Dr Rajiv Desai
An Educational Blog
THE PAIN
THE PAIN:
_
_
Prologue:
Pain, as a proper subject of scientific observation and study, has long been neglected by medical science. Perhaps, like death, it reminds us too much of our failures as physicians. Or perhaps, like sex, it was seen as too private or subjective a subject for objective analysis. Whatever the reason, pain was never mentioned as a topic in my medical school, residency, or internship training. Pain is the most common symptom of disease and the request for its relief probably the most common demand placed on the doctor. Pain is too important a topic to continue to be ignored. Pain is clearly a multidimensional experience. It is neurophysiological, biochemical, psychological, ethnocultural, religious, cognitive, affective and environmental. The topic of pain is extremely complex to say the least. Pain is that pervasive, invasive, all-encompassing personal warning that something-is-wrong signal. Pain is associated with a wide range of injury and disease, and is sometimes the disease itself. It is your friend and yet not your friend. Sometimes it arrives uninvited and unexpectedly in a sneaky manner, creeping up insidiously as it starts to take over everything about you. Sometimes it happens in an accidental manner. It drains you of energy, depletes your creativity, lightens your memory and exhausts you beyond any known definition of the word exhaustion. Your view of the world changes radically and sometimes the world’s view of you changes too. We now recognize that acute pain and chronic pain are distinct entities physiologically, neuroanatomically, and psychologically. Chronic pain is not just acute pain that occurs over a long period of time. Chronic pain can be a soul destroyer. Statistics show that people who suffer from chronic pain are eight times more likely to commit suicide than those who suffer from depression alone. I am surprised to learn that the amount of money spent on chronic pain is greater than the combined cost of heart disease and diabetes.
_
History of pain:
The nature of pain has intrigued philosophers for millennia. The ancient Greeks conceived of pain as an emotion. In the late nineteenth and early twentieth century, the view of pain as sensation became preeminent: it was seen as a direct response to a stimulus. From the mid–twentieth century to the present, these two views have been combined, so medical scientists who study pain now think of it as a subjective experience with distinct discriminative and emotional components. Ancient civilizations understood pain when it was visible, such as a cut or scrape, and recorded their accounts of pain and their treatments on stone tablets. But when pain was internal they related it to magic, demons, and evil thus the responsibility of pain relief, through ceremonies and rites, was left to shamans, priests, and sorcerers. The Greeks (and Romans) were the first to advance the theory that the brain and nervous system have a role in producing the perception of pain – sensation. And they also gave us the word “pain” or Poine, who was the Greek goddess of revenge, who was sent to punish the mortal fools who had angered the gods. The ancient Greek Hippocrates, prescribed chewing willow leaves to women in childbirth for their pain-relieving benefits, and this was the “beginning of aspirin” as willow trees, genus Salix, contains a form of salicylic acid, the active ingredient in aspirin. During the 17th and 18th centuries, the French philosopher Rene Descartes (1664) was the first to describe how pain traveled within the body, now called the pain pathway. He illustrated how particles of fire come into contact with the foot, the sensation traveling to the brain and then he compared pain sensations to the ringing of a bell. From this came the concept that there can be false alarms and we have therefore come to distinguish “psychogenic pain” from “real pain”. This is now known to be a false distinction, but still we hear today the concept of hurt being not the same thing as harm, with the implication that much that hurts may be ignored. It was the 19th century that pain came to dwell under a new domain and huge advances in pain therapy. Physicians discovered that pharmaceuticals, such as opium, morphine, codeine, and cocaine, could be used in pain relief. Another significant discovery was the use of anesthesia for surgery, prior to that, physicians used some fairly unusual techniques, such as putting a wooden bowl over a patient’s head and hammering on the bowl until the patient passed out. Or they would hold a patient’s head over a gas stove, thus inhaling the gas, and wait until they lost consciousness. But it was Queen Victoria who popularized anesthesia – chloroform – for childbirth- she actually made it fashionable. Even though we have made huge leaps in treatments and understanding of pain, some believe that ancient and primitive cultures may have been way before their time. Today, people look at pain treatment as being very objective, rigid, and almost literal – a quick fix – instead of how ancient people saw pain as not only a physical condition but as an emotional and spiritual condition – working with the entire patient.
__
From what we remember from our undergraduate textbooks, when you get hurt, the pain receptors send pain signals up the brain and we sense pain. So if pain is indeed an accurate indication of tissue damage, can it explain the following:
1. Why do 40% of the people (alert, rational & coherent and “not in shock”) admitted to an emergency room with horrific wounds feel no pain or pain of low intensity even after long delays?
2. Why do studies repeatedly show gross abnormalities, like disc bulges, spinal stenosis, herniations, meniscus tears, and so on in 20-70% of people who have no history of pain?
3. Which treatment would help in relieving the pain experienced by amputees in their “missing limb”? And 70% of the amputees report limb pain and sensation even years after the amputation.
4. There are thousands of amputees running with prosthetic limbs and cerebral palsy patients walking with worst gait possible. These folks have more than 100% movement dysfunctions. Why are they not in bed wreathing in pain?
So pain is not merely an indicator of tissue damage even though tissue damage does cause pain.
__
The experience of pain is not easy to understand. Pain is not merely a biologic process. Certainly, pain is a neurobiologic process, but, it also is an emotional experience; it brings up memories of past painful experiences; and, there is an awareness of pain that can be changed or modified. The experience of pain varies from individual to individual and from culture to culture. These cultural influences of pain modify the way peoples of different cultures experience it. The pain of surgery or injury is called acute pain. This pain has a biologic purpose. It tells us where we are hurt so that we may protect ourselves. This pain also activates defensive fight and flight mechanisms which protect us when we are threatened by a hostile environment. The pain that lasts and lasts, in spite of the healing of injury is called chronic pain. This pain is not easy to understand. It has no biologic purpose. It appears that this pain is a disease itself.
_
The task of medicine is to preserve and restore health and to relieve suffering. Understanding pain is essential to both these goals. Because pain is universally understood as a signal of diseases, it is the most common symptom that brings a patient to a physician’s attention. The function of the pain sensory system is to protect the body and maintain homeostasis. Pain is the most common reason for physician consultation in the United States and probably the world. It is a major symptom in many medical conditions, and can significantly interfere with a person’s quality of life and general functioning. Psychological factors such as social support, hypnotic suggestion, excitement, or distraction can significantly modulate pain’s intensity or unpleasantness. Also, sadness can aggravate pain and happiness can reduce pain.
_
Pain management a human right:
The delegates to the International Pain Summit (IPS) of the International Association for the Study of Pain (IASP) (comprising IASP representatives from Chapters in 64 countries plus members in 130 countries, as well as members of the community) have declared that access to Pain Management is a Fundamental Human Right. They found that pain management is inadequate in most of the world because:
1. There is inadequate access to treatment for acute pain caused by trauma, disease, and terminal illness; and failure to recognize that chronic pain is a serious chronic health problem requiring access to management akin to other chronic diseases such as diabetes or chronic heart disease.
2. There are major deficits in knowledge of health care professionals regarding the mechanisms and management of pain.
3. Chronic pain with or without diagnosis is highly stigmatized.
4. Most countries have no national policy at all or very inadequate policies regarding the management of pain as a health problem, including an inadequate level of research and education.
5. Pain Medicine is not recognized as a distinct specialty with a unique body of knowledge and defined scope of practice founded on research and comprehensive training programs.
6.The World Health Organization (WHO) estimates that 5 billion people live in countries with low or no access to controlled medicines and have no or insufficient access to treatment for moderate to severe pain.
7. There are severe restrictions on the availability of opioids and other essential medications, critical to the management of pain.
__
Pain statistics:
New statistics released by IASP and EFIC indicate that one in five people suffer from moderate to severe chronic pain, and that one in three are unable or less able to maintain an independent lifestyle due to their pain. Between one-half and two-thirds of people with chronic pain are less able or unable to exercise, enjoy normal sleep, perform household chores, attend social activities, drive a car, walk or have sexual relations. The effect of pain leads to strained or broken relationship with family and friends in 25 % of chronic pain sufferers, according to the IASP/EFIC data.
_
_
Pain statistics in the U.S.
Pain is a prevalent medical complaint and is one of the primary reasons for which patients seek medical attention in the United States. According to the American Pain Society, 50 million Americans are partially or totally disabled by pain, and 45% of all Americans seek care for persistent pain at some point in their lives. It has been estimated that 50% to 80% of hospitalized patients experience considerable pain regardless of the reason for admission. Despite the introduction of novel analgesics and advances in analgesic delivery systems, pain continues to be an undertreated event in a large proportion of hospitalized patients. Up to 90% of individuals with pain associated with cancer or other terminal illnesses and 50% of patients with acute pain are undertreated.
_
Incidence of pain as compared to major conditions in the U.S.
A hallmark of many chronic conditions, pain affects more Americans than diabetes, heart disease and cancer combined.
_
The chart below depicts the number of chronic pain sufferers compared to other major health conditions.
| Condition | Number of Sufferers | Source |
| Chronic Pain | 116 million Americans | Institute of Medicine of The National Academies |
| Diabetes | 25.8 million Americans |
American Diabetes Association |
| Coronary Heart Disease and Stroke | 16.3 million Americans and 7.0 million Americans respectively |
American Heart Association |
| Cancer | 11.9 million Americans | American Cancer Society |
_
A recent market research report indicates that more than 1.5 billion people worldwide suffer from chronic pain and that approximately 3-4.5% of the global population suffers from neuropathic pain, with incidence rate increasing in complementary to age. Pain is a significant public health problem that costs American society at least $560-$635 billion annually. Pain is the main reason for visiting the emergency department in more than 50% of cases and is present in 30% of family practice visits. Several epidemiological studies from different countries have reported widely varying prevalence rates for chronic pain, ranging from 12-80% of the population. It becomes more common as people approach death. A study of 4,703 patients found that 26% had pain in the last two years of life, increasing to 46% in the last month. A survey of 6,636 children (0–18 years of age) found that, of the 5,424 respondents, 54% had experienced pain in the preceding three months. A quarter reported having experienced recurrent or continuous pain for three months or more, and a third of these reported frequent and intense pain. The intensity of chronic pain was higher for girls, and girls’ reports of chronic pain increased markedly between ages12 and 14.
_
_
Pain costs:
Millions suffer from acute or chronic pain every year and the effects of pain exact a tremendous cost on our country in health care costs, rehabilitation and lost worker productivity, as well as the emotional and financial burden it places on patients and their families. The costs of unrelieved pain can result in longer hospital stays, increased rates of rehospitalization, increased outpatient visits, and decreased ability to function fully leading to lost income and insurance coverage. As such, patient’s unrelieved chronic pain problems often result in an inability to work and maintain health insurance. According to a recent Institute of Medicine Report: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research; pain is a significant public health problem that costs society at least $560-$635 billion annually, an amount equal to about $2,000.00 for everyone living in the U.S.
_
Lost productive time and cost due to Common Pain conditions in the United States Workforce:
1.Over half (52.7%) of the workforce surveyed reported having headache, back pain, arthritis, or other musculoskeletal pain in the past two weeks, and 12.7% of all workforce lost productive time in a two-week period due to pain.
2. Headache (5.4%) was the most common pain condition prompting lost productive time: followed by back pain (3.2%), arthritis pain (2%) and other musculoskeletal pain (2%).
3. Overall, workers lost an average of 4.6 hours per week of productive time due to a pain condition.
4. Other musculoskeletal pain (5.5 hours/week) and arthritis or back pain (5.2 hours/week) produced the largest amounts of lost productive time.
5. Headache produced, on average, 3.5 hours of lost productive time per week.
6. Age did not seem to attenuate the findings.
7. Lost productive time from common painful conditions was estimated to be $61.2 billion per year, while 76.6% of lost productive time was explained by reduced work performance, not absenteeism.
_
Pain cost in Europe:
A new EU-wide study by The Work Foundation (www.theworkfoundation.org) has found that musculoskeletal disorders (MSDs) such as back pain, neck pain and RSI-type conditions, account for nearly half (49%) of all absences from work and 60% of permanent work incapacity in the European Union. The estimated cost to society in Europe is up to €240 billion every year, with 100 million European citizens suffering the misery of MSDs that are serious enough to warrant treatment and absence from work.
_
Effect on functioning:
Experimental subjects challenged by acute pain and patients in chronic pain experience impairments in attention control, working memory, mental flexibility, problem solving, and information processing speed. Acute and chronic pain are also associated with increased depression, anxiety, fear, and anger. If I have matters right, the consequences of pain will include direct physical distress, unemployment, financial difficulties, marital disharmony, and difficulties in concentration and attention…
_
The Neuron (nerve cell):
The neuron is the cell that animals use to detect the outside environment, the internal environment of their own bodies, to formulate behavioral responses to those signals, and to control their bodies based on the chosen responses. The brain, the spinal cord and peripheral ganglia are made up of many cells, including neurons and glial cells. A neuron (nerve cell) is an electrically excitable cell that processes and transmits information by electrical and chemical signaling. Chemical signaling occurs via synapses, specialized connections with other cells. Neurons are cells that send and receive electro-chemical signals to and from the brain and nervous system. There are about 100 billion neurons in the brain. There are many more glial cells; they provide support functions for the neurons, and are far more numerous than neurons. The neuron consists of a cell body (or soma) with branching dendrites (signal receivers) and a projection called an axon, which conduct the nerve signal. At the other end of the axon, the axon terminals transmit the electro-chemical signal across a synapse (the gap between the axon terminal and the receiving cell). A typical neuron has about 1,000 to 10,000 synapses (that is, it communicates with 1,000-10,000 other neurons, muscle cells, glands, etc.). Unlike most other cells, neurons cannot regrow after damage (except neurons from the hippocampus). Glial cells make up 90 percent of the brain’s cells. Glial cells are nerve cells that don’t carry nerve impulses. The various glial (meaning “glue”) cells perform many important functions including: digestion of parts of dead neurons, manufacturing myelin for neurons, providing physical and nutritional support for neurons, and more.
_
_
Neurons in a resting state normally have a membrane potential around -70mV (minus seventy millivolts). This means that the voltage difference between the fluid on the inside of the cell relative to the fluid on the outside of the cell is negative. This negative difference is maintained with ions like Na+, K+, Cl– , and protein anions. The neuron has a pump that actively pumps three Na+ ions out and takes in two K+ ions. This means that a net positive charge flows out of the neuron. This is what gives the cell its negative potential. Ions are also what are responsible for the initiation, and transmission of action potentials. The first step of the action potential is that the Na+ channels open allowing a flood of sodium ions into the cell. This causes the membrane potential to become positive. At some positive membrane potential, the K+ channels open allowing the potassium ions to flow out of the cell. Next the Na+ channels close. This stops inflow of positive charge. But since the K+ channels are still open it allows the outflow of positive charge so that the membrane potential plunges. When the membrane potential begins reaching its resting state, the K+ channels close. Now the sodium/potassium pump does its work and starts transporting sodium out of the cell, and potassium into the cell so that it is ready for the next action potential.
_
_
_
The action potential travels down the length of the axon as a voltage spike. It does this by using the steps just outlined. As a section of the axon undergoes the above process, it increases the membrane potential of the neighboring section and causes it to spike. This is like a mini chain reaction that proceeds down the length of the axon until it reaches the synapse. Thus an action potential is an electric pulse that travels down the axon until it reaches the synapses, where it then causes the release of neurotransmitters. The synapses are extremely close to the dendrites of the target neuron. This allows the neurotransmitters to diffuse across the intervening space and fit into the receptors that are located on the target neuron. This causes some action to take place in that neuron that will either decrease or increase the membrane potential of the neuron. If it increases the membrane potential, then it is exciting the neuron; and if it decreases the membrane potential, then it is inhibiting the neuron. If it causes the membrane potential to pass the firing threshold then it will activate an action potential in the target neuron and send it down its axon. Afferent neurons convey information from outside environment and internal environment (tissues and organs) into the central nervous system and are sometimes called sensory neurons. Efferent neurons transmit signals from the central nervous system to the effector cells and are sometimes called motor neurons. Interneurons connect neurons within specific regions of the central nervous system. Further discussion on wide variety of voltage-gated channels (e.g., Nav, Cav, Kv) that transduce the receptor potential into an action potential or, more commonly, a set of action potentials that encode the intensity of a noxious stimulus applied to nociceptor is discussed later on in the article.
_
The table above shows difference between nociceptive specific (NS) and wide dynamic range (WDR) neurons which transmit pain signal to brain.
_
Definition:
_
The international association for study of pain (IASP) has defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage”. It follows that severity of pain does not correlate with the degree of tissue damage, and that each patient’s experience and expression of pain are different. Pain is a warning that something is wrong, pre-empts other signals, and is associated with an unpleasant affect. Pain is an unpleasant sensation localized to a part of body. It is often described in terms of a penetrating or tissue-destructive process (e.g. stabbing, burning, twisting, tearing, squeezing) and/or of a bodily or emotional reaction (e.g. terrifying, nauseating, sickening). Pain is an unpleasant feeling often caused by intense or damaging stimuli, such as stubbing a toe, burning a finger, putting alcohol on a cut, and bumping the “funny bone.” Further-more, any pain of moderate or severe intensity is accompanied by anxiety and the urge to escape or terminate the feeling. These properties illustrates duality of pain, it is both sensation and emotion. When acute, pain is characteristically associated with behavioral arousal, and a stress response consisting of increased blood pressure, heart rate, pupil diameter, and plasma cortisol levels. In addition, local muscle contraction (e.g. limb flexion, abdominal wall rigidity) is often present. Pain motivates the individual to withdraw from damaging situations, to protect a damaged body part while it heals, and to avoid similar experiences in the future. Most pain resolves promptly once the painful stimulus is removed and the body has healed, but sometimes pain persists despite removal of the stimulus and apparent healing of the body; and sometimes pain arises in the absence of any detectable stimulus, damage or disease.
_
From this definition we see that pain is a perception in the same way that vision and hearing are. By this I mean that its significance is determined by the cerebral cortex in the light of other activity there. It involves sensitivity to chemical changes in the tissues and then interpretation that such changes are, or may be harmful. This perception is real, whether or not harm has occurred or is occurring. Cognition is involved in the formulation of this perception. There are emotional consequences, and behavioral responses to the cognitive and emotional aspects of pain. Pain, in its simplest form, is a warning mechanism that helps protect an organism by influencing it to withdraw from harmful stimuli (such as a pinprick). In its more complex form, such as in the case of a chronic condition accompanied by depression or anxiety, it can be difficult to isolate and treat. Pain receptors, found in the skin and other tissues, are nerve fibers that react to mechanical, thermal, and chemical stimuli. Pain impulses enter the spinal cord and are transmitted to the brain stem and thalamus. The perception of pain is highly variable among individuals; it is influenced by previous experiences, cultural attitudes (including gender stereotypes), and genetic makeup. Pain is more complex than other sensory systems such as vision or hearing because it not only involves the transfer of sensory information to the nervous system, but produces suffering which then leads to aversive corrective behavior. So pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage. It is a protective mechanism for the body and causes a human or animal to react to remove the pain stimulus. It is a complex sensory experience with many subjective components: 1) discriminative; 2) learning and memory—associate pain with certain events; 3) unpleasantness, displeasure and 4) suffering, escape.
_
Pain, as a submodality of somatic sensation, has also been defined as a “complex constellation of unpleasant sensory, emotional and cognitive experiences provoked by real or perceived tissue damage and manifested by certain autonomic, psychological, and behavioral reactions”. The benefit of these unpleasant sensations, however, is underscored by extreme cases: patients lacking the ability to perceive pain due to hereditary neuropathies often maintain unrealized infections; self mutilate, and have curtailed life spans. Normally nociception (vide infra) and the perception of pain are evoked only at pressures and temperatures extreme enough to potentially injure tissues and by toxic molecules and inflammatory mediators. These high threshold physical and noxious chemical stimuli are detected by specialized peripheral sensory neurons (nociceptors). This is in contrast to the high sensitivity of visual, auditory, olfactory, taste, and somatosensory organs to their adequate stimuli. Pain is described as having different qualities and temporal features depending on the modality and locality of the stimulus respectively: first pain is described as lancinating, stabbing, or pricking; second pain is more pervasive and includes burning, throbbing, cramping, and aching and recruits sustained affective components with descriptors such as “sickening”. The intensity of these global reactions underscores the importance of avoiding damaging situations for survival and maintaining homeostasis. As opposed to the relatively more objective nature of other senses, pain is highly individual and subjective and the translation of nociception into pain perception can be curtailed by stress or exacerbated by anticipation.
_
Physiological to pathological pain:
Pain is produced by stimuli that usually cause damage to tissues. It is in the best interest of an organism to detect potential damage to the body so it can take appropriate steps to avoid being harmed. The presence of pain usually forces immobilization of the injured area and prevents additional tissue damage from taking place and allows for the healing and repair processes to occur. Without the aid of the body’s response to tissue damage, survival would be difficult. Pathological pain is considered abnormal given that there is no protective value to the organism. This is the “morphism” in the nervous system that involves plasticity in both the peripheral and central nervous systems. It affects the way the nervous system detects the type of stimuli from the environment – a shift from a normally protective physiological mechanism to an abnormal and possibly pathological mechanism. Consequently, this justifies the concept that pain should also be considered a disease process on its own especially in chronic form.
_
People who sought treatment through the College of Dentistry’s Parker E. Mahan Facial Pain Center, reported that due to their pain, routine tasks such as speaking on the telephone, eating, taking medications and carrying on a conversation, had became daily hassles. Pain is associated with a variety of behaviors. A painful stimulus will arouse us, as in “Pinch me to see if I’m awake.” It can focus our attention on the site of an injury: “I looked down at where it hurt and saw I was bleeding.” It can cue us to try to escape from the cause of an injury or immobilize us so that we do not suffer further damage. In addition, pain causes changes in heart rate and blood pressure, and an endocrine response with elevated stress hormones. For each response elicited by the pain-producing injury, there is a unique central nervous system pathway.
_
The first aspect of pain is that although pain appears to be a simple, homogenous experience, it is actually a complex experience comprising sensory-discriminative, emotional-cognitive, and behavioral components. These components are normally linked together, but they can become disconnected and therefore, much to our astonishment, they can exist separately. The second aspect is that pain, once deprived of all its affective, cognitive, and behavioral components, loses all of its representational and motivational force: it is no longer a signal of threat or injury, and it no longer moves one’s mind or body in any way. The third aspect is that pain deprived of its sensory discriminative components comes to such sensory indeterminacy that it cannot be distinguished from other unpleasant sensations, or sensations of other quality, and loses all informational power with regard to the location, intensity, temporal profile, and nature of harmful stimuli.
_
In everyday life pain is recognized in two forms, namely acute pain and chronic pain. The former has a protective function. It alerts us to damage to the body, it increases our level of arousal, it directs our attention to the cause of the pain, and generates behavior that leads to an escape from it. The chief emotion associated with acute pain is anxiety, and this subsides when pain is relieved and the cause is understood. In contrast, chronic pain does not appear to the sufferer to have any purpose and indeed has negative qualities. It gives rise to feelings of anxiety and at times of depression. The behaviors generated include withdrawal from social activities and a search for relief. The latter may well lead the sufferer to move from one doctor to another and to non-medical practitioners in the hope of pain relief. At times that process itself may generate more physical suffering through unnecessary investigation and the end result is pain, despair, and depression.
__
Since pain is the most complex subject for discussion, let me begin with the nomenclature of pain so that various terms can be understood and interpreted in the right perspective.
_
Pain nomenclature:
_
Pain:
An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.
Note: Pain is always subjective. Biologists recognize that those stimuli which cause pain are liable to damage tissue. Accordingly, pain is that experience we associate with actual or potential tissue damage. It is unquestionably a sensation in a part or parts of the body, but it is also always unpleasant and therefore also an emotional experience. Experiences which resemble pain but are not unpleasant, e.g., pricking, should not be called pain. Unpleasant abnormal experiences (dysesthesias) may also be pain but are not necessarily so because, subjectively, they may not have the usual sensory qualities of pain. Many people report pain in the absence of tissue damage or any likely pathophysiological cause; usually this happens for psychological reasons. There is usually no way to distinguish their experience from that due to tissue damage if we take the subjective report. If they regard their experience as pain, and if they report it in the same ways as pain caused by tissue damage, it should be accepted as pain. This definition avoids tying pain to the stimulus. Activity induced in the nociceptor and nociceptive pathways by a noxious stimulus is not pain, which is always a psychological state, even though we may well appreciate that pain most often has a proximate physical cause.
_
Allodynia: Pain due to a stimulus that does not normally provoke pain.
Note: The stimulus leads to an unexpectedly painful response. This is a clinical term that does not imply a mechanism. Allodynia may be seen after different types of somatosensory stimuli applied to many different tissues. The term allodynia was originally introduced to separate from hyperalgesia and hyperesthesia, the conditions seen in patients with lesions of the nervous system where touch, light pressure, or moderate cold or warmth evoke pain when applied to apparently normal skin. It is important to recognize that allodynia involves a change in the quality of a sensation, whether tactile, thermal, or of any other sort. The original modality is normally nonpainful, but the response is painful. There is thus a loss of specificity of a sensory modality. By contrast, hyperalgesia (vide infra) represents an augmented response in a specific mode, viz., pain. With other cutaneous modalities, hyperesthesia is the term which corresponds to hyperalgesia, and as with hyperalgesia, the quality is not altered. In allodynia, the stimulus mode and the response mode differ, unlike the situation with hyperalgesia. This distinction should not be confused by the fact that allodynia and hyperalgesia can be plotted with overlap along the same continuum of physical intensity in certain circumstances, for example, with pressure or temperature.
_
Analgesia: Absence of pain in response to stimulation which would normally be painful.
_
Anesthesia dolorosa: Pain in an area or region which is anesthetic.
_
Causalgia: A syndrome of sustained burning pain, allodynia, and hyperpathia after a traumatic nerve lesion, often combined with vasomotor and sudomotor dysfunction and later trophic changes.
_
Central pain: Pain initiated or caused by a primary lesion or dysfunction in the central nervous system.
_
Dysesthesia: An unpleasant abnormal sensation, whether spontaneous or evoked.
Note: Special cases of dysesthesia include hyperalgesia and allodynia. A dysesthesia should always be unpleasant and a paresthesia should not be unpleasant, although it is recognized that the borderline may present some difficulties when it comes to deciding as to whether a sensation is pleasant or unpleasant. It should always be specified whether the sensations are spontaneous or evoked.
_
Hyperalgesia: Increased pain from a stimulus that normally provokes pain.
Note: Hyperalgesia reflects increased pain on suprathreshold stimulation. This is a clinical term that does not imply a mechanism. For pain evoked by stimuli that usually are not painful, the term allodynia is preferred, while hyperalgesia is more appropriately used for cases with an increased response at a normal threshold, or at an increased threshold, e.g., in patients with neuropathy. It should also be recognized that with allodynia the stimulus and the response are in different modes, whereas with hyperalgesia they are in the same mode. Current evidence suggests that hyperalgesia is a consequence of perturbation of the nociceptive system with peripheral or central sensitization, or both, but it is important to distinguish between the clinical phenomena, which this definition emphasizes, and the interpretation, which may well change as knowledge advances. Hyperalgesia may be seen after different types of somatosensory stimulation applied to different tissues.
_
Hyperesthesia: Increased sensitivity to stimulation, excluding the special senses.
Note: The stimulus and locus should be specified. Hyperesthesia may refer to various modes of cutaneous sensibility including touch and thermal sensation without pain, as well as to pain. The word is used to indicate both diminished threshold to any stimulus and an increased response to stimuli that are normally recognized. Allodynia is suggested for pain after stimulation which is not normally painful. Hyperesthesia includes both allodynia and hyperalgesia, but the more specific terms should be used wherever they are applicable.
_
Hyperpathia: A painful syndrome characterized by an abnormally painful reaction to a stimulus, especially a repetitive stimulus, as well as an increased threshold.
Note: It may occur with allodynia, hyperesthesia, hyperalgesia, or dysesthesia. Faulty identification and localization of the stimulus, delay, radiating sensation and aftersensation may be present, and the pain is often explosive in character.
_
Hypoalgesia: Diminished pain in response to a normally painful stimulus.
Note: Hypoalgesia was formerly defined as diminished sensitivity to noxious stimulation, making it a particular case of hypoesthesia. However, it now refers only to the occurrence of relatively less pain in response to stimulation that produces pain. Hypoesthesia covers the case of diminished sensitivity to stimulation that is normally painful.
__
|
The implications of some of the above definitions may be summarized for convenience as follows: |
||
| Allodynia | lowered threshold | stimulus and response mode differ |
| Hyperalgesia | increased response | stimulus and response mode are the same |
| Hyperpathia | raised threshold: increased response | stimulus and response mode may be the same or different |
| Hypoalgesia | raised threshold: lowered response | stimulus and response mode are the same |
_
The above essentials of the definitions do not have to be symmetrical and are not symmetrical at present. Lowered threshold may occur with allodynia but is not required. Also, there is no category for lowered threshold and lowered response—if it ever occurs.
_
Hypoesthesia: Decreased sensitivity to stimulation, excluding the special senses.
Note: Stimulation and locus to be specified.
_
Neuralgia: Pain in the distribution of a nerve or nerves.
Note: Common usage, especially in Europe, often implies a paroxysmal quality, but neuralgia should not be reserved for paroxysmal pains.
__
Neuropathic pain: Pain caused by a lesion or disease of the somatosensory nervous system.
Note: Neuropathic pain is a clinical description (and not a diagnosis) which requires a demonstrable lesion or a disease that satisfies established neurological diagnostic criteria. The term ‘lesion’ is commonly used when diagnostic investigations (e.g. imaging, neurophysiology, biopsies, lab tests) reveal an abnormality or when there was obvious trauma. The term ‘disease’ is commonly used when the underlying cause of the lesion is known (e.g. stroke, vasculitis, diabetes mellitus, genetic abnormality). Somatosensory refers to information about the body per se including visceral organs, rather than information about the external world (e.g., vision, hearing, or olfaction). The presence of symptoms or signs (e.g., touch-evoked pain) alone does not justify the use of the term neuropathic. Some disease entities, such as trigeminal neuralgia, are currently defined by their clinical presentation rather than by objective diagnostic testing. Other diagnoses such as postherpetic neuralgia are normally based upon the history. It is common when investigating neuropathic pain that diagnostic testing may yield inconclusive or even inconsistent data. In such instances, clinical judgment is required to reduce the totality of findings in a patient into one putative diagnosis or concise group of diagnoses.
_
Central neuropathic pain: Pain caused by a lesion or disease of the central somatosensory nervous system.
_
Peripheral neuropathic pain: Pain caused by a lesion or disease of the peripheral somatosensory nervous system.
__
Nociception: The neural process of encoding noxious stimuli.
Note: Consequences of encoding may be autonomic (e. g. elevated blood pressure) or behavioral (motor withdrawal reflex or more complex nocifensive behavior). Pain sensation is not necessarily implied.
_
Nociceptive neuron:
A central or peripheral neuron of the somatosensory nervous system that is capable of encoding noxious stimuli.
_
Nociceptive pain: Pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors.
Note: This term is designed to contrast with neuropathic pain. The term is used to describe pain occurring with a normally functioning somatosensory nervous system to contrast with the abnormal function seen in neuropathic pain.
_
Nociceptive stimulus: An actually or potentially tissue-damaging event transduced and encoded by nociceptors.
_
Nociceptor: A high-threshold sensory receptor of the peripheral somatosensory nervous system that is capable of transducing and encoding noxious stimuli.
_
Noxious stimulus: A stimulus that is damaging or threatens damage to normal tissues.
_
Pain threshold: The minimum intensity of a stimulus that is perceived as painful.
Note: Traditionally pain threshold has often been defined as the least stimulus intensity at which a subject perceives pain. Properly defined, the threshold is really the experience of the patient, whereas the intensity measured is an external event. It has been common usage for most pain research workers to define the threshold in terms of the stimulus, and that should be avoided. However, the threshold stimulus can be recognized as such and measured. In psychophysics, threshold is defined as the level at which 50% of stimuli are recognized. In that case, the pain threshold would be the level at which 50% of stimuli would be recognized as painful. The stimulus is not pain and cannot be a measure of pain.
_
Pain tolerance level: The maximum intensity of a pain-producing stimulus that a subject is willing to accept in a given situation.
Note: As with pain threshold, the pain tolerance level is the subjective experience of the individual. The stimuli which are normally measured in relation to its production are the pain tolerance level stimuli and not the level itself. Thus, the same argument applies to pain tolerance level as to pain threshold, and it is not defined in terms of the external stimulation as such.
_
Paresthesia: An abnormal sensation, whether spontaneous or evoked.
Note: After much discussion, it has been agreed to recommend that paresthesia be used to describe an abnormal sensation that is not unpleasant while dysesthesia be used preferentially for an abnormal sensation that is considered to be unpleasant. The use of one term (paresthesia) to indicate spontaneous sensations and the other to refer to evoked sensations is not favored. There is a sense in which, since paresthesia refers to abnormal sensations in general, it might include dysesthesia, but the reverse is not true. Dysesthesia does not include all abnormal sensations, but only those that are unpleasant.
_
Sensitization: Increased responsiveness of nociceptive neurons to their normal input, and/or recruitment of a response to normally subthreshold inputs.
Note: Sensitization can include a drop in threshold and an increase in suprathreshold response. Spontaneous discharges and increases in receptive field size may also occur. This is a neurophysiological term that can only be applied when both input and output of the neural system under study are known, e.g., by controlling the stimulus and measuring the neural event. Clinically, sensitization may only be inferred indirectly from phenomena such as hyperalgesia or allodynia.
_
Central sensitization: Increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input.
Note: This may include increased responsiveness due to dysfunction of endogenous pain control systems. Peripheral neurons are functioning normally; changes in function occur in central neurons only.
_
Peripheral sensitization: Increased responsiveness and reduced threshold of nociceptive neurons in the periphery to the stimulation of their receptive fields.
_ _ _
Duration of pain:
Pain is usually transitory, lasting only until the noxious stimulus is removed or the underlying damage or pathology has healed, but some painful conditions, such as rheumatoid arthritis, peripheral neuropathy, cancer and idiopathic pain, may persist for years. Pain that lasts a long time is called chronic, and pain that resolves quickly is called acute. Traditionally, the distinction between acute and chronic pain has relied upon an arbitrary interval of time from onset; the two most commonly used markers being 3 months and 6 months since the onset of pain. Generally speaking, acute pain lasts for less than 12 weeks and chronic pain lasts for more than 12 weeks. A popular alternative definition of chronic pain, involving no arbitrarily fixed durations is “pain that extends beyond the expected period of healing.” Chronic pain may be classified as cancer pain or benign (non-cancer pain).
_
__
Pain scales:
Pain rating scales, also called pain scales, are used by physicians and other health care providers to evaluate pain and measure pain levels. Pain measurements help determine the severity, type, and duration of the pain, and are used to make an accurate diagnosis, determine a treatment plan, and evaluate the effectiveness of treatment. Types of pain rating scales include verbal scales, numerical scales, and visual analogue scales. Verbal rating scales consist of a series of words commonly used to describe pain (e.g., no pain, mild pain, moderate pain, severe pain). The patient reads the words and chooses the one that best describes the pain he or she is experiencing. A score (e.g., from 0–3) that is assigned to each word is then used to measure pain levels.
_
_
The most commonly used pain scales in healthcare is the numerical rating scale which offers the individual in pain to rate their pain score. It is designed to be used by those over the age of 9. In the numerical scale, the user has the option to verbally rate their scale from 0 to 10 or to place a mark on a line indicating their level of pain. 0 indicates the absence of pain, while 10 represents the most intense pain possible. The numerical rating pain scale allows the healthcare provider to rate pain as mild, moderate or severe, which can indicate a potential disability level. Tracking your pain is a helpful diagnostic tool when dealing with repetitive stress injuries. A visual analog pain scale lets you bypass the cognitive level of your brain and give a truer representation of your pain. The Wong Baker Faces Pain Scale combines pictures and numbers to allow pain to be rated by the user. It can be used in children over the age of 3, and in adults. The faces range from a smiling face to a sad, crying face. A numerical rating is assigned to each of six faces totally.
_
_
Pain classification:
Pain can be categorized according to several variables, including its duration (acute, convalescent, chronic), its pathophysiologic mechanisms (physiologic, nociceptive, neuropathic), and its clinical context (e.g., postsurgical, malignancy related, neuropathic, degenerative). Acute pain follows traumatic tissue injuries, is generally limited in duration, and is associated with temporal reductions in intensity. Chronic pain may be defined as discomfort persisting 3–6 months beyond the expected period of healing. For clinicians dealing with soft tissue trauma, it is helpful to think of pain as being peripheral or central in origin. Peripheral pain originates in muscles, tendons, etc., or in the peripheral nerves themselves. Pain originating in the peripheral nerves, i.e. via trauma to the nerves, is neurogenic pain. Central pain arises from central nervous system pathology … a “primary” CNS dysfuntion. Some of this may arise due to maladaptive thought processes, true “psychogenic” pain. But most of it is due to structural changes in the CNS, e.g., spinal cord injury, multiple sclerosis, stroke and epilepsy. Somatogenic pain is a pain originating from an actual physical cause e.g. trauma, ischaemia etc while Psychogenic pain is pain for which there is no physical cause. It is not however imaginary pain and can be as intense as somatic pain.
_
|
Pain |
|||
|
Nociceptive |
Non-Nociceptive |
||
|
Somatic |
Visceral |
Neuropathic |
Sympathetic |
_
_
Nociceptive pain is pain in which normal nerves transmit information to the central nervous system about trauma to tissues (nocere = to injure, Latin). Nociceptive pain is due to direct stimulation of peripheral nerve endings (e.g. wounds, fractures, burns, angina). It is inflammatory pain which is associated with tissue damage and the infiltration of immune cells. Nociceptive pain may also be divided into “visceral,” “deep somatic” and “superficial somatic” pain.
_
Somatic Pain – a type of nociceptive pain. Pain felt on the skin, muscle, joints, bones and ligaments is called somatic pain. The term musculo-skeletal pain means somatic pain. Somatic pain could be superficial due to stimulation of receptors in skin and deep due to stimulation of receptors in muscles, joints and tendons. The pain receptors are sensitive to temperature (hot/cold), vibration, and stretch (in the muscles). They are also sensitive to inflammation, as would happen if you cut yourself, sprain something that causes tissue damage. Pain as a result of lack of oxygen, as in ischemic muscle cramps, are a type of nociceptive pain. Somatic pain is generally sharp and well localized – if you touch it or move the affected area the pain will worsen.
_
Visceral Pain – a type of nociceptive pain. It is felt in the internal organs and main body cavities. The cavities are divided into the thorax (lungs and heart), abdomen (bowels, spleen, liver and kidneys), and the pelvis (ovaries, bladder, and the uterus). The pain receptors – nociceptors – sense inflammation, stretch and ischemia (oxygen starvation). Afferent fibers from visceral structures reach the CNS via sympathetic and parasympathetic nerves. Their cell bodies are located in the dorsal roots and the homologous cranial nerve ganglia. Specifically, there are visceral afferents in the facial, glossopharyngeal, and vagus nerves; in the thoracic and upper lumbar dorsal roots; and in the sacral roots. There may also be visceral afferent fibers from the eye in the trigeminal nerve.
_
General characteristics of pain due to visceral pathology
1. Is poorly localized
2. Referred to other somatic structures (Referred pain)
3. Is not evoked from all viscera
4. Not necessarily evoked from visceral injury
5. Produces non-specific responses or whole-body moto responses
6. Produces strong autonomic responses (high-blood pressure, increase in heart rate, sweating, etc.)
7. Leads to sensitization of body structures
8. Produces strong affective responses
_
Sympathetic Pain:
The sympathetic nervous system controls our blood flow to our skin and muscles, perspiration (sweating) by the skin, and how quickly the peripheral nervous system works. Sympathetic pain occurs generally after a fracture or a soft tissue injury of the limbs. This pain is non-nociceptive – there are no specific pain receptors. As with neuropathic pain, the nerve is injured, becomes unstable and fires off random, chaotic, abnormal signals to the brain, which interprets them as pain. Generally with this kind of pain the skin and the area around the injury become extremely sensitive. The pain often becomes so intense that the sufferer daren’t use the affected arm or leg. Lack of limb use after a time can cause other problems, such as muscle wasting, osteoporosis, and stiffness in the joints.
__
Another classification:
_
Another researcher divided pain into three classes as per the figure above.
(A) Nociceptive pain represents the sensation associated with the detection of potentially tissue-damaging noxious stimuli and is protective. First, there is the pain that is an early-warning physiological protective system, essential to detect and minimize contact with damaging or noxious stimuli. This is the pain we feel when touching something too hot, cold, or sharp. Because this pain is concerned with the sensing of noxious stimuli, it is called nociceptive pain. The neurobiological apparatus that generates nociceptive pain evolved from the capacity of even the most primitive of nervous systems to signal impending or actual tissue damage from environmental stimuli. Its protective role demands immediate attention and action, which occur by virtue of the withdrawal reflex it activates, the intrinsic unpleasantness of the sensation elicited, and the emotional anguish it engages. Nociceptive pain presents itself as something to avoid now, and when engaged, the system overrules most other neural functions.
(B) Inflammatory pain is associated with tissue damage and the infiltration of immune cells and can promote repair by causing pain hypersensitivity until healing occurs. The second kind of pain is also adaptive and protective. By heightening sensory sensitivity after unavoidable tissue damage, this pain assists in the healing of the injured body part by creating a situation that discourages physical contact and movement. Pain hypersensitivity, or tenderness, reduces further risk of damage and promotes recovery, as after a surgical wound or in an inflamed joint, where normally innocuous stimuli now elicit pain. This pain is caused by activation of the immune system by tissue injury or infection, and is therefore called inflammatory pain; indeed, pain is one of the cardinal features of inflammation. While this pain is adaptive, it still needs to be reduced in patients with ongoing inflammation, as with rheumatoid arthritis or in cases of severe or extensive injury.
(C) Pathological pain is a disease state caused by damage to the nervous system (neuropathic) or by its abnormal function (dysfunctional). Finally, there is the pain that is not protective, but maladaptive, resulting from abnormal functioning of the nervous system. This pathological pain, which is not a symptom of some disorder but rather a disease state of the nervous system, can occur after damage to the nervous system (neuropathic pain), but also in conditions in which there is no such damage or inflammation (dysfunctional pain). Conditions that evoke dysfunctional pain include fibromyalgia, irritable bowel syndrome, tension type headache, temporomandibular joint disease, interstitial cystitis, and other syndromes in which there exists substantial pain but no noxious stimulus, and no or minimal peripheral inflammatory pathology.
_
By analogy, if pain were a fire alarm, the nociceptive type would be activated appropriately only by the presence of intense heat, inflammatory pain would be activated by warm temperatures, and pathological pain would be a false alarm caused by malfunction of the system itself. The net effect in all three cases is the sensation we call pain. However, because the processes that drive each are quite different, treatments must be targeted at the distinct mechanisms responsible.
_
The fundamental difference between nociceptive (including inflammatory) and neuropathic pain is the functioning of central and peripheral nervous system. When pain is felt with intact normally functioning nervous system, it is nociceptive pain but when pain is felt due to dysfunction and/or damage to nervous system, it is neuropathic pain.
_
Neuropathic pain:
Neuropathic pain is due to dysfunction of the pain perception system within the peripheral or central nervous system as a result of injury, disease, or surgical damage (e.g. continuing pain experienced from a limb which has been amputated – phantom limb pain). It is a pathological pain which is a disease state caused by damage or dysfunction of the nervous system. Neuropathic pain is pain in which there are structural and/or functional nervous system adaptations secondary to injury, that take place either centrally or peripherally (Jensen, 1996). Much of what has previously been considered psychogenic pain is now better understood as neuropathic pain of central origin. The IASP defines central pain as “pain initiated or caused by a primary lesion or dysfunction in the central nervous system” (Merskey, and Bogduk, 1994). “Neuropathic” should not be confused with “neurogenic”, a term used to describe pain resulting from injury to a peripheral nerve but without necessarily implying any “neuropathy”.
_
The figure below depicts various neuropathic pain syndromes.
_
Neuropathic pain can be defined as pain initiated or caused by a primary lesion or dysfunction in the nervous system resulting from;
1. Trauma, for example, complex regional pain syndrome, chronic post-surgical pain;
2. Infection, for example, post-herpetic neuralgia;
3. Ischaemia, for example, diabetic neuropathy;
4. Cancer;
5. Chemically induced, for example, as a result of chemotherapy (Farquhar-Smith, 2007).
_
Some types of neuropathic pain may develop when the peripheral nerves have become damaged, causing the pain fibers to transmit pain impulses repetitively and become increasingly sensitive to stimuli. Neuroplasticity may also develop and is characterized by abnormal neuronal sprouting in the peripheral nerves and within the dorsal horn of the spinal cord. This sprouting may result in additional generation of and increased transmission of pain impulses.
_
Characteristics of neuropathic pain:
__
The International Association for the Study of Pain (IASP) classified pain according to specific characteristics:
_
_
_
_
_
_
_
This system has been criticized by some pain researchers as inadequate for guiding research and treatment.
_
Physicians and neuroscientists generally classify pain in the following ways:
1. Acute pain is caused by an injury to the body. It warns of potential damage that requires action by the brain, and it can develop slowly or quickly. It can last for a few minutes to six months and goes away when the injury heals.
2. Chronic pain persists long after the trauma has healed (and in some cases, it occurs in the absence of any trauma). Chronic pain does not warn the body to respond, and it usually lasts longer than six months.
3. Cancer (or malignant) pain is associated with malignant tumors. Tumors invade healthy tissues and exert pressure on nerves or blood vessels, producing pain. Cancer pain can also be associated with invasive procedures or treatments. Some physicians classify cancer pain with chronic pain.
_
_
Note that there is no consensus regarding differentiating acute pain from chronic pain based on duration of pain; whether 3 months or 6 months; and different authors have differing view on this subject.
_
Another way to classify pain is depending on the type of nerve fiber which carries pain impulse.
1. Fast pain transmitted by thinly myelinated A-delta fibers conduct at rates of 12–30 m/s. Fast pain felt within about 0.1 second after a pain stimulus is applied. Felt when a needle is stuck into the skin, when the skin is cut with a knife, or when the skin is acutely burned. Not felt in deeper tissues of the body.
2. Slow pain transmitted by unmyelinated C fibers conduct at low rates of 0.5–2 m/s. Slow pain begins only after 1 second or more and then increases slowly over many seconds and sometimes minutes. Usually associated with tissue destruction.
