Dr Rajiv Desai
An Educational Blog
EBOLA
EBOLA:
________
_______
Prologue:
The most dangerous outbreak of an emerging infectious disease since the appearance of HIV seems to have begun on December 6, 2013, in the village of Meliandou, in Guinea, in West Africa, with the death of a two-year-old boy who was suffering from diarrhea and a fever. We now know that he was infected with Ebola virus. After Ebola infected the boy, it went from him to his mother, who died, to his three-year-old sister, who died, and to their grandmother, who died, and then it left the village and began moving through the human population of Guinea, Liberia, and Sierra Leone. Ebola virus is one of a group of zoonotic viruses that can cause severe disease in humans. The virus is known as a “zoonotic” virus because it’s transmitted to humans from animals. With pressures from a growing global population, climate change, deforestation, urbanization and uneven economic growth, zoonotic diseases are only likely to increase. The current 2014 epidemic is caused by the Zaire strain of Ebola virus, which has a mortality of 50 to 90%. Ebola virus is of great public health importance because of its ability to spread to carers and healthcare workers, the often high case fatality rate (CFR), difficulties in its rapid recognition, and the lack of effective specific treatment. Ebola first appeared in 1976 in a village near the Ebola River in the Democratic Republic of Congo (former Zaire). Forty years ago, Ebola was just the name of a river. It was a small waterway of no particularly sinister character that flowed through northern Zaire, not far from the village hospital where the first known outbreak of a new viral disease had been centered. That river gave its name to the new virus, and now “Ebola” is a global byword for ugly death, misery, and fear of contagion. The always frightening and often contradictory messages – and rumors – prompt patients to avoid going to the hospital due to fear of isolation and lack of effective treatments. It becomes impossible to identify the cases, confirm diagnosis, protect and monitor contacts. Violent protests – with loss of life, involving sometimes the medical staff – have been reported in some outbreaks. The disease threatens humanity by preying on humanity. Without seeing a single Ebola case, I am writing on Ebola as I felt that it is the most important topic that concerns the world as we move from 2014 into 2015.
______
Ebola the virus that scares even scientists:
The figure above shows a researcher working with the Ebola virus while wearing a BSL-4 positive pressure suit to avoid infection. Everything entering or leaving the level 4 laboratory, even the air, is strictly controlled. When a level 4 agent is in the chambers, the air inside is kept at a slightly lower pressure, so a leak causes air to be sucked in rather than blown out. Any changes in pressure, for instance resulting from a pinprick in a rubber glove, cause an alarm to sound. A Russian scientist at a former Soviet biological weapons laboratory in Siberia has died in 2004 after accidentally sticking herself with a needle laced with ebola, the deadly virus for which there is no vaccine or treatment.
______
Abbreviations and synonyms:
EVD = Ebola virus disease = Ebola hemorrhagic fever (EHF) = Ebola
EBVO = Ebola virus
ZEBOV = Zaire ebola virus
SEBOV = Sudan ebola virus
TEBOV = Tai Forest ebola virus
REBOV = Reston ebola virus
BEBOV = Bundibugyo ebola virus
R0 = Ro =RO = R ‘naught’ = Basic reproduction number
GP = Glycoprotein present on the surface of ebola virus
PPE = Personal protective equipment = biohazard suit
_____
In this article, Ebola means EVD caused by ZEBOV unless specified otherwise.
_____
On September 18th 2014, the United Nations Security Council held its first-ever emergency meeting on a health crisis. A Liberian man named Jackson Naimah spoke to the Council via video link from Liberia. Jackson works for Médecins Sans Frontières (MSF), and is a team leader in one of MSF’s Ebola treatment centers in Monrovia. He told the Council that he had lost a niece and a cousin to the virus – both of them nurses infected at work. He said that, as he was speaking to us, sick people were outside the gates of the MSF clinic, begging to be let in and treated. MSF had to turn them away, because they had no more beds. Jackson said, “I feel that the future of my country is hanging in the balance. If the international community does not stand up, we will be wiped out.”
_
We all are familiar with the statistics of what Ebola has done to Liberia, Guinea, and Sierra Leone. More than 10,000 people infected. More than 5,000 people killed, nearly 250 of them health professionals. More than 4,000 children orphaned. Governments of affected countries were initially in denial over the occurrence of the disease. Subsequently, they relinquished responsibility for the care of infected patients to overworked international non-governmental organisations and issued incoherent directives, such as the closure of markets and borders. The Ebola outbreak has now become so serious that health infrastructure is beginning to collapse and hospitals are closing. Without effective medical care patients are dying not only of Ebola but of malaria, diarrhoea, and other conditions. As the Ebola epidemic in West Africa has spiraled out of control, affecting thousands of Liberians, Sierra Leonians, and Guineans, and threatening thousands more, the world’s reaction has been glacially, lethally slow. Only in the past few weeks have heads of state begun to take serious notice. There is no Ebola Vaccine because “the virus previously affected only poor African nations”, WHO chief Dr Margaret Chan says. She criticized drugs companies for turning their backs on markets that cannot pay.
______
Introduction to Ebola:
Ebola typically strikes like the worst and most humiliating flu you could imagine. People get the sweats, along with body aches and pains. Then they start vomiting and having uncontrollable diarrhea. These symptoms can appear anywhere between 2 and 21 days after exposure to the virus. Sometimes, they go into shock. Sometimes, they bleed. In fatal cases, death comes fairly quickly — within a few days or a couple of weeks of getting sick. Survivors return to a normal life after a months-long recovery that can include periods of hair loss, sensory changes, weakness, fatigue, headaches, eye and liver inflammation.
_
Ebola, previously known as Ebola hemorrhagic fever, is a rare and deadly disease caused by infection with one of the Ebola virus strains. Ebola can cause disease in humans and nonhuman primates (monkeys, gorillas, and chimpanzees). Ebola is caused by infection with a virus of the family Filoviridae, genus Ebolavirus. There are five identified Ebola virus species, four of which are known to cause disease in humans: Ebola virus (Zaire ebolavirus); Sudan virus (Sudan ebolavirus); Taï Forest virus (Taï Forest ebolavirus, formerly Côte d’Ivoire ebolavirus); and Bundibugyo virus (Bundibugyo ebolavirus). The fifth, Reston virus (Reston ebolavirus), has caused disease in nonhuman primates, but not in humans. Ebola viruses are found in several African countries. Ebola was first discovered in 1976 near the Ebola River in what is now the Democratic Republic of the Congo. Since then, outbreaks have appeared sporadically in Africa. The natural reservoir host of Ebola virus remains unknown. However, on the basis of evidence and the nature of similar viruses, researchers believe that the virus is animal-borne and that bats are the most likely reservoir. Four of the five virus strains occur in an animal host native to Africa. Ebola hemorrhagic fever (EHF) is one of the most severe viral infections of humans. In outbreaks in central Africa caused by the Zaire species of ebolavirus (ZEBOV), the mortality rate among identified cases has reached 80–90%, while fatalities in epidemics caused by the Sudan species have been in the range of 50–60% (Bwaka et al., 1999; Sanchez et al., 2004). The natural reservoir of these agents has not been identified; humans are only accidental or “dead-end” hosts (Mahanty & Bray, 2004). Ebola virus causes an acute infection. The infection lasts for about two weeks and then it is over. If the patient is lucky, he or she survives, but unfortunately, in many cases, the patients die. But unlike other viruses, including HIV, Ebola virus does not persist in the infected patient. In this aspect it behaves more like the influenza virus. You get ill and a couple of days later it is over—one way or another.
______
What is a virus?
Viruses are particles of nucleic acids, either RNA or DNA, which are surrounded by proteins and sometimes additionally by lipid membranes. With few exemptions, viruses are very small, about 100 times smaller than bacteria. Most importantly, viruses have a parasitic life style and must infect living cells to reproduce. They hijack cellular machineries to amplify their genomes and produce their own proteins and membranes. Virus is alive yet dead, simple yet complex, mindless yet prophetic, seemingly able to anticipate our every move. For scientists who study the evolution and behavior of viruses, the Ebola pathogen is performing true to its vast, ancient and staggeringly diverse kind. By all evidence, researchers say, viruses have been parasitizing living cells since the first cells arose on earth nearly four billion years ago. Some researchers go so far as to suggest that viruses predate their hosts. That they essentially invented cells as a reliable and renewable resource they could then exploit for the sake of making new viral particles. Researchers are deeply impressed by the depth and breadth of the viral universe, or virome. Viruses have managed to infiltrate the cells of every life form known to science. They infect animals, plants, bacteria, slime mold, even larger viruses. As so-called obligate parasites entirely dependent on host cells to replicate their tiny genomes and fabricate their protein packages newborn viruses, or virions, must find their way to fresh hosts or they will quickly fall apart, especially when exposed to sun, air or salt. Viruses are masters at making their way from host to host and cell to cell, using every possible channel. Viruses are also notable for what they lack. They have no ribosomes, the cellular components that fabricate the proteins that do all the work of keeping cells alive. Instead, viruses carry instructions for co-opting the ribosomes of their host, and repurposing them to the job of churning out capsid and other viral proteins. Other host components are enlisted to help copy the instructions for building new viruses, in the form of DNA or RNA, and to install those concise nucleic texts in the newly constructed capsids. Viruses also work tirelessly to evade the immune system that seeks to destroy them. One of the deadliest features of the Ebola virus is its capacity to cripple the body’s first line of defense against a new pathogen, by blocking the release of interferon. That gives the virus a big advantage to grow and spread. Yet the real lethality of Ebola stems from a case of mistaken location, a zoonotic jump from wild animal to human being. The normal host for Ebola virus is the fruit bat, in which the virus replicates at a moderate pace without killing or noticeably sickening the bat. A perfect parasite is able to replicate and not kill its host. The Ebola virus is the perfect parasite for a bat.
_
The virus needs to replicate inside an infected cell. Like many other viruses, Ebola virus brings its own genome replication machinery into the cell. If we understand how this machinery works, we can identify targets for antiviral compounds that block viral replication. This approach has been used successfully for other viruses, such as herpes viruses and HIV. Ebola virus infects specific cells of the immune system that are needed to fight the virus at an early stage of infection. In these cells, Ebola virus blocks antiviral pathways and reprograms the cells in a way that they are not able to respond to the infection effectively. On top of this, the infected cells are used as vessels to transport the virus to almost all organs of the body, where it infects additional cells. This leads to a so-called systemic infection with the devastating consequences for which Ebola virus infections are notorious. Therefore, our goal is to identify antiviral pathways that can be activated in Ebola virus–infected cells, which would lead to the destruction of the infected cells before the virus can spread further. For example, researchers have tested an antiviral drug that selectively induces a suicide program in virus-infected cells. They showed that it kills Ebola virus–infected cells without harming uninfected cells. If the infected cells are eliminated, the virus cannot spread through the body anymore.
_____
The viral haemorrhagic fevers (VHFs) are caused by four types of ribonucleic acid (RNA) virus:
Although agents that cause viral hemorrhagic fever syndrome constitute a geographically diverse group of viruses, all of those identified to date are RNA viruses with a lipid envelope, all are considered zoonoses, all damage the microvasculature (resulting in increased vascular permeability), and all are members of one of the following 4 families:
•Filoviruses cause Ebola and Marburg.
•Arenaviruses cause Lassa fever, Argentine haemorrhagic fever (HF), Bolivian HF, Brazilian HF and Venezuelan HF.
•Bunyaviruses cause Korean HF (Hantavirus), Rift Valley fever (RVF) and Crimean-Congo HF (CCHF).
•Flaviviruses cause yellow fever and dengue fever.
They are all infectious and lead to a potentially fatal disease with fever, malaise, vomiting, mucosal and gastrointestinal bleeding, oedema, and hypotension. Many VHF viruses are virulent, and some are highly infectious (e.g., filoviruses and arenaviruses) with person-to-person transmission from direct contact with infected blood and bodily secretions. Effective therapies and prophylaxis are extremely limited for VHF; therefore, early detection and strict adherence to infection control measures are essential. Ebola virus is one of at least 30 known viruses capable of causing viral hemorrhagic fever syndrome.
_
A New Virus Emerges:
In the summer of 1976, Ngoy Mushola, a doctor from Bumba, Zaire, traveled to Yambuku, a town on the shores of the Ebola river. There, at a local hospital, Mushola recorded the first clinical description of a new disease that was killing almost all of the patients who contracted it. “The illness is characterized with a high temperature of about 39°C, hematemesis [the vomiting of blood], diarrhea with blood, abdominal pain, prostration with “heavy” articulations, and rapid evolution death after a mean of three days,” he wrote in his daily log. The illness, which was later named Ebola hemorrhagic fever after the nearby river, was successfully contained in Zaire over the course of a few months, but not before 318 people contracted the virus. Nearly 90 percent of the victims died within a few days of becoming infected. Hundreds of miles away, in Maridi and Nzara, two cities in the southern tip of Sudan, doctors were witnessing an outbreak, describing patients with high fevers, aches, nausea, bleeding, delirium, and what they termed a “mask-like” or “ghost-like” face. Two hundred and eighty-four were infected and over half died. One of the main risk-factors associated with Ebola virus in the Sudan outbreak was caring for the sick. The disease was spread within hospitals, and many medical care personnel were infected. In several of the Ebola hemorrhagic fever outbreaks that have followed, health care workers have been at risk, and there have been many documented cases of doctors and nurses contracting Ebola virus from the patients they were tending. Scientists and laboratory personnel working with the live virus are also at risk, and a few months after the Sudan outbreak, a scientist working with the virus in England became infected after he accidentally stuck himself with an infected needle.
_
Virulent and Rare:
The virus is intriguing because it acts so quickly. It kills people in two weeks or less. As deadly as Ebola virus is, it has never sustained a large outbreak, probably due to its speed of action and how powerfully sick it makes people. Even as case-fatality can approach 90 percent, infected patients become bed-ridden while they are most infectious, and infection is spread only through direct contact with bodily fluids. Thus, patients are easily quarantined and outbreaks contained. Humans are the unlikely target. Humans are not the natural reservoir for Ebola virus, but merely incidental or accidental hosts. Ebola and Lassa are both non-human viruses. They are persistent in animal populations in the wild, and remain in this animal “reservoir” population because they are not deadly enough to kill the infected animals—an evolutionary advantage for a virus to remain endemic in its host species population.
_
Deadly features of Ebola:
•Its kill rate: In this particular outbreak of 2014, a running tabulation suggests that 54 percent of the infected die, though adjusted numbers suggest that the rate is much higher.
•Its exponential growth: At this point, the number of people infected is doubling approximately every three weeks.
•The gruesomeness with which it kills: by hijacking cells and migrating throughout the body to affect all organs, causing victims to bleed profusely.
•The ease with which it is transmitted: through contact with bodily fluids, including sweat, tears, saliva, blood, urine, vomitus, semen, etc., including objects that have come in contact with bodily fluids (such as bed sheets, clothing, and needles) and corpses.
•The threat of mutation: Prominent figures have expressed serious concerns that this disease will go airborne, and there are many other mechanisms through which mutation might make it much more transmissible.
_
Ebola BSL 4 pathogen:
Because of its high mortality rate, EBOV is listed as a select agent, World Health Organization Risk Group 4 Pathogen (requiring Biosafety Level 4-equivalent containment), a U.S. National Institutes of Health/National Institute of Allergy and Infectious Diseases Category A Priority Pathogen, U.S. CDC Centers for Disease Control and Prevention Category A Bioterrorism Agent, and listed as a Biological Agent for Export Control by the Australia Group. Viruses are ranked on a biosafety level (BSL) scale from 1 – 4, with 4 being the most severe. Ebola is a BSL4 pathogen, for which there are no approved therapeutics or vaccines. The virus is transmitted from one individual to another through the exchange of bodily fluids and enters the body through exposed cuts or mucous membranes, such as an individual’s mouth or nose.
_________
Case definition and contact tracing:
Ebola virus disease (EVD; also Ebola hemorrhagic fever, or EHF), or simply Ebola, is a disease of humans and other primates caused by ebola viruses. Signs and symptoms typically start between two days and three weeks after contracting the virus as a fever, sore throat, muscle pain, and headaches. Then, vomiting, diarrhea and rash usually follow, along with decreased function of the liver and kidneys. At this time some people begin to bleed both internally and externally. Death rates can vary widely, with death occurring in about 50% of cases. This is often due to low blood pressure from fluid loss, and typically follows six to sixteen days after symptoms appear. Surveillance is also a problem. The case definition that was adopted was accurate in the epidemic setting, but it would be much less so in sporadic infections or at the beginning of an epidemic. The finding of copious amounts of Ebola virus antigen in skin opened the way to confirm cases by taking simple skin biopsies, which could be placed in formalin and analyzed later by immunohistochemistry. This obviates the need for cold chain or special precautions while processing or shipping infectious material. One could argue that Ebola diagnostics should be placed at many sites in the potentially endemic areas, but this may be unrealistic given the small number of expected cases and the economics.
_
Case definition of Ebola Virus Disease (EVD):
| Name | Definition |
| Index case | Very first case (probable or confirmed, see below) found to be the origin of the outbreak |
| Alert case | Any person with sudden onset of high fever or sudden death or bleeding or bloody diarrhea or blood in urine |
| Suspect case (person under investigation) | Any person, dead or alive, who present (or presented before the death): |
| (i) fever (>38.5°C or 101.5 °F) with additional symptoms (severe headache, muscle pain, vomiting, diarrhea, abdominal pain, or unexplained hemorrhage) and (ii) epidemiologic risk factors within the past 21 days before the onset of symptoms (close contact with body fluids of a suspect or probable case of EVD, or direct handling of bush animals from disease-endemic areas) | |
| Probable case | Person with symptoms compatible with EVD, as evaluated by a clinician, or a dead person with an epidemiological link with a confirmed case |
| Contacts | Person without suggestive symptom of the disease, but who has been in contact with a suspect or probable case of EVD (living in the same house, provided care during the illness, participated in the burial rites etc.). It should be important to assess the risk level. |
| If laboratory samples are obtained at an appropriate time during the illness, the previous notification categories should be reclassified as “laboratory-confirmed” cases and “not a case” | |
| Confirmed case | Case with positive laboratory response for either PCR or viral isolation or Ebola virus antigen or Ebola antibody |
| “Not a case” | Person with no Ebola-specific detectable antibody or antigen |
_
Contacts of dead or sick animals:
Any person having been exposure to a sick or dead animal in at least one of the following ways:
– has had direct physical contact with the animal
– has had direct contact with the animal’s blood or body fluids
– has carved up the animal
– has eaten raw bush-meat
Provided that this exposure has taken place less than 21 days before the identification as a contact by surveillance teams
_
Laboratory contacts:
Any person having been exposed to biological material in a laboratory in at least one of the following ways:
– has had direct contact with specimens collected from suspected Ebola patients
– has had direct contact with specimens collected from suspected Ebola animal cases
Provided that this exposure has taken place less than 21 days before the identification as a contact by surveillance teams
_
The contact person should be followed for 21 days after exposure. If the contact person is asymptomatic for 21 days after exposure, he is released from follow-up.
_
Contact tracing:
Contact tracing is considered important to contain an outbreak. It involves finding everyone who had close contact with infected individuals and watching for signs of illness for 21 days. If any of these contacts comes down with the disease, they should be isolated, tested and treated. Then the process is repeated by tracing the contacts’ contacts. Social mobilization and culturally appropriate health education efforts are critical to successful case identification and tracking of contacts.
_
Why contact tracing so important for ebola:
The table below shows risk associated with types of contact:
__________
Epidemiology, ecology and outbreak of ebola:
Epidemiology of ebola:
The first cases of filovirus haemorrhagic fever were reported in 1967 in Germany and the former Yugoslavia, and the causative agent was identified as Marburg virus (named after the German city where it was first seen in researchers who caught it from imported non-human primates). Similar cases of haemorrhagic fever were described in 1976 from outbreaks in two neighbouring locations: first in southern Sudan and subsequently in northern Zaire, now Democratic Republic of the Congo (DRC). An unknown causative agent was isolated from patients in both outbreaks and named Ebola virus after a small river in northwestern DRC. These two epidemics were caused by two distinct species of Ebola virus, Sudan Ebola virus and Zaire Ebola virus, a fact not recognised until years later. The third African Ebola virus species, Côte d’Ivoire Ebola virus was discovered in 1994. The virus was isolated from an infected ethnologist who had worked in the Tai Forest reserve in Côte d’Ivoire and had done a necropsy on a chimpanzee. The animal came from a troop that had lost several members to an illness later identified as Ebola haemorrhagic fever. The latest discovery is Bundibugyo Ebola virus, the fourth African species of human-pathogenic Ebola virus found in equatorial Africa (approximate distribution 10° north and south of the equator). An additional Ebola virus species, Reston Ebola virus, is found in the Philippines. It was first described in 1989 and isolated from Cynomolgus monkeys (Macaca fascicularis) housed at a quarantine facility in Reston, VA, USA. These monkeys were imported from the Philippines; an unusually high mortality was noted in infected animals during quarantine, but simian haemorrhagic fever virus co-circulated in the animals. Subsequently, Reston Ebola virus has been found in the Philippines on several occasions with surprising reports documenting infections in pigs.
_
Epidemiologic studies (including a specific search in the Kikwit epidemic) have failed to yield evidence for an important role of airborne particles in human disease. This lack of epidemiologic evidence is surprising and seems to conflict with the viruses’ classification as biosafety level 4 pathogens (which is based in large part on aerosol infectivity) and with formal laboratory assessments showing a high degree of aerosol infectivity for monkeys. Sick humans apparently do not usually generate sufficient amounts of infectious aerosols to pose a significant hazard to those around them. Although numerous die-offs have been reported among chimpanzees and gorillas (some even threatening the viability of these endangered species), these animals (like humans) appear to be sentinels for virus activity. Speculation about the true reservoirs has centered on bats, and preliminary evidence indicates that bats may indeed be the reservoirs of filoviruses. This evidence includes the detection of antibodies and reverse-transcriptase polymerase chain reaction (RT-PCR) products in bats, the epidemiologic findings in subterranean gold mines in Durba (DRC) where Marburg transmission has occurred, and reported associations of human antibody production with the handling of bats. Recent isolation of Marburg virus from Egyptian fruit bats (Rousettus aegyptiacus) captured in Uganda in proximity to cases of human disease further supports bats as reservoirs, but the exact biologic relation and the natural cycle remain to be elucidated.
_
Epidemiologic Investigations:
Health authorities have to gather data on possible transmission chains from hospital records and through interviews with patients in whom EBOV infection was suspected and their contacts, affected families, inhabitants of villages in which deaths occurred, attendants of funerals, public health authorities, and hospital staff members.
_
Ecology of ebola:
Ebola haemorrhagic fever is thought to be a classic zoonosis with persistence of the Ebola virus in a reservoir species generally found in endemic areas. Apes, man, and perhaps other mammalian species that are susceptible to Ebola virus infection are regarded as end hosts and not as reservoir species. Although much effort has been made to identify the natural reservoirs with every large outbreak of Ebola haemorrhagic fever, neither potential hosts nor arthropod vectors have been identified. Rodents and bats have long been thought to be potential reservoir species. This idea was supported by experimental studies in African plants and animals that resulted in productive infection of African fruit and insectivorous bats with Zaire Ebola virus, but a firm link could not be established. The first evidence for the presence of Zaire Ebola virus in naturally infected fruit bats was documented by detection of viral RNA and antibodies in three tree-roosting species: Hypsignathus monstrosus, Epomops franqueti, and Myonycteris torquata. However, despite efforts, Zaire Ebola virus has not been successfully isolated from naturally infected animals. The identification and successful isolation of Marburg virus from the cave-dwelling fruit bat Rousettus aegyptiacus further lends support to the idea of bats as a reservoir species for filoviruses. This finding is reassuring since several of the Marburg virus outbreaks have been associated with caves or mines that are usually heavily infested by bats. Data for potential reservoirs for any of the other four Ebola virus species do not exist. Infections with Ebola virus are rare in equatorial Africa, although probably under-reported. Transmission from the reservoir species to man or other end hosts might therefore be an infrequent event, given the restricted distribution of or restricted contact with the reservoir species. However, bats are frequently encountered in equatorial Africa and hunted for food in many places. Therefore, Ebola virus might persist as an asymptomatic or subclinical infection in the reservoir species, with little or no transmission, and might be sporadically activated through an appropriate stimulus. The stimulus might be stress, co-infection, change in food sources, and pregnancy, as shown experimentally in vivo and in vitro. This hypothesis would explain the sporadic nature and periodicity of outbreaks of Ebola haemorrhagic fever in Africa.
_
Seasonal variation in mortality in chimpanzees of the Tai forest, Ivory Coast, and prevalence of specific antibodies against Zaire ebolavirus virus in febrile patients from East Africa suggests an influence of the climate in the occurrences of Ebola epidemics. Pinzon et al. found a close relationship between the onset of epidemics and particularly dry conditions at the end of the rainy season, leading to a change in the behavior of fruit-eating mammals, particularly sensitive to weather changes, resulting in the increase of virus circulation or human contamination. The seasons punctuate migration of bats, which could explain the emergence of epidemics.
_
Mapping the zoonotic niche of Ebola virus disease in Africa: a 2014 study:
Ebola virus disease (EVD) is a complex zoonosis that is highly virulent in humans. The largest recorded outbreak of EVD is ongoing in West Africa, outside of its previously reported and predicted niche. Authors assembled location data on all recorded zoonotic transmission to humans and Ebola virus infection in bats and primates (1976-2014). Using species distribution models, these occurrence data were paired with environmental covariates to predict a zoonotic transmission niche covering countries across Central and West Africa. Vegetation, elevation, temperature, evapotranspiration, and suspected reservoir bat distributions define this relationship. At-risk areas are inhabited by 22 million people; however, the rarity of human outbreaks emphasizes the very low probability of transmission to humans. Increasing population sizes and international connectivity by air since the first detection of EVD in 1976 suggest that the dynamics of human-to-human secondary transmission in contemporary outbreaks will be very different to those of the past.
___
Ebola Outbreak 2014:
Epidemics usually begin with a single case acquired from an unknown reservoir in nature (bats are suspected) and spread mainly through close contact with sick persons or their body fluids, either at home or in the hospital. Since 1976, there have only been about 20 known Ebola outbreaks. Until last year, the total impact of these outbreaks included 2,357 cases and 1,548 deaths, according to the Centers for Disease Control and Prevention. They all occurred in isolated or remote areas of Africa, and Ebola never had a chance to go very far. And that’s what makes the 2014 outbreak so remarkable: the virus has spread to six countries in Africa plus America, and has already infected more than 13,000 people. It has killed nearly 5,000 people. That is more than six times the sum total of all previous outbreaks combined.
_
_
A complex epidemic of Zaire ebolavirus (EBOV) has been affecting West Africa since approximately December 2013, with the first cases likely occurring in southern Guinea. The causative Ebola strain is closely related to a strain associated with past EBOV outbreaks in Central Africa and could have been circulating in West Africa for about a decade. However, the current epidemic was not identified until March 2014, which facilitated several transmission chains to progress essentially unchecked in the region and to cross porous borders with neighboring Sierra Leone and Liberia and seed a limited outbreak in Nigeria via commercial airplane on 20 July 2014. The World Health Organization declared the Ebola epidemic in West Africa a Public Health Emergency of International Concern on 8 August 2014, with exponential dynamics characterizing the growth in the number of new cases in some areas. Economic and sociocultural factors together with the delay in identifying the outbreak in urban settings have hindered a timely and effective implementation of control efforts in the region.
_
The Situation:
_
_
_
The 2014 Ebola epidemic is centered in the area where Liberia, Sierra Leone and Guinea meet and has infected almost 14,000 victims since December 2013, killing about 5,000. More will die, given a fatality rate of 71 percent in this outbreak. The U.S. Centers for Disease Control and Prevention estimates actual cases are 2.5 times higher and are roughly doubling every month. The epidemic spread to Nigeria and Senegal, which successfully contained it. It reached Mali in late October. Early on, the disease was transmitted by victims who avoided hospitals because of stigma and fear, as well as by unsafe burial practices. As cases increased, efforts to control the epidemic were hampered by a shortage of trained workers at a time when humanitarian groups were dealing with many crises elsewhere. The U.S. responded by sending hundreds of military personnel to Liberia, which in October 2014 began to see a reduction in cases. Ebola jumps to humans through contact with secretions from animals such as chimpanzees, gorillas and bats. It spreads among humans the same way, with medical workers and family members the most at risk. A separate outbreak reported in late August has killed scores of people in the Democratic Republic of Congo. In the first known instance of Ebola transmission outside Africa, medical workers in the U.S. and Spain were infected after caring for people who had contracted Ebola in Africa. Ebola patients infected in Africa have been treated in a number of other European countries.
_
Ebola response roadmap – Situation Report- 3rd December 2014: WHO:
A total of 17,145 confirmed, probable, and suspected cases of Ebola virus disease (EVD) have been reported in five affected countries (Guinea, Liberia, Mali, Sierra Leone, and the United States of America) and three previously affected countries (Nigeria, Senegal and Spain) up to the end of 30 November. There have been 6070 reported deaths. Cases and deaths continue to be under-reported in this outbreak.
_
Some countries have encountered difficulties in their efforts to control the epidemic. In some areas, people have become suspicious of both the government and hospitals, some of which have been attacked by angry protesters who believe either that the disease is a hoax or that the hospitals are responsible for the disease. Many of the areas seriously affected by the outbreak are areas of extreme poverty with limited access to the soap and running water needed to help control the spread of disease. Other factors include reliance on traditional medicine and cultural practices that involve physical contact with the deceased, especially death customs such as washing and kissing the body of the deceased. Some hospitals lack basic supplies and are understaffed, increasing the chance of staff catching the virus themselves. In August, the WHO reported that ten percent of the dead have been health care workers. By the end of August, the WHO reported that the loss of so many health workers was making it difficult for them to provide sufficient numbers of foreign medical staff. In September 2014, the WHO estimated that the countries’ capacity for treating EVD patients was insufficient by the equivalent of 2,122 beds. By the end of October many of the hospitals in the affected area had become dysfunctional or had been closed, leading some health experts to state that the inability to treat other medical needs may be causing “an additional death toll [that is] likely to exceed that of the outbreak itself”. By September 2014, Médecins Sans Frontières/Doctors Without Borders (MSF), the largest NGO working in the affected countries, had grown increasingly critical of the international response. Speaking on 3 September, the president of MSF spoke out concerning the lack of assistance from the United Nations member countries saying, “Six months into the worst Ebola epidemic in history, the world is losing the battle to contain it.” A United Nations spokesperson stated, “They could stop the Ebola outbreak in West Africa in 6 to 9 months, but only if a ‘massive’ global response is implemented.” The Director-General of the WHO, Margaret Chan, called the outbreak “the largest, most complex and most severe we’ve ever seen” and said that it is “racing ahead of control efforts”. In a 26 September statement, the WHO said, “The Ebola epidemic ravaging parts of West Africa is the most severe acute public health emergency seen in modern times.”
_
Ebola tip of the iceberg:
For every four cases of Ebola we know of, there might be six that we don’t:
While official estimates suggest there are already more than 13,000 cases of Ebola this year, the real number is likely much, much higher. The Centers for Disease Control and Prevention estimate that the actual number of Ebola cases is roughly 2.5 times higher than the reported figures — so for every four Ebola cases we know of, there could be six that we don’t. The CDC isn’t alone in this. “There is widespread under-reporting of new cases,” warns the World Health Organization. The WHO has continually said that even its current dire numbers don’t reflect the full reality. The estimated 13,000-plus Ebola cases in West Africa could just be the tip of the iceberg.
_
Could Ebola become endemic worldwide?
_
Global health agencies were too slow in responding to the Ebola crisis:
Ebola is a very preventable disease. We’ve had over 20 previous outbreaks and we managed to contain all of them. It could take months for a full response to get off the ground. But this time, the international response just wasn’t there. There was no mobilization. The World Health Organization didn’t call a public health emergency until August — five months after the first international spread [in March]. It took three months for health officials to identify Ebola as the cause of the epidemic, another five months to declare a public health emergency, and two more months to mount a humanitarian response. In reality, a full response could take several more months still to get off the ground. Part of the reason for the slow response can be attributed to budget cuts at the WHO that have left the agency understaffed and under-resourced. The WHO also now sees itself as a “technical agency,” providing analysis and data, and not as a first responder. But, as an editorial in the journal Nature pointed out: “If the WHO is not the first responder to an emergency such as this, then who is?” The International Health Regulations governing disease responses are also flawed and broken, leaving us unprepared for outbreaks. So this Ebola epidemic has served as a reminder of just how slow and poorly coordinated our global responses to outbreaks are, and this is a problem because any infectious diseases expert will tell you that the best way to stop an outbreak is to contain it early.
_
Health is not free from politics, either. Sadly, the world only seemed to wake up to Ebola after two American missionaries got infected in Liberia. One of them, Dr. Kent Brantly, testified before the Senate in the US to make that point: “This unprecedented outbreak began nine months ago but received very little attention from the international community until the events of mid-July when my friend and colleague, Nancy Writebol, and I became infected.” He added: “The response, however, is still unacceptably out-of-step with the size and scope of the problem now before us.” “Ebola could establish itself as an endemic infection because of a highly inadequate and late global response.” The awakening came too late. Preeminent disease researchers, in an article in the New England Journal of Medicine, wrote, “Ebola has reached the point where it could establish itself as an endemic infection because of a highly inadequate and late global response.” Still, the global health community is now moving aggressively. The director of the World Health Organization called this Ebola epidemic “the greatest peacetime challenge” the world has ever faced. President Barack Obama called the epidemic “not just a threat to regional security… [but] a potential threat to global security.” For this reason, the United States has sent more than 3,000 troops to fight Ebola and has now funded the largest international response in the history of the CDC. In October 2014, the Obama administration appointed Ron Klain it’s first-ever “Ebola Czar” to coordinate the response. In other desperate and unprecedented measures, the United Nations Security Council characterized the virus a threat to international peace and security, holding its second-ever disease-focused meeting and setting up a special UN mission to deal with the epidemic. The Security Council unanimously passed a resolution asking countries around the world to urgently send medical workers and supplies to stop the epidemic. If these measures fail, the world may be faced with something it has never seen before: endemic Ebola.
_
Potential for large outbreaks of Ebola virus disease: a 2014 study:
Outbreaks of Ebola virus can cause substantial morbidity and mortality in affected regions. The largest outbreak of Ebola to date is currently underway with a total of 15,935 confirmed, probable, and suspected cases of Ebola virus disease (EVD) reported in six affected countries till 23 November 2014. To develop a better understanding of Ebola transmission dynamics, authors revisited data from the first known Ebola outbreak, which occurred in 1976 in Zaire (now Democratic Republic of Congo). By fitting a mathematical model to time series stratified by disease onset, outcome and source of infection, they were able to estimate several epidemiological quantities that have previously proved challenging to measure, including the contribution of hospital and community infection to transmission. They found evidence that transmission decreased considerably before the closure of the hospital, suggesting that the decline of the outbreak was most likely the result of changes in host behaviour. Their analysis suggests that the person-to-person reproduction number was 1.34 (95% CI: 0.92–2.11) in the early part of the outbreak. This has profound implications: it suggests that a large outbreak (involving thousands of cases) could have happened even without changing any epidemiological conditions. Authors estimated the probability of such a large outbreak (>1000 cases) to be around 3%. This means that given the same initial conditions, Ebola outbreaks would have been occasionally been large, just by chance. Moreover, a relatively high person-to-person transmission component (R0pp ≈ 1) implied that the 1976 epidemic would have been difficult to control via hospital-based infection control measures alone. If the reduction in community transmission had been smaller, or infection had been seeded into a number of different communities, the outbreak could have continued for some time. The results also suggest that changes in behaviour caused a significant reduction in both hospital-to-community and within-community transmission. Although Yambuku Mission hospital closed on the 30th September, they found that the reduction in transmission occurred well before this point, most likely from susceptible hosts having less contact with infected patients, and making fewer routine outpatient visits to the hospital (Breman et al., 1978). As well as contributing to transmission, infections from syringes also appeared to have a higher case fatality ratio (CFR) than person-to-person infections. This could have been the result of a larger viral inoculum during contact with a contaminated syringe.
_______
Is India prepared to keep Ebola out?
World Health Organisation’s (WHO) India office says it is holding regular meetings with the technical staff at the Union health ministry for developing “appropriate response measures”. It is also guiding the ministry on how to prevent and control the infection “at health facilities, train rapid response teams for laboratory testing, surveillance and emergency contingency planning”. Screening for the virus at the country’s 18 international airports has been stepped up. Scanners that can detect high body temperatures have been placed at the immigration counters. A mandatory health card is distributed to all passengers who have either travelled to the four Ebola-affected countries, Liberia, Guinea, Sierra Leone and Nigeria (now declared Ebola-free), or have transited through these countries during the past 21 days. Travelers are questioned about their contact, if any, in the last 21 days – the incubation period for the virus – with any Ebola patient and whether they worked or visited high-risk areas like hospitals. All flights carrying suspected cases are disinfected before the next batch of passengers is allowed to board. To date, around 22,000 passengers have been screened at airports across India. Of these, 55 were found to be high-risk (those with fever), seven were medium-risk (those with contact history) and 21,737 were low-risk (those who did not have symptoms or history of contact) passengers. The health ministry says the suspected and high-risk cases have all tested negative. Over 1,000 passengers, mostly from Maharashtra, Kerala, Tamil Nadu, Gujarat, West Bengal and Delhi, have also been tracked by the Integrated Disease Surveillance Program (IDSP). High-risk passengers are taken in an ambulance to the designated quarantine facility through a dedicated route without entering the immigration area or mixing with other passengers. Medium-risk passengers have to share their contact details and are tracked actively for at least 21 days by IDSP. Low-risk passengers are provided another health card and advised to contact a helpline if any symptom appears. The immigration staff deputed for Ebola detection has been provided with protective gear. Like at IGI, at the Chhatrapati Shivaji International Airport in Mumbai, a team of trained doctors appointed by the Airport Health Organisation is screening passengers at counters in the pre-immigration arrival area. “Doctors have been instructed to keep a look out for passengers suffering from flu,” says an airport health official. The airport does not have a laboratory, so blood samples of any suspected Ebola case are sent to the National Institute of Virology in Pune for testing. “The test result is ready in 24, at most 48, hours,” says a scientist at the Pune institute. “The virus shows in the blood once the symptoms appear,” he adds. The only other designated laboratory equipped to test for Ebola is the National Centre for Disease Control in Delhi. Now the Indian Council for Medical Research has shortlisted another 10 laboratories to test the virus. At the government’s 24-hour Ebola emergency helplines (011-23061469, 23063205 and 23061302), set up at the health ministry in Delhi, doctors from central government hospitals are on duty round the clock. “We have been getting calls from people wanting to discuss their travel history and risks attached,” says a doctor on duty. Private hospitals too have been calling for details about Ebola symptoms and precautions to be taken. The control room has received about 800 calls since its inception on August 9. The standing instruction is to immediately direct suspected cases to RML Hospital.
_
India quarantines man recovering from Ebola:
India has quarantined a man who was cured of Ebola in Liberia but continued to show traces of the virus in samples of his semen after arriving in the country. The Indian man carried with him documents from Liberia that stated he had been cured. It is not an Ebola case, he is an Ebola-treated patient who is negative in blood but whose body fluid is positive. He has no symptoms. Tests of his semen detected traces of the virus. He will be kept in quarantine until the virus is no longer present in his body. The Indian government has now asked those travelling to India from Ebola-affected countries to carry a certificate stating that there is no evidence of the deadly virus in their body fluids, after this person cured of the disease was found to be carrying the virus in his semen. Peter Piot, a former WHO official who was one of the discoverers of the virus, has in the past expressed concerns about the disease spreading to India. There are nearly 45,000 Indian nationals living in West Africa. Many experts say densely populated India is not adequately prepared to handle any spread of the highly infectious haemorrhagic fever among its 1.2 billion people. Government health services are overburdened and many people in rural areas struggle to get access to even basic health services. Hygiene standards are low, especially in smaller towns and villages, and defecating and urinating in the open are common.
______
Social and cultural aspects of ebola:
Socio-cultural factors:
Socio-cultural factors have not only contributed significantly to Ebola spread, but have also complicated the implementation of control interventions. Specifically, cultural practices involving touching the body of the deceased naturally (and greatly) contribute to the dissemination of the Ebola virus. In particular, the potential for transmission to neighboring and distant areas by exposed funeral attendants could facilitate the development of major epidemics. Moreover, the lack of prior experience or knowledge of the disease can lead communities to deny its existence and to associate illness with witchcraft or conspiracy theories presumably created by governments to gain control of populations or attract resources from the international community. For instance, during the ongoing epidemic in West Africa, a group of individuals looted equipment and potentially contaminated materials in an isolation facility in a quarantined neighborhood. Finally, the stigma carried by Ebola survivors and family members of Ebola victims could exacerbate disease spread. In particular, uninformed families tend to hide relatives and friends infected with Ebola to avoid being shunned by their own communities, which enhances transmission rates. The problem is compounded by the high case fatality ratio of EVD whereby misinformed communities tend to associate case isolation with a death sentence.
_
Ebola is spread through close physical contact with infected people. This is a problem for many in the West African countries currently affected by the outbreak, as practices around religion and death involve close physical contact. Hugging is a normal part of religious worship in Liberia and Sierra Leone, and across the region the ritual preparation of bodies for burial involves washing, touching and kissing. Those with the highest status in society are often charged with washing and preparing the body. For a woman this can include braiding the hair, and for a man shaving the head. If a person has died from Ebola, their body will have a very high viral load. Bleeding is a usual symptom of the disease prior to death. Those who handle the body and come into contact with the blood or other body fluids are at greatest risk of catching the disease. MSF has been trying to make people aware of how their treatment of dead relatives might pose a risk to themselves. It is a very difficult message to get across. All previous outbreaks were much smaller and occurred in places where Ebola was already known – in Uganda and the DR Congo for example. In those places the education message about avoiding contact has had years to enter the collective consciousness. In West Africa, there simply has not been the time for the necessary cultural shift.
_
In the case of Guinea, while the medical teams knew exactly what had to be done to help the population, the implementation of the response plan was hit by poor collaboration with communities. The teams were beaten up and NGOs couldn’t get to the villages to implement the protocols. The transmission of the Ebola virus is not understood as a biological phenomenon in rural parts of the country where traditional beliefs — in particular sorcery — have the upper hand over science. The low literacy level in Guinea — around 25 per cent — and the inadequacy of information channels further hinder the fight against the epidemic. At one point text messages spread a rumour that a Guinean researcher based in Senegal had developed a cure for Ebola based on hot chocolate, milk, sugar and onions. This was enough for these products to run out in various shops around the country, including in Conakry, the capital. We have reached a situation in which people don’t want to hear what they’re being told. In such a difficult situation, it is difficult to find a balance between the fears and resistance of local people and the need to bring the epidemic under control. For example, they have recommended that medical staff stop using the term ‘isolation centers’ to refer to the places where people infected with Ebola are gathered, and instead to use the more reassuring term ‘treatment centers’. At one point, the treatment centers became synonymous with death chambers. People refused to go there, saying that, once you entered, you wouldn’t come out again alive — a reference to the high mortality among victims of Ebola. A study published recently in the New England Journal of Medicine estimates that overall 71 per cent of people who get Ebola do not survive it — and that figure only drops to 64 per cent among those who are hospitalised. In these societies, in which death is accompanied by a set of traditional rituals including the preparation of the corpse and the invocation of the spirits before burial, they don’t understand when we explain that they mustn’t touch the bodies of Ebola victims. From the point of view of their traditions, the corpse must be interrogated to discover the cause of death: whether the person died a natural death or died of ‘sorcery’. It is therefore necessary to touch the body. Yet given the virulence of the Ebola virus, the medical advice is to avoid touching the bodies of people who have died of the disease. We have to find a solution that enabled us to save what is essential: human lives. We have to find a balance, which was to allow the populations to at least see the body and to throw objects into the body bag before the burial. That, at least, would calm their feelings and enabled us to avoid serial contamination of the population. That’s how the principle of secure burials was accepted.
_
Poverty and illiteracy spread Ebola:
Ebola can be stopped. But it takes resources, and a functioning health-care system. The three countries hardest hit by the Ebola epidemic — Guinea, Sierra Leone, and Liberia — all have very weak health systems and little money to spend on health care. That has constrained their ability to stop the epidemic. In most of West Africa, health spending amounts to less than $100 per person per year (in the United States, it’s about $8,000). Guinea, Sierra Leone, and Liberia have some of the worst maternal and child mortality rates on the planet — an indicator of a failing health system. Experts point out that scarce resources make it extremely difficult to contain the Ebola epidemic: “If you’re in a hospital in Sierra Leone or Guinea, it might not be unusual to say, ‘I need gloves to examine this patient,’ and have someone tell you, ‘We don’t have gloves in the hospital today,’ or ‘We’re out of clean needles,’ — all the sorts of things you need to protect against Ebola,” says Daniel Bausch, associate professor at the Tulane University School of Public Health and Tropical Medicine, who is working with the WHO on the outbreak. Bausch would walk into the hospital in the morning and find patients on the floor in pools of vomit, blood, and stool. They had fallen out of their beds during the night, and they were delirious. “What should happen is that a nursing staff or sanitation officer would come and decontaminate the area,” he said. “But when you don’t have that support, obviously it gets more dangerous.” Along with poverty and a health system too weak to combat the virus, illiteracy has contributed to the problem. Guinea, Liberia, and Sierra Leone have some of the lowest literacy rates in the world. Poor literacy has made it much harder for aid workers to mount a public-health information campaign and explain to people how they can stop the spread of Ebola. It also helped to fuel a rumor mill about supposed cures. For example, one persistent myth has been that hot water and salt can stop Ebola.
_
Stigmatization of Some Populations:
Several populations and countries are being stigmatized because of the presence of Ebola within their borders. For former residents of Guinea, Liberia and Sierra Leone now living in the U.S., the fears of stigmatization are very real. Some groups and politicians are advocating that anyone from these countries, sick or not, should not be allowed to come into the U.S. This won’t work. These politicians are also saying that travel into these countries should be severely restricted, which means that aid workers, the U.S. military and medical personnel helping these citizens, cannot enter any of these countries.
_
Survivors of Ebola face second ‘disease’ the stigma:
The doctor has beaten the odds and survived Ebola, but he still has one more problem: The stigma carried by the deadly disease. Even though he is completely healthy, people are afraid to come near him or to have anything to do with him. For example, the man was supposed to give an interview on Guinean radio to describe his triumphant tale. But the station would not allow him into the studio. “We’d prefer he speak by phone from downstairs,” the station’s director told a representative of Doctors Without Borders, while the survivor waited outside in a car. “I can’t take the risk of letting him enter our studio.” For the lucky survivors, the stink of stigma lingers long after the virus has been purged from their bodies. “Thank God, I am cured. But now I have a new disease: the stigmatization that I am a victim of,” said the Guinean doctor, who spoke to The Associated Press but refused to give his name for fear of further problems the publicity would cause him and his family. “This disease (the stigma) is worse than the fever.” Several other people who survived the disease refused to tell their stories when contacted by the AP, either directly or through Doctors Without Borders. Sam Taylor, the Doctors Without Borders spokesman who had taken the doctor to the radio station, confirmed that the man had been infected and survived. The doctor believes he caught Ebola while caring for a friend and colleague who died in Conakry, Guinea’s capital. At the time, he said, he did not know that his friend had Ebola. Shortly after his friend’s death, the doctor got a headache and came down with an intractable fever. And then the vomiting and diarrhea began. “I should have died,” the doctor said, but he responded to care, which includes intensive hydration, and unlike most other Ebola patients, he lived. Surviving Ebola is a matter of staying alive long enough to have the chance to develop enough antibodies to fight off the virus. That’s because it’s typically the symptoms of Ebola — severe fever, hemorrhaging, dehydration, respiratory problems — that kills a patient. Even though he has been cleared of Ebola, the doctor says that people avoid him. “Now, everywhere in my neighborhood, all the looks bore into me like I’m the plague,” he said. People leave places when he shows up. No one will shake his hand or eat with him. His own brothers are accusing him of putting their family in danger.
_
Ebola Deaths Hype:
The Ebola virus is extremely rare. Among the leading causes of death in Africa, it only accounts for a tiny fraction. People are much more likely to die from AIDS, respiratory infections, or diarrhea, as you can see below.
_
Since 1976, Ebola has infected fewer than 5,000 people and killed fewer than 3,000. That’s in Africa, where over 1 billion people live. By contrast, poor, “boring” measles still kills 122,000 people every year and killed over 2 million a year in 1980, before widespread vaccination campaigns. According to the WHO, in 2012, malaria caused an estimated 627,000 deaths, mostly among African children. Also according to the WHO, since the beginning of the AIDS epidemic (which dates back almost as far as the discovery of the Ebola virus), HIV has infected over 75 million and killed 36 million, with approximately 35 million currently living with the infection. None of this means that we shouldn’t take Ebola seriously or that much larger outbreaks couldn’t happen. Nor does noting this difference minimize the deaths of people infected with the disease. We should note from these observations and others, however, that Ebola is unlikely to reach such numbers because it is simply not infectious enough and Ebola outbreaks tend to “burn themselves out” because, unlike HIV or measles (which are also transmissible human-to-human), Ebola virus disease is so rapidly fatal. The public fear of ebola, the latest outbreak of which has killed almost 6,500 people mostly in West Africa, far outweighs concern about other more deadly diseases, such as rabies which has killed 65,000 people in the last year, or emerging dangers like Middle East Respiratory Syndrome (MERS). MERS is a disease in camels and when passed to humans spread quickly between them through the air, while ebola, which originated in bats, requires physical contact to move. Flu-like in nature, MERS has killed more than 190 people since it surfaced in Saudi Arabia two years ago.
_
The majority of Ebola deaths may not be from Ebola in West Africa:
Of this epidemic, the World Bank said Ebola may deal a “potentially catastrophic blow” to the West African countries reeling with the virus. Businesses are shutting down, people aren’t working, and kids aren’t going to school. The epidemic has also led to widespread food insecurity. “The fertile fields of Lofa County, once Liberia’s breadbasket, are now fallow. In that county alone, nearly 170 farmers and their family members have died from Ebola,” the WHO director warned. “In some areas, hunger has become an even greater concern than the virus.” People are going to suffer and die more from other diseases as the already scarce health resources in the region go to Ebola. Speaking at the United Nations, Dr. Joanne Liu, international president of Médecins Sans Frontières, said, “Mounting numbers are dying of other diseases, like malaria, because health systems have collapsed.” Jimmy Whitworth, the head of population health at Britain’s Wellcome Trust, told the Independent in an interview, “People aren’t going to hospitals or clinics because they’re frightened, there aren’t any medical or nursing staff available.” “West Africa will see much more suffering and many more deaths during childbirth and from malaria, tuberculosis, HIV-AIDS, enteric and respiratory illnesses, diabetes, cancer, cardiovascular disease, and mental health during and after the Ebola epidemic,” wrote disease researchers Jeremy Farrar, of the Wellcome Trust, and Peter Piot, of the London School of Hygiene and Tropical Medicine in an article in the New England Journal of Medicine. So this virus has wreaked incalculable damage on not only the bodies of those infected, but on others who are not getting health care they need, and the health systems and economies of West Africa. Dr. Ezie Patrick, with the World Medical Association who is based in Abuja, Nigeria, focused on the simple and disquieting fact that Ebola has also taken the lives of health workers in places where the ratio of doctors per population is abysmally low. “Sadly Ebola is claiming the lives of the few doctors who have decided to work in these challenging health systems thereby worsening the dearth and also increasing the brain drain leading to far fewer doctors in the region.” The disaster could last longer than the epidemic itself. Before the Ebola outbreak, West African nations were seeing promising signs of economic growth. Sierra Leone, for example had the second highest real GDP growth rate in the world. Liberia was 11th in 2013. Now, there’s worry that Ebola will slam the brakes on that development. “A prolonged outbreak could undercut the growth that these countries were finally starting to experience, taking away the resources that would be necessary for improving the health and education systems,” says Jeremy Youde, a professor of political science at the University of Minnesota Duluth. “These countries are generally not starting from a great position as it is, so they don’t have much of a cushion to absorb long-term economic losses. If the international economy turns away from West Africa and brands it as diseased, that could be very problematic.”
