Dr Rajiv Desai
An Educational Blog
OBESITY
OBESITY:
_
_
Prologue:
It only needs a daily excess of energy of 100 kilocalories (the equivalent of a small chocolate bar) to lead to an increase of around 5Kg of fat over 12 months or 50 Kg over 10 years. For thousands of years obesity was rarely seen. Obesity is a modern problem – statistics for it did not even exist 50 years ago. The increase of convenience foods, labor-saving devices, motorized transport and more sedentary jobs means people are getting fatter. It was not until the 20th century that it became common, so much so that in 1997 the World Health Organization (WHO) formally recognized obesity as a global epidemic. The WHO describes the “escalating global epidemic” of obesity as “one of today’s most blatantly visible — yet most neglected — public health problems”. Obesity is a bigger health crisis globally than hunger, and the leading cause of disabilities around the world, according to the British medical journal The Lancet. Obesity is now killing triple the number of people who die from malnutrition as it claims more than three million lives a year worldwide. The world is now obese and faces alarming obesity rates — an increase of 82% globally in the past two decades. Middle Eastern countries are more obese than ever, seeing a 100% increase since 1990. While child hunger has traditionally been the more pressing problem in African countries, researchers say that malnourished, growth-stunted children are turning into overweight adults. This means that countries still wracked by malnutrition, as well as infectious diseases such as malaria, tuberculosis and HIV/AIDS, are facing a battery of chronic health problems such as diabetes and heart disease. A growing number of developing countries shoulder a “double burden” of malnutrition: the persistence of undernutrition, especially among children, along with a rapid rise in overweight and obesity. Many experts believe that obesity in itself is a form of malnutrition that coexists with undernutrition in developing countries. We have gone from a world 20 years ago where people weren’t getting enough to eat to a world now where too much food and unhealthy food even in developing countries that is making us sick.
______
Evidence that obesity epidemic is fairly recent:
A new study by Paul von Hippel and Ramzi Nahhas looks at 60 years of data on child obesity and finds that the increase in obesity rates started with children born in the 1970s and 1980s. Their results lined up pretty well with the conventional wisdom, suggesting that the obesity epidemic is not particularly old but took off in the 1980s.They found that child obesity rates were low and stable among children born in the 1930s, 40s, 50s, 60s, and part of the 70s, and then rose rapidly through the 1980s and 1990s. Unlike Komlos and Brabec, they did not find evidence that the obesity epidemic was underway earlier. They did see some evidence that girls (but not boys) were getting a bit heavier before 1960, but significant numbers of girls didn’t break into the obese category until after 1980. In fact, much of the increase in girls’ weight before 1960 consisted of girls moving out of the underweight category and into a normal weight range. While it may be true that BMIs have been increasing slowly for a long time, the increases observed in recent decades are much faster and have pushed many adults and children over the obesity threshold in a remarkably short time. The trend is distressing, but to reverse it we only need to turn the clock back to 1980. We don’t need to go back to 1900.
_
The map below shows prevalence of obesity worldwide as per WHO:
_
Worldwide obesity has nearly doubled since 1980.
In 2008, more than 1.4 billion adults, 20 and older, were overweight. Of these over 200 million men and nearly 300 million women were obese. 35% of adults aged 20 and over were overweight in 2008, and 11% were obese.
65% of the world’s population lives in countries where overweight and obesity kills more people than underweight.
More than 40 million children under the age of five were overweight in 2011.
Overall, more than 10% of the world’s adult population was obese in 2010.
Growing rates of maternal overweight are leading to higher risks of pregnancy complications, and heavier birth weight and obesity in children.
Obesity is a leading cause of diabetes, ischemic heart disease, and high blood pressure. About 45 per cent of the type 2 diabetes cases are due to excessive body weight.
More than half of the obese people have obstructive sleep apnea, which can cause high blood pressure, heart disease, and road traffic accidents.
Obesity and overweight are major causes of death worldwide—the fifth leading risk for global deaths, in fact. At least 2.8 million adults die each year as a result of being overweight or obese.
The World Health Organization predicts there will be 2.3 billion overweight adults in the world by 2015 and more than 700 million of them will be obese.
_
Epidemiology:
From least to most developed countries, overweight is on the rise:
_
Before the 20th century, obesity was rare; in 1997 the WHO formally recognized obesity as a global epidemic. As of 2008 the WHO estimates that at least 500 million adults (greater than 10%) are obese, with higher rates among women than men. The rate of obesity also increases with age at least up to 50 or 60 years old and severe obesity in the United States, Australia, and Canada is increasing faster than the overall rate of obesity. Once considered a problem only of high-income countries, obesity rates are rising worldwide and affecting both the developed and developing world. These increases have been felt most dramatically in urban settings. The only remaining region of the world where obesity is not common is sub-Saharan Africa.
_
The figure below shows prevalence of overweight people in developed world:
_
In recent years, the number of overweight people in industrialized countries has increased significantly, so much so that the World Health Organization (WHO) has called obesity an epidemic. In the United States, over 65% of the adult population is overweight. Approximately 60 million Americans, nearly one-third of all adults and about one in five children are obese. Researchers at Harvard predict that obesity rates will reach 42% over the next few years. By 2020, 75% of Americans will be overweight or obese. In Canada, about 40% to 60% of adults have a weight problem. Studies of obesity prevalence over time show an increase of 2.3–3.3-fold over about 25 years in the United States and 2.0–2.8-fold over 10 years in the United Kingdom. Current estimates in Australian children and young people indicate that 20%–25% are overweight or obese. People who are obese are at a much higher risk for serious medical conditions such as high blood pressure, heart attack, stroke, diabetes, gallbladder disease, and different cancers than people who have a healthy weight. Obesity is the condition in which the weight and accumulated fat of a person has reached a level that significantly affects their health, longevity, circulatory system, respiratory system, skeletal system and sleep in addition to other parts of their life and body.
_
The figure below shows growth of obesity from 1960 to 2010 in the U.S.
Recently Mexico has overtaken U.S. in obesity prevalence. Experts are putting forward all sorts of reasons why Mexico recently became more obese than the United States — and one of the most overweight countries in the world. Poverty, tacos, urbanization, soda. Those are the widely discussed culprits.
_
A few decades ago, the average weight gain was a half a pound per year and researchers at the time were alarmed. Today, the average adult gains one or two pounds each year, over a lifetime. The majority of people who are overweight see no need to lose weight. The IFIC Foundation Food and Health 2010 Survey found that only 23% of obese Americans think they are obese. 77% of obese Americans think they are merely overweight or not overweight at all. Although 70% of Americans are overweight or obese, a Consumer Reports survey found that only 11% think they are overweight. Also, obese people consistently under-report their food consumption as compared to people of normal weight. This is supported both by tests of people carried out in a calorimeter room and by direct observation.
___________
The fundamental drivers of the obesity epidemic:
Most policy makers do not yet understand that the obesity epidemic is a normal population response to the dramatic reduction in the demand for physical activity and the major changes in the food supply of countries over the last 40 years. A national focus on individual behavior reflects a failure to confront the facts. Thus, the changes in food supply and physical environment are socioeconomically driven, and the health sector simply picks up the consequences. Urbanization alone in China has reduced daily energy expenditure by about 300–400 kcal d−1 and cycling/bussing or going to work by car determines another variation of 200 kcal d−1. Thus, energy demands may have dropped with additional TV/media, mechanization and computerized changes by 400–800 kcal d−1, so weight gain and obesity are inevitable for most or all the population. Food intake should have fallen substantially despite the community’s focus on the value of food after all the food crises of the past. Yet, Chinese fat and sugar intakes are escalating due to primeval biological drive for those commodities with specialized taste buds for fatty acids, meat, sugar and salt. The net result is rise in obesity among Chinese population.
_
Obesity, gender and ethnicity:
There are striking differences in both gender and ethnicity when it comes to rates of obesity. Researchers at the University of North Carolina present a report from the National Longitudinal Survey of Youth, that sheds a light on obesity. By age 36, in this survey, around 26 per cent of US men and 28 per cent of women are already obese. For some unknown reason, black women become obese more than twice as fast as white women, with the rate for Hispanic women being about mid-way between these. Hispanic men become obese 2.5 times faster than men of European ancestry, but there was no difference in obesity rates between white (non-Hispanic) men and black men until age 28 – and then black men become obese 2.2 times faster than white men. More than 80 per cent of those who were obese did not become so until they were about 22, although they put on excess weight at an earlier age. Based on gender, ethnicity, and body mass index at aged 20 to 22, it was possible to predict who would become obese by age 36. The health effects of obesity – increased rates of heart disease and cancer, for example – do not start until many years after a person has become obese. This means there’s time to lose excess weight before health suffers. Knowing more about who is most at risk of obesity may give rise to more effective strategies for tackling the problem.
_
Can obesity be a kind of malnutrition?
Most people, when they hear the word “hunger,” form a mental picture of a skin-and-bones person living in Sudan or India. Perhaps a more precise term to use would be “malnutrition.” As defined by Merriam-Webster, malnutrition means “faulty nutrition due to inadequate or unbalanced intake of nutrients or their impaired assimilation or utilization.” By these criteria, a person can be well-fed calorically, yet suffer from malnutrition because of what he eats, rather than how much he eats. A common form of malnutrition, called “over-nutrition,” is often seen in countries where there is an abundance of fatty foods with plenty of sugar — like those found on a typical American fast food menu. Such over-nutrition frequently causes obesity.
_
Overweight and underweight:
Today we have 1.3 billion overweight people versus only 800 million underweight people. And overweight is increasing and underweight is decreasing, so the figures are splitting and the estimate of 1.3 billion is at the lowest level. There are people that estimate it as double over underweight already. In a book, Burlingame said that 925 million people suffer from hunger, while 1.5 billion people are considered overweight or obese. However, in both of these groups people suffer from micronutrient malnutrition including vitamin A, iron, or iodine deficiency. Overweight and obesity are linked to more deaths worldwide than underweight. 65% of the world’s population lives in a country where overweight and obesity kills more people than underweight. This includes all high-income and middle-income countries. Globally, 44% of diabetes, 23% of ischemic heart disease and 7–41% of certain cancers are attributable to overweight and obesity.
__________
Etymology of the word obesity:
Obesity is from the Latin obesitas, which means “stout, fat, or plump”. Ēsus is the past participle of edere (to eat), with ob (over) added to it. The Oxford English Dictionary documents its first usage in 1611 by Randle Cotgrave.
_
Historical trends:
The Greeks were the first to recognize obesity as a medical disorder. Hippocrates wrote that “Corpulence is not only a disease itself, but the harbinger of others”. The Indian surgeon Sushruta (6th century BCE) related obesity to diabetes and heart disorders. He recommended physical work to help cure it and its side effects. For most of human history mankind struggled with food scarcity. Obesity has thus historically been viewed as a sign of wealth and prosperity. It was common among high officials in Europe in the Middle Ages and the Renaissance as well as in Ancient East Asian civilizations. With the onset of the industrial revolution it was realized that the military and economic might of nations were dependent on both the body size and strength of their soldiers and workers. Increasing the average body mass index from what is now considered underweight to what is now the normal range played a significant role in the development of industrialized societies. Height and weight thus both increased through the 19th century in the developed world. During the 20th century, as populations reached their genetic potential for height, weight began increasing much more than height, resulting in obesity. In the 1950s increasing wealth in the developed world decreased child mortality, but as body weight increased heart and kidney disease became more common. During this time period insurance companies realized the connection between weight and life expectancy and increased premiums for the obese.
_
Obesity and culture:
Many cultures throughout history have viewed obesity as the result of a character flaw. The obesus or fat character in Greek comedy was a glutton and figure of mockery. During Christian times food was viewed as a gateway to the sins of sloth and lust. In modern Western culture, excess weight is often regarded as unattractive, and obesity is commonly associated with various negative stereotypes. People of all ages can face social stigmatization, and may be targeted by bullies or shunned by their peers. Obesity is once again a reason for discrimination.
_
Does culture cause obesity?
A study was conducted to ascertain the cultural perceptions of weight, particularly among women. Brown and Sweeney write: An important recent ethnography of Azawagh Arabs of Niger entitled Feeding Desire (Popenoe, 2004) illustrates these cultural notions to an extreme degree. Here, fatness to the point of voluptuous immobility is encouraged by systematic over-eating in order to hasten puberty, enhance sexuality, and ripen girls for marriage. The people believe that women’s bodies should be fleshy and laced with stretch-marks in order to contrast with thin, male bodies. Men, too, feel the need to gain weight in some cultures. The study cites names like “Notorious B.I.G., Heavy D and the Fat Boys” as examples of culturally accepted icons that are obese, promoting the idea that men need to be large to have power and respect. All of this leads up to the study’s conclusion, which states emphatically that health officials must understand and take into account cultural causes of obesity if they want to effectively address the obesity problem.
______________
Evolution of global obesity epidemic:
For centuries, the human race struggled to overcome food scarcity, disease, and a hostile environment. With the onset of the industrial revolution, the great powers understood that increasing the average body size of the population was an important social and political factor. The military and economic might of countries was critically dependent on the body size and strength of their young generations, from which soldiers and workers were drawn. Moving the body mass index (BMI) distribution of the population from the underweight range toward normality had an important impact on survival and productivity, playing a central role in the economic development of industrialized societies. Historical records from developed countries indicate that height and weight increased progressively, particularly during the 19th century.
_
Until the last decades of the 19th century, developed countries were still struggling with poverty, malnutrition, and communicable diseases. These health problems were considered a major cause of low industrial productivity. In the first decades of the 20th century, studies of poor children indicated that dietary energy supplementation (adding sugar and fat to the usual diet) improved growth, which became an important approach to reduce malnutrition and improve industrial productivity. An influential proponent of improving health and nutrition of the working class as a means to improve overall economic productivity was Boyd-Orr, who later became the founding director of the Food and Agriculture Organization. A major initial goal of this organization was to increase the availability of low-cost calorie sources, primarily edible fats and sugars. Over the following decades, these efforts indeed led to major increases in the availability of dietary energy. According to the Food and Agriculture Organization, global food production by 2002 reached about 2,600 kcal per capita and is projected to reach almost 3,000 kcal by 2030. Major contributors to total calories continue to be refined sugars and vegetable oils. While extreme disparities in access to adequate food availability continue to affect millions of people, there is no question that our ability to ensure stable production of dietary energy is one of the major achievements in human evolution.
_
During the 20th century, as populations from better-off countries began to approach their genetic potential for longitudinal growth, they began to gain proportionally more weight than height, with the resulting increase in average BMI. By the year 2000, the human race reached a sort of historical landmark, when for the first time in human evolution the number of adults with excess weight surpassed the number of those who were underweight. Excess adiposity/body weight is now widely recognized as one of today’s leading health threats in most countries around the world and as a major risk factor for type 2 diabetes, cardiovascular disease, and hypertension.
_
Until relatively recently, obesity was considered a condition associated with high socioeconomic status. Indeed, early in the 20th century, most populations in which obesity became a public health problem were in the developed world, primarily the United States and Europe. In more recent decades, available data show that the most dramatic increases in obesity are in developing countries such as Mexico, China, and Thailand. The global nature of the obesity epidemic was formally recognized by a World Health Organization consultation in 1997. Although few developing countries have nationally representative longitudinal data to assess trends, global estimates using both longitudinal and cross-sectional data indicate that obesity prevalence in countries in intermediate development has increased from 30 percent to 100 percent over the past decade.
_
The emergence of obesity in developing countries initially affected primarily the higher socioeconomic strata of the population. But more recent trends show a shift in prevalence from the higher to the lower socioeconomic level. For example, national surveys in Brazil found that while in 1989 obesity in adults was more prevalent in the higher socioeconomic status, 10 years later the higher prevalence was observed among the lower socioeconomic status. This change increasingly results in the existence of households with an undernourished child and an overweight adult, a situation called the “dual burden” of disease.
_
Of the multiple causal factors associated with the rise in obesity in developing countries, perhaps the two most important are urbanization and globalization of food production and marketing. Urban dwelling has a profound effect on energy balance, particularly on energy expenditure. On the energy output side, urban living is usually associated with lower energy demands compared with rural life. The energy-intense manual labor typical of rural areas may be replaced by a sedentary desk or sidewalk job. Long walks to work or to procure wood or water are replaced by mechanized transportation and public utilities. The global nature of modern commerce, sustained by the technical advances in food production and transportation, has permitted the introduction of low-cost, energy-dense foods in the domestic food market of many developing countries. Marketing campaigns and price incentives have an important impact on food purchasing patterns in developing countries, where as much as 60 percent of household income is spent on food. Consumption of energy-dense foods coupled with reduced energy expenditure facilitates weight gain in adults. In children, the low nutrient content of these foods may not be adequate to sustain normal growth because children require far more nutrients per calorie than adults do. As economic development brings some characteristics of urban lifestyle to rural communities, these populations also begin to show increasing rates of obesity, particularly among women.
_
The changing shape of childhood:
Francine Kaufman traced the differing environments of the child of 40,000 years ago through that experienced today. For the neolithic child, levels of physical activity were high. Approximately one-third of energy intake came from animal protein, typically low in associated fat because animals hunted for meat (as opposed to the high fructose feeds currently used for livestock which greatly increase the saturated fat content of these foods). Half of energy intake was from high-fiber fruits and vegetables, gathered close to home, and the remainder of the diet was high in polyunsaturated fats. Dietary sodium was low. With the advent of agriculture 5,000 years ago, the availability of grain led to greater fat depots in feed animals and began to alter the dietary balance to which humans had evolved. This pattern continued during the development of the civilization of the middle ages and the Renaissance, with the wealthy often exhibiting marked obesity and gluttony being recognized as one of the “seven deadly sins.” Although the lower prevalence of diabetes among European ancestry Caucasians may reflect a diminished need for “thrifty genes,” Kaufman mentioned a current speculation that diabetes did emerge as a health problem during this period in Europe. According to this hypothesis, the European diabetes gene pool decreased as the food supply increased, while other ethnic groups, particularly with the devastation of the subsequent centuries of European colonial rule, faced periods of extreme hunger for which there was survival advantage to the retention of “thrifty genes”.
_
Why we crave for sugar and fatty food?
Fast food isn’t the only factor in rising obesity rates. Anthropologists are now studying why we eat certain things, rather than just what we eat. In a recent issue of AnthroNotes, produced by Smithsonian’s National Museum of Natural History, anthropologists Peter J. Brown and Jennifer Sweeney use culture to explore the behaviors and beliefs in societies that influence weight. They start out by reviewing why humans crave sweet and fatty foods. Calorically dense foods were rare in the pre-agricultural world, where prey animals often carried little extra fat and natural sugars (like honey or ripe fruit). Because they are energy-intense foods, fat and sugar and other problem carbs trip the pleasure and reward meters placed in our brains by evolution over the millions of years during which starvation was an ever-present threat. We’re born enjoying the stimulating sensations these ingredients provide, and exposure strengthens the associations, ensuring that we come to crave them and, all too often, eat more of them than we should. We seem to be genetically predisposed to eat higher calorie foods to store energy. So craving for sugar, sweets and fatty foods is a biological circuit to store energy for use in future scarcity which luckily does not appear in most populations today and hence stored energy get accumulated to obese level.
_
How beverages raise weight evolutionarily?
If you think back for a million years up to 10 to 12 thousand years ago, all that we consumed as a race of hominins, and later Homo sapiens, is water, after maybe consuming for a year or two or three breast milk in infancy. We didn’t evolve to the point that those who consume water would consume less food, so we essentially evolved a system of metabolism where the beverages we consume don’t affect the food we consume. Then all of a sudden you get wine, beer and other alcoholic beverages which we had since around 10,000 B.C. and then in the last 150 to 200 years, all the new beverages—the carbonated beverages, the pasteurized milk and so forth, and the fruit juices that are shipped in boxes, then quick crated, and we see a new generation. But even up to 1950 we consumed very few calories from beverages and in the last 60 years, we’ve gone from consuming almost no calories from beverages to a fifth of our caloric intake in the U.S., and about the same in Mexico and about the same in a dozen other countries—in some less and some more—but the point is, all of those calories we consume doesn’t affect the food that we take in. So if you consume water, you don’t gain weight; if you consume Coke or Pepsi, you gain weight, it’s that simple.
_
Obesity Experiment on monkeys:
For the last 12 years, the Oregon National Primate Research Center at Oregon Health and Science University has been giving rhesus macaque monkeys diets made up of 35 percent fat (typical of the Western diet) to better understand diet-induced obesity in human adults. Shiva belongs to a colony of monkeys whose diets have been overloaded with fat and sugars by scientists studying the twin epidemics of obesity and diabetes. Like many American adults these days, Shiva sits around too much, eats rich, fatty food and drinks sugary sodas. His belly nearly touches the floor – when he is on all fours, that is. Researchers record the amount of food given to monkeys in the obesity study. Overweight monkeys resemble humans not only physiologically but also in their eating habits. The monkeys eat when they are bored, even when they are not really hungry. And unlike humans, who are notorious for fibbing about their daily calorie and carbohydrate intake, monkeys can be monitored closely. Another rhesus monkey, named Fat Albert, weighed nearly 70 pounds, three times normal weight. High-fat diets alone have not tended to make monkeys obese, researchers say, but a high fructose corn-sweetened punch seems to propel weight gain and the development of insulin resistance.
The figure above shows DEXA scans, which are used to measure body composition, show a rhesus macaque whose weight and body fat are normal, left, and an obese monkey on the right.
_
Obesity in pets:
Obesity in pets is common in many countries. Studies from various parts of the world have estimated that between 22 and 44 per cent of dogs are overweight or obese, and these figures are similar for cats. Rates of overweight in dogs in the United States range from 23 to 41% with about 5.1% obese. Rates of obesity in cats were slightly higher at 6.4%. In Australia the rate of obesity among dogs in a veterinary setting has been found to be 7.6%. The main reason that pets become obese is because the food that they eat contains more energy than they use up. This means that if pets eat too much or do not do enough exercise, they may become obese. When people eat, they feed their pets, who gain weight right along with their owners. And given the ingredients of many pet foods, you might as well let them chow down on fast food every day. The risk of obesity in dogs is related to whether or not their owners are obese; however, there is no similar correlation between cats and their owners. Substantial numbers of pet dogs are overweight but you will hardly find a stray dog overweight because stray dog has to work hard to search for food (exercise) and food is not found every day.
___________
Obesity definition:
_
Obesity vs. overweight:
Obesity means having too much body fat. It is not the same as being overweight, which means weighing too much. A person may be overweight from extra muscle, bone, or water, as well as from having too much fat. You can have normal weight but excessive fat (normal weight obesity) and you may be overweight and yet have normal fat.
_
Obesity is a condition caused by the excessive storage of body fat. A person whose weight is over 20 percent greater than what is considered normal for their gender, height, and age is considered obese. Obesity is a medical condition in which excess body fat has accumulated to the extent that it may have an adverse effect on health. The causes of obesity may include genetics, poor diet, lack of exercise, and different underlying illnesses and conditions. Obesity can lead to health problems like diabetes, high blood pressure, coronary artery disease, and stroke, and obesity is associated with increased incidence of certain cancers.
_
Obesity should be defined as excess body fat or adipose tissue; it is this, not weight which is associated with the comorbid conditions. This being the case, the next question is what level of fat should be defined as ‘obese’. Studies of children and adolescents which have examined the relationship between percentage of body fat calculated from skinfold measurements and indicators of biomedical status such as blood pressure and blood lipids, have suggested 30% fat in females and 20–25% in males. There is also evidence of ethnic differences, for example, South Asian people appear to be sensitive to the metabolic consequences of obesity at lower levels than white people. This is further complicated by findings that it is central (also described as intra abdominal or visceral) fat which is more pathogenic. Adults with large waist circumferences have excess morbidity, including back pain, diabetes and CVD risk factors, and although less clear, there is some evidence of health risks associated with excess abdominal fat in children. There is also evidence that the excess fat in obese children and adolescents is likely to accumulate in the abdominal regions. Overall levels, as well as the distribution of fat, differ according to both sex and ethnicity. The android (male, or ‘apple shaped’) fat pattern is represented by relatively greater amounts in the upper body, the gynoid (female, or ‘pear’) pattern by greater amounts in the hip and thigh areas. Female lower body fat is less metabolically active than that in the abdominal region, and is programmed to become mobilized during pregnancy and lactation. In relation to the greater pathogenicity of abdominal fat, it is interesting that mortality rates are higher among females with android fat patterning. Sex differences in fat levels have generally been considered to become manifest during puberty. Thus, in samples followed up through adolescence, levels of fat are higher among females, and of fat-free mass among males. However, more recent studies of pre-pubertal children, some as young as 3 years old, in the US, UK, Germany, Italy and China, have also found higher percentages of body fat and evidence of the gynoid pattern among females. Percentage body fat also appears to be lower in black, perhaps particularly black African children (and adults) compared with Caucasians. In other words, for any given body mass, black African children have higher fat-free and lower fat mass. Levels of abdominal fat also tend to be lower among Black Africans. There is, in addition, some evidence that these differences are more pronounced among females than males. In contrast, many Asian races, and possibly also Hispanics and Chinese, carry a higher percentage fat mass than Caucasians.
_
Overweight and obesity are defined as “abnormal or excessive fat accumulation that may impair health”. Body mass index (BMI) – the weight in kilograms divided by the square of the height in meters (kg/m2) – is a commonly used index to classify overweight and obesity in adults. WHO defines overweight as a BMI equal to or more than 25, and obesity as a BMI equal to or more than 30. BMI is closely related to both percentage body fat and total body fat. In children, a healthy weight varies with age and sex. Obesity in children and adolescents is defined not as an absolute number but in relation to a historical normal group, such that obesity is a BMI greater than the 95th percentile.[vide infra]
_
The figure below shows formula of body mass index (BMI):
_
_
_
As Asian populations develop negative health consequences at a lower BMI than Caucasians, some nations have redefined obesity; the Japanese have defined obesity as any BMI greater than 25 while China uses a BMI of greater than 28.
_
Key terms:
Bariatrics — The branch of medicine that deals with the prevention and treatment of obesity and related disorders.
Hyperplastic obesity — Excessive weight gain in childhood, characterized by the creation of new fat cells.
Hypertrophic obesity — Excessive weight gain in adulthood, characterized by expansion of already existing fat cells.
Ideal weight — Weight corresponding to the lowest death rate for individuals of a specific height, gender, and age.
_
Overweight:
Overweight is defined as a BMI of 25 or more, thus it includes pre-obesity defined as a BMI between 25 and 30 and obesity as defined by a BMI of 30 or more. Pre obese and overweight however are often used interchangeably thus giving overweight a common definition of a BMI of between 25 -30. As much as 64% of the United States adult population is considered either overweight or obese. While the negative health outcomes associated with obesity are accepted within the medical community, the health implications of the overweight category are more controversial. The generally accepted view is that being overweight causes similar health problems to obesity, but to a lesser degree. Adams et al. estimated that the risk of death increases by 20 to 40 percent among overweight people, and the Framingham heart study found that being overweight at age 40 reduced life expectancy by three years. A review in 2013 came to the result that being overweight significantly increases the risk of oligospermia and azoospermia in men. Flegal et al., however, found that the mortality rate for individuals who are classified as overweight (BMI 25 to 30) may actually be lower than for those with an “ideal” weight (BMI 18.5 to 25). [See obesity paradox vide infra]
_
Normal weight obesity:
Normal weight obesity means being fat despite normal weight and normal BMI. There are over 30 million Americans fall into this group. In the study of more than 6,000 Americans with normal body size and body mass index, the doctors showed that people don’t have to be overweight to have excess body fat. Their bodies are normal in weight, but they have too much hidden fat and not enough muscle. Other researchers have used MRI scans of the body to demonstrate that many normal-weight or even underweight individuals have excess and dangerous deposits of body fat, especially in and around their internal organs. A high body fat among normal-weight men and women was associated with an almost 400 per cent increase in the risk for what is called the metabolic syndrome – elevated blood sugar, blood pressure, blood cholesterol, and triglycerides in association with obesity.
_
Obesity as disease:
Obesity has been called many things — an epidemic, a major public health problem, a chronic disorder, a chronic condition — but is it a disease? The American Medical Association (AMA), the largest physician organization in the nation, has recently decided that it is. With the U.S. American Medical Association’s 2013 classification of obesity as chronic disease, it is thought that health insurance companies will more likely pay for obesity treatment, counseling and surgery, and the cost of research and development of fat treatment pills or gene therapy treatments should be more affordable if insurers help to subsidize their cost. The AMA classification is not legally binding, however, so health insurers still have the right to reject coverage for a treatment or procedure.
_
Criticism of obesity as a disease:
Negative side effect of the AMA decision is that the word “disease” is often misinterpreted as meaning that a person has no control over the condition. Critics say that obesity should not be called a “disease” because it is a result of lifestyle choices which people should be able to easily control. This reflects a poor understanding of human behavior. Behavior is not out of our control, but it is not always easy to control either. Our genetics and environment heavily influence our behavior. For example, a host of genetically-driven neurobiological variations influence appetite and even our experience of a food as palatable. This means that some people have intense appetites, some less so. Some people have an intense affinity for sweets and fats, others less so. Humans vary in these factors just as they vary in every other physical characteristic. In terms of environment, the increasing availability of super-palatable foods over the last 3-4 decades strongly impacts how much we eat, which is likely why rates of obesity are climbing since our genes haven’t changed in this timeframe. The food environment is more challenging to contend with for some than others, depending on the hand one is dealt in terms of those neurobiological variations in appetite. Further complicating matters are a host of psychological and physical factors that influence appetite including sleep deprivation, stress, and depression. The assumption that any lifestyle choice should be just as easy for one person as it is for another (i.e., “If I can maintain my weight, everyone should be able to!”) is egocentric because it assumes our brains and environments are identical which is not true for any two people on the planet. On the other hand, AMA’s declaration could help increase funding for future obesity research. Identifying obesity as a disease may also help in reducing the stigma often associated with being overweight.
_
If Obesity is a Disease, who’s going to treat it?
Physicians have:
a) Essentially no training in nutrition and exercise prescription for obesity,
b) Minimal training in the relationship between metabolic health and obesity,
c) Very little time to actually discuss lifestyle intervention in obesity, and
d) Little experience eating right and exercising themselves…
There is a new — and quickly growing — specialty in medicine focusing on obesity, the ABOM (American Board of Obesity Medicine). These physicians are more than equipped to treat obesity. But it needs to be a multidisciplinary approach.
_
Bias against obese people by medical professionals:
The latest study suggests that the one-third of Americans who are obese they may not be getting the proper health care they need — because their doctors are biased against treating them. The study, published in the Journal of Academic Medicine, shows that two out of five medical students have a subconscious bias against obese people. The bias may not be overt, but it can have serious implications for the patient’s health once they leave the doctor’s office. In another study published in the journal Preventive Medicine, researchers documented the close relationship between how doctors think about obesity and how they treat it. That study found that the majority of doctors believed obesity is caused by factors that can be controlled by the obese individual, and therefore preventable. Previous research found that doctors, like the general population, may assume that heavy-set patients won’t follow advice for healthy living as stringently as patients of normal weight. That means they may not be as likely to advise their patients to treat their obesity, or guide them toward the most effective weight management strategies.
_
A 1991 study showed that 80 percent of severely obese people:
- perceive themselves as physically unattractive
- believe that others make disparaging comments about their weight
- dislike being seen in public
- feel discrimination when applying for jobs
- feel that they are treated disrespectfully by their physician
_
Bias, Discrimination, and Obesity:
It has been said that obese persons are the last acceptable targets of discrimination. Anecdotes abound about overweight individuals being ridiculed by teachers, physicians, and complete strangers in public settings, such as supermarkets, restaurants, and shopping areas. Fat jokes and derogatory portrayals of obese people in popular media are common. Overweight people tell stories of receiving poor grades in school, being denied jobs and promotions, losing the opportunity to adopt children, and more. Some who have written on the topic insist that there is a strong and consistent pattern of discrimination, but no systematic review of the scientific evidence has been done.
_
An article reviews information on discriminatory attitudes and behaviors against obese individuals, integrates this to show whether systematic discrimination occurs and why, and discusses needed work in the field. Clear and consistent stigmatization, and in some cases discrimination, can be documented in three important areas of living: employment, education, and health care. Among the findings are that 28% of teachers in one study said that becoming obese is the worst thing that can happen to a person; 24% of nurses said that they are “repulsed” by obese persons; and, controlling for income and grades, parents provide less college support for their overweight than for their thin children. There are also suggestions but not yet documentation of discrimination occurring in adoption proceedings, jury selection, housing, and other areas. Given the vast numbers of people potentially affected, it is important to consider the research-related, educational, and social policy implications of these findings.
_
Most Americans unaware of Health Risks associated with obesity:
When asked whether the participants believed their weight might be considered normal, around half said yes. However, according to the Centers for Disease Control and Prevention, nearly two thirds of American adults are either overweight or obese. This means that a majority of people are probably not completely aware of their own physical health. Many Americans don’t quite understand the full long-term consequences of obesity, according to results from the The Associated Press-NORC Center for Public Affairs Research survey. A surprising number aren’t fully aware of how many chronic diseases and conditions are associated with being obese. Only 5% realize obesity raises cancer and respiratory disease risk.
_
Your idea of obesity is your undoing!
Scientists including an Indian researcher have suggested that whether a person believes that his or her obesity was caused by overeating or by lack of exercise predicts their actual body mass. Researchers Brent McFerran of the Ross School of Business at the University of Michigan and Anirban Mukhopadhyay of Hong Kong University of Science and Technology discovered from an initial survey that people seem to subscribe to one of two major beliefs about the primary cause of obesity. McFerran said that there was a clear demarcation, as some people overwhelmingly implicated poor diet, and a roughly equal number implicated lack of exercise. He said that genetics was placed a distant third. McFerran and Mukhopadhyay then conducted many studies across five countries on three continents. Data from participants in Korea, the United States, and France showed the same overall pattern: Not only did people tend to implicate diet or exercise as the leading cause of obesity, people who implicated diet as the primary cause of obesity actually had lower BMIs than those who implicated lack of exercise. The researchers hypothesized that the link between people’s beliefs and their BMI might have to do with how much they eat. A study with Canadian participants revealed that participants who linked obesity to lack of exercise ate significantly more chocolates than those who linked obesity to diet. And a study with participants in Hong Kong showed that participants who were primed to think about the importance of exercise ate more chocolate than those primed to contemplate diet. The new research has been published in Psychological Science.
__________
Measurement of body fat: degree of estimate of overweight/obesity:
_
Simple weighing:
The person’s weight is measured and compared to an estimated ideal weight. This is the easiest and most common method, but by far the least accurate, as it only measures one quantity (weight) and often does not take into account many factors such as height, body type, and relative amount of muscle mass.
_
Although body weight, particularly at very high levels, tends to be associated with adiposity, weight alone is an insufficient measure of obesity, because it is correlated with height. A number of measures of weight in relation to height have been devised. The simplest is weight for height. Two widely used methods are weight-for-height tables and body mass index (BMI). While both measurements have their limitations, they are reasonable indicators that someone may have a weight problem. One small problem with using weight-for-height tables is that doctors disagree over which is the best table to use. Several versions are available. Many have different weight ranges, and some tables account for a person’s frame size, age and sex, while other tables do not. A significant limitation of all weight-for-height tables is that they do not distinguish between excess fat and muscle. A very muscular person may be classified as obese, according to the tables, when he or she in fact is not. Body Mass Index (BMI), defined as weight (kg)/height squared (m2) is the most frequently used measure of weight in relation to height, but there are others.
_
Body mass index (BMI):
Overweight and obesity are defined by the World Health Organization using the body mass index (BMI). BMI is a measure of body size and is used to indicate level of risk for morbidity (disease risk) and mortality (death rates) at the population level. It is calculated by dividing your weight in kilograms by your height in meters squared. For example, a person who is 165 cm tall and weighs 64 kg would have a BMI of 24. People with a BMI of 25 or more are classified as overweight. People with a BMI of 30 or greater are classified as obese. The degree to which a person is overweight is generally described by body mass index (BMI). There are however several other common ways to measure the amount of adiposity or fat present in an individual’s body. BMI provides a significantly more accurate representation of body fat content than simply measuring a person’s weight. It is only moderately correlated with both body fat percentage and body fat mass (R2 of 0.68.) It does not take into account certain factors such as pregnancy or bodybuilding; however, the BMI is an accurate reflection of fat percentage in the majority of the adult population. BMI, however, does not account extremes of muscle mass, some rare genetic factors, the very young, and a few other individual variations. Thus it is possible for an individual with a BMI of less than 25 to have excess body fat, while others may have a BMI that is significantly higher without falling into this category. Some of methods described below for determining body fat are more accurate than BMI but come with added complexity.
_
The formula for BMI was devised by Belgian mathematician, Adolphe Quetelet, between 1830 and 1850. He warned that the calculation was only meant to be used for large diagnostic studies on general populations and was not accurate for individuals. The new term “body mass index” for the ratio and its popularity date to a paper published in the July edition of 1972 in the Journal of Chronic Diseases by Ancel Keys, which found the BMI to be the best proxy for body fat percentage among ratios of weight and height; the interest in measuring body fat being due to obesity becoming a discernible issue in prosperous Western societies. BMI was explicitly cited by Keys as being appropriate for population studies, and inappropriate for individual diagnosis. Nevertheless, due to its simplicity, it came to be widely used for individual diagnosis, despite its inappropriateness. One basic problem, especially in athletes, is that muscle weight contributes to BMI. Some professional athletes would be overweight or obese according to their BMI, despite carrying little fat, unless the number at which they are considered overweight or obese is adjusted upward in some modified version of the calculation. In children and the elderly, differences in bone density and, thus, in the proportion of bone to total weight can mean the number at which these people are considered underweight should be adjusted downward.
_
Pitfalls of BMI:
The medical establishment has acknowledged major shortcomings of BMI. Because the BMI formula depends only upon weight and height, its assumptions about the distribution between lean mass and adipose tissue are inexact. BMI doesn’t distinguish between body fat and muscle mass, which weighs more than fat. Many NFL players have been labeled “obese” because of their high BMI, when they actually have a low percentage of body fat. BMI generally overestimates adiposity on those with more lean body mass (e.g. athletes) and underestimates excess adiposity on those with less lean body mass. A study in June, 2008 by Romero-Corral et al. examined 13,601 subjects from the United States’ third National Health and Nutrition Examination Survey (NHANES III) and found that BMI-defined obesity (BMI > 30) was present in 21% of men and 31% of women. Using body fat percentages (BF %), however, BF%-defined obesity was found in 50% of men and 62% of women.
_
This graph shows the correlation between body mass index (BMI) and percent body fat (%BF) for 8550 men in NCHS’ NHANES 1994 data. Data in the upper left and lower right quadrants show some limitations of BMI
_
BMI is particularly inaccurate for people who are fit or athletic, as the higher muscle mass tends to put them in the overweight category by BMI, even though their body fat percentages frequently fall in the 10–15% category, which is below that of a more sedentary person of average build who has a normal BMI number. The BMI is not always accurate in elderly adults, who have often lost muscle and bone mass. Although their BMI might be within a normal range, they could still be overweight. BMI may also relate differently to various ethnic groups. For example, Asians may be at risk for health problems at a lower BMI than Caucasians. A further limitation of BMI relates to loss of height through aging. In this situation, BMI will increase without any corresponding increase in weight. BMI does not take into account bone density (bone mass). A person with severe osteoporosis (very low bone density) may have a lower BMI than somebody else of the same height who is healthy, but the person with osteoporosis will have a larger waist, more body fat and weak bones.
_
Body frame and BMI:
BMI also does not account for body frame size; a person may have a small frame and be carrying more fat than optimal, but their BMI reflects that they are normal. Conversely, a large framed individual may be quite healthy with a fairly low body fat percentage, but be classified as overweight by BMI. The standard is to use frame size in conjunction with ideal height/weight charts and add roughly 10% for a large frame or subtract roughly 10% for a smaller frame. For example, a chart may say the ideal weight for a man 5 ft 10 in (178 cm) is 165 pounds (75 kg). But if that man has a slender build (small frame), he may be overweight at 165 pounds (75 kg) and should reduce by 10%, to roughly 150 pounds (68 kg). In the reverse, the man with a larger frame and more solid build can be quite healthy at 180 pounds (82 kg).
_
Criteria and classification of obesity in Japan and Asia:
In 1997 when WHO initiated the formation of the International Obesity Task Force (IOTF), the Task Force proposed the cut-offs for overweight and obesity as BMI 25 and BMI 30, respectively. If we accept the criteria of BMI ≥ 30 to indicate obesity, it would appear that the prevalence of obesity in Japan of less than 3% has changed little during the last 40 years, and we cannot explain the rapid increase in incidence of obesity-associated chronic diseases such as diabetes, hypertension and hyperlipidemia. Thus, JASSO decided to define BMI ≥ 25 as obesity. This cut-off has been proposed for use in the Asia-Oceania Region, and WHO Western Pacific Region noted this proposal. According to this criterion the prevalence of obesity in Japan would average 20%, with a high of 30% in men over 30 years old, and women over 40 years old.
_
BMI-for-age [for children]:
BMI is used differently for children. It is calculated the same way as for adults, but then compared to typical values for other children of the same age. Instead of set thresholds for underweight and overweight, then, the BMI percentile allows comparison with children of the same sex and age. A BMI that is less than the 5th percentile is considered underweight and above the 95th percentile is considered obese for people 20 and under. People under 20 with a BMI between the 85th and 95th percentile are considered to be overweight.
_
| Weight Status Category | BMI-for-Age Percentile |
| Underweight | Less than the 5th percentile |
| Healthy weight | 5th percentile to less than the 85th percentile |
| Overweight | 85th to less than the 95th percentile |
| Obese | Equal to or greater than the 95th percentile |
_
Waist circumference:
Waist circumference is a measure of the distance around the abdomen. Waist circumference is one of the most practical tools to assess abdominal fat for chronic disease risk and during weight loss treatment. A high waist circumference or a greater level of abdominal fat is associated with an increased risk for type 2 diabetes, high cholesterol, high blood pressure and heart disease. Waist circumference may be a better indicator of health risk than BMI alone, especially when used in combination with BMI. Waist circumference is particularly useful for individuals with a BMI of 25-34. According to the United States Department of Health and Human Services (HHS) the following individuals are at increased risk for developing chronic diseases:
•Women with a waist circumference of more than 35 inches.
•Men with a waist circumference of more than 40 inches.
However, lower thresholds for waist circumference have been recommended for Asian populations by the World Health Organization due to recent research findings. Therefore, those at increased risk for developing chronic disease include:
•Asian women with a waist circumference of more than 31 inches.
•Asian men with a waist circumference of more than 35 inches.
_
Accurate measurement of waist circumference is achieved using the following technique:
- Locate the top of the hip bone (iliac crest) and take the measurement just above this bony landmark, just where one finger can fit between the iliac crest and the lowest rib.
- Ensure that the tape measure is positioned horizontally, parallel to the floor.
- Measuring at a level just above the iliac crest, and positioning the tape horizontally, irrespective of whether the umbilicus is above or below the tape, provides the correct waist circumference measurement and should correspond to the maximal abdominal diameter.
- Ensure that the patient is standing erect and has relaxed the abdominal muscles. Measurement is taken at the end of normal expiration.
- Aim to have a snug but not too tight a fit of the tape measure around your waist; do not make compressions in the skin with the tape measure.
- Accuracy can be improved by using a specially designed abdominal circumference tape measure. A constant-tension spring-loaded tape device reduces errors from over-enthusiastic tightening during measurement and improves accuracy and consistency of serial measurements.
It important to recognize when referring to waist circumference measurement, that this should not be considered to be the same as belt size in inches! Men in particular can have a relatively normal belt size, yet can have a significantly increased abdominal circumference above the belt-line. When measuring waist circumference it is important not to be tempted to measure around the narrower part of the abdomen situated below the umbilicus. Using the anatomical landmark of the iliac crest and ensuring that measurement is taken on a horizontal plane just above this level provides the most accurate, reliable and reproducible technique for waist circumference measurement.
_
_
Waist to height ratio ‘more accurate than BMI’:
Your waist should be no more than half the length of your height, according to experts who claim that having too large a trouser size can dramatically shorten your lifespan. Researchers from Oxford Brookes University examined data on patients whose BMI and waist to height ratio were measured in the 1980s. Twenty years later, death rates among the group were much more closely linked to participants’ earlier waist-to-height ratio than their BMI, suggesting it is a more useful tool for identifying health risks at an early stage. By comparing the life expectancies of various groups of people at different waist-to-height ratios, they were able to calculate how many years of life were lost as people’s waistlines increased. For example, a man aged 30 with a waist-to-height ratio of 0.8, representing the largest one in 500 men, stood to lose 16.7 years of life due to their size. A 50-year-old woman with the same ratio, accounting for about one in 150 women of the same age, would lose 8.2 years of life on average. By measuring waist-to-height ratio, it is thought that your doctor is getting a much earlier prediction that something is going wrong, and then the patient can be encouraged to do something about it. Self-assessment of height is usually pretty accurate and the measurement of waist circumference just requires a tape measure. Waist-height ratio can be used for men, women and children of all age groups and ethnicities.
_
Waist–hip ratio:
Waist–hip ratio or waist-to-hip ratio (WHR) is the ratio of the circumference of the waist to that of the hips. According to the World Health Organization’s data gathering protocol, the waist circumference should be measured at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest, using a stretch‐resistant tape that provides a constant 100 g tension. Hip circumference should be measured around the widest portion of the buttocks, with the tape parallel to the floor. Other organizations use slightly different standards. WHO states that abdominal obesity is defined as a waist–hip ratio above 0.90 for males and above 0.85 for females.
_
The table below shows summary of the association of BMI, waist circumference, waist-height ratio and waist-hip ratio with disease risk:
_
Skinfold calipers or “pinch test”:
The skin at several specific points on the body is pinched and the thickness of the resulting fold is measured. This measures the thickness of the layers of fat located under the skin, from which a general measurement of total amount of fat in the body is calculated. This method can be reasonably accurate for many people, but it does assume particular patterns for fat distribution over the body which may not apply to all individuals, and does not account for fat deposits which may not be directly under the skin. Also, as the measurement and analysis generally involves a high degree of practice and interpretation, for an accurate result it must be performed by a professional and cannot generally be done by patients themselves. The skinfold estimation methods are based on a skinfold test, also known as a pinch test, whereby a pinch of skin is precisely measured by calipers at several standardized points on the body to determine the subcutaneous fat layer thickness. These measurements are converted to an estimated body fat percentage by an equation. Some formulas require as few as three measurements, others as many as seven. The accuracy of these estimates is more dependent on a person’s unique body fat distribution than on the number of sites measured. As well, it is of utmost importance to test in a precise location with a fixed pressure. Although it may not give an accurate reading of real body fat percentage, it is a reliable measure of body composition change over a period of time, provided the test is carried out by the same person with the same technique.
_
Bioelectrical impedance analysis:
A small electrical current is passed through the body to measure its electrical resistance. As fat and muscle conduct electricity differently, this method can provide a direct measurement of the body fat percentage, in relation to muscle mass. In the past, this technique could only be performed reliably by trained professionals with specialized equipment, but it is now possible to buy home testing kits which allow people to do this themselves with a minimum of training. Despite the improved simplicity of this process over the years, however, there are a number of factors which can affect the results, including hydration and body temperature, so it still needs some care when taking the test to ensure that the results are accurate.
_
Hydrostatic weighing:
Considered one of the more accurate methods of measuring body fat, this technique involves complete submersion of a person in water, with special equipment to measure the person’s weight while submerged. This weight is then compared with “dry weight” as recorded outside the water to determine overall body density. As fat is less dense than muscle, careful application of this technique can provide a reasonably close estimate of fat content in the body. This technique does, however, require expensive specialized equipment and trained professionals to administer it properly.
_
Dual-energy X-ray absorptiometry (DEXA):
Originally developed to measure bone density, DEXA imaging has also come to be used as a precise way to determine body fat content by using the density of various body tissues to identify which portions of the body are fat. This test is generally considered to be very accurate, but requires a great deal of expensive medical equipment and trained professionals to perform.
_
Body volume index:
The body volume index (BVI) was devised in 2000 as a computer, rather than manual, measurement of the human body for obesity and an alternative to the BMI. Body volume index uses 3D software to create an accurate 3D image of a person so BVI can differentiate between people with the same BMI rating, but who have a different shape and different weight distribution. BVI measures where a person’s weight and the fat are located on the body, rather than total weight or total fat content and places emphasis on the weight carried around the abdomen, commonly known as central obesity. There has been an acceptance in recent years that abdominal fat and weight around the abdomen constitute a greater health risk
_
Ultrasound:
Ultrasound is used extensively to measure tissue structure and has proven to be an accurate technique to measure subcutaneous fat thickness. A-mode and B-mode ultrasound systems are now used and both rely on using tabulated values of tissue sound speed and automated signal analysis to determine fat thickness. By making thickness measurements at multiple sites on the body you can calculate the estimated body fat percentage. Ultrasound techniques can also be used to directly measure muscle thickness and quantify intramuscular fat. Ultrasound equipment is expensive, and not cost-effective solely for body fat measurement, but where equipment is available, as in hospitals, the extra cost for the capability to measure body fat is minimal. Ultrasonographic measuring of fat tissue is currently the favored technique by which one can measure both the subcutaneous and visceral fat tissues. Measurements are carried out using a 7.5- and 3.5-mHz transducer for the subcutaneous and visceral fat tissue, respectively.
_
MRI and CT:
The most accurate method for measuring central obesity is through the use of magnetic resonance imaging or computer-assisted tomographic scanning. Unfortunately, these approaches are too expensive for routine use.
_________
Table below describes the main techniques which have been adopted to measure fat in human subjects. They are categorized as density-based (hydrodensitometry; air displacement plethysmography), scanning (computerized tomography; magnetic resonance imaging; dual-energy x-ray absorptiometry), bioelectrical impedance and anthropometric (skinfold; waist circumference; waist-hip ratio) methods, according to the general principle on which they are based. More complex and detailed descriptions are available. As Table below demonstrates, the majority of these methods are complex and limited to research setting.
|
Table |
||||||
|
Methods to measure fat |
||||||
| General principle | Method | Acronym | Methodology | Method-specific principle | Further comments | |
| Density-based methods | If the density (weight per unit volume) of a human body is known, then the relative proportions of fat and fat-free mass can be estimated using an equation such as those of Siri or Lohman. While mass can be easily determined by weighing, volume measurements are more difficult. | Hydrodensitometry (underwater weighing) | UWW | Weighs the subject while submerged in a large tank (having exhaled maximally) and also outside the tank. | Based on Archimedes’ principle (buoyancy law) that if the density of an object exceeds that of water, it will sink. Given two people of equivalent weight outside the tank, the one with more fat, which is less dense than water, will weigh less in water than the one with more fat-free tissue (such as bone and muscle) which is more dense than water. (In fact, it is unnecessary to actually weight the subject underwater, since their volume can also be assessed via the amount of water displaced when they are submerged.) | Often described as ‘the gold standard’, but time-consuming and requires the subject to submerge themselves, so particularly unsuitable for certain populations, such as children, and limited to research settings. |
| Air Displacement Plethysmography | ADP | Measures the volume of air the subject displaces inside an enclosed chamber. | Given the subject’s volume and weight, their density can be calculated. | Early plethysmographs were complex, inconvenient and required temperature-controlled surroundings. A simple, quick automated plethysmograph has been available since mid 1990s, but is still limited to research settings. | ||
| Scanning methods | Can assess not just overall fat mass, but also its regional distribution. | Computerised Tomography; Magnetic Resonance Imaging | CT; MRI | CT – a series of x-rays pass through the body at different angles. MRI – uses a strong magnetic field and a radio wave antenna which sends signals to the body and then receives them back. These are used to produce internal images. | Both allow for the creation of cross-sectional high-resolution internal images. | Expensive, involve radiation exposure (CT) and limited to research settings. |
| Dual-Energy X-ray Absorptiometry | DEXA or DXA | A series of transverse scans, via low energy x-ray beams, progress inch-by-inch across the body and are collected by an external detector. | The beams are differentially absorbed by the various different tissues (fat, bone, etc) in the body. | Can be used to calculate fat and fat-free mass, and both total and regional body composition in subjects over a wide range of ages and body sizes. Relatively low radiation dose. Validated against UWW and comparison with animal carcasses in the pediatric weight range. Use limited to research settings. | ||
| Bioelectrical impedance methods | Electric currents pass more easily through body fluids in muscle and blood, but encounter resistance (‘bioelectrical impedance’) when they pass through fat, since it contains little water. | Bioelectrical Impedance Analysis | BIA | Conductors are attached to the subject’s body, and a low, safe, current is sent through. Electrodes are generally placed at wrist and ankle; an increasingly commonly used analyzer requires subjects to stand on it in bare feet and hold a handgrip in each hand. Foot-to-foot BIA measures the impedance of the lower body and only requires the subject to stand on pad electrodes. | The resistance between the conductors provides a measure of body fat. | Although less accurate than more sophisticated measurements, some current analyzers are relatively inexpensive, portable, simple and quick, meaning BIA can now be used in the field and with large samples. |
| Anthropometric methods | Direct measurements of various body parameters. | Skinfold measurements | SF | Subcutaneous (but not internal) fat is measured by firmly grasping a fold of skin with calipers and raising it, with no muscle included. Single site measurements, e.g. triceps skinfolds are simplest. An alternative is to add skinfolds from a variety of sites, generally representing both peripheral and trunk areas. | Subcutaneous fat may be taken as an indicator of total fat. Fat distribution can also be determined via the ratio of trunk to peripheral skinfolds. It is also possible to calculate total body fat via equations: Slaughter’s equations predict percent body fat from the sum of triceps plus subscapular, or triceps plus calf in children and young people; more recent equations by Dezenberg use triceps skinfolds plus body weight, sex and ethnicity. | Cheap and fairly simple, but the need to partially undress may put some subjects off, leading to bias. Also difficult to measure reproducibly, particularly if the subject is fat. |
| Waist circumference | WC | Ideally measured using a flexible plastic tape with a sprung handle to ensure reproducible levels of tension. Since a potential source of error is incorrectly positioning the tape, the measurement site is generally specified by reference to specific anatomic landmarks. | WC reflects total and abdominal fat levels, and as an indicator of adiposity is not greatly influenced by height. | WC percentiles for children have been developed in a number of countries. It has also recently been suggested that the ratio of waist to height could be used as a rapid screening tool. | ||
| Waist-hip ratio | WHR | A larger WHR in adults indicates relatively larger amounts of abdominal fat and has been used to describe body fat distribution. However it is influenced by several other bodily factors and there is some evidence that it is a poorer measure of body fat distribution in children. | Infrequently used in studies of children and adolescents. | |||
__________
Visceral fat:
_
Belly fat is made up of two kinds of fat:
1) Subcutaneous fat is below the surface of the skin and can be pinched with fingers, or calipers when measuring body fat.
2) Visceral belly fat is inside the abdominal wall, below the muscles and can’t be measured with calipers. Losing visceral fat will decrease your waist circumference and make you look much leaner around the middle, but it won’t get rid of fat at the umbilical or suprailiac—that’s subcutaneous fat.
• Visceral belly fat is considered a metabolically active “organ” because it releases substances called adipokines, which are cell-to-cell signaling proteins that increase blood pressure, raise LDL, and alter insulin sensitivity, causing diabetes.
• Adipokines released from visceral belly fat actually degrade muscle quality and turn it into fat!
• Diabetes and large amounts of visceral belly fat are generally interrelated health problems that are closely linked with development of cardiovascular disease.
_
Abdominal obesity:
Abdominal obesity, also known as belly fat or clinically as central obesity is excessive abdominal fat around the stomach and abdomen. It can also occur in both children and teenagers if either of their parents have abdominal obesity. There is a strong correlation between central obesity and cardiovascular disease. Visceral and central abdominal fat and waist circumference show a strong association with type 2 diabetes. The effect of abdominal adiposity does not just occur in those who are obese, but also affects people who are non-obese and it also contributes to insulin sensitivity. It is now generally believed that intra-abdominal fat is the depot that conveys the biggest health risk. Visceral fat, also known as organ fat or intra-abdominal fat is located inside the peritoneal cavity, packed in between internal organs and torso, as opposed to subcutaneous fat‚ which is found underneath the skin, and intramuscular fat‚ which is found interspersed in skeletal muscle. Visceral fat is composed of several adipose depots including mesenteric, epididymal white adipose tissue (EWAT) and perirenal fat. An excess of visceral fat is known as central obesity, the “pot belly” or “beer belly” effect, in which the abdomen protrudes excessively. Scientists have come to recognize that body fat, instead of body weight, is the key to evaluating obesity. Techniques such as computed tomography and magnetic resonance imaging made it possible to dissect mass of adipose tissue located at the abdominal level into intra-abdominal fat and subcutaneous fat. Research suggests that fat cells — particularly abdominal fat cells — are biologically active. It’s appropriate to think of fat as an endocrine organ or gland, producing hormones and other substances that can profoundly affect our health. Although scientists are still deciphering the roles of individual hormones, it’s becoming clear that excess body fat, especially abdominal fat, disrupts the normal balance and functioning of these hormones. Visceral fat cells will release their metabolic by-products in the portal circulation, where the blood leads straight to the liver. Thus, the excess of triglycerides and fatty acids created by the visceral fat cells will go into the liver and accumulate there. In the liver, most of it will be stored as fat. This concept is known as ‘lipotoxicity’. Hypertrophy of intra-abdominal adipose cells causes it to be in a hyperlipolytic state in which it is resistant to the antilipolytic effect of insulin. The resulting NEFA (non-esterified fatty acid) flux to the liver causes impairment of liver metabolism which leads to over production of glucose in the liver. Individuals with obesity are more likely to develop NEFA, which can weaken the metabolism of the liver causing high glucose production. Substances released by visceral fat, including free fatty acids, enter the portal vein and travel to the liver, where they can influence the production of blood lipids. Visceral fat is directly linked with higher total cholesterol and LDL (bad) cholesterol, lower HDL (good) cholesterol, and insulin resistance. An individual is at a higher risk of developing ischemic heart disease if they have hyperinsulinemia-dyslipidemia while being abdominal obese. Abdominal adipose tissue is a major source of increased inflammatory Interleukin-6 (IL-6) associated with aging. Induction of cellular senescence by visceral fat contributes to the inflammation. Scientists are also learning that visceral fat pumps out immune system chemicals called cytokines — for example, tumor necrosis factor and interleukin-6 — that can increase the risk of cardiovascular disease. These and other biochemicals are thought to have deleterious effects on cells’ sensitivity to insulin, blood pressure, and blood clotting. Developing asthma due to abdominal obesity is also a main concern.
_
Apple vs. pear shape:
The manner in which fat is distributed in the body is important, as it has been found that fat which is stored in and around internal organs (such as the liver, kidneys, pancreas and heart) tends to be significantly more harmful than fat which is stored peripherally, particularly in the form of subcutaneous (under the skin) fat. People who store fat around internal organs can have a familiar ‘apple’ shape, while those who store fat peripherally can have what is termed a ‘pear’ shape. ‘Apple’ shaped people store fat centrally (around the waist), while ‘pear’ shaped people store fat peripherally (around the hips and buttocks). These shapes become more obvious and pronounced in the overweight and obese categories of BMI. An apple shaped person of the same gender, age and ethnicity as a pear shaped person, and with the same BMI, will tend to have a much greater risk of developing cardiometabolic diseases such as diabetes, dyslipidaemia, hypertension, coronary heart disease and stroke. The reason for this is that internal, visceral fat is more metabolically active than subcutaneous fat, and this activity can have harmful effects such as induced insulin resistance, impaired vascular function and inflammation.
_
What makes visceral fat so dangerous?
The visceral fat cells literally function differently than subcutaneous fat cells. In comparison to visceral cells, subcutaneous cells are greater in number. But the visceral cells are actually larger in size per cell. And they get to be so big that they atrophy themselves—at which point they constantly, 24/7, produce cytokines. Cytokines are a hormone with known inflammatory properties. They promote atherosclerosis, tumor growth, aging, oxidation, hypertension, and cardiovascular disease. That’s quite a laundry list of bad things! Because visceral fat is stored around the liver, it impacts liver function as well. The liver processes fats and because these fats surround it, the liver has to keep processing them—and with them processing a lot of LDL, or bad cholesterol. That means more artherosclerosis—and hardened arteries. If you have this fat you can consider yourself always in a low-level inflammatory state. Visceral fat is known to cause inflammation in the colon and the artery walls, and is a major cause of heart disease, diabetes and some types of cancer. Research even suggests that visceral fat affects mood by increasing production of the stress hormone, cortisol, and reducing levels of feel-good endorphins. So, along with killing you, visceral fat, it seems, can make you feel low.
_
Scientific study on visceral fat:
David was one of 25 obese men and women involved in a pioneering study on this type of fat. They were scanned using ultra-sensitive 3D MRI before and after a three-month program, which involved exercise, a low-fat diet and an over-the- counter slimming pill. The aim was to pick up even the tiniest changes that might occur in visceral fat levels. What surprised researchers was that the rate that visceral fat is shed is significantly greater than for overall body weight. On average, the dieters lost 5.6 per cent of their body weight – but a massive 10.6 per cent of their visceral fat. Some achieved even more impressive results: George Eastcote, 52, shed about 13 per cent of his body weight (15kg) but nearly 34 per cent of his visceral fat – more than three liters in total. (Visceral fat is measured by volume rather than weight.) It’s his ‘before’ and ‘after’ scans that is seen below.
_
You can see that while the subcutaneous fat (shown in –yellow) – the stuff you can pinch – remains largely unchanged, there’s been a significant loss of visceral fat (shown in orange) around the gut, kidneys and liver and right down to the pelvis. The other astonishing finding was that it takes such a short time for visceral fat to be reduced by simple diet and lifestyle changes. This study is very good news for everyone who wants to lose weight for health reasons. It confirms what previous studies suggested: that visceral fat starts disappearing as soon as you go on a diet or start physical activity. Blood tests show that within half an hour of starting exercise, there are already metabolic changes to visceral fat – even though it takes longer for these changes to be picked up on an MRI scan. Why does visceral fat disappear so quickly? It is because it’s intended to be stored as energy. So when the body reduces calorie intake and increases calorie output, this fat begins to be digested. Over several weeks, it appears that any type of weight-loss regime will have a significant impact on visceral fat – apart from liposuction. And this can have a dramatic effect on your health.
_
Subcutaneous Fat:
Subcutaneous fat is found directly under the skin. It’s the fat that’s measured using skin-fold calipers to estimate your total body fat. In terms of overall health, subcutaneous fat in the thighs and buttocks, for instance, may not be as bad and may have some potential benefits. It may not cause as many problems as other types of fat, specifically the deeper, visceral fat. But subcutaneous fat cells on the belly may be another story. There’s emerging evidence that the danger of big bellies lies not only in the deep visceral fat but also the subcutaneous fat.
_
Subcutaneous fat and gender:
Subcutaneous Fat is the fat we find under the skin. Its location is very different in men and women.In women it accumulates very quickly and very easily in those areas known to be feminine: the hips, the “butt” and the breasts. This accumulation is guided by genes and hormones. In moderate amounts, it is very attractive to males. In very large quantities it can be considered disfiguring, although each culture has different values concerning what is attractive and what is not. Female sex hormones are believed to cause fat to be stored in the buttocks, thighs, and hips in women. This typical female fat storage may be essential for normal reproductive function. When women reach menopause and the estrogen produced by ovaries declines, fat migrates from their buttocks, hips‚ and thighs to their belly. In men, subcutaneous fat accumulates under the skin in the abdomen. This accumulation is also guided by genes and hormones. In males, any accumulation of subcutaneous fat is considered unattractive, since males are looked at as fighters or defenders of the tribe.
_
Some causes of central/abdominal obesity:
Some studies indicate that visceral adiposity, together with lipid dysregulation and decreased insulin sensitivity, is related to the excessive consumption of fructose. Other environmental factors, such as maternal smoking, estrogenic compounds in the diet‚ and endocrine-disrupting chemicals may be important also. It has also been shown that the quality protein intake in a 24-hour period and the times achieving the essential amino acid threshold of approximately 10 g is inversely related to percent central abdominal fat. Quality protein uptake is defined as the ratio of essential amino acids to daily dietary protein. A study has shown that alcohol consumption is directly associated with waist circumference and with a higher risk of abdominal obesity in men, but not in women, in the present population. Hypercortisolism, such as in Cushing’s syndrome, also leads to central obesity. Many prescription drugs, such as dexamethasone and other steroids, can also have side effects resulting in central obesity, especially in the presence of elevated insulin levels. Central obesity can be a feature of lipodystrophies, a group of diseases that is either inherited, or due to secondary causes (often protease inhibitors, a group of medications against AIDS). Central obesity is also common in patients with polycystic ovary syndrome (PCOS).
_
Waist measurement is more prone to errors than measuring height and weight. It is recommended to use both standards. BMI will illustrate the best estimate of your total body fatness, while waist measurement gives an estimate of visceral fat and risk of obesity-related disease. The absolute waist circumference [>102 centimeters (40 in) in men and >88 centimeters (35 in) in women] and the waist-hip ratio (>0.9 for men and >0.85 for women) are both used as measures of central obesity. Another measure of central obesity which has shown superiority to BMI in predicting cardiovascular disease risk is the Index of Central Obesity (waist-to-height ratio – WHtR), where a ratio of >=0.5 (i.e. a waist circumference at least half of the individual’s height) is predictive of increased risk. Body Volume Index (BVI) is based upon the principle that excess abdominal weight, measured by part volume as a percentage of total volume, constitutes a greater health risk. Recent validation has concluded that total and regional body volume estimates correlate positively and significantly with biomarkers of cardiovascular risk and BVI calculations correlate significantly with all biomarkers of cardio-vascular risk.
_
Note:
The term ‘abdominal obesity’, ‘central obesity’ and ‘visceral adiposity’ are used interchangeably and these terms denote excess of visceral fat in abdominal cavity and not subcutaneous fat over abdomen. A differential diagnosis of abdominal obesity includes ascites and intestinal bloating.
___________
What are the Signs and Symptoms of Overweight and Obesity?
Weight gain usually happens over time. Most people know when they’ve gained weight. Some of the signs of overweight and obesity include:
• Clothes feeling tight and needing a larger size.
• The scale showing that you’ve gained weight.
• Having extra fat around the waist.
• A higher than normal body mass index and waist circumference.
_
Obesity causes day-to-day problems such as:
- breathlessness
- increased sweating
- snoring or difficulty sleeping
- inability to cope with sudden physical activity
- feeling very tired every day
- back and joint pains.
_
Examination of the Obese Patient:
A comprehensive history relevant to the patient’s obesity should be obtained; this will include the onset of and previous treatment for obesity. Other important issues to consider include:
– Ethnicity,
– Family history,
– Dietary habits, eating pattern and possible presence of an eating disorder (binge eating, binge eating disorder, night eating syndrome, bulimia),
– Presence of depression and other mood disorders,
– Physical activity,
– Other determinants, e.g. genetic, drugs, endocrine abnormalities, psychosocial factors, chronic stress, smoking cessation, etc.,
– Health consequences of obesity,
– Patient’s expectations and motivation for change.
_
Physical Examination:
– Measure weight and height (from which BMI is calculated), waist circumference, blood pressure (appropriate size cuff).
– Assess the presence and impact of obesity-related diseases (diabetes, hypertension, dyslipidaemia, cardiovascular, respiratory, joint diseases, non-alcoholic fatty liver disease (NAFLD), sleep disorders etc.)
– Look for the presence of acanthosis nigricans as a sign of insulin resistance.
_
_
Laboratory Examinations:
The minimum dataset required will include:
– Fasting blood glucose,
– Serum lipid profile (total, HDL and LDL cholesterol, triglycerides),
– Uric acid,
– Thyroid function (TSH level),
– Liver function (hepatic enzymes).
Cardiovascular assessment, if indicated.
Endocrine evaluation if Cushing’s syndrome or hypothalamic disease suspected.
Liver investigation (ultrasound, biopsy) if abnormal liver function tests suggest NAFLD or other liver pathology.
_
The National Institutes of Health (NIH) recommends that doctors assess whether their patients are overweight based on three factors:
1. BMI
2. Waist circumference – a measurement of abdominal fat
3. Risk factors for diseases associated with obesity, such as high blood pressure, high LDL (“bad”) cholesterol, low HDL (“good”) cholesterol, high blood sugar, and smoking.
________________
Physiology and pathophysiology of energy metabolism vis-à-vis obesity:
_
Our bodies, physiology, and genes are much the same as they ever were. Certainly these have not changed much in the decades over which obesity went from rare to pandemic. What has changed is the environment. We are awash in highly-processed, hyper-palatable, glow-in-the-dark foods. We are afloat in constant currents of aggressive food marketing. We are deluged with ever more labor-saving technological advances, while opportunities for daily physical activity dry up. Obesity begins with the accumulation of body fat, and that in turn begins with the conversion of a surplus of daily calories into an energy reserve. That’s exactly what a healthy body is supposed to do with today’s surplus calories: store them against the advent of a rainy (i.e., hungry) day tomorrow. The problem that leads to obesity is that the surplus of calories extends to every day, and tomorrow never comes.
_
Basal metabolic rate (BMR), and the closely related resting metabolic rate (RMR), is the amount of energy expended daily by humans and other animals at rest. About 70% of a human’s total energy expenditure is due to the basal life processes within the organs of the body. About 20% of one’s energy expenditure comes from physical activity and another 10% from thermogenesis, or digestion of food (postprandial thermogenesis). All of these processes require an intake of oxygen along with coenzymes to provide energy for survival (usually from macronutrients like carbohydrates, fats, and proteins) and expel carbon dioxide, due to processing by the Krebs cycle. For the BMR, most of the energy is consumed in maintaining fluid levels in tissues through osmoregulation, and only about one-tenth is consumed for mechanical work, such as digestion, heartbeat, and breathing. The Krebs cycle is a key component of the metabolic pathway by which all aerobic organisms generate energy. Through catabolism of sugars, fats, and proteins, a two carbon organic product acetate in the form of acetyl-CoA is produced. Acetyl-CoA along with two equivalents of water (H2O) is consumed by the Krebs cycle producing two equivalents of carbon dioxide (CO2) and one equivalent of HS-CoA. In addition, one complete turn of the cycle converts three equivalents of nicotinamide adenine dinucleotide (NAD+) into three equivalents of reduced NAD+ (NADH), one equivalent of ubiquinone (Q) into one equivalent of reduced ubiquinone (QH2), and one equivalent each of guanosine diphosphate (GDP) and inorganic phosphate (Pi) into one equivalent of guanosine triphosphate (GTP). The NADH and QH2 generated by the Krebs cycle are in turn used by the oxidative phosphorylation pathway to generate energy-rich adenosine triphosphate (ATP). During vigorous exercise like athlete job, muscle cell have to do extra work and when body is unable to provide extra-oxygen, anaerobic metabolism occurs where glucose is not fully oxidized to carbon dioxide and water, rather it forms lactic acids. In this strategy, energy is still produced but in smaller amount (150 kJ) for each molecule of glucose. Thus the cells use anaerobic methods to generate ATP and keep the muscles functioning.
