Dr Rajiv Desai
An Educational Blog
Novel Approach to Diabetes Mellitus
Novel Approach to Diabetes Mellitus:
________
The figure below shows diabetic patient self-administering insulin injection:
________
My novel hypothesis on diabetes mellitus (DM):
_
Everybody knows that insulin is the only treatment of type 1 diabetes mellitus (T1DM). However in type 2 diabetes mellitus (T2DM) diet, exercise, oral hypoglycemic agents (OHA) precede insulin therapy and only when OHA fails or severe diabetes at onset warrant insulin therapy. There are reports of short term early insulin therapy in all T2DM to protect b-cells of pancreas. I propose a hypothesis that every T2DM patient needs insulin treatment from day one of diagnosis and it ought to be continued indefinitely. By the time diagnosis of diabetes is made, 50 to 80 % of b-cells of pancreas are dead (dysfunctional). The logic is that exogenous insulin will reduce load on surviving b-cells of pancreas, protect surviving b-cells and achieve better glycemic control and reduce complications as rejuvenated surviving b-cells will increase or decrease insulin secretions depending on glucose level and food intake. Exogenous with endogenous insulin will ensure best overall glycemic control irrespective of timing & quantum of food intake. Weight gain occurs as a side effect of insulin therapy only when too much insulin and/or too much food is consumed. Too much insulin provokes hypoglycemia induced over-eating and too much food require more insulin to control blood sugar. When sub-optimal exogenous insulin is used with strict diet control, hypoglycemia and weight gain will be prevented. T2DM ought to be controlled by both exogenous and endogenous insulin. When food intake is lower than normal, endogenous insulin will fall. When food intake is more than normal, endogenous insulin will rise. In other words, hypoglycemia and hyperglycaemia will be prevented reducing complications of DM. Endogenous insulin acts more at liver than peripheral tissue while exogenous insulin act more at peripheral tissue than liver. Endogenous insulin is more physiological than exogenous insulin and therefore all attempts must be made to preserve endogenous insulin by preserving b-cells at any cost. Some OHA (sulfonylurea) stimulate b-cells to increase endogenous insulin secretion but in long run destroys b-cells (about 3-5 years). Also b-cell stimulation by these OHA is non-physiological; if patient has reduced or missed meal, it will still produce sufficient endogenous insulin to cause hypoglycemia. Such OHA must not be used in diabetic therapy as the goal is to conserve b-cells as long as possible. Longer the b-cells last, more physiological is diabetes control and lesser the diabetic complications. The combination of exogenous insulin plus endogenous insulin is better than only exogenous insulin when most b-cells die. Therefore all T2DM must be given at least sufficient exogenous insulin to take care of basal insulin requirement plus partial meal time requirement. All diabetic patients must be assessed for quantum of endogenous insulin secretion (b-cell function) and insulin resistance (i.e. inverse of insulin sensitivity) before starting exogenous insulin. T1DM means almost no endogenous insulin secretion, negligible b-cell function and lifetime full dose exogenous insulin; T2DM can have endogenous insulin secretion ranging from low to high level depending on insulin resistance and b-cell function. Exogenous insulin must be given to all T2DM including those with normal or high endogenous insulin level (insulin resistance) to reduce load on hyper-functioning b-cells and prolong their life. To better utilize insulin (endo/exo), insulin sensitivity must be enhanced (aka reduce insulin resistance) by diet control, weight reduction in obese, exercise, and use of OHA like metformin/ pioglitazone.
_
Hyperglycemia is directly associated with microvascular complications, b-cell dysfunction and insulin resistance. Combining exogenous insulin with endogenous insulin will lead to better control of hyperglycemia than sole exogenous insulin when most b-cells die and sole endogenous insulin when sulfonylurea is used to stimulate b-cells in un-physiologic way. So sub-optimal exogenous insulin is the best treatment of T2DM to control hyperglycemia, reduce microvascular complications, and improve b-cell functions. Macrovascular complications are associated less with hyperglycemia and more with insulin resistance. So whether you give exogenous insulin or sulfonylurea, it would not reduce macrovascular complications. However, metformin and pioglitazone reduce insulin resistance; and combining exogenous insulin with metformin and/or pioglitazone would reduce macrovascular complications as well as reduce insulin requirement.
_
Stages of T2DM:
| Stage | Plasma glucose in mg% | Serum endogenous insulin | Interpretation |
| Zero | FPG 70-100; PPG 100-140 | Slightly lower than normal | Health conscious individual with normal weight, regular exercise and eating low glycemic index carb |
| One | FPG 70-100; PPG 100-140 | Normal insulin level | So called normal people with normal b-cells and no insulin resistance, but eating high glycemic index carb and sedentary life |
| Two | FPG 70-100; PPG 100-140 | High insulin level | Insulin resistance with hyper-functioning b-cells and increased b-cell mass e.g. obesity, no exercise. I would call it pre-diabetes. They are prone to macrovascular complications. |
| Three | FPG > 100; PPG > 140 | High insulin level | Insulin resistance with hyper-functioning b-cells but reduced b-cell mass, I would call it early diabetes mellitus. They are prone to macrovascular and microvascular complications. |
| Four | FPG > 100; PPG > 140 | Normal insulin level | Insulin resistance with some b-cell hyper-function and significant b-cell mass reduction. I would call it established diabetes mellitus. They are prone to macrovascular and microvascular complications. |
| Five | FPG > 100; PPG > 140 | Low insulin level | Insulin resistance with sub-total b-cell loss. I would call it severe diabetes mellitus. They are prone to macrovascular and microvascular complications. |
_
As you can see that you can have normal blood sugar with varying insulin level. You can also have high blood sugar with varying insulin level. This proves that we need to do both blood sugar and blood endogenous insulin level to make accurate pathophysiological diagnosis of diabetes in order to determine insulin secretion and insulin resistance. I want to remove the term ‘impaired glucose tolerance’. Anybody with FPG > 100 mg% and PPG > 140 mg% is diabetic. Pre-diabetes means normal blood sugars but high endogenous insulin levels. A non-diabetic person means normal b-cells mass & function, and no insulin resistance (stage 0/1). Presence or absence of diabetic complications does not determine diagnosis of diabetes mellitus as besides hyperglycemia and insulin resistance, genetic factors also contributes to complications. The moment insulin resistance starts and/or b-cell loss starts with hyperglycemia, you are diabetic (stage 2/3/4/5). In other words, FPG 100 mg% and PPG 140 mg% are cut-off values. Anything less than that is non-diabetic provided their endogenous insulin values are not raised. Anything more than that is diabetic irrespective of endogenous insulin values.
_
So I have put forward novel hypothesis on diabetes. Every patient suspected of diabetes must do, not only FPG, PPG and A1C but also fasting and postprandial serum insulin level for appropriate staging of DM. All DM patients need daily exogenous insulin from day 1 till person dies or in rare cases, till he becomes non-diabetic by life-style changes. Now let me go into the details of insulin therapy literature and various studies on DM vis-à-vis insulin, OHA and diabetic complications to determine whether my hypothesis holds true or fails.
_________
_________
Glucose homeostasis:
Insulin and glucagon are potent regulators of glucose metabolism. Plasma glucose concentration is a function of the rate of glucose entering the circulation (glucose appearance) balanced by the rate of glucose removal from the circulation (glucose disappearance). Circulating glucose is derived from three sources: intestinal absorption during the fed state, glycogenolysis, and gluconeogenesis. The major determinant of how quickly glucose appears in the circulation during the fed state is the rate of gastric emptying. Other sources of circulating glucose are derived chiefly from hepatic processes: glycogenolysis, the breakdown of glycogen, the polymerized storage form of glucose; and gluconeogenesis, the formation of glucose primarily from lactate and amino acids during the fasting state. Glycogenolysis and gluconeogenesis are partly under the control of glucagon, a hormone produced in the α-cells of the pancreas. During the first 8–12 hours of fasting, glycogenolysis is the primary mechanism by which glucose is made available. Glucagon facilitates this process and thus promotes glucose appearance in the circulation. Over longer periods of fasting, glucose, produced by gluconeogenesis, is released from the liver. For nondiabetic individuals in the fasting state, plasma glucose is derived from glycogenolysis under the direction of glucagon. Basal levels of insulin control glucose disposal. Insulin’s role in suppressing gluconeogenesis and glycogenolysis is minimal due to low insulin secretion in the fasting state. For nondiabetic individuals in the fed state, plasma glucose is derived from ingestion of nutrients. In the bi-hormonal model, glucagon secretion is suppressed through the action of endogenous insulin secretion. This action is facilitated through the paracrine route (communication within the islet cells). Additionally, in the fed state, insulin suppresses gluconeogenesis and glycogenolysis in the liver and promotes glucose disposal in the periphery. For individuals with diabetes in the fasting state, plasma glucose is derived from glycogenolysis and gluconeogenesis under the direction of glucagon. Exogenous insulin influences the rate of peripheral glucose disappearance and, because of its deficiency in the portal circulation, does not properly regulate the degree to which hepatic gluconeogenesis and glycogenolysis occur. For individuals with diabetes in the fed state, exogenous insulin is ineffective in suppressing glucagon secretion through the physiological paracrine route, resulting in elevated hepatic glucose production. As a result, the appearance of glucose in the circulation exceeds the rate of glucose disappearance. The net effect is postprandial hyperglycemia.
_
The idealized diagram above shows the fluctuation of blood sugar (red) and the sugar-lowering hormone insulin (blue) in humans during the course of a day containing three meals. In addition, the effect of a sugar-rich versus a starch-rich meal is highlighted. It’s the higher carbohydrate meals that elevate insulin levels the most after a meal. It’s important to note that Insulin is sensitive to both carbohydrate and protein consumed, but not fat. However, of all the food sources, it’s the higher carbohydrate meals that elevate Insulin levels the most after a meal. Even though there are individual differences to insulin sensitivity levels depending upon your metabolic type, the evidence strongly suggests that the leaner and lighter you become the stronger your insulin sensitivity levels will be. This means carbohydrates (and protein to a lesser extent) are producing a bigger and more powerful anabolic effect the closer you get to your weight loss/body fat goals.
__
Hormones that influence blood glucose level:
New understanding of the roles of other pancreatic and incretin hormones has led to a multi-hormonal view of glucose homeostasis besides insulin and glucagon. Glucoregulatory hormones include insulin, glucagon, amylin, GLP-1, glucose-dependent insulinotropic peptide (GIP), epinephrine, cortisol, and growth hormone. Of these, insulin and amylin are derived from the β-cells, glucagon from the α-cells of the pancreas, and GLP-1 and GIP from the L-cells of the intestine.
| Hormone | Tissue of Origin | Metabolic Effect | Effect on Blood Glucose |
| Insulin | Pancreatic β Cells | 1) Enhances entry of glucose into cells; 2) Enhances storage of glucose as glycogen, or conversion to fatty acids; 3) Enhances synthesis of fatty acids and proteins; 4) Suppresses breakdown of proteins into amino acids, of adipose tissue into free fatty acids. | Lowers |
| Somatostatin | Pancreatic δ Cells | 1) Suppresses glucagon release from α cells (acts locally); 2) Suppresses release of Insulin, Pituitary tropic hormones, gastrin and secretin. | Lowers |
| Glucagon | Pancreatic α Cells | 1) Enhances release of glucose from glycogen; 2) Enhances synthesis of glucose from amino acids or fatty acids. | Raises |
| Epinephrine | Adrenal medulla | 1) Enhances release of glucose from glycogen; 2) Enhances release of fatty acids from adipose tissue. | Raises |
| Cortisol | Adrenal cortex | 1) Enhances gluconeogenesis; 2) Antagonizes Insulin. | Raises |
| ACTH | Anterior pituitary | 1) Enhances release of cortisol; 2) Enhances release of fatty acids from adipose tissue. | Raises |
| Growth Hormone | Anterior pituitary | Antagonizes Insulin | Raises |
| Thyroxine | Thyroid | 1) Enhances release of glucose from glycogen; 2) Enhances absorption of sugars from intestine | Raises |
_______
_______
Insulin:
Insulin is a very old protein that may have originated more than a billion years ago. The molecular origins of insulin go at least as far back as the simplest unicellular eukaryotes. Apart from animals, insulin-like proteins are also known to exist in Fungi and Protista kingdoms. Within vertebrates, the amino acid sequence of insulin is strongly conserved. Bovine insulin differs from human in only three amino acid residues, and porcine insulin in one. Even insulin from some species of fish is similar enough to human to be clinically effective in humans. Insulin in some invertebrates is quite similar in sequence to human insulin, and has similar physiological effects. The strong homology seen in the insulin sequence of diverse species suggests that it has been conserved across much of animal evolutionary history.
_
Insulin and pancreas:
Pancreatic beta cells are found in the islets of Langerhans, which are of various size and contain a few hundred to a few thousand endocrine cells. Islets are anatomically and functionally separate from pancreatic exocrine tissue (which secretes pancreatic enzymes and fluid directly into ducts that drain into the duodenum). Normal subjects have about one million islets that in total weigh 1 to 2 grams and constitute 1 to 2 percent of the mass of the pancreas. Islets vary in size from 50 to 300 micrometers in diameter. They are composed of several types of cells. At least 70 percent are beta cells, which are localized in the core of the islet. These cells are surrounded by alpha cells that secrete glucagon, smaller numbers of delta cells that secrete somatostatin, and PP cells that secrete pancreatic polypeptide. All of the cells communicate with each other through extracellular spaces and through gap junctions. This arrangement allows cellular products secreted from one cell type to influence the function of downstream cells. As an example, insulin secreted from beta cells suppresses glucagon secreted from alpha cells. A neurovascular bundle containing arterioles and sympathetic and parasympathetic nerves enters each islet through the central core of beta cells. The arterioles branch to form capillaries that pass between the cells to the periphery of the islet and then enter the portal venous circulation.
_
The human insulin protein is composed of 51 amino acids, and has a molecular mass of 5808 Da. The primary structure of insulin is made from two polypeptide chains named subunit A and B. Subunit A consists of 21 amino acids, whereas subunit B consists of 30 amino acids. These chains are connected by two disulfide bridges as seen in figure below. Insulin also forms quaternary structure by creating diamers using hydrogen bonds and hexamers by bonding with two zinc ions. Insulin’s small size allows it to be a ligand for other proteins appropriately named insulin receptors. Inactive insulin is stored in the body as a hexamer, while the active form is the monomer. The hexamer is an inactive form with long-term stability, which serves as a way to keep the highly reactive insulin protected, yet readily available. The hexamer-monomer conversion is one of the central aspects of insulin formulations for injection. The hexamer is far more stable than the monomer, which is desirable for practical reasons; however, the monomer is a much faster-reacting drug because diffusion rate is inversely related to particle size (e.g., rapid acting insulin analogue). A fast-reacting drug means insulin injections do not have to precede mealtimes by hours, which in turn gives people with diabetes more flexibility in their daily schedules.
_
_
In the beta cells of the pancreas, the insulin molecule is originally produced as a single molecule (preproinsulin) composed of 110 amino acids. It passes through the endoplasmic reticulum and 24 amino acids (“the signal peptide”) are removed by enzyme action from one end of the chain, leaving another form (pro-insulin) behind. The proinsulin folds and binds to give the molecule its final structure. Then the proinsulin passes into vesicles budded off from the Golgi body. Thereafter the middle section (“the C chain”) of 33 amino-acids is removed by the action of the enzymes prohormone convertase 1 and 2, converting it into the final structure with 2 chains, A and B. Further 2 amino acids are removed by another enzyme carboxypeptidase E. Proinsulin is cleaved into equimolar amounts of insulin and C-peptide in the secretory granules. The process of insulin secretion involves fusion of the secretory granules with the cell membrane and exocytosis of insulin, C-peptide, and proinsulin.
_
Insulin is a peptide hormone composed of 51 amino acids that is synthesized, packaged, and secreted in pancreatic beta cells. Insulin is secreted in response to increased blood glucose and amino acids following ingestion of a meal. Like many hormones, insulin exerts its actions through binding to specific receptors present on many cells of the body, including fat, liver, and muscle cells. The primary action of insulin is to stimulate glucose disappearance. Insulin helps control postprandial glucose in three ways. Initially, insulin signals the cells of insulin-sensitive peripheral tissues, primarily skeletal muscle, to increase their uptake of glucose. Secondly, insulin acts on the liver to promote glycogenesis. Finally, insulin simultaneously inhibits glucagon secretion from pancreatic α-cells, thus signalling the liver to stop producing glucose via glycogenolysis and gluconeogenesis. All of these actions reduce blood glucose. Other actions of insulin include the stimulation of fat synthesis, promotion of triglyceride storage in fat cells, promotion of protein synthesis in the liver and muscle, and proliferation of cell growth. Insulin action is carefully regulated in response to circulating glucose concentrations. Insulin is not secreted if the blood glucose concentration is ≤ 3.3 mmol/l, but is secreted in increasing amounts as glucose concentrations increase beyond this threshold. Postprandially, the secretion of insulin occurs in two phases: an initial rapid release of preformed insulin, followed by increased insulin synthesis and release in response to blood glucose. Long-term release of insulin occurs if glucose concentrations remain high. While glucose is the most potent stimulus of insulin, other factors stimulate insulin secretion. These additional stimuli include increased plasma concentrations of some amino acids, especially arginine, leucine, and lysine; GLP-1 and GIP released from the gut following a meal; and parasympathetic stimulation via the vagus nerve.
_
Fully functional beta cells are metabolically very active, shedding and replacing 30–50% of their surface membrane daily in the course of insulin secretion. A lean healthy individual might secrete about 35 units of insulin per day, yet will have about 10 times this amount stored within his pancreas. By contrast, an obese insulin-resistant person might need to produce >100 units daily to maintain normal blood glucose levels. Type 1 diabetes results from progressive beta cell loss by apoptosis, thus increasing the work-load of the residue. A further consequence is loss of beta to beta cell communication and an altered cell-to-cell (paracrine) interaction between beta cells and glucagon-producing alpha cells. Coordinated pulsatile release of insulin deteriorates during the type 1 diabetes prodrome, and results in loss of efficacy in regulating glucose output by liver cells (hepatic insulin resistance). Blood glucose rises, increasing the workload of the remaining beta cells, and may further impair their function by the effect known as glucose toxicity. There are indications that functional defects are present at diagnosis of type 1 diabetes, and may recover to some extent in the period following diagnosis.
_
Oscillations of insulin release:
_
Insulin release from pancreas oscillates with a period of 3–6 minutes.
_
Even during the digestion, in general, one or two hours following a meal, insulin release from the pancreas is not continuous, but oscillates with a period of 3–6 minutes, changing from generating a blood insulin concentration more than about 800 pmol/l to less than 100 pmol/l. This is thought to avoid downregulation of insulin receptors in target cells, and to assist the liver in extracting insulin from the blood. This oscillation is important to consider when administering insulin-stimulating medication, since it is the oscillating blood concentration of insulin release, which should, ideally, be achieved, not a constant high concentration. This may be achieved by delivering insulin rhythmically to the portal vein or by islet cell transplantation to the liver. It is hoped that future insulin pumps will address this characteristic.
_
Basal Insulin Release:
The beta-cells of a healthy person who has not eaten for a while release a small amount of insulin into the blood stream throughout the day and night in the form of very small pulses every few minutes. This is called “basal insulin release.”
Maintaining this steady supply of insulin is important. It allows the cells of the body to utilize blood sugar even if some time has passed since a meal. The steady insulin level as another function, too. A dropping insulin level signals the liver that blood sugar is getting low and that it is time to add more glucose. When this happens, the liver converts the carbohydrate it has stored, (known as glycogen) into glucose, and dumps it into the blood stream. This raises the blood sugar back to its normal level. If a person has exhausted their glycogen stores, as can happen on a low carbohydrate diet, the liver converts protein into glucose to provide the glucose it makes in response to a low level of insulin in the blood. The protein can come from dietary protein or from your body’s own muscles. That is why dieters can lose significant amounts of muscle mass if they don’t get enough protein when they diet. Of course with every meal, there is bolus insulin secreted in addition to basal release to take care of excess glucose.
_
First Phase Insulin Release:
When a health person starts to eat a meal, the beta-cells kick into high gear. Their stored insulin is released immediately. Then, if the blood sugar concentration rises over 100 mg/dl, (5.5 mmol/L) the beta-cells start secreting more insulin into the blood stream. This early release of stored insulin after a meal is called “First Phase Insulin Release.” In a healthy person it keeps the blood sugar from rising very high because it is available to meet most of the glucose that comes from the digestion of the current meal. The amount of insulin secreted in the first phase response to a meal is usually determined by the amount of glucose encountered in the previous meal. In a healthy person, this first phase response peaks a few minutes after you’ve started your meal. The blood sugar rise caused by the meal peaks about half an hour after you start eating.
_
Second Phase Insulin Release:
After completing the first phase insulin release, the beta-cells pause. Then, if blood sugar is still not back under 100 mg/dl (5.5 mmol/L) ten to twenty minutes later, they push out another, smaller second phase insulin response which, in a healthy person, brings the blood sugar back down to its starting level, usually within an hour to an hour and a half after the start of a meal.
_
It is this combination of a robust first phase insulin response followed by a functional second phase insulin response that keeps the blood sugar of a normal person from ever rising over 140 mg/dl(7.8 mmol/L) even after a high carbohydrate meal. When first phase insulin response is completely functional, the blood sugar level at two hours should be back to the normal fasting blood sugar level which is somewhere in the mid 80 mg/dl range (4.5 mmol/L). When first phase release fails, or when second phase insulin response is sluggish, blood sugars start to rise to higher levels after a meal and take longer to return to normal. This condition is called “impaired glucose tolerance.” If the blood sugar rises over 200 mg/dl (11 mmol/L) after a meal the same condition is called “Diabetes.”
_
Incretin hormones GLP-1(Glucagon-like peptide-1) and GIP (Gastric inhibitory polypeptide):
_
_
The intricacies of glucose homeostasis become clearer when considering the role of gut peptides. By the late 1960s, Perley and Kipnis and others demonstrated that ingested food caused a more potent release of insulin than glucose infused intravenously. This effect, termed the “incretin effect,” suggested that signals from the gut are important in the hormonal regulation of glucose disappearance. Additionally, these hormonal signals from the proximal gut seemed to help regulate gastric emptying and gut motility. Several incretin hormones have been characterized, and the dominant ones for glucose homeostasis are GIP and GLP-1. GIP stimulates insulin secretion and regulates fat metabolism, but does not inhibit glucagon secretion or gastric emptying. GIP levels are normal or slightly elevated in people with type 2 diabetes. While GIP is a more potent incretin hormone, GLP-1 is secreted in greater concentrations and is more physiologically relevant in humans. GLP-1 also stimulates glucose-dependent insulin secretion but is significantly reduced postprandially in people with type 2 diabetes or impaired glucose tolerance. GLP-1 stimulates insulin secretion when plasma glucose concentrations are high but not when plasma glucose concentrations approach or fall below the normal range. Derived from the proglucagon molecule in the intestine, GLP-1 is synthesized and secreted by the L-cells found mainly in the ileum and colon. Circulating GLP-1 concentrations are low in the fasting state. However, both GIP and GLP-1 are effectively stimulated by ingestion of a mixed meal or meals enriched with fats and carbohydrates. In contrast to GIP, GLP-1 inhibits glucagon secretion and slows gastric emptying. GLP-1 has many glucoregulatory effects. In the pancreas, GLP-1 stimulates insulin secretion in a glucose-dependent manner while inhibiting glucagon secretion. Animal studies have demonstrated that the action of GLP-1 occurs directly through activation of GLP-1 receptors on the pancreatic β-cells and indirectly through sensory nerves. GLP-1 has a plasma half-life of about 2 minutes, and its disappearance is regulated primarily by the enzyme dipeptidyl peptidase-IV (DPP-IV), which rapidly cleaves and inactivates GLP-1. Infusion of GLP-1 lowers postprandial glucose as well as overnight fasting blood glucose concentrations. The postprandial effect of GLP-1 is partly due to inhibition of glucagon secretion. Yet while GLP-1 inhibits glucagon secretion in the fed state, it does not appear to blunt glucagon’s response to hypoglycemia. GLP-1 helps regulate gastric emptying and gastric acid secretion, perhaps by signalling GLP-1 receptors in the brain and thereby stimulating efferent tracts of the vagus nerve. As gastric emptying slows, the postprandial glucose excursion is reduced. Administration of GLP-1 has been associated with the regulation of feeding behavior and body weight. In addition, there have been reported observations of GLP-1 improving insulin sensitivity and enhancing glucose disposal. Of significant and increasing interest is the role GLP-1 may have in preservation of β-cell function and β-cell proliferation. In animal studies, GLP-1 has been shown to enhance functional β-cell mass.
___
Amylin and Connecting peptide (C-peptide):
In addition to insulin, beta cells also secrete the hormone Amylin and C-peptide, a by-product of insulin production. Amylin slows the rate of glucose entering the bloodstream, making it a more short-term regulator of blood glucose levels. C-peptide is a molecule that helps to prevent neuropathy and other vascular complications by assisting in the repair of the muscular layers of the arteries. It is secreted into the bloodstream in equal quantities (or moles) to insulin.
_
The figure below shows C-peptide component of proinsulin:
_
Amylin:
Isolated from pancreatic amyloid deposits in the islets of Langerhans, amylin was first reported in the literature in 1987. Amylin, a 37–amino acid peptide, is a neuroendocrine hormone co-expressed and co-secreted with insulin by pancreatic β-cells in response to nutrient stimuli. When secreted by the pancreas, the insulin-to-amylin molar ratio in the portal circulation is approximately 50:1. Because of hepatic extraction of insulin, this ratio falls to ∼ 20:1 in the peripheral circulation. Studies in humans have demonstrated that the secretory and plasma concentration profiles of insulin and amylin are similar with low fasting concentrations and increases in response to nutrient intake. In healthy adults, fasting plasma amylin concentrations range from 4 to 8 pmol/l rising as high as 25 pmol/l postprandially. In subjects with diabetes, amylin is deficient in type 1 and impaired in type 2 diabetes. Preclinical findings indicate that amylin works with insulin to help coordinate the rate of glucose appearance and disappearance in the circulation, thereby preventing an abnormal rise in glucose concentrations. Amylin complements the effects of insulin on circulating glucose concentrations via two main mechanisms. Amylin suppresses post-prandial glucagon secretion, thereby decreasing glucagon-stimulated hepatic glucose output following nutrient ingestion. This suppression of post-prandial glucagon secretion is postulated to be centrally mediated via efferent vagal signals. Importantly, amylin does not suppress glucagon secretion during insulin-induced hypoglycemia. Amylin also slows the rate of gastric emptying and, thus, the rate at which nutrients are delivered from the stomach to the small intestine for absorption. In addition to its effects on glucagon secretion and the rate of gastric emptying, amylin dose-dependently reduces food intake and body weight in animal models. Amylin exerts its actions primarily through the central nervous system. Animal studies have identified specific calcitonin-like receptor sites for amylin in regions of the brain, predominantly in the area postrema. The area postrema is a part of the dorsal vagal complex of the brain stem. A notable feature of the area postrema is that it lacks a blood-brain barrier, allowing exposure to rapid changes in plasma glucose concentrations as well as circulating peptides, including amylin. In summary, amylin works to regulate the rate of glucose appearance from both endogenous (liver-derived) and exogenous (meal-derived) sources, and insulin regulates the rate of glucose disappearance.
___
Insulin receptors:
Insulin exerts all of its biological activities, both as a hormone and as a growth factor, by binding to a cell surface receptor complex. The insulin receptor is a member of the membrane-spanning receptor family that harbors intrinsic tyrosine kinase activity. However, the insulin receptor is unique in that it is a heterotetrameric complex composed of two completely extracellular α-peptides that are disulfide bonded to the two transmembrane-spanning β-peptides. Both the α- and β- subunits of the receptor complex are derived from a single gene. When insulin binds to the receptor it activates the intrinsic tyrosine kinase activity of the β-subunits resulting in autophosphorylation of the receptor. The insulin receptor is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of tyrosine kinase receptors. Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis, a functional process that under degenerate conditions may result in a range of clinical manifestations including diabetes and cancer. Tyrosine kinase receptors, including the insulin receptor, mediate their activity by causing the addition of a phosphate group to particular tyrosines on certain proteins within a cell. The “substrate” proteins that are phosphorylated by the Insulin Receptor include a protein called “IRS-1” for “insulin receptor substrate 1”. IRS-1 binding and phosphorylation eventually leads to an increase in the high affinity glucose transporter (Glut4) molecules on the outer membrane of insulin-responsive tissues, including muscle cells and adipose tissue, and therefore to an increase in the uptake of glucose from blood into these tissues. In other words, the glucose transporter Glut4 is transported from cellular vesicles to the cell surface, where it then can mediate the transport of glucose into the cell.
_
Degradation of insulin:
Once an insulin molecule has docked onto the receptor and effected its action, it may be released back into the extracellular environment or it may be degraded by the cell. Degradation normally involves endocytosis of the insulin-receptor complex followed by the action of insulin degrading enzyme. Most insulin molecules are degraded by liver cells. An insulin molecule produced endogenously by the pancreatic beta cells is estimated to be degraded within about one hour after its initial release into circulation (insulin half-life ~ 4–6 minutes).
_____
Insulin actions:
_
The figure below shows synopsis of metabolic actions of insulin:
_
Metabolic actions of insulin help to maintain glucose homeostasis and promote glucose utilization in the body. Insulin increases glucose utilization in peripheral organs (e.g., skeletal muscle and adipose tissue) and suppresses hepatic glucose production (HGP). In addition to these classical metabolic insulin target tissues, there are many other important physiological targets of insulin including the brain, pancreatic beta cells, heart, and vascular endothelium that help to coordinate and couple metabolic and cardiovascular homeostasis under healthy conditions. Insulin has concentration-dependent saturable actions to increase whole-body glucose disposal. The maximal effect of insulin defines “insulin responsiveness” while the insulin concentration required for a half-maximal response defines “insulin sensitivity”. Although, other actions of insulin on fat and amino-acid metabolism, cardiovascular, kidney, and brain function also exhibit a concentration-dependent response, the term “insulin sensitivity” typically refers to insulin’s metabolic actions to promote glucose disposal.
_
Insulin Action and Endothelial Functions:
The metabolic functions of insulin are primarily reflective of its role in glucose and lipid homeostasis in skeletal muscle, adipose tissue, and liver. However, insulin also exerts important functions in other non-classical insulin target tissues such as the brain, pancreas, and the vascular endothelium. The ability of insulin to exert vasodilator action in the vascular endothelium as a result of increased nitric oxide (NO) production is an important component of the ability of this hormone to enhance glucose uptake by skeletal muscle. The insulin-mediated signalling pathway that triggers production of NO in vascular endothelium involves the same signalling proteins (PI3K, PKD, and PKB/Akt) that are components of metabolic regulatory pathways induced by insulin. Therefore, it is understandable why the same disruptions to insulin signalling that lead to insulin resistance caused by excess FFAs and hyperglycemia result in endothelial dysfunction.
_
Insulin potassium relationship:
Serum potassium concentration (K+) reflects total body potassium stores at the steady state, although this relationship can be disturbed in disorders of potassium distribution. Plasma K+ is a major determinant of the resting potential of all cells. Hyperkalemia and hypokalemia are silent yet fatal disturbances because of their arrhythmogenic potentials. Insulin was shown to be an important regulator of potassium homeostasis shortly after its discovery. Insulin causes potassium to shift into the cells thereby decreasing the extracellular K level. That’s why insulin is used in the treatment of hyperkalemia. Basal insulin maintains fasting plasma [K+] within the normal range. When insulin levels are suppressed, plasma [K+] rises and pronounced hyperkalemia develops after a potassium load. Hyperkalemia is often encountered in patients with diabetes. The insulin-deficient state in type 1 diabetes predisposes to hyperkalemia because of an impaired ability of potassium to enter cells. During hyperglycemic hypertonic states in type 1 and type 2 diabetics, potassium is carried out of cells by convective flux as the most abundant intracellular cation. Even at the steady state in a significant portion of type 1 and type 2 diabetics, there is an impaired ability of the distal nephron to excrete potassium because of hyporeninemic hypoaldosteronism or tubular insensitivity to aldosterone. Finally, one wonders whether there is impaired cellular potassium uptake in type 2 diabetes as part of a generalized insulin-resistant state. Level of potassium in the serum also affects insulin secretion from the pancreas. Because the beta cells have an ATP dependent K channel which is when closed leads to retained K inside the beta cell which favors depolarization thereby enhancing Calcium mediated release of secretory granules. Therefore, in hyperkalemia more K will enter the beta cell and insulin secretion will increase and conversely in hypokalemia the K ions are more likely to leave the beta cell and so insulin secretion will decrease. Potassium is a well proven insulin secretagogue in the intact organism and the isolated pancreas. Insulin is a key defender against exogenous potassium load by using intracellular buffering to minimize hyperkalemia before renal excretion.
_
One ponders whether there should be any teleogic reasons of coupling glucose to potassium uptake. In the postprandial state of herbivores or carnivores, caloric and potassium influx are concurrent. In a feast-or-famine situation in hunting carnivores, the magnitude of the load is much exaggerated. The simultaneous shift of glucose and potassium into cells makes physiologic sense with the postprandial outpouring of insulin. Similarly, dietary phosphate frequently accompanies caloric intake, and upon entry into cells, glucose is phosphorylated; thus, simultaneous phosphate uptake also makes physiologic sense. However, if potassium, phosphate, and glucose loads are applied discordantly, the simultaneous cellular uptake will clearly present a homeostatic quandary and some means of dissociation is mandatory. Previous studies have addressed whether potassium and glucose uptake are coupled. DeFronzo et al. observed a relationship between the decline in plasma [K+] and insulin level as well the total amount of glucose taken up by cells. Arslanian et al. concluded that insulin-dependent diabetics have impaired potassium uptake. However, Cohen et al. found independent actions of insulin on glucose and potassium uptake. Another study concluded that glucose and potassium uptake are differentially regulated and that impaired glucose disposal does not affect potassium uptake.
_________
_________
Insulin and C-peptide level:
_
Insulin level:
Elevated level of fasting insulin is the single greatest marker to assess a person’s cardiovascular and diabetic risk factors. This has been consistently demonstrated in the medical literature going back more than 20 years. Elevated levels of insulin are found among those with heart disease, congestive heart failure, insulin resistance, diabetes, high blood pressure, and obesity. Dr. Joseph Mercola says fasting insulin is “the number that may best predict your sudden death.” But what does it mean? So a fasting insulin level should never be 0, which it might be in a person with untreated Type 1 DM. It shouldn’t go below 3 mU/L. But a high insulin level is just as problematic. A high insulin level is a sign of insulin resistance or prediabetes. It can also signify early-stage Type 2 DM. According to Dr. Mercola, too much insulin promotes weight gain by storing fat. It promotes insulin resistance, lowers magnesium levels, and increases inflammation. It also tends to lower HDL (“good”) cholesterol and raise levels of LDL (“bad”) cholesterol. All of these increase the risk of diabetes and heart disease.
_
Serum Insulin and other Cardiovascular Risk Indicators in Children, Adolescents and Young Adults: 1991 study:
Serum insulin correlated positively with body mass index, concentrations of serum triglycerides, and blood pressure, and inversely with the concentration of high density lipoprotein cholesterol. High triglycerides, high systolic blood pressure, and low level of high density lipoprotein cholesterol clustered among subjects within the highest insulin quartile. The results suggest that the insulin resistance phenomenon, caused mainly by obesity and leading to unfavourable levels of other coronary heart disease risk indicators, is already developing in children and young adults. This suggests that preventing obesity in early life is important.
_
Serum insulin or plasma insulin?
The concentration of insulin was measured by double-antibody immunoassay simultaneously in the plasma and serum of thirty-eight fasting and non-fasting individuals. The concentration found in heparin-plasma was consistently higher than that found in serum, and experiments suggest that the high plasma-level is an effect of heparin. Evidence from previous workers (who have measured the hormone’s concentration in plasma and serum separately) would seem to support this. It is proposed that insulin should always be assayed in serum.
_
Insulin secretion is hard to evaluate on the basis of blood samples taken from a peripheral vein. This is because a high proportion of the insulin secreted by the pancreas is cleared on first passage through the liver. The liver is thus exposed to higher concentrations of insulin, delivered in pulses. The systemic circulation sees lower insulin levels with small pulses, since these are largely eliminated in passage through the liver. Furthermore, insulin has a half-life of about 6 minutes in the circulation, so very frequent sampling is needed to monitor its fluctuations. Last but not least, people on insulin typically have low levels of insulin antibodies in the circulation, which complicates accurate measurement in the insulin assay. These limitations can largely be overcome by measuring C-peptide in the plasma or urine. C-peptide is a short peptide sequence released from the proinsulin molecule on a one-to-one ratio as insulin is secreted. C-peptide is not cleared by liver (it is excreted via the kidney) and has a plasma half-life of about 30 minutes. Stressed beta cells accelerate insulin production and secretion, and one consequence is that the proportion of proinsulin to cleaved insulin entering the circulation is increased. A raised proinsulin: insulin ratio is thus a marker of beta cell distress and, since proinsulin has less metabolic effect than cleaved insulin, insulin action is also reduced. The main analytical pitfalls in insulin measurement in serum are related to (a) hemolysis, (b) circulating antiinsulin autoantibodies, and, for the past few years, (c) the reactivity (or lack of reactivity) of rapid- or long-acting pharmacological insulin analogs in sera. Hemolyzed samples contain an insulin-degrading enzyme and should not be analyzed unless they can be handled at 4 °C within ∼2–3 h or an insulinase inhibitor has been added in the blood collection tube to prevent insulin degradation. Antiinsulin antibodies interfere with RIAs and IMAs, yielding overestimated values for free insulin, the biologically active form of insulin, that is not bound to antiinsulin antibodies in serum. Measurement of free insulin requires the removal of antiinsulin antibodies, which can be achieved by polyethylene glycol precipitation. Insulin testing may be ordered with glucose and C-peptide tests. Insulin levels are also sometimes used in conjunction with the glucose tolerance test (GTT). In this situation, blood glucose and insulin levels are measured at pre-established time intervals to evaluate insulin resistance. Physicians ordering insulin assays should be informed in detail about the used assay system in order to interpret the results appropriately.
_
Reference Range of serum insulin:
Insulin is an anabolic hormone that promotes glucose uptake, glycogenesis, lipogenesis, and protein synthesis of skeletal muscle and fat tissue through the tyrosine kinase receptor pathway. In addition, insulin is the most important factor in the regulation of plasma glucose homeostasis, as it counteracts glucagon and other catabolic hormones—epinephrine, glucocorticoid, and growth hormone.
_
One international unit of insulin (1 IU) is defined as the “biological equivalent” of 34.7 μg pure crystalline insulin. This corresponds to the old USP insulin unit, where one unit (U) of insulin was set equal to the amount required to reduce the concentration of blood glucose in a fasting rabbit to 45 mg/dl (2.5 mmol/L). The unit of measurement used in insulin therapy is not part of the International System of Units (abbreviated SI) which is the modern form of the metric system. Instead the pharmacological international unit (IU) is defined by the WHO Expert Committee on Biological Standardization.
Conversion factor:
1 IU = 0.0347 mg insulin human (Ph Eur) or 1 USP unit = 0.0347 mg (USP).
_
Remember, micro-units per milliliter = milli-units per liter.
µU/mL = mU/L
The blood content of insulin can be measured in international units, such as µU/mL or in molar concentration, such as pmol/L, where 1 µU/mL equals 6.945 pmol/L.
_
Reference Range of Insulin Levels in one laboratory:
| Insulin Level | Insulin Level (SI Units) | |
| Fasting | < 25 mU/L | < 174 pmol/L |
| 30 minutes after glucose administration | 30-230 mU/L | 208-1597 pmol/L |
| 1 hour after glucose administration | 18-276 mU/L | 125-1917 pmol/L |
| 2 hour after glucose administration | 16-166 mU/L | 111-1153 pmol/L |
| ≥3 hours after glucose administration | < 25 mU/L | < 174 pmol/L |
The reference range for the normal level for insulin concentrations in serum samples in many laboratories is being given from 5 – 25 µU/mL. This range is only valid for overnight fasted patients (12 hours) with normal weight, without diabetes mellitus, and during normal caloric nutrition and normal insulin sensitivity – which cannot be accurately measured by simple techniques rather than estimated.
_
A typical blood level between meals is 8–11 μIU/mL (57–79 pmol/L). Unfortunately, there isn’t much agreement on what level is ideal. The Web site Health Central says 10–20. Dr. Mercola says less than 5. Fasting serum insulin is quite distinct for males and for females… for males it is an average of 11.5 mU/l and for females it is 8.7 mU/l. Two hours after stimulating the pancreas to release insulin, through what is called the insulin tolerance test in which they give the patient sugar to drink, 120 minutes later insulin circulating in blood is 54.6 and 34.6 for females. A study in Arizona found that women with a fasting insulin level around 8.0 had twice the risk of prediabetes as did women with a level around 5.0. Women with a fasting insulin of 25 or so had five times the risk of prediabetes.
_
How to lower insulin level:
Lowering insulin levels seems pretty similar to lowering glucose. Authorities like Dr. Mercola say the key is to reduce intake of sugar and grains. Those foods stimulate insulin production. Refined grains and fructose-sweetened drinks are the worst, he says. Better to eat fats and proteins. Writing on Livestrong.com, Andy Jackson has a somewhat different take. “Increase your intake of fresh fruits and vegetables, whole grains and lean proteins,” he says. “Avoid processed and fast foods, which are high in sugar, fat, and salt.” Exercise also lowers insulin levels and insulin resistance.
__
Insulin testing may be used to help:
•Diagnose an insulinoma, verify that removal of the tumour has been successful, and/or to monitor for recurrence
•Diagnose the cause of hypoglycemia in an individual with signs and symptoms
•Identify insulin resistance
•Monitor the amount of insulin produced by the beta cells in the pancreas (endogenous); in this case, a C-peptide test may also be done. Insulin and C-peptide are produced by the body at the same rate as part of the conversion of proinsulin to insulin in the pancreas. Both tests may be ordered when a health practitioner wants to evaluate how much insulin in the blood is made by the body and how much is from outside (exogenous) sources such as insulin injections. The test for insulin measures insulin from both sources while the C-peptide test reflects insulin produced by the pancreas.
•Determine when a type 2 diabetic might need to start taking insulin to supplement oral medications
•Determine and monitor the success of an islet cell transplant intended to restore the ability to make insulin, by measuring the insulin-producing capacity of the transplant.
__
C-peptide level in blood:
C-peptide is a chain of proteins that is spun off in the process by which the beta cell makes insulin. During this process, a precursor molecule, proinsulin is split into insulin and C-peptide. So for every molecule of insulin your beta cells produce, they also produce a molecule of C-peptide. C-peptide is removed from the bloodstream by your kidneys while insulin is removed by the liver. This makes a difference in how long these peptides stay in the bloodstream. It takes half an hour until C-peptide is removed, while insulin is gone in five minutes. This means that there should be five times as much C-peptide in your blood at any given time as there is insulin and the longer activity period should smooth out the effects of testing at any one particular moment. However, if there is something wrong with your kidneys they may not remove C-peptide in a normal manner and the result of a C-peptide test may be misleading. If a person is injecting insulin, measuring C-peptide is the only way doctors can determine whether they are also making insulin on their own since lab tests do not distinguish between injected insulin and endogenous insulin. Some doctors prefer to measure C-peptide even in people not injecting insulin because of its longer life in the bloodstream which means you won’t see as much fluctuation from moment to moment in C-peptide levels as you may find with insulin levels.
_
Interpreting the results of the C-Peptide Test:
The C-Peptide test measures how much insulin is released from the pancreas, when stimulated by glucose to do so. According to Mosby’s Manual of Diagnostic and Laboratory Tests – Second Edition, “The exogenously administered insulin suppresses endogenous insulin production.” More accurately, exogenous insulin controls blood glucose concentrations, thus preventing stimulation of endogenous insulin release, which may in effect suppress endogenous insulin production. C-Peptide is not a test for insulin production, but a test for insulin released from the pancreas. To understand how the pancreatic beta cells are working one needs to look at the C-Peptide test and at the blood glucose level at the time of test, and consider all the factors that contribute to hyperglycemia, to arrive at an objective conclusion. As a blood glucose load increases, the C-Peptide levels increase due to endogenous insulin release. In T1DM, C-peptide test is negative as no endogenous insulin secreted. Even in T2DM on exogenous insulin controlling blood sugar, C-peptide test can be negative as endogenous insulin secretion is suppressed and you may falsely label T2DM as T1DM. So to stimulate the pancreas to release insulin before taking the C-Peptide test, increase the blood glucose above normal for at least one hour before taking the blood test. This should help exculpate the pancreas and prove that maybe the beta cells are there and functioning albeit lesser than normal, proving T2DM.
_
C-peptide concentration in ng/mL X 0.331 = C-peptide concentration in nmol/L.
_
Range of C peptide:
Fasting range is: 0.78 – 1.89 ng/ml (0.26 – 0.62 nmol/L)
Range one hour after a glucose load is: 5.00 -12.00 ng/ml (During a glucose tolerance test)
Other labs give the fasting C-peptide assay with a “normal” reference range is between 1.5 – 3.5 ng/mL (0.5-1.15 nmol/L). The complete human C-Peptide range as opposed to the “normal” range is in fact 0.6 – 12.0 ng/ml, keep in mind that the normal values are for a non-diabetic person.