_
Why is containing Ebola proving difficult?
In West Africa, the man-made elements of conflict, confusion and culture have all combined to create a perfect-storm for Ebola. A growing population, decades of civil war, widespread government corruption, dysfunctional health system, a growing distrust in Western medicine and worsening conditions in West Africa contribute to a “perfect storm,” The scientist who first identified Ebola in 1976 gives direct and simple advice on how to contain this latest outbreak: “Soap, gloves, isolating patients, not reusing needles and quarantining the contacts of those who are ill – in theory it should be very easy to contain Ebola,” Dr Peter Piot told the BBC. In practice, this is a much tougher proposition. The main outbreak has emerged in war ravaged West Africa, where much of the health care infrastructure has been totally destroyed. Poverty has combined with fear, ignorance and superstition, particularly in remote communities, where distrust of government is understandably high, and belief in witchcraft and sorcery is interwoven into everyday life. Testing for Ebola often requires multiple blood tests – which is difficult to conduct in areas where strong cultural beliefs prohibit collection of a “life force”. In Liberia, some communities believe the outbreak is a hoax, and that health care workers have been sent to kill them. In one town, health care workers spraying chlorine – a cheap and effective counter to the spread of the disease – were attacked. In Guinea, Medicines Sans Frontiers (MSF) doctors and medics were attacked by villagers who believed the clinical team had brought Ebola to their country. Governmental response has been heavy handed. Liberia’s president threatened to jail anyone sheltering or hiding suspected Ebola cases. An un-coordinated rush by the international community to assist can also complicate efforts, says African governance expert Kim Yi Dionne, especially when it appears that no one is in charge. Already involved in the Ebola response are the local ministries of health for Liberia, Guinea and Sierra Leone, the World Health Organisation, MSF, UNICEF and many other agencies.
_______
Etiology: The Ebola Virus:
Virologists have been using the names Marburg virus and Ebola virus for the type viruses of the genera Marburgvirus and Ebolavirus, respectively, for decades and have not accepted the novel names for these agents (Lake Victoria marburgvirus and Zaire ebolavirus) suggested in the 8th ICTV Report.
_
Taxonomy of ebola virus:
Group: Group V [(-)ssRNA]
Order: Mononegavirales
Family: Filoviridae
Genus: Ebolavirus
Species: Zaire ebolavirus
_
The family Filoviridae resides in the order Mononegavirales and contains the largest genome within the order. This family contains 2 genera: Ebolavirus (containing 5 species) and the antigenically distinct Marburgvirus (containing a single species). EVD in humans is caused by four of five viruses of the genus Ebolavirus. The four are Bundibugyo virus (BEBOV), Sudan virus (SEBOV), Taï Forest virus (TEBOV) and the Zaire Ebola virus (ZEBOV). ZEBOV is the most dangerous of the known EVD-causing viruses, and is responsible for the largest number of outbreaks. The fifth virus, Reston virus (REBOV), is not thought to cause disease in humans, but has caused disease in other primates. All five viruses are closely related to marburgviruses. In patients who have Ebola virus infection, exposure to the virus may be either primary (involving presence in an Ebolavirus -endemic area) or secondary (involving human-to-human or primate-to-human transmission). Physical findings depend on the stage of disease at the time of presentation.
__
Is Ebola an RNA virus? And why is it considered a filovirus? What does that mean?
Yes, Ebola is an RNA virus. It belongs to the family of filoviruses. The family name was derived from the Latin word filum, which alludes to the thread-like appearance of the virions when viewed under an electron microscope. The family Filoviridae comprises two antigenically and genetically distinct genera: Marburgvirus and Ebolavirus. Ebola virus particles are rod-shaped and are surprisingly simply organized. The small viral RNA genome, which consists of only 19 thousand nucleotides—human genomes consist of billions of these nucleotides—is tightly associated with only seven proteins and encased in a membrane. One of the viral proteins sticks out of the membrane and can bind to receptors on the surface of cells. Binding to these receptors helps the virus enter the cell. Inside the cells, Ebola virus replicates itself. Then the new viral particles leave the cells and are ready to infect fresh cells. At some point, the infected cells get exhausted because they have to provide all the material needed to form the virus—and they die. However, Ebola virus–infected cells survive for a pretty long time, making it easy for the virus to spread throughout the body of the infected host.
_
Phylogenetic tree of filoviruses:
Filoviridae, of which Ebola virus is a member, is a family of viruses that contain single, linear, negative-sense ssRNA genomes. Filoviruses have been divided into two genera: Ebola-like viruses with species Zaire, Sudan, Reston, Cote d’Ivoire and Bundibugyo; and Marburg-like viruses with the single species Marburg. All of these are responsible for hemorrhagic fevers in primates that are characterized by often fatal bleeding and coagulation abnormalities.
_
Ebola is a filovirus, and filoviruses appear to have been around in some form for millions of years. The figure above shows phylogenetic tree comparing ebolaviruses and marburgviruses. Numbers indicate percent confidence of branches. Marburgvirus and Ebolavirus are seen to be two different genera. The genus Ebolavirus includes five distinct species. Note that the Yambuku and Kikwit Zaire viruses are virtually identical even though the epidemics for which they were responsible are separated by two decades and hundreds of kilometers. Virtually every virus sequenced from each of those two epidemics is identical over the part of the genome examined. This pattern is typical of that seen with single introductions followed by human-to-human passage via needle or close contact in an African hospital. In the Marburgvirus branch of the tree, there is one major clade with a slightly divergent group characterized by the Ravn 1987 Kenya isolate. All the viruses from the major Angola 2005 outbreak are represented by a single virus because the sequences in this human-to-human epidemic are virtually identical. However, in the outbreak occurring in the Democratic Republic of the Congo (DRC) in 1999 and resulting from multiple independent infections after cave entry, two viruses with slightly different phylogenies are represented within the major group, and there is even another virus within the Ravn subgroup. These sequences were selected from hundreds determined at the U.S. Centers for Disease Control and Prevention and elsewhere.
_
Ebola virus genome:
_
Ebolaviruses contain single-stranded, non-infectious RNA genomes. Ebolavirus genomes are approximately 19 kilobase pairs long and contain seven genes in the order 3′-UTR-NP-VP35-VP40-GP-VP30-VP24-L-5′-UTR. The genomes of the five different ebolaviruses differ in sequence and the number and location of gene overlaps. As all filoviruses, ebolavirions are filamentous particles that may appear in the shape of a shepherd’s crook, of a “U” or of a “6,” and they may be coiled, toroid or branched. In general, ebolavirions are 80 nanometers (nm) in width and may be as long as 14,000 nm (average 800 to 1000 nm). Their life cycle begins with a virion attaching to specific cell-surface receptors, followed by fusion of the virion envelope with cellular membranes and the concomitant release of the virus nucleocapsid into the cytosol. Ebolavirus’ structural glycoprotein (known as GP) is responsible for the virus’ ability to bind to and infect targeted cells. The viral RNA polymerase, encoded by the L gene, partially uncoats the nucleocapsid and transcribes the genes into positive-strand mRNAs, which are then translated into structural and nonstructural proteins. The most abundant protein produced is the nucleoprotein, whose concentration in the host cell determines when L switches from gene transcription to genome replication. Replication of the viral genome results in full-length, positive-strand antigenomes that are, in turn, transcribed into genome copies of negative-strand virus progeny. Newly synthesized structural proteins and genomes self-assemble and accumulate near the inside of the cell membrane. Virions bud off from the cell, gaining their envelopes from the cellular membrane from which they bud from. The mature progeny particles then infect other cells to repeat the cycle. The genetics of the Ebola virus are difficult to study because of EBOV’s virulent characteristics.
_
Figure above shows a protein map of Ebola virus RNA.
_
Ebola is a lipid enveloped, filamentous, negative-sense virus with an RNA genome. The virus is transmitted from one individual to another through exchange of bodily fluids and enters through exposed cuts or mucous membranes (mouth, nose, etc.).
Lipid enveloped:
Lipid enveloped viruses contain a lipid bilayer coat (outer membrane of a cell) that protects their genome and helps them enter (infect) cells. The lipid bilayer of Ebola is composed of the same lipids as human cells and scientists believe this lipid coat may be extracted from lipid rafts of human cells as new virions “bud” or leave cells after intracellular expansion of the virus. Contained within the lipid bilayer of Ebola are virus proteins that help the virus infect new cells and contribute to its replication. All together, the lipid bilayer performs three functions, 1) to cloak the virus from the immune system because it closely resembles normal host cells, 2) to facilitate binding of virus to cells and entry in lipid-to-lipid interactions, and 3) to facilitate viral replication.
Negative-sense RNA:
Mammalian genetic code is DNA to RNA to protein. There are multiple forms of RNA synthesized by mammalian cells, and it is the messenger form of RNA, abbreviated as mRNA, that is translated into protein. Unlike mammals, some viruses (such as Ebola) use RNA rather than DNA as their genetic code. RNA viruses are further classified according to the “sense” or polarity of their RNA. Positive-sense viral RNA is similar to mammalian mRNA and as a result can be immediately translated by the host cell after infection into viral protein. Negative-sense viral RNA is the mirror image of mRNA; consequently it must be converted to positive-sense RNA by an enzyme called RNA polymerase before translation into protein. As such purified RNA of a negative-sense virus is not infectious by itself and needs to be transcribed into positive-sense RNA to make viral protein that can be assembled into new, infectious virus particles. The Ebola genome encodes seven proteins named nucleoprotein, VP24, VP30, VP35, L protein, transmembrane glycoprotein and the matrix protein VP40.
_
Inside each Ebola particle is a tube made of coiled proteins, which runs the length of the particle, like an inner sleeve. Within the inner sleeve of an Ebola particle, invisible even to a powerful microscope, is a strand of RNA, the molecule that contains the virus’s genetic code, or genome. The code is contained in nucleotide bases, or letters, of the RNA. These letters, ordered in their proper sequence, make up the complete set of instructions that enables the virus to make copies of itself. A sample of the Ebola now raging in West Africa has, by recent count, 18,959 letters of code in its genome; this is a small genome, by the measure of living things. Viruses like Ebola, which use RNA for their genetic code, are prone to making errors in the code as they multiply; these are called mutations. Right now, the virus’s code is changing. As Ebola enters a deepening relationship with the human species, the question of how it is mutating has significance for every person on earth.
_
The genome consists of seven genes in the order 3′ leader, nucleoprotein, virion protein (VP) 35, VP40, glycoprotein, VP30, VP24, RNA-dependent RNA polymerase (L)—5′ trailer. With the exception of the glycoprotein gene, all genes are monocistronic, encoding for one structural protein. The inner ribonucleoprotein complex of virion particles consists of the RNA genome encapsulated by the nucleoprotein, which associates with VP35, VP30, and RNA-dependent RNA polymerase to the functional transcriptase—replicase complex. The proteins of the ribonucleoprotein complex have additional functions such as the role of VP35, which is an interferon antagonist. VP40 serves as the matrix protein and mediates particle formation. VP24, another structural protein associated with the membrane, interferes with interferon signaling. The glycoprotein is the only transmembrane surface protein of the virus and forms trimeric spikes consisting of glycoprotein 1 and glycoprotein 2—two disulphide-linked furin-cleavage fragments. An important distinction of Ebola virus from other Mononegavirales is the production of a soluble glycoprotein, which is the primary product of the GP gene, and gets secreted to large quantities from infected cells.
_
_
Ebola actually encodes two forms of its glycoprotein gene. The small, non-structural, dimeric soluble form (sGP) is transcribed directly from the viral mRNA and its function remains mostly unknown. This protein is not found in virus particles, but is instead secreted from infected cells into the blood. A second glycoprotein results from transcriptional editing of the glycoprotein origin of replication and encodes a trimeric, membrane-bound form. This envelope GP spike is expressed at the cell surface, and is incorporated into the virion to drive viral attachment and membrane fusion. It has also been shown as the crucial factor for Ebola virus pathogenicity. GP is actually post-translationally cleaved by the proprotein convertase furin to yield disulphide-linked GP1 and GP2 subunits. GP1 allows for attachment to host cells, while GP2 mediates fusion of viral and host membranes. This protein assembles as a trimer of heterodimers on the viral envelope, and ultimately undergoes an irreversible conformation change to merge the two membranes. The product of the third gene, VP40, is located beneath the viral envelope where it helps to maintain structural integrity. It has also been associated with late endosomes and likely mediates filovirus budding due to its ability to induce its own release from cells in the absence of all other viral proteins. The second matrix protein, VP24, has been shown to suppress interferon production. However, interferon interference may not be its only function. Other experiments have shown that this protein, along with VP35 and NP are sufficient to form nucleocapsid structures. Lastly, VP24 is necessary for the correct assembly of a functional nucleocapsid, as a lack of VP24 leads to reduced transcription/translation of VP30. The remaining structural proteins form the nucleocapsid, and are thus intimately associated with the viral genome. These are the nucleoprotein NP, the polymerase cofactor VP35, the viral-specific transcription activator VP30 and the viral RNA polymerase L. These nucleocapsid proteins have a dual function in the viral replication cycle: they are involved as structural components, but also catalyze replication and transcription of the genome. While NP, VP35 and L are sufficient for replication, transcription initiation will not proceed without VP30.
_
There’s also evidence that the glycoprotein (GP) is what actually kills individual cells. Inserting the gene alone into cells that normally line blood vessels is enough to cause their deaths. Glycoprotein appears to kill cells by blocking their ability to put new proteins on their surface. This causes the cells to lose contact with their neighbors and die. (It also has the side effect of limiting cells’ ability to inform the immune system that they are infected.) Further studies suggest that this effect is level-dependent; moderate amounts of glycoprotein don’t cause cells much difficulty. It’s only the high levels that accumulate later in infections that can kill them. This ensures that high levels of virus are made before their host dies.
_
Viral Replication:
Viruses are unique pathogens in that they use host cell machinery to make their viral proteins and assemble new virus particles, or virions. In other words, they carry their genetic blueprint with them but have the cell they infect do all production and assembly of new virions. Conceptually, they hijack cellular factories in order to replicate. In order for Ebola to infect and replicate it must be able to accomplish two things: it must enter a host cell and it must utilize host cell machinery to produce new virions that can then go on to infect the next individual. This is termed “productive infection.” In the absence of those two things Ebola infection does not spread and would be considered “abortive infection,” meaning the process ends because replication cannot occur.
_
Filovirus Transcription:
During transcription, the RNA genome is transcribed into seven monocistronic mRNAs whose length is determined by highly conserved start and stop signals. These start signals are predicted to form stable stem-loop structures. Just as in other negative sense RNA viruses, the transcription process begins with the binding of the polymerase complex to a single binding site located within the leader region of the genome. The complex then slides along the RNA template and sequentially transcribes the individual genes in their 3’ to 5’ order. However, the polymerase is released from the template following mRNA formation, so reinitiation at downstream genes is attenuated. Thus, the first gene, NP, is transcribed at the highest levels, whereas the last gene, L, is transcribed at the lowest. VP30 is assumed to be a transcription activation factor that is essential for the viral life cycle. While the mechanism is not completely understood, it is suggested that this protein is involved in initiation because VP30-dependent transcription is regulated by RNA of the first transcription start signal. The first 23 nucleotides of this NP mRNA are involved in stem-loop structure formation which might interfere with the progression of transcription by physically hampering polymerase movement. However, the N-terminus of VP30 contains a Zn+2 binding Cys-His motif and is rich in basic amino acids, allowing it to directly interact with RNA. Therefore, the protein could either resolve or cover this secondary structure and allow transcription to proceed. Further research has also shown that VP30 is important in transcription reinitiation, and may bind stem-loops formed by the promoter of each Ebola virus gene. Interestingly, VP24 has also been shown to inhibit transcription and replication of the Ebola virus genome. While the exact mechanism has not yet been elucidated, it is possible that VP24 binds to NP and hampers the function of VP35, VP30 or L. This interference could be important in converting the viral genome from a transcription or replication competent form to one that is ready for virion assembly and egress.
_
Disruption of Cell Adhesion:
Expression of Ebola GP in cultured cells causes a disruption in cell adhesion that results in a loss of cell-cell contacts, as well as a loss of attachment to the culture substrate. The effects of GP are caused by the mucin domain, a highly glycosylated region of GP1 composed of roughly 150 amino acids and containing numerous N- and O- linked glycosylation sites. While this loss of endothelial cell attachment is key for the characteristic hemorrhaging, only recently has a mechanism for the disturbance of cell adhesion been proposed. For years, staining by flow cytometry has associated Ebola infection with a reduction in membrane levels of β1 integrin, a receptor that mediates attachment with the extracellular matrix, as well as major histocompatibility complex 1 (MHC1), a molecule important in immune system recognition. While these effects were previously assumed to result from removal of surface proteins, Francica et al. propose that GP-mediated loss of surface protein recognition occurs via steric shielding of surface epitopes. Observations that this down regulation is relieved by enzymatic removal of carbohydrate modification suggest that the steric occlusion is mediated by N- and O-linked modification of GP. In fact, the O-linked glycosylation of the mucin domain may promote an extended conformation that allows this domain to serve as a 150 residue long flexible rod that can mask domains in the immediate vicinity. Inherent in this mechanism is the fact that GP must localize in close proximity to the affected proteins, possibly explaining the variety of cell receptors regulated by this viral protein. This mechanism also helps the virus in immune system avoidance. The ability of GP to mask MHC1 may be a strategy for avoiding CD8 T cell-mediated killing of Ebola infected cells.
_________
Ebola virus mutation:
Mutations are a way of life for an RNA virus and mutations come and go every time a genome replicates – it is likely that every single genome copy of an RNA virus has a mutation. The key is to determine whether these changes affect any of the biological properties of the virus, such as transmission, stability, or virulence. Recent advances in genomic technologies have been applied to the analysis of blood samples from those infected in the 2014 outbreak. A massively parallel viral sequencing of genetic material collected from 78 patients with confirmed Ebola virus disease, representing more than 70% of cases diagnosed in Sierra Leone from late May to mid-June, 2014 was carried out. This work provided near–real-time insights into the transmission dynamics and genetic evolution, shedding light on the origins of the virus causing the 2014 outbreak in West Africa, and whether the 2014 outbreak is still being fed by new contacts with its natural reservoir (no such evidence was found). Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak and concludes that the current outbreak probably resulted from the spread of the virus from central Africa in the past decade. As is typical of RNA-coded viruses, the Ebola virus was found to mutate rapidly, both within a person during the progression of disease and in the reservoir among the local human population. The observed mutation rate of 2.0 x 10-3 substitutions per site per year is as fast as that of seasonal influenza. This is likely to represent rapid adaptation to human hosts as the virus is repeatedly passed from human to human (as opposed to usually being passed between fruit bats and only occasionally crossing over into humans), and may pose challenges for the development of a vaccine to the virus. The genetic study by Gire and his colleagues (five of whom were dead of Ebola by the time their study appeared) found 341 mutations as of late August, some of which are significant enough to change the virus’s functional identity. The higher the case count in West Africa goes, the more chances for further mutations, and therefore the greater possibility that the virus might adapt somehow to become more transmissible-perhaps by becoming less pathogenic, sickening or killing its victims more slowly and thereby leaving them more time to infect others. That’s why, the Gire group wrote, we need to stop this thing everywhere as soon as possible. Future spillovers of Ebola are bound to occur, but those freshly emerged strains of the virus, direct from the reservoir host, won’t contain any adaptive mutations that the West Africa strain is acquiring now. We need to terminate Ebola 2014 before the virus learns too much about us.
_
Ebola 2014 is mutating as fast as Seasonal Flu: a 2014 study:
The current Ebola 2014 virus is mutating at a similar rate to seasonal flu (Influenza A). This means the current Ebola outbreak has a very high intrinsic rate of viral mutation. The bottom line is that the Ebola virus is changing rapidly, and in the intermediate to long term (3 months to 24 months), Ebola has the potential to evolve. Authors cannot predict exactly what the Ebola virus will look like in 24 months. There is an inherent stochastic randomness to viral evolution which makes predictions on future viral strains difficult, if not impossible. One basic tenet they can rely on is this: Viruses tend to maximize their infectivity (basic reproduction number) within their biological constraints (Nowak, 2006). These evolutionary constraints can be extremely complex, and can include trade-offs between virulence and infectivity, conditions of superinfection, host population dynamics, and even outbreak control measures.
_
Ebola Mutation Rate:
Analysis of the available research suggests that the Ebola 2014 virus is currently mutating at a rate 200% to 300% higher than historically observed (Gire, 2014). Furthermore, the Ebola-2014 virus’s mutation rate of 2.0 x 10−³ subs/site/year is nearly identical to Influenza A’s mutation rate of 1.8 x 10−³ subs/site/year (Jenkins, 2002). This means Ebola 2014 is mutating as fast as seasonal flu.
_
_
Many recent Ebola viral mutations have been synonymous mutations, some have been in intergenic regions, while others are non-synonymous substitutions in protein-coding regions. All have unknown impact at the present time. Such questions should be the subject of future scientific research. Until the Ebola outbreak is brought under control, the Ebola-2014 virus will continue to seed and adapt in its growing pool of West African human hosts. We need to consider that as the weeks and months go on, the rapidly-changing Ebola-2014 virus will undergo repeated export from the West African region to countries around the world. As new Ebola cases grow in West Africa and elsewhere, we are effectively conducting ‘serial passage’ experiments of Ebola-2014 through human hosts. The repeated passage of Ebola-2014 through humans is exerting selection pressure on the Ebola-2014 virus to adapt to our species (instead of fruit bats). The introduction of Ebola-2014 into a large pool of West African human hosts (coupled with the complex dynamics of evolutionary selection pressure) may allow the Ebola-2014 virus to become more transmissible as the months go on, particularly in the absence of effective control interventions.
_
Authors chose to compare Ebola-2014 to Influenza A (Seasonal Flu) because Influenza is one of the fastest-mutating viruses (Jenkins, 2002). Unlike chickenpox (VZV), which people usually only contract once per lifetime, Influenza can infect a single individual many times repeatedly over the years. One of the reasons Influenza is able to re-infect humans each year is because the Influenza’s high mutation rate allows the virus to generate ‘escape mutants’. Escape mutants are Influenza viruses which are no longer recognized by human immune systems. Each winter presents us with a new mutated strain of the Influenza virus. Rapid mutation is beneficial to Influenza genetic fitness (in regards to antigenic regions), because it allows a ‘new’ Influenza virus to circulate year after year. The benefit of a high mutation rate in Ebola 2014 is different — the genetic changes in Ebola-2014 allow for rapid exploration of the entire fitness landscape in a brand new host — humans. We need to be aware that the Ebola-2014 virus is undergoing rapid adaptation. The high mutation rate we see in Ebola-2014 reflects its ability to rapidly explore the fitness landscape. The ability of Ebola to undergo rapid genome substitutions and SNPs, coupled with genetic recombination, will allow ‘survival of the fittest’ in Ebola-2014 genetic variants (on both the intra-host and inter-host levels). New Ebola sub-clades are created with each passing month (there are already four sub-clades as of August 2014). New Ebola genetic variants are created with each new infection, though most are selected against. Rapid adaptation emerges from the high intrinsic Ebola-2014 mutation rate, coupled with the virus’s ability to undergo RNA recombination during superinfection.
_
Figure above shows acquisition of genetic variation of ebola-2014 virus over time. Fifty mutational events (short dashes) and 29 new viral lineages (long dashes) were observed.
_
The diagram above suggests that as the Ebola-infected host pool grows, so does the number of unique Ebola viral lineages. This implies that Ebola acquires genetic diversity as it infects more people, particularly if the virus undergoes recombination during superinfection. The growing number of new Ebola viral lineages will undergo natural selection for some ‘optimum’ balance of virulence, infectivity, tissue tropism, immune suppression, and other parameters which maximize the reproductive fitness of the Ebola virus in humans. What that final virus might eventually look like 2 years from now is anyone’s guess. But the explosion of genetic variation suggests that the Ebola virus will become more difficult to contain as time goes on, which is why early action is important. The idea that the Ebola-2014 Virus jumped species, but is now somehow ‘static’ or ‘frozen in time’ is a mistake. The Ebola-2014 virus is undergoing a period of rapid adaptation in human hosts, as evidenced by the Ebola RNA sequences deposited in Genbank, and other studies. Hopefully, interventions (like contact tracing) will be able to stop Ebola-2014 before the virus optimizes its genotype.
_
As more people become infected, a significant mutation arises that allows for a longer asymptomatic but infectious period, increasing the Ro. Globally, cases continue to double every 16 days, contact tracing infrastructure outside the West becomes saturated, and hospitals are overrun. By early-to-mid 2015, the global pools of Ebola-infected patients are in the millions, mainly centered in West Africa and Southeast Asia with multiple strains of varying virulence. A sudden change in the outbreak epidemiology caused by a recombinant Ebola strain causes confusion about how to respond. Efforts at developing treatments/vaccines become logistically complex and ineffective. The implication of the Ebola 2014 mutation rate is this: A single Ebola mutation doesn’t necessarily mean the virus will become ‘airborne’, or that the virus has altered tissue tropism, or that the virus spreads more easily. But a high intrinsic rate of Ebola mutation means that such changes may become possible in the future. If the number of people infected grows into the hundreds of thousands, or even low millions, then the probability of a significant ‘constellation’ of accumulated Ebola mutations with phenotypic impact becomes more likely. The problem is that accumulated Ebola mutations will scale with the size of the population infected. Conversely, in a small population, such Ebola mutations are not likely to have a significant impact. It’s a bit like the virus is buying lottery tickets… The more lottery tickets the Ebola virus ‘buys’, the more chances it has to ‘win’.
_
Scientists use Mutations to track Ebola 2014 Origins:
In a new paper in Science, researchers reveal that they have sequenced the genomes of Ebola from 78 patients in Sierra Leone who contracted the disease in May and June. Those sequences revealed some 300 mutations specific to this outbreak. The new analysis could help determine if the virus’ behavior has changed — and provide information for future diagnostic tests and treatments. Among their findings, the researchers discovered that the current viral strains come from a related strain that left Central Africa within the past ten years. And the research confirms that the virus likely spread into Sierra Leone when women became infected after attending the funeral of a traditional healer who had been treating Guinean Ebola patients.
_
An expert scientist from the National Institute of Allergy and Infectious Disease (NIAID) has publicly warned that the Ebola strain currently in circulation appears to be far more virulent and infectious than previous strains. Dr. Peter Jahrling has been on the ground in the Liberian capital of Monrovia, studying the disease with a team of researchers, which is also helping to care for and treat patients. He says the viral loads that his team is witnessing exceed what has been observed during previous outbreaks, suggesting that, this time, the disease is far more deadly. Echoing the warnings given by others, Dr. Jahrling believes that this strain of Ebola is not only more deadly than other strains but also mutating at an alarming rate. More of the virus is infecting patients, and it appears to be advancing and spreading more rapidly than usual. “We are using tests now that weren’t [used] in the past, but there seems to be a belief that the virus load is higher in these patients [today] than what we have seen before,” stated Dr. Jahrling to Vox. “If true, that’s a very different bug.”
______
Filoviruses are ancient and integrated into mammalian genomes: a 2010 study:
Integrated elements of filoviruses could indicate a coevolutionary history with a mammalian reservoir, but integration of nonretroviral RNA viruses is thought to be nonexistent or rare for mammalian viruses (such as filoviruses) that lack reverse transcriptase and replication inside the nucleus. Here, authors provide direct evidence of integrated filovirus-like elements in mammalian genomes by sequencing across host-virus gene boundaries and carrying out phylogenetic analyses. Further they test for an association between candidate reservoir status and the integration of filoviral elements and assess the previous age estimate for filoviruses of less than 10,000 years. In 19 of the tested vertebrate species, authors discovered as many as 80 high-confidence examples of genomic DNA sequences that appear to be derived, as long ago as 40 million years, from ancestral members of 4 currently circulating virus families with single strand RNA genomes. Surprisingly, almost all of the sequences are related to only two families in the Order Mononegavirales: the Bornaviruses and the Filoviruses, which cause lethal neurological disease and hemorrhagic fevers, respectively. Phylogenetic and sequencing evidence from gene boundaries was consistent with integration of filoviruses in mammalian genomes. Authors detected integrated filovirus-like elements in the genomes of bats, rodents, shrews, tenrecs and marsupials. Moreover, some filovirus-like elements were transcribed and the detected mammalian elements were homologous to a fragment of the filovirus genome whose expression is known to interfere with the assembly of Ebolavirus. The phylogenetic evidence strongly indicated that the direction of transfer was from virus to mammal. Eutherians other than bats, rodents, and insectivores (i.e., the candidate reservoir taxa for filoviruses) were significantly underrepresented in the taxa with detected integrated filovirus-like elements. The existence of orthologous filovirus-like elements shared among mammalian genera whose divergence dates have been estimated suggests that filoviruses are at least tens of millions of years old. Author’s findings indicate that filovirus infections have been recorded as paleoviral elements in the genomes of small mammals despite extranuclear replication and a requirement for cooption of reverse transcriptase. Author’s results show that the mammal-filovirus association is ancient and has resulted in candidates for functional gene products (RNA or protein).
_
Ancient ebola virus: a 2014 study:
A new study is helping to rewrite Ebola’s family history. It shows that Ebola and Marburg are each members of ancient evolutionary lines, and that these two viruses last shared a common ancestor sometime prior to 16-23 million years ago. The research shows that filoviruses — a family to which Ebola and its similarly lethal relative, Marburg, belong — are at least 16-23 million years old. According to the PeerJ article, knowing more about Ebola and Marburg’s comparative evolution could “affect design of vaccines and programs that identify emerging pathogens.” The research does not address the age of the modern-day Ebolavirus. Instead, it shows that Ebola and Marburg are each members of ancient evolutionary lines, and that these two viruses last shared a common ancestor sometime prior to 16-23 million years ago. The new study builds on the study depicted in previous paragraph, which used viral fossil genes to estimate that the entire family of filoviruses was more than 10 million years old. However, those studies used fossil genes only distantly related to Ebola and Marburg, which prevented the researchers from drawing conclusions about the age of these two viral lines. The current PeerJ publication fills this viral “fossil gap,” enabling the scientists to explore Ebola’s historical relationship with Marburg.
___________
The Filoviridae Journey:
After transmission to a new host, the virus enters a cell through a mechanism that has yet to be determined. Once inside the host cell’s cytoplasm, the filovirus uncoats itself and releases transcriptase (polymerase), which is contained in the virion. Transcriptase transcribes the viral -ssRNA into the complimentary +ssRNA. This positive, single stranded RNA will then be used as the template for the new viral genomes. Soon after the infection, the cell develops cytoplasmic inclusion bodies that contain the highly structured viral nucleocapsid (the nucleocapsid contains the genome and can sometimes have other proteins in it as well). After the nucleocapsid has been formed, the new virus will self-assemble and bud from the cell membrane stealing some of the membrane for its envelope. Not very much is known about the pathogenesis of filoviruses. But we do know that Ebola attacks cells important to the function of lymphatic tissues. It can be found in fibroblastic reticular cells (FRC) among the loose connective tissue under the skin and in the FRC conduit (FRCC) in lymph nodes. This allows the virus to rapidly enter the blood and leads to disruption of lymphocyte homing at high endothelial venules (HEV). Ebola virus seems to be most active in infecting fibroblasts of any type (especially fibroblastic reticular cells). The next most frequent cell types are mononuclear phagocytes with dendritic cells more affected than monocytes or macrophages. Endothelial cells become infected after the connective tissue surrounding them is fully involved. Then, almost as a final insult, epithelial cells of any type are infected. In general, epithelial cells become infected only if they contact other cells that amplify the virus such as fibroblastic reticular cells (FRC) and mononuclear cells. This would be true for skin appendages like hair follicles and sweat glands because they are heavily vascularized and have a lot of FRC networks associated with them. Liver cells and adrenal gland epithelial cells have fibroblastic reticulum as their main connective tissue and both have resident mononuclear cell phagocytes hanging on FRC cells near the blood/epithelial cell interface.
_____
Route of infection:
_
The figure below shows modes of viral entry:
_
Ebola virus seems to enter the host through mucosal surfaces, breaks, and abrasions in the skin, or by parenteral introduction. Most human infections in outbreaks seem to occur by direct contact with infected patients or cadavers. Infectious virus particles or viral RNA have been detected in semen, genital secretions, and in skin of infected patients; they have also been isolated from skin, body fluids, and nasal secretions of experimentally infected non-human primates.
_
Laboratory exposure through needle stick and blood has been reported. Reuse of contaminated needles played an important part in the 1976 outbreaks of Ebola virus in Sudan and Zaire. Butchering of a chimpanzee for food was linked to outbreaks of Zaire Ebola virus in Gabon, and contact exposure was the probable route of transmission. Although proper cooking of foods should inactivate infectious Ebola virus, ingestion of contaminated food cannot wholly be ruled out as a possible route of exposure in natural infections. Notably, handling and consumption of freshly killed bats was associated with an outbreak of Zaire Ebola virus in DRC. Organ infectivity titers in non-human primates infected with Ebola virus are frequently in the range of 107 to 108 pfu/g; thus, exposure through the oral route could invariably be associated with very high infectious doses. In fact, Zaire Ebola virus is highly lethal when given orally to rhesus macaques. The role of aerosol transmission in outbreaks is unknown, but is thought to be rare. In human beings, the route of infection seems to affect the disease course and outcome. The mean incubation period for cases of Zaire Ebola virus infection known to be due to injection is 6·3 days, versus 9·5 days for contact exposures. Moreover, the case-fatality rate in the 1976 outbreak of Zaire Ebola virus was 100% (85 of 85) in cases associated with injection compared with about 80% (119 of 149) in cases of known contact exposure. For non-human primates infected with Zaire Ebola virus, the disease course seems to progress faster in animals exposed by intramuscular or intraperitoneal injection than in animals exposed by aerosol droplets.
_
Virus entry: Macropinocytosis (a type of endocytosis):
As the first step of the viral life cycle, entry into the host cell is a popular drug target as infection could be stopped before replication disrupts cell function. However, the entry mechanism of Ebola virus is poorly understood. Endocytosis offers an efficient way for viruses to cross the significant physical barrier imposed by the plasma membrane and to traverse the underlying cortical matrix. Viruses have also evolved to target distinct endocytic pathways that are capable of delivering the capsid into the cell cytoplasm at sites suitable to initiate replication and to avoid destructive compartments like the lysosome. Understanding the pathway of virus entry and deciphering the mechanism regulating it is important for understanding viral pathogenesis as virus entry into host cell is the first critical step in pathogenesis of infection. While there is ample evidence that ZEBOV enters cells through endocytosis in a pH-dependent manner, the specific endocytic and trafficking pathways have not been clearly defined. Many enveloped viruses, Ebola virus included, rely upon endocytosis to infect cells. Several distinct endocytic mechanisms exist in mammalian cells, and can be distinguished by the type of cargo they carry as well as the proteins involved in their regulation. However, all mechanisms ultimately transport virions through successive endocytic vesicles until a compartment with adequate conditions, low pH in the case of Ebola, is reached. Upon membrane fusion, the capsid moves into the cell cytoplasm at a site where replication proceeds optimally. Recently experiments have been performed with wild-type Ebola virus Zaire that demonstrates that cellular entry involves uptake by a macropinocytosis-like mechanism. Macropinocytosis is one of the path that has already been shown to be important for the uptake of vaccinia virus. This mechanism is associated with outward extensions of the plasma membrane formed by actin polymerization. These so called membrane ruffles can fold back upon themselves and form a macropinosome upon fusion of the distal loop ends. The involvement of macropinocytosis was tested through the use of EIPA (5-(N-ethyl-N-isopropyl amiloride), a potent and specific inhibitor of Na+/H+ exchanger activity important for macropinosome formation. They discovered a dose-dependent inhibition of gfpZEBOV infection as well as severe inhibition of ZEBO-VLP entry in the presence of this amiloride. Further experiments determined that Ebola virions colocalize with internalized dextran, a complex polysaccharide taken in by macropinocytosis, and requires the activity of p53-activated kinase 1, another hallmark of this entry pathway. Lastly, it was noted that gfpZEBO-VLPs were associated with Arp2 and vasodilator-stimulated phosphoprotein (VASP), two actin-associated proteins that promote actin assembly. This also points towards macropinocytosis, since actin is required for the formation of plasma membrane ruffles as well as vesicle trafficking. All of these findings points towards a macropinocytosis-like pathway as the primary internalization method of Ebola. Authors also indicate a role of actin in viral entry and suggest that the virus can actively promote localized actin remodeling through its interaction with Arp2 and VASP. The mechanism by which the virus causes macropinocytosis is not understood, but most likely involves the interaction of GP with cell surface receptors. The receptor tyrosine kinase Ax1 and integrin βI have been implicated as viral receptors, with evidence that several other receptor tyrosine kinases and integrins can trigger macropinocytosis.
_
Virus entry needs two host proteins: NPC1 and TIM-1:
There are two candidates for host cell entry proteins. The first is a cholesterol transporter protein, the host-encoded Niemann–Pick C1 (NPC1), which appears to be essential for entry of Ebola virions into the host cell and for its ultimate replication. In one study, mice with one copy of the NPC1 gene removed showed an 80 percent survival rate fifteen days after exposure to mouse-adapted Ebola virus, while only 10 percent of unmodified mice survived this long. In another study, small molecules were shown to inhibit Ebola virus infection by preventing viral envelope glycoprotein (GP) from binding to NPC1. Hence, NPC1 was shown to be critical to entry of this filovirus, because it mediates infection by binding directly to viral GP. When cells from Niemann Pick Type C patients lacking this transporter were exposed to Ebola virus in the laboratory, the cells survived and appeared impervious to the virus, further indicating that Ebola relies on NPC1 to enter cells; mutations in the NPC1 gene in humans were conjectured as a possible mode to make some individuals resistant to this deadly viral disease. The same studies described similar results regarding NPC1’s role in virus entry for Marburg virus, a related filovirus. A further study has also presented evidence that NPC1 is critical receptor mediating Ebola infection via its direct binding to the viral GP, and that it is the second “lysosomal” domain of NPC1 that mediates this binding.
_
People whose cells lack or don’t have properly functioning NPC1 protein get NPC disease. There are several research groups looking for compounds that can block the protein on the Ebola virus from coming into contact with NPC1 protein. These drugs ultimately could be used as a means to prevent people from getting infected or for making an infection less severe. Parents of children with NPC disease are supporting research into the Ebola-NPC connection. Researchers want to know if Ebola survivors also have mutations on one copy of their NPC gene. And by studying cells from parents who are NPC carriers, they hope to better understand how changes on the NPC gene might lower the risk of dying from Ebola virus. There are an estimated 500 cases of NPC disease diagnosed world-wide, mostly in children. Researchers in the Ebola field say there is still a lot to learn. In mice, at least, they believe that the NPC1 gene plays an essential role in Ebola infection, but how that finding translates into humans remains under study. They also contend that there are a number of different genes and other factors, from the amount of virus to someone’s underlying health, that likely play a role in Ebola survival as well.
_
The second candidate is TIM-1 (aka HAVCR1). T-cell Ig and mucin domain 1 (TIM-1) binds to the receptor binding domain of the Zaire Ebola virus (EBOV) glycoprotein, and ectopic TIM-1 expression in poorly permissive cells enhances EBOV infection by 10- to 30-fold. TIM-1 was shown to bind to the receptor binding domain of the EBOV glycoprotein, to increase the receptivity of Vero cells. Silencing its effect with siRNA prevented infection of Vero cells. TIM-1 expression within the human body is broader than previously appreciated, with expression on mucosal epithelia from the trachea, cornea, and conjunctiva—tissues believed to be important during in vivo transmission of filoviruses. Recognition that TIM-1 serves as a receptor for filoviruses on these mucosal epithelial surfaces provides a mechanistic understanding of routes of entry into the human body via inhalation of aerosol particles or hand-to-eye contact. TIM1 is expressed in tissues known to be seriously impacted by EBOV lysis (trachea, cornea, and conjunctiva). A monoclonal antibody against the IgV domain of TIM-1, ARD5, blocked EBOV binding and infection. Together, these studies suggest NPC1 and TIM-1 may be potential therapeutic targets for an Ebola anti-viral drug and as a basis for a rapid field diagnostic assay.
_
Replication:
_
Being acellular, viruses such as Ebola do not replicate through any type of cell division; rather, they use a combination of host- and virally encoded enzymes, alongside host cell structures, to produce multiple copies of themselves. These then self-assemble into viral macromolecular structures in the host cell. The virus completes a set of steps when infecting each individual cell: The virus begins its attack by attaching to host receptors through the glycoprotein (GP) surface peplomer and is endocytosed into macropinosomes in the host cell. To penetrate the cell, the viral membrane fuses with vesicle membrane, and the nucleocapsid is released into the cytoplasm. Encapsidated, negative-sense genomic ssRNA is used as a template for the synthesis (3′-5′) of polyadenylated, monocistronic mRNAs and, using the host cell’s ribosomes, tRNA molecules, etc., the mRNA is translated into individual viral proteins. These viral proteins are processed, a glycoprotein precursor (GP0) is cleaved to GP1 and GP2, which are then heavily glycosylated using cellular enzymes and substrates. These two molecules assemble, first into heterodimers, and then into trimers to give the surface peplomers. Secreted glycoprotein (sGP) precursor is cleaved to sGP and delta peptide, both of which are released from the cell. As viral protein levels rise, a switch occurs from translation to replication. Using the negative-sense genomic RNA as a template, a complementary +ssRNA is synthesized; this is then used as a template for the synthesis of new genomic (-)ssRNA, which is rapidly encapsidated. The newly formed nucleocapsids and envelope proteins associate at the host cell’s plasma membrane; budding occurs, destroying the cell.
_
The figure below shows replication of ebola virus in host cell:
_
Budding of ebola virus from infected host cell:
_
Once an Ebola particle enters the bloodstream, it drifts until it sticks to a cell. The particle is pulled inside the cell, where it takes control of the cell’s machinery and causes the cell to start making copies of it. Most viruses use the cells of specific tissues to copy themselves. For example, many cold viruses replicate in the sinuses and the throat. Ebola attacks many of the tissues of the body at once, except for the skeletal muscles and the bones. It has a special affinity for the cells lining the blood vessels, particularly in the liver. After about eighteen hours, the infected cell is releasing thousands of new Ebola particles, which sprout from the cell in threads, until the cell has the appearance of a ball of tangled yarn. The particles detach and are carried through the bloodstream, and begin attaching themselves to more cells, everywhere in the body. The infected cells begin spewing out vast numbers of Ebola particles, which infect more cells, until the virus reaches a crescendo of amplification. The infected cells die, which leads to the destruction of tissues throughout the body. This may account for the extreme pain that Ebola victims experience. Multiple organs fail, and the patient goes into a sudden, steep decline that ends in death. In a fatal case, a droplet of blood the size of the “o” in this text could easily contain a hundred million particles of Ebola virus.
_
Target cells and tissues:
Ebola virus has a broad cell tropism, infecting a wide range of cell types. In-situ hybridisation and electron microscopic analyses of tissues from patients with fatal disease or from experimentally infected non-human primates show that monocytes, macrophages, dendritic cells, endothelial cells, fibroblasts, hepatocytes, adrenal cortical cells, and several types of epithelial cells all lend support to replication of these viruses. Temporal studies in non-human primates experimentally infected with Zaire Ebola virus suggest that monocytes, macrophages, and dendritic cells are early and preferred replication sites of these viruses. These cells seem to have pivotal roles in dissemination of the virus as it spreads from the initial infection site via monocytes, macrophages, and dendritic cells to regional lymph nodes, probably through the lymphatic system, and to the liver and spleen through the blood. Monocytes, macrophages, and dendritic cells infected with Ebola virus migrate out of the spleen and lymph nodes to other tissues, thus disseminating the infection.
_______
Ebola virus pathogenesis:
_
Humans infected with ebolaviruses commonly present initially with nonspecific symptoms such as fever, vomiting, and severe diarrhea, with visible hemorrhage occurring in less than half the cases, as in the current outbreak. Owing to poor infrastructure, biosafety concerns associated with processes of patient care and autopsy, and the essential focus on disease containment during outbreaks, there has been little empirical study to elucidate the pathogenesis or pathology of human ebolavirus infection. The closest surrogate disease models are cynomolgus and rhesus macaques, which show clinical signs of viral hemorrhagic fever when infected with most ebolaviruses. Zaire ebolavirus is uniformly lethal in these macaques, and experts have assumed that its pathology and pathophysiology closely resemble those of ebolavirus infections in humans; immunosuppression, increased vascular permeability, and impaired coagulation have been identified as hallmarks of the disease. Evidence of microscopic hemorrhage is usually found, but the degree of bleeding ranges from undetectable to acutely visible. The recently introduced term “Ebola virus disease” may not convey the seriousness of a viral hemorrhagic fever, a clinical syndrome that should trigger isolation guidelines that ensure appropriate case management and implementation of infection-control measures.
_
The figure below shows how infected monocytes release cytokines that damage endothelial cells:
_
Initially during initial stages of infection, the Ebola virus selectively targets dendritic cells, monocytes and macrophages, which spread through the circulatory and lymphatic systems to the liver spleen and lymph nodes. From here the virus can efficiently spread throughout the body. The infected monocytes and macrophages also release massive amounts of cytokines, helping to trigger virus-induced shock by causing damage to the endothelial structures. Infected dendritic cells are prevented from releasing co-stimulatory cytokines necessary for the production of T-cells, preventing sufficient immune response to the infection. The massive release of cytokines and virus particles from monocytes and macrophages impairs the function of endothelial tissue, allowing it to become permeable to water and macromolecules. Virus spreads from the initial infection site (small lesions) to regional lymph nodes, liver, and spleen. Although ebola virus does not infect lymphocytes, their rapid loss by apoptosis is a prominent feature of disease. The direct interaction of lymphocytes with viral proteins cannot be discounted as having a role in their destruction, but the substantial loss of lymphocytes probably results from a combination of factors including infection-mediated impairment of dendritic cells and release of soluble factors from monocytes and macrophages. Soluble factors released from target cells also contribute to the impairment of the vascular system leading to vascular leakage. The systemic virus spread and replication, the general dysregulation of the host immune response, the coagulation abnormalities, the impairment of the vascular system, and hypotension all together finally result in shock and multi-organ failure.
_
Although the endothelium is thought to play an important part in the pathogenesis of Ebola virus, studies defining the molecular mechanisms of endothelial impairment are incomplete. Researchers thought that the virus’ glycoprotein is the primary determinant of vascular-cell injury and that Ebola virus infection of endothelial cells induces structural damage, which could contribute to the haemorrhagic diathesis. However, histological analysis of autopsy tissues from several of the early outbreaks did not identify vascular lesions, and no vascular lesions in any subsequent studies have been reported so far. Similarly, no evidence of substantial vascular lesions in non-human primates infected with Ebola virus exists. In one temporal study in cynomolgus macaques, infection of endothelial cells by Zaire Ebola virus was infrequent and was mainly restricted to the terminal stages of disease.
_
Together with the macrophage-rich lymphoid tissues, the liver and the adrenal gland seem to be important targets for filoviruses, and this tropism probably has an equally important role in the disease pathogenesis. Various degrees of hepatocellular necrosis have been reported in infected people and non-human primates; however, the hepatocellular lesions are generally not serious enough to explain the cause of death. Importantly, haemorrhagic tendencies could be related to decreased synthesis of coagulation and other plasma proteins because of severe hepatocellular necrosis. Adrenocortical infection and necrosis have also been reported in humans and non-human primates infected with Ebola virus. The adrenal cortex plays an important part in control of blood pressure homoeostasis. Impaired secretion of enzymes that synthesise steroids leads to hypotension and sodium loss with hypovolaemia, which are important elements that have been reported in nearly all cases of Ebola haemorrhagic fever. Impairment of adrenocortical function by Ebola virus infection could therefore have an especially important role in the evolution of shock that typifies late stages of Ebola haemorrhagic fever.
_
In addition to sustaining direct damage from viral infection, patients infected with Ebola virus (Zaire) have high circulating levels of proinflammatory cytokines, which presumably contribute to the severity of the illness. In fact, the virus interacts intimately with the cellular cytokine system. It is resistant to the antiviral effects of interferon , although this mediator is amply induced. Viral infection of endothelial cells selectively inhibits the expression of major histocompatibility complex class I molecules and blocks the induction of several genes by the interferons. In addition, glycoprotein expression inhibits V integrin expression, an effect that leads to detachment and subsequent death of endothelial cells in vitro and that correlates with the limited inflammatory response evident in lesions.
_
Acute infection is associated with high levels of circulating virus and viral antigen. Clinical improvement takes place when viral titers decrease concomitant with the onset of a virus-specific immune response, as detected by enzyme-linked immunosorbent assay (ELISA) or fluorescent antibody testing. In fatal cases, there is usually little evidence of an antibody response, and there is extensive depletion of spleen and lymph nodes. Ebola Sudan virus amplification by PCR shows a correlation between serum viral RNA concentration and the likelihood of death. Recovery is apparently mediated by the cellular immune response: convalescent-phase plasma has little in vitro virus-neutralizing capacity and is not protective in humans or in passive transfer experiments in monkey and guinea pig models.
_
Immune System Evasion:
One of the reasons that Ebola is so deadly is that it has multiple ways of interfering with or avoiding the human immune system. While the virus is busy destroying the human body, the immune system is either still in the process of discovering that there is a problem, or is in such disarray that it would be next to impossible to mobilize a unified effort to fight off the invader. Among the five strains of Ebola virus, the Zaire strain appears to be the most virulent, with a mortality rate of up to 90%. Despite extensive research, the molecular basis for this virulence has not been determined. Fatal Ebola infections are marked by unchecked viral replication combined with an inadequate antiviral response. In order for this to occur, the early antiviral innate immune response must be delayed or inhibited. During infection, monocytes/macrophages in the lymphoid tissues are early and sustained targets of this deadly virus. Since these cells usually elicit the response cascade in the acute phage of inflammation, their early infection helps Ebola evade the immune system while subsequently spreading throughout the host. In addition, infected macrophages release increased amounts of nitric oxide (NO), a gaseous hormone that normally functions in cell communication. However, in high concentrations, NO depresses the mitochondrial membrane potential, causing apoptosis in nearby natural killer cells. So far, most of the processes by which Ebola escapes or hampers immune response involve viral structure proteins.
