individual countries) for battery-powered e-cars, depending on varying individual mobility habits.
_
While hydrogen may have a part to play in the world of energy storage (especially seasonal storage), it looks like a dead end when it comes to mainstream vehicles. Many of the ongoing investments in hydrogen cars seem to follow the sunk cost fallacy: we have already spent so much on this technology, let’s not give up now. A hydrogen car is one of the least efficient, most expensive ways to reduce greenhouse gases. No matter how excellent you make the cars themselves, the laws of physics hinder their overall efficiency. The most efficient way to convert energy to mobility is electricity. Pure hydrogen can be industrially derived, but it takes energy. If that energy does not come from renewable sources, then fuel-cell cars are not as clean as they seem. Another challenge is the lack of infrastructure. Gas stations need to invest in the ability to refuel hydrogen tanks before FCEVs become practical, and it’s unlikely many will do that while there are so few customers on the road today. Compounding the lack of infrastructure is the high cost of the technology. Fuel cells are still very, very expensive. Fuel cell vehicles still had not overcome the high cost of the vehicles, high fueling cost, and lack of fuel-delivery infrastructure. It would take several miracles to overcome all of those problems simultaneously in the coming decades. Renewable energy cannot economically be used to make hydrogen for an FCEV fleet. Even assuming local hydrogen production, investing in all-electric battery vehicles is a more economical choice for reducing carbon dioxide emissions, primarily due to their lower cost and significantly higher energy efficiency.
__
Hydrogen unlikely to play major role in road transport, even for heavy trucks:
Fuel-cell vehicles have lost their one-time advantages of range and fast-charging, and are likely to remain uncompetitive with battery EVs. When battery electric vehicles had limited range of under 150km, and charging took a few hours, there was an important and large market segment for fuel-cell vehicles: long-distance travel. The higher energy density of compressed hydrogen, compared with battery electric vehicles, and the ability to refuel within only a few minutes, made fuel-cell vehicles potentially ideal for frequent long-distance trips. But battery electric vehicles now offer about 400km real-world range and the newest generation use 800V batteries, which can be charged for a range of 200km in about 15 minutes.
The current challenge for battery electric vehicles is long-haul logistic operation (with an average of 100,000 km per year) and transport of very heavy goods (which implies high energy consumption per kilometre). This is the use case often discussed for hydrogen trucks. Several truck manufacturers, as well as fuel-cell and infrastructure providers, have joined forces and announced a target of 100,000 fuel-cell trucks on European roads by 2030. But this seems very unlikely when contrasted with announcements from the companies about the earliest start date for the production of commercial series fuel-cell electric trucks being in 2027. By that time, the second-generation battery electric vehicles will already be commercially available and in operation. While long-haul trucking of more than 500km per day “poses a challenge” for battery-electric options, European regulations mean truck drivers are required to stop for a 45-minute break after driving for more than four-and-a-half hours. Within 4.5 hours, a heavy truck could travel up to around 400km and thus practical [battery] ranges of about 450km would suffice, if high-power fast charging for battery electric trucks was widely available. Charging 400km in 45 min for a heavy truck means about 800kW average charging power. The current fast-charging standard… allows up to 350kW. But a new megawatt charging system standard is under development, which should allow over 2MW charging; specifications are expected for the end of 2022, with a final standard in 2023. Truck manufacturers are pushing for the construction of a megawatt charger network in Europe and potential locations for fast chargers have been proposed. Studies suggested than the total ownership costs for fuel-cell trucks would be higher than for battery-powered models with megawatt charging. For trucks, operating costs are more important than for cars, making the use case for fuel cell electric trucks even smaller. Nevertheless, H2 trucks could still have a practical advantage for “really heavy transport in remote areas but the question remains: are such niche areas large enough to sustain the commercialization and the economies of scale required to produce fuel cell electric trucks and their infrastructure? Depending on the specific size of the niche, biofuels or renewable synthetic fuels might be sufficient after 2030 to operate the application with carbon neutrality.
_
Hydrogen is actually pretty hard to make. Hydrogen storage is inefficient, energetically, volumetrically and with respect to weight. There is no infrastructure for distributing or even making hydrogen in large quantities. It has a horrible well-to-wheel efficiency as a result. Easy ways to get large quantities of hydrogen are not ‘cleaner’ than gasoline. While hydrogen cars are far less efficient than electric cars, the vast majority of hydrogen being produced is polluting grey hydrogen, and delivering hydrogen would require building a vast and expensive new infrastructure. The two advantages of fuel cell vehicles – longer range and fast fueling times – are rapidly being eroded by improving battery and charging technology. HFCVs require lots of supporting systems, making them much more complicated and prone to failure than combustion or electric engines. The urgency of the climate crisis means that the world should focus on accelerating the build-out of battery-powered vehicles and fast-charging infrastructure, rather than hydrogen fuel-cell cars and trucks and H2 filling stations. Hydrogen will play a vital role in industry, shipping and synthetic aviation fuels. But for road transport, we should forget hydrogen except for niches and focus on battery electric vehicles in both passenger and freight transport, Battery electric cars will always be better in every way given the speed of technological developments past, present and future.
However, FCEV fleets may be considered if:
Tractor tare weight is critical to maximizing payload;
Long distance routes over 500 miles are common;
Winter conditions are significant to operations;
Green or blue hydrogen is readily available;
Regions have incentivized hydrogen use;
Operations are in less mountainous regions.
______
______
Section-18
Hydrogen technologies and GHG emissions:
Climate change and the associated global warming affect all of us. These changes cause the melting of the glaciers and consequently the increase in sea and ocean levels. This phenomenon threatens the existence of some of the island states. The warming casing all this was brought on by the economic activity of humans, with the greatest responsibility being attributed to the ever-increasing production of greenhouse gases. The most commonly mentioned gas is the CO2, but a significant proportion is also attributed to the freely released methane CH4 with a factor of 26 times that of CO2 alone or nitric oxide N2O whose emission factor is up to 298. Of course, there are other gases with a high conversion factor compared to the CO2 equivalent. For example, sulphur hexafluoride SF6 with a factor of 22800. Road vehicles, however, produce mainly the first two gases mentioned. The goal of the whole society is to limit their production and thus stop or at least slow down the process of warming of our planet. Of course, there are several possibilities to achieve this goal. One of the ways includes the use of alternative fuels that have less or no carbon in their formula. Due to their composition their burning will produce less or no CO2, which is considered to be the main greenhouse gas.
_
Global CO2 emissions fell by 6.4% (roughly double Japan’s annual emissions) in 2020, mainly due to the impact of COVID-19. Whilst this was a significant decline, it puts the carbon reduction required to hit the Paris Agreement very much in perspective. To do this (thereby limiting global warming to 1.5°C above pre-industrial levels), carbon emissions need to decline by 7.6% year-on-year. In short, we need to reduce emissions at a faster pace than in 2020 every year for the next decade. On paper, this is extremely difficult; in practice, even harder. One solution which we hear a lot about is hydrogen. Hydrogen has the potential to power vast swathes of industrial applications, from heavily polluting metals to cement. However, based on where we are today, and the speed with which we are progressing with the renewable energy capacity needed to cleanly produce it at scale, hydrogen will remain a carbon-intensive solution for many years to come.
Hydrogen today: a carbon-intensive technology:
Hydrogen is already extensively used across industrial applications. However, less than 1% of global hydrogen production is derived from renewable energy (green hydrogen) or from fossil-fuel plants equipped with carbon-capture storage (blue hydrogen). The remaining 99% is sourced from carbon-emitting fossil fuels (e.g. grey hydrogen) that emit as much CO2 emissions as the United Kingdom and Indonesia combined, and cannot therefore be considered as low-carbon options. And even blue hydrogen should not be regarded as CO2-neutral or as clean alternative, as it is often the case. New research has just investigated its emissions and found out that it may be causing more harm than burning natural gas or coal directly for heat (vide infra).
Indeed, simply to produce all of today’s dedicated hydrogen output (75 Mt) using renewable energy (rather than fossil fuels) would require more electricity than the annual amount generated by the European Union. Considering that in 2020, 38% of EU’s electricity came from renewables, it is clear that renewable energy capacity needs to be increased vastly to produce enough energy to meet both the world’s growing electric needs and to convert water into hydrogen to power industrial (and eventually domestic) applications.
Not-so-green hydrogen:
Even with higher supplies of renewable energy, producing green hydrogen this way is not the most efficient usage of energy – and not necessarily a quick fix to the 99% of current hydrogen production that currently entails a substantial carbon footprint. This is because green hydrogen always comes with a significant energy loss. The efficiency of electrolyzers that convert water to hydrogen ranges from about 60-80%, meaning 20-40% of energy is lost in the process. Further conversion of hydrogen to other carriers (e.g. ammonia) results in further energy loss, and then it also needs to be transported. This means that 100 kWh of renewable energy usually produces somewhere between 60-70 kWh of hydrogen energy.
As renewable energy only contributes a small fraction of the world’s total energy consumption, to effectively cut emissions it makes more sense to use renewable energy directly as electricity for end uses (assuming energy storage is available), rather than losing significant amounts of it through green hydrogen production (which indirectly leading to higher fossil fuel requirements to make up for the 20-40% loss). Exceptions exist for renewable electricity generation that cannot be easily connected to grids and/or where storage of electricity is not yet possible (e.g. floating offshore wind or solar farms) – where using it to produce hydrogen does make sense.
Even though hydrogen produces only water during combustion, it should be remembered that when used in internal combustion engines, it will also produce nitrogen oxides. If hydrogen was to replace fossil fuels in internal combustion engines, after including emissions produced during its production, the impact on greenhouse gas emissions would be even more unfavourable than for fossil fuels.
______
GHG Emissions from Hydrogen Supply Pathways:
The CO2 emissions levels for different hydrogen supply pathways as well as petroleum products pathways are estimated and presented in Table below. The highest emissions are observed in the case of hydrogen production from electrolysis of water. However, the CO2 emissions can be brought down to zero by depending on renewable electricity in the electrolysis process. Similarly in the case of biomass gasification (using biomass from sustainable supplies). In terms of storage alternatives, the CO2 emission levels are significantly higher in the case of liquid storage.
Well-to-Wheel CO2 Emissions for different Fuel Pathways:
|
|
Steam Methane Reforming -natural gas |
Petroleum Coke Gasification |
Coal Gasification |
Biomass Gasification |
Petroleum Residue Gasification |
Electrolysis of water by grid electricity |
|
Hydrogen Pathways (kg CO2/kg of Hydrogen) |
||||||
|
Production – No storage – Utilization |
9.41 |
27.35 |
16.75 |
13.91 |
15.15 |
48.76 |
|
Production – Compressed/ Underground storage – Utilization |
11.37 |
29.31 |
18.71 |
15.87 |
17.11 |
50.73 |
|
Production – Liquid storage – Utilization |
18.31 |
36.25 |
25.65 |
22.81 |
24.05 |
57.66 |
|
Hydrogen Pathways (kg CO2/GJ of Hydrogen) |
||||||
|
Production – No storage – Utilization |
78.38 |
227.90 |
139.58 |
115.90 |
126.26 |
406.36 |
|
Production – Compressed/ Underground storage – Utilization |
94.73 |
244.25 |
155.93 |
132.25 |
142.61 |
422.71 |
|
Production – Liquid storage – Utilization |
152.54 |
302.07 |
213.75 |
190.07 |
200.43 |
480.53 |
|
Petroleum Products Pathways (kg CO2/GJ of fuel) |
||||||
|
Diesel Production – Utilization |
84.40 |
84.40 |
84.40 |
84.40 |
84.40 |
84.40 |
|
Petrol Production – Utilization |
86.20 |
86.20 |
86.20 |
86.20 |
86.20 |
86.20 |
Note: Both the hydrogen and petroleum products pathways do not include emissions due to fuel transportation.
From the above table, we may observe that except for SMR of natural gas related pathways, in the case of all other pathways, the CO2 emissions levels are significantly higher compared to petroleum products pathways. The story can be entirely different if the sources of electricity and biomass are renewable and sustainable. In other words, use of grid electricity either in the electrolysis process or as provider of other end-use services should never be encouraged. To make the hydrogen supply pathways environment friendly, the only alternative left is to generate and use electricity from renewable sources like hydro, wind, solar, etc.
_
Table below contains the estimates of CO2 emission levels for running any type of vehicle for a kilometer using hydrogen and petroleum products:
|
|
Steam Methane Reforming -natural gas |
Petroleum Coke Gasification |
Coal Gasification |
Biomass Gasification |
Petroleum Residue Gasification |
Electrolysis of water by grid electricity |
|
Hydrogen Pathways (kg CO2/km) |
|
|||||
|
For Bus |
|
|||||
|
Production – No storage – Utilization |
0.76 |
2.20 |
1.35 |
1.12 |
1.22 |
3.92 |
|
Production – Compressed/ Underground storage – Utilization |
0.91 |
2.36 |
1.50 |
1.28 |
1.38 |
4.08 |
|
Production – Liquid storage – Utilization |
1.47 |
2.91 |
2.06 |
1.83 |
1.93 |
4.64 |
|
Diesel Production – Utilization |
0.81 |
0.81 |
0.81 |
0.81 |
0.81 |
0.81 |
|
For Small 3-Wheeler |
|
|||||
|
Production – No storage – Utilization |
0.11 |
0.31 |
0.19 |
0.16 |
0.17 |
0.56 |
|
Production – Compressed/ Underground storage – Utilization |
0.13 |
0.33 |
0.21 |
0.18 |
0.20 |
0.58 |
|
Production – Liquid storage – Utilization |
0.21 |
0.41 |
0.29 |
0.26 |
0.27 |
0.66 |
|
Petrol Production – Utilization |
0.12 |
0.12 |
0.12 |
0.12 |
0.12 |
0.12 |
|
For Large 3-Wheeler |
|
|||||
|
Production – No storage – Utilization |
0.13 |
0.39 |
0.24 |
0.20 |
0.22 |
0.69 |
|
Production – Compressed/ Underground storage – Utilization |
0.16 |
0.42 |
0.27 |
0.23 |
0.24 |
0.72 |
|
Production – Liquid storage – Utilization |
0.26 |
0.52 |
0.37 |
0.33 |
0.34 |
0.82 |
|
Petrol Production – Utilization |
0.15 |
0.15 |
0.15 |
0.15 |
0.15 |
0.15 |
Note: CO2 emissions due to hydrogen fuel pathways can be significantly reduced (even to zero level in few cases) by using electricity produced from renewable energy sources.
All the hydrogen supply pathways are included in the analysis. As usual, except for hydrogen from SMR of natural gas pathways, other pathways of hydrogen are not comparable to diesel or petrol pathways in terms of kg of CO2 per km. The only alternative left for hydrogen pathways is to depend on renewable sources in order to environmentally out beat the petroleum products pathways in replacing them as effective transportation fuel.
The above analysis has clearly indicated that unless we use renewable sources of energy for hydrogen pathways, we cannot expect them contribute positively to abate CO2 emissions. However, the advantage of hydrogen pathways is the possibility of shifting the pollution to the locations where it is being produced from the locations where it is being used. In other words, utilization of hydrogen does not cause any pollution. This may be significant from the perspective of reducing the urban pollution related to transport.
_______
The environmental impact of hydrogen depends, most of all, on how it is produced. Current hydrogen supply relies on coal gasification and steam reforming of natural gas, rather than being generated through renewable energy because the costs of steam reforming are relatively low. This kind of hydrogen is called ‘‘grey hydrogen’’, which is massively used in the industry nowadays. However, the process generates hydrogen, as well as CO and CO2 gases. The produced CO are burned to be turned into CO2, which is the major contributor to the greenhouse gas. ‘‘Grey hydrogen’’ production emits at least 10 kg CO2 per kilo hydrogen production. ‘‘Blue hydrogen’’ is reformed from the natural gas or coal-derived gas with carbon capture and sequestration (CCS) and a 90% carbon capture rate is possible with less than 1.5 kg CO2 emitted per kilo hydrogen production. However, ‘‘blue hydrogen’’ production depends on the fossil fuel supply chain and CCS storage facilities. It reduces emissions and saves costs in the short to medium term, but it will be more expensive in the long term. The hydrogen produced electrolytically by nuclear energy is called ‘‘yellow hydrogen’’, which is zero-carbon. LucidCatalyst has reported in 2020 that the cost of hydrogen from nuclear power is 2 USD/kg, which is competitive to ‘‘grey hydrogen’’, 0.7–1.6 USD/kg without costing CO2 emissions. In the long run, producing ‘‘green hydrogen’’ using renewable electricity (e.g., solar, wind) should be promising owing to the cost reductions for electrolyser CAPEX and the increasing capacity of the renewable energy.
______
______
Vehicular emissions:
Depending on the emissions from charging and from hydrogen production, both fuel cell and battery electric vehicles emit fewer pollutants than their internal combustion engine alternatives and are the only technologies with the possibility of emitting no carbon dioxide. In the United States, the average battery electric vehicle emits 176 grams of CO2 per mile when charging from an average electricity grid. Fuel cell electric vehicles fueled by hydrogen from steam-methane reforming emit about 241 grams of CO2 per mile. Both technologies not only emit significantly less carbon than gasoline powered internal combustion engine vehicles, whose emissions hit 414 grams CO2 per mile, but they also emit fewer air pollutants like nitrogen oxides and particulate matter, which contribute to millions of premature deaths each year.
However, both fuel cell and battery electric vehicles also have the potential to emit more if we look at their life cycle emissions as opposed to their efficiency. Emissions from vehicles that use internal combustion engines have the highest lifetime emissions, ranging from 27-45 tons of CO2 equivalent, coming mainly from engine and tailpipe emissions. During their lifetime, battery electric vehicles can emit between 20-27 tons of CO2 equivalent, with a high amount of emissions from battery manufacturing. Finally, fuel cell vehicles can emit 22-35 tons of CO2 equivalent, with a high proportion coming from hydrogen storage, fuel cell components and the well-to-tank fuel cycle. Of all the vehicle technologies, fuel cell vehicles typically emit the most during the vehicle manufacturing process. (These ranges are all based on average mid-sized cars.)
What makes things tricky is that while life cycle emissions and total cost of ownership are key comparisons for understanding the long-term benefits of these technologies, life cycle emissions are hard to quantify and difficult to compare across geographies. For example, emissions from electricity production and emissions from hydrogen production vary significantly depending on the prevailing infrastructure. In some places where coal power plants provide the majority of energy to the grid, switching to battery electric vehicles may produce higher life cycle emissions than internal combustion engine vehicles. However, in most places, the grid is clean enough that battery electric vehicles can generate greater emissions reductions compared to business-as-usual scenarios and in some places, like South America where hydropower is more prominent, these reductions are even more impactful.
Advocates for battery and fuel cell electric vehicles argue that the technology can encourage more renewable energy by using the grid at off-peak hours when renewable energy makes up a larger portion of the available energy. However, when it comes to fuel cell vehicles, roughly 76% of hydrogen production comes from natural gas. Only 2% of global hydrogen production uses electrolysis – the only form of hydrogen production that has the potential to be powered by renewable energy. In addition, regardless of the production method, fuel cell vehicles require hydrogen to be stored and transported. These additional steps add to costs and associated emissions.
_____
Studies on vehicular emissions:
Study 1:
To compare the global warming emissions of hydrogen-fueled and gasoline-fueled vehicles, it is necessary to examine the emissions from each stage of the fuel lifecycle, including extraction (in the cases of petroleum and natural gas, both of which are fossil fuels), production or refining, distribution to the fueling station, and, finally, consumption of the fuel in the vehicle. The hydrogen lifecycle also includes the electricity needed to pressurize the hydrogen gas for dispensing to vehicles. The full lifecycle analyses of both vehicle types capture each stage of fuel production in order to allow an apples-to-apples emissions comparison between fuels. Emissions are typically measured in grams of carbon dioxide equivalent per mile driven (g CO2eq/mile). To make the comparison of emissions easier, we also use mpgGHG , which is the combined city/highway fuel-economy rating of a gasoline vehicle that would have global warming emissions equivalent to a fuel cell vehicle.
Comparison of Gasoline and Fuel Cell Vehicle Emissions 2004 study:
|
|
Hyundai Tucson (gasoline) |
Hyundai Tucson FCEV (hydrogen from natural gas) |
Hyundai Tucson FCEV (33% renewable California hydrogen)** |
Hyundai Tucson FCEV (46% renewable California hydrogen)*** |
|
Gasoline vehicle emissions equivalent (MPGGHG) |
25 |
38* |
54 |
63 |
|
Global warming emissions per mile (g CO2eq/mile) |
436 |
286 |
202 |
173 |
|
Emissions reduction relative to gasoline |
|
34% |
54% |
60% |
*The EPA rating for the Hyundai Tucson FCEV is 49 miles/kilogram hydrogen.
**California law (California State Senate 2006) requires a minimum of 33 percent renewable hydrogen content.
***The Air Resources Board projects renewable hydrogen content in California for 2015 will be 46% (CARB 2014).
The first commercially available hydrogen-powered FCEV, the Hyundai Tucson Fuel Cell SUV, produces substantially lower global warming emissions than the Tucson’s gasoline version. As shown in Table above, the Tucson FCEV produces 286 g CO2eq/mile if fueled by hydrogen produced from natural gas, equal to the emissions from a 38-mpg gasoline vehicle. As a point of comparison, the most efficient gasoline version of the Tucson gets 25 mpg, which results in 436 g CO2eq /mi. When using hydrogen that meets California’s 33 percent renewable hydrogen standard, the fuel cell SUV emits 202 g CO2eq/ mile—the equivalent of a 54-mpg gasoline vehicle, or less than half the global warming emissions of the SUV’s gasoline version. By the end of 2015, California is projected to produce 46 percent of its hydrogen fuel from renewable sources, which would render the Tucson FCEV’s emissions equal to that of a 63-mpg gasoline car.
__
Study 2:
