Dr Rajiv Desai
An Educational Blog
VACCINE
_____
VACCINE:
The figure above shows a victim of smallpox.
______
Prologue:
“You let a doctor take a dainty, helpless baby, and put that stuff from a cow, which has been scratched and had dirt rubbed into her wound, into that child. Even, the Jennerians now admit that infant vaccination spreads disease among children. More mites die from vaccination than from the disease they are supposed to be inoculated against.” –George Bernard Shaw, 1929. The world has come a long way since George Bernard Shaw fulminated against vaccination in the 1920s. Small pox was declared eradicated from world in 1980 largely due to small pox vaccine. In 2008, Barack Obama called science on vaccines ‘inconclusive’. But in 2015, the same Barack Obama called science on vaccines “indisputable”. Vaccination was voted by readers of the British Medical Journal in 2007 as one of the four most important developments in medicine of the past 150 years, alongside sanitation, antibiotics and anaesthesia. Vaccination currently saves an estimated three million lives per year throughout the world and so topped the list in terms of lives saved, making it one of the most cost-effective health interventions available. Vaccines are widely recognized as one of the greatest public health successes of the last century, significantly reducing morbidity and mortality from a variety of bacteria and viruses. Diseases that were once the cause of many outbreaks, common causes of loss of health and life, are now rarely seen, because they have been prevented by vaccines. However, vaccines can in rare cases themselves cause illness. A rare potential for harm can loom large when people no longer experience or fear the targeted disease. In this regard, the public opinion of vaccines can be a victim of their success. The fact that vaccines are administered to healthy people to prevent diseases which have become rare, largely thanks to vaccination, contributes to concerns about vaccine safety. Because the devastating effects of the diseases are no longer so prominent, public attention is focused on side effects from vaccination. This influences how a person weighs up the risks and benefits of vaccination. Vaccine opponents have questioned the effectiveness, safety, and necessity of all recommended vaccines. Most of the arguments against vaccination appeal to parents’ understandable deep-seated concerns for the health of their children, particularly very young babies. These arguments have reduced vaccination rates in certain communities, resulting in outbreaks of preventable and fatal childhood illnesses. Is vaccine really safe? Is vaccine really effective? What would happen if I don’t vaccinate my child? I attempt to answer these questions by analysing both sides of vaccine story.
________
Note:
This article is about scientific rationale for vaccination amid anti-vaccine movement and not about individual vaccines and hence it is beyond the scope of this article to discuss in detail production, administration, efficacy and safety of individual vaccines. However, whenever necessary individual vaccines are discussed.
_______
Abbreviations and synonyms:
DT= diphtheria toxoid
GBS = Guillain-Barré syndrome
HPV = human papillomavirus
MMR = measles, mumps, and rubella
TD = tetanus and diphtheria toxoids = Td
TDaP = tetanus, diphtheria toxoids and acellular pertussis = DTaP
TDwP = tetanus, diphtheria toxoids and whole cell pertussis = DTwP
TT = tetanus toxoid
Hib = haemophilus influenzae type b
HepB = hepatitis B
IPV = inactivated polio vaccine
OPV = oral polio vaccine
AEFI = adverse event following immunization
MS = multiple sclerosis
PCV= Pneumococcal conjugated vaccine
PPV = Pneumococcal polysaccharide vaccine
WHO = World Health Organization
UNICEF = United Nations Children’s Fund
CDC = Centers for Disease Control and Prevention (U.S.)
GAVI = Global Alliance for Vaccines and Immunization
GIVS = Global Immunization Vision and Strategy
GVAP = Global Vaccination Action Plan
CD = cluster of differentiation
APC = antigen presenting cell
DC = dendritic cell
_______
Edward Jenner and history of vaccination:
As long ago as 429 BC, the Greek historian Thucydides observed that those who survived the smallpox plague in Athens did not become re-infected with the disease. The ancient Greeks knew that people who had recovered from the bubonic plague were resistant to getting it again. Based on this observation, the authorities in Athens used survivors from previous epidemics to nurse sufferers when the same diseases re-emerged. The Chinese were the first to discover and use a primitive form of vaccination, called variolation. It was carried out as early as the 10th century, and particularly between the 14th and 17th centuries. The aim was to prevent smallpox by exposing healthy people to tissue from the scabs caused by the disease. They did this by either putting it under the skin or, more often, inserting powdered scabs from smallpox pustules up the nose. These initial crude attempts at immunization led to further experimentation with immunization by Lady Mary Wortley Montagu in 1718 and Edward Jenner in 1798.
_
_
The word “vaccine” comes from the Latin word vaccinus, which means “pertaining to cows.” Vacca is Latin for cow. What do cows have to do with vaccines? The first vaccine was based on the relatively mild cowpox virus, which infected cows as well as people. This vaccine protected people against the related, but much more dangerous, smallpox virus. More than 200 years ago, Edward Jenner, a country physician practicing in England, noticed that milkmaids rarely suffered from smallpox. The milkmaids often did get cowpox, a related but far less serious disease, and those who did never became ill with smallpox. In an experiment that laid the foundation for modern vaccines, Jenner took a few drops of fluid from a skin sore of a woman who had cowpox and injected the fluid into the arm of a healthy young boy who had never had cowpox or smallpox. Six weeks later, Jenner injected the boy with fluid from a smallpox sore, but the boy remained free of smallpox. Dr. Jenner had discovered one of the fundamental principles of immunization. He had used a relatively harmless foreign substance to evoke an immune response that protected someone from an infectious disease. His discovery would ease the suffering of people around the world and eventually lead to the elimination of smallpox, a disease that killed a million people, mostly children, each year in Europe. These early endeavors have led to the plethora of vaccines that are available today. Although these attempts were successful in providing immunity, the underlying processes required to produce this immunity were unknown. By the beginning of the 20th century, vaccines were in use for diseases that had nothing to do with cows—rabies, diphtheria, typhoid fever, and plague—but the name stuck.
_
Louis Pasteur further developed the technique during the 19th century, extending its use to killed agents protecting against anthrax and rabies. The method Pasteur used entailed treating the agents for those diseases so they lost the ability to infect, whereas inoculation was the hopeful selection of a less virulent form of the disease, and Jenner’s vaccination entailed the substitution of a different and less dangerous disease for the one protected against. Pasteur adopted the name vaccine as a generic term in honor of Jenner’s discovery. Louis Pasteur’s experiments spearheaded the development of live attenuated cholera vaccine and inactivated anthrax vaccine in humans (1897 and 1904, respectively). Plague vaccine was also invented in the late 19th Century. Between 1890 and 1950, bacterial vaccine development proliferated, including the Bacillis-Calmette-Guerin (BCG) vaccination, which is still in use today. In 1923, Alexander Glenny perfected a method to inactivate tetanus toxin with formaldehyde. The same method was used to develop a vaccine against diphtheria in 1926. Pertussis (1914), diphtheria (1926), and tetanus (1938) were combined in 1948 and given as the DTP vaccine. Viral tissue culture methods developed from 1950-1985, and led to the advent of the Salk (inactivated) polio vaccine and the Sabin (live attenuated oral) polio vaccine. Mass polio immunization has now eradicated the disease from many regions around the world. In 1963 the measles vaccine was developed, and by the late 1960s, vaccines were also available to protect against mumps (1967) and rubella (1969). These three vaccines were combined into the MMR vaccine in 1971. Maurice Hilleman was the most prolific vaccine inventor, and developed successful vaccines for measles, mumps, hepatitis A, hepatitis B, chickenpox, meningitis, pneumonia and Haemophilus influenzae. In modern times, the first vaccine-preventable disease targeted for eradication was smallpox. The World Health Organization (WHO) coordinated this global eradication effort. The last naturally occurring case of smallpox occurred in Somalia in 1977. The disease has since been eliminated from natural occurrences in the world, so the vaccine is no longer given. In 1988, the governing body of WHO targeted polio for eradication by 2000. Although the target was missed, eradication is very close. The next disease to be targeted for eradication would most likely be measles, which has declined since the introduction of measles vaccination in 1963. In 2000, the Global Alliance for Vaccines and Immunization (GAVI) was established to strengthen routine vaccinations and introduce new and under-used vaccines in countries with a per capita GDP of under US$1000. GAVI is now entering its second phase of funding, which extends through 2015. The past two decades have seen the application of molecular genetics and its increased insights into immunology, microbiology and genomics applied to vaccinology. Current successes include the development of recombinant hepatitis B vaccines, the less reactogenic acellular pertussis vaccine, and new techniques for seasonal influenza vaccine manufacture. Molecular genetics sets the scene for a bright future for vaccinology, including the development of new vaccine delivery systems (e.g. DNA vaccines, viral vectors, plant vaccines and topical formulations), new adjuvants, the development of more effective tuberculosis vaccines, and vaccines against cytomegalovirus (CMV), herpes simplex virus (HSV), respiratory syncytial virus (RSV), staphylococcal disease, streptococcal disease, pandemic influenza, shigella, HIV, malaria and schistosomiasis among others. Therapeutic vaccines may also soon be available for cancer, allergies, autoimmune diseases and addictions.
__
________
________
Vaccine definition:
Vaccine is an antigenic substance prepared from the causative agent of a disease or a synthetic substitute, used to provide immunity against one or several diseases. A vaccine is a biological preparation that provides active acquired immunity to a particular disease. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins or one of its surface proteins. The agent stimulates the body’s immune system to recognize the agent as a threat, destroy it, and keep a record of it, so that the immune system can more easily recognize and destroy any of these microorganisms that it later encounters. The administration of vaccines is called vaccination. The effectiveness of vaccination has been widely studied and verified; for example, polio vaccine, HPV vaccine, and the chicken pox vaccine. Vaccination is the most effective method of preventing infectious diseases; widespread immunity due to vaccination is largely responsible for the worldwide eradication of smallpox and the restriction of diseases such as polio, measles, and tetanus from much of the world. The World Health Organization (WHO) reports that licensed vaccines are currently available to prevent or contribute to the prevention and control of twenty-five infections. Vaccines can be prophylactic (example: to prevent or ameliorate the effects of a future infection by any natural or “wild” pathogen), or therapeutic (e.g., vaccines against cancer are also being investigated). Many believe vaccines are among the greatest achievements of modern medicine – in industrial nations, they have eliminated naturally occurring cases of smallpox, and nearly eliminated polio, while other diseases, such as typhus, rotavirus, hepatitis A and B and others are well controlled. Conventional vaccines, however, only cover a small number of diseases, and infections that lack effective vaccines kill millions of people every year, with AIDS, hepatitis C and malaria being particularly common.
_
List of Vaccine-Preventable Diseases (2009):
Vaccines are available for all of the following vaccine-preventable diseases (unless otherwise noted):
•Anthrax
•Cervical Cancer (Human Papillomavirus)
•Diphtheria
•Hepatitis A
•Hepatitis B
•Haemophilus influenzae type b (Hib)
•Human Papillomavirus (HPV)
•Influenza (Flu)
• Japanese encephalitis (JE)
• Lyme disease-
•Measles
•Meningococcal
•Monkeypox-There is no monkey pox vaccine. The smallpox vaccine is used for this disease.
•Mumps
•Pertussis
• Pneumococcal
•Polio
•Rabies
•Rotavirus
•Rubella
•Shingles (Herpes Zoster)
•Smallpox
•Tetanus
•Typhoid
•Tuberculosis (TB)
•Varicella (Chickenpox)
•Yellow Fever
_
_
Global Considerations:
Protecting health is a major priority of society, families, and individual parents. Over the past 100 years there has been a revolution in the ability to protect health in the developed world, where there are resources to enable this to happen. In 1900, among every 1,000 babies born in the United States, 100 would die before their first birthday, and five before 5 years of age. By 2007, fewer than seven were expected to die before their first birthday, and only 0.29 per 1,000 before 5 years of age. Diseases severe enough to kill children and adults can also leave survivors disabled in some way, and as mortality has fallen, so has the chance of severe disability from these diseases. Among the dangers for children and adults that have greatly diminished over the past century are infectious diseases. For bacterial diseases, antibiotics have been developed to treat infections before permanent harm can occur. For many viral and bacterial diseases, vaccines now exist. In the early 20th century, smallpox (which has 30 percent mortality and a very high rate of disfigurement and other less common sequelae including blindness and encephalopathy) and rabies (virtually 100 percent fatal) could be prevented with immunization. With the fast growing understanding of microbes and immunity from 1920 onward, the development of immunizations became a race to “conquer” infectious disease. Beginnings with the combination diphtheria, pertussis, and tetanus immunization during World War II and most recently with immunization to prevent cervical cancer (the human papillomavirus vaccine), immunizations have changed our expectations for child and adult health. Infections are less of a terror, and children are expected to survive to adulthood.
_
Immunization is a proven tool for controlling and even eradicating disease. An immunization campaign, carried out by the World Health Organization (WHO) from 1967 to 1977, eradicated smallpox. Eradication of poliomyelitis is within reach. Since Global Polio Eradication Initiative in 1988, infections have fallen by 99%, and some five million people have escaped paralysis. Although international agencies such as the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) and now Global Alliance for Vaccines and Immunization (GAVI) provide extensive support for immunization activities, the success of an immunization program in any country depends more upon local realities and national policies. Successful immunization strategy for the country goes beyond vaccine coverage in that self-reliance in vaccine production, creating epidemiological database for infectious diseases and developing surveillance system are also integral parts of the system. The WHO created the Expanded Program on Immunization (EPI) in 1974 as a means to continue the great success that had been achieved earlier with the eradication of smallpox. At that time less than 5 percent of the world’s children in the developing world were receiving immunizations. The six diseases chosen to be tackled under this new initiative were tuberculosis, diphtheria, tetanus, pertussis, polio, and measles. It was not until 1988 that the WHO recommended that yellow fever vaccine be added to the national immunization programs of those countries with endemic disease (WHO and UNICEF 1996). Later, in 1992, the World Health Assembly recommended hepatitis B vaccination for all infants. Most recently the WHO has recommended that the Haemophilus influenzae type B (Hib) conjugate vaccines be implemented into national immunization programs unless epidemiological evidence exists of low disease burden, lack of benefit, or overwhelming obstacles to implementation (WHO 2006).
_
The World Health Organization (WHO) estimate that vaccination averts 2 to 3 million deaths per year (in all age groups), and up to 1.5 million children die each year due to diseases which could have been prevented by vaccination. They estimate that 29% of deaths of children under five years old in 2013 were vaccine preventable. Global vaccination coverage—the proportion of the world’s children who receive recommended vaccines—has remained steady for the past few years. During 2013, about 84% (112 million) of infants worldwide received 3 doses of diphtheria-tetanus-pertussis (DTP) vaccine, protecting them against infectious diseases that can cause serious illness and disability or be fatal. By 2013, 129 countries had reached at least 90% coverage of DTP vaccine. In 2013, an estimated 21.8 million infants worldwide were not reached with routine immunization services, of whom nearly half live in 3 countries: India, Nigeria and Pakistan. Priority needs to be given to strengthening routine vaccination globally, especially in the countries that are home to the highest number of unvaccinated children. Particular efforts are needed to reach the underserved, especially those in remote areas, in deprived urban settings, in fragile states and strife-torn regions. The American Red Cross, the World Health Organization (WHO), the United Nations Foundation, the United Nations Children’s Fund (UNICEF), and the Centers for Disease Control and Prevention (CDC) are partners in the Measles Initiative, which targeted reduction of worldwide measles deaths by 90% from 2000 to 2010. During 2000–2008, global measles mortality rates declined by 78%—i.e., from an estimated 733,000 deaths in 2000 to 164,000 deaths in 2008. Rotary International, UNICEF, the CDC, and the WHO are leading partners in the global eradication of polio, an endeavor that reduced the annual number of paralytic polio cases from 350,000 in 1988 to <2000 in 2009. The GAVI Alliance and the Bill and Melinda Gates Foundation have brought substantial momentum to global efforts to reduce vaccine-preventable diseases, expanding on earlier efforts by the WHO, UNICEF, and governments in developed and developing countries.
_
_
In 2006, the World Health Organization and UNICEF created the Global Immunization Vision and Strategy (GIVS). This organization created a ten-year strategy with four main goals:
•to immunize more people against more diseases
•to introduce a range of newly available vaccines and technologies
•to integrate other critical health interventions with immunization
•to manage vaccination programs within the context of global interdependence
The Global Vaccination Action Plan (GVAP) was created by the World Health Organization and endorsed by the World Health Assembly in 2012. The plan which is set from 2011-2020 is intended to “strengthen routine immunization to meet vaccination coverage targets; accelerate control of vaccine-preventable diseases with polio eradication as the first milestone; introduce new and improved vaccines and spur research and development for the next generation of vaccines and technologies”. These global actions lead to progression of vaccinations. Living in a globalized world that is extremely connected, diseases that are preventable by vaccinations have become part of a larger public health movement leading to global herd immunity. These task forces and political campaigns that have erected in order to spread availability and knowledge of vaccination are modern attempts to protect the world from vaccination-preventable diseases. The plan was the result of a global collaboration involving governments and elected officials, health professionals, academic institutions, vaccine manufacturers, nongovernmental organizations, and civil society organizations. If the global community meets the plan’s objectives, childhood mortality around the world will be reduced below the targets set by the United Nations Millennium Development Goals.
_
World Immunization Week:
The last week of April each year is marked by WHO and partners as World Immunization Week. It aims to raise public awareness of how immunization saves lives, encouraging people everywhere to vaccinate themselves and their children against deadly diseases. In 2014, under the global slogan “Are you up-to-date?”, more than 180 countries, territories and areas marked the week with activities including vaccination campaigns, training workshops, round-table discussions and public information campaigns. This year’s campaign focuses on closing the immunization gap and reaching equity in immunization levels as outlined in the Global Vaccine Action Plan, which is a framework to prevent millions of deaths by 2020 through universal access to vaccines for people in all communities.
_________
_________
Immunology and vaccinology:
The basic concepts of immunology are an essential component of the foundations of modern vaccinology. To understand the immunology of vaccines, it is important first to examine the key players of the immune system and to understand how they are produced, activated and regulated. Immunology is the study of the structure and function of the immune system. Vaccinology is the science of vaccine development and how the immune system responds to vaccines, but also includes ongoing evaluation of immunization programs and vaccine safety and effectiveness, as well as surveillance of the epidemiology of vaccine-preventable diseases.
_
Human immune system:
The human immune system has evolved over millions of years from both invertebrate and vertebrate organisms to develop sophisticated defense mechanisms to protect the host from microbes and their virulence factors. The normal immune system has three key properties: a highly diverse repertoire of antigen receptors that enables recognition of a nearly infinite range of pathogens; immune memory to mount rapid recall immune responses; and immunologic tolerance to avoid immune damage to normal self-tissues. From invertebrates, humans have inherited the innate immune system, an ancient defense system that uses germ line–encoded proteins to recognize pathogens. Cells of the innate immune system, such as macrophages, dendritic cells, and natural killer (NK) lymphocytes, recognize pathogen-associated molecular patterns (PAMPs) that are highly conserved among many microbes and use a diverse set of pattern recognition receptor molecules (PRRs). Important components of the recognition of microbes by the innate immune system include (1) recognition by germ line–encoded host molecules, (2) recognition of key microbe virulence factors but not recognition of self-molecules, and (3) nonrecognition of benign foreign molecules or microbes. Upon contact with pathogens, macrophages and NK cells may kill pathogens directly or, in concert with dendritic cells, may activate a series of events that both slow the infection and recruit the more recently evolved arm of the human immune system, the adaptive immune system. Adaptive immunity is found only in vertebrates and is based on the generation of antigen receptors on T and B lymphocytes by gene rearrangements, such that individual T or B cells express unique antigen receptors on their surface capable of specifically recognizing diverse antigens of the myriad infectious agents in the environment. Coupled with finely tuned specific recognition mechanisms that maintain tolerance (nonreactivity) to self-antigens, T and B lymphocytes bring both specificity and immune memory to vertebrate host defenses.
_
The immune system can be divided into two main subsystems, the innate/general resistance system and the adaptive system. Both the innate system and the adaptive system continually interact with each other to provide an effective immune response.
_
The figure above shows key players of the immune system. The innate and adaptive immune systems are populated by many different cells that vary in their roles and responsibilities.
_
Innate and adaptive immunity:
All organisms have some form of innate protection against the outside world, which may be as simple as a cell wall or waxy coating. The innate immune system acts as a first line of defense which comprises both cellular and non-cellular effectors. This system provides early containment and defense during the lag time before adaptive immune effectors are available. Innate immunity comprises both soluble (e.g. complement, lysozyme) and cellular effectors (e.g. natural killer [NK] cells, macrophages and dendritic cells [DCs]). The innate and adaptive immune systems are principally bridged by the action of specialised APCs (antigen presenting cells), which translate and transfer information from the body tissues and innate immune system to the adaptive immune system, allowing a systemic response to a localised threat. The innate immune system therefore drives and shapes the development of adaptive immune responses via chemical and molecular signals delivered by APCs to induce the most appropriate type of adaptive response. The adaptive immune system forms the second, antigen-specific line of defense, which is activated and expanded in response to these signals.
_
The innate immune system:
Major Components of the Innate Immune System:
| Pattern recognition receptors (PRR) | C-type lectins, leucine-rich proteins, scavenger receptors, pentraxins, lipid transferases, integrins, inflammasome proteins |
| Antimicrobial peptides | -Defensins, -defensins, cathelin, protegrin, granulysin, histatin, secretory leukoprotease inhibitor, and probiotics |
| Cells | Macrophages, dendritic cells, NK cells, NK-T cells, neutrophils, eosinophils, mast cells, basophils, and epithelial cells |
| Complement components | Classic and alternative complement pathway, and proteins that bind complement components |
| Cytokines | Autocrine, paracrine, endocrine cytokines that mediate host defense and inflammation, as well as recruit, direct, and regulate adaptive immune responses |
_
Cells of innate immune system:
Cells of the innate immune system are produced in the bone marrow and then migrate to different anatomical locations. The innate immune cell repertoire includes tissue-resident cells such as macrophages and immature DCs, and cells which circulate via blood and the lymphatic system, such as monocytes, neutrophils, eosinophils, NK cells and innate T cells. Non-immune system cells at vulnerable locations, including keratinocytes and other epithelial and mucus-producing cells, fibroblasts and endothelial cells, can also exhibit innate defensive behaviours.
_
The innate immune system or general resistance includes a variety of protective measures which are continually functioning and provides a first-line of defense against pathogenic agents. However, these responses are not specific to a particular pathogenic agent. Instead, the innate immune cells are specific for conserved molecular patterns found on all microorganisms. This prevents the innate immune system from inadvertently recognizing host cells and attacking them. However, this prevents the innate immune responses from improving their reactions with repeated exposure to the same pathogenic agent. In other words, the innate immune system does not have memory. The protective defenses of the innate immune system begin with the anatomic barriers such as intact skin and mucous membranes which prevent the entrance of many microorganisms and toxic agents. The skin also has an acidic environment of pH 3-5 which retards the growth of microorganisms. In addition, the normal microorganisms or flora, which inhabit the skin and mucous membranes compete with other microorganisms for nutrients and attachment sites. Further, the mucus and cilia on the mucous membranes aid in trapping microorganisms and propelling them out of the body. Next, the innate immune system includes such physiologic barriers as the normal body temperature, fever, gastric acidity, lysozyme, interferon, and collectins. The normal body temperature range inhibits a variety of microorganisms; and the development of a fever can further inhibit many of these pathogenic organisms. The gastric acidity of the stomach is also quite effective in eliminating many ingested microorganisms. Lysozyme, which is a hydrolytic enzyme found in tears and mucous secretions, can cleave the peptidoglycan layer of the bacterial cell wall thus lysing the microorganism. Interferon(s), which include(s) a group of proteins that are produced by virally infected cells, can bind to noninfected cells and produce a generalized antiviral state. Collectins are surfactant proteins that are present in serum, lung secretions, and on mucosal surfaces. They can directly kill certain pathogenic microorganisms by disrupting their lipid membranes or indirectly by clumping microorganisms to enhance their susceptibility to phagocytosis.
_
Innate immunity:
◦does not depend upon previous exposure to the pathogen
◦does not produce immunologic memory
◦does not improve with repeated exposure to the pathogen.
_
The complement pathways are also a part of the defensive measures of the innate immune system. The complement system consists of approximately 25 proteins that work together to ‘complement’ the action of the adaptive immune response in destroying bacteria. Complement proteins circulate in the blood in an inactive form. Once activated, complement components serve several effector roles including the recruitment of phagocytes, the opsonisation of pathogens to promote phagocytosis, the removal of antibody antigen complexes and the lysis of antibody-coated cells. There are three complement pathways. The classical pathway is triggered when IgM antibodies or certain IgG antibody subclasses bind surface markers/antigens on microorganisms. The alternative or properdin pathway is triggered by the deposition of complement protein, C3b, onto microbial surfaces and does not require antibodies for activation. The third pathway, the lectin pathway, is triggered by the attachment of plasma mannose-binding lectin (MBL) to microbes and does not require antibodies for activation. These three pathways merge into a common pathway which leads to the formation of the membrane attack complex that can form pores in the membrane of targeted cells. The complement pathways are also integral in the opsonization (or increased susceptibility) of particulate antigens to phagocytosis and in triggering a localized inflammatory response.
_
The inflammatory response is another essential part of the innate immune response. The inflammatory response is the body’s reaction to invasion by an infectious agent, antigenic challenge, or any type of physical damage. The inflammatory response allows products of immune system into area of infection or damage and is characterized by the cardinal signs of redness, heat, pain, swelling, and loss of function.
_
In addition to the anatomic and physiologic mechanisms, there are also Pattern recognition receptors or PRRs which contribute to the innate immune response. Pattern recognition receptors are not specific for any given pathogen or antigen, but can provide a rapid response to antigens. PRRs are classified as membrane proteins because they are associated with the cell membrane; and, they can be found in all the membranes of the cells in the innate immune system. Although there are several hundred varieties, all the genes of the PRRs are encoded in the germline to ensure limited variability in their molecular structures. Examples of PRRs include MBL, pulmonary surfactant protein, C-reactive protein, toll-like receptors (TLRs), C-Type lectin, NOD, and MX. The PRRs recognize PAMPs or pathogen associated molecular patterns which can trigger cytokine release. Examples of PAMPs include LPS (endotoxin), peptidoglycan (cell walls), lipoproteins (bacterial capsules), hypomethylated DNA (CpG found in bacteria and parasites), double-stranded DNA (viruses), and flagellin (bacterial flagella). These antigens are produced by microbial cells and not by human cells. Recognition of PAMPs by PRRs leads to complement activation, opsonization, cytokine release, and phagocyte activation. Finally, the mononuclear phagocytes and granulocytic cells are also important to the innate response and help link the innate immune response to the adaptive immune response. Mononuclear phagocytes include monocytes which circulate in the blood and macrophages which are in the tissues. Monocytes and macrophages are highly important in antigen presentation, phagocytosis, cytokine production, and antimicrobial and cytotoxic activities. Upon maturity of the monocytes, the monocytes circulate in the blood for approximately 8 h, then migrate into the tissues and differentiate into specific tissue macrophages or into dendritic cells. There are several types of dendritic cells which are involved in different aspects of immune functions. Many dendritic cells are important in presenting antigen to T-helper cells. However, follicular dendritic cells are found only in lymph follicles and are involved in the binding of antigen–antibody complexes in lymph nodes. Granulocytic cells include neutrophils, eosinophils, and basophils/mast cells. Neutrophils are highly active phagocytic cells and generally arrive first at a site of inflammation. Eosinophils are also phagocytic cells; however, they are more important in resistance to parasites. Basophils in the blood and mast cells in the tissues release histamine and other substances and are important in the development of allergies.
_
Effectors of the innate response:
Under some circumstances, pathogen clearance may be achieved by innate immune effectors without activation of an adaptive immune response. Activated innate cells act as phagocytes, engulfing and destroying the pathogen within intracellular vesicles containing digestive enzymes. To be efficient, this response requires both the recruitment and activation of phagocytes at the site of infection, a process often referred to as the inflammatory response. Cells residing in proximity to the infection site are activated upon recognition of PAMPs, and secrete a large array of soluble mediators, including chemokines and cytokines. Chemokines behave as chemoattractants, favouring the recruitment of innate immune cells to the site of infection, while cytokines (including tumour necrosis factor and interferons) act by increasing the phagocytic activity of cells. Innate immune cells also produce a series of soluble chemical factors (such as peptides) that are able to directly target the invading microbes. Additionally, antigens are taken up by innate cells, with immature DCs the most specialised among them. The antigen is subsequently processed and the DC differentiates into an APC. Antigen-carrying APCs then migrate to the draining lymph node and provide the link between the innate and adaptive immune responses.
_
The innate system may be able to eradicate the pathogenic agent without further assistance from the adaptive system; or, the innate system may stimulate the adaptive immune system to become involved in eradicating the pathogenic agent.
_
Adaptive immune system:
In contrast to the innate immune system, the actions of adaptive immune system are specific to the particular pathogenic agent. This response will take longer to occur than the innate response. However, the adaptive immune system has memory which means that the adaptive immune system will respond more rapidly to that particular pathogen with each successive exposure. The adaptive immune response is composed of the B–cells/antibodies and T-cells. These are the two arms of the adaptive immune system. The B–cells and antibodies compose humoral immunity or antibody-mediated immunity; and, the T-cells compose cell-mediated immunity. As a note, natural killer cells are also from the lymphocyte lineage like B–cells and T-cells; however, natural killer cells are only involved in innate immune responses.
_
Antigen and antibody:
An antigen is a substance that the body recognizes as foreign and that triggers immune responses. The terms immunogen and antigen are often used interchangeably. Antibodies are proteins that are produced in response to antigens introduced into the body.
Antibodies protect the body from disease by:
•binding to the surface of the antigen to block its biological activity (neutralization)
•binding or coating (opsonisation) of the antigen to make it more susceptible to destruction and clearance by phagocytes (phagocytosis)
•opsonisation of special receptors on various cells, allowing them to recognise and respond to the antigen
•activation of the complement system to cause disintegration (lysis) of the pathogen and to enhance phagocytosis.
_
The first arm of the adaptive immune system is humoral immunity, functions against extracellular pathogenic agents and toxins. B–cells are produced in the bone marrow and then travel to the lymph nodes. Within the lymph nodes, naïve B–cells continue to mature and are exposed to pathogenic agents caught in the particular lymph node. Unlike T-cells, B–cells can recognize antigens in their native form which means that B–cells can recognize antigens without requiring that the antigen be processed by an antigen-presenting cell and then presented by a T-helper cell. These antigens are called T-independent antigens because T-cell activation is not required to activate the B–cells. Examples of these T-independent antigens include lipopolysaccharide, dextran, and bacterial polymeric flagellin. These antigens are typically large polymeric molecules with repeating antigenic determinants. These antigens can also induce numerous B–cells to activate; however, the immune response is weaker and the induction of memory is weaker than with T-helper cell activation. In contrast, activation of B–cells with T-helper cell activation results in a much better immune response and more effective memory. This long-term, effective immune response is the type of reaction that is the goal of immunizations. With the binding of the antigen to the Fab region on the B–cell receptor and secondary signaling from cytokines released by T-helper cells, B–cells begin somatic hypermutation at the Fab region which further increases the corresponding fit between the Fab region and the antigen. This process then stimulates the B–cell(s) to mature into a plasma cell(s) which then begins production of the particular antibody with the best corresponding fit to the antigen. From these stimulated B-cells, clones of B-cells with the specificity for the particular antigen will arise. These cells may become plasma cells producing antibodies or memory cells which will remain in the lymph nodes to stimulate a new immune response to that particular antigen. This occurs during the primary immune response when the immune system is first exposed to a particular antigen. This process of clonal selection and expansion will take several days to occur; and, primarily involves the production of IgM. IgM is the first antibody produced during a primary immune response. As the immune response progresses, the activated plasma cells will begin producing IgG specific to the particular antigen. Although IgM is the first antibody produced and is a much larger antibody, IgG is a better neutralizing antibody. IgG binds more effectively to the antigen and aids in opsonization. As a note, other antibodies can be produced by plasma cells. These antibodies include IgD, IgA, and IgE. IgD is primarily found as a receptor bound to the surfaces of mature B–cells. While IgA is the antibody found in secretions such as mucous, saliva, tears, and breast milk; and, IgE is the antibody involved in allergic reactions and parasitic infections. However, the most important antibody for vaccines is IgG. With the memory cells that have been produced with the primary immune response, any succeeding exposures to the antigen will result in a more rapid and effective secondary immune response. With this secondary immune response, the reaction will be quicker, larger, and primarily composed of IgG.
_
As for the other arm of adaptive immunity, cell-mediated immunity, it functions primarily against intracellular pathogens. T-cells mature in the thymus and are then released into the bloodstream. There are two main types of T-cells, CD4 cells and CD8 cells. CD4 cells or T-helper cells have the CD4 co-receptor and only recognize the major histocompatibility complex (MHC) II protein. The MHC II protein is found on all immune cells and acts as a marker of immune cells. CD4 cells are essential for antibody-mediated immunity and in helping B–cells control extracellular pathogens. There are two subsets of CD4 cells, Th1 and Th2. Upon activation by cytokines, B cells differentiate into memory B cells (long-lived antigen-specific B cells) or plasma cells (effector B cells that secrete large quantities of antibodies). Most antigens activate B cells using activated T helper (Th) cells, primarily Th1 and Th2 cells. Th1 cells secrete IFN-γ, which activates macrophages and induces the production of opsonizing antibodies by B cells. The Th1 response leads mainly to a cell-mediated immunity (cellular response), which protects against intracellular pathogens (invasive bacteria, protozoa and viruses). The Th1 response activates cytotoxic T lymphocytes (CTL), a sub-group of T cells, which induce death of cells infected with viruses and other intracellular pathogens. Natural killer (NK) cells are also activated by the Th1 response, these cells play a major role in the induction of apoptosis in tumors and cells infected by viruses. Th2 cells secrete cytokines, including IL-4, which induces B cells to make neutralizing antibodies. Th2 cells generally induce a humoral (antibody) response critical in the defense against extracellular pathogens (helminthes, extracellular microbes and toxins). CD8 cells or T-cytotoxic cells have the CD8 co-receptor and only recognize the major histocompatibility complex (MHC) I protein. The MHC I protein is found on all nucleated body cells except for mature erythrocytes and acts as a marker of body cells. CD8 cells are essential for cell-mediated immunity and in helping control of intracellular pathogens. Unlike B-cells, T-cells can only recognize antigen that has been processed and presented by antigen-presenting cells. There are two types of antigen processing. The first type of antigen processing involves attaching intracellular antigens along with MHC I proteins to the surface of antigen-processing cells. This occurs with viral antigens and tumor cells. The other type of antigen processing involves attaching extracellular antigens along with MHC II proteins to the surface of antigen-presenting cells. This occurs with bacterial and parasitic antigens. Once the T-cell has been activated by the antigen-presenting cell, it begins to carry out its functions depending on whether it is a CD4 cell or a CD8 cell. As with B-cells, activated T-cells also undergo clonal expansion which produces additional effector T-cells for the current infection and memory T-cells for future infections with this antigen.
_
Adaptive immunity is the body’s second level of defense, which develops as a result of infection with a pathogen or following immunization. It defends against a specific pathogen and takes several days to become protective. Adaptive immunity:
◦has the capacity for immunologic memory
◦provides long-term immunity which may persist for a lifetime but may wane over time
◦increases in strength and effectiveness each time it encounters a specific pathogen or antigen.
_
The figure above shows organs and tissues of the immune system. The innate immune system is formed from a combination of physical barriers (skin, mucus), chemical defenses (acids, antimicrobial peptides) and specialised cells capable of responding to pathogens without needing to recognise specific antigens (A). The adaptive immune system consists of a network of primary and secondary organs, where immune cells are either produced or reside until they become activated (B). The primary lymphoid organs (bone marrow and thymus) are where lymphocytes are generated, and the secondary lymphoid organs (peripheral lymph nodes, spleen, tonsils, Peyer’s patches) are where immune responses are initiated and regulated.
_
Summary of differences between the innate and adaptive immune systems:
_
APC:
An antigen-presenting cell (APC) is a cell that displays foreign antigens complexed with major histocompatibility complexes (MHCs) on their surfaces; this process is known as antigen presentation. T-cells may recognize these complexes using their T-cell receptors (TCRs). These cells process antigens and present them to T-cells. T cells cannot recognize, and therefore cannot respond to, ‘free’ antigen. T cells can only ‘see’ an antigen that has been processed and presented by cells via carrier molecules like MHC and CD1 molecules. Most cells in the body can present antigen to CD8+ T cells via MHC class I molecules and, thus, act as “APCs”; however, the term is often limited to specialized cells that can prime T cells (i.e., activate a T cell that has not been exposed to antigen, termed a naive T cell). These cells, in general, express MHC class II as well as MHC class I molecules, and can stimulate CD4+ (“helper”) T cells as well as CD8+ (“cytotoxic”) T cells, respectively. APCs could be dendritic cell (DC), macrophage or certain B cell/epithelial cell.
_
Innate and adaptive immune responses are bridged by the actions of APCs:
The innate immune system provides an essential link between the first encounter with a pathogen at the site of infection and the eventual establishment of immune memory. Innate cells (such as macrophages and DCs) are strategically located at body sites with a high risk of infection (such as epithelia and mucosal surfaces). These types of cells act as both a first line of defense against danger and as key messengers that are able to alert the adaptive immune system. Since naïve T and B cells are not pre-positioned in most organs and tissues of the body, they rely on the innate immune system to sense an infectious event. Among tissue-resident cells, the most efficient APCs are DCs. Immature DCs which have captured antigen become activated and mature into functional APCs, while migrating to the regional draining lymph node or submucosal lymphoid tissue. Mature DCs express high levels of antigen/MHC complexes at the cell surface and undergo morphological changes, which render them highly specialised, to activate naïve T cells. When they arrive in the lymph node, DCs present processed antigen and express co-stimulatory signals. The signals provided by DCs promote T-cell differentiation and proliferation, initiating the adaptive T cell-mediated immune response. APC activation is therefore a necessary prerequisite for an efficient adaptive immune response. DCs not only provide antigen and co-stimulation to naïve T cells, but also contribute to the initial commitment of naïve T helper cells into Th1, Th2 or other subsets. This directs the efficient induction of T helper cells with appropriate cytokine profiles early during infections, without the need for direct contact between antigen-specific T cells and pathogens. Undigested pathogen-derived antigens are also drained by the lymph and transported to the B cell-rich area of the lymph node, where they are exposed to BCR-expressing cells. An adaptive immune response is therefore initiated in a draining lymph node by the concerted action of innate immune cells and free antigens. These activate T and B lymphocytes, respectively, to proliferate and differentiate into effector and memory cells.
_
The table below shows cells of the Innate Immune System and their major roles in triggering Adaptive Immunity:
| Cell Type | Major Role in Innate Immunity | Major Role in Adaptive Immunity |
| Macrophages | Phagocytose and kill bacteria; produce antimicrobial peptides; bind (LPS); produce inflammatory cytokines | Produce IL-1 and TNF- to upregulate lymphocyte adhesion molecules and chemokines to attract antigen-specific lymphocyte. Produce IL-12 to recruit TH1 T helper cell responses; upregulate co-stimulatory and MHC molecules to facilitate T and B lymphocyte recognition and activation. Macrophages and dendritic cells, after LPS signaling, upregulate co-stimulatory molecules B7-1 (CD80) and B7-2 (CD86) that are required for activation of antigen-specific antipathogen T cells. There are also Toll-like proteins on B cells and dendritic cells that, after LPS ligation, induce CD80 and CD86 on these cells for T cell antigen presentation. |
| Plasmacytoid dendritic cells (DCs) of lymphoid lineage | Produce large amounts of interferon- (IFN-), which has antitumor and antiviral activity, and are found in T cell zones of lymphoid organs; they circulate in blood | IFN- is a potent activator of macrophage and mature DCs to phagocytose invading pathogens and present pathogen antigens to T and B cells |
| Myeloid dendritic cells are of two types; interstitial and Langerhans-derived | Interstitial DCs are strong producers of IL-12 and IL-10 and are located in T cell zones of lymphoid organs, circulate in blood, and are present in the interstices of the lung, heart, and kidney; Langerhans DCs are strong producers of IL-12; are located in T cell zones of lymph nodes, skin epithelia, and the thymic medulla; and circulate in blood | Interstitial DCs are potent activators of macrophage and mature DCs to phagocytose invading pathogens and present pathogen antigens to T and B cells |
| Natural killer (NK) cells | Kill foreign and host cells that have low levels of MHC+ self-peptides. Express NK receptors that inhibit NK function in the presence of high expression of self-MHC. | Produce TNF- and IFN-, which recruit TH1 helper T cell responses |
| NK-T cells | Lymphocytes with both T cell and NK surface markers that recognize lipid antigens of intracellular bacteria such as Mycobacterium tuberculosis by CD1 molecules and kill host cells infected with intracellular bacteria. | Produce IL-4 to recruit TH2 helper T cell responses, IgG1 and IgE production |
| Neutrophils | Phagocytose and kill bacteria, produce antimicrobial peptides | Produce nitric oxide synthase and nitric oxide, which inhibit apoptosis in lymphocytes and can prolong adaptive immune responses |
| Eosinophils | Kill invading parasites | Produce IL-5, which recruits Ig-specific antibody responses |
| Mast cells and basophils | Release TNF-, IL-6, and IFN- in response to a variety of bacterial PAMPs | Produce IL-4, which recruits TH2 helper T cell responses and recruit IgG1- and IgE-specific antibody responses |
| Epithelial cells | Produce antimicrobial peptides; tissue-specific epithelia produce mediator of local innate immunity; e.g., lung epithelial cells produce surfactant proteins (proteins within the collectin family) that bind and promote clearance of lung-invading microbes | Produces TGF-, which triggers IgA-specific antibody responses |
LPS, lipopolysaccharide; PAMP, pathogen-associated molecular patterns; TNF-, tumor necrosis factor-alpha; IL-4, IL-5, IL-6, IL-10, and IL-12, interleukin 4, 5, 6, 10, and 12, respectively.
_
Immunological memory:
Immunologic memory is the immune system’s ability to remember its experience with an infectious agent, leading to effective and rapid immune response upon subsequent exposure to the same or similar infectious agents. Development of immunologic memory requires participation of both B and T cells; memory B cell development is dependent on the presentation of antigens by T cells. Irrespective of the type of immune response required for protection, for almost all vaccines long-lasting protection (memory) is a desirable objective. However, while it is easy to state this, it is less certain how it should be achieved, although a great deal has been learnt about immunological memory over the last two decades. During a primary immune response, lymphocytes proliferate and change their phenotype. Memory populations of cells are, therefore, both quantitatively and qualitatively different from those that have not yet encountered antigen. Thus memory consists of expanded clones of lymphocytes with altered function. Among thymus-derived (T) lymphocytes, this is reflected in rapid production of effector cytokines such as IFN-γ or interleukins. Primed cells express higher levels of several adhesion molecules, such as ICAM-1 and integrins, as well as homing molecules such as CD44, CD62L and the cutaneous lymphocyte antigen (CLA). Among B-cells, the hallmark of immunological memory is the production of isotype switched, somatically mutated, high affinity immunoglobulin. It is also clear that memory is a dynamic state. In both man and experimental animals, phenotypically defined memory cells have been shown to divide more rapidly than naive cells. This appears to be an inherent property of memory cells since division continues in the absence of antigen.
_
Constraints on the duration of memory:
In vitro at least, human T lymphocyte clones can only undergo a finite number of cell divisions and, as they approach senescence, no longer express the co-stimulatory molecule CD28, can no longer up-regulate telomerase on activation, and show progressive shortening of telomeres. These mechanisms may limit the duration of memory in the absence of re-exposure to antigen, which would recruit new clones. In addition to these constraints on survival of individual clones, there is also the constraint of space in the memory pool. Although during an acute infection lymphocyte numbers may increase greatly, in the longer term numbers of cells with naive and memory phenotypes change only slowly. Thus every time a new antigen is encountered and a new set of clones undergoes expansion and enters the memory pool, other cells must die to provide space. What factors favour one cell or clone over another in this competition for survival are not known. However, experimental evidence suggests that memory persists longer if the initial clonal expansion is large. Alternatively, persistence of antigen may favour clonal survival as occurs in chronic infections such as EBV or CMV. It is now clear that there is considerable heterogeneity among antigen-specific T-cell populations detected by binding to MHC-peptide tetramers and it is thought that some memory cells may revert to a more slowly dividing state. This suggests two alternative strategies for ensuring persistence of memory. Either vaccines should be designed to ensure maximal clonal expansion by providing an optimal dose of antigen and appropriate adjuvant, or vectors should be chosen to ensure long persistence of antigen.
_
The figure above shows the kinetics of primary and recall (memory) immune responses. On first exposure to a pathogen or antigen (referred to as ‘priming’ in vaccination), the innate immune system must detect, process and translate the threat into a form that can be understood by the adaptive immune system. This occurs via the bridging actions of APCs and takes days/weeks. Following resolution of the challenge, a specialised ‘memory’ cell population remains. The cells within this population are maintained for a long time (months/years) and may remain within the host for the rest of their host’s life. On subsequent exposure to the same antigen (referred to as ‘boosting’ in vaccination), the innate immune response is triggered as before but now the memory cell populations are able to mount a greater and more rapid response as they do not need to undergo the same activation process as naïve cells. The adaptive response on secondary exposure leads to a rapid expansion and differentiation of memory T and B cells into effector cells, and the production of high levels of antibodies. A higher proportion of IgG and other isotypes of antibodies compared with the level of IgM characterises memory antibody responses.
_
By definition, all effective vaccines lead to the development of immune memory, by mimicking the threat of an infection and providing antigens derived from the specific pathogen. The ability to generate immune memory is the key attribute of the adaptive immune system, which is crucial for the long-term protection of individuals and populations. Generating immune memory depends on a high degree of interaction among many different cell types, which maintains higher numbers of T and B cells that were selected as the most useful in the primary immune response. However, while the relative contribution of clonal memory cells to protection can be inferred from the molecules they express and their functional behaviour, the presence of memory cells per se is not indicative of absolute protection against disease.
________
Immune response to vaccine:
_
The figure above shows the flow of information following intramuscular vaccination. An antigen delivered by a vaccine is taken up by macrophages and immature APCs (1). APCs migrate to the lymph node draining the site of vaccination (2). The adaptive immune response is now initiated and effectors, such as CD4 effector T cells, cytotoxic T cells and soluble antibodies (3), are produced which travel throughout the bloodstream and back to the site of vaccination.
_
Vaccines function by stimulating the immune system and prompting a primary immune response to an infecting pathogen or to molecules derived from a particular pathogen. The immune response elicited by this primary exposure to vaccine pathogen creates immunological memory, which involves the generation of a pool of immune cells that will recognize the pathogen and mount a more robust or secondary response upon subsequent exposure to the virus or bacterium. In successful immunization, the secondary immune response is sufficient to prevent disease in the infected individual, as well as prevent the transmission of the pathogen to others. For communicable diseases, immunizations protect not only the individual who receives the immunization, but also others with whom he or she has contact. High levels of vaccination in a community increase the number of people who are less susceptible or resistant to illness and prevent propagation of the infectious agent. Unvaccinated individuals or those who have not developed immunity to this pathogen are afforded an indirect measure of protection because those with immunity reduce the spread of the pathogen throughout the entire population. The larger the proportion of people with immunity, the greater the protection of those without immunity. This effect is called “herd immunity.” [Vide infra] Herd immunity is an important phenomenon as immunization programs rarely achieves 100 percent immunization in a population; and in some cases, previously vaccinated persons may not exhibit effective immunity and disease may result from exposure to the pathogen. For protection, immunization of not only ourselves but also our neighbors is important.
_
As with any challenge to the immune system, the body must first detect the threat whether it is a pathogenic agent or an immunization. This initial detection typically is done by the innate immune system; although, B-cells may also perform this function. This detection process begins when the immune system recognizes epitopes on antigens. Epitopes are small subregions on the antigens that stimulate immune recognition. Multiple components of the innate immune system will then respond to this challenge. These components of innate immunity will opsonize or bind to the agent and aid in its engulfment by antigen-presenting cells such as macrophages or monocytes. These antigen-presenting cell(s) will then process the antigens from this pathogenic agent and insert the processed antigen along with the MHC protein onto the surface on the antigen-presenting cell. If it is a viral antigen, the antigen will be bound with MHC I protein and presented by the antigen-presenting cell to a CD8 cell which will likely trigger cell-mediated immunity. If it is a bacterial or parasitic antigen, the antigen will be bound with MHC II protein and presented by the antigen-presenting cell to a CD4 cell which will likely trigger antibody-mediated immunity.
_
Vaccine-induced immune effectors (see table below) are essentially antibodies—produced by B lymphocytes—and capable of binding specifically to a toxin or a pathogen. Other potential effectors are cytotoxic CD8+ T lymphocytes (CTL) that may limit the spread of infectious agents by recognizing and killing infected cells or secreting specific antiviral cytokines. The generation and maintenance of both B and CD8+ T cell responses is supported by growth factors and signals provided by CD4+ T helper (Th) lymphocytes, which are commonly subdivided into T helper 1 (Th1) and T helper 2 (Th2) subtypes. These effectors are controlled by regulatory T cells (Treg) that are involved in maintaining immune tolerance. Most antigens and vaccines trigger both B and T cell responses, such that there is no rationale in opposing antibody production (‘humoral immunity’) and T cell responses (‘cellular immunity’). In addition, CD4+ T cells are required for most antibody responses, while antibodies exert significant influences on T cell responses to intracellular pathogens.
_
_
How type of vaccine affect immune response:
The nature of the vaccine exerts a direct influence on the type of immune effectors that are predominantly elicited and mediate protective efficacy (see table below). Capsular polysaccharides (PS) elicit B cell responses in what is classically reported as a T-independent manner (e.g. PPV) although increasing evidence supports a role for CD4+ T cells in such (e.g. glycoconjugate vaccines) provides foreign peptide antigens that are presented to the immune system and thus recruits antigen-specific CD4+ Th cells in what is referred to as T-dependent antibody responses (e.g. PCV). A hallmark of T-dependent responses, which are also elicited by toxoid, protein, inactivated or live attenuated viral vaccines, is to induce both higher-affinity antibodies and immune memory. In addition, live attenuated vaccines usually generate CD8+ cytotoxic T cells. The use of live vaccines/vectors or of specific novel delivery systems (e.g. DNA vaccines) appears necessary for the induction of strong CD8+ T cell responses. Most current vaccines mediate their protective efficacy through the induction of vaccine specific antibodies, whereas BCG-induced T cells produce cytokines that contribute to macrophage activation and control of M. tuberculosis.
_
Correlates of vaccine induced immunity:
_
_
_
_
_
How route of vaccine administration affect immune response:
Following injection, the vaccine antigens attract local and systemic dendritic cells, monocytes and neutrophils. Innate immune responses activate these cells by changing their surface receptors and migrate along lymphatic vessels, to the draining lymph nodes where the activation of T and B lymphocytes takes place. In case of killed vaccines, there is only local and unilateral lymph node activation. Conversely for live vaccines, there is multifocal lymph node activation due to microbial replication and dissemination. Consequently the immunogenicity of killed vaccines is lower than the live vaccines; killed vaccines require adjuvants which improve the immune response by producing local inflammation and recruiting higher number of dendritic cells/ monocytes to the injection site. Secondly, the site of administration of killed vaccines is of importance; the intramuscular route which is well vascularised and has a large number of patrolling dendritic cells is preferred over the subcutaneous route. Intradermal route recruits the abundant dendritic cells in the skin and offers the advantage of antigen sparing and early & effective protection but the GMT’s (geometric mean [antibody] titre) are lower than that achieved with IM and may wane faster. The site of administration is usually of little significance for live vaccines. Finally due to focal lymph node activation, multiple killed vaccines may be administered at different sites with a little immunologic interference. Immunologic interference may occur with multiple live vaccines unless they are given on the same day or at least 4 weeks apart or by different routes. Immunological (immune) interference is defined as reduction in the immunogenicity of a vaccine antigen when it is administered as a component of a vaccine that includes multiple vaccine antigens or reduction in the immunogenicity of a vaccine when it is administered separately or concurrent with another vaccine. [see also vaccine interference]
______
Immunological requirement of a vaccine:
1. Identification and selection of the most appropriate antigen:
Vaccines aim to prevent the disease symptoms that are the consequences of a pathogenic infection. In most cases, this does not occur by completely preventing infection but by limiting the consequences of the infection. In other words, vaccine prevents disease and not infection by direct effect, but by indirect herd effect, it also prevents infection and infectiousness. An understanding of the disease pathogenesis and natural immune control is, therefore, very useful when selecting appropriate antigens upon which to base a vaccine. Vaccines developed from pathogens can vary in the complexity of the pathogen-derived material they contain. Our understanding of fundamental immunology, as well as the selection techniques used, has resulted in new vaccines that are better characterised than ever before, and has also initiated a more rational approach to vaccine design.
2. Induction of innate immune responses:
The immune system is triggered by a combination of events and stimuli, as described previously. The requirement for more than the presence of a ‘foreign’ antigen to elicit an immune response must therefore always be considered in vaccine design, particularly when using highly purified or refined antigens. Highly refined subunit antigen formulations, and some inactivated whole pathogens, do not contain many of the molecular features and defensive triggers that are required to alert the innate immune system. These types of antigen are designed to minimize excessive inflammatory responses but, as a result, may be suboptimally immunogenic. Under these circumstances, the addition of adjuvants can mimic the missing innate triggers, restoring the balance between necessary defensive responses and acceptable tolerability.
3. Induction of CD4 T cell help:
The induction of CD4 T cells is essentially controlled by the nature of this initial inflammatory response. Therefore, vaccine adjuvants can play a role in guiding how CD4 T cells are induced and how they further differentiate and influence the quality and quantity of the adaptive immune response.
4. Selection and targeting of effector cells:
It is important to recognise that the dominant immune response to a given pathogen or antigen may not necessarily be the optimum response for inducing protection; indeed through evolution some pathogens have developed strategies to evade or subvert the immune response, as is the case with Neisseria gonorrhoeae, where the dominant antibody response actually facilitates infection by preventing complement-dependent bactericidal activity. Antibody titers are often considered to represent adequate indicators of immune protection but, as discussed above, may not be the actual mechanism by which optimal protection is achieved. Useful specific so-called immune correlates of immunity/protection may be unknown or incompletely characterised. Therefore, modern vaccine design still looks to clinical trials to provide information about clinical efficacy and, if possible, the immunological profiles of protected individuals. Immunogenicity is assessed by laboratory measurement of immune effectors, typically antibodies. Increasingly, however, specific T-cell activation is included in the parameters assessed, as adequate T-cell immunity may be essential for recovery from some infections and improved assay techniques have allowed these evaluations to become more standardised and offer more robust data. This can then open the door to understanding observed clinical efficacy (or lack of) and to defining at least some of the features of vaccine-induced protection. By preferentially targeting the best immunological effectors, vaccines can then hope to mimic or improve on nature’s own response to infection.
_
Booster dose:
In medicine, a booster dose is an extra administration of a vaccine after an earlier dose. After initial immunization, a booster injection or booster dose is a re-exposure to the immunizing antigen. It is intended to increase immunity against that antigen back to protective levels after it has been shown to have decreased or after a specified period. For example, tetanus shot boosters are often recommended every 10 years. If a patient receives a booster dose but already has a high level of antibody, then a reaction called an Arthus reaction could develop, a localized form of Type III hypersensitivity, induced by fixation of complement by preformed circulating antibodies. In severe cases, the degree of complement fixation can be so substantial that it induces local tissue necrosis.
_
Both the innate and adaptive immune subsystems are necessary to provide an effective immune response whether to an actual pathogenic agent or to an immunization. Further, effective immunizations must induce long-term stimulation of both the humoral and cell-mediated arms of the adaptive system by the production of effector cells for the current infection and memory cells for future infections with the pathogenic agent. At least seven different types of vaccines are currently in use or in development that produce this effective immunity and have contributed greatly to the prevention of infectious disease around the world.
______
Immunization:
Immunization is the process whereby a person is made immune or resistant to an infectious disease, typically by the administration of a vaccine. Vaccines stimulate the body’s own immune system to protect the person against subsequent infection or disease. Immunization is a proven tool for controlling and eliminating life-threatening infectious diseases and is estimated to avert between 2 and 3 million deaths each year. It is one of the most cost-effective health investments, with proven strategies that make it accessible to even the most hard-to-reach and vulnerable populations. It has clearly defined target groups; it can be delivered effectively through outreach activities; and vaccination does not require any major lifestyle change. The overwhelming safety and effectiveness of vaccines in current use in preventing serious disease has allowed them to gain their preeminent role in the routine protection of health. Before an immunization is introduced for population-wide use, it is tested for efficacy and safety. However, immunization is not without risks. For example, it is well established that the oral polio vaccine on rare occasion causes paralytic polio and that vaccines sometimes lead to anaphylactic shock. Given the widespread use of vaccines; state mandates requiring vaccination of children for entry into school, college, or day care; and the importance of ensuring that trust in immunization programs is justified, it is essential that safety concerns receive assiduous attention.
_
Immunization is the process by which an individual’s immune system becomes fortified against an agent (known as the immunogen). When this system is exposed to molecules that are foreign to the body, called non-self, it will orchestrate an immune response, and it will also develop the ability to quickly respond to a subsequent encounter because of immunological memory. This is a function of the adaptive immune system. Therefore, by exposing an animal to an immunogen in a controlled way, its body can learn to protect itself: this is called active immunization. The most important elements of the immune system that are improved by immunization are the T cells, B cells, and the antibodies B cells produce. Memory B cells and memory T cells are responsible for a swift response to a second encounter with a foreign molecule. Passive immunization is when these elements are introduced directly into the body, instead of when the body itself has to make these elements. Immunization is done through various techniques, most commonly vaccination. Vaccines against microorganisms that cause diseases can prepare the body’s immune system, thus helping to fight or prevent an infection. The fact that mutations can cause cancer cells to produce proteins or other molecules that are known to the body forms the theoretical basis for therapeutic cancer vaccines. Other molecules can be used for immunization as well, for example in experimental vaccines against nicotine (NicVAX) or the hormone ghrelin in experiments to create an obesity vaccine. Before the introduction of vaccines, the only way people became immune to an infectious disease was by actually getting the disease and surviving it. Smallpox (variola) was prevented in this way by inoculation, which produced a milder effect than the natural disease.
_
Inherited immunity:
Mothers can pass on immunity to their babies across the placenta during the final months of pregnancy. The amount of inherited immunity varies by disease and is an important factor in deciding when a child should be immunized. The neonate is protected against disease by maternal immunoglobulins (Ig). Maternal IgG is transported across the placenta before birth and maternal secretory IgA is present in breast milk and colostrum. These passively acquired antibodies provide protection against pathogens to which the mother was immune. However, protection provided by passively transferred antibodies is short-lived. Passively acquired maternal IgG declines during the first few months of life, and most infants are not breastfed beyond several months of age. More importantly, maternal antibodies offer limited immunologic protection when compared with protection afforded by an infant’s active immune response. A mother’s antibodies may protect a child from measles for 6 to 12 months. But, in the case of diseases such as pertussis, immunity may last only for a few weeks. Tetanus is one example where inherited immunity is critical and the mother must be immunized to offer protection to her newborn.
_
Types of immunization: active and passive immunization:
Immunization can be derived from either passive or active means. These means can be from either natural or artificial sources. Natural sources are due to exposure to the environment, humans, and animals. In contrast, artificial sources are due to medical interventions. Passive immunization occurs with the transfer to preformed antibodies to an unimmunized individual. This individual would then develop a temporary immunity to a particular organism or toxin due to the presence of these preformed antibodies. Once these preformed antibodies have been destroyed, the individual would no longer have immunity to this microorganism or toxin. Passive immunization can occur either naturally or artificially. Excellent examples of natural passive immunization are the passage of maternal antibodies through the placenta to the fetus and the passage of these maternal antibodies to the infant through the colostrum and milk. Excellent examples of artificial passive immunization include the administration of pooled human immune gamma globulin and antivenin. These gamma globulins and antivenins provide temporary immunity to either a particular illness or venom. Passive immunity refers to the process of providing IgG antibodies to protect against infection; it gives immediate, but short-lived protection—several weeks to 3 or 4 months at most. Concurrent with these effects of this temporary immunity from the preformed antibodies, the individual’s own body is likely to be in the early stages of developing its own active immune response. Active immunization occurs with the exposure of an unimmunized individual to a pathogenic agent. The immune system of this individual then begins the process of developing immunity to this agent. In contrast to passive immunization, active immunization typically produces long-term immunity due to the stimulation of the individual’s immune system. Active immunization can occur either naturally or artificially. An excellent example of natural active immunization is exposure to influenza. The body then begins the process of developing long-term immunity to the influenza virus. Excellent examples of artificial active immunization include the different types of vaccines. These immunizations mimic the stimulation necessary for immune development yet do not produce active disease. Wild infection for example with hepatitis A virus (HAV) and subsequent recovery gives rise to a natural active immune response usually leading to lifelong protection. In a similar manner, administration of two doses of hepatitis A vaccine generates an acquired active immune response leading to long-lasting (possibly lifelong) protection. Hepatitis A vaccine has only been licensed since the late 1980s so that follow-up studies of duration of protection are limited to <25 years—hence, the preceding caveat about duration of protection.
_
Immunizing Agents:
Immunizing agents are classified as active or passive, depending on the process by which they confer immunity; prevention of disease through the use of immunizing agents is called immunoprophylaxis. Active immunization is the production of antibodies against a specific agent after exposure to the antigen through vaccination. Active immunizing agents are typically referred to as vaccines. Passive immunization involves the transfer of pre-formed antibodies, generally from one person to another or from an animal product, to provide temporary protection, since transferred antibody degrades over time. It can occur by transplacental transfer of maternal antibodies to the developing foetus, or it can be provided by administration of a passive immunizing agent prepared from the serum of immune individuals or animals.
_
Active immunizing agents – vaccines:
Vaccines are complex biologic products designed to induce a protective immune response effectively and safely. An ideal vaccine is safe with minimal adverse effects, and effective in providing lifelong protection against disease after a single dose that can be administered at birth. Also ideally, it would be inexpensive, stable during shipment and storage, and easy to administer. Some vaccines come closer to fulfilling these criteria than others. Although each vaccine has its own benefits and risks, and indications and contraindications, all vaccines offer protection against the disease for which they were created. In addition to the active component (the antigen), which induces the immune response, vaccines may contain additional ingredients such as preservatives, additives, adjuvants and traces of other substances necessary in the production of the vaccine. Vaccine antigens include: inactivated (killed) or attenuated (weakened) live organisms; products secreted by organisms that are modified to remove their pathogenic effects (e.g., tetanus toxoid); and components of the organism, some of which some are made in the laboratory through recombinant technology.
_
Passive immunizing agents – immune globulins:
Passive immunization with immune globulins provides protection when vaccines for active immunization are unavailable or contraindicated, or in certain instances when unimmunized individuals have been exposed to the infectious agent and rapid protection is required (post-exposure immunoprophylaxis) as vaccine immune response takes time and disease incubation period is short. Passive immunization also has a role in the management of immunocompromised people who may not be able to respond fully to vaccines or for whom live vaccines may be contraindicated. The duration of the beneficial effects provided by passive immunizing agents is relatively short and protection may be incomplete.
The four most commonly used immunoglobulin preparations are as follows.
(i) Hepatitis B Immunoglobulin
(ii) Rabies Immunoglobulin
(iii)Tetanus Immunoglobulin
(iv) Varicella-Zoster Immunoglobulin
_
Monoclonal Antibodies:
Increasingly, technology is being used to generate monoclonal antibodies (MAbs)– “mono” meaning that they are a pure, single type of antibody targeted at a single site on a pathogen, and “clonal” because they are produced from a single parent cell. These antibodies have wide-ranging potential applications to infectious disease and other types of diseases. To date, only one MAb treatment is commercially available for the prevention of an infectious disease. This is a MAb preparation for the prevention of severe disease caused by RSV in high-risk infants. Physicians are also increasingly using MAbs to combat noninfectious diseases, such as certain types of cancer, multiple sclerosis, rheumatoid arthritis, Crohn’s disease, and cardiovascular disease. Scientists are researching other new technologies for producing antibodies in the laboratory, such as recombinant systems using yeast cells or viruses and systems combining human cells and mouse cells, or human DNA and mouse DNA.
Bioterror threats:
In the event of the deliberate release of an infectious biological agent, biosecurity experts have suggested that passive immunization could play a role in emergency response. The advantage of using antibodies rather than vaccines to respond to a bioterror event is that antibodies provide immediate protection, whereas a protective response generated by a vaccine is not immediate and in some cases may depend on a booster dose given at a later date. Candidates for this potential application of passive immunization include botulinum toxin, tularemia, anthrax, and plague. For most of these targets, only animal studies have been conducted, and so the use of passive immunization in potential bioterror events is still in experimental stages.
_
Advantages and Disadvantages of Passive Immunization:
Vaccines typically need time (weeks or months) to produce protective immunity in an individual and may require several doses over a certain period of time to achieve optimum protection. Passive immunization, however, has an advantage in that it is quick acting, producing an immune response within hours or days, faster than a vaccine. Additionally, passive immunization can override a deficient immune system, which is especially helpful in someone who does not respond to immunization. Antibodies, however, have certain disadvantages. First, antibodies can be difficult and costly to produce. Although new techniques can help produce antibodies in the laboratory, in most cases antibodies to infectious diseases must be harvested from the blood of hundreds or thousands of human donors. Or, they must be obtained from the blood of immune animals (as with antibodies that neutralize snake venoms). In the case of antibodies harvested from animals, serious allergic reactions can develop in the recipient. Another disadvantage is that many antibody treatments must be given via intravenous injection, which is a more time-consuming and potentially complicated procedure than the injection of a vaccine. Finally, the immunity conferred by passive immunization is short lived: it does not lead to the formation of long-lasting memory immune cells. In certain cases, passive and active immunity may be used together. For example, a person bitten by a rabid animal might receive rabies antibodies (passive immunization to create an immediate response) and rabies vaccine (active immunity to elicit a long-lasting response to this slowly reproducing virus).
_
What is the difference between antiserum and vaccine?
Simplistically, a vaccine primes your immune system to prevent a future infectious disease. An antiserum either neutralizes the present “infection” or helps your immune system to attack the present infection. Vaccines are generally prophylactic where as antiserums are generally a form of treatment. A vaccine stimulates your immune system to prevent disease, essentially giving you immunity. It generally imparts a long-term immunity that can last years. Specific examples include the polio vaccine which provides immunity to polio virus; or the tetanus vaccine which stimulates your immune system to quickly identify the toxins produced by the clostridium tetani bacteria and to produce the necessary antibodies. An antiserum (sometimes called a serum or the plural antisera) contains preformed specific antibodies (immunoglobulin) to neutralize infection. This provides a temporary immunity, but a long-term immunity generally still requires the use of a vaccine. An example is the tetanus antitoxin which contains antibodies from previously infected animals. If you are infected with clostridium tetani bacteria and you are not immunized, then you would treat the infection with an antiserum. You may also administer a vaccine at the same time (or shortly after) to create immunity to prevent future infections.
_
Vaccination vs. immunization vs. inoculation:
Understanding the difference between vaccines, vaccinations, and immunizations can be tricky. Below is an easy guide that explains how these terms are used:
•A vaccine is a product that produces immunity from a disease and can be administered through needle injections, by mouth, or by aerosol. The administration of vaccines is called vaccination.
•An immunization is the process by which a person or animal becomes protected from a disease. Vaccines cause immunization, and there are also some diseases that cause immunization after an individual recovers from the disease.
_
Most people do not realize that when you receive a shot or a vaccine, it does not mean you are immunized. Many people believe that once you are vaccinated you are completely protected. That belief is wrong. The use of the word “immunization” instead of “vaccination” is found everywhere. Most importantly, news outlets tell the public that immunization is the same as vaccination. However, there is a large difference between the two. No vaccine is 100% effective in preventing disease. Most routine childhood vaccines are effective for 85% to 95% of recipients. Since no vaccine is 100% effective, vaccination does not automatically mean the person is immunized against the disease. Immunization means to make someone immune to something. Vaccination, by contrast, just means to inject a suspension of attenuated or killed microorganisms…administered for prevention of infectious disease. Vaccination does not guarantee immunity. Everyone’s immune system reacts differently. For reasons related to the individual, some will not develop immunity. Also, immunization not only refers to the use of all vaccines but also extends to the use of antitoxin, which contains preformed antibody to e.g. diphtheria or tetanus exotoxins. So vaccination is not synonymous with immunization although both terms are used interchangeably in this article. Natural immunity happens only after one recovers from the actual disease.
__
The terms inoculation, vaccination, immunization and injection are often used synonymously to refer to artificial induction of immunity against various infectious diseases. This is supported by some dictionaries. However, there are some important historical and current differences. In English medicine inoculation referred only to the prevention of smallpox until the very early 1800s. When Edward Jenner introduced smallpox vaccine in 1798 this was initially called cowpox inoculation or vaccine inoculation. Soon, to avoid confusion, smallpox inoculation was referred to as variolation (from variola = smallpox) and cowpox inoculation was referred to as vaccination (from Jenner’s use of Variolae vaccinae = smallpox of the cow). Then, in 1891 Louis Pasteur proposed that the terms vaccine/vaccination should be extended to include the new protective procedures being developed. Inoculation is now more or less synonymous in nontechnical usage with injection etc., and the question e.g. ‘Have you had your flu injection/vaccination/inoculation/immunization?’ should not cause confusion. The focus is on what is being given and why, not the literal meaning of the technique used. Inoculation also has a specific meaning for procedures done in vitro. These include the transfer of microorganisms into and from laboratory apparatus such as test tubes and petri dishes in research and diagnostic laboratories, and also in commercial applications such as brewing, baking and the production of antibiotics. In almost all cases the material inoculated is called the inoculum, or less commonly the inoculant, although the term culture is also used for work done in vitro.
______
Active Immunity in neonates:
Neonates are capable of generating both humoral and cellular immune responses to pathogens at the time of birth. Active immunity in the newborn includes the full range of B-cell responses including the production of IgM, IgG, and secretory and monomeric IgA, as well as the development of helper T-cell (Th) and cytotoxic T-cell responses. In addition, neonates can produce specific Th-cell subsets, including Th1-type cells that participate in cell-mediated immune responses and Th2-type cells that are primarily involved in promoting B-cell responses. The development of active humoral and cellular immune responses in the newborn is necessary to meet the tremendous number of environmental challenges encountered from the moment of birth. When children are born, they emerge from the relatively sterile environment of the uterus into a world teeming with bacteria and other microorganisms. Beginning with the birth process, the newborn is exposed to microbes from the mother’s cervix and birth canal, then the surrounding environment. Within a matter of hours, the gastrointestinal tract of the newborn, initially relatively free of microbes, is heavily colonized with bacteria. The most common of these colonizing bacteria include facultative anaerobic bacteria, such as Escherichia coli and streptococci, and strict anaerobic bacteria, such as Bacteroides and Clostridium. Specific secretory IgA responses directed against these potentially harmful bacteria are produced by the neonate’s intestinal lymphocytes within the first week of life.
_
Functional Differences between Infant and Adult Immune Responses:
Although infants can generate all functional T-cells (i.e., Th1, Th2, and cytotoxic T-cells), infant B-cell responses are deficient when compared with older children and adults. Infants respond well to antigens (such as proteins) that require T-cell help for development. However, until about 2 years of age, the B-cell response to T-cell-independent antigens (such as polysaccharides) is considerably less than that found in adults. For this reason, infants are uniquely susceptible to bacteria that are coated with polysaccharides (such as Haemophilus influenzae type b [Hib] and Streptococcus pneumoniae).
_
Immune response to vaccines by neonates:
The neonate is capable of mounting a protective immune response to vaccines within hours of birth. For example, neonates born to mothers with hepatitis B virus infection mount an excellent protective immune response to hepatitis B vaccine given at birth, even without additional use of hepatitis B virus-specific immunoglobulin. In addition, BCG vaccine given at birth induces circulating T-cells that protect against bacteremia and subsequent development of miliary tuberculosis and tuberculous meningitis.
_
Immune response to vaccines by infants:
The young infant is fully capable of generating protective humoral and cellular immune responses to multiple vaccines simultaneously. Approximately 90% of infants develop active protective immune responses to the primary series of diphtheria-tetanus-acellular-pertussis, hepatitis B, pneumococcus, Hib, and inactivated polio vaccines given between 2 months and 6 months of age. To circumvent the infant’s inability to mount T-cell-independent B-cell responses, polysaccharide vaccines (Hib and S pneumoniae) are linked to proteins (i.e., diphtheria toxoid, diphtheria toxin mutant protein, tetanus toxoid, or meningococcal group B outer-membrane protein) that engage the infant’s Th-cells. By converting a T-cell-independent immune response to a T-cell-dependent response, conjugate vaccines can be recognized by the infant’s B-cells. Conjugate vaccines, therefore, induce protective immune responses in infants that are often greater than those found after natural infection. Bacterial polysaccharide-protein conjugate vaccines (Haemophilus influenzae type b [Hib], pneumococcal and meningococcal conjugates) have revolutionized pediatric vaccination strategies. The widely used carrier proteins are tetanus toxoid (TT), diphtheria toxoid (DT) and diphtheria toxoid variant CRM197 protein.
_
Immune response to vaccines by children with immunodeficiency:
Severely immunocompromised children (specifically, those with T-cell defects) who receive live viral vaccines (e.g., measles or varicella vaccines) or live bacterial vaccines (e.g., BCG vaccine) may develop disseminated infections with these attenuated pathogens. However, the only live vaccine that was routinely given in the United States in the first year of life, the oral polio vaccine (OPV), has now been replaced with inactivated polio vaccine. Therefore, children do not receive their first live viral vaccines until about 12 to 15 months of age. Most children with severe T-cell deficiencies (e.g., severe combined immunodeficiency syndrome) will have been identified by 6 to 8 months of age. However, many children with immunodeficiencies respond well to live viral vaccines. Because the risk of severe infection is greater after natural infection with wild-type viruses than immunization with highly attenuated viruses, the Advisory Committee on Immunization Practices and American Academy of Pediatrics recommend that certain immunocompromised children should receive live viral vaccines. For example, children with human immunodeficiency virus (HIV) infection without severe T-cell deficiencies (Centers for Disease Control and Prevention class N1 or A1 and age-specific percentage of CD4+ lymphocytes greater than 25%) should receive the measles-mumps-rubella (MMR), and varicella vaccines. Immunizations are well-tolerated by this subset of HIV-infected children and confer protective immunity. Immunization with live viral vaccines has also been demonstrated to be safe and effective in certain children with malignancies and in children following bone marrow transplantation.
_
Immune response to vaccine by children with mild, moderate or severe illnesses:
Some parents may be concerned that children with acute illnesses are, in a sense, immunocompromised, and that they are less likely to respond to vaccines or more likely to develop adverse reactions to vaccines than healthy children. Alternatively, parents may believe that children who are ill should not further burden an immune system already committed to fighting an infection. However, vaccine-specific antibody responses and rates of vaccine-associated adverse reactions of children with mild or moderate illnesses are comparable to those of healthy children. For example, the presence of upper respiratory tract infections, otitis media, fever, skin infections, or diarrhea does not affect the level of protective antibodies induced by immunization. Data on the capacity of vaccines to induce protective immune responses in children with severe infections (such as those with bacterial pneumonia or meningitis) are lacking. Although a delay in vaccines is recommended for children with severe illnesses until the symptoms of illness resolve, this recommendation is not based on the likelihood that the child will have an inadequate immune response to the vaccine. Rather, the reason for deferring immunization is to avoid superimposing a reaction to the vaccine on the underlying illness or to mistakenly attribute a manifestation of the underlying illness to the vaccine.
_______
_______
Do vaccines overwhelm the immune system of a child?
1. Infants have the capacity to respond to an enormous number of antigens:
Studies on the diversity of antigen receptors indicate that the immune system has the capacity to respond to extremely large numbers of antigens. Current data suggest that the theoretical capacity determined by diversity of antibody variable gene regions would allow for as many as 109 to 1011 different antibody specificities. But this prediction is limited by the number of circulating B cells and the likely redundancy of antibodies generated by an individual. A more practical way to determine the diversity of the immune response would be to estimate the number of vaccines to which a child could respond at one time. If we assume that 1) approximately 10 ng/mL of antibody is likely to be an effective concentration of antibody per epitope (an immunologically distinct region of a protein or polysaccharide), 2) generation of 10 ng/mL requires approximately 103 B-cells per mL, 3) a single B-cell clone takes about 1 week to reach the 103 progeny B-cells required to secrete 10 ng/mL of antibody (therefore, vaccine-epitope-specific immune responses found about 1 week after immunization can be generated initially from a single B-cell clone per mL), 4) each vaccine contains approximately 100 antigens and 10 epitopes per antigen (i.e., 103 epitopes), and 5) approximately 107 B cells are present per mL of circulating blood, then each infant would have the theoretical capacity to respond to about 10,000 vaccines at any one time (obtained by dividing 107 B cells per mL by 103 epitopes per vaccine). Of course, most vaccines contain far fewer than 100 antigens (for example, the hepatitis B, diphtheria, and tetanus vaccines each contain 1 antigen), so the estimated number of vaccines to which a child could respond is conservative. But using this estimate, we would predict that if 11 vaccines were given to infants at one time, then about 0.1% of the immune system would be “used up.” However, because naive B- and T-cells are constantly replenished, a vaccine never really “uses up” a fraction of the immune system. For example, studies of T-cell population dynamics in HIV-infected patients indicate that the human T-cell compartment is highly productive. Specifically, the immune system has the ability to replenish about 2 billion CD4+ T lymphocytes each day. Although this replacement activity is most likely much higher than needed for the normal (and as yet unknown) CD4+ T-cell turnover rate, it illustrates the enormous capacity of the immune system to generate lymphocytes as needed.