_
Most filovirus proteins are encoded in single reading frames; the surface GP is encoded in 2 frames (open reading frame [ORF] I and ORF II). The ORF I (amino-terminal) of the gene encodes for a small (50-70 kd), soluble, nonstructural secretory glycoprotein (sGP) that is produced in large quantities early in Ebola virus infection. The sGP binds to neutrophil CD16b, a neutrophil-specific Fc g receptor III, and inhibits early neutrophil activation. The sGP also may be responsible for the profound lymphopenia that characterizes Ebola infection. Thus, sGP is believed to play pivotal roles in the ability of Ebola to prevent an early and effective host immune response. One hypothesis is that the lack of sGP production by Marburg virus may explain why this agent is less virulent than African-derived Ebola virus. Leroy et al reported their observations of 24 close contacts of symptomatic patients actively infected with Ebola. Eleven of the 24 contacts developed evidence of asymptomatic infection associated with viral replication. Viral replication was proven by the author’s ability to amplify positive-stranded Ebola virus RNA from the blood of the asymptomatic contacts. A detailed study of these infected but asymptomatic individuals revealed that they had an early (4-6 days after infection) and vigorous immunologic response with production of interleukin (IL) and tumor necrosis factor (TNF), resulting in enhanced cell-mediated and humoral-mediated immunity. In patients who eventually died, proinflammatory cytokines were not detected even after 2-3 days of symptomatic infection. A second, somewhat larger (120-150 kd) GP, transmembrane glycoprotein, is incorporated into the Ebola virion and binds to endothelial cells but not to neutrophils. Ebola virus is known to invade, replicate in, and destroy endothelial cells. Destruction of endothelial surfaces is associated with disseminated intravascular coagulation, and this may contribute to the hemorrhagic manifestations that characterize many, but not all, Ebola infections.
_
Interferon blocking:
Interferon is critical to our ability to defend ourselves against viruses. It makes a variety of responses to viral infection possible, including the self-destruction of infected cells and the blockage of supplies necessary for viral reproduction. The VP24 and VP35 structural proteins of EBOV play a key role in blocking interferon. When a cell is infected with EBOV, receptors located in the cell’s cytosol (such as RIG-I and MDA5) or outside of the cytosol (such as Toll-like receptor 3 (TLR3), TLR7, TLR8 and TLR9), recognize infectious molecules associated with the virus. On TLR activation, proteins including interferon regulatory factor 3 and interferon regulatory factor 7 trigger a signaling cascade that leads to the expression of type 1 interferons. The type 1 interferons are then released and bind to the IFNAR1 and IFNAR2 receptors expressed on the surface of a neighboring cell. Once interferon has bound to its receptors on the neighboring cell, the signaling proteins STAT1 and STAT2 are activated and move to the cell’s nucleus. This triggers the expression of interferon-stimulated genes, which code for proteins with antiviral properties. EBOV’s V24 protein blocks the production of these antiviral proteins by preventing the STAT1 signaling protein in the neighboring cell from entering the nucleus. The VP35 protein directly inhibits the production of interferon-beta. By inhibiting these immune responses, EBOV may quickly spread throughout the body. The Ebola virus VP35 protein inhibits the activation of IRF-3, a critical transcription factor for the induction of early antiviral immunity resulting in high level of virulence of Ebola Virus.
_
Tetherin blocked:
The virus disables a cellular protein called tetherin that normally can block the spread of virus from cell to cell. Tetherin represents a new class of cellular factors that possess a very different means of inhibiting viral replication. Tetherin is the first example of a protein that affects the virus replication cycle after the virus is fully made and prevents the virus from being able to go off and infect the next cell. When a cell is infected with a virus like Ebola, which is deadly to 90 percent of people infected, the cell is pirated by the virus and turned into a production factory that makes massive quantities on new virions. These virions are then released from that cell to infect other cells and promote the spreading infection. Tetherin is one of the immune system’s responses to a viral infection. If working properly, tetherin stops the infected cell from releasing the newly made virus, thus shutting down spread to other cells. A study shows that the Ebola virus has developed a way to disable tetherin, thus blocking the body’s response and allowing the virus to spread. Binding of a protein produced by Ebola to tetherin apparently inactivates this cellular factor. Understanding how the Ebola protein blocks the activity of tetherin may facilitate the design of therapeutics to inhibit this interaction, allowing the cell’s natural defense systems to slow down viral replication and give the animal or person a chance to mount an effective antiviral response and recover. Previous research had found that tetherin plays a role in the immune system’s response to HIV-1, a retrovirus, and that tetherin is also disabled by HIV.
_
Cytokine storm:
Ebola loves not having to deal with the fully functional immune system, because now it can replicate and wreak havoc without interference. This suppression of the innate antiviral immune system then facilitates a cytokine storm. A cytokine storm is basically too many cytokines (a class which interferons belong to), the result of which is a rapid and potentially deadly immune over-response. The production of cytokines is a normal response when the immune system encounters an ordinary invader, and their role is to tell your immune cells to go to some location and do their respective immune cell jobs, and also to produce more cytokines. A healthy or at least in-balance body regulates how many cytokines the immune cells create, so they’re kept in check. And keeping cytokines in check means keeping the body’s immune responses in check. In a cytokine storm, however, the body never gets the message to stop making cytokines. This results in a positive feedback loop in which the body is telling itself to make more and more cytokines without anything ever telling them to stop. The body’s inflammatory immune responses go into hyper-drive, with the results including a skyrocketing fever (potentially deadly in itself) and the rapid build-up of fluids and dead immune cells (pus). Take the shedding of this fluid coupled with a catastrophic decline in the body’s blood clotting ability and the result is Ebola’s infamous blood spewing from orifices, which, in fairness, is really more of an oozing if it occurs at all.
_
The normal job of the immune system is to eliminate infections. But when it’s activated at extreme levels or it’s out of control, it becomes damaging to the host. The most extreme immune attack is the “cytokine storm.” Although many viruses, like bird flu and SARS, can trigger this shock and awe assault, Ebola is probably the best at it. And at the end of an Ebola infection, it’s the cytokine storm that kills you. In essence, a cytokine storm is an SOS signal that causes the immune system to launch its entire arsenal of weapons all at once. This last-ditch, kamikaze attack hurts the virus. But it leaves behind tons of collateral damage. Blood vessels take the brunt of it. The cytokine storm makes the blood vessel walls more permeable. So the arteries, veins and capillaries start to leak blood and plasma. The storm also triggers a big release of nitric oxide, which thins out the blood and damages vessels further. All these factors combine together to reduce blood pressure to dangerous levels. So you don’t die of blood loss, but from something similar to severe septic shock.
_
Results from several studies show an important role for reactive oxygen and nitrogen species in pathogenesis of Ebola haemorrhagic fever. Increased concentrations of nitric oxide in blood were reported in non-human primates experimentally infected with Zaire Ebola virus and were noted in patients infected with Zaire Ebola virus and Sudan Ebola virus. Increased blood concentrations of nitric oxide in patients were associated with mortality. Abnormal production of nitric oxide has been associated with several pathological disorders including apoptosis of bystander lymphocytes, tissue damage, and loss of vascular integrity, which might contribute to virus-induced shock. Nitric oxide is an important mediator of hypotension, and hypotension is a prominent finding in most of the viral haemorrhagic fevers including those caused by Ebola virus.
_
Decoy Strategy:
Ebola also employs a second dastardly trick, another cheat. It releases large amounts of secreted glycoprotein – sGP – into the bloodstreams of its victims. A decoy, sGP looks like the glycoprotein on the exterior of the virus, GP, which should be the immune system’s chief target. By tricking the immune system into seeing it, not GP as the invader, sGP undermines the system’s ability to react effectively to stem the infection.
_
What Ebola does to the human body at a molecular level is far more disturbing:
Once patients contract Ebola, the virus begins to mow down their immune system, killing off the body’s T-lymphocyte cells, the same ones affected by the AIDS virus. However, Ebola is far more aggressive than AIDS and begins tearing through several types of immune cells far more quickly. The virus produces several proteins, one of which blocks immune cells from signaling antibodies to attack. Once the patient’s first line of defense against pathogens is dismantled, the virus can begin replicating. The first symptoms of Ebola are a sudden increase in body temperature, accompanied by strong headaches, joint and muscle pain. Decreased appetite and sore throat are also early indicators of the disease. One of the first tissues the virus settles in is collagen. Collagen keeps the body’s organs in place, like organic glue. Ebola eats away at collagen, causing all kinds of problems. The patient’s upper layer of skin ends up floating on a layer of liquefied tissue, resulting in tiny white blisters and red spots on the surface that can tear off with just a small amount of pressure. Rips in the skin can appear and spontaneous bleeding can occur from several orifices, including the eyes, nose and mouth. At the same time, immune cells called macrophages trigger coagulation, which forms small blood clots throughout the bloodstream, according to a study published in Cell Host & Microbe. Blood vessels all over the body begin to burst and leak, reducing the blood supply to the body’s vital organs. This circulatory failure deprives the organs of oxygen and causes them to shut down. The process is most severe in the liver. All the while, a patient suffering from Ebola will have severe diarrhea and fits of vomiting, both of which will become increasingly filled with blood. The terminal phase of the infection occurs when the immune system, completely thrown out-of-whack, begins attacking a patient’s own body. Large blood blisters appear on the skin and the patient’s eyes become red. Blood pours from the body. Shock sets in as one organ after another fails.
_
Lymphocyte apoptosis in ebola:
Lymphocytes do not support filoviral replication, perhaps because they lack receptors for these viruses. Nevertheless, lymphocyte apoptosis is a characteristic feature of filovirus infections and can be observed in both blood and tissues of infected patients and animals. The apoptotic lymphocytes are not infected and are engulfed by surrounding macrophages. Filovirus-induced lymphocyte apoptosis is also observed in EBOV-infected human PBMCs. The apoptosis of non-infected lymphocytes is not unique to filoviruses. It has been reported in a number of viral infections, including lymphocyte choriomeningitis virus, human immunodeficiency virus, human herpesvirus 6 and Vaccinia virus infections. Although protection from apoptosis of infected cells is a strategy for survival for a number of viruses, induced periods of transient or chronic lymphocytopenia brought about by bystander apoptosis is thought to contribute to the generalized immunosuppression that accompanies some viral infections. The best known example of bystander lymphocyte apoptosis in viral infections is in CD4 T cells during HIV infection. In the case of filovirus infection, not only are CD4 T cells depleted, but CD8 T cells and NK cells as well, suggesting that some generalized mechanisms may contribute to lymphocyte apoptosis in filovirus disease. Using a mouse model of EBOV infection, Bradfute et al. suggested that both intrinsic and extrinsic apoptotic pathways may contribute to lymphocyte depletion. If this is also the case for the disease in humans and non-human primates, then it is likely that multiple mechanisms contribute to the generalized lymphocyte depletion. The loss of lymphocytes has been postulated to contribute to the failure to generate fully protective adaptive immune responses in these species. In fact, extensive lymphocyte apoptosis has been shown to proceed the generation of adaptive responses in humans with fatal disease, making this hypothesis all the more plausible. EBOV-infected cells may secrete TRAIL and increased levels of soluble Fas have been detected in the sera of some EBOV-infected non-human primates. Moreover, increased expression levels of TRAIL and Fas mRNA have been observed in peripheral blood mononuclear cells of infected non-human primates. These could trigger conventional extrinsic pathways of apoptosis in susceptible cells, including T cells. It is also possible that dysregulated DCs and macrophages could contribute in other ways to lymphocyte apoptosis. Infected DCs and macrophages fail to produce regulated cytokine responses, and they are also impaired in their ability to upregulate co-stimulatory molecules, such as CD40 and the B7 family member CD86, consistent with their inability to efficiently prime T cells. Alternatively, T cell apoptosis may result in part from the dysregulated cytokine responses during filovirus infections.
_
Impairment of coagulation:
Defects in blood coagulation and fibrinolysis during Ebola virus infections are manifested as petechiae, ecchymoses, mucosal haemorrhages, congestion, and uncontrolled bleeding at venepuncture sites. However, massive loss of blood is infrequent and, when present, is mainly limited to the gastrointestinal tract. Even in these cases, the amount of blood that is lost is not substantial enough to cause death. Thrombocytopenia, consumption of clotting factors, and increased concentrations of fibrin degradation products are other indicators of the coagulopathy that characterises Ebola virus infections. Results from clinical laboratory data strongly suggest that the coagulation abnormalities that occur during human Ebola haemorrhagic fever are generally consistent with disseminated intravascular coagulation. Furthermore, results from many studies have shown histological and biochemical evidence of disseminated intravascular coagulation during Ebola virus infection in several non-human primate species. The mechanism responsible for triggering the coagulation disorders that typify Ebola haemorrhagic fever are not wholly understood. Results from several studies strongly suggest that expression or release of tissue factor from monocytes and macrophages infected with Ebola virus are key factors that induce the development of coagulation irregularities reported in Ebola haemorrhagic fever. However, coagulopathy noted during Ebola haemorrhagic fever could be caused by several factors, especially during the later stages of disease. For example, rapid reductions in plasma concentrations of the natural anticoagulant protein C were recorded during the course of Zaire Ebola virus infection of cynomolgus monkeys. Together, the data so far suggest that an impaired and ineffective host response leads to high concentrations of virus and proinflammatory mediators in the late stages of disease, which is important in the pathogenesis of haemorrhage and shock. The prevailing hypothesis at this time is that infection and activation of antigen-presenting cells is fundamental to the development of Ebola haemorrhagic fever. The release of proinflammatory cytokines, chemokines, and other mediators from antigen presenting cells, and perhaps other cells, causes impairment of the vascular and coagulation systems leading to multiorgan failure and a syndrome that in some ways resembles septic shock.
_
Global Suppression of the Host Antiviral Response by Ebola- and Marburgviruses: a 2006 study:
In both primate and human models, most of the major organs, including the liver, lymph nodes, and spleen, show high titers of virus, and immunohistochemical analysis has shown that endothelial and mononuclear cells become heavily infected and play central roles in disease progression. Early and sustained infection in monocytes also plays a central role in the occurrence of viral hemorrhagic fever through the expression of proinflammatory and antiviral cytokines, including alpha interferon (IFN-α), interleukin-1 (IL-1), IL-6, IL-8, IL-12, and TNF family members (e.g., TNF-α and TRAIL), and coagulation factors (e.g., tissue factor [TF]), leading to activation of the extrinsic coagulation pathway and ultimately to endothelial cell destruction and permeability. Defective adaptive immune responses, including impaired humoral responses and apoptosis of B and T cells, have been observed in fatal cases of EBOV infection. Interestingly, it has been reported that type I IFN (IFN-α-2b) treatment has little effect on disease progression or pathology in EBOV-infected cynomolgus macaques. Thus, it has been concluded that the progression and ultimate outcome of human clinical filovirus infections are dependent on early antiviral events in EBOV infection that are predicated on the establishment of well-regulated antiviral and immune responses.
_
Filaments are loaded with VP40 and may act as needles that prick neighboring cells and inject the toxic material:
As recently as a decade ago, it wasn’t possible to view live cells under microscopes. Scientists pored over fixed dead ones to puzzle out clues about their behavior. But a new technique called fluorescence dynamics allows researchers to track cells as they grow by tagging them with bright inks. Three people won the Nobel Prize in chemistry this year for their development of super-resolved fluorescence microscopy. Still, it isn’t easy to trace the progress of the infinitesimally small viral particles. Digman and her colleagues created three-dimensional models and now compress hours of raw data to track VP40. There’s no risk from the isolated protein in the cell culture rooms; it needs the disease genome and six other proteins to fully form infectious Ebola. Usually, cells can absorb viruses and other harmful substances and neutralize them with acidic lipids. Not so with Ebola. Digman and the others have discovered that VP40 multiplies unimpeded inside the cell until finally the infected body’s membrane bulges out under the accumulated weight, budding into the needle-like filaments. They’ve learned that the protein hijacks the cell’s motor to form the protrusions. They’ve seen detached filaments floating outside cells. Now they just need to see them pricking other cells. A fluorescent green limb pokes outward from a cell wall under a high-powered microscope. The filament is loaded with VP40, an essential protein in the Ebola virus. The microscope is capturing it budding out in real time. It’s followed by another and another. Those green protrusions may be the means by which the deadly virus races from cell to cell in humans, killing up to 60 percent of those infected. Their hypothesis is that the filaments may act as needles that prick neighboring cells and inject the toxic material, a process that quickly goes viral. If that proves true, it could be key to the development of effective inhibitor treatments.
_________
Protective immune response against ebola:
The components of the immune system that may protect against Ebola virus infection have not been defined. Antibody titers against Ebola virus GPs are readily detectable in patients who recover from Ebola virus infection; however, anecdotal reports have indicated that serum from recovered patients did not consistently protect against infection or exhibit neutralization of virus replication in cell culture. Furthermore, passive transfer of antibodies in animal models only delays the onset of symptoms and does not alter overall survival. More recently, the neutralization of virus replication by selected monoclonal antibodies isolated from the bone marrow of recovered patients was demonstrated in vitro, and monoclonal antibodies that recognize specific epitopes of Ebola virus GP have been shown to confer immune protection in a murine model of Ebola virus infection and in guinea pigs. However, it is relatively easy to protect against infection in the mouse model, and protection of guinea pigs required a high dose of antibody administered very close to the time of virus challenge. Taken together, these results suggest that antibodies alone do not provide protective immunity in a natural context and that cellular immunity is likely to play a significant role in virus clearance. Whether hyperimmune serum from surviving vaccinated animals or certain infrequently occurring antibodies are capable of attenuating infection remains unknown, but such antibodies could potentially contribute to therapy if they can be identified and optimized.
_
A comparison of immune parameters in survivors and nonsurvivors of infection has provided clues into the constituents of an effective immune response. Baize et al. characterized the immune responses of patients in two large Ebola virus outbreaks in Gabon in 1996. There was no significant difference in viral antigen load between survivors and nonsurvivors, but immune responses varied, suggesting that survival is dependent on the initial or innate immune response to infection. Survivors exhibited more significant IgM responses, clearance of viral antigen, and sustained T-cell cytokine responses, as indicated by high levels of T-cell-related mRNA in the peripheral blood. In contrast, antibodies specific for the virus were nearly undetectable in fatal cases, and while gamma interferon (IFN-γ) was detected early after infection, T-cell cytokine RNA levels were more indicative of a failure to develop adaptive immunity in the days preceding death.
_
During infection, there is evidence that both host and viral proteins contribute to the pathogenesis of Ebola virus. Increases in the levels of inflammatory cytokines IFN-γ, IFN-α, interleukin-2 (IL-2), IL-10, and tumor necrosis factor alpha were associated with fatality from Ebola hemorrhagic fever. Moreover, in vitro experiments demonstrated that tumor necrosis factor released from filovirus-infected monocytes and macrophages increased the permeability of cultured human endothelial cell monolayers. However, other reports have observed an association between elevated levels of IFN-γ mRNA and protection from infection, and a protective effect of IFN-α and -γ is suggested by the fact that the virus has evolved at least one protein, VP35, that acts as an IFN-α/β antagonist. Whether the effects of cytokines are protective or damaging may depend not only on the cytokine profile but also may represent a delicate balance influenced by the route and titer of incoming virus as well as factors specific to the individual host immune response.
_
Natural killer (NK) cells confer protection against ebola virus infection:
Natural killer (NK) cells serve as a crucial first line of defense against tumors and a diverse range of pathogens. Recognition of infection by NK cells is accomplished by the activation of receptors on the NK cell surface, which initiate NK cell effector functions. Many of the receptors and ligands involved in NK cell antimicrobial activity have been identified, and we are beginning to appreciate how they function during infection. In addition, NK cells are activated by cytokines (e.g. interleukin 12 and type I interferons), which are products of activated macrophages and dendritic cells. In response to these activating stimuli, NK cells secrete cytokines and chemokines and lyse target cells. Recent studies have focused on the mechanisms by which NK cells recognize and respond to viruses, parasites and bacteria, and on the unique role of NK cells in innate immunity to infection. NK cells are key components of the innate immune system, rapidly responding to invading microbes by exocytosis of perforin and granzymes, which mediate the destruction of infected cells. Additionally, NK cell secretion of cytokines such as IFN-γ, IFN-α/β, and TNF-α serve a dual purpose in that they initiate the immediate activation of antimicrobial pathways in infected cells, followed by modulation of adaptive responses to the pathogen. The induction of cytokines and chemokines by viral infections is also known to trigger NK cell activity. Specifically, virus-induced IFN-α/β enhances NK cell–mediated cytotoxicity. Alternately, the induction of IL-12 by some viral infections is responsible for the production of high levels of IFN-γ by NK cells, as well as the induction of NK cytotoxic activity. NK cells appear to play a critical role in the immune response to Epstein-Barr virus, murine cytomegalovirus (MCMV), and herpes simplex virus-1. The clinical importance of NK cells to antiviral immunity is documented by the fact that recurrent herpesvirus infections have been observed in a NK-deficient patient. NK cell activity is closely regulated by a myriad of activating and inhibiting cell surface receptors, and consequently, viruses have evolved multiple mechanisms to evade or modulate these receptors. Such mechanisms include the up-regulation of HLA-C and HLA-E molecules on the surface of virus-infected cells, expression of viral MHC homologues to trigger NK inhibitory receptors, and/or the release of cytokine homologues with inhibitory activities. In contrast, virus-infected cells often down-regulate MHC class I on their surface, which enhances NK cell–mediated lysis due to removal of the inhibitory signals delivered by the MHC. The innate immune system provides early surveillance and control of viral infections. A study shows that the innate immune response, specifically NK cells, can mediate rapid and complete protection against lethal Ebola virus infection. These observations represent a key advance in understanding the requirements for protective immunity against Ebola virus infection. The identification of NK cells as critical mediators of early protection against Ebola virus infection are an important step forward in the identification of prophylactic and therapeutic interventions against filovirus and other incapacitating acute viral infections. Although the exact application of these findings to therapeutics in treating Ebola virus–infected primates and humans is unclear at this time, therapeutic agents that bolster the innate immune response, including activation of NK cells, should be the target of future studies.
__________
Virulence of ebola:
The reproductive success of a pathogen is dependent upon its ability to replicate itself and to infect new hosts by transfer of its propagules. Rapid replication can increase a pathogens chance of transference, but this requires a greater toll on the hosts system and is likely to lead to an increased chance of host mortality. Due to this, there is believed to be a natural correspondence between a pathogens growth rate and virulence. The relationship between these two factors is explained by the trade-off hypothesis of virulence evolution. This theory largely replaced the commonly accepted idea that a parasite or pathogen should evolve towards avirulence, but it not fully accepted. The avirulence theory assumed that a parasite low virulence would maximize a pathogen’s overall lifetime reproductive success by increasing the time of infection to nearly infinite limits. The reasoning behind this avirulence theory has been explained thusly: The parasite makes a profession out of living at its neighbours’ expenses and all its industry consists of exploiting it with economy, without putting its life in danger. It is like a poor person who needs help to survive, but who nevertheless does not kill its chicken in order to have the eggs. The frequent down trend in virulence from the time a pathogen is introduced to a novel population was offered as evidence for this theory. The trade-off theory developed when evolutionary ecologists began to question the avirulence theory. It proposes that there is a link between ease of transmission and virulence. According to this theory, virulence is an outgrowth of a rapid replication rate in the pathogen, which strains host resources and reduces host fitness (resulting in host mortality). The Trade-off theory links the variables of virulence, transmission and host recovery in a relationship. A high transmission rate will typically go along with a high virulence and low recovery rate. The reproductive success of a pathogen comes from successfully balancing these variables to maximize Ro. High Virulence will allow for high reproduction and transmission, but only up to a point. Natural selection should favor strains that are able to maximize this trade-off. Eventually, virulence can reach a level where the increased transmission is no longer balanced out by the risk of dying along with a host before being able to jump to a new one. This is especially true in isolated host populations or other conditions that limit horizontal transmission, which could possibly explain the low virulence and chronic nature of some infections.
_
Virulence is typically defined as morbidity and mortality of the host organism as a result of parasite or pathogen activity. Measurements of a pathogen’s virulence are traditionally given in terms of parasite induced death rate (PIHD). This definition is suitable for a general discussion of a disease as it includes all deleterious effects on the host. A more specific and narrow definition is required in order to examine selective pressures on the evolution of virulence in a disease, however. The generalized definition, according to Ebert and Bull in their work on virulence evolution, fails to differentiate between virulence’s effects on host and pathogen fitness, and therefore fail to give an accurate assessment of selective pressure on the pathogen’s evolution. For this reason it is important to consider specific aspects of the host/pathogen system (such as means of transference, rate of pathogen growth, etc) before drawing conclusions about the selective pressures for increased or reduced virulence in the pathogen. In the case of the Ebola virus and Ebola Hemorrhagic Fever virulence can be discussed in terms of host death. Unlike with some pathogens, death of the host does not immediately end transmission of the virus. Some studies indicate that the corpse can remain infectious for several days after death. Several epidemics have been traced to contact between the index case and the contaminated remains of a chimpanzee (Ivory Coast 1994, Gabon 1996, Gabon 1996-97) and contaminated monkey meat may have played a role in the index case of the initial 1976 Zaire outbreak (Ebola Haemorrhagic Fever in Zaire 1978). Ebert and Bull define three general stages of evolution in a pathogen transferring to a novel host and the selective pressures involved in each. The first phase includes the initial interactions between a pathogen and the novel host. In some cases this infection is not capable of horizontal transfer between hosts in the novel population. Other situations involve short chains of secondary infection from the index infection. Infections in this phase are likely exposed to great selective pressures, as they are in an entirely new environment, one for which their genes may or may not be particularly suitable. Genes that may not have had a measureable fitness effect in the pathogens normal host environment can suddenly exert great selective pressure. Because of this there is frequently a great range of virulence expressed by different pathogens during this phase. The second phase occurs during the period when a pathogen has established a foothold within the novel population. It follows the epidemic infection model and increases rapidly within the population, because of this rapid growth it is possible for a pathogen to evolve rapidly in this phase. Selective pressure on the host can also be extreme in this phase. The second phase also applies when a mutation in a parasite that has already obtained equilibrium within a host population is significant enough that it gains a selective advantage over other strains and spreads rapidly. Ebert and Bull’s third phase is reached when a pathogen has become firmly established within a host population. Pathogens in this phase are well adapted to the host, but will still experiences selective pressures due to host demographic and environmental changes. The Ebola virus, in human hosts, remains largely within the first phase, although it could be argued that it briefly enters the second phase on a local level during some outbreaks. It causes short lived epidemics when it does infect a human population, but fails to survive long term and become an endemic pathogen. During this initial stage the virus can be exposed to great selective pressure as it is in an unusual host. Evolutionary dynamics within an epidemic scenario, as proposed by Bolker et al, favor pathogens with a high growth and transference rates, and the high virulence that is associated with them, due to the large number of susceptible hosts in the novel population. This differs from a pathogen in later stages, which has reached dynamic equilibrium with the host. These situations tend to select for moderate virulence and longer duration of infection. (Bolker et al). A possible explanation for the extreme virulence in Ebola outbreaks may simply be reporting bias. Many of the early and milder symptoms of Ebola Hemorrhagic Fever are quite similar to those of other diseases endemic to the region, such as malaria, and measles. Some outbreaks are actually mistaken for cases of other diseases until post-infection laboratory tests detect particles of an Ebola strain. A 1994 outbreak in gold mining camps in Gabon (52 cases, 60% mortality) was believed to be a yellow fever epidemic until almost a year after the last case. It is possible that less virulent strains of the virus are simply mistaken for other common infections, treated as such, and never reported. Ebola virus antibodies were detected in sera from 18% of adults in the 1979 Nzara outbreak who were not infected. This is evidence that “It is likely that sporadic infection is more common than can be appreciated from these dramatic outbreaks, which probably represent the extreme of the interaction between man and the virus.” (Baron et al). This fits in with the inherent virulence variance in phase one pathogens suggested by Ebert and Bull above. Other factors that can affect the evolution of virulence in a pathogen are host population density and ease of transmission. These factors are frequently interrelated, as both directly influence the number of susceptible hosts a pathogen is able to infect during its lifespan. A high density of susceptible hosts (such as when a pathogen is emerging in a novel host population) is likely to greatly increase a pathogens reproductive success, and select for pathogens that can replicate quickly and take advantage of the abundant hosts. Likewise, easy transition from one host to the next also selects for pathogens that are able to rapidly replicate and “seize the day”, as it were. Both of these conditions, which favor pathogens with high growth rates, also favor high virulence in accordance with the Trade-off hypothesis (Ebert & Bull 2008).
_
The abovementioned concepts and principles fit in with epidemiological data from outbreaks of Ebola Hemorrhagic Fever. Initial outbreaks of Ebola Hemorrhagic Fever took place within areas with a relatively high concentration of susceptible hosts. The 1976 outbreak centered on the Yambuku Mission Hospital is a good example. This hospital served as the primary medical facility for a local population of around 60,000 as well as travelers. This facility was relatively small, having 17 staff members and holding 120 beds in its crowded wards. It also processed some 6000-12000 outpatients on a monthly basis. Combine this with the five improperly sterilized syringes used to administer injections (the primary dosage method at this facility) and a severe lack of barrier nursing procedures. This would appear to be an optimal situation for the transmission of pathogens that spread through contaminated body fluids. According to the Trade-off Hypothesis and the selective conditions outlined above, pathogen strains that have high reproduction rates (and hence high virulence) would be at a distinct selective advantage. Cases cared for out of the hospital setting would also tend to favor quickly reproducing and more virulent pathogens. Horizontal transfer by physical contact is directly affected by the concentration of virus particles in a contaminated fluid; hence a virus with a higher reproduction rate would be able to successfully exploit a given number of transfer opportunities. This setting lacks the direct viral inoculation by contaminated needle present in the hospital setting, which would perhaps result in less effective transmission. This would also favor more strongly virulent pathogens, which reproduce quickly and successfully exploit transmission opportunities (Ebola Haemorrhagic Fever in Zaire 1978). The conditions present during the 1976 Sudan outbreak were largely similar. Transmission occurred mainly to family members providing nursing care (without barrier nursing techniques) and through contaminated medical equipment and direct contact in a hospital setting. These conditions would also seem to favor more virulent pathogens. Other examples of particularly high virulence outbreaks (in terms of host mortality) also occur under conditions with large amounts of close contact between potential hosts, likely resulting in high transmission. Examples of these situations are found in the 1994 and 1996-97 Gabon outbreaks, which took place at a mining camp and (initially) a remote forest camp respectively. Both of these outbreaks featured transmission of numerous secondary infections through close contact with infected individuals.
_
According to the Trade-off hypothesis, high transmission rates are linked to high levels of virulence. By reducing rate of transmission it may be possible to artificially select for less virulent strains. In the hospital and home care setting, hosts suffering from highly virulent strains with high symptom manifestation (high virulence) are likely to transmit the virus to other hosts, favoring virulent strains. Application of sanitation and barrier nursing practices can reduce transmission of the virulent strains present under these conditions. This could potential favor any less virulent strains, i.e. ones that do not manifest severe symptoms that require hospitalization and are unlikely to be fatal, present in the environment. This could gradually reduce overall virulence over the course of the outbreak. Even if less virulent strains are not present, prevention of transmission is likely to slow and eventually stop the outbreak as the number of remaining susceptible hosts is reduced through various means (Ewald 2004).
_
The Ebola Virus and Ebola Hemorrhagic Fever present an interesting case for evolution of virulence in a pathogen. The periodic outbreaks of the disease offer examples of how selective pressures imposed on a pathogen follow the predictions of the Trade-off hypothesis linking virulence (and attendant host mortality) with rate of transmission. This hypothesis and the conclusions it suggests fit with data observed in outbreaks of virulent Ebola Hemorrhagic Fever. Conditions of dense susceptible host population and rapid and effective transmission seem to demonstrate high incidences of virulence indicating that there may be selective pressure for virulent strains under these conditions. Evidence of strains showing low virulence is suggested by the Ebola virus’ presence in a natural reservoir species and by the formation of antibodies by healthy individuals not linked to current epidemics. Due to this (presumed) variation amongst strains and the relationship between transmission and virulence proposed by the Trade-off hypothesis, reduction of transmission of the virus in hospital and homecare settings may lead to a reduction in strain virulence in prolonged outbreaks.
_
Relationship between the inhibition of antiviral responses and viral fitness:
Viral fitness is a measurement of the ability of a virus to achieve the highest rates of replication and production of progeny virions in a given host. One strategy to increase viral fitness is to control the response of the host cell to infection by affecting cellular gene expression to maximize expression of viral proteins and production of new virus and to minimize the activation of an antiviral state that would increase viral clearance by apoptosis or immune cell killing. In particular, for RNA viruses with relatively small genomes encoding few viral proteins, such as EBOV and influenza virus, control of host cell gene expression is critical for viral fitness. The ability of EBOV and MARV to evade the cellular antiviral response and suppress the immune response during clinical infections demonstrates the profound ability of these viruses to alter host cell gene expression programs. For example, one of the principal components of the innate immune and antiviral responses is the type I IFN response. As a key parameter of viral fitness, IFN antagonists are encoded in many viral genomes, including those of influenza virus, vaccinia virus, and EBOV. Genomics analysis demonstrated that not only IFN induction but also IFN signaling was impaired in ZEBOV- and MARV-infected cells. This significant suppression of the IFN response leads to increased immune evasion, replication, and virulence of ebola virus.
_
Case fatality ratio (CFR) as a measure of virulence of ebola:
A case fatality rate (CFR) – is a relatively simple measurement. It’s the number of people who die from an illness divided by the number of people diagnosed with it. The case fatality ratio (CFR) is calculated as the proportion of deaths among the total number of EVD cases, thereby informing the virulence of the infectious pathogen. EVD can be fatal, but it is important to note that the CFR being ‘almost 100%’ for EVD in general does not rest on any empirical arguments. For the well documented outbreaks of Ebola (excluding only isolated cases who are likely to have acquired infection from animal contact), the expected value of CFR has always been below 90%, with the range from 41% to 89%. The so-called Zaire strain is considered to be slightly more fatal than the Sudan strain. While the CFR for the Sudan strain ranges from 41% to 65%, the CFR for the Zaire strain ranges from 61% to 89%. Considering that the corresponding quartile for the Zaire strain, as determined by the distribution of outbreak-specific estimates, ranges from 73.3% to 84.3%, the CFR of the ongoing 2014 epidemic among cases with definitive recorded clinical outcomes for Guinea, Liberia and Sierra Leone has been consistently estimated at 70.8% (95% CI: 68.6 to 72.8), which is in good agreement with estimates from prior outbreaks. Nevertheless, it must be noted that earlier studies have not addressed ascertainment bias. It is important to follow up the reasons why the estimated 53% (as of 31 August 2014 which involved an underestimation bias due to time delay from illness onset to death) in real-time has been much lower than the published estimate of 70.8% among a portion of cases. Given the potential presence of asymptomatic cases, addressing ascertainment error may be the key to appropriately capture the disease burden for the entire population. The current outbreak has a CFR of about 54% – though it’s subject to change as the outbreak goes on. This figure of 54%, however, is an average taken from several countries. The fatality rate varies from one country to another – in Guinea it’s about 73%, whereas in Liberia its 55%, and in Sierra Leone it’s 41%. Why the variation? The main factors are the level of preparedness and the availability and quality of medical care. Another factor – when it comes to the varying CFR from one outbreak to the next – may be the different strains of the disease. Of the five known ebola strains, the “Zaire” and “Sudan” strains have been responsible for most deaths. The Zaire strain’s average fatality rate is 79% and the Sudan strain’s is 54% – research on the current outbreak, in Guinea, suggests that it is caused by the Zaire strain. The CFR doesn’t tell you how contagious a disease is. Ebola, transmitted through contact with bodily fluids, is much less contagious than airborne diseases such as influenza or measles. What it can do is indicate how serious the disease is, for those patients infected with it. But as we’ve seen, this varies.
_
Overestimation of CFR of ebola:
Is it really true that the fatality rate of ebolavirus is ‘up to 90 percent’?
According to the WHO page on Ebola haemorrhagic fever, Zaïre, Sudan and Bundibugyo species have been associated with large Ebola haemorrhagic fever (EHF) outbreaks in Africa with high case fatality ratio (25–90%) while Côte d’Ivoire and Reston have not. Reston species can infect humans but no serious illness or death in humans has been reported to date.
Till 2011, there have been roughly 1850 recorded cases with over 1200 deaths since ebolavirus was discovered, an average fatality rate of 65%.
But have there been only 1850 human infections till 2011?
The answer is clearly no. The results of several serological surveys have shown that many individuals have antibodies against Zaire ebolavirus – purportedly the most lethal. The results of one study revealed antibodies in 10% of individuals in non epidemic regions of Africa. A similar seroprevalence rate (9.5%) was reported in villages near Kikwit, DRC where an outbreak occurred in 1995. In addition, a 13.2% seroprevalence was detected in the Aka Pygmy population of Central African Republic. No Ebola hemorrhagic fever cases were reported in these areas. A more recent study examined sera from 4,349 individuals in 220 villages in Gabon. Antibodies against Zaire ebolavirus were detected in 15.3% of those tested, with the highest levels in forested regions. The authors believe that the seropositive individuals had mild or asymptomatic ebolavirus infection: The high frequency of ‘immune’ individuals with no disease or outbreak history raises questions as to the real pathogenicity of ZEBOV for humans in ‘natural’ conditions. These findings indicate that the fatality rates of Zaire ebolavirus that are quoted widely are likely to be vast overestimates. Why the infection is more lethal during outbreak conditions is not known. One possibility is related to the size of the viral inoculum received. During outbreaks the virus is spread by contact with the blood, secretions, organs or other body fluids of infected individuals, which contain very large quantities of virus. In contrast, infections in nature – by contact with contaminated fruit, for example – may involve far less virus.
_
The virulence of Ebola virus in man is variable and is dependent on the species or strain; a similar variability seems to recapitulate well in non-human primates. Within the genus Ebola virus, infections with the Zaire Ebola virus species have the highest case-fatality rates (60—90%) followed by those for the Sudan Ebola virus species (40—60%). On the basis of one outbreak, case-fatality rates for Bundibugyo strain infections are estimated to be only 25%. The only reported person infected with Côte d’Ivoire Ebola virus became ill but survived. By comparison, case-fatality rates for Marburg virus infection in Africa are 70—85% but were much lower in the outbreak in Europe in 1967, with a case-fatality rate of only 22%. This low rate has led to speculation that proper intensive care with supportive therapy would increase the survival rate of infected patients. This hypothesis is hard to test because of austere field conditions and ethical dilemmas about not providing care to some patients. Reston Ebola virus is deemed non-pathogenic for man, but laboratory tests have documented the occurrence of infection.
________
Genetic factors of ebola virus responsible ebola virulence:
Ebola virus (EBOV) is the etiological agent of human and primate infections with a high mortality rate; epidemic outbreaks and sporadic cases of this infection are periodically recorded mainly in the Central African region. In spite of increasing evidence points toward bats as natural reservoir of EBOV, the mystery of how it emerges remains unsolved. One of the hypotheses is that avirulent strains of this virus circulate in the populations of natural hosts and change their virulence under certain conditions (Leroy et al., 2004, 2005; Peterson et al., 2004; Pinzon et al., 2004). Indirect evidence for this hypothesis is the presence of anti-EBOV antibodies in individuals having no severe fever. For example, antibodies to EBOV have been detected in populations of Liberia (Knobloch et al., 1982), Cameroon (Bergmann, 1981), Republic of Central Africa (Georges et al., 1989), North Rhodesia, and Republic of Congo (Van der Groen, 1984). This suggests a possible circulation of nonpathogenic EBOV strains in these regions. Examination of serum samples in Zaire in 1972 demonstrated that some EBOV subtypes had circulated there 4 years before the first large-scale outbreak was recorded (Ivanov et al., 1986; Van der Groen, 1984). Similar results were obtained when assaying human blood samples in Ethiopia in 1961–1962 (Tignor et al., 1993). These data suggest a rather wide distribution of EBOV strains nonpathogenic for humans, which cause a subclinical disease. Therefore, genetic factors of ebola virus determine the virulence and range of their variation are of paramount interest for understanding the biology of this pathogen.
_
Elucidating variations in the nucleotide sequence of Ebola virus associated with increasing pathogenicity: a 2014 study: Ebolaviruses causes a severe and often fatal hemorrhagic fever in humans, with some species such as Ebola virus having case fatality rates approaching 90%. Currently the worst Ebola virus outbreak since the disease was discovered is occurring in West Africa. Although thought to be a zoonotic infection, a concern is that with increasing numbers of humans being infected, Ebola virus variants could be selected which are better adapted for human-to-human transmission. To investigate whether genetic changes in Ebola virus become established in response to adaptation in a different host, a guinea pig model of infection was used. In this experimental system, guinea pigs were infected with Ebola virus (EBOV), which initially did not cause disease. To simulate transmission to uninfected individuals, the virus was serially passaged five times in naive animals. As the virus was passaged, virulence increased and clinical effects were observed in the guinea pig. An RNAseq and consensus mapping approach was then used to evaluate potential nucleotide changes in the Ebola virus genome at each passage. Upon passage in the guinea pig model, EBOV become more virulent, RNA editing and also coding changes in key proteins become established. The data suggest that the initial evolutionary trajectory of EBOV in a new host can lead to a gain in virulence. Given the circumstances of the sustained transmission of EBOV in the current outbreak in West Africa, increases in virulence may be associated with prolonged and uncontrolled epidemics of EBOV.
_______
Natural protection against ebola:
Two other features that may play into the outcome of the life or death struggle between humans and Ebola are age and genetic predisposition. A recently published study which tracked case outcomes in Sierra Leone during the current West African outbreak showed a higher survival rate for patients under the age of 21 compared to those over the age of 45. Earlier, a study done based on blood samples from people who had been infected during a 2000 outbreak of Ebola Sudan in Uganda found that certain people were more likely to have milder disease and survive. Another recently published paper looking at the spectrum of disease in mice also suggests genetics play a role in survival. Geisbert is one of the discoverers of an Ebola species known as Ebola Reston, unique among the five types of the viruses because it does not originate in Africa and so far it has not been seen to sicken people. Reston viruses come from the Philippines; on six occasions research monkeys imported from that country have triggered animal outbreaks. It has also been found in pigs, though the animals do not show signs of infection. Ebola Reston is lethal in primates. Research done after animal outbreaks shows that several people have developed antibodies (or “seroconverted”) to Ebola Reston, but did not become noticeably ill.
_
Asymptomatic infection:
Evidence suggests that many Ebola infections are asymptomatic, a factor overlooked by recent outbreak summaries and projections. Particularly, results from one post-Ebola outbreak serosurvey showed that 71% of seropositive individuals did not have the disease; another study reported that 46% of asymptomatic close contacts of patients with Ebola were seropositive. The latter study also found minute concentrations of Ebola virus in these individuals’ blood, suggesting that their antibodies could not be explained by their exposure to dead virus, but that rather they had truly been infected by live virus. Could silent Ebola infections be contagious? Given that Ebola typically spreads through contact with bodily fluids of very sick individuals, who have exceedingly high viral counts, it is very unlikely that silent (asymptomatic) cases can spread the virus with the low levels found in their blood. Although asymptomatic infections are unlikely to be infectious, they might confer protective immunity and thus have important epidemiological consequences. The spread of Ebola in West Africa reveals two truths: The disease is swift, and it is devastating. Amid the chaos of deadly outbreak, researchers say another truth may exist: The disease might be quietly inoculating a significant portion of the population who are exposed to the virus but never succumb to it or show symptoms of being infected. If those individuals have acquired immunity to Ebola, the strategies for the intervention and treatment of the disease need to be reconsidered. If infection without disease protects people from future Ebola infections and illness, the epidemic should decline sooner than currently predicted and affect a smaller number of people.
_
But the question remains, are these asymptomatically infected people immune to future Ebola infection? Since survivors of full-blown Ebola disease seem to become immune to re-infection, we hope that the answer is yes. Immunization of any kind — via vaccine or natural infection — makes people resistant and thereby slows transmission. If silent Ebola infections actually protect against future re-infection, then Ebola is acting as its own vaccine, leaving a large wake of uninfectable people in its path. Importantly, this wake is likely to include healthcare workers who frequently contact patients and are at considerable risk for future exposure. If so, Ebola is simultaneously killing some individuals while protecting others within the population subgroup at highest priority for interventions, such as future vaccines. Widespread acquired immunity would therefore have three important implications. First, outbreak forecasts that do not consider this phenomenon will overestimate the future extent of the outbreak. Second, naturally acquired immunity will amplify the effects of disease control measures, including vaccination. Third, if we can reliably detect immune individuals, then they can safely take on risky health care tasks and thereby prevent spread to non-immune caregivers. If these people are protected from future infections, this would open up new opportunities for controlling the disease. The asymptomatic individuals can help slow the spread of Ebola in two important ways: They can be recruited to work as caregivers in high-risk communities; and their natural immunity may make them prime candidates for donating blood for transfusions. If we can take advantage of natural immunity within the affected communities, we may be able impact the course of the epidemic even before a vaccine becomes available.
_
A surprisingly high proportion of the Gabonese population could have immunity against Ebola. Antibodies to the virus were found in 15.3% of rural communities, whereas these people had never had haemorrhagic fever or other specific symptoms of the disease (such as severe diarrhoea or vomiting). IRD researchers and their partners recently discovered this large number of healthy carriers among Gabonese people, even in areas where there has never been an Ebola outbreak. The scientists consider that these people have somehow come into contact with the virus, probably present in fruit contaminated by saliva from Chiroptera (fruit bats).
_
Human asymptomatic Ebola infection and strong inflammatory response: a 2000 study:
Ebola virus is one of the most virulent pathogens, killing a very high proportion of patients within 5-7 days. Two outbreaks of fulminating haemorrhagic fever occurred in northern Gabon in 1996, with a 70% case-fatality rate. During both outbreaks authors identified some individuals in direct contact with sick patients who never developed symptoms. Authors aimed to determine whether these individuals were indeed infected with Ebola virus, and how they maintained asymptomatic status. 11 of 24 asymptomatic individuals developed both IgM and IgG responses to Ebola antigens, indicating viral infection. Western-blot analysis showed that IgG responses were directed to nucleoprotein and viral protein of 40 kDa. The glycoprotein and viral protein of 24 kDa genes showed no nucleotide differences between symptomatic and asymptomatic individuals. Asymptomatic individuals had a strong inflammatory response characterised by high circulating concentrations of cytokines and chemokines. This study showed that asymptomatic, replicative Ebola infection can and does occur in human beings. The lack of genetic differences between symptomatic and asymptomatic individuals suggests that asymptomatic Ebola infection did not result from viral mutations. Elucidation of the factors related to the genesis of the strong inflammatory response occurring early during the infectious process in these asymptomatic individuals could increase our understanding of the disease.
_
Ebola virus: Genes ‘play significant role in survival’: A 2014 study:
Researchers have found that natural immunity may exist to Ebola, after discovering that some animals get over the disease quickly, without major symptoms. Ebola may not be a deadly disease for everyone, after scientists discovered that some people are likely to be naturally immune to the virus. A study in mice showed that genetic variations govern how ill victims will get after contracting the disease. Some completely resist the disease, while others suffer only a moderate illness. However many still succumb to bleeding, organ failure and shock. The research was conducted in a highly secure, state-of-the-art bio lab in Montana, US. Researchers found that all mice lost weight in the first few days after infection. However, nearly one in five of the mice not only survived, but also fully regained their lost weight within two weeks. After a fortnight they had no evidence of the disease and their livers looked normal. One in nine of the mice were partially resistant and less than half of these died. However susceptible mice had greater than 50 per cent mortality. The authors believe that those who have survived during the recent outbreak in West Africa may have had natural immunity. “Our data suggests that genetic factors play a significant role in disease outcome,” said co-author Dr Michael Katze, of Washington University’s Department of Microbiology. “We hope that medical researchers will be able to rapidly apply these findings to candidate therapeutics and vaccines.” Prof Andrew Easton, Professor of Virology, University of Warwick, said: “This paper demonstrates that the genes of the host play a role in determining the outcome of Ebola infection in terms of the severity of the disease, at least in mice. Prof Jonathan Ball, Professor of Molecular Virology, University of Nottingham, added: “We know that host genetics influences infection outcome for a range of viruses, like HIV and norovirus. We’ve also suspected that genetics plays a role in susceptibility to Ebola virus infection. “It’s a reminder of how infectious diseases shape the evolution of a host; and the human host is no different to any other.” Angela Rasmussen, from the Katze Laboratory at the University of Washington, said the different ways in which the mice were affected mirrored the variety of symptoms seen in humans in the 2014 outbreak. Recent Ebola survivors could have had immunity to this virus or a related virus which may have saved them, for example. This would have meant the disease reacting in a particular way to a host’s genes, which is seen with many other viruses. Andrew Easton, professor of virology at the University of Warwick, said the study provided valuable information, but the data could not be directly applied to humans because they have a much larger variety of genetic combinations than mice.
_
When a person becomes infected with Ebola, the virus depletes the body’s immune cells, which defend against infection. In particular, the Ebola virus depletes immune cells called CD4 and CD8 T lymphocytes, which are crucial to the function of the immune system. But if a person’s immune system can stand up to this initial attack — meaning their immune cells are not as depleted in the first stages of infection — then studies suggest they are more likely to survive the disease. But if the body is not able to fend off this attack, then the immune system becomes less able to regulate itself. This means the immune system is more likely to run out of control and release a “storm” of inflammatory molecules, which cause tiny blood vessels to burst, leading in turn to a drop in blood pressure, multi-organ failure and eventually death. Another marker linked with people’s ability to survive Ebola is a gene called human leukocyte antigen-B, which makes a protein that is important in the immune system. A 2007 study found that people with certain versions of this gene, called B07 and B14, were more likely to survive Ebola, while people with other versions, called B67 and B15, were more likely to die. Finally, some people may be resistant to Ebola infection entirely, if they have a mutation in a gene called NPC1. Studies show that, when researchers take cells from people with the NPC1 mutation and try to infect them with Ebola in a laboratory dish, these cells are resistant to the virus. In European populations, about 1 in 300 to 1 in 400 people have this mutation. But in some populations, this mutation is more common: in Nova Scotia, between 10 and 26 percent of people have this mutation. But the frequency of this mutation in African populations is not known.
________
Natural reservoir of ebola and animal to human transmission:
Reservoir Host:
A reservoir host is one that carries the virus, is asymptomatic (displaying no symptoms of infectious virus), and that transmits the disease to humans or to other animals. The Ebola virus likely originated in African fruit bats. The virus is known as a “zoonotic” virus because it’s transmitted to humans from animals. Humans can also transfer the virus to each other. Other animals known to transmit the virus include:
•chimpanzees
•forest antelopes
•gorillas
•monkeys
•porcupines
_
The natural reservoir for Ebola has yet to be confirmed; however, bats are considered to be the most likely candidate species. Three types of fruit bats (Hypsignathus monstrosus, Epomops franqueti and Myonycteris torquata) were found to possibly carry the virus without getting sick. As of 2013, whether other animals are involved in its spread is not known. Plants, arthropods and birds have also been considered possible viral reservoirs. Bats were known to roost in the cotton factory in which the first cases of the 1976 and 1979 outbreaks were observed, and they have also been implicated in Marburg virus infections in 1975 and 1980. Of 24 plant and 19 vertebrate species experimentally inoculated with EBOV, only bats became infected. The bats displayed no clinical signs of disease, which is considered evidence that these bats are a reservoir species of EBOV. In a 2002–2003 survey of 1,030 animals including 679 bats from Gabon and the Republic of the Congo, 13 fruit bats were found to contain EBOV RNA. Antibodies against Zaire and Reston viruses have been found in fruit bats in Bangladesh, suggesting that these bats are also potential hosts of the virus and that the filoviruses are present in Asia. Between 1976 and 1998, in 30,000 mammals, birds, reptiles, amphibians and arthropods sampled from regions of EBOV outbreaks, no Ebola virus was detected apart from some genetic traces found in six rodents (belonging to the species Mus setulosus and Praomys) and one shrew (Sylvisorex ollula) collected from the Central African Republic. However, further research efforts have not confirmed rodents as a reservoir. Traces of EBOV were detected in the carcasses of gorillas and chimpanzees during outbreaks in 2001 and 2003, which later became the source of human infections. However, the high rates of death in these species resulting from EBOV infection make it unlikely that these species represent a natural reservoir for the virus. More than 100 viruses have been identified in bats, and that number too is rising.