_
Acute pain:
Acute pain has been defined as “pain associated with tissue damage, inflammation, or a disease process that is of relatively brief duration (i.e. hours, days, or even weeks), regardless of its intensity.” Acute pain results from
1. Disease, inflammation, or injury to tissues.
2. Sudden onset e.g., after surgery or trauma
3. May be accompanied by anxiety or emotional distress
The cause can usually be diagnosed and treated confined to a given period of time and severity. Acute pain persists until healing takes place or stops long before healing has been completed. Healing can occur without medical intervention as an injury with acute pain does not overwhelm the body’s reparative mechanisms. Such healing usually takes a few days to a few weeks, and therefore acute pain lasts for the same duration. Additionally, acute pain has been associated with anxiety. The clinical observation that greater the anxiety the greater the perception of an injury as painful appears warranted. However, a clear empirical basis for this simple proposition does not exist. Different studies indicate that anxiety enhances, relieves or has no impact on pain.
_
In the United States alone, nearly 100 million surgeries take place annually. More than 80% of these surgical patients report postoperative pain. Over 70% of emergency department visits are due to pain; acute headache alone accounts for 2.1 million of these visits. Despite substantial advances in pain research in recent decades, inadequate acute pain control is still more the rule than the exception. Numerous studies show that fewer than half of postoperative patients receive adequate pain relief. Patients arriving at emergency departments with significantly painful conditions fare no better, as emergency medicine physicians tend to underuse pain medications. Acute pain is also a common problem in family practice, sports medicine, and especially in internal medicine.
_
Chronic pain:
Chronic pain has been defined as pain that persists for extended periods of time (i.e. months or years), that accompanies a disease process (e.g. rheumatoid arthritis), or that is associated with an injury that has not resolved within an expected period of time (e.g. myofascial pain syndromes, complex regional pain syndrome, and chronic pelvic pain) or persists even when injury has resolved. Chronic pain is widely believed to represent disease itself rather than symptom of a disease.
1. It can be made much worse by unpleasant environmental and psychological factors.
2. It persists over a longer period of time than acute pain.
3. It is resistant to most medical treatments.
4. It causes severe problems for the patient.
5. Chronic pain indicates that the pain has lost its biological role of triggering recuperative behavior.
6. Chronic pain, although triggered by injury or disease, however, has other factors associated with it that prolong its presence. These factors include continued tissue damage, loss of a body part, extensive trauma, or damage to the nervous system as a result of injury.
7. Due to these factors, the pain persists either beyond the expected course of disease, or beyond the time expected for an injury to heal, or it recurs at various times for months or years. In such situations, the injury may exceed the body’s capability to heal. Additionally, intensity of chronic pain may be out of proportion of original injury or damage, and syndromes, such as complex regional pain syndrome, may occur spontaneously without any signs of injury.
8. Chronic pain impairs an individual’s social, vocational and psychological well being. Among psychological factors, chronic pain has been frequently associated with depression, which may vary from minor to severe.
9. Depression also appears to intensify chronic pain. While some patients display depression, others maintain a dispassionate attitude. Patients with a dispassionate attitude appear to have either strong personal or social resources or the pain disorder provides a focus in life that enables them to ignore stressful life challenges, thereby controlling depression.
_
Phantom pain:
Phantom pain is pain from a part of the body that has been lost or from which the brain no longer receives signals. It is a type of neuropathic pain. Phantom limb pain is a common experience of amputees. The prevalence of phantom pain in upper limb amputees is nearly 82%, and in lower limb amputees is 54%. One study found that eight days after amputation, 72 percent of patients had phantom limb pain, and six months later, 65 percent reported it. Some amputees experience continuous pain that varies in intensity or quality; others experience several bouts a day, or it may occur only once every week or two. It is often described as shooting, crushing, burning or cramping. If the pain is continuous for a long period, parts of the intact body may become sensitized, so that touching them evokes pain in the phantom limb, or phantom limb pain may accompany urination or defecation.
_
When a limb is severed, the parts of the nerves past the point of separation are lost along with their nerve endings. Your brain no longer receives much electric impulse from these regions as a result. However, with time, the sensory deprivation undergone by these nerve centers makes them especially sensitive to even mild stimulus, just as your eyes become more sensitive to light after being in a dark room. As a result, even the mildest stimulation, often triggered by memories of past sensations, can be recognized by your brain as much stronger, strong enough in fact to seem very real. It is for this reason that people sometimes describe sensations of pain in limbs that were previously arthritic, or the sensation of resting their limb on the arm of a chair or a table when the limb has long since been removed. It is also for this reason that the sensations may actually seem stronger when some time has passed since the surgery. Phantom pains are not always triggered by memory. Especially severe phantom pains are often the result of an incorrectly performed surgery. Pain in a missing limb is sometimes associated with other sometimes temporary biological factors, as well. Minor to severe illness can cause an escalation in the degree of phantom pain, often to levels that should be reported to a doctor. While analgesics are the most obvious solution to phantom pain, there are other routes that an amputee can take to help relieve the pain. Warmth and exercise are known to increase circulation and to reduce the intensity of your pains.
_
Local anesthetic injections into the nerves or sensitive areas of the stump may relieve pain for days, weeks or, sometimes permanently, despite the drug wearing off in a matter of hours; and small injections of hypertonic saline into the soft tissue between vertebrae produces local pain that radiates into the phantom limb for ten minutes or so and may be followed by hours, weeks or even longer of partial or total relief from phantom pain. Vigorous vibration or electrical stimulation of the stump, or current from electrodes surgically implanted onto the spinal cord all produce relief in some patients. Paraplegia, the loss of sensation and voluntary motor control after serious spinal cord damage, may be accompanied by girdle pain at the level of the spinal cord damage, visceral pain evoked by a filling bladder or bowel, or, in five to ten per cent of paraplegics, phantom body pain in areas of complete sensory loss. This phantom body pain is initially described as burning or tingling but may evolve into severe crushing or pinching pain, fire running down the legs, or a knife twisting in the flesh. Onset may be immediate or may not occur until years after the disabling injury. Surgical treatment rarely provides lasting relief.
_
Mirror therapy, mirror neurons and phantom pain:
Mirror therapy was first reported by Ramachandran in 1996 and is suggested to help phantom limb pain (PLP) by resolving the visual-proprioceptive dissociation in the brain. The patient watches the reflection of their intact limb moving in a mirror placed parasagittally between their arms or legs while simultaneously moving the phantom hand or foot in a manner similar to what they are observing so that the virtual limb replaces the phantom limb. The presence of mirror neurons in the brain is supported by the phenomenon of tactile sensation in the phantom limb elicited by touching the virtual image of the limb in the mirror. When a person with an intact limb observes a person with amputation, he can only “empathize about the amputation” rather than “feel it himself” because of the null input to the mirror neurons from his intact limb. However, a person with an amputation does not receive such null input as the limb is amputated and this result in the activation of mirror neurons which create a perception of tactile sensation. Consequently, since the activation of these mirror neurons modulates somatosensory inputs, their activation may block protopathic pain perception in the phantom limb. A randomized controlled trial of mirror therapy in patients with lower leg amputation has shown significant benefit of PLP versus the control group. Another controlled trial, however, reported that the mirror condition only elicited a significantly greater number of phantom limb movements than the control condition but did not attenuate phantom limb pain and sensations any more than the control condition.
_
Further evidence that the phenomenon of phantom limb is the result of a central representation is the experience of children born without limbs. Such individuals have rich phantom sensations, despite the fact that a limb never developed. This observation suggests that a full representation of the body exists independently of the peripheral elements that are mapped. Based on these results, Ronald Melzack proposed that the loss of a limb generates an internal mismatch between the brain’s representation of the body and the pattern of peripheral tactile input that reaches the neocortex. The consequence would be an illusory sensation that the missing body part is still present and functional. With time, the brain may adapt to this loss and alter its intrinsic somatic representation to better accord with the new configuration of the body. This change could explain why the phantom sensation appears almost immediately after limb loss, but usually decreases in intensity over time.
__
Referred pain:
_
Referred pain (also reflective pain) is pain perceived at a location other than the site of the painful stimulus. An example is the case of ischemia brought on by a myocardial infarction (heart attack), where pain is often felt in the neck, shoulders, and back rather than in the chest, the site of the injury. The International Association for the Study of Pain, as of 2001, has not officially defined the term; hence several authors have defined the term differently. Radiation is different from referred pain. The pain related to a myocardial infarction could either be referred pain or pain radiating from the chest. Classically the pain associated with a myocardial infarction is located in the mid or left side of the chest where the heart is actually located. The pain can radiate to the left side of the jaw and into the left arm. Referred pain is when the pain is located away from or adjacent to the organ involved. Referred pain would be when a person has pain only in their jaw or left arm, but not in the chest. The cardiac general visceral sensory pain fibers follow the sympathetics back to the spinal cord and have their cell bodies located in thoracic dorsal root ganglia 1-4. As a general rule, in the thorax and abdomen, general visceral afferent (GVA) pain fibers follow sympathetic fibers back to the same spinal cord segments that gave rise to the preganglionic sympathetic fibers. The central nervous system (CNS) perceives pain from the heart as coming from the somatic portion of the body supplied by the thoracic spinal cord segments 1-4. Also, the dermatomes of this region of the body wall and upper limb have their neuronal cell bodies in the same dorsal root ganglia (T1-5) and synapse in the same second order neurons in the spinal cord segments (T1-5) as the general visceral sensory fibers from the heart. The CNS does not clearly differentiate whether the pain is coming from the body wall or from the viscera, but it perceives the pain as coming from somewhere on the body wall, i.e. substernal pain, left arm/hand pain, jaw pain.
_
The following are some hypothesis to explain referred pain:
1. Common dermatome hypothesis: When pain is referred, it is usually to a structure that developed from the same embryonic segment or dermatome as the structure in which the pain originates. Radiating pain down the left arm is the result of a myocardial infarction, or pain originating from the shoulder (dermatomes 3-5).
2. Convergence and facilitation theories: Inputs from visceral and skin receptors converge on the same spinal cord neuron (i.e., viscerosomatic neurons). Therefore, visceral pain is referred to skin area because the nociceptors’ terminals from the viscera terminate in the spinal cord on the same neurons that receive input from the skin.
3. Facilitation or irritable focus: Pain impulses from the viscera alone are unable to pass directly from spinal cord neurons to the brain, but create an “irritable focus”. When visceral and skin impulses arrive together, the information transmitted to higher centers and the brain interprets the pain as being from the skin.
4. Learned phenomenon: Visceral information arrives in the CNS. However, the brain interprets that the impulses originate from the site of a previous surgical operation, trauma or localized pathologic process.
_
Breakthrough pain:
Breakthrough pain is pain that comes on suddenly for short periods of time and is not alleviated by the patients’ normal pain management. It is common in cancer patients who often have a background level of pain controlled by medications, but whose pain periodically “breaks through” the medication. The characteristics of breakthrough cancer pain vary from person to person and according to the cause.
_
Incident pain:
Incident pain is pain that arises as a result of activity, such as movement of an arthritic joint, stretching a wound, etc.
_
Pain asymbolia:
Pain without painfulness is found in patients who suffer from a rare neurological syndrome known as pain asymbolia. Characteristically, these patients feel pain upon harmful stimulation, but their pain no longer represents danger or threat to them. These patients do not mind pain at all; indeed, they may even smile or laugh at it.
_
Psychogenic pain:
Psychogenic pain also called psychalgia, is pain caused, increased, or prolonged by mental, emotional, or behavioral factors. Headache, back pain, and stomach pain are sometimes diagnosed as psychogenic. Sufferers are often stigmatized, because both medical professionals and the general public tend to think that pain from a psychological source is not “real”. However, specialists consider that it is no less actual or hurtful than pain from any other source. Recent research in neuroscience suggests that physical pain and psychological pain may share some underlying neurological mechanisms. Of course, the term ‘psychogenic’ assumes that medical diagnosis is so perfect that all organic causes of pain can be detected; regrettably, we are far from such infallibility… All too often, the diagnosis of neurosis as the cause of pain hides our ignorance of many aspects of pain medicine. Also, people with long term pain frequently display psychological disturbance, with elevated scores on the Minnesota Multiphasic Personality Inventory scales of hysteria, depression and hypochondriasis (the “neurotic triad”). Some investigators have argued that it is this neuroticism that causes acute injuries to turn chronic, but clinical evidence points the other way, to chronic pain causing neuroticism. When long term pain is relieved by therapeutic intervention, scores on the neurotic triad and anxiety fall, often to normal levels. Self-esteem, often low in chronic pain patients, also shows improvement once pain has resolved. Emotional pain is a particular kind of psychological pain, more closely related to emotions.
_ _ _
Neurobiology of pain:
The initial concept of pain perception was quite simple: the pain signal directly excited a peripheral nerve, synapsed at the spinal cord, and was perceived in the sensory cortex. There was no signal modulation; it just arrived in the brain as it came from the body. Like much in the rest of medicine, it was found to be not that simple. Neuroanatomical, physiological, psychological, and pharmacological research has shown that the pain pathway is amazingly complex, in keeping with its evolutionary importance to the survival of the organism. In addition, the pain signal is modulated at the site of the pain, at the dorsal horn, and in multiple areas of the central nervous system, both the spinal cord and the brain.
_
_
_
Neurobiology of pain can be divided into neurobiology of nociceptive and neuropathic pain. Also, delineating pain pathway can help alleviate pain by blocking various chemicals or nerves as seen in the table below.
| Pathway | Method of pain control |
| Cortex ^ |
General anesthetic |
| Thalamus ^ |
Opioids |
| Spino-thalamic tracts ^ |
Cordotomy |
| Dorsal horn of spinal cord ^ |
Opioids |
| Sensory (nerve) fiber ^ |
Local anesthetic |
| Nerve endings | Prostaglandin inhibitor |
_
Can one have pain and not know it? Pain vis-à-vis nociception:
It leads me to ponder the distinction between pain and nociception. If you were struggling to explain to someone the difference between pain and nociception, perhaps an easy way of doing so might be to point out that nociception may occur when someone is unconscious, whereas pain by definition cannot. A nociceptive, tissue-threatening stimulus evokes adaptive behavioral responses which aren’t necessarily intentional or reasoned, called nocifensive responses. They are to minimize or escape from noxious stimuli—like the flexor withdrawal response (muscle groups in the leg automatically bending the hip, knee and ankle after stepping on a spiky something). We don’t need to be conscious for nociception to occur. Pain however is an experience of the conscious brain, a sensory and emotional percept. The most potent way we have of suppressing someone’s pain is through general anesthetic, a process which globally eliminates sensory experience. It’s possible that even in an unconscious ‘pain-free’ state that nocifensive responses, like changes in cardiovascular rhythm, persist. So the key ingredient for pain is the conscious brain.
Pain= nociception + consciousness
_
Pain is subjective, and it cannot be ‘seen’ by anyone other than the person experiencing it. Even with neuroimaging we cannot assess it objectively. Imaging experiments tell us that there is a pattern, albeit variable, of spots in the brain that are active when someone is in pain. But no image can tell us that the pain experienced is rated by that brain as a 5 out of 10 pain, around L4/5, and can escalate to 9 out of 10 when you feel sad. The ‘pain neuromatrix’ (vide infra) is too widely distributed for that, and there’s immeasurable capacity for functional reorganization and redundancy in the system.
_
The distinction between pain and nociception provides the basis for focusing on pain as a psychological phenomenon. Nociception refers to the neurophysiologic processing of events that stimulate nociceptors and are capable of being experienced as pain (Turk & Melzack, 2000). Instigation of the nociceptive system and brain processing constitute the biological substrates of the experience. But pain must be appreciated as a psychological phenomenon, rather than a purely physiological phenomenon. Specifically, it represents a perceptual process associated with conscious awareness, selective abstraction, ascribed meaning, appraisal, and learning (Melzack & Casey, 1968). Emotional and motivational states are central to understanding its nature (Price, 2000). Pain requires central integration and modulation of a number of afferent and central processes (i.e., sending message toward the central nervous system and interacting with higher components of the central nervous system) and efferent processes (i.e., sending messages away from higher centers in the central nervous system and toward muscle or gland).
_
We have no ‘pain’ receptors. It is physiologically more correct to call them nociceptors because they are very similar to other receptors which sense temperature, pressure, and chemicals (called non-nociceptive receptors). The only difference between the two is that the nociceptors have a higher threshold than the non-nociceptors, and are only activated when the stimuli is in the higher range. Contrary to what most people believe, they don’t send ‘pain’ signals, they send the same signals as other non-nociceptors but just at a higher threshold.
_
Nociception:
Nociception is defined as “the neural processes of encoding and processing noxious stimuli.” It is the afferent activity produced in the peripheral and central nervous system by stimuli that have the potential to damage tissue. This activity is initiated by nociceptors, (also erroneously called pain receptors), that can detect mechanical, thermal or chemical changes above a set threshold. Once stimulated, a nociceptor transmits a signal along the spinal cord, to the brain. Nociception triggers a variety of autonomic responses and may also result in a subjective experience of pain in sentient beings.
_
Three neuron order for pain transmission:
First order Neuron: Cell body located in a spinal (dorsal root) ganglion, its peripheral process is associated with the receptor (nociceptor), while its central process enters the gray matter of the spinal cord to synapse in the Marginal Nucleus Substantia gelatinosa (lamina II) and Nucleus proprius.
_
Second order Neuron: Cell bodies located in the marginal nucleus and the nucleus proprius. Axons of second order neurons cross the midline and join other axons which also carry pain sensation. These axons form the Spinothalamic Tract in the spinal cord and ascend to brainstem.
_
Third order neuron: The axons of second order neurons synapse on third order neurons in the thalamus. The Thalamus is the crucial relay for the reception and processing of nociceptive information in route to the cortex. Axons terminating in the lateral thalamus mediate discriminative aspects of pain. Axons terminating in the medial thalamus mediate the motivational-affective aspects of pain (emotional aspects of pain; attention to and memory of pain). These third order neurons in the thalamus in turn send their axons to the cerebral cortex. Note: Neurons in the lateral thalamus (for discrimination) project to the somatosensory cortex. Neurons in the medial thalamus (for affective aspects of pain) project to other areas of cortex (prefrontal, insular and cingulate gyrus) and more importantly limbic system. A human becomes aware of painful stimuli at the level of the thalamus; the cerebral cortex is required for localization of the pain to a specific body region.
_
Nociception processes:
Acute pain is a physiological response that warns us of danger. The process of nociception describes the normal processing of pain and the responses to noxious stimuli that are damaging or potentially damaging to normal tissue. There are four basic processes involved in nociception (McCaffery and Pasero, 1999). These are:
1. Transduction;
2. Transmission;
3. Perception;
4. Modulation.
_
_
Nociceptors:
1. Nociceptors are found in all tissues of the body except nervous tissue.
2. Nociceptors convert noxious information into electrical information.
3. Nociceptors are sensitive to chemical, thermal, or mechanical stimuli.
4. At any one time, only 20% of the body’s nociceptors are activated, but in response to tissue injury and inflammation, up to 100% of the body’s nociceptor may be activated.
5. In response to injury, chemical changes occur within nociceptors to self regulate the body’s responses to the injury.
_
Nociceptor:
All nociceptors are specialized free nerve endings that have their cell bodies outside the spinal column in the dorsal root ganglia or trigeminal ganglia and are named according to their appearance at their sensory ends. The trigeminal ganglia are specialized nerves for the face, whereas the dorsal root ganglia associate with the rest of the body. In humans, the detection of peripheral pain begins at free nerve endings. It is here that the polymodal pain receptors TRPA1, TRPV1, TRPV2, ASICs, and high threshold mechanoreceptors detect noxious stimuli such as strong mechanical forces, H+, K+, chemicals, and temperature. Mechanical, thermal, and chemical stimuli are detected by nerve endings (nociceptors), which are found in the skin and on internal surfaces such as the periosteum or joint surfaces. The concentration of nociceptors varies throughout the body, mostly found in the skin and less so in deep internal surfaces. Internal nociceptors are in a variety of organs, such as the muscle, joint, bladder, gut and continuing along the digestive tract. Nociceptors have a certain threshold; that is, they require a minimum level of stimuli before they trigger a signal. Once this threshold is reached a signal is passed along the axon of the nerve into the spinal cord. In some conditions, excitation of pain fibers becomes greater as the pain stimulus continues, leading to a condition called hyperalgesia. The mechanical, thermal or chemical stimuli which activate nociceptors are called noxious stimuli. Activation of these receptors results in nerve impulses which travel along the afferent fibers in the nerves and are transmitted to the central nervous system (CNS). Some nociceptors do not differentiate noxious from non-noxious stimuli, while others respond only to painfully intense stimuli.
_
Transduction:
Transduction begins when the free nerve endings (nociceptors) of C fibers and A-delta fibers of primary afferent neurons respond to noxious stimuli. Nociceptors are exposed to noxious stimuli when tissue damage and inflammation occurs as a result of, for example, trauma, surgery, inflammation, infection, and ischemia.
_
The C fiber and A-delta fibers are associated with different qualities of pain. These are listed in table below depicting characteristics and functions of C fiber and A-delta fibers
| C fibers | A-delta fibers |
Characteristics:
Receptor type:
Pain quality:
|
Characteristics:
Receptor type:
Pain quality:
|
_
Noxious stimuli and responses
There are three categories of noxious stimuli:
1. Mechanical (pressure, swelling, abscess, incision, tumor growth);
2. Thermal (burn, scald);
3. Chemical (excitatory neurotransmitter, toxic substance, ischemia, infection).
_
The cause of stimulation may be internal, such as pressure exerted by a tumor or external, for example, a burn. Chemical mediators that are released after tissue damage or inflammation can either activate or sensitize the nociceptors.
Activators of primary afferent fibers are:
Potassium – from Damaged Cells
Serotonin – from Platelets
Bradykinin – from Plasma Kininogen
Histamine – from Mast Cells
Sensitizers of primary afferent fibers are:
Prostaglandins – from Arachidonic acid
Leukotrienes – from Arachidonic acid
Substance P – Primary afferent
These chemical mediators activate and/or sensitize nociceptors to the noxious stimuli. In order for a pain impulse to be generated, an exchange of sodium and potassium ions (depolarization and repolarization) occurs at the cell membranes of first order sensory neuron.This results in an action potential and generation of a pain impulse. Non-steroidal anti-inflammatory drugs (NSAIDs) prevent pain by inhibiting the metabolism of arachidonic acid to prostaglandins by inhibiting enzyme cyclo-oxygenase; NSAIDs do not effect lipoxygense which is involved in leukotriene production.
_
__
Transduction and ion channels:
Transduction is a physiological process whereby a noxious mechanical, chemical, or thermal stimulus is converted (transduced) via specialized receptors on primary afferents into an electrical impulse (action potential). Transduction of noxious stimuli occurs at nociceptors, a subpopulation of primary sensory neurons that are activated by intense stimuli such as pressure, heat, mechanical insults (a surgical incision) or irritant chemicals (including those which are released by damaged cells). Some of these chemicals such as bradykinin, cholecystokinin and prostaglandins, activate or sensitize nearby nociceptors. Strong physical stimuli (heat or pressure) and disease processes (inflammation) cause tissue damage and release a variety of chemicals. Once released, these chemicals bind and activate specific receptors on the nerve endings of small diameter nerve fibers, increase the excitability of the neuronal cell membrane and lead to generation of propagated action potentials. In addition to this electrochemical reaction, secondary messengers are released and activated, which then interact with the nucleus of the neuron to modulate response properties of affected neurons, leading to a state of peripheral sensitization in certain circumstances. Action potentials can be generated at differing distances from the terminal ending depending on the extent of depolarization of the fiber and resulting inactivation of voltage-gated channels involved in conduction. Theoretically, the depolarizing receptor potential can be accomplished by multiple membrane conductance changes and electrogenic pump activity. Since the electrochemical gradients for sodium (Na+), calcium (Ca+2), and chloride (Cl–) are more positive than the resting membrane potential in sensory neurons, the opening of ion channels permeable to these ions will cause the membrane potential to shift in the positive direction (depolarize). Since the electrochemical gradient for potassium (K+) is more negative than resting potential, closure of active potassium channels not only depolarizes the membrane potential but amplifies current-induced voltage fluctuations due to the resulting increase in membrane resistance.
_
Nociceptors express a wide variety of voltage-gated channels (e.g., Nav, Cav, Kv) that transduce the receptor potential into an action potential or, more commonly, a set of action potentials that encode the intensity of a noxious stimulus applied within their receptive fields. There are 9 known Nav, 10 Cav, and 40 Kv genes in mammals, many of which have multiple splice variants with different functional characteristics. Cell excitability and firing behavior (e.g., threshold for action potential generation, action potential and undershoot amplitude and duration, and maximal firing frequency) depend on the complement of these channels as well as those contributing to frequency modulation (e.g., hyperpolarization-activated cyclic nucleotide-gated cation channel [HCN] and A-type Kv4.3 and Kv3.4 channels). For instance, nociceptors responsive to noxious cold require the expression of the tetrodotoxin-resistant (TTX-resistant) Nav1.8 channel at the peripheral terminal, and mice lacking Nav1.8 and Nav1.7 display deficits in mechanosensation. Peripheral CGRP release by inflammatory mediators is unaffected by TTX, suggesting an important role of TTX-resistant Nav in regulated pain thresholds, consistent with their robust modulation by bradykinin (BK) and PGE2. Since enhanced excitability of primary sensory neurons in inflammatory and pathologic pain states is a major contributor to the perception of pain, specific pharmacological agents that specifically dampen aberrant activity are desirable in the design of pain therapeutics. To this end, an understanding of species-specific differences is critical, as exemplified by the dramatically different phenotypes in mice and humans lacking Nav1.7: although mice lacking Nav1.7 show a mechanosensory (pinch) and formalin-induced (5%) pain phenotype, humans lacking Nav1.7 are insensitive to pain altogether.
_
Na channel (sodium channel):
Voltage-dependent Na+ channels in sensory nerves contribute to the control of membrane excitability and underlie action potential generation. Na+ channel subtypes exhibit a neurone-specific and developmentally regulated pattern of expression, and changes in both channel expression and function are caused by disease. Recent evidence implicates specific roles for Na+ channel subtypes Nav1.3 and Nav1.8 in pain states that are associated with nerve injury and inflammation respectively. Insight into the role of Nav1.8 in pain pathways has been gained by the generation of a null mutant. Moreover, evidence that the Nav1.3 Na+ channel, upregulated in axotomy, plays a significant role in neuropathic pain states is accumulating. The development of selective blockers of Na+ channel subtypes, combined with an analysis of tissue-specific and inducible knockouts of various channel isoforms, should illuminate the possible use of targeting Na+ channels to induce analgesia.
_
Cl channel (chloride channel):
Although glycine receptor Cl− channels (GlyRs) have long been known to mediate inhibitory neurotransmission onto spinal nociceptive neurons, their therapeutic potential for peripheral analgesia has received little attention. However, it has been shown that α3-subunit-containing GlyRs are concentrated into regions of the spinal cord dorsal horn where nociceptive afferents terminate. Furthermore, inflammatory mediators specifically inhibit α3-containing GlyRs, and deletion of the murine α3 gene confers insensitivity to chronic inflammatory pain. This strongly implicates GlyRs in the inflammation-mediated disinhibition of centrally projecting nociceptive neurons. Future therapies aimed at specifically increasing current flux through α3-containing GlyRs may prove effective in providing analgesia.
_
Ca channel (calcium channel):
_
_
The figure above shows potential mechanisms of potentiation, prevention and de-potentiation at synapses between C-fibers and spinal cord projection neurons. Conditioning electrical nerve stimulation or natural noxious stimulation triggers release of glutamate and substance P which causes opening of NMDA receptor channels and T-type voltage-gated Ca2+ channel and Ca2+ release from intracellular stores. This activates Ca2+-dependent signal transduction pathways including protein kinases and transcription factors. Synaptic strength is probably increased by phosphorylation of synaptic proteins including AMPA receptor channels, altered trafficking of synaptic proteins, e.g. increased insertion of AMPA receptors into the sub-synaptic membrane and de-novo protein synthesis. According to this model, LTP can be prevented if release of glutamate and/or substance P is inhibited, for example by activation of pre-synaptic, G-protein-coupled mu-opioid receptors, or if opening of voltage sensitive and Ca2+ permeable ion channels is blocked, e.g. via postsynaptic inhibition by an opioid. Depotentiation could result from de-phosphorylation of synaptic proteins, changes in receptors trafficking and degradation of synaptic proteins.
_
Transmission:
The transmission process occurs in three stages. The pain impulse is transmitted:
1. from the site of transduction along the nociceptor fibers to the dorsal horn in the spinal cord;
2. from the spinal cord to the brain stem;
3. through connections between the thalamus, cortex and higher levels of the brain.
_
Once transduced and generated, action potentials (nerve impulses) are conducted to the central nervous system primarily via two types of primary afferent neurons: thinly myelinated, faster conducting A delta fibers and unmyelinated, slowly conducting C fibers, both termed primary afferents. Action potentials result from activation of specific sodium channels. Nociceptive impulses travel along these peripheral nerve fibers (peripheral transmission) to the dorsal horn of the spinal cord where they synapse with the second order neurons (synaptic transmission). Here, the impulse is further transmitted via neurons which cross the spinal cord and ascend to the thalamus and branches to the brainstem nuclei (central transmission). The nociceptive impulses are then relayed to multiple areas of the brain including the somatosensory cortex, the insula, frontal lobes and limbic system. Once the action potential from the periphery reaches the first synapse at the dorsal horn of the spinal cord, a number of neuroactive substances are released. These include several excitatory and inhibitory neurotransmitters and glial derived chemicals that participate in the immune responses. Astrocytes and microglia in the dorsal horn are also involved and the sum of activity at the synapse results from release of excitatory and inhibitory neurotransmitters, which ultimately lead to generation of action potentials and central transmission of pain signals to higher centers.
__
The C fiber and A-delta fibers terminate in the dorsal horn of the spinal cord. There is a synaptic cleft between the terminal ends of the C fiber and A-delta fibers and the nociceptive dorsal horn neurons (NDHN). In order for the pain impulses to be transmitted across the synaptic cleft to the NDHN, excitatory neurotransmitters are released, which bind to specific receptors in the NDHN. These neurotransmitters are:
Adenosine triphosphate;
Glutamate;
Calcitonin gene-related peptide;
Bradykinin;
Nitrous oxide;
Substance P.
_
Polypeptides contained within the primary afferent nociceptors (substance P, somatostatin, vasoactive intestinal polypeptide and cholecystokinin) are all present in different populations of small diameter unmyelinated primary afferents which terminate in the superficial dorsal horn.
Substance P – excites pain transmission neurons in dorsal horn.
Capsaicin (hot pepper extract) – destroys substance P and reduces pain response.
Peripheral response of Substance P: released from peripheral terminals of nociceptors; found in nerve fibers on blood vessels causes cutaneous wheal and flare reaction of inflammation. Capsaicin as an antagonist of substance P could provide a new approach to control of inflammation and pain; capsaicin cream is now used to treat pain associated with Herpes zoster.
_
Local anesthetics and some antiepileptic drugs block sodium channels and inhibit the production of action potentials along the nociceptive afferents. Opioids bind to presynaptic receptors in the dorsal horn and decrease release of neurotransmitters such as glutamate. Peripheral and spinal nerve blocks interfere with propagation of action potentials and pain transmission into the CNS (at the nociceptors, along the nerve, at the dorsal root ganglion and along the spinothalamic tracts). Epidurally and intrathecally administered analgesics may provide presynaptic and postsynaptic inhibition of receptors at the level of dorsal horn neurons and affect the transmission of nociceptive impulses.
_
Before reaching the brain, the spinothalamic tract splits into the lateral “neospinothalamic” tract and the medial “paleospinothalamic” tract.
1. Neospinothalamic tract:
Fast pain travels via type A-delta fibers to terminate in the dorsal horn of the spinal cord where they synapse on dendrites of the neospinothalamic tract. The axons of these neurons cross the midline (decussate) through the anterior white commissure and ascend contralaterally along the anterolateral columns. These fibers terminate on the ventrobasal complex of the thalamus and synapse with the dendrites of the somatosensory cortex. Fast pain is felt within a tenth of a second of application of the pain stimulus and is a sharp, acute, prickling pain felt in response to mechanical and thermal stimulation. It can be localized easily.
2. Paleospinothalamic tract:
Slow pain is transmitted via slower type C fibers to laminae II and III of the dorsal horns, together known as the substantia gelatinosa. Impulses are then transmitted to nerve fibers that terminate in lamina V, also in the dorsal horn, synapsing with neurons that join fibers from the fast pathway, crossing to the opposite side via the anterior white commissure, and traveling upwards through the anterolateral pathway. These neurons terminate throughout the brain stem, with one tenth of fibers stopping in the thalamus and the rest stopping in the medulla, pons and periaqueductal grey of the midbrain tectum. Slow pain is stimulated by chemical stimulation, is poorly localized and is described as an aching, throbbing or burning pain.
_
__
Perception:
Perception is the process by which a noxious event is recognized as pain by a conscious person. Multiple areas of the brain are involved. There is no one location where perception occurs, although major defining components of pain are attributed to processes that take place in specific areas of the brain. Perception of pain is the end result of the neuronal activity of pain transmission and where pain becomes a conscious multidimensional experience. The multidimensional experience of pain has affective-motivational, sensory-discriminative, emotional and behavioral components. When the painful stimuli are transmitted to the brain stem and thalamus, multiple cortical areas are activated and responses are elicited. These areas are:
1. The reticular system: This is responsible for the autonomic and motor response to pain and for warning the individual to do something, for example, automatically removing a hand when it touches a hot saucepan. It also has a role in the affective-motivational response to pain such as looking at and assessing the injury to the hand once it has been removed from the hot saucepan.
2. Somatosensory cortex: This is involved with the perception and interpretation of sensations. It identifies the intensity, type and location of the pain sensation and relates the sensation to past experiences, memory and cognitive activities. It identifies the nature of the stimulus before it triggers a response, for example, where the pain is, how strong it is and what it feels like.
3. Limbic system: This is responsible for the emotional and behavioral responses to pain for example, attention, mood, and motivation, and also with processing pain and past experiences of pain.
_ _
Picture below shows brain areas functionally related to pain processing.
_
Pain has an inseparable affective-emotional component that defines the response and associated behavior resulting from the initiating noxious event or stimulus. Cognitive-behavioral therapies such as distraction, relaxation, and imagery operate at this level of the pain pathway. Some patients have a dominant affective emotional component and present with increased pain behaviors, anxiety, and depression that must be treated in order to achieve effective pain control.
_
In a nutshell:
The sensation of pain travels from the periphery to the spinal cord along A-delta and C fibers. These first order neurons enter the spinal cord via Lissauer’s tract. Because the A-delta fiber is thicker than the C fiber and is thinly sheathed in an electrically insulating material (myelin), it carries its signal faster (5–30 m/s) than the unmyelinated C fiber (0.5–2 m/s). Pain evoked by the (faster) A-delta fibers is described as sharp and is felt first. This is followed by a duller pain, often described as burning, carried by the C fibers. A-delta and C fibers synapse on second order neurons in substantia gelatinosa (laminae II and III of the dorsal horns). These second order neurons (spinothalamic tract) then decussate, crossing via the anterior white commissure before ascending contralaterally. Before reaching the brain, the spinothalamic tract splits into the lateral neospinothalamic tract and the medial paleospinothalamic tract. Second order neospinothalamic tract neurons carry information from A-delta fibers and terminate at the ventral posterolateral nucleus of the thalamus, where they synapse on third order neurons. Paleospinothalamic neurons carry information from C fibers and terminate throughout the brain stem, a tenth of them in the thalamus and the rest in the medulla, pons and periaqueductal grey matter. Pain-related activity in the thalamus spreads to the insular cortex (thought to embody, among other things, the feeling that distinguishes pain from other homeostatic emotions such as itch and nausea) and anterior cingulate cortex (thought to embody, among other things, the motivational element of pain); and pain that is to be distinctly located activates the primary and secondary somatosensory cortices. In the cortex, the primary and secondary somatosensory areas participate in nociception. The primary somatosensory area consists of postcentral gyrus and the secondary somatosensory area includes the superior lip of the lateral sulcus. The ventrobasal and intralaminar complexes of the thalamus project somatotopically to both primary and secondary somatosensory areas. Most cells in the primary somatosensory area are modality specific: they respond to either light touch, deep pressure, warming, cooling, or tooth pulp stimulation (pain). In the secondary somatosensory area, specific and nonspecific polymodal cells are found.
_
Parallel pathways originating from different nociceptors:
Pain consists of a sensory component (the detection and transmission of painful stimuli) and an affective component (the processing of emotional and behavioral responses). Researchers have shown that different populations of nociceptors — neurons that sense painful stimuli — might engage parallel ascending pathways that target limbic (affective) and motor regions of the brain. A major unanswered question concerning “pain” circuitry is the extent to which different populations of primary afferent nociceptors engage the same or different ascending pathways. In a study, researchers followed the transneuronal transport of a genetically expressed lectin tracer, wheat germ agglutinin, in Na(V)1.8-expressing nociceptors of the nonpeptide class. They found that interneurons of lamina II are at the origin of the major ascending circuits targeted by the nonpeptide nociceptors. These interneurons contact lamina V projection neurons, which in turn predominantly target fourth-order neurons in the amygdala, hypothalamus, bed nucleus of the stria terminalis, and to a remarkable extent, the globus pallidus. These circuits differ greatly from the lamina I-based projection that is targeted by the peptide class of nociceptors. The results indicate that parallel, perhaps independent pain pathways arise from different nociceptor classes and that motor as well as limbic targets predominate in the circuits that originate from the nonpeptide population.
_
Autonomic nervous system (ANS) and pain:
Pain signals can set off autonomic nervous system pathways as they pass through the medulla, causing increased heart rate and blood pressure, rapid breathing and sweating. The extent of these reactions depends upon the intensity of pain, and they can be depressed by brain centers in the cortex through various descending pathways.
_
How brain influence pain perception:
Several observations lead scientists to think that the brain does influence pain perception.
1. The pain from the cut on your hand eventually subsides or reduces to a lower intensity.
2. If you consciously distract yourself, you don’t think about the pain and it bothers you less.
3. People given placebos for pain control often report that the pain ceases or diminishes.
_
Pain is in your brain:
When these ‘warning’ signals from the nociceptors reach the brain, it is up to the brain to decide whether it is indeed a real danger or not. You will not feel pain unless and until the brain believes that there is a threat to the body and hence an action is required. This has been shown in numerous studies both in animals and humans. In other words, it’s not the signals that go to the brain from the body that matters, it’s what the brain decides to do with these signals that matters. This perhaps explains the countless examples we see of how people come to the emergency room with limbs missing and other horrific injuries, but feel no pain whatsoever. The likely explanation is that if the brain indeed thought that the missing limb or the injury was highly threatening, you will be crouched, caring for your wound and will most likely succumb to your injuries. If you think about it, pain does not serve a protective purpose when survival is at stake. However, people coming to emergency room with severed limb without pain would be an exception and not a rule. Also, not feeling pain with severed limb does not necessarily mean that pain has no survival value as world has some weird people who do behave aberrantly.
_
Researchers compared the degree to which people felt pain from similar injuries based on what the individuals thought the pain meant. Those who did not place a major consequence on the pain did not feel as great an intensity of pain as those who thought their pain indicated a life-threatening illness. Do not make the mistake, though, of thinking that this higher intensity of pain is any less real, or that the feeling of depression and anxiety you may see in your loved one, is not real. The person truly feels the pain and fear, and the depression.
_
We now know from imaging and animal studies that persistent pain or pain which lasts for months and years can change the pain pathways – peripheral receptors-spinal cord-brain (physically, functionally and chemically) to make it a lot more sensitive. And this hypersensitivity causes the brain to interpret anything related to those tissues to be highly threatening. Just like the concept, ‘the more your practice, the better you become at performing the skill’, the longer your pain persists, the more efficient the nervous system and the brain becomes in triggering and maintaining pain. Hence in chronic pain, pain has moved up to the nervous system and now has very little to do with the initial damage to the tissues that caused the pain. So some times when folks are in chronic pain, they did not get hurt or they did not re-injure themselves as many think. It is just that your brain and nervous system has become so good at constructing pain at the slightest of triggers –even those that don’t cause damage. These triggers could be in the form of a slight pressure on the affected tissues or nearby tissues or just even the thought of the injury-causing incident. Chronic pain has been a mystery because we were just looking at the tissues and joints while ignoring the nervous system and the brain. But it is in the brain and the nervous system that the action happens!
_
The role of the Cerebral Cortex in pain perception:
_
Brain tissue is not sensitive to pain! The brain itself does not have any receptors for pain. In fact, most brain surgery is performed using a local anesthetic only. The meninges (coverings of the brain), however, are very sensitive to pain. The experience of pain can only be defined in terms of human consciousness. However, anatomical studies in animals together with functional imaging studies in humans have made it possible to identify the main cerebral components of the human nociceptive system. These components comprise at least two main human nociceptive systems working in parallel, called the medial and lateral pain systems. This anatomical evidence has provided a physical construct for the concept of the human pain matrix (Melzack 1990). In addition, other nociceptive pathways have been identified in rodents that have not yet been described in primates.
_
There is a wide spectrum of pain experience, ranging from pain that may closely reflect physical events in tissue (e.g., events leading to excitation of nociceptors and hence nociceptive pain) to pain that is generated without any peripheral physical input (e.g., psychogenic and neuropathic pain). All these pains are equally valid and can only be recorded in terms of the individual’s subjective experience. A number of electroencephalographic (EEG) and functional imaging studies have demonstrated that changing the psychological context of a stimulus, in terms of attentional instruction or anticipation can completely alter neuronal activity within the pain matrix. The brain is therefore acting as a virtual reality system that may or may not be constrained by interactions with the body’s internal and external environment.
_
Electrophysiological pain-evoked potentials, i.e., electroencephalographic (EEG) frequency analysis and magnetoencephalography (MEG), as well as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) provide access to nociceptive processing mainly within the brain. However, recent studies have extended some of these techniques to the spinal cord (Gage et al. 2001). PET has provided the means to measure both metabolic and neurochemical aspects of nociceptive processing. This technique has allowed the identification of receptor systems and changes in their occupation during acute and chronic pain (Jones et al. 1994, 1999; Zubieta et al. 2001) in addition to imaging aspects of cholinergic (Gage et al. 2001) and dopaminergic transmission (Jaaskelainen et al. 2001). The great advantage of fMRI over PET is that it is possible to make repeated measures of nociceptive responses without the constraints imposed by the use of radioactivity, allowing much more complex experimental design. The weakness of all these techniques is that in the final analysis, we are left with significant activation sites in brain volumes without directional information and without information about the ascending or descending nociceptive inputs from which they result. They can therefore only be interpreted with reference to detailed anatomical and pharmacological studies derived from animal studies (Vania et al. 1989a,b,c; Sikes and Vogt 1992) and from human post-mortem studies (Bowsher 1957). Such interpretations, together with information from evoked potentials (Chen et al. 1998) and MEG (Hari et al. 1983) can begin to provide a working model of the circuitry concerned with nociceptive processing.
_
Human pain matrix:
Unfortunately there is no discrete centre where pain is recognized. Pain is so important to survival that almost the whole brain is involved. Pain involves cognition, emotion, and behavior. Therefore it is not surprising that PET scan data obtained during painful stimulation indicates activity in the following cortical areas. Brodmann’s tissue classification follows in brackets.
1. Sensory and motor cortex areas……………………………. (1-4)
2. Premotor cortex (for planning of movement)…………….. (6)
3. Other parts of the parietal cortex……………………………. (7 and 37-40)
4. Other parts of the frontal cortex……………………………… (8-10 and 43-47)
5. Cingulate cortex………………………………………………. (24 and 32)
6. Insula……………………………………………………………… (14)
7. Occipital cortex………………………………………………….. (19)
_
Pain also projects to the following subcortical structures:
Thalamus, putamen, caudate nucleus, hypothalamus, amygdala, periaqueductal grey matter, hippocampus, red nucleus, pulvinar, and vermis of the cerebellum (Wall,1996).
_
All of these support the claim that “the reign of pain is mainly in the brain”. But there is no one center “in control”. Rather we see that pain can be all-pervasive, affecting our thoughts and memories, attitudes and emotions, movements and behavior — and in turn be affected by each and all of them. So all the cortical and subcortical areas involved in pain processing is called “Human Pain Matrix” or Pain Neuromatrix.
_
Many years ago, single-unit recordings in the monkey established that nociceptive pathways in the somatosensory system project to areas 3b and 1 of the primary somatosensory cortex (Kenshalo and Willis 1991), as well as to the secondary somatosensory cortex (S2) and the neighboring posterior parietal cortex (Dong et al. 1989). It has been widely accepted that pain is a multidimensional experience that has sensory-discriminative, affective, motivational, and evaluative components (Melzack and Casey 1968). It has been suggested that these different components are likely to be processed within a “neuromatrix,” rather than in one center (Melzack 1990). Functional imaging experiments have identified such a matrix. A number of cortical structures such as S1 and S2, the anterior insula, and cingulate and dorsolateral prefrontal cortices are reproducibly involved in nociceptive processing (Derbyshire 1999; Peyron et al. 2000), as are subcortical structures including the amygdala (Derbyshire et al. 1997) and the thalamus and hypothalamus (Hsieh et al. 1996, 2001). If pain results from integrating processing within such a matrix, then it should not be surprising that ablation of one component of that matrix may not have immediately obvious effects, if other components of the matrix are able to compensate in some way. A clue to this possibility comes from the predominantly bilateral nociceptive inputs to most cortical components of the matrix on both anatomical and functional grounds (Schlereth et al. 2003; Youell et al. 2004). This parallel processing probably provides for considerable redundancy within this system, which is so essential for species survival.
_
The lateral pain system comprises the lateral thalamic nuclei and the somatosensory cortices. It is fast and somatotopic and may subserve the sensory-discriminative aspects of pain, which include localization, intensity, and duration. The insular cortex also has some somatotopic nociceptive inputs and may be involved in integrating them with inputs from other sensory modalities (Ostrowsky et al. 2002).
_
The medial pain system is slow (polysynaptic) and non-somatotopic, and is thought to process the affective components of pain (Treede et al. 1999). It includes the medial thalamic nuclei, anterior cingulate and dorsolateral prefrontal cortices, and possibly structures concerned with the processing of fear, such as the amygdala.
_
The main division of function between the medial and lateral pain systems is likely to be that of affective and sensory-discriminatory processing, respectively, with intensity probably being processed throughout the matrix.
_
The cingulate cortex, nociception, and the endogenous opioid system:
The role of the cingulate cortex in processing a variety of cognitive, motor, and nociceptive information has been well described (Vogt et al. 1993; Devinsky et al. 1995; Paus et al. 1998; Bush et al. 2000). The observation that the anterior cingulate cortex is the most commonly activated structure in functional imaging studies of pain (Derbyshire 1999) suggests that it has a pivotal role in nociceptive processing. There is substantial interindividual and interstudy variability of the location of anterior cingulate responses to nociceptive stimuli (Vogt et al. 1996; Derbyshire 2000). These locations were shown to extend from the more rostral perigenual cingulate to the caudal mid-cingulate cortex in studies using fMRI, PET, and EEG (Bentley et al. 2002b). The activity within the perigenual cingulate cortex during attention to unpleasantness is consistent with its relatively high concentration of opioid receptors (Jones et al. 1991b; Vogt et al. 1995a) as compared to the more executive areas of the cingulate cortex, and also with the perigenual changes in occupation by endogenous opioid peptides during chronic pain (Jones et al. 1994, 1999; Jones and Derbyshire 1996; Spetea et al. 2002). The perigenual cingulate and associated structures are most likely to be concerned with processing the affective components of pain, whereas the mid-anterior cingulate is more likely to be concerned with executive processing (response selection and monitoring) and control of attention.
