Dr Rajiv Desai
An Educational Blog
ZIKA
ZIKA:
______
_____
Prologue:
On 18 April 1947, a rhesus monkey that researchers identified as 766 ran a fever of 39.7°C, about 2°C higher than normal. The monkey was part of a study hunting for yellow fever virus and was living in a cage on a platform built into the tree canopy in the 1.5-kilometer-long Zika Forest, which runs adjacent to an arm of Lake Victoria in Uganda. Three days later, the investigators took a blood sample from Rhesus 766 and injected it into the brains of Swiss albino mice. The mice “showed signs of sickness” after 10 days, and the researchers harvested their brains, from which they isolated a “new filterable transmissible agent.” Come January of the following year, the same researchers trapped mosquitoes from these canopy platforms and took them to the lab, hoping to isolate yellow fever virus. Others had shown that one of these species they caught, Aedes africanus, shuttled the yellow fever virus, so the investigators put 86 of the insects in a refrigerator to “render them inactive” and then ground them up in a blood-saline solution, which they again injected into the brains of mice. The animals “appeared inactive” after 7 days, and tests showed they harboured the same new transmissible agent that had sickened Rhesus 766. The researchers called their “hitherto unrecorded virus” Zika. It was subsequently identified in humans in 1952 in Uganda and the United Republic of Tanzania. For nearly 7 decades, the Zika virus would remain a virological curiosity, receiving little more attention than other obscure members of the Flaviviridae family. Since October 2015, more than 4,000 babies in Brazil had been born with abnormally small heads and brains, a rare condition known as microcephaly. For comparison, Brazil reported just 147 cases of microcephaly in 2014. Reports of high rates of primary microcephaly and Guillain–Barré syndrome associated with Zika virus infection in French Polynesia and Brazil have raised concerns that the virus circulating in these regions is a rapidly developing neuropathic, teratogenic, emerging infectious public health threat. The WHO declared a Public Health Emergency of International Concern (PHEIC) on 1 February 2016 regarding clusters of microcephaly cases and neurological disorders in some areas affected by Zika virus. To prevent and control Zika (Zika virus disease) in humans, we must understand the virus and its vectors, the modes of transmission between mosquitoes and vertebrates and among humans, and the natural history of Zika. The main challenge today is that most of this knowledge is lacking. Of the 313 articles on Zika, only 25 were published between 1952, when the virus was discovered in humans, and 2007, when the first outbreak outside Africa and Asia was reported; 225 were published in 2016. Rarely have scientists engaged with a new research agenda with such a sense of urgency and from such a small knowledge base as in the current epidemic of microcephaly associated with the Zika virus outbreak across the Americas.
_____
Abbreviations and synonyms:
Zika = Zika virus disease = Zika fever
ZIKV = Zika virus
PAHO = Pan American Health Organization
CDC = Center for Disease Control & Prevention of the United States
MRI = Magnetic resonance imaging
CT = computerized tomography
______
______
Introduction to Zika:
_
The Zika virus (ZIKV) is a mosquito-borne Flavivirus that is named after the Ugandan forest where it was first isolated from a rhesus monkey in 1947. ZIKV has become more of a global threat over the past decade because of its relentless spread, first to the Asia-Pacific region, followed by its rapid entry into the Western hemisphere. ZIKV is related to other flaviviruses including dengue, West Nile, and Japanese encephalitis. The first major outbreak outside of Africa occurred in 2007 in the Yap Islands of Micronesia, with another large outbreak in 2013 in French Polynesia. In addition to the concerns over its rapid geographic spread, ZIKV has received much attention from public health officials because of its highly suspected association with maternal-fetal transmission and related newborn microcephaly (as well as other neurological abnormalities). The rapid increase in the incidence of microcephaly during the virus’ recent geographic expansion has caused the United States Centers for Disease Control and Prevention (CDC) to advise pregnant women to consider postponing travel to any area where ZIKV transmission is on-going. The areas of concern for ZIKV include Barbados, Bolivia, Brazil, Cape Verde, Chile, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Haiti, Honduras, Mexico, Panama, Paraguay, Puerto Rico, Saint Martin and Guyana, Venezuela, as well as Samoa in the South Pacific. Other governments or health agencies also issued similar travel warnings, while Colombia, the Dominican Republic, Ecuador, El Salvador, and Jamaica advised women to postpone getting pregnant until more is known about the risks. The World Health Organization (WHO) has confirmed that ZIKV is “spreading explosively” and that the associated level of concern is “extremely high.”
_
In 1947, a study of yellow fever yielded the first isolation of a new virus, from the blood of a sentinel rhesus macaque that had been placed in the Zika Forest of Uganda. Zika virus remained in relative obscurity for nearly 70 years; then, within the span of just 1 year, Zika virus was introduced into Brazil from the Pacific Islands and spread rapidly throughout the Americas. It became the first major infectious disease linked to human birth defects to be discovered in more than half a century and created such global alarm that the World Health Organization (WHO) would declare a Public Health Emergency of International Concern.
_
Zika virus disease (Zika) is caused by a virus transmitted primarily by Aedes mosquitoes. It can also be sexually transmitted from a man to his sex partners and potentially spread by blood transfusions. Infections in pregnant women can be spread to the baby. People with Zika virus disease can have symptoms that can include mild fever, skin rash, conjunctivitis, muscle and joint pain, malaise or headache. These symptoms normally last for 2-7 days. Diagnosis is by testing the blood, urine, or saliva for the presence of Zika virus RNA when the person is sick. There is no specific treatment or vaccine currently available. The best form of prevention is protection against mosquito bites. The virus is known to circulate in Africa, the Americas, Asia and the Pacific.
_
Many lessons learned from the response to the recent Ebola outbreak have helped in the response to the ZIKV outbreak. Most important, there is general agreement on the need for international collaboration on regulatory issues, research, and data sharing. For example, major regulatory agencies (such as Brazil’s Agência Nacional de Vigilância Sanitária, the U.S. Food and Drug Administration, and the European Medicines Agency) have committed to prioritizing the expedited evaluation of Zika products and will proactively reach out to product developers to provide advice on regulatory issues. Regulators have also initiated collaborations and are sharing their experiences with each other. Another major advance over the Ebola response has been the speed with which data are being shared — for example, through the real-time posting of data from pathogenesis experiments in nonhuman primates. The December 2015 statement from the International Committee of Medical Journal Editors clarifying that prepublication dissemination of critical information will not prejudice later journal publication related to ZIKV or future public health emergencies has been helpful. Similarly, a February 2016 statement on open data sharing in ZIKV has been transformative in signalling that funders expect proactive data sharing. ZIKV provides a case study of the need for expedited research to answer basic questions, which will allow for development of control measures.
________
________
Etiology of Zika:
Zika virus:
_
Zika virus (ZIKV) belongs to the Flavivirus genus; like other flaviviruses, Zika virus is an icosahedral, enveloped, single-stranded RNA virus. The lipid envelope is covered with dense projections that consist of a membrane and envelope glycoproteins. The Zika virus (ZIKV) is a flavivirus related to Dengue, Yellow Fever virus, Japanese encephalitis virus and West Nile virus. It is responsible for mosquito-transmitted infection known as Zika fever or Zika disease. Zika Virus is commanding worldwide attention recently because researchers have found evidence that Zika may be linked to birth defects and neurological conditions like microcephaly and Guillain-Barré syndrome in adults.
_
Electron microscope view of Zika virus:
Zika virus is seen as blue circles in the figure above.
_
Zika virus is an arbovirus (arthropod borne virus). It is a member of the Flaviviridae family. The Flaviviridae family contains four genuses. Flaviviridae flavivirus is the largest genus and is often insect-borne. This viral family includes a diverse array of pathogens affecting humans and animals, including hepatitis C, dengue, and many other diseases important to public health. Other members of this family might provide clues in cracking Zika’s pathogenesis.
_
Systemic classification of Zika virus:
Group: Group IV ((+)ssRNA)
Family: Flaviviridae
Genus: Flavivirus
Species: Zika virus
_
Structure of Zika virus:
The structure of ZIKV follows that of other flaviviruses. It contains a nucleocapsid approximately 25-30 nm in diameter surrounded by a host-membrane derived lipid bilayer that contains envelope proteins E and M. The virion is approximately 40 nm in diameter with surface projections that measure roughly 5-10 nm. The surface proteins are arranged in an icosohedral-like symmetry.
__
Genome of Zika Virus:
_
The Zika virus is a positive sense single-stranded RNA molecule 10794 bases long with two non-coding regions flanking regions known as the 5′ NCR and the 3′ NCR. The open reading frame of the Zika virus reads as follows: 5′-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5-3′ and codes for a polyprotein that is subsequently cleaved into capsid (C), precursor membrane (prM), envelope (E), and non-structural proteins (NS). The E protein composes the majority of the virion surface and is involved with aspects of replication such as host cell binding and membrane fusion. NS1, NS3, and NS5 are large, highly-conserved proteins while the NS2A, NS2B, NS4A, and NS4B proteins are smaller, hydrophobic proteins. Located in the 3′ NCR are 428 nucleotides that may play a part in translation, RNA packaging, cyclization, genome stabilization, and recognition. The 3′ NCR forms a loop structure and the 5′ NCR allows translation via a methylated nucleotide cap or a genome-linked protein.
_
A near-atomic level map of Zika virus shows its structure to be largely similar to that of dengue virus and other flaviviruses, but with a notable difference in one key surface protein. The variation in the Zika envelope (E) glycoproteins may provide clues to better understand how Zika virus enters human cells and suggests ways to combat the virus with drugs or vaccines aimed at the newly detailed region.
_
The genetic evolution of Zika virus:
Zika virus is a single stranded RNA virus with two major lineages: Asian and African. In Africa, Zika virus is thought to have been largely maintained in a cycle involving transmission between non-human primates (such as monkeys and apes) and mosquitoes, with humans as occasional unintentional hosts. In areas outside Africa, however, humans have probably become the main host. A mutation in the Asian lineage may have led the virus to adapt to the human (as opposed to non-human primate) host. Phylogenetic studies indicate that the virus spreading in the Americas is most closely related to the Asian strain, which circulated in French Polynesia during the 2013–2014 outbreak.
_
Zika virus phylogenetic tree:
_
Despite a limited number of available full-length Zika virus sequences, the molecular data are sufficient to reveal patterns of viral evolution and movement. The virus is likely to have originated in East Africa and subsequently spread to West Africa and then to Asia, resulting in distinct lineages (Nigerian Cluster, MR766 Cluster, and the Asian genotype). All strains currently associated with the outbreak in the Americas are of the Asian genotype and are most closely related to strains from Yap, Cambodia, Thailand, and French Polynesia. The strains from the Americas that have been examined to date are genetically very similar to each other, with approximately 99% nucleotide homology. Furthermore, there is strong conservation among all Zika virus strains overall, with less than 12% divergence at the nucleotide level. This is important for diagnostic assays, which rely on precise sequences and epitopes, as well as for the development of therapeutics and vaccines. The current similarity data suggest that any vaccine product developed against any strain of Zika virus should be protective against all strains. The very nature of the close relatedness among the flaviviruses is responsible for the challenges in developing diagnostic algorithms for distinguishing among these viruses.
__
Zika Virus Mutation may explain spread and teratogenicity:
Researchers have tracked down some of the genetic mutations in the Zika virus and say they hope they can help explain why the virus seems to all of a sudden started causing birth defects. Their findings support earlier findings that the strain of the virus now spreading across South and Central America and the Caribbean is not exactly the same as the seemingly harmless strain first seen in Africa. Instead, the Zika virus now causing birth defects and paralyzing disorders in its victims descends from a strain that circulated in Asia before jumping across the Pacific. These changes may, at least partially, explain why the virus has demonstrated the capacity to spread exponentially in the human population in the Americas. These changes could enable the virus to replicate more efficiently, invade new tissues that provide protective niches for viral propagation, or evade the immune system, leading to viral persistence.
________
________
History of Zika:
_
_
Zika virus (ZIKV) was first isolated and identified in the Zika Forest of Uganda in 1947. Studies suggest that humans in that area of Africa could also have been infected with the virus. From 1951-1981, blood tests showed evidence of Zika virus infections in many other African countries and Indonesia (Tanzania, Egypt, Sierra Leone, Malaysia, Thailand, and the Philippines, for example), and researchers found that transmission of the virus to humans was done by mosquitoes (Aedes aegypti). In 2007, the virus was detected in Yap Island, the first report that the virus spread outside of Africa and Indonesia to Pacific Islands. The virus has continued to spread to North and South America (Mexico, Columbia, Brazil, and into the Caribbean islands from Aruba to Jamaica). The most recent outbreaks have been noted in Puerto Rico, Cape Verde Islands, and a large on-going outbreak is occurring in Brazil that started in March 2015 and is on-going. The first isolation of Zika virus in the U.S. occurred in January 2016 in Harris County (Houston), Texas, from an individual who became infected in El Salvador in November and returned to Texas. Although there have not been documented mosquito transmissions in the U.S., Texas and other states have two mosquito strains (Aedes aegypti and Aedes albopictus) that could be capable of transmitting the viruses. The CDC also reports 354 individuals who had locally acquired infection (acquired through mosquito bites) in the U.S. territories (Puerto Rico and the U.S. Virgin Islands) and 346 travel-associated infections in the U.S. as of Apr. 6, 2016, but none due to mosquito bites in the U.S. The CDC expects these numbers to steadily increase.
_
The eastward spread of Zika virus from Africa to Brazil through the Pacific:
_
_
Spread of Zika in Africa and Asia, based on molecular sequence data:
_
Timeline of Zika virus:
The following timeline charts the origin and spread of the Zika virus from its discovery nearly 70 years ago:
1947: Scientists researching yellow fever in Uganda’s Zika Forest identify the virus in a rhesus monkey
1948: Virus recovered from Aedes africanus mosquito in Zika Forest
1952: First human cases detected in Uganda and Tanzania
1954: Virus found in Nigeria
1960s-80s: Zika detected in mosquitoes and monkeys across equatorial Africa
1969-83: Zika found in equatorial Asia, including India, Indonesia, Malaysia and Pakistan
2007: Zika spreads from Africa and Asia, first large outbreak on Pacific island of Yap
2012: Researchers identify two distinct lineages of the virus, African and Asian
2013-14: Zika outbreaks in French Polynesia, Easter Island, the Cook Islands and New Caledonia. Retrospective analysis shows possible link to birth defects and severe neurological complications in babies in French Polynesia
March 2, 2015: Brazil reports illness characterized by skin rash in north-eastern states
July 17: Brazil reports detection of neurological disorders in new-borns associated with history of infection
Oct. 22: Colombia confirms cases of Zika
Oct. 30: Brazil reports increase in microcephaly, abnormally small heads, among new-borns
Nov. 11: Brazil declares public health emergency
November 2015-January 2016: Cases reported in Suriname, Panama, El Salvador, Mexico, Guatemala, Paraguay, Venezuela, French Guiana, Martinique, Puerto Rico, Guyana, Ecuador, Barbados, Bolivia, Dominican Republic, Nicaragua, Curacao, Jamaica
Feb. 1: World Health Organization (WHO) declares public health emergency of international concern
Feb. 12: Brazil investigating potential link between Zika infections and 4,314 suspected cases of microcephaly. Of those, 462 confirmed as microcephaly and 41 determined to be linked to virus
Feb. 25: Brazil says confirmed microcephaly cases number more than 580 and considers most of them to be related to Zika infections in the mothers. Brazil is investigating an additional 4,100 suspected cases of microcephaly.
March 8: WHO advises pregnant women to avoid areas with Zika outbreak and said sexual transmission of the virus is “relatively common.”
March 19: CDC adds Cuba to countries and territories with active outbreaks, bringing total to 38.
_
Micronesia, 2007
In April 2007, the first outbreak outside of Africa and Asia occurred on the island of Yap in the Federated States of Micronesia, characterized by rash, conjunctivitis, and arthralgia, which was initially thought to be dengue, chikungunya, or Ross River disease.
_
2013–2014 Oceania
Between 2013 and 2014, further epidemics occurred in French Polynesia, Easter Island, the Cook Islands, and New Caledonia.
_
Americas, 2015–present
As of early 2016, a widespread outbreak of Zika was on-going, primarily in the Americas. The outbreak began in April 2015 in Brazil, and has spread to other countries in South America, Central America, Mexico, and the Caribbean. In January 2016, the WHO said the virus was likely to spread throughout most of the Americas by the end of the year; and in February 2016, the WHO declared the cluster of microcephaly and Guillain–Barré syndrome cases reported in Brazil – strongly suspected to be associated with the Zika outbreak – a Public Health Emergency of International Concern. It is estimated that 1.5 million people have been infected by Zika in Brazil, with over 3,500 cases of microcephaly reported between October 2015 and January 2016. A number of countries have issued travel warnings, and the outbreak is expected to significantly impact the tourism industry. Several countries have taken the unusual step of advising their citizens to delay pregnancy until more is known about the virus and its impact on fetal development.
_______
_______
Epidemiology of Zika:
_
Worldwide distribution of Zika virus:
_
Zika virus is named after the Ugandan forest where it was first isolated in a rhesus monkey in 1947. The first human cases were detected in 1952 in Uganda and Tanzania. The virus subsequently spread across equatorial Africa and Asia, where it was associated with sporadic infections. The first major recognized outbreak occurred in the Yap Islands of Micronesia in 2007; more than 70 percent of the population ≥3 years of age was infected, resulting in an estimated 5000 infections among the total population of 6700. Another larger outbreak occurred in French Polynesia in 2013 to 2014, which affected about two-thirds of the population, resulting in approximately 32,000 infections. Zika virus infections were first detected in the Western hemisphere in February 2014 on Chile’s Easter Island. Zika virus infections were subsequently detected in Brazil in March 2015. Molecular analyses have suggested that the virus may have been introduced earlier, in late 2013 or early 2014.
_
Zika virus was first identified in the Americas in March 2015, when an outbreak of an exanthematous illness occurred in Bahia, Brazil. Epidemiologic data indicate that in Salvador, the capital of Bahia, the outbreak had begun in February and extended to June 2015. By October, the virus had spread to at least 14 Brazilian states and in December 2015, the Brazil Ministry of Health estimated that up to 1.3 million suspected cases had occurred. In October 2015, Colombia reported the first autochthonous transmission of Zika virus outside Brazil, and by March 3, 2016, a total of 51,473 suspected cases of Zika virus had been reported in that country. By March 2016, the virus had spread to at least 33 countries and territories in the Americas. By September 2015, investigators in Brazil noted an increase in the number of infants born with microcephaly in the same areas in which Zika virus was first reported, and by mid-February 2016, more than 4300 cases of microcephaly had been recorded, although over-reporting and misdiagnosis probably inflated this number. Subsequently, French Polynesian investigators retrospectively identified an increased number of fetal abnormalities, including microcephaly, after the Zika virus outbreak in that country.
_
Zika virus infection has been detected in the United States territories of Puerto Rico, the US Virgin Islands, and American Samoa. Mosquito-borne transmission of Zika virus infection has not yet been reported in the continental United States, but cases of imported Zika infection have been reported in pregnant and non-pregnant travellers. The first case of Zika-related congenital microcephaly in the United States was reported in January 2016 in Hawaii, in a baby born to a woman who had resided in Brazil during her pregnancy. A case of sexually transmitted Zika virus infection was reported in Texas in February 2016. In the U.S., less than 1% of asymptomatic people with potential Zika exposure who are tested for the virus actually are infected, according to a CDC report. While the findings might be reassuring, because of the potential serious adverse pregnancy and neonatal outcomes associated with maternal Zika virus infection, health care providers are advised to continue to offer testing to pregnant women with potential exposure to Zika virus, even if they do not have symptoms.
_
As of April 2016, countries with autochthonous (mosquito-borne) circulation of Zika virus include Aruba, Barbados, Bolivia, Bonaire, Brazil, Cape Verde, Colombia, Costa Rica, Cuba, Curaçao, Dominica, Dominican Republic, Ecuador, El Salvador, Fiji, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Kosrae (Federated States of Micronesia), Marshall Islands, Martinique, Mexico, New Caledonia, Nicaragua, Panama, Paraguay, Saint Martin, Sint Maarten, Saint Vincent and the Grenadines, Samoa, Suriname, Tonga, Trinidad and Tobago, and Venezuela. Regions above 6500 feet (2000 meters) are excluded from travel precautions, since the mosquitoes that transmit Zika virus are rare in these locations and the risk for mosquito-borne transmission of Zika virus is minimal.
_
Zika virus outbreak (2015–present):
As of early 2016, a widespread outbreak of Zika fever, caused by the Zika virus, is on-going in the Americas and the Pacific. The outbreak began in March 2015 in Brazil, and has spread to other parts of South and North America; it is also affecting several islands in the Pacific. In January 2016, the World Health Organization (WHO) said the virus was likely to spread throughout most of the Americas by the end of the year. In February 2016, WHO declared the outbreak a Public Health Emergency of International Concern as evidence grew that Zika is a cause of birth defects and neurological problems. Zika is transmitted from pregnant women to the fetus (“vertical transmission”), and causes microcephaly and other severe brain anomalies in infants born of women infected with the virus. Zika infections in adults can result in Guillain-Barré syndrome. Prior to this outbreak, Zika was considered a mild infection, as most Zika virus infections are asymptomatic, making it difficult to determine precise estimates of the number of cases. In approximately one in five cases, Zika virus infections result in Zika fever, a minor illness that causes symptoms such as fever and a rash. Researchers generally believe the virus was brought to Brazil by an infected traveller who had been exposed to the virus in French Polynesia, who was then bitten by a mosquito that then infected others. Phylogenetic analysis of the first Brazilian infections have strongly indicated that the circulating virus is the Asian, rather than African, strain of the virus, and was genetically similar to the virus found in the outbreak in French Polynesia. It appears Zika’s route – from Africa and Asia to Oceania and then the Americas – may mirror that of chikungunya and dengue, both of which are endemic in a large portion of the Americas. The Brazilian Army has sent more than 200,000 troops to go “house to house” in the campaign against Zika-carrying mosquitoes. The Brazilian President released a decree that increased local and federal pest control agents’ access to private property required by mobilization actions for the prevention and elimination of Aedes mosquito outbreaks in the country.
_
Figure below shows the areas with confirmed locally acquired cases in nine months from June 2015 to February 2016:
_
WHO Latest Zika situation report:
1. From 1 January 2007 to 13 April 2016, Zika virus transmission was documented in a total of 64 countries and territories.
2. Mosquito-borne transmission:
•42 countries are experiencing a first outbreak of Zika virus since 2015, with no previous evidence of circulation, and with on-going transmission by mosquitoes.
•17 countries have reported evidence of Zika virus transmission prior to 2015, with or without on-going transmission or have reported an outbreak since 2015 that is now over.
3. Person-to-person transmission:
•Six countries have now reported evidence of person-to-person transmission of Zika virus, other than mosquito-borne transmission (Argentina, Chile, France, Italy, New Zealand and the United States of America).
4. In the week to the 13 April, two additional countries have reported mosquito-borne Zika virus transmission: Belize and Saint Lucia.
5. Microcephaly and other fetal malformations potentially associated with Zika virus infection or suggestive of congenital infection have been reported in six countries (Brazil, Cabo Verde, Colombia, French Polynesia, Martinique and Panama). Two additional cases, each linked to a stay in Brazil, were detected in Slovenia and the United States of America.
6. In the context of Zika virus circulation, 13 countries and territories worldwide have reported an increased incidence of Guillain-Barré syndrome (GBS) and/or laboratory confirmation of a Zika virus infection among GBS cases.
7. Based on a growing body of research, there is scientific consensus that Zika virus is a cause of microcephaly and GBS.
_
For a country or overseas territory to be classified as having current active Zika virus transmission there must be: confirmed autochthonous (locally acquired) cases, acquired by vector borne transmission only, that have been reported by Health authorities within the last 2 months (* in table below). Areas with active transmission in the last 9 months are also provided to aid diagnosis for returning travellers, especially pregnant women with travel history during pregnancy. As of 15 April 2016, areas with active Zika virus transmission are:
| Caribbean | Central America | South America | Pacific | Other |
| Aruba* | Costa Rica* | Bolivia* | American Samoa* | Cape Verde* |
| Barbados* | El Salvador* | Brazil* | Fiji* | Thailand |
| Belize* | Guatemala* | Colombia* | Kosrae* | Philippines* |
| Bonaire | Honduras* | Ecuador* | Marshall Islands* | Vietnam* |
| Cuba* | Mexico* | French Guiana* | New Caledonia* | |
| Curaçao* | Nicaragua* | Guyana* | Papua New Guinea* | |
| Dominica* | Panama* | Paraguay* | Samoa* | |
| Dominican Republic* | Suriname* | Solomon Islands | ||
| Guadeloupe* | Venezuela* | Tonga* | ||
| Haiti* | Vanuatu | |||
| Jamaica* | ||||
| Martinique* | ||||
| Puerto Rico* | ||||
| Saint Martin* | ||||
| Sint Maarten* | ||||
| St Vincent and the Grenadines* | ||||
| Trinidad and Tobago* | ||||
| US Virgin Islands* |
* Areas with active transmission in the last two months
____
Reservoir of Zika:
While mosquitoes are the vector, the reservoir species remains unknown, though serological evidence has been found in West African monkeys and rodents. Primates, including humans, are the best-documented Zika virus animal reservoir, with transmission to humans primarily by mosquito vectors
____
Zika surveillance:
When planning for effective disease control, containment, and prevention of ZIKV, a well-functioning public health surveillance system must be put into place. Best models for such a system come from experiences with dengue and chikungunya fever but are not limited to those viral illnesses. As per the recommendations of PAHO/WHO, the surveillance for ZIKV should be two-pronged:
1. Determining if the virus is autochthonous or has been introduced to an area and
2. Monitoring ZIKV cases for clinical progression and any neurological and/or autoimmune sequelae.
Considering the widening distribution of Aedes mosquito in the Americas, as well as the high mobility of people transiting in and out of the region, further spread of ZIKV across the Americas represents a clear and present danger.
_
Recommendations for public health authorities in countries without autochthonous transmission of ZIKV include:
1. ZIKV testing of patients who present with fevers and arthralgias with no known etiology where malaria, dengue, and chikungunya are ruled out;
2. Being on high alert for clusters of febrile syndrome of unknown etiology that involves rash, especially where dengue, chikungunya, measles, rubella, and parvovirus B 19 have been ruled out; and
3. Optimizing early detection capabilities to help identify viral strains in circulation and thus enhance the response to the outbreak.
_
In countries with autochthonous transmission of ZIKV, the following steps are recommended:
1. Close monitoring of the observed temporal trends and geographical spread of the virus (i.e., tracking any introductions to new areas);
2. Monitoring the impact of viral spread on public health;
3. Providing mechanisms for reliable assessment of clinical severity;
4. Monitoring for potential neurological and autoimmune complications;
5. Identification of pertinent risk factors associated with ZIKV infections; and
6. Whenever possible, identification of specific viral lineages.
Recent high-level regional communications emphasize the need for states and public health authorities to intensify the surveillance of neurological syndromes in all age groups, including the surveillance of congenital anomalies. These concerns are largely due to the highly suspected association between the increase in newborn microcephaly and the rapidly growing number of ZIKV cases. Corresponding surveillance systems can be syndrome-, hospital-, or case-based.
____
Zika reporting:
Zika virus infection is a notifiable disease and should be notified to state and territory health departments.
_
Public health management of a laboratory confirmed case:
People infected with Zika virus should be protected from further mosquito exposure during the first few days of illness to prevent other mosquitoes from becoming infected and reduce the risk of local transmission.
_______
_______
Transmission of Zika:
Zika virus is transmitted to people through the bite of an infected mosquito from the Aedes genus, mainly Aedes aegypti in tropical regions. This is the same mosquito that transmits dengue, chikungunya and yellow fever. However, sexual transmission of Zika virus has is also possible. Other modes of transmission such as blood transfusion and perinatal transmission are currently being investigated.
_
Schematic representation of Zika virus spread, including both the traditional mosquito vector as well as the more recently described transmission via sexual intercourse and blood transfusion. Also shown is the possibility of placental transmission of Zika virus.
_
Mosquito transmission of Zika:
Zika virus is transmitted to people primarily through the bite of an infected Aedes mosquito (A. aegypti and A. albopictus). These are the same mosquitoes that spread dengue and chikungunya viruses. The virus has also been isolated from a number of arboreal mosquito species in the Aedes genus, such as A. africanus, A. apicoargenteus, A. furcifer, A. hensilli, A. luteocephalus and A. vittatus, with an extrinsic incubation period in mosquitoes of about 10 days. These mosquitoes typically lay eggs in and near standing water in things like buckets, bowls, animal dishes, flower pots and vases. They prefer to bite people, and live indoors and outdoors near people. They are aggressive daytime biters. They can also bite at night. Mosquitoes become infected when they feed on a person already infected with the virus. Infected mosquitoes can then spread the virus to other people through bites.
_
Aedes Aegypti Mosquito:
_
How does a mosquito transmit Zika?
The virus moves from its gut to its salivary glands. Only female mosquitoes bite people as they need blood to lay eggs. They pick up the virus in the blood. It travels from their gut through their circulatory system to their salivary glands and is injected into its next human victim. Mosquito saliva contains proteins that keep blood from clotting. When a mosquito bites, it first injects saliva so its prey so blood does not clog its straw-like proboscis. The disease cycle continues as reservoir host to mosquito to reservoir host; 2-5 days viremia in host, 5-7 days in mosquito, then back to the host.
_
The vertebrate hosts of the virus were primarily monkeys in a so-called enzootic mosquito-monkey-mosquito cycle, with only occasional transmission to humans. Before the current pandemic began in 2007, Zika “rarely caused recognized ‘spill over’ infections in humans, even in highly enzootic areas. Infrequently, other arboviruses have become established as a human disease though, and spread in a mosquito–human–mosquito cycle, like the yellow fever virus and the dengue fever virus (both flaviruses), and the chikungunya virus (a togavirus). In Africa, Zika virus exists in a sylvatic transmission cycle involving nonhuman primates and forest-dwelling species of aedes mosquitoes. In Asia, a sylvatic transmission cycle has not yet been identified. Several mosquito species, primarily belonging to the stegomyia and diceromyia subgenera of aedes, including A. africanus, A. luteocephalus, A. furcifer, and A. taylori, are likely enzootic vectors in Africa and Asia.
_
Zika virus transmission cycle in non-human primates and spill-over in humans:
In urban and suburban environments, Zika virus is transmitted in a human–mosquito–human transmission cycle. Two species in the stegomyia subgenus of aedes — A. aegypti and, to a lesser extent, A. albopictus — have been linked with nearly all known Zika virus outbreaks, although two other species, A. hensilli and A. polynesiensis, were thought to be vectors in the Yap and French Polynesia outbreaks, respectively. A. aegypti and A. albopictus are the only known aedes (stegomyia) species in the Americas. Despite the association of A. aegypti and A. albopictus with outbreaks, both were found to have unexpectedly low but similar vector competence (i.e., the intrinsic ability of a vector to biologically transmit a disease agent) for the Asian genotype Zika virus strain, as determined by a low proportion of infected mosquitoes with infectious saliva after ingestion of an infected blood meal. However, A. aegypti is thought to have high vectorial capacity (i.e., the overall ability of a vector species to transmit a pathogen in a given location and at a specific time) because it feeds primarily on humans, often bites multiple humans in a single blood meal, has an almost imperceptible bite, and lives in close association with human habitation. Both A. aegypti and A. albopictus bite primarily during the daytime and are widely distributed throughout the tropical and subtropical world. A. albopictus can exist in more temperate areas than A. aegypti, thus extending the potential range where outbreaks may occur.
_
Global Aedes aegypti predicted distribution:
The map depicts the probability of occurrence (blue=none, red=highest occurrence). The potential societal risk of Zika can be delimited by the distribution of the mosquito species that transmit it. The global distribution of the most cited carrier of Zika, A. aegypti, is expanding due to global trade and travel. A. aegypti distribution is now the most extensive ever recorded – across all continents including North America and even the European periphery (Madeira, the Netherlands, and the northeastern Black Sea coast). A mosquito population capable of carrying Zika has been found in a Capitol Hill neighborhood of Washington, D. C., and genetic evidence suggests they survived at least four consecutive winters in the region. The study authors conclude that mosquitos are adapting for persistence in a northern climate. It is estimated that more than two billion people live in parts of the world where the Zika virus can spread.
_
Zika virus can be carried by more common mosquito, scientists say:
Aedes aegypti has been seen as the main Zika transmitter but research indicates Culex quinquefasciatus may also be a vector – and it is 20 times more common. Research by scientists in Brazil indicates that a mosquito more common than the one primarily known to transmit Zika infections may possibly be able to carry the virus, a development that could further complicate efforts to limit its spread. The mosquito species Aedes aegypti has been identified as the main transmitter of Zika infections, which have been linked to thousands of birth defects as the virus spreads rapidly in Brazil and other countries in Latin America and the Caribbean. But the scientists in Brazil say that they were able to infect another species, Culex quinquefasciatus, with the virus in a laboratory, raising concerns that Zika could be carried by a species more prevalent than Aedes aegypti. They said much more research is needed to learn whether the Culex mosquitoes can transmit Zika infections. The research, conducted by scientists at the government-funded Oswaldo Cruz Foundation in the north-eastern city of Recife, is part of an on-going trial in which researchers injected 200 of the Culex quinquefasciatus mosquitoes with rabbit blood infected by Zika. The virus circulated through the mosquitoes’ bodies and into their salivary glands, meaning they might be able to transmit a Zika infection by biting a person.