_
The values will be lower in an insulin user and higher in a type 2 oral agent user (agents that promote the release of insulin from the pancreas) when blood glucose levels are controlled. The C-Peptide test is mostly used to diagnose and evaluate patients who are hypoglycemic (produce too much insulin) as well as those who have insulinomas (insulin producing tumors). The test is also used to see if a normal person is secretly using insulin. They may ask patients to fast for this test (to reduce glucose levels in the blood) to establish a baseline in hypoglycemia cases. This is currently a standard procedure for this test. In diabetics however the baseline has to be the opposite, to increase glucose levels in the blood, in order to prove type 2 DM. Fasting may not yield the intended results. Do not fast when testing C-peptide in diabetics. This can be a problem for those of us who have forms of diabetes where our beta cells are able to secrete basal insulin (the slow steady drip of insulin that keeps our blood sugar normal in the fasting state) but are unable to secrete insulin in response to the rising blood glucose that happens at meal time.
_
A very low C-peptide result is the definitive way to diagnose severe Type 1 diabetes–though many people with Type 1 will continue to have a low level of C-peptide in their blood for years after diagnosis as good control started soon after at Type 1 diagnosis appears to keep a small number of their beta cells alive. To derive more meaning for the results of a C-peptide test the lab must know whether it was taken fasting or not fasting and what the blood glucose level was at the moment it was taken. In theory, a high fasting blood sugar with a high C-peptide value should point to Type 2 diabetes primarily caused by insulin resistance. That is because the high C-peptide value would suggest a lot of insulin was being produced but insulin resistance was keeping it from lowering blood sugar. In contrast, a C-peptide value that was normal or below normal taken at the same time as a high fasting glucose would suggest a form of Type 2 where failing beta cells rather than insulin resistance was the primary thing raising blood sugar. In theory, testing C-peptide very few years should also give you some idea of whether or not your beta cells are slowly failing.
_
Normal or High C-Peptide test results may be Good News:
There is some recent research that suggests that C-peptide rather than being an inert by-product of insulin synthesis is, in fact, important for preventing diabetic complications. So any measure that prevents b-cell demise would in long run prevent diabetic complications.
_
The following are some purposes of C-peptide testing:
•A C-peptide test is not ordered to help diagnose diabetes, but when a person has been newly diagnosed with diabetes, it may be ordered by itself or along with an insulin level to help determine how much insulin a person’s pancreas is still producing (endogenous insulin).
•In type 2 diabetes, the body is resistant to the effects of insulin (insulin resistance) and it compensates by producing and releasing more insulin, which can also lead to beta cell damage. Type 2 diabetics usually are treated with oral drugs to stimulate their body to make more insulin and/or to cause their cells to be more sensitive to the insulin that is already being made. Eventually, because of the beta cell damage, type 2 diabetics may make very little insulin and require injections. Any insulin that the body does make will be reflected in the C-peptide level; therefore, the C-peptide test can be used to monitor beta cell activity and capability over time and to help a health practitioner determine when to begin insulin treatment.
•People who are on insulin therapy, regardless of the source of the insulin, may develop antibodies to insulin. These typically interfere with tests for insulin, making it nearly impossible to directly evaluate endogenous insulin production. In these cases, C-peptide measurement is a useful alternative to testing for insulin.
•C-peptide measurements can also be used in conjunction with insulin and glucose levels to help diagnose the cause of documented hypoglycemia and to monitor its treatment. Symptoms of hypoglycemia may be caused by excessive supplementation of insulin, alcohol consumption, inherited liver enzyme deficiencies, liver or kidney disease, or by insulinomas.
•The C-peptide test may be used to help diagnose Insulinomas. These are tumors of the islet cells in the pancreas that can produce uncontrolled amounts of insulin and C-peptide and can cause acute episodes of hypoglycemia. C-peptide tests may be used to monitor the effectiveness of insulinoma treatment and to detect recurrence.
•Sometimes a C-peptide test may be used to help evaluate a person diagnosed with metabolic syndrome, a set of risk factors that includes abdominal obesity, increased blood glucose and/or insulin resistance, unhealthy blood lipid levels, and high blood pressure (hypertension).
•Rarely, when someone has had his pancreas removed or has had pancreas islet cell transplants, intended to restore the ability to make insulin, C-peptide levels may be used to verify the effectiveness of treatment and continued success of the procedure.
_
Postprandial C-peptide Index: The Best Marker of Beta Cell Function? 2014 study:
Authors have recently reported that postprandial serum C-peptide to plasma glucose ratio, called postprandial C-peptide index, was associated with the future need for insulin therapy. C-peptide is a well-established marker of beta cell function. C-peptide is split from insulin and co-secreted with insulin from beta cells at the same molar ratio. While ~50% of insulin is extracted by the liver, C-peptide is not extracted by the liver. Therefore, the measurement of C-peptide more directly reflects beta cell secretory rate compared with insulin, independent of hepatic clearance. Moreover, one of the advantages of C-peptide measurement in clinical settings is that C-peptide can be assessed in patients treated with insulin, which cross-reacts with the insulin measurement. In their study, interestingly, postprandial C-peptide index was superior for predicting the need for future insulin therapy compared with fasting C-peptide index or urinary C-peptide excretion. More recent studies have also confirmed their findings. It has also been reported that postprandial serum C-peptide, but neither fasting C-peptide nor urinary C-peptide, independently predicted successful switching from insulin therapy to liraglutide monotherapy, suggesting that postprandial C-peptide has the best ability to predict treatment efficacy among other C-peptide indices. The reason for this is unknown; however, several possibilities have been postulated. Postprandial C-peptide likely reflects maximal insulin secretion induced by a combination of postprandial hyperglycemia and incretin effects, compared with fasting C-peptide. Besser et al. have reported that in patients with T1DM, 90 min postprandial C-peptide value is highly correlated with peak C- peptide value and area under the curve (AUC) of C-peptide during a mixed-meal tolerance test, which is the gold-standard measure of endogenous insulin secretion in T1DM. Intriguingly, it has been reported that postprandial C-peptide index was more closely correlated with beta cell mass in humans compared with fasting C-peptide index. These results suggest that postprandial C-peptide reflects maximal beta cell functional capacity. Disposition index is a measure of beta cell function adjusted for insulin sensitivity, which reflects “true” beta cell function. Recently, it has been reported that postprandial C-peptide index, but not fasting measures such as fasting C-peptide index and homeostasis model assessment (HOMA)-β is significantly correlated with disposition index assessed by hyperglycemic and euglycemic clamp. The authors speculate that postprandial C-peptide index reflects systemic insulin sensitivity, i.e., mainly glucose disposal in peripheral tissues, whereas fasting C-peptide index or HOMA-β reflects hepatic insulin sensitivity.
Recently, the usefulness of postprandial urinary C-peptide creatinine ratio has also been proposed. It has been reported that postprandial urinary C-peptide creatinine ratio was highly correlated with C-peptide and insulin AUC during an oral glucose tolerance test (OGTT) in non-diabetic subjects. Thong et al. have reported that postprandial urinary C-peptide creatinine ratio weakly correlated with HbA1c change after liraglutide treatment. However, since in patients with chronic kidney disease (CKD), urinary C-peptide creatinine ratio did not correlate with serum C-peptide or insulin, this method cannot be applied to patients with CKD. Authors have also reported that postprandial C-peptide index is inversely associated with future glycemic control and glycemic variability, independently of anti-diabetic medication, indicating a critical role of beta cell function in the management of T2DM. In addition to the UKPDS, recent large scale prospective trials with newer treatment options have also shown that treatment failure is associated with beta cell dysfunction. Therefore, it is important to assess beta cell function in the management of T2DM, and the establishment of better markers of beta cell function in clinical settings is warranted. In this context, postprandial C-peptide index appears to be a simple and useful marker of beta cell function in clinical settings. Remaining issues for the use of postprandial C-peptide index include the timing of sampling, components of the meal, and the impact of renal impairment on the measurement. Future research will be needed to clarify these issues to establish the best marker of beta cell function.
_
Urinary C-Peptide: A simple measure of Integrated Insulin Production with emphasis on the effects of body size, diet, and Corticosteroids: 2015 study:
C-Peptide is secreted from the β-cell in equimolar quantities with insulin. Since a fraction of C-peptide is excreted in the urine, measurement of C-peptide in timed urine collections is a simple indirect measure of integrated insulin production. Normal subjects were studied to determine the effects of diet and oral prednisone on urinary C-peptide excretion. In subjects on a defined diet, there is a positive correlation of urinary C peptide with body weight. When insulin production is increased after oral prednisone, there is also a positive correlation with body mass index and percent ideal body weight. Prednisone increases plasma glucose, immunoreactive insulin, and serum and urinary C-peptide levels beginning 8–12 h after oral administration. This effect of prednisone is most marked in the postprandial state. Diets high in carbohydrate and protein result in significantly more insulin production, as measured by urinary C peptide, than isocaloric diets with low protein or carbohydrate composition.
_________
_________
Hyperinsulinemia:
Hyperinsulinemia is a condition in which there are excess levels of insulin circulating in the blood than expected relative to the level of glucose. While it is often mistaken for diabetes or hyperglycaemia, hyperinsulinemia can result from a variety of metabolic diseases and conditions. While hyperinsulinemia is often seen in people with early stage type 2 diabetes mellitus, it is not the cause of the condition and is only one symptom of the disease. Hyperinsulinemia can be seen in a variety of conditions including diabetes mellitus type 2, in neonates and in drug induced hyperinsulinemia. It can also occur in congenital hyperinsulism, including nesidioblastosis. Hyperinsulinemia is associated with hypertension, obesity, dyslipidemia, and glucose intolerance. These conditions are collectively known as Metabolic syndrome. This close association between hyperinsulinemia and conditions of metabolic syndrome suggest related or common mechanisms of pathogenicity. Hyperinsulinemia has been shown to “play a role in obese hypertension by increasing renal sodium retention”.
_
_
In type 2 diabetes, the cells of the body become resistant to the effects of insulin as the receptors which bind to the hormone become less sensitive to insulin concentrations resulting in hyperinsulinemia and disturbances in insulin release. With a reduced response to insulin, the beta cells of the pancreas secrete increasing amounts of insulin in response to the continued high blood glucose levels resulting in hyperinsulinemia. In insulin resistant tissues, a threshold concentration of insulin is reached causing the cells to uptake glucose and therefore decreases blood glucose levels. Studies have shown that the high levels of insulin resulting from insulin resistance might enhance insulin resistance.
_
Hyperinsulinemia in neonates can be the result of a variety of environmental and genetic factors. If the mother of the infant is a diabetic, and does not properly control her blood glucose levels, the hyperglycemic maternal blood can create a hyperglycemic environment in the fetus. To compensate for the increased blood glucose levels, fetal pancreatic beta cells can undergo hyperplasia. The rapid division of beta cells results in increased levels of insulin being secreted to compensate for the high blood glucose levels. Following birth, the hyperglycemic maternal blood is no longer accessible to the neonate resulting in a rapid drop in the newborn’s blood glucose levels. As insulin levels are still elevated this results in hyperinsulinemia. To treat the condition, high concentration doses of glucose are given to the neonate as required maintaining normal blood glucose levels. The hyperinsulinemia condition subsides after one to two days.
_
Effects of hyperinsulinemia:
•May lead to hypoglycemia or diabetes type 2
•Increased risk of PCOS
•Increased synthesis of VLDL (hypertriglyceridemia) and reduction in HDL
•Hypertension (insulin increases sodium retention by the renal tubules)
•Coronary Artery Disease (increased insulin damages endothelial cells)
•Increased risk of cardiovascular disease
•Weight gain, since insulin promotes the storage of fat
•Lower cellular levels of magnesium, a mineral that is essential for keeping your blood vessels relaxed and your blood circulation efficient
•Increased amounts of inflammatory compounds in your blood, which can cause direct physical damage to your blood vessel walls and encourage the development of blood clots which can lead to heart attacks and strokes
•Possibly a higher risk for cancer due to insulin’s ability to contribute to cell proliferation.
_
Treatment of hyperinsulinemia:
Treatment is typically achieved via diet and exercise, although Metformin may be used to reduce insulin levels in some patients (typically where obesity is present). Another method used to lower excessively high insulin levels is Cinnamon as was demonstrated when supplemented in clinical human trials. A low carbohydrate diet is particularly effective in reducing hyperinsulinism. A healthy diet that is low in simple sugars and processed carbohydrates, and high in fiber, and vegetable protein is often recommended. This includes replacing white bread with whole-grain bread, reducing intake of foods composed primarily of starch such as potatoes, and increasing intake of legumes and green vegetables, particularly soy. Regular monitoring of weight, blood sugar, and insulin are advised, as hyperinsulinemia may develop into diabetes mellitus type 2. It has been shown in many studies that physical exercise improves insulin sensitivity. The mechanism of exercise on improving insulin sensitivity is not well understood however it is thought that exercise causes the glucose receptor GLUT4 to translocate to the membrane. As more GLUT4 receptors are present on the membrane more glucose is taken up into cells decreasing blood glucose levels which then causes decreased insulin secretion and some alleviation of hyperinsulinemia. Another proposed mechanism of improved insulin sensitivity by exercise is through AMPK activity. The beneficial effect of exercise on hyperinsulinemia was shown in a study by Solomon et al. (2009), where they found that improving fitness through exercise significantly decreases blood insulin concentrations.
__________
__________
Exogenous insulin:
Insulin is used to treat a number of disease including diabetes and its acute complications such as diabetic ketoacidosis and hyperosmolar hyperglycemic states. It is also used along with glucose to treat high blood potassium levels.
The three groups of exogenous insulin:
There are three groups of insulin – animal, human (not from humans but produced synthetically to match human insulin) and analogues (where the chemical structure of human insulin has been changed to make the insulin work quicker or last longer). Nowadays, most people use human insulin and insulin analogues, although a small number of people still use animal insulin because they have some evidence that they otherwise lose their awareness of hypos, or they find animal insulin works better for them.
The three types of insulin are:
•Natural animal insulins – derived from pancreases of pigs or cattle
•Synthetic ‘human’ insulins – made in a laboratory by genetic modification: Biosynthetic human insulin for clinical use is manufactured by recombinant DNA technology by inserting human insulin gene into the bacterium Escherichia coli to produce synthetic “human” insulin. Biosynthetic human insulin has increased purity when compared with extractive animal insulin, enhanced purity reducing antibody formation. Researchers have succeeded in introducing the gene for human insulin into plants as another method of producing insulin (“biopharming”) in safflower. This technique is anticipated to reduce production costs.
•Insulin analogues – the latest insulin made by genetically modifying GM ‘human’ insulin: Several analogues of human insulin are available. These insulin analogues are closely related to the human insulin structure, and were developed for specific aspects of glycemic control in terms of fast action (prandial insulins) and long action (basal insulins).
__
Reasons why insulin might not be prescribed sooner if you have type 2 diabetes may include:
•Doctors are concerned about you getting low blood sugar.
•You might not be willing to start insulin or might not have the ability to give yourself injections.
•Primary care providers may perceive that insulin therapy is too complex to manage in their busy practice.
•Prescribing information may be vague, and the provider may be unsure about initial dosing, titration and what kind of insulin to start you on, which may delay making the necessary transition from oral medications to insulin
_
Human insulin versus insulin analogues:
A meta-analysis in 2007 of numerous randomized controlled trials by the international Cochrane Collaboration found only a minor clinical benefit of treatment with long-acting insulin analogues (including two studies of insulin detemir) for patients with diabetes mellitus type 2 while others have examined the same issue in type 1 diabetes. Subsequent meta-analyses undertaken in a number of countries and continents have confirmed Cochrane’s findings. Also if patients know that they are using a different type of insulin, might behave differently (such as testing blood glucose levels more frequently, for example), which leads to bias in the study results, rendering the results inapplicable to the diabetes population at large. Numerous studies have concluded that any increase in testing of blood glucose levels is likely to yield improvements in glycemic control, which raises questions as to whether any improvements observed in the clinical trials for insulin analogues were the result of more frequent testing or due the drug undergoing trials. In 2008, the Canadian Agency for Drugs and Technologies in Health (CADTH) found, in its comparison of the effects of insulin analogues and biosynthetic human insulin, that insulin analogues failed to show any clinically relevant differences, both in terms of glycemic control and adverse reaction profile. A 2011 study indicated that across Type 1 and 2 diabetes, for both rapid- and long-acting analogue insulins, there is no clear advantage over human insulins, with inconsistent statistically significant advantages and lack of clinically important benefits. Analogue insulins have not consistently been demonstrated to be cost-effective, and uncertainty remains regarding the association between analogue insulins and increased cancer risk.
_
There are several problems with insulin as a clinical treatment for diabetes:
•Mode of administration.
•Selecting the ‘right’ dose and timing. Usually one unit of insulin is ~15grams of CHO.
•Selecting an appropriate insulin preparation (typically on ‘speed of onset and duration of action’ grounds).
•Adjusting dosage and timing to fit food intake timing, amounts, and types.
•Adjusting dosage and timing to fit exercise undertaken.
•Adjusting dosage, type, and timing to fit other conditions, for instance the increased stress of illness.
•Variability in absorption into the bloodstream via subcutaneous delivery
•The dosage is non-physiological in that a subcutaneous bolus dose of insulin alone is administered instead of combination of insulin and C-peptide being released gradually and directly into the portal vein.
•It is simply a nuisance for patients to inject whenever they eat carbohydrate or have a high blood glucose reading.
•It is dangerous in case of mistake (most especially ‘too much’ insulin).
_
The central problem for those requiring external insulin is picking the right dose of insulin and the right timing. Physiological regulation of blood glucose, as in the non-diabetic, would be best. Increased blood glucose levels after a meal is a stimulus for prompt release of insulin from the pancreas. The increased insulin level causes glucose absorption and storage in cells, reduces glycogen to glucose conversion, reducing blood glucose levels, and so reducing insulin release. The result is that the blood glucose level rises somewhat after eating, and within an hour or so, returns to the normal ‘fasting’ level. Even the best diabetic treatment with synthetic human insulin or even insulin analogs, however administered, falls far short of normal glucose control in the non-diabetic. Complicating matters is that the composition of the food eaten affects intestinal absorption rates. Glucose from some foods is absorbed more (or less) rapidly than the same amount of glucose in other foods. In addition, fats and proteins cause delays in absorption of glucose from carbohydrates eaten at the same time. As well, exercise reduces the need for insulin even when all other factors remain the same, since working muscle has some ability to take up glucose without the help of insulin. Because of the complex and interacting factors, it is, in principle, impossible to know for certain how much insulin (and which type) is needed to ‘cover’ a particular meal to achieve a reasonable blood glucose level within an hour or two after eating. Non-diabetics’ beta cells routinely and automatically manage this by continual glucose level monitoring and insulin release. All such decisions by a diabetic must be based on experience and training and, further, specifically based on the individual experience of the patient. But it is not straightforward and should never be done by habit or routine. With some care however, it can be done reasonably well in clinical practice. For example, some patients with diabetes require more insulin after drinking skim milk than they do after taking an equivalent amount of fat, protein, carbohydrate, and fluid in some other form. Their particular reaction to skimmed milk is different from other people with diabetes, but the same amount of whole milk is likely to cause a still different reaction even in that person. Whole milk contains considerable fat while skimmed milk has much less. It is a continual balancing act for all people with diabetes, especially for those taking insulin.
_
Basal bolus insulin:
Patients with insulin-dependent diabetes typically require some base level of insulin (basal insulin), as well as short-acting insulin to cover meals (bolus insulin). Maintaining the basal rate and the bolus rate is a continuous balancing act that people with insulin-dependent diabetes must manage each day. This is normally achieved through regular blood tests, although continuous blood sugar testing equipment (Continuous Glucose Monitors or CGMs) are now becoming available which could help to refine this balancing act once widespread usage becomes common.
_
Insulin has 3 characteristics:
•Onset is the length of time before insulin reaches the bloodstream and begins lowering blood glucose.
•Peaktime is the time during which insulin is at maximum strength in terms of lowering blood glucose.
•Duration is how long insulin continues to lower blood glucose.
Each type of insulin has an onset, a peak, and a duration time.
_
The commonly used types of insulin are:
•Fast-acting: Includes the insulin analogues aspart, lispro, and glulisine. These begin to work within 5 to 15 minutes and are active for 3 to 4 hours. Most insulins form hexamers which delay entry into the blood in active form; these analog insulins do not, but have normal insulin activity. Newer varieties are now pending regulatory approval in the U.S. which are designed to work rapidly, but retain the same genetic structure as regular human insulin.
•Short-acting: Includes regular insulin which begins working within 30 minutes and is active about 5 to 8 hours.
•Intermediate-acting: Includes NPH insulin which begins working in 1 to 3 hours and is active 16 to 24 hours.
•Long acting: Includes the analogues glargine and detemir, each of which begins working within 1 to 2 hours and continue to be active, without major peaks or dips, for about 24 hours, although this varies in many individuals.
•Ultra-long acting: Currently only includes the analogue degludec, which begins working within 30–90 minutes, and continues to be active for greater than 24 hours.
•Combination insulin products – Includes a combination of either fast-acting or short-acting insulin with a longer acting insulin, typically an NPH insulin. The combination products begin to work with the shorter acting insulin (5–15 minutes for fast-acting, and 30 minutes for short acting), and remain active for 16 to 24 hours. There are several variations with different proportions of the mixed insulins (e.g. Novolog Mix 70/30 contains 70% aspart protamine [akin to NPH], and 30% aspart.)
__
Insulin administration:
Insulin is usually taken as subcutaneous injections by single-use syringes with needles, via an insulin pump, or by repeated-use insulin pens with disposable needles. Inhaled insulin is also available in U.S. market now. Absorption via the pulmonary route is similar to or faster than subcutaneous injection of rapid-acting insulin, and the effect is longer lasting. Variability of absorption within an individual is small, but substantially affected by intercurrent respiratory conditions; and smoking increases bioavailability. Bioavailability is generally low, however, typically 5-10% of an injection. Thus, pulmonary insulin will be an expensive option. With increased use of insulin, and greater preference for intensive administration schedules, there is much interest in the prospect of needle-free injections and other methods of painless delivery. For individuals who remain uncomfortable with the very fine needles currently available, several needle-free delivery systems can be purchased in the UK, such as the Vitajet and the mhi-500. These devices produce a fine stream of insulin under high pressure to the skin. They can readily deliver small amounts of insulin, but usage so far has been limited. Other approaches to transdermal delivery by iontophoresis and microporation remain in research development. Buccal insulin delivery is under clinical development by Generex and Lilly under the names of Oralin® in Europe and Oralgen® in the USA. Administration is similar to an angina spray, with an aerosol (RapidMist®) delivering a fine spray directly onto the buccal mucosa. Nasal epithelium provides a very low bioavailability of insulin and is sensitive to intercurrent local infections and irritation. Surfactants and other absorption enhancers increase bioavailability, but have not led to a viable delivery system due to disturbances to the integrity of the nasal epithelium. Subcutaneous injections of insulin do not replicate the normal physiological delivery of insulin into the portal circulation so that the liver is exposed to higher insulin concentrations than the periphery. Preferential delivery to the liver is under investigation using insulin linked to thyroxine (thyroxyl-insulin). This is highly protein bound and cannot therefore cross the endothelium, which prevents access to peripheral tissues. However, the liver has open sinusoids which allow free access to the hepatocytes. Insulin has been linked to an organic complex that can be selectively broken by the catalytic aldolase antibody 38C2. The organic complex inactivates the insulin, but administration of the antibody can then be used to provide controlled release of active insulin.
___
Oral insulin:
Unlike many medicines, insulin cannot be taken orally at the present time. Like nearly all other proteins introduced into the gastrointestinal tract, it is reduced to fragments (even single amino acid components), whereupon all ‘insulin activity’ is lost. There has been some research into ways to protect insulin from the digestive tract, so that it can be administered in a pill. So far this is entirely experimental. Oral delivery of insulin may significantly improve the quality of life of diabetes patients who routinely receive insulin by the subcutaneous route. In fact, compared with this administration route, oral delivery of insulin in diabetes treatment offers many advantages: higher patient compliance, rapid hepatic insulinization, and avoidance of peripheral hyperinsulinemia and other adverse effects such as possible hypoglycemia and weight gain. However, the oral delivery of insulin remains a challenge because its oral absorption is limited. The main barriers faced by insulin in the gastrointestinal tract are degradation by proteolytic enzymes and lack of transport across the intestinal epithelium. Several strategies to deliver insulin orally have been proposed, but without much clinical or commercial success. Many attempts to produce an oral insulin formulation have been reported over the last three decades, including liposome-encapsulated insulin and various polymer-wrapped insulins. None has provided both an adequate shield against proteolytic digestion and an effective aid to absorption to give reasonable bioavailability. A recent promising contribution is an alkyl-polyethylene-glycol conjugated hexyl-insulin (HIM2) in clinical trial with Nobex and GSK. Others include a particulate ‘nanocubicle’ emulsion and a polylactide microcapsule. Protein encapsulation into nanoparticles is regarded as a promising alternative to administer insulin orally because they have the ability to promote insulin paracellular or transcellular transport across the intestinal mucosa.
_
Shaking or rolling ‘suspended’ insulin vial:
NPH insulin comes as an insoluble mixture of crystals and liquid and must be resuspended before injection. Researchers at Perugia University in Italy wanted to know what difference it would make if patients didn’t resuspend their NPH insulin by tipping the insulin pen 20 times before injection – and if they didn’t shake it by tipping it back and forth, whether it would matter how they held the needle during the injection. As it turned out, everything mattered. One of the researchers, Dr. Geremia B. Bolli says he was surprised by “the high variability of effects on lowering of blood glucose depending as to whether the NPH pen is properly resuspended or not, and (if it’s not resuspended), even great differences depending on the position of the pen, i.e., horizontal, vertical with tip up or down.” “The same NPH appears as a different insulin in each of these conditions,” The research team reported in the journal Diabetes Care that insulin levels could vary by as much as 23% and blood sugar control could vary by as much as 62% depending on whether NPH insulin is shaken before injecting, or not.
However the “dangers” of over agitating a vial of insulin are:
1) Insulin threads/clumps can be formed and that reduces the overall amount effective of insulin in the vial, thus reducing any doses drawn from that vial.
2) Frosting – over agitation of insulin can cause insulin to stick to the vial, “frosting” it like ice on a window. This frosting may not be visible to the naked eye but it reduces the overall effective insulin in the vial.
3) Micro bubbles could form in the vial and, when drawn into the syringe, would reduce the amount of insulin in the dose.
Consequently, for insulins which require mixing – those in which the insulin is not in solution but is, instead, suspended in the fluid, the safest method of mixing is to roll the vial back and forth with a side to side slanting motion to move the fluid around the entire vial.
___
Matching Insulin to Carbohydrate:
In this approach, insulin dose is based on two factors: the amount of carbohydrate eaten and the difference between actual blood glucose and target blood glucose. Patients work with two ratios: an insulin to carbohydrate and a correction factor, along with a blood glucose target. The insulin to carbohydrate ratio indicates how many carbohydrates one unit of insulin will provide coverage for and the correction factor describes the glucose lowering power of one unit of insulin. If an individual had a insulin to carb ratio of 10 and a correction factor of 50, it would mean:
•She would take one unit of insulin for every 10 grams of carbohydrate eaten
•One unit of insulin would lower her blood glucose 50 points.
This method is more physiological and more flexible than Fixed Dose or Sliding Scale, because it allows for differences both in glucose levels and carbohydrate intake. To use this method you must have adequate arithmetic skills and be willing to count carbohydrate grams or servings. But Insulin to Carb Matching is often worth the extra work because it allows for much more flexibility in food choices while enabling you to become more actively involved in your care.
_
Commonly Used Terms in Insulin Therapy:
| Term | Definition | Calculation |
| Augmentation | Use of either basal or bolus insulin to help improve glucose control in patients with partial beta-cell failure | 0.3 unit per kg |
| Replacement | Use of basal and bolus insulin to control blood glucose when endogenous insulin production is minimal or absent | 0.6 to 1.0 unit per kg |
| Carbohydrate ratio | The number of units of insulin needed to cover for a certain number of grams of carbohydrates ingested | 500 divided by total daily insulin (usually about 1 unit per 10 g) |
| Correction (sensitivity) | How much 1 unit of insulin is expected to decrease the patient’s blood glucose level; when the blood glucose level is above predefined targets, short-acting insulin may be added to the bolus dose or given separately between meals | 1,500 divided by total daily insulin giving figure in mg/dl per unit of insulin |
In my hypothesis I suggested sub-optimal daily insulin for T2DM. That is 0.3 unit/kg body weight daily and is much less than insulin dose for T1DM where there is no endogenous insulin production. Sub-optimal insulin administration will protect b-cells and allow endogenous insulin generation in T2DM for long time.
___
When should Insulin Therapy be started?
Indications for insulin therapy and when to begin it are poorly defined in guidelines and still subject to individual judgment based on a wide range of opinion. Personal beliefs and experience, familiarity with the use of the different insulin preparations and delivery systems, individual preference, patient needle phobia, concern about chronic hyperinsulinemia, risk of hypoglycemia, and difficulties in controlling body weight are some of the many considerations regarding insulin therapy. Each one of these factors can be weighted differently between doctors and between people with diabetes. The expert group proposed that one way to rationalize the approach to insulin treatment could be to consider some clinical scenarios. These could be as follows: 1) the time of diagnosis or early thereafter; 2) in the presence of other emerging medical conditions; and 3) in the course of routine ambulatory diabetes management.
__
Conventional insulin therapy has these characteristics:
1. Insulin injections of a mixture of regular (or rapid) and intermediate acting insulin are performed two times a day, or to improve overnight glucose, mixed in the morning to cover breakfast and lunch, but with regular (or rapid) acting insulin alone for dinner and intermediate acting insulin at bedtime (instead of being mixed in at dinner).
2. Meals are scheduled to match the anticipated peaks in the insulin profiles.
3. The target range for blood glucose levels is higher than is desired in the intensive regimen.
4. Frequent measurements of blood glucose levels were not used.
The down side of this method is that it is difficult to achieve as good results of glycemic control as with intensive insulin therapy. The advantage is that, for diabetics with a regular lifestyle, the regime is less intrusive than the intensive therapy.
_
Intensive insulin therapy:
Intensive insulin therapy is an aggressive treatment approach designed to control your blood sugar levels. Intensive insulin therapy requires close monitoring of blood sugar levels and multiple doses of insulin. In intensive insulin therapy, the amount of insulin can be adjusted flexibly and spontaneously based on a person’s blood sugar levels, how much they eat and how physically active they are. Regular blood sugar monitoring is essential here. You can either inject yourself several times a day or use an insulin pump. To cover your basic insulin needs, longer-acting insulin can be injected once or twice a day. This is often called “basal insulin.” Short-acting insulin is injected before meals to deal with the carbohydrates that are eaten. This is known as a “bolus dose” or “mealtime insulin.” People who have an insulin pump only use short-acting insulin. The pump regularly delivers small amounts of insulin to continuously cover the body’s basic needs. One goal of intensive insulin therapy is to keep blood sugar at almost normal levels. It also aims to allow people to lead a flexible and spontaneous life. There is no need to eat meals at fixed times or make sure you always eat the same amount of carbohydrates. Instead, you inject as much insulin as you need at the time. The amount of insulin needed will also depend on your blood sugar levels, the time of day and whether you plan to be physically active. In DCCT/EDIC clinical trial of 1983, the treatment target of intensive glycaemic control was HbA1c between 4.05–6.05%, FPG between 3.88–6.05 mmol/L and PPG at or below 9.99 mmol/L for T1DM. Different authors have different target glycemic level for intensive insulin therapy. Generally it closely resembles glucose values of normal people. Fortunately, research is ongoing into new methods of blood sugar monitoring and insulin delivery that may make it easier and reduce the risk of intensive insulin therapy. One such method is a closed-loop insulin delivery system that combines continuous blood sugar monitoring with insulin pump delivery. Intensive insulin therapy can prevent or slow the progression of long-term diabetes microvascular complications. However intensive insulin therapy may lead to frequent hypoglycemia and weight gain.
___
Insulin index:
The Insulin Index is a measure used to quantify the typical insulin response to various foods. The index is similar to the Glycemic Index and Glycemic Load, but rather than relying on blood glucose levels, the Insulin Index is based upon blood insulin levels. This measure can be more useful than either the Glycemic Index or the Glycemic Load because certain foods (e.g., lean meats and proteins) cause an insulin response despite there being no carbohydrates present, and some foods cause a disproportionate insulin response relative to their carbohydrate load. The Insulin Index is not the same as a glycemic index, which is all relative to eating 100% glucose, as this index is relative to eating white bread (glycemic index of ~70 to 75).
_
Holt et al. have noted that the glucose and insulin scores of most foods are highly correlated, but high-protein foods and bakery products that are rich in fat and refined carbohydrates “elicit insulin responses that were disproportionately higher than their glycemic responses.” They also conclude that insulin indices may be useful for dietary management and avoidance of non-insulin-dependent diabetes mellitus and hyperlipidemia. Consideration of insulin scores may be relevant to the dietary management and pathogenesis of non-insulin-dependent diabetes mellitus and hyperlipidemia and may help increase the accuracy of estimating preprandial insulin requirements. The reason to be concerned about the Insulin Index is that insulin is essentially a storage hormone, evolved to put aside excess carbohydrate calories and store them in the form of fat in case of future famine. In other words, when we eat too much carbohydrate, we’re sending a hormonal message, via insulin, to the body (actually, to the adipose cells). The message is “Store Fat” It is even worse than that. Not only do increased insulin levels tell the body to store fat; they also tell it to not release any stored fat. This makes it impossible for you to use your own stored body fat for energy. So the excess carbohydrates in your diet not only make you fat, they make sure you stay fat.
_____
Islet transplants:
Type 1 diabetes results from the destruction of insulin-producing cells in the islets of the pancreas. About one third of people with Type 1 diabetes each year will experience a ‘severe’ hypo – meaning that they need someone else to help them. Severe hypos can occur in anyone taking insulin, but they are more likely to occur in people who have had diabetes for more than 15 years and those who are unable to recognise when their blood glucose is low (a problem known as hypoglycaemic unawareness). For these people, an islet transplant can be a life-changing, and sometimes a life-saving, therapy. Islet cell transplantation involves extracting islet cells from the pancreas of a deceased donor and implanting them in the liver of someone with Type 1. This minor procedure is usually done twice for each transplant patient, and can be performed with minimal risk using a needle under local anaesthetic. As of 2008, the UK is fortunate to have the first government-funded islet transplant programme in the world. As of 2013, 95 islet transplants had been performed in 65 people in the UK.
_
Who might be suitable for an islet transplant?
•People with Type 1 diabetes who have experienced two or more severe hypos within the last two years, and have impaired awareness of hypoglycaemia.
•People with Type 1 diabetes and a functioning kidney transplant who experience severe hypos and impaired hypoglycaemia awareness or poor blood glucose control despite the best medical therapy.
Who might not be suitable for an islet transplant?
•People who need a lot of insulin (e.g. more than 50 units per day for a 70kg person).
•People who weigh over 85kg.
•People with poor kidney function.
_
What are the potential benefits?
Islet transplants have been shown to reduce the risk of severe hypos. Results from UK islet transplant patients showed that the frequency of hypos was reduced from 23 per person per year before transplantation to less than one hypo per person per year afterwards. Islet transplants usually also lead to improved awareness of hypoglycaemia, less variability in blood glucose levels, improved average blood glucose, improved quality of life and reduced fear of hypos. Long-term results are good and are improving all the time. For example, the majority of transplant patients can now expect to have a functioning transplant after six years and some people have had more than 10 years of clinical benefit.
_
What risks are involved?
Islet transplants involve a small but increased risk of certain cancers, severe infections and other side-effects related to the medication (immune-suppressants) needed to prevent the islets from being rejected by the body (which is the same medication used by people who receive other kinds of transplants). Islet transplants are unsuitable for people who are desperate to stop their insulin injections. If freedom from insulin injections is achieved, this is usually short-lived, and most people who receive an islet transplant continue to take low-dose insulin therapy. Therefore, islet transplants should not be seen as a cure for diabetes.
_
Infusing patients with islet cells as a treatment for diabetes is not a new idea, however researchers said keeping these cells alive and functioning to moderate blood glucose levels has been difficult. These cells are generally injected into the liver, which is not the most conducive for the survival of the new cells, often rendering the treatment temporary and limiting its use to patients with severe cases of type 1 diabetes. So doctors used a new method of injection, by delivering the cells to the omentum, a tissue that covers the abdominal organs, with a biodegradable scaffolding. The scaffold is made by combining patient’s blood with thrombin, a chemical used to control bleeding during surgery. The two substances form a gel-like material that sticks to the omentum and keeps the islets in place. The gel is absorbed by the body over time, leaving the islet cells in place while new blood vessels form and send oxygen and nutrients to them so they can do their job.
______
Scientists create insulin-producing cells that may treat diabetes:
Currently one of the most promising therapies in the fight against diabetes is the replacement of beta cells. In the replacement therapy for type 1 diabetes, researchers from Université Catholique de Louvain have earlier shown that human pancreatic duct-derived cells (HDDCs) are an attractive source of cells. The cells are found in the adult pancreas and are progenitor cells – cells that have a tendency to differentiate into specific types of cells. In a study, researchers reprogrammed HDDCs to behave like beta cells and secrete insulin within the pancreas, whilst responding to glucose. The researchers used messenger RNA (mRNA) of a transcription factor – a protein that controls which genes are turned off or on in the genome – called MAFA. The mRNA is transformed into protein before binding to cellular DNA in order to orchestrate the changes in cellular functions. Researchers have already developed a mouse model that allows them to transplant their manufactured cells into the diabetic mice and follow-up on their disease.
______
Endogenous insulin vis-à-vis exogenous insulin:
_
Pharmacokinetics and -dynamics of insulin absorption:
Exogenously administered insulin will never be able to exactly mimic the effects of insulin endogenously released by the pancreas. The main reason is that the pancreas releases insulin into the portal vein, so that the insulin passes the liver first. There, more than 50% of insulin is extracted, and as a result hepatic exposure to insulin is high, and peripheral (muscle, fat) exposure to insulin is low. When exogenous insulin is administered -whether i.v., s.c., i.m. or otherwise- it is distributed throughout the circulation, exposing the peripheral organs to relatively high and the liver to relatively low levels of insulin. Another problem is that the pancreas releases insulin in small bouts at short intervals, in direct response to the ambient level of glucose, whereas the dose of exogenously administered insulin is predetermined. The precise effects over time of the various exogenous insulins are also dependent on their pharmacokinetic and pharmacodynamic characteristics and the mode of administration.
_
In normal physiology, β-cell insulin secretion is coupled immediately with changes in the plasma glucose level. The secretory response is rapid (within a minute or two), and because the half-life of insulin is ~5 min, there is little lag time in the glucose regulatory system. Endogenously secreted insulin goes via the portal vein to the liver, where 30–80% of it is either metabolized or used. The portal vein-to-peripheral arterial insulin ratio is ~2:1. The administration of insulin exogenously eliminates the rapid regulation of plasma glucose, since the insulin must be taken up slowly and without regulation from the subcutaneous injection site. The kinetics are determined by the nature of the injected insulin formulation. Additionally it is necessary to create hyperinsulinemia in the periphery to achieve adequate insulin in the liver (portal-to-peripheral insulin levels ~1:2) to appropriately regulate hepatic glucose production and/or glucose uptake.
_
_
Endogenous insulin secretion is regulated by exogenous insulin in two ways:
1. Glucose dependent:
Exogenous insulin reduces blood glucose level which in turn reduces endogenous insulin secretion.
2. Glucose independent:
Exogenous insulin reduces endogenous insulin secretion by negative feedback loop.
Support for inhibition of endogenous insulin secretion by exogenous insulin: A study by Ratzmann KP, Schulz B.
In order to investigate whether or not insulin exerts any influence on endogenous insulin secretion, 6 non-obese healthy volunteers were connected to the artificial beta-cell (BIOSTATOR) for 16 hours. After an overnight fast 0.4 U of intermediate acting insulin (Monotard MC/NOVO INDUSTRIES) per kg body weight were injected subcutaneously. The computer program was set to maintain steady-state plasma glucose concentrations at fasting levels by a variable glucose infusion. This approach is able to prevent completely hypoglycemic episodes. Endogenous insulin secretion was evaluated by measuring plasma C-peptide concentrations. Whereas plasma insulin levels increased markedly in response to insulin injection at the same time C-peptide levels declined correspondingly, indicating an inhibition of insulin secretion. Thus, our results provided support for a negative feedback regulation of insulin secretion by insulin itself.
_
In a nutshell, exogenous insulin administration reduces endogenous insulin secretion by glucose dependent and glucose independent mechanisms and as a corollary, reduces load on b-cells of pancreas.
_________
_________
Insulin vis-à-vis leptin:
High blood glucose levels cause repeated surges in insulin, and this causes one’s cells to become “insulin-resistant” which leads to further high levels of insulin and diabetes. The same happens with leptin. It has been shown that as sugar gets metabolized in fat cells, fat releases surges in leptin, and these surges result in leptin-resistance just as it results in insulin-resistance. Because both insulin resistance and leptin resistance are caused by the same thing: poor diet, the only known way to re-establish proper leptin (and insulin) signalling is to prevent those surges, and the only known way to do that is via proper diet and supplements.
_
In terms of diabetes, leptin may even supersede insulin in importance, for new research is revealing that in the long run glucose and therefore insulin levels may be largely determined by leptin. It had been previously believed that the insulin sensitivity of muscle and fat tissues were the most important factor in determining whether one would become diabetic or not. Elegant new studies are showing that the brain and liver are most important in regulating a person’s blood sugar levels especially in type 2 or insulin resistant diabetes. It should be noted that leptin plays a vital role in regulating your brain’s hypothalamic activity which in turn regulates much of a person’s “autonomic” functions; those functions that you don’t necessarily think about but which determines much of your life (and health) such as body temperature, heart rate, hunger, the stress response, fat burning or storage, reproductive behavior, and newly discovered roles in bone growth and blood sugar levels. Another very recent study reveals leptin’s importance in directly regulating how much sugar that the liver manufactures via gluconeogenesis. Many chronic diseases are now linked to excess inflammation such as heart disease and diabetes. High leptin levels are very pro-inflammatory, and leptin also helps to mediate the manufacture of other very potent inflammatory chemicals from fat cells that also play a significant role in the progression of heart disease and diabetes. A great deal of research indicates that the failure of insulin and leptin to register properly in your brain, along with falling adiponectin levels, creates a highly inflammatory state of affairs that is the prime cause of worsening blood sugar regulation and eventual type 2 diabetes. It has long been known that obesity greatly increased risk for many chronic diseases including heart disease and diabetes, but no one really knew why.
_______
_______
Diabetes mellitus:
As of 2014, an estimated 387 million people have diabetes worldwide, with type 2 diabetes making up about 90% of the cases. This represents 8.3% of the adult population, with equal rates in both women and men. From 2012 to 2014, diabetes is estimated to have resulted in 1.5 to 4.9 million deaths each year. Diabetes at least doubles a person’s risk of death. The number of people with diabetes is expected to rise to 592 million by 2035. The global economic cost of diabetes in 2014 was estimated to be $612 billion USD. In the United States, diabetes cost $245 billion in 2012.
_
Diabetes is defined clinically as an increase in plasma glucose levels. Plasma glucose levels are determined by the sensitivity of tissues to insulin and by the amount of insulin secreted by the pancreatic β cells. A number of factors, including lack of exercise, obesity, and visceral fat are major determinants of insulin resistance. Type 2 diabetes (T2DM) is characterized by insulin resistance and beta cell dysfunction. Recent evidence has emerged that beta cell dysfunction is a common pathogenetic feature of both type 1 and type 2 diabetes, and T2DM never develops without beta cell dysfunction. Therefore, treatment of T2DM should aim to restore beta cell function. The rapid rise in rates of T2DM echoes a similar rise in rates of obesity, which causes insulin resistance and places an increased insulin secretory demand on pancreatic β cells. While diabetes is diagnosed clinically by elevated plasma glucose levels, loss of β-cell function is progressive over time and β-cell dysfunction is far advanced by the time diabetes is diagnosed. Methods for preserving or restoring β-cell function are important for the prevention and treatment of T2DM. Interventions that reduce body fat or that change fat biology provides the best evidence for slowing or arresting the deterioration of β-cell function that causes T2DM. These interventions should form the basis of interventions to prevent and treat T2DM, particularly early in its course. In the UK Prospective Diabetes Study (UKPDS) it was shown that beta cell function in patients with T2DM progressively declined after the diagnosis, and treatment failure was associated with beta cell dysfunction. Since these results suggest that beta cell function predicts treatment efficacy, beta cell function should be assessed for the management of T2DM in clinical settings.
_
Defective beta-cell function, insulin resistance found in newly diagnosed diabetes: a 2015 study:
Defective beta-cell function and insulin resistance are commonly found among patients with newly diagnosed type 2 diabetes, and their presence alone or in combination may be related to phenotype, according to study results presented at the 51st European Association for the Study of Diabetes Annual Meeting. Dauriz and colleagues evaluated 712 glutamic acid decarboxylase antibody (GADA)–negative, drug-naïve patients with newly diagnosed type 2 diabetes from the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS) to determine beta-cell function and insulin resistance. Researchers evaluated insulin sensitivity by euglycemic insulin clamp and beta-cell function by modelling of the prolonged (5 hours) oral glucose tolerance test (OGTT)–derived glucose/C-peptide curves. Overall, 89.8% of participants had beta-cell dysfunction, 87.8% had insulin resistance and 19.7% had only one defect. Eleven percent of participants had isolated beta-cell dysfunction, and 8.9% had isolated insulin resistance. Nearly 80% of participants had coexisting beta-cell dysfunction and insulin resistance. At the time of diagnosis of type 2 diabetes, defective beta-cell function comorbid with insulin resistance was present in a large proportion of VNDS patients,” Dauriz said. “A milder accompanying metabolic phenotype characterized those with only one pathogenic defect. The presence of either insulin resistance or beta-cell dysfunction alone seems to be sufficient to lead to overt diabetes.”
