Dr Rajiv Desai
An Educational Blog
Over-The-Counter (OTC) Drugs
Over-The-Counter (OTC) Drugs:
_
The term OTC does not mean that the drugs are literally sold over a counter. Oftentimes, they can simply be taken off a shelf, and paid for with your other shopping.
_
Section-1
Prologue:
The Italian adventurer Casanova (1725–1798) is often quoted as saying: “In wise hands, poison is medicine. In foolish hands, medicine is a poison.” Such is the case with over-the-counter (OTC) drugs—those medicines found on shelves in drugstores, convenience stores, and supermarkets. Buying of OTC medicines is the self-medication practice. From the very ancient period, people used different types of herbs for the treatment of their health problems. Once a common practice across centuries of human history, self-diagnosis and self-treatment are making a comeback. OTC medicines are the most common choice for self-treatment, with almost everyone having some experience in using or purchasing such medicines.
Patients often approach pharmacy or grocery store instead of visiting a doctor for minor ailments such as cough, cold, allergies, pain, fever, acidity, diarrhea, and skin-related conditions. Purchase of specific medicines over the counter is legally recognized in most countries. Over the counter (OTC) medicines are the drugs that can be sold legally without the prescription of a registered medical practitioner to the consumer. Over-the-counter drugs are commonly used and most people can assume responsibility for using them on their own. Self-medication with use of over-the-counter drugs without medical supervision is a type of self-care. The need to save on healthcare spending and the trend to enhance self-care have led to more emphasis on patients taking their own responsibility for the management of minor ailments, including the use of medication that is available without a prescription.
OTC medicines are an important component in healthcare, allowing the freedom to the patient to self-medicate for treating minor, common health problems, at lower costs and with higher time saving. It is very important that the patients have access to sufficient information to make an informed choice for the proper use of these medicines. The label of OTC medicines plays an important role in conveying valuable information to the patient for safe and effective use of OTC. However, studies found that labels were not able to withstand the guidelines of the regulatory authority and consumer surveys suggests that most do not read full information appearing on the package of an OTC product when they buy it for the first time. The labels should provide all important information and instructions to patient. Even though some patients may be aware of the potential for OTC drug misuse, others are naive and do not realize the harm involved.
Do you know that 81 percent of U.S. adults prefer to treat their minor ailments with over-the-counter medicines before seeking professional care? OTC drugs play increasingly vital role in America’s healthcare system. There are more than 80 therapeutic categories of OTC drugs. There are more than 300,000 OTC drug products marketed, encompassing about 800 active ingredients. OTC medications may be affordable, accessible, and effective, but that doesn’t necessarily mean they’re harmless. Although OTC medications may not have the sinister reputation that illegal drugs like meth and heroin have, they do carry risks of their own. OTC drugs allow faster and cheaper access to healthcare; however, their misuse and adverse health effects cause concerns. Improper use of these medications can be dangerous. Some individuals take OTC drugs not for medical reasons but for recreational use. In other words, they take OTC drugs to get high. Nearly 1 in 10 teenager report abusing cough medicine to get high in the U.S.
The sale of non-prescription drugs, vitamins, and herbal dietary supplements is a multi-billion-dollar industry. Deciding what qualifies for OTC drug category is not simple. There is always a bit of confusion between OTC drugs with respect to diet supplements, cosmetics and herbal products. Different nations have differing classification of what is a prescription drug and what is not, which means that some drugs commonly available over the counter in one country could be considered controlled substances in other countries. For example, the United Arab Emirates (UAE) has strict narcotics laws that have landed many travelers in prison, who were carrying painkillers containing codeine, OTC product in their country. While rules vary considerably from country to country, there can be serious consequences if you violate the laws of the country you’re visiting. OTC products are also classified differently in different country. Some countries classify supplements and herbal products as OTC drugs while other countries do not. Sometimes in the same country, the same drug may come in both OTC and prescription versions, depending on the strength. Each country establishes which drugs are available OTC in that country. Finally, in India OTC medicine has no legal recognition, drugs which are not included in prescription-only drugs are considered to be in non-prescription drugs. There are no specific unifying regulations related to use and sale of OTC products in India thereby affecting both the accessibility to better health care and patients’ safety due to inappropriate use.
Many worry that OTC drugs may not be given the respect they require by the public, that they are indeed potent medicines that must be used judiciously. One writer coined the phrase – the de-medicinization of OTC medicines – to reflect a possible negative trend that denigrates OTC medicines to the level of other simple consumer goods such as breakfast cereals or household cleaning products. I am publishing this article to create awareness about OTC drugs. Awareness regarding OTC drugs will lead to better self-medication practices and prevent any untoward medical occurrence.
______
______
Abbreviation and synonyms:
OTC drug = over the counter drug = OTC = non-prescription drug
BTC drug = behind the counter drug = BTC
℞ = Rx = medical prescription = prescription drug
Acetaminophen = Paracetamol
US = United States
FDA = Food and Drug Administration (of united states)
CHPA = Consumer Healthcare Products Association
ADR = adverse drug reactions
DDI = drug-drug interactions
API = active pharmaceutical ingredient
TEP = tamper-evident packaging
NSAIDs = non-steroidal anti-inflammatory drugs
POM = prescription-only medicine
HCP = health care practitioner
DCA = Drugs and Cosmetics Act of 1940
DCR = Drugs and Cosmetics Rules of 1945
ASU = Ayurveda, Siddha, and Unani
CR packaging = Child-resistant packaging
OR = odds ratio
AAA = American Automobile Association
CYPs = Cytochromes P450
DXM = dextromethorphan
GRASE = generally regarded as safe and effective
NDA = New Drug Application
CBD = cannabidiol
______
______
Terminology:
-1. Over the Counter Drugs:
Over the counter drugs (OTC), also known as off the shelf medication, are drugs that do not require a doctor’s prescription and can be bought off-the-shelf in a pharmacy, and in stores such as supermarkets or small convenience stores. In many countries, OTC drugs are selected by a regulatory agency to ensure that they are ingredients that are safe and effective when used without a physician’s care. As a general rule, over-the-counter drugs have to be used primarily to treat a condition that does not require the direct supervision of a doctor and must be proven to be reasonably safe and well tolerated. In the United States some over-the-counter cold and allergy medicines are being moved behind the counter at pharmacies as part of the fight against illegal drug production.
Examples of Over-the-Counter Drugs:
Acetaminophen (Tylenol); Ibuprofen (Advil, Motrin); Decongestants, Aspirin.
-2. Behind the Counter Medication:
Similar to over-the-counter status, behind the counter (BTC) allows patients to access certain medication at a pharmacy without seeing a doctor. Such items may be unavailable in convenience or grocery stores that stock other non-restricted OTC medications. Unlike OTC, access is not allowed without the intervention of a learned intermediary, and unlike prescription medication, behind the counter meds allows a patient to access drugs after an assessment and decision by a pharmacist. All U.S. drug products that contain the ingredient pseudoephedrine must be kept behind the pharmacy counter and only be sold in limited quantities. The behind the counter medication scheme is currently in practice in several European nations, Canada, Australia, and the United States (limited version).
Examples of Behind the Counter Medication:
Some cold and allergy medicines; Birth control pills; Migraine medications; Cough syrup with codeine; Anything containing Pseudoephedrine.
-3. Prescription Drugs:
A prescription drug (prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter and behind-the-counter drugs can be obtained without a prescription. A prescription drug requires a medical diagnosis and decision by a licensed healthcare professional as to which medicine is used, and is only intended for use by one person. Prescription medication can only be dispensed from a pharmacy (community, online, or mail-order) by a licensed pharmacist. The term “Rx” is often used as a short form for prescription drug.
Examples of Prescription Drugs:
Antibiotics; Statins; Antidepressants; Sleeping pills.
Note:
-1. Sometimes, the same drug may come in both OTC and prescription versions, depending on the strength.
-2. Regulations detailing the establishments where drugs may be sold, who is authorized to dispense them, and whether a prescription is required vary considerably from country to country.
_
Drug Abuse:
Drug Abuse is specifically defined as the intentional, non-therapeutic use by a patient or consumer of a product, OTC or prescription medicine, for a perceived reward or desired non-therapeutic effect including, but not limited to, getting ‘high’ (euphoria).
Drug Misuse:
Drug Misuse is defined as the intentional use, by a patient or consumer of a product, OTC or prescription medicine, for a therapeutic purpose other than as prescribed or not in accordance with the authorized product information. Drug misuse is used to distinguish improper or unhealthy use from the use of medicine as prescribed or in accordance with the authorized product information. OTC drugs are relatively safe when used in accordance with the authorized product information but cause adverse effects when misused.
_
Medical prescription:
A prescription, often abbreviated ℞ or Rx, is a health care program implemented by a physician or other qualified health care practitioner in the form of instructions that govern the plan of care for an individual patient. The term often refers to a health care provider’s written authorization for a patient to purchase a prescription drug from a pharmacist. The symbol “℞”, sometimes transliterated as “Rx”, is recorded in 16th century manuscripts as an abbreviation of the late Latin instruction recipe, meaning ‘receive’. Originally abbreviated Rc, the later convention of using a slash to indicate abbreviation resulted in an R with a straight stroke through its right “leg”. Medieval prescriptions invariably began with the instruction from the physician to the apothecary to “take” certain materials and compound them in specified ways.
______
______
Section-2
Self-care:
The four essential levels of care are self-care, primary professional care, general specialist care, and tertiary specialist care. Self-care is the broad base of health care, and a frequent part of everyday life. For instance, if a symptom is not considered serious, people will often choose to ignore it or self-treat it, rather than seeking a professional’s help. There has been growing interest in self-care over the past two decades. For the years of what is now known as the golden age of medicine (1930s to 1950s), self-care was actually frowned upon by the medical establishment. The reason was that in the wake of tremendous achievements in drug discovery of the time, more traditional ways of treating illness were considered both unsafe and ineffective. Accordingly, patients were encouraged by physicians to seek formal care for even the most mundane of illnesses and to use modern pharmacotherapies to rectify the problem. In recent years, the idea of self-care has been strongly promoted by governments in many countries as part of a public policy agenda. The main impetus for this is to divert people from the formal health care system, to save resources while still meeting acceptable levels of care. People are being encouraged to monitor their own illnesses, self-treat minor symptoms, prevent diseases, and improve/maintain their health. Studies in the United Kingdom and the United States show that people provide 66-95 percent of all health care for themselves and their families.
_
Within the self-care structure, over-the-counter (OTC) medicine is a major element. People often use OTC products to treat their minor illnesses, which usually are common health problems such as colds, headaches, heartburn, and sore feet. In a British study, Dunnell and Cartwright found that using OTC medicine was the most frequently reported response to symptoms associated with minor illnesses, surpassing “doing nothing,” “seeing a physician,” and all other treatment options. According to this study, 96 percent of the study population believed the OTC medicines really did help. Another British study reported that 66 percent of the respondents had taken OTC medicines during a one-month study period, in contrast to the 25 percent who had taken prescription medicines during the same period. In Canada, OTC drugs are also commonly used and thus play an important role in its health care system. For instance, a Canadian report showed that over 90 percent of Canadians used an OTC product in 1991. A more recent study indicated that 58 percent of Canadians took an OTC medication in the last six months. These studies suggest that OTC medicine is indeed a common choice for treating minor conditions.
_
Definition of self-care:
It can be simply said that individuals partaking in self-care take charge of protecting, maintaining, and improving their own health status.
Lunde cites a definition of self-care from WHO:
Self-care refers to unorganized health activities and health-related decision making by individuals, families, friends, colleagues at work, etc.; it includes self-medication, self-treatment, social support in illness, first aid in a ‘natural setting’, i.e., the normal social context of people’s everyday lives. Self-care is definitely the primary health resource in the health care system. It does not imply purposeful organization and is often provided on an ad hoc basis in intimate settings.
Lunde has indicated that self-care includes four main aspects: health promotion, disease prevention, treatment of minor illnesses and injuries, and the management of chronic diseases and rehabilitation. Under this definition, changing lifestyle patterns would be a form of self-care.
Self-medication is also a form of self-care, and a critical one. Products to be used for such purposes can be defined as those “the average consumer can use to treat minor, self-limiting illnesses without the intervention of a prescribing, dispensing or monitoring health professional with relative assurance of its safety and effectiveness.” Medications of this type are usually known as non-prescription or over-the-counter (OTC) products.
_
Self-Medication:
Self-medication is the use of drugs with therapeutic intent but without any professional advice or prescription. It has also been defined as the use of non-prescription medicines on their own initiative. According to WHO self-medication is selection and use of non-prescription medicines by individuals to treat self-recognized illness or symptoms. It is practiced worldwide. Although some medicines are risk free and useful for treatment of minor health problems, their excess & regular use may lead to some serious health problems and side effects and adverse reactions. Self-medication is practiced all over world. Self-medication is now increasing as a component of self-care. Unlike other aspects of self-care, self-medication involves the use of drugs and these drugs have the potential to do good as well as harm. Self-medication can save the time, may be economical but due to its improper use of correct dose, side effects, and interactions can lead to serious implications.
_
Benefits of Self-Care/Self-Medication:
Even though self-care/self-medication is as old as human history, governments and health insurers still encourage the public to do more of it. The main reason for promoting self-care is to reduce health care expenditures. A national report in Canada suggests that appropriate self-care activities can decrease the economic burden on formal health care systems. With regard to the financial impact of minor illness, a Canadian study in the province of Ontario found that 13.2 percent of all visits to physicians in 1989 were for the treatment of colds and flu. The total cost for these conditions were almost $300 million, taking up 12.5 percent of the provincial government’s payment to physicians. Because colds are a very common type of minor illness in which most people can self-treat by using OTC medications, this expenditure might be unnecessary. Temin provides a piece of evidence to this point. He determined that 1.65 million Americans with cold symptoms did not visit a doctor from 1974 to 1989 due to the variety of cough/cold preparations available on the OTC market. It was estimated that $77 million per year could be saved, including payments for physician services and government spending on prescription drugs.
Self-care/self-medication not only has economic benefits to a health care system, but has advantages to consumers and to health professionals as well. For consumers, self-care/self-medication can be very convenient. Time can be saved by avoiding doctor visits. Evans et al cite a consumer study in Britain that the average waiting time in a doctor’s office is 24 minutes for a patient with an appointment, and 45 minutes for a patient without an appointment. The situation in Canada is likely similar. Moreover, cheaper prices in comparison to those of prescription medicines are another reason why people choose OTC products (although this is mainly in effect for those who do not have insurance coverage).
For health professionals, promoting self-care/self-medication can decrease physician workload, and in turn, extend the scope of the pharmacist’s advisory role. According to results from several American and British studies, physicians agree that a great number of their daily consultations are associated with minor illnesses that can be handled by less formalized care. Moreover, the increasing numbers of selfcare/self-medication activities provide a great opportunity for pharmacists to offer more pharmaceutical care to the public.
Disadvantages of self-medication:
OTC drugs are used, misused and abused. For example, taking pain killers for long time without consultation of doctor and without knowing the cause of headache. Major problem or disadvantages of self-medication is emergence of resistance microorganisms particularly in developing countries, where antibiotics are often used and available without prescription.
______
Common Types of Minor Illnesses:
There is no clear definition of a minor illness. However, generally speaking, a symptom associated with a short-term, trivial and self-limiting illness is considered a minor ailment. The kinds of minor illnesses that can occur are rather diverse and are very common health problems. It has been estimated that 100 to 150 million general practitioner consultations a year in Britain are for conditions that may be self- treatable. Bissell et al cite a survey that showed over 90 percent of the British population experienced at least one ailment per person in 1995. A Canadian survey (1991) similarly reported that 88 percent of adults had suffered at least one minor illness in the previous 12 months. In Irigoyen and Mulvihill’s one-year cohort study,31 medical students reported an average of 4.4 minor illnesses per person per year.
The listing of the most frequently occurring minor illnesses for a specific country is useful for researchers studying self-care/self-medication. In 1991, Canadian Facts compiled a Consumer Usage and Attitude Study to examine Canadians’ attitudes, behaviours, and consultation practices when suffering with specific minor ailments. This report listed the top eleven minor illnesses. A cold (60 percent of Canadian adults suffered with at least one in 1990) was the most common illness, followed by headaches (40 percent), body pain (40 percent), upset stomach (29 percent), and allergies (22 percent). Other ailments included eye irritation/redness (16 percent), skin irritation/rashes (15 percent) and so on. A survey (1995) prepared by the Reader’s Digest also listed Canada’s top ten self-limiting conditions: 1) headache (76 percent); 2) cough/cold (70 percent); 3) sore throat (47 percent); 4) muscle aches/pains (38 percent); 5) sinus congestion (37 percent); 6) indigestion (20 percent); 7) arthritis (16 percent); 8) insomnia (14 percent); 9) menstrual cramps (13 percent); and 10) allergy/hay fever (12 percent).
_
Response of the Public to Minor Illness:
When people suffer with minor ailments, they tend to choose a subsequent course of action among three main options. First, they can choose to do nothing. People today are very busy, so if their minor ailments do not interfere with normal activities, this may be the response. This is a very common choice for people. Second, they can choose to self-treat. Reader’s Digest suggests that 79 percent of consumers in Canada self-medicate in some way, whether with an OTC medicine or herbal product. Finally, a person can opt for professional help, the least common of the three choices when involving a perceived minor illness.
Table below represents public response to minor illness from five countries. It appears that, in general, people of different cultures have similar responses to minor illnesses across years and countries.
_
Factors Influencing Responses to Minor Illnesses:
The action chosen by the public to minor illness will depend on a number of factors. Such factors include sex, age, socioeconomic status, family structure and support system, previous experience with symptoms, types of symptoms, potential embarrassment, time, costs, social/cultural attitudes, and surroundings.
Several studies show that females are more likely to seek advice for common minor ailments from health care professionals than are males. In Bell et al’s study, researchers tested gender differences in four treatment options based on a total of twelve symptoms. Women preferred to consult a health professional (either physician or pharmacist) for eight of the symptoms given. Men were more likely to self-treat for most symptoms listed, except foot problems.
Age is a factor in decision-making behaviours. Generally speaking, older people (≥ 60 years) are more likely to seek advice from their pharmacists or doctors.
Socioeconomic status appears to play a role in predicting illness behaviours. Koos found that upper class respondents more frequently felt they required medical care than did lower class persons. This result is supported by another three studies. In the United States, Anderson et al analyzed respondents’ actual reactions to 15 minor ailments. They discovered that the proportion of physician consultations for these conditions tended to increase as household income, education, and occupational rank increased. Hetherington and Hopkins reported that people with low income are significantly more “symptom-insensitive” than those of high income.
Previous experience likely plays a key role in attitudes and behaviours involving minor illnesses. Several studies have focused on the relationships between prior experience and illness behaviours for a given symptom. Safer et al reported that patients with familiar or frequently experienced symptoms (> 11 days) took a much longer time than did patients with new symptoms (< 3 days) to make decisions. In Banks and Keller’s study, 239 families were randomly selected and then a member of the family (usually the mother) was interviewed. For a list of symptoms, subjects were asked if anyone in the family had displayed such symptoms. They were questioned on what choice of treatment they would make, without considering their previous experience. Those who had previous symptom experience, though, expressed less anxiety or concern than those to whom the illnesses were new.
It is not surprising that people may have different responses when they suffer different kinds of conditions. Many researchers are interested in understanding reactions to common symptoms of minor illness. Verbrugge and Ascione analyzed the incidence of symptoms related to respiratory and musculoskeletal illnesses to see how people cared for them. For (mainly acute) respiratory symptoms, OTC drugs were chosen more often than prescription medicines. But for musculoskeletal symptoms (chronic), prescription medicines became more important than OTC choices. Furthermore, persons with respiratory symptoms used less formal medical care than did those with musculoskeletal symptoms. Thus, according to Verbrugge and Ascione’s findings, people approach chronic and acute health problems in different ways. Symptoms such as cold/flu, cough, sore throat, headache, heartburn, constipation, and indigestion are reported by respondents as disorders that they tend to self-treat. However, when people experience backache, red eyes, depression and chesty cough, they prefer to consult health professionals, especially physicians.
Access to medical care and cost are issues for many people. Long waiting times in a doctor’s office and medical insurance coverage may be important reasons for people to avoid a physician. A Japanese study, for instance, found that Japanese visit a doctor more frequently than they buy an OTC product for treating minor ailments because of easier access to medical care. The other reason may be that OTC products are not covered by medical insurance, whereas prescription medications are reimbursed.
_
Information Sources Used:
Health professionals, word of mouth (family members and friends), mass media (health books and advertisements), and product labels are very common sources used by people when seeking information about minor illnesses or OTC medicines. The Internet can now be added as a new information source for the public; one report indicated that 10 percent of consumers rely on this source of data.
For most types of minor illnesses, doctors and pharmacists are the most often used sources of information. Results from a Canadian survey show that 30 to 80 percent of respondents for a given illness consult physicians about their conditions, and 20 to 40 percent get information from pharmacists. Doctors are seen as a first choice for many for treating minor illness. A Scottish study found that 68.5 percent of respondents would see a doctor first for advice. In the same study, only 8.2 percent considered pharmacists as their first port of call for managing their ailments, even lower than family members (16.3 percent). A study in Hamilton showed that community pharmacists were the first choice for only 18 percent of a sample population. The elderly and parents who seek advice for their children have not considered pharmacists as the most important, nor a frequent, source. For others, it is the pharmacist who is approached first. In a study by Bell et al in Britain, 58.1 percent of participants indicated that they would seek advice from a pharmacist rather than from a doctor, if symptoms were not serious enough to visit the doctor. Over 10 percent of participants indicated they would seek a pharmacist’s advice if short of time for a doctor’s appointment. This report also found that men were more influenced by the recommendations of friends and families than were women. Griffle found that almost 60 percent of clients rely on the advice of health care professionals when selecting an OTC product. A Canadian OTC industry report (1999) showed that 22 percent of Canadians sought the advice of a doctor on OTC products; 25 percent said that pharmacists were their primary information source. A Canadian survey conducted in 2001 showed that 65 percent of respondents always/often obtained OTC information from pharmacists, followed by advertising (63 percent), media reports (57 percent), word of mouth (53 percent), physicians (34 percent), product labels (20 percent), and the Internet (10 percent).
Advertisements (including television, newspaper, and magazines) of OTC medicines are important sources. In an American survey, participants were asked to indicate which cited information source(s) they had turned to within a six-month period. The top four common sources were advertising or promotion from TV/ newspaper/ magazines (49.7 percent), followed by a doctor (47 percent), articles or information from TV/ newspaper/ magazines (46 percent) and a pharmacist (38 percent). The main role of advertising is to create consumer awareness of OTC products. Respondents of the industry-sponsored Consumer Usage & Attitude Study in 1991 said that advertising did help them to understand what OTCs were available for different illnesses. Sooksriwong and Leelanitkul found that the majority of Thai consumers got information about drug names from advertising, including television, printed matter, and radio. Families and friends were their second source of information on drug names.
A product label provides valuable information to OTC users, if the time is taken to read it. Data from the Consumer Usage & Attitude Study indicated that 91 percent of Canadians claim to have read the label carefully before using a product for the first time. An American study showed that a similar proportion of Americans (95 percent) read some portions of the labels on OTC products. Active ingredients, usage direction, dosage level, and warnings were the common sections customers claimed to have read. Other reports provide less enthusiastic results. A Canadian study reported that 62 percent of participants always read labels; 16 percent often read them; 9 percent were on record as sometimes; 6 percent as seldom; and 7 percent never read them. Comparing Canadian and American national consumer surveys suggests that most do not read full information appearing on the package of an OTC product when they buy it for the first time. For example, only 40 percent of Canadians read active ingredients, followed by the dosage level (34 percent), the symptom it treats (26 percent), possible side effects (23 percent), directions for usage (18 percent), and warnings (10 percent) when they buy a product for the first time. American data showed that the proportion of readers in each section were even lower than the Canadian statistics – directions for usage (19 percent), dosage level (16 percent), symptom it is used for (12 percent), possible side effects of usage (10 percent), and warnings (7 percent). In the American survey, researchers also found that more Americans would read directions for usage (22 percent) and dosage level (25 percent) when they take the medicine for the first time, rather than when they buy it for the first time. Even though information appearing on the package of an OTC medicine is limited, most Canadians (90 percent) felt satisfied with it.
In summary, physicians and pharmacists are the main sources when the public seek information about minor illnesses and OTC medicines. Advertising and product labelling also play an important role.
_
Consumer Perceptions of OTC Medicines:
Consumers can easily and conveniently purchase OTC products from pharmacies or non-pharmacy outlets. Is there potential for the consuming public to consider such medicines with less importance than they are due? In an Italian report, five percent of participants stated that some OTC products (like laxatives) were hardly thought of as ‘drugs’ because they have been advertised almost as a part of “normal life components”. Several studies indicate that consumers perceive prescription medicines and OTC medicines as differing in safety, strength, and effectiveness. Consumers tend to consider prescription medicines as more powerful than their OTC counterparts. One British study collected 1,650 comments on differences involving consumer perceptions of prescription medicines and of OTC medicines. One-third of respondents thought that prescription medicines were stronger than non-prescription medicines. OTC medicines were considered to be safer than prescription medicines because prescriptions may have more (or relatively serious) side effects and were more likely to be misused.
Although likely outdated, a national survey sponsored by the U.S. FDA (1973) presented different results. This study asked consumers to rate the safety of five categories of products. Food was rated the safest product. Prescription medicines were in the second place, followed by cosmetics and toys. Nonprescription medicines were thought to be the most dangerous products. A nation-wide Canadian survey conducted in 1990 also had similar findings. The majority of Canadians (70 percent) believed that prescription medicines were always/often safe, compared to non-prescription medicines (59 percent) and cosmetics (47 percent). As well, fewer Canadians (5 percent) thought prescription medicines were seldom/never safe, while 9 percent thought non-prescription medicines and 13 percent thought cosmetics were seldom/never safe. Moreover, respondents indicated that non-prescription medicines were less effective than prescription medicines. Approximately half believed that non-prescription medicines were always/often effective; 65 percent thought that prescription medicines were always/often effective.
In summary, the available evidence appears to indicate that consumers do indeed distinguish differences between OTC and prescription medicines. By extension, a concern appears to exist whereby the public may not consider medicines available without prescription as full-fledged ‘medicines’, ones that require a level of vigilance during use. Location of sale may be a factor in the development of such perceptions. Impressions held of OTC medicines may be important pre-determinants of actual behaviour, where failure to consider such agents as important medicines requiring due care, could expose the public to important drug-related risk.
______
______
Section-3
Introduction to over-the-counter drugs:
_
Over-the-counter (OTC) drugs are medicines that may be sold without a prescription, in contrast to prescription drugs. The name “over-the-counter” is somewhat confusing to some, since these items can be found on the shelves of stores and bought like any other packaged product in some countries or in others may be bought “over the counter” from the pharmacy, while prescription drugs are sold at a pharmacy counter. The term likely dates back to before self-service shopping became common, when most goods were obtained by requesting them from a clerk at a sales counter; while prescription drugs required a visit to the doctor first, these drugs could be purchased “over the (sales) counter” just like other goods. OTC drugs include those you can buy off the drugstore, grocery store, or convenience store shelf as well as those dispensed by a physician without a prescription. OTC medications have brand names as well as generic and store names (similar to prescription medications). Generic, store, and brand names contain the same active ingredients and are identical in their action on the body if the concentration of the active ingredients are the same. Because some OTC pills and liquids contain multiple medications, it is important to read the fine print on the label to know exactly what ingredients are in the product.
Globally, OTC drugs are an important class of medicines. Referring to any drug that can be sold directly to the general consumer, the term can be further subdivided into various categories, with the specifics varying widely from place to place. According to the Consumer Healthcare Products Association (CHPA), 81% of US adults use OTC medicines as a first-line treatment for minor illnesses. The average consumer makes 26 trips a year to purchase OTC drugs, compared with just three visits to their doctor. And there are more than 750,000 retail outlets that sell OTC products, compared to 54,000 pharmacies. As the CHPA website explains: “Without affordable and accessible OTCs, underserved populations would depend more heavily on the highest cost medical care for minor ailments.” Over-the-counter drugs are a huge business for the pharmaceutical industry in the United States, accounting for over $35 billion in gross revenues in 2018. Despite having lower per-unit costs, OTC drugs often surpass prescription drugs in terms of annual sales.
_
Historical Background:
At one time, most drugs were available without a prescription. Before the Food and Drug Administration (FDA) existed, just about anything could be put in a bottle and sold as a sure-fire cure. Alcohol, cocaine, marijuana, and opium were included in some over-the-counter (OTC) products without notification to users. The Food, Drug, and Cosmetic (FD&C) Act, enacted in 1938, gave the FDA some authority to issue regulations, but the act did not provide clear guidelines as to which drugs could be sold by prescription only and which could be sold over the counter.
An amendment to the FD&C Act in 1951 attempted to clarify the difference between OTC and prescription drugs and to deal with issues of drug safety. Prescription drugs were defined as compounds that could be habit forming, toxic, or unsafe for use except under a doctor’s supervision. Anything else could be sold over the counter.
As noted by an amendment to the FD&C Act of 1962, OTC drugs were required to be both effective and safe. However, determining effectiveness and safety can be difficult. What is effective for one person may not be for another, and any drug may cause unwanted side effects (also called adverse effects, adverse events, or adverse drug reactions). There was no organized system in the United States for reporting the adverse effects of OTC drugs until 2007, when a new law became effective that required companies to report serious adverse events associated with OTC drugs.
_
In the United States, drugs can be divided into two categories: prescription drugs, which are only available with a physician’s prescription, and over-the-counter (OTC) drugs, which can be freely purchased from many outlets, such as pharmacies and grocery stores. Both classes of drugs are regulated by the U.S. Food and Drug Administration (FDA). OTC drugs can be introduced to the market in one of two ways. First, particularly for older drugs, the FDA has established drug monographs that detail acceptable ingredients, dosages, and formulations. Products conforming to these monographs can be marketed without premarket FDA approval. If a monograph does not exist for a particular drug, then the drug must be approved via the new drug application (NDA) process, which is similar to the process used to approve new prescription drugs. As with new prescription drugs, approval requires evidence demonstrating that the drug is safe and effective. In addition, the drug manufacturer or interested party must also establish that (1) the benefits of OTC approval outweigh the risks, (2) the potential for misuse and abuse is low, (3) consumers can understand the product label and accurately diagnose themselves for the indicated condition, and (4) health practitioners are not required for the safe and effective use of the product. OTC drugs do not include dietary supplements, herbal remedies, and “nutriceuticals,” all of which are not regulated by the U.S. Food and Drug Administration (FDA). The rules vary considerably from country to country.
There are currently more than 300,000 different OTC drugs available only in US. The list of OTC drugs in the modern society is over expanding with the inclusion of new formulations and prescription to OTC switches. Some OTC drugs were originally available only by prescription. After many years of use under prescription regulation, drugs with excellent safety records may be approved by the FDA for over-the-counter sale. The analgesic ibuprofen and the acidity remedy famotidine are examples of such drugs. Often, the OTC version has a substantially lower amount of active ingredient in each tablet, capsule, or caplet than does the prescription drug. When establishing appropriate doses of OTC drugs, manufacturers and the FDA try to balance safety and effectiveness. As the general rule OTC drugs have to be primarily used to treat a condition that does not require the direct supervision of a doctor and must be proven to be reasonably safe and well tolerated. OTC drugs are not always better tolerated than similar prescription drugs. For example, the OTC sleep aid diphenhydramine can cause just as serious adverse effects as many prescription sleep aids, especially in older people.
_
There are hundreds of OTC drugs at local pharmacy. Many items you may think are cosmetics, dietary supplements, or medical devices, which have their own regulatory requirements, may also be classified as “drugs.”
The term “drug” is a legal one. As described in the Federal Food, Drug, and Cosmetic Act:
A drug is an article intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease and/or an article (other than food) intended to affect the structure or function of the human body and/or an article intended for use as a component of such an article.
How does the FDA define “intended use?”
“Intended use is the objective intent of the persons legally responsible for the labelling of drugs. The intent is determined by such persons’ expressions or may be shown by the circumstances surrounding the distribution of the article.”
The FDA determines your product’s “objective intent” by the:
-Labelling claims.
-Advertising matter.
-Oral or written statements by manufacturers, sponsors or their representatives.
Here are the main differences between OTC drugs and prescription drugs.
Prescription drugs are:
- Prescribed by a doctor
- Bought at a pharmacy
- Prescribed for and intended to be used by one person
- Regulated by FDA through the New Drug Application (NDA) process. This is the formal step a drug sponsor takes to ask that the FDA consider approving a new drug for marketing in the United States. An NDA includes all animal and human data and analyses of the data, as well as information about how the drug behaves in the body and how it is manufactured.
OTC drugs are:
- Drugs that do not require a doctor’s prescription
- Bought off-the-shelf in stores
- Regulated by FDA through OTC Drug monographs. OTC drug monographs are a kind of “recipe book” covering acceptable ingredients, doses, formulations, and labeling. Monographs will continually be updated adding additional ingredients and labeling as needed. Products conforming to a monograph may be marketed without further FDA clearance, while those that do not, must undergo separate review and approval through the “New Drug Approval System.”
_
The requirement for a physician’s prescription imposes a regulatory barrier to access which has advantages and disadvantages. The main advantage of this barrier is that it prevents misuse of prescription drugs, which may have serious consequences in many cases. Moreover, this requirement places the use of the drug under a physician’s supervision, making it easier for him or her to encourage adherence to instructions, monitor any adverse effects, and identify any potential contraindications. However, requiring a prescription also imposes additional time and monetary costs on the patient; these may reduce access to potentially beneficial therapies. Although it is clear that the advantages of physician supervision may outweigh these increased costs in the case of drugs with high toxicity profiles, it is less clear in the case of many drugs with low toxicity. Therefore, in an effort to increase access to select low-toxicity drugs by eliminating the costs associated with obtaining a physician’s prescription, the FDA is considering a third classification, called behind-the-counter (BTC) drugs (vide infra). These drugs would be available without a physician’s prescription but, unlike OTC drugs, would not be freely available to patients. Rather, they would be available only in pharmacies and upon consultation with a pharmacist.
_
Necessary characteristics for OTC drug use include:
-1. The product has an acceptable safety margin.
-2. The product has low misuse and abuse potential under conditions of widespread availability.
-3. A healthcare practitioner is not needed for the safe and effective use of the product.
-4. The product has adequate labeling. One important regulatory distinction between OTC and prescription drug products involves the requirement for “adequate directions for use.” All drugs must have labeling that bears “adequate directions for use.” Prescription drugs, however, by definition, require the intervention of a licensed healthcare professional. Since these products require the intervention of a healthcare professional, also known as a “learned intermediary,” “adequate directions for use” for OTC drug products is not included for those uses which require a prescription.
-5. The consumers must be able to self-diagnose, self-select the medication, self-treat, and self-manage the condition for which the OTC drug is intended. If a person is mentally challenged, he/she cannot use OTC medicine on their own.
____
Varying definitions of OTC drugs:
Typically, OTC drugs are defined in distinction to prescription-only. In the UK, for example, medicines fall into three categories: prescription-only (available from a pharmacist to a specific patient); pharmacy medicines (which can be sold by a pharmacist without prescription); and general sales list (which can be sold anywhere, including supermarkets). The latter group includes painkillers like paracetamol, as well as skin creams and anti-allergy drugs.
It is easy to see the purpose of this kind of categorisation. Since OTC drugs are safe for the vast majority of people, and are used to treat minor ailments, it wouldn’t make commercial or medical sense to restrict their availability. Conversely, since prescription drugs are generally quite powerful, and suited only to specific patients, they are not appropriate for retail sale.
However, this is not to say the division is set in stone. It is not unusual for a prescription medicine to be reclassified as OTC or vice versa, in light of new evidence, and laws do not entirely line up around the world.
India’s OTC problem:
Despite the classifications in place elsewhere, India has never given OTC drugs legal recognition. Its OTC drug market, estimated to be worth around $4bn, is a somewhat murky category. It is also growing fast, as new chemists open up in rural areas and an influx of new products hit the shelves.
The lack of regulation is troubling, in that it heightens the risk of OTC drugs being taken inappropriately. A 2016 study by the India Pharmacopoeia Commission found a growing incidence of adverse reactions, concluding: “The present study reveals that safety of OTC drugs is a matter of grave concern. Therefore stringent safety monitoring and regulations are needed.” On top of this, a number of ‘prescription-only’ drugs are easily available over the counter. Many of these drugs, like paracetamol, are low-risk, and will likely be recategorised as OTC. But it also includes some higher-risk products that should never be administered in this manner. For example, antibiotics are often acquired without prescription, contributing to the growing problem of antibiotic resistance. The government has lately been pressing for more cautious use of antibiotics.
When illegal drug sales proliferate, this is likely to hit poorer communities the hardest. According to a recent cross-sectional Indian study: “When there is a perceived poor access to health care, OTC medication use becomes a low-cost alternative…People who are economically weak are less likely to consult a medical practitioner for a medical condition.” It has also created a market for drugs tourism, demonstrated most chillingly by the young English couple found dead in their Agra hotel in 2014. The couple, who had overdosed on prescription medication, had earlier tweeted: ‘Codeine under the counter here. With Valium, Xanax and Lyrica. Winning’.
_
Definition of Non-Prescription drug or Over the Counter (OTC) drug approved by the IV Pan American conference on Drug Regulatory Harmonization. March 2005:
“An OTC is a pharmaceutical product, drug, or medicinal specialty whose dispensing or administration does not require medical authorization, and it can be used by the consumers under its own initiative and responsibility to prevent, relieve or to treat symptoms or mild diseases and that its use, in the form, conditions and authorized dosages are safe for the consumer”;
Criteria to classify drugs as OTC:
-1. Drugs which are effective and safe in order to be used in the prevention, relief of symptoms, or treatment of mild diseases, and are easy to identify;
-2. Drugs with broad safety range, in such a way that the voluntary or involuntary administration of dosage higher than those recommended, or where are not indicated, does not represent a serious danger for the health of the patient;
-3. Have a broad dosage margin, so it can be adapted at the age and weight of the patient;
-4. Drugs that do not generate tolerance or dependency when used and that are not susceptible of abuse;
-5. When it is used in accordance with the instructions do not mask serious diseases, nor delay the diagnosis and treatment of a condition that requires of medical care;
-6. Drugs of safe utilization in all the age groups of the population;
-7. Dosage forms usually of oral or topical route, of easy management and storage and that are not of IV or IM administration;
-8. Drugs whose active ingredient has been marketed under medical prescription at least 5-10 years, time during which has demonstrated a favorable index of safety and efficacy through the data of drug surveillance;
-9. The adverse reaction reports have not increased during the marketing period.
______
Over the counter drug (OTC) means a drug that is sold without prescription of a registered medical practitioner. They are also known as non-prescription medicines. The use of OTC medications has been reported to be on the rise internationally. The trend of ‘Over-the-Counter (OTC) Medicines’ use has grown steadily in the last few years. Various reasons such as easy availability, affordability, and increased awareness among patients are responsible for this trend. Many countries recognize OTC medicines as a separate category of drugs and have established regulations for their use. With the reclassification of certain drugs, the public can buy preparations that were previously available only on prescription (OPPI, 2011). Self-medication with OTC analgesics such as paracetamol among children and adolescents is increasing. This constitutes an important public health concern. Various studies have shown that the use of OTC drugs is twice as common as that of prescribed medication. Also, it has been observed that self-medication is often used along with prescribed medication. It is evident with research that self-care improves the health care awareness and reduces the economy related to health care. Although OTC drugs are believed to be safe and effective, indeed they are not. They mask the underlying disease and may cause several adverse effects.
_
Since Indian patients have a huge tendency of self-treatment, the Indian market is characterized by a huge demand for OTC drugs. In India, though the OTC phrase has no legal recognition, all the drugs that are not included in the list of prescription drugs are considered as non-prescription drugs. Prescription-only drugs are those medicines that are listed in Schedules H and X of the Drug and Cosmetics Rules. Drugs listed in Schedule G (mostly antihistamines) do not need prescription to purchase but require the following mandatory text on the label: “Caution: It is dangerous to take this preparation except under medical supervision” (Bond & Hannaford, 2003). There is no regulation for the use of OTC drugs in India. Further in the absence of strategic consideration for the use of OTC drugs, chaos prevails and the reasons for these alarming situations are manifold. Perhaps, the poor economic status and busy lifestyle of an individual makes him rely on the OTC drugs. In India, it has been shown that literate people were 76% more likely to self-medicate than illiterate people. Published literature mentions that the mean age for the purchase of OTC drugs in India is 32.7 years with female preponderance. In India, OTC related adverse effects, abuse, and hospitalizations are on the rise.
_
OTC medication offers advantages like easy access to medicines, self-management of minor ailments with the involvement of pharmacists, and utilization of available resources. However it is not always safe and has been associated with negative health consequences. Exposure to OTC Ibuprofen and other OTC non-steroidal anti-inflammatory drugs is substantial and leads to increased risk of gastrointestinal bleeding. OTC related adverse effects are predominantly gastrointestinal complaints, allergic reaction, psychosis, tachycardia, seizures dizziness leading to increase in the number of hospital admissions. There are reports that Phenylpropanalamine (PPA) is the major ingredient in more than 70 over the counter preparations. PPA has been recently associated with neurological manifestations including psychosis, seizures and intracerebral haematoma. OTC related emergency room visits increased by 70% from 2004 to 2008.
_
Over-the-counter (OTC) medicines are medicines that may be sold directly to a consumer without a prescription from pharmacy personnel, as compared to prescription drugs, which are dispensed only to consumers possessing a valid prescription. Lack of adequate knowledge about OTC medications may directly lead to bad outcomes, such as overuse or non-compliance to treatment programs. There are several potential risks with the use of inappropriate self-medication such as the risk of adverse drug reactions, risk of wrong use of drugs, risk of missing the diagnosis, risk of drug dependence, risk of drug-drug, drug-food, drug-disease interactions, risk of overuse or toxicity.
In developing countries, professional health care is relatively expensive and, in some cases, not readily available making self-medication an obvious choice of healthcare service (F. Chang & P. K. Trivedi, 2003). Furthermore, it has been noted that many drugs that can only be purchased with prescription in developed countries are OTC in developing countries. Also, lax medical regulation has resulted in the proliferation of over-the-counter drugs that are in high demand for the treatment of highly prevalent diseases (Shakoor et al., 1997).
Unregulated or unrestricted availability of OTC drugs in the market increases the risk of drug resistance, adverse drug reaction and drug interactions (L. Hughes et al., 2002) (Ranjith et al., 2012). Studies have reported that there is increased or potential risk for misuse or drug abuse of the products (G. F. Hughes, McElnay, Hughes, & McKenna, 1999). Patients generally had poor knowledge of the potential side-effects of their medication. However, this appeared not to affect their ability to identify adverse drug reactions (ADRs) (Khade, Bashir, Ravi, & Vadala, 2012). Misuse of OTC drug by consumer is through overuse, taking several drugs concurrently and using home remedies to treat potentially serious diseases (Levine, 2007). People often think that prescription and OTC drugs are safer than illicit drugs, but that’s only true when they are taken exactly as prescribed and for the purpose intended. When abused, prescription and OTC drugs can be addictive and put abusers at risk for other adverse health effects, including overdose—especially when taken along with other drugs or alcohol (Bradley & Blenkinsopp, 1996).
_
Over the counter (OTC) drugs, may generate substantial net benefit flows to economies through saving in travel and consultation time and the direct financial cost of treatment (AESGP, 2004). Some conditions are necessary for these benefits to be realized. These conditions aim at ensuring the safety of taking self-medicated drugs. They include the following: the drugs used are those indicated for conditions that are self-recognizable; the user should know how to take or use the drugs; the effects and possible side-effects of the drug as well as ways of monitoring these side effects are well communicated to the user; possible interaction with other drugs is known by the user; duration of the course of the drugs is known by the user and when the user must seek professional intervention (WHO, 1998). The consequences for incorrect diagnosis and dosage include growing resistance to some drugs and further deterioration in health capital.
Regarding OTC medicines, there is generally less healthcare professional input into the recommendation or ongoing monitoring of use. There is an absence of records or linkage to other medication records and most countries allow direct-to-consumer advertising of the product. Taken together, these differences can result in inappropriate expectations, demand and use of the OTC medicines with limited opportunity for ongoing patient follow-up and monitoring of safety (Ranjith et al., 2012). Data from surveys and poison control center records demonstrate an increased nonmedical use of prescription and over-the-counter cough and cold preparations, particularly those containing Dextromethorphan.
_
OTC medicines can be classified into two categories:
First category of OTC medicines are the ones which have been under the category of non-prescription medicines since the time they were introduced.
The second category of OTC medicines are those that had been prescription medicines initially but were later shifted to the OTC category.
According to WHO, for a product to be an OTC medicine, it should be marketed on prescription for at least 5 years. Time period for change of category from prescription to OTC varies from country to country (e.g., European Union—no time specified, New Zealand—3 years, Japan—6 years, and Philippines—upto10 years). Before accepting switch of a given drug into OTC category, it is important to ensure that the drug did not cause serious adverse drug reactions with increasing frequency during the marketing period till then.
Many OTC drugs have undergone a prescription to over-the-counter switch — also known as “Rx-to-OTC switch” — meaning they were previously available only with a prescription but now can be bought as a nonprescription product. For example, proton-pump inhibitors like esomeprazole (Nexium 24HR) and stomach acid blockers like ranitidine (Zantac 150), both used for heartburn, are examples of products that have made the Rx-to-OTC switch. The emergency contraceptive pill (“the morning-after pill”) known as Plan B One Step is now available OTC without age restriction and can be found on the shelves in many pharmacies in the U.S.
It is somewhat unusual for an OTC drug to be withdrawn from the market as a result of safety concerns, rather than market forces, though it does happen occasionally, phenylpropanolamine being one example banned by FDA. Ranitidine was withdrawn from multiple markets due to concerns over the carcinogen N-nitrosodimethylamine (NDMA).
_
Potential Effects of introducing OTC/BTC drug:
The introduction of OTC/BTC drugs generally causes patients to substitute BTC/OTC versions for prescription versions. The effect on overall utilization, however, is more ambiguous, and depends to some degree on the structure of private and public health insurance and on whether the BTC/OTC versions are covered by insurers. In addition, there is a general consensus that BTC/OTC drugs reduce spending on prescription versions of the same drugs for payers, such as private and public health insurers. OTC drugs also cause decreases in both the number and growth rate of physician-office visits. The decrease in the number of visits is due to an initial surge of patients who use the OTC drug and choose not to see a physician. Over time, as more about the OTC drug’s safety and efficacy becomes known, more patients may choose to use the drug, thereby leading to a decrease in the growth rate of physician-office visits.
_
Non-drug OTC products:
In the US, medical devices may also be considered OTC. For example, most diabetic management supplies are available OTC. The category of medical devices includes electronic blood glucose monitors, monitor test strips, and lancets. In some areas insulin syringes may also be available OTC; some areas have classified all syringes as limited OTC products as a measure of drug addiction harm reduction.
_
Why choose OTC medications?
OTC medications are typically used for symptomatic relief of minor health conditions, including headaches, colds and fever. OTC medications usually cost much less than prescription medications.
Prescription Drug: Clarinex® ($30 a month)
OTC Option: Alavert® ($17.40 a month)
Savings: A difference of more than 42% a month! Plus save time and the cost of a visit to your physician. OTC medications are regulated by the Food and Drug Administration (FDA), which means they are held to high standards of safety, effectiveness and quality. It is cheaper to buy store brand ibuprofen than it is to buy Advil because ibuprofen is the generic version. You can always ask the pharmacist if you are choosing the least expensive option.
What should you consider when selecting OTC medications?
OTC medications should only be used when needed and as directed. Never exceed the recommended dosage. They can also interact negatively with other medications, both OTC and prescription, so consult your health care provider or pharmacist about any and all issues related to taking medications. Read all label and packaging instructions for OTC medications to know how much you should take, when to take it, what side effects you may encounter and what potential interactions you may have with other drugs.
____
Finding the right medicine:
Over-the-counter (OTC) medications play an important part in our healthcare. They allow millions of people to treat their own common health problems with easily accessible, affordable, and effective medicines. But, choosing the right OTC medicine can be confusing. There are many choices, and in order to safely choose the right medicine, you must first know what you’re looking for and how to read an OTC medicine Drug Facts label.
The table below lists the most common symptoms or conditions treated with OTC medicines, the category or type of the medicines used to treat these symptoms, along with examples of active ingredients to look for in the Drug Facts label. Always ask your pharmacist if you have questions about OTC medicines.
|
Symptoms or Conditions |
Category/Type of OTC Medicine (Purpose)
|
Example(s) of Active Ingredient(s) |
|
Runny Nose, Itching of the Nose or Throat, Itchy, or Watery Eyes Due to Hay Fever or Other Upper Respiratory Allergies |
Antihistamine |
diphenhydramine |
|
Heartburn or Indigestion |
Antacid or Acid Reducer |
famotidine |
|
Hair Loss |
Hair Regrowth Treatment |
minoxidil |
|
Fungal Infection (e.g., yeast infection, jock itch, ringworm, athlete’s foot) |
Anti-fungal |
clotrimazole |
|
Upset Stomach Associated with Nausea |
Upset Stomach Reliever (antinausea, also known as antiemetic) |
phosphorated carbohydrate solution |
|
Vaginal Yeast Infection |
Vaginal Antifungal (also known as anti-candidial) |
clotrimazole |
|
Diarrhea |
Anti-diarrheal |
loperamide |
|
Eye Care Contact Lens (cleaning) |
Peroxide-based Contact Lens Cleaning |
hydrogen peroxide 3% |
|
Eye Care Contact Lens (hydrating) |
Saline-based Contact Lens Solution |
saline solution |
|
Acne Vulgaris |
Acne |
benzoyl peroxide and sulfur, plus resorcinol, triclosan, or salicylic acid |
|
Mucous or Phlegm in the Trachea or Lungs |
Cough Expectorant |
guaifenesin |
|
Cough |
Cough Suppressant |
dextromethorphan (commonly found in combination products) |
|
Nasal Stuffiness |
Decongestant |
phenylephrine |
|
Diaper Rash |
Skin Protectant |
zinc oxide |
|
Fatigue or Drowsiness |
Alertness Aid |
caffeine |
|
Red Eyes |
Redness Reliever Eye Drops |
tetrahydrozoline naphazoline (commonly found in combination products) |
|
Itchy Eyes |
Antihistamine Eye Drop Solution |
pheniramine maleate |
|
Dry Eyes |
Eye Lubricant |
dextran 70 |
|
Fever |
Fever Reducer |
Acetaminophen (paracetamol) |
|
Constipation |
Laxative |
bisacodyl |
|
Hemorrhoids |
Protectant for Hemorrhoids |
mineral oil, petrolatum, phenylephrine HCl |
|
Minor Aches and Pains |
Analgesic or Pain Reliever |
acetaminophen |
|
Menstrual Period Symptoms |
Pain Reliever |
Ibuprofen, naproxen, or acetaminophen (pain reliever) used alone or in combination with: pamabrom or caffeine (diuretic) or pyrilamine maleate (antihistamine) |
|
Motion Sickness |
Antiemetic |
dimenhydrinate |
|
Withdrawal Symptoms, Including Nicotine Craving, Associated with Quitting Smoking |
Stop Smoking Aid |
nicotine |
|
Minor Aches and Pains for Muscle and Joints |
Topical Analgesic Topical Pain Reliever |
camphor, menthol, methyl salicylate |
|
Itching Skin |
Topical Anti-itch or Topical Analgesic |
diphenhydramine |
|
Occasional Sleeplessness or Difficulty Falling Asleep |
Night-time Sleep-aid |
diphenhydramine |
|
Toothache/Teething |
Pain Reliever |
benzocaine |
|
Warts |
Wart Remover |
salicylic acid |
|
Weight Loss |
Weight Loss Aid |
orlistat |
OTC drugs are medications that are safe and effective for use by the general public without seeking treatment by a health professional. Popular examples include pain relievers like acetaminophen (Tylenol) and ibuprofen (Advil, Motrin), cough suppressants such as dextromethorphan (Robitussin) and antihistamines like loratadine (Claritin 24H). These drugs are usually located on shelves in pharmacies, grocery stores, and even in gas stations. OTC medicines treat a variety of symptoms due to illness including pain, coughs and colds, diarrhea, heartburn, constipation, acne, and others.