_
_
The number of calories your body uses to carry out the basic functions is known as your basal metabolic rate — what you might call metabolism. Several factors determine your individual basal metabolic rate:
•Your body size and composition. The bodies of people who are larger or have more muscle burn more calories, even at rest.
•Your sex. Men usually have less body fat and more muscle than do women of the same age and weight, burning more calories.
•Your age. As you get older, the amount of muscle tends to decrease and fat accounts for more of your weight, slowing down calorie burning.
_
Energy needs for your body’s basic functions stay fairly consistent and aren’t easily changed. Your basal metabolic rate accounts for about 60 to 75 percent of the calories you burn every day.
In addition to your basal metabolic rate, two other factors determine how many calories your body burns each day:
•Food processing (thermogenesis). Digesting, absorbing, transporting and storing the food you consume also takes calories. This accounts for about 10 percent of the calories used each day. For the most part, your body’s energy requirement to process food stays relatively steady and isn’t easily changed.
•Physical activity. Physical activity and exercise — such as playing tennis, walking to the store, chasing after the dog and any other movement — account for the rest of the calories your body burns up each day. Physical activity is by far the most variable of the factors that determine how many calories you burn each day.
_
The figure below shows fine balance between energy consumed through food and energy used in basal metabolism plus food digestion plus physical activities:
_
Exercise and ATP:
When you exercise or compete in sports, you notice several things about your body. You breathe heavier and faster, your heart beats faster, your muscles hurt and you sweat. The body has an incredibly complex set of processes to meet the demands of working muscles. For your muscles — in fact, for every cell in your body — the source of energy that keeps everything going is called ATP. Adenosine triphosphate (ATP) is the biochemical way to store and use energy.
•Chemically, ATP is an adenine nucleotide bound to three phosphates.
•There is a lot of energy stored in the bond between the second and third phosphate groups that can be used to fuel chemical reactions.
•When a cell needs energy, it breaks this bond to form adenosine diphosphate (ADP) and a free phosphate molecule.
•In some instances, the second phosphate group can also be broken to form adenosine monophosphate (AMP).
•When the cell has excess energy, it stores this energy by forming ATP from ADP and phosphate.
ATP is required for the biochemical reactions involved in any muscle contraction. As the work of the muscle increases, more and more ATP gets consumed and must be replaced in order for the muscle to keep moving. Because ATP is so important, the body has several different systems to create ATP. These systems work together in phases. The interesting thing is that different forms of exercise use different systems, so a sprinter is getting ATP in a completely different way from a marathon runner!
ATP comes from three different biochemical systems in the muscle, in this order:
- phosphagen system
- glycogen-lactic acid system
- aerobic respiration
_
1. A muscle cell has some amount of ATP floating around that it can use immediately, but not very much — only enough to last for about three seconds. To replenish the ATP levels quickly, muscle cells contain a high-energy phosphate compound called creatine phosphate. The phosphate group is removed from creatine phosphate by an enzyme called creatine kinase, and is transferred to ADP to form ATP. The cell turns ATP into ADP, and the phosphagen rapidly turns the ADP back into ATP. As the muscle continues to work, the creatine phosphate levels begin to decrease. Together, the ATP levels and creatine phosphate levels are called the phosphagen system. The phosphagen system can supply the energy needs of working muscle at a high rate, but only for 8 to 10 seconds.
2. Muscles also have big reserves of a complex carbohydrate called glycogen. Glycogen is a chain of glucose molecules. A cell splits glycogen into glucose. Then the cell uses anaerobic metabolism (anaerobic means “without oxygen”) to make ATP and a byproduct called lactic acid from the glucose. About 12 chemical reactions take place to make ATP under this process, so it supplies ATP at a slower rate than the phosphagen system. The system can still act rapidly and produce enough ATP to last about 90 seconds. This system does not need oxygen, which is handy because it takes the heart and lungs some time to get their act together. It is also handy because the rapidly contracting muscle squeezes off its own blood vessels, depriving itself of oxygen-rich blood. There is a definite limit to anaerobic respiration because of the lactic acid. The acid is what makes your muscles hurt. Lactic acid builds up in the muscle tissue and causes the fatigue and soreness you feel in your exercising muscles.
3. By two minutes of exercise, the body responds to supply working muscles with oxygen. When oxygen is present, glucose can be completely broken down into carbon dioxide and water in a process called aerobic respiration. The glucose can come from three different places:
•remaining glycogen supplies in the muscles
•breakdown of the liver’s glycogen into glucose, which gets to working muscle through the bloodstream
•absorption of glucose from food in the intestine, which gets to working muscle through the bloodstream
[Remember, muscle glycogen cannot supply glucose to rest of body like liver glycogen because muscle lacks the enzyme glucose-6-phosphatase. The conversion of glucose-6-phosphate to glucose, which occurs in the liver, kidney and intestine, by the action of glucose-6-phosphatase does not occur in skeletal muscle as these cells lack this enzyme. Therefore, any glucose released from glycogen stores of muscle will be oxidized in the glycolytic pathway. In the liver the action of glucose-6-phosphatase allows glycogenolysis to generate free glucose for maintaining blood glucose levels.]
_
Aerobic respiration can also use fatty acids from fat reserves in muscle and the body to produce ATP. In extreme cases (like starvation), proteins can also be broken down into amino acids and used to make ATP. Aerobic respiration would use carbohydrates first, then fats and finally proteins, if necessary. Aerobic respiration takes even more chemical reactions to produce ATP than either of the above systems. Aerobic respiration produces ATP at the slowest rate of the three systems, but it can continue to supply ATP for several hours or longer, so long as the fuel supply lasts. Aerobic respiration is breakdown of acetyl-CoA derived from glucose, fatty acid or amino acid in Krebs cycle in mitochondria to generate ATP.[vide supra]
_
Energy burning while energy intake (fed-state calories burning) would include calories burned for food digestion & assimilation plus basal metabolism.
Energy burning while physical activity (non-fed state calories burning) would include calories burned for physical activities (exercise) plus basal metabolism.
Rarely humans are doing physical activity (exercise) and eating simultaneously.
_
There is a difference between how food calories (fat or carb) are burned in fed state or non-fed state. Let me discuss non-fed state. Non-fed state burning calories means physical activities. Once ingested, the nutrients, carbohydrates and fats become essential components of providing energy in the body. Carbs provide 4 calories per gram for energy, while fats provide 9. The utilization of carbs or fat as the primary fuel source is largely dependent upon the activity level of an individual. How much of either source is being used at any given time is relative and can be influenced by age, gender, body composition or fitness level.
Intensity and Duration:
The intensity and duration of physical activity are the significant factors influencing carbohydrate or fat oxidation. Oxidation refers to the breakdown of the nutrients into smaller components to be used for energy.
Low Activity Burning:
Although carbs are always being burned for energy, during rest or stages of low activity, fat is the primary fuel source. The volume of fat burning during rest is heavily dependent upon lean body mass. For instance, the more lean muscle you have, the higher your rate of metabolism; thus, the more calories by of fat the body burns.
High Activity Burning:
As physical activity increases, as with exercise, the body becomes more dependent on carbs for energy in the form of blood glucose or muscle glycogen. Glycogen is the carb burned initially during lower intensities. As intensity increases, carbs in the form of blood glucose becomes the primary fuel source.
Oxygen Availability:
Fatty oxidation can only occur in the presence of oxygen. Carbs, on the other hand, can be broken down without oxygen present. This is why as activity levels increase there is a conversion from fat being burned primarily towards carb burning.
Lactate Threshold:
The lactate threshold is the point where the body can no longer supply adequate oxygen to working muscles, thus reducing the contribution of fat as a primary fuel source and increasing the contribution of carbohydrates. This high level of intensity is known as being anaerobic (without oxygen) as opposed to the preceding level, which is considered aerobic (with oxygen). Remember, there is anaerobic respiration in muscles during first 90 seconds of exercise when oxygen is not available to muscles. Then with oxygen supply aerobic respiration occurs in muscles and after high level of exercise when oxygen demand cannot be met, again anaerobic respiration occurs.
_
Now let me discuss calories burning in fed state; meaning when person eats food, the same food provides energy for its digestion as well as basal metabolism before body returns to its energy store (fat + glycogen). Normally people are at rest while feeding and therefore little energy is spent on physical activities. Remember, aerobic respiration in fed-state would use carbohydrates first, then fats and finally proteins, if necessary.
So how this logic can be used in obesity discussion?
It’s important to understand that eating fat will not make you fat. Many people are still convinced that a strict low-fat diet is the best way to lose weight, but many studies have shown that low-carb diets are more effective for weight loss, and here’s why: Your cells need fuel to function, and they can get their fuel in the form of sugar or fat. However, your body must burn all of the available sugar first before it turns to burning fat. So let’s say you eat a big plate of pasta (which turns into sugar in your body) along with a small amount of olive oil and meatballs (fat and protein). Your body must first burn off all of that pasta, and whatever can’t be burned off will eventually be stored as fat. The fat you just ate, meanwhile, also goes into your fat stores. The more carbs and sugar you eat, the more your cells become accustomed to burning sugar as their fuel. After awhile, they will begin to crave it and prefer it to fat. People get fat not so much because they eat fat, but because their bodies have forgotten how to burn it due to high carbohydrate intake (high sugar and high starch intake), and because of poor hormonal communication.
_
When is protein burned for energy?
Protein does not get burned for energy; it gets broken down into amino acids. These amino acids get used on an as-needed basis, and the amino acids that aren’t used either get excreted or converted to glycogen. Different amino acids get converted at different rates, but all amino acids convert to glycogen in the end, providing four kilocalories of energy per gram, which is the same as carbohydrates. The body rarely burns protein as its sole fuel source, and when it does it is usually under conditions of starvation. Interestingly, when no carbohydrate is present in the diet, the body will use the amino acid backbones of protein to form glucose (a carbohydrate) in order to supply the brain with adequate energy. Remember, body prefers to burn carbohydrates, then fat, and finally protein if all else fails.
_
Food energy:
Food energy is energy that animals (including human beings) derive from their food, through the process of cellular respiration, the process of joining oxygen with the molecules of food (aerobic respiration) or of reorganizing the atoms within the molecules for anaerobic respiration. Animals need a minimum intake of food energy in order to sustain their metabolism and drive their muscles. For humans, food energy typically comes from joining oxygen with carbohydrates, fats, proteins, organic acids, polyols, and ethanol present in the diet. Some diet components that provide little or no food energy, such as water, minerals, vitamins and fiber, may still be necessary to health and survival for other reasons. In the International System of Units, energy is measured in joules (J) or its multiples; the kilojoule (kJ) is most often used for food-related quantities. An older metric system unit of energy, still widely used in food-related contexts, is the calorie; more precisely, the “food calorie”, “large calorie” or kilocalorie (kcal or Cal), equal to 4.184 kilojoules. (It should not be confused with the “small calorie” (cal) that is often used in chemistry and physics, equal to 1/1000 of a food calorie.) Fats and ethanol have the greatest amount of food energy per mass, 38 and 30 kJ/g (9 and 7 kcal/g), respectively. Proteins and most carbohydrates have about 17 kJ/g (4 kcal/g). Carbohydrates that are not easily absorbed, such as fiber or lactose in lactose-intolerant individuals, contribute less food energy. Polyols (including sugar alcohols) and organic acids have less than 17 kJ/g (4 kcal/g).
_
Recommended daily intake of food energy:
Recommendations in the United States are 2,700 and 2,100 kcal (11,000 and 8,800 kJ) for men and women (respectively) between 31 and 50, at a physical activity level equivalent to walking about 1.5 to 3 miles per day at 3 to 4 miles per hour on top of the light physical activity associated with typical day-to-day life, with French guidance suggesting roughly the same levels. Children, those with sedentary lifestyles, and older people require less energy; physically active people more. According to the Food and Agriculture Organization of the United Nations, the average minimum energy requirement per person per day is about 1,800 kcal (7,500 kJ).
_
|
Energy Stores in Man |
||||
|
Tissue Fuel |
Provides fuel for |
|||
|
Reserve, grams |
Starvation |
Walking |
Marathon |
|
|
Fat |
9000-15000 |
34 days |
11 days |
3 days |
|
Muscle Glycogen |
350 |
14 hours |
5 hours |
70 minutes |
|
Liver Glycogen |
80 |
3.5 hours |
70 minutes |
18 minutes |
|
Blood/Extracellular Glucose |
20 |
40 minutes |
15 minutes |
4 minutes |
|
Body Protein |
6000 |
15 days |
5 days |
1.3 days |
_
Fats and Energy Storage:
Triglycerides, stored in adipose tissue, are a major form of energy storage both in animals and plants. Migratory birds that must fly long distances without eating use stored energy of triglycerides to fuel their flights. Fats are the primary energy storage form in animals and in plant seeds because energy can be stored more densely in fats than in carbohydrates. That is, metabolic oxidation of fats yields 37 kJ/g (9 kcal/g) whereas carbohydrates and proteins yield only17 kJ/g (4 kcal/g).
A typical 70-kg human may have the following fuel reserves:
400,000 kJ in total human body fat energy
100,000 kJ in total human protein energy (remember proteins are burned last when both sugar and fat are unavailable for energy)
2500 kJ in total human glycogen energy
170 kJ in total human glucose energy
Brain cannot use fats for energy; instead, brain has a specific requirement for glucose. Under conditions of starvation, however, when blood glucose levels decrease, brain can adjust to use ketone bodies, which can be derived from fatty acids.
_
Energy usage in human body:
The human body uses the energy released by respiration for a wide range of purposes: about 20% of the energy is used for brain metabolism, and much of the rest is used for the basal metabolic requirements of other organs and tissues. In cold environments, metabolism may increase simply to produce heat to maintain body temperature. Among the diverse uses for energy, one is the production of mechanical energy by skeletal muscle to maintain posture and produce motion. The conversion efficiency of energy from respiration into mechanical (physical) power depends on the type of food and on the type of physical energy usage (e.g. which muscles are used, whether the muscle is used aerobically or anaerobically). In general, the efficiency of muscles is rather low: only 18 to 26% of the energy available from respiration is converted into mechanical energy. This low efficiency is the result of about 40% efficiency of generating ATP from the respiration of food, losses in converting energy from ATP into mechanical work inside the muscle, and mechanical losses inside the body. The latter two losses are dependent on the type of exercise and the type of muscle fibers being used (fast-twitch or slow-twitch). However, alterations in the structure of the material consumed can cause modifications in the amount of energy that can be derived from the food; i.e. caloric value depends on the surface area and volume of a food. For an overall efficiency of 20%, one watt of mechanical power is equivalent to 4.3 kcal (18 kJ) per hour. For example, a manufacturer of rowing equipment shows calories released from ‘burning’ food as four times the actual mechanical work, plus 300 kcal (1,300 kJ) per hour, which amounts to about 20% efficiency at 250 watts of mechanical output. It can take up to 20 hours of little physical output (e.g. walking) to “burn off” 4,000 kcal (17,000 kJ) more than a body would otherwise consume. For reference, each pound of body fat equates to approximately 3,500 calories. The differing energy density of foods (fat, alcohols, carbohydrates and proteins) lies mainly in their varying proportions of carbon, hydrogen, and oxygen atoms. This then determines the volume of oxygen needed for (aerobic) respiration, approximately 300 kj per mole of molecular oxygen that reacts. In addition, the quality of calories matters because the energy absorption rate of different food with equal amount of calories in human body may vary. It is because some nutrients also have an regulatory effect by signaling in addition to providing energy for the human body. For example, leucine plays an important role in the regulation of protein metabolism and suppresses an individual’s appetite. Saturated fats promote fat synthesis in human body, while PUFAs (Poly Unsaturated Fatty Acids) inhibit fat synthesis and promote fat oxidation doing the opposite. Swings in body temperature – either hotter or cooler – increase the metabolic rate, thus burning more energy. Prolonged exposure to extremely warm or very cold environments increases the basal metabolic rate (BMR). People who live in these types of settings often have BMRs 5–20% higher than those in other climates. Physical activity also significantly increases body temperature, which in turn uses more energy from respiration.
_____________
Carbohydrates, proteins and fats form the three main macronutrients that are essential in every balanced diet. While proteins and fats may be responsible for bodily functions such as the creation of body tissues and insulation, carbohydrates provide calories that are necessary for the production of energy. In fact, carbs provide more than 60 percent of the amount of energy required by the body. The energy is mostly used for normal body functions such as heartbeat, digestion, breathing and body movement. The basic building block of every carbohydrate is a sugar molecule, a simple union of carbon, hydrogen, and oxygen. Simple carbohydrates have one (single) or two (double) sugars. Complex carbohydrates have three or more sugars. The primary function of carbohydrates is to provide energy for the body, especially the brain and the nervous system. An enzyme called amylase helps break down carbohydrates into glucose (blood sugar), which is used for energy by the body. All carbohydrates form glucose when digested. Glucose is transported around the body via blood and taken into cells to be converted into energy. The excess glucose is stored as glycogen in liver & muscles, and fat in adipose tissue.
_
Insulin resistance:
Insulin is a peptide hormone, produced by beta cells of the pancreas, and is central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, skeletal muscles, and fat tissue to absorb glucose from the blood. In the liver and skeletal muscles, glucose is stored as glycogen, and in fat cells (adipocytes) it is stored as triglycerides. Insulin is produced in the pancreas and released when any of several stimuli are detected. These stimuli include ingested protein and glucose in the blood produced from digested food. Insulin resistance (IR) is a physiological condition in which cells fail to respond to the normal actions of the hormone insulin. Insulin resistance in muscle and fat cells reduces glucose uptake (and also local storage of glucose as glycogen and triglycerides, respectively), whereas insulin resistance in liver cells results in reduced glycogen synthesis and storage and a failure to suppress glucose production and release into the blood. Insulin resistance normally refers to reduced glucose-lowering effects of insulin. However, other functions of insulin can also be affected. For example, insulin resistance in fat cells reduces the normal effects of insulin on lipids and results in reduced uptake of circulating lipids and increased hydrolysis of stored triglycerides. Increased mobilization of stored lipids in these cells elevates free fatty acids in the blood plasma. Elevated blood fatty-acid concentrations (associated with insulin resistance and diabetes mellitus Type 2), reduced muscle glucose uptake, and increased liver glucose production all contribute to elevated blood glucose levels. High plasma levels of insulin and glucose due to insulin resistance are a major component of the metabolic syndrome. If insulin resistance exists, more insulin needs to be secreted by the pancreas. If this compensatory increase does not occur, blood glucose concentrations increase and type 2 diabetes occurs. Insulin resistant cells cannot take in glucose, amino acids and fatty acids. Thus, glucose, fatty acids and amino acids ‘leak’ out of the cells.
Can unhealthy life style (junk food and physical inactivity) cause insulin resistance?
Some people may have a genetic predisposition to insulin resistance, while others develop the condition through high stress and unhealthy lifestyles. Unhealthy lifestyles and genetic conditions cause the pancreas to overproduce insulin. The cell, in turn, is overwhelmed by this surplus insulin and protects itself by reducing the number of its insulin receptor sites. This process leaves too few sites for insulin to carry out its normal function, which is to attach itself to the cell wall and act as a “key in a lock” to allow glucose to pass through the cell wall and be converted into energy. The vastly reduced number of receptor sites in insulin resistant people causes an excess of insulin “rejected” by the cell to float freely in the blood stream and thereby elevate plasma insulin level.
Can insulin resistance cause obesity?
Insulin resistance greatly reduces the sensitivity of your cell walls to insulin. So the vital process whereby glucose passes through the cell wall via insulin to be converted into energy is greatly impaired. As a result, excess glucose remains in the blood stream, causing elevated levels of blood sugar, which are sent to the liver. Once there, the sugar is converted into fat and carried via the blood stream throughout the body. This process can lead to weight gain and obesity.
_
How Fat enters your body:
When you eat food that contains fat, mostly triglycerides, it goes through your stomach and intestines. In the intestines, the following happens:
1. Large fat droplets get mixed with bile salts from the gall bladder in a process called emulsification. The mixture breaks up the large droplets into several smaller droplets called micelles, increasing the fat’s surface area.
2. The pancreas secretes enzymes called lipases that attack the surface of each micelle and break the fats down into their parts, glycerol and fatty acids.
3. These parts get absorbed into the cells lining the intestine.
4. In the intestinal cell, the parts are reassembled into packages of fat molecules (triglycerides) with a protein coating called chylomicrons. The protein coating makes the fat dissolve more easily in water.
5. The chylomicrons are released into the lymphatic system — they do not go directly into the bloodstream because they are too big to pass through the wall of the capillary.
6. The lymphatic system eventually merges with the veins, at which point the chylomicrons pass into the bloodstream.
You might be wondering why fat molecules get broken down into glycerol and fatty acids if they’re just going to be rebuilt. This is because fat molecules are too big to easily cross cell membranes. So when passing from the intestine through the intestinal cells into the lymph, or when crossing any cell barrier, the fats must be broken down. But, when fats are being transported in the lymph or blood, it is better to have a few, large fat molecules than many smaller fatty acids, because the larger fats do not “attract” as many excess water molecules by osmosis as many smaller molecules would.
_
Fat Storage:
Chylomicrons do not last long in the bloodstream — only about eight minutes — because enzymes called lipoprotein lipases break the fats into fatty acids. Lipoprotein lipases are found in the walls of blood vessels in fat tissue, muscle tissue and heart muscle.
Insulin:
When you eat a candy bar or a meal, the presence of glucose and amino acids in the intestine stimulates the pancreas to secrete a hormone called insulin. Insulin acts on many cells in your body, especially those in the liver, muscle and fat tissue. Insulin tells the cells to do the following:
•Absorb glucose, fatty acids and amino acids
•Stop breaking down glucose, fatty acids and amino acids; glycogen into glucose; fats into fatty acids and glycerol; and proteins into amino acids
•Start building glycogen from glucose; fats (triglycerides) from glycerol and fatty acids; and proteins from amino acids
The activity of lipoprotein lipases depends upon the levels of insulin in the body. If insulin is high, then the lipases are highly active; if insulin is low, the lipases are inactive. The fatty acids are then absorbed from the blood into fat cells, muscle cells and liver cells. In these cells, under stimulation by insulin, fatty acids are made into fat molecules and stored as fat droplets. It is also possible for fat cells to take up glucose and amino acids, which have been absorbed into the bloodstream after a meal, and convert those into fat molecules. The conversion of carbohydrates or protein into fat is 10 times less efficient than simply storing fat in a fat cell, but the body can do it. If you have 100 extra calories in fat (about 11 grams) floating in your bloodstream, fat cells can store it using only 2.5 calories of energy. On the other hand, if you have 100 extra calories in glucose (about 25 grams) floating in your bloodstream, it takes 23 calories of energy to convert the glucose into fat and then store it. Given a choice, a fat cell will grab the fat and store it rather than the carbohydrates because fat is so much easier to store.
_
Fat plus carb (sugar and starch) is worst combination for fat gain than eating each alone:
We know that insulin promote fat storage but there are three other hormones which do the same. One of them is called acylation stimulating protein (ASP). Where insulin is induced by carbs, ASP is induced by fat (this is not good news for those who say you can’t get fat eating fat), and in an unfortunate fat storing feedback mechanism both ASP and insulin stimulate the secretion of each other. So, in an indirect way, fat does stimulate insulin & insulin stimulates a fat storing helper ASP. The second fat storing hormone is a gut produced signaling molecule called glucose-dependent insulinotrophic peptide (GIP). GIP is induced by carbs & fat and to a much lesser extent protein and fiber. GIP has its own fat storing action on LPL and causes more insulin to be released (here again fat can result in indirect insulin secretion). The hunger hormone ghrelin is also a fat storing hormone. Ghrelin is released when we forgo food. Not only does it induce hunger and cravings for sugary fatty and salty foods, but it also increases the action of LPL and even results in more LPL being made so that when you do eat……you get fatter quicker. This is because ghrelin increases the mRNA expression of LPL. The worst fat storing combination is fat & starch/sugar. It means both carbs and fat cause fat gain and when they are combined together it is far worse than eating them alone.
Carbs Alone= insulin= fat storing
Carbs Alone= GIP= Insulin= Fat storing
Carbs Alone= insulin= ASP= Fat storing
Fat Alone= GIP without hyperglycemia= a little insulin= Little fat storage
Fat Alone= ASP= Fat Storage
Fat with carbs= GIP plus hyperglycemia= excessive insulin= excessive fat storing
Fat with carbs= Double ASP= double insulin= excessive fat storage from independent action of both ASP & insulin
So fat gain by eating fat & carb together is more than sum total of fat gain from eating fat and carb separately.
_
FFA (free fatty acids):
Adipose tissue is biologically highly active and undertakes a range of metabolic and endocrine functions. FFAs released from adipocytes provide the body’s main source of fuel in the post-absorptive state. Although the majority of circulating FFAs arise from outside the intra-abdominal region, FFAs from this source serve as a marker for insulin resistance and associated increases in cardiometabolic risk. Although the liver’s function of glucose production and VLDL synthesis and secretion may be driven to a significant extent by visceral adipose tissue lipolysis, the effects of FFA on muscle, pancreatic β-cells, and the vasculature must be primarily related to upper-body subcutaneous fat. In addition, adipocytes secrete a range of bioactive substances (‘adipokines’) which have the potential to influence metabolism and cardiometabolic function directly. The extent of the contributions of individual adipokines is unclear, although increased secretion of pro-inflammatory mediators and decreased secretion of adiponectin from intra-abdominal adipocytes have been strongly associated with an increased risk of adverse cardiovascular outcomes. The evidence clearly shows that most circulating FFA arise from upper-body subcutaneous adipose tissue. Interventions that reduce body weight in overweight or obese subjects are known to effectively reduce circulating FFA concentrations, especially when plasma insulin concentrations are increased. Such interventions will also improve intra-abdominal adiposity, with the potential for a further improvement in insulin resistance and tendency to normalize FFA and secretion of adipokines. However, given the clear mechanistic links between excess FFA production and adverse metabolic changes, it is reasonable to suppose that improving FFA metabolism alone may improve the overall cardiometabolic risk profile, even where clinically significant weight loss is not achieved. Increased FFA production is associated with multiple adverse cardiometabolic changes, especially in the setting of abdominal obesity and insulin resistance, including promotion of insulin resistance, dyslipidaemia, and impaired β-cell function. These adverse changes are consistent with increased risk of adverse cardiovascular outcomes and an increased risk of developing type 2 diabetes. Therapies that normalize FFA secretion may improve cardiometabolic risk by mechanisms related to and/or independent of weight loss.
_
Breaking down Fat:
When you are not eating, your body is not absorbing food. If your body is not absorbing food, there is little insulin in the blood. However, your body is always using energy; and if you’re not absorbing food, this energy must come from internal stores of complex carbohydrates, fats and proteins. Under these conditions, various organs in your body secrete hormones:
•pancreas – glucagon
•pituitary gland – growth hormone
•pituitary gland – ACTH (adrenocorticotropic hormone)
•adrenal gland – epinephrine (adrenaline)
•thyroid gland – thyroid hormone
These hormones act on cells of the liver, muscle and fat tissue, and have the opposite effects of insulin. When you are not eating, or you are exercising, your body must draw on its internal energy stores. Your body’s prime source of energy is glucose. In fact, some cells in your body, such as brain cells, can get energy only from glucose. The first line of defense in maintaining energy is to break down carbohydrates, or glycogen, into simple glucose molecules — this process is called glycogenolysis. Next, your body breaks down fats into glycerol and fatty acids in the process of lipolysis. The fatty acids can then be broken down directly to get energy, or can be used to make glucose through a multi-step process called gluconeogenesis. In gluconeogenesis, amino acids can also be used to make glucose. In the fat cell, other types of lipases work to break down fats into fatty acids and glycerol. These lipases are activated by various hormones, such as glucagon, epinephrine and growth hormone. The resulting glycerol and fatty acids are released into the blood, and travel to the liver through the bloodstream. Once in the liver, the glycerol and fatty acids can be either further broken down or used to make glucose. Your weight is determined by the rate at which you store energy from the food that you eat, and the rate at which you use that energy. Remember that as your body breaks down fat, the number of fat cells remains the same; each fat cell simply gets smaller.
_
Figure above shows homeostatic regulation of energy balance in mammals. Signals from sites of fat storage communicate the energetic state of the body to the nervous system, which also receives environmental and sensory inputs. The nervous system integrates these signals and responds to alter behavior, physiology and energy uptake, storage and utilization.
__________
Energy efficiency of body may cause obesity:
In earlier paragraph, I have shown that only 18 to 26% of the energy available from respiration is converted into mechanical energy in human body. Yet, it is far more efficient than converting energy generated from gasoline into mechanical energy of a car. For example, a person running a marathon (26 miles or 42 km) burns only about 2,600 calories. In other words, you burn only about 100 calories per mile (about 62 calories per km) when you are running. You can see just how efficient the human body is if you compare your body to a car. A typical car in the United States gets between 15 and 30 miles per gallon of gasoline (6 to 12 km/L). A gallon of gas contains about 31,000 calories. That means that if a human being could drink gasoline instead of eating hamburgers to take in calories, a human being could run 26 miles on about one-twelfth of a gallon of gas (0.3 L). In other words, a human being gets more than 300 miles per gallon (120 km/L)! If you put a human being on a bicycle to increase the efficiency, a human being can get well over 1,000 miles per gallon (more than 500 km/L)! That level of efficiency is the main reason why it is so easy to gain weight.
_____________
What is energy density?
‘Energy density’ is the amount of energy (or calories) per gram of food. Lower energy density foods provide less energy per gram of food so you can eat more of them without consuming too many calories. So this is a good way to help control how much we eat, without going hungry and may also be a great new way to help you lose weight. Low energy density foods include foods with high water content, such as soups & stew, and fruit & vegetables. Foods that are high in fibre, such as wholegrain breads and cereals, and lower fat foods also tend to have a lower energy density. High energy density foods tend to include foods that are high in fat/sugar and have a low water content, for example biscuits and confectionery, crisps, peanuts, butter and cheese. Studies have shown that people tend to consume about the same amount (weight) of food each day, but not necessarily the same amount of energy (or calories). So it is possible to trick ourselves into consuming less energy, without feeling hungrier, by eating a lower energy density diet, which still makes up the same weight of foods overall throughout the course of a day.
_
Very low energy density foods = less than 0.6 calories/gram
Low energy density foods = 0.6 to 1.5 calories/gram
Medium energy density foods = 1.5 to 4 calories/gram
High energy density foods = more than 4 calories/gram
_
Foods that contain a lot of calories are known as energy-dense foods. They tend to be high in fat and/or sugar and can contribute to weight gain.
For example, 100g of chocolate contains around 10 times more calories than 100g of apple:
•100g of milk chocolate = 520 kcals
• 100g of apple = 52 kcals
It can be difficult to control how much energy you are consuming if you eat a lot of energy-dense foods because you only need to eat a small amount to take in a lot of calories. By choosing a diet based on lower energy-dense foods, you can actually eat more food but consume fewer calories. Lower energy-dense foods are high in water and fiber and help us feel fuller for longer.
_
Peanuts may be exception to energy dense concept:
The lower the energy density of your food the better, if you are trying to lose weight, right? Logical though it may seem, this hypothesis is not actually supported by the data, according to one leading food scientist. Analysis of the eating patterns of lean and obese people does not reveal a big variation in the energy density of their diets. There is not a significant correlation between the energy density of the diet and BMI. Epidemiological data consistently shows that people eating a diet high in peanuts – which are extremely energy dense with around 600 calories per 100g compared with apples at 47 cals, white bread at 266 cals, and even glazed donuts at 385 cals – have a lower BMI and a reduced risk of cardiovascular disease and diabetes. Similarly, compliance with weight-loss programs is better when peanuts and peanut butter are included. First, nuts suppress hunger, as well as the desire to eat. Second, recent research shows we don’t absorb all of the energy in nuts, which means we may be overstating their calorie content. When you bite into a nut and break it up into smaller pieces, some of these pass through your digestive system intact, taking the energy contained in them with them. If you analyze the feces [of people that have eaten nuts] you still see lipid-based energy in there. The rigid structure of the nut cell membranes appears to lock in some fat, preventing it from being absorbed in the digestive tract. Third, some research suggests chronic nut consumption is associated with an increase of resting metabolic rate. The extent of the disparity between the actual and stated calorie content depends on the nut, almonds appear to be most out of sync, with a likely 20% overstatement of calories in pack, while whole peanuts (but not peanut butter) are around 10-15% out of sync.
_
Nutrient-Rich vs. Nutrient-Poor:
Foods that provide a lot of kilojoules in only a small volume are called ‘energy-dense’ foods. Foods that are high in fat or sugar, such as take-away foods, pastries, chocolate bars and soft drinks, are considered to be energy-dense. These foods can also be low in important nutrients such as calcium and iron. If children or adults eat too many foods with a high energy density, they can have more than their daily energy requirements. If this extra energy is not burnt through exercise, it will be stored – much of it as fat.
Classical example:
A glass (250ml) of regular milk gives an average girl aged 9 to 11 years who is moderately active:
- 55 per cent of her recommended dietary intake (RDI) for riboflavin
- 28 per cent of her RDI for calcium
- 24 per cent of her RDI for protein
- 20 per cent of her RDI for vitamin A
- 19 per cent of her RDI for phosphorus
- 16 per cent of her RDI for zinc
- 15 per cent of her adequate intake (AI) for potassium
- 11.5 per cent of her RDI for magnesium.
A glass of milk contains 678kJ per 250ml contributing only 8 per cent of her daily energy intake, in comparison to all the nutrients it contains (listed above). This makes it a nutrient-rich food. By contrast, 250ml of soft drink provides approximately 500kJ per 250ml, but contains no protein, vitamins or minerals, making it a waste of kilojoules!
_
Foods that are energy dense have lots of calories per serving. The calories may come from protein, fat, or carbohydrates. Foods that are nutrient dense have high levels of nutrients per serving. Nutrient dense refers to the amount of vitamins, minerals, and/or protein in a food. Some foods can be energy dense and provide few nutrients, while some foods can be nutrient dense but provide little energy or calories. Energy-dense foods are often high in sugar and fat. Vegetables are often nutrient dense but energy densities for most are low.
| Candy is energy dense but provides no vitamins and minerals. |
| Spinach is nutrient dense but provides few calories. |
| Cheese is both energy and nutrient dense. |
_
Examples of Energy- and Nutrient-Dense Foods:
- Whole milk
- Full-fat cheeses
- Creamed soups
- Pudding
- Pasta and vegetables in cream sauce
- Meat with gravy
_
Ideally, the food and drinks children consume should be nutrient-rich. A nutrient-rich food is one that provides a useful amount of key nutrients for relatively few kilojoules. Generally, the core food groups are nutrient rich, including lean meat, fish, fruit and vegetables, nuts, wholegrain breads and cereals. Milk, cheese and yogurt are naturally nutrient-rich and they provide a unique package of essential macronutrients (such as carbohydrate and protein), vitamins and minerals, for relatively few kilojoules. Dairy foods are a healthier alternative to energy-dense, nutrient-poor foods and drinks.
____________
All calories are not equal:
Theoretically, a calorie is a calorie. The big differences are how our body responds to the calories we consume and how those calories make us feel. Consider the following example. Both a 24-ounce sugar-sweetened beverage and a grilled chicken sandwich (3 ounces grilled chicken, small whole wheat bun, lettuce, tomato, onion) contain ~250-275 calories yet the body’s metabolic response of these two items will be very different. How we feel after eating these two items would also be distinct. Because the beverage only contains simple carbohydrates and requires little digestion, it is quickly absorbed into the blood stream, causing a rapid rise in blood glucose. The chicken sandwich, on the other hand, is a combination of complex carbohydrates (including fiber) and protein. The fiber and protein both help to slow digestion and absorption, resulting in a slower, steadier rise in blood glucose. Foods that include calories from fiber and protein also help with satiety, keeping us feeling full for a long period of time. These differences in how the calories are processed and how they make us feel can have significant implications for weight management.
_
Weight management is a simple game of math, some folks argue. To maintain your current weight, you need to consume the same number of calories your body burns each day. To lose a pound, you need to create a caloric deficit of approximately 3,500 calories. Whether you create that deficit by eating less fat, less carbohydrate, less protein or a little less of everything is immaterial. It sounds sensible, but it’s actually not true. A calorie is not a calorie, in more than one sense. Carbohydrate, fat and protein calories are indeed equal by definition in terms of their energy content, but the body processes each in a distinct way, and these differences have real implications for weight management. In addition, food calories of all types may have very different effects on the body depending on when they are eaten and what they are eaten with. Food calories affect the body very differently depending on their source and the overall context in which they are consumed.
1. The energy cost to metabolize fat, carbs and protein is different:
The body must use energy to digest, absorb and metabolize the energy in food. And it so happens that the body uses different amounts of energy to process different energy-containing nutrients. Generally, more energy is required to process protein than carbs, and more energy is required to process carbs than fat. What this means effectively is that a 2,500-calories-a-day high-protein diet adds fewer calories to the body than a 2,500-calories-a-day high-carb diet, which in turn adds fewer calories to the body than a 2,500-calories-a-day high-fat diet. Admittedly, the differences are small. They do not in themselves constitute a rationale to consume a high-protein, low-fat diet for weight management.
2. Calorie restriction slows metabolism:
The biggest problem with using linear calorie equations for fat loss is that the fewer calories you consume, the fewer calories your body burns. Thus, if, based on the 3,500-calorie rule cited above, you decide to cut your daily energy intake by 500 calories in hopes of losing a pound a week (500 calories/day x 7 days = 3,500 calories), you will probably find that you do indeed lose a pound in the first week but less in each subsequent week. This phenomenon is believed to represent a metabolic adaptation to prevent starvation. Your body literally runs cooler to conserve the reduced number of calories you’re eating, thereby effectively increasing the value of each calorie. A 2006 study published in the Journal of the American Medical Association reported that volunteers who maintained a very low-calorie diet for six months exhibited a significantly greater reduction in metabolic rate than could be explain by weight loss alone. A longer-term study on monkeys revealed that monkeys whose food intake was reduced by 30 percent for 11 years exhibited a 13-percent lower metabolic rate than weight loss alone could account for. More relevant for our concerns as athletes is evidence that even small calorie deficits within a single day may alter our metabolism in ways that have negative effects on our body composition. A study involving elite female gymnasts and distance runners found a strong inverse relationship between the number and size of energy deficits throughout the day (that is, periods when the body’s calorie needs exceed the calorie supply from foods) and body fat percentage. In other words, the athletes who did the best job of matching their calorie intake with their calorie needs throughout the day were leaner than those who tended to fall behind. What’s important to note about this study is that the effect of mini calorie deficits was independent of total caloric intake for the day. This means that a woman athlete who requires and consumes X calories a day is likely to have less muscle and more body fat if she does not time her eating well than if she takes in the same total number of calories but distributes them more evenly throughout the day.
3. Protein reduces appetite:
Protein generally reduces appetite more per calorie than fat and carbohydrate. Therefore a person who increases his daily protein intake without making any conscious attempt to eat less is likely to eat less anyway due to reduced appetite. This is another important sense in which protein, carbohydrate and fat calories are not equal. In a recent study from the University of Washington School of Medicine, 19 subjects were fed each of three diets sequentially. For two weeks they followed a weight-maintenance diet comprising 15 percent protein, 35 percent fat, and 50 percent carbohydrate. For the next two weeks they followed a high–protein diet of equal calories. The macronutrient breakdown of this diet was 30 percent protein, 20 percent fat, and 50 percent carbohydrate. Finally, the subjects switched to a high-protein diet with the same macronutrient breakdown but no calorie restriction—subjects were allowed to eat as much or as little as they pleased (or “ad libitum”). They stayed on this last diet for 12 weeks. The authors of the study reported that when subjects switched from the low-protein weight maintenance diet to the high-protein weight maintenance diet, they started feeling much fuller despite the fact that they were consuming the same number of calories. Even more significant, during the unrestricted high-protein diet phase, the subjects voluntarily reduced their daily eating by 441 calories per day and lost almost 11 pounds, including more than eight pounds of body fat, on average.
4. Fiber reduces calorie absorption:
Fiber is a form of carbohydrate that contributes to satiety without contributing calories, because it is not absorbed into the body. Consequently, a 100-calorie high-fiber food will reduce appetite and subsequent eating more than a 100-calorie low-fiber food. Likewise, a person who increases his daily fiber consumption without making any conscious effort to eat less will wind up eating less anyway due to reduced appetite. Thus, a calorie inside a high-fiber food is not equal to a calorie inside a low-calorie food—yet a fourth way in which “a calorie is not a calorie.”
5. Timing of eating affects calorie processing:
Thermic effect of food (TEF) is a fancy name for the energy used up as a result of digesting and absorbing a meal. A study published in the American Journal of Clinical Nutrition found that TEF is higher in the morning than in the evening. Volunteers were given an identical 544-calorie meal at one of three times. In subjects fed at 9 am, TEF increased by 16 percent; in those fed at 5 pm, TEF increased by 13.5 percent; and in those fed at 1 am, TEF increased by only 11 percent. So it’s clear that we burn more calories in the morning. The effect of calories on body composition is also influenced by the size and frequency of meals. For example, a Japanese study found that boxers placed on a six-meals-a-day weight-control diet lowered their body fat percentage significantly more than boxers who ate exactly the same number of calories in just two meals. Generally speaking, food calories are more likely to be stored as fat and less likely to be used immediately for energy, stored as glycogen, or used to synthesize new muscle proteins when they are consumed in excess of short-term needs. This is why six small meals totaling 2,500 calories are not equal to two large meals totaling 2,500 calories.
_
Calories are calories, weight-loss gurus tell us, and to lose weight you need to “eat less, exercise more.” Right? Maybe not. Conventional wisdom argues that a calorie is a calorie, whether it comes from an ice cream cone or a tossed salad. Of course, there’s a huge difference in the nutritional value of these foods. Ice cream is mostly sugar and fat calories, whereas the salad is a trove of vitamins, minerals, antioxidants, and fiber calories. Some people’s bodies will respond differently (metabolism-wise) to 500 calories of sugar than they will to 500 calories of protein. Obviously, this idea has enormous significance for people whose goal is weight loss. It’s in the Insulin. Our bodies’ responses are influenced by insulin, a potent hormone. The body secretes insulin to move glucose from the bloodstream into cells, where it’s either burned for energy or stored as fat. Sugary foods and other processed carbohydrates — think breads, chips, pretzels, white rice and pastas — can trigger a sharp spike in blood glucose levels. That spike is followed by a big jump in insulin. Research tells us that excess insulin also sets the stage for inflammation, has been linked to breast cancer risk, and promotes the accumulation of belly fat. This fat-promoting effect is insulin is well known. In fact, people typically gain weight after they start insulin injections. If all calories were equal, weight loss would be equal on strictly followed low-calorie diets. But such diets, while usually low in fat, tend to be high in carbohydrates — the very foods that increase insulin secretion. And this effect is even more undesirable in the context of something we know about being overweight: According to a study by scientists at the University of Munich (Erdmann et al., 2008), just 10 extra pounds can alter normal insulin function and set the stage for prediabetes and type 2 diabetes. So if you want to lose weight, how should you compose your calories? Fine and Feinman contend that controlling insulin secretion is the key to losing weight. And findings published in the May 16, 2007, Journal of the American Medical Association backs them up: Researchers from Children’s Hospital Boston reported that people with elevated insulin levels were more likely to lose weight on a higher protein, lower carb diet, instead of a traditional low-calorie and high-carb diet. The reason? Fish, chicken, and other quality protein sources barely increase insulin levels. The researchers conclude that the variability in how people lose weight may be partially due to differences in insulin response. And they say that, among people with compromised insulin function, low-glycemic eating may be the best way to achieve weight loss. Regardless of insulin function, they say, eating this way has beneficial effects on cholesterol. None of this means you have to eat a super high-protein diet. Eating lean meat, chicken, and fish, along with high-fiber, nonstarchy vegetables (almost anything except potatoes and corn) helps control both glucose and insulin levels. And may just be the best ticket to weight control, too.
______________
What is sugar?
The meaning of terms often used when talking about dietary sugars:
Caloric sweeteners…
Sweeteners, typically carbohydrates naturally present in or added to foods, caloric sweeteners have approximately 4 calories per gram
Sugars…
Monosaccharides (single sugar units like fructose and glucose) and disaccharides (two sugar units linked together, like sucrose); sometimes called simple sugars
Sucrose…
A disaccharide containing one fructose unit and one glucose unit bonded together (also known as table sugar); contains 50 percent fructose and 50 percent glucose
Glucose…
A monosaccharide; the main source of energy for the body
Fructose…
A monosaccharide; it has the same chemical formula as glucose but different molecular structure; sometimes called fruit sugar since it is the sugar that occurs naturally in fruits, vegetables, and honey,
Added sugars…
Sugars eaten separately, added to foods at the table (such as adding sugar to coffee, tea, or cereal; topping pancakes with syrup, etc.), or used as ingredients in prepared foods; examples include sugar, corn syrup, high fructose corn syrup, honey, and molasses
Table sugar…
Common name for the disaccharide sucrose; obtained from sugar beets and sugar cane
High fructose corn syrup (HFCS)…
A mixture of glucose and fructose produced from corn syrup; the most frequently used types are HFCS 42, common in baking applications, which is 42 percent fructose; and HFCS 55, common in beverage applications, which is 55 percent fructose
______________
The fructose controversy:
Fructose in Diet:
In the average western diet, fructose comes from two sources: as a natural compound in fruit, and as an added ingredient of processed foods. Food companies use fructose because it is sweeter than glucose and helps to stabilize processed foods. The main source of fructose in processed foods in the US is high fructose corn syrup, which is also used to improve the appearance of baked goods because it produces a more consistent browning.
_
Fructose is abundant in fruit. Fruit is fine, but we should think twice before drinking juice or feeding it to our kids. The fiber in whole fruit contributes to a sense of fullness. It is rare to see a child eat more than one orange, but it is common for kids to consume much more sugar and calories as orange juice. Eating fiber also results in less carbohydrate being absorbed in the gut. In addition, fiber consumption allows the brain to receive a satiety signal sooner than it would otherwise, so we stop eating sooner. But failure to limit sugar intake appears to be the most predictive of poor weight control in children. You are not what you eat; you are what you do with what you eat. And what you do with fructose is particularly dangerous as you will see in the following paragraphs.
_
Absorption of fructose:
The digestive and absorptive processes for glucose and fructose are different. When disaccharides such as sucrose or maltose enter the intestine, they are cleaved by disaccharidases. A sodium-glucose cotransporter absorbs the glucose that is formed from cleavage of sucrose. Fructose, in contrast, is absorbed further down in the duodenum and jejunum by a non-sodium-dependent process. After absorption, glucose and fructose enter the portal circulation and both are transported to the liver, where the fructose can be taken up and converted to glucose, or pass into the general circulation. The addition of small, catalytic amounts of fructose to orally ingested glucose increases hepatic glycogen synthesis in human subjects and reduces glycemic responses in subjects with type 2 diabetes mellitus, which suggests the importance of fructose in modulating metabolism in the liver. However, when large amounts of fructose are ingested, they provide a relatively unregulated source of carbon precursors for hepatic lipogenesis.
Fructose and insulin release:
Along with two peptides, glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 released from the gastrointestinal tract, circulating glucose increases insulin release from the pancreas. Fructose does not stimulate insulin secretion in vitro, probably because the β cells of the pancreas lack the fructose transporter Glut-5. Thus, when fructose is given in vivo as part of a mixed meal, the increase in glucose and insulin is much smaller than when a similar amount of glucose is given. However, fructose produces a much larger increase in lactate and a small (1.7%) increase in diet-induced thermogenesis, which again suggests that glucose and fructose have different metabolic effects.
Insulin and leptin:
Insulin release can modulate food intake by at least 2 mechanisms. First, Schwartz et al have argued that insulin concentrations in the central nervous system have a direct inhibitory effect on food intake. In addition, insulin may modify food intake by its effect on leptin secretion, which is mainly regulated by insulin-induced changes in glucose metabolism in fat cells. Insulin increases leptin release with a time delay of several hours. Thus, a low insulin concentration after ingestion of fructose would be associated with lower average leptin concentrations than would be seen after ingestion of glucose. Because leptin inhibits food intake, the lower leptin concentrations induced by fructose would tend to enhance food intake. This is most dramatically illustrated in humans who lack leptin. Persons lacking leptin (homozygotes) are massively obese, and heterozygotes with low but detectable serum leptin concentrations have increased adiposity, which indicates that low leptin concentrations are associated with increased hunger and gains in body fat. Administration of leptin to persons who lack it produces a dramatic decrease in food intake, as expected. Leptin also increases energy expenditure, and during reduced calorie intake, leptin attenuates the decreases in thyroid hormones and 24-h energy expenditure. To the extent that fructose increases in the diet, one might expect less insulin secretion and thus less leptin release and a reduction in the inhibitory effect of leptin on food intake, i.e., an increase in food intake. This was found in the preliminary studies reported by Teff et al. Consumption of high-fructose meals reduced 24-h plasma insulin and leptin concentrations and increased postprandial fasting triacylglycerol concentrations in women but did not suppress circulating ghrelin concentrations.
Fructose and metabolism:
The metabolism of fructose differs from that of glucose in several other ways as well. Glucose enters cells by a transport mechanism (Glut-4) that is insulin dependent in most tissues. Insulin activates the insulin receptor, which in turn increases the density of glucose transporters on the cell surface and thus facilitates the entry of glucose. Once inside the cell, glucose is phosphorylated by glucokinase to become glucose-6-phosphate, from which the intracellular metabolism of glucose begins. Intracellular enzymes can tightly control conversion of glucose-6-phosphate to the glycerol backbone of triacylglycerols through modulation by phosphofructokinase. In contrast with glucose, fructose enters cells via a Glut-5 transporter that does not depend on insulin. This transporter is absent from pancreatic β cells and the brain, which indicates limited entry of fructose into these tissues. Glucose provides “satiety” signals to the brain that fructose cannot provide because it is not transported into the brain. Once inside the cell, fructose is phosphorylated to form fructose-1-phosphate. In this configuration, fructose is readily cleaved by aldolase to form trioses that are the backbone for phospholipid and triacyglycerol synthesis. Fructose also provides carbon atoms for synthesis of long-chain fatty acids, although in humans, the quantity of these carbon atoms is small. Thus, fructose facilitates the biochemical formation of triacylglycerols more efficiently than does glucose. For example, when a diet containing 17% fructose was provided to healthy men and women, the men, but not the women, showed a highly significant increase of 32% in plasma triacylglycerol concentrations. [triacylglycerol means triglyceride]
Overconsumption of sweetened beverages:
One model for producing obesity in rodents is to provide sweetened (sucrose, maltose, etc) beverages for them to drink. In this setting, the desire for the calorically sweetened solution reduces the intake of solid food, but not by enough to prevent a positive caloric balance and the slow development of obesity. Adding the same amount of sucrose or maltose as of a solid in the diet does not produce the same response. Thus, in experimental animals, sweetened beverages appear to enhance caloric consumption.
Fructose and soft drinks:
A similar argument about the role of overconsumption of calorically sweetened beverages may apply to humans. Mattes reported that when humans ingest energy-containing beverages, energy compensation is less precise than when solid foods are ingested. In another study in humans, DiMeglio and Mattes found that when 15 healthy men and women were given a carbohydrate load of 1880 kJ/d (450 kcal/d) as a calorically sweetened soda for 4 wk, they gained significantly more weight than when the same carbohydrate load was given in a solid form as jelly beans. Additional support for our hypothesis that calorically sweetened beverages may contribute to the epidemic of obesity comes from a longitudinal study in adolescents. Ludwig et al showed that in adolescents participating in the Planet Health project, the quantity of sugar-sweetened beverages ingested predicted initial body mass index (BMI; in kg/m2) and gain in BMI during the follow-up period. Raben et al designed a randomized, double-blind study to compare the effect of calorically sweetened beverages with that of diet drinks on weight gain in moderately overweight men and women. This European study found that drinking calorically sweetened beverages resulted in greater weight gain over the 10-wk study than did drinking diet drinks. Compared with the subjects who consumed diet drinks, those who consumed calorically sweetened beverages did not compensate for this consumption by reducing the intake of other beverages and foods and thus gained weight. The beverages in this study were sweetened with sucrose, whereas in the United States almost all calorically sweetened beverages are sweetened with HFCS. Thus, we need a second randomized controlled study that compares sucrose- and HFCS-sweetened beverages. This could establish whether the form of the caloric sweetener played a role in the weight gain observed in the study by Raben et al. The results of the studies by Raben et al and Ludwig et al suggest that the rapid increase in the intake of calorically sweetened soft drinks could be a contributing factor to the epidemic of weight gain. Between 1970, when HFCS was introduced into the marketplace, and 2000, the per capita consumption of HFCS in the United States increased from 0.292 kg per person per year to 33.4 kg per person per year, an increase of > 100-fold. The total consumption of fructose increased nearly 30%. The consumption of free fructose showed a greater increase, which reflected the increasing use of HFCS. During the same interval, the consumption of sucrose decreased nearly 50%, and the intakes of sucrose and HFCS are now nearly identical. Although this shift has clearly led to a major increase in free-fructose consumption, it is unclear how much of the increase in consumption of calorically sweetened soft drinks is a result of the shift to beverages in which one-half of the fructose is free rather than bound with glucose as in sucrose.
_
Several studies over the past few years have come to this conclusion that the primary fuel for obesity epidemic in the west is the excess consumption of high fructose corn syrup (HFCS), including the latest study in Cell Metabolism, in which the researchers note: “Insulin resistance is a common feature of the metabolic syndrome and type 2 diabetes mellitus (T2DM). Both have reached epidemic proportions worldwide with the global adoption of the westernized diet along with increased consumption of fructose, stemming from the wide and increasing use of high-fructose corn syrup sweeteners. It is well established that fructose is more lipogenic than glucose, and high-fructose diets have been linked to hypertriglyceridemia, nonalcoholic fatty liver disease (NAFLD), and insulin resistance”.
_
A team from the University of Barcelona (UB) led by Dr Juan Carlos Laguna published a study in the journal Hepatology that provides clues to the molecular mechanism through which fructose in beverages may alter lipid energy metabolism and cause fatty liver and metabolic syndrome. Fructose is mainly metabolized in the liver, the target organ of the metabolic alterations caused by the consumption of this sugar. In this study, rats receiving fructose-containing beverages presented a pathology similar to metabolic syndrome, which in the short term causes lipid accumulation (hypertriglyceridemia) and fatty liver, and eventually leads to hypertension, resistance to insulin, diabetes and obesity.
_
Fructose effect on the brain may promote obesity – researchers from Yale University School of Medicine compared the effects of fructose and glucose on the brain with MRI scans and found that high fructose diets may be behind the current obesity epidemic. In an article published in JAMA (Journal of the American Medical Association), the authors said they found that regions in the brain that regulate appetite became active when people consumed glucose, but remained inactive when they ingested fructose. When those regions become active, they release hormones that produce feelings of satiety (fullness) – in other words, the hormones tell you to stop eating.
_
Sucrose vs. HFCS:
Each sucrose molecule consists of one molecule of fructose joined to one molecule of glucose. In the gut, these two components are quickly split apart. High-fructose corn syrup is a less expensive mixture of glucose and fructose. There is no point in belaboring the difference. High-fructose corn syrup and sucrose are exactly the same. They’re equally bad. They’re both poison in high doses. Over the past century, Americans have increased their fructose consumption from 15 grams per day to 75 grams per day or more. The trend accelerated beginning about three decades ago, when cheap, easy-to-transport high-fructose corn syrup became widely available. Much of processed food labeled “reduced fat” instead has sugar added to make it more palatable. But when it comes to harmful health effects, sugar is worse than fat. Consumption of either results in elevated levels of artery-clogging fats being made by the liver and deposited in the bloodstream. But fructose causes even further damage to the liver and to structural proteins of the body while fomenting excessive caloric consumption.
_
Is fructose worse than glucose?
- We now know that fructose elevates uric acid, which decreases nitric oxide, raises angiotensin, and causes your smooth muscle cells to contract, thereby raising your blood pressure and potentially damaging your kidneys. Increased uric acid also leads to chronic, low-level inflammation, which has far-reaching consequences for your health. For example, chronically inflamed blood vessels lead to heart attacks and strokes; also, a good deal of evidence exists that some cancers are caused by chronic inflammation
- A 2009 study from the University of California, Davis shows how a high-fructose diet can cause you to build new fat cells around your heart, liver, and digestive organs in just 10 weeks, plunging you into the early stages of diabetes and heart disease (whereas a high glucose diet did not have the same effects)
- Excess fructose consumption is a major contributor to insulin resistance and obesity, hypertension, cardiovascular disease, liver disease, cancer, arthritis, and other diseases
- Fructose is metabolized very differently in your body from glucose; all of the metabolic burden falls on your liver, in much the same way as for alcohol, and your body becomes a sea of toxic byproducts
- Glucose, on the other hand, is your body’s nearly ideal source of fuel, meaning it has none of the damaging metabolic effects of fructose; glucose also suppresses your appetite, unlike fructose, which stimulates your appetite and encourages overeating and the accumulation of excess body fat
_
Fructose and athletes:
For some time bodybuilders have been wary of the consumption of fructose in the belief that it cannot be used by muscle glycogen and is only useful for replenishing liver glycogen levels with an excessive amount easily converted into body fat. A number of studies have been conducted on fructose which highlights its potential to cause insulin resistance, obesity and metabolic syndrome. As a consequence, despite its low glycaemic index, it is generally not advised to be consume liberally. Indeed, large doses of fructose are associated with liver damage said to be equivalent to that seen in alcoholics. Of greater concern to us athletes is what level of fructose is advisable to consume. First, we need to remember that it is almost impossible to consume pure fructose as even fruits and HFCS contain plenty of glucose. In fact, the much demonised HFCS is nearly identical to table sugar (sucrose) in terms of its ratio of glucose to fructose. Sucrose is 50/50 while HFCS is about 55/45. Although some studies show that fructose intake does not increase leptin or satiate appetites, there is other data to show that a fructose preload can lead to lower caloric consumption than a glucose preload in a subsequent meal. At the same time, excessive fructose consumption in one study led to greater food consumption the following day but this was at a dose of 135 grams of pure fructose which would be very high indeed for the average person (equivalent to about 7 soft drink cans per day). The take home message here is that excessive fructose consumption should be avoided. Generally speaking, it is always good advice to focus diet around minimally processed foods as they will be the healthiest for us. A daily fructose consumption of around 50 grams per day for athletes should be fine and plenty enough to replenish liver glycogen and also ensure we get the health benefits of fructose. If we focus our fructose intake on fruits which tend to be only around 50% fructose then it is very unlikely we run into any problems with health.
_________
Glycemic index:
The glycemic index (GI) provides a measure of how quickly blood sugar levels (i.e., levels of glucose in the blood) rise after eating a particular type of food. The effects that different foods have on blood sugar levels vary considerably. The glycemic index estimates how much each gram of available carbohydrate (total carbohydrate minus fiber) in a food raises a person’s blood glucose level following consumption of the food, relative to consumption of pure glucose. Glucose has a glycemic index of 100. Foods with carbohydrates that break down quickly during digestion and release glucose rapidly into the bloodstream tend to have a high GI; foods with carbohydrates that break down more slowly, releasing glucose more gradually into the bloodstream, tend to have a low GI. The glycemic effect of foods depends on a number of factors such as the type of starch (amylose versus amylopectin), physical entrapment of the starch molecules within the food, fat and protein content of the food and organic acids or their salts in the meal — adding vinegar, for example, will lower the GI. The presence of fat or soluble dietary fiber can slow the gastric emptying rate, thus lowering the GI. In general, coarse, grainy breads with higher amounts of fiber have a lower GI value than white breads. However, most breads made with 100% wholewheat or wholemeal flour have a GI not a whole lot different than endosperm only (white) bread. Many brown breads are treated with enzymes to soften the crust, which makes the starch more accessible (high GI). While adding fat or protein will lower the glycemic response to a meal, the relative differences remain. That is, with or without additions, there is still a higher blood glucose curve after a high GI bread than after a low-GI bread such as pumpernickel. Fruits and vegetables tend to have a low glycemic index.
_
The table below shows glycemic index of some foods:
_
High glycemic index foods may lead to insulin resistance:
Carbohydrates come in the form of sugar, starch and fiber. After you eat or drink something with carbs, your body breaks down each type of carbohydrate in essentially the same way, converting it into sugar. The exception is fiber, which passes through your body undigested. The sugar then enters your bloodstream. From there, it enters individual cells throughout your body to provide energy. Extra sugar is stored in your liver and muscles in a form called glycogen.
Two hormones from your pancreas help regulate the level of blood sugar. The hormone insulin moves sugar from your blood into your cells when your blood sugar level is high. The hormone glucagon helps release the sugar stored in your liver when your blood sugar level is low. This process helps keep your body fueled and ensures a natural balance in blood sugar. Some food (high GI) is thought to disrupt this natural balance by creating large spikes in your blood sugar level. When your blood sugar and insulin levels stay high, or cycle up and down rapidly, your body has trouble responding and over time this could contribute to insulin resistance. Insulin resistance is associated with a host of health problems, including:
- Type 2 diabetes
- Obesity
- High blood pressure
- Stroke
- Heart disease
_
Extensive use of sugars and white flour often provide “empty calories” in soda, white bread and “junk” food. That is, these products lack the minerals, vitamins and fiber necessary for good health in spite of their high energy content. It is a fact that “empty calories” are more prevalent in processed food with high GI. Choosing food based on low GI may provide a better nutritional basis not only because low GI food prevents high spikes of insulin but also because low GI food contain minerals, vitamins and fiber necessary for good health.
_
A landmark study demonstrating low glycemic index food reducing obesity:
One of the most interesting studies I have seen concerning glycemic index was done using a group of grossly overweight boys. They started the experiment by eating a breakfast consisting of high, low, or intermediate carbohydrate-rich food. The levels of several nutrients and hormones were followed in their blood over a period of five hours. They were interviewed and their feeling of satiety was monitored. We see that, as expected, blood glucose increased more with the high GI diet than with the low GI breakfast. The intermediate GI breakfast gave a relatively large increase in blood sugar too. The really interesting observation here is that blood sugar fell below the starting values from the 4th hour onwards in the group fed the high GI breakfast. As one might have expected, insulin levels were altered parallel with the changes in blood glucose. Glucagon activates glycogenolysis and gluconeogenesis in the liver and is responsible for maintenance of blood glucose when there is a tendency for this to fall. Here we see a distinct increase in plasma glucagon after the low GI diet while plasma levels of this hormone fall in the other two cases. This tells us clearly that the intestinal absorption of the low GI diet was so slow that uptake of carbohydrate from the diet did not replace the glucose used by the body’s tissues. Glucagon activates both hepatic glucose release and hormone-sensitive lipase in fat. We see a rise in serum free fatty acids from the second hour after breakfast. Hunger is associated with a rise in so-called stress hormones and a fall in blood sugar and insulin. This study demonstrates unmistakably that ingestion of the high GI meal led to a striking increase in both epinephrine (adrenaline) and growth hormone after about 3.5 hours following the test meal. Glucagon also rapidly increased at this time. The boys were hungry after five hours and could choose their own meals. Those who began the study with a high GI meal (and were “starving”) choose high GI food when allowed to eat once more. This probably followed the pronounced rise in stress hormones and fall in blood sugar. The result was that those who started the day with a high GI diet consumed more food (and calories) than the other boys. One could perhaps conclude that a use of food with a low glycemic index might aid in reducing food consumption and weight.
_
Studies that support the view that glycemic index of food is irrelevant in weight management:
A 2008 study out of the University of Cambridge compared a group who consumed mainly high GI foods to one that consumed mainly low GI foods. The result? That the GI of a food had no effect on body weight whatsoever. In another one-year randomized control trial, Das and coworkers (2007) tried to eliminate many of the methodological problems. In their research, subjects were either assigned to a low or high GI eating plan and used a 30% calorie restriction to promote weight loss over time. Food preferences were established and then researchers provided subjects with GI education and all of their food for the first 6 months of the study. This allowed subjects to ‘learn’ proper eating principles prior to attempting these eating plans on their own. Both groups lost weight after one year (8.04 ± 4.1% and 7.81 ± 5.0% for high and low GI, respectively) but there was no difference between groups. So researchers concluded that lowering the glycemic load and glycemic index of weight reduction diets does not provide any added benefit to energy restriction in promoting weight loss in obese subjects.
____________
Is carbohydrate greater evil than fat for the development of obesity?
_
The problem of carbohydrates esp. of high glycemic index rather than fat for development of obesity:
Several populations (e.g. Eskimos and Gabonese) have negligible rates of chronic diseases such as cancer, diabetes, obesity, heart disease, and stroke when eating their native diet. When some members of those populations begin to adopt a Western diet their rates of chronic disease rise up to Western levels while their counterparts who maintain the traditional diet continue to be healthy. Meat-eating seems unlikely to be a cause of cancer as carnivorous Inuit and Masai have extremely low cancer rates, while the vegetarian Hindus had a prevalence of cancers (circa 1910). Refined carbohydrates such as white flour, sugar, white rice, and molasses appear linked to risk of cancer. Diabetes is caused by an overtaxed pancreas (the pancreas produces insulin). Eating sugar and white flour produces insulin spikes, which exert the pancreas. In the 1930′s it was observed that in a number of civilizations, including Sri Lanka, Thailand, Tunisia and China, diabetes existed only among the rich who eat European food and drink sweet wine. In India, the vegetarian Hindus had far higher rates of diabetes than the non-vegetarian Christians and Muslims. Cleave and later Yudkin suggested that diabetes, obesity, heart disease, gall stones, and periodontal disease are linked and are caused by consumption of sugar, flour, and white rice. This theory seems to fit anecdotally, but further experiments are needed to definitely prove it.
_
_
Various tribes and sumo wrestlers induce fattening with high carbohydrate, low-fat foods (ironically, the same nutrient breakdown commonly recommended in the U.S. to lose weight). Experiments showing that rats on a high-fat diet became obese neglected various reports that those rats preferentially chose foods high in both fats and carbohydrates such as sweetened condensed milk, cookies, and bananas. They did not eat to excess high-fat, low-carbohydrate foods such as cheese, pastrami, and peanut butter. Rats, pigs, cattle, and monkeys all fatten when fed high-carbohydrate diets.
_
Many successful low-calorie diet observations may be explained by the corresponding decrease in carbohydrate consumption. If the body does not intake enough glucose to fuel the brain, the liver can synthesis ketone molecules and some additional glucose can be synthesized by breaking down proteins and triglycerides.
_
How carbohydrates promote obesity:
Insulin plays the primary role in the fattening process. Carbohydrates – particularly refined ones – are prime suspects in chronic insulin elevation. The movement of fat to and from the fat tissues has little to do with the amount of fat present in the blood. This movement is instead controlled by hormonal factors (such as insulin). Fat storage is a by-product of carbohydrate metabolism (burning glucose for fuel and store excess glucose as glycogen and fat). Insulin is the only hormone that works to promote fat accumulation; eight others promote fat mobilization and carbohydrate intake drives insulin. Fat cells are more insulin-sensitive than other cells. The levels of circulating insulin are proportional to body fat. Additionally, Sugars and carbohydrates have addictive properties.
_
Is sugar toxic?
- Evidence is mounting that sugar is the primary factor causing not just obesity, but also chronic and lethal disease. According to Dr. Robert Lustig, sugar is toxic to your body, acting as a poison all by itself
- Ending the over-consumption of sugar could have a profoundly beneficial impact on disease rates that currently cost the American health care system a trillion dollars per year
- It’s important to realize that when we talk about “sugar,” all sugars are included. So when you’re evaluating your sugar consumption, don’t stop counting once you’ve accounted for the number of spoons of table sugar you’ve added to foods and beverages. Also include all other types of sweeteners found in various processed foods, such as corn-based sweeteners like high fructose corn syrup (HFCS), honey, and agave
___________
What is the purpose of fat in our body?
Fat is known to have two main purposes, says Susan Fried, PhD, director of the Boston Obesity and Nutrition Research Center at Boston University and a long-time researcher in the field.
- Fat stores excess calories in a safe way so you can mobilize the fat stores when you’re hungry.
- Fat releases hormones that control metabolism.
_
What is fat?
Chemically, fats are triglycerides: triesters of glycerol and any of several fatty acids. “Lipids” is used to refer to both liquid and solid fats, along with other related substances, usually in a medical or biochemical context. Fats serve as energy stores for the body, containing about 37.8 kilojoules (9 Kcal) per gram of fat. They are broken down in the body to release glycerol and free fatty acids (FFA). The glycerol can be converted to glucose by the liver and thus used as a source of energy while FFA are broken down into acetyl-CoA and burned to generate energy.
_
Functions of fat:
The body fat percentage of a person or animal is the total weight of fat divided by total body weight; body fat includes essential body fat and storage body fat. Essential body fat is necessary to maintain life and reproductive functions. Essential fat is the level below which physical and physiological health would be negatively affected. The percentage of essential body fat for women is greater than that for men, due to the demands of childbearing and other hormonal functions. The percentage of essential fat is 2–5% in men, and 10–13% in women. Storage body fat consists of fat accumulation in adipose tissue, part of which protects internal organs in the chest and abdomen. The minimum recommended total body fat percentage exceeds the essential fat percentage value reported above. In men minimal fat is 5% while in women it is 8%. Above average body fat in men is between 16 and 25% and among women is between 24 and 32%. Percentage of fat over 25% in men and 32% in women defines risk of disease. A healthy body requires a minimum amount of fat for the proper functioning of the hormonal, reproductive, and immune systems, as thermal insulation, as shock absorption for sensitive areas, and as energy for future use. But the accumulation of too much storage fat can impair movement and flexibility, and can alter the appearance of the body.