_
A group of 17 European and African tropical disease researchers, ecologists and anthropologists spent three weeks talking to people and capturing bats and other animals near the village of Meliandoua in remote eastern Guinea, where the present epidemic appeared in December 2013. They have concluded that the disease was spread by colonies of migratory fruit bats. If you’re a virus and your primary goal in life is to reproduce and survive, you don’t necessarily want to kill your host really quickly, so bats and viruses have achieved a nice equilibrium. Bats live with Ebola by having certain components of their immune system constantly switched on, so they are prepared before the virus enters their system. What we need to do now is learn how bats tolerate high levels of activation of the immune system, constantly, without any detrimental effects. In contrast, the immune system of humans is only activated after contact with the virus. Initially the virus shuts down the early response which then leads to a deadly overreaction.
_
Ebola cycle:
_
No bats culling to control ebola:
Bats are the primary pollinators of many tropical plants, and they disperse the seeds from fruit they eat. In Africa and Asia, that makes fruit bats responsible for about 50 percent of the tropical rainforest there. You can get sick to death from eating the bats, and the loss of the fruit bat population could hurt other aspects of the environment on which residents depend for their survival. Bats are critical to the health of people and the planet. Best known are the chemical-free pest control and pollination services they perform. Some bats, especially when nursing, can consume more than their body weight in bugs during a single night. If bats are confirmed as the reservoir of the disease, forest communities could try to destroy their vast colonies. That would be an ecological disaster because bats pollinate plants and devour insects. And bat hunts would also only increase human contact with potentially infected animals. It’s tempting to look to culling as the answer to deal with bats as the natural hosts of Ebola. This suggestion was made during the spillover of Hendra virus from bats to horses in Australia. But it is not the answer; bats are an extremely successful group of mammals, making up 20% of all mammalian diversity. They are critical to ecosystems, with roles in insect control and pollination.
_
Unlocking the bat immune system:
Studying how bats control viral replication may unlock alternative mechanisms for tackling Ebola as well as other new and emerging infectious diseases. Increasing antimicrobial resistance of viruses, bacteria and fungi, for instance, is becoming a global concern and we need to think creatively to find solutions. Bats and viruses have achieved an equilibrium that allows them to co-exist. Clues from studies of bat genomes have revealed differences in genes associated with the very early immune response that could help bats respond to infections. These genes appear to be evolving at a faster rate in bats compared with other species, providing evidence that they are likely co-evolving with the viruses that bats carry. Functional differences in the immune system may also play a role. Unlike humans and mice, which activate their immune systems only in response to an infection, bats appear to have certain components of their immune system constantly switched on. This may allow bats to control viral replication much more efficiently compared with other species. Clues are starting to emerge following gene analysis, which suggests bats’ capacity to evade Ebola could be linked with their other stand-out ability – the power of flight. Flying requires the bat metabolism to run at a very high rate, causing stress and potential cell damage, and experts think bats may have developed a mechanism to limit this damage by having parts of their immune system permanently switched on. Researchers found an unexpected concentration of genes for repairing DNA damage, hinting at a link between flying and immunity. This raises the interesting possibility that flight-induced adaptations have had inadvertent effects on bat immune function. If we can redirect the immune responses of other species to behave in a similar manner to that of bats, the high death rate associated with diseases such as Ebola could be a thing of the past. Rather than persecuting bats, we need to unravel the secrets of the success of this group of mammals. Understanding how bats control viral replication would not only assist in developing future therapeutics but may also help predict transmission events from bats into human and animal populations.
_
Experimental inoculation of plants and animals with Ebola virus: 1996 study:
Thirty-three varieties of 24 species of plants and 19 species of vertebrates and invertebrates were experimentally inoculated with Ebola Zaire virus. Fruit and insectivorous bats supported replication and circulation of high titers of virus without necessarily becoming ill; deaths occurred only among bats that had not adapted to the diet fed in the laboratory.
_
Bushmeat:
In Africa, wild animals including fruit bats are hunted for food and are referred to as bushmeat. In equatorial Africa, human consumption of bushmeat has been linked to animal-to-human transmission of diseases, including Ebola. Although it is not entirely clear how Ebola initially spreads from animals to humans, the spread is believed to involve direct contact with an infected wild animal or fruit bat. Scientists at the World Health Organisation believe that fruit bats from the Pteropodidae family are the natural hosts of the ebola virus. The disease infects humans through close contact with infected animals, including chimpanzees, fruit bats and forest antelope. It then spreads between humans by direct contact with infected blood, bodily fluids or organs, or indirectly through contact with contaminated environments. Even funerals of Ebola victims can be a risk, if mourners have direct contact with the body of the deceased.
______
Natural history of ebola viruses:
The emergence, less than 50 years ago, of Ebola virus remains an enigma. It is possible that sporadic cases or limited outbreaks occurred in the past and were unreported due to lack of epidemiological surveillance or appropriate diagnosis. Scattered and minor manifestations of EVD suggest that Ebola viruses circulate in a pathogen complex showing no (or few) contacts with human populations. Destruction of forests and human impact on broader areas could explain the increased frequency and severity of outbreaks. However, we cannot exclude a recent emergence of the virus that would spread rapidly in a susceptible population. According to Polonsky et al., increasing frequency of epidemics may result from the combination of: improvement of monitoring and diagnostic capacities, increase of contact among humans and the natural reservoirs of the virus, and growth of the viral load and prevalence of the virus in reservoirs. Several epidemiological investigations in Central and East Africa have shown circulation of Ebola virus in the human population at a significant rate, but that does not always entail the emergence of an epidemic. The natural reservoir of the virus is not known with certainty. Extensive investigations made in small mammals, even sensitive to the Ebola viruses, were negative during the various epidemics in Central Africa. Subsequent investigations continued outside epidemics. Although viral RNA and specific antibodies have been identified in small mammals, no potential natural host has been acknowledged until 2005. However, initially dismissed due to many negative samples, fruit bats were found with specific viral DNA and antibodies. These animals seem resistant to Filoviridae pathogenicity. The search for potential vectors, especially among arthropods, has always proved negative, including bedbugs (Cimex hemipterus) captured in the beds of infected persons. Deadly outbreaks of Ebola virus have been observed in non-human primates with high mortality. In addition to the contamination of the Swiss primatologist in Ivory Coast, the index case of several outbreaks have been more or less directly associated with hunting or consumption of bush meat, i.e. monkeys, antelopes, bats. Natural infection of bats and sharing their narrow ecological niche with many species of non-human primates are strong arguments in favor of their role as a natural reservoir of the virus. Some species, including Eidolon helvum, Hypsignathus monstrosus, Myonycteris torquata and Epomops franqueti, migrate long distances (>2,500 km), which could explain the multiple remote epidemic clusters.
_
Ebola in Wild animals:
Ebola has a high mortality among primates. Frequent outbreaks of Ebola may have resulted in the deaths of 5,000 gorillas. Outbreaks of Ebola may have been responsible for an 88 percent decline in tracking indices of observed chimpanzee populations in 420 square kilometer Lossi Sanctuary between 2002 and 2003. Transmission among chimpanzees through meat consumption constitutes a significant risk factor, whereas contact between the animals, such as touching dead bodies and grooming, is not. Recovered carcasses from gorillas contain multiple Ebola virus strains, which suggest multiple introductions of the virus. Bodies decompose quickly and carcasses are not infectious after 3 to 4 days. Contact between gorilla groups is rare, suggesting transmission among gorilla groups is unlikely, and that outbreaks result from transmission between viral reservoir and animal populations.
_
Ebola in Domestic animals:
In 2012 it was demonstrated that the virus can travel without contact from pigs to nonhuman primates, although the same study failed to achieve transmission in that manner between primates. Dogs may become infected with EBOV but not develop symptoms. Dogs in some parts of Africa scavenge for food, and they sometimes eat EBOV-infected animals and also the corpses of humans. A 2005 survey of dogs during an EBOV outbreak found that although they remain asymptomatic, about 32 percent of dogs closest to an outbreak showed a seroprevalence for EBOV versus 9 percent of those farther away. The authors concluded that there were “potential implications for preventing and controlling human outbreaks.”
_
Transmission from dogs to human:
Public health officials are concerned about the role of dogs in Ebola virus transmission because there is scientific evidence that another mammal, the bat, is a reservoir for the disease. Based upon a research study in 2005 we know that feral dogs in African villages where there have been large scale epidemics seroconvert to Ebola. Seroconversion means the dogs have been exposed to virus and have produced antibodies specific for Ebola virus. Seroconversion does not imply production of infectious virus that can be transmitted to people or other animals. In other words, this study indicates that Ebola virus breached the dog’s mucosal barrier, was recognized by the canine immune system as being foreign and the body responded by producing anti-Ebola antibodies. In this study, dogs were described as being asymptomatic, and there was no evidence that virus was transmitted between dogs or from dogs to any other host. In summary, there is currently no evidence that exposed dogs become productively infected and shed Ebola virus. As on today, we do not know if the dog’s intracellular machinery can support viral replication, packaging and formation of infectious viral particles, nor do we know how the dog might shed virus for transmission to another host if it is asymptomatic. Extensive research is necessary to answer this question. The American Veterinary Medical Association (AVMA) is currently working on recommendations for handling, testing and treatment of companion animals associated with human cases, and that information will be forthcoming.
__________
Human to human transmission and spread of ebola:
Human-to-human transmission leads to outbreaks, which are often started by a single introduction from the wildlife reservoir or another end host and involve virus variants with little genetic diversity, as in the current outbreak in West Africa. Some recorded outbreaks, on the other hand, have stemmed from multiple introductions, which have resulted in greater genetic viral diversity among the subsequent distinct chains of human-to-human transmission. Within a given species, however, virus variants have been shown to have low genetic diversity, often less than a few percent, as illustrated by the new variant isolated from patients in Guinea. Such limited diversity generally leads to neutralizing cross-reactivity within the species. Biologic characterization of various Zaire ebolaviruses, their case fatality rates, and their virulence in animal models have so far failed to provide convincing evidence of obvious differences in pathogenicity. Thus, it should be assumed that the new West African variant is not more virulent than previous Zaire ebolaviruses; a case fatality rate of about 70%, if confirmed, might even indicate lower virulence. The finding that the Guinea variant resides at a more basal position within the clade than previously known Zaire ebolaviruses argues against an introduction from Central Africa and instead supports the likelihood of distinct evolution in West Africa. These findings reinforce the hypothesis that ebolaviruses have a broader geographic distribution than previously thought.
_
Mode of Transmission:
In an outbreak, it is hypothesized that the first patient becomes infected as a result of contact with an infected animal. Person-to-person transmission occurs via close personal contact with an infected individual or their body fluids during the late stages of infection or after death. Nosocomial infections can occur through contact with infected body fluids for example due to the reuse of unsterilized syringes, needles, or other medical equipment contaminated with these fluids. Humans may be infected by handling sick or dead non-human primates and are also at risk when handling the bodies of deceased humans in preparation for funerals. In laboratory settings, non-human primates exposed to aerosolized ebolavirus from pigs have become infected, however, airborne transmission has not been demonstrated between non-human primates. Viral shedding has been observed in nasopharyngeal secretions and rectal swabs of pigs following experimental inoculation.
_
Communicability:
Communicable as long as blood, body fluids or organs, contain the virus. Ebolavirus has been isolated from semen 61 to 82 days after the onset of illness, and transmission through semen has occurred 7 weeks after clinical recovery
_
What are body fluids?
Basically any kind of fluid that comes from the body is body fluid. Body fluids include blood, saliva, mucus, vomit, feces, sweat, tears, breast milk, urine, and semen.
_
People in West Africa are avoiding hugs and handshakes because the virus can be spread through the sweat on someone’s hand. The uninfected person would have to have a break in the skin of their hand that would allow entry of the virus, CNN’s Dr. Sanjay Gupta says. But “we all have minor breaks in our skin. And there is a possibility that some of the virus can be transmitted that way.” Health care providers — or family and friends — caring for Ebola patients are often at the highest risk of getting sick because they are most likely to come in contact with the body fluids of sick patients, according to the CDC. People with Ebola suffer from extreme vomiting, diarrhea and high fevers, which causes sweating. In the later stages, they may start bleeding from their eyes, mouth or other orifices.
_
What does “direct contact” mean?
Direct contact means that body fluids (blood, saliva, sweat, mucus, vomit, urine, or feces) from an infected person (alive or dead) have touched someone’s eyes, nose, or mouth or an open cut, wound, or abrasion. Ebola is transmitted through contact with body fluids (blood, saliva, semen, vomit, urine, or feces) in much the same way HIV or hepatitis B. Ebola is transmitted from infected human only during symptomatic phase and not in incubation period, and transmission is maximum in advanced stage of disease when patient is gravely ill as his body fluids would contain maximum number of viruses at that time. Although transmission through aerosol has been demonstrated in the laboratory between pigs and primates, it has never been conclusively demonstrated to happen from human to human and the evidence is fairly compelling that it does not. The virus persists on surfaces for days, and only 1-10 virus particles are needed to initiate infection.
_
Human transmission of Ebola viruses:
The contamination of index cases is probably due to contact with an infected animal. Human transmission happens only through close contact with an ill or convalescent person, although at this stage the risk of infection is very small. Studies conducted during the various epidemics have shown that less than one fifth of the people living with a confirmed or probable primary patient have developed the disease. All secondary cases were recorded among people with close contact with the patient and exposed to infected biological fluids. Conversely, people who had no contact with the patient were not sick. Such close contact with the patient throughout care occurs mainly during the illness or burial preparation, including washing the body and funeral ritual that can be long and intimate. The risk greatly increases due to the delay in diagnosis and appropriate management. Ebola viruses have been detected in most patient secretions. They are present in the blood, saliva, feces, breast milk, tears and genital secretions. They have not been isolated from vomit, sputum, sweat or urine. However, the number of tested samples was low. The virus persists in breast milk, genital secretions and eyes during convalescence and up to 13 weeks after recovery. Finally, the risk of transmission from fomites (towels, clothes and sheets from the patient), especially during convalescence, is low and basic protection measures are likely to be sufficient. Nosocomial transmission is behind many hospital outbreaks. Injection materials reused without precaution or inadequately sterilized have been repeatedly denounced and remain a major cause of epidemic spread. This also applies to traditional healers whose practices are often septic.
_
Entry points;
Entry points for the virus include the nose, mouth, eyes, open wounds, cuts and abrasions. Contact with objects contaminated by the virus, particularly needles and syringes, may also transmit the infection. The virus is able to survive on objects for a few hours in a dried state and can survive for a few days within body fluids. The Ebola virus may be able to persist for up to 7 weeks in the semen of survivors after they recovered, which could lead to infections via sexual intercourse. Ebola may also occur in the breast milk of women after recovery, and it is not known when it is safe to breastfeed again. Otherwise, people who have recovered are not infectious.
_
Dead bodies transmitting ebola:
Dead bodies remain infectious; thus, people handling human remains in practices such as traditional burial rituals or more modern processes such as embalming are at risk. Nearly two thirds of the cases of Ebola infections in Guinea during the 2014 outbreak are believed to have been contracted via unprotected (or unsuitably protected) contact with infected corpses during certain Guinean burial rituals.
_
Viral transmission through the air has not been reported to occur during EVD outbreaks. Transmission among rhesus monkeys via breathable 0.8–1.2 μm aerosolized droplets has been demonstrated in the laboratory. The apparent lack of airborne transmission among humans may be due to levels of the virus in the lungs that are insufficient to cause new infections. Spread of EBOV by water or food, other than bushmeat, has also not been observed. No spread by mosquitoes or other insects has been reported. The centre explained that Ebola is not spread through the air, water, or food and a person infected with Ebola can’t spread the disease until symptoms appear. The time from exposure to when signs or symptoms of the disease appear, known as the incubation period, is two to 21 days, but the average time is eight to 10 days. Ebola is spread through direct contact, through broken skin or through eyes, nose, or mouth, via blood and body fluids of a person who is sick with Ebola, or objects, such as needles, that have been contaminated with the blood or body fluids of a person sick with Ebola.
_
_
How does Ebola spread?
When infection occurs in humans, the virus can be spread in several ways to others. Ebola is spread through direct contact (through broken skin or mucous membranes in, for example, the eyes, nose, or mouth) with
•blood or body fluids (including but not limited to urine, saliva, sweat, feces, vomit, breast milk, and semen) of a person who is sick with Ebola
•objects (like needles and syringes) that have been contaminated with the virus
•Ebola is not spread through the air or by water, or in general, by food. However, in Africa, Ebola may be spread as a result of handling bushmeat (wild animals hunted for food) and contact with infected bats.
•Only a few species of mammals (for example, humans, monkeys, and apes) have shown the ability to become infected with and spread Ebola virus. There is no evidence that mosquitoes or other insects can transmit Ebola virus.
_
According to a new Ebola situation assessment issued by the World Health Organization, saliva and tears may also carry some risk. However, the studies implicating these additional bodily fluids were extremely limited in sample size and the science is inconclusive, W.H.O. said. “In studies of saliva, the virus was found most frequently in patients at a severe stage of illness. The whole live virus has never been isolated from sweat.” CDC Director Dr. Tomas Frieden notes that only a person showing symptoms can spread the disease. While the Ebola virus is believed to be able to survive for some days in liquid outside an infected organism, Doctors Without Borders says, agents such as chlorine, heat, direct sunlight, soaps and detergents can kill it.
_
According to the World Health Organization, Ebola spreads through “human-to-human transmission via direct contact (through broken skin or mucous membranes) with blood, secretions, organs or other bodily fluids of infected people.” This means that the most common way Ebola is spread is direct contact with vomit, blood or fecal matter of an infected patient. Individuals who have such contact are at high risk. The Centers for Disease Control and Prevention says “being within approximately 3 feet of an (Ebola) patient or within the patient’s room or care area for a prolonged period of time,” is also potential cause for concern. But the organization noted the risk is low. There is also potential risk of transmission through contaminated surfaces and objects, however the World Health Organization notes the danger is, again, low, and most studies from previous Ebola outbreaks show that “all cases were infected by direct close contact with symptomatic patients.” Finally, experts note that individuals are not infectious — meaning they cannot spread the virus — until they are showing symptoms, which takes between 2 and 21 days. Symptoms start with a fever, followed by vomiting and diarrhea. So to be at a high risk of contracting Ebola, you need to come into contact with the blood, feces or vomit of someone who is showing symptoms. The number of people who find themselves in this situation are relatively small. There is potential for it to spread other ways — such as being in close range with someone who has the disease or touching an object contaminated by an infected person — but the risk is low, because “as an enveloped virus, it has a low tendency to stay viable outside the body,” said Thomas Fekete, a professor of infectious diseases at the Temple University School of Medicine. “And because it is not aerosolized it isn’t especially easy to catch through the respiratory tract.”
_
Touching or kissing the corpses of Ebola victims at funerals is helping to spread the deadly virus. Traditional cultural and religious beliefs in parts of Africa help spread the virus. There are very strong traditional beliefs and traditional funeral rites which require that the whole family touch the dead body and they have a meal in the presence of the dead body. Part of what’s fueling the deadly outbreak is funeral rites that involve touching or kissing the bodies of the deceased; dead bodies can still host the Ebola virus and spread the disease to the living.
______
Ebola transmission through fomites:
If an infected person contaminates an environmental surface with body fluids, it might be possible to acquire infection by touching these surfaces and transferring virus to mucous membranes. This type of transmission is more likely to occur in health care settings where ill patients are shedding large amounts of virus particles. However, transmission of the virus from inanimate objects has rarely been observed in previous outbreaks.
_
Fomite:
A fomite is any object or substance capable of carrying infectious micro-organisms and hence transferring them from one individual to another. Skin cells, hair, clothing, and bedding are common hospital sources of contamination. Fomites are associated particularly with hospital-acquired infections (HAI), as they are possible routes to pass pathogens between patients. Stethoscopes and neckties are two such fomites associated with health care providers. Basic hospital equipment, such as IV drip tubes, catheters, and life support equipment can also be carriers, when the pathogens form biofilms on the surfaces. Careful sterilization of such objects prevents cross-infection. Researchers have discovered that smooth (non-porous) surfaces like door knobs transmit bacteria and viruses better than porous materials like paper money because porous, especially fibrous, materials absorb and trap the contagion, making it harder to contract through simple touch.
_
Fragility of virus:
One reason Ebola is not as contagious as some viruses is that the virus is relatively fragile. That’s in contrast to, say, smallpox, a virus that can remain infectious for long periods even when outside of a host. But Ebola virus left alone, outside a host, on a surface, will begin to disintegrate rather quickly. Ebola is an ‘enveloped’ virus, which means it is surrounded by a lipid membrane. That membrane protects it from its surroundings, but even more importantly is essential to its ability to fuse with and enter living cells. And it’s pretty poor protection, as it is vulnerable to light, heat, dryness, and almost any detergent or alcohol you care to name. The virus in saliva on a counter top will be inactive in minutes. How long will it remain infectious? Hard to say, exactly, though there have been scientific studies on just that question. In one laboratory experiment, scientists couldn’t recover Ebola virus that had contaminated a surface kept at room temperature. In another study, Ebola virus kept at cold temperature was recovered from plastic and glass surfaces after more than three weeks.
_
Susceptibility to Disinfectants:
Ebolavirus is susceptible to 3% acetic acid, 1% glutaraldehyde, alcohol-based products, and dilutions (1:10-1:100 for ≥10 minutes) of 5.25% household bleach (sodium hypochlorite) and calcium hypochlorite (bleach powder). The WHO recommendations for cleaning up spills of blood or body fluids suggest flooding the area with a 1:10 dilutions of 5.25% household bleach for 10 minutes for surfaces that can tolerate stronger bleach solutions (e.g., cement, metal) For surfaces that may corrode or discolour, they recommend careful cleaning to remove visible stains followed by contact with a 1:100 dilution of 5.25% household bleach for more than 10 minutes.
Physical inactivation:
Ebola are moderately thermolabile and can be inactivated by heating for 30 minutes to 60 minutes at 60°C, boiling for 5 minutes, or gamma irradiation (1.2 x10^6 rads to 1.27 x10^6 rads) combined with 1% glutaraldehyde. Ebolavirus has also been determined to be moderately sensitive to UVC radiation.
Survival outside host:
Filoviruses have been reported capable to survive for weeks in blood and can also survive on contaminated surfaces, particularly at low temperatures (4°C). One study could not recover any Ebolavirus from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another study, Ebolavirus dried onto glass, polymeric silicone rubber, or painted aluminum alloy is able to survive in the dark for several hours under ambient conditions (between 20°C and 25°C and 30–40% relative humidity) (amount of virus reduced to 37% after 15.4 hours), but is less stable than some other viral hemorrhagic fevers (Lassa). When dried in tissue culture media onto glass and stored at 4 °C, Zaire ebolavirus survived for over 50 days. This information is based on experimental findings only and not based on observations in nature. This information is intended to be used to support local risk assessments in a laboratory setting.
_______
Assessment of the Risk of Ebola Virus Transmission from Bodily Fluids and Fomites in isolation ward: a 2007 study:
Although Ebola virus (EBOV) is transmitted by unprotected physical contact with infected persons, few data exist on which specific bodily fluids are infected or on the risk of fomite transmission. Therefore, authors tested various clinical specimens from 26 laboratory-confirmed cases of Ebola hemorrhagic fever, as well as environmental specimens collected from an isolation ward, for the presence of EBOV. Virus was detected by culture and/or reverse-transcription polymerase chain reaction in 16 of 54 clinical specimens (including saliva, stool, semen, breast milk, tears, nasal blood, and a skin swab) and in 2 of 33 environmental specimens. Authors conclude that EBOV is shed in a wide variety of bodily fluids during the acute period of illness but that the risk of transmission from fomites in an isolation ward and from convalescent patients is low when currently recommended infection control guidelines for the viral hemorrhagic fevers are followed.
_
The table above shows virus culture and reverse-transcription polymerase chain reaction (RT-PCR) results from 54 clinical samples collected from 26 patients with laboratory-confirmed Ebola hemorrhagic fever. The skin, the sputum, the urine, the sweat and the vomitus showed no virus in this study.
_
The table above shows virus culture and reverse-transcription polymerase chain reaction (RT-PCR) results from 33 environmental samples. All most all fomites (environmental surfaces) except hand gloves and IV insertion site showed no virus in this study.
_
These results suggest that environmental contamination and fomites are not frequent modes of transmission, at least in an isolation ward. However, the infectious dose of EBOV is thought to be low, and neither cell culture nor the RT-PCR assay used for EBOV in this study has not been extensively validated for use in environmental detection. Hence, the sensitivity and specificity are unknown. It is possible that EBOV was present in the environment below the threshold of detection or that environmental surfaces in the isolation ward were, at times, initially contaminated by EBOV but then decontaminated through the daily cleaning routine. However, many of the inanimate objects tested, such as bed frames and bedside chairs, would not routinely be specifically decontaminated with bleach solutions under existing guidelines unless they happened to be visibly contaminated, suggesting that environmental contamination did not occur. Taken together with empirical epidemiological observations during outbreaks, the results suggest that current recommendations for the decontamination of filoviruses in isolation wards are effective. The risk from environmental contamination and fomites might vary in the household or other settings where decontamination would be less frequent and thorough, especially if linens or other household materials were to become visibly soiled by blood. Taken together, the results support the conventional assumptions and field observations that most EBOV transmission comes from direct contact with blood or bodily fluids of an infected patient during the acute phase of illness. The risk of casual contacts with the skin, such as shaking hands, is likely to be low. Environmental contamination and fomites do not appear to pose a significant risk when currently recommended infection control guidelines for the viral hemorrhagic fevers are followed.
_
The absence of EBOV infection in multiple tested urine specimens suggests that the virus may not be efficiently filtered in the kidney. Consequently, exposure to urine appears to be of low risk during both acute illness and convalescence. The absence of EBOV in the urine, low prevalence on the skin, and rapid clearance from the saliva in surviving patients provides some reassurance that the risk of secondary transmission from casual contacts, fomites, or the sharing of toilet facilities in the home after discharge from the hospital is minimal. This conclusion is supported by previous empirical observations.
_______
Another study on fomites:
The table below shows Ebola virus detection by reverse-transcription polymerase chain reaction (RT-PCR) in body fluids collected from EVD patients during an outbreak in Gulu, Uganda and the maximum described persistence after symptom onset described in the literature:
| Body Fluid | Acute phase of illness number detected/number tested (percent) |
Convalescent phase of illness number detected/number tested (percent) |
Last day detected after symptom onset described in the literature |
| Skin | 1/8 (13%) | 0/4 (0%) | 6 |
| Saliva | 8/12 (67%) | 0/4 (0%) | 8 |
| Urine | 0/7 (0%) | 0/4 (0%) | 23 |
| Stool / Feces | 2/4 (50%) | n/d | 29 |
| Breast milk | 1/1 (100%) | 1/1 (100%) | 15 |
| Semen | n/d | 1/2 (50%) | 101 |
| Vaginal fluid | n/d | n/d | 33 |
n/d = not done on specimens from the EVD outbreak in Gulu, Uganda
___
Ebola can survive on surfaces for almost Two Months: Tests reveal certain strains survive for weeks when stored at low temperatures:
For their 2010 paper, ‘The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol’, Sophie Smither and her colleagues tested two particular filoviruses on a variety of surfaces. These were the Lake Victoria marburgvirus (Marv), and ZEBOV. Each was placed into guinea pig tissue samples and tested for their ability to survive in different liquids, and on different surfaces at different temperatures, over a 50-day period. When stored at 4° (39°F), by day 26, viruses from three of the samples were successfully extracted; Zebov on the glass sample, and Marv on both glass and plastic. By day 50, the only sample from which the virus could be recovered was the Zebov from tissue on glass. ‘This study has demonstrated that filoviruses are able to survive and remain infectious, for extended periods when suspended within liquid and dried onto surfaces,’ explained the researchers. ‘Data from this study extend the knowledge on the survival of filoviruses under different conditions and provide a basis with which to inform risk assessments and manage exposure.’ The researchers do stress that these tests were carried out in a controlled lab environment, and not in the real world, but published their findings to highlight the survival rates.
•Research claims certain strains of Ebola can remain on surfaces for 50 days
•It survived the longest on glass surfaces stored at 4° (39°F)
•Center for Disease Control and Prevention claims Ebola typically lives on a ‘dry’ surface for hours – including doorknobs and tables
•But when stored in moist conditions such in mucus, this is extended
•Survival time depends on the surface, and the room temperature
•Virus can be killed using household bleach and people must come into direct contact with the sample to risk infection.
___
How long can Ebola live on a surface?
In one study by the Centers for Disease Control and Prevention, the Ebola virus lived on a surface in a perfectly controlled environment for up to six days. The virus can live on surfaces for a few hours at most. Ebola is a vicious virus inside the body, but it dies very quickly on surfaces. It’s not a hardy virus. It’s a very wimpy virus. Ebola is easily destroyed outside of the body, experts say. UV light, heat and exposure to oxygen all deactivate the virus over time. CDC Director Dr. Thomas Frieden said that while it’s theoretically possible for someone to catch Ebola by touching a surface that an infected patient sneezed on, for example, past outbreaks have shown that direct contact with a patient’s bodily fluids is the way the virus is spread.
What kills the virus?
Health care workers in West Africa rely on bleach. But the CDC says any hospital disinfectant will work on a nonporous surface. Any EPA-approved disinfectant — Chlorox, Lysol, etc. — will work on a nonporous surface. A dishwasher or washing machine will also kill it.
Would any bodily fluids ebola patient flushed contaminate the water system?
The virus wouldn’t survive long in water. The virus depends heavily on its host — either a human or animal — to stay active. Sanitary sewers and waste water treatment system will kill the virus.
How long does the Ebola virus live on contaminated surfaces, such as bed sheets, door knobs, etc.?
It’s different in every set of circumstances. The Ebola virus eventually dries out in the air and dies. It’s not like anthrax, which forms a hard capsule around itself and can survive for months or a year. Ebola is a virus that is meant to live inside blood or fluid in your cells. It’s not meant to live in the open air, so it dies. A sheet that has wet blood in it is more dangerous than one with dried blood, because by then it would have dried out. There’s not one answer, but it is considered to be fairly safe after about 24 hours, certainly in environments that are cleaned regularly like hospitals.
Can I get Ebola from public transportation? As in, if a passenger coughed into their hand and then held onto the pole, and then another passenger held onto that pole and inadvertently wiped their eye?
It is extremely unlikely for the Ebola virus to spread through public transit for several reasons. Not all viruses build up to infectious doses in all bodily fluids. Usually, Ebola does not at first make victims cough or sneeze, although someone who also had the flu could, in theory, spray vomitus or blood. Once Ebola invades the lungs, the body will cough to clear them. But passengers that deathly ill are not likely to be on public transit. According to the recent W.H.O. statement, high levels of Ebola virus in saliva are rare except in the sickest victims, and whole virus has never been found in sweat. The fluids known to build up high viral loads are blood, feces and vomit.
_______
Mother to baby transmission:
The finding of EBOV in breast milk raises the possibility of direct mother-to-child transmission. In fact, breastfed children of the mothers whose milk was later tested died of laboratory-confirmed EHF during early stages of the outbreak. The isolation of virus from breast milk in one case even after clearance from the blood suggests that transmission may occur even during convalescence. It is possible that the mammary gland, like the gonads and chambers of the eye, is an immunologically protected site in which clearance of virus is delayed. It seems prudent to advise breastfeeding mothers who survive EHF to avoid breastfeeding for at least some weeks after recovery and to provide them with alternative means of feeding their infants.
_
Recommendations for Breastfeeding/Infant Feeding in the Context of Ebola:
Key Points
•When safe alternatives to breastfeeding and infant care exist, mothers with probable or confirmed Ebola virus disease should not have close contact with their infants (including breastfeeding).
•In resource-limited settings, non-breastfed infants are at increased risk of death from starvation and other infectious diseases. These risks must be carefully weighed against the risk of Ebola virus disease.
In most situations, breastfeeding is the best choice for feeding an infant, particularly in resource-limited settings. However, for lactating women with probable or confirmed Ebola virus disease, decisions about how to feed their infant must be made on a case-by-case basis by weighing the risk of transmitting the virus to their baby through breastfeeding with the risks of stopping breastfeeding. Mothers infected with Ebola virus may be critically ill and unable to breastfeed. When mothers infected with Ebola virus are able to breastfeed, decisions about whether or not to breastfeed may depend on the age of the infant, the availability and feasibility of safe nutrition and infant care, and overall sanitary conditions. These risks must be balanced against the likely high risk of Ebola virus transmission through breastfeeding, the act of suckling, and close contact with their ill mother. Although Ebola virus has been detected in breast milk, it is not known whether Ebola virus can be transmitted from mothers to their infants through breastfeeding. However, given what is known about transmission of Ebola virus, regardless of breastfeeding status, infants whose mothers are infected with Ebola virus are already at high risk of acquiring Ebola virus infection through close contact with the mother, and are at high risk of death overall. Therefore, when safe replacements to breastfeeding and infant care exist, mothers with probable or confirmed Ebola virus infection should not have close contact with their infants (including breastfeeding). In resource-limited settings; however, because non-breastfed infants are at increased risk of death from starvation and other infectious diseases, such as diarrheal and respiratory diseases, these risks must be carefully weighed against the risk of Ebola virus infection. There is not enough evidence to provide guidance on when it is safe to resume breastfeeding after a mother’s recovery, unless her breast milk can be shown to be Ebola virus-free by laboratory testing. In the one case in which breast milk was tested, Ebola virus was identified in the breast milk of a lactating woman 7 and 15 days after disease onset.
_
Sexual transmission:
In one study the isolation of EBOV from semen 40 days after the onset of illness underscores the risk of sexual transmission of the filoviruses during convalescence. In another study Zaire EBOV has been detected in the semen of convalescent patients by virus isolation (82 days) and RT-PCR (91 days) after disease onset. Marburg virus has also been isolated from the semen and linked conclusively to sexual transmission 13 weeks into convalescence. Abstinence from sex or the use of condoms during sex, as well as avoidance of breastfeeding and contact with the mucous membranes of the eye for at least 3 months after recovery, are still recommended to avoid possible exposure to EBOV in the aforementioned immunologically protected sites.
________
Transmission to health care workers:
Health-care workers treating those who are infected are at greatest risk of getting infected themselves. The risk increases when these workers do not have appropriate protective clothing such as masks, gowns, gloves and eye protection; do not wear it properly; or handle contaminated clothing incorrectly. This risk is particularly common in parts of Africa where health systems function poorly and where the disease mostly occurs. Hospital-acquired transmission has also occurred in some African countries resulting from the reuse of needles. Some health-care centers caring for people with the disease do not have running water. In the United States the spread to two medial workers treating an infected patient prompted criticism of inadequate training and procedures.
__
Why are health care workers most at risk?
Ebola is spread by direct contact with bodily fluids of someone who has the disease. These fluids include blood, vomit and secretions. Health care workers are at higher risk for Ebola infection because they often treat patients who have reached the stage of the infection with the most symptoms, including vomiting, diarrhea and bleeding. This means there is much more potential for exposure. Health care workers also perform procedures that bring a higher risk of contact with bodily fluids, such as kidney dialysis and respiratory intubation.
Is there something “special” about Ebola that makes it more infectious than other diseases?
Although Ebola is not very contagious — that is, it is not easily spread from person to person by causal contact — the virus has a low “infectious dose,” meaning direct contact with even a small amount of virus could cause infection. Just one viral particle could cause an Ebola infection. A minuscule amount of body fluid may contain more than a sufficient amount of Ebola to get an infection.
Why is it that the two nurses got sick, but the family members that ebola patient Duncan was staying with didn’t?
People with Ebola get progressively more infectious as their disease worsens. Although Duncan’s family lived with him when he first showed symptoms, health care workers took care of the patient when the potential for exposure to his blood and other bodily fluids was much higher.
_
Why high death toll of health care workers?
Ebola virus can be transmitted by direct contact with blood, body fluids, or skin of EVD patients or persons who have died of EVD. As of October 23, 2014, 450 healthcare personnel are known to have become infected with Ebola, of whom 244 died. One disturbing feature of the current epidemic is that so many health workers have lost their lives while caring for the sick or trying to spread public-health messages about Ebola. This is partly because Ebola is transmitted through bodily fluids, and no one has more contact with the bodily fluids of an Ebola patient than his or her doctor and nurse. The first reason for high death toll is that health workers haven’t had access to the supplies they need. Since the disease is transmitted through direct exposure to bodily fluids, they are advised to wear face masks, goggles, gowns and gloves while caring for patients. But doctors and nurses in the developing-country context don’t always have that protective gear. Or if they do, they want to use their scarce supplies when absolutely necessary, which brings us to another reason for the alarming loss of health workers: many doctors caring for Ebola patients in West Africa had no idea they were seeing Ebola patients. The disease had never appeared in this part of Africa, and it’s difficult to diagnose, sometimes masquerading as malaria or the flu until symptoms worsen. So doctors and nurses weren’t always protecting themselves as they would from a deadly virus. A third reason for the outsized health-worker death toll is that the total number of people infected with the virus this year is so much greater. In 1976, the death toll was 280 and there were 318 reported cases. In this 2014 outbreak, that denominator is thirty times larger. Finally, the scale of this outbreak requires medical personnel that just weren’t at the ready in West Africa. Sierra Leone has 2.2 doctors for every 100,000 people. The OECD average is 320 per 100,000 population.
________
What is transmission by droplet contact?
Some diseases can be transferred by infected droplets contacting surfaces of the eye, nose, or mouth. This is referred to as droplet contact transmission. Droplets containing microorganisms can be generated when an infected person coughs, sneezes, or talks. Droplets can also be generated during certain medical procedures, such as bronchoscopy. Droplets are too large to be airborne for long periods of time, and quickly settle out of air. Droplet transmission can be reduced with the use of personal protective barriers, such as face masks and goggles. Measles and SARS are examples of diseases capable of droplet contact transmission.
_
What is airborne transmission?
Airborne transmission refers to situations where droplet nuclei (residue from evaporated droplets) or dust particles containing microorganisms can remain suspended in air for long periods of time. These organisms must be capable of surviving for long periods of time outside the body and must be resistant to drying. Airborne transmission allows organisms to enter the upper and lower respiratory tracts. Fortunately, only a limited number of diseases are capable of airborne transmission.
Diseases capable of airborne transmission include:
Tuberculosis
Chickenpox
Measles
_
Air transmission of ebola:
There are two distinct ways a virus can travel in the air. In what’s known as droplet infection, the virus can travel inside droplets of fluid released into the air when, for example, a person coughs. The droplets travel only a few feet and soon fall to the ground. The other way a virus can go into the air is through what is called airborne transmission. In this mode, the virus is carried aloft in tiny droplets that dry out, leaving dust motes, which can float long distances, can remain infective for hours or days, and can be inhaled into the lungs. Particles of measles virus can do this, and have been observed to travel half the length of an enclosed football stadium. Ebola may well be able to infect people through droplets, but there’s no evidence that it infects people by drying out or getting into the lungs on dust particles. Unlike the bacteria that cause tuberculosis or the virus that caused smallpox, the Ebola virus is not airborne. With airborne viruses, infectious particles can remain in the air even after a person leaves the room, the air is still considered infectious. Any droplets of fluid from cough or sneeze that could contain Ebola can travel only about 3 feet before they fall to the ground, because of gravity and therefore not considered airborne. In 1989, a virus known today as Reston, which is a filovirus related to Ebola, erupted in a building full of monkeys in Reston, Virginia, and travelled from cage to cage. One possible way, never proved, is that the virus particles hitched rides in mist driven into the air by high-pressure spray hoses used to clean the cages, and then circulated in the building’s air system. A rule of thumb among Ebola experts is that, if you are not wearing biohazard gear, you should stand at least six feet away from an Ebola patient, as a precaution against flying droplets.
_
_
The question often asked is whether Ebola could evolve to spread through the air in dried particles, entering the body along a pathway into the lungs. In order to become fully airborne, Ebola virus particles would need to be able to survive in a dehydrated state on tiny dust motes that remain suspended in the air and then be able to penetrate cells in the lining of the lungs. Ebola is very unlikely to develop these abilities. That would be like saying that a virus that has evolved to have a certain life style, spreading through direct contact, can evolve all of a sudden to have a totally different life style, spreading in dried form through the air. However, there are many ways by which Ebola could become more contagious even without becoming airborne. For example, it could become less virulent in humans, causing a milder disease and killing maybe twenty per cent of its victims instead of fifty per cent. This could leave more of them sick rather than dead, and perhaps sick for longer. That might be good for Ebola, since the host would live longer and could start even more chains of infection. In the lab in Liberia, Lisa Hensley and her colleagues had noticed something eerie in some of the blood samples they were testing. In those samples, Ebola particles were growing to a concentration much greater than had been seen in samples of human blood from previous outbreaks. Some blood samples seemed to be supercharged with Ebola. This, too, would benefit the virus, by enhancing its odds of reaching the next victim. Higher the number of viruses in body fluids, greater is chance of transmission.
_
There is considerable misunderstanding concerning the potential for aerosol transmission of filoviruses. The data on formal aerosol experiments leave no doubt that Ebola and Marburg viruses are stable and infectious in small-particle aerosols, and experience of transmission between experimental animals in the laboratory supports this. Indeed, during the 1989–1990 epizootic of the Reston subtype of Ebola, there was circumstantial evidence of airborne spread of the virus, and supporting observations included suggestive epidemiology in patterns of spread within rooms and between rooms in the quarantine facility, high concentrations of virus in nasal and oropharyngeal secretions, and ultrastructural visualization of abundant virus particles in alveoli. However, this is far from saying that Ebola viruses are transmitted in the clinical setting by small-particle aerosols generated from an index patient. Indeed patients without any direct exposure to a known EHF case were carefully sought but uncommonly found. The conclusion is that if this mode of spread occurred, it was very minor. What then were the major routes of transmission? Nonhuman primate studies found conjunctival and oral routes of infection to be possible. It seems likely that the increased risk from late-stage patients reflects increased virus excretion as the disease progresses, similar to that seen in monkey models. Thus, mucous-membrane exposure, pharyngeal contamination during swallowing, inoculation via small skin breaks, or even infection from swallowed infectious material may all contribute to virus transmission.
_
WHO insisted that Ebola is not an airborne pathogen, meaning one you can catch by inhaling an infectious dose from “a suspended cloud of small dried droplets. In contrast with viruses like measles and chickenpox, this type of transmission has not been seen in Ebola studies over several decades. Theoretically, wet and bigger droplets from a heavily infected individual, who has respiratory symptoms caused by other conditions or who vomits violently, could transmit the virus—over a short distance—to another nearby person,” the WHO said. But it said it has not seen any studies that demonstrate this type of transmission—whereas solid studies from previous outbreaks show that all cases resulted from “direct close contact with symptomatic patients.”
_
WHO statements:
On 6 October 2014, the World Health Organization (WHO) released a situation assessment reiterating what scientists know thus far about how Ebola is transmitted. The WHO noted that no evidence to date supports the belief that Ebola is airborne and did not indicate in any fashion that rumors suggesting otherwise had any merit:
1. Ebola virus disease is not an airborne infection. Airborne spread among humans implies inhalation of an infectious dose of virus from a suspended cloud of small dried droplets.
2. This mode of transmission has not been observed during extensive studies of the Ebola virus over several decades.
3. Common sense and observation tell us that spread of the virus via coughing or sneezing is rare, if it happens at all. Epidemiological data emerging from the outbreak are not consistent with the pattern of spread seen with airborne viruses, like those that cause measles and chickenpox, or the airborne bacterium that causes tuberculosis.
4. Theoretically, wet and bigger droplets from a heavily infected individual, who has respiratory symptoms caused by other conditions or who vomits violently, could transmit the virus — over a short distance — to another nearby person.
5. This could happen when virus-laden heavy droplets are directly propelled, by coughing or sneezing (which does not mean airborne transmission) onto the mucus membranes or skin with cuts or abrasions of another person.
WHO is not aware of any studies that actually document this mode of transmission. On the contrary, good quality studies from previous Ebola outbreaks show that all cases were infected by direct close contact with symptomatic patients. According to the WHO, no known leaps of a similar nature have occurred with viruses that affect humans:
_
Could ebola mutate becomes airborne?
You can now get Ebola only through direct contact with bodily fluids. If certain mutations occurred, it would mean that just breathing would put one at risk of contracting Ebola. Infections could spread quickly to every part of the globe, as the H1N1 influenza virus did in 2009, after its birth in Mexico. But viruses like Ebola are notoriously sloppy in replicating; meaning the virus entering one person may be genetically different from the virus entering the next. The current Ebola virus’s hyper-evolution is unprecedented; there has been more human-to-human transmission in the past four months than most likely occurred in the last 500 to 1,000 years. When viruses enter a cell, they make copies of their genetic information to assemble new virus particles. Viruses such as Ebola virus, which have genetic information in the form of RNA (not DNA as in other organisms), are notoriously bad at copying their genome. The viral enzyme that copies the RNA makes many errors, perhaps as many as one or two each time the viral genome is reproduced. There is no question that RNA viruses are the masters of mutation. This fact is in part why we need a new influenza virus vaccine every few years. The more hosts infected by a virus, the more mutations will arise. Not all of these mutations will find their way into infectious virus particles because they cause lethal defects. The virus was only discovered to infect humans in 1976, but it surely infected humans long before that. Furthermore, the virus has been replicating, probably for millions of years, in an animal reservoir, possibly bats. There has been ample opportunity for the virus to undergo mutation. If certain mutations occurred, it would mean that just breathing would put one at risk of contracting Ebola. Infections could spread quickly to every part of the globe, as the H1N1 influenza virus did in 2009, after its birth in Mexico. The key phrase here is ‘certain mutations’. We simply don’t know how many mutations, in which viral genes, would be necessary to enable airborne transmission of Ebola virus, or if such mutations would even be compatible with the ability of the virus to propagate. What allows a virus to be transmitted through the air has until recently been unknown. We can’t simply compare viruses that do transmit via aerosols (e.g. influenza virus) with viruses that do not (e.g. HIV-1) because they are too different to allow meaningful conclusions. One approach to this conundrum would be to take a virus that does not transmit among mammals by aerosols – such as avian influenza H5N1 virus – and endow it with that property. This experiment was done by Fouchier and Kawaoka several years ago, and revealed that multiple amino acid changes are required to allow airborne transmission of H5N1 virus among ferrets. These experiments were met with a storm of protest from individuals who thought they were too dangerous. The other important message from the Fouchier-Kawaoka ferret experiments is that the H5N1 virus that could transmit through the air had lost its ability to kill. The message is clear: gain of function (airborne transmission) is accompanied by loss of function (virulence). When it comes to viruses, it is always difficult to predict what they can or cannot do. It is instructive, however, to see what viruses have done in the past, and use that information to guide our thinking. Therefore we can ask: has any human virus ever changed its mode of transmission? The answer is no. We have been studying viruses for over 100 years, and we’ve never seen a human virus change the way it is transmitted. HIV-1 has infected millions of humans since the early 1900s. It is still transmitted among humans by introduction of the virus into the body by sex, contaminated needles, or during childbirth. Hepatitis C virus has infected millions of humans since its discovery in the 1980s. It is still transmitted among humans by introduction of the virus into the body by contaminated needles, blood, and during birth. There is no reason to believe that Ebola virus is any different from any of the viruses that infect humans and have not changed the way that they are spread. Every time a new person gets Ebola, the virus gets another chance to develop new capabilities. Ebola is an RNA virus, which means every time it copies itself; it makes one or two mutations. Experts say the chances are relatively small that Ebola will make that jump from contact transmission to airborne transmission. I am fully aware that we can never rule out what a virus might or might not do. But the likelihood that Ebola virus will go airborne is so remote that we should not use it to frighten people. We need to focus on stopping the epidemic, which in itself is a huge job. Ebola virus infection can also be transmitted if an infected person coughs or vomits at close range. In this case large droplets landing on the mucous membranes can initiate infection. Although these droplets are traveling through the air, this mode of transmission is considered a form of contact transmission, not airborne transmission.
_
Are we sure Ebola isn’t airborne? A 2012 study:
In a porcine transmission experiment animals were infected by dripping virus into the nose, eyes and mouth, and placed in a room with cynomolgous macaques. The pigs were allowed to roam the floor, while the macaques were housed in cages. All of the macaques became infected. They did find aerosolized Ebola in air samples, and some of the macaques did come down with symptoms of Ebola. So, it looked like pigs could spread Ebola through the air, which is something that had already been suggested by the epidemiology of the 2008 pig Ebola outbreak. It’s always nice when experimental data matches up with that observed during a real-life occurrence of the virus.
_
Ebola Believed to be Potentially Airborne, Researchers Report:
Healthcare workers play a very important role in the successful containment of outbreaks of infectious diseases like Ebola. The correct type and level of personal protective equipment (PPE) ensures that healthcare workers remain healthy throughout an outbreak—and with the current rapidly expanding Ebola outbreak in West Africa, it’s imperative to favor more conservative measures. The report goes on to note that any action which can be taken to “reduce risk” of Ebola exposure should not wait until a “scientific certainty” develops. The minimum level of protection in high-risk settings should be a respirator with an assigned protection factor greater than 10. A powered air-purifying respirator (PAPR) with a hood or helmet offers many advantages over an N95 filtering facepiece or similar respirator, being more protective, comfortable, and cost-effective in the long run. The working theory about Ebola transmission from the CDC and the agency’s director Thomas Frieden, is incorrect and “outmoded” according to the report. Virus-laden bodily fluids may be aerosolized and inhaled while a person is in proximity to an infectious person and that a wide range of particle sizes can be inhaled and deposited throughout the respiratory tract. Background information detailing why these experts believes the CDC and WHO are functioning under an outdated mode of thought when it comes to how infectious diseases are transmitted via aerosols is also included in the new report. Medical and infection control professionals have relied for years on a paradigm for aerosol transmission of infectious diseases based on very outmoded research and an overly simplistic interpretation of the data. In the 1940s and 50s, William F. Wells and other ‘aerobiologists’ employed now significantly out-of-date sampling methods (e.g., settling plates) and very blunt analytic approaches (e.g., cell culturing) to understand the movement of bacterial aerosols in healthcare and other settings. Their work, though groundbreaking at the time, provides a very incomplete picture, the report said. According to researchers, early aerobiologists were unable to measure small particles near an infected person and therefore made an assumption that such particles existed on far from the source and airborne transmission could have happened around 3-feet or so from the source.
_
Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus: 1995 study:
The potential of aerogenic infection by Ebola virus was established by using a head-only exposure aerosol system. Virus-containing droplets of 0.8-1.2 microns were generated and administered into the respiratory tract of rhesus monkeys via inhalation. Inhalation of viral doses as low as 400 plaque-forming units of virus caused a rapidly fatal disease in 4-5 days. The illness was clinically identical to that reported for parenteral virus inoculation, except for the occurrence of subcutaneous and venipuncture site bleeding and serosanguineous nasal discharge. Immunocytochemistry revealed cell-associated Ebola virus antigens present in airway epithelium, alveolar pneumocytes, and macrophages in the lung and pulmonary lymph nodes; extracellular antigen was present on mucosal surfaces of the nose, oropharynx and airways. Aggregates of characteristic filamentous virus were present within type I pneumocytes, macrophages, and air spaces of the lung by electron microscopy. Demonstration of fatal aerosol transmission of this virus in monkeys reinforces the importance of taking appropriate precautions to prevent its potential aerosol transmission to humans.