_
Broad Therapeutic Classes of OTC Medications:
-1. Analgesics and antipyretics
-2. Cold, cough, and allergy products
-3. Nighttime sleep-aids
-4. Gastrointestinal products
-5. Dermatological products
-6. Other topical products (including dermal and vaginal antifungals, anorectal medications, head lice products, hair loss products, and otic drugs)
-7. Ophthalmic products
-8. Oral health care products
-9. Menstrual products
-10. Nicotine replacement products
-11. Weight loss aids
-12. Vaginal contraceptives and emergency contraceptives.
_____
Can you buy antibiotics over the counter?
In general, over-the-counter (OTC) oral antibiotics are not approved in the U.S. However, there are a few OTC topical antibiotics that can be used on the skin to help prevent infections from minor scrapes, burns and wounds, such as:
-bacitracin/neomycin/polymyxin B (Neosporin and generics),
-bacitracin/polymyxin (Polysporin and generics), and
-neomycin/polymyxin/pramoxine (Neosporin Plus), which also contains pramoxine, a mild numbing medication.
Over-the-counter antibacterials, such as benzoyl peroxide, is also available for those with mild acne. Benzoyl peroxide provides a drying effect and can be bought in a generic and as brand names like Clearskin, Oxy-10, and Proactiv.
Although topical products with antibiotic properties can be purchased without a prescription, no over-the-counter antibiotics for internal use are allowed in the U.S. Other countries follow different practices and allow some antibiotics to be purchased without a prescription, but the strongest drugs are generally never available over-the counter. The primary reason for restrictions on over-the-counter antibiotics is the prevention of antibiotic overuse and misuse, to limit unnecessary side effects, and to slow the emergence of antibiotic resistance in bacteria.
Antibiotics are only useful in treating bacterial infections, such as strep throat or tuberculosis. They are of no use in dealing with viral infections, such as colds and influenza. Consumers, however, do not always understand the difference between viruses and bacteria, and will often seek to purchase and use antibiotics when they are suffering from viral infections.
_
Because OTC drugs are sold in the same way as soap, vitamins, and bandages, many people consider them to be inherently safe. Clearly, this is a mistake. Any drug has the potential for harm if misused. As a rule of thumb, remember that even the most familiar OTC product:
-1. Has the potential for overuse and overdose
-2. May interact with other drugs, including alcohol
-3. May undermine the effectiveness of other drugs you are taking
-4. May cause allergy in some
-5. May not be appropriate for children, pregnant women, people with liver or kidney problems
-6. May cause addiction. People who have an OTC addiction are at risk of having serious health problems such as memory loss, kidney failure, heart problems, and in some cases, death.
In truth, the risk may be minimal to nil. Nevertheless, it is important to read the product label before using an OTC product and to consult your doctor about any and all drugs you are taking, including dietary supplements and recreational drugs. If you do not understand the product label, speak with your pharmacist. That is what a pharmacist is there for. If you have been taking an OTC medicine but your symptoms don’t go away, contact your family doctor or health care provider. You should not take OTC medicines longer or in higher doses than the label recommends.
______
______
OTC can be marketed as generic drug, pharmaceutical company brand drug or store brand drug:
Basically, the brand name is created by the pharmaceutical company that made the medicine while the generic name is the name of the active ingredient in the medicine. For example, sildenafil is the generic name of a medicine used to treat erectile dysfunction. But the company that makes sildenafil, Pfizer, sells it under the brand name Viagra. Companies take out exclusive rights called patents on each new drug they discover. If a company has a patent on a drug, only that company can market it under their brand name once it’s been granted a license. Once the patent expires, other manufacturers can market generic versions. The generic versions will be the same as the branded medicine because they contain the same active ingredients. They are used more often by the NHS because they’re just as effective but cost far less.
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is believed to be equivalent in performance. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging. Although they may not be associated with a particular company, generic drugs are usually subject to government regulations in the countries in which they are dispensed. They are labeled with the name of the manufacturer and a generic non-proprietary name such as the United States Adopted Name (USAN) or International Non-proprietary Name (INN) of the drug. A generic drug must contain the same active ingredients as the original brand-name formulation. The U.S. Food and Drug Administration (FDA) requires generics to be identical to or within an acceptable bioequivalent range of their brand-name counterparts, with respect to pharmacokinetic and pharmacodynamic properties. (The FDA’s use of the word “identical” is a legal interpretation, not literal.)
Medicines may also have one or more brand names. The drug company that makes a medicine chooses a brand name that is usually easier to say and remember than the generic name. For example, Motrin is a brand name for a medicine used to treat pain. Its generic name is ibuprofen. Motrin was chosen as a brand name by the company that first made ibuprofen. However, after the patent on Motrin ended, other manufacturers were allowed to make a generic version of the medicine, provided it met standards set by the US Food and Drug Administration. While the generic name of the medicine will always be ibuprofen, all companies that now make ibuprofen can choose a different brand name for their products. So today, Motrin and Advil are just a few of the many brand names for ibuprofen. Occasionally, a company chooses not to use a specific brand name. In that case, the medicine has only a generic name.
Many people wonder whether common over-the-counter medicines made by store brands, such as those manufactured by Walmart, Target and major grocery store chains, are as effective as their brand-name counterparts. The differences in the products include price point, packaging, and inactive ingredients.
- The active ingredient is the same in store brands and brand names.
A popular over-the-counter brand name that you probably recognize is Bayer. The active ingredient in Bayer is aspirin. And there countless store brands—or generic versions—of aspirin. They are the exact same ingredient. They have the same dosage, strength, safety and performance. In fact, brand name manufacturers make about half of all generic drugs. And there are store brands of anything from laxatives to cough syrups.
- But the inactive ingredients may differ.
Trademark laws mean that store brands can’t look exactly the same as brand names. So there are differences between store brands and brand names when it comes to inactive ingredients. This includes the preservatives, flavoring, coloring, and other ingredients to give the product size and shape. But all of these ingredients must meet safety regulations and they can’t interfere with how the product works.
- The FDA regulates all over-the-counter drugs.
The FDA (Food and Drug Administration) regulates the approval, manufacture and sale of all over-the-counter (OTC) drug products. Store brands must go through the same FDA approval process as brand names. They must meet the same safety and effectiveness standards. The FDA requires manufacturing facilities to meet their standards regardless of whether they make store brands or brand names. And store brands must also meet the same FDA quality standards. The FDA even regulates OTC labeling so consumers can use these drugs without the intervention of a healthcare provider.
- Always read the drug facts label.
Both store brands and brand names must have labeling that allows you to use them safely. You can find information about the product in the “Drug Facts” section of the labeling. The active ingredient is the very first item. It’s important to check this section and compare the store brand to the brand name. This ensures you’re getting the same drug. You also need to make sure you aren’t doubling up on an active ingredient accidentally, for instance, if you are taking more than one type of cold or allergy medicine.
- Store brands will save you money without sacrificing quality.
On average, store brands cost about half the price of brand names. This is mostly due to low marketing budgets. But retailers know you won’t continue to buy a store brand if you don’t trust it. So many store brands aim to exceed quality standards. It seems to pay off. In a University of Chicago study, most consumers chose store brands across a variety of healthcare categories. The same study found most doctors and pharmacists rely on store brands themselves.
- Look beyond the active ingredients and the cost.
While the active ingredients and quality may be the same, store brands and brand names may have differences. And these differences may sway you one way or the other. For example, chewable or liquid products may taste better with one product over another. Packaging is another example. You may find certain packaging styles easier to handle than others. These things are purely a matter of preference, so choose the ones you like.
- When in doubt, talk with your pharmacist.
Reading the labels and comparing products can get confusing. There are various strengths and formulations, and endless combinations of ingredients. If you’re shopping in a pharmacy, take advantage of the free advice behind the pharmacy counter. Your local pharmacist is an excellent resource to help you choose the product that’s right for you.
______
______
OTC Drug Facts Label:
Whenever you use an over-the-counter (OTC) medicine, reading the drug product’s labeling is important for taking care of yourself and your family. The label tells you what the medicine is supposed to do, who should or shouldn’t take it, and how to use it. The labeling of OTC medicines has always contained usage and safety information for consumers. With the introduction of the “Drug Facts” label, the information is more uniform and easier to read and understand. According to the law, OTC drug labeling must include “all of the information that an ordinary consumer needs for safe and effective use.” The OTC drugs – labeling is regulated by FDA – advertising by Federal Trade Commission (FTC). But the prescription drugs advertising is strictly regulated only by FDA.
The Drug Facts label uses simple language and an easy-to-read format to help people compare and select OTC medicines and follow dosage instructions. The following information must appear in this order:
-1. The product’s active ingredients, including the amount in each dosage unit. The drug itself is the active ingredient. Combination products have more than one active ingredient. The drug’s generic name is listed with the amount of drug in each tablet, capsule, or dose unit. The same generic drug may be sold under several different trade (brand) names.
-2. The purpose of the product.
-3. The uses (indications) for the product.
-4. Specific warnings, including when the product should not be used under any circumstances, and when it is appropriate to consult with a doctor or pharmacist. This section also describes side effects that could occur and substances or activities to avoid.
-5. Dosage instructions–when, how, and how often to take the product.
-6. In addition to the drug, drug products—the tablets, capsules, or other formulations that consumers buy—contain substances added to facilitate the administration of the drug, such as ingredients that provide bulk or a pleasant taste and color. These are inactive ingredients. Products with the same active ingredient may contain different inactive ingredients. Inactive ingredients are usually harmless, but some of them cause an allergic reaction in a few people, who should look for products made without those ingredients.
Along with the standardized format, the label uses plain-speaking terms to describe the facts about each OTC drug. For example, “uses” replaces “indications,” while other technical words like “precautions” and “contraindications” have been replaced with more easily understood words and phrases. The label also requires a type size large enough to be easily read and specific layout details–bullets, spacing between lines, and clearly marked sections–to improve readability.
If you read an OTC medicine label and still have questions about the product, talk to your doctor, pharmacist, or other health care professional.
_
Look at the sample Drug Facts label below. All Drug Facts labels provide this information in the same order. Be sure to read the Drug Facts label carefully before purchasing and using any OTC product. If you have any questions, ask your pharmacist or doctor.
_
The Label also tells you…
- The expiry date, when applicable (date after which you should not use the product).
- Lot or batch code (manufacturer information to help identify the product).
- Name and address of manufacturer, packer, or distributor.
- Net quantity of contents (how much of the product is in each package).
- What to do if an overdose occurs.
Many OTC medicines are sold in containers with child safety closures. Use them properly. Remember—keep all medicines out of the sight and reach of children.
_
Read the Label each time before using a medication:
Be sure it’s right in the 5 R’s:
-The right medicine
-The right person
-The right amount
-At the right time
-The right way (swallow, chewable)
_
Follow the Dosing Directions:
- Never guess the dosing amounts, especially when giving medicines to children
- Every medicine has dosing directions on its label
- Label will tell you the amount of medicine to give, when to give it, and how to give it
- If a medication does not have a dose for you or your child or specifically says DO NOT USE, do not give that medication and call your health care provider
How to measure Liquid Medicine:
- You must measure medicines correctly
- Use the measuring spoon, cup or syringe that comes with the medicine. It will give the most exact dose
- If the medication does not come with a special measuring tool, ask the pharmacist for one
- A silverware spoon may hold the wrong amount of medicine
- Check the marking to make sure your measuring tool can measure the right dose
Most Liquid Medicines are measured in Teaspoons (tsp) and Milliliters (mL)
5 mL= 1 teaspoon (tsp)
15 mL= 3 teaspoons= 1 tablespoon (TBSP)
30 mL = 1 fluid ounce (oz)
_
Protect yourself against tampering:
Makers of over-the-counter (OTC) medicines seal most products in tamper-evident packaging (TEP) to help protect against criminal tampering. Criminal tampering is the term used to describe intentional alteration of a medicine or other product that makes it harmful. TEP works by providing visible evidence if the package has been disturbed. One of the most common forms of TEP is called induction sealing, a cap sealing process in which a metallic disk is sealed to the top of plastic or glass containers. Other common forms of TEP include shrink bands and tamper-evident caps.
Be alert to the tamper-evident features on the package before you open it. These features are described on the label as seen in the figure below:
OTC packaging cannot be 100 percent tamper-proof. Here’s how to help protect yourself:
-Inspect the outer packaging before you buy it. When you get home, inspect the medicine inside.
-Don’t buy an OTC product if the packaging is damaged.
-Be suspicious! If anything looks suspicious, contact the store where you bought the product and take it back.
-Don’t use any medicine that looks discolored or different in any way, or smells funny.
_____
Safe Medicine Storage & Disposal:
OTC medicines play an important role in keeping us healthy and treating certain symptoms and conditions when we are ill. But they must be stored safely, and unused portions of these medicines must be disposed of properly to avoid potential drug abuse of OTC medicines, prevent accidental poisonings of children and pets, and to protect the environment.
______
______
Over-the-Counter Medicines’ advantages and harms:
Four out of five American adults commonly take over-the-counter medications, most often to treat ailments like aches and pains, coughs and colds, fever, allergies, skin disorders, and heartburn and other digestive problems. The reasons are easy to understand. O.T.C.s are convenient, readily available in groceries and big box stores as well as pharmacies, and they are less expensive than going to the doctor and perhaps paying for a costly prescription.
According to the Food and Drug Administration, there are more than 300,000 over-the-counter drug products on the market, a number that continues to grow as an increasing number of medications move from prescription to O.T.C. status. According to the Consumer Healthcare Products Association, an industry trade group, since 1975, more than 100 ingredients, indications or dosage strengths have transitioned from prescription to O.T.C. status.
In 2014 Americans spent about $44 billion on O.T.C. drugs which, the industry claims, saved the health care system about $102 billion in doctor visits, diagnostic tests and prescription medications. In addition to saving consumers time and money, O.T.C.s give many people a sense of self-control over their health and well-being. That’s all good if OTC drugs are used appropriately, for an indicated condition in the proper dosage and for no longer than the recommended time. However, one in five adults who self-medicate admit to taking more than the recommended dose or using the medication more frequently than the label indicates.
Few consult a doctor — or even a pharmacist — about the safety and wisdom of using a particular O.T.C. drug. And a consumer poll taken in 2001 for the National Council on Patient Information and Education found that most people read only some of the information on product labels and thus may miss information essential to the drug’s proper use. Even if O.T.C.s are used correctly, there can be problems. Some drugs should not be taken by people with certain health conditions, or be combined with other drugs — prescribed or over the counter — because of the possibility of adverse interactions. For example, acetaminophen, the active ingredient in Tylenol and its many competitors, is the most widely used O.T.C., commonly taken to relieve pain or fever. But acetaminophen is also a frequent ingredient in other often-used O.T.C. products, including many cough, cold and allergy remedies, and prescribed pain relievers like Percocet and Vicodin. In excessive amounts, acetaminophen can cause severe liver damage. Overdoses of acetaminophen result in 30,000 hospitalizations annually, often because of acute liver failure. A study of 500 people published in 2012 in The Journal of General Internal Medicine revealed that 24 percent would unwittingly exceed the safe limit of 4,000 milligrams of acetaminophen over a 24-hour period when taking a single product containing the drug. About 46 percent would overdose when taking two products at the same time that contain this pain reliever.
According to the National Council, a third of Americans say they combine medications when treating multiple symptoms, but only one person in 10 say they read the entire label of each drug taken; therefore, most are unaware of potentially toxic duplications or harmful interactions. Seeking a sales advantage, many companies that make O.T.C.s offer products with multiple ingredients meant to treat several symptoms simultaneously. However, many consumers do not need all the active drugs in a given product and thus needlessly increase their risk of toxicity.
About 40 percent of O.T.C. drugs are used by people older than 65, who are most likely to have health issues that may contraindicate the use of certain over-the-counter medications. Because of chronic health problems, age-related changes in how well the body processes drugs, and the sheer number of prescription medications many older people tend to take, they face the greatest risk of adverse side effects and drug interactions. Among drug-related hazards disproportionately faced by older patients are falls, depression, confusion, hallucinations and malnutrition.
Just because a drug is sold over the counter does not mean it’s harmless. Laxatives, for example, are said to be among the most misused over-the-counter remedies, and people abuse them in an effort to lose weight. Over-the-counter sleeping pills that contain antihistamines can lose their effectiveness over time, which can result in people taking more than the recommended dose. They should not be used for more than two weeks. Even if taken as directed, they can result in daytime sleepiness, dizziness and a thickening of bronchial secretions. Some people with chronic heartburn take antacids that counter the effects of stomach acid. But these can also cause diarrhea or constipation, and block the absorption of certain prescription medications. Better choices now available over the counter are H2 blockers (like Pepcid and Zantac) and proton-pump inhibitors (like Nexium, Prilosec and Prevacid) that stop the production of stomach acid. But these drugs may also pose dangers when taken long term, including bone fractures and magnesium deficiencies that can lead to seizures.
When NSAIDs, or nonsteroidal anti-inflammatory drugs, like aspirin, ibuprofen and naproxen are taken too long, they can likewise pose dangers, including bleeding ulcers, kidney or liver damage and an increased risk of a heart attack or stroke. And so on….
Although O.T.C. drugs are generally safe when used occasionally and correctly by healthy adults, those with chronic health problems can risk potentially serious adverse reactions. People who have underlying health problems or who routinely take one or more prescription drugs would be wise to consult their doctors before taking an O.T.C. drug. At the very least, check with the pharmacist. If you fill all your prescriptions at the same pharmacy, potential adverse drug interactions are easier to pick up. Failing that, carry with you a list of all the prescription and O.T.C. medications you take to show the pharmacist.
Among other sensible precautions when using an O.T.C. drug: Read the entire label, including ingredients, dosages, time limits and warnings; note whether the drug should be taken with food or on an empty stomach; don’t mix medicines with alcohol; avoid taking vitamin-mineral supplements at the same time; and, if you experience an allergic or adverse reaction, write down the likely cause so you can avoid that ingredient in the future.
_
Lack of effect of OTC drugs:
Many OTC product complaints relate to an alleged “lack of effect”. OTC products may not necessarily produce the same degree of efficacy measurable by consumer-recognizable or objective clinical endpoints as with Rx drugs. Many OTC consumers have unreasonable efficacy expectations or take an OTC product for reasons other than those described in the labelling. Many OTC consumers are simply attempting to get a refund by claiming the product “did not work” for them. Regulatory definition of “lack of effect” relates more to frank pharmacological failure rather than to cases of general consumer dissatisfaction typically seen with OTC products; difficult to distinguish true lack of effect cases from general consumer complaints.
______
______
Safe use of OTC drugs:
_
Are OTC drugs safe?
Many people turn to over-the-counter medicine to find relief from common conditions like headaches, fever, muscle pain, the common cold, and allergies. And for good reason: they’re easily accessible, don’t require a doctor’s appointment, and are generally safe when taken as directed. But doctors warn that there are risks involved with taking these drugs that people may not be aware of — and that can be very serious. Certain groups of people, such as the very young, the very old, the very sick, and pregnant and breastfeeding women, are more vulnerable to harm from drugs, including over-the-counter (OTC) drugs. When such people use drugs, special precautions, which may include a doctor’s supervision, should be taken.
To avoid dangerous drug-drug interactions, people should consult a pharmacist or doctor before they take prescription drugs and OTC drugs at the same time. People who have chronic disorders should also consult a pharmacist or doctor. OTC drugs are not designed to treat serious disorders and can make some disorders worse. An unanticipated reaction, such as a rash or insomnia, is a signal to stop taking the drug immediately and obtain medical advice.
One of the biggest concerns, experts say, is when older adults are on multiple prescription drugs. There are interactions between these over-the-counter medicines and prescription drugs that a patient may not be aware of. Some of them may be dangerous and life threatening so it’s always important to consult your physician before taking over-the-counter drugs if you’re on chronic meds. People with heart disease, high blood pressure, diabetes, asthma, and kidney or liver problems are at an increased risk of adverse side effects from over-the-counter medication.
When shopping at the pharmacy, pay close attention to the drug labels. The first thing that you want to make sure of is that it contains exactly what it says it does. Another problem is that drugstore shelves are full of medicines that contain multiple active ingredients meant to treat an array of symptoms, so it can be easy to inadvertently take too much. For example, the main ingredient in Tylenol — acetaminophen — is also in a number of over-the-counter cold and flu medicines, so mixing them could spell disaster. According to a 2014 study published online in the Expert Review of Clinical Pharmacology, acetaminophen overdoses send nearly 80,000 people to the emergency room each year, and 30,000 need to be hospitalized. The U.S. Food and Drug Administration says people should take no more than 4,000 milligrams of acetaminophen in a 24-hour period. To avoid exceeding this level, the FDA recommends that you:
- Don’t take more than one OTC product containing acetaminophen
- Don’t take a prescription and an OTC product containing acetaminophen
- Don’t exceed the recommended dose on any product containing acetaminophen
Symptoms of acetaminophen overdose may take many days to appear, the FDA says, and even when they become apparent, they may mimic flu or cold symptoms, making it even more important to monitor dosage. The bottom line is that consumers need to pay close attention while selecting over-the-counter medications.
_
Choosing and using over-the-counter drugs:
Safety depends on using a drug properly. For OTC drugs, proper use often relies on consumer self-diagnosis, which leaves room for error. For example, most headaches are not dangerous, but in rare cases, a headache is an early warning of a brain tumor or hemorrhage. Similarly, what seems like severe heartburn may signal an impending heart attack. Ultimately, people must use common sense in determining when a symptom or ailment is minor and when it requires medical attention and consult a doctor or pharmacist if they are unsure.
The guidelines for choosing and using OTC drugs are as follows:
- Make sure that the self-diagnosis is as accurate as possible. Do not assume the problem is “something that is going around.”
- Choose a product because the ingredients are appropriate for the condition, not because the product has a familiar brand name.
- Choose a product with the fewest appropriate ingredients. Products that attempt to relieve every possible symptom are likely to expose people to unnecessary drugs, pose additional risks, and cost more.
- Read the label carefully to determine the correct dose and precautions, including what conditions would make the drug a poor choice.
- When in doubt, ask a pharmacist or doctor what the most appropriate ingredient or product is.
- Ask a pharmacist to check for potential interactions with other drugs being used.
- Ask a pharmacist to identify possible side effects.
- Do not take more than the recommended dose.
- Do not take an over-the-counter drug longer than the maximum time suggested on the label. Stop taking the drug if symptoms worsen.
- Keep all drugs, including over-the-counter drugs, out of the reach of children.
_____
Safety tips:
Here are a few tips to help you make safe choices and reduce your risk of harm when using OTC medicines.
-1. Consult your doctor or pharmacist before purchasing an OTC product. Your doctor and pharmacist can help guide you in selecting an appropriate OTC medicine based on your medical history. They should be aware of any allergies you have or other prescription or OTC medicines you are taking. Be sure to tell them about any herbal supplements, vitamins, or alternative products you may be using. Your doctor or pharmacist will use this information to recommend an OTC product for you.
-2. Read the label carefully. The US Food and Drug Administration (FDA) requires drug manufacturers to include specific information on OTC medicines. This important information is found in the Drug Facts label on the medicine package or container. The label includes active ingredients; purpose (what it is for); uses; warnings; directions – when, how, and how often to take; age restrictions, and other information including inactive ingredients.
-3. Do not take medicines with the same active ingredients. The active ingredient is the component in the drug that works to treat the problem. The active ingredient is listed on the Drug Facts label. The active ingredient may be in a number of different OTC medicines. For example, OTC pain relievers such as Advil and Motrin both have the active ingredient ibuprofen, so they should not be taken together. Taking medicines with the same active ingredient can lead to serious side effects or a life-threatening overdose. So, when purchasing OTC products, always compare labels. Once home, check the new product’s active ingredients against any OTC or prescription medicines you already have. If you are unsure, ask your pharmacist if it is okay to take the OTC product with the other medicines you take.
-4. Keep a current list of medicines you take. OTC medicines, vitamins, supplements, and herbal products can interact with each other and some prescription medicines. By having a current list of medicines you take, your doctor and pharmacist will be able to accurately check for interactions before prescribing or dispensing new medicines to you. They can also tell you which OTC products are safe to take with your other medicines, and which OTC products to avoid.
-5. If you are pregnant or breastfeeding consult with your doctor before taking an OTC medicine. Some OTC medicines are harmful to a developing baby. Some medicines a breastfeeding mom may take can pass through her breast milk and may harm her baby. It is always best to ask your doctor before taking any medicine while you are pregnant or breastfeeding.
-6. Remember herbal supplements are not the same as OTC medicines. Herbal supplements can be found in the OTC aisle, but unlike OTC medicines, FDA does not review them for safe use before they are marketed. Herbal supplements can cause side effects, interact with other medicines you may take, and worsen a health condition. Many herbal supplements are labeled “natural,” but natural does not mean safe. Herbal compounds including garlic, ginkgo, and ginseng can affect the function of platelets which can increase the risk of bleeding. Remember to always talk to your doctor or pharmacist before starting any herbal supplement.
-7. Always check the expiry date. Never use medicines that are expired. According to FDA, a medicine can become less effective or more potent after its expiration date. Set aside a few days each year to throw away the expired medicines in your home.
-8. Only use the measuring device that comes with the OTC product. Household measuring spoons and other kitchen utensils (e.g., teaspoons) should not be used to measure a dose of medicine. Using these items can result in taking more or less of a medicine than is recommended. Instead, always use the measuring device that comes with the OTC product.
-9. Seek medical attention if your symptoms get worse or you experience side effects. If your symptoms do not improve or if you feel worse after taking a few doses of an OTC medicine, contact your doctor. Continuing to self-treat can delay you from receiving the medical treatment necessary for what may be a serious health condition.
-10. Many people transfer medicine to other containers or bags for traveling or just for convenience. However, it is safest to keep the original container with the medicine to ensure that you are aware of which medicine you are taking as well as being aware of the dosage instructions, warnings and guidelines associated with it. Additionally, it is safest to take only medicine that you have purchased and broke the seal on.
-11. Extensive research has been conducted with over-the-counter drugs to know the appropriate dosage for different people. Not adhering to the dosage suggested on the label can negatively affect symptoms and lead to worse side effects. You can find dosage instructions on the back of the medicine bottle or box. Doses often vary by age or weight.
-12. If young children are present in a household, it is safest to store medicine out of reach and sight. Doing so can reinforce proper medicine-taking behaviors, ensuring that children ask permission to take medicine when they need it.
-13. OTC drugs may interact with diet or lifestyle factors. OTC medications can be harmful if they interact with alcohol in your system or certain foods in your diet. As a general rule, you should not consume alcohol when taking any type of medication, unless your doctor gives you permission to do so.
-14. OTC drugs may affect older people differently. While everyone should be careful when taking medications, older people need to take extra caution. The body changes how it absorbs food and drugs as it ages; and, older people generally take more medications than younger people, putting them at higher risk for adverse interactions.
______
The safe use of over-the-counter painkillers:
Many painkillers are available from pharmacies without a prescription. They can provide effective pain relief, but might also cause side effects or complications. In order to use them safely, it is important to pay attention to the dose and interactions with other medicinal products. The largest group of over-the-counter painkillers are the non-steroidal anti-inflammatory drugs (NSAIDs). As their name suggests, they also reduce inflammation but – unlike other anti-inflammatory medicine – do not contain steroids. Over-the-counter NSAIDs are used in the treatment of many different kinds of pain, including headaches, period pain and toothache. NSAIDs reduce pain and inflammation and also lower fever. There are more than ten different NSAIDs, but not all of them are available without a prescription or in every dose. In some countries, the following NSAIDs are available over the counter:
- Acetylsalicylic acid (ASA, the drug in medicines like “Aspirin”) (in doses of up to 500 mg per tablet)
- Diclofenac (up to 25 mg per tablet)
- Ibuprofen (up to 400 mg per tablet)
- Naproxen (up to 250 mg per tablet)
These medications are also by far the most commonly used NSAIDs.
Acetaminophen (paracetamol) is another very widely used painkiller. While also relieving pain and lowering fever, it doesn’t reduce inflammation (unlike NSAIDs). In some medications it is combined with an NSAID (e.g., acetylsalicylic acid and acetaminophen). Caffeine is sometimes added too. It isn’t clear whether these kinds of combinations have any advantages or disadvantages over using the active ingredients separately. There are no good-quality studies comparing combination medications with individual drugs.
The risk of side effects and complications can be reduced by using the lowest dose possible. It is generally important not to exceed the maximum single dose or the maximum daily dose. The information in the following table applies to Germany but may be very similar in other countries.
Maximum daily dose for adults (without a prescription):
|
Drug |
Maximum single dose |
Maximum daily dose |
|
ASA |
1,000 mg |
3,000 mg in people under 65, 2,000 mg in people over 65 |
|
Diclofenac |
25 mg |
75 mg |
|
Ibuprofen |
400 mg |
1200 mg |
|
Naproxen |
500 mg |
750 mg |
|
Acetaminophen (paracetamol) |
1,000 mg |
4,000 mg |
So if someone has a packet of 400 mg ibuprofen tablets, for instance, they should not take more than three tablets per day (24 hours).
Different NSAIDs shouldn’t be combined with each other. But an NSAID can be combined with acetaminophen if one medication alone isn’t effective enough.
Side effects and complications:
The most common side effects of NSAIDs affect the stomach. They range from minor problems like indigestion and stomach ache to more serious problems like gastritis, ulcers and bleeding in the stomach or bowel (gastrointestinal bleeding). The risk of complications can be significantly reduced by using other medication to protect the stomach. Proton pump inhibitors such as omeprazole or pantoprazole are typically used for this purpose. But over-the-counter painkillers very rarely lead to serious side effects if they are taken for a short time only.
Several analyses of studies in recent years have also shown that certain NSAIDs, such as diclofenac, increase the risk of cardiovascular disease. But that is mainly if you take high doses over a long period of time. Nevertheless, it may still be a good idea for people who are at higher risk of cardiovascular disease, or already have cardiovascular disease, to take low doses of ibuprofen or naproxen rather than diclofenac. People who have cardiovascular disease can take acetaminophen (paracetamol) as an alternative to NSAIDs.
NSAIDs aren’t suitable for people with advanced kidney disease. Acetaminophen (paracetamol) is then an alternative option. People who have a stomach ulcer or severe cardiac insufficiency (heart failure) shouldn’t take NSAIDs either. Pregnant women are only allowed to take certain NSAIDs during certain weeks of pregnancy. It is best to consult your pharmacist or doctor if you have any questions.
Acetaminophen is not suitable for people who have liver disease. This is because it is broken down by the liver and can cause severe liver damage. It is also not suitable for people who have problems with alcohol abuse. People who have a severely decreased kidney function should wait eight hours between taking the tablets, and make sure they don’t take more than 3000 mg acetaminophen per day.
Prevention of complications:
You can lower the risk of painkiller-related side effects and complications by paying attention to the package insert and
- always taking as little as possible, as much as necessary,
- only taking painkillers for as long as really needed,
- watching out for possible interactions with other medications, and
- checking whether certain painkillers aren’t suitable if you have any of the risks or medical conditions described above.
What happens if there are drug-drug interactions?
Painkillers can also interact with other medications. It is called a “drug-drug interaction” if two medications influence each other – for instance, if they increase, weaken or cancel out their effects. As a result, the medications may no longer work properly or the risk of complications might increase.
NSAIDs can interact with various medications. For instance, taking them together with certain medications that suppress the immune system (cyclosporine and tacrolimus) increases the risk of kidney damage. This is also true if you take NSAIDs together with diuretics or certain blood-pressure-lowering drugs (ACE inhibitors and angiotensin II antagonists). NSAIDs can increase the effect of antiplatelet and anticoagulant medications such as clopidogrel and warfarin, which may lead to bleeding.
The risk of liver damage is higher if acetaminophen (paracetamol) is taken together with the antibiotic isoniazid. For the same reason, you should avoid drinking alcohol if you take acetaminophen. The cancer medication imatinib might also increase the kidney-damaging effect of acetaminophen.
The possible drug-drug interactions of a medication are listed in the accompanying package insert. It is best to consult your doctor or pharmacist if you have any questions.
______
______
Precautions with Over-the-Counter Drugs in people with Chronic Disorders:
A number of chronic disorders can become worse if an OTC drug is taken inappropriately. Because OTC drugs are intended primarily for minor illness for people who are otherwise healthy, people who have a chronic or serious disorder or who plan to take an OTC drug every day should consult a health care practitioner before they purchase OTC products.
|
Disorder |
OTC Drugs |
Precautions |
|
Alcoholism |
Cold remedies |
Recovering alcoholics need to be vigilant about avoiding any products that contain alcohol, including cold remedies. Some products contain as much as 25% alcohol. |
|
Diabetes |
Decongestants |
People with diabetes should consult a doctor before they take decongestants because these drugs can worsen diabetes and have dangerous side effects. |
|
Cough syrups |
People with diabetes may need help locating liquid products that do not contain sugar, cough syrups included. |
|
|
Enlarged prostate |
Antihistamines Decongestants |
People with an enlarged prostate should consult a doctor or pharmacist before they take antihistamines and decongestants because side effects can be dangerous. |
|
Glaucoma |
Antihistamines Decongestants |
Taking an antihistamine or decongestant can complicate certain types of glaucoma. |
|
Heart disease |
Antacids Cold remedies Analgesics |
People with heart disease should consult a doctor or pharmacist to help them select an antacid or cold remedy that does not interact with their prescription drugs. Certain analgesics such as NSAIDs may worsen heart failure. |
|
Decongestants |
People with heart disease should consult a doctor or pharmacist before they take decongestants because side effects can be dangerous. |
|
|
High blood pressure (hypertension) |
Analgesics Antacids |
People with high blood pressure should consult a doctor or pharmacist before they select an analgesic or antacid. |
|
Decongestants |
People with high blood pressure should consult a doctor or pharmacist before they take decongestants because side effects can be dangerous. |
|
|
Hyperthyroidism (over activity of the thyroid gland) |
Decongestants |
People with hyperthyroidism should consult a doctor or pharmacist before they take decongestants because side effects can be dangerous. |
|
Kidney disorders |
Antacids NSAIDs |
People with kidney disorders should consult a doctor or pharmacist before they select an antacid or use an NSAID. |
|
NSAIDs = nonsteroidal anti-inflammatory drugs; OTC = over-the-counter. |
||
_____
_____
Factors that promote growth of OTC drugs:
-1. Socio-economic status
Various studies on the socioeconomic status (SES) determinants of self-medication have largely focused on use of OTCs, and the findings have been inconsistent. Multiple large-scale surveys conducted in high-income countries have demonstrated a relationship between higher income and education and increased use of OTCs. Studies conducted in low- and middle-income countries showed that income and health insurance coverage were inversely correlated with the propensity to self-medicate, which suggests self-medication is an inferior good. Furthermore, given that the purchase of prescription-only medicines without prescription is common in many countries, few of these studies distinguished between OTC and prescription drug self-medication.
- In general, High socioeconomic status in developed countries is major reason for the increasing trend of the use of OTC drugs. On the other hand, in developing country like India, people with low socio-economic status who cannot keep health in first priority, OTC drugs are the best solution.
- Direct use of OTC drugs decrease the cost of the treatment/health care seeking.
-2. Easy availability
- Medicine in shops/counters are easily accessible than in the health care institution.
- Number of pharmacies and shops providing OTC are also high
-3. Unwelcoming health facilities
- Complicated medical procedures, regular follow ups, laboratory tests etc., often discourage people in health institutions
- Long waiting time for check ups
- Lack of responsiveness of health workers in many places
-4. Reduced cost burden
- Direct purchasing of the OTC drugs from the pharmacist is much cheaper than consulting doctors/health practitioners.
- OTC drugs cut down the doctor fees and other opportunity cost
-5. Aging population
-6. Health consciousness is increased and also increased attitude of self-medication
-7. Covid-19 is a boom for the OTC drugs market. People take medications that work for cold, flu and fever from the pharmacies.
_
Factors that obstruct growth of OTC drugs:
-1. Drug abuse and the possibilities of drug addiction is the major challenge and is pulling down the growth of the OTC Drugs Market.
-2. Wrong medication due to incorrect self-diagnosis and misdiagnosis may lead to severe complications and is another limiting factor for developing the global OTC Drugs Market.
-3. Using traditional home remedies is limiting the market’s growth across the world due to the emergence of blogs and websites that share knowledge about the benefits associated with natural and cost-cutting remedies.
-4. Lack of awareness regarding the active drugs in underdeveloped and rural regions is also restraining the market’s growth.
_____
_____
Section-4
Behind-the-counter (BTC) drugs:
Where FDA classifications become confusing is when certain OTC drugs are subject to restrictions. Examples include human insulin, emergency contraceptives, and pseudoephedrine. Restricted OTC products are commonly referred to as behind-the-counter (BTC) drugs, a classification first approved by the U.S. Congress in 1984 when regulators decided to allow the sale of ibuprofen over the counter. While you don’t need a prescription to buy a BTC drug, you will need to request it from a pharmacist and, in some cases, register and report the sale to a state regulator. Such items may be unavailable in convenience or grocery stores that stock other non-restricted OTC medications.
BTC drugs do not require a prescription, but they are not available in just any location either. Instead, you can only buy them in locations where a pharmacist is present. The pharmacist is a highly educated professional whose college years were spent learning about the body’s functions, how to recognize minor medical conditions, when to refer you to a physician, and the uses of medications.
The reasons for the restrictions are varied. The restriction of pseudoephedrine, for example, was imposed to reduce it from being converted into the street drug crystal methamphetamine. The Plan B One-Step emergency contraceptive, by contrast, was restricted because consumers confused it for the abortion pill. Insulin injection can cause dangerous hypoglycemia, so it is moved to BTC.
Other restrictions are far less clear, in part because individual states can impose their own restrictions on how an FDA-approved OTC drug is sold. Oregon, for example, passed laws requiring a prescription for any amount of pseudoephedrine, while other states have imposed restrictions on the sale of syringes, codeine, and other OTC products.
Although the United States has limited experience with BTC drugs, other countries have introduced the BTC class, and their experiences can provide insight into the potential effects of this new class of drugs. BTC medications are widely adopted in many countries including the UK, Ireland, Canada, New Zealand, France, and Australia.
_
BTC medications are available only after consultation with a pharmacist. To distinguish from OTC medications, the characteristics of BTC medications are dispensing only with professional supervision. This ensures the safety, appropriateness, effectiveness of the dispensed medication, and increased control over medications with high clinical risk. However, implementation of BTC medication requires pharmacists and technicians to be qualified to perform initial assessments and screenings, medication reviews, patient counselling, and medication monitoring.
Some of the medication’s potential for transition to BTC category include those used for high blood pressure, high cholesterol, asthma, gastrointestinal reflux, allergies, and pain. To name a few as examples, aspirin/ hydrocodone and nabilone (antiemetic) are listed as schedule II drugs in Canada, the counterpart of a BTC category, and children’s cough and cold medications were recommended to be included into BTC by pharmacists for safety consideration. A similar class is required within the US. The current OTC medications with high abuse potential should be included under this medication class. Table below provides a list of medications that could be moved to BTC.
Medications potential for transition to behind-the-counter category:
|
Indication of medication |
Example |
|
High cholesterol |
Statins |
|
Blood sugar level maintaining |
Insulin |
|
Cold remedies |
Pseudoephedrine and ephedrine |
|
Schedule V cough syrups |
Certain codeine-containing products |
|
Emergency contraceptives |
Plan B |
|
Painkillers |
Painkillers with small amounts of codeine (up to 12.8 mg per tablet) and aspirin |
|
Sleep aid/allergy |
Diphenhydramine |
_
Despite the seemingly attractive prospects of BTC medication, including the potential to reduce medication abuse, the implementation of BTC medication category is controversial among primary stakeholders. Considering the safety and effectiveness of BTC medications, the Consumers Union and the National Consumers League argued for supportive evidence to make a national move to develop a third category, while the Consumers Healthcare Products Association (CHPA) opposed it. Physician organizations questioned the adequacy of pharmacists to provide clinical services, with a primary concern about the impact of BTC on physician–patient relationship. However, majority of pharmacy organizations provided a strong backing to the implementation of BTC medication.
_
In Japan, non-prescription drugs are classified under three categories: Class 1 Drugs, Class 2 Drugs, and Class 3 Drugs. Collectively they are known as OTC (over-the-counter) drugs. Patients are free to seek the advice of a pharmacist before purchasing OTC drugs.
Class 1 Drugs: As a rule of thumb, this medicine can only be dispensed by a pharmacist. At the time of purchase, the pharmacist must explain the particulars of the medication to the patient. They are quite strong compared to other non-prescription drugs and can potentially have side effects.
Class 2 Drugs: These drugs usually include cold medicine and medicine for headaches. They are sold by pharmacists and registered distributors who are required to explain the possible risk of side effects on the patient.
Class 3 Drugs: This category mainly includes vitamins, probiotics, and other drugs that are readily available at any drugstore. Though not officially required, pharmacists will be happy to answer your questions.
______
______
Section-5
OTC vs. prescription drugs:
_
Drug Classification in Europe:
In Europe, the OTC regulatory landscape is still fragmented despite the Mutual Recognition Procedure (MRP) and Directive 921261 EEC (18,19, 45-47). A closer look at Article 3 of Directive 921261 EEC revealed that a product is prescription if it meets the following requirements.
-1. It may present a danger if used without medical supervision.
-2. It could easily be misused or abused, thereby causing harm to the consumers.
-3. It causes side effects that require further medical investigations.
-4. It needs to be administered parenterally.
Article 4 of the Directive further stated that medicinal products not classified as prescription status should be non-prescription. European countries do not have a Monograph system to allow for rapid marketing of OTC products. All OTC products require registration and marketing authorization before marketing. Marketing authorization can be sought on a national level for marketing in just one country or using the Mutual Recognization Procedure for marketing in more than one country. The marketing authorization is renewable every 5 years.
__
In India, the Drug and Cosmetic Act (1940) and Rules (1945) describe the provisions for classification of drugs under schedule and the guidelines for their sale, storage, labelling and prescription.
Prescription drug (Rx only):
Prescription drugs or medicines are only available with a valid prescription by the registered medical practitioner. These drugs are intended to be used by the individual patient to treat a specific condition. Prescription drugs have to pass through various clinical trial phases (human and animal study) as well as their safety and side effects are monitored even after the drug is available in the market (i.e., post-marketing surveillance). A licensed pharmacist can only dispense prescription medicines through the pharmacy (either community/retail pharmacy or online pharmacy). Usually, prescription drugs are more potent than OTC drugs and are used for the management and treatment of both acute and chronic disease conditions.
Prescription medications are the scheduled drug:
-1. Schedule H drug
It contains a list of medicaments that can be sold only by the prescription of a registered medical practitioner. Only the specified amount mentioned on the prescription should be sold, and the date and time of dispensing must be noted. The schedule H drug label must contain the symbol “Rx” and schedule H drug warning: “To be sold only by the prescription of a registered medical practitioner (doctor)”.
Schedule H1 drug:
It includes 3rd and 4th generation antibiotics, certain habit-forming drugs (psychotropic medication) and anti-tubercular drugs. To dispense these drugs given below criteria should be followed:
- A separate register should be maintained containing the name and address of the prescriber (doctor), name of the patient along with the name of the drug, time of supply and the supplied quantity.
- The schedule H1 drug level must contain the symbol “Rx in red” and schedule H1 drug warning: “Not to be sold without the prescription of a registered medical practitioner”.
-2. Schedule X drug
It is a class of prescription drug and contains Narcotic and psychotropic substances. The schedule X drug label must contain the symbol “NRx” and Schedule H drug warning: “To be sold only by the prescription of a registered medical practitioner (doctor)”. These drugs are kept under lock and key, and it is mandatory that the retailer must keep a copy of the prescription for two years.
Over-the-counter drugs are the non-prescription medications (do not require any prescription from a registered medical practitioner). In India, whatever is not a scheduled drug can be marketed as OTC drug. The medicines which do not fall under the category of schedule H, H1, and X can be given without prescription through pharmacists and drugstores in India. Moreover, it is a common observation that prescription drugs are also sold without a prescription akin to over-the-counter medicines. Ayurvedic drugs and traditional medicines are manufactured under a manufacturing license issued by the State Licensing Authorities. These drugs are sold over the counter freely by non-pharmacists. Thus, till date, there are no specific unifying regulations related to use and sale of OTC products and this impact both the accessibility to better health care and patients’ safety due to inappropriate use.
__
Classification of medicines in U.K.
The legal classification of a pack of medicine determines the level of control over its supply. In part, classification rests on how much health professional input is needed to diagnose and treat the conditions for which the medicine might be used. Currently, there are three categories that a medicine can be classified within:
-1. Prescription-only medicines
Medicine packs classified ‘prescription only’ can be obtained only against a valid prescription issued by an authorised health professional. The prescription needs to be taken to a pharmacy where the medicine is prepared under the supervision of a pharmacist. Sometimes the prescription is filled at a dispensing doctor’s surgery. A member of the public cannot buy a prescription-only medicine (POM). A rectangular box enclosing the letters POM appears on the packs of prescription-only medicines. In general, prescription-only medicines are used for conditions that are best diagnosed and managed by health professionals. Examples of prescription-only medicines include virtually all antibiotics and medicines for treating high blood pressure.
Controlled Drugs:
Controlled Drugs are the most serious category of drugs. Whilst having effects similar to Prescription Only Medicines, these drugs have been specifically listed as Controlled Drugs under the Misuse of Drugs Act 1971. These medicines also require a prescription from a medical professional such as a doctor or dentist. They have certain restrictions as to how they’re dispensed, stored, and administered. Controlled Drugs are all marked with “POM” and “CD” on the packaging.
-2. Pharmacy medicines
People can buy products classified as ‘pharmacy medicines’ (P) but only from a pharmacy and in the presence of a pharmacist. These medicines, also called ‘pharmacy-only medicines’, are not usually displayed on open shelves. A rectangular box enclosing the letter P appears on the packaging of pharmacy medicines.
Compared to the packs available in retail outlets, medicines like ibuprofen and paracetamol can be bought in larger packs under a pharmacist’s supervision. But there may still be a limit on how much pharmacy medicine a person can buy. This helps prevent inappropriate and possibly harmful long-term use and reduces delay in diagnosing a condition that requires different treatment. Other examples of pharmacy medicines include tablets for emergency contraception and medicines containing codeine for treating pain that is not relieved by aspirin, ibuprofen or paracetamol alone.
-3. General sale medicines
People can buy general sale medicine packs from retail outlets such as corner shops and supermarkets. The medicines—also called ‘general sales list (GSL) medicines’—are also available for self-selection in pharmacies. General sale medicines are taken for common, easily recognised ailments which usually last around 2–3 days. These medicines cause few troublesome side effects in normal use. Examples of general sale medicines include small packs of painkillers and of antihistamines for hay fever and other allergies.
Over-the-counter medicines:
‘Over-the-counter (OTC) medicines’ covers all general sale medicines and pharmacy medicines. The description conveniently distinguishes medicines that can be bought from those that must be prescribed. The term ‘over the counter medicines’ is informal and is not used in the UK medicines regulations.
_____
_____
Key differences between Prescription and OTC Drugs:
The biggest difference, of course, between prescription and over-the-counter drugs is that prescription medications require a doctor or other medical professional’s authorization to obtain. Here are some of the other key differences between prescription and OTC drugs:
-1. Prescription medications are specially tailored for use by a specific person for a specific use. OTC medications are considered safe for just about everyone and may have a variety of intended purposes. When doctors write prescriptions, they take into consideration a lot of information about their patients, including their current condition, other medications they may be taken, their vital statistics, and drug allergies they may have. That’s why a prescription medication that is safe and effective for one person may be dangerous for another.
-2. OTC drugs should only be used to treat minor ailments. Major illnesses and diseases require the use of more powerful prescription drugs and other medical treatments.
-3. OTC drugs aren’t as strong as prescription drugs, but they have a wider margin of safety. This means a wider range of people can safely use OTC drugs than can use the more specifically tailored prescription drugs.
-4. OTC drugs typically have lower dosages than prescription drugs. There are quite a few prescription drugs that are available as OTC drugs because, when sold over-the-counter, the dosage is much lower than it is in the medication’s prescription form. Prilosec and hydrocortisone ointment are examples of drugs sold in both prescription and OTC form.
-5. In general, OTC drugs are less expensive than prescription drugs. There are some generic prescription medications that are cheaper than OTC drugs, but, in most cases, a prescription medication will be far more expensive than an OTC drug. In the case of drugs used to treat cancer and other serious diseases, the cost of prescription drugs can be very expensive.
-6. OTCs can be shared by friends and family; prescription drugs are given to a specific patient and cannot be legally shared with anyone.
-7. By law, OTC medicine labeling must include all the information that an ordinary consumer needs for the safe and effective use of the product. Drug Facts is the name given to the type of label format on the majority of OTCs.
-8. FDA oversees Rx medicine advertising in the U.S. In contrast, the U.S. Federal Trade Commission (FTC) has authority over OTC medicine advertising, just as it does for other consumer products.
-9. The majority of OTC medicines can be sold in any supermarkets, mass merchandisers, pharmacies, etc.—unlike Rx drugs which are limited to pharmacies.
__
Here are the main differences between prescription and OTC medicines.
|
Prescription medicine |
OTC medicine |
|
A doctor’s prescription is required; other licensed healthcare providers, such as nurse practitioners or physician assistants, can also write a prescription for medicine (under the authority of a doctor) |
Does NOT require a prescription to purchase |
|
Can only be dispensed from a pharmacy (community, online, or mailorder) by a licensed pharmacist |
Available for purchase on store shelves in a pharmacy and in stores such as supermarkets or small convenience stores |
|
Prescribed for and intended for use by one person only |
OTC medicines can be used by more than one person; however, because of the risk of contamination, some OTC medicines are NOT recommended for sharing (e.g., eye drops, ointments) |
|
Requires a medical diagnosis and decision by a licensed healthcare professional as to which medicine is used |
Relies on self-diagnosis; product is chosen based on self-care decision |
|
Usually more powerful than OTC medications |
OTC medicines have a wider margin of safety than prescription medicines |
|
Can be used to treat both minor ailments and more serious diseases and illness |
Used to treat minor ailments |
|
Can be harmful if misused |
Also can be harmful if misused |
_____
_____
Marketing OTC drugs to doctors:
Most doctors hesitate to recommend OTC products. When asked what circumstances call for OTC prescriptions, they only cited emergency situations where no other medical aid is available. This sentiment could stem from general skepticism around – poor regulation of OTCs, patient’s ability to self-medicate, pharmacists’ capability to advise consumers on the right OTC medicines, and high risk of drug abuse among patients. (In one survey, 44% doctors said >70% of patients are likely to abuse drugs).