_
2. Children are exposed to fewer antigens in Vaccines today than in the past:
Parents who are worried about the increasing number of recommended vaccines may take comfort in knowing that children are exposed to fewer antigens (proteins and polysaccharides) in vaccines today than in the past. Although we now give children more vaccines, the actual number of antigens they receive has declined.
_
Number of Vaccines and Possible Number of Injections over the Past 100 Years
| Year | Number of Vaccines | Possible Number of Injections by 2 Years of Age | Possible Number of Injections at a Single Visit |
| 1900 | 1 | 1 | 1 |
| 1960 | 5 | 8 | 2 |
| 1980 | 7 | 5 | 2 |
| 2000 | 11 | 20 | 5 |
_
Whereas previously 1 vaccine, smallpox, contained about 200 proteins, now the 11 routinely recommended vaccines contain fewer than 130 proteins in total. Two factors account for this decline: first, the worldwide eradication of smallpox obviated the need for that vaccine, and second, advances in protein chemistry have resulted in vaccines containing fewer antigens (e.g., replacement of whole-cell with acellular pertussis vaccine).
_
3. Researchers discovered that:
•In each cubic meter of air, there are between 1.6 million and 40 million viruses.
•In each cubic meter of air, there are between 860,000 and 11 million bacteria.
A child inhales about 5 liters of air per minute (or about .005 cubic meters), so a few hundred thousand viruses and bacteria are inhaled every minute every day of the year. And the researchers discovered that many were unknown species of viruses and bacteria, so the immune system has to adapt to them with each breath. Thus, the 25-30 antigens from vaccines (depending on the age of the child and the number of different flu vaccines that they’ve received) is not even a significant number compared to the millions upon millions of viral and bacterial antigens that enter a child’s lungs every day or week. The tiny number of antigens introduced by vaccines barely register on the immune system’s massive and robust power to deal with antigens.
_
4. Children respond to Multiple Vaccines given at the same time in a manner similar to Individual Vaccines:
If vaccines overwhelmed or weakened the immune system, then one would expect lesser immune responses when vaccines are given at the same time as compared with when they are given at different times. However, the following vaccines induce similar humoral immune responses when given at the same or different times: 1) MMR and varicella, 2) MMR, diphtheria-tetanus-pertussis (DTP), and OPV, 3) hepatitis B, diphtheria-tetanus, and OPV, 4) influenza and pneumococcus, 5) MMR, DTP-Hib, and varicella, 6) MMR and Hib, and 7) DTP and Hib. Achieving similar immune responses by giving vaccines at the same time at different sites may be more easily accomplished than by combining vaccines in the same syringe. Challenges to giving many vaccines in a single injection are based partly on incompatibilities of agents used to buffer or stabilize individual vaccines.
_______
______
Do Vaccines weaken the immune system? Do Vaccines increase the risk of other infections?
Vaccines may cause temporary suppression of delayed-type hypersensitivity skin reactions or alter certain lymphocyte function tests in vitro. However, the short-lived immunosuppression caused by certain vaccines does not result in an increased risk of infections with other pathogens soon after vaccination. Vaccinated children are not at greater risk of subsequent infections with other pathogens than unvaccinated children. On the contrary, in Germany, a study of 496 vaccinated and unvaccinated children found that children who received immunizations against diphtheria, pertussis, tetanus, Hib, and polio within the first 3 months of life had fewer infections with vaccine-related and -unrelated pathogens than the nonvaccinated group. Bacterial and viral infections, on the other hand, often predispose children and adults to severe, invasive infections with other pathogens. For example, patients with pneumococcal pneumonia are more likely to have had a recent influenza infection than matched controls. Similarly, varicella infection increases susceptibility to group A β-hemolytic streptococcal infections such as necrotizing fasciitis, toxic shock syndrome, and bacteremia.
_
Immunological impediments to effective vaccination: tolerance, interference and neutralization:
Other important considerations in vaccine immunology include the phenomena of immune tolerance and immunological/antigenic interference, which can suppress or prevent development of adequate immune responses following vaccination. Immune tolerance refers to the induction of immunological non-responsiveness by repeated exposure to similar antigens, such as polysaccharide antigens; this effect is dose-dependent and may be limited in time as increasing the interval between subsequent doses can partially restore responsiveness. Immunological/antigenic interference occurs when previous or concomitant exposure to another antigen prevents the development of adequate responses to the vaccine antigen, which may be due to previous or concurrent vaccinations. Another potential cause of reduced vaccine efficiency is the presence of passively induced immunity e.g. which transferred from mother to foetus, where the vaccine antigen is neutralised by pre-existing maternally-derived antibody without triggering a host-derived immune response in the infant. These phenomena can be avoided, however, by taking them into account in immunisation schedules.
_
Gut microbiome and vaccines:
Your body contains about 100 trillion bacteria, and bacteriophages (viral components) outnumber the bacteria by 10 to one. All of these bacteria, viruses, and other microorganisms make up your body’s microbiome. While we commonly view all viruses as “bad,” this is really not the case. Some viruses, known as bacteriophages, appear to promote health by infecting and killing bacteria that might otherwise cause disease. There’s a broad and compelling scientific base of evidence showing that a healthy human immune system is the most powerful way to resist infectious diseases or heal after infection and the efficient functioning of your immune system is dependent on gut flora. About 80 percent of your immune system is in your microbiome. Understanding how gut microbes might modulate vaccine responses could help improve the efficiency of certain vaccinations. Clinical trials testing the efficacy of oral vaccines against polio, rotavirus, and cholera have showed a lower immunogenicity of these vaccines in individuals from developing countries when compared to individuals from the developed world. Clinical trials for a killed oral cholera vaccine in Swedish and Nicaraguan children have also shown blunted antibody responses in Nicaraguan children compared to Swedish children. In a study testing a live cholera oral vaccine, Lagos and colleagues demonstrated that excessive bacterial growth in the small intestine of children in less developed countries might contribute to the low antibody response to the vaccine. Different vaccine strains of Shigella flexneri also showed differential protection on individuals from developing countries. In a study testing Bangladeshi adults and children, no significant immune response to this vaccine was mounted, although the same antigen was reactogenic in North American individuals. A recent review article concluded that the composition of your gut microbiome can influence whether a vaccine has an effect in your body. Unhealthy gut microbiome composition (or “dysbiosis”) can lead to inflammation. And that means more bacterial cells pass through the damaged lining of the gut, which stimulates further immune system responses. This is called “leaky gut.” Vaccines may not be as effective because the immune system is already busy dealing with these bacterial cells “leaking” through the gut. On the other hand, having a diverse and “healthy” gut microbiome, and thus no gut inflammation and “leakiness,” might allow a person’s immune system to focus on responding to the vaccine effectively. It is also possible that the gut microbiota of individuals with increased exposure to microorganisms (and therefore antigens) make them more tolerant to vaccination, being unable to mount a proper response compared to individuals living in better socioeconomic conditions. Recent research has also found that the effectiveness of the seasonal flu shot could be enhanced by intestinal bacteria. The immune system detects specific proteins from the bacteria, and this detection seems to increase the immune system’s response to the flu vaccine. Then your body has an easier time mounting an immune response if you are exposed to the real flu virus.
_
Ideal vaccine:
Properties of an ideal vaccine
• Should give life-long immunity
• Should be broadly protective against all variants of an organism
• Should prevent disease transmission, e.g. by preventing shedding
• Should induce effective immunity rapidly
• Should be effective in all vaccinated subjects, including infants and the elderly
• Should transmit maternal protection to the fetus
• Requires few (ideally one) immunisations to induce protection
• Would not need to be administered by injection
• Should be cheap, stable (no requirement for cold chain), and safe
An ideal vaccine is relatively easy to define, but few real vaccines approach the ideal and no vaccines exist for many organisms, for which a vaccine is the only realistic protective strategy in the foreseeable future. Many difficulties account for the failure to produce these vaccines. All micro-organisms deploy evasion mechanisms that interfere with effective immune responses and, for many organisms, it is not clear which immune responses provide effective protection. It is easy to define the properties of an ideal vaccine. Most of these are obvious, but few vaccines approach the ideal. In addition, vaccines do not yet exist for many organisms and it is worth considering why this is so. First it is notable that most successful vaccines are against relatively small organisms. There are excellent vaccines against several viruses and some against bacteria, although several of these do not protect against infection but rather the toxic effects of infection. As yet there are no satisfactory vaccines against parasites. Generally, therefore, successful vaccines are against organisms with smaller genomes although there are of course exceptions to this general rule, for example so far we do not have an effective vaccine against HIV or hepatitis C. Without prior immunisation, most organisms gain a foothold in their host but from very early on in the infectious process must deploy mechanisms to interfere with the host immune response. Even those organisms that rely on rapid multiplication and spread to new hosts must combat innate (non-specific) immune mechanisms. Organisms with a life-style involving co-existence with their host over long periods have also to combat the adaptive (specific) immune response. Thus all micro-organisms have evolved complex defense mechanisms that interfere with every stage of the immune response. Organisms with large genomes have sufficient genetic capacity to carry multiple genes capable of affecting immune response. The sheer magnitude of the enterprise involved in working out all these mechanisms means that there is more complete information available for smaller organisms. A number of viruses have been well studied. Numerous viral gene products that interfere in immune function have been described. These include a large variety of molecules that mimic important regulatory molecules of the immune system, such as interferons, interleukins and chemokines and their receptors. Interference with antigen processing is common and viruses may also prevent apoptosis. Genes dedicated to viral escape may represents at least 10% of viral genomes, indicating the potential magnitude of the task involved in understanding how a complex organism such as a bacterium avoids elimination by the immune system since 10% of a bacterial genome might be 200–400 genes! Smaller organisms do not have the luxury of devoting tens or hundreds of genes to combating the immune system and must adopt other strategies, one of which is rapid change. Many viruses use this method including influenza, HIV and hepatitis C. Larger organisms also employ this strategy including malaria. Most often, the variation takes place after infection of the host. Of course if the organism has a secondary host, change may take place during infection of this species as is thought to occur in the case of influenza virus. Pre-existing immunity can prevent the opportunity for multiplication and development of escape variants such as has been well described for HIV. Thus immunisation against an epidemic strain of influenza virus can provide very effective preventive immunity against spread of that strain but not against future variants. The ability of micro-organisms to deploy escape mechanisms even early in immune responses suggests that, for organisms so far insusceptible to vaccines, we need to decide what the vaccine is intended to do. Do we wish to prevent infection completely, or simply suppress replication of the organisms to an extent compatible with a normal life-span? Is prevention of transmission to other and perhaps more susceptible individuals (for example infants) the objective, or is the aim not the prevention of infection but pathology? Recent understanding of the complex interactions of micro-organisms with their hosts suggests that if we are to make progress in containing many infectious diseases caused by complex organisms, we should better define our objective and tailor our vaccine strategies accordingly. Better understanding of the crucial events in immune responses will help in doing this and may lead to development of new vaccines capable of combating infections in very different ways.
_
Bacterial pathogen genomics and vaccines:
Infectious diseases remain a major cause of deaths and disabilities in the world, the majority of which are caused by bacteria. Although immunisation is the most cost effective and efficient means to control microbial diseases, vaccines are not yet available to prevent many major bacterial infections. Examples include dysentery (shigellosis), gonorrhoea, trachoma, gastric ulcers and cancer (Helicobacter pylori). Improved vaccines are needed to combat some diseases for which current vaccines are inadequate. Tuberculosis, for example, remains rampant throughout most countries in the world and represents a global emergency heightened by the pandemic of HIV. The availability of complete genome sequences has dramatically changed the opportunities for developing novel and improved vaccines and facilitated the efficiency and rapidity of their development. Complete genomic databases provide an inclusive catalogue of all potential candidate vaccines for any bacterial pathogen. In conjunction with adjunct technologies, including bioinformatics, random mutagenesis, microarrays, and proteomics, a systematic and comprehensive approach to identifying vaccine discovery can be undertaken. Genomics must be used in conjunction with population biology to ensure that the vaccine can target all pathogenic strains of a species. A proof in principle of the utility of genomics is provided by the recent exploitation of the complete genome sequence of Neisseria meningitidis group B.
_
In a nutshell:
The human immune system consists of two connected compartments, the innate and the adaptive immune systems which function via the actions of secreted and cellular effectors. The innate and the adaptive immune systems work sequentially to identify invaders and formulate the most appropriate response; this interaction is crucially bridged by specialised antigen-presenting cells (APCs). The innate response, via the action of APCs, sets the scene for the subsequent adaptive response by providing information about the nature of the threat. Primary exposure to a pathogen or antigen induces the production of a population of adaptive immune cells with antigen specificity that are retained for long periods and provide a rapid response upon subsequent exposure. The vaccine concept is based on stimulating the body’s defense mechanisms against a specific pathogen to establish this immunological memory. Current vaccine strategies take advantage of immunological mechanisms, and often target the innate immune system and APCs to induce the desired specific adaptive immune response.
________
________
How Vaccines Work:
To understand how vaccines work you need to understand the story of two 5-year-old children, John and Robert:
John plays with a child in his class who has measles. Ten days later, John develops high fever, runny nose, “pink eye” and a rash. The rash consists of red bumps that start on his face and work their way down to the rest of his body. After two more days, John starts to have trouble breathing. His breaths are short and rapid. John’s mother takes him to the doctor where he gets an X-ray of his chest. The X-ray shows that John has pneumonia (a common complication of measles infection). John is admitted to the hospital where he stays for five days and finally recovers. After having fought off his measles infection, John will never get measles again. Or, said another way, John has immunity to measles. John is immune to measles because he has cells in his body that can make “antibodies” to measles virus. These cells, called “memory B cells,” developed during the infection, and will hang around for the rest of John’s life. Robert also plays with the child who has measles. However, Robert never develops symptoms of measles. He doesn’t get fever, rash or pneumonia. Robert was infected with measles virus, but didn’t get any of the symptoms of measles. This is called an “asymptomatic infection.” Because Robert, like John, also develops “memory B cells,” he too is immune to measles for the rest of his life. Whereas John had to pay a high price for his immunity, Robert didn’t. Robert was lucky. Although some children don’t get severe infections when they are exposed to measles, most do. Before a measles vaccine was developed in 1963, measles would infect about 4 million children each year and kill 3,000.
Vaccines take the luck out of it:
By causing “asymptomatic infections,” vaccines mimic what happened to Robert. This allows children to benefit from the natural immunity that comes with infection without having to suffer the severe, and occasionally fatal, consequences of natural infection. Vaccines remove the element of luck by controlling:
•The potential severity of the pathogen
•The dose of the exposure (smallest amount needed)
•The timing of exposure (before the period of highest risk)
_
Vaccines work at an individual level to protect the immunized person against the specific disease, as well as at a population level to reduce the incidence of the disease in the population, thereby reducing exposure of susceptible persons and consequent illness. Although the primary measure of effectiveness occurs at an individual level, there is also interest in decreasing or even eliminating disease at a population level.
_
How vaccines work at the individual level:
The administration of a vaccine antigen triggers an inflammatory reaction that is initially mediated by the innate immune system and subsequently expands to involve the adaptive immune system through the activation of T and B cells. While the majority of vaccines provide protection through the induction of humoral immunity (primarily through B cells), some vaccines such as Bacille Calmette-Guerin (BCG) and herpes zoster act principally by inducing cell-mediated immunity (primarily though T cells). Long-term immunity requires the persistence of antibodies, and/or the creation and maintenance of antigen-specific memory cells (priming), that can rapidly reactivate to produce an effective immune response upon subsequent exposure to the same or similar antigen.
_
Immunogenicity and markers of protection induced by vaccination:
Immunogenicity means the vaccine’s ability to induce an immune response. Vaccine-induced seroconversion is the development of detectable antigen-specific antibodies in the serum as a result of vaccination; seroprotection is a predetermined antibody level as a result of vaccination, above which the probability of infection is low. The seroprotective antibody level differs depending on the vaccine. A correlate of protection is a specific immune response that is responsible for and statistically linked to protection against infection or disease. Following administration of most vaccines, prevention of infection has been shown to correlate predominantly with the production of antigen-specific antibodies. Serologic markers can be measured using enzyme-linked immunosorbent assays (ELISA), functional antibody activity such as the opsonophagocytic assay (OPA), or both. A surrogate of protection is a substitute immune marker, which may not be linked to protection against infection or disease. For example, serum antibodies may be produced for mucosal vaccines against rotavirus. Although serum antibodies against rotavirus serve as surrogates of protection, they are not necessarily directly protective against infection as this may require mucosal antibodies.
_
How vaccines work at the population level:
Vaccine efficacy:
Vaccine efficacy is defined as the reduction in the incidence of a disease among people who have received a vaccine compared with the incidence in unvaccinated people. Vaccine efficacy refers to the vaccine’s ability to prevent illness in people vaccinated in controlled studies. Vaccine effectiveness refers to the vaccine’s ability to prevent illness in people vaccinated in broader settings (i.e., the “real world”).
_
_
Measuring protection: efficacy versus effectiveness:
In the clinical development of a vaccine, an efficacy study asks the question “Does the vaccine work?” In contrast, an effectiveness study asks the question “Does vaccination help people?” In general, vaccine development proceeds from a study of immunogenicity to a randomized controlled trial that determines vaccine efficacy under ideal conditions. Efficacy studies, however, have several limitations. In an immunogenicity study, when a vaccine is given according to different schedules, the object of the study is not the vaccine itself but the schedules; i.e., what is important is not the “relative immunogenicity” of the vaccine, but which schedule is more protective given the occurrence of the disease that is to be prevented. Furthermore, a clinical trial of vaccine efficacy is unable to predict accurately the level of protection that will be achieved in public health practice. Vaccination effectiveness can be evaluated in a prospective clinical trial, although few such studies have been undertaken. Effectiveness is usually assessed retrospectively, sometimes using a screening test, but more often in a case-control or cohort study. In these studies, rigorous risk adjustment is necessary to ensure the comparability of study populations. Retrospective studies also provide a means for assessing serious but rare vaccine-associated adverse events, an undertaking often needed to maintain public confidence in vaccination programs.
_
Immunization and Herd Immunity:
R0 is the average number of secondary cases produced by a primary case in a wholly susceptible population. Clearly, an infection cannot maintain itself or spread if R0 is less than 1. The larger the value of R0, the harder it is to eradicate the infection from the community in question. A rough estimate of the level of immunization coverage required can be estimated in the following manner: eradication will be achieved if the proportion immunized exceeds a critical value Vc = 1-1/R0. For example, measles has an estimated R0 of 15; therefore, at least 94% (1 minus 1/15 = 94%) of the population needs to be immune to prevent transmission of measles. Thus the larger the R0, the higher the coverage is required to eliminate the infection. Thus the global eradication of measles, with its R0 of 10 to 20 or more, is almost sure to be more difficult to eradicate than smallpox, with its estimated R0 of 4 to 5. Another example is rubella and measles immunization in the US. Rubella has an R0 roughly half that of measles and indeed rubella has been effectively eradicated in the US while the incidences of measles have declined more slowly. Immunization (vaccine) coverage refers to the proportion of the population (either overall or for particular risk groups) that has been immunized against a disease. High immunization coverage is especially required for diseases that have a high reproduction number (R0) to prevent further transmission.
_
Herd immunity:
Herd immunity or herd effect, also called community immunity, population immunity, or social immunity, describes a form of indirect immunity that occurs when large percentages of a population have become immune to an infectious disease, thereby providing a measure of protection for individuals who are not immune. In a population in which a large number of individuals are immune, chains of infection are likely to be disrupted, stopping or slowing the spread of disease. The greater the proportion of individuals in a community who are immune, the smaller the probability that those who are not immune will come into contact with an infectious individual. An individual’s immunity can be gained through recovering from a natural infection or through artificial means such as vaccination. Some individuals cannot become immune due to medical reasons, so it is important to develop herd immunity to protect these individuals. Once a certain threshold has been reached, herd immunity will gradually eliminate a disease from a population. This elimination, if achieved worldwide, results in the eradication of the disease. Herd immunity does not apply to all diseases, just those that are contagious, meaning that they can be transmitted from one individual to another. Tetanus, for example, is infectious but not contagious, so herd immunity does not apply to it. The term “herd immunity” is widely used but carries a variety of meanings. Some authors use it to describe the proportion immune among individuals in a population. Others use it with reference to a particular threshold proportion of immune individuals that should lead to a decline in incidence of infection. Still others use it to refer to a pattern of immunity that should protect a population from invasion of a new infection. A common implication of the term is that the risk of infection among susceptible individuals in a population is reduced by the presence and proximity of immune individuals (this is sometimes referred to as “indirect protection” or a “herd effect”).
_
The top box shows an outbreak in a community in which a few people are ill (shown in red) and the rest are healthy but unimmunized (shown in blue); the illness spreads freely through the population. The middle box shows the same population where a small number have been immunized (shown in yellow); those immunized are unaffected by the illness, but others are not. In the bottom box, a critical proportion of the population has been immunized; this prevents the illness from spreading significantly, even to unimmunized people.
_
Mathematical model of herd immunity:
An important milestone was the recognition by Smith in 1970 and Dietz in 1975 of a simple threshold theorem—that if immunity (i.e., successful vaccination) were delivered at random and if members of a population mixed at random, such that on average each individual contacted R0 individuals in a manner sufficient to transmit the infection, then incidence of the infection would decline if the proportion immune exceeded (R0 − 1)/R0, or 1 –1/R0. This is illustrated in two figures below.
_
Definitions of Terms:
| Term | Symbolic Expression | Definition |
| Basic reproduction number | R0 | Number of secondary cases generated by a typical infectious individual when the rest of the population is susceptible (i.e., at the start of a novel outbreak) |
| Critical vaccination level | Vc | Proportion of the population that must be vaccinated to achieve herd immunity threshold, assuming that vaccination takes place at random |
| Vaccine effectiveness against transmission | E | Reduction in transmission of infection to and from vaccinated compared with control individuals in the same population (analogous to conventional vaccine efficacy but measuring protection against transmission rather than protection against disease). |
_
Diagram above illustrating transmission of an infection with a basic reproduction number R0 = 4 in A, Transmission over 3 generations after introduction into a totally susceptible population (1 case would lead to 4 cases and then to 16 cases). B, Expected transmissions if (R0 − 1)/R0 = 1 − 1/R0 = ¾ of the population is immune. Under this circumstance, all but 1 of the contacts for each case is immune, and so each case leads to only 1 successful transmission of the infection. This implies constant incidence over time. If a greater proportion are immune, then incidence will decline. On this basis, (R0 − 1)/R0 is known as the “herd immunity threshold.” When a critical proportion of the population becomes immune, called the herd immunity threshold (HIT) or herd immunity level (HIL), the disease may no longer persist in the population, ceasing to be endemic.
_
Much of the early theoretical work on herd immunity assumed that vaccines induce solid immunity against infection and that populations mix at random, consistent with the simple herd immunity threshold for random vaccination of Vc = (1−1/R0), using the symbol Vc for the critical minimum proportion to be vaccinated (assuming 100% vaccine effectiveness). More recent research has addressed the complexities of imperfect immunity, heterogeneous populations, nonrandom vaccination, and “freeloaders”. Assuming a vaccine is 100% effective, then the equation used for calculating the herd immunity threshold can be used for calculating the vaccination level needed to eliminate a disease, written as Vc. Vaccines are usually imperfect however, so the effectiveness, E (E standing for effectiveness against infection transmission in the field) of a vaccine must be accounted for:
_
From this equation, it can be observed that if E is less than (1 – 1/R0), then it will be impossible to eliminate a disease even if the entire population is vaccinated. Similarly, waning vaccine-induced immunity, as occurs with acellular pertussis vaccines, requires higher levels of booster vaccination in order to sustain herd immunity. Important among illustrations of this principle are the shifts to multiple doses (up to 20) and to monovalent vaccines in the effort to eliminate polio in India, where the standard trivalent oral polio vaccines and regimens produce low levels of protection. If a disease has ceased to be endemic to a population, then natural infections will no longer contribute to a reduction in the fraction of the population that is susceptible; only vaccination will contribute to this reduction.
_
Herd immunity through vaccination:
The primary way to boost herd immunity is through the use of vaccine. Their use is originally based on the observation that milkmaids exposed to cowpox were immune to smallpox, so the practice of inoculating people with the cowpox virus began as a way to prevent smallpox cases from occurring. Well-developed vaccines provide this protection in a far safer way than natural infections, as vaccines generally do not cause the diseases they protect against and severe adverse effects are significantly less common than complications from natural infections. The immune system does not distinguish between natural infections and vaccines, forming an active response to both, so immunity induced via vaccination is similar to what would have occurred from contracting and recovering from the disease. In order to achieve herd immunity through vaccination, vaccine manufacturers aim to produce vaccines with low failure rates and policy makers aim to encourage their use. After the successful introduction and widespread use of a vaccine, sharp declines in the incidence of diseases it protects against can observed, necessarily decreasing the number of hospitalizations and deaths caused by such diseases.
_
Herd immunity through passive immunization:
The transfer of maternal antibodies, primarily IgG across the placenta helps protect fetuses and newborns from disease. After birth, newborns can also acquire these antibodies from colostrum. Since these antibodies provide some degree of protection, newborns provide a slight boost to herd immunity. This boost, however, is temporary, being gradually lost as the presence of maternal antibodies wanes during the first few months of life. The presence of maternal antibodies in a newborn’s body often, but not always, adversely affects vaccine effectiveness, so additional doses are recommended for some vaccines while others are not first administered to the infant until after such antibodies are no longer present in the body. For some diseases that are particularly severe for fetuses and newborns, such as influenza and tetanus, pregnant women may be immunized in order to transfer antibodies to the child. In contrast to natural passive immunity, acquired passive immunity refers to the process of obtaining serum or plasma from immune individuals, then taking antibodies from this and injecting it to protect certain susceptible persons. High-risk groups, such as certain newborns, pregnant women, organ transplantation recipients, and the immunocompromised, including HIV-seropositive individuals, may receive antibody preparations to prevent infections or to reduce the severity of symptoms. As with natural passive immunity, protection is immediate but wanes over time. Because antibody preparations are capable of producing a certain degree of herd immunity, they have been used to control disease outbreaks.
_
Many examples of herd immunity have been described, illustrating the importance of indirect protection for predicting the short- and long-term impact of vaccination programs, for justifying them economically, and for understanding the nature of the immunity induced by various vaccines. Among the classic examples was the recognition that periodic epidemics of ubiquitous childhood infections such as measles, mumps, rubella, pertussis, chickenpox, and polio, arose because of the accrual of a critical number of susceptible individuals in populations and that epidemics could be delayed or averted by maintaining numbers of susceptible individuals below this critical density (i.e., by maintaining the proportion immune above some threshold). Impressive examples of indirect protection have been observed after the introduction of conjugate vaccines against pneumococcal and Haemophilus infections. Reductions in disease incidence among cohorts too old to have been vaccinated have been responsible for one- to two-thirds of the total disease reduction attributable to these vaccines in some populations. These are due to the ability of conjugate vaccines to protect vaccinees not only against disease but also against nasal carriage, and hence infectiousness.
_
Selective vaccination of groups that are important in transmission can slow transmission in general populations or reduce incidence among population segments that may be at risk of severe consequences of infection. Schools play an important role in community transmission of influenza viruses, and thus there has been discussion of slowing transmission either by closing schools or by vaccinating schoolchildren. Selective vaccination of schoolchildren against influenza was policy in Japan during the 1990s and was shown to have reduced morbidity and mortality among the elderly. Analogous issues relate to vaccination against rubella and human papillomavirus (HPV) in males; for each of these examples the consequences of infection (with rubella or HPV) in males are relatively minor, so the policy issue becomes whether vaccination of males is warranted to protect females, and many societies have decided in favor for rubella but not for HPV. A particularly interesting example of using vaccines to reduce transmission is the potential for “transmission blocking vaccines” for malaria. These vaccines would not protect the individual recipient against infection or disease, but would produce antibodies that block life cycle stages of the malaria parasite in the mosquito. Recent work has shown the biologic feasibility of such vaccines, and models have shown their potential contribution to reducing overall transmission in malaria-endemic communities. They would thus provide the first example of a vaccine that in theory would provide no direct benefit to the recipient. Finally we may refer to eradication programs based on vaccines—globally successful in the case of smallpox and rinderpest, and at least regionally successful to date in the case of wild polio virus. The Americas have been free of wild polio virus circulation for almost 20 years, though the thresholds for herd immunity have proved more elusive in parts of Asia and Africa. Each of these programs has used a combination of routine vaccination, itself successful in some populations, supplemented by campaigns in high-risk regions and populations in order to stop the final chains of transmission. Such examples illustrate how the direct effect of immunity (i.e., successful vaccination) in reducing infection or infectiousness in certain individuals can decrease the risk of infection among those who remain susceptible in the population. Importantly, it is a vaccine’s effect on transmission that is responsible for the indirect effect. If the only effect of a vaccine were to prevent disease but not to alter either the risk of infection or infectiousness, then there would be no indirect effect, and no herd immunity. It was once wrongly argued, for example, that inactivated polio vaccines protected only against paralysis and not against infection. We now know that this is wrong, and that inactivated polio vaccines can decrease both infection risk and infectiousness, as demonstrated in several countries that interrupted wild poliovirus transmission using only these vaccines.
_
Life cycle of virus/bacteria vis-à-vis herd immunity:
The entire concept of herd immunity fails to acknowledge that there is a life cycle of the viruses and the bacteria all on their own, and that what turns them on and off may have nothing to do with the percentage of people who have been infected. All you have to do is look at the SARS outbreak. That virus didn’t infect 70 or 80 percent of the population, which would then impart herd immunity on the 20 or 30 percent that didn’t get the disease. This is because the virus itself had a life cycle of its own. And so it came and went without any percentage of the population being protected. There wasn’t herd immunity, and yet the virus died out on its own. We fail to include that viruses have a life cycle, and that they are in relationship to other organisms and to us. Something activates them and something actually stops them, and it has nothing necessarily to do with the percentage of people who would have the illness or who have been vaccinated.
_
Evolutionary pressure:
Herd immunity itself acts as an evolutionary pressure on certain viruses, influencing viral evolution by encouraging the production of novel strains, in this case referred to as escape mutants, that are able to “escape” from herd immunity and replicate more easily. At the molecular level, viruses escape from herd immunity through antigenic drift, which is when mutations accumulate in the portion of the viral genome that encodes for the virus’s surface antigen, typically a protein of the virus capsid, producing a change in the viral epitope. Alternatively, the reassortment of separate viral genome segments, or antigenic shift, which is more common when there are more strains in circulation, can also produce new serotypes. When either of these occur, memory T cells no longer recognize the virus, the virus becomes resistant to certain existing antiviral drugs, and herd immunity ceases to be relevant to the dominant circulating strain. For both influenza and norovirus, epidemics temporarily induce herd immunity until a new dominant strain emerges, causing successive waves of epidemics. As this evolution poses a challenge to herd immunity, broadly neutralizing antibodies and “universal” vaccines that can provide protection beyond a specific serotype are in development.
_
Why emergence of strains of pathogen resistant to vaccines is rare?
Disease control exerts evolutionary pressures that can lead to the evolution of resistance. This has been seen in a spectacular fashion in the evolution of resistance to antibiotics, anti-virals and anti-parasitics. Despite intense (and often successful) attempts to control infectious diseases through vaccination, there is still rather little evidence of the emergence of strains of pathogen resistant to vaccines. If vaccine induced immunity is less cross-reactive than naturally acquired immunity, there may be a level of vaccine coverage above which a vaccine resistant strain will emerge as a result of the vaccination campaign. This situation is illustrated in following example. Vaccination begins at time 3 years. There follows a period of very low incidence (the honeymoon period) before epidemics of the wild-type strain restart. Note that vaccine efficacy remains at 80% during these post-honeymoon epidemics. The post honeymoon epidemic that starts at time 15 years is a result of the slow accumulation of unvaccinated susceptible. A small number of those who have been vaccinated are also infected because of the incomplete protection conferred by the vaccine. Several decades later, a much larger epidemic occurs and at the same time vaccine efficacy plummets. The vaccine resistant strain has achieved competitive dominance as a result of the growing number of vaccinated individuals. These vaccinated people are well protected against wild-type strain, but have only minimal protection against the vaccine resistant strain. The vaccinated reproductive rate for the vaccine resistant strain is larger than that for the wild-type strain. It takes several decades of accumulation of vaccinated people before this shift in competitive advantage manifests itself in epidemics of the vaccine resistant strain. It is not, however, an unavoidable consequence of vaccination. Highly cross-reactive and immunogenic vaccines can lead to the eradication of both strains at coverage levels below those at which the vaccine resistant strain gains the competitive advantage. A vaccine with greater cross reactivity will not face these problems. Alternatively, low levels of vaccination leave the wild-type strain the competitive superior. If vaccination coverage had been much lower, the vaccine resistant strain would never have gained the competitive advantage. Thus, there are three possible explanations why we have not seen outbreaks of vaccine resistance in response to the major vaccination campaigns against childhood infectious diseases. The first is that we haven’t yet reached several decades of post-vaccination period. The second is that vaccine coverage is too low to give the competitive advantage to resistant strains. The third is that current vaccines give enough cross-immunity so that resistant strains will never emerge.
_
Free riding:
Herd immunity is a public good because it is non-excludable, meaning that there is no way to exclude people from using it, and non-rivalrous, meaning that one person’s use of herd immunity does not restrict others’ use of it. Like other public goods, herd immunity is vulnerable to the free rider problem. Individuals who lack immunity, primarily those who choose not to vaccinate, free ride off of the herd immunity created by those who are immune, enabling them to benefit from herd immunity without contributing to it. Not all free riders are adamantly opposed to vaccination, some may just be hesitant to vaccinate. As the number of free riders in a population increases, outbreaks of preventable diseases become more common. Individuals may choose to free ride for a variety of reasons, including bandwagoning or groupthinking, social norms or peer pressure, religious beliefs, the perceived effectiveness of a vaccine, mistrust of vaccines or public health officials, and flawed assessment of infection and vaccine risks. Most importantly though is that individuals are more likely to free ride if vaccination rates are high enough so as to convince a person that he or she may not need to be immune since a sufficient number of others already are. This makes vaccination itself a social dilemma as individuals can benefit from being selfish by choosing not to vaccinate, but if everyone behaves in this manner, then the entire community suffers. As a major goal of public health officials is to control the spread of infectious diseases, it is necessary to deal with free riders in a responsible manner. The availability of philosophical and personal belief exemptions from vaccination significantly increases the number of free riders over time, jeopardizing herd immunity in certain communities, so efforts should be made to either prevent their use or make their use more difficult. Some free riders can be encouraged to become immune by emphasizing to them the educational, social, and economic benefits of vaccination, such as improved school attendance, decreased health care expenditures, and increased life expectancy. Likewise, encouraging altruism and social responsibility may shift some individuals from being self-interested to doing what is best for the entire community. Many nonvaccinators lack a general understanding of or are unsure about vaccines and the diseases they protect against, so education campaigns have the potential to positively influence these individuals’ vaccination decisions. Punishing nonvaccinators for not vaccinating could undermine trust between public health officials and the community, so creating incentives to become immune and rewards for doing so should be made instead. Some people may not be able to be immune due to medical reasons, in which case ideally only these individuals should be permitted to free ride.
________
My view on infectious disease eradication by vaccines:
Let me start with example.
Suppose you have a vaccine which is 100 % effective and given to 100 % population and no extra-human reservoir for microorganism exists. The logic says that in this case, disease will be eradicated as all people receive vaccine and all are protected. Eradication of a disease is a more demanding goal than control, usually requiring the reduction to zero of cases in a defined geographic area. Eradication of a disease is achieved when its elimination can be sustained without ongoing interventions. Is it possible? No. There is no vaccine that is 100 % effective. No vaccine is given to 100 % population but larger the vaccine recipient population greater the likelihood of disease eradication. Herd immunity says that once a threshold population is vaccinated, the disease outbreak will be prevented and unvaccinated individuals will be protected. If basic reproduction number Ro is less than one, the disease will die out in long run and get spontaneously eradicated without any vaccine. As Ro increases, the disease becomes highly transmissible; the likelihood of eradication fails consequently. R0 is the average number of secondary cases produced by a primary case in a wholly susceptible population. The larger the R0, the higher the vaccine coverage is required to eliminate the infection. If vaccination does not confer solid immunity against infection to all recipients, the herd threshold level of vaccination required to protect a population increases. In other words, if vaccine is not 100 % effective, you need more people to be vaccinated to confer herd immunity; but below a critical level of effectiveness, no disease eradication will be achieved even if 100 % population is vaccinated. So diseases with average R0 and effective vaccine can be eradicated (e.g. small pox). Tuberculosis cannot be eradicated as BCG is not effective. Measles cannot be eradicated despite effective vaccine as R0 is very high. In other words, disease eradication possibility is directly proportional to vaccine efficacy and vaccinated population but inversely proportional to Ro.
_
When vaccine efficacy is 100 % and vaccinated population is 100% and no extra-human reservoir for microorganism exists, disease will be eradicated irrespective of Ro.
When Ro is less than 1, disease will be eradicated irrespective of vaccination.
We are discussing diseases in-between these two extremes where this formula becomes relevant.
_
Let us discuss small pox eradication. The Ro of small pox is between 3.5 to 6 and vaccine efficacy is 95% but WHO did not need to vaccinate majority of world population to eradicate small pox because of unique features of small pox. Smallpox was totally eradicated by a lengthy and painstaking process, which identified all cases and their contacts and ensured that they were all vaccinated. Smallpox eradication was accomplished with a combination of focused surveillance—quickly identifying new smallpox cases—and ring vaccination. “Ring vaccination” meant that anyone who could have been exposed to a smallpox patient was tracked down and vaccinated as quickly as possible, effectively corralling the disease and preventing its further spread. Smallpox was a good candidate for eradication for several reasons. First, the disease is highly visible: smallpox patients develop a rash that is easily recognized. In addition, the time from exposure to the initial appearance of symptoms is fairly short, so that the disease usually can’t spread very far before it’s noticed. Workers from the World Health Organization found smallpox patients in outlying areas by displaying pictures of people with the smallpox rash and asking if anyone nearby had a similar rash. Second, only humans can transmit and catch smallpox. Some diseases have an animal reservoir, meaning they can infect other species besides humans. Yellow fever, for example, infects humans, but can also infect monkeys. If a mosquito capable of spreading yellow fever bites an infected monkey, the mosquito can then give the disease to humans. So even if the entire population of the planet could somehow be vaccinated against yellow fever, its eradication could not be guaranteed. The disease could still be circulating among monkeys, and it could re-emerge if human immunity ever waned. (The discovery of an animal reservoir for yellow fever was in fact what derailed a yellow fever eradication effort in the early 1900s.) Smallpox, however, can infect only humans. In effect, aside from the human population, it has nowhere to hide. WHO trained vaccinators quickly, and they could immunize large groups of people in a short time. Such unique features of small pox are missing in polio/measles and therefore polio/measles is not yet eradicated despite worldwide immunization and effective vaccine. Diseases that are transmissible in incubation period (e.g. measles) do not allow catching contacts fast enough for vaccination. Diseases that are invisible (e.g. 90 % patients infected with polio virus) would prevent detection of new cases and hinder vaccination efforts based on the location of new cases and potential exposure to other individuals. Measles has Ro of 14 to 18 and therefore despite effective vaccine and worldwide immunization, measles eradication is a dream.
_
Vaccine and virus mutation:
The purpose of vaccines is not to prevent a virus from mutating. The purpose of a vaccine is to prevent infection/disease by a virus. However, with certain viruses, like smallpox, effective vaccination programs can eliminate the virus before it has a chance to mutate. This works best with viruses which are slow to mutate (e.g., DNA viruses), only infect humans and cannot survive for long outside of the host. The faster a virus mutates, the more likely a person is to be reinfected (e.g., a person can catch the flu every year or potentially even multiple times within the same season) and the more often a vaccine is reformulated and administered (e.g., flu vaccine requires reformulation and readministration every year). If someone is infected, the immune system will try to stop the infection by killing the viruses. There is potential for some of the viruses to survive long enough to spread to another host. Those viruses can have some slight mutations that allowed them to survive long enough to reproduce. So, the only real way to stop a virus from mutating is to kill every last one before it can reproduce and spread to a new host. Thus far, only vaccines have allowed us to accomplish this feat (e.g., smallpox). During natural infection, it takes time for immune system to kill virus and that time allows virus multiplication and subsequent mutation and spread of mutated virus while during infection of immunized individual, the immune response is quick and robust, thereby killing viruses fast before it has chance to mutate. In other words, vaccination has a greater ability to prevent virus mutation as compared to natural infection.
______
Determinants of vaccine response in individuals:
The strength and duration of the immune system’s response to a vaccine is determined by a number of factors as outlined in Table below.
| Vaccine type | The type of vaccine antigen and its immunogenicity directly influence the nature of the immune response that is induced to provide protection:
|
| Vaccine adjuvants and carrier proteins |
|
| Optimal dose of antigen |
|
| Interval between doses |
|
| Age of vaccine recipient |
|
| Pre-existent antibodies |
|
| Status of the immune system |
|
_
Vaccine-induced seropositivity:
Vaccine-induced seropositivity or VISP is the phenomenon wherein a person who has received a vaccine against a disease would thereafter give a positive or reactive test result for having that disease when tested for it, despite not actually having the disease. This happens because many vaccines encourage the body to produce antibodies against a particular disease, and blood tests often determine whether a person has those antibodies, regardless of whether they came from the infection or just a vaccination. VISP is especially a concern in vaccine trials for HIV vaccine research because people who give a positive result in an HIV test, even if that result is because of a vaccine and not because of an infection, may face discrimination because of HIV infection.
________
Influenza vaccine:
What does vaccine “match” and “mismatch” mean?
Vaccines against seasonal influenza must be frequently updated and the process for selecting the viruses and manufacturing the influenza vaccines starts several months before the influenza season begins. Detailed, timely data on viruses that are circulating and infecting humans globally are gathered, shared among countries and scientists, and are eventually used to formulate the upcoming seasonal influenza vaccines. Influenza viruses are constantly changing, including during the time between vaccine virus selection and the influenza season. If these changes lead to antigenic differences between the circulating seasonal influenza viruses and those viruses that are included in the seasonal influenza vaccine, then the vaccine and circulating viruses may not be closely related. The degree of similarity or difference between the circulating viruses and the viruses in the vaccines is often referred to as “vaccine match” or “vaccine mismatch”.
_
Can I get vaccinated and still get the influenza?
Yes.
It is possible to get influenza-like illness even if you have been vaccinated. This is possible for the following reasons:
• Antibodies that protect against infection take approximately two weeks to develop after vaccination. You may be exposed to an influenza virus shortly before or after getting vaccinated. This exposure may result in your becoming ill before the vaccine begins to protect you.
• The influenza vaccine is made to protect against viruses that were identified in the previous influenza season as likely to become widespread. You may be exposed to a virus not included in the vaccine and develop illness.
• The effectiveness of influenza vaccines can vary widely. Moreover, a person’s susceptibility to infection and response to vaccination are influenced by numerous factors. In general, the influenza vaccine works best among healthy younger adults and older children. Influenza vaccination is not a perfect tool, but it is the best available way to protect against influenza infection.
• Respiratory pathogens that are not related to influenza viruses can cause “flu-like” symptoms. The influenza vaccine does not protect you against these pathogens. You won’t know for sure that you are infected with influenza virus unless you are tested.
_
What is the vaccine effectiveness of seasonal influenza vaccines?
The vaccine effectiveness of seasonal influenza vaccines is a measure of how well the seasonal influenza vaccine prevents influenza virus infection in the general population during a given influenza season. If the vaccine effectiveness is high, it indicates that individuals who have received the seasonal influenza vaccine are less likely to have an influenza illness. If the vaccine effectiveness is low, it indicates that the seasonal influenza vaccine may not be as likely to prevent influenza illness in the vaccinated population. It is important to remember that even with low vaccine effectiveness, substantial numbers of influenza-related illnesses can still be prevented. Each season, studies are conducted in some countries to measure the effectiveness of influenza vaccine. During seasons when most circulating influenza viruses are similar to the viruses in the influenza vaccine, the vaccine can reduce the risk of illness caused by influenza virus infection by about 50-60% among the overall population. The efficacy of influenza vaccine in the year 2014 was only 30 %.
_
M2e-based Universal Influenza Vaccines:
The successful isolation of a human influenza virus in 1933 was soon followed by the first attempts to develop an influenza vaccine. Nowadays, vaccination is still the most effective method to prevent human influenza disease. However, licensed influenza vaccines offer protection against antigenically matching viruses, and the composition of these vaccines needs to be updated nearly every year. Vaccines that target conserved epitopes of influenza viruses would in principle not require such updating and would probably have a considerable positive impact on global human health in case of a pandemic outbreak. The extracellular domain of Matrix 2 (M2e) protein is an evolutionarily conserved region in influenza A viruses and a promising epitope for designing a universal influenza vaccine. Such a universal influenza vaccine could be used to prevent seasonal influenza, provided that it proves to be non-inferior to the existing seasonal influenza vaccines that mainly rely on the induction of strain-specific virus neutralizing antibodies. M2e is a highly conserved target for universal influenza A vaccine development. Different types of M2e-based vaccine, such as DNA vaccine, protein vaccine, VLPs vaccine, and vectored vaccine, are all able to provide a certain level of broad-spectrum protection in animal models. M2e-specific antibodies, mainly IgG, are the main actors in immune protection and do so by engaging Fc Receptor expressing immune cells such as alveolar macrophages. It is also well documented that mucosal immunization with M2e-based vaccines offers better protection in mouse models compared to parenteral immunization strategies. This improved protection may be attributable to the induction of M2e-specific IgA. The infection-permissive character of M2e-based vaccines can be considered as an advantage when vaccinating immunologically naïve individuals. Because M2e-immunity does not neutralize the virus, the limited virus replication still induces cross-reactive T cell responses against other conserved viral antigens such as NP and M1. However, M2e will likely not be a complete substitute for the currently licensed influenza vaccines that are able to confer much stronger protection, be it against a very narrow antigenic range of viruses. In the future, with many other universal influenza vaccine candidates on the horizon, M2e-conjugate vaccines will likely find a place as part of a vaccine that is a blend of different conserved epitopes that together may offer strong, long lasting, and foremost broad immune protection. Whether such a vaccine will perform better clinically than a fully antigenic matched seasonal vaccine remains to be seen. However, such universal vaccines would prove their value in the case of a pandemic.
_
Polio vaccine:
Two polio vaccines are used throughout the world to combat poliomyelitis (or polio). The first was developed by Jonas Salk through the use of HeLa cells and first tested in 1952. Announced to the world by Dr Thomas Francis Jr. on 12 April 1955, it consists of an injected dose of inactivated (dead) poliovirus. An oral vaccine was developed by Albert Sabin using attenuated poliovirus. Human trials of Sabin’s vaccine began in 1957, and it was licensed in 1962. There is no long term carrier state for poliovirus in immunocompetent individuals, polioviruses have no non-primate reservoir in nature (although they have been induced in transgenic mice), and survival of the virus in the environment for an extended period of time appears to be remote. Therefore, interruption of person to person transmission of the virus by vaccination is the critical step in global polio eradication. The two vaccines have eradicated polio from most countries in the world, and reduced the worldwide incidence from an estimated 350,000 cases in 1988 to just 223 cases in 2012. This represents a 99.9% reduction, but recently there has been an alarming bounce back in some countries towards more cases. In November 2013, the World Health Organization announced a polio outbreak in Syria. May 2014, WHO declared a global health emergency for only the second time since regulations permitting it to do so were adopted in 2007, due to a spread of polio. As per the WHO, Pakistan, Syria and Cameroon have recently allowed the virus to spread—to Afghanistan, Iraq and Equatorial Guinea, respectively.
_
_
HIV vaccine:
An HIV vaccine is a vaccine which would either protect individuals who do not have HIV from contracting that virus, or otherwise may have a therapeutic effect for persons who have or later contract HIV/AIDS. Currently, there is no effective HIV vaccine but many research projects managing clinical trials seek to create one. There is evidence that a vaccine may be possible. Work with monoclonal antibodies (MAb) has shown or proven that the human body can defend itself against HIV, and certain individuals remain asymptomatic for decades after HIV infection. Potential candidates for antibodies and early stage results from clinical trials have been announced. One HIV vaccine candidate which showed some efficacy was studied in RV 144, which was a trial in Thailand beginning in 2003 and first reporting a positive result in 2009. Many trials have shown no efficacy, including the STEP study and HVTN 505 trials. The urgency of the search for a vaccine against HIV stems from the AIDS-related death toll of over 25 million people since 1981. Indeed, in 2002, AIDS became the primary cause of mortality due to an infectious agent in Africa.
_
There are a number of factors that cause development of an HIV vaccine to differ from the development of other classic vaccines:
•Classic vaccines mimic natural immunity against reinfection generally seen in individuals recovered from infection; there are almost no recovered AIDS patients.
•Most vaccines protect against disease, not against infection; HIV infection may remain latent for long periods before causing AIDS.
•Most effective vaccines are whole-killed or live-attenuated organisms; killed HIV-1 does not retain antigenicity and the use of a live retrovirus vaccine raises safety issues.
_
HIV structure:
The epitopes of the HIV viral envelope are more variable than those of many other viruses. Furthermore, the functionally important epitopes of the gp120 protein are masked by glycosylation, trimerisation and receptor-induced conformational changes making it difficult to block with neutralising antibodies.
The ineffectiveness of previously developed vaccines primarily stems from two related factors.
•First, HIV is highly mutable. Because of the virus’ ability to rapidly respond to selective pressures imposed by the immune system, the population of virus in an infected individual typically evolves so that it can evade the two major arms of the adaptive immune system; humoral (antibody-mediated) and cellular (mediated by T cells) immunity.
•Second, HIV isolates are themselves highly variable. HIV can be categorized into multiple clades and subtypes with a high degree of genetic divergence. Therefore, the immune responses raised by any vaccine need to be broad enough to account for this variability. Any vaccine that lacks this breadth is unlikely to be effective.
The difficulties in stimulating a reliable antibody response have led to the attempts to develop a vaccine that stimulates a response by cytotoxic T-lymphocytes. Another response to the challenge has been to create a single peptide that contains the least variable components of all the known HIV strains.
_
According to Gary J. Nabel of the Vaccine Research Center, NIH, in Bethesda, Maryland, several hurdles must be overcome before scientific research will culminate in a definitive AIDS vaccine. First, greater translation between animal models and human trials must be established. Second, new, more effective, and more easily produced vectors must be identified. Finally, and most importantly, there must arise a robust understanding of the immune response to potential vaccine candidates. Emerging technologies that enable the identification of T-cell-receptor specificities and cytokine profiles will prove valuable in hastening this process. In July 2012 a science group speculated that an effective vaccine for HIV would be completed in 2019. A killed whole HIV vaccine, SAV001, that has had success in the US FDA phase 1 human clinical trial in Sep. 2013. This HIV vaccine uses a “dead” version of HIV-1 for the first time. The outcome of the phase 1 human clinical trial has turned out that the vaccine has shown no serious adverse effects while boosting HIV-1 specific antibody. According to Dr. Chil-Yong Kang of Western University’s Schulich School of Medicine & Dentistry in Canada, the developer of this vaccine, the antibody against gp120 surface antigen and p24 capsid antigen increased to 8-fold and 64-fold, respectively after vaccination. There have been reports that HIV patients coinfected with GBV-C can survive longer than those without GBV-C, but the patients may be different in other ways. There is current active research into the virus’ effects on the immune system in patients coinfected with GBV-C and HIV. A promising new approach to a live attenuated HIV-1 vaccine is being pursued by scientists, using a genetically modified form of the HIV virus. The new method involves manipulating the virus’ codons, this is a sequence of three nucleotides that form genetic code, to rely on an unnatural amino acid for proper protein translation, which allows it to replicate. Because this amino acid is foreign to the human body, the virus cannot continue to reproduce.
_
Types of HIV vaccine:
To date, over 40 different HIV vaccines have been tested in several thousand volunteers. Most of this research has consisted of early safety and efficacy studies of recombinant proteins, produced in a variety of different systems. Despite some encouraging evidence of immune responses in people, it is unclear whether many of these would prevent HIV infection. Typically, vaccines are administered to large numbers of people at high risk of infection. After a certain time, the vaccinated participants’ experiences are compared to those of people who received a placebo. As described in what an HIV vaccine would have to do, this may involve assessing the antibodies present in their blood, or the response of their CD8 T-cells to HIV in the test tube, or looking for HIV seroconversions in the trial participants. Researchers have explored a number of strategies that they hope will produce protective immune responses.
These include:
•Live attenuated vaccines.
•Inactivated vaccines.
•Recombinant sub-unit vaccines.
•Modified envelope vaccines.
•Peptide vaccines.
•DNA vaccines.
•Recombinant vectored vaccines.
•Other vectors.
•Replicons.
•Vaccines against viral toxins.
More often than not, studies use a combination of the above types of vaccine in ‘prime and boost’ vaccines, in which two or more different vaccines are used to try and broaden or intensify immune responses. Examples include a vector virus to prime a T-cell response with a subunit (peptide) booster or DNA vaccine to produce antibodies, or two different vector viruses expressing the same gene sequence.
_
Malaria:
One of the most frustrating quests has been for a malaria vaccine. The most common parasites responsible for malaria (plasmodia) have demonstrated an impressive ability to circumvent eradication efforts by becoming drug-resistant. The fact that the WHO recently announced that it was exceedingly pleased with a new vaccine that protects just 30 percent of those immunized indicates the immense difficulty of producing a malaria vaccine. Although this percentage is very low compared with other vaccines, given the severity of malaria worldwide and the fact that it kills more than one million and infects more than 300 million children a year, even such limited coverage could save thousands if not millions of lives in the hardest-hit areas of the globe.
_
Types of malaria vaccines:
Early malaria vaccine development efforts focused on the parasite’s pre-erythrocytic stage—the period during which the organism, in the form of a sporozoite, enters a person’s blood stream and heads for the liver, where it matures and begins a prolific multiplication process. Today, vaccine developers are trying to develop three types of vaccines:
•Pre-erythrocytic vaccine candidates
•Blood-stage vaccine candidates
•Transmission-blocking vaccine candidates
_
1. Pre-erythrocytic vaccine candidates:
Pre-erythrocytic vaccine candidates aim to protect against the early stage of malaria infection—the stage at which the parasite enters or matures in an infected person’s liver cells. These vaccines would elicit an immune response that would either prevent infection or attack the infected liver cell if infection does occur. These candidates include:
•Recombinant or genetically engineered proteins or antigens from the surface of the parasite or from the infected liver cell.
•DNA vaccines that contain the genetic information for producing the vaccine antigen in the vaccine recipient.
•Live, attenuated vaccines that consist of a weakened form of the whole parasite (the sporozoite) as the vaccine’s main component.
2. Blood-stage vaccine candidates:
Blood-stage vaccine candidates target the malaria parasite at its most destructive stage—the rapid replication of the organism in human red blood cells. Blood-stage vaccines do not aim to block all infection. They are expected to decrease the number of parasites in the blood, and in so doing, reduce the severity of disease. Evidence suggests that people who have survived regular exposure to malaria develop natural immunity over time. The goal of a vaccine that contains antigens or proteins from the surface of the blood-stage parasite (the merozoite) would be to allow the body to develop that natural immunity with much less risk of getting ill.
3. Transmission-blocking vaccine candidates:
Transmission-blocking vaccine candidates seek to interrupt the life cycle of the parasite by inducing antibodies that prevent the parasite from maturing in the mosquito after it takes a blood meal from a vaccinated person. These vaccines would not prevent a person from getting malaria, nor would they lessen the symptoms of disease. They would, however, limit the spread of infection by preventing mosquitoes that fed on an infected person from spreading malaria to new hosts. A successful transmission-blocking vaccine would be expected to reduce deaths and illness related to malaria in at-risk communities.
_
Ebola vaccine:
Ebola vaccines currently in trials include:
•A DNA-based plasmid vaccine that primes host cells with some of the Ebola proteins.
•A vaccine based on a replication incompetent chimpanzee respiratory virus engineered to express a key Ebola protein.
•A live attenuated virus from the same family of viruses that causes rabies, also engineered to express a critical Ebola protein.
•A vaccine based on a vaccinia virus and engineered to express a critical Ebola protein.
Each of those strategies has drawbacks in terms of safety and delivery.
Whole virus vaccines have long been used to successfully prevent serious human diseases, including polio, influenza, hepatitis and human papillomavirus-mediated cervical cancer. The advantage conferred by inactivated whole virus vaccines such as the one devised by Halfmann, Kawaoka and their colleagues is that they present the complete range of proteins and genetic material to the host immune system, which is then more likely to trigger a broader and more robust immune response. An Ebola whole virus vaccine, constructed using a novel experimental platform, has been shown to effectively protect monkeys exposed to the often fatal virus. The vaccine described in the journal Science (march 2015), was developed by a group led by Yoshihiro Kawaoka, a University of Wisconsin-Madison expert on avian influenza, Ebola and other viruses of medical importance. It differs from other Ebola vaccines because as an inactivated whole virus vaccine, it primes the host immune system with the full complement of Ebola viral proteins and genes, potentially conferring greater protection. In terms of efficacy, this affords excellent protection. It is also a very safe vaccine. The vaccine was constructed on an experimental platform first devised in 2008 by Peter Halfmann, a research scientist in Kawaoka’s lab. The system allows researchers to safely work with the virus thanks to the deletion of a key gene known as VP30, which the Ebola virus uses to make a protein required for it to reproduce in host cells. Ebola virus has only seven genes and, like most viruses, depends on the molecular machinery of host cells to grow and become infectious. By engineering monkey kidney cells to express the VP30 protein, the virus can be safely studied in the lab and be used as a basis for devising countermeasures like a whole virus vaccine. The vaccine reported by Kawaoka and his colleagues was additionally chemically inactivated using hydrogen peroxide, according to the new Science report.
_
Dengue vaccine:
While no licensed dengue vaccine is available, several vaccine candidates are currently being evaluated in clinical studies.
In light of the increasing rate of dengue infections throughout the world despite vector-control measures, several dengue vaccine candidates are in development. The candidate currently at the most advanced clinical development stage, a live-attenuated tetravalent vaccine based on chimeric yellow fever-dengue virus (CYD-TDV), has progressed to phase III efficacy studies. Potential dengue vaccines must be tetravalent to induce an immune response against each of dengue’s four serotypes. Results from a phase III multicentric efficacy study in Latin America have been published in November 2014. Sanofi Pasteur, the vaccines division of Sanofi, announced the publication of the detailed results of the final landmark phase III clinical efficacy study in Latin America in The New England Journal of Medicine. Overall efficacy against any symptomatic dengue disease was 60.8 percent in children and adolescents 9-16 years old who received three doses of the vaccine. Analyses show a 95.5 percent protection against severe dengue and an 80.3 percent reduction in the risk of hospitalization during the study. The results of this second phase III efficacy study confirm the high efficacy against severe dengue and the reduction in hospitalization observed during the 25-month active surveillance period of the first phase III efficacy study conducted in Asia, highlighting the consistency of the results across the world. However, efficacy questions linger over the results from the Asian phase III trials. The three dose regimen was least effective against Dengue-2, the most prevalent strain in Asia, and efficacy increased with the child’s age. Due to different epidemiologic profiles for dengue in Asia and Latin America, Sanofi is proposing vaccine roll-out tailored to each region. For example, dengue infection among children is not as common in Latin America as it is in Asia—so, it would make little sense to include a dengue vaccine in the Expanded Program on Immunization (EPI) in Latin America. Sanofi hopes to reduce dengue mortality by 50% and morbidity by 25% before 2020. Other pharmaceutical companies such as Novartis, Merck, and GlaxoSmithKline also have dengue candidates in development, although none have progressed to phase III clinical trials to date. The Sanofi Dengue vaccine is expected to be licensed sometime this year.
_________
_________
Classification of vaccines: types of vaccine:
There are two basic types of vaccines: live attenuated and inactivated. The characteristics of live and inactivated vaccines are different, and these characteristics determine how the vaccine is used. Live attenuated vaccines are produced by modifying a disease-producing (“wild”) virus or bacteria in a laboratory. The resulting vaccine organism retains the ability to replicate (grow) and produce immunity, but usually does not cause illness. Live attenuated vaccines include live viruses and live bacteria.
Inactivated vaccines can be composed of either whole viruses or bacteria, or fractions of either:
• Fractional vaccines are either protein-based or polysaccharide-based.
• Protein-based vaccines include toxoids (inactivated bacterial toxin), and subunit or subvirion products.
• Most polysaccharide-based vaccines are composed of pure cell-wall polysaccharide from bacteria.
• Conjugate polysaccharide vaccines are those in which the polysaccharide is chemically linked to a protein. This linkage makes the polysaccharide a more potent vaccine.
_
However, recent advances in molecular biology had provided alternative methods for producing vaccines.
Listed below are the possibilities;-
1. Subunit vaccines – purified or recombinant viral antigen
2. Recombinant virus vaccines
3. Anti-idiotype antibodies
4. DNA vaccines
_
Why are some vaccines live and some dead?
The bottom line is that the decision is entirely driven by the science. If scientists can make a killed vaccine that is effective, that is what they will do. It’s all about trial and error. Most viral diseases require live-attenuated vaccines, but the vast majority of bacterial illnesses are prevented with inactivated vaccines. There are some exceptions to this rule, though. For example:
•Some travelers to less-developed countries get the vaccine to prevent typhoid fever. There are live and killed forms of this vaccine.
•Rabies is a viral infection that is 100 percent fatal once it has progressed. The disease is simply too dangerous to give, even in a weakened state. Fortunately, science allowed the development of an inactivated rabies vaccine.
_
There are a variety of vaccine types that are either currently in use or in development for the prevention of infectious diseases. Under ideal conditions, vaccines should trigger the innate immune system and both arms of the adaptive immune system. However, each vaccine type has both advantages and disadvantages which can affect the stimulation of the immune system and thus limit the usefulness of the vaccine type. First, live, attenuated vaccines as exemplified by the vaccines against measles, mumps, and chickenpox contain laboratory-weakened versions of the original pathogenic agent. Therefore, these vaccines produce a strong cellular and antibody responses and typically produce long-term immunity with only one to two doses of vaccine. Typically, it is less difficult to create live, attenuated vaccines with viruses rather than bacteria because viruses have fewer genes so it is easier to control the viral characteristics. However, because these vaccines contain living microorganisms, refrigeration is required to preserve potency; and, there is the possibility of reversion to the original virulent form of the pathogenic agent. In addition, live vaccines cannot be given to individuals with weakened immune systems because the vaccine produces actual disease. Inactivated vaccines as exemplified by the inactivated influenza vaccine are produced by destroying a pathogenic agent with chemicals, heat, or radiation. This inactivation of the microorganism makes the vaccine more stable. These vaccines do not require refrigeration and can be freeze-dried for transport. However, these vaccines produce weaker immune responses therefore additional booster shots are required to maintain immunity. In experiments with mice by Raz et al., a vaccine made from irradiated Listeria monocytogenes bacteria, rather than heat-killed bacteria, showed protection against a challenge with live Listeria. The irradiated vaccine also stimulated a protective response from T-cells which previously had only been shown to occur with vaccines made from live, weakened Listeria bacteria. Subunit vaccines as exemplified by the recombinant hepatitis B vaccine include only epitopes (specific parts of antigens to which antibodies or T-cells recognize and bind) that most readily stimulate the immune system. Because these vaccines only use a few specific antigens, this reduces the likelihood of adverse reactions; however, this specificity increases the difficulty of determining which antigens should be included in the vaccine. Toxoid vaccines as exemplified by the diphtheria and tetanus vaccines are produced by inactivating bacterial toxins with formalin. These toxoids stimulate an immune response against the bacterial toxins. Conjugate vaccines as exemplified by the Haemophilus influenzae type B (Hib) vaccine are a special type of subunit vaccine. In a conjugate vaccine, antigens or toxoids from a microbe are linked to polysaccharides from the outer coating of that microbe to stimulate immunity (especially in infants). Naked DNA vaccines are still in the experimental stages of development. These vaccines would use DNA specific for microbial antigens to stimulate immunity. This DNA would be administered by injection and then body cells would take up the DNA. These body cells would then start producing the antigen and displaying it on their surfaces which would then stimulate the immune system. These vaccines would produce both a strong antibody response to the free antigen and a strong cellular response to the microbial antigens displayed on the cell surfaces. These vaccines are also considered relatively easy and inexpensive to create and produce. Naked DNA vaccines for influenza and herpes are still in the developmental stages.
_
| Vaccine type | Childhood (ages 0-6) Immunization Schedule |
| Live, attenuated | Measles, mumps, rubella (MMR combined vaccine) Varicella (chickenpox) Influenza (nasal spray) Rotavirus |
| Inactivated/Killed | Polio (IPV) Hepatitis A |
| Toxoid (inactivated toxin) | Diphtheria, tetanus (part of DTaP combined immunization) |
| Subunit/conjugate | Hepatitis B Influenza (injection) Haemophilus influenza type b (Hib) Pertussis (part of DTaP combined immunization) Pneumococcal Meningococcal |
_
| Vaccine type | Other available vaccines |
| Live, attenuated | Zoster (shingles)Yellow fever |
| Inactivated/Killed | Rabies |
| Subunit/conjugate | Human papillomavirus (HPV) |
_
Live attenuated vaccine (LAV):
Some vaccines contain live, attenuated microorganisms. Many of these are active viruses that have been cultivated under conditions that disable their virulent properties, or that use closely related but less dangerous organisms to produce a broad immune response. Although most attenuated vaccines are viral, some are bacterial in nature. Examples include the viral diseases yellow fever, measles, rubella, and mumps, and the bacterial disease typhoid. The live Mycobacterium tuberculosis vaccine developed by Calmette and Guérin is not made of a contagious strain, but contains a virulently modified strain called “BCG” used to elicit an immune response to the vaccine. The live attenuated vaccine-containing strain Yersinia pestis EV is used for plague immunization. Attenuated vaccines have some advantages and disadvantages. They typically provoke more durable immunological responses and are the preferred type for healthy adults. But they may not be safe for use in immunocompromised individuals, and may rarely mutate to a virulent form and cause disease.
_
Viruses are simple microbes containing a small number of genes, and scientists can therefore more readily control their characteristics. Viruses often are attenuated through a method of growing generations of them in cells in which they do not reproduce very well. This hostile environment takes the fight out of viruses: As they evolve to adapt to the new environment, they become weaker with respect to their natural host, human beings. Fully potent viruses (known as natural or ‘wild-type’ viruses) cause disease by reproducing themselves many thousands or millions of times in the body’s cells. However, vaccine viruses usually reproduce fewer than 20 times. Vaccine viruses replicate just well enough to cause the immune system to produce protective antibodies and to make very long-lived ‘memory B cells’ that remember the infection and produce more antibodies if the natural infectious virus is encountered in the future. Live, attenuated vaccines are more difficult to create for bacteria. Bacteria have hundreds of genes and thus are much harder to control. Scientists working on a live vaccine for a bacterium, however, might be able to use recombinant DNA technology to remove several key genes. This approach has been used to create a vaccine against the bacterium that causes cholera, Vibrio cholerae, although the live cholera vaccine has not been licensed in the United States.
_
Safety and stability:
Since LAVs contain living organisms, there is a degree of unpredictability raising some safety and stability concerns.
•Attenuated pathogens have the very rare potential to revert to a pathogenic form and cause disease in vaccinees or their contacts. Examples for this are the very rare, serious adverse events of:
◦vaccine-associated paralytic poliomyelitis (VAPP) and
◦disease-causing vaccine-derived poliovirus (VDPV) associated with oral polio vaccine (OPV).
•Functional immune systems eliminate attenuated pathogens in their immune response. Individuals with compromised immune systems, such as HIV-infected patients may not be able to respond adequately to the attenuated antigens.
•Sustained infection, for example tuberculosis (BCG) vaccination can result in local lymphadenitis or a disseminated infection.
•If the vaccine is grown in a contaminated tissue culture it can be contaminated by other viruses (e.g. retro viruses with measles vaccine).
•As a precaution, LAVs tend not to be administered during pregnancy. However, the actual potential for fetal damage remains theoretical. For example, numerous studies have demonstrated that accidental rubella vaccination during pregnancy did not result in an increased risk of birth defects.
•LAVs can have increased potential for immunization errors:
◦Some LAVs come in lyophilized (powder) form. They must be reconstituted with a specific diluent before administration, which carries the potential for programmatic errors if the wrong diluent or a drug is used.
◦Many LAVs require strict attention to the cold chain for the vaccine to be active and are subject to program failure when this is not adhered to.
_
Live vs. dead (inactivated) vaccine:
| Feature | Live | Dead |
| Dose | Low | High |
| Number of doses | Single | Multiple |
| Need for adjuvant | No | Yes |
| Duration of immunity | Many years | Less |
| Antibody responses | IgG | IgA, IgG |
| Cell mediated immunity | Good | Poor |
| Reversion to virulence | Possible | Impossible |
_
Inactivated vaccine:
Some vaccines contain inactivated, but previously virulent, micro-organisms that have been destroyed with chemicals, heat, radioactivity, or antibiotics. Examples are influenza, cholera, bubonic plague, polio, hepatitis A, and rabies. The term killed generally refers to bacterial vaccines, whereas inactivated relates to viral vaccines. Typhoid was one of the first killed vaccines to be produced and was used among the British troops at the end of the 19th century. Polio and hepatitis A are currently the principal inactivated vaccines used and in many countries, whole cell pertussis vaccine continues to be the most widely used killed vaccine. The adaptive immune response to a killed/inactivated vaccine is very similar to a toxoid vaccine with the exception that the antibody response generated is directed against a much broader range of antigens. Thus, following injection, the whole organism is phagocytosed by immature dendritic cells; digestion within the phagolysosome produces a number of different antigenic fragments which are presented on the cell surface as separate MHC II: antigenic fragment complexes. Within the draining lymph node, a number of TH2, each with a TCR for a separate antigenic fragment, will be activated through presentation by the activated mature dendritic cell. B cells, each with a BCR for a separate antigenic fragment, will bind antigens that drain along lymph channels. Release by the TH2 of IL2, IL4, IL5 and IL6 induces B-cell activation, differentiation and proliferation with subsequent isotype switch (IgM to IgG) and memory cell formation. This process takes a minimum of 10–14 days but on subsequent exposure to the organism, a secondary response through activation of the various memory B cells is induced which leads to high levels of the different IgG molecules within 24–48 h. Hepatitis A is an example of an inactivated vaccine that might be used by occupational health practitioners. It is a formalin inactivated, cell culture adapted, strain of HAV; vaccination generates neutralizing antibodies and protective efficacy is in excess of 90%. Vaccination should be considered for laboratory workers working with HAV and sanitation workers in contact with sewage. Additionally, staff working with children who are not toilet trained or in residential situations where hygiene standards are poor may also be offered vaccination. Primary immunization with a booster between 6 and 12 months after the first should provide a minimum 25 years protection.
_
Such vaccines are more stable and safer than live vaccines: The dead microbes can’t mutate back to their disease-causing state. Inactivated vaccines usually don’t require refrigeration, and they can be easily stored and transported in a freeze-dried form, which makes them accessible to people in developing countries. Killed/inactivated vaccines have a number of disadvantages. They usually require several doses because the microbes are unable to multiply in the host and so one dose does not give a strong signal to the adaptive immune system; approaches to overcome this include the use of several doses and giving the vaccine with an adjuvant. This could be a drawback in areas where people don’t have regular access to health care and can’t get booster shots on time. Local reactions at the vaccine site are more common—this is often due to the adjuvant. Using killed microbes for vaccines is inefficient because some of the antibodies will be produced against parts of the pathogen that play no role in causing disease. Some of the antigens contained within the vaccine, particularly proteins on the surface, may actually down-regulate the body’s adaptive response—presumably, their presence is an evolutionary development that helps the pathogen overcome the body’s defenses. And finally, killed/inactivated vaccines do not give rise to cytotoxic T cells which can be important for stopping infections by intracellular pathogens, particularly viruses.
___
Toxoid Vaccines:
For bacteria that secrete toxins, or harmful chemicals, a toxoid vaccine might be the answer. These vaccines are used when a bacterial toxin is the main cause of illness. Scientists have found that they can inactivate toxins by treating them with formalin, a solution of formaldehyde and sterilized water. Such “detoxified” toxins, called toxoids, are safe for use in vaccines. When the immune system receives a vaccine containing a harmless toxoid, it learns how to fight off the natural toxin. The immune system produces antibodies that lock onto and block the toxin. Vaccines against diphtheria and tetanus are examples of toxoid vaccines. Tetanus toxoid vaccine is manufactured by growing a highly toxigenic strain of Clostridium tetani in a semi-synthetic medium: bacterial growth and subsequent lysis release the toxin into the supernatant and formaldehyde treatment converts the toxin to a toxoid by altering particular amino acids and inducing minor molecular conformational changes. Ultrafiltration then removes unnecessary proteins left as a residual from the manufacturing process to produce the final product. The toxoid is physico-chemically similar to the native toxin thus inducing cross-reacting antibodies but the changes induced by formaldehyde treatment render it non-toxigenic. Following deep subcutaneous/intramuscular (sc/im) administration of tetanus vaccine, the toxoid molecules are taken up at the vaccination site by immature dendritic cells: within this cell, they are processed through the endosomal pathway (involving the phagolysosome) where they are bound to major histocompatibility complex type II (MHC II) molecules; the MHC II:toxoid complex then migrates to the cell surface. While this process is happening within the cell, the now activated mature dendritic cell migrates along lymph channels to the draining lymph node where they encounter naive T helper type 2 cells (TH2), each with their own unique T-cell receptor (TCR). Identifying and then binding of the MHC II:toxoid to the specific TH2 receptor then activates the naive T cell, causing it to proliferate. Simultaneously, toxoid molecules not taken up by dendritic cells pass along lymph channels to the same draining lymph nodes where they come into contact with B cells, each with their own unique B-cell receptor (BCR). Binding to the B cell through the specific immunoglobulin receptor that recognizes tetanus toxoid results in the internalization of toxoid, processing through the endosomal pathway and presentation on the cell surface as an MHC II:toxoid complex as happens in the dendritic cell. These two processes occur in the same part of the lymph node with the result that the B cell with the MHC II:toxoid complex on its surface now comes into contact with the activated TH2 whose receptors are specific for this complex. The process, termed linked recognition, results in the TH2 activating the B cell to become a plasma cell with the production initially of IgM, and then there is an isotype switch to IgG; in addition, a subset of B cells becomes memory cells. The above mechanism describes the adaptive immune response to a protein antigen-like tetanus toxoid; such antigens are termed T-dependent vaccines since the involvement of T helper cells is essential for the immune response generated. Polysaccharide antigens in contrast generate a somewhat different response as will be described in the section on subunit vaccines. The rationale for tetanus vaccination is thus based on generating antibodies against the toxoid which have an enhanced ability to bind toxin compared with the toxin receptor binding sites on nerve cells; in the event of exposure to C. tetani, this large toxin:antibody complex is then unable to bind to the receptor so neutralizing the toxin and preventing disease development. Diphtheria and pertussis toxoid (in acellular pertussis vaccines) are two commercially available toxoid vaccines against which antibodies are produced in an exactly analogous manner as described above. Toxoid vaccines tend not to be highly immunogenic unless large amounts or multiple doses are used: one problem with using larger doses is that tolerance can be induced to the antigen. In order therefore to ensure that the adaptive immune response is sufficiently effective to provide long-lasting immunity, an adjuvant is included in the vaccine. For diphtheria, tetanus and acellular pertussis vaccines, an aluminium salt (either the hydroxide or phosphate) is used; this works by forming a depot at the injection site resulting in sustained release of antigen over a longer period of time, activating cells involved in the adaptive immune response. Aluminium adjuvants are also readily taken up by immature dendritic cells and facilitate antigen processing in the spleen/lymph nodes where the necessary cell–cell interactions take place that lead to the development of high-affinity clones of antibody producing B cells.