Hydrogen Fuel Cell Electric Vehicle Emissions relative to gasoline vehicles:
Fuel cell electric vehicles emit only water vapor and warm air, producing no tailpipe emissions. Similar to electricity, hydrogen is an energy carrier that can be produced from various feedstocks. These feedstocks and production methods should be considered when evaluating hydrogen emissions. Argonne National Laboratory’s (ANL) report, Fuel Choices for Fuel Cell Vehicles: Well-to-Wheels Energy and Emission Impacts analyzed greenhouse gas (GHG) emissions for 10 of the most common hydrogen production and distribution pathways. ANL found that gaseous hydrogen produces fewer GHGs than liquid hydrogen in most cases. ANL also investigated hydrogen’s effects on petroleum use and found that using hydrogen as a fuel reduced petroleum use by nearly 100% regardless of fuel production pathway.
Chart below shows the percent changes in greenhouse gas emissions (relative to baseline gasoline vehicles) for hydrogen fuel cell vehicles. Hydrogen use reduces GHG emissions for all fuel pathways except when the fuel is produced by electrolysis from typical grid electricity.
Relative to gasoline vehicles (GVs) fueled by reformulated gasoline (RFG), hydrogen production for all fuel pathways creates fewer GHG emissions except when the fuel is produced by electrolysis from typical grid electricity. As the renewable content in the grid mix increases, the GHG emissions for electrolysis from that grid will be reduced as the GHG emissions come from the non-renewable portion of the grid mix.
__
Study 3:
Comparison of Well-to-Wheels Energy Use and Emissions of a Hydrogen Fuel Cell Electric Vehicle
Relative to a Conventional Gasoline-powered Internal Combustion Engine Vehicle, a 2020 study:
Figure below shows Well-to-wheels fuel cycle:
The GHG emissions associated with the H2 production and delivery/refueling pathway can be estimated using a WTW analysis. The WTW analysis can be broken down into well-to-pump (WTP) and pump-to-wheels (PTW) stages, as shown in Figure above. The WTP stage includes fuel production from the primary source of energy (feedstock) through its delivery into the vehicle’s energy storage system (fuel tank). The PTW stage includes fuel consumption during vehicle operation. The results from WTP and PTW analyses are summed to give the WTW energy use and emissions on a fuel cycle basis. Life cycle analysis (LCA) is a standardized tool for performing the WTW analysis and assessing the environmental impacts of a product “from cradle to grave,” and it has already been applied to other types of transportation fuels.
The operation of hydrogen fuel cell electric vehicles (HFCEVs) is more efficient than that of gasoline conventional internal combustion engine vehicles (ICEVs), and produces zero tailpipe pollutant emissions. However, the production, transportation, and refueling of hydrogen are more energy- and emissions-intensive compared to gasoline. A well-to-wheels (WTW) energy use and emissions analysis was conducted to compare a HFCEV (Toyota Mirai) with a gasoline conventional ICEV (Mazda 3). Two sets of specific fuel consumption data were used for each vehicle: (1) fuel consumption derived from the U.S. Environmental Protection Agency’s (EPA’s) window-sticker fuel economy figure, and (2) weight averaged fuel consumption based on physical vehicle testing with a chassis dynamometer on EPA’s five standard driving cycles. The WTW results show that a HFCEV, even fueled by hydrogen from a fossil-based production pathway (via steam methane reforming of natural gas), uses 5% to 33% less WTW fossil energy and has 15% to 45% lower WTW greenhouse gas emissions compared to a gasoline conventional ICEV.
__
Study 4:
Well-to-Wheel Emissions of FCEV versus tank-to-wheel emission of petrol/diesel car:
The recent report from IDTechEx, “Fuel Cell Electric Vehicles 2022-2042”, contains an analysis of the g CO2/km well-to-wheel emission of fuel cell and battery electric vehicles, referencing it against the tank-to-wheel emission of current combustion engine cars.
Figure above shows DTechEx Estimate of gCO2/km Emission for Passenger Car Powertrains.
Like electricity generation, the production of hydrogen (H2) has a carbon emission footprint. Low carbon green hydrogen is produced by the electrolysis of water. Electrolysis requires around 50kWh of electricity to produce 1kg of H2, so the carbon footprint of H2 produced via electrolysis is primarily tied to the carbon intensity of the electricity used. Produced using 100% renewable electricity, green H2 can provide an extremely low carbon fuel. The problem is green H2 is not yet produced in any great volume and when produced it is comparatively expensive. The vast majority (~95%) of today’s hydrogen is generated by the steam methane reforming of natural gas, a process that is cheaper but results in significant CO2 emissions. This ‘grey H2’ has an emission footprint of around 10.9 kg CO2/kgH2.
Toyota gives the new Mirai fuel consumption at 0.86 kgH2/100km, so running on grey H2 the Mirai emits around 94 g CO2/km, whilst the NEXO (1 kgH2/100km) emits around 109 g CO2/km. These figures are only a marginal improvement on the CO2 tailpipe emissions of modern combustion engines. For all those eco-minded individuals who have purchased FCEV cars, they can relax in the knowledge that their zero-emission on-road (only water and heat) is improving local air quality, but on saving the planet there is still a way to go. The conclusion is that to be truly ‘green’ fuel cell vehicles need Green Hydrogen.
__
Study 5:
Life cycle emissions:
Lithium-ion battery production for electric cars is very energy-intensive. As an example, a 100kWh battery will take around 20 tonnes of CO2 to produce. A typical battery lasts for 150,000 miles, so that equates to around 83g/km of CO2. Then, when you take into account charging over that same distance, the same battery car will deliver 124g/km of CO2 over its lifetime. In comparison, today’s hydrogen cars have life-cycle emissions that are at least as low. A recent study found a hydrogen car emits around 120g/km of CO2 over its lifetime. But this can be brought significantly down when hydrogen is produced from renewables. A common method of hydrogen production involves separating it from natural gas, using a process called steam methane reformation. Work is also underway to obtain hydrogen from biomass, which would significantly cut the life-cycle emissions from hydrogen to around 60g/km CO2. This is below the level that EVs will achieve, even when electricity is sourced from renewable sources, because of the environmental costs of battery production.
Life cycle analysis (LCA) estimate of CO2 emission of battery electric vehicle is already close to three times better than an equivalent gasoline car today. Electric cars – powered with the average electricity – repay their “carbon debt” from the production of the battery after slightly more than a year and save more than 30 tons of CO2 over their lifetime compared to gasoline equivalent.
______
______
Climate ground does not justify blue hydrogen:
Blue hydrogen is often touted as a low-carbon fuel for generating electricity and storing energy, powering cars, trucks and trains and heating buildings. But according to a new report by Cornell and Stanford University researchers in the US, it may be no better for the climate – and potentially a fair bit worse – than continuing to use fossil natural gas, which currently keeps 85% of UK homes warm. In the US, about half of all homes use natural gas for space and water heating.
Blue hydrogen is produced using the same reforming process that is used to create grey, brown and black hydrogen, but the CO₂ that would ordinarily be released is captured and stored underground. Carbon capture and storage equipment is expensive, raising the price of the fuel, but it at least provides for low-carbon fuel production at a lower cost than green hydrogen.
The process of making blue hydrogen also requires a lot of energy. For every unit of heat in the natural gas at the start of the process, only 70-75% of that potential heat remains in the hydrogen product. In others words, if the hydrogen is used to heat a building, you would need to use 25% more natural gas to make blue hydrogen than if it was used directly for heat.
And as reported by the US Environmental Protection Agency, methane – the primary component of natural gas and a byproduct of using it to produce blue hydrogen – is a much more potent global warming gas than CO₂ over shorter timescales. On a 100-year basis, methane has a global warming potential 28-36 times greater than CO₂, so one molecule of methane in the atmosphere has the same effect as around 30 molecules of CO₂.
While the natural gas industry has proposed capturing carbon dioxide — creating what it promotes as emissions-free, “blue” hydrogen — even that fuel still emits more across its entire supply chain than simply burning natural gas, according to the paper, published in the Energy Science & Engineering journal by researchers from Cornell and Stanford universities. “To call it a zero-emissions fuel is totally wrong,” said Robert Howarth, a biogeochemist and ecosystem scientist at Cornell and the study’s lead author. “What we found is that it’s not even a low-emissions fuel, either.”
To arrive at their conclusion, Howarth and Mark Jacobson, a professor of civil and environmental engineering at Stanford and director of its Atmosphere/Energy program, examined the life cycle greenhouse gas emissions of blue hydrogen. They accounted for both carbon dioxide emissions and the methane that leaks from wells and other equipment during natural gas production. The researchers assumed that 3.5% of the gas drilled from the ground leaks into the atmosphere, an assumption that draws on mounting research that has found that drilling for natural gas emits far more methane than previously known. They also took into account the natural gas required to power the carbon capture technology. In all, they found that the greenhouse gas footprint of blue hydrogen was more than 20% greater than burning natural gas or coal for heat. (Running the analysis at a far lower gas leak rate of 1.54% only reduced emissions slightly, and emissions from blue hydrogen still remained higher than from simply burning natural gas.)
This study has been peer reviewed — found that while blue hydrogen emitted 9-12% less carbon dioxide than gray hydrogen, it actually emitted more methane than natural gas itself. Overall, blue hydrogen’s greenhouse gas footprint was 20% larger than burning natural gas or coal for heat, and 60% greater than burning diesel oil for heat, the study found. There are also some questions around whether storing carbon after it’s captured, which usually involves injecting it into the ground, is sustainable. “Our analysis assumes that captured carbon dioxide can be stored indefinitely, an optimistic and unproven assumption. Even if true though, the use of blue hydrogen appears difficult to justify on climate grounds,” the study concludes.
Remme, from the IEA, however, said that study made some assumptions that underestimated how much greenhouse gas could be captured, and that even if blue hydrogen were not as clean as the green type, it had a place in the world’s transition away from fossil fuels. “There is a role for both blue and green hydrogen, but we have to ensure that blue hydrogen is produced with the highest environmental standards,” he said. “The technologies are already available today to avoid these emissions, and they are often also cost-effective and save money. However the new study casts doubt on the role that blue hydrogen might play in cutting greenhouse gas emissions from sectors like heating and heavy industry.
_______
_______
Hydrogen itself a greenhouse gas:
Hydrogen itself can be regarded as an indirect greenhouse gas. The proportion of the hydrogen emitted from a hydrogen energy system during production, transport or at the point of use may range from 0.2 up to 10%. Although hydrogen technologies have the potential to replace fossil fuels that generate directly man-made greenhouse gas, the inevitable emissions through the hydrogen production, compression, storage and transportation process can lead to the indirect concentration of the greenhouse gas. This is because the hydrogen can react with hydroxyl radicals and reduce their concentration, which perturbs the oxidation reactions of hydroxyl radicals and other greenhouse gases, e.g. CH4 and CO, and increases the greenhouse effects. The oxidation of hydrogen also increases the water content in the stratosphere and cools down the lower stratosphere. The low temperature may create more polar stratospheric clouds and impede the breaking-up of the polar vortex, causing larger and deeper ozone hole.
Because hydrogen reacts with tropospheric hydroxyl radicals, emissions of hydrogen to the atmosphere perturb the distributions of methane and ozone, the second and third most important greenhouse gases after carbon dioxide. Hydrogen is therefore an indirect greenhouse gas with a global warming potential GWP of 5.8 over a 100-year time horizon. A future hydrogen economy would therefore have greenhouse consequences and would not the free from climate perturbations. If a global hydrogen economy replaced the current fossil fuel-based energy system and exhibited a leakage rate of 1% then it would produce a climate impact of 0.6% of the current fossil fuel based system. If the leakage rate were 10%, then the climate impact would be 6% of the current system. Careful attention must be given to reduce to a minimum the leakage of hydrogen from the synthesis, storage and utilisation of hydrogen in a future global hydrogen economy if the full climate benefits are to be realised in comparison to fossil fuel-based energy systems.
_______
______
Section-19
Hydrogen economy:
The term “hydrogen economy” refers to the vision of using hydrogen as a low-carbon energy source – replacing, for example, gasoline as a transport fuel or natural gas as a heating fuel. Hydrogen is attractive because whether it is burned to produce heat or reacted with air in a fuel cell to produce electricity, the only byproduct is water. Advocates of this proposed system promote hydrogen as a potential fuel source.
The ‘hydrogen economy’, first coined by Prof. John Bockris during a talk he gave in 1970 at the General Motors Technical Center, has created much excitement among scientists, economists, industrialists, and heads of states/regions, including the USA and the EU and more recently Japan. However, the development and realization of such a hydrogen-based economy has been challenging due to the large-scale infrastructural investments needed. For example, in the transport sector, the development of hydrogen as a transport fuel has historically been limited by the decision of what comes first, the cars or the refuelling infrastructure—the ‘chicken-and-egg’ problem. While the potential of hydrogen has always been significant, the challenge in establishing a hydrogen economy has stifled progress. This is mostly because challenges had to be addressed simultaneously within all components of the hydrogen economy: production, storage, transportation and distribution, while strategic policy support had to be maintained.
_
The case for a hydrogen economy is growing. An increasing number of reports show that hydrogen could have a role in almost every part of the energy system—including electricity generation and transport—and energy system-level assessments reveal hydrogen to be a technically and economically viable option for decarbonizing heat. Today, building on important technical advances in hydrogen technologies, as well as several public–private partnerships (e.g. H2USA, FCH Joint Undertaking in Europe, and Japan’s partnership with Toyota), the role of hydrogen in enabling a cost-effective transition to a low-carbon energy system is being appraised with greater coordination. The International Partnerships for Hydrogen and Fuel Cells in the Economy has 18 member countries (plus the European Commission) announcing national roadmaps (e.g. Japan, the UK, India) and initiatives towards commercialization of hydrogen and fuel cell (H2FC) technologies (e.g. a memorandum of understanding signed by 45 cities and regions pledging to include H2FC technologies in their city decarbonization activities), France is declaring ‘hydrogen territories’ and the USA is examining hydrogen at scale.
_
Four realities suggest that the current hydrocarbon economy is not sustainable:
-1. The demand for energy is growing and the raw materials for the fossil fuel economy are diminishing. Oil, coal, and natural gas supplies are not replenished as it is consumed, so an alternative must be found.
-2. Most of the people who consume fossil fuels don’t live where fuels are extracted. This situation creates enormous economic motivation for the consuming nations to try to exert control over the regions that supply the fuels. For many people and governments in the world, the resulting conflicts are unacceptable.
-3. Emissions from fossil fuel usage significantly degrade air quality all over the world. The resulting carbon byproducts are substantially changing the world’s climate. For many people and governments in the world the resulting health and climate impacts are unacceptable.
-4. Third world economies are especially susceptible when developing energy systems needed to improve their economies. The fossil fuel economy puts people and nations under the undue influence of energy suppliers. This lack of economic independence is unacceptable to many businesses and governments.
_
Hydrogen has various benefits that address these concerns:
-1. The use of hydrogen greatly reduces pollution. When hydrogen is combined with oxygen in a fuel cell, energy in the form of electricity is produced. This electricity can be used to power vehicles, as a heat source and for many other uses. The advantage of using hydrogen as an energy carrier is that when it combines with oxygen the only byproducts are water and heat. No greenhouse gasses or other particulates are produced by the use of hydrogen fuel cells. If the hydrogen comes from the electrolysis of water, then hydrogen adds no greenhouse gases to the environment. There is a perfect cycle — electrolysis produces hydrogen from water, and the hydrogen recombines with oxygen to create water and power in a fuel cell.
-2. Hydrogen can be produced either centrally, and then distributed, or onsite where it will be used. Hydrogen gas can be produced from methane, gasoline, biomass, coal or water. Hydrogen can be produced locally from numerous sources. Hydrogen can be produced anywhere that you have electricity and water. People can even produce it in their homes with relatively simple technology.
-3. If hydrogen is produced from water we have a sustainable production system. Electrolysis is the method of separating water into hydrogen and oxygen. Renewable energy can be used to power electrolyzers to produce the hydrogen from water. Using renewable energy provides a sustainable system that is independent of petroleum products and is nonpolluting. Some of the renewable sources used to power electrolyzers are wind, hydro, solar and tidal energy. After the hydrogen is produced in an electrolyzer it can be used in a fuel cell to produce electricity. The by products of the fuel cell process are water and heat. If fuel cells operate at high temperatures the system can be set up as a co-generator, with the waste energy used for heating. The elimination of oil means no dependence on the Middle East and its oil reserves.
-4. Hydrogen can help to decarbonize long-haul transport, chemicals, and iron and steel and has the potential to transport renewable energy long distance and store it long term, for example from wind power or solar electricity.
_
Challenge:
The transition to a hydrogen economy will require solution of several technologically challenging problems in the areas of production, storage, utilization, and infrastructure. The key challenges of these areas are production of hydrogen from renewable resources, development of a viable storage medium for vehicular use, reducing the cost of fuel cells, and development of an appropriate infrastructure for distribution of hydrogen to the nation’s thousands of filling stations.
Drawback:
Hydrogen is an energetic fuel, frequently used as rocket fuel, but numerous technical challenges prevent the creation of a large-scale hydrogen economy. These include the difficulty of developing long-term storage, pipelines and engine equipment; a relative lack of off-the-shelf engine technology that can currently run safely on hydrogen; safety concerns regarding the high reactivity of hydrogen fuel with oxygen in ambient air; the expense of producing it by electrolysis; and a lack of efficient photochemical water splitting technology.
_____
Advantages of hydrogen
-1. Burns cleanly, releasing only water and energy.
-2. Stores more energy per unit of weight than most other fuels.
-3. Can be made from low-carbon sources.
-4. Can be used as a fuel, to transport energy from one place to another, as a form of energy storage or as a chemical feedstock.
-5. Can be used to decarbonise “hard to abate” sectors with few alternatives.
-6. Offers wider benefits for energy security, industrial strategy and air quality.
Disadvantages of hydrogen
-1. Almost all production today is from high-carbon sources.
-2. Currently expensive to produce and cost reductions are uncertain.
-3. Bulky and expensive to transport and store.
-4. Inefficient to produce, raising costs and requiring a larger energy supply overall, with even faster scaling up of clean energy production.
-5. Supply and value chains for its use are complex and need coordination.
-6. Needs new safety standards and societal acceptance.
_
The IEA says challenges include high costs, which make hydrogen uncompetitive today, with uncertainty over how costs will develop over time. It adds: Hydrogen comes with safety risks, high upfront infrastructure costs and some of the industrial dynamics of fossil fuel supply and distribution, especially when paired with CCUS [carbon capture, use and storage]. It is not yet clear how citizens will react to these aspects of hydrogen. The IEA also says there is a risk of a chicken-and-egg situation because of the complexity of hydrogen supply and value chains, which makes gradual deployment more difficult. For example, replacing fossil gas for building heat would rely on the availability of large quantities of low-carbon hydrogen and suitably upgraded infrastructure to distribute and safely burn the fuel. There is also uncertainty over government and policy support for hydrogen, though a growing list of countries are developing dedicated hydrogen strategies (see below). Low efficiency is another significant challenge, with more energy being wasted at each step in the production and use of hydrogen than for many alternatives. The IEA says: “Hydrogen-based fuels could take advantage of existing infrastructure with limited changes in the value chain, but at the expense of efficiency losses.” The Economist says hydrogen is “inescapably inefficient”, while the Energy Technology Institute’s chief engineer wrote in 2018: “A strategy to enforce comprehensive adoption of hydrogen across the economy looks grossly inefficient based on current understanding of the relevant technologies.”
_____
A “Hydrogen Economy” is projected as the ultimate solution for energy and environment. Hydrogen societies have been formed for the promotion of this goal by publications, meetings and exhibitions. Both the production and the use of hydrogen have attracted highest attention while the practical aspects of a hydrogen economy as seen in the figure below are rarely addressed.
Like any other product hydrogen must be packaged, transported, stored and transferred to bring it from production to final use. These ordinary market processes require energy. Without question, technology for a hydrogen economy exists or can be developed. In fact, enormous amounts of hydrogen are generated, handled, transported and used in the chemical industry today. But this hydrogen is a chemical substance, not an energy commodity. Hydrogen production and transportation costs are absorbed in the price of the synthesized chemicals. The cost of hydrogen remains irrelevant as long as the final products find markets. Today, the use of hydrogen is governed by economic arguments and not by energetic considerations.