_
Pain modulation:
The body possesses an endogenous analgesia (endogenous opioids) system, which can be supplemented with analgesic drugs to regulate nociception and pain. There is both an analgesia system in the central nervous system and peripheral receptors that decreases the grade in which nociception reaches the higher brain areas. The degree of pain can be modified by the periaqueductal gray before it reaches the thalamus and consciousness. According to gate control theory of pain, this area can also reduce pain when non-painful stimuli are received in conjunction with nociception.
1. Central:
The central analgesia system is mediated by 3 major components: the periaquaductal grey matter, the nucleus raphe magnus and the nociception inhibitory neurons within the dorsal horns of the spinal cord, which act to inhibit nociception-transmitting neurons also located in the spinal dorsal horn.
2. Peripheral:
The peripheral regulation consists of several different types of opioid receptors that are activated in response to the binding of the body’s endorphins. These receptors, which exist in a variety of areas in the body, inhibit firing of neurons that would otherwise be stimulated to do so by nociceptors.
_
Opioid Receptors:
Opiate receptors – or binding sites in the central nervous system – were discovered in 1973. These sites are areas of neuronal membrane to which opioids attach themselves and bring about inhibition in the underlying cell. It was noted that morphine attaches to specific binding sites which suggested that there must be naturally-occurring opioids within the body (Bowsher 1987). It is now known that there are four distinct types of opioid receptor, mu, kappa and delta and sigma. There is a widespread distribution of opiate receptors within the Central Nervous System. These are numerous in the paramedian system’s intralaminar thalamic nuclei, reticular formation and limbic structures. There are only a few in the neospinothalamic system’s ventrobasal thalamus and post-central gyrus. In the dorsal horn there are large numbers situated postsynaptically on neuronal membranes and there are some situated presynaptically on the intraspinal part of C afferent nerve fibres (Baldry 1993). Thus opioid binding sites (receptors) occur at many synaptic and non synaptic sites in the CNS, notably the spino-reticular system, and inhibition results from the action of exogenous opioids (e.g. morphine) or endogenous opioids (e.g. enkephalins, endorphins) at these sites (Bowsher 1987). Studies of the distribution of endogenous opioid peptides have shown that there are high levels of enkephalins and dynorphins in the limbic structures, periaqueductal grey area, the nucleus raphe magnus and the substantia gelatinosa of the dorsal horn (with spill-over into the cerebrospinal fluid); and anterior pituitary and adrenal medulla, with release into the plasma. Plasma enkephalins are released from the adrenal gland, the gut, sympathetic ganglia and peripheral autonomic neurons (Baldry 1993).
_
Endogenous opioid substances are endorphins, enkephalins(met-Enk and leu-Enk) and dynorphins synthesized by nerve cells.
1. Endorphin: anterior and intermediate pituitary lobes; hypothalamus
2. Enkephalin: periaqueductal gray matter; rostral medulla (raphe nucleus); dorsal gray horn of spinal cord
3. Dynorphin: hippocampus; deep layers of cerebral cortex; caudate; putamen; globus pallidus
These bind opioid receptors and action is blocked by the opioid receptor antagonist naloxone. These endogenous opioids are coded by separate genes and present in different cell populations of the brain, pituitary, adrenal gland, gut and sympathetic nervous system. The endorphin-mediated analgesia system (EMAS) demonstrates how narcotic analgesics relieve pain and drugs like morphine, pethidine and codeine mimic actions of endorphins at synapses in pain-modulating networks.
_
Following are some situations and conditions which are thought to stimulate endorphin production.
1) Emergency situations requiring the person to perform physical actions despite pain and injury.
2) Intense physical activity. Moderate physical activity over an extended period of time can also increase the supply of endorphin.
3) Positive beliefs and expectations (e.g., the “placebo effect”).
4) Positive emotional states such as happiness, joy, laughter, and love.
What this means is that psychological factors, including positive mental attitudes and positive emotional states can have a beneficial effect on your brain chemistry, which in turn can reduce your experience of pain.
_
Pain Modulation System:
The pain modulation system consists of endogenous opioid system, the serotonergic system and the descending pain pathways; all of them are inter-connected for pain modulation. Much of the pain modulation is carried out by the endogenous opioids. The electrical stimulation of rat brains produced a phenomenon called stimulation produced analgesia (SPA) which is a function of endogenous opioid release. Endogenous opioids caused selective reduction in pain with few side effects (warmth and/or sleepiness). Serotonin and norepinephrine are important neurotransmitters involved in modulating the pain response particularly because they can be manipulated by a variety of pharmacological agents.
_
Descending pain Modulation:
_
Descending Pain Modulation Pathways means pain modulating neurons from the cortex, hypothalamus, midbrain periaqueductal gray and rostral medulla alter pain response in the dorsal horn of the spinal cord. Descending input from the brainstem influences central nociceptive transmission in the spinal cord. Specific brainstem nuclei send projections to the dorsal horn of the spinal cord and when activated by ascending nociceptive impulses and other influences from the brain result in descending modulation. Just as there are ascending pain pathways from the body to the brain, there are also descending pain pathways communicating from the brain to the body which inhibit pain. The most important descending pathways begin in the periaqueductal gray (PAG). Stimulation of the PAG has been shown to produce analgesia, but no change in the ability to detect temperature, pressure, or touch. The neurons beginning in the PAG end on cells in the medulla, including the serotonergic cell bodies of the raphe nuclei. The serotonergic neurons then descend into the spinal cord to inhibit cell firing. Other cells in the PAG terminate close to the locus coeruleus in the brainstem. Thus, there are at least two major pathways that descend to the spinal cord to inhibit the projection of pain. Though not completely understood, modulation results in descending inhibition of nociception through the release of neurotransmitters such as serotonin, norepinephrine and endogenous opioids. Modulatory processes can also increase descending facilitation of nociception and consequently pain. Psychological factors such as fear and anxiety exert facilitatory influences through these modulatory systems.
_
The modulation of pain involves changing or inhibiting transmission of pain impulses in the spinal cord. The multiple, complex pathways involved in the modulation of pain are referred to as the descending modulatory pain pathways (DMPP) and these can lead to either an increase in the transmission of pain impulses (excitatory) or a decrease in transmission (inhibition). Descending inhibition involves the release of inhibitory neurotransmitters that block or partially block the transmission of pain impulses, and therefore produce analgesia. Inhibitory neurotransmitters involved with the modulation of pain include:
Endogenous opioids (enkephalins and endorphins)
Serotonin (5-HT)
Norepinephirine (noradrenalin)
Gamma-aminobutyric acid (GABA)
Neurotensin
Acetylcholine
Oxytocin
Endogenous pain modulation helps to explain the wide variations in the perception of pain in different people as individuals produce different amounts of inhibitory neurotransmitters. Endogenous opioids are found throughout the central nervous system (CNS) and prevent the release of some excitatory neurotransmitters, for example, substance P, therefore, inhibiting the transmission of pain impulses.
_
Certain antidepressants such as the tricylics and selective norepinephrine reuptake inhibitors (SNRIs) that inhibit the reuptake of serotonin and norepinephrine enhance descending inhibition. This may explain their effectiveness in neuropathic and other types of pain. Opioid analgesics have direct effects on descending modulation. Cognitive behavioral therapies are thought to modulate pain by reducing fear and anxiety. Spinal cord stimulation, epidural and intrathecal delivery of drugs such as opioids also modify descending modulation.
_
The Gate Control Theory:
_
Melzack and Wall (1965), developed their now-famous theory on pain mechanisms, which postulated that in each dorsal horn of the spinal cord, there is a gate-like mechanism which inhibits or facilitates the flow of afferent impulses into the spinal cord before it evokes pain perception and response. The gate control theory proposes that the substantia gelatinosa, which caps the grey matter of the spinal horn in the spinal cord, is the essential site of control. The control mechanism is referred to as a ‘gate’ and is operated by external and internal influences. Pain impulses can only pass through when the gate is open, and not when it is closed (Davis 1993). The theory, as originally propounded, stated that the opening or closing of the ‘gate’ is dependent on the relative activity in the large diameter (A-beta ) and small diameter fibers (A-delta and C), with activity in the large diameter fibers tending to close the ‘gate’, and activity in the small diameter fibers tending to open it (Baldry 1993). So if nociceptive input exceeds A-beta fiber input, then the gate is open and the pain impulse ascends the spinal cord to the brain. If A-beta fiber input exceeds nociceptive input, then the gate is closed and the pain impulse is stopped or diminished due to the action of the inhibitory neurotransmitters and, therefore, does not pass up the spinal cord (Davis 1993). An essential part of the theory ever since the time it was first put forward is that the position of the ‘gate’ is in addition influenced by the brain’s descending inhibitory system. Recent research by Garrison and Foreman (1994) supports this theory insofar as their study shows that dorsal horn neurons which can potentially transmit noxious information to supraspinal levels, can have their cell activity decreased during TENS (Transcutaneous electrical nerve stimulation ) application to somatic receptive fields. These findings are consistent with the concept of the ‘gate control theory of pain’ in that less noxious information would be involved in the pain perception process (Garrison and Foreman 1994). They also showed that there is a differential effect in that more cells respond to conventional high frequency, low intensity (TENS) variables than they do to low frequency, high intensity (ALTENS) variables. So entry into the central nervous system can be visualized as a gate, which is opened by pain-generated impulses and closed by low-intensity stimuli such as rubbing or mild electric stimulation (TENS), furthermore, it can also be closed by endogenous opioid mechanisms which can be activated from the brain or peripherally by acupuncture (Bowsher 1987) or by gentle rubbing, massage, electrical stimulation and hot or cold therapies. The gate control theory of pain postulates that nociception (pain) is “gated” by non-nociception stimuli such as vibration. Thus, rubbing a bumped knee seems to relieve pain by preventing its transmission to the brain. Pain is also “gated” by signals that descend from the brain to the spinal cord to suppress (and in other cases enhance) incoming nociception (pain) information.
_
_
Let’s go through the theory step by step:
1. Without any stimulation, both large and small nerve fibers are quiet and the inhibitory interneuron (I) blocks the signal in the projection neuron (P) that connects to the brain. The “gate is closed” and therefore no pain.
2. With non-painful stimulation, large nerve fibers (A-beta) are activated primarily. This activates the projection neuron (P), but it also activates the inhibitory interneuron (I) which then blocks the signal in the projection neuron (P) that connects to the brain. The “gate is closed” and therefore no pain.
3. With pain stimulation, small nerve fibers (A-delta and C) become active. They activate the projection neurons (P) and block the inhibitory interneuron (I). Because activity of the inhibitory interneuron is blocked, it cannot block the output of the projection neuron that connects with the brain. The “gate is open”, therefore, pain!!
_
Thoughts, emotions and “circuitry” can affect both ascending and descending pain pathways. So, several factors, physiological and psychological, can influence pain perception:
1. Age (vide infra) — Brain circuitry generally degenerates with age, so older people have lower pain thresholds and have more problems dealing with pain.
2. Gender (vide infra) — Research shows that women have a higher sensitivity to pain than men do. This could be because of sex-linked genetic traits and hormonal changes that might alter the pain perception system. Psychosocial factors could be at work, too — men are expected not to show or report their pain.
3. Fatigue — We often experience more pain when our body is stressed from lack of sleep.
4. Memory — How we have experienced pain in the past can influence neural responses (memory comes from the limbic system).
_
Wind-up:
Example: frequently touching the hot plate becomes painful
Low frequency repetitive stimulation of C-fibers produces a gradual increase in the discharge frequency of WDR neurons until they are in a state of nearly continuous discharge. In this state there are augmented responses to input and enlarged receptive fields. Input from areas that previously did not activate the WDR neuron now evoke a prominent response, and
Low threshold stimulation is able to drive the neurons. Wind-up is elicited by any prolonged or intense C-fiber input.
_
Pain sensitization and hypersensitivity:
Pain systems need to be sensitive enough to detect potentially harmful stimuli. But often they become too sensitive, causing us pain that provides no benefit. This hypersensitivity arises because our pain pathways actually increase in sensitivity when they relay pain messages, and the mechanisms of this sensitization are beginning to be revealed. Pain hypersensitivity takes two forms:
1. Thresholds are lowered so that stimuli that would normally not produce pain now begin to (allodynia).
2. Responsiveness is increased, so that noxious stimuli produce an exaggerated and prolonged pain (hyperalgesia).
Pain hypersensitivity after an injury helps healing by ensuring that contact with the injured tissue is minimized until repair is complete – an adaptive response. However, pain hypersensitivity may persist long after an injury has healed or occur in the absence of any injury. In this case, pain provides us with no benefits, and is a manifestation of pathological change in the nervous system.
_
Pain receptors become sensitized after tissue damage. When tissue is damaged or a noxious stimulus is repeated, nociceptors exhibit sensitization such that there can be a reduction in the threshold for activation, an increase in response to a given stimulus, or the appearance of spontaneous activity. This sensitization results from the actions of second messenger systems activated by the release of inflammatory mediators (bradykinin, histamine, prostaglandins and serotonin) at the site of injury. This causes some of the features of hyperalgesia produced by tissue damage or by pathological processes. Hyperalgesia after cutaneous injury can be divided into two phenomena: Primary hyperalgesia occurs at the site of injury and is characterized by hyperalgesia to mechanical and heat stimuli. Secondary hyperalgesia occurs outside the injury site and is characterized by mechanical hyperalgesia only. Hyperalgesia in inflammatory processes corresponds to primary hyperalgesia. Hyperalgesia in referred pain and neuropathic pain resembles secondary hyperalgesia,
_
Sensitization could be peripheral and/or central sensitization. ‘Sensitization’ here means an increase in the excitability of neurons, so they are more sensitive to stimuli or sensory inputs.
1.Peripheral sensitization:
Peripheral sensitization is a reduction in threshold and an increase in responsiveness of the peripheral ends of nociceptors, the high-threshold peripheral sensory neurons that transfer input from peripheral targets (skin, muscle, joints and the viscera) through peripheral nerves to the central nervous system (spinal cord and brainstem). Peripheral sensitization is the result of changes in key proteins and ion channels (known as transduction proteins) that determine the excitability of the nociceptor terminal. The transduction proteins are the means by which a noxious stimulus, for example excessive heat, is converted into electrical activity. Normally for heat this only occurs at around 42°C, the heat pain threshold. After peripheral inflammation, though, the threshold falls considerably. Peripheral sensitization contributes to the pain hypersensitivity found at the site of tissue damage and inflammation. A good example of this is the change in heat sensitivity after sunburn, when a normally warm stimulus such as a shower feels burning hot in the sunburned areas. Sensitization arises due to the action of inflammatory chemicals or mediators released around the site of tissue damage or inflammation. Some of these, such as ATP, can directly activate the ends of the peripheral nociceptors, signaling the presence of inflamed tissue and producing pain. Other chemical mediators are produced by activated inflammatory cells, such as neutrophils (a type of white blood cell). When activated, these cells begin making an enzyme known as COX-2, which leads to the production and secretion of prostaglandin PGE2. This mediator act as a sensitizer, altering pain sensitivity by increasing the responsiveness of peripheral nociceptors. Aspirin-like pain-killing drugs act by inhibiting Cox-2 and prostaglandin production.
_
Tissue damage results in a drop in pH and release of chemicals, e.g. histamines and bradykinin, to which small non-myelinated C fibers are sensitive. Fitzgerald and Woolf (1984) have hypothesized that C fibers are primarily chemical sensors, although they do respond also to high level mechanical and thermal stimulation. The C fibers respond by generating an electrical impulse which travels along the nerve to the dorsal horn of the spinal cord. Activity of the C fibers may be up-regulated peripherally by serotonin, prostaglandins, thromboxane, and leucotrienes in the damaged tissues. This is referred to as peripheral sensitization in contrast to central sensitization which occurs at the dorsal horn. Both occur in chronic pain. Substance P may also be released peripherally with resultant increase in peripheral vasodilation and further sensitization of the C fiber’s peripheral ending. Even chemical products of tissue breakdown may sometimes enter the neuron and be transported centrally to exert an effect at the dorsal horn synapse.
_
2. Central sensitization:
_
_
Central sensitization is an increase in the excitability of neurons within the central nervous system, so that normal inputs begin to produce abnormal responses. Unlike other sensory stimuli, relatively brief trains of activity in peripheral nociceptors have the ability to trigger long term changes in CNS circuitry and cause prolonged states of hypersensitivity. This ‘central sensitization’ contributes to an amplification of the noxious input and a spread of pain outside the original damaged region (secondary hyperalgesia) and the onset of pain from normally innocuous stimuli (allodynia). The central sensitization arises from increases in membrane excitability, strengthened excitatory synaptic outputs and reduction of inhibitory interneuronal activity, which in turn are regulated by shifts in gene expression, the production and trafficking of key receptors, channels, and downstream neuronal signaling pathways. The first phase depends on changes to existing proteins involved in synaptic transmission of nerve impulse while the second phase relies on new gene expression. The increased excitability is typically triggered by a burst of activity in nociceptors (such as that evoked by an injury), which alter the strength of synaptic connections between the nociceptor and the neurons of the spinal cord (so-called activity-dependent synaptic plasticity). Low-threshold sensory fibers activated by very light touch of the skin, for example, begin to activate neurons in the spinal cord (for inputs from the body) or in the brainstem (for inputs from the head) that normally only respond to noxious stimuli. As a result, an input that would normally evoke an innocuous sensation now produces pain. In effect, the synaptic changes increase the ‘gain’ of the system. Although the pain feels as if it originates in the periphery, it is actually a manifestation of abnormal sensory processing within the central nervous system. Central sensitization is responsible for tactile allodynia (pain in response to light brushing of the skin) and for the spread of pain hypersensitivity beyond an area of tissue damage so that adjacent non-damaged tissue is tender. Central sensitization can also occur after surgery, contributing to pain on movement or touch, in migraine attacks where brushing hair is often painful, and in some patients with nerve damage where even blowing on the skin produces excruciating burning pain.
_
_
There are treatments that may actually aggravate central sensitization pains–oxycodone working on kappa receptors, prolonged high dose opioid metabolites, excessive triptans or ergotamine for migraines, and untreated pain itself. It is also becoming clear there are some kinds of treatment that may have more specific advantages in treating central sensitization: gabapentin, pregabalin, tramadol, amitriptyline, buprenorphine, and ketamine, for example.
_
Mechanism of pain transmission at synapse in various pain conditions:
1. Normosensitivity
_
2. Central sensitization
_
3. Neuropathic
_
4. Hyperalgesia
_
5. Allodynia
_
The picture below shows excellent illustration of what happens to pain response following injury.
_
1. Normal pain response curve shows zero pain intensity with lower intensity stimuli (e.g. light touch does not elicit pain normally – innocuous stimuli) and as stimulus intensity increases (e.g. squeezing or pinching harder) one begins to feel pain with increasing intensity.
2. Following injury, the pain response curve shifts to the left.
3. Due to shift of pain response curve to left, a stimulus which would cause a low level of pain would now cause a much higher level of pain. This is the definition of hyperalgesia.
4. Normally innocuous stimuli now becomes painful – definition of allodynia.
_
The mechanisms of pain and the ability to control pain may alter in different pain states. Pain is a unique constellation of peripheral and central plastic changes. Central neuroplasticity occur due the phenomenon of central sensitization, whereby neuronal activity within the spinal cord is increased. This is linked to painful sensations such as hyperalgesia and allodynia and the generation of spontaneous pain. Also observed are increased peripheral receptive fields of individual spinal neurones, lowered threshold to peripheral stimuli and increased spontaneous firing. This plasticity is of great importance in consideration of a rational basis for the treatment of both inflammatory and neuropathic pain where the damage to tissue and nerve leads to alterations in both the peripheral and central mechanisms of pain signaling. In terms of existing drug therapies, this plasticity, the ability of the system to change in the face of a particular pain syndrome is the reason for the effectiveness of NSAIDs in inflammatory conditions yet also responsible for some limitations in the effectiveness of opioids in neuropathic pain.
_
Hypoalgesia:
Hypoalgesia denotes a decreased sensitivity to painful stimuli. Hypoalgesia occurs when nociceptive (painful) stimuli are interrupted or decreased somewhere along the path between the input (nociceptors), and the places where they are processed and recognized as pain in the conscious mind. Hypoalgesic effects can be mild, such as massaging a stubbed toe to make it hurt less or taking aspirin to decrease a headache, or they can be severe, like being under strong anesthesia. Hypoalgesia can be caused by exogenous chemicals such as opioids, as well as by chemicals produced by the body in phenomena such as fear- and exercise- induced hypoalgesia. Hypoalgesia can also be associated with diseases, such as CIP or in less severe cases with diabetes or other diseases associated with hypertension. Based on the existing evidence, hypoalgesia is argued to be a correlate of dysregulation of central nervous system structures involved in both pain control and cardiovascular regulation in individuals who are genetically predisposed to develop high blood pressure. As such, hypoalgesia may serve as a valuable method of identifying individuals at greatest risk for hypertension.
_
Pain perception in animals:
The most reliable method for assessing pain in most humans is by asking a question: a person may report pain that cannot be detected by any known physiological measure. However, like infants (Latin infans meaning “unable to speak”), non-human animals cannot answer questions about whether they feel pain; thus the defining criterion for pain in humans cannot be applied to them. Experts currently believe that all vertebrates can feel pain, and that certain invertebrates, like the octopus, might too. Nociception has been documented in non-mammalian animals, including fishes and a wide range of invertebrates, including leeches, nematode worms, sea slugs, and fruit flies. As in mammals, nociceptive neurons in these species are typically characterized by responding preferentially to high temperature (40º Celsius or more), low pH, capsaicin, and tissue damage. As for other animals, plants, or other entities, their ability to feel physical pain is at present a question beyond scientific reach, since no mechanism is known by which they could have such a feeling. In particular, there are no known nociceptors in groups such as plants, fungi, and most insects, except for instance in fruit flies. In vertebrates, endogenous opioids are neurochemicals that moderate pain by interacting with opiate receptors. Opioids and opiate receptors occur naturally in crustaceans and, although at present no certain conclusion can be drawn, their presence indicates that lobsters may be able to experience pain. Opioids may mediate their pain in the same way as in vertebrates. Veterinary medicine uses, for actual or potential animal pain, the same analgesics and anesthetics as used in humans.
_
It’s sometimes said that, “…animals don’t feel pain the way we do.” This is wrong. Anyone who’s stepped on a dog’s foot is quite well aware that higher beings (which certainly include all mammals and birds, and probably almost all vertebrates) have a similar reaction to such stimuli. If a man drops a rock on his toe we say he “screeches in pain,” but in animals, scientists hedge their bets (and avoid the cardinal sin of attributing human emotions to animals) by using the term “exhibits a pain-like response,” for the same noise, elicited by the same stimulus. This is quibbling: an animal clearly is capable of feeling “pain” in the same way we do.
_
The table below shows signs of pain in animals.
_
Almost all organisms, including bacteria, will attempt to escape from an aversive stimulus. Because bacteria are not thought to be capable of feeling pain (e.g. they lack a nervous system), possessing an escape response to an aversive stimulus is not enough evidence to demonstrate that a species is capable of feeling pain. To infer that a non-human vertebrate (mammals, birds and reptiles) is in pain, researchers rely on the vocalizations and physiological responses (e.g. the release of stress hormones) that an animal produces when faced with an aversive stimulus. Because these responses are similar to our own when we are in pain, researchers argue that, by analogy, animals showing these responses are also in pain. This technique cannot be used with invertebrates. Invertebrate physiology is different from our own. The invertebrates diverged from that of vertebrates hundreds of millions of years ago. Most, if not all, invertebrates have the capacity to detect and respond to noxious or aversive stimuli. That is, like vertebrates, they are capable of “nociception.” Examples of aversive stimuli include changes in temperature beyond the animal’s normal range, contact with noxious chemicals, mechanical interference, or electric shock. Under certain conditions, all of these might be expected to cause pain in humans. In general, invertebrates, like vertebrates, respond to such stimuli by withdrawing or escaping so as to reduce the likelihood that they will be damaged by the noxious conditions. Some invertebrates, like vertebrates, also have special sensory receptors called nociceptors, which respond specifically to noxious stimulation. Such nociceptive nerve cells have been found in the segmental ganglia of the medicinal leech, Hirudo medicinalis (Nicholls and Baylot, 1968). Nerve impulses are generated in these cells, which Nicholls and Baylor called N cells, specifically in response to noxious mechanical stimulation, such as such as pinching, squeezing, or cutting of the body wall. What evidence might help in distinguishing between nociceptive “responsiveness” and the perception of pain? At the outset, it should be pointed out that because pain is a subjective experience, it is highly unlikely that any clear-cut, definitive criteria will ever be found to decide this question. The “relatively simple organization” of the insect central nervous system, Elsemann et al. argue, “raises the question of whether any experience akin to human pain could be generated” in these animals (and by implication in other invertebrates with a similar or less complex nervous organization). On the analysis of Gould and Gould (1982), the answer to such a question would be “no,” for these authors can find no evidence for conscious experience in insects. Certainly, on the limited amount of evidence presented here, it seems very difficult to imagine that insects and the other simpler invertebrates mentioned above can “suffer” pain in anything like the vertebrate sense. Nevertheless, the issue certainly is not closed, and further questions should be asked. It might be inferred, incorrectly, that certain invertebrates experience pain simply because they bear a (superficial) resemblance to vertebrates-the animals with which humans can identify with most clearly. Equally, pain might incorrectly be denied in certain invertebrates simply because they are so different from us and because we cannot imagine pain experienced in anything other than the vertebrate or, specifically, human sense. The question of pain in invertebrates will be extremely difficult to resolve–if, indeed, it is resolvable. Although it is impossible to know the subjective experience of another animal with certainty, the balance of the evidence suggests that most invertebrates do not feel pain. The evidence is most robust for insects, and the consensus is that they do not feel pain. Perhaps the question of pain in invertebrates could be more easily settled where the “most highly organized of all invertebrates” (Russell-Hunter, 1979), the cephalopods, are concerned. These animals, which include cuttlefish, squid, and octopuses, “have the largest brains of all invertebrates” (Wells, 1962). The ratio of brain-weight to body-weight of many cephalopods also exceeds that of most fish and reptiles (Packard, 1972). The cephalopod brain has a “hierarchical” organization (see Boycott, 1961), the “higher” centers of the brain being concerned with sensory analysis, memory, learning, and decision-making. It has been suggested that these areas of the cephalopod brain might be regarded as analogous to the cerebral cortex of higher vertebrates (Russell-Hunter, 1979). According to Wells (1978), since in Octopus the brain “represents only the more specialized sensory integrative, higher movement control and learning parts of a rather diffuse nervous system…it becomes clear that one is dealing with an animal that might well be expected to possess a central nervous capability approaching that or exceeding that of many birds and mammals.” The evidence seems to suggest that at least some of the cephalopods might have a nervous organization that would allow them to experience something like pain. It is unclear, however, whether cephalopods are able to “suffer” pain.
_
In vertebrates, nociceptive information is collated and augmented in the brain and signals are relayed down the nervous system to alter the intensity of pain. All vertebrates possess the primitive areas of the brain to process nociceptive information, namely the medulla, thalamus and limbic system. However, one area of great importance for pain perception in humans is the cortex and its relative size decreases as we descend the evolutionary tree. For instance, in relative terms, the cortex gets smaller going from humans, through primates, mammals, birds, reptiles, amphibia and finally to fish, which possess only a rudimentary cortex. Although comparatively simple, fish have recently been shown to possess sensory neurons that are sensitive to damaging stimuli and are physiologically identical to human nociceptors. Fish show several responses to a painful event: they adopt guarding behaviors, become unresponsive to external stimuli and their respiration increases. These responses disappear when the fish are given morphine – evidence that they are, mechanistically at least, directly analogous to pain responses in more complex animals.
_
Some animals show reflex responses similar to our own. For example, when we accidentally touch a hot iron we respond almost immediately by retracting our hand. There is a lag period following this when no adverse sensations are felt but, if left untreated, the burn begins to throb and we alter our behavior to guard the affected area. Other animals respond to painful damage in a similar way. Their responses comprise several behavioral and physiological changes: they eat less food, their normal behavior is disrupted, their social behavior is suppressed and they may adopt unusual behavior patterns (typically, highly repetitive or stereotyped behaviors, such as rocking to and fro), they may emit characteristic distress calls, and they experience respiratory and cardiovascular changes, as well as inflammation and release of stress hormones.
_
Emotional pain in animals:
Are animals capable of feeling emotional pain? Humans can certainly feel pain without physical damage – after the loss of a loved one, or the break-up of a relationship, for example. Some scientists suggest that only primates and humans can feel emotional pain, as they are the only animals that have a neocortex – the ‘thinking area’ of the cortex found only in mammals. However, research has provided evidence that monkeys, dogs, cats and birds can show signs of emotional pain and display behaviors associated with depression during painful experience, i.e. lack of motivation, lethargy, anorexia, and unresponsiveness to other animals. Thomas Nagel concluded that unless we can get inside the head of an animal and actually be it, we will never know exactly how that animal feels.
_
Pain tolerance and pain threshold:
Pain tolerance is the maximum level of pain that a person is able to tolerate. Pain tolerance is distinct from pain threshold (the point at which pain begins to be felt). Pain Threshold is the level at which a person first begins to experience pain from a stimulus, either artificial or biological. A person’s Pain Tolerance level, is the overall level of pain a person can tolerate before breaking down either physically or mentally. This is the logic used by some police officers to employ third degree torture of the accused to extract truth and confession, believing that extreme pain will overwhelm pain tolerance of the accused and force him to speak the truth. While human Pain Threshold is relatively low for most people (it doesn’t take much to feel pain – even a needle or pin prick will do), Pain Tolerance is another matter. The human body can withstand a lot of pain before breaking down, and for those who deal with constant levels of high pain on a regular basis every day, the tolerance level is very high, almost dangerously so. It is widely believed that regular exposure to painful stimuli will increase pain tolerance – i.e. increase the ability of the individual to handle pain by becoming more conditioned to it. However, this is not true – the greater exposure to pain will result in more painful future exposures. Repeated exposure bombards pain synapses with repetitive input, increasing their responsiveness to later stimuli, through a process similar to learning. Therefore, although the individual may learn cognitive methods of coping with pain, these methods may not be sufficient to cope with the boosted response to future painful stimuli. An intense barrage of painful stimuli potentiates the cells responsive to pain so that they respond more vigorously to minor stimulation in the future. Because of this, trauma victims (or patients in pain) are given painkillers (such as morphine) as soon as possible – to prevent pain sensitization. Kalat suggests that morphine should be taken before surgery as people who begin taking morphine before surgery need less of it afterward.
_
_
Swearing increases pain tolerance:
Swearing occurs in most cultures – people swear to let off steam, or to shock or insult others. It is also a common response to a painful experience. We’ve all done it: after stubbing our toe, or hitting our thumb with a hammer, we draw a sharp breath and mutter a swear word. Until now, though, whether swearing actually alters our perception of pain had not been investigated. But according to a new study, swearing increases pain tolerance, enabling us to withstand at least one form of pain for longer. The authors suggest that it is because swearing induces negative emotions. It is well known that pain has a strong emotional aspect to it. Fear of pain, for example, is known to enhance pain perception, possibly by activating pathways which descend from the brain and modulate noxious stimuli entering the spinal cord. Swearing, too, is known to induce negative emotions (according to Steven Pinker, it taps into the “deep and ancient parts of the emotional brain”). It may therefore trigger a physiological alarm reaction known as the fight or flight response, which accelerates the heart rate and reduces sensitivity to pain.
_
Laughter increases pain Tolerance:
We all know how good it feels to have a hearty laugh, and now a new study suggests that a guffaw can also reduce the feeling of pain. University of Oxford researchers found that a good laugh is linked with feeling less pain, and it’s likely because laughing spurs the body to release feel-good chemicals called endorphins, which can also act as painkillers. In a 2007 study in the journal Evidence-based Complementary and Alternative Medicine, University of California, Los Angeles researchers also showed that funny videos helped kids tolerate pain for longer, suggesting getting kids to “humorous distraction” could help distract kids when they’re undergoing painful procedures.
_
Why does Pain Tolerance differ among people?
Research has been done that claims the source is genetic, psychological, or even gender-based. The first, and most evidentially-supported, argument—the genetic explanation—revolves around the gene that codes for COMT, an enzyme that metabolizes, or breaks down, the neurotransmitter dopamine. COMT depletes the dopamine supply in the brain, freeing receptors in the brain to which the dopamine was bound so that they are available to bind to endorphins, which lead to pain relief. The researchers caution that pain tolerance cannot logically be explained by a single gene, an argument supported by the fact that COMT has other functions in the body; however, COMT must play a very large part in the differences seen in individuals. The psychological research done on this topic operates under the understanding that pain can be manifested in negative emotions, such as anxiety, depression and anger, to name a few. These researchers argue that these negative emotional responses to pain stimuli can be counterbalanced by positive emotional responses; in one very compelling study, the positive emotional responses were produced by sexual fantasies. The pleasant emotions produced by the thought of a sexual fantasy counteract the unpleasant thoughts that are a result of pain. The implications of this are that if a person enduring a painful experience imagines something that evokes in them positive emotions, they are able to cope with the pain better, and actually report experiencing less pain. Conversely, if a person experiences negative sensations from sources other than the painful experience in combination with the painful experience, the subject cannot endure as much pain and reports experiencing more pain than other subjects. Researchers have found that estrogen can act as a natural painkiller. Higher estrogen levels result in a higher pain tolerance, and lower estrogen levels cause effectively lower pain tolerance in subjects. Granted, the study was done only in women, but it is curious that hormones can affect how one deals with pain.
_
Pain in clinical medicine:
_
_
Pain as an aid to diagnosis:
Pain is a symptom of many medical conditions. Knowing the time of onset, location, intensity, pattern of occurrence (continuous, intermittent, etc.), exacerbating and relieving factors, and quality (burning, sharp, etc.) of the pain will help the examining physician to accurately diagnose the problem. For example, chest pain described as extreme heaviness may indicate myocardial infarction, while chest pain described as tearing may indicate aortic dissection.
_
Under-treatment of pain:
Inadequate treatment of pain is widespread throughout surgical wards, intensive care units, accident and emergency departments, in general practice, in the management of all forms of chronic pain including cancer pain, and in end of life care. This neglect is extended to all ages, from neonates to the frail elderly. In September 2008, the World Health Organization (WHO) estimated that approximately 80 percent of the world population has either no or insufficient access to treatment for moderate to severe pain. Every year tens of millions of people around the world, including around four million cancer patients and 0.8 million HIV/AIDS patients at the end of their lives suffer from such pain without treatment. Yet the medications to treat pain are cheap, safe, effective, generally straightforward to administer, and international law obliges countries to make adequate pain medications available. Reasons for deficiencies in pain management include cultural, societal, religious, and political attitudes, including acceptance of torture. Moreover, the biomedical model of disease, focused on pathophysiology rather than quality of life, reinforces entrenched attitudes that marginalize pain management as a priority. Other reasons may have to do with inadequate training, personal biases or fear of prescription drug abuse. This failure to adequately treat pain may be due to physicians’ fear of being accused of over-prescribing, despite the relative rarity of prosecutions, or physicians’ poor understanding of the health risks attached to opioid prescription. Current strategies for improvement in pain management include framing it as an ethical issue; promoting pain management as a legal right; providing constitutional guarantees and statutory regulations that span negligence law, criminal law, and elder abuse; defining pain management as a fundamental human right; categorizing failure to provide pain management as professional misconduct, and issuing guidelines and standards of practice by professional bodies.
_
Pain, tissue damage and disability:
Pain is the most common reason for patients to enter health‐care settings and the most common reason given for self‐medication. Pain interrupts all other activity and arrests current behaviour. It functions to prime escape or protective behaviour. As it is an everyday and frequent experience, there is also a common understanding of pain, both lay and professional, that it is a useful signal of damage. Indeed, in the majority of cases pain is a relatively reliable signal of damage and one that refers well to its spatial location. Also, the intensity of pain often refers well to the extent of damage. For example, extracting two teeth hurts about twice as much as extracting one tooth.There is, however, a number of cases where the extent of damage does not refer well to the experience of pain. For example, some people report pain that has no identifiable lesion, as in many cases of back pain, headache and angina. It is also possible to have tissue damage without any pain. For example, up to 40% of patients with established reversible myocardial ischemia do not report pain. More recently, it has been recognized that it is possible to experience pain in a location distal to the damage or to experience pain in a missing or extra limb or location. Even under laboratory conditions, where we can control the intensity of the pain‐inducing stimulus, there is a great deal of variability in patient response. We should be mindful of the fact that pain is not a reliable indicator of tissue damage and that tissue damage is not a reliable indicator of pain. There is also a number of cases where the extent of damage and the extent of pain together do not refer well to the experience of disability. Some patients appear not to be disabled by extensive damage and pain, whereas other patients respond with extensive disability to seemingly minor damage and pain. This variability can be witnessed in everyday practice. Anyone doctor who is in the business of invasive procedure as part of their routine work will understand that different people respond differently to the same procedure under the same circumstances, and that the same people respond to the same procedure differently at different times or under different circumstances. A brief and unscientific survey of colleagues or friends as to their choice of analgesia during dental procedures will quickly exemplify this variability.
_
It seems that several factors can affect how you experience and interpret pain:
1. Emotional and psychological state;
2. Memories of previous pain;
3. Upbringing;
4. Expectations of and attitudes towards pain;
5. Beliefs and values;
6. Age;
7. Sex; and
8. Social and cultural influences.
_
Understanding differential responding:
We can successfully conclude that people are different and respond differently to pain‐inducing stimuli and to attempts at pain management. If we can understand what predicts these differences, we may be able to improve treatment delivery and effectiveness.
_
Personality:
A number of studies have attempted to describe or uncover what may be thought of as a pain‐prone personality. It was thought that those who were less hardy or less robust to the hardships of the world would show less tolerance of pain stimuli and would be more complaining of pain. In addition, there was also the idea that the pain expressed by patients was a manifestation of guilt or of loss, or that pain revealed a self‐destructive, sadomasochistic style of sexual development. There is no evidence, however, to support these ideas. The experience of pain does not prevent personality disorders but neither is it thought to be a mask or alternative manifestation of them.
_
Gender (vide infra):
In an excellent recent review of this field, Anita Unruh reported that ‘In most studies, women report more severe levels of pain, more frequent pain and pain of longer duration than do men.’ Women are more likely to experience recurrent pain, have moderate and severe pain from menstruation & childbirth and may be at increased risk of disability arising from pain. Unruh also reported that, despite the fact that women report more pain than men, women are at greater risk of being labeled as having a psychogenic disorder and are more vulnerable to pain being explained as a purely psychological (used negatively in this case to mean unreal) phenomenon.
_
Age (vide infra):
Very little is known about the specific effects of age and ageing and about the psychology of pain for specific age groups. For example, effective pain management in children has been hampered by the erroneous beliefs that neonates and infants could not feel pain and that children would respond addictively to opioid analgesia. We now know these ideas to be without support. An important but unresearched area is the effects of emotional and cognitive development upon the experience of pain for children and adolescents. At the other end of the lifespan, we are also only now beginning to learn about the effects of cognitive impairment on pain experience.
_
Culture (vide infra):
Early studies of the effect of culture focused upon the reports of ethnic differences in pain expression. However, the study of culture extends further than the ethnic group membership of patients. For example, a recent interesting study showed that ethnic differences (in a US sample) did not affect the report of post‐operative pain or patient‐controlled analgesia for post‐operative pain, but did, however, affect physician prescribing behavior. More recently, the study of cultural influences has extended to the broader study of the cultural construction of pain and has started to embrace the use of anthropological and sociological methods.
_
In nonverbal patients:
When a person is non-verbal and cannot self report pain, observation becomes critical, and specific behaviors can be monitored as pain indicators. Behaviors such as facial grimacing and guarding indicate pain, as well as an increase or decrease in vocalizations, changes in routine behavior patterns and mental status changes. Patients experiencing pain may exhibit withdrawn social behavior and possibly experience a decreased appetite and decreased nutritional intake. A change in condition that deviates from baseline such as moaning with movement or when manipulating a body part and limited range of motion are also potential pain indicators. In patients who possess language but are incapable of expressing themselves effectively, such as those with dementia, an increase in confusion or display of aggressive behaviors or agitation may signal that discomfort exists, and further assessment is necessary. Infants feel pain but they lack the language needed to report it, so communicate distress by crying. A non-verbal pain assessment should be conducted involving the parents, who will notice changes in the infant not obvious to the health care provider. Pre-term babies are more sensitive to painful stimuli than full term babies.
_
Pains you shouldn’t ignore:
Worst headache of your life:
Get medical attention immediately. If you have a cold, it could be a sinus headache but you could have a brain hemorrhage or brain tumor. With any pain, unless you’re sure of what caused it, get it checked out.
_
Pain or discomfort in the chest, throat, jaw, shoulder, arm, or abdomen:
Chest pain could be pneumonia or a heart attack. But be aware that heart conditions typically appear as discomfort, not pain. Heart patients talk about pressure. They’ll clench their fist and put it over their chest or say it’s like an elephant sitting on their chest. It is heaviness in chest rather than pain but do not wait for pain to suspect heart attack.
_
Pain in lower back or between shoulder blades:
Most often it’s arthritis. Other possibilities include a heart attack or abdominal problems. One danger is aortic dissection, which can appear as either a nagging or sudden pain.
_
Severe abdominal pain:
Still have your appendix? Don’t flirt with the possibility of a rupture. Gallbladder and pancreas problems, stomach ulcers, and intestinal blockages are some other possible causes of abdominal pain that need attention.
_
Calf pain:
One of the lesser known dangers is deep vein thrombosis (DVT), a blood clot that can occur in the leg’s deep veins. It can be life-threatening. Cancer, obesity, immobility due to prolonged bed rest or long-distance travel, pregnancy, and advanced age are among the risk factors.
_
Burning feet or legs:
Nearly one-quarter of the 27 million Americans who have diabetes are undiagnosed, according to the American Diabetes Association. In some people who don’t know they have diabetes, peripheral neuropathy could be one of the first signs.
_
It is neither possible nor desirable to discuss different pain syndromes in this article, nevertheless it is worthwhile to discuss few words about headache. Headache disorders are the most prevalent of the neurological conditions and among the most frequent of medical complaints seen in general practice. They take many forms, including migraine, tension-type headache, trigemino-autonomic cephalalgia (cluster headache), primary stabbing headache, primary sex headache, or rarer conditions such as trigeminal neuralgia or persistent idiopathic facial pain. Half of the general population experience headache during any given year, and more than 90% report a lifetime history of head pain. The most severely disabled 3% experience a headache such as chronic migraine and chronic tension-type headache at least 15 days per month. Headache disorders contribute to a considerable loss of work time and productivity.
_
Pain reporting by aged:
With about 20% of adults suffering from chronic pain, it is a substantial proportion of elderly patients who have this problem. We know that sensitivity to pain may be reduced in older adults, yet that does not mean that these people experience less pain. In the elderly, it is important to know that age-associated psychosocial phenomena, such as loss of family and friends and loss of independence, may contribute to pain and suffering. An aging adult may not respond to pain in the way that a younger person would. Their ability to recognize pain may be blunted by illness or the use of multiple prescription drugs. In the elderly, it is important to know that age-associated psychosocial phenomena, such as loss of family and friends and loss of independence, may contribute to pain and suffering. It is important to know that other symptoms such as depression and anxiety, sleep disturbances, weight loss and cognitive impairment may be related to pain, and even be a manifestation of pain in older persons. Pain is not only a verbal report, but pain behaviors such as guarding, agitation, facial expression and altered mobility could represent pain, for example, in Alzheimer’s disease. Depression may also keep the older adult from reporting they are in pain. The older adult may also quit doing activities they love because it hurts too much. Decline in self-care activities (dressing, grooming, walking, etc.) may also be indicators that the older adult is experiencing pain. The older adult may refrain from reporting pain because they are afraid they will have to undergo surgery or will be put on a drug they become addicted to. They may not want others to see them as weak, or may feel there is something impolite or shameful in complaining about pain, or they may feel the pain is deserved punishment for past transgressions.
_
The management of pain in older persons represents a particular challenge. Pain in many elderly patients is under treated. We know little about the pain manifestations in dementia, but it is often under treated and a proactive approach in treating these patients is often necessary. Pharmacological treatment of pain is particularly important. Many elderly patients tolerate analgesics such as opioids, anti-inflammatory drugs and adjunctive agents like tricyclic antidepressants less well than younger people. The result is often sedation & confusion, and NSAIDs produce more side effects in elderly. It is therefore important to recognize that there are other treatments than pharmacology available for this group of patients. For example, multidisciplinary pain programs combining pharmacological and non-pharmacological treatment are efficacious in the management of longstanding pain in older persons.
_
When to call doctor for child with pain?
Parents should notify their pediatrician if any of the following occurs in a child:
1. The child is in severe pain.
2. The child has pain that lasts for more than three days.
3. Parents have questions or concerns about their child’s treatment or condition.
4. The child is in the hospital and the parent thinks he or she is in pain. The sooner the pain is treated, the easier it is to control.
_
Understanding the anatomical pathways and neurochemical mediators involved in noxious transmission and pain perception is the key to optimizing the management of acute and chronic pain. Although acute pain and associated responses can be unpleasant and often debilitating, they serve important adaptive purposes. They identify and localize noxious stimuli, initiate withdrawal responses that limit tissue injury, inhibit mobility thereby enhancing wound healing, and initiate motivational and affective responses that modify future behavior. Nevertheless, intense and prolonged pain transmission, as well as analgesic undermedication, can increase postsurgical/traumatic morbidity, delay recovery, and lead to development of chronic pain.
Commonly observed pathophysiologic changes include, but are not limited to, the following:
(1) Neurohumoral alterations termed peripheral sensitization occurring at the site and in regions immediately adjacent to injury,
(2) Alterations in synaptic function and nociceptive processing occurring within spinal cord and limbic cortex,
(3) Sympathoadrenal activation resulting in an elevation of heart rate & blood pressure and a diminution in regional blood flow, and
(4) Neuroendocrine responses mediating hyperglycemia and a negative nitrogen balance.