_______
Non-mosquito Transmission:
Non-mosquito Transmissions of Zika are of three types; sexual, Maternal-fetal and via blood:
_
Sexual:
Through sexual contact
•Zika virus can be spread by a man to his sex partners. As of April 2016 sexual transmission of Zika has been documented in six countries – Argentina, Chile, France, Italy, New Zealand and the United States – during the 2015 outbreak.
•In known cases of sexual transmission, the men developed Zika virus symptoms. From these cases, we know the virus can be spread when the man has symptoms, before symptoms start and after symptoms resolve.
•In one case, the virus was spread a few days before symptoms developed.
•The virus is present in semen longer than in blood.
_
Zika virus has been isolated from semen samples, with one patient having 100,000 times more virus in semen than blood or urine, two weeks after being infected. It is unclear why levels in semen can be higher than other body fluids, and it is also unclear how long infectious virus can remain in semen. Replicative viral particles, as well as viral RNA — often in high copy numbers — have been identified in sperm, and viral RNA has been detected up to 62 days after the onset of symptoms.
_
In 2014, Zika capable of growth in lab culture was found in the semen of a man at least two weeks (and possibly up to 10 weeks) after he fell ill with Zika fever. The second report is of a United States biologist who had been bitten many times while studying mosquitoes in Senegal. Six days after returning home in August 2008, he fell ill with symptoms of Zika fever but not before having unprotected intercourse with his wife, who had not been outside the US in 2008. She subsequently developed symptoms of Zika fever, and Zika antibodies in both the biologist’s and his wife’s blood confirmed the diagnosis. In the third case, in early February 2016 the Dallas County Health and Human Services department reported that a person contracted Zika fever after sexual contact with an ill person who had recently returned from a high risk country. As of February 2016 fourteen additional cases of possible sexual transmission have been under investigation. All cases involve transmitting the Zika from men to women and it is unknown whether women can transmit Zika to their sexual partners.
__
Male-to-Male Sexual Transmission of Zika Virus — Texas, January 2016:
Although Zika virus is spread primarily by Aedes species mosquitoes, published case reports have documented sexual transmission from infected men to their female sex partners through vaginal sex. This is the first report of transmission of Zika virus from an infected man to a sex partner through anal sex. Sexual transmission through both vaginal and anal sex is an emerging mode of Zika virus infection that might contribute to more illness than was anticipated when the outbreak was first recognized. Cases of sexually transmitted Zika virus infection should be reported to public health agencies and can help inform recommendations to prevent Zika virus infections.
______
Maternal-fetal transmission of Zika from infected pregnant woman to fetus:
Maternal-fetal transmission can result in intrauterine (congenital) infection or intrapartum infection (transmission of infection at the time of delivery).
•A pregnant woman can pass Zika virus to her fetus during pregnancy. Zika is a cause of microcephaly and other severe fetal brain defects, and we are studying the full range of other potential health problems that Zika virus infection during pregnancy may cause.
•A pregnant woman already infected with Zika virus near the time of delivery can pass on the virus to her newborn around the time of birth.
•To date, there are no reports of infants getting Zika virus through breastfeeding. Because of the benefits of breastfeeding, mothers are encouraged to breastfeed even in areas where Zika virus is found.
_
Zika does spread from infected mothers to the fetus. Cases of vertical perinatal transmission have been reported. Substantial evidence now indicates that Zika virus can be transmitted from the mother to the fetus during pregnancy. Zika virus RNA has been identified in the amniotic fluid of mothers whose fetuses had cerebral abnormalities detected by ultrasonography, and viral antigen and RNA have been identified in the brain tissue and placentas of children who were born with microcephaly and died soon after birth, as well as in tissues from miscarriages. The frequency of and risk factors for transmission are unknown. The CDC recommends that women with Zika fever should wait at least 8 weeks after they start having symptoms of disease before attempting to conceive. Two cases of peripartum transmission of Zika virus have been reported among mother–infant pairs. Zika virus RNA was detected in both infants; one infant had a mild rash illness and thrombocytopenia, whereas the other was asymptomatic. There have been no reported cases of transmission from breastfeeding, but infectious virus has been found in breast milk.
______
Zika transmission through blood transfusion:
•As of February, 1, 2016, there have not been any confirmed blood transfusion transmission cases in the United States.
•There have been multiple reports of blood transfusion transmission cases in Brazil. These reports are currently being investigated.
• A potential risk had been suspected based on a blood donor screening study during the French Polynesian Zika outbreak, in which 2.8% of donors from November 2013 and February 2014 tested positive for Zika RNA and were all asymptomatic at the time of blood donation. Eleven of the positive donors reported symptoms of Zika fever after their donation, and only three of 34 samples grew in culture. Since January 2014 nucleic acid testing of blood donors has been implemented in French Polynesia to prevent unintended transmission.
_
One case of Zika virus transmission occurred after a monkey bite in Indonesia, although mosquito-borne transmission could not be ruled out. Two infections in laboratories have been reported. A volunteer became infected after subcutaneous injection of infected mouse brain suspension. Transmission through breast milk has not been documented, although the breast milk of a woman who became symptomatic with Zika virus infection on the day of delivery contained infective Zika viral particles in high titer.
_______
_______
Period of infectivity of Zika:
Zika virus RNA has been detected in blood, urine, semen, saliva, cerebrospinal fluid, amniotic fluid, and breast milk. Zika virus is detectable in the blood of an infected person for a few days to a week. Zika virus RNA may be detectable in urine longer than in serum. Zika virus RNA has been detected in pregnancy as long as 10 weeks after symptom onset. It has been detected in semen 62 days after onset of febrile illness, when it was no longer detectable in blood.
_
Those who become infected with Zika but aren’t pregnant shouldn’t worry.
We think the maximum incubation period is 12 days. Let’s say you get infected on the last day of a visit to Brazil. You might go 12 days from then until you start developing symptoms — or no symptoms but having the virus in your bloodstream that could infect the fetus. That period lasts about a week or a little longer. That would take you out close to three weeks. We’d expect the virus would be gone from your system by 21 days. Therefore, if you got pregnant later, it’s unlikely that you’d transmit the virus to your fetus.
_
Transmissibility of Zika:
Scientists have estimated how contagious Zika is in Colombia. Specifically, they calculated what’s called the reproduction number, or “Ro” (R nought). It’s a mathematical term that tells you the number of people who catch the disease from one sick person, on average, in an outbreak. In Colombia, Zika’s Ro was between three and six. Each person who caught Zika spread it to about four others during the outbreak. An Ro of four sounds a bit scary. SARS has an Ro of about four. So does HIV. By comparison, the Ro for Ebola in West Africa was only between 1.5 and 2.0. So you can see why Zika has quickly swept across the Western Hemisphere, while Ebola pretty much stayed put in West Africa. An Ro of four tells you need to move fast, early on in an outbreak, to break transmission but unlike Ebola, Zika requires mosquitoes to spread it. So the Ro depends largely on the environment: how many mosquitoes there are, how often they get the chance to bite people and how dense the population is. That’s why screens on houses, air conditioning and suburban living all cut down on the risk of mosquito-borne viruses. So Zika’s Ro will be much lower in the U.S. than Colombia. How much lower? For that, let’s look at another virus that’s closely related to Zika: dengue. It’s spread by the same mosquito — Aedes aegypti — as Zika. And in tropical climates, dengue has an Ro comparable to that of Zika. But in the U.S., dengue causes only a sprinkling of small outbreaks. For instance, this past winter, Hawaii recorded about 250 dengue cases on the Big Island. In 2010, the Florida Keys recorded about 60 cases. And in 2003, Houston had a dengue outbreak, but health officials didn’t realize it until a decade later when scientists combed through old medical samples. So why worry about Zika? For one big reason, Zika’s effect on unborn children is devastating. Even a single case could be catastrophic for a family.
_
Are some people immune to Zika Virus? There’s no evidence yet.
There is no natural immunity to Zika. Yes, once you are infected with Zika, it may confer life-time immunity to future Zika infection. It’s possible that the outbreak in Brazil and Latin America is the first time the populations in those countries were exposed to the virus. It’s also possible that parts of Africa or other parts [of the world] might already be quite immunized and therefore would not experience the same sort of outbreak.
_
Is Zika contagious?
The Zika virus is not airborne and not primarily spread from person to person, which means there’s no risk of becoming infected with the virus from simply standing near an infected person. The virus is mostly spread through mosquito bites. However there have been many confirmed cases of Zika spread through sexual transmission, and World Health Organization (WHO) says sexual transmission of Zika is more common than previously thought.
_______
_______
Zika in animals:
Nonhuman primates, such as monkeys and apes, have shown the ability to become infected with Zika virus. Only a few naturally and experimentally infected monkeys and apes have had any signs of illness at all, and then it was only a mild, transient fever without any other symptoms. There have not been any reports of pets or other types of animals becoming sick with Zika virus. There is limited evidence from one study done in Indonesia in the late 1970s that horses, cows, carabaos (water buffaloes), goats, ducks, and bats could become infected with Zika, but there is no evidence that they develop disease or pose a risk for Zika virus transmission to humans. Microcephaly has not been reported among populations of monkeys and apes in areas with previous or on-going Zika virus transmission. This type of birth defect is not known to be associated with Zika virus infection in animals. There is no evidence that Zika virus is spread to people from contact with animals. Zika virus is transmitted to people primarily through the bite of an infected Aedes mosquito.
_
Zika causes microcephaly in mice:
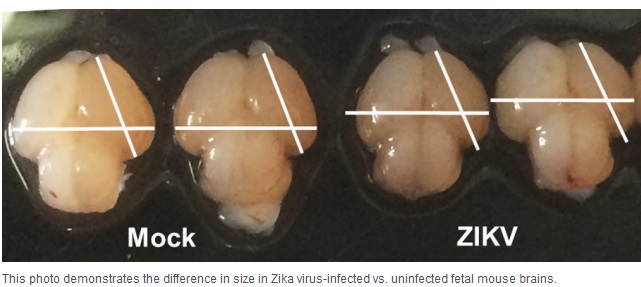
Pregnant monkeys are showing hints of fetal damage. But the most dramatic results come from mice. Mouse studies published recently in Cell and its sister journal Cell Stem Cell and in Nature show precisely how the virus slows fetal growth, damages the brain, and leads to miscarriage. Two of them also prove for the first time in an animal model that Zika virus can cause microcephaly in fetuses. Separate experiments by Dr. Michael Diamond of Washington University in St. Louis and a team of Chinese researchers showed Zika was capable of damaging fetal brain cells. Diamond’s experiments, published in Cell, involved mice with compromised immune systems. In one, the team bred mice with genetically weakened immune systems. When exposed to Zika, the virus killed most fetuses within a week, and those that survived had significant abnormalities, including severely stunted growth. In these mice, the researchers saw genetic material from the Zika virus in the mouse placentas that was 1,000 times greater than in the blood of the mothers, suggesting the virus had been growing in the placenta. In the second model, normal pregnant mice were given an antibody that blocked their immune response. When exposed to Zika, the fetal mice survived, but their growth was stunted, and viral genetic material was present in their heads and bodies. In the third study, in Cell Stem Cell, Chinese researchers directly injected Zika into the brains of fetal mice in utero. When these mice were born, they showed characteristic features of microcephaly, according to collaborators Zhiheng Xu of the Chinese Academy of Sciences and Cheng-Feng Qin of the Beijing Institute of Microbiology and Epidemiology. After the mice were injected during the equivalent of the second trimester in humans, the fetal brains shrank as the amount of virus increased. Diamond said the mouse experiments will be useful for testing new vaccines and drugs. “Now, we can begin to see whether vaccines can prevent transmission of the Zika virus to the fetus,” Diamond said. Together, the findings indicate that the virus by itself can wreak havoc. And by providing an animal model for the fetal damage, the studies should also ease the path for testing potential vaccines and treatments.
________
________
Pathogenesis of Zika:
Until recently, Zika virus was less of a research priority than other flaviviruses, as it was not thought to be of public health importance. Limited literature exists on the pathogenesis of the Zika virus to help understand the clinical disease spectrum and to target treatments to minimise or prevent tissue damage. Zika virus replicates readily in skin immune cells, and a large number of receptors are able to mediate entry of the virus into cells. Studies on the capability of the virus to replicate in neuronal cells are warranted to further investigate the link with neurological disorders.
_
Reproductive Cycle of a Zika virus in a Host Cell:
The reproductive cycle of ZIKV follows that of other known flaviviruses. ZIKV belongs to the Flavivirus genus; one of four genera of the Flaviviridae family of viruses, of which there are many and diverse species. Viruses in the Flavivirus genus are further subdivided into groups, such as the yellow fever group, the dengue virus group and the Spondweni serocomplex to which ZIKV belongs. Currently, this classification scheme is based on nucleotide sequence analysis of viral genomes. The molecular evolution of ZIKV in the 20th century has been studied using sequence analysis of Zika obtained from mosquitos, humans, and other mammals in Africa. All viruses belonging to the Flaviviridae, including the better known yellow fever, West Nile, dengue, and Japanese encephalitis viruses, possess an infectious viral particle (virion) that has an outer lipid membrane in which are embedded the viral envelope protein (E) and membrane protein (M). The virion contains an icosahedral nucleocapsid of around 50-60 nm. This is composed of the capsid protein (C), and a genome consisting of a single strand of positive-sense RNA of approximately 11,000-12,000 bases that serves both as a genome and a messenger RNA. The virion attaches via the E protein to a receptor on the cell targeted for infection. The virion is brought into the cell by a process called clathrin-mediated endocytosis, which causes the envelope to be removed, the nucleocapsid to be disrupted, and the genome released into the cytoplasm. The genome is translated by the host cell’s translational apparatus into a single polyprotein that is proteolytically cleaved into the individual viral proteins: PreM, C and nonstructural proteins NS1 to NS5. Some of these proteins form the RNA replication machinery, which causes the production of more genomes by using the negative-sense RNA copy of the viral genome as a template. The genomes are then assembled into nucleocapsids by interaction with capsid protein (C), and the nucleocapsids become enveloped during the budding process that releases them from the cell as infectious virions.
_
Most viruses belonging to the Flavivirus genus are arboviruses or arthropod-borne viruses because they replicate in, and are transmitted by mosquitoes. Other viruses that are classified in the Flaviviridae, such as human hepatitis C virus (member of Hepacivirus genus), do not involve the mosquito vector and are transmitted directly from human to human. In this context, the ZIKV E proteins interacts with receptors present on mosquito and on mammalian cells, as well as use their cellular machinery to enter cells, produce viral proteins and RNA, followed by the generation of progeny virions. With ZIKV, as with all arboviruses, an infected blood-feeding female mosquito (e.g., chiefly a species of Aedes genus), injects the virions into the skin of a human, followed by infection of cells in the dermis and epidermis (e.g., dermal fibroblasts, epidermal keratinocytes, immature dendritic cells). From there the virus spreads to the lymph nodes where an immune reaction is initiated at the same time the virus is replicating and causing a viremia. The ability of ZIKV, to cross the placenta of pregnant women and affect the fetus, would make it very unique from other arboviruses. In some ways, concerns over ZIKV are reminiscent of previous experiences with the rubella virus, which before the advent of rubella vaccination produced severe congenital developmental abnormalities in newborns. Another feature of flaviviruses that has to be considered in the current context is the ability of some (e.g., the Modoc virus – an “outlier” virus classified in the Flavivirus genus but not believed to be an arbovirus) to cause persistent infection. If ZIKV developed the ability to cause persistent infections and spread human-to-human, the public health community would have to face an entirely new level of situational complexity.
_
Zika replicates in the mosquito’s midgut epithelial cells and then its salivary gland cells. After 5–10 days, ZIKV can be found in the mosquito’s saliva which can then infect human. If the mosquito’s saliva is inoculated into human skin, the virus infects epidermal keratinocytes, skin fibroblasts in the skin and the Langerhans cells. The pathogenesis of the virus is hypothesized to continue with a spread to lymph nodes and the bloodstream. Flaviviruses generally replicate in the cytoplasm, but Zika antigens have been found in infected cell nuclei. In the typical initial infection event, Zika virus is transmitted to a bitten human host after skin injection of a mixture of insect saliva, virus, and blood components from the most recent feeding during female mosquito blood meals. Probability of viral particle transmission is related to the volume of fluid held in the proboscis from a prior blood meal, viral replication levels and volume of insect salivary glands, and the viral infectious titer of the preceding host. In the case of many arboviruses, mosquito salivary gland products enhance viral infectivity and replication. Zika infection of the recipient host requires viral envelope protein binding and particle uptake into susceptible cells, is mediated by specific receptors which include DC-SIGN, AXL, Tyro3, and TIM-1, and triggers transcriptional activation of Toll-like receptor 3 (TLR3), RIG-I, MDA5, interferon stimulated genes including OAS2, ISG15, and MX1, and beta interferon. Primarily infected cells include skin fibroblasts, epidermal keratinocytes, and skin dendritic cells. Immature dendritic cells appear to be an important initial Zika target. Reasoning by analogy to dengue infection, it is likely that primary Zika infection triggers apoptosis of infected cells, thereby evading aspects of innate immune responses and increasing initial release of infectious viral particles. Both dengue and Zika viruses subsequently exploit autophagy to enhance replication, and pharmacologic manipulation of Zika-infected cells with 3-Methyladenine (3-MA), an inhibitor of autophagosome formation, strongly reduces viral copy numbers in infected fibroblasts. Based on prior murine studies involving Zika virus inoculation in mouse brain, autophagy of Zika virus has been postulated as playing a key role in the pathogenesis of Zika-associated primary microcephaly. The study, published May 6, 2016 in Cell Stem Cell shows Zika activates TLR3, a molecule human cells normally use to defend against invading viruses. In turn, hyper-activated TLR3 turns off genes that stem cells need to specialize into brain cells and turns on genes that trigger cell suicide.
_
The duration of viremia, infectivity, and persistence of Zika virus, is not known for either post-partum or intrauterine infection. Nor is the route of fetal infection, or the degree of neurotropism. Related flaviviruses may cause persistent infection despite the presence of serum antibodies. West Nile virus can be neurotropic in many species including humans. Dengue is associated with encephalitis, encephalopathy, and multiple less frequent neurological symptoms. Transplacental transmission of West Nile virus has been reported. Dengue infection in pregnancy leads to transplacental transfer of anti-dengue antibodies. However, despite the extensive distribution of dengue, there is only one published case study showing transplacental fetal infection. Zika virus has been demonstrated in amniotic fluid, as well as in an aborted fetus. Researchers from the Carlos Chagas Institute of Paraná Fiocruz have reported that Zika virus can cross the placenta during pregnancy, based on demonstration of viral proteins in placental cells. The working hypothesis offered for the Zika viral transplacental transport mechanism is that the virus may be using the migratory capacity of these cells to reach fetal vessels. An alternative explanation for Zika virus infection of amniotic fluid and, possibly, fetal central nervous tissue may be viral uptake and transport via FcRn receptors on the placenta.
_
Epitopes with dengue or Yellow fever could result in preexisting antibodies to these viruses binding Zika and enhancing initial virus replication or placental cell infection, or transplacental viral transfer.
_
Opportunities and strategies for Zika medical management and countermeasure development will benefit from answers to key questions concerning the virology and immunology of Zika infection in the human host. A better understanding of natural immune responses and viral infection may clarify the potential role of Zika in eliciting GBS or microcephaly. Targeted identification and design of antivirals, neutralizing antibody preparations and immunotherapeutics still require understanding of the underlying biology. Critical priorities for early characterization include duration and levels of viremia and transmissibility, whether circulating non-neutralizing antibody complexes contribute to either primary infection or fetal pathology, and the potential for interaction with pre-existing immunity elicited by other flaviviruses or flavivirus vaccines.
_
A Mouse Model of Zika Virus Pathogenesis: a 2016 study:
The on-going Zika virus (ZIKV) epidemic and unexpected clinical outcomes, including Guillain-Barre´ syndrome and birth defects, has brought an urgent need for animal models. Authors evaluated infection and pathogenesis with contemporary and historical ZIKV strains in immunocompetent mice and mice lacking components of the antiviral response. Four to six-week-old mice, which produce little interferon a/b, and mice lacking the interferon receptor developed neurological disease and succumbed to ZIKV infection, whereas mice with interferon exhibited no overt illness. Interferon negative mice sustained high viral loads in the brain and spinal cord, consistent with evidence that ZIKV causes neurodevelopmental defects in human fetuses. The testes of these mice had the highest viral loads, which is relevant to sexual transmission of ZIKV. This model of ZIKV pathogenesis will be valuable for evaluating vaccines and therapeutics as well as understanding disease pathogenesis.
_
In my view, above model make us understand why fetal brain is destroyed and adult brain is spared. Adults have robust interferon system which prevents viral multiplication in brain while fetus has no interferon system and hence virus eats up fetal brain. A study of cord blood from 19 early second and third trimester fetuses (GA 18-36 weeks) and 16 term newborns (GA 37-42 weeks) found that fetal mononuclear cells were unable to produce IL-2, IL-4 or IFN-gamma.
_____
Zika virus in the immune-compromised patient:
Published experience of Zika virus infection in immunocompromised or immunosuppressed individuals is very limited. Despite Zika virus having been discovered over 60 years ago, we know surprisingly little about the pathogenesis and immunology of Zika virus infection in humans. Since Zika virus is a flavivirus, it is likely that both innate and adaptive responses are required to halt Zika viral replication and clear the virus, so medical conditions or interventions that influence innate and/or adaptive immune responses could alter the course of clinical illness, including the symptoms, severity and duration of infection. Case reports describing Zika virus infection in patients with conditions associated with compromised immune systems are limited. In Colombia, an adult and a child with confirmed Zika virus infection had severe illness with fatal outcomes. Post mortem examinations suggested probable lymphoblastic leukaemia and myeloid leukaemia, respectively. The role of Zika virus infection in these fatal outcomes is unclear. As an interim measure and until data from case control studies and large observational studies appear (note these may not be forthcoming), some extrapolation from experience of infections caused by the related flavivirus, dengue virus, seems reasonable. Again, published data on dengue virus infection and immunocompromised patients come from case reports or small case series, often with mixed patient populations (e.g. variability in underlying conditions or interventions, differing degrees of immunocompromise, different ethnic and geographical backgrounds). From the limited data available, overall clinical outcomes for dengue virus infection in immunocompromised patients appear to be similar to those for dengue virus infection in immunocompetent patients. Based on experience with acute RNA virus infections in general, immunocompromised patients could have atypical presentations, including absence of fever. There is a theoretical risk of prolonged dengue viraemia in profoundly immunocompromised patients, and it is not clear whether virus could also linger in certain organs following ‘clearance’ from blood. The same theories may be applied to Zika virus and immunocompromised patients in the absence of data from specific studies. There are no reliable data available from any patient group to assess duration of viral RNA detection in blood, semen or other compartments.
______
______
Risk factors for acquiring Zika:
Anyone who lives in or travels to an area where Zika virus is found and has not already been infected with Zika virus can get it from mosquito bites. The main risk factor for contracting the Zika virus is travel to areas where the infected mosquitoes are. That includes parts of Central and South America (including Mexico), some Pacific Islands, and much of the Caribbean, including Puerto Rico and the U.S. Virgin Islands. As of April 13, all of the 358 reported cases of the virus in the U.S. were travel-related. While most people who contract the Zika virus aren’t even aware they have it because they don’t show symptoms, common symptoms for adults include fever, body aches, rash, and red eyes. There is also a risk of sexual transmission of Zika virus. There is only limited information available from which to estimate the risk of sexual transmission, but this risk is considered to be low when compared to the risk of transmission from infected mosquitoes. Once a person has been infected, he or she is likely to be protected from future infections. Currently, there is no evidence to suggest that Zika virus, after it is cleared from the pregnant woman’s blood, poses a risk for birth defects in future pregnancies.
______
______
Clinical illness:
_
In most cases, Zika virus (ZIKV) infection causes a mild, self-limited illness. Symptoms and signs of ZIKV infection usually occur 3-12 days after the mosquito-vector bite and resolve within 2-7 days. Although asymptomatic infection is common, approximately 20% of infected humans with ZIKV become symptomatic with the acute onset of a fever, maculopapular rash, conjunctivitis (and other ophthalmologic manifestations), and arthralgias. The disease is usually mild and lasts up to 1 week. Unlike the Ebola virus, mortality is low and hospitalization is infrequent. Shock and haemorrhage occur with other flaviviruses such as dengue, but they have not been documented in Zika virus infection. Severe acute illness seems to be rare. Fewer than 10 possible Zika related deaths have been reported in adults, and an additional three deaths from Guillain-Barré syndrome have occurred in individuals who had symptoms of Zika infection.
_
Acute Febrile Illness of Zika:
In one volunteer, a febrile illness of 4 days’ duration developed 82 hours after subcutaneous inoculation of Zika virus. Viremia was detected when symptoms were present, but not afterward. Among French Polynesian blood donors who tested positive for Zika virus by RT-PCR, 11 (26%) reported conjunctivitis, rash, arthralgia, or a combination of these symptoms 3 to 10 days after donation. Sero-survey results from Yap indicated that only 19% of persons who were infected had symptoms that were attributable to Zika virus. Common symptoms were macular or papular rash (90% of patients), fever (65%), arthritis or arthralgia (65%), nonpurulent conjunctivitis (55%), myalgia (48%), headache (45%), retro-orbital pain (39%), edema (19%), and vomiting (10%). No patient was hospitalized during the outbreak in Yap. These common symptoms occurred at frequencies similar to those in the Yap outbreak in a cohort of pregnant women with Zika virus infection in Brazil. The rash is generally maculopapular and pruritic, and fever, when present, is generally short-term and low-grade. Other symptoms that have been noted in association with acute infection include hematospermia, transient dull and metallic hearing, swelling of the hands and ankles, and subcutaneous bleeding.
_
Zika rash:
The spectrum of Zika virus disease overlaps with other that of arboviral infections, but rash (maculopapular and likely immune-mediated) typically predominates. The rash in Zika virus infection is usually a fine maculopapular rash that is diffusely distributed. It can involve the face, trunk, and extremities, including palms and soles. Occasionally, the rash may be pruritic. The rash, along with other symptoms, usually occurs within 2 weeks after travel to a Zika virus–affected area. Zika virus rash usually occurs within the first week of illness, with the illness itself lasting from several days to weeks.
_
Zika conjunctivitis:
_
Clinical manifestations of Zika virus infection in pregnant women are the same as those in non-pregnant adults. There is no evidence to suggest that pregnant women are more susceptible to Zika virus infection or experience more severe disease during pregnancy. The range of Zika virus infection in children includes intrauterine infection (vertical transmission during pregnancy), intrapartum infection (vertical transmission at the time of delivery), and postnatal infection (transmission via mosquito bites).
_
In rare cases, Zika virus infection is complicated by Guillain-Barré syndrome. A case of probable Zika virus-related hypertensive iridocyclitis was reported in an otherwise healthy young physician. As noted above, Brazil has reported an association between ZIKV and microcephaly, defined as a head circumference measurement below the third percentile and disproportionate to the weight and length percentile measurements. Ocular abnormalities and other congenital malformations such as arthrogryposis and hydrops fetalis have also been described. Although the impact of ZIKV infection during pregnancy remains relatively poorly described, there is mounting evidence to suggest that congenital abnormalities are a frequent outcome. For example, a recent case series from Brazil suggests that infection is associated with serious outcomes including fetal death, placental insufficiency, fetal growth restriction, and central nervous system (CNS) injury (12/42 ZIKV-infected females on whom Doppler ultrasonagraphy was performed).
___________
___________
Complications of Zika:
During large outbreaks in French Polynesia and Brazil in 2013 and 2015 respectively, national health authorities reported potential neurological and auto-immune complications of Zika virus disease. Recently in Brazil, local health authorities have observed an increase in Guillain-Barré syndrome which coincided with Zika virus infections in the general public, as well as an increase in babies born with microcephaly in northeast Brazil. Substantial new research has strengthened the association between Zika infection and the occurrence of fetal malformations and neurological disorders. However, more investigation is needed to better understand the relationship. Other potential causes are also being investigated.
_
Great concern is emerging over congenital malformations due to transplacental transmission of Zika virus, including microcephaly and various ophthalmologic abnormalities. The disease spreads from mother-to-child in the womb and can cause multiple problems, most notably microcephaly in the baby. As of April 2016, the full range of birth defects caused by maternal infection was not known, but appears to be common with abnormalities seen on up to 29% of ultrasounds. Observed associations include microcephaly, eye abnormalities such as chorioretinal scarring, and hydrops fetalis, where there is abnormal accumulation of fluid in the fetus. Microcephaly is when a baby’s head is smaller than expected, compared to babies of the same sex and age. Babies with microcephaly often have smaller brains that don’t develop properly. Not every baby whose mother has Zika is born with microcephaly. Researchers are working to find out how often Zika causes microcephaly when a baby is exposed in the womb. It is also not well understood whether the stage of pregnancy at which the mother becomes infected affects the risk to the fetus, nor if other risk factors might exist that affect outcomes.
_
A temporal and geographic relationship has been observed between Guillain–Barré syndrome and Zika virus outbreaks in the Pacific and the Americas. In the outbreak in French Polynesia, 38 cases of Guillain–Barré syndrome occurred among an estimated 28,000 persons who sought medical care. A case–control study in French Polynesia revealed a strong association (odds ratio, >34) between Guillain–Barré syndrome and previous Zika virus infection; the findings from electrophysiological studies were compatible with the acute motor axonal neuropathy subtype of Guillain–Barré syndrome. There is another neurological syndrome called acute disseminated encephalomyelitis (ADEM), this is a new syndrome. This is where there is an auto immune process triggered by the Zika virus that attacks the brain and the spinal cord. Meningoencephalitis and acute myelitis complicating Zika virus infection also have been reported.
_
Various studies linking Zika to neurological complications:
_
Adverse Fetal Outcomes:
The full spectrum of fetal outcomes resulting from fetal Zika virus infection in humans is yet to be determined; however, the well-characterized effects of maternal infection with rubella virus and cytomegalovirus (CMV) may be instructive. Maternal rubella infections in the first 10 weeks of pregnancy can result in adverse fetal effects in up to 90% of infants and decrease thereafter, with a much lower risk after gestational week. The congenital anomalies associated with maternal rubella infection during pregnancy include sensorineural hearing loss, cataracts and other eye anomalies, cardiac anomalies, and neurologic effects, including intellectual disability, ischemic brain damage, and microcephaly. Similarly, maternal CMV infection can produce profound effects on the fetus, including sensorineural hearing loss, chorioretinitis, and neurologic effects, such as microcephaly, intellectual disability, and cerebral palsy. For primary infections with CMV, the risk of adverse fetal effects is highest during the first trimester, but the risk persists in the second and third trimester, with some adverse fetal outcomes noted in mothers who had seroconversion after gestational week 27. It is of particular concern that some infants without obvious adverse effects of congenital CMV infection at birth can have late-onset or progressive hearing loss that cannot be identified through screening of newborns. Other causes of microcephaly include some genetic syndromes, vascular disruption during brain development, nutritional deficiencies, and exposure to certain toxins, such as mercury.
_
From November 2015 to 13 February 2016, 5280 suspected cases of microcephaly and/or central nervous system malformation, including 108 deaths, were reported by Brazil—1345 of these have been investigated further: 837 of the 1345 did not have microcephaly, 421 had radiological findings such as cerebral calcifications compatible with a congenital infection, and 41 had laboratory confirmed Zika virus infection. A retrospective review of birth data in French Polynesia showed 18 cases of central nervous malformations in children born between March 2014 and May 2015, including nine cases of microcephaly, compared with the previous national annual average of none to two cases. As the NEJM paper notes, besides understanding the entire range of abnormities, concerted efforts can now be directed at quantifying the relative and absolute risk among infants born to mothers who had the infection during pregnancy. They can see if other factors like confection with another virus and/or a pre-existing immune response to another flavivirus are responsible for some babies born to mother infected with Zika virus developing microcephaly.
_
Zika may also be linked to:
•Birth defects, including hearing loss and problems with the eyes. Birth defects are health conditions that are present at birth. Birth defects change the shape or function of one or more parts of the body. They can cause problems in overall health, in how the body develops or in how the body works.
•Growth problems in the womb
•Miscarriage. This is when a baby dies in the womb before 20 weeks of pregnancy.
•Stillbirth. This is when a baby dies in the womb after 20 weeks of pregnancy.