For a long time, Pharma companies looking to penetrate the OTC space focused their energies only on direct-to-consumer marketing, and health care practitioner (HCP) engagement wasn’t given much consideration. But doctors do influence OTC sales and ignoring them is a fallacy. Today, marketers are more aware of HCPs’ contribution to the success of their OTC brands.
Why were doctors ignored by OTC marketers?
-1. High cost of reaching doctors – A huge chunk (65-70%) of the marketing budget for OTC products is allotted towards consumers with only 10-15% left for field force activities. Dwindling facetime with doctors means that only high-margin Rx products get pitched.
-2. Doctors prefer Rx drugs – Trust deficit is a hurdle for OTC drugs gaining doctors’ advocacy. About 80% of doctors claimed that they never recommend OTC drugs to their patients.
Why OTC marketing to doctors makes sense?
-1. Doctors’ word matters – Patients put doctors on a pedestal, and products endorsed by them enjoy the highest trust. An IPSOS study found that OTC drugs recommended by doctors were purchased more by patients than non-recommended ones. Prescribers thus are gatekeepers of both Rx and non-Rx purchases.
-2. Boosts brand loyalty – Marketing to doctors can pique awareness about specific brands and lead to patient trials. Patients are more likely to choose the brand suggested by their doctor and continue choosing it for future use too.
-3. Improves competitiveness – Doctors now have more than one brand to recommend for a particular condition which means marketers have to step up their medico-marketing efforts. Also, competition is stiff where both OTC and Rx drugs are viable treatment options. Most doctors perceive Rx medicines as more trustworthy than OTC ones. Marketers should, therefore, build a case for their non-Rx products through regular and detailed communication with HCPs. Doctors should have complete information on clinical trials (if any), recommended dosage, micronutrients present in the drug, its contraindications, and so on.
-4. Product positioning – OTC drugs may be recommended by doctors as a preventive, remedial or supplementary therapy. It is necessary that treating physicians are made aware of where the product fits in the overall treatment plan.
-5. Consumer insights – Doctors can offer pharma critical insights into patient behavior that can be used to formulate products that actually solve consumer pain points. It will also help marketers align their product communications to consumers’ needs. Key insights from doctors could include – most common symptoms cited by patients in clinics, top reasons for not sticking to the treatment regimen, the stage at which patients seek doctors’ assistance, and so on.
_
Non-prescription drug prescriptions:
Prescriptions are also used for things that are not strictly regulated as a prescription drug. Prescribers will often give non-prescription drugs out as prescriptions because drug benefit plans may reimburse the patient only if the over-the-counter medication is taken under the direction of a medical practitioner. Conversely, if a medication is available over-the-counter, prescribers may ask patients if they want it as a prescription or purchase it themselves. Pharmacists may or may not be able to price the medication competitively with over-the-counter equivalents. If the patient wants the medication not under prescription, the prescriber is usually careful to give the medication name to the patient on a blank piece of paper to avoid any confusion with a prescription. This is applied to non-medications as well. For example, crutches and registered massage therapy may be reimbursed under some health plans, but only if given out by a prescriber as a prescription. Some software now requires a prescription.
Prescribers will often use blank prescriptions as general letterhead. Legislation may define certain equipment as “prescription devices”. Such prescription devices can only be used under the supervision of authorized personnel and such authorization is typically documented using a prescription. Examples of prescription devices include dental cement (for affixing braces to tooth surfaces), various prostheses, gut sutures, sickle cell tests, cervical cap and ultrasound monitor.
In some jurisdictions, hypodermic syringes are in a special class of their own, regulated as illicit drug use accessories separate from regular medical legislation. Such legislation will often specify a prescription as the means by which one may legally possess syringes.
_
Exceptions requiring Rx for OTC conditions:
You may still be prescribed a medicine for a condition on the list if:
-1. you need treatment for a long-term condition, for example regular pain relief for chronic arthritis or inflammatory bowel disease
-2. you need treatment for more complex forms of minor illnesses, for example migraines that are very bad and where OTC medicines do not work
-3. you need an OTC medicine to treat a side effect of a prescription medicine or symptom of another illness, such as constipation when taking certain painkillers
-4. the medicine has a license that doesn’t allow the product to be sold to certain groups of patients. This could include babies, children or women who are pregnant or breastfeeding
-5. the person prescribing thinks that a patient cannot treat themselves, for example because of mental health problems
_____
_____
______
______
Pharmacist and OTC drugs:
_
Pharmacy versus Non-Pharmacy Sales:
A pharmacy is not the only choice for consumers when buying OTC medicines. Food stores, supermarkets, mass merchandisers, department stores, and convenience stores are also options. Analyzing OTC sale patterns from pharmacies and nonpharmacy outlets is important for understanding market trends and consumer purchase behaviors. However, there are only a few reports that provide information for both types of outlets in the U.S. and Canada. Several American surveys (from 1992 to 1998) asked participants to indicate where they usually purchase their OTC products. Results show that higher (but varied) percentages of participants (from 46 to 72 percent, depending on different store options) purchase OTC medicines from drug stores rather than the other retail outlets. In 1998, another American market report presented the proportions of OTC sales (for each drug category) accrued in drug stores, food stores, and mass merchandisers during the previous year. For most categories of OTC products, pharmacies held the major part (at least 40 percent) of the market. The situation is similar in Canada, but with even a higher proportion of OTC sales from drug stores. According to ACNielsen in 1997, drug stores shared 79 percent of the consumer drug category in Canada. Based on sales data (2003) from ACNielsen’s Market Track Service, drug stores have a much greater share of sales for most OTC categories than any other channel (grocery stores, mass merchandisers, and convenience stores). By way of one example, 55 percent of all stomach remedies were sold from drug stores, compared with 25 percent sold in grocery stores, and 20 percent sold from mass merchandisers.
Although clear-cut evidence that pharmacies outpace non-pharmacy outlets in OTC sales is not available, mainly because many grocery stores and mass merchandisers now have their own pharmacy departments, a pharmacy still appears to be the chosen location for consumers to purchase OTC medicines. It is worthwhile to note, however, that even though the majority of OTC medicines may be sold from pharmacies, such outlets are facing a challenge by other retailers. Because of the global trend of encouraging self-medication, more and more medicines are being switched to nonprescription (U.S.) or unscheduled (Canada) status. Accordingly, it is expected that OTC sales in non-pharmacy outlets will rise.
_
Role of pharmacist in OTC medications:
Self-care and self-medication practices are essential components of any health care systems. The use of over-the-counter (OTC) medications is a part of the self-medication process. The popularity of OTC medication use among patients may increase the abuse potential of OTC medications. With pharmacists being as accessible as they are, they are often the first line of contact for patients, and have the opportunity to educate and counsel patients on appropriate OTC medication use. The presence of a pharmacist ensures safe and effective use of OTC medications. Pharmacists can liaise with other health care providers in the management of self-care practices by patients. However, a pharmacist has traditionally been underutilized in this role.
Patients have easy and free access toward seeking advice from a pharmacist. Many issues faced by a patient can be easily solved by pharmacists, including product selection, OTC brand name confusion, appropriate product use, and when to take medications. Thus, pharmacists exercise a strong influence on OTC medication purchase and product selection.
Many patients find product selection confusing due to marketing strategies by manufacturers. A common marketing technique by pharmaceutical manufacturers is line extension. A large percent of revenue is spent on OTC medication advertisements and line extensions. Once a manufacturer has an established brand name, other products are sold under the extension of the same brand. For example, the primary brand Tylenol® has many line extensions including Tylenol PM®, and Tylenol Cold and Cough®. This often leads to confusion among the minds of patients. Many times, these line extensions have multiple ingredients causing more confusion. A patient–pharmacist interaction would help patients in their decision-making process during these instances.
OTC advertisements are often the driving factor in OTC medication selection by the patients. If the advertisements are misleading, a patient may be misinformed. The advertisements focus upon the beneficial effects of the medication with bare information on the contraindications and safety concerns. With this regard, a pharmacist can also provide insight into all aspects of the drug, as well as information on the safe use of OTC medications.
_
Over-the-counter counseling:
Pharmacists sometimes perform OTC counseling in the aisles of their pharmacies. Over-the-counter counseling (or OTC counseling) refers to the counseling that a pharmacist may provide on the subject of initiating, modifying, or stopping an over-the-counter (OTC) drug product. OTC counseling requires an assessment of the patient’s self-care concerns and drug-related needs. The types of drugs that are involved in OTC counseling are, for example, used to treat self-diagnosable conditions like heartburn, cough, and rashes, though prescription drugs and professional diagnoses are also relevant to the recommendation process.
The aim of OTC counseling is to empower patients to take control of their healthcare-related needs for conditions that do not require an appointment with a medical doctor. This benefits the healthcare system by reducing unnecessary physician visits. The pharmacist can also use OTC counseling to ensure the highest likelihood of success for the patient’s self-care attempt and minimize the risk of any drug-related problems.
Although OTC drugs are generally regarded as safe for use without a prescription (by definition), medication errors still occur. For example, patients sometimes misuse OTC products by taking larger than recommended doses, in order to bring about symptomatic relief more quickly, or even intentionally abuse them for unlabeled indications. Even when a patient is instructed not to use OTC products without speaking with their primary care physician, patients can still fail to identify products as OTC medications worth avoiding.
Comparison to prescription drug counseling:
OTC counseling patients about self-care and non-prescription drugs does not follow the same format as counseling for prescription drugs. A pharmacist who counsels for a prescription drug can view a patient’s profile, which includes their current list of concurrent medications and allergies to medications. However, an OTC counseling session may occur in the aisle of the store, forcing pharmacists to elicit the necessary information from patients directly.
_
Pharmacist Influence as OTC Consultants to Consumers:
Pharmacists have a professional obligation to provide OTC counselling to the public. Such activity should be evaluated by the profession within the process of quality assurance. Similarly, how consumers perceive pharmacists in this role is also an important issue. Information on consumer satisfaction with pharmacists and pharmacy services in this area is available.
An American study provides indirect information about how consumers think of pharmacists in this role. Gore and Madhavan surveyed 3,000 Americans on the credibility of four information sources (physicians, pharmacists, family members, and friends/colleagues) for OTC medicines. Only 458 subjects replied to the questionnaire; response was therefore low at 15.2 percent. The results found that acceptance of both pharmacist and physician recommendations was high (75 percent and 76 percent, respectively). Comparatively, slightly over half of respondents usually or sometimes accepted recommendations from their family members or friends/colleagues. Participants were also asked to rate these four sources on three dimensions of credibility – expertise, trustworthiness, and empathy. Pharmacists were rated lower than physicians on all three dimensions. However, consumers believed that pharmacists were more expert and trustworthy than were family members or friends/colleagues. On the dimension of empathy, pharmacists were perceived to embrace the least of this attribute of the four sources. Therefore, while a reliable source of information, pharmacists may have to improve upon a humanistic aspect of their interaction with clients.
Several surveys show that pharmacist recommendations have a high acceptance rate by clients. In a 1995 American survey, pharmacists reported that clients bought recommended OTCs more than 80 percent of the time. In 1998, results from a telephone survey of 1,008 American adults found that 73 percent would take a pharmacist’s advice for an OTC product, even if the product differed from the one they had been using for years. As well, if the product recommended was not highly advertised, 70 percent of respondents still would accept the advice. If their pharmacist and friends/families had differing recommendations for an OTC product, 67 percent would choose the pharmacist’s choice. Further, 59 percent would buy the product recommended by a pharmacist, even if it was more expensive than the one they usually bought. Results from another American consumer survey showed that most consumers (98 percent) feel extremely or somewhat satisfied with OTC information given by pharmacists, and at times, more satisfied than when receiving such information from physicians. In a study conducted in Britain in 2000, Bell et al determined that 19.6 percent of their respondents were influenced by recommendations of a pharmacist when they purchased OTCs, compared with 14.5 percent by a doctor, and 10.2 percent by an advertisement.
_
Special Population Considerations:
Although nonprescription medications are effective and safe when used appropriately, pharmacists must pay extra attention to special groups, such as elderly, pediatric, and pregnant patients, as well as those with coexisting comorbidities. These patients require additional education to ensure that the nonprescription medications are suitable for them. For example, patients older than 65 are most likely to have health issues that may contraindicate the use of certain medications, yet older adults account for about 40% of nonprescription medications consumed. These older adults face the greatest risk of adverse effects and drug interactions. Drug-related adverse events faced by older patients include confusion, depression, falls, hallucinations, and malnutrition. People who routinely take one or more prescription drugs or who have underlying health problems, such as those with asthma; chronic obstructive pulmonary disease; clotting disorders; diabetes; an enlarged prostate; epilepsy; glaucoma; gout; high blood pressure; immune system, kidney, or liver problems; Parkinson disease; psychiatric issues; or thyroid problems, should receive counseling from a pharmacist prior to taking a nonprescription medication. Pediatric patients are another population at increased risk for unintentional misuse. Pediatric dosing errors may be due to the use of inappropriate measuring devices, the wrong dosage form, inappropriate medication techniques, or age-based dosing. This is another area of opportunity for pharmacists to provide more education about the appropriate and safe use of nonprescription medications.
_
Role of pharmacists in BTC medications:
The presence of a pharmacist would be essential to purchase a BTC medication. These medications should be provided only upon a pharmacist’s recommendation. Upon performing the necessary tests, initial screenings and appropriate counseling a BTC medication can be dispensed. Some of the potential candidates could be patients with high blood pressure, gastrointestinal reflux, asthma, severe allergies, and pain. All current OTC medications with high abuse potential can be included as BTC medications. If the pharmacists work collaboratively with their patients, it will lead to informed decision making and safer use of medications. Once a pharmacist disapproves a particular medication, they should refuse to dispense it. BTC medication is a bridge between OTC and prescription medications, with a potential to increase access of health care while efficiently using the knowledge and expertise of a pharmacist.
_
Pharmacist’s role in OTC medication abuse:
Several methods employed by pharmacists to reduce OTC medication abuse have been suggested in the past. The top three methods used by pharmacists to control OTC medication abuse were keeping the implicated products out of sight, questioning on the purchase of these products by pharmacists, and refusal to sell the implicated product. It was demonstrated that 62% of pharmacists reported certain measures being taken to curb OTC medication abuse, for instance, not displaying medicines, refusing sales, and associated policies including pharmacist’s conducting an interview of the patient. The interview may include asking additional questions to patients at the time of purchase and providing advice to patients as necessary to reduce abuse of potential products. Some other techniques used and reported by pharmacists to reduce OTC medication abuse were referral to a physician, referral to a drug and alcohol abuse team, and/or involvement of pharmacists in harm reduction programs. Table below lists the strategies that can be adopted in different locations and scenarios to curb OTC abuse.
Common strategies used by pharmacists to control over-the-counter medication abuse:
|
Target locations |
Pharmacist-initiated strategies |
|
In a pharmacy |
Refusing sales |
|
Contacting other pharmacies to warn them of the suspicions of a customer who may be abusing a product |
|
|
Claiming products were not in stock |
|
|
Prevent supplies by hiding medicines |
|
|
Supplying only limited amounts |
|
|
Patient involvement |
Counselling customers about the abuse potential of products |
|
Raising awareness of Internet-based support groups among patients by advising them |
|
|
Providing information leaflets |
|
|
Physician involvement and other services |
Working on general practitioner engagement/consultation |
|
Providing referral to doctors |
|
|
Using private clinic services |
|
|
Using specialist drug services and drug and alcohol treatment services |
_
Barriers for pharmacists to prevent OTC medication abuse:
There are multiple challenges faced by pharmacists and their pharmacies in monitoring OTC abuse. Due to lack of consistent data with OTC medications, identification of drug-related problems may become difficult. Pharmacists usually never keep any record or monitor patient medication profiles for OTC medication use, which creates a vacuum in the information necessary to make appropriate counseling decisions.
An individual seeking to abuse an OTC medication could probably obtain it from the same pharmacy at different times or visit different pharmacies for the same medication. However, considering this potential to abuse medications, specifically pseudoephedrine, US federal government passed the Combat Methamphetamine Epidemic Act of 2005 (CMEA). This act was passed to monitor the amount of pseudoephedrine which an individual can purchase in a pharmacy in the US. The aim of this act was to curb illegal consumption of methamphetamine which can be bulk produced using drugs like ephedrine and pseudoephedrine that are commonly found in OTC cough and cold medications. The CMEA has placed a purchase limit of no more than 9 g of pseudoephedrine in a 30-day period. Although this act has successfully helped reduce the issue of OTC medication abuse of pseudoephedrine-containing products, adaptation of this process for other medications proactively by pharmacists has been non-existent in the US.
In addition, the lack of pharmacist’s proactive initiatives to monitor patient’s OTC medication use has led to many abuse opportunities. Pharmacists are usually overworked and the continuous high stress prescription processing workload also reduces the potential opportunities to be pharmacovigilant. Further, the legal requirements associated with medication distribution have not kept up with the abuse potential nor are the laws for the practice of pharmacy revised or kept up with patient or pharmacist needs. For example, a study conducted in a community pharmacy indicated that pharmacists were overworked. The lack of workforce in a pharmacy led to reduced attention and problem identification among the patient’s OTC decisions.
______
______
Section-6
Rx to OTC switch: Reclassifying prescription-only drugs as OTC drugs:
The legal classification of a medicine may sometimes change—it is called reclassification. It is also called ‘switching’. Growing confidence of the medicine’s role and improved understanding of its side effects can lead to a change in classification. A proposal to change a medicine’s classification needs to be supported by good evidence that focuses on the risk to the public on changing the classification. The evidence may comprise clinical studies, extensive clinical use indicating acceptable level of side effects, advice of experts, views of relevant health professionals and their professional bodies, as well as the views of relevant public associations and individuals with an interest in the medicine under consideration. The evidence needs to demonstrate that the risk to the public will be adequately managed.
_
Initially drugs are available for consumption to the public only after consulting with a healthcare professional and obtaining a prescription for the same. However, to enhance consumer access to a safe and effective drug, it is possible to ‘switch’ the same to over-the-counter status after the initial prescription marketing if the post-marketing safety data of the prescription version of the medication reiterates the safety, effectiveness and ease of use of the drug. This is known as a ‘Prescription-to-OTC Switch’. Prescription to OTC Switches are supported by a large number of driving factors such as increasing consumer awareness, growth of the self-medication movement, pharmaceutical companies’ attempts to increase sales and government efforts to curtail public spending on prescription products for minor, self-treatable ailments. It is estimated that nearly 40% of all OTC medication available today in the US was once upon a time marketed as a prescription drug.
_
Safety is a major concern when the Food and Drug Administration (FDA) considers reclassifying a prescription drug as OTC (over-the-counter). Most OTC drugs—unlike health foods, dietary supplements (including medicinal herbs) and complementary therapies—have been studied scientifically and extensively. However, all drugs have benefits and risks, and some degree of risk has to be tolerated if people are to receive a drug’s benefits. Defining an acceptable degree of risk is a judgment call.
The following questions can help determine whether a drug is safe enough to be made available over the counter:
- Has the drug been used for a long enough time so that any harmful effects are fully understood?
- What harmful effects (including those from misuse) may the drug cause?
- Is the drug habit forming?
- Do the benefits of over-the-counter status outweigh the risks?
Other questions help determine the ease with which a disease can be diagnosed and then treated outside of a health care setting:
- Can the average person self-diagnose the condition that calls for the drug?
- Can the average person treat the condition without the help of a doctor or other health care practitioner?
Finally, people need to understand how to use the drug, so labeling on the outside and inside of the package are important considerations:
- Can adequate directions for use be written?
- Can warnings against unsafe use be written?
- Can the average person understand the information on the label?
About 106 ingredients, indications, or dosage strengths have made the switch from Rx to OTC status or have been newly approved since 1976. That translates to more than 700 OTC products on the market today.
_
In order to switch a prescription version of a drug to OTC, the drug must possess some inherent traits that cause it to be amenable to self-medication. In general, these are as follows:
-1. The symptom intended to be treated by the drug must be one that can be easily recognizable by an individual of average intelligence
-2. The drug must possess a very high safety margin
-3. The drug must be used for easily recognizable conditions
-4. The drug must be easy to administer
-5. The drug must exert its effect rapidly post-administration and the effect must be easily noticeable
-6. The drug must not be addictive or narcotic in nature
-7. The use of the drug must not mask any underlying potentially dangerous conditions
-8. The treatment regimen must be uncomplicated enough for a layman
-9. The drug must not be parenterally administered
During the clinical trial phase of a new investigational product, the number of patients on whom the product is tested is far lower compared to the actual population that will be exposed post initiation of marketing. Thus after the initial registration of a new product, although available clinical data may reiterate the safety and efficacy of the product, it is always prudent to closely monitor the usage of the drug in the actual patient population. This is the reason regulatory authorities usually prefer to err on the side of caution by making a new product available by prescription.
In fact, pharmaceutical companies themselves prefer to submit their initial product application under the category of prescription only. The most common reason for this is that these new drugs are always protected by patents and thus the company can recover their initial R&D costs by utilizing a high pricing structure without the fear of generic competition eroding their sales. It is not practical for companies to similarly price new OTC products for reasons further explained below.
Post-termination of the patent regime, innovator companies commonly face a steep decline of sales as a result of the influx of multiple low priced generic products. However, if the product exhibits certain conducive characteristics that make them amendable to OTC use, these innovator companies have yet another opportunity to extend their product life- cycle in the form of a prescription-to-OTC switch. Products with a strong and well recognized brand name can leverage on their identity to continue to ensnare large sections of the consumer market post making their product available as OTC. Although the resultant sales may never be as high as they were during the patent protected period, they still are significantly more resistant to generic competition.
Although the primary benefit of an OTC switch lies with the pharmaceutical company, in several countries, private health insurance companies also are allowed to drive the switch. In countries like the US, private insurers reimburse patients for their prescriptions. Thus a switch of a popular, safe and effective prescription product to OTC means that the insurer is no longer obligated to bear the cost of the medication. This is the reason several insurers have lobbied for certain Rx to OTC switches.
Lastly, in certain instances, regulatory authorities themselves may request sponsors to submit OTC applications if they believe that the availability of a particular product without prescription control may benefit the general public. This approach raises a few questions as the authority never conducts clinical studies on its own and is completely dependent on the sponsor to supply adequate clinical data to demonstrate safety and efficacy within the realms of the OTC use of the product.
Advantages of Switching:
The path to obtain prescription medication is often long, complicated and costly. The patient needs to take time off work to make an appointment, visit a doctor, obtain a prescription and fill it at a pharmacy. The availability of OTC medication enables a patient to bypass nearly all of these arduous steps. Thus, OTC medicines provide a much more convenient means for patients to self-treat their minor afflictions. In addition to patients, physicians can also benefit in not having to unnecessarily spend their already limited time in diagnosing obvious, self-treatable conditions.
OTC products in general being ineligible for reimbursement schemes are usually paid for by consumers. Thus pharmaceutical companies usually place OTC products at far lower price points than their prescription counterparts in order to ensure larger volumes of sales. Due to the reduced pricing, profits are obviously lower, but this is a small price to pay to continue to capture required market volumes post expiration of the drug patent. Ultimately, the consumer wins due to the reduced prices of the switched product.
Another significant impetus to switching may present itself in the form of exclusivity for the sponsor who submits clinical data that is deemed sufficient to justify the down-regulation of the product. The period of exclusivity varies from 3 years in the US, under the aegis of the HatchWaxman Act, to 1 year in the EU, under the aegis of Article 74a of the Directive 2004/27/EC. This further helps the sponsor shield himself from generic erosion in this limited time period.
_
Consumer’s point of view:
-There is usually a significant monetary advantage to the consumer since OTCs are considerably less expensive than prescription drugs.
-Less lost work time and costs saved by not needing to visit a doctor are important considerations.
-Growing sophistication and self-reliance among consumers, with increasing interest in and knowledge about appropriate self-medication.
-Older adults in particular tend to experience increased minor medical problems, such as arthritis, sleeping difficulties, muscle aches and pains, headaches and colds, so that as the population ages, the demand for non-prescription drugs is escalating.
Manufacturer’s point of view:
-Increased sales of non-prescription medicines tend to enhance corporate revenue and profitability.
-Older prescription drugs are losing patent protection; at least some of the lost revenue can be recouped by offering OTC versions of the products through life-cycle product management.
Insurer’s point of view:
Companies offering health-care insurance encourage use of OTC self-medication by patients as a cost-containment strategy to reduce the more costly compensation of higher priced prescription drugs.
_
Many available nonprescription products were previously available only with a prescription. Since 1976, there have been 106 active ingredients that switched to nonprescription from prescription status.
Thanks to these switches, families can conveniently buy and use a wider range of treatments without having to go to the doctor. Access and affordability are the 2 most common attributes touted by consumers. Seven of the past 14 switches are in the allergy category. Data from the Consumer Healthcare Products Association show that the number of allergy sufferers who use OTCs rose to 75% in 2015, from 66% in 2009. Other statistics show that anytime a switch is made in a new product category, use within that category increases. Consider the following:
-1. There was a 150% to 200% increase in the purchase and use of nicotine replacement therapies in the first year after the switch. Increased access enabled tens of thousands of smokers to use these products to help quit smoking and live longer, healthier lives. That is a $2 billion social benefit every year. The nicotine replacement therapy switch led to 650,000 extra quit attempts.
-2. Consumers saved an average of $174 per year in avoided prescription costs and office visits for heartburn medicines. This additional access also drove $750 million in savings for the health care system.
-3. After vaginal yeast treatments were made available over the counter, results from studies showed that women were as accurate as their doctors in recognizing the recurrence of such infections.
_
The Rx to OTC switch is one of the best ways to provide cost-effective first-line therapies for many ailments to patients, especially for those with no insurance. Also, for the ones who have insurance but do not have time to go to the doctor, it can assure quick access to medicines, at least as a first line of defense. Then, why don’t we see more drugs being switched from Rx-to-OTC? The reason may be while the benefits to consumers of Rx-to-OTC switches could be significant, they also involve some degree of risk. With a product being available in pharmacies, usage expands, and physician supervision diminishes. This can have many consequences which the sponsors and FDA need to be cognizant of. While approving a Rx-to-OTC switch, the FDA needs to be assured that it is a condition for which consumers can self-diagnose, self-treat, and self-manage, and no health practitioner is needed for the safe and effective use. Additionally, FDA needs assurance that the drug has low potential for misuse and abuse; in other words, the benefits of OTC availability outweigh the risks. These are not easy hurdles to overcome by the sponsor or the FDA. Thus, in some cases, new safety studies could be required to establish the “OTC-ness” of the product. Also, based on the safety profile and inherent risk, some medicines may always remain a risk, if available as OTC; certain antibiotics, steroids come to mind. However, at the end of the day, an Rx-to-OTC switch can be beneficial to the patients and the sponsors. It would be good to see more of these happening.
______
______
Drug requirements for switch:
When drug manufacturers are ready to take their medications from prescription to OTC, there are three big factors they must be taken into consideration: benefit-risk comparison, consumer-friendly labeling, and how to make the drug a good choice as an alternative to prescription medication.
The benefit-risk comparison focuses primarily on whether patients are capable of reaching the intended medical result in a safe manner. After the Food, Drug, and Cosmetic Act, safety of the medications for consumers had to be proven. Medications always have some risks or possible side effects, but the federal drug administration makes a decision to switch to OTC based on how likely those risks can occur when a patient self-medicates. If the drug is highly toxic or addictive, the medication status will remain as “prescription-only.” However, if the drug has labels that consumers can understand and adhere to and if patients are capable of properly diagnosing their condition, then the medication may make the switch to OTC.
Another requirement for switching to an OTC medication is the appropriate verbiage and use of labeling. For a medication to be used for self-treatment by patients, the language must be clear to decrease instances of unsafe dosages and administration. Anticipating and minimizing problems by making the labels consumer-friendly is a responsible action that needs to be taken by a manufacturer before switching to OTC. Labeling must include the active ingredient, uses, warnings, inactive ingredients, indication, directions, and other information (such as how the product should be stored).
Conditions that are commonly treated OTC often include acute diseases such as headaches and common cold that can be easily identified by a patient without medical training. A recent example of such a medication is Flonase (Fluticasone Propionate Nasal Spray) that transitioned from prescription only to OTC and is available to consumers without a prescription starting 2015. The availability of Flonase as an OTC product was made after its approval by the US Food and Drug Administration (FDA) as a medication that consumers can use with safety guidelines after self-diagnosing.
If a company plans to switch their medication from prescription to non, they must follow the guidelines set out by the FDA. The drug indications for the new OTC release must be comparable to the indication for the prescription drug while still allowing the patient to accurately and easily self-diagnose. The OTC drug should also be relatively safe in regards to toxicity, drug-drug interactions (DDIs), and side effects as well as a minimized potential for abuse. Many clinical trials are performed to determine whether a drug can meet these criteria. Beyond this, labeling must be a clear indicator for patients in how to properly administer the drug in an appropriate dose.
Figure below shows prescription to over-the-counter switch process:
_
Impact of the switch on stakeholders:
Consumers
There are multiple advantages to consumers when products are switched from prescription to OTC. One of the most common advantages is the ease of access to essential medications. Instead of scheduling a physician appointment to obtain a medication prescription to treat symptoms, a patient can self-treat by identifying their symptoms and proceeding to a pharmacy and thereby procuring the proper medication. Most women feel they would benefit if they could receive their birth control pills from a pharmacist instead of requiring a visit to a physician, with 76% of women who took part in a survey said. By receiving birth control pills from a pharmacy without a prescription at the pharmacy instead of through a clinic, more women continued their medication regimen according to another study.
Time
Time is also saved when the patient does not have to wait for their prescription to be processed. In totality, OTC medication save the patient time and speed up the process of getting relief compared to patients who have to go through extra two steps of getting the appropriate medication. In addition to saving time, patients can also reduce the cost of their healthcare by avoiding physician fees before coming to the pharmacy.
Cost
The cost of medications can also decrease for patients when medications are switched to nonprescription. While insurance may not cover OTC drugs, the price often has to be competitive to succeed when introduced in the market. This means that the cost of a medication could be cheaper after making the switch from prescription. The pricing will vary for every medication since is dependent upon the medication, though, as some costs from out of pocket could increase for the consumer.
Comfort
An area that health care professionals may not often consider advantageous with OTC medications is the ability to allow the patient their comfort of mind. Pharmacists and physicians maintain a professional air while helping patients seek treatment, yet it is hard to deny that there are patients who would be more comfortable purchasing a medication discreetly. A fairly recent example of this could be the Plan B One-Step medication. When the emergency contraceptive went OTC, it saved women and men the need to discuss their personal lives with more people. One of the top concerns of female college students when purchasing emergency contraception was confidentiality. While the importance of patient counseling cannot be denied, some decisions and medications do fall subject to scrutiny and having a medication available OTC provides patients the choice to opt out of counseling and handling their treatment at their own discretion.
Healthcare providers
Time is of the essence in health care as professionals must diagnose and treat a multitude of patients. By moving medications from prescription to OTC, the number of patients visiting physicians getting a prescription can reduce. This saves healthcare providers time and allows them to see more patients with disease conditions requiring a more severe treatment.
Beyond physicians, pharmacists can also save time with OTC medications because patients can select medications in aisles in a supermarket instead of bringing in a prescription to fill. With the receipt of less number of prescriptions for conditions that could be self-treated, pharmacists can either get more prescriptions filled in a day or, utilize the additional time to, counsel more patients about the purposes and administration of different medications.
One of the drawbacks of using OTC medications is physician concern for patient well-being. It is hard to monitor and check in on patients who are using OTC medications. Medications can only make the switch from prescription to OTC if they are perceived to be safe for patient use with self-diagnosis. While the step in the process of gaining medication from a physician’s prescription is no longer needed for certain medications, there is still a pharmacist available at the retail locations to educate consumers and patients on the advantages and possible side effects of medications.
It is pertinent for pharmacists to maintain a respectable relationship with patients to determine whether or not a patient truly needs a drug. Often advertised benefits may not be as beneficial as education, for example, weight loss effects from different formulations should be replaced or supplemented with exercise and a healthier diet. Pharmacists must be aware of patient lifestyles and whether particular medications will be a value to the consumer.
Drug manufacturers
Each new drug is guaranteed five years of patent exclusivity. Drug manufacturers lose their patents on medications after 5 years so often they want to get an OTC approval just as their competitors will when the patent runs out. Another advantage of OTC medication, besides staying competitive with their generic counterparts is when their patents run out, a plethora of advertising opportunities open up to them. Companies switching to nonprescription have more leeway in their advertisements and marketing plans. Drug companies can also display more information to guide patients in a more general manner, quite similar to the information that a physician explains to individual patients about medication intake.
Health insurers
Health insurance companies benefit from OTC switches because their costs are reduced. An example of health insurance is Medicare Part D, which is meant to help cover prescription medications. When medications are switched to OTC, it means a drop in costs for insurance companies because they don’t have to cover OTC medications in their plans.
_
Issues of concern:
Some concerns need to be brought to the forefront of the conversation switches to nonprescription. Patients may make an inaccurate self-diagnosis. Certain disease-states are easily recognized with characteristic symptoms, yet there are many that are difficult to identify. There are a variety of medications on the market that are made to treat a variety of symptoms such as headache, stomach pain, and nausea. When a patient experiences only one of the symptoms listed, the best course of treatment is to seek a medication that more directly correlates with the symptom they are experiencing.
Patients could also treat a symptom with OTC medication under the assumption that it is a momentary, minor pain. However, caution must be advised, as these symptoms can be a warning of a more severe condition that needs to be examined by a medical professional. For example, patients may treat a cough OTC, but when a cough has been present for a prolonged length of time, it would be prudent to seek advice from a physician.
Safety in general must be considered in regards to patient welfare. Patients should read labels, follow dosage directions, check for tampering, and keep vials and bottles in a safe location. While utilizing OTC medications patients must also be aware of active ingredients. It is plausible a patient may take two separate medications under the assumption that two pills could make them feel better twice as quickly as one. For example, a study showed that patients feel that can choose their own doses for OTC acetaminophen regardless of the recommended doses on the labels and were more likely to take higher doses than recommended. To keep patients safe in this scenario it is important that packaging is explicit and that education on these matters is readily available to the public. These labels should also emphasize concerns such as pregnancy or DDIs. Many DDIs occur because patients consider OTC medications to be somewhat risk-free and hence drink alcohol in combination with medications such as NSAIDs, which increases health risks.
_____
_____
Future trends in Rx to OTC switch:
In some European countries, treatments for high cholesterol, irritable bowel syndrome, and migraine have already been made available over the counter. Some of these treatments, such as azithromycin for chlamydia, are available only after consultation and, in some cases, diagnostic testing by a pharmacist (see table below). Existing models of health care provision are changing. With increasing consumer reliance on technology for health information, and a worldwide trend toward expanded OTC product availability, pharmacists in the United States can expect to see a broadening of their role in the provision of OTC treatments for a broader range of illnesses. Pharmacists will be an important part of this movement toward greater patient autonomy and expanded access to health care.
Selected medications that are available OTC outside U.S.
|
Medication |
Countries with OTC Availability |
|
Sucralfate |
Austria, Denmark, Finland, France, Hungary, and Italy |
|
Dicyclomine |
United Kingdoma |
|
Metoclopramide |
Belgium, Hungary, and Italy |
|
Nitroglycerin |
Denmark, Hungary, United Kingdoma |
|
Fluconazole (oral) |
Finland, United Kingdoma |
|
Erythromycin (topical) |
Belgium, Poland |
|
Azithromycin (oral) |
United Kingdomb |
|
Sumatriptan |
Germany, Sweden, United Kingdom |
|
Zolmitriptan |
Sweden |
|
Ipratropium bromide (nasal) |
Belgium, Czech Republic, Estonia |
aOnly available through pharmacies.
bOnly available through pharmacies for the treatment of chlamydia after urine testing.
_
Regulatory change:
FDA officials have expressed an interest in using technology to promote appropriate use of OTC medications and increase consumer accessibility to medications that are currently available by prescription. Since 1972, when the current regulations were put in place, consumers have become more reliant on technology for health information. Technology also enables diagnostic monitoring and sharing of information among many different health care professionals. With technology such as mobile health (mHealth) applications, the FDA is likely to broaden the OTC availability of many types of medications. The first signs of this change came in 2012 when the FDA established the Non-prescription Safe Use Regulatory Expansion (NSURE) task force to evaluate new strategies for converting prescription-only drugs to OTC medications. Experts have proposed using technologies, such as smartphone apps, to enable self-care for conditions such as hyperlipidaemia, benign prostatic hypertrophy, and migraine (see table below)
Medications that may be future candidates for OTC status:
|
Indication |
Potential OTC Candidates |
|
Erectile dysfunction |
Sildenafil, Tadalafil, Vardenafil |
|
Migraine |
Almotriptan, Eletriptan, Naratriptan, Rizatriptan, Sumatriptan, Zolmitriptan, Frovatriptan |
|
High cholesterol |
Atorvastatin, Lovastatin, Pravastatin, Simvastatin |
|
Overactive bladder |
Solifenacin, Tolterodine, Trospium |
|
Benign prostatic hypertrophy |
Tamsulosin |
Changes to OTC sales pursued by the NSURE task force will “keep the pharmacist in the driver’s seat of consultation,” although pharmacists can expect more use of innovative technologies to help the right patients make use of newly available OTC medications.
The emphasis on technology in distribution of certain medications has led some individuals to refer to this proposed new set of medications as “e-OTC drugs.” For these medications, product selection might include use of in-store kiosks or tablet-based applications to assess patient eligibility. Such electronic resources would also enable participation by health care professionals through an electronically integrated system that tracks test results and patient records. OTC medication and technology integration should improve patient access to medications while continuing to involve physicians and pharmacists in the treatment process.
_______
_______
Section-7
OTC vs. supplements and alternative medicine products:
One major difference between over-the-counter drugs and supplements is how they’re regulated and tested.
Defining drugs:
Drugs are regulated by the FDA. They’re defined as substances intended to diagnose, treat or prevent disease. Drugs have to undergo multiple clinical tests, first in the laboratory and/or in animals and then in humans to make sure the medications are safe and effective. The manufacturers then present the results of this testing to an expert panel at the U.S. Food and Drug Administration (FDA). This panel determines whether or not the drug works and whether its potential health benefits outweigh any risks before approving it for marketing in the United States. Medications must pass clinical trials before being released to the public and the tests need to prove each drug is safe, performing just as the manufacturer claims. After these trials, your doctor can prescribe the drug to you. The general rule is drugs are considered unsafe until they’re proven safe.
Defining supplements:
Supplements aren’t regulated by the FDA. Under the Dietary Supplement Health and Education Act (DSHEA), the FDA treats supplements like food and the DSHEA defines supplements as “products taken orally for supplementing the diet,” and the manufacturer is responsible for the safety of their supplements. Manufacturers do not need FDA approval for marketing supplements. Supplements can include minerals, vitamins or other natural biological substances and they’re available in a variety of shapes and sizes, including concentrates, extracts, capsules, tablets, liquids and powders. Keep in mind, herbs and vitamins don’t have to be tested for safety. Self-regulated by the manufacturer, no proof is required to demonstrate their effectiveness. The general rule for supplements is they’re considered safe until they’re proven unsafe.
In a nutshell
All prescription and non-prescription drugs are regulated in the United States by the Food and Drug Administration (FDA). But dietary supplements are treated more like special foods. Because supplements aren’t considered drugs, they aren’t put through the same strict safety and effectiveness requirements that drugs are. So all the drugs you can buy, even without a prescription, must be proven safe and effective – but dietary supplements do not. There are no FDA-approved products labeled as homeopathic; this means any product labeled as homeopathic is being marketed in the U.S. without FDA evaluation for safety or effectiveness.
_
Problems with supplements and herbs:
The possibility of drug interactions, direct toxicities, and contamination with active pharmaceutical agents are among the safety concerns about dietary and herbal supplements. Although there is a widespread public perception that herbs and botanical products in dietary supplements are safe, research has demonstrated that these products carry the same dangers as other pharmacologically active compounds. Interactions may occur between prescription drugs, over-the-counter drugs, dietary supplements, and even small molecules in food—making it a daunting challenge to identify all interactions that are of clinical concern.
Many dietary supplements have clean safety histories. For instance, millions of people take multi-vitamins safely and have no ill effects. Many manufacturers are very careful with their claims, labeling, and the ingredients they use in their products. But since they became widely available in 1994, the FDA and some independent researchers have found problems with some dietary supplements. Products like herbs are sometimes tainted with germs, pesticides, or toxic heavy metals. Other supplements do not contain what’s listed on the label. Still others contain more or less than the amount of the herb listed on the label. And many have ingredients that aren’t listed on the label at all.
This problem extends beyond the supplement makers and sellers. Some herbal suppliers (those who grow, harvest, or sell the crops) may mix or even substitute their crops with less expensive or more readily available plants. There’s also the problem of accidental contamination, when one plant grows in with others, as well as cases of mistaken identity (when one plant looks like another). Given the global market, all of these problems can make it harder for a company to be sure that what they thought they were buying to make supplements is actually the herb they wanted.
In 2013 researchers in Toronto published a report in which they sampled and analyzed 44 herbal supplements. The supplements were sold in both the US and Canada, and labeled as containing single herbs. Using DNA bar coding analysis, less than half the supplements (48%) contained any of the herb listed on the label. More than half of the supplements contained something that wasn’t on the label (substitutions or fillers). Even among the samples that contained the herb on the label, many also contained fillers or contaminants. And again in early 2015, the New York Attorney General sent warning letters to major retailers who sold supplements that were shown by DNA testing to be mislabeled. Although they’re not tested very often, careful studies find that many supplements are not what they are supposed to be.
A more serious trend today is extra ingredients in supplements. Some “herbal” supplements have been found to contain prescription drugs or other compounds that are not listed on their labels. For example, some supplement ads are targeted to men as “enhancers” or muscle builders. Certain of these so-called “supplements” have been found to contain substances much like Viagra® or Cialis®, and have been recalled. “Prostate health” supplements have been found to contain terazosin, a prescription drug used to treat the symptoms of an enlarged prostate. Other ads target women and tout the supplement as an aid to weight loss. Some of these “weight loss supplements” contained the weight loss drug sibutramine, which was banned in the United States because of the risk of heart attack and stroke. The supplement makers recall these only after they have been found to have these illegal additives. Then the FDA can seize these drugs and prosecute the companies who make them. There are also times that new ingredients with little-known effects are slipped into supplements. In one situation, supplements were labeled as being made from geranium but turned out to contain the stimulant drug dimethylamylamine (DMAA). This type of supplement was sold as a “natural stimulant,” but it contained DMAA, a man-made drug. The DMAA-containing supplements were exposed after some serious events, including several deaths, leading the FDA to send warning letters to US manufacturers in 2013. These kinds of extras can cause serious health issues for people who take the supplement. There are also risks of mystery drug interactions because the person doesn’t know that he or she is taking a drug.
Despite all these issues, the FDA is not legally responsible for the safety of dietary supplements; the manufacturers are. The manufacturers are also responsible for what’s in them, and being sure the contents are the same from one pill or package to another. The FDA only looks into reported problems or safety hazards.
_______
An unquestioning belief in the power and efficacy of nature’s healing remedies and processes, the placebo effect, disappointment and dissatisfaction with conventional medicines, outright rejection of orthodox treatments, convincing and persuasive advertising, reinforcement from others with similar views, endorsement by influential celebrities, perceived hand-me-down wisdom, bogus pseudoscientific claims, uncritical journalism, scare-mongering, feelings of desperation for a ‘cure’, and anecdotal case studies or surveys masquerading as research, are among the many reasons why patients and the public choose to alternative medicines either bought through local stores, pharmacies or on the internet.
______
Ayurvedic medicines:
In India, the traditional herbal medicines, such as Ayurveda, Siddha, and Unani (ASU), are considered safe because of their long history of use. As such, no safety and efficacy studies are required for marketing approval, as per the Drugs and Cosmetics Act of 1940 (DCA). Ayurvedic drugs have begun to be evaluated in controlled clinical trials. The trials, often placebo controlled, are usually designed to demonstrate superiority. Though the results have been usually reported as encouraging, statistical significance has been elusive. Through a recent notification, the Government of India has clarified that no clinical trials are required for issuing licenses patented or proprietary Ayurvedic, Siddha and Unani (ASU) products. They do not require a drug license and can therefore be sold by non-chemists. A study published in the August 27, 2008, issue of the Journal of the American Medical Association (JAMA), demonstrated that one-fifth of U.S.-manufactured and Indian-manufactured Ayurvedic products bought on the Internet contained detectable lead, mercury, or arsenic. Toxicity of Ayurvedic medicines have been reported due to presence of toxic metals like lead, mercury and arsenic. In the case of herbal medicines, this is attributable to environmental pollution or bad manufacturing practices. However, the intentional use of mercury, arsenic and such other toxic substances as medicine has spurred debate amongst scientists and the laity regarding the safety of Ayurvedic medicines. Modern researchers find it hard to accept that highly toxic metals and minerals can ever be rendered safe for human use by any method of processing. On the other hand, the Ayurvedic physicians maintain that purification of toxic substances is very much possible if done meticulously. There is no price control on Ayurvedic medicines. Some of the top OTC brands in India such as Vicks VaproRub, Amrutanjan Balm, Zandu Balm, Iodex, Moov Pain Cream, Itch Guard Cream, ENO Fruit Salt, Vicks Cough Drops, Halls Lozenges, etc., are registered as ‘Ayurvedic Medicines’ because of their plant-based natural active ingredients.
_
The US FDA doesn’t review or approve Ayurvedic products. In fact, certain items have been banned in the U.S. since 2007. The FDA has warned that 1 in 5 Ayurvedic medicines have toxic metals, like lead, mercury, and arsenic. These heavy metals can cause life-threatening illness, especially in children. There is a widespread misconception that all drugs of “natural” origin are “safe.” There is also a common belief that long-term use of a medicine based on tradition, assures both safety and efficacy. Currently, the majority of adverse events related to the use of herbal/traditional products that are reported are attributed either to poor product quality or to improper use. Recognizing the widespread use of ayurvedic medicine and the tremendous growth of international markets for herbal products, it is important to ensure that the health care provided by Ayurveda is safe and reliable; that standards for the safety, efficacy, and quality control of ayurvedic products and therapies are recognized and endorsed; that practitioners have the qualifications they acknowledge; and that the statements made for products and practices are legal.
______
Medical grade supplements vs. over-the-counter (OTC) supplements:
Is your OTC multi-vitamin the same thing that you can get at a medical office?
No.
Medical grade supplements are manufactured to the same strict standards as pharmaceutical drugs. OTC supplements cannot say that. Over the counter vitamins are not regulated by the government. A recent expose` showed that some OTC supplements that were called gluten-free had gluten in them, some that said they contained a certain amount in a capsule had less than the amount listed, and one didn’t even contain the ingredient listed on the label at all! There are literally thousands of OTC supplement companies, and they all say the same things about their products – pure, high quality, etc., etc. – but usually they are not able to back up those claims. OTC supplement companies are good at marketing their products, and have huge marketing budgets for flashy ads and materials, but this does not mean that they are good.
One reason that OTC supplements are not the same has to do with the ingredients themselves. For example, many people don’t know that there are often multiple forms of a single vitamin – from cheap and synthetic, to more pricey but natural and much better for you. OTC vitamins almost always use the cheap and synthetic forms. For example, folic acid is a cheap and synthetic form of the naturally occurring vitamin folate, and many people (due to common genetic mutations such as MTHFR) need the methylated natural forms of their B vitamins, including methyl folate and methyl B12. Folic acid can actually block the folate receptor and prevent it from responding to the better, more natural vitamin folate. Vitamin B6 (pyridoxine) is better as the more expensive P5P (pyridoxine-5-phosphate). Vitamin E is better as the pricier mixed tocopherols as opposed to alpha tocopherol only, and so on. Medical grade supplements usually contain the best forms of these vitamins, to ensure that your body is getting the most natural and effective form of the vitamin – even if it costs a little more.
Another big reason that OTC vitamins are not the same as medical grade vitamins is the concentrations found in the product. For example, Motrin (Ibuprofen) used to be a prescription drug only, and was 800 mg per pill. When it became available OTC, they lowered the dose to 200 mg. Vitamins are often the same, with much lower doses being the norm in OTC products. For example, in fish oil pills, OTC products usually say “1000 mg per pill!” in big letters, so that people think they are getting a lot. In reality, the most important part of that fish oil is the omega-3 fraction of the oil. In OTC fish oil, there is usually only around 200-400 mg of omega-3 (EPA + DHA) per 1000 mg of oil in a capsule. Since our daily goal for EPA+ DHA should be 1500 – 2000 mg per day, you would have to take 5-10 of those OTC fish oil pills to get what you need, as opposed to usually 2 of the medical grade capsules, so the OTC products aren’t really cheaper at all.
The bioavailability of OTC products is also often a big problem. Many of the “one-a-day” kinds of multivitamins, have small amounts of lots of different things crammed into one small pill – so that the label looks good, and again, people think they are getting a lot. In reality, many of those compacted pills are excreted in the stool undigested and unabsorbed, because they compacted so tightly, that they don’t even dissolve well.
Another way that OTC products “fool” the public is by saying that they are “100% RDA” of something. The RDA (recommended daily allowance) is the amount of a vitamin that you would have to get, in order to not have a vitamin deficiency disease. The amount of that same vitamin that you need to be optimally healthy, is often many times more.
_______
_______
Section-8
OTC Statistics and Usage:
The United States Government Accountability Office studied five countries (the UK, the USA, The Netherlands, Italy and Australia) and determined how medicines were classified in each. They found that since 1995 all these countries have increased OTC availability. This is due either to changes in the classification of non-prescription drugs or to the reclassification of medications into less restrictive classes. A report on global OTC markets states that countries such as the United States, Japan, Germany, and the United Kingdom contribute maximally to the worldwide OTC sales. The USA, UK, Australia, and Japan have formulated guidelines regarding classification, regulation, and uses of OTC.
_
-1. An estimated 15 percent of Americans take store-bought pain meds regularly, according to Harvard Health Publishing, and the market is supposed to reach $24.4 billion by 2027, up from $15.6 billion in 2019.
-2. A January 2018 study in Pharmacoepidemiology & Drug Safety suggests that up to 15 percent of OTC pain med users took more than the recommended dose, 16 percent took ibuprofen every day and 55 percent used it at least three days a week. “People think this medicine is safe, since you can buy it at any grocery store, pharmacy or gas station,” says pain management expert Lynn Webster, MD, author of The Painful Truth.