_
The body’s reaction to unrelieved pain includes:
1. Increased heart rate and blood pressure
2. Changes to blood gases, namely reduced oxygen and increased carbon dioxide
3. Higher levels of stress hormones including cortisol and adrenaline
4. Gastrointestinal problems such as slowed digestion
5. Musculoskeletal problems such as tension and fatigue
6. Emotional problems such as anxiety and depression.
__
Pain cycle:
Let’s look at an example of how pain works as a process within the body. If you think about pricking your finger, this is where you get the pain sensation and a message is sent back quickly from your finger up to your brain and the brain responds by sending a message quickly back to say the pain is there, move your finger or take the needle out of your finger. But if you have a strong constant pain, the message from the nerve endings keeps sending up messages to the brain and the cycle of pain begins.
_
What factors affect the pattern of nociceptive processing in the brain?
1. Anticipation:
In terms of responses to experimental pain it is now very clear that the psychological context of the stimulus in terms of anticipation and attention may be as important as the stimulus parameters.
2. Chronicity (acute versus chronic pain):
It has been assumed for many years that the medial and lateral systems might respectively be concerned with processing chronic and acute pain (Albe-Fessard et al. 1985). Functional imaging studies have provided unequivocal evidence that this is not the case. Both systems are involved in acute nociceptive processing and at least one type of chronic pain (neuropathic pain), and these are processed in parallel (Jones 1999).
3. Empathy:
Recent studies have addressed the issue of pain empathy in terms of empathy for a partner experiencing pain. An elegant experiment compared brain activations when a volunteer or her partner, who was seated next to an MRI scanner, experienced pain. The results showed activation of mid-cingulate and anterior insular cortices, brainstem, and cerebellum during pain empathizing (Singer et al. 2004). The cortical components of these responses are within the medial pain system, providing a further example of segmentation of function within the pain matrix.
4. Sex.
So far there is evidence for subtle differences in cortical processing of pain between age-matched men and women, with the most convincing differences being a shift of processing in women within the cingulate cortex toward the perigenual cingulate cortex. Interestingly, this is one of the areas of the cortex with the highest levels of opioid receptors (Vogt et al. 1995a). Higher opioid receptor binding in women has been found in the temporal cortex, amygdala, and thalamus, but not so far within the cingulate cortex (Zubieta et al. 1999).[further discussion vide infra in gender and pain]
5. Learning.
Aversive conditioning in animals has been defined within circuitry comprising the hippocampus, anterior and posterior cingulate cortices, amygdala, striatum, and medial and anterior thalamic nuclei (Gabriel 1990, 1993; Uylings et al. 1990; Maren et al. 1991; Vogt et al. 1993). The motor outputs for these responses are by way of premotor components of the cingulate cortex and the striatum. The amygdala, thalamus, and cingulate cortices are involved in the acquisition of conditioned responses, forming a template that is compared with new sensory inputs. If there is a match, the appropriate motor response is initiated by way of the cingulate and motor cortices and the primed striatum. If there is a mismatch, the hippocampus is activated to block any motor response. Damage to the amygdala has been associated with impairment of acquisition of conditioned autonomic responses and with impairment of affective memories without significant impairment of nonaffective cognitive functions (Cahill et al. 1995). Functional MRI studies have allowed preliminary access to some of the processes that may be taking place in humans during aversive conditioning. Temporal difference models that allow the modeling of prediction error during the acquisition of aversive conditioning have identified the ventral striatum, anterior insular, and anterior cingulate cortices as being important in these processes (Ploghaus et al. 2000; Seymour et al. 2004).
_
Why Cancer Pain?
For the more than 10 million people worldwide who are diagnosed with some form of cancer each year, pain associated with their condition is a serious concern. Although pain is not necessarily inevitable for everyone with cancer, it is common. Approximately one-third of adults who are actively receiving treatment for cancer and two-thirds of those with advanced malignant disease experience pain. Children with cancer have similar pain experiences. While increasing numbers of medical professionals and governments are beginning to place more attention on the pain suffered by long-term survivors of cancer, much more research is needed. The consequences of unrelieved cancer pain are devastating and can include functional impairment, immobility, social isolation, and emotional and spiritual distress. In some cases, cancer pain that is not managed can lead to the cessation of potentially curative therapies, ultimately having a negative impact on the patient’s survival. Cancer patients express greater fear of dying in pain (i.e., suffering) than dying. Family and friends also suffer as they witness the pain and anguish experienced by a loved one with cancer.
_
Painful sex:
Intercourse pain, or dyspareunia, can cause problems in a couple’s sexual relationship. In addition to the physically painful sex, there is also the possibility of negative emotional effects. For both men and women, pain can occur in the pelvic area during or soon after sexual intercourse. It can happen at any time during sex — for example, at the time of penetration, erection, or ejaculation — or after sexual activity. Eventually, ongoing pain may cause a person to lose interest in any sexual activity. In many cases, a woman can experience painful sex if there is not sufficient vaginal lubrication. When this occurs, the pain can be resolved if the female becomes more relaxed, if the amount of foreplay is increased, or if the couple uses a sexual lubricant. In some cases, a woman can experience painful intercourse if one of the following conditions is present:
1. Vaginismus. This is a common condition in which there is a spasm in the vaginal muscles, mainly caused by the fear of being hurt.
2. Vaginal infections. These conditions are common and include yeast infections.
3. Problems with the cervix (opening to the uterus). In this case, the penis can reach the cervix at maximum penetration, so problems with the cervix (such as infections) can cause pain during deep penetration.
4. Problems with the uterus. These problems may include fibroids that can cause deep intercourse pain.
5. Endometriosis. A condition in which the endometrium (tissue lining the uterus) grows outside the uterus.
6. Problems with the ovaries. Such problems might include cysts on the ovaries.
7. Pelvic inflammatory disease. The tissues deep inside become badly inflamed and the pressure of intercourse causes deep pain.
8. Ectopic pregnancy. A pregnancy in which a fertilized egg develops outside of the uterus.
9. Menopause. The vaginal lining can lose its normal moisture and become dry.
10. Intercourse too soon after surgery or childbirth.
11. Sexually transmitted diseases. These may include genital warts, herpes sores, or other STDs.
12. Injury to the vulva or vagina. These injuries may include a tear from childbirth or from a cut (episiotomy) in the perenium (area of skin between the vagina and the anus) that is made during labor.
13. A diaphragm that does not fit properly
14. Sexual abuse or rape
_
Causes of painful intercourse in men include:
1. Hemorrhoids
2. Herpes sores, genital warts, or other sexually transmitted infections (STIs)
3. Prostatitis — inflammation of the prostate
4. Reaction to the latex of a diaphragm or condom
_
Can you die from pain?
Yes, but hardly ever. In a patient with a heart or cerebrovascular condition, a severe pain could cause death by inducing a fatal stroke, infarction or arrhythmia. In some cases, the increase in the sympathetic outflow caused by the distress of the pain would stress the cardiovascular system to a point in which a fatal tachyarrhythmia is precipitated or an already damaged blood vessels could break in the brain causing death. Occasionally the vaso-vagal reflex could cause a lethal bradyarrhythmia.
_
Pain and emotions:
_
_
For more than 60 years, the experience of pain has been reported to be associated with various negative emotional states, including depression, anxiety, fear, and anger (Chapman et al., 1946; Hemphill et al., 1952; Ramzy & Wallerstein, 1958; Schachter, 1957; Webb & Lascelles, 1962). Studies have generally found that higher levels of negative emotions are associated with greater acute and chronic pain intensity (e.g., Bruehl et al., 2002; Janssen, 2002; Linton, 2000; Staud, 2004), and possibly with increased risk of developing chronic pain (Linton, 2005). Although these positive associations between pain and negative emotional states undoubtedly exist, underlying mechanisms remain only incompletely understood. From an evolutionary perspective, an association between emotions and pain responses might be expected, given that pain in times past was often the result of situations threatening survival of the organism (e.g., attack). Anger and fear would be the two emotions most likely to be elicited under such threatening circumstances, as they reflect motivational states underlying the fight and flight responses, respectively (Averill, 1983; Fendt & Fanselow, 1999; Sewards & Sewards, 2002). Consistent with the idea of anger as a primary emotional reaction to physical pain, human experimental studies confirm that acute physical discomfort triggers significantly increased anger and anger-related thoughts, even more so than fearful reactions (Berkowitz, 1990). In addition to pain triggering emotional reactions, acute emotional states can also affect pain, and in some cases are even associated with analgesia. For example, there is a significant animal literature indicating that acute fear reactions to threat are associated with subsequent reductions in pain sensitivity, in part via endogenous opioid mechanisms deriving from the periaqueductal gray (De Oca et al., 1998; Lichtman & Fanselow, 1990; Rau et al., 2005). Similarly, interconnections between neural systems underlying anger and pain might be expected, although the nature of these interactions may be complex. Literature is reviewed indicating that greater tendency to manage anger via direct verbal or physical expression (trait anger-out) is associated with increased acute and chronic pain responsiveness. Neuroimaging data are overviewed supporting overlapping neural circuits underlying regulation of both pain and anger, consisting of brain regions including the rostral anterior cingulate cortex, orbitofrontal cortex, anterior insula, amygdala, and periaqueductal gray. These circuits provide a potential neural basis for observed positive associations between anger-out and pain responsiveness. Endogenous opioids appear to modulate activity in this brain network. Results of a series of studies support the hypothesis that the hyperalgesic effects of elevated trait anger-out derive in part from deficient endogenous opioid activity, most likely resulting from altered activity or connectivity in these brain regions.
_
Empathy through Pain perception:
There are patients with congenital insensitivity to pain (CIP) which is a rare condition. They don’t feel pain; cognition and sensation is otherwise normal; for instance they can still feel discriminative touch (though not always temperature), and there is no detectable physical abnormality. They offer a unique opportunity to test the model of empathy. Does the lack of self-pain representation influence the perception of others’ pain? CIP patients globally underestimate the pain of others when emotional cues were lacking, and that their pain judgments, in contrast with those of control subjects, are strongly related to interindividual differences in empathy trait. In other words, if you cannot feel the pain yourself, you would not feel the pain of others provided all other biological variables are same.
_
Pain and psychology:
Pain itself, and the ensuing disruption in normal lifestyle, can lead to depression. When people feel helpless and hopeless their pain is more intense. In fact, the emotional effects of chronic pain-including depression, anger and anxiety-may do more damage to long-term health than the actual physical degree of discomfort, report researchers in recent issues of Cranio and The Clinical Journal of Pain. In studies of patients with chronic facial pain, researchers found that the psychological effects of being in pain were more disruptive to patients’ daily lives than the pain itself.
_
_
Both acute and chronic forms of pain are familiar, but in addition pain occurs in two other quite different situations. It may occur as a symptom in a depressive illness. In other words it is not, as is commonly thought in such situations, that depression has developed because pain is being experienced but, in fact, the pain is part of a primary depressive illness. Up to half of those who develop depressive illnesses experience physical symptoms unrelated to any obvious underlying pathology, and of those symptoms pain is the most common. The failure of doctors to appreciate this fact does occasionally lead to a prolonged search for a physical cause for pain because its presence overshadows other features of a depressive illness. Pain occurs in individuals experiencing anxiety, or emotional tension. For example, tension headaches are very common. The presence of anxiety in a pain sufferer tends both to increase the severity of pain experienced and to reduce the individual’s tolerance or ability to cope with it. Pain may occur in the absence of an obvious physical cause and where the features of a mental illness are not detectable. Individuals with this type of pain may have had a trivial injury but the level of pain and disability with which they present is out of all proportion to the severity of that injury. In addition, the behavior shown by the sufferer reveals considerable dependence upon others, loss of willingness to take responsibility for themselves, their home, and their work, and a preoccupation with a search for a ‘cure’ for the pain, which they regard firmly as physical in origin.
_
Specific psychological factors:
Although early theories focused on global factors, more recent areas of study have developed our understanding of specific psychological traits or specific states of experience that affect the report of pain and suffering.
1. Fear:
Pain functions to threaten danger and invoke an escape or ameliorative response. This threat component of pain is not an addition to the sensory component, nor does it follow from the sensory aspects. Instead, it is a primary and central component as it urges analgesic behavior. Fear and anxiety processes have been studied from a number of perspectives, although they cover essentially the same issue. The most relevant to clinical practice are reviewed here.
_
2. Attention and vigilance:
Threatening pain is a stimulus that orients attention to both the source of pain and the potential for escape or analgesia. Some people have increased or heightened attention to pain sensation. In particular, where the threat of pain is constant or recurrent, a pattern of vigilance to pain can develop. McCracken developed a measure of vigilance to pain with a sample of chronic low back pain patients and found that patients who report high levels of attention to pain also report higher pain intensity, increased use of health‐care resources and more emotional distress. Vigilance to pain was a significant predictor of disability, distress and use of health‐care resources. Hypervigilance or excessive attention to threat has also been offered as a possible explanation for the dominant anxiety and poor concentration observed in patients with diffuse idiopathic or fibromyalgia pain. One test of this hypothesis found that fibromyalgia patients reported a lower threshold and higher tolerance to an experimentally induced pain than did a sample of patients with rheumatoid arthritis, who, in turn, reported lower threshold and higher tolerance than a non‐pain control sample.
_
3. Catastrophizing and worry:
The consequences of repeated attention to threat may be the development of a fixed pattern of responding to threatening stimuli and pain. One particular response to threatening pain, which is proving to be predictive of the severity of complaint of pain, has been termed ‘catastrophic thinking’ or ‘catastrophizing’. Put simply, this is a habitual, almost immediate, appraisal of a situation as extremely and globally catastrophic. Sullivan and colleagues have developed a measure of catastrophic thinking about pain that assesses the extent to which we magnify the outcome and effects of pain, consider ourselves helpless to respond, and have little control over whether we think this way or not. They conducted two experiments, the first with pain‐free students, who they subjected to a cold‐pressor procedure, and the second with patients undergoing an aversive medical procedure. They found that catastrophizers reported significantly more negative pain‐related thoughts, more distress and higher pain intensity compared with non‐catastrophizers. Keefe and colleagues have used a different measure of pain control and catastrophizing in studying clinical populations. For example, they studied patients with rheumatoid arthritis who had undergone knee replacement surgery and found that those who rarely catastrophized had much lower levels of pain and disability than patients who catastrophized often. So now we have well-described phenomenon of pain catastrophizing, or the maladaptive responses to pain (tendency to focus on and magnify pain sensations with an intense sense of unbearable suffering and helplessness) that plays an extremely important role in how pain is perceived and processed. Pain catastrophizing now accounts for a substantial proportion of pain-related disability. Studies in patients with fibromyalgia show that pain catastrophizing is associated with increased activity in brain areas related to anticipation of pain (medial frontal cortex, cerebellum), attention to pain (dorsal ACC, dorsolateral prefrontal cortex), emotional aspects of pain (claustrum, closely connected to amygdala) and motor control. Thus, catastrophizing influences pain perception through altering attention and anticipation, and heightening emotional responses to pain.
_
4. Avoidance:
One consequence of the urgency effect of pain, the fact that pain demands a change of behavior, is that patients with pain avoid pain‐inducing activity. A number of studies now show that the pain alone is insufficient to explain disability and avoidance. McCracken and colleagues, for example, demonstrated that the fear of pain made a unique and significant contribution to the prediction of disability. Taking this further, some authors have argued that the fear of pain is more disabling than pain itself. In a recent study of this idea, Crombez and colleagues replicated the finding that pain‐related fear is a better predictor of disability than pain, but also extended the findings to a behavioral performance test. They showed that, when instructed to engage in a behavioral performance task that involves musculoskeletal loading, chronic low back pain patients performed poorly on the task. Poor behavioral performance was predicted by elevated levels of fear of (re)injury due to movement and the fear of the effect that physical activity would have on the pain. Pain‐related fear is thought to mediate the effects of pain upon performance. A recent authoritative review of this emerging field argues that the avoidance of pain or injury‐inducing activity is a normal mechanism of survival. However, when pain becomes chronic, those with marked fear of pain chronically avoid activity that leads to disability. Counter‐intuitively, in many cases of chronic non‐malignant pain, it may be healthier to confront or engage in physical activity that, in the short term, produces pain and the fear of pain & (re)injury, but in the long term relieves pain and disability.
_
5. Depression (vide infra):
The experience of pain and the threat of pain can lead to negative or low affect. Chronic low affect, including persistent feelings of frustration and anger & negative or destructive self‐appraisal are common effects of persistent pain. Unsurprisingly, the majority of adult chronic pain patients who present for treatment at pain clinics are also depressed to some degree. However, this depression is not brought about directly by the pain severity but by the disabling consequences of how one reacts to the chronic pain. There are a number of facets of depression that are important in understanding the pain patient.
_
6. Anger:
Anger is not always associated with depression. However, it is included here as the angry pain patient is often poorly understood. Anger is a relatively common experience for pain patients and so, in turn, for the pain professional. Where there is no clear immediate object of anger (e.g. an aggressive other person or an immediate agent of injustice), it is often associated with global frustration and hostility, feelings of aggression and a feeling of being blamed. Anger in chronic pain patients is often unrecognized as a means by which patients attempt to claim self‐control or self‐esteem. Anger and hostility can have significant deleterious effects upon both health and treatment effectiveness.Treatment of the very angry patient requires a high degree of trust and honesty in an environment of cynicism and hostility. Aggression and overt anger often increase the probability of treatment ineffectiveness as either patient or therapist will withdraw from therapeutic contact, thereby fuelling anger. Treatments designed for the chronic pain patient should directly address in some form the effects of anger and frustration.
_
7. Self‐denigration:
A key component of depression is the extent to which individuals appraise their self‐worth and abilities negatively (e.g. ‘I’m useless and pathetic’, ‘I’ll never be able to control this pain’). Early research suggested that negative self‐appraisal may promote a self‐fulfilling prophecy in which patients learn to be helpless and hopeless. Research with rheumatological patients did not find any convincing evidence for this case. Rather, recent evidence indirectly suggests that what may be important about depression in chronic pain is the extent to which the pain refers critical judgment onto the self. Recent experimental studies demonstrate that patients have specific, not global, memory biases for pain information that refers negatively to the self. Although a focus on the specific self‐denigrating effects of depression and pain is only now being developed and data are certainly needed, it could have far‐reaching effects on current self‐management approaches to chronic pain. Simply instructing patients that the route to successful management of pain lies with them may be an invitation to fail. Indeed, many pain patients, when presented with the idea of self‐management, first understand this to mean a threat to their worthiness for treatment.
_
8. Coping:
The term ‘coping’ is often used to denote two similar events. First, it is understood to mean anything that one does in response to a stressful event, regardless of its efficacy in removing the stressor or in relieving the stress response. Secondly, it is understood to mean a positive effect of either removing the stressor or relieving the stress response. Here the first meaning is taken. Whenever we are faced with a stressful event such as pain, or the fear of pain, we respond. This response can have both positive and negative effects. The personality variables discussed above will have a strong effect on the response people make to pain and/or the fear of pain. However, the search for patterns of responding or types of responding has also included other ideas worth mentioning.
_
9. Action and control:
First, the idea that there are passive and/or active ways of responding is commonly held. Patients who are passive in response to threat show greater distress and disability than patients who attempt to solve problems. Similarly, those who believe that they have the personal ability to have control over pain also show improved function and fitness. One interesting investigation found that if women in active labor are given some control over parts of the delivery process, positive effects can be seen in terms of reduced pain, reduced tiredness and increased energy even if this control is only at the level of monitoring. Taking some control over the cause of pain or the method of analgesia has a beneficial effect. Those who respond actively to pain or the fear of pain are more likely to adjust effectively.
_
10. Information and predictability:
Related to whether one takes action or takes part in analgesic procedures is the effect of whether one seeks to predict the effects of pain or whether one prefers to be distracted. Many experimental studies of the possible effects of distraction from, or attention to, pain and analgesia have been conducted. The key finding is that both approaches can be effective. However, the most important finding suggests that only those strategies that fit with a person’s preferred or habitual method will be effective. For example, if someone is used to managing the pain of dentistry by thinking of anything else but dentistry, giving the patient detailed information about the procedure will simply undermine an effective strategy. Crombez and colleagues reported an interesting study of what information it might be useful to have for those who pay attention to the pain. They found that information about the sensation of the expected pain did not improve the reaction to the pain. However, knowing how long a pain will last did improve the reaction to the pain.
_
11. Making sense of the pain:
People are intrinsically motivated to make sense of experience. Except in extreme cases of depression or in specific circumstances of prolonged restriction or incarceration, people are motivated to reach an understanding of personal events. Until a pain is understood within a system of knowledge, it will interrupt current thinking and promote worry and concern. Knowing what has caused a pain and what it may mean and does not mean is critical for effective coping. Those patients who are most difficult to help are those who repeatedly present with problems that have no known etiology. Not knowing compounds distress and an uncertain diagnosis leads to an increased belief in illness.
_
Clinical implications of psychological factors in pain management:
Most clinicians ignore these factors and do not attempt to harness their effects. Worse still, there is a large industry dedicated to the eradication of these effects as they pollute otherwise neat designs for testing the effects of pharmacological agents upon an analgesic response. For it is these effects that make up the placebo element of all analgesics. One could suggest that in most acute pain situations, these factors take care of themselves and do not need attending to. However, psychological factors are central to the experience of pain, the delivery of effective analgesia and for the specific treatment of chronic pain and disability.
_
Pain and depression:
_
_
A strong association between chronic pain and depression has long been suggested. Not surprisingly, serotonin and norepinephrine, the neurotransmitters most associated with depression, play key roles in the modulation of pain. Pain and depression are closely related. Depression can cause pain — and pain can cause depression. Sometimes pain and depression create a vicious cycle in which pain worsens symptoms of depression, and then the resulting depression worsens feelings of pain. In many people, depression causes unexplained physical symptoms such as back pain or headaches. Sometimes this kind of pain is the first or the only sign of depression. Pain and the problems it causes can wear you down over time, and may begin to affect your mood. Chronic pain causes a number of problems that can lead to depression, such as trouble sleeping and stress. Disabling pain can cause low self-esteem due to work, legal or financial issues. Depression doesn’t just occur with pain resulting from an injury. It’s also common in people who have pain linked to a health condition such as diabetes or migraines.
_
Physical symptoms are common in depression, and, in fact, vague aches and pain are often the presenting symptoms of depression. These symptoms include chronic joint pain, limb pain, back pain, gastrointestinal problems, tiredness, sleep disturbances, psychomotor activity changes, and appetite changes. A high percentage of patients with depression who seek treatment in a primary care setting report only physical symptoms, which can make depression very difficult to diagnose. Physical pain and depression have a deeper biological connection than simple cause and effect; the neurotransmitters that influence both pain and mood are serotonin and norepinephrine. Dysregulation of these transmitters is linked to both depression and pain. Antidepressants that inhibit the reuptake of both serotonin and norepinephrine may be used as first-line treatments in depressed patients who present with physical symptoms.
_
The prevalence of pain indepressed cohorts and depression in pain cohorts are higherthan when these conditions are individually examined. The presenceof pain negatively affects the recognition and treatment ofdepression. On average, 65% of patientswith depression experience one or more pain complaints, anddepression is present in 5% to 85% (depending on the study setting)of patients with pain conditions. Depression is most prevalentin pain, psychiatric, and specialty clinics v/s population-basedor primary care studies. When pain is moderate to severe, impairs function,and/or is refractory to treatment, it is associated with moredepressive symptoms and worse depression outcomes (e.g., lowerquality of life, decreased work function, and increased healthcare utilization). Similarly, depression in patients with painis associated with more pain complaints and greater impairment.Depression and pain share biological pathways and neurotransmitters,which has implications for the treatment of both concurrently.
_
The biochemical theory of depression posits that depression is the result of a neurochemical imbalance or a functional deficiency of key neurotransmitters, the monoamines: serotonin, norepinephrine, and dopamine. A common theory holds that depression and painful symptoms follow the same descending pathways of the central nervous system. Eight studies described the biological link between depression and pain. Although nociceptive fibers transmitting pain signals from the periphery of the body through the dorsal horn to the medulla, midbrain, hypothalamus, thalamus, limbic cortical areas (anterior cingulate and insular cortex), somatosensory cortex, and posterior parietal cortex have been carefully mapped, there is an increasing interest in the neuroanatomy of a descending system of pain modulation (vide supra). The increasing knowledge about this system allows scientists and physicians to better understand mechanisms of pain modulation via medications as well as psychological mechanisms such as expectation, attention and distraction, and negative and positive affect.
_
Pain transmission occurs via ascending (excitatory) and descending (inhibitory) pathways involving norepinephrine and serotonin, or 5-HT. Serotoninergic neurons originate in the brain stem raphe region and project throughout the central nervous system (CNS), including descending projections to the spinal cord, which result in suppression of sensory input. They also project to areas of the brain including the frontal cortex (mediating mood), hypothalamus (mediating appetite and sleep), and amygdala (mediating anxiety and fear response). A reduction of presynaptic serotonin release and a compensatory upregulation of 5-HT2, a postsynaptic serotonin neuron, have been found in depressed patients. Limited research has also suggested that pain may increase the turnover of serotonin.
_
Brain pathways that handle the reception of pain signals, including the seat of emotions in the limbic region, use some of the same neurotransmitters involved in the regulation of mood, especially serotonin and norepinephrine. When regulation fails, pain is intensified along with sadness, hopelessness, and anxiety. And chronic pain, like chronic depression, can alter the functioning of the nervous system and perpetuate itself. The mysterious disorder known as fibromyalgia may illustrate these biological links between pain and depression. Its symptoms include widespread muscle pain and tenderness at certain pressure points, with no evidence of tissue damage. Brain scans of people with fibromyalgia show highly active pain centers, and the disorder is more closely associated with depression than most other medical conditions. Fibromyalgia could be caused by a brain malfunction that heightens sensitivity to both physical discomfort and mood changes.
_
The presence of pain negatively affects the recognitionand treatment of depression. Depression is often under recognizedand thus frequently undertreated. At least 75% of primary carepatients with depression present with physical complaints exclusively and seldom attribute their pain symptoms to depression orother psychiatric illness. These physical complaints may bedue to amplification of chronic physical disease and remainmedically unexplained after extensive workup. As a result, providersfrequently assess for physical causes of pain and treat medicallyinstead of exploring the pain symptoms in a broader, biopsychosocialcontext.Primary care providers should recognize that pain is a commonsymptom of depression, that depression and painful conditionsfrequently coexist, and that evaluation and treatment of bothare important.
_
To get symptoms of pain and depression under control, you may need separate treatment for each condition. However, some treatments may help with both.
1. Because of shared chemical messengers in the brain, antidepressant medications can relieve both pain and depression.
2. Psychological counseling (psychotherapy) can be effective in treating both conditions.
3. Stress-reduction techniques, meditation, staying active, journaling and other strategies also may help.
Treatment for co-occurring pain and depression may be most effective when it involves a combination of treatments.
_
The two major types of antidepressants, tricyclics and selective serotonin reuptake inhibitors (SSRIs), may have different roles in the treatment of pain. Amitriptyline, a tricyclic, is one of the antidepressants most often recommended as an analgesic, partly because its sedative qualities can be helpful for people in pain. SSRIs such as fluoxetine and sertraline may not be quite so effective as pain relievers, but their side effects are usually better tolerated, and they are less risky than tricyclic drugs. Some physicians prescribe an SSRI during the day and amitriptyline at bedtime for pain patients. Both drug classes act in brain pathways that regulate mood and the perception of pain. Tricyclics heighten the activity of the neurotransmitters norepinephrine and serotonin; SSRIs act more selectively on serotonin. Some researchers and clinicians believe that a newer antidepressant which acts strongly on both neurotransmitters, the so-called dual action drug venlafaxine , is superior to both tricyclics and SSRIs for treating pain. Physicians and psychiatrists are also considering the uncertain potential of the anticonvulsant drug gabapentin and drugs that block the activity of substance P, another neurotransmitter involved in the regulation of both pain and depression. Electroconvulsive therapy (ECT), a standard treatment for severe depression, may have independent analgesic effects.
_
A differing study:
Treat chronic pain and depression independently:
In a study appearing in the May 2005 issue of Arthritis & Rheumatism, the researchers used functional magnetic resonance imaging of the brain to determine that in patients with fibromyalgia, their level of depression has little influence on the intensity of pain they experience. “We have seen that if you give antidepressants to the average patient with fibromyalgia, they’ll come back a couple of months later and say, ‘My pain isn’t any better, but I don’t feel so sad about it,’ ” researcher said. “Our research provides further evidence that these pathways are quite independent.” While other clinical research has supported the idea that pain and depression should be treated independently of each other, the researchers say this is the first time it has been shown using functional imaging brain scans. There is an incorrect impression among many doctors that if you treat a patient’s depression, it will make their pain better. Not so. If someone has pain and depression, you have to treat both. Approximately 30% to 54% of people with chronic pain also have a major depressive disorder.
_
Pain and cognition:
Research suggests that pain-related negative emotions and stress potentially impact cognitive functioning independent of the effects of pain intensity. The anterior cingulate cortex is likely an integral component of the neural system that mediates the impact of pain-related distress on cognitive functions, such as the allocation of attentional resources. A maladaptive physiologic stress response is another plausible cause of cognitive impairment in patients with chronic pain, but a direct role for dysregulation of the hypothalamic-pituitary-adrenocortical axis has not been systematically investigated. Pain catastrophyzing as discussed earlier is a type of cognitive error developed by people in pain. In other words they develop an unnecessarily negative view of their condition and its likely outcome. In such a state they tend to focus to an extent upon the negative features of their disorder.
__
Pain, society and culture:
It has been acknowledged that pain (like the brain itself) occupies a strange liminal position between biology and culture. We know that expression of personal response to pain (even when measured objectively under test conditions) is bewilderly varied, not only between individuals but also between cultures. It seems that our particular sensitivity to pain, is not simply a matter of genetics, physiology and circumstance, but also one of learned “hermeneutics” , the way we interpret our pain is all important for the mode of our suffering of it.
_
As a consequence of the need to encompass the physical, psychological, and social aspects of pain experience, clinicians and pain researchers have developed what is known as the biopsychosocial model of pain. It is based upon what we know about the generation and control of pain within the nervous system, and also its psychological aspects and the social factors that influence the thinking of individuals about pain and their behavior. This approach to pain has lead to the development of powerful psychological tools for pain management, which come under the broad heading of cognitive-behavioral theory and practice.
_
_
_
Cultural barriers can also keep a person from telling someone they are in pain. Religious beliefs may prevent the individual from seeking help. They may feel certain pain treatment is against their religion. They may not report pain because they feel it is a sign that death is near. Many people fear the stigma of addiction and avoid pain treatment so as not to be prescribed addicting drugs. Many Asians do not want to lose respect in society by admitting they are in pain and need help, believing the pain should be borne in silence, while other cultures (e.g. Jewish) feel they should report pain right away and get immediate relief.
_
The nature or meaning of physical pain has been diversely understood by religious or secular traditions from antiquity to modern times. Physical pain is an important political topic in relation to various issues, including pain management policy, drug control, animal rights or animal welfare, torture, and pain compliance. In various contexts, the deliberate infliction of pain in the form of corporal punishment is used as retribution for an offence, or for the purpose of disciplining or reforming a wrongdoer, or to deter attitudes or behavior deemed unacceptable. In some cultures, extreme practices such as mortification of the flesh or painful rites of passage are highly regarded.
_
Pain is so unique from other sensations such as touch, smell and taste that pain is defined as an ‘emotion or experience’. Pain, just like your emotions, is influenced by your thoughts, culture, beliefs and attitude. In the 1950’s Henry Beecher, a military doctor in World War II, looked at the magnitude of injury and the morphine dose soldiers took to control pain. As expected, the greater the injury, the greater the morphine dose. And hence he concluded that there is no influence of your emotions and thinking on your pain. To apply these finding to civilians, he did the same study on civilians. And he found the same: the greater the injury, the greater the morphine. But there was one critical difference – for the same amount of tissue damage, the civilians took three times more morphine that the soldiers! How the heck is that possible? For a soldier, the injury meant he survived the war and he can recover and go back home. However, a civilian looked at the injuries from a completely different and negative perspective. For the civilian, the injury meant an awful situation which will dramatically change their life for the worse. Their emotions, attitudes and beliefs influenced how the brain perceived the threat level of the injury and modulated pain accordingly. It is now clear from brain imaging studies that that there is no single ‘pain centre in the brain’ as we used to believe. Many areas in the brain are actively involved in constructing and modulating this multisensory experience called pain (known as the Pain Neuromatrix). It is very appropriate to say that pain is an output constructed in the brain and not an input to the brain as we used to believe.
_
Consideration of socio-cultural and learning factors reveals that learning about pain takes place within a definite social context, and the way each of us behaves when in pain reflects that fact. At a national level it is customary in general for those who are from Northern European countries to regard complaints about pain, especially amongst men, as a weakness of character. In contrast, in Southern European countries to complain about pain is regarded as beneficial to the sufferer. These are very broad generalizations but do have some basis in fact. An important psychological mechanism by which we learn the behaviors we exhibit when in pain is defined as operant learning. It is a process by which overt behavioral responses to a stimulus are significantly influenced by their consequences, including the responses of others to them.
_
Operant learning & conditioning:
Operant learning is well illustrated by the effects of a simple injection upon a child. The sight of the needle and the pain experienced is an ‘unconditioned stimulus’ and as a response to it the child cries. On the next occasion the child cries at the sight of the syringe and needle, which has become ‘the conditioned stimulus’. If crying leads to the abandonment of the injection the child has developed a ‘conditioned escape response’. Seeing another child crying before an injection which is then not given leads to another type of learning — ‘an observational learning model’.
_
In some individuals such mechanisms lead to the development of pain behaviors that have a negative effect upon their lives — for example, the excessive use of rest to relieve pain, or the abuse of powerful narcotic-related drugs may actually lead to increasing chronicity of pain and disability. To counter such developments, psychologists have developed techniques based upon operant conditioning, which are designed to reverse maladaptive pain behaviors and to replace them by adaptive behaviors. In other words, their techniques involve the use of learning of behavior designed to lead to coping with pain and everyday life rather than withdrawing from them. Put in simple terms, ‘good behavior is rewarded and bad behavior is punished’.
_
Operant conditioning has been criticized on the grounds that it does not take sufficient account of mental activity. In other words, individuals have thoughts about pain and attitudes towards it. They draw on memories of past experience when in pain, and this leads to thinking and behavior, which is the result of those experiences. Such thoughts and attitudes, or cognitions, as they are called, cannot be ignored when a clinician is evaluating a person in pain and planning their treatment. For this reason, a purely behavioral approach has been replaced by a cognitive-behavioral approach to pain analysis and management. The main cognitive elements that have been identified include beliefs about pain and its causes, beliefs about the extent to which the individual feels he or she has control over pain, and the extent to which individuals believe that they are able to function despite pain. Therefore, self-efficiency is a significant factor in determining ability to cope
_
Pain Management:
The Cartesian model of thinking was proposed by the philosopher Descartes almost 450 years back. Descartes wrote, “The flame particle jumps from the fire, touches the toe, moves up the spinal cord until a little bell goes off in the brain and says, ‘ouch, it hurt’.” Now we believe that pain is perceived, processed and felt as pain by brain.
_
_
The figure above shows the old Cartesian model of pain and the new model which shows pain as a multisensory experience affected by both bottom-up and top-down inputs.
_
Bottom-Up Approach (Nociceptive Mechanisms):
This involves any treatments which lowers or inhibits the nociceptive signals (bottom) to the brain (hence called bottom-up approach). Most current pain therapies targeting the tissues and joints are based on this ‘bottom-up’ approach. A simple example would applying ice or heat to the damaged area. Another example would be lowering the body weight for if you have knee or low back pain. McKenzie’s method, Postural correction, Sahrmann’s movement impairment syndrome, Trigger Point therapy, ART, Functional Movement Screens and all come under this category. But the problem with this ‘bottom up’ approach is that the treatment is rationalized in a context which reinforces the belief that there is something wrong in their tissues and joints (and thereby raising the threat level) and may only bring temporary relief.
_
Top-Down Approach (Non-Nociceptive Mechanisms):
This is done by educating the person about the physiology of pain, the role of brain in pain, and ”how pain does not mean harm” (hence called top-down approach). If we explain pain based on our structural-pathology model, every time people feel pain they think they got hurt or re-injured themselves and naturally try to avoid pain-causing behaviors. This thinking process heightens the threat level in the brain leading to pain persistence (fear-avoidance belief model). The fear-avoidance model is now seen as a central mechanism of how acute pain turns into chronic pain. Pain education should make them understand that “pain does not mean harm”. Most of our current treatments based on the structural-pathology model may provide temporary pain relief, but pain explained based on our current model only helps to heighten fear of pain and anxiety in the patient. It has been shown in recent studies that teaching patients about modern pain biology can change beliefs and attitudes about pain and lower the pain sensitivity. Further, when education about pain physiology is included into physiotherapy treatment of patients with chronic pain, pain and disability are reduced.
_
It would be more scientifically correct to include both methods in pain management.
_
Pain management (also called pain medicine or algiatry) is a branch of medicine employing an interdisciplinary approach for easing the suffering and improving the quality of life of those living with pain. The typical pain management team includes medical practitioners, clinical psychologists, physiotherapists, occupational therapists, and nurse practitioners. Pain sometimes resolves promptly once the underlying trauma or pathology has healed, and is treated by one practitioner, with drugs such as analgesics and (occasionally) anxiolytics. Effective management of long term pain, however, frequently requires the coordinated efforts of the management team. Treatment approaches to long term pain include pharmacologic measures, such as analgesics, tricyclic antidepressants and anticonvulsants, interventional procedures, physical therapy, physical exercise, application of ice and/or heat, and psychological measures, such as biofeedback and cognitive behavioral therapy. Pain management practitioners come from all fields of medicine. Most often, pain fellowship trained physicians are anesthesiologists, neurologists, physiatrists or psychiatrists. Palliative care doctors are also specialists in pain management. As well as medical practitioners, the area of pain management may often benefit from the input of physiotherapists, chiropractors, clinical psychologists and occupational therapists, amongst others. Together the multidisciplinary team can help create a package of care suitable to the patient.
_
There is now growing recognition that poorly relieved acute pain increases the occurrence of cognitive dysfunction, immune suppression, and chronic postsurgical pain. Consequently, perioperative treatments may have long-term implications on patient outcome and quality of life. The figure below shows that earlier the pain is controlled, better it is.
_
Acute pain management variables:
Acute pain management is influenced by a number of patient variables that have been shown to affect the intensity, duration, and interpretation of pain, as well as the safety and efficacy of analgesic therapy. To develop an appropriate plan for acute pain management, factors such as patient age, race, sex, pharmacogenomics, and surgical or medical comorbidites must be considered. In general, traditional as-needed (PRN) dosing regimens have difficulty accounting for variabilities in analgesic response and interindividual differences in pain, perception, and coping skills. Patient variables also influence the safety and effectiveness of more modern and sophisticated forms of analgesic administration, such as patient-controlled analgesia (PCA), neuraxial opioids, and peripheral neural blockade. Age is among the most important patient variables influencing analgesic response. Advancing age can alter analgesic dose response in several ways. A decrease in hepatic enzymes, particularly cytochrome P450 (CYP450), microsomes and glucoronidases, as well as diminished hepatic blood flow, can reduce opioid and local anesthetic metabolism and delay drug elimination. With regard to opioids, age-related reductions in plasma albumin may increase the fraction of unbound or active drug, whereas diminished pain transmission and central nervous system (CNS) activity may significantly reduce perception and subsequent processing of pain. Because of these factors, a negative correlation between age and postoperative opioid consumption is commonly observed.
_
Pain management misconceptions:
The biggest misconception that I see in pain management is that some patients believe that medications are the primary way to alleviate their pain. In reality, patients need a comprehensive treatment plan, including cognitive, behavioral, pharmacological, surgical, and rehabilitative modalities, in order to successfully address their painful symptoms. Many patients are not aware of the multitude of treatment options available to help decrease their pain, and improve their overall quality of life. Another misconception is that the patient thinks that years of neglecting their body can be remedied in one or two visits. They will almost always say, “All I did was to bend over to pick up the . . . and my back went out.” Yet, another misconception I see regularly is that the pain can be “cured” or eliminated, just because people are entitled to have this relief. For the vast majority of chronic pain patients, this is unrealistic. Pain management is not like the ads on TV: “I had pain, I took a pill, my pain went away!” Our culture is so sold on the idea that you can assign blame for something and then demand (and receive) complete relief (in the legal as well as the medical sense) from what they see on TV that the realities of actual pain management are truly inconceivable to them.
_
Analgesia and anesthesia:
Analgesia refers to the reduction or relief of pain. This is what commonly happens for example when you take paracetamol (acetaminophen) for a headache or when patients are given morphine after an operation for pain. Analgesia implies pain relief – but does not imply that all sensation is taken away. In other words, analgesia does not mean numbness. Anesthesia refers to lack of sensation. This can be accomplished with local anesthetics, for example the injection that your dentist gives you when you have a tooth filled. Almost always this also results in a lack of pain – therefore analgesia usually accompanies anesthesia. Note that when local anesthetics are used to provide anesthesia they also frequently cause muscle weakness or muscle relaxation. As you can see, there is a clear difference between pure analgesia (pain relief, sensation and muscle ability normal) and anesthesia (lack of sensation, might be accompanied by muscle weakness or relaxation). The analgesic drugs work in the peripheral and central nervous systems of the human body. Examples of such drugs that are considered to be analgesic are paracetamol, anti-inflammatory drugs (salicylates or ibuprofen), morphine, Flupirtine, and others. Anesthesia, meanwhile, has only three major classifications and that is the local anesthesia, the regional anesthesia and the general anesthesia. Each group of anesthetics focuses mostly on removing the sensation and feeling from the one part of the body or perhaps the entire human body, depending on the extent of the surgery. Analgesia is taken only after the effects of anesthesia have worn of. Anesthesia should be properly administered by a licensed physician while analgesia can easily be taken orally. General anesthetics work by shutting down the forebrain regions whose activity regulates cycles of arousal and quiescence. There are several kinds of general anesthetics, but those most commonly used enhance or mimic the action of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA).In contrast, a local anesthetic works by blocking the transmission of the pain message along a primary afferent nociceptor’s axon. The message thus never reaches the central nervous system.
__
Graded Exposure Approach or Activity:
In this approach, the person is gradually exposed to feared activities without causing pain and thereby lowering the threat level in the brain. These feared activities could be imagined movements, exercise, or daily functions. Many researchers believe that a large part of pain relief seen with exercise and other rehabilitation methods is from lowering the threat level in the brain using the graded approach. So when your patients talk about how they have less pain after lifting weights, it is because they were just gradually exposed in a graded manner to the threatening exercise.
_
Inadequate treatment of pain is widespread throughout surgical wards, intensive care units, accident and emergency departments, in general practice, in the management of all forms of chronic pain including cancer pain, and in end of life care. This neglect is extended to all ages, from neonates to the frail elderly. African and Hispanic Americans are more likely than others to suffer needlessly in the hands of a physician; and women’s pain is more likely to be undertreated than men’s. The International Association for the Study of Pain advocates that the relief of pain should be recognized as a human right, that chronic pain should be considered a disease in its own right, and that pain medicine should have the full status of a specialty. It is a specialty only in China and Australia at this time. Elsewhere, pain medicine is a subspecialty under disciplines such as anesthesiology, physiatry, neurology, palliative medicine and psychiatry.
_
Rest:
Resting can sound difficult, especially if you are being asked to rest when you are experiencing pain, or have plenty of tasks that you want to do, but learning relaxation techniques can relieve tension and ultimately constant pain messages to the brain. Fatigue will affect how you perceive pain and how you cope with it. Learning ways to relax completely can be a real trial for some people, others are gifted at it! You need to find the best way to help you relax. Some people find certain types of music help, others like absolute quiet. It is useful to try resting on a bed for a short time each day when the pain is bad; lying in a dark room, without any additional noises or distractions. A short sleep may even be worthwhile. Set an alarm to ensure that you only sleep for a certain period of time so that over time you don’t lose your normal night sleep patterns.
_
Exercise
The balance of rest, relaxation and exercise can improve pain control and maintain mobility. For most types of arthritis exercise is essential. This is because joints and muscles need to move regularly and muscles need to maintain their muscle strength and bulk. Some of the pain experienced in arthritis is related to the stiffness that can be experienced following periods of inactivity or early morning stiffness (EMS). The different types of exercise may vary according to the type of problems you have and the difficulties you are experiencing. If you are seeing a rheumatologist they will refer you to a physiotherapist to advise you on an exercise regime. However it is important to know whether you are doing ‘active exercise’ or ‘passive exercise’.
a) Active Exercise:
Active exercise is what most people do to keep fit and reduce weight. It involves things like going for a walk or gardening. They often require the body and the joints to bear weight and work against a force – such as lifting or digging in a garden.
b) Passive Exercise:
This is where the joints and muscles are worked but not against a specific weight or force. It involves moving the muscles and joints through a wide range of movements, this can be done while lying on the bed or sitting in a chair.
_
Many sedentary people believe that all exercisers have to experience pain if they are to become fitter: this belief is encouraged by the well known training motto: ‘No pain, no gain’. Sensible people interpret this as meaning that it takes effort, dedication, and commitment to improve fitness; it does not mean that exercise has to be a masochistic ordeal. On the contrary, if real pain is experienced, an exerciser should stop. Of course, an athlete who is training for top-class competition must be able to tolerate greater discomfort than a recreational athlete, but even an elite athlete must learn to distinguish between real pain and discomfort. Real pain acts as an important warning signal that something is wrong. If the signal is ignored, serious injury may result.
_
Weight Loss:
It is very difficult to lose weight, particularly when problems with mobility significantly reduce opportunities to undertake vigorous exercise regimes. However, it is worthwhile knowing that people with arthritis who are overweight have greater damage to the joints and increased difficulties with mobility and exercising. Losing weight can be a very effective way to reduce your pain. It may be worth seeking advice about a suitable diet that will help you lose weight yet ensure you maintain a healthy balanced diet.
_
The Use of Aids (walking sticks, joint protection, footwear):
For healthcare professionals, encouraging individuals to use various aids or assistive devices is often the hardest thing to achieve, yet it is one of the simplest ways of reducing fatigue, improving mobility, reducing pain and protecting joints. This is partly because most of us simply do not wish to consider the use of any form of aid, seeing it as recognition of a problem or as ‘giving in’. Yet the use of some equipment can be liberating, allowing more energy and time for other activities. Coming to terms with using any form of aid is often most effective when supported by help and advice from a member of the multidisciplinary team.
_
Walking Aids:
The way we use our body and our joints is taken for granted when everything works well. When a joint fails or is painful it is common for people to continue to walk, go up and down stairs etc but with pain and increasing difficulty. It is often achieved without the use of aids but usually is achieved at the cost of the other joints having to take the extra weight. This means that other joints will take the weight but not in a way that they are designed to. This means the weight will be distributed differently and add an extra load to healthier or less painful joints. An example of this is when you have a painful knee. To continue to walk you protect the painful joint by throwing your weight onto the ‘good’ knee. This results in your body changing the mechanics of how you position your back before movement, distributing the weight onto the ‘good’ knee joint. The good knee has to cope with the abnormal load by distributing the force through other joints. This can result in not only a painful knee but in addition a painful ankle or hip too. To minimize the risk of this, a walking stick could help by taking some of that additional load and maintaining a better body posture. When the knee settles and pain & function improves, you can then put the walking stick away.