_
There are three principal advantages of toxoid vaccines. First, they are safe because they cannot cause the disease they prevent and there is no possibility of reversion to virulence. Second, because the vaccine antigens are not actively multiplying, they cannot spread to unimmunized individuals. Third, they are usually stable and long lasting as they are less susceptible to changes in temperature, humidity and light which can result when vaccines are used out in the community. Toxoid vaccines have two disadvantages. First, they usually need an adjuvant and require several doses for the reasons discussed above. Second, local reactions at the vaccine site are more common—this may be due to the adjuvant or a type III (Arthus) reaction—the latter generally start as redness and induration at the injection site several hours after the vaccination and resolve usually within 48–72 h. The reaction results from excess antibody at the site complexing with toxoid molecules and activating complement by the classical pathway causing an acute local inflammatory reaction.
_
Subunit Vaccines:
Subunit vaccines are a development of the killed vaccine approach: however, instead of generating antibodies against all the antigens in the pathogen, a particular antigen (or antigens) is used such that when the antibody produced by a B cell binds to it, infection is prevented; the key therefore to an effective subunit vaccine is to identify that particular antigen or combination of antigens. Instead of the entire microbe, subunit vaccines include only the antigens that best stimulate the immune system. In some cases, these vaccines use epitopes—the very specific parts of the antigen that antibodies or T cells recognize and bind to. Because subunit vaccines contain only the essential antigens and not all the other molecules that make up the microbe, the chances of adverse reactions to the vaccine are lower. Subunit vaccines can contain anywhere from 1 to 20 or more antigens. Of course, identifying which antigens best stimulate the immune system is a tricky, time-consuming process. Once scientists do that, however, they can make subunit vaccines in one of two ways:
1. They can grow the microbe in the laboratory and then use chemicals to break it apart and gather the important antigens.
2. They can manufacture the antigen molecules from the microbe using recombinant DNA technology. Vaccines produced this way are called “recombinant subunit vaccines.” A recombinant subunit vaccine has been made for the hepatitis B virus. Scientists inserted hepatitis B genes that code for important antigens into common baker’s yeast. The yeast then produced the antigens, which the scientists collected and purified for use in the vaccine. Research is continuing on a recombinant subunit vaccine against hepatitis C virus.
_
Subunit vaccines can be further categorized into:
1. Protein-based vaccines
2. Polysaccharide vaccines
3. Conjugate vaccines
_
Hepatitis B and Haemophilus influenzae b (Hib) are examples of subunit vaccines that use only one antigen; influenza is an example of a subunit vaccine with two antigens (haemagglutinin and neuraminidase). The adaptive immune response to a subunit vaccine varies according to whether the vaccine antigen is a protein or a polysaccharide—subunit vaccines based on protein antigens, for example hepatitis B and influenza, are T-dependent vaccines like toxoid vaccines (as previously discussed) whereas polysaccharides generate a T-independent response. An example of a T-independent subunit vaccine that might be administered in the occupational setting is PPV 23 made up of the capsular polysaccharide from 23 common pneumococcal serotypes which uses the capsular polysaccharide as the vaccine antigen. The vaccine is administered into the deep subcutaneous tissue or intramuscularly. At the injection site, some polysaccharide molecules are phagocytosed by immature dendritic cells (and macrophages), which subsequently migrate to the local lymph nodes where they encounter naive TH2. However, the TCR only recognizes protein molecules and so even though presented by a mature dendritic cell and displayed on MHC II molecules, the TH2 is not activated. Simultaneously, non-phagocytosed polysaccharide molecules pass along lymph channels to the same draining lymph nodes where they encounter B cells, each with their own unique BCR. Because the vaccine antigen consists of linear repeats of the same high molecular weight capsular polysaccharide, it binds with high avidity to multiple receptors on a B cell with the appropriate specificity. Such multivalent binding is able to activate the B cell without the need for TH2 involvement, leading to the production of IgM. Because, however, the TH2 is not involved, there is only limited isotype switching so that only small amounts of IgG are produced and few memory B cells formed. In an adequately immunized individual, when Streptococcus pneumoniae crosses mucosal barriers, specific IgM antibody in serum will bind to the pathogen’s capsular polysaccharide facilitating complement-mediated lysis. IgM is highly effective at activating complement; it is significantly less able to act as a neutralizing or opsonizing antibody. PPV 23 should be offered to workers with chronic respiratory, heart, renal and liver disease, asplenia or hyposplenia, immunosuppression or the potential for a CSF leak: for those individuals with chronic renal disease and splenic dysfunction, where attenuation of the immune response may be expected further doses every 5 years are recommended. T-independent vaccines can be converted to efficient T-dependent vaccines by covalently binding them (a process termed conjugation) to a protein molecule. Following phagocytosis by immature dendritic cells, the conjugated protein and polysaccharide molecules are presented both as MHC II:protein and MHC II:polysaccharide complexes at the cell surface. Migration to the draining lymph node will bring this activated mature dendritic cell into the T-cell-rich area and lead to activation of a TH2 with high specificity for the carrier protein. Simultaneous passage of vaccine antigen along draining lymph channels to the B-cell-rich area of draining lymph nodes results in binding between the polysaccharide:protein conjugate and a B cell whose BCR has a high specificity for the polysaccharide. The polysaccharide:protein complex is internalized, phagocytosed and the protein is expressed as a cell surface complex with MHC II. There is then linked recognition between the activated TH2 with high specificity for the carrier protein and this B cell. TH2 involvement leads to co-stimulation and cytokine release resulting in IgM then IgG and generation of memory cells.
_
The advantages of subunit vaccines are the same as toxoid vaccines with the added benefit that one can distinguish vaccinated people from infected people—for example with hepatitis B vaccination, only an adaptive immune response to the surface antigen is possible whereas with infection core and e antigen responses occur. Subunit vaccines share the same disadvantages as toxoid vaccines, namely the need for an adjuvant (and often multiple doses), together with the frequent occurrence of local reactions at the injection site.
__
| Vaccine Type | Disease | Advantage | Disadvantage |
| Live, weakened vaccines | Measles, mumps, rubella (German measles), polio (Sabin vaccine) and chicken pox | Produces a strong immune response so can provide life-long immunity with 1-2 doses. | Not safe for people with compromised immune systems. Needs refrigeration to stay potent. |
| Inactivated or “killed” vaccines | Cholera, flu, hepatitis A, rabies, polio (Salk vaccine) | Safe for people with compromised immune systems. Easily stored and transported; does not require refrigeration. | Usually requires booster shots every few years to remain effective. |
| Subunit Vaccines | Hepatitis B | Lower chance of adverse reaction. | Research can be time-consuming and difficult. |
| Conjugate Vaccines | Haemophilus influenzae B (or Hib) and pneumococcal vaccine | Safe for people with immune compromised systems. | Usually requires booster shots every few years to remain effective. |
_
While most vaccines are created using inactivated or attenuated compounds from micro-organisms, synthetic vaccines are composed mainly or wholly of synthetic peptides, carbohydrates, or antigens.
_
Synthetic Peptides:
The development of synthetic peptides that might be useful as vaccines depends on the identification of immunogenic sites. Several methods have been used. The best known example is foot and mouth disease, where protection was achieved by immunizing animals with a linear sequence of 20 aminoacids. Synthetic peptide vaccines would have many advantages. Their antigens are precisely defined and free from unnecessary components which may be associated with side effects. They are stable and relatively cheap to manufacture. Furthermore, less quality assurance is required. Changes due to natural variation of the virus can be readily accommodated, which would be a great advantage for unstable viruses such as influenza. Synthetic peptides do not readily stimulate T cells. It was generally assumed that, because of their small size, peptides would behave like haptens and would therefore require coupling to a protein carrier which is recognized by T-cells. It is now known that synthetic peptides can be highly immunogenic in their free form provided they contain, in addition to the B cell epitope, T- cell epitopes recognized by T-helper cells. Such T-cell epitopes can be provided by carrier protein molecules, foreign antigens or within the synthetic peptide molecule itself. Synthetic peptides are not applicable to all viruses. This approach did not work in the case of polioviruses because the important antigenic sites were made up of 2 or more different viral capsid proteins so that it was in a concise 3-D conformation.
_
Anti-idiotype antibodies:
The ability of anti-idiotype antibodies to mimic foreign antigens has led to their development as vaccines to induce immunity against viruses, bacteria and protozoa in experimental animals. Anti-idiotypes have many potential uses as viral vaccines, particularly when the antigen is difficult to grow or hazardous. They have been used to induce immunity against a wide range of viruses, including HBV, rabies, Newcastle disease virus and FeLV, reoviruses and polioviruses.
_
Another classification of vaccines:
Valence:
Vaccines may be monovalent (also called univalent) or multivalent (also called polyvalent). A monovalent vaccine is designed to immunize against a single antigen or single microorganism. A multivalent or polyvalent vaccine is designed to immunize against two or more strains of the same microorganism, or against two or more microorganisms. The valency of a multivalent vaccine may be denoted with a Greek or Latin prefix (e.g., tetravalent or quadrivalent). In certain cases a monovalent vaccine may be preferable for rapidly developing a strong immune response. A monovalent vaccine contains a single strain of a single antigen (e.g. Measles vaccine), whereas polyvalent vaccine contains two or more strains/serotypes of the same antigen (e.g. OPV).
_
Combination vaccines:
Combination vaccines consist of two or more antigens in the same preparation. This approach has been used for over 50 years in many vaccines such as DTaP and MMR. Combination vaccines can be useful to overcome logistic constraints of multiple injections, and accommodate for a children’s fear of needles and pain. Combination products simplify vaccine administration and allow for the introduction of new vaccines without requiring additional health clinic visit and injections. It is very important, however, that combination vaccines are carefully tested before introduction. For instance, adjuvants are pharmacological agent (e.g., aluminum salt, oil-in-water emulsions) that modifies the effect of other agents, such as a drug or vaccine, while having few if any direct effects when given by itself. Adjuvants are often included in vaccines to enhance the recipient’s immune response to a supplied antigen, while keeping the injected foreign material to a minimum. Adjuvants in a combination vaccine could reduce the activity of one antigen and excessively increase the reactivity of another antigen. There could also be interactions with other vaccine components such as buffers. Buffers are substances that minimize changes in the acidity of a solution when an acid or base is added to the solution. Buffers are used in the manufacturing process of some vaccines. Stabilizers are compounds that are used to help vaccine maintain its effectiveness during storage. Vaccine stability is essential, particularly where the cold chain is unreliable. Factors affecting stability are temperature and pH and preservatives. With all combinations, manufacturers must therefore evaluate the potency. Potency is measure of strength or immunogenicity in vaccines. Potency of each antigenic component and the effectiveness of the vaccine components when combined to induce immunity, risk of possible reversion to toxicity, and reaction with other vaccine components must be evaluated. Licensed combination vaccines undergo extensive testing before approval by national regulatory authorities to assure that the products are safe, effective, and of acceptable quality.
_
Heterotypic vaccines:
Also known as Heterologous or “Jennerian” vaccines, these are vaccines that are pathogens of other animals that either do not cause disease or cause mild disease in the organism being treated. The classic example is Jenner’s use of cowpox to protect against smallpox. A current example is the use of BCG vaccine made from Mycobacterium bovis to protect against human tuberculosis.
___
A number of innovative vaccines are also in development and in use:
•Dendritic cell vaccines combine dendritic cells with antigens in order to present the antigens to the body’s white blood cells, thus stimulating an immune reaction. These vaccines have shown some positive preliminary results for treating brain tumors.
•Recombinant Vector – by combining the physiology of one micro-organism and the DNA of the other, immunity can be created against diseases that have complex infection processes (vide infra).
•DNA vaccination – an alternative, experimental approach to vaccination called DNA vaccination, created from an infectious agent’s DNA, is under development (vide infra).
•T-cell receptor peptide vaccines are under development for several diseases using models of Valley Fever, stomatitis, and atopic dermatitis. These peptides have been shown to modulate cytokine production and improve cell mediated immunity.
•Targeting of identified bacterial proteins that are involved in complement inhibition would neutralize the key bacterial virulence mechanism.
_
Recombinant (antigen) vaccines:
Vaccine antigens may also be produced by genetic engineering technology. These products are sometimes referred to as recombinant vaccines. There are four genetically-engineered vaccines are currently available:
• Hepatitis B vaccines are produced by insertion of a segment of the hepatitis B virus gene into the gene of a yeast cell. The modified yeast cell produces pure hepatitis B surface antigen when it grows.
• Human papillomavirus vaccines are produced by inserting genes for a viral coat protein into either yeast (as the hepatitis B vaccines) or into insect cell lines. Viral-like particles are produced and these induce a protective immune response.
• Live typhoid vaccine (Ty21a) is Salmonella typhi bacteria that has been genetically modified to not cause illness.
• Live attenuated influenza vaccine (LAIV) has been engineered to replicate effectively in the mucosa of the nasopharynx but not in the lungs.
_
Recombinant Vector Vaccines:
Recombinant vector vaccines are experimental vaccines that use either an attenuated virus or microbe to introduce microbial DNA into body cells. “Vector” refers to the virus or bacterium used as the carrier. In nature, viruses latch on to cells and inject their genetic material into them. In the lab, scientists have taken advantage of this process. They have figured out how to take the roomy genomes of certain harmless or attenuated viruses and insert portions of the genetic material from other microbes into them. The carrier viruses then ferry that microbial DNA to cells. These viral vaccines would readily mimic a natural infection thus stimulating the immune system. Attenuated bacteria could also have genetic material for antigens from a pathogenic microbe inserted. These antigens from the pathogenic microbe would then be displayed on the harmless microbe this mimicking the pathogen and stimulating the immune system. Recombinant vector vaccines closely mimic a natural infection and therefore do a good job of stimulating the immune system. Both bacterial and viral-based recombinant vectors vaccines for HIV, rabies, and measles are in the experimental stages. Recombinant vector vaccines are experimental vaccines similar to DNA vaccines, but they use an attenuated virus or bacterium to introduce microbial DNA to cells of the body.
_
In this approach, a gene encoding a major viral antigen (that is a target for neutralizing Ab) is inserted (cloned) into another, non-virulent viral vector so that the cloned gene is expressed and the protein produced during viral infection. Animals are then infected with the recombinant virus, and mount an immune response (both humoral and cellular) against the introduced antigen. This is approach is somewhat analagous to an attenuated virus, except that the vector virus can be unrelated to the original virus except for the introduced genes. In addition to eliciting both humoral and cellular immune response, recombinant viruses can also induce secretory immunity if administered via an appropriate route. Examples of vectors that are being tried are adenovirus and vaccinia virus. It may even be possible to introduce genes from more than one pathogenic virus into the same vector (poxviruses can accommodate up to 25 kb of DNA) in order to make a vaccine strain that will protect against several pathogens. Like attenuated viruses, a potential problem with recombinant viruses is that they could cause disease in immunocompromised animals.
_
Hybrid virus vaccine:
An alternative application of recombinant DNA technology is the production of hybrid virus vaccines. The best known example is vaccinia; the DNA sequence coding for the foreign gene is inserted into the plasmid vector along with a vaccinia virus promoter and vaccinia thymidine kinase sequences. The resultant recombination vector is then introduced into cells infected with vaccinia virus to generate a virus that expresses the foreign gene. The recombinant virus vaccine can then multiply in infected cells and produce the antigens of a wide range of viruses. The genes of several viruses can be inserted, so the potential exists for producing polyvalent live vaccines. HBsAg, rabies, HSV and other viruses have been expressed in vaccinia. Hybrid virus vaccines are stable and stimulate both cellular and humoral immunity. They are relatively cheap and simple to produce. Being live vaccines, smaller quantities are required for immunization. As yet, there are no accepted laboratory markers of attenuation or virulence of vaccinia virus for man. Alterations in the genome of vaccinia virus during the selection of recombinant may alter the virulence of the virus. The use of vaccinia also carries the risk of adverse reactions associated with the vaccine and the virus may spread to susceptible contacts. At present, efforts are being made to attenuate vaccinia virus further and the possibility of using other recombinant vectors is being explored, such as attenuated poliovirus and adenovirus.
_
Vaccine generations:
From whole organism vaccine (live or dead) to DNA vaccine:
First generation vaccines are whole-organism vaccines – either live and weakened, or killed forms. Live, attenuated vaccines, such as smallpox and polio vaccines, are able to induce killer T-cell (TC or CTL) responses, helper T-cell (TH) responses and antibody immunity. However, there is a small risk that attenuated forms of a pathogen can revert to a dangerous form, and may still be able to cause disease in immunocompromised vaccine recipients (such as those with AIDS). While killed vaccines do not have this risk, they cannot generate specific killer T cell responses, and may not work at all for some diseases. In order to minimise these risks, so-called second generation vaccines were developed. These are subunit vaccines, consisting of defined protein antigens (such as tetanus or diphtheria toxoid) or recombinant protein components (such as the hepatitis B surface antigen). These, too, are able to generate TH and antibody responses, but not killer T cell responses. DNA vaccines are third generation vaccines, and are made up of a small, circular piece of bacterial DNA (called a plasmid) that has been genetically engineered to produce one or two specific proteins (antigens) from a pathogen. The vaccine DNA is injected into the cells of the body, where the “inner machinery” of the host cells “reads” the DNA and uses it to synthesize the pathogen’s proteins. Because these proteins are recognised as foreign, when they are processed by the host cells and displayed on their surface, the immune system is alerted, which then triggers a range of immune responses. These DNA vaccines were developed from “failed” gene therapy experiments. The first demonstration of a plasmid-induced immune response was when mice inoculated with a plasmid expressing human growth hormone elicited antibodies instead of altering growth.
_
DNA Vaccines:
In this approach, genes (DNA) encoding specific viral proteins are injected into an animal (either in muscle or skin). The DNA is then taken up by cells, where it is transcribed into mRNA which is then translated to give rise to the viral protein. This protein is expressed on the surface of cells, either alone or in association with MHC molecules. It is recognized as a foreign molecule by the immune system, and elicits an immune response. It was discovered almost 20 years ago that plasmid DNA, when injected into the skin or muscle of mice, could induce immune responses to encoded antigens. Since that time, there has since been much progress in understanding the basic biology behind this deceptively simple vaccine platform and much technological advancement to enhance immune potency. Among these advancements are improved formulations and improved physical methods of delivery, which increase the uptake of vaccine plasmids by cells; optimization of vaccine vectors and encoded antigens; and the development of novel formulations and adjuvants to augment and direct the host immune response. The ability of the current, or second-generation, DNA vaccines to induce more-potent cellular and humoral responses opens up this platform to be examined in both preventative and therapeutic arenas. So-called naked DNA vaccines consist of DNA that is administered directly into the body. These vaccines can be administered with a needle and syringe or with a needle-less device that uses high-pressure gas to shoot microscopic gold particles coated with DNA directly into cells. Sometimes, the DNA is mixed with molecules that facilitate its uptake by the body’s cells. Naked DNA vaccines being tested in humans include those against the viruses that cause influenza and herpes. As of 2015, DNA vaccination is still experimental and is not approved for human use. This approach has several advantages, including the following:
1) Since no infectious agent (attenuated or inactivated) is involved, there is no chance of producing an infection, even in immunocompromised animals.
2) Expression of the protein is relatively long-term (the gene may integrate into the cell DNA and be stably expressed) so that long term immunity may be solicited.
3) Since the antigen is endogenously synthesized inside cells, it elicits a strong cellular immune response.
4) Once a gene is cloned, DNA is inexpensive to make and is stable, so the vaccines should be inexpensive.
5) No adjuvant is required.
_
DNA vaccines for non-infectious diseases offer new treatments for tumour and allergy. Vaccines against allergies need to suppress or alter an unwanted immune response, while a cancer DNA vaccine has to overcome tolerance and/or immune suppression and initiate a powerful immune response.
_
Delivering antibody genes:
Another method that can provide lasting antibodies is gene transfer. This method involves using DNA or a viral vector to deliver a gene for the monoclonal antibody into a person’s cells. The DNA or vector carries instructions for making the antibody inside a person’s cells, allowing them to make the HIV-specific antibodies on their own, rather than getting injections of them. This method is similar to that used with DNA vaccines and viral vector vaccines. The major difference is that in this case a copy of an antibody gene is delivered and for vaccines, a copy of an HIV gene is delivered. Studies with broadly neutralizing antibodies may lead directly to a strategy to prevent HIV. They could also tell us which antibodies work and what amounts are needed to prevent HIV infection. From there, scientists can work to develop future vaccines to reproduce this response.
_
Genetically modified (GM) vaccine:
Today we have several different types of GM vaccines in production, development or research phases, such as:
1. DNA vaccines & Naked DNA vaccines
2. Recombinant Vector vaccines
3. Recombinant (antigen) vaccine
GM vaccines are already in use and being administered to people; these include vaccines for hepatitis B, rotavirus and HPV, among others. There are experimental GM vaccines being developed that use tumorigenic cancer cells and cells from humans, dogs, monkeys, cows, pigs, rodents, birds and insects. Use of foreign DNA in various forms has the potential to cause a great deal of trouble, not only because there is the potential for it to recombine with our own DNA, but also there is the potential for it to turn the DNA “switches,” the epigenetic parts of the DNA, on and off.
_
Humans and animals receiving certain live virus-vectored vaccines will be shedding and transmitting genetically modified vaccine strains that may pose unpredictable risks to the vaccinated, close contacts and environment. For example, vaccine developers creating an experimental AIDS vaccine by genetically engineering the live-attenuated measles virus to express a fusion protein containing HIV-1 antigens, face challenges in trying to limit shedding and transmission of infectious virus by the recently vaccinated. These very real risks should be thoroughly quantified before licensure and widespread use of GMO vaccines because the ability of vaccine strain viruses to recombine with wild-type viruses and produce new hybrid viruses with potentially serious side effects.
_______
3D vaccine:
Cancer cells are generally ignored by the immune system. This is because—for the most part—they more closely resemble cells that belong in the body than pathogens such as bacterial cells or viruses. The goal of cancer vaccines is to provoke the immune system to recognize cancer cells as foreign and attack them. One way to do this is by manipulating dendritic cells, the coordinators of immune system behavior. Dendritic cells constantly patrol the body, sampling bits of protein found on the surface of cells or viruses called antigens. When a dendritic cell comes in contact with an antigen that it deems foreign, it carries it to the lymph nodes, where it instructs the rest of the immune system to attack anything in the body displaying that antigen. Though similar to healthy cells, cancer cells often display unique antigens on their surface, which can be exploited to develop cancer immunotherapies. For example, in dendritic cell therapy, white blood cells are removed from a patient’s blood, stimulated in the lab to turn into dendritic cells, and then incubated with an antigen that is specific to a patient’s tumor, along with other compounds to activate and mature the dendritic cells. These “programmed” cells are then injected back into the bloodstream with the hopes that they will travel to the lymph nodes and present the tumor antigen to the rest of the immune system cells. While this approach has had some clinical success, in most cases, the immune response resulting from dendritic cell vaccines is short-lived and not robust enough to keep tumors at bay over the long run. In addition, cell therapies such as this, which require removing cells from patients and manipulating them in the lab, are costly and not easily regulated. To overcome these limitations, Mooney’s lab has been experimenting with a newer approach that involves reprogramming immune cells from inside the body using implantable biomaterials.
_
_
The idea is to introduce a biodegradable scaffold under the skin that temporarily creates an “infection-mimicking microenvironment”, capable of attracting, housing, and reprogramming millions of dendritic cells over a period of several weeks. In a 2009 paper published in Nature Materials, Mooney demonstrated that this could be achieved by loading a porous scaffold—about the size of a dime—with tumor antigen as well as a combination of biological and chemical components meant to attract and activate dendritic cells. Once implanted, the scaffold’s contents slowly diffused outward, recruiting a steady stream of dendritic cells, which temporarily sought residence inside the scaffold while being simultaneously exposed to tumor antigen and activating factors. When the scaffold was implanted in mice, it achieved a 90% survival rate in animals that otherwise die from cancer within 25 days. Now, Mooney and his team have taken this approach a step further, creating an injectable scaffold that can spontaneously assemble once inside the body. This second generation vaccine would prevent patients from having to undergo surgery to implant the scaffold and would also make it easier for clinicians to administer it. The new 3D vaccine is made up of many microsized, porous silica rods dispersed in liquid. When injected under the skin, the liquid quickly diffuses, leaving the rods behind to form a randomly assembled three-dimensional structure resembling a haystack. The spaces in between the rods are large enough to house dendritic cells and other immune cells, and the rods have nano-sized pores that can be loaded with a combination of antigens and drugs. When injected into mice that were then given a subsequent injection of lymphoma cells, the 3D vaccine generated a potent immune response and delayed tumor growth. Compared to a bolus injection containing the same drugs and antigens (but no scaffold), the 3D vaccine was more effective at preventing tumor growth, with 90% of mice receiving the 3D vaccine still alive at 30 days compared with only 60% of mice given the bolus injection. While the 3D injectable scaffold is being tested in mice as a potential cancer vaccine, any combination of different antigens and drugs could be loaded into the scaffold, meaning it could also be used to treat infectious diseases that may be resistant to conventional treatments. Mooney says that in addition to continuing to develop the cancer vaccine, he also plans to explore how the injectable scaffold can be used to both treat and prevent infectious diseases. More broadly, Mooney predicts that spontaneously assembling particles will be adopted by many fields in the future.
_
Cancer vaccine:
Cancer vaccines are medicines that belong to a class of substances known as biological response modifiers. Biological response modifiers work by stimulating or restoring the immune system’s ability to fight infections and disease. Cancer vaccines are designed to boost the body’s natural ability to protect itself, through the immune system, from dangers posed by damaged or abnormal cells such as cancer cells. There are two broad types of cancer vaccines:
1. Preventive (or prophylactic) vaccines, which are intended to prevent cancer from developing in healthy people; and
2. Treatment (or therapeutic) vaccines, which are intended to treat an existing cancer by strengthening the body’s natural defenses against the cancer.
The U.S. Food and Drug Administration (FDA) has approved two types of vaccines to prevent cancer: vaccines against the hepatitis B virus, which can cause liver cancer, and vaccines against human papillomavirus types 16 and 18, which are responsible for about 70 percent of cervical cancer cases. Cancer cells can carry both self antigens and cancer-associated antigens. The cancer-associated antigens mark the cancer cells as abnormal, or foreign, and can cause B cells and killer T cells to mount an attack against them. Cancer cells may also make much larger amounts of certain self antigens than normal cells. Because of their high abundance, these self antigens may be viewed by the immune system as being foreign and, therefore, may trigger an immune response against the cancer cells. The FDA has approved one cancer treatment vaccine for certain men with metastatic prostate cancer. Researchers are developing treatment vaccines against many types of cancer and testing them in clinical trials. Several studies have suggested that cancer treatment vaccines may be most effective when given in combination with other forms of cancer therapy. In addition, in some clinical trials, cancer treatment vaccines have appeared to increase the effectiveness of other cancer therapies. The most commonly reported side effect of cancer vaccines is inflammation at the site of injection, including redness, pain, swelling, warming of the skin, itchiness, and occasionally a rash. People sometimes experience flu-like symptoms after receiving a cancer vaccine, including fever, chills, weakness, dizziness, nausea or vomiting, muscle ache, fatigue, headache, and occasional breathing difficulties. Blood pressure may also be affected.
_
Antigens are often not strong enough inducers of the immune response to make effective cancer treatment vaccines. Researchers often add extra ingredients, known as adjuvants, to treatment vaccines. These substances serve to boost immune responses that have been set in motion by exposure to antigens or other means. Patients undergoing experimental treatment with a cancer vaccine sometimes receive adjuvants separately from the vaccine itself. Adjuvants used for cancer vaccines come from many different sources. Some microbes, such as the bacterium Bacillus Calmette-Guérin (BCG) originally used as a vaccine against tuberculosis, can serve as adjuvants. Substances produced by bacteria, such as Detox B, are also frequently used. Biological products derived from nonmicrobial organisms can be used as adjuvants, too. One example is keyhole limpet hemocyanin (KLH), which is a large protein produced by a sea animal. Attaching antigens to KLH has been shown to increase their ability to stimulate immune responses. Even some nonbiological substances, such as an emulsified oil known as montanide ISA–51, can be used as adjuvants. Natural or synthetic cytokines can also be used as adjuvants. Cytokines are substances that are naturally produced by white blood cells to regulate and fine-tune immune responses. Some cytokines increase the activity of B cells and killer T cells, whereas other cytokines suppress the activities of these cells. Cytokines frequently used in cancer treatment vaccines or given together with them include interleukin 2 (IL2), interferon alpha (INF–a), and GM–CSF, also known as sargramostim.
_
Although researchers have identified many cancer-associated antigens, these molecules vary widely in their capacity to stimulate a strong anticancer immune response. Two major areas of research aimed at developing better cancer treatment vaccines involve the identification of novel cancer-associated antigens that may prove more effective in stimulating immune responses than the already known antigens and the development of methods to enhance the ability of cancer-associated antigens to stimulate the immune system. Research is also under way to determine how to combine multiple antigens within a single cancer treatment vaccine to produce optimal anticancer immune responses. Perhaps the most promising avenue of cancer vaccine research is aimed at better understanding the basic biology underlying how immune system cells and cancer cells interact. New technologies are being created as part of this effort. For example, a new type of imaging technology allows researchers to observe killer T cells and cancer cells interacting inside the body. Researchers are also trying to identify the mechanisms by which cancer cells evade or suppress anticancer immune responses. A better understanding of how cancer cells manipulate the immune system could lead to the development of new drugs that block those processes and thereby improve the effectiveness of cancer treatment vaccines. For example, some cancer cells produce chemical signals that attract white blood cells known as regulatory T cells, or Tregs, to a tumor site. Tregs often release cytokines that suppress the activity of nearby killer T cells. The combination of a cancer treatment vaccine with a drug that would block the negative effects of one or more of these suppressive cytokines on killer T cells might improve the vaccine’s effectiveness in generating potent killer T cell antitumor responses.
_
Limitations of cancer treatment vaccines:
Developing successful cancer treatment vaccines is difficult. Some limitations of these vaccines are:
•Cancer cells suppress the immune system; this is how the cancer is able to grow and develop in the first place. Researchers are using adjuvants in vaccines to try to overcome this problem.
•Because cancer cells develop from a person’s own healthy cells, they may not “look” harmful to the immune system. Therefore, instead of being identified as harmful to the body and eliminated, the cancer cells are ignored.
•Larger or more advanced tumors are hard to eliminate using only a vaccine. This is one reason why cancer vaccines are usually given in addition to other treatments.
•The immune systems of people who are sick or older may not be able to produce a strong immune response following vaccination, limiting the vaccine’s effectiveness. Also, some cancer treatments may damage a person’s immune system, limiting its ability to respond to a vaccine.
Because of these reasons, some researchers think that a cancer treatment vaccine may be more effective for patients with smaller tumors or early-stage cancers.
_
Autologous Vaccine delays progression following surgery for Ovarian Cancer:
Treatment with the immunotherapy Vigil delayed time to progression in all patients with stage III/IV ovarian cancer who were treated with the autologous tumor cell vaccine compared with those who were not, according to an open-label phase II trial. In the 31-patient trial, which was presented at the 2015 SGO Annual Meeting, patients were randomized to receive the vaccine or no treatment following surgery. Of the 20 patients who received the vaccine, a median time to progression had not yet been reached compared with a median of 14.5 months in those who were not treated. Additionally, the Vigil vaccine, composed of granulocyte macrophage colony-stimulating factor [GM-CSF] bi-shRNAi furin vector-transfected autologous tumor cells, demonstrated an acceptable safety profile, and participants showed a high rate of immune response via T-cell activation.
_
Measles Vaccine cures woman of Cancer:
Mayo Clinic researchers employing “virotherapy”—or virus-based treatment—completely eradicated a 49-year-old woman’s blood cancer using an extremely heavy dose of the measles vaccine (enough to vaccinate 100 million people), according to a newly released report in the journal Mayo Clinic Proceedings. The study team injected two cancer patients with “the highest possible dose” of an engineered measles virus. (Past research had shown the virus was capable of killing myeloma-infected plasma cells while sparing normal tissue.) Both patients responded to the treatment and showed reductions in bone marrow cancer and myeloma protein. One of the patients, Stacy Erholtz, experienced complete remission and has been cancer-free for 6 months. This is the first study to show that this type of virotherapy may be effective when it comes to some types of cancers. Viruses naturally destroy tissues and the measles virus appears to cause cancer cells to group together and “explode” which not only destroys them but also helps alert the patient’s immune system to their presence. While the second myeloma patient did not experience such a dramatic recovery, the virotherapy was still effective in targeting and treating sites of her tumor growth, the Mayo researchers say. The two women included in the study were chosen because their cancer had failed to respond to other treatments, and so they were out of options, the study authors say. Also, neither of the women had much previous exposure to measles, which means they had few antibodies to the virus. While a lot more work has to be done to develop the treatment for other cancer sufferers, Russell says the ultimate goal for this therapy is “a single-shot cure for cancer.”
_
US scientists report promising new melanoma vaccines:
Experimental tailor-made vaccines targeting melanoma patients’ individual genetic mutations have given encouraging preliminary results, researchers have said. The clinical test on three patients with this form of aggressive skin cancer in an advanced stage is unprecedented in the United States. The vaccines appear to boost the number and diversity of T-cells, which are key to the human immune system and attack tumors, researchers said in a report published in the journal Science. Melanoma accounts for around five percent of all new cancer cases diagnosed in the United States, but that proportion is rising. Last year 76,000 Americans were diagnosed with melanoma and nearly 10,000 died of it, according to the National Cancer Institute. The vaccines were developed by sequencing the genomes of the three patients’ tumors and comparing them to samples of healthy tissue to identify proteins that had mutated. These are known as neoantigens, and are unique to cancer cells. The researchers then used computer programs and laboratory trials to predict and test the neoantigens most likely to trigger a strong immune response and thus be added to the vaccine. The vaccine was administered to patients whose tumors had been removed but without preventing cancer cells from spreading to the lymph nodes, which is an indication that the melanoma is going to reappear. The initial clinical results have been good enough to start a phase 1 clinical trial approved by the US Food and Drug Administration on six patients. If this broader test proves the vaccines work, it would pave the way for immunotherapy that prevents melanoma from resurfacing in patients. The study was led by Gerald Linette, an oncologist at the University of Washington in St. Louis, Missouri. Although the test was preliminary, it was based on the breadth and diversity of the T-cells, meaning these vaccines are promising as a therapy, he said. But the researchers cautioned that it was too early to say if these vaccines would continue to work long-term. None of the three patients tested so far have suffered major negative side effects. Immunotherapy, already used with success against melanoma, is a promising new strategy against very aggressive cancer cells for which there is currently no effective treatment.
_
Cervical cancer vaccine:
Worldwide, it is estimated that 274,000 women died from cervical cancer in 2002. The cause of cervical cancer is almost 100 percent attributable to genital infection with the human papillomavirus (HPV). HPVinfection is also the cause of other anogenital cancers, recurrent respiratory papillomatosis, and genital warts in both men and women. Given that HPV is sexually transmitted, the prevalence of HPV infection in the population peaks among persons in their late teens or early twenties during the years following sexual debut. Up to 70 percent of women will acquire genital HPV infection sometime during their lifetime. Most women clear the infection; however, some experience persistent infections that can lead to cervical cancer. The progression from persistent infection to cervical cancer typically evolves slowly, often over a period of 20 years or longer. During this time, the disease develops through a precancerous stage (i.e., cervical intraepithelial neoplasia (CIN)) that can be detected through regular cytologic screening of the cervix with a Papanicolaou test. If screening confirms an abnormality (i.e., dysplasia), then additional testing and treatment can usually eliminate disease. Countries that have adopted organized cervical cancer screening programs have significantly reduced the morbidity and mortality associated with cervical cancer in the population. Recently, a number of landmark clinical studies have demonstrated that a prophylactic HPV vaccine can prevent HPV infection and disease.
| Human papillomavirus (HPV) vaccines | Gardasil 9 | Gardasil | Cervarix |
| Who makes it? | Merck & Co., Inc. | Merck & Co., Inc. | GlaxoSmithKline plc |
| What kinds of HPV does it protect against? | HPV types 16, 18, 6, 11, 31, 33, 45, 52, and 58 which cause several types of cancer and genital warts | HPV types 16, 18, 6, and 11, which cause several types of cancer and genital warts | HPV types 16, 18, which cause several types of cancer |
| Who should get the vaccine?* |
|
|
|
|
|||
| When should the vaccine be given? |
|
||
CDC has carefully studied the risks and benefits of HPV vaccination. HPV vaccination is recommended because the benefits, such as prevention of cancer, far outweigh the risks of possible side effects. The cervical cancer vaccine is most effective in women who have never been exposed to HPV types 16 and 18 infections. Current data showed that it is 100% effective in preventing precancerous changes (CIN) caused by HPV types 16 and 18. However, HPV 16 and 18 infections account for 70% of cervical cancer. Therefore, vaccinated women can still be infected or have CIN caused by other HPV types. As the vaccines cannot protect against infection by other high risk types of HPV, nor can they clear the virus in those who are already infected, they cannot eliminate the need of cervical screening.
_
Therapeutic vaccine for HPV associated cancer:
Inovio was given accolades by its industry peers for “Best Therapeutic Vaccine” for its DNA-based immunotherapy, VGX-3100, which was designed to treat HPV-associated precancers and cancers. In a large, controlled phase II efficacy trial Inovio reported top line data demonstrating regression of disease and clearance of the underlying cause of the condition – the HPV virus. Inovio expects to publish the complete data set in a peer-reviewed journal this year, is advancing this product into a phase III trial early next year, and has expanded studies of this immunotherapy to include cervical and head and neck cancer.
_
Therapeutic vaccine:
Vaccines have classically been developed to prevent infectious diseases. Thanks to a technological revolution in molecular biology, immunology and other vaccine-related techniques along with an enhanced understanding of disease mechanisms, it is possible to consider immune-related approaches to the treatment of various diseases rather than limiting immune approaches to the prevention of infectious diseases. In theory, multiple immunotherapy products can now be envisaged for every major therapeutic category from anti-infectives to autoimmune disorders, oncology, cardiovascular or neurological conditions. Therapeutic cancer vaccines are already discussed above and now I discuss therapeutic vaccine for many chronic diseases. The next great frontier for vaccine development will be vaccines against chronic diseases such as peptic ulcer disease, atherosclerotic heart disease, type I and II diabetes, and Alzheimer’s disease, to mention a few. In some instances the feasibility for vaccination is based on the discovery that infection with a specific pathogen is (or is likely) responsible for the chronic disease. Whereas the association between hepatitis B virus and hepatocellular carcinoma has long been known, some pathogens that have more recently been associated with chronic diseases include human papilloma virus with cervical cancer, Helicobacter pylori with peptic ulcer disease and gastric carcinoma, an association between Chlamydia and atherosclerotic heart disease (and perhaps with cervical cancer). In other instances, vaccine development is based on immunisation with chemical moieties that play a role in the pathogenesis of the chronic disease. Thus, immunisation against certain lipids may be an approach to prevent atherosclerotic heart disease and vaccination with β-amyloid protein may thwart the progression of Alzheimer’s dementia.
_
Vaccines for non-infectious illness could help developing nations tackle the growing burden of chronic disease. Chronic, non-communicable diseases are responsible for almost 60 per cent of all deaths annually worldwide, with half of these from cardiovascular disease. Non-communicable diseases of old age and poor lifestyles, such as heart disease, cancer and diabetes, are the biggest killers in the developing world. Certainly, vaccines have long been a mainstay in the arsenal against infectious diseases. But recently there have been murmurs about a new type of vaccine, designed for non-infectious diseases. These therapeutic vaccines still use the immune system to attack the disease, but, as the name suggests, they’re designed to treat rather than prevent illness. The idea isn’t as outlandish as it may sound. In 1999, the US-based Institute of Medicine ranked chronic illnesses such as type one diabetes and melanoma as promising vaccine candidates, labeling the development of these vaccines a matter of public health urgency. Biotechnology companies took the hint, and since then, several vaccines targeting cancers, cardiovascular disease and hypertension have made it to phase I and II clinical trials. Two companies, Switzerland-based Cytos and UK-based Protherics, are testing hypertension vaccines in phase II trials. The vaccine is designed to prompt the immune system to produce antibodies against the hormone angiotensin, which constricts blood vessels and raises blood pressure. The vaccine will need to be administered once or twice a year. A vaccine against atherosclerosis the build-up of fatty plaques of cholesterol on artery walls, which can lead to heart attack or stroke is in phase I trials. Developed by Swedish company Bioinvent, in conjunction with US company Genentech, the vaccine is made up of the human antibody BI-204 that, when injected in the body, is designed to recognise a type of cholesterol (low-density lipoprotein, or LDL) that forms plaques as foreign and attack it. The company hopes the vaccine will prevent heart attacks in patients with acute coronary artery disease. These heart disease vaccines are still in the early stages of development.
_
Diabetes vaccine:
There are also a number of vaccines in progress for type 1 diabetes. Diabetes is an autoimmune disease that usually strikes in adolescence or young adulthood. The cause is unknown but the process can be detected years before overt diabetes by measuring antibody forming against the islet cells. In time the islet cells are destroyed and blood sugar rises as insulin is no longer produced or produced in adequate amounts. In Australia, scientists have been developing a nasal vaccine which desensitizes the immune response to insulin. Phase 1 studies are complete and later stage trials in Australia, New Zealand and Germany are underway. One vaccine, from Selecta Biosciences given nasally to young people with antibody but not overt disease, showed a depressed immune response. The investigators believe that this may be an approach to multiple autoimmune diseases. The idea is to develop an antigen-specific tolerogenic vaccine while not damaging the normal immune response to pathogens and other foreign invaders. Theoretically if a person recently diagnosed with type 1 diabetes was vaccinated, there might be hope for preserving whatever islet cells still existed and perhaps for some regrowth. Or, should stem cell therapies prove effective, the vaccine would prevent the autoimmune mechanism from destroying the newly implanted stem cells and their daughter cells. A study in 80 patients, published in the journal Science Translational Medicine in 2013, showed a vaccine could retrain their immune system. Experts described the results as a “significant step”. In patients with type 1 diabetes, the immune system destroys beta cells in the pancreas. This means the body is unable to produce enough insulin and regular injections of the hormone are needed throughout life. The vaccine was targeted to the specific white blood cells which attack beta cells. After patients were given weekly injections for three months, the levels of those white blood cells fell. Blood tests also suggested that beta cell function was better in patients given the vaccine than in those treated only with insulin. The research is at an early stage and trials in larger groups of people, which measure the long-term effect of the vaccine, are still needed.
_
Life-style vaccine:
Life-style vaccines are defined as vaccines to manage chronic conditions in healthy individuals. Three major examples of such candidate vaccines are discussed: contraceptive vaccines, vaccines to treat drug addiction, and anti-caries vaccines.
1. Contraceptive vaccines:
Current approaches to contraception are essentially based on hormonal control, condoms and surgery. Vaccination against hormones controlling reproduction is a promising immunological approach to contraception. It may rely on hormones that control the production of gametes or are involved in the survival of the fertilized egg. On the other hand, contraceptive vaccines could also induce antibodies against surface proteins of the gametes in order to block fertilization of ova by sperm.
2. Vaccination and drug addiction:
Two examples of drug addiction will be described here: addiction to cocaine and nicotine. Cocaine addiction and nicotine dependence are major health concerns, and new strategies for the treatment of drug abuse are urgently needed. Addiction usually depends on activation of receptors expressed by cells of the central nervous system. Vaccination is expected to induce antibodies against systemic drug molecules and thereby block their further uptake into the brain. However, cocaine and nicotine are molecules too small to be immunogenic; they can be considered as haptens and need to be linked to a carrier. Moreover, a vaccine has to be formulated with appropriate adjuvants in order to induce a high and long-lasting antibody response to neutralize these drugs.
TA-CD:
TA-CD is an active vaccine developed by the Xenova Group which is used to negate the effects of cocaine, making it suitable for use in treatment of addiction. It is created by combining norcocaine with inactivated cholera toxin. It works in much the same way as a regular vaccine. A large protein molecule attaches to cocaine, which stimulates response from antibodies which destroy the molecule. This also prevents the cocaine from crossing the blood–brain barrier, negating the euphoric high and rewarding effect of cocaine caused from stimulation of dopamine release in the mesolimbic reward pathway. The vaccine does not affect the users “desire” for cocaine, only the physical effects of the drug.
3. Vaccination and dental caries:
Dental caries is the most common infectious disease affecting humans. The main causative agents are a group of streptococcal species collectively referred to as the mutans streptococci. Streptococcus mutans has been identified as the major etiological agent of human dental caries. The first step in the initiation of infection by this pathogenic bacterium is its attachment to a suitable receptor. Two groups of proteins from mutans streptococci represent primary candidates for a human caries vaccine: (i) glucosyltransferase enzymes, which synthesize adhesive glucans and allow microbial accumulation; and (ii) cell-surface fibrillar proteins that mediate adherence to the salivary pellicle. It is hypothesized that a mucosal vaccine against a combination of S. mutans surface proteins would protect against dental caries by inducing specific salivary immunoglobulin A (IgA) antibodies. These IgAs may reduce bacterial pathogenesis and adhesion to the tooth surface by affecting several adhesins simultaneously.
__________
__________
Vaccine production, adjutants and preservatives:
_
Vaccine production:
There are only about 30 different vaccine types (but many more product formulations) compared with approximately 20,000 drugs. Accordingly, there are relatively few vaccine manufacturers and a limited number of countries where vaccines are produced. Most countries use vaccines that are imported from elsewhere. To support countries with limited national regulatory (NRA) capacity, WHO provides a system of vaccine prequalification that has been adopted as a standard for procurement by United Nations agencies and some countries. Alternatively, countries can procure their vaccines directly on the domestic or international market.
_
Vaccines are made using the disease-causing virus or bacteria, but in a form that will not harm your child. Instead, the weakened, killed, or partial virus or bacteria prompts your baby’s immune system to develop antibodies, or defenders, against the disease. Once it is determined how the virus and bacteria will be modified, vaccines are created through a general three-step process:
1. Antigen is generated. Viruses are grown in primary cells (i.e. chicken eggs for the influenza vaccine), or on continuous cell lines (i.e. human cultured cells for hepatitis b vaccine); bacteria is grown in bioreactors (i.e. Hib vaccine).
2. Antigen is isolated from the cells used to create it.
3. Vaccine is made by adding adjuvant, stabilizers and preservatives. Adjuvants increase immune response of the antigen; stabilizers increase the vaccine’s storage life; and preservatives allow for the use of multi-dose vials.
It is important to remember that vaccines undergo rigorous safety testing prior to FDA approval and are continually monitored for safety. The vaccine production process involves several vaccine manufacturer-funded testing phases over many years to ensure that it is safe to administer. The vaccines are also studied to be administered in groups, to work together to protect your child.
_
Three ways to make a vaccine:
While processes may differ slightly from company to company, here are the basic steps in these three methods:
_
Combination vaccines are harder to develop and produce, because of potential incompatibilities and interactions among the antigens and other ingredients involved. Vaccine production techniques are evolving. Cultured mammalian cells are expected to become increasingly important, compared to conventional options such as chicken eggs, due to greater productivity and low incidence of problems with contamination. Recombination technology that produces genetically detoxified vaccine is expected to grow in popularity for the production of bacterial vaccines that use toxoids. Combination vaccines are expected to reduce the quantities of antigens they contain, and thereby decrease undesirable interactions, by using pathogen-associated molecular patterns. In 2010, India produced 60 percent of the world’s vaccine worth about $900 million(€670 million).
_
How influenza vaccine is produced:
Influenza vaccine is the best available protection against the disease. Among all vaccines, however, the process of making influenza vaccines is considered uniquely complicated and difficult. One reason is that the constantly evolving nature of influenza viruses requires continuous global monitoring and frequent reformulation of the vaccine strains. Another reason is that the rapid spread of these viruses during seasonal epidemics, as well as the occasional pandemic, means that each step in the vaccine process must be completed within very tight time frames if vaccine is to be manufactured and delivered in time. In response to the realities imposed by influenza, a highly functional process has evolved over decades in which the public and private sectors work together to develop and produce influenza vaccine.
_
Manufacturing methods of flu vaccine:
1. For the inactivated vaccines, the virus is grown by injecting it, along with some antibiotics, into fertilized chicken eggs. About one to two eggs are needed to make each dose of vaccine. The virus replicates within the allantois of the embryo, which is the equivalent of the placenta in mammals. The fluid in this structure is removed and the virus purified from this fluid by methods such as filtration or centrifugation. The purified viruses are then inactivated (“killed”) with a small amount of a disinfectant. The inactivated virus is treated with detergent to break up the virus into particles, and the broken capsule segments and released proteins are concentrated by centrifugation. The final preparation is suspended in sterile phosphate buffered saline ready for injection. This vaccine mainly contains the killed virus but might also contain tiny amounts of egg protein and the antibiotics, disinfectant and detergent used in the manufacturing process. In multi-dose versions of the vaccine, the preservative thimerosal is added to prevent growth of bacteria. In some versions of the vaccine used in Europe and Canada, an adjuvant is also added, this contains a fish oil called squalene, vitamin E and an emulsifier called polysorbate 80.
2. For the live vaccine, the virus is first adapted to grow at 25 °C (77 °F) and then grown at this temperature until it loses the ability to cause illness in humans, which would require the virus to grow at our normal body temperature of 37 °C (99 °F). Multiple mutations are needed for the virus to grow at cold temperatures, so this process is effectively irreversible and once the virus has lost virulence (become “attenuated”), it will not regain the ability to infect people. To make the vaccine, the attenuated virus is grown in chicken eggs as before. The virus-containing fluid is harvested and the virus purified by filtration; this step also removes any contaminating bacteria. The filtered preparation is then diluted into a solution that stabilizes the virus. This solution contains monosodium glutamate, potassium phosphate, gelatin, the antibiotic gentamicin, and sugar. A new method of producing influenza virus is used to produce the Novartis vaccine Optaflu. In this vaccine the virus is grown in cell culture instead of in eggs. This method is faster than the classic egg-based system and produces a purer final product. Importantly, there are no traces of egg proteins in the final product, so the vaccine is safe for people with egg allergies.
_
What viruses are recommended by WHO to be included in influenza vaccines for use in the 2015-16 northern hemisphere influenza season?
WHO recommends that influenza vaccines for use in the 2015-16 northern hemisphere influenza season contain the following viruses:
– an A/California/7/2009 (H1N1)pdm09-like virus
– an A/Switzerland/9715293/2013 (H3N2)-like virus
– a B/Phuket/3073/2013-like virus.
It is recommended that quadrivalent vaccines containing two influenza B viruses contain the above three viruses and a B/Brisbane/60/2008-like virus.
_
Vaccine antigen production in transgenic plants: strategies, gene constructs and perspectives:
Stable integration of a gene into the plant nuclear or chloroplast genome can transform higher plants (e.g. tobacco, potato, tomato, banana) into bioreactors for the production of subunit vaccines for oral or parental administration. This can also be achieved by using recombinant plant viruses as transient expression vectors in infected plants. The use of plant-derived vaccines may overcome some of the major problems encountered with traditional vaccination against infectious diseases, autoimmune diseases and tumours. They also offer a convenient tool against the threat of bio-terrorism.
____
Vaccine components:
Any vaccine consists of two parts, active ingredient and excipients. Active ingredient means immunogen (antigen) that stimulates immunity and excipient is any vaccine component besides immunogen that help improve vaccine efficacy, safety and shelf life.
_
Vaccine Excipients (components of vaccine besides immunogen):
Beside the active vaccine itself, the following excipients are commonly present in vaccine preparations:
| Type of Ingredient | Examples | Purpose |
| Preservatives | Thimerosal (only in multi-dose vials of flu vaccine) | To prevent contamination |
| Adjuvants | Aluminum salts | To help stimulate the body’s response to the antigens |
| Stabilizers | Sugars, gelatin | To keep the vaccine potent during transportation and storage |
| Residual cell culture materials | Egg protein | To grow enough of the virus or bacteria to make the vaccine |
| Residual inactivating ingredients | Formaldehyde | To kill viruses or inactivate toxins during the manufacturing process |
| Residual antibiotics | Neomycin | To prevent contamination by bacteria during the vaccine manufacturing process |
_
Vaccines contain live viruses, killed viruses, purified viral proteins, inactivated bacterial toxins, or bacterial polysaccharides. In addition to these immunogens, vaccines often contain other substances. For example, vaccines may contain preservatives that prevent bacterial or fungal contamination (e.g., thimerosal); adjuvants that enhance antigen-specific immune responses (e.g., aluminum salts); or additives that stabilize live, attenuated viruses (e.g., gelatin, human serum albumin). Furthermore, vaccines may contain residual quantities of substances used during the manufacturing process (e.g., formaldehyde, antibiotics, egg proteins, yeast proteins). Researchers reviewed data on thimerosal, aluminum, gelatin, human serum albumin, formaldehyde, antibiotics, egg proteins, and yeast proteins. Both gelatin and egg proteins are contained in vaccines in quantities sufficient to induce rare instances of severe, immediate-type hypersensitivity reactions. However, quantities of mercury, aluminum, formaldehyde, human serum albumin, antibiotics, and yeast proteins in vaccines have not been found to be harmful in humans or experimental animals. Parents should be reassured that quantities of mercury, aluminum, and formaldehyde contained in vaccines are likely to be harmless on the basis of exposure studies in humans or experimental studies in animals. Although severe anaphylactic reactions may occur rarely after receipt of vaccines that contain sufficient quantities of egg proteins (e.g., influenza, yellow fever) or gelatin (e.g., MMR), children who are at risk for severe infection with influenza can be desensitized to influenza vaccine, and gelatin-specific allergies are very rare. Immediate-type hypersensitivity reactions to neomycin or yeast proteins have not been clearly documented and remain theoretical.
_______
Preservative:
Preservatives may be defined as compounds that kill or prevent the growth of microorganisms, particularly bacteria and fungi. They are used in vaccines to prevent microbial growth in the event that the vaccine is accidentally contaminated, as might occur with repeated puncture of multi-dose vials. In some cases, preservatives are added during manufacture to prevent microbial growth; with changes in manufacturing technology, however, the need to add preservatives during the manufacturing process has decreased markedly. The United States Code of Federal Regulations (the CFR) requires, in general, the addition of a preservative to multi-dose vials of vaccines; indeed, worldwide, preservatives are routinely added to multi-dose vials of vaccine. Tragic consequences have followed the use of multi-dose vials that did not contain a preservative and have served as the impetus for this requirement. One particularly telling incident from Australia is described by Sir Graham S. Wilson in his classic book, The Hazards of Immunization. In January 1928, in the early stages of an immunization campaign against diphtheria, Dr. Ewing George Thomson, Medical Officer of Health of Bundaberg, began the injection of children with toxin-antitoxin mixture. The material was taken from an India-rubber-capped bottle containing 10 mL of TAM. On the 17th, 20th, 21, and 24th January, Dr. Thomson injected subcutaneously a total of 21 children without ill effect. On the 27th a further 21 children were injected. Of these children, eleven died on the 28th and one on the 29th. This disaster was investigated by a Royal Commission and the final sentence in the summary of their findings reads as follows: The consideration of all possible evidence concerning the deaths at Bundeberg points to the injection of living staphylococci as the cause of the fatalities. From this experience, the Royal Commission recommended that biological products in which the growth of a pathogenic organism is possible should not be issued in containers for repeated use unless there is a sufficient concentration of antiseptic (preservative) to inhibit bacterial growth.
_
Several preservatives are available, including thiomersal, phenoxyethanol, and formaldehyde. Thiomersal is more effective against bacteria, has a better shelf-life, and improves vaccine stability, potency, and safety; but, in the U.S., the European Union, and a few other affluent countries, it is no longer used as a preservative in childhood vaccines, as a precautionary measure due to its mercury content. Although controversial claims have been made that thiomersal contributes to autism, no convincing scientific evidence supports these claims. Over the past several years, because of an increasing awareness of the theoretical potential for neurotoxicity of even low levels of organomercurials and because of the increased number of thimerosal containing vaccines that had been added to the infant immunization schedule, concerns about the use of thimerosal in vaccines and other products have been raised. Indeed, because of these concerns, the Food and Drug Administration has worked with, and continues to work with, vaccine manufacturers to reduce or eliminate thimerosal from vaccines. Thimerosal has been removed from or reduced to trace amounts in all vaccines routinely recommended for children 6 years of age and younger, with the exception of inactivated influenza vaccine. A preservative-free version of the inactivated influenza vaccine (contains trace amounts of thimerosal) is available in limited supply at this time for use in infants, children and pregnant women. Some vaccines such as Td, which is indicated for older children (≥ 7 years of age) and adults, are also now available in formulations that are free of thimerosal or contain only trace amounts. Vaccines with trace amounts of thimerosal contain 1 microgram or less of mercury per dose.
_
Thimerosal (thiomersal):
•Thimerosal is a preservative that is used in the manufacturing process of some vaccines and other medicines to prevent the growth of bacteria and fungi, which could otherwise cause illness or injury.
•It metabolizes into ethylmercury, not methylmercury, a mistake commonly made by anti-vaxxers who claim that the amount of mercury that used to be in vaccine exceeded EPA exposure guidelines of 0.1mcg/kg/day. Those guidelines are for methylmercury, a compound that has a half-life in the body of several weeks to months and is often found in fish or other environmental exposures. Ethylmercury, on the other hand, has a half-life of a few days to about a week, meaning that it is not in the body long enough for it to build up to toxic levels from vaccination to vaccination.
•Concern has been raised about ethylmercury in thimerosal causing damage to the brain. However, this compound does not readily cross the blood-brain barrier. Some may counter this by talking about inorganic mercury, but this form of mercury, also, does not readily cross the barrier; only prolonged (regular) exposure to mercury leads to accumulation in the central nervous system. Moreover, total mercury levels that do accumulate in the brain are cleared much more rapidly after ethylmercury exposure than after methylmercury exposure (though limitations of the linked study are that it was done in animals and overall dosing may not accurately reflect dosing in humans).
•Using the EPA guidelines for methylmercury, a 3.2 kg newborn could be exposed to 0.32mcg of methylmercury every day without adverse health effects. This amounts to 116.8mcg of methylmercury in the course of a year, assuming an exposure of 0.1mcg/kg every single day. This also assumes that the child does not gain any weight over the course of that year, which would drive the adverse effect-free exposure limit higher. Keeping in mind that ethylmercury is eliminated significantly faster than methylmercury, the maximum 25mcg/dose of ethylmercury in a thimerosal-containing flu shot is much lower than the EPA one-year exposure. Therefore, unless the child is regularly exposed to other sources of mercury, it is highly unlikely that the minute amounts in a flu vaccine will cause any adverse developmental effects. But, for those who are still concerned about thimerosal, thimerosal-free versions of the flu vaccine are available.
•Some people say that you get much less mercury when you eat it than when you inject it. Looking at the most common form of ingested mercury (methylmercury), which is found in varying amounts in nearly all seafood, we will see that there is actually greater exposure from eating 6 oz. of white tuna, for example, than receiving one flu shot, the only recommended vaccine that has greater than trace amounts, though thimerosal-free versions are available. According to the DHHS Agency for Toxic Substances and Disease Registry, roughly 95% of ingested methylmercury is absorbed via the gastrointestinal tract (stomach and intestines), from whence it can then spread to other body organs. White albacore tuna contains about 0.407 ppm (mcg/g) methylmercury. A 6 oz. (170 g) can of white tuna would then contain on average about 69.19 mcg of mercury (170 g X 0.407 mcg/g = 69.19 mcg). Eating the full 6 oz. can, then, would mean that you are absorbing 65.73 mcg of methylmercury. That’s over two and a half times the amount of mecury from a thimerosal-containing flu vaccine (which tops out at 25 mcg/dose). And remember, the methylmercury from the tuna sticks around much longer than the ethylmercury from the vaccine.
•It was removed from the final product of nearly all U.S. vaccines around 2001/2002. This was a political move, due in large part to public pressure, rather than based on sound science. This was a recommendation rather than a regulatory requirement. A handful of studies that suggested problems with thimerosal, but which were inconclusive, prompted a “better safe than sorry” approach from the FDA while the issue was investigated by FDA, CDC and others. No follow-up studies have found any health risks beyond local hypersensitivity.
•Some vaccines still use it during the manufacturing process, but remove it from the final product, leaving, at most, trace amounts. The influenza vaccine still uses thimerosal, though thimerosal-free versions are available.
•Despite the removal of thimerosal from vaccines, resulting in exposure levels lower than anytime in the past, autism rates have not declined, suggesting that there is no connection between thimerosal and autism.
•To date, no properly controlled study has shown a causal link between thimerosal and autism.
_
Thimerosal was removed from all childhood vaccines in 2001 with the exception of inactivated flu vaccine in multi-dose vials. However, thimerosal has been removed from all single-dose preparations of flu vaccine for children and adults. There has never been thimerosal in live attenuated flu vaccine or recombinant flu vaccine. No acceptable alternative preservative has yet been identified for multi-dose flu vaccine vials.
_
Thiomersal is a toxic compound, there is no denying that. But let’s get back to math. The toxicity of compounds is measured through an analysis called the dose-response relationship, which describes the change in effect on an organism caused by differing doses of a compound after a certain exposure time. Table salt is tasty and safe in small amounts, but could kill you if taken in huge amounts. The dose-response relationship provides a graph that mathematically establishes what amounts of a compound causes what effects. This would seem to be a logical, and easily understood concept, but for many individuals, a bad substance is always bad. First of all, the half-life of thiomersal in blood is around 2.2 days. That might seem long, but it means half is gone in a couple of days, cleared out by the kidneys. It does not accumulate. But the math is even more telling. This flu vaccine, given once a year, has a maximum dose of 25 micrograms of mercury (but not elemental mercury). According to the thiomersal Material Safety Data Sheet (MSDS), the LD50, that is, the approximate dose at which 50% of organisms will die (in this case a mouse), is 5011 mg/kg body weight. Suppose a 20 kg child would get 25 micrograms of non-elemental mercury in one injection once a year. The theoretical LD50 dose for that same child would be around 100 grams of thiomersal, or about 4 million times higher than the amount of thiomersal in one vaccine dose–if vaccines used in children actually had thiomersal, which it doesn’t. So, you would have to inject your child 4 million times a day, every day, to make it potentially toxic. And dose-response relationships are not linear. That doesn’t mean that there’s some tiny risk of death from even a small dose of thiomersal–there is actually no risk. And again, since there’s no thiomersal in pediatric vaccines this argument is ridiculous. But more than all that, we have solid scientific data that show us that thiomersal is totally unrelated to autism, and is completely safe in vaccines. This illogical removal of thiomersal from vaccines makes it nearly impossible to have multi-use vials, so every vaccine has to be in a single-use prefilled syringe, which has rapidly driven up the costs of vaccines.
_
A recent study published in the Lancet medical journal showed the blood mercury levels of infants who received vaccines that contained thimerosal were well below all the safety levels set by government agencies. The Lancet study looked at 61 infants, most having blood-mercury levels below 2 nanograms per milliliter after vaccination; the highest safety limit, set by the Environmental Protection Agency, is 5.8 nanograms. Some critics counter that the study was too small and that delays in testing some of the infants may have missed the peak blood-mercury levels. In an effort to address some of these issues, another study of 200 children is going on in Argentina.
_________
Adjuvant:
An adjuvant (from Latin, adiuvare: to aid) is a pharmacological and/or immunological agent that modifies the effect of other agents. Adjuvants may be added to vaccine to modify the immune response by boosting it such as to give a higher amount of antibodies and a longer lasting protection, thus minimizing the amount of injected foreign material. Adjuvants may also be used to enhance the efficacy of vaccine by helping to subvert the immune response to particular cells type of immune system, for example by activating the T cells instead of antibody-secreting B cells depending on the type of the vaccine. Adjuvants are also used in the production of antibodies from immunized animals. The adjuvants would fall into two classes, either delivery systems (such as cationic microparticles) or immune potentiators (such as cytokines or PRRs). The delivery systems would possibly be used to concentrate and display antigens in repetitious patterns, to assist in localizing antigens and immune potentiators, and to target the antigens in the vaccine to the antigen-presenting cells. While, the immune potentiators would be used activate the innate immune system directly. There are different classes of adjuvants that can push immune response in different directions, but the most commonly used adjuvants include aluminum hydroxide and paraffin oil.
_
Immunologic adjuvants are added to vaccines to stimulate the immune system’s response to the target antigen, but do not in themselves confer immunity. Adjuvants can act in various ways in presenting an antigen to the immune system. Adjuvants can act as a depot for the antigen, presenting the antigen over a long period of time, thus maximizing the immune response before the body clears the antigen. Examples of depot type adjuvants are oil emulsions. Adjuvants can also act as an irritant which causes the body to recruit and amplify its immune response. A tetanus, diphtheria, and pertussis vaccine, for example, contains minute quantities of toxins produced by each of the target bacteria, but also contains some aluminium hydroxide. Such aluminium salts are common adjuvants in vaccines and have been used in vaccines for over 80 years. The body’s immune system develops an antitoxin to the bacteria’s toxins, not to the aluminium, but would not respond enough without the help of the aluminium adjuvant.
_
Types of adjuvants:
•Inorganic compounds: alum, aluminum hydroxide, aluminum phosphate, calcium phosphate hydroxide
•Mineral oil: paraffin oil
•Bacterial products: killed bacteria Bordetella pertussis, Mycobacterium bovis, toxoids
•Nonbacterial organics: squalene, thimerosal
•Delivery systems: detergents (Quil A)
•Cytokines: IL-1, IL-2, IL-12
•Combination: Freund’s complete adjuvant, Freund’s incomplete adjuvant
_
Alum as an adjuvant:
Alum is the most commonly used adjuvant in human vaccination. It is found in numerous vaccines, including diphtheria-tetanus-pertussis, human papillomavirus, and hepatitis vaccines. For almost 80 years, aluminium salts (referred to as ‘alum’) have been the only adjuvant in use in human vaccines. Only in the last two decades, have novel adjuvants (MF59®, AS04) been introduced in the formulation of new licensed vaccines. As our understanding of the mechanisms of ‘immunogenicity’ and ‘adjuvancy’ increases, new adjuvants and adjuvant formulations are being developed.
_
Mechanisms of adjuvant action:
Adjuvants may exert their effects through different mechanisms. Some adjuvants, such as alum and emulsions (e.g. MF59®), function as delivery systems by generating depots that trap antigens at the injection site, providing slow release in order to continue the stimulation of the immune system. These adjuvants enhance the antigen persistence at the injection site and increase recruitment and activation of antigen presenting cells (APCs). Particulate adjuvants (e.g. alum) have the capability to bind antigens to form multi-molecular aggregates which will encourage uptake by APCs. Some adjuvants are also capable of directing antigen presentation by the major histocompatibility complexes (MHC). Other adjuvants, essentially ligands for pattern recognition receptors (PRR), act by inducing the innate immunity, predominantly targeting the APCs and consequently influencing the adaptative immune response. Adjuvants accomplish this task by mimicking specific sets of evolutionarily conserved molecules, so called PAMPs, which include liposomes, lipopolysaccharide (LPS), molecular cages for antigen, components of bacterial cell walls, and endocytosed nucleic acids such as double-stranded RNA (dsRNA), single-stranded DNA (ssDNA), and unmethylated CpG dinucleotide-containing DNA. Because immune systems have evolved to recognize these specific antigenic moieties, the presence of an adjuvant in conjunction with the vaccine can greatly increase the innate immune response to the antigen by augmenting the activities of dendritic cells (DCs), lymphocytes, and macrophages by mimicking a natural infection. Alum is the most commonly used adjuvant in human vaccination. It is found in numerous vaccines, including diphtheria-tetanus-pertussis, human papillomavirus and hepatitis vaccines. Alum provokes a strong Th2 response, but is rather ineffective against pathogens that require Th1–cell-mediated immunity. Alum induces the immune response by a depot effect and activation of APCs. Recently, the NLRP3 inflammasome has been linked to the immunostimulatory properties of alum although its role in adjuvant-induced antibody responses remains controversial. Emulsions (either oil-in-water or water-in-oil), such as Freund’s Incomplete Adjuvant (IFA) and MF59®, can trigger depot generation and induction of MHC responses. IFA induces a predominantly Th2 biased response with some Th1 cellular response. MF59® is a potent stimulator of both cellular (Th1) and humoral (Th2) immune responses. However, the precise mode of action of emulsion-based adjuvants is still unclear. A complication with emulsion-based adjuvants is their potential to induce autoimmunity.
__
Aluminum salts include aluminum hydroxide, aluminum phosphate, and potassium aluminum sulfate (alum). Aluminum-containing vaccines are prepared by adsorption of antigens onto aluminum hydroxide or aluminum phosphate gels or by precipitation of antigens in a solution of alum. Aluminum salts were found initially to enhance immune responses after immunization with diphtheria and tetanus toxoids in studies performed in the 1930s, 1940s, and 1950s.The safety of aluminum has been established by experience during the past 80 years, with hundreds of millions of people inoculated with aluminum-containing vaccines. Adverse reactions including erythema, subcutaneous nodules, contact hypersensitivity, and granulomatous inflammation have been observed rarely. Aluminum-containing vaccines are not the only source of aluminum exposure for infants. Because aluminum is one of the most abundant elements in the earth’s crust and is present in air, food, and water, all infants are exposed to aluminum in the environment. For example, breast milk contains approximately 40 μg of aluminum per liter, and infant formulas contain an average of approximately 225 μg of aluminum per liter. Vaccines contain quantities of aluminum similar to those contained in infant formulas. However, because large quantities of aluminum can cause serious neurologic effects in humans, guidelines are established.
_
For determining the quantity of aluminum below which safety is likely, data were generated in mice that were inoculated orally with various quantities of aluminum lactate. No adverse reactions were observed when mice were fed quantities of aluminum as high as 62 mg/kg/day. By applying uncertainty factors of 3 (for extrapolation to humans) and 10 (for human variability), the ATSDR concluded that the minimum risk level for exposure to aluminum was 2 mg/kg/day. The half-life of elimination of aluminum from the body is approximately 24 hours. Therefore, the burden of aluminum to which infants are exposed in food and vaccines is clearly less than the guideline established by the ATSDR and far less than that found to be safe in experimental animals.
_
Vaccines contain aluminum in a salt form. Anti-vaxers claim this is toxic, and some will cite that 4ppm will cause blood to coagulate. However, individuals are not exposed to such amounts of aluminum in a single vaccination visit. Below are the vaccines containing aluminum, with the corresponding parts per million (ppm) for an infant (~251 mL of blood in the body) and an 80lb. child (~4000 mL of blood); note the two numbers for DTaP represent extreme ranges of aluminum content:
| Vaccine | ppm in infant | ppm in child | age received (in months) |
| DTaP (170mcg) | 0.677 | 0.043 | 2, 4, 6, w/ final ~4-6 yrs |
| DTaP(625mcg) | 2.490 | 0.156 | |
| Hep A | 0.996 | 0.063 | 12 w/ final ~6 mo. later |
| Hep B | 0.996 | 0.063 | birth, 1 or 2, final at 6+ |
| HiB | 0.896 | 0.056 | 2, 4 |
| HPV | 0.896 | 0.056 | 11 or 12 yrs., then 2, 6 mo. |
| Pediatrix | 3.386 | 0.213 | 2, 4, 6 (in lieu of DTaP, IPV and Hep B) |
| Pentacel | 1.315 | 0.083 | 2, 4, 6, 15-18 (in lieu of DTaP, IPV and HiB) |
| Pneumococcus | 0.498 | 0.031 | 2, 4, 6, 12-15 |
_
CFR 610.15 lists the maximum amount of aluminum per dose in vaccines, depending on the method of calculation. This ranges from 0.85mg (that’s milligrams) to 1.25 mg. HepB vaccine contains 250 mcg (that’s micrograms) per dose, or 0.25mg.
_
This table lists main categories of adjuvants and formulations evaluated in humans:
| Adjuvant/formulations | Pathogen (antigen) | Trial results |
| MINERAL SALTS | ||
| Aluminium salts (hydroxide, phosphate, alum) | Numerous antigens | Licensed for human use. Induction of strong antibody responses |
| Calcium phosphate | DT | Was found to be better than Al(OH)3 in a booster trial |
| SBAS-4/ASO4 (alum + MPL) | HBV (HBs antigen), HSV (gD) | Increased antibody titres and lymphoproliferative responses when compared with alum, increased seroconversion rate after 2 immunizations |
| EMULSIONS | ||
| MF59 (stabilized squalene/sater) | Flu (split trivalent) | Component of a licensed influenza vaccine. Increase vaccine immunogenicity in young adults and in elderly (HAI titre). Safe (only mild local reactions), even after repeated injections in elderly |
| HBV(rPreS2-S) | More immunogenic than alum-adsorbed licensed hepatitis B vaccine | |
| HSV-2 (rgB + rgD) | Prophylactic vaccination: humoral and cellular immunity after 3 injections is superior to natural immunity after HSV-2 infection. A therapeutic vaccination trial in patients with recurrent genital herpes showed no improvement in rate of recurrence but both severity and duration of 1st outbreak were reduced | |
| HIV1 (gp120), CMV (rgB) | Improved immunogenicity over alum | |
| MF59 + MTP-PE | Flu (trivalent split), HIV1 (env) | MTP-PE increases reactogenicity, with no overall improvement in terms of immunogenicity (equivalent to MF59) |
| QS21 (purified saponin from Quillaja saponaria) | Malaria (SPf), HIV (gp120), melanoma, pneumo conj | Some local reactions. Enhanced antibody responses. Limited cellular responses in humans, despite good results obtained in animal models. QS21 enhances by 2-fold the booster effect (antibody response) of second dose of conjugate polysaccharide vaccine against Neisseria pneumoniae |
| SBAS-2/ASO2 (squalene/water + MPL + QS21) | Malaria (RTS,S) | High anti-CSP titres (better than with squalene/water or with MPL + alum) after 3 immunizations. Short-lived protection (less than 6 months) of 7 out of 8 naive individuals against challenge (infected mosquito bites). RTS,S-specific lymphoproliferative and antibody responses but no induction of CD8+ CTLs |
| HIV-1 (rgp120) | Increased seroconversion rate in seronegative subjects after single immunization (superior to MPL + QS21 or alum). Strong cell-mediated immunity (T-cell proliferation; superior to MPL + QS21), but no CD8+ CTLs. No detectable neutralizing antibodies against primary isolates | |
| Incomplete Freund adjuvant (IFA, stabilized water/Drakeol) | gp120-depleted inactivated HIV-1 | REMUNE vaccine. Increased anti-p24 titres and DTH responses. In seropositive subjects: increased lymphoproliferation and β-chemokine (Rantes, MIP-1α, MIP-1β) production following p24 stimulation |
| Melanoma (gp100) | Induction of T-cell responses (evaluated by ELISPOT/IFN production) against gp100 HLA A2 restricted epitopes | |
| Montanide ISA51 (stabilized water/Drakeol) | HIV-1 (Tat toxoid) | Well tolerated. Increased anti-Tat antibody titres in 100% of the subjects. DTH response and lymphoproliferation to Tat in 50% of the subjects |
| Montanide ISA720 (stabilized water/squalene) | Malaria (MSP1, MSP2, Resa AMA1) | Well tolerated (minor local effects – tenderness, swelling and discomfort of use). Low antibody responses (equivalent to alum, despite superior antibody responses observed in animals). Strong lymphoproliferation |
| NATURAL/SYNTHETIC BACTERIAL PRODUCTS | ||
| Monophosphoryl lipid A (MPL) | Various antigens | Well tolerated in humans when administered in association with bacterial antigens or TAAs. Limited increase of cellular responses |
| Detox (stabilized squalene/water + MPL + CWS) | Malaria (R32NS18) | Some side-effects in malaria naive individuals (tenderness, induration, oedema + malaise and fever). Induction of anti-CSP antibodies after 3 immunizations (better than alum). Protection of 2/11 naive individuals against challenge with infected mosquitoes |
| Melanoma cell lysates | Induction of cellular and humoral responses against melanoma associated antigens. Increase in survival in patients with metastatic melanoma. Vaccine (Melacine) has been registered for this indication in Canada | |
| RC-529 (synthetic MPL-like acylated monosaccharide) | HBV (HBs) | Th1 and mucosal adjuvant in mice. Found to enhance, in association with alum, antibody responses against HBs antigen in humans (faster and stronger seroconversion) |
| OM-174 (lipid A derivative, E. coli), OM triacyl | Malaria (CSP), cancer | OM-174 was found to be safe in a phase I study in cancer patients (i.m. route). OM triacyl adjuvants are synthetic analogues based on a common triacyl motif, which induce maturation of human dendritic cells in vitro |
| Holotoxins (CT, PT, LT) | Various antigens | Utilization of detoxified bacterial toxins (mutated toxins or B subunits) devoided of ADP-ribosyltransferase activity. Enhancement of serous and mucosal IgA production. On-going evaluation of CT and LT as adjuvants in patch-based transcutaneous immunization. A flu vaccine with LT mutants is about to be tested intranasally in humans |
| CpG oligonucleotides | Hepatitis B (HBs) | Act as potent Th1 adjuvants in mice, chimpanzees and orang utangs. Two phase I trials conducted in humans (in association with alum) have shown enhanced antibody responses against the HBs antigen. CTL responses not documented. Based on the motif and chemical backbone, three classes of oligonucleotides are now defined with respect to their distinct capacity to activate either human B-, NK- or dendritic cells in vitro |
| IMMUNOADJUVANTS | ||
| Cytokines (IL-2, IL-12, GM-CSF) | TAAs, malaria (CSP, MSP1), hepatitis A and B | Utilization of cytokines as recombinant proteins, with limitations including short biological half-life and some severe toxicity (vascular leak syndrome, hepatotoxicity for IL-2 and IL-12, respectively). Enhancement of antibody responses with GM-CSF. More recently, utilization of recombinant vectors expressing locally (intratumourally) immunostimulatory cytokines (e.g. poxviruses) |
| Accessory molecules (B7.1) | Colorectal cancer (CEA) | The accessory molecule (B7.1), which provides co-stimulatory signals to T lymphocytes, has been included in association with the CEA antigen within the canarypox vector ALVAC, thereby enhancing cellular responses |
| PARTICULATE FORMULATIONS | ||
| Liposomes (DMPC/Chol) | Flu (monovalent split) | Well tolerated. No increase in antibody titers (equivalent to vaccine alone). Slight increase in CD8+ CTL response |
| DC Chol | H. pylori (urease) | Despite enhanced antibody and Th2/Th1 responses in animal models, no significant enhancement of cellular immune responses in humans |
| Virosomes | Hepatitis A, flu | Well tolerated. Rapid seroconversion leading to protective anti-hepatitis A or anti-influenza virus antibodies |
| ISCOMS (structured complex of saponins and lipids) | Flu (trivalent split), HPV16 (E6/E7) | Increase of influenza-specific CD8+ CTL response (when compared with flu vaccine alone) |
| PLGA | TT | PLGA particles were shown to elicit Th1 (presentation of CTL epitopes) and Th2 responses in mice. On-going trial with the tetanus toxoid: a difficulty is to prepare GMP-grade PLGA particles under aseptic conditions |
CSP, P. falciparum circumsporozoite; CWS, cell wall skeleton from Mycobacterium phlei; DT, diphtheria toxoid; MTP-PE, muramyl tripeptide dipalmitoyl phosphatidyl ethanolamine; PLGA, poly-(D,L)-lactide-co-glycolic acid; TAAs, tumour associated antigens; TT, tetanus toxoid.