_
Patients with type 1 diabetes depend on external insulin (most commonly injected subcutaneously) for their survival because the hormone is no longer produced internally. Patients with type 2 diabetes are often insulin resistant and, because of such resistance, may suffer from a “relative” insulin deficiency. Some patients with type 2 diabetes may eventually require insulin if dietary modifications or other medications fail to control blood glucose levels adequately. Over 40% of those with Type 2 diabetes require insulin as part of their diabetes management plan.
______
Type 2 diabetes is characterized by progressive decline in pancreatic beta cell function and persistent insulin resistance. Type 2 diabetes is characterized by hyperglycemia caused by defects in insulin secretion (impaired β-cell function) and insulin action (insulin resistance by the liver and muscle tissue). These defects occur early in the course of the disease and are often present before diagnosis. Decreased beta cell mass and amyloid deposits in the islet are the pathological hallmark of the disease. Preserved beta cell function is the most important determinant of glucose disposal, even after adjustment for insulin sensitivity, which might modulate beta cell function. Besides, beta cell dysfunction is also responsible for several functional abnormalities in type 2 diabetes. These defects include:
• Impaired first and second phase insulin response.
• Decreased pulsatile and oscillatory insulin response.
• Increased release of Pro-insulin like molecules
• Impaired ability to compensate for superimposed insulin resistance
______
______
As you can see in the figure above, at the onset of T2DM, insulin resistance is established, and although serum insulin is high, b-cell failure is set in.
_____
_____
The figure above shows that in established T2DM, insulin levels during oral glucose tolerance test (OGTT) is lower than normal people and people with impaired glucose tolerance (IGT) indicating advanced b-cell failure.
_____
_____
Why Fasting Blood Sugar Levels are often the last to deteriorate in T2DM:
As you become more diabetic, and your second phase insulin response grows weaker, it may take four or five hours for your beta-cells to secrete enough insulin to bring your blood sugar level down to its fasting level. And, in fact, during the day your blood sugar may never get back to its fasting level because the glucose coming in from your next meal comes into the bloodstream before the glucose from the previous meal has completely cleared. Only at night, while you are sleeping, may your beta-cells finally secrete enough insulin to get your blood sugar down low enough that you wake up with a normal fasting blood sugar. However, since it took all the insulin your beta-cells could make to get back to that normal blood sugar and they will have had no chance to store any extra insulin to take care of your breakfast. As soon as you eat breakfast, blood glucose will rise, and once again your beta-cells will have to spend many hours trying to bring it back down. Eventually, even the long hours of the night will not be enough time for your beta-cells to produce enough insulin to bring your blood sugar back to normal, and now, perhaps a decade after you achieved diabetic post-meal numbers, you will finally start seeing diabetic fasting blood sugar levels. This process explains why for many people who become diabetic–particularly middle-aged women, the fasting blood sugar level is the very last measurement to become abnormal. Only when a whole night isn’t long enough for your beta-cells to bring your blood sugar back down to normal or near-normal levels will you become diabetic by a fasting blood sugar test.
_
Higher than 110 mg/dl fasting blood sugar and its risk:
A study of 344 people published in November 2007 examined the relationship of their fasting blood sugar to the presence of metabolic syndrome. They broke their study subjects into four groups by fasting blood sugar rather than the usual three. The groups were: Normal (<101 mg/dl or 5.6 mmol/L), FBG1 (101-109 mg/dl 5.6 and 6.0 mmol/L), FBG2 (110-124 mg/dl 6.1-6.9 mmol/L) and Diabetic (>125 mg/dl 7 mmol/L). This is unusual, because most studies will lump people with fasting blood sugars between 100 and 110 mg/dl (the FBG1 group) with the either the normal or the pre-diabetic group. By breaking that group out separately it was possible to discover a relationship between fasting blood sugar and health that might have otherwise been missed. And that is exactly what happened. This study found that people in the FBG2 group had the same cardiovascular and metabolic syndrome incidence as people with diabetes. Which backs up what we have seen above: for most people, the deterioration of fasting blood sugar over 110 mg/dl occurs only after many years of exposure to very high post-meal blood sugars and by the time fasting blood sugar deteriorates this much, diabetic complications, most notably heart disease are well established. In contrast, the intermediate FBG1 group was a lot more normal as far as cardiovascular and metabolic syndrome markers went. This suggests that the fasting blood sugar between 100 mg/dl and 110 mg/dl, should be treated as a major watershed and that if you test into this fasting blood sugar range on a screening, you should take aggressive steps to lower your post-meal blood sugars, because you have caught the abnormality early enough to be able to prevent cardiovascular deterioration.
_
Type 2 Diabetes and the Aging Pancreatic Beta Cell: Diabetes and Age:
An increased incidence of diabetes is observed with age, and there are many possibly reasons for this. One of these is that the beta cell has reduced proliferative capacity and in diabetic individuals this is further confounded by higher rates of beta cell apoptosis. The currently known underlying mechanisms behind the reduction in beta cell proliferation observed with age include reduced expression of cell cycle activators, increased expression of cell cycle inhibitors, reduced pdx1 expression, and increased amylin aggregation. Studying aging in the non-diabetic rodent and human models is currently a developing field; therefore very few broad conclusions can be drawn. Further study in these areas is important as they could indicate targets for preventing or slowing the progression of diabetes with age.
_______
______
Oral hypoglycaemic agents (OHA):
The emerging epidemic of type 2 diabetes, coupled with finite health resources, requires the treatment of hyperglycaemia to be simple and efficiently managed. Type 2 diabetes is a progressive disease and eventually almost all patients will require insulin to maintain good glycaemic control. Knowing when and how to start insulin in general practice is central to the optimal management of type 2 diabetes. The need to start insulin therapy in a newly diagnosed patient with type 2 diabetes is relatively uncommon. It should be considered when there is considerable weight loss, severe symptoms of hyperglycaemia or the presence of significant ketonuria. Many of these patients can be converted back to oral drugs once glycaemic control has been established and there is some recovery of pancreatic β cell function. A more common problem is when and how to commence insulin in patients with type 2 diabetes who are in ‘secondary failure’. The term secondary failure refers to the ‘failure’ of oral hypoglycaemic drugs to maintain glycaemic control. The United Kingdom Prospective Diabetes Study (UKPDS) clearly showed that most people with type 2 diabetes will experience progressive pancreatic β cell dysfunction, despite excellent control. The secondary failure rate in this study was 44% after six years of diabetes. Since the time of the UKPDS, targets for glycaemic control have become increasingly stringent so secondary failure of oral hypoglycaemic drugs now occurs much sooner and is almost invariable.
_
Traditionally metformin plus a sulfonylurea has been the mainstay of oral treatment. Patients understandably often want to know whether they should try adding a third drug or begin insulin. The addition of acarbose can usually only decrease the HbA1c by 0.5% at best, so one would only consider its use if a slight improvement in control is needed. Repaglinide and sulfonylureas should not be used in combination, as they are both insulin secretagogues. The response to therapy with a thiazolidinedione (pioglitazone or rosiglitazone) can be more profound with improvements in HbA1c of 1-2%.The decision whether to start insulin or to add a thiazolidinedione would depend on factors such as patient acceptance, coexisting conditions (thiazolidinediones are contraindicated in oedematous states and heart failure) and access to medicines. At this stage it matters less which drug or ‘pathway’ is used, but more that the patient’s glycaemic target is reached. Sometimes it is necessary to let patients try triple oral drug therapy. If nothing else, it serves to convince them that insulin is indeed necessary. In this situation, it is important not to delay insulin therapy for more than a few months. A trial of triple therapy for two months should be sufficient to assess whether it is likely to be effective or not.
_
Almost all patients with type 2 diabetes will eventually fail to respond adequately to oral hypoglycaemic drugs and will require insulin therapy. A regimen of bedtime intermediate-acting insulin in combination with daytime oral drugs is acceptable to patients, simple to start and results in rapid improvement in glycaemic control. It can be started safely in general practice and is the most practical way of implementing insulin in the face of a worldwide epidemic of type 2 diabetes.
_
Clinical Efficacy of Oral Hypoglycemic Agents:
| Class of hypoglycemic agents | Reduction in HbA1c(%) | Reduction in FPG (mg per dL [mmol per L]) |
| Sulfonylureas | 0.8 to 2.0 | 60 to 70 [3.3 to 3.9] |
| Meglitinides | 0.5 to 2.0 | 65 to 75 [3.6 to 4.2] |
| Biguanides | 1.5 to 2.0 | 50 to 70 [2.8 to 3.9] |
| Thiazolidinediones | 0.5 to 1.5 | 25 to 50 [1.4 to 2.8] |
| Alpha-glucosidase inhibitors | 0.7 to 1.0 | 35 to 40 [1.9 to 2.2] |
DPP4 inhibitors lower A1C levels by about 0.5% to 1 %, so they are not big blockbusters in terms of A1c reduction. Reductions in A1C have been reported when SGLT-2 inhibitors were used ranged from 0.58 % to 0.89% with dapagliflozin compared with 0.23% with placebo. For every 1 percent reduction in results of HbA1c, the risk of developing eye, kidney, and nerve disease (microvascular complications) is reduced by 40 percent while the risk of heart attack (macrovascular complications) is reduced by 14 percent.
________
Consensus treatment of T2DM:
Consensus statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) 2012:
_
DPP-4-i, DPP-4 inhibitor; Fx’s, bone fractures; GI, gastrointestinal; GLP-1-RA, GLP-1 receptor agonist; HF, heart failure; SU, sulfonylurea. a, Consider beginning at this stage in patients with very high HbA1c (e.g., ≥ 9 %). b, Consider rapid-acting, nonsulfonylurea secretagogues (meglitinides) in patients with irregular meal schedules or who develop late postprandial hypoglycemia on sulfonylureas. c, additional potential adverse effects and risks. d, Usually a basal insulin (NPH, glargine, detemir) in combination with noninsulin agents. e, Certain noninsulin agents may be continued with insulin (see text). Consider beginning at this stage if patient presents with severe hyperglycemia (≥16.7–19.4 mmol/L [≥300–350 mg/dL]; HbA1c ≥10.0 %–12.0 %) with or without catabolic features (weight loss, ketosis, etc.).
_
The Continuation of Oral Medications with the Initiation of Insulin Therapy in Type 2 Diabetes: A Review of the Evidence: 2010:
The combination of oral medications with insulin is inevitable in the treatment of type 2 diabetes. Unfortunately, there are no consensus statements available to guide the physician on the continuation or discontinuation of specific therapies. The clinician treating the type 2 diabetic patient must be aware of the literature regarding use of oral medications with insulin with a specific focus on glucose lowering efficacy, hypoglycemia, weight gain, and cost. The use of metformin should be encouraged and continued once insulin is initiated given overall benefits in glucose reduction, no major increase risk of hypoglycemia, amelioration of weight gain, and the overall cost to the patient. Use of sulfonylureas with the combination of basal insulin should be considered for overall reduction in glucose with close monitoring for signs and symptoms of hypoglycemia. Continuation of sulfonylurea with the addition of bolus insulin cannot be supported by the current literature due to overall lack of efficacy, increased weight, and risk of hypoglycemia. Use of insulin in combination with TZDs is beneficial in the reduction of A1C and total insulin dose. Continuation should be patient specific with consideration for risk of fluid retention and the burden of the cost of the therapy to the patient. Use of incretin therapy with insulin cannot be recommended at this time due to lack of sufficient data and the overall cost of therapy to the patient. Use of alpha-glucosidase inhibitors in combination with insulin have been shown to be beneficial in regards to glucose control and weight stability. The use of alpha-glucosidase inhibitors with insulin have to be balanced with the gastrointestinal side effects and the cost to the patient.
_
There has been a recent shift from a uniform treatment targeting HbA1c to a patient centered approach due to disappointing results of intensified glucose control in megatrials such as VADT, ADVANCE, and ACCORD. In addition, morbidity and mortality has been substantially reduced since the UKPDS leading to an overestimation of the absolute risk for cardiovascular complications in randomized controlled trials. With substantial progress in prevention of cardiovascular complications, patients with type 2 diabetes now survive long enough to face diabetes-related complications and cancer risk. This requires rethinking of antidiabetic treatment strategies as exemplified by a recent consensus statement of the EASD and ADA, calling for a more patient centered treatment.
_________
_________
Insulin resistance (inverse of insulin sensitivity):
_
Insulin resistance (IR) is defined as a reduced tissue response to the effects of insulin on glucose metabolism, including reduced glucose uptake in muscle and fat tissue, reduced liver glycogen formation and accelerated liver glucose production. Other insulin-stimulated processes may or may not be reduced. Insulin is a key regulator of glucose homeostasis. Insulin resistance is established by genetic and environmental factors. Insulin resistance leads to impaired glucose tolerance, and plays an important pathophysiological role in the development of diabetes. There is considerable racial, individual and tissue variation in the spectrum of effects of insulin resistance. In fat cells, for example, insulin also stimulates triglyceride formation and inhibits fat breakdown. If these processes are not insulin resistant, hyperinsulinemia will stimulate fat accumulation and weight gain. If these processes are insulin resistant, weight gain will not be a feature of insulin resistance. The liver may have a greater, lesser or similar degree of insulin resistance compared with the muscles. Insulin resistance is primarily a complex genetic condition which increases slowly with age and is exacerbated at puberty and during pregnancy. It is aggravated by oral contraceptives, presumably as a result of liver exposure to pregnancy-levels of sex hormones immediately after absorption into the portal circulation. It is also aggravated by obesity, partly from the increased levels of free fatty acids and probably from locally acting hormones (i.e., cytokines such as tumour necrosis factor-a and resistin) secreted from enlarged fat cells within the muscle mass. The main reason for the rapid rise in the manifestations of insulin resistance throughout the world is the enormous reduction in exercise associated with modern life, along with the development of foods that are both high in energy and low in fibre. Rapidly digested starch in many processed foods leads to an exaggerated postprandial insulin response, aggravating obesity and insulin resistance.
_
Insulin resistance can be broadly defined as a subnormal biological response to normal insulin concentrations. By this definition, it may pertain to many biological actions of insulin in many tissues of the body. Typically, however, in clinical practice, insulin resistance refers to a state in which a given concentration of insulin is associated with a subnormal glucose response. The term first came into use several years after the introduction of insulin therapy in 1922 to describe occasional diabetic patients who required increasingly large doses of insulin to control hyperglycemia. Most of these patients developed insulin resistance secondary to antibodies directed against the therapeutic insulin, which at that time was both impure and derived from non-human species. So insulin resistance that time was arbitrarily defined as the requirement of 200 or more units of insulin per day to attain glycemic control and to prevent ketosis. Antiinsulin antibodies are rare in patients treated with recombinant human insulin, and the spectrum of clinical disorders in which insulin resistance plays a major role has changed markedly. Insulin resistance, rather than being a rare complication of the treatment of diabetes, is now recognized as a component of several disorders, including the following:
●Extreme insulin-resistance syndromes, such as the type B syndrome with autoantibodies against the insulin receptor, and rare inherited disorders, such as Leprechaunism with insulin-receptor mutations and the lipodystrophic states.
●Impaired glucose tolerance and type 2 diabetes mellitus.
●Obesity, stress, infection, uremia, acromegaly, glucocorticoid excess, and pregnancy, which cause secondary insulin resistance.
●Common disorders such as the metabolic syndrome, hypertension, hyperlipidemia, coronary artery disease, the polycystic ovary syndrome (PCOS), and ovarian hyperthecosis, in which the mechanism of the associated hyperinsulinemia is unknown.
_
Insulin resistance plays a major patho-physiological role in type 2 diabetes and is tightly associated with major public health problems including obesity, hypertension, coronary artery disease, dyslipidemias, and a cluster of metabolic and cardiovascular abnormalities that define the metabolic syndrome. A global epidemic of obesity is driving the increased incidence and prevalence of type 2 diabetes and its cardiovascular complications. Insulin resistance is commonly associated with visceral adiposity, glucose intolerance, hypertension, dyslipidemia, hypercoaguable state, endothelial dysfunction, and/or elevated markers of inflammation. Therefore, the presence of these clinical abnormalities is usually characteristic of an insulin resistant state. In addition to clinical manifestations of the “Insulin Resistance Syndrome,” insulin resistance predisposes to accelerated cardiovascular disease (CVD). Therefore, it is of great importance to develop tools for quantifying insulin sensitivity/resistance in humans that may be used to appropriately investigate the epidemiology, pathophysiological mechanisms, outcomes of therapeutic interventions, and clinical course of patients with insulin resistance. Combinations of causes are common. For example, obesity, the most common cause of insulin resistance, is associated mainly with postreceptor abnormality but is also associated with a decreased number of insulin receptors.
_____
Insulin sensitivity:
Whereas insulin resistance is the measure of how much insulin is required to achieve a given glucose metabolic effect, insulin sensitivity is the measure of how much glucose metabolic effect is achieved from a given amount of insulin. That is, insulin sensitivity is the inverse of insulin resistance:
Insulin sensitivity = 1/insulin resistance
Insulin resistance = 1/ insulin sensitivity
_
Insulin sensitivity is a general phenomenon in the body, and can be measured a few ways through studies. The pancreas (an organ that regulates blood sugar) secretes insulin in response to high blood sugar, and cells (like muscle or fat cells) can absorb blood sugar when stimulated by insulin. Insulin sensitivity is the relationship between how much insulin needs to be produced in order to deposit a certain amount of glucose. You are insulin sensitive if a small amount of insulin needs to be secreted to deposit a certain amount of glucose, and insulin resistant if a lot of insulin needs to be secreted to deposit the same amount of glucose. Insulin sensitivity is seen as the opposite of insulin resistance, is a major risk factor for the development of Type II diabetes.
Types of Insulin Sensitivity:
There are three main types of insulin sensitivity; peripheral insulin sensitivity, hepatic insulin sensitivity, and pancreatic insulin sensitivity.
1. Peripheral insulin sensitivity is how readily body cells in your periphery tissue, such as muscle and fat, can absorb glucose; either on their own (muscle can absorb glucose when contracted) or when insulin stimulates them. It is the most well-known form of insulin resistance.
2. Hepatic insulin sensitivity is related to the process of gluconeogenesis, the production of new blood sugar. Usually inflammatory factors prevent insulin from acting in the liver via inducing insulin resistance, and insulin’s actions are unable to tell the liver to ‘stop’ producing glucose.
3. Pancreatic insulin sensitivity is the functioning of the cells that secrete insulin, the beta-cells. B-cells themselves are sensitive to insulin by possessing receptors that interact with insulin.
Insulin sensitivity is how effective the body is as using insulin to reduce elevated blood glucose levels, with a greater efficacy being more ‘sensitivity’ and poorer efficacy being more ‘resistant’. When the body becomes too poor at using insulin to reduce blood glucose levels, type II diabetes ensues.
_
Plasma insulin and whole body glucose disposal:
The above figure shows schematic representation of concentration-response relationships between plasma insulin concentrations and insulin-mediated whole body glucose disposal. Curve a: normal insulin sensitivity and responsiveness. Curve b: rightward shift in insulin concentration-response curve. This represents decreased insulin sensitivity (increased EC50) with normal insulin responsiveness. Curve c: Decreased insulin sensitivity (increased EC50) and reduced insulin responsiveness. Curve d: Leftward shift in the insulin concentration-response response curve. This represents increased insulin sensitivity (decreased EC50) with normal insulin responsiveness.
_
Clues to Insulin Resistance:
•Mid-body weight gain. People who carry extra weight in their abdomen often have insulin resistance. Some folks are genetically programmed in an “apple” shape, with a full belly and sculpted, thin arms and legs. Other folks notice a change in middle age, with a preferential middle body weight gain not present when they were younger. Everyone’s waist should be smaller than their hips. For women a waist measurement should be no more than 75% of your hip size, and for men no more than 90% of your hip size. So for a man with a 36 inch waist: if he can’t find some place lower on his body that measures 40 inches, there’s a good chance he has some insulin resistance. (Being overweight with a normal waist to hip size suggests that your weight is well-proportioned, balanced with muscle, and less likely to cause insulin resistance or subsequent diabetes.)
•Blood sugar problems are probably the most common indicator of excessive insulin secretion that can then lead to insulin resistance. If you get very agitated or excited after eating sugar, or find yourself sleepy or hungry again soon after meals, you might have the cycle referred to as “sugar blues”: first your high carbohydrate meal causes your blood sugar to climb sky high (which might feel like a mild caffeine effect) followed by your body making lots of insulin, causing your blood sugar to fall (sleepiness). The repetition of that cycle leads to insulin resistance.
•High blood pressure. High levels of insulin are associated with increased levels of aldosterone which causes fluid retention and elevated blood pressure.
•Fatty liver is being found even in children and teens and is always a serious indicator of insulin resistance. Insulin resistant fatty liver is almost always reversible with careful food choices.
•Skin changes can indicate insulin resistance, including excessive skin tags and darkened skin in skin fold areas.
•Menstrual and reproductive problems in women, particularly polycystic ovarian syndrome, can indicate insulin resistance, even in women who are not overweight. Women with a history of gestational diabetes, or babies weighing over 9 pounds, have a significantly increased risk of developing insulin resistance.
•Cataracts are increased in patients with both type 1 and type 2 diabetes, and early cataracts (before the age of 70) can be seen in people with insulin resistance.
•Frequent infections can both indicate and increase pre-existing insulin resistance.
_
Acanthosis Nigricans and insulin resistance:
Acanthosis Nigricans, or the dark patchy skin on the neck, underarms, groin and knuckles, is caused by extra skin cells in these areas that increase pigmentation, thus making the skin appear darker. There are several reasons for the presence of excessive skin cells. The most common cause is due to hormonal imbalance, especially of the hormone insulin that regulates blood glucose levels. When insulin fails in its primary function of lowering blood glucose level efficiently (insulin resistance), the body produces it in excess. The increased insulin interacts with skin cells, especially those in body folds, causing them to multiply, thereby making the skin look darker. Other reasons for excessive skin cells could be genetics, drug intake, or in rare cases, cancer. A sedentary lifestyle, excessive consumption of unhealthy, high-carb junk food, lack of exercise and obesity are all factors that could trigger this condition. Several health-related issues such as diabetes, hyperthyroidism and Polycystic Ovary Syndrome (PCOS) could also cause Acanthosis Nigricans.
_
Just need one Blood Test:
If your FPG is less than 100 mg% and fasting insulin is less than 5 milliunit/liter, you are not insulin resistant.
_
Insulin sensitivity and secretion are reciprocally related; thus, insulin resistance results in increased insulin secretion to maintain normal glucose and lipid homeostasis. The mathematical relation between sensitivity and secretion is curvilinear or hyperbolic. Several mediators are thought to signal the pancreatic B cells to respond to insulin resistance; failure of the signals or of the B cells to adapt adequately in relation to insulin sensitivity results in inappropriate insulin levels, impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and type 2 diabetes. These potential signalling mediators include glucose, free fatty acids, autonomic nerves, fat-derived hormones (e.g., adiponectin), and the gut hormone glucagonlike peptide 1 (GLP-1). GLP-1 is an incretin hormone that stimulates insulin secretion, causes B-cell mitosis while inhibiting apoptosis, inhibits glucagon secretion, and delays gastric emptying with overall antidiabetic effects. The mechanisms responsible for insulin resistance syndromes include genetic or primary target cell defects, autoantibodies to insulin, and accelerated insulin degradation. Given that glucose and lipid metabolism largely depend on mitochondria to generate energy in cells, mitochondrial dysfunction may play an important role in the development of insulin resistance and associated complications. Obesity, the most common cause of insulin resistance, is associated with a decreased number of receptors and with postreceptor failure to activate tyrosine kinase. While adiposity and insulin resistance are related, they are not necessarily synonymous, and each may make independent and different contributions to increasing the risk of cardiovascular disease. Leptin and ghrelin are two hormones that have a major influence on energy balance. Leptin is a long-term regulator of energy balance, suppressing food intake and thereby inducing weight loss, while ghrelin is a fast-acting hormone, seemingly playing a role in meal initiation. Obese subjects tend to be leptin resistant; their circulating levels of the anorexigenic hormone leptin are increased, but the levels of the orexigenic hormone ghrelin are decreased.
_
Insulin resistance plays a major pathogenic role in the development of the metabolic syndrome, which may include any or all of the following:
• Hyperinsulinemia
• Type 2 diabetes or glucose intolerance
• Central obesity
• Hypertension
• Dyslipidemia that includes high triglyceride levels
• Low HDL-C level and small, dense low-density lipoprotein (LDL) particles
• Hypercoagulability characterized by an increased plasminogen activator inhibitor 1 (PAI-1) level
_
The figure below shows how insulin resistance, hyperinsulinemia and metabolic syndrome increase cardiovascular risk:
_
Inflammation and adipocytokines probably play some role in the etiopathogenesis of metabolic syndrome. Increased levels of the acute-phase inflammatory marker C-reactive protein (CRP) are related to insulin resistance and the metabolic syndrome, suggesting a role for chronic, low-grade inflammation. In a number of prospective studies, increased levels of CRP predict the development of diabetes and cardiovascular disease. Reduced serum levels of adiponectin (a hormone made by fat tissue) and elevated leptin concentration are also features of conditions associated with the metabolic syndrome or cardiovascular disease. Omentin, a novel adipokine, is a protein expressed and secreted from visceral but not subcutaneous adipose tissue that increases insulin sensitivity in adipocytes. Plasma levels of omentin-1, the major circulating isoform, are inversely correlated with body mass index (BMI), waist circumference, leptin levels, and insulin resistance syndrome and are positively correlated with adiponectin and high-density lipoprotein (HDL) levels. Insulin resistance, the compensatory hyperinsulinemia, and other components are associated with increased risk of cardiovascular disease; endothelial dysfunction is a prominent feature of insulin resistance syndrome. Type 2 diabetes is characterized by increased hepatic glucose output, increased peripheral resistance to insulin action (due to receptor and postreceptor defects), and impaired insulin secretion. In skeletal muscle, various abnormalities, including defective glucose transport, may cause insulin resistance. Glucose transporter 4 (GLUT-4) is the main insulin-responsive transporter. Insulin and IGFs are important regulators of ovarian function. Insulin resistance and hyperinsulinemia are thought to be responsible for the hyperandrogenism that is characteristic of the polycystic ovary syndrome (PCOS). Other distinct manifestations of insulin resistance syndrome or related conditions involve various organs, as well as the skin. Two major variants of insulin receptor abnormalities associated with acanthosis nigricans have been described, the classic type A insulin resistance syndrome, which is due to an absent or dysfunctional receptor, and type B insulin resistance syndrome, which results from autoantibodies to the insulin receptor. Both syndromes are associated with hyperinsulinemia. Hypoglycemia may still occur in some individuals with insulin resistance syndrome because of an agonist effect of autoantibodies on the insulin receptor. In some patients with insulin-binding antibodies, hypoglycemia may occur when insulin dissociates from the antibodies several hours after a meal.
_
Novel Mechanism of Insulin Resistance in Type 2 Diabetes: a 2015 study:
Now, scientists from Sweden’s Karolinska Institute have discovered a mechanism that explains how insulin-producing cells can be insulin resistant and insulin sensitive at the same time. The findings are being published in the journal Cell Reports, and may lead to future novel treatment strategies for type 2 diabetes. Insulin is critical in lowering blood glucose concentration. Individuals with type 2 diabetes suffer from insulin resistance and this means that their cells/organs are insensitive to insulin. In type 2 diabetes the body tries to compensate by producing more insulin, and also by increasing the number of insulin-producing cells. Finding new treatment strategies is only possible by gaining a greater understanding of what happens in the body of a diabetic patient. One scientific challenge is to explain how a cell/organ at the same time can be insulin resistant in one biological function and insulin sensitive in another. Drs Barbara Leibiger and Ingo Leibiger, both members of Professor Per-Olof Berggren’s research group at the Department of Molecular Medicine and Surgery, Karolinska Institutet, are particularly interested in the insulin-producing beta cells. “The beta cell must have insulin to work properly,” says Barbara Leibiger, PhD, Associate Professor, and lead author of the current study. “In a person with diabetes, the beta cells become insensitive to insulin.” The researchers have previously shown that the beta cell has two receptors with different biological functions, insulin receptor A and insulin receptor B. In the current study, they found that under diabetic conditions, even though insulin receptor B is insulin insensitive for one signalling pathway, insulin can under these conditions instead activate a different signalling pathway, leading to beta cell proliferation. The researchers also identified the factor, PI3K-C2α, which caused the switch from one signalling pathway to another. “The results are important since it explains how the beta cell can go from a differentiated state to a proliferative state,” says Ingo Leibiger, PhD, Associate Professor, who co-supervised the current study with Professor Berggren. “This means that the cells change from being glucose-responsive to instead increase in number.”
_
Puberty and Insulin Resistance:
A Transient insulin resistant state occurs during puberty and is part of normal human development. Insulin resistance increases immediately at the beginning of puberty, peaks at mid-puberty, and then declines to nearly prepubertal levels by early adulthood. Girls are more insulin resistant than boys during puberty which is related in part to differences in adiposity between the sexes. Glucose homeostasis is maintained during puberty through compensatory hyperinsulinemia. While resistance to insulin’s effect on glucose metabolism is present, insulin-stimulated protein metabolism is normal. Therefore, a physiologic consequence of insulin resistance during puberty may be augmentation of rapid growth by promotion of protein anabolism. The causes of physiological insulin resistance during puberty have not been definitively established. Gonadal sex steroids do not appear to play a central role. There is strong evidence, however, that GH/IGF-I activity contributes to pubertal insulin resistance. GH and IGF-I levels increase and then decrease during puberty, following a pattern similar to that of insulin resistance. A significant association between IGF-I levels and pubertal insulin resistance has been demonstrated in many studies.
____
Sleep and insulin resistance:
Sleep deprivation and T2DM:
Figure above provides a schematic representation of the mechanisms by which sleep deprivation may result in increased risk of insulin resistance and diabetes either by directly affecting parameters of glucose tolerance or indirectly via a disturbance in appetite regulation, leading to increased food intake and weight gain. Studies show that untreated sleep problems, especially sleep apnea, can increase the risk of obesity, insulin resistance, and type 2 diabetes. Night shift workers may also be at increased risk for these problems.
_
Sleep duration associated with insulin sensitivity in men, beta cell function in women: a 2015 study:
Men who are short or long sleepers have lower insulin sensitivity when compared with men who sleep about 7 to 8 hours per night, according to study findings presented at the 51st European Association for the Study of Diabetes Annual Meeting. In a cross-sectional study evaluating the association between sleep duration, insulin sensitivity and beta-cell function in healthy men and women, researchers found no association between sleep duration and insulin sensitivity in women, but noted an association between short and long sleep duration with increased beta cell function. Researchers found that men who were short or long sleepers had lower insulin sensitivity compared to men who sleep about 8 hours, observing a positive, U-shaped association between sleep duration and insulin sensitivity. No such association was observed in women. In women, researchers observed a negative, U-shaped association between sleep duration and beta cell glucose sensitivity. No such association was observed in men.
_______
_______
Differentiation between the Insulin Resistance Syndrome and type 2 diabetes:
_
The figure below differentiates T2DM from insulin resistance:
As long as there is adequate insulin response to insulin resistance, T2DM does not occur. In other words, inadequate insulin response (b-cell failure) is the hallmark of T2DM.
_
Sensitivity to insulin-mediated glucose disposal varies widely in the population at large. When insulin resistant individuals cannot maintain the degree of hyperinsulinemia needed to overcome the resistance, type 2 diabetes develops. However, even when insulin resistant individuals secrete enough insulin to remain nondiabetic, they remain at increased risk to develop a cluster of abnormalities. As depicted in figure above, some individuals with the Insulin Resistance Syndrome will eventually develop diabetes because they lose the ability to secrete the large amount of insulin needed to overcome the insulin resistance. However, while the majority of insulin resistant individuals do not become frankly diabetic, they remain at increased risk to develop CVD (cardiovascular diseases) and all of the other clinical consequences of insulin resistance/compensatory hyperinsulinemia. Since CVD is also the major cause of morbidity and mortality in patients with type 2 diabetes, and because the vast majority of individuals with CVD and/or type 2 diabetes are also insulin resistant, it could be argued that the differentiation between the two clinical syndromes outlined in figure above is inappropriate. However, the diagnosis of type 2 diabetes is relatively straightforward and based primarily upon the degree of hyperglycemia that increases risk of diabetic microangiopathy. An approach to identifying those individuals who do not have diabetes, but who do have the Insulin Resistance Syndrome, is not so simple.
_______
Events associated with insulin resistance:
The figure above shows number of clinical events observed, as a function of insulin resistance tertile at baseline. CA, cancer; CHD, coronary heart disease; HT, hypertension; SSPG, steady-state plasma glucose (determined by continuous infusion of somatostatin, insulin, and glucose during a 75-g oral glucose tolerance test). Plasma glucose and insulin concentrations were drawn every 10 min during the last 30 min of the infusion. The average of the last four measurements was used to define the SSPG. There were 28 events in the highest tertile (SSPG >7.8 mmol/l), 12 in the intermediate tertile (SSPG > 4.4 < 7.8 mmol/l), and none in the most insulin-sensitive tertile (SSPG <4.4 mmol/l). As you can see that hypertension, coronary heart disease and cancer risk increased with insulin resistance without development of T2DM.
____
Insulin resistance and CVS risk:
Relationship between obesity, insulin resistance, and coronary heart disease risk: a 2002 study:
Conclusions:
The results presented have reaffirmed the fact that the greater the BMI, the more insulin-resistant the individual. At the same time results show that overweight/obese individuals can be insulin-sensitive and that normal weight subjects can be insulin-resistant. In addition, authors have differentiated between the relative impact of overweight/obesity and insulin resistance on coronary heart disease (CHD) risk factors, demonstrating that insulin resistance at any given BMI accentuates the risks of both type 2 diabetes and CHD. Implications of these findings are self-evident; the most intensive efforts to reduce risk of type 2 diabetes and CHD in overweight/obese individuals should be focused on those individuals who are also insulin-resistant.
_
Postprandial insulin resistance as an early predictor of cardiovascular risk: a 2007 study:
Insulin resistance, hyperglycemia, hyperinsulinemia, hyperlipidemia and oxidative stress are risk factors related to cardiovascular diseases including congestive heart failure, myocardial infarction, ventricular hypertrophy, endothelial nitric oxide impairment in systemic blood vessels and the heart, atherosclerosis, and hypercoagulability of blood. The traditional focus on insulin sensitivity and blood levels of markers of risk determined in the fasted state is inconsistent with the large volume of recent data that indicates that the metabolic defect in the pre-diabetic and diabetic condition relates more strongly to postprandial deficiency than to the fasting state. Risk factors for adverse cardiovascular events can be detected in the pre-diabetic insulin-resistant subject based upon the metabolic response to a test meal even in the absence of altered fasting parameters.
____
The conventional treatment of insulin resistance: (also read treatment of hyperinsulinemia discussed earlier):
Weight loss and exercise are considered the best treatments for restoring the body’s ability to respond to insulin normally. Since smoking contributes to insulin resistance, quitting is recommended to bring the condition under control (as well as to improve your overall health). The FDA has not approved any drug specifically for the treatment of insulin resistance or pre-diabetes. However, in an effort to lower blood glucose levels and restore the body’s normal response to insulin, physicians may prescribe drugs normally used to treat type 2 diabetes. These include two classes of pharmaceuticals, known as biguanides and thiazolidinediones, that sensitize muscle and other tissues to the effects of insulin. Metformin (a biguanide) can help reduce the risk of developing diabetes in those with insulin resistance but is not as effective as losing weight and increasing activity. Other medications used for diabetes that act by different mechanisms may also be prescribed. These include alpha-glucosidase inhibitors, which restrict or delay the absorption of carbohydrates after eating, resulting in a slower rise of blood glucose levels, as well as sulfonylureas and meglitinides, which act directly on the pancreas and are sometimes prescribed to increase insulin production.
_
Exercise and insulin resistance:
Considerable information regarding the postexercise increase in insulin sensitivity phenomenon has accumulated since it was first reported by Ruderman’s group in 1982. This information includes these findings: 1) the increase in sensitivity is not just to insulin but also to stimulation of glucose transport by the contraction/hypoxia pathway; 2) the increase in sensitivity is mediated by translocation of more GLUT4 to the cell surface in response to a submaximal stimulus; 3) it requires the presence of serum during the period of stimulation by contractions, hypoxia, or AICAR; and 4) the increase in sensitivity does not require protein synthesis. A recent study in the International Journal of Behavioral Nutrition and Physical Activity looked at how insulin sensitivity is mediated by exercise. The results suggest the amount of time spent exercising may be an important part of improving the body’s response to glucose. Insulin resistance was improved by 25% going from no exercise to 60 minutes of exercise per day. It improved 50% from no exercise to 120 minutes of exercise. In addition to the more vigorous types of exercise, there was a trend for higher insulin sensitivity even doing light exercise such as walking, but not a significant one. It could be that the stimulus for improved glucose metabolism requires glucose utilization.
_
Aerobic exercise, or exercise that you can maintain for a prolonged period of time, seems to be able to acutely improve insulin resistance by increasing uptake of glucose into cells. It can increase insulin sensitivity immediately, as a session of 25-60 minutes (at 60-95% VO2 max) for 3-5 days. Improvements can also be seen after a week of aerobic training, when doing mostly 2 short sessions of 25 minutes of walking at 70% VO2 max. Interestingly, the opposite is also true. Voluntary restriction of activity or a drastic increase in sedentary activity can reduce insulin sensitivity in as little as 2 weeks. Over the long term, aerobic exercise done routinely can preserve beneficial changes in insulin sensitivity. Insulin sensitivity as a result of exercise can occur independent of weight loss. This is not to say that aerobic exercise will not lead to weight loss, as it may. The function of weight loss seems to be a blend of activity and diet, whereas insulin sensitivity increases could occur without changes in the diet. In regards to hepatic insulin resistance, it has been seen over time periods of 12 weeks light aerobic activity but studies lasting 1 week have sometimes noted no difference.
_______
_______
Fat, carbohydrates, insulin and energy metabolism:
_
Fat and insulin resistance:
Effects of Fat on Glucose Uptake and Utilization in Patients with Non-Insulin-dependent Diabetes: a 1995 study:
Authors conclude that physiologically elevated levels of FFA (free fatty acids) could potentially be responsible for a large part of the peripheral insulin resistance in patients with non-insulin-dependent diabetes mellitus.
_
Insulin Resistance and High Fat Diet:
Visit almost any laboratory on the planet that studies insulin resistance in animals or in humans and you’ll notice one simple technique that achieves insulin resistance in a repeatable fashion – eating a diet high in fat. Prolonged exposure of skeletal muscle and myocytes to high levels of fatty acids leads to severe insulin resistance. Among the different types of fatty acids, saturated long-chain fatty acids such as palmitic and stearic acids were demonstrated to be potent inducers of insulin resistance. Several mechanisms have been suggested to explain how saturated fatty acids impair insulin actions such as the Randle cycle, accumulation of intracellular lipid derivatives (diacylglycerol and ceramides), oxidative stress, modulation of gene transcription, inflammation and mitochondrial dysfunction. Saturated fatty acids are the most potent influencers of insulin resistance.
Direct effects of saturated fatty acid intake on muscle and liver include:
Mitochondrial dysfunction in the muscle and liver
The production of free radicals in the muscle and liver
Cellular inflammation in the muscle and liver
_
When excess fat accumulates it the muscle and liver tissue, the ability of glucose to enter both tissues is significantly compromised. An overaccumulation of unoxidized long-chain fatty acids can saturate the storage capacity of adipose tissue, resulting in a lipid ‘spill over’ to non-adipose tissues, such as the liver, muscle, heart, and pancreatic-islets. Under these circumstances, such ectopic lipid deposition can have deleterious effects. The excess lipids are driven into alternative non-oxidative pathways, which result in the formation of reactive lipid moieties that promote metabolically relevant cellular dysfunction (lipotoxicity) and programmed cell-death (lipoapoptosis). Several investigators have shed light on the ability of fatty acids to induce low-grade inflammation, which acts as an initial step in a series of events leading to damaged blood vessels, liver disease, heart disease and hypertension: Elevated free fatty acid levels (due to obesity or to high-fat feeding) cause insulin resistance in skeletal muscle and liver, which contributes to the development of type 2 diabetes mellitus (T2DM), and produce low-grade inflammation, which contributes to the development of atherosclerotic vascular diseases and NAFLD (non-alcoholic fatty liver disease).
_
Carbohydrates and insulin resistance:
Consumption of excess carbohydrates can lead to disastrous health effects, including (but not limited to):
Increased appetite and overeating
Increased fat synthesis in the liver
Unwanted weight gain
Insulin resistance, high blood sugar and diabetes
The metabolic syndrome
Systemic inflammation
In the past decade, a large body of evidence has begun to uncover the potent effects of refined carbohydrates on decreased cardiovascular health, diabetes health, liver health and unwanted weight gain. Statements taken from these articles include: “High fructose exposure during critical periods of development of the fetus, neonate and infant can act as an obesogen by affecting lifelong neuroendocrine function, appetite control, feeding behaviour, adipogenesis, fat distribution and metabolic systems. These changes ultimately favour the long-term development of obesity and associated metabolic risk.” “High consumption of refined grains, particularly white rice, has been reported to be associated with a higher risk of type 2 diabetes…These results suggest that high consumption of rice and noodles may contribute to hyperglycaemia through greater insulin resistance and that this relationship is independent of adiposity and systemic inflammation.”
_
Fat Storage and Insulin:
The most significant factor in fat storage is the level of insulin in the blood. Insulin has many effects on the body. With respect to fat storage, insulin increases the storage of fat in fat cells and prevents fat cells from releasing fat for energy. Eight hormones stimulate fat utilization: epinephrine, norepinephrine, adrenocorticotrophic hormone (ACTH), glucagon, thyroid-stimulating hormone, melanocyte-stimulating hormone, vasopressin and growth hormone. One hormone prevents fat utilization: insulin. The pancreas releases insulin when blood sugar levels rise above normal. Optimal fasting blood sugar should be between 70 and 90 mg/dL. Following a meal, blood sugars rise in relation to the amount and type of carbohydrates consumed. Processed carbohydrates are absorbed faster, and tend to cause faster and greater rises in blood sugar. The pancreas releases insulin, which tells the muscle, liver and fat cells to take up the blood sugar (and fat if it’s available) and remove it from the blood. This is a normal process because elevated blood sugar is toxic for the body. With a moderate amount of carbohydrates consumed each day, the pancreas can gently say to the muscles, liver and fat cells, “Please grab that sugar.” The pancreas is happy because it doesn’t have to work too hard to get its message across. The muscle cells, liver cells and fat cells are happy because they’ve got room to store the moderate amount of sugar. The individual is happy because energy levels throughout the day stay pretty consistent and he or she stays relatively lean. Over time, when individuals eat excessive amounts of carbohydrates, especially processed carbohydrates, on a daily basis, the muscle and fat stop listening to the pancreas’ release of insulin, which is called insulin resistance. The liver and muscle cells have a limited capacity to store glucose. Once they’re full, they shut the door and it all gets sent to the fat cells. Since blood sugar levels are not brought down to the level they should be, the pancreas “talks louder.” It releases more insulin than it should have to. With more insulin released, glucose levels can be brought down to a safe level again, at least for a while. Over time, fasting blood sugar levels start to creep up. Fasting blood sugar levels that start trending over 90 mg/dL can be a sign of early insulin resistance. Once they get above 100 mg/dL, most physicians will tell their patients they have insulin resistance. As the years go by and excessive carbohydrate consumption continues, blood sugar levels get higher and higher. The pancreas has to yell at the fat cells by secreting even higher levels of insulin. Eventually the cells with receptors for insulin stop listening and/or the pancreas stops secreting insulin. Blood sugar levels rise, and when they get above 125 mg/dL, type 2 diabetes is usually diagnosed.
_
Those who are insulin resistant will have elevated insulin levels throughout the day, which means their fat cells won’t be able to release fat for fuel. In Gary Taubes’ book Good Calories, Bad Calories, he explains that people become sedentary because they are gaining fat, they don’t gain fat because they are sedentary. The opposite way to look at this is once people control their insulin levels, they have the energy to become much more active and then lose body fat.
_
Here are some extracts regarding insulin and calories and their roles in fat storage.
•Insulin has many roles, including the ability store fat under certain conditions. To label insulin as the “fat storage hormone”, however would be an oversimplification.
•Insulin does not appear to directly cause fat gain under low calorie conditions
•Lower insulin response to a meal surprisingly does not consistently correlate with greater satiety.
•Reasonably cutting calories improves insulin metabolism over a wide range of carbohydrate levels. Also, insulin levels decrease with decreases in body weight regardless of calorie composition.
•Glycemic index studies are conflicting with the Cochrane Collaboration concluding low GI diets to be effective, there are other studies that don’t show any difference in body fat or satiety when calories are equal.
•Many people have found success on low glycemic index/load and otherwise carb-controlled diets. Eating lower glycemic index and keeping refined grains to a minimum is a good idea in spite of the inconclusive data relating to their long term effectiveness in controlling weight.
•Even while low carbing, however, calories can and do matter at some point in the weight loss journey.
•Insulin, blood sugar and calories aside, eating foods higher in nutrients is never a bad idea and these are typically found in low glycemic foods.
In a nutshell, instead of being hung up on whether insulin resistance causes overeating or the other way around, just focus on making good choices one meal at a time.