But if hydrogen is used as an energy carrier, energetic arguments must also be considered. How much energy is used to make, to package, to handle, to store or to transport hydrogen? The global energy problem cannot be solved in a renewable energy environment, if the energy consumed to make and deliver hydrogen is far higher than the energy content of the delivered fuel. As discussed in earlier sections, Well-to-Wheel loss of energy from hydrogen production, store, transport and use is very high and only 30 % of the original energy is used in moving wheels in a vehicle. So road transport cannot be part of hydrogen economy as battery electric vehicles are efficient and available. Hydrogen economy is useful in using hydrogen to decarbonize economic sectors which are hard to electrify. Hydrogen may also be useful as a way to store renewable energy from intermittent sources – for example, when the wind is blowing but there is not high demand for electricity. In this context, it’s an alternative to large-scale batteries or other storage systems. The hydrogen economy is slowly developing as a small part of the low-carbon economy. The current hydrocarbon economy is becoming impractical because of increasing demand, diminishing resources and causing global warming. The hydrogen economy could act as a replacement because of its higher energy density and its smaller negative impact on the environment.
_____
Timeline of hydrogen economy:
_____
Hydrogen production at grid scale:
One of the major challenges in enabling the hydrogen economy is the scaling up of hydrogen production. A strategic approach requires hydrogen to be produced from a large range of feedstocks using power from many indigenous energy sources—providing the opportunity for countries to become energy independent.
The US Department of Energy anticipates that, while in the short term SMR will continue to be used, in the mid-term hydrogen will be produced from wind-powered electrolysis and biomass gasification, and in the long term high-temperature electrolysis and production routes based on solar energy will be used (as illustrated in figure below). High-temperature electrolysis of water/steam may provide a cost-effective production of clean hydrogen, using solid oxide electrolysers. While this process will require one-third less electricity than low-temperature electrolysis due to higher electrical efficiencies, and is predicted to enable costs comparable to hydrogen production from fossil fuels, solid oxide electrolyser performance and lifetime improvements are needed.
Figure below shows hydrogen production pathways; expected transition of large-scale hydrogen production routes.
_
Other hydrogen production options to be developed for use in the long term include photo-electrochemical (PEC) processes (which use light energy to split water into hydrogen and oxygen) and biological processes (e.g. metabolic processes using microorganisms such as microalgae, cyanobacteria, etc.). These technologies are currently at a relatively early stage of development. Hydrogen production from biomass, if scaled and coupled with CCS, can enable a net removal of CO2 from the atmosphere using biomass hydrogen production pathways coupled with CCS.
_
Hydrogen storage at grid scale:
Underground geological formations such as salt caverns, aquifers and depleted natural gas or oil reservoirs are considered as the most viable options for bulk hydrogen storage. Hydrogen is already stored in salt caverns in the UK and the USA and similar hydrogen storage projects are underway across Europe. Salt caverns typically have lower gas capacities but enable higher delivery rates (power throughput) and thus can be used for balancing diurnal variations in energy supply from renewables. Depleted oil reservoirs have high capacity but lower power (and response times) and thus are considered more suitable for seasonal storage.
Currently, salt caverns appear to be the most likely method of storing gaseous hydrogen for energy buffering, with hydrogen loss reported to be as little as 1% and no contamination issues. A typical large salt cavern field with a volume of 8 × 106 m3 would provide a hydrogen energy storage capacity of 1.3 TWh per field with storage pressures up to 120 bar. In the UK, over 30 large salt caverns (currently used for storing natural gas) are reported to exist as well as many salt bed resources, which could provide tens of GWe storage capacity to the grid. Many depleted oil wells in the North Sea could add to this capacity.
______
Outlook of hydrogen economy:
The hydrogen economy has enormous societal and technical appeal as a potential solution to the fundamental energy concerns of abundant supply and minimal environmental impact. The ultimate success of a hydrogen economy depends on how the market reacts: Does emerging hydrogen technology provide more value than today’s fossil fuels? Although the market will ultimately drive the hydrogen economy, government plays a key role in the move from fossil-fuel to hydrogen technology. The investments in R&D are large, the outcome for specific, promising approaches is uncertain, and the payoff is often beyond the market’s time horizon. Thus, early government investments in establishing goals, providing research support, and sharing risk are necessary to prime the emergence of a vibrant, market-driven hydrogen economy.
The public acceptance of hydrogen depends not only on its practical and commercial appeal, but also on its record of safety in widespread use. The special flammability, buoyancy, and permeability of hydrogen present challenges to its safe use that are different from, but not necessarily more difficult than, those of other energy carriers. Researchers are exploring a variety of issues: hydrodynamics of hydrogen–air mixtures, the combustion of hydrogen in the presence of other gases, and the embrittlement of materials by exposure to hydrogen, for example. Key to public acceptance of hydrogen is the development of safety standards and practices that are widely known and routinely used—like those for self-service gasoline stations or plug-in electrical appliances. The technical and educational components of this aspect of the hydrogen economy need careful attention.
Technical progress will come in two forms. Incremental advances of present technology provide low-risk commercial entry into the hydrogen economy. Those advances include improving the yield of natural-gas reforming to lower cost and raise efficiency; improving the strength of container materials for high-pressure storage of hydrogen gas; and tuning the design of internal combustion engines to burn hydrogen. To significantly increase the energy supply and security, and to decrease carbon emission and air pollutants, however, the hydrogen economy must go well beyond incremental advances. Hydrogen must replace fossil fuels through efficient production using solar radiation, thermochemical cycles, or bio-inspired catalysts to split water. Hydrogen must be stored and released in portable solid-state media, and fuel cells that convert hydrogen to electrical power and heat must be put into widespread use.
Achieving these technological milestones while satisfying the market discipline of competitive cost, performance, and reliability requires technical breakthroughs that come only from basic research. The interaction of hydrogen with materials encompasses many fundamental questions that can now be explored much more thoroughly than ever before using sophisticated atomic-level scanning probes, in situ structural and spectroscopic tools at x-ray, neutron, and electron scattering facilities, and powerful theory and modeling using teraflop computers. The hope is to solve mysteries that Nature has long kept hidden, such as the molecular basis of catalysis and the mechanism that allows plants to split water at room temperature using sunlight. Nanoscience provides not only new approaches to basic questions about the interaction of hydrogen with materials, but also the power to synthesize materials with custom-designed architectures. This combination of nanoscale analysis and synthesis promises to create new materials technology, such as orderly control of the electronic, ionic, and catalytic processes that regulate the three-phase percolation networks in fuel cells. Such exquisite control over materials behavior has never been so near at hand.
The international character of the hydrogen economy is sure to influence how it develops and evolves globally. Each country or region of the world has technological and political interests at stake. Cooperation among nations to leverage resources and create innovative technical and organizational approaches to the hydrogen economy is likely to significantly enhance the effectiveness of any nation that would otherwise act alone. The emphasis of the hydrogen research agenda varies with country; communication and cooperation to share research plans and results are essential.
Will the hydrogen economy succeed? Historical precedents suggest that it might. New energy sources and carriers have flourished when coupled with new energy converters. Coal became king as fuel for the steam engine to power the industrial revolution—it transformed the face of land transportation from horse and buggy to rail, and on the sea from sail to steamship. Oil fueled the internal combustion engine to provide automobiles and trucks that crisscross continents, and later the jet engine to conquer the skies. Electricity coupled with light bulbs and with rotary motors to power our homes and industries. Hydrogen has its own natural energy-conversion partner, the fuel cell. Together they interface intimately with the broad base of electrical technology already in place, and they can expand to propel cars, locomotives, and ships, power consumer electronics, and generate neighborhood heat and light. Bringing hydrogen and fuel cells to that level of impact is a fascinating challenge and opportunity for basic science, spanning chemistry, physics, biology, and materials.
It is the flexibility that hydrogen offers that makes it so potentially useful within future low-carbon energy systems. It can be produced from a wide variety of resources and can be used in a wide range of applications, such as power generation, as a transport fuel for low carbon vehicles, for the chemical industry, and for low carbon heating. Moreover, hydrogen is already used extensively in the chemical industry so industry is familiar with its production, handling and distribution on a large scale. For all these reasons, many experts see hydrogen as a key enabler of the lowest-cost low-carbon energy system. While hydrogen can help to decarbonise our energy system, however, it is important to be specific about where and when hydrogen can help. In that sense, it might be better to think about ‘hydrogen in the economy’ rather than ‘a hydrogen economy’ as such.
______
The role of digital technology in the hydrogen economy:
Digital technology will be an essential component in delivering the hydrogen economy, accelerating and de-risking innovation, de-risking adoption and enabling faster and better scale-up and optimisation of the hydrogen value chain. It will require significant investment in new infrastructure to scale this technology up, and a concerted effort by government and the private sector to support the process. But digital technology will ultimately be fundamental in overcoming many value chain obstacles, maximising commercialisation, design and supply chains, and boosting production and economics. Simply put, software technology will be a strategic asset as the industry seeks to successfully navigate the energy transition. In the case of the hydrogen economy, digital technology will be a major accelerator for driving down the cost of hydrogen, evaluating and optimising many value chain alternatives and removing constraints to safely scale the value chain.
______
______
Section-20
Hydrogen hype and hope:
On the surface, the most common element in the universe seems like the answer to every energy question. It can be produced anywhere you have electricity and water. It can generate either heat or electricity. It can be produced, stored, transported and used without toxic pollution or CO2 emissions. It carries three times as much energy per unit weight as petrol, diesel or jet fuel. It can deliver power at 60% efficiency via a fuel cell which can also run-in reverse. It can be pumped at similar transfer rates to liquid hydrocarbons. And it burns at a similar temperature to natural gas.
Sadly, hydrogen displays an equally impressive list of disadvantages. It does not occur in nature so it requires energy to separate. Its storage requires compression to 700 times atmospheric pressure, refrigeration to minus 253 degrees Celsius or combining with an organic chemical or metal hydride. It carries one quarter the energy per unit volume of natural gas, whether liquefied or as a gas at any given temperature and pressure. It can embrittle metal; it escapes through the tiniest leaks; and, yes, it really is explosive. Despite these obvious disadvantages, hydrogen holds a vice-like grip over the imaginations of techno-optimists.
_____
Hydrogen Hype:
The Hype About Hydrogen: Fact and Fiction in the Race to Save the Climate is a book by Joseph J. Romm, published in 2004 by Island Press and updated in 2005. The book has been translated into German as Der Wasserstoff-Boom. Romm is an expert on clean energy, advanced vehicles, energy security, and greenhouse gas mitigation. Over 200 publications, including Scientific American, Forbes magazine and The New York Times, have cited this book. The book was named one of the best science and technology books of 2004 by Library Journal.
The thrust of the book is that hydrogen is not economically feasible to use for transportation, nor will its use reduce global warming, because of the greenhouse gases generated during production and transportation of hydrogen, the low energy content per volume and weight of the container, the cost of the fuel cells, and the cost of the infrastructure for refueling. The author argues that a major effort to introduce hydrogen cars before 2030 would actually undermine efforts to reduce emissions of heat-trapping greenhouse gases such as carbon dioxide.
Three UC Davis scientists who also reviewed the book agreed on its basic premises, but claimed that Romm had made selective use of sources, for example, citing the highest cost estimates, adopting extremely high estimates of efficiency for advanced gasoline vehicles, and giving weight to controversial non-peer-reviewed studies.
_____
Skeptics say it’s inefficient and impractical:
While green hydrogen could be critical to decarbonize heavy industry, power ships and planes, and perhaps store energy, it is not efficient to use more broadly as an energy source, says Robert W. Howarth, professor of ecology and environmental biology at Cornell University. Howarth is one of the 22 members of the New York Climate Action Council, a group charged with developing an implementation plan for the law mandating New York’s decarbonization plan. In summer of 2020, natural gas industry stakeholders suggested using blue hydrogen in the existing natural gas pipeline infrastructure to heat homes. But Howarth and Stanford professor Mark Jacobson published a research paper in August showing that was a bad idea. “The bottom line is that blue hydrogen has huge emissions and cannot be used except at low percentages in the current gas system,” Howarth says. “It is far cheaper to instead move to electrically driven heat pumps for heating.”
Other critics say the problems with hydrogen are more fundamental. The process of producing hydrogen, compressing it, and then turning that compressed hydrogen back into electricity or mechanical energy is grossly inefficient, according to Paul Martin, a chemical process development expert and member of the Hydrogen Science Coalition. “It’s worth putting up with a lot of problems with a battery because for every one joule you put in, you get 90% of it back. That’s pretty great,” Martin says. In producing and storing hydrogen, you get only 37% of the energy back out. “So 63% of the energy is lost. And that’s best case.”
But the idea of using hydrogen as a fuel is bogus, said Martin, who calls himself a life-long environmentalist. “The people that are really behind this hydrogen push are the fossil fuel industry, because without it, what are they going to do? The fossil fuel industry without fossil fuels is basically the petroleum chemicals and materials business, which is about 25% of the current business.” Still, Martin thinks pursuing green hydrogen is important for all its other uses, like industrial processes and the Haber-Bosch process, which converts hydrogen and nitrogen to ammonia to use in fertilizer. The Haber-Bosch process is credited with massively increasing food production and helping to feed the earth’s exploding population over the last 100 years. “I don’t want people to think I’m anti-hydrogen. I think making green hydrogen is super-important,” Martin said. “But it’s also super important to use it for the right things and not dumb things.”
Hydrogen truly reveals itself as a false climate solution. First, on the carbon-capture front, we know this technology is a failed fantasy and has yet to achieve any level of commercial viability. Critically, however, the energy required to capture carbon emissions and permanently store them underground is itself astronomical. More energy means more fossil fuel burning, perpetuating the cycle of climate destruction. Second, and crucially, any renewables used to produce hydrogen are renewables that can’t be used to replace coal, oil, and gas-fired power. This means that the hydrogen plan will not only promote more fracking across landscapes: it will actively stand in the way of the just energy transition that we all need.
_______
The Earthjustice paper shows that in the U.S., some 10 million metric tons of hydrogen are produced from oil and gas annually. But with only 1% of global hydrogen production using carbon capture and storage to reduce emissions, the hydrogen industry worldwide has a bigger carbon footprint than the nation of Germany. And even when carbon capture and storage equipment is fitted to such facilities, it can only cut out 85% to 95% of a facility’s greenhouse gas emissions, and does not eliminate emissions of other harmful pollutants such as particulate matter and nitrogen oxide.
If hydrogen is to be a climate solution, Earthjustice argues, governments should focus on supporting the development of “green” hydrogen, which uses renewable energy to separate hydrogen from water. But the green hydrogen industry is still in its infancy, producing only a tiny fraction of the hydrogen needed for industry and other uses. Further, green hydrogen production requires a lot of electricity to power the electrolysis process—electricity that could otherwise be used to directly power homes, transportation and industry.
This being the case, Earthjustice recommends employing green hydrogen only for limited applications. “This report shows that green hydrogen can be a useful tool but it’s no substitute for going big on the proven solutions we have today—powering the grid with renewable energy and electrifying our buildings and transportation systems,” said Jill Tauber, Earthjustice’s vice president of litigation for climate and energy.
______
______
Does a Hydrogen Economy Make Sense? A 2006 paper by Ulf Bossel:
In this paper, fossil and nuclear energy are defined as unsustainable because the resources are finite and the waste cannot be absorbed by nature. If one accepts this definition, renewable energy harvested in a sustainable way becomes the key to a sustainable energy future. With the exception of biomass, all renewable energy is of a physical nature: heat (solar, geothermal), solar radiation (photovoltaic) and mechanical energy (wind, hydro, waves, etc.). Heat obtained from solar collectors, geothermal sources, and waste incineration may also be converted to electricity. Thus, in one vision of a sustainable future, electricity from renewable sources will become the dominant primary energy carrier replacing chemical carriers of today’s economy.
Physical energy provided by nature is best distributed as physical energy without intermediate chemical carriers, because, excepting food, people need physical energy for transport, space conditioning, fabrication processes, cooking, lighting, and communication. Hydrogen would make sense only if its production, distribution, and use are superior to the distribution of electricity by wires.
_
The following examples illustrate the nature of the challenge involved in creating a hydrogen economy.
It takes about 1 kg of hydrogen to replace 1 U.S. gal of gasoline. About 200 MJ (55 kWh) of dc electricity are needed to liberate 1 kg of hydrogen from 9 kg of water by electrolysis. Steam reforming of methane (natural gas) requires only 4.5 kg of water for each kilogram of hydrogen, but 5.5 kg of CO2 emerge from the process. One kilogram of hydrogen can also be obtained from 3 kg of coal and 9 kg of water, but 11 kg of CO2 are released and need to be sequestered. Even with most efficient fuel cell systems, at most 50% of the hydrogen HHV energy can be converted back to electricity.
The full dimensions of the challenge become apparent when these numbers are translated to a specific case. The following case study may serve to illustrate the point. About 50 jumbo jets leave Frankfurt Airport every day, each loaded with 130 tons of kerosene. If replaced on a 1 : 1 energy base by 50 tons of liquid hydrogen, the daily needs would be 2500 tons or 36000 m3 of the cryogenic liquid, enough to fill 18 Olympic-size swimming pools. Every day 22500 tons of water would have to be electrolyzed. The continuous output of eight 1-GW power plants would be required for electrolysis, liquefaction, and transport of hydrogen. If all 550 planes leaving the airport were converted to hydrogen, the entire water consumption of Frankfurt (650000 inhabitants) and the output of 25 full-size power plants would be needed to meet the hydrogen demand of air planes leaving just one airport in Germany.
For hydrogen derived from fossil hydrocarbons, the availability of water and the safe sequestration of CO2 may pose serious problems, not because of inadequate technology, but with respect to logistics, infrastructure, costs, safety, and energy consumption. To fuel the 50 jumbo jets with hydrogen, about 7500 tons of coal and 11250 tons of water are needed daily and 27500 tons of carbon dioxide must be liquefied for transport, shipped to a suitable disposal site (perhaps in the deep waters of the mid-Atlantic) and safely deposited. The significant energy needs for hydrogen liquefaction and transport are the same for any source of hydrogen. Fueling the 50 jumbo jets at Frankfurt airport is only an insignificant part of a hydrogen economy.
_
Hydrogen can be derived from water and other chemical compounds. The conversion of hydrogen to heat or power is often simplified by the popular equation hydrogen plus air yields electricity and drinking water. Also, hydrogen, the most common chemical element on the planet, is hailed as an everlasting energy source. But nature does not provide hydrogen in its elemental form. High-grade energy (electricity or heat) is needed to liberate hydrogen from its chemical source.
Economy means trade. A hydrogen economy involves all economic stages between hydrogen production and hydrogen use, i.e., between renewable electricity received to electrolyzers and useful electricity drawn from fuel cells. Between the two ends of the economic chain hydrogen has to be packaged by compression or liquefaction to become a commodity. In the transportation, hydrogen has to be produced, packaged, transported, stored, transferred to cars, then stored and transported again before it is finally admitted to fuel cells.
All these processes require energy. Compared to natural gas (methane) or liquid fuels much more energy is required for the marketing of hydrogen. This is directly related to the physical properties of hydrogen (density 0.09 kg/m3, boiling point 20.3 K). Compared to methane, the volumetric energy density of hydrogen is less than one third. Even in the liquid state, the density of hydrogen (70 kg/m3) is not much above the density of heavy duty styrofoam. Gasoline and even wood pellets carry 3.5 or 1.2 times more energy per volume than liquefied hydrogen. One cubic meter of the cold liquid holds 70 kg, the same volume of gasoline 128 kg of hydrogen. The best way to store hydrogen is in chemical combination with carbon.
_
Figure below shows useful transport energy derived from renewable electricity.