_
Joint Protection:
Some types of arthritis or functional problems can be improved with the use of joint protection. It is quite common for people who have some types of arthritis (e.g. rheumatoid arthritis) to be assessed by an occupational therapist or rheumatology practitioner. The therapist or practitioner will assess the activities you undertake and see where stress on joints can be reduced to protect the joints and keep them in their normal alignment. The process of assessment will often include providing practical advice on how to avoid abnormal stresses or forces on the joints and can include additional pieces of equipment such as kettle tippers, reducing the need to lift a full kettle of water. When joints are more effectively protected pain can be reduced. The occupational therapist can provide information on the benefits of splinting and how to protect your joints.
_
Footwear:
Podiatrists are specialists trained in managing foot problems and other difficulties related to the foot and diseases that affect the feet. For many forms of arthritis early practical advice on footwear can be very effective. Our joints work as shock absorbers taking up the impact and reducing the force applied to the joints. When we are young there is a tissue called cartilage that lines all healthy joints and is nice and spongy and provides an effective cushioning for movement. As we grow older this cartilage becomes less flexible and in osteoarthritis may become worn away allowing two joints to move against each other without the cushioning effect of cartilage. One way of improving the ‘shock absorber’ or cushioning effects that used to be achieved by our young healthy joints is to ensure that shoes have good cushioning included in them. Trainers are a good example of cushioning support that a shoe can provide. However, there are a number of problems that might be experienced with different types of arthritis. A thorough foot assessment often proves very useful. The assessment will look at whether the arch support of your foot is maintained, and that you are bearing you weight down effectively onto your ankle and foot. Sometimes the use of an arch support can be very effective.
_
I have damaged meniscus in each knee and I found use of “knee support” and sports shoes excellent knee protector and pain reliever. My advice to any patient with mechanical/degenerative knee problem (osteoarthritis) is to maintain ideal body weight, not to sit on floor, not to climb stairs, wear knee support and a good pair of sports shoes; all of these will reduce pain substantially without any pain killer and prevent further damage to knees.
_
Analgesics:
An analgesic (also known as a painkiller) is any member of the group of drugs used to relieve pain (achieve analgesia). The word analgesic derives from Greek an- (“without”) and algos – (“pain”). Analgesic drugs act in various ways on the peripheral and central nervous systems; they include paracetamol (also known in the US as acetaminophen), the non-steroidal anti-inflammatory drugs (NSAIDs) such as the salicylates & ibuprofen, and opioid drugs such as morphine and opium. They are distinct from anesthetics, which reversibly eliminate sensation. Acute pain is usually managed with medications such as analgesics and anesthetics. Management of chronic pain, however, is much more difficult and may require the coordinated efforts of a pain management team, which typically includes medical practitioners, clinical psychologists, physiotherapists, occupational therapists, physician assistants, and nurse practitioners.
_
The types of drugs that you need to treat your pain depend on what type of pain you have. Everybody who has pain should consider taking painkillers but different painkillers work better for different types of pain. For pain associated with inflammation, such as acute back pain or headaches, paracetamol and anti-inflammatory medicines work best. If the pain is caused by sensitive or damaged nerves, as is the case with shingles or sciatica, it is usually treated with tablets that are also used for epilepsy and depression. These tablets change the way the central nervous system works.
_
Pain, inflammation and NSAIDs:
Cyclooxygenase (COX) is an enzyme that is responsible for formation of important biological mediators called prostanoids, including prostaglandins, prostacyclin and thromboxane. COX-1 is considered a constitutive enzyme, being found in most mammalian cells. COX-2, on the other hand, is undetectable in most normal tissues. It is an inducible enzyme, becoming abundant in activated macrophages and other cells at sites of inflammation. Let’s say you hit your finger with a hammer. The part of your finger that is damaged has nerve endings in it having nociceptors which get stimulated by mechanical force. The damaged tissue in your finger also releases some chemicals that make those nerve endings register the crushing shock even stronger — like turning up the volume on your stereo so you can hear it better. Some of these chemicals are prostaglandins, and working cells in the damaged tissues make these chemicals using an enzyme called cyclooxygenase 2 (COX-2). Because of the prostaglandins, the nerve endings that are involved now send a strong signal through nerves in your hand, then through your arm, up your neck and into your brain, where your mind decides this signal means, “pain”. The prostaglandins seem to contribute just a portion of the total signal that means pain, but this portion is an important one. In addition, prostaglandins not only help you to feel the pain of the damaged finger, but they also cause the finger to swell up (this is called inflammation) to bathe the tissues in fluid from your blood that will protect it and help it to heal. Remember this is a simplified version of the pain story; lots of chemicals seem to be involved in this process, not just prostaglandins. Pharmacological inhibition of COX can provide relief from the symptoms of inflammation and pain. Aspirin, ibuprofen and naproxen treat pain, swelling and fever by blocking enzyme COX-2 reducing prostaglandins produced in response to inflammation. These medicines are called the non-steroidal antiinflammatory drugs (NSAIDs) because they decrease swelling but they aren’t steroids, which are the most potent antiinflammatory chemicals we have. Non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin and ibuprofen, exert their effects through inhibition of COX. Older generation NSAIDs block both COX-1 and COX-2. The problem with the fact that NSAIDs go through your entire bloodstream is that your body needs prostaglandins for some reasons. One place they are useful is in the stomach; it turns out that COX-1 makes a prostaglandin that seems to keep your stomach lining nice and thick. NSAIDs also block COX-1 from working, and your stomach lining gets thin, allowing the digestive juice inside to irritate it. Selectivity for COX-2 is the main feature of celecoxib, rofecoxib, and other members of this drug class. Because COX-2 is usually specific to inflamed tissue, there is much less gastric irritation associated with COX-2 inhibitors, with a decreased risk of peptic ulceration. The selectivity of COX-2 does not seem to negate other side-effects of NSAIDs, most notably an increased risk of renal failure, and there is evidence that indicates an increase in the risk for heart attack, thrombosis, and stroke through an increase of thromboxane unbalanced by prostacyclin (which is reduced by COX-2 inhibition). Rofecoxib was withdrawn in 2004 because of such concerns. Some other COX-2 selective NSAIDs, such as celecoxib, and etoricoxib, are still on the market. Another family of medicines related to aspirin includes acetaminophen (paracetamol), which decreases fevers and pain, but it doesn’t affect either swelling or your stomach as much as the true NSAIDs do. Recent findings do suggest that it is highly selective for COX-2. While it has analgesic and antipyretic properties comparable to those of aspirin or other NSAIDs, its peripheral anti-inflammatory activity is usually limited by several factors, one of which is the high level of peroxides present in inflammatory lesions.
_
NSAIDs are considered more effective because of their better patient compliance due to the better dosing regimens and because they contain a higher effective dose. Also, parenteral administration of NSAIDs may allow them to be used for treatment of pain syndromes associated vomiting or because of the severity of the pain; e.g. renal and biliary colic. Also, various studies demonstrate that NSAIDs may be effective in the treatment of severe pain syndromes. NSAIDs are recently used to prevent post-operative pain by prophylactic administration before surgery decreasing inflammation via blocking prostaglandin synthesis and prevent sensitization of the peripheral terminals of nociceptive afferents.
_
Two NSAIDs are not better than one:
Millions of people take NSAIDs every day for arthritis, acute injury, and menstrual cramps. According to research presented at the 2007 Annual Scientific Meeting of the American College of Gastroenterology, patients underreport their use of over-the-counter NSAIDs. Survey results have shown that patients who don’t report over-the-counter NSAID use believe the drugs are insignificant because they are available without a prescription. Taking two different NSAIDs is not good — it can increase the risk of undesirable side effects and serious adverse events. It has also been found to worsen health-related quality of life (the ability to perform usual daily activities). The increased risk associated with taking two NSAIDs is significant. A study published in the European Journal of Clinical Pharmacology concluded that the risk of severe drug reactions causing injury to the liver and acute renal failure was 6 to 7 times higher in reported cases of simultaneous use of two NSAIDs. Don’t let that happen to you. I know many doctors prescribing two strong NSAIDs for acute pain in the hope that patient will be relieved quickly but it is unscientific and dangerous practice. Taking two NSAIDs do not add up their analgesic potential but it will certainly add up their side effects. However, it is commonly found that when one NSAID fails, replacing it with another may work. This is because all the variables in NSAIDs pharmacokinetics and prostaglandin metabolism are not known to us. So if anybody says that by taking two NSAIDs, his pain is relieved, it would mean that one of the two NSAID is actually relieving pain and the other is only contributing to side effects.
_
NSAIDs and GI side effects:
The major adverse drug reaction (ADR) associated with NSAIDs relate to gastrointestinal (GI) effects. These effects are dose-dependent, and in many cases severe enough to pose the risk of ulcer perforation, upper gastrointestinal bleeding, and death, limiting the use of NSAID therapy. An estimated 10-20% of NSAID patients experience dyspepsia, and NSAID-associated upper gastrointestinal adverse events are estimated to result in 103,000 hospitalizations and 16,500 deaths per year in the United States, and represent 43% of drug-related emergency visits. A US study puts the human impact of NSAID-related gastrointestinal deaths into perspective: the rate is higher than that found from cervical cancer, asthma or malignant melanoma. Through systemic and topical effects, NSAIDs impair the mucosal barrier to gastric acid, which, together with the action of pepsin, may result in upper GI symptoms, peptic ulcers and ulcer complications. Although COX-2 selective NSAIDs are associated with a lower risk of peptic ulceration compared with non-selective NSAIDs, patients with risk factors and those taking concomitant low-dose acetylsalicylic acid (ASA) therapy for cardioprotection remain at risk. NSAIDs cause ulcers in some people. Some of those who have ulcers also have symptoms, which include bleeding. In some of those who have bleeding ulcers, the bleeding is sufficiently severe to result in hospital admission, and may cause death. This is a fairly simplified version of events, and many of the papers in this field have as many as 10 different classifications of upper gastrointestinal complaints from which to classify an event. Clearly, the important issue is the overall incidence of severe adverse events, including hospital admission and death, however much we might like information about the risk of any particular event happening to any particular patient. The variables are drug and dose, duration of exposure, and patient characteristics. Most of the publications referenced in this focus have realm about the scale of the problem of NSAID-related GI problems. They make good reading, and would repay the effort if someone were to pull the information together. But for those with little time, a flavor is given in Table below:
_
NSAID-related deaths and admissions to hospital:
| Event | UK | USA | Canada |
| Annual NSAID prescriptions | 25 million | 70 million | 10 million |
| NSAID-related admissions | 12,000 | 100,000 | 3,900 |
| NSAID-related deaths | 2,600 | 16,500 | 365 |
_
Both Helicobacter pylori and NSAIDs cause ulcers, so there may be some interactions. The evidence up to now has been unclear, perhaps because there are so many different things going on in epidemiological studies. A randomized trial indicates that perhaps eradicating the bug in people on NSAIDs may be a good thing. The evidence we have is that using paracetamol as a first-line agent is sensible. It is an effective and safe analgesic at therapeutic doses. It is worth remembering that NSAIDs given by topical routes are not associated with any of the gastrointestinal adverse effects seen with the oral route. Meta-analysis has also shown them to be effective, with NNTs of about 3 in chronic conditions. Thereafter the rule would seem to be to use ibuprofen for preference, at the lowest effective dose, and with mucosoprotective agents for those at highest risk of developing severe adverse gastrointestinal effects. Of course, combining NSAIDs with proton pump inhibitors (omeprazole group) would reduce GI side effects and many studies have proved it.
_
Effect of pain and NSAIDs on blood pressure:
Acute pain increases blood pressure by increasing sympathetic activity, but the role of chronic pain on blood pressure is less well understood. Hypertension and co-existing musculoskeletal problems are two of the common conditions for which antihypertensives and analgesics are prescribed together. Among analgesics, NSAIDs are most frequently prescribed. NSAIDs decrease the synthesis of prostaglandins (PG) by inhibiting cyclo-oxygenase, an enzyme essential for transformation of arachidonic acid into PGs. The PGs are important in control of blood pressure by virtue of their effects on the kidney and blood vessels. Two meta-analyses have demonstrated that, after pooling data drawn from published reports of randomized trials of younger adults, NSAID use produces a clinically significant increment in mean blood pressure of 5 mm Hg, most marked in patients with controlled hypertension. Stratification by NSAID type revealed that piroxicam, naproxen and indomethacin had the greatest, and sulindac the smallest, pressure effect. The NSAIDs antagonize the antihypertensive effect of diuretics, beta-blockers and ACE inhibitors more than that of calcium-channel blockers. The elderly and those with salt-sensitive hypertension experience greater rise in blood pressure with NSAIDs. Physicians should avoid NSAIDs and instead use alternative analgesics such as paracetamol and physical therapy for control of pain. If necessary, the dose of the antihypertensive medications may have to be increased for better control of blood pressure. Since both pain and hypertension are common, it is important that their relationship be well understood by the primary care physicians.
_
Analgesic nephropathy:
Analgesic nephropathy involves damage to one or both kidneys caused by overexposure to mixtures of medications, especially over-the-counter pain remedies (analgesics). Analgesic nephropathy involves damage within the internal structures of the kidney. The specific kidney injuries induced by analgesics are renal papillary necrosis and chronic interstitial nephritis. They appear to result from decreased blood flow to the kidney, rapid consumption of antioxidants, and subsequent oxidative damage to the kidney. This kidney damage may lead to progressive chronic renal failure, abnormal urinalysis results, high blood pressure, and anemia. A small proportion of individuals with analgesic nephropathy may develop end-stage kidney disease. It is caused by long-term use of analgesics, especially over-the-counter (OTC) medications that contain phenacetin or acetaminophen and NSAIDs such as aspirin or ibuprofen. About 6 or more pills per day for 3 years increases the risk for this problem. This frequently occurs as a result of self-medicating, often for some type of chronic pain. Analgesic nephropathy occurs in about 4 out of 100,000 people, mostly women over 30. The rate has decreased significantly since phenacetin is no longer widely available in OTC preparations.
_
Hearing loss and analgesic use:
Regular analgesic use was independently associated with an increased risk of hearing loss. The increased risk of hearing loss seen with regular analgesic use was greatest among younger men, particularly those below age 60 years. In men aged 60 years and above, there was no relation observed between the risk of hearing loss and regular aspirin use, and the relation between regular use of NSAIDs and acetaminophen (paracetamol) was attenuated. The risk of hearing loss increased with longer duration of analgesic use for both NSAIDs and acetaminophen. The ototoxic effects of high-dose salicylates, reversible hearing loss and tinnitus, are well documented. In animal models, salicylate administration results in abnormal outer hair cell function and decreased cochlear blood flow. Salicylates induce biochemical and electrophysiological changes that alter membrane conductance of outer hair cells and vasoconstriction in auditory microvasculature, possibly mediated by antiprostaglandin activity. Similar to salicylates, other NSAIDs inhibit cyclooxygenase and decrease prostaglandin activity, potentially reducing cochlear blood flow.
_
Asthma and analgesics:
The development of wheezing, sometimes accompanied by rhinorrhoea and urticaria, in some asthmatic patients shortly after taking aspirin has been recognized for at least 50 years. Aspirin, indomethacin, fenamates, and phenylbutazone have different chemical structures but in one study, all of them inhibited biosynthesis of prostaglandins and all of them also precipitated the asthma attacks in aspirin-sensitive patients. Recent data have suggested that frequent use of paracetamol (acetaminophen) may contribute to a deterioration of respiratory function in asthma. A small proportion of patients with asthma who are NSAID-intolerant experience short-lived deterioration in respiratory function with the use of high doses of paracetamol but this is uncommon and has not been implicated in life-threatening reactions. Routine warnings about paracetamol use in asthma are, therefore, not warranted. Medical personnel, however, should be aware of the potential for worsening of symptoms in some individuals with asthma using paracetamol and institute formal investigation or withdrawal of the drug if they suspect such a reaction.
_
Common painkillers can raise heart risk:
Taking daily doses of over-the-counter painkillers can increase the risk of heart attacks and stroke by up to 40 per cent, researchers have warned. Patients who regularly use diclofenac, a common anti-inflammatory drug, were shown to have a two-fifths higher chance of suffering heart problems. Ibuprofen, one of the most common painkillers, was linked to an 18 per cent higher risk of heart attack and stroke. The figures were published in a new paper compiling evidence from 51 international studies into the impact of a range of NSAIDs on more than 2.7 million patients.
_
Opioids:
‘Narcotic’ is a somewhat imprecise term because it suggests “narcosis”, which is indicated of a somnolent state or “sleepy” state. “Opioid analgesic” therefore is a more appropriate term emphasizing the clinically important analgesic property which is the pharmacological property of importance in the therapeutic application of these agents. Opioids are defined as all natural/semisynthetic opium alkaloid derivatives, synthetic agents, and other agents whose opioid-like effects are blocked by classical opioid antagonists, such as naloxone or naltrexone. Opium is derived from plant opium poppy (Papaver somniferum). Opium is obtained following drying the milky juice from unripe seed pod. Opium has a characteristic odor and bitter-taste with its chief active ingredient being morphine. Also present are codeine, thebaine (a non-analgesic agent), noscapine and papaverine, a non-analgesic vasodilator. Even though the words opioid and opiate are used interchangeably, opiates strictly means natural opioid substances derived from opium plant.
_
Endogenous Opioid Peptides:
The rationale for endogenous opioid peptides came from the idea that opioid receptors are present in the body for the purpose of interacting with endogenous or naturally occurring substances. As a consequence, research proceeded to attempt identification of these naturally occurring substances now known as beta-endorphins and related peptides. Morphine (and related agents) causes analgesia by acting on opioid receptors in nervous system. Various opioid receptor subtypes are depicted in the table below.
_
| Drug | Mu (m) | Delta (d) | Kappa (k) |
| Opioid Peptides | |||
| Enkephalins | Antagonist | Agonist | |
| beta-endorphin | Agonist | Agonist | |
| Dynorphin | Weak Agonist | Agonist | |
|
Agonists |
|||
| Codeine | Weak Agonist | Weak Agonist | |
| etorphine | Agonist | Agonist | Agonist |
| fentanyl | Agonist | ||
| meperidine | Agonist | ||
| methadone | Agonist | ||
| Morphine | Agonist | Weak Agonist | |
|
Agonist-antagonists |
|||
| Buprenorphine | Partial Agonist | ||
| dezocine | Partial Agonist | Agonist | |
| nalbuphine | Antagonist | Agonist | |
| pentazocine | Antagonist or Partial Agonist | Agonist | |
| Antagonist: naloxone | Antagonist | Antagonist | Antagonist |
_
The site of opioid action is at spinal cord. It reduces neurotransmitter release; by closing a voltage-gated Ca2+ channel on presynaptic neuronal terminals and inhibit postsynaptic neurons (hyperpolarization) by increasing K+ channel conductance. Reduced transmitter released– affects acetylcholine, norepinephrine, glutamate, serotonin, substance P.
_
The most dangerous side effect of opioids is respiratory depression. Minor degrees of respiratory depression may be detected following standard doses of opioids, but this is not clinically important. With higher doses or in frail patients, the respiratory rate decreases (less than 12 per minute), the patient becomes increasingly sedated, and the pupils very small.
_
Tramadol:
Tramadol is commonly referred to as an atypical centrally acting analgesic because of its combined effects as an opioid agonist and a serotonin and noradrenaline reuptake inhibitor (Lotsch, 2005). Although an effective analgesic, it may not provide adequate pain relief if used as the sole agent for the management of moderate to severe acute pain in the currently recommended doses (Thevenin et al, 2008 Level II). Tramadol is an effective treatment of neuropathic pain with an NNT of 3.8 (Hollingshead et al, 2006 Level I).
_
The figure above shows role of NSAIDS and opioids in inflammatory pain.
_
NSAIDs and opioids both alter the pain pathway in different ways, so combining the two classes of drugs to enhance analgesia makes sense. Also, opioids and tricyclic antidepressants combination would enhance opiate analgesia by potentiating biogenic amine links in the endorphin circuit. Also, NSAID plus tricyclic antidepressant can be used for refractory pain with inflammatory component.
_
Pain killer comparison charts:
OVER – THE – COUNTER (OTC) Pain Medicine:
|
generic name |
Dose |
PAIN |
Uses According |
Best Uses |
Problems |
|
aspirin |
81 mg |
1 |
Minor aches, pains cold, headache, muscular ache |
minor aches and pains | aspirin allergy, asthma, stomach bleeding, Reye’s Syndrome |
|
acetaminophen |
500 mg |
2 |
Headache | fever, if allergic to aspirin, arthritis, rheumatism, musculo-skeletal |
June 2009, again 7-04-06- paracetamol Liver Damage “paracetamol is the No. 1 cause of acute liver failure in the U.S.” “[Acetaminophen] is a leading cause of death from pharmaceuticals,” |
|
Combination of |
325 mg |
2.25 |
Headache Muscle ache |
headache | same as above not for children and teens |
|
ibuprofen |
200 mg |
2.5 |
Aches, pains, colds, toothache, muscle aches, backache, menstrual, fever |
fever, muscle ache |
aspirin allergy, asthma, upset stomach, not for last trimester |
|
naproxen sodium |
220 mg |
3 |
Joint and muscle pain | arthritis, rheumatism, musculo-skeletal |
Naproxen heart risk upset stomach, not for nursing mothers |
|
ibuprofen |
800 mg |
3.5 |
(listed above) | (as above) | (as above) |
|
ketoprofen |
12.5 mg |
3.6? |
Anti-inflammatory, analgesic |
arthritis | Ketoprofen side effects |
_
Note:
More Warnings on OTC Drugs:
NSAIDs increase blood pressure
Aspirin and NSAIDS cause stomach bleeding resulting in death.
NSAIDS Linked to Erectile Dysfunction.
_
Prescription pain killers:
|
generic name |
Dose |
PAIN |
Uses According |
Problems |
|
celecoxib |
100 mg |
3.7 |
Osteoarthritis, rheumatoid arthritis, short term pain, painful menstruation |
increased heart risk, stomach bleeding, stroke |
|
tramadol |
50 mg |
3.8 |
Moderate pain | nausea, constipation, dizziness, headache, drowsiness, vomiting |
|
meperidine HCl |
50 mg |
4 |
The opiates and synthetic opiates (from 4 down to 10) are the most effective pain killers, but they are subject to the most abuse, and prescription pain killer addiction. All the opiates and derivatives are habit-forming.The inclusion of high amounts of acetaminophen with all these medications is to increase opioid analgesia but one has to watch for liver side effects of acetaminophen (paracetamol). | |
|
hydrocodone and |
5-500 mg |
5 |
||
|
hydrocodone and |
5-600 mg |
5 |
||
|
hydrocodone and |
7.5-650 mg |
5.5 |
||
|
hydrocodone and |
7.5-750 mg |
6 |
||
|
hydrocodone-APAP, |
5-325 mg |
6.5 |
||
|
hydrocodone and |
10-500 mg |
7 |
||
|
hydrocodone and |
10-660 mg |
7 |
||
|
oxycodone HCl with acetaminophen |
2.5-325 mg |
7 |
Oxycodone is very effective. | |
|
oxycodone HCl, |
4.50 mg 0.38 mg 325 mg |
7 |
||
|
oxycodone HCl |
40 mg |
8.5 |
||
|
morphine sulfate |
15 mg |
9 |
Morphine may be one of the strongest pain relief medicines available. | |
|
fentanyl |
Skin patch Lollipop |
10 |
Fentanyl is very effective for gunshot wounds, and fragmentation wounds. However, there have been serious problems with fentanyl dosing (overdosing). Death has resulted from simply handling the medication with the fingers. One must use great care when handling fentanyl. | |
|
hydromorphone |
1 mg |
11 |
It is more effective than morphine, and that it has fewer side effects, although just as serious. | |
|
oxymorphone extended release |
5 mg 5 mg |
? |
3 hours (also, injection and suppository)5 – 8 hours | |
__
DISCLAIMER:
The above mentioned OTC and prescription pain killers are discussed for the purpose of education and awareness. No advice is intended. Consult your doctor for personal pain reliever advice. Although I have tried to be as accurate as possible, errors and misstatements are possible.
_
Creams/ patches:
These can be applied to painful joints, sprains and strains, particularly useful for smaller joints, e.g. knuckles. It is important to know that when creams are applied too frequently or liberally they can be absorbed by the body and have effects throughout. Some of the anti-inflammatory creams can be bought over the counter at the chemist. They have been shown to be effective for pain relief in the short term (up to two weeks). There are also creams that reduce pain sensors on the skin and at the fine nerve endings which recognize the pain and reduce the pain message that the brain receives from the painful area. These creams can sometimes take several weeks before the pain relief is felt and when first used can cause an initial burning sensation. Some people find this counter irritant effect quite soothing and the burning sensation itself does reduce when you get used to the cream. Examples include Ketoprofen (an anti-inflammatory cream) and Capsaicin cream (reduces pain sensors). Capsaicin is the active ingredient in chili peppers that makes them hot. Capsaicin is used in medicated creams and lotions to relieve muscle or joint pain. Capsaicin used on the body causes a sensation of heat that activates certain nerve cells. With regular use of capsaicin, this heating effect reduces the amount of substance P, a chemical that acts as a pain messenger in the body. Capsaicin topical is used for temporary relief of muscle or joint pain caused by strains, sprains, arthritis, bruising, or backaches. Topical gels and creams are often combined with NSAIDs and narcotic analgesics for severe pain due to sprains, strains, joint pains, and for other types of arthritis. A higher concentration Capsaicin topical is used to treat nerve pain (neuralgia) in people who have had herpes zoster, or “shingles.”
_
Fentanyl skin patches are very strong narcotic painkillers that may cause death from overdose.
_
Adjuvant analgesics & potentiators:
Adjuvant analgesics (co-analgesics) are primarily used for treating some other condition, but they also relieve pain. These compounds are useful in treating neuropathic pain (chronic pain that comes from injury to the central nervous system). They include the following:
1. Anti-epileptic drugs reduce membrane excitability and action potential conduction in neurons of the central nervous system.
2. Tricyclic antidepressants affect synaptic transmission of serotonin and norepinephrine neurons in the central nervous system, thereby affecting pain-modulating pathways.
3. Anesthetics block action potential transmission by interfering with sodium and potassium channels in nerve cell membranes. Examples include lidocaine, novocaine and benzocaine.
_
Drugs which have been introduced for uses other than analgesics are also used in pain management. Both first-generation (such as amitriptyline) and newer anti-depressants (such as duloxetine) are used alongside NSAIDs and opioids for pain involving nerve damage and similar problems. Antidepressants like imipramine and doxepin reduce depression and therefore pain associated with depression; also produce analgesia by acting directly upon pain modulation systems. Tricyclic Antidepressants are the most useful group of psychotropic drugs used for pain management by blocking serotonin and norepinephrine (NE) reuptake, increasing the levels of 5-HT and NE, which are part of the endorphin-mediated analgesia system (EMAS). Tricyclics can also produce analgesia directly or by enhancing the action of opiates. Other agents directly potentiate the effects of analgesics, such as using hydroxyzine, promethazine, carisoprodol or tripelennamine to increase the pain-killing ability of a given dose of opioid analgesic. Adjuvant analgesics, also called atypical analgesics, include nefopam, orphenadrine, pregabalin, gabapentin, cyclobenzaprine, scopolamine, and other drugs possessing anticonvulsant, anticholinergic and/or antispasmodic properties, as well as many other drugs with CNS actions. These drugs are used along with analgesics to modulate and/or modify the action of opioids when used against pain, especially of neuropathic origin. Dextromethorphan has been noted to slow the development of tolerance to opioids and exert additional analgesia by acting upon the NMDA receptors; some analgesics such as methadone and ketobemidone and perhaps piritramide have intrinsic NMDA action. Phenothaizines are usually used as adjuncts to narcotic analgesics.
_
Anticonvulsants relieves pain by
1. Blocks Na Channels: Examples include Carbamazepine, Oxcarbazepine, Phenytoin, Valproic Acid and Lamotrigine
2. Blocks Ca Channels: Examples include Gabapentin and Pregabalin
3. Inhibits glutamine release + others e.g.Topiramate
_
Pregabalin is a substituted analogue of gabapentin that binds to a 2-d protein subunit of voltage gated calcium channels and reduces glutamate, substance P, NE.
_
Epidural and neuraxial analgesia:
The use of epidural analgesia has shifted from a purely obstetrical practice to managing pain in patients undergoing multiple types of surgery, including thoracic, gastrointestinal, urologic, gynecologic, orthopedic, and vascular procedures. Epidural analgesia is the second most common form of pain management used in the United States after systemic opioids. More importantly, epidural analgesia has been shown to provide superior postoperative analgesia both at rest and with activity compared with systemic opioid administration. Epidural infusion provides more consistent pain relief resulting in a lower overall consumption of opioid and decreased related side effects. Patients mobilize faster, are less sedated, and have improved respiratory functions compared to those receiving systemic analgesia only. Neuraxial analgesia defines the administration of opioids alone, or in combination, with local anesthetics and, occasionally, clonidine into the spinal or epidural space. This form of analgesic delivery provides powerful and highly efficient anesthetic augmentation and pain relief in a variety of clinical settings. Following epidural and spinal injection, a small fraction of opioid molecules leaves the cerebrospinal fluid (CSF) and binds to receptors in dorsal horn. Activation of these receptors effectively suppresses afferent noxious transmission at the first synapse with cells in the CNS. Epidural and intrathecally administered opioids provide greater analgesic potency than similar doses administered parenterally.
_
Patient-controlled analgesia:
Patient-controlled analgesia (PCA) describes the conceptual framework for on-demand, intermittent administration of opioid and nonopioid analgesics under patient control. The broader concept of PCA should neither be restricted to a single route or mode of administration, nor should PCA imply a mandatory need for a sophisticated or expensive infusion device.
_
Multimodal analgesia:
_
The combination of multiple modalities of analgesic therapies otherwise called balanced analgesia or multimodal analgesia, has been proposed as the rational approach to pain management and many consider this concept as the most effective way in managing acute postoperative pain; this practice has evolved rapidly. The rationale behind this concept has been that analgesic drugs, acting through different mechanisms, result in additive or synergistic analgesia.
_
WHO pain ladder for cancer relief:
The cornerstone of the WHO document rests on five simple recommendations for the correct use of analgesics to make the prescribed treatments effective. This advice is applicable today, not only for cancer patients with pain, but also for all patients with either acute or chronic pain who require analgesics. The five points for the correct use of analgesics are as follows:
1. Oral administration of analgesics. The oral form of medication should be privileged whenever possible.
2. Analgesics should be given at regular intervals. To relieve pain adequately, it is necessary to respect the duration of the medication’s efficacy and to prescribe the dosage to be taken at definite intervals in accordance with the patient’s level of pain. The dosage of medication should be adjusted until the patient is comfortable.
3. Analgesics should be prescribed according to pain intensity as evaluated by a scale of intensity of pain. This point is important because pain-relief medications should be prescribed after clinical examination and adequate assessment of the pain. The prescription must be given according to the level of the patient’s pain and not according to the medical staff’s perception of the pain. If the patient says that he has pain, it is important to believe him. This point makes reference to the levels of the analgesic ladder that will be explained in detail further below.
4. Dosing of pain medication should be adapted to the individual. There is no standardized dosage in the treatment of pain. Every patient will respond differently. The correct dosage is one that will allow adequate relief of pain. The posology should be adapted to achieve the best balance between the analgesic effect and the side effects.
5. Analgesics should be prescribed with a constant concern for detail. The regularity of analgesic administration is crucial for the adequate treatment of pain. Once the distribution of medication over a day is established, it is ideal to provide a written personal program to the patient. In this way the patient, his family, and medical staff will all have the necessary information about when and how to administer the medications.
_
_
Three different types of painkillers are set out in an ’analgesic ladder’.
1. Mild pain – Mild painkillers e.g. paracetamol or anti-inflammatory drugs e.g. ibuprofen, diclofenac sodium or celecoxib.
2. Moderate pain – Weak opioid painkillers e.g. dihydrocodeine, codeine phosphate or tramadol.
3. Severe pain – Strong opioid painkillers e.g. morphine, oxycodone, fentanyl or diamorphine.
The idea behind the analgesic ladder is that if a person’s pain is not controlled by the painkillers on one level, their doctor should prescribe a drug from the next level rather than try a different painkiller from the same group. For example, if you’re taking a painkiller such as ibuprofen but are still getting pain, or if your pain gets worse, your doctor should prescribe a weak opioid (moderate) painkiller such as dihydrocodeine, codeine phosphate or tramadol. If the pain still isn’t controlled or if it increases, your doctor could then prescribe a strong opioid painkiller.To calm fears and anxiety, additional drugs – “adjuvants” – should be used. To maintain freedom from pain, drugs should be given “by the clock”, that is every 3-6 hours, rather than “on demand” This three-step approach of administering the right drug in the right dose at the right time is inexpensive and 80-90% effective. Surgical intervention on appropriate nerves may provide further pain relief if drugs are not wholly effective.
_
_
Cancer pain management:
Cancer pain is often very complex, but the most intractable pain is often neuropathic in origin, arising from tumor invasion of the meninges, spinal cord & dura, nerve roots, plexuses and peripheral nerves. Multimodal therapies are necessary. It is recognized that the World Health Organization (WHO) analgesic ladder, whilst providing relief of cancer pain towards the end of life for many sufferers worldwide, may have limitations in the context of long-term survival and increasing disease complexity. The neurophysiology of cancer pain is complex: it involves inflammatory, neuropathic, ischaemic and compression mechanisms at multiple sites. Knowledge of these mechanisms and the ability to decide whether a pain is nociceptive, neuropathic or a combination will lead to best practice in pain management. Opioids remain the mainstay of cancer pain management, but the long-term consequences of tolerance, dependency, hyperalgesia and the suppression of the hypothalamic/pituitary axis should be acknowledged and managed in both non-cancer and cancer pain, in addition to the well-known side effects such as constipation. NSAIDs, antiepileptic drugs, tricyclic antidepressants, NMDA antagonists, sodium channel blockers, topical agents and the neuraxial route of drug administration all have their place in the management of complex cancer pain. Psychological distress increases with the intensity of cancer pain. Cancer pain is often under-reported and under-treated for a variety of complex reasons, partly due to a number of beliefs held by patients, families and healthcare professionals. There is evidence that cognitive behavioral techniques that address catastrophizing and promote self-efficacy lead to improved pain management. Group format pain management programs could contribute to the care of cancer survivors with persistent pain. Physiotherapists and Occupational Therapists have an important role in the management of cancer pain and have specific skills which enable them to be both patient-focused and holistic. Therapists utilize strategies which aim to improve patient functioning and quality of life, but the challenge remains for them to practice in an evidence-based way and more research is urgently needed in this field.
_
Transcutaneous Electrical Stimulation (TENS):
Action of TENS is based on premise that large diameter myelinated axons have a lower electrical threshold than smaller diameter axons and do not produce pain when activated, it is possible to stimulate them selectively without producing discomfort and stimulation of large diameter fibers causes inhibition of small diameter pain fibers and modulation of pain response (gate control theory). TENS machines have two small electrode pads that need to be attached to the skin. The TENS machine works by providing mild electrical impulses that stimulate the touch sensors in the skin reducing the pain messages to the brain. A small battery powers the electrical impulses, the level and type of impulses can be adjusted. TENS machines can be purchased from chemists. TENS is most effective when stimulation is applied to the injured nerve proximal to the site of injury; also used for spinal cord injury. It is used routinely used for postoperative pain. Although TENS machines may be useful to some people it might be worth taking specialist advice from the hospital physiotherapist/practitioner about whether you might benefit from using one. Research evidence to date has not proved conclusive evidence of the value of TENS for chronic pain.
__
Physiatry:
Physical medicine and rehabilitation (physiatry/physiotherapy) employs diverse physical techniques such as thermal agents and electrotherapy, as well as therapeutic exercise and behavioral therapy, alone or in tandem with interventional techniques and conventional pharmacotherapy to treat pain, usually as part of an interdisciplinary or multidisciplinary program.
_
Common uses of Physiotherapy for Pain Relief:
Physiotherapy involves treatment that focuses on prevention of injuries or disabilities. Physiotherapy helps to relieve pain, promote healing and restore function and movement. Physiotherapy is practiced by professionally trained physiotherapists who are specialists, skilled and educated specifically in proper rehabilitation. Right from minor injuries to major injuries, physiotherapy has been established to be an effective tool against injuries and pain. Various methods of physiotherapy are applied for different injuries in various regions of the body. Particularly beneficial for sufferers of lower back pain, physiotherapy is used to release tension and so reduce pain in the back. Various tactics are used such as exercise, traction and massage and the process helps millions of people around the world overcome constant back pains. Utilized to rehabilitate patients after a stroke or hip replacement, physiotherapy is also used to reduce pain induced by sports injuries, and various methods are used to make it possible for the patient to lead a normal life again. Patients may also be shown various methods for lifting, for instance, to avoid future strain on the lower back. A therapist may focus on decreasing pain with either passive or active therapy.
Examples of passive techniques include:
1. Heat/ice packs
2. TENS units
3. Ultrasound
Examples of active techniques include:
1. Stretching and range of motion exercises
2. Strengthening exercises
3. Pain-relief exercises
4. Low-impact aerobic conditioning
_
Psychological therapy of pain:
Individuals with more social support experience less cancer pain, take less pain medication, report less labor pain and are less likely to use epidural anesthesia during childbirth or suffer from chest pain after coronary artery bypass surgery. Suggestion can significantly affect pain intensity. About 35% of people report marked relief after receiving a saline injection they believe to have been morphine. This “placebo” effect is more pronounced in people who are prone to anxiety, so anxiety reduction may account for some of the effect, but it does not account for all of the effect. Placebos are more effective in intense pain than mild pain; and they produce progressively weaker effects with repeated administration. It is possible for many chronic pain sufferers to become so absorbed in an activity or entertainment that the pain is no longer felt, or is greatly diminished. Cognitive behavioral therapy (CBT) is effective in reducing the suffering associated with chronic pain in some patients but the reduction in suffering is quite modest, and the CBT method employed seems to have no effect on outcome. Evidence for the usefulness of behavioral therapy (BT) and cognitive behavioral therapy (CBT) in the management of adult chronic pain is generally weak, due partly to the proliferation of techniques of doubtful quality, and the poor quality of reporting in clinical trials. The crucial content of individual interventions has not been isolated and the important contextual elements, such as therapist training and development of treatment manuals, have not been determined. The widely varying nature of the resulting data makes useful systematic review and meta-analysis within the field very difficult. Other psychologically oriented pain management includes hypnosis, biofeedback, guided imagery and psychotherapy. However, many patients unwilling to take this approach, or unable to pay for it. Since chronic pain produces anxiety, depression and other emotional problems, it is useful to evaluate these patients psychiatrically; psychiatric factors are difficult to quantify and it is difficult to determine efficacy of psychiatric therapy.
_
The manipulation of coping mechanisms is of great significance when considering the management of pain and especially of chronic pain. We are all familiar with coping strategies, some of which are regarded as active — for example, indulging in active and distracting behavior, whereas others are passive — for example, taking rest or medicines. If the strategy used maximizes function in the presence of pain and reduces anxiety, then it is said to be adaptive. On the other hand, if the strategies used involve too much rest, too great a dependence on medication or on others, or conversely too much activity which provokes excessive pain, they are maladaptive. Cognitive therapies involve changing thoughts and attitudes about pain with a view to changing self-management in the direction of adaptive behavior: a change which often leads to a lessening of pain.
__
Complementary and Alternative medicine (CAM):
Pain is the most common reason for people to use complementary and alternative medicine. An analysis of the 13 highest quality studies of pain treatment with acupuncture, published in January 2009 in the British Medical Journal, concluded that there is little difference in the effect of real, sham and no acupuncture. There is interest in the relationship between vitamin D and pain, but the evidence so far from controlled trials for such a relationship, other than in osteomalacia, is unconvincing. A 2007 review of 13 studies found evidence for the efficacy of hypnosis in the reduction of pain in some conditions, though the number of patients enrolled in the studies was low, bringing up issues of power to detect group differences, and most lacked credible controls for placebo and/or expectation. The authors concluded that although the findings provide support for the general applicability of hypnosis in the treatment of chronic pain, considerably more research will be needed to fully determine the effects of hypnosis for different chronic-pain conditions. A 2003 meta-analysis of randomized clinical trials found that spinal manipulation was “more effective than sham therapy but was no more or less effective than general practitioner care, analgesics, physical therapy, exercise, or back school” in the treatment of low back pain.
_
_
These CAM approaches do not involve drugs or surgery.
1. Chiropracty manipulates joints to relieve compression of nerves.
2. Massage stimulates blood flow, relieves muscle spasms and increases somatosensory information, which can relieve pain through the gate control theory.
3. Hot applications increase blood flow, and cold applications reduce inflammation, which contributes to pain.
4. Stimulation of the skin with small electrodes can close the gate to pain.
5. Acupuncture may stimulate nerve cells and release endorphins. The increased stimulation might also close the gate to pain.
6. Mental control techniques rely on the ability of the mind and emotions to control and alleviate pain through descending neural pathways. They include relaxation techniques, hypnosis, biofeedback and distraction techniques.
_
About Scientific Evidence on CAM Therapies:
Scientific evidence on CAM therapies includes results from laboratory research (e.g., animal studies) as well as clinical trials (studies in people). It encompasses both “positive” findings (evidence that a therapy may work) and “negative” findings (evidence that it probably does not work or that it may be unsafe). Scientific journals publish study results, as well as review articles that evaluate the evidence as it accumulates; fact sheets from the National Center for Complementary and Alternative Medicine (NCCAM) base information about CAM research primarily on the most rigorous review articles, known as systematic reviews and meta-analyses. Authors of such reviews often conclude that more research and/or better designed studies are needed.
_
| Promising Evidence of Potential Benefit | Limited, Mixed, or No Evidence To Support Use | |
| Low-Back Pain | ||
| Acupuncture |
√ |
|
| Massage |
√ |
|
| Spinal Manipulation |
√ |
|
| Progressive Relaxation |
√ |
|
| Yoga |
√ |
|
| Prolotherapy |
√ |
|
| Herbal Remedies |
√ |
|
| Arthritis | ||
| Acupuncture |
√ |
|
| Glucosamine/Chondroitin |
√ |
|
| Gamma Linolenic Acid (GLA) |
√ |
|
| Herbal Remedies |
√ |
|
| Balneotherapy (Mineral Baths) |
√ |
|
| Tai Chi |
√ |
|
| Headache | ||
| Acupuncture |
√ |
|
| Relaxation training |
√ |
|
| Feverfew |
√ |
|
| Neck Pain | ||
| Acupuncture |
√ |
|
| Spinal Manipulation |
√ |
|
| Scientific Evidence on CAM for Pain | ||
_
Caution:
If you are considering CAM for Chronic Pain, do not use a CAM therapy as a replacement for conventional care or to postpone seeing a doctor about chronic pain or any other medical problem. Learn about the therapy you are considering, especially the scientific evidence on its safety and whether it works. If you are considering CAM, keep in mind that they can act in the same way as medications. They can cause medical problems if not used correctly, and some may interact with prescription or nonprescription medications or other dietary supplements you take. Your health care provider can advise you. If you are pregnant or nursing a child, or if you are considering giving a child a dietary supplement, it is especially important to consult your health care provider.
_
Acupuncture and pain:
Despite the favorable research, some experts caution that it’s difficult to test acupuncture in a clinical setting. In part, this is because any valid clinical study will include a control group that is given a sham treatment (placebo). In the case of acupuncture, the placebo consists of needles inserted in random points, rather than at actual pressure points. This can lead to what’s called the placebo effect — when study participants believe that they’ve received the real treatment and expect their symptoms to improve. As evidence, a 2006 study in the “British Medical Journal” found that acupuncture reduced the number of days patients suffered from tension headaches, but sham acupuncture in the study had almost the exact same results. Also, the quality of the research conducted so far on acupuncture hasn’t been consistent. Many of the studies in the past have been small, and have focused on short-term, rather than long-term results.
_
Hypnotherapy for the management of Chronic Pain:
Thirteen studies, excluding studies of headaches, were identified that compared outcomes from hypnosis for the treatment of chronic pain to either baseline data or a control condition. The findings indicate that hypnosis interventions consistently produce significant decreases in pain associated with a variety of chronic-pain problems. Also, hypnosis was generally found to be more effective than nonhypnotic interventions such as attention, physical therapy, and education. Most of the hypnosis interventions for chronic pain include instructions in self-hypnosis. However, there is a lack of standardization of the hypnotic interventions examined in clinical trials, and the number of patients enrolled in the studies has tended to be low and lacking long-term follow-up.
_
Magnet therapy:
How do Magnets Work? Magnets do not block pain signals, they treat the cause of the pain. Joint wear and tear, chronic damage from earlier injuries or chronic inflammation can cause pressure upon nerves. The pressure upon the nerves is usually caused by swelling or inflammation around the injury, this extra fluid causes the tissues to swell and thus places pressure upon the nerve endings. Compression of the nerves causes constant pain stimuli to be sent to the brain. This causes the chronic constant pain that is often associated with long term ailments. To relieve the pressure on the compressed nerves, the excess fluid in the tissues must be removed. Once the pressure has been removed the pain will subside. Magnets do reduce the inflammation in the tissues therefore they are very effective at reducing pain at the point of injury. Because the cause of the pain has been removed ( i.e. the inflammation), the pain relieving results will last for a much longer period of time than pain killers, which are just blocking the signal. Whilst the magnetic field is reducing the inflammation, it is also improving blood supply to the injured area. The extra blood flow brings fresh rich oxygen, nutrients and hormones. One of these hormones is endorphin. Endorphin is known as the “happy” hormone as it is responsible for mood enhancement. The other function of endorphin is to kill pain naturally. As increased blood flow reaches the injured area the concentration of endorphins increases and pain is reduced. To be frank, magnet therapy appears to be science fiction to me but who knows when fiction becomes fact. Also, thousands of patients undergo MRI daily but none report pain relief even coincidentally.
_
Non-specific effects:
Some of the pain relief with every pain treatment could also be attributed to the effects which are not specific to the treatment. These could include the person’s beliefs, expectations and experiences with other illnesses, previous use of the current treatment or other treatments, context and the interaction between the patient and the practitioner and so on (placebo effect). But, mind you, they all work by affecting how the brain perceives pain. Every pain treatment out there, whether it is acupuncture, postural correction, movement dysfunctions, trigger point therapy, stretching, active release technique, manual therapy, McKenzie methods, meditation, yoga and so forth, works by the above mechanisms to affect the brain since the experience of pain comes from your brain and may have very little to do with eliminating the ‘pathology’ in the body as claimed. While many people do have “issues in the tissues”, that’s far from the only consideration.