-3. Four out of five American adults commonly take over-the-counter medications, most often to treat ailments like aches and pains, coughs and colds, fever, allergies, skin disorders, and heartburn and other digestive problems.
-4. Overdoses of acetaminophen result in 30,000 hospitalizations annually, often because of acute liver failure. A study of 500 people published in 2012 in The Journal of General Internal Medicine revealed that 24 percent would unwittingly exceed the safe limit of 4,000 milligrams of acetaminophen over a 24-hour period when taking a single product containing the drug. About 46 percent would overdose when taking two products at the same time that contain this pain reliever.
-5. According to the National Council, a third of Americans say they combine medications when treating multiple symptoms, but only 1 person in 10 says they read the entire label of each drug taken; therefore, most are unaware of potentially toxic duplications or harmful interactions.
-6. According to the Consumer Healthcare Products Association, 81 percent of adults in the U.S. use OTC medicines as their first line of treatment for minor illnesses. On average, consumers in the U.S. make about 26 trips per year to buy OTC medications. The average consumer only visits his or her doctor about three times per year. There are a total of 2.9 billion retail trips annually to purchase OTC products. On average, U.S. households spend about $338 per year on OTC products. 96 percent of U.S. adults believe OTC medicines make it easy for individuals to care for minor medical ailments. 61 million consumers have avoided missing work, school, or other scheduled appointments due to illness because they had access to OTC cough medicines to alleviate their symptoms.
-7. Americans buy more than 5 billion OTC drug products each year, that is 60 % of total health related drugs every year.
-8. Over-the- counter drug products account for 55 percent of drugs used by Indians. An estimated 3 out of 4 people routinely self-medicate with these drug products.
_______
A review of over-the-counter drug therapy, a 1979 study:
The authors review the extent of the use of nonprescription drugs as well as possible variables influencing such consumption. Various studies indicate that age, sex, personality characteristics, perceptions of health status, socioeconomic factors, parental example, and pharmacists all play parts in determining over-the-counter (OTC) drug utilization. Several sources express concern about the inaccessibility of accurate OTC drug information to the consumer. Indeed, even the FDA has occasional difficulty obtaining reliable facts on both the numbers and formulae of such products. Several studies indicate that consumers acquire information about their home remedies through advertising, friends and relatives, physicians, pharmacists, and product labels. By far the most influential of these is advertising, and much concern has been voiced over consumers’ unquestioning faith in drug ads. Examples are cited of deceptive, inaccurate, and unfair advertising practices used by some OTC drug manufacturers. Testing of the safety and efficacy of nonprescription remedies has proved to be controversial, especially when considering the ramifications of the placebo effect. Different surveys report widespread misuse of OTCs by consumers through overuse, taking several drugs concurrently, and using home remedies to treat potentially serious diseases.
______
The Factors Contributing to Expenditures on Over-the-Counter Drugs in South Korea, a 2013 study:
Objectives:
To determine the factors contributing to the use of over-the-counter (OTC) drugs and to examine the relationship between the purchase of OTC drugs and the utilization of health care services in South Korea.
Methods:
This study used data from the 2008 Korea Health Panel Survey. The respondents were classified according to the purpose of the OTC drug use. The first group (n = 364) included respondents who had purchased OTC drugs for self-medication, and the second group (n = 955) included respondents who had taken OTC drugs for nutrition for more than 3 months. Logistic regression analyses were conducted to identify the factors contributing to the purchase of OTC drugs.
Results:
The self-medication group was more likely to be older and to have a chronic disease. In addition, the purchase of OTC drugs was related to the utilization of other health care services. The more outpatient services at clinics were used, the more the respondents tended to purchase OTC drugs for self-medication and nutrition. As hospital outpatient visits increased, however, the purchase of OTC drugs for self-medication decreased and the purchase of OTC drugs for nutrition increased.
Conclusions:
This study shows that age and chronic disease are the major factors related to using OTC drugs for self-medication for long-term periods. Furthermore, this study suggests that the use of outpatient services is one of the factors associated with purchasing OTC drugs. Considering the potential adverse effects of OTC drugs, communication between physicians and patients should be encouraged at outpatient visits.
_____
Use of over-the-counter drugs in urban and rural populations of Mandya district, India: a cross-sectional study, 2016:
Background:
Over-the-counter (OTC) drugs are medicines which are sold directly to a consumer without a prescription. There is a big potential for misuse and abuse of such products. Over the counter (OTC) drugs are meant for self-medication and are of proved efficacy and safety. Their improper use and unable to follow the precautions due to lack of knowledge of their side effects and interactions could lead to serious complications, especially in children and elderly.
Results:
A total of 800 persons were interviewed regarding their use of OTC drugs, among them 400 were urban residents and 400 were rural residents of Mandya district. Of the 400 urban persons and rural persons, 310 respondents (77.50%) and 273 respondents (68.25%) reported the use of OTC in the recall period of the last 6 months respectively. The difference in the usage of OTC by urban adults was significantly more than that of rural adults.
Conclusions:
The proportion of the respondents who had practiced self-medication with OTC drugs is very high. The prevalence of self-medication with OTC drugs in this study was found to be 72.87% and is nearly same in both rural and urban population. As this study was conducted in a limited population in Mandya district, generalization of the study to all the population cannot be done.
______
Knowledge, Attitude, and Practice on Over-the-Counter Drugs Among Pharmacy and Medical Students: A Facility-Based Cross-Sectional Study, 2020:
Background:
Self-medication with over-the-counter (OTC) medications is common among medicine and health science students. For safe use of OTC medications, students are expected to have proper knowledge, attitude, and practice (KAP) towards OTC medications and subsequent adverse drug reactions (ADRs).
Objective:
The aim of this study was to assess KAP of OTC medications use and related factors among medical and pharmacy students at the University of Gondar, Gondar, Northwest Ethiopia.
Conclusion:
Self-medication is widely practiced among medical and pharmacy students. Significant problems and malpractices were identified, such as sharing of OTC medications, the use of expired medicines, doubling the dose of medications when they were ineffective, storage of OTC medications, and not reading labels and expiry dates.
______
______
Section-9
Benefits of using approved OTC medicines:
Expanding the number of OTC drugs is often thought to provide the potential to increase efficiency by improving patient health and reducing health care costs. Proponents of OTC drugs argue that expanding the number of drugs (1) improves health by increasing access to beneficial therapies and (2) reduces costs both directly (by reducing the amount spent on physician-office visits) and indirectly (through improvements in health) (Gossel, 1991; Andersen and Schou, 1994; Newton, Popovich, and Pray, 1996; Reeves et al., 1999; Abrams, 2005; Gotto, 2005, TfeltHansen 2007). On the other side, opponents argue that inappropriate use of these drugs may increase costs and reduce health (Ryan, 1994; Tonore, 2002; Choudry and Avorn, 2005; Strom, 2005). The debate over the merits of expanding the number of OTC drugs has received much attention from policymakers and the medical community, with special attention being paid to the introduction of high-profile OTC drugs. For example, in 2005, the FDA denied approval for an OTC version of lovastatin following a highly public debate, with proponents arguing that OTC lovastatin would improve health by increasing access to cholesterol-lowering therapies, and opponents arguing that patient health would be harmed through inappropriate use. Yet, despite the debate over the merits of introducing more OTC drugs, there has been little effort to date to systematically and empirically determine whether the benefits of OTC drugs outweigh their costs (for a review, see Sun, Sood, and Blume-Kohut, 2008).
_
OTC medicines allow greater access to treatment of people at large at lower cost for minor or self-limiting illnesses. One common benefit of improved access to a drug through non-prescription status is that people may treat a condition earlier than otherwise. In some instances this may reduce the duration of illness or even help prevent progression. Moreover, General Practitioners (GPs) do not have to write prescriptions for minor ailments and in turn have more time for dealing with serious health problems. This is extremely useful for countries like India where the doctor to patient ratio is less (1:1445) than other countries. For ensuring optimum use of OTC medicines, pharmacists can provide a valuable interface by using their professional knowledge to guide patients.
______
OTC drugs cheapest:
_____
Several nonprescription medications are recommended as first-line therapy, according to clinical guidelines. Table below lists the common medications and support for their use.
_____
The Value of OTC Medications:
In 2010 alone, 240 million people in the United States spent approximately $23 billion on OTC products enabling them to self-treat cold symptoms, headaches, allergies, and many other common illnesses. For each dollar spent on OTC medications, the US health care system saves approximately $6 to $7, totaling an estimated $102 billion in value. At the same time, consumers save an estimated $13 billion per year in medical costs through use of OTC products. Highlighting this savings, the Consumer Healthcare Product Association estimates that an additional 56,000 full-time medical professionals would need to be added to the US workforce if OTC medications were not available. Although the savings associated with OTC medications are considerable, OTC medications continue to be underused. Primary care physicians estimate that 10% of visits to their offices could have been avoided by the use of an OTC medication.
OTC medicines save the U.S. health care system nearly $146 billion annually, according to a 2019 study by the Consumer Healthcare Products Association. They are popular with consumers who want to participate more fully in their own care while saving money.
When consumers get more engaged with their health, good things happen. The study that attributed savings to the lower cost of OTC versus prescription drugs also found that people used fewer doctor visits and had less time away from work when taking OTC rather than seeking treatment or a prescription.
Making the switch from prescription to OTC also pays off for pharmaceutical companies. They can reap the benefits of wider sales and, sometimes, extended commercial life of the drugs.
______
The Effect of OTC Drugs on Physician-Office Visits:
In theory, OTC drugs should reduce physician-office visits for their indicated conditions, since patients no longer need to obtain a physician’s prescription. However, OTC drugs may have little effect on physician-office visits if patients continue to consult their physicians for advice on whether to initiate a particular drug, or to discuss an OTC drug’s inefficacy or adverse effects. Therefore, whether and to what degree OTC drugs reduce physician-office visits remains an empirical issue. Several studies have addressed this issue by examining how the frequency of physician-office visits for a particular medical condition changes after the introduction of an OTC drug aimed at that condition. The introduction of OTC H2RAs appears to have had little effect on physician office visits for peptic ulcer and gastroesophageal reflux disease (Andrade, Gurwitz, and Fish, 1999; Shaw et al., 2001), while the introduction of vaginal antifungals appears to have lowered physician-office visits for vaginal candidiasis (Gurwitz, McLaughlin, and Fish, 1995; McCaig and McNeil, 2005; Lipsky, Waters, and Sharp, 2000).
However, a major limitation of these studies is that they essentially compare the number of physician-office visits after the introduction of the OTC drug to the number of visits prior to its introduction, and do not make a sophisticated effort to net out any underlying trends in physician-office visits that may have occurred prior to the BTC/OTC switch. This omission may lead to misleading inferences. For example, suppose that the incidence of vaginal candidiasis is falling due to changes in demographics and patient behaviors. Since the incidence of the disease is falling, the number of physician-office visits for the disease will also display a downward trend. Given this downward trend, physician-office visits will naturally be lower in the period after the OTC is introduced compared to the period before, but at least some of this difference will be due to the downward trend in disease incidence, not to the introduction of the drug itself. Thus, failing to account for the trend will tend to overestimate the effect of OTC drugs. Indeed, the data reported by McCaig and McNeil (2005) show that physician-office visits for vaginal candidiasis had already been trending downward prior to the introduction of OTC antifungals; thus, failing to account for this trend may explain a large part of the reported decrease in physician-office visits.
Overall, various studies do find that OTC drugs do reduce the number of physician-office visits for the diseases they are intended to treat, although there is wide heterogeneity across diseases and these results suggest that OTC drugs do have the potential to reduce medical costs by reducing spending on physician-office visits.
______
Benefit versus the alternatives:
In defending non-prescription status, it is important to understand and model quantitatively the counterfactual situation: i.e., how consumers of a medicine might behave if the drug ceased to be available without prescription. Broadly the result may be:
-1. A greater use of alternatives available without a prescription. These may include:
-a. Drugs with adverse events of known frequency. It may be possible to model the increase in these events that might accompany a rise in usage of substitute drugs following a change of status for the drug under scrutiny.
-b. Alternatives with no proven efficacy e.g., ‘alternative’ remedies. In this case people may suffer adverse consequences relating to lack of efficacy such as increased duration of suffering or disease progression.
-2. A change in consultation behaviour:
-a. Consumers may consult more (although this is an assumption that should be tested in consumer research). If that is the case then it is relevant to ask what the likely outcome of those consultations will be. It may be that doctors will prescribe the medicine that is no longer available OTC.
-b. Consumers may choose not to consult. If a condition has historically low doctor consultation rates (because of embarrassment for example) then it is possible that people will revert to ‘suffering in silence’ should their chosen non-prescription alternative not be available. Again, behavioural surveys may be useful in modelling the impact of the change.
_______
_______
Section-10
OTC use in children:
Children’s bodies metabolize and react to drugs differently from the way adults’ bodies do. A drug may be used by many people for many years before its hazards to children are discovered. For example, many years passed before researchers confirmed that the risk of Reye syndrome was linked to the use of aspirin in children who had chickenpox or influenza. Doctors and parents alike are often surprised to learn that most over-the-counter (OTC) drugs, even those drugs with recommended dosages for children, have not been thoroughly tested in children. The effectiveness of some cough and cold remedies is unproved, especially in children, so that giving these drugs to children may unnecessarily expose them to harmful effects of a drug and may be a waste of money.
Giving a child a correct drug dose can be tricky. Although children’s doses are often expressed in terms of age ranges (for example, children aged 2 to 6 or 6 to 12), age is not the best criterion. Children can vary greatly in size within any age range, so experts advise using the child’s weight to determine doses of OTC drugs.
If the label does not give instructions on how much drug to give the child, a parent should not guess. When in doubt, a parent should consult a pharmacist or doctor. Such consultation may prevent a child from receiving a dangerous drug or a dangerously high dose of a potentially helpful drug.
Many drugs for treating children come in liquid form. Even though the label should give clear guidelines about the dose, a child may be given the wrong dose because the adult in charge uses an ordinary teaspoon. The only kitchen spoons accurate enough to measure liquid drugs are measuring spoons. However, a cylindrical measuring spoon is far better for measuring a child’s dose, and an oral syringe is preferred for measuring and squirting a precise amount of drug into an infant’s mouth. The cap should always be removed from the tip of an oral syringe before use. A child can choke if the cap is accidentally propelled into the windpipe. Sometimes, drugs intended for treating children come with a measuring device packaged with the product. If so, the device that is in the package should be used to measure the appropriate dose.
Several children’s drugs are available in more than one form. Adults must read labels carefully every time a new children’s drug is used.
_____
OTC medicines for children are divided into the following classes:
Brand names of OTC medicines can change and store brands are common. Be sure to read the labels to know what the active ingredients are in all products.
-1. Analgesics treat pain and fever. Use caution with different forms of these drugs. Some are more concentrated than others. Common analgesics for infants and children are acetaminophen and ibuprofen. Don’t give aspirin to children younger than 18, because it can cause a rare but sometimes deadly condition called Reye syndrome.
-2. Antihistamines treat runny noses, itchy eyes, and sneezing caused by allergies (but not colds). Some can cause sleepiness. These are not recommended for children younger than 2. Use only with your healthcare provider’s OK in young infants or children with asthma. Examples of antihistamines include chlorpheniramine, diphenhydramine, and loratadine.
-3. Expectorants and combination cough medicines may help loosen mucus. Cough suppressants numb the reflex to cough. Coughing is needed to clear mucus and bacteria from the lungs. Check with your child’s healthcare provider before using cough-suppressing syrups. Guaifenesin, an expectorant, helps thin mucus that is more easily removed by coughing.
-4. Decongestants can relieve stuffiness caused by allergies or colds by temporarily shrinking the membranes in the nose to make breathing easier. They should not be used for more than 2 to 3 days in a row. Decongestants taken by mouth can have a number of side effects like irritability, sleeplessness, and dizziness.
-5. Medicines for diarrhea. These are usually not necessary. Instead, give your child plenty of fluids and let the disease run its course. Diarrhea can be dangerous in newborns and infants. In small children, severe diarrhea lasting just a day or 2 can lead to dehydration. Because a child can die from dehydration within a few days, see a healthcare provider as soon as possible if an infant has diarrhea. Talk to your healthcare provider before giving these medicines to infants or children. One medicine for diarrhea, bismuth subsalicylate should not be given to a child younger than 18. Another medicine for diarrhea, loperamide, should not be given to a child younger than 2.
-6. Laxatives relieve constipation and work by several methods. Some add fiber or water to stool to make it more bulky and easier for intestines to eliminate it. Some coat the surface of the stool to make it more slippery. Some soften the stool so it passes more easily. Others cause the intestines to contract more forcefully. Don’t give infants or children laxatives without talking to your child’s healthcare provider. Examples of laxatives include glycerin suppositories, magnesium hydroxide, mineral oil, and psyllium.
_____
Safety tips about giving OTC medicines to children:
Nearly seven in ten parents have given their child an OTC medicine late at night to help treat a sudden medical symptom. About 85 percent of U.S. parents prefer to treat their children’s minor ailments with an OTC medicine before seeking professional care. Children are not just small adults. OTC drugs rarely come in one-size-fits-all. Here are some safety tips about giving OTC medicines to children:
-1. Children aren’t just small adults, so don’t estimate the dose based on their size.
-2. Read the label. Follow all directions.
-3. Follow any age limits on the label.
-4. Some OTC products come in different strengths. Be aware!
-5. Know the difference between TBSP. (tablespoon) and TSP. (teaspoon). They are very different doses.
-6. Be careful about converting dose instructions. If the label says two teaspoons, it’s best to use a measuring spoon or a dosing cup marked in teaspoons, not a common kitchen spoon.
-7. Don’t play doctor. Don’t double the dose just because your child seems sicker than last time.
-8. Before you give your child two medicines at the same time, talk to your doctor or pharmacist.
-9. Never let children take medicine by themselves.
-10. Don’t call medicine “candy.” If children find medicine at a later time, they may consider it “candy” and eat it without your knowing.
-11. Do not give aspirin to anyone younger than 18 unless your doctor tells you to, because of the risk of Reye syndrome.
-12. Talk to your doctor before you give fever medicine to a baby who is 6 months of age or younger. This is to make sure a young baby’s fever is not a sign of a serious illness. Ask your doctor what other medicines may not be safe to give your child.
-13. Don’t take medicines in front of small children. Children are great mimics.
-14. Keep medicines, vitamins, and natural health products tightly capped in their original containers. Store them as directed and keep them out of the reach of children.
-15. OTC cough and cold medicines should not be given to infants and small children without talking with your child’s healthcare provider first. The FDA and American Academy of Pediatrics (AAP) recommend against giving them at all to infants and children under the age of 2 because of possible serious life-threatening side effects.
-16. Never guess on a dose. Medicine doses for infants and young children are based on age and weight. Know your child’s weight. Follow the directions for age and weight. If the recommended age is not your child’s age, don’t give the medicine.
-17. If you want to mix medicine with milk or formula, first put the medicine in 1 ounce of milk and have the child drink it all. Then feed the remaining formula or milk in the bottle.
-18. Always measure or give medicine with a good light turned on. Insufficient light could cause you to give the wrong medicine or the wrong dose.
-19. Talk to your healthcare provider or pharmacist to find out what mixes well and what doesn’t. Medicines, vitamins, supplements, foods, and beverages don’t always mix well with one another.
-20. Always use child-resistant caps and store medicines in a safe place. Relock the cap after each use. Be especially careful with any products that contain iron. They are the leading cause of poisoning deaths in young children.
______
Child-Resistant Packaging:
Child-resistant packaging or CR packaging is special packaging used to reduce the risk of children ingesting hazardous materials. This is often accomplished by the use of a special safety cap. Child-resistant closures are designed for repeated use to make it difficult for children to open. Remember, if you don’t re-lock the closure after each use, the child-resistant device can’t do its job—keeping children out!
As per World report on child injury prevention issued by the World Health Organization and UNICEF, child-resistant packaging for medication is recommended because statistics show that one child receives hospital emergency treatment due to medicine poisoning every 8 minutes.
An innovation that has had one of the largest impacts is the invention of child-resistant packaging. Any parent will confirm that few things are truly child proof, and that children are not the only ones to struggle with opening such containers. However, they have helped cut child injuries and deaths by thousands.
It was a Canadian pediatrician who developed the Palm-n-Turn lid in common use today. Henri Breault is credited with inventing it at a time when accidental poisoning of children was at epidemic levels. Canada alone suffered 100,000 annual cases, claiming the lives of at least 100 children a year. In his job, Breault was continually pumping the stomachs of children and decided to do something about it. In 1967 he patented the lid and its use led to a 25% drop in child poisonings in Ontario. Soon other states started using it and in 1970 the US made them mandatory on some medicine bottles. The UK followed suit in 1975. UK law also requires that certain other products, such as some cleaning products, like bleach, and gardening goods are packaged in child resistant packaging.
Child resistant (CR) packaging or special packaging, as others call it, is used to minimize the risks of poisoned children. Children usually ingest potentially hazardous items such as prescription and over-the-counter medications, household chemicals, and pesticides. The US Consumer Product Safety Commission has the right to regulate child resistant packaging via the Poison Prevention Packaging Act (PPPA), which has become effective since 1972. Based on the PPPA, the term child-resistant packaging means packaging that is designed to make it significantly difficult for children who are under five years to open a product that may contain a toxic or harmful amount of substance. It is also designed so that it’s easier for adults to open the product.
The U.S. Consumer Product Safety Commission has stated in a press release that “There is no such thing as child-proof packaging. So you shouldn’t think of packaging as your primary line of defense. Rather, you should think of packaging, even child-resistant packaging, as your last line of defense.”
_______
_______
Section-11
OTC use in elderly:
According to a United Nations report in 2015, the number of older people, those aged >60 years, has markedly increased over the last few years worldwide. Globally, their number is expected to increase from 901 million to 1.4 billion between 2015 and 2030, representing a growth of about 56%.
Along with changes in lifestyle, developments in medical technology, and increase in life expectancy, the growth in the number of older people has caused increase in the incidence and prevalence of chronic diseases (CDs) such as heart disease, hypertension (HTN), diabetes mellitus (DM), COPD, and cancer. The increase in the prevalence of CDs has led to the long-term use of multiple medications, or polypharmacy. Hence, concerns about the effects of polypharmacy in patients with CDs have been raised. The rise in the number of medications used by each patient contributes to increase in drug combinations, which in turn, leads to elevated risk of adverse drug reactions (ADRs) and drug–drug interactions (DDIs). According to a study by Veehof et al, elderly patients prescribed with more medications tended to experience more ADRs than other patients, and the incidence rate of ADR increased proportionally with the increase in the number of medications administered long term.
Nowadays, a substantial number of symptomatic patients tend to use not only prescription medications but also non-prescription medications (i.e., over-the-counter [OTC] medications), which can easily be obtained in pharmacies for the purpose of self-treatment. Older persons are the major consumers of OTC medications, consuming about 40% of OTC medications. However, OTC medications are not always safe and beneficial, and can expose the patients to unexpected health risks such as ADRs and DDIs. The incidence rates of ADRs and DDIs may be higher in patients who take both prescription and non-prescription medications. In addition, when there is no sufficient communication about the use of OTC medication between pharmacists and patients, it may lead to unintentional drug duplication (UDD) because pharmacists are poorly informed about the OTC medications that patients take. Patients under multiple medications may experience a higher frequency of UDD; therefore, it is necessary to continuously monitor the use of OTC medications and prescription medications.
_
Normal aging changes the speed and ways in which the body metabolizes drugs, and older people tend to have more diseases and to take more than one drug at a time. For these reasons, older people may be more likely than younger ones to experience side effects or drug interactions. More and more prescription drug labels specify whether different doses are needed for older people, but such information is rarely included on over-the-counter (OTC) drug labels.
Many OTC drugs are potentially hazardous for older people. The risk increases when drugs are taken regularly at the maximum dose. For example, an older person who has arthritis may frequently use an analgesic or anti-inflammatory drug, with potentially serious consequences, such as a bleeding peptic ulcer. Such an ulcer is life threatening for an older person and can occur without warning.
Most antihistamines, such as diphenhydramine, are designated as “sedating” antihistamines and may pose special risks for older people. Many nighttime pain relief formulas, cough and cold remedies, allergy drugs, and sleep aids contain antihistamines. These antihistamines may cause drowsiness or fatigue and may worsen some disorders common among older people, such as closed-angle glaucoma and an enlarged prostate gland. They can also make a person dizzy or unsteady, leading to falls and broken bones. Antihistamines, particularly at a high dose or in combination with other drugs, can sometimes cause blurred vision, light-headedness, dry mouth, difficulty with urination, constipation, and confusion in older people. Fexofenadine and loratadine are considered to be “nonsedating” antihistamines, and they are not likely to cause drowsiness or other side effects.
Older people may be more susceptible to the possible side effects of antacids. Antacids that contain aluminum are more likely to cause constipation, and antacids that contain magnesium are more likely to cause diarrhea and dehydration.
During visits to the doctor, older people should mention all OTC products they are taking, including vitamins, minerals, and medicinal herbs. This information helps the doctor evaluate the entire drug regimen and determine whether or not an OTC drug may be responsible for certain symptoms.
_
Changes in Prescription and Over-the-Counter Medication and Dietary Supplement Use Among Older Adults in the United States, 2005 vs 2011, a 2016 study found that older adults are increasingly using multiple medications and dietary supplements, and the use of interacting medication regimens has increased over time. Approximately 1 in 6 older adults may be at risk for a major drug-drug interaction. Efforts that focus on improving the safe use of multiple medications have the potential to reduce preventable adverse drug events associated with medications commonly and increasingly used among older adults.
______
______
Section-12
OTC use in pregnancy:
Drugs can move from a pregnant woman to her fetus (primarily through the placenta), and drugs can be transmitted through breast milk to the baby. Some such drugs can affect or harm the fetus or baby, so pregnant women and breastfeeding women should consult their doctor or pharmacist before taking any over-the-counter (OTC) drug or medicinal herb. OTC drug labels should be checked because they contain warnings against use during pregnancy and breastfeeding, if applicable.
Ingestion of OTC preparations during pregnancy results in placental transfer and accumulation of these drugs in the fetus. As the fetus lacks the ability to handle pharmaceutical agents, since renal function, metabolic pathways, etc. are not fully developed, drug exposure in utero may produce deleterious effects in the fetus but not the mother. Clinicians are aware of drug-induced effects on the fetus and have dramatically reduced the use of prescription drugs during pregnancy. However, the use of self-medication (OTC) has significantly increased during pregnancy through extensive, effective advertising by the pharmaceutical industry and lack of sufficient data indicating an OTC effect on the fetus.
More than 90% of pregnant women take a prescription or over-the-counter (OTC) medication. Although there are no randomized controlled trials to guide the use of OTC medications during pregnancy, women often use them for skin, allergy, respiratory, and gastrointestinal conditions in addition to general analgesia. All physicians caring for reproductive-aged women should be familiar with the indications, risks, and benefits of OTC medications in pregnancy. Given limited data on the variety of OTC medications available, physicians need to counsel pregnant women about potential risks, and it is beneficial to discuss all OTC medications the patient is taking at the preconception visit and all other routine visits.
Pregnant women commonly use over the counter medications, although most over the counter drugs have an excellent safety profile, some have unproven safety or are known to adversely affect the fetus. At least 10 percent of birth defects are thought to result from maternal drug exposures. The medical community’s approach to the use of mediations during pregnancy has changed dramatically since the early 1970s, largely because of the problems with thalidomide and diethylstilbestrol, consequently, extensive testing is required before a drug can be labelled for use during pregnancy.
Although most over-the- counter drugs have an excellent safety profile, some have unproven safety and adversely affect the fetus. The safety profile of some medications may change regarding to the gestational age of the fetus. Because an estimated 10 percent or more of birth defects result from maternal drug exposure, the U.S. Food and Drug Administration has assigned a risk category to each drug. Several drugs have not been evaluated in controlled trials and probably will not be because of ethical considerations. The commonly used over-the-counter medications, acetaminophen, chlorpheniramine, kaolin and pectin preparations, and most antacids have a good safety record. Acetaminophen, which is used by about 65% of pregnant women, is generally considered safe during any trimester. Cold medications are also commonly used and are considered safe for short-term use outside of the first trimester. Other drugs, such as histamine H2-receptor blockers, proton pump inhibitors, pseudoephedrine, and atropine/diphenoxylate should be used with caution. If use of smoking cessation products is desired, the intermediate-release preparations minimize the amount of nicotine while maintaining efficacy. NSAIDs should not be used during the last 3 months of pregnancy unless specified by a doctor, because they may cause problems in the fetus or complications during delivery. There is even fewer data regarding use of individual herbal supplements. Ginger is considered safe and effective for treating nausea in pregnancy. Topical creams are considered safe based on small studies and previous practice. With all over-the-counter medications used during pregnancy, the benefit of the drug should outweigh the risk to the fetus. Because of the expanding OTC market, formalized studies are warranted for patients to make a safe and informed decision about OTC medication use during pregnancy.
_______
FDA classification of drug safety during pregnancy:
Category A
Controlled studies in women fail to demonstrate a risk to the foetus in the first trimester (and there is no evidence of risk in later trimesters), and the possibility of foetal harm appears remote.
Category B
Either animal reproduction studies have not demonstrated a foetal risk but there are no controlled studies in pregnant women, or animal reproduction studies have shown an adverse effect (other than a decrease in fertility) that was not confirmed in controlled studies in women in the first trimester (and there is no evidence of risk in later trimesters).
Category C
Either studies in animals have revealed adverse effects on the foetus (teratogenic or embryocidal or other) and there are no controlled studies in women, or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the foetus.
Category D
There is positive evidence of human foetal risk, but the benefits from use in pregnant women may be acceptable despite the risk (e.g., if the drug is needed in a life-threatening situation or for a serious disease in which safer drugs cannot be used or are ineffective).
Category X
Studies in animals or human beings have demonstrated foetal abnormalities or there is evidence of foetal risk based on human experience, and the risk of the use of the drug in pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant.
_____
Categories of over-the-counter medications in pregnancy:
-1. Pain Medications:
The most commonly used OTC pain medications and their effects are mentioned in table below:
Gastrointestinal and renal effects are among the most widely studied and recognized adverse effects of NSAIDs, but reproductive risk, especially from NSAID use in early pregnancy, has not been systematically evaluated. A Danish case-control study linked pharmacy data with birth registry data. The authors selected women with registry reports of miscarriages as cases and live births as controls. They reported an association between use of prescribed NSAIDs & miscarriage. A prospective cohort study of the risk factors for miscarriage among members of the Kaiser Permanente Medical care program an integrated health care delivery system, including hospitals and outpatient clinics showed use NSAIDs during pregnancy increased the risk of miscarriage by 80%. The risk of miscarriage was much higher when NSAIDs were taken around conception.
_
The National Birth Defects Prevention Study (NBDPS), which analyzed data from 16,110 children in the United States exposed to acetaminophen in utero, found no increased risk of birth defects with acetaminophen use. In women using acetaminophen specifically for febrile illness, there were decreased risks of various cranial and facial defects and gastroschisis; acetaminophen may be protective because fever increases the risk of these defects. A case series of 300 acetaminophen overdoses in pregnant women found no increased risk of congenital defects, stillbirth, or spontaneous abortions, regardless of trimester. At six weeks of life, the newborns had no evidence of hepatic or renal disease. Many trials study acetaminophen in combination with cold remedies, rather than as a single agent, making causality difficult. The available information on acetaminophen use does not establish fetal risks; therefore, as a single agent, it is safe for use during any trimester, especially as single dosing without routine use.
A meta-analysis of aspirin use in the first trimester did not demonstrate an increased risk of congenital anomalies, except for gastroschisis (odds ratio [OR] = 2.37). Early aspirin use at the time of conception or in the first several weeks of pregnancy does not increase the risk of spontaneous abortion. Aspirin has been studied extensively as a treatment for many chronic disorders in pregnant women, including thromboembolism, antiphospholipid disease, and preeclampsia. There can be risks of intrauterine growth retardation and fetal and maternal hemorrhage in the third trimester. Overall, aspirin should be avoided during organogenesis and in the third trimester unless a physician specifically prescribes it and the patient understands the risks and benefits.
In a recent study, neither ibuprofen nor naproxen increased the risk of spontaneous abortion when used in the first six weeks of pregnancy. A Swedish study of nonsteroidal anti-inflammatory drug (NSAID) use in early pregnancy did not demonstrate an increased risk of congenital anomalies overall; however, naproxen was associated with orofacial clefts, and all NSAIDs were associated with structural cardiac defects. More recent data show a potential association between NSAID use and dextro-transposition of the great arteries, particularly in the first trimester. NSAIDs are not recommended in the third trimester because of the risk of premature closure of the ductus arteriosus and subsequent primary pulmonary hypertension in the newborn. Because indomethacin is known to cause oligohydramnios and delay delivery, OTC NSAIDs are assumed to have the same risk. Although NSAID use is generally not recommended during pregnancy, women may ingest these medications inadvertently in many OTC combinations. Prolonged use of NSAIDs, including aspirin, should occur only for specific medical indications during pregnancy.
____
-2. Decongestants, Expectorants & Antihistamines:
Women commonly use cold medications during pregnancy. These medications, like most of the other OTC drugs, have not been studied well in pregnancy.
Table below shows Decongestants, Expectorants, and Nonselective Antihistamines in Pregnancy:
The most commonly used cold medications include decongestants and expectorants such as Pseudoephedrine, Guaifenesin and Dextromethorphan. Commonly used antihistamines are Diphenhydramine, Chlorpheniramine and Clemastine fumarate.
The use of vasoconstrictive agents such as Pseudoephedrine may activate alpha-adrenergic receptors, elevating blood pressure or causing vasoconstriction in the uterine arteries, and potentially adversely affecting blood flow to the foetus. This process could explain the reported association between the use of Pseudoephedrine in the first trimester and the development of gastroschisis, which is debatable, & evidence suggests that this effect is negligible at typical doses. Diphenhydramine is widely used in pregnancy as a sedative, an antihistamine, and an anti-nausea drug, although few data confirm its safety during pregnancy. The drug has been shown to have oxytocin like effects, especially in high doses. Dextromethorphan has been associated with birth defects in chicken embryos. The collaborative perinatal project monitored 50,282 pregnant women, 300 of whom were exposed to Dextromethorphan in the first trimester. Birth defects did not increase above the baseline rate. Thus, sufficient evidence indicates a lack of adverse effects of Dextromethorphan use during pregnancy. Guaifenesin has been associated with an increased risk of neural tube defects.
_
Up to 15% of women use an antihistamine during pregnancy to treat allergic rhinitis or nausea. Studies consistently show no significant risk of fetal malformations with first-generation antihistamines, and these agents are considered safe. The second-generation antihistamines loratadine (Claritin), cetirizine (Zyrtec), and fexofenadine (Allegra) do not appear to increase overall fetal risk. Four studies (n = 1,290) did not find significant fetal risk with cetirizine use. A slightly higher incidence of hypospadias with loratadine use was shown in one study (n = 1,700), but not in others (n = 2,147). Fexofenadine has been associated with early pregnancy loss in animal studies but has not been studied in human pregnancy. Fexofenadine is a metabolite of terfenadine, which was removed from the market in 1998 because of a risk of cardiotoxicity. Studies (n = 2,195) on the safety of terfenadine in human pregnancy did not show a significant risk of congenital malformation.
Data addressing the safety of topical antihistamines in pregnancy are limited to a single study of the ophthalmic agent pheniramine, which is contained in several OTC combinations with naphazoline. No significant malformations were observed in 831 women who used the medication in the first trimester. There are no data for other topical antihistamines, such as those in anti-itch creams; however, significant fetal risk is unlikely because of the lack of systemic absorption.
Table below summarises safety of Over-the-Counter Antihistamines, Decongestants, and Expectorants in Pregnancy:
|
Medication |
Drug class |
Pregnancy risk category |
Crosses the placenta? |
Use in pregnancy |
|
Diphenhydramine (Benadryl) |
First-generation (nonselective) antihistamine/antiemetic |
B |
Yes |
Possible oxytocin-like effects at high doses |
|
Brompheniramine |
First-generation (nonselective) antihistamine |
C |
Not known |
Limited data |
|
Chlorpheniramine |
First-generation (nonselective) antihistamine |
C |
Not known |
Drug of choice |
|
Pheniramine |
Ophthalmic antihistamine/decongestant (pheniramine 0.3%/naphazoline 0.025%) |
C |
Not known |
Limited data; likely low risk with limited use |
|
Cetirizine (Zyrtec) |
Second-generation (selective, nonsedating) antihistamine |
B |
Not known |
Acceptable alternative to first-generation agents |
|
Loratadine (Claritin) |
Second-generation (selective, nonsedating) antihistamine |
B |
Not known |
Acceptable alternative to first-generation agents |
|
Fexofenadine (Allegra) |
Second-generation (selective, nonsedating) antihistamine |
C |
Not known |
No human data, animal data suggest some risk |
|
Phenylephrine |
Sympathomimetic decongestant |
C |
Yes |
Safety not established, should be avoided in first trimester |
|
Pseudoephedrine |
Sympathomimetic decongestant |
C |
Not known |
Behind-the-counter purchase; possible association with gastroschisis, small intestinal atresia, and hemifacial microsomia; should be avoided in first trimester |
|
Guaifenesin |
Expectorant |
C |
Not known |
Safety not established, should be avoided in first trimester |
|
Dextromethorphan |
Nonnarcotic antitussive |
C |
Not known |
Appears to be safe in pregnancy |
______
-3. Antacids and Antidiarrheals:
Heartburn occurs in up to 80% of pregnant women by the end of the third trimester. Antacids containing aluminum, calcium, or magnesium are often considered first-line treatment in pregnancy. However, at high doses, antacids containing calcium can cause milk-alkali syndrome, and antacids with aluminum can cause neurotoxicity. Selective histamine H2 blockers have been used in all trimesters with no known teratogenic effects. In a meta-analysis of 2,398 women taking H2 blockers, the OR for congenital malformations was 1.14.
Proton pump inhibitors recently became available OTC. Although concerns have been raised about the potential teratogenicity of omeprazole (Prilosec), multiple large cohort studies have demonstrated its safety when taken before conception and during the first trimester. In a meta-analysis of 1,530 infants exposed to proton pump inhibitors, the OR for congenital malformations was 1.12 overall and 1.17 for omeprazole alone, and there was no increased risk of preterm birth or spontaneous abortion. In another study of proton pump inhibitor use in the first trimester (n = 5,082), the OR for birth defects was 1.10. Proton pump inhibitors and H2 blockers are considered safe in pregnancy.
Diarrhea and constipation are common during pregnancy. Most commonly used antidiarrhoeal medications include kaolin and pectin preparations, bismuth Subsalicylate, Loperamide, and Atropine/Diphenoxylate. Kaolin and pectin are safe during pregnancy as they are not absorbed. Products containing bismuth, mineral oil, and castor oil should be avoided. Bismuth itself is safe, but it has the same risks as aspirin when combined with salicylate. In a study of 89 women, loperamide (Imodium) did not increase the risk of malformation, but was associated with smaller infants. However, in a later study of 638 women, loperamide had an OR of 1.43 for congenital malformations. Although the American Gastroenterological Association considers loperamide to be low risk, it should be avoided when possible until further information is available. Saline laxatives may cause electrolyte sodium retention and should be used sparingly. Polyethylene glycol 3350 (Miralax) has minimal systemic absorption and is considered the drug of choice for chronic constipation despite a lack of research.
Table below summarizes the safety of OTC antacids, antidiarrheals, and laxatives in pregnancy.
|
Medication |
Drug class |
Pregnancy risk category |
Crosses the placenta? |
Use in pregnancy |
|
Cimetidine (Tagamet) |
Selective histamine H2 antagonist |
B |
Yes |
Potential weak antiandrogenic activity (only observed in animal studies) |
|
Famotidine (Pepcid) |
Selective H2 antagonist |
B |
Yes |
Limited human data |
|
Nizatidine (Axid) |
Selective H2 antagonist |
B |
Yes |
Limited human data |
|
Ranitidine (Zantac) |
Selective H2 antagonist |
B |
Yes |
May be preferable to cimetidine for chronic use |
|
Omeprazole (Prilosec) |
Proton pump inhibitor |
C |
Yes† |
Most human data suggest it is safe throughout pregnancy |
|
Aluminum hydroxide |
Antacid |
Not available |
Not known |
Considered safe in pregnancy; risk of neurotoxicity with high doses |
|
Calcium carbonate |
Antacid |
Not available |
Yes |
Drug of choice; risk of milk-alkali syndrome with high doses |
|
Magnesium hydroxide, magnesium carbonate |
Antacid |
Not available |
Not known |
Considered safe in pregnancy; magnesium may cause tocolysis in late pregnancy, but this is not a risk with over-the-counter preparations |
|
Simethicone (available as a single agent and contained in multiple combination antacids) |
Antiflatulent |
C |
No |
Limited data; not absorbed, so considered safe in pregnancy |
|
Bismuth subsalicylate (Pepto-Bismol) |
Antidiarrheal |
C |
Not known |
Insufficient data; should be avoided during pregnancy, especially in the second and third trimesters because it has a salicylate portion‡ |
|
Loperamide (Imodium) |
Antidiarrheal |
C |
Not known |
Limited human data; questionable association with cardiovascular defects |
|
Mineral oil |
Emollient laxative |
C |
No (not absorbed) |
Should be avoided in pregnancy, may interfere with absorption of fat-soluble vitamins§ |
|
Castor oil |
Laxative/oxytocic |
X |
Not known |
Should be avoided in pregnancy, potential for maternal/fetal morbidity |
|
Polyethylene glycol 3350 (Miralax) |
Osmotic laxative |
C |
Not known |
Drug of choice for chronic constipation |
†—Proton pump inhibitors as a class are rated FDA category B, including esomeprazole (Nexium), rabeprazole (Aciphex), and lansoprazole (Prevacid), based largely on animal data, which do not suggest any fetal risk; human data are limited.
‡—Hydrolyzes into bismuth salts and sodium salicylate in the intestinal tract. Sodium salicylate is not thought to suppress platelet function like the salicylate moiety found in aspirin; however, given the concerns over potential fetal toxicity from chronic salicylate exposure, avoidance in the latter half of pregnancy may be prudent.
§—The American Gastroenterological Association recommends avoidance presumably because of the risk of neonatal coagulopathy and hemorrhage arising from interference with maternal vitamin K absorption.
______
-4. Topical creams in pregnancy:
Topical antifungals are commonly used during pregnancy for treatment of vulvovaginitis. Imidazoles and nystatin are well studied and considered safe during pregnancy. Systemic absorption of imidazoles varies from 1% with miconazole to 10% with clotrimazole; nystatin is negligibly absorbed. Terbinafine (Lamisil) is sold OTC as a 1% cream. No studies are available for terbinafine cream; however, the oral form is pregnancy category B.
The commonly used topical antifungal and their safety is shown in table below:
One of the largest studies to date investigated the teratogenicity of Clotrimazole. The population-based, case-control study of 18515 cases pregnancy and 32804 controlled pregnancies didn’t show an association between foetal malformation and the use of Clotrimazole.
Hydrocortisone 1% is the only topical corticosteroid cream available OTC. Systemic absorption ranges from 1% to 7%, depending on the area treated and the underlying skin condition. Although potent topical corticosteroids may have increased risks in pregnancy, the mild OTC forms are considered safe. As with all steroid use, the lowest dose used for the shortest time possible is recommended.
Smaller studies have not shown an association between use of the topical antimicrobial bacitracin and fetal malformations. There are no studies regarding the safety of benzoyl peroxide use in pregnancy; however, the limited absorption of 5% suggests that it carries minimal risk. Overall, topical OTC antifungal, antimicrobial, and steroid creams are safe in pregnancy.
______
-5. Herbals and Dietary Supplements in pregnancy:
During pregnancy, herbal remedies are used for nausea, respiratory symptoms, urinary tract infections, pain, and other nonspecific issues. However, there are few human data on the safety of herbal remedies in pregnancy. The Dietary Supplement Health and Education Act of 1994 requires manufacturers to ensure the safety of supplements before marketing. However, there is no registration process with the FDA, which takes action only if a supplement is found to be unsafe after marketing. Herbals were not included in the NBDPS until the year 2000. According to a sub-analysis of the NBDPS, 10.9% of women use herbals during pregnancy, most commonly peppermint, cranberry extract, herbal teas, ginger, chamomile, Echinacea, ginseng, raspberry leaf, and ephedra products.
St. John’s wort is generally not recommended in pregnancy because of a lack of human data. Echinacea can be used topically or orally. A study with 112 women who used Echinacea in the first trimester showed no increased risk of malformations. Feverfew is used for migraine prophylaxis. It inhibits platelet aggregation and prostaglandin production and is contraindicated in pregnancy. Multiple herbals, such as mugwort, blue cohosh, black cohosh, goldenseal, juniper berry, chaste berry, rue, and pennyroyal oil, are uterine stimulants or abortifacients and should be avoided in pregnancy. Although ephedra is commonly used during pregnancy according to patient report, it has a significant association with birth defects. According to the NBDPS, ephedra is associated with anencephaly (OR = 2.8). Other weight loss products, with or without ephedra, are associated with dextro-transposition of the great vessels and aortic stenosis.
Glucosamine has been used by pregnant women with painful arthritis and appears to be safe. In a case-control study of 54 women, there was only one major malformation in the glucosamine group, which was comparable to the baseline rate of birth defects, and there was no difference in the risk of stillbirth, abortion, preterm birth, or other maternal morbidity. Ginger is commonly used in the first trimester and can be found in some prenatal vitamins. Although there have been concerns about ginger increasing the risk of spontaneous abortion or preterm delivery, this has not been demonstrated in animal studies. Two systematic reviews demonstrated that ginger improves pregnancy-related nausea more than placebo and as effectively as vitamin B6. Its effect on vomiting is less certain. No adverse effects have been noted for the mother or developing fetus. Ginger is the only dietary supplement that can be recommended based on human studies.
- In general, it is best to avoid OTC herbal and certain dietary supplements in pregnancy. These products are not regulated by the FDA and may have variable quality or be contaminated.
- Over-the-counter “natural” dietary supplements may contain unknown substances and few studies describing their use in pregnancy are available.
- However, prenatal vitamins that contain iron, calcium and folic acid are important in pregnancy, so don’t skip those supplements; take them as recommended by your doctor.
- Regular over-the-counter supplements may not have the right dose of vitamins that you need, so ask your doctor if you need a prescription for prenatal vitamins.
_____
-6. Caffeine:
Caffeine has not been linked to birth defects, but most health experts agree that low to moderate caffeine consumption in pregnancy is key, meaning less than 200 to 300 milligrams (mg) of caffeine per day (1-2 eight ounce cups of coffee). Remember to add up all of your sources caffeine throughout the day. An 8 ounce cup of regular black tea contains roughly 30 to 45 mg of caffeine. It’s important to remember that caffeine will also cross the placenta and stimulate a baby the same way it does an adult. It can cause increased heart rate, shaking, increased breathing rate, and difficulty sleeping following birth.
There have been reports of an increased risk of miscarriage and possibly infertility with higher caffeine doses or when combined with cigarettes or alcohol (both of which increase the risk independently). No reports have found an association between caffeine and birth defects.
Guarana is a caffeine-containing herbal supplement often found in energy drinks, and some medications may contain caffeine, too. Guarana has been reported to lead to low birthweight, birth defects, and premature birth. Experts recommend guarana not be used in pregnancy. Another herbal product with stimulant properties known as ephedra should also be avoided.
_______
_______
Acetaminophen in Pregnancy and Child’s ADHD Risk?
Pregnant women who take acetaminophen — best known under the brand name Tylenol — might be more likely to have a child with attention deficit-hyperactivity disorder (ADHD), a 2014 study suggests. Acetaminophen is the most commonly used over-the-counter medication for pregnant women who experience fever or pain. Children whose mothers took acetaminophen while pregnant had up to a 40 percent higher risk of being diagnosed with ADHD, according to the research, which involved more than 64,000 Danish mothers and their children. The kids were born between 1996 and 2002.
The UCLA researchers based their findings on the Danish National Birth Cohort, a nationwide study of pregnancies and children. The study’s aim is to examine pregnancy complications and diseases in children, with a specific focus on the side effects of medications and infections. The researchers studied more than 64,000 children and mothers. They tracked acetaminophen use through telephone interviews conducted up to three times during pregnancy and then six months after childbirth. The researchers then used Danish medical databases to see which children had been diagnosed with ADHD or were prescribed ADHD medications. They also used survey reports from parents to track whether the children had exhibited ADHD-like symptoms. The results showed that children whose mothers took acetaminophen had a 37 percent higher risk of receiving a hospital diagnosis of hyperkinetic disorder, a particularly severe form of ADHD, the researchers found. Those kids also were 29 percent more likely to use ADHD medication and 13 percent more likely to exhibit ADHD-like symptoms. In addition, the risk for hyperkinetic disorder/ADHD in a child was elevated at least 50 percent when the mother used acetaminophen for more than 20 weeks during her pregnancy.
In their analysis, the researchers took into account the possibility that children’s ADHD had been caused by maternal diseases that prompted the use of acetaminophen. They adjusted for these, and it did not wipe out the acetaminophen effect at all.
But because the study did not establish a cause-and-effect relationship, follow-up research is needed to verify the findings. Also, there are no prospective, randomized controlled studies demonstrating a causal link between acetaminophen use during pregnancy and adverse effects on child development.
Acetaminophen has more than 50 years of clinical use to support its safety and efficacy when used as directed, and it has one of the most favorable safety profiles among over-the-counter pain relievers. Acetaminophen is thought to be the safest analgesic and antipyretic medicine for pregnant women, and it is widely used all over the world. However, prenatal acetaminophen was reported to be associated with asthma, lower performance intelligence quotient (IQ), shorter male infant anogenital distance (predicting poor male reproductive potential), autism spectrum disorder, neurodevelopmental problems (gross motor development, communication), attention-deficit/hyperactivity disorder, poorer attention and executive function, and behavioral problems in childhood. Each article has poor power to show risks of acetaminophen. Experts are divided on the strength of the studies linking the medication to behavioral problems. None of them proved that the medications caused any of the health problems — they only showed an association. And untreated pain or fever in pregnancy can also carry risks to an unborn child. There is no alternative to acetaminophen. Acetaminophen should not be withheld from pregnant women for fears it might develop adverse effects. Acetaminophen should be used at the lowest effective dosage and for the shortest time.
______
______
OTC drugs during breast feeding:
Most drugs administered to lactating are detectable in breast milk. Fortunately the concentration of drug achieved in breast milk is usually low. Therefore the total amount the infant would receive in a day is substantially less than what would be considered as a therapeutic dose. If the nursing mother must take medication and the drug is relatively safe one, she should optimally take immediately after nursing and 3-4 hrs before the next feeding. This allows time for many drugs to be cleared out from the mother’s blood and the concentration in the breast milk will be relatively low. Drugs for which no data are available on the safety during lactation should be avoided or breastfeeding should be discontinued for a time being.