_
Antigen particulate formulations:
Apart from simply admixing the antigen with the adjuvant, formulation strategies may aim to facilitate the capture and the entry of the antigen into antigen presenting cells. For example, formulating T-cell antigens, expressed as peptides, proteins, plasmid DNA or even RNA into cationic liposomes appears to increase CTL responses in vivo in animal models. Liposomes are artificial, spherical, closed vesicles which consist of one or more lipid bilayers. Liposome-encapsulated antigens are delivered more efficiently to the cytoplasm of APCs, presumably as a result of membrane fusion. Usually, liposomes are made from ester phospholipids. More recently, polar phospholipids from archebacteriae have also been used, leading to so-called ‘archeosomes’. The latter are based on regularly branched phytanyl chains, with 20 or 40 carbon length. Archeosomes demonstrate better stabilities to high temperature, alkaline pH, serum proteins, when compared with conventional liposomes. Other formulations being explored include spherulites (multilamellar vesicles made of biocompatible amphiphiles) and transfersomes (highly deformable vesicles which can deliver small molecules non-invasively through the skin). One liposome-based approach has proven successful in humans: in this approach, antigens derived from the hepatitis A or influenza virus have been incorporated into a mixture of natural and synthetic phospholipids, called virosomes . Such vaccines were shown to be well tolerated and to induce both a 100% seroconversion rate and high antibody titers within 2 weeks.
_
Immunostimulating complexes (ISCOMS):
1. An alternative vaccine vehicle
2. The antigen is presented in an accessible, multimeric, physically well defined complex
3. Composed of adjuvant (Quil A) and antigen held in a cage like structure
4. Adjuvant is held to the antigen by lipids
5. Can stimulate CMI
6. Mean diameter 35nm
In the most successful procedure, a mixture of the plant glycoside saponin, cholesterol and phosphatidylcholine provides a vehicle for presentation of several copies of the protein on a cage-like structure. Such a multimeric presentation mimics the natural situation of antigens on microorganisms. These immunostimulating complexes have activities equivalent to those of the virus particles from which the proteins are derived, thus holding out great promise for the presentation of genetically engineered proteins. Similar considerations apply to the presentation of peptides. It has been shown that by building the peptide into a framework of lysine residues so that 8 copies instead of 1 copy are present, the immune response induced was of a much greater magnitude. A novel approach involves the presentation of the peptide in a polymeric form combined with T cell epitopes. The sequence coding for the foot and mouth disease virus peptide was expressed as part of a fusion protein with the gene coding for the Hepatitis B core protein. The hybrid protein, which forms spherical particles 22nm in diameter, elicited levels of neutralizing antibodies against foot and mouth disease virus that were at least a hundred times greater than those produced by the monomeric peptide.
_
Additives:
Additives are used to prevent antigens from adhering to the sides of glass vials with a resultant loss in immunogenicity. The types of additives used in vaccines include sugars (e.g., sucrose, lactose), amino acids (e.g., glycine, monosodium salt of glutamic acid), and proteins (e.g., gelatin or human serum albumin). Three issues surround the use of protein additives in vaccines: 1) the observation that immediate-type hypersensitivity reactions are a rare consequence of receiving gelatin-containing vaccines, 2) the theoretical concern that human serum albumin might contain infectious agents, and 3) the theoretical concern that bovine-derived materials used in vaccines might contain the agent associated with bovine spongiform encephalopathy (“mad-cow” disease).
_
Stabilizers:
Stabilizers are used to help the vaccine maintain its effectiveness during storage. Vaccine stability is essential, particularly where the cold chain is unreliable. Instability can cause loss of antigenicity and decreased infectivity of LAV. Factors affecting stability are temperature and acidity or alkalinity of the vaccine (pH). Bacterial vaccines can become unstable due to hydrolysis and aggregation of protein and carbohydrate molecules. Stabilizing agents include MgCl2 (for OPV), MgSO4 (for measles), lactose-sorbitol and sorbitol-gelatine.
_
Diluents:
A diluent is a liquid used to dilute a vaccine to the proper concentration. In vaccines, this is usually sterile saline or water.
_______
Manufacturing residuals:
Residuals are substances used in the production of vaccine that remain as residual quantity in the final product. They are inactivating agents (e.g., formaldehyde), antibiotics, and cellular residuals (e.g., egg and yeast proteins).
_
Inactivating Agents:
Inactivating agents separate a pathogen’s immunogenicity from its virulence by eliminating the harmful effects of bacterial toxins or ablating the capacity of infectious viruses to replicate. Examples of inactivating agents include formaldehyde, which is used to inactivate influenza virus, poliovirus, and diphtheria and tetanus toxins; β-propiolactone, which is used to inactivate rabies virus; and glutaraldehyde, which is used to inactivate toxins contained in acellular pertussis vaccines. Formaldehyde deserves special consideration.
_
Formaldehyde:
Concerns about the safety of formaldehyde have centered on the observation that high concentrations of formaldehyde can damage DNA and cause cancerous changes in cells in vitro. Although formaldehyde is diluted during the manufacturing process, residual quantities of formaldehyde may be found in several current vaccines. Fortunately, formaldehyde does not seem to be a cause of cancer in humans and animals that are exposed to large quantities of formaldehyde (a single dose of 25 mg/kg or chronic exposure at doses of 80–100 mg/kg/day) do not develop malignancies. The quantity of formaldehyde contained in individual vaccines does not exceed 0.1 mg. This quantity of formaldehyde is considered to be safe for 2 reasons. First, formaldehyde is an essential intermediate in human metabolism and is required for the synthesis of thymidine, purines, and amino acids. Therefore, all humans have detectable quantities of formaldehyde in their circulation (approximately 2.5 μg of formaldehyde/mL of blood). Assuming an average weight of a 2-month-old of 5 kg and an average blood volume of 85 mL/kg, the total quantity of formaldehyde found naturally in an infant’s circulation would be approximately 1.1 mg—a value at least 10-fold greater than that contained in any individual vaccine. Second, quantities of formaldehyde at least 600-fold greater than that contained in vaccines have been given safely to animals.
_
We are exposed to formaldehyde anyway:
Our primary route of exposure is breathing it, indoors or outdoors. Much of this inhaled formaldehyde comes from car exhaust, tobacco smoke, power plants, forest fires and wood stoves. Outdoors, we are exposed to anywhere from 0 to 100 parts per billion (ppb) every day. Indoors, it can be as much as 500 to 2,000 ppb (temporary housing such as that used after hurricane Katrina measured from 3-590 ppb). To a smaller degree, we ingest it in our food and water (the average American diet contains about 10-20mg of formaldehyde from things like apples, carrots, pears, milk, etc.), as well as some exposure via cosmetics. According to the U.S. Environmental Protection Agency, humans can consume 0.2mg of formaldehyde per kilogram of weight every day without seeing any adverse effects. When setting these levels, the EPA uses a safety buffer of about 10-100 times, meaning that the true safe level for daily exposure is likely around 2-20mg/kg every day.
_
Looking at the recommended schedule of vaccines from the CDC, let’s pick the vaccines from that list that a child might receive in their first 6 years of life (picking the highest amounts, just for illustration):
•HepB – Recombivax – 3 doses (birth, 1-2 mos. and 6-18 mos.) – 7.5μg/dose
•DTaP – Infanrix – 5 doses (2 mos., 4 mos., 6 mos., 15-18 mos. and 4-6 yrs.) – 100μg/dose
•Hib – ActHIB – 3 doses (2 mos., 4 mos. and 12-15 mos.) – 0.5μg/dose
•IPV – IPOL – 4 doses (2 mos., 4 mos., 6-18 mos. and 4-6 yrs.) – 100μg/dose
•Influenza – Fluzone – 7 doses (6 mos., 12 mos. and yearly 2-6 yrs.) – 100μg/dose
•HepA – Havrix – 2 doses (12 mos. and 6-18 mos. after first dose) – 100μg/dose
That’s all of the vaccines on the recommended schedule for 0-6 years that contain formaldehyde. If a child got all of those doses all at once (which they never would), they would get a total of 1,824μg, or 1.824mg, of formaldehyde. A 3.2kg (~7lb) newborn with an average blood volume of 83.3mL/kg would naturally have, at any given time, about 575-862μg of formaldehyde circulating in their blood. By the time they are 6 years old (~46lb or 21kg), they’ll naturally have 3,562-5,342μg of formaldehyde in their blood. Bear in mind that the formaldehyde from each shot will not build up in their bodies from shot to shot, as it is very rapidly (within hours) metabolized and eliminated as formate in the urine or breathed out as CO2. So what’s the most a child might get in a single office visit? That would probably be at their 6 month visit (when they are, on average, 16.5lbs or 7.5kg) with HepB, DTaP, IPV and flu, for a total of 307.5μg. That is about 160 times less than the total amount their body naturally produces every single day. Compare that to the 428.4-1,516.4μg of formaldehyde in a single apple.
_
Antibiotics:
Antibiotics are present in some vaccines to prevent bacterial contamination during the manufacturing process. Because antibiotics can cause immediate-type hypersensitivity reactions in children, some parents are concerned that antibiotics that are contained in vaccines might be harmful. However, antibiotics that are most likely to cause immediate-type hypersensitivity reactions (e.g., penicillins, cephalosporins, sulfonamides) are not contained in vaccines. Antibiotics that are used during vaccine manufacture include neomycin, streptomycin, polymyxin B, chlortetracyline, and amphotericin B. Only neomycin is contained in vaccines in detectable quantities. However, immediate-type hypersensitivity reactions to the small quantities of neomycin contained in vaccines has not been clearly documented. Although neomycin-containing products have been found to cause delayed-type hypersensitivity reactions, these reactions are not a contraindication to receiving vaccines.
_
Cellular Residuals:
Egg Proteins:
Egg allergies occur in approximately 0.5% of the population and in approximately 5% of atopic children. Because influenza and yellow fever vaccines both are propagated in the allantoic sacs of chick embryos (eggs), egg proteins (primarily ovalbumin) are present in the final product. Residual quantities of egg proteins found in the influenza vaccine (approximately 0.02–1.0 μg/dose) are sufficient to induce severe and rarely fatal hypersensitivity reactions in children with egg allergies. Unfortunately, children with egg allergies also have other diseases (e.g., asthma) that are associated with a high risk of severe and occasionally fatal influenza infection. For this reason, children who have egg allergies and are at high risk of severe influenza infection should be given influenza vaccine via a strict protocol. In contrast to influenza vaccine, measles and mumps vaccines are propagated in chick embryo fibroblast cells in culture. The quantity of residual egg proteins found in measles- and mumps-containing vaccines is approximately 40 pg—a quantity at least 500-fold less than those found for influenza vaccines. The quantity of egg proteins found in measles- and mumps-containing vaccines is not sufficient to induce immediate-type hypersensitivity reactions, and children with severe egg allergies can receive these vaccines safely.
Yeast Proteins:
Hepatitis B vaccines are made by transfecting cells of Saccharomyces cerevisiae (baker’s yeast) with the gene that encodes hepatitis B surface antigen, and residual quantities of yeast proteins are contained in the final product. Engerix-B (GlaxoSmithKline) contains no more than 5 mg/mL and Recombivax HB (Merck and Co) contains no more than 1 mg/mL yeast proteins. Immediate-type hypersensitivity reactions have been observed rarely after receipt of hepatitis B vaccine (approximately 1 case per 600 000 doses). However, yeast-specific IgE has not been detected in patients with immediate-type hypersensitivity or in nonallergic patients after receipt of hepatitis B vaccine. Therefore, the risk of anaphylaxis after receipt of hepatitis B vaccine as a result of allergy to baker’s yeast is theoretical.
_
The table below shows various excipients (components besides immunogen) contained in various vaccines:
__________
__________
Animal experiments and clinical trials for vaccine safety and efficacy:
_
Development of New Vaccines:
The general stages of the development cycle of a vaccine are:
•Exploratory stage
•Pre-clinical stage
•Clinical development
•Regulatory review and approval
•Manufacturing
•Quality control
_
Nonclinical evaluation of vaccines:
Vaccines are administered to healthy humans, often in the first year of life. The demands for safety and efficacy are therefore very high. Nonclinical testing is a prerequisite to moving a candidate vaccine from the laboratory to the clinic and includes all aspects of testing, product characterization, proof of concept/immunogenicity studies and safety testing in animals conducted prior to clinical testing of the product in humans. The nonclinical evaluation of vaccines includes the initial testing of candidate formulations in animal models. In vivo and in vitro toxicity studies conducted before the start of clinical trials (preclinical) identify potential safety concerns and serve to avoid possible harm to human subjects. Potential concerns include toxicity due to the active ingredients or excipients, reactions to trace impurities such as production substrates, and interactions between components of other vaccines administered simultaneously. Studies designed to determine the right dose to induce an immune response in appropriate animal models can provide valuable information on the immune response that can be expected in humans, and guide the determination whether the candidate vaccine will be beneficial to both the human study participant and the wider population once marketed. But it must be recognized that there are limitations in animal testing; susceptibility to infection by viruses, bacteria, and other microorganisms are often highly specific, and the immune responses in an animal model, particularly at the elevated doses used for nonclinical testing, may not be predictive of what will ultimately occur in humans. Nevertheless, few people would accept the administration of a candidate medicinal product without some level of assurance of its acceptability in a living animal. Therefore, nonclinical testing continues to be a balance between the desire to reduce the use of animals for testing purposes against the rights of humans to be administered safe and effective vaccines. International harmonization of testing requirements is therefore an essential tool needed to establish uniform approaches to the determination of the safety and efficacy of medicinal products, as well as to restrict animal testing to those critical areas where it cannot be replaced by alternative means.
_
Nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines:
Over the past decades, strategies and approaches for the development and delivery of vaccine antigens have been expanded. Some of these antigens are weakly immunogenic and require the presence of adjuvants for the induction or enhancement of an adequate immune response. Vaccines with aluminium-based adjuvants have been used extensively in immunization programs worldwide and a significant body of safety information has accumulated for them. As the knowledge of immunology and the mechanisms of vaccine adjuvant action have developed, the number of vaccines containing novel adjuvants being evaluated in clinical trials has increased. Vaccines containing adjuvants other than aluminium-containing compounds have been authorized for use in many countries (e.g., human papillomavirus and hepatitis B vaccines), and a number of vaccines with novel adjuvants are currently under development, including, but not limited to, vaccines against human immunodeficiency virus (HIV), malaria and tuberculosis, as well as new-generation vaccines against influenza and other diseases.
_
The vaccine development paradigm:
Considerable attention has been focused in recent years on the versatile advances in modern biotechnology that are giving rise to the exciting new candidates that fill the upstream portion of the vaccine development pipeline. On the other hand, less notice has generally been paid to the series of sophisticated clinical vaccine studies that must be properly executed to advance a vaccine candidate, incrementally, towards ultimate licensure, based on proof of the vaccine’s safety, immunogenicity and efficacy in target populations.
_
Prelicensure Evaluations of Vaccine Safety:
Before vaccines are licensed by the FDA (any regulatory agency), they are evaluated in clinical trials with volunteers. These trials are conducted in three progressive phases:
Phase I trials:
Phase I trials preliminarily examine the candidate’s safety and immunogenicity in small numbers of healthy adults. Such early dose/response tests detect common adverse reactions and provide an initial glimpse of whether relevant immune responses are generated.
Phase II trials:
In Phase II, the clinical study is expanded and vaccine is given to people who have characteristics (such as age and physical health) similar to those for whom the new vaccine is intended. Phase II trials assess the vaccine in increasingly larger numbers of subjects, are typically placebo-controlled to better measure the rate of adverse reactions versus background rates of complaints. The level of shedding of a live viral or bacterial vaccine or of a recombinant strain is often intensively examined in phase II trials, as is its propensity to be transmitted to household contacts and to survive in the environment. For vaccines that will ultimately be used in infants and children, phase I and II trials must be undertaken in progressively younger subjects. Particularly demanding is the design of phase II clinical trials to evaluate the reactogenicity and immunogenicity of the new multivalent combination vaccines in infants. The ultimate objective of combining vaccine antigens into a single inoculation is worthy, but experience has shown that interactions may occur that depress the immune response to some antigens or that enhance the overall reactogenicity. Thus, phase II clinical trials must rigorously demonstrate that acceptable immune responses to all antigens can indeed be stimulated without undue reactogenicity. Phase I and II trials of certain candidate vaccines require special considerations (e.g. vaccines against RSV and group A Streptococcus pyogenes) because of safety concerns.
Experimental challenge studies:
In some instances, as with candidate vaccines to prevent influenza, Shigella dysentery, cholera or Plasmodium falciparum malaria, preliminary assessments of vaccine efficacy can be obtained through carefully performed experimental challenge studies with wild-type organisms in fully informed, consenting, adult community volunteers.
Phase III trials:
Large-scale, randomized, controlled phase III field trials remain the gold standard for demonstrating the efficacy of a vaccine. Such trials tend to be expensive, require several years to complete and are subject to the vagaries of year-to-year variation in disease incidence. Moreover, in prelicensure efficacy trials, the protective activity of a vaccine is measured under idealized conditions where extra personnel participate in the vaccination and only fully vaccinated subjects are included in calculations of efficacy; therefore, the practicality of programmatic use of the vaccine is not readily estimated. Epidemiological methods to estimate vaccine ‘efficacy’ (i.e. effectiveness) after licensure and large-scale use have also been developed.
Phase IV trials:
Many vaccines undergo Phase IV formal, ongoing studies after the vaccine is approved and licensed. Phase IV trial are optional studies that drug companies may conduct after a vaccine is released. The manufacturer may continue to test the vaccine for safety, efficacy, and other potential uses. Most phase IV assessments involve case/control studies which are relatively inexpensive and simple to perform, but have inherent limitations that can distort the estimation of ‘efficacy’. Nevertheless, a few controlled phase IV post-licensure selective vaccination trials have been performed that directly measure effectiveness of vaccine used under real-life, programmatic conditions.
_
_
A long journey fraught with many potential pitfalls and considerable attrition awaits any vaccine candidate as it attempts to run the gauntlet from inventive concept to licensed product and public health tool. Few of the vaccines that enter phase I trials reach the point of a phase III efficacy trial, and only a handful of vaccine candidates ultimately become licensed by regulatory agencies. Moreover, the step-wise paradigm by which vaccine candidates are advanced from initial phase I dose response safety/immunogenicity trials to phase II reactogenicity/immunogenicity trials in larger numbers of subjects, and finally to large-scale phase III efficacy trials is becoming increasingly complex and expensive. In particular, the cost of generating clinical trial data while strictly adhering to the rules and regulations of Good Clinical Practice and of performing quality assurance and monitoring to verify the validity of such data has greatly escalated during the past decade.
_
_
Postlicensure Monitoring of Vaccine Safety:
After licensure, a vaccine’s safety is assessed by several mechanisms. The NCVIA of 1986 requires health care providers to report certain adverse events that follow vaccination of children. As a mechanism for that reporting, the Vaccine Adverse Event Reporting System (VAERS) was established in 1990 and is jointly managed by the CDC and the FDA. This safety surveillance system collects reports of adverse events associated with vaccines currently licensed in the United States. Adverse events are defined as health effects that occur after immunization and that may or may not be related to the vaccine. While VAERS was established in response to the NCVIA, any adverse event following vaccination—whether in a child or an adult, and whether or not it is believed to have been caused by vaccination—may be reported through VAERS. In 2008, VAERS received >25,000 reports of adverse events following vaccination. Of those, 9.5% were reportedly serious, causing disability, hospitalization, life-threatening illness, or death. VAER related issues are discussed later on. Enhanced post-licensure epidemiological surveillance has proven its value by demonstrating herd immunity effects (as with H. influenzae type b and meningococcal C conjugate vaccines), non-target consequences of vaccine use (e.g. the rare occurrence of vaccine-associated paralytic poliomyelitis in household contacts of infants who have received Sabin live oral polio vaccine) and rare vaccine-associated adverse events.
_
Vaccine policy:
The following questions should be asked when a vaccination policy against a particular virus is being developed.
- What proportion of the population should be immunized to achieve eradication.
- What is the best age to immunize?
- How is this affected by birth rates and other factors
- How does immunization affect the age distribution of susceptible individuals, particularly those in age-classes most at risk of serious disease?
- How significant are genetic, social, or spatial heterogeneities in susceptibility to infection?
- How does this affect herd immunity?
_______
_______
Vaccine schedule for children, adolescents and adults:
Scheduling vaccine:
A vaccination schedule is a series of vaccinations, including the timing of all doses, which may be either recommended or compulsory, depending on the country of residence. A vaccine is an antigenic preparation used to produce active immunity to a disease, in order to prevent or reduce the effects of infection by any natural or “wild” pathogen. Many vaccines require multiple doses for maximum effectiveness, either to produce sufficient initial immune response or to boost response that fades over time. For example, tetanus vaccine boosters are often recommended every 10 years. Vaccine schedules are developed by governmental agencies or physicians groups to achieve maximum effectiveness using required and recommended vaccines for a locality while minimizing the number of health care system interactions. Over the past two decades, the recommended vaccination schedule has grown rapidly and become more complicated as many new vaccines have been developed. Some vaccines are recommended only in certain areas (countries, subnational areas, or at-risk populations) where a disease is common. For instance, yellow fever vaccination is on the routine vaccine schedule of French Guiana, is recommended in certain regions of Brazil but in the United States is only given to travelers heading to countries with a history of the disease. In developing countries, vaccine recommendations also take into account the level of health care access, the cost of vaccines and issues with vaccine availability and storage. Sample vaccination schedules discussed by the World Health Organization show a developed country using a schedule which extends over the first five years of a child’s life and uses vaccines which cost over $700 including administration costs while a developing country uses a schedule providing vaccines in the first 9 months of life and costing only $25. This difference is due to the lower cost of health care, the lower cost of many vaccines provided to developing nations, and that more expensive vaccines, often for less common diseases, are not utilized.
_
Until recently, most vaccines were aimed at babies and children alone. Now more and more vaccines are developed for use among elderly, pregnant mothers, adolescents, travelers and adults in a population. In addition, vaccines are increasingly being administered in form of combination of more than one component. Vaccinations of animals are being used both to prevent their contracting diseases and to prevent transmission of disease to humans. In 1900, the smallpox vaccine was the only one administered to children. By the early 1950s, children routinely received three vaccines, for protection against diphtheria, pertussis, tetanus and smallpox, and as many as five shots by two years of age. Since the mid-1980s, many vaccines have been added to the schedule. As of 2009, the US Centers for Disease Control and Prevention (CDC) now recommends vaccination against at least fourteen diseases. By two years of age, U.S. children receive as many as 24 vaccine injections, and might receive up to five shots during one visit to the doctor. The use of combination vaccine products means that, as of 2013, the United Kingdom’s immunization program consists of 10 injections by the age of two, rather than 25 if vaccination for each disease was given as a separate injection.
_
Main objectives of scheduling of vaccines are to achieve maximum effectiveness using recommended vaccines for a country while minimizing the number of health care system interactions. Epidemiological, immunological and programmatic aspects are taken into account while scheduling vaccines. In past two decades, many new vaccines have been developed, vaccination schedule is undergoing rapid changes and has become more complex. Traditionally, public sector in developing countries is slow to incorporate newer vaccines as compared to private sector after the vaccine is licensed for use. Cost effectiveness, safety and effectiveness for a given region are important issues for introduction of newer vaccines. As such vaccination schedule in public sector has lesser number of vaccines as compared to those developed by private sector. It often becomes a matter of debate what is the best schedule, but the knowledge of principles that go behind making each schedule will help pediatricians to build an informed opinion.
_
The figure below shows vaccine schedule in U.S. for immunization up to 18 years of age (2015):
BCG is not generally recommended for use in the United States because of the low risk of infection with Mycobacterium tuberculosis, the variable effectiveness of the vaccine against adult pulmonary TB, and the vaccine’s potential interference with tuberculin skin test reactivity.
_
Vaccination schedule recommended by Indian Academy of Pediatrics (IAP) 2013 include BCG:
| Birth – 15 days | BCG + OPV (zero dose) +HepB1 |
| 6 weeks – 8 weeks | OPV1 +IPV1 + DPT1* + HepB2 + Hib1 + Rotavirus1 + PCV1 |
| 10 weeks- 12 weeks | OPV2 + IPV2 + DPT2* + Hib2 + Rotavirus2 + PCV2 |
| 14 weeks – 16 weeks | OPV3 + IPV3 + DPT3* + Hib3 + Rotavirus3# + PCV3 |
| 6 months | HepB3 + OPV1 |
| 9 months (completed) | Measles vaccine + OPV2 |
| 12 months | Hepatitis A1 |
| 15 months | MMR1 + Varicella + PCV booster |
| 18 months | OPV4 + IPV booster1 + DPT*booster1 + Hib booster1 + Hepatitis A2 |
| 2 years | Typhoid1 (give repeat shots every 3 years) |
| 5 years | OPV5 + DPT* booster2 +MMR2^ + Varicella2$$ |
| 10 – 12 years | Tdap/Td (Every 10 years then give Td)+ HPV** |
*DPT: It is given either as DPaT or DPwT
**HPV is given only in females (3 doses at 0,1-2 months and 6 months interval)
#Rotavirus 3rd dose may be required only with one brand
^ MMR 2nd dose can be given at any time 4-8 weeks after the first dose
$$ Varicella 2nd dose can be given anytime 3 months from the first dose
PCV= Pneumococcal conjugated vaccine, IPV= Injectable polio vaccine, Td = Tetanus toxoid + adult dose of pertussis toxoid, HPV= Human papillomavirus
_
Adolescents:
Vaccinations and booster shots are recommended for pre-teens and adolescents. Teenagers are more vulnerable than smaller children to exposure to such diseases as HPV and meningitis. If a teen is behind on their immunizations there is a catch-up schedule that can be followed to protect him or her from the diseases that can still do harm.
•Young adults planning to live in a dormitory situation should be vaccinated against meningococcal disease.
•Tetanus, diphtheria toxoids and acellular pertussis vaccine (Tdap) should be given to 11-12 year olds who have completed the childhood series; 13-18 year olds who missed the 11-12 year old Tdap dose or who received Td instead a dose of Tdap should be given the vaccine five years after the last Td or DTaP dose.
•HPV (Human Papillomavirus Vaccine) is important for females at ages 13-18 to reduce the risk of contracting HPV which can increase the risk of cervical cancer later in life.
•Influenza vaccine should be attained yearly prior to flu season to protect against the anticipated flu viruses in circulation. It is impossible to get the flu from a flu shot. The flu shot takes approximately two weeks to be effective so it is important to get it as early as possible.
•Hepatitis B is a disease that many adults don’t know that they have contracted. While often thought of as a strictly sexually transmitted disease, it has been shown that this disease can be transmitted by the exchange of saliva as in situations where teenagers share food and drink or kissing. The vaccine is very effective at preventing this disease which can lead to liver cancer in later life.
•Inactivated Polio is a vaccine that an adolescent should get if he or she failed to get this immunization as a child. Polio still exists in some parts of the world and this vaccine will protect the adolescent from it being brought back into the country by a traveler.
•Measles, mumps and rubella vaccine is very important if the adolescent has not previously had this vaccine. Mumps can sterilize an adolescent male. Measles hospitalizes one out of five people who contract the disease. Rubella can spread to a pregnant woman and cause fetal damage. This is a very important combination for an adolescent to receive if he or she did not get it as a child.
_
Administration of Vaccines:
_
Routes of administration:
_
The route of administration is the path by which a vaccine (or drug) is brought into contact with the body. This is a critical factor for success of the immunization. A substance must be transported from the site of entry to the part of the body where its action is desired to take place. Using the body’s transport mechanisms for this purpose, however, is not trivial. Intramuscular (IM) injection administers the vaccine into the muscle mass. Vaccines containing adjuvants should be injected IM to reduce adverse local effects. Subcutaneous (SC) injection administers the vaccine into the subcutaneous layer above the muscle and below the skin. Intradermal (ID) injection administers the vaccine in the topmost layer of the skin. BCG is the only vaccine with this route of administration. Intradermal injection of BCG vaccine reduces the risk of neurovascular injury. Health workers say that BCG is the most difficult vaccine to administer due to the small size of newborns’ arms. A short narrow needle (15 mm, 26 gauge) is needed for BCG vaccine. All other vaccines are given with a longer, wider needle (commonly 25 mm, 23 gauge), either SC or IM. Oral administration of vaccine makes immunization easier by eliminating the need for a needle and syringe. Intranasal spray application of a flu vaccine offers a needle free approach through the nasal mucosa of the vaccinee.
_
Various routes of administration used by various vaccines:
_
General instructions on immunization:
1. Vaccination at birth means as early as possible within 24 to 72 hours after birth or at least not later than one week after birth.
2. Whenever multiple vaccinations are to be given simultaneously, they should be given within 24 hours if simultaneous administration is not feasible due to some reasons.
3. The recommended age in weeks/months/years mean completed weeks/months/years.
4. Any dose not administered at the recommended age should be administered at a subsequent visit, when indicated and feasible.
5. The use of a combination vaccine generally is preferred over separate injections of its equivalent component vaccines.
When two or more live parenteral/intranasal vaccines are not administered on the same day, they should be given at least 28 days (4 weeks) apart; this rule does not apply to live oral vaccines.
6. If given <4 weeks apart, the vaccine given 2nd should be repeated.
7. The minimum interval between 2 doses of inactivated vaccines is usually 4 weeks (exception rabies).
8. Vaccine doses administered up to 4 days before the minimum interval or age can be counted as valid (exception rabies). If the vaccine is administered > 5 days before minimum period, it is counted as invalid dose.
9. Any number of antigens can be given on the same day.
10. Changing needles between drawing vaccine into the syringe and injecting it into the child is not necessary.
11. Different vaccines should not be mixed in the same syringe unless specifically licensed and labeled for such use.
12. Patients should be observed for an allergic reaction for 15 to 20 minutes after receiving immunization(s).
13. When necessary, 2 vaccines can be given in the same limb at a single visit.
14. The anterolateral aspect of the thigh is the preferred site for 2 simultaneous IM injections because of its greater muscle mass.
15. The distance separating the 2 injections is arbitrary but should be at least 1 inch so that local reactions are unlikely to overlap.
16. Although most experts recommend “aspiration” by gently pulling back on the syringe before the injection is given, there are no data to document the necessity for this procedure. If blood appears after negative pressure, the needle should be withdrawn and another site should be selected using a new needle.
17. A previous immunization with a dose that was less than the standard dose or one administered by a nonstandard route should not be counted, and the person should be reimmunized as appropriate for age.
_
Aspiration is the process of pulling back on the plunger of the syringe after skin penetration but prior to injection to ensure that the contents of the syringe are not injected into a blood vessel. Although this practice is advocated by some experts, aspiration is not required because of the lack of large blood vessels at the recommended vaccine injection sites. Multiple vaccines can be administered at the same visit; indeed, administration of all needed vaccines at one visit is encouraged. Studies have shown that vaccines are as effective when administered simultaneously as they are individually, and simultaneous administration of multiple vaccines is not associated with an increased risk of adverse effects. If more than one vaccine must be administered in the same limb, the injection sites should be separated by 1–2 inches so that any local reactions can be differentiated. If a vaccine and an immune globulin preparation are administered simultaneously (e.g., Td vaccine and tetanus immune globulin), a separate anatomic site should be used for each injection.
_
For certain vaccines (e.g., HPV vaccine and hepatitis B vaccine), multiple doses are required for an adequate and persistent antibody response. The recommended vaccination schedule specifies the interval between doses. Many adults who receive the first dose in a multiple-dose vaccine series do not complete the series or do not receive subsequent doses within the recommended interval; in these circumstances, vaccine efficacy and/or the duration of protection may be compromised. Providers should implement recall systems that will prompt patients to return for subsequent doses in a vaccination series at the appropriate intervals. With the exception of oral typhoid vaccination, an interruption in the schedule does not require restarting of the entire series or the addition of extra doses.
_
The desirability of administering vaccines by non-parenteral routes:
The Sabin oral polio vaccine set a precedent among vaccines for practicality and ease of administration to subjects of any age. There is great interest to identify ways to administer other vaccines by non-parenteral routes, e.g. orally, nasally or transcutaneously. Certain live vector vaccines, antigen delivery systems and powerful adjuvants offer promise as strategies to successfully administer vaccines via mucosal and transcutaneous surfaces. There already exists considerable experience with several other oral and intranasal vaccines including: Ty21a live oral typhoid vaccine; a live oral cholera vaccine (CVD 103-HgR) and a non-living oral cholera vaccine (whole vibrio cells plus B subunit); and a live (cold adapted) and a non-living (virosomes plus LT adjuvant) intranasal influenza vaccine. From this considerable experience, several observations have been made:
•In most populations, oral or intranasal vaccines are preferred over parenteral vaccines, thereby increasing compliance.
•Mucosal immunisation precludes problems of injection safety found in some non-industrialised countries where the sporadic use of non-sterile needles and syringes can result in the inadvertent spread of hepatitis B, hepatitis C and HIV.
•Specialized microfold cells overlying mucosa-associated lymphoid tissues found both along the intestine and in the nose constitute competent portals of entry to inductive sites for immune responses.
•Because they elicit secretory IgA (usually in addition to systemic immune responses), mucosal vaccines are particularly attractive for pathogens that primarily cause mucosal infection of the gastrointestinal, respiratory or genito-urinary tracts or that invade via the mucosa lining those tracts.
•Properly formulated, mucosally administered vaccines can be adapted to stimulate any relevant type of immune response, in addition to secretory IgA, including serum IgG neutralizing antibodies (against toxins and viruses) and a variety of cell-mediated responses including lymphocyte proliferation accompanied by release of cytokines, and classical MHC I-restricted CD8+ lymphocytes.
•Some mucosal vaccines (e.g. Ty21a) have stimulated long-term protection enduring for up to 7 years.
•Mucosal immunisation is not a panacea. Problems that require research include the observation that several oral vaccines are less immunogenic in subjects living in under-privileged conditions in non-industrialised countries and whether oral immunisation with certain vaccines (such as live rotavirus strains) increases the risk of intussusception during a short period of time immediately following vaccination.
_
Why do children receive so many vaccinations?
Vaccines are our best defense against many diseases, which often result in serious complications such as pneumonia, meningitis (swelling of the lining of the brain), liver cancer, bloodstream infections, and even death. Vaccination is recommended to protect children against many infectious diseases, including measles, mumps, rubella (German measles), varicella (chickenpox), hepatitis B, diphtheria, tetanus, pertussis (whooping cough), Haemophilus influenza type B (Hib), polio, influenza (flu), and pneumococcal disease.
Why are these vaccines given at such a young age? Wouldn’t it be safer to wait?
Children are given vaccines at a young age because this is when they are most vulnerable to certain diseases. Newborn babies are immune to some diseases because they have antibodies given to them from their mothers. However, this immunity only lasts a few months. Further, most young children do not have maternal immunity to diphtheria, whooping cough, polio, tetanus, hepatitis B, or Hib. If a child is not vaccinated and is exposed to a disease, the child’s body may not be strong enough to fight the disease. An infant’s immune system is more than ready to respond to the very small number of weakened and killed infectious agents (antigens) in vaccines. From the time they are born, babies are exposed to thousands of germs and other antigens in the environment and their immune systems are readily able to respond to these large numbers of antigenic stimuli.
Why vaccines are administered in combination or simultaneously?
A combination vaccine consists of two or more different vaccines that have been combined into a single shot. Combination vaccines have been in use in the United States since the mid-1940’s. Examples of combination vaccines in current use are: DTaP (diphtheria-tetanus-pertussis), trivalent IPV (three strains of inactivated polio vaccine), MMR (measles-mumps-rubella), DTaP-Hib, and Hib-Hep B (hepatitis B). Simultaneous vaccination is when more than one vaccine shot is administered during the same doctor’s visit, usually in separate limbs (e.g. one in each arm). An example of simultaneous vaccination might be administering DTap in one arm or leg and IPV in another arm or leg during the same visit. Giving a child several vaccinations during the same visit offers two practical advantages. First, we want to immunize children as quickly as possible to give them protection during the vulnerable early months of their lives. Second, giving several vaccinations at the same time means fewer office visits. This saves parents both time and money, and may be less traumatic for the child
Is simultaneous vaccination with multiple vaccinations safe? Wouldn’t it be safer to separate vaccines and spread them out, vaccinating against just one disease at a time?
The available scientific data show that simultaneous vaccination with multiple vaccines has no adverse effect on the normal childhood immune system. A number of studies have been conducted to examine the effects of giving various combinations of vaccines simultaneously. These studies have shown that the recommended vaccines are as effective in combination as they are individually, and that such combinations carry no greater risk for adverse side effects. Consequently, both the Advisory Committee on Immunization Practices and the American Academy of Pediatrics recommend simultaneous administration of all routine childhood vaccines when appropriate. Research is underway to find methods to combine more antigens in a single vaccine injection (for example, MMR and chickenpox).
Can so many vaccines, given so early in life, overwhelm a child’s immune system, suppressing it so it does not function correctly?
No evidence suggests that the recommended childhood vaccines can “overload” the immune system. In contrast, from the moment babies are born, they are exposed to numerous bacteria and viruses on a daily basis. Eating food introduces new bacteria into the body; numerous bacteria live in the mouth and nose; and an infant places his or her hands or other objects in his or her mouth hundreds of times every hour, exposing the immune system to still more antigens. When a child has a cold they are exposed to at least 4 to 10 antigens and exposure to “strep throat” is about 25 to 50 antigens. In the face of these normal events, it seems unlikely that the number of separate antigens contained in childhood vaccines …would represent an appreciable added burden on the immune system that would be immunosuppressive.
Why do children need so many doses of certain vaccines?
Most vaccines require at least 2 doses. With inactivated (killed) vaccines, each dose of vaccine contains a fixed amount of disease antigen (virus or bacteria). Immunity is built in phases with each dose boosting immunity to a protective level. Live vaccines are different, in that the antigen in the vaccine reproduces and spreads throughout the body. One dose produces satisfactory immunity in most children. But a second dose is recommended, because not all children respond to the first one.
Can a child get a disease even after being vaccinated?
It isn’t very common, but it can happen. About 1% to 5% of the time, depending on the vaccine, a child who is vaccinated fails to develop immunity. If these children are exposed to that disease they could get sick. Sometimes giving an additional vaccine dose will stimulate an immune response in a child who didn’t respond to one dose. For example, a single dose of measles vaccine protects about 95% of children, but after two doses almost 100% are immune. Sometimes a child is exposed to a disease just prior to being vaccinated, and gets sick before the vaccine has time to work. Sometimes a child gets sick with something that is similar to a disease they have been vaccinated against. This often happens with flu. Many viruses cause symptoms that look like flu, and people even call some of them flu, even though they are really something else. Flu vaccine doesn’t protect from these viruses.
Can a child actually get the disease from a vaccine?
Almost never. With inactivated (killed) vaccines, it isn’t possible. A dead virus or bacteria, or part of a virus or bacteria, can’t cause disease. With live vaccines, some children get what appears to be a mild case of disease (for example what looks like a measles or chickenpox rash but with only a few spots). This isn’t harmful, and can actually show that the vaccine is working. A vaccine causing full-blown disease would be extremely unlikely. One exception was the live oral polio vaccine, which could very rarely mutate and actually cause a case of polio. This was a rare but tragic side effect of this otherwise effective vaccine. Oral polio vaccine is no longer used in the U.S.
Why does the government require children to be vaccinated to attend school in the U.S.?
School immunization laws are not imposed by the federal government, but by the individual states. But that doesn’t answer the question, which is often asked by people who see this as a violation of their individual rights. The mission of a public health system, as its name implies, is to protect the health of the public — that is, everybody. Remember that vaccines protect not only the person being vaccinated but also people around them. Immunization laws exist not only to protect individual children, but to protect all children. If vaccines were not mandatory, fewer people would get their children vaccinated — they would forget; they would put it off; they would feel they couldn’t afford it; they wouldn’t have time. This would lead to levels of immunity dropping below what are needed for herd immunity, which would lead in turn to outbreaks of disease. So mandatory vaccination, while it might not be a perfect solution, is at least a practical solution to a difficult problem. In a sense, school immunization laws are like traffic laws. We’re not allowed to drive as fast as we want on crowded streets or to disobey traffic signals. This could be seen as an imposition on individual rights too. However, these laws are not so much to prevent drivers from harming themselves, which you could argue is their right, but to prevent them from harming others, which is not.
_
Why aren’t vaccines available for all diseases?
The procedure for developing a vaccine takes many years and even more money, often hundreds of millions of dollars. It’s because of this that vaccines are prioritized in this order:
•Vaccines that fight diseases that cause the most deaths and damage, like meningitis
•Vaccines that prevent severe diseases like measles and influenza
•Vaccines, like the one for rotavirus, that prevent significant suffering
Additionally, vaccines are studied and produced by companies, so the return on investment must be significant in order to justify the large expense. Vaccines are currently in development to prevent malaria. The malaria vaccine has been slighted in the past because the financial return was not worth the investment the industry had to make. Another reason that vaccines can be tricky to produce is that some viruses mutate so quickly that traditional vaccines are ineffective. A prime example is the HIV/AIDS virus. Despite these hurdles, there is currently a tremendous movement to develop a vaccine to fight HIV/AIDS.
_
Regular, Alternative, Selective Vaccine Schedules:
The regular vaccine schedule for children aged 0-6 is approved by the CDC, American Academy of Pediatrics (AAP), and the American Academy of Family Physicians in the United States. It recommends 25 shots in the first 15 months of life. The shots immunize against whooping cough (pertussis), diphtheria, tetanus, mumps, measles, rubella, rotavirus, polio, hepatitis B, and other diseases. The alternative and selective vaccination schedules aren’t reviewed or approved by the CDC or other public health group. They come solely from Sears. Sears’ alternative vaccine schedule spreads the shots out over a longer period of time, up to age 5-6 years. For instance, he recommends not giving kids more than two vaccines at a time. It also changes the order of vaccines, prioritizing what Sears believes are the most crucial vaccines to get, based on how common and severe the diseases are. Many countries have regular vaccine schedule approved by their academy of pediatrics or WHO. Any deviation from regular schedule is alternative schedule.
_
Delaying Vaccines increases risks—with no added Benefits:
Some parents delay vaccines out of a misinformed belief that it’s safer, but that decision actually increases the risk of a seizure after vaccination and leaves children at risk for disease longer. Children receiving delayed vaccinations tend to fall into one of two groups: those whose parents intentionally delay vaccines and those whose families have difficulty getting vaccines on time.
No benefit to waiting to vaccinate: two studies:
2010 study:
No evidence to date reveals any benefits to delaying vaccines. A study in 2010 showed that children who received delayed vaccinations performed no better at ages seven to 10 on behavioral and cognitive assessments than children who received their vaccines on time. There was not a single outcome for which the delayed group did better. Authors note that delaying vaccines leaves children at risk for disease longer, and that many parents have little firsthand experience with those diseases. In this context, any potential side effect—real or perceived—may be enough to convince a parent that it’s safe to defer vaccines. However, that is not a safe choice, especially as vaccine-preventable diseases like measles are making a comeback.
2014 study:
The study published in Pediatrics, found that administering the MMR shot or the less frequently used MMRV one (which includes the varicella, or chickenpox vaccine) later, between 16 and 23 months, doubles the child’s risk of developing a fever-caused, or febrile seizure as a reaction to the vaccine. The risk of a febrile seizure following the MMR is approximately one case in 3,000 doses for children aged 12 to 15 months but one case in 1,500 doses for children aged 16 to 23 months. This study adds to the evidence that the best way to prevent disease and minimize side effects from vaccines is to vaccinate on the recommended schedule and an undervaccinated child is left at risk of infectious disease for a longer period. Delaying also makes for increased visits to the doctor’s office along with the time and hassle and risk of exposure to other infectious diseases in the doctor’s office. It’s not clear why the MMR and MMRV vaccines increase febrile seizure risk in the older children, but it may be simply that they receive the vaccines when they are already more susceptible to the seizures.
______
Parenteral administration in adults:
Parenteral vaccines recommended for routine administration to adults are given by either the IM or the SC route. Most parenteral vaccines are given to adults by the IM route. Vaccines given by the SC route include live-virus vaccines such as varicella, zoster, and MMR vaccines as well as the inactivated meningococcal polysaccharide vaccine. The 23-valent pneumococcal polysaccharide vaccine may be given by either of these routes, but IM administration is preferred because it is associated with a lower risk of injection-site reactions. Vaccines given to adults by the SC route are administered with a 5/8-inch needle into the upper outer-triceps area as seen in the figure below. Vaccines administered to adults by the IM route are injected into the deltoid muscle with a needle whose length should be selected on the basis of the recipient’s sex and weight to ensure adequate penetration into the muscle. Current guidelines indicate that, for men and women weighing <130 lbs (<60 kg), a 5/8-inch needle is sufficient; for women weighing 130–200 lbs (60–90 kg) and men weighing 130–260 lbs (60–118 kg), a 1- to 1.5-inch needle is needed; and for women weighing >200 lbs (>90 kg) and men weighing >260 lbs (>118 kg), a 1.5-inch needle is required.
_
Enhancing Immunization in Adults:
Although immunization has become a centerpiece of routine pediatric medical visits, it has not been as well integrated into routine health care visits for adults. Accumulating evidence suggests that immunization coverage can be increased through efforts directed at consumer-, provider-, institution-, and system-level factors. The literature suggests that the application of multiple strategies is more effective at raising coverage rates than is the use of any single strategy.
_
The figure below shows immunization schedule from age 19 years onwards till elderly:
_
Some recommended adult immunization schedules:
Influenza vaccination:
Annual vaccination against influenza is recommended for all persons aged 6 months and older, including all adults. Healthy, nonpregnant adults aged less than 50 years without high-risk medical conditions can receive either intranasally administered live, attenuated influenza vaccine (FluMist), or inactivated vaccine. Other persons should receive the inactivated vaccine. Adults aged 65 years and older can receive the standard influenza vaccine or the high-dose (Fluzone) influenza vaccine. The US Food and Drug Administration (FDA) recently approved several new flu vaccines, including trivalent (three strain) and quadrivalent (four strain) vaccines. The available choices this year will include:
| Standard three-strain flu vaccine. This year’s version includes influenza strains H1N1 and H3N2, and an influenza B virus | Egg-free vaccine (FluBlok), in which the influenza virus’ were grown in caterpillar cells instead of chicken eggs |
| Quadrivalent, or four-strain vaccine, which includes two A class of viruses and two from the B class, which tends to cause illness primarily in young children | High-dose vaccines, promoted for seniors aged 65 and over |
| Nasal spray, called FluMist. This year it will contain four strains opposed to three, matching the quadrivalent injection | Intradermal vaccine, promoted for those afraid of needles. The vaccine is delivered through a panel of micro-needles rather than a single needle |
_
Tetanus, diphtheria, and acellular pertussis (Td/Tdap) vaccination:
Administer a one-time dose of Tdap to adults aged less than 65 years who have not received Tdap previously or for whom vaccine status is unknown to replace one of the 10-year Td boosters, and as soon as feasible to all 1) postpartum women, 2) close contacts of infants younger than age 12 months (e.g., grandparents and child-care providers), and 3) healthcare personnel with direct patient contact. Adults aged 65 years and older who have not previously received Tdap and who have close contact with an infant aged less than 12 months also should be vaccinated. Other adults aged 65 years and older may receive Tdap.
_
Pneumococcal polysaccharide (PPV 23) vaccination:
Vaccinate all persons with the following indications:
Medical: Chronic lung disease (including asthma); chronic cardiovascular diseases; diabetes mellitus; chronic liver diseases; cirrhosis; chronic alcoholism; functional or anatomic asplenia (e.g., sickle cell disease or splenectomy [if elective splenectomy is planned, vaccinate at least 2 weeks before surgery]); immunocompromising conditions (including chronic renal failure or nephrotic syndrome); and cochlear implants and cerebrospinal fluid leaks. Vaccinate as close to HIV diagnosis as possible.
Other: Residents of nursing homes or long-term care facilities and persons who smoke cigarettes. Routine use of PPSV is not recommended for American Indians/Alaska Natives or persons aged less than 65 years unless they have underlying medical conditions that are PPSV indications. However, public health authorities may consider recommending PPSV for American Indians/Alaska Natives and persons aged 50 through 64 years who are living in areas where the risk for invasive pneumococcal disease is increased
Revaccination with PPV 23 (booster PPV 23):
One-time revaccination after 5 years is recommended for persons aged 19 through 64 years with chronic renal failure or nephrotic syndrome; functional or anatomic asplenia (e.g., sickle cell disease or splenectomy); and for persons with immunocompromising conditions. For persons aged 65 years and older, one-time revaccination is recommended if they were vaccinated 5 or more years previously and were aged less than 65 years at the time of primary vaccination.
_
PPV 23 made up of the capsular polysaccharide from 23 common pneumococcal serotypes which uses the capsular polysaccharide as the vaccine antigen. PPV 23 is T-independent vaccine and its efficacy can be increased to efficient T-dependent vaccines by covalently binding them (a process termed conjugation) to a protein molecule. That is 13-valent pneumococcal conjugate vaccine (PCV 13). PPV23 is licensed only for individuals aged >2 years as T independent immunity is absent below age of 2 years. According to the ACIP recommendations published in September 2014, both pneumococcal conjugate vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPV23) should be administered routinely in a series to all adults age 65 years and older. The two vaccines should not be given at the same visit. PCV13 is recommended to be given first because of the immune response to the vaccine when given in this sequence. An evaluation of immune response after a second pneumococcal vaccination administered 1 year after an initial dose showed that subjects who received PPSV23 as the initial dose had lower antibody responses after subsequent administration of PCV13 than those who had received PCV13 as the initial dose followed by a dose of PPSV23.
______
Vaccine Information Statements (VIS):
Vaccine Information Statements (VISs) are information sheets produced by the Centers for Disease Control and Prevention (CDC). VISs explain both the benefits and risks of a vaccine to adult vaccine recipients and the parents or legal representatives of vaccinees who are children and adolescents. Federal law requires that VISs be handed out whenever certain vaccinations are given (before each dose). As required under the National Childhood Vaccine Injury Act, all health care providers in the United States who administer, to any child or adult, any of the following vaccines – diphtheria, tetanus, pertussis, measles, mumps, rubella, polio, hepatitis A, hepatitis B, Haemophilus influenzae type b (Hib), trivalent influenza, pneumococcal conjugate, meningococcal, rotavirus, human papillomavirus (HPV), or varicella (chickenpox) – shall, prior to administration of each dose of the vaccine, provide VIS. If there is not a single VIS for a combination vaccine, use the VISs for all component vaccines. VISs should be supplemented with visual presentations or oral explanations as appropriate
_______
Vaccination in pregnancy:
Immunization during pregnancy, that is the administration of a vaccine to a pregnant woman, is not a routine event as it is generally preferred to administer vaccines either prior to conception or in the postpartum period. When widespread vaccination is used, the risk for an unvaccinated pregnant patient to be exposed to a related infection is low, allowing for postponement, in general, of routine vaccinations to the postpartum period. Nevertheless, immunization during pregnancy may occur either inadvertently, or be indicated in a special situation, when it appears prudent to reduce the risk of a specific disease for a potentially exposed pregnant woman or her fetus. As a rule of thumb the vaccination with live virus or bacteria is contraindicated in pregnancy as live virus or bacteria can have adverse effect on developing fetus. Also during pregnancy immune system is downgraded to allow fetus growth and live organisms could disseminate.
_
_
Toxoids:
Tetanus toxoids appear safe during pregnancy and are administered in many countries of the world to prevent neonatal tetanus. The World Health Organization (WHO) states more than 180,000 newborns die and over 30,000 women die each year from tetanus. It is recommended by the American Congress of Obstetrics and Gynecologists (ACOG) the following schedule for pregnant women to receive the vaccine–
•Schedule if never immunized: three doses in 0, 4, and 6-12 months
•Schedule if unknown immunization: at least two doses in the late second or third trimester. The National Business Group on Health (NBGH) states an analysis of pregnant women who received at least two doses had 98% effectiveness of the tetanus vaccine (NBGH, 2011).
One of the doses during pregnancy should be the Tdap (ACOG, 2012).
_
Immune globulins:
Immune globulins are used for post exposure prophylaxis and not associated with reports that harm is done to the fetus. Such agents are considered in pregnant women exposed to hepatitis B, rabies, tetanus, varicella, and hepatitis A.
_
The following vaccines are considered safe to give to women who may be at risk of infection:
• Hepatitis B: Pregnant women who are at high risk for this disease and have tested negative for the virus can receive this vaccine. It is used to protect the mother and baby against infection both before and after delivery. A series of three doses is required to have immunity. The 2nd and 3rd doses are given 1 and 6 months after the first dose.
• Influenza (Inactivated): This vaccine can prevent serious illness in the mother during pregnancy. All women who will be pregnant (any trimester) during the flu season should be offered this vaccine. Talk to your doctor to see if this applies to you.
• Tetanus/Diphtheria/Pertussis (Tdap): Tdap is recommended during pregnancy, preferably between 27 and 36 weeks’ gestation, to protect baby from whooping cough. If not administered during pregnancy, Tdap should be administered immediately after the birth of your baby.
_
What vaccinations are not recommended during pregnancy?
Don’t get these vaccines during pregnancy:
•BCG (tuberculosis)
•Meningococcal
•MMR
•Nasal spray flu vaccine (called LAIV) (Pregnant women can get the flu shot, which is made with killed viruses.)
•Typhoid
•Varicella
Wait at least 1 month after getting any of these vaccinations before you try to get pregnant.
_
Flu vaccines safe for Pregnant Moms:
A review of data from the 2009 flu season showed that the use of flu vaccines can help prevent fetal death, a major concern for pregnant mothers. For years, pregnant women have been unsure about whether getting the flu shot could harm their unborn child. The report, published in the New England Journal of Medicine, also confirmed the safety of flu vaccinations for women in the later stages of pregnancy.
___________
Vaccination to health care workers, travelers and people suffering from various medical disorders:
Vaccine to health care worker:
Is there anything different that health-care workers need to do compared with non-health-care workers?
Health-care workers are treated a little differently than other adults for two reasons. First, a health-care worker is more likely to be exposed to certain risks of infection (such as hepatitis B) than the normal population. Second, if a health-care worker becomes infected, they may transmit those infections to their patients (chickenpox, pertussis).
Special recommendations:
•Tetanus/diphtheria/pertussis (Td/Tdap):
◦It is recommended that any health-care worker who may have patient contact receive a Tdap shot if they have not received one as an adolescent (as long as it has been two years since their last Td shot). This helps prevent the spread of pertussis.
•Hepatitis B:
◦Health-care workers who have not been vaccinated should receive the three-dose series and obtain anti-hepatitis B serology testing one to two months after their third dose.
•Measles/mumps/rubella (MMR):
If there is no serologic evidence of immunity, the health-care worker should receive two doses of MMR separated by 28 days or more.
•Varicella:
◦All health care workers must have a history of varicella disease (chickenpox), prior vaccination, or serologic evidence of immunity. If not, the worker should receive two doses of vaccine 28 days apart.
•Influenza:
◦Health-care workers should receive one dose of either the flu shot or the nasal flu vaccine annually.
___________
Vaccine to foreign traveler:
_
Traveler’s vaccination:
_
According to the World Tourism Organization, international tourist arrivals grew exponentially from 25 million in 1950 to >900 million in 2008. Not only are more people traveling; travelers are seeking more exotic and remote destinations. Travel from industrialized to developing regions has been increasing, with Asia and the Pacific, Africa, and the Middle East now emerging destinations. Studies show that 50–75% of short-term travelers to the tropics or subtropics report some health impairment. Most of these health problems are minor: only 5% require medical attention, and <1% require hospitalization. Although infectious agents contribute substantially to morbidity among travelers, these pathogens account for only 1% of deaths in this population. Immunizations for travel fall into three broad categories: routine (childhood/adult boosters that are necessary regardless of travel) as listed in the figure above, required (immunizations that are mandated by international regulations for entry into certain areas or for border crossings), and recommended (immunizations that are desirable because of travel-related risks). Required and recommended vaccines commonly given to travelers are listed in Table below:
_
Vaccines Commonly Used for Travel:
| Vaccine | Primary Series | Booster Interval |
| Cholera, live oral (CVD 103 – HgR) | 1 dose | 6 months |
| Hepatitis A | 2 doses, 6–12 months apart, IM | None required |
| Hepatitis A/B combined (Twinrix) | 3 doses at 0, 1, and 6–12 months or 0, 7, and 21 days plus booster at 1 year, IM | None required except 12 months (once only, for accelerated schedule) |
| Hepatitis B (Engerix B): accelerated schedule | 3 doses at 0, 1, and 2 months or 0, 7, and 21 days plus booster at 1 year, IM | 12 months, once only |
| Hepatitis B (Engerix B or Recombivax): standard schedule | 3 doses at 0, 1, and 6 months, IM | None required |
| Immune globulin (hepatitis A prevention) | 1 dose IM | Intervals of 3–5 months, depending on initial dose |
| Japanese encephalitis (JE-VAX) | 3 doses, 1 week apart, SC | 12–18 months (first booster), then 4 years |
| Japanese encephalitis (Ixiaro) | 2 doses, 1 month apart, SC | Optimal booster schedule not yet determined |
| Meningococcus, quadrivalent [Menimmune (polysaccharide), Menactra, Menveo (conjugate)] | 1 dose SC | >3 years (optimal booster schedule not yet determined) |
| Rabies (HDCV), rabies vaccine absorbed (RVA), or purified chick embryo cell vaccine (PCEC) | 3 doses at 0, 7, and 21 or 28 days, IM | None required except with exposure |
| Typhoid Ty21a, oral live attenuated (Vivotif) | 1 capsule every other day x 4 doses | 5 years |
| Typhoid Vi capsular polysaccharide, injectable (Typhim Vi) | 1 dose IM | 2 years |
| Yellow fever | 1 dose SC | 10 years |
____________
____________
The figure below shows vaccine schedule for adults suffering from various medical disorders:
________
________
Vaccine storage, handling, cold chain and delivery system:
Storage and Handling:
Injectable vaccines are packaged in multidose vials, single-dose vials, or manufacturer-filled single-dose syringes. The live attenuated nasal-spray influenza vaccine is packaged in single-dose sprayers. Oral typhoid vaccine is packaged in capsules. Some vaccines, such as MMR, varicella, zoster, and meningococcal polysaccharide vaccines, come as lyophilized (freeze-dried) powders that must be reconstituted (i.e., mixed with a liquid diluent) before use. The lyophilized powder and the diluent come in separate vials. Diluents are not interchangeable but rather are specifically formulated for each type of vaccine; only the specific diluent provided by the manufacturer for each type of vaccine should be used. Once lyophilized vaccines have been reconstituted, their shelf-life is limited and they must be stored under appropriate temperature and light conditions. For example, varicella and zoster vaccines must be protected from light and administered within 30 minutes of reconstitution; MMR vaccine likewise must be protected from light but can be used up to 8 h after reconstitution. Single-dose vials of meningococcal polysaccharide vaccine must be used within 30 minutes of reconstitution, while multidose vials must be used within 35 days.
_
Vaccines are stored either at refrigerator temperature (2–8°C) or at freezer temperature (–15°C or colder). In general, inactivated vaccines (e.g., inactivated influenza, pneumococcal polysaccharide, and meningococcal conjugate vaccines) are stored at refrigerator temperature, while vials of lyophilized-powder live-virus vaccines (e.g., varicella, zoster, and MMR vaccines) are stored at freezer temperature. Diluents for lyophilized vaccines may be stored at refrigerator or room temperature. Live attenuated influenza vaccine—a live-virus liquid formulation administered by nasal spray—is stored at refrigerator temperature. To avoid temperature fluctuations, vaccines should be placed in the body of a refrigerator and not in the door, in vegetable bins, on the floor, next to the wall, or next to the freezer—locations where temperatures may differ significantly. Frequent opening of a refrigerator door to retrieve food items can adversely affect the internal temperature of the unit and damage vaccines; thus food and drink should not be stored in the same refrigerator as vaccines. Frozen vaccines must be stored in the body (not the door) of a freezer that has its own external door separate from the refrigerator. They should not be stored in small “dormitory-style” refrigerators. The temperature of refrigerators and freezers used for vaccine storage must be monitored and the temperature recorded at least twice a day. Ideally, continuous thermometers are used that measure and record temperature all day and all night.
_
What is the Cold Chain?
“Cold chain” refers to the process used to maintain optimal conditions during the transport, storage, and handling of vaccines, starting at the manufacturer and ending with the administration of the vaccine to the client. The optimum temperature for refrigerated vaccines is between +2°C and +8°C (e.g. OPV, HepB vaccine). For frozen vaccines the optimum temperature is -15°C or lower. In addition, protection from light is a necessary condition for some vaccines.
_
An estimated 17% to 37% of providers expose vaccines to improper storage temperatures, and refrigerator temperatures are more commonly kept too cold than too warm. One study involving site visits showed that 15% of refrigeration units had temperatures of +1°C or lower. Freezing temperatures can irreversibly reduce the potency of vaccines required to be stored at 35°F to 46°F (2°C to 8°C). Certain freeze-sensitive vaccines contain an aluminum adjuvant that precipitates when exposed to freezing temperatures. This results in loss of the adjuvant effect and vaccine potency. Physical changes are not always apparent after exposure to freezing temperatures and visible signs of freezing are not necessary to result in a decrease in vaccine potency. Although the potency of the majority of vaccines can be affected adversely by storage temperatures that are too warm, these effects are usually more gradual, predictable, and smaller in magnitude than losses from temperatures that are too cold. In contrast, varicella vaccine and LAIV are required to be stored in continuously frozen states and lose potency when stored above the recommended temperature range.
_
Importance of maintaining the Cold Chain:
Vaccines are sensitive biological products which may become less effective, or even destroyed, when exposed to temperatures outside the recommended range. As India is witnessing wastage of at least 50 per cent of vaccines stocked by various healthcare agencies owing to heat exposure, the Central Drugs Standard Control Organisation (CDSO) has decided to explore the idea of developing thermostable vaccines. Cold-sensitive vaccines experience an immediate loss of potency following freezing. Vaccines exposed to temperatures above the recommended temperature range experience some loss of potency with each episode of exposure. Repetitive exposure to heat episodes results in a cumulative loss of potency that is not reversible. However, information on vaccine degradation is sparse and multipoint stability studies on vaccines are difficult to perform. In addition, information from manufacturers is not always available, so it can be difficult to assess the potency of a mishandled vaccine.
Maintaining the potency of vaccines is important for several reasons.
1. There is a need to ensure that an effective product is being used. Vaccine failures caused by administration of compromised vaccine may result in the re-emergence or occurrence of vaccine preventable disease.
2. Careful management of resources is important. Vaccines are expensive and can be in short supply. Loss of vaccines may result in the cancellation of immunization clinics resulting in lost opportunities to immunize.
3. Revaccination of people who have received an ineffective vaccine is professionally uncomfortable and may cause a loss of public confidence in vaccines and/or the health care system.
_
Vaccine vial monitor:
A vaccine vial monitor (VVM) is a thermochromic label put on vials containing vaccines which gives a visual indication of whether the vaccine has been kept at a temperature which preserves its potency. The labels were designed in response to the problem of delivering vaccines to developing countries where the cold chain is difficult to preserve, and where formerly vaccines were being rendered inactive and administered ineffectively due to their having been denatured by exposure to ambient temperature. A vaccine vial monitor (VVM) is a label containing a heat sensitive material which is placed on a vaccine vial to register cumulative heat exposure over time. VVM is the only tool among all time temperature indicators that is available at any time in the process of distribution and at the time a vaccine is administered indicating whether the vaccine has been exposed to a combination of excessive temperature over time and whether it is likely to have been damaged. It clearly indicates to health workers whether a vaccine can be used. The combined effects of time and temperature cause the inner square of the VVM to darken, gradually and irreversibly. A direct relationship exists between the rate of colour change and temperature:
-The lower the temperature, the slower the colour change.
-The higher the temperature, the faster the colour change.
_
_
The World Health Organization has described VVMs as crucial in the spread of polio vaccination programs. Critically, VVMs have been shown to save lives in a cost-effective way. A joint study by WHO and PATH from 2006 identified more than 23 million doses of vaccine that had been overexposed to heat, and therefore were not administered to children. This is critical, as it clearly identified that these children could then be targeted for a future vaccine intervention, to ensure they were administered with an effective vaccine, as opposed to being marked in data as having received vaccine (when in fact, the vaccine could have been ineffective). In this way, VVMs help ascertain a clearer picture of the true vaccination coverage in a given population. The same study identified 31 million doses that had been exposed to potentially-damaging heat, but were still useable – thus greatly avoiding vaccine wastage. WHO estimates that over the next ten years, VVMs will enable the delivery of an additional 140 million doses of vaccine, saving 140,000 lives and decreasing the morbidity rates for countless others.
_
Pitfalls of VVM:
Studies have shown that health workers without proper training sometimes do not understand what a VVM is or how it works. A 2007 study in urban areas of Valsad in India showed that vaccine administrators were unaware of the purpose of the monitors. A study was done in the context of the temperatures in the states of Uttar Pradesh and Bihar in India where polio has been difficult to control and where summer temperatures rise to 45°C routinely and sometimes go as high as 50°C. Its findings suggest that the VVMs are not reliable when exposed to high environmental temperatures. Previous studies have shown deterioration in virus levels resulting from thaw-freeze cycles which are not indicated by the VVMs. This makes the practice of returning vials exposed to ambient temperatures, to the freezer for storage at night and reuse later, particularly risky.
_
Comparable technology:
Electronic time–temperature indicators can detect all temperature changes, including issues of freezing vaccines which heat-detecting VVMs would not detect.
_______
Public Health Reporting and Outbreak Detection & Control:
Outbreak Detection & Control:
Clusters of cases of a vaccine-preventable disease detected in an institution, a medical practice, or a community may signal important changes in the pathogen, vaccine, or environment. Several factors can give rise to increases in vaccine-preventable disease, including (1) low rates of immunization that result in an accumulation of susceptible people (e.g., measles resurgence among vaccination abstainers); (2) changes in the infectious agent that permit it to escape vaccine-induced protection (e.g., nonvaccine-type pneumococci); (3) waning of vaccine-induced immunity (e.g., pertussis among adolescents and adults vaccinated in early childhood); and (4) point-source introductions of large inocula (e.g., food-borne exposure to hepatitis A virus). Reporting episodes of outbreak-prone diseases to public health authorities can facilitate recognition of clusters that require further interventions.
_
Public Health Reporting:
Recognition of suspected cases of diseases targeted for elimination or eradication—along with other diseases that require urgent public health interventions, such as contact tracing, administration of chemo- or immunoprophylaxis, or epidemiologic investigation for common-source exposure)—is typically associated with special reporting requirements. Clinicians and laboratory staff have a responsibility to report some vaccine-preventable disease (notifiable diseases) occurrences to local or state public health authorities according to specific case-definition criteria. All providers should be aware of state or city disease-reporting requirements and the best ways to contact public health authorities. A prompt response to vaccine-preventable disease outbreaks can greatly enhance the effectiveness of control measures.
______
______
Vaccine benefits, effectiveness and impact:
_
Effectiveness of vaccine:
Vaccines have historically been the most effective means to fight and eradicate infectious diseases. Limitations to their effectiveness, nevertheless, exist. Sometimes, protection fails because the host’s immune system simply does not respond adequately or at all. Lack of response commonly results from clinical factors such as diabetes, steroid use, HIV infection or age. However it also might fail for genetic reasons if the host’s immune system includes no strains of B cells that can generate antibodies suited to reacting effectively and binding to the antigens associated with the pathogen. Even if the host does develop antibodies, protection might not be adequate; immunity might develop too slowly to be effective in time, the antibodies might not disable the pathogen completely, or there might be multiple strains of the pathogen, not all of which are equally susceptible to the immune reaction. However, even a partial, late, or weak immunity, such as a one resulting from cross-immunity to a strain other than the target strain, may mitigate an infection, resulting in a lower mortality rate, lower morbidity and faster recovery. If a vaccinated individual does develop the disease vaccinated against, the disease is likely to be less virulent than in unvaccinated victims. The following are important considerations in the effectiveness of a vaccination program:
1. careful modeling to anticipate the impact that an immunization campaign will have on the epidemiology of the disease in the medium to long term
2. ongoing surveillance for the relevant disease following introduction of a new vaccine
3. maintenance of high immunization rates, even when a disease has become rare.
__
The efficacy or performance of the vaccine is dependent on a number of factors:
•the disease itself (for some diseases vaccination performs better than for others)
•the strain of vaccine (some vaccines are specific to, or at least most effective against, particular strains of the disease)
•whether the vaccination schedule has been properly observed
•idiosyncratic response to vaccination; some individuals are “non-responders” to certain vaccines, meaning that they do not generate antibodies even after being vaccinated correctly
•assorted factors such as ethnicity, age, or genetic predisposition
_
Control, Elimination, and Eradication of Vaccine-Preventable Diseases:
Immunization programs are associated with the goals of controlling, eliminating, or eradicating a disease. Control of a vaccine-preventable disease reduces illness outcomes and often limits the disruptive impacts associated with outbreaks of disease in communities, schools, and institutions. Control programs can also reduce absences from work for ill persons and for parents caring for sick children, decrease absences from school, and limit health care utilization associated with treatment visits. Elimination of a disease is a more demanding goal than control, usually requiring the reduction to zero of cases in a defined geographic area but sometimes defined as reduction in the indigenous sustained transmission of an infection in a geographic area. As of 2010, the United States had eliminated indigenous transmission of measles, rubella, poliomyelitis, and diphtheria. Importation of pathogens from other parts of the world continues to be important, and public health efforts are intended to react promptly to such cases and to limit forward spread of the infectious agent. Eradication of a disease is achieved when its elimination can be sustained without ongoing interventions. The only vaccine-preventable disease that has been globally eradicated thus far is smallpox. Although smallpox vaccine is no longer given routinely, the disease has not naturally reemerged because all chains of human transmission were interrupted through earlier vaccination efforts and humans were the only natural reservoir of the virus. Currently, a major health initiative is targeting the global eradication of polio. Sustained transmission of polio has been eliminated from most nations but has never been interrupted in four countries: Afghanistan, India, Nigeria, and Pakistan. Detection of a case of disease that has been targeted for eradication or elimination is considered a sentinel event that could permit the infectious agent to become reestablished in the community or region. Hence, such episodes must be promptly reported to public health authorities.
_
Vaccine-preventable diseases and vaccine-preventable deaths:
A vaccine-preventable disease is an infectious disease for which an effective preventive vaccine exists. If a person acquires a vaccine-preventable disease and dies from it, the death is considered a vaccine-preventable death. The most common and serious vaccine-preventable diseases tracked by the World Health Organization (WHO) are: diphtheria, Haemophilus influenzae serotype b infection, hepatitis B, measles, meningitis, mumps, pertussis, poliomyelitis, rubella, tetanus, tuberculosis, and yellow fever. The WHO reports licensed vaccines being available to prevent, or contribute to the prevention and control of, 25 vaccine-preventable infections. Vaccine-preventable deaths are usually caused by a failure to obtain the vaccine in a timely manner. This may be due to financial constraints or to lack of access to the vaccine.
_
Progress against diseases for which vaccines already exist and deaths from diseases for which vaccines might be developed:
| Annual deaths (all ages) if no immunization |
Prevented | Occurring | % prevented | |
| Smallpox | 5.0 million | 5.0 million | — | 100 |
| Diphtheria | 260,000 | 223,000 | 37,000 | 86 |
| Whooping cough | 990,000 | 630,000 | 360,000 | 64 |
| Measles | 2.7 million | 1.6 million | 1.1 million | 60 |
| Neonatal tetanus | 1.2 million | 0.7 million | 0.5 million | 58 |
| Hepatitis B | 1.2 million | 0.4 million | 0.8 million | 33 |
| Tuberculosis | 3.2 million | 0.2 million | 3.0 million | 6 |
| Polio (cases of lifelong paralysis) | 640,000 | 550,000 | 90,000 | 86 |
| Malaria/other parasitic infections | 2.2 million | — | 2.2 million | 0 |
| HIV/sexually transmitted diseases | 1.3 million | — | 1.3 million | 0 |
| Diarrhoea/enteric fevers | 3.0 million | — | 3.0 million | 0 |
| Acute respiratory infections | 3.7 million | — | 3.7 million | 0 |
SOURCE Estimates supplied by Children’s Vaccine Initiative, Geneva, February 1996.
_
Decline in death rates due to reduction in vaccine preventable diseases in the U.S.:
_
Reduction in mortality after introduction of immunization:
_
Routine vaccines save lives, says science. A study from CDC researchers led by Anne Schuchat analyzed what happened to disease rates as childhood vaccination rates increased starting in the early 1990s. The researchers used these findings to model the resulting effect over the kids’ lifetimes. In the analysis, the researchers factored in most routine vaccines recommended for children below age 6 (among them the MMR and whooping cough vaccines). Their findings: Routine childhood vaccinations given between 1994 and 2013 would save 732,000 lives and prevent 322 million cases of illness and 21 million hospitalizations over the course of the children’s lifetimes.
_
Other vaccine successes:
•In North America, diphtheria vaccines reduced the diphtheria-related deaths by more than 99%.
•Before the varicella-zoster virus (VZV) vaccine was introduced, almost 350,000 cases of chickenpox occurred annually in Canada. There were 53 deaths due to chickenpox between 1987 and 1996. A vaccine is now available.
•Thanks to vaccines, there has not been a single case of smallpox in the world since 1977.
•The discovery and use of polio vaccines has all but eliminated polio in the Americas. In 1960, there were 2,525 cases of paralytic polio in the United States. By 1965, there were 61. Between 1980 and 1990, cases averaged 8 per year, and most of those were induced by vaccination! There has not been a single case of polio caused by the wild virus since 1979, with a rare case reported each year from persons coming into the country carrying the virus. In 1994, polio was declared eradicated in all of the Americas.
_
About 1.5 million children die each year from vaccine-preventable diseases. More than 70 percent of the world’s unvaccinated children live in 10 countries with large populations and weak immunization systems. Vaccines save millions of lives each year and are among the most cost-effective health interventions ever developed. Immunization has led to the eradication of smallpox, a 74 percent reduction in childhood deaths from measles over the past decade, and the near-eradication of polio. Despite these great strides, there remains an urgent need to reach all children with life-saving vaccines. One in five children worldwide are not fully protected with even the most basic vaccines. As a result, an estimated 1.5 million children die each year—one every 20 seconds—from vaccine-preventable diseases such as diarrhea and pneumonia. Tens of thousands of other children suffer from severe or permanently disabling illnesses. Vaccines are often expensive for the world’s poorest countries, and supply shortages and a lack of trained health workers are challenges as well. Unreliable transportation systems and storage facilities also make it difficult to preserve high-quality vaccines that require refrigeration.