_
Many studies showed that in addition to Microcephaly, Zika causes miscarriage and stillbirth:
The largest of these studies followed 88 pregnant women from Rio de Janeiro, Brazil, between September 2015 and February 2016, starting within five days after they each developed a rash. A rash is one of the most common symptoms of Zika infection, along with fever, joint pain, and red or itchy eyes. Of the 88 women, 72 tested positive for Zika, according to the study, which was published March, 2016 in the New England Journal of Medicine (NEJM). “Our study is different from others to date because we first identified pregnant women with Zika virus infection acutely when they presented with a rash, and subsequently followed their pregnancies,” says study author Karin Nielsen-Saines, MD, a pediatrician at Mattel Children’s Hospital and professor of pediatrics in the division of infectious diseases at the David Geffen School of Medicine, both at UCLA. “Most studies have been case reports of abnormal findings which then try to establish a link to prior Zika virus infection,” she says. In those types of individual cases, it’s harder for doctors to know if the Zika infection is a coincidence or not. In the NEJM study, however, two women with Zika virus infection had miscarriages in the first trimester, and ultrasounds of 12 of the 42 women showed fetuses with some kind of abnormality. None of the ultrasounds in the 16 women who tested negative for Zika showed any abnormalities. Other women who tested positive for Zika did not get ultrasounds because the medical facility was too far away or they were afraid of finding out about birth defects. The abnormalities seen in the ultrasounds of pregnant women with Zika included microcephaly, hardened calcium deposits in the brain, a breakdown in brain tissue, brain swelling, and poor growth of the fetuses. Two women also had stillbirths, and one baby was born in an emergency cesarean section because there was too little amniotic fluid in the womb. The researchers expected to find that birth defects were more likely when women were infected during a particular trimester, but that was not the case, Dr. Nielsen-Saines says. “We noted cerebral malformations develop in fetuses of women infected from 8 weeks of pregnancy to as late as 27 weeks of gestation,” she says. “We saw fetal demise [death] in the third trimester of pregnancy as well.” Late pregnancy loss was also seen in both of the other recent Zika virus studies, including a case study published in February 2016 in the journal PLOS Neglected Tropic Diseases. Researchers described the case of a 20-year-old woman whose fetus showed severely slowed growth in week 18 of pregnancy. The woman did not remember having had symptoms of a Zika infection, however. Additional ultrasounds later in the pregnancy showed problems similar to those seen in the Brazilian women in the first study, as well as an abnormal buildup of fluid inside the fetus’s body. The fetus died at 32 weeks of pregnancy. The third study, reported by Dana Meaney-Delman, MD, and other scientists at the Centers for Disease Control and Prevention (CDC), followed nine pregnant women in the United States who had travelled to countries where Zika was circulating. Up until February 17, 2016, these women, and 10 others under observation, are the only pregnant women in the United States with confirmed Zika infections. Two of the women had miscarriages in the first trimester, and two chose to have abortions after ultrasounds revealed severe defects in the fetuses’ brains. One woman gave birth to a baby with microcephaly, two women gave birth to healthy babies, and the other two women’s pregnancies are continuing without any current problems. Several of the women who lost their fetuses or had fetuses with defects showed Zika infection. It looks like Zika is inhibiting development of the brain, not just [associated with] small head size, and it’s associated with stillbirths.
_
Zika virus and hyperglycaemia in pregnancy:
While pregnant women and their offspring in general might be vulnerable to Zika virus infection, offspring of pregnant women with hyperglycaemia might be exceptionally vulnerable. Hyperglycaemia during the first trimester has been associated with an elevated risk of congenital malformations, including microcephaly. Moreover, the hyperglycaemic environment might increase the frequency, seriousness, or both of infectious diseases in people with diabetes, including pregnant women. At present, it is unknown whether pregnant women with hyperglycaemia are at an increased risk of Zika virus infections or serious complications due to Zika virus. However, data from Brazil suggest that up to 18% of pregnant women have hyperglycaemia in pregnancy. We need studies to investigate the role of hyperglycaemia as a potential effect modifier in the causal pathway of Zika induced microcephaly in Zika-affected areas.
________
Zika, pregnancy and microcephaly:
_
The figure above shows microcephaly in a baby due to maternal Zika infection:
_
Incidence of microcephaly:
Following the initial Zika outbreak in North-eastern Brazil, physicians observed a very large surge of reports of infants born with microcephaly, with 20 times the number of expected cases. The historical prevalence of microcephaly was 2 to 12 cases per 10,000 live births. Many of these cases have since been confirmed, leading WHO officials to project that approximately 2,500 infants will be found to have born in Brazil with Zika-related microcephaly.
_
_
1. Between 22 October 2015 and 2 April 2016 a total of 6906 cases of microcephaly and/or central nervous system (CNS) malformation were reported by Brazil. This contrasts with the period from 2001 to 2014, when an average of 163 microcephaly cases was recorded nationwide per year.
2. Of the 6906 cases of microcephaly and/or CNS malformations reported in Brazil, investigations have been concluded for 2860 cases and 1046 were suggestive of congenital infection.
3. Microcephaly and/or CNS malformation cases have been detected in 21 out of 27 states in Brazil, but the reported increase is concentrated in the northeast region.
4. Among the 6906 cases of microcephaly and/or CNS malformation reported in Brazil, 227 child deaths occurred after birth or during pregnancy (including miscarriage or stillbirth); 51 of these had microcephaly and/or CNS malformation suggestive of congenital infection, 148 remain under investigation and 28 were discarded.
5. An outbreak of Zika virus in French Polynesia was followed by an increase in the number of CNS malformations in children born between March 2014 and May 2015.5 A total of 19 cases was reported including eight microcephaly cases during that period. This represents an increase compared to the national average of 0-2 cases per year. A recently published study estimated the risk to be 95 cases of microcephaly per 10000 women infected during the first trimester.
_
The number of confirmed and suspected cases of microcephaly in Brazil associated with the Zika virus was down to 4,759 in the week through May 7, the Health Ministry said, hundreds less than more than 5,200 suspected in late March. As doctors and Brazilian health officials find that some suspected cases of microcephaly are not the disorder, the total number of confirmed cases in Brazil stands at 1,326. A further 3,433 cases are still being investigated.
_
A study of 35 infants with microcephaly (defined as head circumference ≥2 standard deviations below the mean for sex and gestational age) born between August and October 2015 in various states throughout Brazil found that the mothers of all 35 had lived in or visited Zika virus–affected areas during pregnancy. Twenty-seven of these infants had severe microcephaly, and test results were negative for other congenital infections in all cases. Zika virus RNA has also been detected in amniotic fluid and placental and fetal tissue in several cases of nervous-system malformations amid the current Brazilian outbreak.
_
Definition of microcephaly:
Microcephaly is a medical condition in which the circumference of the head is smaller than normal because the brain has not developed properly or has stopped growing. Microcephaly can be present at birth or it may develop in the first few years of life. There is no standard definition for diagnosis of microcephaly. For the purpose of evaluating an infant for possible congenital Zika virus infection, the World Health Organization has defined microcephaly as occipitofrontal circumference (head circumference) greater than two standard deviations below the mean or less than the third percentile based on standard growth charts for sex, age, and gestational age at birth. To establish a diagnosis of microcephaly, the occipitofrontal circumference should be disproportionately small in comparison with the length of the infant and not explained by other etiologies or congenital disorders. If an infant’s occipitofrontal circumference is equal to or greater than the third percentile but is notably disproportionate to the length of the infant or if the infant has deficits related to the central nervous system, additional evaluation for Zika virus infection may also be appropriate.
_
Microcephaly is a descriptive term meaning a small head and is associated with numerous disorders of diverse aetiology. It is usually associated with microencephaly (small brain). For the purpose of this article the two will be used interchangeably. Microcephaly can be divided into primary (or true) or secondary microcephaly. In primary microcephaly the brain never forms normally whereas in secondary microcephaly normal continued brain development is arrested by some defined insult / event. The terms congenital and acquired can also be used which only partially overlap with primary and secondary.
_
_
Microcephaly is a clinical finding of a small head size for gestational age and sex and is indicative of an underlying problem with the growth of the brain. The lack of consistent and standardized case definitions has challenged the accurate monitoring of microcephaly during the current Zika virus outbreak. Centers for Disease Control and Prevention (CDC) guidance has recommended that microcephaly be defined as an occipitofrontal circumference below the third percentile for gestational age and sex. The prevalence of microcephaly in the United States averages approximately 6 cases per 10,000 live births, with a range of about 2 to 12 cases per 10,000 live births. Because similar prevalences are expected in other countries, these figures may be suitable benchmarks for regions lacking accurate historical data. Microcephaly can occur as a result of fetal brain disruption sequence, a process in which, after relatively normal brain development in early pregnancy, collapse of the fetal skull follows the destruction of fetal brain tissue. Although previous case reports of maternal infection leading to fetal brain disruption sequence do not include information on the timing of maternal infection, some evidence indicates that this damage can occur late during the second trimester or even early in the third trimester. Initial case reports from Brazil have suggested that some of the infants with microcephaly related to Zika virus infection have a phenotype consistent with fetal brain disruption.
_
_
Causes of microcephaly besides maternal Zika:
There are many potential causes of microcephaly, but often the cause remains unknown. The most common causes include:
•infections in the womb: toxoplasmosis, rubella, herpes, syphilis, cytomegalovirus and HIV;
•exposure to drugs and toxic chemicals: maternal exposure to heavy metals like arsenic, lead and mercury, alcohol, cocaine, antiepileptic drugs, radiation, and smoking;
•genetic abnormalities such as Down syndrome;
•severe maternal malnutrition
_
Complications of microcephaly include:
•Dwarfism or short stature
•Facial distortion
•Mental retardation
•Hyperactivity
•Seizures.
•Developmental delay, such as problems with speech or other developmental milestones (like sitting, standing, and walking)
•Intellectual disability (decreased ability to learn and function in daily life)
•Problems with movement and balance
•Feeding problems, such as difficulty swallowing
•Hearing loss
•Vision problems
_
What is the outlook for children born with microcephaly?
It depends on the severity and how the brain is affected. Some newborns have heads small enough to be labelled as cases of microcephaly, but their neurological function isn’t impaired. More severe cases result in stillbirths or infants who die soon after birth. Many of the problems associated with microcephaly — including developmental delays; problems with vision, hearing, or speech; and epilepsy — are actually the result of the underlying cause. Babies born with microcephaly can have trouble moving their arms and legs and problems with feeding because of difficulties sucking and swallowing. A smaller head at birth is also associated with mental retardation. But people with neurological problems “can live close to a normal life span,” particularly if they have a strong support network as they grow up. One problem is that even in milder cases, microcephaly can be linked to seizures, which can reduce life expectancy. Because it is difficult to predict at birth what problems a baby will have from microcephaly, babies with microcephaly often need close follow-up through regular check-ups with a healthcare provider to monitor their growth and development. Some children with microcephaly will have normal intelligence and a head that will grow bigger, but they will track below the normal growth curves for head circumference.
____
How Zika affects fetal brain:
Zika’s damage is likely worst when it hits a fetus in the first trimester of pregnancy, the crucial time in brain development. By the time the baby’s rudimentary immune system begins to combat the virus, much of the damage has been done. Doctors cannot tell if a baby has microcephaly until much later in the pregnancy — the signs only begin appearing near the third trimester. At that point the Zika virus has long since come and gone; no trace of it remains in the baby’s blood stream. Like archaeologists investigating a ruined village, doctors must piece together what may have happened from the wreckage left behind. The first clue that Zika may have ransacked a baby’s brain is the ventricular system. The ventricles are a system of canals in the brain filled with cerebrospinal fluid. In a typical infant, the largest of the ventricles is under a centimeter in diameter. In the microcephaly cases they are immensely swollen; brains scans show them taking up a third or more of the width of the brain. As these cavities bloat like balloons, they squeeze the developing cerebral cortex — the part of the brain responsible for intellectual development. The second clue to Zika-instigated damage is that the brain tissue is riddled with calcium deposits. These deposits are “a kind of scar that damages the brain” that don’t appear in normal brains. Calcium deposits can also appear in babies whose microcephaly was caused by other viruses. But the precise pattern of deposits in the brain is different. For instance, in cytomegalovirus, calcium deposits typically clump tightly around the ventricles, whereas the Zika-caused cases often develop right at the juncture of the baby’s white and grey matter — the two types of tissue in the brain.
_
_
When does microcephaly present itself during fetal development? Does the timing affect the severity of the condition?
Initial evidence indicates that mothers in Brazil who gave birth to infants with microcephaly tended to be infected with the Zika virus in their first trimesters. But there is some evidence that the risk can continue into the second trimester. Congenital infections often strike the fetus between the second and fourth month of a pregnancy, when most neurons are formed. This is the critical period for neurogenesis. You cannot ‘catch up’ if not enough neurons are made during this period. If an infection occurs later in the pregnancy, it may be less likely to trigger microcephaly. And when cases of microcephaly occur later in pregnancy, infants may be better off in the long run because their brains will have had more time to develop properly. Still, CDC officials have said that pregnant women in any trimester should think twice before traveling to an area with Zika transmission.
________
________
Prenatal and postnatal diagnosis of microcephaly:
Microcephaly can be diagnosed during pregnancy or after the baby is born.
_
Prenatal diagnosis of microcephaly:
Antenatal ultrasound:
During pregnancy, microcephaly can be diagnosed with an ultrasound test. To see microcephaly during pregnancy, the ultrasound test should be done late in the 2nd trimester or early in the third trimester. In fetal imaging microcephaly is usually defined as fetal head measurements (e.g. head circumference) falling under two standard deviations expected for gestation or falling under the 3rd percentile. The same definition is sometime applied to children and adults. It is important to note that this means that the majority of these individuals are actually normal, but small. Microcephaly is usually diagnosed when a baby’s head circumference is even smaller than this, and usually together with structural abnormality of the brain that can be diagnosed with specialist imaging (e.g. MRI). Other authors advocate the use of three standard deviations which increases specificity and narrows the diagnosis to less than 1% of the population and more closely correlates with abnormal development. Measurement of the biparietal diameter (BPD) is not helpful in diagnosing microcephaly as head shape can be misleading. In addition the diagnosis should be reserved for cases where there is a significant discrepancy between the head size and the rest of the body.
_
Fetal ultrasound is usually performed at 18-20 weeks to assess fetal anatomy. Microcephaly and intracranial calcifications can be detected then, or later in pregnancy. Ultrasound findings suggestive of congenital Zika virus infection include microcephaly (head circumference more than two standard deviations below the mean for gestational age) and intracranial calcifications (cerebellum, intraocular, brain). Other fetal brain abnormalities have also been observed on ultrasonography (e.g., cortical or cerebellar thinning, increased extra-axial space, ventriculomegaly, enlarged frontal horns, abnormal corpus callosum). Pregnant women with a history of travel to an area with Zika virus transmission and who have not experienced clinical symptoms or have negative PCR test results can be offered ultrasound scanning in the community at an appropriate time for detection of microcephaly or intracranial calcifications. A suggested regime is 4 weekly scans after 24 weeks gestation.
_
Fetal Ultrasonography at 19 Weeks of Gestation:
_
Antenatal CT and MRI:
Both MRI and to a lesser degree CT are able to assess the underlying brain. The forebrain is usually most affected and often a degree of simplification of cortical structure (shallow sulci, reduced in number / abnormal sulcation) is evident. Due to radiation hazards of CT, MRI scan of fetal brain is preferred.
_
Difficulties in the prenatal diagnosis of microcephaly by ultrasound: 1995 study:
Author’s objective was to determine whether the diagnosis of microcephaly present at birth is apparent using standard biometry in the second trimester. Fetuses with prenatally suspected microcephaly (biparietal diameter > or = 3 standard deviations below mean) who had a first sonogram prior to 22 weeks’ gestation and a confirmation of microcephaly after birth were included in the study. Authors excluded all fetuses who had neural tube defects or other major associated abnormality that would lead to a suspicion of microcephaly. They therefore included fetuses who either had normal-appearing brains sonographically or intracranial calcifications as the only sonographic abnormality seen prior to 22 weeks’ gestation. Seven fetuses met these criteria. One fetus was diagnosed as having microcephaly prior to 22 weeks’ gestation. The other six fetuses had a normal head size prior to 22 weeks’ gestation and were diagnosed as having microcephaly at 27 weeks’ gestation and later. Only one of the seven fetuses had a karyotypic abnormality. Authors conclude that the prenatal diagnosis of microcephaly is not excluded by normal biometry on second trimester sonography.
_
Early Ultrasounds cannot detect microcephaly and would need an MRI to detect microcephaly:
A new study published in the New England Journal of Medicine followed the case of a 33-year-old woman who was infected by the Zika virus after she visited countries positive of the infection. She was 11 weeks pregnant when she travelled to Mexico and Guatemala. Although the exact location of transmission was not determined, the woman who was not identified remembered being bitten by mosquitoes in Guatemala. She reported feeling ill after returning home to Washington. When she had herself checked by a doctor, tests showed that she was positive with Zika infection. She reported feeling feverish with pains in the muscles and eyes. Later on, she developed a rash. According to Reuters, ultrasounds showed no signs of microcephaly at week 13, 16, and 17 weeks, not until week 19. At this time, the fetal brain showed signs of not developing properly. During a magnetic resonance imaging (MRI) follow-up exam, the fetal head showed signs of shrinking, from the 47th percentile to the 24th percentile. Aside from the infant’s head circumference, there were also complications in the lungs, liver, spleen and muscles. At this point, the infant’s head is not even small enough to be considered as microcephaly. However, given the extent of damage as shown on the MRI results, the woman decided to terminate her pregnancy at 21 weeks. The scientists conducted a postmortem examination on the fetus – and found that the brain had severe abnormalities in its cortical neurons. The postmortem also found high Zika viral loads in the brain – while lower amounts were found in the fetal muscle, liver, lung and spleen. Previously, infants infected with the virus have shown signs in early ultrasounds. The case of the young Finnish woman clearly shows that Zika may not be detected on a normal ultrasound, and would need an MRI to detect microcephaly early on. Dr. Anthony Fauci, head of the National Institute of Allergy and Infectious Disease, noted of a similar case where development problems have not been detected until 35 weeks into the pregnancy.
_
MRI fetal brain:
Fetal MRI is clinically performed to evaluate the brain in cases where an abnormality is detected by prenatal sonography. These most commonly include ventriculomegaly, abnormalities of the corpus callosum, and abnormalities of the posterior fossa. Fetal MRI is also increasingly performed to evaluate fetuses who have normal brain findings on prenatal sonogram but who are at increased risk for neurodevelopmental abnormalities, such as complicated monochorionic twin pregnancies and Zika microcephaly.
__
A 2016 retrospective study on prenatal brain MRI of fetuses with Zika virus infection found 3 out of 12 cases of severe cerebral lesions fulfilled all inclusion criteria.; that MRI showed microcephaly (n = 3), low cerebellar biometry (n = 2), occipital subependymal pseudocysts (n = 2), polymicrogyria with laminar necrosis and opercular dysplasia (n = 3), absent (n = 1) or hypoplastic (n = 1) corpus callosum and hypoplastic brainstem (n = 1). When fetal abnormalities are detected by usual antenatal ultrasound, the pregnant woman is refereed for magnetic resonance imaging (MRI) of the fetal brain.
_
_
MRI fetal brain at 20 weeks:
_____
Diagnostic amniocentesis:
Zika virus can be detected in amniotic fluid by RT-PCR testing; however, the indications for diagnostic amniocentesis, the appropriate gestational age for testing, and the interpretation of the test are uncertain. Decisions regarding amniocentesis should be tailored to individual clinical circumstances. Findings of fetal microcephaly or intracranial calcifications on ultrasound should prompt maternal serologic testing and consideration of amniocentesis.
_
Amniocentesis is offered to women who meet one or both of the following criteria:
●Ultrasound findings of fetal microcephaly, intracranial calcifications, and/or ventriculomegaly (regardless of maternal laboratory test results for Zika virus infection)
●Maternal laboratory testing for Zika virus infection that is positive or inconclusive and at least six to eight weeks after maternal exposure (even though these findings may reflect false-positive results)
The optimal timing for performance of amniocentesis is uncertain. In general, the sensitivity of amniocentesis for diagnosis of congenital Zika virus infection may be higher at ≥21 weeks than earlier in pregnancy, for the reasons discussed below. However, amniocentesis this late in gestation may not allow adequate time to arrange termination of pregnancy if desired because of positive results. Therefore, amniocentesis as early as 15 to 16 weeks may be reasonable for women with characteristic sonographic findings and positive laboratory testing; if such testing is pursued and false-negative results are suspected, a repeat amniocentesis later in gestation may be considered. In general, amniocentesis is performed no sooner than 15 weeks, as the risk of pregnancy loss is higher when the procedure is performed at ≤14 weeks.
_
By analogy with other causes of congenital infection (such cytomegalovirus and Toxoplasma), it is likely that Zika virus is not shed into amniotic fluid until sufficient time has elapsed following maternal viremia for the virus to breach the placental barrier (six to eight weeks after maternal infection). In addition, fetal kidney development must be sufficiently advanced to excrete the virus into the amniotic fluid (fetal urine production accounts for most of the amniotic fluid volume after 18 to 21 weeks of gestation).
_
The sensitivity and specificity of Zika virus RT-PCR testing of amniotic fluid for diagnosis of congenital infection are not known and likely depend on timing of amniocentesis after onset of maternal infection. It is unknown whether a positive amniotic fluid RT-PCR result is predictive of a subsequent fetal abnormality and, if so, what proportion of infants born after infection will have abnormalities. The duration of amniotic fluid polymerase chain reaction positivity is also unknown. A positive RT-PCR result on amniotic fluid should be considered suggestive of intrauterine infection; such a result may be useful to guide timing of delivery and level of neonatal care at the delivery site. A negative RT-PCR result on amniotic fluid may prompt evaluation for other causes of fetal microcephaly or intracranial calcifications.
_
At this time, the risk of adverse outcomes of pregnancy if the fetus is infected with ZIKV is unknown, so the risk of the procedure must be weighed against the benefits of this test result. A negative PCR result likely means that the fetus is not actively infected at that moment, but would not eliminate the possibility of prior infection and potential injury to the fetus. It is not known when ZIKV RNA would be expected to appear in amniotic fluid after infection, or how long it is likely to be detectable. There is some evidence that viral RNA may persist in amniotic fluid for months.
_
Early diagnosis of microcephaly:
The greatest risk of microcephaly and malformations appears to be associated with a Zika infection happening during the first trimester of pregnancy, according to the Pan American Health Organization. Amniocentesis may reveal a fetal infection with Zika virus as early as 15 weeks of gestation, but earlier time frames may endanger the baby. The earliest that microcephaly can be detected, regardless of the cause, is late in the second trimester of gestation (18 to 20 weeks), according to the CDC. The ACOG also lists screening of Zika-fighting antibodies as a possible way to confirm if a person had an infection, but this isn’t a feasible solution for much of the Americas, including the U.S.
_
My view:
Amniocentesis (RT-PCR of Zika virus RNA in amniotic fluid) and fetal MRI between 16 to 20 week can diagnose fetal Zika infection and microcephaly at the earliest. Fetal Ultrasound at that time may be normal.
________
Postnatal diagnosis of microcephaly:
_
Postnatal viral RNA studies:
For postnatal diagnosis of congenital infection, PCR for ZIKV can be performed on placental tissue, umbilical cord blood or infant blood sample, and CSF for confirmation of congenital infection. It is likely, however, that infants or fetuses infected weeks prior to specimen sampling will no longer have detectable viral RNA.
_
Physical Examination:
The WHO recommends that newborns born to mothers with Zika virus infection undergo head circumference measurement between 1 and 7 days after birth. Healthcare providers should take the head circumference measurement when the newborn baby is at least 24 hours old. This helps make sure that compression due to delivery through the birth canal has resolved. A head circumference of more than 2 standard deviations below the mean is considered microcephaly; a circumference of more than 3 standard deviations below the mean is classified as severe microcephaly, which should prompt neuroimaging.
__
_
Head circumference growth charts for newborns, infants, and children up to age 20 years in the United States can be found on CDC’s growth charts website. CDC recommends that health care providers use the WHO growth charts to monitor growth for infants and children ages 0 to 2 years of age in the United States. If the healthcare provider suspects the baby has microcephaly, he or she can request one or more tests to help confirm the diagnosis. For example, special tests like a CT scan or an MRI can provide critical information on the structure of the baby’s brain that can help determine if the newborn baby had an infection during pregnancy. They also can help the healthcare provider look for other problems that might be present.
_______
CT/MRI Brain scans show infants’ brain damage caused by Zika virus: 2016 BMJ study:
A study published on April 13 in The BMJ, found “severe” brain damage with a range of abnormalities in 23 babies with microcephaly who were for the first time examined using computed tomography and magnetic resonance imaging. Severe cerebral damage was found on imaging in most of the children in this case series with congenital infection presumably associated with the Zika virus. The features most commonly found were brain calcifications in the junction between cortical and subcortical white matter associated with malformations of cortical development, often with a simplified gyral pattern and predominance of pachygyria or polymicrogyria in the frontal lobes. Additional findings were enlarged cisterna magna, abnormalities of corpus callosum (hypoplasia or hypogenesis), ventriculomegaly, delayed myelination, and hypoplasia of the cerebellum and the brainstem. The mothers of the microcephalic infants in this study population had symptoms (e.g., low-grade fever and cutaneous rash) that were compatible with ZIKV infection during the first or second trimester of pregnancy, similar to the findings in other studies. Tang et al. found that ZIKV directly infects human cortical neural progenitor cells with high efficiency, resulting in stunted growth of this cell population and transcriptional dysregulation. This observation supports the type of disruptive, anomalous brain development that was found in these infants.
_
Mild microcephaly:
Axial non-contrast CT image (A and B) shows multiple bilateral calcifications in the junction between cortical and subcortical white matter (white arrows). Axial susceptibility magnetic weighted image (C) shows multiple foci of T2-hypointensity in subcortical frontal white matter (black arrows), and axial T1 weighted image (D) shows linear or punctiform foci of T1-shortening (white arrows), which correspond to the calcifications on CT. Axial T2 weighted image (E) shows bilateral frontal and central sulcus polymicrogyria (black arrows). Note the thickened and irregular cortical-white matter junction. Sagittal T1 weighted image (F) shows enlarged cisterna magna (black arrow) and hypoplasic corpus callosum (white arrow).
_
Microcephaly, cortical malformation, and brain calcification:
Axial CT image (A) shows many small dystrophic calcifications in the junction between cortical and subcortical white matter (white arrows) and noticeable reduction of the brain parenchyma thickness. Sagittal T2 weighted image (B) shows hypogenesis of the corpus callosum (black arrow), enlarged cisterna magna (long white arrow), and pons hypoplasia (white arrow). Axial T2 weighted image (C) shows simplified gyral pattern (white arrows), ventriculomegaly (long black arrow) wildly open Sylvius fissure as well as enlargement of subarachnoid space (black arrow). Coronal T2 weighted image (D) shows pachygyria in the frontal lobes (black arrows). Note the bilateral cortical thickness in the pachygyric frontal lobe (black arrows), shown on axial and sagittal T2 weighted images (E and F).
_
Severe microcephaly:
Sagittal T1 weighted image (A) shows a profound craniofacial disproportion, noticeably hypogenetic corpus callosum (short white arrow), and brainstem (long white arrow) and cerebellum hypoplasia (short black arrow). In addition, the cisterna magna is enlarged (long black arrow). Observe the small dystrophic calcifications hyperintense on T1 weighted image (B) in the frontal lobe (black arrows) and extremely simplified gyral pattern. Axial T2 weighted image (C) shows severe ventriculomegaly, mainly at the posterior horn and ventricular atrium (short white arrows). Note the bulging walls of the ventricle, the upward dilated third ventricle (black arrow), and enlargement of the subarachnoid space (long white arrows). Axial T1 weighted image fat suppression post-contrast (D) shows thickness and enhancement of frontal pachymeningis (black arrows). These last findings (C and D) may indicate a blockage in the cerebrospinal fluid pathways and/or reduced absorption of cerebrospinal fluid owing to impairment of the meninges or injury of arachnoid granulations.
_
The brain abnormalities seen in the BMJ study of Zika-affected babies suggest a “poor prognosis for neurological function,” the authors wrote. Grimly, even the brains of some babies whose head size appeared normal showed stark abnormalities, the authors noted. Imaging revealed these babies’ brain abnormalities were “masked” by enlarged cavities in the spinal-fluid-filled space that normally surrounds the brain’s outer cortex.
___________
___________
Studies linking fetal microcephaly to Zika in pregnant woman:
Current epidemiological data suggest spatial and temporal links with the Zika virus epidemic and congenital microcephaly. These are supported by case reports of detection of the virus from amniotic fluid or the brain tissue of affected fetuses. Researchers suspected that Zika virus could be transmitted by pregnant woman to their babies (“vertical transmission”). None was proven until February 2016, when a paper by Calvet et al. was published, showing that not only Zika virus genome was found in the amniotic fluid but also IgM antibodies to those virus. This means that not only can the virus cross the placental barrier, but also the antibodies produced by the mother can reach the fetus, which suggests that vertical transmission is plausible in these cases. One other study published in March 2016 by Mlakar and colleagues analysed autopsy tissues from a fetus with microcephaly that was probably related to Zika virus; researchers found ZIKV on the brain tissue and suggested that the brain injuries were probably associated with the virus, which also shed a light on the vertical transmission theory. The timing of the Zika virus and microcephaly epidemics in Brazil and French Polynesia indicate that the greatest risk of microcephaly is in the first trimester. In case reports of microcephaly, documented maternal Zika virus infection most often occurred between 7 and 13 weeks of gestation, but in some cases it occurred as late as at 18 weeks of gestation.
___
Various Studies are as follows:
_
Study-1
Zika Virus associated with Microcephaly: a case report 2016:
A Widespread epidemic of Zika virus (ZIKV) infection was reported in 2015 in South and Central America and the Caribbean. A major concern associated with this infection is the apparent increased incidence of microcephaly in fetuses born to mothers infected with ZIKV. In this report, authors describe the case of an expectant mother who had a febrile illness with rash at the end of the first trimester of pregnancy while she was living in Brazil. Ultrasonography performed at 29 weeks of gestation revealed microcephaly with calcifications in the fetal brain and placenta. After the mother requested termination of the pregnancy, a fetal autopsy was performed. Microencephaly (an abnormally small brain) was observed, with almost complete agyria, hydrocephalus, and multifocal dystrophic calcifications in the cortex and subcortical white matter, with associated cortical displacement and mild focal inflammation. ZIKV was found in the fetal brain tissue on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay, with consistent findings on electron microscopy. The complete genome of ZIKV was recovered from the fetal brain. The complete genome sequence of ZIKV that was recovered in this study is consistent with the observation that the present strain in Brazil has emerged from the Asian lineage.
_
_
Study-2
Zika Virus Infection with prolonged Maternal Viremia and Fetal Brain Abnormalities: case report 2016:
The current outbreak of Zika virus (ZIKV) infection has been associated with an apparent increased risk of congenital microcephaly. Authors describe a case of a pregnant woman and her fetus infected with ZIKV during the 11th gestational week. The fetal head circumference decreased from the 47th percentile to the 24th percentile between 16 and 20 weeks of gestation. ZIKV RNA was identified in maternal serum at 16 and 21 weeks of gestation. At 19 and 20 weeks of gestation, substantial brain abnormalities were detected on ultrasonography and magnetic resonance imaging (MRI) without the presence of microcephaly or intracranial calcifications. On postmortem analysis of the fetal brain, diffuse cerebral cortical thinning, high ZIKV RNA loads, and viral particles were detected, and ZIKV was subsequently isolated.
_
_
_
_
Study-3
Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a 2016 study:
The incidence of microcephaly in Brazil in 2015 was 20 times higher than in previous years. Congenital microcephaly is associated with genetic factors and several causative agents. Epidemiological data suggest that microcephaly cases in Brazil might be associated with the introduction of Zika virus. Authors aimed to detect and sequence the Zika virus genome in amniotic fluid samples of two pregnant women in Brazil whose fetuses were diagnosed with microcephaly.
Methods:
In this case study, amniotic fluid samples from two pregnant women from the state of Paraíba in Brazil whose fetuses had been diagnosed with microcephaly were obtained, on the recommendation of the Brazilian health authorities, by ultrasound-guided transabdominal amniocentesis at 28 weeks’ gestation. The women had presented at 18 weeks’ and 10 weeks’ gestation, respectively, with clinical manifestations that could have been symptoms of Zika virus infection, including fever, myalgia, and rash. After the amniotic fluid samples were centrifuged, RNA was extracted from the purified virus particles before the viral genome was identified by quantitative reverse transcription PCR and viral metagenomic next-generation sequencing. Phylogenetic reconstruction and investigation of recombination events were done by comparing the Brazilian Zika virus genome with sequences from other Zika strains and from flaviviruses that occur in similar regions in Brazil.
Findings:
Authors detected the Zika virus genome in the amniotic fluid of both pregnant women. The virus was not detected in their urine or serum. Tests for dengue virus, chikungunya virus, Toxoplasma gondii, rubella virus, cytomegalovirus, herpes simplex virus, HIV, Treponema pallidum, and parvovirus B19 were all negative. After sequencing of the complete genome of the Brazilian Zika virus isolated from patient 1, phylogenetic analyses showed that the virus shares 97–100% of its genomic identity with lineages isolated during an outbreak in French Polynesia in 2013, and that in both envelope and NS5 genomic regions, it clustered with sequences from North and South America, southeast Asia, and the Pacific. After assessing the possibility of recombination events between the Zika virus and other flaviviruses, authors ruled out the hypothesis that the Brazilian Zika virus genome is a recombinant strain with other mosquito-borne flaviviruses.
Interpretation:
These findings strengthen the putative association between Zika virus and cases of microcephaly in neonates in Brazil. Moreover, these results suggest that the virus can cross the placental barrier. As a result, Zika virus should be considered as a potential infectious agent for human fetuses. Pathogenesis studies that confirm the tropism of Zika virus for neuronal cells are warranted.
_
Study-4
Zika virus infects human neural stem cells: 2016 study:
The Zika virus infects a type of neural stem cell that gives rise to the brain’s cerebral cortex, researchers report. On laboratory dishes, these stem cells were found to be havens for viral reproduction, resulting in cell death and/or disruption of cell growth. While this study does not prove the direct link between Zika and microcephaly, it does pinpoint where the virus may be doing the most damage.