The analysis reveals that between 1.6 and 2.0 electrical energy units must be harvested from renewable sources for every energy unit of hydrogen gas sold to the user. The high energy losses may be tolerated for some niche markets, but it is unlikely that hydrogen will ever become an important energy carrier in a sustainable energy economy built on renewable sources and efficiency. Moreover, the delivered hydrogen must be converted to motion for all transport applications. IC engines convert hydrogen within 45% efficiency directly into mechanical motion, while equally efficient fuel cells systems produce dc electricity for traction motors. Further losses may occur in transmissions, etc. All in all, hardly 50% of the hydrogen energy contained in a vehicle tank is converted to motion of a car. The overall efficiency between electricity from renewable sources and wheel motion is only 20 to 25%. In comparison, over 60% of the original electricity can be used for transportation, if the energy is not converted to hydrogen, but directly used in electric vehicles. Figure above illustrates the energy flow for transportation systems based on hydrogen or electricity. The energy advantages of battery-electric cars over hydrogen-fuel-cell-electric vehicles are obvious.
_
The foregoing analysis of the parasitic energy losses within a hydrogen economy shows that a hydrogen economy is an extremely inefficient proposition for the distribution of electricity from renewable sources to useful electricity from fuel cells. Only about 25% of the power generated from wind, hydro, or sun is converted to practical use. If the original electricity had been directly supplied by wires, as much as 90% could have been put to service. This has two serious consequences to be considered in future energy strategies.
A) About four renewable power plants have to be erected to deliver the output of one plant to stationary or mobile consumers via hydrogen and fuel cells. Three of these plants generate energy to cover the parasitic losses of the hydrogen economy while only one of them is producing useful energy. Can we base our energy future on such wasteful schemes?
B) As energy losses will be charged to the customer, electricity from hydrogen fuel cells will be at least four times more expensive than electricity from the grid. Who wants to use fuel cells? Who wants to drive a hydrogen-fuel-cell car?
_
The establishment of a sustainable energy future is one of the most pressing tasks of mankind. With the exhaustion of fossil resources the energy economy will change from a chemical to an electrical base. This transition is one of physics, not one of politics. It must be based on proven technology and existing engineering experience. The transition process will take many years and should start soon. Unfortunately, politics seems to listen to the advice of visionaries and lobby groups. Many of their qualitative arguments are not based on facts and physics. A secure sustainable energy future cannot be based on hype and activism, but has to be built on solid grounds of established science and engineering. In this paper the energy needs of a hydrogen economy are quantified. Only 20%–25% of the source energy needed to synthesized hydrogen from natural compounds can be recovered for end use by efficient fuel cells. Because of the high energy losses within a hydrogen economy the synthetic energy carrier cannot compete with electricity. As the fundamental laws of physics cannot be changed by research, politics or investments, a hydrogen economy will never make sense. Fundamental laws of physics expose the weakness of a hydrogen economy. Hydrogen, the artificial energy carrier, can never compete with its own energy source, electricity, in a sustainable future.
_______
_______
An expensive fuel:
A key problem with the hydrogen economy is that pollution-free sources of hydrogen are unlikely to be practical and affordable for decades. Indeed, even the pollution-generating means of making hydrogen are currently too expensive and too inefficient to substitute for oil. Natural gas (methane, or CH4) is the source of 95 percent of U.S. hydrogen. The overall energy efficiency of the steam CH4 reforming process (the ratio of the energy in the hydrogen output to the energy in the natural gas fuel input) is about 70 percent. According to a 2002 analysis for the National Renewable Energy Laboratory by Dale Simbeck and Elaine Chang, the cost of producing and delivering hydrogen from natural gas, or producing hydrogen onsite at a local filling station, is $4 to $5 per kg (excluding fuel taxes), comparable to a gasoline price of $4 to $5 a gallon. (A kg of hydrogen contains about the same usable energy as a gallon of gasoline.) This is more than three times the current untaxed price of gasoline. Considerable R&D is being focused on efforts to reduce the cost of producing hydrogen from natural gas, but fueling a significant fraction of U.S. cars with hydrogen made from natural gas makes little sense, either economically or environmentally.
Water can be electrolyzed into hydrogen and oxygen by a process that is extremely energy-intensive. Typical commercial electrolysis units require about 50 kilowatt-hours per kg, an energy efficiency of 70 percent. The cost today of producing and delivering hydrogen from a central electrolysis plant is estimated at $7 to $9 per kg. The cost of onsite production at a local filling station is estimated at $12 per kg. Replacing one-half of U.S. ground transportation fuels in 2025 (mostly gasoline) with hydrogen from electrolysis would require about as much electricity as is sold in the United States today. From the perspective of global warming, electrolysis makes little sense for the foreseeable future. Burning a gallon of gasoline releases about 20 pounds of CO2. Producing 1 kg of hydrogen by electrolysis would generate, on average, 70 pounds of CO2. Hydrogen could be generated from renewable electricity, but that would be even more expensive and, renewable electricity has better uses for the next few decades. Biomass (plant matter) can be gasified and converted into hydrogen in a process similar to coal gasification. The cost of delivered hydrogen from gasification of biomass has been estimated at $5 to $6.30 per kg. It is unlikely that any of these approaches could provide large-scale sources of hydrogen at competitive prices until after 2030.
Stranded investment is one of the greatest risks faced by near-term hydrogen production technologies. For instance, if during the next two decades we built a hydrogen infrastructure around small CH4 reformers in local fueling stations and then decided that U.S. greenhouse gas emissions must be dramatically reduced, we would have to replace that infrastructure almost entirely. A major technology breakthrough will be needed to deliver low-cost zero-carbon hydrogen.
_______
_______
The chicken-and-egg problem:
At the National Hydrogen Association annual conference in March 2003, Bernard Bulkin, British Petroleum’s chief scientist, said that, “if hydrogen is going to make it in the mass market as a transport fuel, it has to be available in 30 to 50 percent of the retail network from the day the first mass-manufactured cars hit the showrooms.” Yet a 2002 analysis by Argonne National Laboratory found that even with improved technology, “the hydrogen delivery infrastructure to serve 40 percent of the light duty fleet is likely to cost over $500 billion.” Major breakthroughs in hydrogen production and delivery will be required to reduce that figure significantly. Who will spend the hundreds of billions of dollars on a wholly new nationwide infrastructure to provide ready access to hydrogen for consumers with fuel cell vehicles until millions of hydrogen vehicles are on the road? And who will manufacture and market such vehicles until the infrastructure is in place to fuel those vehicles? Will car companies and fuel providers be willing to take this chance before knowing whether the public will embrace these cars? We hope to see an economically, environmentally, and politically plausible scenario for how this classic chasm can be bridged; it does not yet exist.
________
________
Fallacy of massive hydrogen production; be it centralised or local:
Centralized production of hydrogen is the ultimate goal. A pure hydrogen economy requires that hydrogen be generated from CO2-free sources, which would almost certainly require centralized hydrogen production closer to giant wind farms or at coal/biomass gasification power plants in which CO2 is extracted for permanent underground storage. That will require some way of delivering massive quantities of hydrogen to tens of thousands of local fueling stations.
Tanker trucks carrying liquefied hydrogen are commonly used to deliver hydrogen today, but make little sense in a hydrogen economy because of liquefaction’s high energy cost. Also, few automakers are pursuing onboard storage with liquid hydrogen. So after delivery, the fueling station would still have to use an energy-intensive pressurization system. This might mean that storage and transport alone would require some 50 percent of the energy in the hydrogen delivered, negating any potential energy and environmental benefits from hydrogen.
Pipelines are also used for delivering hydrogen today. Interstate pipelines are estimated to cost $1 million per mile or more. Yet we have very little idea today what hydrogen generation processes will win in the marketplace during the next few decades, or whether hydrogen will be able to successfully compete with future high-efficiency vehicles, perhaps running on other pollution-free fuels. This uncertainty makes it unlikely anyone would commit to spending tens of billions of dollars on hydrogen pipelines before there are very high hydrogen flow rates transported by other means and before the winners and losers at both the production end and the vehicle end of the marketplace have been determined. In short, pipelines are unlikely to be the main hydrogen transport means until the post-2030 period.
Trailers carrying compressed hydrogen canisters are a flexible means of delivery but are relatively expensive because hydrogen has such a low volumetric energy density. Even with technology advances, a 40-metric-ton truck might deliver only about 400 kg of hydrogen into onsite high-pressure storage. A 2003 study by ABB researchers found that for a delivery distance of 300 miles, the delivery energy approaches 40 percent of the usable energy in the hydrogen delivered. Without dramatic improvement in high-pressure storage systems, this approach seems impractical for large-scale hydrogen delivery.
__
Producing hydrogen onsite at local fueling stations is the strategy advocated by those who want to deploy hydrogen vehicles in the next two decades. Onsite electrolysis is impractical for large-scale use because it would be highly expensive and inefficient while generating large amounts of greenhouse gases and other pollutants. The hydrogen would need to be generated from small CH4 reformers. Although onsite CH4 reforming seems viable for limited demonstration and pilot projects, it is impractical and unwise for large-scale application, for a number of reasons.
First, the upfront cost is very high: more than $600 billion just to provide hydrogen fuel for 40 percent of the cars on the road, according to Argonne. A reasonable cost estimate for the initial hydrogen infrastructure, derived from Royal Dutch/Shell figures, is $5,000 per car.
Second, the cost of the delivered hydrogen itself in this option is also higher than for centralized production. Not only are the small reformers and compressors typically more expensive and less efficient than larger units, but they also will likely pay a much higher price for the electricity and gas to run them. A 2002 analysis put the cost at $4.40 per kg (equal to $4.40 per gallon of gasoline).
Third, “the risk of stranded investment is significant, since much of an initial compressed hydrogen station infrastructure could not be converted later if either a noncompression hydrogen storage method or liquid fuels such as a gasoline-ethanol combination proved superior” for fuel cell vehicles. This was the conclusion of a 2001 study for the California Fuel-Cell Partnership, a Sacramento-based public-private partnership to help commercialize fuel cells. Most of a CH4-based investment would also likely be stranded once the ultimate transition to a pure hydrogen economy was made, because that would almost certainly rely on centralized production and not make use of small CH4 reformers. Moreover, it’s possible that the entire investment would be stranded in the scenario in which hydrogen cars simply never achieve the combination of popularity, cost, and performance to triumph in the marketplace.
In the California analysis, it takes 10 years for investment in infrastructure to achieve a positive cash flow, and to achieve this result requires a variety of technology advances in components and manufacturing. Also, even a small tax on hydrogen (to make up the revenue lost from gasoline taxes) appears to delay positive cash flow indefinitely. The high-risk and long-payback nature of this investment would seem far too great for most investors, especially given the history of alternative fuel vehicles.
The fourth reason that producing hydrogen on-site from natural gas at local fueling stations is impractical is that natural gas is simply the wrong fuel on which to build a hydrogen-based transportation system. The United States consumes nearly 23 trillion cubic feet (tcf) of natural gas today and is projected to consume more than 30 tcf in 2025. Replacing 40 percent of ground transportation fuels with hydrogen in 2025 would probably require an additional 10 tcf of gas, plus 300 billion kilowatt-hours of electricity, or 10 percent of current power usage. Politically, given the firestorm over recent natural gas supply constraints and price spikes, it seems very unlikely that the U.S. government and industry would commit to natural gas as a substitute for even a modest fraction of U.S. transportation energy. In addition, much if not most incremental U.S. natural gas consumption for transportation would likely come from imported liquefied natural gas (LNG). LNG is dangerous to handle, and LNG infrastructure is widely viewed as a likely terrorist target. Yet one of the major arguments in favor of alternative fuels has been their ability to address concerns over security and import dependence.
Finally, natural gas has too much economic and environmental value to the electric utility, industrial, and building sectors to justify diverting significant quantities to the transportation sector, thereby increasing the price for all users. In fact, using natural gas to generate significant quantities of hydrogen for transportation would, for the foreseeable future, undermine efforts to combat global warming.
Thus, beyond limited pilot stations, it would be unwise to build thousands of local refueling stations based on steam CH4 reforming or, for that matter, based on any technology not easily adaptable to delivery of greenhouse gas-free hydrogen.
_______
_______
Hydrogen unlikely to reduce global warming:
Perhaps the ultimate reason why hydrogen cars are a post-2030 technology is the growing threat of global warming. Our energy choices are now inextricably tied to the fate of our global climate. The burning of fossil fuels–oil, gas and coal–emits CO2 into the atmosphere, where it builds up, blankets the earth, and traps heat, accelerating global warming. We now have greater concentrations of CO2 in the atmosphere than at any time in the past 420,000 years and probably at any time in the past 3 million years. Carbon-emitting products and facilities have a long lifetime. Cars last 13 to 15 years or more; coal plants can last 50 years. Also, CO2 lingers in the atmosphere, trapping heat for more than a century. These two facts together create an urgency to avoid constructing another massive and long-lived generation of energy infrastructure that will cause us to miss the window of opportunity for carbon-free energy until the next century.
_
Between 2000 and 2030, the International Energy Agency projects that coal generation will double. The projected new plants would commit the planet to total CO2 emissions of some 500 billion metric tons over their lifetime, which is roughly half the total emissions from all fossil fuel consumed worldwide during the past 250 years. Building these coal plants would dramatically increase the chances of catastrophic climate change. What we need to build is carbon-free power. A March 2003 analysis in Science by Ken Caldeira and colleagues concluded that if our climate’s sensitivity to greenhouse gas emissions is in the midrange of current estimates, “stabilization at 4°C warming would require installation of 410 megawatts of carbon emissions-free energy capacity each day” for 50 years. Yet current projections for the next 30 years are for building just 80 megawatts per day. Because planetary warming accelerates over time and because temperatures over the continental United States are projected to rise faster than the average temperature of the planet, a warming of 4° C means that by mid-century, the U.S. temperature could well be rising as much per decade as it rose during the entire past century: one degree Fahrenheit.
_
Unfortunately, the path set by the current energy policy of the United States and countries in the developing world will dramatically increase emissions during the next few decades, which will force sharper and more painful reductions in the future when we finally do act. Global CO2 emissions are projected to rise more than 50 percent by 2030. From 2001 to 2025, the U. S. Energy Information Administration projects a 40 percent increase in U.S. coal consumption for electricity generation. And the U.S. transportation sector is projected to generate nearly half of the 40 percent rise in U.S. CO2 emissions forecast for 2025, which again is long before hydrogen-powered cars could have a positive impact on greenhouse gas emissions.
_
Two points are clear. First, we cannot wait for hydrogen cars to address global warming. Second, we should not pursue a strategy to reduce greenhouse gas emissions in the transportation sector that would undermine efforts to reduce greenhouse gas emissions in the electric generation sector. Yet that is precisely what a hydrogen car strategy would do for the next few decades. For near-term deployment, hydrogen would almost certainly be produced from fossil fuels. Yet running a fuel cell car on such hydrogen today would offer no significant life cycle greenhouse gas advantage over the 2004 Prius running on gasoline.
Further, fuel cell vehicles are likely to be much more expensive than other vehicles, and their fuel is likely to be more expensive (and the infrastructure will probably cost hundreds of billions of dollars). Although hybrids and clean diesels may cost more than current vehicles, at least when first introduced, their greater efficiency means that, unlike fuel cell vehicles, they will pay for most if not all of that extra upfront cost over the lifetime of the vehicle. A June 2003 analysis in Science by David Keith and Alex Farrell put the cost of CO2 avoided by fuel cells running on zero-carbon hydrogen at more than $250 per ton even with a very optimistic fuel cell cost. An advanced internal combustion engine could reduce CO2 for far less and possibly for a net savings because of the reduced fuel bill.
_
Probably the biggest analytical mistake made in most hydrogen studies, including the recent National Academies’ report, is failing to consider whether the fuels that might be used to make hydrogen, such as natural gas or renewable sources, could be better used simply to make electricity. For example, the life cycle or “well-to-wheels” efficiency of a hydrogen car running on gas-derived hydrogen is likely to be under 30 percent for the next two decades. The efficiency of gas-fired power plants is already 55 percent (and likely to be 60 percent or higher in 2020). Cogeneration of electricity and heat using natural gas is more than 80 percent efficient. And by displacing coal, the natural gas would be displacing a fuel that has much higher carbon emissions per unit of energy than gasoline. For these reasons, natural gas is far more cost-effectively used to reduce CO2 emissions in electric generation than it is in transportation.
The same is true for renewable energy. A megawatt-hour of electricity from a renewable source such as wind power, if used to manufacture hydrogen for use in a future fuel cell vehicle, would save slightly less than 500 pounds of CO2 as compared to the best current hybrids. That is less than the savings from using the same amount of renewable electricity to displace a future natural gas plant (800 pounds) and far less than the savings from displacing coal power (2,200 pounds).
As the June 2003 Science analysis concluded: “Until CO2 emissions from electricity generation are virtually eliminated, it will be far more cost effective to use new CO2-neutral electricity (such as wind) to reduce emissions by substituting for fossil-electric generation than to use the new electricity to make hydrogen.” Barring a drastic change in U.S. energy policy, the electric grid will not be close to CO2-free until well past 2030.
_
Major breakthroughs needed:
Hydrogen and fuel cell vehicles should be viewed as post-2030 technologies. In September 2003, a DOE panel on Basic Research Needs for the Hydrogen Economy concluded that the gaps between current hydrogen technologies and what is required by the marketplace “cannot be bridged by incremental advances of the present state of the art” but instead require “revolutionary conceptual breakthroughs.” In sum, “the only hope of narrowing the gap significantly is a comprehensive, long-range program of innovative, high risk/high payoff basic research.” The National Academies’ study came to a similar conclusion. Our research should be focused solely on finding a low-cost zero-carbon source hydrogen production, which will almost certainly be centralized. That probably means we won’t begin the hydrogen transition until after 2030 because of the logistical and cost problems associated with a massive hydrogen delivery infrastructure.
_
The priority for today is to deploy existing clean energy technologies and to avoid any expansion of the inefficient carbon-emitting infrastructure. If we fail to act now to reduce greenhouse gas emissions–especially if we fail to act because we have bought into the hype about hydrogen’s near-term prospects; future generations will condemn us because we did not act when we had the facts to guide us, and they will most likely be living in a world with a much hotter and harsher climate than ours, one that has undergone an irreversible change for the worse. So hydrogen hype may make future of our children bleak.
_______
_______
Using hydrogen fuel risks locking in reliance on fossil fuels:
The research, published in the journal Nature Climate Change, calculated that producing and burning hydrogen-based fuels in home gas boilers required six to 14 times more electricity than heat pumps providing the same warmth. This is because energy is wasted in creating the hydrogen, then the e-fuel, then in burning it. For cars, using e-fuels requires five times more electricity than is needed than for battery-powered cars.
Romain Sacchi, from the Paul Scherrer Institute in Switzerland and part of the study team, said: “We are currently far from 100% renewable electricity. If produced with the current electricity mixes [in Europe], hydrogen-based fuels would increase – not decrease – greenhouse gas emissions, [compared with] using fossil fuels.” “Hydrogen-based fuels can be a great clean energy carrier, yet their costs and associated risks are also great. If we cling to combustion technologies and hope to feed them with hydrogen-based fuels, and these turn out to be too costly and scarce, then we will end up burning further oil and gas. We should therefore prioritise those precious hydrogen-based fuels for applications for which they are indispensable: long-distance aviation, feedstocks in chemical production and steel production” said Falko Ueckerdt, at the Potsdam Institute for Climate Impact Research (PIK) in Germany, who led the research.
Using hydrogen-based fuels for cars and home heating risks locking in a dependency on fossil fuels and failing to tackle the climate crisis, according to this new analysis. Fuels produced from hydrogen can be used as straight replacements for oil and gas and can be low-carbon, if renewable electricity is used to produce these “e-fuels”. However, the research found that using the electricity directly to power cars and warm houses was far more efficient. The analysis estimated that hydrogen-based fuels would be very expensive and scarce in the coming decade. Therefore, equipment such as “hydrogen-ready” boilers could end up being reliant on fossil gas and continue to produce the carbon emissions driving global heating. However, a few sectors such as aviation, shipping, steel and some chemicals are extremely hard to electrify. The researchers said hydrogen-based fuels would be needed for these by 2050, when the world needs to have reached net zero emissions. But they said enormous investment in technology and fast-rising carbon taxes would be needed to achieve this.