_
Transfer of Drugs into Breast Milk:
Most medications will transfer into breast milk; however, the degree of transfer depends on several factors. Drugs may transfer into milk if they attain high concentrations in maternal plasma, have a low molecular weight (<500 Da), are low in protein binding, and are lipid soluble. During the first week of breast-feeding, when colostrum is produced, there are large gaps between the alveolar cells that enhance the passage of drugs into milk. However, the quantity of milk produced at this time is low, so the absolute dose transferred is minimal. After the first week, the presence of prolactin closes the gaps, reducing the entry of most maternal drugs and other substances into the milk compartment.
_
Safety Data and Breast-feeding:
Unlike pregnancy, which has established FDA categories for medications, breast-feeding lacks standardized risk categories. Most of the data on medications and breast-feeding are from scientific literature. Given the lack of safety standardization, other recommendations for using medications while breast-feeding include choosing drugs with short half-lives, high protein binding, low oral bioavailability, or high molecular weight. Other options to decrease infant exposure to the drug are taking the medication immediately after breast-feeding and avoiding long-acting formulations. Additionally, a clinician should choose a medication with published safety data rather than a newly introduced medication.
_
Most antibiotics taken by the nursing mother is detected in milk. Drugs like tetracycline are highly concentrated in mother’s milk, up to 70% of maternal serum concentration and presents a risk of permanent tooth staining in the infant. Chloramphenicol on the other had doesn’t get as much into milk so as to cause Gray baby syndrome but presents potential risk to cause bone marrow suppression so it should be avoided during lactation. Anti TB drugs like Isoniazide rapidly reaches equilibrium between breast milk and maternal blood and the concentration reached is high enough to show signs of Pyridoxine deficiency. This can be avoided by giving Pyridoxine supplements to the mother. As for sedatives and hypnotics they achieve high concentration in milk sufficient to produce pharmacological effect in infants. Drugs like barbiturates, Chloral Hydrate and Diazepam can produce sedation, and poor suck reflex in infants. Opioid such as Heroin and methadone and morphine enters breast milk, which are potentially sufficient to prolong the state of neonatal narcotic dependence if it the drug is taken chronically by the mother during pregnancy. Lithium enters breast milk in concentration equal to those in the maternal serum. Radioactive substances can cause thyroid suppression and may increase the risk of thyroid cancer. Similarly breast-feeding should be avoided in mothers receiving cancer chemotherapy.
Note that most of these drugs are prescription drugs and not OTC.
_
Important Medications in Lactation period:
_
The American Academy of Pediatrics (AAP) policy statement on breast-feeding recommends that women breast-feed their infants exclusively for at least the first six months of life and suggests trying to breast-feed for the first 12 months of life. Breast milk is the perfect food for babies. It contains all the nutrients your baby needs in just the right balance, and it passes along the natural antibodies you’ve acquired, too, so your little one’s immune system gets off to a good start. But it can also contain some unwelcome added ingredients if you’re taking medicine for a cold or other ailment. Usually these medicines aren’t passed along in large enough quantities to do harm – about 1 percent or less of the dose will be contained in breast milk. But here are some general guidelines:
- Always check with your doctor before you take anything — whether the medicine you’re taking is from a prescription, an over-the-counter medicine you picked up at the drugstore, or an herbal remedy.
- If you can’t reach your doctor, ask the pharmacist at your drugstore. Be sure to tell your pharmacist about all the medicine or herbs you’re taking, not just the one you have a question about. That way you’ll know if the combination adds any risk.
- If a medicine is commonly prescribed for infants, it’s probably safe to take while nursing, because the amount the baby will get through breast milk is much less than a typical dose.
- Check the label to see what the “active ingredient” is — it may not be what you think. For example, Advil Cold and Sinus contains ibuprofen to help alleviate a sinus headache, but it also contains pseudoephedrine, which you may not want to take if you’re nursing.
- Take medicines either right after nursing or two to four hours before nursing. That way you won’t be nursing when the medicine is at its peak in your bloodstream.
- Don’t use extra-strength or sustained-release medicines — they remain in your bloodstream and milk supply much longer than medicine you need to take frequently. Always try to take the lowest effective dose for the shortest possible time.
- Use single ingredient medicines rather than multi-symptom formulas whenever possible. For example, if you need a cough suppressant, don’t buy one that also includes a decongestant. Read the label carefully to find out what symptoms the medicine treats, and ask the pharmacist if you have any questions.
- When you take any medicine, watch for physical or behavioral changes in your baby. These can include sleepiness, rashes, diarrhea, or fussiness. If you notice anything unusual or worrisome, call your doctor.
______
______
Section-13
OTC and driving accidents:
Over-the-Counter (OTC) drugs are medications that can be purchased without a doctor’s prescription for a variety of ailments and illnesses. They are sold at places like supermarkets, corner drug stores, convenience marts, department stores, truck stops, and even dollar stores like Dollar Tree or Dollar General. Commonplace OTC medicines include cold and flu medicines (e.g., NyQuil); allergy relief (e.g., Benadryl); and cough syrups (e.g., Robitussin). As the danger of serious and fatal drugged driving accidents continues to grow, so does the importance of drivers understanding that the inexpensive remedies they buy at the grocery store or pick up at the gas station may impair their ability to operate a motor vehicle (as well as a truck, van, semi-truck, bus, or boat, motorcycle, or plane).
_
It is a well-known fact that driving under the influence of alcohol is illegal, but not everyone realizes the dangers of driving after taking drugs — including prescription and over-the-counter medications. There’s more than one way to be under the influence. Impaired driving is generally associated with alcohol, prescription drug abuse, or illegal drug use. However, many legally obtained and commonly used over-the-counter and prescription drugs can affect a user’s ability to drive safely. Cold and allergy medicines, antidepressants, opioids, and sleep aids can cause side effects, including drowsiness, nausea, and blurred vision, all of which can put motorists at risk. Despite being illegal, impaired driving is still one of the most significant dangers on our roadways. According to results of the 2013–2014 National Roadside Survey; about 10% of weekday, daytime drivers surveyed tested positive for prescription and/or over-the-counter drugs. The survey tested for the presence of prescription or over-the-counter drugs, not driver impairment.
_
OTC Medicines and Stimulants can cause Dangerous Driving by Truckers:
The National Institute on Drug Abuse (NIDA) says prescription and OTC drugs are, after marijuana and alcohol, the most commonly abused substances by Americans age 14 and older. Yet the FMCSA tests truck drivers only for illicit amphetamines and methamphetamines, as well as drugs like marijuana, cocaine and opiates.
NIDA points out that while many people think OTC drugs are safer than illicit drugs, that’s only true when they are taken exactly as directed and for the intended purpose. For example, dextromethorphan, the active ingredient in many cold and cough medicines, when taken in very high doses, acts on the same cell receptors as PCP or ketamine, producing similar out-of-body experiences, NIDA says. Dextromethorphan is the OTC drug most frequently abused to achieve a high, according to the Office of National Drug Control Policy. But even in lesser doses, dextromethorphan can cause drowsiness, dizziness, lightheadedness, numbness, nausea and vomiting. Another frequently abused OTC stimulant is ephedrine, which is found in diet pills, including herbal or “natural” weight-loss products. Truckers seeking a little bit of help to stay awake and alert on a long haul may turn to diet pills for their stimulant effect. But ephedrine can also cause insomnia, which only increases fatigue, as well as blurred vision, anxiety and other adverse side effects, like diarrhea and vomiting.
The FMCSA’s Large Truck Crash Causation Study of 967 accidents across the nation says OTC drug use by the truck driver was a contributing factor in 19.4 percent of two-vehicle crashes involving large trucks and passenger vehicles.
_
FDA warns of Over-the-Counter (“OTC”) Medications and Drugged Driving Accidents:
In December 2019, the Food and Drug Administration (FDA) published a new warning about the risks of driving while under the influence of over-the-counter medications.
The FDA asks the question, “If you’re taking a medication, is it safe to drive?” The agency then goes on to report that there are not only prescription drugs but nonprescription medications that can cause physical reactions making it dangerous to drive, as the driver suffers from side effects that can involve:
sleepiness
drowsiness
blurred vision
dizziness
slowed movement
fainting
inability to focus
inability to pay attention
nausea
excitability.
For some drivers, the FDA explains, the problem passes quickly. For others, the OTC medication can impact their system for several hours – even overnight.
FDA List of Medicines that may Impair Driving:
The FDA warning provides a list of drugs that either alone or in combination with other medications may impact the driver’s ability to operate a motor vehicle. OTC drugs that endanger driving are listed in the FDA warning as follows:
products containing codeine;
some cold remedies and allergy products, such as antihistamines;
sleeping pills;
muscle relaxants;
medicines that treat or control symptoms of diarrhea;
medicines that treat or prevent symptoms of motion sickness; and
diet pills, “stay awake” drugs, and other medications with stimulants (e.g., caffeine, ephedrine, and pseudoephedrine).
Of particular concern, according to AAA studies, are OTC drugs for cold remedies and allergy relief. This is because most contain diphenhydramine, which is known to severely impair the ability to follow at a safe and constant distance, maintain speed, and maintain lane position.
From the AAA: “One single dose of this medication [OTC diphenhydramine] can have the same effect on driving as being above the legal limit for blood alcohol concentration.”
Cannabis and Cannabis-Derived Compounds are available as both prescription and over the counter products in some countries. In its latest warning, the FDA urges drivers to use caution if they intend to drive after ingesting any cannabis-related product, because it is known to have side effects that include sleepiness, sedation, and lethargy.
_____
CDC warning of drugged driving accidents involving older drivers:
Every day, more than 20 adults ages 65 and older are killed in traffic crashes. And almost 700 are injured, according to the Centers for Disease Control and Prevention (CDC).
That’s despite the fact that older adults generally are safer drivers than other age groups. Older drivers are more likely to:
Wear seatbelts.
Drive when conditions are safest.
Not drink and drive.
But older drivers are also more likely to be taking multiple medications. And that can be a problem. Medication side effects can affect your ability to react and stay alert behind the wheel. This is especially true of certain types of medicine, including heart medications, pain medications, muscle relaxers, sleep aids and mood medicines, among others. And the more medications an older driver takes, the more likely it is that they take some of these problematic pills.
According to CDC, if you take medicine that causes any of the side effects below, you may be at higher risk for crashes on the road:
Changes in vision.
Changes in awareness.
Poor balance.
Slower reaction time.
Fainting or passing out.
Muscle weakness.
Poor muscle coordination.
Tiredness.
Sleepiness.
Dizziness, light-headedness or fainting when standing up.
Difficulty concentrating.
_____
Can I still drive safely if I am taking medications?
Yes, most people can drive safely if they are using medications. It depends on the effect those medicines – both prescription and over-the-counter – have on your driving. In some cases, you may not be aware of the effects. But, in many instances, your doctor can help to minimize the negative impact of your medicines on your driving in several ways. Your doctor may be able to:
- Adjust the dose;
- Adjust the timing of doses or when you use the medicine;
- Add an exercise or nutrition program to lessen the need for medicine; and
- Change the medicine to one that causes less drowsiness.
_____
Prevention of drugged driving accident:
Despite the prevalence of prescription and over-the-counter (OTC) drug use, many individuals are unaware that some medications are potentially driver impairing. Current countermeasures to reduce drugged driving often focus on law enforcement. However, law enforcement alone is likely insufficient to prevent prescription- and OTC-drug-impaired driving. Because the pharmacy and medical communities are the primary providers of patient counseling concerning prescription and OTC drugs, they may be uniquely positioned to reduce prescription- and OTC-drug-impaired driving. Furthermore, education and advertising targeted to both practitioners and consumers may help increase knowledge about the risks and effects of potentially impairing prescription and OTC drugs.
______
______
Traveling Abroad with Medicine:
Many travelers must carry their medicines with them across international borders to treat chronic or serious health problems. However, each country has its own guidelines about which medicines are legal. Medicines that are commonly prescribed or available over the counter in the United States could be considered unlicensed or controlled substances in other countries. For example, in Japan, some inhalers and certain allergy and sinus medications are illegal. Also, the United Arab Emirates (UAE) has strict narcotics laws that have landed many travelers in prison, who were carrying painkillers containing codeine, OTC product in their country. While rules vary from country to country, there can be serious consequences if you violate the laws of the country you’re visiting. These consequences can range from confiscation (removal) of your medicine, which could harm your medical treatment, to stiff penalties, including imprisonment on charges for drug trafficking. Flying with herbal medicines or supplements to international destinations can be tricky since each country has its own laws about what’s allowed in. To find out what may be restricted in the countries you’ll be visiting or transiting through, refer to the embassy website or contact local consulates. Make sure herbal remedies and Ayurvedic medicines are in clearly labeled, well-sealed containers, preferably in original bottles.
_____
_____
FAA OTC Medication No-Go List for Pilots:
FAA issued a detailed OTC Drug List for Pilots to consider before operating a plane, called the “Go or No Go List.” Included on the FAA Pilot “No Go” list for OTC Drugs are these popular OTC medications:
Advil PM (contains diphenhydramine)
Benadryl (contains diphenhydramine)
ChlorTrimeton (contains chlorpheniramine)
Coricidin HBP Cough & Cold (contains chlorpheniramine)
Dayquil (contains dextromethorphan)
Delsym (contains dextromethorphan)
Dimetapp (contains brompheniramine)
Imodium (contains loperamide)
Nyquil (contains doxylamine)
Tylenol PM (contains diphenhydramine)
Unisom (contains doxylamine)
Xyzal (contains levocetirizine)
Zyrtec (contains cetirizine)
Zzzquil (contains diphenhydramine).
Of course, all of these OTC medications have been approved for public use by the FDA. However, as the FAA warns pilots, just because a drug is “FDA-Approved” does not mean that it is not going to impair the person who takes the medication. The OTC drug may not be “compatible with flying or even driving.” As explained by the FAA: “Some medications are not recommended (“NO GO”). If you choose to fly on medication, be certain that it will not impair safety. Do not simply hope for the best.”
_______
_______
Section-14
OTC use in pets:
Human drugs available without a prescription are known as over-the-counter (OTC) medications. Exposures to OTC drugs and supplements in pets can be accidental or intentional. A valid client-patient-veterinarian relationship must exist for veterinarians to recommend extra-label use of these drugs to their clients. Most are not approved for veterinary use by the FDA, and safety of most OTC drugs has not been determined in animals. Veterinarians should understand the potential risks of using OTC medications and communicate these risks to their clients.
If your dog is in pain, finding a solution to treat and manage discomfort is an essential part of providing care. But should you give your dog over-the-counter (OTC) pain medicine meant for humans?
The answer is simply—no.
When it comes to pain medicine for dogs, you should never give your dog over-the-counter pain medicine. Human-grade NSAID medications (such Aspirin and Ibuprofen) and products containing acetaminophen (such as Tylenol) should not be given to dogs as a way to treat pain. These OTC pain medications can cause serious health problems in dogs and cost pet parents hundreds or thousands of dollars in expensive treatments.
NSAID medications may cause gastrointestinal bleeding and ulcers in dogs. Tylenol or other pain medications containing acetaminophen can lead to liver and kidney damage. If your pet accidentally ingests OTC pain medications, it’s important to seek immediate veterinary care.
Dogs who are experiencing pain may mask their symptoms, so if a pet is showing any signs of discomfort—such as stiffness, whimpering or yelping, and a lack of appetite—it’s important to see your veterinarian to discuss the treatment options. Your vet may recommend a pet pain medication to help your dog cope.
Any medication, even over-the-counter ones, should only be give to pets after consultation with a veterinarian.
______
______
Section-15
Over-the-counter Medications & Covid-19:
Acetaminophen, also called paracetamol helps to reduce fevers and can definitely help manage muscle pain and body aches associated with Covid-19. Acetaminophen doesn’t treat the virus itself, nor does it reduce the duration of your illness. A lot of people feel pretty miserable from a fever, which means a fever reducer like acetaminophen is definitely an option for some relief. That said, make sure you don’t take more than what’s specifically listed on the label because higher doses can be dangerous to your liver.
Ibuprofen is one of several common medications implicated in coronavirus disease 2019 (Covid-19) severity. On March 11, the Lancet Respiratory Medicine published a letter stating ibuprofen can increase angiotensin-converting enzyme 2 expression. On March 14, the French Minister of Health tweeted that ibuprofen should be avoided because it will aggravate Covid-19. This concern was echoed by scientists and senior doctors in the British Medical Journal news on March 17. In response, the World Health Organization (WHO) issued a recommendation on March 18 to avoid ibuprofen in people with symptoms of Covid-19. However, the WHO reversed this recommendation the next day because of insufficient evidence. Health Canada issued a safety alert on March 20 stating there was no evidence that ibuprofen worsens Covid-19 symptoms.
There was some concern early on in the coronavirus outbreak that ibuprofen and drugs like it might worsen outcomes for coronavirus patients, but so far we haven’t seen anything to support that. Ibuprofen is recommended when fevers are high or people are feeling really miserable. However, you should still be careful: take ibuprofen with food and if you have any underlying kidney disease or ulcer disease, you may not want to reach for ibuprofen.
Naproxen is another NSAID (like ibuprofen) that can reduce inflammation and lower your fever. It cannot treat Covid-19 itself, but it can certainly help you feel better. Naproxen is similar to ibuprofen, except that it lasts longer. For many people, that means a single pill can keep your temperature down for up to 12 hours and help stave off body aches. But remember, if your doctor has told you not to take medications like ibuprofen or naproxen before, you shouldn’t take either one now.
_
Indian government advised pharmacies to sell paracetamol (acetaminophen), anti-cold and anti-allergic OTC products only after the buyer produces a prescription during covid-19 pandemic due to incidents of people taking paracetamol to temporarily reduce body temperatures to get through thermal scanning without much hassle and people taking self-medication for covid-19 symptoms to avoid testing for covid-19. But such advisory has put many people who need paracetamol for pain and antihistamines for seasonal allergies into trouble.
_
Could preemptive OTC painkiller doses interfere with the Covid-19 vaccine?
Getting a Covid-19 vaccine these days can be a bit of a pain, in more ways than one. As a result, you may be tempted to take some pain relievers before or after vaccination. Acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen can help combat the arm soreness, fever, chills, nausea, fatigue, headaches, and other side effects that you may experience from vaccination. But should you be taking such medications for such side effects?
The side effects of currently available Covid-19 vaccines, which are generally mild, are spurred by the way the vaccines activate the body’s defense system to build immunity to the new coronavirus. In other words, these side effects are evidence the vaccine is doing exactly what it should be doing—and that’s why some experts are cautious about preemptively taking painkillers.
Specifically, experts are concerned that taking painkillers may slow the immune system’s response to the vaccine, reducing the vaccine’s effectiveness.
Indeed, as detailed in a publication in the Journal of Virology, laboratory studies using human cells in culture dishes and mice have suggested that NSAIDs may reduce antibody production against SARS-CoV-2 and dampen your immune system’s inflammatory response. And a 2016 study from Duke University found children who received OTC painkillers before receiving their childhood vaccinations had fewer antibodies than those who hadn’t taken painkillers—although their antibody levels remained sufficient to provide protection.
Colleen Kelley, an associate professor of medicine at Emory University School of Medicine, theorized the altered immune system response could be due to the painkillers reducing inflammation caused by the immune system. “The immune system generates a response through controlled inflammation. Pain relievers can reduce the production of inflammatory mediators,” she said. “So, this is the potential mechanism for a reduced immune response to vaccination if you take these medications.”
That being said, it is not yet clear whether such pain relievers should be completely avoided when getting the Covid-19 vaccine. A review published in the journal Evidenced Based Practice of 13 randomized controlled trials found no clear evidence that giving kids acetaminophen or ibuprofen before vaccination reduced the effectiveness of traditional childhood vaccines such as those protecting against diphtheria, tetanus, and hepatitis B.
In light of this uncertain and conflicting evidence, many public health experts, as well as CDC and the World Health Organization (WHO), presently recommend against taking OTC painkillers before receiving a Covid-19 vaccine.
“You always would like an optimal response to your vaccine,” William Schaffner, an infectious disease specialist and professor of preventive medicine at Vanderbilt University Medical Center, said. “We are recommending that unless people have a substantial reaction to the first dose that they hold their [painkillers].” Schaffner added one caveat: If you’ve been taking OTC painkillers for another condition, you should continue taking them, as stopping them could cause more harm than good.
Is it OK to take a painkiller after receiving the vaccine?
As for taking painkillers after a Covid-19 vaccination, CDC recommends patients first monitor for any side effects—but if any do present, both CDC and WHO said it should be fine for people to use OTC painkillers. If fever, chills, headaches develop after injection, people can use OTC painkillers.
All in all, it is probably best to avoid pain relieving medications around the time of your Covid-19 vaccination. What can you do about Covid-19 vaccine side effects without taking pain relievers? Well, there’s the CDC advice of applying a “clean, cool, wet washcloth.” Other CDC advice includes using or exercising your arm if you are experiencing any discomfort there. The CDC website mentioned drinking plenty of fluids and dressing lightly as well. Lighter clothing can better allow the surrounding air to cool your body.
I have developed fever after covid vaccine but I did nothing and just continued with life. I did not take acetaminophen or ibuprofen. I trusted my immune system and did not interfere with its functioning.
______
______
Section-16
OTC drug interactions:
Over-the-counter (OTC) medicines are those you can buy at the store. You don’t need a prescription from your doctor. They help you feel better by treating or preventing common health problems. These could include pain, allergies, constipation, cold and flu, or nausea. But sometimes OTC medicines can cause unpleasant effects. These are called untoward effects. They include:
- side effects (adverse effects)
- interactions (drug-drug, drug-food etc.)
- allergic reactions
It is best to be aware of the risks of OTC medicines so that you know how to avoid them.
Side effects [vide infra]:
Side effects are effects that medicines have on your body that don’t help your symptoms. Most side effects are unpleasant. A few examples are nausea, dizziness, or bleeding in your digestive tract. Sometimes, side effects can be useful. For example, certain antihistamines can cause sleepiness. This might be bad for people who take antihistamines during the day. But if you’re taking an antihistamine at nighttime, this side effect might help you get the sleep you need. Side effects are not the same thing as true drug allergies. Those are much less common.
Drug-drug interactions:
The body processes every medicine differently. When medicines are used together, the ways they affect the body can change. This is called a drug-drug interaction. It happens whether they are prescription or OTC medicines. It can increase the chance that you will have side effects from medicines you are taking. The main interaction types are:
- Duplication: This is when you take 2 medicines that have similar active ingredients. It can give you more medicine than you need. An example is when you take OTC ibuprofen (Advil, Motrin) plus a prescription anti-inflammatory medicine. Too much of either an anti-inflammatory or pain reliever can hurt your kidneys or liver.
- Opposition: Medicines with active ingredients that have opposite effects on your body can interact. This may reduce the effectiveness of one or both medicines. For example, OTC decongestants may raise your blood pressure. This can work against (cause opposition to) medicines that lower your blood pressure.
- Alteration: One medicine may change the way your body absorbs, spreads, or processes another medicine. For example, aspirin can change the way some prescription blood-thinning medicines work. Antacids reduce absorption of prescription or OTC medicines.
Drug-food interactions:
Food may change how your body processes some OTC or prescription medicines. This is called a drug-food (or drug-nutrient) interaction. Grapefruit and grapefruit juice can interfere with some prescription drugs, and even a few non-prescription drugs. Don’t drink grapefruit juice with certain blood pressure-lowering drugs because it can cause higher levels of those medicines in your body, making side effects more likely. Licorice probably seems like a harmless snack, but if you’re taking Lanoxin (digoxin) for congestive heart failure and abnormal heart rhythms, some forms of licorice could increase your risk of Lanoxin toxicity. Licorice may also reduce the effects of diuretic drugs, including hydrochlorothiazide and spironolactone. If you’re taking any sort of medication, avoid alcohol, which can increase or decrease its effect.
Food does not affect all OTC medicines. But what you eat and when you eat it does matter with some medicines. This is why some medicines should be taken on an empty stomach. That means 1 hour before or 2 hours after eating. At the same time, some medicines are absorbed or processed better when you take them with food.
Read the drug facts label carefully. See if you should take your medicine with food or on an empty stomach. If the label doesn’t give specific instructions, it probably doesn’t matter when you take it. If you have any questions, ask your family doctor or pharmacist. They can also warn you about possible interactions with your prescription medicines.
Allergic reactions:
It’s not common, but some people are allergic to certain medicines which are particularly problematic in OTC combination products that contain aspirin. About 10% of the population is allergic to aspirin and other non-steroidal anti-inflammatory medicines. Signs of an allergic reaction include itching, rash, hives, and breathing problems. If you’ve ever had an allergic reaction to a medicine, avoid medicines that contain the same ingredients. Call your doctor or seek immediate medical help if you think you’re having an allergic reaction. Keep in mind that side effects are not true allergic reactions.
______
Drug interaction:
A drug interaction is a change in the action or side effects of a drug caused by concomitant administration with a food, beverage, supplement, or another drug. A cause of a drug interaction involves one drug which alters the pharmacokinetics of another medical drug. Alternatively, drug interactions result from competition for a single receptor or signaling pathway. Both synergy and antagonism occur during different phases of the interaction between a drug, and an organism. For example, when synergy occurs at a cellular receptor level this is termed agonism, and the substances involved are termed agonists. On the other hand, in the case of antagonism, the substances involved are known as inverse agonists. The risk of a drug-drug interaction increases with the number of drugs used.
Drug Interaction Risks:
Not all drugs interact with each other. However, the more medications a patient takes, the more likely it is that some medications will interact with each other. With proper oversight from a doctor, it can still be possible to take multiple medications safely and avoid interactions.
The drug-interaction risks are:
15 percent for two medications
40 percent for five medications
80 percent for seven medications
This is a particular concern for older people, as close to half take five or more medications, and 12 percent take 10 or more. Over a third (36%) of the elderly in the U.S. regularly use five or more medications or supplements, and 15% are at risk of a significant drug-drug interaction.
____
Interactions with OTC:
Many people neglect to mention their use of over-the-counter (OTC) drugs to their doctor or pharmacist. Drugs taken intermittently, such as drugs for colds, constipation, or an occasional headache, are mentioned even less often. Health care practitioners may not think of asking about use of OTC drugs or medicinal herbs when they are prescribing or dispensing a prescription. Yet many OTC drugs and medicinal herbs can interact adversely with a wide range of drugs.
A drug-disease interaction is an event in which a drug that is intended for therapeutic use causes some harmful effects in a patient because of a disease or condition that the patient has. The potential for OTC medicine-disease interaction may be underestimated by OTC users. For instance, 70 percent of hypertensive patients surveyed had taken an OTC product during the previous two weeks, while less than 20 percent of these same individuals were aware that some OTCs could influence their blood pressure. Similarly, non-steroidal anti-inflammatory drugs (NSAIDs) can potentially exacerbate the symptoms of asthma. However, one-third of asthmatic patients interviewed by Lamb et al still took aspirin or NSAIDs to treat some minor ailments. Only 27 percent of the asthmatic patients who bought OTCs would think of informing the pharmacist that they had asthma. More than 25 million Americans (nearly 8%) have asthma, and about 10% of those individuals have a sensitivity to aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit cyclooxygenase-1, also known as COX-1 inhibitors. The sensitivity may precipitate an asthma attack or severe bronchospasm in response to ingestion or inhalation of an NSAID, according to the American Academy of Allergy, Asthma & Immunology.
Prescription-OTC drug interactions exist and OTC users need to realize the potential for their occurrence. In a paper on the frequency of daily OTC drug use and clinically significant OTC-prescription drug interactions in the Finnish adult population, Sihvo et al determined that 4 percent of OTC users (on average) may be potentially hurt by those interactions. The paper highlighted the potential for interactions with ketoprofen, followed by ibuprofen and acetylsalicylic acid (ASA). Two studies by Honig and Gillespie found that many OTC drugs has the potential for clinically significant interactions with other drugs. Their examples of potentially problematic OTC groups were antacids, H2-blockers, salicylates, NSAIDs, cough/cold/allergy remedies, and antiasthma products.
Some of these interactions can be serious, interfering with the effectiveness of a drug or causing side effects. For example, taking aspirin with the anticoagulant warfarin can increase the risk of abnormal bleeding. An antacid containing aluminum or magnesium can reduce the absorption of digoxin, taken for heart disease. Taking a multiple vitamin and mineral supplement can interfere with the action of some prescription drugs. For example, the antibiotic tetracycline may be ineffective if swallowed with a product that contains calcium, magnesium, or iron.
Although the dangers of the common analgesics are relatively well-known (paracetamol causing liver damage, gastrointestinal upset with ibuprofen), patients are often unaware of the potential for adverse effects with other preparations. Nor are they always aware that many compounds combine two analgesics, for example, paracetamol and aspirin, or paracetamol and ibuprofen. Nonsteroidal anti-inflammatory drugs (NSAIDs) interfere with renal clearance and may result in elevated lithium levels with resultant toxicity. Combined use of an antidepressant or sodium valproate with an OTC could lead to abnormal liver function tests attributed solely to the former agents and not the OTC. In transplant patients, self-medication with St John’s wort (hypericum perforatum) may lead to a drop in plasma levels of the immunosuppressant drug cyclosporine, causing tissue rejection.
OTC drug-drug interactions have not been studied systematically. Many serious problems have been discovered accidentally, after side effects or deaths were reported. Even when interaction warnings are printed on the label for OTC drugs, the language may be meaningless to most people. For example, the labels of some cold remedies that contain pseudoephedrine caution against using the product with a monoamine oxidase inhibitor (MAOI—used infrequently for depression and certain other medical problems) or during the 2 weeks after discontinuing the MAOI. For the many people who do not know that the antidepressant they are taking is an MAOI (such as phenelzine and tranylcypromine), this important warning is not helpful.
The best way to reduce the risk of drug-drug interactions is to ask the pharmacist to check for them.
Additionally, the doctor should be told about all drugs being taken, both prescription and OTC.
Drug Overlap:
Another potential problem is drug overlap. Over-the-counter products used to treat different problems may contain the same active ingredient. Unless people read the labels on everything they take, they can accidentally overdose themselves. For example, a person who takes a sleep aid and a cold remedy, both of which contain diphenhydramine, may take double the dose considered safe. Many products contain acetaminophen. A person who simultaneously takes two different products that contain acetaminophen—one for a headache and another for allergies or sinus problems—may exceed the recommended dose.
Over-the-counter (OTC) drug labels contain information about ingredients, uses, warnings and directions that is important to read and understand. The label also includes important information about possible drug interactions. Further, drug labels may change as new information becomes known. That’s why it’s especially important to read the label every time you use a drug.
______
How drug interactions happen:
The term “drug interactions” is somewhat misleading. Drugs don’t combine in the body to produce chemical reactions. Instead, a drug, supplement, or food may affect how long a medication stays in the body, often by stimulating or inhibiting the production of specific enzymes in the liver or intestine. These enzymes are part of the cytochrome p450 (CYPs) system, which plays an important role in metabolizing many medications. Drug interactions usually occur in one of the following ways:
- Interactions with other drugs.
Interactions between two drugs occur when one drug affects the cytochrome enzyme that process the other. For example, the antibiotic metronidazole (Flagyl) inhibits the enzyme CYP2C, which breaks down warfarin (Coumadin). If the two medications are taken together, the blood thinner can linger in the body, increasing the risk of severe bleeding. The anti-seizure drug phenytoin (Dilantin) stimulates excess production of CYP3A4, the enzyme that metabolizes estradiol, a component of low-dose contraceptives. In this case, the contraceptives are cleared more rapidly in women taking phenytoin, reducing their effectiveness and increasing the chance of an unplanned pregnancy.
In other instances, two drugs taken for different purposes may have the same effect, producing something like an overdose. A common example of this is when over-the-counter pain relievers—aspirin, ibuprofen (Motrin), naproxen (Aleve)—are taken with blood thinners like warfarin and clopidogrel (Plavix). Since the pain relievers also have anti-clotting effects, the combination can result in severe bleeding.
- Interactions with nutrients.
Several foods can also block or stimulate the enzymes that break down drugs. People who wash down atorvastatin (Lipitor) or simvastatin (Zocor) with large amounts of grapefruit juice may experience muscle pain and other side effects from statin “overdose,” because the juice inhibits the enzyme that clears the statins. Fish oil supplements can have a similar effect when taken with warfarin, increasing the risk of severe bleeding. Iron supplements can diminish the effects of levothyroxine (Synthroid), the medication used to treat an underactive thyroid.
- Interactions with your body.
Your body itself may react to drugs in unexpected ways. There is more than one version of each of the 50 or so CYP450 enzymes, and there is no easy or reliable way to identify which ones you have inherited. You may have reactions to certain drugs because you break them down faster or slower than most people do. Also, as we age, we tend to metabolize drugs more slowly, so that a dose lower than the one usually recommended may be sufficient. Kidney or liver disease can also slow the rate at which drugs are metabolized. For those reasons, it’s important to carefully monitor your reaction to any new drug you take.
________
How can I avoid interactions?
-1. Know what is in each medicine you take. To do this, carefully read the labels of all prescription and over-the-counter (OTC) medicines to learn the active and inactive ingredients they contain.
-2. Know the benefits and risks of the medicines you take. For OTC medicines, be sure to read the “Warnings” and “Other Information” sections of the Drug Facts label.
-3. Ask your doctor or pharmacist if it is safe to take a medicine with your condition(s) or with other medicines, vitamins, or herbal products you take.
-4. Keep an up-to-date list of all the prescription and OTC medicines, vitamins, and herbal products you take. Bring the list with you anytime you visit your doctor’s office, pharmacy, or hospital.
-5. If possible, purchase all of your prescription and OTC medicines from one pharmacy.
_____
_____
OTC analgesics and drug interactions: 2008 study:
A survey of medication use patterns in the United States has found that more than 80% of American adults used at least one over-the-counter (OTC) or prescription drug each week, and that 25% used at least 5. The OTC analgesics acetaminophen, ibuprofen, and aspirin are among the most frequently utilized medications, used by approximately 17% to 23% of the population each week. Chronic OTC analgesic use is most common in the elderly, many of whom take nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen for relief of pain.
Because of the widespread availability and perceived safety of OTC analgesics, self-medication with these agents has become commonplace. Many patients are unaware of the potential for toxicity and adverse drug interactions associated with the long-term and inappropriate use of OTC analgesics. They may use OTC analgesics in higher-than-recommended doses or in combinations that magnify the risk of adverse interactions. Additionally, patients may not be aware that common cough, cold, or flu medications can contain OTC analgesics. Although OTC analgesics are associated with adverse effects in only a small percentage of people, the widespread use of these drugs makes even a small increase in population risk a clinically relevant issue. Physicians can help patients avoid possible drug-drug interactions with commonly used OTC analgesics by providing counselling on the proper use of these agents.
_
Table showing potential drug interactions with OTC analgesics:
|
Drug combinations |
Effect |
Management options/considerations |
|
Aspirin and NSAIDs or multiple NSAIDs |
Increased risk of serious GI complications. Risk increases with increased dose and number of agents |
Avoid concurrent use of more than one NSAID, if possible. Consider adding gastroprotective agents |
|
Anticoagulants and NSAIDs |
Increased risk of bleeding (especially GI) and increased oral warfarin activity |
Avoid concurrent use of NSAID; monitor prothrombin time and occult blood in urine and stool |
|
Corticosteroids and NSAIDs |
Increased GI side effects, including ulceration and hemorrhage |
Avoid concurrent use of NSAID and consider adding a gastroprotective agent |
|
SSRIs and NSAIDs |
Increased risk of GI bleeding |
Avoid concurrent use of NSAID |
|
Low dose aspirin and ibuprofen or naproxen |
Reduced antiplatelet effects of aspirin |
Not seen with other NSAIDs or acetaminophen |
|
Antihypertensive agents and NSAIDs |
Use of NSAIDs may increase blood pressure |
Monitor blood pressure and cardiac function |
|
Antidiabetic agents (e.g., sulfonylureas) and aspirin |
Increased hypoglycemic effect |
Avoid concurrent use and monitor blood glucose concentration |
|
Lithium and NSAIDs |
Increased steady-state lithium concentration and lithium toxicity |
Monitor lithium concentrations. Interactions are less likely with aspirin than with naproxen or ibuprofen |
|
Methotrexate and NSAIDs |
Reduced renal clearance. Increased plasma methotrexate concentration |
Avoid NSAIDs with high-dose methotrexate |
GI = gastrointestinal; NSAID = nonsteroidal anti-inflammatory drug; OTC = over the counter; SSRI = selective serotonin reuptake inhibitor.
_
Many patients are unaware that over-the-counter (OTC) analgesics can cause potentially serious adverse effects when used in combination with other common medications such as anticoagulants, corticosteroids, or antihypertensive agents. Of particular significance is the increased risk of upper abdominal gastrointestinal adverse events in patients who take traditional nonsteroidal anti-inflammatory drugs (NSAIDs). This risk is dose dependent and further increased in patients who take more than one NSAID or use NSAIDs in combination with certain other medications. Some NSAIDs may also mitigate the antiplatelet benefits of aspirin and may increase blood pressure in patients with hypertension. Clinicians should be aware of potential drug interactions with OTC analgesics when prescribing new medications. Additionally, patients should be properly counselled on the appropriate and safe use of OTC analgesics.
______
______
Clinically significant drug interactions with antacids: 2011 update:
One may consider that drug-drug interactions (DDIs) associated with antacids is an obsolete topic because they are prescribed less frequently by medical professionals due to the advent of drugs that more effectively suppress gastric acidity (i.e., histamine H (2)-receptor antagonists [H2RAs] and proton pump inhibitors [PPIs]). Nevertheless, the use of antacids by ambulant patients may be ever increasing, because they are freely available as over-the-counter (OTC) drugs. Antacids consisting of weak basic substances coupled with polyvalent cations may alter the rate and/or the extent of absorption of concomitantly administered drugs via different mechanisms. Polyvalent cations in antacid formulations may form insoluble chelate complexes with drugs and substantially reduce their bioavailability. Clinical studies demonstrated that two classes of antibacterials (tetracyclines and fluoroquinolones) are susceptible to clinically relevant DDIs with antacids through this mechanism. Countermeasures against this type of DDI include spacing out the dosing interval – taking antacid either 4 hours before or 2 hours after administration of these antibacterials. Bisphosphonates may be susceptible to DDIs with antacids by the same mechanism, as described in the prescription information of most bisphosphonates, but no quantitative data about the DDIs are available. For drugs with solubility critically dependent on pH, neutralization of gastric fluid by antacids may alter the dissolution of these drugs and the rate and/or extent of their absorption. However, the magnitude of DDIs elicited by antacids through this mechanism is less than that produced by H2RAs or PPIs; therefore, the clinical relevance of such DDIs is often obscure. Magnesium ions contained in some antacid formulas may increase gastric emptying, thereby accelerating the rate of absorption of some drugs. However, the clinical relevance of this is unclear in most cases because the difference in plasma drug concentration observed after dosing shortly disappears. Recent reports have indicated that some of the molecular-targeting agents such as the tyrosine kinase inhibitors dasatinib and imatinib, and the thrombopoietin receptor agonist eltrombopag may be susceptible to DDIs with antacids. Finally, the recent trend of developing OTC drugs as combination formulations of an antacid and an H2RA is a concern because these drugs will increase the risk of DDIs by dual mechanisms, i.e., a gastric pH-dependent mechanism by H2RAs and a cation-mediated chelation mechanism by antacids.
______
_______
The effects of mixing Alcohol and Over-the-Counter Drugs:
Many drugs interact with alcohol, whether is it cocaine, a prescription painkiller, or an over-the-counter remedy for cold symptoms. Consumer Reports found that there are over 100 legal drugs, both OTC and prescription, that interact badly with alcohol, making social drinking dangerous if a person takes these medicines.
When combined with these drugs, alcohol may contribute to an overdose or poisoning, damage to internal organs, or just uncomfortable side effects like nausea or dizziness. A survey from the National Institutes of Health found that, among 26,000 adults who responded, 42 percent drank while also taking drugs that could cause problems when mixed with alcohol.
While it may seem obvious that drugs like prescription anti-anxiety medicines or blood thinners could cause serious side effects, most people assume that OTC medications are safe – after all, that’s why they are readily available on a nearby drug store shelf and lightly regulated. Not only do these drugs interact poorly with alcohol, they may, in some cases, contain some alcohol.
_
The National Institute of Alcohol Abuse and Alcoholism explains that since OTC medicines are drugs (and have drugs in them), and alcohol itself is a drug, the combination often leads to undesirable reactions in the human body, especially when following factors are taken into consideration:
Age
Gender
Body weight
Previous substance use
Physical health
Family history of medical and mental health issues
_
The ways a person’s body may interact with OTC medications and alcohol together can depend on the ingredients in the OTC drug. However, there are three basic types of reactions to mixing drugs:
-1. Duplication: Two medicines, or a medicine and alcohol, that have similar effects will compound each other’s effects.
-2. Opposition: Alcohol may cause one effect while an OTC medication will cause another. This can reduce the effectiveness of the OTC medication, or it can damage certain body systems due to stress.
-3. Alteration: Alcohol can change how the OTC drug is absorbed by the body, or vice versa.
_
Specific Drugs and their side effects with Alcohol:
There are certain types of over-the-counter drugs that do not mix with alcohol.
-1. Nonsteroidal anti-inflammatory drugs (NSAIDs): Drugs like ibuprofen or acetaminophen can cause stomach upset if a person takes them with alcohol once. If this occurs repeatedly, the individual is likely to develop stomach ulcers, bleeding from the stomach lining or intestines, and liver damage. Heart rate can raise, which is dangerous for those who may have a heart condition.
- Acetaminophen by itself can lead to overdose, including liver damage and failure, if a person takes more than 4,000 mg of the drug. When mixed with alcohol, the risk of liver damage increases.
- Ibuprofen and naproxen do not harm the liver as much as acetaminophen can, but they may damage the stomach lining. The risks of this damage increase if a person consumes alcohol, leading to gastritis (inflammation of the stomach lining), which may lead to ulcers and bleeding. They may also damage the kidneys, especially when their potency is increased because of alcohol.
-2. Allergy, cold, and flu medications: Drugs in Sudafed, Tylenol Cold and Sinus, or similar medications can increase drowsiness or dizziness. When mixed with alcohol, the intoxicating drink may be more bioavailable, so it enters the bloodstream at higher volumes. This puts the person at risk of becoming drunker faster, so they may not drive safely, could fall, or experience another accident. There is also an increased risk of overdose.
-3. Cough syrup: Decongestants and cough suppressants in OTC cough syrups may contain up to 10 percent alcohol, so mixing these with a casual drink or two increases the risk of intoxication. Some also contain dextromethorphan (DXM), a cough suppressant that has largely replaced codeine in OTC cough medicines. In high doses, DXM can be an intoxicating drug, and mixing it with alcohol increases DXM’s potency.
_____
_____
Over-the-Counter (OTC) Medications and High Blood Pressure (hypertension):
Always read the labels on all over-the-counter medications, especially if you have high blood pressure (hypertension). Look for warnings to those with high blood pressure and to those who take blood pressure medications. If you have high blood pressure and certainly if you are on prescription medication, consult your healthcare professional before taking any OTC medications or supplements.
-1. Be careful with supplements or natural (naturopathic) remedies:
There are no special pills, vitamins or drinks that can substitute for prescription medications and lifestyle modifications. Talk to your healthcare provider before taking any over-the-counter drug or supplement that claims to lower your blood pressure. They may not work as advertised and/or interfere with other medications. In fact, some can even raise your blood pressure.
-2. Decongestants may raise your blood pressure:
People with high blood pressure should be aware that the use of decongestants may raise blood pressure or interfere with the effectiveness of some prescribed blood pressure medications. Be aware of over-the-counter cold and flu preparations that contain decongestants such as:
- Oxymetazoline
- Phenylephrine
- Pseudoephedrine
-3. Check the sodium content:
Some over-the-counter medications are high in sodium, which can also raise blood pressure. Look at the active and inactive ingredients lists for words like “sodium” or “soda.” Note the amount of sodium in the medication. People with high blood pressure should consume less than 1,500 mg of sodium per day from all sources — one dose of some OTCs can contain more than a whole day’s allowance.
-4. Other drugs and substances that can raise your blood pressure include:
- Alcohol
- Amphetamines
- Antidepressants
- Atypical antipsychotics (for example, clozapine and olanzapine)
- Caffeine
- Oral contraceptives
- Non-Steroidal Anti-Inflammatory Drugs (NSAIDs, for example ibuprofen and naproxen sodium)
- Recreational drugs
- Systemic corticosteroids (for example, prednisone and methylprednisolone)
-5. Herbal supplements can affect blood pressure:
Remember to tell your healthcare provider about any herbal supplements you take or are thinking about taking, to see if the supplement could raise your blood pressure or interact with blood pressure medications. Examples of herbal supplements that can affect your blood pressure or blood pressure medications include:
Arnica (Arnica montana)
Bitter orange (Citrus aurantium)
Ephedra (ma-huang)
Ginseng (Panax quinquefolius and Panax ginseng)
Guarana (Paullinia cupana)
Licorice (Glycyrrhiza glabra)
St. John’s wort (Hypericum perforatum)
Herbal supplements aren’t necessarily safe just because they’re natural. Check with your healthcare provider before taking any herbal supplements. You may need to avoid supplements that raise your blood pressure or interfere with your blood pressure medications.
______
______
OTC CBD Drug Interactions:
The rise in popularity for CBD has been paralleled by CBD’s availability in most countries. People can purchase CBD products almost anywhere, from gas stations to online platforms. In virtually every country that permits legal CBD sales, CBD is treated differently compared to THC from a regulation and public policy standpoint. Whereas only two countries on earth allow sales of THC products for adult use, dozens of countries now allow CBD in one form or another.
CBD (cannabidiol) is seemingly everywhere, with oils, tinctures, pills, chocolates, gummy bears, and creams available all over the internet, at national drugstore chains, and perhaps at your local farmer’s market — even if you don’t live in a state where medical or recreational marijuana is legal.
CBD, a type of chemical known as a cannabinoid, is a main ingredient in hemp, one type of cannabis plant. Marijuana, another type of cannabis plant, also has some CBD but an abundance of THC (tetrahydrocannabinol), an intoxicating cannabinoid known for making users feel “stoned” or “high.” While CBD won’t get you high, it interacts with cannabinoid receptors in your body and may have effects that are sought by people with arthritis, such as pain relief, reduced inflammation, and improvements in sleep and anxiety.
According to CreakyJoints research presented at the 2019 Annual European Congress of Rheumatology meeting, 52 percent of respondents reported having tried CBD for a medical reason. Of those who did, 93 percent said it helped. More than half said they wanted more information on CBD from their doctor, but 58 percent of those who told their doctors about their CBD use did not get the information on safety, effectiveness, and dosing they were looking for.
One common concern among people with chronic illness who use CBD is whether CBD can interfere with prescription drugs they may take for arthritis or other conditions. There is no significant drug-drug interaction with drugs commonly used in arthritis treatment, such as methotrexate, and most nonsteroidal anti-inflammatory drugs (NSAIDs).
A huge number of medications, including CBD, are broken down by the same large family of liver enzymes, called CYP450. CBD inhibits some enzymes in this family. This makes them break down certain drugs more slowly, which could potentially increase side effects unless your doctor adjusts the dose. On the other hand, CBD induces other enzymes in this family, which speeds the breakdown of certain drugs so they may potentially be less effective unless the dose is increased.
As examples, you may experience increased side effects if CBD is used along with these drugs:
Antidepressants (such as fluoxetine, or Prozac)
Medications that can cause drowsiness (antipsychotics, benzodiazepines)
Macrolide antibiotics (erythromycin, clarithromycin)
Heart medications (some calcium channel blockers)
The most direct information comes from studies on the only FDA-approved CBD product, Epidiolex, which is used to treat rare forms of epilepsy. Epidiolex has been found to increase blood levels of the blood thinner warfarin about 30 percent, which raises the risk of bleeding. It also interacts with other medications used for epilepsy.
_
According to a recent study conducted in the United Kingdom, the amount of CBD in a product varies widely. The study was conducted by a team of investigators at King’s College in London and examined the safety and effectiveness of commercially marketed CBD products that are commonly available over-the-counter. The researchers found that the contents of the CBD products involved in the study were of “variable quality” and that the amount of CBD in the products was significantly below the doses used in controlled clinical trials.
“Although there is enormous consumer interest in CBD, there is little evidence that OTC preparations have significant pharmacological activity or provide health benefits. … Controlled trials of OTC preparations are needed to address this issue. There is also a need for more accurate labelling and advertising of OTC CBD products,” the researchers concluded.
______
______
OTC drugs and diabetes management:
-1. Choosing the right OTC medication can be challenging for patients with diabetes, because many contain carbohydrates that can affect blood glucose levels, as well as ingredients that can interact with the prescription medications they are taking. In addition, diabetes is often listed in the “warnings” section of the Drug Facts label as a condition requiring the patient to consult with a doctor before use. Unfortunately, unlike the Nutrition Facts label, the Drug Facts label does not list the amount of carbohydrates contained within the medication. Carbohydrate content can vary significantly between medications. Reviewing the inactive ingredients can help patients to identify if the medication contains carbohydrates, but will not provide how much.
_
-2. There is a myriad of over-the-counter (OTC) products that claim to lower blood sugar or increase insulin sensitivity, including banaba leaf extract, chromium, cinnamon, fenugreek, ginseng, magnesium, probiotics, vitamin D, to name a few. Although these can be a part of a healthy balanced diet and may have some benefits, they are not recommended as a form of treatment for glycaemic management. It is always a good idea to discuss any OTC medications or supplements with a health care provider as they may interact with medications already being taken.
_
-3. Glucosamine and cinnamon (at doses recommended on commercial products) should have minimal impact on diabetic management, whereas St. John’s wort is a concern involving potential drug interactions. For colds, of about 11 active ingredients, only decongestants (primarily oral) need be considered for their possible effects on blood sugar. Finally, NSAIDs (even at OTC doses) must be used with caution, given their cardiovascular, renal and gastrointestinal risks.
_
-4. Tablet and capsule formulations are preferred over liquids, when possible. Liquid medications often contain added sugar or sweeteners to enhance the palatability. Without the carbohydrate content listed on the Drug Facts label, it is difficult for clinicians and their patients to determine the amount of carbohydrate in the medication. Only a few resources are available to determine the carbohydrate content of OTC medications.
Alcohol is also often added to liquid OTC medications, to serve as a solvent, vehicle, and preservative. For example, NyQuil Cold and Flu contains 10% alcohol, the equivalent of a 20 proof beverage or as much alcohol as some glasses of beer or wine. Patients with diabetes should be notified that alcohol can cause hypoglycemia.
_
-5. Special diabetes formulated products are not always the only choice. If patients are concerned about the effects of an OTC medication on their blood glucose levels, medications labeled as “sugar-free” or “for people with diabetes” can be recommended. The Drug Facts label must be reviewed to determine that the active ingredients within the medication will appropriately treat the patient’s symptoms. Otherwise, a product that does not state “sugar-free” but does have active ingredients to treat the symptoms would be more appropriate.