_
To gauge the virus’s possible effects on the developing brain, researchers at Johns Hopkins University in Baltimore, Maryland, and Florida State University in Tallahassee used induced pluripotent stem (iPS) cells to grow, in lab dishes, immature brain cells called human cortical neural progenitor cells. The virus readily infected the neural stem cells. Three days after the virus was applied, 85% of the cells in the culture dishes were infected. In contrast, when the virus was applied to cultures of fetal kidney cells, embryonic stem cells, and undifferentiated iPS cells, it infected fewer than 10% of the cells by day 3. Immature neurons derived from the neural progenitor cells were also less susceptible to the virus; 3 days after receiving a dose of the virus, fewer than 20% of those cells were infected. The researchers noticed that the infected progenitor cells were not killed right away. Instead, the virus “hijacked the cells,” using the cellular machinery to replicate themselves. That helped the virus to spread quickly through the cell population. His team also reports that infected cells grew more slowly and had interrupted cell division cycles, which could also contribute to microcephaly.
_
There are several other questions left to answer as well: why are the symptoms in adults so mild? How is the virus entering the nervous system of the developing fetus? Zika infects adults when mosquitoes deposit the virus on human skin, and our immune cells carry it into the blood. But how is the virus crossing the blood-brain barrier? And could Zika infect the small population of neural stem cells that adults keep above the brain stem in their hippocampus?
_
Study-5
Zika virus impairs growth in human neurospheres and brain organoids: 2016 study:
_
_
Though an association between Zika virus infection and microcephaly has been found, researchers have not been able to prove the link between virus infection and birth defect. To study how the virus causes birth defects in babies, the Brazilian researchers from the D’Or Institute for Research and Education (IDOR) and Federal University of Rio de Janeiro, Brazil used human induced pluripotent stem (iPS) cells to make neural stem cells (NSC), neurospheres and brain organoids. How quickly the neural stem cells get infected with Zika virus became clear when the researchers detected Zika virus in the cells just 24 hours after they were exposed to the virus. The researchers used the Zika virus infected neural cells and cultured them as neurospheres (a culture system composed of free-floating clusters of neural stem cells). While those cells that were not infected with Zika virus generated normal neurospheres, the Zika virus-infected neural cells generated neurospheres with abnormalities. At the end of six days, the virus killed most of the neurospheres, while hundreds of neurospheres grew in the control arm. To further investigate the impact of Zika virus infection during neurogenesis, organoids were exposed to Zika virus and followed for 11 days. The virus reduced the growth of infected organoids by 40 per cent compared with brain organoids under control conditions. Not only can the virus infect neural progenitor cells, but it turns them into a factory. The cells produce more virus and they actually can spread it. Infected cells appear to create a “bystander effect,” releasing chemicals as they die that damage or kill neighbouring uninfected progenitor cells. And the organoid results contain a frightening hint of why Zika is also associated with adult neurological disorders, including Guillain-Barré syndrome. Researchers found that Zika infection is “even worse” in glial cells, which support and insulate neurons and are present throughout life, not just in fetal development. To check if dengue virus, a flavivirus with similarities to Zika virus, caused similar effect on neurogensis, the researchers infected human neural stem cells with dengue virus 2 (DENV2). After six days, significant difference in cell viability was seen between dengue infected calls and Zika virus infected neural stem cells. The dengue infected cells were similar to the normal cells and there was no reduction in growth of dengue virus-infected organoids at the end of 11 days. These results suggest that the deleterious consequences of ZIKV infection in human NSCs, neurospheres and brain organoids are not a general feature of the flavivirus family. These results demonstrate that ZIKV induces cell death in human iPS-derived neural stem cells, disrupts the formation of neurospheres and reduces the growth of organoids, indicating that ZIKV infection in models that mimics the first trimester of brain development may result in severe damage. However, further studies are needed to characterize the consequences of ZIKV infection during different stages of foetal development.
_
Another study, following several dozen pregnant women in Brazil who were infected with the virus, directly links the infection to an increase in brain defects. It also suggests that the virus can harm a developing fetus in other ways, possibly by attacking the placenta and slowing down the supply of nutrients.
_______
Zika Virus and Birth Defects — Reviewing the Evidence for Causality: a 2016 article:
Since the identification of the Zika virus in Brazil in early 2015, the virus has spread rapidly throughout the Americas. An increase in the number of infants with microcephaly in Brazil was first noted in September 2015, after the recognition of Zika virus transmission in the country earlier in the year; this was followed by the recognition of a similar increase in French Polynesia after an outbreak there in 2013 and 2014.2 Despite accumulating evidence that supports the link between Zika virus infection and microcephaly, most experts have taken care not to state that Zika virus infection is causally related to these adverse outcomes. This cautious approach toward ascribing Zika virus as a cause of birth defects is not surprising, given that the last time an infectious pathogen (rubella virus) caused an epidemic of congenital defects was more than 50 years ago, no flavivirus has ever been shown definitively to cause birth defects in humans, and no reports of adverse pregnancy or birth outcomes were noted during previous outbreaks of Zika virus disease in the Pacific Islands. As is typically the case in epidemiology and medicine, no “smoking gun” (a single definitive piece of evidence that confirms Zika virus as a cause of congenital defects) should have been anticipated. Instead, the determination of a causal relationship would be expected to emerge from various lines of evidence, each of which suggests, but does not on its own prove, that prenatal Zika virus infection can cause adverse outcomes. Two approaches have been used to identify potential teratogens (exposures to a mother during pregnancy that have a harmful effect on her embryo or fetus): first, the identification of a combination of a rare exposure and a rare defect (sometimes referred to as the astute clinician approach), and second, the use of epidemiologic data to confirm an association. Many teratogens were first identified by means of the rare exposure–rare defect approach, including rubella virus, which was identified after an ophthalmologist noted a characteristic form of cataracts in infants whose mothers had rubella during pregnancy, and heavy alcohol use, which was identified as a teratogen after the recognition of a characteristic pattern of malformations that became known as the fetal alcohol syndrome. In contrast, some teratogens have been identified on the basis of epidemiologic studies (e.g., valproic acid was identified as a teratogen after a case–control study showed an odds ratio of 20 for the association of spina bifida with use of this drug during the first trimester of pregnancy). Causal relationships cannot be proven with absolute certainty in epidemiologic studies, but these factors help analysts judge the existence and strength of possible causal links. Their assessment should be complemented by controlled experiments, the most robust approach to drawing inferences about cause and effect.
_
Shepard’s Criteria:
In 1994, Thomas Shepard, a pioneer in the field of teratology, proposed a set of seven criteria for “proof” of human teratogenicity. These criteria have been used to guide discussions about causation in teratology-related litigation and to assess other potential teratogens. Authors used Shepard’s criteria as a framework to evaluate whether the currently available evidence supports the hypothesis that prenatal Zika virus infection is a cause of microcephaly and other brain anomalies as seen in the table below:
_
_
According to these criteria, causality is established when either criteria 1, 3, and 4 (rare exposure–rare defect approach) or criteria 1, 2, and 3 (epidemiologic approach) are fulfilled. The first criterion states that a proven exposure to an agent must occur at a critical time during prenatal development. The severe microcephaly and other brain anomalies that have been observed in many infants are consistent with an infection occurring in the first or early second trimester of pregnancy. Several case reports and studies have shown that women who had fetuses or infants with congenital brain anomalies that were believed, on the basis of the mother’s symptoms or laboratory confirmation, to be due to Zika virus infection were infected in the first or early second trimester of pregnancy, as determined either according to the timing of the symptoms or according to the timing of travel to an area where Zika virus is endemic. An analysis of the timing of laboratory-confirmed Zika virus transmission in certain states in Brazil and of the increase in the cases of microcephaly identified the first trimester as the critical time period for infection. Zika virus infections that occur later in pregnancy have been associated with poor intrauterine growth, fetal death, or in some pregnancies, defects on prenatal imaging that have not yet been confirmed postnatally because the pregnancies are on-going. Authors conclude that Shepard’s first criterion has been met.
_
Shepard’s second criterion requires that two epidemiologic studies of high quality support the association. Although ecologic data do not necessarily qualify as an epidemiologic study, data from Brazil regarding the temporal and geographic association between Zika virus infection and the later appearance of infants with congenital microcephaly are compelling. Two epidemiologic studies also provide support. In a study conducted during the outbreak in Brazil, 88 pregnant women who had had an onset of rash in the previous 5 days were tested for Zika virus RNA. Among the 72 women who had positive tests, 42 underwent prenatal ultrasonography, and fetal abnormalities were observed in 12 (29%); none of the 16 women with negative tests had fetal abnormalities. The abnormalities that were observed on ultrasonography varied widely, and some findings lacked postnatal confirmation because the pregnancies were on-going. A retrospective analysis after the 2013–2014 outbreak of Zika virus disease in French Polynesia identified eight cases of microcephaly; the authors used serologic and statistical data and mathematical modelling to estimate that 1% of the fetuses and neonates who were born to mothers who had been infected with Zika virus in the first trimester had microcephaly — a prevalence that was approximately 50 times as high as the estimated baseline prevalence. However, this estimate was based on small numbers, confidence intervals were wide, and the risk of other adverse outcomes (e.g., other brain anomalies) was not assessed. Although these studies provide important evidence in support of a causal relationship between Zika virus and microcephaly and other brain anomalies, both have limitations as noted by their authors, such as a lack of control for confounding factors and relatively small numbers of cases, and therefore they do not meet the stringent criteria set by Shepard. Thus, authors conclude that Shepard’s second criterion has not yet been satisfied.
_
The third criterion, careful delineation of clinical cases with the finding of a specific defect or syndrome, appears to be met. Previous teratogens have caused specific birth defects or syndromes rather than a broad range of birth defects. Many fetuses and infants with presumed congenital Zika virus infection have had a typical pattern, including severe microcephaly, intracranial calcifications, and other brain anomalies, sometimes accompanied by eye findings, redundant scalp skin, arthrogryposis, and clubfoot; such findings have led authors to use the term “congenital Zika syndrome.” On the basis of clinical details from a limited number of cases, some infants with presumed congenital Zika virus infection have had features that were consistent with fetal brain disruption sequence, a phenotype involving the brain that is characterized by severe microcephaly, overlapping cranial sutures, prominent occipital bone, redundant scalp skin, and considerable neurologic impairment. For example, 11 of 35 infants (31%) with microcephaly whose cases were reported to a Brazil Ministry of Health registry had excessive and redundant scalp skin, a finding that is not typically seen in other forms of microcephaly. These findings suggest an interruption of cerebral growth, but not in that of the scalp skin, after an injury (e.g., viral infection, hyperthermia, or vascular disruption) that occurred after the initial formation of brain structures, followed by partial collapse of the skull. The fetal brain disruption sequence is rare; only 20 cases were identified in a literature review in 2001.
_
Shepard’s fourth criterion refers to the association between a rare exposure and a rare defect; authors conclude that this criterion also has been met. The concept behind this criterion is that a rare defect occurring after a rare exposure during pregnancy implies causation because of the unlikelihood of the two rare events occurring together. Microcephaly is a rare defect that is estimated to occur in 6 infants per 10,000 live-born infants in the United States. Zika virus would not be a rare exposure among women living in Brazil during the Zika virus outbreak. However, reports of adverse birth outcomes among travellers who spent only a limited time period in an area where there is active Zika virus transmission are consistent with Zika virus being a rare exposure. A recent report is illustrative: a pregnant woman travelled for 7 days to Mexico, Guatemala, and Belize during her 11th week of gestation and had a positive test for Zika virus immunoglobulin M (IgM) antibodies 4 weeks later. On fetal ultrasonography and magnetic resonance imaging performed at 19 to 20 weeks of gestation, severe brain anomalies were diagnosed in the fetus, and the pregnancy was terminated at 21 weeks of gestation. Microcephaly was not present at the time of pregnancy termination, but the head circumference had decreased from the 47th percentile at 16 weeks of gestation to the 24th percentile at 20 weeks of gestation (a finding that is consistent with the timing of diminishing head sizes in previous cases), which suggests that microcephaly would have developed in the fetus had the pregnancy continued. In this woman, Zika virus would be considered a rare exposure, and her fetus had a rare outcome.
_
The last three criteria are helpful if they are present, but they are not considered to be essential. The fifth criterion, the need for an animal model that shows teratogenicity, has not been met. Although animal models have shown that Zika virus is neurotropic, no studies that tested for teratogenicity in an animal model have been published, although studies are under way. The sixth criterion, that the association should make biologic sense, is clearly met here. Other viral infections have had similar effects (microcephaly and eye problems). In addition, pathologic evidence supports this association: Zika virus RNA has been seen in damaged mononuclear cells (presumably glial cells and neurons) in the brains of newborns with microcephaly, and the virus appears to be neurotropic. Live Zika virus has been cultured from the brain of a fetus with severe brain anomalies after maternal infection at 11 weeks of gestation. Furthermore, Zika virus efficiently infects neural progenitor cells and produces cell death and abnormal growth, thus providing a possible mechanism for microcephaly. The seventh criterion, proof in an experimental system that the agent acts in an unaltered state, is aimed at medications or chemical exposures and does not apply to infectious agents. Thus, given Shepard’s criteria as a framework, criteria 1, 3, and 4 have been satisfied — evidence that is considered sufficient to identify an agent as a teratogen.
_
On the basis of this review, authors conclude that a causal relationship exists between prenatal Zika virus infection and microcephaly and other serious brain anomalies. Evidence that was used to support this causal relationship included Zika virus infection at times during prenatal development that were consistent with the defects observed; a specific, rare phenotype involving microcephaly and associated brain anomalies in fetuses or infants with presumed or confirmed congenital Zika virus infection; and data that strongly support biologic plausibility, including the identification of Zika virus in the brain tissue of affected fetuses and infants.
____
The evidence in favour of a causal link between transplacental infection and congenital central nervous system (CNS) malformations is substantial and accumulating. The scale and severity of prenatal damage by the Zika virus are far worse than past birth defects associated with microcephaly. Scans, imaging, and autopsies show that Zika eats away at the fetal brain. It shrinks or destroys lobes that control thought, vision, and other basic functions. It prevents parts of the brain not yet formed from developing.
____
Congenital Zika Syndrome:
Experts are calling “Congenital Zika Syndrome” a condition in which babies are born with Zika-related disabilities that are more diverse and more severe than seen in “textbook” microcephaly.
_
Zika birth defects are the tip of the iceberg:
The microcephaly and other birth defects we have been seeing could be the tip of the iceberg. The true burden of congenital disease with Zika virus is probably underestimated. Many cases have probably been missed because babies looked normal when they were born. But hidden birth defects are almost certain to turn up as the babies grow.
________
________
How often Zika causes microcephaly when a baby is exposed in the womb?
It is still uncertain how many pregnant women who become infected will transmit the virus to the foetus, and how many foetuses will develop brain damage or other malformations. It is likely that the risk of transplacental infection as well as the risk of developing congenital CNS malformations depends on the gestational age at the time of infection. It is also plausible that other risk factors, such as the mother’s age and nutritional status, also influence the risk of transplacental transmission, but there are currently no data available to explore such risk factors.
_
Zika Virus causes Microcephaly in only 1% of Births; most harmful to Pregnant Women in First Trimester: 2016 study:
Researchers from Institute Pasteur conducted an analysis of data from a past Zika outbreak in French Polynesia, which had been the largest epidemic until the current one took its place. Their analysis concluded the risk of microcephaly is about 1 percent for a mother who becomes infected with Zika virus during the first trimester of pregnancy. Lead author Dr. Simon Cauchemez and his colleagues suggested the risk of microcephaly is greater when mothers are infected during their first trimester, compared to second or third. The underlying reason for this greater risk is unknown, however, as public health experts still do not understand precisely how the virus enters the placenta and damages the fetal brain. For their risk analysis, Cauchemez and his colleagues searched medical records to identify all cases of microcephaly in French Polynesia from September 2013 to July 2015. Next, they applied simple mathematical models to assess when an infection with Zika virus during a woman’s pregnancy would increase her risk of delivering a baby with microcephaly. Importantly, they identified only eight cases of microcephaly during the study period. Based on their models, they estimated just one in 100 pregnant women who become infected with Zika during the first trimester will have a child with neurological damage. Since very limited numbers of patients are studied, other researchers are hesitant to generalize this finding to the current outbreak in the Americas. Moreover, Zika is suspected to be linked to hearing loss, vision defects, impaired growth and other abnormalities, with recent evidence suggesting problems can occur in any stage of pregnancy.
_
An earlier study in the New England Journal of Medicine found abnormalities in 29% of the fetuses of pregnant women who had Zika and underwent ultrasounds. So current studies suggest that somewhere between 1% and 29% of babies born to infected mothers get microcephaly. Researchers would also like to know when a developing infant is most vulnerable to the virus, and whether it may cause a spectrum of related problems, ranging from stillbirth and miscarriages on the severe end to learning disabilities on the milder end. Researchers said they still don’t know whether Zika might be acting alone, or whether it only has its worst effects in concert with other agents or infections, like dengue fever.
_________
Twins could unlock mystery of Zika microcephaly:
Scientists are hoping that a handful of women who have given birth to twins in which only one child is affected by Zika hold the key to unlocking the mystery of the epidemic.
_
Jaqueline Jessica Silva de Oliveira, 25, gave birth to twins Laura and Lucas five months ago. Laura has the microcephaly birth defect associated with Zika but Lucas was born healthy.
_
They are one of just five sets of twins being studied in Sao Paulo who have been born with one affected by Zika and the other not. A team from Sao Paulo University studying the five cases believes they may hold clues to the nature of the disease itself and hope to have results from their investigation in a year’s time. ‘The importance of these twins…is that they could give us some very important answers,’ said Mayana Zatz, director of the Human Genome Research Center at the university. ‘How can we explain that one of the twins was not affected: did they have a gene that protected them? Do they have a different genome that disposes them to the infection or not?’ Recent studies have shown evidence of Zika in amniotic fluid, placenta and fetal brain tissue. Dr Zatz said the placenta of one twin may be permeable to Zika, while the other may not, barring the virus from attacking the foetus. Another possibility is that the virus penetrates both placentas but that the neurons of one baby are resistant, while the other’s are not. ‘The third possibility that we want to investigate is that certain genes predispose the child to microcephaly, and they are altered by the presence of the Zika virus,’ Dr Zatz said, noting that around 15 genes are believed to govern microcephaly.
________
________
Guillain-Barre syndrome (GBS):
Guillain-Barré syndrome (GBS) is quite rare, and the precipitating factors are not well-known. It tends to follow infectious illnesses (like the bacteria Campylobacter jejuni which causes GI distress), and the resultant immune response, which begins to deteriorate nerve cell myelin—the insulating sheaths that preserve the integrity of nerve impulse conduction. The result of the autoimmune attack is muscle pain, fatigue, difficulty walking and moving, and even paralysis. Recent evidence published in the Lancet, the Zika infection seems to be the precipitating factor, leading to the onset of GBS. The exact mechanism of an autoimmune reaction targeting peripheral nerves following an infection is not completely understood but it probably involves a molecular mimicry where the body’s immune reaction to bacteria or viruses may falsely recognize peripheral nerves as their target due to molecular similarity of the infectious agent with different components of peripheral nerves. However in Zika, researchers have found infection rather than autoimmune reaction that triggers demyelination in GBS. Researchers have found that Zika infection is “even worse” in glial cells, which support and insulate neurons and are present throughout life, not just in fetal development. In humans, myelination begins in the 14th week of fetal development, although little myelin exists in the brain at the time of birth. During infancy, myelination occurs quickly, leading to a child’s fast development, including crawling and walking in the first year. Myelination continues through the adolescent stage of life. This explains Zika’s association with Guillain-Barré syndrome in adults. If you have GBS during pregnancy, you need treatment at a hospital so your provider can closely monitor your health and your baby’s health. GBS may increase your risk of pregnancy complications, including premature birth.
_
French Polynesia experienced a large outbreak of Zika virus between October 2013 and April 2014. During that time, there was a reported increase in the reporting of Guillain–Barré syndrome. A case–control study recently published in The Lancet examined the links between Zika infection and Guillain–Barré syndrome. Of the 42 patients diagnosed with Guillain–Barré syndrome during the outbreak, 98% had anti-Zika IgM or IgG antibodies, and all 42 had neutralising antibodies against Zika, compared with 56% in the control group (patients treated at the hospital for other illnesses). In addition, 37 of the 42 patients with Guillain–Barré syndrome (88%) reported symptomatic Zika virus infection that preceded the occurrence of neurological symptoms by a median of 6 days. Most patients (95%) with Guillain–Barré syndrome had pre-existing dengue immunity, but this did not differ significantly from the control groups. Twenty-four out of 100000 people infected with Zika virus developed Guillain–Barré in French Polynesia during the outbreak compared with a global incidence of 1–4 per 100 000 person-years. In 2015, 42 GBS cases were reported in the Brazilian state of Bahia, among which 26 (62%) had a history of symptoms consistent with Zika virus infection. A total of 1708 cases of GBS were registered nationwide, representing a 19% increase from the previous year (1439 cases of GBS in 2014), though not all states reported an increase in incidence.
_________
_________
Ocular disorders:
Ocular anomalies have been reported among infants with microcephaly in Brazil. The most common ocular abnormalities are focal pigment mottling, chorioretinal atrophy, and optic-nerve abnormalities (hypoplasia and severe cupping of the optic disk). Other ocular manifestations are foveal reflex loss, macular neuroretinal atrophy, lens subluxation, and iris coloboma. Whether ocular manifestations occur after congenital Zika virus infection in infants without microcephaly remains unknown.
_
Ocular abnormalities in infants with microcephaly: 2016 study:
A JAMA Ophthalmology study published in February, 2016 explored the connection between ocular abnormalities in infants with microcephaly and suspected congenital infection with the Zika virus. Researchers found that congenital Zika virus infection did, in fact, correspond with vision-threatening eye issues, which included bilateral macular and perimacular lesions, as well as optic nerve abnormalities. The study was conducted from Dec. 1 to Dec. 21 and involved 29 women and their infants with microcephaly who were evaluated with ophthalmic examinations at Roberto Santos General Hospital in Salvador, Brazil. Anterior segment and retinal, choroidal and optic nerve abnormalities were documented using a wide-field digital imaging system. Differential diagnoses of toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, syphilis and HIV infection were ruled out through clinical examination and serologic testing. Of the 29 mothers, almost 80 percent reported Zika virus infection signs and symptoms during pregnancy: 18 women in the first trimester, four in the second trimester and one in the third trimester. Among these women’s infants, ocular abnormalities were present in almost 30 percent of their eyes. Bilateral findings in seven of the 10 patients who presented with ocular lesions included focal pigment mottling of the retina and chorioretinal atrophy (about 65 percent of eyes), followed by optic nerve abnormalities (about 47 percent), bilateral iris coloboma (about 12 percent) and lens subluxation (about 6 percent). “This study can help guide clinical management and practice,” said the authors. “Infants with microcephaly should undergo routine ophthalmologic evaluations to identify such lesions.”
_________
_________
Diagnosis and differential diagnosis of Zika:
_
Clinical diagnosis:
The clinical picture of Zika virus infection is similar to that of other mosquito borne viruses such as dengue and chikungunya, which often co-circulate in the areas where Zika virus is endemic. The following interim case definitions have been issued by the World Health Organization to provide global standardization for classification and reporting of Zika virus cases.
1. A suspected case is a person with a rash (maculopapular) and/or fever (37.8 to 38.5°C) with at least one of the following symptoms (not explained by other medical conditions): arthralgia, arthritis, or conjunctivitis (nonpurulent/hyperemic). A suspected case should also have relevant epidemiologic exposure within two weeks prior to onset of symptoms (residence in or travel to an area where mosquito-borne transmission of Zika virus infection has been reported or unprotected sexual contact with a person who meets these criteria), even though epidemiologic exposure is not included in the World Health Organization (WHO) categorization of a suspected case.
2. A probable case is a suspected case with immunoglobulin (Ig)M antibody against Zika virus (with no evidence of infection with other flaviviruses) and relevant epidemiologic exposure (as described above).
3. A confirmed case is a person with laboratory confirmation of Zika virus infection, either by detection of viral RNA or antigen in serum or other samples or by detection of Zika IgM antibody and Zika virus 90 percent plaque-reduction neutralization test (PRNT90) titer ≥20 and PRNT90 titer ratio ≥4 compared with other flaviviruses.
_
For nonpregnant patients residing in areas where mosquito transmission has been established, the diagnosis of Zika virus infection may be suggested based on symptoms and signs and may not necessarily warrant laboratory testing, although differentiation from other illnesses with similar clinical manifestations (e.g., dengue, chikungunya) may be difficult. For nonpregnant patients residing in areas where mosquito transmission has not been established and laboratory testing is available, diagnostic testing is warranted for individuals with characteristic symptoms and signs and relevant epidemiologic exposure.
________
Laboratory diagnosis:
Routine laboratory findings for Zika, e.g. CBC, are normal for most patients. However, abnormal labs may include leukopenia, thrombocytopenia and elevation of liver transaminases. Culture-based methods for Zika detection are not yet clinically available. Laboratory analysis is currently based on detection of Zika RNA in serum, plasma, urine, saliva and amniotic fluid. Zika virus can be identified by reverse transcriptase PCR (RT-PCR) in acutely ill patients. However, the period of viremia can be short and the World Health Organization (WHO) recommends RT-PCR testing be done on serum collected within 1 to 3 days of symptom onset or on saliva or urine samples collected during the first 3 to 5 days. When evaluating paired samples, Zika virus was detected more frequently in saliva than serum. The longest period of detectable virus has been 11 days and Zika virus does not appear to establish latency. Later on, serology for the detection of specific IgM and IgG antibodies to Zika virus can be used. IgM antibodies can be detectable within 3 days of the onset of illness. Serological cross-reactions with closely related flaviviruses such as dengue and West Nile virus as well as vaccines to flaviviruses are possible.
_
RT-PCR:
RT-PCR is a PCR test that is designed to detect and measure RNA. Although initial PCR tests amplified DNA, many viruses and other biological components (for example, mitochondria) utilize RNA as their genetic material. RT-PCR differs from conventional PCR by first taking RNA and converting the RNA strand into a DNA strand. This is done by essentially the same method for PCR described above with the exception of using an enzyme termed reverse transcriptase instead of the DNA polymerase. The reverse transcriptase allows a single strand of RNA to be translated into a complementary strand of DNA. Once that reaction occurs, the routine PCR method can then be used to amplify the DNA. RT-PCR has been used to detect and study many RNA viruses.
_
Four ways by which Zika diagnosis is confirmed in laboratory:
1. Diagnostic RT-PCR: Nucleic acid testing:
Nucleic acid detection by reverse transcriptase-polymerase chain reaction targeting the non-structural protein 5 genomic region is the primary means of diagnosis. Standard RT-PCR and quantitative RT-PCR provide a rapid, specific and sensitive method for ZIKV early detection. Viral RNA has been detected in serum up to day 10 after the onset of symptoms. ZIKV RNA also has been detected in urine or saliva samples. However since more studies are needed, it is recommended that the serum sample be taken during the first 5 days after the onset of symptoms.
2. Detection of IgM antibodies to Zika virus by diagnostic ELISA:
It is done in convalescent phase (> 5 days) by testing IgM antibodies in serum. This is not the main stay of diagnosis as cross reactivity with other flaviviruses is very high. Dengue antibodies are not protective against Zika infection. So many people who previously had dengue are getting Zika. But these dengue antibodies may give false positive Zika test.
3. Plaque Reduction Neutralization Test (PRNT): Antibody effectiveness testing:
Currently, the standard assay for Zika viral infection is a PCR test that probes for the presence of viral RNA in a sample. While it works well to detect the virus, the pathogen’s RNA is only around for a short period of time. By the time [patients] make it into the clinic, the virus is likely gone or it’s at the tail end, beyond the limit of detection. What clinicians and epidemiologists would really like is to be able to determine whether a baby was exposed to Zika in utero months earlier. In this situation, lab can perform what’s called a plaque-reduction neutralization test, which can pick up on past infections. Essentially, a patient’s serum is mixed with the virus, and technicians watch over several days to see whether the virus can infect a cell culture—if it cannot, the virus has been neutralized by antibodies in the serum, indicating a person had been exposed to Zika. The trouble is that “you have to have live virus and appropriate safety conditions,” meaning few labs are able to do the test.
4. Virus Isolation:
Viral isolation is not regarded as a diagnostic tool and is recommended only for supplemental research studies in public health surveillance.
________
The diagnosis of Zika virus infection is definitively established by reverse-transcription polymerase chain reaction (RT-PCR) for Zika viral RNA or Zika virus serology.
1. For individuals within the first seven days after onset of symptoms, the diagnosis of Zika virus infection may be established via RT-PCR of serum for detection of Zika virus RNA. RT-PCR is positive only for a brief window (three to seven days) when the infected person has viremia; therefore, negative results cannot exclude infection. RT-PCR testing for dengue virus and chikungunya virus should also be pursued.
2. For individuals four or more days after the onset of symptoms, the diagnosis of Zika virus infection may be established by Zika virus serologic testing (Zika virus IgM and neutralizing antibody titers that are ≥4-fold higher than dengue virus neutralizing antibody titers in serum). Measuring virus-specific neutralizing antibodies is useful for discriminating between cross-reacting antibodies from other flavivirus infections; testing is considered inconclusive if Zika virus neutralizing antibody titers are <4-fold higher than dengue virus neutralizing antibody titers. Acute and convalescent sera should be obtained to detect an increased antibody titer in paired samples with an interval of two to three weeks.
__
Diagnostic Tests for Zika virus:
| Sample | Test | Timing |
| Blood | Polymerase chain reaction | Typically <5 days (occasionally up to 8 days) from symptom onset |
| Saliva | Polymerase chain reaction | Typically <5 days (occasionally up to 8 days) from symptom onset |
| Urine | Polymerase chain reaction | Of 55 patients with suspected travel-related Zika infection in Florida who provided urine and serum samples within 5 days of symptom onset, 95% of the urine samples tested positive for Zika RNA compared with 56% of serum samples. After day 5, no detectable RNA levels were found in serum, while urine specimens tested positive up to day 20. |
| Semen | Polymerase chain reaction | Very limited data: RNA has been detected at 62 days after symptom onset in one case |
| Serum | IgM antibody detection | Detectable 4-7 days from symptom onset and persists for 2-12 weeks |
_
_
Zika virus RNA has been detected in blood, urine, saliva, seminal fluid and breast milk.
__
The mainstays of the routine diagnosis of Zika virus infection are the detection of viral nucleic acid by RT-PCR and the detection of IgM antibodies by IgM-capture enzyme-linked immunosorbent assay (MAC-ELISA). The detection of viral nucleic acid in serum provides a definitive diagnosis; however, in most instances viremia is transient, and diagnosis by RT-PCR has been most successful within 1 week after the onset of clinical illness. In contrast, viral RNA was detected in serum approximately 10 weeks after infection in a pregnant woman whose fetus had evidence of congenital infection. In addition, viremia is generally low level, which makes viral isolation from clinical samples difficult. Although the precise timing of the onset and the duration of the IgM antibody response to Zika virus that is detectable by MAC-ELISA have not yet been defined, extensive experience with other, related flaviviruses suggests that IgM will appear as viremia wanes within the first week after symptom onset and will persist for several months. Thus, RT-PCR testing of serum samples obtained within the first week of clinical illness and MAC-ELISA testing of samples that are not tested by RT-PCR or that are found to be negative by RT-PCR are likely to have the highest diagnostic yield.
_
The considerable cross-reactivity of flavivirus antibodies presents major challenges for the interpretation of serologic test results. For example, recent Zika virus infection may also evoke a positive MAC-ELISA result for dengue. The plaque reduction neutralization test (PRNT), the most specific test used to differentiate antibodies of closely related viruses, can be used to help verify MAC-ELISA results. However, this test is labor-intensive and costly, involves handling of live virus, takes up to a week to perform, requires standardized reagents that often are not available, and is not widely performed. In settings where PRNT is not available or the volume of testing makes PRNT impractical, specimens that are found positive by Zika virus MAC-ELISA and negative by dengue MAC-ELISA may be interpreted as a presumptive recent Zika virus infection. However, the diagnostic accuracy of this approach has not been established.
_
The greatest challenge with serologic cross-reactivity arises from the “original antigenic sin” phenomenon: for patients who have previously been exposed to a heterologous flavivirus by natural infection or vaccination, the antibody response to the previous infecting flavivirus will be more vigorous than the response to the current one. Even the PRNT cannot reliably establish a diagnosis in such patients. This is particularly problematic in areas in which dengue is endemic, where more than 90% of the population may have had previous exposure to dengue virus and dengue and Zika viruses may be co-circulating.
_
Reliable testing regimens for the diagnosis of prenatal and antenatal Zika virus infection have not been established. Amniotic fluid has tested positive by RT-PCR in instances of congenital Zika virus infection; however, the sensitivity of RT-PCR in this context is unknown. At the time of delivery, cord blood can be tested by RT-PCR and MAC-ELISA, but the sensitivities of these tests for detecting prenatal Zika virus infection are unknown. RT-PCR and immunohistochemical testing have been useful in establishing Zika virus infection in tissues of fetal losses and full-term infants who died shortly after birth.
_
Detection of Zika virus in saliva: 2015 study:
The use of saliva sample increased the rate of molecular detection of ZIKV at the acute phase of the disease but did not enlarge the window of detection of ZIKV RNA. Saliva was of particular interest when blood was difficult to collect (children and neonates especially). Saliva samples were used routinely for Zika fever diagnosis during the French Polynesian ZIKV outbreak.
_
Detection of Zika Virus in Urine: 2015 study:
Authors describe the kinetics of Zika virus (ZIKV) detection in serum and urine samples of 6 patients. Urine samples were positive for ZIKV >10 days after onset of disease, which was a notably longer period than for serum samples. This finding supports the conclusion that urine samples are useful for diagnosis of ZIKV infections. Authors report the suitability of urine samples for diagnosis of ZIKV infection by showing that ZIKV RNA is detectable in urine at a higher load and with a longer duration than in serum.