_
Through coordination, insulin is the main hormone that essentially tells the body what to do with the food energy just eaten. For example, when carbohydrate or protein is eaten, insulin directs the body to burn the carbohydrate or protein instead of using fat. And when mostly fat is eaten, the lack of an insulin response directs the body to burn the fat just eaten. Either way, you burn what you eat, and when that runs out, you go back to burning stored fat. This process is easily misinterpreted because one of insulin’s main functions following a meal is to shut down fat release from fat cells while the body burns carbohydrate and protein. Insulin’s role is really that of a traffic cop— signalling where nutrients should go (carbs, protein, and fat) just after meals. Overall, however, insulin does not promote fat accumulation in the long run. Keep in mind that insulin also promotes the transport of glucose and amino acid into muscle for synthesis of glycogen and proteins after meals. At the end of the day, the total 24-hour ‘flux’ of fat in and out of fat cells does not appear to depend on these insulin spikes.
________
Aetiology of obesity-associated insulin resistance:
_
The figure below shows that healthy obese and normal weight people have normal blood sugar while healthy obese have high insulin level compared to normal weight people suggesting insulin resistance in healthy obese:
_
How does obesity cause insulin resistance?
Researchers and clinicians have been attempting to answer this question for many years, and the current opinion is that there are many factors involved in the aetiology of obesity-related insulin resistance. Primarily, the adipocyte is the source of a number of factors that have been shown to effect insulin sensitivity. These factors include free fatty acids, tumour necrosis factor alpha, interleukin 6 and adiponectin. In addition, triglyceride deposition within the muscle is increased in obese subjects and the level of intramyocellular triglyceride has been shown to correlate negatively with insulin sensitivity. Thus, there are numerous clinical and in vitro studies demonstrating the ability of adipose tissue to increase insulin resistance. Furthermore obesity, via its ability to increase insulin resistance, is thought to play a prime role in the aetiology of the metabolic syndrome. This disorder is characterised by dyslipidaemia, glucose intolerance, insulin resistance, hypertension and abdominal obesity. However, the latest guidelines published by the National Cholesterol Education Program (NCEP) for the diagnosis of this syndrome recommend that insulin resistance be assessed using fasting plasma glucose rather than fasting serum insulin levels. This highlights the lack of clinical data supporting the use of HOMA, QUICKI or insulin levels for defining insulin resistance.
_
Cellular Energy Excess:
There is an interesting paper in 2009 titled “Insulin Resistance is an Antioxidant Defense Mechanism”. The authors presented compelling evidence that exposure to excess nutrients causes cells to produce excess reactive oxygen species, which in turn shuts down insulin signalling. This was presumably due to the fact that the mitochondria, the cells’ tiny furnaces, were overloaded with energy. Adding powerful antioxidants to the cells prevented insulin resistance because it blocked this signal. Insulin resistance protects the mitochondria, and hence the cell, from damage due to energy excess. This is consistent with countless other studies showing that exposing cells to excess nutrients, particularly free fatty acids, causes insulin resistance. Increasing circulating free fatty acids in humans rapidly induces insulin resistance. Suppressing free fatty acid levels restores insulin sensitivity in obese people with insulin resistance, including type 2 diabetics. Energy balance (in and out) has a powerful effect on insulin sensitivity. Experimental overfeeding studies in humans have shown that increasing ‘energy in’ causes insulin resistance in parallel with fat gain. Reducing calorie intake and losing body fat via virtually any diet imaginable, including simple calorie restriction, low-fat diets, and low-carbohydrate diets, causes an apparent increase in insulin sensitivity, and so does exercise, which increases the ‘out’ side of the equation. People who practice long-term calorie restriction for life extension have very low fasting insulin and glucose, suggesting high insulin sensitivity. The overfeeding and resulting fat gain causes insulin resistance in animals and humans, and insulin resistance is a major diabetes risk factor. So there is a robust link between excess energy intake, insulin resistance, and the development of diabetes. In summary, a variety of lines of evidence suggest that insulin resistance, in large part, is a cellular defence mechanism against energy excess. Cellular energy excess is caused primarily by the chronic consumption of energy in excess of what is expended. Fat tissue can mop up the excess energy for a while, but if the excess is chronic and fat tissue enlarges (particularly abdominal fat), other tissues will be exposed to progressively more energy (fatty acids and glucose), and cells will act to protect themselves by reducing insulin sensitivity.
_
In a nutshell, high fat and high carb diet (overfeeding) leads to insulin resistance and obesity, and obesity in turn leads to further increase in insulin resistance. It is akin to insulin resistance leading to hyperinsulinemia and hyperinsulinemia further aggravates insulin resistance. There is a robust link between excess energy intake, insulin resistance, and the development of diabetes type 2. Diet control and physical exercise can break these vicious cycles.
________
Insulin resistance in T1DM:
Type 1 diabetes is an autoimmune disease in which destruction of pancreatic islet beta cells leads to insulin deficiency. In first-degree relatives of Type 1 diabetes probands, the presence of circulating autoantibodies to the islet antigens insulin, GAD or tyrosine phosphatase-like insulinoma antigen 2 (IA2) denotes underlying islet autoimmune pathology and an increased risk of diabetes. Risk has been shown to be related to the number of antigen specificities, the level of islet antibodies, specific HLA alleles, and the degree of impaired insulin secretion, but prediction remains inexact. Improved prediction would facilitate the counselling of at-risk individuals and the selection of subjects for intervention trials to prevent clinical disease. Glucose disposal and prevailing glycaemia are determined not only by insulin secretion but also by insulin action. In contrast to in Type 2 diabetes, impaired insulin action or insulin resistance has not been considered to play a major role in the pathogenesis of Type 1 diabetes. Nevertheless, insulin resistance has been documented in established Type 1 diabetes and physiological states of insulin resistance such as puberty and pregnancy are associated with the onset of Type 1 diabetes. First-degree relatives positive for islet antibodies who are at increased risk of Type 1 diabetes have varying degrees of insulin resistance, but whether insulin resistance influences progression to clinical disease has not been documented. Interestingly, in the non-obese diabetic mouse model of Type 1 diabetes, hyperinsulinemia, a marker of insulin resistance, antedates clinical disease, and the incidence of diabetes has been reduced by treatment with rosiglitazone, a thiazolidinedione drug that decreases insulin resistance. If insulin resistance is found to play a role in the progression to human Type 1 diabetes, this would have important implications not only for risk prediction but also for intervention to reduce insulin resistance and delay the onset of diabetes. Therefore, in a cohort of first-degree Type 1 diabetes relatives positive for islet antibodies, authors in a 2004 study prospectively examined measures of insulin resistance and insulin secretion to determine whether insulin resistance is, in itself, a risk factor for progression to clinical disease. They concluded that relatives positive for islet antibodies who progress most rapidly to diabetes have a subtle disturbance of insulin–glucose homeostasis years before the onset of symptoms, distinguished by greater insulin resistance for their level of insulin secretion. Taking steps to reduce this insulin resistance could therefore delay the development of Type 1 diabetes.
__________
__________
B-cell functioning:
The main function of a beta cell is to produce and secrete insulin – the hormone responsible for regulating levels of glucose in the blood. When blood glucose levels start to rise (e.g. during digestion), beta cells quickly respond by secreting some of their stored insulin while at the same time increasing production of the hormone. This quick response to a spike in blood glucose usually takes about ten minutes. In people with diabetes, however, these cells are destroyed by the immune system (type 1 diabetes), or are unable to produce a sufficient amount of insulin needed for blood sugar control (type 2 diabetes).
_
The pancreatic beta cell function and mass are decreased from the clinical onset of both types of diabetes mellitus (DM) and this is accompanied by a correspondent deterioration of glycaemic control. In type 1 diabetes (T1DM), the phenomenon is more severe and is mainly due to the autoimmune attack of auto reactive T cells against islet beta cells. It is assumed that about 70-90% of the beta cell mass is lost at the time of clinical presentation, which is usually abrupt, with acute metabolic decompensation. In type 2 diabetes (T2DM), the pathogenesis is complex, and in most cases, the reduction of beta cell function and mass is associated with different degrees of insulin resistance. The clinical presentation of patients with T2DM varies widely; from very asymptomatic to symptoms of ketoacidosis and accordingly, blood glucose concentration at diagnosis may range from mildly increased to severe hyperglycaemia. In daily practice, it is sometimes difficult to discern between T1DM and T2DM because it has become apparent that they substantially overlap. Several clinical and laboratory criteria at onset, such as age, severity of symptoms, degree of hyperglycaemia, presence of ketosis, body mass index (BMI), perceived need for insulin therapy and (when possible to be measured) presence of diabetes-specific autoantibodies are used, but none of these criteria have definite thresholds that would allow a precise distinction between the two types, nor are they exclusively correlated with either type. In fact, it has been suggested that DM is a continuous spectrum of diseases with the autoimmune-mediated destruction of beta cells in childhood at one end and age-related metabolic deterioration at the other end of it. Moreover, the “accelerator hypothesis” suggests that although on different genetic backgrounds, T1DM and T2DM are basically one and the same disorder distinguished only by the rate of beta cell destruction and the causal factors (“accelerators”) leading to beta cell loss: high intrinsic rate of apoptosis, insulin resistance and autoimmunity, which act in various degrees in different individuals. Other factors, such as incretin hormones, adiponectin or islet amyloid are also involved. It has been proposed that the accelerators are interconnected, in the sense that for example, insulin resistance may increase apoptosis (possibly through pro-inflammatory mediators and metabolic up-regulation of beta cells), followed by the release of beta cell antigens and onset of autoimmune attack in genetically susceptible subjects. Insulin resistance is central for the development of T2DM, although some patients with T1DM may also have a certain degree of insulin resistance. Insulin sensitivity decreases with age, but other factors are known to influence it: nutrients (glucose and lipids), inflammation, adipokines or disordered insulin secretion. Beta cell dysfunctional patterns such as delayed insulin secretory rate to a stimulus or loss of pulsatile secretion of insulin may be indicative of disease progression. Although apoptosis seem to be the main mechanism responsible for pancreatic beta cell death, studies indicate that necrosis is also implicated in the development and progression of diabetes.
_
Type 2 diabetes frequently results from progressive failure of pancreatic b-cells in a setting of chronic insulin resistance. Whether b-cell defect and insulin resistance are simply coincident or are causally related is unknown. The distinction has important implications for the prevention of type 2 diabetes with interventions that ameliorate insulin resistance. If type 2 diabetes develops when preprogramed b-cell failure occurs in people who also happen to be insulin resistant, then treatment of insulin resistance will only delay the inevitable onset of b -cell failure and hyperglycemia. If, on the other hand, insulin resistance causes or accelerates b-cell failure, then treatment of resistance could preserve b-cell function and truly prevent diabetes over relatively long periods of time. In 2002 study, researchers observed 50% reduction in the incidence of type 2 diabetes in young Hispanic women with recent GDM who were treated with an insulin sensitizing drug. Protection from diabetes persisted 8 months after the drug was stopped, and it was associated with preservation of b-cell compensation for stable insulin resistance. Perhaps most importantly, protection required an improvement in serum insulin soon after initiation of treatment, but was most closely linked to a reduction in the amount of insulin required from b-cells. These findings provide strong evidence that insulin resistance contributes to b-cell dysfunction in susceptible individuals by increasing secretory demands on b-cells. Reducing those demands can preserve b-cell function and prevent type 2 diabetes for relatively long periods of time.
_
The prevalence of type 2 diabetes mellitus (T2DM) is increasing worldwide. T2DM is sustained by insulin resistance and impaired insulin secretion. Impaired insulin secretion due to either beta-cell dysfunction and/or beta-cell loss is now recognized in the pathogenesis and progression of diabetes. The loss of beta-cell mass and the progressive decline in beta-cell function is an early feature of the natural history of diabetes and it is detectable prior to diagnosis. The United Kingdom Prospective Diabetes Study (UKPDS) showed that beta-cell function, as evaluated by the homeostatic model assessment (HOMA-B) index, was already decreased by 50% by the time of the diagnosis and that it continued to decline over the 6-year observation period, even with on-going hypoglycemic therapy. The relative increase of α-cells mass, another typical defect on Langerhans islets of diabetic subjects, may even precede beta-cells loss, being already observed in normoglycaemic baboons with different degrees of obesity. Beta-cell mass is influenced by a balance between proliferative and pro-apoptotic signals, which may be modulated by various growth factors, cytokines, and hormones, whose specific role in the rate of beta-cell decline remains unclear. High levels of glucose and free fatty acids (gluco-and lipotoxicity), islet amyloid polypeptide deposition, and circulating inflammatory cytokines have been all implicated in beta-cell apoptosis. Thus, in vitro studies have demonstrated that high glucose levels induce an over-expression of pro-apoptotic genes of the Bcl family in cultured pancreatic Islets of Langerhans, and the important role of islet amyloid (IA) in the pathogenesis of beta cell dysfunction has been demonstrated in rodent models, non human primates, as well as in human studies; also the influence of inflammation on beta cell function and peripheral insulin resistance has been increasingly recognized. At any rate, whenever it appears, impaired beta-cell function leads to the progressive failure of islet cells to secrete sufficient amounts of insulin to overcome peripheral insulin resistance, ultimately resulting in failure to maintain normal glucose homeostasis over time. However, the rate of beta-cell failure is unpredictable and not all persons with T2DM will need insulin therapy to maintain their blood glucose levels. Several factors may have a bearing on beta-cell function, including lifestyle, clinical, and metabolic factors, as well as ongoing therapies. The early identification of these clinical predictors could provide clues to developing strategies to ameliorate the long-term management of T2DM.
_
Several years ago, Ralph DeFronzo and colleagues did a very interesting study that looked at beta-cell function in nondiabetic subjects, and those with IGT impaired glucose tolerance and type 2 diabetes. They compared the glucose area under the curve (AUC) (how high the glucose rose) in individuals with impaired glucose tolerance or with type 2 diabetes. What they looked at was a range of impaired glucose tolerance; they assessed people with glucose AUC between 140 mg/dL and 160 mg/dL, 160 mg/dL and 180 mg/dL, and 180 mg/dL and ≤ 200 mg/dL. These people, by definition, had impaired glucose tolerance (2-hour PPG ≥ 140 mg/dL and < 200 mg/dL). Their glucose AUC (how much glucose they still had in their blood after an acute glucose challenge) was increased. They showed that with progressive increases in glucose AUC, there is a progressive fall-off in the insulin AUC, so that for in those people with blood glucose values in the upper range, there is a relative fall-off in the insulin secretion. Then as one progresses to type 2 diabetes, in the different quartiles, there is a progressive fall-off in beta-cell function. So the dysfunction, or the failure to achieve adequate compensatory function, happens early in the development of IGT. Subsequently, there is a progressive fall-off in beta-cell function. Ralph DeFronzo suggested in his 2009 Banting Lecture that by the time you are diagnosed and have a postprandial blood sugar over 200 mg/dl at 2hrs you will have lost 80% of your beta cell function. There is no longer doubt that preservation of beta-cell function plays an important role in preventing diabetes. In their recent review of this topic, DeFronzo and Abdul-Ghani identified genetic and acquired factors that contribute to the demise of beta-cell function. Diabetes is diagnosed clinically by elevated plasma glucose levels, however, loss of β-cell function is progressive over time and β-cell dysfunction is far advanced by the time diabetes is diagnosed clinically. Patients with impaired glucose tolerance have <50 % of normal β-cell function and patients with T2DM have <15 % of normal β-cell function for their degree of insulin resistance, demonstrating the progressive nature of β-cell dysfunction in the course of T2DM. Therefore, methods for preserving or restoring β-cell function are important in our attempts to prevent and treat T2DM.
_
How many Beta-Cells have to die to cause diabetes?
This question was answered by a series of autopsies performed on pancreases taken from Mayo Clinic patients whose medical histories were known. They found that patients diagnosed as diabetic had 63% less beta cell mass than normal people–which they attributed to beta cell death, not shrinking in the size of the individual cells. Obese people who were not diabetic had 50% more beta cells than normal. They also found that the pancreases of obese patients not diabetic who were diagnosed as having impaired fasting glucose–defined as fasting blood sugars between 110 mg/dl and 125 mg/dl (6.1 and 6.9 mmol/L)had also lost significant amounts of cells–40% of them. The same study found that lean people with type 2 diabetes had more dead beta cells in their islets than obese people with diabetes.
_
Use this understanding to stop your Diabetes from progressing:
Even after you have been diagnosed as having a type 2 diabetic fasting plasma glucose, you may still have a good number of beta-cells left–anywhere from 40 to 60%. If you can reduce your insulin resistance through weight loss, exercise, and the use of drugs that counter insulin resistance, and if you keep your carb intake low to avoid blood sugar spiking, those cells may be able to produce enough insulin to control your blood sugar. Even more important, if you keep your blood sugar under the damage-limit of 140 mg/dl (7.8 mmol/L) at all times, you may be able to keep glucotoxicity from murdering the rest of those cells.
_
Several clinical and experimental studies have clearly shown that even minimally preserved beta cell function is metabolically beneficial. This leads to lower HbA1C levels, lower insulin dosage and lesser metabolic decompensation after insulin withdrawal. Using hyperglycemic clamp studies for quantitative assessment of beta cell function has clearly shown that it is the most important determinant of glucose disposal. It has also been found to be a major contributor to oral glucose tolerance even in high risk relatives of type 2 diabetic patients in different ethnic groups. Beta cell function is quantified as the ratio of the incremental insulin to glucose responses over the first 30 minutes during the Oral Glucose Tolerance Test (OGTT) was found to be more important in determining glucose disposal. This result was valid even after adjustment for insulin sensitivity, which might modulate beta cell function. Beta cell dysfunction is responsible for various defects observed in type 2 diabetics, individuals with impaired glucose tolerance (IGT), impaired fasting glucose (IFG) and includes those genetically predisposed to develop type 2 diabetes. These defects include:
a. Diminished first and second phase insulin release
b. Decreased pulsatile or oscillatory insulin release
c. Increased release of proinsulin-like molecules and
d. Impaired ability to compensate for superimposed tissue insulin resistance.
_
Natural History of Pancreatic Islet B-Cell Function in Type 2 Diabetes Mellitus Studied over Six Years by Homeostasis Model Assessment: a 2009 study:
Islet B-cell function and insulin sensitivity were estimated with the aid of a mathematical model from repeated fasting plasma glucose and insulin measurements over a 6 year period in 131 patients with type 2 diabetes mellitus who could be managed satisfactorily on dietary therapy alone. The estimated islet B-cell function, expressed as a percentage of normal, decreased significantly (p<0.05) at a rate of 1.5% per year, with no statistically significant change in insulin sensitivity. Extrapolation suggests the reduction in B-cell function predated the departure of fasting plasma glucose from the normal range.
___
Deficit of Beta Cell function and mass in T2DM:
T2DM is characterized by insulin resistance and beta cell dysfunction. Since plasma insulin level is often higher in patients with T2DM compared with non-diabetic individuals, T2DM is characterized by obesity, hyperinsulinemia, and insulin resistance; however, the significance of beta cell dysfunction in patients with T2DM has been often ignored. However, recent studies have consistently shown the presence of beta cell dysfunction in patients with T2DM. Since insulin secretion shows a compensatory increase in the presence of insulin resistance, true beta cell function should be evaluated based on insulin secretion adjusted by insulin sensitivity, the so-called disposition index (vide infra). Using the disposition index, DeFronzo et al. have reported that beta cell function is already decreased by approximately 80% in patients with impaired glucose tolerance (IGT) compared with non-diabetic subjects. Moreover, recent studies have shown that not only beta cell function, but also beta cell mass is decreased in patients with T2DM. Butler et al. conducted histological analysis of beta cell mass in the pancreas obtained from autopsy subjects with or without T2DM. As a result, they found that beta cell mass was decreased by approximately 40% and 65% in lean and obese individuals with T2DM, respectively, compared to age- and BMI-matched non-diabetic individuals. Other histological studies have also confirmed reduced beta cell mass in patients with T2DM. Taken together, the current evidence consistently shows that there is reduced functional mass of beta cells in patients with T2DM. Since type 1 diabetes (T1DM) is characterized by beta cell loss due to autoimmune attack, the current evidence indicates that reduced beta cell mass is a common pathophysiological feature of both type 1 and type 2 diabetes.
__
The magnitude of the increased demand for insulin due to insulin resistance caused by excess caloric intake and physical inactivity exceeds the magnitude of beta cell mass expansion, resulting in an increase in beta cell workload. In individuals who are susceptible to type 2 diabetes (T2DM), increased beta cell workload may lead to beta cell failure and the development of T2DM.
_
In the figure above, although insulin level is high in IGT and early T2DM, b-cell mass and b-cell function calculated by disposition index is lower than normal individuals.
________
________
The main factors and mechanisms associated with reduction of beta cell function/mass are hyperglycaemia/ glucotoxicity, lipotoxicity, autoimmunity, inflammation, islet amyloid, adipokines, incretins and insulin resistance.
_
1. Hyperglycemia/glucotoxicity:
Pancreatic beta cells are extremely sensitive to the blood glucose concentrations and changes in its homeostasis influence beta cell function and population dynamics. Indeed, chronic exposure to abnormally high blood glucose has detrimental effects on insulin synthesis/secretion, cell survival and insulin sensitivity through multiple mechanisms (“glucotoxicity”), which in turn lead to hyperglycaemia and finally to the vicious circle of continuous deterioration of beta cell function. As opposed to beta cell desensitization (a temporary state of cellular refractoriness to glucose stimulation) or beta cell exhaustion (depletion of insulin stores reversible with cell rest), glucotoxicity implies irreversible changes to the cellular components of insulin production/secretion. It is possible however, that islet cells are in various states: desensitization, exhaustion or apoptosis in the same time. Therefore, after glucose normalization (e.g. with initiation of insulin therapy), some beta cells recover and improve their insulin secretion. Multiple pathways and mechanisms through which chronic hyperglycaemia may impair beta cell function and cause beta cell apoptosis have been suggested.
_
Relationship between plasma glucose level and insulin secretion in type 2 diabetic patients: a 2003 study:
It is concluded that hyperglycemia (fasting or postprandial glucose) of type-2 diabetic patients has considerably destructive effect on dynamic insulin secretion.
_
Hyperglycemia causing b-cell apoptosis:
_
2. Lipotoxicity:
Diabetes is often associated with changes in lipoprotein profiles and increased FFA concentrations. Prolonged exposure to increased FFA levels (derived from adipocyte lipolysis or from lipoprotein hydrolysis) may have negative effects on beta cell function (i.e. attenuate GSIS) and lead to accumulation of toxic FA metabolites in the islet cells (“lipotoxicity”). Several observations suggest that the FFA toxicity on beta cells depends on several parameters (concentration of unbound FFA, level of unsaturation, nature of the isomers or chain length). However, there are reports (mainly coming from in vitro experiments and some animal studies) indicating that the deleterious effects of lipids on beta cells occur only in the presence of hyperglycaemia, when elevated FA are not oxidized in mitochondria and are shunted towards the esterification pathways, with accumulation of long-chain acyl-CoA esters in the cytoplasm (“glucolipotoxicity”).
_
3. Autoimmunity and inflammation:
The role of autoimmunity in T1DM has been long recognized. During the progression of the disease, autoimmune-mediated apoptosis of beta cells leads to a continuous reduction of insulin secretion and beta cell mass. Some phenotypic T2DM subjects also present markers of autoimmunity and these are correlated with beta cell function, suggesting a possible role of immune system in the pathogenesis of T2DM. Reports have indicated that about 10% of individuals with T2DM have diabetes-specific autoantibodies, but the percentage is higher in younger-age and in leaner groups. More recent data identified T2DM subjects with islet-reactive T cell responses (with or without autoantibodies) and these were correlated with a lower beta cell function.
_
4. Adipokines:
Adipokines are hormone-like peptides/proteins released from adipose tissue with a role in the “adipo-insular” axis: some (such as leptin) act as pro-inflammatory cytokines and contribute to beta cell failure, while others have protective effects on beta cell function and survival. It has been shown that leptin may induce inflammatory reactions and apoptosis upon chronic exposure. Leptin exerts direct effects on pancreatic beta cells that express leptin receptors, where it activates multiple signalling pathways leading to inhibition of GSIS and decreased pre-proinsulin levels. Other cytokines released by adipocytes (e.g. IL-6 and TNFα) may also modulate beta cell survival, although it has not been clarified if the quantities released in circulation are enough to cause beta cell dysfunction. One recent report indicated the TNFα increase the expression of amylin (and not proinsulin) in beta cells. Newer adipocyte-derived secretory molecules, like resistin and apelin, have been shown to reduce insulin secretion. Adiponectin, on the other hand, is structurally related with TNFα, but has a number of beneficial effects: it exerts insulin sensitising actions (mainly in the liver), promotes beta cell function and survival, has local and systemic anti-inflammatory effects. Under detrimental metabolic conditions, adiponectin is down-regulated, resulting in low plasma concentrations. At the level of beta cells adiponectin acts through its receptors and appears to increase the GSIS. The major effect on pancreatic beta cells seems however to be the anti-apoptotic one, by activation of pro-survival kinases, reduction of intracellular ceramide levels and increase of sphingosine-1 phosphate (an anti-apoptotic metabolite) concentrations.
_
5. Incretins:
The positive effects of incretin hormones on beta cell function and mass are known. Through binding on specific receptors on beta cells and increases of intracellular cyclic AMP (cAMP) and calcium, incretins stimulate the GSIS, improving both first- and second-phase responses and restoring the biphasic profile. They also up-regulate several beta cell-specific genes, including proinsulin, glucokinase and glucose transporters. Because the stimulation of insulin synthesis occurs by up-regulation of insulin transcription and translation and stabilization of insulin mRNA, the beta cell secretory capacity and insulin stores are preserved. It has also been shown that glucagon-like peptide-1 (GLP-1) prevents glucolipotoxicity in beta cells via PKB activation. Through these effects incretins protect and improve the function of beta cells. Moreover, the chronic effects of incretin hormones seem to favor the maintenance of beta cell mass, by stimulating the regeneration, proliferation and neogenesis while enhancing resistance to apoptosis, as indicated by animal and in vitro studies. In T2DM there is both an impairment of incretin release and a resistance to their action, which contributes significantly to insulin deficiency and beta cell dysfunction. Hyperglycemia further amplifies the alteration of the incretin effect, in part by down-regulating or de-sensitization of their specific receptors.
_
6. Insulin resistance:
There is a large body of literature emphasizing that insulin resistance places an augmented demand on beta cells to increase the insulin secretion in order to compensate the defect in insulin action, and this leads to a progressive beta cell dysfunction and failure. The mechanisms by which insulin resistance contributes to beta cell failure are not fully elucidated. One hypothesis is that insulin hypersecretion finally causes beta cells exhaustion, while other suggests that the factors that lead to insulin resistance (e.g. lipotoxicity) also result in beta cell failure.
____
Factors Associated with Beta-Cell Dysfunction in Type 2 Diabetes: The BETADECLINE Study: 2014:
T2DM is characterized by insulin resistance and various degrees of beta-cell impairment. Several lines of evidence strongly suggest that preserving beta-cell function is critical to prevent loss of glucose control and progression from prediabetes to overt diabetes or from use of OHAs to insulin therapy in subjects with long-standing disease. Nonetheless, insulin therapy is not an inexorable end, and the rate of progression in T2DM appears to differ individually. Because such unpredictability may reflect individual variability in the rate of beta-cell decline, identifying the clinical factors that can predict it holds importance for diabetes management. In this analysis of baseline data from the BetaDecline study, authors examined the degree of beta-cell dysfunction and its potential associations with demographic, clinical, treatment variables, and markers of inflammation and lipotoxicity in a large cohort of T2DM outpatients on stable treatment with diet and/or OHAs for more than 1 year. In conclusion, in this large cohort of type 2 diabetic outpatients, baseline higher (proinsulin/insulin) PI/I values, a marker of beta-cell dysfunction, were more frequent in men and those using secretagogues.
_
Beta-Cell Rest:
Some studies mostly in cell-cultures and animal models have demonstrated that giving stressed beta-cells a rest can sometimes restore function. A few studies suggest this can also be done in humans. One way of “resting” beta-cells is to use injected insulin as soon as type 2 diabetes is diagnosed, particularly if your blood sugars are very high at the time of diagnosis. If you take the burden off your beta-cells by supplementing insulin, there’s some suggestion that they may recover some of their ability to produce insulin later on so that you can go off insulin and retain much better control. You’ll still have to limit carbs and address any problems you have with insulin resistance through weight loss, exercise, and insulin-sensitizing drugs.
_
Beta Cell Function and Glycemic Control:
If reduced functional beta cell mass is a common pathophysiological feature of both type 1 and type 2 diabetes, what is the clinical relevance of beta cell dysfunction in T2DM? Since beta cell function is already reduced in patients with IGT, preceding the onset of T2DM, beta cell dysfunction has an important role in the deterioration of glucose tolerance. Thus, preservation or recovery of beta cell function could be an effective therapeutic strategy to prevent T2DM. Recent studies also suggest the importance of beta cell function in the management of hyperglycemia in patients with T2DM. In the UK Prospective Diabetes Study (UKPDS) and a Diabetes Outcome Progression Trial (ADOPT), treatment failure was associated with a progressive decline of beta cell function. The association between beta cell dysfunction and treatment failure is not only observed in adult patients, but was also shown in adolescent patients with T2DM. The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) trial showed that lower beta cell function at baseline was associated with poorer glycemic control after four years in adolescent patients with T2DM who were treated with metformin or metformin plus rosiglitazone. Another study found that patients with lower beta cell function at baseline were more likely to subsequently need insulin therapy compared with those with higher beta cell function. More surprisingly, even with more frequent insulin therapy, patients with lower beta cell function at baseline showed higher glycated hemoglobin (HbA1c) and glycated albumin (GA) levels after 2 years. These results indicate that beta cell function is significantly associated with clinical outcome, i.e., glycemic control, in patients with T2DM.
_
Preservation of B-Cell Function: The Key to Diabetes Prevention: 2011 study:
Individuals in the upper tertile of IGT are maximally/near-maximally insulin resistant, have lost 70–80% of their b-cell function, and have approximately a 10% incidence of diabetic retinopathy. Therefore, preservation of the remaining 20–30% of b-cell function is critical to prevent future development of T2DM. Authors concluded that pharmacological intervention with a variety of agents (thiazolidinediones, metformin, acarbose, glucagon-like peptide-1 analogs) consistently reduces the rate of conversion of IGT to T2DM.
________
Measures to protect b-cells:
_
The figure below shows measures to protect b-cells by targeting factors that cause b-cell demise:
_
Glucotoxicity can be ameliorated if hyperglycemia is reduced. This is obtained by improvement of peripheral glucose uptake (with metformin or thiazolidindione (TZD)), reduction of hepatic glucose production (with metformin, TZD, incretins), improvement of insulin secretion (with sulphonylureas (SU) or incretins), exogenous administration of insulin. Moreover, some of the agents (TZD, insulin, incretins) have direct protective effects on beta cell function. Both TZDs and incretins have been shown to decrease beta cell apoptosis, promote beta cell proliferation, increase insulin synthesis and secretion and improve the insulin secretion pattern. Some studies indicated beneficial effect of early insulin therapy on beta cells in patients with latent autoimmune diabetes of adults (LADA. Similarly, early intensive insulin therapy with multiple injections or continuous subcutaneous insulin infusion in subjects with newly diagnosed T2DM has favorable outcomes in terms of recovery and maintenance of beta cell function (especially restoration of acute insulin response). The exact mechanisms behind the beneficial effects of exogenous insulin administration on beta cells are not completely elucidated, but it is though that it allows cells rest, at least in part by down-regulating their metabolism and/or by releasing them from the hyperglycemic stress. Insulin therapy may also protect pancreatic beta cells by decreasing the severity of insulitis, suppressing the inflammatory processes, reducing antigen expression and subsequent amelioration of T cell responses/number of infiltrative cells in the islets. Prolonged exposure to SU on the other hand has been associated with beta cell exhaustion, desensitisation and possibly acceleration of oxidative stress and apoptosis, leading to a progressive reduction of insulin production capacity and deterioration of glycemic control over time. TZDs and insulin are also potent suppressors of lipolysis and thus reduce the amount of FFA released in circulation. TZD also mobilize the fat out of beta cells, liver and muscle. These processes ameliorate lipotoxicity. Incretins have also been shown to correct glucolipotoxicity, by reducing glucose and FFA levels or possibly though mechanisms related to PKB activation and NF-kB pathway. The anti-inflammatory effects of insulin have already been mentioned, but these have been also demonstrated for TZDs and metformin, as well as for other (non-hypoglycemic) drugs that are used by subjects with diabetes (e.g. statins, fibrates, angiotensin converting enzyme inhibitors, angiotensin receptor blockers and even high-dose aspirin). With regards to immune interventions targeted towards autoimmunity and aimed to protect remaining insulin-producing beta cells and their function, these have been used only in clinical trials of T1DM (and some of LADA), but unfortunately, the promising results of phase 2 studies could not be confirmed in phase 3 trials for all agents. So far, there is limited literature regarding agents that would ameliorate islet amyloid deposition and its toxic effects on beta cells, although some recent reports suggest that rosiglitazone (a TZD) and exendin-4 (a GLP-1 receptor agonist) may have protective effects via PI-3K/AKT pathway. The role of incretin therapy in T2DM and the positive effects of incretins on beta cells have been extensively studied. Finally, insulin sensitivity is improved by agents that have potent insulin sensitizing effects (metformin, TZD), but possibly the reduction of hyperglycaemia per se may also improve it. In addition to drug therapy, other therapeutical options like intensive lifestyle intervention and gastric bariatric surgery (by Roux-en-Y gastric bypass method)result in decrease of body weight and improvement of beta cell function, while pancreatic islet transplantation increase the insulin-secreting cell mass. All these pathophysiological aspects should be considered when choosing the treatment intervention, in order to better protect and preserve beta cells and obtain long-term benefits. Possibly, a combination of two/more agents that concomitantly target more of these mechanisms/factors would render most benefits.
_
Animal studies support the idea of β-cell mass and/or function as modifiable parameters, especially in earlier stages of the disease. In vitro studies have similarly suggested that the shorter the period of antecedent glucose exposure, the more likely cultured β-cells are to recover full function. Clinical evidence is suggestive as well. The Finnish Diabetes Prevention Study and the US Diabetes Prevention Program, both showed a 58% reduction in diabetes less incidence among individuals with IGT treated with diet and exercise for ∼3 years. Also, diabetes rates in pre-diabetic patients treated with metformin for the same period declined by 31% (46), and, in high-risk patients treated with acarbose in the STOP-NIDDM randomized trial, by 25%. Although these findings are consistent with the concept that early intervention to lower glucose levels may check disease progression, they may reflect delays in, rather than prevention of progression, because cumulative incidence rates of diabetes rose at the end of these studies in both treatment and control groups.
_
Several clinical trials have shown that the DPP-IV inhibitors sitagliptin and vildagliptin, improve both postprandial and fasting β-cell function in patients with T2DM for up to 24 weeks. Another recent study using a homeostasis model assessment found those first- and second-phase insulin responses, as well as both fasting and stimulated β-cell function, returned to normal after overnight administration of GLP-1. Similarly, intravenous administration of the GLP-1 receptor agonist, exenatide, maintained for 30 minutes, restored normal insulin secretory pattern after glucose challenge in T2DM patients lacking a first-phase insulin secretion in the absence of exenatide. Another GLP-1 receptor agonist, liraglutude administered via a single subcutaneous injection to adults with well-controlled T2DM showed increased insulin and C-peptide levels and dramatically improved insulin secretory response attributed to restored β-cell responsiveness. With a significantly longer half-life than exenatide, liraglutide can be dosed once daily. Once-daily subcutaneous administration for 1 week significantly lowered overall glycemia while improving first-phase insulin response and nearly doubling the disposition index.
_
_
Intensive Lifestyle Modification:
Obesity is a major risk factor for T2DM, and the increased prevalence of obesity is largely responsible for the concomitant increase in T2DM. Obesity and lack of physical activity cause insulin resistance and increase the workload on β cells. Weight loss and exercise interventions increase insulin sensitivity and unload the secretory demand on β-cells. The United States Diabetes Prevention Program (DPP) showed that implementing a program that achieved at least a 7 % reduction in body weight through diet and exercise reduced the incidence of T2DM by 58 % in patients with impaired glucose tolerance (IGT). The effect of lifestyle intervention on reducing the incidence of T2DM was related to overall improvements in β-cell function driven by its gains in insulin sensitivity, such that the hyperbola describing the relationship between insulin secretion and in insulin sensitivity was shifted to the right. The Finnish Diabetes Prevention Study (DPS) also showed a 58 % reduction in the incidence of T2DM in individuals with IGT who were assigned to a lifestyle intervention that included weight loss and increased physical activity. A recent analysis of the DPS indicated that lifestyle intervention helps to preserve β-cell function and prevent the development of T2DM through improvements in insulin sensitivity.
_
Beta-Cell Preservation…Is weight loss the answer? A 2011 study:
Obesity is associated with an increased risk of type 2 diabetes (T2DM). Pancreatic beta-cell failure is an early event in the development of glucose dysregulation and diabetes. Interventions to halt beta-cell failure in T2DM include diet modification, exercise, and use of pharmacologic agents. There is evidence that abdominal obesity may contribute to diabetes through insulin resistance and beta-cell impairment. Pivotal long-term studies into the prevention of T2DM have shown the importance of weight loss beside diet, lifestyle, and medication. The Finnish Diabetes Prevention Program (DPP) showed that weight loss gradually reduces the risk of diabetes, and that even modest weight loss can significantly reduce the incidence of T2DM. Similarly, in the US DPP, weight loss as part of intensive lifestyle modification was the major factor in reducing the incidence of T2DM in high-risk subjects, being more effective than drug intervention. While understanding the relationship between obesity and diabetes is complex, we know that weight loss has positive effects on adipose tissue. It causes an increase in the beneficial fat cell hormone adiponectin, and a decrease in adipose tissue inflammation. Also, it is associated with reduced insulin resistance and a consequential reduction in glucolipotoxicity, which can improve beta-cell function. Various pivotal studies suggest that weight loss prevents diabetes, and in turn preserves beta-cell function. At present, it is assumed that an increase in the intracellular deposition of triglycerides (TG) caused by visceral adipocytes flooding the portal circulation with free fatty acids leads to ectopic TG accumulation in muscles, liver, and pancreas. In subjects prone to diabetes, this event retards glucose metabolism by interfering with insulin signalling and insulin secretion, and finally leads to beta-cell dysfunction, IFG, and IGT. A strategy to reduce the excessive fat outflow from the abdominal depots and to prevent ectopic fat deposition is to reduce the volume of visceral fat depots by weight loss. In summary, weight loss improves glycemic control and thereby mitigates diabetes symptoms and complications, possibly through the preservation of beta-cell function. Therefore, efforts to prevent diabetes and preserve beta-cell function in patients with T2DM should more rigorously emphasize and target weight loss.
_
_
Bariatric Surgery:
Bariatric surgery is an effective and durable treatment for obesity and provides substantial improvements in glycemic control in obese patients with T2DM. Four bariatric surgical procedures are used conventionally, including Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric banding (LAGB), biliopancreatic diversion (BPD), and laparoscopic sleeve gastrectomy (LSG), and RYGB and LAGB are the most widely used surgical procedures. A meta-analysis indicated that bariatric surgery results in resolution of diabetes in 78 % of patients with T2DM demonstrating its significant impact on T2DM remission. The effects of bariatric surgery on β-cell function were recently summarized in a review by Bradley et al, and indicate that LAGB surgery increases the disposition index following modest weight loss, RYGB surgery increases the disposition index after significant weight loss, BPD surgery increases early insulin secretion and disposition index in patients with T2DM, and LSG surgery increases early insulin secretion in patients with T2DM, but its effects on disposition index are currently unknown. Three randomized controlled trials have compared the effects of bariatric surgery to medical therapy on remission of T2DM, and all 3 studies showed that bariatric surgery was significantly more effective than medical therapy in achieving remission of T2DM within 1 to 2 years following surgery. The long- term effects of bariatric surgery on T2DM remission were examined in the Swedish Obese Subjects Study (SOS), a nonrandomized prospective case-control study in over 4,000 obese patients who underwent bariatric surgery. They found that 72 % of T2DM patients achieved remission at 2 years after surgery, and 36 % had maintained T2DM remission at 10 years after surgery. Similar results were found in another smaller study which showed durable remission of T2DM (>5 years) in over half of the 89 % of patients who had achieved early following RYBG remission, and a meta-analysis which reported that 62 % of patients with T2DM remained free of diabetes for more than 2 years following bariatric surgery.
__
Do Beta Cells regenerate after diagnosis of DM?
Other than glucotoxicity, negative contribution of saturated fatty acids, lipoproteins, leptin, and circulating and locally produced cytokines will burn out the b-cells further, inducing apoptosis and/or necrosis. Studies with early short-term insulin treatment revealed that beta cell function could be re-gained with improved quality of insulin secretion as shown by better proinsulin/ insulin ratios and restored first phase. Numerous in vitro and clinical studies have also demonstrated that insulin therapy has potential anti-inflammatory benefits independent of its ability to save beta cells from gluco- and lipotoxicity, probably via its action on IRS proteins. Preclinical and clinical studies suggest that thiazolidinediones can decrease b-cell apoptosis, maintain b-cell neogenesis and prevent islet amyloidosis, decrease risk of diabetes in prediabetic patients. In vitro studies of human islets have demonstrated that chronic hyperglycemia desensitizes b-cells to glucose being accompanied by three major Ca2+ abnormalities: elevated basal Ca2+, loss of glucose-induced rise in Ca2+, and oscillatory activity disturbance with a decrease in glucose induced slow oscillations. In the studies with ‘’beta cell rest’’ with diazoxide and insulin it preserved residual insulin secretion for up to 18 months compared to placebo, both groups having multiple insulin injections. Diazoxide could also have antiapoptotic effect because it was demonstrated that incubation of murine pancreatic islets and b-cells with glucose induced apoptosis of b-cells that is Ca2+ dependent, because introduction to the culture medium of diazoxide, which blocked glucose-induced Ca2+ increase, inhibited apoptosis. The closure of KATP channels by the sulfonylureas tolbutamide and glibenclamide may also induce Ca2+-dependent b-cell apoptosis in rodent and human islets. Alvarsson et al., compared insulin and gIibenclamide treatment of type 2 diabetics who were diagnosed for less than 2 years, and showed that treatment with insulin given twice daily as premixed insulin preserved b- cell function more effectively than glibenclamide for 2 years follow-up, measured as glucagon-stimulated C-peptide response. Therefore, short-acting insulin secretagogues may be preferred to long-acting ones, so that patients will be exposed to proapoptotic stimuli less during prolonged treatment with short-acting agents. But this was according to in vitro studies of human islets, and in vivo confirmation is necessary . GLP-1R agonists have been associated with expansion of b-cell mass via stimulation of b-cell proliferation, promotion of islet cell neogenesis, and inhibition of b-cell apoptosis in animal studies. In human culture cells, GLP-1 prevented glucose- and palmitate-induced apoptosis via activation of the phosphatidylinositol-3 kinase/protein kinase B signalling pathway. Studies in humans have demonstrated that exenatide enhances first- and second-phase insulin secretion in diabetics and liraglutide improves first and second-phase insulin response and increases glucose sensitivity. As shown in animal studies, liraglutide may increase b-cell number and function, but more evidence with clinical studies are needed. Animal studies with mice has demonstrated that 8–12 wk of treatment with an analog of sitagliptin (des-fluorositagliptin) produced restoration of b-cell mass and morphology, increased islet insulin content, and improved glucosestimulated insulin secretion in isolated islets, similar to GLP-1 or GLP-1R analogs.
_
Beta Cell Proliferation and Compensation counters Beta Cell Demise and Dysfunction and promotes Beta Cell Preservation:
Cytokine-mediated oxidative stress and inflammation, inherent in obesity and insulin resistance, induces beta cell death therefore the beta cell population declines contributing to the manifestation of beta cell dysfunction. Similarly peripheral FFA-induced inflammation, as in obesity and insulin resistance, leads to beta cell destruction and dysfunction. Further, within the pancreas, saturated FFA induce islet inflammation and increase cytokine expression in beta cells thereby inducing beta cell dysfunction.
_
The figure above shows beta cell proliferation and compensation counters beta cell demise and dysfunction and promotes beta cell survival. Factors are encircled; proliferating factors are in bold. Metabolites, hormones, and transcription factors stimulate beta cell proliferation to sustain beta cell compensation. Beta cell compensation is a critical intervention process: it both inhibits beta cell demise and dysfunction; and protects beta cell structure and function. Proliferative agents, novel compounds, and healthy lifestyles help to preserve beta cells by maintaining beta cell integrity and function. Therefore with an adequate (and functional) beta cell population, beta cell physiology is maintained. Apart from feeding beta cell compensation, beta cell proliferation also restores beta cell population (when replenishment > death) thereby contributing to beta cell maintenance.