_
Music therapy for pain:
Music therapy acts as an intervention for pain perception by affecting pain in all of its facets, impacting one’s quality of life in the moment. It is known that emotions, thoughts and feelings are a result of neurons firing, as is nociception (pain). Perhaps the messages instigated by the “mind” are able to overpower the message of nociception. PET scans (positron emission topography) reveal that the endocrine system and the limbic system are stimulated by music releasing endorphins (body’s natural pain killers) and dopamine (pleasure) into the body. Low levels of dopamine results in feelings of anxiety, worthlessness and fatigue, all factors likely to contribute to an increase in pain perception. Serotonin is also controlled by the limbic system; it is responsible for regulating mood and enhancing the affects of analgesics. Research measuring serotonin pre and post music therapy sessions would be useful in determining serotonin’s role in the effects of music therapy as an intervention for pain perception. The parallels between music therapy and pain are evident in this research. Pain is a subjective experience and the perception of that pain can be influenced in a music therapy session. Patients are able to benefit from interventions, such as music therapy, that offer flexibility within routine yet unpredictable venues, such as hospitals. The treatment, unlike pharmaceuticals, does not have any time restrictions or contraindications. This flexibility provides an opportunity for patients to regain some control over their pain management by not being restricted from when they can or cannot take part in the therapy. Music therapy as an intervention for pain perception is able to affect the multiple variables involved in pain while providing a more pleasant environment for fellow patients and staff. It is cost effective, without side effects, and improves one’s overall sense of well being, contributing to quality of life.
_
Backache:
About 80 percent of the adults in the U.S. have been bothered by back pain at some point. Lower-back pain disrupts many aspects of life. In one survey, 46 percent said that it interfered with their sleep, 31 percent reported that it thwarted their efforts to maintain a healthy weight, and 24 percent said that it hampered their sex life. The percentage of people highly (completely or very) satisfied with their back-pain treatments and advice varied by practitioner visited.
_
| Professional | Highly satisfied |
| Chiropractor |
59% |
| Physical therapist |
55% |
| Acupuncturist |
53% |
| Physician, specialist |
44% |
| Physician, primary-care doctor |
34% |
_
Interventional pain management for backache:
The goal of interventional pain management is to find the source of a person’s pain, then to treat it in the most effective, least invasive way possible. Finding the cause of the pain is crucial in thoroughly treating it. Medication can sometimes be effective in pain relief, but it doesn’t address the underlying cause. It’s not that you shouldn’t use pain medications but it’s that medications treat the symptom of pain rather than the source of it. And there are risks with any medication. Interventional pain techniques can provide other effective and safe options. Because interventional pain management is so comprehensive, there are a variety of treatments available. The type of treatment any given patient receives depends on his or her unique condition. However, interventional pain doctors typically start with the least invasive treatments, then work their way through other treatments if necessary. The following are effective intervention methods for back pain:
Trigger point injections:
Facet Joint Injections:
Medial Branch Nerve Blocks:
Radiofrequency Neurotomy:
Epidural Steroid Injections:
Cryoablation of Peripheral Nerves:
Botox Injections for Muscle Spasms and Pain
Intradiscal Electrothermal Therapy
Percutaneous Disc Decompression
Percutaneous Vertebroplasty/Kyphoplasty
Spinal Cord Stimulation
Intrathecal Drug Delivery:
_
Nonsurgical spinal decompression therapy for chronic pain:
Spinal decompression therapy is an FDA cleared revolutionary, nonoperative neck and back pain treatment that has shown excellent results for decreasing pain. Spinal decompression therapy is a treatment based on computerized intermittent traction that can allow patients to become pain-free and avoid surgery with the following painful conditions:
Neck pain
Arm and Leg Pain
Sciatica
Degenerative Disc Disease
Facet Syndrome
Spinal Stenosis
Low Back Pain
Herniated or Bulging Discs
Failed Neck or Back Surgery
The research has shown that over 75% of patients achieved good to excellent outcomes. The treatment works by providing an intermittent traction that is gentle and provides pressure relief for the spine. This works well for both that pain issues along with back pain problems. The spinal decompression machine provides a gradual application and release of traction which works by tricking the muscles around the spine not to spasm or guard. This allows increased blood flow into the spine along with a significant amount of nutrients and oxygen. This can permit the injured disc to heal or can even pull back in a bulging or herniated disc backward supposed to be anatomically. It also provides substantial pain relief for spinal arthritis conditions such as spinal stenosis or facet syndrome and even degenerative disc disease.
_
RFA:
Another technique to alleviate chronic pain is Radiofrequency ablation (RFA). Radiofrequency ablation represents a cutting-edge treatment that is often able to reduce the amount of pain medications that are necessary and provide typically a much longer pain relief then a standard cortisone injection. Research studies looking at radiofrequency ablation have shown anywhere between a 50% to 90% good to excellent results in both the neck and the low back. One study performed in the last few years showed average pain relief of 470 days with the treatment which is approximately 16 months.
_
Other interventional modalities include:
1. Surgical Lesions –disruption of the spinothalamic tract
2. Sympathetic Blocks and TENS: use independent modulatory mechanisms and can be used together to enhance analgesia. Sympathetic block reduces abnormal excitatory influences on peripheral nociceptors; sympathetic therapy may be enhanced using catecholamine depleting agents such as reserpine or guanethidinne and only proven to work when given by regional perfusion in high concentrations.
_
Counter irritation:
Counter irritation is one of the earliest forms of healing. It has long been thought that pain can be relieved and healing promoted by irritating an area of the skin. A counterirritant is a substance which creates inflammation in one location with the goal of lessening the inflammation in another location. Capsaicin is a counterirritant during initial phase but later on it blocks substance P by actions on nerve cells.
Methods of Counter Irritation:
Many things from noxious chemicals to sharp instruments have been used to provide the irritant stimulus. The following is a list of a few:
1. Cupping: The air inside a small jar is heated and the mouth of the jar is placed on the skin, as the air inside cools a vacuum is created. This draws blood and other body fluid to the surface of the skin creating bruising and swelling.
2. Scarification: use of a sharp instrument to scratch the skin.
3. Blistering: use of heat or very irritant chemical to produce blisters on the skin.
4. Massage: if deep enough massage will produce reddening and minor& transient damage to the underlying tissue, this in turn will cause minor irritation. Deep massage generally has greater therapeutic benefit than the light surface variety.
5. Irritant chemicals: Very many of these have been used over the years. Those commonly used are belladonna (deadly nightshade) and mustard plasters, both of these will cause blisters if left on long enough. Many embrocations are mildly irritant substances which cause low level inflammation; examples are menthol, turpentine and camphor.
_
How Counter Irritation Works:
The most popular view involves the gate theory of pain. This postulates that sensation from non-pain nerve endings such as those which transmit heat and pressure sensation will, if given enough stimulation, cause an overload in the nerve traffic up the spinal cord to the brain. When this happens it is more difficult for signals from pain receptors (nociceptors) to reach the brain. Since pain is only felt when nerve impulses from pain receptors get to the brain, pain will be diminished or not felt at all.
_
Nutritional Approaches:
Nutritional approaches to pain management can involve both changes in diet and the use of dietary supplements, including vitamins, minerals, enzymes and other substances. These strategies can be used to prevent pain, such as migraine headache, or promote the relief of pain and inflammation as part of a comprehensive pain management strategy. Like the herbal remedies, research in nutritional approaches is in an early phase. Although nutritional therapies are often perceived to have fewer side effects than pharmaceuticals, this may or may not be true and some nutrients are known to be unsafe in certain circumstances or at certain doses. The use of these therapies should be discussed with a health professional.
_
1. Dietary manipulation may influence pain perception or inflammation, and there have been numerous studies to evaluate the outcomes associated with different quantities of food or specific dietary factors. Although the data are limited, studies have begun to assess the effects of fasting, food allergies, and various dietary constituents, such as a glycemic diet, lectins, dietary blue green algae, and green tea polyphenols. Recently, animal studies have measured the effect of phytoestrogens (plant estrogens) in soy-containing diets on pain physiology. Green tea polyphenols are flavonoids, and like other food substances (e.g., grape seed proanthocyanidins and citrus flavonoids) may have anti-oxidant effects. These anti-oxidant effects could potentially have a variety of health benefits, including a treatment effect in painful inflammatory disorders. Vegetarian diets have been advocated for fibromyalgia (Kaartinen, 2000) and rheumatoid arthritis (Kjeldsen-Kragh, 1994, 1999).
_
2.Essential fatty acids (omega-3 and omega-6) perform a variety of functions in the body, including the regulation of immune and inflammatory responses. Recent research suggests that the levels of these chemicals, and the balance between them, could be involved in human disease, such as arthritis and other inflammatory conditions. Common sources of omega-3 fatty acids are coldwater fish such as salmon, herring, sardines and halibut, and nonhydrogenated oils such as canola, grape seed, walnut, hemp and flaxseed oils. Common sources of omega-6 fatty acids include cottonseed, corn and other types of vegetable oils that are used in most processed foods. Studies have suggested that the use of a dietary supplement containing specific essential fatty acids could have favorable effects on pain in patients with osteoarthritis or inflammatory disorders. They may enable patients with osteoarthritis to reduce their use of nonsteroidal anti-inflammatory drugs and allow those with rheumatoid arthritis to reduce the use of other medications. Studies also have shown that higher intake of omega-3 correlates with reduced menstrual pain.
_
3.Gamma-linolenic acid (GLA) is an unsaturated fatty acid derived from plant seed oils, such as evening primrose and borage seed oils. A number of studies have suggested that it reduces inflammation and produces improvement in patients with rheumatoid arthritis. Improvements include decreases in the duration of morning stiffness, joint pain and swelling, and the ability to reduce other medication usage. In addition GLA’s may be useful in the preventive approach to migraine headaches by reducing the severity, frequency and duration of migraine attacks. Since GLA is an omega-6 fatty acid, it is optimally taken with a balance of omega-3s.
_
4.Amino acid tryptophan is converted into 5-hydroxytrytophan, which is converted to serotonin, a neurotransmitter that plays a role in pain and inflammation. Increasing tyrptophan levels in the body may help to reduce chronic pain and reduce episodes of migraine headaches in those who are predisposed to migraines. Foods that contain high levels of tryptophan include salmon, tuna, garbanzo beans, cashew nuts, sunflower seeds, turkey, yogurt, bananas and dairy products.
_
5.Vitamin deficiencies are associated with a variety of diseases, some of which (like neuropathy) may be painful. The recommended daily allowances of vitamins are calculated to prevent deficiency, and a vitamin supplement should be taken if the ability of the diet to provide these substances is in question. There is limited evidence that the additional use of specific vitamins may be therapeutic in some disorders. Vitamins B6 and B2 may play a role in reducing muscle spasms and cramps, preventing migraine, and reducing the symptoms of carpal tunnel syndrome. Vitamin B12, which helps to maintain the sheath that surrounds and protects nerve fibers, may help pain from peripheral neuropathies and low back pain with sciatica. The use of vitamin E, an anti-oxidant, may be effective in relieving pain and improving mobility in those osteoarthritis and a mixed anti-oxidant supplement containing vitamin C, vitamin E, selenium and L-methionine has shown promise in patients with pancreatic inflammatory pain. In Germany, alpha-lipoic acid, another anti-oxidant found mostly in red meat, is an approved treatment for peripheral neuropathy. Administered intravenously, this substance appears to decrease the pain, burning, and numbness associated with neuropathy.
_
6.Minerals also may have therapeutic uses. Zinc and copper may help in wound healing and reduce pain and inflammation. Magnesium is analgesic for neuropathic pain in animal studies and has shown clinical benefit in the treatment of migraine, cluster and tension headaches. It is unclear whether magnesium can reduce pain related to surgery. Magnesium’s mechanism of action in pain management may be partly due to NMDA blockade.
_
7.Enzymes are substances that influence chemical reactions. Bromelain, a complex enzyme of pineapple, is commonly used in Europe as an anti-inflammatory compound for many forms of musculoskeletal injury, arthritis, cramps, post-surgery and post-traumatic swelling. It has been shown to be beneficial in reducing swelling, inflammation and pain by blocking the creation of proinflammatory compounds like prostaglandins, decreasing the production of kinins, and inhibiting fibrin production. Although it is generally well tolerated, it can aggravate ulcers or esophagitis, and can interact with antiplatelet agents.
_
8.Glucosamine sulfate and chondroitin sulfate may favorably influence cartilage and have been studied as treatments for arthritis. Although some studies have had negative results, others suggest significant benefit from both these agents. Glucosamine sulfate has been shown to reduce symptoms of osteoarthritis and temporomandibular joint pain. The onset of improvement may take from one to three months; side effects are mild. Chondroitin sulfate was shown to be significantly superior to placebo in reducing pain in osteoarthritic joints, producing at least 50% improvement compared to placebo. Further studies are needed to clarify its role in the treatment of arthritis and other pain conditions.
_
Foods that fight pain:
1. Whole grains: whole grains are a good source of magnesium, a mineral that has been shown in animal studies to short-circuit muscle pain.
2. Ginger: Ginger is the best pain killer having analgesic properties like the popular ibuprofen. It contains a quartet, gingerols, paradols, shogaols, and zingerone which are active ingredients to reduce pain. Drink ginger tea in monsoons and winter to get relief from that recurring pain.
3. Turmeric: It contains curcumin that helps nip pain
4. Olive oil: It is rich in antioxidant polyphenols that help reduce common pain-causing mechanism in the body. But use it carefully as it has 120 calories per tablespoon.
5. Salmon and mackerel: It is rich in pain-busting omega-3 fatty acids and a great source of another pain fighter: vitamin D.
6. Nuts: Almonds, walnuts are great source of omega 3 fatty acids and anti oxidants that help in pain control.
7. Strawberries: Strawberries are full of vitamin C, an antioxidant with powerful pain-reducing properties, according to research.
8. Dairy: Milk and yogurt contain two bone-building nutrients – calcium and vitamin D. Vitamin D can diminish chronic pain according to research findings.
9. Grapes: Resveratrol in grapes and grape juice can often have an analgesic effect similar to aspirin, according to a handful of studies.
_
Alcohol as pain killer:
High-alcohol liquor, two forms of which were in the US Pharmacopoeia up until 1916 and in common use by physicians well into the 1930s, has been used in the past as an agent for dulling pain, due to the CNS depressant effects of ethyl alcohol, a notable example being the American Civil War. However, the ability of alcohol to relieve severe pain is likely inferior to many analgesics used today (e.g. morphine, codeine). As such, the idea of alcohol for analgesia is generally considered a primitive practice in virtually all industrialized countries today.
_
Alcohol and pain killer:
_
There is a common misconception that you can safely consume over the counter painkillers and alcohol. The effects of mixing alcohol and painkillers are variable for every painkiller and are also affected by the amount of alcohol that has been consumed by the patient. The reason that painkillers and alcohol should not be taken at the same time is that both the substances are depressants, i.e., they affect the nervous system. The double effect of both the substances is not very healthy for the human nervous system and respiratory system. The respiratory system and the nervous system, due to the effects of both depressants, get relaxed and eventually slow down. The rate of breathing reduces, thus decreasing the volume of essential oxygen in the body. The second effect is that these substances also tend to affect the digestive system, especially the liver.
How does alcohol interact with painkillers?
1. Anti-convulsants: Combining alcohol with an anticonvulsant can put you at risk for seizures, even if you are taking an anticonvulsant for chronic pain. The combination can also cause severe drowsiness or make you lightheaded.
2. Opioids: Mixing alcohol and opioids can be lethal. The combination can make you drowsy or cause memory problems. In some cases, mixing the two causes breathing problems or leads to an accidental overdose.
3. NSAIDs: Mixing alcohol with over-the-counter or prescription NSAIDs is not necessarily dangerous in the short term. However, it can increase your risk for developing ulcers or liver damage.
4. Antidepressants: When combined with antidepressants, alcohol can increase feelings of hopelessness and suicidal thoughts, especially in adolescents. Mixing the two can also cause drowsiness and dizziness, as well as lead to an accidental overdose.
_
Novel agents to relieve pain:
_
Flupirtine:
Flupirtine is a centrally acting K+ channel opener with weak NMDA antagonist properties. It is used in Europe for moderate to strong pain and migraine and its muscle relaxant properties. It has no anticholinergic properties and is believed be devoid of any activity on dopamine, serotonin or histamine receptors. It is not addictive and tolerance usually does not develop. However, tolerance may develop in occasional cases.
_
Bone cement with local anesthetics:
Bone cement is used for fixation of artificial joints/implants to the skeleton. It acts as a filler between the bone and the implant. It consists of poly-methyl methacrylate (PMMA), which is a transparent thermoplastic. It is a synthetic polymer of methyl methacrylate. Addition of crystals of barium sulfate gives it radiopacity. Incorporation of substances such as local anesthetics, antibiotics, anti-inflammatory drugs, immunomodulators, osteogenic promoters, and diagnostic substances such as radioactive tracers into bone cement has been attempted. The combination of a local anesthetic with methyl methacrylate cement offers the promise of selective analgesia following surgery or injury to bone. Analgesia from incorporated local anesthetic is thought to be due to the elution of the drug into the surgical site. However, much research into the efficacy, duration of analgesia, and side effects needs to be done. Some elution behavior of local anesthetics has been preliminarily studied.
_
CR845:
CR845 is a novel peripherally restricted, all-d-amino acid tetrapeptide kappa opioid selective agonist under development for the treatment of acute and chronic pain. It belongs to a novel class of opioid ligands which has a reduced ability to cross the blood–brain barrier and therefore acts without inducing central side effects. It is currently available as an intravenous formulation in a sterile isotonic 0.04 M acetate buffer of pH 4.5 composed of acetic acid, sodium acetate trihydrate, sodium chloride, and water, with addition of hydrochloric acid for pH adjustments. CR845 acts to reduce pain by selectively activating kappa opioid receptors located on peripheral nerve terminals, and kappa receptors on certain immune cells. CR845 has no activity at the mu or delta subtypes of opioid receptors, or other known receptors. Stimulation of kappa receptors by CR845 results in the initiation of an intracellular cascade that leads to the inhibition of ion channels necessary for peripheral nerve activity and culminates in reduced afferent nerve activity. Activation of kappa receptors on immune cells results in reduced release of nerve-sensitizing pro-inflammatory molecules.
_
Cannabis derivative:
Cannabis sativa is the genus and species name of a flowering plant which has been used medicinally for thousands of years. Delta-9-tetrahydrocannabinol (THC) is responsible for almost all the psychoactive effects of cannabis, but is only one of more than 60 similar compounds found in cannabis which, together, are collectively known as cannabinoids. Cannabinoid agonists that share the basic chemical structure of delta-9-tetrahydrocannabinol have been increasingly studied in recent years for potential benefits in various types of pain and pain syndromes. Delta-9-tetrahydrocanabinol (THC), the active ligand in Cannabis sativa extract, was thought to exert its pharmacological effects by activating cannabinoid receptors in the central nervous system. These receptors were identified in 1988, and endogenous ligands termed endocannabinoids were isolated in 1992. Two major cannabinoid receptor subtypes termed CB1 and CB2 have been characterized. Central CB1 receptors are primarily localized in neocortex and the limbic system, and are responsible for many of the behavioral and analgesic effects of cannabinoid agonists. Peripheral CB2 receptors and some CB1 subtypes are primarily localized in immune cells such as leukocytes and mast cells, which have been shown to be involved in pain and inflammatory responses. CB2 receptors are not present in the CNS; however, they are also found in peripheral nerve fibers and are particularly concentrated in injured neural endings. Marijuana (cannabis) contains a principle active ingredient THC which binds to the same receptors as the body’s natural endogenous cannabinoids. This fact has made marijuana the subject of heated debate in the last decade because THC is able to mimic the action of natural cannabinoids that the body produces in signaling cascades in response to a peripheral pain stimulus. THC binds to cannabinoid receptors called “CB1” on cells of the spinal cord and pain-modulating centers of the brain to decrease sensitivity to pain. Patients with multiple sclerosis, cancer, AIDS, and a number of other conditions have sought marijuana for years to treat their various symptoms.
_
Note:
Many species of plants produce biologically active ingredients to protect against herbivores that feed on the plants’ tissues. For example the milky juice of milkweeds is very bitter, toxic, and sticky. An insect feeding on the milkweed can be trapped in the sticky juice, repelled or killed by the chemicals. THC is most likely a repellent to many insects and grazing animals. So cannabis plant has THC for its own survival and not for human pain relief. The way marijuana contain THC and the way opium contain morphine; and both of these have biological receptors in human brain; and the way body produces endogenous opioids & cannabinoids which target the same receptors; makes me think that during evolution of species over millions of years, some cross-connection exist between plant kingdom and animal kingdom at the basic level of DNA, genes, proteins and molecular biology and therefore presence of these receptors in human brain which readily accept plant based chemicals is not a mere coincidence but an evolutionary design.
_
Injectable capsaicin:
The injectable analgesic Adlea™ is a concentrated and purified form of capsaicin (8-methyl-N-vanillyl-6-nonenamide). It is an unapproved preparation, currently in phase 3 trials for relief of post-surgical acute pain, nerve trauma-induced neuropathic pain, and for chronic musculoskeletal, tendon-related, and arthritic pain. Capsaicin is an alkaloid derived from plants and has been a common ingredient in food and spices for many centuries. It is concentrated in the seeds and inner stem of chili peppers and is the active ingredient responsible for making them taste hot.
_
TRPV:
Transient receptor potential vanilloid (TRPV) channel blockers (antagonists) are new drugs, which are not currently approved for clinical use, yet have great analgesic potential. An example of this class of drugs is capsazepine, a synthetic analog of capsaicin, which inactivates TRPV1 channels by competitively occupying TRPV1 binding sites in sensory neurons. Although capsazepine, the first TRPV antagonist, exhibited certain analgesic and anti-inflammatory properties, its effects were of low potency as well as variable within different species. As a result, capsazepine never reached the clinical arena. It is used now in the laboratory as an investigating tool for the study of other TRPV antagonists. The next compound studied was iodo-resiniferatoxin (I-RTX), an analog of resiniferatoxin (RTX). This drug was more potent than capsazepine and more specific for TRPV receptors but lacked consistent analgesic effects. The functional importance of the TRPV channels in pain was further elucidated with the cloning of the vanilloid receptor. The search for the perfect TRPV antagonist has been intensified and currently there are several drugs undergoing clinical trials. The new generation of TRPV antagonists has structures completely different from the agonists and therefore they are devoid of partial agonistic effect.
_
Neramexane:
Neramexane is a central-acting N-methy-d-aspartate receptor antagonist which first underwent preclinal trials in Germany. In 1998, it was investigated for both neuroprotective and alcoloholism effects. Neramexane is an open-channel blocker against the NMDA receptor. It has moderate affinity to the NMDA receptor, with strong voltage-dependency and fast-onset blocking kinetics. It is investigated for role in treating neuropathic pain. Metabolic pathways, drug clearance and elimination: in the phase I study, an oral dose (5–40 mg) demonstrated a plasma half-life of approximately 29–42 hours. Metabolism has not been clearly elucidated yet. After administration of a single dose, 30–40% of the drug was excreted from the kidney unchanged.
_
Nociceptin:
Nociceptin (orphanin FQ) is a novel neuropeptide whose actions have yet to be completely elucidated. However, stimulation of the nociceptin receptor has been implicated in the modulation of many neurobehavioral processes, including pain, substance dependence, sexual behavior, anxiety, locomotor activity, learning capacity, memory, and adaptation to stressful stimulus. Continued research in this area has the potential to lead to a new class of drugs for pain medicine and beyond.
_
BL-7050 for neuropathic pain:
A new drug developed by Tel Aviv University researchers, known as BL-7050, is offering new hope to patients with neuropathic pain. The medication works by targeting a group of proteins which act as a channel for potassium. Potassium has a crucial role in the excitability of cells, specifically those in the nervous system and the heart. When potassium channels don’t function properly, cells are prone to hyper-excitability, leading to neurological and cardiovascular disorders such as epilepsy and arrhythmias. These are also the channels that convey pain signals caused by nerve damage, known as neuropathic pain. In both in-vitro and in-vivo experiments measuring electrical activity of neurons, the compound has been shown to prevent the hyper-excitability of neurons — protecting not only against neuropathic pain, but epileptic seizures as well.
_
Local adenosine:
Researchers have published a new method to eliminate pain using acupuncture point location as a basis for the procedure. It all started when researchers measured that adenosine, an inhibitory neurotransmitter with antinociceptive properties, is produced by acupuncture stimulation. Nociceptors are blocked by adenosine. Acupuncture stimulates the natural production of adenosine within the body. Based on these findings, the researchers decided to inject prostatic acid phosphatase (PAP) into an acupuncture point. PAP is an ectonucleotidase that converts AMP to adenosine. The results showed a powerful dose dependent antinociceptive response in mice. Antinociception was boosted by adding additional AMP and was blocked with adenosine antagonists. The researchers say that this approach to pain management would “bypass side-effects associated with opioid-based analgesics, and hence could provide a novel abuse-resistant way to treat pain.”
_
Neurotoxin for chronic pain:
Management of chronic pain is often challenging since commonly prescribed analgesics do not have direct effects on damaged nerves, but, rather, nonspecific effects on pain receptors and other mediators of pain. Neurotoxins are a new class of medications that are being evaluated as potential analgesics. Using these medications, researchers hope to achieve pain relief by suppressing the activity of injured nerve tissue and directly targeting the source of some types of chronic pain. A challenge in developing these medications is that in addition to affecting nerves causing pain, they could also damage other nerves, including those in the brain and spinal cord. Neurotoxins could therefore cause serious harm related to unintended nerve damage, and are undergoing careful testing to understand how well they selectively affect already damaged nerves.
Anti-nerve growth factor is the first highly selective neurotoxin to be investigated for use in humans. Discovered in the 1950s, investigators found that this immunotoxin stunts neural growth.
_
Various ways in which pain can be controlled:
|
Method |
Possible Mechanism(s) |
Uses/Examples |
| Aspirin | Acts mostly in peripheral nervous system. Reduces inflammation. | Headache, musculoskeletal pain |
| Morphine | Acts in central nervous system (brain and spinal cord) to block pain messages. Activates pain-modulating systems in the brain that project to the spinal cord. | Post-operative pain; other pain conditions |
| Other pain reducing drugs |
Act on a variety of neurotransmitter systems. | Variety of pain conditions |
| Hypnosis |
|
Dental procedures, childbirth, burns, headache |
| Acupuncture |
|
Back pain, minor surgical operations |
| Placebo |
|
Headache, post-operative pain |
| Transcutaneous Electrical Nerve Stimulation (TENS) |
|
Post-operative pain, arthritis, cancer pain |
| Neurosurgery | Removal or blockade of painful signals | Cancer pain |
| Stress |
|
Football player continues to play regardless of injury. Soldier continues to fight regardless of wounds. |
_
Pain and obesity:
Pain is one of the most common and debilitating problems in patients challenged by severe obesity. Not just a consequence of mechanical complications of obesity (osteoarthritis, back pain, plantar fasciitis, fibromyalgia, etc.), pain is often a key barrier to physical activity and thus weight management. In fact, excess pain can promote psychological (e.g. depression, anxiety) and behavioral (e.g. binge eating) factors that may further promote weight gain. The relationship between pain catastrophizing and eating behavior is of particular interest, as high-fat and high-sucrose foods have been shown to increase pain tolerance. Thus, binging on highly-palatable foods may be a compensatory response to emotional distress and pain. It is not difficult to see how patients can enter into a vicious cycle of pain, increased eating, weight gain, more pain, more eating, and so on.
_
Difference between tolerance and addiction to pain killers:
Needing a higher dose of painkillers to manage your pain does not mean you’re addicted. But it’s important to be able to distinguish between addiction and tolerance. People who use opioid painkillers for months or years often develop a tolerance to the drugs — which means they need higher doses of the medication to achieve the same results. Many people who take painkillers worry about their risk of addiction and see signs of tolerance as the early hint of a downward spiral, but this is not necessarily an accurate. Tolerance is an absolutely normal expected phenomenon when people take many pharmacological substances on a regular basis. As your body adjusts, you need more of a dose to get the effect you’re looking for. Your body, primarily your liver, starts to process the medication more efficiently while your brain requires more of the medication to achieve the same effects. If you need a painkiller for months or years, the dose that was adequate at the beginning might be five- or ten-fold less than what you will need after those months or years. Addiction, on the other hand, leads to less rational behaviors. For example, a person developing an addiction might start to argue with himself, day after day, telling himself that taking an extra dose “because of a hard day” is okay without calling the doctor. Where people get into trouble is when they start to self-medicate. A classic sign of addiction from the doctor’s perspective is when a person whose pain is under control asks for more painkillers. Doctors have various ways of determining whether pain is under control or not beyond a patient’s self report. Addiction is when we organize our lives around a substance and continue it despite it causing problems, or when we use more than we plan or intend to. It’s important for people taking painkillers to know that tolerance will keep building as long as you are taking the medication, but if you stop for some time, it will begin to fade.
_
Pain killer addiction:
_
_
Opioid addiction and opioid dependence:
Addiction to prescription painkillers is a disease that has become increasingly prevalent in the United States and elsewhere. Opiate, or narcotic pain medications are commonly prescribed by physicians to treat pain. Often, patients continue taking their medication as prescribed and become physically dependent upon the drug. However, physical dependency and addiction are different. Psychiatry defines addiction as compulsive use of a substance despite negative consequences—and it is this craving, impairment and loss of control over substance use. People who are addicted to opioids take opioids not for pain killing potential but because they are addicted to it. Physical dependence to a drug can be identified by withdrawal symptoms if the drug is abruptly stopped or decreased. While physical dependence may be a component of addiction, it is not, in and of itself, addiction. In fact, physical dependence is a consequence of many medications. For example, certain blood pressure medications can cause physical dependence. Yet, these medications do not lead to addiction. Dependence is a physical state that occurs when the lack of a drug causes the body to have a reaction. Physical dependence is solely a physical state indicating that the body has grown so adapted to having the drug present that sudden removal of it will lead to negative consequences such as a withdrawal reaction. This can occur with almost any kind of drug. A good example of dependence is a heavy coffee drinker’s use of caffeine. If you are used to drinking several cups of coffee each day, you soon learn about physical dependence when you miss a day or two. This does not mean you are addicted to the caffeine; it only means your body is surprised not to see what it has come to expect. Addiction occurs when a patient begins to raise their dose without first consulting their doctor, they find multiple sources for the drug without their primary doctors knowledge (doctor shopping), they lie about their usage, and they will not stop using the medication when the doctor stops the treatment. Addiction is both a biological and psychological condition whereby a person exhibits compulsive behavior to satisfy their craving for pain medication even when there are negative consequences associated with taking the drug. Dependence is an unavoidable consequence of opioid therapy. An opioid-dependent pain patient has improved function with the use of the drug while an opioid-addicted patient does not. Dependence happens because of the following physical process:
1. The brain has responded to the presence of the pain medicine by increasing the number of receptors for the drug, and the nerve cells in the brain cease to function normally.
2. The body stops producing endorphins (the body’s natural painkillers) because it is receiving opiates instead.
3. The degeneration of the nerve cells in the brain causes a physical dependency on an external supply of opiates, and reducing or stopping intake of the drug causes a painful series of physical changes called the withdrawal syndrome. At this point the patient may continue taking the pain medication to avoid the withdrawal symptoms, rather than taking it to treat the pain that caused them to take the medicine initially. When this occurs the patient is considered to be dependent on the prescription pain medicine. Addiction, on the other hand has genetic/familial component to it and many addicts have a prior history of psychiatric illness.
_
_
Prescription Drug Abuse Facts from the Office of National Drug Control Policy (ONDCP):
1. Prescription drugs are the second-most abused category of drugs in the United States, following marijuana.
2. Among 12th graders, pharmaceutical drugs used non-medically are six of the ten most-used substances.
3. From 1998 to 2008, the proportion of all substance abuse treatment admissions age 12 or older who reported any pain reliever abuse increased more than fourfold.
4. Prescription painkillers are considered a major contributor to the total number of drug deaths. In 2007, for example, nearly 28,000 Americans died from unintentional drug poisoning, and of these, nearly 12,000 involved prescription pain relievers.
5. Nearly one-third (29 percent) of people age 12 or older who used illicit drugs for the first time in the past year began by using prescription drugs non-medically.
6. According to a 2008 Department of Defense survey, about one in nine active-duty service members (11 percent) reported past-month prescription drug misuse.
7. The estimated number of emergency department visits linked to non-medical use of prescription pain relievers nearly doubled between 2004 and 2009.
8. In 2009, the number of first-time, non-medical users of psychotherapeutics (prescription opioid pain relievers, tranquilizers, sedatives, and stimulants) was about the same as the number of first-time marijuana users.
9. Approximately two million adults age 50 and older (2.1 percent of adults in that age range) used prescription-type drugs non-medically in the past year.
10. Substance abuse treatment admissions for individuals age 50 or older nearly doubled from 1992 to 2008, climbing from 6.6 percent of all admissions to 12.2 percent. The percentage of primary admissions for prescription drug abuse among older individuals increased from 0.7 percent to 3.5 percent over the same time period.
__
Why people abuse Prescription Painkillers:
1. Painkillers numb physical pain very effectively
2. Non-Drug pain management services are inaccessible
3. Painkillers distance you from emotional pain
4. Painkillers can be Pleasurable. Opioids, in particular, have a side effect of euphoria.
5. Painkillers induce relaxation
6. Tolerance builds up quickly
7. Physical neglect intensifies pain: physical behaviors such as overuse of an injured part of the body, poor posture resulting from a lack of sensation when in positions that would otherwise be uncomfortable, and a lack of moderate exercise that would otherwise strengthen the weakened area are bad habits resulting in the person will often just take more painkillers, creating a vicious cycle of physical neglect being concealed by the effects of the drugs.
8. Withdrawal is very unpleasant
9. Painkillers are Legally Available
10. Addiction leads to stigma, which leads to Illicit Drug Use.
___
Newborns addicted to pain killers:
Neonatal withdrawal syndrome or neonatal abstinence syndrome is when a group of problems occur in a newborn that was exposed to drug abuse in the womb. There are two types of neonatal abstinence syndrome: neonatal abstinence syndrome due to prenatal or maternal use of substances that result in withdrawal symptoms in the newborn and postnatal neonatal abstinence syndrome secondary to discontinuation of medications such as fentanyl or morphine used for pain therapy in the newborn. Neonatal abstinence syndrome mostly occurs when the mother takes addictive illicit or prescription drugs. About three percent of the 4.1 million of women able to bear a child who abuse drugs are believed to continue drug abuse during pregnancy. Opiates & narcotics, Selective serotonin reuptake inhibitors (SSRIs), Antihistaminics (Diphenhydramine, Hydroxyzine), Marijuana, Ethchlorvynol, Nicotine, or Meprobamate etc are offending drugs. Symptoms depend on the specific drug the mother used during pregnancy, the amount of drug used, whether the baby was born premature or full-term, and how long the drug was used. Some symptoms can occur one to three days after birth or five to ten days and they include: diarrhea, blotchy skin coloring, vomiting, seizures, excessive crying, sweating, trembling, sleep problems, sneezing (stuffy nose), irritability, or poor feeding. Treatment depends on the baby’s overall health and symptoms. Babies are usually watched for vomiting and dehydrating and sometimes receive fluids through a vein. Some need medicine for withdrawal; Benzodiazepines for alcohol withdrawal and Methadone for heroin and other opiate withdrawal. Some doctors prescribe the baby whatever drug they were born addicted to and slowly wean the baby off by lowering dose. Babies who experience feeding problems need high calorie formula that provides more nutrition and smaller portions more often. Treatment relieves symptoms but how well the baby does depends partially on whether the mother continues to abuse drugs.
_
Pain killers in pregnancy:
Concern remains about common painkiller use during pregnancy. There doesn’t seem to be any evidence that paracetamol, if used occasionally, increases the risk of problems for the baby, but you should always be careful about taking them more than two or three times a week and see your doctor if you need to use them regularly. It’s probably best to avoid ibuprofen and codeine in early pregnancy and they shouldn’t be taken at all later in pregnancy, as the potential risks to the fetus are well known. Aspirin is also best avoided, especially towards the end of pregnancy, although there’s some research looking at whether in low doses, in the early stages, it may help to prevent recurrent miscarriage in some women.
_
Now new research published in the journal Human Reproduction suggests that mild painkillers such as aspirin, acetaminophen and ibuprofen may be linked to an increase in male reproductive disorders. The scientists from Denmark, Finland and France found that women who either took a combination of mild analgesics during pregnancy or took them during the second trimester of pregnancy had a greater risk of giving birth to baby boys with undescended testicles, a condition known as cryptorchidism, which may forecast poor semen quality and testicular cancer. Specifically, the researchers found that women who used more than one painkiller at the same time had a seven-fold increased risk of having sons with some form of cryptorchidism in comparison with women who took no analgesics. In the second trimester, any painkiller use more than doubled the risk of cryptorchidism, and simultaneous painkiller use increased the risk 16-fold. The scientists also pointed to work conducted on rats by two of the researchers who found that analgesics toyed with androgen production and led to decreased levels of testosterone when male fetal organs were forming. Researchers aren’t sure why that happens. The most important message is that if you take the occasional paracetamol, it’s not going to do your baby any harm.
_
Opioids and pregnancy:
Moms-to-be who take prescription opioid painkillers such as codeine, hydrocodone or oxycodone may increase the risk of birth defects in their newborns, according to a new U.S. government report. For women who took prescription pain killers, the risk of having a baby with hypoplastic left heart syndrome — a critical heart defect was about double. Taking these types of analgesics just prior to pregnancy or in the early stages of pregnancy was linked to a modest risk of congenital heart defects in an ongoing population study, according to the national Centers for Disease Control and Prevention. The risk for spina bifida, hydrocephaly, congenital glaucoma and gastroschisis was also heightened, the report said.
_
Labor pain:
Pain experienced during labor is probably the most painful event in the lives of women. Labor pain is a complex and subjective interaction between multiple physiologic, psychosocial, environmental plus cultural factors and a woman’s interpretation of labor stimuli. It must be emphasized that a women’s internal experience of pain is affected by the environment in which she is laboring. Environment itself influences a mother’s experience of pain. Environment can influence pain perception in women. Tension and stress resulting from pregnancy crisis and labor increase when the mother is hospitalized, which is concomitant with stressful situations and factors that affect pain perception during labor. A study found significant positive correlations between pain and tension from environmental factors in primiparous (r=0.16, p<0.01) and in multiparous (r=0.22, p<0.05) women. Furthermore, primiparous women believed that a crowded delivery room (70%) and restriction of movement and mobility (67%) contributed to their environmental stresses. Multiparous women believed that noise in the delivery ward (84%) and restrict of fluid intake (78%) increased their stresses. Performance of routine diagnostic tests in hospitalized pregnant woman, provision of invasive medical care during labor process and a noisy & crowded environment all influence the mother’s experience and perception of pain. Therefore, the medical staffs seem to play a great role in alleviating labor pain by reducing stressors, especially the objective ones that are more stressful.
_
Hot fomentation and cold fomentation:
A fomentation, from the Latin word fomentum, which means soothing application or poultice, is any warm, moist application that delivers heat to the body for healing. Nowadays, fomentation means applying or extracting heat from tissues by using various methods. Cold and hot fomentations have been used for many years to treat people with various ailments. Hot fomentations are more common than cold fomentations. Pain is the most common problem that is treated with hot fomentations. In the early stage of an injury cold fomentations can be given to treat the condition. As the condition becomes sub acute, hot fomentations replace the cold fomentations. For epistaxis (bleeding from nose), I used to advise ice packs (cold fomentation) to arrest bleeding as cold temperature will cause vasoconstriction and reduce bleeding from the blood vessel.
_
For acute injury, hot fomentation and massage will harm the patient. Massage will injure more number of blood vessels and prevent arrest of bleeding; which may enhance the swelling and worsen the pain. If a fracture is already present the gap will widen creating more problems to manage. Hot fomentation will cause vasodilation and prevent arrest of bleeding in acute injury. Also, hot fomentation will dilate the blood vessels more and help to increase the swelling by more extravasations of blood. That will give rise to more pain and swelling. So the rationale for icing an acute injury initially is to decrease the inflammation. This clearly helps. After 48-72 hours it is generally recommended that heat be applied to the area of injury. The impression is that this (heat) helps with pain relief in the majority of patients 48-72 hours after injury. After 48 to 72 hours, increased temperature given by hot fomentations will cause an increase in the blood flow in the body of the person affected. The increased blood flow causes the transport of nutrients from the other parts of the body to the wounded site and so helps in increasing the rate of healing. The increased blood supply to the injured part also helps in removing all the debris from the affected area. For sub-acute or chronic injury, heat can also help the tissue to be relaxed. Hot fomentations help to relieve these muscle spasms.
_
Indications for heat applications:
1. Muscle spasm, including infant colic caused by muscle tightness
2. Poor local circulation
3. Musculoskeletal pain (muscle soreness, stiff joints, arthritis pain, chronic back pain)
4. Muscle tightness
5. Warming of tissue to make it more pliable and stretchable before massage, especially for athletic persons with very dense tissue or areas with sensitive scar tissue
6. Soreness after deep massage
7. Menstrual cramps
8. Active trigger points
9. When derivation (a tissue-shifting effect) is desired moving blood toward the hot application and away from congested areas. Useful for migraine headache.
10. Nervous tension
11. Chilled local area
12. Chilled client
_
Local contraindications for heat application:
1. Loss of sensation (lack of feeling), which can be caused by spinal cord injury, diabetic neuropathy, other medical condition, or the use of some medications. These conditions can render the person unable to feel the pain of a too-hot application. Never put a hot application on a numb area.
2. Rash or other skin condition that could be made worse by heat
3. Inflammation
4. Swelling
5. Broken skin—burn, wound, sore, cracked skin from eczema or severe chapping
6. Malignancy
7. Implanted device such as cardiac pacemaker, stomach band, and infusion pump
8. Diabetics should not receive hot applications to the legs or feet.
9. Peripheral vascular disease, including diabetes, Buerger’s disease, and arteriosclerosis of the lower extremities
10. Sensitivity to heat, especially in those with thin skin, such as the elderly and small children, who might burn more easily
11. Any acute injury of less than 48 hours duration.
_
Caution for heat application:
1. Elderly (over 60 years old)
2. Children
_
A child’s pain perception: Plasticity and complexity:
A child’s pain perception can be regarded as plastic from a psychological, as well as biological, perspective. Tissue damage initiates a sequence of neural events that may lead to pain, but many developmental, social, and psychological factors can intervene to alter the sequence of nociceptive transmission and thereby modify a child’s pain. Child characteristics, such as cognitive level, sex, gender, temperament, previous pain experience, family, and cultural background generally shape how children interpret and cope with pain (Katz et al. 1980; Blount et al. 1991; Bennett-Branson and Craig 1993; Schanberg et al. 1998; Peterson et al. 1999; Chen et al. 2000; Chambers et al. 2002).
_
Pain in children:
Few studies reliably document the prevalence of pain in children. Research on the neurobiology of pain in early development has shown that infants, and children of all ages, have the capacity to perceive pain (Fitzgerald 2005). We must therefore assume that any experience expected to be painful for an adult is likely to cause pain in infants and children. Indeed, many factors increase the impact of pain in children, who are also often exposed disproportionately to pain.
_
Everyday pain: Minor bumps and bruises are very common in children in the course of active play and sport. They are usually not medically significant, nor can they all be reasonably avoided, but each episode provides an opportunity for learning about how to cope with pain, either magnifying or reducing the distress.
_
Short-term pain: lasting minutes, hours, or days may be caused by illness, trauma, or medical procedures such as immunization, blood tests, and surgery. Equivalently intrusive events are reported to be more painful by younger than by older children. Children are generally unable either to refuse or to rationalize painful experiences, so they suffer especially from invasive diagnostic procedures. There have been improvements in management of acute pain in some pediatric hospitals and units, but pain management techniques in many centers around the world have not progressed in the past 20 years. Advances in health care, such as new knowledge of pain prevention strategies, have not been consistently translated into decreased prevalence or intensity of pain experienced by children in hospitals.
_
Recurrent pain: in childhood arises from complex and multifaceted causes. Stomachaches, headaches, limb pain (“growing pains”), and chest or back pain are experienced occasionally to frequently by up to 30% of children. Even when such pain seems relatively minor in intensity, it may interfere with school and family life, causing both emotional and financial stress. So-called “functional” pains that lack an obvious pathological basis may be caused by subtle neurophysiological dysfunctions that are not easily characterized by everyday medical investigations, and thus are frequently minimized by clinicians.
_
Disease-related and chronic pain: Pain is the initial symptom of many childhood diseases. Cancer pain is a well-recognized concern in developed countries, and much of the focus of non-governmental organizations worldwide has been in this area (WHO 1998). Many children in the world live in developing countries and suffer an additional burden of pain from malaria, HIV/AIDS, sickle cell disease, and other conditions endemic in poorer countries with inadequate health care resources. For example, available options to man-age sickle cell pain vary significantly from country to country. Some children have diseases such as juvenile arthritis, migraine, and inflammatory bowel disease that cause chronic daily or near-daily pain. Children, like adults, can suffer neuropathic pain from complex regional pain syndrome or peripheral nerve or spinal cord injury. Children with chronic pain experience high levels of pain-associated disability. We still do not fully understand the risk factors for development of chronic pain in childhood, but prior untreated pain must be regarded as a likely candidate. Children’s chronic pain affects and is influenced by family factors. Children with chronic pain may be at higher risk for chronic pain in adulthood. Children with terminal illness experience pain from the cumulative effects of progressive disease, invasive procedures, treatment, and psychological distress. Special challenges arise in managing their pain and the side effects of pain treatment in the home setting.
_
Pain in children undertreated:
Two studies highlight how children’s pain problems were undertreated during this period. In 1968, Swafford and Allan surveyed analgesic use for all children treated in an intensive care unit during a 4-month period. Only 14% of children (26 of 180) had received any opioids for pain relief. Moreover, only 3% of children received analgesics after general surgery, presumably because “Pediatric patients seldom need relief of pain after general surgery. They tolerate discomfort well” (Swafford and Allan 1968). Subsequently, Eland (1974) compared medication use for 18 adults and 25 children with similar medical conditions during their hospitalization. While 372 opioid doses and 299 non-opioid doses were administered to adults, only 24 analgesic doses were administered to children. In fact, more than half of the children did not receive any analgesics, despite undergoing major trauma including amputation of the foot, excision of neck mass, and heminephrectomy.