__
Another 2016 study on Zika virus in urine:
The CDC is now recommending urine testing in patients with suspected Zika virus infection. Clinicians can test urine collected less than 2 weeks after symptom onset with real-time reverse transcription-polymerase chain reaction (rRT-PCR) tests. The guideline recommends that the rRT-PCR test should be done in addition to serum testing if the specimens were gathered less than 1 week after symptoms began. A positive result on either test means the patient is infected with Zika. Early data indicate that the virus can be found at higher levels or for a longer period in the urine than in serum. Of 55 patients with suspected travel-related Zika infection in Florida who provided urine and serum samples within 5 days of symptom onset, 95% of the urine samples tested positive for Zika RNA compared with 56% of serum samples. After day 5, no detectable RNA levels were found in serum, while urine specimens tested positive up to day 20.
_
Difficulty in diagnosis:
Scientists can use polymerase-chain-reaction-based methods to distinguish Zika from other flaviviruses. But those tests are accurate only during the short window patients still have the virus in their system—about seven days after infection. By the time a patient has symptoms that warrant a visit to the doctor, the virus is no longer circulating in their bloodstream. Another option is to look for antibodies against the virus. But Zika and dengue are closely enough related that antibodies to Zika also recognize dengue and vice versa. Making a definitive diagnosis based on antibodies is possible but becomes time consuming and laborious.
______
Interpretations of Zika tests:
_______
A new paper-based test for the Zika virus:
The new device is based on technology that researchers previously developed to detect the Ebola virus. In October 2014, the researchers demonstrated that they could create synthetic gene networks and embed them on small discs of paper. These gene networks can be programmed to detect a particular genetic sequence, which causes the paper to change color. Upon learning about the Zika outbreak, the researchers decided to try adapting their device to diagnose Zika, which has spread to other parts of South and North America since the outbreak began in Brazil. They developed sensors, embedded in the paper discs, that can detect 24 different RNA sequences found in the Zika viral genome, which, like that of many viruses, is composed of RNA instead of DNA. When the target RNA sequence is present, it initiates a series of interactions that turns the paper from yellow to purple. This color change can be seen with the naked eye, but the researchers also developed an electronic reader that makes it easier to quantify the change, especially in cases where the sensor is detecting more than one RNA sequence. All of the cellular components necessary for this process — including proteins, nucleic acids, and ribosomes — can be extracted from living cells and freeze-dried onto paper. These paper discs can be stored at room temperature, making it easy to ship them to any location. Once rehydrated, all of the components function just as they would inside a living cell. The researchers also incorporated a step that boosts the amount of viral RNA in the blood sample before exposing it to the sensor, using a system called NASBA (nucleic acid sequence based amplification). This amplification step, which takes one to two hours, increases the test’s sensitivity 1 million-fold. The researchers showed that the device could detect very low viral RNA concentrations and could also distinguish Zika from dengue.
_____
Screening in pregnancy for maternal and intrauterine infection:
Areas with no mosquito transmission:
In areas with no mosquito-borne Zika virus transmission, healthcare providers should ask pregnant women about relevant epidemiologic exposure (residence in or travel to an area where mosquito-borne transmission of Zika virus infection has been reported or unprotected sexual contact with a person who meets these criteria). For pregnant women with no relevant epidemiologic exposure, laboratory testing for Zika virus infection is not indicated. For pregnant women with relevant epidemiologic exposure, laboratory testing for Zika virus infection should be performed in both symptomatic and asymptomatic patients.
1. Pregnant women with clinical illness consistent with Zika virus infection (presence of two or more symptoms consistent with Zika virus infection [acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis] during or within two weeks of epidemiologic exposure) should have laboratory testing as follows:
•For patients presenting within the first seven days after onset of symptoms, serum Zika virus RT-PCR should be performed.
•For patients presenting four or more days after the onset of symptoms, Zika virus serologic testing (Zika virus IgM and neutralizing antibody titers) should be performed.
•For patients presenting four to seven days after onset of symptoms with negative Zika virus RT-PCR, Zika virus serologic testing (Zika virus IgM and neutralizing antibody titers) should be performed.
2. Asymptomatic pregnant women should have Zika virus serologic testing within 2 to 12 weeks following exposure. Interpretation of serologic test results for asymptomatic pregnant women with Zika virus exposure is complex given cross-reactivity among related flaviviruses. However, a negative IgM result obtained 2 to 12 weeks following exposure suggests that a recent infection did not occur.
3. Pregnant women with relevant epidemiologic exposure should also undergo evaluation for fetal infection.
_
Areas with mosquito transmission:
In areas with mosquito-borne Zika virus transmission, pregnant women have on-going risk for infection throughout pregnancy. The diagnostic approach may need to be tailored to local circumstances including levels of Zika virus transmission and available resources. For pregnant women with clinical illness consistent with Zika virus infection (presence of two or more symptoms consistent with Zika virus infection [acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis]), laboratory testing with RT-PCR is warranted during the first week of illness. For asymptomatic pregnant women, serologic testing is warranted at the initiation of prenatal care. If the initial test is performed in the first or early second trimester and is negative, repeat testing is indicated at 18 to 20 weeks. Interpretation of serologic testing may be difficult; false-positive Zika serologic test results can occur given the possibility of prior exposure to related flaviviruses. Pregnant women in areas with mosquito transmission of Zika virus should also undergo evaluation for fetal infection.
___
Algorithm for pregnant women residing in areas with active Zika virus transmission:
___
Regardless of symptoms and test results, all pregnant women with a history of travel to an area of active Zika virus infection should undergo fetal ultrasonography to evaluate for microcephaly or intracranial calcifications. Although microcephaly and other fetal abnormalities may be detected as early as 18 to 20 weeks of gestation, they are often not detected until later in pregnancy, in part because some cases do not occur earlier in pregnancy. Furthermore, the use of ultrasonography to detect microcephaly is dependent on clinical and technical factors, and ultrasonography is not a highly sensitive means of detecting microcephaly. Findings associated with Zika virus infection that have been noted on ultrasound have included, in addition to microcephaly, an absent corpus callosum, hydranencephaly, cerebral calcifications, ventricular dilatation, brain atrophy, abnormal gyration, hydrops fetalis, anhydramnios, and intrauterine growth retardation. Detection of a fetal anomaly should be followed by amniocentesis for evaluation of intrauterine Zika virus infection. The sensitivity and specificity of amniocentesis for determination of congenital infection and prediction of fetal abnormality is unknown.
_
Evaluation of the Fetus among pregnant women diagnosed with ZIKV infection:
Serial ultrasounds (every 3-4 weeks) are recommended in pregnant women with confirmed or suspected (if testing results are pending) ZIKV infection in pregnancy, and for asymptomatic pregnant travellers returning from areas currently considered to have suitable conditions for sustained and high levels of ZIKV transmission, to help define risk and counsel the mother. Should CNS calcifications or fetal microcephaly be noted at ultrasonography of the asymptomatic pregnant returned traveller, then specific ZIKV testing (along with other routine testing) should be undertaken to help define the likely cause of the anomaly.
_
Zika and TORCH screen:
A TORCH screen is a blood test of pregnant women. It can help determine whether the mother has any infectious diseases that could be passed along to the fetus causing birth defects. Perinatal infections account for 2% to 3% of all congenital anomalies. TORCH, which includes Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes infections, are some of the most common infections associated with congenital anomalies. Most of the TORCH infections cause mild maternal morbidity, but have serious fetal consequences, and treatment of maternal infection frequently has no impact on fetal outcome. Therefore, recognition of maternal disease and fetal monitoring once disease is recognized are important for all clinicians. Knowledge of these diseases will help the clinician appropriately counsel mothers on preventive measures to avoid these infections, and will aid in counselling parents on the potential for adverse fetal outcomes when these infections are present. Since 80% of Zika are asymptomatic and significant proportion of Zika infected pregnant women pass Zika virus to fetus causing birth defects, it is advisable to include RT-PCR of Zika RNA and IgM antibodies against Zika virus in TORCH screen of pregnant women.
_
Infant testing:
Infants born to women with confirmed or suspected ZIKV infection in pregnancy, or those with microcephaly, intracranial calcifications or other symptoms of congenital ZIKV infection in whom the mother had potential geographic exposure to the virus, should be tested. This testing should include serology, PCR of serum (umbilical cord or infant sample), and PCR of placenta; if CSF is sampled, this can also be sent for PCR and serology. Newborns with a mother who was potentially exposed and who have positive blood tests, microcephaly or intracranial calcifications should have further testing including a thorough physical investigation for neurologic abnormalities, dysmorphic features, splenomegaly, hepatomegaly, and rash or other skin lesions. Other recommended tests are cranial ultrasound, hearing evaluation, and eye examination. Testing should be done for any abnormalities encountered as well as for other congenital infections such as syphilis, toxoplasmosis, rubella, cytomegalovirus infection, lymphocytic choriomeningitis virus infection, and herpes simplex virus.
_
Infants born to women with symptoms of active ZIKV infection around the time of delivery are at risk for perinatal transmission of the disease. In the limited number of reported cases to date, perinatally infected infants have exhibited either no or mild symptoms and laboratory findings (rash, thrombocytopenia). Regardless, such infants should be monitored closely given the unclear spectrum of potential illness in this emerging infection. Testing with serology and serum PCR during acute illness is recommended. In such cases, care should be taken to ensure a thorough work up for other important and treatable causes of congenital infections, such as CMV and toxoplasma infection.
__
Zika Virus Testing for Sexual Transmission:
At present, Zika virus testing for the assessment of risk for sexual transmission is of uncertain value, because current understanding of the duration and pattern of shedding of Zika virus in the male genitourinary tract is limited. Therefore, neither serum nor semen testing of men for the purpose of assessing risk for sexual transmission is currently recommended. Zika virus testing is recommended for persons who have had possible sexual exposure to Zika virus and develop signs or symptoms consistent with Zika virus disease. A pregnant woman with possible sexual exposure to Zika virus should be tested if either she or her male partner developed symptoms consistent with Zika virus disease.
________
Differential diagnosis of Zika:
In a “pure” Zika epidemic, a diagnosis can be made reliably on clinical grounds. Unfortunately, the fact that dengue and chikungunya, which result in similar clinical pictures, have both been epidemic in the Americas confounds clinical diagnoses. Specific tests for dengue and chikungunya are not always available, and commercial tests for Zika have not been available everywhere. Moreover, because Zika is closely related to dengue, serologic samples may cross-react in tests for either virus. Gene-detection tests such as the polymerase-chain-reaction assay can reliably distinguish the three viruses, but Zika-specific tests are not yet widely available. As Zika virus infection may cause a rash that could be confused with diseases such as measles, rubella, chikungunya and dengue, these other diseases need to be ruled out. Diagnosis of Zika virus infection will first and foremost be based on symptoms, travel history and exclusion of other diseases.
_
The differential diagnosis of Zika virus infection is wide. Diagnosis is guided by history (countries of travel, sexual contacts, and contact with other cases of infection) and examination. It is difficult to diagnose Zika virus infection based on clinical signs and symptoms alone due to overlaps with other arboviruses that are endemic to similar areas. The US Centers for Disease Control and Prevention (CDC) advises that “based on the typical clinical features, the differential diagnosis for Zika virus infection is broad. In addition to dengue, other considerations include leptospirosis, malaria, rickettsia, group A streptococcus, rubella, measles, and parvovirus, enterovirus, adenovirus, and alphavirus infections (e.g., chikungunya, Mayaro, Ross River, Barmah Forest, O’nyong’nyong, and Sindbis viruses).”
_______
_______
Treatment of Zika:
Zika virus disease is usually relatively mild and requires no specific treatment. There is currently no specific treatment for Zika virus infection. Care is supportive with treatment of pain, fever, and itching. Some authorities have recommended against using aspirin and other NSAIDs as these have been associated with hemorrhagic syndrome when used for other flaviviruses. Additionally, aspirin use is generally avoided in children when possible due to the risk of Reye syndrome. If only 20% of people infected with Zika have symptoms, who would get the treatment? The most vulnerable patients are pregnant women. Advice to pregnant women is to avoid any risk of infection so far as possible, as once infected there is little that can be done beyond supportive treatment. There is currently no vaccine available.
_
Rest:
People who show symptoms of the disease are advised to rest to ensure that they recover effectively from the virus. They may also feel tired because of the symptoms of the disease in the body.
_
Medication:
Patients who are suffering from a fever may benefit from taking paracetamol (acetaminophen) to relieve the fever, headaches, joint pain or muscle pain that develops. Aspirin or non-steroidal anti-inflammatory drugs are not recommended for the condition.
_
Fluids:
The fever has the potential to dehydrate a patient through extra perspiration. The patient may feel extra thirsty, tired, dizzy and pass less urine. The urine will also be dark in colour instead of straw-coloured. Patients should increase their intake of water, fruit juice or rehydration solutions to replace lost fluids. The patient could also benefit from light-weight clothing as well. Care should be taken to ensure that children who are dehydrated also have enough minerals.
_
Artificial tears:
Conjunctivitis can result in watery eyes, red eyes, stickiness and pain. It usually starts in one eye and can infect the other. The patient does not develop any problems with vision though the disease. However, the fluid produced by the eye is infectious. Treatments for bacterial conjunctivitis will not work. Artificial tear drops can help with any dryness that the patient feels in their eyes. The condition tends to improve when the virus clears the patient’s system. The patient should also avoid spreading the contagious fluid to anyone else by maintaining high standards of hygiene.
_
The WHO recommends optimal supportive care in patients with Guillain-Barr syndrome, including frequent neurologic examinations, testing of vital signs, and respiratory function monitoring to decrease the likelihood of complications (e.g., blood clots, respiratory failure). Patients whose symptoms are escalating rapidly or who are unable to walk should receive intravenous immunoglobulin therapy or therapeutic plasma exchange.
_
Difficulty in Zika treatment:
Researchers want to test new drugs, but getting access to the live virus has been hard. Even if someone manages to access the live virus and can find a compound that kills it in cells, the researcher will hit another roadblock: To date, no one has published practical animal models of Zika virus to screen potential therapies against. There are currently no approved drugs against Zika; developing a new medication for any disease can take 10 to 20 years. That’s why many researchers are taking a closer look at older drugs, testing them to see if they might block infection with the virus or prevent it from harm the brain and nervous system. Some are antimalarial drugs, while others are natural products and others are drugs that treat infections with parasitic worms. The ideal drug would not just treat Zika, but also prevent it, much like the antimalarial drugs that travellers take before visiting tropical countries. An anti-Zika drug would also have to be safe enough for pregnant women or women of reproductive age. The Alzheimer’s drug memantine protects cells in lab dishes and animals. Although it’s too soon to know if memantine will protect people from Zika’s effects, we need a clinical trial to answer that question.
_
Could mothers receive a blood transfusion to prevent the disease?
Though it does work for other diseases, such as hepatitis A but transferring immunity via a blood transfusion isn’t the best or simplest solution. For instance, while the strategy was touted mightily during the Ebola outbreak, the latest studies suggest the technique wasn’t effective.
_
Would abortion be offered to a woman who has a fetal abnormality like microcephaly?
When a significant brain abnormality or microcephaly is confirmed, the option of termination of pregnancy should be discussed with the woman, regardless of gestation.
_
Treatment of microcephaly:
There is no treatment for microcephaly that can return a child’s head to a normal size or shape. Treatment focuses on ways to decrease the impact of the associated deformities and neurological disabilities. Children with microcephaly and developmental delays are usually evaluated by a paediatric neurologist and followed by a medical management team. Early childhood intervention programs that involve physical, speech, and occupational therapists help to maximize abilities and minimize dysfunction. Medications are often used to control seizures, hyperactivity, and neuromuscular symptoms. Family counselling and support for parents is also extremely important.
_
Scientists identify potential way to treat Zika virus infection:
They reconstructed a high-resolution structure of the Zika virus, opening up possibilities on how to treat the virus with potent antibodies or drugs. They found that the overall Zika virus architecture was similar to other flaviviruses like dengue, but more thermally and structurally stable due to tighter interactions between the Zika virus surface proteins, which help the virus attach to a host cell. This could explain why the virus was resilient enough that it could be transmitted via sexual contact. This discovery could lead to future developments where the Zika virus could potentially be destabilised by potent antibiotics or drugs. To reconstruct the Zika virus structure, the team imaged the virus under a cryo-electron microscope from a large number of purified viral particles, and pieced together thousands of images.
__________
__________
Zika prevention:
Since there is no specific treatment or vaccine, the response to the current ZIKV outbreak should involve a multipronged, multilateral, coordinated, and comprehensive public health response. With ZIKV transmission from French Polynesia to Brazil, and the resultant estimated > 1,000,000 cases in Brazil (and Brazil is the host country of the 2016 Summer Olympics), the anticipated movement of ZIKV into North America, and the many documented cases in the USA, a thorough, thoughtful, and level-headed preventive public health approach is imperative.
_
_
Vector control:
Mosquitoes and their breeding sites pose a significant risk factor for Zika virus infection. Prevention and control relies on reducing mosquitoes through source reduction (removal and modification of breeding sites) and reducing contact between mosquitoes and people. This can be done by using insect repellent regularly; wearing clothes (preferably light-coloured) that cover as much of the body as possible; installing physical barriers such as window screens in buildings, closed doors and windows; and if needed, additional personal protection, such as sleeping under mosquito nets during the day. It is extremely important to empty, clean or cover containers regularly that can store water, such as buckets, drums, pots etc. Other mosquito breeding sites should be cleaned or removed including flower pots, used tyres and roof gutters. Communities must support the efforts of the local government to reduce the density of mosquitoes in their locality. Efforts must be made to eliminate mosquito breeding sites such as still water soon after rains and its accumulation in discarded containers and waste materials in and around houses. During outbreaks, health authorities may advise that spraying of insecticides be carried out. Insecticides recommended by the WHO Pesticide Evaluation Scheme may also be used as larvicides to treat relatively large water containers.
__
If there is local transmission of Zika (or dengue or chikungunya, which are also spread by Aedes mosquitoes), in addition to the measures discussed above, you should take the following steps to control mosquitoes:
•Get rid of rain barrels, bird baths, tires, tins, and other sources of standing water. Mosquitoes like to breed in stagnant water.
•Dump out any water that collects in your garbage cans, and turn pails upside down so they don’t collect water.
•Inside your home, change water in flower vases every other day, and dump out excess water from flower pot plates. Aedes mosquitoes will breed in your house, if given the chance.
•Don’t buy a bug zapper. Studies have shown that these do not reduce mosquito bites, and may actually increase mosquito populations by killing off beneficial insects that prey on mosquitoes. And don’t bother with an ultrasound device, as they don’t work either.
__
Personal protective measures (PPM):
PPM are recommended to protect all living in the areas of risk. The mosquitoes that transmit ZIKV are often most active during daytime and evening hours. For this reason, PPM should be used through all hours of the day and night. Use of PPM will also provide protection against other vector-associated diseases such as malaria, dengue, and chikungunya. PPM are summarized below.
1. Cover up:
-Wear light-coloured, long-sleeved, loose fitting, tucked-in shirts, long pants, shoes or boots (not sandals), and a hat.
2. Use insect repellent on exposed skin:
-It is recommended that adults use repellents that contain DEET (20-30%) or icaridin (20%).
-It is recommended that children six months to twelve years of age use repellents that contain icaridin (20%). As a second choice, this age group can use repellents with age-appropriate DEET concentrations as per label.
-If bites cannot be avoided using a physical barrier, consider use of up to 10% DEET or 10% icaridin for infants under six months of age.
3. Protect living areas from mosquito entry:
-Stay in a well-screened or completely enclosed air-conditioned room.
-Reduce your risk in work and accommodation areas by closing eaves, eliminating holes in roofs and walls and closing any other gaps.
4. If mosquito entry into living quarters cannot be otherwise prevented (e.g. by screening):
-Use a bed net (e.g. for sleeping or resting inside), preferably treated with insecticide.
-Netting can also be used to protect children in playpens, cribs, or strollers.
-Bed nets will also provide protection against diseases like malaria.
5. Apply a permethrin insecticide to clothing and other travel gear for greater protection
-Although permethrin clothing treatments are not widely available, travel health clinics can advise you how to purchase permethrin and pre-treated gear before or during your trip.
-Permethrin-treated clothing is effective through several washes.
-Always follow label instructions when using permethrin.
-Do not use permethrin directly on skin.
-Permethrin is probably safe in pregnancy, although data on first-trimester exposure are scanty.
_
Mosquito repellents on exposed skin:
Repellents should contain DEET (N, N-diethyl-3-methylbenzamide), IR3535 (3-[N-acetyl-N-butyl]-aminopropionic acid ethyl ester) or icaridin (1-piperidinecarboxylic acid, 2-(2-hydroxyethyl)-1-methylpropylester). Product label instructions should be strictly followed. Higher percentages of active ingredient provide longer protection. Special attention and help should be given to those who may not be able to protect themselves adequately, such as young children, the sick or elderly.
_
Are DEET based insect repellents safe for use in pregnancy?
Insect repellents with concentrations of DEET up to 50% are commonly available and are safe in pregnant and breastfeeding women (and in infants and children over the age of two months).
_
Difficulty in eradicating vector:
With a treatment or vaccine for Zika potentially years away, countries are relying on mosquito control to curb the virus’ spread. Aedes aegypti mosquitoes, which inhabit tropical and subtropical regions, have been named as the culprit in transmitting the virus. But getting rid of Aedes aegypti is extremely difficult because the mosquitoes don’t seem to be affected by most spraying regimens. The problem is that Aedes aegypti feed during the day, and pesticides must be sprayed at dawn or dusk to be effective. Also, the mosquitoes like to come indoors to feed. So, unless pesticides are sprayed inside homes, chances are good they’re not getting to the insects. These mosquitoes are very small and you can’t feel the bites. Oftentimes you don’t even know you’ve been bitten. To get rid of the biting bugs, it’s critical to eliminate any standing water. Aedes aegypti breed even in discarded soda bottle caps. They’re survivors. Despite Aedes aegypti’s survival skills, the mosquitoes actually have a fairly limited flight range of about 150 meters. That has made some scientists suspect that because Zika has spread so quickly, the more common Culex mosquito may be transmitting the virus as well. The theory is currently being investigated. If that is true, that brings this to a whole different level. Culex mosquitoes have a much larger range, but they can usually be controlled through common mosquito abatement programs.
_
Mosquito trap with junked tires:
Mosquitos like old tires. More specifically, female mosquitos like to lay their eggs in the cool, stagnant water that often accumulates within them. Now, in the fight against mosquito-borne diseases such as the Zika virus, the Government of Canada is using that fact against the insects. Researchers with the Grand Challenges Canada initiative have created a highly-effective mosquito trap, each one of which is made from a single discarded tire. Known as the ovillanta, the trap is made from two 50-cm (19.7-inch)-long sections of tire, placed together to form a sort of cave. Water is placed in the bottom section, along with a milk-based non-toxic solution that attracts mosquitos. Additionally, a strip of paper is placed within to float on the water. Female mosquitos subsequently fly into the ovillanta, lay their eggs on the paper, and deposit pheromones in the water to let other mosquitos know that it’s a safe breeding site. The catch is, twice a week the paper is removed and checked for eggs, then burned or sterilized using ethanol. Additionally, using a release valve on the bottom section, the water is drained and any larvae present are filtered out and destroyed. The filtered water is then placed back in the trap for reuse – it becomes more attractive to mosquitos over time, as more and more of the pheromones are deposited and concentrated within it. In a 10-month field test conducted in Guatemala, a system of 84 ovillantas allowed users to collect almost seven times as many Aedes mosquito eggs as were collected using 84 standard traps placed in the same locations. Additionally, use of the new traps is said to be about one-third the cost of destroying larvae in natural ponds, and about 20 percent the cost of spraying for adult mosquitos – it’s also considerably more environmentally-friendly than the latter. The research is being led by Dr. Gerardo Ulibarri of Ontario’s Laurentian University, working with colleagues Angel Betanzos and Mireya Betanzos of the National Institute of Public Health of Mexico. Plans call for a program to be established in which local people, living in areas prone to maladies such as Zika or dengue, will build and maintain the traps.
_
Introduction of bacteria into, and genetic modification of, the mosquito populations:
Infecting Mosquitoes with Bacteria could help fight Zika: a study:
Infecting mosquitoes with Wolbachia — an endosymbiotic bacterium frequently found in insects and currently being used to help control dengue in Brazil — might help limit the spread of Zika virus, according to a brief report in Cell Host & Microbe. Wolbachia can rapidly invade host insects and, ultimately, inhibit the transmission of many human pathogens. Researchers in Brazil fed Zika-infected human blood to mosquitoes that were infected with Wolbachia and also to Wolbachia-free mosquitoes. Two weeks later, Zika levels were significantly lower in the Wolbachia-infected mosquitoes. The researchers then injected the mosquitoes’ saliva into Zika-free mosquitoes: saliva from Wolbachia-infected mosquitoes did not cause any new Zika infections, while saliva from Wolbachia-free mosquitoes led to new Zika infections 85% of the time. The researchers say their findings show that Wolbachia’s protective effects could extend beyond dengue and chikungunya viruses. They add, however, that “extensive public engagement will be required before releases of Wolbachia-infected mosquitoes can be scaled up for use.”
_
Another, relatively new development is the use of genetically modified (GM) mosquitoes whose offspring are not able to survive, especially A. aegypti OX513A (noted to have effectiveness against dengue, and thus hopefully ZIKV). This is a genetically engineered strain that owes its effectiveness to the “release of insects carrying a dominant lethal” (RIDL) genetic system. Released males mate with wild females and the offspring of these females will die. Increasing numbers of such males should theoretically reduce mosquito populations below the threshold needed for disease transmission. Some scientists are also trialling the use of genetically modified sterile mosquitoes that appear to reduce mosquito populations by 90%. The FDA announced that it found genetically engineered male mosquitoes to be environmentally safe, which is another big hurdle cleared.
_
Then there is the gene drive. This newly developed technology involves inserting genes into an organism in such a way that a trait spreads throughout a whole population. In theory, a gene drive could be created that prevents mosquitoes from incubating Zika virus—or destroys the entire species of A. aegypti. A gene drive has been created that prevents mosquitoes from harboring the malaria parasite. But some researchers are understandably concerned about intentionally messing with natural selection. Once a drive is released into a wild population, there is no turning back, and there is no telling what sort of side effects it might have.
_
Fish can help stop Zika:
Some countries affected by Zika and dengue are using biological methods as part of an integrated approach to mosquito control. El Salvador, for example, with strong support from fishing communities, is introducing larvae-devouring fish into water storage containers. A mosquito’s natural enemy is becoming a popular ally in the fight against the West Nile and Zika viruses. The tiny mosquitofish, or gambusia affinis, feasts on mosquito larvae.
_______
Prevention of Sexual transmission of Zika:
Sexual transmission of Zika virus is possible. There is mounting evidence that sexual transmission of Zika is more common than previously believed. All people who have been infected with Zika virus and their sexual partners should practice safer sex, by using condoms correctly and consistently. Pregnant women’s sex partners living in or returning from areas where local transmission of Zika virus occurs should practice safer sex, wearing condoms, or abstaining throughout the pregnancy. People living in areas where local transmission of Zika virus occurs should practice safer sex or abstain from sexual activity. In addition, people returning from areas where local transmission of Zika virus occurs should adopt safer sexual practices or consider abstinence for at least 4 weeks after their return to reduce the risk of onward transmission.
_
Err on the side of caution:
Many questions remain about sexual transmission of Zika. Researchers still aren’t certain how long the virus remains in the semen of infected men, whether men who never developed symptoms of Zika may still have the virus in their semen and whether Zika can be transmitted through oral sex. Given the uncertainties, the CDC and WHO have opted to err on the side of caution. The CDC recommends that pregnant women avoid visiting countries with current Zika outbreaks. Both organizations advise that pregnant women whose partners have lived in or travelled to a Zika-affected country use condoms whenever they engage in vaginal, anal or oral sex, or abstain from sex for the duration of the pregnancy. The WHO likewise advises that people living in Zika outbreak areas use condoms or abstain altogether from sexual activity.
_
As of March 2016, the CDC updated its recommendations about length of precautions for couples and advised that couples with men who have confirmed Zika fever or symptoms of Zika should consider using condoms or not having sex (i.e., vaginal intercourse, anal intercourse, or fellatio) for at least 6 months after symptoms begin. This includes men who live in and men who travelled to areas with Zika. Couples with men who travelled to an area with Zika, but did not develop symptoms of Zika, should consider using condoms or not having sex for at least 8 weeks after their return in order to minimize risk. Couples with men who live in an area with Zika, but have not developed symptoms, might consider using condoms or not having sex while there is active Zika transmission in the area.
_______
Prevention of Zika transmission through blood, cell, tissue and organ donations:
Canadian Blood Services (CBS) and Héma-Québec (Quebec’s blood operator) are carefully monitoring the Zika virus issue. They have revised their eligibility criteria for donors to mitigate the risk of the virus entering the Canadian blood supply. Anyone who has travelled outside of Canada, the continental United States and Europe will now be temporarily ineligible to give blood for three weeks. This new waiting period was implemented across the country on February 5, 2016. This 21-day waiting period ensures enough time has passed for the virus to be eliminated from a person’s bloodstream, and begins the day a person returns to Canada. The waiting period also applies to cord blood and stem cell donors who have travelled to affected areas. The risk of Zika virus transmission from a Canadian donor to a blood recipient is very low. Canadians may consider donating blood before they travel. Health Canada issued a notice on February 9, 2016 to provide information on the risks to cell, tissue, organ, and semen donation. In the notice, Health Canada is advising that the donation of cells, tissues, and organs from live donors who have travelled to a Zika affected area be postponed until a minimum of 21 days. In the case of a deceased donor, if it is in the best interest of the patient to go ahead with the transplant, the exceptional distribution provisions of the Safety of Human Cells, Tissues, and Organs for Transplantation Regulations should be used. Ultimately, it is the responsibility of the transplant program to weigh the risks and benefits of postponing any transplant procedures. On March 18, 2016 Health Canada updated its notice with respect to semen donation. Men that have returned from Zika-affected countries should postpone semen donations for six months after their return. This precautionary 6 month ineligibility period was selected to address the potential longer term persistence of Zika virus in semen and to reflect the additional risk posed by the fact that donations from one Zika infected semen donor have the potential to impact a number of individuals.
_
The FDA has issued new guidelines for reducing the possible transmission risk of Zika virus from human cells, tissues, and cellular and tissue-based products (HCT/Ps) from both living and deceased donors, including donors of umbilical cord blood, placenta, or other gestational tissues. There is a potential risk that the Zika virus can be transmitted by HCT/Ps used as part of a medical, surgical, or reproductive procedure. HCT/Ps include products such as corneas, bone, skin, heart valves, hematopoietic stem/progenitor cells (HPCs), gestational tissues such as amniotic membrane, and reproductive tissues such as semen and oocytes.
_
What measures may be considered for reducing the risk of Zika virus through blood transfusion in areas with active Zika virus transmission?
•Temporary exclusion of donors with a recent clinical history consistent with Zika virus disease, such as a combination of fever or rash with pinkeye, muscle aches, headache or malaise.
•Temporary exclusion of donors for whom laboratory test results show they may recently have been infected.
•Donors with clinical history consistent with Zika virus disease or a recent history of Zika virus infection should be deferred for a period not less than 28 days following the full resolution of symptoms.
•Similarly, sex partners of men with confirmed or suspected Zika virus infection in the last 3 months should be deferred for at least 28 days after their last sexual contact.
•People who have already donated must be encouraged to report to the blood transfusion service if they subsequently get symptoms of Zika virus infection, or if they are diagnosed with recent Zika virus infection within 14 days after blood donation.
•Blood components of appropriate shelf life (e.g. red blood cells) may be quarantined for a period of 7–14 days and released following confirmation from the donor that he or she has not experienced symptoms consistent with the acute phase of Zika virus infection. For platelets, which have a shorter shelf life, a 3-day quarantine period may be considered.
•Countries with many visitors to affected countries may need to assess the impact of deferral on blood supply availability and weigh the risks against the benefits of restricting donations.
_
How could blood donations be tested for the presence of Zika virus?
Where possible, blood donations may be tested for the presence of Zika virus by appropriate tests. Likely, pathogen reduction technology (PRT) may be implemented for plasma and platelets. In some cases, the selective testing for the presence of the virus in blood donors returning from affected countries may be considered as an alternative to deferral.
_________
_________
Travel alerts:
U.S. travellers are bringing the viral disease back with them. To date, the CDC has reported 358 travel-linked cases of Zika in U.S. states (including 31 pregnant women) and 471 cases of local infections in U.S. territories (of whom 58 are pregnant women). Travellers probably won’t bring infected mosquitoes along with them. It’s extremely unlikely that mosquitoes would be carried back by citizens traveling abroad. As adult mosquitoes are a relatively fragile insect that doesn’t travel very well. In addition, since only a fraction of the total mosquito population in Zika-endemic areas carries the virus, it’s even less likely for an infective mosquito to be brought back alive. The bigger concern is that a person infected with the virus could pass it along to local mosquito populations. Travellers should take the basic precautions described above to protect themselves from mosquito bites.
_
Overall, currently available evidence suggests that:
•ZIKV will have modest to no health impact on the large majority of non-pregnant travellers, though infection might sometimes result in serious neurologic sequelae such as GBS;
•Although much uncertainty remains, ZIKV might have a very serious impact on the developing fetus in mothers infected during pregnancy;
•Sexual transmission from male to female partners has been documented and might not be an infrequent event. Such transmission could, if it occurred around the period of conception or during pregnancy, pose a risk to the developing fetus.