_
Body fat percentages for males and females and their classification
| Males | Females | Rating |
| 5-10 | 8-15 | Athletic |
| 11-14 | 16-23 | Good |
| 15-20 | 24-30 | Acceptable |
| 21-24 | 31-36 | Overweight |
| >24 | >37 | Obese |
_
Adipose tissue (AT):
In biology, adipose tissue or body fat or just fat is loose connective tissue composed mostly of adipocytes. In addition to adipocytes, adipose tissue contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and a variety of immune cells [i.e. adipose tissue macrophages (ATMs)]. Adipose tissue is derived from preadipocytes. Its main role is to store energy in the form of lipids, although it also cushions and insulates the body. Far from hormonally inert, adipose tissue has in recent years been recognized as a major endocrine organ, as it produces hormones such as leptin, estrogen, resistin, and the cytokine TNFα. Moreover, adipose tissue can affect other organ systems of the body and may lead to disease. Obesity or being overweight in humans and most animals does not depend on body weight, but on the amount of body fat—to be specific, adipose tissue. The two types of adipose tissue are white adipose tissue (WAT) and brown adipose tissue (BAT). The formation of adipose tissue appears to be controlled in part by the adipose gene. Adipose tissue, more specifically brown adipose tissue, was first identified by the Swiss naturalist Conrad Gessner in 1551. In humans, adipose tissue is located beneath the skin (subcutaneous fat), around internal organs (visceral fat), in bone marrow (yellow bone marrow) and in breast tissue.
_
Brown fat:
A specialized form of adipose tissue in humans, most rodents and small mammals, and some hibernating animals, is brown fat or brown adipose tissue. It is located mainly around the neck and large blood vessels of the thorax. When you are first born, your body does not have much white fat to help insulate and retain body heat; although there are white fat cells, there is not much fat stored in them. Brown fat cells are somewhat smaller than white, are composed of several smaller fat droplets and are loaded with mitochondria, which can generate heat. A newborn baby produces heat (a process called thermogenesis) primarily by breaking down fat molecules into fatty acids in brown fat cells. Instead of those fatty acids leaving the brown fat cell, as happens in white fat cells, they get further broken down in the mitochondria and their energy is released directly as heat. This same process occurs in hibernating animals, which have more brown fat than humans. Once the newborn baby starts eating more, developing layers of white fat, the brown fat goes away. This specialized tissue can generate heat by “uncoupling” the respiratory chain of oxidative phosphorylation within mitochondria. The process of uncoupling means, when protons transit down the electrochemical gradient across the inner mitochondrial membrane, the energy from this process is released as heat rather than being used to generate ATP. This thermogenic process may be vital in neonates exposed to cold, which then require this thermogenesis to keep warm, as they are unable to shiver, or take other actions to keep themselves warm. Beta-3-Adrenergic receptors are found on brown adipocytes, and treatment with b3-selective agonists markedly increases energy expenditure and decreases obesity in rodents. Whether b3-selective agonists will be effective anti-obesity agents in humans is presently under investigation. It’s known that children have more brown fat than adults, and it’s what helps them keep warm. Brown fat stores decline in adults but still help with warmth. Brown fat is now thought to be more like muscle than like white fat. When activated, brown fat burns white fat. Although leaner adults have more brown fat than heavier people, even their brown fat cells are greatly outnumbered by white fat cells. A 150-pound person might have 20 or 30 pounds of fat and they are only going to have 2 or 3 ounces of brown fat. But that 2 ounces if maximally stimulated, could burn off 300 to 500 calories a day — enough to lose up to a pound in a week. Brown fat cells, being the ones that produce heat in the body, are protective against obesity as well as type 2 diabetes.
_
White Fat:
White fat is much more plentiful than brown, experts agree. The job of white fat is to store energy and produce hormones that are then secreted into the bloodstream. Small fat cells produce a “good guy” hormone called adiponectin, which makes the liver and muscles sensitive to the hormone insulin, in the process making us less susceptible to diabetes and heart disease. When people become fat, the production of adiponectin slows down or shuts down, setting them up for disease.
_
Making white fat cells more like brown fat cells:
Scientists from the Perelman School of Medicine found a protein switch that determines whether precursor fat cells become white or brown fat cells. Brown fat cells burn calories, they produce heat. White fat cells store calories in the all-too-obvious deposits that plague the growing numbers of obese people. They wonder whether it might be possible to reprogram white fat cells to become a little more like brown fat cells.
_
Free fatty acids are liberated from lipoproteins by lipoprotein lipase (LPL) and enter the adipocyte, where they are reassembled into triglycerides by esterifying it onto glycerol. Human fat tissue contains about 87% lipids. There is a constant flux of FFA (Free Fatty Acids) entering and leaving adipose tissue. The net direction of this flux is controlled by insulin and leptin – if insulin is elevated there is a net inward flux of FFA and only when insulin is low can FFA leave adipose tissue. Insulin secretion is stimulated by high blood sugar which results from consuming carbohydrates. In humans, lipolysis (hydrolysis of triglycerides into free fatty acids) is controlled through the balanced control of lipolytic B-adrenergic receptors and A-adrenergic receptor-mediated antilipolysis. Fat cells have an important physiological role in maintaining triglyceride and free fatty acid levels, as well as determining insulin resistance. Abdominal fat has a different metabolic profile—being more prone to induce insulin resistance. This explains to a large degree why central obesity is a marker of impaired glucose tolerance and is an independent risk factor for cardiovascular disease (even in the absence of diabetes mellitus and hypertension).
__
Adipose tissue as endocrine organ:
_
_
_
Obesity is characterized by increased storage of fatty acids in an expanded adipose tissue mass and is closely associated with the development of insulin resistance in peripheral tissues such as skeletal muscle and the liver. In addition to being the largest source of fuel in the body, adipose tissue and resident macrophages are also the source of a number of secreted proteins. Cloning of the obese gene and the identification of its product, leptin, was one of the first discoveries of an adipocyte-derived signaling molecule and established an important role for adipose tissue as an endocrine organ. Since then, leptin has been found to have a profound role in the regulation of whole-body metabolism by stimulating energy expenditure, inhibiting food intake and restoring euglycemia, however, in most cases of obesity leptin resistance limits its biological efficacy. In contrast to leptin, adiponectin secretion is often diminished in obesity. Adiponectin acts to increase insulin sensitivity, fatty acid oxidation, as well as energy expenditure and reduces the production of glucose by the liver. Resistin and retinol binding protein-4 are less well described. Their expression levels are positively correlated with adiposity and they are both implicated in the development of insulin resistance. More recently it has been acknowledged that macrophages are an important part of the secretory function of adipose tissue and the main source of inflammatory cyokines, such as TNF alpha and IL-6. An increase in circulating levels of these macrophage-derived factors in obesity leads to a chronic low-grade inflammatory state that has been linked to the development of insulin resistance and diabetes. These proteins commonly known as adipokines are central to the dynamic control of energy metabolism, communicating the nutrient status of the organism with the tissues responsible for controlling both energy intake and expenditure as well as insulin sensitivity.
_
_
Leptin:
In 1995, Jeffrey Friedman, in his residency at Rockefeller University, together with Rudolph Leibel, Douglas Coleman et al. discovered the protein leptin that the genetically obese mouse lacked. Leptin is produced in the white adipose tissue and signals to the hypothalamus. When leptin levels drop, the body interprets this as loss of energy, and hunger increases. Mice lacking this protein eat until they are four times their normal size. Leptin, however, plays a different role in diet-induced obesity in rodents and humans. Because adipocytes produce leptin, leptin levels are elevated in the obese. However, hunger remains, and, when leptin levels drop due to weight loss, hunger increases. The drop of leptin is better viewed as a starvation signal than the rise of leptin as a satiety signal. However, elevated leptin in obesity is known as leptin resistance. The changes that occur in the hypothalamus to result in leptin resistance in obesity are currently the focus of obesity research. Gene defects in the leptin gene (ob) are rare in human obesity.
_
_
_
Ghrelin:
Ghrelin is a 28 amino acid hunger-stimulating peptide and hormone that is produced mainly by P/D1 cells lining the fundus of the human stomach and epsilon cells of the pancreas. Ghrelin levels increase before meals and decrease after meals. It is considered the counterpart of the hormone leptin, produced by adipose tissue, which induces satiation when present at higher levels. In some bariatric procedures, the level of ghrelin is reduced in patients, thus causing satiation before it would normally occur. Ghrelin and synthetic ghrelin mimetics (the growth hormone secretagogues) increase food intake and increase fat mass by an action exerted at the level of the hypothalamus. They activate cells in the arcuate nucleus that include the orexigenic neuropeptide Y (NPY) neurons. Ghrelin-responsiveness of these neurons is both leptin- and insulin-sensitive. Ghrelin also activates the mesolimbic cholinergic-dopaminergic reward link, a circuit that communicates the hedonic and reinforcing aspects of natural rewards, such as food, as well as of addictive drugs, such as ethanol. Indeed, central ghrelin signalling is required for reward from alcohol and palatable/rewarding foods. There is also strong evidence that ghrelin has a peripheral appetite modulatory effect on satiety by affecting the mechanosensitivity of gastric vagal afferents, making them less sensitive to distension resulting in over eating. Ghrelin levels in the plasma of obese individuals are lower than those in leaner individuals, suggesting that ghrelin does not contribute to obesity, except in the cases of Prader-Willi syndrome-induced obesity where high ghrelin levels are correlated with increased food intake. At least one study found that gastric bypass surgery not only reduces the gut’s capacity for food but also dramatically lowers ghrelin levels compared to both lean controls and those that lost weight through dieting alone.
_
Leptin and ghrelin are considered to be complementary in their influence on appetite, with ghrelin produced by the stomach modulating short-term appetitive control (i.e. to eat when the stomach is empty and to stop when the stomach is stretched). Leptin is produced by adipose tissue to signal fat storage reserves in the body, and mediates long-term appetitive controls (i.e. to eat more when fat storages are low and less when fat storages are high). Although administration of leptin may be effective in a small subset of obese individuals who are leptin deficient, most obese individuals are thought to be leptin resistant and have been found to have high levels of leptin. This resistance is thought to explain in part why administration of leptin has not been shown to be effective in suppressing appetite in most obese people. While leptin and ghrelin are produced peripherally, they control appetite through their actions on the central nervous system. In particular, they and other appetite-related hormones act on the hypothalamus, a region of the brain central to the regulation of food intake and energy expenditure. There are several circuits within the hypothalamus that contribute to its role in integrating appetite, the melanocortin pathway being the most well understood. The circuit begins with an area of the hypothalamus, the arcuate nucleus, that has outputs to the lateral hypothalamus (LH) and ventromedial hypothalamus (VMH), the brain’s feeding and satiety centers, respectively. The arcuate nucleus contains two distinct groups of neurons. The first group coexpresses neuropeptide Y (NPY) and agouti-related peptide (AgRP) and has stimulatory inputs to the LH and inhibitory inputs to the VMH. The second group coexpresses pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) and has stimulatory inputs to the VMH and inhibitory inputs to the LH. Consequently, NPY/AgRP neurons stimulate feeding and inhibit satiety, while POMC/CART neurons stimulate satiety and inhibit feeding. Both groups of arcuate nucleus neurons are regulated in part by leptin. Leptin inhibits the NPY/AgRP group while stimulating the POMC/CART group. Thus a deficiency in leptin signaling, either via leptin deficiency or leptin resistance, leads to overfeeding and may account for some genetic and acquired forms of obesity.
_
Ghrelin, leptin and thyroid function:
The stomach releases the peptide ghrelin, which further acts as an appetite stimulator and meal initiation signal. These changes result in increases in peptide Y and agouti-related protein in the arcuate nucleus area, along with the decrease here of alpha-melanocyte stimulating hormone. All this now results in a decrease of melanocortin-4 receptor, accompanied by a decrease in thyrotropin-releasing hormone. These activities also lead to an increase in melanin-concentrating hormone in the lateral hypothalamic area. The result: metabolism slows down and food intake increases. in an attempt to restore the previously burned-off fat. On the other hand, leptin attenuates the decreases in thyroid hormones and 24-h energy expenditure.
_
Insulin and Leptin:
In the 1990s, Lustig worked with children diagnosed with hypothalamic obesity, a disorder that can occur after brain tumor surgery. The children were making more insulin than was necessary for normal energy storage in fat cells. Lustig thought the kids were not receiving signals from leptin, which helps send a message that the appetite has been sated. Lustig concluded that the children’s brains were fooled into thinking that they were starving. Lustig administered a drug called octreotide, known to block insulin release. Insulin levels fell; the children ate less, lost weight, spontaneously became more active and improved their quality of life. Lustig tried the same treatment with obese adults, and found that a subset responded in the same way as the children with hypothalamic obesity. Eating stimulates secretion of insulin and leptin. The conventional view holds that insulin, like leptin, feeds back in the brain to limit food intake, Lustig explains. But Lustig does not think that chronically elevated insulin levels feed back negatively to curb eating. Instead, chronically elevated insulin blocks leptin’s negative feedback signal, Lustig believes. “Most people think insulin does the same thing as leptin,” he says. “I think it does just the opposite.” Lustig believes that fructose generates greater insulin resistance than other foodstuffs, and that fructose calories, therefore, fail to blunt appetite in the same way as other foods.
_
Adiponectin:
Adiponectin is a protein which in humans is encoded by the ADIPOQ gene. It is involved in regulating glucose levels as well as fatty acid breakdown. Adiponectin is a protein hormone that modulates a number of metabolic processes, including glucose regulation and fatty acid oxidation. Adiponectin is exclusively secreted from adipose tissue (and also from the placenta in pregnancy) into the bloodstream and is very abundant in plasma relative to many hormones. Contrary to expectations, despite being produced in adipose tissue, adiponectin was found to be decreased in obesity. This downregulation has not been fully explained. The gene was localised to chromosome 3q27, a region highlighted as affecting genetic susceptibility to type 2 diabetes and obesity. Adiponectin is a 30 kDa protein specifically expressed in adipocytes, plasma levels of which negatively correlate with adiposity, insulin resistance, coronary artery disease and dyslipidemia in both mice and humans. In mice, deletion of adiponectin results in insulin resistance, dyslipidemia and increased neointimal proliferation, whereas overexpression or pharmacological administration of adiponectin improves insulin sensitivity and protects against atherosclerosis. Supplementation by differing forms of adiponectin were able to improve insulin control, blood glucose and triglyceride levels in mouse models. Recently, a protective role for adiponectin in cardiomyopathy was demonstrated: adiponectin deletion enhances cardiac hypertrophy, whereas overexpression attenuates it; furthermore, in vitro, adiponectin modulates hypertrophic signals in cardiomyocytes. Adiponectin also stimulates angiogenesis and is important for recovery from ischaemic injury. Under different conditions, however, adiponectin can also be antiangiogenic. Adiponectin is thought to directly affect a wide variety of target cells, including hepatocytes, myocytes, endothelial cells, macrophages and smooth muscle cells; AMPK has been identified as a key intracellular mediator of adiponectin function. Recently, the notion of a primarily peripheral action of adiponectin has been challenged by the finding that central injection of adiponectin modulates energy expenditure, resulting in decreased body weight. It will be important to determine whether central effects of adiponectin also contribute to its effects on glucose metabolism and cardiovascular function.
_
Adiponectin: action, regulation and association to insulin sensitivity:
Adiponectin is a novel adipocyte-specific protein, which, it has been suggested, plays a role in the development of insulin resistance and atherosclerosis. Although it circulates in high concentrations, adiponectin levels are lower in obese subjects than in lean subjects. Apart from negative correlations with measures of adiposity, adiponectin levels are also reduced in association with insulin resistance and type 2 diabetes. Visceral adiposity has been shown to be an independent negative predictor of adiponectin. Thus, most features of the metabolic syndrome’s negative associations with adiponectin have been shown. Adiponectin levels seem to be reduced prior to the development of type 2 diabetes, and administration of adiponectin has been accompanied by lower plasma glucose levels as well as increased insulin sensitivity. Furthermore, reduced expression of adiponectin has been associated with some degree of insulin resistance in animal studies indicating a role for hypoadiponectinaemia in relation to insulin resistance. The primary mechanisms by which adiponectin enhance insulin sensitivity appears to be through increased fatty acid oxidation and inhibition of hepatic glucose production. Adiponectin levels are increased by thiazoledinedione treatment, and this effect might be important for the enhanced insulin sensitivity induced by thiazolidinediones. In contrast, adiponectin levels are reduced by pro-inflammatory cytokines especially tumour necrosis factor-alpha. In summary, adiponectin in addition to possible anti-inflammatory and anti-atherogenic effects appears to be an insulin enhancer, with potential as a new pharmacologic treatment modality of the metabolic syndrome and type 2 diabetes.
_
_
Obesity and chronic inflammation:
_
_
The adipose tissue secretes a large number of bioactive substances, adipocytokines, which may be involved in a variety of physiologic and pathologic processes. Unbalanced production of pro- and anti-inflammatory adipocytokines seen in visceral fat obesity contributes critically to the development of the metabolic syndrome. Evidence has accumulated indicating that obesity is associated with a state of chronic, low-grade inflammation, suggesting that inflammation may be a potential mechanism, whereby obesity leads to insulin resistance. Indeed, obese adipose tissue is characterized by adipocyte hypertrophy, followed by increased angiogenesis, immune cell infiltration, extracellular matrix overproduction, and thus, increased production of proinflammatory adipocytokines during the progression of chronic inflammation. The dynamic change found in the adipose tissue can be referred to as “adipose tissue remodeling,” in which stromal cells change dramatically in number and cell type during the course of obesity. Among stromal cells, infiltration of macrophages in the adipose tissue precedes the development of insulin resistance in animal models, suggesting that they are crucial for obesity-related adipose tissue inflammation. Researchers have demonstrated that a paracrine loop involving saturated fatty acids and TNF-α derived from adipocytes and macrophages, respectively, aggravates obesity-induced adipose tissue inflammation. Notably, saturated fatty acids, which are released from hypertrophied adipocytes via the macrophage-induced lipolysis, serve as a naturally occurring ligand for TLR4 complex, thereby activating macrophages. Understanding the molecular mechanism underlying adipose tissue remodeling may lead to the identification of novel, therapeutic strategies to prevent or treat obesity-induced adipose tissue inflammation. The adipose tissue communicates with multiple organs or tissues by virtue of a large number of adipocytokines and thus, influences a variety of physiologic and pathophysiologic processes. Obesity may be viewed as a chronic, low-grade inflammatory as well as a metabolic disease; chronic inflammation within the adipose tissue or adipose tissue remodeling results in the dysregulation of adipocytokine production, thereby contributing to the pathophysiology of the metabolic syndrome. Among stromal cells, macrophages should play a critical role in obesity-related adipose tissue inflammation. Understanding the molecular mechanism underlying homeostatic inflammation of obese adipose tissue may lead to novel, therapeutic strategies to prevent or treat obesity-induced adipose tissue inflammation.
_
Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance:
Insulin resistance arises from the inability of insulin to act normally in regulating nutrient metabolism in peripheral tissues. Increasing evidence from human population studies and animal research has established correlative as well as causative links between chronic inflammation and insulin resistance. However, the underlying molecular pathways are largely unknown. In this report, researchers show that many inflammation and macrophage-specific genes are dramatically upregulated in white adipose tissue (WAT) in mouse models of genetic and high-fat diet-induced obesity (DIO). The upregulation is progressively increased in WAT of mice with DIO and precedes a dramatic increase in circulating-insulin level. Upon treatment with rosiglitazone, an insulin-sensitizing drug, these macrophage-originated genes are downregulated. Histologically, there is evidence of significant infiltration of macrophages, but not neutrophils and lymphocytes, into WAT of obese mice, with signs of adipocyte lipolysis and formation of multinucleate giant cells. These data suggest that macrophages in WAT play an active role in morbid obesity and that macrophage-related inflammatory activities may contribute to the pathogenesis of obesity-induced insulin resistance. Authors propose that obesity-related insulin resistance is, at least in part, a chronic inflammatory disease initiated in adipose tissue.
_
The proinflammatory cytokine TNF-α has been demonstrated to mediate insulin resistance as a result of obesity in many rodent obesity models. TNF-α was overexpressed in white adipose tissue (WAT) in obese and insulin-resistant states; mice lacking the TNF-α ligand or the p55 TNF receptor were partially protected from obesity-induced insulin resistance. Recently, the chemokine monocyte chemotactic protein-1 (MCP-1) was also shown to impair adipocyte insulin sensitivity. In recent years, a large number of human population studies have linked insulin resistance to systemic inflammation. For example, in one recent report, the acute-phase response was studied in Caucasian subjects with T2DM. There was a significant graded increase of serum sialic acid, a marker of the acute-phase response; α-1 acid glycoprotein; IL-6; and urinary albumin-excretion rate among three groups, with the lowest levels in nondiabetic subjects, intermediate levels in T2DM patients without metabolic syndrome, and the highest levels in T2DM patients with metabolic syndrome. In a larger study, the relation of C-reactive protein (CRP), fibrinogen, and white cell count to components of insulin resistance syndrome was evaluated in the nondiabetic population of the Insulin Resistance Atherosclerosis Study (n = 1,008). CRP, a predictor of cardiovascular events in previous reports, was found to be independently related to insulin insensitivity. Salicylates, including sodium salicylate and aspirin, are used to treat inflammatory conditions such as rheumatic fever and rheumatoid arthritis. Historically, it has also been known that high doses of salicylates are able to lower blood glucose concentrations.
_
Macrophage accumulation is likely a direct response to the abnormal fat metabolism caused by the increasing adiposity. Adipocytes are known to secrete hormones, cytokines, and FFAs, most of which have been shown to play some role in inflammation and systemic insulin resistance. Adiponectin can inhibit adhesion of macrophages to endothelial cells, an essential process in the pathogenesis of atherosclerosis. The decrease in adiponectin therefore might have contributed to the increased macrophage activity in obese adipose tissue. Leptin, which increases in proportion to fat mass, promotes cholesterol ester synthesis in macrophages in a hyperglycemic environment, an important process in the formation of foam cells in atherosclerosis . This would suggest that lack of leptin signaling in the ob/ob and db/db models might have some protective effect against the inflammatory response. Once macrophage activation and infiltration is initiated, it is reasonable to expect that this macrophage-mediated inflammatory response would lead to impaired insulin response in adipocytes. Macrophages, upon activation, secrete numerous cytokines and chemokines, such as TNF-α, IL-1, IL-6, and MCP-1, that are known to cause insulin resistance in adipocytes. These cytokines and chemokines further activate macrophages to increase lymphokine production and secretion. These feedback loops are schematically summarized in the figure below. As a consequence, insulin signaling in adipocytes could become increasingly impaired, eventually leading to massive adipocyte lipolysis, necrosis, and systemic insulin resistance.
_
The figure above shows hypothetical model of chronic inflammation and adipocyte insulin resistance. When adiposity reaches a certain threshold, factors derived from adipocytes induce macrophage activation and infiltration. Activated macrophages secrete cytokines that can impair adipocyte insulin sensitivity and stimulate further activation and infiltration of peripheral monocytes and macrophages into fat. Preadipocytes can also secrete chemokines under the stimulation of TNF-α, which can contribute to macrophage infiltration. These amplifying signals increasingly impair adipocyte insulin signaling and eventually cause systemic insulin resistance.
_
The figure below shows how oxidative stress leads to metabolic syndrome:
_
Hypertrophy versus hyperplasia?
One amazing fact is that fat cells generally do not generate after puberty — as your body stores more fat, the number of fat cells remains the same. Each fat cell simply gets bigger! (There are two exceptions: the body might produce more fat cells if an adult gains a significant amount of weight or has liposuction performed.)
_
Life span of adipocyte:
Two distinct mechanisms can lead to increased AT size: hypertrophy (an increase in adipocyte volume) or hyperplasia (an increase in adipocyte cell number). Although we recognize that adipocyte hypertrophy prevails in obesity, there remains some debate as to whether the adipocyte number remains constant in an adult individual, or whether the ability to undergo hyperplasia is age dependent. Key questions to take into consideration are: what is the actual half-life of an adipocyte? Can certain processes alter the rate of adipocyte turnover? And if so, is the adipocyte indeed programmed to survive for a certain period of time, or can its fate be derailed under extreme metabolic oscillations? Using incorporation of environmental 14C as a tracer, Spalding and colleagues documented that “new adipocytes form constantly to replace lost adipocytes” and estimated the half-life of the average adipocyte to be in the order of 8.3 years. This group postulated that adipocyte cell number is relatively fixed by early adulthood, and that any alterations in fat mass during adulthood are merely credited to alterations in adipocyte hypertrophy. The rate of appearance of newly emerging adipocytes is balanced by adipocyte death, with the total number of adipocytes being tightly controlled, while the whole system is in a state of constant flux. This hypothesis suggests that adipocyte progenitor cells are either recruited into the stromal vascular fraction of adult AT or propagated as precursor cells into mature AT, thus allowing them to differentiate into lipid-laden mature adipocytes at the same rate that existing adipocytes undergo cell death. These studies, however, raise further questions: given that obesity is associated with a higher adipocyte turnover due to an elevated rate of cell death, are there conditions in which a higher rate of preadipocyte recruitment, rather than loss of mature adipocytes through cell death, can occur? Can we ever tip the balance to have an uneven “apoptotic-to-adipogenic ratio” and what would be the metabolic consequences? Moreover, what are the signals that trigger an influx of preadipocytes and enhanced recruitment of preadipocytes towards the adipogenic axis? One plausible mechanism may be that once adipocytes reach a critical volume, they secrete factors that recruit new adipocytes, however the details of these processes are far from understood.
_______________
Obesity and brain:
When you eat, you eat because the brain tells you that you need more energy. Now, normally when you have got enough energy stored, then your brain says `it’s enough’ and the way it does this is by creating satiety. However, some obese people did not have properly functioning dopamine receptors, which meant they had to eat more food before their brains told them they were full. By gaining an understanding of this difference the researchers could help discover new weight-loss treatments for obese people.
_
_
Appetite regulation:
The figure below shows various mechanisms of appetite regulation and food intake:
_
Gut-brain connection:
_
_
In addition to the obvious role of the gut in the digestion and absorption of nutrients, the intestine and associated visceral organs, including the pancreas, liver, and visceral adipose depots, have important sensing and signaling roles in the regulation of energy homeostasis. To accomplish this role, the gut uses neural and endocrine pathways to communicate with controllers of energy balance in the hypothalamus and hindbrain. The brain integrates long-term energy balance. Peripheral signals relating to long-term energy stores are produced by adipose tissue (leptin) and the pancreas (insulin). Feedback relating to recent nutritional state takes the form of absorbed nutrients, neuronal signals, and gut peptides. Neuronal pathways, primarily by way of the vagus nerve, relate information about stomach distention and chemical and hormonal milieu in the upper small bowel to the NTS within the dorsal vagal complex (DVC). Hormones released by the gut have incretin-, hunger-, and satiety stimulating actions. The incretin hormones GLP-1, GIP, and potentially OXM improve the response of the endocrine pancreas to absorbed nutrients. GLP-1 and OXM also reduce food intake. Ghrelin is released by the stomach and stimulates appetite. Gut hormones stimulating satiety include CCK released from the gut to feedback by way of vagus nerves. OXM and PYY are released from the lower gastrointestinal tract and PP is released from the islets of Langerhans. A new understanding of the role of the gut in obesity and energy balance has recently developed. The list of peptide hormones emanating from the gastrointestinal system and influencing energy balance continues to grow, and it seems likely that additional gut hormones will be identified. Although loss-of function studies indicate a high degree of redundancy, this does not preclude the potential efficacy of obesity-regulating drugs acting on these targets. Indeed, the likely introduction of GLP-1 agonists as diabetes treatments with weight loss potential heralds a new era of antiobesity therapy. Moreover, the apparent importance of alterations in the gut hormonal milieu caused by surgical intervention on the gastrointestinal tract could lead to new approaches to surgery or devices. Of course, many unanswered questions remain. Among these are the possible roles of gut pathways in the genetic etiology of obesity and diabetes, a better understanding of the receptors and signals that sense specific nutrients in the gut, and a better understanding of the hierarchy and interactions between different gut signals and between gut signals and those related to long-term energy stores, such as leptin.
_
The lines of communication between the gastrointestinal (GI) tract and central nervous system (CNS) form a key component in a recently established model of appetite regulation. This gut-brain axis has both neural and humoral components that relay information to important CNS centers, including the hypothalamus and the brainstem. These CNS structures have extensive reciprocal connections and both receive neuronal input from the periphery, with the brainstem-vagus nerve complex being of particular significance in the control of feeding. Neuronal activity in hypothalamic and brainstem nuclei is susceptible to influence by circulating hormones. In the hypothalamic arcuate nucleus (Arc), signals from the periphery result in changes in the relative activity of two subpopulations of neurons: an orexigenic population co-expressing the neurotransmitters neuropeptide Y and agouti-related peptide and an anorexigenic population co-expressing pro-opiomelanocortin and cocaine- and amphetamine-regulated transcript. Alterations in the release of these neuropeptides affect feeding behavior and energy expenditure, resulting in the maintenance of energy homeostasis. The GI-pancreatic complex is the largest endocrine organ in the body and a source of important regulatory peptides. Cholecystokinin was the first to be implicated in the short-term control of food intake, and other appetite-regulating hormones have subsequently been characterized. Of these, ghrelin is the only known orexigenic gut hormone, whereas a number of satiety factors exist, including glucagon-like peptide (GLP)-1, oxyntomodulin (OXM), peptide YY (PYY), and pancreatic polypeptide (PP). Unlike leptin, which is thought to signal longer-term energy status, these gut hormones appear to act as meal initiators and terminators. Alterations in levels of gut hormones after bariatric surgery may contribute to the appetite suppression and sustained weight loss seen in patients undergoing this procedure and supports the development of these hormones as therapeutic targets.
_
The figure above is a schematic diagram of the gastrointestinal tract illustrating where particular gut hormones are concentrated and their major putative functions. The gastrointestinal tract releases a number of hormones, including ghrelin and gastrin from the stomach, insulin, glucagon, pancreatic polypeptide and amylin from the pancreas, cholecystokinin, secretin, GIP and motilin from the small intestine, and GLP-1, GLP-2, oxyntomodulin and PYY3–36 from the large intestine. These hormones signal to the periphery and to the central nervous system to regulate a number of biological processes. The recognition that several gastrointestinal hormones, released in response to nutritional stimuli are important regulators of appetite (PYY, oxyntomodulin, GLP-1 and decreased secretion of ghrelin), offers a strategy for the development of more effective anti-obesity agents….treatment of obesity could involved a combination of hormones, e.g. GLP-1, PYY, and/or oxyntomodulin to produce a superior appetite suppressing hormone profile that may result in a weight loss far exceeding that seen in single-agent trials. Using several hormones in low doses and incorporating lifestyle interventions might maximize the clinical effect while minimizing the side effects.
_
_
_
Gut Microbiota and its possible relationship with Obesity:
Obesity results from alterations in the body’s regulation of energy intake, expenditure, and storage. Recent evidence, primarily from investigations in animal models, suggests that the gut microbiota affects nutrient acquisition and energy regulation. Its composition has also been shown to differ in lean vs. obese animals and humans. In an article, researchers review the published evidence supporting the potential role of the gut microbiota in the development of obesity and explore the role that modifying the gut microbiota may play in its future treatment. Evidence suggests that the metabolic activities of the gut microbiota facilitate the extraction of calories from ingested dietary substances and help to store these calories in host adipose tissue for later use. Furthermore, the gut bacterial flora of obese mice and humans include fewer Bacteroidetes and correspondingly more Firmicutes than that of their lean counterparts, suggesting that differences in caloric extraction of ingested food substances may be due to the composition of the gut microbiota. Bacterial lipopolysaccharide derived from the intestinal microbiota may act as a triggering factor linking inflammation to high-fat diet-induced metabolic syndrome. Interactions among microorganisms in the gut appear to have an important role in host energy homeostasis, with hydrogen-oxidizing methanogens enhancing the metabolism of fermentative bacteria. Existing evidence warrants further investigation of the microbial ecology of the human gut and points to modification of the gut microbiota as one means to treat people who are overweight or obese.
_
Are there any innate mechanisms to prevent weight gain or weight loss?
Yes, enzyme FBPase and neuropeptide Y:
_
Liver regulates fat by talking to brain:
Researchers from the University of Melbourne and Austin Health have come one step closer to understanding how our bodies regulate fat and weight gain. Dr Barbara Fam from the University’s Molecular Obesity Laboratory group at Austin Health with Associate Professor Sof Andrikopoulos have discovered that the liver can directly talk to the brain to control the amount of food we eat. The results have demonstrated that the liver, which has never been classed as an important organ in controlling body weight before, is in fact a major player and should be considered a target for treatment of weight gain. Test on mice showed that over-expression of a specific enzyme in the liver resulted in 50% less fat and the subjects ate less food than mice without the extra enzyme. Needed in the production of glucose, the enzyme is called FBPase. The really striking result was that the genes in the brain, important in making us increase our food intake were actually reduced. The results suggest that consumption of a diet high in fat causes increase in liver FBPase that was likely put in place as a negative feedback mechanism to limit further weight gain. Importantly, FBPase does not function to control body weight under normal physiological circumstances but acts only when the system is exposed to excess nutrients such as fat. When people eat diets loaded with fat and sugars particularly over the long term, it can have a number of different effects on the body but it appears that we actually have in place an innate system that protects us from any further weight gain that could happen while eating these types of diets.
_
The brain circuit that makes it hard for obese people to lose weight:
That’s what happens when obese people diet – the less food they eat, the less energy they burn, and the less weight they lose. Dr Shu Lin, Dr Yanchuan Shi and Professor Herbert Herzog and his team have been studying the complex processes behind energy balance using various mouse models. They have shown that the neurotransmitter Neuropeptide Y (NPY), known for stimulating appetite, also plays a major role in controlling whether the body burns or conserves energy. The researchers found that NPY produced in a particular region of the brain – the arcuate nucleus (Arc) of the hypothalamus – inhibits the activation of ‘brown fat’, one of the primary tissues where the body generates heat.“This study is the first to identify the neurotransmitters and neural pathways that carry signals generated by NPY in the brain to brown fat cells in the body. It is also the first to show a direct connection between Arc NPY, the sympathetic nervous system and the control of energy expenditure.” said Professor Herzog. “We know that NPY also influences other aspects of the sympathetic nervous system – such as heart rate and gut function – but its control of heat generation through brown fat seems to be the most critical factor in the control of energy expenditure.” “When you don’t eat, or dramatically curtail your calorie intake, levels of NPY rise sharply. High levels of NPY signal to the body that it is in ‘starvation mode’ and should try to replenish and conserve as much energy as possible. As a result, the body reduces processes that are not absolutely necessary for survival.” “Evolution has provided us with these mechanisms to help us survive famine, and they are strictly controlled. When people had to survive by finding food or hunting game, they could not afford to run out of energy and die of exhaustion, so their bodies evolved to cope.” “Until the twentieth century, there were no fast food chains and people did not have ready access to high fat, high sugar, foods. So in evolutionary terms, it was unlikely that people were going to get very fat and mechanisms were only put in place to prevent you losing weight.” “Obesity is a modern epidemic, and the challenge will be to find ways of tricking the body into losing weight – and that will mean somehow circumventing or manipulating this NPY circuit, probably with drugs.”
__________
Obesity as food addiction:
On average a physically active man needs around 2,500 calories per day, while a woman needs 2,000. If we eat any more, the extra energy is stored for later use, mostly as fat. This mechanism was life-saving during our hunter-gatherer days when food was often scarce. However, the boom in plentiful, cheap food, coupled with a general decrease in physical activity, means that those stores of fat are rarely called on. Instead they continue to grow. So why don’t people just stop eating foods high in fat and sugar if they know they can cause physical problems? Scientists are still searching for the answers, but it appears that our brains have been wired to encourage the consumption of calorie-rich foods, even at the expense of good health. Quite simply, these foods bring us pleasure.
_
Could candy be addictive, like cocaine or nicotine? Until recently, many believed overeating and obesity were caused by a lack of “willpower”. But new research suggests that certain diets — those high in fat and sugar — can lead to changes in the brain that are similar to those seen with drug addiction. These findings are changing our understanding of what may drive overeating. Scientists are finding high-fat, high-sugar foods can trigger lasting brain changes that might make it difficult to resist overeating. Furthermore, those changes resemble what happens in the brain when someone is addicted to drugs, such as nicotine, alcohol, and cocaine. One of the central players in the regulation of appetite is the hormone leptin. Produced in the body’s fat cells, leptin lets the brain know when there is enough energy stored in those cells. It tells the brain when you can stop eating, at least for a while. Some evidence suggests the brains of obese individuals are less sensitive to the hormone. In extremely rare cases of obesity — those in which the body produces no leptin because of a genetic mutation — overeating was curtailed and weight was lost after leptin injections. Scientists learned early on that leptin acted in the hypothalamus, a brain structure involved in the regulation and control of hunger. More recently, they discovered leptin also influences appetite by acting in a midbrain region known as the ventral tegmental area (VTA). This region contains dopamine neurons, which play a key role in the brain’s reward system — the source of the feeling of pleasure we get when we eat certain foods — and in the development of drug addictions.
_
High GI food leads to stimulation of nucleus accumbens of dopamine reward system:
A study shows that food can trigger addictive behaviors just like drugs can. Dr. David Ludwig, director of New Balance Foundation Obesity Center at Boston’s Children’s Hospital studied 12 obese men after they drank two milk shakes. Each shake contained the same calories and sugar levels, but one had a significantly higher glycemic index from the carbohydrates. The higher glycemic shake resulted in spiked blood sugar levels as expected. Then they crashed hours later and felt the strong pang of hunger. By scanning the men’s brains, Ludwig was able to determine the higher glycemic shakes sparked the nucleus accumbens, which is also triggered by drugs and addictive behaviors like gambling. The study is published in the American Journal of Clinical Nutrition. While they studied only men and the sample size was small, they used a gold-standard “randomized, blinded, crossover design” and the results were large enough to overcome the sample size. One milkshake rapidly raised glucose levels in the blood (high-GI) while the other (low-GI) did not. GI is glycemic index and processed foods tend to be high GI while whole foods tend to be low GI. Processed junk foods shoot quick jolts of glucose into the bloodstream. The results are clear: you consume processed high-GI food and by the time you are ready for your next meal your blood sugar will have dropped as a consequence of the initial jolt, you are feeling hungrier, and the pleasure/reward/craving center in your brain is firing like mad. Apparently, your brain is adapting to the lowered glucose levels in your blood by getting you ready to consume more, and then more and more. How hungry you are at dinner depends not just on how much you ate for lunch but on what you ate for lunch. The same calories at the junk food drive-thru will prime you for a bigger dinner than had you been able to eat a lunch-time meal that avoided industrialized calorie-delivery systems. What you eat matters.
_
Overlapping neuronal circuits in drug addiction and food addiction (obesity):
_
_
Drugs and food exert their reinforcing effects in part by increasing dopamine (DA) in limbic regions, which has generated interest in understanding how drug abuse/addiction relates to obesity. Here, researchers integrate findings from positron emission tomography imaging studies on DA’s role in drug abuse/addiction and in obesity and propose a common model for these two conditions. Both in abuse/addiction and in obesity, there is an enhanced value of one type of reinforcer (drugs and food, respectively) at the expense of other reinforcers, which is a consequence of conditioned learning and resetting of reward thresholds secondary to repeated stimulation by drugs (abuse/addiction) and by large quantities of palatable food (obesity) in vulnerable individuals (i.e. genetic factors). In this model, during exposure to the reinforcer or to conditioned cues, the expected reward (processed by memory circuits) overactivates the reward and motivation circuits while inhibiting the cognitive control circuit, resulting in an inability to inhibit the drive to consume the drug or food despite attempts to do so. These neuronal circuits, which are modulated by DA, interact with one another so that disruption in one circuit can be buffered by another, which highlights the need of multiprong approaches in the treatment of addiction and obesity.
_
_
Researchers have used PET to evaluate these conditioned responses in healthy controls. They hypothesize that food cues would increase extracellular DA in striatum and that these increases would predict the desire for food. Food-deprived subjects were studied while stimulated with a neutral or food-related stimulus (conditioned cues). To amplify the DA changes, they pretreated the subjects with MP (20 mg orally), a stimulant drug that blocks DA transporters (the main mechanism for the removal of extracellular DA; Giros et al. 1996). Food stimulation significantly increased DA in striatum and these increases correlated with the increases in self reports of hunger and desire for food as seen in figure above (Volkow et al. 2002b;). Similar findings were reported when food cues were presented to healthy controls without pretreatment with MP. These findings corroborate the involvement of striatal DA signaling in conditioned responses to food and the participation of this pathway in food motivation in humans. Since these responses were obtained when subjects did not consume the food, this identifies these responses as distinct from the role of DA in regulating reward through NAc.
_
Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats:
Researchers found that development of obesity was coupled with emergence of a progressively worsening deficit in neural reward responses. Similar changes in reward homeostasis induced by cocaine or heroin are considered to be crucial in triggering the transition from casual to compulsive drug-taking. Accordingly, they detected compulsive-like feeding behavior in obese but not lean rats, measured as palatable food consumption that was resistant to disruption by an aversive conditioned stimulus. Striatal dopamine D2 receptors (D2Rs) were downregulated in obese rats, as has been reported in humans addicted to drugs. Moreover, lentivirus-mediated knockdown of striatal D2Rs rapidly accelerated the development of addiction-like reward deficits and the onset of compulsive-like food seeking in rats with extended access to palatable high-fat food. These data demonstrate that overconsumption of palatable food triggers addiction-like neuroadaptive responses in brain reward circuits and drives the development of compulsive eating. Common hedonic mechanisms may therefore underlie obesity and drug addiction.
_
Dopamine reward and junk food: [junk food vide infra]
The research by Johnson and Kenny examined whether exposure to the kind of high-fat, super high-calorie foods that floods the junk-food market are responsible for creating food-addicts in a similar way to drugs that alter the brain in ways that make stopping more difficult. The study took three groups of rats and gave them either the regular chow diet lab animals are used to or the worse kind of birthday party food: bacon, sausage, cheesecake, pound cake, frosting and chocolate. You can imagine the party going on in the rat cages that got to eat that! Of the two groups that got to eat the crazy-fat food, one had unlimited access while the other got to binge for only one hour a day. The bottom line: Only the rats that got unlimited access to the fat-party food developed compulsive eating habits that resulted in roughly twice the weight gain of the other two groups and the ability to continue eating even in the face of signals for punishment (a light that they were trained to associate with shocks). When the researchers looked deeper, they found that the brains of these rats suffered a significant reduction in the density of a specific kind of dopamine receptor (D2) in a brain part known as the striatum, the same kind of reduction common in drug addicted people and obese individuals. This receptor type is often thought to be important for regulation of impulses, both physical and otherwise. It therefore makes sense that losing this type of function would cause uncontrollable eating or drug taking. While this research isn’t saying that compulsive eating, or obesity, are the same as drug addiction, it does strongly suggest that there are common mechanisms in both. More importantly, it reveals a common process that unfolds when over-exposure to the reward, in this case food, occurs. This tells us that there can likely be common pathways to these different addictive disorders, though whether any specific person ended up a food- or drug-addict because of this kind of process is still an open question.
_
In other research, scientists discovered another similarity with drug addiction. When rats fed junk food were suddenly switched back to a healthier diet, they avoided anxiety-inducing situations — just as they do when going through withdrawal from cocaine and other addictive drugs. Tests showed these rats had five times the normal amount of corticotropin-releasing factor (CRF) mRNA in their brains during this denial period. CRF helps regulate fear, anxiety, and stress. Once the rats were put back on a junk food diet, they no longer avoided stressful situations, and their CRF levels returned to normal. They also began to overeat even more than before — a finding that may suggest why people who go on and off diets tend to find it increasingly difficult to lose weight.
_
Through other intriguing research, scientists discovered that a junk food diet can alter the production of dopamine and other genes in the brain’s reward system — even after the diet is abandoned for a healthier one. Studies also found these brain changes can be passed on from pregnant mice to their offspring, making the mice pups more vulnerable to both obesity and addictive-like behaviors in adulthood. Such findings may help explain why overcoming obesity is much more complicated than “saying no” to junk food and may point to possible interventions to help people struggling to make positive behavioral change.
_
Pleasure eating triggers body’s reward system and may stimulate overeating: Hedonic hunger and obesity:
When eating is motivated by pleasure, rather than hunger, endogenous rewarding chemical signals are activated which can lead to overeating, according to a recent study in The Endocrine Society’s Journal of Clinical Endocrinology & Metabolism (JCEM). The phenomenon ultimately affects body mass and may be a factor in the continuing rise of obesity. Hedonic hunger refers to the desire to eat for pleasure, and to enjoy the taste, rather than to restore the body’s energy needs. For example, desiring and eating a piece of cake even after a satiating meal is consumption driven by pleasure and not by energy deprivation. The physiological process underlying hedonic eating is not fully understood, but it is likely that endogenous substances regulating reward mechanisms like the hormone ghrelin and chemical compounds such as 2-arachidonoylglycerol (2-AG) are involved. In this study, researchers assessed eight satiated healthy adults, aged 21–33 years, feeding them each their personal favorite food and, later, a less-palatable food of equal caloric and nutrient value. Researchers periodically measured 2-AG and ghrelin levels. The plasma levels of ghrelin and 2-AG increased during hedonic eating, with the favorite foods, but not with non-hedonic eating. This increase suggests an activation of the chemical reward system, which overrides the body’s signal that enough has been eaten to restore energy. Hedonic hunger may powerfully stimulate overeating in an environment where highly palatable foods are omnipresent, and contribute to the surge in obesity. Understanding the physiological mechanisms underlying this eating behaviour may shed some light on the obesity epidemic. Further research should confirm and extend our results to patients with obesity or with other eating disorders in order to better understand the phenomenon of hedonic eating.
_
Addictive Genes and Obesity:
Our brains are programmed to activate the pleasure centers of the brain on visualizing food and then eating it, to remember the food that was eaten in specialized memory neurons, and to activate our digestive processes including chewing and hormone secretion in preparation for digesting a delicious meal. Eating food is a life-giving event in the wild and so it is perfectly reasonable that this activity would be highly valued within our inherited biological control mechanisms. However, when the stimuli are linked in susceptible individuals to increase in reward circuitry activity as detected by changes in dopamine and glucose metabolism, then neuronal plasticity could be altered in ways that both increase the tendency to eating disorders and obesity as well as drug addiction. There is increasing evidence that the same brain reward circuits involved in perpetuating drug abuse are involved in the hedonic urges and food cravings observed clinically in overweight and obese subjects. A polymorphism of the D2 dopamine receptor which renders it less sensitive to dopamine stimulation has been proposed to promote self-stimulatory behavior such as consuming alcohol, abusing drugs, or binging on foods. It is important to determine how this polymorphism may interact with other well-known candidate genes for obesity including polymorphisms of the leptin receptor gene and the opiomelanocortin gene.
______________
_
The figure below shows factors affecting weight:
Body weight is the result of genes, metabolism, behavior, environment, culture, and socioeconomic status. Behavior and environment play a large role causing people to be overweight and obese. These are the greatest areas for prevention and treatment actions.
_
Causes of obesity:
_
_
At an individual level, a combination of excessive food energy intake and a lack of physical activity is thought to explain most cases of obesity. A limited number of cases are due primarily to genetics, medical reasons, or psychiatric illness. In contrast, increasing rates of obesity at a societal level are felt to be due to an easily accessible and palatable diet, increased reliance on cars, and mechanized manufacturing. There are many complex behavioral and societal factors that combine to contribute to the causes of obesity. The Foresight report (2007) referred to a “complex web of societal and biological factors that have, in recent decades, exposed our inherent human vulnerability to weight gain”. The report presented an obesity system map with energy balance at its centre. Around this, over 100 variables directly or indirectly influence energy balance.
_
•Biology: an individual’s starting point – the influence of genetics and ill health;
•Activity environment: the influence of the environment on an individual’s activity behavior, for example a decision to cycle to work may be influenced by road safety, air pollution or provision of a cycle shelter and showers;
•Physical Activity: the type, frequency and intensity of activities an individual carries out, such as cycling vigorously to work every day;
•Societal influences: the impact of society, for example the influence of the media, education, peer pressure or culture;
•Individual psychology: for example a person’s individual psychological drive for particular foods and consumption patterns, or physical activity patterns or preferences;
•Food environment: the influence of the food environment on an individual’s food choices, for example a decision to eat more fruit and vegetables may be influenced by the availability and quality of fruit and vegetables near home;
•Food consumption: the quality, quantity (portion sizes) and frequency (snacking patterns) of an individual’s diet.
_
_
Besides increased food energy intake and lack of physical activities, a 2006 review identified ten other possible contributors to the recent increase of obesity:
(1) Insufficient sleep,
(2) Endocrine disruptors (environmental pollutants that interfere with lipid metabolism),
(3) Decreased variability in ambient temperature,
(4) Decreased rates of smoking, because smoking suppresses appetite,
(5) Increased use of medications that can cause weight gain (e.g., atypical antipsychotics),
(6) Proportional increases in ethnic and age groups that tend to be heavier,
(7) Pregnancy at a later age (which may cause susceptibility to obesity in children),
(8) Epigenetic risk factors passed on generationally,
(9) Natural selection for higher BMI, and
(10) Assortative mating leading to increased concentration of obesity risk factors (this would increase the number of obese people by increasing population variance in weight).
_
Diet:
_
The figure below shows that diet energy per person per day increased significantly since 1961 to 2003 worldwide.
_
The per capita dietary energy supply varies markedly between different regions and countries. It has also changed significantly over time. From the early 1970s to the late 1990s the average calories available per person per day (the amount of food bought) increased in all parts of the world except Eastern Europe. The United States had the highest availability with 3,654 calories per person in 1996. This increased further in 2003 to 3,754. During the late 1990s Europeans had 3,394 calories per person, in the developing areas of Asia there were 2,648 calories per person, and in sub-Saharan Africa people had 2,176 calories per person. Total calorie consumption has been found to be related to obesity. The widespread availability of nutritional guidelines has done little to address the problems of overeating and poor dietary choice. From 1971 to 2000, obesity rates in the United States increased from 14.5% to 30.9%. During the same period, an increase occurred in the average amount of food energy consumed. For women, the average increase was 335 calories per day (1,542 calories in 1971 and 1,877 calories in 2004), while for men the average increase was 168 calories per day (2,450 calories in 1971 and 2,618 calories in 2004). Most of this extra food energy came from an increase in carbohydrate consumption rather than fat consumption. The primary sources of these extra carbohydrates are sweetened beverages, which now account for almost 25 percent of daily food energy in young adults in America, and potato chips. Consumption of sweetened drinks is believed to be contributing to the rising rates of obesity. As societies become increasingly reliant on energy-dense, big-portions, and fast-food meals, the association between fast-food consumption and obesity becomes more concerning. In the United States consumption of fast-food meals tripled and food energy intake from these meals quadrupled between 1977 and 1995. Agricultural policy and techniques in the United States and Europe have led to lower food prices. In the United States, subsidization of corn, soy, wheat, and rice through the U.S. farm bill has made the main sources of processed food cheap compared to fruits and vegetables.
_
Eating Habits:
We need to eat a certain way to maintain a healthy weight. But what is the perfect way? Nobody really knows, but there are some eating patterns which are clearly not good for weight control. So your eating habits may be contributing to your weight problem. There are a number of eating styles, which are not compatible with good weight control. Some of these are:
•Fasting and feasting: Poor (low) calorie intake for most of the day followed by hunger (and sometimes excessive or even unbearable hunger) and then catches up eating, that never seems to satisfy. Some people, knowing that calories increase your weight try skipping meals. Then they start eating later in the day and then don’t seem to be able to stop eating. They are “fasting and feasting”. This eating style is terrible for weight control.
•Quick eaters: The speed of eating is closely tied to weight control. People who eat their food quickly are more likely to be obese. This makes sense when you think of fullness. It stakes around 10 to 20 minutes to feel full, no matter what you have eaten. If in that time you have eaten a large meal and gone back for seconds or thirds and then had desert, you will have eaten much more than the person who is still tucking into their first course. Also, you are less likely to remember what or how much you have eaten if it goes down very quickly. If food tastes really nice, and we want more, we are likely to eat less and therefore less calories if we savour every mouthful and eat slowly. So eating slowly is like a substitute for having more.
•Emotional Eaters: Basically eating is associated with various emotions. We are emotional creatures, but if emotions are connected strongly to eating this makes weight control more challenging.
•Pleasure Eaters: It can be hard to limit eating when it is so pleasurable!
•Habitual Eaters: This is eating the same food over and over again, because (so you think) you are “addicted” to it. What is really happening here is that a well-worn habit of eating a certain food is giving you an excess of that food, and of course the calories that go with it. It can be very hard to break a food habit! It also refers to people who eat for reasons that have nothing to do with hunger, such as having a cake with a cuppa, just because…
•Non-Pragmatic Eaters: Pragmatic eaters eat only those foods that they have calculated their body needs. E.g. “I need to eat more protein today, because I went to the gym and did weight training.”, “I haven’t eaten my serve of fruit today. I’ll have an apple.” These people are very unlikely to have a weight problem. We should all eat like this to a certain extent. Non-pragmatic eaters never think like this and are more likely to struggle with their weight.
_
Fast food and junk food invasion:
There has been an “invasion” of children’s diets by soft drinks, fast food, and high-calorie poor-quality snacks, with per capita soda consumption having increased fivefold over the past 50 years and now comprising >10% of caloric intake for the average adolescent. Fast food has increased from 2 to 15% of calories in children’s diets in all aspects of society since the 1970s, leading to food high in refined carbohydrates, high in trans fatty acids, low in fiber, and low in vitamins. In a study comparing the daily ingestion of 1,150 ml sugar versus artificially sweetened drinks, the latter decreased calorie intake. Over 10 weeks, sugar-sweetened diets led to a 5-lb weight gain. Epidemiologic analysis suggests that each additional serving of sugar-sweetened drink per day increases the risk of obesity by 60%. So a high intake of calorically sweetened beverages can be regarded as a determinant for obesity. In diet recall studies of 6,000 children ages 4–19 from 1994 to 1998, one-third ate “fast food” on any given day, in association with caloric intake of 2,236 cal, as opposed to that of 2,049 cal on days without such food products. Fast food eaters have higher BMI and greater risk of developing features of the insulin resistance syndrome. Provision of larger portions leads, not unsurprisingly, to greater calorie intake, suggesting the adverse effect of the common sales technique of “supersizing,” where for a small additional fee the purchaser can increase caloric intake from, for example, 150 to 600 cal for typical beverages. Worryingly, the proportion of children eating dinner with their families declines with age and has decreased over time, although such meals are associated with healthier nutrient intake.
_
Junk food: [junk food as addiction discussed vide supra]
Junk food is a derisive slang term for food that is of little nutritional value and often high in fat, sugar, salt, and calories. Junk foods typically contain high levels of calories from sugar or fat with little protein, vitamins or minerals. Foods commonly considered junk foods include salted snack foods, gum, candy, sweet desserts, fried fast food, and sugary carbonated beverages. Many foods such as hamburgers, pizza, and tacos can be considered either healthy or junk food depending on their ingredients and preparation methods with the more highly processed items usually falling under the junk food category. What is and is not junk food can also depend on the person’s class and social status, with wealthier people tending to have a broader definition while lower-income consumers may see fewer foods as junk food, especially certain ethnic foods. A study by Paul Johnson and Paul Kenny at the Scripps Research Institute in 2008 suggested that junk food consumption alters brain activity in a manner similar to addictive drugs like cocaine or heroin. After many weeks with unlimited access to junk food, the pleasure centers of rat brains became desensitized, requiring more food for pleasure. After the junk food was taken away and replaced with a healthy diet, the rats starved for two weeks instead of eating nutritious fare. A 2007 British Journal of Nutrition study found that female rats who eat junk food during pregnancy increased the likelihood of unhealthy eating habits in their offspring. A report published in the Journal of the Federation of American Societies for Experimental Biology suggests that babies of mothers with a high-sugar and high-fat diet while pregnant are more prone to junk food themselves. The study was conducted on rats and suggests that pups “whose mothers eat excessive amounts of high-fat, high-sugar junk foods when pregnant or breastfeeding are likely to have a greater preference for these foods later in life.”
_
Intake of sugar-sweetened beverages and weight gain: a systematic review:
Consumption of sugar-sweetened beverages (SSBs), particularly carbonated soft drinks, may be a key contributor to the epidemic of overweight and obesity, by virtue of these beverages’ high added sugar content, low satiety, and incomplete compensation for total energy. Whether an association exists between SSB intake and weight gain is unclear. Authors searched English-language MEDLINE publications from 1966 through May 2005 for cross-sectional, prospective cohort, and experimental studies of the relation between SSBs and the risk of weight gain (i.e., overweight, obesity, or both). Thirty publications (15 cross-sectional, 10 prospective, and 5 experimental) were selected on the basis of relevance and quality of design and methods. Findings from large cross-sectional studies, in conjunction with those from well-powered prospective cohort studies with long periods of follow-up, show a positive association between greater intakes of SSBs and weight gain and obesity in both children and adults. Findings from short-term feeding trials in adults also support an induction of positive energy balance and weight gain by intake of sugar-sweetened sodas, but these trials are few. A school-based intervention found significantly less soft-drink consumption and prevalence of obese and overweight children in the intervention group than in control subjects after 12 months, and a recent 25-week randomized controlled trial in adolescents found further evidence linking SSB intake to body weight. The weight of epidemiologic and experimental evidence indicates that a greater consumption of SSBs is associated with weight gain and obesity. Although more research is needed, sufficient evidence exists for public health strategies to discourage consumption of sugary drinks as part of a healthy lifestyle.
_
Fast food:
Fast food is the term given to food that can be prepared and served very quickly. While any meal with low preparation time can be considered to be fast food, typically the term refers to food sold in a restaurant or store with preheated or precooked ingredients, and served to the customer in a packaged form for take-out/take-away. A typical fast food meal in the United States includes a hamburger, french fries, and a soft drink. McDonald’s, Kentucky Fried Chicken and Pizza Hut are typical fast food restaurants. Pizza is a common fast food category in the United States. Fish and chip shops are a form of fast food popular in the United Kingdom, Australia and New Zealand. Fish is battered and then deep fried. Chinese takeaways/takeout restaurants are particularly popular. They normally offer a wide variety of Asian food (not always Chinese), which has normally been fried. Most options are some form of noodles, rice, or meat. The concept of ready-cooked food for sale is closely connected with urban development. In Ancient Rome cities had street stands that sold bread, sausages and wine. Fast food outlets are take-away or take-out providers, often with a “drive-through” service which allows customers to order and pick up food from their cars, but most also have indoor and/or outdoor seating areas in which the customers can eat the food on-site. Many petrol/gas stations have convenience stores which sell pre-packaged sandwiches, doughnuts, and hot food and they also sell frozen foods and have microwaves on the premises in which to prepare them. Fast food is part to blame for this obesity epidemic. Fast food dominates in the food industry allowing our society to think it is okay to eat such unhealthy food. Fast food contains foods that are high in gluten, sodium, sugars, and fats. Consuming too much of it can lead to obesity and cause other diseases which are damaging to one’s health. Also, the food portions that are standardized by fast food companies are to attribute for obesity as well. Places like McDonald’s, Chik-fil-a and Taco Bell make it seem normal to ingest such large portion sizes which can lead to obesity. A study done in Jeddah has shown that current fast food habits are related to the increase of overweight and obesity among adolescents in Saudi Arabia.
_
How fast food cause obesity: one example is sufficient:
At rest (for example, while sitting and watching television), the human body burns only about 12 calories per pound of body weight per day (26 calories per kilogram). That means that if you weigh 150 pounds (68 kg), your body uses only about:
150 X 12 = 1,800 calories per day
Twelve calories per pound per day is a rough estimate.
Those 1,800 calories are used to do everything you need to stay alive:
•They keep your heart beating and lungs breathing.
•They keep your internal organs operating properly.
•They keep your brain running.
•They keep your body warm.
The 1,800 calories that a typical person at rest needs per day is just not that many. For example, if you go to your neighborhood McDonald’s restaurant and order a meal, you will get a hamburger sandwich, a large order of french fries and a large Coke. This meal contains:
•710 calories in the hamburger sandwich
•540 calories in the french fries
•310 calories in the drink
In other words, just this one meal provides 1,560 calories you need during a day.
_
Fast foods are typically:-
• high in calories
• high in fat
• high in saturated and trans fat
• high in sugar
• high in simple carbohydrates
• high in sodium (salt)
_
Underestimation of caloric intake by fast food eaters:
Apparently, fast-food frequenters have no idea how many calories they’re ordering up at the counter. Researchers conducted a large cross-sectional study of 1,877 adults and 330 school-age kids who regularly visited fast-food chains including McDonald’s, Burger King, Wendy’s, KFC, Subway and Dunkin’ Donuts. The investigators collected receipts from the participants in order to calculate how many calories the participants consumed from their meals. They also asked the volunteers to estimate the number of calories they had just ordered. At the time of the study, none of the restaurant chains included calorie information on their menus, as many now do. Reporting in the BMJ, the researchers found that on average, adults consumed 836 calories with each order, adolescents ate 756 and kids downed 733 calories. Not only was that a relatively large amount to consume in a single meal, but the participants also consistently underestimated how dense their meals were by an average of more than 100 calories. Adults and kids underestimated their meals by 175 calories, and adolescents by 259 calories. The more calories the meals contained, the more the participants underestimated their content. Interestingly, the greatest disparity in calorie estimations was among Subway diners. Adults and adolescents who ate at the sandwich chain underestimated their meals by 20% to 25% more than the participants who ate at McDonald’s, possibly because the Subway choices have an aura of being lighter and healthier than those at fast-food chains.
_
Is fast food junk food?
Junk food is any food that may be comforting and appealing but is of low nutritional value and contains a lot of bad stuff like fat, salt and sugar. “Fast food” literally just means food that takes little time to prepare, but it’s now almost always associated with retail food outlets such as burger restaurants, to contrast the sort of service they offer with that of a traditional restaurant where “proper” meals take longer to prepare. Food eaten for convenience is not necessarily lacking in nutritional value or a risk to health. Some of the most convenient foods like a piece of fruit, a wholemeal salad sandwich, or baked beans on toast, are all healthful ways of eating. Also all food needs to be considered in the context of the total diet, rather than any single occasion of eating. Unhealthful fast foods (convenience foods sold ready to eat such as battered fish and chips, hamburgers, hot dogs and meat pies) are generally fatty, often fried, salty and low in dietary fiber (roughage). But fast foods do not have to be unhealthful. For example, lean hamburger meat can be served with plentiful salad in a low-salt, wholemeal bread roll with little butter or margarine. Fish can be grilled instead of fried, eaten with lemon or vinegar rather than salt, and the chips can be thick rather than thin so that they absorb less fat on frying. The term ‘junk food’ is not the same as convenience food or fast food. It can be used to mean foods which are relatively low in nutrients compared to their energy (kilojoule) content. Generally junk foods rely on flavors such as salt or sugar and artificial colors for their appeal.
__
Overeating of fast food:
In the study, from the Children’s Hospital in Boston, teens age 13-17 were given three types of fast-food meals (all including chicken nuggets, French fries, and cola). In one meal, the teens were served a lot of food at once. In another, a lot of food was served at the same time, but in smaller portions. And in the third test meal, a lot of food was served, but in smaller portions over 15-minute intervals. The researchers found that it didn’t seem to matter how much food was served — the teens still took in about half of their daily calorie needs in that one meal. The researchers suggested that certain factors inherent to fast food might promote overeating:
- It’s low in fiber.
- It’s high in palatability (that is, it tastes good).
- It offers a high number of calories in a small volume.
- It’s high in fat.
- It’s high in sugar in liquid form.
__
Fast foods, energy density and obesity:
Fast foods are frequently linked to the epidemic of obesity, but there has been very little scientific appraisal of a possible causal role. Here authors review a series of studies demonstrating that the energy density of foods is a key determinant of energy intake. These studies show that humans have a weak innate ability to recognize foods with a high energy density and to appropriately down-regulate the bulk of food eaten in order to maintain energy balance. This induces so called ‘passive over-consumption’. Composition data from leading fast food company websites are then used to illustrate that most fast foods have an extremely high energy density. At some typical outlets the average energy density of the entire menus is approximately 1100 kJ per 100g. This is 65% higher than the average British diet (approximately 670 kJ per 100 g) and more than twice the energy density of recommended healthy diets (approximately 525 kJ per 100 g). It is 145% higher than traditional African diets (approximately 450 kJ per 100 g) that probably represent the levels against which human weight regulatory mechanisms have evolved. Authors conclude that the high energy densities of many fast foods challenge human appetite control systems with conditions for which they were never designed. Among regular consumers they are likely to result in the accidental consumption of excess energy and hence to promote weight gain and obesity.
__
Food poundage:
Interestingly, research shows that most humans eat around 3-5 pounds of food per day. Indeed, as we approach 4 pounds of food intake for the day, most of us are feeling pretty satisfied. Now, this can be 4 pounds of celery. Or it can be 4 pounds of candy bars. It’s not the food or calorie content that matters. It’s the volume/poundage that counts. And obviously, there are some big nutrient differences between celery and candy bars. 4 pounds of raw veggies will provide 400 Kcal, 4 pounds of raw fruits will provide 1000 Kcal, 4 pounds of cooked whole grains/legumes provides 1600 Kcal, 4 pounds of nuts/seeds provides about 10,000 Kcal and 4 pounds of Lucky Charms, Pop Tarts, Cheese provides about 10,000 Kcal.
_
The energy density of a recommended healthy diets is 125 Kcal per 100gms while energy density of typical fast food is 260 Kcal per 100 gms. Since humans consume almost the same quantity of food by weight daily, fast food consumption would lead to obesity.
_
The Relationship between Obesity and the Prevalence of Fast Food Restaurants: State-Level Analysis:
Obesity accounts for approximately 300,000 deaths a year in the United States, and prevalence rates have been increasing over the past decade. The nutrition environment may be contributing to this epidemic. A study examined the relationship between fast food restaurants and obesity on a state-wide basis. The results indicate a correlational relationship between both the number of residents per fast food restaurant and the square miles per fast food restaurants with state-level obesity prevalence.
_
Can fast food end obesity?
The fast-food industry is highly motivated to provide enticing and healthful offerings and has been doing so for many years, with some success. It should be encouraged to move strongly in this direction. The trick is to get people to eat healthfully incrementally, slowly, in stealthy ways, in the places where they actually eat now. Don’t try to get a McDonald’s customer to shop at a farm market. Keep him coming to McDonalds, but improve his diet when he’s there. Don’t tell him he’s eating something that’s healthful! It actually inhibits sales. Chef Dan Coudreaut, the head of culinary innovation at McDonald’s said that he can create processed food that is far more healthful or he can reduce the fat content in a dish. The healthful dish is simply more processed — but that isn’t a bad thing. Science has developed healthful ways to create food that is just as appetizing and satisfying as junk food. It’s doing it by breaking down the sensory characteristics of a meal. Companies are now devoted to increasing the length of time a flavor lingers on the tongue — which is part of how fat affects flavor. It’s called a food’s “temporal profile.” Textures can be mimicked successfully by a company called Tic Gums. Tic serves an in-development version of a low-fat salad dressing that is better than any you’ve ever had. And Domino Foods now sells a low-cal combo of sugar and stevia that takes away the “licorice note” in stevia, a natural sweetener gaining widespread acceptance. Bottom line: lower-calorie processed food that’s actually healthful and nutritious can be offered to consumers through the channels they love. Also tell the consumer how good a healthful dish actually tastes and save the fact that it’s healthful for the foot notes. Studies have shown that consumers don’t like to be lectured when they’re hungry. All they want is the satisfaction. Helping them lose weight is just a bonus.
_
Sedentary lifestyle:
A sedentary lifestyle plays a significant role in obesity. Worldwide there has been a large shift towards less physically demanding work, and currently at least 30% of the world’s population gets insufficient exercise. This is primarily due to increasing use of mechanized transportation and a greater prevalence of labor-saving technology in the home. In children, there appear to be declines in levels of physical activity due to less walking and physical education. World trends in active leisure time physical activity are less clear. The World Health Organization indicates people worldwide are taking up less active recreational pursuits, while a study from Finland found an increase and a study from the United States found leisure-time physical activity has not changed significantly. With the arrival of televisions, computers, video games, remote controls, washing machines, dish washers and other modern convenience devices, the majority of people are leading a much more sedentary lifestyle compared to their parents and grandparents. Some decades ago shopping consisted of walking down the road to the high street where one could find the grocers, bakers, banks, etc. As large out-of-town supermarkets and shopping malls started to appear, people moved from using their feet to driving their cars to get their provisions. In some countries, such as the USA, dependence on the car has become so strong that many people will drive even if their destination is only half-a-mile away. The less you move around the fewer calories you burn. However, this is not only a question of calories. Physical activity has an effect on how your hormones work, and hormones have an effect on how your body deals with food. Several studies have shown that physical activity has a beneficial effect on your insulin levels – keeping them stable. Unstable insulin levels are closely associated with weight gain.
_
Increased urbanization has resulted in several environmental factors which may discourage participation in physical activity such as:
- violence,
- high-density traffic,
- low air quality, pollution,
- lack of parks, sidewalks and sports / recreation facilities.
_
Computers and television viewing associated with obesity risk:
In fact this has already been proved to a certain extent, a study published in Pediatric Obesity by researchers from the University of Alberta concluded that children with electronic devices in their bedroom, such as televisions or computers are at a much higher risk of becoming obese compared to those who don’t. In both children and adults, there is an association between television viewing time and the risk of obesity. A review found 63 of 73 studies (86%) showed an increased rate of childhood obesity with increased media exposure, with rates increasing proportionally to time spent watching television.