_
Deaths due to vaccine-preventable diseases:
Total number of children who died from diseases preventable by vaccines currently recommended by WHO are 1.5 million:
Hib: 199 000
Pertussis: 195 000
Measles: 118 000
Neonatal tetanus: 59 000
Tetanus (non-neonatal): 2 000
Pneumococcal disease: 476 000
Rotavirus: 453 000
_____
Benefits of immunization:
Immunization is one of the most important advances in public health and is estimated to have saved more lives in the world over the past 50 years than any other health intervention. Before vaccines became available, many children died from diseases such as diphtheria, measles and polio that are now preventable by immunization. Immunization programs are responsible for the elimination, containment or control of infectious diseases that were once common; however, the viruses and bacteria that cause vaccine preventable diseases still exist globally and can be transmitted to people who are not protected by immunization. If immunization programs were reduced or stopped, diseases that are now rarely seen because they are controlled through immunization would re-appear, resulting in epidemics of diseases causing sickness and death. This phenomenon has been seen in many countries; for example, large epidemics of diphtheria and measles have occurred in Europe in recent decades after immunization rates declined. Immunization is important in all stages of life. Infants and young children are particularly susceptible to vaccine preventable diseases because their immune systems are not mature enough to fight infection; as a result, they require timely immunization. Older children and adults also require immunization to restore waning immunity and to build new immunity against diseases that are more common in adults. Immunization directly protects individuals who receive vaccines. Through herd immunity, immunization against many diseases also prevents the spread of infection in the community and indirectly protects:
•infants who are too young to be vaccinated,
•people who cannot be vaccinated for medical reasons (e.g., certain immune-suppressed people who cannot receive live vaccines),
•people who may not adequately respond to immunization (e.g. the elderly).
_
Without doubt, vaccines are among the most efficient tools for promoting individual and public health and deserve better press:
Disease control benefits:
Eradication:
Unless an environmental reservoir exists, an eradicated pathogen cannot re-emerge, unless accidentally or malevolently reintroduced by humans, allowing vaccination or other preventive measures to be discontinued. While eradication may be an ideal goal for an immunization program, to date only smallpox has been eradicated, allowing discontinuation of routine smallpox immunization globally. Potentially, other infectious diseases with no extrahuman reservoir can be eradicated provided an effective vaccine and specific diagnostic tests are available. Eradication requires high levels of population immunity in all regions of the world over a prolonged period with adequate surveillance in place. The next disease targeted for eradication is polio, which is still a global challenge. Although high coverage with oral polio vaccine (OPV) has eliminated type 2 poliovirus globally, transmission of types 1 and 3 continues in limited areas in a few countries. OPV-caused paralytic disease, directly or by reversion to virulence, and persistent vaccine-virus excretion in immunodeficient individuals are problems yet to be solved. Global use of monovalent type 1 and type 3 OPV and inactivated polio vaccine (IPV) may eventually be required.
Elimination:
Diseases can be eliminated locally without global eradication of the causative microorganism. In four of six WHO regions, substantial progress has been made in measles elimination; transmission no longer occurs indigenously and importation does not result in sustained spread of the virus. Key to this achievement is more than 95% population immunity through a two-dose vaccination regimen. Combined measles, mumps and rubella (MMR) vaccine could also eliminate and eventually eradicate rubella and mumps. Increasing measles immunization levels in Africa, where coverage averaged only 67% in 2004, is essential for eradication of this disease. Already, elimination of measles from the Americas, and of measles, mumps and rubella in Finland has been achieved, providing proof in principle of the feasibility of their ultimate global eradication. It may also be possible to eliminate Haemophilus influenzae type b (Hib) disease through well implemented national programs, as experience in the West has shown. Local elimination does not remove the danger of reintroduction, such as in Botswana, polio-free since 1991, with importation of type 1 poliovirus from Nigeria in 2004, and in the United States of America (USA) with measles reintroduced to Indiana in 2005 by a traveler from Romania. For diseases with an environmental reservoir such as tetanus, or animal reservoirs such as Japanese encephalitis and rabies, eradication may not be possible, but global disease elimination is a feasible objective if vaccination of humans (and animals for rabies) is maintained at high levels.
_
Control of mortality, morbidity and complications:
For the individual:
Efficacious vaccines protect individuals if administered before exposure. Pre-exposure vaccination of infants with several antigens is the cornerstone of successful immunization programs against a cluster of childhood diseases. Vaccine efficacy against invasive Hib disease of more than 90% was demonstrated in European, Native American, Chilean and African children in large clinical studies in the 1990s. In the United Kingdom, no infant given three doses developed Hib disease in the short-term (boosters may be required for long-term protection), and recent postmarketing studies have confirmed the high effectiveness of vaccination of infants against Hib in Germany and pertussis in Sweden. Many vaccines can also protect when administered after exposure – examples are rabies, hepatitis B, hepatitis A, measles and varicella.
For society:
Ehreth estimates that vaccines annually prevent almost 6 million deaths worldwide. In the USA, there has been a 99% decrease in incidence for the nine diseases for which vaccines have been recommended for decades, accompanied by a similar decline in mortality and disease sequelae. Complications such as congenital rubella syndrome, liver cirrhosis and cancer caused by chronic hepatitis B infection or neurological lesions secondary to measles or mumps can have a greater long-term impact than the acute disease. Up to 40% of children who survive meningitis due to Hib may have life-long neurological defects. In field trials, mortality and morbidity reductions were seen for pneumococcal disease in sub-Saharan Africa and rotavirus in Latin America. Specific vaccines have also been used to protect those in greatest need of protection against infectious diseases, such as pregnant women, cancer patients and the immunocompromised.
_
Mitigation of disease severity:
Disease may occur in previously vaccinated individuals. Such breakthroughs are either primary – due to vaccine failure – or secondary. In such cases, the disease is usually milder than in the non-vaccinated. In a German efficacy study of an acellular pertussis vaccine, vaccinated individuals who developed whooping cough had a significantly shorter duration of chronic cough than controls. Such findings were confirmed in Senegal. Varicella breakthroughs exhibit little fever, fewer skin lesions and fewer complications than unvaccinated cases. Milder disease in vaccinees was also reported for rotavirus vaccine.
_
Prevention of infection:
Many vaccines are primarily intended to prevent disease and do not necessarily protect against infection. Some vaccines protect against infection as well. Hepatitis A vaccine has been shown to be equally efficacious (over 90% protection) against symptomatic disease and asymptomatic infections. Complete prevention of persistent vaccine-type infection has been demonstrated for human papillomavirus (HPV) vaccine. Such protection is referred to as “sterilizing immunity”. Sterilizing immunity may wane in the long term, but protection against disease usually persists because immune memory minimizes the consequences of infection.
_
Protection of the unvaccinated population:
Herd protection:
Efficacious vaccines not only protect the immunized, but can also reduce disease among unimmunized individuals in the community through “indirect effects” or “herd protection”. Hib vaccine coverage of less than 70% in the Gambia was sufficient to eliminate Hib disease, with similar findings seen in Navajo populations. Another example of herd protection is a measles outbreak among preschool-age children in the USA in which the attack rate decreased faster than coverage increased. Herd protection may also be conferred by vaccines against diarrhoeal diseases, as has been demonstrated for oral cholera vaccines. “Herd protection” of the unvaccinated occurs when a sufficient proportion of the group is immune. The decline of disease incidence is greater than the proportion of individuals immunized because vaccination reduces the spread of an infectious agent by reducing the amount and/or duration of pathogen shedding by vaccinees, retarding transmission. Herd protection as observed with OPV involves the additional mechanism of “contact immunization” – vaccine viruses infect more individuals than those administered vaccine. The coverage rate necessary to stop transmission depends on the basic reproduction number (R0), defined as the average number of transmissions expected from a single primary case introduced into a totally susceptible population. Diseases with high R0 (e.g. measles) require higher coverage to attain herd protection than a disease with a lower R0 (e.g. rubella, polio and Hib). Because of herd protection, some diseases can be eliminated without 100% immunization coverage.
Source drying:
Source drying is a related concept to herd protection. If a particular subgroup is identified as the reservoir of infection, targeted vaccination will decrease disease in the whole population. In North Queensland, Australia, there was a high incidence of hepatitis A in the indigenous population. Vaccination of indigenous toddlers, with catch-up up to the sixth birthday, had a rapid and dramatic impact in eliminating the disease in the indigenous population and in the much larger non-indigenous population (who were not vaccinated) across the whole of Queensland. Similar approaches have been very successfully applied in several other larger settings, including Israel and the USA. The success of source drying justifies vaccination of special occupational groups, such as food handlers, to control typhoid and hepatitis A. Pertussis vaccine boosters for close contacts (such as parents, grandparents, nannies, siblings and baby unit nurses), who are the most common sources of transmission to infants, protect those too young to be given primary vaccination with a surrounding “pertussis-free cocoon”.
_
Prevention of related diseases and cancer:
Protection against related diseases:
Vaccines will also protect against diseases related to the targeted disease. For example, in Finland, the USA and elsewhere, influenza vaccination has been found protective for acute otitis media in children, with a vaccine efficacy of more than 30%. Measles vaccination protects against multiple complications such as dysentery, bacterial pneumonia, keratomalacia and malnutrition. An enterotoxic Escherichia coli vaccine demonstrated protection against diarrhoea due to Salmonella enterica.
Cancer prevention:
Infective agents cause several cancers. Chronic hepatitis B infection leads to liver cancer. Vaccination against such pathogens should prevent the associated cancer as already observed for hepatocellular carcinoma in Taiwan, China. These results could be replicated in Africa. Reduction of the incidence of cervical cancer is expected with the use of HPV vaccines against serotypes 16 and 18, responsible for over 70% of the global cervical cancer burden, as reduction in precancerous lesions has been demonstrated in vaccinees.
_
Societal and other benefits:
Health-care and other savings for society:
Immunization programs require funding for infrastructure (e.g. cold-chain maintenance), purchase of vaccines and adequate staffing. However, the mortality and morbidity prevented translates into long-term cost savings and potential economic growth. Globally, the savings from vaccines were estimated by Ehreth in 2003 to be of the order of tens of billions of US dollars of direct savings. Malaria (for which there are currently several promising vaccines in development) costs sub-Saharan Africa US$ 100 billion worth of lost annual gross domestic product (GDP). Savings are enhanced if several antigens are delivered in a single vaccine. Combination vaccines bring the added benefit of better compliance, coverage, and injection safety. Introduction of a new antigen is facilitated with combination vaccines, ensuring early high coverage by maintaining previous immunization schedules, without compromising (and sometimes improving) immunogenicity and reactogenicity. When taking into account indirect costs, savings are higher for common diseases with lower mortality and morbidity (such as varicella) than for more severe diseases (such as polio). Indirect costs, such as lost productivity (as well as direct medical costs) have been emphasized by eminent health economists in assessing the full value of vaccination. Immunization programs, compared to other common public health interventions such as wearing seat-belts and chlorination of drinking water, are a good investment and more cost effective than, for example, advice on smoking cessation. Cost savings will be achieved with the new live-attenuated rotavirus and conjugated pneumococcal vaccines, as well as wider use of hepatitis B and Hib vaccines.
_
Preventing development of antibiotic resistance:
By reducing the need for antibiotics, vaccines may reduce the prevalence and hinder the development of resistant strains. Introduction of a conjugate pneumococcal vaccine for infants in the USA in 2000 saw a 57% decline in invasive disease caused by penicillin-resistant strains and a 59% decline in strains resistant to multiple antibiotics by 2004 across a broad age spectrum: 81% among children under 2 years of age and 49% among persons aged 65 years and older. Vaccines against typhoid can prevent primary infection and the spread of antibiotic-sensitive as well as multidrug-resistant strains. The development of new vaccines against infectious pathogens where antibiotic resistance is a global threat (e.g. Staphylococcus aureus) is viewed as a better long-term option to control the problem of increasing resistance.
_
Extending life expectancy:
Vaccines can increase life expectancy by protecting against diseases against which one would not expect benefit. Elderly individuals given influenza vaccine in the USA had approximately 20% less chance of suffering cardiovascular and cerebrovascular disease and 50% lower risk of mortality from all causes compared to their unvaccinated counterparts. In Sweden, administration of polysaccharide pneumococcal vaccine and inactivated influenza vaccine significantly reduced the risk of in-hospital mortality for pneumonia and cardiac failure among elderly persons, with an additive effect when both vaccines had been administered.
_
Safe travel and mobility:
With global air travel rising, there is an increased risk of exposure to infectious diseases abroad. Travelers transmit and disseminate disease, as has been observed in the case of polio and in the dispersal of meningococcal strains by returning pilgrims from Saudi Arabia. In the case of the Muslim Hajj (the largest annual human gathering in the world), local authorities require meningococcal vaccination and recommend various other vaccinations, such as influenza and hepatitis B, for pilgrims. The most common vaccine-preventable diseases among travelers are influenza and hepatitis A. Other vaccines to consider for travel include rabies, hepatitis B, typhoid, cholera, yellow fever, Japanese encephalitis and measles. Many vaccines can be given by flexible accelerated schedules to ensure early protection. Thus the traveler seeking health advice, even within a few weeks of departure, can travel overseas without vaccine-preventable health risks to themselves and others.
_
Other public health benefits:
In developing countries, vaccination programs are cornerstones of primary health-care services. The infrastructure and personnel required for an effective and sustainable immunization program give opportunities for better primary health-care services, particularly in the critical perinatal and early infancy period.
_
Empowerment of women:
With improvements in infant and child mortality, women tend to opt for fewer children as the need to have many children to ensure that some will reach adulthood is reduced. This has significant health, educational, social and economic benefits.
_
Protection against bioterrorism:
The current concern about the potential use of smallpox virus in bioterror is due to the cessation of vaccination (and of vaccine manufacture) following the monumental achievement of smallpox eradication. The potential of vaccines to protect populations from bioterrorism threats such as smallpox and anthrax has led many governments to ensure an adequate supply of the necessary vaccines in preparation against such an attack. Surveillance and response systems for vaccine-preventable and other diseases play a critical role in identification, characterization and response to biological weapons.
_
Promoting economic growth:
Poor health has been shown to stunt economic growth while good health can promote social development and economic growth. Health is fundamental to economic growth for developing countries and vaccinations form the bedrock of their public health programmes. The annual return on investment in vaccination has been calculated to be in the range of 12% to 18%, but the economic benefits of improved health continue to be largely underestimated.
_
Enhancing equity:
The burden of infectious, including vaccine-preventable, diseases falls disproportionately on the disadvantaged. Vaccines have clear benefits for the disadvantaged. Pneumococcal immunization programs in the USA have at least temporarily removed racial and socioeconomic disparities in invasive pneumococcal disease incidence, while in Bangladesh, measles vaccination has enhanced equity between high- and low-socioeconomic groups.
_
Promoting peace:
There were at least seven United Nations Children’s Fund (UNICEF) vaccine-mediated ceasefires during civil conflicts. These conflicts were in diverse parts of the world, from Liberia to Afghanistan, where even warring factions see the benefit of immunization programs. During protracted conflict it is possible to ensure that vaccination coverage remains high. This is seen in Sri Lanka, where despite unrest for the last two decades coverage in 2005 for both three doses of diphtheria–tetanus–pertussis vaccine and one dose of measles vaccine was 99%. The high cost-effectiveness and multiple benefits of relatively modest resource investments in immunization contrast starkly with profligate global military expenditures, currently over US$ 1 trillion annually.
_
In a nutshell:
The benefits of vaccination extend beyond prevention of specific diseases in individuals. They enable a rich, multifaceted harvest for societies and nations. Vaccination makes good economic sense, and meets the need to care for the weakest members of societies. Reducing global child mortality by facilitating universal access to safe vaccines of proven efficacy is a moral obligation for the international community as it is a human right for every individual to have the opportunity to live a healthier and fuller life. Achievement of the Millennium Development Goal 4 (two-thirds reduction in 1990 under-5 child mortality by 2015) will be greatly advanced by, and unlikely to be achieved without, expanded and timely global access to key life-saving immunizations such as measles, Hib, rotavirus and pneumococcal vaccines. So a comprehensive vaccination program is a cornerstone of good public health and will reduce inequities and poverty.
_
The accidental advantages of vaccines:
The Bacille Calmette-Guérin vaccination was given to protect you from tuberculosis. What we are only just realising is that, in common with several other vaccines, it may have done far more than that. There is growing evidence that vaccines have a wider-ranging influence on the immune system than we thought. In Africa, for instance, studies have shown that measles vaccine cuts deaths from all other infections combined by a third, mainly by protecting against pneumonia, sepsis and diarrhoea. Even in the West, where it is far less common for children to die from infectious illnesses, there are still surprising benefits: some vaccines seem to reduce our susceptibility to eczema and asthma. Exactly what causes these “non-specific effects”, as they are termed, is a mystery. But some scientists are arguing that, despite the uncertainties, it is time to start harnessing them more effectively. The World Health Organization, which is the main provider of vaccines in developing countries, has asked a group of vaccine experts to get to the bottom of it. “This could have huge implications for global healthcare,” says Christine Benn, a senior researcher at the Statens Serum Institute in Denmark and a member of the WHO committee. “Vaccines have been a fantastic success, but we can probably do much better by taking non-specific effects into account. An examination of these issues is long overdue.” Considering vaccines have been used since the 1800s and are the central plank of our public health system, it may seem hard to believe that such profound effects could have gone ignored all this time. In fact, an early 20th century Swedish physician called Carl Näslund did notice something was up after the BCG vaccine was introduced in his country. Vaccinated children had a much higher chance of reaching their first birthday – even though TB normally kills older children. In the 1940s and 50s, trials in the US and UK suggested that BCG-vaccinated children had a 25 per cent lower death rate from diseases other than TB. But no one took much notice until 30 years ago, when a Danish anthropologist called Peter Aaby began working in the West African state of Guinea-Bissau. In 1979 he witnessed a severe measles outbreak that killed 1 in 4 infants affected. Aaby arranged for measles vaccination to be introduced, but was surprised to see that even after the epidemic abated, immunised children were more likely to survive childhood. Aaby began digging, and discovered studies from elsewhere in Africa, as well as Bangladesh and Haiti, that also suggested measles vaccine gives a wider kind of protection. “We are collecting more and more data consistent with non-specific effects being very important,” says Aaby. What could the explanation be? Several lines of evidence suggest that our immune systems can be affected by many factors, including past encounters with microbes. Those microbes can be in the environment or a vaccine syringe. “If infections can alter the immunological milieu, it is not a major leap to suggest that vaccines might also do so,” said Andrew Pollard, head of the Oxford Vaccine Centre at the University of Oxford, in an editorial about Aaby’s work. According to the old view of vaccines, they work by priming what is known as our adaptive immune system. This consists of various defense cells circulating in the blood, which make antibodies and other molecules that recognise and latch on to specific foreign proteins on bacteria, viruses or other germs. It is this lock-and-key specificity that is responsible for our immune memory. On our first encounter with the measles virus, say, the immune cells that make potent antibodies to it reproduce, giving rise to successive generations of daughter cells that make progressively more powerful antibodies. The end product is highly proficient measles-killing machines that linger in our bodies for years. That’s why, if we re-encounter the virus, it is defeated so quickly we don’t even notice. But that may not be the whole story. Another, evolutionarily older, branch of our defences known as the innate immune system might also be playing a role. These cells are programmed to react to anything unfamiliar or untoward, such as the chemicals released when tissues are damaged, attacking any molecules or microorganisms that might pose a threat. Last year, surprising evidence emerged that BCG stimulates the innate immune system as well as the adaptive one. In people who received the shot, certain kinds of innate immune cells responded more strongly to bacterial and fungal pathogens completely unrelated to the TB bug. This is the first indication that the innate immune system reacts to vaccines, and the researchers suggested it could explain some of the general immune-boosting effects of BCG. “It’s quite preliminary data, but it’s very important,” says Nigel Curtis, head of infectious diseases at the Royal Children’s Hospital Melbourne and the University of Melbourne, Australia, who studies BCG. The discovery may be only one part of the explanation for BCG’s mysterious powers, though. For starters, it emerged recently that even memory cells of the adaptive immune system can target unrelated microbes, if there is sufficient cross-reactivity with a germ we have previously vanquished.
Tipping the balance:
But the theory that probably has most evidence behind it concerns two competing arms of the adaptive immune system, known as type 1 and type 2 helper T-cells. Broadly, type 1 cells promote immune reactions against bacteria and viruses, while type 2 cells are geared towards fighting off parasitic worms in the gut. Both BCG and the measles vaccine seem to tip the balance to type 1, according to studies of the antibodies released into the bloodstream after vaccination. Whatever the explanation is, we might be able to maximise the benefits, either by designing new vaccines or augmenting the effects of existing ones. But the WHO committee has another line of inquiry: there are suggestions that one vaccine could have harmful non-specific effects. The vaccine under suspicion is DTP, which prevents diphtheria, tetanus and pertussis, otherwise known as whooping cough. It was Aaby, again, who first drew attention to this. These days, he works as a vaccine researcher for the Danish Statens Serum Institute, but he is still based mainly in Guinea-Bissau. For several months in 2001 and 2002, health centers in the capital city, Bissau, ran out of DTP, and some infants never got their shot. Aaby noted that, among children who had been admitted to hospital for some reason, those who had had the shot were over twice as likely to die during their hospital stay. Further studies showed that the effect was particularly pronounced for girls. What no one knows is why DTP might have such an effect. One possible explanation is that the pertussis component is made from killed whooping cough bacteria. There are other ways to make vaccines, including using live but weakened bacteria or viruses, with both BCG and the measles shot being this type. Killed vaccines, on the other hand, seem to tip the type 1/type 2 balance away from the bacteria and virus-fighting type 1 arm. Animal studies show that, for unknown reasons, females have a naturally stronger type 2 bias, which could explain the sex difference in mortality seen in Guinea-Bissau. No one is suggesting we stop giving the DTP vaccine. Its protection from diptheria, tetanus and whooping cough is hugely beneficial – especially in the West.
_________
_________
Vaccine failure, interference and spread of disease:
_
There are two main reasons for failure of immunizations:
(1) Failure of the vaccine delivery system to provide potent vaccines properly to persons in need; and
(2) Failure of the immune response, whether due to inadequacies of the vaccine or factors inherent in the host.
_
Vaccine failure:
Vaccine failure is when disease occurs in a person despite being vaccinated for it. It is of two types:
1. Primary vaccine failure: This is when a person fails to produce antibodies (at detectable levels) or does not produce enough antibodies considered necessary to protect from the disease.
2. Secondary vaccine failure: This is when a person does produce antibodies in response to vaccination however the levels wane and decline at a faster rate than normally expected. However, antibodies to almost all vaccines decline over time, even after booster shots, so secondary vaccine failure in outbreaks of disease amongst the vaccinated is frequent.
_
Vaccine failure stems from poor vaccine efficacy. No vaccine is 100% efficacious meaning few percent people who received vaccine can still get disease. In the past three flu seasons, the CDC rated the influenza vaccine’s overall effectiveness between 47 and 62 percent meaning between 38 to 53 % population who received vaccine would still get influenza. A study, which involved nearly 9,000 high school students, found that by the age of 15, about 15 percent of teens who received the full series of hepatitis B shots as infants tested positive for hepatitis B surface antigen (HBsAg) as vaccinees may have lost their immunological memories against HBsAg.
_
Vaccine failure of Tdap vaccine:
An analysis of Washington state’s 2012 pertussis epidemic, the worst since 1942, found that the vaccine to prevent the disease waned sharply and quickly in teens who were fully inoculated. A new analysis of that epidemic finds the vaccine used to prevent pertussis waned quickly and sharply in adolescents, likely contributing to a surge of cases among those who already had their shots. Effectiveness of the Tdap vaccine — tetanus, diphtheria and acellular pertussis — was only about 64 percent overall, and it dropped to about 34 percent within two to four years after it was given, according to a study led by Dr. Anna Acosta, an epidemiologist with the Centers for Disease Control and Prevention (CDC). That helps explain why even kids who received all the CDC-recommended doses by age 11 were part of a spike in cases during the epidemic, the worst in Washington since 1942. The study confirms what others suggested, that a switch from whole-cell pertussis vaccine to acellular types in 1997 took a toll on the vaccine efficacy. The change was made because there was an “unacceptably high” level of reactions to the whole-cell shots, including febrile seizures.
_
Vaccine interference:
Vaccine interference may be intra- or inter-vaccine in nature. Intra-vaccine interference is determined by the nature and dose of the individual vaccine valences, the nature and quality of any additives and the pharmaceutical formulation of the product. Additionally, vaccinee factors including the presence of pre-existing immunity, the stage of immunological maturation, genetic and environmental background may also determine interference. The vaccine schedule and mode of delivery are further contributory factors. In practice, the phenomenon of vaccine interference argues that individual vaccines should not be combined or associated in the absence of specific data sheet recommendations to do so. Live-attenuated vaccines replicate at low concentrations and elicit protective immunity without causing disease. This strategy has proven to be successful when the vaccine targets one pathogen, as is the case for vaccines against yellow fever and Japanese encephalitis viruses. Translation of this straightforward idea to target dengue has proven frustrating, because dengue is a complex flaviviral disease that is caused by not one, but four antigenically distinct dengue viruses (DENV-1, 2, 3, and 4) and in tetravalent dengue vaccine, the DEN-3 serotype was found to predominate and suppress the response to DEN-1, −2 and −4 serotypes. When two or more vaccines are mixed together in the same formulation, the two vaccines can interfere. This most frequently occurs with live attenuated vaccines, where one of the vaccine components is more robust than the others and suppresses the growth and immune response to the other components. This phenomenon was first noted in the trivalent Sabin polio vaccine, where the amount of serotype 2 virus in the vaccine had to be reduced to stop it from interfering with the “take” of the serotype 1 and 3 viruses in the vaccine.
_
Does flu vaccine make you more susceptible to influenza? 2015 study:
While a subject may be stimulating their immune system to build up specific antibodies, they are only doing this for a specific strain of virus. This allows other strains of influenza to have more influence when a subject comes in contact with them in real time. The flu shot also encourages viruses to mutate faster to survive, making flu shot subjects more susceptible to more powerful strains in the future. The Canadian research involves four studies and about 2,000 people. The observations were telling. The people who were subjected to seasonal flu vaccines in the past were most likely to come down with the H1N1 virus in the future. By focusing on one strain of virus, flu vaccines subject the body to future danger, facilitating the entry of other virus strains into the body. Dengue fever is another virus that commonly takes advantage of the seasonally vaccinated. The truth about flu vaccines is becoming so apparent that many health authorities in Quebec have considered canceling their recommendations for seasonal flu shots for healthy individuals.
_
Microbial adaptation following vaccination:
The widespread use of vaccinations may trigger bacterial adaptations leading to antibiotic-resistant bacterial diseases and vaccine-resistant viral diseases.
• This can happen through several mechanisms. These include mutation (Hepatitis B vaccine), reversion to virulence (Oral Polio vaccine) or strain replacement (PCV 7). Strain replacement, in the case of PCV 7, meant that following widespread vaccination with PCV 7, other pneumococcal strains that were not included in the vaccine became much more likely to cause pneumococcal infections. One of these, 19S, was known to be multi-antibiotic resistant.
• Hemophilus influenzae cases also increased, filling in the niche that had been created by PCV, and about 40% of these infections are multi-drug resistant.
• When PCV, a vaccine used against pneumonia, meningitis, and bloodstream infections, was first introduced, it protected against seven pneumococcal strains, but was soon linked to an increase in rates of antibiotic-resistant infections due to 19S pneumococci and hemophilus influenzae. The vaccine was then modified to include 13 pneumococcal strains to combat the 19S problem. However, the new vaccine will not improve the increase in hemophilus infections, and may make that problem worse.
• In the United States ear infections, sinus infections, bronchitis, pneumonia and meningitis, which are often caused by pneumococcal bacteria or hemophilus, have become much harder and more expensive to treat because of increasing resistance to antibiotics. This is due in part to the widespread use of the PCV vaccine.
• Vaccines have also been implicated in causing new, vaccine-resistant strains of whooping cough, hepatitis and polio.
_
Other cases noted in the literature include:
•Whooping Cough: In Australia, dangerous new strains of whooping cough bacteria were reported in March 2012. The vaccine, researchers said, was responsible. The reason for this is because, while whooping cough is primarily attributed to Bordetella pertussis infection, it is also caused by another closely related pathogen called B. parapertussis, which the vaccine does not protect against. Two years earlier, scientists at Penn State had already reported that receiving the pertussis vaccine significantly enhanced nasal colonization of B. parapertussis, thereby promoting vaccine-resistant whooping cough outbreaks.
•Hepatitis B: In 2007, immunologists discovered mutated vaccine-resistant viruses were causing disease.
_
Polio by polio vaccine:
The oral polio vaccine, which is still used in many third-world countries, is made from three live polio viruses, and carries a risk of causing polio. The viruses in the vaccine can also mutate or recombine into a deadlier version, igniting new outbreaks. The U.S. Centers for Disease Control and Prevention (CDC) admits that 154 cases of polio in the US that occurred between 1980 and 1999 were vaccine-associated, or on average 8 cases per year in the U.S. According to Nature, poliovirus reverts to virulence in 2 to 4 babies per million vaccinated. And, according to an article in Clinical Infectious Diseases, the risk of vaccine-associated polio ranged from 0 to 9 per million persons vaccinated for each of the three Sabin strains. The World Health Organization (WHO) acknowledges: “In very rare cases, the administration of OPV [oral polio vaccine] results in vaccine-associated paralysis associated with a reversion of the vaccine strains to the more neurovirulent profile of wild poliovirus. In a few instances, such vaccine strains have become both neurovirulent and transmissible and have resulted in infectious poliomyelitis. This problem is so significant that oral polio vaccines are no longer used in the developed world. (They were stopped in the U.S. in 2000 and replaced by injected vaccines that were not live.) However, because they are cheaper to produce than injected vaccines, they are still used in the “less developed” world.
_
Recently Vaccinated individuals found to spread virus:
You can shed live virus in body fluids whether you have a viral infection or have gotten a live attenuated viral vaccine. The Johns Hopkins Patient Guide for immunocompromised patients used to mention avoiding “contact with children who are recently vaccinated” You can be an asymptomatic carrier of a viral infection (acquired naturally or via vaccination), so while you may show no symptoms, you may still be able to transmit the virus to others. As of March 2015, the guide has been revised and this language has been removed. Live attenuated viral vaccines (LAV) that use live viruses try to, in essence, fool your immune system into believing that you’ve come into contact with a real virus, thereby stimulating the antibody response that will protect you. When you get these live viral vaccines, you shed live virus in your body fluids. Just like when you get a viral infection, you shed live virus. That’s how viral infections are transmitted. Because viruses, unlike bacteria, need a living host… in order to multiply. Scientific evidence demonstrates that individuals vaccinated with live virus vaccines such as MMR (measles, mumps and rubella), rotavirus, chicken pox, shingles and influenza can shed the virus for many weeks or months afterwards and infect the vaccinated and unvaccinated alike. However, shedding of viruses in vaccines typically occurs in lower amounts than during shedding of wild-type viruses. In other words, weakened viruses in live attenuated vaccines can shed, but in weakened amounts. Thus, because weakened viruses in vaccines cause mild or no disease, shed weakened viruses also cause mild or no disease. Furthermore, vaccine recipients can carry diseases in the back of their throat and infect others while displaying no symptoms of a disease.
_
Vaccinated Individuals can be asymptomatic carriers of disease:
One of the dangers of any viral disease outbreak is that people often fail to realize is that you can be an asymptomatic carrier of a viral infection; so while you show no symptoms or only mild symptoms, you may still be able to transmit the virus to others. Even fewer people understand that this is also true for live-virus vaccines. In an animal study, while whole cell DPT and acellular-pertussis-vaccinated baboons did not develop serious clinical disease symptoms—such as loss of appetite and cough—when they were exposed to the B. pertussis bacteria, they still colonized B. pertussis in their throats and were capable of transmitting the infection to other baboons. The study’s lead author Tod Merkel also explained that when exposed to B. pertussis after recently getting vaccinated, you could be an asymptomatic carrier and infect others, saying: “When you’re newly vaccinated, you are an asymptomatic carrier, which is good for you, but not for the population.”
______
______
Vaccine safety, adverse events and autism:
So far I discussed vaccine efficacy, benefits and impact. I also discussed vaccine failure. Now I will discuss the most contentious issues of vaccines, vaccine safety. This is so because vaccine is given to a healthy child who is at the mercy of parents and parents certainly don’t want to do anything that harms child. The adverse effect of vaccine gets too much importance & attention compared to adverse effect of any drug because vaccine is administered to a healthy child while drug is given to a sick child.
_
What is a vaccine safety crisis?
You may not be able to define it, but you certainly know when you are in one! Crises in vaccine safety are characterized by an unexpected series of events that initially seem to be out of control. The outcome is usually uncertain when the crisis is first identified, and there is a threat to the success of a vaccine or immunization program. A crisis may have a “real” basis arising from genuine vaccine reactions or immunization errors, or it may have no foundation in reality and be triggered entirely by mistaken rumours. Often a crisis in vaccine safety originates in the identification of AEFIs, but is aggravated by negative rumours. Whether a rumour triggers a series of events that build into a crisis depends on the nature of the rumour, how fast it spreads and whether prompt and effective action is taken to address it. When approaching a crisis, keep in mind that this may not only be a challenge, but also an opportunity to improve the communication on immunization issues. You have the opportunity to dispel negative rumours, to take action to upgrade policies and procedures if required, and to correct any errors or lapses in best practice.
_
Some examples of vaccine crisis:
17 Children die after receiving Hepatitis B Vaccine in china in 2013:
Over a period of two months, eight infants in China died within hours, and in some cases minutes, of receiving hepatitis B vaccines. Nine other deaths among Chinese children aged 5 and younger were also recently reported following hepatitis B vaccination. Six of the deaths occurred in infants who had received the vaccine made by Shenzhen Kangtai Biological Products, while two occurred after hepatitis B vaccine produced by drug maker Beijing Tiantan Biological Product. Health authorities in China have since launched an investigation and have suspended the use of millions of doses of hepatitis B vaccine made by Shenzhen Kangtai. Serious questions regarding effectiveness, low transmission rates among babies and the steep risk of side effects make the hepatitis B vaccine’s use very hard to justify for healthy newborns. It’s interesting to note that US pharmaceutical giant Merck actually helped the Chinese build Shenzhen Kangtai in the 1990s. Merck also granted the company the biological technology to produce a hepatitis B vaccine royalty free in what the New York Times described as an “unusual joint venture aimed at improving health standards in China.”
_
Young Tribal Girls died following involvement in HPV Vaccine trial in India in 2009:
In 2009, tests had been carried out on 16,000 tribal school children in Andhra Pradesh, India, using the human papiloma virus (HPV) vaccine, Gardasil. According to the report, within a month of receiving the vaccine, many of the children fell ill and by 2010, five of them had died. A further two children were reported to have died in Vadodara, Gujarat, where an estimated 14,000 tribal children were vaccinated with another brand of the HPV vaccine, Cervarix, manufactured by GlaxoSmitheKline (GSK). According to a report, a total of 120 girls had been taken ill, suffering from a variety of symptoms, including “epileptic seizures, severe stomach aches, headaches and mood swings.” It said it was disturbed to find that ‘all the seven deaths were summarily dismissed as unrelated to vaccinations without in-depth investigations …’ the speculative causes were suicides, accidental drowning in well (why not suicide?), malaria, viral infections, subarachnoid hemorrhage (without autopsy) etc.”
_
Adverse effects following meningococcal A vaccine trial in Africa 2012:
In December 2012, in the small village of Gouro, Chad, Africa, situated on the edge of the Sahara Desert, five hundred children were locked into their school, threatened that if they did not agree to being force-vaccinated with a meningitis A vaccine, they would receive no further education. These children were vaccinated without their parents’ knowledge. This vaccine was an unlicensed product still going through the third and fourth phases of testing. Within hours, one hundred and six children began to suffer from headaches, vomiting, severe uncontrollable convulsions and paralysis. The children’s wait for a doctor began. They had to wait one full week for a doctor to arrive while the team of vaccinators proceeded to vaccinate others in the village. When the doctor finally came, he could do nothing for the children. The team of vaccinators, upon seeing what had happened, fled the village in fear. Forty children were finally transferred to a hospital in Faya and later taken by plane to two hospitals in N’Djamena, the capital city of Chad. After being shuttled around like cattle, many of these sick, weak children were finally dumped back in their village without a diagnosis and each family was given an unconfirmed sum of £1000 by the government. No forms were signed and no documentation was seen. They were informed that their children had not suffered a vaccine injury. However, if this were true, why would their government award each family £1000 in what has been described as hush money?
_
Why Japan banned MMR vaccine:
Japan stopped using the MMR vaccine seven years ago – virtually the only developed nation to turn its back on the jab. Government health chiefs claim a four-year experiment with it has had serious financial and human costs. Of the 3,969 medical compensation claims relating to vaccines in the last 30 years, a quarter had been made by those badly affected by the combined measles, mumps and rubella vaccine, they say. The triple jab was banned in Japan in 1993 after 1.8 million children had been given two types of MMR and a record number developed non-viral meningitis and other adverse reactions. Official figures show there were three deaths while eight children were left with permanent handicaps ranging from damaged hearing and blindness to loss of control of limbs. The government reconsidered using MMR in 1999 but decided it was safer to keep the ban and continue using individual vaccines for measles, mumps and rubella. The British Department of Health said Japan had used a type of MMR which included a strain of mumps vaccine that had particular problems and was discontinued in the UK because of safety concerns. The Japanese government realised there was a problem with MMR soon after its introduction in April 1989 when vaccination was compulsory. Parents who refused had to pay a small fine. An analysis of vaccinations over a three-month period showed one in every 900 children was experiencing problems. This was over 2,000 times higher than the expected rate of one child in every 100,000 to 200,000. The ministry switched to another MMR vaccine in October 1991 but the incidence was still high with one in 1,755 children affected. No separate record has been kept of claims involving autism. Tests on the spinal fluid of 125 children affected were carried out to see if the vaccine had got into the children’s nervous systems. They found one confirmed case and two further suspected cases. In 1993, after a public outcry fuelled by worries over the flu vaccine, the government dropped the requirement for children to be vaccinated against measles or rubella. Dr Hiroki Nakatani, director of the Infectious Disease Division at Japan’s Ministry of Health and Welfare said that giving individual vaccines cost twice as much as MMR ‘but we believe it is worth it’. In some areas parents have to pay, while in others health authorities foot the bill. However, he admitted the MMR scare has left its mark. With vaccination rates low, there have been measles outbreaks which have claimed 94 lives in the last five years.
_
Japan no longer recommends HPV vaccine:
Japan issued the first suspension of the government’s recommendation to get vaccinated against HPV in June 2013. The Japanese Government and Health authorities then organized a symposium on HPV vaccines, which occurred in February 2014. Important testimony was delivered by one doctor who had treated over 20 cases of multiple sclerosis (MS) after Gardasil vaccination. Pharmaceutical representatives were trying to say that such side effects are psychogenic, but how can a psychogenic disorder cause MS lesions in a person’s brain—and in a girl who was perfectly healthy prior to vaccination? They didn’t have an answer to that. All these problems started in temporal association with the vaccine. Just out of precautionary principle, you would think that they would have the common sense to at least halt the use of the vaccine until more research is done. But no, they just want to force it, and they parrot that it is safe. They do not have any proof of safety other than manipulated research. This symposium was followed by a large press conference, attended by Dr. Tomljenovic and research colleagues from France and the US. Since then, attempts by the makers of HPV vaccines to reinstate active recommendation of HPV vaccination by the Japanese Government have all failed, and Merck—which manufactures Gardasil—warned investors that Japan’s decision would have “a significant negative impact” on sales. GlaxoSmithKline’s HPV vaccine Cervarix also saw a downturn in sales immediately following the original suspension.
_
All above examples of vaccine harms are scary. I do not know the truth. Let us examine vaccine adverse reaction in detail.
_
Vaccine adverse effects: All Vaccines carry risks:
Vaccination given during childhood is generally safe. Adverse effects if any are generally mild. Most reactions to vaccines are mild and self-limited. Most reactions subside in 24-48 hours. The most common reactions involve inflammation at the site of vaccination – like redness, swelling or pain as well as fever. These vary by vaccine but are common affecting no more than 10 % of vaccines. Fortunately the reactions are usually mild and self limited lasting only a few days. Uncommon (from 0.1 to <1% of vaccinees) and rare (<1 in 1000 doses) reactions may occur – it is best to get specific information for each. With any medicine, there is always a small chance that someone might have an allergic reaction. True anaphylaxis reactions are rare (1 in 100,000 to 1 in a million doses) but are reversible with proper treatment. The reactions usually onset shortly after immunization is received. For this reason, a nurse or doctor will need to watch your child for 15 – 30 minutes (depending on the vaccine) after receiving a vaccine. It is important that you stay in the clinic for that period of time and watch for signs of an allergic reaction such as breathing problems or severe swelling and blotchy skin on your child’s body or around the mouth. If you see any of these symptoms or are concern about your child’s status, talk to your doctor or nurse immediately. It’s important to understand that all vaccines carry a risk for provoking an immediate acute adverse reaction, such as anaphylactic shock, fainting, or having a seizure. Further, vaccines can impair and alter immune system responses and can also cause brain inflammation (encephalopathy) that may lead to permanent brain damage. In addition, as Institute of Medicine Committees have pointed out in published reports, some individuals are more susceptible to suffering harm from vaccines because of biological, genetic, and environmental risk factors but, most of the time, doctors cannot predict who will be harmed because there are few scientific studies that have evaluated vaccine risks for individuals. Here are just some of the ways vaccines can impair or alter immune responses and brain function:
•Some components in vaccines are neurotoxic, including heavy metals such as mercury preservatives and aluminum adjuvants; residual toxins like endotoxin and bioactive pertussis toxin; and chemicals like formaldehyde and phenooxyethanol.
•The lab-altered and genetically engineered viruses and bacteria in vaccines may impair immune responses and do not stimulate the same kind of immunity that occurs when the body responds to an infectious disease
•Foreign DNA/RNA from human, animal and insect cell substrates used to produce vaccines may trigger serious health problems for some people
•Vaccines may alter your T-cell function and lead to chronic illness
•Vaccines can trigger allergies by introducing large foreign protein molecules into your body that have not been properly broken down by your digestive tract (since they are injected). Your body can have an allergic reaction to these foreign particles.
_
Vaccination induces immunity by causing the recipient’s immune system to react to antigens contained in the vaccine. Local and systemic reactions such as pain or fever can occur as part of the immune response. In addition, other vaccine components like adjuvants, stabilizers and preservative also contribute to adverse event. A successful vaccine keeps even minor reactions to a minimum while producing the best possible immune response. There is low public tolerance of vaccine adverse reactions. Vaccines are therefore only licensed when the frequency of severe reactions is very rare and when only minor, self-limiting reactions are reported.
_
Can we compare vaccine risk with other daily risks of life?
Vaccines prevent six million deaths worldwide every year, CNN’s Dr. Sanjay Gupta writes. And there’s basically no reason not to get them. Only one in a million children has a serious adverse reaction. Those are great odds. You’re 100 times more likely to get struck by lightning than have an allergic reaction to a vaccine, Gupta says. Taking aspirin, for example, is much more likely to cause bleeding in brain.
_
_
Risk of disease is far greater than risk of vaccine:
_
_
Not all reported vaccine adverse events are indeed caused by vaccine:
____
Adverse event following immunization (AEFI):
Although vaccines are proven to be extremely safe, there is a potential risk of an adverse reaction, as with any other drug or medication. The Adverse Event Following Immunization (AEFI) is defined as “a medical incident that takes place after immunization, causes concern and is believed to be caused by the immunization”. Any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the use of the vaccine is clubbed under AEFI. The adverse event may be any unfavorable or unintended sign, an abnormal laboratory finding, a symptom or a disease. This risk of AEFI with vaccination is always weighed against the risk of not immunizing a child. It is only when the benefit outweighs the risk, that a vaccine is considered safe. However, even at a relatively low rate, because of the high absolute number of beneficiaries, there is risk of a few serious adverse events in the vaccinated children. These events may be recognized during clinical trials or during post-marketing surveillance e.g. intussusceptions following rotavirus vaccine. Tolerance to vaccine associated adverse events is generally lower as these are administered to healthy children unlike other pharmaceutical products used in morbid populations. Vaccine associated adverse events are more likely to be noticed and communicated and can often significantly impact immunization programs as noticed with MMR and pertussis vaccines. The vaccines are foreign for human bodies, given to healthy infants and children. In the natural process of developing immunity, a vaccine may cause fever, erythema, local pain, etc. Besides, there is a slight risk of foreign body reaction to the components in the vaccines. These factors are likely to cause some concerns in the caregivers/parents. Whatever the cause, an AEFI may upset people to the extent that they may refuse further vaccination for their children. This may lead to the children much more likely to get a vaccine preventable disease, become seriously ill, disabled, and risk death. AEFI surveillance, therefore, helps to preserve public confidence in the immunization program. Though, the majorities of AEFIs are mild, settle without treatment, and have no long-term consequences; very rarely, serious adverse reaction can occur. The vaccination programs work in a ‘paradox’ meaning thereby that the focus of attention changes with the implementation of immunization program—when the vaccination coverage increases and disease burden reduces drastically, more cases of AEFI attract the attention of the people than the disease in the community. Figure below depicts how AEFI impacts an ongoing immunization program.
_
As rate of adverse event peaks, vaccine coverage falls and disease resurges followed by increase vaccine coverage:
_
Classification of AEFI:
For the programmatic purpose, the AEFIs are classified in five broad categories. Table below provides brief description of each reaction.
_
Vaccine product-related reaction (vaccine reaction):
An AEFI that is caused or precipitated by a vaccine due to one or more of the inherent properties of the vaccine product e.g. extensive limb swelling following DTP vaccination. Vaccine reaction is untoward event caused or precipitated by the vaccine when given correctly, caused by the inherent properties of the vaccine. These reactions are caused by a constituent of the vaccine. In some cases this will be the vaccine antigen (the substance that generates immunity), and is thus a side effect of the immunological process of generating immunity. In other cases it will be caused by other vaccine constituents (e.g. preservatives, stabilisers, antibiotics, or residual substance from the manufacturing process) or the adjuvant that is added to boost the vaccine’s immunogenicity. Vaccine reactions can be categorised into two types:
• Common, usually minor and self-limiting
• Rare and more serious
_
Minor reactions:
1. Usually occur within a few hours of injection.
2. Resolve after short period of time and pose little danger.
3. Local (or localized): Restricted or limited to a specific body part or region and includes pain, swelling or redness at the site of injection.
4. Systemic: Relating to a system, or affecting the entire body or an entire organism and includes fever, malaise, muscle pain, headache or loss of appetite.
_
Local reaction: swelling/redness at the site of injection.
_
Serious event:
An AEFI will be considered serious, if it:
•results in death,
•is life-threatening,
•requires in-patient hospitalization or prolongation of existing hospitalization,
•results in persistent or significant disability/incapacity,
•is a congenital anomaly/birth defect, or
•requires intervention to prevent permanent impairment or damage.
_
Severe vaccine reactions, onset interval, and rates associated with selected childhood vaccines:
| Vaccine | Reaction | Onset interval | Frequency per doses given |
| BCG | Fatal dissemination of BCG infection | 1 – 12 months | 0.19 – 1.56/1,000,000 |
| OPV | Vaccine associated paralytic poliomyelitis | 4 – 30 days | 2 – 4/1,000,000 |
| DTwP | Prolonged crying and seizures | 0 – 24 hours | < 1/100 |
| HHE | 0 – 24 hours | < 1/1,000 – 2/1,000 | |
| Measles | Febrile seizures | 6 – 12 days | 1/3,000 |
| Thrombocytopenia | 15 – 35 days | 1/30,000 | |
| Anaphylaxis | 1 hour | 1/100,000 |
_
_
Vaccine quality defect-related reaction:
An AEFI that is caused or precipitated by a vaccine that is due to one or more quality defects of the vaccine product including its administration device as provided by the manufacturer.
Example: Failure by the manufacturer to completely inactivate polio virus in inactivated polio vaccine (IPV) leading to cases of paralytic polio.
_
Immunization error-related reaction:
An AEFI that is caused by inappropriate vaccine handling, storage, prescribing, preparation or administration and thus by its nature is preventable and often constitute the greatest proportion of AEFIs. Example: Transmission of infection by contaminated multidose vial. They are preventable and detract from the overall benefit of the immunization program. The identification and correction of these incorrect immunization practices are of great importance.
Examples of immunization errors and possible AEFIs:
| Immunization error | Possible AEFI |
Non-sterile injection
|
|
Reconstitution error
|
|
Injection at incorrect site
|
|
| Vaccine transported/stored incorrectly |
|
_
Immunization anxiety-related reaction:
An AEFI arising from anxiety about the immunization. Individuals can react in anticipation to and as a result of an injection of any kind. These reactions are not related to the vaccine, but to fear of the injection. It could be syncope, vomiting, hyperventilation or even rarely convulsion.
_
Coincidental event:
Coincidental events occur after a vaccination has been given but are not caused by the vaccine or its administration.
Vaccinations are normally scheduled in infancy and early childhood, when illnesses are common and congenital or early neurological conditions become apparent. Coincidental events are inevitable when vaccinating children in these age groups, especially during a mass campaign. Applying the normal incidence of disease and death in these age groups along with the coverage and timing of immunizations allows estimation of the expected numbers of coincidental events after immunization.
_
Knowing the background mortality of the AEFI that coincidentally follow vaccination is key when responding to AEFI reports:
Expected coincidental deaths following DTP vaccination in selected countries:
| Country | Infant Mortality Rate per 1000 live births (IMR) | Number of births per year (N) | Number of infant death during year in | ||
| Month after immunization | Week after immunization | Day after immunization | |||
| = (IMRxN/12)×nv×ppv | = (IMR×N/52)×nv×ppv | = (IMR×N/365)×nv×ppv | |||
| Australia | 5 | 267,000 | 300 | 69 | 10 |
| Cambodia | 69 | 361,000 | 5,605 | 1,293 | 185 |
| China | 18 | 18,134,000 | 73,443 | 16,948 | 2,421 |
| Japan | 3 | 1,034,000 | 698 | 161 | 23 |
| Laos | 48 | 170,000 | 1,836 | 424 | 61 |
| New Zealand | 5 | 58,000 | 65 | 15 | 2 |
| Philippines | 26 | 2,236,000 | 13,081 | 3,019 | 431 |
Note: Assumes uniform distribution of deaths and that children who are near death will still be immunized.
nv = number of immunization doses: assumed here to be three dose schedule; 3.
ppv= proportion of population vaccinated: assumed here to be 90% for each dose; 0.9.
_
Based on the data in the table above, 2421 infant deaths are expected to occur coincidentally (i.e. not linked to the vaccine) in China the day after immunization with DTP. In other words, if more than 2421 infants died next day following DPT, that extra deaths can be attributed to vaccine.
_
One of the main challenges in surveillance of AEFIs is to differentiate coincidental events from events that are caused by a reaction to a vaccine or its components. Observing the rate of an adverse event in the vaccinated population and comparing it with the rate of this event among the unvaccinated population can help to distinguish genuine vaccine reactions. The following graphic shows how comparing the background rate with the observed rate of an event can help to determine the vaccine reaction rate (i.e. the rate of events that are actually caused by the vaccine).
_
Example: Fever following vaccination; in the graph above, 5 children got fever after vaccination per 1000 vaccination but only 2 were due to vaccine per se and remaining 3 was background fever rate.
_
| Terminology | How is this measured | Example |
| Background rate | Background rates can be determined in a population prior to the introduction of a new vaccine or simultaneously in non-vaccinated people. | If we measured the temperatures of a population of 1 000 unvaccinated children during one week, some children would present a fever (defined as >38°C) during the time of observation (e.g., infections). For example: a rate of 3 cases of fever per 1 000 children per week. |
| Observed (reported) rate | The observed rate can be measured in pre-licensure clinical trials or post-licensure studies. | If we observe the same population of 1 000 children but we now vaccinate all children and measure their temperatures daily there will be greater rate of fever. Thus, the rate of fever may increase to 5/1,000 children per week, with the increase concentrated in the 72 hours that follow vaccination. |
| Vaccine reaction rate (attributable rate) | Randomised clinical trials which are placebo controlled. Post-licensure studies – passive surveillance. |
Thus, the vaccine attributable rate of fever will be 2/1 000 vaccinated children (that is the observed rate minus the background rate). |
_
Imagine that rumours begin to circulate about a vaccine when cases of convulsions following immunization occur amongst vaccinated infants. The background rate of convulsions in this population is 1:1000 infants. The observed rate in vaccinated infants is 1.2:1000. The vaccine attributable rate derived from these figures is 2 additional cases in every 10000 vaccinations, compared with the background rate.
_
Comparing observed with “expected” rates of adverse events:
If the background rate of a particular adverse event is not known in a community (as is often the case), you will need to compare the observed rate in your population with the ‘expected rate’ published by the vaccine regulatory authorities. For example, the information from WHO shows the expected rates of AEFIs following some childhood vaccines:
| Vaccine | Estimated rate of severe reactions |
| BCG | 1 in 1000 to 1 in 50000 doses |
| OPV (oral polio vaccine) | 1 in 2–3 million doses (or 1 in 750000 doses for the first dose) |
| Measles | 1 in 1 million doses |
| DTP | 1 in 750000 doses |
_
Other factors to consider when comparing rates of AEFIs: confounding variables:
Keep in mind the other confounding factors that may influence the comparison of rates of adverse events. Confounding variable or factor is interference by a third variable so as to distort the association being studied between two other variables, because of a strong relationship with both of the other variables. A confounding variable can adversely affect the relation between the independent variable (cause) and dependent variable (outcome/effect). This may cause the researcher to analyze the results incorrectly. The results may show a false correlation between the dependent and independent variables, leading to an incorrect rejection of the null hypothesis. Here are some factors to consider when comparing one observed AEFI rate with another:
Vaccines:
Although a vaccine may have the same antigens, different manufacturers may produce vaccines (or ‘lots’ of the same vaccine) that differ substantially in their composition, including the presence of an adjuvant or other components. These variations result in vaccines with different reactogenicity (the ability to cause vaccine reactions), which in turn affects the comparison of their vaccine attributable rates.
Age:
The same vaccine given to different age groups may result in different vaccine-attributable rates. For example, MMR vaccine given to infants may cause febrile convulsions. This symptom does, however, not occur in adolescents who are given the same vaccine.
Vaccine doses:
The same vaccine given as a ‘primary dose’ may have a different reactogenicity profile than when it is given as a ‘booster dose’. For example, the DTaP vaccine given as a primary dose is less likely to result in extensive limb swelling when compared with this same vaccine given as a booster dose.
Case definitions:
Adverse events may be defined differently in research studies that do not stick to the same case definition. Not using standardized case definitions may consequently affect the estimation of the AEFI rate.
Surveillance methods:
The way that surveillance data is collected may alter the rate. For example, surveillance data may be collected actively or passively, using pre- or post-licensure clinical trials, with or without randomization and placebo controls.
___
Did vaccine indeed cause AEFI?
The figure below shows 5 factors to be considered for establishment of causality:
_
1. Consistency:
The association of a purported AEFI with the administration of vaccine should be consistent. The findings should be replicable in different localities, by different investigators not unduly influencing one another, and by different methods of investigation, all leading to the same conclusion.
2. Temporal relation:
There should be a temporal relationship between the vaccine and the adverse event. The vaccine should precede the earliest manifestation of event.
3. Biological plausibility:
The association should be coherent, plausible and explicable according to known facts in the natural history and biology of the disease.
4. Specificity:
The association should be distinctive. The adverse event should be linked specifically or uniquely with the vaccine concerned rather than occurring frequently, spontaneously or commonly in association with other external stimuli or conditions.
5. Strength of association:
The association between AEFI and the vaccine should be strong in terms of magnitude and also in dose-response relationship of the vaccine with the adverse event.
_
_
_
Anaphylactic Hypersensitivity to Egg and Egg-Related Antigens:
Egg allergy is one of the most common food allergies of childhood, with a prevalence of 1% to 3% in children under 3 years of age. It is often associated with eczema in infants and asthma in young children. As most children outgrow their egg allergy, the prevalence in adulthood is much lower and is estimated at 0.1%. The most common egg allergy is to egg white. Cross-sensitivity with egg yolk and chicken protein has been described. Vaccines that contain small quantities of egg protein can cause hypersensitivity reactions in some people with allergies to eggs. There are several vaccines manufactured by processes involving hens’ eggs or their derivatives, such as chick cell cultures. This manufacturing process may result in the following vaccines containing trace amounts of residual egg and chicken protein:
•measles-mumps-rubella (MMR) vaccines
•measles-mumps-rubella-varicella (MMRV) vaccine
•influenza vaccines
•tick-borne encephalitis (TBE) vaccine
•RabAvert®rabies vaccine
•yellow fever (YF) vaccine
Hypersensitivity reactions occurring following receipt of these vaccines varies considerably in relation to the amount of residual egg and chicken protein in the vaccine. Anaphylaxis after vaccination is rare. It may occur in people with anaphylactic hypersensitivity to eggs and in those with no history of egg allergy, due to other components in the vaccine. Due to this lack of predictability, immunization should always be performed by personnel with the capability and facilities to manage anaphylaxis post-vaccination. Individuals should be asked about allergies to egg or chicken prior to vaccination with influenza, TBE, YF, or RabAvert®rabies vaccines. Prior egg ingestion is not a prerequisite for immunization with egg protein-containing vaccine. It should be noted that any vaccine is contraindicated in people who have had an anaphylactic reaction to a previous dose of the vaccine. Referral to an allergy specialist is recommended. Atopic diseases are not a contraindication to immunization with egg protein-containing vaccine.
_
Can people with severe egg allergies still get an annual influenza vaccination?
The new vaccine, recombinant hemagglutinin influenza vaccine (RIV), is not made using eggs. This vaccine is safe for patients with egg allergy. Nasal-spray flu vaccines appear to be safe for children age 2 or older who have egg allergies or asthma, according to English researchers.
_
Vaccine-derived polioviruses (VDPV):
Vaccine-derived polioviruses (VDPVs) are rare strains of poliovirus that have genetically mutated from the strain contained in the oral polio vaccine. The oral polio vaccine contains a live, attenuated (weakened) vaccine-virus. When a child is vaccinated, the weakened vaccine-virus replicates in the intestine and enters into the bloodstream, triggering a protective immune response in the child. Like wild poliovirus, the child excretes the vaccine-virus for a period of six to eight weeks. Importantly, as it is excreted, some of the vaccine-virus may no longer be the same as the original vaccine-virus as it has genetically altered during replication. This is called a vaccine-derived poliovirus. Very rarely, vaccine-derived poliovirus can cause paralysis. Vaccine-associated paralytic poliomyelitis (VAPP) occurs in an estimated 1 in 2.7 million children receiving their first dose of oral polio vaccine. All cases of acute flaccid paralysis (AFP) among children under fifteen years of age are reported and tested for wild poliovirus or vaccine-derived polioviruses within 48 hours of onset. In 2009, the Global Polio Laboratory Network started using a new method to routinely screen for vaccine-derived polioviruses. The method is based on real-time reverse transcription-polymerase chain reaction (rRT-PCR), which targets nucleotide substitutions that occur early in the emergence of the virus. Circulating vaccine-derived polioviruses must be managed in the same way as wild poliovirus outbreaks. The solution is the same for all polio outbreaks: immunize every child several times with the oral vaccine to stop polio transmission, regardless of whether the virus is wild or vaccine-derived. Vaccine-derived polioviruses appear to be less transmissible than wild poliovirus. Outbreaks are usually self-limiting or rapidly stopped with 2–3 rounds of high-quality supplementary immunization activities. Once wild poliovirus transmission has been stopped globally, the vaccine-viruses will be the only source of live polioviruses in the community and could potentially lead to the re-emergence of polio. Use of the oral polio vaccine in routine immunization programs will therefore be phased out to eliminate the rare risks posed by vaccine-derived polioviruses.
_
Eczema Vaccinatum:
Eczema vaccinatum is a serious complication that occurs when people with eczema or atopic dermatitis get vaccinated. The lesion spreads to skin that is currently affected or has recently been affected by eczema. This complication can occur even if the eczema or atopic dermatitis is not active at the time, and requires immediate medical attention. Prior to 1960, eczema vaccinatum occurred in 10 to 39 people per 1 million people vaccinated. Vaccine Immune Globulin (VIG) is felt to be a useful treatment for eczema vaccinatum.
_
Bell palsy following intranasal vaccination:
Results from a case–control study and a case-series analysis indicate a significantly increased risk of Bell palsy developing following intranasal immunization with a new vaccine. This inactivated influenza vaccine, composed of influenza antigens in a virosomal formulation with E. coli derived LT adjuvant, was licensed in Switzerland in October 2000. Following spontaneous reports of Bell palsy, the company decided not to market the vaccine during the following season. In general, the etiology and pathogenesis of Bell palsy remain inadequately understood. The greater risk of Bell palsy following immunization with this vaccine may be due to specific vaccine components such as LT toxin, influenza antigens or virosomes, or simply to use of the intranasal administration route. It is thus possible that such complications of vaccine administration may also apply to other nasal vaccines. GACVS therefore recommends that any novel vaccine for nasal administration should be tested on a sufficiently large number of subjects before licensing and submitted to active post-marketing surveillance studies. Since the average time to onset of Bell palsy following intranasal immunization with this new vaccine was as much as 60–90 days, GACVS recommends that the follow-up period in the context of clinical trials should be routinely extended to 3 months following administration of a new intranasal vaccine.
_
Vaccine and mad cow disease:
Because vaccines are a natural product, they sometimes require the use of animal cells during production. This process is strictly controlled so that it does not pose a risk to people. No brain cells are used in manufacturing vaccines. During the manufacturing process, the vaccines are purified, and all animal cells are removed. However, each batch of vaccine is tested to ensure that it is free from infectious agents. For some vaccines, material derived from cows (for example, gelatin and lactose) have been used in the manufacturing process, and this has raised the question of whether vaccines can transmit “mad cow disease” to humans. Scientists in several countries have studied this risk and estimated that, in theory, there could be a risk of one person in 40 billion being exposed to the disease through a vaccine. Even though the risk is extremely small, vaccine manufacturers are working to find alternatives to these components.
_
OPV AIDS hypothesis:
The oral polio vaccine (OPV) AIDS hypothesis suggests that the AIDS pandemic originated from live polio vaccines prepared in rhesus macaque tissue cultures and then administered to up to one million Africans between 1957 and 1960 in experimental mass vaccination campaigns. Data analyses in molecular biology and phylogenetic studies contradict the OPV AIDS hypothesis; consequently, scientific consensus regards the hypothesis as disproven. The journal Nature has described the hypothesis as “refuted”.
________
________
Vaccine and autism:
Autism Spectrum Disorder (ASD) is really a collection of several disorders that have three abnormal areas in common: social skills, communication skills, and repetitive or obsessive traits. In the 1980s, one in 10,000 kids was diagnosed with autism. Today, one in 150 American 8-year-olds has some form of autism. Boys outnumber girls four to one. The United States is not the only country seeing this trend. It is increasingly diagnosed worldwide. For starters, is it really an epidemic? Or, are more people being diagnosed? Many children who were diagnosed with mental retardation 30 years ago are children who are diagnosed with classic autism today. And mildly disabled ASD kids today are children who never would have had a diagnosis 30 years ago. Those verbal, but socially awkward, children account for the majority of new ASD cases.
_
Although child vaccination rates remain high, some parental concern persists that vaccines might cause autism. Three specific hypotheses have been proposed: (1) the combination measles-mumps-rubella vaccine causes autism by damaging the intestinal lining, which allows the entrance of encephalopathic proteins; (2) thimerosal, an ethylmercury-containing preservative in some vaccines, is toxic to the central nervous system; and (3) the simultaneous administration of multiple vaccines overwhelms or weakens the immune system. A worldwide increase in the rate of autism diagnoses—likely driven by broadened diagnostic criteria and increased awareness—has fueled concerns that an environmental exposure like vaccines might cause autism. Theories for this putative association have centered on the measles-mumps-rubella (MMR) vaccine, thimerosal, and the large number of vaccines currently administered. However, both epidemiological and biological studies fail to support these claims.
_
The MMR vaccine controversy started with the 1998 publication of a fraudulent research paper in the medical journal The Lancet that lent support to the later discredited claim that colitis and autism spectrum disorders are linked to the combined measles, mumps and rubella (MMR) vaccine. The media have been criticized for their naïve reporting and for lending undue credibility to the architect of the fraud, Andrew Wakefield. Andrew Wakefield, the author of the original research paper, had multiple undeclared conflicts of interest, had manipulated evidence, and had broken other ethical codes. The Lancet paper was partially retracted in 2004, and fully retracted in 2010, when The Lancet’s editor-in-chief Richard Horton described it as “utterly false” and said that the journal had been “deceived.” Wakefield was found guilty by the General Medical Council of serious professional misconduct in May 2010 and was struck off the Medical Register, meaning he could no longer practice as a doctor in the UK. In 2011, Deer provided further information on Wakefield’s improper research practices to the British medical journal, BMJ, which in a signed editorial described the original paper as fraudulent. The BMJ editors conclude that Wakefield deliberately faked the study. “Is it possible that he was wrong but not dishonest: that he was so incompetent that he was unable to fairly describe the project or to report even one of the 12 children’s cases correctly?” they ask. “No. A great deal of thought and effort must have gone into drafting the paper to achieve the results he wanted.” The scientific consensus is that no evidence links the MMR vaccine to the development of autism, and that this vaccine’s benefits greatly outweigh its risks. Following the initial claims in 1998, multiple large epidemiological studies were undertaken. Reviews of the evidence by the Centers for Disease Control and Prevention, the American Academy of Pediatrics, the Institute of Medicine of the US National Academy of Sciences, the UK National Health Service, and the Cochrane Library all found no link between the MMR vaccine and autism. While the Cochrane review expressed a need for improved design and reporting of safety outcomes in MMR vaccine studies, it concluded that the evidence of the safety and effectiveness of MMR in the prevention of diseases that still carry a heavy burden of morbidity and mortality justifies its global use, and that the lack of confidence in the vaccine has damaged public health.
_
The medical community has relied mainly on epidemiology – the statistical study of large populations. These studies have overwhelmingly found no link between autism and MMR. Opponents claim that some of these studies might have flaws, but there are over a dozen epidemiological studies in different countries that use different techniques that have reached the same conclusion. At the very least, these studies show that the large increases in rates of autism that have been reported in many countries around the world cannot be due to MMR. However, the opponents of the vaccine point out that epidemiology can’t rule out an increased risk to a small number of children – a vulnerable subset. But perhaps the most crucial question is whether the measles virus really is persisting in the bodies of autistic children; and now that question too has been investigated. A new, unpublished study has examined blood samples from a group of 100 autistic children and 200 children without autism. These samples have been examined using the most sensitive methods available. They found 99% of the samples contained no trace of the measles virus, and the samples that did contain the virus were just as likely to be from non-autistic children. The study therefore found no evidence of any link between MMR and autism.
_
Studies that fail to support an association between measles-mumps-rubella vaccine and autism.
_
A large and growing body of scientific evidence has shown no connection between vaccines and autism. Parents can be confident that the medical and public health communities – including the prestigious Institute of Medicine (IOM), American Academy of Pediatrics (AAP), American Medical Association (AMA), World Health Organization (WHO), National Institutes of Health (NIH), Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) – strongly support the safety and benefits of immunizations.
_
New evidence clears measles vaccine of autism link: 2015 study:
Receipt of the measles, mumps, and rubella (MMR) vaccine is not associated with increased autism risk even among high-risk children, a JAMA study finds. Researchers retrospectively studied over 95,000 children who were continuously enrolled in a large U.S. health plan from birth until at least age 5 years who also had older siblings enrolled in the health plan. Some 2% of the children had an older sibling with autism spectrum disorder (ASD). Overall, 1% of the children were diagnosed with ASD during follow-up. Children who received the MMR vaccine were no more likely to be diagnosed with ASD than unvaccinated children — a finding that held true even among children whose older siblings had autism. An editorialist notes: “Taken together, some dozen studies have now shown that the age of onset of ASD does not differ between vaccinated and unvaccinated children, the severity or course of ASD does not differ between vaccinated and unvaccinated children, and now the risk of ASD recurrence in families does not differ between vaccinated and unvaccinated children.”
_
If the MMR vaccine doesn’t cause autism, why is the diagnosis made around the same time as the vaccination?
One of the criteria used to make a diagnosis of autism is a language delay. Because children do not have significant expressive language under a year of age, doctors have to wait until 15 to 18 months to confirm a language delay and make the diagnosis. That’s about the same time as the MMR vaccination, which leads some parents to wonder about autism and vaccination.
_
Does this have anything to do with thimerosal or mercury in vaccines?
No. Thimerosal is a mercury-based preservative. It cannot be used in live-virus vaccines such as the MMR. There has never been thimerosal in MMR vaccines. Research shows that thimerosal does not cause ASD. In fact, a 2004 scientific review by the IOM concluded that “the evidence favors rejection of a causal relationship between thimerosal–containing vaccines and autism.” Since 2003, there have been nine CDC-funded or conducted studies that have found no link between thimerosal-containing vaccines and ASD, as well as no link between the measles, mumps, and rubella (MMR) vaccine and ASD in children. Between 1999 and 2001, thimerosal was removed or reduced to trace amounts in all childhood vaccines except for some flu vaccines. This was done as part of a broader national effort to reduce all types of mercury exposure in children before studies were conducted that determined that thimerosal was not harmful. It was done as a precaution. Currently, the only childhood vaccines that contain thimerosal are flu vaccines packaged in multidose vials. Thimerosal-free alternatives are also available for flu vaccine.
_
Italian court rules mercury and aluminum in vaccines cause autism:
An Italian court in Milan awarded compensation to the family of a young boy who developed autism from a six-in-one hexavalent vaccine manufactured by British drug giant GlaxoSmithKline. On September 24, 2014, Italy’s version of the National Vaccine Injury Compensation Program agreed that GSK’s “INFANRIX Hexa” vaccine for polio, diphtheria, tetanus, hepatitis B, pertussis and haemophilus influenza type B induced permanent autism and brain damage in the previously healthy child, whose name has been kept private for safety. The vaccine, which contains multiple antigens, thimerosal (mercury), multiple forms of aluminum, formaldehyde, recombinant (genetically modified) viral components and various chemical preservatives, demonstrably caused the young boy to regress into autism shortly after he received all three doses of the vaccine, prompting his family to petition the case before Italy’s Ministry of Health. When the Ministry rejected it, the family proceeded to file a lawsuit. After listening to expert medical testimony, the Italian court remarkably concluded that the boy suffered permanent harm as a result of the vaccine, and particularly its neurotoxic mercury and aluminum components. Also presented as evidence was a 1,271-page confidential GSK report revealing that the drug giant knew full well from human clinical trials that INFANRIX Hexa causes autism, but the company chose to release the vaccine anyway. At least five known cases of autism arising from the jab are listed in the report on page 626, in fact: At the conclusion of this damning report, GSK admits that INFANRIX Hexa can cause a wide range of deadly illnesses but insists that its risk-benefit profile “continues to be favourable.”
_
Anecdotal report does not mean scientific evidence and legal culpability is different than medical culpability. Studies that fail to support an association between thimerosal in vaccines and autism are listed in the table below:
_
The table below shows difference between autism and mercury poisoning:
_
Too many vaccines and autism:
The notion that children might be receiving too many vaccines too soon and that these vaccines either overwhelm an immature immune system or generate a pathologic, autism-inducing autoimmune response is flawed for several reasons:
1. Vaccines do not overwhelm the immune system. Although the infant immune system is relatively naive, it is immediately capable of generating a vast array of protective responses; even conservative estimates predict the capacity to respond to thousands of vaccines simultaneously. Consistent with this theoretical exercise, combinations of vaccines induce immune responses comparable to those given individually. Also, although the number of recommended childhood vaccines has increased during the past 30 years, with advances in protein chemistry and recombinant DNA technology, the immunologic load has actually decreased. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with >3000 of these immunological components in the 7 vaccines administered in 1980. Further, vaccines represent a minute fraction of what a child’s immune system routinely navigates; the average child is infected with 4–6 viruses per year. The immune response elicited from the vast antigen exposure of unattenuated viral replication supersedes that of even multiple, simultaneous vaccines.
2. Multiple vaccinations do not weaken the immune system. Vaccinated and unvaccinated children do not differ in their susceptibility to infections not prevented by vaccines. In other words, vaccination does not suppress the immune system in a clinically relevant manner. However, infections with some vaccine-preventable diseases predispose children to severe, invasive infections with other pathogens. Therefore, the available data suggest that vaccines do not weaken the immune system.
3. Autism is not an immune-mediated disease. Unlike autoimmune diseases such as multiple sclerosis, there is no evidence of immune activation or inflammatory lesions in the CNS of people with autism. In fact, current data suggest that genetic variation in neuronal circuitry that affects synaptic development might in part account for autistic behavior. Thus, speculation that an exaggerated or inappropriate immune response to vaccination precipitates autism is at variance with current scientific data that address the pathogenesis of autism.
4. No studies have compared the incidence of autism in vaccinated, unvaccinated, or alternatively vaccinated children (i.e., schedules that spread out vaccines, avoid combination vaccines, or include only select vaccines). These studies would be difficult to perform because of the likely differences among these 3 groups in health care seeking behavior and the ethics of experimentally studying children who have not received vaccines.
_
From science to law:
Do childhood vaccines cause autism? This scientific question has now become a legal one — perhaps inevitable in our society. Some families with autistic children are pursuing legal channels in an effort to prove that vaccines are responsible for their children’s condition. Most of them allege that the cause is the mercury-containing preservative thimerosal, which was formerly used in many vaccines in the United States and elsewhere. Others argue that the culprit is the measles, mumps, and rubella (MMR) vaccine itself or perhaps the vaccine in combination with thimerosal. Although most experts have concluded that there is no proof of a causal tie between autism and thimerosal or the MMR vaccine, some doctors and scientists, some groups representing families with autistic children, and many parents fervently believe there is a connection. Claimants not only want to prove that the federal government, the Institute of Medicine, vaccine makers, and mainstream science are wrong; they also want money. A child with autism is likely to require extraordinarily expensive services — and to have very limited employment prospects in adulthood. Besides, many parents of autistic children may feel better psychologically if they can blame profit-seeking drug companies for their children’s problems. More than 5000 such families have filed claims with the federal Vaccine Injury Compensation Program (VICP).
_
Vaccine and SIDS:
What about reports that vaccines are linked to chronic diseases or problems such as sudden infant death syndrome (SIDS)?
Vaccines do not cause SIDS. Fortunately, we have learned that other factors, such as sleeping position and second-hand smoke, are linked with SIDS, and successful public education campaigns about these factors have helped to reduce the rate of SIDS. Vaccines are sometimes blamed for conditions that are poorly understood. A child’s first year of life is a time of tremendous growth and development, and it is a time when serious problems may start to appear. It is also the time when most vaccines are given, but this does not mean that vaccines cause these problems. Many of our vaccines have been in use for decades with no evidence of long-term adverse effects. Still, research to ensure the safety of vaccines continues. Anti-vaccine books and web sites claim that vaccines cause autism, seizure disorders, multiple sclerosis (MS) or Crohn’s disease, among other health problems. These connections have never held up to scientific scrutiny. Recent research using the best scientific methods and reviews of studies from around the world provide very strong evidence that
•MMR vaccine does not cause autism or inflammatory bowel disease;
•Hepatitis B vaccine does not cause multiple sclerosis or relapses of pre-existing MS;
•Pertussis vaccine does not cause brain damage;
•Childhood vaccines do not increase the risk of asthma.
_______
_______
Vaccine Safety Monitoring and Adverse Event Reporting:
Historical Perspective:
Fortunately the scientific community takes even the slightest suggestion that a vaccine causes an affliction seriously. Every time that a vaccine is accused of a side effect or of causing damage to the person receiving the vaccine the scientific vaccine community goes into full research mode. When there is found to be a relationship between a side effect and a vaccine, the scientific community is alerted while the vaccine’s safety is reviewed. The vaccine may be temporarily or permanently suspended from use. For example, RotaShield®, was a rotavirus vaccine that was licensed by the Food and Drug Administration (FDA) in August 1998 and recommended for use in the United States by the ACIP. In July of 1999 with almost 1 million children having been immunized with the vaccine, it was noticed that an increase in the number of children who developed a serious bowel disease called “intussusception” was occurring. The common thread was the RotaShield® vaccine, and so the Centers for Disease Control and Prevention (CDC) recommended that use of the vaccine be suspended. Scientific investigation estimated that the risk of intussusception attributable to the vaccine was about one per 10,000 (or less) among vaccinated infants, which was significantly higher than for children who were not vaccinated with that vaccine. Action was quickly taken, and the vaccine was voluntarily withdrawn from the market by the manufacturer in October 1999. Further investigation showed that those who received the RotaShield® vaccine in 1998 and 1999 were not at continuing risk of developing intussusception. This shows that the surveillance systems put in place by both the government and the scientific community work and that the continuous monitoring of vaccines and the diligence of the scientific community provides us with the safest vaccines possible today. Additionally, rigorous questioning of the safety of vaccines leads researchers to find new ways to develop and manufacture vaccines.