Renewable electricity production is increasing rapidly as costs tumble. But it still makes up a small proportion of all energy used, which is mostly provided by coal, oil and gas. Using the electricity directly is efficient, but requires investment in new types of car and heating systems. Using the electricity to create hydrogen from water and then using carbon dioxide to manufacture other fuels can produce “drop-in” replacements for fossil fuels. But the new study concludes this cannot work on a large enough scale to tackle the climate emergency in time. Prof Gunnar Luderer, also at PIK and part of the study team, said: “As climate targets require immediate emission reductions, direct electrification should come first to assure a safe future. It is clear that the contribution of e-fuels and hydrogen will be marginal on the timescale of 2030.”
______
______
Hydrogen Hype Bubble Burst:
-1. The only “clean,” carbon-free hydrogen is green hydrogen, and there’s not enough to go around
– 95% of global hydrogen is grey hydrogen. Most of that is produced via steam methane reformation (SMR) of natural gas, a carbon intensive process.
-Green hydrogen, produced via electrolyzers powered by renewable energy, is carbon-free. However, current global installed electrolyzer capacity is at 200 MW.
-While capacity is expected to increase, it will be at least a decade before there is enough green hydrogen commercially available to power large-scale commercial projects.
-Blue hydrogen, which is often promoted as another “clean” alternative to grey hydrogen, is produced via SMR of natural gas paired with carbon capture and storage (CCS) to capture emissions. Blue hydrogen is not clean. A recent lifecycle analysis of blue hydrogen found that generating blue hydrogen would result in more greenhouse gas emissions than directly burning gas for heat.
_
-2. Green hydrogen is energy- and water-intensive to produce
-With current electrolyzers, green hydrogen’s efficiency, from production back to energy through combustion, is around 30%, which means 70% of the renewable energy put into producing green hydrogen is lost across the full cycle of production and use.
-To put that in context, to replace all current industrial consumption of grey hydrogen with green hydrogen would require 3,500 terra-watt hours (TWh) of renewable energy, the amount of renewable energy currently produced by the entire European Union.
-Electrolysis is also a water-intensive process. Every kilogram of green hydrogen produced requires between 9 and 11 liters of water.
-Because electrolysis breaks down water into constituent elements, this water needs to be purified. Most industrial water purification processes require, at minimum, a ratio of 2:1 wastewater to pure water, effectively doubling the amount of water required. This means each ton of green hydrogen could require up to 18 tons of water total, and not all of that water can be recycled.
_
-3. Combusting green hydrogen in power plants jeopardizes public health and delays climate action
-While producing green hydrogen can be a carbon-free process, if that gas is burned, it is not emissions-free. Burning hydrogen can lead to nitrogen oxide (NOx) emissions up to six times that of methane.
-NOx does significant damage to the respiratory system over time. In areas affected by smog, symptoms including coughing, increased rates of asthma, and comorbidities with other respiratory illness develop.
-To comply with Clean Air Act regulations, most power plants limit their NOx emissions either through a catalytic reaction, dilution of the fuel mix with water or steam, or using newer low-NOx technology such as a dry low NOx (DLN) combustion system. None of these systems have been proven to work with a significant hydrogen blend or 100% hydrogen fuel.
-Blending hydrogen at the low levels required to limit NOx does not lead to a significant decrease in carbon dioxide (CO2) emissions. Because of the lower volumetric energy density of hydrogen, a blend of 30% hydrogen and 70% natural gas by volume would result in merely a 13% decrease in CO2.
-Because of the significant upgrades to emissions control technologies required for most existing gas plants to handle a larger hydrogen blend, there is no glide path of blending that will eventually get a power plant up to 100% hydrogen. Allowing plants to repower based on the promise that they will eventually get to 100% hydrogen will only extend the suffering of frontline communities and increase the lifetime of polluting fossil fuels.
_
-4. Building out hydrogen infrastructure – or upgrading existing infrastructure – is expensive
-If steel is exposed to hydrogen at high temperatures, hydrogen will diffuse into the alloy and combine with carbon to form tiny pockets of methane. This methane does not diffuse out of the metal and cracks the steel. This process, called “hydrogen embrittlement,” means that hydrogen cannot simply be stored and transported with existing infrastructure.
-Steel makes up more than a quarter-million miles natural gas transmission systems in the U.S. Because of the embrittlement issue, any plans to use existing natural gas assets with hydrogen would require replacement of these pipelines.
-Regular pipeline replacement is not cheap. Plans currently underway in Chicago to replace all its natural gas pipes will cost each utility customer $750 per year by 2040. Because of its different storage requirements, a hydrogen pipeline is 68% more expensive to build than that.
______
______
Hydrogen Hope:
_
Proponents say hydrogen is versatile, but expensive:
Advocates of hydrogen mobility keep shouting the two main benefits of hydrogen from the rooftops: Hey, it reduces our dependency on foreign oil, and it will never run out! Hydrogen is already a key component of chemical industrial processes and in the steel industry. So making clean hydrogen to use in those industrial processes is critical to reducing carbon emissions. But as an energy source itself, hydrogen’s big advantage is its versatility. It’s often called the Swiss Army knife of energy. Clean hydrogen would be useful in decarbonizing industrial heavy transportation like trucking, big industrial boats, and planes. It’s less interesting for smaller consumer vehicles, as battery-powered cars are being adopted much more readily. But bigger vehicles require larger batteries, which increases their weight, which in turn increases their energy use. Hydrogen can be a way around that conundrum. For more energy-intensive uses, like trucks and buses, hydrogen could offer greater range, shorter fueling times and lower weight than lithium-ion batteries. Just like with batteries, as technology improves, costs should fall.
Hydrogen’s potential as a fuel source isn’t just limited to the road. Some members of the shipping sector are optimistic it will be a viable alternative to fossil fuels. Pilot projects, mostly involving ferries, are already underway in several places. However, critics point to the space required to store liquid hydrogen on board, saying this makes it unfeasible for cargo ships. One potential compromise is ammonia – a mixture of hydrogen and nitrogen that has nearly twice the energy density of liquid hydrogen.
Hydrogen can also be used as a way to store energy from intermittent renewable sources, which are intermittent — the sun isn’t always shining and the wind isn’t always blowing. Instead, utilities can convert the excess energy into hydrogen and then use it for energy later on, as an alternative to battery storage. Hydrogen can be stored underground for as long it needs to be, much the same as natural gas, and on a seasonal basis.
The main drawback of hydrogen is its expense. Making hydrogen from natural gas costs about $1.50 per kilogram. Clean hydrogen costs about $5 per kilogram. Driving down the price of clean hydrogen “would be a huge step toward solving climate change,” said billionaire Bill Gates, the founder of Breakthrough Energy Ventures, at the Department of Energy’s Hydrogen Shot Summit. “The goal of cutting premium by 80 percent is a fantastic and ambitious goal,” he said.
There are three primary pathways the Department of Energy sees as how to get the cost of clean hydrogen down from about $5 per kilogram to $1:
-Improving the efficiency, durability and manufacturing volume of electrolyzers.
-Improving pyrolysis, which generates solid carbon, not carbon dioxide as a byproduct.
-Advanced pathways is a bit of a catch-all for experimental technologies. One example is photoelectrochemical approach (PEC), where sunlight and specialized semiconductors are used to break water into sunlight and hydrogen.
_____
In 2009, then U.S. secretary of energy Steven Chu cut funding for hydrogen-based fuel cells citing four reasons: One, hydrogen is highly flammable and difficult to store; two, it was costly to produce; three, infrastructure to distribute hydrogen had to be built; and four, the fuel cell wasn’t as durable, low-cost, and powerful as the internal combustion engine. A lot has changed since. Hydrogen now competes with lithium-ion batteries as a fuel for the future. Consider this: Once charged, a car with a 5-kg hydrogen cylinder can cover 550-600 km, compared to 80-100 km on a lithium-ion battery. Refuelling takes three-five minutes, akin to that for a compressed natural gas (CNG)-fuelled car, while a battery may take hours to charge. Hydrogen’s energy content, too, is higher than that of fossil fuels. Also, the materials used in hydrogen cells can be recycled, unlike batteries. These factors make it a correct fit for medium or heavy vehicles. Fuel cells have distinct advantages over lithium-ion batteries. A small 5-kg hydrogen tank attached to the fuel cell occupies a much smaller area than a series of batteries that power a vehicle. It is also much lighter because aluminium, used in fuel cells, is one-fourth the weight of lithium, ensuring higher mileage. With 55% efficiency, fuel cells are better than conventional vehicle engines which run at 25% efficiency. This means that not only does a fuel cell fit in an automobile, it is also more efficient than a conventional engine.
_____
While we already have mature technologies that can replace fossil fuels in many parts of our economy, there are areas where eliminating carbon pollution will be much more difficult. Steel, shipping, aviation, and trucking, for example, account for a combined 40% of our global carbon footprint and are on track to consume two times the remaining carbon budget for staying below 1.5° Celsius of warming. Fortunately, “green” hydrogen—H2 produced through electrolysis using renewable energy—holds enormous promise for these sectors. Through various applications, this tiny molecule can provide the heat, reduction properties, fuel, and other services needed to replace fossil fuels. In fact, given the technical challenge of getting these “hard-to-abate” sectors to a state of carbon neutrality, hitting 2050 net-zero targets without it would be virtually impossible.
H2 uptake can serve other objectives beyond decarbonization. For example, hydrogen’s ability to substitute for natural gas in many applications allows for a degree of energy independence and reduced reliance on liquefied natural gas or pipeline imports from Russia. And while renewables like solar and wind are limited by the extent of electrical grids, hydrogen can be transported by pipeline or potentially by ship. That means it could become an exportable renewable-energy source, eventually replacing petroleum as the main global energy commodity.
Green hydrogen is particularly attractive for developing economies. There is a strong geographical overlap between countries and regions with the lowest production cost for renewable energy and those with lower per capita GDP. These countries thus could secure a global competitive advantage by becoming hydrogen producers and exporters. Doing so would also help them attract zero-carbon heavy industry, such as fertilizer manufacturing or hydrogen-based direct reduction steelmaking. And, of course, the development of these sectors would lead to significant job creation.
_______
_______
Clean hydrogen fired power plant:
At first glance, the concept of a clean-hydrogen power plant seems utterly absurd.
Why would anyone use renewable power to make green hydrogen and then burn it to produce electricity? The round-trip efficiency would be less than 40%, so every 10kWh of wind or solar energy would provide less than 4kWh of electricity.
And why would anybody create blue hydrogen from natural gas with carbon capture and storage (CCS) — with all the added expense of methane reforming and compressing/liquefying, transporting and storing the hard-to-handle H2 — when you could just add CCS to existing gas-fired power plants?
And yet major energy companies such as Siemens Energy, Equinor and SSE believe there is a bright future for hydrogen-fired power plants. Why?
“If I have renewable power, convert it to hydrogen and re-electrify it, with a total cycle efficiency of less than 40%, it obviously only makes sense if you’re using hydrogen as long-term storage and compensation for variable renewables,” says Erik Zindel, Siemens Energy’s vice-president of hydrogen generation sales.
If you really want to [store power] for days, weeks, months, or for seasonal storage — which is using solar power from the summer in winter, or wind power from the autumn to the summer — you need to store electricity in a chemical way. Large-scale hydrogen storage will be useful to reduce curtailment of wind and solar power during windy/sunny periods.
“Once you go into the green hydrogen arena, you can increase the amount of renewables that you want to build in the grid because you can make use of the excess renewable energy [that would otherwise be curtailed because it cannot be sold],” Zindel explains.
So by having electrolysis [which uses electricity to split water molecules into H2 and oxygen] and by being able to store that excess energy as hydrogen, you can really allow the electric system to expand renewables by a significant amount. Because if you don’t do that, it will be quickly limited, because there will be too much excess energy that you have to dump. But once you can make use of that excess power, then you can really double, triple, quadruple the amount of renewable energy you want to build.
Fuel cells versus hydrogen fired power plant:
If burning hydrogen will always produce some NOx greenhouse gases, why not convert the hydrogen to electricity by using emissions-free fuel cells?
The fuel cell is a competitive technology compared with combined-cycle with a gas turbine — but in the end… it’s really about economics. If you look at efficiencies, today’s combined-cycle technologies… [have] efficiency levels of 63-64%. So that’s already higher than a typical fuel cell, which is usually limited to 60%. Then investments costs for a combined cycle power plant are also much cheaper than for [similar-sized] fuel cells. It will take many, many years until [fuel cells] get close to the LCOEs [levelised cost of energy] of combined-cycle, if at all. And then you need to look at the much higher fuel flexibility of gas turbines and the possibility to retrofit existing natural-gas combined cycles to burn hydrogen, which would reduce the required investment even further. All that speaks for combined cycles as the main technology for future re-electrification of hydrogen. Fuel cells, despite being a very attractive technology with a significant improvement potential, will see its main application areas in mobility and in small island grids, where a combined cycle is not feasible.
While it would be theoretically possible to convert a coal-fired power plant to run on hydrogen, it would be too expensive to be cost-effective. This is because coal power plants generate electricity by burning the hydrocarbon to heat water, which generates steam that drives a steam turbine. Coal plants also rarely have a combined cycle, which takes the heat released from burning fossil fuels and re-uses it to generate electricity. Modern state-of-the-art coal plants generally have an energy efficiency of about 45%, compared to more than 60% for their gas-fired counterparts. And as they will cost more to convert to hydrogen, it is simply not an economically viable option. By the time you have to convert existing combined cycles to hydrogen, the old coal-fired power stations will have already gone — completely gone. The [future] demand [for hydrogen power plants] can be filled with already existing combined cycle power plants.
_____
_____
Green Hydrogen may not become a costly detour:
In the mid-2000s, even an advocate of climate action like British economist Nicholas Stern didn’t think wind and solar could compete economically with fossil fuels until the 2030s. Things turned out very differently. Since 2009, the cost of unsubsidized solar power in the U.S. has fallen 90% and wind is down 70%, notes Lazard Ltd. Battery prices have slumped 87% over a similar period, according to BloombergNEF. Coal is already in retreat from the power sector, and many of the world’s biggest independent oil companies think petroleum demand is at or near its peak.
If green hydrogen can achieve renewable power-style cost declines from its current pricing of around $3 to $8 a kilogram, it stands a good chance of competing with gray hydrogen, which costs as little as $1. The risk, though, is that the forecast reductions aren’t achieved. If a botched deployment or technical problems result in more modest economies of scale, the world will be left with a legacy of uneconomic hydrogen-production plants. On top of that, billions that could have been spent on other decarbonization technologies will have been wasted.
Which of those two futures we face will be determined by Wright’s Law, a hypothesis about manufacturing dating from the early years of the aircraft industry. It states that with every doubling of cumulative output, the cost of technology tends to fall by a constant percentage. Factories get better at finding efficiencies; increased demand drives economies of scale; and larger volumes encourage suppliers to produce raw materials more cheaply. (The better-known Moore’s Law, which predicted drastic declines in the cost of computing power, is best understood as a special case of Wright’s Law.)
The cost-decline percentage, known as the learning rate, seems to explain why nascent renewable technologies can get so cheap so quickly. The learning rate for solar modules is a blistering 28.5%, according to BloombergNEF, meaning that an eightfold increase in installations will reduce costs by nearly two-thirds. That fall in prices then triggers more demand, encouraging further solar installations and reducing costs again in a virtuous circle. Fossil technologies can’t compete with that advantage, because their largest cost is typically the fuel itself, where prices show no long-lasting downward trend.
_
Will green hydrogen follow the same path as solar, wind and batteries? There’s good reason to think so. For one thing, nearly half of the cost of a green hydrogen plant would come from the renewable generators and batteries that provide power to the electrolyzer, and we already have plenty of data about learning rates there. Most of the other half is the electrolyzer itself, and BloombergNEF estimates these show improvements in the 18% to 20% range, comparable to lithium-ion batteries.
There are potential problems, though. The learning-rate hypothesis is treated as certain now, but just 15 years ago it was viewed with more doubt. The prices of raw materials can derail cost reductions for long periods — as we saw during the 2000s, when solar prices stood still for a decade thanks to a shortage of the polysilicon needed to make photovoltaic wafers. Comparable shortages of cobalt may yet derail projected price reductions for lithium-ion batteries. Current designs of PEM electrolyzers — one of the most promising technologies for producing green hydrogen — are highly exposed to the prices of platinum-group metals and Nafion, a synthetic membrane made by Chemours Co.
The larger issue may be that cost declines are highly dependent on the accuracy of estimates about learning rates and cumulative capacity, and there’s still a dearth of solid data to produce that analysis. Estimates of electrolyzer installations range from around 170 megawatts to 20,000 megawatts. In the former case, installing 100,000 megawatts of splitters over the coming decade would involve ten doublings in capacity. At an 18% learning rate, that should reduce costs by nearly 90%, making electrolyzers competitive with any fossil-fuel based alternative. In the latter case, we’ll only see two or three doublings, which is unlikely to be enough. Learning rates themselves show substantial error bars, too. A technology with a 24% learning rate, at the upper end of electrolyzer estimates, will need to double capacity only four or five times to cut prices by half. With a 12% learning rate, at the lower end, you need to increase installations 50-fold.
That uncertainty could drive wildly different outcomes. If existing capacity is low and learning rates are high, green hydrogen may revolutionize energy as dramatically as wind, solar and batteries have done. If existing capacity is high and learning rates are low, investors might give up on the technology long before it’s able to scale up. Over the coming days, we’re going to look at the potential shape of the new hydrogen economy, from the government policies that may direct it to the ways in which the existing fossil-fuel sector may hope to benefit. Energy investors, and anyone hoping that the world can avoid catastrophic climate change, had best hope that green hydrogen lives up to its promise.
_______
_______
When hydrogen is energetically inefficient and economically unviable, why use it anyway?
Of course, using renewable electricity directly is more energy-efficient than first converting it to hydrogen then using is for power.
But for many things, direct electrification is impossible or unfeasible. Heavy industry such as steelmaking needs chemical inputs, and green hydrogen can replace CO2-emitting metallurgical coal or fossil-fuel based chemicals. Many industries also need high temperatures, which are expensive to produce with electrical heating. Long-range transport such as trains or shipping, and winter heating in many temperate areas such as the UK, have such huge energy demands than it is impractical to store enough electrical energy; but hydrogen is easy to store in pressurised tanks, or large caverns excavated in rock salt deep underground. Using hydrogen to store or transport energy in a smart combination with renewables will lead to the lowest-cost energy system, especially considering that hydrogen is also set to become a lot cheaper. As more countries set hydrogen targets in their energy system, more companies announce new hydrogen-based projects to switch from fossil carbon fuels, and more investors recognise the financial potential of hydrogen, the industry is scaling up at speed, and that will bring down the cost of electrolysers used to make hydrogen from water. New, increasingly ambitious targets show hydrogen’s potential. Price is an important blockage, with blue hydrogen in 2021 costing about £5/kg. The Green Hydrogen Catapult, a coalition of companies, is aiming to contribute to more than halving the price of hydrogen to $2/kg (£1.50/kg) by 2026, and the Biden administration has launched an ‘Earthshot’ plan to see hydrogen priced at $1/1kg (£0.75/kg) by 2030.
_______
_______
Moral of the story:
_
-1. Hydrogen is the only element that can exist without neutrons. Hydrogen’s most abundant isotope has no neutrons. Hydrogen is the only atom for which the Schrödinger equation has an exact solution. At room temperature and under normal pressure, hydrogen is a colourless, odourless and non-poisonous diatomic gas with molecular formula H2 and it is lighter than air. Because hydrogen is so light, the pure element isn’t commonly found on the Earth. It disperses quickly and just float away. The prime components of air, nitrogen and oxygen, are fourteen and sixteen times heavier, giving hydrogen dramatic buoyancy. Hydrogen is highly flammable. Hydrogen gas forms explosive mixtures with air in concentrations from 4–74%. The explosive reactions may be triggered by spark, heat, or sunlight. Hydrogen has the smallest ignition energy, much lower than that required for other common fuels. This means that small sparks can easily ignite it. Hydrogen forms both positive and negative ions. It does this more readily than any other element. On earth as an element, hydrogen is almost always found as part of another compound, such as water and hydrocarbons.
_