_
Television viewing and obesity in adult females: A study:
Researchers measured the relation between time spent watching television per week and obesity in 4,771 adult females. After controlling for age, education, cigarette smoking, length of work week, and weekly duration of exercise, females who reported three to four hours of TV viewing per day showed almost twice the prevalence of obesity (body fat greater than 30 percent), and those who reported more than four hours of TV watching per day showed more than double the prevalence of obesity, compared to the reference group (less than 1 hr/day). Part of the TV/obesity association was a function of differences in exercise duration among the four TV viewing categories.
_
A study found that the risk of obesity increased 12% per weekly hour of television viewing, while it is decreased 10% per weekly hour of moderate physical activity.
_
Are fast foods and Television viewing contributing to obesity epidemic?
This study explored the hypothesis that TV viewing and fast food eating may contribute to obesity in the United States. The data for women were generally consistent with the hypotheses. Hours of TV viewing per day and meals eaten at fast food restaurants per week were both positively associated with body mass index cross-sectionally. TV viewing also predicted weight gain in high-income women. The finding that energy intake and percentage of energy from fat were also positively associated with TV viewing and fast food eating; would seem to strengthen the inference of causation by identifying a plausible intervening mechanism. The associations between TV-viewing and obesity seen for women in this study are consistent with previous research in adults. The observation of a stronger link between TV viewing and obesity in low-income women is also consistent with the only previous study, in children, to examine the issue. The important negative findings in this study merit further comment. First is the lack of an association between TV viewing or fast food eating and body mass index in men. One possible explanation is that these men were a special group (i.e., volunteers for a study of weight gain prevention). Another is that the relationships between TV viewing, fast food eating, and weight are not the same in adult men and women, perhaps as a result of differences in occupational or social roles.
__
Not sleeping enough give rise to obesity:
If you do not sleep enough your risk of becoming obese doubles, according to research carried out at Warwick Medical School at the University of Warwick. The risk applies to both adults and children. Professor Francesco Cappuccio and team reviewed evidence in over 28,000 children and 15,000 adults. Their evidence clearly showed that sleep deprivation significantly increased obesity risk in both groups. Professor Cappuccio said, “The ‘epidemic’ of obesity is paralleled by a ‘silent epidemic’ of reduced sleep duration with short sleep duration linked to increased risk of obesity both in adults and in children. These trends are detectable in adults as well as in children as young as 5 years. Professor Cappuccio explains that sleep deprivation may lead to obesity through increased appetite as a result of hormonal changes. If you do not sleep enough you produce Ghrelin, a hormone that stimulates appetite. Lack of sleep also results in your body producing less Leptin, a hormone that suppresses appetite. The purpose of ghrelin is basically the exact opposite of leptin: It tells your brain when you need to eat, when it should stop burning calories and when it should store energy as fat. During sleep, levels of ghrelin decrease, because sleep requires far less energy than being awake does. People who don’t sleep enough end up with too much ghrelin in their system, so the body thinks it’s hungry and it needs more calories, and it stops burning those calories because it thinks there’s a shortage. During sleep, leptin levels increase, telling your brain you have plenty of energy for the time being and there’s no need to trigger the feeling of hunger or the burning of calories. When you don’t get enough sleep, you end up with too little leptin in your body, which, through a series of steps, makes your brain think you don’t have enough energy for your needs. So your brain tells you you’re hungry, even though you don’t actually need food at that time, and it takes steps to store the calories you eat as fat so you’ll have enough energy the next time you need it. The decrease in leptin brought on by sleep deprivation can result in a constant feeling of hunger and a general slow-down of your metabolism. Sleep deprivation has also been found to increase levels of stress hormones and resistance to insulin, both of which also contribute to weight gain. Insulin resistance can also lead to type 2 diabetes.
_
Genetics in obesity:
Obesity is a multifactorial condition. Environmental risk factors related to a sedentary life-style and unlimited access to food applies constant pressure in subjects with a genetic predisposition to gain weight. The fact that genetic defects can result in human obesity has been unequivocally established over the past 3 years with the identification of the genetic defects responsible for different monogenic forms of human obesity: the leptin, leptin receptor, pro-opiomelanocortin, pro-hormone convertase-1 and melanocortin-4 receptor genes. The common forms of obesity are, however, polygenic. The examination of specific genes for involvement in the susceptibility to common obesity has not yet yielded convincing results. Obesity is a complex trait influenced by diet, developmental stage, age, physical activity and genes (Brockmann and Bevova, 2002; Friedman, 2003). Genetic predisposition is a key contributing factor in obesity as demonstrated by familial aggregation, twin and adoption studies (Allison et al., 1996; Friedman, 2003; Stunkard et al., 1990). Estimates for the genetic basis of phenotypic variations in obesity range from approximately 40 to 70%. This matches or exceeds the accepted genetic contribution to height (Friedman, 2003). The idea that genetic loci alter body fat content has been substantiated by identification of mutations that cause low- or high-fat phenotypes in rodents and humans (Brockmann and Bevova, 2002; Delrue and Michaud, 2004). There is convincing experimental evidence showing that the balance between energy intake (food consumption) and energy expenditure (basal metabolic rate, i.e. biochemical processes required to maintain cellular viability, physical activity and adaptive thermogenesis) is tightly regulated. A homeostatic network maintains energy stores through a complex interplay between the feeding regulatory centers in the central nervous system (CNS), particularly in the hypothalamus and the regulated storage and mobilization of fat stores. Thus, genes that encode the molecular components of this system may underlie obesity and related disorders.
__
Twin studies:
Twin and adoption studies show consistently that variation in body mass index has a strong genetic component. One study assessed the heritability of body mass index in over 20 000 young adult twin pairs from eight European countries, with data collected from 1963 to 2002 (although mostly from 1980 onwards). The correlation of body mass index between identical twins in the eight countries ranged from 0.65 to 0.83 and was consistently stronger than that between non-identical same sex twins (correlation 0.31 to 0.58). The estimated genetic effects, correcting for age and sex differences, were 60-70%. In a recent systematic review of five adoption studies with several hundred parent-biological child and parent-adoptee comparisons, children’s body mass index was consistently more strongly correlated with that of their biological parents than of their adoptive parents. Intrauterine “programming” did not account for the differences because the correlations were similar for father-biological child pairs and mother-biological child pairs. Strong genetic effects remain even in contemporary environments. In a study of over 5000 twin pairs born in the UK during 1994-97, the correlation of body mass index at age 8-11 years between identical twins (0.86, 95% confidence interval 0.85 to 0.87) was stronger than that between non-identical same sex twins (0.51, 0.47 to 0.53). Genetic effects were estimated as 77%. Another study of over 2000 very young twin pairs found that appetite, estimated from parental questionnaires, had a genetic component. Correlations between appetite measures in one twin and weight of the other twin were stronger in identical than non-identical twin pairs.
_
Studies that have focused on inheritance patterns rather than on specific genes have found that 80% of the offspring of two obese parents were also obese, in contrast to less than 10% of the offspring of two parents who were of normal weight. A study from the American Journal of Clinical Nutrition found that children with two obese parents are 12 times more likely to become obese themselves.
_
FTO gene:
People with two copies of the FTO gene (fat mass and obesity associated gene) have been found on average to weigh 3–4 kg more and have a 1.67-fold greater risk of obesity compared with those without the risk allele. FTO is an enzyme that in humans is encoded by the FTO gene located on chromosome 16. Increases in hypothalamic expression of FTO are associated with the regulation of energy intake but not feeding reward.
_
FTO gene linked to Ghrelin:
Researchers think they’ve hit on why a common obesity gene causes weight gain: Those who carry a version of it don’t feel full after eating and take in extra calories. That’s because the variant of the FTO gene in question, which one in six individuals carry, leads to higher levels of ghrelin, a hormone involved in mediating appetite and the body’s response to food, researchers have discovered. While most studies on FTO have relied on mice, the new work analyzed blood samples and brain scans from humans. Hattersley was part of a team that in 2007 reported that people who had one version of the FTO gene, called AA, weighed an average of 3 kilograms more than those with the TT version of the gene. Since then, studies in mice have shown that in everyone, there are high levels of the FTO protein in brain areas that control energy balance. Researchers have also found that animals with the AA version tend to eat more and prefer high-fat food compared with those with the TT version. But why FTO had this effect wasn’t known. Rachel Batterham, an endocrine and obesity researcher at University College London, thought that gut hormones that mediate the body’s response to eating could be the missing link between FTO and food intake. One such hormone is ghrelin, known to be produced by gut cells to stimulate hunger. So Batterham and her colleagues measured levels of ghrelin in the blood of nonobese men with the AA or TT versions of FTO. In those with the TT variant, ghrelin levels rose before a meal, when the person experienced hunger, and fell after eating, as expected. But in those with the obesity-associated AA version, ghrelin levels stayed relatively high even after eating. Moreover, the AA individuals reported a faster increase in hunger after a test meal. And MRI scans revealed that, when the test subjects were shown images of food before or after eating, brain activity in areas associated with motivation and rewards remained high before and after the meal in AA individuals. This suggests that the increased ghrelin levels were impacting the brain’s response to food—which “fits very well with what we already know the effects of ghrelin,” Batterham says. The FTO protein actually alters the ghrelin gene, causing methyl chemical groups to be removed, so-called epigenetic modification that impacts how much protein the ghrelin gene produces. The AA gene variant, the researchers report in The Journal of Clinical Investigation, removed more methyl groups from the gene, leading to increased levels of the hunger hormone.
_
Science shows that genetics plays a role in obesity. Genes can directly cause obesity in disorders such as Bardet-Biedl syndrome and Prader-Willi syndrome. In people with early-onset severe obesity (defined by an onset before 10 years of age and body mass index over three standard deviations above normal), 7% harbor a single point DNA mutation. Occurrences of monogenic types of obesity are evidence that obesity may be caused by genetic mutations; however, as yet, only 78 cases worldwide have been attributed to mutations of seven distinct genes. The most common forms of obesity are probably the result of variations within a large number of genes (polygenic). Sequence variations within a pool of 56 different genes have been reported as being related to obesity phenotypes, however, only ten of those genes showed positive results in at least five different studies. Polymorphisms in various genes controlling appetite and metabolism predispose to obesity when sufficient food energy present. As of 2006, more than 41 of these sites on the human genome have been linked to the development of obesity when a favorable environment is present. The percentage of obesity that can be attributed to genetics varies, depending on the population examined, from 6% to 85%.
_
Selected genes with variants that have been associated with obesity
|
Gene symbol |
Gene name |
Gene product’s role in energy balance |
| ADIPOQ | Adipocyte-, C1q-, and collagen domain-containing | Produced by fat cells, adiponectin promotes energy expenditure |
| FTO | Fat mass- and obesity-associated gene | Promotes food intake |
| LEP | Leptin | When leptin levels drops hunger increases |
| LEPR | Leptin receptor | When bound by leptin, inhibits appetite |
| INSIG2 | Insulin-induced gene 2 | Regulation of cholesterol and fatty acid synthesis |
| MC4R | Melanocortin 4 receptor | When bound by alpha-melanocyte stimulating hormone, stimulates appetite |
| PCSK1 | Proprotein convertase subtilisin/kexin type 1 | Regulates insulin biosynthesis |
| PPARG | Peroxisome proliferator-activated receptor gamma | Stimulates lipid uptake and development of fat tissue |
__
Thrifty genes:
Survival of Homo sapiens during evolution was dependent on the procurement of food, which in turn was dependent on physical activity. However, food supply was never consistent. Thus it is contended that the ancient hunter-gatherer had cycles of feast and famine, punctuated with obligate periods of physical activity and rest. Hence, gene selection in the Late-Paleolithic era was probably influenced by physical activity and rest. To ensure survival during periods of famine, certain genes evolved to regulate efficient intake and utilization of fuel stores. Such genes were termed “thrifty genes” in 1962. The thrifty gene hypothesis postulates that, due to dietary scarcity during human evolution, people are prone to obesity. Their ability to take advantage of rare periods of abundance by storing energy as fat would be advantageous during times of varying food availability, and individuals with greater adipose reserves would be more likely to survive famine. This tendency to store fat, however, would be maladaptive in societies with stable food supplies. Furthermore, convincing evidence shows that this ancient genome has remained essentially unchanged over the past 10,000 years and certainly not changed in the past 40-100 years. Although the absolute caloric intake of modern-day humans is likely lower compared with our hunter-gatherer ancestors, it is nevertheless in positive caloric balance in the majority of the US adult population mainly due to the increased sedentary lifestyle in present society. Authors contend that the combination of continuous food abundance and physical inactivity eliminates the evolutionarily programmed biochemical cycles emanating from feast-famine and physical activity-rest cycles, which in turn abrogates the cycling of certain metabolic processes, ultimately resulting in metabolic derangements such as obesity and Type 2 diabetes. In this context, authors postulate that perhaps a crucial mechanism to break the stall of the metabolic processes would be via exercise through the regulation of “physical activity genes,” some of which may also be potential candidates for the “thrifty genes” of our hunter-gatherer ancestors. Therefore, the identification of such “thrifty gene” candidates would help provide insight into the pathogenetic processes of the numerous physical inactivity-mediated disorders.
__
Medical illnesses:
Medical illnesses that increase obesity risk includes several rare genetic syndromes (listed above) as well as some congenital or acquired conditions: hypothyroidism, Cushing’s syndrome, growth hormone deficiency, and the eating disorders: binge eating disorder and night eating syndrome.
_
Mental illnesses:
Obesity is not regarded as a psychiatric disorder, and therefore is not listed in the DSM-IVR as a psychiatric illness. The risk of overweight and obesity is higher in patients with psychiatric disorders than in persons without psychiatric disorders.
_
Weight gain and immobility:
Some medical problems, such as arthritis & fractures, can lead to the decrease in activity, which usually results in weight gain.
_
Evidence that certain pharmaceuticals increase weight:
Weight gain is induced by many psychotropic medications (antipsychotics, antidepressants, mood stabilizers), anticonvulsants (phenytoin, valproate), antidiabetics, antihypertensives, steroid hormones and contraceptives, antihistamines and protease inhibitors. Selective serotonin reuptake inhibitors (antidepressants) may also produce weight gain, but data are less consistent. Almost all atypical antipsychotics produce markedly more weight gain than placebo or traditional antipsychotics. For olanzapine and clozapine, mean weight gains were over 4 kg at 10 weeks. These drugs are active at many receptors involved in body weight regulation and these findings were reproduced in animal models. Most antidiabetics, including insulin, sulfonylureas and thiazolidinediones also promote adiposity, especially the newer thiazolidinediones, which promote adipocyte proliferation. Beta-blockers induce a mean weight gain of approximately 1.2 kg. Data are less consistent for oral contraceptives, but one study estimated a mean weight gain of approximately 5 kg at 2 years. Antihistamines also appear to induce weight gain, with more potent antihistamines producing greater weight gain. Human immunodeficiency virus antiretroviral drugs and protease inhibitors also produce weight gain and increased abdominal adiposity.
_
Obesity and the polycystic ovary syndrome:
The polycystic ovary syndrome (PCOS) is a condition characterized by hyperandrogenism and chronic oligo-anovulation. However, many features of the metabolic syndrome are inconsistently present in the majority of women with PCOS. Approximately 50% of PCOS women are overweight or obese and most of them have the abdominal phenotype. Obesity may play a pathogenetic role in the development of the syndrome in susceptible individuals. In fact, insulin possesses true gonadotrophic function and increased insulin availability at the level of ovarian tissue may favour excess androgen synthesis. Obesity, particularly the abdominal phenotype, may be partly responsible for insulin resistance and associated hyperinsulinemia in women with PCOS. Therefore, obesity-related hyperinsulinemia may play a key role in favouring hyperandrogenism in these women. Other factors such as increased estrogen production rate, increased activity of the opioid system and of the hypothalamic-pituitary-adrenal axis, decreased sex hormone binding globulin synthesis and, possibly, high dietary lipid intake, may be additional mechanisms by which obesity favours the development of hyperandrogenism in PCOS. Irrespective of the pathogenetic mechanism involved, obese PCOS women have more severe hyperandrogenism and related clinical features (such as hirsutism, menstrual abnormalities and anovulation) than normal-weight PCOS women. This picture tends to be more pronounced in obese PCOS women with the abdominal phenotype. Body weight loss is associated with beneficial effects on hormones, metabolism and clinical features. A further clinical and endocrinological improvement can also be achieved by adding insulin-sensitizing agents and/or antiandrogens to weight reduction programs. These obviously emphasize the role of obesity in the pathophysiology of PCOS.
_
Pregnancy and weight gain:
Eating a healthy, balanced diet will help your baby get the nutrients he or she needs and grow at a healthy rate. But how many extra calories do you really need? Though you do need some extra calories, it’s not necessary to ”eat for two.” The average pregnant woman needs only about 300 healthy calories more a day than she did before she was pregnant. This will help her gain the right amount of weight during pregnancy. Pregnancy can also be a catalyst in becoming obese. During pregnancy, a woman’s weight necessarily increases for development of a baby. Some women find this weight difficult to lose after the baby is born. This weight gain may contribute to the development of obesity in women. If a woman is very overweight when she gets pregnant, her doctor may want her to lose weight. She should only lose weight under her doctor’s care. But in most cases, women should not try to lose weight or diet during pregnancy.
_
_
Evidence that greater gravida age increases risk of offspring obesity:
Wilkinson et al. studied obese British children and found that a common risk factor was having an elderly mother. Patterson et al. studied girls aged 9–10 years and found that the odds of obesity increased 14.4% for every 5-year increment in maternal age. Biological data support these findings. Symonds et al. observed a correlation between maternal age and fat deposition in sheep, in part related to uncoupling protein levels. This is in part related to an accelerated loss of the brown adipose uncoupling protein 1 levels in the offspring of adult primiparous mothers after birth, which may act to increase white adipose tissue deposition in later life.
_
It is possible that the extremes of energy imbalance in utero (overfeeding and low birth weight) may contribute to obesity. We may now be seeing the transgenerational obesogenic effects of environmental changes initiated one or more generations ago. Forebodingly, obesity’s prevalence could increase further if children of the current generation’s overweight or obese parents are thereby predisposed further still.
_
Obesity could be caused by gut bacteria rather than over-eating: a study:
•Study suggests the bacteria plays a larger role than eating too much or not exercising enough
•Researchers found diet that altered gut bacteria caused dramatic weight loss
Researchers in Shanghai studied mice who had been bred to be resistant to obesity. These mice remained slim despite being fed a rich diet and being kept from exercising. However, when some of these mice were injected with the human bacterium enterobacter, they quickly became obese. Enterobacter was first linked with obesity after being found in high quantities in the gut of a morbidly obese human volunteer, said the report from Shanghai’s Jiaotong University. The mice were injected with the bacterium for up to 10 weeks as part of the experiment. The experiments show that the bacterium ‘may causatively contribute to the development of obesity’ in humans, according to the paper published in the International Society for Microbial Ecology. The researchers added that a patient lost 4stone 7lbs in nine weeks after being placed on a diet of ‘whole grains, traditional Chinese medicinal foods and prebiotics’, and said this was because it had reduced the bacterium’s presence in the patient’s gut to ‘undetectable’ levels.
_
C-sections lead to obese kids, study finds:
Based on an analysis involving more than 10,000 British babies, roughly 9 percent of whom were born via C-section, researchers found that babies delivered through alternate route tend to be larger overall than babies born the natural way. To arrive at this conclusion, Dr. Jan Blustein, Ph.D., M.D., from the New York University (NYU) School of Medicine evaluated data on 10,219 children born in Great Britain between 1991 and 1992. Initially, the children born via C-section weighed about 0.125 pounds less, on average, than those born vaginally. But after just a few weeks, the tables turned. After just six weeks, children born via C-section were found to already be heavier than those born vaginally, and this trend reportedly continued as the children got older. By the time the children reached age 11, those born via C-section were found weigh a shocking 83 percent more than those born vaginally, which would help explain why an increasing number of children today are obese. “There may be long-term consequences to children that we don’t know about,” says Dr. Blustein about the findings, noting that prevalence of C-sections has also risen quite substantially throughout the past several decades. C-section babies miss out on natural transference of bacterial flora, which could explain their tendency towards obesity. Published in the International Journal of Obesity, the study also found that babies born via C-section generally lack the appropriate balance of gut bacteria due to the fact that they are not delivered through their mothers’ birth canals. A normal vaginal birth imparts the necessary colonization of “friendly” bacteria in the gut that later helps regulate weight. But C-section babies miss out on this. “Generally, the early colonization and establishment of the intestine with bacteria seems very important,” adds Teresa Ajslev from the Institute of Preventive Medicine in Frederiksberg, Denmark. “Yet, much more work is needed before we can explain the mechanisms of the early bacterial colonization.” Many readers are already aware of the vital role gut flora plays in food digestion, nutrient absorption, and weight maintenance. When this bacterial balance is thrown off kilter, or when it never properly develops in the first place, health problems can ensue. For many people, accumulation of belly fat is one potential consequence of this imbalance.
_
Elderly and obesity:
Obesity can occur at any age, even in young children. But as you age, hormonal changes and a less active lifestyle increase your risk of obesity. In addition, the amount of muscle in your body tends to decrease with age. This lower muscle mass leads to a decrease in metabolism. These changes also reduce calorie needs and can make it harder to keep off excess weight. If you don’t control what you eat as you age, you’ll likely gain weight.
_
Miscellaneous causes of obesity:
In the United States the number of children a person has is related to their risk of obesity. A woman’s risk increases by 7% per child, while a man’s risk increases by 4% per child. This could be partly explained by the fact that having dependent children decreases physical activity in Western parents. In the developing world urbanization is playing a role in increasing rate of obesity. In China overall rates of obesity are below 5%; however, in some cities rates of obesity are greater than 20%. Malnutrition in early life is believed to play a role in the rising rates of obesity in the developing world. Endocrine changes that occur during periods of malnutrition may promote the storage of fat once more food energy becomes available.
__
Smoking and obesity:
Smoking has a significant effect on an individual’s weight. Those who quit smoking gain an average of 4.4 kilograms (9.7 lb) for men and 5.0 kilograms (11.0 lb) for women over ten years. However, changing rates of smoking have had little effect on the overall rates of obesity.
_
_
A study was conducted to critically evaluate the relations among smoking, body weight, body fat distribution, and insulin resistance as reported in the literature. In the short term, nicotine increases energy expenditure and could reduce appetite, which may explain why smokers tend to have lower body weight than do nonsmokers and why smoking cessation is frequently followed by weight gain. In contrast, heavy smokers tend to have greater body weight than do light smokers or nonsmokers, which likely reflect a clustering of risky behaviors (e.g., low degree of physical activity, alcoholism, eats poorly etc) that is conducive to weight gain. Other factors, such as weight cycling, could also be involved. In addition, smoking increases insulin resistance and is associated with central fat accumulation. As a result, smoking increases the risk of metabolic syndrome and diabetes, and these factors increase risk of cardiovascular disease. In the context of the worldwide obesity epidemic and a high prevalence of smoking, the greater risk of (central) obesity and insulin resistance among smokers is a matter of major concern.
_
Alcohol and obesity:
Alcohol represents an important source of energy. Despite its comparatively high energy content of 7.1 g/kcal, it is still controversial whether moderate amounts of alcohol represent a risk factor for weight gain and obesity. Many people are not aware of the calories contained in alcoholic drinks. Epidemiologic data showed a positive, negative, or no relationship between alcohol intake and body weight. Alcohol contains calories, but drinking alcohol doesn’t lead to weight gain, according to extensive medical research, and many studies report a reduction in weight for drinkers, especially women. The medical evidence of this is based on a large number of studies of thousands of people around the world. Some of these studies are very large; one involved nearly 80,000 and another included 140,000 subjects. The reason that alcohol doesn’t increase weight is unclear, but research suggests that alcohol energy is not efficiently used. Alcohol also appears to increase metabolic rate significantly, thus causing more calories to be burned rather than stored in the body as fat. Moderate amounts of alcohol enhance energy intake due to the caloric content of the alcohol as well as its appetite-enhancing effects. Alcohol consumption can lead to an increase in food intake. Alcohol-induced thermogenesis is approximately 20% in healthy nonalcoholic subjects, i.e., moderate alcohol consumers, which is higher than for other energy substrates but considerably lower than in heavy alcohol consumers. This would suggest that a major fraction of the alcohol energy represents unavailable energy source for ATP synthesis in moderate non-daily alcohol consumers. Experimental evidence from several metabolic studies showed a suppression of lipid oxidation by alcohol and thus the enhancement of a positive fat balance. The nonoxidized fat is preferentially deposited in the abdominal area. The experimental metabolic evidence suggests that the consumption of moderate amounts of alcohol has to be accounted for in the energy-balance equation and may represent a risk factor for the development of a positive energy balance and thus weight gain. In the heavy alcohol consumer and eventually also in daily moderate alcohol consumers, a larger fraction of the alcohol energy might not be an available source of energy due to the induction of the microsomal ethanol-oxidizing system (MEOS). Experimental data in combination with epidemiologic findings suggest that alcohol energy counts more in moderate nondaily alcohol consumers than in some moderate daily and all heavy consumers. Accordingly the question is not “Whether alcohol calories do count” but “How much do alcohol calories count?” There seems to be a large individual variability according to the absolute amount of alcohol consumed, the drinking frequency as well as genetic factors. Presently it can be said that alcohol calories count more in moderate nondaily consumers than in daily (heavy) consumers. Further, they count more in combination with a high-fat diet and in overweight and obese subjects. The associations between alcohol and obesity are heavily influenced by lifestyle, genetic and social factors.
_
My experience in India shows that moderate drinkers from upper class people have abdominal obesity while moderate as well heavy drinkers from lower class people are malnourished and underweight as alcohol is replaced as food and after spending money on alcohol, these lower class people do not have money for nutritious food. I have seen patients who can be labeled as “alcoholic cachexia’.
_
Do stress reactions cause abdominal obesity and co-morbidities?
‘Stress’ embraces the reaction to a multitude of poorly defined factors that disturb homeostasis or allostasis. In a study, the activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system have been utilized as objective measurements of stress reactions. Although long-term activation of the sympathetic nervous system is followed by primary hypertension, consequences of similar activation of the HPA axis have not been clearly defined. In population studies adrenal hormones show strong statistical associations to centralization of body fat as well as to obesity. There is considerable evidence from clinical to cellular and molecular studies that elevated cortisol, particularly when combined with secondary inhibition of sex steroids and growth hormone secretions, is causing accumulation of fat in visceral adipose tissues as well as metabolic abnormalities (The Metabolic Syndrome). Hypertension is probably due to a parallel activation of the central sympathetic nervous system. Depression and ‘the small baby syndrome’ as well as stress exposure in men and non-human primates are followed with time by similar central and peripheral abnormalities. Glucocorticoid exposure is also followed by increased food intake and ‘leptin resistant’ obesity, perhaps disrupting the balance between leptin and neuropeptide Y to the advantage of the latter. The consequence might be ‘stress-eating’, which, however, is a poorly defined entity. Factors activating the stress centers in humans include psychosocial and socioeconomic handicaps, depressive and anxiety traits, alcohol and smoking, with some differences in profile between personalities and genders. Polymorphisms have been defined in several genes associated with the cascade of events along the stress axes. Based on this evidence it is suggested that environmental, perinatal and genetic factors induce neuroendocrine perturbations followed by abdominal obesity with its associated co-morbidities.
_
Evidence that endocrine disruptors can increase adiposity:
Endocrine disruptors (EDs) are lipophilic, environmentally stable, industrially produced substances that can affect endocrine function and include dichlorodiphenyltrichloroethane, some polychlorinated biphenols and some alkylphenols. By disturbing endogenous hormonal regulation, EDs may fatten through multiple pathways. Consider the effect of estrogen on white adipose tissue: in rodents, white adipose is increased by ovariectomy and decreased by estrogen replacement therapy. Similarly, postmenopausal women have increased white adipose tissue, which is reduced by estrogen replacement therapy. The estrogen receptor-alpha knockout mouse has increased white adipose tissue in mice of both sexes. Some EDs directly bind to nuclear receptors, including the peroxisome proliferator-activated receptor italic gamma and the retinoic acid X receptor. Kanayama et al. found that the organontin EDs are high-affinity agonists for the peroxisome proliferator-activated receptor italic gamma and retinoic acid X receptor and stimulate adipocyte proliferation. Other EDs are antagonists of certain nuclear receptors. For example, vinclozolin is a dicarboximide fungicide and an androgen receptor antagonist. Some EDs are antiandrogens and may thereby alter nutrient partitioning toward a more fatty body composition. Endocrine disruptors can also inhibit aromatases and the aromatase knockout mouse has increased adiposity. In humans, body ED burden and BMI or fat mass are positively correlated, even when normalized to total body triglyceride.
_
Obesity and social class:
_
The figure below shows that lower income Americans have double the obesity rate than wealthy Americans:
People with lower levels of education and lower incomes are more likely to be overweight or obese. This may be because they have less opportunity to eat healthy foods and take part in physical activities. Much research has focused on the dietary choices and food availability among those with lower incomes. The cheapest foods contain high levels of refined grains and added sugars and fat, and tend to include processed ingredients and high-fructose corn syrup, proven to cause obesity. In addition, sodas and sugar-sweetened drinks have become more common, adding calories to daily diets. We tend to think about weight as a personal choice: we assume that people make the choice to eat healthy food or to eat junk food. In reality it costs more to eat healthy food than it does to eat junk food. Fast food is easily accessible and it’s filling, but there are many more fat calories than in fresh fruit and vegetables. But fresh fruit and vegetables cost more. Many families don’t have the money to make healthy food choices. So they buy bulk processed food so their kids don’t go hungry.
_
Factors that link low income groups to obesity include:-
- Lack of awareness of nutritious foods.
- Increased calorie intake from junk and fast foods with low nutritious value.
- Low-income neighbourhoods usually lack full-service grocery stores and farmers’ markets that can provide fruits, vegetables, whole grains, and low-fat dairy products. As an alternative there are more convenience stores.
- Healthy foods when available are usually more expensive. Alternatives of refined grains, added sugars, and fats are inexpensive and readily available in low-income communities.
- Lack of filling and nutritious foods also means eating less or skipping meals. This also means that when food is available there is overeating. This leads to cycles of food restriction or deprivation followed by overeating.
- Lack or limited access to healthcare. This results in lack of diagnosis and treatment of emerging obesity.
- Lack of physical activity is also common among low income neighbourhoods. There are fewer parks, gymnasiums, bike paths. Unsafe neighbourhoods also mean children get less time to spend out of doors playing.
- Low-income families also face high levels of stress due to food insecurity, financial pressures, lack of access to health care, inadequate transportation, poor housing and surrounding neighbourhood violence. Stress may lead to weight gain and obesity as well.
_
Few years ago, an obesity researcher at the University of Washington named Adam Drewnowski ventured into the supermarket to solve a mystery. He wanted to figure out why it is that the most reliable predictor of obesity in America today is a person’s wealth. For most of history, after all, the poor have typically suffered from a shortage of calories, not a surfeit. So how is it that today the people with the least amount of money to spend on food are the ones most likely to be overweight? Drewnowski gave himself a hypothetical dollar to spend, using it to purchase as many calories as he possibly could. He discovered that he could buy the most calories per dollar in the middle aisles of the supermarket, among the towering canyons of processed food and soft drink. (In the typical American supermarket, the fresh foods — dairy, meat, fish and produce — line the perimeter walls, while the imperishable packaged goods dominate the center.) Drewnowski found that a dollar could buy 1,200 calories of cookies or potato chips but only 250 calories of carrots. Looking for something to wash down those chips, he discovered that his dollar bought 875 calories of soda but only 170 calories of orange juice. As a rule, processed foods are more “energy dense” than fresh foods: they contain less water and fiber but more added fat and sugar, which makes them both less filling and more fattening. These particular calories also happen to be the least healthful ones in the marketplace, which is why we call the foods that contain them “junk.” Drewnowski concluded that the rules of the food game in America are organized in such a way that if you are eating on a budget, the most rational economic strategy is to eat badly — and get fat.
_
Energy density vs. energy cost:
The highest rates of obesity in the United States occur among population groups with the highest poverty rates and the least education. The impact of socioeconomic variables on obesity may be mediated, in part, by the low cost of energy-dense foods. The observed inverse relationship between energy density of foods, defined as available energy per unit weight (kilocalories per gram or megajoules per kilogram), and energy cost (dollars per kilocalorie or dollars per megajoule) means that diets based on refined grains, added sugars, and added fats are more affordable than the recommended diets based on lean meats, fish, fresh vegetables, and fruit. Refined grains, added sugars, and added fats are among the lowest-cost sources of dietary energy. They are inexpensive, good tasting, and convenient. In contrast, the more nutrient-dense lean meats, fish, fresh vegetables, and fruit generally cost more. Taste and convenience of added sugars and added fats can also skew food choices in the direction of prepared and prepackaged foods. Paradoxically, attempting to reduce diet costs may lead to the selection of energy-dense foods, increased energy intakes, and overweight. Economic decisions affecting food choice may have physiologic consequences. Laboratory studies suggest that energy-dense foods and energy-dense diets have a lower satiating power and may result in passive overeating and therefore weight gain. Epidemiologic analyses suggest that the low-cost energy-dense diets also tend to be nutrient poor. If the rise in obesity rates is related to the growing price disparity between healthy and unhealthy foods, then the current strategies for obesity prevention may need to be revised. The present energy-cost framework provides an economic explanation for the observed links between obesity and the food environment, with diet cost as the principal intervening variable. If higher food costs represent both a real and perceived barrier to dietary change, especially for lower-income families, then the ability to adopt healthier diets may have less to do with psychosocial factors, self-efficacy, or readiness to change than with household economic resources and the food environment. Continuing to recommend costly diets to low-income families as a public health measure can only generate frustration and culpability among the poor and less-well educated. Obesity in America is, to a large extent, an economic issue.
_
The correlation between social class and BMI varies globally. A review in 1989 found that in developed countries women of a high social class were less likely to be obese. No significant differences were seen among men of different social classes. In the developing world, women, men, and children from high social classes had greater rates of obesity. An update of this review carried out in 2007 found the same relationships, but they were weaker. The decrease in strength of correlation was felt to be due to the effects of globalization. Among developed countries, levels of adult obesity, and percentage of teenage children who are overweight, are correlated with income inequality. A similar relationship is seen among US states: more adults, even in higher social classes, are obese in more unequal states. Many explanations have been put forth for associations between BMI and social class. It is thought that in developed countries, the wealthy are able to afford more nutritious food, they are under greater social pressure to remain slim, and have more opportunities along with greater expectations for physical fitness. In undeveloped countries the ability to afford food, high energy expenditure with physical labor, and cultural values favoring a larger body size are believed to contribute to the observed patterns. Attitudes toward body mass held by people in one’s life may also play a role in obesity. A correlation in BMI changes over time has been found among friends, siblings, and spouses. Stress and perceived low social status appear to increase risk of obesity.
_
Socioeconomic status and obesity: A review of the literature:
The relationship among malnutrition, infectious disease, and poverty, and the relationship among obesity, chronic disease, and economic well-being are no longer applicable in the developed countries and are being reduced daily in the developing countries in the region. In peri-urban areas it is normal to find a family in which the father has high blood pressure, may be fat or not, is short, and has a probable history of malnutrition; the mother is anemic, probably obese, and short; and the children suffer frequent infections and show stunting. A review of 144 published studies of the relationship between socioeconomic status (SES) and obesity reveals a strong inverse relationship among women in developed societies. The relationship is inconsistent for men and children in developed societies. In developing societies, however, a strong direct relationship exists between SES and obesity among men, women, and children. A review of social attitudes toward obesity and thinness reveals values congruent with the distribution of obesity by SES in different societies. Several variables may mediate the influence of attitudes toward obesity and thinness among women in developed societies that result in the inverse relationship between SES and obesity. They include dietary restraint, physical activity, social mobility, and inheritance.
_
_
Poverty and obesity:
Another Latin American study found that the poor do not eat what they want, or what they know they should eat, but what they can afford. Restrictions on access to food determine two simultaneous phenomena that are two sides of the same coin: the poor are malnourished because they do not have enough to feed themselves and they are obese because they eat poorly, with a significant energy imbalance. The foods available to them are industrialized, massproduced, undifferentiated, and inexpensive products. In the Buenos Aires metropolitan area, Argentina’s major urban conglomerate, prices for fruits and vegetables, lean meats, and dairy products tend to increase faster than the average rate of inflation. Given this situation, the poor choose foods that are rich in carbohydrates, fats, and sugars, which do not provide them with adequate nutrition, but do satisfy their appetites and are easily incorporated in their traditional consumption patterns and their standards of commensalism (group meals). For its part, the food industry fosters this behavior by compartmentalizing supply and marketing mass, low-quality products with a higher fat and sugar content that are targeted to sectors with less purchasing power.
_
Poverty and obesity: the role of energy density and energy costs: a review:
Many health disparities in the United States are linked to inequalities in education and income. This review focuses on the relation between obesity and diet quality, dietary energy density, and energy costs. Evidence is provided to support the following points. First, the highest rates of obesity occur among population groups with the highest poverty rates and the least education. Second, there is an inverse relation between energy density (MJ/kg) and energy cost ($/MJ), such that energy-dense foods composed of refined grains, added sugars, or fats may represent the lowest-cost option to the consumer. Third, the high energy density and palatability of sweets and fats are associated with higher energy intakes, at least in clinical and laboratory studies. Fourth, poverty and food insecurity are associated with lower food expenditures, low fruit and vegetable consumption, and lower-quality diets. A reduction in diet costs in linear programming models leads to high-fat, energy-dense diets that are similar in composition to those consumed by low-income groups. Such diets are more affordable than are prudent diets based on lean meats, fish, fresh vegetables, and fruit. The association between poverty and obesity may be mediated, in part, by the low cost of energy-dense foods and may be reinforced by the high palatability of sugar and fat. This economic framework provides an explanation for the observed links between socioeconomic variables and obesity when taste, dietary energy density, and diet costs are used as intervening variables. More and more Americans are becoming overweight and obese while consuming more added sugars and fats and spending a lower percentage of their disposable income on food.
__
Air conditioning:
You have to burn calories if your environment is too hot or too cold for comfort. But more people than ever live and work in temperature-controlled homes and offices and therefore burn less calories and get fat.
_
A Common Virus may contribute to Obesity:
Scientists have found new evidence linking a common kind of viral infection to obesity. The human adenovirus-36 (Ad-36) — a cause of respiratory infections and pinkeye — may also be a contributing factor to obesity, as it’s been found to transform adult stem cells into fat cells, capable of storing additional fat. Their findings were reported at the 234th national meeting and exposition of the American Chemical Society in Boston, Massachusetts. In a previous epidemiologic study, the researchers found that about 30 percent of obese people were infected with the Ad-36 virus, compared to 11 percent of lean individuals. Exactly how the virus causes obesity in people is still unknown, but it is believed that a specific gene, called E4Orfl, in the virus may be the culprit that promotes the obesity effect. The team is now trying to figure out the factors that predispose some people with the virus to develop obesity, whereas others remain unaffected. Researchers hope the findings may lead to a future vaccine or antiviral obesity medication.
_
Body fat and metabolic syndrome:
The metabolic syndrome is a cluster of risk factors for atherosclerosis. Although a universally accepted definition is still lacking because available classifications present slightly different diagnostic criteria, the metabolic syndrome is now recognized as a serious public health problem that affects up to 45% of the population >50 years of age in the United States and ~20 to 25% of the adult population in Europe. To diagnose the metabolic syndrome, the concomitant presence of at least three components, among them visceral obesity defined by the measurement of the waist circumference, elevated blood pressure, hyperglycemia, hypertriglyceridemia, or reduced high-density lipoprotein cholesterol levels, is required. The concomitant presence of these risk factors, and thus the presence of the metabolic syndrome, is associated with inflammatory and hypercoagulable states that through increased levels of coagulation factors, reduction in fibrinolysis, endothelial dysfunction, and platelet hyperreactivity may predispose patients to develop cardiovascular events. Several studies have consistently shown that patients with the metabolic syndrome are at significantly increased risk of diabetes, coronary artery disease, and ischemic stroke. A few recent studies suggest that the metabolic syndrome may also play a role in the pathogenesis of venous thromboembolism, but this latter finding needs confirmation by large clinical studies.
_
The metabolic syndrome is a constellation of metabolic risk factors that consist of the following:
- Atherogenic dyslipidemia [serum elevations of triglycerides, apolipoprotein B (apo B), and small low-density lipoprotein (LDL) particles plus low high-density lipoprotein (HDL) cholesterol]
- Elevated blood pressure
- Elevated glucose associated with insulin resistance
- Prothrombotic state
- Proinflammatory state
Many of these factors can be identified through special testing but are not measured in clinical practice. Recently the National Cholesterol Education Program Adult Treatment Panel III report proposed a simple scheme for the routine diagnosis of metabolic syndrome. According to this scheme, a diagnosis of metabolic syndrome can be made if a person has three of the following five features:
- Increased waist circumference (≥102 cm in men and ≥ 88 cm in women)
- Elevated triglycerides (≥150 mg/dl)
- Reduced HDL cholesterol (<40 mg/dl in men and < 50 mg/dl in women)
- Elevated blood pressure (≥130/85 mm Hg or on treatment for hypertension)
- Elevated glucose (≥100 mg/dl)
_
Our understanding of the relation between obesity and metabolic risk factors is growing rapidly. This understanding is based on the discovery of multiple products released from adipocytes. In the presence of obesity, these products are released in abnormal amounts. Each of these products has been implicated in the causation of one or another of the metabolic risk factors. The following is a list of the factors most implicated in the development of metabolic syndrome:
- Nonesterified fatty acids (NEFAs)
- Inflammatory cytokines
- PAI-1
- Adiponectin
- Leptin
- Resistin
_
Vitamin D and obesity:
Does obesity cause vitamin D deficiency or the other way around?
A new study published in the journal PLOS Medicine provides some of the strongest evidence yet that obesity is indeed a cause of vitamin D deficiency. The large study was a collaborative effort between U.S. and European researchers, and was funded by the British Heart Foundation and the UK Medical Research Council. Many prior studies have found a strong correlation between obesity and low levels of vitamin D. According to the researchers, however, the new study is the first that has actually been able to show a causal link, confirming that obesity causes vitamin D deficiency rather than the other way around (and rather than both conditions being caused by some third factor). The researchers examined 21 separate studies on a total of 42,024 adults of European ancestry to collect data on not just vitamin D levels and body mass index (BMI, a measure of obesity), but also on 12 separate genetic variations related to BMI and four genetic variations related to vitamin D levels. Based on prior research into these genetic variations, all participants were assigned scores approximating their genetic predispositions to obesity and to lower vitamin D levels. The researchers hypothesized that if obesity is a cause of vitamin D deficiency, then people with a high genetic predisposition to obesity should be more likely to have a lower vitamin D levels. In contrast, if it is vitamin D deficiency that causes obesity, then a genetic predisposition to vitamin deficiency should be associated with higher obesity rates. Confirming the findings of previous studies, the researchers found that every 1 kg/m2 increase in BMI was associated with a 1.15 percent lower vitamin D blood concentration. In a separate statistical analysis, the researchers also found that a 10 percent increase in BMI led to a 4.2 percent decrease in vitamin D levels. All statistical analysis was controlled for the influence of potential confounding factors. Furthermore, the researchers found that people with a higher genetic predisposition to obesity had both a higher BMI and lower vitamin D levels. Yet while people who were genetically predisposed to vitamin D deficiency did indeed have lower vitamin D levels, they did not have higher BMIs than less predisposed individuals. The latter finding was further confirmed in an analysis of 123,864 people taking part in the Genetic Investigation of Anthropometric Traits (GIANT) study. This strongly suggests that obesity is a cause of vitamin D deficiency, not the other way around.
_
15% of urban children are obese in Maharashtra, India; and it is predicted to double in the next 5 years. Obese children face an increased risk of Vitamin D deficiency because they tend to absorb vitamin D in their fat stores, which prevents it from being utilized in their blood. Vitamin D is one of the most important “vitamins” to overall human health and vital functioning of human body. Inadequate exposure to sunlight also acts as a contributing factor causing Vitamin D deficiency. With rapidly changing lifestyle, children too prefer to remain indoors engaging in activities like watching television, playing video games or spending a lot of time with computers that makes them obese. Vitamin D is fat soluble, excess body fat will pull vitamin D out of circulation thus contributing to deficiency. Research shows that about 75% of human body’s supply of Vitamin D is generated by our skin’s exposure to sunlight (UV-B rays in particular). Further, vitamin D deficiency plays a big role in problem related to metabolism and weak metabolism eventually leads to obesity. Also 10% increase in Body Mass Index reduces 4.2% level of vitamin D in human body. Therefore, for obese children it becomes inevitable to expose to sunlight and raise the vitamin D level. Maintaining appropriate levels of vitamin D is critical because it influences nearly 3,000 of the roughly 25,000 genes in the human body. Sufficient vitamin D levels assist the body in not only reducing bad cholesterol and increasing good cholesterol but also help in a multitude of essential repair and maintenance activities. Vitamin D deficiency plus obesity, combined, increases your risk of developing diabetes, heart diseases and some types of cancer much more than just obesity or just low Vitamin D.
_
How government contributes to obesity epidemic?
One example is sufficient. Look at federal subsidies for food production in the U.S.:
- Meat/Dairy — 73.8 percent
- Grains — 13.2 percent
- Sugar/Oil/Starch/Alcohol — 10.7 percent
- Nuts/Legumes — 1.9 percent
- Vegetables/Fruits — 0.4 percent
That’s right – just 1.9 percent for nuts and legumes and 0.4 percent for fruits and vegetables. As a result, a salad often costs you more than a Big Mac.
_
Eating disorders and obesity: two sides of the same coin?
The eating disorders anorexia and bulimia nervosa have traditionally been regarded as entirely separate from obesity. Eating disorders have been regarded as Western culture-bound syndromes, arising in societies with excessive emphasis on weight, shape and appearance, and best treated by psychological therapies, in particular cognitive behavioural therapy or family-based interventions. In contrast, obesity has been considered a medical illness with metabolic and genetic origins, and thought to be best treated by mainstream medicine, involving dietary, drug or surgical treatment. Some researchers believe that this polarisation is fundamentally flawed, and research and treatment of both types of disorder would be better served by greater appreciation of the psychosocial components of obesity and the biological and genetic components of eating disorders. There are similarities in phenotype (such as excessive attempts at weight control, binge eating behaviours) and in risk factors (such as low self-esteem, external locus of control, childhood abuse and neglect, dieting, media exposure, body image dissatisfaction, weight-related teasing and shared susceptibility genes). One example of shared genetic risk is the brain-derived neurotrophic factor (BNDF) gene, in which the valine allele of the Val66Met amino acid polymorphism predisposes to obesity, whereas the methionine allele predisposes to eating disorders. Thus the evidence suggests that these disorders will have both shared and distinct susceptibility factors; some will predispose to both types of disorder, some will push in opposite directions, and some will separate them.
__________
Is obesity self-perpetuating?
The longer a person is overweight, the harder it becomes for them to lose weight. Many have wondered whether obesity itself becomes a permanent state, i.e. does obesity promote obesity? Researchers from the University of Michigan and the National Council of Science and Technology (COINCET) in Argentina, reported in the Journal of Clinical Investigation that in animal experiments, obesity seems to become a self-perpetuating state. They found that the “normal” body weight of mice that become obese starts going up; their bodies’ perception of normal weight becomes a heavier than before, regardless of whether they are made to go on diets which had made them lose weight. Senior author, Malcolm J. Low, M.D., Ph.D., said “Our model demonstrates that obesity is in part a self-perpetuating disorder and the results further emphasize the importance of early intervention in childhood to try to prevent the condition whose effects can last a lifetime. Our new animal model will be used in pinpointing the reasons why most adults find it exceedingly difficult to maintain meaningful weight loss from dieting and exercise alone.”
______________
Childhood obesity:
_
_
Childhood obesity is one of the most serious public health challenges of the 21st century. Overweight children are likely to become obese adults. They are more likely than non-overweight children to develop diabetes and cardiovascular diseases at a younger age, which in turn are associated with a higher chance of premature death and disability. Children’s choices, diet and physical activity habits are influenced by their surrounding environment. Childhood obesity has reached epidemic proportions in 21st century, with rising rates in both the developed and developing world. Rates of obesity in Canadian boys have increased from 11% in 1980s to over 30% in 1990s, while during this same time period rates increased from 4 to 14% in Brazilian children. Childhood obesity has more than doubled in children and tripled in adolescents in the past 30 years. In 2010, more than one third of children and adolescents were overweight or obese in the U.S. Rates of childhood obesity have increased greatly between 1980 and 2010. Currently 10% of children worldwide are either overweight or obese. Poor diet is one risk factor for childhood obesity that we all know. Overeating causes a child to take in more calories than he or she needs to function, which leads to weight gain. The large (too big) portion sizes you find on restaurant plates don’t help. Foods that are packed with sugar, fat and calories, such as candy, fast food and soft drinks, mean excess calories, too. Not getting enough physical activity is another risk factor. Thanks to television, video games and recreational computer use, children are finding more reasons to settle into a comfy chair. They aren’t moving around in physical education classes, either. In fact, as of 2003, just 28 percent of adolescents took part in daily physical education classes in the U.S.
_
Birth factors:
Some studies suggest that a person is more likely to become obese later in life if they experienced poor nutrition in utero, maternal smoking, or had a low birth weight However, other studies show that high birth weight (especially above 4,000 g) is a stronger risk for becoming overweight. There is some evidence showing that breastfeeding infants compared with formula feeding is associated with a reduced risk of becoming obese.
_
Obesity predicted by parent’s weight:
A child’s risk of becoming an obese adult is strongly influenced by parental obesity in either the mother or father. For children under 10 years old, whether obese or not, having an obese parent more than doubles the risk of adult obesity. The toddler who’s overweight but whose parents are of normal weight has a 90% chance of not being an obese adult, or a 10% chance of adult obesity. But toddlers who are overweight and have at least one parent who’s overweight, their risk goes up to 40%.
_
Obesity risk could be passed on through Sperm, shows mice study:
A new study out of the University of Adelaide has found “molecular signals” in the sperm of obese mice fathers that could pass on obesity and/or metabolic disease to their children. This effect was found to last two generations to the mice’s grandchildren, even if the progeny were healthy eaters. With obese fathers, the changes in their sperm – in their microRNA molecules – might program the embryo for obesity or metabolic disease later in life. What they have also found is that there is an increased chance of both male and female offspring developing metabolic disease similar to type 2 diabetes. Even mice fathers who were obese but did not show any signs of diabetes were found to pass on the risk of this metabolic disease down to two generations. Though the obesity seen in the second generation was not as “severe” as the first generation of offspring, these results were found regardless of the eating habits of either generation.
_
Genetics in childhood obesity:
Childhood obesity is often the result of interplay between many genetic and environmental factors. Polymorphisms in various genes controlling appetite and metabolism predispose individuals to obesity when sufficient calories are present.
As such obesity is a major feature of a number of rare genetic conditions that often present in childhood. These are:
Prader-Willi syndrome with an incidence between 1 in 12,000 and 1 in 15,000 live births is characterized by hyperphagia and food preoccupations which leads to rapid weight gain in those affected.
Bardet-Biedl syndrome
MOMO syndrome
Leptin receptor mutations
Congenital leptin deficiency
Melanocortin receptor mutations
In children with early-onset severe obesity (defined by an onset before ten years of age and body mass index over three standard deviations above normal), 7% harbor a single locus mutation.
__
Lack of breastfeeding support:
Breastfeeding protects against childhood overweight and obesity. However, in the United States, while 75% of mothers start out breastfeeding, only 13% of babies are exclusively breastfed at the end of 6 months. The success rate among mothers who want to breastfeed can be improved through active support from their families, friends, communities, clinicians, health care leaders, employers, and policymakers.
_
The figure below shows increased prevalence of childhood obesity since 1974 till 2004 in the U.S.
_
BMI in children:
Rapid changes in BMI occur in normal growth, and BMI varies with age and sex. It rises in the first year of life, then falls during preschool years, before rising again into adolescence. The point at which BMI starts to rise again (usually around 4–6 years of age) is termed “adiposity rebound”. Thus, calculated BMI values need to be compared with age and sex reference standards. For clinical use, the expert working group has recommended the BMI-for-age percentile charts. [vide supra]
_
Television and media:
Children 8—18 years of age spend an average of 7.5 hours a day using entertainment media, including TV, computers, video games, cell phones, and movies. Of those 7.5 hours, about 4.5 hours is dedicated to viewing TV. Eighty-three percent of children from 6 months to less than 6 years of age view TV or videos about 1 hour and 57 minutes a day. TV viewing is a contributing factor to childhood obesity because it may take away from the time children spend in physical activities; lead to increased energy intake through snacking and eating meals in front of the TV; and, influence children to make unhealthy food choices through exposure to food advertisements.
_
Calorie-rich drinks and foods are readily available to children. Consumption of sugar-laden soft drinks may contribute to childhood obesity. In a study of 548 children over a 19 month period the likelihood of obesity increased 1.6 times for every additional soft drink consumed per day. High consumption of sugary drinks, which have few, if any, nutrients, has been associated with obesity. On a typical day, 80% of youth drink sugary drinks. Calorie-dense, prepared snacks are available in many locations frequented by children. High-energy-dense foods are ones that have a lot of calories in each bite. A recent study among children showed that a high-energy-dense diet is associated with a higher risk for excess body fat during childhood. Portion sizes of less healthy foods and beverages have increased over time in restaurants, grocery stores, and vending machines. Research shows that children eat more without realizing it if they are served larger portions. This can mean they are consuming a lot of extra calories, especially when eating high-calorie foods.
_
Besides genetic factors, environmental factors (TV viewing, physical inactivity) and diet (high fat foods, sweet drinks); Other risk factors for obesity in childhood and adolescence include:
- Early infant feeding: Breastfeeding is possibly protective for the development of obesity.
- Parental obesity, eating patterns, and attitudes: Parental obesity more than doubles the risk of adult obesity among both obese and non-obese children. Dietary disinhibition in the mothers of preschoolers is associated with subsequent excess weight gain in their daughters, and a 6-year outcome study of children showed that parental dietary disinhibition is associated with greater increases in body fatness. Parents who strongly encourage their children to eat have heavier children.
- Early adiposity rebound: Earlier adiposity rebound is associated with increased body fatness in adolescence.
- Socioeconomic status: In some developed countries, poorer children or those who live in rural settings are more at risk of obesity, whereas in countries undergoing economic transition childhood obesity is associated with a more affluent lifestyle and with living in urban regions.
- Ethnicity: Data from the United States show that there is an increased risk of obesity in Native Americans and Hispanic Americans compared with white Americans, although these differences may be largely related to differences in socioeconomic status.
- Underlying medical disorders: Secondary obesity may occur with medical conditions, including hypothyroidism, hypercortisolism, growth hormone deficiency and hypothalamic damage.
- Prescription drugs: Some drugs may contribute to obesity. These include glucocorticoids, antipsychotic drugs (eg, risperidone) and some antiepileptic medications.
_
Obesity can be predicted in 2-month old infants:
Researchers have found that infants as young as two months old already exhibit growth patterns that can predict the child’s weight by age 5. “Almost from birth, we quickly saw this growth pattern emerge in our curves and growth charts for weight over height,” Susan Ludington, the study’s lead investigator and the Carl W. and Margaret David Walter Professor of Pediatric Nursing at Case Western Reserve University, said. Analyzing well-child records, normal-weight babies with a body-mass index (BMI) in the 17 percentile were found to have plateaued at about two months and rarely deviated over the next five years, she said. Overweight or obese babies crossed the 17 percentile many months later (about age 14 months) and continued an upward climb when BMI growth patterns were monitored. The researchers found that, by age 5, normal-weight children developed differently from birth than those considered overweight, obese or severely obese. The researchers suspect, based on prior research findings by others, how a mother ate during pregnancy might have contributed to a baby’s hormones and the ability to satisfy a baby’s hunger. By graphing, a pattern emerged that found both girls and boys known to be obese at 5 begin to show significantly higher weight over height than normal weight babies as early as 2-4 months of age. Because such patterns emerge before children generally start eating solid food, early life growth patterns may provide important information about a person’s future health issues, Ludington said. The researchers also questioned using the BMI index as a guide to growth, which is based on European babies primarily breast-fed in the first year. In the United States, many babies have only formula feedings. These findings could potentially change the age at which obesity is typically diagnosed, which is now at or after age two. The findings are published in the journal Clinical Pediatrics.
_
Pushing kids to eat may cause obesity later:
Researchers found that between 50 and 60% of parents from our sample reported requiring that their child eat all of the food on their plate at a meal. Further, they found that between 30-40% of parents from within our sample reported encouraging their child to continue eating even after their child stated that they were full. While these pressure-to-eat behaviors were more frequent among parents of non-overweight adolescents, they were still endorsed quite frequently by parents of overweight and obese adolescents, indicating that many parents endorse these behaviors regardless of their child’s current weight status. Researchers also found dads were more likely than moms to pressure their sons and daughters to eat, and adolescent boys were pressured more than adolescent girls. Parental pressure to eat can be detrimental to children because it takes away from a child’s ability to respond naturally to their own hunger. Instead, (it) encourages them to respond to cues in their environment which can lead to unhealthy weight gain over time. The data also showed that restricting food from kids was a common practice of either parent, in both boys and girls. Research has shown that when a parent places a restriction on a particular food item (i.e. no treats) that a child becomes more interested in consuming that food item and will often overeat that food when given the opportunity. Instead, parents should be encouraged to allow their children to eat all foods in moderation. Investigators believe that parents should keep an eye on their child’s weight and make an effort to better understand good eating practices, instead of worrying about whether their kids clean their plates or have a cookie now and then. Study authors recommended such practices as eating regular family meals, having nutritious snacks at home, choosing healthy foods and encouraging young people to make better food choices as a way to fight weight problems. And most importantly parents should also work hard to model healthy eating and a healthy relationship with food to their child by eating a well-balanced diet.
_
Why do parents let their children get fat?
Obesity experts say parents are struggling with a multitude of problems when it comes to their child’s weight. They range from a lack of education about food, limited cooking skills and limited money to buy healthier food to longer working hours and marketing campaigns for junk food aimed at kids. This starts with ‘clean your plate’ syndrome – my mother put down huge portions and made us carry on eating long after we were full”. Many parents don’t realize their child is fat when it might be obvious to other people. According to studies, 75% of parents underestimated the size of an overweight child, while 50% underestimated the size of an obese child.
_
What can be targeted for control and prevention of childhood obesity?
_
Suggestions to parents for the promotion of healthy nutrition at home:
For infants and young children:
- exclusively breastfeed;
- avoid the use of added sugars and starches when feeding formula;
- accept the child’s ability to regulate energy intake rather than feeding until the plate is empty;
- assure the appropriate micronutrient intake needed to promote optimal linear growth.
For children and adolescents:
- provide healthy breakfast before each school day;
- serve healthy school snacks to children (whole-grain, vegetables, fruits);
- promote intake of fruits and vegetables;
- restrict intake of energy-dense, micronutrient-poor foods (e.g. packaged snacks);
- restrict intake of sugars-sweetened soft drinks;
- ensure opportunity for family meals;
- limit exposure to marketing practices (e.g. limit television-viewing);
- teach children to resist temptation and marketing strategies;
- provide information and skills to make healthy food choices.
_
Role of schools:
Suggestions to promote healthy diets in Schools:
- provide health education to help students acquire knowledge, attitudes, beliefs and skills which are needed to make informed decisions, practice healthy behaviors and create conditions that are conducive to health;
- provide school food programs to increase the availability of healthy food in schools (e.g. breakfast, lunch and/or snacks at reduced price);
- have vending machines only if they sell healthy options like water, milks, juices, fruits and vegetables, sandwiches and low-fat snacks;
- ensure that food served in schools adheres to minimum nutrition standards;
- provide school health services for students and staff of the school to help foster health and well-being as well as prevent, reduce, monitor, treat and refer important health problems or conditions for students and staff of the school;
- use school gardens as a tool to develop awareness about food origins;
- promote parental involvement.
___
Another viewpoint on sweet drink and obesity in children:
In an analysis of the CSFII data, workers reported that overweight children consume more total drinks, including sugar-sweetened drinks, milk, cordials, and fruit drinks. However, the slight increase of 200 g/day for soft drinks is of little practical importance. Therefore, consumption of soft drinks might be an indicator for other dietary changes, rather than a cause of the obesity per se. Soft drink consumption is a marker for increased consumption of other foods.
_
Could skimmed milk be contributing to obesity epidemic?
Low-fat dairy can encourage weight gain, say experts. Experts say that eating low calorie foods does not necessarily mean lower calorie intake – in some cases it can make you eat or drink more. They say there is very little data to back up the idea that skimmed milk promotes weight loss or management and that because reduced fat foods might not be as filling, they could lead consumers to compensate by eating and drinking more. Study found that children who drank skimmed milk in childhood grew up to be larger than those brought up on whole milk. Reduced-fat milk products are often pumped with sugar to make them taste better – One glass of low-fat chocolate milk contains 158 calories – 68 of them coming from solid fats and added sugars – while a glass of unflavoured, semi-skimmed milk has 122 calories, with 37 of them coming from solid fats and sugars. Full-fat milk only contains three to four per cent fat. Finally, it should not be forgotten that research has shown that skimmed milk also provides less nutrients than whole. Full-fat dairy is a vital source of the fat-soluble vitamins A, D, E and K as well as calcium and phosphorus, the minerals that work with vitamin D for build strong bones. But the term ‘fat-soluble’ means that these vitamins need to be delivered in or with fat for the nutrients to be available to the body. Taking the fat out makes it difficult or even impossible to absorb them.
_
Does milk promote weight loss?
Amy Joy Lanou of the University of North Carolina at Asheville and Neal Barnard with the Physicians Committee for Responsible Medicine in Washington, DC, evaluated evidence from 49 clinical trials from 1966 to 2007 that assessed the effect of milk, dairy products, or calcium intake on body weight and BMI, with or without the use of dieting. Evidence from the trials showed that neither dairy products nor calcium supplements helped people lose weight. Of the 49 clinical trials, 41 showed no effect, two demonstrated weight gain, one showed a lower rate of weight gain, and only five showed weight loss.
_
Effectiveness of intervention on physical activity of children: systematic review and meta-analysis of controlled trials with objectively measured outcomes:
This review provides strong evidence that physical activity interventions have had only a small effect (approximately 4 minutes more walking or running per day) on children’s overall activity levels. This finding may explain, in part, why such interventions have had limited success in reducing the body mass index or body fat of children.
_
ADHD may prime boys for Obesity:
In the study published in Pediatrics, researchers connected the impulsive behavior that can characterize attention-deficit/hyperactivity disorder (ADHD) with the overeating that contributes to calorie overload. In the 33-year study that tracked boys with ADHD into adulthood, men who were hyperactive as children were twice more likely to have higher body-mass-index readings and rates of obesity than men who didn’t have the condition as children. Of men diagnosed with ADHD as kids, 41% were obese compared with 22% of men who didn’t have ADHD as children. The average rate of obesity for men in this age group was 24%. It’s not entirely clear why the disorder, which can make focusing and concentrating on tasks more difficult, would lead men with the disorder to weigh so much more than their peers. But the researchers suspect that impulsivity and poor decision making skills played a role. We live in a society with supersized amounts of food. If someone has less than the average amount of self-control because of the ADHD, they are less able to withstand the temptations of food.
_
BPA linked to obesity in young girls: [discussed vide supra: endocrine disruptors]
The environmental culprit, according to the study published in the journal PLOS ONE, may be bisphenol-a, a chemical commonly found in plastic and cans. Li and colleagues studied 1,326 school-age children in Shanghai, China, and measured BPA levels in their urine. In girls ages 9 to 12, higher BPA urine levels were associated with a doubled risk of obesity. And as BPA urine levels increased, so did the girls’ obesity risk – measured using their weight in reference to weight distribution in the population. But strikingly, only girls in this age group were affected, the research showed. Neither girls outside of the 9-12 age range nor boys experienced a risk of being overweight or obese, even with high levels of BPA in their urine. Girls seem to be more sensitive to environmental impact, and authors don’t know exactly why.
_
Undernourished mother and obese child:
Scientists from Auckland University’s Liggins Institute have discovered that genetic pre-disposition to obesity can be reversed through good nutrition in early childhood. Their research shows that when a mother is undernourished, her child’s body is pre-set to cope with a life of scarcity. And remember, undernourished does not necessarily mean not eating enough food. Many overeaters in the western world are severely undernourished due to an appalling diet of junk food. Not only are their children likely to become fat because they eat the same foods as their parents, but also because they are biologically storing for not enough nutrients….a double whammy. In laboratory tests, newborn offspring from both well-fed and undernourished rats were given leptin, a hormone that signals to the body when it has eaten enough. When they became adults, the long-term effects were measured by looking at genes that regulate metabolism in the liver. Rats from well-fed mothers reacted to leptin in the opposite way to those from undernourished mothers. The researchers urge mothers to eat a more balanced diet (with the right amounts of protein and vitamins) during pregnancy. However, if the fetus is under-nourished in the womb, the long-term effects can still be corrected through good nutrition in early childhood.
_
What are the consequences of childhood obesity?
Health risks now:
•Childhood obesity can have a harmful effect on the body in a variety of ways. Obese children are more likely to have– ◦High blood pressure and high cholesterol, which are risk factors for cardiovascular disease (CVD). In one study, 70% of obese children had at least one CVD risk factor, and 39% had two or more.
◦Increased risk of impaired glucose tolerance, insulin resistance and type 2 diabetes.
◦Breathing problems, such as sleep apnea, and asthma.
◦Joint problems and musculoskeletal discomfort.
◦Fatty liver disease, gallstones, and gastro-esophageal reflux (i.e., heartburn).
◦Obese children and adolescents have a greater risk of social and psychological problems, such as discrimination and poor self-esteem, which can continue into adulthood.
Health risks later:
• Obese children and adolescents are more likely to become obese as adults. For example, one study found that approximately 80% of children who were overweight at aged 10–15 years were obese adults at age 25 years. Another study found that 25% of obese adults were overweight as children. The latter study also found that if overweight begins before 8 years of age, obesity in adulthood is likely to be more severe. A study has also found that tackling childhood obesity will not necessarily lead to eating disorders later in life. Adult obesity is associated with a number of serious health conditions including heart disease, diabetes, and some cancers.
_
Childhood obesity management:
Because childhood obesity often persists into adulthood and is associated with numerous chronic illnesses, children who are obese are often tested for hypertension, diabetes, hyperlipidemia, and fatty liver. Treatments used in children are primarily lifestyle interventions and behavioral techniques, although efforts to increase activity in children have had little success. In the United States, medications are not FDA approved for use in this age group. Social and economic development as well as policies in the areas of agriculture, transport, urban planning, environment, education, food processing, distribution and marketing influence children’s dietary habits and preferences as well as their physical activity patterns. Increasingly, these influences are promoting unhealthy weight gain leading to a steady rise in the prevalence of childhood obesity
_
A study of 1800 children aged 2 to 12 in Colac, Australia tested a program of restricted diet (no carbonated drinks or sweets) and increased exercise. Interim results included a 68% increase in after school activity programs, 21% reduction in television viewing, and an average of 1 kg weight reduction compared to a control group.
_
The two best steps to take for getting to your goal are to improve your child’s diet and increase physical activity. Your health care provider can give you detailed guidance, but below are a few diet and activity suggestions to get you started:
- Watch portion sizes and limit access to foods high in sugar.
- Add lots of fruits and vegetables to your child’s diet.
- Select whole-grain foods.
- Target healthy protein sources through beans, fish, poultry or lean meat.
- Create opportunities for a variety of exercises that are fun, such as dancing, swimming, hiking and jumping rope.
- Limit sedentary activities, such as watching television or playing video games.
- Make it a family affair; get everyone moving together
_
Prevention of childhood obesity:
- Healthy lifestyle habits, including healthy eating and physical activity, can lower the risk of becoming obese and developing related diseases.
- The dietary and physical activity behaviors of children and adolescents are influenced by many sectors of society, including families, communities, schools, child care settings, medical care providers, faith-based institutions, government agencies, the media, and the food and beverage industries and entertainment industries.
- Schools play a particularly critical role by establishing a safe and supportive environment with policies and practices that support healthy behaviors. Schools also provide opportunities for students to learn about and practice healthy eating and physical activity behaviors.
______________
Obesity and fertility, conception and pregnancy:
_
Obesity and sex hormones:
Obesity leads to decrease in testosterone, follicle stimulating hormone, inhibin B and sex hormone binding globulin. This leads to low sperm count and quality in obese men. In obese women there is increase in androgen metabolism and elevated estrogen levels. Obese men show low estrogen and low testosterone levels as well.
_
Obesity and infertility:
One of the best established connections between obesity and reproductive problems is the link between obesity and infertility. Obesity decreases the rates of successful pregnancy in natural conception cycles. In women who are undergoing reproductive therapies by accelerating and augmenting their ovulation cycles for better chances of conception, obesity may reduce the rates of pregnancy as well. High levels of leptin and low levels of adiponectin may also reduce rates of conception. Fertility can be partially restored if weight loss can be achieved.
_
Obesity and anovulation:
Obesity is likely to cause insulin resistance that is linked to anovulation or failure of a woman to produce the egg from each ovary each month. Insulin levels and obesity also lead to altered sex hormones, high androgens (male hormones), high levels of free growth factor 1 etc. Studies show that women having anovulation related infertility is 30% higher in women with BMI ranged between 24 and 31 as compared to that of normal weight women. Furthermore for those with BMI over 31, the chance is even 170% higher. Only 5% loss of body weight can increase in ovulation rates and reduces biochemical abnormalities. Studies show that in obese women with anovulation, the underlying cause is most likely due to polycystic ovary syndrome (PCOS). PCOS is associated with obesity or overweight along with symptoms of high male hormone secretion such as hirsutism, acne, high cholesterol level and insulin resistance.
__
Obesity and complications in pregnancy:
In the United States, more than one third of women are obese according to the recent National Health and Nutrition Examination Survey. This means that more than one half of pregnant women are overweight or obese. As obesity is an increasing problem among fertile women, it is crucial that specialists involved in the treatment of these women be aware of the risks of complications and know how to deal with them. Complications associated with obesity in pregnancy are gestational diabetes mellitus, hypertensive disorders, and thromboembolic complications. Complications associated with obesity in labor are augmentation, early amniotomy, cephalopelvic disproportion, cesarean section, and perioperative morbidity. Complications associated with obesity in children are macrosomia, shoulder dystocia, small for gestational age, late fetal death, and congenital malformations, especially neural tube defects.