_
Vaccine Adverse Event Reporting System (VAERS) in the U.S.:
Vaccines are developed with the highest standards of safety. However, as with any medical procedure, vaccination has some risks. Individuals react differently to vaccines, and there is no way to predict how individuals will react to a particular vaccine. The National Childhood Vaccine Injury Act (NCVIA) requires health care providers to report adverse events (possible side effects) that occur following vaccination, so the Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) established the Vaccine Adverse Events Reporting System (VAERS) in 1990. VAERS is a national passive reporting system that accepts reports from the public on adverse events associated with vaccines licensed in the United States.
VAERS data are monitored to–
•Detect new, unusual, or rare vaccine adverse events
•Monitor increases in known adverse events
•Identify potential patient risk factors for particular types of adverse events
•Identify vaccine lots with increased numbers or types of reported adverse events
•Assess the safety of newly licensed vaccines
Approximately 30,000 VAERS reports are filed annually, with 10-15% classified as serious (resulting in permanent disability, hospitalization, life-threatening illnesses or death). Anyone can file a VAERS report, including health care providers, manufacturers, and vaccine recipients or their parents or guardians. The VAERS form requests the following information:
•The type of vaccine received
•The timing of the vaccination
•The onset of the adverse event
•Current illnesses or medication
•Past history of adverse events following vaccination
•Demographic information about the recipient
_
While the VAERS provides useful information on vaccine safety, this passive reporting system has important limitations. One is that it only collects information about events following vaccination; it does not assess whether a given type of event occurs more often than expected after vaccination. A second is that event reporting is incomplete and is biased toward events that are believed to be more likely to be due to vaccination and that occur relatively soon after vaccination. To obtain more systematic information on adverse events occurring in both vaccinated and unvaccinated persons, the Vaccine Safety Datalink project was initiated in 1991. Directed by the CDC, this project includes eight managed-care organizations in the United States; member databases include information on immunizations, medical conditions, demographics, laboratory results, and medication prescriptions. The Department of Defense oversees a similar system monitoring the safety of immunizations among active-duty military personnel. In addition, postlicensure evaluations of vaccine safety may be conducted by the vaccine manufacturer. In fact, such evaluations are often required by the FDA as a condition of vaccine licensure.
_
Institute of medicine (IOM) report:
The report was released by the Institute of Medicine (IOM) in 2011, which is part of the National Academy of Science.
Over a period of three years, they reviewed over 1,000 studies on vaccines. Interestingly, they excluded studies funded by the pharmaceutical industry, although some of the studies were funded by government agencies independently.
The review focused on eight vaccines:
| Hepatitis A-hepatitis B | Measles, mumps, and rubella vaccine | Meningococcal vaccine | Pneumococcal vaccine |
| Diphtheria, tetanus, and acellular pertussis, also known as DTaP or Tdap | Varicella zoster (chickenpox) | HPV vaccine | Influenza vaccine |
_
Some of those serious health problems included:
| Multiple sclerosis | Lupus | Encephalitis (brain inflammation) |
| Rheumatoid arthritis | Autism | Encephalopathy, involving permanent brain damage |
_
Perhaps the most important thing IOM did in this review is that they looked at two categories of science:
1. Epidemiological research (large studies comparing different groups of people against each other)
2. Bench science (research into the biological mechanisms at work within cells and molecules)
This is very important because a lot of the studies that the CDC relies on as evidence that vaccines don’t cause any problems are epidemiological studies. This report is important because they looked at both kinds of science. The most shocking conclusion of this report is that for more than a hundred bad health outcomes that have been reported after these eight vaccines have been given to people, they could not come to a conclusion as to whether or not those vaccines did or did not cause those adverse events!
Individual Susceptibility was discussed as a Co-Factor:
The IOM report also discussed individual susceptibility; the fact that some people are more susceptible for biological reasons, including genetic reasons, to having an adverse event after a vaccination. According to the report, both epidemiologic and mechanistic research suggests that most individuals who experience an adverse reaction to vaccines have a preexisting susceptibility. However, the report also states that in most cases they don’t know what those individual susceptibilities are.
Potential predispositions suggested in the report include:
•Genetic variation
•Age
•Coinciding illness
•Environmental factors
Every physician who gives a vaccine should read this 600-page report. That it is their responsibility because this is the latest report on the science of vaccination; of what’s in the published literature.
_
Safety of Vaccines Used for Routine Immunization of US Children: A Systematic Review 2014:
Concerns about vaccine safety have led some parents to decline recommended vaccination of their children, leading to the resurgence of diseases. Reassurance of vaccine safety remains critical for population health. This study systematically reviewed the literature on the safety of routine vaccines recommended for children in the United States. Strength of evidence was high for measles/mumps/rubella (MMR) vaccine and febrile seizures; the varicella vaccine was associated with complications in immunodeficient individuals. There is strong evidence that MMR vaccine is not associated with autism. There is moderate evidence that rotavirus vaccines are associated with intussusception. Limitations of the study include that the majority of studies did not investigate or identify risk factors for AEFIs; and the severity of AEFIs was inconsistently reported. Authors found evidence that some vaccines are associated with serious AEFIs; however, these events are extremely rare and must be weighed against the protective benefits that vaccines provide.
_
Vaccine Regulation in the U.S.:
Because vaccines are given to healthy individuals, they undergo a more rigorous approval process than drugs which are given to cure sick people. Licensing of vaccines typically takes 15 years and an average of $800 million of manufacturers’ money. The Food and Drug Administration (FDA) ensures the safety, purity, potency and effectiveness of vaccines.
But it doesn’t stop there:
•Post-licensing monitoring is conducted – tracking any side-effects from the vaccine.
•Samples of every lot of medicine must be submitted to the FDA before it is sold. This ensures that each batch is as safe and effective as the last.
•Since 1990, the Vaccine Safety Datalink (VSD) has collected statistics from more than 7 million people in health plans who have received vaccines.
•In 1990, the Centers for Disease Control and Prevention (CDC) and the FDA established the Vaccine Adverse Event Reporting System (VAERS), which gathers information about any side effects patients have experienced. VAERS accepts any reported information without determining a cause and effect relationship. Clinical Immunization Safety Assessment Centers (CISA) were started in 2001. They conduct clinical research about vaccine adverse events (VAE) and the role of individual variation; provide clinicians with evidence-based counsel and empower them to make informed immunization decisions.
_
How is a vaccine or a batch recalled?
Vaccine recalls or withdrawals are almost always voluntary by the manufacturer. Only in rare cases will the Food and Drug Administration (FDA) request a recall. But in every case, FDA’s role is to oversee a manufacturer’s strategy and assess the adequacy of the recall.
Why would a vaccine or batch of vaccine be withdrawn or recalled?
There have been only a few vaccine recalls or withdrawals, most due to concerns about the vaccine’s effectiveness, not its safety. When the strength of a vaccine lot has been reduced, those vaccines may not produce an immune response that is strong enough to protect against disease. Although those vaccines may not be effective, they are still safe. Vaccines are tested carefully and monitored continuously before and after they are licensed for use. If a vaccine lot is found to be unsafe, the FDA recalls it immediately.
_
The Vaccine Injury Compensation Program (VICP) in the U.S.:
This legislation was adopted by Congress in 1988 in response to a somewhat similar scare over the pertussis portion of the diphtheria–pertussis–tetanus (DPT) vaccine. Alerted to a possible link by British researchers, many observers feared that the vaccine was causing some children grave neurologic harm — claims that were later generally discredited. Yet the alarm was so great that droves of British families refused the pertussis vaccine, substantial numbers of children became ill with whooping cough, and some 70 children died. In the United States, several parents sued the manufacturers of DPT vaccines. Even though most public health officials believed that the claims of side effects were unfounded, some families won substantial awards from sympathetic juries who were convinced otherwise. As a result, most companies making the DPT vaccine ceased production, and the remaining major manufacturer threatened to do so. Health officials feared the loss of herd immunity, and Congress responded by creating the VICP. This program provides compensation to children who have serious adverse effects from any childhood vaccine. The compensation covers medical and related expenses, lost future income, and up to $250,000 for pain and suffering. The funding for paying successful claims regarding vaccines administered before 1988 came from the U.S. Treasury. For claims regarding later vaccinations, funding comes from a patient fee of 75 cents per vaccination. The VICP trust fund currently contains more than $2 billion. About 7000 claims have been filed for adverse effects other than autism, and so far about 2000 have resulted in compensation, in amounts averaging about $850,000. Approximately 700 claims remain unresolved, since the VICP frequently takes more than 2 years to process a petition. To win a VICP award, the claimant does not need to prove everything that is required to hold a vaccine maker liable in a product liability lawsuit. But a causal connection must be shown. If medical records show that a child had one of several listed adverse effects within a short period after vaccination, the VICP presumes that it was caused by the vaccine (although the government can seek to prove otherwise). An advisory committee helps to amend the list of adverse effects as the consensus view changes with the availability of new studies. If families claim that a vaccine caused an adverse effect that is not on the list, the burden of proof rests with them. Autism is not on the list for any vaccine, and the VICP has rejected about 300 such claims outright. In 2011, the US Supreme Court ruled that vaccines are “unavoidably unsafe” and that the federal Vaccine Injury Compensation Program (VICP) should be the sole remedy for all vaccine injury claims. Most claims are now filed by adults suffering vaccine injury after receiving a flu vaccine.
________
________
Vaccine contraindications, concerns and exemptions:
Contraindications and precautions:
Before vaccination, all patients should be screened for contraindications and precautions. A contraindication is a condition that is believed to substantially increase the risk of a serious adverse reaction to vaccination. A contraindication is a situation in a vaccine should not be used because the risk outweighs any potential therapeutic benefit. A vaccine should not be administered when a contraindication is documented. For example, a history of an anaphylactic reaction to a dose of vaccine or to a vaccine component is a contraindication for further doses. A precaution is a condition that may increase the risk of an adverse reaction following immunization or that may compromise the ability of the vaccine to produce immunity. In general, vaccines are deferred when a precaution is present. However, there may be circumstances when the benefits of giving the vaccine outweigh the potential harm, or when reduced vaccine immunogenicity may still result in significant benefit to a susceptible, immunocompromised host. In some cases, contraindications and precautions are temporary and may lead to mere deferral of vaccination until a later time. For example, moderate or severe febrile illnesses are generally considered transient precautions to vaccination and result in postponement of vaccine administration until the acute phase has resolved; thus the superimposition of adverse effects of vaccination on the underlying illness and the mistaken attribution of a manifestation of the underlying illness to the vaccine are avoided. It is important to recognize conditions that are not contraindications in order not to miss opportunities for vaccination. For example, in most cases, mild acute illness (with or without low-grade fever), a history of a mild to moderate local reaction to a previous dose of the vaccine, and breast-feeding are not contraindications to vaccination.
_
There are two types of contraindications (reasons not to give a vaccine): permanent and temporary.
•The following are permanent contraindications to vaccination:
1. Severe allergic reaction to a vaccine component (animal proteins [eggs], antibiotic, stabilizer, or preservative) or following a previous dose of the vaccine;
2. Encephalopathy within seven days of a pertussis vaccination (not from another identifiable cause).
•The following are precautions/temporary contraindications to vaccination:
1. Pregnancy: Although the risk of vaccination during pregnancy is mostly theoretical, caution is advised. Therefore, women who are known to be pregnant should not receive any of the live vaccines. Inactivated vaccines are considered generally safe during pregnancy and should be used when indicated.
2. Immunosuppression: People with active cancer, leukemia, or lymphoma (or people taking high doses of steroids) should not receive live vaccines but can receive inactivated vaccines.
◦Human immunodeficiency virus (HIV): Vaccination depends on the severity of the illness. In asymptomatic (without symptoms) individuals, many vaccines are considered safe. In general, the inactivated vaccines are safe for both symptomatic and asymptomatic individuals infected with HIV.
◦Moderate to severe illness: If someone is ill with more than a simple cold, earache, diarrhea, or other minor illness, vaccination should be postponed until the illness is over.
_
| Vaccine Formulation | Contraindications and Precautions |
| All vaccines | Contraindication:
Severe allergic reaction (e.g., anaphylaxis) after a previous vaccine dose or to a vaccine component Precaution: Moderate or severe acute illness with or without fever; defer vaccination until illness resolves |
| Td | Precautions:
GBS within 6 weeks after a previous dose of TT-containing vaccine History of Arthus-type hypersensitivity reactions after a previous dose of TT-containing vaccine; defer vaccination until at least 10 years have elapsed since the last dose |
| Tdap | Contraindication:
History of encephalopathy (e.g., coma or prolonged seizures) not attributable to another identifiable cause within 7 days of administration of a vaccine with pertussis components, such as DTaP or Tdap Precautions: GBS within 6 weeks after a previous dose of TT-containing vaccine Unstable neurologic condition (e.g., cerebrovascular events and acute encephalopathic conditions) History of Arthus-type hypersensitivity reactions after a previous dose of TT-containing and/or DT-containing vaccine, including MCV4; defer vaccination until at least 10 years have elapsed since the last dose Pregnancy |
| HPV | Contraindication:
History of immediate hypersensitivity to yeast (for Gardasil) Precaution: Pregnancy. If a woman is found to be pregnant after initiation of the vaccination series, the remainder of the 3-dose regimen should be delayed until after completion of the pregnancy. If a vaccine dose has been administered during pregnancy, no intervention is needed. A vaccine-in-pregnancy registry has been established for Gardasil; patients and health care providers should report any exposure to quadrivalent HPV vaccine during pregnancy. |
| MMR | Contraindications:
History of immediate hypersensitivity reaction to gelatin or neomycin Pregnancy Known severe immunodeficiency (e.g., hematologic and solid tumors; chemotherapy; congenital immunodeficiency; long-term immunosuppressive therapy; severe immunocompromise due to HIV infection) Precaution: Recent (within 11 months) receipt of antibody-containing blood product |
| Varicella | Contraindications:
Pregnancy Known severe immunodeficiency History of immediate hypersensitivity reaction to gelatin or neomycin Precaution: Recent (within 11 months) receipt of antibody-containing blood product |
| Influenza, injectable, trivalent | Contraindication:
History of immediate hypersensitivity reaction to eggs Precautions: History of GBS within 6 weeks after a previous influenza vaccine dose Pregnancy is not a contraindication or precaution. This vaccine is recommended for women who will be pregnant during influenza season. |
| Influenza, live attenuated | Contraindications:
History of immediate hypersensitivity reaction to eggs Age 50 years Pregnancy Immunosuppression, including that caused by medications or by HIV infection; known severe immunodeficiency (e.g., hematologic and solid tumors; chemotherapy; congenital immunodeficiency; long-term immunosuppressive therapy; severe immunocompromise due to HIV infection) Certain chronic medical conditions, such as diabetes mellitus; chronic pulmonary disease (including asthma); chronic cardiovascular disease (except hypertension); renal, hepatic, neurologic/neuromuscular, hematologic, or metabolic disorders Close contact with severely immunosuppressed persons who require a protected environment, such as isolation in a bone marrow transplantation unit Close contact with persons with lesser degrees of immunosuppression (e.g., persons receiving chemotherapy or radiation therapy who are not being cared for in a protective environment; persons with HIV infection) is not a contraindication or precaution. Precaution: History of GBS within 6 weeks of a previous influenza vaccine dose |
| Pneumococcal polysaccharide | None |
| Hepatitis A | Precaution:
Pregnancy |
| Hepatitis B | Contraindication:
History of immediate hypersensitivity to yeast |
| Meningococcal conjugate | Contraindications:
Age >55 years (licensed for use only among persons 2–55 years of age) History of severe allergic reaction to dry natural rubber (latex) or to DT-containing vaccines Precautions: History of GBS |
| Meningococcal polysaccharide | Contraindication:
History of severe allergic reaction to dry natural rubber (latex) |
| Zoster | Contraindications:
Age <60 years Pregnancy Known severe immunodeficiency History of immediate hypersensitivity reaction to gelatin or neomycin |
_
History of Immediate Hypersensitivity to a Vaccine Component:
A severe allergic reaction (e.g., anaphylaxis) to a previous dose of a vaccine or to one of its components is a contraindication to vaccination. While most vaccines have many components, substances to which individuals are most likely to have had a severe allergic reaction include egg protein, gelatin, and yeast. In addition, although natural rubber (latex) is not a vaccine component, some vaccines are supplied in vials or syringes that contain natural rubber. These vaccines can be identified by the product insert and should not be administered to persons who report a severe (anaphylactic) allergy to latex. The much more common local or contact hypersensitivity to latex is not a contraindication to administration of a vaccine supplied in a vial or syringe that contains latex.
_
Immunosuppression:
Live-virus vaccines elicit an immune response due to replication of the attenuated (weakened) vaccine virus that is contained by the recipient’s immune system. In persons with compromised immune function, enhanced replication of vaccine viruses is possible and could lead to disseminated infection with the vaccine virus. For this reason, live-virus vaccines are contraindicated for persons with severe immunosuppression, defined according to the specific vaccine on the basis—at least in part—of differences in the prevalence of conditions causing immunosuppression at the time of vaccine recommendation issuance. Severe immunosuppression may be caused by many disease conditions, including HIV infection and hematologic or generalized malignancy. In some of these conditions, all affected persons are severely immunocompromised. In others (e.g., HIV infection), the degree to which the immune system is compromised depends on the severity of the condition, which in turn depends on the stage of disease or treatment. Severe immunosuppression may also be due to therapy with immunosuppressive agents, including high-dose glucocorticoids. In this situation, the dose, duration, and route of administration may influence the degree of immunosuppression. The potential risks of live vaccines need to be weighed against the benefits in immunocompromised clients who may be particularly vulnerable to the vaccine-preventable disease. Concerns are that they may not respond adequately to subunit and inactivated vaccination and that LAV vaccines are potentially pathogenic. Routine childhood vaccinations – except BCG vaccination are not contraindicated in children with asymptomatic HIV-infection; however, timing of vaccination may be earlier or more frequent in this subgroup. In symptomatic HIV/AIDS, LAV vaccines are contraindicated, e.g. measles and yellow fever vaccines should not be given.
_
OPV and diarrhea:
It is recommended that even during diarrhea OPV drops should be administered, but, this dose should not be counted and then the OPV should be administered again when diarrhea is controlled. As it is known that OPV administered during an episode of diarrhea may not produce the optimum effect, so there is a need to repeat the dose. Then why should a child with diarrhea be taken to the Immunization Center causing inconvenience to the child and the mother, as the OPV dose administered will not be very effective?
OPV can be administered to a child with mild diarrhea. Since the effect may not be optimum, at the next opportunity, give another dose and do not count the earlier dose. The point is, when the child is available for a dose of OPV, give it in spite of mild diarrhea, for we do not know for sure that the child will be brought back for another dose of OPV later. So the child gets the benefit of at least the present dose, even if its effect is not as good as in a well child. If the child is available again, repeat the dose. All these guidelines are for giving the best possible protection to the child and not to be stingy about a dose of OPV that might not perhaps give the best result. Look at it another way. The child takes a dose of OPV, but develops mild diarrhea the same day. Will the child come to harm? No. Should the dose be repeated? Ideally, yes. Remember, diarrhea as a minor adverse effect due to oral polio vaccine per se occurs in 10 % recipients. During pulse polio campaigns, keep the contraindications to the minimum, namely very sick children in hospitals. Even there, there is no harm in giving vaccine to all those who are recovering, have chronic illness, are on antimicrobials, etc. but, they can be immunized just before sending home rather than giving it in the ward itself, where the probability of wild poliovirus transmission is very low.
_
Severe asthma and vaccination:
Asthma should be optimized before giving any vaccine. LAIV (live attenuated influenza vaccine) should not be administered to individuals with severe asthma (defined as currently on oral or high dose inhaled glucocorticosteriods or active wheezing) or those with medically attended wheezing in the seven days prior to vaccination. LAIV can be used in stable, non-severe asthmatics.
_
Congenital malformation of gastrointestinal tract or history of intussusceptions:
Rotavirus vaccine is contraindicated in infants with a history of intussusception or uncorrected congenital malformation of the gastrointestinal tract that would predispose for intussusception.
_
Guillain-Barré syndrome (GBS) with onset within 6 weeks of immunization:
Cases of GBS or polyneuritis have been reported following administration of tetanus toxoid-containing vaccine and there has been one case report of relapsing GBS following each of three doses of vaccine. However, population studies have not supported a causal association. Cases of GBS or polyneuritis have also been reported following receipt of diphtheria toxoid-containing vaccine. While some evidence favours a causal relationship between tetanus toxoid and GBS, there is little evidence to support an independent association between receipt of diphtheria toxoid and GBS. Persons who develop GBS within 6 weeks of receipt of tetanus toxoid-containing vaccine should not receive a further dose. Those who develop GBS outside the 6-week interval may receive subsequent doses of the vaccine. If there is a history of both Campylobacter infection (which has been associated with GBS) and receipt of a tetanus and diphtheria toxoid-containing vaccine within the 6 weeks before the onset of GBS, consultation with an infectious disease specialist is advised. In a review of studies between 1976 and 2005, the United States Institute of Medicine concluded that the 1976 swine flu vaccine was associated with an elevated risk of Guillain-Barré Syndrome (GBS). However, evidence was inadequate to accept or reject a causal relation between GBS in adults and seasonal influenza vaccination. More recent studies suggest that the absolute risk of GBS in the period following seasonal and H1N1-pdm09 influenza vaccination is about one excess case per 1 million vaccines. The risk of GBS associated with influenza vaccination must be balanced against the risk of GBS associated with influenza infection itself. In general, it is recommended to avoid subsequent influenza vaccination of persons known to have had GBS within six weeks of a previous influenza vaccination.
_
Tuberculosis and vaccination:
MMR, MMRV, varicella, and herpes zoster vaccines are contraindicated in individuals with active, untreated tuberculosis as a precautionary measure. Although tuberculosis may be exacerbated by natural measles infection, there is no evidence that measles or varicella-containing vaccines have such an effect. BCG vaccine is contraindicated for individuals with a positive tuberculin skin test, although immunization of tuberculin reactors has occurred frequently without complications.
_____
Acute illness (with or without fever) and vaccination:
In general, people with minor or moderate acute illness may receive vaccines. There is no increase in risk of adverse events following immunization and no interference with response to vaccine. There are three exceptions –
1. If significant nasal congestion is present that might impede delivery of LAIV to the nasopharyngeal mucosa, TIV can be administered or LAIV could be deferred until resolution of the illness.
2. In infants with moderate-to-severe gastroenteritis, rotavirus vaccine should be deferred until the condition improves unless deferral will result in scheduling of the first dose beyond the recommended age limit.
3. Administration of oral cholera and travelers’ diarrhea vaccine should be postponed in persons with acute gastrointestinal illness.
The risks and benefits of vaccinating a severely ill person need to be carefully assessed. The benefits of protection in a high risk exposure situation or when the window of opportunity is short (i.e., when travel or immunocompromise are imminent) need to be assessed against the risks that a vaccine-related adverse event (particularly fever) could complicate the management of the person. It is possible that systemic adverse events may complicate the medical management of an acute illness or that events associated with the acute illness may be misperceived as vaccine-related adverse events. Expert opinion is strongly recommended in this situation.
_
Febrile seizure or syncope following immunization:
Parents may hesitate to have their child have a vaccine if he/she had a history of a post-immunization febrile seizure. Likewise, people may hesitate to have a vaccine if they had an episode of syncope following a previous vaccine (such as may occur with HPV vaccine in a young girl). Vaccines are safe to given when there is a history of a febrile seizure. For MMR vaccine for example, the risk of febrile seizures within 2 weeks following MMR vaccination was 1.56 per 1000 children overall, 3.97 per 1000 for siblings of children with a history of febrile seizures, and 19.47 per 1000 for children with a personal history of febrile seizures. This means when a child has a history of a febrile seizure 98% will not have a febrile seizure following an MMR vaccine. Children with a history of febrile seizures have no increased risk of developing a seizure disorder, such as epilepsy. Oral analgesics/antipyretics (such as acetaminophen or ibuprofen) can be used for treatment of minor adverse reactions such as fever or injection site discomfort that might occur following vaccination. There is no evidence that antipyretics prevent febrile seizures. Vaccines are safe to give when there is a history of fainting after a vaccine. The likelihood of fainting can be reduced by measures that lower stress in those awaiting immunization, such as short waiting times, comfortable room temperature, preparation of vaccines out of view of recipients, and privacy during the procedure. People should be immunized while seated.
_
Inconsolable crying following immunization:
Persistent or inconsolable crying and an unusual, high-pitched cry (most typically after pertussis vaccination) are not associated with any sequelae and are likely to be pain responses at the site of injection in young infants.
_
Ocular-respiratory syndrome (ORS):
Oculo-respiratory syndrome is a usually transient condition characterized by bilateral conjunctivitis, facial edema, and upper respiratory symptoms that has been known to occur primarily after receiving influenza immunization. Symptoms typically appear 2 to 24 hours after vaccination and resolve within 48 hours of onset. If ORS occurred with lower respiratory symptoms, subsequent influenza vaccine is contraindicated.
_
Bleeding disorder:
People with bleeding disorders should receive all recommended immunizations according to routine schedules when appropriate safety measures have been taken. Control of bleeding disorders should be optimized prior to immunization. Vaccine providers should ensure that there are no symptoms or signs compatible with an undiagnosed bleeding disorder (e.g. unexplained bruising). If such indicators are present before immunization, a diagnosis should be established before commencing immunization. When administering a parenteral vaccine, consider use of a small gauge needle and apply pressure for 5-10 minutes after the immunization.
_
Breastfeeding:
Following routine immunization of either a mother or her infant during breastfeeding there is no reduction in maternal or infant response to vaccines and no increase in the risk of adverse events for either mother or infant. There is some evidence that breastfeeding may have a beneficial effect in infants after vaccination and is associated with less fever and pain. BCG, smallpox and yellow fever vaccines are generally contraindicated in breastfeeding women but may be considered in high-risk situations.
_
Antibiotic therapy:
Antibiotic therapy, does not interfere with response to inactivated vaccines or most live vaccines with the following exceptions: Live, oral typhoid vaccine should be delayed until 48 to 72 hours after receipt of the last dose of antibiotics active against Salmonella typhi. BCG vaccine should not be administered to individuals receiving drugs with anti-tuberculous activity, including fluoroquinolones.
Antiviral therapy:
Antiviral therapy does not interfere with response to inactivated vaccines or most live vaccines with the following exceptions:
1. Varicella vaccine and herpes zoster vaccine may have reduced effectiveness if given concurrently with antivirals active against varicella zoster virus (such as acyclovir, valacyclovir, famciclovir). People taking long-term antiviral therapy should discontinue these drugs, if possible, from at least 24 hours before administration of varicella or herpes zoster vaccine and should not restart antiviral therapy until 14 days after vaccination.
2. LAIV should not be administered until 48 hours after antiviral agents active against influenza (e.g., oseltamivir and zanamivir) are stopped, and antiviral agents should not be administered until at least 14 days after receipt of LAIV unless medically indicated. If antiviral agents are administered within this time frame (from 48 hours before to 14 days after LAIV), revaccination should take place at least 48 hours after the antivirals are stopped.
Recent administration of blood products containing antibodies:
Passive immunization with human immune globulin or receipt of most blood products can interfere with the immune response to certain live vaccines. Measles-containing or varicella vaccines should be given at least 14 days prior to administration of an immune globulin preparation or blood product, or delayed until the antibodies in the immune globulin preparation or blood product have degraded. A risk-benefit assessment is needed for post-partum women who have received Rh immune globulin (RhIg) and require MMR or varicella vaccine. Herpes zoster vaccine should be delayed until 3 months after a dose of intravenous immune globulin.
_
Premature birth:
Premature infants respond adequately to vaccines used in infancy and are not at significantly increased risk of adverse events. In general, immunize premature infants per the routine immunization schedule, according to child’s chronological age with the exception of hepatitis B vaccination of preterm infants with a birth weight of less than 2,000 grams. The response to hepatitis B vaccine may be diminished in such infants. Hospitalized premature infants should have continuous cardiac and respiratory monitoring for 48 hours after their first immunization.
_
Skin Disorders:
Vaccines are generally safe for people with skin disorders. For comfort, administer vaccine into non-affected area. There are two exceptions to this. Smallpox vaccine is contraindicated in those with eczema (atopic dermatitis) in non-outbreak situation. BCG vaccine is contraindicated when there is extensive skin disease or burns.
_______
Vaccinate household contacts of pregnant women, immunocompromised persons, and neonates:
Vaccination provides protection at both an individual and population level. Some people may have conditions that preclude vaccination, but they can be protected by having the people around them vaccinated. Immunization of household contacts of immunosuppressed persons, pregnant women, and neonates provides important protection against transmission of disease in the household. Up-to-date routine immunizations are recommended for household contacts of pregnant women, immunocompromised persons, and neonates with the following exceptions:
•TIV is preferred over LAIV for those in close contact with severely immunocompromised persons.
•If there are household contacts who have received live, oral polio vaccine in another country within the last 6 weeks, they should not have contact with immunocompromised persons.
•If a vaccine recipient develops a varicella-like rash, the rash should be covered and the vaccinee should avoid direct contact with the immunocompromised person for the duration of the rash
•Smallpox vaccine should not be administered to household contacts of an immunocompromised person in a non-emergency situation.
•Special precautions should be taken for unvaccinated pregnant household and other close contacts of persons receiving smallpox vaccine in order to eliminate viral transfer to these contacts.
________
What are invalid reasons for postponing vaccination?
Vaccination should not be postponed for any of the following reasons:
•Mild illness: Low-grade fever, colds, upper respiratory-tract infections, and mild diarrhea are not reasons to put off vaccination.
•Antibiotics: The current administration of antibiotics is not a reason to put off vaccination.
•Household contacts of pregnant women or immunosuppressed patients: Living in a house with a pregnant woman or an immunosuppressed patient is not a reason to put off vaccination. Two exceptions are the live attenuated nasal influenza vaccine and smallpox vaccine.
•Breastfeeding: Breastfeeding is not a reason for either the mother or baby to put off vaccination.
•Preterm birth: Preterm birth is not a reason to put off vaccination.
•Generalized allergies: Children with allergies, but no history of reactions to vaccine components, should receive vaccines as recommended.
•Family history: Having a family member who had an adverse reaction to a vaccine is not a reason to put off vaccination.
__
Mythical contraindications to immunization:
•Has already had the disease (applies only to BCG vaccine)
•Personal or family history of atopy
•Personal or family history of epilepsy
•Minor upper respiratory tract symptoms at the time of immunisation
•Significant reaction to another vaccine
________
________
Opposition to vaccine:
Health and medical scholars have described vaccination as one of the top ten achievements of public health in the 20th century. Yet, opposition to vaccination has existed as long as vaccination itself (indeed, the pre-vaccination practice of variolation came under criticism as well). Critics of vaccination have taken a variety of positions, including opposition to the smallpox vaccine in England and the United States in the mid to late 1800s, and the resulting anti-vaccination leagues; as well as more recent vaccination controversies such as those surrounding the safety and efficacy of the diphtheria, tetanus, and pertussis (DTP) immunization, the measles, mumps, and rubella (MMR) vaccine, and the use of a mercury-containing preservative called thimerosal. Although the time periods have changed, the emotions and deep-rooted beliefs—whether philosophical, political, or spiritual—that underlie vaccine opposition have remained relatively consistent since Edward Jenner introduced vaccination.
_
The anti-vaccination movement (also known as anti-vaxxers) is an irrational trend of mistrust of vaccination that is almost as old as the technique itself. The movement blames vaccines or their ingredients for a range of maladies whose mechanisms are rejected or have not been explained by current scientific research. Some of these maladies can often be childhood illnesses in order to increase the emotive factor of the argument. The ubiquity of vaccination often makes it an easy target for blame. Vaccine-preventable diseases have been a major cause of illness, death, and disability throughout human history. The advent of the modern vaccine era has changed this significantly; most North Americans and Europeans have little memory of a pre-vaccine era where diseases such as mumps and measles — to say nothing of smallpox or polio — were common and often deadly. In more recent times, there has been much debate in the press and in the doctor’s office regarding vaccine safety — namely what possible side-effect vaccines cause and whether these outweigh the risks of leaving a population without a vaccination schedule. Vaccines have been alleged to cause all manner of illnesses; autism is a prominent example, as its direct causes are still fairly mysterious and probably very wide-ranging, with no single cause or lifestyle risk-factor being identified. Some prominent Americans have spoken out vociferously about the supposed danger of vaccines. Fears about vaccines were excusable when they were new, but present concerns are groundless. Anti-vaccination rhetoric is usually presented in lots of scary, little “facts.”
_
There are many ideas which are not supported by reviewed and accepted scientific evidence that vaccines are inherently harmful. For example, it is claimed that specific vaccines such as MMR (mumps, measles and rubella), or specific ingredients like thiomersal are causative factors leading to disease. Some claims are more vague, based on the feeling that vaccines are “unnatural,” that they are somehow “useless,” or that the diseases they prevent “aren’t that bad anyway.” Anti-vaccination campaigners often use the language of being for “freedom” in whether to be vaccinated, such as with MMR, where the campaign was the “choice” to take a non-combined vaccine. These beliefs often stem from other ideological positions; for instance, vaccination programs are seen as excessive government interference, or as an implementation of socialized medicine, although it’s hardly just a conservative thing. Similarly, those against “artificial” interference will also shun vaccination regardless of efficacy. It could be argued that these ideologies are the root causes of anti-vaccination positions, and bias which specific concern an individual will be attracted to.
_
Vaccines are considered one of the greatest achievements of biomedical science and public health. However, during the last few decades an increasingly vocal anti-vaccination movement has challenged the safety and effectiveness of recommended vaccines. The extent of concern in the United States was highlighted by a national survey that found that although the majority of parents supported vaccination, 25% believed that too many vaccinations could weaken children’s immune systems and 23% believed that children get too many immunizations. Recent studies indicate that 66% of US adults (137 million) are now online and that 80% of all adults online use the Internet to look for health information. Furthermore, 52% of those who have visited online health sites believe that “almost all” or “most” of the health information they find online is credible. Individuals searching for vaccination information may find themselves visiting anti-vaccination sites. A study explored the content and design attributes of anti-vaccination sites that an individual might encounter doing a typical Web search, with the goal of enhancing our understanding of concerns raised on these sites.
Content and Design Attributes of Anti-vaccination Web Sites: A 2002 study:
The most commonly found content claims were that vaccines cause idiopathic illness (100% of sites), vaccines erode immunity (95%), adverse vaccine reactions are underreported (95%), and vaccination policy is motivated by profit (91%). The most common design attributes were the presence of links to other anti vaccination sites (100% of sites), information for legally avoiding immunizations (64%), and the use of emotionally charged stories of children who had allegedly been killed or harmed by vaccines (55%). In a nutshell, anti-vaccination Web sites express a range of concerns related to vaccine safety and varying levels of distrust in medicine. The sites rely heavily on emotional appeal to convey their message.
_
My website does not present one side of the story. Let me show you the other side of vaccines.
_
A 1992 study published in The American Journal of Epidemiology shows that children die at a rate 8 times greater than normal within three days after getting a DPT vaccination. A preliminary study by the Center for Disease Control (CDC) found children who received the HiB vaccine … were found to be 5 times more likely to contract the disease than children who had not received the vaccine. In the New England Journal of Medicine July 1994 issue a study found that over 80% of children under 5 years of age who had contracted whooping cough had been fully vaccinated. In 1977 Dr Jonas Salk (inventor of the Salk polio vaccine) testified with other scientists that 87% of the polio cases which occurred in the US since 1970 were the by-product of the polio vaccine. The Sabin oral polio vaccine (OPV) is the only known cause of polio in the U.S. today. The February 1981 issue of the Journal of the American Medical Association found that 90% of obstetricians and 66% of pediatricians refused to take the rubella vaccine. The pro-vaccination side is all that is offered in the media, schools, doctor’s offices, PHS, and all government publications. This is a biased one-sided view of vaccinations based much on manufacturer’s studies and writings. The other side is rarely discussed and adverse events after vaccination are dismissed as a one-in-a-million chance which is a necessary risk we all have to take. The truth is that the risks are far greater than they are telling us, and there are no mandatory vaccines. Extreme pressures are placed on parents for not signing permission and accepting all responsibility for the toxic vaccines. Yet, doctors cannot guarantee the safety of vaccines or that they will even work. Many vaccinations fail to achieve their intended level of immunity and many cause horrible complications (including death) which one will have to suffer for the rest of their life. The trade-off is not worth the risk. Mumps and measles are innocuous childhood diseases, but the vaccines have caused cancer, diabetes, brain damage, leukemia, autism, and even death (SIDS).
_
Were Vaccines really the ‘Savior’ against Past Diseases?
Conventional medicine teaches that the polio and the smallpox epidemics went away because of the vaccines, and that most of the diseases that we faced in the 20th century were brought down because of the power, strength and the implementation of the vaccine policy. Meanwhile, there are a significant number of studies in the medical literature that actually show there were many other reasons that these infectious diseases went away. For example, one article published in 2000 in the Pediatrics Journal describes how, before the World War II, the majority of the infectious diseases the US was faced with – such as diphtheria, tetanus, polio, pertussis, measles, influenza, parapertussis, tuberculosis and scarlet fever – were all reduced before World War II and before there were antibiotics and vaccinations available to treat or to vaccinate against these diseases. The reasons for the reductions in incidence rates and mortality of these diseases were predominantly due to the implementation of public health strategies, including:
•Clean water
•Better living conditions
•Improved sanitation
•Improved nutrition
Natural exposure to whatever diseases are lurking in the world is the only way for the body to develop permanent antibodies that will forever protect against disease. Eating fresh, nutrient-dense organic food and living a healthy lifestyle also helps boost your immune system, allowing you to overcome and develop resistance to diseases naturally.
_
Have the proper safety studies actually been done?
So, why is there such a vast difference among intelligent, scientifically oriented, committed and objective scientists and physicians about the safety and efficacy of vaccines? Dr. Palevsky says: “I think that if you ask most of my colleagues where they get their information, they will say that they read it from the American Academy of Pediatrics, from the AMA, from the CDC, and in their journals. But I would like to challenge most of my colleagues to look through the studies themselves to actually see if the proper scientific studies were done using a proper study group and a proper control group. Were the ingredients in vaccines properly studied? Is there a difference between being exposed to a virus, bacteria, heavy metal or toxin through the air, food, your intestines and your skin, versus when it’s injected into your body? Have we really looked at what happens to vaccine materials once injected into a child? Is an antibody sufficient to provide protection for a child against disease? More and more studies are coming out to show that:
•The proper studies haven’t been done
•Antibodies are not the final way in which your body is protected
•There is a difference between how children process material through air and food versus through injection
•There are particles in vaccines that do accumulate in your body and cause impairments in your immune system
•There are particles in the vaccines that get into your brain, and
•There are foreign DNA particles that get into your body
For many health professionals it is a shock to discover that there is such a lack of information on the safety and efficacy, and a mounting degree of information that actually raises suspicions about the safety and effectiveness of vaccines, and whether or not they have been properly studied. What we currently have is a one-sided policy; a one way of thinking that is impossible to really allow for the appropriate debate. Science is truly a field where you ask a question, you find an answer, and you don’t have the biases or the influences that change the way an answer or a conclusion is made. We are not seeing that with vaccines.
_
The Concept of Herd Immunity challenged:
The fact is that vaccination does not stop you from carrying bacteria or viruses in your nose, in your throat, in your intestines, in your airway, on your skin, or in your body. But many do not understand the significance of this fact, and have been made to believe that if you’re vaccinated, you won’t carry viruses, and therefore, others will be protected because you’re vaccinated. If, in fact, children are vaccinated, then why are parents and public health authorities afraid that non-vaccinated children are somehow carrying something that their children are not, when they should feel comfortable that their children are vaccinated? You can’t have it both ways. You can’t vaccinate believing that your children are protected and then feel that your children are not protected because somehow, some non-vaccinated child is carrying some secret organism that no one else is carrying. It just doesn’t make any sense.
_
Vaccinated vs. Unvaccinated: Survey Reveals Who’s Healthier:
In December 2010, a survey was initiated by VaccineInjury.info to compare the health of vaccinated children with unvaccinated children. To date, over 7,850 surveys have been submitted, and the study is ongoing. Though this is obviously not a double-blind controlled study, and depends on the individuals submitting the data to give accurate information, it is still revealing. So far, the results show:
| Health Condition | Prevalence in Vaccinated Children | Prevalence in Unvaccinated Children |
| Allergies | 40% report at least one allergy | Less than 10% |
| Asthma | 6% | 2.5% |
| Hay fever | 10.7% of German children | 2.5% |
| Neurodermatitis (an autoimmune disorder) | 13% of German children | 7% |
| ADHD | 8% of German children, and another nearly 6% with borderline cases | 1-2% |
| Middle ear infections | 11% of German children | Less than 0.5% |
| Sinusitis | Over 32% of German children | Less than 1% |
| Autism | Approximately 1 in 100 | Only 4 cases out of 7,800+ surveys (one child tested very high for metals, and another’s mother tested very high for mercury) |
Unvaccinated children are generally healthier. International studies looking at the health outcomes of unvaccinated children compared to their vaccinated peers have repeatedly shown that the unjabbed are generally less afflicted with allergies, autism, behavioral disorders, autoimmune dysfunction and respiratory ailments. Concerning the flu vaccine, for instance, a study published in the journal Clinical Infectious Diseases found that individuals jabbed for influenza are 550 percent more likely to have respiratory problems.
__
Alternative medicine and vaccination:
Many forms of alternative medicine are based on philosophies that oppose vaccination and have practitioners who voice their opposition. These include some elements of the chiropractic community, some homeopaths, and naturopaths. The reasons for this negative vaccination view are complicated and rest at least in part on the early philosophies that shape the foundation of these groups. Several surveys have shown that some practitioners of homeopathy, particularly homeopaths without any medical training, advise patients against vaccination. For example, a survey of registered homeopaths in Austria found that only 28% considered immunization an important preventive measure, and 83% of homeopaths surveyed in Sydney, Australia, did not recommend vaccination. Many practitioners of naturopathy also oppose vaccination. Homeopathic “vaccines” (nosodes) are ineffective because they do not contain any active ingredients and thus do not stimulate the immune system. They can be dangerous if they take the place of effective treatments.
_
Financial motives:
Critics have accused the vaccine industry of misrepresenting the safety and effectiveness of vaccines, covering up and suppressing information, and influencing health policy decisions for financial gain. Vaccines are highly profitable for drug companies, which aren’t held liable for damages. Conversely, many groups profit by promoting the controversiality of vaccines, such as lawyers who receive fees often totaling millions of dollars, expert witnesses paid to provide testimony and to speak at conferences, and practitioners of alternative medicine offering ineffective and expensive medications, supplements, and procedures such as chelation therapy and hyperbaric oxygen therapy. In the late 20th century, vaccines were a product with low profit margins, and the number of companies involved in vaccine manufacture declined. In addition to low profits and liability risks, manufacturers complained about low prices paid for vaccines by the CDC and other US government agencies. In the early 21st century, the vaccine market greatly improved with the approval of the vaccine Prevnar, along with a small number of other high-priced blockbuster vaccines, such as Gardasil and Pediarix, which each had sales revenues of over $1 billion in 2008.
__
Research frauds:
In 2010, two Merck virologists filed a federal lawsuit under the False Claims Act against their former employer, alleging the vaccine maker lied about the effectiveness of their mumps vaccine
Falsified mumps vaccine tests to fabricate a 95% efficacy rate:
According to Stephen Krahling and Joan Wlochowski, both former Merck virologists, the Merck company engaged in all the following behavior:
• Merck knowingly falsified its mumps vaccine test results to fabricate a “95% efficacy rate.”
• In order to do this, Merck spiked the blood test with animal antibodies to artificially inflate the appearance of immune system antibodies. Merck also added animal antibodies to blood samples to achieve more favorable test results, though it knew that the human immune system would never produce such antibodies, and that the antibodies created a laboratory testing scenario that did not in any way correspond to, correlate with, or represent real life … virus neutralization in vaccinated people.
• Merck then used the falsified trial results to swindle the U.S. government out of “hundreds of millions of dollars for a vaccine that does not provide adequate immunization.”
• Merck’s vaccine fraud has actually contributed to the continuation of mumps across America, causing more children to become infected with mumps. Yes, the vaccine spreads disease, they say.
• Merck used its false claims of “95 percent effectiveness” to monopolize the vaccine market and eliminate possible competitors.
• The Merck vaccine fraud has been going on since the late 1990’s, say the Merck virologists.
• Testing of Merck’s vaccine was never done against “real-world” mumps viruses in the wild. Instead, test results were simply falsified to achieve the desired outcome.
• This entire fraud took place “with the knowledge, authority and approval of Merck’s senior management.”
• Merck scientists “witnessed firsthand the improper testing and data falsification in which Merck engaged to artificially inflate the vaccine’s efficacy findings,” according to court documents.
_
AIDS vaccine research fraud:
In related vaccine news, Dong-Pyou Han, assistant professor of biomedical sciences at Iowa State University, recently resigned after faking AIDS vaccine test results. The researcher apparently added human blood that contained HIV antibodies to rabbit blood to skew the results. The human HIV antibodies in the rabbit blood made it appear as though the experimental AIDS vaccine was working and prompting the animals to build defenses against HIV. Not only were the results presented at scientific meetings over a period of several years, but the findings were instrumental in helping the research team gain $19 million in federal grants ($10 million of which was awarded after the fraudulent results were reported). It just goes to show you, again, that even scientific “truths” can be falsified, and even work from widely respected university researchers must be closely examined and supported before being accepted as fact…
_
Research fraud is the biggest argument against vaccines but in my view, it is not widespread. Occasional research fraud report does not mean that all vaccine scientists & researchers are fraudulent. Nonetheless, big pharma corporations do manipulate research to sell drugs and vaccines. The biggest manipulation is withholding data on adverse effects. Therefore, instead of drug companies, independent researchers should conduct clinical trials on safety and efficacy of vaccines.
____
There is, of course, well developed published data on the complication rates of vaccines and this data plays an essential role of the approval process vaccines must pass in order to be licensed for sale, and recommended for use. For vaccination recommendations by governments or advisory institutions (i.e. the World Health Organization) to change to an anti-vaccination stance, it must be fully demonstrated that the harms caused directly by the vaccine are greater than the harms caused by withholding the vaccine from circulation. This needs to be demonstrated at a population level, with solid and significant statistics. When faced with this, anti-vaccination activists often argue that the reporting system is not robust enough, resulting in these figures being misleading. The reality is that unexpected side-effects can occur, just as a parachute may fail to open, but the regulatory process ensures that such events are rare and within a risk boundary that means vaccination is statistically safer than non-vaccination. This particular pseudoscientific belief is unlikely to go away soon, despite the evidence attesting to the efficacy and safety of vaccines. It has several important factors that make it a very durable belief:
1. It involves harm to children.
2. It involves science that most people do not understand.
3. It involves using a painful method (a needle) to inject a foreign substance into our bodies. Some of them even have remnants of inactive viruses!
4. It is frightening.
5. It seems on its face to “make sense,” especially to make sense of an otherwise unexplainable tragedy.
_
The common argument by anti-vaxxer is “natural” immunity (i.e., immunity gained by infection with the disease) is better or lasts longer than immunity gained by a vaccine. This is not necessarily true. For example, natural immunity to pertussis, wears off after about 4-20 years and the vaccine-induced immunity wears off after 4-12 years. Therefore, even if an individual had pertussis as a child, they may still become infected as an adult, suffering the full effects and passing it on to others. Some anti-vaxxers claim there is antifreeze in vaccines. This is false. Antifreeze is ethylene glycol. Vaccines use polyethylene glycol. These are different substances, the latter of which is not toxic. As such, these generally progressive “anti-vaxxers,” for all their educated airs, bear a resemblance to religious conservatives who reject the theory of evolution. Both groups regard the scientific authorities as just another untrustworthy source — and find charlatans with degrees to contradict them.
_
Robert Kennedy Jr. warns of vaccine-linked ‘holocaust’:
Prominent vaccine skeptic Robert F. Kennedy Jr. arrived at the Sacramento screening of a film linking autism to the vaccine preservative thimerosal and warned that public health officials cannot be trusted. “They can put anything they want in that vaccine and they have no accountability for it,” said Kennedy, who walked onto and left a Crest Theater stage to standing ovations, of the federal Centers for Disease Control and Prevention. The overwhelming scientific consensus supports vaccine use and dismisses any serious side effects. Multiple studies have rejected any link between the mercury-containing chemical thimerosal and autism. Nevertheless, vaccine manufacturers have removed thimerosal from nearly all childhood vaccines (except multi-dose influenza vaccine) and California bill further barred thimerosal content. In light of these facts, I feel Kennedy’s continued activism disingenuous. I think it is dangerous that he is spreading misinformation about something that’s very important for public health. Autism rates have continued to rise even though we are not using thimerosal in vaccines for children. We still haven’t figured out exactly what causes autism. We do know it’s not vaccines.
_
Today, the spectrum of anti-vaccinationists ranges from people who are simply ignorant about science (or “innumerate” — unable to understand and incorporate concepts of risk and probability into science-grounded decision making) to a radical fringe element who use deliberate mistruths, intimidation, falsified data, and threats of violence in efforts to prevent the use of vaccines and to silence critics. Anti-vaccinationists tend toward complete mistrust of government and manufacturers, conspiratorial thinking, denialism, low cognitive complexity in thinking patterns, reasoning flaws, and a habit of substituting emotional anecdotes for data. Their efforts have had disruptive and costly effects, including damage to individual and community well-being from outbreaks of previously controlled diseases, withdrawal of vaccine manufacturers from the market, compromising of national security (in the case of anthrax and smallpox vaccines), and lost productivity.
_
In the face of such a legacy, what can we do to counter anti-vaccination campaigns?
1. First, we must continue to fund and publish high-quality studies to investigate concerns about vaccine safety.
2. Second, we must maintain, if not improve, monitoring programs, such as the Vaccine Adverse Events Reporting System (VAERS) and the Clinical Immunization Safety Assessment Network, to ensure coverage of real but rare adverse events that may be related to vaccination, and we should expand the VAERS to make compensation available to anyone, regardless of age, who is legitimately injured by a vaccine.
3. Third, we must teach health care professionals, parents, and patients how to counter anti-vaccinationists’ false and injurious claims. The scientific method must inform evidence-based decision making and a numerate society if good public policy decisions are to be made and the public health held safe. Syncretism between the scientific method and unorthodox medicine can be dangerous.
4. Fourth, we must enhance public education and public persuasion. Patients and parents are seeking to balance risks and benefits. This process must start with increasing scientific literacy at all levels of education. In addition, public–private partnerships of scientists and physicians could be developed to make accurate vaccine information accessible to the public in multiple languages, on a range of reading levels, and through various media. We must counter misinformation where it is transmitted and consider using legal remedies when appropriate.
_
Vaccines DO NOT contain Fetal Tissue:
Of the many lies told by anti-vaccination advocates, this is one of the worst, because it hits on a real moral issue. However, anyone with a modicum of training in biology will tell you that it is impossible for vaccines (or any other injected medicine) to contain human tissue. The reason is simple: if you are injected with anything containing tissue from another person, your body will immediately recognize it as an invader and begin attacking it. This immune response is often quite radical and can easily lead to death! This is why blood from a donor to a recipient must be carefully matched before the recipient can receive it. Thus, there is no human tissue of any kind in vaccines. For any lie to be successful there must be a grain of truth in it. This lie is no exception. There is a tangential connection between some vaccines and abortion. The hepatitis A vaccine, the rubella portion of the MMR vaccine, the chicken pox vaccine, and the shingles vaccine all contain viruses (weakened or inactivated) that were grown in human cells. A virus must be given a medium in which to propagate. Many vaccines use viruses that can propagate in several kinds of mammal cells, but some viruses are so specific that they can only propagate in human cells. The viruses used in the above-listed vaccines are that specific. Thus, they must be grown in human cells. Where do the vaccine companies get the cells for these vaccines? They get them from companies like Coriell Cell Repositories. This company has many cell lines, which are cultures of self-perpetuating cells. Each culture of cells is continually reproducing, making more cells. Those cells are sold to researchers, drug companies, and other medical technology firms. The specific cell lines used in vaccines are the MRC-5 and WI-38 cell lines, and they have been supplying medical research of all types for more than 50 years. Where do these cell lines come from? That’s where the grain of truth in this lie comes from. Both of these cell lines were cultured from cells taken from two abortions, one (MRC-5) that was performed in September,1966 and one (WI-38) that was performed in July, 1962.
_
The Anti-Immunization Activists: A pattern of deception, misrepresentation and misinterpretation:
The most sensational claims for vaccine ineffectiveness (for example, 29,972 smallpox deaths in Japan, all in vaccinated people) are referenced only to the writings of old anti-immunization activists. If they were true, you would be able to find something in a refereed medical textbook, or even a history book. Anti-vaxxers show a pattern of misrepresentation, misinterpretation and deception. Here are some examples:
1. Lancet 338: 715, 1991
The anti-vaxxer cites this article to claim that polio vaccine is ineffective. He says, “In 1989, the country of Oman experienced a widespread polio outbreak six months after achieving complete vaccination.”
This is clearly untrue. If you will examine the article, you’ll discover:
The epidemic actually began in January, 1988. Because of immunization, Oman had experienced a dramatic drop in its incidence of polio in the early 1980’s. However, there was only 88% coverage by 1987, just before the epidemic began. In October through December, 1988, the government undertook an aggressive immunization program, and the epidemic stopped ended in March, 1989. This is apparently where the anti-immunization activist got the six-months business. But there is nothing in the article to indicate that complete immunization was ever achieved before, during, or after the epidemic. The vaccine mostly did what it was supposed to do, protecting most of the children from paralysis. “A primary series of OPV (3 doses) reduced the risk of paralysis by 91% (adjusted estimate); two doses reduced the risk by 80%.”
2. NEJM 332: 500, 1995.
The anti-vaxxer cites “a very recent study in the New England Journal of Medicine which revealed that a substantial number of Romanian children were contracting polio from the vaccine.” The reference, however, is to the Washington Post. If the real reference had been given, it would have been easier for readers to find the author’s misrepresentation. It is known that following oral polio vaccine, there is a very low rate of paralytic polio. In Rumania, though, the risk of 1 in 196,000 was 5-17 times higher than everywhere else in the world. The authors found the obvious explanation: in Romania, it was customary to give intramuscular injections of antibiotics to children with fever. It has been known for generations that intramuscular injections during the prodromal phase of polio increase the risk of its becoming a paralytic disease.
3. AJDC 145: 1379, 1991.
The anti-vaxxer cites the article in support of his statement that outbreaks of Hib have occurred despite immunization. Again, examining the actual article shows how the activist is trying to trick you. Before the Hib vaccine, the annual incidence of Hib disease (meningitis, cellulitis, septicemia) among children under age 2 was 100 per 100,000. Following the introduction of the Hib vaccine, failures were reportable, and reporting was strongly encouraged. The Hib vaccine was improved in Dec. 1987 (PRP-D), and this study actually demonstrates that effectiveness was increased by 2/3. In fact, the government could find only 26 failures in the whole country.
4. Br. Med. J. 283: 696, 1981
The anti-vaxxer cites this study of whooping cough and adults and states, “England actually saw a drop in pertussis deaths when vaccination rates dropped from 80% to 30% in the mid 70’s. Swedish epidemiologist B. Trollfors’ study [this one] of pertussis vaccine efficacy and toxicity around the world found that ‘pertussis-associated mortality is currently very low in industrialized countries and no difference can be discerned when countries with high, low, and zero immunization rates are compared.’ He also found that England, Wales, and West Germany had more pertussis fatalities in 1970 when the immunization rate was high than during the last half of 1980, when rates had fallen.” Once again, examining the actual article shows that it has been misrepresented. The cause of the increase in whooping cough in the early 1970’s in Sweden was faulty production of the vaccine. A pertussis vaccine giving 90% immunity was introduced in Sweden during the late 1940s. From the early 1960s about 90% of all infants were vaccinated and pertussis became rare. In the first years of the 1970’s whooping cough returned, and since 1974 the disease has been endemic. The return of the disease seems to have been related to changes in production of the vaccine at the beginning of the decade. The reason there was a tremendous amount of whooping cough in Sweden during the 1970’s was that adults’ immunization had worn off and they were catching it from unimmunized children. The statement that England saw a drop in pertussis deaths after immunization rates dropped doesn’t appear in the article either. If it means “total deaths”, then it’s very surprising; and if it were true, the author would have a genuine scientific reference. If it means “percentage of pertussis patients that died”, it’s probably true. When immunization rates are high, the disease occurs primarily among very young babies, who have not been immunized. Very young babies are more likely to die. Thanks to the anti-immunization campaigns, the disease became much more common, affecting lots of older people who were more likely to survive and take the disease home to the babies.
_
Does natural HPV infection prevent cancer?
A study published in the Journal of Infectious Diseases has revealed that HPV infection, resulting in naturally acquired human papilloma virus (HPV) antibodies, reduces the risk for new infection and cervical abnormalities linked to cancer in non-HPV vaccinated subjects. In addition to this startling finding, another study revealed that the HPV vaccine may not protect women against high-grade squamous intraepithelial lesions, dysplasias. If, in fact, the HPV vaccines do not work as widely advertised, and natural HPV infectious exposures actually protect against the progression of HPV linked cervical changes to cancer, then taken together, both these findings challenge the most fundamental assumptions within vaccine science, and render highly dubious the oft repeated rhetoric that natural HPV infection is juggernaut –like deadly force the best defense against which are universal immunization campaigns. The commonly held notion that naturally transmitted HPV infection and subsequent elevation of antibody titers is a disease process that leads inevitably to tissue pathology and possibly precancer or cancer, rather than an instance of the immune system effectively meeting the HPV viral challenging and responding with an appropriate antibody response, conferring lasting immunity, is debunked by this new study. Clearly, natural infection not only prevents reinfection, but even reduces the risk of HPV’s potential induction of dysplastic cellular changes associated with cancer. My gut feeling says that the study may be flawed. Viral infections are known to cause cancer and not prevent cancer. Viruses invade us to survive, multiply and propagate at our cost as they are obligate intracellular parasites. To say that viral infection prevents cancer is to say that the Sun rises in West. I would check my eyes rather than the Sun.
_______
Individual liberty and compulsory vaccination:
To eliminate the risk of disease outbreaks, at various times governments and other institutions established policies requiring vaccination. For example, an 1853 law required universal vaccination against smallpox in England and Wales, with fines levied on people who did not comply. Unlike children in Canada and the European Union, American children must get dozens of doses of vaccines or they can’t get a public school education. Government and medical trade officials have narrowed medical contraindications to vaccination after Congress shielded doctors and vaccine manufacturers from vaccine injury lawsuits. In the United States, the Supreme Court ruled in Jacobson v. Massachusetts (1905) that states could compel vaccination for the common good. Contemporary U.S. policies usually require children receive vaccinations before entering school, although many states allow for religious and personal exemptions due to philosophical or health reasons. A few other countries also follow this practice. Compulsory vaccination greatly reduces infection rates for associated diseases. Beginning with nineteenth century early vaccination, these policies stirred resistance from a variety of groups, collectively called anti-vaccinationists, who objected on ethical, political, medical safety, religious, and other grounds. Common objections included claims of “excessive government intervention in personal matters” or that proposed vaccinations were not sufficiently safe. Many modern vaccination policies allow exemptions for people with compromised immune systems, allergies to vaccination components, or strongly held objections. Compulsory vaccination policies have provoked opposition at various times from people who say that governments should not infringe on an individual’s freedom to choose medications, even if that choice increases the risk of disease to themselves and others. If a vaccination program successfully reduces the disease threat, it may reduce the perceived risk of disease enough that an individual’s optimal strategy is to refuse vaccination at coverage levels below those optimal for the community. Exempting some people from mandatory vaccination results in a free-rider problem, in which a few individuals gain the advantage of herd immunity without paying the cost; decreased rates of vaccinations such as through exemptions may cause loss of herd immunity, substantially increasing risks even to certain vaccinated individuals.
_
Ethical issues pertaining to public health, and specifically immunization activities, are important in the implementation of and the public’s response to mandatory vaccination programs. Often, some ethical principles are in conflict with others, or at the very least, are required to be given more weight than others, when mandatory vaccination campaigns are implemented. Efforts to minimize conflicts among the relevant ethical principles are important because such conflicts can feed anti-vaccination movements. Vaccinations are one of public health’s greatest achievements. However, an ethical dilemma lies in the balance of personal autonomy and choice versus protection of the entire at risk population. Vaccines have become readily available in most parts of the world, yet debates continue as to the appropriateness of requirements for vaccinations, including legal mandates of vaccinations during public health emergencies and more routinely for school entry. In countries where freedom and personal liberty are valued above all else, a more subtle approach may be needed; for example, by limiting exemptions to vaccination. In California, all that is required is a signature on a form printed from the internet to obtain a personal belief exemption. These loopholes allow parents to refuse vaccines on unscientific and unproven grounds. Instead, pediatricians should do more to counsel parents about the safety and efficacy of vaccines, and more education should be provided to school age children. Hopefully, such educational plans can raise vaccine rates without imposing punishments. If parents still refuse vaccination, they should be held accountable. Perhaps they should pay a fine, similar to what Massachusetts instituted in the 1800s smallpox vaccination campaign. At the very least, unvaccinated children must be kept out of school in the event of an outbreak. Religious exemptions should only be offered to parents who demonstrate membership of a religion that genuinely opposes vaccination, of which there are few. The reality is that many parents use religious exemptions to justify vaccine refusal, even if their religion says nothing about vaccines, or even supports vaccination. Yet, legislators in the United States would likely avoid association with anything viewed as impinging on religious freedom. It is highly likely that religious exemptions will remain, thus governments must do more to strictly enforce their legitimacy.
_
Keeping the balance:
If a country does decide to enact and enforce mandatory vaccination, it will be important to determine which vaccines will be required. Although some vaccines should be mandated on the grounds that the diseases they protect against are highly contagious or extremely debilitating or deadly, the decision is less straightforward for other vaccines. For example, hepatitis B requires direct exposure to blood or open sores and therefore is relatively not contagious for children. Yet if it is contracted, it can cause severe disease.
Where should a government draw the line?
Should citizens be required to be vaccinated for every disease for which scientists develop a vaccine?
Probably not.
Democracies are built on freedom and we should have the freedom to make choices about our healthcare. But we cannot risk making choices that endanger the lives of others. Governments must step in and tighten up rules on vaccination to protect our children and our future. The World Health Organization (WHO) has no official policy on mandatory vaccinations, Alison Brunier, communications officer for Immunizations, Vaccines and Biologicals at the WHO says. “While it is preferable that high community demand and acceptance make compulsory vaccination programs unnecessary, WHO understands that some countries may wish to move in that direction when faced with declining vaccination rates and outbreaks of disease.” But WHO is “very interested in learning from the experience of countries who introduce compulsory vaccination in order to better understand the impact on immunization coverage and the strengths and weaknesses of such approaches,” she adds.
_
Individual versus group goals:
Rational individuals will attempt to minimize the risk of illness, and will seek vaccination for themselves or their children if they perceive a high threat of disease and a low risk to vaccination. However, if a vaccination program successfully reduces the disease threat, it may reduce the perceived risk of disease enough so that an individual’s optimal strategy is to encourage everyone but their family to be vaccinated, or (more generally) to refuse vaccination at coverage levels below those optimal for the community. For example, a 2003 study found that a bioterrorist attack using smallpox would result in conditions where voluntary vaccination would be unlikely to reach the optimum level for the U.S. as a whole, and a 2007 study found that severe influenza epidemics cannot be prevented by voluntary vaccination without offering certain incentives. Governments often allow exemptions to mandatory vaccination for religious or philosophical reasons, but decreased rates of vaccination may cause loss of herd immunity, substantially increasing risks even to vaccinated individuals.
_
No harm principle:
Diekema and Marcuse have put forth a more direct approach for evaluating and resolving ethical issues around mandatory vaccination programs. Their approach is based upon the often cited medical maxim commonly translated as: “first, do no harm.” When applied to vaccination activities, this maxim has the following implications: the vaccination should be of benefit to the subject being vaccinated; care should be taken to prevent any harm that might accrue from the vaccination; compared to other procedures for addressing the same issue, the vaccination should be the best opportunity for successfully preventing disease as compared to the risk for harm; and if harm does result from the vaccination, the benefit of vaccination to the subject should at least compensate for the harm incurred. Because vaccination provides not only a direct benefit (immunity to disease) to the person being vaccinated but also provides a benefit to others in the community via herd immunity, Diekema and Marcuse remind us that unvaccinated persons can be viewed as “harming” the community. It, therefore, follows that for serious and highly communicable diseases, there is a role for compulsory vaccination programs. Diekema and Marcuse cite the utilitarian philosopher John Stuart Mill who held that: “The only purpose for which power can rightfully be exercised over any member of a civilized community, against his will, is to prevent harm to others. His own good, either physical or moral, is not sufficient warrant.” This principle, known as the “harm principle,” can be used to expand the application of the maxim: “first, do no harm” to the community interests that result from vaccination programs. It, therefore, follows that the “harm principle” can be used to justify compulsory vaccination programs in specific instances where the community interests or benefits are deemed to be significant. Often, the issue is in determining what is considered significant.
_
Why keep high vaccination rates:
First, not everyone is able to be immunized, due to a variety of medical reasons (e.g., egg allergies, age, etc.). Second, vaccines are not 100% effective, though most are very close. This means that in order to prevent an outbreak, a high number of individuals needs to be immunized so that a virus or bacteria does not have enough potential hosts to sustain itself. There is a small possibility that even with vaccination, you will not gain immunity. Finally, there are some individuals (the elderly, AIDS patients, transplant recipients, some cancer patients, etc.) for whom vaccines just will not work or not work as well, because their immune system does not, or cannot, mount a full response to it. These individuals are also unlikely to gain immunity from infection, either. For all of these reasons, it is very important to keep vaccination rates up, so that those who do not or cannot benefit from vaccines are protected by herd immunity.
________
________
Consequences of vaccine refusal:
Since the mid-1990s, marked progress has been made to increase overall vaccination rates in the United States. Despite this success, pockets of low vaccination coverage persist, especially among groups of religious or philosophic objectors to vaccination. Historically, these groups have experienced periodic outbreaks of vaccine-preventable diseases, e.g., measles, pertussis, and varicella. In recent years, concerns about the safety of vaccines have been highlighted by the news media and may have influenced additional parents to refuse vaccination for their children. Recent studies suggest that a major factor contributing to this decision is the sense that the vaccines are more dangerous than the diseases against which they protect. Other factors include the beliefs that the diseases are rare because of herd immunity and that parents can protect their children from contracting the disease and from experiencing disease-related complications.
_
Unvaccinated children have a much greater chance of getting disease than children who have received the vaccine. Two recent studies of disease outbreaks in the U.S. illustrate this concern. Children whose parents chose not to have them immunized against measles were 22 to 35 times more likely to get measles than were immunized children. Children who did not receive the vaccine for pertussis (whooping cough) were almost 6 times more likely to get whooping cough than immunized children; the risks were even higher for the younger children (children < 11 years old), who were 62 times more likely to get measles if they were not immunized and 16 times more likely to get pertussis in these outbreaks. Unimmunized children also add to the risk for children who cannot receive vaccinations or for whom the vaccine did not provide full protection from disease. People who are not immunized can be carriers of disease and pose a risk to those around them, even if they do not get sick themselves.
_
Why should we keep vaccinating against diseases that we will probably never see?”
Vaccines don’t just protect yourself:
Most vaccine-preventable diseases are spread from person to person. If one person in a community gets an infectious disease, he can spread it to others who are not immune. But a person who is immune to a disease because she has been vaccinated can’t get that disease and can’t spread it to others. The more people who are vaccinated, the fewer opportunities a disease has to spread (herd immunity). If only some get vaccinated, the virus spreads. If most get vaccinated, spreading is contained. If one or two cases of disease are introduced into a community where most people are not vaccinated, outbreaks will occur. In 2013, for example, several measles outbreaks occurred around the country, including large outbreaks in New York City and Texas – mainly among groups with low vaccination rates. If vaccination rates dropped to low levels nationally, diseases could become as common as they were before vaccines.
Diseases haven’t disappeared:
The United States has very low rates of vaccine-preventable diseases, but this isn’t true everywhere in the world. Only one disease — smallpox — has been totally erased from the planet. Polio no longer occurs in the U.S., but it is still paralyzing children in several African/Asian countries. More than 350,000 cases of measles were reported from around the world in 2011, with outbreaks in the Pacific, Asia, Africa, and Europe. In that same year, 90% of measles cases in the U.S. were associated with cases imported from another country. Only the fact that most Americans are vaccinated against measles prevented these clusters of cases from becoming epidemics. Disease rates are low in the United States today. But if Americans let themselves become vulnerable by not vaccinating, a case that could touch off an outbreak of some disease that is currently under control is just a plane ride away.
_
What would happen if we stopped Vaccinations?
We could soon find ourselves battling epidemics of diseases we thought we had conquered decades ago.
We know that a disease that is apparently under control can suddenly return, because we have seen it happen, in countries like Japan, Australia, and Sweden. Here is an example from Japan. In 1974, about 80% of Japanese children were getting pertussis (whooping cough) vaccine. That year there were only 393 cases of whooping cough in the entire country, and not a single pertussis-related death. Then immunization rates began to drop, until only about 10% of children were being vaccinated. In 1979, more than 13,000 people got whooping cough and 41 died. When routine vaccination was resumed, the disease numbers dropped again. The chances of your child getting a case of measles or chickenpox or whooping cough might be quite low today. But vaccinations are not just for protecting ourselves, and are not just for today. They also protect the people around us (some of whom may be unable to get certain vaccines, or might have failed to respond to a vaccine, or might be susceptible for other reasons). And they also protect our children’s children and their children by keeping diseases that we have almost defeated from making a comeback.
_
Diseases that have been all but eradicated in the past decades are suddenly coming back. Measles, mumps and whooping cough are completely preventable in the U.S., or at least they are supposed to be. But as an increasing number of parents decide not to vaccinate their children, the “herd immunity” is lost which needs the majority of a community to be vaccinated in order to eliminate possible chain infections. This is no longer an issue of “I’ll worry about my kids, you worry about yours” — not vaccinating your children can have seriously dangerous implications for the entire population. We’re not talking about hypothetical situations, but real cases. The map below charts the resurgence of diseases that were essentially eradicated in the U.S.:
_
Here are three of the diseases that have come back in the US due to the anti-vaccine movement:
1. Measles:
After the measles vaccine was introduced in 1963, incidences of the diseases dropped by 99%. Since then, there have been an average of 60 measles cases per year in the U.S., but that number increased to 222 cases in 2011. Outbreaks have been reported in New York, Boston, Philadelphia, San Francisco and Los Angeles.
2. Mumps:
An ongoing outbreak at Ohio State University has resulted in 23 confirmed cases, and there was a similar outbreak at New York’s Fordham University, with over a dozen students affected. Before the vaccine was introduced in 1967, there were 300,000 cases of mumps per year. Although that number sharply declined in later years to 200 incidences per year, in 2006 alone, there were 6,584 cases of mumps. Another outbreak in 2009 led to 3,400 cases.
3. Whooping cough:
At the height of the epidemic, whooping cough afflicted more than 250,000 children every year and killed 9,000. Luckily, a very effective vaccine was introduced in the ’40s; there were just 1,000 new cases annually by 1976. But in 2012, that number increased exponentially again to 17,000 cases, resulting in 10 children’s deaths.
_
Science loses ground to pseudo-science because the latter seems to offer more comfort. That may not sound like much, but a recent study by the Los Angeles Times indicates that the impact can be devastating. The Times found that even though only about 2 percent of California’s kindergartners are unvaccinated (10,000 kids, or about twice the number as in 1997), they tend to be clustered, disproportionately increasing the risk of an outbreak of such largely eradicated diseases as measles, mumps, and pertussis (whooping cough). The clustering means almost 10 percent of elementary schools statewide may already be at risk. The New England Journal of Medicine laid the blame for clusters of disease outbreaks throughout the US squarely at the feet of declining vaccination rates, while nonprofit health care provider Kaiser Permanente reported that unvaccinated children were 23 times more likely to get pertussis, a highly contagious bacterial disease that causes violent coughing and is potentially lethal to infants. In the issue of the journal Pediatrics, Jason Glanz, an epidemiologist at Kaiser’s Institute for Health Research, revealed that the number of reported pertussis cases jumped from 1,000 in 1976 to 26,000 in 2004. A disease that vaccines made rare, in other words, is making a comeback. “This study helps dispel one of the commonly held beliefs among vaccine-refusing parents: that their children are not at risk for vaccine-preventable diseases,” Glanz says.
_
Experience from other countries shows that diseases quickly return when fewer people are immunized:
•Ireland saw measles soar to more than 1,200 cases in the year 2000, as compared with just 148 the previous year, because immunization rates fell to around 76%. Several children died in this outbreak.
•A large outbreak of rubella (German measles) occurred in Nebraska in 1999. All 83 cases in this outbreak involved adults who had not been immunized. Most of them came from countries where rubella immunization is not routine. The outbreak spread from a meat-packing plant to the general community, including several pregnant women and two day care centers. The greatest danger from rubella is to infants, who may be born with congenital rubella syndrome if their mothers are infected during pregnancy.
•In 1994, there were 5,000 deaths due to diphtheria in Russia after the organized immunization system was suspended. Previously, Russia (like Canada) had had only a few cases of diphtheria each year and no deaths. Diphtheria toxoid came into routine use in the 1930s, but even today diphtheria remains a severe disease. About one person in 10 with diphtheria still dies in spite of medical treatment.
•In the U.K., a major drop in rates of immunization against pertussis (whooping cough) in 1974 was followed by an epidemic of more than 100,000 cases and 36 deaths by 1978.
• Japan had 13,000 cases and 41 deaths from whooping cough in 1979, after only 30% of children received pertussis immunization. In earlier years, when most children received vaccine, Japan had only a few hundred cases of whooping cough and no deaths.
• Sweden had a similar experience with pertussis. When vaccination programs restarted, the number of cases fell once again.
_
Enforcement of mandatory immunization requirements for children entering childcare facilities and schools has resulted in high immunization coverage levels in the U.S. While all states and the District of Columbia allow exemptions from the requirements for medical reasons, and all but two offer exemptions to accommodate religious beliefs, 19 states allow exemptions based on parents’ personal beliefs. Several recent outbreaks of measles, pertussis, and varicella (chickenpox) have been traced to pockets of unvaccinated children in states that allow personal belief exemptions.
_
Objection to Vaccination as a risk for Tetanus among children younger than 15 Years: A study:
A study found that the majority of recent cases of tetanus among children in the United States were in unvaccinated children whose parents objected to vaccination. Parents who choose not to vaccinate their children should be advised of the seriousness of the disease and be informed that tetanus is not preventable by means other than vaccination. Tetanus is unique among vaccine-preventable diseases in that it is not contagious and the causative agent, Clostridium tetani is ubiquitous in the environment. Herd immunity plays no part in protecting individuals or the community. Tetanus toxoid-containing vaccines have demonstrated high effectiveness. Thus, most cases of tetanus are in people who are unvaccinated, are partially vaccinated, or have waning immunity.
______
______
Vaccine and society:
Complication risk: disease vs. vaccination:
Complications are more likely to arise from illness than from vaccination. Children who get measles have a 1 in 20 chance of developing a serious complication; however, serious complications from the vaccine number 1 or 2 per million, according to the Institute for Vaccine Safety at Johns Hopkins University. Before the introduction of measles vaccination, there were about half a million cases per year in the United States, while only 89 cases were diagnosed in 1998. Historically, the mortality rate for measles in the U.S. was about 1 to 3 deaths per every 1000 cases, with young children suffering the highest mortality rates. Most deaths occur as a result of pneumonia or encephalitis. After the introduction of polio vaccination, cases in the United States decreased from almost 30,000 in 1955 — many of which led to paralysis or death — to 910 cases by 1962. New cases of polio in the U.S. are now a thing of the past. Of the two types of polio vaccine, the oral vaccine, while effective, is the less safe of the two. As there are no more naturally acquired cases in the U.S., the oral vaccine had become the only cause of polio (8-9 cases). Since the vaccine risk, however small, eventually exceeded the disease risk, the oral vaccine was abandoned in favor of the killed vaccine. In regions where polio is still a major problem, the oral vaccine is still a better choice, as it can enter the water supply and vaccinate others passively. In regions with high HIV rates, this may be less true, as live vaccines are usually avoided in patients with compromised immune systems. According to Willem van Panhuis et. al. 2013, “a total of 103.1 million cases of these contagious diseases have been prevented since 1924 on the basis of median weekly prevaccine incidence rates.” In Britain, there was concern in the early 70s about the pertussis vaccine, where it was blamed for several cases of encephalitis. Despite the connection never being proven outright, vaccination rates still dropped from 77% to 39%. Following this drop in immunization, the UK was hit by two large whooping cough epidemics (one in 1978, and another in 1982), both of which resulted in many deaths. Unfortunately, these sorts of consequences from vaccine denial aren’t confined to history; whooping cough also hit the American northwest, generating one of the worst outbreaks in 70 years, with a 1300% increase in cases in 2012 entirely blamed on recent hysteria over vaccines. The rates of complication from vaccines are so low that the benefit of vaccines for each individual child is higher than the risk of a poor outcome, so it is not true that the few children with adverse events are being sacrificed for the health of others.