_________
Infectivity, transmissibility and mortality:
_
The basic reproduction number, R0:
The most important epidemiological quantity to estimate for an infectious disease is typically the basic reproductive ratio, R0, defined as the expected number of secondary cases produced per primary case early in the epidemic. When R0 is greater than 1, the expectation is that a new epidemic will eventually infect a significant percentage of the population if it is not stopped by interventions or chance extinction; conversely, when R0 is less than 1, chance events may lead to a large number of cases, but these are always expected to be much less numerous than the total population size. The basic reproduction number, R0, is interpreted as the average number of secondary cases caused by a typical infected individual throughout its entire course of infection in a completely susceptible population and in the absence of control interventions. In the context of a partially susceptible population owing to prior exposure or vaccination, the (effective) reproduction number, R, quantifies the potential for infectious disease transmission. If R <1, transmission chains are not self-sustaining and are unable to generate a major epidemic. By contrast, an epidemic is likely to occur whenever R >1. When measured over time t, the effective reproduction number Rt, can be helpful to quantify the time-dependent transmission potential and evaluate the effect of control interventions in almost ‘real time’. In summary, R0 is regarded as a summary measure of the transmissibility of infectious diseases, playing a key role in determining the required control effort (for example, intensity of quarantine and isolation strategies). R0 could also be useful for guiding the numbers of antivirals and vaccines that would be needed to achieve control whenever these are available.
_
R0 estimates for prior Ebola outbreaks in Central Africa:
R0 has been estimated for prior EVD outbreaks in Central Africa using mathematical modeling and epidemiological data for two Ebola outbreaks, namely the 1995 outbreak in Democratic Republic of Congo and the 2000 Uganda outbreak, respectively. Unlike the ongoing epidemic in West Africa, past outbreaks in Central Africa have been confined to relatively rural and isolated areas without spreading to urban sectors which facilitated the effective implementation of control interventions. Using a homogenous mixing SEIR (Susceptible-Exposed-Infectious-Removed) model that accounted for a gradual decay in the transmission rate at the start of interventions, Chowell et al. estimated R0 at 1.83 for Congo and 1.34 for Uganda. Using the same epidemic model but employing a Bayesian estimation method, Lekone and Finkenstadt estimated slightly lower values at 1.33 to 1.35 for the outbreak in Uganda. Legrand et al. employed a different modeling approach: while allowing for homogeneous mixing, the study took into account three different transmission settings, that is, transmissions in community, hospital settings and during funerals. R0 was estimated at 2.7 for Congo, 1995 and 2.7 for Uganda, 2000, but estimates showed substantial uncertainty. Transmission from burials alone accounted for 1.8 secondary transmissions in Congo while community transmission in Uganda accounted for 2.6 secondary transmissions. Variability in R0 estimates across studies can be attributed to differences in model structure and underlying assumptions.
_
An assessment of R0 based on the growth rate of the 2014 Ebola epidemic in West Africa:
Assuming that the mean generation time is 12 days (with standard deviation 5.2 days) based on contact tracing data from an outbreak in Uganda 2000, R0 for Liberia is estimated at 1.96 (95% CI: 1.92, 2.01). For Sierra Leone, R0 is 3.07 (95% CI: 2.85, 3.32) and 1.30 (95% CI: 1.26, 1.33) for the early and late phases, respectively.
_
Comparing R0 with other infectious diseases:
_
Fortunately, it is harder to contract Ebola:
Ebola, SARS and chicken pox take roughly the same amount of time to pass from one sick person to the next group of people, called a “generation.” If Ebola were as easy to catch as chicken pox, thousands of people would have been sickened by the fourth generation.
Four generations of Ebola:
Infected 15 people, killed 11 people. A carrier can infect up to 2 people every 9 to 15 days.
Four generations of SARS:
Infected 15 people, killed 2 people. A carrier can infect up to 3 people every 8 to 9 days.
Four generations of Chicken pox:
Infected 5,220 people, killed 0 people. A carrier can infect up to 17 people every 14 to 16 days.
_
The average reproductive ratio is a measure of how easily a disease travels from person to person. A rate of 5 means each sick person passes the disease to an average of five other people. When the number is below 1, the epidemic dies out. The average serial interval is the length of time between the first patient showing symptoms and a secondary case showing symptoms. The longer the better, because that allows time to find and isolate people who have been exposed before they spread the virus.
_
Infectivity:
The virus is extremely infectious. Experiments suggest that if one particle of Ebola enters a person’s bloodstream it can cause a fatal infection. This may explain why many of the medical workers who came down with Ebola couldn’t remember making any mistakes that might have exposed them. One common route of entry is thought to be the wet membrane on the inner surface of the eyelid, which a person might touch with a contaminated fingertip. The virus is believed to be transmitted, in particular, through contact with sweat and blood, which contain high concentrations of Ebola particles. People with Ebola sweat profusely, and in some instances they have internal hemorrhages, along with effusions of vomit and diarrhea containing blood.
_
Ebola spreads slower but it is deadly:
It means it is not easily transmitted from one infected person to another although case fatality rate is high. Compared to other infectious diseases, Ebola spreads slowly and to relatively few people. But it is extremely deadly: The World Health Organization said 70 percent of cases in this year’s outbreak are fatal.
_
The figure below shows that even though case fatality rates of HIV (untreated) and tuberculosis (untreated) are similar to Ebola, HIV and tuberculosis are far more contagious than Ebola.
_
Since we have effective treatment of HIV and TB, CFR of treated HIV and TB would be far lower than untreated cases.
_
Where and How Much?
The speed and degree to which Ebola manages to overcome an individual depends on a couple of factors, scientists who study the virus say. If you are unlucky enough to be infected with Ebola, the amount (or dose) of virus to which you are exposed and the route by which the virus makes its way into your body could mean the difference between whether you live or die. In the world of Ebola, less is better but even a very little is bad. Scientists have differing views on the sometimes cited claim that a single virion – just one virus – is sufficient to trigger infection. While that may, or may not be the true figure for the minimum infectious dose for humans, it is likely that infection can occur from contact with small amounts of virus. Nevertheless, a low-dose exposure may prove less lethal if it allows the immune system to get into gear before the viruses have a chance to disable too many of the early responders. How you get infected likely also plays a role in how sick you become. An exposure that delivers the virus into the blood stream – for example a needle-stick injury, dreaded in the filovirus research world – is more damaging than when viruses are introduced via the mucus membranes surrounding the eyes, nose and in the mouth. Onset of symptoms is quickest with direct-to-blood exposures; they typically account for the short end of the incubation period range, two to 21 days. Most infections become apparent within eight to 10 days of exposure. If you get a direct injection with a lot of virus particles, I don’t think anything’s going to save you, because you’re just overwhelmed. In the 1976 Ebola epidemic that brought the disease to the attention of the world, 85 people were known to have been infected through the reuse of contaminated syringes. All 85 died, along with nearly 200 others in and around Yambuku, in the former Zaire (now the Democratic Republic of Congo).
_
The Ebola virus is highly infectious but not very transmissible. That may sound to a lay person’s ear like a contradiction. What this means is that very little virus – in animal experiments, as few as 10 virus particles (virions) – can potentially lead to a fatal infection. That’s the “infectious” part of the equation. But it’s not easy for that virus to be transmitted. Ebola is much less contagious than measles or influenza. It’s not an airborne virus. It’s transmitted through bodily fluids. The overwhelming majority of people who have been infected with Ebola are people who have directly cared for a person who is actively sick with the disease or have handled the body of someone who have died from it. Experts call it “a caregivers disease,” and note that children don’t typically get it even though they are running around touching many objects and surfaces. They don’t care for sick people as a rule. Ebola is terrifying but not as easy to catch as people think. The cases have had direct, intimate interactions with sick people or bodies: taking care of someone at home, treating a patient without protection, or preparing the body of an Ebola victim for burial. We’re talking about very sick people with vomit and stool on their skin and clothes in a house without running water or a toilet, not someone with a runny nose on a public bus. Bodies are dangerous because the virus inside is protected for a few days, and the cleaning and preparation for burial of a body requires very close interaction with it. And we see relatively few kids with Ebola, when any parent knows that kids run around touching everything and never washing their hands. If it was on surfaces or as contagious as something like measles then we’d see a lot more sick kids.
_
The figure below shows that ebola patient becomes more contagious as disease worsens and dead body is maximally contagious as recently dead bears maximum virus in body fluids:
_
Note:
Please avoid confusion between infectiousness and contagiousness even though they are used synonymously. Infectiousness (infectivity) is a measure of the ability of a microorganism to establish itself in the host and refers to the individual dose or numbers of the microorganisms required to infect susceptible host. Infectiousness is quantified by infectious dose; lesser the number of organisms required to cause infection, greater is infectiousness (infectivity). Contagiousness means ease of transmission from one person to another by any means.
____________
Risk factors for ebola:
For most people, the risk of getting Ebola is low. The risk increases if you:
•Travel to Africa. You’re at increased risk if you visit or work in areas where Ebola virus outbreaks have occurred.
•Conduct animal research. People are more likely to contract the Ebola virus if they conduct animal research with monkeys imported from Africa or the Philippines.
•Provide medical or personal care. Family members are often infected as they care for sick relatives. Medical personnel also can be infected if they don’t use protective gear, such as surgical masks and gloves.
•Prepare people for burial. The bodies of people who have died of Ebola hemorrhagic fever are still contagious. Helping prepare these bodies for burial can increase your risk of developing the disease.
_
Individuals at the highest risk for Ebola infection include the following:
•Anyone who has had direct contact with the blood and body fluids of an individual diagnosed with Ebola
•Anyone who has had close physical contact with an individual diagnosed with Ebola
•Anyone who lived with or visited an Ebola-diagnosed patient while the patient was ill
_
The Ebola age divide:
A study found that age is a key factor establishing the fatality rate; for over 45s it is 94% while for those aged up to 21 it is 57%.
_
Who is most at risk?
During an outbreak, those at higher risk of infection are:
* health care workers;
* family members or others in close contact with infected people;
* mourners who have direct contact with the bodies of the deceased as part of burial ceremonies; and
* hunters in the rain forest who come into contact with dead animals found lying in the forest.
More research is needed to understand if some groups, such as immuno-compromised people or those with other underlying health conditions, are more susceptible than others to contracting the virus. Exposure to the virus can be controlled through the use of protective measures in clinics and hospitals, at community gatherings, or at home.
_
Definition and assessment of risk exposure:
| Risk level | Definition |
| High-risk exposure | • Percutaneous injury, e.g. needlestick, or mucous membrane exposure to body fluids of an EVD patient |
| • Direct care or exposure to body fluids of an EVD patient without appropriate personal protective equipment (PPE) | |
| • Laboratory worker processing body fluids of confirmed EVD patients without appropriate PPE or standard biosafety precautions | |
| • Participation in funeral rites that include direct contact with human remains in the geographic area where an outbreak is occurring without appropriate PPE | |
| Low-risk exposure | • Household member or other casual contact with an EVD patient |
| • Providing patient care or casual contact without high-risk exposure with EVD patients in health care facilities in EVD outbreak affected countries | |
| No known exposure | Persons with no known exposure were present in an EVD outbreak affected country in the past 21 days with no low-risk or high-risk exposures |
| Casual contact is defined as (i) being within approximately 3 feet (1 meter) or within the room or care area for a prolonged period of time (e.g. healthcare personnel, household members) while not wearing recommended personal protective equipment; or (ii) having direct brief contact (e.g., shaking hands) with an EVD case while not wearing recommended personal protective equipment |
_
Public health workers will use these risk levels along with assessing symptoms to decide how best to monitor for symptoms and what other restrictions may be needed.
Recommended actions for people without Ebola symptoms:
| RISK LEVEL | PUBLIC HEALTH ACTION | ||
| Active Monitoring | Travel Restrictions | Restricted Public Activities | |
| HIGH risk | Yes—direct active | Yes | Yes |
| SOME risk | Yes—direct active | Possible | Possible |
| LOW risk | Yes—direct active for some*; active for others | No | No |
| NO risk | No | No | No |
*Direct active monitoring is recommended for healthcare workers caring for sick Ebola patients while wearing appropriate PPE correctly and for travelers on an airplane who were seated within 3 feet of a person sick with Ebola.
_________
Clinical manifestation of ebola:
_
The following two types of exposure history are recognized:
•Primary exposure: This typically involves travel to or work in an Ebola-endemic area
•Secondary exposure: This refers to human-to-human exposure (e.g., medical caregivers, family caregivers, or persons who prepared deceased patients for burial), primate-to-human exposure (e.g., animal care workers who provide care for primates), or persons who collect or prepare bush meat for human consumption.
_
Infectious dose:
Ebola fever has an infectious dose of only 1 to 10 viral particles.
_
Incubation period:
2 to 21 days (average 10 days).
_
Signs and symptoms typically begin abruptly within 5 to 10 days of infection with Ebola virus.
Early signs and symptoms include:
•Fever
•Severe headache
•Joint and muscle aches
•Chills
•Weakness
Over time, symptoms become increasingly severe and may include:
•Nausea and vomiting
•Diarrhea (may be bloody)
•Red eyes
•Raised rash
•Chest pain and cough
•Stomach pain
•Severe weight loss
•Bleeding, usually from the eyes, and bruising (people near death may bleed from other orifices, such as ears, nose and rectum)
•Internal bleeding
_
_
Physical Examination:
Physical findings depend on the stage of disease at the time of presentation. Early in the disease, patients may present with fever, pharyngitis, and severe constitutional signs and symptoms. A maculopapular rash, more easily seen on white skin than on dark skin, may be present around day 5 of infection and is most evident on the trunk. Bilateral conjunctival injection is also common. Late in the disease, patients often develop an expressionless facies. At this point, bleeding from intravenous (IV) puncture sites and mucous membranes is common. It is worth noting that in the 1976 Ebola outbreak, bleeding was seen in most cases, whereas in the 1995 Ebola outbreak, bleeding occurred in only half of the patients. Myocarditis and pulmonary edema also are seen in the later stages of the disease. Terminally ill patients often die tachypneic, hypotensive, anuric, and in a coma.
_
The different species of Ebola virus seem to cause somewhat different clinical syndromes, but opportunities for close observation of the diseases under good conditions have been rare. Generally, the abrupt onset of Ebola haemorrhagic fever follows an incubation period of 2—21 days (mean 4—10) and is characterised by fever, chills, malaise, and myalgia. The subsequent signs and symptoms indicate multisystem involvement and include systemic (prostration), gastrointestinal (anorexia, nausea, vomiting, abdominal pain, diarrhoea), respiratory (chest pain, shortness of breath, cough, nasal discharge), vascular (conjunctival injection, postural hypotension, oedema), and neurological (headache, confusion, coma) manifestations. Haemorrhagic manifestations arise during the peak of the illness and include petechiae, ecchymoses, uncontrolled oozing from venepuncture sites, mucosal haemorrhages, and post-mortem evidence of visceral haemorrhagic effusions. A macropapular rash associated with varying severity of erythema and desquamate can often be noted by day 5—7 of the illness; this symptom is a valuable differential diagnostic feature and is usually followed by desquamation in survivors. Abdominal pain is sometimes associated with hyperamylasaemia and true pancreatitis. In later stages, shock, convulsions, severe metabolic disturbances, and, in more than half the cases, diffuse coagulopathy supervene.
_
_
Patients with fatal disease develop clinical signs early during infection and die typically between day 6 and 16 with hypovolaemic shock and multiorgan failure. Haemorrhages can be severe but are only present in fewer than half of patients. In non-fatal cases, patients have fever for several days and improve typically around day 6—11, about the time that the humoral antibody response is noted. Patients with non-fatal or asymptomatic disease mount specific IgM and IgG responses that seem to be associated with a temporary early and strong inflammatory response, including interleukin β, interleukin 6, and tumour necrosis factor α (TNFα). However, whether this is the mechanism for protection from fatal disease remains to be proven. Convalescence is extended and often associated with sequelae such as myelitis, recurrent hepatitis, psychosis, or uveitis. Pregnant women have an increased risk of miscarriage, and clinical findings suggest a high death rate for children of infected mothers. This high death rate could be due to transmission from the infected mother to the child during breastfeeding, either through milk or close contact.
_
A typical ebola case:
A “typical” case of EVD begins after an incubation period of about 1 week with the abrupt onset of fever, chills, and a variety of nonspecific signs and symptoms. At the time of presentation, the pulse and blood pressure may be within normal limits. Virus is detectable in the blood at the onset of illness, and persists at high levels in fatal cases. An erythematous, maculopapular rash may be seen during the first week, more commonly in light-skinned individuals. A variety of minor hemorrhagic manifestations, such as petechiae, ecchymoses, and persistent bleeding from needle punctures, are also seen. Laboratory abnormalities include leukopenia and lymphocytopenia, with an increased percentage of neutrophils; thrombocytopenia; and increased serum AST and ALT levels, with AST > ALT. Leukocytosis with a left shift and the appearance of atypical lymphocytes are seen as the disease progresses. Worsening illness is characterized by the development of hypotension, renal insufficiency, and shock, and in many cases by major bleeding from the gastrointestinal tract. Fatally infected patients usually die during the second week of illness, without developing a detectable antibody response. Extensive hemorrhage, hepatocellular necrosis, and a diffuse loss of lymphocytes are the principal findings at autopsy. Survivors undergo a prolonged convalescence, with persistent asthenia, weight loss, and sloughing of skin and hair. Infectious virus may persist for weeks to months in the anterior chamber of the eye or the testes, with the potential for sexual transmission.
__
Fever not a surefire sign of infection:
Ebola study finds that in nearly 13% of confirmed and probable cases tracked, there was no fever. Some experts say the assumption that Ebola spreads only when an infected person has fever should be reassessed. For public health workers screening more than 1,000 air travelers who arrive each week in the United States from Ebola-stricken West Africa, one symptom above all others is supposed to signal danger: fever. So long as an individual’s temperature does not exceed 101.5 degrees and there are no visible symptoms of Ebola, health authorities say it should be assumed the person is not infectious. Yet the largest study of the current outbreak found that in nearly 13% of “confirmed and probable” cases in Liberia, Sierra Leone, Guinea and elsewhere, those infected did not have fevers. The study, sponsored by the World Health Organization and published by the New England Journal of Medicine, analyzed data on 3,343 confirmed and 667 probable cases of Ebola. The finding that 87.1% of those infected exhibited fever — but 12.9% did not — illustrates the challenges confronting health authorities as they struggle to contain the epidemic. U.S. health officials have repeatedly emphasized that fever is a reliable sign of infectiousness. As a defense against the spread of the virus to this country, the Obama administration has ordered that passengers arriving from West Africa at five U.S. airports be checked for fever. Dr. Thomas Frieden, director of the U.S. Centers for Disease Control and Prevention, underlined the importance of fever in discussing the case of Thomas E. Duncan, a Liberian who traveled by air to Dallas and was diagnosed with Ebola. He died recently. Referring to those who had close contact with Duncan, Frieden said a week ago: “The only thing we need to ensure is that their temperature is monitored, and if they develop a fever, that they are immediately assessed, isolated and if found to be positive, then appropriately cared for.” Frieden has said that about 150 air passengers per day — or 1,050 per week — enter the U.S. from Liberia, Sierra Leone and Guinea, the countries at the heart of the outbreak. Dr. Anthony Fauci, who is helping to shape the U.S. response to Ebola as director of the National Institute of Allergy and Infectious Diseases, was asked by a CNN interviewer on Oct. 4 whether a person could be “contagious without having a fever.” Fauci replied that “the answer to that is no.” He continued: “You never say 100% but it’s essentially 100%. … In biology nothing is 100%, but that’s quite a reasonable conclusion to make.” Asked in the same interview about screening of air travelers, Fauci said, “Almost invariably, fever is the thing that signals the onset.”
_
Duration of Illness in Fatal Cases:
In 25 well-documented fatal cases of Marburg and Ebola HF, the majority of deaths occurred during the second week of illness, with a median survival of 9 days from onset of illness to death. The only patient who died after day 16 suffered a terminal intracerebral hemorrhage while being treated in an intensive care unit. The observation that persons who live through the second week of illness are likely to recover is consistent with a report from the 1995 Ebola Zaire HF outbreak that showed that patients who were still alive on day 14 had a >75% chance of survival.
_
Convalescence:
All descriptions of survivors of ebola agree that recovery is prolonged, lasting weeks to months. Sequelae of the acute illness include asthenia, weight loss, headache, dysesthesias, migratory arthralgias, sloughing of skin, loss of scalp hair, and persistent anemia. In a number of instances, acute orchitis or uveitis has developed weeks after the resolution of acute illness, and virus was detected in samples of semen or aqueous humor. During the 1967 Marburg HF outbreak, a convalescent male patient transmitted the virus to his wife, apparently through sexual intercourse.
_
Complications:
Ebola hemorrhagic fevers leads to death for a high percentage of people who are affected. As the illness progresses, it can cause:
- Multiple organ failure
- Severe bleeding
- Jaundice
- Delirium
- Seizures
- Coma
- Shock
For people who survive, recovery is slow. It may take months to regain weight and strength, and the viruses remain in the body for weeks. People may experience:
- Hair loss
- Sensory changes
- Liver inflammation (hepatitis)
- Weakness
- Fatigue
- Headaches
- Eye symptoms (Iritis, iridocyclitis, choroiditis, blindness)
- Testicular inflammation
EBOV may be able to persist in the semen of some survivors for up to seven weeks, which could give rise to infections and disease via sexual intercourse.
_
How doctors know an Ebola patient is no longer contagious:
The blood, or serologic, tests are typically done to assess if a patient with an infectious disease is still sick. Blood test results of someone recovering from the Ebola virus will indicate a decreased viral load compared to someone with an acute infection. Doctors will also look to see if the patient has developed certain antibodies that indicate the body is fighting off the infection. In addition to lab work, doctors also rely on less high-tech approaches such as basic physical exams. The standard is if the symptoms have resolved and the patient has clinically improved that means the patient has recovered from the acute infection. There is some indication that a patient may continue to shed the virus even after a doctor determines the acute infection has subsided. Most likely, the rate at which the virus clears the body varies just as much as the incubation period, which is between 2 and 21 days. According to the World Health Organization, in one instance, a lab worker who contracted Ebola on the job and survived was found to have traces of the virus in his semen 61 days after the initial infection. This could theoretically mean a man could infect his partner during sexual intercourse weeks after he’s declared disease-free, although no such cases have been documented.
____
Prognosis:
EVD has a high risk of death in those infected which varies between 25 percent and 90 percent of those infected. Death, if it occurs, follows typically six to sixteen days after symptoms appear and is often due to low blood pressure from fluid loss. Early supportive care to prevent dehydration may reduce the risk of death. If an infected person survives, recovery may be quick and complete. Prolonged cases are often complicated by the occurrence of long-term problems, such as inflammation of the testicles, joint pains, muscle pains, skin peeling, or hair loss. Eye symptoms, such as light sensitivity, excess tearing, iritis, iridocyclitis, choroiditis, and blindness have also been described.
________
Laboratory findings:
Laboratory variables are less characteristic but the following findings are often associated with Ebola virus disease: early leucopenia (as low as 1000 cells per μL) with lymphopenia and subsequent neutrophilia, left shift with atypical lymphocytes, thrombocytopenia (50 000—100 000 cells per μL), highly raised serum aminotransferase concentrations (aspartate aminotransferase typically exceeding alanine aminotransferase), hyperproteinaemia, and proteinuria. Prothrombin and partial thromboplastin times are extended and fibrin split products are detectable, indicating diffuse intravascular coagulopathy. In a later stage, secondary bacterial infection might lead to raised counts of white blood cells.
_
In patients, the diagnosis is carried out by the detection of viral antigens through ELISA, identification of nucleic acid by PCR, specific antibody titer, or virus isolation. Specific IgM and IgG antibodies appear during the second week following the first clinical signs (about 15 to 20 days after the infection). IgM titers persist about two months whereas IgG titers remain several years after the end of the disease. Virus isolation can be achieved by inoculation in mice, guinea pigs or non-human primates in whom the disease is very close to what is observed in humans. The virus grows on kidney cell lines from African green monkey Cercopithecus aethiops. The diagnosis is made by optical microscopy examination of cytopathic effects, visualization of the virions by electron microscopy, identification of specific proteins by ELISA, or RNA detection by PCR. IgG titration allows retrospective diagnosis in convalescents or exposed persons, or epidemiological investigations even years after the epidemic.
_
Specific Ebola laboratory diagnostic tests:
The diagnosis of EVD is confirmed by isolating the virus, detecting its RNA or proteins, or detecting antibodies against the virus in a person’s blood. Isolating the virus by cell culture, detecting the viral RNA by polymerase chain reaction (PCR) and detecting proteins by enzyme-linked immunosorbent assay (ELISA) are methods best used in the early stages of the disease and also for detecting the virus in human remains. Detecting antibodies against the virus is most reliable in the later stages of the disease and in those who recover. During an outbreak, isolation of the virus via cell culture methods is often not feasible. In field or mobile hospitals, the most common and sensitive diagnostic methods are real-time PCR and ELISA. In 2014, with new mobile testing facilities deployed in parts of Liberia, test results were obtained 3–5 hours after sample submission. Filovirions, such as EBOV, may be identified by their unique filamentous shapes in cell cultures examined with electron microscopy, but this method cannot distinguish the various filoviruses.
__
Viremia:
There is no evidence that persons infected with Ebola or Marburg virus are viremic during the incubation period, but virus has been detected in blood samples on the day of illness onset. Serum levels of viral genomes, as detected by reverse transcription–polymerase chain reaction (RT-PCR), and of viral antigen, as detected by enzyme-linked immunosorbent assay (ELISA), increase during the first week of illness, and in fatal cases remain elevated until death. Studies performed during outbreaks in Gabon and Uganda found that titers of circulating viral genomes were significantly higher in fatal than in nonfatal cases. In patients who survive infection, viremia usually becomes undetectable by the end of the second week of illness. However, infectious virus may persist in certain anatomic sites, such as the testes, breasts or the anterior chamber of the eye.
_
RT-PCR ebola:
Reverse transcription polymerase chain reaction (RT-PCR) is one of many variants of polymerase chain reaction (PCR). This technique is commonly used in molecular biology to detect RNA expression. RT-PCR is often confused with real-time polymerase chain reaction (qPCR) by students and scientists alike. However, they are separate and distinct techniques. While RT-PCR is used to qualitatively detect gene expression through creation of complementary DNA (cDNA) transcripts from RNA, qPCR is used to quantitatively measure the amplification of DNA using fluorescent probes. The laboratory uses antigen capture and reverses transcription-PCR (RT-PCR) to diagnose ebolavirus infection in suspect patients. The RT-PCR and antigen-capture diagnostic assays proved very effective for detecting ebolavirus in patient serum, plasma, and whole blood. In samples collected very early in the course of infection, the RT-PCR assay could detect ebolavirus 24 to 48 h prior to detection by antigen capture.
_
Viral Isolation:
About 100 μl of serum samples are used to inoculate Vero E6 cells maintained in 25-cm2 flasks in Dulbecco’s modified Eagle’s medium containing 2 to 5% fetal-calf serum and penicillin–streptomycin. Cells and supernatant were passaged several times. Virus growth in the cells was verified on immunofluorescence with the use of polyclonal mouse anti-EBOV–specific antibodies in the serum of mice challenged with EBOV or on the basis of an increase in viral levels in the cell-culture supernatant over several orders of magnitude, as measured on RT-PCR.
_
Electron Microscopy:
Specimens from patients are prepared for electron microscopy with the use of a conventional negative-staining procedure. In brief, a drop of 1:10 diluted serum was adsorbed to a glow-discharged carbon-coated copper grid and stained with freshly prepared 1% phosphotungstic acid (Agar Scientific). Images are taken at room temperature with the use of a Tecnai Spirit electron microscope (FEI) equipped with a LaB6 filament and operated at an acceleration voltage of 80 kV.
_
_
Antibody Response:
Because immunofluorescence assays have a high positive background rate, the detection of antifilovirus antibodies has been based on ELISA since the 1995 Kikwit outbreak. Experience during several large epidemics has shown that most fatally infected patients fail to develop an antibody response; the detection of virus-specific immunoglobin M (IgM) or G (IgG) in a serum specimen is therefore a favorable prognostic sign. When an IgM response occurs, it is generally detectable during the first week of illness, and peaks during the second week. Virus-specific IgG appears soon after the IgM. Limited reports indicate that IgG can be detected by ELISA in disease survivors for as long as 11 years.
_
The diagnosis of EVD is confirmed definitively in a laboratory through several types of tests:
- by isolating the virus by cell culture,
- detecting viral RNA by polymerase chain reaction (PCR)
- detecting viral antigens by enzyme-linked immunosorbent assay (ELISA),
- detecting antibodies against the virus in a person’s blood.
_
These testing methods take anywhere from 12 hours to four days to show results. These tests are not entirely foolproof, though. One test for Ebola, the indirect fluorescence assay, is known to have a rather low specificity, and therefore a rather high false negative rate. PCR testing has also been known to miss cases of affliction. In other words, it is possible for an Ebola test to be negative when the person actually does have Ebola. Dr. Brantly’s first Ebola test was negative: According to Dr. Brantly’s employer, Samaritan’s Purse, a U.S.-based international relief organization that has operated in Liberia for 13 years, Dr. Brantly first felt ill July 23 but tested negative. Despite that negative result, he was placed into isolation – a provident decision. His symptoms soon worsened, and a repeat test showed evidence of the virus.
_
Laboratory tests used in diagnosis include:
| Timeline of Infection | Diagnostic tests available |
| Within a few days after symptoms begin |
|
| Later in disease course or after recovery |
|
| Retrospectively in deceased patients |
|
___
Laboratory diagnosis for viral haemorrhagic fevers is generally done in national and international reference centers, which should be contacted immediately on suspicion for advice on sampling, sample preparation, and sample transport. Laboratory diagnosis of Ebola virus is achieved in two ways: measurement of host-specific immune responses to infection and detection of viral particles, or particle components in infected individuals. Nowadays, RT-PCR and antigen detection ELISA are the primary assays to diagnose an acute infection. Viral antigen and nucleic acid can be detected in blood from day 3 up to 7—16 days after onset of symptoms. For antibody detection the most generally used assays are direct IgG and IgM ELISAs and IgM capture ELISA. IgM antibodies can appear as early as 2 days post onset of symptoms and disappear between 30 and 168 days after infection. IgG-specific antibodies develop between day 6 and 18 after onset and persist for many years. A IgM or rising IgG titre constitutes a strong presumptive diagnosis. Decreasing IgM, or increasing IgG titers (four-fold), or both, in successive paired serum samples are highly suggestive of a recent infection. All these assays can be done on materials that have been rendered non-infectious. An efficient way to inactivate the virus for antigen and antibody detection is the use of gamma irradiation from a cobalt-60 source or heat inactivation. Similarly, the nucleic acid can be amplified by purification of the virus RNA from materials treated with guanidinium isothiocyanate—a chemical chaotrope that denatures the proteins of the virus and renders the sample non-infectious.
______
Fast ebola tests:
Genalyte’s $10 10-minute finger prick test:
Genalyte has spent seven years developing a totally new approach. By placing an array of sensors on one tiny silicon chip, it is able to avoid the many manual (and thus variable and messy) steps involved in sample prep that comprise the bulk of testing time. There’s no chemistry involved. It’s just straightforward watching the compounds and binding is seen directly. So it’s a fundamental new diagnostic technology. Eliminating the sample prep is “revolutionary” – and it should be able to test not just for Ebola, but for all types of infectious disease (including influenza), as well as allergy testing and autoimmune testing.
_
Ebola diagnosis in 10 minutes: researchers working on tool to curb global epidemic:
Corgenix researchers say the test they’re developing works like a pregnancy test and can quickly identify whether or not a patient has contracted Ebola using a blood sample in only 15 minutes. However, researchers are “at a standstill” until their certain the tests are 100 percent accurate.
_
Fast, simple diagnostic test specific to 2014 Ebola outbreak by Primerdesign:
Viruses all have a unique genetic fingerprint the same as we do. Ours is encoded in DNA but the Ebola virus uses RNA (Ribonucleic acid). So the kit is designed to specifically detect the Ebola RNA in a patient blood sample.
Process:
1. Blood sample is taken from patient
2. RNA is extracted with a few simple steps
3. RNA is placed in a tube with our kit ingredients
4. Tube goes in to machine
5. Analysis complete within 90 minutes
Primerdesign is a spinoff company from the University of Southampton specialising in Real-Time PCR technology. Real-Time PCR, also known as ‘qPCR’ is a mature technology based on the same DNA testing technology of ‘CSI’ fame.
_
This slip of paper can detect the Ebola virus in less than an hour:
Two teams of researchers at Harvard University’s Wyss Institute for Biologically Inspired Engineering have devised a way to embed synthetic gene networks onto pocket-sized matrices of paper. These slips can be freeze-dried and stored at room temperature for up to a year and rehydrated with water whenever they’re needed. This is noteworthy because these slips of paper can essentially be “programmed” to give visual cues when it detects certain bacteria or viruses. A very timely use case involves a strain-specific Ebola sensor, which produces certain proteins only if it detects the particular Ebola strain. In this instance, the protein stains the paper with a dark purple in less than an hour if one of two Ebola strains are detected. One of the major benefits of this innovation, laid out in a paper titled “Paper-Based Synthetic Gene Networks,” is the extension of synthetic biology beyond laboratories. “We’ve harnessed the genetic machinery of cells and embedded them in the fiber matrix of paper, which can then be freeze dried for storage and transport — we can now take synthetic biology out of the lab and use it anywhere to better understand our health and the environment,” says lead author and Wyss staff scientist Keith Pardee, Ph.D. These paper-based tests are also very cheap. James Collins, an author of the paper and a synthetic biologist at Boston University, estimates that each slip of this detection paper could be designed and produced in about a day and would cost between 4 and 65 cents. There’s still room for improvement, especially when it comes to the ability to detect low concentrations of molecules. “We’re now comfortably in the picomolar range, which is getting close to where you want to be, but I think we’d like to be even lower,” Collins says.
_
Development of an Immunofiltration-Based Antigen-Detection Assay for Rapid Diagnosis of Ebola Virus Infection:
Ebola virus (EBOV) has caused outbreaks of severe viral hemorrhagic fever in regions of Central Africa where medical facilities are ill equipped and diagnostic capabilities are limited. To obtain a reliable test that can be implemented easily under these conditions, monoclonal antibodies to the EBOV matrix protein (VP40), which previously had been found to work in a conventional enzyme-linked immunosorbent assay, were used to develop an immunofiltration assay for the detection of EBOV antigen in chemically inactivated clinical specimens. The assay was evaluated by use of defined virus stocks and specimens from experimentally infected animals. Its field application was tested during an outbreak of Ebola hemorrhagic fever in 2003. Although the original goal was to develop an assay that would detect all EBOV species, only the Zaire and Sudan species were detected in practice. The assay represents a first-generation rapid field test for the detection of EBOV antigen that can be performed in 30 min without electrical power or expensive or sensitive equipment.
_
Fast ebola test problems:
Many faster tests use lateral flow strips (think of a pregnancy test) where antibodies bind to an Ebola virus protein. All that’s required is a finger prick of blood on the strip and a tube with a solution that reveals a line if the virus is present. These tests can take as few as 15 minutes, but like home pregnancy tests, they’re not nearly as accurate. In the case of Ebola, false positives could place people without Ebola in contact with other infected patients, while false negatives could release Ebola patients back into the public.
________
Biomarkers of Inflammation:
Cytokines and chemokines are a diverse group of proteins that modulate the immune response and have been extensively studied in many different disease processes. Serum samples from this outbreak have been analyzed in the past, and increased levels of IL-10 were reported in patients with fatal outcomes. The acute-phase response refers to a constellation of host responses that occur during infection and other inflammatory processes. These responses are classically triggered by proinflammatory cytokines and lead to increased levels of acute-phase reactants. These markers of inflammation are often used clinically to assist in diagnosis and to track a patient’s response to therapy in many infectious or inflammatory processes. The acute-phase reactants —SAA, C-reactive protein, ferritin, and IgG—are elevated. Given the role of the endothelium in maintaining vascular integrity and modulation of hemodynamic stability and the vascular leakage seen in EVD, several markers of endothelial function sICAM, sVCAM, and soluble E-selectin need to be measured. The frequent presence of hemorrhagic manifestations in EVD patients warrants an examination of the measureable factors that control coagulation and fibrinolysis. PAI-1, fibrinogen, tissue plasminogen activator, D-dimer, thrombomodulin, and TF need to be measured in patient samples.
_____
Diagnosis of EVD:
When EVD is suspected in a person, his or her travel and work history, along with an exposure to wildlife, are important factors to consider for possible further medical examination. Most patients acutely ill as a result of infection with Ebola or Marburg virus have high concentrations of virus in blood. Antigen-detection ELISA is a sensitive, robust diagnostic modality. Virus isolation and reverse-transcription PCR are also effective and provide additional sensitivity needed in some cases. Recovering patients develop IgM and IgG antibodies that are readily detected by ELISA. The indirect fluorescent antibody test with paired sera is an effective diagnostic tool in most acute cases but is extremely misleading in population-based serologic surveys for Ebola virus activity. RT-PCR is extremely useful in detecting the need for quarantine or geographic spread. Skin biopsies are an extremely useful adjunct in postmortem diagnosis of infection with Ebola virus (and, to a lesser extent, Marburg virus) because of the presence of large amounts of viral antigen, the relatively low risk posed by sample collection, and the lack of cold-chain requirements for formalin-fixed tissues.
________
Findings at autopsy:
Pathologic changes in fatal cases of filoviral HF are known from a few autopsies performed during the 1967 Marburg HF outbreak and in single cases and epidemics in Africa; they have been summarized by Zaki and Goldsmith. In both Marburg and Ebola HF, the principal gross abnormality is the presence of multiple foci of hemorrhage. The most characteristic histopathologic finding is extensive hepatocellular necrosis, with eosinophilic inclusion bodies corresponding to aggregates of nucleocapsids seen by electron microscopy. The spleen and lymph nodes show a marked decrease in lymphocytes, variously described as follicular “necrosis” or “atrophy,” leaving residual cellular debris. Evidence of acute tubular necrosis, consistent with hypovolemic shock, is seen in the kidneys. Other organs show scattered foci of necrosis, edema, and other nonspecific changes. Few autopsies have been performed on people who have died from Ebola virus disease, because of the high risk posed by the procedures. In fact, a scientific review published in October 2014 identified only 30 human cases where an autopsy or post-mortem biopsies were performed.
______
Differential diagnosis of ebola:
When considering the diagnosis of EVD, other, more common diseases should not be overlooked; for example, malaria, typhoid fever, shigellosis, cholera, leptospirosis, plague, rickettsiosis, relapsing fever, meningitis, hepatitis and other viral haemorrhagic fevers.
_
_
The complete differential diagnosis is extensive and requires consideration of many other infectious diseases such as typhoid fever, shigellosis, rickettsial diseases, cholera, sepsis, borreliosis, EHEC enteritis, leptospirosis, scrub typhus, plague, Q fever, candidiasis, histoplasmosis, trypanosomiasis, visceral leishmaniasis, measles and viral hepatitis among others. Non-infectious diseases that may result in symptoms similar to those of EVD include acute promyelocytic leukemia, hemolytic uremic syndrome, snake envenomation, clotting factor deficiencies/platelet disorders, thrombotic thrombocytopenic purpura, hereditary hemorrhagic telangiectasia, Kawasaki disease and warfarin poisoning.
_______
Treatment of ebola:
Case management is based on isolation of patients and use of strict barrier nursing procedures, such as protective clothing and respirators. These procedures have been sufficient to rapidly interrupt transmission in hospital settings in rural Africa. For members of rural African communities, cadavers are residual risks and should be handled accordingly. Traditional funeral and caretaking methods contribute to the spread of the virus and potentiate outbreaks. Methods to achieve barrier nursing, waste disposal, and other key elements inexpensively and practically in Africa have been devised, and field-tested manuals are available. Important elements for outbreak prevention are provision of sterile equipment for injections, which is remarkably and tragically missing in Africa, and personal protective equipment to doctors, nurses, and caretakers, who are at high risk of contraction of infections in hospitals. As a part of their contingency plans, many developed countries have established proper isolation and intensive care units to deal with imported cases. Whether patients with viral haemorrhagic fever should be transported at later stages of disease is a persistent debate. Nevertheless, any hospital should be safely capable of minimum management of Ebola and other viral haemorrhagic fevers, and should prioritise an initial crucial assessment and an early rapid diagnosis.
_
No specific treatment is currently approved. However, survival is improved by early supportive care with rehydration and symptomatic treatment. Treatment is primarily supportive in nature. These measures may include management of pain, nausea, fever and anxiety, as well as rehydration via the oral or by intravenous route. The World Health Organization recommends avoiding the use of aspirin or ibuprofen for pain due to the bleeding risk associated with use of these medications. Blood products such as packed red blood cells, platelets or fresh frozen plasma may also be used. Other regulators of coagulation have also been tried including heparin in an effort to prevent disseminated intravascular coagulation and clotting factors to decrease bleeding. Antimalarial medications and antibiotics are often used before the diagnosis is confirmed, though there is no evidence to suggest such treatment is in any way helpful. Interferon therapies have been tried as a form of treatment for EVD, but were found to be ineffective. If professional care is not possible, guidelines by WHO for care at home have been relatively successful. In such situations, recommendations include using towels soaked in bleach solutions when moving infected people or bodies and applying bleach on stains. It is also recommended that the caregivers wash hands with bleach solutions and cover their mouth and nose with a cloth.
_
Supportive Treatment:
The mainstay of treatment for Ebola virus disease involves supportive care while the immune system mobilizes an immune response. The most important aspect of supportive care involves preventing intravascular volume depletion, correcting profound electrolyte abnormalities, and avoiding the complications of shock:
●Patients require careful hemodynamic monitoring, and intravenous fluid repletion requirements may be high (e.g., 5 to 10 liters per day). Ebola virus disease may result in reduced effective arterial blood volume despite extracellular fluid volume overload (i.e., “third spacing”).
●Patients may develop significant electrolyte disturbances (e.g., hyponatremia, hypokalemia, and hypocalcemia) and may require frequent repletion of electrolytes to prevent cardiac arrhythmias.
●Patients may require nutrition support.
●Intensive nursing may be required in order to respond to the patient’s changing clinical situation.
Additional measures may include correction of severe coagulopathy, and symptomatic management of fever, nausea, vomiting, diarrhea, and abdominal pain. If dialysis is required, clinicians should refer to the CDC document on how to safely perform acute hemodialysis in patients with Ebola virus disease.
_
Infected patients may also require evaluation and/or treatment of other concomitant infections; for example:
●Patients should be evaluated and treated for concomitant malaria if they are at risk for this disease.
●Empiric antimicrobial treatment should be considered when patients develop vomiting, diarrhea, and/or other signs of severe gastrointestinal dysfunction and/or signs of sepsis. However, the importance of gastrointestinal bacteria in the clinical course of patients with Ebola virus disease is uncertain.
_
Intensive care:
Intensive care is often used in the developed world. This may include maintaining blood volume and electrolytes (salts) balance as well as treating any bacterial infections that may develop. Dialysis may be needed for kidney failure, and extracorporeal membrane oxygenation may be used for lung dysfunction.
______
Rationale of experimental treatment of ebola without trial: Europe vs. USA:
A consortium including European universities and medical groups plans to give experimental drugs to West African Ebola patients without assigning some to a placebo group, touching off an intense trans-Atlantic quarrel over what is ethical and effective in treating the virus. Academics and medical groups in the U.K. and France, such as Oxford University, the Wellcome Trust, Doctors Without Borders and Institute Pasteur of France, have decided to give the drugs to sick African patients without randomly assigning other patients to a control group not getting the medicines. They say that in a ghastly epidemic, it is unethical to hold back treatment from anyone. That has put them at odds with senior U.S. officials at the Food and Drug Administration and the National Institutes of Health. Dr. Luciana L. Borio, FDA assistant commissioner for counterterrorism and emerging threats, told public-health officials at the World Health Organization in Geneva this month she was extremely concerned by the plans to give the medicines to patients without better evidence they work and aren’t highly toxic. “This is too urgent an issue for us not to start out with what we know is scientifically best,” said Dr. Borio. “The fastest and most definitive way to get answers about what are the best products is a randomized clinical trial.” U.S. officials recommend a gold-standard study in which all patients get best possible care; one group also would get a drug, and the other group a placebo. In the case of Ebola, standard care includes aggressively replacing fluids in patients since they can vomit and have diarrhea. Without randomly assigning some patients to the placebo group, scientists say, it can’t be known whether the drugs used to treat the epidemic are saving lives or killing people. Dr. Piero Olliaro, an infectious-diseases doctor at Oxford University who also works at a WHO-affiliated group, is among the leaders of the European group proposing to give experimental drugs to all patients. Among the drugs that could be used, according to Dr. Olliaro, would be experimental medicines brincidofovir from Chimerix Inc. and one called Avigan, or T-705, from Fujifilm Holdings Corp’s Toyama Chemical unit. Doctors from some African countries have joined the efforts of the European coalition. The number of cases in the current Ebola outbreak neared 16,000, the World Health Organization said recently. “What we want to do is not cutting corners. It’s perfectly acceptable,” said Dr. Olliaro. “The problem is where there’s no treatment, people are going to die. Has any American been offered placebo? How would that go down with the American public?” Seven patients were given the experimental drug ZMapp from Mapp Biopharmaceutical, and two died. It isn’t known what effect ZMapp had on the patients’ outcomes. The drug’s limited supply was exhausted in August, and the company and partners are now manufacturing more. Brincidofovir is an experimental anti-viral drug that has worked against Ebola in test-tube cell cultures and now has been used in at least three patients in the U.S. A relatively high dosage in a clinical trial of bone-marrow transplant patients was linked to a high rate of severe diarrhea. Avigan was approved in Japan for influenza, but is still experimental in the U.S. It has shown some anti-Ebola activity in mice and cell cultures, and has been used in some Ebola patients in Europe. Officials at Chimerix and Fujifilm have said they are willing to supply their drugs for use in clinical trials in Africa, but declined to discuss the design of those trials. Fujifilm said it is providing Avigan for use in a clinical trial in Ebola patients in Guinea that is being planned by French and Guinea officials. The European group says plans are in place to start the trials in Africa this year, and some African health officials have publicly said they support the move. European regulators generally require randomized clinical trials before they will approve drugs, as do U.S. regulators. Ideally, in clinical trials, doctors and patients don’t know who is getting the real drug. An independent committee can stop the trial if it discovers the drug is a winner, or dangerous. Dr. Olliaro’s view echoes arguments made in the late 1980s by AIDS activists who wanted access to experimental AIDS drugs. AIDS was then a death sentence, so even an experimental drug was worth trying to some patients. Death rates from Ebola range from 40% to 70% throughout West Africa, Dr. Olliaro said. Dr. Olliaro says a placebo group is unnecessary because in given villages or clinics, doctors will know roughly what the death rate has been there. They can use that rate as a historical control where patients getting a drug are compared with past experience. Historical controls are widely criticized by medical experts because historical results change. A village could improve its medical care, for example. Defenders of the European plan argued in The Lancet that the death rate among Ebola patients is high enough to justify unusual measures. “When conventional care means such a high probability of death, it is problematic to insist on randomizing patients to [placebo] when the intervention arm holds out at least the possibility of benefit,” they wrote. At the NIH’s National Institute for Allergy and Infectious Diseases, Deputy Director for Research H. Clifford Lane is working with the FDA and officials in West Africa to get a randomized, controlled study going of several yet-to-be-determined Ebola drugs. “We want to get the most effective treatments into as many people as quickly as possible, and the only way to do that is a randomized clinical trial,” Dr. Lane said. Without a control group, he said, “you could hurt people.”
_______
Experimental therapies in brief:
There are no approved therapies for patients who have developed Ebola or Marburg virus disease. However, several different treatments have been used during the 2014 outbreak in West Africa. Several experimental treatments for Ebola are being developed, which have shown promising results in monkeys when given up to five days after infection. However, they have not been tested in more than a handful of people and none has been licensed. These strategies have included the use of antibody preparations, small RNA particles, and novel antiviral agents. [vide infra]
1. Antibodies preparations:
Two types of antibody preparations have been used:
●A “cocktail” of three monoclonal antibodies directed against the Ebola viral glycoprotein (“ZMapp”) has been administered to several patients. ZMapp was administered to two United States healthcare workers, both of whom survived and recovered. Two other severely ill healthcare workers given ZMapp did not survive, possibly due to the late initiation of therapy. This agent has been found to prevent the death of Ebola-infected macaques, even when initiated after the animals had developed fever, viremia, and abnormalities in white blood cell counts and blood chemistry.
●The use of whole blood or serum from convalescent Ebola virus disease survivors is being used for the treatment of affected patients. The World Health Organization has issued interim guidance for the collection and administration of convalescent whole blood or plasma for treatment of Ebola virus disease during an outbreak.
2. Small RNA particles:
●An RNA interference agent (TKM-Ebola) that suppresses the production of viral proteins. This agent decreases the occurrence of Ebola virus disease when administered to nonhuman primates following filovirus challenge. Antisense phosphorodiamidate morpholino oligomers that also target viral messenger RNA encoding Ebola virus proteins are being evaluated. Small interfering RNAs and phosphorodiamidate morpholino oligomers have been found to decrease the occurrence of disease when administered to nonhuman primates following filovirus challenge. When small interfering RNA molecules targeting three different viral genes were delivered to macaques 30 to 60 minutes after virus challenge, 60 to 100 percent of animals survived infection, whereas all controls died. In a related approach, antisense oligonucleotides also resulted in the survival of most animals.
3. Antiviral agents:
●A novel antiviral agent, brincidofovir, which is undergoing evaluation as a treatment for cytomegalovirus. This agent has been reported to have in vitro activity against the Ebola virus.
●The synthetic small molecule drug, BCX4430, inhibits viral RNA polymerase function, acting as a non-obligate RNA chain terminator. BCX4430 protected macaques against disease when treatment was begun as late as 48 hours after infection.
_________
Antivirals:
A number of antiviral medications are being studied.
•Favipiravir, approved in Japan for stockpiling against influenza pandemics, appears to be useful in a mouse model of Ebola. On 4 October 2014, it was reported that a French nun who contracted Ebola while volunteering in Liberia was cured with Favipiravir treatment.
•BCX4430 is a broad-spectrum small molecule antiviral drug developed by BioCryst Pharmaceuticals and undergoing animal testing as a potential human treatment for Ebola by USAMRIID. The drug has been approved to progress to Phase 1 trials, expected late in 2014.
•Brincidofovir is a broad-spectrum antiviral drug. Its maker has been granted FDA approval to proceed with a trial to test its safety and efficacy in Ebola patients. It has been used to treat the first patient diagnosed with Ebola in the USA, after he had recently returned from Liberia.
•Lamivudine, usually used to treat HIV/AIDS, was reported in September 2014 to have been used successfully to treat 13 out of 15 Ebola-infected patients by a doctor in Liberia, as part of a combination therapy also involving intravenous fluids and antibiotics to combat opportunistic bacterial infection of Ebola-compromised internal organs. Western virologists have however expressed caution about the results, due to the small number of patients treated and confounding factors present. Researchers at the NIH stated that lamivudine had so far failed to demonstrate anti-Ebola activity in preliminary in vitro tests, but that they would continue to test it under different conditions and would progress it to trials if even slight evidence for efficacy is found.
•JK-05 is developed by the Chinese company Sihuan Pharmaceutical along with the Chinese Academy of Military Medical Sciences. It is reportedly being fast tracked through human trials for Ebola treatment after successful tests in mice.
•Lack of available treatment options has spurred research into a number of other possible antivirals targeted against Ebola,including natural products such as scytovirin and griffithsin, as well as synthetic drugs including DZNep, FGI-103, FGI-104, FGI-106, dUY11 and LJ-001, and other newer agents.