_
High-fat diet during pregnancy makes offspring fat:
Exposure to a high-fat diet in the womb and after birth can permanently change the cells in the brain that control food intake, predisposing offspring to overeating and an increased preference for fatty and sugary foods, a new study has revealed. The study, funded by the National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases, found that male offspring of maternal monkeys that ate a high-fat diet had increased body weight, compared with the offspring of mothers that ate a low-fat diet. The study’s lead author, Juliana Gastao Franco, PhD, a postdoctoral fellow at Oregon Health and Science University, said that studies in humans have demonstrated that maternal obesity during pregnancy is a strong determinant of offspring body mass index. Franco and her co-investigators studied monkeys born to females that consumed either a low-fat diet, consisting of 14 percent of calories from fat, or a high-fat diet in which 36 percent of calories came from fat. After weaning, 20 offspring of female monkeys on the high-fat diet either received the same high-fat diet (8 monkeys) or were switched to the control diet (12 monkeys). Seven offspring of the control monkeys continued to receive the control diet. It was found that all male offspring that had fetal exposure to a high-fat diet had increased body weight, despite having no changes in their metabolic rate and regardless of what they ate after weaning. Also, the offspring that were switched to the control diet displayed, on average, greater overall food intake and increased binge eating of food with high sugar and fat, compared with either those maintained on a high-fat diet or the controls offspring.
_
Maternal Obesity has little effect on the Immediate Offspring but impacts on the Next Generation:
Maternal obesity during pregnancy has been linked to an increased risk of obesity and cardiometabolic disease in the offspring, a phenomenon attributed to developmental programming. Programming effects may be transmissible across generations through both maternal and paternal inheritance, although the mechanisms remain unclear. Using a mouse model, authors explored the effects of moderate maternal diet-induced obesity (DIO) on weight gain and glucose-insulin homeostasis in first-generation (F1) and second-generation (F2) offspring. DIO was associated with insulin resistance, hyperglycemia and dyslipidemia before pregnancy. Birth weight was reduced in female offspring of DIO mothers (by 6%, P = .039), and DIO offspring were heavier than controls at weaning (males by 47%, females by 27%), however there were no differences in glucose tolerance, plasma lipids, or hepatic gene expression at 6 months. Despite the relative lack of effects in the F1 generation, authors found clear fetal growth restriction and persistent metabolic changes in otherwise unmanipulated F2 offspring with effects on birth weight, insulin levels, and hepatic gene expression that were transmitted through both maternal and paternal lines. This suggests that the consequences of the current dietary obesity epidemic may also have an impact on the descendants of obese individuals, even when the phenotype of the first generation appears largely unaffected.
_
Management of pregnancy with obesity: a study:
Dietary and lifestyle interventions in obese pregnancy are effective in reducing gestational weight gain without any adverse effect on the risk of babies small for gestational age. Compared with physical activity and a mixed approach, dietary interventions were associated with the greatest reduction in weight gain in pregnancy. Interventions also resulted in significant reduction in the risk of pre-eclampsia. There was an overall trend towards reduction in gestational diabetes, gestational hypertension, preterm birth, and intrauterine death with intervention compared with control. Diet in particular, significantly reduced the risk of pre-eclampsia, gestational diabetes, gestational hypertension, and preterm births compared with any other intervention. The interventions had less effect on outcomes related to fetal weight and other morbidity and mortality. Furthermore, there was no evidence that the interventions reduced the rates of caesarean section or induction of labour. The rating of evidence quality was moderate for the lack of effect observed with interventions on size for gestational age. The quality of evidence for the benefit observed with interventions on gestational weight gain was moderate but low for clinical outcomes.
______________
Obesity disease risk:
_
Pathogenesis of obesity effects and complications:
_
_
Health consequences fall into two broad categories: those attributable to the effects of increased fat mass (such as osteoarthritis, obstructive sleep apnea, social stigmatization) and those due to the increased release of peptides from enlarged and/or increased fat cells (diabetes, cancer, cardiovascular disease, non-alcoholic fatty liver disease). Increases in body fat alter the body’s response to insulin, potentially leading to insulin resistance. Increased fat also creates a proinflammatory state, and a prothrombotic state.
_
Obesity effects:
_
Psychological Effects:
Very overweight and obese people have reduced psychological health. Their self-esteem and social interaction may be impaired, they may have a poor body image and anxiety and depression. Many studies show that depression is especially present in females who are obese and that there is more depression present in those who are obese than persons of normal weight. Studies have also shown that binge eating is common amongst severely obese adolescents and is related to high levels of anxiety and depression, as well as to low levels of self-esteem and body attitude. A young female is more vulnerable to the psychological effects and is more likely to exhibit abnormal behaviour such as binge-eating.
Social Effects:
Obese people suffer from prejudice and discrimination in many situations. Society can have a negative opinion of overweight and obese people and they can be perceived in a negative way. They can encounter prejudice and discrimination in, for example, the fields of employment travel, schooling, healthcare, retail and so on. They may have great difficulty in buying clothes and fitting into seats on planes, incurring extra costs in requiring tailor-made clothes and double seats when travelling. Difficulty in getting or keeping a job (with reduced pay and promotion prospects) may lead to poverty reinforced low self-esteem and reduced quality of life.
Effects on Society:
As well as detrimental effect on the individual, obesity places a financial burden on society as a whole. There is a huge cost to the economy from obesity-related ill-health, premature retirement, unemployment, premature death, benefit payments, productivity and loss of working days. The direct and indirect costs of obesity in England (2002) have been conservatively estimated at £3.3 to £3.7 billion. With the rise in obesity, this figure is likely to increase.[obesity and economy-vide infra]
_
Obesity, Depression, and Quality of Life:
The high rates of obesity and depression, and their individual links with cardiovascular disease, have prompted many investigators to explore the relationship between weight and mood. An analysis of 17 cross-sectional studies found that people who were obese were more likely to have depression than people with healthy weights. Since the studies included in the analysis assessed weight and mood only at one point in time, the investigators could not say whether obesity increases the risk of depression or depression increases the risk of obesity. Studies have shown that among middle-aged women, obesity is strongly associated with depression. This association among populations remains consistent even after adjusting for other factors such as race, marital status, educational attainment, tobacco use or antidepressant use. Depression is further associated with significantly lower physical activity levels and higher caloric intake. New evidence confirms that the relationship between obesity and depression may be a two-way street: A meta-analysis of 15 long-term studies that followed 58,000 participants for up to 28 years found that people who were obese at the start of the study had a 55 percent higher risk of developing depression by the end of the follow-up period, and people who had depression at the start of the study had a 58 percent higher risk of becoming obese. Although a biological link between obesity and depression has not yet been definitively identified, possible mechanisms include activation of inflammation, changes in the hypothalamic–pituitary–adrenal axis, insulin resistance, and social or cultural factors. Obesity also raises the risk of panic disorder or agoraphobia, particularly among females. Excess alcohol consumption, poor diet and physical inactivity have been associated with depression as well as obesity.
_
Medical conditions associated with obesity:
_
|
Diabetes |
Polycystic ovarian syndrome |
Urinary incontinence |
Pickwickian syndrome |
|
Cancer |
Gastro-esophageal reflux disease |
Chronic renal failure |
Depression |
|
Congestive heart failure |
Fatty liver disease |
Lymph edema |
Osteoarthritis |
|
Enlarged heart |
Hernia |
Cellulitis |
Gout |
|
Pulmonary embolism |
Erectile dysfunction |
Stroke |
Gallbladder disease |
__
_
Gender differences in the health risks of Obesity:
While excess weight is linked to poor health and a greater risk of disease in men and women, there are some gender differences. The prevalence of obesity among men and women in the US is similar, 35.5% for men and 35.8% for women, and has not changed significantly from 2003-2008. Worldwide, gender differences are more pronounced, with 10% of men and 14% of women obese. Prevalence of overweight is similar. Men are more likely to carry excess fat around their abdomen (referred to as “apple shaped”), which is riskier than carrying weight around the hips and thighs (referred to as “pear shaped”) as many women do. Overweight men also tend to have more visceral fat which substantially increases the risk of heart disease, metabolic syndrome and diabetes. However, after menopause, visceral fat levels rise rapidly in overweight women to comparable levels found in heavier men. This rise evens out the health risks of obesity between genders later in life. As such, calls to action to reduce the prevalence of obesity generally do not differentiate between men and women.
_
Morbidity associated with obesity:
Excessive body weight is associated with various diseases, particularly cardiovascular diseases, diabetes mellitus type 2, obstructive sleep apnea, certain types of cancer, osteoarthritis and asthma. As a result, obesity has been found to reduce life expectancy. Obesity increases the risk of many physical and mental conditions. These comorbidities are most commonly shown in metabolic syndrome, a combination of medical disorders which includes: diabetes mellitus type 2, high blood pressure, high blood cholesterol, and high triglyceride levels. Complications are either directly caused by obesity or indirectly related through mechanisms sharing a common cause such as a poor diet or a sedentary lifestyle. The strength of the link between obesity and specific conditions varies. One of the strongest is the link with type 2 diabetes. Excess body fat underlies 64% of cases of diabetes in men and 77% of cases in women.
__
Obesity is an epidemic disease that threatens to inundate health care resources by increasing the incidence of diabetes, heart disease, hypertension, and cancer. These effects of obesity result from two factors: the increased mass of adipose tissue and the increased secretion of pathogenetic products from enlarged fat cells. This concept of the pathogenesis of obesity as a disease allows an easy division of disadvantages of obesity into those produced by the mass of fat and those produced by the metabolic effects of fat cells. In the former category are the social disabilities resulting from the stigma associated with obesity, sleep apnea that results in part from increased parapharyngeal fat deposits, and osteoarthritis resulting from the wear and tear on joints from carrying an increased mass of fat. The second category includes the metabolic factors associated with distant effects of products released from enlarged fat cells. The insulin-resistant state that is so common in obesity probably reflects the effects of increased release of fatty acids from fat cells that are then stored in the liver or muscle. When the secretory capacity of the pancreas is overwhelmed by battling insulin resistance, diabetes develops. The strong association of increased fat, especially visceral fat, with diabetes makes this consequence particularly ominous for health care costs. The release of cytokines, particularly IL-6, from the fat cell may stimulate the proinflammatory state that characterizes obesity. The increased secretion of prothrombin activator inhibitor-1 from fat cells may play a role in the procoagulant state of obesity and, along with changes in endothelial function, may be responsible for the increased risk of cardiovascular disease and hypertension. For cancer, the production of estrogens by the enlarged stromal mass plays a role in the risk for breast cancer. Increased cytokine release may play a role in other forms of proliferative growth. The combined effect of these pathogenetic consequences of increased fat stores is an increased risk of shortened life expectancy.
_
Generally speaking, the more body fat you’re carrying, the higher the health risk. However, the amount of weight gained throughout your adult years also contributes to the risk. Some of the many chronic conditions and diseases associated with obesity include:
- Insulin resistance
- High blood pressure
- Atherosclerosis
- Cardiovascular disease
- Stroke
- Some cancers including breast, endometrial and colon cancer
- Type 2 diabetes (non-insulin dependent diabetes mellitus)
- Gall bladder disease
- Polycystic ovarian syndrome
- Musculoskeletal problems such as osteoarthritis and back pain
- Gout
- Cataracts
- Stress incontinence
- Sleep apnoea.
_
Obesity and Coronary Artery Disease: a meta-analysis:
Numerous studies have demonstrated a direct association between excess body weight and coronary artery disease (CAD). The BMI–CAD Collaboration Investigators conducted a meta-analysis of 21 long-term studies that followed more than 300,000 participants for an average of 16 years. Study participants who were overweight had a 32 percent higher risk of developing CAD, compared with participants who were at a normal weight; those who were obese had an 81 percent higher risk. Although adjustment for blood pressure and cholesterol levels slightly lowered the risk estimates, they remained highly significant for obesity. The investigators estimated that the effect of excess weight on blood pressure and blood cholesterol accounts for only about half of the obesity-related increased risk of coronary heart disease.
_
Obesity alone raises risk of fatal heart attack, study finds:
Obese men face a dramatically higher risk of dying from a heart attack, regardless of whether or not they have other known risk factors for cardiovascular disease, a new study reveals. The finding stems from an analysis involving roughly 6,000 middle-aged men, and it suggests that there is something about carrying around excess weight that contributes to heart disease independent of risk factors such as high blood pressure, diabetes, high cholesterol and arterial disease. What exactly that something is, however, remains unclear, although the researchers suggest that the chronic inflammation that typically accompanies significant weight gain might be the driving force behind the increased risk. Obesity is an independent risk factor for cardiovascular disease, and is associated with elevated levels of several proinflammatory cytokines, such as interleukin 6 (IL-6), interleukin 8 (IL-8), and C-reactive protein (CRP), a marker of inflammation. Markers of low-grade inflammation are positively associated with endothelial dysfunction in human obesity. There is mounting evidence that inflammation plays a role in the development of coronary heart disease (CHD).
_
Long-Term Obesity among young leads to increased Heart Risk:
Young adults who remain obese for two decades or more double their risk of developing a marker of heart disease in middle age, researchers said. Every year of obesity raises the risk of developing coronary artery calcification, a silent predictor of heart disease with mild to no symptoms, by 2 percent to 4 percent, according to study published in the Journal of the American Medical Association. Those in the study who had obesity and abdominal obesity over two decades or more also had their coronary artery calcification progress in their heart.
__
Obesity and Cardiovascular Death:
In a meta-analysis of 26 observational studies that included 390,000 men and women, several racial and ethnic groups, and samples from the U.S. and other countries, obesity was significantly associated with death from CAD and cardiovascular disease. Women with BMIs of 30 or higher had a 62 percent greater risk of dying early from CAD and also had a 53 percent higher risk of dying early from any type of cardiovascular disease, compared with women who had BMIs in the normal range (18.5 to 24.9). Men with BMIs of 30 or higher had similarly elevated risks
_
Obesity cardiomyopathy:
The pathophysiology of obesity‐induced cardiomyopathy is now well defined, largely as a result of studies in non‐hypertensive individuals awaiting bariatric surgery (of some importance since the general morbidly obese population may be somewhat less fit then this preselected group). Obesity is associated with an increase in blood volume and cardiac output, the latter rising by 20–30 ml per kilogram of excess body fat. The increase in cardiac output is largely a result of ventricular dilation and an increase in stroke volume. The ventricular dilation results in increased left ventricle wall stress, leading to hypertrophy. Such eccentric left ventricular hypertrophy results in reduced compliance and left ventricular diastolic function, i.e. impairment of ventricular filling, leading to elevated LVEDP and pulmonary oedema. The capacity of the dilated ventricle to hypertrophy is limited so, when left ventricular wall thickening fails to keep pace with dilation, systolic dysfunction ensues (‘obesity cardiomyopathy’). The problem is often compounded by superimposed hypertension and ischaemic heart disease. Ventricular hypertrophy and dysfunction worsen with increasing duration of obesity and improve to some extent with weight loss.
_
Obesity and Stroke:
Ischemic (clot-caused) stroke and coronary artery disease share many of the same disease processes and risk factors. A meta-analysis of 25 prospective cohort studies with 2.3 million participants demonstrated a direct, graded association between excess weight and stroke risk. Overweight increased the risk of ischemic stroke by 22 percent, and obesity increased it by 64 percent. There was no significant relationship between overweight or obesity and hemorrhagic (bleeding-caused) stroke, however. A repeat analysis that statistically accounted for blood pressure, cholesterol, and diabetes weakened the associations, suggesting that these factors mediate the effect of obesity on stroke.
_
Insulin resistance:
Insulin is necessary for the transport of blood glucose (sugar) into the cells of muscle and fat (which is then used for energy). By transporting glucose into cells, insulin keeps the blood glucose levels in the normal range. Insulin resistance (IR) is the condition whereby the effectiveness of insulin in transporting glucose (sugar) into cells is diminished. Fat cells are more insulin resistant than muscle cells; therefore, one important cause of insulin resistance is obesity. The pancreas initially responds to insulin resistance by producing more insulin. As long as the pancreas can produce enough insulin to overcome this resistance, blood glucose levels remain normal. This insulin resistance state (characterized by normal blood glucose levels and high insulin levels) can last for years. Once the pancreas can no longer keep up with producing high levels of insulin, blood glucose levels begin to rise, resulting in type 2 diabetes, thus insulin resistance is a pre-diabetes condition.
_
Obesity and Diabetes:
The condition most strongly influenced by body weight is type 2 diabetes. In the Nurses’ Health Study, which followed 114,000 middle-age women for 14 years, the risk of developing diabetes was 93 times higher among women who had a body mass index (BMI) of 35 or higher at the start of the study, compared with women with BMIs lower than 22. Weight gain during adulthood also increased diabetes risk, even among women with BMIs in the healthy range. The Health Professionals Follow-Up Study found a similar association in men. More recently, investigators conducted a systematic review of 89 studies on weight-related diseases and then did a statistical summary, or meta-analysis, of the data. Of the 18 weight-related diseases they studied, diabetes was at the top of the risk list: Compared with men and women in the normal weight range (BMI lower than 25), men with BMIs of 30 or higher had a sevenfold higher risk of developing type 2 diabetes, and women with BMIs of 30 or higher had a 12-fold higher risk. Fat cells—especially those stored around the waist—secrete hormones and other substances that fire inflammation. Although inflammation is an essential component of the immune system and part of the healing process, inappropriate inflammation causes a variety of health problems. Inflammation can make the body less responsive to insulin and change the way the body metabolizes fats and carbohydrates, leading to higher blood sugar levels and, eventually, to diabetes and its many complications. Several large trials have shown that moderate weight loss can prevent or delay the start of diabetes in people who are at high risk.
_
How Obesity increases the risk for Diabetes: ER (endoplasmic reticulum) stress:
The Salk team, led by Marc Montminy, Ph.D., a professor in the Clayton Foundation Laboratories for Peptide Biology, discovered how a condition known as ER (endoplasmic reticulum) stress, which is induced by a high fat diet and is overly activated in obese people, triggers aberrant glucose production in the liver, an important step on the path to insulin resistance. In healthy people, a “fasting switch” only flips on glucose production when blood glucose levels run low during fasting. “The existence of a second cellular signaling cascade—like an alternate route from A to B—that can modulate glucose production, presents the potential to identify new classes of drugs that might help to lower blood sugar by disrupting this alternative pathway,” says Montminy. It had been well established that obesity promotes insulin resistance through the inappropriate inactivation of a process called gluconeogenesis, where the liver creates glucose for fuel and which ordinarily occurs only in times of fasting. Yet, not all obese people become insulin resistant, and insulin resistance occurs in non-obese individuals, leading Montminy and his colleagues to suspect that fasting-induced glucose production was only half the story. “When a cell starts to sense stress a red light goes on, which slows down the production of proteins,” explains Montminy. “This process, which is known as ER stress response, is abnormally active in livers of obese individuals, where it contributes to the development of hyperglycemia, or high blood glucose levels. We asked whether chronic ER stress in obesity leads to abnormal activation of the fasting switch that normally controls glucose production in the liver.” The ER, short for endoplasmic reticulum, is a protein factory within the cell. To test this hypothesis the Salk team asked whether ER stress can induce gluconeogenesis in lean mice. Glucose production is turned on by a transcriptional switch called CRTC2, which normally sits outside the nucleus waiting for the signal that allows it to slip inside and do its work. Once in the nucleus, it teams up with a protein called CREB and together they switch on the genes necessary to increase glucose output. In insulin-resistant mice, however, the CRTC2 switch seems to get stuck in the “on” position and the cells start churning out glucose like sugar factories in overdrive. Surprisingly, when postdoctoral researcher and first author Yiguo Wang, Ph.D., mimicked the conditions of ER stress in mice, CRTC2 moved to the nucleus but failed to activate gluconeogenesis. Instead, it switched on genes important for combating stress and returning cells to health. On closer inspection, Wang found that in this scenario CRTC2 did not bind to CREB but instead joined forces with another factor, called ATF6a. What’s more, when CREB and ATF6a compete for teaming up with CRTC2’s —the more ATF6a is bound to CRTC2, the less there is for CREB to bind to. “This clever mechanism ensures that a cell in survival mode automatically shuts down glucose production, thus saving energy,” says Wang. This observation led the researcher to ask what happens to ATF6a following the kind of persistent stress presented by obesity? They found that the levels of ATF6a go down when ER stress is chronically activated, compromising the cells’ survival pathway and favoring the glucose production pathway; hyperglycemia wins in conditions of persistent stress. Explains Wang, “Our study helps to explain why obese people have a stronger tendency to become diabetic. When ER stress signaling is abnormal glucose output is actually increased.” “It is possible that mutations in the highly conserved CRTC2 lead to a predisposition to inappropriate gluconeogenesis,” says Montminy, who is now trying to identify natural mutations in CRTC2 that may lead to insulin resistance in carriers. In addition to Drs. Wang and Montminy, researchers contributing to this study include research technician Liliana Vera, and Wolfgang H. Fischer, Ph.D., director of the Mass Spectrometry Core Facility.
_
Obesity and high blood pressure:
Studies have shown that the rise of high blood pressure sufferers is seen in conjunction with a dramatic increase in the prevalence of overweight and obesity. This risk has been estimated by the Framingham Heart Study that suggests that approximately 78% of the hypertension cases in men and 65% in women can be directly attributed to obesity. Further the connection with high blood pressure is also present with body fat distribution in obesity. Abdominal obesity has been linked to hypertension in studies. The Normative Aging Study for example showed that in men over 18 years of the study hypertension risk increased approximately threefold with a one-unit change in the abdominal circumference/hip breadth ratio. The Framingham Heart Study revealed that a 5% weight gain increases hypertension risk by 30% in a 4-year time period. However weight loss reduces both systolic and diastolic blood pressures. It has been seen that in obese patients with hypertension, there is an increased absorption of sodium from the kidney and an increase in blood volume. This could be due to the activated sympathetic nervous system or the renin–angiotensin system and high pressures within the kidney.
_
Nonalcoholic fatty liver disease:
If there is some twisted bright spot on this list of top diseases caused by obesity, it’s one of the most common disease, fatty liver disease, isn’t deadly in its most common form. In fact, quite a bit of the population is likely to have fatty liver disease, which will go undetected, because in its early and mid-level stages it doesn’t present any symptoms. Outside of scarring of the liver, a person wouldn’t even notice. The danger is if this nonalcoholic fatty liver disease progresses into what’s known as nonalcoholic steatohepatitis, or silent liver disease, which can lead to cirrhosis (i.e., permanent scarring), and eventually complete failure, of the liver. The downside to silent liver disease is that there is no current standard of treatment — at least as it pertains to drugs. The most logical way to treat the disease is by inducing weight loss as quickly as possible, but even that offers no guarantee of success.
_
Obesity and respiratory system:
Why do respiratory problems occur with obesity?
It is hypothesized that deposition of fat tissue in the abdominal wall and around the abdominal organs hampers movement of the diaphragm and reduce the lung expansion during inspiration and reduced lung capacity. The function of the respiratory muscles also deteriorates in obese patients much like in respiratory diseases like chronic obstructive lung disease (COPD). With the rise of weight and BMI the lung volumes decreases. This leads to more restricted air entry. There is lowered
- forced expiratory volume in 1 second (FEV1)
- forced vital capacity (FVC)
- functional residual capacity (FRC)
- expiratory reserve volume (ERV)
- residual volume (RV)
- total lung capacity (TLC)
_
Respiratory function in eucapnic obesity
- Decreased chest wall and lung compliance
- Small airway dysfunction and expiratory flow limitation
- Preservation of the forced expiratory volume in 1s/forced vital capacity ratio
- Variable reduction of (or preserved) ventilatory muscle strength and endurance
- Increased work and oxygen cost of breathing
- Normal or increased carbon monoxide transfer factor
- Abnormal ventilation/perfusion relations and arterial oxygen desaturation
- Normal or increased central respiratory drive
_
Obesity affects the respiratory system health adversely in more ways than one. Some of the health effects of obesity on respiratory system include diseases like:-
• Exertional dyspnea – This is basically severe breathlessness caused due to minor exertions. This is a common feature among obese individuals.
•Obstructive sleep apnea syndrome – This condition leads to closing or narrowing of the airways during sleep leading to snoring, repeated waking and lack of adequate and restful sleep.
•Chronic obstructive pulmonary disease (COPD) — Chronic obstructive pulmonary disease (COPD) and obesity are common and disabling chronic health conditions with increasing prevalence worldwide. A relationship between COPD and obesity is increasingly recognized, although the nature of this association remains unknown.
_
Above figure shows mechanistic links between chronic obstructive pulmonary disease, body composition, adipose tissue dysfunction, systemic inflammation and insulin resistance.
_
•Asthma – Obese patients are more at risk of asthma exacerbations. Studies show the prevalence of asthma is higher by 38% in overweight patients and by 92% in obese patients. Obese patients with asthma also get more acute attacks, need more asthma medication, need more frequent visits to the emergency department (ED), and have more hospital admissions than non obese patients with asthma. People with obesity are also more likely to be hospitalized for asthma. A study has stated that 75% of patients treated for asthma in the emergency room were either overweight or obese.
•Obesity hypoventilation syndrome – Obese individuals have low lung reserve and may thus have difficulty in providing enough oxygen for their body. This may lead to hypoxia or low oxygenation of the body.
•Pulmonary embolism – This is a serious condition where a blood clot gets lodged in the blood vessels of the lungs leading to a life threatening medical emergency. Pulmonary embolism may lead to failure and death.
• Aspiration pneumonia – Due to the short and narrowed airways there is a possibility of the stomach contents moving into the lungs. This causes severe pneumonia caused by the harmful stomach acids.
• Pickwickian syndrome is characterized by obesity, hypersomnolence, hypoxia, hypercapnia, right ventricular failure and polycythaemia.
_
Obesity and asthma: Study finds a link in the genes:
Genes linked to chronic inflammation in asthma may be more active in people who are obese, according to new research that uncovers several biological ties between obesity and asthma. In the comparative study, the scientists found that four genes associated with chronic inflammation in asthma were more active in obese and morbidly obese people, by more than 100 percent in some cases. The highest activity was found in the morbidly obese. This increased gene expression matters because it can cause white blood cells called mononuclear cells to produce far greater amounts of inflammatory factors like interleukin 4, LIGHT and lymphotoxinβ receptor which contribute to allergic inflammation and other abnormalities in the bronchial passages in asthma. The scientists also found higher concentrations of two asthma-related compounds in the plasma of obese and morbidly obese patients: MMP-9, which is associated with inflammation, and nitric oxide metabolites (NOM), which are an indicator of oxidative stress. Following gastric bypass surgery in morbidly obese diabetic patients, MMP-9 and NOM levels dropped, along with the expression of six asthma-related genes including the key factors, interleukin 4, LIGHT, lymphotoxinβ and interleukin 33 in parallel with weight loss and improvements in the status of their diabetes. The research established a connection between Type 2 diabetes, obesity and asthma based on biological mechanisms. This is important because obesity and Type 2 diabetes are associated with a more than 100 percent increase in the prevalence of asthma.
_
Obesity and risk of Chronic Renal Failure:
Few large-scale epidemiologic studies have quantified the possible link between obesity and chronic renal failure (CRF). A study analyzed anthropometric data from a nationwide, population-based, case-control study of incident, moderately severe CRF. Eligible as cases were all native Swedes who were aged 18 to 74 yr and had CRF and whose serum creatinine for the first time and permanently exceeded 3.4 mg/dl (men) or 2.8 mg/dl (women) during the study period. A total of 926 case patients and 998 control subjects, randomly drawn from the study base, were enrolled. Face-to-face interviews, supplemented with self-administered questionnaires, provided information about anthropometric measures and other lifestyle factors. Logistic regression models with adjustments for several co-factors estimated the relative risk for CRF in relation to body mass index (BMI). Overweight (BMI ≥ 25 kg/m2) at age 20 was associated with a significant three-fold excess risk for CRF, relative to BMI <25. Obesity (BMI ≥30) among men and morbid obesity (BMI ≥ 35) among women anytime during lifetime was linked to three- to four-fold increases in risk. The strongest association was with diabetic nephropathy, but two- to three-fold risk elevations were observed for all major subtypes of CRF. Analyses that were confined to strata without hypertension or diabetes revealed a three-fold increased risk among patients who were overweight at age 20, whereas the two-fold observed risk elevation among those who had a highest lifetime BMI of >35 was statistically nonsignificant. Obesity seems to be an important—and potentially preventable—risk factor for CRF. Although hypertension and type 2 diabetes are important mediators, additional pathways also may exist.
_
Assessment of body mass index and hand anthropometric measurements as independent risk factors for carpal tunnel syndrome:
The goal of this study was to clarify the role of body mass index and hand anthropometric measurements as independent risk determinants in the development of carpal tunnel syndrome (CTS) and their relationship to the severity of CTS. A total of 131 patients with clinical symptoms of CTS and 131 normal subjects were enrolled, of whom 121 were female both in the CTS cases and the controls. All cases were electrodiagnostically confirmed and assigned to three severity groups. BMI, wrist ratio, shape index, digit index and hand length/height ratio were measured in all participants. Mean values for each item were compared between cases and controls and severity subgroups. A logistic regression analysis was performed to determine independent CTS risk factors. The mean values of BMI, wrist ratio and shape index were significantly higher in all CTS patients and females compared to controls, whereas in males only BMI and wrist ratio were higher. The patients in the mild severity subgroup had a significantly lower age and wrist ratio. BMI, wrist ratio and shape index were found to be independent risk factors of CTS development in all patients and females. This study showed BMI, wrist ratio and shape index as independent risk factors for CTS. These findings are of potential anatomical and clinical importance and outline the risk factors of anatomical malfunction of the wrist in CTS.
_
Obesity is a major preventable cause of cancer:
_
_
Major studies confirm that being overweight or obese increases your risk of various cancers. The World Health Organisation (WHO) says that overweight and obesity are the most important known avoidable causes of cancer after tobacco. Scientists have estimated that anywhere between 7% and 15% of breast cancer cases in developed countries are caused by obesity. Over a hundred studies show that women who are overweight or obese and have been through the menopause have higher breast cancer risks. Obesity is one of the most important causes of bowel cancer. Some groups have estimated that obesity causes about 11-14% of bowel cancer cases. Studies have consistently found that obese people are three to four times more likely to develop womb cancer than people with a healthy bodyweight. Being overweight or obese increases the risk of a type of oesophageal cancer called “oesophageal adenocarcinoma”.
_
Obesity increases risk of many other types of cancer:
Studies have consistently found that people who are overweight or obese are also more likely to develop pancreatic, kidney, and gallbladder cancers. Studies have estimated that having a high body weight accounts for nearly a quarter of kidney and gallbladder cancers. And there is more and more evidence that being overweight or obese could increase the risk of many other types of cancer, including:
•brain cancer
•leukaemia
•liver cancer
•multiple myeloma
•non-Hodgkin lymphoma
•ovarian cancer, before the menopause
•aggressive prostate cancer
•thyroid cancer
_
Obesity most likely increases the risk of cancer by raising levels of hormones such as estrogen and insulin. Obesity could also cause cancer through other means, including:
•increasing the risk of oesophageal cancer by causing ‘gastric acid reflux’, a condition where the stomach’s acids are briefly pushed back into the throat. This damages the lining of the oesophagus.
•increasing the risk of gallstones, which in turn increase the risk of gallbladder cancer.
•being associated with physical inactivity or unhealthy diets.
_
Several possible mechanisms have been suggested to explain the association of obesity with increased risk of certain cancers:
◦Fat tissue produces excess amounts of estrogen, high levels of which have been associated with the risk of breast, endometrial, and some other cancers.
◦Obese people often have increased levels of insulin and insulin-like growth factor-1 (IGF-1) in their blood (a condition known as hyperinsulinemia or insulin resistance), which may promote the development of certain tumors.
◦Fat cells produce hormones, called adipokines that may stimulate or inhibit cell growth. For example, leptin, which is more abundant in obese people, seems to promote cell proliferation, whereas adiponectin, which is less abundant in obese people, may have antiproliferative effects.
◦Fat cells may also have direct and indirect effects on other tumor growth regulators, including mammalian target of rapamycin (mTOR) and AMP-activated protein kinase.
◦Obese people often have chronic low-level, or “subacute,” inflammation, which has been associated with increased cancer risk.
_
Overweight and health problems of the lower extremities: osteoarthritis (OA), pain and disability:
A study found that there is a strong association between overweight/obesity and health problems of the lower extremities, i.e. OA, pain and disability. The increasing prevalence of overweight and obesity worldwide urges for public health action not only for diabetes and heart disease, but also OA.
_
Why OA?
The basic pathology behind obesity and knee osteoarthritis is the weight burden that has to be borne by the knees in an obese and overweight individual. While walking or bearing the weight on a single leg with each step while walking, a force of 3 to 6 times that of body weight is transmitted across the knee joint. Similarly the force exerted across the hip is 3 times that of body weight. These forces are increased several times over during high-impact activities. In fact only 10 pounds overweight can increase the force on the knee by 30-60 pounds with each step. In addition there are excessive fat tissues that produce hormones and other factors that affect the articular or joint cartilage metabolism and cause inflammation of the joints giving rise to joint pathology. Leptin is one of the hormones associated with obesity induced knee osteoarthritis.
_
Obesity and bone health in adults and the elderly:
The incidence and prevalence of obesity among the elderly is higher than in younger adults. The trends are prominent in developed nations like United States and also in other nations. With age the natural risk of brittle bones and easily fractured bones due to conditions like osteoporosis is common. Osteoporosis may be major contributing factor to fractures among the obese and overweight elderly individuals. Risk of fractures is also high among the elderly with a low body mass index. There are several factors that may contribute to increased risk of fractures among the low body weight elderly. This includes low bone mineral density (BMD), less soft tissue that may protect bone from impact of the injury and increased fall risk resulting from muscle weakness. They are more at risk of hip fractures. The interest in the association between overweight and obese elderly individuals still gains precedence over low weight individuals because of the rising prevalence of obesity. Less than 1% elderly individuals may have a low BMI related fracture whereas obese elderly form nearly three quarters of all obese individuals in developed nations. Elderly obese individuals are also at risk of osteoporosis and fracture of the spine or vertebra. This has been demonstrated in several studies. BMI and total body weight are the most common body size measurements evaluated in association with fracture risk. Other measures include fat mass, hip girth, and waist to hip ratio etc.
_
Obesity and deep vein thrombosis (DVT) & pulmonary embolism (PE):
Obesity is an important risk factor for DVT/PE in both men and women. Obesity raises and alters the levels of factors that affect coagulation and blood clotting. Obese individuals may be producing more adipokines such as leptin, develop insulin resistance and a chronic inflammatory state. These also increase platelet activity. Platelets are blood cells responsible for beginning blood coagulation and clot formation. Obesity also leads to overproduction of plasminogen activator inhibitor-1 that comes from adipocytes or fat cells and hepatocytes or liver cells. These cells are driven by increased blood levels of free fatty acids, cytokines, adipokines and relative hypoxia or lack of oxygen in adipose tissue in obesity. The plasminogen activator inhibitor-1 leads to inhibition of clot break down or fibrinolysis promoting clot formation and raising the risk of DVT and PE. Studies have shown that obese individuals have nearly twice the risk of both PE and DVT and obese patients less than 40 years have nearly a fivefold risk than those who are not obese. The risk of development of PE is nearly six fold high among women with a BMI of 35 kg/m2 or more. Obesity also raises the other known risk factors for developing DVT. For example, obesity is connected to genetic mutations F5 G1691A (Factor V Leiden) and F2 G20210A (prothrombin). These are blood coagulation factors that are imbalanced in DVT and PE. Obesity doubles the risk of such imbalances.
_
Obesity and Reproduction:
Obesity can influence various aspects of reproduction, from sexual activity to conception. Among women, the association between obesity and infertility, primarily ovulatory infertility, is represented by a classic U-shaped curve. In the Nurses’ Health Study, infertility was lowest in women with BMIs between 20 and 24, and increased with lower and higher BMIs. This study suggests that 25 percent of ovulatory infertility in the United States may be attributable to obesity. During pregnancy, obesity increases the risk of early and late miscarriage, gestational diabetes, preeclampsia, and complications during labor and delivery. It also slightly increases the chances of bearing a child with congenital anomalies. One small randomized trial suggests that modest weight loss improves fertility in obese women. The impact of obesity on male fertility is less clear. In a study by Hammoud and colleagues, the incidence of low sperm count (oligospermia) and poor sperm motility (asthenospermia) increased with BMI, from 5.3 and 4.5 percent, respectively, in normal-weight men to 15.6 and 13.3 percent in obese men. In contrast, a study by Chavarro and colleagues found little effect of body weight on semen quality except at the highest BMIs (above 35), despite major differences in reproductive hormone levels with increasing weight. Sexual function may also be affected by obesity. Data from the Health Professionals Follow-Up Study, the National Health and Nutrition Examination Survey (NHANES), and the Massachusetts Male Aging Study indicate that the odds of developing erectile dysfunction increase with increasing BMI. Of note, weight loss appears to be mildly helpful in maintaining erectile function. The effect of obesity on female sexual function is less clear. In a recent French study, obese women were less likely than normal-weight women to report having had a sexual partner in the preceding 12 months, but the prevalence of sexual dysfunction was similar in both groups. In a smaller survey of 118 women, Esposito and colleagues found that obese women had lower scores on the Female Sexual Function Index, with strong correlations between increasing BMI and problems with arousal, lubrication, orgasm, and satisfaction.
_
Obesity, Memory, and Cognitive Function:
Alzheimer’s disease and dementia are scourges of populations that enjoy a long life span. In the United States, these diseases affect more than 7.5 million people, most of them over age 65. At 65, the estimated lifetime risk for Alzheimer’s disease is 17.2 percent in women and 9.1 percent in men. Body weight is a potentially modifiable risk factor for Alzheimer’s disease and dementia. A meta-analysis of 10 prospective cohort studies that included almost 42,000 subjects followed for three to 36 years demonstrated a U-shaped association between BMI and Alzheimer’s disease. Compared with being in the normal weight range, being underweight was associated with a 36 percent higher risk of Alzheimer’s disease while being obese was associated with a 42 percent higher risk. The associations were stronger in studies with longer follow-up. A more recent meta-analysis demonstrated a similarly strong association between obesity and Alzheimer’s disease.
_
A review of the association between obesity and cognitive function across the lifespan:
Recent research suggests that increased adiposity is associated with poor cognitive performance, independently of associated medical conditions. The evidence regarding this relationship is examined in a review article. A relatively consistent finding across the lifespan is that obesity is associated with cognitive deficits, especially in executive function, in children, adolescents and adults. However, as illustrated by contradictory studies, the relationship between obesity and cognition is uncertain in the elderly, partly because of inaccuracy of body mass index as a measure of adiposity as body composition changes with aging. It is also unclear whether obesity is a cause or a consequence of these cognitive deficits, acknowledging the possible bidirectional relationship. Author’s investigations suggest that weight gain results, at least in part, from a neurological predisposition characterized by reduced executive function, and in turn obesity itself has a compounding negative impact on the brain via mechanisms currently attributed to low-grade systemic inflammation, elevated lipids and/or insulin resistance. The possible role of cognitive remediation treatment strategies to prevent and/or treat obesity is contemplated.
_
Effects of obesity on the skin:
Effects on skin barrier function-
Obesity increases water loss across the skin to a great extent. In morbidly obese patients skin is significantly dry and skin repair after wounds is impaired.
Sebaceous glands and sebum production-
Sebum plays a major role in acne development. It is an oily substance that is produced to keep the skin moisturized and supple. Acne occurs when the sebaceous channels are blocked and infected. Acne is clearly exacerbated by obesity-associated disorders. In obese individuals androgens (male hormones), insulin, growth hormone, and insulin like growth factors are raised. These are all known risk factors for acne.
Sweat glands-
Obese patients have larger skin folds and tend to sweat more profusely due to thick layers of subcutaneous fat.
Lymph channels-
Obesity impedes or slow lymphatic flow. This leads to collection of protein-rich lymphatic fluid in the subcutaneous tissue. This is called lymphedema.
Blood vessels of the skin-
Obesity changes blood circulation of the skin leading to obesity-related microangiopathy and hypertension. Blood flow in the skin is increased in obese individuals.
Collagen structure and function and wound healing-
Obesity is also associated with changes in collagen structure. Collagen forms the structure of the skin and helps in wound healing. However, obese patients rarely manifest facial wrinkles and weakening of the skin or skin laxity due to increased subcutaneous fat.
Subcutaneous fat-
Subcutaneous fat is made up almost entirely of white adipose tissue. In normal humans it provides insulation and serves as energy storage. This adipose tissue contains adipocytes that secrete endocrine hormonal peptides like leptin and tumor necrosis factor. Obese individuals have excess subcutaneous fat.
_
Skin manifestations in obese and overweight individuals:
Obesity is associated with a number of skin conditions. These include:
Acanthosis nigricans:
This is a most common skin problem in obesity. Acanthosis nigricans appears as velvety, symmetriacal dark patches. It is most commonly observed in the arm pits, groin, back of the neck, elbows, knuckles, and face. Acanthosis nigricans is associated with insulin resistance.
Acrochordons:
These are soft brown papules or growths seen commonly on the neck and in the armpits and groin. They are frequently seen in association with acanthosis nigricans
Keratosis pilaris:
These lead to formation of spiny papules or growths on the external surface of the arms.
Hyperandrogenism and hirsutism:
This is caused by high levels of male hormones. These features are common with polycystic ovarian disease and insulin resistance.
Striae distensae:
These are commonly called stretch marks. They are long plaques found in areas with greatest tension and are commonly found on the breasts, buttocks, abdomen, and thighs. They appear as red marks turning violet, then finally becoming white depressed plaques.
Adiposis dolorosa:
This is a rare condition with multiple, painful, subcutaneous lipomas seen in obese and postmenopausal women.
_
Skin diseases aggravated by obesity
Some skin diseases are aggravated by obesity and overweight. These include:-
• lymphedema
•psoriasis
• chronic venous insufficiency
• insulin resistance syndrome
•cellulites
• skin infections including fungal infections
• plantar hyperkeratosis
• hidradenitis suppurativa
• tophaceous gout
• intertrigo
_
Obesity and urinary incontinence:
Several studies have shown that obesity and overweight is directly associated with urinary incontinence. Obesity is an independent risk factor for stress related and mixed urinary incontinence and is the most important risk factor for daily urinary incontinence compared to any other factor. Some studies suggest that excess body weight increases abdominal pressure. This in turn increases bladder pressure and mobility of the urethra. This leads to stress urinary incontinence. This also causes an overactive bladder.
_
Obesity and periodontal disease:
The first report connecting obesity and periodontal disease appeared in 1977 among laboratory rats. It was noted that under healthy oral conditions, obesity per se does not promote pathologic periodontal changes. The periodontal changes and severe damage is seen in response to bacterial plaque accumulation. Human reports first came in 1998 among Japanese adults. Studies show that increasing body mass index (BMI) and waist to hip ratio was associated with increasing risk of periodontitis.
__
Anesthesia risks for obese individuals:
Anesthesia may pose several risks for the obese individuals. Some of these include:-
Risks to the respiratory system:
Obese individuals are at a risk of obstructive sleep apnea and several other respiratory problems. They may have airway obstruction as well. An anaesthetist should also assess the patient’s ability to breathe deeply, an action that would ensure a good ventilation and oxygenation while under anesthesia. Because of the lack of space in the back of the throat, intubation with an endotracheal tube that helps in breathing and ventilation during surgery may be difficult for obese and morbidly obese individuals. Fibreoptic intubation is the safest course of action for these patients. There is also a risk of lowered oxygenation among obese individuals during surgery. Obese patients are also at risk of lung infections and other lung complications after anesthesia. Due to the problems with general anesthesia regional anaesthetic techniques, such as peripheral nerve blocks and epidural blockade may be preferred for obese individuals.
Risks to the cardiovascular system:
The heart is under pressure in an obese individual. There is a higher risk of heart attacks, angina pain, and lack of oxygenation of the heart muscles, stroke and high blood pressure in obese individuals. Anesthesia may raise the risk of cardiovascular adverse events in the obese. For example, mild to moderate hypertension is seen in 50–60% of obese patients and severe hypertension in 5–10% patients. In addition there may be insulin resistance as well. Total blood volume is increased in the obese but the blood flow to the brain and kidneys are normal. Obese individuals are also at risk of developing cardiac rhythm abnormalities due to low oxygenation, electrolyte disturbance caused by diuretic therapy, coronary artery disease, obstructive sleep apnea, myocardial hypertrophy etc. During anesthesia, each of these risk factors is aggravated. There may be ventricular impairment, heart failure or arrhythmias precipitated by anesthetic agents. After inducing anesthesia workings of the heart may deteriorate in an obese individual, blood pressure regulation may be deranged and there may be a risk of heart attacks as well.
_
Prevention of anesthetic complications and practical considerations:
• Patient is usually advised to lose as much weight as possible before surgery.
• Regional anesthesia and awake intubation is preferred over general anesthesia.
•Opioid and sedative drugs may cause respiratory depression and should be avoided.
• Aspiration pneumonia, due to the back flow of the stomach’s contents into the lungs, is a common lung complication among obese people. To prevent this there should be adequate measures like medications and keeping the patient on a completely empty stomach for at least 12 hours before surgery.
• Risk of deep vein thrombosis after surgery is larger among obese individuals. This may be prevented by suitable blood clot dissolving medications like heparin.
• Most operating tables are designed for patients of up to 120–140 kg in weight. For those who weigh more than this limit, specially designed tables may be needed.
• Position should be maintained to prevent nerve compressions and pressure sores. Tilting or turning the patient to their right may compress large blood vessels like the inferior vena cava in the abdomen leading to complications – this needs to be avoided.
• Getting an intravenous line in an obese patient may be difficult due to high amount of subcutaneous fat. In case a line cannot be established, a central venous line is considered.
_
Trauma and the obese patient:
It is a widely held belief that the outcome of trauma in obese patients is poor, but data to support this are scarce. Boulanger and colleagues examined retrospectively the pattern of blunt trauma in obese and non‐obese individuals over a 4 yr period. The obese group tended to be involved more in car crashes (62.7% vs 54.1%) and to have better GCS scores, and they were more likely to have rib fractures, pulmonary contusions, pelvic fractures and extremity fractures. They were less likely to have suffered head trauma or liver injuries. Smith‐Choban and colleagues reported an eight‐fold increase in mortality following blunt trauma in morbidly obese patients compared with the non‐obese. The metabolic response to severe trauma appears to be different in obese and non‐obese subjects. Jeevanandam and colleagues showed that traumatized obese patients mobilized relatively more protein and less fat than non‐obese victims. In other words, they were unable to use their most abundant fuel source. They suggest that the nutritional management of obese trauma victims should provide enough glucose calories to spare protein. Care of the morbidly obese trauma victim in the resuscitation room is likely to prove difficult. Given the high probability of underlying cardiorespiratory impairment, such patients are likely to require high inspired oxygen fractions, early intubation and respiratory support, meticulous fluid resuscitation with invasive monitoring, and adequate personnel to transport them around the emergency department. Bleeding is likely to produce early cardiovascular decompensation and so should be vigorously sought and treated. Portable radiographs may be of poor quality because of overlying soft tissue, and clinical signs may be difficult to elicit. More sophisticated imaging techniques, such as CT scanning, may be needed, although many CT tables have weight restrictions of about 160 kg. The attending physician should always consider the possibility of covert pathology in the obese trauma patient.
_
The obese patient on the intensive care unit:
Few data are available on the morbidity and mortality of obese patients in the intensive care setting, but again it is widely held that the outcome is poor. Despite this, morbid obesity was not included as a comorbid variable in the development of the APACHE (acute physiology and chronic health evaluation) II and III prognostic indices. Obese patients are more likely to be admitted to the intensive care unit. Rose and colleagues reported that acute postoperative pulmonary events were twice as likely in the obese as in the non‐obese, and that hospitalized obese patients were at an increased risk of developing respiratory complications. The alterations in pulmonary function that have already been outlined are important when considering mechanical ventilation in the obese patient. A tidal volume based on the patient’s actual body weight is likely to produce alveolar over‐distension and high airway pressure, so increasing the risk of barotrauma. An initial tidal volume based on IBW should be used which can then be adjusted according to inflation pressures and blood gas analysis. The use of PEEP may help to prevent airway closure and atelectasis but may be at the expense of the cardiac output. Weaning from mechanical ventilation may be difficult because of high oxygen requirements, increased work of breathing, reduced lung volumes and ventilation–perfusion mismatching. Burns and colleagues showed that a position of 45° head‐up tilt resulted in a larger tidal volume and a lower respiratory rate than either the 0° or 90° position, and so may be of benefit during weaning. If a long stay in the ICU is envisaged, early tracheostomy may assist weaning, although the percutaneous approach is likely to prove difficult. One must always consider the possibility of the obese patient requiring emergency re‐intubation and so extubation should be planned when adequate personnel are available, especially if the initial intubation was problematic. The morbidly obese patient is likely to have significant cardiovascular impairment and to tolerate fluid loading poorly. Invasive haemodynamic monitoring may assist in titrating fluid replacement and assessing cardiac performance. Siting of central venous catheters may be difficult, resulting in a higher incidence of catheter misplacement and local complications such as infection and thrombosis. Femoral vein catheterization may be impossible owing to local intertrigo. Doppler ultrasound has been shown to improve the success rate of central vein cannulation in high‐risk groups with a reduced rate of complications. Despite having excess body fat stores and increased lean body mass, obese individuals are at risk from developing protein malnutrition during periods of metabolic stress. Weight reduction during episodes of critical illness is not beneficial and appropriate nutritional support should not be withheld. Obese individuals are not able to mobilize their fat stores during critical illness and tend to rely on carbohydrate instead. The increased carbohydrate use increases the respiratory quotient and accelerates protein breakdown further to fuel gluconeogenesis. Energy expenditure equations tend to be unreliable in critical illness, especially in the obese. An indirect calorimeter should be used to calculate energy expenditure, but if one is not available, then patients should receive 20–30 kcal per kg of IBW per day. Most of the calories should be given as carbohydrate, with fats given to prevent essential fatty acid deficiency. Protein requirements may be difficult to assess because of an increased lean body mass, but 1.5–2.0 g per kg of IBW should achieve nitrogen balance. Expert advice should be sought from a dietician. Clearly the morbidly obese patient will present the emergency room and intensive care staff with a formidable challenge. Better understanding of the pathophysiology and complications that accompany obesity may improve their care and outcome. More research on the outcome of the morbidly obese in these settings is required.
_
The health risks of Obesity: Worse than Smoking, Drinking, or Poverty:
Obesity is widely recognized as a health risk. The negative effects of obesity and other known health risks, such as smoking, heavy drinking, and poverty, have been well documented. But until now, no one has compared them. Is one problem worse than another? Or are they all equally risky? Two RAND researchers, health economist Roland Sturm and psychiatrist Kenneth Wells examined the comparative effects of obesity, smoking, heavy drinking, and poverty on chronic health conditions and health expenditures. Their finding: Obesity is the most serious problem. It is linked to a big increase in chronic health conditions and significantly higher health expenditures. And it affects more people than smoking, heavy drinking, or poverty.
____________
Mortality due to obesity:
_
_
_
Obesity is one of the leading preventable causes of death worldwide. Large-scale American and European studies have found that mortality risk is lowest at a BMI of 20–25 kg/m2 in non-smokers and at 24–27 kg/m2 in current smokers, with risk increasing along with changes in either direction. A BMI above 32 kg/m2 has been associated with a doubled mortality rate among women over a 16-year period. In the United States obesity is estimated to cause 111,909 to 365,000 deaths per year, while 1 million (7.7%) of deaths in Europe are attributed to excess weight. On average, obesity reduces life expectancy by six to seven years, a BMI of 30–35 kg/m2 reduces life expectancy by two to four years, while severe obesity (BMI > 40 kg/m2) reduces life expectancy by ten years (see the meta-analysis below).
_
Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies:
Obesity shaves two to four years off the average lifespan, while being very obese can shorten your lifespan by 8 to 10 years, according to a new analysis of 57 studies including nearly 900,000 people. The study, published in the journal The Lancet, was conducted in part by the eminent epidemiologist Sir Richard Peto of the University of Oxford. Peto and his colleagues in the Prospective Studies Collaboration, a team of dozens of researchers from around the world, say they did the new study to figure out exactly how body mass index (BMI) relates to mortality. Researchers also investigated how smoking influenced this relationship and how excess weight affected death risk from specific causes. In both sexes, mortality was lowest at about 22·5–25 kg/m2. Above this range, positive associations were recorded for several specific causes and inverse associations for none, the absolute excess risks for higher BMI and smoking were roughly additive, and each 5 kg/m2 higher BMI was on average associated with about 30% higher overall mortality (hazard ratio per 5 kg/m2 [HR] 1·29 [95% CI 1·27–1·32]): 40% for vascular mortality (HR 1·41 [1·37–1·45]); 60–120% for diabetic, renal, and hepatic mortality (HRs 2·16 [1·89–2·46], 1·59 [1·27–1·99], and 1·82 [1·59–2·09], respectively); 10% for neoplastic mortality (HR 1·10 [1·06–1·15]); and 20% for respiratory and for all other mortality (HRs 1·20 [1·07–1·34] and 1·20 [1·16–1·25], respectively). Below the range 22·5–25 kg/m2, BMI was associated inversely with overall mortality, mainly because of strong inverse associations with respiratory disease and lung cancer. These inverse associations were much stronger for smokers than for non-smokers, despite cigarette consumption per smoker varying little with BMI. Although other anthropometric measures (e.g., waist circumference, waist-to-hip ratio) could well add extra information to BMI, and BMI to them, BMI is in itself a strong predictor of overall mortality both above and below the apparent optimum of about 22·5–25 kg/m2. The progressive excess mortality above this range is due mainly to vascular disease and is probably largely causal. At 30–35 kg/m2, median survival is reduced by 2–4 years; at 40–45 kg/m2, it is reduced by 8–10 years (which is comparable with the effects of smoking). The definite excess mortality below 22·5 kg/m2 is due mainly to smoking-related diseases, and is not fully explained.
_
Higher death rates associated with increasing degrees of obesity:
This multi-center study of more than 90,000 women published today in the Journal of the American Medical Association, shows extremely obese women to be at significantly higher risk of dying than women at normal weight and links the risk of death to specific categories of obesity. To better quantify the health risks associated with obesity, the Pitt researchers, collaborating with researchers from six other institutions, examined the rates of death and of newly diagnosed coronary heart disease over a seven year period of 90,185 women in five specific weight categories. The women, all participants of the Women’s Health Initiative-Observational Study, were assigned to the weight categories based on body mass index (BMI): normal (BMI 18.5 24.9), overweight (BMI 25.0 29.9), obesity 1 (BMI 30.0 34.9), obesity 2 (BMI 35.0 ¨C 39.9) and extreme obesity (BMI 40). The study showed that white women in the obesity 1 category (approximately 60 pounds above a normal weight for a 5-foot, 5-inch tall woman) to have a 12 percent higher risk of death over the seven year follow-up period, but extremely obese women (approximately 110 pounds above a normal weight for a 5-foot, 5-inch tall woman) had an alarming 86 percent higher risk of death than their normal weight counterparts. Death rates increased substantially with increasing weight category, ranging from 68.39 deaths per 10,000 person-years in women with normal BMIs to 116.85 deaths per 10,000 person-years in extremely obese women.
_
Landmark study finds obesity to be more dangerous to health than smoking:
Being seriously overweight may reduce a person’s life expectancy even more than smoking, according to a report by 250 leading scientists. According to the Foresight Report on obesity in the United Kingdom, having a body mass index higher than 30 (the definition used for “obese”) decreases a person’s life expectancy by an average of 9 years. Men with a body mass index higher than 45; however, have their life expectancy reduced by 13 years. In contrast, being a smoker reduces life expectancy by an average of 10 years. Currently, 25 percent of adults in the United Kingdom are classified as obese. But the report projected that if current trends continue, 60 percent of British men will be obese by the year 2050, along with 50 percent of women and 25 percent of children. Along with this increase, the report projected a 70 percent increase in Type-2 Diabetes, a 30 percent increase in strokes, a 20 percent increase in cases of coronary disease, and an increase in the incidence of certain cancers. The report warned that the obesity crisis is exacerbated by the fact that being overweight is coming to be seen as “normal.” Another study published in The Lancet shows that severe obesity shortens a person’s expected lifespan by 10 years, comparable to the effect of a lifetime spent smoking. Data collected from over 60,000 Canadians show that obesity leads to more doctor visits than smoking. If obesity were not a factor, doctor visits would decrease by 10 percent or more.
______________
Obesity and mortality paradox:
The rising level of obesity, along with diabetes, cardiovascular disease and other related diseases, is predicted to slow down or reverse the decline in mortality seen in most Western countries in recent decades. But closer examination shows the picture is more complicated than that. Obesity rates have been increasing in developed countries since the 1950s (albeit slowly at first). And since the 1960s, deaths from disease have steadily decreased in most developed countries while life expectancy has consistently increased. It seems obesity’s negative impact on mortality may be outweighed by other factors favorably influencing life expectancy. In the United States, for instance, cholesterol, smoking, and physical activity levels have all improved in recent years. Indeed, the rate of decline in mortality might have been even faster if it weren’t for the increasing prevalence of diabetes. But some researchers predict that the negative effects of increasing levels of obesity will soon outweigh the benefits from reductions in smoking. Improved medical treatment of some of the pathways linking obesity to mortality may have also blunted obesity’s negative impact. Hypertension, for example, has been better managed in recent years. The impact of obesity may itself have been overestimated because its main adverse effects are experienced by a minority of the population. The best estimates of the association between body mass index (BMI) and mortality suggest that the mortality risk from excess body weight increases from a BMI of 25 but isn’t substantial until BMI exceeds 32 or 35. Between 15% and 25% of the US population have BMIs in this range. While this is a significant proportion, it is nevertheless a minority. And the relationship between obesity and health appears to reverse in old age. In old age, people who have low body weight are at higher risk of disability and mortality. But this reversal may be due to weight loss in old age due to disease. Indeed, body weight may not be a significant risk factor for mortality in itself. Instead it might simply be a surrogate marker for a particular lifestyle, or a particular diet, physical activity level, and genetic factors. If this were so, obese individuals would represent a heterogeneous group of people with high body weight for different reasons, some of which may not be strongly related to morbidity or mortality.
______________
Obesity survival paradox:
This obesity paradox encompasses two basic premises. One of these includes the fact that obese individuals tend to survive longer and better after a major cardiac surgery or cardiac event like a heart attack or heart failure. Another paradox is the fact the low income, hungry populations seem to suffer more from obesity than the high income affluent populations.
_
_
Although the negative health consequences of obesity in the general population are well supported by the available evidence, health outcomes in certain subgroups seem to be improved at an increased BMI, a phenomenon known as the obesity survival paradox. The paradox was first described in 1999 in overweight and obese people undergoing hemodialysis, and has subsequently been found in those with heart failure and peripheral artery disease (PAD). In 2001, A. Mosterd and colleagues from the Netherlands studied the prognosis of patients diagnosed with heart failure. They did statistical analyses on more than 5,000 patients, some of whom had heart failure. They found that patients with low BMIs and low blood pressure had more in-hospital deaths than patients with higher BMIs. The researchers claimed that their discoveries supported similar findings from a 1993 study in Massachusetts, and since 2001, at least eight studies have supported the findings. So, even though obesity is a well-known risk factor for heart failure and would be expected to cause problems for obese heart-failure patients, it seems that the opposite could be true. In people with heart failure, those with a BMI between 30.0 and 34.9 had lower mortality than those with a normal weight. This has been attributed to the fact that people often lose weight as they become progressively more ill. Similar findings have been made in other types of heart disease. People with class I obesity and heart disease do not have greater rates of further heart problems than people of normal weight who also have heart disease. In people with greater degrees of obesity, however, the risk of further cardiovascular events is increased. Even after cardiac bypass surgery, no increase in mortality is seen in the overweight and obese. One study found that the improved survival could be explained by the more aggressive treatment obese people receive after a cardiac event. Another found that if one takes into account chronic obstructive pulmonary disease (COPD) in those with PAD, the benefit of obesity no longer exists. The obesity paradox extends to other conditions besides heart failure. Patients with chronic kidney disease most often undergo hemodialysis, where a machine filters impurities out of the blood. About 20 percent of dialysis patients die each year from cardiovascular complications. Studies by researchers at UCLA Medical Center have shown that dialysis patients with higher BMIs have a better chance of survival than those with lower BMIs. Better nutrition may be a partial explanation, or it may be that in uremic milieu, excessive fat and surplus calories might confer some survival advantage. The “surplus calorie theory” as a potential mechanism for the paradox is of great interest. If proven to be correct, it might explain why peritoneal dialysis patients who receive excessive calories through dialysis do not exhibit the paradox and, secondly and more importantly, therapy could be directed to enhance a greater caloric intake by renal failure patients to engender a better survival outcome.
_
To summarize, the obesity paradox goes something like this. Obesity is a major risk factor for cardiovascular disease (like hypertension, congestive heart failure, coronary artery disease) and chronic renal disease. However, in patients with these chronic diseases, it appears that obesity is associated with better survival. If this finding is actually true, it could have important implications for how physicians treat patients with chronic diseases. Doctors could conceivably stop putting patients on diets and recommending that they lose weight.
_
The UCLA researchers have posed some biological explanations for it:
1. The adverse effects of obesity take more time to develop than those of chronic disease (heart failure, kidney disease). Therefore, the consequences of wasting kill patients much faster than obesity does.
2. In both CHF and chronic kidney disease, malnutrition and inflammation are common. These conditions alone could reduce the survival of these patients. Therefore, weight gain could be an indicator of better nutrition and, therefore, improved chances of survival in obese patients.
__
Diabetes and the Obesity survival Paradox:
Type 2 diabetes, a condition widely thought of as a disease of the overweight and sedentary, also develops in people who aren’t overweight. And it may be deadlier in these normal-weight people, a new study shows. In the study, which appeared in The Journal of the American Medical Association, researchers reviewed data involving more than 2,500 people with Type 2 diabetes, some of whom were followed for decades. The scientists found that those who were of normal weight around the time of their diagnoses were twice as likely to die during the study period, compared with those who were overweight or obese. The researchers could not explain why having a greater body mass index, or B.M.I., might protect someone with diabetes. But they did point out that some doctors may be prone to treating thin diabetics differently from their obese counterparts, and may be less likely to push them to make diet and exercise changes that could improve their survival. “Normal-weight people may be treated less aggressively,” said Mercedes R. Carnethon, an author of the study and an associate professor of preventive medicine at the Northwestern University Feinberg School of Medicine. “This really is an argument to treat a normal-weight person with diabetes as aggressively as you would treat an overweight or obese person with diabetes.” The findings also provide evidence that patients with Type 2 diabetes may display what researchers call the obesity paradox, the observation that people with certain chronic diseases tend to have lower mortality rates if they carry excess pounds. The phenomenon has been documented previously in people with heart failure, hypertension and kidney disease (vide supra).
_______________
Economic impact of obesity:
In addition to its health impacts, obesity leads to many problems including disadvantages in employment and increased business costs. These effects are felt by all levels of society from individuals, to corporations, to governments. In 2005, the medical costs attributable to obesity in the US were an estimated $190.2 billion or 20.6% of all medical expenditures, while the cost of obesity in Canada was estimated at CA$2 billion in 1997 (2.4% of total health costs). The total annual direct cost of overweight and obesity in Australia in 2005 was A$21 billion. Overweight and obese Australians also received A$35.6 billion in government subsidies. The estimate range for annual expenditures on diet products is $40 billion to $100 billion in the US alone. Full-time workers in the U.S. who are overweight or obese and have other chronic health conditions miss an estimated 450 million additional days of work each year compared with healthy workers — resulting in an estimated cost of more than $153 billion in lost productivity annually, according to a 2011 Gallup Poll.
_
_
Healthcare costs due to Obesity:
Overweight and obesity and their associated health problems have a significant economic impact on the U.S. health care system. Medical costs associated with overweight and obesity may involve direct and indirect costs. Direct medical costs may include preventive, diagnostic, and treatment services related to obesity. Indirect costs relate to morbidity and mortality costs. Morbidity costs are defined as the value of income lost from decreased productivity, restricted activity, absenteeism, and bed days. Mortality costs are the value of future income lost by premature death. The medical care costs of obesity in the United States are staggering. In 2008 dollars, these costs totaled about $147 billion. Obesity costs both the individual and society as whole much more in terms of medical costs. On average, people who are considered obese pay $1,429 (42%) more in health care costs than normal-weight individuals. Medical expenses for obese employees are 42 percent higher than for a person with a healthy weight, according to the Centers for Disease Control.
_
Social stigma and employment issue:
Obesity can lead to social stigmatization and disadvantages in employment. When compared to their normal weight counterparts, obese workers on average have higher rates of absenteeism from work and take more disability leave, thus increasing costs for employers and decreasing productivity. A study examining Duke University employees found that people with a BMI over 40 kg/m2 filed twice as many workers’ compensation claims as those whose BMI was 18.5–24.9 kg/m2. They also had more than 12 times as many lost work days. The most common injuries in this group were due to falls and lifting, thus affecting the lower extremities, wrists or hands, and backs. The Alabama State Employees’ Insurance Board approved a controversial plan to charge obese workers $25 a month for health insurance that would otherwise be free unless they take steps to lose weight and improve their health. These measures started in January 2010 and apply to those state workers whose BMI exceeds 35 kg/m2 and who fail to make improvements in their health after one year. Some research shows that obese people are less likely to be hired for a job and are less likely to be promoted. Obese people are also paid less than their non-obese counterparts for an equivalent job; obese women on average make 6% less and obese men make 3% less. Specific industries, such as the airline, healthcare and food industries, have special concerns. Due to rising rates of obesity, airlines face higher fuel costs and pressures to increase seating width. In 2000, the extra weight of obese passengers cost airlines US$275 million. The healthcare industry has had to invest in special facilities for handling severely obese patients, including special lifting equipment and bariatric ambulances. Costs for restaurants are increased by litigation accusing them of causing obesity. In 2005 the US Congress discussed legislation to prevent civil law suits against the food industry in relation to obesity; however, it did not become law.
_
Obesity status and sick leave: a systematic review:
This review identified 36 studies on the relation between obesity status and sick leave. Pooling of effect estimates was not possible due to great heterogeneity between studies regarding definition of sick leave (short-term/long-term), measure of obesity (body mass index/waist circumference/percentage body fat), definition of obesity status (World Health Organization standards/other), study population (sex/age/occupation/country) and exposure and outcome ascertainment (self-reported/objectively assessed). Nevertheless, a clear trend towards greater sick leave among obese compared with normal weight workers could be discerned, especially for spells of longer duration. In studies from the USA, which consistently reported about five times lower number of sick leave days per person-year than European, obese workers had about 1–3 extra days of absence per person-year compared with their normal weight counterparts. In European studies, the corresponding difference was about 10 days. For overweight workers the data were conflicting, indicating either increased or neutral level of sick leave compared with normal weight. Regarding underweight, the studies were very few and concerns regarding direction of causality were greater. Finally, in all four interventional studies identified substantial weight loss in obese subjects resulted in reduced sick leave, at least temporarily. In conclusion, increasing obesity in children and adults is likely to negatively affect future productivity as obesity increases the risk of sick leave, disability pension and death.
______________
Obesity management:
_
Weight loss benefits:
_
_
Guide to obesity treatment:
_________________
Obesity is difficult to tackle because of the many contributing factors. The International Obesity Taskforce suggests:
- Helping families to understand how to provide a healthy environment for themselves and their children. This would include decisions about activity and eating habits
- Identifying high-risk groups in the community
- Changing city planning to include venues for safe, accessible and affordable physical activities
- Improving the nutritional value of processed foods
- Reducing food marketing to children
- Reducing the price of healthy foods such as fruits, vegetables and wholegrain products
- Improving the nutrition and variety of food available at school canteens and in workplaces
- Improving opportunities for physical activity in schools and workplaces
- Increasing education for health professionals on how to recognize and manage weight problems in patients
- Investing in community education programs on weight management.
_
Eating a healthy diet can help prevent obesity
People can:
1) maintain a healthy weight
2) limit total fat intake and shift fat consumption away from saturated fats to unsaturated fats
3) increase consumption of fruit, vegetables, pulses, whole grains and nuts
4) limit the intake of sugar and salt
But healthy diets are more expensive than junk food.
_
•Even though medications and diets can help, the treatment of obesity cannot be a short-term “fix” but has to be a life-long commitment to proper diet habits, increased physical activity, and regular exercise.
•The goal of treatment should be to achieve and maintain a “healthier weight,” not necessarily an ideal weight.
•Even a modest weight loss of 5%-10% of initial weight and the long-term maintenance of that weight loss can bring significant health benefits by lowering blood pressure and lowering the risks of diabetes and heart disease.
•The chances of long-term successful weight loss are enhanced if the doctor works with a team of professionals, including dietitians, psychologists, and exercise professionals.
_
Trying to lose weight quickly by crash-dieting carries the following risks:
- You may develop health problems
- You will probably experience vitamin deficiencies
- You chances of failure are significantly higher
_
Recently, The New England Journal of Medicine (NEJM) published a study comparing the effects of different diets on weight loss. Their conclusion? It doesn’t matter what you eat, only how much you eat. So, pick a diet you can stick with, as that’s all that really matters. It’s only partially true. How much you weigh is a balance between calories in–how many you eat–and calories out–how many you burn, i.e., how much you exercise. The laws of thermodynamics haven’t changed recently. However, it is emphatically not true that all foods are equally healthful. In general, losing weight is a good thing for those who are overweight, but it’s important to lose weight in a way that enhances your health rather than one that may compromise it.
_
Simple carbs:
Glucose, fructose and galactose are referred to as monosaccharides. Lactose, sucrose and maltose are called disaccharides (they contain two monosaccharides). Monosaccharides and disaccharides are called simple carbohydrates. Foods that contain simple carbohydrates include table sugar, products with white flour, honey, milk, yoghurt, candy, chocolate, fruit, fruit juice, cake, jam, biscuits, molasses, soda and packaged cereals. Despite the fact that simple carbohydrates do not contain enough essential nutrients, some foodstuffs such as fruits may still be good for you.