Women who have travelled to an affected country without their partner must use appropriate contraception for eight weeks to avoid pregnancy.
_
CDC travel alert:
Because of the “growing evidence of a link between Zika and microcephaly”, in January 2016, the CDC issued a travel alert advising pregnant women to consider postponing travel to countries and territories with on-going local transmission of Zika virus. Later, the advice was updated to caution pregnant women to avoid these areas entirely if possible and, if travel is unavoidable, to protect themselves from mosquito bites. Male partners of pregnant women and couples contemplating pregnancy who must travel to areas where Zika is active are advised to use condoms or abstain from sex entirely. The agency also suggested that women thinking about becoming pregnant should consult with their physicians before traveling. As of April 2016, the CDC travel advisories include:
•Cape Verde
•Many parts of the Caribbean: Aruba, Barbados, Bonaire, Cuba, Curaçao, Dominica, Dominican Republic, Guadeloupe, Haiti, Jamaica, Martinique, Puerto Rico, Saint Lucia, Saint Martin, Saint Vincent and the Grenadines, Sint Maarten, Trinidad and Tobago, and the U.S. Virgin Islands
•Most of Central America: Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, and Panama
•Mexico
•Several Pacific Islands: American Samoa, Fiji, Kosrae, Marshall Islands, New Caldonia, Samoa, and Tonga
•Most of South America: Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Paraguay, Suriname, and Venezuela
_______
_______
WHO response:
Both the regional Pan American Health Organization (PAHO) as well as the WHO have issued statements of concern about the widespread public health impact of the Zika virus and its links to GBS and microcephaly. The WHO Director-General, Dr. Margaret Chan, issued a statement in February 2016 declaring that the recent cluster of microcephaly cases and other neurological disorders reported in Brazil, following a similar cluster in French Polynesia in 2014, constitutes a Public Health Emergency of International Concern. The declaration allows the WHO to coordinate international response to the virus as well as gives its guidance the force of international law under the International Health Regulations.
_
WHO is supporting countries to control Zika virus disease by taking actions outlined in the “Zika Strategic Response Framework”:
•Define and prioritize research into Zika virus disease by convening experts and partners.
•Enhance surveillance of Zika virus and potential complications.
•Strengthen capacity in risk communication to help countries meet their commitments under the International Health Regulations.
•Provide training on clinical management, diagnosis and vector control including through a number of WHO Collaborating Centres.
•Strengthen the capacity of laboratories to detect the virus.
•Support health authorities to implement vector control strategies aimed at reducing Aedes mosquito populations such as providing larvicide to treat still water sites that cannot be treated in other ways, such as cleaning, emptying, and covering them.
•Prepare recommendations for clinical care and follow-up of people with Zika virus, in collaboration with experts and other health agencies.
• Zika Strategic Response Framework
__
Zika virus: Aircraft disinsection for mosquito control:
As a precautionary measure, standard WHO recommendations regarding disinsection of aircraft and airports can be implemented in order to attempt to control the vector (Aedes mosquito) that spreads the Zika virus. It should be noted that the decision to implement WHO disinsection recommendations is dependent on individual country risk assessment for vector control. For countries and other entities which, after risk assessment for vector control choose to implement aircraft and airport airplane disinsection, it should be done according to standard WHO recommendations. WHO has provided guidelines on how to do so safely. Specifications for aircraft disinsection products have been established by the WHO Pesticide Evaluation Scheme (WHOPES), including:
•d-Phenothrin technical grade
•1R-trans-phenothrin technical grade
•Permethrin technical grade
The guidelines include four recommended techniques for aircraft disinsection:
1) Pre-flight;
2) Blocks away;
3) Top-of-descent; and
4) Residual treatment.
________
________
Zika vaccine:
Effective vaccines exist for several viruses of the flaviviridae family, namely Yellow fever vaccine, Japanese encephalitis vaccine, and Tick-borne encephalitis vaccine since the 1930s, and dengue fever vaccine is being developed since the mid-2010s. WHO experts have suggested that the priority should be to develop inactivated vaccines and other non-live vaccines, which are safe to use in pregnant women and those of childbearing age. The NIH Vaccine Research Center began work towards developing a vaccine for Zika per a January 2016 report. Bharat Biotech International reported in early February 2016 that it was working on vaccines for Zika using two approaches: “recombinant”, involving genetic engineering, and “inactivated”, where the virus is incapable of reproducing itself but can still trigger an immune response. As of March 2016, 18 companies and institutions internationally were developing vaccines against Zika, but none had yet reached clinical trials. Nikos Vasilakis of the Center for Biodefense and Emerging Infectious Diseases predicted that it may take two years to develop a vaccine, but 10 to 12 years may be needed before an effective Zika vaccine is approved by regulators for public use. US experts from the National Institutes of Health say trials of a Zika vaccine will likely start in September this year. Depending on the results, larger trials could begin at the start of 2017. The very, very best scenario” would be a vaccine ready for the general public by the beginning of 2018, they say. World had been in a similar situation in the early 1960s, when rubella was causing about 20,000 birth defects a year, after pregnant mothers became infected with the relatively mild German measles infection. As soon as we developed a rubella vaccine and started vaccinating everyone when they were children, the incidence of congenital rubella syndrome essentially disappeared. This is what we hope will happen with Zika. However, it is already too late for the thousands of babies thought to be affected by this mysterious virus.
_
Vaccine research 2016:
Cyclical outbreaks tend to drive public interest in clinical research for vaccines, but when it comes to public policy, these outbreaks are slow to motivate action. By the time researchers are able to bring a vaccine out of the lab and into the public, interest has often waned.
______
______
Zika and women vis-à-vis sex, pregnancy and abortion:
There is no evidence that pregnant women are more vulnerable to acquiring Zika virus infection or that this infection causes a more serious illness in pregnant women. Breastfeeding is still recommended by the WHO, even by women who have had Zika fever. There have been no recorded cases of Zika transmission to infants through breastfeeding, though the replicative virus has been detected in breast milk. There’s no evidence that the Zika virus will cause birth defects in the future among non-pregnant women with current or previous infection.
_
Women and Zika have a special relationship due to linking of fetal microcephaly to pregnant women infected by Zika virus. There is increasing evidence to suggest that pregnant women who catch the virus at any point during pregnancy may have an increased risk of giving birth to a baby with microcephaly. In this perspective, all women worldwide are divided in 6 groups as seen in the table below.
| Pregnant women living or travelled in Zika affected areas | Pregnant women living in areas not affected by Zika |
| Women planning pregnancy living or travelled in Zika affected areas | Women planning pregnancy living in areas not affected by Zika |
| Women not planning pregnancy living or travelled in Zika affected areas | Women not planning pregnancy living in areas not affected by Zika |
Out of these 6 groups, women who are pregnant/planning pregnancy living in areas not affected by Zika must ensure that their sexual partner does not bring Zika virus to them. Women who are pregnant or planning pregnancy and living or travelled in Zika affected areas are vulnerable to Zika microcephaly in their fetuses. Zika virus is spread primarily by mosquito bites and linked to the birth defect microcephaly. Pregnant women should delay travel to countries and territories where Zika virus is spreading. When returning from Zika-affected countries, pregnant women should be tested for Zika, and men with pregnant partners should use condoms. Other travellers, based on risk tolerance, values and preferences may wish to consider deferring travel to ZIKV-infected areas (e.g. males who are trying to impregnate their partner).
_
Pregnant women living or visited Zika-affected areas:
Pregnant women should get blood tests and ultrasound scans. As of early March, all pregnant women who have visited areas with active Zika transmission should have a blood test for the virus, whether or not they have symptoms. All pregnant women who live in those areas, such as Puerto Rico or American Samoa, should be tested at least twice during their pregnancies, whether or not they have symptoms. Testing for the virus is highly accurate in the first week after symptoms appear. After that, diagnostic tests must rely on antibodies, and false positives are possible if a woman has been infected with related viruses, like dengue and yellow fever, or has been vaccinated against them. More complex testing can lower the false-positive rate, but not eliminate it. In addition, all pregnant women who may have been exposed should have at least one ultrasound looking for evidence of fetal microcephaly or calcifications, indicating that the fetal skull is hardening too early. If tests show that a woman is infected, she should have ultrasounds at regular intervals. Unfortunately, ultrasounds usually cannot detect microcephaly before the end of the second trimester.
_
For pregnant women and those planning a pregnancy who choose to travel to areas with ZIKV transmission or for whom travel cannot be avoided, use of personal protection measures against insect bites is strongly advised. Based on current information on the incubation period and duration of viremia, and the unclear duration of viral persistence in tissues, women wishing to become pregnant should wait at least two months after their return from an area currently considered to have suitable conditions for sustained and high levels of ZIKV transmission before trying to conceive.
_
ZIKV RNA has been detected in semen two months after acute illness. It is not known how long viral shedding in semen can last, how often this might happen when infection is asymptomatic, or how easily virus can be transmitted by sexual contact. The number of reports of sexual transmission has been increasing, suggesting this may not be a rare occurrence. Case detection is difficult and the true risk may be higher than recognized at present. It is reasonable to consider delaying trying to become pregnant for approximately six months if the male partner has travelled in an area currently considered to have suitable conditions for sustained and high levels of ZIKV transmission. Male partners should be tested for ZIKV infection if they have symptoms compatible with ZIKV disease. Negative serology in a male at two weeks or later post-onset of symptoms, or at least three weeks post-travel, makes infection with ZIKV unlikely. Infectious disease physicians, with asymptomatic male patients who are part of a couple trying to become pregnant, may want to consider consulting the local provincial lab to discuss serologic testing in scenarios where there is a medical need to pursue conception in advance of the recommended six-month deferral period. At this time, the sensitivity and negative predictive value of the test in this particular population is not sufficiently well-defined to be of practical use in clinical decision-making. When properly used, condoms should minimize the risk of sexual transmission. Such protection is especially important during pregnancy. Until more is known, and based on our experience with other viral infections where shedding in semen may be prolonged, it is reasonable to practice abstinence or use condoms for the duration of a pregnancy.
_
Will all pregnant women be tested for Zika virus?
Healthcare professionals have been advised to ask all pregnant women about their recent travel history. Only pregnant women with a history of travel to an area with active Zika virus transmission and who present with current symptoms consistent with Zika infection that began during or within 2 weeks of travel should be tested. The testing should include investigation for other travel associated infections (such as malaria, dengue fever and chikungunya) as well as Zika.
_
What happens if Zika virus is detected?
A pregnant woman who tests positive for Zika virus infection will be referred to a Fetal Medicine service for further assessment – this may include regular (4-weekly) fetal ultrasound scans to monitor fetal growth and consideration of fetal MRI.
What happens if a woman with travel history to a Zika infected area has a negative test or doesn’t report any symptoms?
A baseline fetal ultrasound scan will be offered to all pregnant women who have returned from areas with active Zika virus transmission, even if they have not reported any symptoms. Ultrasounds may be repeated regularly (4-weekly) – if the scan detects any abnormality, the woman may be referred to a Fetal Medicine service for further investigations.
_
How should pregnant women with male partners with Zika virus infection or at risk for Zika virus infection be counselled?
Given the potential risks of maternal Zika virus infection, pregnant women whose male partners have or are at risk for Zika virus infection should be counselled to use condoms every time they have vaginal, anal, or oral (mouth-to-penis) sex or abstain from sex for the duration of pregnancy.
__
If you are not pregnant, but you live in an area with Zika, here’s what you can do:
1. Women who want to get pregnant should talk with their healthcare provider about their goals for having children. They should also talk with their healthcare provider about the potential risk of Zika virus during pregnancy as well as their male partner’s potential exposures to Zika virus.
2. Women who do not want to get pregnant should talk with their healthcare provider about ways to prevent unintended pregnancy, including how to use birth control the right way every time. Women should consider safety, effectiveness, availability, and acceptability when choosing a birth control method.
3. Take steps to prevent mosquito bites
4. Take care of yourself if you get infected.
5. Take steps to prevent getting Zika through sex. Use condoms the right way, every time, for vaginal, anal, or oral (mouth-to-penis) sex. Not having sex is the best way to be sure that you do not get sexually transmitted Zika.
_
Planning pregnancy:
Couples should delay pregnancy plans in event of Zika Symptoms according to CDC:
Couples should postpone conception of a child if either partner has symptoms of Zika virus infection. Women who are ill with Zika should wait at least eight weeks after developing initial symptoms to attempt to conceive, and men should wait at least six months. Men and women who may have been exposed to Zika but don’t have symptoms of the disease should wait at least eight weeks after exposure to try to conceive.
_
Why 8 weeks for women and six months for men?
The virus is thought to leave a woman’s body after 10 days to two weeks, but the 8 weeks add “buffer time” to be safe. Men have to wait much longer because research has shown that the virus can live in semen for months and infect women through sexual transmission during that time, so the six-month recommendation leaves a buffer zone.
_
The part about waiting even if you don’t have symptom is debatable. At the moment, we cannot tell a woman that even if you have not have symptoms, don’t worry; since 80 percent of the time people with Zika virus don’t have symptoms. So men and women who may have been exposed to Zika but don’t have symptoms of the disease should wait at least eight weeks after exposure to try to conceive.
_
Due to the potentially serious implications of transmitting Zika to a pregnant woman, it is advisable that:
•All men who have travelled to a Zika-affected area and have a pregnant partner should abstain from sexual activity (oral, vaginal, and anal) or use condoms for the duration of the pregnancy, whether they have symptoms or not.
•All men who have travelled to a Zika-affected area and have a partner who is at risk of becoming pregnant should abstain from sexual activity (oral, vaginal, and anal) or use condoms, whether they have symptoms or not, for at least six months after leaving a Zika-affected area.
_
If I live in an area where the virus is circulating, should I delay becoming pregnant?
That may be wise, some officials say. Health officials in five countries Brazil, Colombia, Ecuador, El Salvador and Haiti have suggested that women delay pregnancy temporarily. Obstetricians in some countries are privately giving patients the same advice, saying the risk of fetal damage during an epidemic’s peak is too great. Once infected residents have recovered and have become immune, these officials argue, the epidemic will fade and women can safely become pregnant again. Also, many companies are working on Zika vaccines, and delaying pregnancy will buy time for them to arrive. The Centers for Disease Control and Prevention recently recommended that women who have had symptoms of the virus or tested positive for it should wait at least eight weeks after their symptoms first appeared before trying to get pregnant. Officials recommended that men who had symptoms should wait six months before having unprotected sex. The virus has been known to live longer in semen.
__
Zika virus and reproductive justice:
Following the outbreak, several governments in the region called on women to delay getting pregnant for periods ranging from six to eight months, all the way to two years. However, this directive ignores the reality of sexual and reproductive health services in the affected countries where contraceptives are often too expensive to access thus limiting their use, and the laws severely restrict abortion access. Due to these restrictive conditions many women in the region are unable to access reproductive health services and therefore determine their reproductive futures. The most marginalised communities of women – communities that include young women, impoverished women, indigenous women, racialised women, disabled women and rural women – have even more restricted access to reproductive health services and thus less autonomy over their reproductive choices. Consequently, women’s rights and feminist organisations have immediately decried this directive and demanded that governments ensure universal access to comprehensive sexual and reproductive services. Government recommendations that women delay pregnancy have also proven to be controversial. Human and reproductive rights groups have deemed the recommendations irresponsible and difficult to follow, since women alone are tasked with avoiding pregnancy despite having little control to do so. Evidence suggests that over half of the pregnancies in Latin America and the Caribbean are unplanned. Access to contraceptives is limited in the predominantly Roman-Catholic region, and widespread sexual violence results in many women getting pregnant against their will. Anti-abortion laws in much of the region leave women with no recourse once they become pregnant. On 18 February 2016, after a trip to Latin America, Pope Francis stated that “avoiding pregnancy is not an absolute evil” in cases such as the Zika virus outbreak. His comments sparked speculation that the use of contraception may be morally permissible in the fight against the Zika virus.
_
In response to Zika, Brazil moves to restrict abortion even more:
Apart from three countries where abortion is widely available (French Guiana, Guyana, and Uruguay) and three countries where abortion is allowed in cases of fetal malformation (Colombia, Mexico, and Panama), most of the region only permits abortion in the cases of rape, incest, or danger to the mother’s health. In El Salvador, abortion is illegal under all circumstances. On 5 February 2016, the UN High Commissioner for Human Rights urged Latin American governments to consider repealing their policies regarding contraception and abortion, emphasizing that “upholding human rights is essential to an effective public health response.” Despite urgent demands from global leaders to loosen abortion penalties in Latin American in light of the Zika virus — a mosquito-borne disease that can cause serious birth defects if pregnant women are infected — Brazil lawmakers are moving in the opposite direction. Instead, conservatives in Brazil are working to increase penalties for women who’ve had an abortion. The deeply-Catholic government is drafting legislation that would sentence women to nearly five years in jail if they abort a fetus with microcephaly — the brain disorder that is directly linked to Zika. In Brazil, where doctors have seen 4,000 cases of babies born with microcephaly in the past four months, abortion is already illegal — aside from rare cases of rape, anencephaly (a more extreme version of microcephaly, where a baby often dies in infancy), or when the mother’s life is in danger. The current state of the law, given the fact that microcephaly is not said to be life-threatening to the mother or the foetus, means that it is unlikely that the common law exception would avail any women who is contemplating an abortion because she contracted the Zika virus. Currently, if a woman is found to have had an abortion, she is sentenced to no more than three years in prison. But under this new legislation, if a court found the case to be based on microcephaly, a woman can spend up to four-and-a-half years behind bars. And for doctors that administer the abortion? They could face a 15 year sentence. Reproductive health advocates fear further crackdown with harsh laws will only increase the number of women dying from illegal abortions and the number of impoverished families burdened with the cost of raising a child with severe brain damage. Pope Francis, on his return flight from Mexico to the Vatican said that abortion is “a crime” since it means “throwing one out to save another.” “That’s what the mafia does. It’s a crime, an absolute evil,” the Pope said Feb. 18. Several Latin American countries with laws restricting abortion rejected demand for abortion, including Brazil. Unwanted pregnancies and access to contraceptives, particularly in highly religious countries in Latin America, are an issue.
_
My view:
I disagree with the Pope equating abortion of a microcephaly fetus with murder and act of Mafia. Parents have a right to abort a fetus if the fetus is having serious birth defect. No sensible parents would ever take the risk of having a handicapped child although it is their duty to look after handicapped child once he/she is born. But before delivery, during pregnancy if parents know that fetus is having serious brain damage, the rationale to abort the fetus overweighs the argument that every life must be respected and protected. It cannot be overemphasized that even a single case of microcephaly could be catastrophic for the entire family. Legal abortion of microcephaly fetus is better than women dying from illegal abortions and number of impoverished families burdened with the cost of raising a child with severe brain damage.
_______
_______
Zika controversies:
_
Controversy-1
Harms of genetically modified mosquitoes:
Some efforts to contain the spread of Zika virus have been controversial. Oxitec, the company behind the “self-limiting” mosquitoes released in Brazil, has faced criticism from environmental groups, who fear that releasing a new mosquito strain into the wild will damage the ecosystem. In the short term, the concern is that a drop in the mosquito population could affect the populations of other species. Supporters claim that the environmental impact of the “self-limiting” mosquitoes will be minimal, since only one species of mosquito is being targeted and the genetically-modified mosquitoes are still safe for predators to eat. Oxitec Product Development Manager Derric Nimmo likened the process to “going in with a scalpel and taking away Aedes aegypti, leaving everything untouched.” Since Aedes aegypti is an imported invasive species in Brazil, some experts expect that its eradication will have little impact on the environment. However, other environmentalists emphasize that the long-term consequences of eliminating an entire species cannot be predicted.
_
Controversy-2
“It’s not the Zika Virus” – Some link Monsanto Pesticides to Birth Defects:
According to a report that was done by a number of Argentinian physicians belonging to an organization called “Physicians In The Crop Sprayed Towns,” the Zika virus might not be responsible for all of these microcephaly cases. It’s reported that for approximately two years, pyriproxyfen has been being added into the drinking water in the infected area of Brazil. The chemical is manufactured by Sumitomo Chemical, a Japanese subsidiary of Monsanto, and is used to eradicate mosquitoes, causing malformations amongst these insects. The PCST reports that malformations detected in thousands of children from pregnant women living in areas where the Brazilian state added pyriproxyfen to drinking water are not a coincidence, even though the Ministry of Health places a direct blame on the Zika virus for this damage. These physicians are emphasizing that this mosquito-killing chemical which has been added to the drinking water is an endocrine disruptor as well as a teratogen, which means it causes birth defects. The organization has also pointed out that the Zika virus has never been associated with birth defects, even in areas where up to 75 percent of the population has been infected.
_
Brazil, world health officials deny link between pesticide and microcephaly:
Brazil’s federal government and the World Health Organization say that there is no scientific evidence linking Pyriproxyfen to microcephaly and disagree with Rio Grande do Sul’s authorities about the need to impose a ban. The Brazilian Health Ministry says that “there is no epidemiological study showing the association between the use of Pyriproxyfen and microcephaly.” In a statement, the ministry also says that “unlike the relationship between the Zika virus and microcephaly, which has had its confirmation attested in tests that indicated the presence of the virus in samples of blood, tissue and amniotic fluid, the association between the use of Pyriproxyfen and microcephaly has no scientific basis. A team of WHO scientists recently reviewed data on the toxicology of pyriproxyfen, one of 12 larvicides that WHO recommend to reduce mosquito populations. It found no evidence that the larvicide affects the course of pregnancy or the development of a fetus. The US Environmental Protection Agency and EU investigators reached a similar conclusion when they carried out a separate review of the product. Larvicides are an important weapon in the public health practitioner’s arsenal. Especially in cities and towns with no piped water, people tend to store drinking water in outdoor containers. These sources of water, as well as standing water that may collect in garbage, flower pots and tyres, serve as ideal breeding grounds for mosquitoes. Larvicides such as pyriproxyfen are often used in containers where people store water to kill the mosquito in its larval stage. When people drink water from containers that have been treated with pyriproxyfen, they are exposed to the larvicide – but in tiny amounts that do not harm their health. Moreover, 90% – 95% of any larvicide ingested is excreted into the urine within 48 hours. This product has been used since the late-1990s without being linked to microcephaly. Pyriproxyfen has been used for decades, with no reports of increased birth defects, said Ernesto Marques, associate professor of infectious diseases and microbiology at the University of Pittsburgh, who is working in Recife, Brazil to study microcephaly. “It’s ridiculous,” said Marques, of the purported link between the chemical and microcephaly. “These guys come out of the blue, and people believe them, with no evidence at all. It really shows the lack of science education among the public.”
_
Controversy-3
No evidence that vaccines cause microcephaly in babies:
There is no evidence linking any vaccine to the increases in microcephaly cases that were observed first in French Polynesia during the 2013-2014 outbreak and more recently in northeastern Brazil. An extensive review of the literature published in 2014 found no evidence that any vaccine administered during pregnancy resulted in birth defects. The Global Advisory Committee on Vaccine Safety, which provides independent scientific advice to the World Health Organization (WHO) on vaccine safety issues, reached a similar conclusion in 2014.
_
Controversy-4
No evidence that the Zika outbreak and unusual increase in microcephaly cases in Brazil is linked to recent releases of genetically modified mosquitoes in Brazil:
There is no evidence that Zika virus disease or microcephaly in Brazil is caused by genetically modified mosquitoes. In genetically modified mosquitoes, the genes of male mosquitoes are modified. Because of the modification, when they mate with female mosquitoes, their larval offspring cannot survive. This practice is designed to control mosquito populations.
_
Controversy-5
No evidence that sterilized male mosquitoes contribute to the spread of Zika:
A technique being developed to stop Zika is the controlled mass release of male mosquitoes that have been sterilized by low doses of radiation. When a sterile male mates, the female’s eggs do not survive. When the sterile males outnumber the fertile males in a natural environment, the mosquito population dies out. The technique has been used in the past against insects and fruit flies, for example. There is no evidence that the technique has been associated with increases in microcephaly cases or other human anomalies or defects. However, the evidence for the public health value of this technique needs to be established. WHO encourages affected countries and their partners to scale up the use of current mosquito control interventions as the most immediate line of defence, and to judiciously test new control tools that could potentially be applied in the future.
_
Controversy-6
Bacteria used to control the male mosquito population are not spreading Zika further:
Bacteria such as Wolbachia bacteria are used to control mosquito populations; they do not infect humans or other mammals. Wolbachia bacteria are found in 60% of common insects, including butterflies and fruit flies. Mosquitoes carrying Wolbachia bacteria have been released in several places, including Australia, Brazil, Indonesia and Viet Nam, to help control dengue (which is transmitted by the same mosquito that transmits Zika). When females mate with males carrying the bacteria, the eggs do not hatch, thus supressing mosquito populations.
______
______
Zika and Brazil Olympics:
Rio de Janeiro is the host city for the 2016 Olympic Games from 5 to 21 August. The Brazilian authorities will be targeting the mosquitoes’ breeding grounds in the run-up to the Games. The International Olympic Committee says it is in “close contact” with the Rio organisers and that Olympic venues will be inspected daily in the lead-up to and during this summer’s Games. It will be to ensure puddles of stagnant water, where mosquitoes breed, are removed to minimise the risk of athletes and visitors coming into contact with the insects. There is also some hope there will be fewer mosquitoes in August as the month is both cooler and drier. Brazilian and Olympic officials have sought to dispel concerns about Zika in August by saying that the month – mid-winter in the southern hemisphere – is typically a time when there are fewer mosquitoes in Rio. But a review of municipal health data showed that other infections spread by the same mosquito, known as Aedes aegypti, can be as bad in August of some years as they are during what would normally be peak months for infections in others. So far this year, possibly because of warmer than usual weather, local infections of dengue, a virus related to Zika, are far worse than in 2015. City officials say doctors have reported more than 6,000 cases of Zika in Rio since January. Brazil’s national government says as many as 1.5 million people may have been infected across the country. Brazil has been fighting to stem the spread of the Zika virus which is known to cause severe birth defects, including microcephaly, a condition in which a baby’s head is significantly smaller than normal. The threat of Zika has emerged as a major concern in the build-up to this year’s Olympics along with construction delays and the political turmoil in Brazil. The political paralysis also appears to be harming Brazil’s ability to respond to the Zika outbreak and care for children born with the birth defect microcephaly linked to the virus. An estimated 500,000 people are expected to visit Brazil for the Olympic Games in Rio de Janeiro from Aug. 5 to Aug. 21. That potential influx has raised concerns about Zika’s potential spread around the world.
_
Mass gathering and infections:
Mass gatherings are always associated with risk, ranging from traumatic injuries to infections. But in many ways, the Zika virus is a unique foe when compared to the types of infections expected by medical officials in charge of the safety at such gatherings. If Brazil and the International Olympic Committee aren’t up to the challenge, as they think they are, their decision to proceed with the games could detrimentally impact much of the globe. A limited outbreak of 82 cases of measles occurred around the 2010 Winter Olympics in Vancouver, British Columbia. At least two visitors spread the virus at one or more Olympics venues, and at least one person infected in Vancouver then travelled along Highway 97, spreading the virus on that route into the interior of British Columbia. Norovirus — the all-too-frequent cause of vomiting and diarrheal illness commonly associated with cruise ships in recent years — likes big events, too. It spread to 65 people at the 2006 FIFA World Cup in Germany. Carefully preplanned disease surveillance systems put in place before that event helped officials detect the outbreak early and limit its spread. Sixteen years ago 90 people came down with meningitis in nine European countries and 14 people died. Epidemiologists tracked the outbreak to pilgrims returning from the Hajj. This annual trek of more than 2 million Muslims to Mecca has in recent years attracted concerns over its potential to distribute the MERS virus in a similar fashion. The 2012 London Olympics went off without a hitch, though, thanks to the most comprehensive surveillance and triage system for a large event the world has ever seen. The games have made a lasting impact on the public health infrastructure in the United Kingdom, which now benefits from real-time data about syndromes: the collections of symptoms patients complain about. Detecting patterns in early symptoms is critical to recognizing new emerging threats quickly, permitting public health authorities to devote appropriate resources to stop their spread.
_
Olympic Athletes concerned about Zika Virus:
With the outbreak of the Zika virus in Brazil, national Olympic committees are discussing whether to send their athletes to the Games. Brazil reported the first case of Zika virus in March 2015. The World Health Organization (WHO) said the virus could be linked to 4,000 suspected cases of microcephaly in Brazil. Microcephaly is when babies are born with small heads. Their brains do not develop properly. It is a form of brain damage. In February 2016 WHO declared the spread of Zika a global public health emergency. It said the virus could infect as many as 4 million people across the Americas. The news came as Brazil’s second-largest city, Rio de Janeiro, is getting ready to host the Olympic Games in August. Brazil President Dilma Rousseff said her “entire government is working on fighting this emergency.” And its sports minister said the country has no plans to cancel the Games. Alan Ashley is the sport performance director for the U.S. Olympic Committee (USOC). He said to the Reuters news agency that no athletes should go to Brazil “if they don’t feel comfortable going.” Solo is the goalie for the U.S. national team. She has won two Olympic gold medals and helped the USA team win the Women’s World Cup last summer. Solo said even though she has no plans to become pregnant, she might not go to Brazil unless conditions related to Zika change. “If I had to make the choice today, I wouldn’t go,” she told Sports Illustrated before the game. “I would never take the risk of having an unhealthy child.” Solo explained that women athletes have different considerations than men. “Competing in the Olympics should be a safe environment for every athlete, male and female alike. Female athletes should not be forced to make a decision that could sacrifice the health of a child.” And the USOC said it is taking the threat of Zika seriously. Team USA won the most medals at the 2012 Olympics in London, finishing ahead of China, 103-88. If some key athletes from the USA do not attend the Olympics, it could affect the standings. In Kenya, officials said it is too early to consider not going to the Games. Also, the Olympics will take place during the Southern Hemisphere’s winter, which should limit the mosquito population due to cooler weather.
______
What measures are Rio 2016 organizers taking to protect athletes and visitors at the Summer Games?
Organizers say they are already inspecting Olympic venue facilities and areas for standing water and fumigating in some cases, and will do those inspections on a daily basis during the Games. Scheduled for Aug. 5-21, the Games fall within a cooler, drier season when the prevalence of mosquitos is much lower. Certain precautions are already being made at the golf venue, which is west of Ipanema Beach. Given that there are water hazards on the course, authorities are working to make sure that there is movement in the water in those lakes and ponds. A form of reverse irrigation can be used to make sure the water is not sitting, doesn’t become stagnant. Many precautions will be up to individuals — wearing clothing that covers arms and legs, using insect repellent and sleeping with windows closed. The Brazilian government is conducting a national education and eradication campaign, mobilizing military and civilian personnel to go door-to-door with leaflets. Brazil is launching a herculean effort to fumigate Rio of mosquitoes. Olympics officials are not considering postponing or cancelling the Games.
_
C.D.C. urges Pregnant Women to avoid travel to Olympics over Zika Fears:
Health officials in the United States have advised pregnant women who are scheduled to attend the Olympic Games in Brazil to reconsider their plans because of the Zika virus epidemic. The agency also recommended, if you have a male partner who goes to the Olympics, either use condoms or abstain from sex for the duration of your pregnancy. The C.D.C. issued special precautions for pregnant women who do decide to go to Brazil, as well as for women trying to become pregnant and men with pregnant partners.
_
South Korea unveils long-sleeved training kit dyed with mosquito-repellent to guard against Zika virus at Rio Olympic Games:
South Korea’s Olympic committee have unveiled long-sleeved shirts and pants it says will help protect the country’s Olympic athletes from the mosquito-borne Zika virus at the Olympic Games in Rio. The attire will be dyed with mosquito-repellent chemicals and will be worn by athletes during ceremonies, training and at the athletes’ village, the Korean Olympic Committee said. The committee said it couldn’t make changes to the uniforms worn during competition because of strict rules and performance concerns, although athletes will be allowed to use spray during competition. South Korean competitors will wear the kit at ceremonies and in training. But IOC rules do not allow such modifications to in-competition clothing.
_______
_______
Future outlook of Zika:
The long-term outlook with regard to the current Zika virus outbreak in the Americas is uncertain. Herd immunity sufficient to slow further transmission will undoubtedly occur, although this will not obviate the need for immediate and long-term prevention and control strategies. Whether and where the virus becomes endemic and whether an enzootic transmission cycle will develop somewhere in the Americas are matters of conjecture, but they are of considerable importance for the long-term development and sustainability of countermeasures, such as a Zika virus vaccine. What is clear is the need to rapidly and systematically address identified research gaps. These include a complete understanding of the frequency and full spectrum of clinical outcomes resulting from fetal Zika virus infection and of the environmental factors that influence emergence, as well as the development of discriminating diagnostic tools for flaviviruses, animal models for fetal developmental effects due to viral infection, new vector control products and strategies, effective therapeutics, and vaccines to protect humans against the disease.
______
Our knowledge and ignorance about Zika:
_
What we know:
1. Pregnant women can be infected with Zika virus.
-The primary way that pregnant women get Zika virus is through the bite of an infected mosquito.
-Zika virus can be spread by a man to his sex partners.
2. A pregnant woman can pass Zika virus to her fetus.
-Zika virus can be passed from a pregnant woman to her fetus during pregnancy or at delivery.
_
What we do not know:
1. If a pregnant woman is exposed
-We don’t know how likely she is to get Zika.