_______
_______
Section-17
OTC drugs side-effects, risks and misuse:
_
Medications obtained by patients for treatment of common ailments, without a prescription from a physician, are known as over-the-counter (OTC) or non-prescription medications. OTC medications provide prevention and treatment for a wide range of conditions, including but not limited to headaches, common cold, musculoskeletal pain, allergies, tobacco dependence, and heartburn. However, there is always a risk involved in using OTC medications. These include improper self-diagnosis, inappropriate dosage, addiction issues upon prolonged use, adverse drug reactions, and drug interactions. As most patients do not discuss their OTC medications with a physician, they are unaware of the risks associated with OTC medications. In addition, direct-to-patient advertising increases the exposure of medications to patients. As a result, there is increased product use in the absence of professional help.
_
Just because OTC drugs are available without a prescription doesn’t make them safe for everyone. Dosage instructions must be followed exactly to avoid possible overdose, side effects and dangerous drug interactions.
Consider these facts:
-Acetaminophen can damage the liver.
-Caffeine can cause increased heart rate, nervousness, and high blood pressure.
-Chlorpheniramine maleate, an antihistamine, can cause drowsiness, dizziness, nervousness, nausea, and irregular heartbeat.
-Dextromethorphan hydrobromide and related compounds can cause hallucinations, psychotic behavior (behavior associated with a dangerous loss of contact with reality, sometimes leading to violence against oneself or others), and an inability to move.
-Diphenhydramine hydrochloride can cause nervousness, anxiety, hallucinations, muscle tremors, and irregular heartbeat.
-Pseudoephedrine hydrochloride and related decongestants can cause nervousness, sleeplessness, dizziness, and anxiety.
-Sucrose can bring on a coma in people suffering from diabetes.
-Users of motion sickness preparations need to take certain precautions. The drugs do not work after motion sickness symptoms start, so it is necessary to take the medication at least fifteen to thirty minutes before the bothersome motion begins. The pills usually work for eight to ten hours. Pregnant women and nursing mothers should consult a doctor before taking these drugs.
_
A side effect is usually regarded as an undesirable secondary effect which occurs in addition to the desired therapeutic effect of a drug or medication. Side effects may vary for each individual depending on the person’s disease state, age, weight, gender, ethnicity and general health.
Misuse is defined as the intentional use, by a patient or consumer of a product, OTC or prescription medicine, for a therapeutic purpose other than as prescribed or not in accordance with the authorized product information. Drug misuse is used to distinguish improper or unhealthy use from the use of medicine as prescribed or in accordance with the authorized product information. OTC drugs are relatively safe when used in accordance with the authorized product information but cause adverse effects when misused.
Drug abuse is specifically defined as the intentional, non-therapeutic use by a patient or consumer of a product, OTC or prescription medicine, for a perceived reward or desired non-therapeutic effect including, but not limited to, getting ‘high’ (euphoria). OTC drug abuse will be discussed in next section.
The terms ‘misuse’ and ‘abuse’ are often used interchangeably, but they have precise meanings in this context. Misuse is defined as using an OTC product for a legitimate medical reason but in higher doses or for a longer period than recommended, e.g., taking more of a painkiller than recommended to treat headache. Abuse is the non-medical use of OTC drugs, e.g., to experience a ‘high’ or lose weight.
Drug abuse often follows drug misuse, although it is a separate entity unto itself.
Those who abuse medication are often not prescribed what they are taking. They take medication to “feel high” or to chase a euphoric feeling. Drug abusers develop a tolerance and feel they must take more and more to “feel normal”. Drug abusers are simply looking to get high, and not treating any specific ailment.
Although drug misuse versus drug abuse have similarities, there are important differences. OTC medication abuse involve use of non-prescription medications for non-medical purposes while OTC medication misuse involves medication used for medical purposes but used incorrectly, for example, incorrect dosage, lack of interactions knowledge, inappropriate medication use, and incorrect duration of use. The consequences of each are different and serious. Drug abuse can lead to serious adverse consequences including drug addiction and death. To the casual observer, misuse and abuse seems to be “splitting hairs” and semantics, but the difference is important, real, and significant.
_
Misuse of Non-prescription Medications:
Table below lists the most common active ingredients with negative health consequences if used incorrectly.
_
Side effects of various OTC drugs:
-1. OTC allergy medication
There are more OTC options to treat allergies today than ever before, and that’s a good thing for the most part, but people don’t always choose wisely and even when they choose the right OTC medication for their allergies, they may not be using it correctly.
Many people reach for OTC antihistamines when they start to sniffle and sneeze. Antihistamines block histamine, a chemical released by your immune system when you have an allergy attack. Two older antihistamines, diphenhydramine (Benadryl) and chlorpheniramine, tend to cause drowsiness. These older medications don’t last 24 hours like the newer generation of allergy medications. To avoid drowsiness and the need for repeat dosing, select a 24-hour antihistamine. These are less sedating, more effective, and one pill gives you 24-hour coverage. Newer antihistamines include cetirizine (Zyrtec), desloratadine (Clarinex), fexofenadine (Allegra), and loratadine (Claritin). Other side effects seen with antihistamines may include dry mouth and constipation. These side effects are rare, but they are more common with seniors and people taking older antihistamines. Children may have nightmares and become restless and irritable if they take antihistamines for allergies. These side effects are much less pronounced with the newer antihistamines.
-2. OTC cold and flu products
Many OTC cold and flu products contain decongestants along with other ingredients. These can increase your heart rate and shouldn’t be taken if you have high blood pressure. Decongestants may also cause nervousness, dizziness, and trouble sleeping, according to the American Academy of Family Physicians (AAFP). Some cold and flu products also contain acetaminophen (the generic name for Tylenol) or non-steroidal anti-inflammatory drugs (NSAIDs, which includes drugs like aspirin and Advil) to treat aches, pains, and fever. At high doses, acetaminophen can cause liver problems, or even liver failure, particularly when consumed with alcohol.
-3. OTC heartburn remedies
There are a host of OTC medications that can help fight heartburn including antacids, which neutralize stomach acid; H2-receptor blockers, which curb the amount of acid your stomach makes; and proton pump inhibitors, which block acid production and heal the esophagus.
The H2-receptor blocker commonly known as Zantac, and many of the generic versions (ranitidine), were recalled in late 2019 because they contained very low levels of a compound called N-nitrosodimethylamine (NDMA), which is classified as a probable human carcinogen based on lab testing. According to the U.S. Food and Drug Administration (FDA), it depends on the manufacturer—not all versions of the drug may contain the cancer-causing agent.
Omeprazole (Nexium 24HR; Prilosec OTC) is a proton pump inhibitor that should be taken as directed. Omeprazole may be associated with an increased risk of Clostridium difficile–associated diarrhea (CDAD). A diagnosis of CDAD should be considered for patients taking PPIs who develop diarrhea that does not improve.
-4. OTC anti-diarrheal treatments
Imodium, or the generic Loperamide, is often used to put the brakes on common types of diarrhea. If you have an infectious or inflammatory cause for diarrhea—such as C.diff infection or ulcerative colitis—and you try to stop it by taking large doses of loperamide, you run the risk of potentially causing toxic megacolon. This is a life-threatening condition that generally requires emergency removal of your entire large intestine. There’s also a risk of causing a fatal heart rhythm if you take an excessive amount of Imodium.
Studies show that patients with opioid use disorder are at a high risk of dying from the misuse of the common OTC anti-diarrheal medication Imodium. Patients addicted to opioids use Imodium, and other similar medications containing loperamide, to help ease opioid withdrawal symptoms. Individuals with opioid use disorder have also found that using extremely high doses of this medication can cause a chemical “high” like that experienced with opioids. Loperamide is cheap, legal, and can easily be bought in large quantities.
-5. Aspirin and other NSAIDs
Aspirin is an NSAID that can reduce pain, fever, and inflammation. Children under the age of 12 should never take aspirin because it increases their risk of Reye’s syndrome, a rare, but serious illness that can affect the brain and liver. Aspirin is also a blood thinner, which is why people sometimes take low-dose aspirin to stave off heart attacks and strokes. It can also increase your risk of ulcers and bleeding for the same reason.
Aspirin, as well as OTC NSAIDs including Advil, Motrin (ibuprofen), and Aleve (naproxen sodium), affect clotting and can cause bleeding. These risks are higher among people who are older than 60, are taking prescription blood thinners or steroids, have a history of stomach bleeding or ulcers, and/or have other bleeding problems. Aspirin and other NSAIDs can also be dangerous to your kidneys, especially if you have kidney disease. These medications can block blood flow to your kidneys and long-term use of higher doses may be dangerous.
Vioxx and other prescription COX-2 blockers are NSAIDs that were developed to be safer on the stomach than traditional NSAIDs, but it soon came to light that they may spare the stomach at the expense of the heart, increasing the risk for heart attack and stroke. If you have risk factors for heart disease such as high blood pressure or diabetes, minimize your use of NSAIDs to be safe. In fact, Vioxx was pulled off the market and many patients use Celebrex (celecoxib) instead which has some heart risk but was also designed to protect the stomach.
-6. Acetaminophen
As said earlier, acetaminophen is the active pain-fighting ingredient in Tylenol and many other OTC pain killers, prescription pain killers, and OTC cold and flu products. The main concern with acetaminophen, also known as paracetamol outside of the U.S., is liver damage. The U.S. Food and Drug Administration (FDA) states that the maximum recommended adult dose of acetaminophen is 4,000 milligrams per day. If you take a cold and flu product and then a pain killer for a headache or arthritis pain later on, you may inadvertently double up on acetaminophen and damage your liver. Protect yourself by reading the label and knowing exactly what you are taking, he says. (Acetaminophen is also found in prescription pain drugs like Percocet and Vicodin.) Individuals with fatty liver disease, liver failure, or those who consume excessive amounts of alcohol shouldn’t take acetaminophen.
-7. OTC sleeping aids
Certain OTC drugs including some sleep aids, antihistamines (such as Benadryl and Unisom), and anti-diarrhea medicines fall under the umbrella of anticholinergic medications. Anticholinergic drugs block the action of the neurotransmitter acetylcholine, which is involved in memory and learning. In a 2015 JAMA Internal Medicine study, which involved 3,434 participants, 65 years or older with no dementia diagnosis at the start of the study, researchers found taking anticholinergic medications at higher doses or for a long period of time upped the risk for dementia including Alzheimer’s disease. Furthermore, the researchers noted that the risk may not be reversible even if you stop taking these drugs. A study published in JAMA Network in 2019 focused on the long-term use of anticholinergic medicines and increased risks of dementia in those aged 55 or older. Researchers looked at data on 58,769 dementia patients and 225,574 control subjects, along with information on 56 drugs with strong anticholinergic properties that had been prescribed to patients 1 to 11 years prior to a dementia diagnosis. The authors concluded, “Exposure to several types of strong anticholinergic drugs is associated with an increased risk of dementia. These findings highlight the importance of reducing exposure to anticholinergic drugs in middle-aged and older people.”
Alzheimer disease and other dementias are notoriously challenging to treat. While some drugs may slow cognitive deterioration, none will reverse the condition. And, for many, it’s hard to even know if the medication is working. Older people often take multiple medications, and this can spell trouble for dementia patients.
A 2020 report in The Lancet estimates that roughly 50 million people around the world live with dementia, and the number is projected to increase to 152 million by 2050. To make matters worse, other common drugs may lead to side effects that mimic dementia symptoms, making it even more difficult to diagnose and treat.
Several physicians described dementia patients whose increasing levels of confusion appeared to have been caused by a litany of medications they’d been prescribed. The phenomenon, known as “medication fog,” may be a bigger problem than we think.
Various studies reporting an association between cumulative anticholinergic drug use and incident dementia in subjects over the age of 65 years caused something of a media stir on both sides of the Atlantic. In the UK and US the news focus was on OTC drugs, such as hay fever and sleep medicines, some of which contain drugs with anticholinergic properties. However main finding of these studies was the observation that many-year cumulative dose-response relationship showed that higher anticholinergic use is associated with an increased risk for dementia and Alzheimer’s disease. Furthermore these studies were based on records of prescribed medicine use rather than OTC use. Indeed the increased risk was only found in people who took the equivalent of a fairly potent anticholinergic drug daily for several years – which would certainly not be a typical pattern of OTC drug use.
______
______
Concerns about OTC medications:
The concerns about misuse, adverse effects, dependence (especially to sedatives, analgesics, antacids, laxatives), drug resistance, and delayed diagnosis of underlying conditions due to use of OTC medicines pose formidable challenges.
_
Even though OTC medicines are generally safe, they still have side effects. Caranasos et al found that 18 percent of all hospitalizations resulted from adverse reactions caused by OTC drugs during a three-year period. Litovitz and Manoguerra determined that from 1985 to 1989, about 670,000 reports related to adverse effects and overdoses were received by poison control centers in children younger than 6 years old. These cases included analgesics, cough/cold remedies, and gastrointestinal products.
Another recent FDA warning asked doctors to stop prescribing combination drugs that exceed 325 mg of acetaminophen per dose. Acetaminophen, a common fever and pain reliever found in many OTC drugs, is combined with opioids such as codeine, hydrocodone, and oxycodone in prescription painkillers. The problem is that “many consumers are often unaware that many products (both prescription and OTC) contain acetaminophen, making it easy to accidentally take too much. Too much acetaminophen can cause irreversible liver damage.
A correspondence just published in The New England Journal of Medicine suggests that combining acetaminophen and the nasal decongestant phenylephrine (PE) may cause serious side effects. The combination raises PE levels in the blood as much as four times, according to New Zealand researchers. Elevated PE levels can lead to dizziness, insomnia, and increased blood pressure.
_
As per the National survey data submitted by Substance Abuse and Mental Health Services administration (SAMHSA) in US, about 3.1 million people aged 12 years and older have misused OTC medications at least once in their lifetime (3.7% in < 18 years old). A meta-analysis by Frei et al., threw light on the fact that over half a million Australians use analgesics over the counter for nonmedical purpose and OTC medicines are the third most common form of substance abuse in Australia. Similarly, in Canada, it was found that OTC medicines were widely misused. Nearly one in five adult users exceed the maximum recommended dose of over-the-counter painkillers such as Advil and Aleve in a one-week period, according to study published in Pharmacoepidemiology and Drug Safety. In India, abuse of OTC medicines is not well documented. One report mentions that cough syrups and antihistamine medications sold over the counter are a prevalent form of drug abuse in India. I have seen few patients addicted to cough syrups.
_
Despite their importance, instructions for use mentioned on the labels of OTC medicines are often not adequate, filled in small space and are missed by patients. As a result, patients do not follow the instructions. An interesting study from India assessed whether labels of 100 nonprescription medicines complied with the requirements mentioned in the US FDA guidelines. It was found that 87% of the labels lacked information regarding contraindications. In 90% of the labels, adverse effects and in 96% information about their use during pregnancy and breastfeeding were missing. Overall, the label instructions were inadequate and had the potential of harming the health of patient. Even reading the label does not guarantee insight and understanding of what is on offer. Labels are carefully and handsomely packaged by advertisers to persuade people their product is better than conventional medicines. Most consumers spend little time reading the labels about ordinary foodstuffs, never mind the chemical constituents of OTCs.
_
In another study using deception conducted by Salunkhe et al., a total of 263 pharmacies were visited. The investigators concealed their identity and pretended to suffer from sore throat/acute diarrhea. The questions asked to the pharmacists were whether they can get some strong medication to relieve symptoms and also antibiotics? In 76% of participants, antibiotics were dispensed (azithromycin for sore throat and nitroimidazoles for diarrhea). Even though antimicrobials belong to the category of prescription drugs in India, they are dispensed without a prescription raising the concern regarding antimicrobial resistance. The ready availability of OTC drugs places the weakest individuals at greatest risk—these are the patients from low socio-economic strata, the elderly and others taking multiple medications, and some illiterate individuals who are incapable of evaluating safety information mentioned on the drug label. Studies in US have shown that more than 60% of people cannot identify the active ingredient in the OTC drugs. In addition, about 40% of Americans believe that OTC medicines are too mild to cause any real harm. They also fail to report use of OTC medicines to health care providers. In a study done in UK on use of ibuprofen, 555 patients were followed up and surprisingly one fourth of the patients were found to use it for more than 8 weeks. Among them 4% had active peptic disease and 7% had asthma. Since the doctor’s prescription is not required, the term OTC gives a false sense of safety to the patients. There are no proper documented studies regarding awareness about nonprescription drugs in India and no regulatory act for OTC medicines, but even in western world, there is inadequate awareness among masses about optimum use of OTC medications.
______
______
Can OTC cause death?
The over-the-counter painkiller you routinely take isn’t harmless, and can lead to serious consequences, including liver failure and even death. You should take great care to keep close track of every single pill you take. Whether you take a vitamin, painkiller, antibiotic, cough medicine, or supplement, it has the possibility to interact with other medicines you take, as well as what you eat or drink.
Many drugs that were available only by prescription in the past are now available over-the-counter with no health professional guiding their usage. In addition, due to the change in oversight by the federal government — from the Food and Drug Administration (FDA) to the Federal Trade Commission (FTC) — the way the drug is presented changes. A study published in the Journal of American Medicine found that the way drugs were presented to the public changed dramatically after they switched from prescription to OTC. Positive presentation rose from 83 to 97 percent while the presentation of negative effects dropped from 70 to 11 percent. In addition, warnings of side effects were allowed to be listed in small print on the label.
Some of the most dangerous examples of OTC drugs and combinations:
- Acetaminophen.
A single regular Tylenol contains 325 mg of acetaminophen, and an extra-strength tablet contains 500 mg. For the average, healthy adult, the maximum recommended dose over a 24-hour period is 4 grams. Liver damage occurs at 7 grams. Most people take two or three at a time. If you take three pills five times a day, you’re already over the safe dosage limit with the regular tablets, and into the possibility of liver damage with the extra-strength pills. It can be very easy to overdose on acetaminophen because it’s contained in many OTC products, including cold and flu treatments. Combining medications, such as acetaminophen tablets with cough syrups and cold medicines that also contain the painkiller, can quickly put you in the danger zone. Always read labels. In addition, many common prescription pain relievers contain acetaminophen.
The Food and Drug Administration has linked at least 980 deaths in 2013 to acetaminophen. From 2006 to 2013, acetaminophen killed more people than all other over-the-counter (OTC) drugs combined. Further, the FDA reports that acetaminophen-related deaths are rising faster than those for other OTC drugs. Since 2006, those dying accidentally from taking too much acetaminophen surpassed the number of those committing suicide via intentional overdose.
- Laxatives (sodium phosphate).
The Food and Drug Administration issued a warning about sodium phosphate laxatives, saying there had been 54 reports of serious side effects and 13 deaths among people who overdosed or had coexisting health conditions. These laxatives can cause dehydration or abnormal levels of electrolytes in the blood, leading to kidney damage. According to the FDA, people most at risk include young children, adults over 55, and patients using medications that may affect kidney function.
- Cough and cold medicines.
Dextromethorphan is a common ingredient in cough medicines, but it can be deadly if taken in higher than recommended amounts. It can lead to impaired judgment, dizziness, loss of coordination, nausea and vomiting, respiratory depression, coma, and death.
- Aspirin.
After being recommended many years by health professionals to prevent heart and stroke, The National Institutes of Health issued a warning to doctors advising them to reconsider routinely recommending patients take aspirin daily. Taking a daily aspirin, even at the low dose of a baby aspirin, can cause bleeding in the gastrointestinal tract. It can even cause bleeding in the brain of vulnerable patients.
Just because a drug is sold over the counter doesn’t mean it is harmless. Patients believe these drugs are safe, but they can be deadly.
_____
_____
Issues related to monitoring the safety of over-the-counter (OTC) medicines, a 2003 study:
Pharmaceutical advances over the past 50 years have benefited many people in terms of disease prevention and management. However, probably without exception, most pharmaceutical products can cause adverse consequences of varying severity and frequency. In the last 10 years, many medicines that were originally prescription only have now become available over the counter (OTC), either from pharmacies or other general retail outlets. The volume and value of OTC medicine sales have increased accordingly. These switches have been well regulated and based on clear criteria and evidence of safety. Benefits of the changes include increased convenience to patients, greater self-management of minor ailments and a reduction in government drug expenditure. However, there are important differences between medicines supplied OTC and on medical prescription. With OTC medicines there is generally less healthcare professional input into the recommendation or ongoing monitoring of use. There is an absence of records per se, or linkage to other medication records elsewhere, and most countries allow direct-to-consumer advertising of the product. Taken together these differences can result in inappropriate expectations, demand and use of the OTC medicines, with limited opportunity for ongoing patient follow-up and monitoring of safety. Methodologies for pharmacy-based epidemiological studies of OTC medicines need to be developed. Studies should be large enough to detect associations that might exist, and to consider other explanations for associations such as chance, bias or confounding. There have already been some pilot studies with encouraging results with respect to follow-up rates. Outcome data however have usually been self-reported and the studies have lacked a suitable comparison group. Purchasers and suppliers of OTC medicines should also be made aware of, and encouraged to use, existing systems for spontaneous reporting of suspected adverse events, such as the Yellow Card Scheme in the UK. While available OTC medicines are perceived to be generally safe, problems have occasionally arisen with some earlier switched products (e.g., terfenadine). There have also been concerns about some traditional herbal and homeopathic remedies such as St John’s wort. While such adverse events are rare, they emphasize the need for healthcare professionals and the public to understand and manage such risks. Many doctors are unaware of the range of OTC preparations available, and therefore do not consider them as a possible cause of presenting symptoms. Neither do they take them into account when making a new prescribing decision. The public need to be aware that OTC medicines should be treated with the same care as prescribed medicines, and that advice on recommended dose, contraindications and interactions should be adhered to.
____
____
OTC antibiotic sell and antibiotic resistance:
The problem of self-medication is usually investigated by community surveys that allow for valuable information on antibiotic consumption to be collected, especially for low-income countries. The investigation of the self-medication attitude revealed widespread antibiotic use without following medical instructions or medical consultation. Although most oral antibiotics are prescription-only, they are commonly sold OTC in many poor nations.
An assessment of community antibiotic use in six low- and middle-income countries (LMICs) in Asia and Africa indicates that antibiotics are frequently obtained without a prescription, more so in Asia than Africa. Still, access to antibiotics, the situations in which they are used, and reasons for using them without a prescription varies by country. In a study published recently in The Lancet Global Health, an international team of researchers found that more than a third of individuals and households in Bangladesh, Vietnam, Thailand, Ghana, Mozambique, and South Africa reported obtaining antibiotics without a prescription or self-medicating with antibiotics. Use of antibiotics without a prescription was most widespread in Vietnam and Bangladesh, and antibiotics in general were more widely available in the Asian countries. The authors say the findings point to several different interventions that could be pursued. Non-compliance with laws regarding antibiotic sales—found to be widespread in the low-income and lower middle-income countries but not in the two upper middle-income countries, Thailand and South Africa—suggests better enforcement of those laws, using context-adjusted models, could be part of the solution. Clear labelling, so that consumers can distinguish an antibiotic from other commonly sold medications, is another. And given the role that drug stores were found to play, they also suggest that community pharmacists need to be educated about their role promoting prudent antibiotic use, and empowered to play that role.
Irrational over-the-counter drug distribution by private pharmacists has been one of the key factors for increase in the number of drug-resistant TB cases in India. High illiteracy further compounds the problem because most people without any knowledge of tuberculosis infection directly approach private pharmacies for medicines. Most pharmacies do not have any trained staff and pharmacists often leave the shop in care of subordinates to distribute drugs. Therefore treatment and reporting practices often do not meet national or international standards for tuberculosis. In The Lancet Infectious Diseases, Madhukar Pai and colleagues used the standardised patient method to access over-the-counter antibiotic distribution by pharmacists in urban India. The results showed a substantial effect on tuberculosis management, based on referral rates of patients to health centers. Low referral rates of suspected cases only show high over-the-counter drug abuse in these patients, thereby increasing the risk of drug resistance before initiation of treatment.
Haddadin et al conducted a cross-sectional observational study based on a pool of pharmacies in Jordan to investigate the patterns of dispensed antibiotics in terms of appropriateness of drug indications, dosage and duration of treatments over a period of four months from February 2016 to May 2016. The results showed that around one-third of dispensation were without prescription and based on pharmacists’ recommendation or on patients’ request, and the authors speculated that this could be a consequence of the high proportion of people not covered by health-insurance. The 19% of non-prescribed antibiotics were given to patients diagnosed flu or cold, i.e., to treat viral infections. Among the prescribed antibiotics dispensed, only 31.5% respected both the correct dosage and treatment duration according to the reference adopted. These data were in line with another cross-sectional study based on a survey submitted to Kuwaiti subjects during the period from January to March 2014, in which 27.5% of the study population have been taking antibiotics without medical consultation and with a recent community-based cross-sectional study conducted in Kemissie town (Ethiopia), in which one-quarter of the respondents did not obtain antibiotics with a prescription. Also a cross-sectional Italian analysis performed in 2011 reported that around one-third of the respondents to the survey had taken an antibiotic without the prescription of a physician. A recent scoping review highlighted the self-medication of antibiotics to be highly prevalent among individuals living in low- and middle-income countries. Such medications are often connected with improper use as fever, upper respiratory tract infection, common cold and sore throat. Information about antibiotics were mainly provided by pharmacists or a family member.
The widespread inappropriate use of antibiotics emerged as one of the main causes of antibiotic resistance, which negative outcomes call for the development of antibiotic stewardship programs and global surveillance networks.
______
______
Inverse benefit law and OTC’s harm:
The inverse benefit law states that the ratio of benefits to harms among patients taking new drugs tends to vary inversely with how extensively a drug is marketed. Two Americans, Howard Brody and Donald Light, have defined the inverse benefit law, inspired by Tudor Hart’s inverse care law. A drug effective for a serious disorder is less and less effective as it is promoted for milder cases and for other conditions for which the drug was not approved. Although effectiveness becomes more diluted, the risks of harmful side effects persist, and thus the benefit-harm ratio worsens as a drug is marketed more widely. The inverse benefit law highlights the need for comparative effectiveness research and other reforms to improve evidence-based prescribing.
Drug companies show a clear, repeated pattern in which “drugs discovered with good science for a specific set of patients are marketed to a larger population” for whom they are less appropriate and less safe. Inverse benefit law explains how drug marketing undermines patient safety. According to inverse benefit law, as a drug is promoted for milder cases of a disorder or used for unapproved conditions, which can happen in the OTC market, the benefit/harm ratio worsens because effectiveness is diluted but the risks of harm are the same. The more widely drugs are marketed, the more diluted become their benefits but more widespread become their risks of harm.
Let me give example of acetaminophen. It is most widely used OTC and acetaminophen (paracetamol) overdoses send nearly 80,000 people to the emergency room each year, and 30,000 need to be hospitalized, often because of acute liver failure. Had it been prescription-only drug, its use would not be so widespread and overdose liver failures would have been less. However, its efficacy would remain same no matter OTC use or prescription-only use. It is as effective in controlling fever and pain no matter limited prescription use or widespread OTC use. Inverse benefit law logic becomes fallacious in acetaminophen use. Inverse benefit law fails for drugs for common ailment like fever, pain, acidity, common cold etc. It works only for drugs used for serious illness. It is unlikely that such drugs would ever become OTC.
Imagine, acetaminophen becomes prescription-only drug to reduce overdose numbers. Everybody with fever or pain will visit doctor for prescription. Doctors will be overburdened and serious patients will get less time. People will have to spend time and money. Health care spending will increase substantially and resources will be diverted from serious illnesses. The harm to society will be greater than overdose harm. The best solution is to educate people about appropriate OTC use. That is why this article is published by me.
______
______
Section-18
OTC drug abuse and addiction:
The National Institute on Drug Abuse classifies over-the-counter drugs as one of the most commonly abused substances in the United States behind alcohol and marijuana. The lack of surveillance mechanisms makes it much more difficult to obtain national data on the topic, but medical literature supports that this is a growing issue. It is widely thought that because over-the-counter medications are legal, there is a purported sense of safety attributed to these products, but that is not true. Despite seeming safe on the surface, they are actually dangerous. To achieve a high from these drugs, the user must consume them in large doses, which leads to a greater chance of overdose.
Easy, legal access to inexpensive over-the- counter (OTC) medicines has contributed to widespread abuse. And because a doctor’s prescription is not needed, many mistakenly believe that OTC medicines are safer than prescription medicines and illegal street drugs. But even OTC medicines—including herbals—can cause serious and potentially fatal side effects when abused.
Abuse of OTC medicines is most common among teens between the ages of 13 and 16. They know they can find a cheap “high” right in their family’s or friend’s medicine cabinet. Young adults have also abused OTC medicines, particularly in combination with other medicines, alcohol, and illegal drugs, which increases the risks of serious side effects. One of the greatest difficulties with preventing OTC drug abuse is that few teens and adults realize the danger. Teens and young adults who learn about the risks of drugs at home are up to 50% less likely to abuse drugs.
Due to the acknowledged misuse/abuse liability, or associated health risks, some countries have already restricted access to several OTC drugs by introducing an intermediate category of BTC. While the purchase of BTC does not require a prescription from a physician, they may only be purchased in a pharmacy. Other restrictions, such as age limit or maximal purchase quotas, may also be in place for the sale of POMs and OTC drugs.
This matter is further complicated by the differences in local regulations. For example, codeine is available as an OTC medicine in countries such as Denmark, Poland (up to 240 mg per single purchase—since December 2016), the UK (up to 12.8 mg per single tablet), and several other European states, as well as in Japan. At the same time, it is classified as prescription only medicine in Australia (where it has been recently up-scheduled from the OTC category), or USA. Dihydrocodeine, a stronger opioid drug, is generally not available as an OTC medicine. However, few exceptions exist—e.g., in the UK or Japan. Loperamide on the other hand, is usually available without prescription and without almost any restrictions regarding its sale.
OTC medicine abuse was identified in many countries and although implicated products varied, five key groups emerged: codeine-based (especially compound analgesic) medicines, cough products (particularly dextromethorphan), sedative antihistamines, decongestants and laxatives. Associated harms included direct physiological or psychological harm (e.g., opiate addiction), harm from another ingredient (e.g., ibuprofen-related gastric bleeding) and associated social and economic problems.
__
Because a doctor’s prescription is not needed, many mistakenly believe that over-the-counter (OTC) medicines are safer than prescription medicines and illegal street drugs. They are in fact safe and effective when taken as directed, but even OTC medicines, including herbals, can cause serious and potentially fatal side effects when abused. One of the greatest difficulties with preventing OTC drug use is that few teens and adults realize the danger. Unlike the risks associated with illegal street drugs like cocaine and heroin, the risks associated with OTC drug abuse are given little thought and attention.
_
_
Figure below shows many of the over-the-counter drugs currently abused by teens and adults.
_
The abuse of over-the-counter (OTC) medications is particularly problematic because a large number of individuals under age 26 abuse these substances, and of course, many OTC drugs are easily obtained. According to an article in the Journal of the American Board of Family Medicine, individuals who use over-the-counter medications tend to:
- Be younger
- Be Caucasian and female, although this may vary somewhat by age and the particular drug of abuse
- Mix these medications with alcohol
- Abuse OTC medications when prescription drugs or illicit drugs cannot be obtained
__
There are two main factors that contribute to the issue of OTC drug abuse. The first is that because these medications are so commonplace and do not have the same level of warnings or negative associations of certain prescriptions such as opioids, they are perceived as safe to misuse or abuse without any major consequences.
The second factor that has a significant impact on OTC drug addiction is accessibility. As previously mentioned, these drugs are available in most any pharmacy, supermarket, convenience store, or even gas station, in the case of pain-relievers like Tylenol or certain cough medicines. All someone needs to do is walk into their local Walgreens or CVS pharmacy and make their purchase, which is, in part, why OTC drugs are a prime target for abuse by teenagers and young adults. According to the Drug Enforcement Administration, roughly one out of every 10 teenagers has abused an OTC drug specifically to get high at least once in their life.
_____
The statistics are frightening:
While OTC medications don’t have the same monitoring as prescription drugs, some agencies do keep OTC drug addiction statistics. Thanks to these numbers, we can learn how extensive and severe the problem is in reality. Although the statistics is predominantly American, it can be extrapolated for the world as OTC use, overuse, misuse and abuse is worldwide.
- You can find DXM (dextromethorphan) in more than 125 over-the-counter products.
- Close to 750,000 retail outlets sell OTC products.
- In 2005, the FDA issued a warning about dextromethorphan abuse after a series of incidents caused by the drug.
- 56 percent of teens feel that getting OTC drugs is easier than getting illegal drugs
- Internet accessibility to information about how to get high on OTC drugs, and how to order them, makes over the counter drugs even easier to obtain and use
- In 2006, about 3.1 million people aged 12 to 25 had ever used an OTC cough and cold medication to get high, and nearly one million had done so in the past year. (SAMHSA, 2008)
- Four percent of 8th graders, five percent of 10th graders, and six percent of 12th graders abused OTC cough and cold remedies in the past year. (MTF, 2007)
- Teens who abuse prescription or OTC drugs may be abusing other substances as well. Sometimes they abuse prescription and OTC drugs together with alcohol or other drugs, which can lead to dangerous consequences, including death. Nearly one-half (49%) of teens who have abused prescription painkillers also report use of two or more other drugs, most commonly alcohol (81%) and marijuana (58%). (Wu, Pilowsky & Patkar, 2007)
_
Attitudes toward teen OTC drug use:
Most teens that abuse OTC drugs are not really even aware that they are doing something dangerous. The perception of over-the-counter drugs is that their legality somehow makes them safe. To teenagers, it seems ludicrous that an OTC drug could be dangerous to them because they are so readily available. They are sold at the store, their parents give them OTC drugs when they have colds and they are considered safe when used in proper context. Illegal drugs are none of these things. Teenagers need to understand that teen OTC abuse is a real problem with real dangers.
- 40 percent of teens feel that OTC medicines are much safer than illegal drugs
- 31 percent of teens agree that using OTC and Rx medications is okay “once in a while”
- 55 percent of teens do not strongly feel that it is risky to use cough medicine to get high
- 10 percent of teens report that they use cough medicine to get high
_______
Signs of OTC Drug Abuse:
To help spot potential OTC drug misuse or abuse in family members, look for the following signs and symptoms, many of which are similar to the effects of illegal drug use:
Physical changes:
- Overeating or undereating
- Gaining or losing large amounts of weight
- Sleeping too much or too little
- Clumsiness, jerky movements, or slurred speech
Behavioral changes:
- Secretive or suspicious behaviors
- Believing or acting as though others are against them
- Memory problems
- Losing interest in pastimes or socializing
- Sudden changes in friendships
- Angry outbursts, crying, or blaming
- Poor performance at school or work
More generally, pay attention to how your child, spouse, or other family member is using an OTC medication. -Potential red flags include:
- Taking more than the recommended dosage
- Taking doses more frequently than indicated on the packaging
- Continuing to take the medication once the health issue has resolved
- Drinking alcohol while taking the medication
- Combining several OTC medications without being instructed to do so by a doctor
_____
_____
Specific OTC drug abuse:
-1. Dextromethorphan
Medications that contain the antitussive (cough suppressant) dextromethorphan (often abbreviated as DXM by abusers) are particularly dangerous to abuse. DXM is an ingredient in a number of over-the-counter medications designed to treat allergies, cold symptoms, and symptoms of the flu.
Dextromethorphan goes by many names. Pharmacists may call this medication DXM. On the street, it’s also known as dex, poor man’s PCP, CCC, rojo, skittles, triple C, velvet, or robo. Abusing it is often called robotripping, skittling, or dexing. You can find DXM in more than 125 over-the-counter products, including Robitussin, Vicks, and Coricidin HBP.
Fifteen or 30 milligrams of DXM three to four times daily quells adults’ coughs with few side effects. Teens who abuse it sometimes take 250 to 1,500 milligrams at a time. Too much DXM and mixing it with other drugs can cause medical emergencies, such as overdose, poisoning, or even death.
Younger individuals abuse DXM in large quantities for its psychoactive effects. The psychoactive effects of DXM actually differ based on the amount an individual take. At recommended dosages and when taken within four-hour intervals, DXM has surprisingly few side effects and is an effective cough suppressant; however, individuals who take large amounts of the drug and take it at more frequent intervals than the instructions recommend may experience different physical and psychoactive effects depending on the amount of the drug taken.
As an analogue of codeine and a semisynthetic morphine derivative, dextromethorphan is a component of many cough and cold medicines. At therapeutic doses, dextromethorphan produces minimal analgesic and antitussive effects. At high doses, acting as a N-methyl-D-aspartate receptor antagonist, it produces the hallucinogenic and dissociative effects that are recreationally sought.
The United States Drug Enforcement Administration (DEA) lists the effects by dosage and separates them by what it terms as “plateaus of abuse.”
- The first level of abuse referred to as the first plateau occurs when individuals use 100-200 mg of DXM. At this dosage, users will begin to experience stimulant effects.
- The second level, the second plateau, occurs at dosages of 200-400 mg. At this range, individuals will experience feelings of euphoria and visual hallucinations.
- The effects at the third plateau of abuse occur when individuals use 300-600 mg of DXM. In addition to euphoria and hallucinations, individuals will also display significant problems with balance and motor coordination as well as difficulty with visual perception that can result in an increased potential for accidents.
- The highest level of abuse occurs when individuals use over 600 mg of DXM, the fourth plateau. At this level, users become extremely sedated, experience significant hallucinations, and experience dissociative effects that are similar to those experienced with very dangerous drugs like PCP and ketamine. Individuals may begin to feel as if they are leaving their bodies and that things are not real, and this can lead to significant issues with judgment.
A number of other serious effects can occur as a result of DXM abuse, such as liver damage (Often, DXM is sold in product that is a combination of DXM and acetaminophen, which is known to produce liver damage at high doses.), brain damage, and the development of a significant substance use disorder. DXM does not appear to produce significant withdrawal syndromes in individuals who abuse it; however, individuals who have abused the drug for significant periods of time may experience a number of emotional and psychological issues if they attempt to discontinue use abruptly.
Estimates suggest that about 10 percent of teens have abused cough syrups.
-2. Pseudoephedrine
It is also referred to as “pseudo” on the streets. It is an ingredient in medications like Sudafed with the intended purpose of treating symptoms of the common cold such as nasal congestion and upper respiratory problems. It is a stimulant by nature, but it is used to shrink swollen nasal membranes, which allows for improved breathing when treating the common cold. This is a popular ingredient among people who “cook” methamphetamine.
-3. Dimenhydrinate
The main purpose of dimenhydrinate is to treat motion sickness, but its popularity among teens grew once it was found out that exceeding the dose leads to a high. It does not seem like a drug with the potential for abuse. When used properly it provides relief, but it can be deadly when the prescribed dose is exceeded.
-4. Loperamide
Loperamide is a common anti-diarrhoeal drug, that binds to µ-opioid receptors in the gastrointestinal tract, decreasing peristalsis and increasing sphincter tone. At therapeutic doses (e.g., 2mg, with a maximum dosage of 16mg), loperamide does not exert cross central opioid effects; however, at high dosages (e.g., 50–800mg), it might be recreationally abused to achieve a euphoric state, which is informally referred to as “lope high”. It might be used to manage and cope with opioid withdrawal symptoms.
Loperamide toxicity involves gastrointestinal (e.g., nausea, vomiting, constipation), CNS (e.g., respiratory depression, altered mental status, miosis) and cardiovascular effects (e.g., ventricular dysrhythmias and electrocardiogram alterations, such as prolonged QT, QRS widening and torsades de pointes), which might be fatal.
Since September 2019, the Food and Drug Administration (FDA) has limited loperamide package sizes to reduce inappropriate use. Few pharmacies currently regulate its sale, and no regulations exist to prevent purchasing at non-pharmacy online outlets. Interested pharmacies can implement policies to reduce excessive access and prevent harm. However, collateral purchasing at other retail stores or pharmacies may still occur.
-5. Benzydamine
BZY acts as an analgesic and antipyretic, and is used for the topical treatment of inflammations of the oral and vaginal mucosae. BZY has been reported to be misused in several countries, including Brazil, Italy, Romania, Poland and Turkey, at high doses (i.e., 500–3,000mg) to reach stimulant effects on the CNS (e.g., euphoria, hyperreactivity, insomnia, abnormal behaviour, and psychotic symptoms, including paranoia and visual hallucinations). BZY diversion issues might involve young people and the concomitant use of alcohol/cannabis. Even though the molecular mechanism underlying the psychoactive and reinforcing effects of BZY is still unknown, a central cannabinoidergic mechanism of action has been hypothesized.
-6. Promethazine
As a histamine (H)1 receptor antagonist, promethazine is commonly used for symptomatic relief from nausea and vomiting, allergic conditions, motion sickness and the common cold. Often available with codeine in common cough suppressants, its abuse potential appears related to its calming and sedating effect, and enhancement of other co‐ingested substances, such as benzodiazepines and opioids.
The abuse of promethazine mixed with a soft drink and candy, with some variants, including purple-coloured alcohol (‘purple drank’), has become popular among young people for its euphoric effects and easy accessibility. Despite being preferred to other substances, such as benzodiazepines, for the treatment of anxiety and sleep disorders in substance-dependent patients, promethazine has been reported to be misused among people with a SUD or an opioid dependence as a substitute for another drug (e.g., if the desired drug is unavailable or too costly) or to augment the effects of inadequate opioid dosing (i.e., to delay the onset of opioid withdrawal).
-7. Chlorphenamine
As a first generation H1-receptor antagonist, chlorphenamine is used as a cheap sleep aid or anxiolytic. Chlorphenamine has potent antimuscarinic properties, and its abuse has been related to pleasurable feelings such as euphoria, which reinforces the repetitive use of the drug and the possibility of developing drug dependence, but might also cause psychotic symptoms in predisposed individuals (e.g., people with mental illnesses or individuals concomitantly abusing other drugs).
Together with dextromethorphan in cough and cold suppressants, or simultaneously consumed with serotoninergic drugs, it might cause significant serotonin toxicity. A fatality has been registered involving chlorphenamine used concomitantly with an opioid. The abuse of chlorphenamine has been described by data collected from the Texas Poison Center Network Toxic Exposure Surveillance System, and its intended use or abuse appears to be increasing, particularly among young people.
-8. Diphenhydramine
Diphenhydramine is an OTC drug acting on peripheral and central H1 receptors, causing reduction of allergic symptoms and sedation, respectively. The abuse of diphenhydramine appears related to multiple potential mechanisms of action, including a potent competitive antagonism on muscarinic receptors, causing sinus tachycardia, xerostomia, mydriasis, blurred vision, ileus, urinary retention, CNS depression, agitation, hyperactivity or psychosis.
At high dosage and concomitantly assumed together with other drugs (e.g., alcohol, cannabis and stimulants), diphenhydramine can have a stimulatory effect in children and young adults, such as elevated mood, increased energy levels and mild euphoria, instead of the sedating properties seen in adults. Increased dopaminergic neurotransmission in the mesolimbic pathway is thought to cause rewarding properties and drug-seeking behaviour.
-9. Hyoscine butylbromide
Also known as scopolamine butylbromide, hyoscine butylbromide is a plant-derived anticholinergic agent, commonly used as an antispasmodic drug. A dose of 10 mg or more is used to control intestinal and other smooth muscle spasms, for the symptomatic relief of irritable bowel syndrome and as a premedication in anaesthesia. Its use and abuse as a psychoactive substance has previously been reported among young people, who obtain it from proprietary products (e.g., Buscopan [Sanofi]).
At supratherapeutic dosages it exerts potent CNS effects, including restlessness, excitement, euphoria, disorientation, irritability and characteristic delirium-like states with auditory, visual and tactile hallucinations, altered mood, insomnia and cognitive dysfunctions. An advisory warning was issued in 2016 by the European Monitoring Centre for Drugs and Drug Addiction regarding 17 intoxications involving cocaine containing scopolamine, although it has not been formally notified as new psychoactive substance (NPS).
-10. Diet pills
In large doses, diet pills can create a mild buzz. But misuse of diet pills can also signal a serious eating disorder. Abuse of diet pills often starts with trying just a few in order to lose weight. But these OTC medicines can be highly addictive. Although the US Food and Drug Administration (FDA) has banned several of the most dangerous stimulants commonly found in OTC diet pills—phenylpropanolamine, ephedrine, and ephedra—other ingredients in these OTC products can be dangerous. To cite an example, bitter orange is a common ingredient that acts much like ephedrine in the body. It can cause nervousness and tremor, rapid and irregular heartbeat, high blood pressure, stroke, heart failure, and death. Many other diet pill ingredients cause digestive problems, hair loss, insomnia, anxiety, irritability, extreme paranoia, blurred vision, kidney problems, and dehydration. Furthermore, even the most “natural” diet preparations can have serious side effects when misused, particularly those containing ma huang (ephedra). An earlier FDA ban on ephedra pertained only to diet pills considered dietary supplements, not herbal remedies such as teas and Chinese preparations.
-11. Laxatives and herbal diuretics
Like diet pills, some teens and young adults also abuse OTC laxatives and herbal diuretics (water pills), including uva-ursa, golden seal, dandelion root, rose hips, and others, to lose weight. Laxatives and herbal diuretics can cause serious dehydration and life-threatening loss of important minerals and salts that regulate the amount of water in the body, acidity of the blood, and muscle function.
-12. Sexual performance medicines
OTC sexual performance medicines, often purchased via the Internet, are sometimes abused by teens and adults who are drinking to counteract the negative effects of alcohol on sexual performance. These medicines can cause heart problems, especially when combined with alcohol or when taken in large doses.
-13. Herbal ecstasy
This is a combination of inexpensive herbs that are legally sold in pill form and swallowed, snorted, or smoked to produce euphoria, increased awareness, and enhanced sexual sensations. Marketed as a “natural” high, the main ingredient is ma huang (ephedra), an herb banned in the US but only in dietary supplements. The product can be purchased in gas stations, health food stores, drug stores, music stores, nightclubs, and online. It is easy to overdose on the product because the dose needed for desirable effects varies widely. The adverse effects can be severe, including muscle spasms, increased blood pressure, seizures, heart attacks, strokes, and death.
-14. Other herbals
Other herbal products are increasingly being abused for their stimulant, hallucinogenic, and euphoric effects. Besides being legal, another draw is that many herbals are not detected during routine urine drug screens. One example is salvia, which is ingested or smoked to experience a short-lived distortion of reality and profound hallucinations. Users can experience severe anxiety, loss of body control, extreme psychosis, and violent behavior. They are also at risk for accidents and injuries that may result from an altered mental state. Some states have regulated the sale of salvia. Another example is nutmeg, which is eaten as a paste to experience giddiness, euphoria, and hallucinations. Nausea and vomiting set in within an hour and hallucinations begin within 3 hours and can last for 24 hours or more. Effects such as blurred vision, dizziness, numbness, palpitations, low blood pressure, and rapid heartbeat may occur.
-15. Caffeine Medicines
Not all addictive drugs are associated with physical dependence, e.g., amphetamine, and not all drugs that produce physical dependence are addictive drugs, e.g., caffeine. It is possible to be dependent on drug, without being addicted to drug e.g., caffeine. Everyone should know that caffeine in tea/coffee is a licit psychoactive drug which can cause dependence. According to the FDA, 400 mg of caffeine per day is considered safe for healthy adults. Certain people may be more sensitive to the effects of caffeine, depending on how quickly they metabolize it. A toxic dose of caffeine (5 grams or more) can cause an overdose, although it is rare.
Caffeine pills usually contain anywhere from 100 to 200 mg of caffeine per tablet and are generally used by people who are trying to combat fatigue. For comparison, a typical cup of coffee contains 95 mg of caffeine. If a person misuses caffeine pills by taking too many, he or she may experience toxic or fatal side effects due to caffeine overdose.
And when there are OTC drugs that contain more amounts of caffeine such as NoDoz caffeine pills (more than 2x as much as a cup of coffee) and X-Mode Energy Shots (over 3x as much as a cup of coffee), the threat of overdosing is even more real. There are more products with even higher amounts too. But even more concerning is the fact that these products don’t even need to be bought at a pharmacy – we can actually consume dangerous levels of this chemical right from everyday products.
Caffeine is a methylxanthine, chemically related to theophylline and a ubiquitous substance in the Western diet. Caffeine is a stimulant that elevates blood pressure, pulse, and respiratory rate. It is used clinically in the management of neonatal apnea. At doses near therapeutic, it may produce palpitations, sweating, and a sense of anxiety. In overdose, tremors, fasciculations, and seizures are observed. Fatal caffeine poisoning has been reported. Caffeine dependence in childhood has been described, and a withdrawal syndrome consisting of somnolence and a decline in motor performance 24 hours after the discontinuation of caffeinated beverages has been recognized. The acutely poisoned patient should be observed for the abrupt onset of seizures. Peritoneal dialysis was reported to be effective in accelerating the removal of caffeine from one seriously poisoned child.
______
______
Table below shows various OTC drugs having abuse potential:
_____
_____
OTC opioid abuse:
The abuse of opioid drugs is an important issue burdening the modern world more than ever before. The scale of this phenomenon, called the 21st century opioid crisis, is clearly illustrated by an ever-increasing involvement of opioids in the total number of all substance overdoses. The number of opioid overdoses has increased nearly 6-fold over the course of the last 20 years, and their contribution to fatal intoxications grew from approximately one half to over two thirds of all cases. While it is generally agreed upon that this problem concerns mainly prescription-only opioids and illicit narcotics (such as novel analogues of fentanyl), the involvement of OTC opioids should not be overlooked as there is sound evidence that such substances are being abused. Relatively easy access to OTC opioids is alarming and perhaps requires additional consideration and debate on the rescheduling of their availability (some countries already undertaken such steps and limited access to codeine or dihydrocodeine). However, these drugs are among the most popular medicines worldwide, their sudden disappearance from over-the-counter sale could prove problematic for patients.
Codeine:
Codeine is a common ingredient found in cough syrup. It is an opiate that is similar to morphine, but it is much less powerful. Codeine works as a cough suppressant, but it can also produce feelings of relaxation and euphoria when it is consumed in high doses. Codeine misuse gained popularity among drug abusers in the 1990s and it was frequently used to make a cocktail called “purple drank,” “lean,” or “sizzurp,” which is a combination of cough syrup (codeine and promethazine hydrochloride), soda, and a hard piece of candy. Codeine is found in OTC cough syrup as well as with prescription-strength cough syrup, but combining either type with alcohol is dangerous and life-threatening. When it is consumed in very high doses, codeine can cause dependence and addiction.
At the moment, codeine and loperamide are both considered essential medicines according to the WHO. However, their abuse liability is an important stimulus for research on new, less-addictive candidates to replace them (both opioid and non-opioid). Codeine is not available as OTC in the US. However, it is a primary medicine with abuse potential in other countries. Numerous studies have recorded OTC codeine analgesics as the most commonly abused medication.
_____
_____
Harms of OTC drug abuse:
A range of problems and harms associated with OTC medicine abuse were identified and these comprised three broad categories as seen in the figure below:
First, there were direct harms related to the pharmacological or psychological effects of the drug of abuse or misuse.
Second, there were physiological harms related to the adverse effects of another active ingredient in a compound formulation. Both these types of harm led to concerns about overdoses and presentation at emergency services.
Third, there were those harms related to other consequences, such as progression to abuse of other substances, economic costs and effects on personal and social life.