_
Complex carbs:
Complex carbohydrates consist of a chemical structure that is made up of three or more sugars, which are usually linked together to form a chain. These sugars are mostly rich in fiber, vitamins and minerals. Due to their complexity, they take a little longer to digest, and they don’t raise the sugar levels in the blood as quickly as simple carbohydrates. Complex carbohydrates act as the body’s fuel, and they contribute significantly to energy production. In fact, carbs provide more than 60 percent of the amount of energy required by the body. A complex carbohydrate is made up of chains of glucose molecules. Starches are the way plants store energy — plants produce glucose and chain the glucose molecules together to form starch. Most grains (wheat, corn, oats, and rice) and things like potatoes and plantains are high in starch. Your digestive system breaks a complex carbohydrate (starch) back down into its component glucose molecules so that the glucose can enter your bloodstream. It takes a lot longer to break down a starch, however. If you drink a can of soda full of sugar, glucose will enter the bloodstream at a rate of something like 30 calories per minute. A complex carbohydrate is digested more slowly, so glucose enters the bloodstream at a rate of only 2 calories per minute. Complex carbohydrates are commonly found in vegetables, whole-meal bread and cereals. Examples of foods that contain complex carbohydrates include spinach, yams, broccoli, beans, zucchini, lentils, skimmed milk, whole grains and many other leguminous plants and vegetables.
_
Note: carb means carbohydrate colloquially.
_
General diet guidelines for achieving and (just as importantly) maintaining a healthy weight:
•A safe and effective long-term weight reduction and maintenance diet has to contain balanced, nutritious foods to avoid vitamin deficiencies and other diseases of malnutrition.
•Eat more nutritious foods that have “low energy density.” Low energy dense foods contain relatively few calories per unit weight (fewer calories in a large amount of food). Examples of low energy dense foods include vegetables, fruits, lean meat, fish, grains, and beans. For example, you can eat a large volume of celery or carrots without taking in many calories.
•Eat less “energy dense foods.” Energy dense foods are high in fats and simple sugars. They generally have a high calorie value in a small amount of food. The United States government currently recommends that a healthy diet should have less than 30% fat. Fat contains twice as many calories per unit weight than protein or carbohydrates. Examples of high-energy dense foods include red meat, egg yolks, fried foods, high fat/sugar fast foods, sweets, pastries, butter, and high-fat salad dressings. Also cut down on foods that provide calories but very little nutrition, such as alcohol, non-diet soft drinks, and many packaged high-calorie snack foods.
•About 55% of calories in the diet should be from complex carbohydrates. Eat more complex carbohydrates such as brown rice, whole-grain bread, fruits, and vegetables. Avoid simple carbohydrates such as table sugars, sweets, doughnuts, cakes, and muffins. Cut down on non-diet soft drinks, these sugary soft drinks are loaded with simple carbohydrates and calories. Simple carbohydrates cause excessive insulin release by the pancreas, and insulin promotes growth of fat tissue.
•Educate yourself in reading food labels and estimating calories and serving sizes.
•Consult your doctor before starting any dietary changes. You doctor should prescribe the amount of daily calories in your diet.
_
Does eating more frequent meals really rev up your Metabolism?
You’ve probably heard that eating smaller meals, several times a day will stimulate your metabolism, and keep it revved to burn more calories throughout your day. Although some studies have found modest health benefits to eating smaller meals, the research usually involved extremes. Many weight-loss books and fad diets claim six meals a day is a more realistic approach. You’ve probably heard this advice many times: Eat smaller meals more frequently to lose weight. Unfortunately, science is still split on this issue. Some studies show a benefit to eating this way, while others find no discernible biological differences whatsoever. One study that carefully demonstrated this, published in 2009 in The British Journal of Nutrition, involved groups of overweight men and women who were randomly assigned to very strict low-calorie diets and followed for eight weeks. Each subject consumed the same number of calories per day, but one group took in three meals a day and the other six. Both groups lost significant and equivalent amounts of weight. There was no difference between them in fat loss, appetite control or measurements of hormones that signal hunger and satiety. Other studies have had similar results. Exercise, on the other hand, seems to effectively increase metabolism according to studies. Another French study published in the journal Forum of Nutrition in 2003 found that people whose habitual diet pattern included a fourth meal – the so-called “goûter” or snack commonly eaten at 4 pm in France – had demonstrable benefits on Body Mass Index and metabolic profile, even though their total energy intake for the day is not greater than those who skip this meal. In the end, perhaps the most prudent recommendation is to simply let your hunger dictate when to eat. An important caveat here though is to remember that what you eat is essential. If your body gets the nutrients it needs, your hunger will be a reliable indicator for when you need to eat. However, many people today are in fact undernourished, despite being overweight. Consuming junk food and fast food that does not feed your body the nutrients it needs and will often lead to eating far more calories than you need simply because your insulin- and other hormonal balances are out of whack.
_
Obese women in particular shouldn’t skip breakfast:
Overweight women, who skip breakfast, experience acute, or rapid-onset, insulin resistance, a condition that, when chronic, is a risk factor for diabetes, a new study has revealed. The study funded by the Endocrine Fellows Foundation in Washington, D.C., the National Institutes of Health and the Colorado Nutrition Obesity Research Center, suggested that regularly skipping breakfast over time may lead to chronic insulin resistance and thus could increase an individual’s risk for type 2 diabetes. Elizabeth Thomas, MD, an endocrinology fellow at the University of Colorado School of Medicine in Aurora, and co-workers studied nine nondiabetic women, with an average age of 29, who were overweight or obese. Subjects were randomly assigned to receive either breakfast or no breakfast at the first visit and the opposite at the second visit. Four hours later, all subjects ate the same standardized lunch at each visit. They had blood samples taken every 30 minutes after lunch for three hours to test their insulin and glucose levels. The researchers found that the women’s insulin and glucose levels after lunch were significantly higher on the day they skipped breakfast than on the day when participants ate breakfast. According to Thomas, the higher levels demonstrated acute insulin resistance because of skipping breakfast.
_
The influence of food portion size and energy density on energy intake: implications for weight management:
The increase in the prevalence of obesity has coincided with an increase in portion sizes of foods both inside and outside the home, suggesting that larger portions may play a role in the obesity epidemic. Although it will be difficult to establish a causal relationship between increasing portion size and obesity, data indicate that portion size does influence energy intake. Several well-controlled, laboratory-based studies have shown that providing older children and adults with larger food portions can lead to significant increases in energy intake. This effect has been demonstrated for snacks and a variety of single meals and shown to persist over a 2-d period. Despite increases in intake, individuals presented with large portions generally do not report or respond to increased levels of fullness, suggesting that hunger and satiety signals are ignored or overridden. One strategy to address the effect of portion size is decreasing the energy density (kilojoules per gram; kilocalories per gram) of foods. Several studies have demonstrated that eating low-energy-dense foods (such as fruits, vegetables, and soups) maintains satiety while reducing energy intake. In a clinical trial, advising individuals to eat portions of low-energy-dense foods was a more successful weight loss strategy than fat reduction coupled with restriction of portion sizes. Eating satisfying portions of low-energy-dense foods can help to enhance satiety and control hunger while restricting energy intake for weight management.
_
Multiple, well-controlled studies have shown that providing subjects with larger portions of food in a laboratory setting leads to significantly higher energy intakes. For example, when adults were served 4 different portions of macaroni and cheese on different days, subjects consumed 30% more energy (676 kJ) when offered the largest portion (1000 g) compared to the smallest portion (500 g). The effect was seen both when the portion on the plate was determined by the investigator and when the participants served themselves from bowls containing different size portions. Despite the difference in intake, participants reported similar ratings of hunger and fullness after eating. After the study was over, more than half (55%) of the subjects did not notice that there were differences in the portions served. It is surprising that in a controlled laboratory setting, where the main focus was food and eating, a majority of participants in the study appeared to be unaware of the changes in the amount of food offered and the subsequent effect on their intake, hunger, and satiety. It seems likely that when individuals are in situations where there are more distractions, such as when eating out, they would be even less aware of portion size.
_
It’s not just what you eat, it’s how you eat:
Healthy eating is about more than the food on your plate—it is also about how you think about food. Healthy eating habits can be learned and it is important to slow down and think about food as nourishment rather than just something to gulp down in between meetings or on the way to pick up the kids.
- Eat with others whenever possible. Eating with other people has numerous social and emotional benefits—particularly for children—and allows you to model healthy eating habits. Eating in front of the TV or computer often leads to mindless overeating.
- Take time to chew your food and enjoy mealtimes. Chew your food slowly, savoring every bite. We tend to rush though our meals, forgetting to actually taste the flavors and feel the textures of our food. Reconnect with the joy of eating.
- Listen to your body. Ask yourself if you are really hungry, or have a glass of water to see if you are thirsty instead of hungry. During a meal, stop eating before you feel full. It actually takes a few minutes for your brain to tell your body that it has had enough food, so eat slowly.
- Eat breakfast. A healthy breakfast can jumpstart your metabolism.
- Avoid eating at night. Try to eat dinner earlier in the day and then fast for 14-16 hours until breakfast the next morning. Early studies suggest that this simple dietary adjustment—eating only when you’re most active and giving your digestive system a long break each day—may help to regulate weight. After-dinner snacks tend to be high in fat and calories so are best avoided, anyway.
_
Low-calorie diets (LCDs):
LCDs (low caloric diet) are recommended for weight loss in overweight and obese persons. An LCD is designed to create an energy deficit of 500–1,000 kcal/day and induce a weight loss of 0.5–1 kg/week. The NHLBI/ NAASO guide recommends LCDs of 1,000–1,200 kcal/day for most overweight women and 1,200–1,600 kcal/day for overweight men (and for women who exercise regularly or weigh ≥75 kg). The recommended macronutrient composition of these diets is shown in table below.
_
Low calories diet:
_
Very-low-calorie diets (VLCDs):
VLCDs are typically recommended for patients with a BMI ≥30 kg/m2 who have failed to lose weight using an LCD. VLCDs provide 200–800 kcal/day, with large amounts of protein (70–100 g/day) to preserve lean body mass. Liquid formula diets are supplemented with vitamins and minerals that also must be taken if a diet of lean meat, fish, or fowl is consumed. VLCDs should only be used under appropriate medical supervision. These diets produce weight losses of 15–25% in 8–16 weeks, but are not as widely used today as a decade ago. This can be attributed to their cost (i.e., approximately $3,000 for a 6-month program) and to findings of significant weight regain. Several randomized trials found VLCDs to be no more effective than LCDs 1 year after treatment. These findings led an expert panel convened by NHLBI not to recommend the use of VLCDs.
_
Low-calorie diets can lower total body weight by an average of 8% in the short term. These diets are well-tolerated and characterize successful strategies in maintaining significant weight loss over a 5-year period. Very-low-calorie diets produce a more rapid weight loss but should only be used for fewer than 16 weeks because of clinical adverse effects. Diets that are severely restricted in carbohydrates (3%–10% of total energy intake) and do not emphasize a reduction of energy intake may be effective in reducing weight in the short term, but there is no evidence that they are sustainable or innocuous in the long term because their high saturated-fat content may be atherogenic. Fat restriction in a weight-loss regimen is beneficial, but the optimal percentage has yet to be determined.
_
Low-carbohydrate diets:
Low-carbohydrate diets are relatively high in fat and protein content and are not recommended by the American Heart Association. Included in this category are the Protein Power diet and the Atkins diet. The Atkins diet has drawn the attention of consumers since its inception in the 1970s. Atkins states that most people consume carbohydrates that are absorbed rapidly, which leads to increases in glycemia and insulin secretion followed by lowered blood glucose levels and increased cravings result, whereby refined snack foods are usually consumed, which perpetuates the cycle and leads to body fat storage. A key premise of the Atkins diet is that the total energy intake is not important. Carbohydrate intake is severely restricted (3%–10% of total daily energy intake), whereas fat and protein can be consumed to satisfaction. Exercise is mandatory, and a daily vitamin, mineral and fatty acid supplement is recommended. In a review of 94 trials on the efficacy of low-carbohydrate diets from 1966 to 2003, Bravata and colleagues reported that weight loss was principally associated with reduced energy intake and not reduced carbohydrate intake. These observations conform with the principles of thermodynamics. They are also in agreement with the results of 4 recent randomized trials designed to test the main principles of the Atkins diet, in which low-carbohydrate diets (< 30 g/d of carbohydrates, < 10% of total daily energy intake from carbohydrates, ad libitum protein and fat intake) were compared with the conventional low-fat, energy-restricted diet. Brehm and colleagues reported that the mean daily energy intake at the end of the 6-month study period was 5468 kJ on the low-carbohydrate diet and 5237 kJ on the low-calorie diet. Both the low-calorie and low-carbohydrate diets result in weight loss. The Atkins diet, promote a diet that is high in protein and low in carbs, but also very high in saturated fat. There is no doubt that saturated fat should be limited in our diets as it has been shown to have a variety of negative effects on the body, including increasing the risk of heart disease, raising blood pressure, increasing risk of diabetes and causing weight gain. High-protein, low-fat diets represent a hybrid between two current schools of thought in weight loss and maintenance: low-carb diets and low-fat diets. Low-carb, high protein diets minimize hunger during weight loss, while low-fat diets may protect heart health. Both types of diet are effective for weight loss and maintenance, and some people choose to combine the two to maximize their results. High-protein, low-fat diets are effective for weight loss and favorably alter biomarkers in healthy adults.
_
A review of 94 studies of low-carbohydrate diets concluded that there is not enough evidence to recommend for or against the use of such diets. The reviewed study diets supplied various amounts of carbohydrate, with some as low as 20 g/day. Participant weight loss was principally associated with decreased caloric intake and increased duration of the diet, but not with reduced carbohydrate content. We do not have long-term studies with respect to efficacy, safety, and weight maintenance. A special caution, the National Academy of Sciences’ Institute of Medicine has recommended a minimum daily intake of 100 g of carbohydrate for optimal brain function.
_
Low-carbohydrate high protein diets have been widely promoted in recent years as an effective approach to losing weight. These diets generally recommend dieters receive 30% to 50% of their total calories from protein. However, high protein, low-carb diets can cause a number of health problems, including:
- Kidney failure. Consuming too much protein puts a strain on the kidneys, which can make a person susceptible to kidney disease.
- High cholesterol. It is well known that high-protein diets (consisting of red meat, whole dairy products, and other high fat foods) are linked to high cholesterol. Studies have linked high cholesterol levels to an increased risk of developing heart disease, stroke, and cancer.
- Osteoporosis and kidney stones. High-protein diets have also been shown to cause people to excrete a large amount of calcium in their urine. Over a prolonged period of time, this can increase a person’s risk of osteoporosis and kidney stones. A diet that increases protein at the expense of a very restrictive intake of plant carbohydrates may be bad for bones, but not necessarily a high-protein intake alone.
- Cancer. One of the reasons high-protein diets increase the risks of certain health problems is because of the avoidance of carbohydrate-containing foods and the vitamins, minerals, fiber, and antioxidants they contain. It is therefore important to obtain your protein from a diet rich in whole grains, fruits, and vegetables. Not only are your needs for protein being met, but you are also helping to reduce your risk of developing cancer.
- Unhealthy metabolic state (ketosis). Low-carb diets can cause your body to go into a dangerous metabolic state called ketosis since your body burns fat instead of glucose for energy. During ketosis, the body forms substances known as ketones, which can cause organs to fail and result in gout, kidney stones, or kidney failure. Ketones can also dull a person’s appetite, cause nausea and bad breath. Ketosis can be prevented by eating at least 100 grams of carbohydrates a day.
__
Ketosis and insulin synthesis: what is normal?
At the heart of the debate about most low carbohydrate diets are fundamental questions about what a normal diet is and how the human body is supposed to operate. These questions can be outlined as follows:
The diets of most people in modern Western nations, especially the United States, contain large amounts of starches and often substantial amounts of sugars, including fructose. Most westerners seldom exhaust stored glycogen supplies and hence rarely go into ketosis. This has been regarded by the majority of the medical community in the last century as normal for humans. Ketosis had been confused with ketoacidosis, a dangerous and extreme ketotic condition associated with diabetes, and had been regarded by the medical community as harmful and potentially life-threatening, who believe it unnecessarily stresses the liver and causes destruction of muscle tissues. A perception developed that getting energy chiefly from dietary protein rather than carbohydrates causes liver damage and that getting energy chiefly from dietary fats rather than carbohydrates causes heart disease and other health problems. This view is still held by the majority of those in the medical and nutritional science communities. However, it is now widely recognized that periodic ketosis is in fact normal, and that ketosis provides a number of surprising benefits, including neuroprotection against diverse types of cellular injury. People who eschew low carbohydrate diets cite hypoglycemia and ketoacidosis as a risk factor. While mild acidosis may be a side effect when beginning a ketogenic diet, it is benign and should not be confused with diabetic ketoacidosis, which can be life threatening. A diet very low in starches and sugars induces several adaptive responses. Low blood glucose causes the pancreas to produce glucagon, which stimulates the liver to convert stored glycogen into glucose and release it into the blood. When liver glycogen stores are exhausted, the body starts utilizing fatty acids instead of glucose. The brain cannot use fatty acids for energy, and instead uses ketones produced from fatty acids by the liver. By using fatty acids and ketones as energy sources, supplemented by conversion of proteins to glucose (gluconeogenesis), the body can maintain normal levels of blood glucose without dietary carbohydrates. Most advocates of low-carbohydrate diets, such as the Atkins Diet, argue that the human body is adapted to function primarily in ketosis. They argue that high insulin levels can cause many health problems, most significantly fat storage and weight gain. They argue that the purported dangers of ketosis are unsubstantiated (some of the arguments against ketosis result from confusion between ketosis and ketoacidosis which is a mostly diabetic condition unrelated to dieting or low-carbohydrate intake). They also argue that fat in the diet only contributes to heart disease in the presence of high insulin levels and that if the diet is instead adjusted to induce ketosis, fat and cholesterol in the diet are beneficial. Also, most low carb diets plans discourage consumption of trans fat.
_
Low carb diet from plant source better than animal source:
A study of more than 100,000 people over more than 20 years within the Nurses’ Health Study observationally concluded that a low-carbohydrate diet high in vegetables, with a large proportion of proteins and oils coming from plant sources, decreases mortality with a hazard ratio of 0.8. In contrast, a low-carbohydrate diet with largely animal sources of protein and fat increases mortality, with a hazard ratio of 1.1. This study, however, has been met with criticism, due to the unreliability of the self-administered food frequency questionnaire, as compared to food journaling, as well as classifying “low-carbohydrate” diets based on comparisons to the group as a whole (decile method) rather than surveying dieters following established low-carb dietary guidelines like the Atkins or Paleo diet.
_
High protein diet for weight loss:
A high-protein diet is often recommended by bodybuilders and nutritionists to help efforts to build muscle and lose fat. It should not be confused with low-carb diets such as the Atkins Diet, which are not calorie-controlled and which often contain large amounts of fat. We’ve long known that protein is essential to a healthy diet, but this deserves a new exclamation point. A high protein diet is essential to a slimmer, happier and healthier society. Increase protein, lose pounds. High protein diets may contribute to weight loss in a variety of ways, for example it has been suggested that by reducing carbohydrates, the brain may receive less hunger stimulating hormones, resulting in a reduced appetite. High protein diets have been shown to increase satiety, and result in the consumption of fewer calories in followers, which in turn leads to weight loss. The Recommended Dietary Allowance for men is 56 grams per day and 46 grams for women, values that the majority of Americans already exceed. For weight loss benefit however, it is thought that around 120g of protein should be eaten daily. Referred to as the world’s largest diet study, a team of Danish researchers utilized eight European research centers to monitor the diets of 772 random European families, comprising 938 adults and 827 children. Their findings demonstrate the role of protein in combating the obesity epidemic. “If you want to lose weight, your best bet is to maintain a diet high in protein-rich foods — such as lean meat, low-fat dairy products and beans — and low in refined starches, such as white bread and white rice,” read a statement announcing the research. “With this diet, you can eat until you are full without counting calories and without gaining weight.” Overweight adults were first put through a low calorie diet for eight weeks, losing an average of 24 pounds. They were then assigned to one of five different diets for six months to determine which was most effective at preventing weight increase. Those on the high protein diet with a low glycemic index (a measure of the effects of carbohydrates on blood sugar levels) were the only ones that maintained their weight after the initial weight loss. Those on the low protein, high glycemic index diet regained the most. The research is particularly important in solving child obesity. The 827 children, of which 45% were overweight, followed the same diet as their parents during the six month period. In the group of kids on the high protein, low glycemic index diet, the obese rate dropped by 15 percent. In goes the protein, out goes the blues. Extreme protein intake (in excess of 200 g per day), coupled with inadequate intake of other calorie sources (fat or carbohydrates), can cause a form of metabolic disturbance and death commonly known as rabbit starvation.
_
The Many Benefits of a Mediterranean Diet:
Key components of the Mediterranean diet include:
-Eating a generous amount of fruits and vegetables
-Consuming healthy fats such as olive oil and canola oil
-Eating small portions of nuts -Drinking red wine, in moderation, for some -Consuming very little red meat -Eating fish on a regular basis
Basically, at the top of the Mediterranean food pyramid are grains, fresh fruits and vegetables, olive oil, cheeses, yogurt, nuts, and legumes, all of which are consumed on a daily basis. Foods eaten on a weekly basis are fish and seafood, poultry, eggs, and sweets. Mediterranean people consume red meat less often on a monthly basis, and red wine about 1-2 glasses per day. If you’re looking for a heart-healthy eating plan, the Mediterranean diet might be right for you. The Mediterranean diet incorporates the basics of healthy eating, plus a splash of flavorful olive oil and perhaps a glass of good red wine, among other components characterizing the traditional cooking style of countries bordering the Mediterranean Sea. Most healthy diets include fruits and vegetables, fish and whole grains, and limit unhealthy fats. While these fundamental parts of a healthy diet remain tried and true, subtle variations or differences in proportions of certain foods may make a difference in your risk of heart disease. Seafood plays a very important part in the diet, and portion sizes of meat in particular, as you can see above are often smaller. The Mediterranean eating style significantly reduces the risk of further heart disease in individuals who had already had a heart attack. Remarkably, this benefit was not related to any significant difference in cholesterol levels — rather other components of the diet seem to work in concert to protect the body. Actually, the Mediterranean diet is not really a set diet. It is simply a healthy eating pattern – a pattern close to the dietary guidelines recommended by the American Heart Association. This diet is high in the good fats (monounsaturated and polyunsaturated fats) as present in fish, olive oil and nuts; and low in saturated fats and trans fats. It provides excellent source of fiber and antioxidants through encouragement of eating lots of plant-based foods. Many Mediterranean cultures adopt a laid-back attitude toward life, which decreases daily stress. Evidence from epidemiological studies supports a protective effect of this dietary pattern on weight gain and the development of type 2 diabetes. Many researchers concluded that dietary patterns associated with a high intake of fruits and vegetables in Mediterranean populations may reduce long-term risk of subsequent weight gain and obesity among adults.
_
Low glycemic index (healthy carbohydrates) diet best for weight reduction: a study:
A diet based on healthy carbohydrates—rather than a low-fat or low-carbohydrate diet—offers the best chance of keeping weight off without bringing unwanted side effects, a study published in the Journal of the American Medical Association suggests. Study participants following a low-glycemic-index diet, which is similar to a Mediterranean diet and focuses on fish, fruit, vegetables, nuts and whole grains, also saw improved cholesterol levels and other important markers that lower the risks of developing heart disease and diabetes. Such a diet might include minimally processed oatmeal, almonds, brown rice, beans and healthy fats like olive oil, among other foods.
Participants were placed on one of three diets for a month:
A low-fat diet, which included mostly whole grains, fruits, and vegetables, where 60% of daily calories came from carbohydrates, 20% from fats, and 20% from protein.
A low-glycemic-index diet, which included minimally processed grains, vegetables, legumes, and healthy fats, where 40% of calories came from carbohydrates, 40% from fat, and 20% from protein.
A very-low-carb diet, modeled after the Atkins plan, where 10% of calories came from carbohydrates, 60% from fats, and 30% from protein.
Participants were then switched to the other two diets during two additional four-week periods. “The low-fat diet had the worst effect” on energy expenditure. Participants on that diet also had increases in triglycerides, and lower levels of so-called good cholesterol. We should avoid severely restricting any major nutrient and focus on the quality of the nutrient. Those on the low-carb diet had the biggest boost in total energy expenditure, burning about 300 calories more per day than those on the low-fat diet—about the same as an hour of moderate exercise. But that bump came at a cost: increases in cortisol, a stress hormone, and a measure of inflammation called CRP, which can raise the risk of developing heart disease and diabetes. Those on the low-glycemic-index diet burned about 150 calories a day more than those on the low-fat diet without any negative impacts on cholesterol levels or various hormones, making it the ideal diet. The team found that a low-glycemic index diet had similar metabolic benefits to a very low-carb diet – but without negative effects of stress and inflammation as seen by participants consuming the very low-carb diet. Researchers said that because they reduce the surge in blood sugar after a meal, both the low-glycemic index and the very-low carbohydrate–diet may be preferable to a low-fat diet for those trying to achieve lasting weight loss. In addition to the benefits noted in this study, low-glycemic-index diets are easier to stick to on a day-to-day basis, compared to low-carb and low-fat diets, which many people find limiting. Unlike low-fat and low-very-carbohydrate diets, a low-glycemic-index diet doesn’t eliminate entire classes of food, likely making it easier to follow and more sustainable. Resting energy expenditure and total energy expenditure were reduced more with a low-carbohydrate diet or low–glycemic index diet compared to a low-fat diet, despite all participants consuming the same amount of calories. The researchers suggest that a strategy to reduce glycemic load rather than dietary fat may be advantageous for weight-loss maintenance and cardiovascular disease prevention.
_
_
Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men: The New England journal of medicine:
Some foods — vegetables, nuts, fruits, and whole grains — were associated with less weight gain when consumption was actually increased. Obviously, such foods provide calories and cannot violate thermodynamic laws. Their inverse associations with weight gain suggest that the increase in their consumption reduced the intake of other foods to a greater (caloric) extent, decreasing the overall amount of energy consumed. Higher fiber content and slower digestion of these foods would augment satiety, and their increased consumption would also displace other, more highly processed foods in the diet, providing plausible biologic mechanisms whereby persons who eat more fruits, nuts, vegetables, and whole grains would gain less weight over time. Yogurt consumption was also associated with less weight gain in all three cohorts. Potential mechanisms for these findings are unclear; intriguing evidence suggests that changes in colonic bacteria might influence weight gain. It is also possible that there is an unmeasured confounding factor that tracks with yogurt consumption (e.g., people who change their yogurt consumption may have other weight-influencing behaviors that were not measured by our instruments). The present findings suggest that the relationship between alcohol use and weight change is complex, and further analyses are needed that address potential heterogeneity with respect to sex, beverage type, baseline intake, direction of change, and duration of follow-up. Short-term controlled trials suggest that liquids are less satiating than solid foods, increasing the total amount of energy consumed. Overall, the analysis showed that changes in the consumption of all liquids except milk were positively associated with weight gain; the findings for high-carbohydrate beverages were consistent with those for refined carbohydrates and starches consumed in foods. Temporal trends render the findings especially relevant: between 1965 and 2002, U.S. beverage consumption increased from 11.8 to 21.0% of all calories consumed — 222 more kilocalories per person per day — with sugar-sweetened beverages and alcohol accounting for 60% and 32% of the increase, respectively.
__
Food variety and weight loss:
What is food variety?
Food variety refers to the consumption of a mixture of foods from the entire range of food groups (that is, vegetables, fruit, cereals, meat, fish and dairy products). The word variety indicates that a range of foods from each food group should be consumed. For example, instead of choosing one cereal product to eat, it is better to choose a selection of cereal products and alternate, say between wheat, oat, rye, rice, or barley. It is recommended that a variety of foods be eaten because the nutritional content of each food is very different. This is true even for foods that are from the same food group. Eating a variety of foods balances the vitamins, minerals, fats, carbohydrates, complete and incomplete proteins. Since there are more than 40 essential nutrients we must consume to maintain good health, and since no food contains every nutrient you need, this makes a case for eating a wide variety of foods.
_
There are clear benefits to eating a wider variety of foods:
•Access to a wider variety of micronutrients and phytochemicals. Think of all the various antioxidants associated with the greens, reds, yellows, purples, and oranges in fruits and vegetables. Think of how vitamin and mineral content differs between foods.
•Dilution of food toxins. Food toxins usually operate in a dose-dependent manner, so keeping a variety would help keep the doses low and spread thin.
•Food enjoyment. Eating the same three things is a sure path to food boredom. Eating should come with a serving of sensory enjoyment.
•You could develop food allergies or intolerances from eating the same foods all the time.
_
Let’s use an example. If you eat 1,200 calories per day, you should lose weight. If those calories come only from jelly beans, it won’t be a very healthy way to lose weight. Jelly beans contain calories but very few vitamins, minerals, and other nutrients the body needs. However, if you consult dietician, he will advise a weight loss plan that contains generous amounts of vegetables and fruits, whole grain carbohydrates, lean sources of protein, heart healthy fats, and just a small amount of a sweet, such as jelly beans. These types of foods contain vitamins, minerals, fiber, and many other nutrients and phytonutrients (different types of nutrients found in plants). These types of foods have a tremendous amount of evidence supporting their beneficial effects on health. To promote effective weight loss, your diet should lower calorie intake. To promote good health, it should include a variety of healthy food choices.
_
Can food variety cause weight gain?
However, there’s more than one side to the food variety story. Ongoing research suggests that narrowing your choices and eating a smaller variety of foods might actually make it easier to lose fat! Greater variety does not automatically translate into better fat loss. If calories remain equal, then “more variety = more fat loss” is not true. To the contrary, many people inadvertently eat more calories as a result of following the food variety guideline too literally without paying attention to portion sizes. In fact, there’s a large body of evidence showing that if you have less variety in your diet, you will usually eat less and lose more fat! In a study from Tufts University, 71 men and women, with ages ranging from 20 to 80, were analyzed for their food intake patterns and body composition. The more variety there was in their diets, the higher their caloric intake. In particular, a wide variety of sweets, carbohydrates, condiments, entrees and snack foods were most associated with increased calorie consumption. Research from Brown University in Providence, Rhode Island studied weight loss maintenance in a group called the National Weight Control Registry (NWCR). The NWCR is the largest ongoing observational study examining long term weight loss maintainers. Registry members reported consuming a diet with very low variety in all food groups. The only exceptions were fruits and vegetables. The researchers concluded that restricting variety may help with consuming a low-energy diet and maintaining long-term weight loss.
_
Stimulus narrowing and the buffet effect:
Stimulus narrowing means a lot of people find it easier to keep track of calories if they eat the same thing every day. Also, when mostly the same foods are eaten every day, it becomes habitual and habit patterns run unconsciously without much work or effort. Eating the same when you start a fat loss program for the first time also helps you establish a baseline very easily. When you have a baseline, it’s easy to make changes one at a time and measure the effects of those changes in a systematic fashion. This is helpful in the early learning stages. If your nutrition is random – the foods you eat, your meal frequency or anything at all – it’s difficult to keep track of calories unless you faithfully log every morsel into a written or electronic journal. It’s also harder to track the effects of dietary manipulations because you never had a baseline.
Another phenomenon we see in the research is what scientists call “the buffet effect,” which means that if you’re given a lot of choices at one meal — literally, as at a buffet line, a potluck dinner or even a menu that stays on the table – you almost always eat more. Researcher Brian Wansink and his team from Cornell University food and brand laboratory observed the eating behaviors of diners at 11 all-you-can-eat buffets. As you might guess, the subjects all ate more and ate more quickly. Calorie intake was also positively associated with being seated nearby and facing the buffet.
_
Less variety, less pleasurable eating:
It’s been proposed that if you decrease the variety in your meals, it reduces calorie consumption due to sensory-specific satiety. Sensory specific satiety means that there is a reduction in hedonic ratings of the foods eaten in a meal. In other words, if eating a food is not very pleasurable, for any reason, you tend to eat less.
_
Meal substitutes:
When used as substitutes for regular meals, meal substitutes are a convenient way to reduce calories as part of a low-calorie diet plan. A typical meal substitute available in powder and liquid form is Slim-Fast. Ensure is another meal substitute available in both liquid and bars. Meal substitutes should provide protein and be low in fat and calories. The label should include the amount of calories per serving and the percentages of protein, carbohydrates, and fat. The total number of calories per serving is predetermined so it is easier to keep track of the daily consumption of calories.
_
Artificial sweeteners:
Saccharin and aspartame are sugar substitutes that provide little or no calories. They may be used as a substitute for table sugar. Using saccharin instead of a teaspoonful of sugar eliminates 33 calories from the diet. People with phenylketonuria (a serious genetic disease in which an individual is unable to break down and eliminate an amino acid, phenylalanine) should not use aspartame because it contains phenylalanine. Fructose, sorbitol, and xylitol may be used as alternatives to sugar, but they provide more calories than saccharin and aspartame. Excessive use of sorbitol also may cause diarrhea.
_
Can ASB [artificially sweetened beverages] promote weight gain?
In an opinion piece in the journal Trends in Endocrinology and Metabolism, Purdue University professor Susan Swithers writes that drinks containing such chemicals as aspartame, sucralose, and saccharin have been found to contribute to excessive weight gain, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Her piece summarizes studies on the health effects of artificial sweeteners: Recent data from humans and rodent models have provided little support for ASB [artificially sweetened beverages] in promoting weight loss or preventing negative health outcomes such as [type 2 diabetes], metabolic syndrome, and cardiovascular events. Instead, a number of studies suggest people who regularly consume ASB are at increased risk compared with those that do not. How is this possible? Swithers describes a number of theories, some of them relating to the effects of such sweeteners on metabolism. “Sweet tastes are known to evoke numerous physiological responses,” she writes. “By weakening the validity of sweet taste as a signal for caloric post-ingestive outcomes, consumption of artificial sweeteners could impair energy and body weight regulation.” The findings of the San Antonio Heart Study, which pointed to a strong link between diet soda consumption and weight gain over time. “On average, for each diet soft drink our participants drank per day, they were 65 percent more likely to become overweight during the next seven to eight years” said Sharon Fowler, in a release announcing the findings several years back. Another bit of evidence: A multi-ethnic study, which included some 5,000 men and women, found that diet soda consumption was linked to a significantly increased risk of both type-2 diabetes and metabolic syndrome. Also, it’s also worth remembering that some scientists have found that artificial sweeteners can be toxic. However, researchers at Boston Children’s Hospital found that overweight teens did well when they switched from sugar-laden drinks to zero-calorie options such as diet soda. If you’re choosing between a diet soda and a regular soda, then it’s probably healthier to go for the former. But these studies are a reminder that such a choice won’t keep you healthy.
_
Eat more healthy carbs and whole grains (low GI food):
Choose healthy carbohydrates and fiber sources, especially whole grains, for long lasting energy. In addition to being delicious and satisfying, whole grains are rich in phytochemicals and antioxidants, which help to protect against coronary heart disease, certain cancers, and diabetes. Studies have shown people who eat more whole grains tend to have a healthier heart.
A quick definition of healthy carbs and unhealthy carbs:
Healthy carbs (sometimes known as good carbs) include whole grains, beans, fruits, and vegetables. Healthy carbs are digested slowly, helping you feel full longer and keeping blood sugar and insulin levels stable.
Unhealthy carbs (or bad carbs) are foods such as white flour, refined sugar, and white rice that have been stripped of all bran, fiber, and nutrients. Unhealthy carbs digest quickly and cause spikes in blood sugar levels and energy.
_
Try different types of protein. Whether or not you are a vegetarian, trying different protein sources—such as beans, nuts, seeds, peas, tofu, and soy products—will open up new options for healthy mealtimes.
- Beans: Black beans, navy beans, garbanzos, and lentils are good options.
- Nuts: Almonds, walnuts, pistachios, and pecans are great choices.
- Soy products: Try tofu, soy milk, tempeh, and veggie burgers for a change.
- Avoid salted or sugary nuts and refried beans.
Downsize your portions of protein. Many people in the West eat too much protein. Try to move away from protein being the center of your meal. Focus on equal servings of protein, whole grains, and vegetables. Focus on quality sources of protein, like fresh fish, chicken or turkey, tofu, eggs, beans, or nuts. When you are having meat, chicken, or turkey, buy meat that is free of hormones and antibiotics.
_
Limit sugar and salt:
If you succeed in planning your diet around fiber-rich fruits, vegetables, whole grains, lean protein, and good fats, you may find yourself naturally cutting back on foods that can get in the way of your healthy diet—sugar and salt.
Sugar:
Sugar causes energy ups and downs and can add to health and weight problems. Unfortunately, reducing the amount of candy, cakes, and desserts we eat is only part of the solution. Often you may not even be aware of the amount of sugar you’re consuming each day. Large amounts of added sugar can be hidden in foods such as bread, canned soups and vegetables, pasta sauce, margarine, instant mashed potatoes, frozen dinners, fast food, soy sauce, and ketchup. Here are some tips:
- Avoid sugary drinks. One 12-oz soda has about 10 teaspoons of sugar in it, more than the daily recommended limit! Try sparkling water with lemon or a splash of fruit juice.
- Eat naturally sweet food such as fruit, peppers, or natural peanut butter to satisfy your sweet tooth.
How sugar is hidden on food labels:
Check food labels carefully. Sugar is often disguised using terms such as:
|
|
Limiting foods and drinks with added sugars, like high-fructose corn syrup, is important. Added sugars will give you extra calories without nutrients like vitamins and minerals. Added sugars are found in many desserts, canned fruit packed in syrup, fruit drinks, and nondiet drinks.
Salt:
Most of us consume too much salt in our diets. Eating too much salt can cause high blood pressure and lead to other health problems. Try to limit sodium intake to 1,500 to 2,300 mg per day, the equivalent of one teaspoon of salt.
- Avoid processed or pre-packaged foods. Processed foods like canned soups or frozen dinners contain hidden sodium that quickly surpasses the recommended limit.
- Be careful when eating out. Most restaurant and fast food meals are loaded with sodium.
- Opt for fresh or frozen vegetables instead of canned vegetables.
- Cut back on salty snacks such as potato chips, nuts, and pretzels.
- Choose low-salt or reduced-sodium products.
- Try slowly reducing the salt in your diet to give your taste buds time to adjust.
_
Enjoy healthy fats & avoid unhealthy fats
Good sources of healthy fat are needed to nourish your brain, heart, and cells, as well as your hair, skin, and nails. Foods rich in certain omega-3 fats called EPA and DHA are particularly important and can reduce cardiovascular disease, improve your mood, and help prevent dementia.
Add to your healthy diet:
- Monounsaturated fats, from plant oils like canola oil, peanut oil, and olive oil, as well as avocados, nuts (like almonds, hazelnuts, and pecans), and seeds (such as pumpkin, sesame).
- Polyunsaturated fats, including Omega-3 and Omega-6 fatty acids, found in fatty fish such as salmon, herring, mackerel, anchovies, sardines, and some cold water fish oil supplements. Other sources of polyunsaturated fats are unheated sunflower, corn, soybean, flaxseed oils, and walnuts.
Reduce or eliminate from your diet:
- Saturated fats, found primarily in animal sources including red meat and whole milk dairy products.
- Trans fats, found in vegetable shortenings, some margarines, crackers, candies, cookies, snack foods, fried foods, baked goods, and other processed foods made with partially hydrogenated vegetable oils.
_
Food pyramid:
The food pyramid, also known as dietary guidelines is designed to reduce obesity in men, women and children. At the bottom are things everybody should eat/do every day and on the top are things that we should avoid to do/eat. There are different pyramids designed for different food cultures and the figure below is one of the food pyramid.
_
How religious fasting can reduce body fat:
Glycogen is the unused and stored form of carbohydrates in muscles. When glycogen stores begin to peak from eating plenty of carbs, the body upgrades its fat-storing ability. Conversely, as glycogen stores are depleted, fat-burning increases. One way to kick-start the fat-burning process is to go extremely low-carb on two consecutive days every couple of weeks (fasting). This ensures that you tap into your glycogen stores for fuel, which signals the body to burn more fat. Limit your total carbs on two consecutive days every two weeks or so to less than 100 grams per day. This will require you to know how many grams of carbohydrates are in the foods you eat and have the discipline to be very strict on your intake. Your diligence will be rewarded with a noticeable difference in body fat. After two days, you can return to a more normal, though not excessive, carb intake. So while doing religious fasting, ensure that you eat less than 100 gms of carbohydrates per day.
_
What is best breakfast? Fruits & wholegrains or eggs & nuts?
Contrary to popular belief, consumption of granola, skimmed milk, fruit juice and wholegrains for breakfast is not conducive to fat loss. These typical “healthy” breakfast options are all high in carbohydrates and low in both fat and protein. This is in fact the complete opposite of what we need first thing in the morning for optimal body composition. We wake up in the morning in a fasted fat burning state. We have high levels of the hormones cortisol and growth hormone and low levels of insulin. Our bodies are breaking down triglycerides into free fatty acids for metabolism (we are burning our body fat for energy!). The last thing we need to do at this point is consume a large amount of carbohydrates. What this does is raise insulin levels and shuts down fat burning. Some research shows that fat burning remains vastly reduced throughout the whole day as a result of this insulin spike. Additionally, blood glucose levels will fall, causing the body to crave sweet foods throughout the morning to bring them back up. In years gone by we have been led to believe that eliminating fat from our diet is the most effective way to lose body fat. At first glance this makes sense, however when we consider the essential roles of dietary fat within the body and what we are replacing dietary fats with (more carbohydrates generally), we can begin to understand why this is an ineffective approach. Consuming good sources of fat, contrary to popular belief, will actually aid fat loss rather than oppose it. The body needs fat for many important functions, such as building hormones and healthy cell membranes. The addition of specific forms of dietary fat, such as medium chain triglycerides (found abundantly in coconut oil), have been shown to significantly increase our ability to utilise fat for fuel. Looking now at protein, it is important to consume a high quality source of protein at breakfast because you need the amino acids to build and maintain muscle mass (muscle mass is essential for maximising metabolic rate, remaining insulin sensitive and preventing just about every health issue from joint problems to cardiovascular disease!). Additionally, it will make you feel fuller throughout the morning, stabilise your blood sugar levels and increase your metabolic rate (it requires more energy to digest protein than carbohydrates). We can now see that the optimal breakfast for body composition should be low in carbohydrate and higher in protein and fat. This means we should avoid bread, cereal and excessive fruit consumption and favor eggs, meats and nuts.
_
Limiting soda sizes may increase obesity, boost sugary drink consumption instead of reduce it: a study:
These are the findings of a new study out of the University of Missouri – Kansas City, which found that people are more likely to spend more money on, and drink more, sugary beverages when they are sold only in smaller containers than when they are sold in typical varying sizes. These data suggest that a sugary drink restriction may not be effective in reducing consumption when businesses are able to sell bundles of soda that add up to the original, larger drink sizes. Even with the restriction, those who want to drink more will still be able to go ahead and have two. Offering bundled beverages was also found to generate more profits than offering beverages in the multiple sizes, which means businesses in restricted areas actually have the potential to boost both their profits and the volume of sugary beverages they sell. In other words, beverage size bans will most assuredly increase sugary beverage consumption rates, create more disease, and boost profits for the junk food industry, all of which are the opposite of what beverage size bans were intended to accomplish.
_
Why Americans are fat?
The US Dept. of Agriculture estimates that the average person in the United States eats 0.5 lbs of meat, 1.6 lbs of dairy products, 0.2 lbs of fats and oils, 0.8 lbs of fruits, 0.7 lbs. of vegetables, 0.5 lbs of grains, and 0.4 lbs of sugars per day for a total of 4.7 lbs. of food per day. No wonder Americans are so fat!
_
The Dietary Guidelines for Americans 2010 from the U.S. Department of Agriculture and the U.S. Department of Health and Human Services recommend eating the following amounts of food if you are eating 2,000 calories per day. Remember to adjust the amounts depending on your daily calorie level.
How many Vegetables each day?
A person who eats 2,000 calories daily should have 2 ½ cups of vegetables a day. This might include a half-cup each of broccoli, tomatoes, cauliflower, and a sweet potato. Aim for lots of color on your plate as a way to get a variety of vegetables each day.
How much Fruit each day?
A person who consumes about 2,000 calories daily should plan to eat 2 cups of fruit a day. This might include one large banana, one-half cup of strawberries and a half-cup of orange juice. To help you get enough fiber, most of your daily fruit intake should be in the form of whole fruits rather than fruit juices.
How many Grain Foods each day?
A person who eats 2,000 calories per day should eat 6 ounces of grain foods daily. At least half (3 ounces) of the grain foods eaten should be whole grains. Approximately one ounce of grain foods counts as a serving. This is about one slice of bread, one roll, or one small muffin. It is also about one cup of dry flaked cereal or a half-cup of cooked rice, pasta, or cereal.
How much Dairy each day?
Dairy products are another important part of eating well. A person who consumes 2,000 calories daily should have the equivalent of 3 cups of low-fat or fat-free milk, yogurt, or other dairy products daily. One cup of yogurt contains about the same amount of calcium as 1 cup of milk. Eating 1½ ounces of natural cheese or 2 ounces of processed cheese counts as drinking 1 cup of milk.
How much Protein each day?
A person who consumes 2,000 calories daily should eat about 5½ ounces of protein each day. You can get protein from seafood, lean meat and poultry, as well as eggs, beans and peas, tofu, nuts, and seeds. One egg or one-fourth cup of cooked dry beans or tofu counts as 1 ounce of meat, poultry, or seafood. One tablespoon of peanut butter or a half-ounce of nuts or seeds also is the same as 1 ounce of meat, poultry, or seafood.
How much Oil each day?
Oils are fats that are liquid at room temperature, like the vegetable oils used in cooking. Use mainly polyunsaturated and monounsaturated oils like those that come from olive or canola oil. A person who eats 2,000 calories daily should not consume more than the equivalent of 6 teaspoons of oil daily.
_______________
Physical activity to reduce obesity:
_
Regular physical activity helps maintain a healthy body:
Physical activity should not be mistaken for sport. Physical activity is any bodily movement produced by the skeletal muscles that uses energy. This includes sports, exercise and other activities such as playing, walking, doing household chores, gardening, and dancing. People should engage in adequate levels of physical activity throughout their lives. At least 30 minutes of regular, moderate-intensity physical activity on most days reduces the risk of cardiovascular disease, diabetes, colon cancer and breast cancer. Muscle strengthening and balance training can reduce falls and improve mobility among older adults. More activity may be required for weight control.
_
In “real life,” Galassetti noted, people do not maintain constant levels of exercise, but rather exercise intensively but intermittently, with intermediate periods of resting. Fat storage during periods of increased energy intake is normally balanced by periods of fat utilization with exercise. For example, African Pigmies utilize large amounts of energy when walking away from their villages for hunting. “We maintain the genes for that wide range of foraging,” Galassetti stated, and so “humans maintain their fat depots.” The regulation of body composition involves fat storage in adipocytes, with exercise involving the physiologic reduction in insulin levels and release of epinephrine and growth hormone resulting in lipolysis.
_
A study comparing moderate-intensity (65% Vo2max) long-duration or high-intensity (>75% Vo2max) shorter-duration physical training found the latter somewhat more effective in improving fitness despite similar caloric expenditure, with visceral adipose tissue decreasing similarly and improvements in lipids (HDL, triglyceride, and LDL size) with both physical training interventions.
_
Physical activity is recommended as part of a comprehensive weight loss therapy and weight control program because it:
(1) modestly contributes to weight loss in overweight and obese adults (Evidence Category A),
(2) may decrease abdominal fat (Evidence Category B),
(3) increases cardiorespiratory fitness (Evidence Category A), and
(4) may help with maintenance of weight loss (Evidence Category C).
Physical activity should be an integral part of weight loss therapy and weight maintenance. Initially, moderate levels of physical activity for 30 to 45 minutes, 3 to 5 days a week, should be encouraged. All adults should set a long-term goal to accumulate at least 30 minutes or more of moderate intensity physical activity on most, and preferably all, days of the week. Exercise burns only a modest amount of calories. But it does have other benefits. Exercise improves insulin sensitivity in skeletal muscle, lowering insulin levels in the bloodstream. Exercise reduces stress and, therefore, reduces stress-induced eating. Lastly, exercise increases metabolic rate. The directive to balance active play with computer, video and TV time is the most difficult one to comply with.
__
General exercise recommendations:
•Perform 20-30 minutes of moderate exercise five to seven days a week, preferably daily. Types of exercise include walking, stationary bicycling, walking or jogging on a treadmill, stair climbing machines, jogging, and swimming.
•Exercise can be broken up into smaller 10-minute sessions.
•Start slowly and progress gradually to avoid injury, excessive soreness, or fatigue. Over time, build up to 30 to 60 minutes of moderate to vigorous exercise every day.
•People are never too old to start exercising. Even frail, elderly individuals (70-90 years of age) can improve their strength and balance.
_
Benefits of exercise:
Physical activity and exercise help burn calories. The amount of calories burned depends on the type, duration, and intensity of the activity. It also depends on the weight of the person. A 200-pound person will burn more calories running 1 mile than a 120-pound person, because the work of carrying those extra 80 pounds must be factored in. But exercise as a treatment for obesity is most effective when combined with a diet and weight-loss program. Exercise alone without dietary changes will have a limited effect on weight because one has to exercise a lot to simply lose 1 pound. However regular exercise is an important part of a healthy lifestyle to maintain a healthy weight for the long term. Another advantage of regular exercise as part of a weight-loss program is a greater loss of body fat versus lean muscle compared to those who diet alone.
Other benefits of exercise include
•improved blood sugar control and increased insulin sensitivity (decreased insulin resistance),
•reduced triglyceride levels and increased “good” HDL cholesterol levels,
•lowered blood pressure,
•a reduction in abdominal fat,
•reduced risk of heart disease.
Remember, these health benefits can occur independently (with or without) achieving weight loss. Before starting an exercise program, you should talk to your doctor about the type and intensity of the exercise program.
_
Diet plus physical activity:
The combination of a reduced calorie diet and increased physical activity is recommended since it produces weight loss that may also result in decreases in abdominal fat and increases in cardiorespiratory fitness (Evidence Category A). A low-calorie diet that triggers weight loss of one to two pounds per week is generally considered the healthiest way to secure permanent weight loss. Increased physical activity should include 150 minutes per week of moderate to intense physical activity. To combat severe obesity, patients may have to perform as much as 250 to 300 minutes of exercise per week.
_
Exercise is important, too, but we sometimes overemphasize how important exercise is:
Tacking regular exercise onto a diet program typically doesn’t help obese children lose more weight, a new review suggests. Researchers analyzed 14 trials that assigned overweight and obese youth to a diet and exercise program or a diet-only plan. Most studies found that kids tended to have a lower body mass index and a smaller percentage of body fat after completing either type of intervention. Adding aerobic exercise such as jogging or dance to a restricted-calorie diet had little effect on weight loss. However, kids who did resistance training lost more body fat than those who didn’t exercise. Strength training was tied to an extra drop in body fat and a greater increase in muscle. The reviewers found that some measures of cholesterol and blood sugar, including insulin and ”good” cholesterol, improved with the addition of regular exercise. But changes in other levels, such as “bad” cholesterol, were greater with a diet plan alone. In many studies, kids regained weight once the programs ended.
_
Exercise is equally important as dieting: a study: Reducing calories alone is not enough:
New research demonstrates that simply reducing caloric intake is not enough to cause significant weight loss. This could be the result of a natural compensatory mechanism that reduces physical activity following a reduction in calories. Diet and exercise must be combined to achieve weight loss goals, according to the study. The research was conducted on 18 female rhesus macaque monkeys. They were fed high-calorie diet for several years, then given a 30 percent reduction in calories. Not only did they lose no weight, but physical activity levels for the monkeys began to drop shortly after the reduced-calorie diet was introduced. Another comparison group of three monkeys was trained to exercise for one hour daily on a treadmill. This group did lose weight. These interesting new findings suggest that when you start to consume fewer calories, your body automatically responds by reducing your activity levels. In generations past, when finding your next meal was not as easy as opening your fridge, this mechanism could help your body to hold on to valuable energy stores and survive longer in times of famine. The key to healthy weight loss is to use a combined approach of both diet and exercise together. This is the strategy that will lead to gradual weight loss and a healthier lifestyle that you can actually sustain and live with.
_
Women who are lean but inactive have higher mortality risk: a study:
Another revealing study also found that both diet and fitness levels are crucial for lifelong health. After analyzing more than 115,000 nurses, results showed that being overweight or being sedentary increased your risk of death independently:
1. Women who were obese and inactive had a 2.5 times higher mortality rate than women who were lean and active
2. Those women who were active (despite being obese) were twice as likely to die a premature death than those who were lean and active
3. Lean women who exercised under 3.5 hours a week increased their risk of premature death by 55 percent, compared to those women who worked out more often
4. Obese women who worked out for at least 3.5 hours a week experienced a death rate that was 91 percent higher than lean women who exercised similarly
5. The premature death rate was 142 times greater for obese, inactive women
So while being overweight or obese certainly increases many health risks, those who were lean and inactive also had their share of risks. And as you might suspect, the most significant risks apply to those who are both overweight/obese and also sedentary. The moral of the story, of course, is that you want to carefully tailor both your dietary and your exercise habits in ways that will support your health and well-being.
_
The recommended weekly volume of 150 minutes of aerobic conditioning for fitness improvement serves as a minimum for the treatment of obesity. For long-term weight loss and for prevention of weight regain in obese individuals, exercise duration should progress to 200–300 minutes per week. Recently revised guidelines stress the dose effect of physical activity and emphasize greater weight loss and prevention of regain at the level of 250–300 minutes per week. These guidelines also reiterate the importance of calorie restriction. This level of training obviously poses a challenge, and progression should proceed slowly along with behavioral strategies. The consensus concerning intensity is that moderate-intensity exercise at 55% to 69% of maximum heart rate is appropriate for the management of body weight rather than activity of more vigorous intensity. The value of intermittent exercise bouts is inconclusive at this time.
_
Do you burn fat or carbohydrate while exercising?
The body never shuts off one fuel source when it turns on the other. Your body burns either fat or carbs depending on the intensity of your activity. The body relies on both fat and carbs for energy all the time, albeit in different ratios. In fact, as you read my article, you may be burning about 50-60 percent fat and 50-40 percent carbohydrates. You’re not using much of either, however, because the amount of Kcal you need probably amounts to about one or two Kcal a minute. If you were to get up and start jogging in place, your body would need to supply you with some quick energy to do so, so the metabolism ratio might shift to drawing upon more carbohydrates, say 70 percent, and less fat, say 30 percent. If you were to continue jogging, then, in order to preserve the carbs (which can run out since you have limited stores in the body), your body would gradually shift its metabolism ratio again to say, 60 percent fat and 40 percent carbohydrates. From an energy efficiency point of view, it pays to be fit. The endurance athlete would be able to make the shift sooner, and his fat-burning percentage might be 65-75 percent. However, in practical terms this is purely technotalk, and these ratios don’t make a big difference when it comes to losing weight and decreasing your body fat. For the most part, athletes are often leaner not because they might rely on slightly more fat for fuel, but because they practice their sport two to three, or more, hours a day — this burns a lot of calories. If you had the time, energy, and fitness level to work out three hours a day, being overweight would probably not be an issue. To lose weight, you need to burn more calories than your body consumes and uses every day. Exercise is one main way to burn a lot of calories. But when it comes to weight loss, what matters is how many calories you burn, not so much whether they are fat or carbohydrate calories. Remember, it is the burning of as many calories as you can is the best way to lose weight, and therefore jogging and sprinting are going to give the best results, even though their fat-burning quota is on the low end of the ratio.
_
The table below shows fat burning quota of various activities:
|
Activity |
Calories Burned |
Fat Percentage |
Calories from Fat |
| Watching TV for 20 minutes | 40 calories | 60 percent | 24 calories |
| Walking for 20 minutes | 100 calories | 65 percent | 65 calories |
| Jogging & sprinting for 20 minutes | 250 calories | 40 percent | 100 calories |
_
Aerobic exercise (cardio) is the best way to burn Fat, not Weight Lifting:
Aerobic exercise has been ruled the best type of exercise for eliminating fat, according to a study by a group of experts from Duke University who explored the comparison between resistance training and aerobic training. This study, published in the Journal of Applied Physiology, is the biggest randomized trial to measure revisions in body composition from three different types of exercise in overweight adults who do not have diabetes. Aerobic exercise, commonly known as “cardio”, including running, walking, and swimming, has historically been established as a good way to lose weight. Recently, however, recommendations have pointed out that resistance training, such as weight lifting to build and perpetuate muscle mass, could also contribute to weight loss via boosting a person’s resting metabolic level.
_
Walking:
Walking is a popular and convenient form of exercise that can play an important role in weight management. Effective weight management requires an accurate knowledge of how much metabolic energy is expended during exercise. Obese individuals expend much more metabolic energy during walking than normal-weight individuals. Total body weight is the primary determinant of the cost of walking. Your weight and the distance you walk determine the energy calories burned while walking. Walking speed matters less than distance and weight. A rule of thumb is 100 calories per mile for a 180-pound person and 65 for a 120-pound person.
_
Exercise and visceral fat:
According to researchers from Duke University Medical Center, exercise can significantly reduce the amount of visceral fat you carry around. The more exercise you do, the more of this type of dangerous fat you will lose.
The team studied 175 men and women, they were all overweight and led sedentary lives – they were all beginning to show signs of lipid problems. Four groups were created and the 175 people were placed into them at random. Each group carried out different levels of activity/inactivity:
a. No exercise at all.
b. Low dose moderate intensity activity (walking 12 miles per week
c. Low dose vigorous intensity activity (12 miles jogging per week)
d. High dose vigorous intensity activity (20 miles jogging each week)
All 175 people were told not to alter their diets. The aim of the study was to see what impact exercise alone might have.
Researchers in this study said that extra exercise can reverse the amount you have, while some moderate exercise can stop your visceral fat mounting up. If, on the other hand, you remain inactive, more likely than not you will pile on the weight at a rate of four pounds per year, say researchers. Computed Tomography (CT) was used at the beginning and end of the trial (to determine the extent and distribution of fat change). Slentz found there was no significant difference in visceral fat levels among the low exercise groups. He concluded that mild exercise helps stop the increase, but does not reduce. The inactive group experienced increases in visceral fat levels. He found that the more people exercised, and the higher their intensity, the faster they lost their excess visceral fat. He also added that jogging 17 to 20 miles a week may seem like a lot. However, all the participants were soon able to run those amounts each week quite comfortably. The most active group saw visceral fat levels drop by 6.9% in six months, subcutaneous fat levels dropped by 7%.
_
Exercise precautions:
The following people should consult a doctor before vigorous exercise:
•Men over age 40 or women over age 50
•Individuals with heart or lung disease, asthma, arthritis, or osteoporosis
•Individuals who experience chest pressure or pain with exertion, or who develop fatigue or shortness of breath easily
•Individuals with conditions or lifestyle factors that increase their risk of developing coronary heart disease, such as high blood pressure, diabetes, cigarette smoking, high blood cholesterol, or having family members with early onset heart attacks and coronary heart disease
•A patient who is obese
_
Which is better for weight loss — cutting calories or increasing exercise?
For most people, it’s probably too difficult to eliminate the amount of calories through exercise that you could through dieting. Cutting calories through dietary changes seems to promote weight loss more effectively than does exercise and physical activity. But physical activity also is important in weight control. Exercise also is important because it can help you maintain your weight loss. Studies show that people who lose weight and keep it off over the long term get regular physical activity. If you lose weight by crash dieting or by drastically restricting yourself to 400 to 800 calories a day, you’re more likely to regain weight quickly, often within six months after you stop dieting. Getting regular exercise also can help prevent excess weight gain in the first place.
_
Groundbreaking Scientific study proving dieting more effective and efficient than exercise for weight loss:
Anthropologists leading one of the new studies began with a research trip to Tanzania. There, they recruited volunteers from the Hadza tribe, whose members still live by hunting and gathering. Providing these tribespeople with a crash course in modern field-study technology, the researchers fitted them with GPS units, to scrupulously measure how many miles each walked daily while searching for food. They also asked them to swallow so-called doubly labeled water, a liquid in which the normal hydrogen and oxygen molecules have been replaced with versions containing tracers. By studying these elements later in a person’s urine, researchers can precisely determine someone’s energy expenditure and metabolic rate. The researchers gathered data for 11 days, then calculated the participants’ typical daily physical activity, energy expenditure and resting metabolic rates. They then compared those numbers with the same measures for an average male and female Westerner. It’s long been believed that a hunter-gatherer lifestyle involves considerable physical activity and therefore burns many calories, far more than are incinerated by your average American office worker each day. And it was true, the scientists determined, that the Hadza people in general moved more than many Americans do, with the men walking about seven miles a day and the women about three. But it was not true that they were burning far more calories. In fact, the scientists calculated, the Hadza’s average metabolic rate, or the number of calories that they were burning over the course of a day, was about the same as the average metabolic rate for Westerners. The implication, the scientists concluded, is that “active, ‘traditional’ lifestyles may not protect against obesity if diets change to promote increased caloric consumption.” That is, even active people will pack on pounds if they eat like most of us in the West. The underlying and rather disheartening message of that finding, of course, is that physical activity by itself is not going to make and keep you thin. (It’s worth noting that the Hadza people were almost uniformly slight.)
_____________
Behavioral Changes:
Changing your behaviors or habits related to food and physical activity is important for losing weight. The first step is to understand which habits lead you to overeat or have an inactive lifestyle. The next step is to change these habits. Below are some simple tips to help you adopt healthier habits.
Change your surroundings. You might be more likely to overeat when watching TV, when treats are available at work, or when you’re with a certain friend. You also might find it hard to motivate yourself to be physically active. However, you can change these habits.
- Instead of watching TV, dance to music in your living room or go for a walk.
- Leave the office break room right after you get a cup of coffee.
- Bring a change of clothes to work. Head straight to an exercise class on the way home from work.
- Put a note on your calendar to remind yourself to take a walk or go to your exercise class.
Keep a record. A record of your food intake and the amount of physical activity that you do each day will help inspire you. You also can keep track of your weight. For example, when the record shows that you’ve been meeting your physical activity goals, you’ll want to keep it up. A record also is an easy way to track how you’re doing, especially if you’re working with a registered dietitian or nutritionist.
____________
Long-term weight loss maintenance:
As many as 85% of dieters who do not exercise on a regular basis regain their lost weight within two years. In five years, the figure rises to 90%. There is a general perception that almost no one succeeds in long-term maintenance of weight loss. However, research has shown that ≈20% of overweight individuals are successful at long-term weight loss when defined as losing at least 10% of initial body weight and maintaining the loss for at least 1 y. To maintain their weight loss, members report engaging in high levels of physical activity (≈1 h/d), eating a low-calorie, low-fat diet, eating breakfast regularly, self-monitoring weight, and maintaining a consistent eating pattern across weekdays and weekends. Moreover, weight loss maintenance may get easier over time; after individuals have successfully maintained their weight loss for 2–5 y, the chance of longer-term success greatly increases. Continued adherence to diet and exercise strategies, low levels of depression and disinhibition, and medical triggers for weight loss are also associated with long-term success. Repeatedly losing and regaining weight encourages the body to store fat and may increase a patient’s risk of developing heart disease. The primary factor in achieving and maintaining weight loss is a life-long commitment to regular exercise and sensible eating habits.
_____________
Anti-obesity medications:
_
_
_
Anti-obesity drugs may be used in adult patients at medical risk from obesity (BMI 30 or greater), or overweight patients with established comorbidities (BMI 27) if the drug license permits, where dietary and lifestyle modifications have been unsuccessful in achieving a 10% weight reduction after at least three months of supervised care. Not all obese patients respond to drug therapy. An anti-obesity drug should therefore be prescribed for no longer than 12 weeks in the first instance and weight loss should then be measured. The drug treatment should be stopped in those obese patients who have not achieved a 5% weight reduction after 12 weeks of drug treatment. If a 5% weight loss is attained then the drug may be continued beyond this initial period, provided body weight is continually monitored and weight is not regained. Rapid weight regain is common after short-term use of anti-obesity drugs (12 weeks or less). Weight loss drugs should never be used without concomitant lifestyle modifications. Continual assessment of drug therapy for efficacy and safety is necessary. If the drug is efficacious in helping the patient to lose and/or maintain weight loss and there are no serious adverse effects, it can be continued. If not, it should be discontinued.
_
Anti-obesity medication or weight loss drugs are all pharmacological agents that reduce or control weight. These drugs alter one of the fundamental processes of the human body, weight regulation, by either altering appetite, or absorption of calories. The main treatment modalities for overweight and obese individuals remain dieting and physical exercise. Only one anti-obesity medications orlistat is currently approved by the FDA for long term use. It reduces intestinal fat absorption by inhibiting pancreatic lipase. As fat blockers like orlistat remove excess fats via the intestines, they may cause uncomfortable cramping, gas and diarrhea. Because these drugs also reduce the body’s absorption of essential vitamins and nutrients, people who take orlistat are advised to take a daily multivitamin supplement. The combination of phentermine and topiramate was approved by the U.S. FDA on July 17, 2012, as an obestity treatment complementary to a diet and exercise regimen. Phentermine suppresses appetite by causing a release of norepinephrine in the body. Phentermine alone is still available for treatment of obesity but only on a short-term basis (a few weeks). The common side effects of phentermine include headache, insomnia, irritability, and nervousness. Fenfluramine and dexfenfluramine suppress appetite mainly by increasing release of serotonin by the cells. Both fenfluramine and dexfenfluramine were withdrawn from the market in September 1997 because of association of these two medications with pulmonary hypertension (a rare but serious disease of the arteries in the lungs) and association of fen/phen with damage to the heart valves. Rimonabant a second drug, works via a specific blockade of the endocannabinoid system. It has been developed from the knowledge that cannabis smokers often experience hunger, which is often referred to as “the munchies”. It had been approved in Europe for the treatment of obesity but has not received approval in the United States or Canada due to safety concerns. The European Medicines Agency in October 2008 recommended the suspension of the sale of rimonabant as the risks seem to be greater than the benefits. Sibutramine which acts in the brain to inhibit deactivation of the neurotransmitters, thereby decreasing appetite was withdrawn from the United States and Canadian markets in October 2010 due to cardiovascular concerns. Lorcaserin was approved June 28, 2012 for obesity with other co-morbidities. Lorcaserin is a selective 5-hydroxytryptamine receptor 2c agonist developed as a weight-loss drug. The average weight loss by study participants was modest, but the most common side effects of the drug are considered benign. In people with Diabetes mellitus type 2, the drug metformin can reduce weight. Metformin limits the amount of glucose that is produced by the liver as well as increases muscle consumption of glucose.
_
Meta-analysis of drugs:
Author’s meta-analysis of one to four year randomized controlled trials of orlistat, sibutramine, and rimonabant in adults showed that each drug results in average placebo subtracted weight reductions of less than 5 kg. They found no data on the effect of these agents on mortality or cardiovascular morbidity. Weight maintenance studies for each drug reported similar amounts of weight regained in active and placebo arms, such that the original weight differential between groups was maintained. They found differing effects on secondary end points and adverse effect profiles. These updated results are consistent with the results of previous reviewsbut more precisely define the long term effects of current agents on weight and secondary end points and describe each drug’s unique adverse effect profile.
_
Contraindications of anti-obesity drugs:
New drugs:
The ideal anti-obesity drug would produce sustained weight loss with minimal side effects. The mechanisms that regulate energy balance have substantial built-in redundancy, overlap considerably with other physiological functions, and are influenced by social, hedonic and psychological factors that limit the effectiveness of pharmacological interventions. It is therefore unsurprising that anti-obesity drug discovery programs have been littered with false starts, failures in clinical development, and withdrawals due to adverse effects that were not fully appreciated at the time of launch. Drugs that target pathways in metabolic tissues, such as adipocytes, liver and skeletal muscle, have shown potential in preclinical studies but none has yet reached clinical development. Recent improvements in the understanding of peptidergic signaling of hunger and satiety from the gastrointestinal tract mediated by ghrelin, cholecystokinin (CCK), peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), and of homeostatic mechanisms related to leptin and its upstream pathways in the hypothalamus, have opened up new possibilities. Although some have now reached clinical development, it is uncertain whether they will meet the strict regulatory hurdles required for licensing of an anti-obesity drug. The large number of potential new therapies should not be surprising given a projected market size of US$3.7 billion for a safe and effective anti-obesity drug (Vickers and Cheetham, 2007). It is beyond the scope of this article to review all the potential therapies listed in the table below.
Rimonabant (Acomplia) is sometimes referred to as the “munchies drug,” because it acts on the brain in the opposite way of marijuana. Scientists developed the drug after noticing that people who smoked marijuana often experienced food cravings, or “the munchies.” These food cravings occur when substances in marijuana called cannabinoids act on cannabinoid receptors in the brain. Scientists figured out that by blocking those receptors, they could control food cravings. In a study of 1,500 obese or overweight people, participants who took 20 milligrams of rimonabant for a year and cut 600 calories out of their diet lost about 14 pounds, compared with only 4 pounds among people who took a placebo. The participants also lowered their cholesterol, triglycerides, and insulin resistance (a condition in which the body does not effectively take glucose, or sugar, into the cells).
_
British researchers are working on an anti-fat injection containing the hormone oxyntomodulin, which is naturally found in the small intestine and lets the brain know that the body is full. Researchers believe that injections of this hormone could help obese individuals eat less. In one small study, people who took oxyntomodulin lost about 4 ½ pounds more than people who took a placebo. Researchers say they will need to conduct more studies to confirm whether this treatment is effective. Although oxyntomodulin is sometimes called a “fat vaccine” in the media, it is not a true vaccine because it does not involve the immune system.
_
The German-based Noxxon Pharma is working on another drug called Spiegemler that, like the CYT009-GhrQb vaccine, targets ghrelin to battle obesity. But unlike the vaccine, Spiegelmer does not involve the immune system. Instead, it binds to and neutralizes ghrelin in the blood to control appetite. In a seven-day study, mice that were given the drug lost more weight than a control group.
_
AOD 9604: A new type of anti-obesity drug that mimics the effects of exercise by increasing the rate at which fat is metabolized. AOD 9604 is a synthetic form of part of human growth hormone. Researcher believes this part of the hormone is only responsible for controlling the rate of fat metabolism and nothing else, and therefore that the drug is unlikely to have bad side effects. The drug is currently undergoing human trials.