_
Researchers identify new pathway to regenerate insulin-producing cells: a 2015 study:
Researchers at the University of Massachusetts Medical School have discovered a new pathway that triggers regeneration of beta cells in the pancreas, a key development that may aid in the development of diabetes treatments. The research was published online in The Journal of Clinical Investigation. Insulin, a hormone that regulates blood sugar, is made in pancreatic beta cells. When insulin demand in the body exceeds beta cell insulin production capacity, prompted by events such as beta cell loss (type 1 diabetes) or weight gain (type 2 diabetes), beta cells proliferate to try to increase in number. Until now, it was unclear how beta cells sense the demand to more make insulin. In an innovative study in mice, Laura Alonso, MD, associate professor of medicine and the George F. and Sybil H. Fuller Foundation Term Chair in Diabetes, and Rohit Sharma, PhD, a postdoctoral associate, discovered how the pancreas knows that more insulin-producing beta cells are needed. When there’s an increase in insulin demand, there’s a corresponding increase in insulin production in the endoplasmic reticulum (ER) of the beta cell, which causes some stress to the ER system. “Although it was previously known that too much ER stress is bad for beta cells, causing them to die, this study found that a modest amount of stress is actually good for them, because it provides pressure to increase beta cell number to produce more insulin and keep blood sugar regulated,” Alonso said. The research also uncovered a surprising twist. The study found ER stress pathways actually send a signal to the cell nucleus, via an ER protein called ATF6 that says “proliferate.” Importantly, the study also found that the key elements of this pathway are active in human beta cells too. According to Alonso, these results explain why beta cells are so sensitive to stress, because they need to sense stress in order to determine how many beta cells are needed. “This may also explain the conundrum of why there do not appear to be stem cells in the pancreas,” Alonso added. “Only fully mature insulin-producing beta cells are able to use this stress mechanism to sense that the body needs more insulin than the current allotment of beta cells can provide.”
_________
_________
B-cell dysfunction vis-à-vis insulin resistance (insulin secretion vis-à-vis insulin action):
It is well known that the hyperglycemia that characterizes type 2 diabetes is caused by both IR and beta-cell failure. Although it is generally assumed that both causes are present in the typical diabetes patient, the relative contributions of each cause could have important ramifications for both prevention and treatment. Emphasizing weight loss, for example, in patients who are at an appropriate weight but with little beta-cell function would not be an effective prevention strategy. Similarly, selection of the most effective antihyperglycemic agent would be enhanced if we could identify precisely what was driving the hyperglycemia. One might also speculate that the differential contributions of beta-cell dysfunction and IR might help explain findings such as the apparent U-shaped relationship between glycated hemoglobin (A1c) and cardiovascular disease and mortality.
____
Insulin resistance and hyperinsulinemia relationship: a 2008 study:
Insulin resistance, recently recognized as a strong predictor of disease in adults, has become the leading element of the metabolic syndrome and renewed as a focus of research. The condition exists when insulin levels are higher than expected relative to the level of glucose. Thus, insulin resistance is by definition tethered to hyperinsulinemia. The rising prevalence of medical conditions where insulin resistance is common has energized research into the causes. Many causes and consequences have been identified, but the direct contributions of insulin itself in causing or sustaining insulin resistance have received little sustained attention. Authors examine situations where insulin itself appears to be a proximate and important quantitative contributor to insulin resistance. 1) Mice transfected with extra copies of the insulin gene produce basal and stimulated insulin levels that are two to four times elevated. The mice are of normal weight but show insulin resistance, hyperglycemia, and hypertriglyceridemia. 2) Somogyi described patients with unusually high doses of insulin and hyperglycemia. Episodes of hypoglycemia with release of glucose-raising hormones, postulated as the culprits in early studies, have largely been excluded by studies including continuous glucose monitoring. 3) Rats and humans treated with escalating doses of insulin show both hyperinsulinemia and insulin resistance. 4) The pulsatile administration of insulin (rather than continuous) results in reduced requirements for insulin. 5) Many patients with insulinoma who have elevated basal levels of insulin have reduced (but not absent) responsiveness to administered insulin. In summary, hyperinsulinemia is often both a result and a driver of insulin resistance. Hyperinsulinemia desensitizes receptors and causes worsening of situation in a vicious cycle of insulin resistance and hyperglycemia.
_
Hyperinsulinemia in the basal state of any origin produces widespread insulin resistance. All tissues that have insulin receptor pathways will be affected, including the pancreatic β-cell, and possibly the brain. Defective insulin signalling at the β-cell impairs glucose-stimulated insulin release. At steady state, basal hyperinsulinemia generates and sustains insulin resistance, irrespective of where the pathology started. Hyperinsulinemia, insulin resistance, and impairment of glucose-stimulated insulin release are intertwined biologically. A single process (hyperinsulinemia) could generate all three simultaneously. There are several factors that contribute to insulin release. In the basal state, free fatty acid levels act in part to stimulate the release of insulin. Obese subjects have high levels of free fatty acids, and this might be a major contributor to the hyperinsulinemia present in these patients. Basal insulin levels are such an important determinant of insulin sensitivity, it is important to understand the multiple elements that are driving the hyperinsulinemia in the basal state. It may be that the stimulus for basal hyperinsulinemia varies by patient and/or disease state. One of the characteristics of hyperinsulinemic states, specifically the metabolic syndrome, is an elevated level of inflammatory markers, including cytokines and C-reactive protein. Many cells of the innate immune system are sensitive to insulin and reduce their output of cytokines at insulin concentrations that are commonly encountered in vivo. It could be that the inflammatory background associated with obesity may be due to insulin resistance at the level of the inflammatory cells. Basal hyperinsulinemia perpetuates insulin resistance by a wide range of mechanisms. Free fatty acids and other stimulators of insulin secretion have been defined, and these factors might be the initial insults that drive the excess insulin secretion. Understanding the pathophysiology of the basal hyperinsulinemia may provide guidance in the development of effective and specific therapies.
_
A widely recommended approach to the control of hyperglycemia, which authors endorse, is to add a parenteral insulin preparation that provides a constant background addition to circulating insulin for 12–24 h to augment or replace in vivo basal insulin secretion. Authors accept that empirically this practice improves control of glycemia. They hypothesize that were it possible to administer basal insulin as intermittent brief boluses, control of glycemia would be equal (or better), achieved with smaller doses of insulin and less insulin resistance systemically. Parathyroid hormone provides another striking example of how homologous hormone regulates target cell sensitivity. Continuous hormone (e.g. with hyperparathyroidism) melts bones, while intermittent administration builds bones dramatically. Hypothalamic obesity appears to be another example of insulin resistance where hyperinsulinemia may be driving insulin resistance.
______
β-Cell dysfunction vs insulin resistance in type 2 diabetes: the eternal “chicken and egg” question: a 2011 paper:
The idea that type 2 diabetes (T2DM) is mainly due to insulin resistance stems from the 1930s, but became dominating from the 1980s. However, evidence since the 1960s indicates that insulin response to glucose is markedly diminished from the earliest signs of glucose intolerance. Insulin pump treatment induces near-normoglycemia in T2DM with doses similar to type 1 diabetes, indicating that hyperglycemia is caused by lack of insulin, insulin resistance acting as an amplifier. Insulin secretion is genetically controlled. T2DM risk gene polymorphisms hint toward mechanisms of reduced insulin secretion in diabetes-prone subjects, in whom insulin response decreases as the number of diabetic alleles increases. Author hypothesize that the genetic background of the β cell determines its adaptation capacity to increased insulin demand imposed by augmented caloric intake and insulin resistance; failure to adapt eventually leads to T2DM. Therefore, author regards the “prediabetic” β cell as a normal cell with limited adaptability, diabetes risk being entirely context-dependent (nutritional load and insulin sensitivity). Once hyperglycemia is established, β cells are exposed to continuous nutrient stimulation, with consequent oxidative and endoplasmic reticulum (ER) stresses. The result is increasing functional deficiencies and β-cell apoptosis, hence reduced β-cell mass. An intriguing as yet unanswered question is whether the mechanisms of β-cell deficit in the diabetic environment operate before hyperglycemia in overfed, insulin-resistant subjects. Therapeutic agents preventing β-cell oxidative and ER stress could stop the progression and perhaps initiation of T2DM.
_
The figure below shows that genetic background leads to reduced b-cell mass with insulin resistance merely augmenting the process that lead to T2DM:
_
Hyperglycemia augmenting B-cell dysfunction and insulin resistance:
Beta cell dysfunction is the critical determinant for type 2 diabetes which is compounded by insulin resistance. The interplay between beta cell dysfunction and insulin resistance remains highly complex. The onset of hyperglycemia can trigger both beta cell dysfunction and insulin resistance as seen in the figure below. Beta cell dysfunction is more severe than insulin resistance. With beta cell dysfunction, insulin secretion is impaired whereas with insulin resistance, insulin may still be secreted but insulin insensitivity manifests in target tissues. As beta cell dysfunction and insulin resistance exacerbate, hyperglycemia amplifies leading to the progression to type 2 diabetes.
_
The figure above shows hyperglycemia-induced beta cell dysfunction, insulin resistance, and type 2 diabetes.
_______
_______
Disposition index:
Normally, increases in insulin resistance are matched by a compensatory increase in insulin secretion by the β cells, and the relationship between insulin resistance and insulin secretion is defined by a hyperbola. Based on this hyperbolic relationship, β-cell compensation can be determined by the disposition index, defined as the product of insulin secretion and insulin sensitivity. As long as the product of insulin secretion times insulin sensitivity remains constant, glucose tolerance is preserved. For example, in a lean, insulin sensitive individual, less insulin secretion is required to maintain normal glucose levels. An obese, insulin resistant individual requires a compensatory increase in insulin secretion in order to maintain normal glucose levels. Inadequate β-cell compensation for insulin resistance results in impaired glucose homeostasis and eventually to T2DM. Longitudinal studies have shown that reduced β-cell function as reflected in the disposition index is a powerful predictor of conversion from normal glucose tolerance to T2DM in at-risk populations. The disposition index gives you an idea of the relationship between insulin sensitivity and secretion — how well your body can utilize insulin and the insulin secretion (the acute insulin response to an acute load of glucose). The important thing to realize is that this is a hyperbolic curve. This hyperbolic curve tells us that, for the most part, as insulin sensitivity goes down or insulin resistance goes up, beta-cell function increases. But with severe insulin resistance, the beta cell has to start working very hard and produce large amounts of insulin. This relationship tells us a few things. All you have to do is change your insulin sensitivity just a small amount when you are very insulin resistant, and you can remarkably decrease the amount of insulin secretion that you need to maintain normal blood glucose levels. So what we need to do is try to get people back on this normal curve, and try to get their insulin sensitivity improved so that their beta cells don’t have to work so hard. The index is a quantitative, convenient, and accurate tool in analyzing epidemiologic data and identifying incipient impaired glucose tolerance. Separate major issues remain, however: the causes of insulin resistance, the causes of the failure of adequate β-cell compensation in type 2 diabetes, and the nature of the signal(s) from insulin-resistant tissues that fail to elicit the appropriate β-cell increment in sensitivity to glucose and other stimuli. The disposition index is likely to remain the most accurate physiologic measure for testing hypotheses and solutions to these challenges in whole organisms.
__
The Disposition Index (DI) is the product of insulin secretion and insulin sensitivity. Normally, increases in insulin resistance (due to factors such as weight gain and inactivity) are matched by a compensatory increase in insulin secretion in a hyperbolic relationship (A to B) and DI remains constant. Inadequate insulin secretion to compensate for insulin resistance results in a reduction in DI and impaired glucose homeostasis (A to C) and eventually to type 2 diabetes (A to D). Consider a normal, insulin-sensitive subject, with an insulin sensitivity index (SI) equal to 2.0 × 10−5 min−1 per pmol/l. Such an insulin-sensitive individual would not require a massive insulin response to glucose administration to maintain normal blood glucose and glucose tolerance; first-phase β-cell response in this individual might be 400 pmol/l, yielding a disposition index of 800. A similar individual, experiencing a reduction in insulin sensitivity of 80% due to one of many possible causes (puberty, pregnancy, infection, increased adiposity), would be predicted to mount a fivefold greater insulin response (to 2,000 pmol/l), exhibiting the identical disposition index of 800. The single parameter, the disposition index (DI), can thus be envisioned to predict the normal β-cell response adequate for any degree of insulin resistance. The DI is a measure of the ability of the β-cells to compensate for insulin resistance. It can be considered a measure of the functionality of the pancreas in the intact individual.
___
Insulin enhances glucose-stimulated insulin secretion in healthy humans: 2009 study:
Islet β-cells express both insulin receptors and insulin-signalling proteins. Recent evidence from rodents in vivo and from islets isolated from rodents or humans suggests that the insulin signalling pathway is physiologically important for glucose sensing. Authors evaluated whether insulin regulates β-cell function in healthy humans in vivo. Glucose-induced insulin secretion was assessed in healthy humans following 4-h saline (low insulin/sham clamp) or isoglycemic-hyperinsulinemic (high insulin) clamps using B28-Asp insulin that could be immunologically distinguished from endogenous insulin. Insulin and C-peptide clearance were evaluated to understand the impact of hyperinsulinemia on estimates of β-cell function. Preexposure to exogenous insulin increased the endogenous insulin secretory response to glucose by ≈40%. C-peptide response also increased, although not to the level predicted by insulin. Insulin clearance was not saturated at hyperinsulinemia, but metabolic clearance of C-peptide, assessed by infusion of stable isotope–labelled C-peptide, increased modestly during hyperinsulinemic clamp. These studies demonstrate that insulin potentiates glucose-stimulated insulin secretion in vivo in healthy humans. In addition, hyperinsulinemia increases C-peptide clearance, which may lead to modest underestimation of β-cell secretory response when using these methods during prolonged dynamic testing. In conclusion, these findings demonstrate that insulin potentiates the β-cell insulin secretory response to glucose in healthy, insulin-sensitive persons. These findings are consistent with the hypothesis that the human β-cell is an insulin-responsive tissue. Finally, authors hypothesize that insulin regulation of glucose-stimulated insulin secretion might be altered in persons with insulin resistance or T2DM and might contribute to the progressive loss of β-cell function in individuals with T2DM.
_
Insulin resistance and insulin secretion correlation:
Insulin resistance precedes T2DM. β-Cell dysfunction also precedes T2DM, and many genes that confer diabetes risk have physiologic importance for β-cell function. Insulin secretion and action are coupled; with increasing insulin resistance, there is a proportional greater secretory response to maintain similar glycemia. Diminished insulin secretion and action have been considered separate processes; yet the presence and functional activity of the insulin receptor and its signalling proteins within the β-cell suggest that insulin could regulate its own secretion. Insulin resistance and insulin secretory defects contribute to the pathogenesis of type 2 diabetes mellitus (T2D). These processes have largely been thought of as separate. Insulin and insulin-like growth factor–1 (IGF1) receptors and major insulin receptor substrates, IRS-1 through IRS-4, are present and functional in islets and/or β-cells. Furthermore, β-cell–specific insulin receptor knockout (βIRKO) mice manifest defective glucose-stimulated insulin secretion and progressive glucose intolerance, leading to overt diabetes in some animals. Likewise, deletion of IRS-1, IRS-2, or Akt2 alters glucose sensing and β-cell growth. These murine models provide evidence implicating insulin signalling in the regulation of insulin secretion within β-cells and suggest a potential link between insulin resistance and the insulin secretory defect manifest in T2D.
_
Although insulin sensitivity and insulin secretion are thought to be independent processes connected only by blood glucose level (deceased insulin sensitivity leads to high blood glucose level leads to higher insulin secretion and vice versa), presence and functional activity of the insulin receptor and its signalling proteins within the β-cell suggest that insulin could regulate its own secretion. So insulin resistance is not only at liver, adipose tissue and muscles but also at b-cells themselves.
_________
_________
Measurement of insulin secretion and resistance (insulin sensitivity is inverse of resistance):
_
The concept of insulin resistance is relatively easy to understand, but determining precisely who is insulin resistant is more complicated. The relationship between glucose and insulin is quite complex and involves the interaction of many metabolic and regulatory factors. Normal insulin sensitivity varies widely and is influenced by age, ethnicity, and obesity. Simply put, not all people with impaired insulin sensitivity are necessarily suffering from a disorder, and pregnancy is a perfect example of this. A World Health Organization consensus group recently concluded that the insulin sensitivity index (SI) of the lowest 25% of a general population can be considered insulin resistant. The European Group for the Study of Insulin Resistance took a more restricted view, defining insulin resistance as the SI of the lowest 10% of a non-obese, nondiabetic, normotensive Caucasian population. Insulin resistance is one of the major aggravating factors for metabolic syndrome. There are many methods available for estimation of insulin resistance which range from complex techniques down to simple indices. For all methods of assessing insulin resistance it is essential that their validity and reliability is established before using them as investigations. The reference techniques of hyperinsulinaemic euglycaemic clamp and its alternative the frequently sampled intravenous glucose tolerance test are the most reliable methods available for estimating insulin resistance. However, many simple methods, from which indices can be derived, have been assessed and validated e.g. homeostasis model assessment (HOMA), quantitative insulin sensitivity check index (QUICKI). Given the increasing number of simple indices of IR it may be difficult for clinicians and researchers to select the most appropriate index for their studies.
_
Surrogate Methods to measure insulin resistance (IR):
The quantitative assessment of insulin sensitivity is not routinely used during biochemical investigations for diagnostic purposes, but the emerging importance of insulin resistance has led to its wider application research studies. Evaluation of a number of clinical states where insulin sensitivity is compromised calls for assessment of insulin resistance. Insulin resistance is increasingly being assessed in various disease conditions where it aids in examining their pathogenesis, etiology and consequences. The hyperinsulinemic euglycemic glucose clamp is the gold standard method for the determination of insulin sensitivity, but is impractical as it is labor- and time-intensive. A number of surrogate indices have therefore been employed to simplify and improve the determination of insulin resistance.
_
__
Hyperinsulinemic euglycemic glucose clamp:
The hyperinsulinemic euglycemic glucose clamp technique has been described as the gold standard method for quantifying insulin sensitivity. It is the reference method for quantifying insulin sensitivity in humans because it directly measures the effects of insulin in promoting glucose utilization under steady-state conditions in vivo. Direct estimation of IR by means of the euglycemic clamp technique and insulin suppression test (IST) is experimentally demanding, complicated, and impractical when large scale epidemiological studies are involved. These methods are laborious, painstaking and expensive, are therefore rarely used in larger-scale clinical research and, as such, are irrelevant for clinical practice. Consequently, over the years, a number of surrogate indices for insulin sensitivity or insulin resistance have been developed. The glucose clamp is difficult to apply in large scale investigations because of the chaotic procedure, which involves intra-venous infusion of insulin, taking frequent blood samples over a 3 h period, and the continuous adjustment of a glucose infusion. Two types of clamps are quite commonly used. The hyperglycemic clamp, which requires maintaining a high blood sugar level by perfusion or infusion with glucose, is a way to quantify how fast beta-cells respond to glucose. The hyperinsulinemic clamp, which requires maintaining a high insulin level by perfusion or infusion with insulin, is a way to quantify how sensitive the tissue is to insulin. The hyperinsulinemic clamp is also called euglycemic clamp, meaning a normal blood sugar level is maintained.
_
Insulin sensitivity test (IST):
IST involves IV infusion of a defined glucose load and a fixed-rate infusion of insulin over approximately 3 hours. Somatostatin may be infused simultaneously to prevent insulin secretion, inhibit hepatic gluconeogenesis, and delay secretion of counter-regulatory hormones— particularly glucagon, growth hormone, cortisol, and catecholamines. Fewer blood samples are required for this test, compared to clamp techniques. The mean plasma glucose concentration over the last 30 minutes of the test reflects insulin sensitivity. Although lengthy, IST is less labor intensive than clamp techniques and the FSIVGTT.
_
Insulin tolerance test (ITT):
A simplified version of IST, ITT measures the decline in serum glucose after an IV bolus of regular insulin (0.1–0.5 U/kg) is administered. Several insulin and glucose levels are sampled over the following 15 minutes (depending on the protocol used). The ITT primarily measures insulin-stimulated uptake of glucose into skeletal muscle. Because this test is so brief, there’s very little danger of counter-regulatory hormones interfering with its results. IV access should be established for insulin injection, blood sampling, and for rapid administration of D50W should severe hypoglycemia occur. Normal values for women with PCOS have not been published to date, but normal ranges for insulin sensitivity in a general population have been published for persons with a body mass index below 30 kg/m2 and for obese subjects (BMI >30 kg/m2) at 0.026 to 0.085 mmol/L /minute and 0.012 to 0.017 mmol/L /minute respectively. These values reflect the rate of decline of log transformed glucose values.
_
Continuous infusion of glucose with model assessment (CIGMA):
Like ITT, CIGMA requires fewer venepunctures and is less laborious than clamp techniques. A constant IV glucose infusion is administered, and samples for glucose and insulin are drawn at 50, 55, and 60 minutes. A mathematical model is then used to calculate SI. The results are reasonably compatible with clamp techniques; however, few laboratories have used CIGMA for insulin sensitivity testing in diabetic patients and there is no substantive data using the CIGMA technique in women with PCOS.
_
Fasting insulin:
Measurement of the fasting insulin level has long been considered the most practical approach for the measurement of insulin resistance. It correlates well with insulin resistance. A considerable correlation has been found between fasting insulin levels and insulin action as measured by the clamp technique. A substantial overlap between insulin-resistant and normal subjects is a constraint, as there is a lack of standardization of the insulin assay procedure. Nevertheless, with a reliable insulin assay, insulin resistance can be detected early, before clinical disease appears. As glucose levels change rapidly in the postprandial state, the use of fasting insulin for estimating IR should be done after an overnight fast, since the variable levels of glucose confound the simultaneous measure of insulin. In healthy subjects, increased fasting insulin levels (with normal fasting glucose levels) correspond to insulin resistance. In this population 1/(fasting insulin) can substituted for insulin sensitivity that decreases as subjects become more insulin resistant (and fasting insulin levels rise). However, it does not cover the inappropriately low insulin secretion in the face of hyperglycemia seen in diabetic subjects or glucose-intolerant subjects. Use of fasting insulin levels for assessment of IR is limited because of a high proportion of false-positive results and by lack of standardization. To overcome this issue, standardization of insulin assay has been recommended by the ADA Task Force, to be certified by a central laboratory. A high plasma insulin value in individuals with normal glucose tolerance reflects insulin resistance, and high insulin levels presage the development of diabetes. Fasting serum insulin is an inexpensive assay, and does not require any mathematical calculations. A fasting insulin >20 µU/mL in Caucasian women and >23 µU/mL in Mexican-American women probably indicates insulin resistance in women with PCOS. At least one researcher has advocated averaging two or three readings to account for day-to-day variability. The fasting serum insulin concentration provides information about the subject’s insulin sensitivity, but not about reductions in either beta cell mass or function. Serum insulin and blood glucose must be measured at the same time to evaluate insulin secretion properly. As an example, many patients with type 2 diabetes have higher fasting serum insulin concentrations than normal subjects, suggesting that they are oversecreting insulin. However, when the blood glucose concentration in the normal subject is raised to that of the patient with type 2 diabetes, the serum insulin concentration is much higher than in the diabetic patient. The degree of insulin resistance also needs to be considered; obese subjects with normal fasting blood glucose concentrations have fasting serum insulin concentrations several times higher than lean subjects with similar blood glucose concentrations.
_
Pitfalls of serum fasting insulin:
Insulin secretion is pulsatile. To overcome this inherent inaccuracy, it is wise to take a mean value of three fasting specimens for insulin collected over 10 minutes. Prospective mortality studies have shown insulin measures do not predict cardiovascular mortality when metabolic syndrome characteristics, such as lipids, are taken into account. Furthermore, the relationship between clamp insulin resistance and fasting serum insulin level is weak: fasting serum insulin level explains only 36% of variance in insulin resistance, even less when glucose metabolism is impaired. Measurement of serum insulin levels has been described as “fraught with errors and inconsistencies”; a clinician who ordered this inappropriate test would be overwhelmed by poor 120-minute reproducibility, among other problems. Some researchers understand insulin responses to an oral glucose tolerance test to be more a test of insulin secretion rather than resistance (and even then of low validity). A vigorous insulin response during an oral glucose tolerance test reflects gastric emptying and beta-cell insulin secretion, more than insulin action. A flat insulin response in a patient with insulin resistance may indicate a beta-cell secretory deficit.
_
Glucose/insulin ratio:
The Glucose/insulin (G/I) ratio has been employed in a number of studies as an index of insulin resistance. In a study conducted by Legro et al fasting G/I ratio was compared to insulin sensitivity measured by the FSIVGTT. It was found that fasting G/I ratio is a highly sensitive and specific measurement of insulin sensitivity. The G/I ratio has become very popular since its first description in 1998 as an accurate index of insulin sensitivity in women with PCOS. The ratio of glucose to insulin is easily calculated, with lower values depicting higher degrees of insulin resistance. A G/I ratio of less than 4.5 has been shown to be sensitive (95%) and specific (84%) for insulin resistance in a group of women with PCOS, when compared to a control group.
_
HOMA:
Homeostatic model assessment (HOMA) is a method for assessing β-cell function and insulin resistance (IR) from basal (fasting) glucose and insulin or C-peptide concentrations. It has been reported in >500 publications, 20 times more frequently for the estimation of IR than β-cell function. Insulin levels depend on the pancreatic β-cell effect to glucose concentrations while, glucose concentrations are regulated by insulin-mediated glucose production via the liver. Thus, deficient β-cell function will echo a diminished response of β-cell to glucose-stimulated insulin secretion. Similarly, insulin resistance is reflected by the diminished suppressive effect of insulin on hepatic glucose production. The HOMA model has proved to be a robust clinical and epidemiological tool for the assessment of insulin resistance. HOMA describes this glucose-insulin homeostasis by means of a set of simple, mathematically-derived nonlinear equations. The approximating equation for insulin resistance has been simplified, and uses a fasting blood sample. It is derived from the use of the insulin glucose product, divided by a constant.
The product of FPG (fasting plasma glucose) × FPI (fasting plasma insulin) is an index of hepatic insulin resistance.
HOMA-IR = (glucose × insulin) / 22.5
Insulin concentration is reported in μU/L and glucose in mmol/L.
The constant of 22.5 is a normalizing factor; i.e., the product of normal fasting plasma insulin of 5 μU/mL, and the normal fasting plasma glucose of 4.5 mmol/L typical of a “normal” healthy individual = 22.5.
Whereas the β-cell function is also calculated by another equation using fasting insulin and glucose values.
HOMA1 – %B = (20 × FPI) / (FPG – 3.5)
_
_
Estimation with the help of HOMA model parallels equally with that of the euglycemic clamp method (r = 0.88). HOMA-IR has been observed to have a linear correlation with the glucose clamp and minimal model estimates of insulin sensitivity/resistance in various studies of distinct populations. Derived from a mathematical assessment of the interaction between b-cell function and IR, the HOMA model is used to compute steady-state insulin and glucose concentrations. C-peptide, a measure of insulin secretion (not insulin action), can be used in HOMA modelling of both b-cell function and IR. A new version of HOMA, called HOMA 2 is recalibrated to modern insulin assays. It can be calculated with software from Oxford University called HOMA Calculator, however equations have not been published.
_
Glucose insulin (GI) product:
Application of the product of the plasma glucose and insulin concentrations during the OGTT has also been supported by few researchers as an index of whole-body insulin sensitivity. IR can be envisaged by increased plasma insulin in spite of normal or increased plasma glucose concentrations. The product of the plasma glucose and insulin concentrations provides the better index of insulin sensitivity. Furthermore, the higher the plasma glucose level, along with a higher plasma insulin response, the more severe is the state of insulin resistance. The lower the GI product, the more responsive are the tissues of the body to insulin..
_
Fasting insulin resistance index:
The fasting insulin resistance index (FIRI) was formulated by Duncan et al in search of a distinct marker, as the use or ratio of glucose and insulin might not be reliable for the estimation of IR. Increased insulin secretion to restore a normal level of plasma glucose leads to persistent elevation of insulin and probably of glucose also.
FIRI is calculated as FIRI = (fasting glucose × fasting insulin)/25.
_______
The analysis presented by Lorenzo et al. indicates that surrogate measures of insulin resistance containing a fasting insulin value provide potentially useful information about interindividual variation in insulin resistance at one point in time. A small amount of additional information can be obtained by adding multiple measures of insulin from OGTTs. Thus, simple surrogate measures of insulin resistance may be appropriate for large-scale clinical and epidemiological studies with cross-sectional design. The loss of accuracy compared with the clamp and minimal model approaches can be counterbalanced by large sample sizes, convenience, and cost savings. However, good correlations in cross-sectional settings don’t justify the use of surrogate measures of insulin resistance in other types of studies. Surrogates have already been shown to perform poorly in genetic association studies and in longitudinal metabolic studies. They need to be validated before they can be applied in any setting where cross-sectional variability in insulin resistance from all sources is not the primary characteristic of interest.
________
Imminent markers of insulin resistance:
With the passing of time and ongoing intensified research, many newer particles are gaining attention as surrogate markers in assessment of IR. In recent times, inflammatory markers have gained popularity in terms of assessment of insulin resistance.
Imminent markers of insulin resistance are as follows:
1. Insulin growth factor binding protein-1 (IGFBP-1)
2. sCD36 (solubleCD36)
3. C-reactive protein (CRP)
4. Ferritin
5. Adiponectin
6. Tumour necrosis factor (TNF alpha)
7. Resistin
8. C3 complement
9. Glycosylated hemoglobin (Hb)A1c
10. Protein kinase C (PKC) in microangiopathy
11. Sex hormone-binding globulin (SHBG) in hyperandrogenic Syndrome
_
Tools to detect insulin resistance include:
•Tests showing the degree of pancreatic output and what could be defined as “pancreatic stress”. These include both fasting insulin and fasting glucose, a Homeostasis Model Assessment (HOMA) that measures beta cell function and insulin sensitivity, a C-Peptide test and a pro-insulin test;
•Measurements of lipid hormones such as leptin and adiponectin. These biomarkers can give insight into a person’s unique communication between fat metabolism and insulin.
•Tests that evaluate a person’s degree of inflammation. These biomarkers include a cardiac-specific C-reactive protein measurement (CRP) and a sedimentation rate.
•Measurements that quantify fatty acid metabolism and the fatty acids released by the patient. These can also give particle size and number as well as an average inflammatory number.
Companies offering these kinds of screening tests include Genova Diagnostics and Metabolon. The ideal candidate for this in-depth testing is a person in whom there is strong family history of diabetes, who may not be fat, yet presents other features that point in the direction of metabolic imbalance, such as elevated cholesterol or hypertension. It is also a great resource for patient education and motivation in order to start their lifestyle change sooner rather than later.
_______
Assessment of beta cell function:
Since functional beta cell mass cannot be directly measured in patients with diabetes, methods that evaluate the beta cell function are used instead. This evaluation in physiologic conditions is however difficult because it is interrelated with other variables, mainly insulin sensitivity. Changes in insulin sensitivity (e.g. insulin resistance augmentation with increased body weight) demand compensation through a proportionate adjustment of insulin secretion by beta cells in order to maintain glucose homeostasis. In fact, it was proven that between insulin secretion and insulin sensitivity there is a non-linear, hyperbolic relationship, and the product of the two variables is constant (“disposition index”). Therefore, a correct evaluation of beta cell function requires quantification of insulin secretion in relation with insulin sensitivity (evaluation of the disposition index). In addition, the problem of hepatic extraction and non-constant clearance of insulin has to be considered. For this reason, C peptide values could be measured instead, because C peptide is co-secreted in equimolar amounts with insulin, undergoes non-significant hepatic extraction, has a relatively constant kinetics and thus reflects more accurately the pancreatic insulin secretion. Moreover, a good assessment of beta cell function requires an evaluation of the dynamic insulin secretory response to a stimulus, which, if not standardized, needs an appropriate method of normalization. It should be mentioned though that the normalization methods necessitate a specialized software and expertise. The gold-standard methods for evaluation of insulin secretion and sensitivity are considered hyperglycemic and euglycemic-hyperinsulinemic clamp, respectively, but they have many inconveniences (high cost, time consuming, complicated technique requiring trained personnel) which make their use in clinical practice difficult (even for some research purposes). Therefore, simpler methods are used in practice, such as oral stimulation tests (with glucose or mixed meals), intravenous stimulation tests (with glucose, glucagon or arginin) or simply surrogate markers derived from basal measurements (such as homeostasis model assessment (HOMA) indexes)
_
Surrogate methods for evaluation of beta cell function:
There is considerable overlap between methods for evaluation of b-cell function and methods to measure insulin resistance. The tests that evaluate beta cell function and insulin sensitivity may be used for a better understanding of the fundamental mechanisms that interconnect the two variables and to identify the factors that signal beta cell adaptation to changes of insulin sensitivity.
_
Current progress in Non-Invasive Imaging of Beta Cell Mass of the Endocrine Pancreas:
The increasing incidence of diabetes requires a better understanding of the pathogenesis of the clinical disease. Studies in prevention and treatment have been hampered by the single end-point of diagnosis of diabetes and hyperglycemia. The common pathology in both type 1 and type 2 diabetes is insufficient beta-cell mass to meet the metabolic demand. Unfortunately, current diagnostic methods rely on metabolic responses that do not accurately reflect true beta-cell mass. Recent advances in beta-cell imaging have utilized multiple modalities in experimental and clinical settings. While no “gold-standard” exists to measure beta-cell mass, modalities such as single photon emission computed tomography, optical and fluorescent imaging, magnetic resonance imaging, and positron emission tomography have been used with mixed success. Many of the methods are limited by the inability to translate to the clinical setting, poor discrimination between the exocrine and endocrine pancreas, or a poor measurement of beta-cell mass. However, promising new “neurofunctional imaging” approaches have emerged as improved measures of beta-cell mass.
__________
__________
Insulin treatment of DM:
_
Rationale of early insulin therapy (EIT) in beta cell preservation:
The current paradigm of management of type 2diabetes is one of sequential addition of treatment modalities starting from medical nutrition therapy, exercise, single or combination oral hypoglycemic agents (OHAs) and finally insulin administration with or without OHAs. This strategy has miserably failed in achieving recommended glycemic goals to prevent micro vascular as well as macro vascular complications. Besides it does not address the fundamental issues of progressive beta cell dysfunction and several other pathogenetic mechanisms including first phase insulin response, inflammation, glucotoxicity, lipotoxicity, inflammation etc. Patients continue to have glycosylated hemoglobin over 8% for more than 5 years before appropriate treatment decisions are made. Despite the development of new drugs and therapeutic strategies for treating type 2 diabetes mellitus (T2DM), achieving long-term glycemic control remains a challenge. Results from the United Kingdom Prospective Diabetes Study (UKPDS) suggest that deterioration of glycemic control can be largely attributed to progressive β-cell loss, irrespective of the nature of pharmacological intervention. Therefore, treatments that can preserve or improve β-cell function are of great interest in the field of T2DM therapeutics. Insulin administration is uniquely suitable to address most of these issues, provided it is started early in the natural history of type 2 diabetes. Short-term intensive insulin administration quickly restores normoglycemia, provides rest to the stressed beta cells, allowing them to regenerate and helps in maintaining long term glycemic control with diet, exercise and insulin sensitizers. It also prevents deposition of islet amyloid which is inherent with sulfonylureas administration, the most prescribed drug in the treatment of type 2 diabetes. Early initiation of insulin addresses the issues of glucotoxicity, lipotoxicity, inflammation, insulin resistance, first phase insulin response and many others. Data from preclinical studies also suggest that insulin exerts antiapoptotic effects via its action on IRS proteins. Using an IRS-1-knockout mouse model, Hennige and colleagues demonstrated that insulin plays an important antiapoptotic role and may promote b-cell growth. Backed by incontrovertible pathophysiological rationale and evidenced by elegant animal and human studies, it sounds prudent to shift the paradigm of insulin administration in type 2 diabetes from one of ‘last resort’ to ‘first assault’.
_
Insulin is widely thought of as a treatment of last resort for patients with type 2 diabetes, but new research may help change that thinking. Early insulin therapy resulted in better control than that seen with oral medications. Researchers suggest that early insulin therapy can help patients maintain the ability to produce their own insulin longer. In addition, early insulin therapy is not associated with hypoglycemia or weight gain and does not negatively affect volunteers’ cholesterol levels, researchers say. These are all common reasons doctors delay prescribing insulin therapy. Researchers say the message is not that all, or even most, people with type 2 diabetes should be on insulin from the start, but that doctors should not delay giving insulin to patients who may need it.
_
While several strategies can be employed to tackle many of these factors contributing to beta cell decline, insulin alone has the most salutary effect on majority of them. Both acute and prolonged hyperglycemia adversely affects beta cell function. Glucotoxicity leads to impaired gene transcription, down-regulation of glucose transporters and alteration of transporter function induced by oxidative stress. Early use of insulin results in increased insulin gene expression and insulin synthesis. It provides rest to beta cells, already stretched to their capacity and helps them regenerate over time. Beta cells are most stressed and therefore most vulnerable to programmed cell death (apoptosis) during the first few months following the clinical onset of diabetes. Quick restoration of euglycemia by early insulin therapy at this stage will naturally preserve beta cell function on a long term basis. This has been demonstrated in several experimental and clinical studies. In Chinese hamster, a spontaneous and selectively inbred animal model for non-obese type 2 diabetics, two weeks of normalization of glycemia resulted in marked improvement in beta cell function. This was characterized by improved beta cell signalling induced by the cyclic AMP protein kinase A pathway. This was also associated with improved islet insulin content and beta cell morphology as demonstrated by immunocytochemistry. In patients with Latent Autoimmune Diabetes of Adults (LADA), early initiation of insulin has been shown to preserve beta cell function. This was evidenced by preserved C-peptide response compared to baseline in insulin treated group, as compared to Sulfonylurea (SU) group, which showed significantly lesser C-peptide after two years. This worsened further at the end of three years. It has also been demonstrated that short term glycemic control by intravenous insulin infusion restores SU sensitivity in significant proportion of non-obese SU nonresponsive type 2 diabetic subjects. These patients showed a significant improvement of metabolic control and beta cell secretion. During the 6-month follow up period they could be managed with glibenclamide alone. Metabolic improvement was associated with improvement in fasting and post meal C-peptide levels as well.
_
Loss of first phase insulin response (FPIR) has emerged as one of the most important factor in the pathogenesis of type 2 diabetes. Its magnitude correlates with the degree of beta cell dysfunction. Its consequences include:
a. Inadequate inhibition of endogenous glucose production.
b. Rise in non esterified fatty acids (NEFA) due to inadequate antilipolytic action of insulin.
c. Inadequate priming of insulin sensitive tissues leading to decreased glucose disposal.
d. Altered signalling capacity of hormones leading to insulin resistance.
e. Enhanced stimulatory action of glucagon on gluconeogenesis.
f. Enhanced post prandial hyperglycemia.
g. Increased risk of micro and macrovascular complications.
It is also important to understand the correlation between levels of glycemia and loss of FPIR:
• FPIR is mostly absent when the FPG is more than 109 mg/dl
• When FPG is > 140 mg/dl 75% of beta cell function is lost.
• When FPG is > 180 mg/dl there is complete loss of FPIR.
• When 2 hrs PG values are > 200 mg/dl there is marked reduction in FPIR.
• Even in subjects with IGT there is marked reduction in the FPIR.
Considering these facts it seems prudent that all attempts be made to restore FPIR. This would logically correct or mitigate all the consequences mentioned above. Additional benefits would include beta cell rest, reduced hyperinsulinemia of the late phase after ingestion of a meal, reduced production of islet amyloid peptide and improved insulin secretion over time. Excessive accumulation of amyloid deposits between islet cells and capillaries leads to destruction of islet endocrine cells and progressive worsening of beta cell function. Current paradigm of using sulfonylureas (SU) in majority of type 2 diabetic patients leads to increased deposition of amyloid deposits and faster decline in beta cell function. However insulin sparing sulfonylureas (Glimepiride) and non-sulfonylureas secretagogues (Repaglinide and Nateglinide) may not produce this undesired effect. Bruttomesso et al have shown that intra venous infusion of insulin during the first 30 minutes of an OGTT markedly improved glucose tolerance by restoring FPIR. It was clearly demonstrated in this study that neither a continuous infusion of insulin nor delaying the infusion beyond 30 minutes achieved similar benefit. This implies the importance of the timing of insulin administration. Several other studies have used subcutaneous Lispro, Aspart and others to demonstrate similar benefits; thus assuring translation of these benefits of restoring FPIR in clinical practice. These studies have shown that the restoration of FPIR by intensive insulin treatment leads to improved insulin secretion and long term glycemic control. This may pave way to withdraw insulin for several years.
_
A number of in vitro and animal studies have demonstrated that chronic elevation of free fatty acid impairs beta cell
function (Lipotoxicity). Free fatty acid (FFA) also antagonizes the action of insulin, both on glucose production and glucose utilization. It also promotes Gluconeogenesis and enhanced Glucose 6-phosphatase gene expression, which directly increases glucose production. Increased beta cell concentration of fatty acid co-A, TNF-alpha, resistin, leptin, adipsin and amylin and tissue accumulation of lipids all contribute to the inexorable decline in beta cell function. Early insulin therapy is known to mitigate the deleterious effects of these molecules directly or indirectly.
_
Clinical studies have shown beneficial effects of early short-term (2–3 weeks) intensive insulin therapy in patients with newly diagnosed T2DM. A multi-center randomized trial showed that early, short-term intensive insulin treatment at the time of T2DM diagnosis led to improved β-cell function and greater diabetes remission rates at 1 year compared with early, short-term treatment with oral hypoglycemic agents. A recent randomized control trial evaluated β-cell function preservation after 3.5 years of intensive therapy with insulin plus metformin compared with triple oral therapy with metformin, glyburide, and pioglitazone after an initial 3-month insulin treatment period and found that both approaches were effective in preserving β-cell function. Based on these results, the authors suggested that patients with newly diagnosed T2DM should be treated with an initial period of intensive insulin therapy to maximize β-cell recovery and then continued either on insulin therapy or switched to a combination of oral agents with complementary mechanisms of action. The Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial recently reported results of the comparison between treatment with insulin glargine to normalize fasting plasma glucose levels compared with standard care on cardiovascular outcomes, cancer, and incident diabetes in over 12,000 patients with cardiovascular disease risk factors plus IFG or T2DM who were followed for a median of 6.2 years. They found that glargine had a neutral effect on cardiovascular outcomes and cancers, but that it reduced new-onset diabetes among the participants without diabetes at randomization providing support for the idea that the early use of insulin may preserve β-cell function in patients with impaired glucose homeostasis.
_
Currently, T2DM is managed by a progressive stepwise introduction of lifestyle interventions, followed by the use of oral agents alone and in combination and, finally, insulin. While some evidence indicates that oral agents, such as metformin and TZDs, may preserve b-cell function, patients using metformin still exhibit a continuous loss of b-cell function over time and TZDs are associated with significant adverse effects. In addition, despite the fact that sulfonylureas remain the most common initial drug choice in T2DM, their use may increase the risk of b-cell apoptosis. The impact of incretin mimetics and DPP-IV inhibitors on b-cell function in a clinical setting is still under investigation. Moreover, some residual b-cell function is required for these agents to provide glycemic control. Since early insulin initiation has been shown to preserve b-cell function, it is likely that the current practice of initiating T2DM therapy with oral agents may, eventually, be replaced with early intensive insulin therapy. Lifestyle changes will be strongly encouraged, together with adjunctive therapies, to control weight, improve insulin resistance and protect and/or restore b-cell function. In addition to A1C, FPG and postprandial glucose (PPG), b-cell function will be routinely monitored as a means of assessing T2DM progression. Closer monitoring of blood glucose levels by patients will also be recommended to minimize glycemic variability and glucose swings. In the new paradigm, intensive insulin-induction therapy will be the focus of early T2DM therapy instead of the use of oral insulin secretagogues and sensitizers. The goal of such therapy would be twofold: first, to ‘rest’ the b-cell while there is still potential to reverse deterioration; and second, to establish tight glycemic control immediately after T2DM diagnosis. Once tight control is established, scheduled insulin-replacement therapy can be instituted to maintain A1C as close to normal as possible, with the goal of slowing progression of T2DM and its long-term adverse health-related consequences.
_
Many of the studies demonstrating the benefits of early intensive insulin treatment used CSII or MDI to initiate insulin therapy. As such regimens are expensive and complex—and therefore unlikely to be adopted in clinical practice—Zeng et al. conducted a randomized, open-label, parallel-group study to assess the effects of early insulin therapy using basal insulin monotherapy, compared with CSII, in 59 people with newly diagnosed T2DM. Following 2 weeks of treatment, both insulin regimens significantly improved glycemic control, β-cell function, and plasma lipid profiles compared with baseline, and there were no significant differences between the two groups, except that the time to achieve the fasting glycemic target (defined as a fasting capillary blood glucose level between 4.4 and 6.1 mmol/L, regardless of postprandial blood glucose levels) was significantly shorter with CSII than with insulin monotherapy (P<0.01). The results therefore suggest that basal insulin monotherapy might be a reasonable alternative to CSII for initial insulin therapy in people with newly diagnosed T2DM. The importance of correcting basal insulin levels for diurnal control has long been recognized. The Treating-to-Target in Type 2 diabetes (4-T) study showed that initiating insulin-based treatment with a basal insulin (once/twice daily insulin detemir) provides considerable benefits over a prandial insulin (three times daily insulin aspart) or premixed insulin (twice daily biphasic insulin aspart) regimen in people with T2DM.48,49 At 3 years, a greater proportion of people randomized to basal or prandial insulin, versus premixed insulin, had reached an HbA1c target of ≤7.0%, whereas treatment with basal insulin detemir was associated with significantly lower rates of hypoglycemia and less weight gain compared with prandial and premixed insulin regimens.