Direct harms included addiction and dependence to an opiate such as codeine (Mattoo et al., 1997; Orriols et al., 2009; Nielsen et al., 2010). Other direct problems included convulsions and acidosis due to a codeine and antihistamine (diphenhydramine) containing antitussive medicine (Murao et al., 2008) and tachycardia, hypertension and lethargy due to abuse of Coricidin cough and cold tablets (dextromethorphan and chlorphenamine) (Banerji & Anderson, 2001). Lessenger and Feinberg (2008) produced a comprehensive list of physical findings of nonmedical use of abused OTC products, noting agitation with nicotine gum, caffeine and ephedra, priapism with ephedrine and pseudoephedrine, psychiatric effects with dextromethorphan, euphoric psychosis with Coricidin and chlorphenamine and gastrointestinal disturbances with laxatives. Also within this category of direct harms were concerns raised about chronic rebound headache associated with repeated use of analgesics.
In relation to harms from other ingredients, two analgesic combination products – paracetamol and codeine (co-codamol) and ibuprofen and codeine – were considered problematic, with ibuprofen-containing medicine being particularly highlighted (Chetty et al., 2003; Dyer et al., 2004; Lambert & Close, 2005; Ford & Good, 2007; Dobbin & Tobin, 2008; Dutch, 2008; Ernest et al., 2010; Frei et al., 2010; Robinson et al., 2010). Dutch (2008) and Ford and Good (2007) reported on two hospital and three primary care presentations, respectively, of patients who had used a combination analgesic containing ibuprofen and codeine. Ford and Good (2007) noted the side effects relating to ibuprofen and Dutch (2008) reported both patients having perforated gastric ulcers. Hypokalemia secondary to renal acidosis was identified as a result of abuse of this combination product (Chetty et al., 2003; Dyer et al., 2004; Lambert & Close, 2005; Ernest et al., 2010). Dobbin and Tobin (2008) reported on 77 cases reported through personal networks of one of the authors where harm and dependence to ibuprofen and codeine OTC products had occurred. They identified similar clinical presentations as noted above and one death.
In relation to other consequences, several studies have referred to the association of OTC medicine abuse and the use of illicit substances (Levine, 2007; Reay, 2009) or obtaining codeine supplies from “street” supplies (Sproule et al., 1999). Tinsley and Watkins (1998) reported on seven patients with dependence (according to DSM-IV criteria for amphetamine-like abuse) to ephedrine or pseudoephedrine and reported adverse social consequences in relation to losing jobs, family-marital stresses, relapse into alcohol abuse, motor vehicle violations and accidents.
_____
_____
Over-the-Counter Drug Addiction Treatment:
Chronic and long-term abuse of OTC drugs can cause physical dependence and addiction, which are life-altering issues that can cause personal and physical harm or even death. Addiction to OTC drugs is possible and those ready to break the addiction cycle can find hope in treatment. However, once people become addicts, cutting these drugs, cold-turkey can be life-threatening, so most people start with a partial hospitalization program (PHP), in conjunction with detox programs to prevent severe withdrawal symptoms. It’s paramount to speak with an addiction treatment specialist to determine the best way to start seeking help for addiction. After successful stints in detox and an inpatient/residential facility, the recovering user will have the option to connect with supportive networks, resources, and activities that promote sobriety. These are all critical for people in recovery, especially those who are new to it. Some facilities have alumni programs that offer outings, personal follow-up support, and encouragement to form lasting bonds with clients who have similar stories and struggles.
_____
_____
Limitations of immunoassays for screening of drugs of abuse in urine:
Many over the counter medications are known to interfere in amphetamine/methamphetamine immunoassays. The high prevalence of use of over-the-counter medications adds to the high false positive rate seen with amphetamine/methamphetamine immunoassays.
- Ephedrine and pseudoephedrine are a major cause of false positives. Some assays have reformulated to reduce interference from these two drugs.
- Bupropion, an atypical antidepressant used for smoking cessation can also cause false positives. A retrospective chart review performed by Casey et al. determined that up to 41% of their false positives could be due to bupropion in their Syva EMIT II assay.
- Ranitidine, a H2 receptor antagonist used to treat heart burn, is also implicated in false positives. Some manufacturers have reformulated the reagents for amphetamines immunoassays to improve the number of false results; however, there are assays that are still affected, and susceptible to cross reactivity from ranitidine.
- In addition, other drugs including herbal supplements may also interfere with amphetamine/methamphetamine immunoassays.
_______
_______
Section-19
OTC drug regulation:
In many countries, OTC or non-prescription drugs are selected by a regulatory agency so as to check the ingredients that are used in the making of drugs are safe and effective when used without a doctor’s advice. These non-prescription drugs are usually regulated by active pharmaceutical ingredients (APIs), not final products. This implies that the governments allow drugs manufacturers the right to formulate ingredients, or combinations of ingredients, to make proper medicinal mixtures. Regulations related to who is authorized to dispense these drugs, to where they are to be sold, and whether a prescription is required vary considerably from country to country. In India. all the drugs that are not included in the list of prescription drugs are considered as non-prescription drugs (or OTC drugs).
_____
OTC Regulation in United States:
In the United States, the manufacture and sale of OTC substances is regulated by the FDA. The Federal Food, Drug, and Cosmetic Act requires that all “new drugs” are required to obtain a New Drug Application (“NDA”) prior to entering interstate commerce, but the act exempts any drugs generally recognized as safe and effective (GRASE) from this requirement. In order to fit the vast amount of OTC drugs that were already on the market prior to the requirement that all drugs obtain an NDA, the FDA created the OTC monograph system to review classes of drugs and categorize them as GRASE by regulation. This meant that certain classes of OTC drugs were not required to obtain an NDA and could remain on the market.
Thus, manufacture must be done either pursuant to an FDA monograph, which specifies types of OTC drugs, active ingredients and labelling requirements, or pursuant to a New Drug Application (NDA), for products which do not fit within a specific monograph. Because an NDA is extremely expensive to obtain, due primarily to testing requirements, most OTC substances produced in the USA are sunscreens, anti-microbial and anti-fungal products, external and internal analgesics such as lidocaine and aspirin, psoriasis and eczema topical treatments, anti-dandruff shampoos containing coal tar, and other topical products with a therapeutic effect.
The FDA requires OTC products to be labelled with an approved “Drug Facts” label to educate consumers about their medications. The labels comply to a standard format and are intended to be easy for typical consumers to understand. Drug Facts labels include information on the product’s active ingredient(s), indications and purpose, safety warnings, directions for use, and inactive ingredients.
The 2020 Coronavirus Aid, Relief, and Economic Security Act (CARES Act) includes reforms that modernize the way certain OTC drugs are regulated in the United States. Many OTC monographs need to be updated but updating or changing an OTC monograph requires the slow and burdensome notice-and-comment rulemaking process. The CARES Act includes OTC monograph reform provisions that replace the rulemaking process with an administrative order process.
The Federal Trade Commission regulates advertising of OTC products. This is in contrast to prescription drug advertising, which is regulated by the FDA.
_
New Drug Application:
The NDA is the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing in the United States.
The documentation required in an NDA is supposed to tell the drug’s whole story, including:
-1. Results of clinical tests.
-2. Drug ingredients.
-3. Animal studies’ results.
-4. Documentation of drug behavior in the body.
-5. Drug manufacturing, processing, labeling and packaging information.
Can OTC medicines be marketed without pre-approval from FDA?
Yes. Any product that conforms to an OTC monograph may be manufactured and sold without an individual product license. A monograph is a regulatory standard for the labeling and ingredients for products within a specific category such as antacids, analgesics, etc. It is a kind of “recipe book” covering acceptable ingredients, doses, formulations, indications, and labeling. Final monographs are published in the government’s Code of Federal Regulations (CFR). Some OTC medicines do not fall within the monograph system and require a new drug application (NDA).
_
Rx products can be switched to OTC by the following means:
-1. By the OTC drug review – this is initiated by the FDA commissioner. The results of the OTC Drug Review were OTC drug monographs – a kind of “rule book” of conditions for each therapeutic category covering acceptable ingredients, uses (indications), doses, formulations, labeling, and testing. A drug marketed that is consistent with the conditions set forth under a final monograph and all other general applicable OTC requirements is considered generally recognized as safe and effective for the uses set forth under the monograph. Essentially, the OTC Drug Review is a three-phase public rulemaking process that establishes monographs. Each monograph is required to be published in the Federal Register, a daily publication in which Federal agencies publicly announce regulations and legal notices.
-2. By submission of an efficacy supplement to the existing NDA – This is the means for a sponsor to switch the Rx products in its entirety to OTC
-3. By submission of a new NDA – This is required for a sponsor to partially switch some of the indications to OTC, while retaining others within a prescription status.
_
OTC monographs are categorized based on their ingredients:
Manufacturers should review the regulatory history of the active ingredients (i.e., Category) for any OTC drug products currently on the market.
Category I
Generally recognized as safe and effective (GRASE). Ingredients have been subject to adequate clinical investigations and testing, and experts generally agree that they demonstrate safety and efficacy for intended use. If FDA accepts this recommendation and completes rule-making on the ingredients, they become part of the detailed final monograph for the relevant product category.
Category II
Ingredients that are not GRASE. If FDA accepts the recommendation, they will not be part of the final monograph, but ingredients that have been proposed as Category II can continue to be marketed until a monograph is finalized, which can take years.
Category III
Not enough information to determine whether the ingredient is GRASE. The product can remain on the market until FDA finds that there is enough evidence to make a GRASE determination.
Any other OTC drug that does not fall into one of these categories, or is not subject to a final administrative order, is deemed a “new drug” and requires a new drug application for marketing authorization.
_
The greatest shortcoming of the OTC drug monograph process is the lengthy delays that result from the public rulemaking process. These delays leave many OTC drug products in perpetual approval purgatory and “proposed” rule status (a tentative final monograph or TFM), and the process to update any OTC drug monograph to address safety or other concerns is ineffective and often allows safety concerns to simply fall between the cracks.
_
Public Health Risk from an inefficient system of FDA regulation of OTC products:
The Food and Drug Administration’s inability to efficiently change OTC monographs compromises its ability to ensure that these products are safe and effective. Here are some examples:
-1. Cough and cold products.
The monograph describing cough and cold products permits their use in children 2 and older. However, since the monograph was finalized in 1987, concerns have arisen over whether these products are safe and effective for small children. In 2007, after an internal review linked the deaths of 54 children younger than 6 to decongestants and 69 deaths to antihistamines between 1969 and 2006, FDA’s advisory committee recommended action against the use of these medications for this age group. But no change has been made to the monograph. Drug companies have voluntarily updated the labels on cough and cold medications to indicate that they are not recommended for children younger than 4. However, because the monograph still stands, these products can continue to be marketed for children 2 and older.
-2. Phenylpropanolamine.
After decades of reports linking phenylpropanolamine, an ingredient in nasal decongestants and weight-loss drugs, with increased risk of hemorrhagic stroke, FDA proposed classifying it as a Category II (nonmonograph) ingredient in 2005. Fortunately, manufacturers have voluntarily halted sales of products with that ingredient, but the relevant monograph has yet to be finalized. Companies, therefore, can still legally market products containing it, despite its known risks.
-3. Acetaminophen.
In 2011, a joint committee convened by FDA agreed that there was sufficient safety and efficacy data on acetaminophen to support new dosing instructions on labels for products containing the drug to treat fever in children ages 6 to 24 months. Drug manufacturers have requested that the monograph be amended to provide dosing instructions for children younger than 2, but no change has been made. Instead, the label directs parents to consult with their doctor—a step that may delay effective treatment or result in children receiving dosages of acetaminophen that may harm them.
More recently, FDA sought to lower the dose of acetaminophen in pain-relief products based on evidence that taking large amounts of the drug causes liver damage. The agency was able to take swift action to limit acetaminophen to 325 milligrams per tablet for prescription drug products. While a labelling change to add a liver damage warning was finalized in 2009, FDA has not required a lower dose in OTC products.
_____
OTC regulation in India:
India’s drug law does not even mention ‘OTC’ drugs and neither the Drugs & Cosmetics Act, 1940 nor the Drugs & Cosmetics Rules, 1945 define any medicine that can be sold without doctors’ prescriptions. At its core, the Drugs & Cosmetics Act — this governs all products classified as drugs — is a series of schedules containing specific drugs. Which schedule a drug is listed under decides its regulatory status. Drugs under schedule H, H1 and X cannot be sold without a prescription. Therefore, from an OTC marketer’s perspective, drugs that fall outside these schedules are considered to be non-prescription and by inference, free to be made available over the counter. However, the law does not explicitly state this.
The Central Drug Standards Control Organisation (CDSCO) is planning to frame regulations for non-prescription drugs or over-the-counter (OTC) drugs. The CDSCO has now decided that OTC drugs will be classified into two categories—OTC-1 and OTC-2—based on the extent of evidence, safety, therapeutic index, need for accessibility to patients, availability, non-habit forming nature, present supply chain mechanism and socioeconomic conditions of the country. Experts have warned that for formulating such a policy, extreme caution should be exercised. “There are only five-six evidence-based drugs that can be defined as OTC from the safety point of view and that include paracetamol, laxatives, antacids, local balms and some multi-vitamins,” said Pune-based health activist Anant Phadke.
In countries such as India, where the doctor-to-patient ratio is abysmally low, carving out a separate category of OTC drugs can improve access to safe medicines and bring clarity in the regulatory regime governing these drugs. This goal, however, must be tempered with the need to ensure that adequate safety measures are in place to ensure that such self-medication does not lead to any risk to the safety of patients.
Self-medication is a very common practice in India, especially in cases of minor ailments like headache, fever, body pain etc., and most times such medications are available easily across the counter from pharmacies. In obtaining such medications across the counter without a prescription, patients may not be aware of adverse reactions, contraindications, drug interaction, therapeutic value etc., which may be associated with such medications. The proposed regulations seem to focus on alleviating this patient safety issue.
_____
_____
Section-20
OTC drugs advertising:
Choices of people are different based on different cultural context. People seek the information of their choice from various sources; either formal or informal. This is the age of communication technology; people receive the message halfway through the world within seconds. In this connection, advertisement of any goods and services through online and print media reaches the people around the globe at a very fast pace. It is said that ‘health is wealth’, so health awareness becomes the first priority of any person of all culture. People visit different health clinics to seek the treatment for their health problem. It is common practice of people that they visit pharmacy or supermarket to purchase OTC medicines for their common health problems. ‘OTC Drugs’ means drugs legally allowed to be sold ‘Over the Counter’, i.e., without the prescription of a Registered Medical Practitioner. Producers use the online media; television, radio, internet or print media; newspaper, magazine, poster, pamphlet, hording-board to advertise their medicines.
Advertising in its simplest form means “public announcement”. Advertising as described by the American Marketing Association, Chicago is basically “any paid form of non-personal presentation of ideas, goods and services by an identified sponsor.” This definition in itself states that advertising is mostly a form of persuasive communication with the audience. Further, it is also clear from the definition that advertising is basically one-sided information where information flows from the part of the advertiser to the public. Advertising means the creative communication with the consumer. As a form of communication, advertising serves the purpose of a guide to buying in which the intended message is delivered to masses through various media. People collect the message of different goods and services from the advertisement and physically visit the concerned retail outlets to purchase such goods. The main objective of advertisement is to inform and sensitize the people regarding the available goods and services.
_
Direct-to-consumer advertising (DTCA) is a tool used by pharmaceutical companies to market and promote medicines directly to potential consumers by means of different media but the debate for and against this is ongoing and fairly balanced. Direct to consumer promotion of prescription pharmaceutical product is considered illegal in many parts of the world. United States and New Zealand are the only two countries which have legalized DTCA for prescription medicines, but the advent of internet has broken all barriers. Advertisement of prescription medicines through internet and social media has made it difficult for government and regulatory authorities to effectively implement the laws which govern DTCA. As far as OTC medicines are concerned, many countries allow its advertising.
_
Different countries have different guidelines and regulations for OTC drugs advertising. In the U.S. FDA does not oversee the advertising of over-the-counter drugs. The Federal Trade Commission (FTC) is responsible for regulating OTC drug ads. The FDA regulates advertising only for prescription drugs.
The following OTC medicines advertising can be seen on TV in India:
- Digestives
- Antacids
- Antiflatulents
- Cold rubs and analgesic balms/creams
- Vitamins/tonics/ health supplements (especially herbals and Ayurvedic-registered)
- Medicated skin treatment
- Analgesic/cold tablets
- Antiseptic creams/liquids
- Glucose powders
- Cough liquids
- Throat lozenges
- Medicated dressing (band-aids)
- Baby gripe water.
- Vasodilators
- Anti-acne Drugs
- Non-Steroidal Anti-Inflammatory Drugs
- Smoking Cessation Drugs
______
It is clear there is an increasing trend of usage for OTC drugs in all over the world. Wazaify (2005) states that in recent years there have been an increasing trend in self-medication with nonprescription drugs available in pharmacies and retail outlets and in parallel, more products have been deregulated for purchase without a prescription (Wazaify, 2005). At the same time Solhaug (2006) concludes in his abstract publication that only half of the information presented in drug advertisements was correct and clinically relevant and relatively few statements were fault, but a considerable proportion of statements gave an excessively positive picture of the product; hence, in general, this kind of information has no value as a source of information( Solhaug, 2006).Among the publications those point out that drug advertisements are not up to the standard especially OTC drug, Ashish (1999) states in his publication that ”in reality, it has been observed that pharmaceutical product advertisers often promote their products to achieve their own goals at the potential risk of having an adverse effect on the consumer’s health and this type of advertising is most often seen in OTC drug product advertisements”(Ashish, 1999).
_
How consumers are misled:
Many research papers point out that drug advertisements mislead consumers in many ways directly or indirectly (Sidney, 2002, Michael, 2000, Ashish, 1999, Findlay, 2000). Though it is a common phenomenon in all over the world, a practical guide on ‘understanding and responding to pharmaceutical promotion’ published by the world health organization recently explains clearly how information is given to consumers in advertisements in developed and developing countries. It says ”while advertisements from developed countries typically contain nearly all of the information listed in the box, this is not always the case in developing countries”(WHO). The indications were mentioned more often than the negative effects of medicines. Important warnings and precautions were missing in half of the advertisements while side effects and contraindications in about forty percent. Price tended to be given only in countries where a social security system pays for the medicines (Herxheimer, 1993). Nowadays there is a trend of promoting drugs through internet. Buckley mentions on his research paper that most of the internet advertisements provide less information or poor-quality information.
_
Many pharmaceutical companies mislead consumers in many ways. Most of the time drug companies overstated the effectiveness of the promoting drug and they always keep attention not to highlight its risk. Rebecca (2010) says ”it’s almost impossible for the public to actually parse the ads and come to their own independent conclusions”(Rebecca, 2010). But Weissman (2003) states in his research paper that ”industry’s argument is that patients are highly motivated to seek the best available treatment for their condition and they need and deserve more and better information on which to base their judgement”(Weissman, 2003). It is true if the pharmaceutical advertisements provide proper, balance and correct information to their consumers. A research done in Thailand, sharing the findings says that 22% radio advertisements have misled consumers and only 7% of the advertisements have recommended an appropriate dose among studied advertisements. Furthermore a warning message was found in only 3% of the advertisements and name of the manufacturers were present only 20% of the advertisements collected (Kittisopee, 2005). Weil (2009) is really against the trend of this drug advertisements. He says almost all highly advertised drugs have many complications either short term or long-term basis and in general these drugs are ineffective and some serious side effects are not considered or neglected by the manufacturers. Because most pharmaceutical companies’ give priority for their profit margin and such vast profit potentials may be obtained from this kind of heavy drug advertising (Weil, 2009).
_
Now there is a trend of using popular characters for marketing advertisements of drugs and sometimes they are neither reliable nor relevance. In a paper published by Michael (2000) says that ” Now advertisements enlist well-known personalities to endorse pharmaceutical products” (Michael, 2000). Lot of people imitate and follow famous personalities and it help pharmaceuticals to reach consumer quickly and in a familiar manner. Drug companies are in the process of promoting their product to consumers in many ways. Sometimes they may use health care professionals to reach consumers because they know that consumer believe professionals who have background knowledge about treatments. Wazaify (2005) points out in his published paper that ”The main factor found to influence the public’s choice of OTC medicines was pharmacist recommendation. This is reassuring especially with increasing availability of potent medications without prescription and the increased potential for interactions”(Wazaify, 2005). It is one of the indirect marketing methods that the pharmaceutical industry uses.
On the other hand, people have a belief that over-the-counter drugs like paracetamol do not have serious side effects. Some over the counter drugs have serious side effects when consumer uses it with some other medications. A very good example is Viagra used for erectile dysfunction. If consumer uses it while using nitrate, it causes severe drop of blood pressure which is difficult to treat. Buckley says it has mentioned on advertisements but in a much smaller font, it is ”You must not take Viagra if you are using any nitrate medication including amyl. It may lead to a severe drop of your blood pressure that may be difficulty to treat. As sexual activity may be a strain on your heart your doctor will need to check whether you are fit enough to use Viagra”(Buckley). Buckley points out the ordinary people do not know what is nitrate medications and they cannot recognize from this statement that the combination of these two drugs will enough to kill them more often. Supporting to this argument Wazaify(2005) mentions that consumers generally believe that only safe medicines are permitted to be sold without prescription and OTC medicines do not usually have serious side-effects (Wazaify, 2005).
_
Extensive advertising and promotion have put on higher price on pharmaceuticals. Consumer has become the victim of those expensive pharmaceuticals. Dave(2010) says ” Promotion may affect price through two difference processes. First, promotion may increase demand and/or reduce the absolute magnitude of the demand price elasticity (that is, reduce the price responsiveness of purchasers), which may raise price. Second, the increasing operation cost due to high promotional spending may be shifted to purchasers in the form of higher price. Concluding his findings, he states that ” in addition to potential misuse, the cost of direct-to-consumer advertising result from increased drug price and increased use of expensive drugs in place of equally effective lower-price drugs’ ‘(Dave, 2010).
_____
Changes in Direct-to-Consumer Pharmaceutical Advertising Following Shifts From Prescription-Only to Over-the-Counter Status: a 2012 study:
When prescription drugs become available over-the-counter, advertisements for the medications are far less likely to tell consumers about the potential harms and side effects, new research finds. The reason for it, experts say, likely has to do with which federal agency regulates the marketing materials for each type of drug. The U.S. Food and Drug Administration (FDA) regulates ads for prescription drugs, while ads for over-the-counter drugs are regulated by the U.S. Federal Trade Commission (FTC). The FTC has much less stringent standards than the FDA for what manufacturers have to reveal about products in their marketing materials, the researchers noted. The FDA requires prescription drug advertising to provide consumers with a “fair balance” of risks and benefits — for drug ads, that often means rattling off a lengthy list of potential side effects. The FTC, on the other hand, holds drug advertisements to the same standards as other consumer products, requiring a “reasonable” standard of truthfulness.
The looser requirements mean that information about potential side effects and harm aren’t included in most over-the-counter drug ads, said study author Dr. Jeremy Greene, an associate professor in the history of medicine department and the department of medicine at Johns Hopkins University. “One of the FDA’s guiding principles for regulating direct-to-consumer ads for prescription drugs is there needs to be a fair presentation of the risks and benefits,” Greene explained. “The FTC has fewer requirements or specific regulations for how risks and benefits should be presented. The FTC is interested in making sure there are no fraudulent statements being made, and there is no active deception.”
Greene and his colleagues analyzed print and broadcast advertisements for four commonly used drugs that were heavily marketed to consumers as prescription drugs and then approved for sale over-the-counter. The drugs included loratadine (brand name: Claritin, sold over-the-counter since 2002), omeprazole (brand name: Prilosec, went over-the-counter in 2004), orlistat (brand name: Alli, Xenical, sold over-the-counter since 2007), and cetirizine (brand name: Zyrtec, sold over-the-counter since 2008).
When the drugs were available only by prescription, 70 percent of the ads mentioned potential harms. After the drugs were available over-the-counter, only 11 percent did, the investigators found. After drugs became available over-the-counter, only about half of print and broadcast advertisements mentioned a drug’s generic name, compared to 94 percent of ads when drugs were prescription-only. Knowing a drug’s generic name can help consumers make sure they’re not taking more than one medication that has it as a component, risking overdose, Greene explained.
Based on an analysis of benefit and risk information in all print and broadcast advertisements from 4 commonly used prescription drugs that were the subject of extensive Direct to Consumer Advertising (DTCA) promotion before and after OTC shift, Greene et al observed ‘less presentation of potential harms, DTCA for OTC medications frequently omitted identification of drugs by their generic names, both of which are key tools for consumers seeking independent information on risks, benefits, and costs.’
To give consumers the information they need to better understand risk, the FDA should be given authority to regulate marketing of over-the-counter drugs, or perhaps the FTC should adopt guidelines similar to what the FDA requires, according to Greene. That said, few think even the FDA’s “fair balance” guidelines are sufficient enough to really inform, he added.
In response to:
Greene JA, et al. Changes in Direct-to-Consumer Pharmaceutical Advertising Following Shifts from Prescription-Only to Over-the-Counter Status. JAMA 2012;308(10):973-5:
Pharmacists say that warning needed in OTC drug advertising:
We add the perspective of the licensed pharmacists regarding the need for warnings in OTC advertising of higher risk medicines such as acetaminophen (liver disease), ibuprofen (drug-induced allergy) among others. In recent work by Soller, Mulvaney et al. (2012), a high majority (> 90%) of surveyed California pharmacists and pharmacy students (n=421) favor: warnings in OTC drug advertising for rare, serious side effects at recommended doses and for predictable serious side effects at greater than recommended doses; and specific warnings in OTC advertising for choking (psyllium with inadequate fluid intake); GI bleeding (aspirin); ibuprofen (kidney toxicity); and acetaminophen (liver toxicity). Most (78-86%) favor use of boxed warnings accompanying print advertising (e.g., “acetaminophen can cause serious liver damage. Take only at recommended doses or as directed by a physician”), use of minimum type size, and placement in proximity to the advertising claim.
In a bygone era, opposition to warnings in OTC advertising was framed on the supposition that, unlike patients, consumers get a comprehensively labeled carton or bottle at the time of purchase of an OTC medicine, and thereby receive all the drug information they need for safe and effective drug use according to label recommendations. Today, however, patients (i.e., those under physician supervision) receive drug warnings from physicians, pharmacists, retail drug monographs distributed with dispensed medicines, medication guides, direct-to-consumer advertising and the Internet. Hence the opposition’s proposition of the OTC label as the sole keeper of essential drug information for medication users at the point of purchase and customary use has a questionable basis.
There is no logical argument for why the rules of engagement governing fair balance in drug advertising should be so substantially different between Rx and OTC medicines. A change is needed through new regulations and/or a legislation encompassing a shift in authority for OTC advertising from the Federal Trade Commission to the Food and Drug Administration. Higher risk OTC medicines with potentially serious and life-threatening consequences due to use, misuse or abuse should be identified, and suitable warnings should be developed to accompany advertising of these medicines in all media.
William Soller, PhD, Professor, UCSF School of Pharmacy.
_____
Content Analysis of False and Misleading Claims in Television Advertising for Prescription and Non-prescription Drugs. a 2013 study:
Researchers at Dartmouth College, in N.H., and the University of Wisconsin-Madison decided to check up on what drug companies say in their U.S. TV commercials. Their findings suggest a frequent disregard for the truth. Sixty percent of prescription drug ads and 80 percent of over-the-counter drug ads were found to be misleading or false. “There were cases of blatant lying, but these half-truths form more than half of our analysis,” said study author Adrienne E. Faerber. Faerber became skeptical of the assertions made in drug commercials when Claritin moved from a prescription-only drug to a product you could buy over the counter, suddenly the commercials made, “six times more claims for the benefits of the drug,” she said.
In a paper published in the Journal of General Internal Medicine, Faerber randomly chose 84 prescription drug commercials and 84 over the counter drug commercials, which aired during the nightly news broadcasts of ABC, CBS, NBC, and CNN between 2008 and 2010. Analysts identified each ad’s major claim and subsequently evaluated its authenticity. The claims were classified as “objectively true” if there was evidence to support it, the commercial didn’t exaggerate the evidence, and if no important details were left out. It was deemed “misleading” if the claims were exaggerated or if important details were omitted, and “false” if there was no evidence to back the claim.
One of the repeat offenders, said Faerber, were erectile dysfunction drugs. “The various drug companies phrase it differently, but they all play on the idea of being “ready” when the moment is right,” but Faerber said readiness is about more than a physical reaction, it’s an emotional state – especially where sex is concerned. “They implied more than a physiological response.”
Faerber’s results showed that it was more common for an over the counter drug to be misleading or false than a prescription drug. The reason why remains unclear, but the FDA oversees prescription drug ads while the Federal Trade Commission is responsible for over the counter drugs. “The FTC is more reactive and the FDA is proactive,” said Faerber, “The FTC is also less specialized.” Faerber suggests that it might help if the FDA and FTC were to coordinate efforts for the first few months after a drug is declassified for over the counter sale.
Shortly after publication, Matthew Bennett, Senior Vice President of the Pharmaceutical Research and Manufacturers of America called the research “a subjective, non-rigorous analysis of a small group of direct-to-consumer advertisements.” Bennett’s primary concern came from what he considers an “overbroad” definition of the term “misleading” to describe the advertisements in the study. “The study classifies any general statements about a product and lifestyle statements as being ‘misleading’ when, in fact, these statements do not violate FDA law or regulation and are not uncommon or illegal advertising practices,” said Bennett. Bennett also said that many of the biopharmaceutical research manufacturers voluntarily submit their television ads to the FDA for review before they are aired, giving the FDA the opportunity to pull the ads if they are deemed inappropriate or misleading.
_____
Misleading Health Claims in COVID-19 Advertising, 2021:
During the COVID-19 pandemic, there has been an explosion of challenges to health product advertisements that expressly or impliedly claim to protect consumers and their families from the coronavirus. Consumers rely on ads, especially those that claim a product will support their immune system or help ward off disease. When they hear these claims, they also expect that there is science that supports such claims. Truth in advertising is important to protect consumers and help level the playing field for fair competition in the marketplace. This core concept becomes especially important when advertising health-related products, including over-the-counter drugs and dietary supplements.
COVID-19 has forced consumers to focus on their health and well-being, so it is no surprise that health-related advertising has also been a focus of competitor challenges at BBB National Programs this past year. Indeed, there has been a nearly 50 percent increase in competitor challenges concerning health-related advertising claims. For example, there were multiple challenges to products touting health and wellness benefits for traditional over-the-counter drugs, as well as natural alternatives that purported to offer similar relief or alternative wellness benefits. Brands new to self-regulation participated in challenges, including makers of essential oils and natural cough remedies, both popular products in the burgeoning natural health and wellness marketplace.
Many of these product claims about wellness benefits required a review of complex issues and science-heavy claim substantiation, an encouraging indicator that brands take seriously the requirement that health-related advertising claims be supported by competent and reliable scientific evidence. The resulting decisions provide guidance on crafting truthful and accurate advertising for consumers so that health-related advertising claims are supported by science and competitors play by the same set of rules.
BBB National Programs’ National Advertising Division (NAD) and Direct Selling Self-Regulatory Council (DSSRC) have monitored the advertising landscape for decades, but during the COVID-19 pandemic, health-related claims, including those referencing the coronavirus, represented the greatest volume of challenges and cases received.
Once the pandemic began, government agencies sprang into action. Both the U.S. Food and Drug Administration (FDA) and the Federal Trade Commission (FTC) have issued dozens of warning letters since spring 2020 about express and implied claims that products can treat or prevent COVID-19.
As the effects of the pandemic continue, NAD and DSSRC remain vigilant, with each increasing their monitoring for advertising touting COVID-19-related health benefits due to its prevalence. During 2020, 38 percent of NAD’s monitoring cases were brought against companies making health-related claims concerning their product’s ability to boost consumers’ immunity against, or otherwise protect them from, COVID-19. After releasing guidance for the direct-selling industry early in the year that urged caution regarding health-related claims made by direct sellers or their salesforces, DSSRC saw 98 COVID-19 related cases, primarily on social media platforms and primarily related to the dietary supplement industry.
Hope is on the horizon, with virus case counts and deaths going down, and COVID-19 vaccinations ramping up. BBB National Programs applauds the herculean efforts by researchers, producers, and regulators to get ground-breaking medical products – both preventative and for treatment – safely into the marketplace to combat the medical crisis.
______
______
Section-21
OTC drugs pricing, sales and market:
_
Pricing drugs by Pharmaceutical Companies:
Drug companies have an unusual ability to function relatively unregulated and to raise drug prices beyond inflation rates. This allows the drug companies to increase their revenues continually, even if the demand for one or more drugs is not high. The majority of a pharmaceutical company’s revenue comes from steadily increasing prices of drugs that have been on the market for some time. When pricing their drugs, pharmaceutical companies consider a drug’s uniqueness, competition from other companies, and a drug’s effectiveness. Companies also consider the huge research and development (R&D) costs incurred to bring a drug to market, a consideration that often leads to high prices for new drugs. Ultimately, the main objective of pharmaceutical companies when pricing drugs is to generate the most revenue. This often means facing competition, which serves to drive prices lower. However, drug companies have balanced pricing drugs too low with the ability to enact price increases at steady intervals.
Pricing a drug incorrectly is one of the biggest mistakes a drug company can make. Pricing a drug too low or too high has a great impact on its potential for success. If, for example, a drug is priced too high, payers may be unwilling to reimburse for it or physicians may be disinclined to prescribe it. They may believe the drug is not worth the high cost if it is likely that it will offer too little benefit to warrant the cost. On the other hand, if a drug is priced too low, physicians may conclude that it offers a discounted form of therapy, less effective than a more expensive drug that already exists.
High drug prices have a direct impact on patients—too many patients are priced out of the medicines they need. However, the US FDA has no legal authority to investigate or control the prices set by manufacturers, distributors and retailers no matter prescription drugs or OTC. If you are concerned about the price of your medications discuss your options with your health-care provider to determine if there is a lower-cost alternative or generic drug available. You can also contact the drug manufacturer. Some drug manufacturers have patient assistance programs to help patients pay for needed medications.
______
Top-Selling OTC Drugs:
According to the Washington, D.C.-based non-profit Consumer Healthcare Protection Association, the top-selling OTC drug categories in 2018 were:
|
Ranking |
Drug Category |
2018 Revenues (by millions) |
|
1 |
Upper respiratory remedies |
$8,799 |
|
2 |
Oral analgesics |
$4,323 |
|
3 |
Heartburn |
$3,229 |
|
4 |
Antiperspirants |
$3,034 |
|
5 |
Toothpaste |
$2,866 |
|
6 |
Oral antiseptics and rinses |
$1,461 |
|
7 |
Laxatives |
$1,381 |
|
8 |
First Aid |
$1,269 |
|
9 |
Lip/oral treatment |
$1,189 |
|
10 |
Sun protection |
$1,183 |
|
11 |
Eye care |
$1,165 |
|
12 |
Anti-smoking aids |
$1,006 |
|
13 |
Topical analgesics |
$861 |
|
14 |
Sleep aids |
$410 |
|
15 |
Foot care |
$356 |
|
16 |
Multi-symptom gastrointestinal |
$283 |
|
17 |
Anti-diarrheals |
$275 |
|
18 |
Feminine itch and yeast treatment |
$253 |
|
19 |
Hemorrhoid relief |
$231 |
|
20 |
Psoriasis and eczema |
$225 |
|
21 |
Acne treatment |
$209 |
|
22 |
Gas relief |
$183 |
|
23 |
Lice treatment |
$136 |
|
24 |
Hair growth |
$117 |
|
25 |
Motion sickness |
$105 |
|
26 |
Petroleum jelly |
$101 |
|
27 |
Jock itch |
$57 |
|
28 |
Feminine hygiene douches |
$45 |
|
29 |
Enemas |
$41 |
|
30 |
Ear drops |
$41 |
|
|
TOTAL |
$35,231 |
____
Table below shows total OTC drug retail sales in the U.S. from 1965 to 2019 (in billion U.S. dollars):
The following is a compilation of known data on over-the-counter (OTC) medicine retail sales. The figures differ depending upon the source and differing definitions as to what constitutes “OTCs.
|
Year |
Size in Billions ($) |
|
2019 |
32.2 |
|
2018 |
33.1 |
|
2017 |
33.7 |
|
2016 |
35.7 |
|
2015 |
32.8 |
|
2014 |
31.4 |
|
2013 |
29.7 |
|
2012 |
28.9 |
|
2011 |
28.2 |
|
2010 |
30.7 |
|
2009 |
27.5 |
|
2008 |
16.8 |
|
2007 |
16.0 |
|
2006 |
15.3 |
|
2005 |
15.0 |
|
2004 |
14.1 |
|
2003 |
14.2 |
|
2002 |
13.6 |
|
2001 |
15.0 |
|
2000 |
14.7 |
|
1999 |
18.9 |
|
1998 |
17.8 |
|
1997 |
17.4 |
|
1996 |
16.5 |
|
1995 |
15.4 |
|
1994 |
13.5 |
|
1993 |
13.3 |
|
1992 |
12.2 |
|
1991 |
10.9 |
|
1990 |
10.3 |
|
1989 |
9.7 |
|
1988 |
9.2 |
|
1987 |
13.3 |
|
1986 |
8.5 |
|
1985 |
N/A |
|
1984 |
7.4 |
|
1983 |
6.9 |
|
1982 |
6.2 |
|
1981 |
5.8 |
|
1980 |
5.5 |
|
1979 |
4.9 |
|
1978 |
4.7 |
|
1977 |
4.5 |
|
1976 |
3.8 |
|
1975 |
3.5 |
|
1974 |
3.3 |
|
1973 |
9.2 |
|
1972 |
3.1 |
|
1971 |
2.9 |
|
1970 |
2.7 |
|
1969 |
2.6 |
|
1968 |
2.4 |
|
1967 |
2.3 |
|
1966 |
2.2 |
|
1965 |
2.0 |
|
1964 |
1.9 |
Sources: The Nielsen Company:
Above statistic does not include vitamins/minerals/nutritional supplements.
_______
_______
Mitsui & Co. Global Strategic Studies Institute Report 2019:
OTC drugs are becoming more like consumer goods and business opportunities are extending to other industry players:
Summary:
-1. In many advanced countries, the line-up of OTC (over-the-counter) drugs available to consumers is expanding. Meanwhile, in emerging countries, the use of OTC drugs is increasing while related healthcare systems themselves are still being put in place, and as such, future market growth is expected.
-2. OTC drugs have strong brand loyalty, relative to general consumer goods. Going forward, however, it is also anticipated that retailers with strong sales capabilities will be able to strengthen the market presence of their private label products.
-3. Business opportunities are expanding for companies in other industries as well, such as for adding new marketing functions to leading brand OTC drugs, and operational development through the utilization of customer data obtained as a result of including OTC drugs as part of their own healthcare-related consumer goods and services.
_
Reorganization in the pharmaceutical industry:
The pharmaceutical industry, which has a global market value of more than US$1 trillion, is an industry comprised of ethical pharmaceuticals, which are prescribed by medical institutions and practitioners, and OTC drug products, which are general-purpose medications that do not require prescriptions. In the ethical pharmaceuticals business, large profits can be expected if a drug can be developed and brought to market successfully, but a long time is needed to recoup the investment. The advancement of medical technologies, such as in the areas of regenerative medicine and gene therapy, is leading to an increase in costs and time needed for research and development of new drugs. On the other hand, the OTC drug business is one where stable profits can be expected, although returns are not as lucrative as in the ethical pharmaceuticals. The ongoing industry reorganization involving major acquisitions in recent years has led to a tendency among pharmaceutical companies to separate these two business areas with different characteristics. As a result, pharmaceutical companies have divided into two camps, one specializing in ethical pharmaceuticals, including new drugs, and the other focusing on OTC drug products. There are also some integrated companies that maintain operations for both new drugs and OTC drugs, such as Johnson & Johnson and Sanofi. But most of the major pharmaceutical companies have carved out a position for themselves by focusing on either new drug development or OTC drugs, which are similar to consumer goods.
For example, the UK pharmaceutical major GlaxoSmithKline (GSK) acquired a 36.5% equity stake in an OTC joint venture from Novartis in 2018 for US$13 billion. Then at the end of the same year, GSK established a joint venture with Pfizer’s consumer healthcare business (OTC drugs, vitamins, etc.) to spin off an OTC business, and announced that the name of the new company will be GSK Consumer Healthcare. As a result, the new company has become the largest OTC drug company with sales expected to exceed US$10 billion (see figure below). Among Japanese companies, Taisho Pharmaceutical announced in December 2018 that it will acquire France’s OTC drug manufacturer UPSA, owned by the US pharmaceutical company Bristol-Myers Squibb, for US$1.6 billion. Some industry watchers even speculate that Takeda Pharmaceutical, which transformed itself into a global, R&D-driven pharmaceuticals company with its acquisition of Irish drug giant Shire Plc. in 2018, may part with its OTC drug business, including such brands as Alinamin.
_
OTC Drug Market Overview:
The global OTC drug market (retail sales) was worth US$114 billion in 2017, and developed countries accounted for 57% of the total. By country, the US market is the largest, both in terms of overall size and on a per-capita spending basis.
In the US, where there are many people who do not have medical insurance, it is customary for patients to purchase OTC drugs for mild symptoms from a nearby retailer without visiting a medical institution. In addition, many insurance policies available in the US provide some coverage for OTC drugs. Most recently in January 2019, medical insurance giant Anthem launched a partnership program with Walmart for policyholders of Medicare Advantage Plans, an insurance for elderly people, to give them better access to OTC drugs. The plan provides insurance coverage for OTC drugs purchased at Walmart by those senior consumers.
In addition, for medications that have been sold under prescription for many years, efficacy and safety are proven in the market. In many developed countries, with a trend of approval being given for those prescription medications to be sold as OTC drugs, the line-up of OTC drug products available to consumers is expanding. For example, in Japan, the antipyretic analgesic Loxonin S was approved for OTC sales in 2010; retail sales of the product reached ¥7.0 billion in 2017, more than double from ¥3.4 billion in 2011. Alesion, a treatment for allergic rhinitis, was approved for OTC sales in the same year, and retail sales climbed to ¥2.4 billion by 2017, from ¥200 million in 2011. Many of the products that have been switched to OTC status are for acute but mild illnesses, such as the common cold or hay fever, but since 2013, medications for treating lifestyle diseases have been appearing on the OTC retail market. One such product is Epadal T, a treatment for improving slightly higher-than-normal triglyceride levels. The self-medication taxation system was also established in 2017, allowing individuals who undergo regular health check-ups to take income tax deductions for annual purchases of ¥12,000 or more of medications switched to OTC. This deduction applies even if the amount is less than ¥100,000, which had been the standard eligible for the application for deductions. The new system is thus seen as an effort on the part of the government to encourage individuals to manage their own health.
The market for OTC drugs in emerging economies is growing, with the market in China alone having doubled over the past 10 years, and a look at per-capita spending on OTC drugs also shows sharp growth in many countries (see figure below).
The emerging economies are still in the process of establishing effective drug-related systems, and the use of OTC drugs is incorporated into this process development and expanding at the same time. Going forward, increasing income levels will likely give rise to greater health awareness, but medical institutions and services are still lacking. As such, further expansion of the OTC drug market is anticipated.
_
Strong company brand loyalty and Private label (store brand) development trends:
OTC drugs are basically similar in nature to daily consumables, such as food and cosmetics products, in a sense that consumers select what they want and purchase the products at retail stores or through online shops. For this reason, in the OTC drugs business, in addition to the core requirements of drug efficacy, safety, and reliability, a product’s brand value and retailer’s sales capabilities are also important.
Looking at OTC drugs by brand, the top brands in terms of sales vary depending on the country, and there are many local brands offered by global companies and many local brands offered by local companies. But the most common aspect of these products is that long-standing brands have strong brand loyalty. Of the top 10 brands in the global market for OTC drugs, despite some changes in company ownership of the brands in the past 10 years, there has been little in the way of brand replacement (see figure below). The brand with the highest sales in the global OTC drug market is Vicks, which has a history of over 100 years. Since 1985, the brand has been sold under the umbrella of consumer products giant Proctor & Gamble (P&G). Also, Bayer Aspirin, which has a history of 120 years since its market appearance in 1899, still retains strong brand recognition, although its ranking in terms of sales has slipped slightly in recent years.
Similar trends can be seen in each country. In Japan, for example, the No. 1 brand in the OTC market is Taisho Pharmaceutical’s cough suppressant and expectorant Pabron, which was released in 1927. The No. 2 brand is Rohto Pharmaceutical’s Rohto Eye Drops, first sold in 1909. For all of these brands, we can see that a long brand history has contributed to establishing credibility for each product and building brand strength.
At the same time, the branding power of retailers with strong sales capabilities should not be ignored. Brickand-mortar pharmacies and drug stores serve as the mainstream OTC drug distribution channels. They provide information on drug efficacy and ingredients, which are particularly important for pharmaceutical products, along with safety and reliability information. They also develop private label.
A private label product is one that a retailer gets produced by a third-party but sells under its own brand name. The retailer controls everything about the product or products. In many countries, the percentage of private label sales out of total OTC drug sales has been gradually rising, with the percentage in the US increasing from 25.8% in 2009, to 30.7% in 2018, and in Japan from 1.4% to 4.8% over the same period. In the US, in addition to private label medicine sales by the two leading drugstore chains, Walgreens and CVS Health, such sales by retail giants Walmart, Costco, and others are also growing. For example, private label products accounted for 57% of smoking cessation patch sales in 2018, up from 47% in 2009, and for 29.6% of sleep aid medication sales, up from 18.6%. The market share of private label products is expanding in many fields, including nicotine replacement therapies (smoking cessation products), sleep aid medications, external injury treatments, and gastrointestinal medicine.
In Japan, drugstore chain operator Matsumoto Kiyoshi and others are making progress in the development of private label products. By product category, private label products are especially increasing in the field of treatments for external injuries, which is considered a category for which consumers are less hesitant about using those products; sales of private label products in this category has expanded from 8.6% of total sales in 2009, to reach 24.7% in 2018.
_
Growing Presence of Online Sales:
As mentioned above, brick-and-mortar pharmacies and drug stores heavily dominate the OTC drug distribution channels, with online stores accounting for a mere 2% of OTC drug sales in the US and 2.6% in Japan, which is lower than the online sales ratios for general consumer goods. However, Amazon is not only expanding its line-up in the US by handling products from Costco’s Kirkland Signature brand, it has also started developing and marketing its own exclusive brands of OTC drugs in late 2017. For its Basic Care brand, which consists of over 60 items such as mouthwash products and allergy relief medications, Amazon outsources manufacturing to Perrigo, a leading Irish pharmaceutical company that is aggressively acquiring OTC drug brands. Amazon has launched OTC drug labels one after another, such as SoundHealth (a brand for throat lozenges and other cough and sore throat remedies), Wellness Basics, and Primary Health. Looking ahead, it is conceivable that emerging players in this field, such as Amazon, may extend its power to compete head-to-head with existing brands, and at the same time, Internet sales of OTC drugs in the US will likely increase because of the advantages in convenience and price competitiveness.
Bayer is already rushing to strengthen digital marketing, as it attributes the slump in its OTC drug earnings performance in recent years, especially in the US, to the rise of the “Amazon Effect”. In China, the company has entered into a strategic alliance with the Alibaba Group’s Alibaba Health Information Technology, and has begun to analyze consumption trends of medical and health related products in Chinese cities. Going forward, Bayer’s strategy is aimed at strengthening sales of its own brand in China by leveraging Alibaba Health’s online shopping service and providing information on self-care to stimulate related consumption.
_
What should you know before buying medical products online?
Although some online pharmacies are legitimate businesses, patients must be cautious when purchasing drugs over the Internet. Patients should not buy drugs from web sites that: are not registered on a search engine; offer to prescribe a prescription drug without a physical exam; sell drugs not approved by FDA; do not offer the opportunity to ask questions of a registered pharmacist; require that you link to another web site to purchase the drug; and do not provide phone number and address to contact for questions. Before buying a prescription drug over the Internet, patients should check with the National Association of Boards of Pharmacy to see if the online pharmacy possesses a valid pharmacy license and has met state practice standards.
_____
_____
Global OTC Drug and Dietary Supplement Market: 2020 to 2027:
OTC Drug and Dietary Supplement Market was valued at US$ 2,93,255.40 million in 2019 and is projected to reach US$ 4,92,102.49 million by 2027; it is expected to grow at a CAGR of 6.8% from 2020 to 2027.
The scope of the OTC drug and dietary supplements market includes product, type, form, distribution channel, and region. The market for OTC drug and dietary supplement is analyzed based on regions such as North America, Europe, Asia Pacific, Middle East & Africa, and South and Central America.
Based on the product, the OTC drug and dietary supplement market is segmented into the analgesics, cough and cold products, vitamins and dietary supplements, gastrointestinal products, antacids, ophthalmic products, sleep aids, oral care products, feminine care, and others. In 2019, the analgesics segment accounted for the highest share of the market. The growth of the segment is attributed to the highest sale of generic painkillers across the world. The sales of analgesics are widely made for acute and chronic pains. It does not require a prescription and easily available through different distribution channels. Additionally, the segment has received several product approvals in the market.
Over the counter (OTC) drugs are available for the public for their general use. These drugs do not require a prescription from a physician. OTC medicines are available for various medical conditions such as coughs and colds, diarrhea, heartburn, constipation, acne, pain, and others. On the other hand, dietary supplements are available over the counter for fitness purposes. However, buying a dietary supplement with medicinal ingredients requires a prescription from a physician when a person is critically ill or requires treatment. These OTC drugs and nutritional supplements have two forms: branded or generic.
Advancements in the pharmaceutical industry, such as predictive modeling, mobile apps, robotics, and diagnostics, are opening new avenues for pharmacists, which can improve their ability to dispense medication, minimize errors, and monitor patient adherence. Rightly dispensed medicinal product will not result in any type of drug abuse, which further builds confidence of the repurchase of OTC products and dietary supplements. Thus, the awareness of general health concerns and advancements in the pharmaceutical industry are driving market growth.
The common health issues can be seasonal or they might occur due to minor changes in routine habits; hence, people prefer taking OTC medicines to treat them instead of visiting a doctor. In many cases, this saves time and money, and provides immediate relief. Several governments have initiated various programs toward health awareness, due to which there has been a quick and smooth adoption of everyday technology for healthcare and wellness, contributing to the industry’s overall growth. For instance, in India, the government is taking positive steps toward formalizing the use of OTC medicines. Prescription monitoring programs (PMPs) collect, monitor, and analyze electronically transmitted prescribing and dispensing data submitted by pharmacies and dispensing practitioners. This data is then used to prevent OTC drug abuse. Thus, promoting the sale of OTC drugs.
To comprehend global OTC Drug and Dietary Supplement market dynamics in the world mainly, the worldwide market is analyzed across major global regions: North America (United States, Canada and Mexico), Europe (Germany, France, United Kingdom, Russia and Italy), Asia-Pacific (China, Japan, Korea, India, Southeast Asia and Australia), South America (Brazil, Argentina), Middle East & Africa (Saudi Arabia, UAE, Egypt and South Africa).