_
Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model: a 2014 study:
Outbreaks of Ebola hemorrhagic fever in sub-Saharan Africa are associated with case fatality rates of up to 90%. Currently, neither a vaccine nor an effective antiviral treatment is available for use in humans. Here, authors evaluated the efficacy of the pyrazinecarboxamide derivative T-705 (favipiravir) against Zaire Ebola virus (EBOV) in vitro and in vivo. T-705 suppressed replication of Zaire EBOV in cell culture by 4 log units with an IC90 of 110 μM. Mice lacking the type I interferon receptor (IFNAR−/−) were used as in vivo model for Zaire EBOV-induced disease. Initiation of T-705 administration at day 6 post infection induced rapid virus clearance, reduced biochemical parameters of disease severity, and prevented a lethal outcome in 100% of the animals. The findings suggest that T-705 is a candidate for treatment of Ebola hemorrhagic fever.
_
Antiviral Drug Therapy of Filovirus Infections: S-Adenosylhomocysteine Hydrolase Inhibitors Inhibit Ebola Virus in vitro and in a Lethal Mouse Model:
Ebola (subtype Zaire) viral replication was inhibited in vitro by a series of nine nucleoside analogue inhibitors of S-adenosylhomocysteine hydrolase, an important target for antiviral drug development. Adult BALB/c mice lethally infected with mouse-adapted Ebola virus die 5–7 days after infection. Treatment initiated on day 0 or 1 resulted in dose-dependent protection, with mortality completely prevented at doses ⩾0.7 mg/kg every 8 h. There was significant protection (90%) when treatment was begun on day 2, at which time, the liver had an average titer of 3 × 105 pfu/g virus and the spleen had 2 × 106 pfu/g. Treatment with 2.2 mg/kg initiated on day 3, when the liver had an average titer of 2 × 107 pfu/g virus and the spleen had 2 × 108 pfu/g, resulted in 40% survival. As reported here, Carbocyclic 3-deazaadenosine is the first compound demonstrated to cure animals from this otherwise lethal Ebola virus infection. S-adenosylhomocysteine (SAH) hydrolase is a cell-encoded enzyme that is an important intracellular target for antiviral drug development. Inhibitors of the cellular enzyme indirectly inhibit transmethylation reactions by a feedback mechanism. Several transmethylation reactions involved in viral replication are potential targets. Inhibition of SAH hydrolase by a drug effectively shuts down methylation and any steps in viral replication that are dependent upon methylation.
__
Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430: a 2014 study:
In the journal Nature, scientists — who conducted much of their work in the secretive, high-containment biological laboratory maintained by USAMRIID at Fort Detrick, Maryland — have reported the discovery of a small molecule that rescues rodents and monkeys from various hemorrhagic fevers. Even more, the drug exhibited activity against a wide range of viruses. The molecule, named BCX4430, resembles the famous “A” found in DNA: adenosine. (Recall that DNA is made of Adenosine, Thymidine, Cytidine and Guanosine.) The RNA-based filoviruses also use “A” in their genomes. BCX4430, because it resembles “A”, can be accidentally used by the virus when it is trying to grow inside of our cells. For the virus, this is a fatal mistake. BCX4430 blocks further growth and reproduction. Hold up a minute, you’re probably thinking. Don’t all cells use “A” too? Shouldn’t this drug be expected to hurt not only the virus, but humans as well? That would be a reasonable expectation, but for some reason, BCX4430 appears to only hurt the virus. Human cells appear not to be fooled by BCX4430 and do just fine in its presence. The most compelling experiment the research team ran involved the infection of cynomolgus macaque monkeys with deadly Marburg virus. Macaques were given twice daily doses of BCX4430 for 14 days beginning 1 hour, 24 hours, or 48 hours post-infection. All of the monkeys that did not receive BCX4430 died by day 12. However, every monkey (except for one) that received a dose of BCX4430 survived, even if the initial dose came 48 hours after infection. In total, 17 out of 18 treated monkeys lived. Amazingly, in vitro experiments showed that BCX4430 could potentially work against a wide range of viruses, including SARS, MERS, influenza, dengue, and measles. The Nature paper demonstrated that BCX-4430 given after a viral challenge could stop Ebola and related Marburg infections from taking hold in rodents. Most notably, BCX-4430 given 48 hours after Marburg virus infection confers complete protection to cynmologous monkeys. Biocryst’s BCX-4430 is a much smaller molecule intended for broad spectrum anti-viral use, described by the company as “an RNA dependent-RNA polymerase inhibitor that has demonstrated broad-spectrum.
_____
Chimerix announces Emergency Investigational New Drug Applications for Brincidofovir (CMX001) authorized by FDA for Patients With Ebola Virus Disease:
Chimerix’s lead product candidate, brincidofovir, is an oral nucleotide analog that has shown broad-spectrum in vitro antiviral activity against all five families of DNA viruses that affect humans, including viruses in the herpes virus family and adenovirus. Brincidofovir has shown no evidence of kidney or bone marrow toxicity in nearly 900 patients treated to date. Building on the positive Phase 2 results in cytomegalovirus (CMV) prevention, Chimerix initiated the Phase 3 SUPPRESS trial in 2013. If positive, data from SUPPRESS will support Chimerix’s initial regulatory submission for brincidofovir for the prevention of CMV infection in adult hematopoietic cell transplant (HCT) recipients. Chimerix recently initiated AdVise, a Phase 3 trial in adenovirus, which is an often-fatal viral infection with no approved treatment; enrollment is ongoing for the pilot portion of the trial. Chimerix is also working with the Biomedical Advanced Research and Development Authority (BARDA) to develop brincidofovir as a medical countermeasure against smallpox. Brincidofovir has received Fast Track designation from the FDA for CMV, adenovirus, and smallpox.
__________
Antisense technology:
Advanced antisense therapies for postexposure protection against lethal filovirus infections: a 2010 study:
Currently, no vaccines or therapeutics are licensed to counter Ebola or Marburg viruses, highly pathogenic filoviruses that are causative agents of viral hemorrhagic fever. Here authors show that administration of positively charged phosphorodiamidate morpholino oligomers (PMOplus), delivered by various dosing strategies initiated 30–60 min after infection, protects >60% of rhesus monkeys against lethal Zaire Ebola virus (ZEBOV) and 100% of cynomolgus monkeys against Lake Victoria Marburg virus (MARV) infection. PMOplus may be useful for treating these and other highly pathogenic viruses in humans. Both small interfering RNAs (siRNAs) and phosphorodiamidate morpholino oligomers (PMOs) targeting EBOV RNA polymerase L protein may prevent disease in nonhuman primates. Sarepta Therapeutics has completed a Phase I clinical trial with its PMO protecting up to 80-100 percent of the nonhuman primates tested.
_
TKM-Ebola:
TKM-Ebola is a small interfering RNA compound, currently being tested in a Phase I clinical trial in humans. The drug, being developed by Tekmira Pharmaceuticals of British Columbia, was in the initial phase of human testing, which is on healthy volunteers, when the F.D.A. halted the trial because side effects were observed. In May 2010, a series of studies demonstrating the ability of an RNAi therapeutic utilizing Tekmira’s LNP technology to protect non-human primates from the Ebola virus were published in The Lancet. Tekmira conducted the studies in collaboration with infectious disease researchers from Boston University and the United States Army Medical Research Institute for Infectious Diseases (USAMRIID). These studies were funded in part by the U.S. Department of Defense’s (DoD) Joint Project Manager Transformational Medical Technologies (JPM-TMT) Office. The results of these preclinical studies demonstrated that when siRNA – delivered by Tekmira’s LNP technology – targeted the Ebola virus to treat previously infected non-human primates, the result was 100 percent protection from an otherwise lethal dose of Zaire Ebola virus.
_
TKM-Ebola is an intravenously infused RNAi Therapeutic that has been demonstrated to save the lives of monkeys infected with an otherwise fatal dose of Ebola. It is a cocktail of three Small interfering RNAs that target L protein, VP24, and VP35 genes of Zaire Ebola virus (ZEBOV), encapsulated by a specific formulation of Liposomes (also known as stabilized nucleic lipid particles (SNALPs)). Each Ebola viral particle contains a non-infectious RNA genome of roughly 19 kbp that encodes 7 structural proteins. The L protein and VP35 make up the polymerase complex, which transcribes and replicates the EBOV genome. The L protein provides the RNA-dependent RNA polymerase activity of the complex. VP24 and VP35 are involved (although not fully understood) in the inhibition of the host type 1 interferon response. Clearly, targeting these 3 proteins would most likely be the most effective strategy to treat the disease. In a series of studies, the team from Tekmira were able to show that siRNA targeting these proteins could indeed be an effective therapeutic strategy. In order to deliver the metabolically-sensitive siRNA into the cytoplasm of the infected cells, they use a lipid-based encapsulation and delivery strategy. They incorporated the siRNA cocktail in their patented formulation of liposomes, which (in case of TKM-Ebola) are made up of cholesterol, DMPC, PEG2000-c-DMA and cationic DLinDMA. Using this delivery system, they were able to show significant reduction in the expression of said proteins in in-vitro studies and hence prevent further proliferation of ZEBOV. Early non-primate studies, with this cocktail, showed 100% protection against ZEBOV, in macaques, when treated with 7 doses of drug, post-exposure to the virus. (Interestingly, only 2 out of 3 rhesus monkeys survived when treated with 4 doses, post-exposure to virus). Unfortunately, in the midst of the outbreak, the FDA instituted a Clinical Hold on the TKM-Ebola safety study because a case of dangerously high cytokine elevations was observed at the highest dose planned in this dose-escalating/dose-finding study (0.5mg/kg). The company argues that the pharmacologic corresponding dose to those curing the monkeys is lower than 0.5mg/kg. Secondly, a side effect that is not tolerable in healthy volunteers (usually ~20-year old male students) could be well tolerated in subjects with a 70-90% likelihood of dying from a disease in a matter of days.
__________
Passive Immunity in Prevention and Treatment of Infectious Diseases:
Antibodies have been used for over a century in the prevention and treatment of infectious disease. They are used most commonly for the prevention of measles, hepatitis A, hepatitis B, tetanus, varicella, rabies, and vaccinia. Although their use in the treatment of bacterial infection has largely been supplanted by antibiotics, antibodies remain a critical component of the treatment of diptheria, tetanus, and botulism. High-dose intravenous immunoglobulin can be used to treat certain viral infections in immunocompromised patients (e.g., cytomegalovirus, parvovirus B19, and enterovirus infections). Antibodies may also be of value in toxic shock syndrome, Ebola virus, and refractory staphylococcal infections. Palivizumab, the first monoclonal antibody licensed (in 1998) for an infectious disease, can prevent respiratory syncytial virus infection in high-risk infants. The development and use of additional monoclonal antibodies to key epitopes of microbial pathogens may further define protective humoral responses and lead to new approaches for the prevention and treatment of infectious diseases. Antibodies have been used for a century for the prevention and treatment of infectious diseases. In bacterial disease, antibodies neutralize toxins, facilitate opsonization, and, with complement, promote bacteriolysis; in viral disease, antibodies block viral entry into uninfected cells, promote antibody-directed cell-mediated cytotoxicity by natural killer cells, and neutralize virus alone or with the participation of complement. Prior to the use of antibiotics, antibodies were the only specific agents for the treatment of certain infections. Although this role has largely been supplanted by antibiotics, there still remains a crucial role for antibody in the treatment of certain infectious diseases. Antibodies can be administered as human or animal plasma or serum, as pooled human immunoglobulin for intravenous (IVIG) or intramuscular (IG) use, as high-titer human IVIG or IG from immunized or convalescing donors, and as monoclonal antibodies (MAb). The therapeutic use of MAb is increasing dramatically, but only one (palivizumab for respiratory syncytial virus) has been licensed for prophylaxis of an infectious disease.
_
Rodent models of filovirus infection have been developed and used particularly to investigate immune system correlates of protection. Passive transfer of neutralizing Abs protects guinea pigs from Ebola virus and Marburg virus infection. Vaccination with recombinant vaccinia virus expressing Ebola virus glycoprotein (GP) confers partial protection in guinea pigs that is not observed with constructs expressing other Ebola virus proteins. These studies imply an important role for antibody in protection against filovirus challenge. Other studies suggest that cell-mediated immunity is important. DNA vaccination with constructs expressing either GP or nucleocapsid protein (NP) protects mice from lethal challenge with Ebola virus. Protection of guinea pigs by DNA vaccination was correlated with antibody and cell mediated responses to GP. The extent to which the rodent models are representative of human filovirus infection is not known. Considerable viral adaptation may be involved in the model. For instance, Ebola virus must undergo eightfold serial passage through mice to produce a virus lethal for these animals. It is therefore important to carry out studies in nonhuman primates. One detailed study has been carried out to evaluate the efficacy of passively administered antibody in protection against Ebola virus in macaques. The Ab used was an immunoglobulin G (IgG) preparation from a horse that had been hyperimmunized with Ebola virus and had a high neutralizing-Ab titer as assessed in a plaque reduction assay. The antibody did delay the onset of clinical symptoms and viremia, but 11 of 12 infected monkeys eventually died. As noted by the authors of that study, the polyclonal equine IgG has a number of limitations, suggesting that it may be valuable to investigate the protective and therapeutic benefit of human monoclonal IgGs. The limitations include the inherently rather low specific activity achievable by passive administration of a polyclonal Ab compared to a monoclonal Ab and the unfavorable pharmacokinetics and diminished effector function activity of an equine IgG in macaques. Human IgGs are very similar to macaques IgGs and are expected to show good pharmacokinetics and effector function activity in the macaques. However, although the use of potent neutralizing human Abs to filoviruses could potentially answer a number of questions, it is not clear that such Abs are produced in natural infection as opposed to the hyperimmunization method used to generate equine IgG as described above. Neutralizing-Ab titers in serum of patients recovering from Ebola virus infection are typically low. These could reflect low concentrations of potent neutralizing Abs in serum or higher concentrations of weakly neutralizing Abs. The latter are unlikely to be effective against the virus, given the results of the studies with macaques. On the other hand, potent neutralizing Abs would signal potential approaches for vaccine development and might prove useful in prophylactic or therapeutic reagents.
_
Treatment of Ebola Hemorrhagic Fever with Blood Transfusions from Convalescent Patients: a1999 study:
Between 6 and 22 June 1995, 8 patients in Kikwit, Democratic Republic of the Congo, who met the case definition used in Kikwit for Ebola (EBO) hemorrhagic fever, were transfused with blood donated by 5 convalescent patients. The donated blood contained IgG EBO antibodies but no EBO antigen. EBO antigens were detected in all the transfusion recipients just before transfusion. The 8 transfused patients had clinical symptoms similar to those of other EBO patients seen during the epidemic. All were seriously ill with severe asthenia, 4 presented with hemorrhagic manifestations, and 2 became comatose as their disease progressed. Only 1 transfused patient (12.5%) died; this number is significantly lower than the overall case fatality rate (80%) for the EBO epidemic in Kikwit and than the rates for other EBO epidemics. The reason for this low fatality rate remains to be explained. The transfused patients did receive better care than those in the initial phase of the epidemic. Plans should be made to prepare for a more thorough evaluation of passive immune therapy during a new EBO outbreak.
_
Blood products for ebola:
The WHO has stated that transfusion of whole blood or purified serum from Ebola survivors is the therapy with the greatest potential to be implemented immediately, although there is little information as to its efficacy. September 2014, WHO issued an interim guideline for this therapy. The blood serum from those who have survived an infection is currently being studied to see if it is an effective treatment. During a meeting arranged by WHO, this research was deemed to be a top priority. Seven of eight people with Ebola survived after receiving a transfusion of blood donated by individuals who had previously survived the infection in a 1999 outbreak in the Democratic Republic of the Congo. This treatment, however, was started late in the disease meaning they may have already been recovering on their own and the rest of their care was better than usual. Thus this potential treatment remains controversial. Intravenous antibodies appear to be protective in nonhuman primates who have been exposed to large doses of Ebola. The WHO has approved the use of convalescent serum and whole blood products to treat people with Ebola. Post-exposure management should consider either passive immunotherapy, or administering drugs that block the action of the virus or its replication. Convalescent sera, thought to contain natural specific protective antibodies developed during the disease, have been used exceptionally, with some success. It is noteworthy that these patients had also received better symptomatic treatments than regular patients, and were not representative. However, despite this indisputable bias, several experimental studies have confirmed, particularly in non-human primates, the effectiveness of such approach. Transfusions are probably useful for the treatment or prevention of shock and may provide coagulation factors to stop or to prevent bleeding. However, because of the small number of patients studied and the lack of control subjects, we cannot conclude that the neutralizing antibodies in transfused convalescent blood improve the outcome for EHF patients.
_
Fortunately, there are examples of provocative new findings that may provide therapy for filovirus infections. Simple convalescent serum has generally had low neutralizing capacity in vitro and has not conferred protection on passive transfer. Nevertheless, high-titered hyperimmune horse anti-Ebola serum has been produced and been found protective in baboons challenged with Ebola virus. This product has been confirmed to be efficacious in guinea pigs, but it is not as useful in rhesus monkeys or the mouse model of Ebola virus infection. The production of human monoclonal antibodies against Ebola virus surface protein from mRNA extracted from bone marrow of Kikwit survivors raises the possibility that an improved, standardized, safe, and replenishable source of therapeutic antibodies could be developed.
_
Points against ebola survivor’s blood transfusion to ebola patient:
It seemed obvious to all at first that the active components in blood transfusions from ebola survivors must be anti-ebola antibodies. Such antibodies would neutralize the virus and help the immune system clear it out. And in fact, a 1999 study reported that seven patients who survived the 1995 outbreak in the Democratic Republic of the Congo after receiving transfusions from survivors had anti-ebola antibodies circulating in their blood. One kind of antibody, called IgM, was absent in another patient who received a transfusion and died. This very small study seemed to indicate that transfusions could work against ebola and that antibodies are key to making them work. (You may be wondering why, if the transfusions worked, more patients weren’t treated this way during the 1995 outbreak. One important reason is that that blood cannot be transfused unless the donor and recipient blood types are compatible. So the treatment is limited by the number of willing survivors and their blood types.) This idea was challenged by a 2007 study done in a nonhuman primate species, rhesus macaques. For this study, researchers drew blood from a rare few monkeys who had survived ebola infection four or five years earlier and a second “boost” infection 30 days earlier. They transferred the blood into other, recently-infected animals, and none of them survived…even those that made a lot of antibody. There are, of course, caveats. Monkeys are not humans, after all. It is possible that they fight the virus differently. And in the discussion of the paper, the researchers admit the experiments that had successfully transferred antibody-mediated immunity in guinea pigs had not worked in rhesus macaques. There are also caveats to the human study though. The main one being that it can’t account for the better treatment transfusion patients received compared to other patients. The seven may have survived simply because they received better care in the clinics. The boost of cells, fluids, proteins and electrolytes that come along with blood transfusions may also have helped. In spite of it all, the World Health Organization is behind blood transfusions and transfer of plasma from ebola survivors.
_________
ZMapp:
The biotech product, called ZMapp™, is indeed still experimental – it’s not yet approved for human use, and not yet even in phase I clinical trials – it’s far from secret. The three-antibody mixture originated with Mapp Biopharmaceuticals, a small San Diego-based company established in 2003 and led by Larry Zeitlin, Ph.D., a Johns Hopkins-trained reproductive biologist who became an expert in “plantibodies,” antibody therapeutics produced in, and purified from, bioengineered plants. The ZMapp three-antibody cocktail isn’t a vaccine. Instead, it provides an artifical immune response against sugar-tagged proteins on the outside of the Ebolavirus (GP). The tobacco species, Nicotiana bethamiana, is a common plant molecular biology tool. There is a process of agroinfiltration, where a solution of a recombinant agrobacterium is used to insert new genetic material into the plant. This general biotherapeutic approach is called passive immunity. By injecting the patient with ready-made antibodies raised in the laboratory to latch onto specific parts of an infectious agent, their body can mount an immediate immune response. Passive immunity is therefore different from a vaccine that might require weeks for the person to make their own antibodies against the virus. These three antibodies represent a clever strategy against the virus. One of the antibodies binds up a form of Ebolavirus protein that seems to be sent off by the virus as a decoy against the immune response.
_
ZMapp is a cocktail of three monoclonal antibodies, which are immune system proteins that can isolate and neutralize an invading pathogen. The antibodies were initially harvested from mice exposed to a protein from Ebola, then genetically engineered to make them more like human antibodies. The resulting antibodies are then manufactured in genetically engineered tobacco plants. To make ZMapp, the genes encoding for the antibodies were extracted from the hybridomas, genetically engineered to replace mouse components with human components, and transfected into tobacco plants. Like intravenous immunoglobulin therapy, ZMapp contains neutralizing antibodies that provide passive immunity to the virus by directly and specifically reacting with it in a “lock and key” fashion.
_
A blend of three monoclonal antibodies has completely protected monkeys against a lethal dose of Ebola virus. Unlike other post-infection therapies, the treatment works even at advanced stages of the disease. These include small ‘interfering’ RNAs (known as TKM-Ebola9) and various combinations of antibodies. But these treatments need to be administered within 2 days of exposure to the virus. So although these approaches were highly important and can be used to treat known exposures, the need for treatments that protect at later times after infection was paramount. Further development and improvement of the antibody-based strategies led to a cocktail of monoclonal antibodies that protected 43% of monkeys when given as late as 5 days after Ebola exposure — a time at which the clinical signs of disease are apparent. Another therapy that combines monoclonal antibodies with interferon-α (a protein that stimulates an antiviral response) provides almost complete protection of macaques when given 3 days after exposure, at which point the virus can be detected but clinical signs are only just beginning to be seen in some animals.
_
The composite drug is being developed by Leaf Biopharmaceutical (LeafBio, Inc.), a San Diego based arm of Mapp Biopharmaceutical. LeafBio created ZMapp in collaboration with its parent and Defyrus Inc., each of which had developed its own cocktail of antibodies, called MB-003 and ZMab.
MB-003:
MB-003 is a cocktail of three humanized or human–mouse chimeric mAbs: c13C6, h13F6 and c6D8. A study published in September 2012 found that rhesus macaques infected with Ebola virus (EBOV) survived when receiving MB-003 (mixture of 3 chimeric monoclonal antibodies) one hour after infection. When treated 24 or 48 hours after infection, four of six animals survived and had little to no viremia and few, if any, clinical symptoms. MB-003 was created by Mapp Biopharmaceutical, based in San Diego, with years of funding from US government agencies including the National Institute of Allergy and Infectious Disease, Biomedical Advanced Research and Development Authority, and the Defense Threat Reduction Agency.
ZMAb:
ZMAb is a mixture of three mouse mAbs: m1H3, m2G4 and m4G7. A study published in November 2013 found that EBOV-infected macaque monkeys survived after being given a therapy with a combination of three EBOV surface glycoprotein (EBOV-GP)-specific monoclonal antibodies (ZMAb) within 24 hours of infection. The authors concluded that post-exposure treatment and a second lethal exposure after 10 and 13 weeks resulted in a robust immune response. ZMab was created by Defyrus, a Toronto-based biodefense company, funded by the Public Health Agency of Canada. The identification of the optimal components from MB-003 and ZMab was carried out at the Public Health Agency of Canada’s National Microbiology Laboratory in Winnipeg.
_
A 2014 paper described how Mapp and its collaborators, including investigators at Public Health Agency of Canada, Kentucky BioProcessing, and the National Institute of Allergy and Infectious Diseases, first chimerized the three antibodies comprising ZMAb, then tested combinations of MB-003 and the chimeric ZMAb antibodies in guinea pigs and then primates to determine the best combination, which turned out to be c13C6 from MB-003 and two chimeric mAbs from ZMAb, c2G4 and c4G7. This is ZMapp. In an experiment also published in the 2014 paper, 21 rhesus macaque primates were infected with the Kikwit Congolese variant of EBOV. Three primates in the control arm were given a non-functional antibody, and the 18 in the treatment arm were divided into three groups of six. All primates in the treatment arm received three doses of ZMapp, spaced 3 days apart. The first treatment group received its first dose on 3rd day after being infected; the second group on the 4th day after being infected, and the third group, on the 5th day after being infected. All three primates in the control group died; all 18 primates in the treatment arm survived. Mapp then went on to show that ZMapp inhibits replication of a Guinean strain of EBOV in cell cultures.
_
The drug was first tested in humans during the 2014 West Africa Ebola virus outbreak, but has not been subjected to a randomized controlled trial to determine whether it works, and whether it is safe enough to allow on the market. As of October 2014, ZMapp had been used to treat 7 individuals infected with the Ebola virus. Although some of them have recovered, the outcome is not considered to be statistically significant. Mapp announced on August 11, 2014, that its supplies of ZMapp had been exhausted. The lack of drugs and unavailability of experimental treatment in the most affected regions of the West African Ebola virus outbreak spurred some controversy. The fact that the drug was first given to Americans and a European and not to Africans, according to the Los Angeles Times, “provoked outrage, feeding into African perceptions of Western insensitivity and arrogance, with a deep sense of mistrust and betrayal still lingering over the exploitation and abuses of the colonial era”. Salim S. Abdool Karim, the director of an AIDS research center in South Africa, placed the issue in the context of the history of exploitation and abuses. Responding to a question on how people may have reacted if ZMapp and other drugs would have been used first on Africans, said, “It would have been the front-page screaming headline: ‘Africans used as guinea pigs for American drug company’s medicine'”.
_
Before there is widespread elation that a cure is at hand, health officials tried to inject some sober reality. World Health Organization spokesman Gregory Hartl says that health authorities “cannot start using untested drugs in the middle of an outbreak, for various reasons.” Doctors Without Borders issued a statement saying: “It is important to keep in mind that a large-scale provision of treatments and vaccines that are in very early stages of development has a series of scientific and ethical implications. As doctors, trying an untested drug on patients is a very difficult choice since our first priority is to do no harm, and we would not be sure that the experimental treatment would do more harm than good.” There is normally a long, complicated process before a drug can be given to patients under FDA regulations. But the FDA does have a “compassionate use” regulation that allows experimental drugs to be administered in emergencies such as this one.
_
Researchers in Thailand claim to have developed an antibody-based treatment for Ebola using synthesized fragments of the virus. It has not been tested against Ebola itself. Scientists from the WHO and NIH have offered to test the treatment against live Ebola virus, but there is still a great deal of development needed before human trials.
_______
Ebola Treatment is very expensive: Treatment for Ebola can cost up to $500,000:
Nina Pham, a Dallas nurse who contracted Ebola while caring for the first person in the U.S. to have the virus, is now Ebola-free. Getting a clean bill of health took 13 days in the hospital and an estimated $110,000. Daily hospital care for Ebola can easily reach $8,500 per day, according to Lockton, a global insurance company in Kansas City, Mo.—$6,000 a day in an intensive care unit, plus $2,500 per day for additional costs, such as physician fees and aggressive support therapy. Costs may even be higher than that, says Eric Justin, Lockton’s chief medical officer, if there are other health complications. That was the case with the U.S.’s first Ebola patient, Thomas Eric Duncan, who was also on dialysis. Based on a two-week confinement, Lockton estimates that the minimum expense for an intensive care unit alone at any hospital would reach $100,000. Experimental medication expenses or care in a specialized biocontainment facility could easily push costs to $500,000 or more. That doesn’t account for potential complications like loss of hearing or vision, bringing disability or long-term health care costs into play. So who would pay? For now, standard health insurance policies would respond, although it’s unclear exactly how much care they may pay for, says Logan Payne of Lockton’s international practice. Most health-care policies, for example, wouldn’t cover experimental treatments, but Justin says patients can’t be charged for such drugs as ZMapp, which has been given to several Ebola victims, until after the FDA approves them. Thus, “for an indeterminate period of time when those experimental drugs are used,” there likely won’t be a cost for the medication, he adds.
_______
Other treatments:
Two selective estrogen receptor modulators usually used to treat infertility and breast cancer (clomiphene and toremifene) have been found to inhibit the progress of Ebola virus in vitro as well as in infected mice. Ninety percent of the mice treated with clomiphene and 50 percent of those treated with toremifene survived the tests. These drugs inhibit of virus-host cell fusion and viral entry. The study authors conclude that given their oral availability and history of human use, these drugs would be candidates for treating Ebola virus infection in remote geographical locations, either on their own or together with other antiviral drugs. A 2014 study found that three ion channel blockers used in the treatment of heart arrhythmias, amiodarone, dronedarone and verapamil, block the entry of Ebola virus into cells in vitro. Other potential anti-ebloa drugs include antifungal terconazole, antidepressant amitriptyline and some anticancer drugs.
____
Inhibition of Lassa virus and Ebola virus infection in host cells treated with the kinase inhibitors genistein and tyrphostin:
Arenaviruses and filoviruses are capable of causing hemorrhagic fever syndrome in humans. Limited therapeutic and/or prophylactic options are available for humans suffering from viral hemorrhagic fever. In this report, authors demonstrate that pre-treatment of host cells with the kinase inhibitors genistein and tyrphostin AG1478 leads to inhibition of infection or transduction in cells infected with Ebola virus, Marburg virus, and Lassa virus. In all, the results demonstrate that a kinase inhibitor cocktail consisting of genistein and tyrphostin AG1478 is a broad-spectrum antiviral that may be used as a therapeutic or prophylactic against arenavirus and filovirus hemorrhagic fever.
Where does Genistein come from?
While primarily found in soy products, especially fermented soy foods, wherein beneficial microbes cause the biotransformation of the precursor phytocompund genistin into genistein, it is also found in fava beans, kudzu, coffee and red clover, and many other lesser known medicinal plants.
__
Resolving cytokine storms with Selenium:
The highly pathogenic Zaire strain of the Ebola virus may be dependent on the trace mineral selenium (Se), due to the presence in the Ebola genome of several open reading frames (ORFs) containing clusters of up to 17 inframe UGA codons, which potentially encode the rare amino acid selenocysteine (SeC). This raises the possibility that Se deficiency in host populations may actually foster viral replication, possibly triggering outbreaks linked and perhaps even facilitating the emergence of more virulent viral strains. Selenium is a strong antioxidant and anti-inflammatory that can control the cytokine storms provoked from out of control infections. The clinical investigations in sepsis studies indicate that higher doses of selenium are well tolerated as continuous infusions of selenium as sodium selenite (4,000 μg selenium as sodium selenite pentahydrate on the first day, 1,000 μg selenium/day on the nine following days) and had no reported toxicity issues. In view of this new information, Biosyn introduced the 1,000 µg dose vials for such high selenium clinical usage. Revici used a special molecular form of selenium (bivalent-negative selenium) incorporated in a molecule of fatty acid. In this form, he can administer up to 1 gram of selenium per day, which corresponds to 1 million micrograms per day, reportedly with no toxic side effects. In contrast, too much selenite (hexavalent-positive selenium) has toxic effects on animals, so human intake of commercial selenite is limited to a dosage of only 100 to 150 micrograms by mouth. The last 25 years the average daily selenium intake has fallen from 60µg/day to 35µg/day. The UK government has established a Reference Nutrient Intake (RNI) level of selenium at 75µg/day. Therefore a nutritional gap now exists between the actual recommended level of daily selenium and what people are actually achieving through their diets. Selenium-deficient lymphocytes are less able to proliferate in response to mitogen, and in macrophages, leukotriene B4 synthesis, which is essential for neutrophil chemotaxis, is impaired by this deficiency. These processes can be improved by selenium supplementation. The humoral system is also affected by selenium deficiency; for example, IgM, IgG and IgA titers are decreased in rats, and IgG and IgM titers are decreased in humans. In endothelial cells from asthmatics, there is a marked selenium deficiency that results in an increase in expression of adhesion molecules, which causes greater adhesion of neutrophils. Selenium is also involved in several key metabolic activities through its selenoprotein enzymes that protect against oxidative damage. Further, selenium deficiency may allow invading viruses to mutate and cause longer-lasting, more severe illness. Animal research has shown selenium and vitamin E have synergistic effects, enhancing the body’s response to bacterial and parasitic infections.
_
Hemorrhagic Infections resolved with Vitamin C:
To date, no viral infection has been demonstrated to be resistant to the proper dosing of vitamin C as classically demonstrated by Klenner. However, not all viruses have been treated with Klenner-sized vitamin C doses, or at least the results have not been published. Ebola viral infection and the other acute viral hemorrhagic fevers appear to be diseases that fall into this category. Because of the seemingly exceptional ability of these viruses to rapidly deplete vitamin C stores, even larger doses of vitamin C would likely be required in order to effectively reverse and eventually cure infections caused by these viruses. Cathcart (1981), who introduced the concept of bowel tolerance to vitamin C hypothesized that Ebola and the other acute viral hemorrhagic fevers may well require 500,000 mg of vitamin C daily to reach bowel tolerance! Whether this estimate is accurate, it seems clear as evidenced by the scurvy-like clinical manifestations of these infections that vitamin C dosing must be vigorous and given in extremely high doses. If the disease seems to be winning, then even more vitamin C should be given until symptoms begin to lessen. Obviously, these are viral diseases that would absolutely require high doses of vitamin C intravenously as the initial therapy. The oral administration should begin simultaneously, but the intravenous route should not be abandoned until the clinical response is complete. Death occurs too quickly with the hemorrhagic fevers to be conservative when dosing the vitamin C. Viral hemorrhagic fevers typically only take hold and reach epidemic proportions in those populations that would already be expected to have low body stores of vitamin C, such as are found in many of the severely malnourished Africans. In such individuals, an infecting hemorrhagic virus will often wipe out any remaining vitamin C stores before the immune systems can get the upper hand and initiate recovery. When the vitamin C stores are rapidly depleted by large infecting doses of an aggressive virus, the immune system gets similarly depleted and compromised. Intravenous vitamin C is a powerful treatment when people are on the edge between life and death from hemorrhagic fevers with the power to bring people back from the brink. Vitamin C (ascorbic acid) is known to perform many critical functions within the body involving detoxification, tissue building, immune enhancement, pain control, and controlling or killing pathogenic organisms. The Ebola virus kills by way of free radicals which can be neutralized by massive doses of sodium ascorbate intravenously.
_
Vitamin D – Perfect Helpmate to Vitamin C:
Vitamin D reduces the risk of dying from a viral infection. Researchers from Winthrop University Hospital in Mineola, New York found that giving supplements of vitamin D to a group of volunteers reduced episodes of infection with colds and flu by 70% over three years. The researchers said that vitamin D stimulates “innate immunity” to viruses and bacteria. Very few have any idea that Vitamin D can be taken in high dosages like Vitamin C can.
_
Glutathione:
Glutathione is required in many of the intricate steps needed to carry out an immune response. It is needed for the lymphocytes to multiply in order to develop a strong immune response, and for killer lymphocytes to be able to kill undesirable cells such as cancer cells or virally infected cells. The importance of glutathione cannot be overstated. It has multiple roles as indicated and, indeed, as one examines each system or organ more closely, the necessity for glutathione becomes increasingly evident. Glutathione values decline with age and higher values in older people are seen to correlate with better health, underscoring the importance of this remarkable substance for maintaining a healthy, well-functioning body.”
_
Antimalarial drug Chloroquine shows promise against Ebola:
Several research groups have been scouring medicine cabinets for FDA approved drugs that have activity against Ebola viral replication. The most interesting among these is the antiviral activity of chloroquine and related compound hydroxychloroquine both of which are also used in the treatment of systemic lupus erythematosus and rheumatoid arthritis. These antimalarial agents accumulate in the endosomes and prevent their acidification maturation, which are crucial steps in the activation of not only lysosomal enzymes but also innate immune receptors TLR7 and TLR9. Now it turns out the Ebola and Marburg viruses also pass through the endosomes and their acidication is essential for exiting the endosomes and chloroquine and hydroxychloroquine trap the viral particles preventing their escape. Among monkeys treated with chloroquine 80-90 percent survived challenge with Ebola and Marburg viruses according to one study. Intraperitoneal administration improved the survival to 100%. Since these drugs target host proteins, the virus is unlikely to develop drug resistance.
_
Ozone Therapy:
Ebola hijacks your immune system and suppresses it. Once your immune system realizes the virus is there, it launches a cytokine storm, and it is this cytokine storm that leads to massive tissue destruction and capillary leakage. This is what causes the hemorrhaging associated with Ebola. Ozone modulates the cytokine storm. It’s anti-infective. It improves circulation and blood flow, oxygen delivery, and likely upregulates mitochondrial respiration, thereby generating more energy in your cells. Oxygen is one of the most important things your body needs for tissue healing when you’re riddled with infection. With bacteria, ozone works by puncturing the membrane of the bacteria, causing it to spill its contents and die. It also inactivates viruses, and does so 10 times faster than chlorine. Ozone also has the advantage of stimulating the immune system, and modulating it—either up or down depending on what your system requires. Ozone is only hard on the lungs, but it can be given in other ways. It can be given intravenously. It can be given in the bladder, in the vagina, in the rectum, via injection – anywhere. It’s just [that] it can be tough on the lungs. You don’t want to be breathing it.
_
Red Algae extract defends against Ebola:
Mannose-binding lectins are produced in the human body via a DNA sequence, called the MBL2. When our genes are working properly, these lectins flood the bloodstream and scourge disease – including unwanted fungi, bacteria, and even parasites, which utilize glycoprotein shells to protect themselves. They also inhibit virus growth. They do this by breaking apart the surface of the unwanted microbe and breaking them down, allowing the body’s additional immune cells to kill off the virus or toxin and prevent it from replicating. Research over the past five years has found that low levels of mannose-binding lectins increases the risk of respiratory infections, including syncytial virus infections, pneumonia and others. Ebola, as many other viruses do, comes with a glycoprotein shell that must be broken down in order for someone who is in contact with it to remain healthy. Furthermore, the glycoprotein shell of the Ebola virus produces glycoproteins that damage cells, allowing the virus to penetrate and replicate within the cell – but red algae has been shown to keep this from happening. The reason for this mutation/switch-off has yet to be fully understood. Red algae produces mannose-binding lectins plentifully, which allow the plants to protect themselves from invasion by viruses. The most promising form of mannose-binding lectins is a component of the Scytonema varium red algae called Scytovirin. The protein extract was isolated by researchers from the National Cancer Institute at Frederick, Maryland in 2003. It contains 95 amino acids, and was found to bind to HIV-1 viral shells. Another antiviral protein in red algea broke down the glycoprotein shells of HIV and HCV. And yet another anti-viral extract found in a New Zealand red algae species, Griffithsia was beneficial in treating mice with epidemic-potentiated SARS and also HIV-1. It stopped the viruses from replicating. In 2010, Harvard researchers tested a recombinant version of Griffithsin – called rhMBL – against Ebola, and found that it broke down those pesky viral shells while giving mice complete immunity to the virus. Other animals have since been tested with similar results. So, even if you aren’t certain red algae will keep you from contracting Ebola, you can boost your immune system and protect against a number of diseases, from Ebola to SARS, simply by taking a red seaweed supplement.
________
Ebola survivors:
Surviving Ebola: For those who live through it, what lies ahead?
Medical experts say most people who manage to recover from an acute Ebola infection will likely be able to return to their life and resume normal activities. But unfortunately, Ebola survivors do often develop certain chronic inflammatory conditions that affect the joints and eyes, problems that can follow a survivor through the remainder of their life. Ebola survivors are at risk for arthralgia, a type of joint and bone pain that can feel similar to arthritis. Ebola survivors also frequently report complications with eyes and vision, an inflammatory condition known as uveitis which can cause excess tearing, eye sensitivity, eye inflammation and even blindness. It’s not completely known how long a person can continue to shed the virus once the acute infection has subsided. It’s likely that the recovery from Ebola varies as much as the incubation period of the virus, which can last anywhere between 2 to 21 days. According to the World Health Organization, a lab worker who contracted Ebola on the job was found to have traces of the virus in his semen 61 days after the initial infection. Though it has not been documented, this could theoretically mean a man could infect his partner during sexual intercourse weeks after he seemed to get over the disease. Researchers are still trying to understand what factors help some patients survive Ebola when so many others do not.
___
Ebola survivors are involved in the following aspect of life:
1. Donate their blood/plasma for patients suffering from EVD
2. Can become care-taker of patients suffering from EVD
3. May suffer from chronic complications of EVD
4. May face stigmatization by society
5. Have to abstain from sex or practice safe sex with condoms, not breastfed their children and avoid contact with mucous membrane of eyes; all for at least three months from recovery.
____
Ebola Survivors are likely to be immune to ebola virus:
There is strong epidemiological evidence that once an individual has resolved an Ebola virus infection, they are immune to that strain. Studies of blood samples taken from Ebola survivors a few years after they became infected with the virus show that these people have developed antibodies that can neutralize the Ebola virus. This suggests that Ebola survivors are immune to the disease, and will not get infected again. However, no one has tested what really happens if a survivor is exposed to the virus for a second time. It is not clear whether survivors become immune to all strains of the Ebola virus or only the one that infected them, nor is it clear how long this immunity lasts. There has not been a single case of a person who has been infected who has recovered and has been infected again in the same epidemic. There are five known species of the Ebola virus. The current outbreak is caused by Zaire Ebola virus, which is the deadliest type. In previous outbreaks involving this strain, only 10 percent of patients have survived the infection. In the current outbreak, about 47 percent of people infected with the virus have survived, according to the World Health Organization. It is possible that early treatment efforts have played a role in improving survival rates in this outbreak. It is not clear which biological factors may determine a person’s chance of surviving Ebola, but a stronger immune system appears to be one important factor. Also, laboratory evidence suggests that some people with a genetic mutation may be entirely resistant to Ebola infection. The doctors still don’t know if the experimental drug played any role in helping Ebola patients survive, but patients’ better nutrition and stronger immune systems may have helped their recover.
_
Ebola Survivors could be the Best Weapon for fighting the Killer Virus:
Train immune ebola survivors to help medics, says UK nurse Will Pooley. Many public health authorities believe Pooley and others who have survived Ebola could have a significant advantage when interacting with those who have the disease. There are no known cases of a survivor becoming reinfected, and in laboratory tests monkeys have stayed immune to Ebola for several years. Pooley knows this. “It doesn’t seem likely that I would contract it again,” he said. Compassionate care might not be the only benefit Pooley could bring to those suffering from Ebola. After recovering, Pooley traveled to the United States to donate blood to an American doctor named Kent Brantly, who was also infected in Sierra Leone. The experimental treatment is called “convalescent serum” and involves giving patient antibodies from an Ebola survivor’s blood. The serum has not been through any clinical trials, but Brantly is now Ebola free. Pooley is one of several people who beat Ebola and are now ready and willing to care for the sick. In Liberia, the WHO has been training Ebola survivors in a mock treatment center so they can be deployed to new and existing Ebola clinics. Whether or not they’re immune, they are uniquely equipped to help people who are sick because of their experiences.
_
Ebola Survivors and Sex: better safe than sorry:
Doctors Without Borders, which oversees many Ebola clinics in west Africa, is sending home recovered Ebola patients with a stack of condoms, and health workers are urging them to only engage in protected sex for at least three months after recovery. The virus has been found in the semen and vaginal fluids of convalescents for weeks or even months after symptoms of Ebola have abated, setting off concern that the virus could be spread via sexual contact with otherwise healthy individuals. In men, one study found that Ebola continued to persist in semen for 90 days. U.S. health officials are echoing this caution as a small number of patients have been released from American hospitals. To date, however, there has not been a single documented case of Ebola transmission from sexual activity. Moreover, simply detecting the genetic presence of the virus in recovering patients does not automatically mean that disease transmission could or would take place—especially if the virus is only present in relatively low concentrations. Although a whole, functioning virus is needed to transmit an infection to another person, current testing methods are also so sensitive they also detect nucleic acids from the virus that continue to lurk in bodily fluids during recovery. That may be why one 1999 study in the Democratic Republic of the Congo, which followed 29 people recovering from Ebola and their household contacts (including sex partners) for up to 21 months, found that although four of the five tested convalescents had at least one semen sample with detected Ebola virus inside it, none of their sexual partners developed symptoms of Ebola, even if they had unprotected sex during that period. So why the “safe sex” warning when thousands of patients have survived Ebola and may have gone on to have sex, apparently without infecting their partners? Extreme caution is not an overreaction with this disease. Studies by Bausch and others have also detected live Ebola virus in sexual fluids that can successfully grow in cell culture, suggesting it could also lead to infections in other individuals. It is possible that sexually transmitted Ebola may have flown under the radar because there has been a dearth of data from outbreaks in years past. Also, although extremely unlikely, it is possible that mild Ebola—with very minor symptoms that were not recognized as such—has developed in patients’ sexual partners. Thus, the CDC warns that convalescing patients must either abstain from intercourse and oral sex for three months or use condoms for that entire time. With any infectious disease, when patients have a high viral load in their bodily fluids, it increases the risk they will pass disease to someone else through direct contact with those fluids. With HIV, for example, the risk of passing the disease between partners increases with higher viral load: For every 10-fold increase in viral concentration, one 2012 study suggests there is about a threefold increase in the risk of transmission per sexual act. And with HIV, condoms are a highly effective mode of blocking disease transmission because the virus is primarily spread via contact with sexual fluids or blood. As with HIV, when Ebola progresses, a patient’s viral loads inch upward and that boosts the chance of disease transmission via contact with bodily fluids. Moreover, a certain degree of natural immunological protection for certain body parts—the central nervous system, eyes and gonads—makes it difficult for virus to exit those bodily parts, which may lead to the virus continuing to be present even after the virus was cleared from the blood, according to Bausch. And if an Ebola patient’s disease proves fatal, his viral load at death is particularly high, which boosts the risk of contracting the disease from interacting with the corpse. Ebola virus manages to thrive in a variety of bodily fluids. It is found in its highest concentrations in blood, vomit and feces. But coming into direct contact with semen, vaginal fluids, saliva or even sweat could still be risky while a patient is symptomatic. (Although it’s not likely patients in the throes of illness would be engaging in sex. And live Ebola virus, according to WHO, has never been isolated in human sweat.) Just how infectious those fluids may be after recovery, however, remains a series of question marks. Studies in this area have been extremely small and continue to be largely inconclusive. Thus far, there are no recorded cases of sexual transmission of Ebola. With more than 16,000 cases currently in west Africa right now, however, public health officials do not want to take any chances. Bruce Ribner, the clinician who led the Emory University Hospital team that treated patients Kent Brantly and Nancy Writebol, said in a recent interview with Scientific American that although studies have shown Ebola patients shed genetic material from the pathogen into their sexual fluids there is scant evidence they are often shedding viable virus that could infect others. Yet even Ribner advised his patients about the recommended CDC guidelines of not having unprotected sex for three months. For now, it’s better safe than sorry.
_______
Isolation and quarantine:
Isolation and quarantine help protect the public by preventing exposure to people who have or may have a contagious disease.
•Isolation separates sick people with a contagious disease from people who are not sick. In health care, isolation refers to various measures taken to prevent contagious diseases from being spread from a patient to other patients, health care workers, and visitors, or from others to a particular patient.
•Quarantine separates and restricts the movement of people who were exposed to a contagious disease to see if they become sick. This is often used in connection to disease and illness, such as those who may possibly have been exposed to a communicable disease until they either show signs of the disease (when they will be isolated) or are no longer at risk. Quarantine is usually effective in decreasing spread. Governments often quarantine areas where the disease is occurring or individuals who may transmit the disease outside of an initial area. When someone has been exposed to a contagious disease and it is not yet known if they have caught it, they may be quarantined or separated from others who have not been exposed to the disease. For example, they may be asked to remain at home to prevent further potential spread of the illness. They also receive special care and observation for any early signs of the illness.
_
Sartwell conducted one of the first systematic studies on the incubation time for human pathogens and found that a broad spectrum of agents had incubation time distributions that could be modeled as lognormal (although alternative distributional forms were not tested). Leclerc et al. examined the incubation time distribution of a variety of plant pathogens and observed that they could be fit (depending on the pathogen and the plant age) by either the gamma, lognormal, or Weibull distributions. All of these three are skewed right. Williams looked at the theoretical incubation time distribution for pathogens conforming to a stochastic in vivo birth-death process and found that they could also be characterized by a skewed distribution. In general none of the often used incubation time distributions have a maximum upper limit. In other words, therefore there is no quarantine time that will provide absolute assurance of no residual risk from contagion. Nishiura pointed out the importance of examining the upper tail of the incubation time distribution when assessing the quarantine period following exposure to smallpox. This was also discussed in the context of the SARS corona virus outbreak. Both of these previous authors noted the importance of the distributional form in assessing the upper tail probability, and the influence that data truncation may have on such estimates. To make use of this approach, an acceptable residual risk needs to be set. To do this, one needs to balance out the costs and benefits of quarantine and risk reduction. For contagious diseases this will require coupling the risk of premature release from quarantine to a disease transmission model such as Legrand et al. Clearly for pathogens that have a high degree of transmissibility and/or a high degree of severity, the quarantine time should be greater than for agents with lower transmissibility and/or severity.
_
Ebola, Quarantine of nurses and travel ban:
The governors of a number of states, including New York and New Jersey, recently imposed 21-day quarantines on health care workers returning to the United States from regions of the world where they may have cared for patients with Ebola virus disease. The motivation for this policy is to protect the citizens of their states from contracting this often-fatal illness. Health care professionals treating patients with this illness have learned that transmission arises from contact with bodily fluids of a person who is symptomatic — that is, has a fever, vomiting, diarrhea, and malaise. We have very strong reason to believe that transmission occurs when the viral load in bodily fluids is high, on the order of millions of virions per microliter. This recognition has led to the dictum that an asymptomatic person is not contagious; field experience in West Africa has shown that conclusion to be valid. Therefore, an asymptomatic health care worker returning from treating patients with Ebola, even if he or she were infected, would not be contagious. Furthermore, we now know that fever precedes the contagious stage, allowing workers who are unknowingly infected to identify themselves before they become a threat to their community. This understanding is based on more than clinical observation: the sensitive blood polymerase-chain-reaction (PCR) test for Ebola is often negative on the day when fever or other symptoms begin and only becomes reliably positive 2 to 3 days after symptom onset. This point is supported by the fact that of the nurses caring for Thomas Eric Duncan, the man who died from Ebola virus disease in Texas in October, only those who cared for him at the end of his life, when the number of virions he was shedding was likely to be very high, became infected. Notably, Duncan’s family members who were living in the same household for days as he was at the start of his illness did not become infected. A cynic would say that all these “facts” are derived from observation and that it pays to be 100% safe and to isolate anyone with a remote chance of carrying the virus. What harm can that approach do besides inconveniencing a few health care workers? Hundreds of years of experience show that to stop an epidemic of this type requires controlling it at its source. We need tens of thousands of additional volunteers to control the epidemic. We are far short of that goal, so the need for workers on the ground is great. These responsible, skilled health care workers who are risking their lives to help others are also helping by stemming the epidemic at its source. If we add barriers making it harder for volunteers to return to their community, it will discourage future volunteers to travel to West Africa to control epidemic and thereby worsen epidemic. However, some experts say that maybe volunteers aren’t that effective anyway, and that their lack of actual experience may make them more likely to catch Ebola. The key to stopping Ebola worldwide is stopping it in Africa. Unfortunately, restricting the movement of people — whether through travel bans or forced quarantines of potentially exposed health workers — while politically expedient in a time of panic isn’t likely to stop Ebola spread. In fact, it could have the opposite of the desire effect. To completely seal off and don’t let planes in or out of the West African countries involved, then you could paradoxically make things much worse in the sense that you can’t get supplies in, you can’t get help in, you can’t get the kinds of things in there that we need to contain the epidemic. So restricting travel would render useless the two best methods we have for stopping Ebola: tracking the movements and contacts of potential Ebola cases, and getting aid and resources to West Africa to stop Ebola at the source. Plus, evidence and experience suggests flight restrictions didn’t work to stop HIV/AIDS, SARS, and Swine Flu. This is why health officials unanimously agree that a travel ban is a bad idea.