_
Natural gut hormones may provide a treatment for obesity:
Researchers from the Garvan Institute of Medical Research have shown that a hormone released naturally from the gut could be used to treat obesity and Type 2 diabetes. After a meal, the hormone peptide YY (PYY) is released into the blood from the gastrointestinal tract. PYY then acts on the brain, contributing to a feeling of satiety and inhibiting the desire to continue eating. These effects of PYY suggested that it could be used as a weight loss medication. The major unknown was whether or not the effects would last beyond a few weeks, one of the critical requirements of weight loss medications. Using mice genetically engineered to produce more PYY, Professor Herbert Herzog, Head of Garvan’s Neuroscience Program, and Dr Amanda Sainsbury-Salis, a senior scientist within the Neuroscience Program, showed that long-term increases in PYY can induce and maintain lower body fat levels in mice. The results were published in the international journal Neuropeptides. “At a time when people are considering radical treatments for obesity, including surgical intervention, we’re very pleased to have identified a more natural alternative,” said Dr Sainsbury-Salis. “If people respond to PYY in the same way as mice, supplements of the hormone should reduce body fat significantly over time.” “The other exciting thing about PYY is that it significantly improves a person’s ability to clear glucose, from the blood. It should therefore have the ability to prevent glucose intolerance, a known precursor of Type 2 diabetes. In addition to reducing body fat and improving glucose tolerance, the team showed that elevated PYY also increases thyroid function, which in turn increases body temperature and metabolic rate. So when a PYY-overproducing mouse is fed the same diet as a control mouse, it has less body fat.
_
Good gut bacteria could provide new treatment for obesity and diabetes:
•Study found that Akkermansia muciniphila bacteria helped burn fat and improve blood sugar levels
•Experts now hope that discovery will help pave way for new medical treatments for obesity and diabetes
_
Anti-obesity vaccine:
The “fat vaccine” is under investigation by Cytos, a Swiss biotechnology company. Currently called CYT009-GhrQb, the vaccine’s purpose is to create an immune response in the body against ghrelin, a peptide released by cells in the stomach. Scientists don’t know exactly how ghrelin works, but several studies have shown that it stimulates appetite. In one study of anorexic patients, people given ghrelin were hungrier and ate more than those given a placebo. Blood levels of ghrelin quickly rise after people lose weight, which may be why so many people have trouble keeping the weight off. Studies have also indicated that bariatric surgery works, in part, because ghrelin levels drop when the stomach has been reduced. The CYT009-GhrQb vaccine instructs the immune system to release antibodies that attach to ghrelin and hold it in the bloodstream. This keeps the peptide from making its way into the brain and triggering the feeling of hunger. The other vaccine works in a similar way, creating antibodies against a hormone called somatostatin. This hormone is created by the brain and digestive system and causes the metabolism to slow by interacting with other hormones. By creating antibodies against this hormone the metabolism speeds up, allowing the patient to eat more calories and still lose weight. It was reported that levels of other hormones remain unchanged for both treatments. The somatostatin treatment is only in the early stages of development, but tests on mice showed a 10 percent reduction in weight after just one shot. A further “booster” shot after three weeks helped to keep their weight in check. Although further studies are necessary to discover the long-term implications of these vaccines, treatment of human obesity with vaccination could provide physicians with a drug and a surgery-free option against the weight epidemic.
_____________
Bariatric surgery:
_
Bariatric surgery, or weight loss surgery, is a type of procedure performed on people who are dangerously obese, for the purpose of losing weight. This weight loss is usually achieved by reducing the size of the stomach with an implanted medical device (gastric banding) or through removal of a portion of the stomach (sleeve gastrectomy or biliopancreatic diversion with duodenal switch) or by resecting and re-routing the small intestines to a small stomach pouch (gastric bypass surgery). Long-term studies show the procedures cause significant long-term loss of weight, recovery from diabetes, improvement in cardiovascular risk factors, and a reduction in mortality of 23% to 40%.
_
_
Currently, there are basically two types of bariatric surgery:
Restrictive surgeries:
These surgeries restrict the size of the stomach and slow down digestion.
Malabsorptive/restrictive surgeries:
These surgeries restrict the size of the stomach but also bypass or remove part of your digestive system to decrease absorption of food/calories.
_
In general, gastric bypass and other weight-loss surgeries could be an option for you if:
- Your body mass index (BMI) is 40 or higher (extreme obesity).
- Your BMI is 35 to 39.9 (obesity), and you have a serious weight-related health problem, such as type 2 diabetes, high blood pressure or severe sleep apnea. In some cases, you may qualify for certain types of weight-loss surgery if your BMI is 30 to 34 and you have serious weight-related health problems.
But gastric bypass isn’t for everyone who is severely overweight. You may need to meet certain medical guidelines to qualify for weight-loss surgery. Patients should be referred to high-volume centers with surgeons experienced in bariatric surgery. The American Society for Metabolic & Bariatric Surgery says obesity surgery is safe and that the death rate is less than 1 percent, lower than for gallbladder and hip replacement surgery.
_
In addition to weight loss, gastric bypass surgery may improve or resolve conditions often related to being overweight, including:
- Gastroesophageal reflux disease
- Heart disease
- High blood pressure
- Severe sleep apnea
- Type 2 diabetes
- Stroke
Gastric bypass surgery can also improve your ability to perform routine daily activities, which could help improve your quality of life.
_
Longer term risks and complications of weight-loss surgery vary depending on the type of surgery. They can include:
- Bowel obstruction
- Dumping syndrome, causing diarrhea, nausea or vomiting
- Gallstones
- Hernias
- Low blood sugar (hypoglycemia)
- Malnutrition, In fact, an astounding 98% of bariatric surgery patients exhibit micronutrient deficiencies within two years of surgery.
- Stomach perforation
- Ulcers
- Vomiting
- Death (rare)
_
You may experience changes as your body reacts to the rapid weight loss in the first three to six months after gastric bypass or other weight-loss surgery, including:
- Body aches
- Feeling tired, as if you have the flu
- Feeling cold
- Dry skin
- Hair thinning and hair loss
- Mood changes
_
Bariatric surgery in children/adolescent:
Indication for bariatric surgery in adolescents and children could be considered in centers with extensive experience of such treatment in adults and who are able to offer a true multidisciplinary approach, which involves pediatric skills relating to surgery, dietetics and psychological management.
In adolescents with severe obesity, bariatric surgery can be considered if the patient
- Has a BMI >40 (or 99.5 percentile for respective age) and at least one comorbidity
- Has followed at least 6 months of organized weight reducing attempts in a specialized centre
- Shows skeletal and developmental maturity
- Is capable to commit to comprehensive medical and psychological evaluation before and after surgery.
- Is willing to participate in a postoperative multidisciplinary treatment program.
- Can access surgery in a unit with specialist pediatric support (nursing, anesthesia, psychology, postoperative care).
_
Contraindications specific for bariatric surgery
- Absence of a period of identifiable medical management
- Patient who is unable to participate in prolonged medical follow-up
- Non-stabilized psychotic disorders, severe depression and personality disorders, unless specifically advised by a psychiatrist experienced in obesity
- Alcohol abuse and/or drug dependencies
- Diseases threatening life in the short term
- Patients who are unable to care for themselves and have no long-term family or social support that will warrant such care
_
Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery:
Weight loss is associated with short-term amelioration and prevention of metabolic and cardiovascular risk, but whether these benefits persist over time is unknown. As reported in NEJM, As compared with conventional therapy, bariatric surgery appears to be a viable option for the treatment of severe obesity, resulting in long-term weight loss, improved lifestyle, and, except for hypercholesterolemia, amelioration in risk factors that were elevated at baseline.
_
Bariatric surgery is one of the most effective treatments for achieving long-term weight loss in morbidly obese patients. Bariatric surgery causes weight loss through substantial decline of hunger and increased satiety. Recently our understanding of neuroendocrine regulation of food intake and weight gain, especially regarding the role of gut hormones, has significantly increased. The changes in these hormones following bariatric surgery can partly explain the mechanism behind weight loss achieved through these procedures. The average weight loss in clinical practice after 2years for adjustable gastric banding (GB) is 20%, Roux-en-Y gastric bypass (RYGB) is 30% and biliopancreatic diversion or duodenal switch (BPD) is 35%. The exact mechanism involved in weight loss after the bariatric surgery is not yet clear. However, a number of studies have demonstrated that gut hormones may play a role in weight loss following these procedures. It appears that while the early beneficial effect of bariatric surgery on glycemic control may well be largely mediated by caloric restriction, the changes in gut hormones may well explain why patients with surgery are more successful in the long term in their ability to continue adhering to lower caloric intake and keeping the weight off. The hormone Ghrelin produced by stomach is reduced in bariatric surgery due to reduced stomach size that may be partly responsible for weight loss.
_
Gut hormones as mediators of appetite and weight loss after Roux-en-Y Gastric Bypass:
A study found that the attenuated appetite after gastric bypass is associated with elevated PYY and GLP-1 concentrations, and appetite returns when the release of gut hormones is inhibited. The results suggest a role for gut hormones in the mechanism of weight loss after gastric bypass and may have implications for the treatment of obesity.
__
Safety and Effectiveness of the Intragastric Balloon for Obesity: A Meta-Analysis:
The objective of a study is to determine the safety, efficacy, and effectiveness of the most widely used balloon, BioEnterics Intragastric Balloon (BIB), to treat obesity. Authors pooled 15 articles (3,608 patients) to estimate BIB effectiveness. The estimates for weight lost at balloon removal for BIB were the following: 14.7 kg, 12.2% of initial weight, 5.7 kg/m2, and 32.1% of excess weight. However, data were scant after balloon removal. Yet, efficacy at balloon removal was estimated with a meta-analysis of two randomized controlled trials (75 patients) that compared balloon versus placebo, indicating the balloon group lost more weight than the placebo group. These differences in weight lost were 6.7 kg, 1.5% of initial weight, 3.2 kg/m2, and 17.6% of excess weight. Regarding BIB safety, the majority of complications were mild and the early removal rate was 4.2%. The use of the BIB, within a multidisciplinary weight management program, is a short-term effective treatment to lose weight, but it is not yet possible to verify its capacity to maintain the weight lost over a long period of time.
_
Scientists try to explain how bariatric surgery helps reverse Type 2 diabetes
Scientists are unraveling the mystery of why weight-loss surgery such as gastric bypass is so effective at dropping pounds and reversing Type 2 diabetes. For years, experts assumed that weight-loss surgery worked so well because it reduced the size of the stomach and bypassed part of the intestine to limit calorie absorption. But that’s not the case. The problem with those mechanical explanations is that they’re wrong. The surgery works because it changes the nature of communication, or signaling, between the gut and the brain, pancreas and liver. After surgery, hormones in the gut signal the pancreas to secrete more insulin and reduce the amount of glucose made by the liver. Researchers revealed that a key part of that communication involves bile acids, which break down fat and play a role in fat absorption, but also act as hormones. Identifying the significance of bile acids as being responsible for inducing many of the benefits of surgery, is “a real step forward. Researchers genetically engineered the animals so that they wouldn’t have the key protein responsible for bile acids’ hormonal action. Then, they performed surgery. The result was that there was no weight loss and no improvement in glucose levels. This suggests the reason surgery works has less to do with mechanical changes to the digestive system, than it does with signaling from hormones in the gut.
_
Role of reduced BCAA due to bariatric surgery may improve diabetes:
Researchers at Duke University Medical Center and St. Luke’s and Roosevelt Hospital Center, Columbia University, have uncovered a new clue for why bariatric surgery is more effective than dietary remedies alone at controlling glucose levels. This discovery, and facts gleaned from their previous studies, provides even more evidence that branched-chain amino acids are biomarkers that deserve careful scrutiny in the development and treatment of diabetes. Physicians have observed that bariatric surgery results in improved blood sugar levels in up to 80 percent of cases, but the reason has not been entirely clear. Although considerable weight loss is part of that success, gastric bypass surgery (GBP) improves glycemic control in type 2 diabetes even before significant weight loss has occurred, which suggests alternate mechanisms related to biochemical and/or hormonal changes. The current study showed that obese people with Type 2 diabetes undergoing GBP surgery have much lower levels of circulating branched-chain amino acids (BCAA) and the aromatic amino acids phenylalanine (Phe) and tyrosine (Tyr), compared to a matched group of obese patients with diabetes who lost an equal amount of weight by following a diet. The results were published in Science Translational Medicine. This enhanced reduction in BCAA and aromatic amino acids Phe and Tyr was linked to better improvement in glycemic (blood sugar) control in the GBP group.
_
Changes in intestine after gastric bypass surgery cause improvement in diabetes:
Researchers at Boston Children’s Hospital, curious what organ or bodily process could account for the body’s sudden improved regulation of blood sugar, put rats that had undergone a gastric bypass procedure into a PET scanner that tracks where sugar is in the body. In the scanner, one part of the rat’s body glowed with activity: the small intestine. The intestine “uses so much energy after gastric bypass, because it has to work harder.” The researchers studied the biomolecular changes in the intestine after surgery to try and pick apart what precise alterations could account for its new role as a hotspot for regulating blood sugar. What they found surprised them, because it isn’t typically found in the intestines of adults: a molecule that acts as a glucose transporter and ferries sugar out of the blood and into cells. Understanding those changes in detail lays the groundwork for the search for effective diabetes therapies. Now, scientists can examine whether it is possible to use a drug or other intervention to trigger the body to generate that transporter in the intestine. Researchers say that the majority of the surgery’s effect on diabetes was due to the changes in the intestine, with about 36 percent of the effect accounted for by other changes such as weight loss. Work published earlier this year found that transplanting the gut microbes from a mouse that has received gastric bypass surgery into another mouse will help spur weight loss.
_
Bariatric Surgery to treat Diabetes in Non-morbidly Obese Patients:
Surgery offers better short-term metabolic outcomes than medical management, but cost, postoperative complications, and lack of long-term follow-up data support caution. Because gastric bypass and other forms of bariatric surgery lower risk for diabetes and cardiovascular-related mortality in morbidly obese patients, attention now has focused on nonmorbidly obese patients with diabetes. Results of a recent randomized trial and a systematic review suggest potential benefits, but many uncertainties remain. In a trial conducted in the U.S. and China, 120 adults (mean age, 49; mean body-mass index, 35 kg/m2) with uncontrolled diabetes were randomized to Roux-en-Y gastric bypass surgery or intensive medical management consisting of 30 diet and exercise counseling sessions during 12 months. Surgery patients received usual lifestyle-modification counseling and postoperative eating guidance. Most enrollees also were taking medications for hypertension and hyperlipidemia. At 12 months, 11 medical-management patients and 28 surgical patients had achieved the composite outcome of glycosylated hemoglobin (HbA1c) <7.0%, LDL cholesterol level <100 mg/dL, and systolic blood pressure <130 mm Hg. The medical-management group attained a mean weight loss of 8%, compared with 26% in the surgical group. Among surgical patients, 10 had serious early or late postoperative complications (e.g., anastomotic leak, stricture, small bowel obstruction). Other adverse events were similar in the two groups. In a systematic review, three randomized clinical trials of surgery versus medical management in 290 nonmorbidly obese patients with diabetes or metabolic syndrome favored various bariatric surgery procedures for weight loss and glycemic control after 1 to 2 years of follow-up. Bariatric surgery also was favored over intensive medical management in observational studies. No follow-up data beyond 5 years were available in any surgical study, whereas medical-management studies showed benefits after 10 or more years of follow-up. Surgery-related mortality ranged from 0.3% to 1.0%, and almost all surgical studies were from single centers. Comparing surgical and medical approaches to achieve weight loss and glycemic control and to lower cardiovascular risk in nonmorbidly obese patients is still in an early and uncertain phase. Short-term improvement in metabolic complications of obesity favors surgery, but the incidence of surgical complications is not trivial. Long-term follow-up data (for ≥10 years) are needed to understand the consequences of surgically altering intestinal function and nutrient absorption and to guide decision making. For many primary care physicians, recommending major surgery for clinical entities that traditionally have been treated medically is unsettling.
_
Gut bacteria and bariatric surgery:
Now, a study by Ling-Chun Kong and colleagues from Paris, France, in a paper published in the American Journal of Clinical Nutrition supports the notion that bariatric surgery may have additional effects on energy homeostasis by affecting the gut microbiome. The researchers profiled the gut microbiota from fecal samples and adipose tissue samples in severely obese individuals, before as well as three and six months after roux-en-Y gastric bypass surgery. In these patients, surgery resulted in a remarkable increase in richness of gut microbiota (previous studies have shown that gut microbiota phylogenetic richness is lower in obese than in lean subjects), whereby almost 40% of the increase in gut bacteria belonged to the phylum Proteobacteria. Most of these changes occurred early, with no further differences noted between the three and six month samples. The researchers also found significant associations between gut microbiota composition and adipose tissue gene expression (including numerous genes related to metabolism and inflammation) as well as clinical phenotype – as substantial proportion of which were independent of any changes in caloric intake. As to the cause or clinical implications of these changes, the authors can only speculate. As they point out, some of these changes may be due to alterations in diet composition, eating behavior (e.g. increased chewing), or biological changes that include differences in pH and other aspects of intestinal milieu. Although such “correlational” studies cannot prove cause and effect, this study does document profound changes in the gut bacteriome with gastric bypass surgery and given our understanding that gut bugs may very much influence energy metabolism, it will be certainly be of considerable interest to examine whether these changes contribute to the success of bariatric surgery.
_
Mom’s obesity surgery may help her children’s genes work differently making them lean:
Obese mothers tend to have kids who become obese. Now provocative research suggests weight-loss surgery may help break that unhealthy cycle in an unexpected way – by affecting how their children’s genes behave. In a first-of-a-kind study, Canadian researchers tested children born to obese women, plus their brothers and sisters who were conceived after the mother had obesity surgery. Youngsters born after mom lost lots of weight were slimmer than their siblings. They also had fewer risk factors for diabetes or heart disease later in life. More intriguing, the researchers discovered that numerous genes linked to obesity-related health problems worked differently in the younger siblings than in their older brothers and sisters. It’s not that mom passed on different genes, but how those genes operate in her child’s body. Researchers say factors inside the womb seem to affect the dimmer switches that develop on the genes of a fetus – chemical changes that make genes speed up or slow down or switch on and off. That in turn can greatly influence health. The findings are reported in the journal Proceedings of the National Academy of Sciences.
____________
Adipose tissue Lipolysis treats Obesity, Chronic Inflammation, Diabetes and offers weight-loss alternative to Liposuction, Gastric Bypass:
This minimally-invasive obesity treatment is an injection that inhibits immune response in a specific population of immune cells, triggering fat loss. Obesity provokes an inflammatory immune response, causing insulin resistance in adipose (i.e. fat) tissue. In addition to causing fat retention, this can lead to diabetes and other metabolic disorders. Researchers at Columbia decreased a specific population of immune cells with an injection. This prevented inflammation and subsequent insulin resistance, and it led to a higher rate of fat breakdown in tests on mice. This treatment carries far fewer inherent risks than liposuction or gastric bypass surgeries and would be much less expensive. Additionally, this technology could potentially treat diabetes and other obesity-associated metabolic diseases, such as hypertension, fatty liver disease, arthritis, some cancers, and Alzheimer’s disease.
____________
Liposuction: a cosmetic surgery:
Liposuction is a type of cosmetic surgery used to remove unwanted body fat. The operation is also known as liposculpture or suction-assisted lipectomy. Liposuction is carried out on areas of the body where deposits of fat tend to collect, such as the buttocks, hips, thighs and abdomen. Other popular areas for liposuction are under the chin, neck, upper arms, breasts, knees, calves or ankles. Liposuction permanently removes fat cells and can alter body shape. However, the remaining fat cells can grow, so weight loss is not necessarily permanent, especially if you eat an unhealthy diet and don’t exercise after the operation. Liposuction is not a treatment for obesity, and it will not remove cellulite or stretch marks. There is a limit to the amount of fat that can safely be removed, and the surgery carries a number of risks, such as infection, scarring and numbness.
_____________
Your doctor may send you to other health care specialists if you need expert care. These specialists may include:
- An endocrinologist if you need to be treated for type 2 diabetes or a hormone problem, such as an underactive thyroid.
- A registered dietitian or nutritionist to work with you on ways to change your eating habits.
- An exercise physiologist or trainer to figure out your level of fitness and show you how to do physical activities suitable for you.
- A bariatric surgeon if weight-loss surgery is an option for you.
- A psychiatrist, psychologist, or clinical social worker to help treat depression or stress.
_____________
Alternative treatment:
The Chinese herb ephedra (Ephedra sinica), combined with caffeine, exercise, and a low-fat diet in physician-supervised weight-loss programs, can cause at least a temporary increase in weight loss. Diuretic herbs, which increase urine production, can cause short-term weight loss but cannot help patients achieve lasting weight control. The body responds to heightened urine output by increasing thirst to replace lost fluids, and patients who use diuretics for an extended period of time eventually start retaining water again anyway. In moderate doses, psyllium, a mucilaginous herb available in bulk-forming laxatives like Metamucil, absorbs fluid and makes patients feel as if they have eaten enough. Red peppers and mustard help patients lose weight more quickly by accelerating the metabolic rate. They also make people more thirsty, so they crave water instead of food. Walnuts contain serotonin, the brain chemical that tells the body it has eaten enough. Dandelion (Taraxacum officinale) can raise metabolism and counter a desire for sugary foods. Acupressure and acupuncture can also suppress food cravings. Visualization and meditation can create and reinforce a positive self-image that enhances the patient’s determination to lose weight. By improving physical strength, mental concentration, and emotional serenity, yoga can provide the same benefits. Also, patients who play soft, slow music during meals often find that they eat less food but enjoy it more.
_
Garcinia Cambogia:
Garcinia Cambogia is a small pumpkin shaped fruit found in Southeast Asia and clinically proven to inhibit the absorption of fat and stop weight gain. It contains a very important active compound called Hydroxycitric Acid (HCA). The HCA from garcinia cambogia supplement aids in weight loss by doing two things: It helps to block fat and suppresses your appetite. These two actions work together to inhibit the absorption of fat and stop weight gain. A study published in the journal of Diabetes, Obesity and Metabolism demonstrated that in the right concentration, HCA causes significant weight loss, lowers food intake and body weight gain as well as tackling factors such as cholesterol, low-density lipoproteins, triglycerides and serum leptin levels. HCA blocks fat by inhibiting a key enzyme (Citrate lyase) that your body uses to turn glucose into fat. HCA stops the fat-making process and the production of LDL (bad cholesterol) and triglycerides decrease. HCA is a known appetite suppressant that reduces cravings and decreases the urge to consume calories. HCA may stimulate the production of serotonin levels in the brain, elevating mood and promoting well being.
_______________
Domestic laws in the fight against obesity:
Domestic laws can be used by a government to influence the availability and accessibility of food products. They can therefore be used to help to achieve public health nutrition objectives. Domestic laws can be used as a public health policy instrument to support NPAN by regulating the availability and conditions of sale of certain foods.
There are three such regulatory approaches:
1. Using pricing controls on foods such as imposing tariffs, providing for domestic subsidies or imposing or increasing domestic taxes on particular food commodities;
2. Placing restrictions on the supply of particular foods such as banning their import, prohibiting the domestic sale of specific foods or requiring certain composition standards; and
3. Mandating labeling requirements for foods sold in the domestic market, such as labels containing warning statements, nutrient claims and nutrition information panels.
____________
Obesity as Public health concern:
The World Health Organization (WHO) predicts that overweight and obesity may soon replace more traditional public health concerns such as undernutrition and infectious diseases as the most significant cause of poor health. Obesity is a public health and policy problem because of its prevalence, costs, and health effects. The United States Preventive Services Task Force recommends screening for all adults followed by behavioral interventions in those who are obese. Public health efforts seek to understand and correct the environmental factors responsible for the increasing prevalence of obesity in the population. Solutions look at changing the factors that cause excess food energy consumption and inhibit physical activity. Efforts include federally reimbursed meal programs in schools, limiting direct junk food marketing to children, and decreasing access to sugar-sweetened beverages in schools. When constructing urban environments, efforts have been made to increase access to parks and to develop pedestrian routes. Many countries and groups have published reports pertaining to obesity. In 1998 the first US Federal guidelines were published, titled “Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report”. In 2006 the Canadian Obesity Network published the “Canadian Clinical Practice Guidelines (CPG) on the Management and Prevention of Obesity in Adults and Children”. This is a comprehensive evidence-based guideline to address the management and prevention of overweight and obesity in adults and children. In 2004, the United Kingdom Royal College of Physicians, the Faculty of Public Health and the Royal College of Paediatrics and Child Health released the report “Storing up Problems”, which highlighted the growing problem of obesity in the UK. The same year, the House of Commons Health Select Committee published its “most comprehensive inquiry […] ever undertaken” into the impact of obesity on health and society in the UK and possible approaches to the problem. In 2006, the National Institute for Health and Clinical Excellence (NICE) issued a guideline on the diagnosis and management of obesity, as well as policy implications for non-healthcare organizations such as local councils. A 2007 report produced by Sir Derek Wanless for the King’s Fund warned that unless further action was taken, obesity had the capacity to cripple the National Health Service financially. Comprehensive approaches are being looked at to address the rising rates of obesity. The Obesity Policy Action (OPA) framework divides measure into ‘upstream’ policies, ‘midstream’ policies, ‘downstream’ policies. ‘Upstream’ policies look at changing society, ‘midstream’ policies try to alter individuals’ behavior to prevent obesity, and ‘downstream’ policies try to treat currently afflicted people. Governments, international partners, civil society, nongovernmental organizations and the private sector all have vital roles to play in contributing to obesity prevention. Curbing the global obesity epidemic requires a population-based multisectoral, multi-disciplinary, and culturally relevant approach.
___________
Obesity prevention:
Why prevent obesity?
The rationale behind obesity prevention is several folds. First, obesity develops over time and, once it has done so, is very difficult to treat. A number of analyses have identified the failure of obesity treatments (with possible exception of gastric surgery in the seriously obese) to achieve long-term success. Second, the health consequences associated with obesity result from the cumulative metabolic and physical stress of excess weight over a long period of time and may not be fully reversible by weight loss. Third, the proportion of the population that is either overweight or obese in many developed countries is now so large that there are no longer sufficient health care resources to offer treatment to all. It can be argued, therefore, that the prevention of weight gain (or the reversal of small gains) would be easier, less expensive, and potentially more effective than to treat obesity after it has fully developed.
_
Perhaps the strongest evidence for the preventability of obesity comes from the successful management of childhood obesity. A number of researchers have shown that effective management and support of overweight and obese children can significantly reduce the number of children who carry their weight problem into adulthood. The long term prevention of weight gain in these studies was achieved during the difficult transition periods of childhood and adolescence when weight gain can be a major problem. It is also interesting that in studies where children were treated together with their parents, the children were successful in reducing and maintaining their weight loss whilst over time the adults returned to their pre-study body weight.
_
How can Overweight and Obesity be prevented?
Following a healthy lifestyle can help you prevent overweight and obesity. Many lifestyle habits begin during childhood. Thus, parents and families should encourage their children to make healthy choices, such as following a healthy diet and being physically active. Make following a healthy lifestyle a family goal. For example:
- Follow a healthy eating plan. Make healthy food choices, keep your calorie needs and your family’s calorie needs in mind, and focus on the balance of energy in and energy out.
- Focus on portion size. Watch the portion sizes in fast food and other restaurants. The portions served often are enough for two or three people. Children’s portion sizes should be smaller than those for adults. Cutting back on portion size will help you balance energy in and energy out.
- Be active. Make personal and family time active. Find activities that everyone will enjoy. For example, go for a brisk walk, bike or rollerblade, or train together for a walk or run.
- Reduce screen time. Limit the use of TVs, computers, DVDs, and videogames because they limit time for physical activity. Health experts recommend 2 hours or less a day of screen time that’s not work- or homework-related.
- Keep track of your weight, body mass index, and waist circumference. Also, keep track of your children’s growth.
_
Primary Prevention:
Primary prevention means preventing the illness before it happens. In the case of obesity, primary prevention efforts include focusing on healthy lifestyle behaviors related to maintaining a normal weight. Practicing good nutrition and having regular physical activity are methods of preventing obesity. Policies that promote good nutrition and physical activity are other measures of primary prevention.
Secondary prevention:
In the case of obesity, secondary prevention means person is already overweight/obese but yet not developed complications like diabetes, hypertension, osteoarthritis, heart disease etc. In the secondary prevention of obesity, simply eating more fruit and vegetables or walking more steps will not be enough. It is likely going to take far more drastic changes to your diet and to your activity levels to halt progression or reverse your condition. Effective weight management is neither easy nor simple. Now that you already have the problem, you will need special attention, special dedication, and perhaps even special treatments to stop gaining more weight and hopefully lose some of that excess weight and keep it off. To some readers, secondary prevention may sound much more like treatment than prevention – this is because secondary prevention is in fact far closer to treatment than prevention. Indeed, moving to secondary prevention requires a drastic rethinking in how we address the overweight and obesity epidemic at a population level. The question no longer is how to help thin people stay thin. The question now is, how to help overweight and obese people not gain any more weight and perhaps receive treatments that will help them lose some of that excess weight and keep it off
Tertiary prevention:
Tertiary prevention means person is already obese, has co-morbidities like diabetes, hypertension etc and you want to prevent further weight gain and prevent more complications. Basically it amounts to treatment of obesity and its complications. The word prevention here is rather misnomer.
_____________
Are there any advantages of obesity?
1) Certain types of work are only catered to the obese:
There are jobs where an obese individual is suited for more than a normal weight person or someone who’s just overweight. An example of this could be an acting job. Say there’s a story with a character who is obese playing a big part. The only person who’ll probably fill the role of that character is… someone who’s obese.
2) Stronger Bones/Muscles:
This can be considered as one of the advantages of obesity. The reason being that any attempt at exercising, even a casual walk can contribute to this. Certain gym activities such as lifting weights also help with obese people having strong bones and muscles.
3) More warmth is possible in winter:
This is due to the excessive body fat especially in subcutaneous area prevent heat loss from obese person’s body into the air in cold environments by insulating the core of body. Also, fat has a tendency to keep some blood from getting closest to the skin where it can lose heat rapidly.
4) Size… does it matter?
This seems to be more of a feature than one of the advantages of obesity but there are some people who are proud of their hugeness and put it to some use. That makes it an advantage for them.
___________
Some Airlines make obese passengers buy Two Seats:
You’re paying more to travel, and not just for your plane ticket: Every pound counts as the number of carriers charging for all checked luggage racks up. So it stands to reason that the public might be wondering why the airlines don’t charge passengers with significant overweight. Southwest calls them “customers of size.” Medical professionals would use the term “obese.”
_
Questioning safety of heavy passengers on Planes:
More than six decades ago, when the federal standards on the strength of airplane seats and seat belts were written, government regulations specified that seats be designed for a passenger weight of 170 pounds. But now the average American man weighs nearly 194 pounds and the average woman 165. Now, some engineers and scientists have raised questions about whether airplane seats, tested with crash dummies that reflect the 170-pound rule, are strong enough to protect heavy travelers. “If a heavier person completely fills a seat, the seat is not likely to behave as intended during a crash,” said Robert Salzar, the principal scientist at the Center for Applied Biomechanics at the University of Virginia. “The energy absorption that is built into the aircraft seat is likely to be overwhelmed and the occupants will not be protected optimally.” Nor would the injury necessarily be confined to that passenger, Dr. Salzar said. If seats collapse or belts fail, he said, those seated nearby could be endangered from “the unrestrained motion of the passenger.” Yoshihiro Ozawa, an engineer whose company, Jasti Ltd. in Japan, has been making crash dummies for 20 years, raised similar concerns. He said he worried that there was no data proving that “seats and seat belts are safe enough” for larger passengers. “If we don’t test with heavier dummies, we won’t know if it is safe enough,” Mr. Ozawa said. “There is no regulation that says they have to test for heavier.”
____________
Obesity myths:
Many beliefs about obesity persist in the absence of supporting scientific evidence (presumptions); some persist despite contradicting evidence (myths). The promulgation of unsupported beliefs may yield poorly informed policy decisions, inaccurate clinical and public health recommendations, and an unproductive allocation of research resources and may divert attention away from useful, evidence-based facts.
_
Myth: Small sustained changes in energy intake or expenditure produce large, long-term weight changes.
Fact: According to researchers, predictions suggesting that large changes in weight will accumulate indefinitely in response to small sustained lifestyle modifications rely on the half-century-old 3500-kcal rule, which equates a weight alteration of 0.45 kg (1 lb) to a 3500-kcal cumulative deficit or increment [1 kg weight loss equal to deficit of 7200 Kcal]. However, the original model was derived from short-term experiments predominantly performed in men on very-low-calorie diets. Recent studies have shown that individual variability affects changes in body composition in response to changes in energy intake and expenditure, with analyses predicting substantially smaller changes in weight (often by an order of magnitude across extended periods). For example, whereas the 3500-kcal rule predicts that a person who increases daily energy expenditure by 100 kcal by walking 1.6 km (1 mile) per day will lose 22.7 kg (>50 lb) over a period of 5 years, the true weight loss is only about 4.5 kg (10 lb), assuming no compensatory increase in caloric intake. Taking small steps to reduce calories is fine, but it doesn’t have the same effect over time. As you lose more weight, you need to exercise more and eat fewer calories to keep the weight off. However, making big changes or many changes all at once can be overwhelming.
_
Myth: Large, rapid weight loss is associated with poorer long-term weight-loss outcomes, as compared with slow, gradual weight loss.
Fact: A review of weight-loss trials found that rapid weight loss using very-low-calorie diets (less than 800 calories) led to more weight loss than low-calorie diets (800-1200 calories) after 6 months, and there was no significant difference in weight loss between the very-low-calorie and the low- calorie diets in long-term studies that followed up dieters for at least a year. Diets that are fewer than 800 calories are restrictive and hard to stick to over time. Studies that follow up at 2 years would be a better indication of long-term results.
_
Myth: All calories are created equal.
Fact: You’ve heard “a calorie is a calorie,” meaning your body processes them all the same way regardless of where they come from. But not so fast: 100 calories of chocolate cake are not the same as 100 calories of carrots. As it turns out, your body burns nearly 50% more calories after eating a meal packed with whole foods versus an equivalent meal made of processed fare, according to a 2010 study published in the health journal Food & Nutrition Research. During manufacturing, processed foods are broken down and stripped of many nutrients, making it easier for the body to digest them. On the other hand, whole foods, such as multigrain bread, apples or zucchini, contain good-for-you nutrients like fiber that the body has to work overtime to break down, temporarily boosting metabolism. Plus, eating smarter calories via foods packed with filling fiber or satisfying protein, like a chicken breast instead of potato chips, will help you naturally eat less over time.
_
Myth: It is important to set realistic goals for weight loss.
Fact: Empirical data indicate no consistent negative association between ambitious goals and program completion or weight loss. Indeed, several studies have shown that more ambitious goals are associated with better weight-loss outcomes. Furthermore, two studies showed that interventions designed to improve weight-loss outcomes by altering unrealistic goals resulted in more realistic weight-loss expectations but did not improve outcomes.
_
Myth: Physical-education classes, in their current form, play an important role in reducing or preventing childhood obesity.
Fact: Physical education, as typically provided, has not been shown to reduce or prevent obesity. Findings in three studies that focused on expanded time in physical education indicated an increase in the number of days children attended physical-education classes, but the effects on body-mass index (BMI) were inconsistent across sexes and age groups. Two meta-analyses showed that even specialized school-based programs that promoted physical activity were ineffective in reducing BMI or the incidence of obesity. There is almost certainly a level of physical activity (a specific combination of frequency, intensity, and duration) that would be effective in reducing or preventing obesity. Whether that level is plausibly achievable in conventional school settings is unknown, although the dose-response relationship between physical activity and weight warrants investigation in clinical trials.
_
Myth: Breast-feeding is protective against development of obesity.
Fact: Breastfeeding as a protective factor against weight gain has been examined in at least 20 studies involving nearly 40 000 subjects. Five studies (including the two largest) found a protective effect, two found that breastfeeding predicted obesity, and the remainder found no relationships. There are probably multiple effects of confounding in these studies; however, the reduction in the risk of developing obesity observed in the two largest studies was substantial (20-37%). Promoting breastfeeding has many benefits, the prevention of childhood obesity probably being one of them.
_
Myth: Sexual activity is a good form of exercise. A bout of sexual activity burns 100 to 300 Kcal of energy for each partner.
Fact: It may be intense, but it’s not long-lasting enough to be much better than watching television, calorically speaking. A man having intense sexual activity and who weighs 154 lb would burn about 3.5 Kcal per minute. Since this activity lasts about 6 minutes on average, totaling 21 Kcal, and about 7 Kcal are burned just lying down watching TV, the grand total of calories burned during sex is about 14 Kcal. Please do not be disappointed by this calculation. Apart from burning calories, sex reduces stress, helps you sleep better; raises your heart rate, improves blood flow, tones your muscles, changes more of your bad cholesterol (LDL) to good cholesterol (HDL), rejuvenates your mind and body. The increased activity helps women to produce more estrogen, which make them feel attractive, beautiful and sexy. Also it certainly makes you feel happier, rested and contented. And not to forget the bonding and intimacy that develops between partners.
_
Myth: Celery, cucumbers and iceberg lettuce have negative calories.
Fact: The concept goes something like this: some veggies are so low in calories that they require more energy to digest than they contain. The result? Eating celery, cucumbers or iceberg lettuce can give you a “negative calorie balance.” Sounds great in theory, but the calories you need for digestion won’t ever exceed the number of calories any type of food contains. A stalk of celery provides 6 calories to the body, but the body expends only half of a single calorie digesting it. However, these non-starchy, low-calorie veggies can still help you lose weight since their fiber and water content will keep you feeling full for longer. So go ahead and pile them on generously when you hit the salad bar for lunch.
_
Note: Water is the only beverage that could be called a “negative calorie” beverage: Cold water will expend a greater number of calories because the body has to warm the liquid to body temperature, although a single glass of ice water at 0°C would only burn 8.8kcal. Drinking one such glass a day, it would take a person over a year to lose a single pound of weight.
_
Myth: Eating more fruits and vegetables will result in weight loss or less weight gain, regardless of whether any other changes to one’s behavior or environment are made.
Fact: Eating fruits and vegetables is healthy, but it needs to be accompanied by eating fewer calories from other foods so it doesn’t lead to weight gain. Fruits and vegetables are nutritious and contain fiber and water, which can help fill you up. With few exceptions, they are low in fat and calories. If these foods are part of your meals and replace higher-calorie snacks, they can help with weight control.
_
Myth: Snacking contributes to weight gain and obesity.
Fact: Evidence does not consistently show that snacking leads to weight gain or obesity. It depends on the snack. High-calorie, high-fat and high-sugar snacks eaten frequently and in large amount can contribute to weight gain. Healthy snacks such as fruit, veggies, low-fat yogurt, and a handful of nuts are reasonable snacks to eat as part of a weight- loss plan.
_
Myth: Skipping a meal in a day could help you lose weight and subsequently improve your health.
Fact: This is as far from the truth as it can get. Skipping meals will slow down the metabolism rate of your body and this would mean that the body will begin reserving its energy stores so that it has enough food to make do with, when it comes to daily functions. If you do end up skipping meals, it will put you at the risk of binge eating when you do finally get down to having a decent meal. Instead of skipping meal, eat less at meal time.
_
Myth: Doing cardio on an empty stomach burns more total fat for the day.
Fact: It sounds like it makes sense: Your body needs energy for a morning run, so not eating beforehand forces your body to dip into its fat stores for fuel, allowing you to burn more fat. Exercise physiologist Brad Schoenfeld, CSCS, author of Women’s Home Workout Bible, spent years researching the theory, hoping to confirm it as fact. Instead, he found that while you do burn more calories from fat if you exercise sans snack, ultimately it doesn’t matter because, as he notes in the Strength and Conditioning Journal, “if you burn more fat during a workout, your body physiologically adjusts to burn less fat post-exercise—and vice versa. So it all evens out.” Sports nutritionist Cassie Dimmick, RD, adds, to eat or not to eat before a workout is a personal preference, but “most experts advocate pre-gym noshing because it provides the fuel you need to exercise longer & harder and therefore burn more calories.” She recommends opting for a filling, nutrient-rich snack, such as a piece of fruit, applesauce or a slice of whole wheat toast with peanut butter. Remember, refined carbs boost insulin levels, hampering fat-burning during the workout. So avoid refined carbs altogether before exercise.
_
Myth: Always work out in the fat burning zone.
Fact: Why work harder, when you can take it easy and burn more fat, right? This is the reason why the fat burning zone myth is so attractive. The truth is at best, the fat burning zone is very misleading, and at worst, it’s complete misinformation. Fat burning zone of exercise means exercise burns predominantly fat and not carb or protein. It’s easy to say that a 30-minute jog burned 300 calories, but it doesn’t tell us if we burned 300 calories from the breakdown of fats, carbs, or muscle. We could have easily burned 300 calories from the breakdown of carbohydrates or worse — we could have burned 300 calories from lean muscle tissue (protein), which is the last thing we want to do. The “fat burning zone” function on cardio machines keeps you working out at a slow, steady pace—around 60% to 70% of your maximum heart rate—and this low-intensity form of exercise is thought to help your body burn a higher percentage of calories from fat. (So if you burn 100 total calories, 60 of those may come from fat and 40 from carbohydrates in your body.) The problem? The total number of calories burned is the only thing that matters—not what type of calories—and working out at a low intensity ultimately burns fewer calories since you’re not pushing yourself as hard as you should be. In order to maximize calorie burn (and, ultimately, fat loss) in less time, do high intensity interval training instead. To try it, alternate one or two minutes of easy running (or pedaling) with a quick one-minute burst of speed (you should be breathing heavily at the end of the interval.) Repeat intervals for a total of 20 minutes, and do two to three interval workouts per week for the best results. Bonus: Studies show intense workout sessions stoke metabolism for up to 24 hours after you’ve left the gym, burning at least 100 extra calories throughout the day.
_
Note: A food calorie means Kcal (kilocalorie). There is a lot of confusion when the word calorie is used in nutrition, obesity and exercise. I request all researchers and authors to use word Kcal while discussing energy gain or loss in their discussions.
_
Myth: To lose weight, you should only focus on cardio.
Fact: When it comes to dropping pounds, the first thing many of us think about is sweating it out by running or cycling. However, strength training also has an effect on helping you lose weight similar to cardio albeit less efficiently. There’s a reason that if you go into a gym, you’ll see lean people lifting weights. Building lean muscle raises your metabolism, allowing you to burn more calories when you’re doing anything, whether that’s running or just sitting at your desk. But that doesn’t mean that you should abandon your cardio routine. Cardio workouts keep your heart-health in check and burn lots of calories in little time, so just keep in mind that a routine that mixes cardio and one or two strength workouts a week is the best way to maximize results.
_
Myth: Exercise done at a low intensity, such as walking, is better at fat burning than other high-intensity activities, like running or cardio activities where you push yourself very hard.
Fact: In a strict scientific sense, these claims are true because working at a lower intensity requires less quick energy and a higher percentage of fat is burned. But you’ll also burn fewer calories than you would if, for the same amount of time, you work out at a harder intensity (running versus walking). If you’re trying to lose weight, even though a higher percentage of fat is being used, a lower total amount of fat is lost. Whether increased fat burning will result in actual weight loss is dependent upon several variables, including the total calories burned (which include both fat and carbohydrate calories) and the total fat calories burned. If you do work at a low intensity, you need to increase the time spent exercising to burn more calories. What matters most is the total number of calories burned. If you burned 250 calories every day from a short, fast jog, you’d see a bigger difference in weight and fat loss than if you walked every day for the same amount of time. The number of fat calories you burn isn’t that important, because even if you burn a lot of carb calories, these need to be replaced both by the carbs you eat in your diet and also within your body. Your fat stores will be broken down and transformed into carbohydrates when you need fuel. Even if you’re burning lots of carb calories and less fat calories through exercise, your fat still inevitably gets used. See the table below:
| 30 Minutes of Exercise | Fat Calories Burned | Glycogen Calories Burned | Total Calories Burned |
| Low Intensity Group | 120 | 80 | 200 |
| High Intensity Group | 140 | 260 | 400 |
High intensity exercise burned 260 glycogen calories which needs to be replaced by dietary glucose or form fat store, either way it will help reduce fat.
_
Myth: Eating six small meals a day boosts your metabolism.
Fact: While most of us were raised with the notion that we should eat three square meals a day, many people now believe that it’s better to eat smaller portions more frequently in order to help keep your metabolism stoked all day. But does grazing on six mini meals really burn more calories? While conflicting evidence does exist, a 2009 study published in the British Journal of Nutrition found no differences in weight loss among dieters who ate three or six times a day (total daily calories was the same for both groups). And, after reviewing 18 studies on the topic, the International Society of Sports Nutrition concluded that meal frequency doesn’t boost metabolism or encourage weight loss. However, researchers did note that eating frequently may help keep between-meal hunger at bay. Bottom line? Settle on an eating plan that keeps you satisfied and full so you’re less likely to binge due to hunger.
_
Myth: Working out in cold weather burns more calories.
Fact: There is some truth in it because shivering from cold temperatures revs up calorie burn, you will torch more as your body works to heat itself up. However, the difference is negligible at best. Trying to shiver away calories is not a smart or effective strategy. So when the mercury plummets, be smart and bundle up—the miniscule bump in calorie burn isn’t worth increasing your risk of frostbite or hypothermia.
_
Myth: You have to burn 250 calories every time you work out in order to lose weight.
Fact: To lose a pound a week, you have to cut 500 calories a day, and some health experts suggest achieving that by eating 250 fewer calories while burning 250 more daily. However, losing weight isn’t about what you burn day-to-day, but rather what you do over the course of a week—or even a month—allowing you the flexibility to make up for days when your diet gets derailed. That means if you’re not feeling well one day and skips a workout; it won’t make a big difference in the long run. The next day, just stay at the gym 10 minutes longer or try a higher-intensity yoga class. As long as you’re burning more calories in the long term, you’ll lose weight.
_
Myth: Spot exercise (that is, exercising a specific muscle or location of the body) most effectively burns fat at the desired location.
Fact: Spot exercise is beneficial for building specific muscles, but it has little effect, if any, on fat in that area of the body, or on the body’s distribution of body fat. The same logic applies to sit-ups and belly fat. Sit-ups, crunches and other abdominal exercises are useful in building the abdominal muscles, but they have little effect, if any, on the adipose tissue located there. The appearance of a more muscular region may give the impression of reduced fat, when this is not the case. So if you have a pot belly, low calorie diet with regular general exercise will help rather than spot exercise at the abdominal region.
_
Allison and colleagues wondered why the widespread acceptance of obesity myths and presumptions is so common. The authors pointed out several factors that seem to play a part in this phenomenon.
- One is what experts refer to as the “mere exposure effect” – repeating an idea frequently enough that people start to believe it is true.
- Another factor is that people find some ideas so desirable that they do not want to let them go, despite evidence against them.
- And the other is “confirmation bias” – when one seeks out information only to confirm an opinion that is already held.
__________
Obesity studies and research: science or pseudoscience:
As you read this article, you will find many contradictory studies, many contradictory claims and of course, the great obesity survival paradox. Obesity studies and research is marred with mixture of science and pseudoscience. On one hand it is recommended that to lose one pound weight, you must have 3500 Kcal deficit and on other hand individual variability affects changes in body composition in response to changes in energy intake and expenditure resulting in lesser weight loss despite 3500 Kcal deficit. On one hand high carb low fat diet recommended for weight loss and on the other hand similar diet promote weight gain. On one hand fructose is proclaimed as sole offender of obesity epidemic in America and on the other hand, experts advise to eat fruits (contain fructose) to reduce weight. Then experts differentiate fructose in fruits as natural fiber-bound (low GI) which makes it less toxic than fructose of HFCS in junk food. On one hand exercise is recommended to promote weight loss and on the other hand obesity studies in children show no benefit of exercise in promoting weight loss. I am certain that all the variables in the genesis of obesity are not known and also we do not exactly know how the known variable affect the weight.
_
Energy Balance in the Development of Obesity: conventional theory, alternative theory and my novel adipostat theory:
_
Conventional theory:
Obesity = increased food intake + reduced physical activities (i.e. positive energy balance)
_
_
Obesity can result from a minor energy imbalance, which lead to a gradual but persistent weight gain over a considerable period. Some researchers have hypothesized that energy imbalance is the result of inherited metabolic characteristics; whereas others believe it is caused by poor eating and lifestyle habits, that is “gluttony and sloth”. Positive energy balance occurs when energy intake is greater than energy expenditure and promotes weight gain. Conversely, negative energy balance promotes decrease in body fat stores and weight loss. Body weight is regulated by a series of physiological processes, which have the capacity to maintain weight within a relatively narrow range (stable weight). It is thought that the body exerts a stronger defense against undernutrition and weight loss than it does against over-consumption and weight gain. Figure above suggests that positive energy balance and weight gains are influenced by powerful societal and environmental forces which may overwhelm the physiological regulatory mechanisms that operate to keep weight stable. These include increasing automation, lack of recreational facilities and opportunities, increase in food variety and availability. Moreover, the susceptibility of individuals to these influences is affected by genetic and other biological factors such as sex, age and hormonal activities, over which they have little or no control (WHO 1998). Dietary intake and physical activity are important contributing factors in the development of obesity. If calorie intake is in excess of requirement it will be stored mainly as body fat. If the stored body fat is not utilized over time, it will lead to overweight or obesity. Inter-individual variations in energy intake, basal metabolic rate, spontaneous physical activity, the relative rates of carbohydrate-to-fat oxidation, and the degree of insulin sensitivity seem to be closely involved in energy balance and in determining body weight in some individuals (Ravussin 1993).
_
Excess calories + lack of exercise = obesity. Right? May be wrong. Read alternative theory of obesity below:
_
Alternative theory (insulin involvement causing obesity):
An article published recently in the BMJ argues that we have been pursuing the wrong hypothesis on the causes of obesity. Along with substandard science, this wrongheadedness has apparently exacerbated the obesity crisis. Author Gary Taubes asserts that obesity is probably not caused by a positive energy balance (more energy is consumed than spent). A promising rival hypothesis has been forgotten without having been properly investigated. According to that hypothesis, obesity is a hormonal, regulatory disorder. Energy imbalance is only a consequence of that underlying hormonal factor. The problem is not that we’re eating too much, it’s what we’re eating. And the probable culprit is carbohydrates. But this is yet to be definitively proven. This alternative hypothesis of obesity constitutes three distinct propositions. First, is the basic proposition that obesity is caused by a regulatory defect in fat metabolism, and so a defect in the distribution of energy rather than an imbalance of energy intake and expenditure. The second is that insulin plays a primary role in this fattening process, and the compensatory behaviors of hunger and lethargy. The third is that carbohydrates, and particularly refined carbohydrates– and perhaps the fructose content as well, and thus perhaps the amount of sugars consumed– are the prime suspects in the chronic elevation of insulin; hence, they are the ultimate cause of common obesity.
_
Both conventional theory and alternative theory are challenged in the following arguments:
_
A.) Leptin signaling, and the circuits in the brain regulate body fat mass. According to literally thousands of publications spanning nearly two centuries, the brain is the only organ that is known to regulate body fat mass in humans and other animals– neither fat tissue itself, nor the insulin-secreting pancreas have the ability to regulate body fat mass as far as we currently know. Leptin is the system that Drs. Jules Hirsch and Rudy Leibel have shown in carefully controlled human studies is responsible for the metabolic defect.
B.) Insulin has many functions throughout the body. The primary role of insulin is to manage circulating concentrations of nutrients (principally glucose, amino acids, and fatty acids, the body’s three main fuels), keeping them within an optimal range, and coordinating the shift between metabolic fuels that is required when a person consumes more of one or the other. Any time insulin suppresses fat burning, it increases carbohydrate and/or protein burning by an equivalent amount. The reason insulin suppresses fat burning is because it’s a signal of glucose abundance. It’s telling tissues to stop burning fat because carbohydrate is the available fuel. If you eat a meal of 500 calories of carbohydrate, you will burn that carbohydrate under the direction of insulin, which will also make sure body fat mostly stays inside your fat cells during the process. If you eat a meal of 500 calories of fat, you will burn fat instead of carbohydrate, but since you just ate fat, you aren’t dipping into your body fat stores any more than you were when you ate carbohydrate. So even though insulin temporarily suppresses fat burning and the release of fat from fat cells when you eat carbohydrate, at the end of the day if you ate the same number of calories you end up with the same amount of fat in your fat cells either way. Let’s look at the effect of insulin on food intake. To keep it as realistic as possible, let’s compare satiety and subsequent food intake among foods that raise insulin to varying degrees. If calories and protein are kept the same, high-carbohydrate meals cause equal or greater satiety than high-fat meals, and equal or less subsequent food intake, despite a much larger insulin response. Due to the insulin-stimulating effect of protein, low-carbohydrate high-protein meals can sometimes stimulate insulin to an equal or greater degree than high-carbohydrate meals, yet even in these cases higher insulin release is associated with increased satiety. Experiments in which investigators feed volunteers protein foods that stimulate insulin to different degrees show that the amount of satiety is positively correlated with the degree of insulin release, which is not consistent with the idea that insulin stimulates food intake. In the long term, low-carbohydrate diets suppress appetite in many overweight/obese people; however this is unlikely to be related to insulin.
_
If elevated insulin leads to increased fat storage and increased food intake, then experimentally elevating insulin in animals should replicate this (since insulin acts on fat cells in the same manner in humans and non-human mammals). However, this is not observed. Insulin injections at a dose that does not cause frank hypoglycemia do not increase food intake, and in some cases they even reduce it. Chronically increasing circulating insulin without causing hypoglycemia reduces food intake and body weight in non-diabetic animals, without causing illness, contrary to what this idea would predict. If anything, insulin constrains food intake and body fatness, and research indicates that this action occurs via the brain. Insulin infused into the brains of baboons causes a suppression of appetite and fat loss, which is consistent with the fact that insulin and leptin have overlapping functions in the brain. Knocking out insulin receptors in the brain leads to increased fat mass in rodents, suggesting that its normal function involves constraining fat mass. Insulin is also co-secreted with amylin, which suppresses food intake and body weight. This is why insulin is viewed by some obesity researchers as an anti-obesity hormone. Now let’s look at energy expenditure. If insulin is increasing fat accumulation due to a decrease in energy expenditure (presumably because elevated insulin is locking fat away inside fat cells), then people with higher fasting insulin should have lower resting energy expenditure. Lucky for us, that hypothesis has been tested. At least two studies have shown that higher fasting insulin is associated with a higher resting energy expenditure, independent of body fatness, not a lower expenditure. If anything, this is the opposite of what the hypothesis would predict. How about post-meal insulin spikes due to eating carbohydrate? A number of studies have consistently shown that under isocaloric controlled conditions, substantially different carbohydrate:fat ratios do not influence energy expenditure in any measurable way, even over long periods of time. Therefore, if insulin doesn’t increase energy intake (if anything, the combination of insulin and amylin that the pancreas releases in response to carbohydrate decreases it), and doesn’t decrease energy expenditure, then how exactly is it supposed to cause energy accumulation in the body as fat? There is no energy fairy. Obese people are obese despite having higher fasting insulin, not because of it. The fact is, insulin spikes after meals temporarily decrease fat release from fat cells, but if you look at total 24 hour energy balance, insulin spikes, in conjunction with all the other hormones that are released in response to food ingestion, do not cause fat accumulation. This is exactly how you would expect the system to work if it were designed to constructively handle a wide variety of macronutrient ratios, which it is. Just as cholesterol did not evolve to give us heart attacks, insulin did not evolve to make us fat.
_
Now let’s address the common sense arguments that are used to support the insulin hypothesis of obesity. These include:
1. Type I diabetics, who don’t produce enough insulin, lose fat.
2. Animals lacking insulin receptors on fat cells are resistant to fat gain.
3. Insulin therapy often causes fat gain in diabetics.
4. Repeated insulin injection into the same site causes fat accumulation at that site (lipoma).
5. Two drugs that suppress insulin secretion, diazoxide and octreotide, sometimes cause weight loss in controlled trials.
These observations are all accurate, and at a glance, they seem to support the hypothesis. Manipulating insulin signaling can change fat mass, and obese people have higher insulin, so it must be involved in obesity, right? Unfortunately these arguments fall apart upon closer scrutiny, not because they’re based on inaccurate observations, but because they’re irrelevant to common obesity. In obesity as in most other conditions where insulin is high, elevated insulin is a symptom of insulin resistance, and the two occur in parallel. The pancreas secretes more insulin because the tissues can’t “hear” it as well, so they need more of it to do the job. The more insulin resistance, the more insulin. The key point here is that elevated insulin in obesity is a compensatory response to insulin resistance, i.e. a reduced insulin signal. The cells are not seeing more insulin signaling, because they’re insulin resistant, so it makes no sense to invoke increased insulin action to explain common obesity. Arguments 1-5 listed above are cases where insulin levels and/or insulin sensitivity are changing independently of one another, either through a pathological process (islet autoimmunity), genetic manipulation (fat cell insulin receptor knockout), or through drugs. This is why they’re irrelevant to common obesity, where insulin levels and insulin resistance rise in parallel, such that total insulin action is either maintained or diminished. If we want to do an experiment that’s actually relevant to the question, we can use animal models that are genetically manipulated to maintain insulin sensitivity in response to fattening diets, which as expected eliminates the increase in insulin that is typically observed on these diets. These experiments show that fat mass accumulation does not consistently differ between animals that experience an increase in insulin, and those that don’t– they all get fat at approximately the same rate. Also both diazoxide and octreotide (argument #5) are extremely nonspecific drugs that have actions in the hypothalamus (brain) that would be expected to influence fat mass, so we actually have no idea if they act by reducing circulating insulin levels or through some other mechanism. The idea of fat gain in insulin-treated diabetics (argument #3) is not as airtight as it might at first seem. On average, diabetics do gain fat when they initiate insulin therapy using short-acting insulins. This is partially because insulin keeps them from peeing out glucose (glycosuria) to the tune of a couple hundred calories a day. It’s also because there isn’t enough insulin around to restrain the release of fat from fat cells (lipolysis), which is one of insulin’s jobs, as described above. When you correct this insulin deficiency (absolute or relative), obviously a diabetic person will typically gain weight. In addition, short-acting insulins are hard to control, and often create episodes where glucose drops too low (hypoglycemia), which is a potent trigger for food intake and fat gain. So what happens when you administer insulin to less severe diabetics that don’t have much glycosuria, and you use a type of insulin that is more stable in the bloodstream and so causes fewer hypoglycemic episodes? This was recently addressed by the massive ORIGIN trial. Investigators randomized 12,537 diabetic or pre-diabetic people to insulin therapy or treatment as usual, and followed them for 6 years. The insulin group received insulin glargine, a form of long-acting “basal” insulin that elevates baseline insulin throughout the day and night. In this study, insulin treatment brought fasting glucose from 125 to 93 mg/dL on average, so it was clearly a high enough dosage to have meaningful biological effects. After 6 years of divergent insulin levels, the difference in body weight was only 4.6 lbs (2.1 kg), which is at least partially explained by the fact that the insulin group had more hypoglycemic episodes, and took less metformin (a diabetes drug that causes fat loss). A previous study found that three different kinds of long-acting insulin actually caused a slight weight loss over three months. This is rather difficult to reconcile with the idea that elevated fasting insulin is as fattening as claimed.
_
In obesity, fat tissue is insulin resistant. The fat tissue of obese people doesn’t suppress fatty acid release in response to experimentally elevated insulin or mixed meals as effectively as the fat tissue of a lean individual. In fact, obese people release an equal or larger amount of fatty acids from their fat tissue than lean people under basal conditions as well. If this is true, then why do they remain obese? It’s simple: the long-term rate of fat entering the fat cells is equal to the rate exiting, or higher. There is no defect in the ability of fat cells to release fat in obesity, the problem is that the fat that is released is not being oxidized (burned) at a rate that exceeds what is coming in from the diet; therefore it all ends up back in the fat tissue. While we’re on the subject, let’s address the idea of “internal starvation”. Taubes suggests that people overeat because they can’t access their fat stores due to elevated insulin. However, obese people have normal or elevated levels of circulating fat, so how is that possible? The internal starvation model was interesting, if speculative, at the time it was proposed, however the evidence for it has simply failed to materialize. If anything, obesity is a condition of “internal excess”. Let’s also address the claim that obese people don’t necessarily eat more than lean people. Food records are notoriously inaccurate, however there is at least one way to measure total energy intake in a precise and unbiased manner. It is called the “doubly labeled water method” (DLW). DLW studies have shown that after controlling for confounding factors (gender, age, physical activity), obese people almost invariably expend more, and consume more calories than lean people. Weight stable obese people have a higher energy flux out of fat cells, and a higher metabolic rate, but it is not enough to overcome the higher calorie intake that is also observed. That has been repeatedly confirmed and it is simply a fact at this point.
_
If elevated insulin leads to fat gain, then this should be scientifically observable. All we have to do is look for people with different levels of circulating insulin (controlling for baseline fat mass), and see if it predicts fat gain over time. Fortunately for us, this has been studied many times. In most studies, people with higher fasting insulin at baseline actually gain less fat over time that people with lower fasting insulin. In the most recent study, higher insulin (and insulin resistance) at baseline was associated with less fat gain over time, but this relationship was eliminated by adjusting for baseline fat mass, leaving no relationship between insulin and fat gain after adjustment. Again, this proves that elevated fasting insulin is not the cause of common obesity.
_
Therefore, the insulin hypothesis is not consistent with basic thermodynamics, it’s not consistent with research on the biological functions of insulin, and it’s not consistent with observational studies. Obese people do not have a defect in the ability to release fat from fat cells and burn it, to the contrary, they release more fat from fat cells than lean people, and burn more of it. However, this is compensated for by a higher energy intake, and a higher rate of fat incorporation into fat cells that counterbalances the increased expenditure. This shows that insulin does not cause obesity by acting directly on fat cells to cause fat storage. To understand obesity, we have to understand what causes increased food intake, and that factor is not insulin. If insulin action on fat cells is a dominant factor in obesity, why don’t genes linked to insulin signaling show up at the top of the list in these studies? There are enough proteins that regulate insulin secretion in the pancreas and insulin signaling in fat cells that one would expect genetic variability in these genes to turn up frequently if they were important regulators of fat mass, but instead the list is dominated by genes that relate to the brain, and leptin signaling in particular. This is consistent with a huge body of literature implicating the brain in body fat mass regulation and the development of obesity.
C.) Carbohydrate, particularly refined carbohydrate and sugar, cause fat accumulation by increasing Insulin. Is it true?
It has been already demonstrated that it makes no sense to invoke insulin as a mechanism between carbohydrate consumption and body fatness. Another problem with the hypothesis is a thing called the insulinogenic index (II). The II is simply a measure of how much eating a food increases insulin, per unit calorie. It turns out, it doesn’t correspond with the carbohydrate content of a food very well. In particular, protein-rich foods such as beef can increase insulin secretion as much as certain starch foods such as pasta, or more. High-protein diets, as many of you know, aid with weight loss. Some have suggested that this is because of glucagon release by the pancreas in response to protein. That may well play a role, but if we are going to invoke glucagon, then aren’t we acknowledging that other signals besides insulin play an important role in this process? So you can’t just look at insulin, you have to consider amylin, glucagon, GLP-1, ghrelin, leptin, stomach distension, and all of the other short- and long-term signals that are activated in response to nutrient ingestion and changes in body fat mass. These collectively regulate food intake and long-term body fatness via the brain. The other problem is that refined and unrefined carbohydrates often have a similar II. Pasta made from white and whole-grain wheat have the same II, and the same goes for white and whole-grain bread. Doughnuts and cookies are on par with whole grain bread. So post-meal insulin is not a compelling explanation for the potentially different effects of protein, unrefined carbohydrate, refined carbohydrate and sugar on body fatness.
_
It’s likely that refined carbohydrate and sugar can contribute to obesity, but by what mechanism? Insulin is not a compelling explanation. But let’s forget about insulin for a minute. Without worrying about the mechanism, let’s simply consider the hypothesis that carbohydrate consumption per se causes body fat accumulation. Now some will say all carbohydrate is fattening, although refined carbohydrate is more fattening. To address this hypothesis, first let’s find some cultures that have a very high carbohydrate intake and see how fat they are:
The New Guinea highland tribe at Tukisenta that was studied extensively in the 1960s and 70s: They ate 94 percent of their calories as carbohydrate, mostly from sweet potatoes, for a total calorie intake of 2,300 kcal/day in men and 1,770 kcal/day in women. Investigators found them to be fit, lean and muscular, with no sign of protein deficiency (Trowell and Burkitt. Western Diseases. 1981):
West Nile district, Uganda, 1940s: The diet consisted of millet, cassava, corn, lentils, peanuts, bananas and vegetables (Trowell and Burkitt. Western Diseases. 1981). Despite food abundance, “in the 1940s it was quite unusual to see a stout man or woman.” “In recent years, however, a fair number of upper-class middle-aged West Nile women have begun to look rather stout, and some men have become very obese, especially those who hold lucrative posts and can purchase whatever food they like.” This corresponded with an increase in “sugar, cooking oils, milk, fish and meat” and a corresponding decrease in “home-grown starchy staple foods.” This same scenario has happened to hundreds, if not thousands of African communities whose traditional diets are very high in carbohydrate.
Northern Cameroon, 1980s: The Massas tribe (also spelled Massa) is known for its overfeeding ritual called Guru Walla: The Massa tribe of northern Cameroon fattens their males using both milk and a porridge made from sorghum, a corn-like grain that provides sweet syrup from the stalk. One man gained seventy-five pounds on a ceremonial binge. The average weight gain tends to be fifteen to twenty pounds using milk and porridge. The Massa are cattle herders and their staple diet is primarily milk. This fattening comes about by the addition of carbohydrates (sorghum) almost exclusively. These people are lean on their typical high-carbohydrate fare until they deliberately overconsume a mixture of sorghum and milk.
Most of Asia, 20th century: Many Asian countries, including China, Japan, Taiwan and India, have a traditional diet that is very high in carbohydrate. In many cases, the dominant carbohydrate was white rice, a refined carbohydrate. Yet traditional Japanese, Chinese and Southern Indians eating mostly white rice were renowned for their leanness. Any plausible hypothesis of obesity needs to account for these observations.
Kitava, 1990s: Dr. Staffan Lindeberg showed that the Kitavan diet is 69% carbohydrate, mostly from taro, breadfruit, sweet potatoes and cassava. Thus, their diet would have had a high glycemic load and high II. They also obtain 50 g/day of carbohydrate from fruit, most of which would presumably been sugar. Yet there was no obesity on the island, and only a few individuals that were slightly overweight. Fasting serum insulin was low, consistent with other high-carbohydrate cultures. Dietary carbohydrate does not cause insulin resistance.
Pima, 20th century: The Pima of New Mexico currently have one of the highest obesity rates in the world, on par with Nauru. The Pima were first contacted in 1539 by the Spanish, who apparently found them to be lean and healthy. At the time, they were eating a high-carbohydrate, low-fat diet based on corn, beans, starchy squash, and a modest amount of gathered animal and plant foods from the forest and rivers in the area. In 1869, the Gila river went dry for the first time, and 1886 was the last year water flowed onto their land, due to upstream river diversion by settlers. They suffered famine, and were rescued by government rations consisting of white flour, sugar, lard, canned meats, salt and other canned and processed goods. They subsequently became obese and have remained that way ever since. Their diet consisted mostly of bread cooked in lard, sweetened beverages and canned goods, and they also received salt. More recently, their diet has modernized but still relies heavily on processed food.
The above discussion proves that despite very high carbohydrate consumption in many tribes & cultures, they did not become obese and remained lean. One important caveat is that all these people ate natural carbohydrates rather than processed carbohydrates. None of them drank sweetened beverages and ate junk/fast food. Also, all of them were physically active as compared to modern people. The corollary is that sweetened beverages and junk/fast food do cause obesity, if not by insulin action, then by other means.
_
Old adipostat hypothesis:
Adipostat is a hypothetical mechanism which keeps the level of body fat of most people within a narrow range despite considerable variations in dietary fat intake and physical activity. The adipostat is thought to consist of a complicated network of brain cells, hormones, and organs that regulate body weight. Researchers at the Oregon Health Science University have identified two neuronal pathways in the hypothalamus that might be the neuronal basis. One pathway, called the NPY/GRP, stimulates feeding; the other pathway, called the MSH, inhibits feeding and is involved in the maintenance of metabolic rates. It is thought that some people may become obese because of a malfunction in the adipostat, but this hypothesis has not gained general support in the scientific community. Three mechanisms for an adipostat have been proposed. The first proposal likens the adipostat to a thermostat. When fat stores exceed genetically determined limits, the adipostat switches on metabolic processes (called futile cycles) that convert excess fat into heat. Conversely, when fat stores run low, the adipostat switches off the futile cycles. Thus fat stores are regulated by the adipostat increasing or decreasing fat metabolism. The second proposal is that a hormone is released from adipose tissues affecting the appetite control centre in the brain. The third proposal is that the activity of special, metabolically active fatty tissue increases when fat stores exceed the normal level, increasing heat output and burning off the excess fat.
_
Thermogenesis challenges the old adipostat hypothesis for body-weight control:
According to the adipostat hypothesis for body-weight control, alterations in body weight should always be compensated by adequate alterations in food intake and thermogenesis. Thus, increased thermogenesis should not be able to counteract obesity because food intake would be increased. However a study has found that thermogenesis in different forms (through artificial uncouplers, exercise, cold exposure) may counteract obesity and is not always fully compensated by increased food intake. Correspondingly, a decreased capacity for metaboloregulatory thermogenesis (i.e. non-functional brown adipose tissue) may in itself lead to obesity. This is evident in mice and may be valid for human subjects, as a substantial proportion of adults possess brown adipose tissue, and those with less or without brown adipose tissue would seem to be more prone to obesity. Thus, increased thermogenesis may counteract obesity, without dietary intervention.