_
Treating Children’s Pain
Most pain can be prevented, treated, or at least reduced using inexpensive pharmacological, psychological, and physical techniques. Yet even in developed countries, deficiencies in children’s pain management persist (Ellis 2002; Jacob and Puntillo 2000; Johnston 2005), and it is certain that most children in the developing world receive inadequate pain care. A substantial body of research shows the effectiveness of cognitive and behavioral techniques, such as information giving, paced breathing, progressive muscle relaxation, hypnosis, and imagery for reducing children’s pain and distress from invasive procedures (Kuttner and Culbert 2003). Despite this evidence, few children and parents are taught anxiety-reducing skills to prevent or reduce pain during and after procedures. There is broad clinical experience and considerable research on pharmacological treatment for children’s acute pain in pediatric specialty settings in developed countries, although data on chronic pain is sparse. However, this knowledge is applied inconsistently in North America, Europe, and other developed countries, and hardly at all in many low and middle- income countries of the developing world (Forgeron et al. in press). Health care providers and parents often worry that pain medication will be dangerous for children. In fact, developmental concerns with analgesic treatment are minimal once the neo-natal period is past, other than dosing appropriate for the size of the child. There is no evidence that children become addicted to strong opioids that are prescribed appropriately for pain treatment.
_
Distraction is a simple and very effective way to reduce pain for infants and children:
Distraction tends to work best for mild pain and how you distract your child will depend on his or her age. Babies can be distracted with colorful mobiles and mirrors. Younger children can be distracted with blowing bubbles or party blowers, reading a favorite book, playing with a musical toy or with the use of virtual reality glasses. Older children can choose what they wish to be distracted with, a hand-held video game, for example.
_
Sucrose for analgesia in newborn infants undergoing painful procedures:
Forty-four studies enrolling 3,496 infants were included in a meta-analysis. Sucrose significantly reduced duration of total crying time (seconds). There were no differences in adverse effects between sucrose and control groups. Sucrose is safe and effective for reducing procedural pain from single events. An optimal dose could not be identified due to inconsistency in effective sucrose dosage among studies.
_
Fetal pain:
Fetal pain has so many implications that it requires a scientific appraisal independent of the heated controversies regarding abortions, women’s rights, or the beginnings of human life. These implications include pain perception in preterm neonates, anesthesia for fetal surgery or intra-uterine procedures, and the long-term consequences of perinatal anesthesia/analgesia on brain development. The available scientific evidence makes it possible, even probable, that fetal pain perception occurs well before late gestation. Those attempting to deny or delay its occurrence must offer conclusive evidence for the absence of fetal pain at given levels of maturity. When developmental time is translated across animal species to humans, it is clear that functionally effective patterns of sensory processing develop during the second trimester. Thalamocortical interactions located in the subplate zone persist into maturity, thus providing a functional template for subsequent cortical processing. Several lines of evidence indicate that consciousness depends on a subcortical system and that certain contents of consciousness are located in cortical areas. These subcortical structures, which develop much earlier than the cortex, may play a pivotal role in sensory perception. Our current understanding of development provides the anatomical structures, the physiological mechanisms, and the functional evidence for pain perception developing in the second trimester, certainly not in the first trimester, but well before the third trimester of human gestation.
_
Gender difference in pain perception:
Traditionally, biomedical research in the field of pain has been conducted with male animals and subjects. Over the past 20–30 yr, it has been increasingly recognized that this narrow approach has missed an important variable: sex. An ever-increasing number of studies have established sex differences in response to pain and analgesics. These studies have demonstrated that the differences between the sexes appear to have a biological and psychological basis. The terms “sex” and “gender” are not synonymous. According to the Institute of Medicine definition, sex is “the classifications of living things, generally as male or female according to their reproductive organs and function assigned by the chromosomal complement.” Gender is “a person’s self-representation as male or female, or how that person is responded to by social institutions on the basis of the individual’s gender presentation.” The lack of semantic precision in the literature can and has led to confusion and faulty conclusions.
_
The study of neonatal gender differences in pain expression is important since neonatal pain behavior occurs prior to any learned reaction pattern. The objective of this study was to verify the presence of gender differences in pain expression in preterm and term newborn infants. Sixty-five consecutive neonates (37 female and 28 male infants) with gestational age between 28 & 42 weeks and with 25 &120 hrs of life were studied. In conclusion, recently born female neonates of all gestational ages expressed more facial features of pain than male infants, during the capillary puncture and 1 min afterwards. Maybe differences in pain processing and/or pain expression among genders may explain this finding.
_
Science is providing new insights into pain relief, anesthesia and, oddly enough, redheads. Females are more sensitive to pain, less tolerant and more able to discriminate different levels of pain than males. This is true in studies of both humans and animals. Women are also much more likely to suffer from chronic pain conditions than men. Researchers originally suspected that this was primarily due to the fact that they are more likely to seek medical care in general. But while women do indeed seek more care, they’re also genuinely more likely to develop painful conditions like fibromyalgia, rheumatoid arthritis and migraines. For example, 80 to 90 percent of people with fibromyalgia are women, according to the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Scientists have discovered that women’s experience of pain may be not only quantitatively different, but qualitatively as well. Pregnancy actually is the exception to the rule. While pregnant, women do become progressively less sensitive to pain as they get closer to giving birth. Natural painkillers like endorphins are elevated during pregnancy and labor, helping fight pain.
_
Picture above shows while experiencing pain, the right amygdala is activated more in men, and it has more connections to regions of the brain involved with external environment. In women, the experience of pain activates the left amygdala more, which has more connections to regions of the brain associated with internal functions.
_
Center for Neurovisceral Sciences & Women’s Health (CNS) at UCLA, which conducted the study and published in the June 2003 issue of the journal Gastroenterology, the study examined 26 women and 24 men with IBS. UCLA researchers took positron emission tomography (PET) brain scans of patients during mild pain stimuli. Although researchers found some overlapping areas of brain activation in men and women, several areas of male and female brains reacted differently when given the same pain stimulus. The female brain showed greater activity in limbic regions, which are emotion-based centers. In men, the cognitive regions, or analytical centers, showed greater activity. The reason for the two different brain responses may date back to primitive days, when the roles of men and women were more distinct. These gender differences in brain responses to pain may have evolved as part of a more general difference in stress responses between men and women. Men’s cognitive areas may be more highly triggered because of the early male role in defending the homestead, where in response to stress and pain, the brain launched a calculated fight-or-flight reaction. The female limbic regions may be more responsive under threat because of their importance in triggering a nurturing and protecting response for the young, leading to a more emotion-based response in facing pain and stress. Researchers noted that both responses have advantages and neither is better. In fact, under conditions of external threat, the different responses may lead to complementary behaviors between men and women. Men were more unwilling than women to report pain, and that women seek medical advice more often than men as this difference may be an evolutionary survival advantage for women, rather than a perceived weakness, some researchers speculate. Because pain drives most people to seek treatment in the first place, women may reduce the chance of significant tissue damage if they get checked out early.
_
Biological explanations for gender based pain differences:
A number of scientists have hypothesized about potential biological explanations for gender pain differences. Berkley described three aspects of male and female biology that plainly differ: the pelvic reproductive organs, types of circulating hormones, and cyclical changes in hormone levels. Other biological explanations for the differences in pain response include mechanisms of analgesia having to do with opioid receptors in the body, mechanisms of nerve growth factor, and sex-based differences in sympathetic nervous system function (e.g., sex-based differences in areas of the brain associated with reproduction). That these differences could result in men and women experiencing different emotional responses to pain (e.g., anxiety, fear, depression, or hostility). A number of studies have added to the body of literature on the influence of reproductive hormones on biological pain differences. Berkley concluded that the reproductive hormones appear to influence sex-based pain differences through the action of a number of neuroactive agents, such as dopamine and serotonin. Giamberardino and colleagues found that a woman’s pain sensitivity increases and decreases throughout her menstrual cycle, with skin, subcutaneous tissue, and muscles being affected differently by female hormonal fluctuations. They also found that sex-based differences in pain response may depend on the proximity of the stimulus to external reproductive organs. Fillingim and colleagues found that the menstrual cycle produced greater effects on ischemic (i.e., lack of blood flow and oxygen), compared with thermal, pain sensitivity. The authors suggest that opiate receptors could be desensitized by reproductive hormones during certain phases of a woman’s menstrual cycle, thus increasing pain sensitivity (particularly ischemic pain sensitivity) at those times. Some research has shown differences in the brain and central nervous system of men and women that may contribute to differences in pain response. For example, Fillingim and Maixner describe neural mechanisms that contribute to sex based differences in the perceptual, emotional, and behavioral responses to noxious stimuli. These include peripheral afferents (impulses sent to the brain), brain and central nervous system networks, and peripheral efferents (commands sent from the brain to the muscles). The authors note differences in female tissue thickness and sensory receptor density as one example of structural differences in females that may contribute to enhanced perception o sensation to the skin.
_
Culture, gender, and pain:
There are [so many] overlays of societal and cultural norms and other factors that go into the reporting of pain that it may not have a biological basis at all. The interplay between behavior and the value systems of a culture is complex and may influence pain perception in many ways. Children are socialized from a very young age to think about pain and to react to painful events in certain ways. In many societies, boys are actively discouraged from expressing emotions. When a boy falls down and cries due to pain; parents say, don’t cry, don’t behave like a girl. So in many cultures, boys must bear pain and not express it, otherwise they will be ridiculed.
_
Gender and Pain Management
There is a growing body of literature that indicates women are more likely than men to be undertreated for their pain. It appears that gender affects not only pain perception, pain coping, and pain reporting, but also pain-related behaviors, including use of healthcare and the social welfare system. It is also probable that men and women differ systematically in their responses to pain treatments, although further research is needed in this area. Whatever the pain prevalence differences for men and women, most studies show that women seek healthcare for pain at a higher rate than men. On the other hand, studies have shown differences in the attitudes of healthcare providers toward men’s and women’s experiences of pain with neglect of women’s pain by physicians. Historically, medical literature has portrayed women as hysterical and oversensitive. By extension, physicians often view women’s statements as emotional, rather than objective. In one study of patients with chronic pain, female patients were more likely than their male counterparts to be diagnosed with histrionic disorder, excessive emotionality, and attention-seeking behavior. Also, regarding physician’s perception of female patients with pain, Hadjistavropoulos and colleagues found that physicians distinguished between their “attractive” and “unattractive” patients. Attractive female patients were perceived as experiencing less pain than unattractive female patients, evidencing a “healthy is beautiful” stereotype. One study indicated that women are more likely to be given sedatives for their pain and men to be given analgesics.
_
A striking discovery is that the painkiller morphine works differently in the brains and spinal cords of males and females. Animal experiments have revealed that females require twice the dosage as males to experience the same amount of pain relief. Morphine binds to mu opioid receptors – cell membrane proteins that influence the behavior of cells – in order to decrease pain. Previously, studies of opioid receptors had focused exclusively on males. Although this protein is located in the same brain region, males seem to have higher levels of the receptor than females, and it is expressed differently. Researchers suspect that this distinction may account for sex differences in pain relief.
_
Pain and race:
Past research has shown that race can affect the way that adults express their pain. A 2002 study published in the International Journal of Intercultural Relations found that black patients were less likely to disclose the fact that they were in pain than their white counterparts. When they did discuss their pain they were less likely to describe its intensity. And doctors might also be less skilled in recognizing the pain of certain races. Specifically, doctors were almost twice as likely to underestimate the pain of black patients compared to other ethnicities in a 2007 study from the University Of Tennessee College Of Medicine. Whether either of these findings applies to pain in children is simply not known. However, a new study found that black children seen in the emergency department for abdominal pain are less likely to receive pain medication than white children.
_
Few words about of chronic pain:
_
_
_
Chronic pain has been increasing in prevalence. It is considered the most underestimated health care problem impacting quality of life. A number of epidemiological studies conducted in different parts of the world, reported prevalence rates of chronic pain ranging from 12-80%. Chronic pain has detrimental effects on physical and mental health, daily activities, family relationships, employment, and economic well-being of the sufferers and family caregivers.
_
The table above shows magnitude of chronic pain
_
The figure above shows domains of chronic pain.
_ ___
The exact mechanisms involved in the pathophysiology of chronic pain are complex and remain unclear. It is believed that following injury, rapid and long-term changes occur in parts of the CNS that are involved in the transmission and modulation of pain (nociceptive information) (Ko and Zhuo, 2004). Central nociceptor pathways are sensitized and reorganized if pain is prolonged and tissue is damaged. A central mechanism in the spinal cord, called ‘wind-up’, also referred to as hypersensitivity or hyperexcitability, may occur. Wind-up occurs when repeated, prolonged, noxious stimulation causes the dorsal horn neurones to transmit progressively increasing numbers of pain impulses. The patient can feel intense pain in response to a stimulus that is not usually associated with pain, for example, touch. This is called allodynia. This abnormal processing of pain within the PNS and CNS may become independent of the original painful event. In some cases, for example, amputation, the original injury may have occurred in the peripheral nerves, but the mechanisms that underlie the phantom pain are generated in both the PNS and the CNS.
___
Chronic pain (also called persistent pain) can be caused by ongoing tissue damage, such as in osteoarthritis. However, in some cases no physical cause for the pain can be found or pain persists long after the injury has healed. In many cases chronic pain is a disorder in itself rather than being the symptom of a disease process.
At the cellular level, several processes can contribute to pain becoming chronic.
1. Pain receptors and neurones along the pain pathway may become too easily activated.
2. Connections between the neurons in the pathway can be altered.
3. The brain and spinal cord may fail to dampen down the pain signals.
4. Pain receptors that are normally silent (dormant) can become activated by inflammation.
5. After nerve injury, nerves may regrow but function abnormally.
_
The table above shows possible mechanisms of chronic pain
_
The figure above shows how chronic pain builds up and persists.
_
The figures below shows gray matter loss in chronic pain:
_
Chronic pain and sex:
Sexual intercourse usually causes some degree of discomfort in people who suffer from chronic pain. If some part of the body is very painful, then, whether you’re a man or a woman, sex is bound to suffer. The extent to which your sex life is affected depends on how widespread the pain is and which part of your body is affected. If you take medication to control your pain, try to time sex for when your medicine’s therapeutic effect is at its peak. Experiment with different positions that lessen physical strain, such as lying side by side. It can help to warm the bed in advance with an electric blanket to ease muscle and joint discomfort. Also, do some gentle stretches and use polyester or silk sheets to make it easier to turn and move in bed. Try touching, cuddling, massaging and kissing, without intercourse initially. Talk openly and honestly to your partner about how pain affects your enjoyment of sex, and what you want & need from your relationship and each other. On the other hand, research suggests that sexual activity, when comfortable, is often followed by several hours of pain relief. The key is to return to some form of sexual activity as soon as possible. The longer you avoid sex, the bigger the fear of resuming sex becomes, and a downward spiral sets in. The lack of intimacy can damage your relationship.
_
Heritability of pain:
From a Darwinian perspective, nociceptive pain is neither possible to eliminate nor is it desirable to do so. Nociception is essential for survival and, if a variation in a pain mechanism gene alters the function of a nociception-related molecule, the survival rate would be diminished. For instance, without pain mechanisms, people would not recognize the danger of leaving their hand on a hot stove. Mutations leading to decreased pain sensitivity occur in well under 1% of the population and lead to frequent injuries and inadvertent self-mutilation, which are incompatible with longevity or transmission to offspring.
_
The genetics of human pain has been the subject of intense study in the past decade. The completion of the human genome project in 2003 has provided a better understanding of why patients may experience pain syndromes. Substantial evidence from preclinical models suggests that basal nociceptive sensitivity—neural processes of encoding and noxious stimuli—as well as antinociceptive responses to drugs show significant heritability. Evidence of genetic influences on pain sensitivity in humans has yet to be clinically applied. Polymorphisms—the normal differences in DNA sequences among individuals in a population—of receptors, transporters, metabolizing enzymes, and targets of pharmacotherapy are under investigation. Substantial evidence indicates that a large component of the pain experience, such as acute pain thresholds or efficacy of analgesics, is inherited. The involvement of channelopathies in human pain conditions has been highlighted by evidence from analysis of pain phenotypes in transgenic animal models. Aberrant channel expression has also been linked to chronic pain evoked by physical insults. Hence understanding the ion channels involved in the pathophysiology of human pain conditions could provide opportunities for the development of novel therapeutic agents as well as furthering our insights into the functioning of the nervous system.
_
_
The figure above shows schematic of ion channels in nociceptor function. The cell bodies of nociceptors are contained within the dorsal root ganglia and terminate as free endings in peripheral tissues. The peripheral terminals respond to noxious stimuli or tissue damage through receptors and ion channels including TRP channels, acid-sensing ion channels (ASIC), serotonin (5-HT) receptors, ATP-gated P2X receptors, tyrosine kinase receptor A (TRKA), and numerous GPCRs that indirectly activate ion channels. Receptors at the terminals respond to noxious stimuli such as heat or pressure. When a defined threshold of depolarization is reached, voltage-gated sodium channels are activated and an action potential is generated. During an action potential, an IFM-inactivating segment moves to block the channel within 0.5–1 ms. In this inactivated state, the channel cannot be opened. Meanwhile, potassium channels open, acting to repolarize the membrane. As the membrane repolarizes, the sodium channel gate is closed and inactivating segment is displaced, returning the sodium channel to a resting closed state. This process is repeated to propagate the action potential along the axon. The action potential is propagated along the axon to the presynaptic terminals synapses with second-order neurons in the dorsal horn. Calcium influx through voltage-gated calcium channels (VGCC) triggers the release of neurotransmitters such as glutamate from presynaptic terminals. Glutamate activates ionotropic AMPA, NMDA receptor (NDMAR), and metabotropic glutamate receptors (mGluR) on the postsynaptic terminals in the spinal cord, and the signal is transmitted through the ascending pathways to higher centers in the brain.
_
Biophysical properties of ion channels can determine nociceptor excitability, and hence abnormal channel function or expression could lead to chronic or neuropathic pain. The time and voltage dependence of activation and inactivation processes modulate duration and frequency of action potentials in nociceptors. The Na+ channels Nav1.7 (SCN9A) and Nav1.8 (SCN10A) have been shown to be important in nociceptive pathways. As importantly, slowly inactivating Na channels contribute to sub-threshold potentials that determine whether a spike is generated in response to a depolarizing noxious stimulus. Nav1.9, preferentially expressed by nociceptors, has slow activation and inactivation kinetics that allow it to amplify response to sub-threshold stimuli. Nav1.7 has fast activation and inactivation kinetics but is also able to respond to slow depolarizations because of the properties of the channel in the closed state, and hence can amplify sub-threshold potentials. Thus the biophysical properties of ion channels can determine nociceptor responses to noxious stimuli and ultimately the level of pain experienced. Many inherited human sodium channelopathies impact the activation and inactivation of these channels, resulting in altered neuronal response to stimuli.
_
_
Familial hemiplegic migraine (FHM), Congenital insensitivity to pain (CIP), Paroxysmal extreme pain disorder (PIPS) and Familial episodic pain syndrome (FEPS) are few disorders inherited as monogenic channelopathy. The table above shows a synopsis of monogenic channelopathy–associated human pain syndromes. Although they are extremely rare, study of these familial pain syndromes has provided a unique insight into the ion channels involved in human pain. In addition, discussion on transgenic animal models has broadened our knowledge of the functional role of ion channels in pain transduction and the mechanisms of pathological pain. Translating data obtained from animal models to human pain pathology remains challenging, because there are subtle differences in pain mechanisms between mice and humans. For instance, antagonists of substance P acting at the NK1 receptor are analgesic in mice but not humans. The use of transgenic mouse models is nonetheless generally useful in advancing the molecular understanding of pain sensation and the development of novel therapeutics, because there are broad similarities in pain processing in mice and humans. The central role of ion channels in chronic pain and in diseases of excitability such as epilepsy is underscored by the utility of similar drugs for both pathologies. The study of human heritable disorders of pain has suggested new analgesic drug targets, but many mouse knockout studies of ion channels implicated in pain have yet to be linked to human pain conditions. Results from ion channel gene deletion studies in mice are informative but highly dependent on a comprehensive analysis of phenotype. Understanding subtle changes in function and the levels and spatiotemporal patterns of ion channel expression holds the key to controlling neuronal excitability in altered pain states. Thus, information from monogenic human pain syndromes coupled to human pain GWASs, together with in-depth phenotyping of targeted mutant mouse models will help identify channels and regulatory genes that contribute to pathological pain.
_
CIP:
Congenital insensitivity to pain (CIP), also known as congenital analgesia, is one or more rare conditions where a person cannot feel (and has never felt) physical pain. For patients with this disorder, cognition and sensation are otherwise normal; for instance patients can still feel discriminative touch (though not always temperature), and there are no detectable physical abnormalities. Children with this condition often suffer oral cavity damage both in and around the oral cavity (such as having bitten off the tip of their tongue) or fractures to bones. Unnoticed infections and corneal damage due to foreign objects in the eye are also seen. Because the child cannot feel pain they may not respond to problems, thus being at a higher risk of more severe diseases or otherwise. This disorder can be due to mutations in the voltage-gated sodium channel SCN9A (NaV1.7). Patients with such mutations are congenitally insensitive to pain.The disorder is primarily found in homogeneous societies. For example, it is found in Vittangi, a village in Kiruna Municipality in northern Sweden, where nearly 40 cases have been reported. Also, Ashkenazi Jews have been found to have a higher risk, though it is still unusual.
_
Mu opioid receptor (MOR):
The mu receptor OPRM1 is the primary target site for opioid medications. The gene for this receptor is located on chromosome 6 and is a significant factor in the variability of how a patient responds to opioids. The most common single nucleotide polymorphism (SNP), or base-pair change, of the MOR gene consists of a change of adenine to guanine in the 118 position that leads to a substitution of the amino acid asparagine for aspartate. This substitution affects the function of the receptor by increasing its binding affinity for β-endorphins and subsequently affects the action of opioids at the receptor site.
_
Pain research:
Research on pain is focused on neurobiology studies concerning neuronal plasticity development, nociceptors molecular identity, signaling mechanisms, ionic channels involved in the generation, modulation & propagation of action potentials in all type of excitable cells. All these findings open the possibility for developing new therapeutic treatment. The interest of researchers for receptors, neurotransmitters, second messengers, transcription factors involved in neuronal processing, in spinal cord and in cortical areas, increased dramatically. There are evidences clarifying the origin of chronic pain.
_
Recent breakthroughs in our understanding of pain mechanisms can be traced to the introduction of molecular biology techniques into pain research. New ion channel proteins involved in generating, modulating, and propagating action potentials along nociceptor axons have been cloned and characterized using molecular biology techniques. These techniques continue to reveal nociceptive mechanisms involving molecules, receptors, and neural networks that underlie neural reorganization (“plasticity”) in the spinal cord and brainstem after peripheral tissue damage or nerve injury. At the latest IASP World Congress, in his presidential address Professor Besson voiced concern that clinical and basic research appear to be diverging, even as dramatic new insights into fundamental mechanisms of nociception promise therapeutic breakthroughs. It is normal for considerable time to elapse between the development of new information from basic research and its application in the clinic.
_
Cravatt has been studying how fatty acid amines that are found in neural tissue and fluids can reduce sensitivity to pain. He and his researchers have been testing mice to see if they are less sensitive to pain in response to changing amide levels. On the molecular level, Cravatt’s lab is the first to actually manipulate the entire fatty acid communications system. During the course of his investigations, Cravatt came across fatty acid amide hydrolase (FAAH), an amino acid membrane-bound enzyme that is a target for pain therapy not only because it breaks down the molecules that provide the pain relief but also because it turns out that FAAH seems to be the only enzyme responsible for doing so.
_
Pain and Peptides:
TSRI Professor Tamas Bartfai, Ph.D., Director of the Harold L. Dorris Neurological Research Center is interested in the neuropeptide galanin. This chemical exists specifically in hippocampus neurons and is released into the gaps, or synapses, between two neurons during the signaling from one neuron to another that takes place during cognitive processes. Galanin also controls the pain threshold at the spinal cord level through the same neuronal action in the spinal cord that morphine uses – hyperpolarizing primary sensory neurons. Transgenic models with no galanin receptors have different pain thresholds. One possible application for this is to develop a class of galanin receptor agonists – non-opiate pain relievers that could be taken with morphine, for instance, to lower required doses of morphine.
_
Neuronal heterogeneity and pain processing:
Everyone knows how it feels to bite into a hot chili pepper or burn the roof of one’s mouth with a hot drink. This activates nerve cells that relay the potential threat to the brain, which then causes the person in question to perceive pain. Over 14 years ago, researchers discovered the first receptor molecule that reacts to heat as well as to capsaicin, the active substance in chili extracts. At the time it was believed that science had come a big step closer to understanding the emergence of pain and its treatment with medicine. The disappointment was great when it was found several years later that laboratory mice from whom the gene of this receptor had been artificially removed still perceived pain. Despite repeated attempts to explain the causes of this observation, it has remained a mystery until now. Researchers at the University of Freiburg have now deciphered basic mechanisms governing the perception of pain. Their findings, published in the journal PLoS One, demonstrate that even simple organisms possess sensor systems with multiple safeguards for the perception of painful stimuli like heat. The roundworm only has 302 nerve cells at its disposal, but this small number is sufficient for complex behaviors and even for learning processes. The study shows that the worm uses at least six of these cells as sensors to detect dangerous heat. In order to perceive pain, however, the worm needs to use more than one of them at a time. In addition, one and the same cell can react to the painful stimulus with two different molecular mechanisms. According to the researchers, the findings can now be used to discover and understand the interactions between the various mechanisms for these processes in humans.
_
N type calcium channel and opioids:
At the synapse — the point of connection between nerve cells — N-type calcium channels control the release of neurotransmitters. These chemicals carry messages between nerve cells — messages that include sensations of pain. So if you block N-type channels, you can block pain. In 2004, Lipscombe and her colleagues discovered a unique form of the N-type channel at synapse between first order sensory neuron and second order sensory neurons responsible for nociception. These are the channels that opioids act on. Opiods act on a special form of N-type calcium channel, the cellular gatekeepers that help control pain messages passed between nerve cells. All N-type channels are made up of a string of about 2,400 amino acids. In nociceptor N-type channels, that string differs by a mere 14 amino acids. This small difference in molecular make-up makes these channels much more sensitive to the pain-blocking action of opioids. By blocking these channels, pain signals are inhibited.
_
LTP:
Long-term potentiation (LTP) at synapses of nociceptive nerve fibers is a proposed cellular mechanism underlying some forms of hyperalgesia. LTP at synapses between primary afferent C-fibers and a group of nociceptive neurons in spinal cord lamina I which express the NK1 receptor for substance P is a potential mechanism underlying some forms of pain amplification in behaving animals and perhaps human subjects. Both, LTP and hyperalgesia involve the same essential elements, i.e. primary afferent C-fibers and lamina I neurons which express the NK1 receptor. Further, induction protocols, pharmacological profile and signal transduction pathways are virtually identical.
_
The capsaicin receptor: a heat-activated ion channel in the pain pathway:
Capsaicin, the main pungent ingredient in ‘hot’ chilli peppers, elicits a sensation of burning pain by selectively activating sensory neurons that convey information about noxious stimuli to the central nervous system. Researchers have used an expression cloning strategy based on calcium influx to isolate a functional cDNA encoding a capsaicin receptor from sensory neurons. This receptor is a non-selective cation channel that is structurally related to members of the TRP family of ion channels. The cloned capsaicin receptor is also activated by increases in temperature in the noxious range, suggesting that it functions as a transducer of painful thermal stimuli in vivo.
_
Anticipating Pain:
For some people, anticipating pain can be as bad as the real thing. Dread can be a real pain describes research conducted by Dr. Gregory Berns of Emory University. The brain activity measured during the anticipation phase was actually occurring in the brain’s pain center, according to fMRI used to show dread responses in brain: The scans showed that brain activity related to dread was localized in the areas of the brain associated with pain. Dread was found in the parts of the pain network linked to attention. This is important because it suggests that dread is not as simple as fear or anxiety, which are emotions controlled by different brain regions. The findings also showed that the mild and extreme dreaders had different patterns of brain activity. The extreme dreaders had more activity in the attentional parts of the pain matrix, and this activity was seen much earlier in each trial compared to the mild dreaders. Marketers have long used graphic representations of pain in advertisements. Research on whether an ad can trigger similar dread feelings or create activity in the same pain centers hasn’t been published yet, but it seems likely. Between mirror neurons “simulating” an observed (or even heard) action by another, and pain centers being triggered by thinking about future pain, it’s clear that marketers may have the opportunity to create discomfort among their targets.
_
Stress induced analgesia:
Scientists have long known about the phenomenon of stress-induced analgesia (SIA), best exemplified by the many reported cases of wounded soldiers and injured athletes who feel no pain while the battle or the game is on, but do feel it afterward, as soon as they are back in conditions of safety and calm. From an evolutionary standpoint, stress-induced analgesia can be regarded as a component of the fight-or-flight response. It would not be highly adaptive if pain from injuries could prevent us from fighting or fleeing even when our lives depended on it. But once the threat of death has passed, our normal pain-sensing mechanisms have to do their work in order to immobilize the injured part of the body and prevent the injury from getting worse. Research done on the mechanisms of the descending control pathways for pain since the early 1980s has now given us a better understanding of stress-induced analgesia. We now know that the tendency to experience this phenomenon varies from one individual to another and is influenced by variables such as age, sex, degree of sensitivity to opiates, and past stressful experiences. The mechanisms by which stress-induced analgesia inhibits pain seem to involve the descending systems of the midbrain, applying both opioid and non-opioid mechanisms. Research is also tending to show that neurotransmitters associated with stress, such as norepinephrine, and brain structures associated with fear reactions, such as the amygdala, are also involved. Many other endogenous substances, such as anandamide and its cannabinoid receptors, also seem to play a role, in this case in the non-opioid effect in the periaqueductal grey matter.
_
Current scientific view on pain:
Neuroscientists nowadays think of the nervous system as plastic. They no longer believe the relay of pain information to be based on an immutable relationship between a painful stimulus and the sensory output of pain. Rather, the perception of pain results from the integration of information from a variety of sources. Of course information is relayed from the injured tissue or organ in the periphery, but the strength of this signal can be modified by emotional and behavioral information coming down from the brain, as well as by inputs from other peripheral sensations. Furthermore, biologists now think that the integration of these signals actually takes place in the spinal cord, not in the brain, and that the integrated information is then carried up to the brain for further processing. C. Woolf and M. Salter (Science 2000 288:1765) recently enumerated the three general levels at which neural information could be modified in response to chronic pain. They noted that the extent and duration of the response to the stimulus at the periphery could be modified. Alterations can also take place at a chemical level within any one or several of the neurons along the pain-conduction pathway. These include changes in the number or sensitivity of receptors, ion channels and internal signaling molecules. Finally, chronic pain can induce a modulation of the neurotransmitters that affect the flow of information from one neuron to the next, or it can even alter the anatomical features of these neurons and their interconnections. The set of alterations described by Woolf and Salter may lead to long-term changes in the connectivity and organization among nerve cells. This, in turn, may lead to a “pain memory”, not much different from ordinary memory in the brain.
__
Pain benefits:
Although pain is something that we invariably want to escape or to stop, it serves several very important functions. Pain protects us by triggering a reflexive withdrawal from something damaging before we can suffer further injury, such as when we drop a hot pan before we sustain extensive burns. It is also a warning system that lets us know when an injury is about to occur: the burning ache in our muscles during extreme exertion warns us to stop using them. Pain forces us to immobilize or protect an injured part, such as a broken ankle, thus giving it a chance to heal. Pain also lets us know when we need to seek medical help, and teaches us what behaviors to avoid in the future.
_
Pain not only a warning but could help recovery:
The intense pain of a heart attack could actually help patients, researchers have discovered. They have found that during an attack – when a blood clot blocks an artery that is serving the heart with oxygen – pain signals from cardiac nerves help to attract stem cells to repair the damage. Heart attack patients are routinely treated with morphine to ease the intense pain, but morphine operates by blocking pain-inducing substances, including the one that stimulates stem cell activity in artery walls. Its use could therefore have serious implications for a patient’s long-term recovery. A key molecule in the body’s ability to sense pain, called Substance P, is released from nerve terminals in the heart during a heart attack. Substance P then mobilizes stem cells from the bone marrow to the site of the artery blockage. These stem cells have the ability to generate new blood vessels to bypass the blockage and restore some of the blood flow. Pain is a very complicated process. It’s not just the body’s way of warning you that something is wrong; when we feel pain, it can also be a sign that the body is doing what it can to fix the problem.
_
Pain vis-à-vis pleasure, evolution and duality of existence:
On the evolutionary hypothesis, the fact that pleasures are generally associated with beneficial, and pains with detrimental, experiences, is the result of natural selection among random variations: those individuals who happen to have an association of this kind have higher biological fitness than those who have no such association, or the reverse association. The fact that pain states are associated with damaging experiences is the result of natural selection. Pain is an adaptive trait and improves the survival value.
_
Evolutionary and behavioral role:
Pain is part of the body’s defense system, producing a reflexive retraction from the painful stimulus, and tendencies to protect the affected body part while it heals, and avoid that harmful situation in the future. It is an important part of animal life, vital to healthy survival. People with congenital insensitivity to pain have reduced life expectancy. The most fit creature would be the one whose pains are well balanced. Those pains which mean certain death when ignored will become the most powerfully felt. The relative intensities of pain, then, may resemble the relative importance of that risk to our ancestors (lack of food, too much cold, or serious injuries are felt as agony, whereas minor damage is felt as mere discomfort). This resemblance will not be perfect, however, because natural selection can be a poor designer. The result is often glitches in animals, including supernormal stimuli. Such glitches help explain pains which are not, or at least no longer directly adaptive (e.g. perhaps some forms of toothache, or injury to fingernails). Idiopathic pain (pain that persists after the trauma or pathology has healed, or that arises without any apparent cause), may be an exception to the idea that pain is helpful to survival, although some psychodynamic psychologists argue that such pain is psychogenic, enlisted as a protective distraction to keep dangerous emotions unconscious.
_
There is strong evidence of biological connections between the neurochemical pathways used for the perception of both pain and pleasure, as well as other psychological rewards. One approach to evaluating the relationship between pain and pleasure is to consider these two systems as a reward-punishment based system. When pleasure is perceived, one associates it with reward. When pain is perceived, one associates with punishment. Evolutionarily, this makes sense, because often times, actions that result in pleasure or chemicals that induce pleasure work towards restoring homeostasis in the body. For example, when the body is hungry, the pleasure of rewarding food to one-self restores the body back to a balanced state of replenished energy. Like so, this can also be applied to pain, because the ability to perceive pain enhances both avoidance and defensive mechanisms that were, and still are, necessary for survival. On an anatomical level, it can be shown the source for the modulation of both pain and pleasure originates from neurons in the same locations, including the amygdala, the pallidum, and the nucleus accumbens. At the systems level, the major regions that have been implicated in pain and reward processing by functional imaging studies and direct brain stimulation in humans, as well as by electrophysiology and tracing studies in animals, show striking overlap. Not only have Leknes and Tracey, two leading neuroscientists in the study of pain and pleasure, concluded that pain and reward processing involve many of the same regions of the brain, but also that the functional relationship lies in that pain decreases pleasure and rewards increase analgesia, which is the relief from pain. There is also scientific evidence that one may have opposing effects on the other. Pain and pleasure are powerful motivators of behavior and have historically been considered opposites. Emerging evidence from the pain and reward research fields points to extensive similarities in the anatomical substrate of painful and pleasant sensations. Recent molecular-imaging and animal studies have demonstrated the important role of the opioid and dopamine systems in modulating both pain and pleasure. Understanding the mutually inhibitory effects that pain and reward processing have on each other; and the neural mechanisms that underpin such modulation; is important for alleviating unnecessary suffering and improving well-being. So why would it be evolutionarily advantageous to human beings to develop a relationship between the two perceptions at all? Dr. Kringelbach suggests that this relationship between pain and pleasure would be evolutionarily efficient, because it was necessary to know whether or not to avoid or approach something for survival. According to Dr. Norman Doidge, the brain is limited in the sense that it tends to focus on the most used pathways. Therefore, having a common pathway for pain and pleasure could have simplified the way in which human beings have interacted with the environment.
___
Tonic dopamine signaling:
_
To maintain homeostasis, animals must aim for the ‘Golden Mean’ — that is, the right balance between pleasure-seeking and pain-avoidance. Tonic dopamine activity refers to the level of extrasynaptic dopamine that is present at a steady-state concentration in the extracellular space. The responsiveness of the phasic dopamine system (a system which is caused by brief bursts of neuronal firing and relates to reward motivation and prediction error) is important for the regulation of appetitive and aversive behaviors. Impulsive behavior and schizophrenia have been linked to an excessively responsive phasic dopamine system, whereas depression, chronic pain and anhedonia have been associated with low responsiveness to reward cues. Increased tonic dopamine is known to result from prolonged stress or pain as shown in the figure above, a mechanism that might have evolved to ensure rest and low activity levels during injury. Unfortunately, the same mechanism is thought to cause increased pain sensitivity in certain pain syndromes through its inhibition of endogenous phasic dopamine antinociception. Abstinence from addictive drugs has also been associated with hyperalgesia and increased tonic signaling. The resulting inhibition of phasic signaling is thought to underpin reduced responsiveness to pleasure (anhedonia) during abstinence, and can be reversed by re-administering the addictive drug. At the other extreme, decreased tonic dopamine, causing hyper-responsiveness of phasic dopamine, has been related to positive symptoms in schizophrenia. Impulsivity in schizophrenia is associated with excessive pleasure-seeking and substance abuse.
_
Pain-pleasure inhibition:
_
Figure above shows schematic illustration of pain–pleasure inhibition. The Motivation-Decision Model of pain posits that anything of potentially greater importance than pain should have antinociceptive effects (be it a greater threat or the possibility of a reward). By the same evolutionary-psychology rationale, it is clear that anything that is potentially more important than a reward (such as an even greater reward or a threat for which action is needed) should similarly decrease its pleasantness, thus allowing for the appropriate avoidance or approach behaviors. The opioid and mesolimbic dopamine systems are the prime candidates for systems that transmit signals relating to motivational and hedonic aspects of both pain and pleasure and, in particular, their interactions, as illustrated above.
a) Both pain and pleasure have been shown to elicit opioid release in the orbitofrontal cortex (OFC), the amygdala (Amy), the nucleus accumbens (NAc) and the ventral pallidum (VP). Pleasure and reward expectation are also associated with increased phasic dopamine signaling from the ventral tegmental area (VTA) to the NAc and VP42, which in turn causes increased -opioid release in the NAc55. Pain has been associated with both increases and decreases in mesolimbic dopamine signaling, depending on the type of measurement and pain model that have been used.
b) opioid receptor antagonists, such as naloxone, reverse pleasure-related analgesia.
c) opioid receptor agonists, such as morphine, have been shown to re-enable pleasure that has been previously reduced by concomitant pain.
__
Read my article on “The anger”. Anger and fear are the two sides of the same coin of primitive survival instinct. Anger is associated with fierceness, daring, possessiveness and taking risks. Fear is exactly opposite. The primitive survival instinct is in dual mode, fight (anger) or flight (fear); depending on the assessment of circumstances by the organism. This fact substantiates my theory of “Duality of Existence” in living organisms. From an evolutionary perspective, an association between emotions and pain responses might be expected, given that pain in times past was often the result of situations threatening survival of the organism (e.g., attack). Anger and fear would be the two emotions most likely to be elicited under such threatening circumstances, as they reflect motivational states underlying the fight and flight responses, respectively. On the other hand, from an evolutionary standpoint, stress-induced analgesia can be regarded as a component of the fight-or-flight response. It would not be highly adaptive if pain from injuries could prevent us from fighting or fleeing even when our lives depended on it. But once the threat of death has passed, our normal pain-sensing mechanisms have to do their work in order to immobilize the injured part of the body and prevent the injury from getting worse. So stress response, basic emotions anger & fear (fight or flight), and pain & pleasure are all correlated evolutionarily, neurobiochemically and genetically. Higher the organism evolutionarily, greater the correlation. Since humans are highest on evolutionary ladder, we have highest correlation. Non-human animals have lower correlation. Invertebrates have least correlation. My theory is that pain and pleasure are two sides of the same coin of organism survival based on duality of existence just like anger (fight) and fear (flight) reaction. Pleasure encourages organism to perform the task and pain provokes organism to avoid the task, both for survival.
_
Myths about the risks and dangers of analgesics:
_
Myth1: Toughing it out is always better than relying on painkillers.
Fact: Although many pride themselves on their toughness, those who refuse medications despite severe pain may be putting their health— and their jobs and relationships— at risk. Uncontrolled pain is associated with adverse consequences in terms of daily functioning, mood, sleep, overall quality of life, energy level, the ability to work and marital relationships. Newer studies actually show that persistent pain causes changes in the brain and spinal cord that begets more pain. Some animal studies suggest that controlling pain could help prevent these problems.
_
Myth 2: People on opioids are always impaired—and cannot drive safely or work in demanding jobs.
Fact: Studies of drivers on steady doses of opioids do not find impairment. In fact, at least one study by Finnish researchers showed that impairment on standard driving measures was more correlated with poorly controlled pain than with taking medication for it.
_
Myth 3: When taken as directed, opioids are more likely to kill you than aspirin, ibuprofen or naproxen.
Fact: When taken as directed, opioids are safe drugs. The vast majority of opioid-related deaths occur amongst recreational users or deliberate suicides. Deaths amongst pain patients are rare— in fact, recent research finds that even for people with advanced illnesses, use of high-dose opioids does not significantly increase risk of death. Nearly three times as many people die from complications of correctly taking painkillers like aspirin and ibuprofen— known as non-steroidal anti-inflammatory drugs—than die from opioid overdose. More people die from gastro-intestinal bleeding from NSAIDs taken in correct doses than from inadvertent opioid overdose.
_
Myth 4: Accidental overdose is common amongst pain patients on opioids.
Fact: Most opioid overdoses do not result from medical use. As patients take opioids over weeks and months, they develop a tolerance to the respiratory depressive effect, which is the thing that can cause death. This means that even if people forget they’ve taken their medication already and accidentally double their dose— unless they have dementia and do this rapidly and repeatedly— the risk of death is low. Instead, the vast majority of opioid overdoses involve combinations of drugs that cause sedation— typically alcohol and sleeping pills or anti-anxiety medications like benzodiazepines. At least 80 percent of opioid overdoses are actually caused by such drug mixing—and while some severe pain patients need both benzodiazepines and opioids, they are prescribed together with great caution. In many overdose deaths, use is obviously non-medical because the victims injected or snorted drugs meant to be taken orally.
_
Myth 5: Most people who get addicted to painkillers are “accidental” addicts who sought pain treatment and had no prior history of drug problems.
Fact: The vast majority of people who become addicted to prescription opioids have significant prior histories of drug problems. Nearly 80 percent of Oxycodone addicts have taken cocaine, for example, according to large national survey research. This means either that pain patients prescribed Oxycodone suddenly start using cocaine—or, more plausibly, that most people who misuse opioids have a past or current drug problem. Even having chronic medical problems—which includes chronic pain—did not increase risk for Oxycodone addiction. If you do not have a personal or family history of addiction—especially if you have never suffered psychiatric problems like depression, schizophrenia or bipolar disorder, and especially if you are middle-aged or older—your risk for developing addiction during pain treatment is very low.
_
Myth 6: Addiction is inevitable if opioids are taken long-term or in high dose.
Fact: This myth stems from confusion about the nature of addiction. Many people believe that addiction is simply needing a substance to function—but if this were the case, everyone would have to be considered addicted to food, air and water. Average people view addiction as physical dependence. In fact, psychiatry defines addiction as compulsive use of a substance despite negative consequences—and it is this craving, impairment and loss of control that people fear. However, while most people who take opioids for long enough will develop physical dependence and suffer withdrawal if the drugs are stopped abruptly, addiction in pain patients is rare. The reality is that addiction appears to be distinctly uncommon in patients without a prior history of addiction or a family history of addiction.
_
Myth 7: Because most people don’t get addicted to painkillers, I can use them as I please.
Fact: You need to use prescription painkillers (and any other drug) properly. It’s not something patients should tinker with themselves. They definitely have an addiction potential. Use prescription pain medicines as prescribed by your doctor and report your responses — positive and negative — to your doctor.
_
Myth 8: I’m a strong person. I won’t get addicted.
Fact: Addiction isn’t about willpower, and it’s not a moral failure. It’s a chronic disease, and some people are genetically more vulnerable than others. The main risk factor for addiction is genetic predisposition.
_
Myth 9: If I need higher doses or have withdrawal symptoms when I quit, I’m addicted.
Fact: That might sound like addiction to you, but it’s not how doctors and addiction specialists define addiction. Everybody can become tolerant and dependent to a medication, and that does not mean that they are addicted. Tolerance and dependence don’t just happen with prescription pain drugs, they occur in drugs that aren’t addictive at all, and they occur in drugs that are addictive. So it’s independent of addiction. Many people mistakenly use the term “addiction” to refer to physical dependence. That includes doctors. Physical dependence, which can include tolerance and withdrawal, is different. Even though it is a part of addiction but it can happen without someone being addicted. In other words, dependence may be a component of addiction but dependence is not synonymous with addiction. Addiction is uncommon while dependence is common after long term opioid use.
_
Myth 10: Opioid withdrawal is extremely debilitating and potentially deadly.
Fact: We’ve all seen the movies: the desperate addict shivering, shaking and vomiting from heroin withdrawal, pleading for relief. While opioid withdrawal can be unpleasant, it doesn’t have to be. You can probably take 80 percent of people off opioids by decreasing the dose 50 percent every other day and they will be asymptomatic. In fact, many patients go through withdrawal without even realizing that their “flu symptoms” are linked to the fact that they decided to stop their pain medication suddenly. The severity of withdrawal also appears to have a genetic component—some people are susceptible to miserable symptoms, while others suffer few or even no effects. While withdrawal from alcohol or barbiturates is potentially fatal if not properly managed, even the worst opioid withdrawal is unlikely to be deadly. However, withdrawal can be risky if the patient is still in pain or on other drugs. Managed incorrectly and in concert with other drugs, it can be very dangerous. The main reason people suffer withdrawal has “nothing to do with medicine, but rather to societal pressures that have led to laws that the Drug Enforcement Agency is required to enforce.” For example, it is illegal for a doctor to prescribe opioids for addiction outside of certain settings, so some physicians are afraid to taper patients’ doses for fear of being arrested for having “maintained” an addict. Similarly, doctors may drop patients suddenly if they suspect addiction, without tapering their medications.
_
Myth 11: All that matters is easing my pain.
Fact: Pain relief is key, but it’s not the only goal. The focus is on functional restoration when analgesic or any intervention is prescribed to control patient’s pain.
_
Myth 12: My doctor will steer me clear of addiction.
Fact: Doctors certainly don’t want their patients to get addicted. But they may not have much training in addiction, or in pain management. You need a specialist trained in de-addiction measures.
_
The moral of the story:
_
1. Pain is a biopsychosocial phenomenon.
_
2. When we look at the scientific research on pain, we see that the science of pain has increasingly conceived of pain as less like perception of an objective reality and more like emotions by first drawing the sensory/affective distinction and then emphasizing more and more its affective aspect. There are sufficient scientific grounds to state that pain is a homeostatic emotion.