_
The table below compares the risks of death due to three vaccine-preventable diseases and the risks of adverse events following immunization with the approved vaccines.
_
Risks of illnesses and risks associated to the corresponding vaccines:
| Measles | Death:
|
| Diphtheria | Death: 1 in 20 cases. |
| Tetanus | Death: 25 – 70 in 100 cases overall. (10 – 20 in 100 cases with good intensive care management.) |
| Measles vaccine | Encephalitis or severe allergic reaction: 1 in 1,000,000 cases. |
| DTP vaccine | Continuous crying, then full recovery: 1 in 1000 cases. |
| Tetanus toxoid vaccine |
|
_
Impact of rumours & scares:
The history of immunization is not only characterized by its unique success at achieving huge reductions in mortality (deaths) and morbidity (illness and disability) from vaccine-preventable infections and the global eradication of smallpox. It is also notable for the emergence of vaccine skeptics who firmly believe that vaccines are harmful and lobby against them. This – often very vocal – opposition has been a persistent challenge to immunization programs since they first began over two centuries ago.
Example 1: Whole-cell pertussis “scare”:
Many recent immunization programs have suffered setbacks from immunization scares. Children have been needlessly put into danger by frightened parents that refused immunization for their children after “scare stories” about particular vaccines. The rumours about the pertussis whole-cell vaccine from about 1960 onwards in four different locations affected the vaccine coverage entailing a rise in the incidence of pertussis. These examples also show how negative beliefs about a particular vaccine can spread around the world and reduce public confidence in its safety.
Example 2: MMR and autism controversy in the UK:
In 2008, 14 years after the local transmission of measles was halted in the UK, the Health Protection Agency for England and Wales declared it had once again become endemic, i.e. continuously circulating in the population. This was seen as a result of almost a decade of low MMR vaccination coverage across the UK. Burgess, Burgess and Leask (2006) analysed how a report of a hypothesized link between measles-mumps-rubella vaccination and autism in 1998 became a major public health issue in the United Kingdom, leaving most experts surprised by its overwhelming influence on public opinion about MMR vaccination. Effectively communicating with parents of autistic children and members of the general public who believed that the truth about the vaccine was being concealed would have been critical to avoid the reduction of vaccination coverage.
_
Health-damaging outcomes of negative rumours are not confined to high-income countries. There are many other cases from all over the world. For example, in 2009, the death of a 7-year-old child in Taiwan, following his vaccination against the H1N1 strain of influenza virus, led to rumours that the vaccine was responsible. These rumours were followed by a 30% drop in the number of children receiving it. The main reason for less tolerance towards vaccines, making them more likely to be the subject of negative rumours and “scare stories” than is the case for medical drugs is public tolerance towards adverse reactions is lower compared to side-effects of drugs as vaccines are given to healthy people.
_
Public perception vs. expert perception:
Health experts do not view the risks associated with a medical procedure (such as vaccination) in the same way as members of the public (parents, patients and vaccinees). Experts understand risks in terms of numerical values and rates. In contrast to the perception of experts, parents, guardians and vaccinees rather want to know whether they or their child could be the “one in a million” who develops encephalitis following immunization with measles vaccine.
_
Other factors that may influence the way public tends to see risk, include:
Negligence of the danger of the disease:
Most adults in high-income countries with high vaccination coverage have never seen a case of measles or any of the other vaccine-preventable childhood diseases. As a consequence, they may underestimate the probability of harm if the disease does develop.
Influence by individual context:
The public is likely to perceive risk in broad religious, social or personal contexts. For example, some will distrust the medical system due to a personal prejudice against “experts” and a desire not to be influenced by them; others will uncritically accept all instructions from health workers because they feel intimidated or inferior.
Aversion to medicine:
Adverse personal experiences from the past (e.g. the memory of a painful injection or a sore/swollen arm) may also negatively influence attitudes to vaccine-associated risk. The thought of being injected with a foreign substance derived from disease-causing organisms can induce fear and dread. Clients may feel reluctant to come to a clinic or other health facility, or to bring their children if the environment feels intimidating and the health workers are not reassuring or welcoming.
For all these reasons, it is important to understand the concerns of your target audience and the different approaches required to communicate effectively with persons planning to receive a vaccine, the public and your expert colleagues.
_
Public complacency due to low prevalence of vaccine preventable diseases:
While the measles vaccine protects virtually everyone who is inoculated, not all vaccines have the same rate of success. But even if a vaccine is effective for only 70, 80 or 90 percent of those who take it, the other 30, 20 or 10 percent who don’t get the full benefit of the vaccine are usually still not at risk. That’s because most of the people around the partially protected are immune, so the disease can’t sustain transmission long enough to spread. But when people decide to forgo vaccination, they threaten the entire system. They increase their own risk and the risk of those in the community, including babies too young to be vaccinated and people with immune systems impaired by disease or chemotherapy. They are also free-riding on the willingness of others to get vaccinated, which makes a decision to avoid vaccines out of fear or personal belief a lot safer. Of course it is the very success of modern vaccines that makes this complacency possible. In previous generations, when epidemic disease swept through schools and neighborhoods, it was easy to persuade parents that the small risks associated with vaccination were worth it. When those epidemics stopped–because of widespread vaccinations–it became easy to forget that we still live in a dangerous world.
_
Anti-vaccine campaigners: please see the impact of vaccines:
The graphs below shows that the number of infected people, measured over 70-some years and across all 50 states in the United States, generally declined after vaccines were introduced.
The maps below show number of cases per 100,000 people.
_
Measles:
_
Hepatitis A:
_
Mumps:
_
Pertussis (whooping cough):
The vaccine was introduced in 1914.
_
Polio:
_
Rubella:
_
Small pox:
The vaccine for smallpox was introduced in 1800.
_
Vaccines have almost totally eliminated 14 Infectious Diseases in the U.S. as seen in the graph below:
In the two centuries since vaccines were first developed, over a dozen of what used to be the most common infectious diseases have practically been eradicated. “Morbidity” in figure above refers to the number of people getting sick from, but not necessarily dying of, the diseases.
_
Overwhelming evidence shows benefits and safety of routine childhood vaccination. Many parents, however, worry about the risks from some vaccines. Although this concern is mistaken, these are genuine worries and should be treated seriously and sympathetically. Health professionals have a responsibility to provide parents with accurate information on which to base their decision.
_
Vaccines have been purportedly blamed for causing autism but in fact now we will have a vaccine against autism:
Vaccine against autism: A 2013 study:
The groundbreaking study by Brittany Pequegnat and Guelph chemistry professor Mario Monteiro appeared in the journal Vaccine. They developed a carbohydrate-based vaccine against the gut bug Clostridium bolteae. C. bolteae is known to play a role in gastrointestinal disorders, and it often shows up in higher numbers in the GI tracts of autistic children than in those of healthy kids. More than 90 per cent of children with autism spectrum disorders suffer from chronic, severe gastrointestinal symptoms. Of those, about 75 per cent suffer from diarrhea, according to current literature. “Little is known about the factors that predispose autistic children to C. bolteae,” said Monteiro. Although most infections are handled by some antibiotics, he said, a vaccine would improve current treatment. “This is the first vaccine designed to control constipation and diarrhea caused by C. bolteae and perhaps control autism-related symptoms associated with this microbe,” he said. The new anti- C. bolteae vaccine targets the specific complex polysaccharides, or carbohydrates, on the surface of the bug. The vaccine effectively raised C. bolteae-specific antibodies in rabbits. Doctors could also use the vaccine-induced antibodies to quickly detect the bug in a clinical setting, said Monteiro. The vaccine might take more than 10 years to work through preclinical and human trials, and it may take even longer before a drug is ready for market, Monteiro said. “But this is a significant first step in the design of a multivalent vaccine against several autism-related gut bacteria,” he said. Monteiro has studied sugar-based vaccines for two other gastric pathogens: Campylobacter jejuni, which causes travelers’ diarrhea; and Clostridium difficile, which causes antibiotic-associated diarrhea. The research was supported by the Natural Sciences and Engineering Research Council.
___________
Vaccine and economy:
One challenge in vaccine development is economic: Many of the diseases most demanding a vaccine, including HIV, malaria and tuberculosis, exist principally in poor countries. Pharmaceutical firms and biotechnology companies have little incentive to develop vaccines for these diseases, because there is little revenue potential. Even in more affluent countries, financial returns are usually minimal and the financial and other risks are great. Most vaccine development to date has relied on “push” funding by government, universities and non-profit organizations. Many vaccines have been highly cost effective and beneficial for public health. The number of vaccines actually administered has risen dramatically in recent decades. This increase, particularly in the number of different vaccines administered to children before entry into schools may be due to government mandates and support, rather than economic incentive.
_
Cost benefit of vaccines:
Vaccine preventable diseases result in significant costs to individuals, the health care system, and society, including costs associated with visits to health care providers, hospitalizations, and premature deaths. Parents may lose time from work to care for sick children and sick children lose time at school. For example, the societal cost for each case of rotavirus requiring a visit to the emergency room is estimated to be $675. The cost-benefit of vaccine is strongly influenced by the price of the vaccines used. Many vaccines, such as measles-mumps-rubella vaccine for children, provide both health benefits and savings in health care costs as seen in the table below. This means that the cost of implementing the immunization program is less than the cost of treating the illness or injury that would occur if the program had not been implemented. Because immunization with these vaccines improves health and results in cost savings, the decision to include these vaccines in publicly funded immunization programs is straightforward. In developing public health programs, international organizations such as the World Health Organization, United Nations Children’s Fund and the World Bank recommend that immunization be given high priority because of its high cost-effectiveness.
Cost savings achieved through selected immunization programs:
| Immunization program | Cost saving per $1 spent |
| Influenza for adults 65 years of age and older | $45 |
| Measles, mumps, rubella for children | $16 |
| Pneumococcal polysaccharide for adults 65 years of age and older | $8 |
| Diphtheria, pertussis, tetanus for children | $6 |
_
Vaccines provide high public-health bang for the buck. The CDC researchers weighed the benefits of the vaccinations (“savings in direct and indirect costs that accrued from averting illnesses, hospitalizations, and deaths”) against costs (“program costs included vaccine, administration, vaccine adverse events, and parent travel and work time lost”). In 2009 alone, the researchers determined, each $1 spent on vaccines and their administration yielded $10 in benefits to society. And the vaccinations from 1994-2013, the researchers found, will save society a net $1.38 trillion, both directly (by reducing health expenses) and indirectly (via the economic activity that is saved from avoided illnesses). That’s almost 10 percent of the U.S. economy’s gross domestic product. The United States saves about $27 per $1 invested in DTaP vaccination, and $13 per $1 spent on MMR vaccination. UNICEF estimates that $6.2 billion could be saved in treatment costs if vaccines were more prominent in the world’s poorest countries. According to the International Vaccines Access Center at Johns Hopkins Bloomberg School of Public Health, $62.9 billion could be saved by providing Hib, pneumococcal, and rotavirus vaccinations to the 73 poorest countries: $1.4 billion in treatment costs, $300 million in lost caretaker wages, $6.2 billion in lifetime productivity loss due to disability, and $55 billion in lifetime productivity loss because of death. The cost-effectiveness of immunisation has made vaccines increasingly affordable for low-income countries.
_
Newer vaccines tend to be costlier and may not be cost-saving, so the decision to introduce them into publicly funded immunization programs is determined by society’s willingness to pay for their anticipated health benefits. In general, such programs compare very favourably to other public health interventions in terms of cost per life year saved as seen in the table below.
Cost per life year saved for selected immunization programs and other public health interventions
| Public health intervention | Cost per life year saved# |
| Vaccines | |
| Hepatitis B screening in pregnancy and immunization of children of carriers | $164 |
| Human papillomavirus vaccine for 12 year old girls in a school-based immunization program | $12,921 |
| Varicella vaccine for children | $16,000 |
| Pneumococcal conjugate vaccine for children | $125,000 |
| Other interventions | |
| Mandatory seat belt law | $69 |
| Chlorination of drinking water | $3,100 |
| Smoking cessation counseling | $1,000 to $10,000 |
| Annual screening for cervical cancer | $40,000 |
| Driver and passenger air bags/manual lap belts (vs. airbag for driver only and belts) | $61,000 |
| Smoke detectors in homes | $210,000 |
| Crossing control arm for school buses | $410,000 |
| Radiation emission standard for nuclear power plants | $100,000,000 |
# Monetary resources required to save one year of “statistical” life
_
In 2005, Harvard University scientists calculated that spending on GAVI’s program to expand vaccine coverage in eligible countries would deliver a rate of return of 18% by 2020 – higher than most other health interventions, and similar to primary education. Harvard study finds that the benefits of vaccination have been greatly underestimated. The economic impacts of immunization stem from the fact that immunization protects individuals not only against getting an illness per se, but also against the long-term effects of that illness on their physical, emotional, and cognitive development,” Although new vaccines supported by GAVI cost more than other vaccines that have long been included in national immunisation programs, they remain cost-effective compared with many other interventions.
_
Cost-effectiveness and DALYs:
In low-income countries, life expectancy is shorter and more lifetime is spent in poor health. To measure the lost value of a healthy life year free of illness and disability, public health refers to disability-adjusted life years or DALYs. A DALY combines the years of life lost due to premature death (mortality) and loss of full health due to illness and disability (morbidity).WHO’s Commission on Macroeconomics and Health has classified interventions that gain a year of healthy life (i.e., a DALY averted) at a cost that is less than GDP per capita as very cost-effective. Those averting each DALY at a cost between one and three times the GDP per capita are cost effective. A 2006 study estimated the cost per DALY averted with the traditional EPI vaccines ranges from US$ 7 to US$ 438. The cost per death averted ranges from US$ 205 in South Asia and Sub-Saharan Africa to US$ 3,540 in Europe and Central Asia.
_
Estimated Economic Benefits during the ‘Decade of Vaccines’ include Treatment Savings, Gains in Labor Productivity: A 2011 study:
In 2010 the Bill & Melinda Gates Foundation announced a $10 billion commitment over the next ten years to increase access to childhood vaccines in the world’s poorest countries. The effort was labeled the “Decade of Vaccines.” This study estimates both the short- and long-term economic benefits from the introduction and increased use of six vaccines in seventy-two of the world’s poorest countries from 2011 to 2020. Increased rates of vaccination against pneumococcal and Haemophilus influenzae type b pneumonia and meningitis, rotavirus, pertussis, measles, and malaria over the next ten years would save 6.4 million lives and avert 426 million cases of illness, $6.2 billion in treatment costs, and $145 billion in productivity losses. Monetary estimates based on this type of analysis can be used to determine the return on investment in immunization from both the international community and local governments, and they should be considered in policy making.
_______
_______
Veterinary vaccines:
Vaccinations of animals are used both to prevent their contracting diseases and to prevent transmission of disease to humans. Both animals kept as pets and animals raised as livestock are routinely vaccinated. It is estimated that veterinary vaccines are available for over 400 diseases affecting mammals, birds and fish, including farm animals, pets and wildlife. Though revenues from the global human vaccine market are over 30 times that of veterinary vaccines, veterinary vaccines are very widely used with over two billion doses of foot-and-mouth disease (FMD) vaccine used per year, and poultry vaccines given on an even greater scale. In some instances, wild populations may be vaccinated. This is sometimes accomplished with vaccine-laced food spread in a disease-prone area and has been used to attempt to control rabies in raccoons. Where rabies occurs, rabies vaccination of dogs may be required by law. Other canine vaccines include canine distemper, canine parvovirus, infectious canine hepatitis, adenovirus-2, leptospirosis, bordatella, canine parainfluenza virus, and Lyme disease, among others.
_
Despite the universal importance of vaccines, approaches to human and veterinary vaccine evaluation differ markedly. For human vaccines, vaccine efficacy is the proportion of vaccinated individuals protected by the vaccine against a defined outcome under ideal conditions, whereas for veterinary vaccines the term is used for a range of measures of vaccine protection. The evaluation of vaccine effectiveness, vaccine protection assessed under routine program conditions, is largely limited to human vaccines. Challenge studies under controlled conditions and sero-conversion studies are widely used when evaluating veterinary vaccines, whereas human vaccines are generally evaluated in terms of protection against natural challenge assessed in trials or post-marketing observational studies. Although challenge studies provide a standardized platform on which to compare different vaccines, they do not capture the variation that occurs under field conditions. Field studies of vaccine effectiveness are needed to assess the performance of a vaccination program. However, if vaccination is performed without central co-ordination, as is often the case for veterinary vaccines, evaluation will be limited.
_
Core vaccines are defined as those vaccines which all pets, regardless of circumstances, should receive. Core vaccines protect animals from severe, life-threatening diseases which have global distribution. Non-core vaccines are those that are required by only those animals whose geographical location, local environment or lifestyle places them at risk of contracting specific infections. Pet owners should talk to a veterinarian about which vaccines are necessary (core) and which are optional (non-core), because there is some variation depending on where you live and whether or not your pet goes outdoors.
•Canine core vaccines: distemper, parvovirus, hepatitis and rabies
•Canine non-core: measles, canine adenovirus-2, parainfluenza, bordetella, leptospirosis, coronavirus and lyme
•Feline core: distemper, feline viral rhinotracheitis, rabies feline and calicivirus
•Feline non-core: feline leukemia, ringworm, feline infectious peritonitis, bordatella and chlamydia
Some debate is heating up between people who think that animals need yearly revaccinations and those that think it’s unnecessary. Unfortunately, there’s not enough evidence to prove beyond a reasonable doubt that immunity lasts more than a year.
_
The Rabies Vaccine for Dogs and Cats: What you need to know:
The rabies vaccine is the only shot required by law for dogs and cats in the United States. This is primarily to protect humans from getting rabies from their pets. Consequences for not vaccinating against rabies depend on the Animal Control laws in your area. At the very least, you won’t be able to board your pet, participate in training classes or shows, or use a professional groomer. Many vets will insist on vaccination before boarding or treating your pet. And if your dog or cat bites or scratches anyone, or is picked up by Animal Control, there will surely be a stiff fine and your pet will be impounded and vaccinated (or worse). Most localities require vaccination every three years even though studies in France and blood antibody tests in the U.S. show that the rabies vaccine’s immunity lasts for seven years. Worse yet, whether because of habit, ignorance or greed, some localities require annual vaccination–although the “three year” shot is guaranteed by manufacturers to give immunity for three years. The one-year shot is not safer than the three-year shot, and has to be given more often, making it potentially more dangerous. Puppies are generally required to get their first shot around four months, then again one year later and thereafter as required by local law. After rabies vaccination, your dog may experience fever, malaise or even life-threatening anaphylactic shot. Non-immediate reactions days or even months after vaccination (called “vaccinosis”).
_
DIVA vaccines:
DIVA (Differentiating Infected from Vaccinated Animals) vaccines make it possible to differentiate between infected and vaccinated animals. DIVA vaccines carry at least one epitope less than the microorganisms circulating in the field. An accompanying diagnostic test that detects antibody against that epitope allows us to actually make that differentiation. The DIVA strategy has been applied in various countries and successfully eradicated pseudorabies virus. Swine populations were intensively vaccinated and monitored by the companion diagnostic test and subsequently the infected pigs were removed from the population. Bovine herpesvirus 1 DIVA vaccines are also widely used in practice. Scientists have put and still are putting much effort in applying the DIVA principle to a wide range of infectious diseases, such as, for example, classical swine fever, avian influenza, Actinobacillus pleuropneumonia and Salmonella infections in pigs.
_______
_______
Research on vaccines:
Vaccine development & research has several trends:
Principles that govern the immune response can now be used in tailor-made vaccines against many noninfectious human diseases, such as cancers and autoimmune disorders. For example, the experimental vaccine CYT006-AngQb has been investigated as a possible treatment for high blood pressure. Factors that have impact on the trends of vaccine development include progress in translatory medicine, demographics, regulatory science, political, cultural, and social responses. Combinations of vaccines are becoming more common; vaccines containing five or more components are used in many parts of the world. Two hexavalent diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type B, poliovirus and hepatitis B (DTaP-Hib-IPV-HepB) combination vaccines were licensed and introduced in Europe in 2000. A new heptavalent diphtheria–tetanus–whole cell pertussis–hepatitis B–Haemophilus influenzae type b–Neisseria meningitidis serogroups A and C vaccine is also developed. New methods of administering vaccines are being developed, such as skin patches, aerosols via inhalation devices, and eating genetically engineered plants. Vaccines are being developed to defend against bioterrorist attacks such as anthrax, plague, and smallpox. Scientists are now trying to develop synthetic vaccines by reconstructing the outside structure of a virus.
_
Plants as bioreactors for vaccine production:
Transgenic plants have been identified as promising expression systems for vaccine production. Complex plants such as tobacco, potato, tomato, and banana can have genes inserted that cause them to produce vaccines usable for humans. Bananas have been developed that produce a human vaccine against Hepatitis B. Another example is the expression of a fusion protein in alfalfa transgenic plants for the selective directioning to antigen presenting cells, therefore increasing vaccine potency against Bovine Viral Diarrhea Virus (BVDV).
_
Flying syringe:
Flying syringe is a phrase that is used to refer to proposed, but not yet created, genetically modified mosquitoes that inject vaccines into people when they bite them. In 2008 the Gates Foundation awarded $100,000 to Hiroyuki Matsuoka of Jichi Medical University in Japan to do research on them, with a condition that any discoveries that were funded by the grant must be made available at affordable prices in the developing world. If Matsuoka proves that his idea has merit, he will be eligible for an additional $1 million of funding.
_
Microbial complement inhibitors as vaccines:
Complements inhibiting surface proteins of pathogenic bacteria provide candidates for vaccines because of two reasons. First, an immune response against them would recognize the microbes and secondly, it would neutralize the key bacterial virulence mechanism. Prerequisites for a vaccine protein include the following: (i) it should show limited variability, (ii) it should be immunogenic and the immune response against it should cover a sufficiently broad range of microbial strains, (iii) it should not be hidden beneath a capsule, long LPS O-polysaccharide side chains or a protein coat and (iv) it should not raise unwanted immune responses against host structures. Bacterial complement inhibitors often act by binding the soluble inhibitors factor H or C4 binding protein, by blocking C3 or C5 activation or by enzymatically cleaving key complement components. Inhibitors have been found from all major types of pathogens and may offer promise as rational vaccine candidates for preventing diseases such as meningococcal meningitis, systemic pneumococcal or group B streptococcal disease and Lyme borreliosis.
_
Immunoprophylaxis by gene transfer (I.G.T):
In Feb 2015, a team of scientists announced what could prove to be an enormous step forward in the fight against HIV. Scientists at Scripps Research Institute said they had developed an artificial antibody that, once in the blood, grabbed hold of the virus and inactivated it. The molecule can eliminate HIV from infected monkeys and protect them from future infections. But this treatment is not a vaccine, not in any ordinary sense. By delivering synthetic genes into the muscles of the monkeys, the scientists are essentially re-engineering the animals to resist disease. Researchers are testing this novel approach not just against HIV, but also Ebola, malaria, influenza and hepatitis. The first human trial based on this strategy — called immunoprophylaxis by gene transfer, or I.G.T. — is underway, and several new ones are planned. It could revolutionize the way we immunize against public health threats in the future. Conventional vaccines prompt the immune system to learn how to make antibodies by introducing it to weakened or dead pathogens, or even just their molecular fragments. Here immune cells produce a range of antibodies, some of which can fight these infections.
_
Safer vaccine created without virus:
British scientists have developed a “holy grail” vaccine for foot-and-mouth disease that is safer and more resilient than current vaccines. At the moment, animals are given a small dose of live infectious virus to stimulate the body’s immune system into producing antibodies that recognise and destroy the pathogen whenever it appears in the bloodstream. Now scientists believe they can produce an entirely synthetic vaccine thanks to atomic analysis of the virus using Britain’s particle accelerator, the Diamond Light Source, near Oxford in southern England. Using the data, a team of scientists from Oxford and Reading Universities and the Pirbright Institute were able to reconstruct the outside structure of the virus, which triggers the production of antibodies. The hollow shell contains no pathogenic RNA — the genetic material viruses use to replicate themselves — so there is no chance of accidental infection during vaccination. The scientists were also able to tweak the structure to make it stronger. Preclinical trials found it to be stable at temperatures up to 56 degrees Celsius for at least two hours. The technology should also be transferable to other viruses from the same family, such as poliovirus and hand, foot and mouth disease, a human virus endemic in Southeast Asia. Foot-and-mouth disease is endemic in central Africa, parts of the Middle East and Asia.
_
Reverse vaccinology:
The science of genomics has provided scientists with the complete genome sequences of more than 300 bacterial species – most of them responsible for human disease. Researchers use an organism’s genome to pick out the genes most likely to correspond to conserved antigens that could be used in a vaccine. Once identified, the genes can be combined and inserted into a different, rapidly multiplying organism – such as yeast – to produce candidate antigens, which are then screened for their ability to produce protective immune responses. This approach is known as “reverse vaccinology”: it starts with a genetic blueprint of an organism and rapidly generates antigens of interest. In contrast, the more time-consuming conventional approach starts with the pathogenic organism itself, which is grown in the laboratory (a lengthy process made more complex by the fact that some pathogens cannot easily be grown in a laboratory), and from which a limited number of antigens are isolated. These are then tested for their ability to induce potentially protective immune responses. Reverse vaccinology has not yet produced a licensed vaccine but researchers have used it to develop several candidate vaccines, some of which are currently in the late stages of clinical testing (for example, a candidate vaccine against group B meningococcus).
_
Reverse Vaccinology with Meningococcus B:
Attempts at reverse vaccinology first began with Meningococcus B (MenB). Meningococcus B caused over 50% of meningococcal meningitis, and scientists had been unable to create a successful vaccine for the pathogen because of the bacterium’s unique structure. This bacterium’s polysaccharide shell is identical to that of a human self-antigen, but its surface proteins vary greatly; and the lack of information about the surface proteins caused developing a vaccine to be extremely difficult. As a result, Rino Rappuoli and other scientists turned towards bioinformatics to design a functional vaccine. Rappuoli and others at the J. Craig Venter Institute first sequenced the MenB genome. Then, they scanned the sequenced genome for potential antigens. They found over 600 possible antigens, which were tested by expression in Escherichia coli. The most universally applicable antigens were used in the prototype vaccines. Several proved to function successfully in mice, however, these proteins alone did not effectively interact with the human immune system due to not inducing a good immune response in order for the protection to be achieved. Later, by addition of outer membrane vesicles that contain lipopolysaccharides from the purification of blebs on gram negative cultures; this adjuvant (previously identified by using conventional vaccinology approaches) enhanced immune response to the level that was required. Later, the vaccine was proven to be safe and effective in adult humans.
_
Pros and cons of reverse vaccinology:
The major advantage for reverse vaccinology is finding vaccine targets quickly and efficiently. Traditional methods may take decades to unravel pathogens and antigens, diseases and immunity. However, in silico can be very fast, allowing to identify new vaccines for testing in only a few years. The downside is that only proteins can be targeted using this process. Whereas, conventional vaccinology approaches can find other biomolecular targets such as polysaccharides.
_
Comparison between Traditional and Reverse Vaccinology:
| Traditional Vaccinology | Reverse Vaccinology | |
| Antigens available | 10-25 identified by biochemical or genetic tools, such as knocking out the important genes. | Virtually all antigens encoded by the genome are available and could be sifted using computer algorithm. However, this could result in a data overload, which makes choosing the right target more challenging. |
| Property of antigens | The most abundant antigens, the most immunogenic during disease, only from cultivable microorganisms. | All antigens are available, even if not highly immunogenic during disease. Antigens from noncultivable microorganisms can be identified. |
| Immunology of the antigens | Highly immunogenic antigens, often variable in sequence, because of immune selective pressure. Some may contain domains mimicking self-antigens and may induce autoimmunity. | The most conserved protective antigens can be identified. Usually these are not the most immunogenic during infection. The novel antigens are screened against the human genome, and antigens with homology to self-antigens are removed upfront, allowing to overcome the bottleneck where stimulating the immune response could give the wrong outcome. |
| Polysaccharide antigens | A major target of traditional bacterial vaccines. | Cannot be identified by reverse vaccinology; however, operons coding for the biosynthesis of polysaccharides can be identified. This can lead to discovery of novel carbohydrate antigens. |
| T cell epitopes | Known epitopes limited to the known antigens. | Virtually every single T cell epitope is available. Screening of the total T cell immunity can be done by overlapping peptides. |
______
Vaccine delivery systems:
The development of new delivery systems raises the hope of vaccines that are safer and more efficient to deliver and administer. Lines of research include liposomes and ISCOM (immune stimulating complex). Notable developments in vaccine delivery technologies have included oral vaccines. A microneedle approach, which is still in stages of development, uses “pointed projections fabricated into arrays that can create vaccine delivery pathways through the skin”. An experimental needle-free vaccine delivery system is undergoing animal testing. A stamp-size patch similar to an adhesive bandage contains about 20,000 microscopic projections per square inch. This dermal administration potentially increases the effectiveness of vaccination, while requiring less vaccine than injection.
_
Why do we need innovative delivery technologies?
There are currently several factors that are creating pressure to improve delivery systems for vaccines. First, in the current regulatory environment, there is a growing requirement to develop vaccines that are very well defined in molecular terms. Thus, as opposed to using whole-inactivated pathogens presenting a complex range of antigens, most newly developed vaccines are rather based on selected target antigens. In some cases these may be single molecules, or even fragments thereof, derived from an infectious micro-organism, a tumour cell, an allergen or an auto-antigen. The target molecule may be administered as a purified protein or as a peptide(s), or may be expressed from plasmid DNA or a recombinant virus. Often, such molecular vaccines are poorly immunogenic, implying a need for an adjuvant, a specific formulation or a vector system of enhanced immunogenicity. Second, although in the past most vaccines have been designed to stimulate antibody responses against surface molecules of bacteria or viruses, new generation vaccines are increasingly designed to elicit cellular immune responses, especially of the Th1 type. Such responses are considered paramount for targeting chronic infectious diseases that may have an intracellular stage (associated for example with HIV1, herpes viruses, hepatitis C virus, Helicobacter pylori, Plasmodium falciparum, Mycobacterium tuberculosis), but also for the development of therapeutic vaccines against cancer, autoimmune diseases or allergies. New vaccines are also being developed to elicit mucosal immune responses in humans, for example to protect against pathogens such as influenza virus, HIV1, HSV or human oncogenic or wart-associated papilloma viruses. Unlike most of the traditional vaccines, these efforts require the recruitment of cellular or mucosal immune effector mechanisms and necessitate the exploration of new routes of administration, new formulations, and new adjuvant systems. Third, improving vaccine administration generally, either for the physician, or more importantly for the customer, towards pain-free and safe needle-less devices is likely to represent a major driver in the future vaccine market. Innovative delivery technologies include three categories of delivery systems: (i) adjuvants and formulations; (ii) antigen vectors, including live attenuated micro-organisms and synthetic vectors; and (iii) novel devices for vaccine administration.
_
Virosome:
A virosome is a drug or vaccine delivery mechanism consisting of unilamellar phospholipid membrane (either a mono- or bi-layer) vesicle incorporating virus derived proteins to allow the virosomes to fuse with target cells. Virosomes are not able to replicate but are pure fusion-active vesicles.
Influenza virosomes:
In contrast to liposomes, virosomes contain functional viral envelope glycoproteins: influenza virus hemagglutinin (HA) and neuraminidase (NA) intercalated in the phospholipid bilayer membrane. They have a typical mean diameter of 150 nm. Essentially, virosomes represent reconstituted empty influenza virus envelopes, devoid of the nucleocapsid including the genetic material of the source virus. The unique properties of virosomes partially relate to the presence of biologically active influenza HA in their membrane. This viral protein not only confers structural stability and homogeneity to virosome-based formulations, but it significantly contributes to the immunological properties of virosomes, which are clearly distinct from other liposomal and proteoliposomal carrier systems. It has been shown that a physical association between the virosome and the antigen of interest is necessary for the full adjuvant effect of virosomes. Such physical association can be achieved by a variety of methods, depending on the properties of the antigen. Antigens can be incorporated into virosomes, adsorbed to the virosome surface, or integrated into the lipid membrane, either via hydrophobic domains or lipid moieties cross-linked to the antigen. Virosomes therefore represent an innovative, broadly applicable adjuvant and carrier system with prospective applications in areas beyond conventional vaccines.
Non-influenza virosomes:
They are also being considered for HIV-1 vaccine research.
_
Devices:
A number of new needle-free or modified needle devices, which carry potentially a number of advantages over conventional needle injection, are being developed for vaccine administration. Such advantages include increased safety, acceptability and, therefore, treatment compliance, as well as potentially increased efficacy linked to a broader, or modified, tissue distribution of the antigen, ease of use leading to self-administration, administration of smaller doses of the antigen and adjuvants, as well as delivery via either the mucosal (nasal or oral), subcutaneous or intradermal route. Table below summarizes devices which have been (or are being tested in humans). The Macroflux microneedle system allows administration of the antigen dry-coated onto microneedles. When pressed onto the skin, the microprojections create mechanical pathways through the superficial skin, allowing intracutaneous delivery of the antigen to an average depth of 100 μm. The antigen dose administered can be controlled by the formulation, wearing time, and system size. The largest experience in the field of needle-free delivery to humans has been gained with a variety of jet-injectors able to deliver vaccines by the subcutaneous route. These devices use forces derived from two sources of power, either a spring or compressed gas, to propel the vaccine through the skin. Needle-free injection was found to increase immune responses to both conventional and DNA-based vaccines: for example, seroconversion rates as well as antibody titers elicited in humans by a hepatitis A vaccine or a trivalent influenza vaccine were found to be increased by at least 10% when using needle-free injections, as opposed to needle and syringe administration.
_
New devices for vaccine administration:
| Device | Antigen(s) | Comments |
| Minineedles (e.g. Macroflux microprojection array) | Various antigens | In this system, a titanium microprojection array with an adhesive patch backing is used. The antigen is adsorbed as a powder onto the minineedles and injected subcutaneously (at a depth of about 100 μm) by patch application to the skin |
| Needle-less injection | ||
| Spring powered (Advantajet, Injex, Vitajet 3, Medi-Jector) | Hepatitis A, flu antigens, hepatitis B | Spring-powered needle-free devices have been initially designed and used in humans for the administration of insulin or growth hormones. Volumes of 20–500 μl can be administered subcutaneously. Changing the orifice size modulates the administration pressure, in relation to differences in the thickness of skin between patients |
| Gas powered (Biojector 2000, Penjet, J-Tip, Powderject system) | Many DNA plasmids plasmids | Gas-powered systems include nitrogen, CO2 or helium gas powered systems, allowing IM, subcutaneous or intradermal administration. Such systems allow the administration of volumes of up to 1 ml. Although most systems have been designed to administer antigens as a liquid, one system (Powderject) relies on a pre-filled helium-powered system in which dry-powder formulations, stable at room temperature (e.g. plasmid DNA) are precipitated onto small (3 μm diameter) gold particles for administration through the skin. |
| Patches for transcutaneous immunization | Shigella or Salmonella antigens, CS6 (E. coli), LT | The antigen in combination with an adjuvant (e.g. CT or LT) are administered onto hydrated skin. In various animal models, and more recently in humans, this approach was found to elicit antibodies (both IgGs and IgAs), as well as a strong lymphoproliferative response, in the absence of any adverse event. |
| Aerosol for delivery of powder vaccines | Measles | In order to produce fine particles without damaging activity of the virus, a live attenuated measles virus is micronized by jet milling to generate particles with the appropriate size for pulmonary delivery (1–5 μm). Particles are blended with an inert carrier to improve aerosol dispersion with a nebulizer. |
_____
Nanopatch vaccine delivery:
Australian scientists have developed a cheap and painless ’needle-free’ vaccination device that can be self-administered. A team of 20 researchers led by Professor Mark Kendall, from the Australian Institute for Bioengineering and Nanotechnology at The University of Queensland, have developed the Nanopatch, a stamp-sized vaccine delivery device that could make vaccination programs globally simpler and cheaper. The Nanopatch, having 20,000 micro projections per square centimeter, is designed to directly place vaccine into the human skin, which is rich in immune cells. And unlike the needle and syringe, which places vaccine into the muscle – which has very few immune cells – the Nanopatch puts it into immune spot. And by doing this vaccines work a lot better. The Nanopatch potential lies in it being cheap, painless, very effective being transported without refrigeration – and can be given without the need for extensive training. The removal of the need for refrigeration is achieved by dry-coating vaccine to the Nanopatch, which could have huge potential for developing countries like India, and many within Africa. The World Health Organisation estimates 50 per cent of vaccines in Africa do not work properly because the ’cold chain’ has been broken. In a pandemic, the reduced dose would also make it easier for governments to supply sufficient vaccine to the public. The new device is simple as it does not need a trained practitioner to administer the vaccine. The Nanopatch has to be worn to just 2 minutes or even less, thus giving a pain-free immunisation. The vaccine could hit markets in next 10 years. Vaxxas has just signed an agreement with the World Health Organisation (WHO) to trial its Nanopatch delivery system for polio vaccines, which the company hopes will progress the technology through the next stage of clinical trials.
____
New microneedle patch for measles vaccination designed:
Administering measles vaccine with a microneedle patch may be much easier than getting an immunisation with a hypodermic needle, according to researchers at Georgia Institute of Technology and the Centers for Disease Control and Prevention (CDC). The microneedle patch is designed to be administered by minimally trained workers and to simplify storage, distribution, and disposal compared with conventional vaccines. The patch under development measures about a square centimeter and is administered with the press of a thumb. The underside of the patch is lined with 100 solid, conical microneedles made of polymer, sugar, and vaccine that are a fraction of a millimetre long. When the patch is applied, the microneedles press into the upper layers of the skin; they dissolve within a few minutes, releasing the vaccine. The patch can then be discarded. Because microneedles dissolve in the skin, there is no disposal of needles, reducing the risk of accidental needlesticks. The researchers are also testing if microneedles could be used to administer inactivated polio vaccine. Researchers are also studying microneedle-administration of the influenza, rotavirus and tuberculosis vaccines.
_
Nanoparticle-coated bacteria can deliver cancer vaccine:
Marking an important step in the development of immunotherapy cancer treatment, scientists have demonstrated that nanoparticle-coated bacteria can effectively deliver an oral DNA vaccine that stimulates the body’s own immune system to destroy its cancer cells. This is the first time that a nanoparticle coating has been used for bacterial delivery of oral DNA vaccination in vivo. Compared with uncoated bacteria, coated bacteria can bypass many of the roadblocks (phagocytosis by macrophages and acid in stomach) that have so far limited the immune response and that currently pose the biggest challenge to DNA vaccinations against cancer.
_
_
Nanoparticle-coated bacteria can act as a vaccine vector, delivering vaccines that evoke an antitumor immune response. As shown here, the nanoparticles are made from cationic polymers and the bacteria’s plasmid DNA. The positively charged nanoparticles self-assemble onto the negatively charged bacterial cell walls. One oral DNA vaccine that researchers have been working on is called NP/SAL, which suppresses tumor angiogenesis (blood vessel formation). Many tumors secrete angiogenic factors such as vascular endothelial growth factor (VEGF) to promote blood vessel formation, which can eventually lead to tumor metastasis. The NP/SAL vaccine stimulates the immune system to produce T cells (white blood cells) and cytokines (chemical messengers), which in turn interfere with the VEGF pathway, reducing blood vessel formation and ultimately inhibiting tumor growth. The researchers found that 60% of mice vaccinated with NP/SAL carried by nanoparticle-coated bacteria survived the full 35-day study duration without tumor growth. The mice also lost almost no body weight, reflecting the low toxicity of the vaccine. The researchers expect that this strategy of using nanoparticle-coated bacteria as DNA vaccine vectors can be applied to a wide variety of vaccines, and potentially used to treat a wide spectrum of cancers.
_______
_______
Myths and FAQ:
Frequently asked questions (FAQ):
Are nosodes vaccines and do they work?
Often called homeopathic vaccine, nosodes are made using saliva, feces, mucus or other material infected with a particular disease or ailment. The substance is mixed with alcohol and diluted. However, they are usually diluted so much that little or no active ingredients are left in the final product. The solution is sometimes turned into a sugar pill and taken orally. Nosodes are not vaccines and there is no scientific evidence to show they work.
_
Is it better to get the disease than be vaccinated?
No, it is not better to get the disease than be vaccinated. The benefits of being vaccinated far outweigh those of infection with the pathogen. The rates of complications, both short- and longer-term, are much higher and generally more severe after natural infections than the rates of side effects associated with the corresponding vaccines. For example, one in 15 patients with diphtheria die from the disease, whereas serious side effects from the diphtheria vaccine are very rare. Similarly, approximately one in four patients chronically infected with hepatitis B will die from cirrhosis of the liver or from liver cancer; this risk is reduced to almost zero after hepatitis B immunisation. Vaccines have the added advantage of offering more effective protection against subsequent exposure to certain pathogens. Examples of diseases that do not always generate protective immunity include tetanus and whooping cough. In the case of tetanus, the tiny amount of toxin needed to produce life-threatening disease is too small to generate sufficient levels of protective antibodies to neutralise the toxin. To achieve protective antibody levels, it is necessary to give a much larger dose of toxin, which requires the use of vaccine.
_
Do vaccines cause autoimmune disease?
Over the past 30 years, the number of people who develop autoimmune diseases has been increasing, particularly in societies where rates of infectious disease have declined. This has raised the question of whether vaccine use is contributing to the reported rise in certain autoimmune disorders. With the exception of the two rare diseases mentioned below, the answer is no. This conclusion is based on the stringent monitoring procedures put in place for detecting side effects of vaccination. The first exception is the small increase in risk of developing the rare condition known as idiopathic thrombocytopenic purpura (ITP), which has been reported after the MMR vaccine. However, the risk of developing this disorder associated with measles infection is more than 10 times greater than associated with the vaccine. The other exception is Guillain–Barré syndrome – but again, the risk of developing the disease after influenza vaccination is much lower than after the actual infection. Barring two exceptions, there is no credible scientific evidence to suggest that any vaccine in current use can cause these particular autoimmune diseases. In addition, the vast majority of people (mainly adults) who develop autoimmune diseases have no recent history of being vaccinated.
_
Do vaccines cause allergic disease?
Like autoimmune diseases, allergic diseases such as asthma have become more common in the developed world over the past 30 years. However, there is no significant evidence that vaccines cause allergic diseases. The question asked is whether vaccines can precipitate attacks of serious allergic reactions in susceptible children or adults. Overall, the rate of severe allergic reactions following vaccination is extremely low; between 0.02 and 4.52 per 100,000 doses. Nevertheless, precautions should always be taken by people with a past history of reaction to a specific vaccine or a strong family history of allergic disease.
_
Why do we still need vaccines if the diseases they prevent have disappeared from our part of the world?
It is important to continue vaccine programs for four basic reasons:
•First, unless a disease has completely disappeared, there is a real risk that small outbreaks can turn into large epidemics if most of the community is not protected. The only disease that has been entirely eliminated in the world so far is smallpox. Some diseases, such as tetanus, are caused by bacteria that live naturally in the soil. The risk of diseases like tetanus will never disappear, so continued immunization is important.
•Second, no vaccine is 100% effective. There will always be some people who are not immune, even though they have had their shots. This small minority will be protected as long as people around them are immunized.
•Third, there are a small number of people who cannot receive vaccines. These may be people who have previously had a severe allergic reaction to a component of the vaccine, or they have a medical condition that makes receiving vaccines too risky for them. These people are not protected from disease, and for some diseases it is very important that people around them are immune and cannot pass disease along to them. By protecting themselves, immunized people can also protect those around them who are vulnerable to disease.
•And fourth, most vaccine-preventable diseases are still common in other parts of the world. Travelers can carry them from country to country. If we are not protected by immunization, these diseases will quickly spread. For example, most cases of measles in Canada today can be traced to someone who travelled there from a country where measles is more common.
_
Why aren’t all vaccines 100% effective?
Vaccines are designed to generate an immune response that will protect the vaccinated individual during future exposures to the disease. Individual immune systems, however, are different enough that in some cases, a person’s immune system will not generate an adequate response. As a result, he or she will not be effectively protected after immunization. That said, the effectiveness of most vaccines is high. After receiving the second dose of the MMR vaccine (measles, mumps and rubella) or the standalone measles vaccine, 99.7% of vaccinated individuals are immune to measles. The inactivated polio vaccine offers 99% effectiveness after three doses. The varicella (chickenpox) vaccine is between 85% and 90% effective in preventing all varicella infections, but 100% effective in preventing moderate and severe chicken pox.
_
Why do some vaccines require boosters?
It’s not completely understood why the length of acquired immunity varies with different vaccines. Some offer lifelong immunity with only one dose, while others require boosters in order to maintain immunity. Recent research has suggested that the persistence of immunity against a particular disease may depend on the speed with which that disease typically progresses through the body. If a disease progresses very rapidly, the immune system’s memory response (that is, the “watchdog antibodies” generated after a previous infection or vaccination) may not be able to respond quickly enough to prevent infection—unless they’ve been “reminded” about the disease fairly recently and are already watching for it. Boosters serve as a “reminder” to your immune system.
_
Isn’t it true that better hygiene and nutrition were responsible for decreases in deaths and disease rates, rather than vaccines?
Improved hygiene and nutrition, among other factors, can certainly lower the incidence of some diseases. Some argue that improved health and hygiene have caused the dramatic decline in infectious diseases over the last century, not vaccines. To support this argument, graphs are used to depict declining disease death rates before the introduction of vaccines and no visible impact from vaccination. These graphs always show death rates overall rather than disease incidence and hide the true effect of vaccines. Dr. Isaac Golden presents us with a graph that shows a declining incidence rate of pertussis and an arrow to represent the introduction of the vaccine in 1948, which by this time in the graph the incidence rate is relatively low. There are a number of problems with this. Most importantly, the arrow is pointing to the wrong time of pertussis vaccine use. The 1948 mark points to the introduction of the Diphtheria, Tetanus, and Pertussis (DTaP or DTP) vaccine. Regular pertussis vaccines, however, have been around since the 1920’s when Louis W. Sauer created a successful vaccine. Others were successful and used the vaccines to help control outbreaks. Dr. Viera Scheibner presents another graph to claim the same thing about diphtheria. In this case, and many like it, the graph is deceptive in the fact that it shows a limited time span and, most importantly, is measuring the deaths attributed to that disease. This hides the true success of vaccines, as decreases in incidence rates are much more telling. Data documenting the number of cases of a disease before and after the introduction of a vaccine, however, demonstrate that vaccines are overwhelmingly responsible for the largest drops in disease rates. Measles cases, for example, numbered anywhere from 300,000 to 800,000 a year in the United States between 1950 and 1963, when a newly licensed measles vaccine went into widespread use. By 1965, U.S. measles cases were beginning a dramatic drop. In 1968 about 22,000 cases were reported (a drop of 97.25% from the height of 800,000 cases in just three years); by 1998, the number of cases averaged about 100 per year or less. A similar post-vaccination drop occurred with most diseases for which vaccines are available. Perhaps the best evidence that vaccines, and not hygiene and nutrition, are responsible for the sharp drop in disease and death rates is chicken pox. If hygiene and nutrition alone were enough to prevent infectious diseases, chicken pox rates would have dropped long before the introduction of the varicella vaccine, which was not available until the mid-1990s. Instead, the number of chicken pox cases in the United States in the early 1990s, before the vaccine was introduced in 1995, was about four million a year. By 2004, the disease incidence had dropped by about 85%. Higher standards of living and sanitation alone unfortunately do not ensure protection from infectious diseases. With short travel times over large distances, infectious diseases can be carried from countries with greater disease prevalence. Cases have occurred in unimmunized people all around the world as a result of travel to or from areas where vaccine-preventable diseases are still very common.
_
Why can’t we eradicate other diseases, as we did with smallpox?
In theory, nearly any infectious disease for which an effective vaccine exists should be eradicable. With sufficient vaccination levels and coordination between public health organizations, a disease can be prevented from gaining a foothold anywhere; eventually, without anyone to infect, it must die off. (A notable exception is tetanus, which is infectious but not contagious: it’s caused by a bacterium commonly found in environment. It does not need human/animal host for survival. Thus, tetanus could not be eradicated without completely removing the Clostridium tetani bacterium from the planet.) Smallpox is unusual, however, in the set of characteristics that made it susceptible to eradication. Unlike many other infectious diseases, smallpox has no animal reservoir. People with smallpox were highly visible: the smallpox rash was easily recognizable, so that new cases could be detected quickly. Vaccination efforts could be focused based on the location of the cases and potential exposure to other individuals. Polio, by contrast, causes no visible symptoms in about 90% of the people it infects. As a result, tracking the spread of the polio virus is extremely difficult, which makes it a difficult eradication target. Perhaps most importantly, smallpox patients generally did not reach their highest level of infectivity (that is, their ability to infect others) until after the appearance of the smallpox rash. As a result, quick action to quarantine infected individuals upon the eruption of the rash usually left enough time to vaccinate anyone who had already been exposed, and prevent additional exposures. Many infectious diseases do not allow for this kind of reaction time. Measles patients, for example, can become infectious up to four days before the appearance of the measles rash. As a result, they can pass the virus on to many, many other people before anyone even knows that they are infected.
________
________
Myths:
_
Myth: Vaccines are unsafe.
Fact: In general, no pharmacologic agent, including vaccines, can be considered 100 per cent safe. However, all vaccines currently available must pass stringent safety testing before being approved for use by the regulatory authority. The majority of problems thought to be related to the administration of a vaccine are actually not due to the vaccine itself. Many are coincidental events that just happen to occur at the same time as vaccination. This is particularly the case in the first year of a child’s life, when vaccines are given regularly. Events that occur in the child’s first year of life may therefore coincide with the time that a vaccine has been received. A good example of this is a six-month-old infant having a seizure. If the seizure started one hour after a vaccination, it would be natural to think differently about why it may have occurred than if it commenced one hour before the vaccination. Vaccines may produce some undesirable side effects, such as pain and redness at the injection site or fever, but most reactions are mild and resolve quickly. It is usually not possible to predict who may have a mild reaction and who may have a rarer, serious reaction to a vaccine. However, the risk of adverse effects can be minimized by following guidelines regarding when vaccines should and shouldn’t be used.
_
Myth: Vaccines are cultured on cell lines from aborted fetuses.
Fact: Although bacteria can, under the right supportive conditions, survive and replicate on their own, viruses require cells in order to replicate and can only be grown in the laboratory in cells or ‘cell lines’. A cell line is a specific population of cells that is maintained in culture for extended periods. Cell lines have an unlimited lifespan and represent a renewable and predictable system for growing viruses used in the production of vaccines. The best cell types in which to grow human-specific viruses are often cell lines derived originally from a sample of human tissue. It is very hard to grow some viruses that infect humans in any other type of cell. Certain cell lines (human diploid cell lines WI-38 and MRC-5) originated from fetal tissue obtained from two elective abortions indicated for medical reasons in the 1960s. These cell lines have been growing under laboratory conditions for more than 50 years. There has been no further tissue obtained from fetuses since the 1960s. Abortions have not been conducted specifically for the purpose of harvesting cell lines. Vaccines which are manufactured using cell lines originally derived from fetal tissue include rubella-containing vaccines (MMR and MMRV), hepatitis A vaccines, varicella vaccines and rabies vaccine.
_
Myth: Immunisation is unnatural and natural immunity is better than vaccine induced immunity:
Fact: Vaccines use a person’s natural response to disease to stimulate the immune system so that if someone is exposed to that specific pathogen in the future, their immune system can ‘remember it’ and mount an effective response to either stop disease developing or reduce the severity of disease. Choosing to remain unvaccinated, and have the disease rather than prevent it, can have serious consequences. Diseases such as tetanus and meningitis can kill and maim, whereas the vaccines against these diseases are generally well tolerated with minor side effects. Vaccines provide the same stimulus to the immune system as an infection and can potentially offer more effective protection against certain pathogens. While it’s true that natural immunity lasts longer in some cases than vaccine-induced immunity can, the risks of natural infection outweigh the risks of immunization for every recommended vaccine. For example, wild measles infection causes encephalitis (inflammation of the brain) for one in 1,000 infected individuals, and, for every 1,000 reported measles cases, two individuals die. The combination MMR (measles, mumps, and rubella) vaccine, however, results in encephalitis or a severe allergic reaction only once in every million vaccinated individuals, while preventing measles infection. The benefits of vaccine-acquired immunity extraordinarily outweigh the serious risks of natural infection, even in cases where boosters are required to maintain immunity. Additionally, the Hib (Haemophilus Influenzae type b) and tetanus vaccines actually provide more effective immunity than natural infection. Most importantly, protection through vaccination avoids the complications associated with having the disease. The benefits of vaccination far outweigh those of infection with a vaccine-preventable disease. The diseases we prevent with vaccines can kill and seriously harm children. Vaccines are safe; it’s much safer to get vaccinated than to get the disease.
_
Myth: Breastfeeding children mean they do not need to be vaccinated.
Fact: Maternal antibodies alone, such as those provided through breastfeeding, are not sufficient to protect a baby against all infections. Maternal antibodies do provide some protection to the newborn but the amount of protection varies with different diseases and the presence of maternal antibodies is dependent on the mother’s prior exposure to the actual disease or antigen. For example, mothers who have not recently been immunised or infected with pertussis (whooping cough) generally only pass on minimal protection against pertussis to their baby. In addition, the low amount of antibody that is transferred rapidly wanes during the first weeks, leaving the infant vulnerable to infection if they are exposed to pertussis. On the other hand, maternal antibodies against measles may provide protection to the infant for up to 12 months. These factors are taken into account when vaccine schedules are planned. Without a doubt, breastfeeding has proven benefits, like enhancing the protection of infants against some colds, ear infections, and diarrhea. However, breastfeeding does not prevent the diseases that vaccines do prevent. Unlike vaccines, breast milk does not stimulate the infant’s own immune system to produce the antibodies needed to fight very specific diseases. Vaccines and breastfeeding do not interfere with each other, and together make an excellent way to keep your child healthy. Importantly, for certain diseases, such as influenza, vaccination of a woman during her pregnancy protects her against this disease, as well as protecting her baby in the baby’s first few months of life (due to the passage of high levels of maternal antibodies across the placenta prior to birth).
_
Myth: Vaccines cause or spread the diseases they are supposed to prevent.
Fact: The majority of vaccines are inactivated or prepared from only part of the pathogen. This means the components of the vaccine are not living and therefore cannot cause disease. An exception to this is live attenuated viral vaccines which contain weakened (or ‘attenuated’) forms of the virus that the vaccine aims to protect against. The weakened virus does replicate in the host to create an immune response, but cannot cause disease, except on very rare occasions (e.g. OPV). There are also other types of live vaccines which contain a naturally occurring organism that does not itself cause disease in humans but which is closely related to (and can therefore induce protection against) the human pathogen which can cause disease. After most natural infections and most vaccines, the infecting organism or antigens do not persist in the body because they are eliminated by the immune response they induce. An exception to this is the virus that causes chickenpox and then remains dormant in sensory nerves to (sometimes) reactivate later in life and cause herpes zoster (shingles). Similar to what happens following natural infection, in some people vaccinated with the live attenuated varicella vaccine, the vaccine virus will reactivate later in life to cause shingles. However, this occurs at a much lower rate than following natural varicella infection, and reported cases have been mild. Similarly, if a vesicular skin rash occurs at the injection site of a varicella vaccine (which occurs in five people out of every 100 people who receive the vaccine), there is the potential to transmit the vaccine virus to someone else through direct contact with the rash. However, transmission of vaccine virus in this way is extremely rare. In the United States, where more than 56 million doses of varicella vaccine have been distributed over 10 years, there have been only six documented cases of transmission of the vaccine virus from an immunocompetent vaccinated person to others. The MMR vaccine can also cause a transient rash 7 to 10 days after vaccination, but it is non-infectious.
_
Myth: People who are vaccinated can still get the disease.
Fact: Some people argue that since cases of vaccine-preventable disease do occur in those who have been vaccinated, vaccines are not effective. However, this is not completely true. There is a relationship between vaccination rates, vaccine effectiveness and apparent vaccine failures. Where vaccination rates are high and an outbreak of disease occurs, the numbers of cases in vaccinated people can appear to be high in relation to the number of cases among those who are unvaccinated. This apparent paradox is because of two factors:
•First, no vaccine is 100 per cent effective. To make vaccines safer than the disease, the bacteria or virus is killed or weakened (attenuated). For reasons related to individuals’ genetics, not all vaccinated people develop immunity. Most routine childhood vaccines are effective in 85 to 95 per cent of recipients. That means that in every 100 people who receive a vaccine, between 5 and 15 of them may not develop protective immunity.
•Second, in developed nations, people who have been vaccinated against the common childhood vaccine-preventable diseases vastly outnumber those who have not.
How these two factors work together to bring about a situation where the majority of cases in an outbreak occur in those who have been vaccinated is explained using the following hypothetical scenario. In a high school of 1,000 students, none has ever had measles disease. All but five of the students have had two doses of measles vaccine, and so are fully immunised. The entire student body is exposed to measles, and every susceptible student becomes infected. The five unvaccinated students will be infected, of course. But of the 995 who have been vaccinated, we would expect several to have not responded to the vaccine. The efficacy rate for two doses of measles vaccine can be as high as 99 per cent so, in this school, ten students will have not responded to the vaccine, and they too become infected. Therefore, 10 of 15, or about 67 per cent, of the cases will be in students who have been fully vaccinated. However, this doesn’t prove the vaccine didn’t work – only that most of the children in the school had been vaccinated, so those who were vaccinated and did not respond outnumbered those who had not been vaccinated. Looking at it another way, 100 per cent of the children who had not been vaccinated got measles, compared with around one per cent of those who had been vaccinated. Measles vaccine protected most of the students. If nobody in the school had been vaccinated, there would probably have been 1,000 cases of measles. So measles vaccine is highly effective despite 10 vaccinated students getting measles.
_
Myth: Vaccines are linked to Guillain-Barré syndrome.
Fact: Guillain-Barré syndrome (GBS) is a rare neurologic disorder involving inflammatory demyelination of peripheral nerves. It is estimated that every year there are one to two newly diagnosed cases of GBS for every 100,000 people in the population (0.001–0.002%). The most severe cases of GBS result in paralysis, sometimes requiring respiratory support if the chest wall muscles are affected. GBS can occur spontaneously (without any identified cause) or after certain events such as infections, including influenza. In the United States in 1976, receipt of that year’s seasonal influenza vaccine formulation was associated with an increased risk of getting GBS. Several studies have been done to evaluate if other influenza vaccines since 1976 have been associated with GBS. These long-term studies have only found a very small increase in GBS following influenza vaccination of approximately one additional case for every one million people vaccinated against influenza (above the number that would have occurred anyway without vaccination). More importantly, risk of getting GBS after natural influenza infection is higher than influenza vaccine. Isolated case reports have suggested a possible association of GBS with several other vaccines including oral polio, MMR, tetanus toxoid-containing and hepatitis B vaccines. However, robust epidemiologic studies have not demonstrated any link. In the United States, a possible association between GBS and a quadrivalent meningococcal conjugate vaccine used in teenagers was reported to the United States Vaccine Adverse Events Reporting System (VAERS). However, a subsequent investigation found that this vaccine was not associated with an increased risk of GBS.
_
Myth: Vaccines cause sudden infant death syndrome (SIDS).
Fact: Sudden infant death syndrome (SIDS) is defined as the sudden and otherwise unexplained death of an infant under one year of age. The incidence of SIDS peaks at two months of age, the age at which children are recommended to receive their first vaccines. This apparent ‘association’ between the timing of vaccination and SIDS deaths has been examined to determine whether there is a causal link. A number of well-controlled studies in the last 20 years have found, almost unanimously, that the number of SIDS deaths associated in time with DTP vaccination was within the range expected to occur by chance and irrespective of vaccination. This data is important to highlight when chance associations do occur. For example, a study using Australian data from 1997–2001 calculated that, by chance alone, approximately two of the 130 SIDS cases per year would have occurred within the 24-hour period after vaccination. To date all of the published evidence suggests that vaccination does not increase the risk of SIDS, and some studies have suggested that vaccination may lower the risk. There are several well-established risk factors for SIDS, such as putting the baby into bed in a prone (face-down) position and smoking by the parents. Major reductions in SIDS deaths can be attributed to successful campaigns that have focused on reducing these risk factors.
_
Myth: Hepatitis B vaccine causes multiple sclerosis.
Fact: There is no evidence that hepatitis B vaccine, or any other vaccine, causes multiple sclerosis (MS). There was concern about hepatitis B vaccination and MS in France in the 1990s. There were reports of MS or MS-like illness occurring after administration of hepatitis B vaccines in a large-scale vaccination program of adolescents/young adults, an age group where MS often first presents. The French government initially stopped the vaccination program. However, on further study, the rate of MS in vaccinated people was found to be the same as the usual rate of MS in the population. Numerous other studies performed around the world, and expert panels from the World Health Organization, the Institute of Medicine and the Centers for Disease Control and Prevention in the United States, agree that there is no evidence to support the theory that vaccination with hepatitis B vaccine, or any other vaccine, is associated with an increased risk of multiple sclerosis. There is also evidence that vaccination does not worsen the symptoms or cause relapses of MS.
_
Myth: Vaccination of young children can cause seizures.
Fact: Febrile convulsions (sometimes referred to as seizures) are a relatively common response to fever of any cause in young children. In most cases, these seizures are mild and improve on their own. Overall, by the age of five years, approximately three in every 100 children will have experienced a febrile convulsion, irrespective of whether a vaccine is given. As fever is a well-documented adverse event following the administration of many common childhood vaccines, it is not unexpected that febrile convulsions may occur following vaccination, although it is still very rare. The risk is higher following administration of certain vaccines, such as influenza, MMR and MMRV vaccines. Giving the seasonal influenza vaccine and 13-valent pneumococcal conjugate vaccine (PCV 13) at the same time may also be associated with an increased risk of febrile convulsions in young children, compared to when either vaccine is given separately. However, as this risk is still relatively low (18 additional cases for every 100,000 doses of these vaccines administered together), PCV 13 and seasonal influenza vaccine can still be given to children at the same visit. Health professionals should ensure that parents are aware of the risk and offer alternative vaccination options, such as vaccination on separate days, if parents are concerned.
_
Myth: There is a link between rotavirus vaccine and intussusceptions.
Fact: Intussusception is a “telescoping” of the intestine where one section slides inside another section. This can cut off the blood supply, block the intestine, and cause tears, infections, and death. Most cases are in young children. They have severe abdominal pain (intermittent at first), and pass blood in the stool, typically mixed with mucus and having the appearance of currant jelly. The baseline incidence of intussusception in children is on the order of 1-4 per 1,000. Most cases have no identified cause, but the most plausible candidate is hypertrophied lymphoid tissue resulting from viral illnesses, especially rotavirus infections. Rotavirus is the leading cause of severe diarrhea in infants and children worldwide, leading to more than half a million deaths each year in children under the age of 5. The first rotavirus vaccine, RotaShield, was found to have an association with intussusception. Two newer vaccines, Rotateq and Rotarix, were thought not to carry that risk, but two new trials have shown that they do. Still, the risk is small and the benefits of the vaccines are great. The first rotavirus vaccine, Rotashield, was introduced in 1999. It was withdrawn from the market within a year because post-marketing surveillance found 1-2 excess cases of intussusception per 10,000 recipients. Newer vaccines, Rotateq and Rotarix, were licensed only after testing (in over 60,000 infants each) failed to find any association with intussusception. Those trials were designed to have enough statistical power to detect a risk similar to that of RotaShield. Both new vaccines contain live, attenuated strains of virus and are given orally. Rotateq is a pentavalent (prepared from 5 strains) vaccine given in 3 doses at age 2, 4, and 6 months. Rotarix is monovalent (prepared from 1 strain) and is given in 2 doses at age 2 and 4 months. Either is recommended, but about 10 times more doses of the pentavalent vaccine have been administered. After the new vaccines came into common use, studies in other countries pointed to a small increase in intussusception with the newer vaccines, although at a much lower rate than with Rotashield. It is reasonable to conclude that intussusception can occur with either vaccine, but that the risk is low, on the order of 1-5 cases per 100,000 infants. As always, risks must be weighed against benefits, and the benefits of these vaccines are indisputable. An editorial in the NEJM estimated that each year in the US the vaccines have prevented 53,000 hospitalizations and 170,000 ER visits at the cost of 45 to 213 cases of intussusception. The ACIP estimates that 14 infant deaths are prevented each year in the US, and in Mexico deaths from diarrhea decreased by 40% after the vaccine program was implemented. Even the unvaccinated may benefit as they are exposed to fewer rotavirus infections in the community. The greatest benefits will be seen in Third World countries, but there is greater risk there too. If intussusception is promptly diagnosed and treated, it is relatively benign and has no lasting consequences; but adequate medical care may not be readily available in Third World countries. Another consideration: it’s possible that some infants may be protected from intussusceptions caused by the rotavirus itself. The benefits of rotavirus vaccination outweigh the risks associated with it, and a review of the risks and benefits carried out by the WHO reached the same conclusion. Rotavirus vaccines therefore continue to be recommended globally on the basis of this positive benefit to risk profile. Health professionals should ensure that rotavirus vaccine is not given to infants above specified upper age limits; the benefit and safety profile of vaccination in older children has not been established. They should also inform parents and carers of the rare risk of intussusception, how to be alert for the signs and symptoms of the condition, and what action to take.
_
Myth: Early batches of the polio virus were contaminated with SV40, a monkey virus that can cause cancer in humans.
Fact: It is true that early polio vaccine batches were contaminated with SV40. Polio can only replicate in cells, so monkey cells were used to get high enough numbers of the virus that vaccines could be made from. This accident resulted in spreading SV40 to millions of US residents between 1955 and 1963. The problem with the claim, however, is that there is no good evidence that these viruses cause cancer in humans. The present review of recent studies showed that the earlier results describing the recovery of SV40 DNA sequences from a large proportion of tumors were not reproducible and that most studies were negative. Contamination with laboratory plasmids was identified as a possible source of false positive results in some previous studies. The low-level immunoreactivity of human sera to SV40 was very likely the result of cross-reactivity with antibodies to the SV40-related human polyomaviruses BKV and JCV, rather than of authentic SV40 infection. SV40 sero-reactivity in patients with the suspect tumors was no greater than that in controls. In epidemiologic studies, the increased incidence of some of the suspect tumors in the 1970s to 1980s was not related to the risk of exposure to SV40-contaminated vaccines. In summary, the most recent evidence does not support the notion that SV40 contributed to the development of human cancers.
_
Myth: Vaccines contain preservatives that are dangerous.
Fact: Until recently, many vaccine concerns centered on the safety of thimerosal, a compound that prevents the vaccine from being contaminated by bacteria and contains a form of mercury called ethylmercury. Mercury in large quantities is known to be harmful to a child’s developing brain. Worries about thimerosal’s effect on children prompted its removal from nearly all childhood vaccines in 1999. (Thimerosal is still present in multi-dose flu vaccine—though you can ask your doctor for a thimerosal-free shot.) Yet it’s become clearer since then that ethylmercury does not pose the same health hazard as its cousin, methylmercury, a metal found in the environment that’s known to accumulate in the body and cause harm to developing children. The body is able to eliminate ethylmercury much more quickly than it can eliminate methylmercury. University of Rochester researchers confirmed that when they compared mercury concentrations in the urine, blood and stools of children who got vaccines containing thimerosal with those of kids who received only thimerosal-free vaccines. All the children had mercury levels well below the EPA’s most stringent public safety limits. Even if your baby received a vaccine that contained thimerosal, the overwhelming majority of data support a lack of association between the substance and neurological problems. Children are exposed to mercury from many environmental sources. The reality that a lot of people seem to miss is that the largest source of organic mercury is the environment: the air we breathe, the water we drink and the fish we eat. The total amount of mercury released by mankind in 2010 was estimated to be 1960 metric tons. The majority of this comes from coal burning and gold mining. It often contaminates air and water when emitted from factories as part of industrial waste. Because mercury contaminates waterways, it often ends up in fish. You can lessen your child’s mercury exposure by limiting the amount of fish he/she eats.
_
Myth: Vaccines are contraindicated in immunocompromised individuals.
Fact: The potential risks of live vaccines need to be weighed against the benefits in immunocompromised clients who may be particularly vulnerable to the vaccine-preventable disease. Concerns are that they may not respond adequately to subunit and inactivated vaccination and that live attenuated vaccines are potentially pathogenic. Although immunocompromised individuals who receive live viral vaccines (e.g., measles or varicella vaccines) or live bacterial vaccines (e.g., BCG vaccine) may develop disseminated infections with these attenuated pathogens, the risk of severe infection is greater after natural infection with wild-type viruses than immunization with highly attenuated viruses; therefore certain immunocompromised children should receive live viral vaccines. For example, children with human immunodeficiency virus (HIV) infection without severe T-cell deficiencies (Centers for Disease Control and Prevention class N1 or A1 and age-specific percentage of CD4+ lymphocytes greater than 25%) should receive the measles-mumps-rubella (MMR), and varicella vaccines.
_
Myth: It’s best to wait until children are older before starting to give them vaccines.
Fact: Immunization schedules are designed to protect the most vulnerable patients from disease. If you wait to give the vaccine, you may miss the window when a child is most vulnerable. When you get off the schedule, you really put your child at risk. Some parents delay vaccines out of a misinformed belief that it’s safer, but that decision actually increases the risk of a seizure after vaccination and leaves children at risk for disease longer. The risk of a febrile seizure following the MMR is approximately one case in 3,000 doses for children aged 12 to 15 months but one case in 1,500 doses for children aged 16 to 23 months. The best way to prevent disease and minimize side effects from vaccines is to vaccinate on the recommended schedule and an undervaccinated child is left at risk of infectious disease for a longer period. Delaying also makes for increased visits to the doctor’s office along with the time and hassle and risk of exposure to other infectious diseases in the doctor’s office.
_
Myth: Antibodies, the end product of vaccines, do not offer protection against pathogens. HIV is an example of this because those who are infected are diagnosed by screening for antibodies against the virus.
Fact: It is true that antibodies against HIV in people infected with it are not protective against the disease. Let me take you to brief synopsis of human immune system. There is humoral immunity and cell mediated immunity. Humoral immunity generates antibodies against foreign antigens of invading microbes. There are two types of antibodies, protective and non-protective antibodies. Protective antibodies against infectious agents are often neutralizing. They protect by neutralizing a function of the pathogen such as the harmful part of a toxin or a viral coat protein which binds to a cell-surface viral receptor on the host cells. Other protective antibodies are opsonizing and complement-fixating. Non-protective antibodies cannot eliminate pathogen although they bind on some antigen on microbes. Both protective and non-protective antibodies are used in diagnostic tests to diagnose infection. Humoral antibodies act on extracellular pathogens and their antigens. Cell mediated immunity acts on intracellular pathogens and their antigens. Both humoral and cell mediated immunity act in synergy with each other rather than independent of each other. Cell mediated immunity consists of helper T cells (CD4) and cytotoxic T cells (CD8). Helper T cells are Th1 and Th2 types. Antibody response using helper T cell is T cell dependent antibody response. Antibody response without using helper T cell is T cell independent antibody response which is weaker, shorter and without immune memory. Th1 type responses are considered paramount for targeting chronic infectious diseases that may have an intracellular stage for example with HIV, herpes viruses, hepatitis C virus, Helicobacter pylori, Plasmodium falciparum and Mycobacterium tuberculosis. HIV targets helper T cells and thereby prevents neutralizing antibody production. That is why antibodies generated against HIV are non-protective and useful only for diagnostic purpose. A vaccine-preventable disease is an infectious disease for which an effective preventive vaccine exists but not all infectious diseases are vaccine preventable. Many infectious diseases need vaccine than generate strong Th1 response rather than mere antibodies although all vaccines in principle stimulate both humoral and cellular component of adaptive immunity via engaging innate immunity. Another factor is a virus’s mutation rate. Out of thousands of viruses released per cell destroyed, there could be few having different versions of one antigen. If the antigen the body uses to recognize the pathogen changes enough, the virus could escape detection. That means although you have protective antibody, virus has mutated and protective antibody becomes non-protective.
_________
_________
The moral of the story:
_
1. A vaccine is a biological preparation that contains an agent made from weakened or killed forms of the disease causing microorganism, its toxins or one of its surface proteins and provides active adaptive immunity to prevent infectious disease. These are prophylactic vaccines. The administration of vaccines is called vaccination. Vaccines are administered to healthy humans often in the first year of life; therefore demands for safety and efficacy are very high.
_
2. Vaccines have classically been developed to prevent infectious diseases. However, now it is possible to consider immune-related approaches to the treatment of various diseases including autoimmune disorders, oncology, addiction, cardiovascular or neurological conditions. Therapeutic vaccines are meant to combat existing disease rather than offer lasting protection against infectious diseases as traditional vaccines do.
_
3. Vaccination averts 2 to 3 million deaths per year (in all age groups) worldwide and yet, up to 1.5 million children die each year due to diseases which could have been prevented by vaccination. One in five children worldwide is not protected with even the most basic vaccines. Half of unvaccinated children live in 3 countries: India, Nigeria and Pakistan.
_
4. Vaccine generate primary adaptive immune response with immunological memory (akin to asymptomatic/ inapparent natural infection e.g. hepatitis A/Polio) and prime body to mount robust secondary response upon subsequent exposure to the virus or bacterium to prevent disease. Vaccine prevents disease and not infection by priming immune system in the vaccine recipient, and through herd immunity prevents infection in unvaccinated individuals and reduces transmissibility (contagiousness) of infectious disease.
_
5. The immune system does not distinguish between natural infections and vaccines, forming an active response to both, so immunity induced via vaccination is similar to what would have occurred from contracting and recovering from the disease. Well-developed vaccines provide protection in a far safer way than natural infections, as vaccines generally do not cause the diseases they protect against and severe adverse effects are significantly less common than complications from natural infections. Bacterial and viral infections often predispose children and adults to severe, invasive infections with other pathogens. So vaccine not only prevents vaccine preventable disease but also prevents infections with other pathogens.
_
6. Vaccines give a wider kind of protection rather than preventing specific infectious disease due to wider-ranging influence on the immune system which include innate immune cells responding more strongly to bacterial and fungal pathogens completely unrelated to the microbe against which vaccine is used; and tipping of balance between Th1 and Th2 type of helper T-cells of adaptive immune system towards Th1 type helper T cell activation. That is why some vaccines reduce deaths from all other infections as well as reduce susceptibility to eczema and asthma. In Germany, a study of 496 vaccinated and unvaccinated children found that children who received immunizations against diphtheria, pertussis, tetanus, Hib, and polio within the first 3 months of life had fewer infections with vaccine-related and -unrelated pathogens than the unvaccinated group.
_
7. Herd immunity means the risk of infection among susceptible individuals in a population is reduced by presence and proximity of immune individuals through immunization. In other words, herd immunity means immune population protecting non-immune population by reducing transmissibility (contagiousness) of infectious disease. Herd immunity does not apply to all diseases, just those that are contagious, meaning that they can be transmitted from one individual to another. Tetanus, for example, is infectious but not contagious, so herd immunity does not apply to it. The logic of herd immunity is that greater the proportion of individuals in a community who are immune, the smaller the probability that those who are not immune will come into contact with an infectious individual. Infectious (contagious) diseases spread from one person to another person. If you are immune to disease by vaccination, you will neither get disease nor spread disease to anyone. The more people who are vaccinated, the fewer opportunities a disease has to spread. When vaccination coverage of population reaches herd immunity threshold level, the disease will cease to be endemic and by maintaining proportion of immune population above herd immunity threshold level, epidemics can be averted. Additionally many vaccines prevent carrier state thereby prevent spread of disease. The introduction of conjugate vaccines against Pneumococcal and Haemophilus infections in infants reduced incidence of disease among unvaccinated cohorts as conjugate vaccine not only protect against disease but also prevent nasal carriage thereby reducing contagiousness. Also, OPV viruses are transmitted by fecal-oral route to unvaccinated individuals and immunize them (contact immunization). Source drying is a related concept to herd immunity. If a particular subgroup is identified as the reservoir of infection, targeted vaccination will decrease disease in the whole population. The success of source drying justifies vaccination of special occupational groups, such as food handlers to control typhoid and hepatitis A.