_
Why has the quarantine period for someone suspected of being infected with Ebola virus set at 21 days?
The length of the quarantine period is based on the incubation period, the time before symptoms of an infection appear. For Ebola virus, the incubation period is 2-21 days after infection. If ebola contact does not get symptoms (fever) in 21 days, he/she does not have ebola virus disease. Of course there are silent ebola infections where quarantine logic fails but they are not contagious anyway.
______
Prevention of ebola in general:
_
Standard precautions for all patients:
It is not always possible to identify patients with EVD early in the course of their illness because initial symptoms may be non-specific. For this reason, it is important that health care workers (HCWs) at all levels carefully apply standard precautions on a consistent basis, with all patients – regardless of their diagnosis – in all practices and at all times.
These include:
1. hand hygiene
2. use of disposable medical examination gloves before contact with body fluids, mucous membrane, non-intact skin and contaminated items, and
3. gown and eye protection before procedures and patient-care activities likely to involve contact with or projection of blood or body fluids.
In addition, regular application of best practices for injection safety and safe handling and disposal of sharp instruments, safe cleaning and disinfection of the environment and of reusable equipment, and safe laundry and waste management should be a high priority in the health care facility (HCF).
_
WHO hand hygiene guidelines for infectious diseases:
_
There are three key preventive interventions:
1. The first is meticulous infection control in health care settings. The greatest risk of transmission is not from patients with diagnosed infection but from delayed detection and isolation. Since the early symptoms of EVD — fever, nausea, vomiting, diarrhea, and weakness — are nonspecific and common, patients may expose family caregivers, health care workers, and other patients before the infection is diagnosed.
2. Second, educating and supporting the community to modify long-standing local funeral practices to prevent contact with body fluids of people who have died from EVD, at least temporarily until the outbreak is controlled, will close the second major route of propagation of the virus. This is a culturally sensitive issue that requires culturally appropriate outreach and education.
3. And third, avoiding handling of bush meat (wild animals hunted for sustenance) and contact with bats (which may be the primary reservoir of Ebola virus) can reduce the risk of initial introduction of Ebola virus into humans. Bush meat consumption could be reduced through socioeconomic development that increases access to affordable protein sources. Where bush meat consumption continues, safer slaughter and handling can be encouraged. The potential effect of deforestation and other environmental changes on increasing human–bat contacts needs to be further studied and addressed.
These are straightforward interventions, but Ebola virus is a formidable enemy. If a single case is missed, a single contact becomes ill and isn’t isolated, or a single lapse in infection control or funeral-practice safety occurs, another chain of transmission can start.
_
Good outbreak control relies on applying a package of interventions, namely case management, surveillance and contact tracing, a good laboratory service, safe burials and social mobilisation. Community engagement is key to successfully controlling outbreaks. Raising awareness of risk factors for Ebola infection and protective measures that individuals can take is an effective way to reduce human transmission. Risk reduction messaging should focus on several factors:
1. Reducing the risk of wildlife-to-human transmission from contact with infected fruit bats or monkeys/apes and the consumption of their raw meat. Animals should be handled with gloves and other appropriate protective clothing. Animal products (blood and meat) should be thoroughly cooked before consumption.
2. Reducing the risk of human-to-human transmission from direct or close contact with people with Ebola symptoms, particularly with their bodily fluids. Gloves and appropriate personal protective equipment should be worn when taking care of ill patients at home. Regular hand washing is required after visiting patients in hospital, as well as after taking care of patients at home.
3. Outbreak containment measures including prompt and safe burial of the dead, identifying people who may have been in contact with someone infected with Ebola, monitoring the health of contacts for 21 days, the importance of separating the healthy from the sick to prevent further spread, the importance of good hygiene and maintaining a clean environment.
_
Infection control:
Recommendations include isolation of hospitalized patients with known or suspected Ebola virus disease; the use of standard, contact, and droplet precautions; and the correct use of appropriate personal protective equipment (PPE). If possible, aerosol-generating procedures should be avoided. However, if they must be performed, patients should be placed in an airborne infection isolation room. The type of PPE used and the careful placement and removal (i.e., donning and doffing) of such equipment are critical to prevent nosocomial transmission of Ebola virus. During the 2014 outbreak in West Africa, several patients have been cared for in the United States. The staff at Emory University used full body suits and powered air purifying respirators (PAPR) to help staff work for extended periods, decrease the physical discomfort of working in multi-component PPE, and avoid difficulties such as fogged face shields. The donning and doffing of PPE was always observed by another staff member. Infection control includes isolation and treatment of patients, contact tracing, and monitoring each contact for 21 days after exposure. It is difficult to isolate and care for patients with EVD, not because the disease is particularly infectious or the virus particularly hardy, but because a single lapse can be devastating. Neither negative air flow nor special respirators are essential; meticulous attention to gown, glove, mask, and eye protection and great care while removing protective equipment are key. Improved hospital infection control throughout the region would prevent a substantial number of EVD and other illnesses. Soap and water or alcohol-based hand sanitizers readily disrupt the envelope of this single-stranded RNA virus, and decontamination with dilute bleach is effective and readily available even in remote settings.
_
Preventing Ebola at individual level:
Individuals can take several precautions to protect against Ebola. These steps include:
•avoiding contact with blood and body fluids
•educating themselves on recognizing the disease and preventing it
•practicing careful hand hygiene, including washing hands with soap and water or an alcohol-based hand sanitizer
•refraining from engaging in burial rituals that involve handling the body of a person who died from Ebola
•refraining from handling items a person with Ebola has handled, including clothing, bedding, needles, or medical equipment
Healthcare workers and lab technicians also must practice very careful precautions. This includes isolating people with Ebola and wearing protective gowns, gloves, masks, and eye shields when coming in contact with the infected person or their belongings. Careful protocol and disposal of these protective materials is also vital for infection prevention. Cleaning crews should use a bleach solution to clean floors and surfaces that may have come in contact with the Ebola virus.
_
_
Is hand hygiene important in ebola?
Hand hygiene is essential and should be performed:
- before donning gloves and wearing PPE on entry to the isolation room/area;
- before any clean or aseptic procedures is being performed on a patient;
- after any exposure risk or actual exposure with a patient’s blood or body fluids;
- after touching (even potentially) contaminated surfaces, items, or equipment in the patient’s surroundings; and
- after removal of PPE, upon leaving the isolation area.
It is important to note that neglecting to perform hand hygiene after removing PPE will reduce or negate any benefits of the PPE. Either an alcohol-based hand rub or soap and running water can be used for hand hygiene, applying the correct technique recommended by WHO. It is important to always perform hand hygiene with soap and running water when hands are visibly soiled. Alcohol-based hand rubs should be made available at every point of care (at the entrance and within the isolation rooms and areas); running water, soap, and single use towels should also be always available.
______
Preventing ebola in health care setting:
Health-care workers should always take standard precautions when caring for patients, regardless of their presumed diagnosis. These include basic hand hygiene, respiratory hygiene, use of personal protective equipment (to block splashes or other contact with infected materials), safe injection practices and safe burial practices. Health-care workers caring for patients with suspected or confirmed Ebola virus should apply extra infection control measures to prevent contact with the patient’s blood and body fluids and contaminated surfaces or materials such as clothing and bedding. When in close contact (within 1 meter) of patients with EBV, health-care workers should wear face protection (a face shield or a medical mask and goggles), a clean, non-sterile long-sleeved gown, and gloves (sterile gloves for some procedures). Laboratory workers are also at risk. Samples taken from humans and animals for investigation of Ebola infection should be handled by trained staff and processed in suitably equipped laboratories.
_
_
All HCWs (including aides and cleaners) and visitors should be trained/instructed to use personal protective equipment (PPE) and perform hand hygiene. Instructions should be displayed at the entry of the isolation room/area. Personal clothing should not be worn while working in the patient care areas. Scrub or medical suits should be worn.
_
Use of the personal protective equipment (PPE):
Experts agreed that it was most important to have PPE that protects the mucosae – mouth, nose and eyes – from contaminated droplets and fluids. Given that hands are known to transmit pathogens to other parts of the body, as well as to other individuals, hand hygiene and gloves are essential, both to protect the health worker and to prevent transmission to others. Face cover, protective foot wear, gowns or coveralls, and head cover were also considered essential to prevent transmission to healthcare workers. Although PPE is the most visible control used to prevent transmission, it is effective only if applied together with other controls including facilities for barrier nursing and work organization, water and sanitation, hand hygiene, and waste management. Benefits derived from PPE depend not only on choice of PPE, but also adherence to protocol on use of the equipment.
_
Health worker with protective ebola suit:
Doffing of personal protective equipment (PPE) is more difficult than donning. A buddy system involving a safety officer with a checklist is recommended for both donning and doffing of PPE. Clinicians must be sure that none of their skin is exposed. Doffing is more difficult than donning because the PPE may be contaminated with blood and bodily fluids from the Ebola patient at that point, and even a small exposure can lead to transmission of the disease. There is no room for error when removing PPE.
_
Surgical cap:
The cap forms part of a protective hood covering the head and neck. It offers medical workers an added layer of protection, ensuring that they cannot touch any part of their face whilst in the treatment centre.
Goggles:
Goggles, or eye visors, are used to provide cover to the eyes, protecting them from splashes. The goggles are sprayed with an anti-fogging solution before being worn. In the new guidelines, health workers are advised to use a single use disposable full face shield as goggles may not provide complete skin coverage.
Medical mask:
It covers the mouth to protect from sprays of blood or body fluids from patients. When wearing a respirator, the medical worker must tear this outer mask to allow the respirator through.
Respirator:
A respirator such as N95 respirators or powered air purifying respirators is worn to protect the wearer from a patient’s coughs. According to guidelines from the medical charity Medecins Sans Frontieres (MSF), the respirator should be put on second, right after donning the overalls.
Medical Scrubs:
A surgical scrub suit, durable hospital clothing that absorbs liquid and is easily cleaned, is worn as a baselayer underneath the overalls. It is normally tucked into rubber boots to ensure no skin is exposed.
Overalls:
The overalls are placed on top of the scrubs. These suits are similar to hazardous material (hazmat) suits worn in toxic environments. The team member supervising the process should check that the equipment is not damaged.
Double gloves:
A minimum two sets of gloves are required, covering the suit cuff. When putting on the gloves, care must be taken to ensure that no skin is exposed and that they are worn in such a way that any fluid on the sleeve will run off the suit and glove. Medical workers must change gloves between patients, performing thorough hand hygiene before donning a new pair. Heavy duty gloves are used whenever workers need to handle infectious waste.
Apron:
A waterproof apron is placed on top of the overalls as a final layer of protective clothing. A waterproof apron covers the torso to the level of the mid-calf for use if patients have vomiting or diarrhea.
Boots:
Ebola health workers typically wear rubber boots, with the scrubs tucked into the footwear. If boots are unavailable, workers must wear closed, puncture and fluid-resistant shoes.
_
The figure below shows health care workers treating ebola patient in Africa wearing PPE:
_
A fundamental principle guiding the selection of different types of PPE was the effort to strike a balance between the best possible protection against infection while allowing health workers to provide the best possible care to patients with maximum ease, dexterity, comfort and minimal heat-associated stress.
__
Healthcare workers who may be exposed to people with Ebola should follow these steps:
•Wear appropriate PPE.
•Practice proper infection control and sterilization measures.
•Isolate patients with Ebola from other patients.
•Avoid direct contact with the bodies of people who have died from Ebola.
•Notify health officials if you have had direct contact with the blood or body fluids, such as but not limited to, feces, saliva, urine, vomit, and semen of a person who is sick with Ebola. The virus can enter the body through broken skin or unprotected mucous membranes in, for example, the eyes, nose, or mouth.
________
Global health security:
In addition to implementing stringent control efforts, we need to accelerate development and deployment of vaccines and antiviral treatment. In addition to acting to stop this outbreak, we should put systems in place to prevent another one. We have three critical advances that will enable further action: increased societal commitment on a global scale; new technologies that allow us to work better, faster, and cheaper; and successes, ranging from better control of EVD in Africa to the rapid and effective response to H7N9 influenza in China. The current EVD outbreak is a tragic illustration of the importance of improving global health security. The components of this strategy — prevent wherever possible, detect rapidly, and respond effectively — match the framework for stopping the EVD outbreak. EVD is a painful reminder that an outbreak anywhere can be a risk everywhere. The Global Health Security Agenda aims to strengthen public health systems in countries that need it most in order to stop outbreaks before they become emergencies. I believe that stopping outbreaks in a way that leaves behind stronger systems to identify, stop, and prevent future health threats is a moral imperative.
_
__________
Prevention of ebola while traveling:
If you travel to or are in an area affected by an Ebola outbreak, make sure to do the following:
•Practice careful hygiene. For example, wash your hands with soap and water or an alcohol-based hand sanitizer and avoid contact with blood and body fluids.
•Do not handle items that may have come in contact with an infected person’s blood or body fluids (such as clothes, bedding, needles, and medical equipment).
•Avoid funeral or burial rituals that require handling the body of someone who has died from Ebola.
•Avoid contact with bats and nonhuman primates or blood, fluids, and raw meat prepared from these animals.
•Avoid hospitals in West Africa where Ebola patients are being treated. The U.S. embassy or consulate is often able to provide advice on facilities.
•After you return, monitor your health for 21 days and seek medical care immediately if you develop symptoms of Ebola.
•Avoid nonessential travel to Liberia, Guinea, and Sierra Leone.
_
Exit screening for Ebola:
Current exit screening of all persons departing affected countries through international airports, seaports and major land crossings is recommended by WHO and can reduce the numbers of people with symptoms from travelling from the countries with high levels of Ebola transmission. While screening upon entry into non-affected countries may provide an opportunity to further increase public awareness about Ebola, such screening also can require significant resources including staff, facilities and systems to care for ill travelers who might be suspected of having Ebola.
_
Is there any evidence that screening travelers arriving at international airports is an effective way of identifying those with serious infections?
The data from Canada, which introduced airport screening during the SARS (severe acute respiratory syndrome) epidemic, are not encouraging. A total of 677 494 people arriving in Canada returned completed questionnaires, of whom 2478 answered “yes” to one or more question. A specially trained nurse referred each of these for in-depth questioning and temperature measurement; none of them had SARS. Thermal scanners were installed at six major airports. Of the 467 870 people screened, 95 were referred to a nurse for further assessment. None of them was confirmed to have a raised temperature. The cost of this unsuccessful program was $15millon. Why was this measure so ineffective, and could it work now? During the SARS epidemic a simple model was used to assess the fraction of cases that could be detected by entrance screening. Assuming that people with symptoms are not allowed to board, entrance screening can only pick up those who develop symptoms while travelling. The longer the incubation period in relation to the flight duration, the lower the chance that this will happen, and the lower the yield from entrance screening. Updating the model using data on Ebola (incubation time 9.1±7.3 days; direct flight from Freetown to London 6.42 hours), researchers estimate that, if everyone with symptoms was denied boarding, about 7 out of 100 people infected with Ebola travelling to the UK would have symptoms on arrival and hence be detectable by entrance screening (95% confidence interval 3 to 13). The other 93% would enter the UK unimpeded. If passengers arriving via Paris or Brussels (journey time about 13 hours) were not screened in transit, entrance screening in the UK could detect up to 13% of infected people (95% CI 7% to 21%). The majority would still enter the UK before developing symptoms. Only if patients are allowed to fly irrespective of symptoms would entrance screening be able to detect a substantial fraction of cases (43% if there is no direct flight, 95% CI 34% to 53%). People who know they are at risk and develop symptoms will want to seek care immediately, as they will fear for their lives. The priority should be to provide information to all those who may be at risk on how and where to seek care. This would be as effective as screening at a fraction of the cost. Adopting the policy of “enhanced screening” gives a false sense of reassurance. This simple calculations show that an entrance screening policy will have no meaningful effect on the risk of importing Ebola into the UK. Better use of the UK’s resources would be to immediately scale-up our presence in West Africa—building new treatment centers at a rate that outstrips the epidemic, thereby averting a looming humanitarian crisis of frightening proportions. In so doing, we would not only help the people of these affected countries but also reduce the risk of importation to the UK.
_
These scientific studies show that airport Ebola screenings are largely ineffective:
The Department of Homeland Security imposed new travel restrictions for anyone arriving from Liberia, Sierra Leone and Guinea, requiring those passengers to come through one of five major U.S. airports in Atlanta, Chicago, New Jersey, New York and Virginia. Those travelers now have to submit to temperature checks and questioning. But scientific studies published by the National Institutes of Health have shown that similar protocols were largely ineffective during an outbreak of Swine Flu in 2009. A study of screenings at Australia’s Sydney Airport during the Swine Flu pandemic found that fever was detected in 5,845 passengers during the roughly two-month period covered by the analysis. Only three of those individuals ended up having the virus, which is known in the scientific community as H1N1. Researchers determined that 45 patients who acquired the illness overseas would have “probably passed through the airport” during the roughly two-month period covered in the study. That means the screeners likely missed the vast majority of individuals who arrived at the facility with Swine Flu, despite grabbing thousands of travelers who showed signs of fever. The Department of Homeland Security requires temperature checks of air passengers arriving from Ebola-ravaged nations, but studies have determined that the method is largely ineffective at detecting individuals who are infected. The report said only 0.5 percent of H1N1 cases in New South Wales, Australia, were detected at the airport, whereas 76 percent were identified in emergency rooms and at general-practice medical centers. Ultimately, researchers concluded that airport temperature checks were “ineffective in detecting cases of H1N1 flu.” Similarly, a study of fever screening in Japan during the pandemic determined that “reliance on fever alone is unlikely to be feasible as an entry screening measure.” Indeed, temperature checks didn’t work for Liberian Thomas Eric Duncan, who died from Ebola this month after arriving in Dallas. Duncan did not have a fever when he landed in Texas on Sept. 28, 2014; and he said he had not been in contact with Ebola patients in his native country, although that later proved to be a false statement. The Australian study concluded that officials should consider “more effective interventions, such as contact tracing in the community.” The findings are in line with what federal officials have said: That the best way to prevent Ebola from spreading is to identify everyone whom infected individuals have contacted.
_
WHO: Air travel is low-risk for Ebola transmission:
The World Health Organization (WHO) reiterated its position that the risk of transmission of Ebola virus disease during air travel remains low. Unlike infections such as influenza or tuberculosis, Ebola is not airborne. It can only be transmitted by direct contact with the body fluids of a person who is sick with the disease. On the small chance that someone on the plane is sick with Ebola, the likelihood of other passengers and crew having contact with their body fluids is even smaller. Usually when someone is sick with Ebola, they are so unwell that they cannot travel. WHO is therefore advising against travel bans to and from affected countries.
___________
Safe burial:
Dead Bodies still contain high levels of the Ebola virus. At least 20% of new infections occur during burials, WHO says. If a person with Ebola disease dies, direct contact with the body should be avoided. There is risk of transmission in the health facility when ebola patient dies because the bodies and body fluids of deceased patients remain contagious for several days after death. Traditional practices regarding patient care and burial rituals often involve high risk conducts, such as washing and preparation of the body for exposure for several days, during which family and friends pay tribute by stroking or hugging the deceased. Safe burial process involves observing rituals differently, such as “dry ablution” Volunteers with full protective clothing are trained to handle and disinfect bodies. Family and community members are at risk if burial practices involve touching and washing the body. Burial should take place as soon as possible after the body is prepared in the health facility. Health facility staff should prepare the body safely. Be aware of the family’s cultural practices and religious beliefs. Help the family understand why some practices cannot be done because they place the family or others at risk for exposure. Certain burial rituals, which may have included making various direct contacts with a dead body, require reformulation such that they consistently maintain a proper protective barrier between the dead body and the living. Social anthropologists may help find alternatives to traditional rules for burials.
_
_
The grave should be at least 2 meters deep. Explain to the family that viewing the body is not possible. Help them to understand the reason for limiting the burial ceremony to family only. Disinfect the vehicle after transporting the Body.
_
Cremation vs. Burial vis-à-vis Ebola:
Even as Liberians fall ill and die of Ebola, many beds in treatment centers are empty because of the government’s order that the bodies of all suspected Ebola victims in the capital be cremated. Cremation violates values and cultural practices in the western African country. The order has so disturbed people that the sick are often kept at home and, if they die, are being secretly buried, increasing the risk of more infections. Burial, especially in a body bag or coffin, is just as effective at ending transmission as cremation. The danger with Ebola is in handling the corpse of a person who died recently. The world has never seen a serious, grave and complex crisis of this nature where people are dying every day with unsafe burial practices. Once it is buried, the danger is largely over, unless someone digs it up quickly. The virus attacks living cells and does not go on reproducing indefinitely. Once a body is buried, bacteria, which are able to digest dead flesh, quickly overwhelm the corpse.
_______
Ebola vaccine:
Many Ebola vaccine candidates had been developed in the decade prior to 2014, but as of October 2014, none had yet been approved by the United States Food and Drug Administration (FDA) for clinical use in humans. Several promising vaccine candidates have been shown to protect nonhuman primates (usually macaques) against lethal infection. These include replication-deficient adenovirus vectors, replication-competent vesicular stomatitis (VSV) and human parainfluenza (HPIV-3) vectors, and virus-like particle preparations. Conventional trials to study efficacy by exposure of humans to the pathogen after immunization are obviously not feasible in this case. For such situations, the FDA has established the “animal rule” allowing licensure to be approved on the basis of animal model studies that replicate human disease, combined with evidence of safety and a potentially potent immune response (antibodies in the blood) from humans given the vaccine. At the 8th Vaccine and ISV Conference in Philadelphia on 27−28 October 2014, Novavax Inc. reported the development in a “few weeks” of a glycoprotein (GP) nanoparticle Ebola virus (EBOV GP) vaccine using their proprietary recombinant technology. A recombinant protein is a protein that whose code is carried by recombinant DNA. The vaccine is based on the newly published genetic sequence of the 2014 Guinea Ebola strain that is responsible for the current Ebola disease epidemic in West Africa. In “preclinical models”, a useful immune response was induced, and was found to be enhanced ten to a hundred-fold by the company’s “Matrix-M” immunologic adjuvant. A study of the response of non-human primate to the vaccine had been initiated. Attractive features of such a vaccine could be no need for frozen storage, and the possibility of rapid scaling to manufacture of large dose quantities. The Health Ministry of Russia also claims to have developed a vaccine called Triazoverin, which is said to be effective against both Ebola and Marburg filoviruses, and might be available for clinical trials in West Africa as soon as the start of 2015. Phase I clinical trials involve the administration of the vaccine to healthy human subjects to evaluate the immune response, identify any side effects and determine the appropriate dosage.
_
_
Two experimental vaccines against Ebola are currently being tested to see whether they are safe to use in people, and health officials have said that millions of doses could be available by the end of next year.
But how do the vaccines work?
Both vaccines essentially consist of a harmless virus that has been “spiked” with a protein from the Ebola virus. If a person is given the vaccine, the body thinks it’s being infected with this rather innocuous virus, [and] part of the virus happens to be the Ebola protein. This prompts an immune response, and the body develops antibodies against the Ebola protein. Ideally, if a vaccinated person were later exposed to the real Ebola virus, these antibodies would be ready to fight off the infection before it could take hold.
1. First vaccine, which began safety testing this summer, is being developed by the National Institute of Allergy and Infectious Diseases (NIAID) and GlaxoSmithKline. It consists of a type of cold virus called an adenovirus that affects chimpanzees and has genetic material from two strains of Ebola: Zaire Ebola (which is causing the current outbreak in West Africa) and Sudan Ebola, according to NIAID. The engineered adenovirus can’t replicate in the human body. It’s used to deliver the Ebola gene to a person’s cells, which, in turn, produce a single Ebola protein. If the vaccine works as it should, this protein will cause an immune response. But in any case, it cannot cause Ebola virus disease, according to the NIAID. In September 2014, two Phase I clinical trials began for the vaccine cAd3-EBO Z, which is based on an attenuated version of a chimpanzee adenovirus (cAd3) that has been genetically altered so that it is unable to replicate in humans. For the trial designated VRC 20, 20 volunteers were recruited by the NIAID in Bethesda, Maryland, while three dose-specific groups of 20 volunteers each were recruited for trial EBL01 by University of Oxford, U.K.
_
The first test of an Ebola vaccine in people shows it’s safe and appears to be working as designed. A look at the first 20 people injected with the vaccine, which has been shown to protect monkeys from Ebola, shows no dangerous side effects. And it seems to be producing an immune response that would be expected to protect them from infection. This response is very comparable to the level of the response that actually protected the animals. There’s no ethical way to vaccinate people and then expose them to Ebola on purpose, of course, so the trial is designed to see if the vaccine is safe and if the immune system responds in a way that would be expected to protect them. It did, the NIAID researchers report in the New England Journal of Medicine. They especially looked at immune cells called CD8 T-cells. From previous studies in non-human primates it is known that CD8 T-cells played a crucial role in protecting animals that had been vaccinated with this NIAID/GSK vaccine and then exposed to otherwise lethal amounts of Ebola virus. The size and quality of the CD8 T-cell response researchers saw in this trial are similar to that observed in non-human primates vaccinated with the candidate vaccine. The real test will come if and when the vaccine is used to protect doctors, nurses and other health care workers who treat actual Ebola patients.
_
2. The second vaccine, called VSV-ZEBOV, consists of a virus that mainly infects animals (including rodents, cattle, swine and horses), called the vesicular stomatitis virus (VSV). In the vaccine, one gene of VSV has been replaced with the gene for the outer protein of the Zaire Ebola virus, according to the National Institutes of Health. Safety testing of the VSV-ZEBOV vaccine began recently at the NIH. The study involves 39 healthy adults who will be given either a low dose of the vaccine, a higher dose of the vaccine or a placebo. VSV-ZEBOV was developed by the Public Health Agency of Canada and was licensed to the biopharmaceutical company NewLink Genetics Corp. On 20 October, the Public Health Agency of Canada began air shipment of 800 doses of the VSV-EBOV vaccine to the WHO in Geneva. The WHO has recruited 250 volunteers ready to begin Phase I clinical trials in four locations: Switzerland, Germany, Gabon and Kenya. If the results of this and following trials show that the earlier results in nonhuman primates are replicable in humans, this vaccine could be deployed in areas such as West Africa and would be expected to require only a single dose. Also, its efficacy in protecting nonhuman primates when administered even after viral exposure has occurred may help protect health-care workers after a suspected exposure. The second round of testing is known as Phase 2 trials, which will further test vaccines’ safety, and also look at its effectiveness. If the vaccines are effective, pharmaceutical companies could manufacture several hundred thousand doses in the first half of 2015, and millions of doses by the end of that year, WHO said. The pharmaceutical multinational Merck announced that it will pay $50 million for commercial rights to manufacture and develop the vaccine, invented at the federal government’s National Microbiological Laboratory in Winnipeg. Europe and America have belatedly come to realize that Ebola is not just an African disease. What is odd, however, is that the money goes not to the Canadian publicly owned entity that developed the vaccine but to a small U.S. middleman that appears to have done little.
_
Experimental Ebola vaccine protects monkeys for 10 months: a 2014 study:
An experimental Ebola vaccine similar to one being developed by GlaxoSmithKline is effective for at least five weeks in lab monkeys but requires boosting with an additional vaccine to extend its protection to 10 months, according to a study published. The findings offer an early hint of which, if any, of the Ebola vaccines in development will prove effective, and in what form. Johnson & Johnson and NewLink Genetics are also among the firms accelerating their efforts to provide Ebola vaccines and treatments as the worst known outbreak of the virus ravages West Africa, killing more than 6,000 people. The results of the new study suggest, for instance, that a GSK vaccine now being tested on healthy volunteers will protect against Ebola infection in the short term, but may have to be augmented for long-term protection. The study, published in Nature Medicine, is the first to report that a vaccine regimen produced “durable immunity” against Ebola, protecting four out of four monkeys for 10 months. The vaccine uses a chimp adenovirus, closely related to a human version that causes upper respiratory tract infections, into which scientists spliced an Ebola gene. The adenovirus infects cells in a vaccinated animal, causing them to take up the gene and produce Ebola proteins. That primes the immune system to attack the proteins of Ebola viruses when an infection occurs. The vaccine in the study is similar to competing vaccines being developed by GSK, which began human safety trials recently, and by J&J, which aims to start safety trials in early 2015. A third experimental Ebola vaccine uses a different delivery system, a livestock pathogen called vesicular stomatitis virus (VSV). A version developed by the Public Health Agency of Canada and licensed to NewLink Genetics is scheduled to be tested for safety in healthy volunteers this fall. Profectus BioSciences is also developing a VSV vaccine.
_
Nasal spray vaccine has potential for long-lasting protection from Ebola virus: a 2014 study:
A nasal vaccine in development by researchers at The University of Texas at Austin has been shown to provide long-term protection for non-human primates against the deadly Ebola virus. Results from a small pre-clinical study represent the only proof to date that a single dose of a non-injectable vaccine platform for Ebola is long-lasting, which could have significant global implications in controlling future outbreaks. This work is being presented at the 2014 American Association of Pharmaceutical Scientists (AAPS) Annual Meeting and Exposition, the world’s largest pharmaceutical sciences meeting, in San Diego. Maria Croyle, a professor in the College of Pharmacy at The University of Texas at Austin, Kristina Jonsson-Schmunk, a graduate student in pharmacy, and colleagues at the university developed a nasal formulation that improved survival of immunized non-human primates from 67 percent (2 out of 3) to 100 percent (3 out of 3) after challenge with 1,000 plaque forming units of Ebola Zaire 150 days after immunization. This is important since only 50 percent of the primates given the vaccine by the standard route (intramuscular injection) survived challenge. The main advantage of this vaccine platform over the others in clinical testing is the long-lasting protection after a single intranasal dose. This is important since the longevity of other vaccines for Ebola that are currently being evaluated is not fully understood. Moreover, the nasal spray immunization method is more attractive than a needle vaccine given the costs associated with syringe distribution and safety.
________
Ebola bioterrorism:
Ebolavirus is classified as a biosafety level 4 agent, as well as a Category A bioterrorism agent by the Centers for Disease Control and Prevention. It has the potential to be weaponized for use in biological warfare, and was investigated by Biopreparat for such use, but might be difficult to prepare as a weapon of mass destruction because the virus becomes ineffective quickly in open air. Fake emails pretending to be Ebola information from the WHO or the Mexican Government have in 2014 been misused to spread computer malware. In the case of a bioterror attack employing Ebola virus, patients with no possible exposure to an Ebola patient would develop the same disease and would be seen in doctors’ offices or hospital emergency rooms. The appearance of multiple patients with a similar, rapidly progressive illness would be especially suggestive of bioterrorism. Any clinician suspecting that such an event is unfolding should report it promptly to local and state health authorities. These high-priority bioterrorism agents are defined by their ability to be easily disseminated or transmitted, their high mortality rates or capacity to generate major public health impacts, their potential for causing mass panic and social disruption, and the requirement for government action to ensure public preparedness. Moreover, there is a paucity of FDA-approved therapeutic options for the bacterial agents and no approved therapeutics for the viral pathogens. The threat of these biological agents is exacerbated by the incessant risk that these agents could become resistant to current therapeutic agents by conventional as well as genetic means. In addition, there is no effective way to address the threats of emerging, engineered, or advanced pathogens in a timely manner, as the current drug discovery and development paradigm takes up to 20 years for introduction of a new, approved drug into the market. Thus, the current de novo drug discovery and development paradigm is ineffective for dealing with biological threat agents.
_
______
Economic impact of ebola:
The economic impact of the Ebola epidemic could reach $32.6 billion by the end of next year if the disease ravaging Guinea, Liberia and Sierra Leone spreads to neighboring countries in West Africa. The World Bank’s assessment said the economic impact of Ebola is already serious in the three countries and could be catastrophic if it becomes a more regional health crisis. However, if the spread of the disease is contained within about the next six months, the economic impact would be limited to about $3.8 billion in the region, the World Bank estimated. Analysts at Barclays are warning that the continued spread of the deadly Ebola virus beyond the confines of West Africa could have a “significant” impact on the financial markets. If consumers and businesses retrench by reducing flights on airplanes, changing vacation plans, or altering business connections in a globally interdependent world, G.D.P. growth rates will fall farther. The price of cocoa beans spiked more than 10% last month due to fears that Ebola could spread to the Ivory Coast, the world’s largest producer of chocolate’s main ingredient. Ivory Coast shares a border with Guinea and Liberia, two of the three countries (the third being Sierra Leone) that are most affected by the virus. Not only is this West African region ground zero for Ebola, but it’s also home of 70% of the world’s cocoa supply. Airline stocks are already down about 7% due to fears of a global health crisis. Meanwhile, the World Travel and Tourism Council that represents airlines, hotels, and other travel companies is reporting a 30% plunge in early bookings to Africa, where the disease is deeply entrenched. Nobody has yet to calculate the fallout of the Ebola virus on the health care system. But what’s clear is that the money being poured into the fight against the disease (training, testing, treatment, waste disposal) — not to mention the money lost as hospital beds sit unused in isolation areas — will certainly affect the industry.
_
The virus is claiming new victims—African tourism and football:
Safari tents remain zipped, hotel pools are empty, game guides idle among lions and elephants. Tour operators across Africa are reporting the biggest drop in business in living memory. A specialist travel agency, SafariBookings.com, says a survey of 500 operators in September 2014 showed a fall in bookings of between 20% and 70%. Since then the trend has accelerated, especially in Botswana, Kenya, South Africa and Tanzania. Several American and European agents have stopped offering African tours for the time being. The reason is the outbreak of the Ebola virus in West Africa, which has killed more than 6,000 people. The epidemic is taking place far from the big safari destinations in eastern and southern Africa—as far or farther than the homes of many European tourists. There are more air links from West Africa to Europe than to the rest of the continent, whose airlines have in any case largely suspended flights. Moreover Ebola is hardly the biggest killer disease in Africa (AIDS and malaria are bigger). Yet, in the mind of many visitors, all of Africa is a single country. One despairing tour operator calls it an “epidemic of ignorance”. Directly and indirectly, tourism accounts for almost 10% of sub-Saharan Africa’s GDP and pays the salaries of millions of people. The industry is worth about $170 billion a year. In 2013 more than 36 million people visited Africa, a figure that had been growing by 6% per year. Now many safari lodges are closer to extinction than the animals that surround them. Redundant workers might eventually turn to poaching. Fear of Ebola is growing among Africans, too. Morocco said it would not host the African Cup of Nations, the premier football event on the continent, due to start on January 17th 2015. Morocco had sought a year-long postponement, citing the danger of the virus spreading at large gatherings. Miffed, the Confederation of African Football barred Morocco, which has not had a single Ebola case, from the tournament. The three worst-affected countries—Liberia, Sierra Leone and Guinea—have not, or not yet qualified. Organisers are scrambling to find an alternative host. African football may be the next victim of Ebola.
_________
Myths of ebola:
Myth: You will not get Ebola if you have a healthy immune system.
Fact: The state of your immune system does not dictate if you can or cannot get infected. A healthy immune system is definitely very important to prevent diseases, but the Ebola virus has become so virulent that it can infect people with even the most robust immune system. Since the virus interferes with and avoids immune system and also uses person’s immune response to spread, the health of one’s immune system does not stop this virus from infecting a person. Nonetheless, early and effective immune response is most important factor of ebola survival. Therefore people with immunodeficiency due to any cause are unlikely to survive ebola but more studies are required to confirm this hypothesis. There is no study that compares ebola fatality between normal people and HIV infected people.
_
Myth: A patient presenting with symptoms of Ebola and travel to Liberia within the past 21 days can be safely removed from isolation after a negative serum test.
Fact: An initially negative reverse transcription polymerase chain reaction (RT-PCR) test result for Ebola virus does not rule out Ebola virus infection. If an initial test is negative in a person under investigation for Ebola, repeat testing is indicated in 72 hours. It takes about three days once a person starts showing symptoms for the Ebola virus to accurately show up on tests. In other words, it is possible for an Ebola test to be negative when the person actually does have Ebola. This is critical, as many of those admitting themselves to hospitals with possible Ebola symptoms are doing so the day they start exhibiting them, or possibly the day after at the latest. Unless medical personnel across the world fully understand this, many Ebola patients will be discharged with false negatives, only to spread the disease to their friends and family.
_
Myth: Ebola is not transmissible before a person is symptomatic, so there is no scientific basis for quarantine of contacts.
Fact: Quarantine is not a strategy to prevent spread of diseases that may be transmissible prior to the onset of symptoms. Quarantine separates and restricts the movement of people who were exposed to a contagious disease to see if they become sick. If they indeed become sick, isolate them, treat them and trace all their contacts. This is especially true of ebola that carries 50 to 70 % mortality with the best available treatment. Contact tracing is most important for ebola as even one missed contact can keep the outbreak going. Also contact tracing finds new cases quickly so that they can be isolated to prevent spread of disease. But there is a downside to overdoing it. Quarantine may have had limited effectiveness when widely used in Canada and Asia in a 2003 SARS outbreak. With several hundred thousand individuals placed in quarantine and relatively few of them later developing SARS, local public health officials later acknowledged that the response was disproportionate to the threat. Thus, the paradox of quarantine and other social distancing measures is that they may be effective in fighting a disease outbreak, but they can be applied too broadly, resulting in a variety of social harms, including economic disruption, personal isolation, and even violence. There are silent ebola infections where quarantine logic fails but they are not contagious anyway. Of course the issue is how you define quarantine. The word quarantine comes from the Italian quaranti giorni, meaning ’40 days’. When bubonic plague swept through Europe in the 14th century, the government of Venice required ships to anchor away from the city for 40 days before they could unload passengers or cargo. The authorities thought 40 days would be enough time for any disease to be identified and either treated or pass through its normal course. All ships under quarantine had to fly a yellow flag.
_
Myth: Travel bans would keep Ebola from spreading.
Fact: In the past, some nations have banned flights from other countries in hopes of blocking the entry of viruses, including SARS and H1N1 (swine flu). None of the bans were effective, and the viruses spread regardless of government measures took to keep them out. The key to stopping Ebola worldwide is stopping it in Africa. Unfortunately, restricting the movement of people through travel bans isn’t likely to stop Ebola spread. In fact, it could have the opposite of the desire effect. To completely seal off and don’t let planes in or out of the West African countries involved, then you could paradoxically make things much worse in the sense that you can’t get supplies in, you can’t get help in, you can’t get the kinds of things in there that we need to contain the epidemic. For aid workers, it is already difficult to go to affected countries. Stigma makes it even harder for those who want to help. Without human resources, we cannot run our operations; and if we cannot run our operations, we cannot stop Ebola. Travel ban might actually make things worse, because it could encourage people to lie about their travel to West Africa. And without that crucial information, people infected with Ebola could slip into other countries without the health authorities being able to track and monitor them for symptoms and it is even harder to track them systematically.
_
Myth: Ebola is a highly contagious disease.
Fact: Compared with other diseases such as tuberculosis, smallpox, measles, chicken pox, influenza, SARS and polio, Ebola is not particularly contagious. Infection requires a lot of contact with the virus, such as coming in contact with bodily fluids (blood, vomit, saliva, feces, urine, sweat, etc.) Ebola is not easily spread; for example, it does not spread by casual contact — no household contacts of the first Ebola patient in the U.S. Liberian Thomas Eric Duncan contracted the disease. It is only when the patient is critically ill with a high viral load in body fluids that he becomes contagious as ebola is spread via contact with blood and other bodily fluids. That is why nurse caring for Duncan got infected. This is the rationale for the high degree of precaution, including monitored donning and doffing of personal protective equipment (PPE), when caring for such a patient. There is no evidence for airborne transmission of Ebola. While some experts have suggested that Ebola could mutate to become airborne, scientific consensus is that this would be extremely unlikely. Also, ebola is a fragile virus outside host and killed easily by common household disinfectants.
_
Myth: A patient presenting with fever and travel to West Africa within the past 21 days is Ebola.
Fact: In travelers from sub-Saharan Africa, diseases with short incubation periods, such as malaria and typhoid fever, also present with fever and must be considered in the differential diagnosis of Ebola. Malaria is much more common than Ebola. However, a positive malaria test does not rule out Ebola, as malaria is extremely prevalent in this population and the diseases could coexist.
_
Myth: Cardiopulmonary resuscitation is indicated for Ebola patients in cardiac arrest.
Fact: Prevailing expert opinion is that if a patient has loss of cardiac output due to multisystem organ failure from septic shock in the setting of Ebola, resuscitative efforts would be futile and also extremely risky for the clinicians performing the procedures. Some Ebola centers have requested that patients sign a do-not-resuscitate (DNR) order. Self-sacrifice by health care professionals that results in no offsetting clinical benefits for the patient is not required by the professional virtue of self-sacrifice. However, health workers might apply the argument even to people who show no signs of Ebola but might have come in contact with an Ebola patient. The efficacy of other invasive procedures such as intubation and dialysis are still being debated, with anecdotal reports arising in the Western world of good outcomes after their application.
_
Myth: Silent ebola infection means ebola survivor.
Fact: Silent ebola infection means person infected with ebola virus but did not develop ebola virus disease and therefore person is asymptomatic and non-contagious. Ebola survivor is a person who suffered ebola virus disease with all symptoms & signs, and was contagious during illness but managed to survive by eliminating ebola infection. Ebola survivor may harbor ebola virus in eye, gonads and breast for few months even after eliminating virus in blood.
__________
My novel theory of deadliness of infectious diseases:
Deadliness of any infectious disease = infectivity X transmissibility X mortality X susceptible population
In other words, the deadliness of any infectious disease is directly proportional to infectivity (infectiousness), transmissibility (contagiousness), mortality (case fatality rate with treatment) and magnitude of susceptible population. Infectivity means infectious dose in number of organism required for infecting host (N) and transmissibility is basic reproduction number (R0). Lesser the number of organisms (N) required for infection, greater is infectivity.
_
_
Let us compare Deadly Index of Influenza A and Ebola:
Influenza A (common flu) has CFR of 0.05 %
It has R0 = 3
Infectious dose of approximately 790 organisms via the nasopharyngeal route
Deadly Index = 0.00018987 X susceptible population
_
Zaire Ebola of 2014 has calculated CFR 54 % (WHO estimated 70 %)
It has R0 = 2
Infectious dose of 10 organisms by contact transmission
Deadly Index = 10.8 X susceptible population
_
If susceptible population for influenza A and Ebola is same, Ebola is 57,000 times (10.8/0.00018987) deadlier than influenza A. The only variable in our hand is susceptible population. So to reduce mortality, we have to reduce susceptible population by reducing transmission (isolation, sanitation, barrier nursing, and use of PPE) including quarantine all contacts, and vaccinating the world. Today we have no vaccine, so reducing transmission is the only option. Quarantine of all ebola contact for 21 days is mandatory. Airport exit screening in Ebola infected West African nations along with airport entry screening at all international airports worldwide is mandatory. In order to stop the epidemic, the rate of transmission would have to be cut in half. In order to reduce Ebola deadliness to make it equivalent to influenza deadliness, the ebola transmission rate ought to be reduced by 1/57,000 times of the present rate which is impossible. Of course, giving better treatment would also reduce Ebola deadliness but today we have no effective treatment and experimental ebola therapies like ZMapp, BCX4430 and TKM-Ebola are very expensive and beyond reach of ordinary citizen of third world.
_________
_________
The moral of the story:
_
1. Ebola virus disease (EVD) or simply Ebola, previously known as Ebola hemorrhagic fever (EHF) is an acute, rare and severe infection caused by one of the ebola virus strains being transmitted from animal to human and subsequently human to human. Unlike HIV, ebola virus does not persist in infected person, either you die or survive without virus.
_
2. Migratory fruit bats are natural reservoir of ebola viruses. Bats carry silent ebola infections and transmit ebola to other animals and humans. Bats supported replication and circulation of high titers of virus without becoming ill. A perfect parasite is able to replicate and not kill its host which is an evolutionary advantage to remain endemic in its host species population. The ebola virus is the perfect parasite for a bat. Humans, apes and other mammals are incidental/accidental hosts. Human infection is a case of mistaken location, a zoonotic jump from wild animal to human being and human lethality in not in favor of survival of ebola virus species.
_
3. Phylogenetic and sequencing evidence suggests that ebola viruses are ancient (at least 23 million years old) and ebola virus-like elements are integrated into genome of bats, rodents, shrews, tenrecs and marsupials. No wonder bats are natural reservoir of ebola virus.
_
4. Mutations are a way of life for an RNA virus. Scientists have found the origins of the current Ebola 2014 outbreak by tracking its mutations and the current viral strains come from a related strain that left Central Africa within the past ten years. The Ebola-2014 virus’s mutation rate of 2.0 x 10−³ subs/site/year is nearly identical to Influenza A’s mutation rate of 1.8 x 10−³ subs/site/year. This means Ebola-2014 is mutating as fast as seasonal flu. The Influenza’s high mutation rate allows the virus to generate ‘escape mutants’ which are no longer recognized by human immune systems and therefore we need influenza vaccine every year to immunize us against mutant virus. The high mutation rate of Ebola-2014 may confer survival fitness and more adaptability to human host at both the intra-host and inter-host levels. Ebola acquires genetic diversity as it infects more people. The growing number of new ebola viral lineages will undergo natural selection for some ‘optimum’ balance of virulence, infectivity, tissue tropism, immune suppression, and other parameters which maximize the reproductive fitness of the ebola virus in humans. Longer the 2014 ebola outbreak lasts, greater the reproductive fitness of ebola and lesser the efficacy of treatment/vaccine. Ebola strain currently in circulation appears to be far more virulent and contagious than previous strains. Viral loads observed in 2014 outbreak exceed what has been observed during previous outbreaks. More of the virus is infecting patients, and it appears to be advancing and spreading more rapidly than usual. With increasing numbers of humans being infected, Ebola virus variants (due to mutations) could be selected which are better adapted for human-to-human transmission. The repeated passage of Ebola-2014 through humans is exerting selection pressure on the Ebola-2014 virus to adapt to our species (instead of fruit bats) which may allow the Ebola-2014 virus to become more transmissible and less virulent as the months go on to make ebola endemic, particularly in the absence of effective control interventions.
_
5. Ebola outbreak of 2014 is more than six times the sum total of all previous outbreaks combined. One disturbing feature of the current epidemic is that so many health workers have lost their lives while caring for the sick or trying to spread public-health messages about Ebola. Ebola epidemic of 2014 is unlikely to reach the numbers of HIV pandemic because Ebola outbreaks tend to “burn themselves out” as Ebola virus disease is rapidly fatal.
_
6. The reported cases of ebola in 2014 outbreak are tip of the iceberg. There is widespread under-reporting of new cases in Africa and actual cases are 2.5 times higher and are roughly doubling every month. There are numerous reports of symptomatic persons evading diagnosis and treatment, of laboratory diagnoses that have not been included in national databases, and of persons with suspected Ebola virus disease who were buried without a diagnosis having been made.
_
7. Ebola epidemic 2014 has served as a reminder of just how slow and poorly coordinated the responses to outbreaks are. It took three months for health officials to identify Ebola as the cause of the epidemic, another five months to declare a public health emergency, and two more months to mount a humanitarian response. And the best way to stop an outbreak is to contain it early. Ebola has reached the point where it could establish itself as an endemic infection because of a highly inadequate and late response.
_
8. Ebola is a disease of poverty, weak health care system, nonexistent hospital infection control, poor personal hygiene, illiteracy, fear, ignorance, superstition, and cultural practice of touching dead body. Guinea, Sierra Leone, and Liberia have all these to account for 2014 ebola outbreak but even densely populated India has all these factors. I am afraid if Ebola spreads in India, it would be killing million people.
_
9. Ebola epidemic of 2014 in West Africa caused collapse of health care system, economic slowdown and widespread food insecurity in affected countries. Ebola has also taken the lives of health care workers in places where the ratio of doctors & nurses per population is abysmally low. All these caused even more deaths due to malaria, tuberculosis, HIV, diarrhea and chest infections.
_
10. The key to stopping Ebola worldwide is stopping it in Africa. Tracking the movements and contacts of potential Ebola cases, and getting aid & resources to West Africa to stop Ebola at the source are the best methods of stopping ebola in Africa.
_
11. 2014 Ebola outbreak must be stopped in a way that leaves behind stronger systems to prevent, identify and stop future Ebola outbreaks.
_
12. Ebola is transmitted from infected human only during symptomatic phase and not in incubation period, and transmission is maximum in advanced stage of disease when patient is gravely ill as his body fluids would contain maximum number of viruses at that time. The viral load isn’t static. It increases as the disease progresses. That is why a person who is infected but without symptoms (incubation period) will not spread the virus initially as there is very little virus present in the blood, and it is not yet present in other bodily fluids. Ebola get progressively more contagious as the disease worsens. Ebola transmission occurs when the viral load in bodily fluids is high, on the order of millions of virions per microliter. Dying patient has highest number of virus in body fluid and so dead body has maximum number of virus in body fluids and thereby highly contagious. At least 20% of new infections occur during burials. Therefore safe burial practice of ebola victims is of paramount importance.
_
13. Ebola is spread through direct contact, through broken skin or through eyes, nose, or mouth; via blood and body fluids of a person who is sick with Ebola, or objects, such as needles, that have been contaminated with the blood or body fluids of a person sick with Ebola. Direct contact through broken skin or mucous membranes is the key. Soap, gloves, isolating patients, not reusing needles/syringes and quarantining the contacts of those who are ill should be very easy to contain Ebola but in practice, this is a much tougher proposition in developing countries.
_
14. Researchers have concluded that airport temperature checks are ineffective in detecting cases of infectious diseases including influenza or ebola. Also the risk of transmission of Ebola during air travel remains low as Ebola is not airborne unlike infections such as influenza or tuberculosis.
_
15. Ebola virus infection can be transmitted if an infected person coughs, sneezes or vomits at close range. In this case large wet droplets landing on the mucous membranes of susceptible host can initiate infection. Although these droplets are traveling through the air, this mode of transmission is considered a form of contact transmission (droplet contact transmission), not airborne transmission. Any droplets of fluid from cough or sneeze that could contain ebola can travel only about 3 feet before they fall to the ground because of gravity as there droplets are large, wet and heavy; and therefore not considered airborne. Airborne transmission means small light dried airborne particles containing ebola viruses that remain suspended in air for several hours even after ebola patient has left the room and that travel hundreds of meter by wind and can transmit infection to another human. Ebola is an ‘enveloped’ virus and its lipid membrane is vulnerable to light, heat and dryness. Such virus is highly unlikely to survive airborne. No human virus has ever changed its mode of transmission. Ebola virus is RNA virus that constantly mutate while replicating but jump from contact transmission to airborne transmission is highly improbable though not impossible. However any action which can be taken to “reduce risk” of Ebola exposure should not wait until a “scientific certainty” develops especially for health care workers. Today there is no scientific certainty that ebola is airborne or not, and therefore better safe than sorry. All ebola healthcare workers right from doctors, nurses and laboratory technicians must wear personal protective equipment (PPE) equipped to protect against airborne transmission while treating ebola patients in hospital. If you are not wearing PPE, then you should stand at least six feet away from an Ebola patient, as a precaution against flying wet droplets.