_
3. About 35% of people report marked pain relief after receiving a saline injection they believe to have been morphine. This is not only a placebo effect (of which CAM practitioners take advantage to claim their efficacy); but makes clear that pain is not merely a sensation or perception but a basic emotion just like lust, anger etc.
_
4. Evolutionary biologically, acute pain is essential for survival of a species.
_
5. Women are more sensitive to pain, less pain tolerant, more pain reactive and more able to discriminate different levels of pain than men, which is due to evolutionary higher survival value as they bear and nurture children.
_
6. Access to pain management is a fundamental human right and it must be included in the constitution of various nations.
_
7. One in five people suffers from moderate to severe chronic pain, and that one in three is unable or less able to maintain an independent lifestyle due to their pain.
_
8. Pain is a significant public health problem that costs American society at least $560-$635 billion and European society up to €240 billion annually. Statistics from developing world is unavailable.
_
9. It is widely believed that regular exposure to painful stimuli will increase pain tolerance but this is not true – the greater exposure to pain will result in more painful future exposures. Repeated exposure bombards pain synapses with repetitive input, increasing their responsiveness to later stimuli. Therefore, although the individual may learn cognitive methods of coping with pain, these methods may not be sufficient to cope with the boosted response to future painful stimuli. Because of this, trauma victims (or patients in pain) are given painkillers as soon as possible – to prevent pain sensitization. Researchers suggest that analgesics should be taken before surgery as people who begin taking analgesics before surgery need less of it afterward.
_
10. Analgesic under-medication is one of the most important factor for delaying recovery in acute injury/inflammation and leads to chronic pain.
_
11. Unnecessary legal/societal restrictions on opioid use for the treatment of pain must be removed.
_
12. Hot fomentation should not be given in any acute injury as it will worsen inflammation, increase swelling and increase pain. Cold application (ice application) will help relieve pain in acute injury. Hot fomentation may be given 48 hours after injury to speed up healing.
_
13.The way marijuana (cannabis) contain THC and the way opium contain morphine; and both of these have biological receptors in human brain; and the way body produces endogenous opioids & cannabinoids which target the same receptors; makes me think that during evolution of species over millions of years, some cross-connection exist between plant kingdom and animal kingdom at the basic level of DNA, genes and molecular biology; and therefore presence of these receptors in human brain which readily accept plant based chemicals which plants synthesized for its own survival, is not a mere coincidence but an evolutionary design, the intent of which ought to researched.
_
14. Pain and pleasure coexist in same brain pathways as two sides of the same coin of task performance for survival. Pleasure encourages organism to perform the task and pain provokes organism to avoid the task, both for survival. This validates the theory of ‘Duality of existence” in living organisms.
_
15. I axiom that the spinal cord plays a greater role in pain modulation than researched so far. I propose a theory of spinal consciousness which contributes to overall consciousness (cortex + sub-cortical structures) of humans but plays a more significant role in animals who lack neocortex in their brain.
_
Dr. Rajiv Desai. MD.
May 5, 2012
_
Postscript:
This is the most complex subject I have discussed so far. My endeavor has been to provide genuine and accurate information to the people along with innovative ideas. However, the subject is so vast that it is not possible to cover all aspects. Any inadequacy or inaccuracy is deeply regretted.


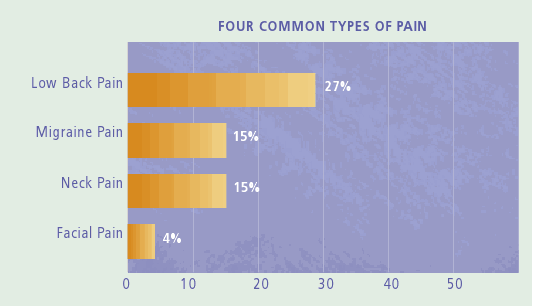
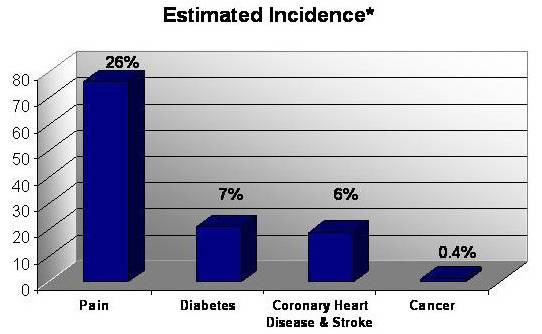
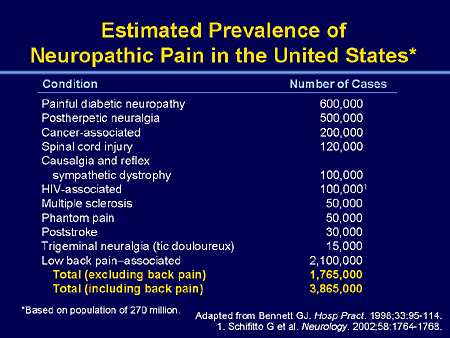
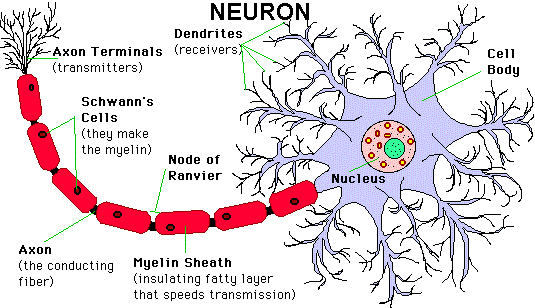
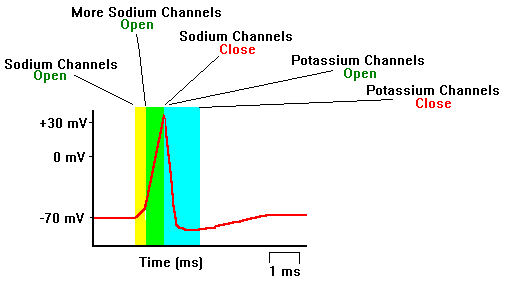
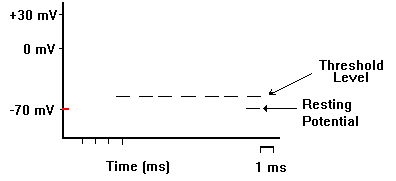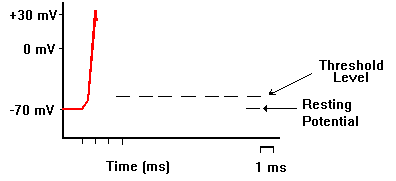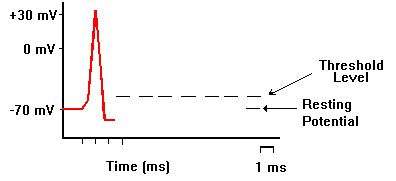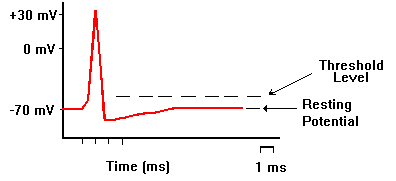
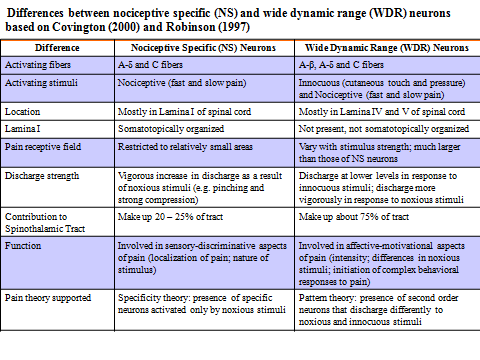


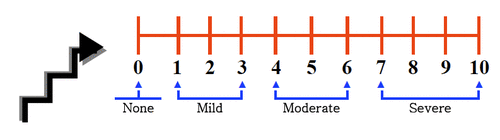
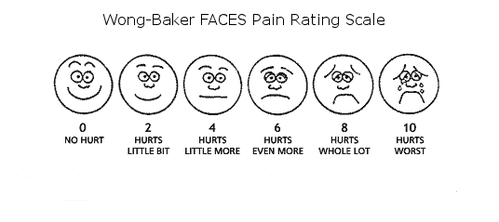
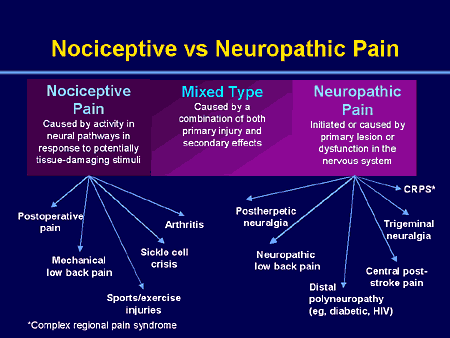
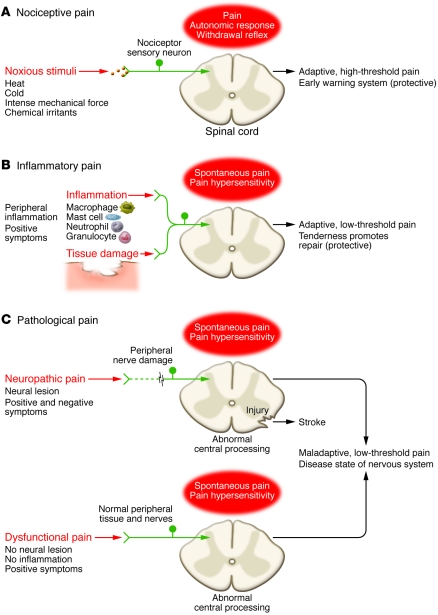
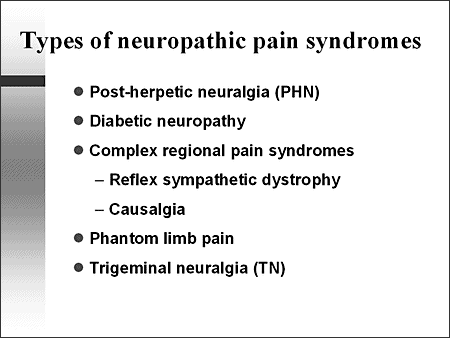
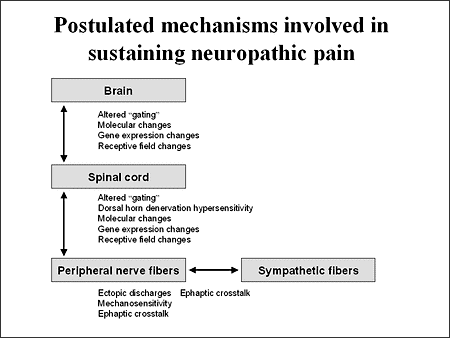
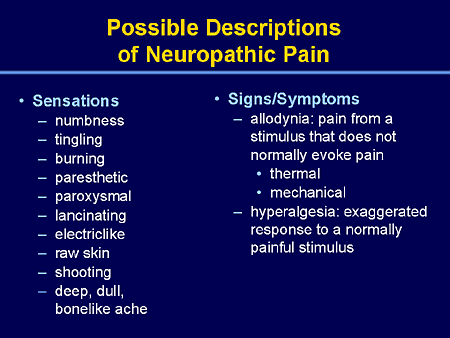

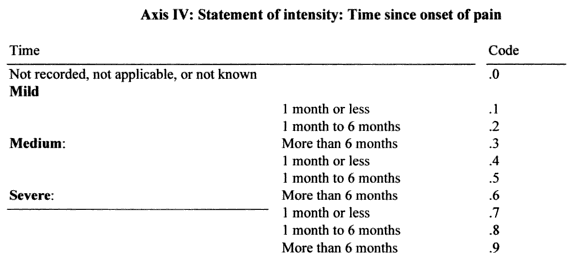
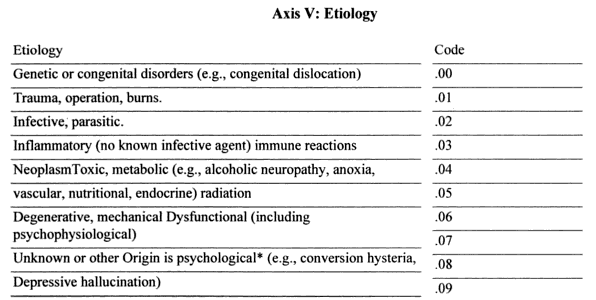
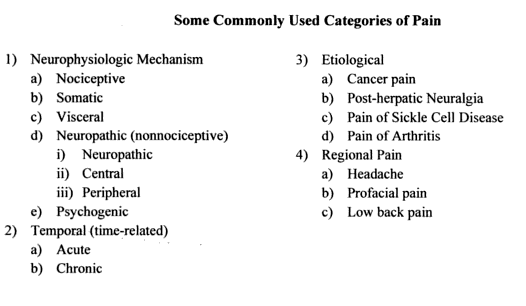
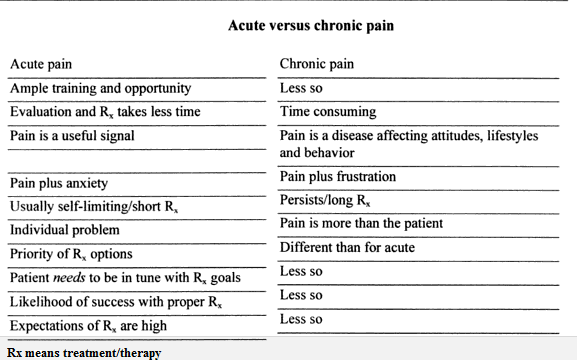
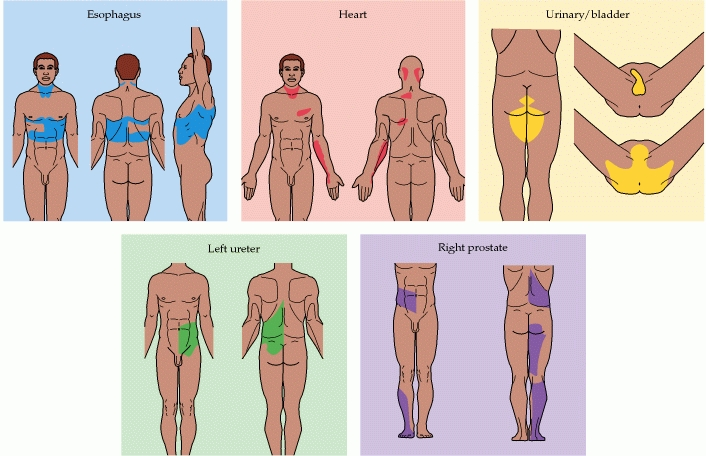
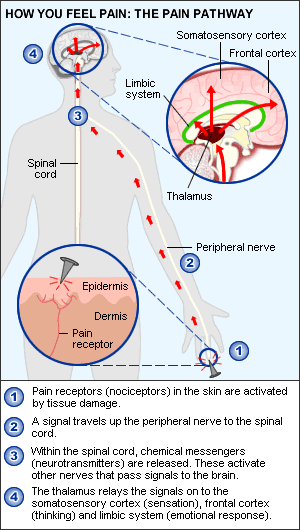
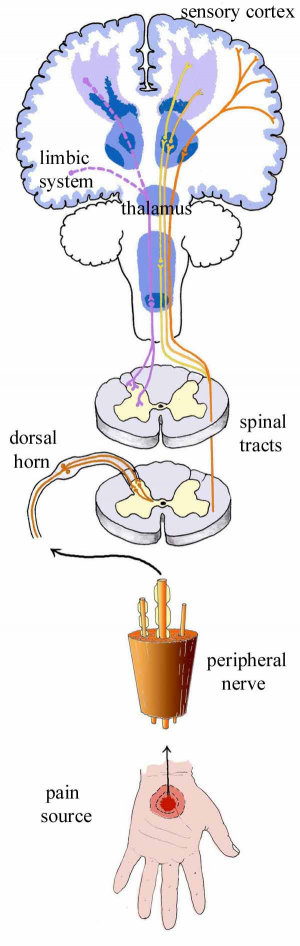
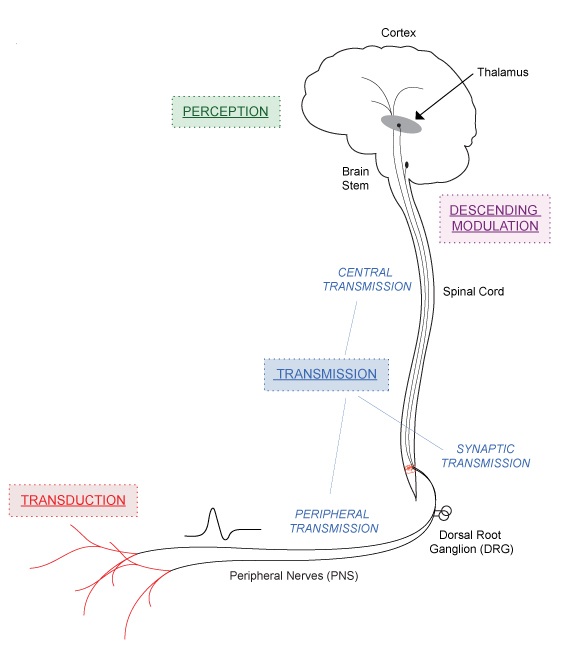
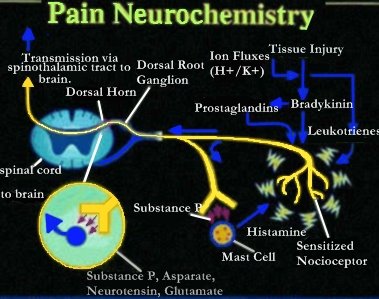
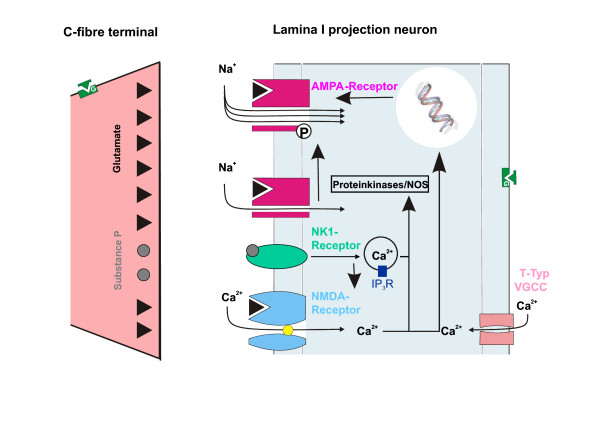
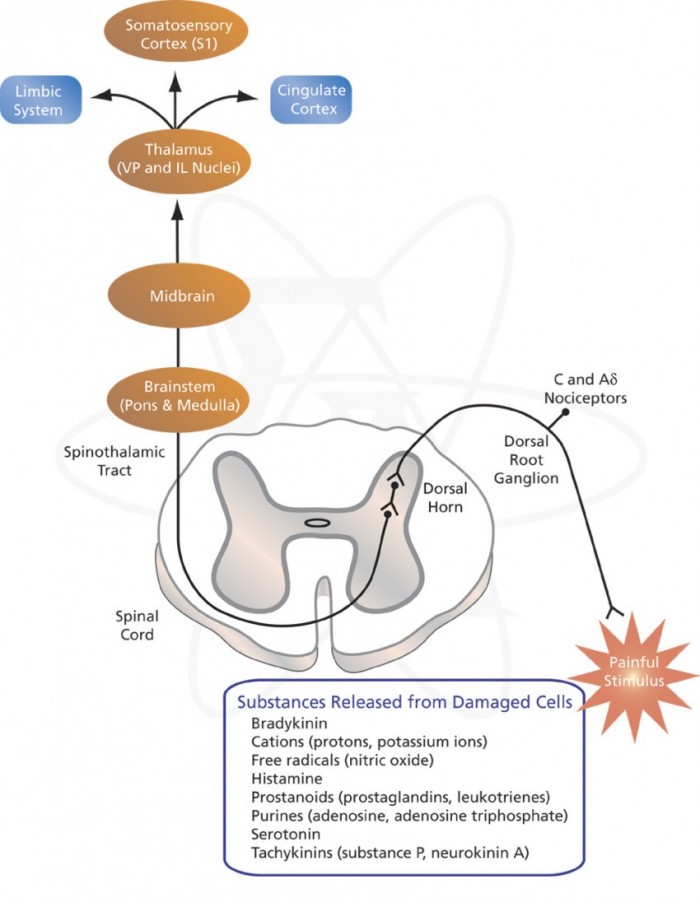
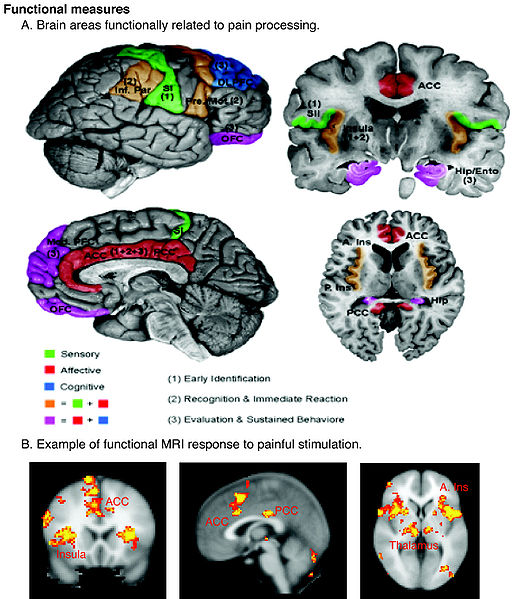
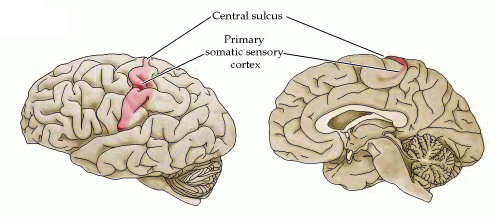
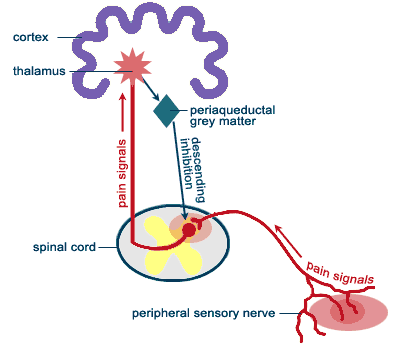
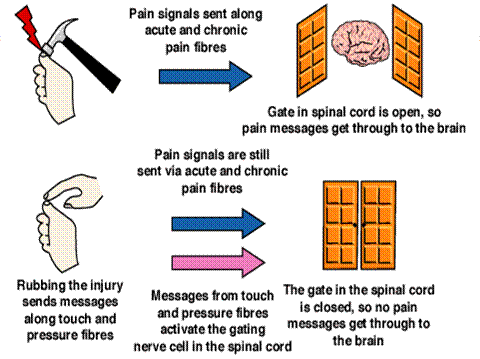
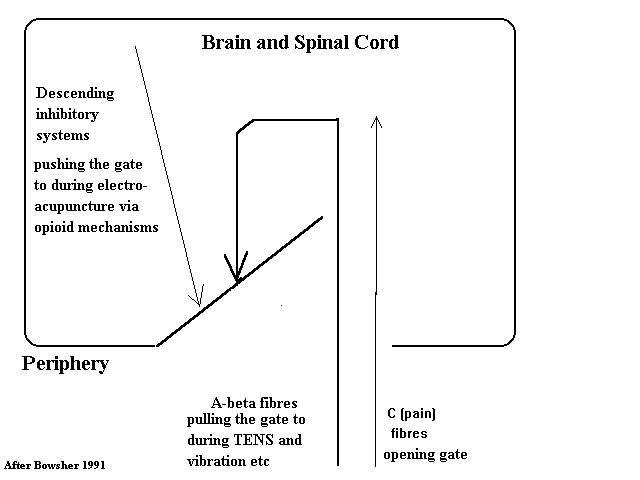
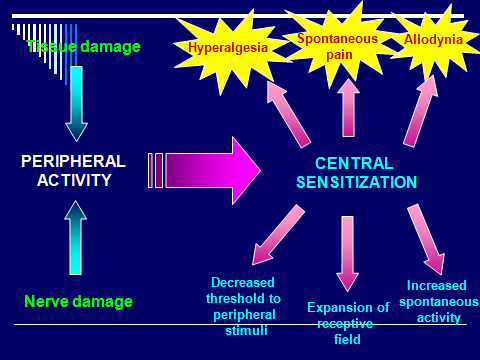
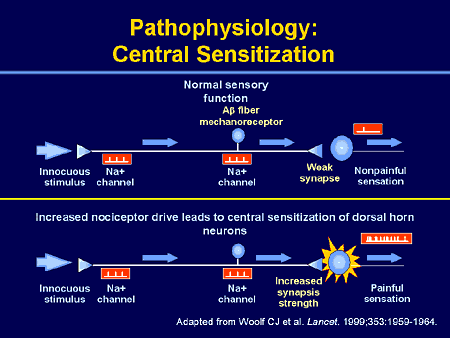
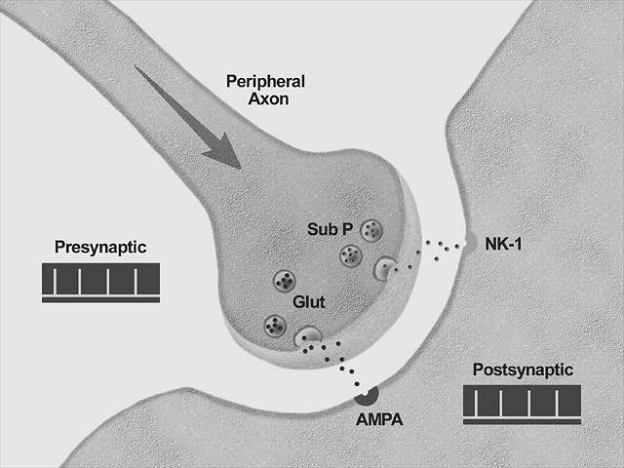
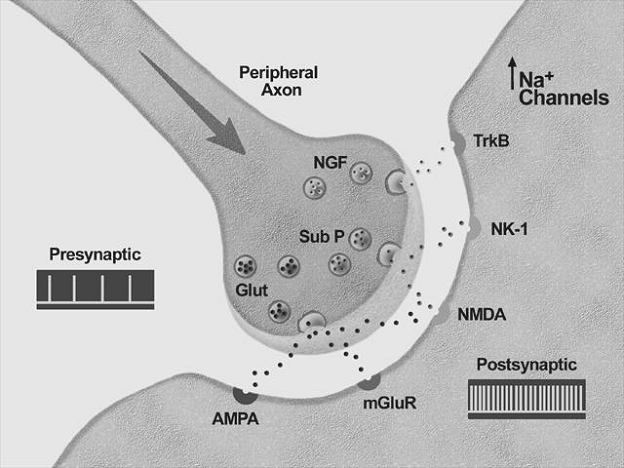
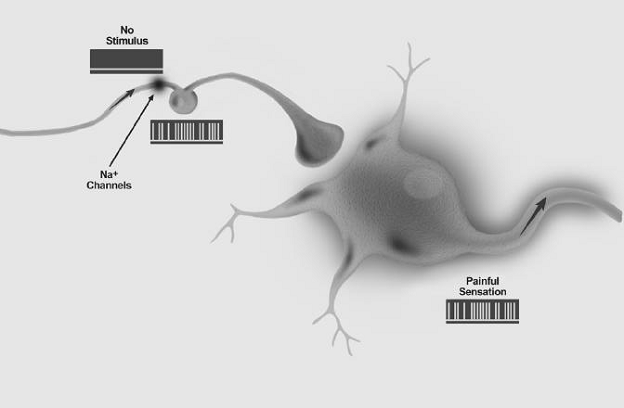
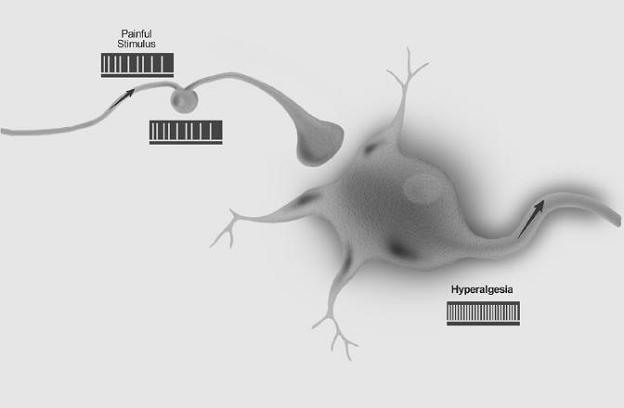
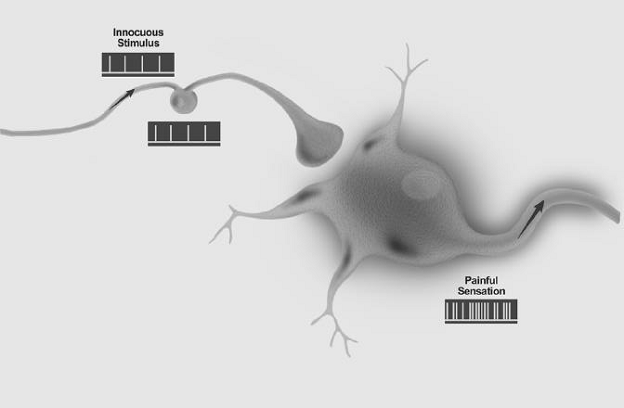
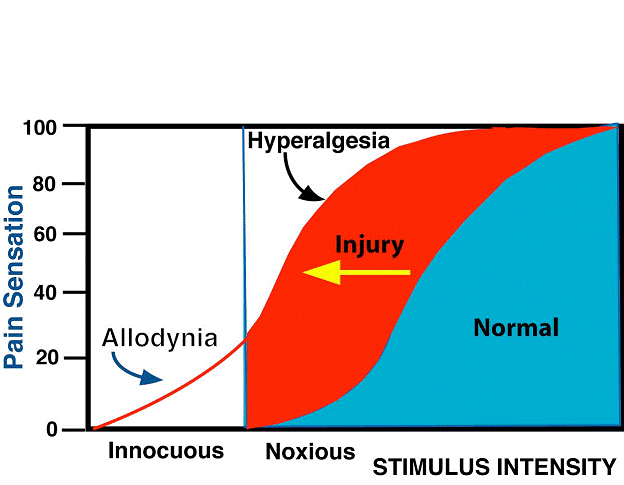
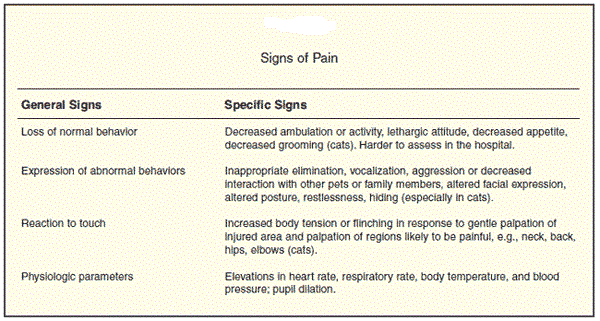
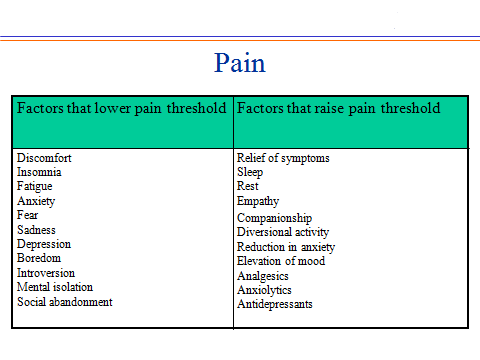

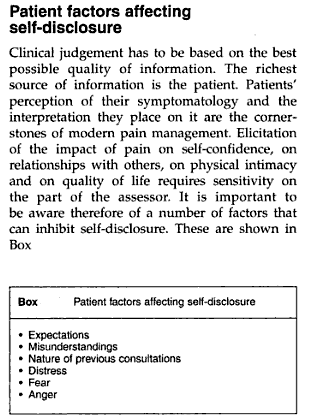
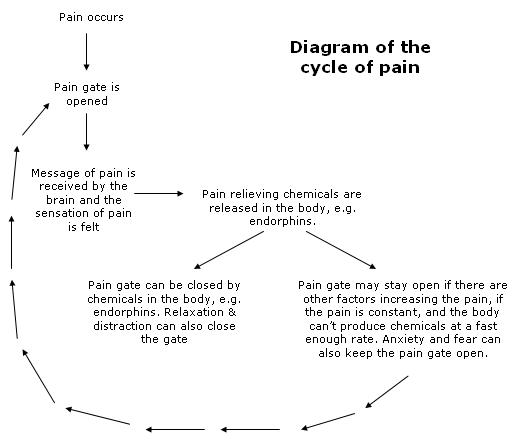
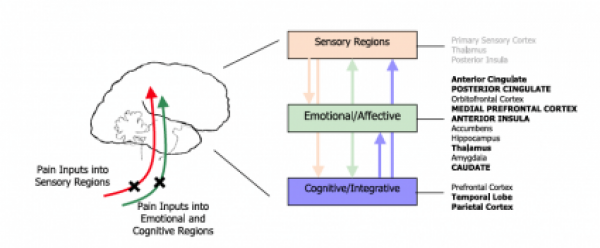

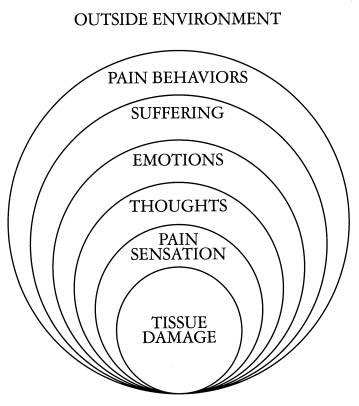
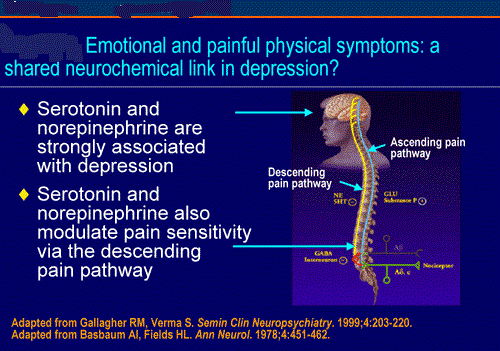


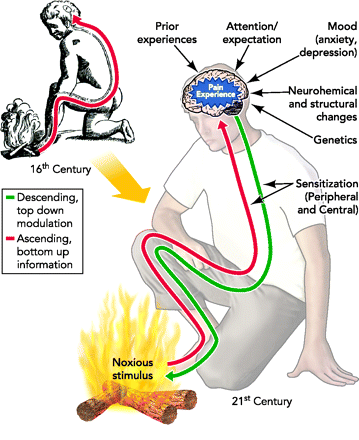
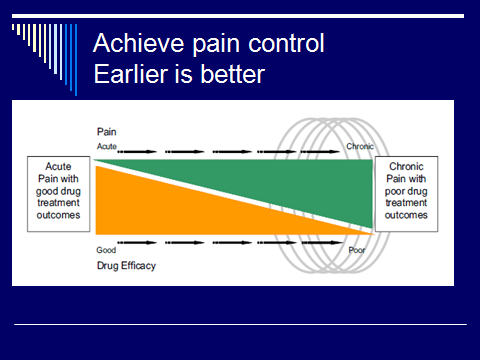
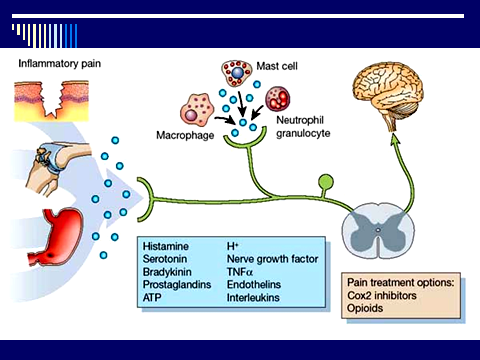
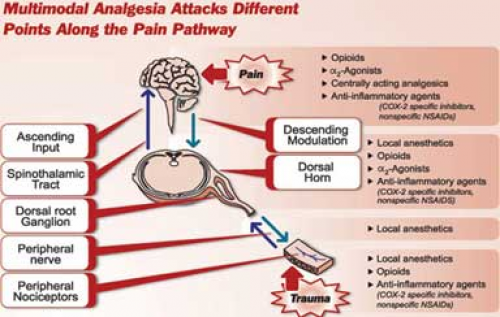
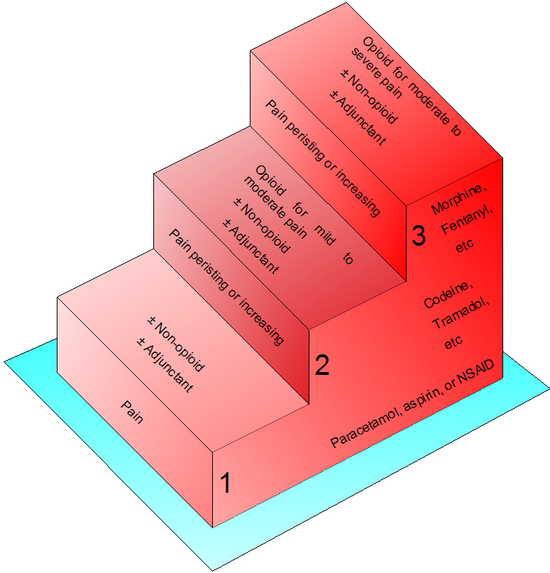
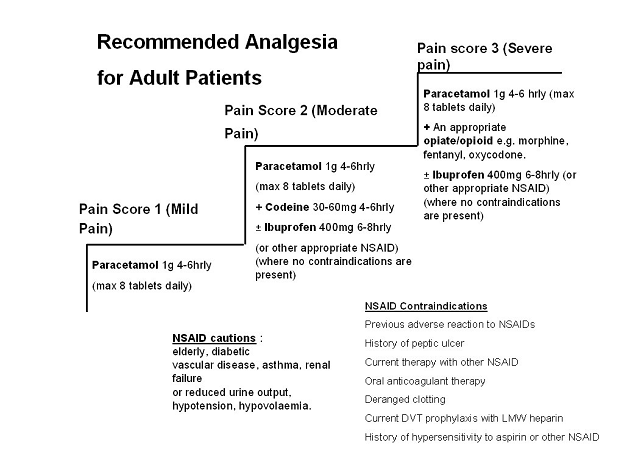

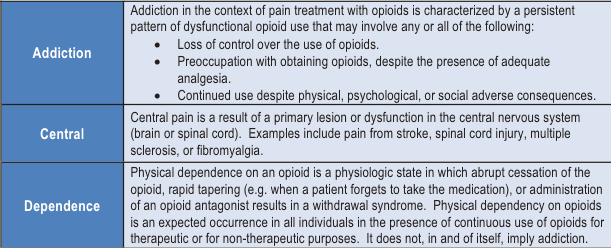

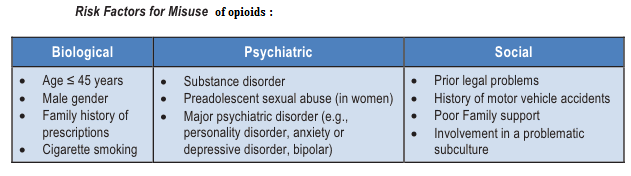
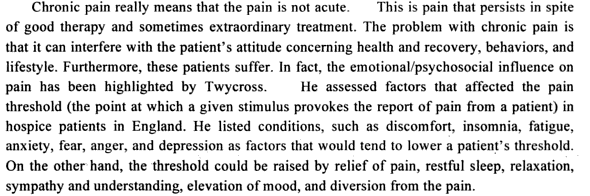

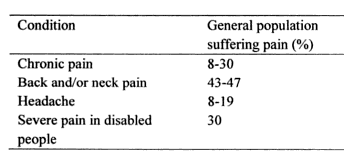
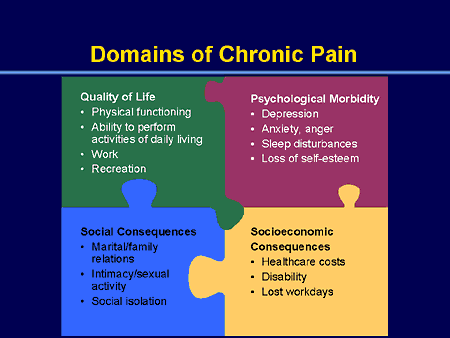
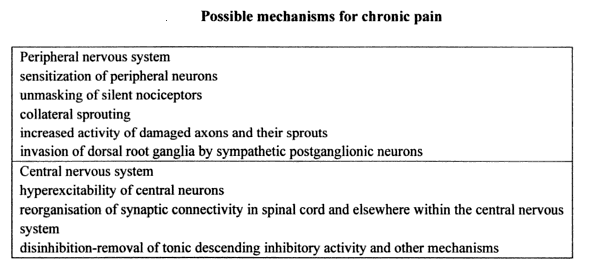
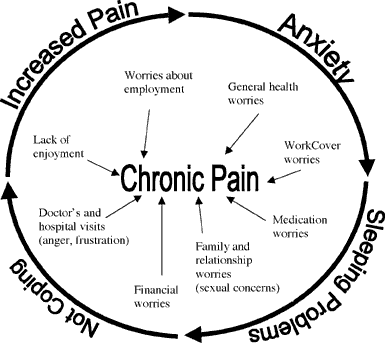
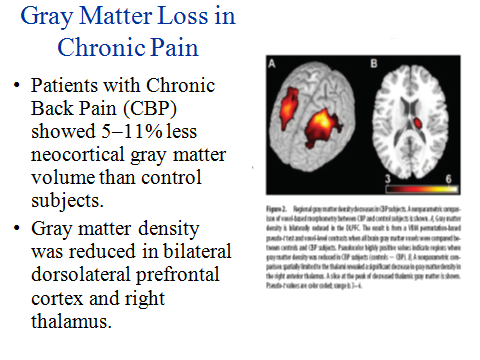
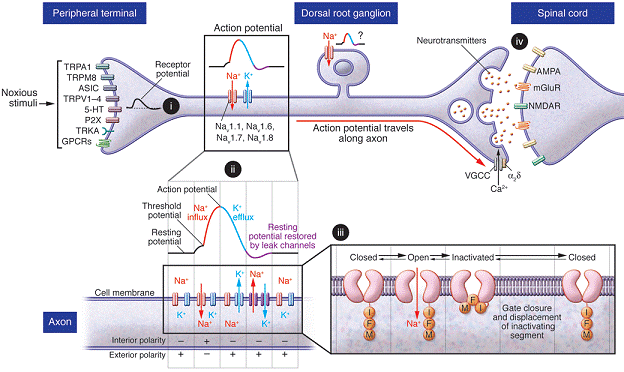
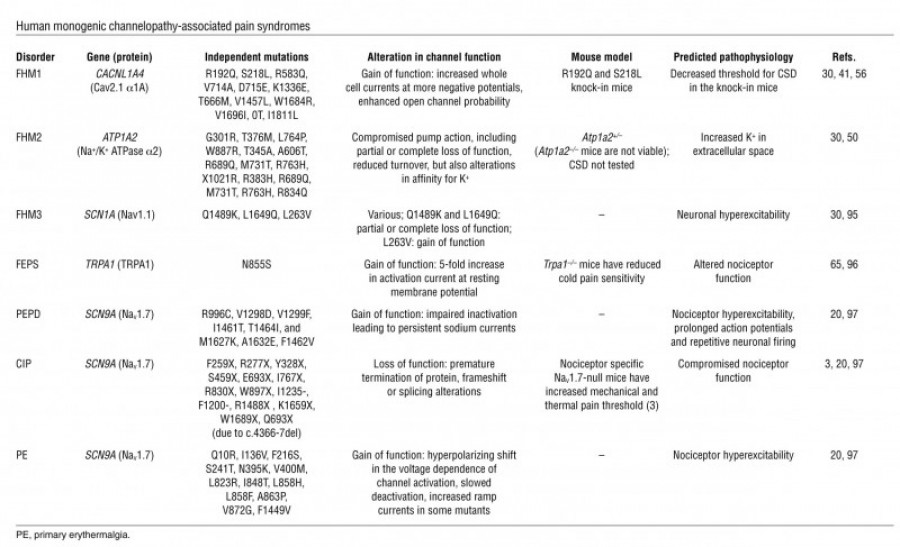
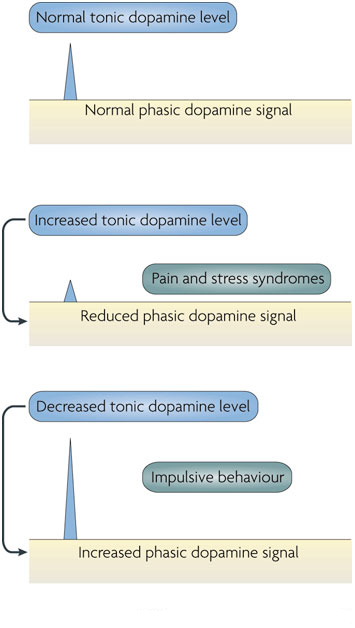
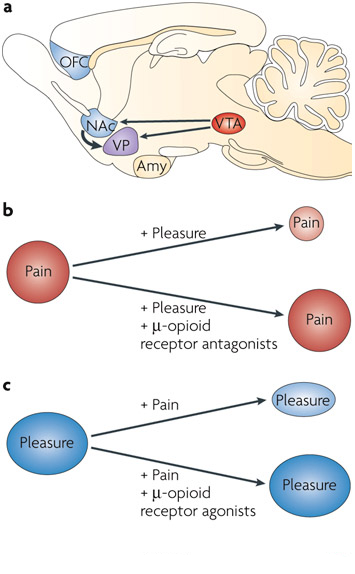
Fantastic post however I was wondering if you could write a litte more on this topic? I’d be very grateful if you could elaborate a little bit further. Thank you!|
The information shared is of top quality which has to get appreciated at all levels. Well done…
Your excitement urges others to do their ideal. You are an remarkable problem-solver.
Why people still make use of to read news papers when in this technological world all is presented on net?|
Yes, quite
_ _ _ _ _ _ _ _ _ _ _ _ _ _
Некулицы Иван prometheus github
You should be a part of a contest for one of the greatest sites on the net. I will highly recommend this web site!
I do not even know how I ended up here, but I thought this post was good. I don’t know who you are but certainly you’re going to a famous blogger if you are not already 😉 Cheers!
Ever feel ashamed and frustrated by the weight you’ve gained because your body no longer works like it used to years ago? And no matter which time-tested techniques you try, you just can’t get rid of pounds and pounds of stubborn excess fat? Click here for more :> https://cutt.ly/redteadetox
Fantastic blog article.Really looking forward to read more. Much obliged.
You actually make it seem so easy with your presentation but I find this topic to be actually something that I think I would never
understand. It seems too complicated and extremely broad for me.
I’m looking forward for your next post, I will try to get the hang of it!
Cheapest is the dearest.
Really enjoyed this blog. Fantastic.