_
8. Eradication of a disease is a more demanding goal than disease control, usually requiring reduction to zero cases in a defined geographic area. Eradication of a disease is achieved when its elimination can be sustained without ongoing interventions. Eradication of disease ought to be differentiated from elimination of disease. Elimination of disease means zero cases in a defined geographic area but need constant immunization program to sustain it. Small pox is eradicated so no small pox vaccine for population while polio is eliminated from most countries but yet need childhood polio vaccination in the same countries to sustain elimination. No vaccine is 100 % efficacious and no vaccine can be administered to 100 % population. Infectious disease eradication possibility is directly proportional to vaccine efficacy and quantum of vaccinated population but inversely proportional to basic reproduction number (Ro) of infectious disease. Additional factors are unique features of each disease, microbial life cycle and non-human reservoir of microorganism. Tetanus is unique among vaccine-preventable infectious diseases in that it is not contagious, herd immunity plays no part in protecting individuals or the community and the causative agent Clostridium tetani is ubiquitous in the environment not needing human/animal host thereby making tetanus eradication impossible.
_
9. Vaccination is not synonymous with immunization as vaccination does not guarantee immunity because everyone’s immune system reacts differently. Also, immunization does include the use of antitoxin/antisera, which contains preformed antibody (immunoglobulin) against e.g. diphtheria or tetanus exotoxins. Vaccination by definition is induction of active immunity while immunization includes both active and passive immunity. Nonetheless, vaccination and immunization are used interchangeably worldwide.
_
10. Immunogenicity of a vaccine is determined by measurement of specific antibodies and specific T cell activation. However, although antibody titers are often considered to represent adequate indicators of immune protection, they may not be the actual mechanism by which optimal protection is achieved. All micro-organisms have evolved complex defense mechanisms that interfere with every stage of the immune response and therefore vaccine must induce adaptive immune response that circumvents microbial defense rather than mere production of antibodies. There are protective and non-protective antibodies and if vaccine generates non-protective antibodies, it will not prevent disease despite conferring vaccine-induced seropositivity. Therefore, clinical trials are required to provide information about vaccine efficacy besides providing information about vaccine safety.
_
11. There are three immunological impediments to vaccine efficacy: tolerance, interference and neutralization. Immune tolerance refers to the induction of immunological non-responsiveness by repeated exposure to similar antigens. Immunological (immune) interference is defined as reduction in the immunogenicity of a vaccine antigen when it is administered as a component of a vaccine that includes multiple vaccine antigens or when it is administered separately or concurrently with another vaccine. Neutralization refers to presence of passively induced immunity e.g. which transferred from mother to foetus, where the vaccine antigen is neutralised by pre-existing maternally-derived antibody without triggering a host-derived immune response in the infant. Vaccine production, vaccine schedule and vaccine administration must ensure to reduce immunological impediments.
_
12. The immune response to vaccine is dependent on many factors including disease itself, match to circulating strains, type & dose of antigen, status of recipient immune system, biological including genetic factors of recipient, gut flora of recipient, coexisting diseases in recipient, age of immunization, prior exposure, time since vaccination, route of vaccine, type of vaccine, type of excipient (adjuvant, preservative, and stabilizer), use of carrier protein or vector, cold chain maintenance, vaccine schedule and immunological impediments to vaccine efficacy (tolerance, interference and neutralization).
_
13. In case of killed (inactivated) vaccines, there is only local and unilateral lymph node activation. Conversely for live attenuated vaccines (LAV), there is multifocal lymph node activation due to microbial replication and dissemination. Consequently the immunogenicity of killed vaccines is lower than the live vaccines; killed vaccines require adjuvants which improve the immune response by producing local inflammation and recruiting higher number of dendritic cells/ monocytes to the injection site. LAV vaccines produce a strong cellular and antibody responses and typically produce long-term immunity with only one to two doses of vaccine. However, because these vaccines contain living microorganisms, refrigeration is required to preserve potency; and there is a remote possibility of reversion to the original virulent form of the pathogenic agent. Inactivated/killed vaccines do not require refrigeration and can be freeze-dried for transport but these vaccines produce weaker immune responses, therefore additional booster shots are required to maintain immunity. This could be a drawback in areas where people don’t have regular access to health care and can’t get booster shots on time. Local reactions at the vaccine site are more common—this is often due to the adjuvant used to boost immune response.
_
14. Oral vaccines against polio, rotavirus, and cholera have showed a lower immunogenicity in individuals from developing countries as compared to individuals from developed countries because gut flora (microbiome) is unhealthy in people from lower socioeconomic strata due to excessive exposure to microorganisms.
_
15. Most routine childhood vaccines are effective in 85 to 95 per cent of recipients with higher efficacy in live attenuated vaccine and lower efficacy in inactivated/killed vaccines. That means that in every 100 children who receive a vaccine, between 5 and 15 of them may not develop protective immunity. But these can be protected by herd immunity provided the population of those who developed protective immunity is large enough to confer herd immunity.
_
16. Administration of plenty of vaccines does not overwhelm the immune system of a child. A child has the theoretical capacity to respond to about 10,000 vaccines at any one time. Although we now give children more vaccines, the actual number of antigens they receive has declined. Children respond to multiple vaccines given at the same time in a manner similar to individual vaccines. The 11 routinely recommended vaccines contain fewer than 130 antigens in total which is not even a significant number compared to the millions of viral and bacterial antigens that enter a child’s lungs every day.
_
17. The biggest advance in vaccine administration technique is needle-free vaccine patches. Besides preventing needle prick pain and its associated complications thereby increasing compliance, vaccine patches gives better immune response increasing vaccine efficacy without any need of cold chain.
_
18. Scheduling of vaccines maximize vaccine effectiveness and minimize number of interactions with health care system. Combination vaccines bring the added benefit of better compliance, coverage, and injection safety.
_
19. Vaccination with live attenuated virus or bacteria is contraindicated in pregnancy as live virus or bacteria can have adverse effect on developing fetus which may get infected with microbial replication. However, the actual potential for fetal damage remains theoretical. For example, numerous studies have demonstrated that accidental rubella vaccination during pregnancy did not result in an increased risk of birth defects. Also during pregnancy, immune system of mother is downgraded to allow fetal growth and live organisms could disseminate in mother.
_
20. An adverse event following immunization (AEFI) is any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavourable or unintended sign, abnormal laboratory finding, symptom or disease. AEFI can undermine confidence in a vaccine and ultimately have dramatic consequences for immunization coverage and disease incidence.
_
21. In 2011 the US Supreme Court ruled that vaccines are “unavoidably unsafe”. It is illogical to assume that every single adverse reaction following vaccination is caused by the vaccine, but it is also equally illogical to assume that none of them are caused by the vaccine. However, anecdotal report does not mean scientific evidence and legal culpability is different than medical culpability. In my view, court judges are ignorant about medical science and they do not understand interplay of thousands of biological variables in any medical event; some of the variables are known to us and some of them unknown; some of them are under our control and some are not. For example, viruses are constantly mutating and recombining with each other to generate novel strain of virus in order to survive i.e. circumvent host cell defense.
_
22. Whether vaccine indeed caused AEFI is determined by consistency, temporal relationship, biological plausibility, specificity and strength of association between administration of vaccine and adverse event; not to overlook confounding variables like vaccine production process & excipient, age, doses, case definitions and surveillance methods; and not to overlook background rate of adverse event in order to account for coincidental events. Background rate is rate of untoward medical events occurring in non-vaccinated population.
_
23. Although vaccines must undergo stringent safety tests before distribution, the trials typically don’t enroll enough people to catch risks on the order of one case per 10,000–100,000 people. The only way to find such side effects is to deploy the vaccine in the population and watch.
_
24. Risk of vaccine adverse event is far smaller than other daily risks of life like road traffic accident, lightening and adverse effects of common drugs; and much smaller than risk of disease itself. Vaccine is considered safe when vaccine benefit outweighs vaccine risk. Some vaccines are associated with serious AEFIs; however, these events are extremely rare and must be weighed against the protective benefits that vaccines provide. Experts understand risks in terms of numerical values and rates. In contrast to the perception of experts, parents want to know whether their healthy child could be the “one in a million” who develops encephalitis following immunization with measles vaccine.
_
25. When epidemic disease swept through schools, neighborhoods and communities, it was easy to persuade parents that the small risks associated with vaccination were worth it. When those epidemics stopped–because of widespread vaccinations–we forget that we still live in a dangerous world and it becomes very difficult to convince parents that the small risks associated with vaccination are worth it.
_
26. The adverse effect of vaccine gets too much attention and much less tolerated compared to adverse effect of any drug because vaccine is administered to a healthy child while drug is given to a sick child. Vaccines are therefore only licensed when the frequency of severe reactions is very rare and when only minor, self-limiting reactions are reported. A successful vaccine keeps even minor reactions to a minimum while producing the best possible immune response. The principle of ‘first do no harm’ has to be the responsibility of every doctor/nurse giving a vaccine to a healthy child. However no vaccine is 100 % safe and therefore if I enforce this principle, there would be no vaccination in the world. We have to balance between harm and benefits. If the probability of serious adverse event due to vaccination is lower than the probability of acquiring vaccine preventable disease with serious morbidity/complication, then vaccination is justified. For example, Vaccine-associated paralytic poliomyelitis (VAPP) occurs in an estimated 1 in 2.7 million children receiving their first dose of oral polio vaccine. If your chance of getting paralytic polio is more than 1 in 2.7 million, then taking OPV is justified. Polio was eradicated from the United State in 1979. From 1980 through 1999, there were 152 confirmed cases of paralytic polio reported in the USA. Of the 152 cases, 6 cases were imported from outside the USA, 2 were indeterminate, and the remaining 144 cases were vaccine-induced polio from the Oral Polio Vaccine (OPV). Therefore OPV was replaced in the USA by the Inactivated Polio Vaccine (IPV) in 2000. IPV cannot cause paralytic polio as virus is killed as oppose to OPV which contains live attenuated polio virus. However the same rationale cannot be implemented in many third world countries where sporadic polio cases do occur and where personal hygiene and sanitation are poor resulting in fecal-oral transmission of polio. Therefore OPV is justified despite slight risk of paralytic polio as transmission of OPV viruses via fecal-oral route will increase vaccine coverage and immunize more people.
_
Vaccine balance:
_
27. Both epidemiologic studies and biological research suggests that most individuals who experience a vaccine reaction (adverse reaction due to inherent properties of vaccine) have a preexisting susceptibility predominantly due to biological factors including genetic factors.
_
28. Vaccine-specific antibody responses and rates of vaccine-associated adverse reactions of children with mild or moderate illnesses are comparable to those of healthy children. Although a delay in vaccines is recommended for children with severe illnesses until the symptoms of illness resolve, this recommendation is not based on the likelihood that the child will have an inadequate immune response to the vaccine. Rather, the reason for deferring immunization is to avoid superimposing a reaction to the vaccine on the underlying illness or to mistakenly attribute a manifestation of the underlying illness to the vaccine.
_
29. Vaccines do not cause autism. Andrew Wakefield deliberately faked the study. Both epidemiological and biological studies fail to support these claims. A worldwide increase in the rate of autism diagnosis is likely driven by broadened diagnostic criteria and increased awareness.
_
30. Neither thiomersal (thimerosal) nor formaldehyde nor aluminum salts are harmful to children as very minute concentrations are present in vaccines. In fact children do ingest higher amount of mercury, formaldehyde and aluminum from other sources.
_
31. Robert Kennedy Jr. is spreading disinformation campaign against vaccines in United States. Multiple studies have rejected any link between the mercury-containing chemical thimerosal and autism. Autism rates have continued to rise even though we are not using thimerosal in vaccines for children except in multi-dose influenza vaccine. Children are exposed to mercury from many environmental sources. The reality that a lot of people seem to miss is that the largest source of organic mercury is the environment: the air we breathe, the water we drink and the fish we eat. The total amount of mercury released by mankind in 2010 was estimated to be 1960 metric tons. The majority of this comes from coal burning and gold mining. It often contaminates air and water when emitted from factories as part of industrial waste. Because mercury contaminates waterways, it often ends up in fish. If thimerosal caused autism due to its mercury content, then the air you breathe, the water you drink and the fish you eat also causes autism.
_
32. Anti-vaccination campaigners show a pattern of misrepresenting facts, reasoning flaws, falsifying data, mistrusting competent agencies (e.g. WHO, CDC), conspiratorial thinking, denialism, anecdotalism and scientific ignorance; and in my view the hallmark of anti-vaccine campaign is substitution of logic by emotions. Vaccination is not the only issue where logic is substituted by emotions. Sports, entertainment, politics and religion are other areas where logic is substituted by emotions.
_
33. Whether you have the viral infection or you get the live attenuated vaccine, you shed live virus in your body fluids and you are able to transmit the virus to other people who come in contact with your body fluids. However, shedding of viruses in vaccines typically occurs in lower amounts than during shedding of wild-type viruses and shed viruses cause mild or no disease as they are attenuated. Severely immunocompromised patients should avoid contact with people who have recently received live attenuated vaccine. Also some vaccines although prevent disease cannot prevent asymptomatic carrier state (e.g. pertussis vaccine) and may lead to spread of disease.
_
34. There are claims of research fraud on vaccine efficacy and withholding data on vaccine reactions by vaccine manufacturers; therefore independent researchers should conduct clinical trials on safety and efficacy of vaccines rather than manufacturer of vaccine.
_
35. Vaccine failure must be distinguished from vaccine resistance. Vaccine failure means vaccine failed to evoke immune response against microorganism for which it was developed but vaccine resistance means microorganism itself has changed (mutated or replaced by another strain) to evade immune response. When a subject is stimulating their immune system by influenza vaccine to build up specific antibodies; they are only doing this for a specific strain of influenza virus. This allows other strains of influenza to have more influence when a subject comes in contact with them in real time. The influenza vaccine may also encourage viruses to mutate faster to survive; making vaccine recipients more susceptible to more powerful strains in the future. Both vaccine failure and vaccine resistance reduce vaccine efficacy.
_
36. Although microbial adaptation may occur leading to spread of vaccine resistant strains by mutation (Hepatitis B vaccine), reversion to virulence (OPV) or strain replacement (PCV 7), it is rare. On the other hand, during natural infection, it takes time for immune system to kill virus and that time allows virus multiplication and subsequent mutation and spread of mutated virus while during infection of immunized individual, the immune response is quick and robust, thereby killing viruses fast before it has chance to mutate. In other words, vaccination has a greater ability to prevent virus mutation as compared to natural infection.
_
37. Relationship between vaccination and antibiotic resistance is complex. On one hand vaccination reduces development of antibiotic resistance by decreasing the likelihood that bacteria targeted by certain vaccines would be exposed to antimicrobial agents. On the other hand, following widespread vaccination with PCV 7, other pneumococcal strains that were not included in the vaccine became much more likely to cause pneumococcal infections. One of these, 19S, was known to be multi-antibiotic resistant. In other words, in order to prevent development of antibiotic resistant strains of bacteria, we need a vaccine that covers most of the prevalent strains of bacteria including antibiotic resistant strain.
_
38. For any new vaccine, it takes about 8 to 17 years from research to registration which include animal and human studies (phase I, II and III) and costs about ½ to 1 billion dollar to vaccine manufacturer. Safety, efficacy and cost effectiveness for a given region are important issues for introduction of newer vaccines.
_
39. Vaccination is the most cost effective public health measure and $1 spent in vaccine could yield anywhere from $10 to $45 in benefits to society. The cost-effectiveness of immunization has made vaccines increasingly affordable for low-income countries. Harvard school of public health study in 2005 finds vaccines boost the economies of poor countries.
_
40. The benefits of vaccination extend beyond prevention of specific diseases in individuals. They enable a rich, multifaceted harvest for societies and nations. Vaccines help disease control, elimination and eradication; control of mortality, morbidity, severity and complications of diseases; prevent infection and diseases in unvaccinated population; decrease health care expenditures; prevent antibiotic resistance; increase life expectancy; ensure safe travel; improve school attendance; promote economic growth, empower women and enhance social equity.
_
41. Although improved hygiene, nutrition, sanitation and standard of living does lower incidence of some infectious diseases, vaccines are overwhelmingly responsible for the largest drops in infectious disease rates worldwide. Polio is spread by fecal-oral route and despite poor hygiene & poor sanitation in India, Polio is eliminated from India due to pulse polio campaign established by government administering free OPV to all children. On the other hand, Hepatitis A spread by the same fecal-oral route is prevalent in India as its vaccine is not given freely by government and only those who can afford it uses it.
_
42. Vaccination should not be stopped as vaccine preventable diseases have become rare due to following reasons:
a. Vaccines do not protect only you but also protect your community through herd immunity.
b. Herd immunity is important as no vaccine is 100 % effective and 5 to 15 % people who are not immunized despite vaccination need protection plus there are small number of people who cannot receive vaccines due to various reasons.
c. Vaccine preventable diseases may be rare in your community but exist elsewhere in world and can be imported in your community.
d. Unless a disease has completely disappeared, there is a real risk that small outbreaks can turn into large epidemics if most of the community is not protected.
e. Vaccination is not only for today but also to protect future generations.
f. Anti-vaccine movement in the United States has brought back clusters of measles, mumps, whooping cough and chickenpox in the US. Rare diseases have made come back. We could soon find ourselves battling epidemics of diseases we thought we had conquered if vaccination is stopped.
g. Although tetanus is rare, its bacteria clostridium tetani survive in environment and do not need humans/animal host, so immunization against tetanus must be continued indefinitely even if you don’t see a single case of tetanus.
h. Unvaccinated children have a much greater chance of getting disease than children who have received the vaccine. For example, it has been shown in the United States that unvaccinated children have 22 to 35 times the risk of contracting measles and 6 times the risk of contracting whooping cough than vaccinated children. Moreover unvaccinated children may transmit contagious diseases to children who cannot receive the vaccine or to those who have only partial immunity, especially infants. The larger the population of unvaccinated children, the weaker the herd immunity, and if the percentage of vaccinated children falls below a certain point herd immunity basically collapses.
i. People who are not immunized can be carriers of disease and pose a risk to those around them, even if they do not get sick themselves.
_
43. A free-rider is someone who accepts a benefit without paying for it. Free-rider in vaccination is unvaccinated individual getting protection from disease due to herd immunity. So others get vaccinated, also get some adverse effects of vaccines and free-rider gets immunity without spending anything or risking anything. Individuals may choose to free ride for a variety of reasons including bandwagoning or groupthinking, social norms or peer pressure, religious beliefs, perceived effectiveness of a vaccine, mistrust of vaccines or public health officials, flawed assessment of infection and vaccine risks. Most importantly though is that individuals are more likely to free ride if vaccination rates are high enough so as to convince a person that he or she may not need to be immune since a sufficient number of others already are.
_
44. Democracies are built on freedom & liberty and we should have the freedom to make choices about our healthcare. However, we cannot risk making choices that endanger lives of others. By refusing vaccination on any non-medical ground, we are reducing herd immunity, substantially increasing risks even to vaccinated individuals as no vaccine is 100% effective and most routine childhood vaccines are effective for 85% to 95% of recipients. No one should say that few children with adverse events are being sacrificed for the health of others because rates of complication from vaccines are so low that the benefit of vaccines for each individual child is higher than the risk of a poor outcome as complications are more likely to arise from illness rather than from vaccination. At the same time, enforced mandatory vaccination should be selective for certain diseases that pose threat to society (highly communicable and serious disease) rather than all vaccines invented by scientists. No parent should be punished for not vaccinating their child. Punishing people for not vaccinating could undermine trust between public health officials and community. Instead emphasis should be on educating parents and giving incentives for vaccination. It cannot be overemphasized that unvaccinated children must be kept out of school in the event of an outbreak.
__________
__________
Dr. Rajiv Desai. MD.
May 8, 2015
________
Postscript:
India is world’s largest manufacturer of vaccines, but it is sad that the country has the largest number of unvaccinated children close to seven million. I request every parent to read this article on ‘Vaccine’ and give all vaccines to their children as per vaccine schedule. Please do not get emotionally misled by pseudoscience of anti-vaccine campaign. Science on vaccination is imperfect but imperfection is better than quackery.
_________



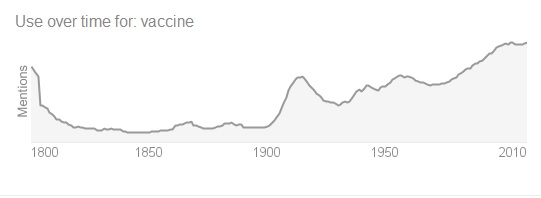
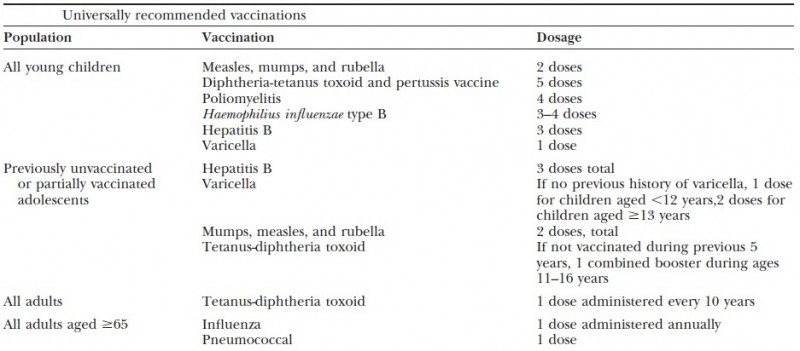
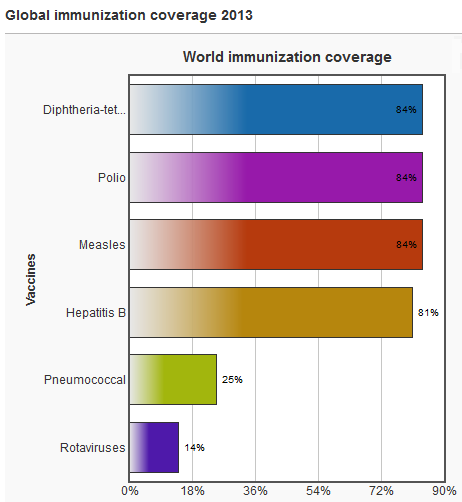
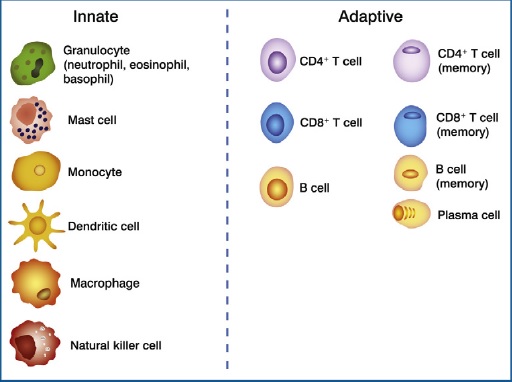
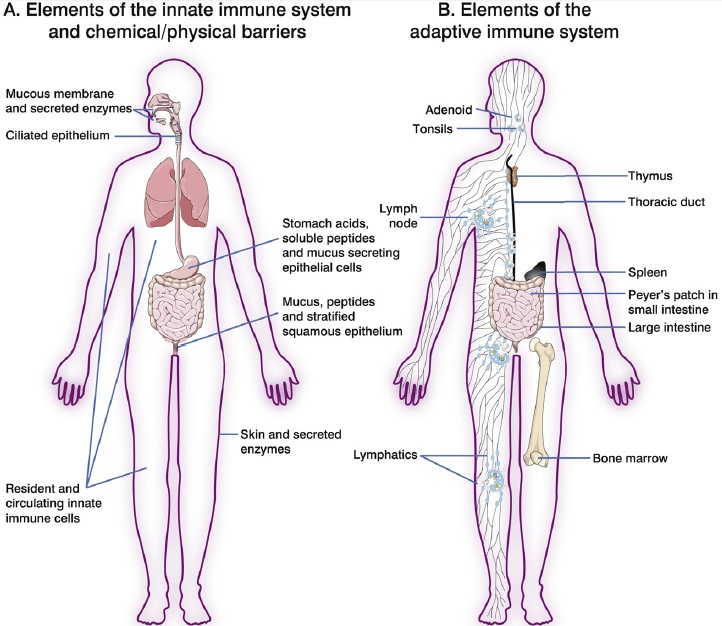
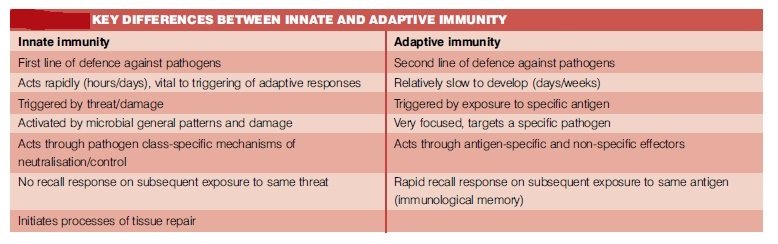
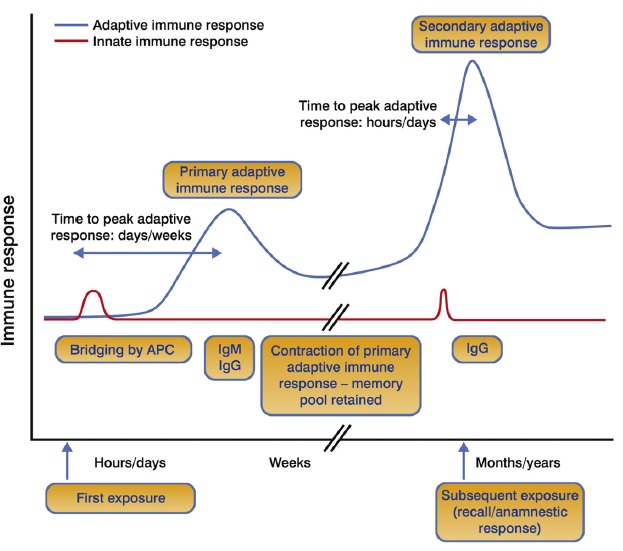
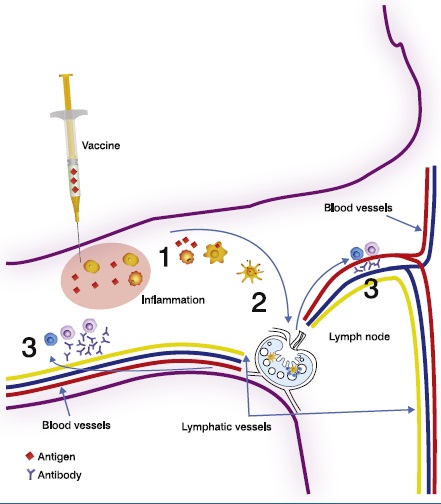
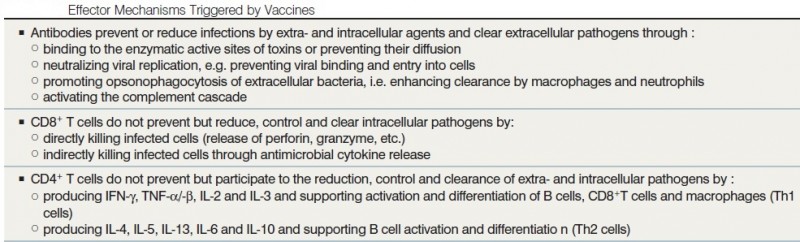
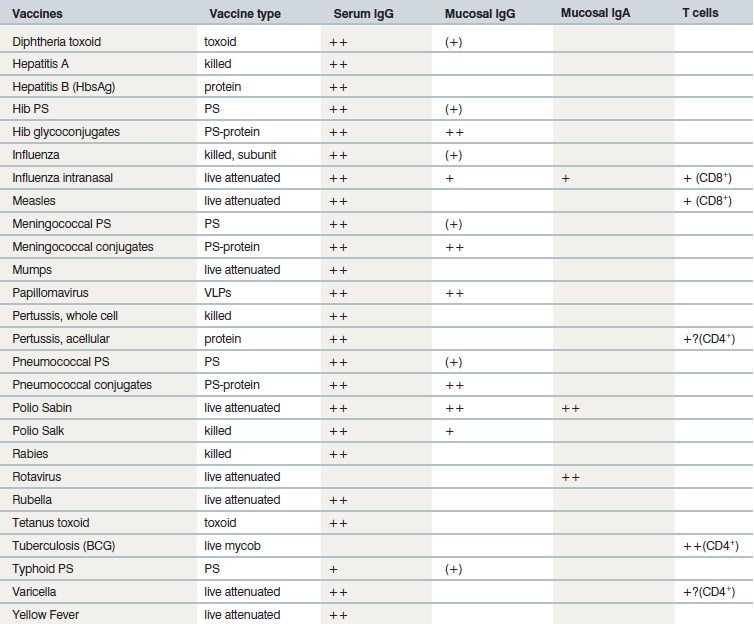
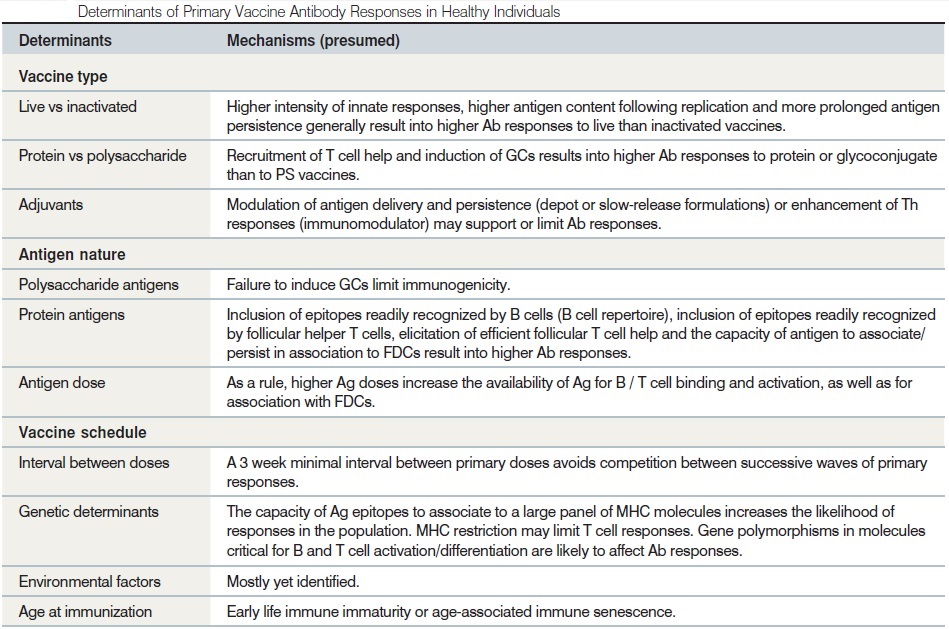
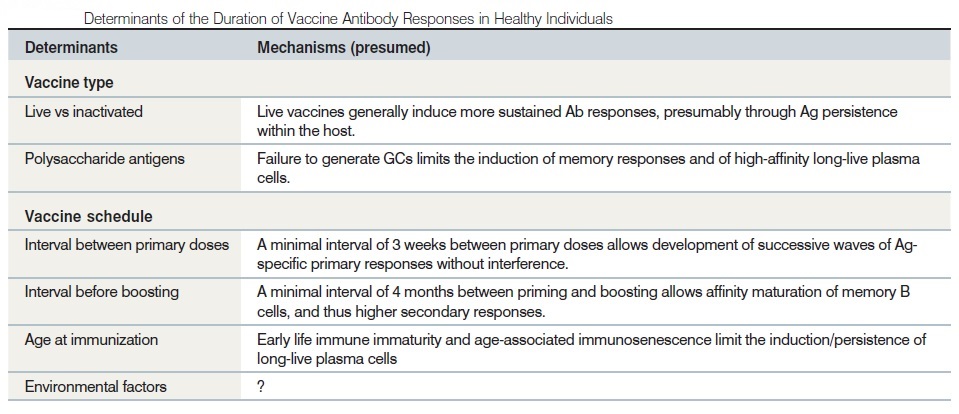
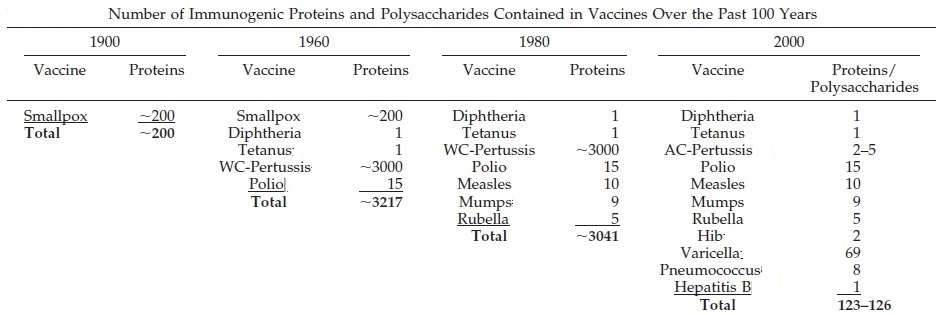
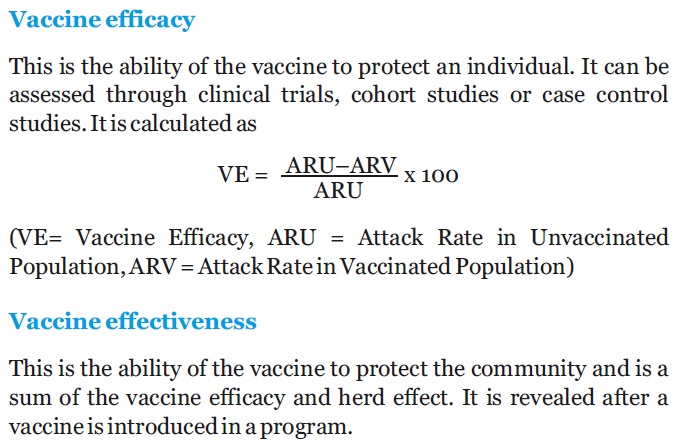
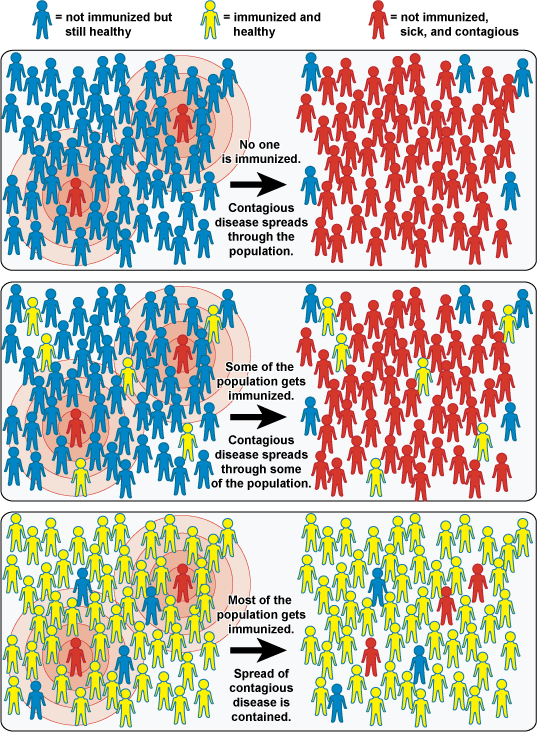
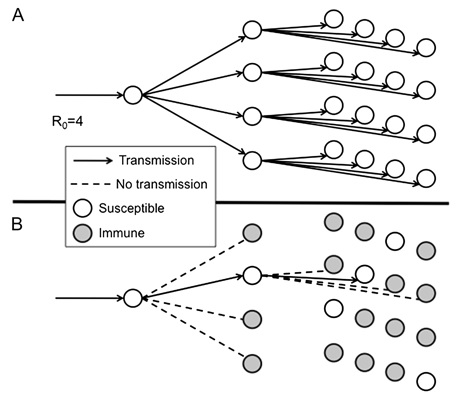
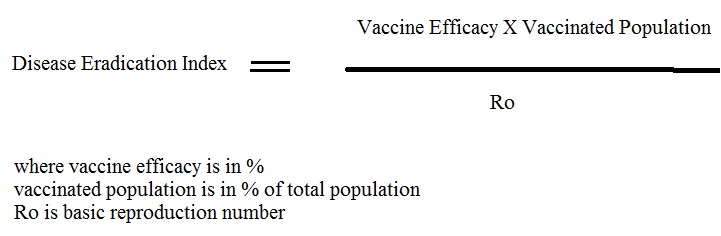
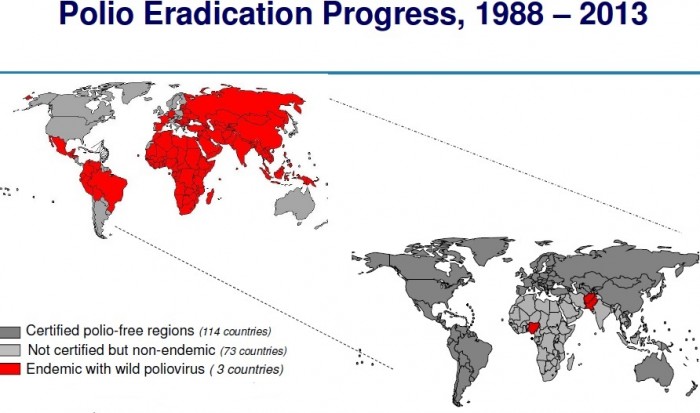
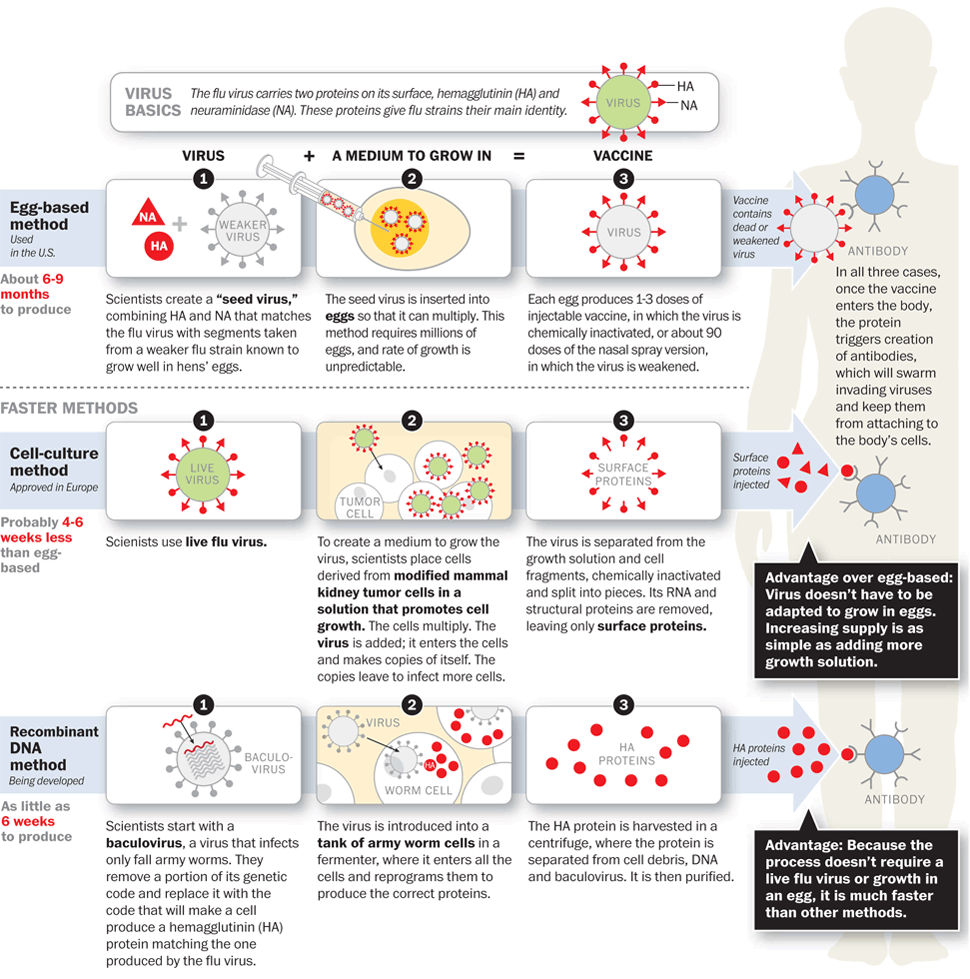
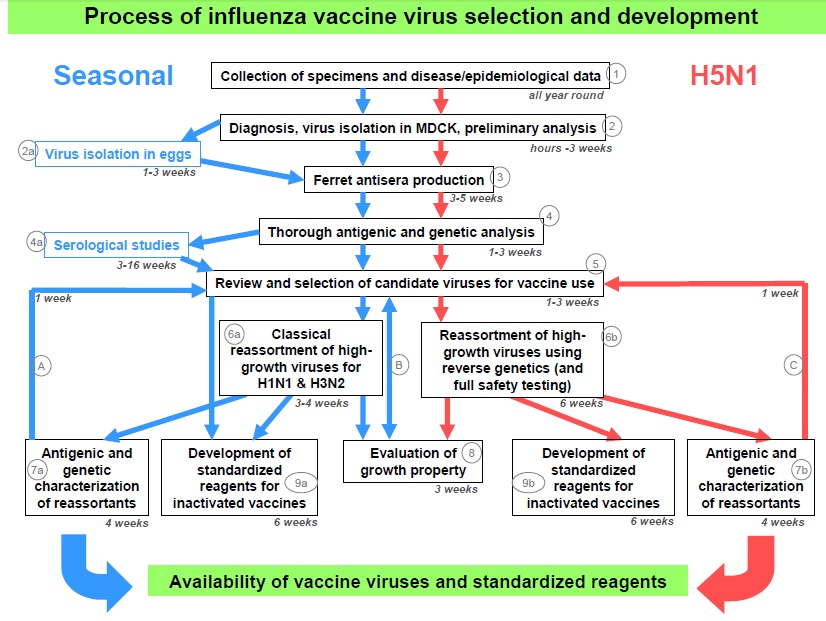
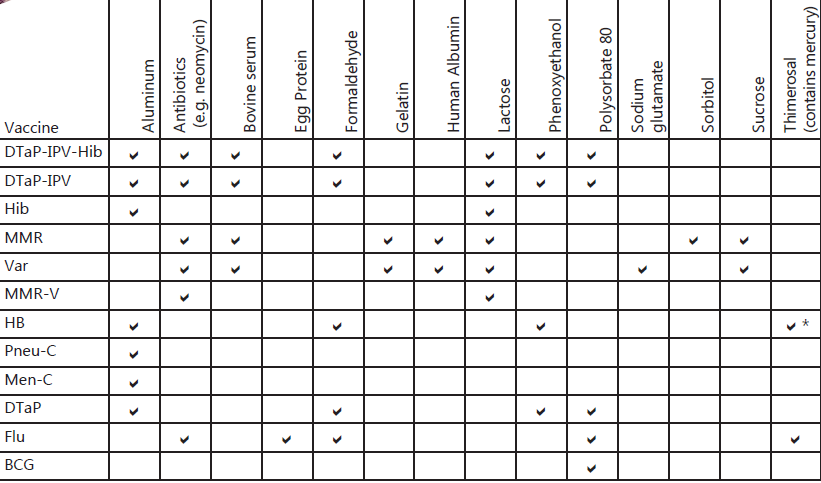

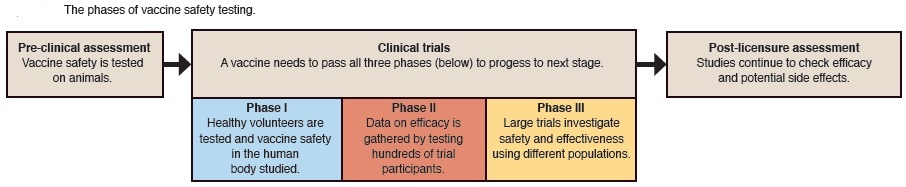
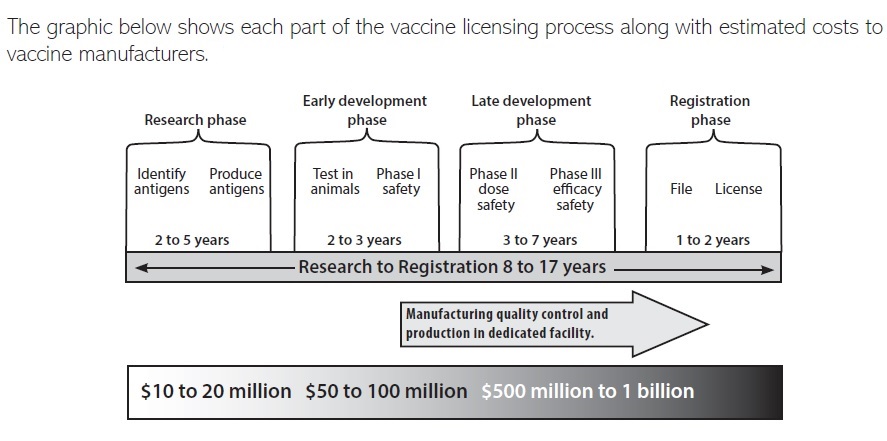
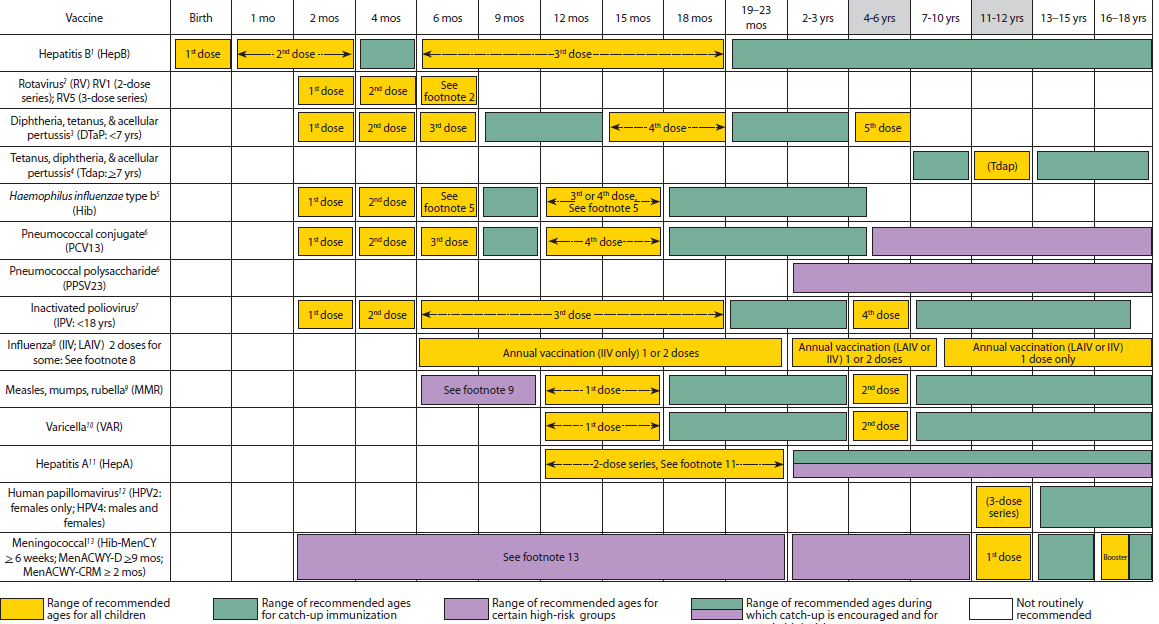
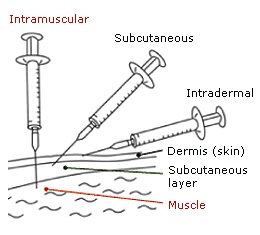
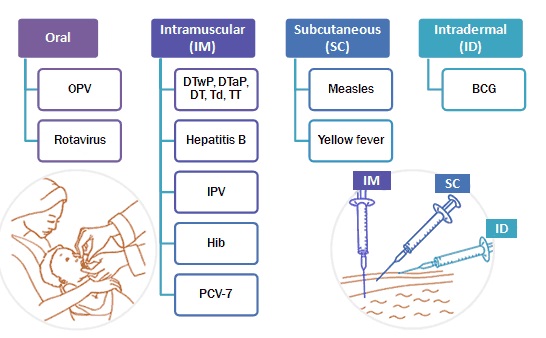
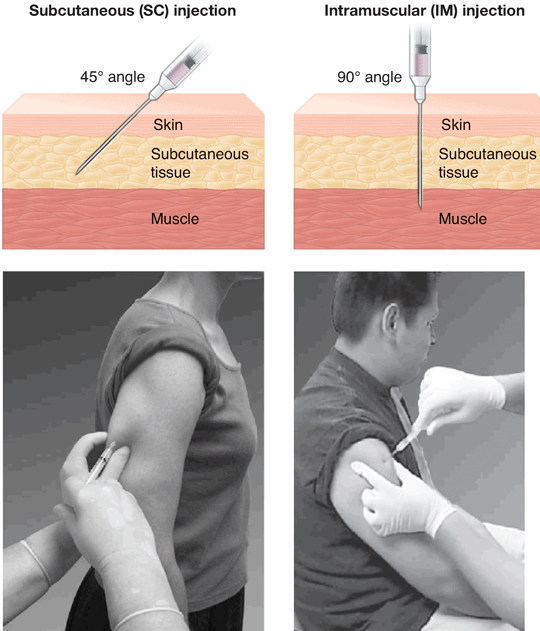
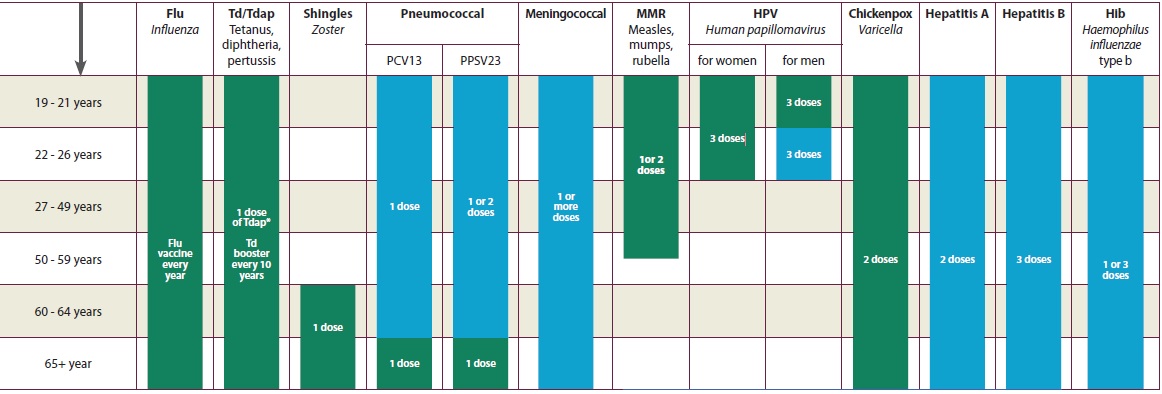

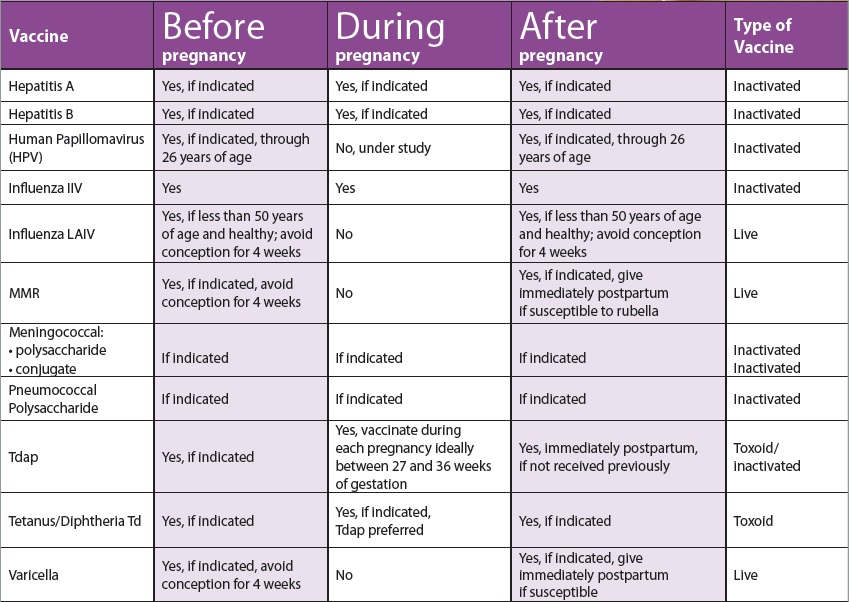
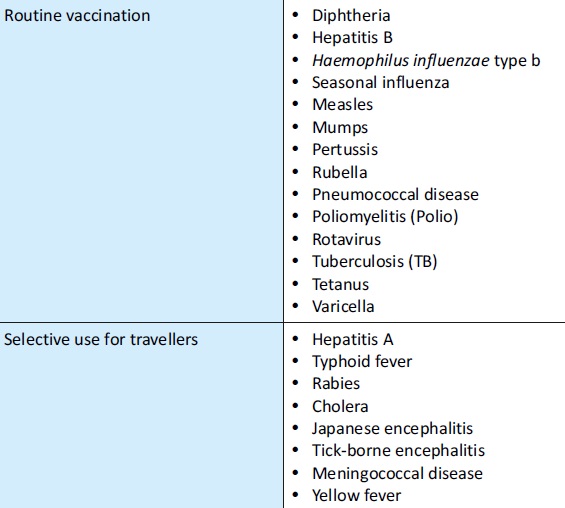
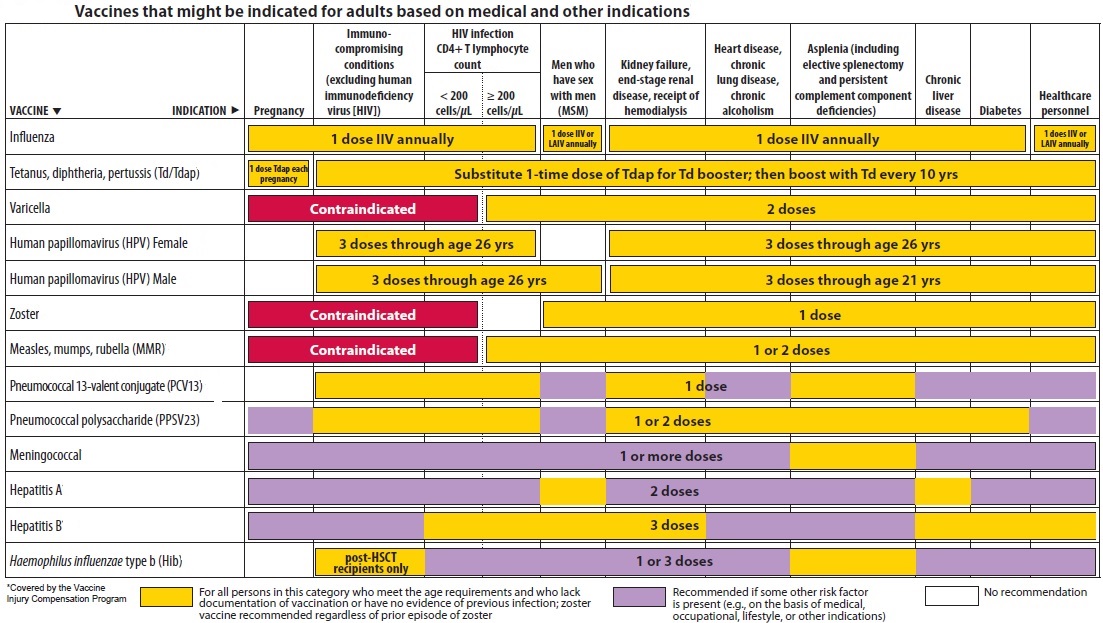
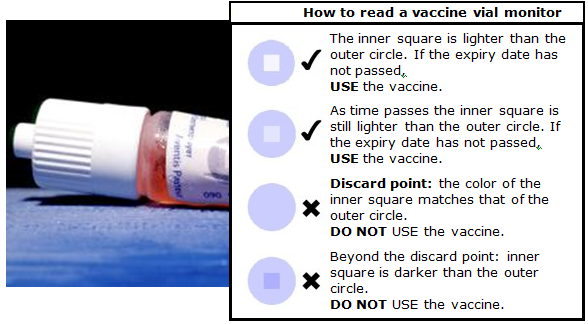
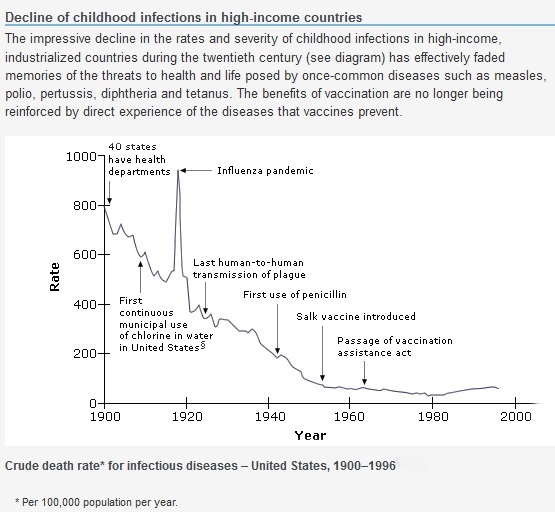
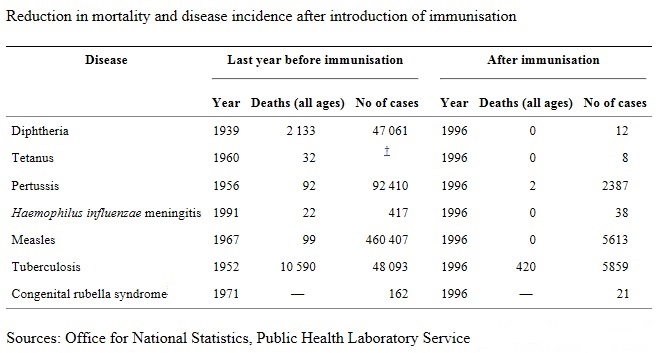

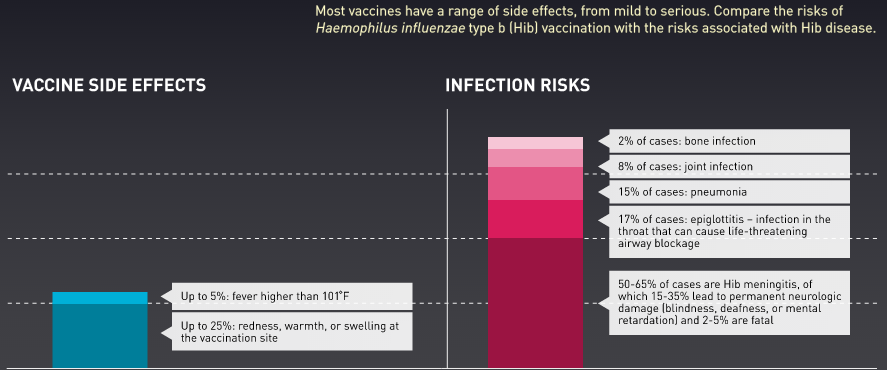
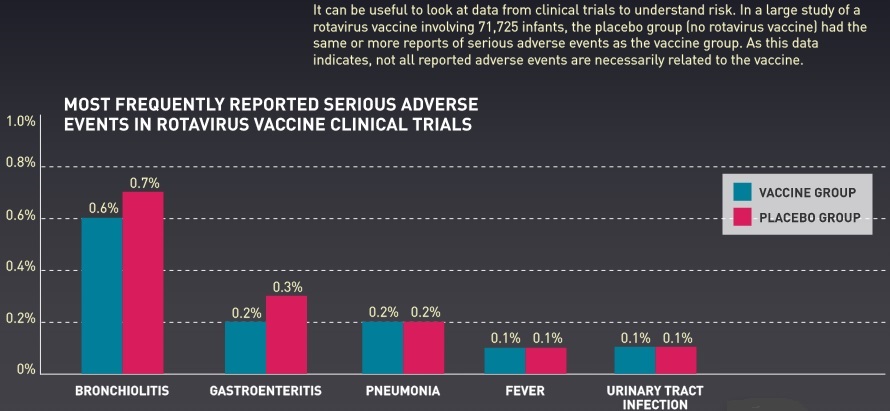
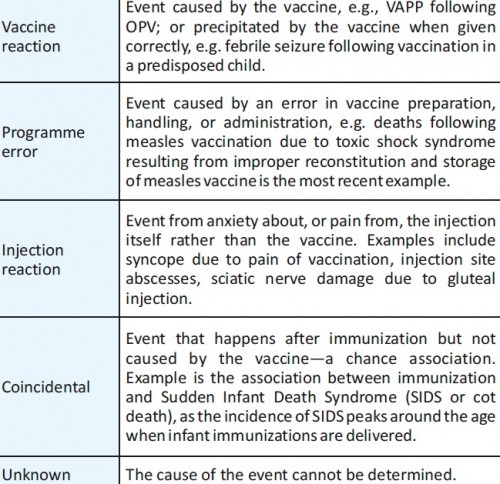
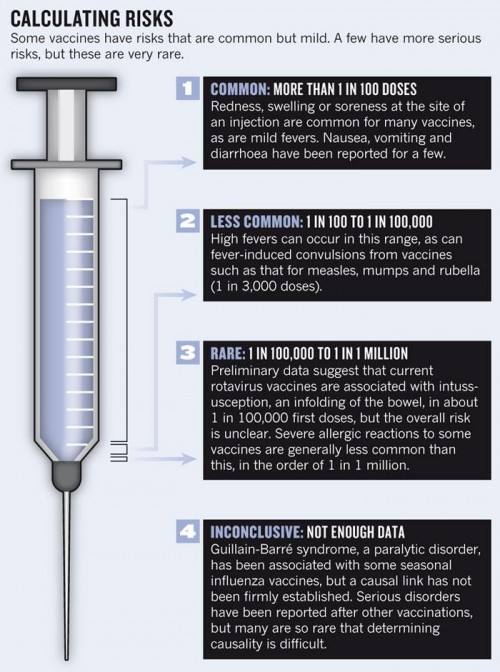
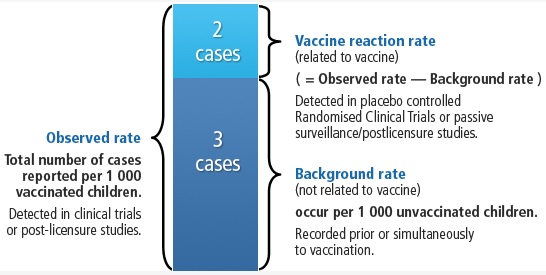
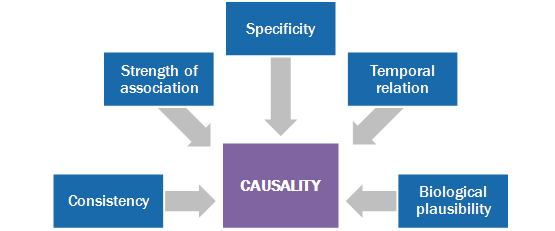
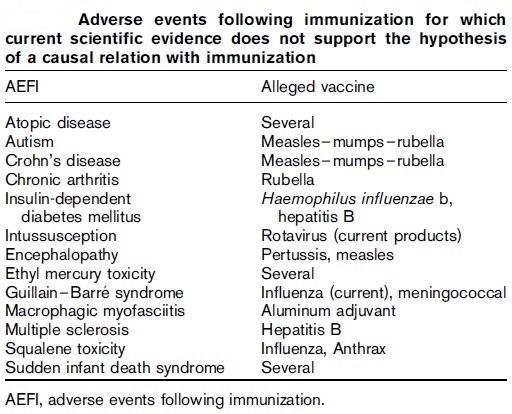
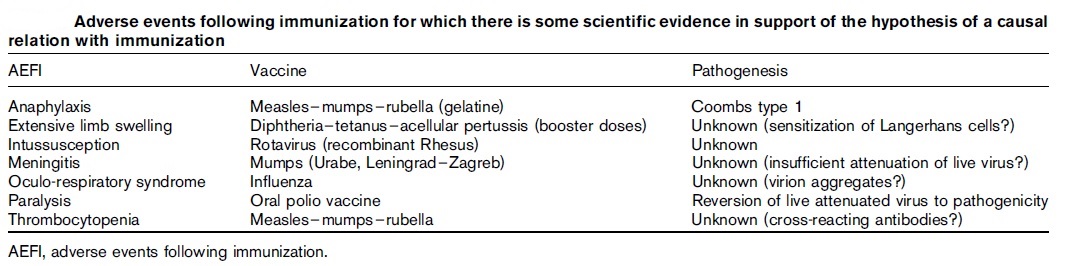
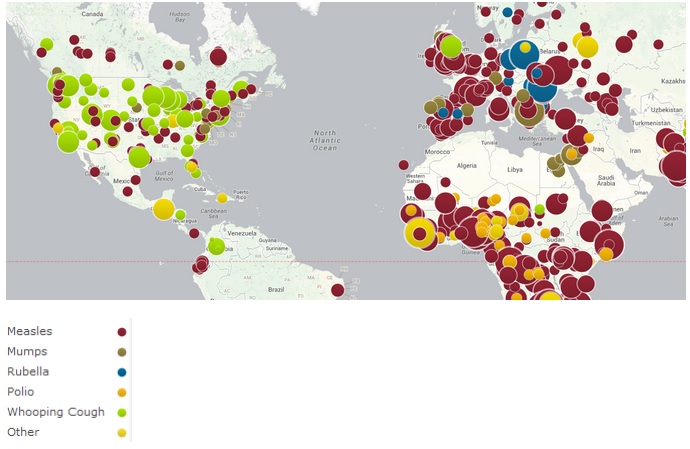

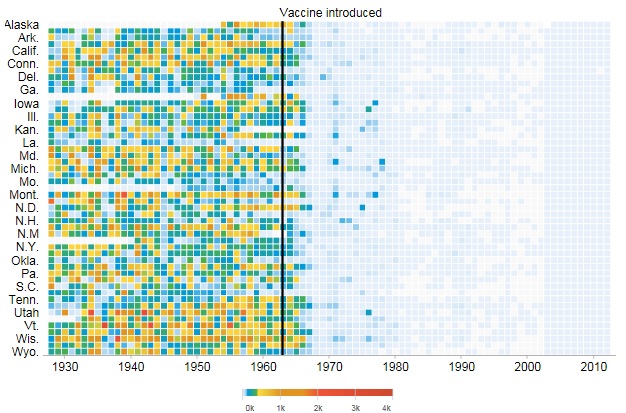
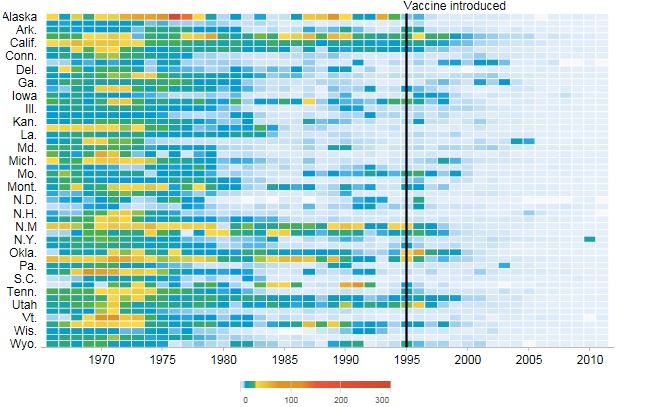
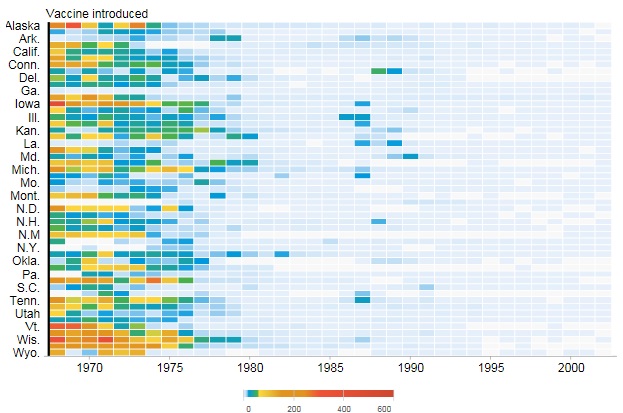
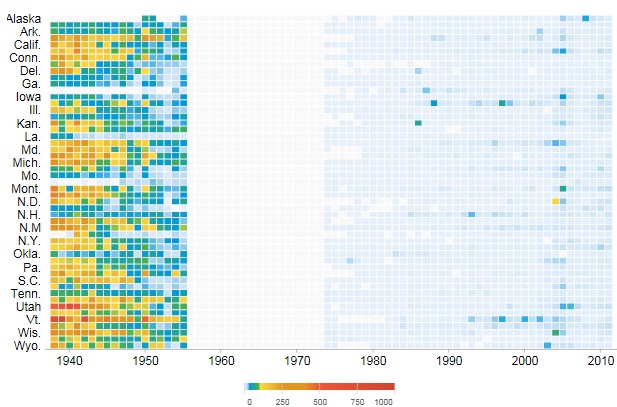
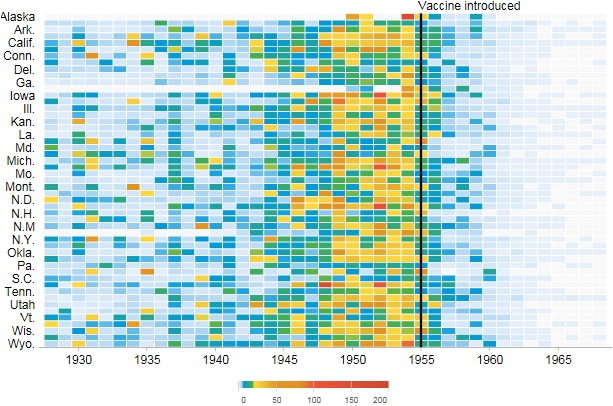
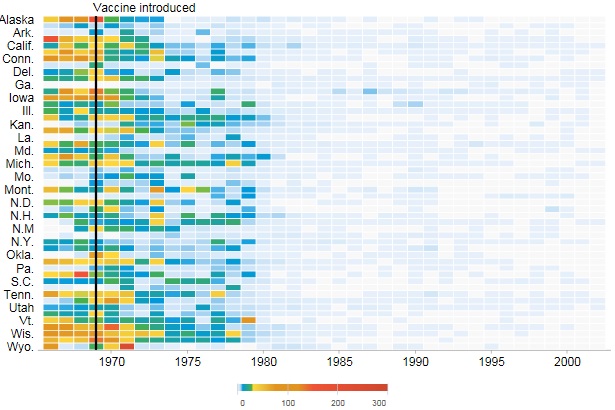
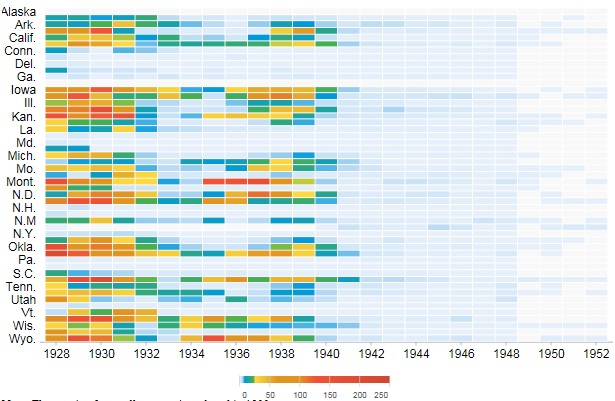
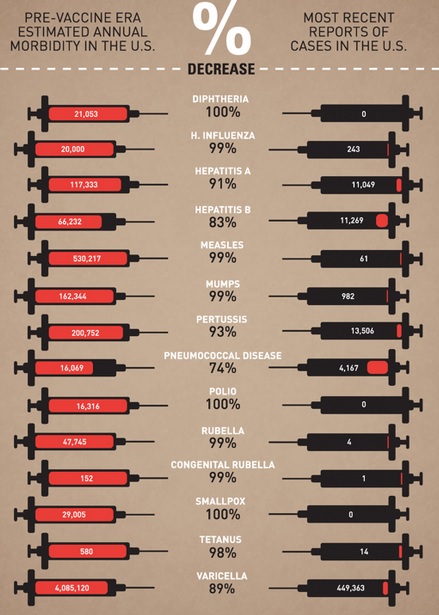
When some one searches for his required thing, therefore he/she desires
to be available that in detail, so that thing is
maintained over here.
I read this paragraph fully about the difference
of newest and previous technologies, it’s amazing article.
An outstanding share! I’ve just forwarded this onto a colleague who was doing a little research on this. And he in fact bought me breakfast because I stumbled upon it for him… lol. So let me reword this…. Thanks for the meal!! But yeah, thanks for spending the time to talk about this issue here on your blog.
Paragraph writing is also a excitement, if you be familiar with after that you can writeif not it is difficult to write.
wow, awesome blog.Much thanks again. Great.
You made some really good points there. I looked on the web to find
out more about the issue and found most people will go along with your views on this site.
Just desire to say your article iis as astounding. The clarity in your submit is just cool and i
could suppose you’re an expert in this subject. Well together with your permission let me
to take hold of your RSSfeed to stay up to date witth forthcoming post.
Thank you one million and please continue the enjoyable work.
Your method of describing all in his post is actuzlly fastidious,
all can without difficulty be aware of it, Thanks a lot.
Magnificent beat ! I wish to apprentice while you amend your web site, how could i subscribe for
a blog site? The account aided me a applicable deal.
I were a little bit familiar of this your broadcast offered shiny
transparent concept
Exceptional post however I was wondering if you could write a litte
more on this subject? I’d be very thankful if you could elaborate a
little bit further. Thank you!
Very energetic blog, I loved that bit. Will there be a part 2?
I agree with you
Excellent post. I was checking constantly this blog and I’m
impressed! Very useful info specifically the last part
🙂 I care for such info much. I was looking for this particular info for
a long time. Thank you and best of luck.
I agree with you
This post is worth everyone’s attention. How can I find
out more?
This is my first time pay a quick visit at here and i am really impressed to read everthing at
alone place.
Really appreciate you sharing this article post. Fantastic.
Excellent web site you have here.. It’s difficult to find excellent writing like
yours nowadays. I really appreciate people like you!
Take care!!
Ahaa, its fastidious dialogue regarding this piece of writing here
at this website, I have read all that, so at this time me also commenting here.
very good submit, i certainly love this website, keep on it
Sweet blog! I found it while browsing on Yahoo News. Do you have any suggestions on how to get listed
in Yahoo News? I’ve been trying for a while but I never seem
to get there! Cheers
Thanks for the good writeup. It in reality was a enjoyment account
it. Glance complex to far added agreeable from you!
However, how can we keep in touch?
I think this is among the most important info for me.
And i am glad reading your article. But should remark on few general things, The web site
style is ideal, the articles is really excellent : D.
Good job, cheers
Somebody essentially help to make severely articles I might state.
This is the first time I frequented your website page and thus far?
I surprised with the research you made to make this actual post incredible.
Wonderful task!
Article writing is also a excitement, if you know after that you
can write otherwise it is difficult to write.
Great article post.Much thanks again. Really Great.
Thanks so much for the blog. Cool.
“Wow, marvelous blog format! How lengthy have you ever been running a blog for? you made running a blog glance easy. The total glance of your web site is fantastic, let alone the content material!”
Looking forward to reading more. Great blog post.Really looking forward to read more. Want more.
Say, you got a nice blog.Thanks Again. Great.
Very informative article post.Really thank you!
Very good article post.Really looking forward to read more. Fantastic.
Thanks for the blog post.Really thank you! Cool.
Great, thanks for sharing this post. Awesome.
“Enjoyed every bit of your blog.Thanks Again. Fantastic.”
Does your website have a contact page? I’m having a tough time locating it but, I’d
like to shoot you an email. I’ve got some creative
ideas for your blog you might be interested in hearing. Either way, great blog and I look forward
to seeing it improve over time.
“Thanks so much for the blog post.Really looking forward to read more. Really Great.”
I have been examinating out many of your stories and it’s pretty good stuff.
I will surely bookmark your site.
Hello colleagues, how is all, and what you want
to say about this piece of writing, in my view its really remarkable designed for me.
I read this post completely about the difference of most recent and previous technologies,
it’s amazing article.
I am extremely impressed with your writing skills and also with the layout
on your weblog. Is this a paid theme or did you modify it yourself?
Either way keep up the excellent quality writing, it is rare to see a great blog like this
one today.
Awesome write-up. I am a normal visitor of your website and appreciate you taking the time to maintain the excellent site. I’ll be a regular visitor for a long time.
I’m basically pleased with this particular blog and I wished to take the
time to thank you from the bottom of my heart!
I really like reading sites similar to this! Causes me to feel a warm enjoyable experience and also educated!
Thank you so much!
Normally I don’t learn article on blogs, but I wish to say that this write-up very forced
me to try and do it! Your writing taste has been amazed me.
Thank you, quite nice post.
What’s up, yup this paragraph is genuinely pleasant and I have
learned lot of things from it concerning blogging. thanks.
Stunning! That you don’t come by info such
as this easily and that I am not so ungrateful!
Maintain it up folks!