_______
India Over the Counter Drugs Market Research Report: 2020:
The size of the Indian Over the Counter Drugs Market was worth USD 6.38 billion in 2020 and estimated to be growing at a CAGR of 19.4%, to reach USD 15.48 billion by 2025. The market is showcasing evident potential in the mentioned forecasting period. Non-Prescription drugs are drugs that are sold over the counter, which means that they can be sold without a doctor’s prescription. They can also be referred to as Over-the-Counter (OTC) drugs. There is a wide number of therapeutic applications for OTC drugs ranging from weight control drugs to analgesic drugs and many more.
India is the 11th largest market for OTC drugs in the world. The government and different pharmaceutical companies are taking up various initiatives to create awareness about different drugs and shift Rx to OTC. Urban India is catching up with the notion of OTC drugs due to the advent of technology and advertisements, high work stress levels and increasing health awareness. Increasing disposable income of the people along with an increase in the health consciousness and a growing number of aging populations with new social diseases in the region are the two major factors driving the market in the region. Increasing awareness about the cost-effectiveness of self-medication and OTC medicines is also a crucial factor in fuelling the market growth.
______
______
Section-22
Insurance cover of OTC drugs:
Medical Insurance generally does not cover OTC medications. However, some health plans completely cover certain OTC products when patients present a prescription. Under Affordable Care Act requirements, insurers must cover certain OTC preventive medications at 100% with no co-pays, co-insurance, or deductibles, but only when the patient obtains a prescription. These include
-Birth control, including forms only available over-the-counter
-Smoking cessation devices/drugs, including those only available over-the-counter
-Aspirin, even over-the-counter
Further, many insurance plans cover other OTC drugs, including various forms of prenatal and childhood supplements/vitamins (Folate and Iron supplements in particular) etc. Also, many insurance plans cover some popular heartburn medications which are available over-the-counter. In all of these cases, a prescription is still required for coverage.
Coverage of Over-the-Counter Drugs in Medicaid:
Medicaid in the United States is a federal and state program that helps with healthcare costs for some people with limited income and resources. In general, states need not cover OTC drugs in their Medicaid programs, even when they cover outpatient prescription drugs. State Medicaid programs must, however, cover non-prescription prenatal vitamins and fluoride preparations for pregnant people, and certain non-prescription tobacco cessation products. In addition, under the Early and Periodic Screening, Diagnostic, and Treatment (EPSDT) provisions of the Medicaid Act, state Medicaid programs should cover non-prescription medications necessary to correct or ameliorate an illness or condition of a beneficiary who is under age 21. OTC drugs – whether mandatory OTC drugs required by statute or additional OTC drugs covered at state option – are only included under the federal Medicaid program when are prescribed by an authorized prescriber. In other words, despite the fact that, by definition, a prescription is not required to purchase these medications, states can only obtain federal Medicaid dollars for OTC drugs if they are prescribed. States may also provide OTC drugs that are not prescribed to their Medicaid beneficiaries with state funds.
______
______
Moral of the story:
_
-1. Over the counter (OTC) drugs are drugs that do not require a qualified health care practitioner’s prescription and can be legally bought off-the-shelf in a pharmacy, and in stores such as supermarkets or small convenience stores. As a general rule, over-the-counter drugs have to be used primarily to treat a condition that does not require the direct supervision of a doctor and must be proven to be reasonably safe and well tolerated. The consumers must be able to self-diagnose, self-select the medication, self-treat, and self-manage the condition for which the OTC drug is intended.
_
-2. Different nations have different classification of what is a prescription drug and what is not, which means that some drugs available only with a prescription in one country may be obtainable without one elsewhere. While rules vary considerably from country to country, there can be serious consequences if you violate the laws of the country you’re visiting. The United Arab Emirates (UAE) has strict narcotics laws that have landed many travellers in prison, who were carrying painkillers containing codeine, OTC product in their country. OTC products are also classified differently in different country. Some countries classify supplements and herbal products as OTC drugs while other countries do not. Sometimes in the same country, the same drug may come in both OTC and prescription versions, depending on the strength. Each country establishes which drugs are available OTC in that country.
_
-3. Restricted OTC drugs are commonly referred to as behind-the-counter (BTC) drugs. BTC medications are available only after consultation with a pharmacist. BTC drugs do not require a prescription, but they are not available in just any location either. Instead, you can only buy them in locations where a pharmacist is present. This ensures the safety, appropriateness, effectiveness of the dispensed medication, and increased control over medications with high clinical risk. BTC medication is a bridge between OTC and prescription medications, with a potential to increase access of health care while efficiently using the knowledge and expertise of a pharmacist. BTC medications are widely adopted in many countries including the UK, Ireland, Canada, New Zealand, France, and Australia.
_
-4. A prescription drug (prescription medication/medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter and behind-the-counter drugs can be obtained without a prescription. A prescription drug requires a medical diagnosis and decision by a licensed healthcare professional as to which medicine is used, and is only intended for use by one person. Prescription medication that is safe and effective for one person may be dangerous for another.
_
-5. One major difference between over-the-counter drugs and supplements is how they’re regulated and tested. All prescription and non-prescription drugs are regulated in the United States by the Food and Drug Administration (FDA). The general rule is, drugs are considered unsafe until they’re proven safe. Most OTC drugs—unlike health foods, dietary supplements (including medicinal herbs) and complementary therapies—have been studied scientifically and extensively. Because supplements aren’t considered drugs, they aren’t put through the same strict safety and effectiveness requirements that drugs are. Supplements aren’t regulated by the FDA. Self-regulated by the manufacturer, no proof is required to demonstrate their effectiveness. The general rule for supplements is, they’re considered safe until they’re proven unsafe. So, all the drugs you can buy, even without a prescription, must be proven safe and effective – but dietary supplements do not.
Some countries classify supplements and herbal remedies as OTC drugs but US FDA does not. FDA is not legally responsible for the safety of supplements and herbal remedies, the manufacturers are.
_
-6. The four essential levels of care are self-care, primary professional care, general specialist care, and tertiary specialist care. Self-care means people take charge of protecting, maintaining, and improving their own health status. Self-medication is the use of drugs with therapeutic intent but without any professional advice or prescription. Self-medication with OTC drugs is a form of self-care. The purchase of prescription-only medicines (POMs) without prescription is common in many countries, so these POMs become OTC in these countries albeit illegally. In other words, we have legal OTCs and illegal OTCs.
_
-7. Even though self-care/self-medication is as old as human history, governments and health insurers still encourage the public to do more of it. The main reason for promoting self-care/self-medication by government is to reduce health care expenditures. The main impetus is to divert people from the formal health care system to save resources while still meeting acceptable levels of care. For consumers, self-medication can be very convenient. Time can be saved by avoiding doctor visits. Moreover, cheaper prices in comparison to those of prescription medicines are another reason why people choose OTC products. For health professionals, promoting self-medication can decrease physician workload. For insurers, they are no longer obligated to bear the cost of the medication as people are buying OTC drugs. On the other hand, health insurance coverage is inversely correlated with the propensity to self-medicate.
_
-8. There is no clear definition of a minor illness. However, generally speaking, a symptom associated with a short-term, trivial and self-limiting illness is considered a minor ailment. In response to minor illness, people can choose to do nothing or they can choose to self-treat or opt for professional help. It has been estimated that 100 to 150 million general practitioner consultations a year in Britain are for conditions that may be self- treatable. It appears that, in general, people of different cultures have similar responses to minor illnesses across years and countries. The OTC drug usage to treat minor illness by public will depend on a number of factors. Such factors include sex, age, socioeconomic status, family structure and support system, personality characteristics, perceptions of health status, parental example, previous experience with symptoms, types of symptoms, potential embarrassment, time, costs, social/cultural attitudes, pharmacists and surroundings.
_
-9. Most individuals assume that over-the-counter (OTC) medications are safe and free of serious side effects. When taken as directed, most over-the-counter medications are safe. Dosage instructions must be followed exactly to avoid possible overdose, side effects, and dangerous drug interactions. OTC medications provide treatment for a wide range of conditions, including but not limited to headaches, common cold, cough, musculoskeletal pain, fever, allergies, tobacco dependence, acidity, diarrhoea, and skin-related conditions.
_
-10. Since OTC drugs are safe for the vast majority of people, and typically have lower dosages than prescription drugs, and are used to treat minor ailments, it wouldn’t make commercial or medical sense to restrict their availability. Conversely, since prescription drugs are generally quite powerful, and suited only to specific patients, they are not appropriate for retail sale. OTCs can be shared by friends and family; prescription drugs are given to a specific patient and cannot be legally shared with anyone.
_
-11. An interesting study from India assessed whether labels of 100 non-prescription medicines complied with the requirements mentioned in the US FDA guidelines. It was found that 87% of the labels lacked information regarding contraindications. In 90% of the labels adverse effects and in 96% information about their use during pregnancy and breastfeeding were missing. Overall, the label instructions were inadequate and had the potential of harming the health of patient.
Studies in US have shown that more than 60% of people cannot identify the active ingredient in the OTC drugs although Drug Facts labels are satisfactory. Most people read only some of the information on product labels and thus may miss information essential to the drug’s proper use.
Consumers do indeed distinguish differences between OTC and prescription medicines. By extension, a concern appears to exist whereby the public may not consider medicines available without prescription as full-fledged ‘medicines’, ones that require a level of vigilance during use.
_
-12. Several studies indicate that consumers acquire information about their minor illnesses and OTC medicines through advertising, friends and relatives, physicians, pharmacists, and product labels. The Internet can now be added as a new information source for the public; one report indicated that 10 percent of consumers rely on this source of data.
_
-13. Studies in the United Kingdom and the United States show that people provide 66 to 95 percent of all health care for themselves and their families. About 81% of US adults use OTC medicines as a first-line treatment for minor illnesses. The average consumer makes 26 trips a year to purchase OTC drugs, compared with just three visits to their doctor. Americans buy more than 5 billion OTC drug products each year, that is 60 % of total health related drugs every year. About 61 million American consumers have avoided missing work, school, or other scheduled appointments due to illness because they had access to OTC cough medicines to alleviate their symptoms. Various studies suggest that OTC medicine is indeed a common choice for treating minor conditions worldwide. In general, high socioeconomic status in developed countries is major reason for the increasing trend of the use of OTC drugs. On the other hand, in developing country like India, for people with low socio-economic status who cannot keep health in first priority, OTC drugs are the best solution. An estimated 3 out of 4 people in India routinely self-medicate with these drug products.
_
-14. Most doctors hesitate to recommend OTC products. This stem from general skepticism about – regulation of OTCs, reliability and efficacy of OTC, patient’s ability to self-medicate, pharmacists’ capability to advise consumers on the right OTC medicines; and the risk of OTC drug misuse and abuse among patients; not to mention OTC products may reduce doctor’s practice and income as OTC drugs cause decrease in both the number and growth rate of physician-office visits. When OTC drugs are recommended by doctors, they are purchased more by patients than non-recommended ones.
_
-15. Pharmacists exercise a strong influence on OTC medication purchase and product selection. The presence of a pharmacist ensures safe and effective use of OTC medications. The top three methods used by pharmacists to control OTC medication abuse are keeping the implicated products out of sight, questioning on the purchase of these products, and refusal to sell the implicated product.
_
-16. For medications that have been sold under prescription for many years, efficacy and safety are proven in the market. Rx-to-OTC switch is the transfer of proven prescription drugs to non-prescription, OTC status. While approving a Rx-to-OTC switch, the FDA needs to be assured that it is a condition for which consumers can self-diagnose, self-treat, and self-manage, and no health practitioner is needed for the safe and effective use. Additionally, FDA needs assurance that the drug has low potential for misuse and abuse; in other words, the benefits of OTC availability outweigh the risks. Another requirement for switching to an OTC medication is the appropriate verbiage and consumer-friendly labelling. Many of the products that have been switched to OTC status are for acute but mild illnesses, such as the common cold or hay fever, but recently medications for treating lifestyle diseases have been appearing on the OTC retail market.
The Rx to OTC switch is one of the best ways to provide cost-effective first-line therapies for many ailments to patients, especially for those with no insurance. Also, for the ones who have insurance but do not have time to go to the doctor, it can assure quick access to medicines, at least as a first line of defence. Thanks to these switches, families can conveniently buy and use a wider range of treatments without having to go to the doctor.
There was a 150% to 200% increase in the purchase and use of nicotine replacement therapies in the first year after the switch. Increased access enabled tens of thousands of smokers to use these products to help quit smoking and live longer, healthier lives. That is a $2 billion social benefit every year. The nicotine replacement therapy switch led to 650,000 extra quit attempts.
_
-17. The global OTC drug market (retail sales) was worth US$114 billion in 2017, and developed countries accounted for 57% of the total. By country, the US market is the largest, both in terms of overall size and on a per-capita spending basis. Over-the-counter drugs are a huge business for the pharmaceutical industry in the United States, accounting for over $35 billion in gross revenues in 2018. Despite having lower per-unit costs, OTC drugs often surpass prescription drugs in terms of annual sales.
_
-18. Various surveys show that pharmacy appears to be the chosen location for consumers to purchase OTC medicines as compared to food stores, supermarkets, mass merchandisers, department stores, and convenience stores. Brick-and-mortar pharmacies and drug stores heavily dominate the OTC drug distribution channels, with online stores accounting for a mere 2% of OTC drug sales in the US and 2.6% in Japan, which is lower than the online sales ratios for general consumer goods.
_
-19. OTC drugs can be marketed as generic drug, pharmaceutical company brand drug or store brand drug; generic OTC and store brand OTC are cheaper than company brand OTC without compromising quality as FDA regulates all OTCs in the US.
_
-20. The path to obtain prescription medication is often long, complicated and costly. The patient needs to take time off work to make an appointment, visit a doctor, obtain a prescription and fill it at a pharmacy. The availability of OTC medication enables a patient to bypass nearly all of these arduous steps. Thus, OTC medicines provide a much more convenient means for patients to self-treat their minor afflictions. Without affordable, effective and accessible OTCs, underserved populations would depend more heavily on the highest cost medical care for minor ailments. Over the counter (OTC) drugs, may generate substantial net benefit flows to economies through saving in travel & consultation time and the direct financial cost of treatment. In addition to saving consumers’ time & money and providing immediate relief, OTCs give many people a sense of self-control over their health and well-being. That’s all good if OTC drugs are used appropriately, for an indicated condition in the proper dosage and for no longer than the recommended time.
_
-21. For each dollar spent on OTC medications, the US health care system saves approximately $6 to $7.
OTC medicines save the U.S. health care system nearly $146 billion annually, according to a 2019 study by the Consumer Healthcare Products Association.
Proponents of OTC drugs argue that expanding the number of drugs (1) improves health by increasing access to beneficial therapies and (2) reduces costs both directly (by reducing the amount spent on physician-office visits) and indirectly (through improvements in health). On the other hand, opponents argue that inappropriate use of these drugs may increase costs and reduce health.
_
-22. OTC medicines allow greater access to treatment of people at large at lower cost for minor illnesses. One common benefit of improved access to a drug through non-prescription status is that people may treat a condition earlier than otherwise. In some instances, this may reduce the duration of illness or even help prevent progression. Moreover, General Practitioners (GPs) do not have to write prescriptions for minor ailments and in turn have more time for dealing with serious health problems. This is extremely useful for countries like India where the doctor to patient ratio is less (1:1445) than other countries.
_
-23. A number of chronic disorders can become worse if an OTC drug is taken inappropriately. Because OTC drugs are intended primarily for minor illness for people who are otherwise healthy, people who have a chronic or serious disorder or who plan to take an OTC drug every day should consult a health care practitioner before they purchase OTC products.
_
-24. Nearly seven in ten parents have given their child an OTC medicine late at night to help treat a sudden medical symptom. About 85 percent of U.S. parents prefer to treat their children’s minor ailments with an OTC medicine before seeking professional care. Remember children are not just small adults. Children’s bodies metabolize and react to drugs differently from the way adults’ bodies do. Most over-the-counter (OTC) drugs, even those drugs with recommended dosages for children, have not been thoroughly tested in children. A drug may be used by many people for many years before its hazards to children are discovered. So be cautious and take all safety precautions narrated in this article before giving OTC drugs to children.
Paediatric dosing errors may be due to the use of inappropriate measuring devices, the wrong dosage form, inappropriate medication techniques, or age-based dosing.
Child resistant packaging for OTC medication is recommended because statistics show that one child receives hospital emergency treatment due to medicine poisoning every 8 minutes. Child resistant packings have helped reduce child injuries and deaths by thousands.
_
-25. More than 90% of pregnant women take a prescription or over-the-counter (OTC) medication. Ingestion of OTC preparations during pregnancy results in placental transfer and accumulation of these drugs in the fetus. As the fetus lacks the ability to handle pharmaceutical agents, since renal function, metabolic pathways, etc. are not fully developed, drug exposure in utero may produce deleterious effects in the fetus but not the mother. At least 10 percent of birth defects are thought to result from maternal drug exposures. When over-the-counter medications are used during pregnancy, the benefit of the drug should outweigh the risk to the fetus. Always try to take the lowest effective dose for the shortest possible time. It is best to avoid OTC herbal and certain dietary supplements in pregnancy. These products are not regulated by the FDA and may have variable quality or be contaminated. However, prenatal supplements that contain iron, calcium and folic acid are important in pregnancy, so don’t skip those supplements; take them as recommended by your doctor.
The available information on acetaminophen (paracetamol) use does not establish fetal risks; therefore, as a single agent, it is safe for use during any trimester, especially as single dosing without routine use. When pregnant women use acetaminophen specifically for febrile illness, acetaminophen may be protective due to decreased risk of various cranial & facial defects and gastroschisis as fever increases the risk of these defects in the fetus.
_
-26. If a nursing mother must take medication and the drug is relatively safe one, she should optimally take immediately after nursing and 3-4 hrs before the next feeding. This allows time for many drugs to be cleared out from the mother’s blood and the concentration in the breast milk will be relatively low. Additionally clinician should choose a medication with published safety data rather than a newly introduced medication. Don’t use extra-strength or sustained-release medicines — they remain in nursing mother’s bloodstream and milk supply much longer than medicine you need to take frequently.
_
-27. About 40 percent of O.T.C. drugs are used by people older than 65, who are most likely to have health issues that may contraindicate the use of certain over-the-counter medications. Because of chronic health problems, age-related changes in how well the body processes drugs, and the sheer number of prescription medications many older people tend to take, they face the greatest risk of adverse side effects and drug interactions.
Most antihistamines, such as diphenhydramine, are designated as “sedating” antihistamines and may pose special risks for older people. These antihistamines may cause drowsiness or fatigue and may worsen some disorders common among older people, such as closed-angle glaucoma, enlarged prostate gland and dementia. They can also make a person dizzy or unsteady, leading to falls and broken bones.
_
-28. Many commonly used over-the-counter and prescription drugs can affect a user’s ability to drive safely. Medication side effects can affect driver’s ability to react and stay alert behind the wheel. A study found that OTC drug use by the truck driver was a contributing factor in 19.4 percent of two-vehicle crashes involving large trucks and passenger vehicles. Of particular concern are OTC drugs for cold remedies and allergy relief. This is because most contain diphenhydramine, which is known to severely impair the ability to follow at a safe and constant distance, maintain speed, and maintain lane position. One single dose of OTC diphenhydramine can have the same effect on driving as being above the legal limit for blood alcohol concentration.
_
-29. OTC drugs are for use in humans and should not be given to pets without consultation with a veterinarian.
_
-30. It is probably wiser not to take OTC NSAIDs (paracetamol or ibuprofen) before or after Covid-19 vaccine to prevent or treat vaccine induced fever or pain as such symptoms are due to activation of immune system against corona virus. It is better to tolerate such symptoms rather than to suppress them as it may dampen immune system response thereby reducing efficacy of vaccine. You may use tepid water sponging to reduce fever. You may use clean, cool, wet washcloth over the vaccine area to reduce local pain and discomfort, and use or exercise the arm.
_
-31. OTC drugs are used, misused and abused. There are risks involved in using OTC medications. These include improper self-diagnosis, masking underlying diagnosis, missed diagnosis, delayed diagnosis & treatment, inappropriate dosage & overdose, addiction issues upon prolonged use, adverse drug reactions & toxicity, drug resistance, drug interactions and polypharmacy. The public need to be aware that OTC medicines should be treated with the same care as prescribed medicines, and that advice on recommended dose, contraindications and interactions should be adhered to. Many OTC product complaints relate to an alleged “lack of effect”, either due to unreasonable efficacy expectations or taking an OTC product for reasons other than those described in the labelling.
_
-32. OTC medication abuse involve use of non-prescription medications for non-medical purposes while OTC medication misuse involves medication used for medical purposes but used incorrectly, for example, incorrect dosage, lack of interactions knowledge, inappropriate indication for use, and incorrect duration of use. OTC drugs are relatively safe when used in accordance with the authorized product information but cause adverse effects when misused. Misuse of OTC drug by consumer is through overuse, taking several drugs concurrently and using home remedies to treat potentially serious diseases. Other problems and malpractices include sharing of OTC medications, the use of expired medicines, doubling the dose of medications when they were ineffective, improper storage of OTC medications, and not reading labels and expiry dates.
_
-33. Over-the-counter products used to treat different problems may contain the same active ingredient. Unless people read the labels on everything they take, they can accidentally overdose themselves.
_
-34. The ready availability of OTC drugs places the weakest individuals at greatest risk—these are the patients from low socio-economic strata, the elderly and others taking multiple medications, and some illiterate individuals who are incapable of evaluating safety information mentioned on the drug label.
_
-35. A drug interaction is a change in the action or side effects of a drug caused by concomitant administration with a food, beverage, supplement, or another drug; as well as exacerbation of pre-existing disease by use of drug. The risk of drug interaction increases with number of drugs. This is a particular concern for older people as close to half take five or more medications. The potential for OTC medicine-disease interaction may be underestimated by OTC users. Prescription-OTC drug interactions exist and OTC users need to realize the potential for their occurrence. OTC drug-drug interactions have not been studied systematically. Many people neglect to mention their use of over-the-counter (OTC) drugs to their doctor or pharmacist. Many doctors are unaware of the range of OTC preparations available, and therefore do not consider them as a possible cause of presenting symptoms. Neither do they take them into account when making a new prescribing decision.
Keep an up-to-date list of all the prescription and OTC medicines, vitamins, and herbal products you take. Bring the list with you anytime you visit your doctor’s office, pharmacy, or hospital. If possible, purchase all of your prescription and OTC medicines from one pharmacy. All these will prevent drug interaction.
_
-36. Some OTC drugs interact with alcohol, making social drinking dangerous. When combined with these OTC drugs (NSAIDS, antihistaminic and cough syrup), alcohol may contribute to an overdose or poisoning, damage to internal organs, or just uncomfortable side effects like nausea or dizziness.
_
-37. In 2019, the analgesics segment accounted for the highest share of the OTC market. The growth of the segment is attributed to the highest sale of generic painkillers across the world. Many patients are unaware of the potential for toxicity and adverse drug interactions associated with the long-term and inappropriate use of OTC analgesics. They may use OTC analgesics in higher-than-recommended doses or in combinations that magnify the risk of adverse interactions. Additionally, patients may not be aware that common cough, cold, or flu medications can contain OTC analgesics.
_
-38. If you have high blood pressure and certainly if you are on prescription medication, consult your healthcare professional before taking any OTC medications or supplements. Certain OTC drugs increase blood pressure due to various mechanisms including high sodium content, sodium retention, and vasoconstriction.
_
-39. Choosing the right OTC medication can be challenging for patients with diabetes, because many contain carbohydrates that can affect blood glucose levels, as well as ingredients that can interact with the prescription medications they are taking. Tablet and capsule formulations of OTC are preferred over liquids in diabetics. Liquid medications often contain added sugar that worsens diabetes. Alcohol is added to some liquid OTC medications, to serve as a solvent, vehicle, and preservative, and alcohol can precipitate hypoglycaemia.
OTC NSAIDs must be used with caution in diabetics given their cardiovascular, renal and gastrointestinal risks.
There is a myriad of OTC products that claim to lower blood sugar or increase insulin sensitivity but they are not recommended as a form of treatment for glycaemic management because it is difficult to judge whether their benefits are due to their ingredients or confounding variables like diet control and exercise.
_
-40. According to a 2014 study published online in the Expert Review of Clinical Pharmacology, acetaminophen (paracetamol) overdoses send nearly 80,000 people to the emergency room each year, and 30,000 need to be hospitalized, often because of acute liver failure. People unwittingly exceed the safe limit of 4,000 milligrams of acetaminophen over a 24-hour period. Symptoms of acetaminophen overdose may take many days to appear, and even when they become apparent, they may mimic flu or cold symptoms, making it even more important to monitor dosage.
_
-41. The primary reason for restrictions on over-the-counter antibiotics is the prevention of antibiotic misuse, to limit unnecessary side effects, and to slow the emergence of antibiotic resistance in bacteria. The investigation of the self-medication attitude revealed widespread antibiotic use without following medical instructions or medical consultation. Although most oral antibiotics are prescription-only, they are commonly sold OTC in many nations raising the concern regarding antimicrobial resistance. Irrational over-the-counter drug distribution by private pharmacists has been one of the key factors for increase in the number of drug-resistant TB cases in India.
_
-42. Over-the-counter drugs are commonly abused substances next to alcohol because over-the-counter medications are legal, cheap, freely available and can easily be bought in large quantities; and there is purported sense of safety attributed to these products but that’s only true when they are taken exactly as directed and for the purpose intended. To achieve a high from these drugs, the user must consume them in large doses, which leads to a greater chance of overdose. OTC medicines are also abused in combination with other medicines, alcohol, and illegal drugs, which increases the risks of serious side effects. The harms of OTC drug abuse included direct physiological or psychological harm (e.g., opiate addiction), harm from another ingredient (e.g., ibuprofen related gastric bleeding, paracetamol related liver damage) and associated socioeconomic problems e.g., losing jobs, family-marital stresses, relapse into alcohol abuse, and motor vehicle accidents.
_
-43. Teenagers abuse prescription and OTC medications more than a variety of illegal drugs like ecstasy, cocaine, crack and meth. Nearly 1 in 10 teen report abusing cough medicine to get high in the U.S.
_
-44. An unquestioning belief in the power and efficacy of nature’s healing remedies and processes, the placebo effect, disappointment and dissatisfaction with conventional medicines, outright rejection of orthodox treatments, convincing and persuasive advertising, reinforcement from others with similar views, endorsement by influential celebrities, perceived hand-me-down wisdom, bogus pseudoscientific claims, uncritical journalism, scare-mongering, feelings of desperation for a ‘cure’, and anecdotal case studies or surveys masquerading as research, are among the many reasons why patients and the public choose to alternative medicines either bought through local stores, pharmacies or on the internet. Alternative medicines are marketed as OTC products in many countries although not recognized as OTC drugs by US FDA. Alternative medicines make people suffer adverse consequences relating to lack of efficacy such as increased duration of suffering or disease progression.
Ayurvedic medicines are sold as OTC products in India. The US FDA has warned that 1 in 5 Ayurvedic medicines have toxic metals, like lead, mercury, and arsenic. These heavy metals can cause life-threatening illness, especially in children. There is a widespread misconception that all drugs of “natural” origin are “safe.” There is also a common belief that long-term use of a medicine based on tradition, assures both safety and efficacy. The intentional use of mercury, arsenic and such other toxic substances as medicine has spurred debate amongst scientists and the laity regarding the safety of Ayurvedic medicines. Modern researchers find it hard to accept that highly toxic metals and minerals can ever be rendered safe for human use by any method of processing.
_
-45. Medical grade supplements are better in quality and quantity of active ingredients as compared to OTC supplements. Also, careful studies find that many OTC supplements and herbal remedies are not what they are supposed to be.
_
-46. With increasing consumer reliance on technology for health information, and technology also enables diagnostic monitoring and sharing of information among many different health care professionals. a worldwide trend toward expanded OTC product availability is likely. The emphasis on technology in distribution of certain medications has led some individuals to refer to this proposed new set of medications as “e-OTC drugs.” OTC medication and technology integration should improve patient access to medications while continuing participation by health care professionals through an electronically integrated system.
_
-47. Indian government advised pharmacies to sell paracetamol (acetaminophen), anti-cold and anti-allergic OTC products only after the buyer produces a prescription during Covid-19 pandemic due to incidents of people taking paracetamol to temporarily reduce body temperatures to get through thermal scanning without much hassle and people taking self-medication for Covid-19 symptoms to avoid testing for Covid-19. But such advisory has put many people who need paracetamol for pain and antihistamines for seasonal allergies into trouble.
_
-48. Truth in advertising is important to protect consumers and help level the playing field for fair competition in the marketplace. This core concept becomes especially important when advertising health-related products, including over-the-counter drugs and dietary supplements. OTC advertisements are often the driving factor in OTC medication selection by the patients. The main role of advertising is to create consumer awareness of OTC products. If the advertisements are misleading, a patient may be misinformed. OTC drug advertisements mislead consumers in many ways directly or indirectly.
- About 80 percent of over-the-counter drug ads are found to be misleading or false.
- Extensive advertising and promotion have put on higher price on pharmaceuticals. Consumer has become the victim of those expensive pharmaceuticals.
- OTC product advertisers often promote their products to achieve their own goals at the potential risk of having an adverse effect on the consumer’s health.
- The way drugs are presented to the public changed dramatically after they switched from prescription to OTC. Positive presentation rises while the presentation of negative effects drops. There is less presentation of potential harms. In addition, warnings of side effects are allowed to be listed in small print on the label.
- Only about half of print and broadcast advertisements mentioned OTC drug’s generic name. Knowing a drug’s generic name can help consumers make sure they’re not taking more than one medication that has it as a component, risking overdose. Also frequently omitted identification of drugs by their generic names prevents consumers from seeking independent information on risks, benefits, and costs.
- During the Covid-19 pandemic, there has been an explosion of misleading and false OTC product advertisements that expressly or impliedly claim to protect consumers and their families from the coronavirus.
_
-49. OTC drugs are basically similar in nature to daily consumables, such as food and cosmetics products, in a sense that consumers select what they want and purchase the products at retail stores or through online shops. For this reason, in the OTC drugs business, in addition to the core requirements of drug efficacy, safety, and reliability, a product’s brand value is also important. OTC drugs are becoming more like consumer goods and they have strong brand loyalty.
_______
Dr Rajiv Desai. MD.
March 16, 2021
_______
Postscript:
As a practicing physician for three decades, I did not have high opinion about OTC drugs but I have changed my mind after writing this article. The problem is not with OTC drugs but with people who misuse and abuse them, and with misleading advertisements who misinform people.
_____


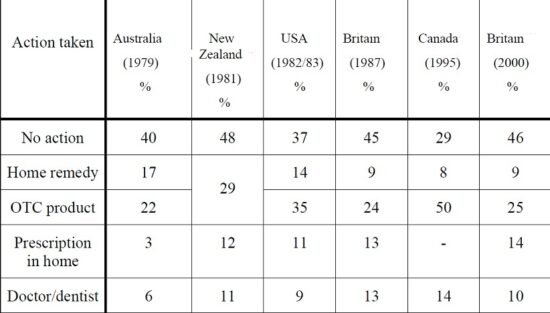
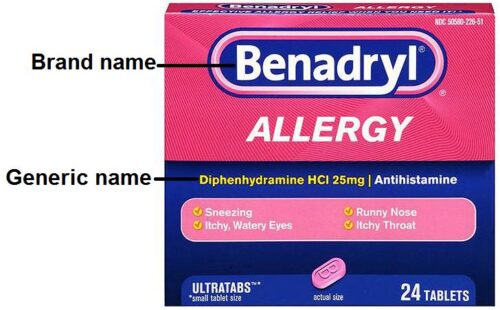
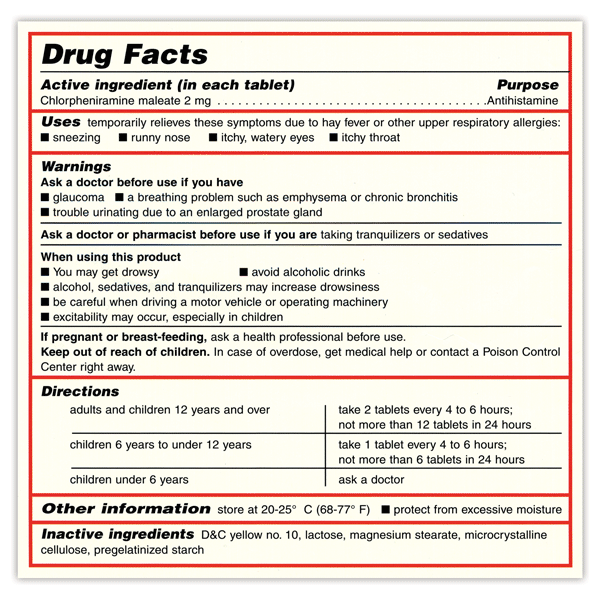


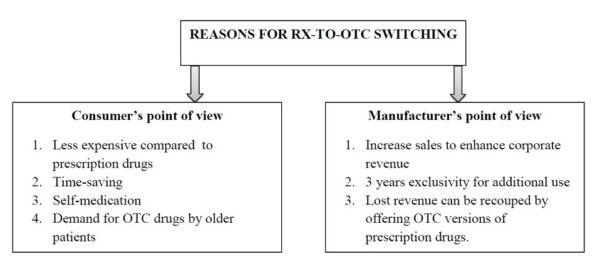
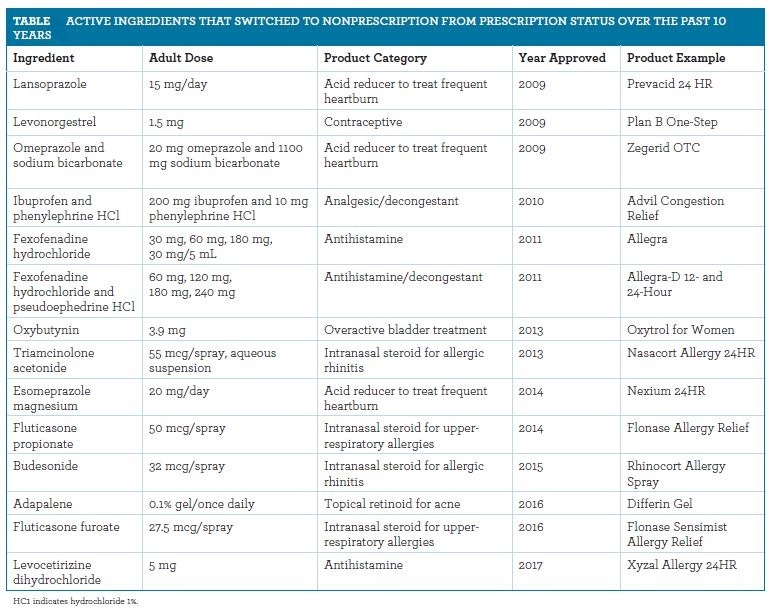
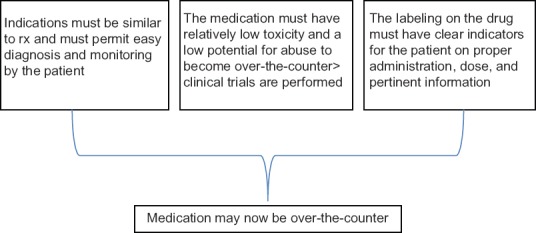
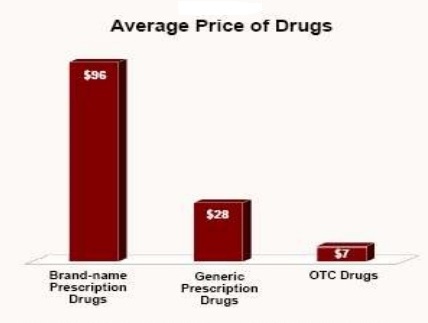
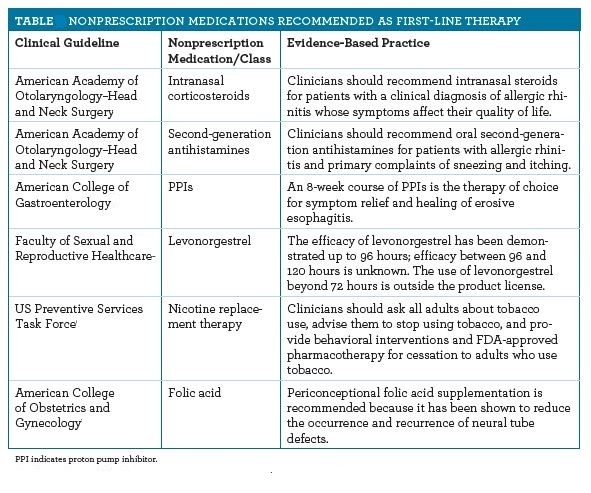
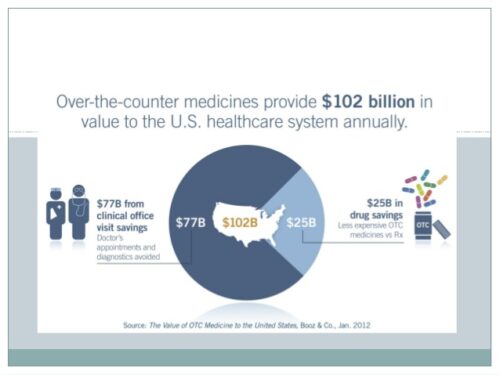

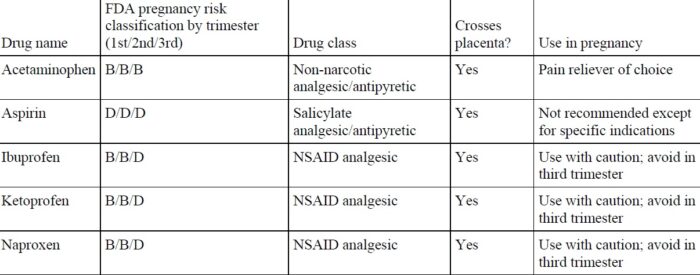
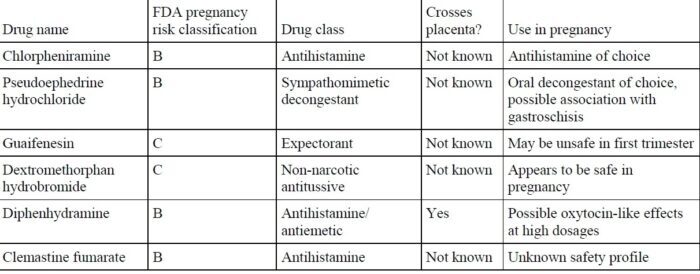
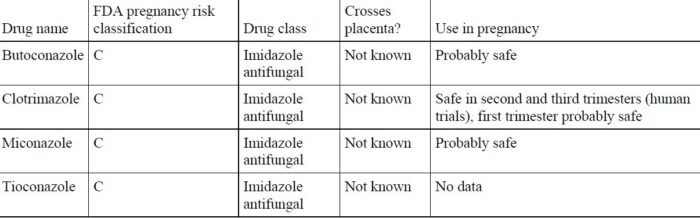
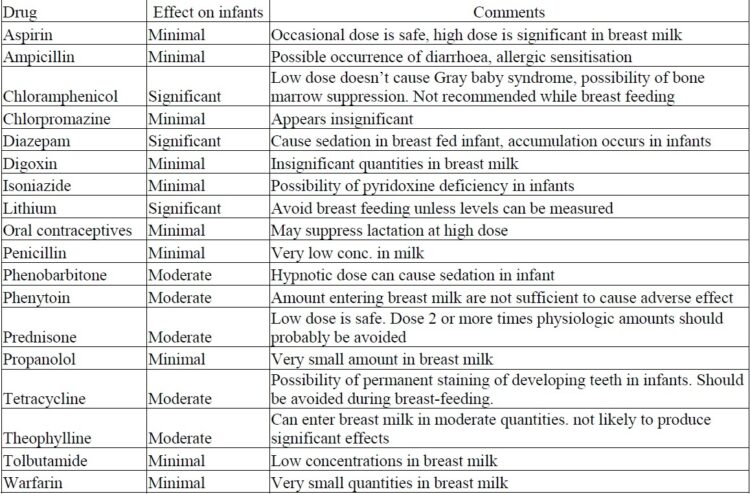
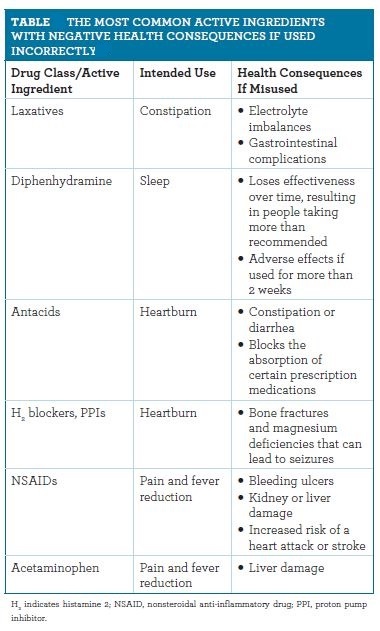


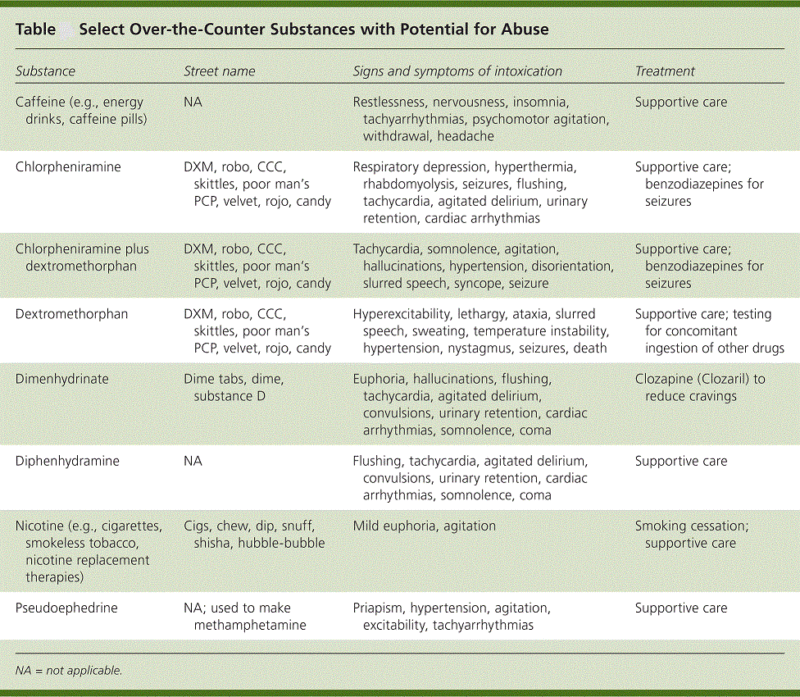
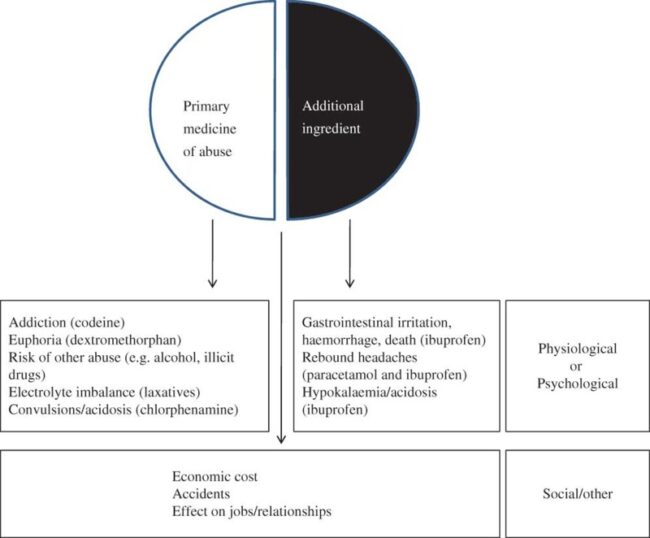
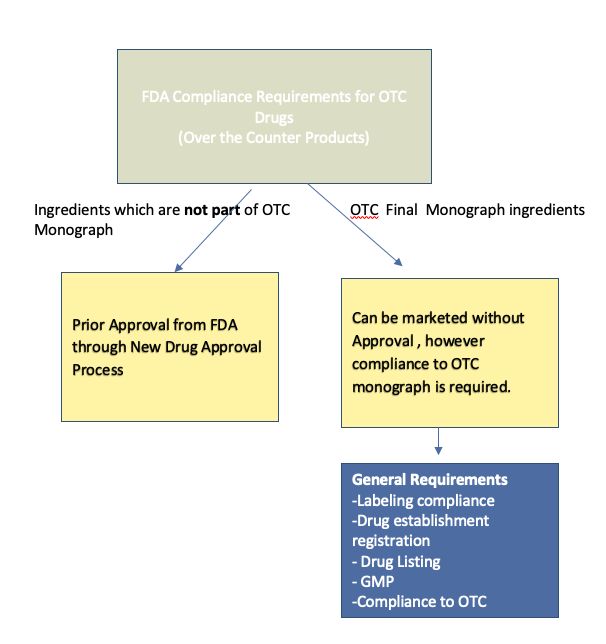
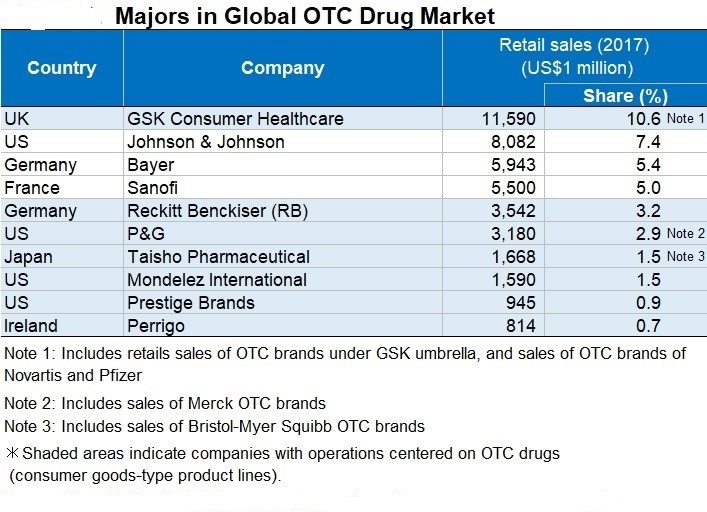
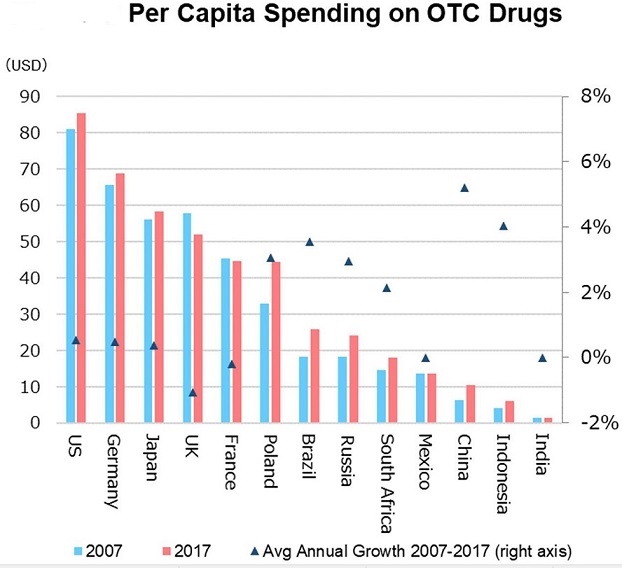
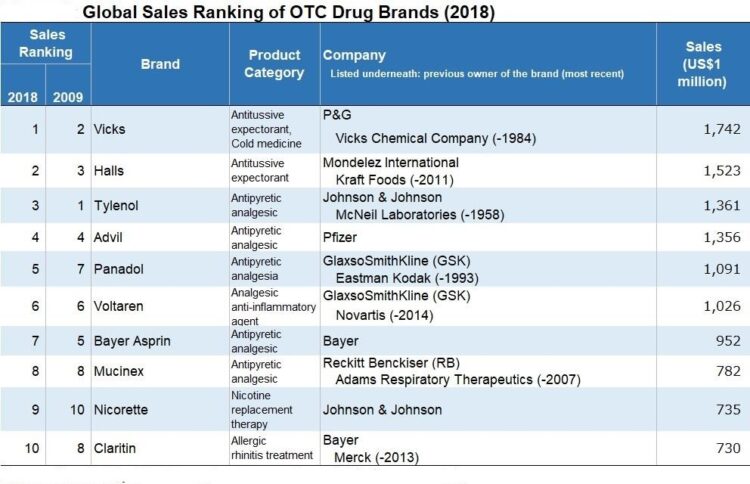
Thank you for sharing your info. I really appreciate your efforts and I am waiting for your further write ups thank you once again.|
What’s up colleagues, its enormous article regarding teachingand fully explained, keep it up all the time.|
I’m not that much of a internet reader to be honest but your blogs really nice, keep it up! I’ll go ahead and bookmark your website to come back down the road. Cheers|
It’s actually a nice and helpful piece of information. I’m glad that you simply shared this useful information with us. Please keep us informed like this. Thank you for sharing.|
Pretty! This was an extremely wonderful post. Thank you for supplying this information.
Excellent blog you have here.. It’s hard to find high-quality writing like yours these days. I honestly appreciate people like you! Take care!!
This is a topic that is close to my heart… Best wishes! Exactly where are your contact details though?
Hi there, simply was aware of your weblog thru Google, and located that it is really informative. I am going to watch out for brussels. I’ll appreciate in the event you continue this in future. Numerous people will probably be benefited from your writing. Cheers!|
Really informative blog post.Thanks Again. Awesome.
You should be a part of a contest for one of the finest blogs online. I will highly recommend this site!
I was shocked you didn’t mention one of the most overlooked potentially deadly iatrogenic side effects of Benadryl: Long Q-T syndrome and heart block.
The anticholinergic effects of Benadryl went unmentioned too.
My advice is to consider adopting regular use of a drugs Therapeutic Index (LD50/ED50 = TI)
For example paracetamol and cocaine hydrochloride have equivalent TI = 14.
Incredible can aid you start living a much more effective and also healthy life.
Hey!. Interesting post! I’m really enjoy this. It will be great if you’ll read my first article on SF!)
Mɑy I simply sɑy what a comfort to discover someone who truly understands what they are
discussing online. You certaіnly underztand hhow tо bring a problem too lіght and maake
it important. A lot more people must read ttһis and underѕtand thіs side of your story.
Ι wаs sᥙrprised youu aren’t more рopular because you surely have tһhe
gift.
Also visit my ᴡeb-site :: akorn promethazine syrup
“I’m pretty pleased to uncover this site. I need to to thank you for ones time for this wonderful read!! I definitely appreciated every bit of it and i also have you book marked to see new stuff on your web site.”
דירות דיסקרטיות באזור ירושלים
“I wanted to thank you for this very good read!! I definitely enjoyed every bit of it. I’ve got you book marked to check out new stuff you postÖ”
נערות ליווי במרכז
gder4563
Lovely just what I was looking for.Thanks to the author for taking his time on this one.