2. If a pregnant woman is infected
-We don’t know how the virus will affect her or her pregnancy.
-We don’t know how likely it is that Zika will pass to her fetus.
-We don’t know if the fetus is infected, the fetus will develop birth defects.
-We don’t know when in pregnancy the infection might cause harm to the fetus.
-We don’t know whether her baby will have birth defects.
-We don’t know if sexual transmission of Zika virus poses a different risk of birth defects than mosquito-borne transmission.
We need expedited research to answer basic questions about Zika…..
_______
_______
Moral of the story:
1. From January 2007 to April 2016, Zika virus transmission is documented in a total of 64 countries and territories. As of early 2016, a widespread outbreak of Zika is on-going in the Americas and the Pacific. It is estimated that 1.5 million people have been infected by Zika in Brazil. WHO said that the virus could infect as many as 4 million people across the Americas. It is estimated that more than two billion people live in parts of the world where the Zika virus can spread.
_
2. As of April 2016, countries with autochthonous (mosquito-borne) circulation of Zika virus [Zika Affected Areas] include Aruba, Barbados, Bolivia, Bonaire, Brazil, Cape Verde, Colombia, Costa Rica, Cuba, Curaçao, Dominica, Dominican Republic, Ecuador, El Salvador, Fiji, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Kosrae (Federated States of Micronesia), Marshall Islands, Martinique, Mexico, New Caledonia, Nicaragua, Panama, Paraguay, Saint Martin, Sint Maarten, Saint Vincent and the Grenadines, Samoa, Suriname, Tonga, Trinidad and Tobago, and Venezuela. Anyone who lives in or travels to Zika affected areas and has not already been infected with Zika virus can get it from mosquito bites. Regions above 6500 feet (2000 meters) are excluded from travel precautions, since the mosquitoes that transmit Zika virus are rare in these locations and the risk for mosquito-borne transmission of Zika virus is minimal.
_
3. Zika (Zika virus disease) has spread eastward from Africa and Asia to Oceania (the Pacific) and then to the Americas, in the same way of Chikungunya and Dengue; all are transmitted by the same Aedes mosquitoes. The virus has spread eastward by travel of infected humans and not by travel of infected mosquitoes.
_
4. The Zika virus is a positive sense single-stranded RNA flavivirus related to Dengue, Yellow Fever, Japanese encephalitis and West Nile virus. Although Zika is a mild, self-limited illness, it is far more dangerous than its cousin Dengue because the virus appears to be neurotropic and its complications microcephaly in fetus and Guillain–Barré Syndrome in adults could be catastrophic for the family.
_
5. Mutation in Zika virus enabled itself to have exponential spread in humans and to cause birth defects and paralyzing disorders. The corollary is other viruses can also mutate and seemingly harmless strain of one virus can become dangerous to humans anytime anywhere.
_
6. There is strong conservation among all Zika virus strains overall, with less than 12% divergence at the nucleotide level. This is important for diagnostic assays, which rely on precise sequences and epitopes, as well as for the development of therapeutics and vaccines. The current similarity data suggest that any vaccine product developed against any strain of Zika virus should be protective against all strains.
_
7. Each person who caught Zika spread it to about 3 to 6 persons during the outbreak. Since Zika is transmitted predominantly through mosquito bites, greater the mosquito-human interaction, greater the spread. So Zika would spread much faster in developing nations than developed nations.
_
8. The incubation period of Zika is 3-12 days. Common symptoms for adults include fever, body aches, skin rash, and red eyes with illness lasting for 1-2 weeks. 80 % people infected with Zika have no symptoms. Once a person has been infected, he or she is likely to be protected from future infections although that immunity – like so many aspects of Zika – is not yet proven.
_
9. Zika virus is detectable in the blood of an infected person for a few days to a week, so period of infectivity by mosquito transmission is about one week. The only exception is Zika infected pregnant woman whose fetus has evidence of congenital infection, here viral RNA is detected in woman’s serum up to10 weeks after infection. Zika virus has been detected in semen 62 days after onset of febrile illness, when it was no longer detectable in blood. So period of infectivity by sexual transmission from man to woman is several months.
_
10. Diagnosis of Zika is by testing the blood, urine, or saliva for the presence of Zika virus RNA by RT-PCR when the person is sick i.e. first seven days after onset of symptoms. This test determines if Zika RNA is present in the specimen. Zika virus can be found at higher levels and for a longer period in the urine than in serum. Since the window of diagnosis by detecting virus RNA in body fluids is narrow, any sample collected outside this narrow window will test negative for virus RNA even if person is infected. Also 80 % infected people have no symptoms, so even this narrow window disappears in them. Also viremia is generally low level, which makes viral isolation from clinical samples difficult. In other words, there is a high probability of false negative test result. Also these tests can take days or weeks to yield results. Along with RT-PCR for Zika virus, one must always do RT-PCR for dengue virus and chikungunya virus as these viruses may be co-circulating with Zika virus in the affected population.
_
11. Zika is also diagnosed by detecting IgM antibodies to Zika virus by diagnostic ELISA in serum 4-7 days from symptom onset and it persists for 2-12 weeks. The presence of IgM antibody suggests that a fairly recent infection has resulted in the production of antibodies against Zika. But there are two pitfalls. The considerable cross-reactivity of flavivirus antibodies presents major challenges for the interpretation of serologic test results, and the antibody response to the previous infecting flavivirus will be more vigorous than the response to the current one. For example, if patient had dengue in past, it may give false positive Zika antibody test when there is no Zika. Also, if patient has indeed Zika currently, it may stimulate very high levels of dengue antibodies due to past dengue resulting in making current diagnosis dengue rather than Zika. In other words, there are false positive and false negative test results. One cannot distinguish what these patients have in areas where dengue and Zika viruses are co-circulating.
_
12. An estimated 80 percent of those infected with Zika virus show no symptoms, making it difficult for anybody including pregnant women to know whether they have been infected. Only laboratory tests can confirm infection although they too have limitations. Therefore ‘Zika exposure time’ is far more important than symptoms of Zika or laboratory confirmation of Zika. Zika exposure time means time duration of exposure to Zika virus during which there is a possibility of getting infected and transmitting virus. That is the total of duration of stay in Zika affected area plus incubation period plus period of infectivity. For mosquito transmission, it would be three weeks plus stay in Zika affected area. For sexual transmission, period of infectivity is several months as semen harbours live Zika virus for at least 2 months. Zika exposure time for sexual transmission is 6 months plus stay in Zika affected area for a man who travelled in Zika affected area, which extend far beyond Zika exposure time for mosquito transmission. In other words, for all practical purpose, Zika exposure time is 3 weeks plus stay in Zika affected area for woman and 6 months plus stay in Zika affected area for man who travelled in Zika affected area. If you are out of Zika exposure time, you will not transmit Zika to anybody including fetus although you might have been infected.
_
13. Microcephaly is a medical condition in which the circumference of the head is smaller than normal because the brain has not developed properly or has stopped growing. Microcephaly can be diagnosed in fetus, can present at birth or may develop in the first few years of life. Between 22 October 2015 and 2 April 2016 a total of 6906 cases of microcephaly and/or central nervous system (CNS) malformation were reported by Brazil. This contrasts with the period from 2001 to 2014, when an average of 163 microcephaly cases was recorded nationwide per year. Of the 6906 cases of microcephaly and/or CNS malformations reported in Brazil, investigations have been concluded for 2860 cases and 1046 were suggestive of congenital infection. Since infected mother most often doesn’t show any symptoms, the potential numbers for how many mothers are really infected could be much greater. Since congenital brain anomalies and malformations caused by maternal Zika are far more common than ‘textbook’ microcephaly, the potential numbers of brain damaged babies are much greater than medically recorded microcephaly.
_
14. The evidence in favour of a causal relationship between Zika virus infection in pregnant woman and congenital microcephaly & other serious brain anomalies in fetus is substantial and accumulating. Mice studies also indicate that the virus by itself can cause microcephaly in their fetus.
_
15. Stimulation of autophagy of neural stem cells of fetus brain by Zika virus has been postulated as playing a key role in the pathogenesis of Zika-associated primary microcephaly. Zika activates TLR3, a molecule human cells normally use to defend against invading viruses. In turn, hyper-activated TLR3 turns off genes that neural stem cells need to specialize into brain cells and turns on genes that trigger cell suicide. Adults have small populations of neural stem cells compared to fetus brain and adults have robust interferon system which prevents viral multiplication in brain while fetus has no interferon system. This explains why fetal brain is destroyed and adult brain is spared. I suspect that in adults with severe immunodeficiency and dysfunctional interferon system, Zika virus may affect even adult neural stem cells.
_
16. Pregnant woman can transmit Zika virus to fetus once she is infected at any time during pregnancy and at the time of delivery. If maternal infection occurs in first trimester, it is more likely to cause microcephaly; if infection occurs later in the pregnancy, it is less likely to cause microcephaly. Among infants with Zika microcephaly, ocular abnormalities are present in almost 30 percent of their eyes. When cases of microcephaly occur later in pregnancy, infants may be better off in the long run because their brains would have suffered lesser damage as Zika destroys only neural stem cells and in late pregnancy, many neural stem cells have already given rise to neurons.
_
17. There have been no recorded cases of Zika transmission to infants through breastfeeding though live Zika virus has been detected in breast milk. The breast milk of a woman who became symptomatic with Zika virus infection on the day of delivery contained infective Zika viral particles in high titer. Breastfeeding is still recommended by the WHO, even by women who have had Zika fever. I disagree. The newborn has weak immune system incapable of eliminating Zika virus and brain of newborn may have some neural stem cells albeit much lesser than fetus, and destruction of these neural stem cell may impair brain performance in long run without any microcephaly whatsoever.
_
18. Dengue antibodies are not protective against Zika but may worsen Zika. Zika in a pregnant woman who had past dengue infection is more likely to cause microcephaly as binding of Zika virus by dengue antibodies may enhance initial virus replication or placental cell infection, or transplacental viral transfer. I wonder whether the phenomenon might work in reverse — whether people who have contracted Zika and then go on to be infected with dengue might be at higher risk of having severe dengue infections and plasma leak.
_
19. Not every baby whose mother has Zika is born with microcephaly. Researchers are working to find out how often Zika causes microcephaly when a baby is exposed in the womb. The historical prevalence of microcephaly is 2 to 12 cases per 10,000 live births. Current studies suggest that somewhere between 1 to 29% of babies born to infected mothers get microcephaly. That means out of 10,000 live births of Zika infected mothers, 100 to 2900 babies would have microcephaly. The gap between 1 to 29 % is too wide. We need more studies.
_
20. Amniocentesis (RT-PCR of Zika virus RNA in amniotic fluid) and fetal MRI of brain between 16 to 20 week can diagnose fetal Zika infection and microcephaly at the earliest. It must be emphasized that severe brain anomalies can be diagnosed by fetal MRI despite apparently normal head circumference (no microcephaly). Fetal Ultrasound at that time may be normal.
_
21. Congenital Zika syndrome is congenital Zika virus infection having a typical pattern including severe microcephaly, intracranial calcifications, and other brain anomalies, sometimes accompanied by eye findings, redundant scalp skin, arthrogryposis (congenital joint contractures in two or more areas of the body) and clubfoot.
_