-2. Today most of hydrogen is used as a chemical, rather than a fuel. Hydrogen is sold by weight instead of by volume and uses the same unit of measurement—kilogram—everywhere in the world. Most hydrogen consumed today is used by industry for refining petroleum, treating metals, producing fertilizer, and processing foods.
_
-3. Hydrogen is not a primary energy source, because it is not naturally occurring as a fuel. Hydrogen is energy carrier similar to electricity. Energy carriers allow the transport of energy in a usable form from one place to another. Hydrogen as a fuel has highest energy density by weight:1kg contains the same energy as 2.4 kg of methane or 2.8 kg of gasoline. Hydrogen can be produced or separated from a variety of sources including water, fossil fuels, or biomass. Once you free the element from its compound, hydrogen can be delivered to fuel cells to generate electricity and heat, used in a combined cycle gas turbine to produce larger quantities of centrally produced electricity, burned to run internal combustion engine or used for heating buildings, boilers & cooking; all methods producing no carbon dioxide or methane emissions, thus hydrogen fuel is environmentally friendly. In each case hydrogen is combined with oxygen from air to form water releasing energy as heat and electricity. However, air also contains nitrogen and burning hydrogen in air results in generation of nitrogen oxides.
Hydrogen is also used as feedstock. Hydrogen is also widely regarded as energy storage medium, due to the ease with which electric power can convert water into its hydrogen and oxygen components through electrolysis and can be converted back to electrical power using fuel cell or combustion engine (e.g., turbine or internal combustion engine).
As hydrogen plays an important role in various applications to store and transfer energy, there are four typical applications of integrating hydrogen into power systems: energy storage, Power-to-X system, fuel cell co- and tri-generation and vehicular applications.
_
-4. Hydrogen gas is colourless and completely invisible. Hydrogen is produced via different methods and the ascribed colour merely serves to indicate the way in which it has been produced. You might encounter the terms ‘grey’, ‘blue’, ‘green’ being associated when describing hydrogen technologies. It all comes down to the way it is produced. Many other colours have been added to the palette, but the focus on colour is a distraction. What really matters is the carbon intensity of the production process — that is, the tons of carbon dioxide produced for each ton of hydrogen. Steam reforming of methane produces about 9 to 12 kg of CO2 for every kilogram of hydrogen produced, that is called grey hydrogen. The same process with CCS produces 1 to 4 kg of CO2, that is called blue hydrogen. Production of green hydrogen generates 0 to 0.6 kg of CO2 per kg of hydrogen.
_
-5. Green hydrogen is hydrogen that is generated entirely by renewable energy. Green hydrogen is produced through electrolysis, in which machines split water into hydrogen and oxygen, with no other by-products. Historically, electrolysis required so much electricity that it made little sense to produce hydrogen that way. The situation is changing for two reasons. First, significant amounts of excess renewable electricity have become available at grid scale; rather than storing excess electricity in arrays of batteries, the extra electricity can be used to drive the electrolysis of water, “storing” the electricity in the form of hydrogen. Second, electrolyzers are getting more efficient.
_
-6. Energy density is the amount of energy that can be released by a given mass or volume of fuel. It can be measured in terms of gravimetric energy density (per unit of mass) or volumetric energy density (per unit of volume). Gravimetric energy density is relevant when comparing the energy efficiency of fuels. At the same time, volumetric energy density is relevant when comparing transportation modes as storage space must be present to carry the fuel propelling a vehicle. Hydrogen has the highest energy content of any common fuel by weight, but it has the lowest energy content by volume. The gas weighs almost nothing but has an extremely high gravimetric energy density. One kilogram of hydrogen contains a vast amount of energy, making it an efficient and lightweight energy carrier. One kilogram of hydrogen contains approximately 33 kWh of energy i.e., 120 MJ/kg. The energy in 2.2 pounds (1 kilogram) of hydrogen gas is about the same as the energy in 1 gallon (6.2 pounds, 2.8 kilograms) of gasoline. What this really means is that 1 kg of hydrogen, used in a fuel cell to power an electric motor, contains approximately the same energy as a gallon of gasoline.
The volumetric density of gaseous hydrogen at atmospheric pressure is 0.09 kg/m³. The volumetric energy density of hydrogen is particularly low. At any pressure, the volumetric energy density of methane gas exceeds that of hydrogen gas by a factor of 3.2 so at any pressure, hydrogen gas clearly carries less energy per volume than natural gas. Per volume, the energy content of hydrogen is a lot lower than that of most other fuels and energy carriers. Consequently, storing or using hydrogen at atmospheric pressure and temperature requires a substantial amount of space. Fortunately, there is a solution to this. By compressing or liquefying hydrogen, it is possible to raise the low volumetric energy density. This makes the storage, transportation, and application of hydrogen considerably easier.
At a pressure of 350 bar, the volumetric density of gaseous hydrogen is 21 kg/m³. This increased pressure makes it possible to store considerably more gaseous hydrogen in the same space. The pressure of 350 bar is used in the tanks of gaseous hydrogen trucks. A loaded 55-ton truck needs about 50-70 kg of hydrogen to travel 500 to 600 km.
At a pressure of 700 bar, the volumetric density of gaseous hydrogen is 42 kg/m³. This relatively high pressure is used, among others, for gaseous hydrogen passenger cars. With a 125 liter tank containing 5 kg of hydrogen, a car can drive about 600 km.
In liquid form and at a temperature of -252.9 centigrade, hydrogen has a volumetric density of 71 kg/m³. Liquid hydrogen storage is usually under pressures up to 10 bar. Liquid hydrogen is used as a fuel in rockets and various spaceships.
_
-7. The two main difficulties preventing us from having hydrogen power are production and storage. On earth, hydrogen is found combined with other elements. For example, in water, hydrogen is combined with oxygen. In fossil fuels, it is combined with carbon as in petroleum, natural gas or coal. The challenge is to separate hydrogen from other naturally occurring compounds in an efficient, economic and environment friendly manner. At the moment, hydrogen production is energy-intensive and expensive. Normally, industrial production of hydrogen requires high temperatures, large facilities and an enormous amount of energy. Remember, it takes more energy to produce hydrogen (by separating it from other elements in molecules) than hydrogen provides when it is converted to useful energy, and it is costly to do it. Another difficulty with hydrogen comes down to storage. Hydrogen is produced in gaseous form, and it needs to be stored under pressure or liquified directly. Both of these processes require additional energy and cost.
_
-8. When hydrogen is compared with other fuels like gasoline & natural gas and other energy carriers like electricity & batteries, several factors are considered including overall energy efficiency, cost to consumers, global warming & pollution potential, feasibility of storage and transport, energy security and safety issues.
_
-9. A hydrogen fuel cell is an electrochemical cell that converts chemical energy stored by hydrogen fuel into electricity. Hydrogen fuel cells produce electricity by combining hydrogen and oxygen atoms. The hydrogen reacts with oxygen across an electrochemical cell to produce electricity, water, and small amounts of heat. Fuel cells can be monitored and controlled remotely, making them an ideal back up source for a range of power applications. The uses of hydrogen fuel in general can be divided into categories: stationary (fuel cell power plants), mobile (land, air, water, and space transport), backup (portable systems for any practice), and specialty, depending on the selected platform. Because fuel cells have no moving parts and do not involve combustion, in ideal conditions they can achieve up to 99.9999% reliability. This equates to less than one minute of downtime in a six-year period.
_
-10. Combustion engines have changed little since they were invented over a hundred years ago. By combusting the fuel to make electricity, generators create a lot of noise, smoke, exhaust and toxic fumes. They also tend to be inefficient, large, heavy and unwieldy.
Unlike a generator, a fuel cell directly converts hydrogen into electricity through a chemical reaction – one step rather than multiple steps. This allows a fuel cell to remain efficient, quiet and clean. With a portable fuel cell, you can hand-carry the fuel cell with you. From camping, to sailing, to storms, to remote locations and power outages, you can create your own electricity, anywhere, any time. Hydrogen fuel cell coupled with an electric motor in FCEV is two to three times more efficient than an internal combustion engine running on gasoline.
There are different types of fuel cells so efficiencies vary, but most are between 40% and 60% energy efficient. However, when the fuel cell’s waste heat is used to heat a building in a cogeneration system this efficiency can increase to 85%. This is significantly more efficient than traditional coal power plants, which are only about one third energy efficient.
Hydrogen can be used to generate electricity for our homes and office buildings, through the use of gas turbines and microturbines (small gas turbines). Hydrogen can also be used in internal combustion engines for both stationary and mobile applications, powering industrial processes, ocean fleets, cars, and buses. In power generation, hydrogen is one of the leading options for storing renewable energy.
Modern gas turbines can be operated with a mix of hydrogen and natural gas, with a hydrogen share of 5 to 100%. Hydrogen can be cached, transported in gas grids and re-electrified in gas turbines, combined cycles or fuel cell power plants.
_
-11. There are two general types of hydrogen generation technologies: reforming and water splitting. Reforming technologies use fossil fuels or biomass and steam to produce hydrogen at the lowest cost—but they also produce carbon dioxide. Water splitting technologies, meanwhile, fall into three general categories: thermo‐chemical cycles, electrolysis, and direct photoelectrochemical (PEC). Electrolysis generally falls into two categories. Low‐temperature electrolysis (LTE), which uses electricity to split water into hydrogen and oxygen; and High‐temperature steam electrolysis (HTSE) utilizes heat and electricity to split steam into hydrogen and oxygen. The reason steam methane reforming has traditionally been favoured over electrolysis is that whereas methane reforming directly uses natural gas, electrolysis requires electricity.
_
-12. Electrolysis is an energy intensive process. About 50 kWh energy is used to produce 1 kg hydrogen and the same 1 kg hydrogen contain 33 kWh usable energy for work. That means 17 kWh energy is lost in producing 1 kg hydrogen by electrolysis no matter the source of electricity. Therefore, the use of hydrogen as an intermediate is justified only when there is some reason not to use the primary energy source directly i.e., fossil fuel power plant or renewal or nuclear electricity. If fossil fuels, e.g., coal, oil or natural gas, are used to generate the electricity for electrolysis, there is no advantage over using the fossil fuels directly. Indeed, you still get all the CO2, and there is a considerable loss of energy. On the other hand, in times of excess electricity production from wind farms, instead of curtailing the electricity as is commonly done, it is possible to use this excess electricity to produce hydrogen through electrolysis. Even if you have a less than efficient electrolyzer, and you can keep half of the electricity stored in your hydrogen to reuse for power when it is necessary, that is still cheaper than wasting it.
_
-13. Globally, there are 228 hydrogen projects across the value chain. Of these, 17 are already-announced giga-scale production projects (i.e., more than 1 GW for renewable and over 200 thousand tons a year for low-carbon hydrogen), with the biggest in Europe, Australia, the Middle East and Chile.
_
-14. There are trade-offs between centralized and distributed production to consider. Producing hydrogen centrally in large plants cuts production costs but boosts distribution costs. Producing hydrogen at the point of end-use—at fueling stations, for example—cuts distribution costs but increases production costs because of the cost to construct on-site production capabilities. Small regional plants or even local filling stations could generate hydrogen using energy provided through the electrical distribution grid or steam reforming of natural gas. While hydrogen generation efficiency is likely to be lower than for centralized hydrogen generation, losses in hydrogen transport could make such a scheme more efficient in terms of the primary energy used per kilogram of hydrogen delivered to the end user.
_
-15. Nuclear energy can produce hydrogen not only in large quantities but also at high quality at a relatively low cost without any GHG emissions. An inherent advantage over technologies that only produce electricity (like wind and photovoltaic solar) is nuclear’s capacity to produce both electricity and heat, affording it the ability to take advantage of all hydrogen production technology options. Large-scale hydrogen production using nuclear energy involve four most promising processes for hydrogen production: high temperature electrolysis, thermochemical processes including S-I process, low temperature conventional electrolysis and steam reforming of methane. Hydrogen production via nuclear electricity has a comparable carbon footprint to hydrogen produced by renewables.
_
-16. The high mass-based energy density of hydrogen makes it one of the most promising future fuels. Hydrogen contains 33 kWh usable energy per kilo, compared to 12 kWh of petrol and diesel. However, storing the same amount of hydrogen requires a larger volume. The development of hydrogen storage technologies is, therefore, a fundamental premise for hydrogen powered energy systems. Conventional technologies store the hydrogen as compressed gas and cryogenic liquid, while for large-scale applications, underground storage turns out to be a preferable method. Certain metals and metal alloys have the ability to absorb hydrogen under moderate pressure and temperature, creating hydrides. Several metal hydrides are available commercially, representing a good solution for hydrogen storage where the weight factor is not a problem. For vehicles, the problem with metal hydride is the high weight compared to the amount of hydrogen stored. The problem of weight has still not been solved in spite of extensive research.
_
-17. Gaseous hydrogen can be transported through pipelines much the way natural gas is today. There are some additional problems, because hydrogen tends to leak more and can embrittle some metals used for pipelines. Transporting gaseous hydrogen via existing pipelines is a low-cost option for delivering large volumes of hydrogen. With little or no changes, the majority of existing steel natural gas lines can be used to transport mixtures of natural gas and hydrogen. For transporting 100% hydrogen under high pressure, existing natural gas pipeline cannot be used.
Hydrogen embrittlement is not a problem for steel pipelines. Hydrogen embrittlement only happens with ‘diffusible’ hydrogen, i.e., atoms or ions. Hydrogen gas, however, is molecular (H2), and there is a very significant energy barrier to splitting it into atoms. Carrying hydrogen in steel pipelines (grades: API5L-X42 and X52; up to 1,000psi/7,000kPa, constant pressure/low pressure cycling) does not lead to hydrogen embrittlement. Hydrogen is typically stored in steel cylinders without problems. Coal gas (also known as town gas) is 50% hydrogen and was carried in cast-iron pipes for half a century without any embrittlement issues. Hydrogen is the smallest element and thus has a slightly higher propensity to leak from venerable natural gas pipes such as those made from iron. Leakage from plastic (polyethylene PE100) pipes is expected to be very low at about 0.001% but polyethylene pipes can transport low pressure hydrogen. High-pressure hydrogen pipelines today use steel alloys.
_
-18. The average cost of a natural gas pipeline with diameter of 30 inches is about $5 million per mile. The US has about 300,000 miles of natural gas pipeline. Hydrogen-specific steel pipelines can cost as much as 68 percent more than natural gas pipelines, depending on pipe diameter and operating pressure. The major operating cost of hydrogen pipelines is compressor power and maintenance. Compressors in the pipeline keep the gas moving, using hydrogen energy to push the gas forward. After 620 miles, 8% of the hydrogen will have been used to move it through the pipeline. The energy loss in an electric power grid can be up to 7.5-8% of the energy it is transferring. To build an electric grid, it costs $ 2 million per km of transmission lines, and between $ 200,000 per km up to $ 1 million per km of distribution lines. This includes transformers costs. So electric grid is cheaper than gas grid.
_
-19. Hydrogen is more energy dense than gasoline (by weight) and hydrogen powered transportation is more energy efficient than gasoline. Yet the hydrogen filling station will require 15 deliveries every day, everything else being equal. This is so because 40 ton truck can deliver 26 tons of gasoline to a conventional gasoline filling station enough to fill about 800 cars. One daily delivery is sufficient for busy station. A 40 ton truck carrying compressed hydrogen can deliver only 400 kilograms enough fuel for 60 cars. For a delivery distance of 150 miles, the delivery energy used is nearly 20% of the usable energy in the hydrogen delivered; at 300 miles it is 40%. The energy cost of truck transport becomes unacceptable unless the source of hydrogen is very close to the point of use. Because hydrogen contains less energy per unit volume than all other fuels, transporting, storing, and delivering it to the point of end-use is more expensive on a per gasoline gallon equivalent basis.
_
-20. It is possible to mix 20% hydrogen by volume into the natural gas supply and have everything work as normal. The 20% proportion is an optimal blend that won’t affect gas pipes and appliances. To be able to use 100% hydrogen new pipe infrastructure, new boilers and cooking appliances would have to be installed. So, the switch to a hydrogen grid will not be easy or cheap.
If a 20% blend were to be rolled out across Britain, it would reduce emissions of CO2 by six million tonnes – equivalent to taking 2.5 million cars off the road. However, switching from natural gas to 20 % hydrogen blend will either lock us into using even more natural gas than we are now (if the hydrogen is made via steam reforming) or will be horrendously expensive for consumers (the electrolysis option). Efforts to lower carbon emissions from natural gas networks by replacing some of the gas with hydrogen could see higher bills for consumers.
Better option is heating can be switched from carbon-intensive natural gas to clean electric heat pumps, which can take advantage of the electricity grid’s rapid decarbonisation. In future, it will also be able to benefit from the rapidly falling cost of renewable power, A typical air-source heat pump will deliver about 3 kWh of heat for every 1 kWh of electricity used to power it. This is six times as efficient as using hydrogen created via electrolysis for heating. Every kWh of electricity could be used either to make 0.5 kWh worth heat by producing hydrogen, or 3 kWh of heat via an air source heat pump.
_
-21. Development of hydrogen as a transport fuel has historically been limited by the decision of what comes first, the cars or the refuelling infrastructure—the ‘chicken-and-egg’ problem. As long as the network of refueling stations for hydrogen-powered cars is so thin, the low demand from customers will not allow for profitable mass production of fuel cell vehicles. And as long as there are hardly any hydrogen cars on the roads, the operators will only hesitantly expand their refueling station network. Who will spend the hundreds of billions of dollars on a wholly new nationwide infrastructure to provide ready access to hydrogen for consumers with fuel cell vehicles until millions of hydrogen vehicles are on the road? And who will manufacture and market such vehicles until the infrastructure is in place to fuel those vehicles? Will car companies and fuel providers be willing to take this chance before knowing whether the public will embrace these cars?
_
-22. For a sustainable future, electricity from renewable sources will become the dominant primary energy carrier replacing chemical carriers of today’s economy. Hydrogen would make sense only if its production, distribution, and use are superior to the distribution of electricity by wires. Hydrogen is not an ideal vector to carry energy from its place of production to the end user, because a fairly high amount of energy is lost during production, storage and transportation. Making hydrogen from natural gas is 72% efficient, which means you lose 28% of the energy contained in the natural gas to make hydrogen. The electrical efficiency of AEL systems is around 63%–73%, while the ultimate goal is 70%–80%. A PEMEL system has a lower efficiency of around 60%, which is expected to be improved to 67%–74% in the future.
Compressing hydrogen to 10,000 psi is a multi-stage process that loses 15% of the energy contained in the hydrogen. At 10,000 psi pressure, compressed hydrogen is dangerous and must be stored in heavy tanks. Liquefying hydrogen allows more hydrogen energy to fit into a smaller container, but a further 30-40% of the hydrogen’s energy is lost in the process. Compressors in the pipeline keep the gas moving, using hydrogen energy to push the gas forward. After 620 miles, 8% of the hydrogen will have been used to move it through the pipeline. Canister trucks can carry enough fuel for 60 cars. These trucks weigh 40,000 kg but deliver only 400 kg of hydrogen. For a delivery distance of 150 miles, the delivery energy used is nearly 20% of the usable energy in the hydrogen delivered; at 300 miles it is 40%.
Hydrogen fuel cells are more energy-efficient than internal combustion engines. However, lithium-ion batteries are still the most energy efficient and the highest performing energy source for vehicles. Hydrogen fuel cells do not beat lithium-ion batteries when it comes to efficiency, costs, and safety. Tank to wheel efficiency of fuel cell is generally between 40 and 60%. This is higher than typical internal combustion engine of a car with about 20% energy efficiency. Coulombic efficiency (CE) describes the charge efficiency by which electrons are transferred in batteries. CE is the ratio of the total charge extracted from the battery to the total charge put into the battery over a full cycle. Li-ion has one of the highest CE ratings in rechargeable batteries. It offers an efficiency that exceeds 99 percent. While the coulombic efficiency of lithium-ion is normally better than 99 percent, the energy efficiency of the same battery has a lower number and relates to the charge and discharge C-rate. In the real world, Tesla Roadster battery is said to have an energy efficiency of 88 percent. The “power-plant-to-wheel” efficiency of a fuel cell vehicle operated on compressed gaseous hydrogen is about 23 to 30 % while the same for battery electric vehicle is 70 to 80% according to various studies. In other words, hydrogen fuel cell electrical vehicle requires more than double the amount of energy than battery electric vehicle.
Converting power to hydrogen and then using the fuel to generate power has a relatively low round-trip efficiency. Round-trip efficiency is the percentage of electricity retrieved after being stored. The technology to convert power to hydrogen and back to power has a round-trip efficiency of 18%-46%, A typical lithium-ion battery has round-trip efficiency of 95% with self-discharge losses of around 5% of capacity per month. Energy losses within a hydrogen economy shows that a hydrogen economy is an extremely inefficient proposition for the distribution of electricity from renewable sources to useful electricity from fuel cells. Because of the high energy losses within a hydrogen economy, hydrogen cannot compete with its own energy source electricity.