_
United Kingdom Prospective Diabetes Study (UKPDS) study started 35 years ago with 5012 people with mean fasting plasma glucose at diagnosis 209 mg/dl for men and 223 mg/dl for women, and the corresponding HbA1c values were 9.0% and 9.3%, respectively. The trial showed that sulfonylureas, metformin, and insulin were equally effective in reducing microvascular complications. Meanwhile, in contrary to fears about sulfonylureas and insulin, none of these agents caused harm through cardiovascular or other toxicity. However in an analysis of 4,209 subjects followed for the first 6 years after randomization, all the treatment groups showed deterioration of glycemic control over time with the rate of increase of HbA1c remarkably similar in all the groups, about 0.2–0.3% of HbA1c yearly. Analysis of the available data suggests that the rise of HbA1c was mainly due to a progressive decline of beta-cell function rather than worsening of insulin resistance or lack of adherence to medications. These observations showed that diabetes itself is a progressive disorder despite any drug used in UKPDS, therefore most people need combination treatments by time, and many people will need insulin treatments generally with multiple injections. The UKPDS follow-up 10 years after the end of the original study showed that a period of good glycemic control early in the course of the disease has a lasting benefit independent of subsequent glycemic control. The relative risks of non-fatal MI, death related to diabetes, and all-cause mortality were 15% (P=0.01), 17% (P=0.01), and 13% (P=0.007) lower in individuals originally treated with intensive therapy compared with conventional therapy. The reduction in the risk of microvascular complications with intensive therapy was also maintained (24%; P=0.001). These benefits were achieved despite the fact that the difference in HbA1c levels apparent at the end of intervention was lost within 1 year and that HbA1c levels were comparable over the remaining follow-up period. The results of the UKPDS mandate that treatment of T2DM include aggressive efforts to lower blood glucose to as close to normal as possible early in the disease process. The lasting benefit could partly be due to metabolic memory, thereby reducing vascular stresses in diabetes despite deteriorating glycemic control. A legacy effect or “metabolic memory” is believed to be associated, in part, with the increased formation of advanced glycation end-products evident in T2DM. Advanced glycation end-products modify mitochondrial DNA respiratory proteins, resulting in excess ROS, which cause further mitochondrial DNA damage and functional respiratory chain decline. This process perpetuates ROS generation and overall cellular injury, maintaining oxidative stress signalling even after normoglycemia is achieved. Intensive insulin treatment is associated with significantly lower levels of advanced glycation end-products than conventional treatment, supporting the long-term benefits observed with an intensive management strategy. Owing to the favorable outcomes observed in the UKPDS study, several large-scale studies have been undertaken that assessed the effects of intensive treatment regimens on vascular outcomes in people with advanced T2DM. These include ACCORD (Action to Control Cardiovascular Risk in Diabetes), ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation), and VADT (Veterans Affairs Diabetes Trial). In all three of these studies, an intensive control regimen resulted in better glycemic outcomes, including significantly lower HbA1c, than standard therapy. The benefits of intensive glycemic control in people with T2DM have been confirmed by several meta-analyses. These suggest that intensive glycemic control does not significantly affect all-cause mortality or cardiovascular (CV) death but is associated with significant reductions in the risk of non-fatal MI, with an increase in the risk of severe hypoglycemia, compared with standard treatment. Based on these observed benefits Duckworth et al. suggested that intensive glycemic control would provide maximal benefits if initiated earlier in the disease, particularly if severe hypoglycemia was avoided.
__
The figure below shows that even 1 year after early insulin therapy in T2DM, endogenous insulin levels are high suggesting improved b-cell function and prevention of progression of DM:
_____
_____
Early Insulin Treatment in Type 2 Diabetes: a 2009 review:
Baseline characteristics and outcomes of patients with type 2 diabetes receiving temporary insulin therapy at disease diagnosis:
| n | Age | BMI | Baseline A1C (%) | Insulin dose (units · kg−1 · day−1) | Days to glycemic control | Duration insulin therapy (weeks) | % Early responders | % Sustained responders | Weight change | |
| Ilkova et al. | 13 | 50 | 27 | 11.2 | 0.61 | 1.9 | 2 | 92 | 69 (26 months) | 0.4 kg |
| Li et al. | 126 | 50 | 25 | 10.0 | 0.7 | 6.3 | 2 | 90 | 42 (24 months) | −0.04 kg/m2 |
| Ryan et al. | 16 | 52 | 31 | 11.8 | 0.37–0.73 | <14 | 2–3 | 88 | 44 (12 months) | −0.5 kg/m2 |
Early responders are subjects who achieved euglycemia with insulin treatment, and late responders are subjects who maintained long-term euglycemia without pharmacotherapy after the initial insulin treatment. In summary, aggressive and often temporary use of insulin therapy at disease onset in type 2 diabetes is associated with effective glycemic control with minimal weight gain and hypoglycemia. Early restitution of physiologic insulin secretion and glycemic control could be, in theory, followed by therapies to prolong maintenance of euglycemia, such as thiazolidinediones or glucagons-like peptide 1–based interventions. A more timely and selective introduction of insulin replacement therapy, as β-cell function progresses, could facilitate the achievement and maintenance of euglycemia and thus reduce disease-associated complications.
_
The Effect of Early Insulin Therapy on Pancreatic β-Cell Function and Long-Term Glycemic Control in Newly Diagnosed Type 2 Diabetic Patients: 2010 study:
Authors retrospectively evaluated the effect of EIT on pancreatic β-cell function and long-term glycemic control in newly diagnosed patients who had T2DM undergoing treatment in a general outpatient clinic. Insulin was applied in biphasic or prandial fashion with or without basal insulin, approaches that are commonly employed in general practice. Their results suggest that regardless of the method of delivery, EIT significantly improves β-cell function and facilitates long-term glycemic control in patients with newly diagnosed type 2 diabetes mellitus.
_
β-Cell Function Preservation after 3.5 Years of Intensive Diabetes Therapy: a 2012 study:
Objective was to assess β-cell function preservation after 3.5 years of intensive therapy with insulin plus metformin (INS group) versus triple oral therapy (TOT group) with metformin, glyburide, and pioglitazone. The study shows that it is possible to preserve β-cell function long after the initial diagnosis of type 2 diabetes if therapy is initiated in a timely and intensive manner. Instead of starting with diet and/or monotherapy followed by stepwise treatment escalation when failure is achieved, patients should be treated with an initial period of intensive insulin therapy to maximize β-cell recovery and then either continued on an insulin-based regimen or switched to multiple hypoglycemic medications with complementary mechanisms of action. Either of these strategies will stabilize β-cell function and maintain excellent long-term glucose control, a desirable disease-modifying effect. In addition, this can be achieved safely, and authors have previously shown that both treatment strategies have high patient satisfaction ratings with improved quality of life. In conclusion, intensive insulin therapy at the time of diagnosis of type 2 diabetes followed by either an insulin-based regimen or multiple oral hypoglycemic agents preserves both glycemic control and β-cell function for at least 3.5 years with no significant difference in the adverse-effect profile.
_
Use of insulin in type 2 diabetes: What we learned from recent clinical trials on the benefits of early insulin initiation: 2014 review:
The majority of people with type 2 diabetes mellitus (T2DM) require insulin therapy to maintain HbA1clevels < 7% during the first decade of diagnosis. Large prospective trials investigating the cardiovascular (CV) benefits of intensive glycaemic control have produced inconsistent results; however, meta-analyses have suggested that intensive glycaemic control provides both micro- and macrovascular benefits. The ORIGIN study investigated the impact of basal insulin glargine therapy targeting ≤ 5.3 mmol/L for fasting plasma glucose compared with standard care on CV outcomes in people with pre- or early diabetes, and demonstrated a neutral effect on CV outcomes with long-term use of insulin glargine early in the course of diabetes, with a low rate of severe hypoglycaemia and modest weight gain. The EARLY, GLORY and EASIE studies also demonstrated that insulin use earlier in the treatment pathway led to improved glycaemic control, reduced weight gain and fewer hypoglycaemic episodes than when insulin was added later in the course of disease. The beneficial effect of early transient intensive insulin therapy (TIIT) at diagnosis has been demonstrated in a number of trials; it rapidly limits the damage caused by gluco- and lipotoxicity, improving residual b-cell function and potentially slowing disease progression. The evidence suggests that people newly diagnosed with T2DM and HbA1c> 9% should be given early TIIT to achieve normoglycemia within weeks, after which standard care should then be adopted. Insulin use earlier in the treatment pathway should be considered, as it reduces the risk of hypoglycaemia as well as allows b-cell rest, which can help preserve b-cell function.
_
Early insulin therapy (EIT) studies:
_
Milestones in clinical research of early insulinization for preservation of β-cell function: 2014:
Correction of hyperglycemia with insulin increases peripheral sensitivity and improves residual β-cell function. The hypothesis of β-cell protection by early insulin therapy was tested by several clinical studies, beginning with noncontrolled studies in patients with severe hyperglycemia and followed by randomized controlled studies in severely uncontrolled newly diagnosed patients using short-term and longer-term insulin therapy as well as well-controlled type 2 diabetic patients using long-term insulin therapy as seen in the figure below.
_
When one considers initiation of insulin therapy in a type 2 diabetic patient with the intention to preserve β-cell function, the level of evidence supporting this decision is relatively high.
_
For the subgroup of patients with severe symptomatic hyperglycemia, there is strong evidence, in addition to guideline recommendations (American Diabetes Association/ European Association for the Study of Diabetes, International Diabetes Federation, American Association of Clinical Endocrinologists, Canadian, and National Institute for Health and Care Excellence), to support initiation of short-term insulin therapy. Insulin therapy is an effective way to reverse short-term glucotoxicity and lipotoxicity and shows evidence of midterm β-cell preservation. Short-term insulin treatment is safe, with low incidence of hypoglycemia and less concern for weight gain. The place of long-term early insulin treatment in well-controlled type 2 diabetic patients is still debatable. Although the ORIGIN study demonstrated that early insulin therapy with insulin glargine is both safe and feasible, there are still the pros and cons of this treatment to consider. The benefits are as follows. 1) Insulin therapy can achieve near-normal FPG. 2) The achievement of such goals, especially in newly-onset diabetes, is relatively easy and can be maintained for many years. 3) Early treatment with insulin is safe with respect to both cardiovascular and tumor genesis. Among the drawbacks to consider regarding early insulin therapy are 1) weight gain (even though weight gain might be mild, its clinical consequences are yet unknown), 2) increased risk of hypoglycemia, and 3) patients’ preference for different treatments. In light of the above, who will be a good candidate for early insulin therapy? Beyond the consensus concerning severely hyperglycemic patients, other patients who may benefit from early insulin therapy may be those who have a more prominent increase in FPG compared with their increase in postprandial glucose. Another group may be the leaner type 2 diabetic patients, since increased weight is less of a risk, and they are more likely to be insulin deficient. Lastly, we may consider early insulin therapy in obese type 2 diabetes in combination with GLP-1 analogs or in patients treated with GLP-1 analog when FPG still remains high.
________
Insulin resistance occurs in parallel with sensory neuropathy in streptozotocin-induced diabetes in rats: differential response to early vs late insulin supplementation: a 2012 study:
Authors investigated whether progressive sensory neuropathy was accompanied by changes in whole-body insulin sensitivity (WBIS) in rats made diabetic by streptozotocin (STZ). The effects of early and late insulin supplementation were also studied. The STZ-treated rats failed to gain weight and exhibited stable hyperglycemia and low plasma insulin levels with a decrease in nerve conduction velocity (NCV) measured in A and C fibers of the saphenous nerve. A decreased sensory neuropeptide (SNP) release such as that of substance P, somatostatin, and calcitonin gene–related peptide determined from organ fluid of tracheal preparations subjected to electrical field stimulation also occurred in diabetic animals. These features were accompanied by a decrease in WBIS measured by hyperinsulinemic-euglycemic glucose clamping and a decrease in insulin-stimulated glucose uptake in cardiac and gastrocnemius muscle. When insulin supplementation with slow-release implants (2 IU/d) was started 4 weeks after STZ injection, blood glucose level normalized. Both insulin sensitivity and sensory nerve function reflected in either NCV or SNP release completely recovered by the 12th post-STZ week. When the insulin implants were applied from the eighth post-STZ week, both WBIS and glucose uptake remained significantly decreased, with a seriously impaired NCV and SNP release with strong hyperglycemia. Late insulin supplementation, however, even by using double implantation from the 10th post-STZ week, was unable to restore blood glucose, WBIS, NCV, and SNP release by the 12th week. Insulin resistance occurs in parallel with sensory neuropathy in STZ-diabetic rats. Both can be improved by early but not late insulin supplementation. The rationale of the study is early insulin therapy reduces insulin resistance and prevents diabetic complication as opposed to late insulin therapy.
________
Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients: a 2008 study:
The role of intensive insulin therapy in medical surgical intensive care patients remains unclear. The objective of this study was to examine the effect of intensive insulin therapy on mortality in medical surgical intensive care unit patients. A total of 523 patients were randomly assigned to receive intensive insulin therapy (target blood glucose 4.4-6.1 mmol/L or 80-110 mg/dL) or conventional insulin therapy (target blood glucose 10-11.1 mmol/L or 180-200 mg/dL). Intensive insulin therapy was not associated with improved survival among medical surgical intensive care unit patients and was associated with increased occurrence of hypoglycemia. Based on these results, authors do not advocate universal application of intensive insulin therapy in intensive care unit patients.
_
The original trial of intensive insulin therapy in surgical intensive care unit patients by Van den Berghe et al. demonstrating improved morbidity and mortality led to widespread adoption of this therapy worldwide. The failure of a subsequent trial to show improvement which was stopped early due to an increased risk of hypoglycemia, the VISEP trial, and the failure to improve mortality in a second trial by Van den Berghe et al. of intensive insulin therapy in medical intensive care unit patients have raised serious concerns about the general applicability of intensive insulin therapy.
_
Intensive versus Conventional Glucose Control in Critically Ill Patients: a 2009 study:
Within 24 hours after admission to an intensive care unit (ICU), adults who were expected to require treatment in the ICU on 3 or more consecutive days were randomly assigned to undergo either intensive glucose control, with a target blood glucose range of 81 to 108 mg per deciliter (4.5 to 6.0 mmol per liter), or conventional glucose control, with a target of 180 mg or less per deciliter (10.0 mmol or less per liter). Authors defined the primary end point as death from any cause within 90 days after randomization. In this large, international, randomized trial, authors found that intensive glucose control increased mortality among adults in the ICU: a blood glucose target of 180 mg or less per deciliter resulted in lower mortality than did a target of 81 to 108 mg per deciliter.
_
The pros and cons of intensive insulin therapy to prevent surgical site infections: 2014 study:
To summarize the results of the existing international literature, Kao and associates performed a Cochrane review concerning the effect of intensive insulin therapy in the perioperative period on the incidence of surgical site infections, incidence of hypoglycemia, level of glycemic control, all-cause and infection-related mortalities, and hospital stay. Furthermore, Kao and associates investigated the effects of various levels of glycemic control. Only1 trial (out of 5 randomized, controlled trials) demonstrated a significant reduction in surgical site infections with strict glycemic control, but the quality of this trial was difficult to assess as a result of poor reporting. Kao and associates concluded that there was insufficient evidence to support strict glycemic control versus conventional management (maintenance of glucose < 200 mg/dL) for the prevention of surgical site infections.
_
Targeting intensive versus conventional glycaemic control for type 1 diabetes mellitus: a systematic review with meta-analyses and trial sequential analyses of randomised clinical trials: a 2014 study:
To assess the benefits and harms of targeting intensive versus conventional glycaemic control in patients with type 1 diabetes mellitus, a systematic review with meta-analyses and trial sequential analyses of randomised clinical trials.
Results:
18 randomised clinical trials included 2254 participants with type 1 diabetes mellitus. All trials had high risk of bias. There was no statistically significant effect of targeting intensive glycaemic control on all-cause mortality (risk ratio 1.16, 95% CI 0.65 to 2.08) or cardiovascular mortality (0.49, 0.19 to 1.24). Targeting intensive glycaemic control reduced the relative risks for the composite macrovascular outcome (0.63, 0.41 to 0.96; p=0.03), and nephropathy (0.37, 0.27 to 0.50; p<0.00001. The effect estimates of retinopathy, ketoacidosis and retinal photocoagulation were not consistently statistically significant between random and fixed effects models. The risk of severe hypoglycaemia was significantly increased with intensive glycaemic targets (1.40, 1.01 to 1.94). Trial sequential analyses showed that the amount of data needed to demonstrate a relative risk reduction of 10% were, in general, inadequate.
Conclusions:
There was no significant effect towards improved all-cause mortality when targeting intensive glycaemic control compared with conventional glycaemic control. However, there may be beneficial effects of targeting intensive glycaemic control on the composite macrovascular outcome and on nephropathy, and detrimental effects on severe hypoglycaemia. Notably, the data for retinopathy and ketoacidosis were inconsistent. There was a severe lack of reporting on patient relevant outcomes, and all trials had poor bias control.
_______
Multifaceted benefit of insulin:
Aside from glycemic control, insulin treatment can potentially provide additional benefits. The anti-inflammatory and antioxidant effects of insulin may contribute to protection against endothelial dysfunction and vascular disease. These effects include suppression of reactive oxygen species (ROS) and adhesion molecule expression. Insulin has also been demonstrated to induce endothelial nitric oxide synthase expression in endothelial cells, causing vascular dilatation due to increased production of nitric oxide. In general, T2DM is associated with progressive deterioration of β-cell mass and function, and one of the key goals of therapy is to preserve β-cells. Factors that are thought to promote β-cell loss include insulin resistance, glucotoxicity and lipotoxicity, inflammation, and obesity. It has been known for nearly 40 years that insulin therapy improves β-cell function as determined by an enhanced insulin response to glucose. Clinical evidence demonstrates that early insulin therapy in T2DM maximizes the potential to nearly normalize glucose control, to prevent progression of glucose intolerance, to restore β-cell function, to improve metabolic memory, to offer long-term protection to end organs, and to enhance individuals’ quality of life. Recent clinical data from the ORIGIN trial confirm the efficacy and safety of early insulin therapy with only modest increases in hypoglycemia and weight gain. Treatment with insulin may also provide independent protection against vascular endothelial dysfunction through its anti-inflammatory and antioxidative stress actions; however, longer-term follow-up of trials is needed to determine whether this protection results in clinically relevant changes to outcomes.
______
Endogenous insulin secretion in diabetic management:
Guidelines for treatment in Type 1 and Type 2 diabetes differ greatly predominantly reflecting differences in endogenous insulin secretion. Within both major subgroups of diabetes there is both between individual variation and with time intra-individual reduction in a patient’s endogenous insulin secretion which results in differing treatment requirements. Traditionally, in clinical practice, endogenous insulin secretion is not measured and treatment decisions are made on the basis of glycaemic control and clinical diagnosis of diabetes subtype. There is some evidence supporting direct measurement of endogenous insulin secretion to assess the most appropriate treatment for a patient, particularly in the context of predicting response to oral therapy. Little is known regarding whether measuring endogenous insulin secretion can assist choice of insulin regimen. It is possible to measure endogenous insulin secretion in clinical practice using C-peptide, which is secreted in equimolar amounts to insulin. 90 minute C-peptide in a formal mixed meal test is a robust assessment of insulin response in insulin treated patients. One area where underlying insulin secretion is likely to affect treatment requirements is requirement for prandial exogenous insulin. Intensive insulin regimens with prandial rapid or short acting insulin are clearly appropriate in Type 1 diabetes outside the honeymoon period where there is absolute insulin deficiency. However in Type 2 diabetes where endogenous insulin secretion is preserved, excellent glycaemic control can be achieved using basal (intermediate or long acting) insulin without rapid or short acting prandial insulin. Researchers hypothesised that in insulin treated diabetes, patients with higher endogenous insulin secretion will have a lower rise in glucose after meals and will respond less to prandial insulin. So they aimed to assess this in a mixed population of Type 1 and Type 2 diabetes with a wide spectrum of insulin secretion.
_
Assessment of endogenous insulin secretion in insulin treated diabetes predicts postprandial glucose and treatment response to prandial insulin: a 2012 study:
Authors assessed endogenous insulin secretion in 102 participants with insulin treated diabetes (58 Type 1) following a standardised mixed meal without exogenous insulin. They tested the relationship between endogenous insulin secretion and post meal hyperglycaemia. In 80 participants treated with fast acting breakfast insulin they repeated the mixed meal with participants usual insulin given and assessed the impact of endogenous insulin secretion on response to exogenous prandial insulin. Post meal glucose increment was inversely correlated with endogenous insulin secretion (90 minute C-peptide. Similar doses of exogenous prandial insulin lowered glucose increment more when patients had less endogenous insulin; by 6.4(4.2-11.1) verses 1.2(0.03-2.88) mmol/L (p<0.001) for patients in the lowest verses highest tertiles of endogenous insulin. In insulin treated patients, the measurement of endogenous insulin secretion may help predict the degree of postprandial hyperglycaemia and the likely response to prandial insulin. Authors have shown that patients with less endogenous insulin secretion have greater post meal hyperglycaemia and greater response to prandial exogenous insulin. In this study of insulin treated patients with Type 1 and Type 2 diabetes postprandial hyperglycaemia is inversely related to endogenous insulin secretion. This is consistent with previous research in non insulin treated Type 2 diabetes has shown that those with low insulin secretion have higher glucose increment after oral glucose tolerance test and higher glycaemic variability.
__________
__________
Insulin: Misconceptions and Reality:
The lack of adequate insulin secretion characterizes all hyperglycemic states. When insulin action is normal, as in type 1 diabetes, there is a near total loss of insulin secretory function. In type 2 diabetes, the abnormalities in insulin secretion are multiple. One of the initial defects is a loss of the early phase of meal-stimulated insulin secretion. This is followed by an inability of the β-cell to increase insulin secretion sufficient to overcome hepatic and peripheral insulin resistance. Type 2 diabetes is characterized by a progressive decrease in both β-cell mass and secretory function so that, in most individuals, absolute insulin deficiency occurs in the late stages of the disease. It would seem logical that the ideal treatment for type 2 diabetes should be early and continuing insulin therapy. Unfortunately, there are several misconceptions of insulin treatment and insulin action in type 2 diabetes that limit the usefulness of insulin treatment and that suggest that chronic insulin therapy is best used in the later stages of diabetes when there is an absolute deficiency of insulin. I attempt to clear these misconceptions:
_
Insulin therapy and insulin resistance:
Contrary to some beliefs, there is no evidence that doses of insulin used in clinical practice exacerbate insulin resistance. It has long been recognized that the chronic hyperglycemia associated with type 2 diabetes (glucose toxicity) leads to impairment in insulin secretion and a possible defect in glycogen synthesis. In a study of intensive insulin therapy in patients with type 2 diabetes, the use of insulin partially reversed the postbinding defect in peripheral insulin action, produced near-normal basal hepatic glucose output, and enhanced insulin secretion, thereby maintaining lower glucose values. In addition, the mean daily insulin requirement fell by 23% after ∼2 weeks of therapy, levelling off thereafter.
_
Insulin therapy and weight gain:
The use of insulin has been associated with weight gain, which in turn has been considered a major factor in insulin resistance. The magnitude of the weight gain is influenced by the level of the initial glycemic control, the treatment glycemic control achieved, the duration of insulin therapy, the insulin regimen used, and which combination of oral agents are concomitantly used. For example, in a study normalizing the HbA1c during 6 months of intensive multiple-dose insulin therapy, the mean weight gain was 8.7 kg. The 3-year data from the 4-T trial of insulin treatment regimens added to oral agents showed a 1.4% decrease in HbA1c and 6.4-kg weight gain in the patients assigned to prandial insulin treatment and a 1.3% decrease in HbA1c and 5.7-kg weight gain in the patients assigned to mixed insulin twice a day. In the ACCORD study, 28% of the intensively treated cohort and 14% of the ordinarily treated cohort gained >10 kg during a mean treatment period of ~3.5 years. In the UKPDS, patients in intensive therapy gained more weight than those in conventional therapy groups; patients taking insulin gained ∼4 kg compared to 2.6 kg for those on chlorpropamide and 1.7 kg for those on glibenclamide. Yet patients in the intensive therapy groups also had fewer microvascular complications, suggesting that tight glycemic control may be more important in therapeutic decision-making. The use of metformin as an adjunct to insulin therapy provides effective glycemic control without significant weight gain.
_
A type 2 diabetics has a common story: “I was never fat until after my doctor started me on insulin.” Usually these people have been following high-carbohydrate diets and so must inject large doses of insulin to effect a modicum of blood sugar control. Insulin, remember, is the principal fat-building hormone of the body. Although a type 2 diabetic may be resistant to insulin-facilitated glucose transport (from blood to tissues), that resistance doesn’t diminish insulin’s capacity for fat-building. In other words, insulin can be great at making you fat even though it may be, for those with insulin resistance, inefficient at lowering your blood sugar. Since excess insulin causes insulin resistance, the more you take, the more you’ll need, and the fatter you’ll get. This is not an argument against the use of insulin; rather it supports the conclusion that high levels of dietary carbohydrate—which, in turn, require large amounts of insulin— usually make blood sugar control (and weight reduction) impossible. Doctors have witnessed, over and over, dramatic weight loss and blood sugar improvement in people who have merely been shown how to reduce their carbohydrate intake and therefore their insulin doses.
_
Insulin therapy and hypoglycemia:
Hypoglycemia may occur from a mismatch between insulin and carbohydrate intake, exercise, or alcohol consumption. Hypoglycemia has been associated with an increased risk of dementia and may have implications in cardiac arrhythmia. Clinicians often cite hypoglycemia as an adverse effect that might preclude the use of insulin. Indeed, in the DCCT study of type 1 diabetes, tighter control produced a risk of severe hypoglycemia three times higher than that of conventional therapy. This must be viewed, however, in the context of substantially reduced microvascular and neurological complications. Furthermore, the rates of severe hypoglycemia are quite low in type 2 diabetes. In the Kumamoto study of type 2 diabetes, average A1C results were 7.1 and 9.4% for tight and conventional groups, respectively. However, only mild hypoglycemic reactions occurred and at similar rates in both groups. The UKPDS found that the rate of major hypoglycemic episodes (defined as an episode in which help from another person or medical intervention was necessary) was higher for patients taking insulin (2.3%) than for patients undergoing any other intensive therapy or conventional treatment (<1%). The rate of any hypoglycemic episode (including episodes that the patient was able to treat unaided) was 36.5% with insulin treatment, compared to 11, 17.7, and 1.2% for chlorpropamide, glibenclamide, and diet, respectively. In a secondary analysis of obese patients on intensive glycemic control, the rate of major hypoglycemic events per year for intensive insulin treatment was 2.5% versus <1% for other treatments. Despite the increased risk of mild hypoglycemia, aggressive therapy that combines patient education and self-management with a form of exogenous insulin that closely mimics normal insulin secretion can help to reduce the morbidity and mortality associated with type 2 diabetes. In many trials, intensive glycemic control greatly increased the incidence of severe hypoglycemia. The occurrence of one or more severe hypoglycemic episode in either intensively or moderately controlled patients increased the hazard ratio (HR) for mortality during the studies. A similar phenomenon was noted in the Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study, in which intensive insulin therapy to achieve normal blood glucose levels in intensive care patients resulted in a 90-day mortality that was increased by 14% compared with individuals treated to blood glucose levels in the moderate range (~140 mg/dL). In a nutshell, it is intensive insulin therapy that causes severe hypoglycemia and mortality. I am proposing sub-optimal insulin therapy from day1 where severe hypoglycemia and mortality wold be very unlikely.
_
Insulin therapy and cardiovascular diseases:
Although cardiovascular disease is not specific to diabetes, it is more prevalent among patients with type 1 or type 2 diabetes than among those without diabetes. Type 1 diabetes is associated with at least a 10-fold increase in cardiovascular disease as compared with an age-matched nondiabetic population. An association between hyperglycemia and cardiovascular disease has been suggested by some, but not all, studies of patients with type 1 diabetes. However, controlled clinical trials of patients with type 1 or type 2 diabetes have not demonstrated a reduction in the occurrence of cardiovascular disease with long-term intensive diabetes therapy. During the DCCT, fewer cardiovascular events occurred in the intensive-treatment group than in the conventional-treatment group, but the small number of events in the relatively young cohort precluded a determination of whether the use of intensive diabetes therapy affected the risk of cardiovascular disease.
_
The incidence of heart disease and ischemic heart mortality is up to four times higher in people with diabetes. Ischemic heart disease, other heart disease, and cerebrovascular disease account for 40, 15, and 10% of all deaths in this population, respectively. A population-based survey of almost 3,000 people identified high prevalence rates for a constellation of disorders known as syndrome X, metabolic syndrome, and insulin resistance syndrome interchangeably—abdominal obesity, type 2 diabetes, glucose intolerance, hypertension, hypertriglyceridemia, and hypercholesterolemia—that far exceeded the rates for each disorder alone or in pairs. For each of these conditions, fasting and post-glucose hyperinsulinemia was a common thread that suggested the presence of insulin resistance. When insulin-resistant subjects were compared to control subjects, significantly lower HDL cholesterol levels were also seen. Because of the connection among hyperinsulinemia, insulin resistance, and cardiovascular risk factors, the UKPDS compared cardiovascular events among patients randomized to conventional lifestyle and dietary management and those on a tight glycemic control regimen with sulfonylureas, metformin (in overweight patients), or insulin. No adverse effects on cardiovascular outcomes were seen with any of the treatments, including insulin.
_
Effect of Insulin Therapy on Macrovascular Risk Factors in Type 2 Diabetes: a 1999 study:
Many patients with type 2 diabetes require insulin therapy for improved glycemic control after b-cell failure. However, many physicians are reluctant to institute insulin therapy in type 2 diabetes for fear of accelerating atherosclerosis. The epidemiological evidence is reasonably sound that hyperinsulinism correlates with increased cardiovascular disease in nondiabetic people and those with early type 2 diabetes. It is much less clear, however, that insulin concentration plays a negative role when less well controlled diabetes is considered. The data are more consistent, in fact, with the glucose hypothesis, i.e., that hyperglycemia is a risk factor, although the magnitude of the glucose effect is not well defined. Certainly, the dysmetabolism associated with poor glycemic control could increase the risk of macrovascular events through well-known mechanisms. There is direct evidence that insulin therapy can reduce the risk of macrovascular events by improving glycemic control and diabetes-associated dyslipidemias, although the beneficial effects may be significantly compromised by excessive weight gain. Insulin therapy does not appear to induce hypertension independent of changes in body weight. Clinicians with a patient in poor diabetic control, when dietary, lifestyle, and oral medication options have been exhausted, must decide between two potentially unattractive alternatives: allowing continued poor control of diabetes or starting insulin therapy. The most conclusive evidence for choosing good glycemic control with insulin is that doing so will retard or prevent diabetic retinopathy, neuropathy, and nephropathy. But the weight of evidence also supports the conclusion that insulin therapy will improve, rather than adversely affect, the risk of CVD. In uncontrolled diabetes, the elements of dysmetabolism that accompany hyperglycemia, particularly the abnormalities of lipid metabolism, could well be the most important influences on CVD. These metabolic abnormalities of poorly controlled diabetes are improved by adequate insulinization. It is concluded that optimal glycemic control confers a known benefit and can only be achieved with insulin therapy in some people with type 2 diabetes. In these circumstances, the use of insulin has a net benefit on cardiovascular risk, mediated primarily through improvement in dyslipidemia and glycemia itself.
_
The Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction study assessed the effect of acute insulin-glucose infusion followed by long-term intensive (multidose) insulin treatment in diabetic patients who have had an acute myocardial infarction. Among patients who had received an acute infusion, there was a significant decrease in glucose. After 1 year, there was a significant reduction in mortality in the group who received intensive insulin treatment, particularly in patients who had a low cardiovascular risk profile and were insulin naive. These effects persisted after a mean follow-up of 3.4 years; the absolute reduction in mortality was 11%. Other studies have reported improvement or neutral effects on other cardiovascular risk factors—total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, or hypertension—with insulin, even among obese patients.
_
Insulin therapy and CV (cardiovascular) risk: ORIGIN trial:
The Outcome Reduction With an Initial Glargine Intervention (ORIGIN) trial randomized 12 537 patients with CVD risk factors and prediabetes or diabetes to insulin glargine or standard care. Metformin (28%) and sulfonylurea (29%) use was similar in both groups, but insulin reached only 11% in the standard group by study end. After a median of 6 years, there was no difference in the incidence of cardiovascular death, myocardial infarction, or stroke between groups (2.94 vs 2.85 per 100 person-years, respectively; hazard ratio, 1.02; 95% CI, 0.94-1.11).34 There was also no difference in the incidence of cancer or cancer death. However, patients in the insulin group had more weight gain and hypoglycemic events.
_
_
In summary, based on the available data it is not possible to ascertain whether the lack of positive effects on CV outcomes with insulin glargine may be due to inability, on top of modest improvement of glycemic control, to assure concomitant amelioration of insulin sensitivity, plasma FFA, or lipid profile. Alternatively, one could argue that the beneficial effect generated by the modest glycemic improvement could be offset by potential atherogenic impact of chronic hyperinsulinemia. Experimental data have shown that modest elevation of plasma insulin levels, mimicking fasting hyperinsulinemia of insulin-resistant states, abrogates endothelium-dependent vasodilation in large conduit arteries, probably by increasing oxidative stress. Moreover, in the presence of insulin resistance, hyperinsulinemia, at least in the in vitro setting, can overstimulate the intracellular mitogen (mitogen-activated protein kinase dependent) signalling pathway in endothelial cells, which, together with impaired phosphatidylinositol 3-kinase activation of nitric oxide synthase, could yield an atherogenic state. The ORIGIN trial could not document CV benefits from early insulin treatment in high-risk patients with recent-onset diabetes while it increases severe and non-severe hypoglycemia. On the other hand, one could read the trial’s results to conclude that insulin treatment in high-risk CV patients is not associated with increased CV or neoplastic event rate. Given prior concern associated with insulin use, this may be seen as a reassuring finding. Insulin glargine also slowed progression from prediabetes to diabetes.
__
Is impaired insulin secretion a risk factor for cardiovascular disease (CVD)?
Yes.
We know that CVD risk in type I diabetes are high and we encounter with impaired insulin secretion in this situation. Also in many published papers, impaired insulin Secretion is considered as a risk factor of CVD.
_
Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes: a 2005 study:
Conclusion: Intensive diabetes therapy has long-term beneficial effects on the risk of cardiovascular disease in patients with type 1 diabetes.
_
Insulin Therapy improves Endothelial Function in Type 2 Diabetes: a 2012 study:
A total of 75 in vivo endothelial function tests (intrabrachial artery infusions of endothelium-dependent [acetylcholine] and -independent [sodium nitroprusside] vasoactive agents) were performed in 18 type 2 diabetic patients (aged 58±2 years, body mass index 28.5±0.6 kg/m2, and fasting plasma glucose 229±11 mg/dL) and 27 matched normal subjects. These tests were performed before and 6 months after combination therapy with insulin and metformin and before and 6 months after metformin therapy only. Before insulin therapy, blood flow responses to acetylcholine (15 μg/min) were significantly blunted in type 2 diabetic patients (7.5±0.7 mL · dL−1 · min−1) compared with normal subjects (11.6±0.9 mL · dL−1 · min−1, P<0.01). During insulin therapy, the acetylcholine response increased by 44% to 10.8±1.6 mL · dL−1 · min−1 (P<0.05). Insulin therapy also significantly increased the blood flow responses to both low and high doses of sodium nitroprusside. Authors conclude that insulin therapy improves endothelium-dependent and -independent vasodilatation. These data support the idea that insulin therapy has beneficial rather than harmful effects on vascular function.
_
Some studies showing adverse cardiovascular impact of insulin therapy:
Insulin-sensitizing therapies and cardiovascular benefits in specific populations of type 2 diabetic patients:
Two studies (one a retrospective analysis of patients with type 2 diabetes after an acute myocardial infarction and one a subanalysis of the Sibrafiban versus aspirin to yield Maximum Protection from ischemic heart events post-acute coronary syndrome -SYMPHONY study) suggested that survival and clinical outcomes after acute coronary artery disease is better after a treatment strategy emphasizing insulin-sensitizing therapies than insulin-providing therapies. Clinical outcomes in the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI-2D) trial were shown to be better in patients with type 2 diabetes and coronary heart disease undergoing coronary bypass surgery and treated with an insulin-sensitizing strategy than in patients treated with an insulin-providing strategy. These data are somewhat tenuous but still suggest that insulin-providing strategies may not be as good in some patients with type 2 diabetes as insulin-sensitizing strategies.
_
A 2014 study found that among patients with diabetes who were receiving metformin, the addition of insulin compared with a sulfonylurea was associated with an increased hazard of a composite of nonfatal cardiovascular outcomes and all-cause mortality. There is consensus that metformin is first-line diabetes treatment; however, uncertainty remains regarding additional therapy after inadequate control with metformin. Among the options, intensification with either insulin or a sulfonylurea is considered a high-efficacy strategy with reasonable costs. Two large randomized trials demonstrated that regimens including greater insulin use and tighter control did not reduce cardiovascular events compared with standard care. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, patients were randomized to intensive control (target HbA1c level <6%) or standard care. Approximately 77% of patients in the intensive group received insulin compared with 55% in the standard group. ACCORD was stopped when interim analyses found more all-cause deaths in the intensive vs standard group (5.0% vs 4.0%; hazard ratio, 1.22; 95% CI, 1.01-1.46). Most excess mortality was due to cardiovascular deaths (2.63% vs 1.83% during a mean 3.5 observation years; P = .02). Whether insulin itself or other effects of intense treatment such as hypoglycemia contributed to the increased mortality remains unknown. Several observational studies have also reported no cardiovascular benefit of insulin relative to noninsulin comparators, and some have suggested worse outcomes. A Canadian study reported increased all-cause mortality among insulin users compared with nonusers in a dose-response manner. Similarly, a study of primary care patients in the UK General Practice Research database determined that metformin + insulin was associated with an elevated risk of all-cause mortality and cardiovascular- and cancer-related outcomes compared with metformin monotherapy. However, these studies did not address confounding by disease severity adequately. The first did not control for HbA1c, and the second compared more intensive therapies such as insulin (alone or in combination) with metformin monotherapy.
_
Some epidemiological studies have shown that the use of insulin is associated with an increased risk of cardiovascular events, cancer and all cause mortality in comparison with other glucose-lowering therapies. In one of these studies (a retrospective cohort study using data from the UK General Practice Research Database) mortality and other diabetes- related outcomes for just under 85,000 individuals were examined in relation to five diabetes therapies monotherapy with either metformin, sulfonylurea or insulin, metformin plus sulfonylurea or insulin plus metformin. Treatment with insulin alone conferred a statistically significantly increased risk of a first major cardiac event or a first diagnosis of cancer ranging from 1.8 to 2.6 times the risk seen in those treated with metformin alone (the comparison group). This excess risk was lower in the group treated with insulin plus metformin than in the insulin monotherapy group, though was still significant at 1.3 times the risk of the comparison group. However, even though these results are consistent and despite the use of statistical adjustment, observational data such as these should be interpreted with caution due to the risk of a form of analytical bias that is termed ‘confounding by indication’. This form of confounding is a common feature of studies of outcomes in relation to drug and other therapies simply because the reasons patients have been prescribed the therapies may themselves be related to the perceived risks of any particular outcome occurring. In other words, people may be prescribed insulin partly because they are perceived of being at risk to adverse outcomes, including risk of cardiovascular disease.
_
Risk of cancer with insulin therapy:
Type 2 diabetes is associated with an increased risk of mortality from a range of solid tumours, including cancers of the colon, breast and pancreas. Similar associations have been noted with central obesity and other conditions associated with increased levels of circulating insulin. These observations have given rise to the hypothesis that growth of these tumours, which are characterised by abnormal expression and function of the insulin–IGF-1 series of receptors, is promoted by the trophic action of insulin interacting with these receptors. The cancer risk associated with diabetes may also be influenced by therapy: for example, the risk of colon cancer is higher in individuals on insulin, patients on metformin are less likely to be diagnosed with cancer, and the risk of mortality from solid tumours is lower for metformin than for exogenous insulin or sulfonylureas. As recognition dawns that cancer should be numbered among the complications of diabetes, the possibility that therapies for diabetes may influence tumour progression is likely to attract increasing interest and concern. Furthermore, the observation that both endogenous insulin and exogenous insulin therapy are associated with tumour progression raises questions as to the safety of the insulin analogues, which have subtly modified receptor binding properties and accelerate the growth and proliferation. Not all studies implicate insulin treatment as the potential cause of the increase in cancers. A recent publication reported that in Chinese patients with type 2 diabetes, hyperglycemia predicts cancer incidence and insulin treatment was associated with better glycemic control and a reduced cancer risk.
_________
Other side effects of insulin administration:
Allergy to Insulin products is rare with a prevalence of about 2%, of which most reactions are not due to the Insulin itself but to preservatives added to insulin such as zinc, protamine, and meta-cresol. Most reactions are Type I hypersensitivity reactions and rarely cause anaphylaxis. A suspected allergy to Insulin can be confirmed by skin prick testing, patch testing and occasionally skin biopsy. Allergic reactions are less common with the newer insulins. First line therapy against Insulin hypersensitivity reactions includes symptomatic therapy with antihistamines. The affected persons are then switched to a preparation that does not contain the specific agent they are reacting to or undergo desensitization. Other adverse effects of insulin include loss or overgrowth of fat tissue at injection sites, and insulin resistance. If you inject in the same area over and over again, you may develop fat deposits there, which reduce insulin absorption. This effect is less common with the types of insulin used today, and you can prevent it by rotating injection sites. Insulin resistance, which may be caused by the formation of antibodies against insulin, is usually managed by increasing the insulin dose.
__________
Insulin as a drug of abuse in body building:
An extensive literature search identified very few cases of insulin abuse. However, from the few cases that have been published, it is apparent that the problem of insulin abuse may be much more widespread than these few isolated cases. A source within the body building community revealed that “at least 10%” of his 450 regular patients admitted to using insulin and that most of them obtained insulin from diabetic friends. As insulin has a half-life of four minutes in the human body, it vanishes rapidly and would be very difficult to detect. Even when detected it is impossible to distinguish from the athlete’s own insulin. It is thus a very attractive potential drug of abuse. The primary source of carbohydrate during exercise is muscle glycogen stores. The greater the muscle glycogen stores, the longer the exercise time to exhaustion. Insulin works in synergy with steroids. Steroids spawn new muscle whereas insulin inhibits catabolism in muscle and liver by increasing the synthesis of glycogen and proteins and promoting the entry of glucose and amino acids into muscle cells before an event, thereby improving stamina. This is achieved by taking glucose and insulin simultaneously for a couple of hours using a technique called a hyperinsulinemic clamp. Insulin has been a prescription only medicine in the United Kingdom since 1998, and its use is prohibited, in non-diabetic athletes, by the International Olympic Committee. However, there is little to prevent diabetics giving or selling their insulin to athletes and body builders. To help to appreciate the magnitude of drug abuse in body builders, Tricker et al1 identified that 54% of male and 10% of female body builders admitted to using steroids on a regular basis, and Rich et al stated that in excess of one million elite and recreational athletes use performance enhancing drugs in the United States and that as many as 25% of anabolic androgenic steroid abusers concurrently abuse insulin. There were two apparent reasons why steroid use is so prevalent. Firstly, steroid use was “perceived to be an important factor in winning competitions”, and secondly “significant gains in strength” could be achieved by including anabolic steroid as part of the training regimen despite the reported adverse side effects”. The method of insulin abuse appears to be relatively simple and spread by word of mouth. Most users inject 10 IU regular insulin and then consume sugar-containing foods and drinks. Hypoglycaemic events are thus usually avoided. The anabolic properties of insulin used in the hypoinsulinemic (diabetic) patient are well recognised; however, the concept of a hyperinsulinemia induced anabolic state is much less well supported. Physiological hyperinsulinemia reportedly stimulates amino acid transport in human skeletal muscle. Banadonna et al state that this may have a role in determining the overall concomitant response of muscle amino acid/protein metabolism to insulin. Although insulin inhibits protein breakdown, stimulation of bulk protein synthesis during hyperinsulinemia is observed only when concomitant hyperaminoacidaemia occurs. Insulin abuse in body builders is an increasing problem overlooking potential dangers that may befall those who abuse insulin without medical supervision.
_______
_______
Insulin versus oral hypoglycemic agents (OHA):
_
Comparison of Insulin With or Without Continuation of Oral Hypoglycemic Agents in the Treatment of Secondary Failure in NIDDM Patients: a 1994 study:
In NIDDM patients with secondary OHA failure, therapy with a combination of OHAs and insulin and with insulin alone was equally effective and well tolerated. However, combination therapy was associated with a lower insulin dose and less weight gain. Combination treatment may be considered when OHA failure occurs as a potential intermediate stage before full insulin replacement.
_
Oral Sulfonylurea Hypoglycemic Agents prevent Ischemic Preconditioning in Human Myocardium: 1997:
Patients receiving oral hypoglycemic agents for diabetes mellitus are at increased risk of cardiovascular mortality. Oral hypoglycemic agents are inhibitors of the ATP-sensitive potassium (KATP) channel. Ischemic preconditioning is mediated by KATP channel activation. Authors therefore hypothesized that myocardium from patients taking long-term oral hypoglycemic agents would be resistant to the protection by ischemic preconditioning. The study concluded that human myocardium from patients without long-term exposure to oral hypoglycemic agents is functionally protected by preconditioning. Long-term oral hypoglycemic intake blocks the protection by preconditioning. These data suggest that ischemic preconditioning in human myocardium relies on KATP channels, and long-term inhibition of KATP channels with oral hypoglycemic agents may explain the excess cardiovascular mortality in these patients.
_
Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes–UK Prospective Diabetes Study (UKPDS) Group study 1998:
Improved blood-glucose control decreases the progression of diabetic microvascular disease, but the effect on macrovascular complications is unknown. There is concern that sulfonylureas may increase cardiovascular mortality in patients with type 2 diabetes and that high insulin concentrations may enhance atheroma formation. Authors compared the effects of intensive blood-glucose control with either sulfonylurea or insulin and conventional treatment on the risk of microvascular and macrovascular complications in patients with type 2 diabetes in a randomised controlled trial.