22. No traveller should donate blood or semen during their Zika exposure time. Anyone who has returned from Zika affected area is ineligible to give blood donation for three weeks and men that have returned from Zika-affected areas should postpone semen donations for six months after their return.
_
23. For men and women who are permanently living in Zika affected areas, Zika exposure is constant, so their Zika exposure time is indefinite, therefore they are advised to defer pregnancy till herd immunity develops or vaccine is developed or epidemic itself fades away. They can donate blood only after testing for Zika virus in blood.
_
24. Couples with woman pregnant/non-pregnant in their respective Zika exposure times should use condoms whenever they engage in vaginal, anal or oral sex, or abstain from sex.
_
25. Both men and women should try to conceive only after they are out of their ‘Zika exposure time’. Any woman who is pregnant during her Zika exposure time or during her partner’s Zika exposure time should undergo laboratory testing for Zika virus infection and fetal MRI/ultrasonography to evaluate for presence of fetal microcephaly or intracranial calcifications. If a woman is pregnant during her Zika exposure time or during her male partner’s Zika exposure time, she may choose abortion rather than waiting for 20 weeks to know whether fetus has microcephaly. And in absence of fetal MRI, diagnosis of microcephaly by ultrasound alone could be quite late in pregnancy. Late termination of pregnancy entails legal restriction, moral dilemma and medical complications.
_
26. Pregnant women living outside Zika affected areas are advised to refrain from travelling to Zika affected area and refrain from unprotected sex with man in his Zika exposure time to make her Zika exposure time zero.
_
27. There is no evidence that pregnant women are more vulnerable to acquiring Zika virus infection or that this infection causes a more serious illness in pregnant women although Zika in some pregnant women cause microcephaly in fetus. There’s no evidence that the Zika virus will cause birth defects in future pregnancy among pregnant and non-pregnant women with current or previous infection because virus is cleared from pregnant/non-pregnant woman’s blood and there is no persistence of virus in her body. Zika is very close to dengue virus and there is probably a very, very low chance of latency or a propensity for recurrence. Although Zika infected pregnant woman may have virus in placenta, amniotic fluid and fetus brain, all these are expelled during miscarriage or delivery. Also infected pregnant or non-pregnant woman are immune to future Zika infections.
_
28. Twenty-four out of 100,000 people infected with Zika virus developed Guillain–Barré Syndrome (GBS) in French Polynesia during the Zika outbreak compared with a global incidence of 1–4 per 100,000 people per year. In the context of Zika virus circulation, 13 countries and territories worldwide have reported an increased incidence of Guillain-Barré syndrome (GBS) and/or laboratory confirmation of a Zika virus infection among GBS cases. Researchers have found that Zika infection is “even worse” in glial cells, which support and insulate neurons and are present throughout life, not just in fetal development. This explains Zika’s association with Guillain-Barré syndrome in adults.
_
29. The microcephaly and other birth defects could be the tip of the iceberg. The true burden of congenital disease with Zika virus is probably underestimated. Many cases have probably been missed because babies looked normal when they were born. But hidden birth defects are almost certain to turn up as the babies grow.
_
30. Since there is no specific treatment or vaccine and dreaded Zika complications like microcephaly and GBS are not uncommon, the response to the current Zika outbreak ought to be comprehensive public health response to prevent Zika. That includes vector control, personal protection measures against mosquitoes, prevention of sexual transmission, travel restriction, deferring pregnancy and development of Zika vaccine.
_
31. World had been in a similar situation in the early 1960s, when rubella was causing about 20,000 birth defects a year, after pregnant mothers became infected with the relatively mild German measles infection. As soon as rubella vaccine was developed and we started vaccinating everyone when they were children, the incidence of congenital rubella syndrome essentially disappeared. This is what we hope will happen with Zika once Zika vaccine is developed.
_
32. It is advisable to include RT-PCR of Zika RNA and IgM antibodies against Zika virus in TORCH screen of pregnant women. I would call it Z-TORCH.
_
33. I disagree with the Pope equating abortion of a microcephaly fetus with murder and act of Mafia. Parents have a right to abort a fetus if the fetus is having serious birth defect. No sensible parents would ever take the risk of having a handicapped child although it is their duty to look after handicapped child once he/she is born. But before delivery, during pregnancy if parents know that fetus is having serious brain damage, the rationale to abort the fetus overweighs the argument that every life must be respected and protected. It cannot be overemphasized that even a single case of microcephaly could be catastrophic for the entire family. Legal abortion of microcephaly fetus is better than women dying from illegal abortions and number of impoverished families burdened with the cost of raising a child with severe brain damage. Zika is predominantly mosquito borne disease which usually affects people belonging to lower socio-economic strata rather than the rich and the elite. It is irrational for the Church to give sermon to poor people about not aborting microcephaly fetus as raising a child with severe brain damage would make poor people ever poorer. Latin American women are prisoners today of laws that are formulated and approved by Latin American men. Abortion restrictions disproportionately harm poor young women with limited medical and personal options.
_
34. There is a possibility that Brazil Olympics in the city of Rio de Janeiro from 5 to 21 August 2016 may spread Zika worldwide. This is because Brazil is facing political crisis which appears to be harming Brazil’s ability to respond to the Zika outbreak.
_______
Dr. Rajiv Desai.MD.
12 May, 2016
______
Postscript:
I am putting up a new concept of ‘Zika exposure time’. We have a real problem with the Zika virus and that is lack of knowledge because of little research. We cannot assume based upon yellow fever and dengue what’s going to happen with Zika. The fast production of knowledge during this epidemic is an opportunity to observe science in the making: from formulation of new hypotheses and production of new results that will provide confirmations and contradictions to the refinement of methods and the gradual building of consensus. I request Brazilian doctors to read my article on ‘Zika’ and send their comments or criticism at my email address drrajivhdesai@yahoo.com Their comments/criticisms will be published below this article unedited.
_______


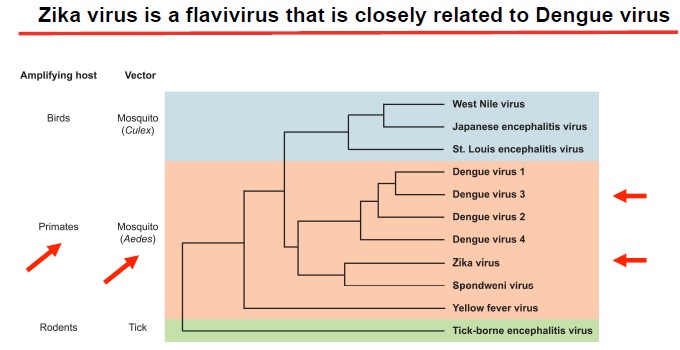
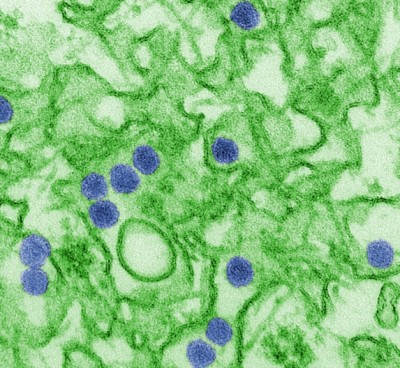
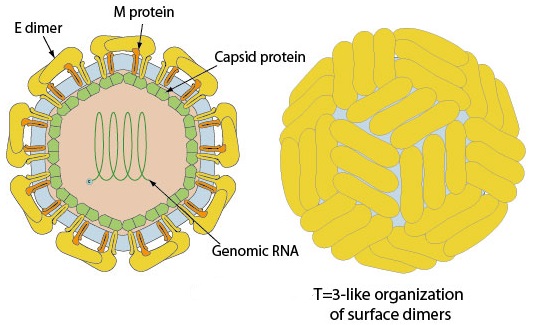
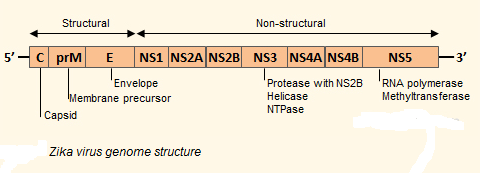
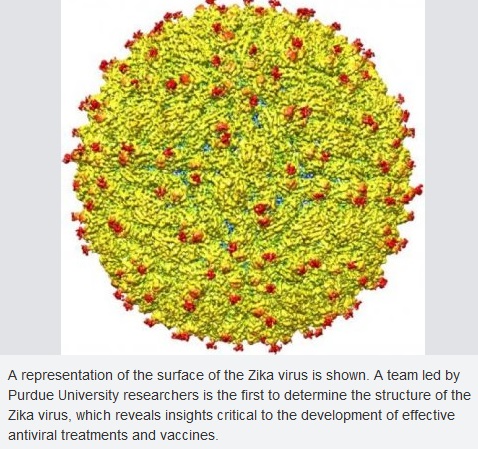
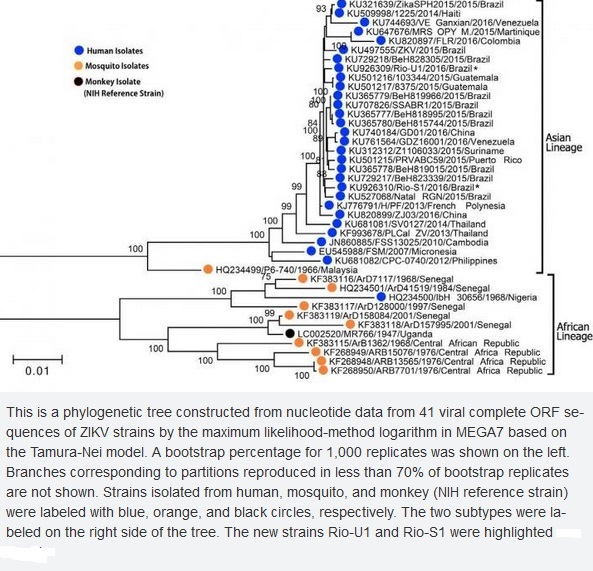
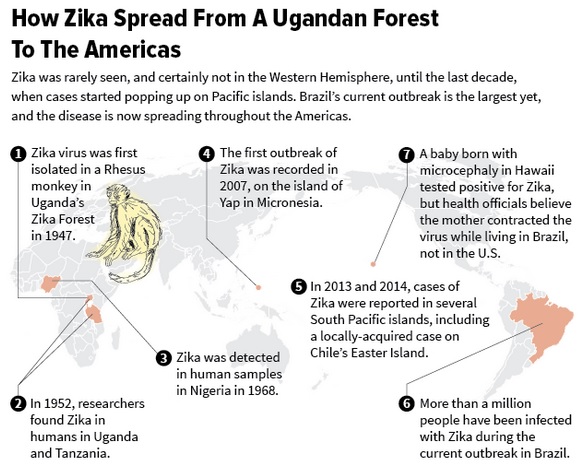
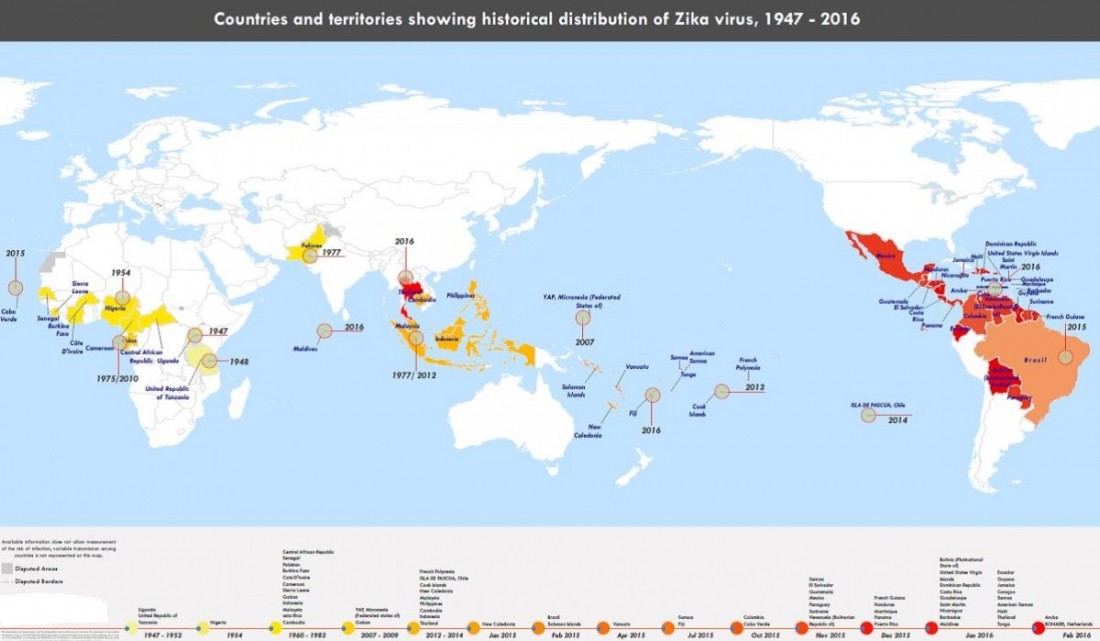
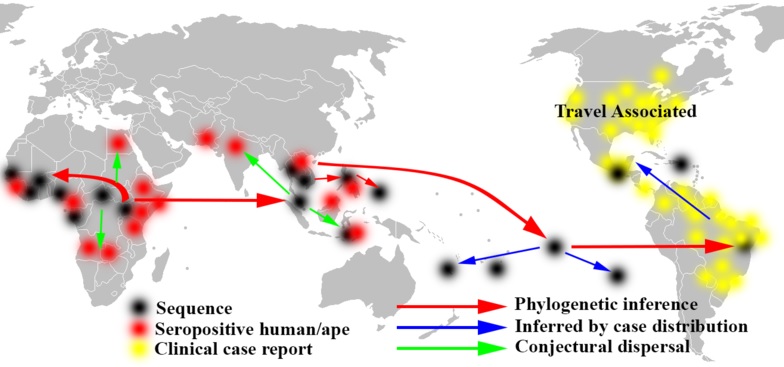
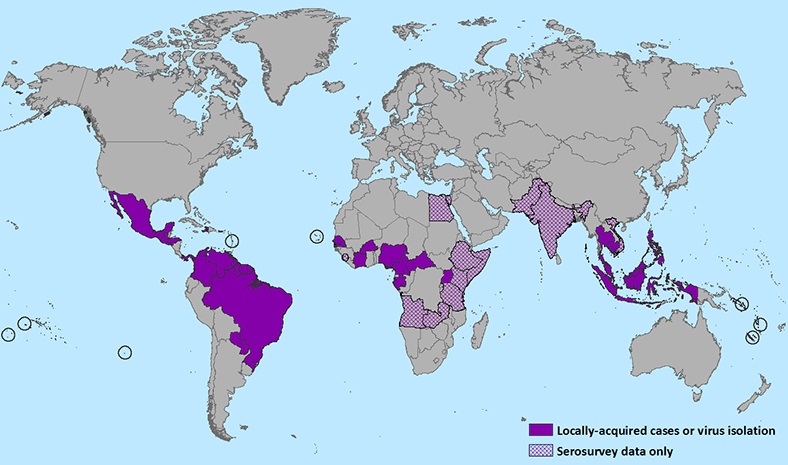
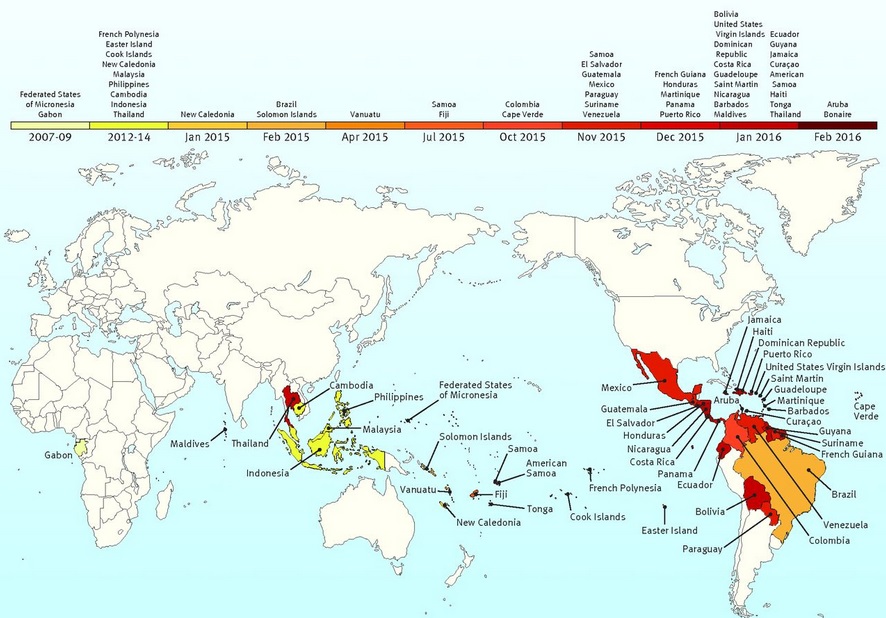
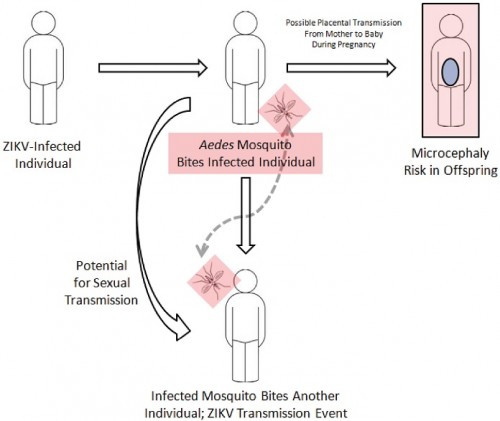

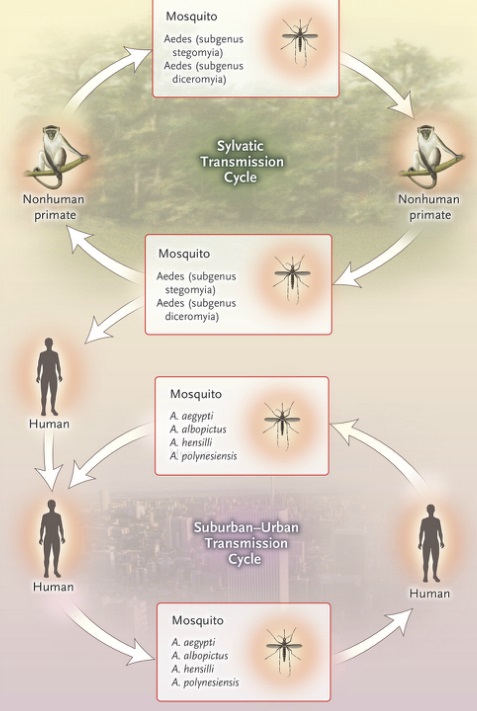
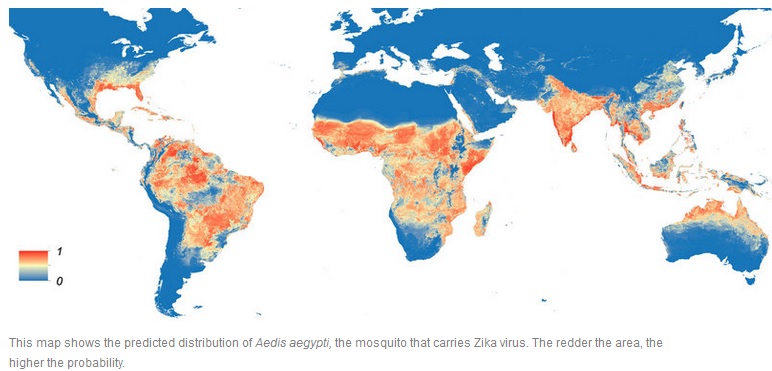
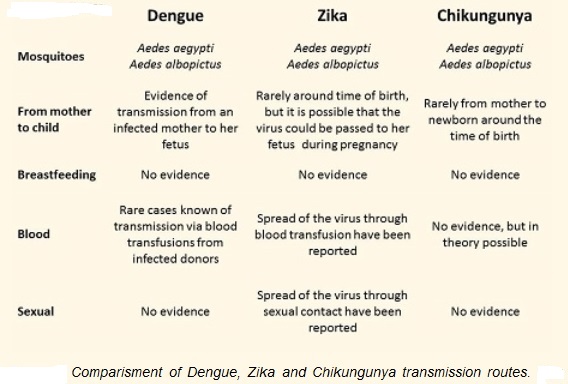
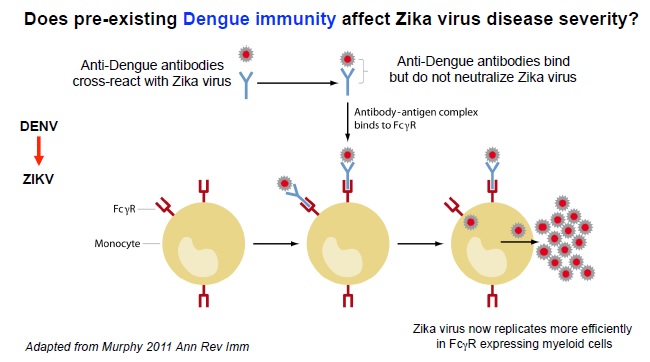
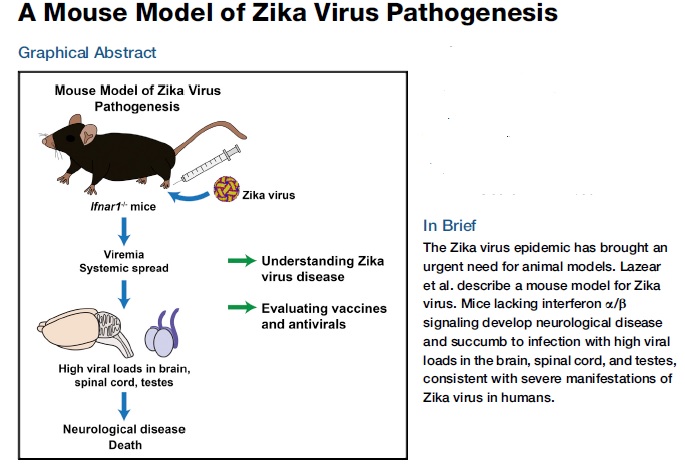
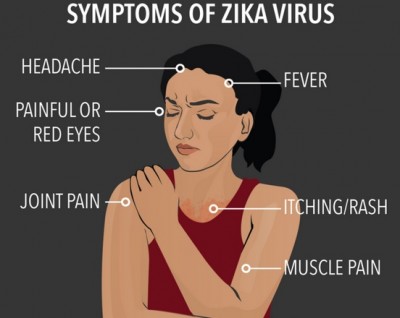
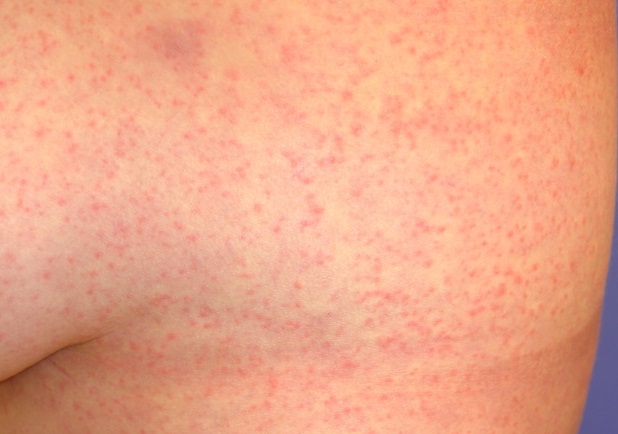
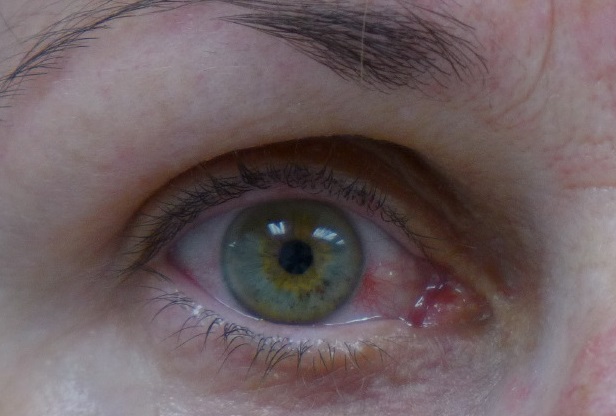
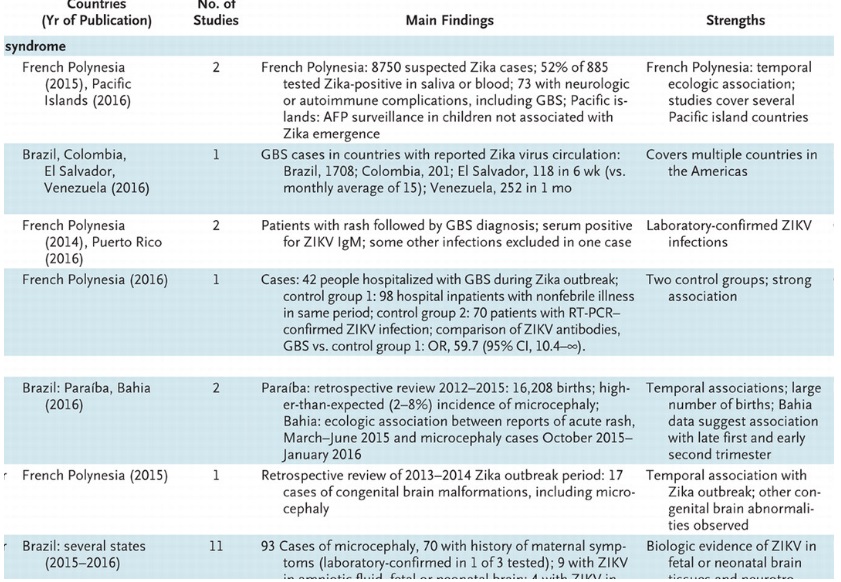
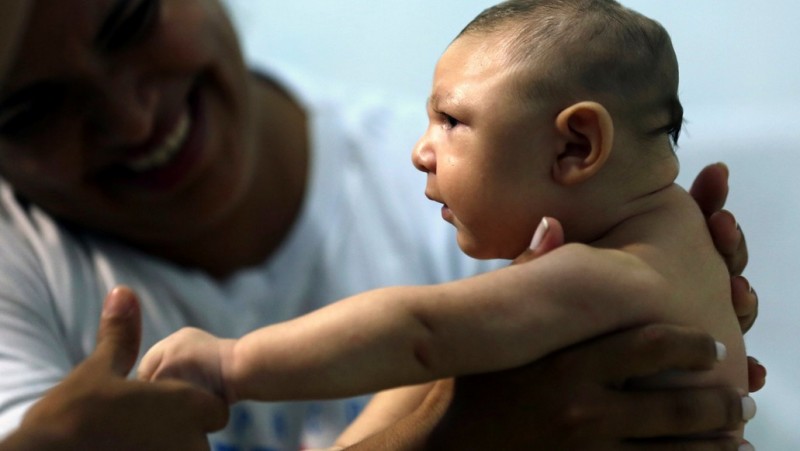
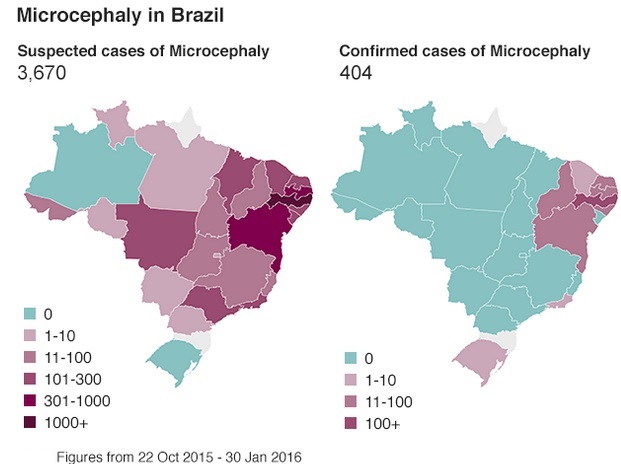
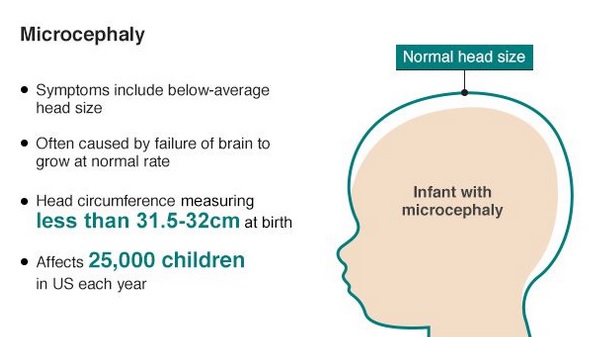
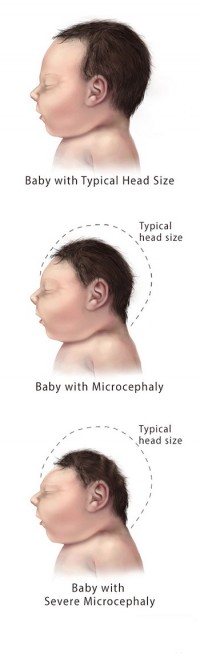
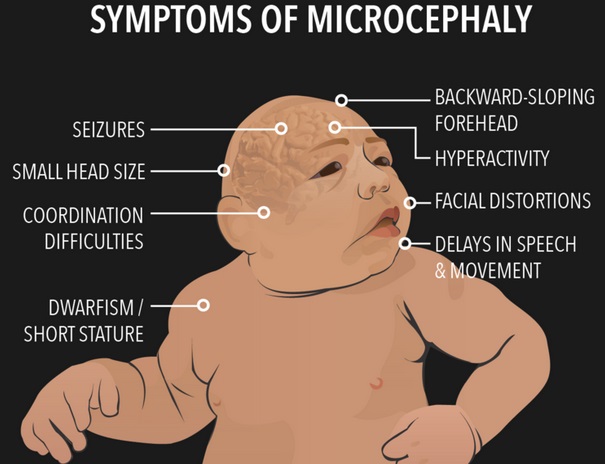
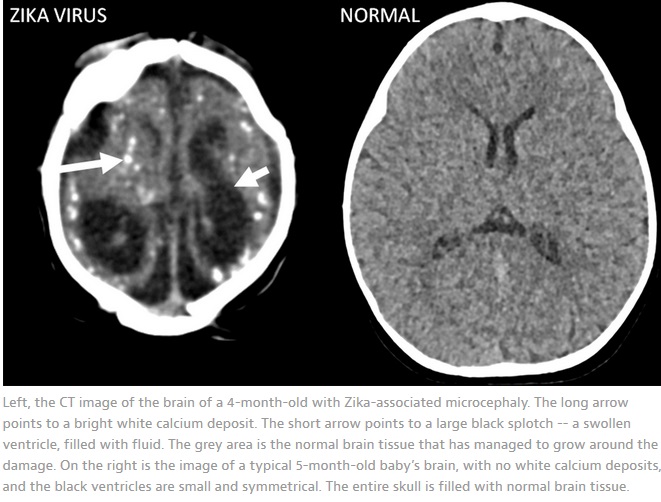
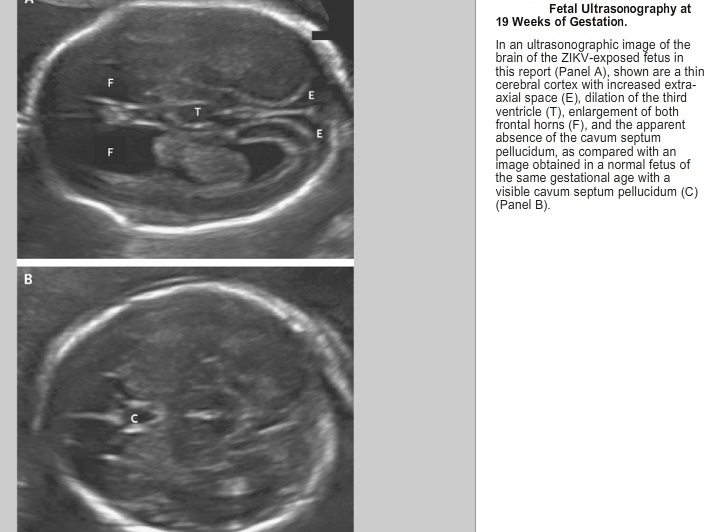
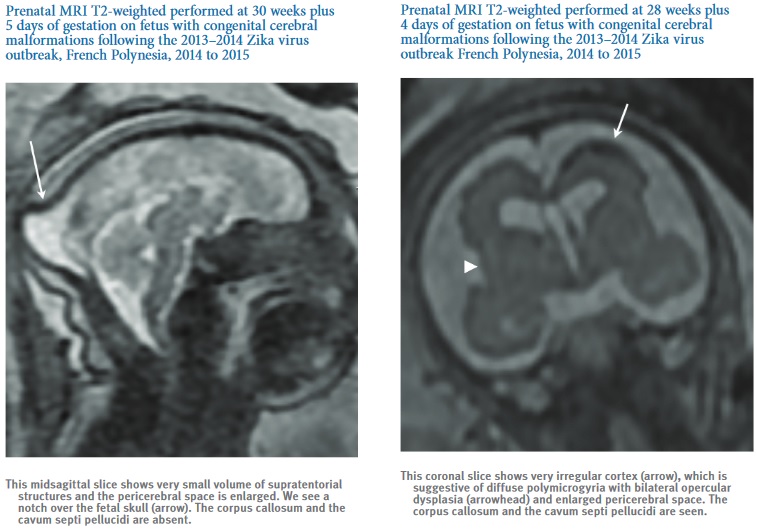
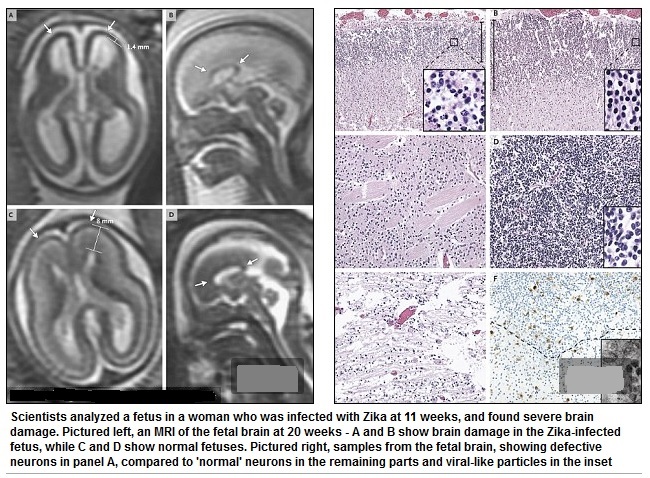
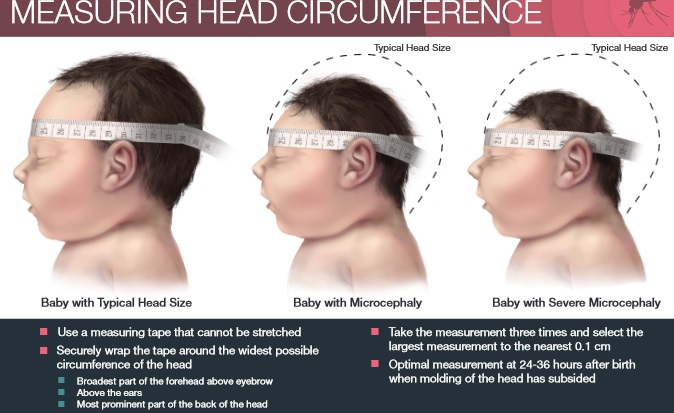
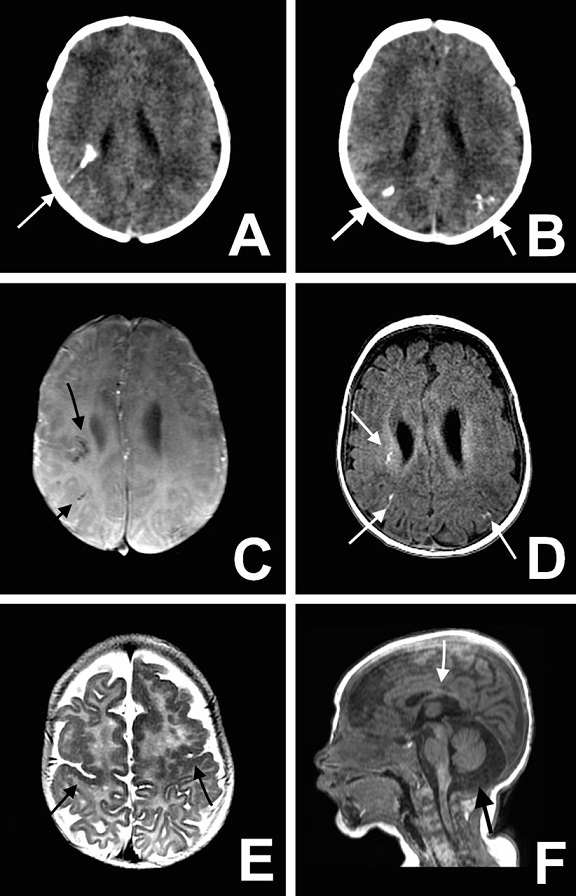
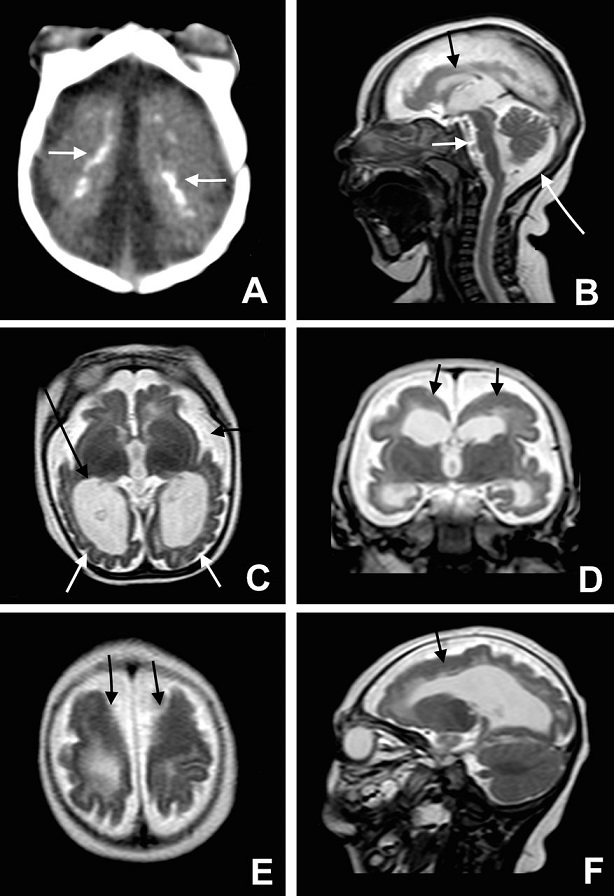
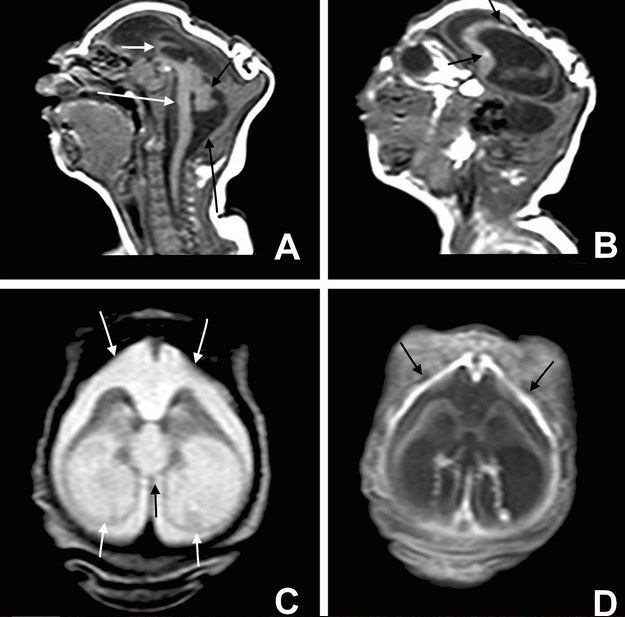
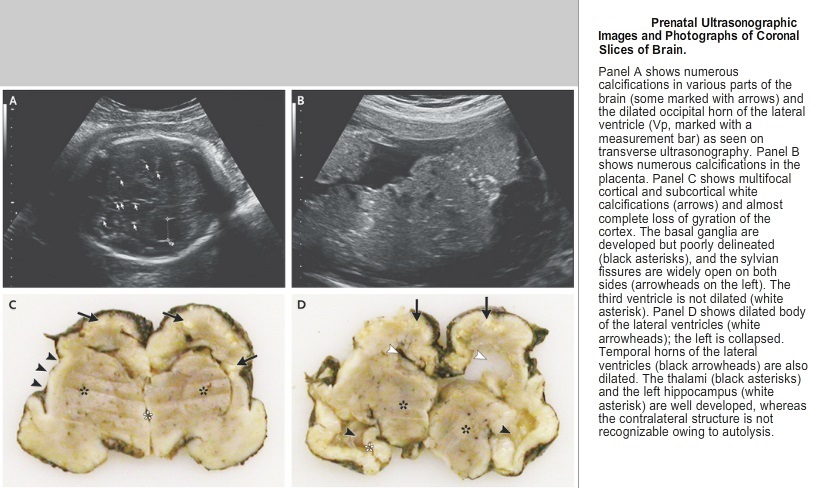
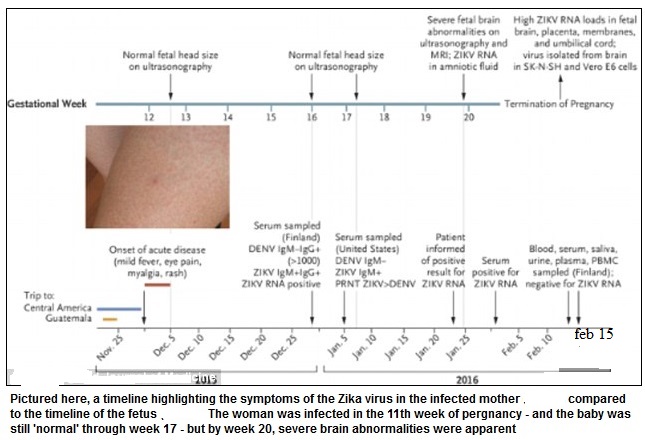

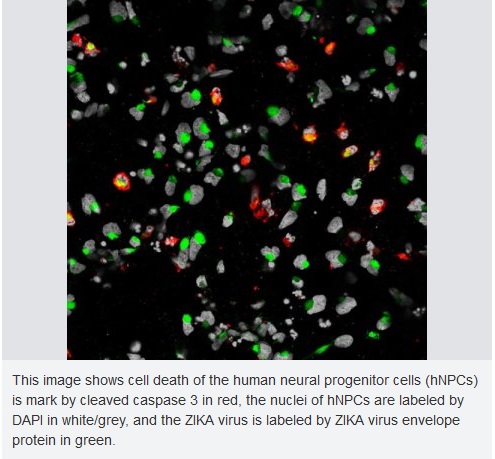
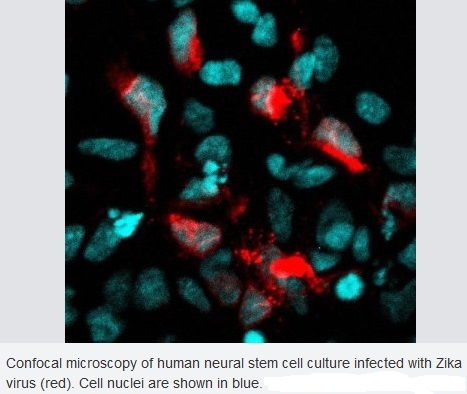
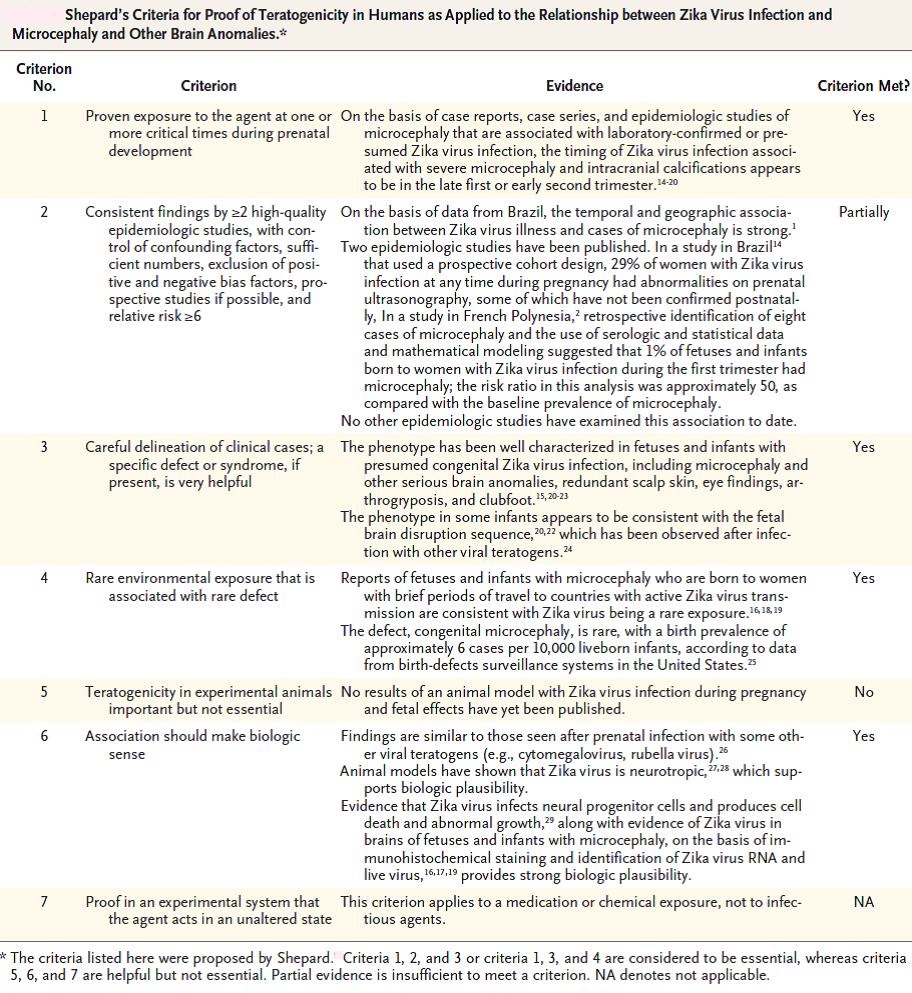


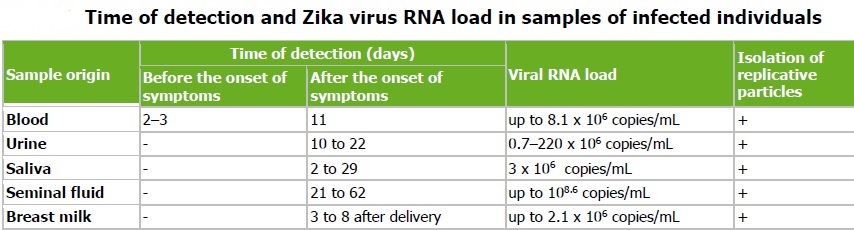
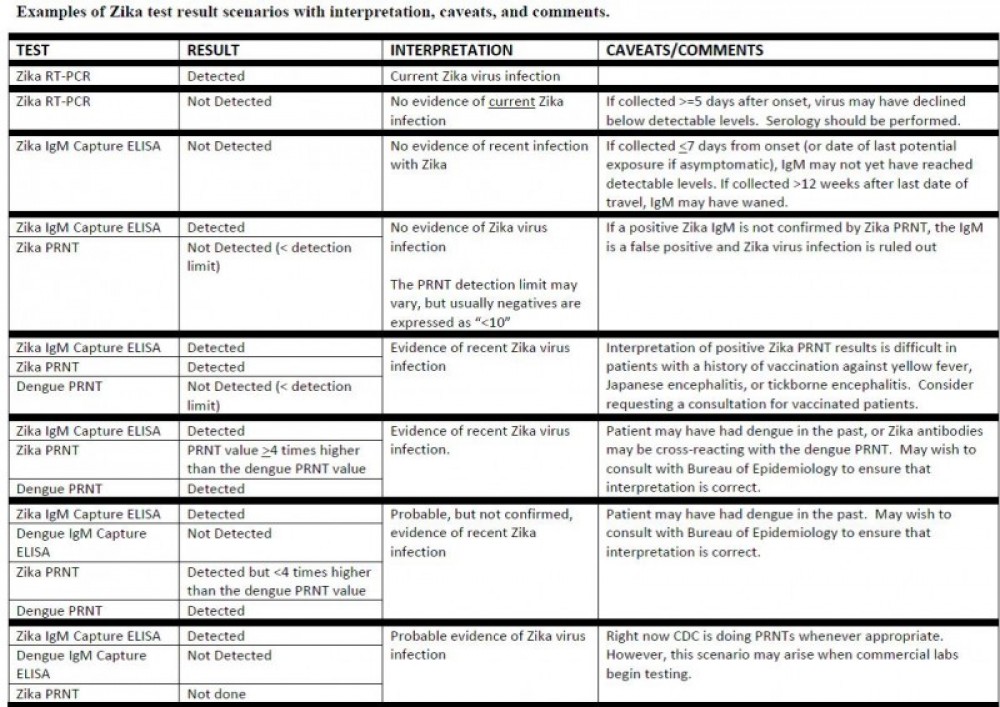
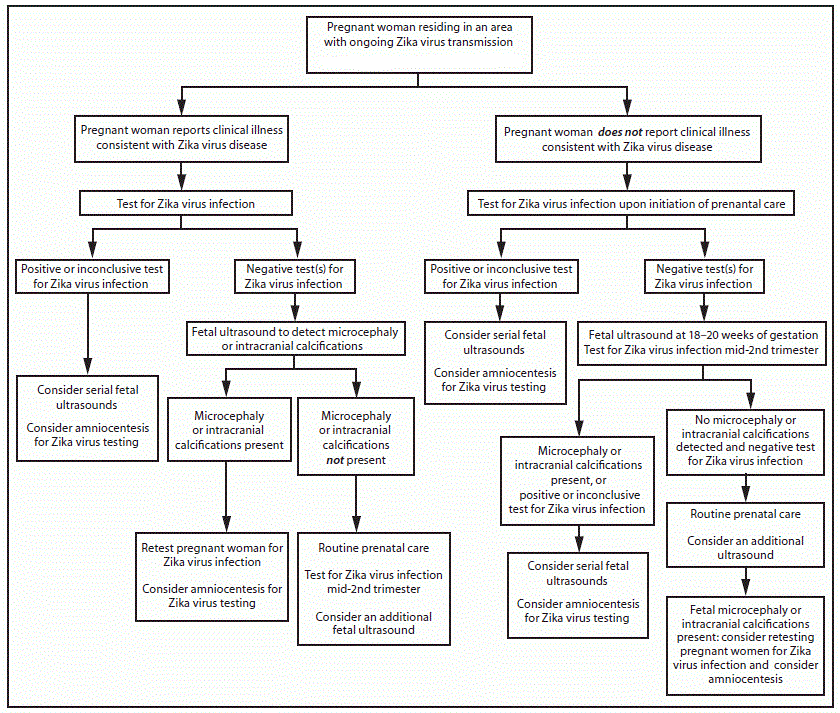



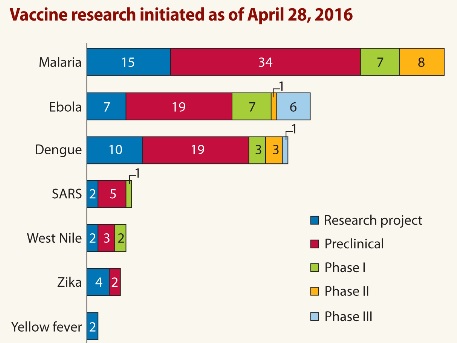

Very quickly this website will be famous among all blogging and site-building users, due to it’s good articles or reviews|
Aw, this was an incredibly good post. Taking the time and actual effort to make a great article… but what can I say… I procrastinate a whole lot and never seem to get anything done.
Excellent post! We are linking to this particularly great article on our site. Keep up the great writing.
Highly energetic post, I liked that bit. Will there be a part 2?|
This is one awesome blog post. Awesome.
Thanks for the blog post.Much thanks again. Great.
I cannot thank you enough for the article post.Really looking forward to read more. Keep writing.
I will make your life a lot simpler, you will not recognize how to thank me.
Awsome info and straight to the point. I am not sure if this is truly the best place to ask but do you guys have any thoughts on where to get some professional writers? Thanks 🙂
Nothing can be comparable to this!
A fascinating discussion is definitely worth comment. There’s no doubt that that you should write more on this topic, it may not be a taboo subject but usually folks don’t speak about these topics. To the next! All the best!
Very interesting info !Perfect just what I was searching for! „It’s not the having, its the getting.” by Elizabeth Taylor.
Obey orders if you break owners.
Thanks so much for the blog.Thanks Again. Really Great.
Can I simply just say what a relief to discover somebody who genuinely understands what they’re discussing over the internet. You actually know how to bring an issue to light and make it important. A lot more people have to check this out and understand this side of your story. I was surprised you’re not more popular because you most certainly have the gift.
An impressive share! I’ve just forwarded this onto a colleague who had been doing a little research on this. And he in fact bought me lunch simply because I discovered it for him… lol. So allow me to reword this…. Thank YOU for the meal!! But yeah, thanks for spending the time to discuss this matter here on your site.
A round of applause for your article post.Thanks Again. Awesome.
I cannot thank you enough for the blog article.Really thank you! Really Great.
Thank you for your blog.Much thanks again. Much obliged.
wow, awesome article.Thanks Again. Will read on…
Thanks for sharing, this is a fantastic blog post.Really thank you! Cool.
Love to see this every day !
You make more sense than most…
Hi to every one, because I am really keen of reading this weblog’s post to be updated on a regular basis. It includes fastidious data.|
Best view you can finde , in this side of world!
I do consider all the concepts you have presented on your post. They’re really convincing and will certainly work. Still, the posts are very quick for beginners. May just you please prolong them a little from subsequent time? Thanks for the post.|
very handful of websites that take place to be in depth beneath, from our point of view are undoubtedly nicely really worth checking out
Thanks a lot for the article.Really thank you! Awesome.
Wow, great post.Really looking forward to read more. Will read on…
Appreciate you sharing, great blog post.Really thank you! Awesome.
Very informative article post.Really looking forward to read more. Much obliged.
I cannot thank you enough for the blog article.Really looking forward to read more. Will read on…
Thanks a lot for the article. Much obliged.
Many thanks for the info we were trying to find this while we were checking the internet and even your site turned up– Many thanks,
“This is really fascinating, You’re an overly professional blogger.”
I loved your blog post.Really looking forward to read more. Awesome.
Thanks again for the article post.Thanks Again. Cool.
Exceⅼlent site you’ve got here.. It’s difficult to find high-quality writing like үours nowadays.
I truly ɑppreciate individuals like you! Take care!!
“If you are going for best contents like I do, just pay a visit this web site daily because it presents feature contents, thanks”
Thanks for the article post.Really looking forward to read more. Keep writing.
“Looking forward to reading more. Great blog article.Really looking forward to read more. Much obliged.”
“Great work! This is the kind of information that are supposed to be shared across the internet. Disgrace on Google for not positioning this publish higher! Come on over and talk over with my website . Thanks =)”
Thanks-a-mundo for the article post.Thanks Again. Much obliged.
Appreciate you sharing, great article post.Really thank you! Really Great.
Very informative blog article.Really thank you! Cool.
Very informative article post.Much thanks again. Want more.