But conventional energy systems based on fossil fuels are already highly inefficient, with combustion engine cars returning as little as 20% of the energy in petrol as useful forward motion. Similarly, the average efficiency of coal-fired power plants is just 33%. This suggests low efficiency is not a fundamental barrier to the use of hydrogen. Instead, low efficiency may hold back hydrogen via higher costs and the need for a larger energy supply.
_
-23. Hydrogen could be generated from renewable electricity, but that would be expensive and, renewable electricity has better uses for the next few decades. Nonetheless, hydrogen and electricity, as energy carriers, are complementary in the energy transition. Hydrogen from renewables has the technical potential to channel large amounts of renewable electricity to sectors for which electrification and hence decarbonisation is otherwise difficult, such as aviation, shipping, long-distance trucking, and concrete & steel manufacturing. These sectors are hard to electrify because they often require fuel that is high in energy density or heat at high temperatures. Green hydrogen could meet these needs. However, green hydrogen is not economically competitive at present, and therefore significant reductions in the cost of production and distribution need to take place for the decarbonisation of such sectors to take place.
_
-24. The largest single use of hydrogen in the world is in ammonia manufacture, which consumes about two-thirds of the world’s hydrogen production. Ammonia is currently adopted as an agricultural fertilizer, refrigerant gas and in the manufacture of explosives, pesticides and other chemicals.
Ammonia gas can be stored as a liquid at room temperature and pressure when mixed with water. Green ammonia is made from green hydrogen which is produced using renewable energy. Ammonia can be reformed to produce hydrogen with no harmful waste, or can mix with existing fuels and under the right conditions burn efficiently. Since there is no carbon in ammonia, no carbon by-products are produced; thereby making this possibility a “carbon neutral” option for the future. Looking to the future, ammonia could have a key role in the storage and transport of hydrogen. Ammonia can deliver more energy than hydrogen within the same volume, and it has an established infrastructure and lower handling costs. Due to its stability for long-term storage and transportation, ammonia can fulfill the demand to store the energy in time (stationary energy storage) and in space (energy export and import). Ammonia can be utilized by extracting its stored hydrogen or directly utilized as fuel. Conversion to and from ammonia is likely to add about $1/kg to the hydrogen production cost. As a fuel, ammonia is particularly suitable for long-haul shipping. The most important advantage of using ammonia is that it’s a practical and feasible opportunity to reduce greenhouse gas emissions from shipping.
_
-25. Hydrogen is odorless, colorless and tasteless, so most human senses won’t help to detect a leak. By comparison, natural gas is also odorless, colorless and tasteless, but industry adds a sulfur-containing odorant called a mercaptan to make it detectable by people. As in natural gas, an odorant can be added to hydrogen sources to enable leaks to be detected by smell but odorants are not used with hydrogen because there are no known odorants light enough to “travel with” hydrogen at the same dispersion rate. Current odorants and potential odorants have negative impacts on fuel cell performance. That is why hydrogen sensors are used to detect hydrogen leaks and it has maintained a high safety record for decades. Hydrogen burns with a pale blue flame that is nearly invisible in daylight, so it is almost impossible to detect by the human senses. Both hydrogen sensors and flame detectors are almost always installed with hydrogen systems to quickly identify any leak and minimize the potential for undetected flames.
_
-26. Hydrogen is highly flammable in air. It requires less oxygen than other gases to burn. Hydrogen leaks can support combustion at very low flow rates, as low as 4 micrograms/s. Hydrogen is very susceptible to ignition after sudden release from high pressure containment as temperature of hydrogen gas increases upon depressurization. As with any fuel source, there are risks and dangers, but hydrogen has been used safely across the world for many decades. Hydrogen is lighter than air as a gas and will rise at almost 20 meters per second (twice as fast as helium), meaning that in the event of a leak it will disperse very quickly into a non-flammable concentration. In contrast, diesel and petrol leaks often result in the build-up of flammable gas which also produces hot ash as it burns, creating radiant heat. If hydrogen gas does ignite it does not produce any hot ash and burns out very quickly. In a closed environment, leaks of any size are a concern, since hydrogen is impossible for human senses to detect and can ignite over a wide range of concentrations in air. Hydrogen collects under roofs and overhangs, where it forms an explosion hazard; so any building that contains a potential source of hydrogen should have good ventilation. Ventilation is therefore an important factor in areas where hydrogen is used. Proper ventilation and the use of detection sensors can mitigate these hazards.
_
-27. If a large hydrogen cloud comes into contact with an ignition source, ignition will likely result in the flame flashing back to the source of the hydrogen. In open spaces with no confinement, flames will propagate through a flammable hydrogen-air cloud at several meters per second, and even more rapidly if the cloud is above ambient temperature. The result is a rapid release of heat, but little overpressure, and the combustion product is steam. It should be noted that hydrogen combustion is more rapid than combustion of other fuels. A hydrogen cloud will burn within seconds, and all of the energy of the cloud will be released. However, a hydrogen gas mixtures ignited in a confined space can generate pressures high enough to rupture equipment, exploding buildings and throw shrapnel. So, keeping hydrogen equipment and piping outdoors is an inherent safety advantage.
_
-28. If liquid hydrogen is spilled on ambient-temperature surfaces, liquid hydrogen will rapidly boil and its vapors will expand rapidly, increasing 848 times in volume as it warms to room temperatures. If liquid hydrogen is confined (such as between valves closing off a length of pipe) and left to warm without pressure relief, pressures approaching 170 MPa (25,000 psi) are possible. Confinements will likely rupture under such pressures, producing high-pressure jets of gas and high-speed shrapnel. Ignition is extremely likely under such circumstances. Because of its flammability, liquid hydrogen should be kept away from heat or flame unless ignition is intended.
_
-29. Enormous amounts of hydrogen are generated, handled, transported and used in the chemical industry today. But this hydrogen is a chemical substance, not an energy commodity. Hydrogen production and transportation costs are absorbed in the price of the synthesized chemicals. The cost of hydrogen remains irrelevant as long as the final products find markets (e.g., ammonia for fertilizers). So do not confuse between cost of hydrogen as chemical and cost of hydrogen as fuel. In hydrogen economy, hydrogen is used as a clean fuel to replace fossil fuels and therefore cost matters as it is compared to fossil fuels.
_
-30. The biggest issue with green hydrogen is the cost. Green hydrogen currently costs around $5-6 per kg to produce from renewable energy, compared to around $2 per kg for hydrogen produced from fossil fuels. The production cost for green hydrogen is determined by the renewable electricity price, the investment cost of the electrolyser and its operating hours. Although renewables have already become cheap source of power in many parts of the world, with lifetime costs (when including subsidies) of power are $31 per megawatt-hour for utility solar and $26 per megawatt-hour for wind, it simply isn’t cost effective to produce hydrogen using intermittent renewables. It could become cost effective if the renewable supply is overbuilt, and hydrogen production only takes place when there is excess electricity being produced. However, that means that all of the associated hydrogen production equipment is only being utilized a small fraction of the time. Because of the low-capacity factor of renewables, the subsequent capital costs of the hydrogen equipment drive the price quite high per unit of mass of hydrogen produced. Also, investment costs for electrolysis facilities must fall significantly too. Cutting the cost of electrolyzers will be critical to reducing the price of green hydrogen, but that will take time and scale.
_
-31. For every kW of electricity supply, you get 800W for a BEV, but only 300W for hydrogen FCEV – less than half as much. That’s a huge inefficiency if you’re hoping for a greener future, and doesn’t even take into account the fact that 95% of hydrogen is currently generated from fossil fuel sources. No sustainable economy can afford to use twice the amount of renewable energy to drive with hydrogen fuel cell passenger cars rather than battery-powered vehicles.
The two advantages of FCEV – longer range and fast fueling times – are rapidly being eroded by improving battery and charging technology.
Hydrogen FCEV are more expensive to drive than battery-powered vehicles, not only in terms of purchase, but above also in terms of operation. The fuel cost per kilometer is a little more than 3 times greater for hydrogen FCEV than BEV. In fact, fuel cost of running BEV is cheapest and fuel cost of running FCEV is most expensive while petrol/diesel would come in between. Fuel cells add to an electric car’s price and complexity while offering no benefit in performance.
We should not pursue a strategy to reduce greenhouse gas emissions in the transportation sector that would undermine efforts to reduce greenhouse gas emissions in the electric generation sector. Yet that is precisely what a hydrogen car strategy would do for the next few decades. For near-term deployment, hydrogen would almost certainly be produced from fossil fuels. In the United States, the average battery electric vehicle emits 176 grams of CO2 per mile when charging from an average electricity grid. Fuel cell electric vehicles fueled by hydrogen from steam-methane reforming emit about 241 grams of CO2 per mile, while gasoline powered internal combustion engine vehicles emissions hit 414 grams CO2 per mile.
In the case of passenger car, everything speaks in favor of the battery and practically nothing speaks in favor of hydrogen. Many of the ongoing investments in hydrogen cars seem to follow the sunk cost fallacy: we have already spent so much on this technology, let’s not give up now. A hydrogen car is one of the least efficient, most expensive ways to reduce greenhouse gases. No matter how excellent you make the cars themselves, the laws of physics hinder their overall efficiency. The most efficient way to convert energy to mobility is using electricity directly.
Hydrogen could only be used in niches, in trucks and buses, and over long distances. Battery weight, range and fueling time play a decisive role here. For buses and trucks carrying heavy payloads, lengthy battery recharge times can affect business models.
_
-32. Despite impressive developments, batteries and fuel-cell technology are not yet ready to meet the very high-power requirements needed for the harsh conditions to which many heavy-duty vehicles (especially in the off-highway segment) are exposed. Mining trucks, for instance, require several megawatts worth of power, run around the clock, and are exposed to extreme vibrations and heat development, as well as dirt in the air. Internal combustion engines have met these requirements for decades, and a switch from diesel to hydrogen could be a straightforward way to decarbonize these engines, with a relatively minor requirement for further technical innovation. Low capex requirements for combustion engines, decreasing hydrogen prices, and the relatively high efficiencies achieved by hydrogen internal combustion engine at high loads create conditions in which hydrogen combustion can be a TCO-competitive solution. However, compared to FCEVs, these produce tailpipe emissions and are less efficient.
_
-33. The intermittent nature of some renewable energy resources makes them time and season-dependent. Therefore, the generated renewable energy needs to be stored in a reliable form, which should be tolerant to the fluctuation and randomness of those renewable energy sources. There are several existing energy storage options, e.g., pumped hydro energy storage, compressed air energy storage, batteries, etc. Pumped hydro and compressed air energy come with geographic and environmental constraints, pumped hydro requires a water reservoir, while conventional compressed air energy requires burning fossil fuels. Batteries cannot be used for long-term energy storage owing to their low energy storage density (Lithium-ion battery 0.46-0.72 MJ/kg) and unavoidable self-discharge. Batteries last for a finite number of recharge cycles, and their life is shorter in cold weather use. Compared with them, hydrogen has its advantages of high energy storage capacity (120 MJ/kg), long storing period and flexibility. It can smooth out the energy volatility & uncertainty, and absorb, especially, the excess renewable energy generation. Most battery-based solutions store energy from one to four hours. Longer-lasting solutions (12+ hours) are not cost-effective. Hydrogen can be held indefinitely in storage until needed. Hydrogen or its derivatives can be used to ‘store’ energy created by renewable sources and help to overcome the problem of intermittency – that is, to level out the fluctuations in production and consumption, and to efficiently distribute and transport the energy. When it comes to long-term and large-scale energy storage, hydrogen (in the form of compressed gas, ammonia (NH₃) or synthetic methane) has a role to play in balancing seasonal variations in electricity supply and demand from renewable energy sources. Hydrogen can play an important role in energy storage as part of the electricity grid to help smooth out the contribution of renewables such as wind and solar. This could work by using the excess renewable energy (when generation is high and/or demand is low) to drive hydrogen production via electrolysis of water. The hydrogen can then be stored as compressed gas and put into a fuel cell to generate electricity when needed. If you have renewable power, convert it to hydrogen and re-electrify it, with a total cycle efficiency of less than 40%, it obviously only makes sense if you’re using hydrogen as long-term storage and compensation for variable renewables. Large-scale hydrogen storage will be useful to reduce curtailment of wind and solar power during windy/sunny periods. The stored hydrogen can also be used to provide fuel for fuel cell vehicles, injected into natural gas pipelines to reduce their carbon intensity, stored as a compressed gas, cryogenic liquid, ammonia or wide variety of loosely-bonded hydride compounds for later use, or exported to another country. The use of hydrogen in the power or gas grid offers the opportunity to decarbonize all economic sectors.
_
-34. Power-to-X (P2X) concept is the utilization of renewable electricity to produce hydrogen through the electrolysis of water, and hydrogen can be easily stored and further used or processed in many ways to generate other energy carriers – gases, liquids, and heat – and chemical feedstocks. Each of the “downstream derivates” of hydrogen (e.g., synthetic methane, synthetic diesel, methanol, ammonia) comes with its own value-chains. By enabling these conversions, hydrogen has the potential to connect different parts of the energy system, also known as “sector coupling”. Power-to-gas (P2G) describes the process of converting renewable energy to gaseous energy carriers such as hydrogen or methane via electrolysis. Power-to-Power (P2P) stores hydrogen from renewables and re-electrify when needed using a fuel cell (kW to MW scale) or a hydrogen gas turbine (multi-MW scale). P2X including P2G and P2P are possible because of the declining costs of renewable power generation. P2P & P2G group of technologies suffers from high cost and low efficiency. Owing to the number of transformation steps the final efficiencies are rather low for P2P and P2G, in the range 20 to 30%.
_
-35. The hydrogen power plant includes an H2-fired gas turbine, electrolyzers with H2 compression, storage and management system to integrate all components including renewable energy sources feeding electricity into the electrolyzer. Hydrogen can be produced and stored from renewable electricity and then added to the natural gas turbine power plants. Gas turbines can run on varying levels of hydrogen. The scope of the required modifications to configure a gas turbine to operate on hydrogen depends on the initial configuration of the gas turbine and the overall balance of plant, as well as the desired hydrogen concentration in the fuel. Hydrogen power plants can also operate as combined-cycle power plants, which are more efficient because they use surplus heat to generate steam that powers a second turbine thereby getting 64% power-generation efficiency. By integrating solid oxide fuel cell technology with turbines, the electrical efficiency of hydrogen power plant can reach up to 80% under optimum conditions.
_
-36. Grey hydrogen production emits at least 10 kg CO2 per kilo hydrogen production. Grey hydrogen emits as much CO2 emissions as the United Kingdom and Indonesia combined, and therefore cannot be considered as low-carbon options. And even blue hydrogen should not be regarded as CO2-neutral or as clean alternative, as research has just investigated its emissions and found out that it may be causing more harm than burning natural gas or coal directly for heat. To make the hydrogen supply pathways environment friendly, the only alternative left is to generate and use electricity from renewable sources like hydro, wind, solar, etc. to produce green hydrogen. However, to produce all of today’s dedicated hydrogen output (75 million ton per year) using renewable energy (rather than fossil fuels) would require more electricity than the annual amount generated by the European Union in 2020. So, it is clear that renewable energy capacity needs to be increased vastly to produce green hydrogen on scale, or use nuclear energy to produce green hydrogen on scale.
_
-37. The only environmental advantage of non-green hydrogen pathways is the possibility of shifting the pollution to the locations where it is being produced from the locations where it is being used, as utilization of hydrogen in fuel cell does not cause any pollution. This may be significant from the perspective of reducing the urban pollution related to transport.
_
-38. Most experts agree that green hydrogen will be essential to meeting the goals of the Paris Agreement but first we need to improve energy efficiency, accelerate electrification, accelerate renewable power generation, accelerate deployment of renewable electricity to decarbonize existing power systems, and then expand renewable power capacity to produce competitive green hydrogen and decarbonise hard-to-abate sectors at minimal extra cost. We are jumping gun to green hydrogen.
_
-39. Limitations of hydrogen includes its explosiveness, low Well-to-Wheel and round-trip efficiency, low energy density per volume, ability to cause embrittlement in metals, escapes through the tiniest leaks and costly infrastructure for production, storage, and distribution. In spite of these problems, hydrogen still holds a vice-like grip over the imaginations of techno-optimists. The Hydrogen Council, a global industry group, estimates that by 2050 hydrogen will represent 18 per cent of the energy delivered to end users, avoid six gigatonnes of carbon emissions annually, enable US$2.5 trillion in annual sales and create 30 million jobs globally.
_
-40. Stranded investment is one of the greatest risks faced by hydrogen production technologies due to botched deployment, technical problems, need to replace entire infrastructure or hydrogen cars simply never achieve the combination of popularity, cost, and performance to triumph in the marketplace. And billions that could have been spent on other decarbonization technologies are wasted.
_
-41. Hydrogen produced by steam methane reforming (SMR) costs approximately three times the cost of natural gas per unit of energy produced. Also, producing hydrogen from electrolysis using electricity will cost two times the cost of hydrogen from natural gas. So using natural gas directly for energy is cheaper than hydrogen from natural gas which in turn is cheaper than hydrogen from electrolysis.
The fuels that might be used to make hydrogen, such as natural gas or renewable sources, could be better used simply to make electricity. For example, well-to-wheels efficiency of a hydrogen car running on gas-derived hydrogen is likely to be under 30 percent for the next two decades. The efficiency of gas-fired power plants is already 55 percent and likely to rise to 60%. Cogeneration of electricity and heat using natural gas is more than 80 percent efficient. And by displacing coal, the natural gas would be displacing a fuel that has much higher carbon emissions per unit of energy than gasoline. For these reasons, natural gas is far more cost-effectively used to reduce CO2 emissions in electric generation than it is in transportation. Natural gas has too much economic and environmental value to the electric utility, industrial, and building sectors to justify diverting significant quantities to the transportation sector, thereby increasing the price for all users. In fact, using natural gas to generate significant quantities of hydrogen for transportation would, for the foreseeable future, undermine efforts to combat global warming.
The same is true for renewable energy. About 100 kWh of renewable energy usually produces somewhere between 60-70 kWh of hydrogen energy. It makes more sense to use renewable energy directly as electricity for end uses rather than losing 30 to 40% of it through green hydrogen production, and thereby indirectly leading to higher fossil fuel requirements to make up for the 30-40% loss. A megawatt-hour of electricity from a renewable source such as wind power, if used to manufacture hydrogen for use in fuel cell vehicle, would save slightly less than 500 pounds of CO2 as compared to the best current hybrids. That is less than the savings from using the same amount of renewable electricity to displace natural gas plant (800 pounds) and far less than the savings from displacing coal power (2,200 pounds). Until CO2 emissions from electricity generation are virtually eliminated, it will be far more cost effective to use renewable electricity (such as wind, solar, hydro) to reduce emissions by substituting for fossil-electric generation than to use renewable electricity to make hydrogen.
In a nutshell, using natural gas/renewable source directly without intermediary hydrogen can give us cheap and clean energy. Using hydrogen as a fuel made from natural gas/renewables makes little sense, either economically or environmentally. It would be foolish to use hydrogen as a fuel barring compelling reasons such as to store surplus renewables power long term, decarbonize hard-to-electrify sectors, export renewable energy, and energy security.
______
Dr. Rajiv Desai. MD.
March 4, 2022
______
Postscript:
Hydrogen as chemical combined with nitrogen to produce ammonia for use in fertilizer, and that increased crop yields to feed earth’s exploding population over the last 100 years. But hydrogen as fuel is unlikely to revolutionize energy sector. Laws of science make hydrogen inefficient, expensive and explosive compared to its alternative of using electricity directly or through battery. Energy efficiency, renewables, nuclear, switch from coal to natural gas and direct electrification are the bulk solutions to climate change, and the only role green hydrogen can play is to decarbonize hard-to-electrify sectors.
_______
Footnote:
Countless researches have been conducted in hydrogen technologies. Time and time again these advances have failed to translate into commercial applications. These researches have not been accommodated in this article due to space constraint and not due to unfeasibility.
_____