Conclusion:
Intensive blood-glucose control by either sulfonylureas or insulin substantially decreases the risk of microvascular complications, but not macrovascular disease, in patients with type 2 diabetes. None of the individual drugs had an adverse effect on cardiovascular outcomes. All intensive treatment increased the risk of hypoglycaemia.
_
Insulin monotherapy and insulin combined with oral hypoglycemic agents provide similar glycemic control: a 2005 study:
In patients with type 2 diabetes mellitus and inadequate glycemic control, how do insulin monotherapy and insulin combined with oral hypoglycemic agents (OHAs) compare?
22 articles reporting 20 RCTs (n = 1811, mean age 60 y, 54% women, mean known duration of diabetes 9.6 y) met the inclusion criteria. Study quality was generally low (mean score 2.6). No RCTs reported diabetes-related morbidity, mortality, or all-cause mortality. 13 RCTs (21 comparisons) provided sufficient data on glycemic control to be pooled. 5 comparisons of a single evening injection with evening insulin combined with sulfonylurea showed less lowering from baseline of glycated hemoglobin (HbA1c) with insulin monotherapy (Table). Of 4 other comparisons that could not be included in the meta-analysis, 2 showed better outcome with combination therapy and 2 showed no difference. Twice-daily insulin monotherapy was compared with combination insulin and OHAs in 10 comparisons. Greater lowering of HbA1c levels was seen with twice-daily insulin monotherapy than morning NPH insulin plus sulfonylurea or plus sulfonylurea and metformin (4 comparisons). Among RCTs comparing multiple daily insulin injections with insulin plus OHAs, no difference was seen for lowering of HbA1c levels (5 comparisons). Of 14 RCTs (22 comparisons) that assessed hypoglycemia, all but 1 comparison showed no difference between monotherapy and combination therapy: 1 comparison showed more hypoglycemic events with monotherapy. Combination therapy resulted in a 46% weighted mean relative reduction in daily insulin requirement. Few RCTs showed a difference in weight gain: 1 comparison showed 3.7 kg less weight gain with bedtime NPH insulin plus metformin compared with twice-daily monotherapy, and 3 comparisons showed 1.1 kg less weight gain with combination therapy compared with multiple daily monotherapy.
Conclusion:
In patients with type 2 diabetes, insulin monotherapy and insulin combined with oral hypoglycemic agents provide similar improvements in glycemic control.
_
The Acute Effect of Preprandial Exogenous and Endogenous Sulfonylurea-stimulated Insulin Secretion on Postprandial Glucose Excursions in Patients with Type 2 Diabetes: 2009 study:
The total amount of insulin secreted seems more important than the timing of the insulin release for the postprandial glucose tolerance in Type 2 diabetic subjects. Neither endogenous Sulfonylurea-stimulated insulin secretion nor peripheral pre-meal supply of insulin could normalize postprandial glucose excursions in patients with Type 2 diabetes.
_
The safety of triple therapy with oral antidiabetics versus insulin in type 2 diabetes: a 2010 study:
Minor hypoglycaemic episodes occurred in 34.85% of the patients in insulin groups and 26.53% in three oral drugs group. The most frequent adverse event in both groups was gastrointestinal disturbance (28.57% of insulin vs 61.11% of oral triple therapy group). Adverse events occurring in both group included flatulence, abdominal discomfort, diarrhoea, and nausea. The insulin group had 59.09% incidence of drug interactions as compared with 69.39% in oral triple therapy groups. So patient treated with three oral drugs is subjected to an additive risk of adverse events and drug interaction compared with insulin therapy.
_
Insulin-based versus Triple Oral Therapy for Newly Diagnosed Type 2 Diabetes: Which is Better? 2009 study:
Of 29 patients randomly assigned into each group, 83% (insulin group) and 72% (oral group) completed this 3-year study. At study completion, A1C was 6.1 ± 0.6% (insulin group) versus 6.0 ± 0.8% (oral group). Weight increased similarly in both groups (P = 0.09) by 4.47 kg (95% CI 0.89–8.04 kg) (insulin group) and 7.15 kg (95% CI 4.18–10.13 kg) (orals group). Hypoglycemic events did not differ between groups (mild 0.51 event/person-month in the insulin group vs. 0.68 event/person-month in the orals group, P = 0.18 and severe 0.04 event/person-year in the insulin group vs. 0.09 event/person-year in the orals group, P = 0.53). Compliance, QoL, and treatment satisfaction were similar between groups, with 100% of patients randomly assigned to insulin willing to continue such treatment.
Conclusions:
When compared with a clinically equivalent treatment regimen, insulin-based therapy is effective and did not cause greater weight gain or hypoglycemia nor decrease compliance, treatment satisfaction, or QoL. Insulin is safe, well-accepted, and effective for ongoing treatment of patients with newly diagnosed type 2 diabetes.
__
Insulin versus Oral Hypoglycemic Agent as Initial Therapy for Newly Diagnosed Diabetes Mellitus Type 2: A Systematic Review and Meta-Analysis of 2013:
All four studies showed lower post treatment BMI among participants in the insulin treatment arm. An opposite finding was expected as insulin is known to cause weight gain. Main adverse effect was hypoglycemia. Among newly diagnosed type 2 DM patients, there is insufficient evidence for or against the use of insulin compared to oral hypoglycemic agents as initial management in terms of improvement in glycemic control, decrease in insulin resistance, and improvement in beta cell function.
___
Effect of intensive glucose lowering treatment on all-cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials: 2011:
13 studies were included. Of 34 533 patients, 18 315 received intensive glucose lowering treatment and 16 218 standard treatment. Intensive treatment did not significantly affect all-cause mortality (risk ratio 1.04, 99% confidence interval 0.91 to 1.19) or cardiovascular death (1.11, 0.86 to 1.43). Intensive therapy was, however, associated with reductions in the risk of non-fatal myocardial infarction (0.85, 0.74 to 0.96, P<0.001), and microalbuminuria (0.90, 0.85 to 0.96, P<0.001) but a more than twofold increase in the risk of severe hypoglycaemia (2.33, 21.62 to 3.36, P<0.001). Over a treatment period of five years, 117 to 150 patients would need to be treated to avoid one myocardial infarction and 32 to 142 patients to avoid one episode of microalbuminuria, whereas one severe episode of hypoglycaemia would occur for every 15 to 52 patients. In analysis restricted to high quality studies (Jadad score >3), intensive treatment was not associated with any significant risk of reductions but resulted in a 47% increase in risk of congestive heart failure (P<0.001).
Conclusions:
The overall results of this meta-analysis show limited benefits of intensive glucose lowering treatment on all-cause mortality and deaths from cardiovascular causes. Authors cannot exclude a 9% reduction or a 19% increase in all-cause mortality and a 14% reduction or a 43% increase in cardiovascular death. The benefit: risk ratio of intensive glucose lowering treatment in the prevention of macrovascular and microvascular events remains uncertain. The harm associated with severe hypoglycaemia might counterbalance the potential benefit of intensive glucose lowering treatment. More double blind randomised controlled trials are needed to establish the best therapeutic approach in people with type 2 diabetes.
What is already known on this topic:
•Glucose lowering treatments are widely used to treat type 2 diabetes and to prevent long term cardiovascular complications as well as impairment of renal and visual functions
•The benefit of such treatment on clinical end points has not been shown unambiguously
What this study adds:
•Overall results of this meta-analysis show that there are no benefits of intensive glucose lowering treatment on all-cause mortality and deaths from cardiovascular causes in adults with type 2 diabetes
•Intensive glucose lowering treatment of type 2 diabetes should be considered with caution and therapeutic escalation should be limited
________
Insulin versus insulin plus metformin studies:
Combination of insulin and metformin in the treatment of type 2 diabetes: a 2002 study:
In type 2 diabetic patients who are intensively treated with insulin, the combination of insulin and metformin results in superior glycemic control compared with insulin therapy alone, while insulin requirements and weight gain are less.
_
Safety Factor: Insulin vs Oral Antidiabetic in Type 2 Diabetes: a 2012 study:
In a previous study by Kahn and colleagues, a thiazolidinedione was associated with a longer duration of efficacy as monotherapy vs metformin and a sulfonylurea. However, the thiazolidinedione was associated with a higher risk for cardiac events vs the sulfonylurea, and the sulfonylurea promoted hypoglycemia. In the current study by Ekstram and colleagues, metformin was associated with superior results for cardiovascular events and mortality outcomes vs insulin among patients with type 2 diabetes mellitus. Metformin did not promote higher rates of acidosis/serious infection vs insulin or other oral hypoglycemic agents, even among patients with substantial renal impairment.
_
Comparison of metformin and insulin versus insulin alone for type 2 diabetes: 2012 systematic review of randomised clinical trials with meta-analyses and trial sequential analyses:
There was no evidence or even a trend towards improved all-cause mortality or cardiovascular mortality with metformin and insulin, compared with insulin alone in type 2 diabetes. Data were limited by the severe lack of data reported by trials for patient relevant outcomes and by poor bias control. After combining all the evidence available from randomised clinical trials, authors were unable to find any evidence or even a trend towards improved all-cause mortality or cardiovascular mortality with metformin and insulin, compared with insulin alone.
What is already known on this topic:
– Because of the progressive nature of type 2 diabetes, a substantial proportion of patients end up receiving insulin treatment
– Current guidelines for diabetes treatment recommend combining metformin with insulin instead of using insulin alone
– Previous meta-analyses have only included a few trials comparing metformin and insulin with insulin alone
What this study adds:
– The reporting of patient relevant outcomes was sparse
– An influence of metformin and insulin versus insulin alone on all-cause mortality or cardiovascular mortality could not be established, and more trials are needed to provide firm evidence for an effect or absence of an effect
– Metformin and insulin treatment, compared with insulin alone, seems to be associated with a reduction in HbA1c, weight gain, and insulin dose
– Metformin and insulin seems to increase the risk of severe hypoglycaemia compared with insulin alone
________
________
Impact of various therapies on A1C reduction:
_
DPP4 inhibitors lower A1C levels by about 0.5% to 1 % and SGLT-2 inhibitors lower A1C levels by about were 0.58 % to 0.89%. GLP-1 receptor agonist exenatide reduces A1C by about 0.5 to 1%. For every 1 percent reduction in results of HbA1c, the risk of developing eye, kidney, and nerve disease (microvascular complications) is reduced by 40 percent while the risk of heart attack (macrovascular complications) is reduced by 14 percent.
_
In a nutshell, only insulin lowers A1C levels by more than 5 % compared to any OHAs or their combinations, and corollary is that only insulin can reduce diabetic complications more than any other therapy. Insulin plus diet control plus exercise could theoretically reduce A1C level by about 7%.
________
________
Pregnancy and lactation:
_
Gestational diabetes is a complication in about 5% of pregnancies, is increasing in prevalence, and is associated with complications to the pregnancy and a long-term risk of diabetes in both mother and offspring. Intervention to change lifestyle and, if maternal hyperglycemia persists, treatment with additional insulin have been shown to improve perinatal outcomes. Women who begin insulin therapy require education to ensure the safe administration of insulin. Use of insulin is also associated with hypoglycemia and weight gain. The use of safe and effective oral agents may offer advantages over insulin. Oral metformin is a logical option for women with gestational diabetes mellitus. It improves insulin sensitivity, probably by activating AMP kinase, and is not associated with weight gain or hypoglycemia. Reported outcomes of its use during pregnancy have been favorable except for one small, retrospective cohort study that showed increased rates of perinatal loss and preeclampsia as compared with insulin treatment. Metformin crosses the placenta and could affect fetal physiology directly. Its use in pregnancy remains controversial; to our knowledge, only two small, randomized trials comparing metformin with insulin have been reported to date.
_
Metformin versus Insulin for the Treatment of Gestational Diabetes: a 2008 study:
Authors found no significant increase in a composite measure of neonatal complications among women with gestational diabetes mellitus who were randomly assigned to metformin as compared with those who were assigned to insulin. Rates of neonatal hypoglycemia, one of the components of the composite end point, were similar in the two groups, but severe hypoglycemia (<1.6 mmol of glucose per liter [28.8 mg per deciliter]) occurred significantly less often in infants of women taking metformin. Prematurity was included as part of the composite outcome. The rationale was that if metformin had any unanticipated adverse effect on fetal growth or well-being, there would be more iatrogenic preterm births. The frequency of preterm birth was higher in the metformin group than in the insulin group, but the difference was associated with a greater frequency of spontaneous (rather than iatrogenic) preterm births that could be due to chance or to an unrecognized effect of metformin on the labor process. The increased rate of preterm birth was not associated with higher rates of other complications, probably because the difference between the two groups in mean gestational age at delivery was clinically insignificant. In a previous cohort of women with gestational diabetes mellitus who were treated with either insulin or glyburide, the rates of preterm delivery were 13% for the insulin group and 12% for the glyburide group, but the causes of preterm birth were not documented. In this study, 46.3% of women taking metformin required supplemental insulin. This proportion is likely to vary in other populations, depending on patient characteristics and target levels of glucose. How do the effects of metformin in women with gestational diabetes mellitus compare with those reported for glyburide? In a randomized trial comparing glyburide and insulin in 404 women with gestational diabetes mellitus, glycemic control and pregnancy outcomes were similar between groups (although the trial was underpowered to address neonatal complications). Subsequent experience with glyburide shows that approximately 20% of women change to insulin. There are no published trials comparing metformin with glyburide, and comparisons among available data are limited by differences in study populations and glycemic aims. Although this study was not designed to compare the outcome of combined treatment with that of either treatment alone, the rate of neonatal complications did not differ significantly between women who required supplemental insulin and those who received metformin alone. Moreover, women receiving combined treatment required less insulin and gained less weight than those taking insulin alone.
_
Let’s consider the literature on glyburide and metformin separately. All three studies comparing glyburide and insulin demonstrated a higher incidence of neonatal hypoglycemia among women taking glyburide, with one of those studies demonstrating a statistically significant effect. In contrast, the single large randomized, controlled study comparing metformin and insulin showed a non-significant reduction in the rate of neonatal hypoglycemia for women taking metformin. The combined effect of the oral agents in the meta-analysis is a nonsignificant increase in neonatal hypoglycemia, but the odds ratio is 1.59, which certainly has some clinical meaning. A similar clinical heterogeneity of the effect on birth weight is seen when the different oral agents are compared with insulin. Overall, data on glyburide are confusing. Most studies find glyburide and insulin to be essentially equivalent for glycemic control, but insulin leads to better outcomes (although this effect is not significant, probably owing to insufficient power). The bias among many researchers exploring the use of glyburide for GDM is obvious. One study observed that glyburide was associated with a greater likelihood of neonatal hypoglycemia and higher birth weight than insulin, but still concluded that glyburide is a reasonable first-line therapy for GDM.
_
Metformin vs Insulin in the Management of Gestational Diabetes: A Meta-Analysis: 2013:
Metformin is comparable with insulin in glycemic control and neonatal outcomes. It might be more suitable for women with mild GDM. This meta-analysis also provides some significant benefits and risks of the use of metformin in GDM and help to inform further development of management guidelines. In conclusion, metformin could be used in women with GDM in view of the comparative glycemic control and neonatal outcomes, especially for those mild GDM patients. However, the risk of preterm birth could not be ignored. Clinicians should weigh in practice according to the condition of the patients. Further studies with larger sample sizes must be completely designed to assess maternal and neonatal complications and to evaluate long-term follow-up of children for the safety of metformin as a universal treatment in GDM patients.
_____
Lactation:
Should breastfeeding Mom resume her oral hypoglycemic?
The current standard of treatment is to keep women with Type 2 diabetes on insulin from the time they conceive through the postpartum period if they’re breastfeeding. The reason for this is to prevent the baby from developing hypoglycemia. Insulin doesn’t cross the placenta or transfer into breast milk like oral agents can. In fact, early studies on oral hypoglycemics showed that after a single oral dose of first-generation sulfonylureas like chlorpropamide and tolbutamide, there was a drug concentration of 3 – 18 mcg/ml in breast milk, depending on which drug was given. However, a small study of breastfeeding women who received the second-generation sulfonylureas—like glyburide and glipizide —had no drug detected in their milk. Part of the reason may be that these newer hypoglycemic agents are 98% – 99% bound by plasma proteins, limiting their ability to cross into either the placenta or breast milk.
________
________
Preventing and reversing diabetes:
_
Breaking up prolonged sitting reduces Postprandial Glucose and Insulin Responses: a 2012 study:
Observational studies show breaking up prolonged sitting has beneficial associations with cardiometabolic risk markers, but intervention studies are required to investigate causality. Authors examined the acute effects on postprandial glucose and insulin levels of uninterrupted sitting compared with sitting interrupted by brief bouts of light- or moderate-intensity walking. The study found that interrupting sitting time with short bouts of light- or moderate-intensity walking lowers postprandial glucose and insulin levels in overweight/obese adults. This may improve glucose metabolism and potentially be an important public health and clinical intervention strategy for reducing cardiovascular risk. These findings provide initial experimental confirmation of hypotheses generated by epidemiologic observational studies on the deleterious health consequences of prolonged sedentary time (too much sitting as distinct from too little exercise). Of particular importance is the potential for reducing cardiovascular disease risk by briefly breaking up prolonged periods of sitting with activity of at least light intensity. Brief interruptions to sitting led to significant reductions in postprandial glucose and insulin, irrespective of the activity intensity. The 24–30% lowering in the plasma glucose iAUC and the 23% lowering in the insulin iAUC seen after the activity break conditions, at the very least, is comparable in magnitude to an acute bout of moderate-intensity aerobic or resistance exercise in overweight/obese individuals. These findings support the hypothesis that brief interruptions to sedentary time with a minimum of light-intensity physical activity can attenuate acute postprandial plasma glucose and serum insulin response during prolonged sitting. Importantly, the brevity of the interruptions to sitting (2 min) indicates that such breaks would not count toward the minimum amount of aerobic activity necessary for substantial health benefits within current physical activity guidelines because at least 10-min episodes of activity are stipulated. However, consistent with studies of the effects of continuous exercise on glucose metabolism, the sum of total activity time over a 5-h period was 28 min. A logical next step would be to build on these findings to design a study that would provide a head-to-head comparison of a single continuous exercise bout to the breaking up of prolonged sitting protocol used in this study.
_
Diet + Exercise still Best for Diabetes Prevention: a 2015 study:
Diet and exercise interventions continue to be the best way to prevent type 2 diabetes, according to long-term follow-up of Diabetes Prevention Program (DPP) participants, published in the Lancet Diabetes and Endocrinology. Nearly 2800 adults who participated in the DPP randomized trial agreed to longer follow-up in the outcomes study. In the randomized trial, participants were assigned to a behavioural lifestyle intervention (diet and exercise), metformin, or placebo; the interventions lasted for about 3 years. Then, in the outcomes study, all participants were offered maintenance lifestyle sessions; those originally assigned to metformin received the drug unmasked, and those assigned to the lifestyle intervention were offered supplemental group sessions. During a total 15 years’ follow-up, diabetes incidence was reduced by 27% in the lifestyle group and 18% in the metformin group, relative to the placebo group. The differences among the groups, however, declined throughout follow-up. In women only, the lifestyle intervention reduced risk for microvascular complications — by about 20% relative to metformin or placebo.
_
What can you do with your food and lifestyle choices to support healthy blood sugar and insulin levels?
1. Make non-starchy vegetables the foundation of your diet. Dark green leafy lettuce, tomatoes, celery, cucumber, cabbage, kale, Swiss chard, bok choy, zucchini, broccoli, cauliflower, and all unmentioned green vegetables are excellent choices.
2. Reduce or eliminate your intake of sugar and all foods that contain sugar. Some of the most concentrated sources of sugar are soda, cookies, chocolate bars, donuts, pastries, ice cream, and ketchup.
3. Reduce or eliminate your use of sweeteners like molasses, corn syrup, high fructose corn syrup, pasteurized/heated honey, and maple syrup.
4. Limit intake of fruit juices. Even freshly squeezed fruit juice taken over the long term can lead to high blood sugar and insulin levels. If you want to taste fruit, eat whole fruit, not the juice. The fiber, vitamins, and minerals that come with whole fruit help to slow down the pace at which the natural sugars from fruit enter your bloodstream.
5. Do activities and exercises that build or maintain your muscles. Muscle tissue acts as a storage site for extra sugar. The more muscle tissue you have, the better you can regulate your blood sugar and insulin level.
__
Overfeeding leads to insulin resistance very quickly:
Diabetes has become an increasingly big problem in the Western world. In the United States, the number of new cases in 1980 compared to now has more than tripled. Studies have shown that type 2 diabetes is strongly linked to a poor diet high in sugar. Now, a new study out of Temple University, suggests the effect of diabetes can be seen just a few days after starting a high calorie diet. This is much quicker than previously thought. The study was conducted on six men of normal weight who were fed 6,000 calories per day for a week. The men gained an average of eight pounds and each reached a state of metabolic insulin resistance within two days of beginning the experiment. The meals consisted of what an average American meal would look like but in very large portions. To put this into perspective, the Institute of Medicine recommends a daily intake of 2,200 calories per day for men of the age used in the study. The scientists conducting the study took tissue samples of the men to see how their bodies reacted to the increased food intake. What they found was that the men in the study developed an insulin resistance, which is when the cells in the body cannot use insulin efficiently. This occurs when there is a large intake of carbohydrates and the pancreas has to work harder to produce more insulin to regulate the metabolism of the carbohydrates. When very large amounts of insulin are needed, cells can work up a resistance to normal concentrations of it and the result is that more insulin is needed putting stress on the pancreas. Eventually the pancreas ceases in its ability to produce adequate amounts of insulin and blood sugar levels rise. Insulin resistance is a precursor to type 2 diabetes. Unfortunately, the effects of the high calorie diet go beyond insulin resistance. The mitochondria, which are responsible for breaking down glucose for energy, were also under stress. They couldn’t keep up with the high calorie intake and as a result produced toxic byproducts. This process is known as oxidative stress. Oxidative stress can actually lead to insulin resistance through negative effects on important proteins. According to the team, oxidative stress leads to changes in a protein called GLUT4, which is involved in glucose transport. Changes in this protein may be associated with insulin resistance. This study is a reminder of how important a healthy diet is and shows us that diabetes is a big threat to our health and must be taken seriously.
________
Reversibility of T2DM:
Type 2 diabetes has long been regarded as a chronic progressive condition, capable of amelioration but not cure. A steady rise in plasma glucose occurs irrespective of the degree of control or type of treatment. Beta cell function declines linearly with time, and after 10 years more than 50% of individuals require insulin therapy. The underlying changes in beta cell function have been well described, and beta-cell mass decreases steadily during the course of type 2 diabetes. Overall, there is strong evidence that type 2 diabetes is inexorably progressive, with a high likelihood of insulin therapy being eventually required to maintain good glycaemic control. However, type 2 diabetes is clearly reversible following bariatric surgery. The normalisation of plasma glucose concentration follows within days of surgery, long before major weight loss has occurred, and it has become widely assumed that the protective effects of gastrointestinal surgery are mediated by altered secretion of incretin hormones. Improved control of blood glucose in type 2 diabetes by moderate energy restriction has been demonstrated by others. Authors have hypothesised that the profound effect of a sudden negative energy balance on the metabolism could explain the post-bariatric surgery effect and, specifically, that the decrease in the intracellular fatty acid concentrations in the liver would lead to a lower export of lipoprotein triacylglycerol to the pancreas, with the release of beta cells from the chronic inhibitory effects of excess fatty acid exposure.
_
Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol: a 2011 study:
Aims/hypothesis:
Type 2 diabetes is regarded as inevitably progressive, with irreversible beta cell failure. The hypothesis was tested that both beta cell failure and insulin resistance can be reversed by dietary restriction of energy intake.
Methods:
Eleven people with type 2 diabetes (49.5± 2.5 years, BMI 33.6±1.2 kg/m2, nine male and two female) were studied before and after 1, 4 and 8 weeks of a 2.5 MJ (600 kcal)/day diet. Basal hepatic glucose output, hepatic and peripheral insulin sensitivity and beta cell function were measured. Pancreas and liver triacylglycerol content was measured using three-point Dixon magnetic resonance imaging. An age-, sex- and weight-matched group of eight non-diabetic participants was studied.
Results:
After 1 week of restricted energy intake, fasting plasma glucose normalised in the diabetic group (from 9.2± 0.4 to 5.9±0.4 mmol/l; p=0.003). Insulin suppression of hepatic glucose output improved from 43±4% to 74±5% (p= 0.003 vs baseline; controls 68±5%). Hepatic triacylglycerol content fell from 12.8±2.4% in the diabetic group to 2.9± 0.2% by week 8 (p=0.003). The first-phase insulin response increased during the study period (0.19±0.02 to 0.46± 0.07 nmol min−1 m−2; p<0.001) and approached control values (0.62±0.15 nmol min−1 m−2; p=0.42). Maximal insulin response became supranormal at 8 weeks (1.37± 0.27 vs controls 1.15±0.18 nmol min−1 m−2). Pancreatic triacylglycerol decreased from 8.0±1.6% to 6.2±1.1% (p=0.03).
Conclusions/interpretation:
Normalisation of both beta cell function and hepatic insulin sensitivity in type 2 diabetes was achieved by dietary energy restriction alone. This was associated with decreased pancreatic and liver triacylglycerol stores. The abnormalities underlying type 2 diabetes are reversible by reducing dietary energy intake.
_
This study demonstrates that the twin defects of beta cell failure and insulin resistance that underlie type 2 diabetes can be reversed by acute negative energy balance alone. A hierarchy of response was observed, with a very early change in hepatic insulin sensitivity and a slower change in beta cell function. In the first 7 days of the reduced energy intake, fasting blood glucose and hepatic insulin sensitivity fell to normal, and intrahepatic lipid decreased by 30%. Over the 8 weeks of dietary energy restriction, beta cell function increased towards normal and pancreatic fat decreased. Following the intervention, participants gained 3.1±1.0 kg body weight over 12 weeks, but their HbA1c remained steady while the fat content of both pancreas and liver did not increase. The data are consistent with the hypothesis that the abnormalities of insulin secretion and insulin resistance that underlie type 2 diabetes have a single, common aetiology, i.e. excess lipid accumulation in the liver and pancreas. This provides a unified hypothesis to explain a common disease that previously appeared to require separate disease processes affecting the pancreas and insulin-sensitive tissues. Absence of rapid insulin secretion in response to a rise in plasma glucose is the hallmark of type 2 diabetes, and the decline in beta cell function determines the progression towards a need for insulin therapy. However, conventional therapy, even with sulfonylurea, fails to produce more than a small increase in the first-phase insulin response. As a consequence, the rapidity and extent of return of beta cell function in response to dietary energy restriction in the present study is striking. It supports the accumulating information on the inhibitory effect of fatty acids on insulin secretion in vitro and in vivo and is the first direct evidence in humans that the beta cell defect of type 2 diabetes is reversible by sustained negative energy balance. Prolonged elevation of plasma fatty acids in humans decreases insulin secretion, and it has previously been shown that there is an association between pancreatic fat content and type 2 diabetes. Prior to the onset of spontaneous diabetes in rodents, both total pancreatic fat and islet triacylglycerol content increase sharply. In vitro, chronic saturated fatty acid exposure of beta cells inhibits the acute insulin response to glucose, and removal of fatty acids allows recovery of this response. The present data provide clear evidence that decreasing total pancreatic fat is associated with a return of beta cell function. However, it is probable that the negative effect on beta cell function is exerted by toxic intermediaries such as diacylglycerol and ceramides, which change rapidly in response to acute metabolic changes, rather than by stored triacylglycerol per se, which acts as an index of fatty acid intermediary concentration. There was no correlation between indices of insulin secretion and pancreatic fat, which suggests that there are individual thresholds of tolerance for such toxic intermediaries rather than a simple dose–response relationship within the pancreas.
________
________
Connecting dots:
I have proposed a novel hypothesis on diabetes. Every patient suspected of diabetes must do, not only FPG, PPG and A1C but also fasting and postprandial serum insulin level for appropriate staging of DM. All DM patients need daily exogenous insulin from day 1 till person dies or in rare cases, till he becomes non-diabetic by life-style changes. Insulin is the best treatment of diabetes mellitus from day1. I have reviewed literature and gone through most studies on the subject. Now let me connect dots to substantiate my hypothesis or reject it.
________
Dot one:
Insulin physiology:
Insulin’s role is really that of a traffic cop— signalling where nutrients should go (carbs, protein, and fat) just after meals. When carbohydrate or protein is eaten, insulin directs the body to burn the carbohydrate or protein instead of using fat. And when mostly fat is eaten, the lack of an insulin response directs the body to burn the fat just eaten. Either way, you burn what you eat, and when that runs out, you go back to burning stored fat. To label insulin as the “fat storage hormone” would be an oversimplification and misrepresentation. It’s important to note that Insulin is sensitive to both carbohydrate and protein consumed, but not fat. However, of all the food sources, it’s the higher carbohydrate meals that elevate Insulin levels the most after a meal.
________
Dot two:
Serum insulin level measurement:
Insulin should always be assayed in serum and not plasma. Although there isn’t much agreement on what level is ideal, fasting serum insulin level in a healthy adult should be between 2 to 8 milliU/L and 2 hour post-prandial level between 16 to 60 milliU/L. C-peptide is a short peptide sequence released from the proinsulin molecule on a one-to-one ratio as insulin is secreted. C-Peptide is secreted from the β-cell in equimolar quantities with insulin. While ~50% of insulin is extracted by the liver, C-peptide is not extracted by the liver. Therefore, the measurement of C-peptide more directly reflects beta cell secretory rate compared with insulin, independent of hepatic clearance. The complete human C-Peptide range as opposed to the “normal” range is in fact 0.6 – 12.0 ng/ml, keeping in mind that the normal values are for a non-diabetic person. The postprandial serum C-peptide to plasma glucose ratio is called postprandial C-peptide index. Postprandial C-peptide reflects maximal insulin secretion induced by a combination of postprandial hyperglycemia and incretin effects, compared with fasting C-peptide. Postprandial C-peptide index is more closely correlated with beta cell mass in humans compared with fasting C-peptide index. Therefore postprandial C-peptide index appears to be a simple and useful marker of beta cell function in clinical settings. Stressed beta cells accelerate insulin production and secretion, and one consequence is that the proportion of proinsulin to cleaved insulin entering the circulation is increased. A raised proinsulin: insulin ratio (PI/I ratio) is thus a marker of beta cell distress and, since proinsulin has less metabolic effect than cleaved insulin, insulin action is also reduced.
______
Dot three:
Why endogenous insulin secretion is so important in diabetic patients?
1. For individuals with diabetes in the fasting state, plasma glucose is derived from glycogenolysis and gluconeogenesis under the direction of glucagon. Exogenous insulin influences the rate of peripheral glucose disappearance and, because of its deficiency in the portal circulation, does not properly regulate the degree to which hepatic gluconeogenesis and glycogenolysis occur. For individuals with diabetes in the fed state, exogenous insulin is ineffective in suppressing glucagon secretion through the physiological paracrine route, resulting in elevated hepatic glucose production. As a result, the appearance of glucose in the circulation exceeds the rate of glucose disappearance. The net effect is postprandial hyperglycemia.
2. Following a meal, endogenous insulin release from the pancreas is not continuous, but oscillates with a period of 3–6 minutes, changing from generating a blood insulin concentration more than about 800 pmol/l to less than 100 pmol/l. This is thought to avoid downregulation of insulin receptors in target cells, and to assist the liver in extracting insulin from the blood. Exogenous insulin cannot be given in oscillating levels.
3. B-cells co-secrete Amylin and C-peptide along with endogenous insulin. Amylin reduces glucagon secretion and delay gastric emptying postprandial thereby regulate the rate of glucose appearance from both endogenous (liver-derived) and exogenous (meal-derived) sources. C-peptide is a molecule that helps to prevent neuropathy and other vascular complications by assisting in the repair of the muscular layers of the arteries. These tasks cannot be achieved by exogenous insulin.
Due to these three reasons, endogenous insulin secretion ought to be preserved in diabetics as long as possible by any means.
______
Dot four:
Role of b-cells in diabetes mellitus:
The beta cell dysfunction is a common pathogenetic feature of both type 1 and type 2 diabetes, and T2DM never develops without beta cell dysfunction. Therefore, treatment of T2DM should aim to restore beta cell function. Patients with impaired glucose tolerance have <50 % of normal β-cell function and by the time you are diagnosed and have a postprandial blood sugar over 200 mg/dl at 2hrs you will have lost 80% of your beta cell function relative to the degree of insulin resistance. Autopsy studies found that diabetic had 63% less beta cell mass than normal people–which they attributed to beta cell death, not shrinking in the size of the individual cells. The United Kingdom Prospective Diabetes Study (UKPDS) clearly showed that most people with type 2 diabetes will experience progressive pancreatic β cell dysfunction, despite excellent control. Beta cell function is the most important determinant of glucose disposal valid even after adjustment for insulin sensitivity, which might modulate beta cell function. Beta cell function is significantly associated with clinical outcome, i.e., glycemic control, in patients with T2DM. In the UKPDS and a Diabetes Outcome Progression Trial (ADOPT), treatment failure (poor glycemic control) was associated with a progressive decline of beta cell function. The results of the UKPDS mandate that treatment of T2DM include aggressive efforts to lower blood glucose to as close to normal as possible early in the disease process because a period of good glycemic control early in the course of the disease has a lasting benefit independent of subsequent glycemic control due to regeneration of b-cells. Several clinical and experimental studies have clearly shown that even minimally preserved beta cell function is metabolically beneficial. This leads to lower HbA1C levels, lower insulin dosage and lesser metabolic decompensation after insulin withdrawal.
______
Connecting dot 3 and dot 4:
B-cells generate endogenous insulin. Since endogenous insulin secretion must to be preserved in diabetics as long as possible by any means and since T2DM never develops without beta cell dysfunction and since beta cell function is significantly associated with clinical outcome; the goal of diabetic treatment is to preserve, protect and rejuvenate b-cell function.
______
Dot 5:
Insulin resistance:
1. Although insulin sensitivity and insulin secretion are thought to be independent processes connected only by blood glucose level (deceased insulin sensitivity leads to high blood glucose level leads to higher insulin secretion and vice versa), presence and functional activity of the insulin receptor and its signalling proteins within the β-cell suggest that insulin could regulate its own secretion. So insulin resistance is not only at liver, adipose tissue and muscles but also at b-cells themselves.
2. Insulin resistance is defined as a subnormal glucose response to insulin compared to healthy normal individual. Although insulin is inefficient at lowering blood sugar for those with insulin resistance, insulin resistance doesn’t diminish insulin’s capacity for fat-building.
3. As long as there is adequate insulin response to insulin resistance, T2DM does not occur. In other words, inadequate insulin response (b-cell failure) is the hallmark of T2DM.
4. Genetic factors also contribute to b-cell dysfunction and here insulin resistance may act as amplifier of the process leading toT2DM.
5. If your FPG is less than 100 mg% and fasting insulin is less than 5 milliunit/liter, you are not insulin resistant. Anything more you could be insulin resistant. A high plasma insulin value in individuals with normal glucose tolerance reflects insulin resistance, and high insulin levels presage the development of diabetes. The fasting serum insulin concentration provides information about the subject’s insulin sensitivity, but not about reductions in either beta cell mass or function. Serum insulin and blood glucose must be measured at the same time to evaluate insulin secretion properly. As an example, many patients with type 2 diabetes have higher fasting serum insulin concentrations than normal subjects, suggesting that they are oversecreting insulin. However, when the blood glucose concentration in the normal subject is raised to that of the patient with type 2 diabetes, the serum insulin concentration is much higher than in the diabetic patient.
6. At the onset of T2DM, insulin resistance is established with hyperinsulinemia (b-cell hyperfunction). However, since insulin secretion shows a compensatory increase in the presence of insulin resistance, true beta cell function should be evaluated based on insulin secretion adjusted to insulin sensitivity, the so-called disposition index. A correct evaluation of beta cell function requires quantification of insulin secretion in relation with insulin sensitivity. The Disposition Index (DI) is the product of insulin secretion and insulin sensitivity. Normally, increases in insulin resistance are matched by a compensatory increase in insulin secretion in a hyperbolic relationship and DI remains constant. DI falls in IGT and T2DM. Using the disposition index, DeFronzo et al. have reported that beta cell function is already decreased by approximately 80% in patients with impaired glucose tolerance (IGT) compared with non-diabetic subjects. In established T2DM, insulin level during oral glucose tolerance test (OGTT) is lower than normal people and people with impaired glucose tolerance (IGT) indicating b-cell failure. Although insulin level is high in IGT and early T2DM, b-cell mass and b-cell function calculated by disposition index is lower than normal individuals.
7. People in the FPG level between 110 – 124 mg/dl have the same cardiovascular and metabolic syndrome incidence as people with diabetes proving macrovascular complication linked to insulin resistance and hyperinsulinemia. Postprandial insulin resistance is an early predictor of cardiovascular risk rather than fasting parameters.
_______
Connecting dot 2 and dot 5:
Postprandial C-peptide index appears to be a simple and useful marker of beta cell function in clinical settings. The fasting serum insulin concentration in relation to fasting plasma glucose provides information about the subject’s insulin sensitivity. Elevated level of fasting insulin is the single greatest marker to assess a person’s cardiovascular and diabetic risk factors. So in clinical practice, we must measure not only fasting & post prandial plasma glucose and A1C but also fasting serum insulin and post prandial C-peptide level in every new diabetic patient to determine endogenous insulin secretion (b-cell function) and insulin resistance.
_______
Dot six:
Diabetes is a disease of vicious cycles:
High fat and high carb diet (overfeeding) leads to insulin resistance and obesity, and obesity in turn leads to further increase in insulin resistance. Insulin resistance leads to hyperinsulinemia and hyperinsulinemia further aggravates insulin resistance. Although insulin resistance is by definition tethered to hyperinsulinemia, hyperinsulinemia is often both a result and a driver of insulin resistance. Hyperinsulinemia desensitizes receptors and causes worsening of situation in a vicious cycle of insulin resistance and hyperglycemia. Hyperglycemia itself augments both B-cell dysfunction and insulin resistance leading to the progression to type 2 diabetes and further hyperglycemia. There is a robust link between excess energy intake, insulin resistance, and the development of diabetes type 2. Diet control and physical exercise can break these vicious cycles.
_______
Dot seven:
Advantages of exogenous insulin therapy in diabetes mellitus:
1. Exogenous insulin administration initially reduces endogenous insulin secretion by glucose dependent and glucose independent mechanisms by reducing load on b-cells of pancreas. Insulin therapy allows b-cells rest, at least in part by down-regulating their metabolism and/or by releasing them from the hyperglycemic stress.
2. Once b-cells are rested, preserved and regenerated, and patient is doing diet control and physical exercise to reduce insulin resistance, exogenous insulin requirement falls as now insulin resistance is less and rejuvenated b-cells are secreting endogenous insulin. The use of exogenous insulin also partially reverses the post-binding defect in peripheral insulin action reducing insulin resistance.
3. In studies of insulin treated patients with Type 1 and Type 2 diabetes, postprandial hyperglycaemia is inversely related to endogenous insulin secretion. In other words, greater the b-cell function in insulin treated DM patients, lesser is postprandial hyperglycemia.
4. Studies with early short-term insulin treatment revealed that beta cell function could be re-gained with improved quality of insulin secretion as shown by better proinsulin/ insulin ratios and restored first phase. Early initiation of insulin addresses the issues of glucotoxicity, lipotoxicity, inflammation, insulin resistance, first phase insulin response and prevents deposition of islet amyloid. Early use of insulin results in increased insulin gene expression and insulin synthesis.
5. The anti-inflammatory and antioxidant effects of insulin may contribute to protection against endothelial dysfunction and vascular disease. These effects include suppression of reactive oxygen species (ROS) and adhesion molecule expression.
6. Various studies demonstrated that insulin use earlier in the treatment pathway led to improved glycaemic control, reduced weight gain and fewer hypoglycaemic episodes than when insulin was added later in the course of disease. Early insulin therapy reduces insulin resistance and prevents diabetic complication as opposed to late insulin therapy.
7. There is no clear advantage of analogue insulin over human insulin.
8. When one considers initiation of insulin therapy in a type 2 diabetic patient with the intention to preserve β-cell function, the level of evidence supporting this decision is relatively high.
9. The effect of use of insulin in type 2 diabetes from the time of diagnosis has been evaluated in clinical trials, notably the UK Prospective Diabetes Study (UKPDS) and Outcome Reduction With Initial Glargine Intervention (ORIGIN). UKPDS showed that early and continued glucose control can reduce microvascular complications and, in the long-term, improve cardiovascular prognosis. The beneficial effect of insulin therapy is further supported by studies in type 1 diabetes where it is apparent that if insulin therapy is used effectively to induce early glycemic control, both micro- and macrovascular protection is achieved.
10. I suggest sub-optimal daily exogenous insulin for T2DM which is 0.3 unit/kg body weight from day 1 of diagnosis and it is much less than insulin dose for T1DM where there is no endogenous insulin production. Sub-optimal insulin administration will protect b-cells and allow endogenous insulin generation in T2DM for long time.
_______
Dot eight:
Misconceptions of insulin therapy cleared:
When compared with a clinically equivalent treatment regimen, insulin-based therapy is effective and did not cause greater weight gain or hypoglycemia. It is intensive therapy that gained more weight than those in conventional therapy group. It is faulty diet associated with insulin therapy that causes weight gain; and dramatic weight loss and blood sugar improvement occur in people who have merely been shown how to reduce their carbohydrate intake and therefore their insulin doses. All intensive treatment increased the risk of hypoglycaemia. It is intensive insulin therapy that causes severe hypoglycemia and mortality. There is no incontrovertible evidence to show that insulin therapy increases cardiovascular risk and cancer risk. It is the insulin resistance that leads to increased cardiovascular risk rather than insulin treatment. It must be acknowledged that although the safety and benefit of moderate doses of insulin in the long-term are clear, the use of high-dose insulin therapy, especially with mealtime injections, for people with obesity and severe insulin resistance might still be associated with adverse outcomes. Hence in such people, it is mandatory to reduce body weight by diet control, exercise and metformin to reduce insulin resistance and improve outcome.
______
Connecting dot 7 and dot 8:
I am proposing sub-optimal insulin therapy from day1 i.e. 0.3 IU/Kg daily in T2DM which is very unlikely to cause weight gain provided carbohydrate intake is curtailed and unlikely to cause severe hypoglycemia.
_______
Dot nine:
Insulin versus oral hypoglycemic agents (OHA):
1. Although sulfonylurea (SU) control hyperglycemia and reduce glucotoxicity and thereby protect b-cells, prolonged exposure to SU on the other hand has been associated with beta cell exhaustion, desensitisation and possibly acceleration of oxidative stress and apoptosis, leading to a progressive reduction of insulin production capacity and deterioration of glycemic control over time. Current paradigm of using sulfonylureas in majority of type 2 diabetic patients leads to increased deposition of amyloid deposits and faster decline in beta cell function.
2. B-cell stimulation by sulfonylurea is non-physiological; if patient has reduced or missed meal, it will still produce sufficient endogenous insulin to cause hypoglycemia. Any hypoglycemic event on OHA means that some b-cell function is still present generating endogenous insulin. Similarly if patient stops taking OHA due to any reason and there is rebound hyperglycemia, it would also mean presence of some b-cell function. Such patients with some residual b-cell function would benefit from insulin therapy.
3. Authors are unable to find any evidence or even a trend towards improved all-cause mortality or cardiovascular mortality with metformin and insulin, compared with insulin alone.
4. In patients with type 2 diabetes, insulin monotherapy and insulin combined with oral hypoglycemic agents provide similar improvements in glycemic control.
5. Most studies find glyburide and insulin to be essentially equivalent for glycemic control in GDM, but insulin leads to better outcomes. Although metformin can be used instead of insulin in GDM, almost half women eventually did need insulin and metformin is associated with increased risk of preterm birth. Given the limitations of the studies conducted so far, I believe that insulin should remain first-line therapy in the treatment of gestational diabetes mellitus (GDM). The goal of treatment is not to achieve good control eventually, as metformin does, but to quickly achieve a state of euglycemia to minimize the effects of hyperglycemia on the foetus. As far as pregnant women with T2DM are concerned, they must be kept on insulin from the time they conceive through the postpartum period if they’re breastfeeding.
6. Although DPP-IV inhibitors (e.g. sitagliptin), GLP-1 receptor agonist (e.g. exenatide) and pioglitazone do improve b-cell function but their potential to reduce A1C is limited as compared to insulin and they also have adverse effects. On the top, they cannot be used in T1DM.
________
Connecting dot 7, dot 8 and dot 9:
Insulin alone is better than OHA or insulin plus OHA or GLP-1 receptor agonist in all diabetes with the possible exception of obese patients who need metformin to reduce weight and reduce insulin resistance.
________
Dot ten:
Reversibility of T2DM:
Overall, there is strong evidence that type 2 diabetes is inexorably progressive, with a high likelihood of insulin therapy being eventually required to maintain good glycaemic control. However, type 2 diabetes is clearly reversible following bariatric surgery. Bariatric surgery results in resolution of diabetes in 78 % of patients with T2DM demonstrating its significant impact on T2DM remission. Overfeeding (high calorie diet) leads to insulin resistance in a matter of days. On the other hand, sudden dietary energy restriction (negative energy balance) alone can reduce insulin resistance and improve b-cell function. In other words, although early insulin therapy preserves b-cells, insulin with positive energy balance (overfeeding) would nullify protective effects of insulin on b-cells and would lead to worsening diabetes, obesity and worsening diabetic complications.