_
16. Even though infectiousness is synonymous with contagiousness, I would like to differentiate between infectiousness (infectivity) and contagiousness. Infectiousness (infectivity) is a measure of the ability of a microorganism to establish itself in the host and refers to the individual dose or numbers of the microorganisms required to infect susceptible host. Contagiousness means ease of transmission from one person to another by any means. The ebola virus is extremely infectious (infective). Experiments suggest that even if one particle of Ebola enters a person’s bloodstream it can cause a fatal infection (e.g. accidental needle prick). Even 3 ebola particles can transmit infection by direct contact (e.g. splash of vomitus). This may explain why many of the medical workers who came down with Ebola couldn’t remember making any mistakes that might have exposed them. But it is harder to contract Ebola. One ebola patient can infect up to 2 people every 9 to 15 days while one chicken pox patient can infect up to 17 people every 14 to 16 days. One cannot overlook the fact that lesser the number of microorganisms required for acquiring infection, greater the ease of transmission. Despite this fact, ebola spreads slowly means had infectious dose been thousands of viruses, ebola would not be contagious at all. Even though ebola spreads slowly and to relatively few people, it kills more than other diseases. The World Health Organization said 70 percent of cases in 2014 outbreak are fatal. Virulence is a measure of the disease producing ability of microorganisms. Higher the virulence of microorganism, greater the morbidity and mortality of susceptible host. Faster the replication rate of pathogenic microorganism, greater the depletion of host resources and lesser the host fitness, resulting in higher virulence and higher host mortality. However, mere higher replication rate cannot be correlated with severity of disease as equally important is significance of target organ. Ebola virus specifically targets immune cells, endothelial cells and hepatocytes. Ebola has high mortality not only because of faster replication rates but also because of targeting vital systems of body. In a nutshell, ebola is highly virulent, highly infectious (infective) but averagely contagious disease. Compared with other diseases such as tuberculosis, smallpox, measles, chicken pox, influenza, SARS and polio, Ebola is not particularly contagious.
_
17. Although high transmission rate will typically go along with a high virulence (high reproduction/replication rate) because rapid replication can increase a pathogens chance of transference as higher the number of viruses in body fluids, greater is chance of transmission; ebola is not highly transmissible despite highly virulent and despite highly infectious; because it is not airborne, because it is a fragile virus outside host and because it is transmitted through body fluids of a severely ill patient late in the disease. The severely ill patients late in disease carry maximum viruses in body fluids but they are bedridden and therefore confine viruses to a place where they lie. In order to reduce virulence of ebola to reduce mortality, ebola transmission rate must be further reduced by isolation, sanitation and barrier nursing of ebola patient because reduced transmission would result in selection of lower virulent ebola strains over higher virulent ebola strains. Lower virulent strain would cause a milder disease and killing maybe twenty per cent of its victims instead of seventy per cent. This could leave more of them sick rather than dead, and perhaps sick for longer. That might be good for Ebola, since the host would live longer and could start even more chains of infection. Even if less virulent strains are not present, prevention of transmission is likely to slow and eventually stop the outbreak as the number of remaining susceptible hosts is reduced through various means. In order to stop the epidemic, the rate of transmission would have to be cut in half. This would be equivalent to vaccinating 50% of the susceptible population.
_
18. We the humans are in Catch-22 situation. Higher the Ebola mortality, more human lives are lost but outbreak burns itself out. Lesser the human mortality, virus would become endemic and persist at low level all the time.
_
19. The overwhelming majority of people who have been infected with Ebola are people who have directly cared for a person who is actively sick with the disease or have handled the body of someone who have died from it. Ebola is a caregiver’s disease and children don’t typically get it even though they are running around touching many objects and surfaces because they are not taking care of sick people as a rule. Studies conducted during the various epidemics have shown that less than one fifth of the people living with a confirmed or probable primary patient have developed the disease. All secondary cases were recorded among people with close contact with the patient and exposed to infected biological fluids. The World Health Organization says 588 health care workers have been infected with Ebola and 337 have died of it. Conversely, people who had no contact with the patient were not sick.
_
20. Contact is a person who was/is in direct contact with ebola patient alive or dead, direct contact with sick or dead animal suffering from ebola and direct contact with specimens collected from suspected Ebola patients/animals; provided that this exposure has taken place less than 21 days before the identification as a contact by surveillance teams. Direct contact means physical contact of skin or mucous membrane (eye, mouth, and nose) of anybody with infected (alive/dead) person’s body (skin/mucous membrane) and/or body fluids (blood, saliva, mucus, tears, genital secretions, breast milk, vomitus, urine, sweat or feces) either directly and/or indirectly through fomites. Contact tracing is most important for ebola as even one missed contact can keep the outbreak going. Also contact tracing finds new cases quickly so that they can be isolated to prevent spread of disease. Contacts are watched for signs of illness for 21 days. If any of these contacts comes down with the disease, they should be isolated, tested and treated. Then the process is repeated by tracing the contacts’ contacts. The best way to prevent Ebola from spreading is to identify everyone whom infected individual has contacted either when alive or after death in the course of funeral/burial rituals. Liberian Thomas Eric Duncan died from Ebola after arriving in Dallas. Duncan said he had not been in contact with Ebola patients in his native country, although that later proved to be a false statement. Since people habitually lie, how on earth can you identify a contact anyway?
_
21. Quarantine is restricting movement of contacts for the incubation period of the contagious disease to see if they become sick. If they indeed become sick, isolate them, treat them and trace all their contacts. If not then set them free. Quarantine is not a strategy to prevent spread of diseases that may be transmissible prior to the onset of symptoms. Ebola is not transmissible in incubation period but we still do need to quarantine ebola contacts in incubation period. There are silent ebola infections where quarantine logic fails but they are not contagious anyway. Isolation refers to separating those who are having contagious disease from those who are not to prevent spread of disease. Theoretically there is no quarantine time that will provide absolute assurance of no residual risk from contagion. Practically one needs to balance out costs and benefits of quarantine and risk reduction. The paradox of quarantine and other social distancing measures is that they may be effective in fighting a disease outbreak, but they can be applied too broadly, resulting in a variety of social harms, including economic disruption, personal isolation, and even violence.
_
22. A study on 3,343 confirmed and 667 probable cases of Ebola in 2014 ebola outbreak found that 87.1% of those infected exhibited fever but 12.9% did not. So fever is not a guaranteed symptom of ebola infection. However, fever precedes the contagious stage, allowing contacts to identify themselves as infected before they become a threat to the community. This is so because the sensitive blood polymerase-chain-reaction (PCR) test for Ebola is often negative on the day when fever or other symptoms begin and only becomes reliably positive 2 to 3 days after symptom onset. Do not confuse Ebola patient without fever (afebrile Ebola) with asymptomatic Ebola (silent Ebola). Ebola patient without fever is suffering from ebola virus disease and he/she may have symptoms (vomiting, diarrhea, headache etc) except fever. Asymptomatic Ebola is not suffering from ebola virus disease, has no symptoms what so ever, but only infected with ebola virus for some time. Ebola virus disease occurs due to infection with ebola virus; but infection with ebola virus does not necessarily mean Ebola virus disease. Disease will produce some symptoms but infection without disease is silent.
_
23. The RT-PCR and antigen-capture ELISA are very effective for detecting ebola virus in patient’s serum, plasma, and whole blood during acute phase of Ebola. In samples collected very early in the course of infection, the RT-PCR assay could detect ebola virus 24 to 48 h prior to detection by antigen capture. Detection of significant IgM antibody response during the first week of illness suggests ebola survival as fatal cases do not develop antibody response.
_
24. The accuracy of diagnostic test for ebola is of paramount importance. False positives could place people without Ebola in contact with other infected patients, while false negatives could release Ebola patients back into the public. Ebola should never be diagnosed or ruled out by a single laboratory test.
_
25. Timing of diagnostic test for Ebola is of paramount importance. It takes about three days once a person starts showing symptoms for the Ebola virus to accurately show up on tests. In other words, it is possible for an Ebola test to be negative when the person actually does have Ebola. This is critical, as many of those admitting themselves to hospitals with possible Ebola symptoms are doing so the day they start exhibiting them, or possibly the day after at the latest. Unless medical personnel across the world fully understand this, many Ebola patients will be discharged with false negatives, only to spread the disease to their friends and family.
_
26. Survival in ebola virus disease is dependent on species of ebola virus (Sudan vs. Zaire), quantum of viral load, route of infection (parenteral vs. direct contact), age, genetic predisposition, mutation in a gene called NPC1, level of preparedness & availability & quality of medical care, and immune response (protective vs. suppressed). Out of all survival factors, an early and effective immune response (innate and acquired) is the most important. Ebola is so deadly because it has multiple ways of interfering with or avoiding the human immune system. Ebola not only invades and evades immune system but also uses immune cells as a vehicle for dissemination throughout human body. Survivors exhibited more significant IgM responses, clearance of viral antigen, and sustained T-cell cytokine responses, as indicated by high levels of T-cell-related mRNA in the peripheral blood. A detailed study of infected but asymptomatic individuals revealed that they had an early (4-6 days after infection) and vigorous immunologic response with production of interleukin (IL) and tumor necrosis factor (TNF), resulting in enhanced cell-mediated and humoral immunity. A study showed that the innate immune response, specifically natural killer (NK) cells, can mediate rapid and complete protection against lethal Ebola virus infection. In contrast, antibodies specific for the virus were nearly undetectable in fatal cases, and while gamma interferon (IFN-γ) was detected early after infection, T-cell cytokine RNA levels were more indicative of a failure to develop adaptive immunity in the days preceding death. Genomics analysis demonstrated that not only IFN induction but also IFN signaling was impaired in ZEBOV-infected cells as ebola viruses alter host cell gene expression programs. This significant suppression of the IFN response leads to increased immune evasion, replication, and virulence of ebola virus.
_
27. The best experimental therapy for ebola virus disease include the use of whole blood, serum or plasma from convalescent Ebola virus disease survivors, a cocktail of three monoclonal antibodies directed against the Ebola viral glycoprotein (ZMapp), a novel broad-spectrum nucleoside analogue BCX4430 which inhibit RNA polymerase and three small interfering RNAs (siRNAs) that target L protein, VP24, and VP35 genes of Ebola virus (TKM-Ebola). I don’t want to comment on ethical aspects of experimental therapy and use of drugs without randomized double blind controlled trials in predominantly fatal illnesses. I am a practicing doctor and if my patient has 70 % chance of death despite best available treatment, I would use experimental therapy with the knowledge and the consent of the patient and his/her family. Provision of supportive care, particularly fluid and electrolyte management and treatment of bacterial superinfections can significantly improve survival.
_
28. Mammary gland (breast), gonads (testes &ovaries) and chambers of the eye are immunologically protected site in which clearance of ebola virus is delayed in ebola survivors and therefore ebola survivors must avoid breast feeding, unprotected sex and contact with the mucous membranes of the eye for 3 months following recovery from ebola. Inadvertent rubbing of eyes by hand is common and therefore ebola survivor must make conscious efforts to prevent hands touching eyes. Although studies have shown Ebola patients shed genetic material from the virus into their sexual fluids, there is scant evidence that they are often shedding viable virus that could infect others. Nonetheless, extreme caution is not an overreaction with the deadly disease.
_
29. We need Ebola Vaccine for pre-exposure and post-exposure prophylaxis of health care workers, household contacts and susceptible population for following reasons:
a) High ebola mortality
b) Very few viral particles can transmit infection
c) Direct contact transmission
d) Lack of effective treatment
e) Very expensive available treatment
f) Prevent endemic ebola
The only reason we don’t have ebola vaccine today is because ebola in past affected poor African nations and it was not cost-effective for big pharmaceutical corporations to spend money on ebola vaccine development. Now ebola has entered United States and we see sea-change in the thinking of pharmaceutical industry and sea-change in the political will of the West.
_
30. The most important point overlooked by the world in recent 2014 ebola outbreak is asymptomatic (silent) ebola infections. Results from one post-ebola outbreak study showed that 71% of seropositive individuals did not have the disease; another study reported that 46% of asymptomatic close contacts of patients with Ebola were seropositive. The latter study also found minute concentrations of Ebola virus in these individuals’ blood, suggesting that their antibodies could not be explained by their exposure to dead virus, but that rather they had truly been infected by live virus. A study found that asymptomatic Ebola infection did not result from viral mutations. Asymptomatic cases are likely to have a little bit of virus for a little bit of time and then fight it off. Rough approximations tell us that as many as 50 percent of infections are silent. So for every case of severe Ebola, there may be another person who is infected without ever knowing it. That means case fatality rate (CFR) of ebola is overestimated as plenty of silent infections are not taken into account. Ebola typically spreads through contact with bodily fluids of very sick individuals, who have exceedingly high viral counts; therefore it is very unlikely that silent (asymptomatic) cases can spread the virus with the low levels found in their blood for some time. Although asymptomatic infections are unlikely to be contagious, they might confer protective immunity and thus have important epidemiological consequences. Epidemics are fueled by susceptible people. The more there are, the bigger an epidemic can become. Immunization of any kind — via vaccine or natural infection — makes people resistant and thereby slows transmission. The asymptomatic individuals can also help slow the spread of Ebola by being recruited to work as caregivers in high-risk communities. Also if infection without disease protects people from future Ebola infections and illness, the epidemic should decline sooner than currently predicted and affect a smaller number of people. Also naturally acquired immunity will amplify the effects of disease control measures, including vaccination. I want researchers to conduct studies to find out commonality between silently infected people and people who survived ebola virus disease.
_
31. According to my theory of deadliness of infectious diseases, Ebola is 57,000 times deadlier than influenza (common flu). The only variable in our hand is susceptible population. So to reduce mortality, we have to reduce susceptible population by reducing transmission (isolation, sanitation, barrier nursing, and use of PPE) including quarantine all contacts, and vaccinating the world. Of course, giving better treatment would also reduce Ebola deadliness but today we have no effective treatment and experimental ebola therapies like ZMapp, BCX4430 and TKM-Ebola are very expensive and beyond reach of ordinary citizen of third world.
_________
_________
Dr. Rajiv Desai. MD.
December 6, 2014
_________
Postscript:
Ebola is not transmitted by the air. Fear and ignorance are transmitted by the air. It is the duty of media to tell truth rather than spread fear. I would like to emphasize that isolation, sanitation and barrier nursing of Ebola patients would definitely prevent Ebola spread substantially. I strongly recommend quarantine of all Ebola contacts for 21 days till effective Ebola Vaccine is available. Intravenous fluid administration to correct dehydration and maintenance of circulatory blood volume would significantly reduce mortality. I have already proved that intravenous normal saline in adequate doses at appropriate time saves life in severe Dengue patients and the same logic can be deduced for Ebola. Ebola is my last article of 2014. I wish Merry Christmas and hope that the year 2015 shall bring peace, prosperity, security and justice throughout the world.
________





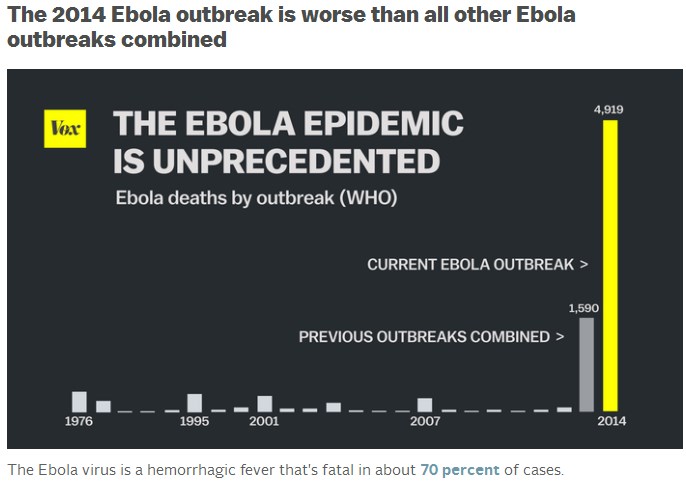
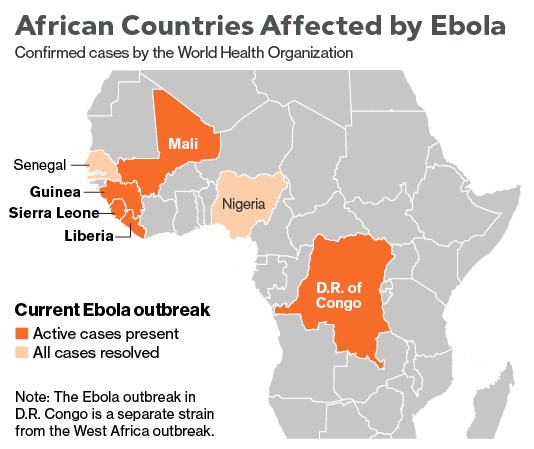
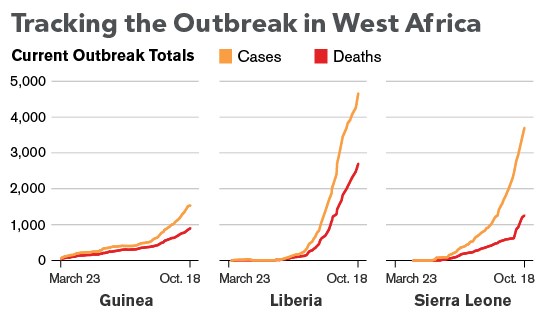
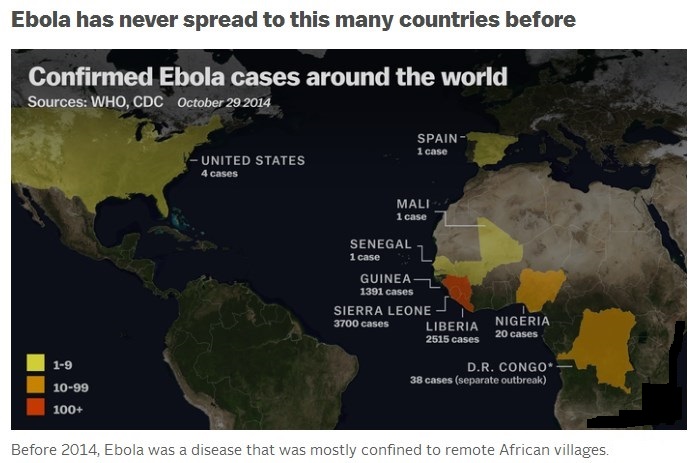
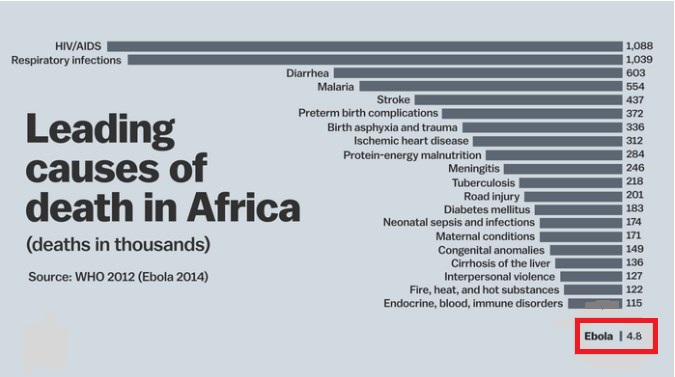
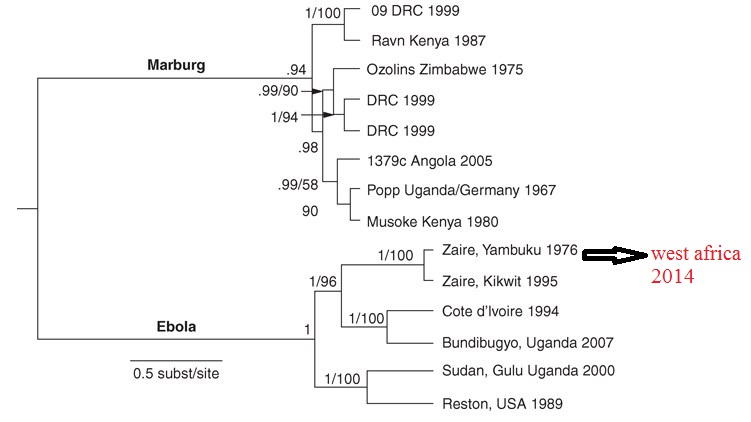
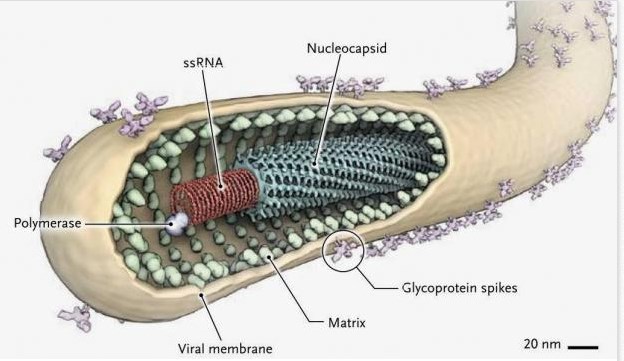
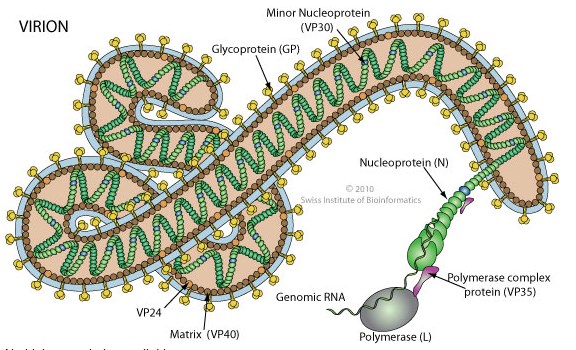
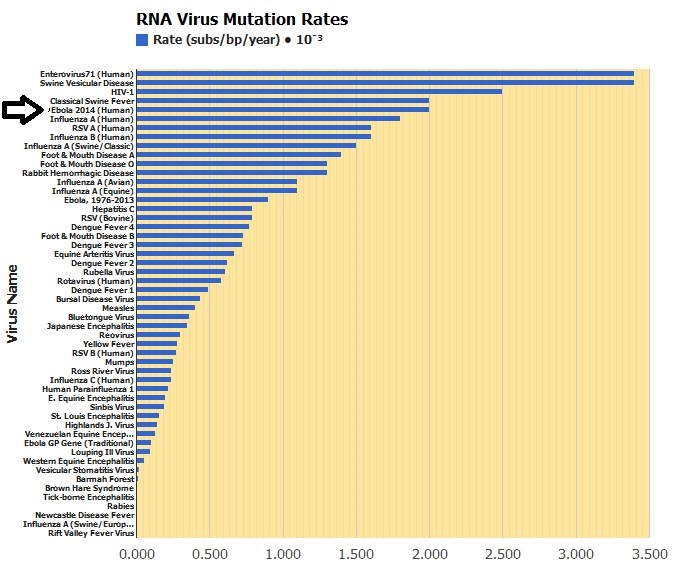
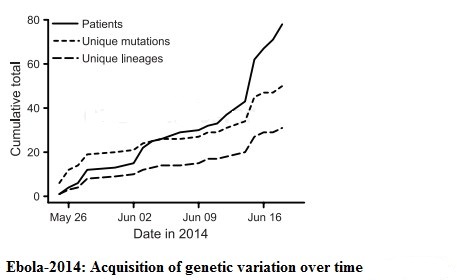
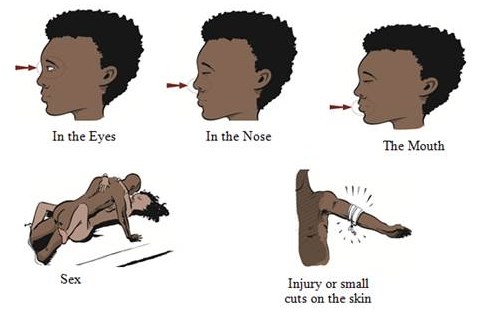
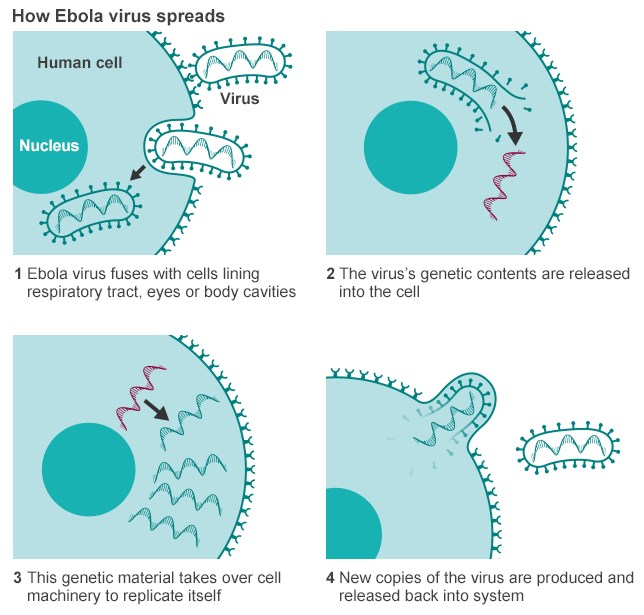
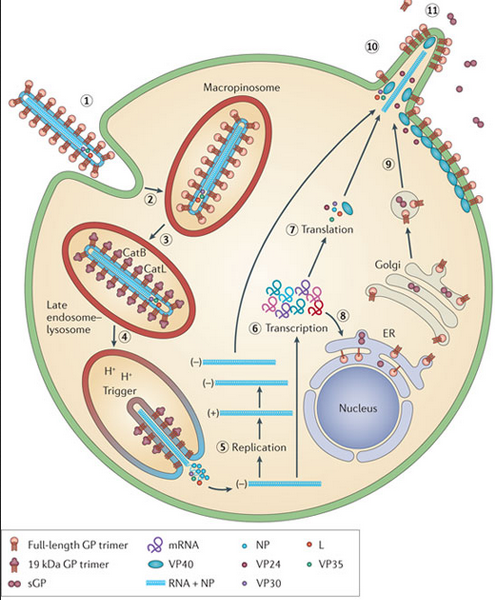
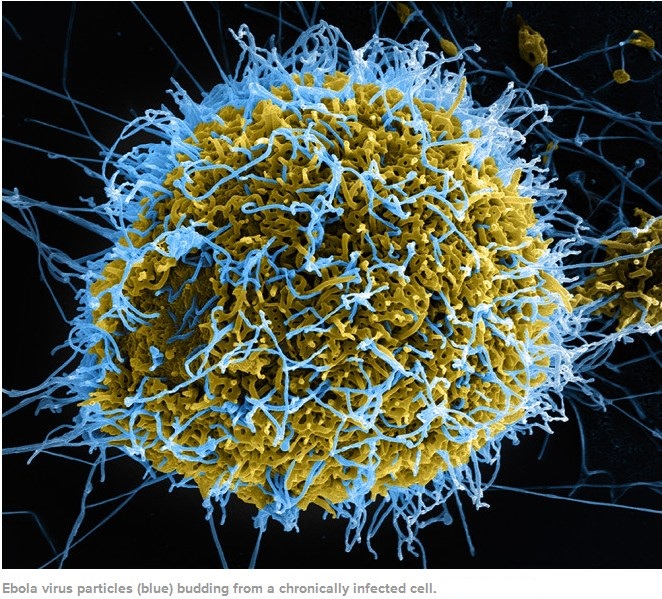
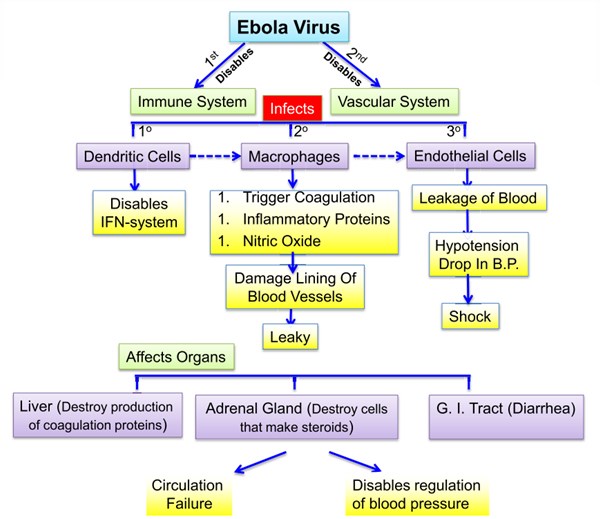
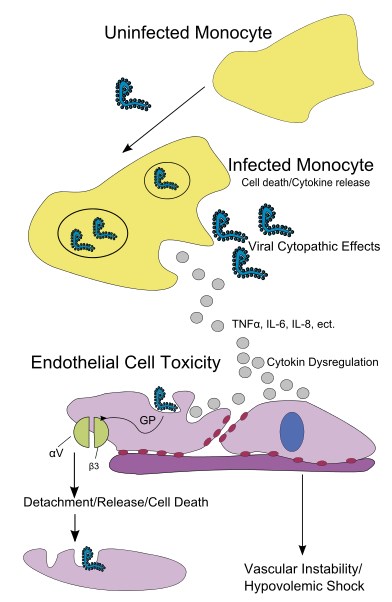
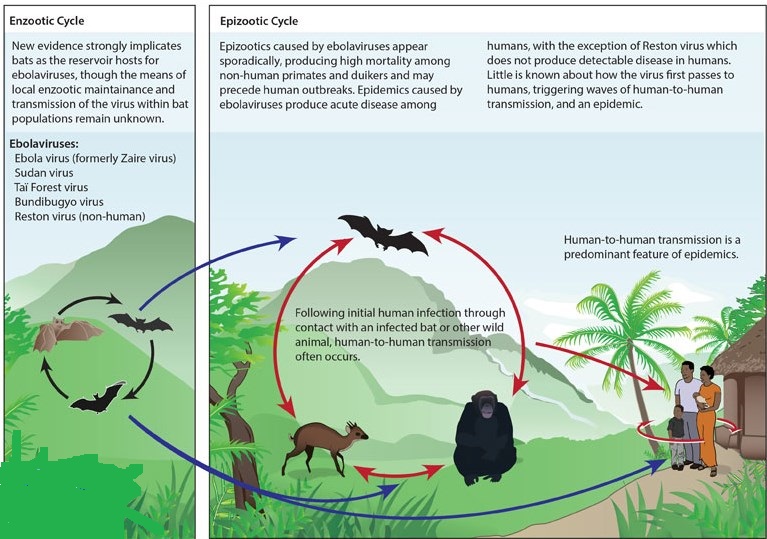

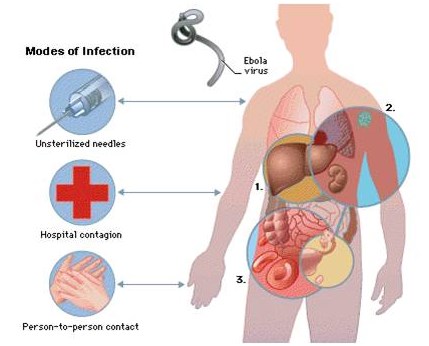
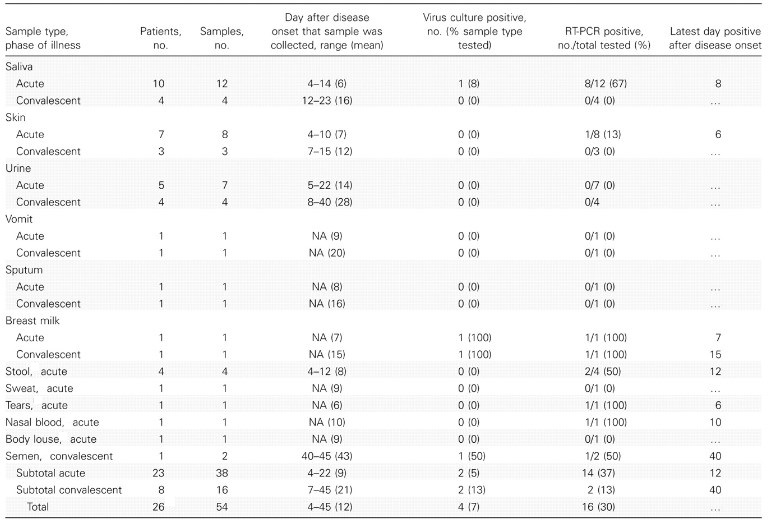
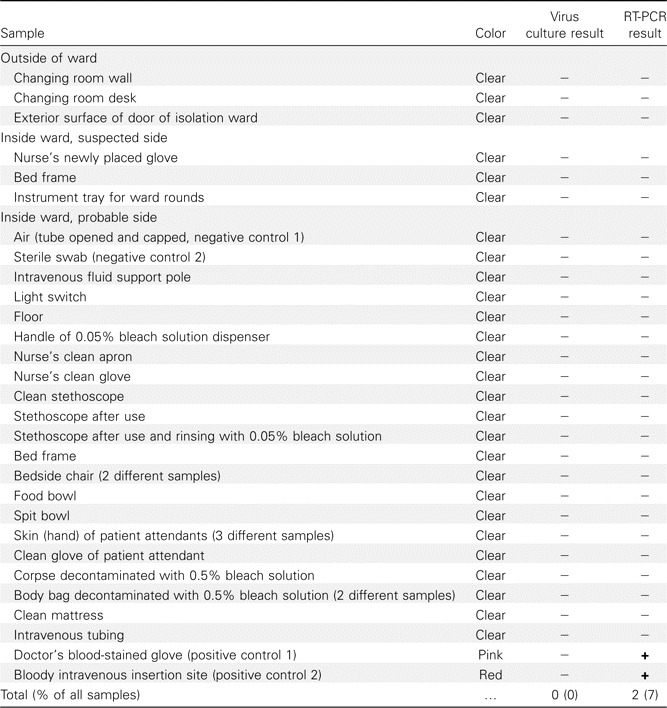
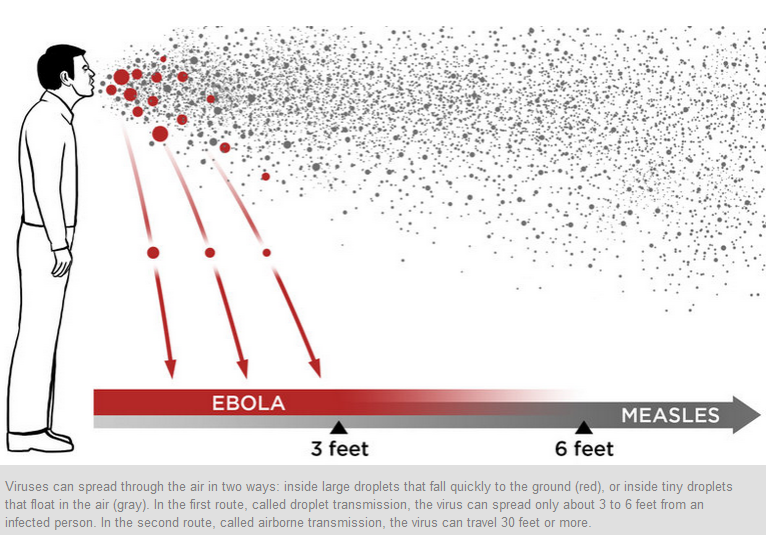
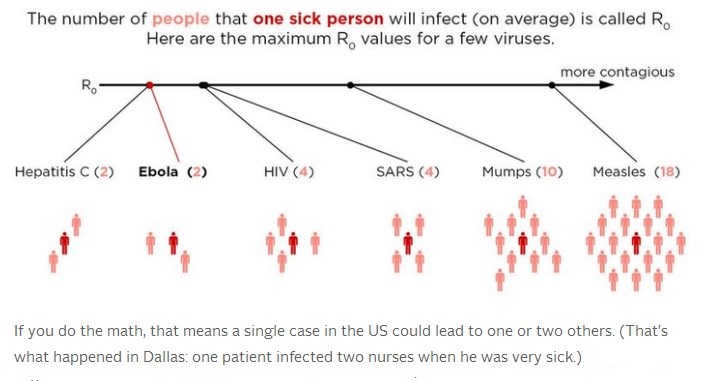
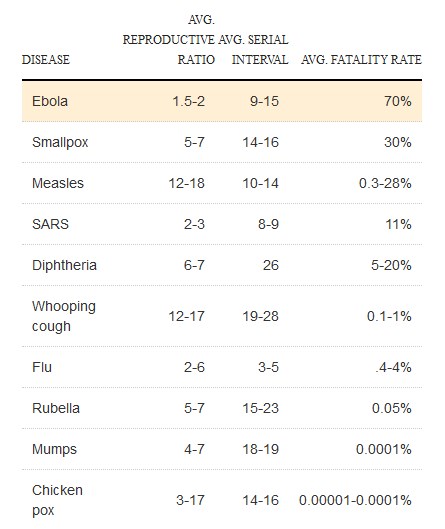
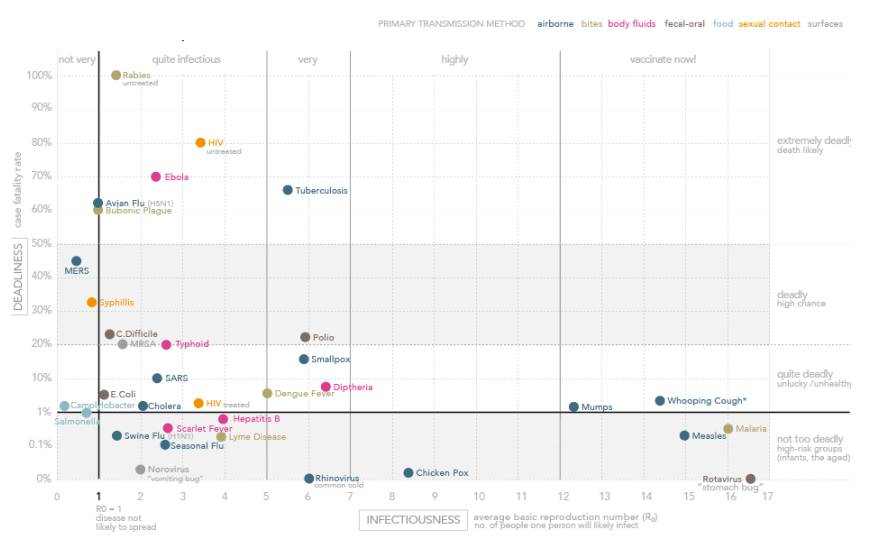
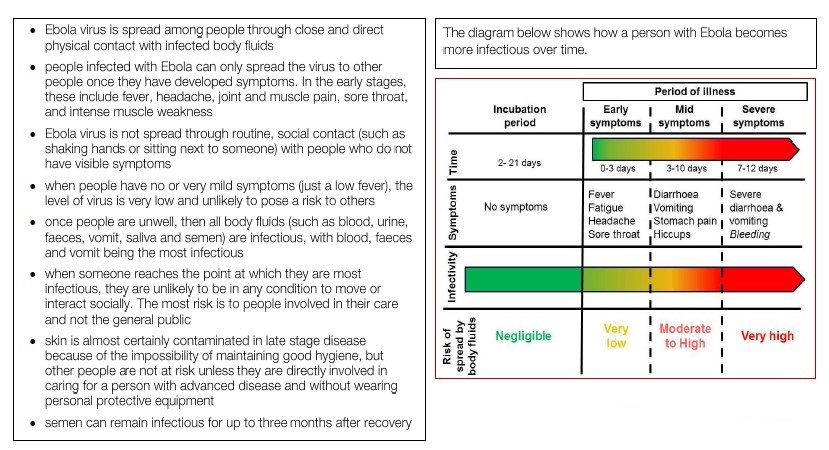
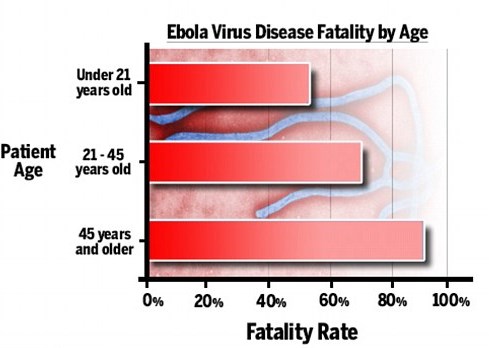
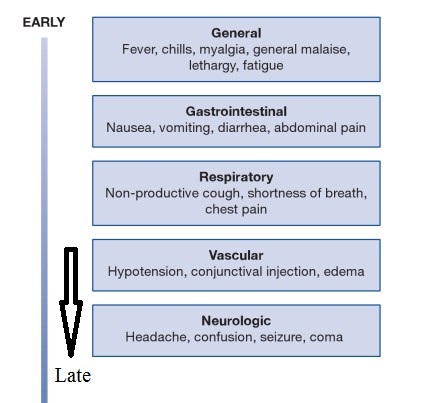
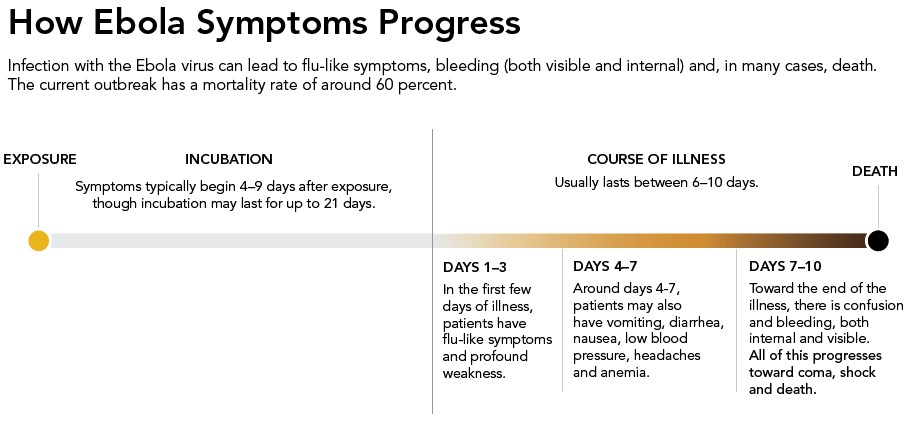
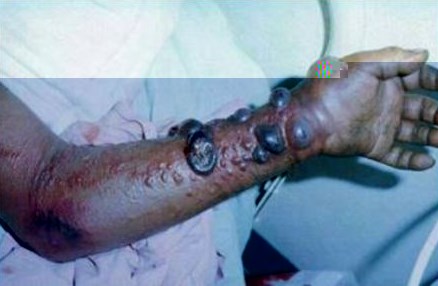
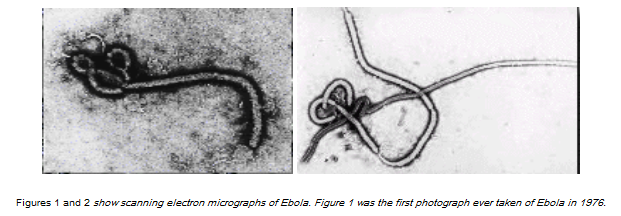

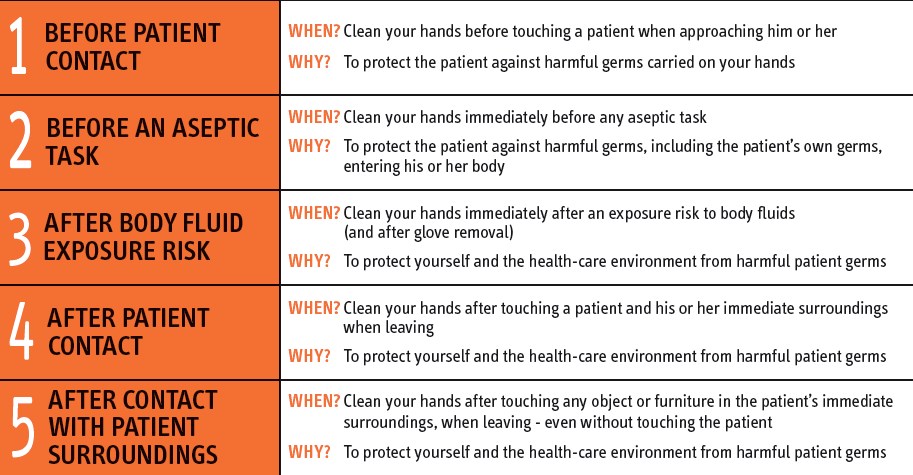

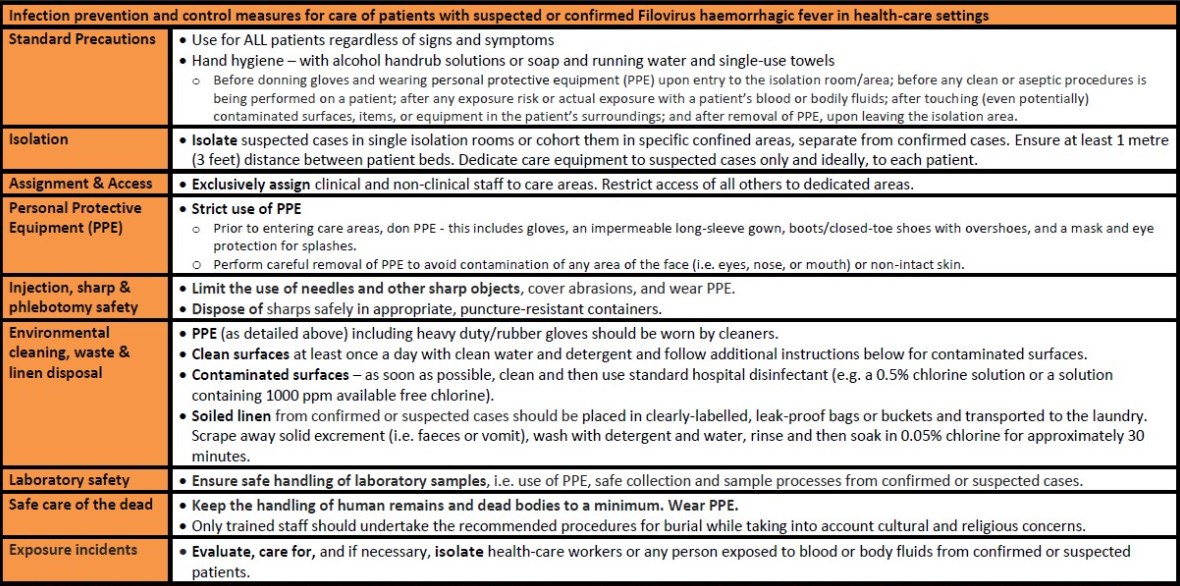
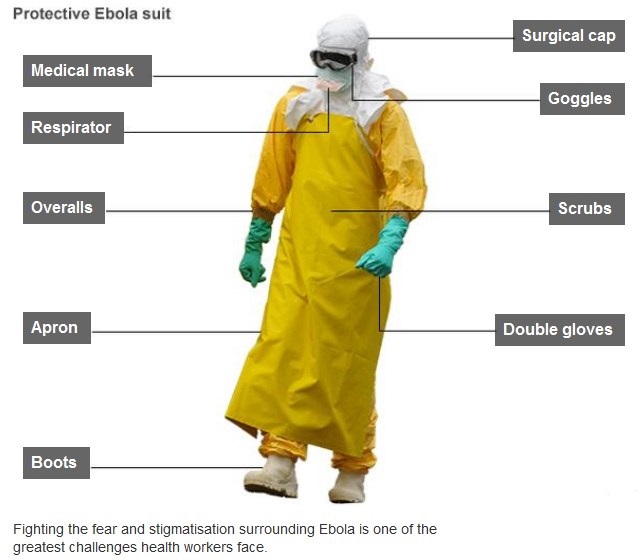
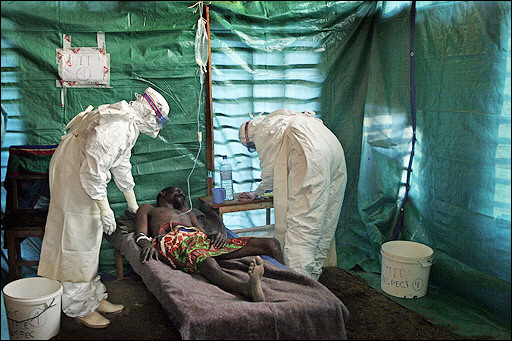
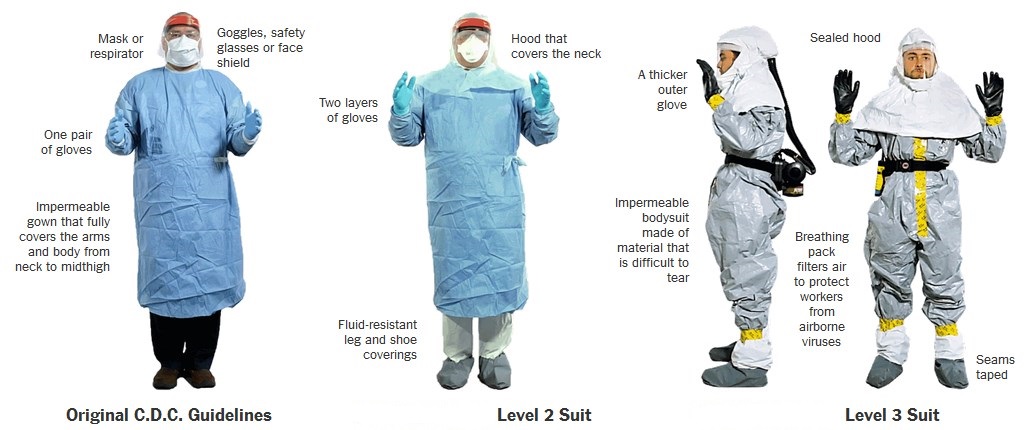
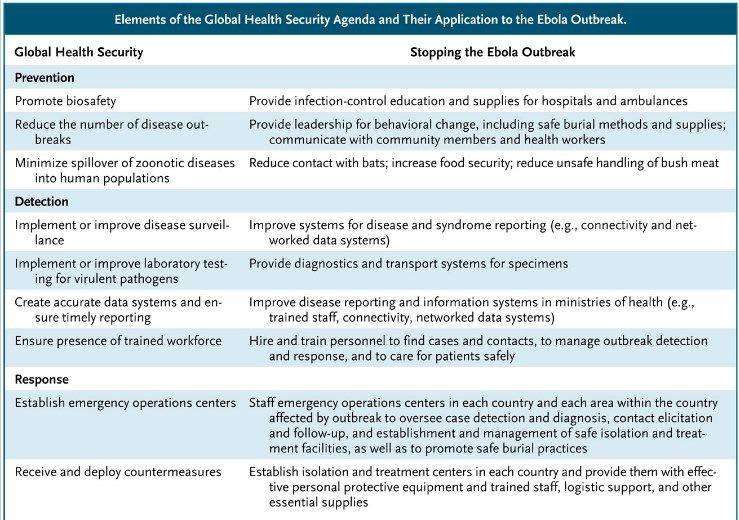

Hey There. I found your blog using msn. This is a really well written article. I?ll make sure to bookmark it and return to read more of your useful information. Thanks for the post. I?ll definitely return.
Great, thanks for sharing this article post. Keep writing.
and what you desire to say about this piece of writing,セックス ドールin my view its actually amazing designed for me.
Everything is very open with a very clear explanation of the challenges. It was truly informative. Your website is very useful. Thank you for sharing.
Wonderful post! We will be linking to this great post on our website. Keep up the good writing.
Highly energetic post, I loved that a lot. Will there be a part 2?|
Way cool! Some very valid points! I appreciate you writing this article and also the rest of the site is extremely good.
You have a way of making difficult jobs seem very easy. Your excitement encourages others to do their ideal.
Your blog is a true gem in the vast expanse of the online world. Your consistent delivery of high-quality content is truly commendable. Thank you for consistently going above and beyond in providing valuable insights. Keep up the fantastic work!
Your assistance in finishing this task was indispensable. We really could not have done it without your aid. Thanks for belonging to the team!
Thank you for the good writeup. It in fact was a amusement
account it. Look advanced to far added agreeable from you!
By the way, how could we communicate?
Pretty! This was an incredibly wonderful post. Thank you for supplying this info.
Hi there! I simply want to offer you a huge thumbs up for your great info you have here on this post. I will be coming back to your web site for more soon.
I couldnít resist commenting. Very well written!
Very neat article.Much thanks again.