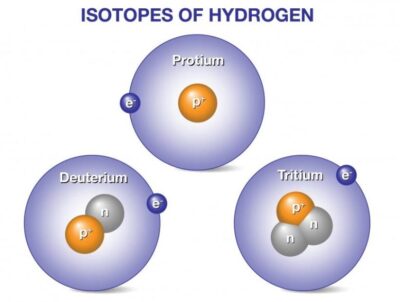

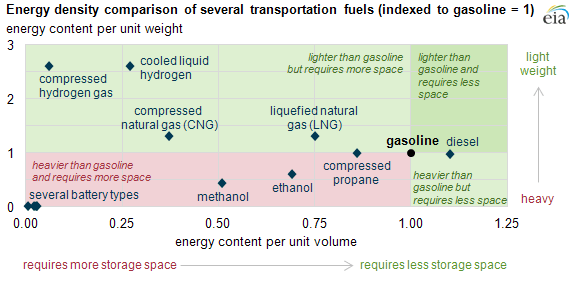
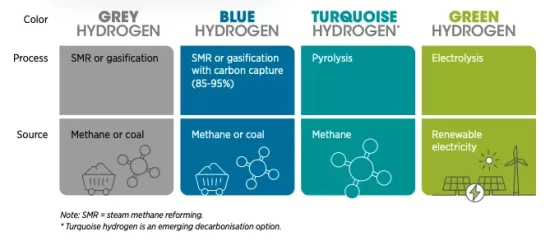
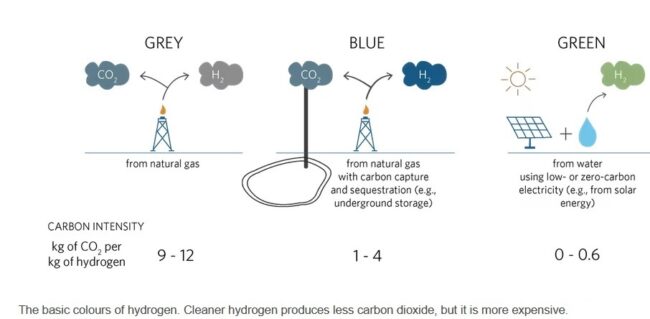
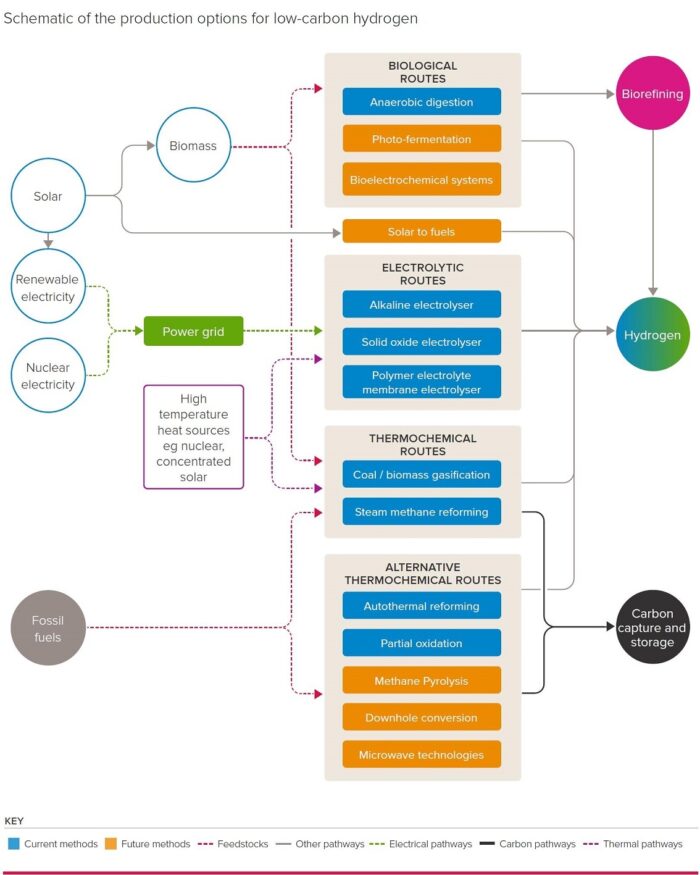
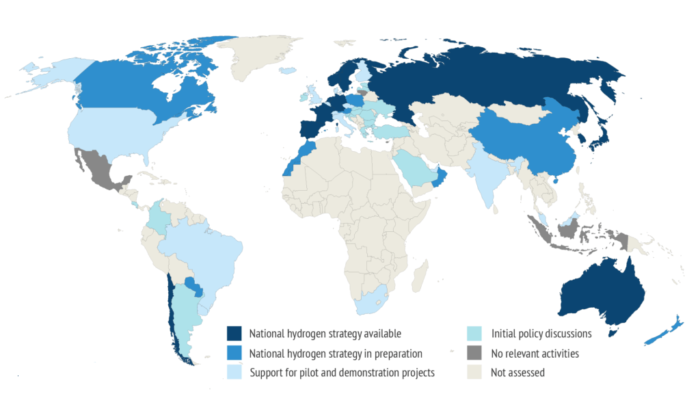
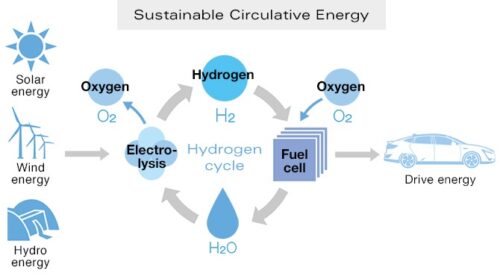

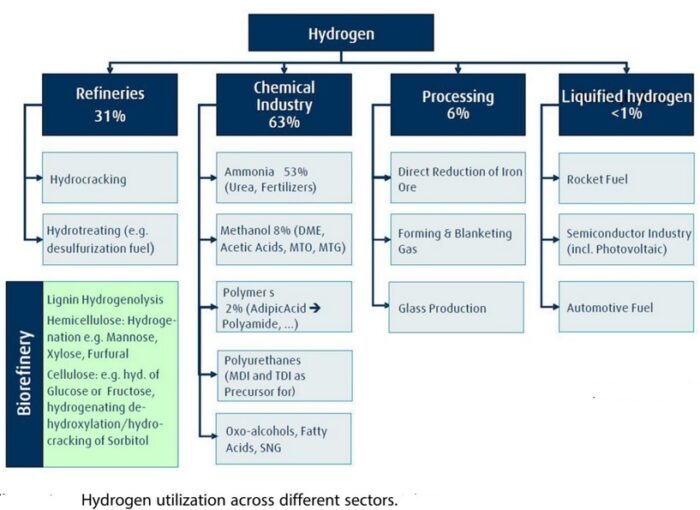


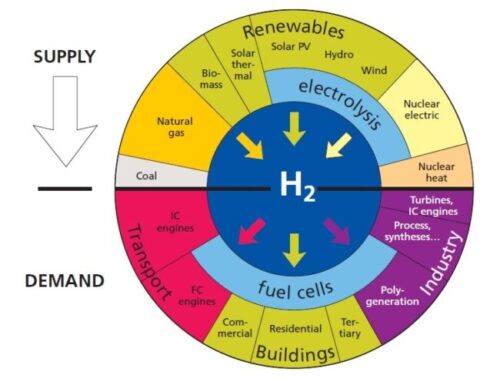

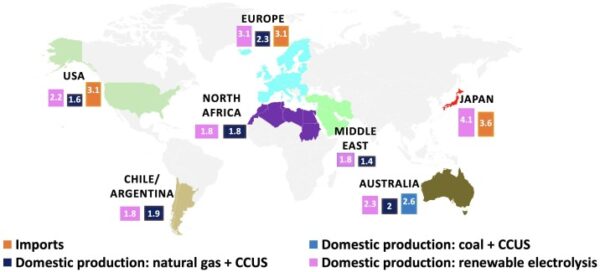

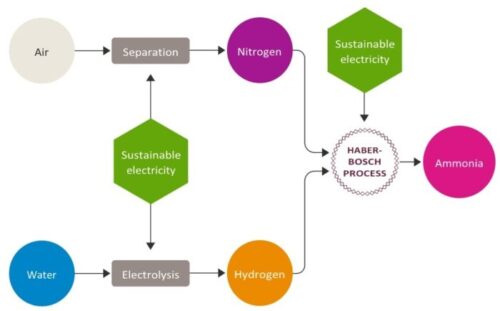
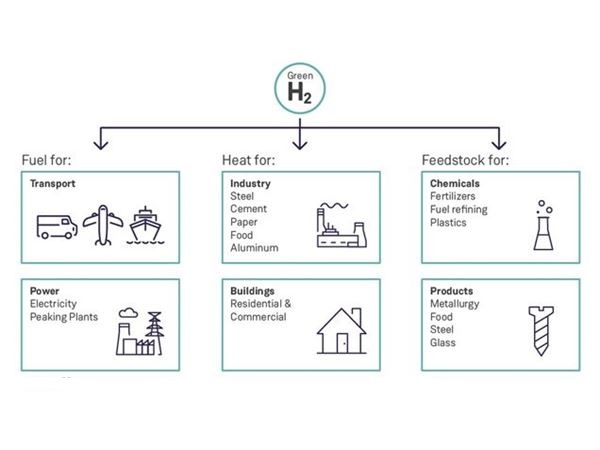


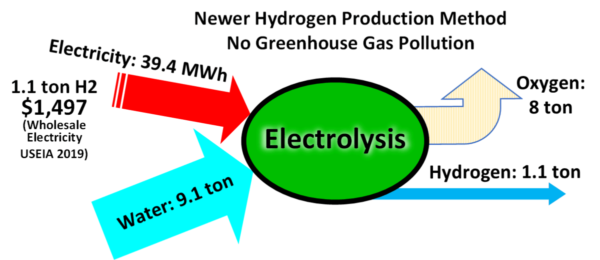
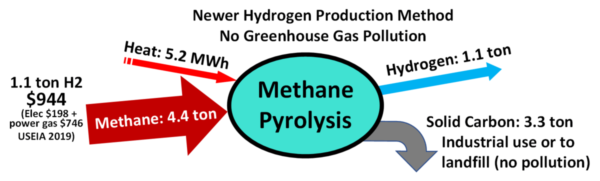
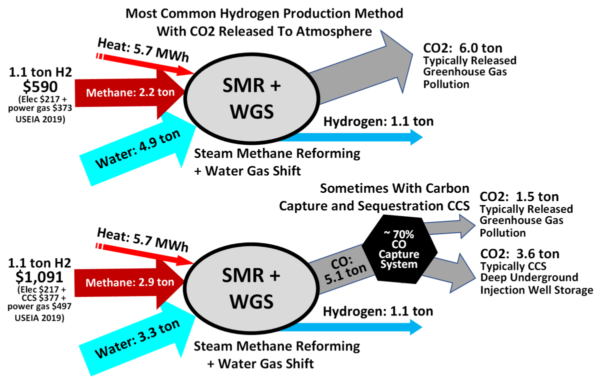

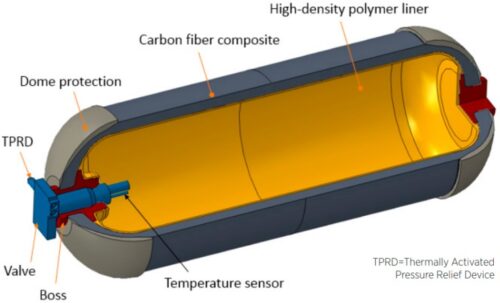


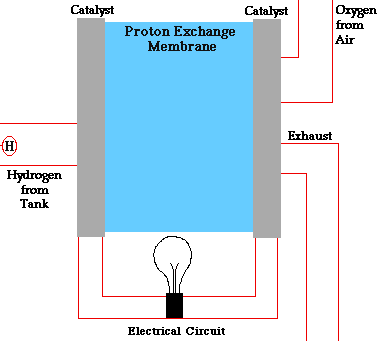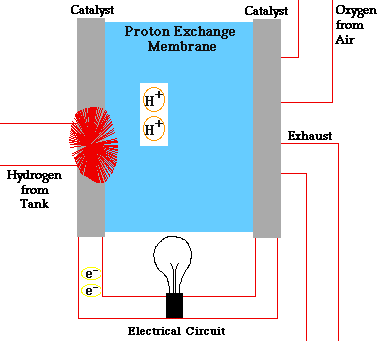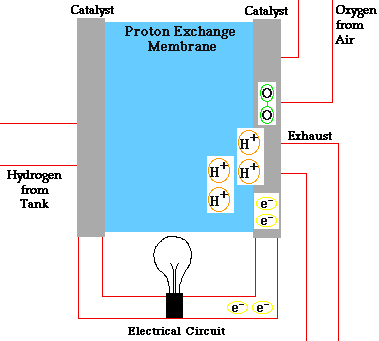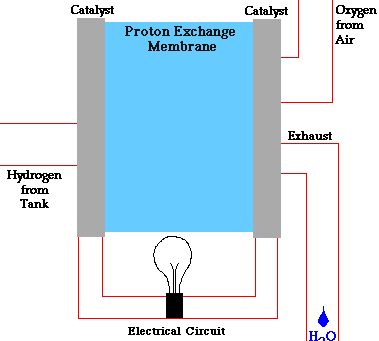
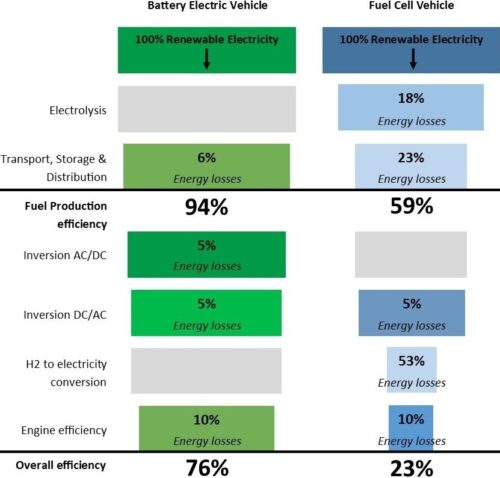
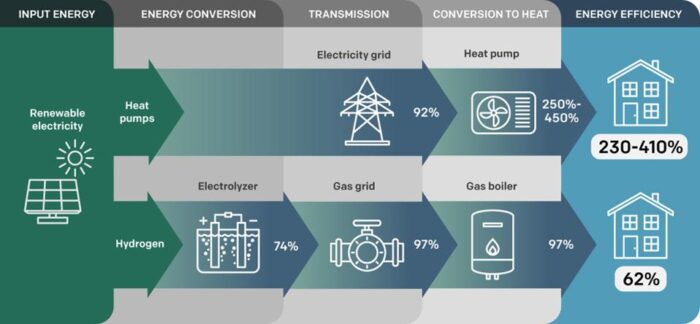

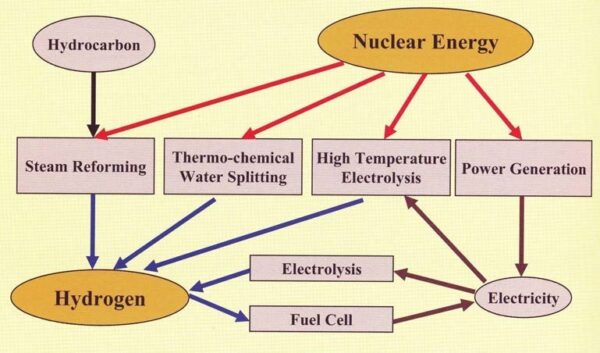

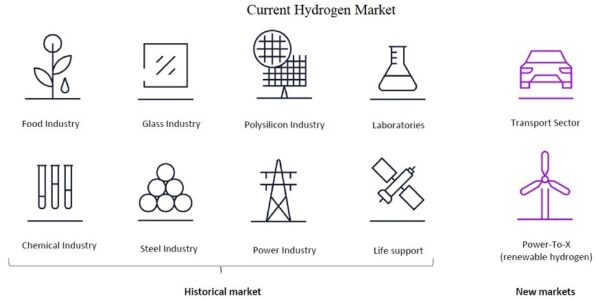
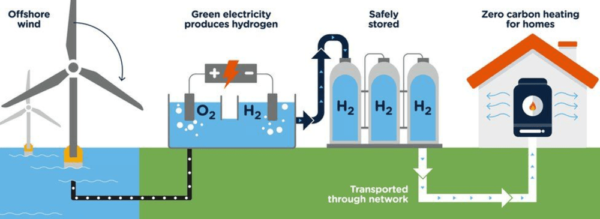
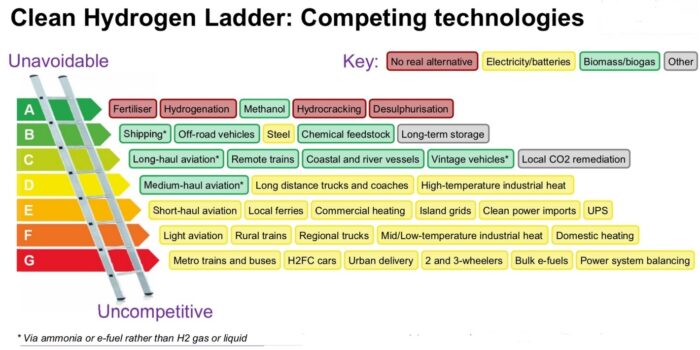

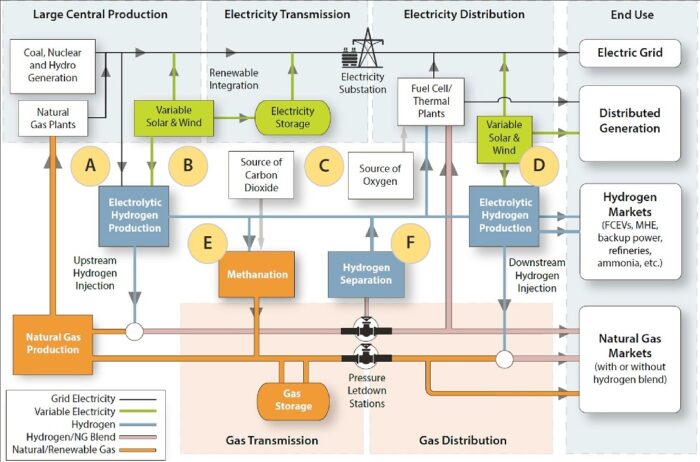


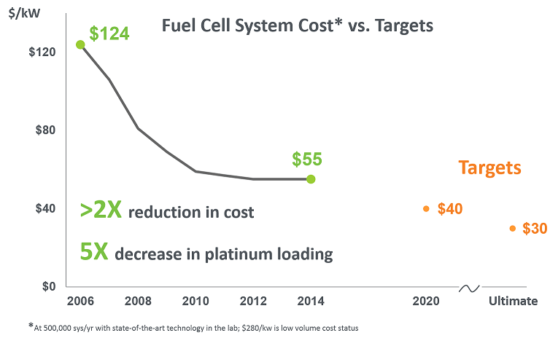


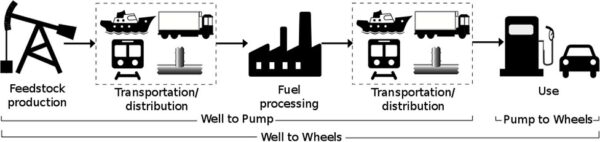



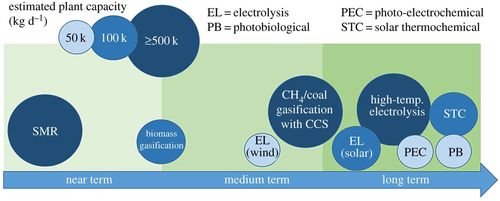
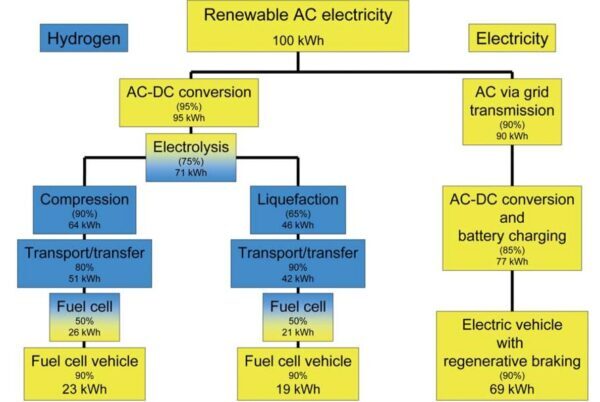
Really appreciate you sharing this blog article.Really thank you! Awesome.
%%
This is a topic that is near to my heart… Cheers! Exactly where are your contact details though?