________
Connecting dot 6, dot 7, dot 8, dot 9 and dot 10:
If T2DM patient is very obese requiring bariatric surgery, do bariatric surgery first rather than insulin therapy. All T2DM patients taking insulin from day 1 must adhere to diabetic diet and physical exercise to reduce excess weight, reduce insulin resistance and break various vicious cycles discussed in dot 6.
________
Dot eleven:
Diabetic complications:
1. Intensive blood-glucose control by either sulfonylureas or insulin substantially decreases the risk of microvascular complications, but not macrovascular disease, in patients with type 2 diabetes.
2. Hyperglycemia per se is linked to microvascular complication with or without insulin resistance. Insulin resistance is linked to macrovascular complications with or without hyperglycemia. Genetic factors also contribute to diabetic complications independent of degree of hyperglycemia and independent of insulin resistance.
3. For every 1 percent reduction in results of HbA1c, the risk of developing eye, kidney, and nerve disease (microvascular complications) is reduced by 40 percent while the risk of heart attack (macrovascular complications) is reduced by 14 percent.
4. Only insulin lowers A1C levels by more than 5 % compared to any OHAs or their combinations, and corollary is that only insulin can reduce diabetic complications more than any other therapy. Although it is acknowledged that achieving HbA1c < 7.0% is a difficult task, improvement of glycemic control with insulin is associated with improved patient well-being even if the HbA1c target is not achieved.
5. Insulin has been used longer than any other medication for treating diabetes, and the body of evidence supporting a favourable balance of benefits to risks of its use continues to grow. Some large, long-term clinical trials, including the UKPDS, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC), and ORIGIN, have shown that both microvascular and cardiovascular benefits can be obtained.
6. There is apparent U-shaped relationship between glycated hemoglobin (A1C) and cardiovascular disease and mortality. A study found that patients with an average A1C ≤6% are 20% more likely to experience a cardiovascular event than the group with an average A1C between 6 to 8%. Patients with an average A1C >8% experience a 16% increase in the likelihood of a cardiovascular event. Too tight A1C control or too lax A1C control increases cardiovascular mortality, therefore moderation is a key in diabetic management and sub-optimal insulin therapy from day 1 proposed by me ought to be seen as moderation. There are no benefits of intensive glucose lowering treatment on all-cause mortality and deaths from cardiovascular causes in adults with type 2 diabetes. Intensive insulin therapy (target blood glucose 80-110 mg/dL) is also not associated with improved survival among critically ill patients.
_______
Connecting all eleven dots:
The ideal treatment for type 1 DM and type 2 DM is early and continuing insulin therapy accompanied by diet control, physical exercise, reducing excess weight and use of metformin in obese individuals. Insulin therapy lasts lifelong in T1DM, probably lifelong in T2DM with exception of candidates for bariatric surgery and rare cases of reversible DM following negative energy balance (weight reduction) with exercise. Sub-optimal insulin dose is best for T2DM so that both exogenous and endogenous insulin work in tandem.
______
______
Final comments:
My hypothesis does hold water at the end of the day. In clinical practice, we must measure not only fasting & post prandial plasma glucose and A1C but also fasting serum insulin and post prandial C-peptide level in every new diabetic patient to determine endogenous insulin secretion (b-cell function) and insulin resistance. Insulin is the best treatment of all types of diabetes mellitus. Insulin therapy must start from day 1 and continued indefinitely, and must be accompanied by diet control, physical exercise and weight reduction & metformin use in obese patients. Only very obese individuals who are candidates for bariatric surgery must be treated by bariatric surgery and not lifetime insulin.
______
______
Dr. Rajiv Desai. MD.
October 6, 2015
______
Postscript:
I request diabetes researchers to read this article. Their comments and criticisms may be conveyed to me at my email ID [email protected]
________
Footnote:
In my article ‘Self-measurement of blood glucose (SMBG)’ posted last year, I have stated that it is the arterial blood glucose that secretes insulin and it is the high arterial blood glucose that causes diabetic complications when insulin secretion and/or action is reduced. Then why rely on venous blood glucose for diagnosis of diabetes? Since it is difficult to puncture artery every time for blood glucose test and since arterial blood glucose is almost same as capillary blood glucose, why not capillary blood glucose for diagnosis of diabetes mellitus? Of course we have to change cutoff values for diabetes diagnosis but it would be more rational, more physiological and correlate well with diabetic complications. Of course there is marked difference between capillary blood glucose and venous blood glucose in non-diabetic individual post prandial, but since capillary (surrogate arterial) blood glucose determines diabetic complications, cutoff value for diagnosis of diabetes, both fasting and post prandial, must be based on capillary blood glucose measurement (SMBG) rather than venous glucose. This article ‘Novel Approach to Diabetes Mellitus’ complements the article ‘Self-measurement of blood glucose (SMBG)’ posted last year.
_________

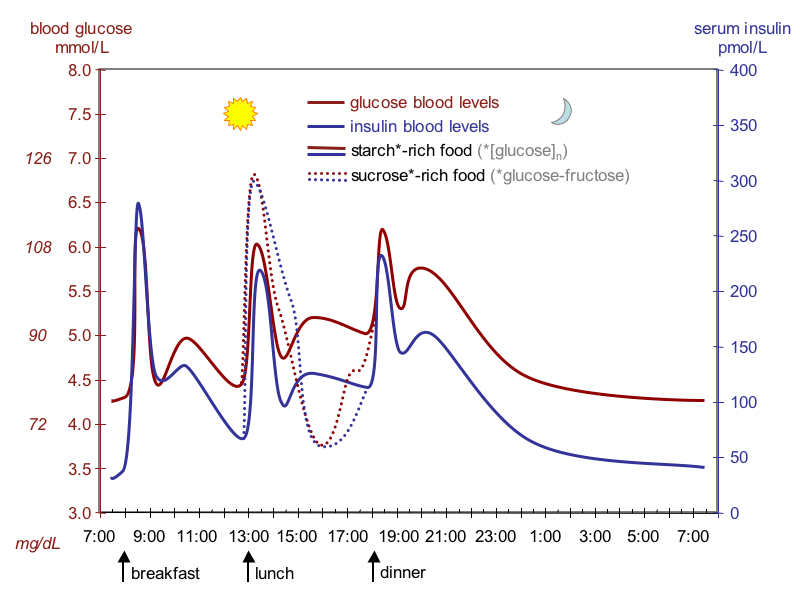
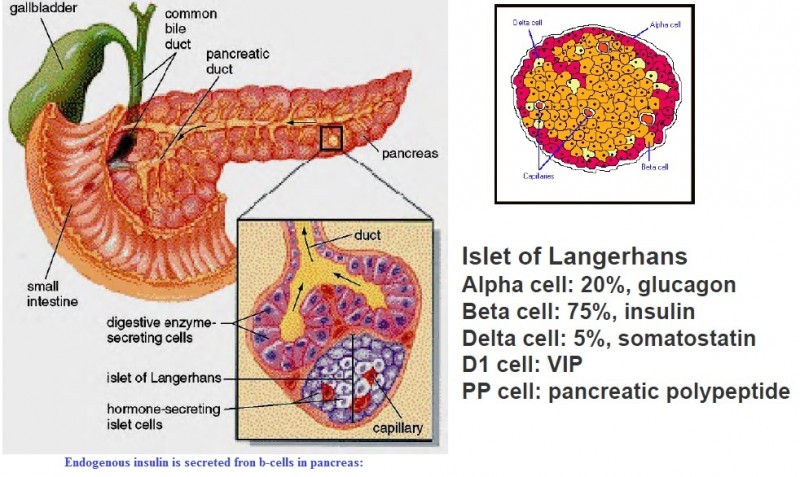
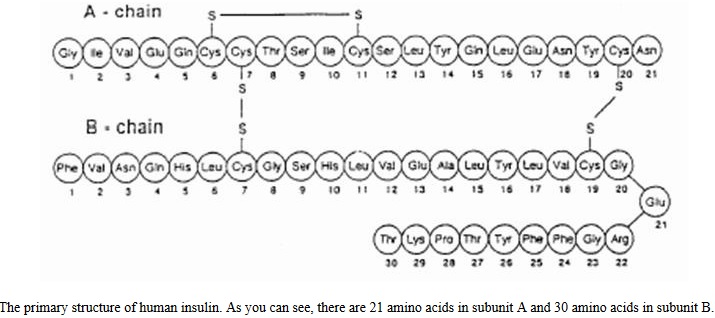
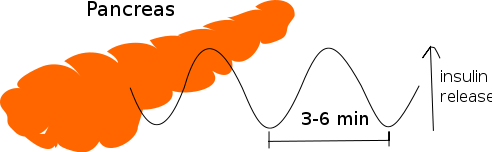

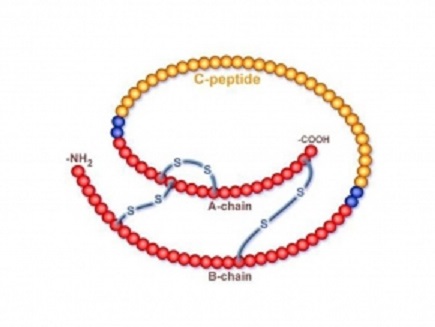
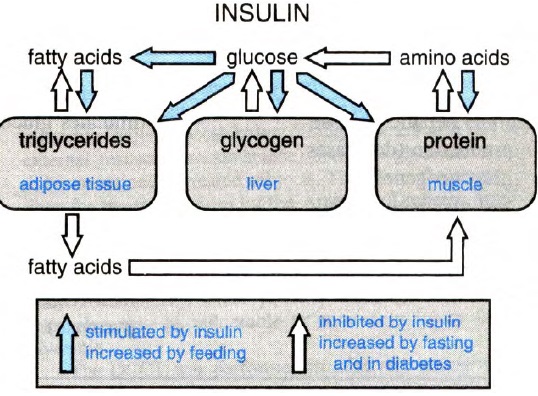
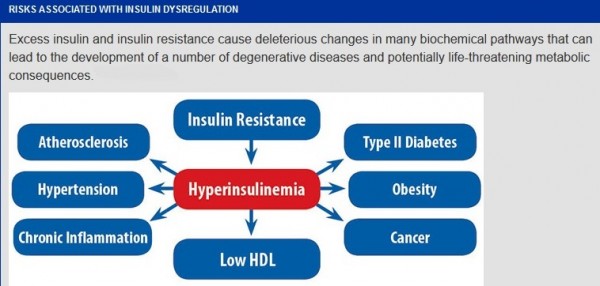
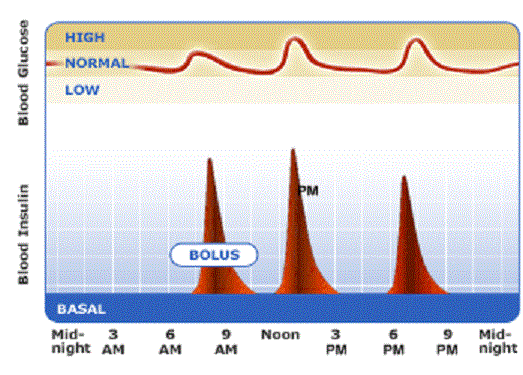
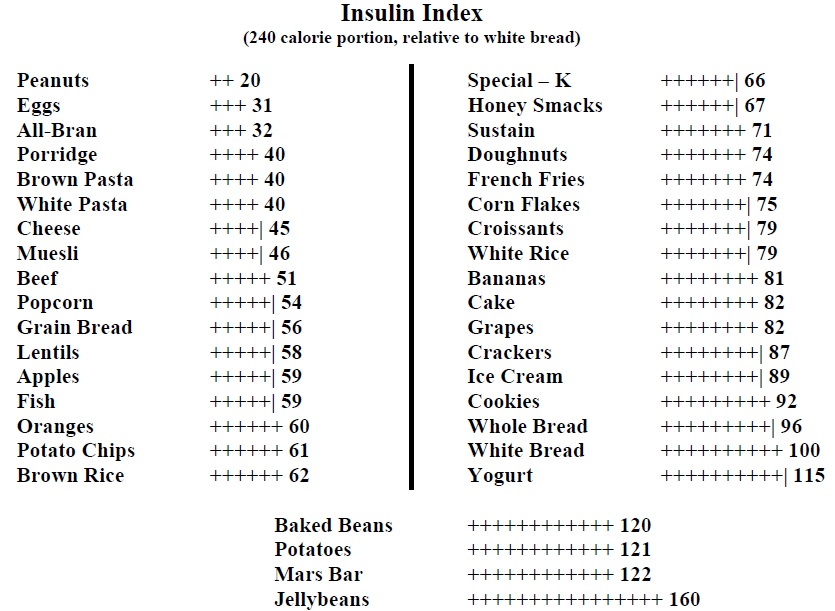
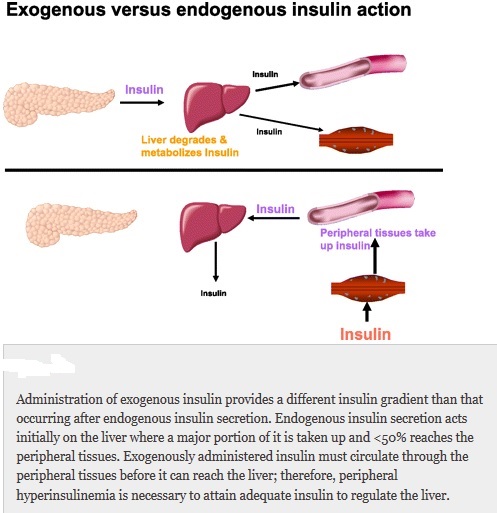
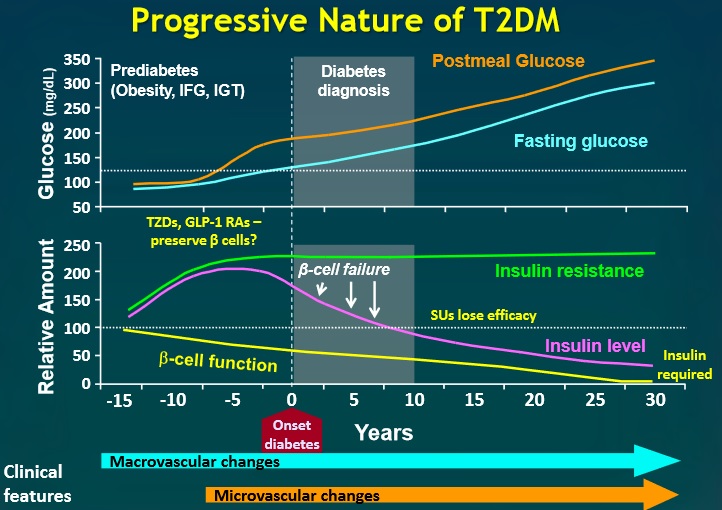
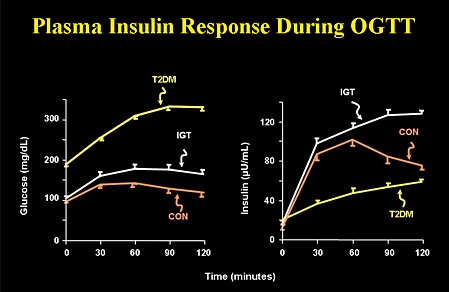
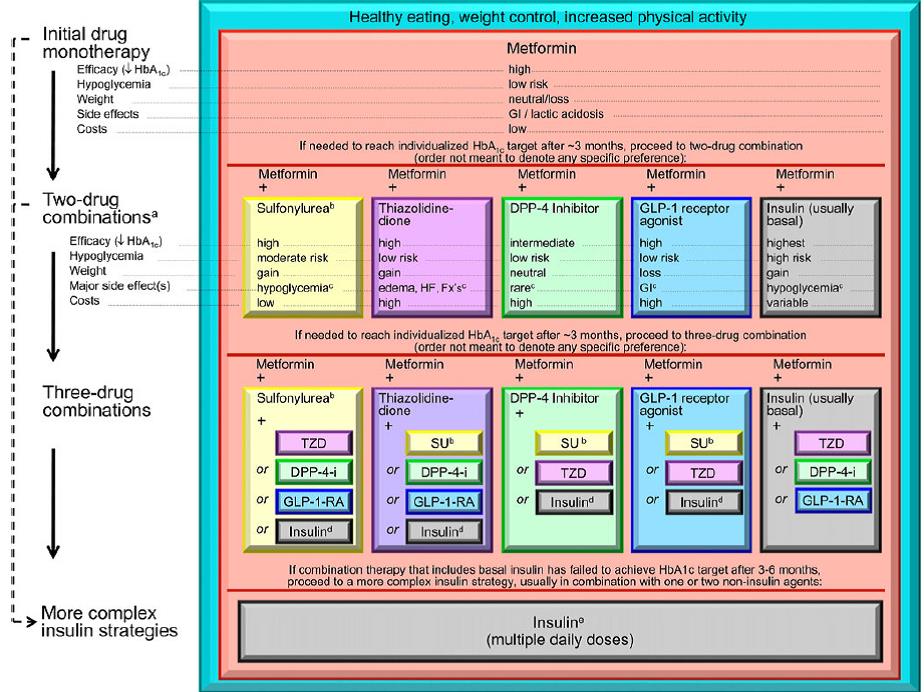
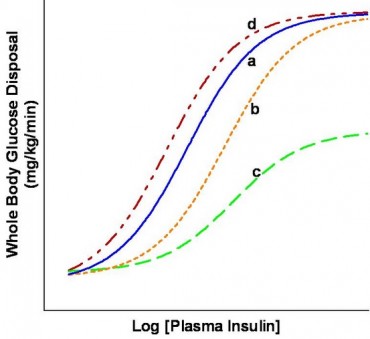
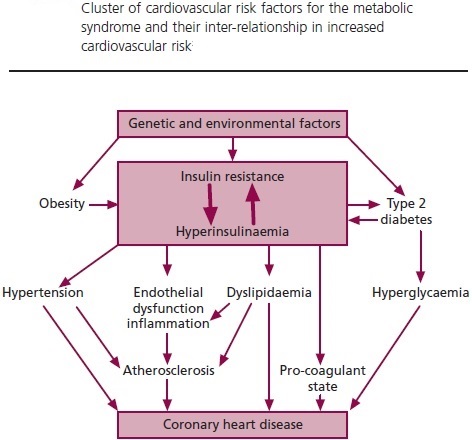
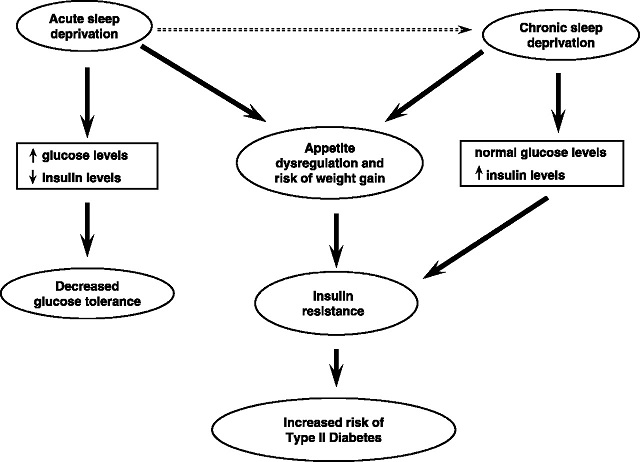
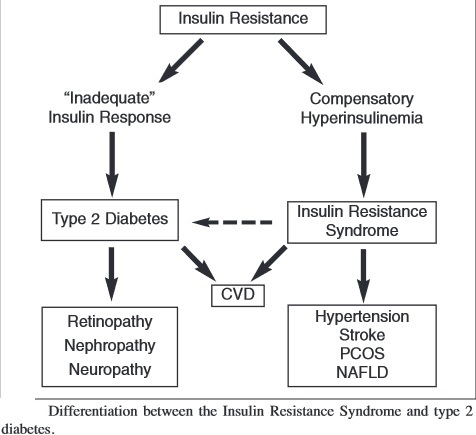
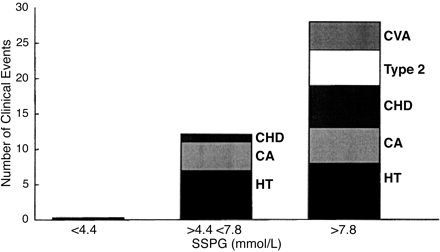
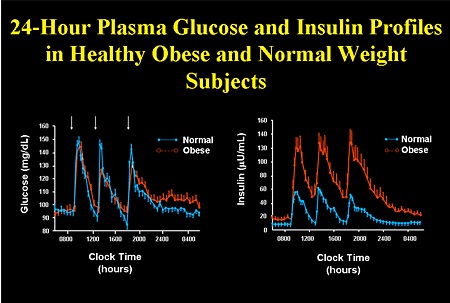
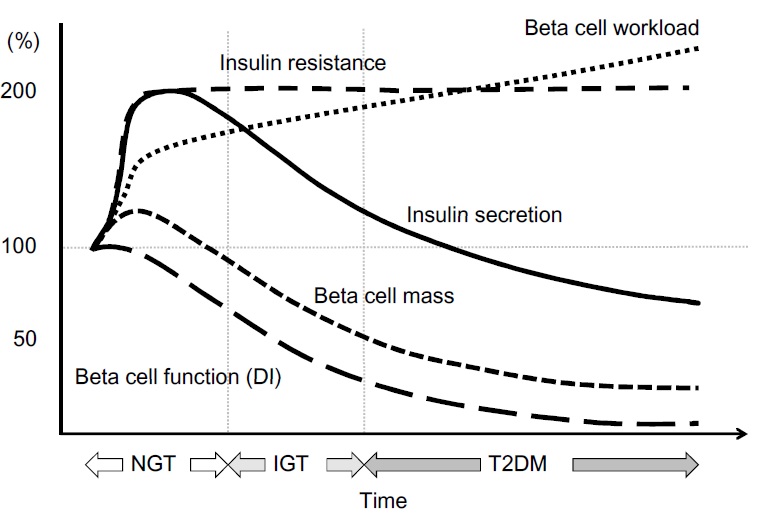
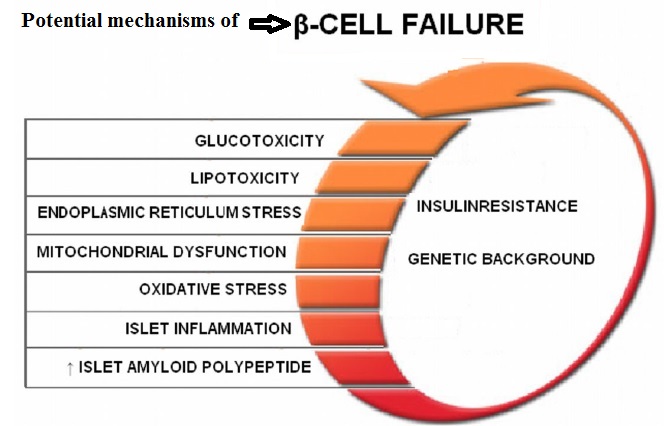
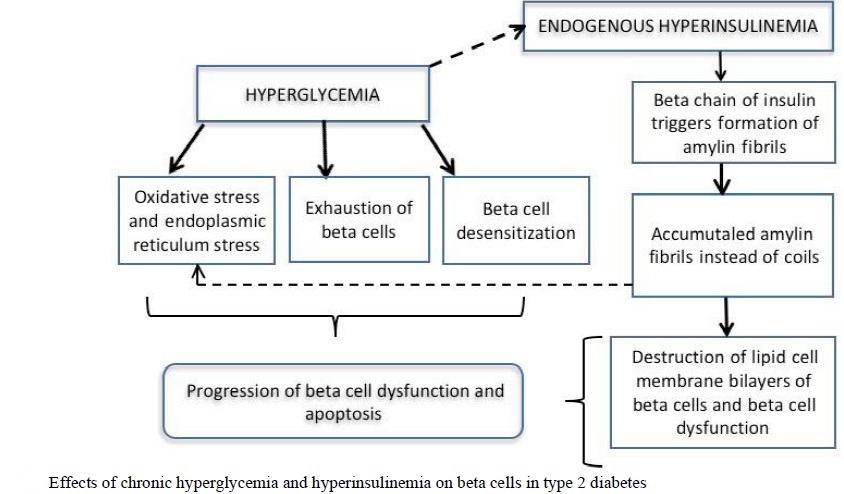
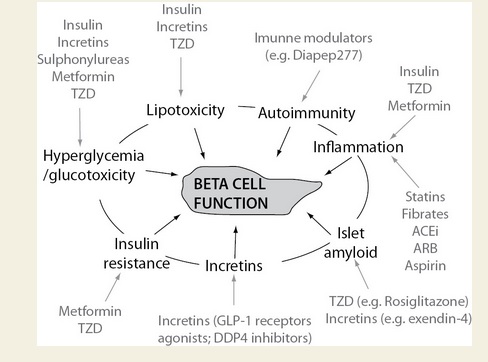

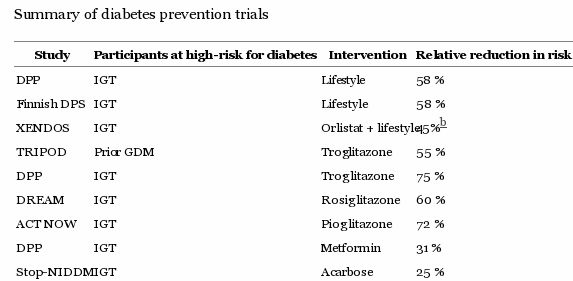
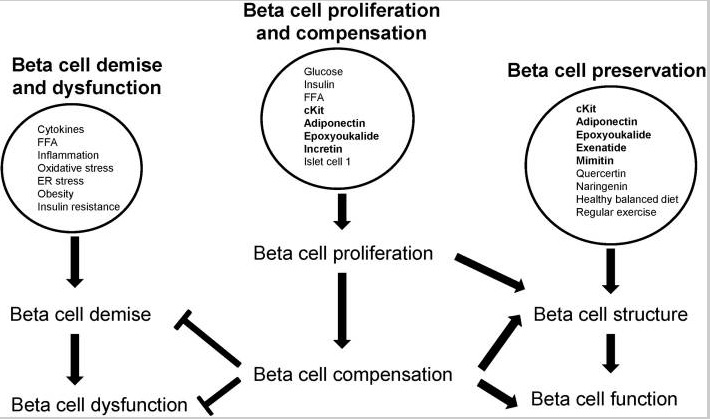
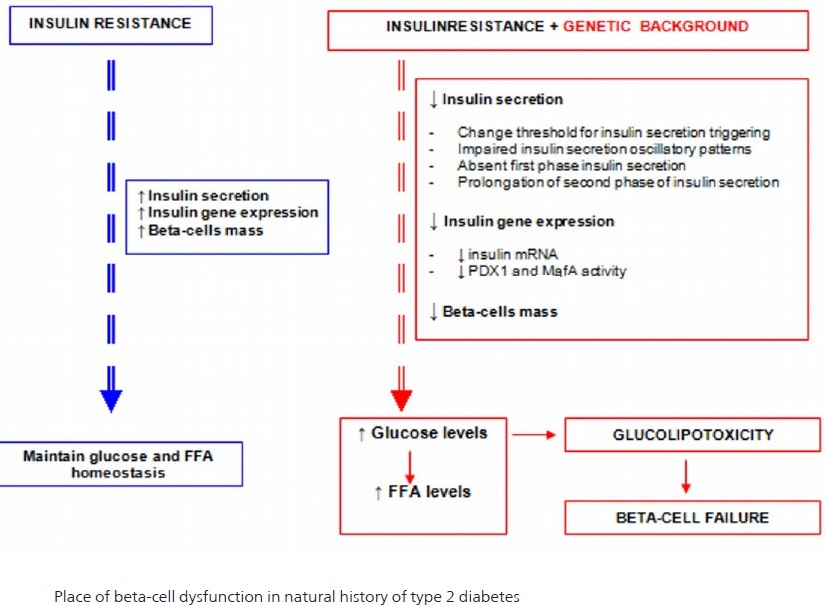
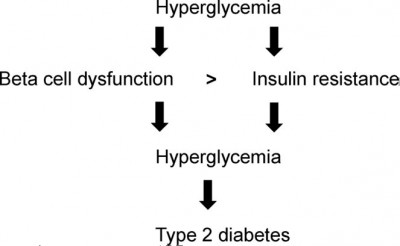
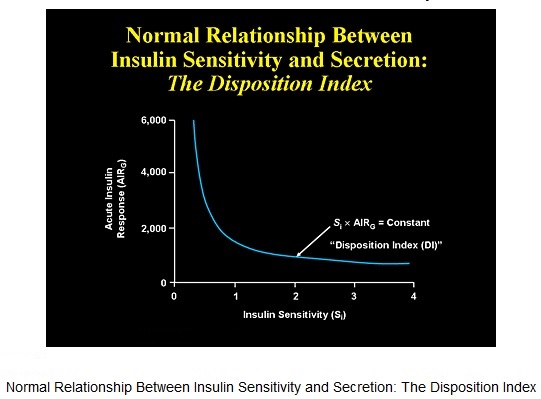
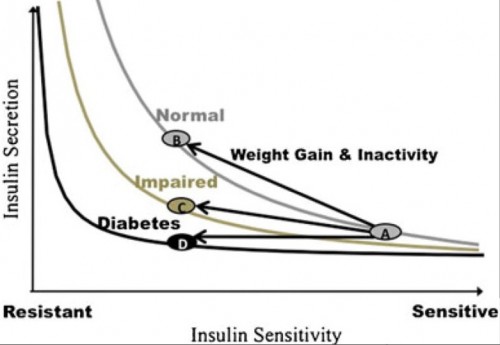
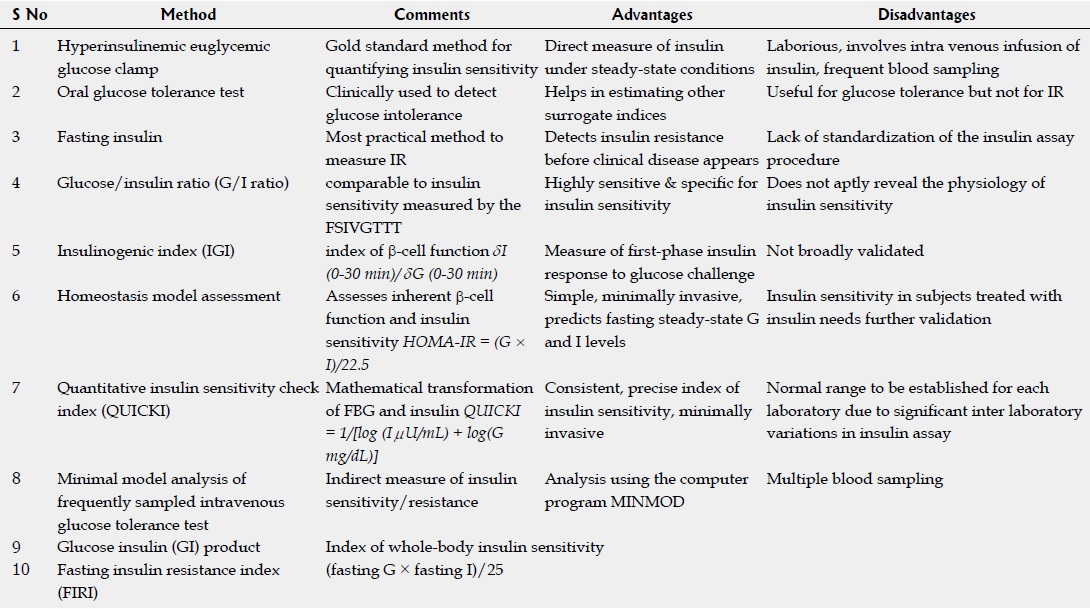
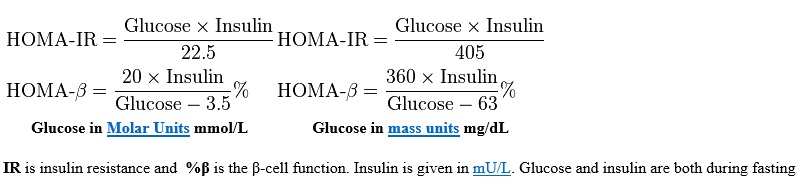
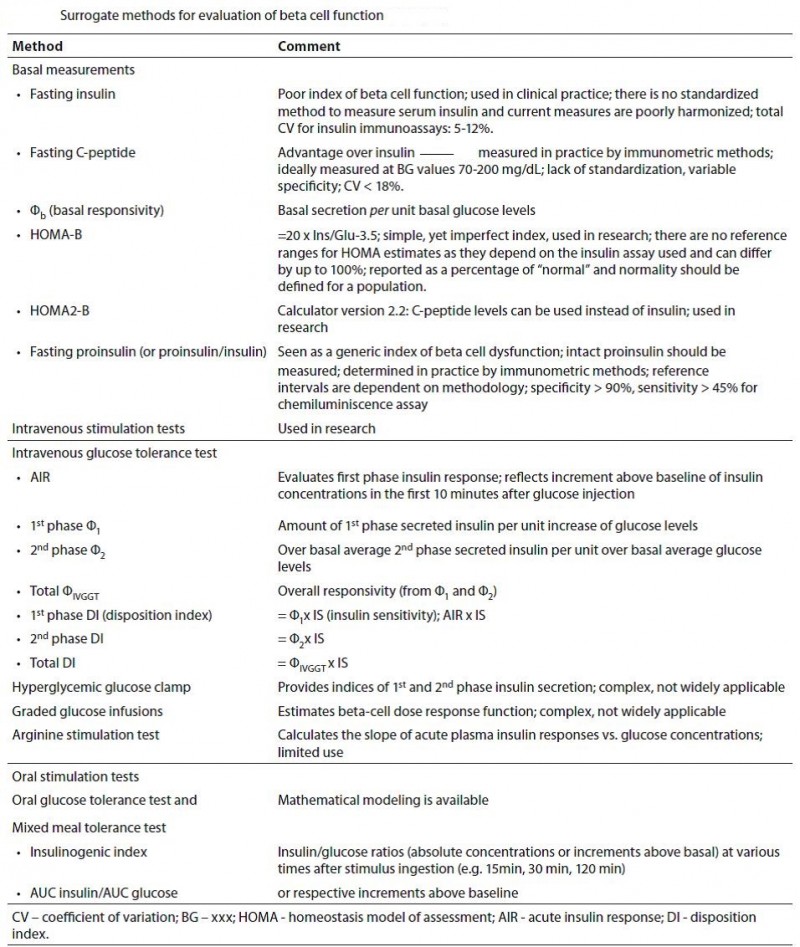
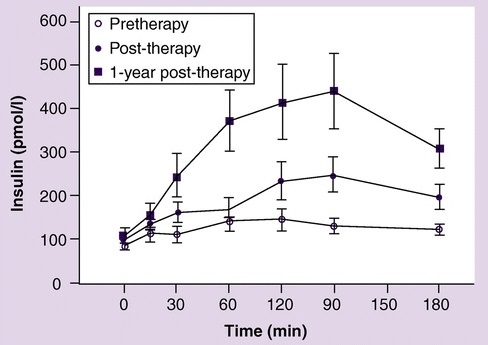
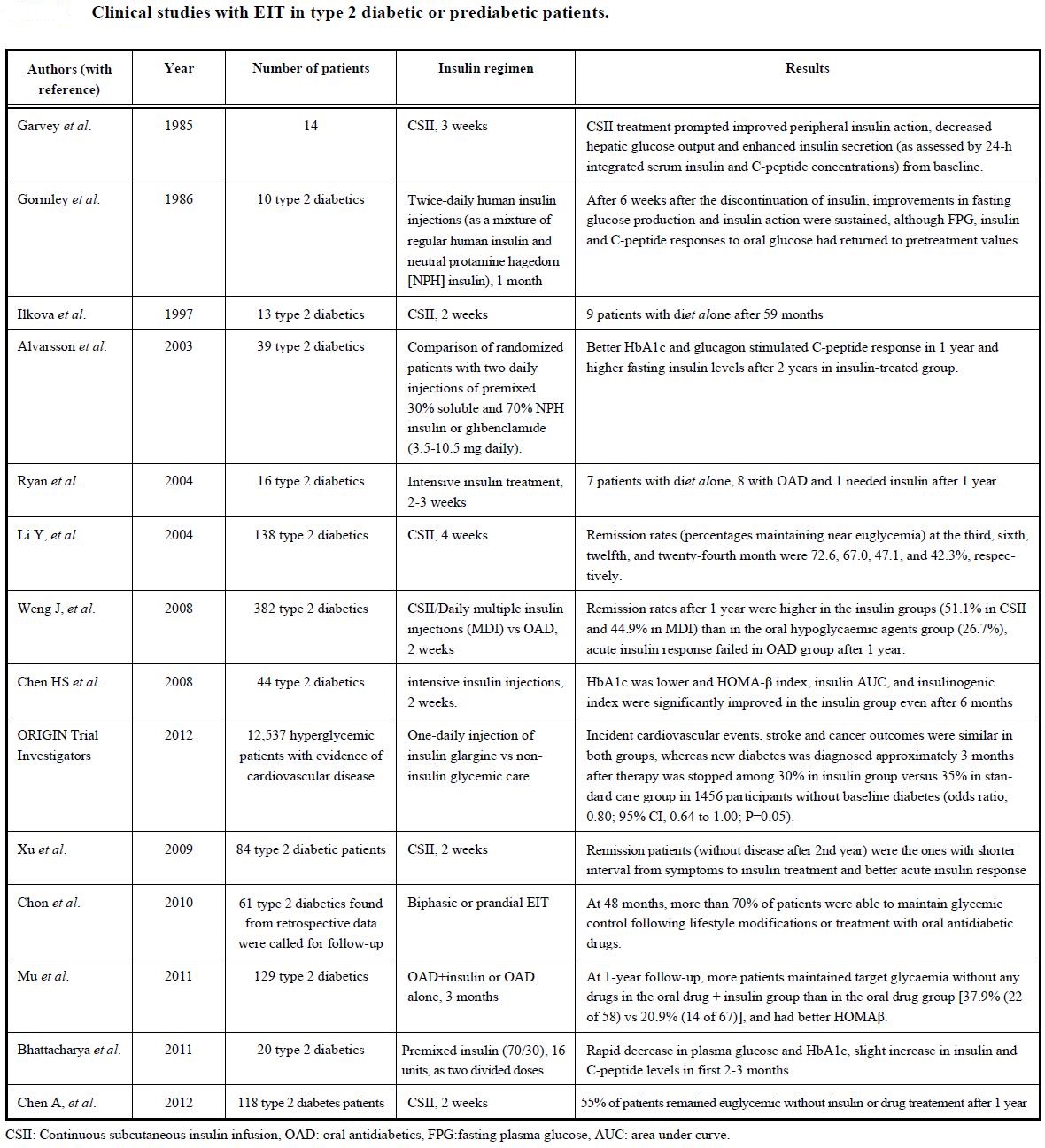
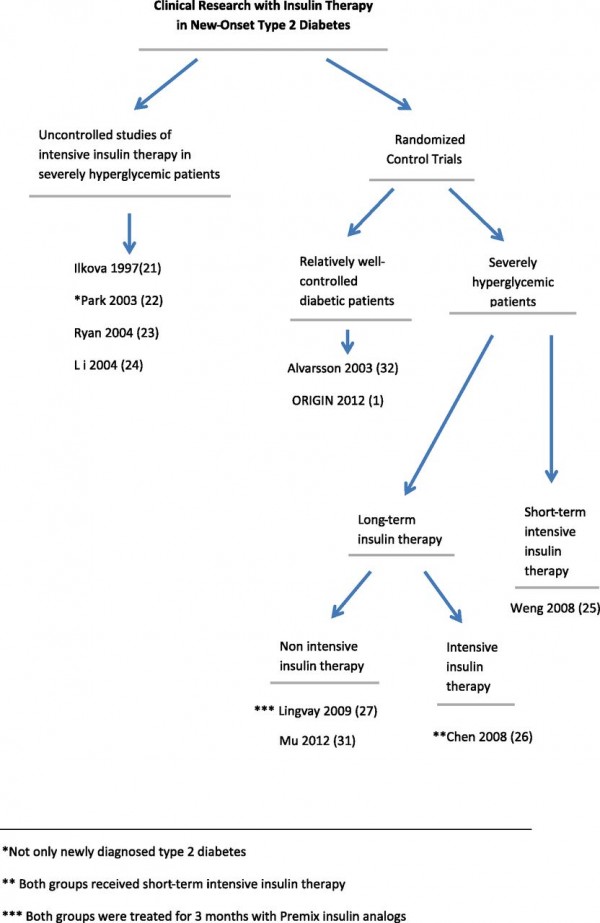
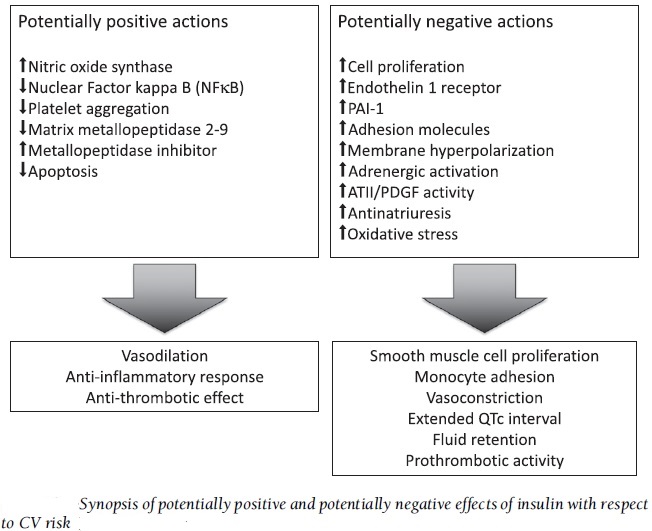
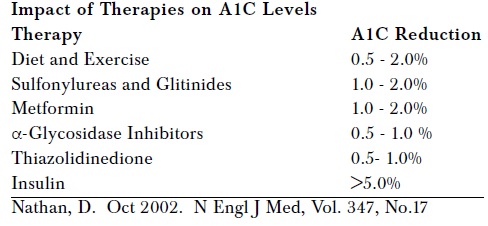
Hey, thanks for the article post.Much thanks again. Want more.
I really liked your blog.Thanks Again. Will read on…
I really enjoy the blog.Thanks Again. Cool.
Greetings! Very useful advice within this post! It’s the little changes that will make the greatest changes. Thanks a lot for sharing!
I was wondering if you ever thought of changing the structure of your website? Its very well written; I love what youve got to say. But maybe you could a little more in the way of content so people could connect with it better. Youve got an awful lot of text for only having 1 or 2 pictures. Maybe you could space it out better?
There is certainly a great deal to learn about this subject. I really like all of the points you made.
This site was… how do I say it? Relevant!! Finally I have found something that helped me. Thank you!
This is a topic that’s near to my heart… Cheers! Where are your contact details though?
Excellent blog you have here.. Itís difficult to find good quality writing like yours these days. I honestly appreciate people like you! Take care!!
Very well written story. It will be useful to anybody who utilizes it, as well as yours truly :). Keep doing what you are doing – can’r wait to read more posts.
It’s hard to find experienced people about this subject,but you seem like you know what you’re talkingabout! Thanks
It is actually a nice and useful piece of info. I’m satisfied that you just shared this useful information with us. Please keep us up to date like this. Thank you for sharing.
It’s an awesome article for all the online people;they will take benefit from it I am sure.
Hi! I simply would like to give you a big thumbs upfor the great info you have right here on this post. Iwill be coming back to your blog for more soon.
Major thankies for the article.Really thank you! Fantastic.
You’re so awesome! I don’t believe I’ve read through a single thing like this before. So good to find somebody with a few genuine thoughts on this topic. Really.. thank you for starting this up. This site is something that is needed on the internet, someone with a little originality!
This website was… how do I say it? Relevant!! Finally I have found something which helped me. Appreciate it!
This blog was… how do you say it? Relevant!! Finally I have found something that helped me. Many thanks!
Very good article! We are linking to this great article on our website. Keep up the good writing.
You’re so interesting! I don’t believe I have read a single thing like that before. So nice to find somebody with some unique thoughts on this subject. Really.. thank you for starting this up. This web site is one thing that is required on the internet, someone with a bit of originality!
There’s definately a lot to find out about this issue. I love all of the points you’ve made.
Good day! I could have sworn Iíve been to this site before but after browsing through some of the posts I realized itís new to me. Anyways, Iím definitely happy I found it and Iíll be bookmarking it and checking back often!
bookmarked!!, I like your blog!
I love it when individuals come together and share ideas. Great website, keep it up!
After looking into a handful of the articles on your website, I honestly like your technique of writing a blog. I book marked it to my bookmark site list and will be checking back in the near future. Take a look at my website as well and let me know how you feel.
You are so cool! I do not think I have read a single thing like this before. So wonderful to discover someone with original thoughts on this topic. Really.. thank you for starting this up. This site is something that is required on the web, someone with a little originality!
I couldnít refrain from commenting. Exceptionally well written!
Hi, I do believe this is an excellent website. I stumbledupon it 😉 I will revisit yet again since I saved as a favorite it. Money and freedom is the greatest way to change, may you be rich and continue to guide other people.
Thank you for the auspicious writeup. It in fact was a amusement account it. Look advanced to more added agreeable from you! However, how could we communicate?
You’re consequently amazing. Oh my Our god. The almighty bless you.
Whoa. Guru. You’re the guru. Give thanks to you.
The only thing I regret is not having found your blog site earlier.
It’s a envious article. It’s very wonderful and innovative. Who are usually you to write this specific special article?