_
Novel Adipostat theory proposed by me:
The brain is the primary homeostatic regulator of fat mass, just as it homeostatically regulates blood pressure, breathing rate, and body temperature. This has been suspected since the early brain lesion studies of the 1940s and even before, and the discovery of leptin in 1994 cemented leptin’s role as the main player in body fat homeostasis. The setpoint around which the body defends various variables can be changed (e.g., hypothalamic set point elevated in fever). Even though carbohydrates, fat and protein are partially inter-convertible, energy is mainly stored as fat. Body fat is essential for survival as it provides energy in event of starvation or lack of food due to any cause for weeks. I propose that brain has adipostat, a center for fat homeostasis that is set to maintain body fat in a normal range. It is evolutionary biologically hardwired in brain for survival as millions of years ago; our ancestors did not get food every day and every time. This adipostat regulate eating behavior and physical activities. Scientific studies have found that caloric restriction in diet leads to a natural compensatory mechanism that reduces physical activity to save energy; proving that there exists a center in brain that regulates food intake along with physical activities. That is why obese people must combine dietary restriction with exercise to reduce weight. This adipostat is influence by genes, dietary factors, exercise, gut hormones, adipokines, cytokines, hedonic hunger, willpower etc. When fat storage is less than adequate, its set point is lowered encouraging person to eat more and take rest; and when fat storage is more than adequate, its set point is elevated making person to eat less and exert more. This is how adipostat worked in our ancestors thousands of years ago. However, in modern times person eats everyday fully and so there is no chance of lowering of set point and over the time, due to constant bombardment of gut hormones & adipokines, due to ample & continuous supply of macronutrients, adipostat becomes dysfunctional sensing higher body fat as normal. So despite having excess fat, adipostat is not elevated. Dysfunctionally lowered adipostat set point means excess fat in body is perceived by brain as normal and therefore brain makes no efforts to suppress hunger. That is why most obese people do not feel that they are obese and make no efforts to reduce food intake. We have to persuade brains of obese people to eat less; do exercise etc to reduce weight but most of the time it fails in long run as adipostat set point is dysfunctionally lowered. This also explains why obesity has become self-perpetuating disorder because adipostat perceives high fat content as normal regardless of whether they are made to go on a diet which had made them lose weight earlier, meaning weight loss did not correct adipostat, and that is why most adults find it exceedingly difficult to maintain meaningful weight loss from dieting and exercise alone. Bariatric surgery work by reducing gut hormone effect on adipostat and some medications work by influencing adipostat directly, so these interventions do successfully reduce weight as it resets adipostat set point. The scientific treatment of obesity is to reset adipostat. Of course, theoretically, strong will power can control diet, do exercise and reset adipostat but how many of us have strong willpower?
_____________
Is obesity poor predictor of health?
Yes, there are certain health risks associated with having an elevated BMI, such as Type II diabetes and heart disease. More broadly, a higher BMI is associated with a greater risk of cardiometabolic abnormalities, as measured by blood pressure, triglycerides, cholesterol, glucose, insulin resistance and inflammation. Nonetheless, almost one quarter of “normal weight” people also have metabolic abnormalities, and more than half of “overweight” and almost one third of “obese” people have normal profiles, according to a 2008 study. One explanation for this discrepancy is that physical fitness and/or nutrition – rather than weight per se – may be what really matters. Several studies have shown that physically fit “obese” individuals have lower incidence of heart disease and mortality from all causes than do sedentary people of “normal” weight. A recent clinical trial published in the New England Journal of Medicine showed that adopting a Mediterranean diet reduced cardiovascular risk independent of weight loss. Some assume that the problem lies with BMI as a measure, which does not distinguish between fat, muscle, or bone. While BMI is indeed a flawed measure, it is not clear that there are better ones. A 2009 study, using the National Health and Nutrition Examination Survey, estimated excess deaths for standard BMI levels as well as for comparable levels of percentage body fat, waist circumference, hip and arm circumferences, waist:hip ratio, the sum of 4 skinfold thicknesses, and waist:stature ratio. They found no systematic differences between BMI and other variables. In other words, it is not just that BMI is a poor measure of obesity but that obesity is a poor predictor of health.
_
Consistently, in various studies, physical inactivity was a better predictor of all-cause mortality than being overweight or obese:
Study-1:
Groundbreaking work on fitness and weight has been done by [epidemiologist Steven] Blair and colleagues at the Cooper Institute. They have shown that the advantages of being fit are striking and that people can be fit even if they are fat … and thus have lowered risk of disease. A remarkable finding is that heavy people who are fit have lower risk than thin people who are unfit.
Study 2:
Fit man carrying 50 pounds of body fat had a death rate less than one-half that of an unfit man with only 25 pounds of body fat.
-Harvard Health Policy Review, 2003
Study-3:
Unfit, lean men had twice the risk of all-cause mortality as did fit, lean men and also had higher risk of all-cause mortality when compared with fit, obese men. The all-cause mortality rate of fit, obese men was not significantly different from that of fit, lean men … In summary; we found that obesity did not appear to increase mortality risk in fit men. For long-term health benefits we should focus on improving fitness by increasing physical activity rather than relying only on diet for weight control.
-American Journal of Clinical Nutrition, 1999
study-4:
The report from the Aerobics Center Longitudinal Study presents convincing evidence that fitness is a more potent risk factor for mortality than is fatness … an effect of fitness that was statistically independent of the level of fatness was confirmed. The effect of fatness independent of fitness was less clear.
-American Journal of Epidemiology, 2002
_
_
“If the height/weight charts say you are 5 pounds too heavy, or even 50 pounds or more too heavy, it is of little or no consequence healthwise-as long as you are physically fit. On the other hand, if you are a couch potato, being thin provides absolutely no assurance of good health, and does nothing to increase your chances of living a long life.”
-Steven Blair, P.E.D., Cooper Institute for Aerobics Research, 1997
_
Many large people are very healthy; many thin people are unhealthy. As another part of the big picture, largely being ignored today, are life-threatening health risks for the many painfully thin girls and women in our culture who are under nourished because of their desperate fear of fat. Underweight and nutrient deficiencies present severe problems in our culture. So it’s important that weight concerns not overshadow the need for growing children and adults to be fully nourished, moderately active and live in a nurturing environment. In the big picture it is total wellness that counts. Despite the risks related to obesity, it is a mistake to exaggerate those risks.
_
Some hard questions and honest answers:
The first question:
1. How strong is the link between obesity and health risks?
The answer came shortly. In 2005, in a rigorous analysis of actual deaths in the U.S. over a 30-year period, research scientists with CDC’s National Center for Health Statistics, showed a figure closer to 112,000 deaths. Their study revealed a great deal more. Yes, these were serious health risks. But rather than being spread across the two categories of overweight and obese, they were confined to a relatively small segment of the population. The high risk came at the level of severe obesity, called grade 3 – a body mass index (BMI) of 40 or more. Fewer than five percent of U.S. adults reach this level. For the one-third of the population in the overweight group no risk at all was found. In fact, it had the lowest health risk of any group, lower than the category labeled normal weight and often called healthy weight. The researchers found seriously high risks in the underweight group. Further, their report of higher risks for the normal group supported a great deal of other research that suggests a BMI of 24 to 30 as the point of lowest risk.
2. Does obesity cause the diseases associated with it?
For several decades research has shown an association between obesity and certain diseases. All that time it has been assumed that obesity causes these diseases. This is a leap not allowed in credible science. But somehow health scientists seemed not to notice. Association does not prove cause. This is basic science. When two factors are related – or linked – it is necessary to go a step farther and look independently a third factor (or factors) that may be causing both. In this case the most likely third factors are genetics, nutritionally poor but energy dense food and inactivity. In fact, in my view, genetics does not matter as it is inconceivable that human genome changed so much in 50 years that obesity from rarity became epidemic. Although genetic factors play a role, heritability is not destiny; calculations show that moderate environmental changes can promote as much weight loss. Yet, inexplicably, most studies that look at the risks of obesity fail to consider the role of inactivity in disease. This has led to much confusion and inappropriate health care. When scientists do look at activity, they find it is more closely related to health risks than is obesity. Researchers with the long-running fitness studies at the Cooper Institute Aerobics Center in Dallas, TX. find that when obese persons are moderately or highly fit their mortality risk is no higher than for similarly active persons of normal weight.
3. Does weight loss improve health and longevity?
Weight loss is widely recommended for larger people. However, efforts to lose weight in a lasting way are almost invariably unsuccessful. Any lost weight is soon regained. Weight loss efforts may have immediate detrimental effects, and it is by no means clear that weight loss improves health or mortality risk. An increasing body of evidence casts this in doubt. Furthermore, it may enable “setpoint creep.” so that weight is ratcheted upward after each weight loss bout. Beauty, health and strength come in all sizes. However, society’s disapproval can be cruel. Size prejudice is found in health care, education, social relationships and many other aspects of daily living. In their work and careers, large people often meet size prejudice in hiring, advancement and salary. Being overweight or obese can be a severe social handicap. Children who are teased, labeled and stigmatized may have long-term damage to self-esteem and body concept. Some experts say that for children the most detrimental consequences of excess weight are probably not health risks, but emotional and psychological damage. They point out that, while cultural bias is powerful, it is not a reason to change the child, but rather to change the culture. Large children would be much happier in an accepting, nurturing environment, which they may not find in school, and sometimes not even in the home. Research tells us that large people are no different than thin people. Each is an individual and deserves to be regarded this way and treated with respect and acceptance. For most large people the key to good health may be simply leaving weight alone, and instead focusing on habits of moderate, regular physical activity and other aspects of healthy living. The scientists at the Cooper Center advocate this kind of reasonable solution. They summarize the data and conclude: “We believe that public health would be better served with more comprehensive attempts to increase population levels of physical activity, rather than emphasizing ideal weight ranges and raising an alarm about increasing prevalence rates of obesity.”
_
Healthy obese:
The definition of obesity — a body mass index, of 30 or above — is a crude metric. An estimated 30 percent of those classified as obese are actually healthy in terms of their cardio-metabolic profile, including such things as circulating glucose, insulin, and cholesterol levels. In this group of “healthy obese,” prescribing weight loss may actually be harmful. Furthermore, in the most detailed analysis of data worldwide carried out to date, having a BMI of 30 – <35 kg/m2 was not associated with a higher death rate. Only at levels of extreme obesity (35 kg/m2 or greater) does one see an increase in the death rate. Those who are overweight (BMI of 25-30) actually have the lowest death rates.
_
In the study, researchers at the Albert Einstein College of Medicine, Bronx, N.Y., and colleagues assessed body weight and metabolic abnormalities (including high blood pressure, elevated triglycerides and low HDL or “good” cholesterol) in 5,440 people. Participants were considered metabolically healthy if they had none or one abnormality and metabolically abnormal if they had two or more abnormalities. The result:
- About 23 percent of normal-weight adults were metabolically abnormal.
- About 51 percent of overweight adults were metabolically normal.
- About 31 percent obese adults were metabolically normal.
Normal-weight people with metabolic abnormalities tended to be older, less physically active and have larger waists than healthy normal-weight individuals. Obese people with no metabolic abnormalities were more likely to be younger, black, more physically active and have smaller waists than those with metabolic risk factors. The bottom line recapitulates what other data show: it’s far better to be active and overweight, even obese, than sedentary and normal weight. The goal of optimum health is not to be thin, but to be as healthy as you can, regardless of weight. It’s very clear that some people are genetically programmed to carry extra pounds, and as long as they maintain their cardiovascular health through exercise, they should indeed be considered healthy.
_
In the past years it became clear that up to 30% of obese patients are metabolically healthy with insulin sensitivity similar to healthy lean individuals, lower liver fat content, and lower intima media thickness of the carotid artery than the majority of metabolically ‘unhealthy’ obese patients. Recent studies suggest that protection against development of hepatic steatosis, ectopic fat deposition, inflammation of visceral adipose tissue, and adipose tissue dysfunction contributes to healthy obesity. The evidence shows that morbid obesity is associated with an increased likelihood of developing disease and suffering from early mortality, but it also shows that those who are a few pounds overweight don’t need to panic. What’s more, it is clear that everyone, fat or thin, will benefit from regular exercise regardless of whether they lose weight.
_
Defining obesity as a disease makes little medical sense, since, rather than judging a person’s health based solely on his/her BMI, a physician needs to examine each patient as an individual, take a detailed history, and assess clinical parameters, such as blood insulin, cholesterol, triglycerides, etc. If these are somewhat outside of the desirable bounds, behavior & life style changes may have the desired effect. If the values are more extreme and if behavior & life style changes are less of a realistic option, then drugs can be considered. But the decision needs to be made based on the complete profile of the individual, not on some arbitrary cut-point in a proxy variable.
_
Publishing bias against obesity:
Ioannidis defines publishing bias as the combination of various design, data, analysis, and presentation factors that tend to produce research findings when they should not be produced, and can entail manipulation in the analysis, and selective or distorted reporting of the findings. Much publishing bias in the obesity field involves one or more of the following:
Assuming that association proves cause:
Obesity is being blamed for much illness and disease for which there is little or no evidence it is causal. Making the leap from association to cause is a violation of science not permitted for other health conditions. Yet for obesity it is widely assumed, reported and believed.
Conclusion and abstract differs from body of study:
Determining at the outset how a study will turn out is not responsible science, but happens often in this field. In its least subtle form, a negating paragraph may simply be tacked on, for example, to a report that shows the ineffectiveness or harmful effects of dieting, but says, in effect, “In spite of all this, keep dieting.”
“Cherry picking.”
In a practice known as cherry picking, a subset is found that gives the desired results. The research report is based on this subset, rather than the larger study population.
Short-term intervention:
By its nature, weight is lost easily at first, but not sustained. Typically, weight regain follows until all lost weight, and perhaps more, is regained. Therefore, a short-term study is irrelevant because it fails to show true results. A one-year study does not represent lasting change.
Undisclosed side effects:
A full reporting of trial results is needed, including detrimental effects, but these are often unavailable. Not publishing the whole story can lead to inappropriate clinical interventions.
High dropout rate:
High dropout rates indicate high failure rates, so are important in understanding a weight loss intervention. It is irresponsible to report on the final, smaller number of “successes” remaining in the study as if they represent the total group enrolled.
Non-representative sample of subjects:
The sample needs to accurately represent the group being studied, and the results not extrapolated beyond that population. This is often violated in the obesity field when results from small, specialized groups are reported as if they apply to the general population, or only half the findings are reported.
Self-reported data; mailed-in questionnaires:
Self-reported answers are not always reliable, especially in regard to weight, height and weight loss or maintenance. Further, a low reply rate weakens the study, as respondents may be self-selected in a way that skews results.
_________________
How data can be manipulated to change outcome vis-à-vis obesity and mortality risk?
From overweight people die earlier to overweight people live longer!!!
Two large and well-conducted studies in the Lancet and New England Journal of Medicine examined this issue by gathering data on more than two million people who had their BMI calculated and were followed for their risk of dying over a defined time period. The large numbers of participants and detailed individual data in these studies means the researchers were able to look at how small differences in BMI relate to the risk of death, accounting for a range of other factors known to influence this relationship, including illnesses that could potentially affect BMI. Despite the diversity of populations covered by the studies, and the differences in methods, their findings are remarkably consistent: people with a BMI at the upper end of the WHO “normal” level (22.5 to 24.9) have the lowest death rates. You can see this visually represented by the J-shaped curve in the graph below:
_
_
As BMI goes up in increments of 2.5 above and below the 22.5-to-24.9 category, so do death rates. People with BMIs above this optimal level have an increased risk of dying, especially from heart disease, most likely due to increases in blood pressure, cholesterol levels and diabetes caused by excess body fat. They also have an increased risk of dying of cancer. People with BMI levels below the optimal level also have increased death rates, particularly from respiratory diseases (such as chronic bronchitis) and cancer. The increased risk of death in the people with lower BMIs may also be because chronic illness has caused them to lose weight.
_
But the JAMA paper reached a different conclusion:
It comes down to the way the data from each of the studies have been collected and presented. The JAMA review used broad classifications for underweight, normal weight, overweight, and obese and very obese, rather than the 2.5 increments of BMI. People with a BMI of 18.5 to 24.9 were included in the “normal weight” category; we can see from the graph above that this broad category includes people with the lowest risk of death, combined with people with a higher risk of death. The “normal weight” category was then used as the comparison group for the studies, and has an average risk of death that is higher than the risk in the broad “overweight” category. This skews the optimal weight finding and changes the shape of the curve, from J-shaped to tick-shaped as seen below.
_
_
The result?
One that suggests being overweight makes you live longer.
There are varying reasons why the researchers might have used these broad groups, including the fact that many studies are too small to be able to present statistically reliable results according to finer gradations in BMI. Also, low body weight often results from chronic disease, rather than being a cause of chronic disease. The weight loss may have been unintentional as a result of the underlying disease process; or the weight loss may have been intentional, because patients with serious conditions often become motivated for the first time to lose weight. Regardless, because of this phenomenon, people with a BMI below 25 are a mix of healthy individuals and those who are ill and have lost weight due to their disease. Leaner people are also more likely to smoke than their heavier counterparts. These factors will artificially inflate mortality rates among lean people, thus diminishing the harmful impact of overweight and obesity.
_
Nonetheless, the fundamental point is that the same data can give contradictory and differing results depending on the methods of the processing of data. All medical researchers must understand this fact about the fallibility of scientific studies on obesity.
_______________________________
The moral of the story:
_
1. Obesity means having too much body fat which usually leads to weight gain. However, you can have normal weight but excessive fat (normal weight obesity), and you may be overweight and yet have normal fat. Remember glucose, fat and protein are partially inter-convertible with each other but energy is mainly stored as fat because energy can be stored more densely in fats than carbohydrates. That is, metabolic oxidation of fats yields 37 KJ/g (9 kcal/g) whereas carbohydrates and proteins yield only 17 KJ/g (4 kcal/g).
_
2. One third of world’s adult population is overweight and one tenth obese. Today 925 million people suffer from hunger, while 1.5 billion people are considered overweight or obese. As countries get richer, they trade hunger for obesity.
_
3. Body mass index (BMI) – the weight in kilograms divided by the square of the height in meters (kg/m2) – is a commonly used index to classify overweight and obesity in adults. WHO defines overweight as a BMI equal to or more than 25, and obesity as a BMI equal to or more than 30. BMI is used because, for most adult people, it correlates with their amount of body fat even though BMI does not directly measure body fat. A child’s weight status is determined using an age- and sex-specific percentile for BMI rather than the BMI categories used for adults because children’s body composition varies as they age and varies between boys and girls. Regrettably, BMI has some serious flaws.
_
4. In men minimal fat is 5% while in women it is 8% of body weight. Above average body fat in men is between 16 and 25% and among women is between 24 and 32%. Percentage of fat over 25% in men and 32% in women defines risk of disease (true obesity).
_
5. Your waist should be no more than half the length of your height and waist-to- height ratio is a more useful tool for identifying health risks at an early stage than BMI.
_
6. Moderate obesity reduces life expectancy by about 3 years while severe obesity reduces it by about 10 years, similar to life expectancy of lifelong smokers. Obesity is now killing triple the number of people who die from malnutrition worldwide and 65% of the world’s population lives in a country where obesity kills more people than underweight.
_
7. Majority of obese people think that they are not obese and see no need to lose weight. Obese people consistently under-report their food consumption as compared to people of normal weight and they are unaware of the health risks of obesity.
__
8. Carbohydrate, fat and protein calories are indeed equal by definition in terms of their energy content, but the body processes each in a distinct way and they have very different effects on the body depending on when they are eaten, with what they are eaten, which hormones are secreted or suppressed by them and how these hormones interact with brain. So all calories are not equal when we consume them as food.
_
9. Your cells need fuel to function, and they can get their fuel in the form of sugar or fat. [Protein is burned as fuel when neither carb nor fat is available as fuel.] However, the body never shuts off one fuel source when it turns on the other. The body relies on both fat and carbs for energy all the time, albeit in different ratios but these ratios don’t make a big difference when it comes to losing weight and decreasing your body fat. Even if you’re burning lots of carb calories and less fat calories through exercise, your fat still inevitably gets used because burned carb calories need to be replaced both by the carbs you eat in your diet and your fat stores that will be broken down and transformed into carbohydrates. So we must do exercise that burns maximum calories in short time no matter whether those calories came from carbs or fat. What matters most is the total number of calories burned. [Energy burning while physical activity (non-fed state calories burning)]
_
10. Your cells need fuel to function, and they can get their fuel from diet in the form of sugar or fat. However, your body must burn all of the available sugar first before it turns to burning fat. For example, if you eat sugar with butter, your body will try to burn sugar first and whatever can’t be burned off will eventually be stored as fat albeit inefficiently as 100 extra calories in glucose (about 25 grams) floating in your bloodstream need 23 calories of energy to convert the glucose into fat and then store it. The butter you just ate, meanwhile, also goes into your fat stores. That is why low fat diet fails in weight reduction as high carbs will be stored as fat even though fat intake is low. [Energy burning while energy intake (fed-state calories burning)]
_
11. Both carbohydrates (sugar & starch) and fat cause fat gain but when they are combined together it is far worse than eating them alone due to hormonal effects. That means fat gain by eating fat & carbohydrates together is more than sum total of fat gain from eating fat and carbohydrates separately. In other words, energy is stored as fat very efficiently when fat and carbohydrates are eaten together. The worst fat storing combination is fat & starch/sugar. [No Pepsi/Coke with French fries]
_
12. There is evidence to suggest that high fructose intake either in the form of sucrose (table sugar) or high fructose corn syrup (HFCS) does lead to hypertriglyceridemia, nonalcoholic fatty liver disease (NAFLD), insulin resistance and obesity. Fructose is metabolized very differently in your body from glucose and fructose is more lipogenic than glucose.
_
13. Calorie rich foods are necessary for survival (they supply energy) and therefore evolutionary biologically they are hardwired into dopamine reward system to bring pleasure and so we crave for it. Leptin influences appetite by acting on ventral tegmental area (VTA) of brain containing dopamine neurons. High glycemic index food especially highly palatable, highly processed junk food (e.g. sugar containing beverages) cause stimulation of nucleus accumbens in brain involved in dopamine reward system (same system involved in drug addiction) leading to addiction to junk foods by reduction and down-regulation of dopamine receptors (D2), thereby compelling individual to consume junk food frequently to get the same pleasure, ultimately leading to obesity. Junk food diet does alter the production of dopamine and other genes in the brain’s reward system. If addicting drug cocaine can be banned for public consumption, why not sugar containing beverages (Pepsi, Coke etc)?
_
14. Consumption of sugar-sweetened beverages (SSBs), particularly carbonated soft drinks, may be a key contributor to the epidemic of overweight and obesity, by virtue of these beverages’ high added sugar content, addicting potential and less satiating than solid foods, increasing the total amount of energy consumed. Studies have found that those who consumed calorically sweetened beverages did not compensate for this consumption by reducing the intake of other foods and thus gained weight.
_
15. On a typical day, 80% of youth drink sugary soft drinks. The likelihood of obesity increased 1.6 times for every additional soft drink consumed per day.
_
16. The energy density of a recommended healthy diets is 125 Kcal per 100gms while energy density of typical fast food is 260 Kcal per 100 gms. Since humans consume almost the same quantity of food by weight daily, fast food consumption would lead to obesity.
_
17. Drinking milk is not associated with weight gain and whole milk is better than skimmed milk nutritionally. In fact, low-fat milk can encourage weight gain as reduced fat in milk might not be as filling, so it could lead consumers to compensate by eating and drinking more. Also, reduced-fat milk products are often pumped with added sugar to make them taste better, which lead to weight gain. So drink whole milk and be healthy. However, there is no evidence to show that milk promote weight loss in obesity.
_
18. The best diet to cause weight loss in obesity is low calorie diet containing all macronutrients with carbohydrates from low glycemic index group and fat from unsaturated group without trans fat. Alternative good diets for weight loss are high protein low glycemic index diet and high protein low fat diet. High carbohydrate low fat diet and low carbohydrate diet including Atkins diet are not recommended for weight loss. No matter which diet you choose to reduce weight, eat plenty of non-starchy fruits & vegetables as they have water, fibers, nutrients, fewer calories and keep you full longer.
_
19. TV viewing is a contributing factor to obesity because it may take away from the time you spend in physical activities; lead to increased energy intake through snacking and eating meals in front of the TV; and, influence you to make unhealthy food choices through exposure to food advertisements. TV viewing is directly linked to obesity in children and women but not in men possibly due to differences in occupational or social role. Women who reported three to four hours of TV viewing per day showed almost twice the prevalence of obesity as compared to women viewing TV for less than one hour per day.
_
20. Visceral fat is linked to insulin resistance, increased LDL level and chronic low-grade inflammatory response increasing cardiovascular events. Exercise can significantly reduce the amount of visceral fat in few weeks and higher the intensity of exercise, faster the loss of excess visceral fat.
_
21. Exercise alone without dietary changes will have a limited effect on weight because one has to exercise a lot to simply lose 1 pound. On the other hand, when you start to consume fewer calories by dieting, your body automatically responds by reducing your activity levels. In generations past, when finding your next meal was not as easy and this mechanism could help your body to hold on to valuable energy stores and survive longer in times of famine. Therefore the key to healthy weight loss is to use a combined approach of both diet and exercise together. However, cutting calories through dietary changes seems to promote weight loss more effectively than does exercise & physical activity; and physical activity by itself is not going to make and keep you thin. Regardless of body weight or weight loss, an increased level of exercise increases health.
_
22. For normal people, exercise will not prevent obesity if diet is promoting increased caloric consumption.
_
23. Spot exercise is beneficial for building specific muscles, but it has little effect, if any, on fat in that area of the body, or on the body’s distribution of body fat, and it will not burn fat at the desired location. So sit-ups will not reduce subcutaneous fat over abdomen.
_
24. Bariatric surgery is not only recommended for severe obesity with co-morbidities when lifestyle intervention has failed, but also recommended for treatment of type 2 diabetes in an obese patient without any co-morbidity.
_
25. The association between poverty and obesity may be mediated, in part, by the low cost of energy-dense foods and may be reinforced by the high palatability of sugar and fat. Poor do not eat what they want, or what they know they should eat, but what they can afford. Given this situation, the poor choose foods that are rich in carbohydrates, fats, and sugars, which do not provide them with adequate nutrition, but do satisfy their appetites and are easily incorporated in their traditional consumption. A dollar can buy 1,200 Kcal of cookies or potato chips but only 250 Kcal of carrots; and the same dollar buys 875 Kcal of soda but only 170 Kcal of orange juice. On the top of it, potato chips and soda are tastier than carrots and orange juice. Also, poor may be less educated and unaware of the difference between junk food and healthy food. No wonder poor would become obese and malnourished.
_
26. Fat laden hypertrophic adipocytes of adipose tissue have abnormal adipokine secretion and also activate macrophages to produce inflammation; both of these ultimately cause insulin resistance. But there could be other mechanisms of insulin resistance as lean individual with normal fat can be insulin resistant and almost one third of all obese individuals are insulin sensitive. Some researchers show that obese people are obese despite having higher fasting insulin, not because of it, although other researchers hypothesize that insulin resistance cause obesity. Whether obesity caused insulin resistance or whether insulin resistance cause obesity or whether increased sugar intake caused both insulin resistance & obesity; makes no difference to therapeutic implications as we have to reduce sugar intake and reduce consumption of refined carbohydrates.
_
27. Physically fit “obese” individuals have lower incidence of heart disease and mortality from all causes than do sedentary people of “normal” weight and therefore obesity per se is a poor predictor of health. Consistently, in various studies, physical inactivity was a better predictor of all-cause mortality than being overweight or obese. We all need to be adequately nourished and moderately physically active with an eye on weight rather than weight being the sole parameter of good health. The high health risk comes at the level of severe obesity, called grade 3 – a body mass index (BMI) of 40 or more. People should not be swayed by the exaggerations, publishing bias and fear mongering so common in the field of obesity.
_
28. Healthy obese are those whose cardio-metabolic profile, including such things as circulating glucose, insulin, and cholesterol levels are normal, they have lower liver fat content, and lower intima media thickness of the carotid artery than the majority of metabolically ‘unhealthy’ obese patients, and they are very active & physically fit. Prescribing weight loss to such individuals may actually be counter-productive but we need more research.
_
29. The relationship between body mass index (BMI) and mortality is curvilinear; with very high and low BMIs are associated with increased odds of dying. The way you collect data and present it in your study affects the result of your study vis-à-vis obesity and mortality risk in such a way that lowest mortality is seen in overweight group (BMI 25 to 30) and higher mortality seen in normal weight group (BMI 18.5 to 24.9), rather than the other way around.
_
30. There are three theories of obesity development: conventional theory suggests positive energy balance as cause of obesity, alternative theory suggests high intake of sugar and refined carbohydrates with resultant increased insulin action as cause of obesity and the third is novel adipostat theory being proposed by me. The setpoint around which the body defends various variables can be changed (e.g., hypothalamic set point elevated in fever). Body fat is essential for survival as it provides energy in event of starvation or lack of food due to any cause for weeks. I propose that brain has adipostat, a center for fat homeostasis that is set to maintain body fat in a normal range. It is evolutionary biologically hardwired in brain for survival as millions of years ago; our ancestors did not get food every day and every time. This adipostat regulate eating behavior and physical activities. When fat storage is less than adequate, its set point is lowered encouraging person to eat more and take rest; and when fat storage is more than adequate, its set point is elevated making person to eat less and exert more. This is how adipostat worked in our ancestors thousands of years ago. However, in modern times person eats everyday fully and so there is no chance of lowering of set point and over the time, due to constant bombardment of gut hormones & adipokines, due to ample & continuous supply of macronutrients, adipostat becomes dysfunctional sensing higher body fat as normal. So despite having excess fat, adipostat is not elevated. Dysfunctionally lowered adipostat set point means excess fat in body is perceived by brain as normal and therefore brain makes no efforts to suppress hunger. That is why most obese people do not feel that they are obese and make no efforts to reduce food intake. The scientific treatment of obesity is to reset adipostat. Of course, theoretically, strong will power can control diet, do exercise and reset adipostat but how many of us have strong willpower?
_
31. I am certain that all the variables in the genesis of obesity are not known and also we do not exactly know how the known variables affect the weight; and therefore obesity studies & research will be confounding at least for some time. Only one thing is clear. Since the genes and the physiology of humans cannot change in merely 30 years, the only reason for obesity epidemic is change of life style over last 30 years. Large portions, highly tasty, energy-dense, micronutrient-poor foods and beverages with physical inactivity is the root cause of obesity epidemic. This combination also causes various co-morbidities associated with obesity rather than obesity per se causing such co-morbidities.
_________________
Dr. Rajiv Desai. MD.
August 1, 2013
________________
Postscript:
Every day I weigh myself for last many years and that is the best way to prevent obesity. I have noted that whenever I have eaten plenty of junk food/fast food/fried carbs, my weight increases by 300 to 500 gms next day and I cut down my intake next day to come to my baseline weight. In my view, the best way to maintain your weight is to weigh yourself daily on the same weighing scale at the same time.

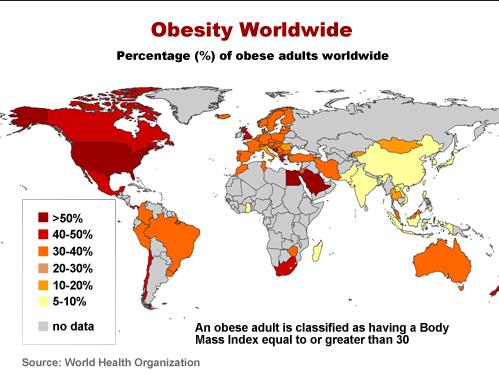
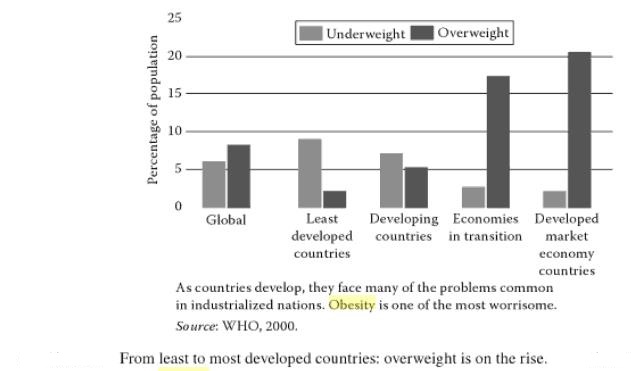
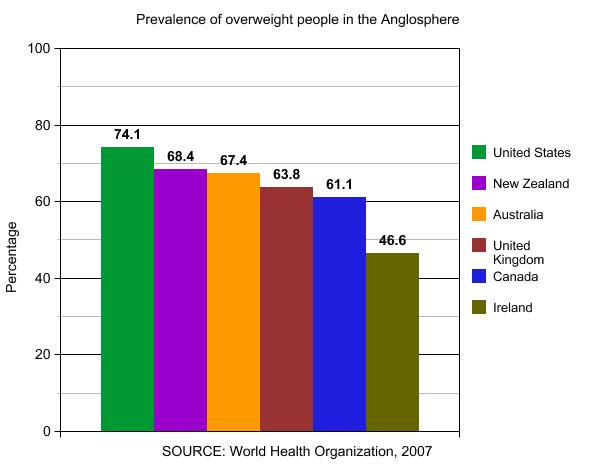
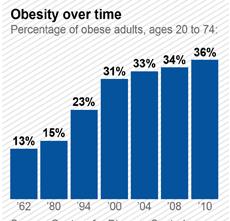
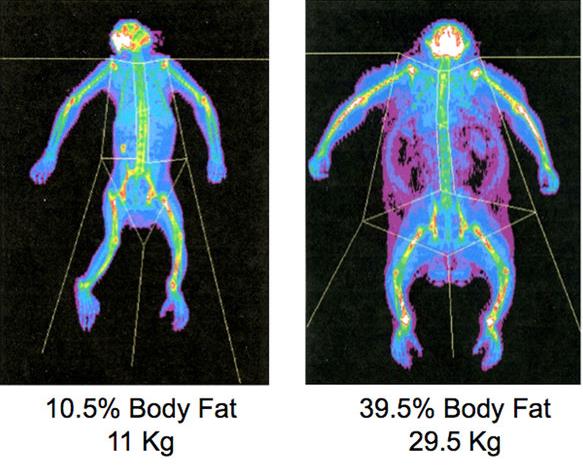

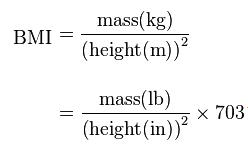
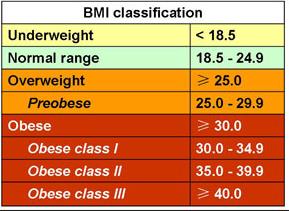
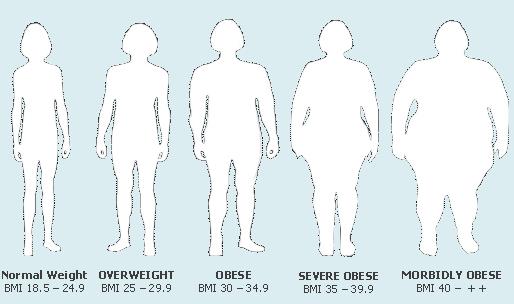
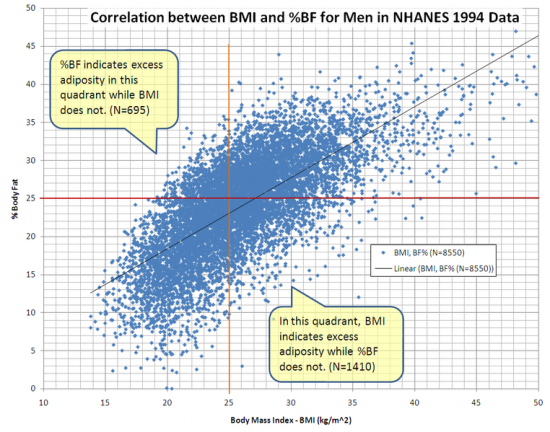
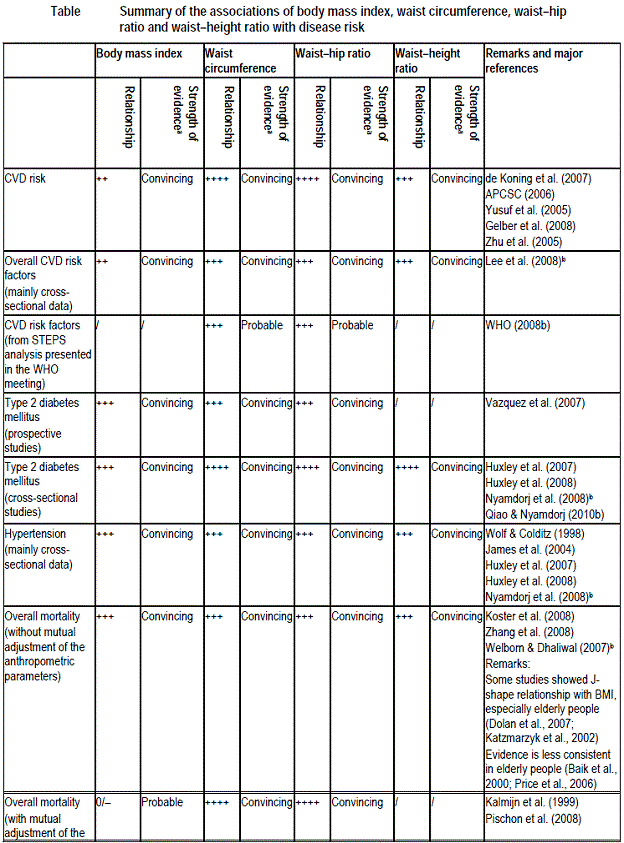
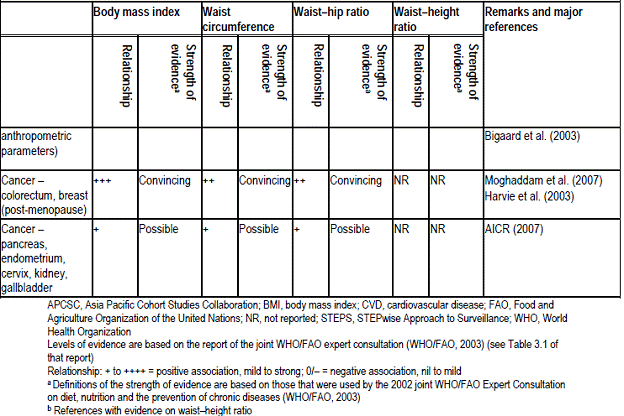
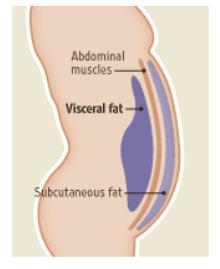
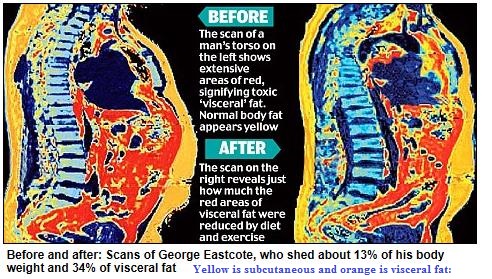
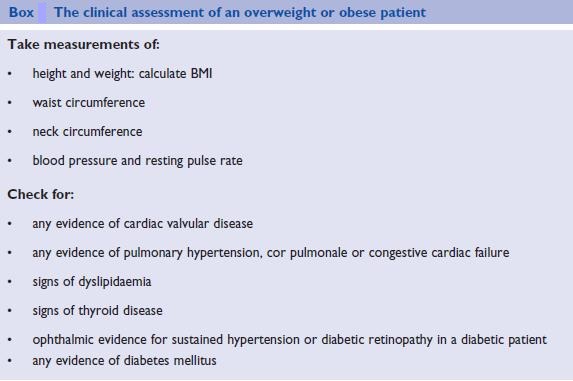

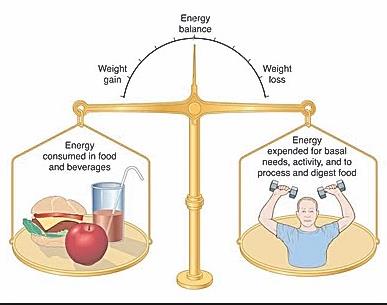
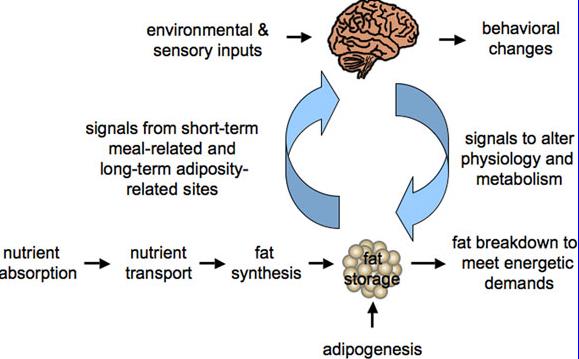

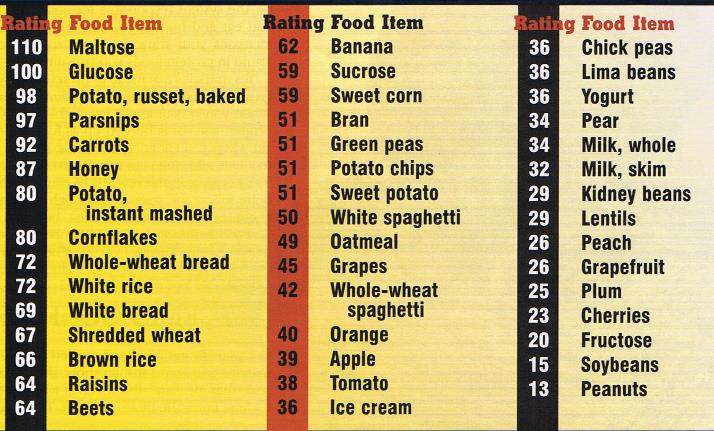
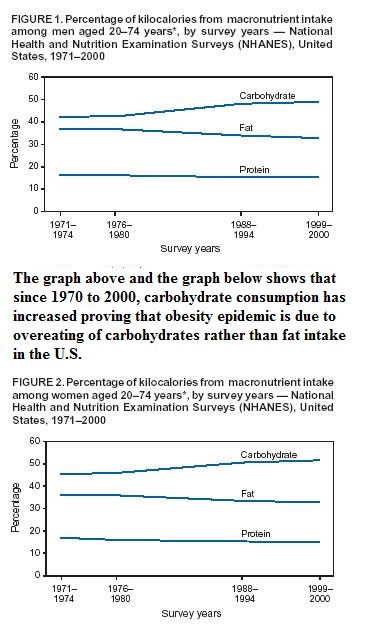
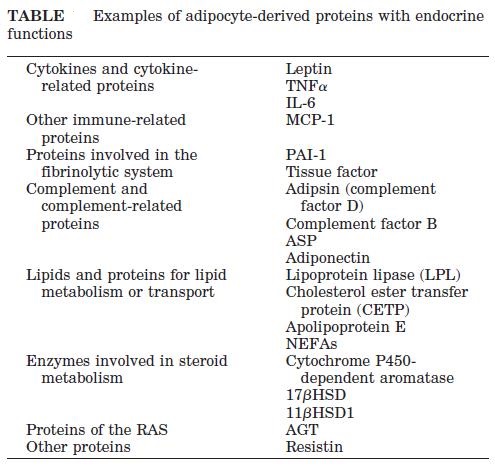
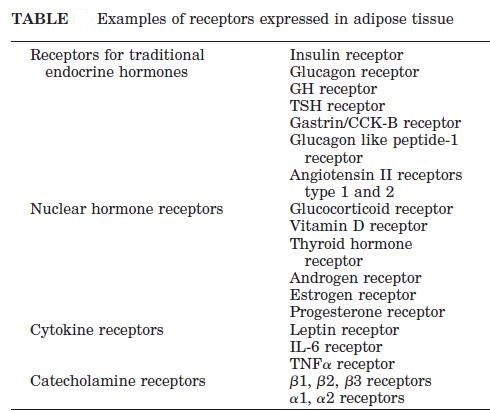
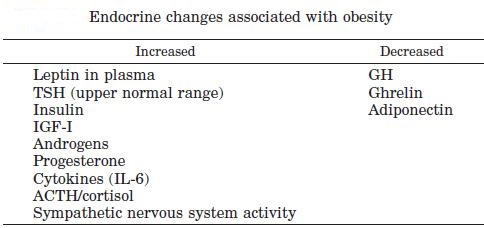
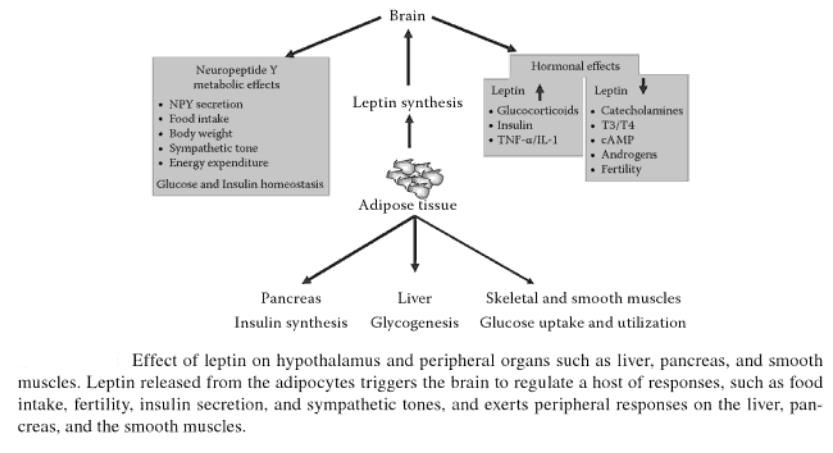
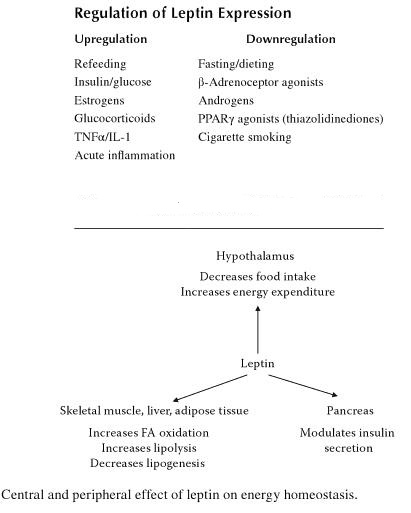
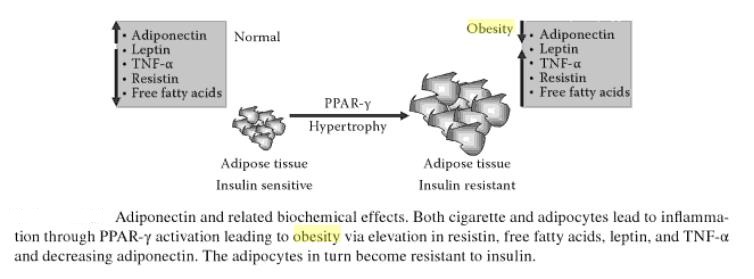
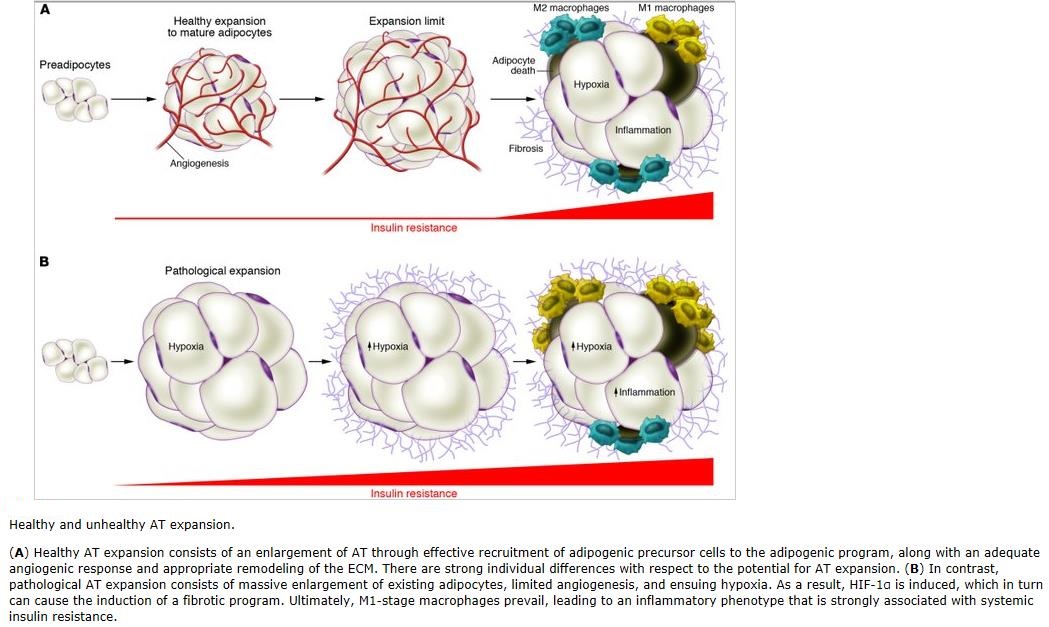
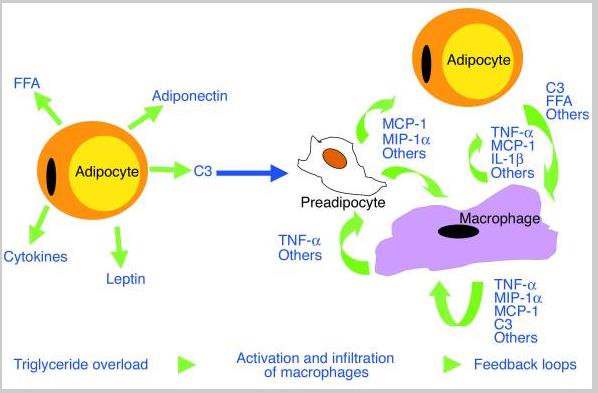
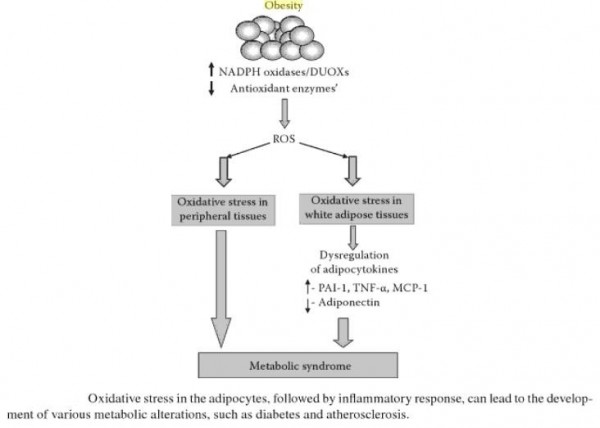
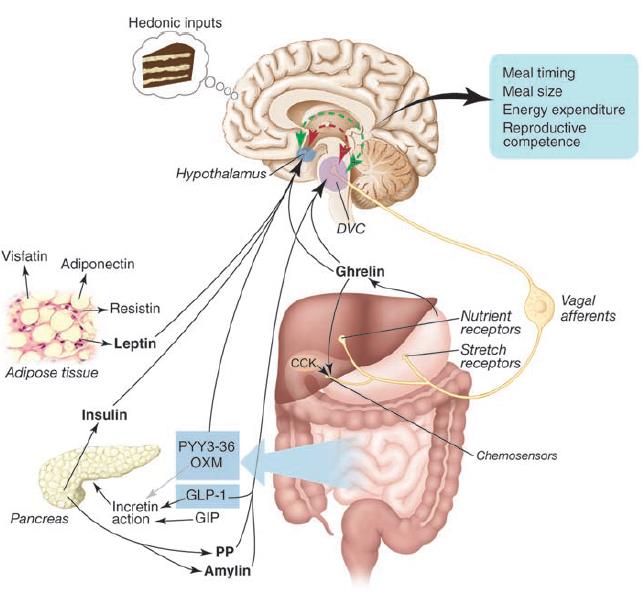
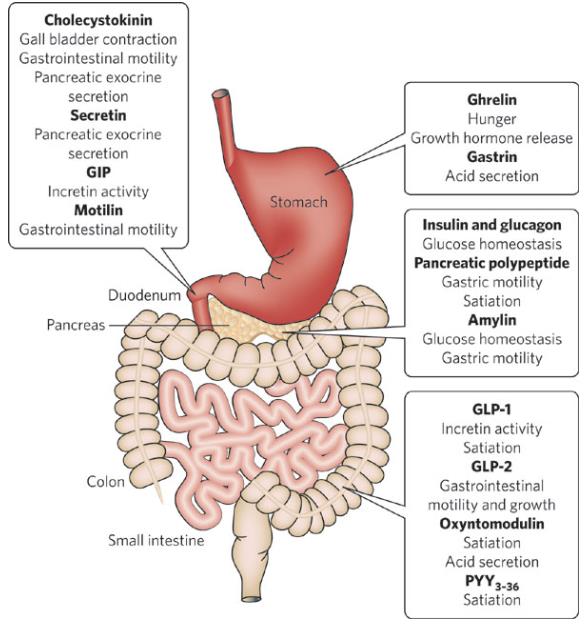
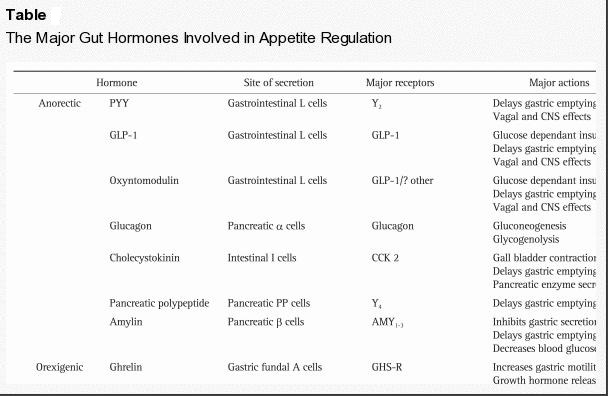
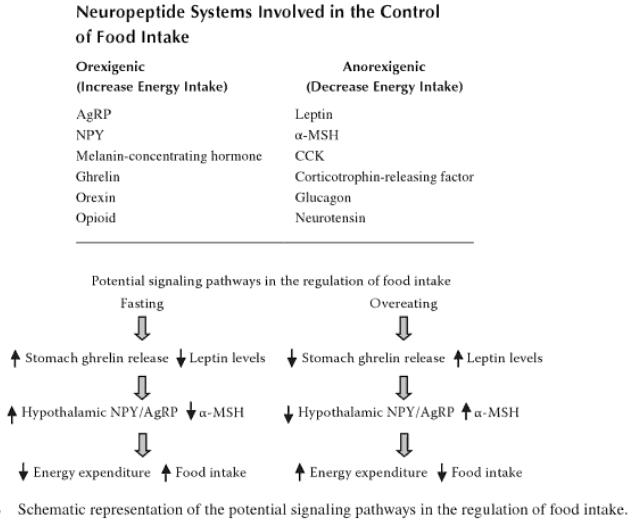
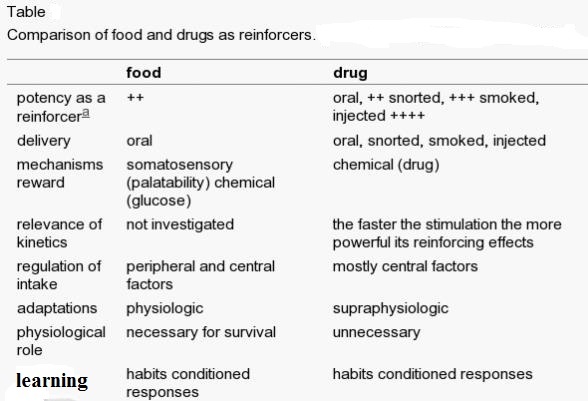
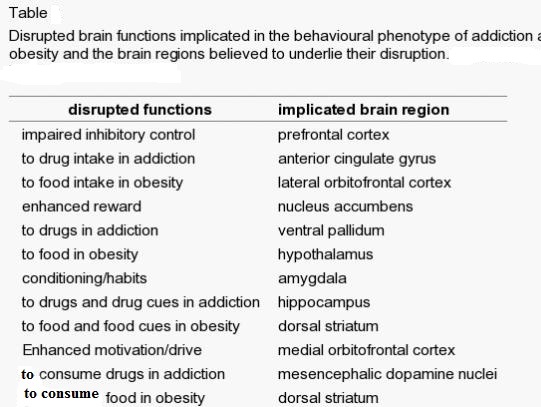
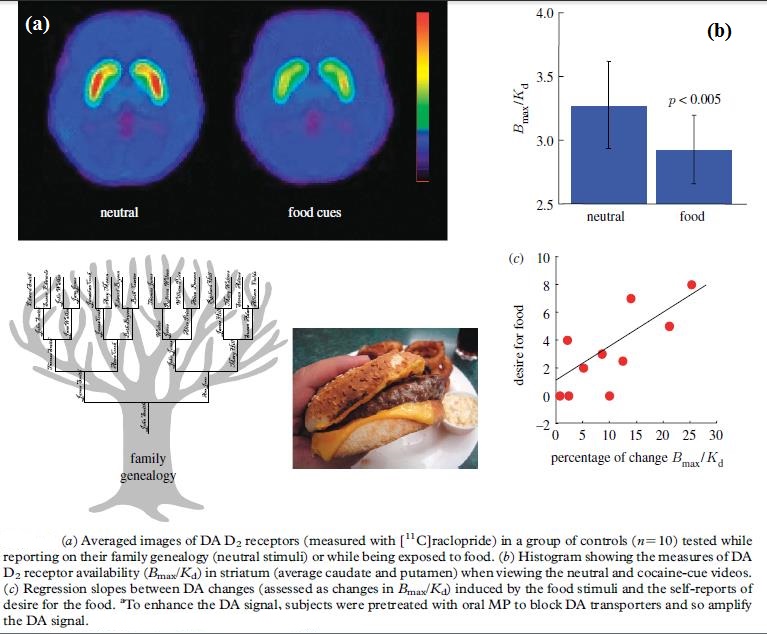
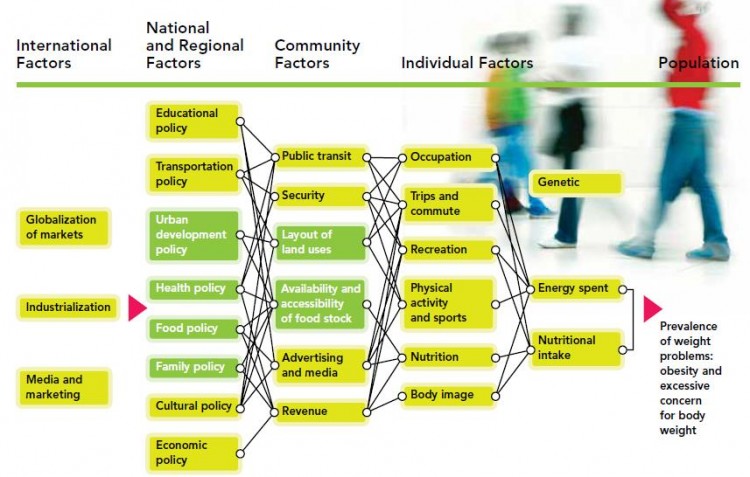
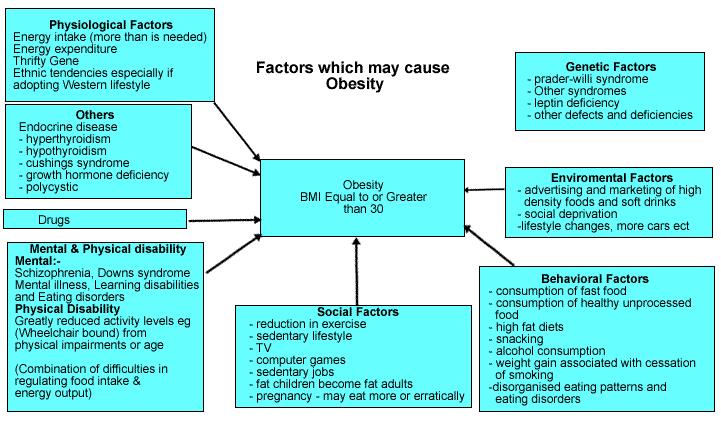
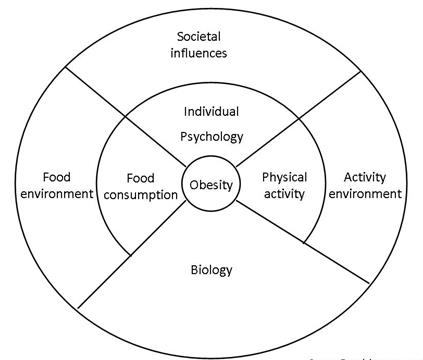

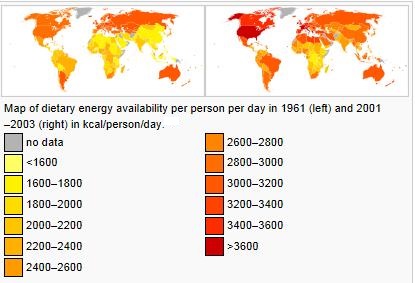

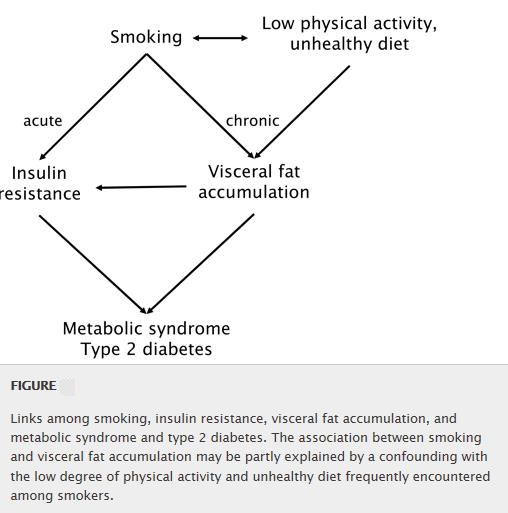
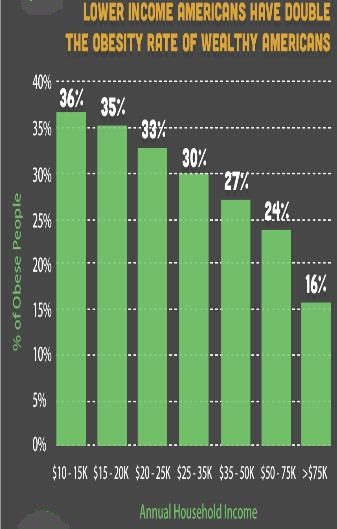
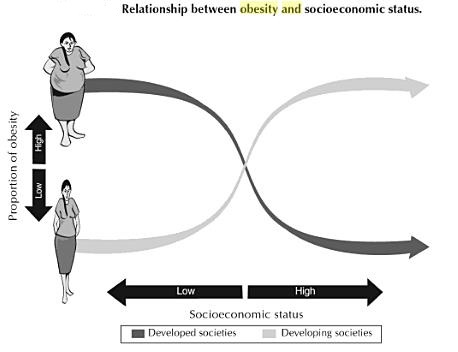

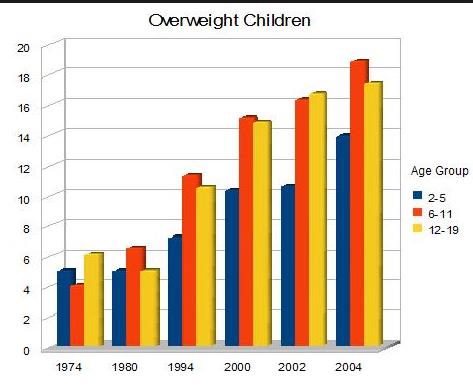
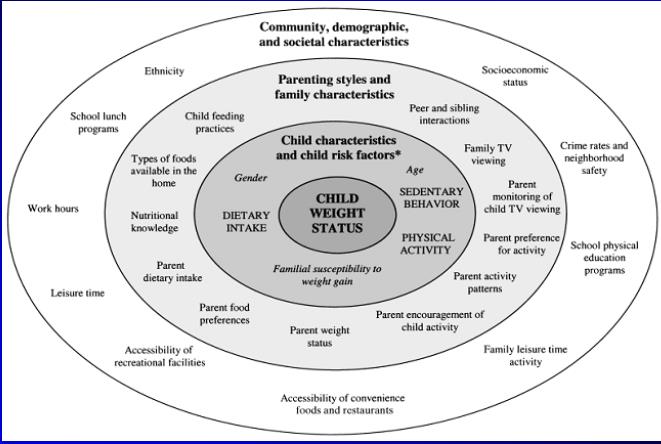
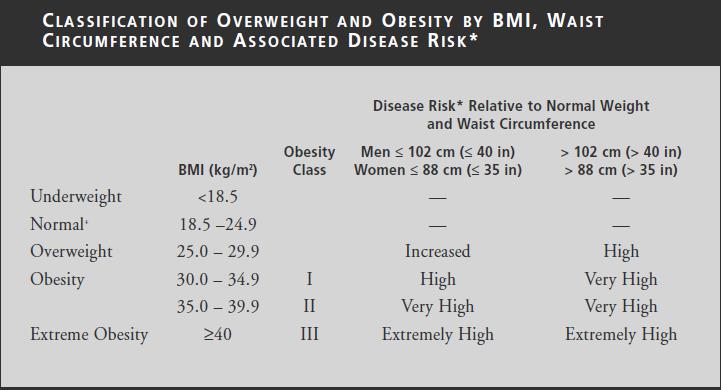
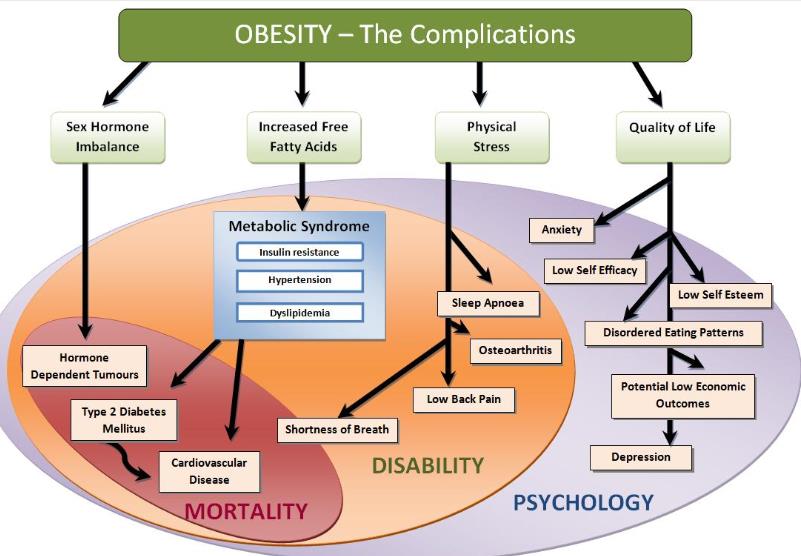
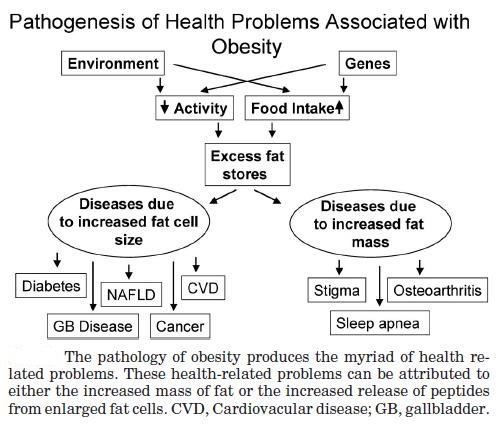
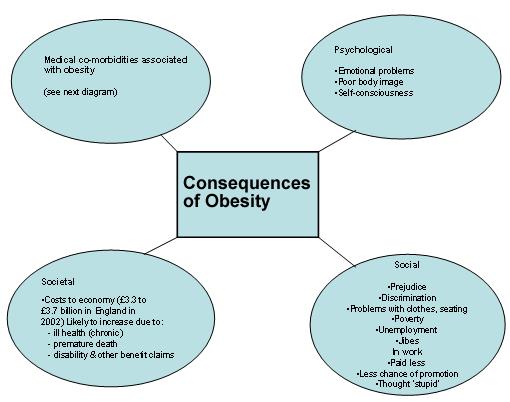
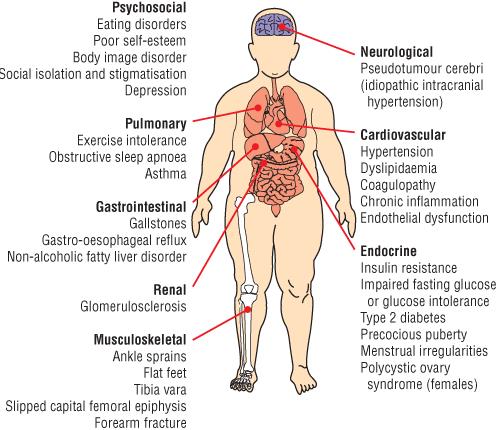

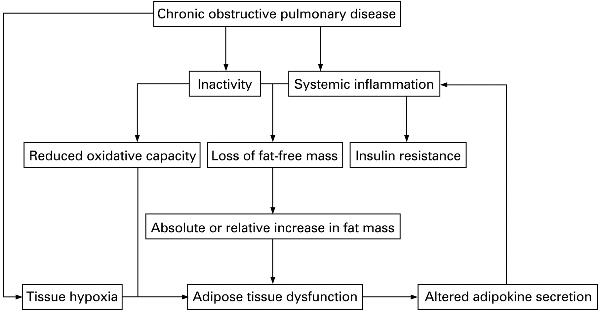
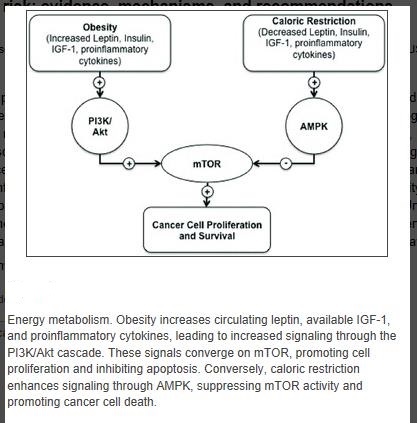
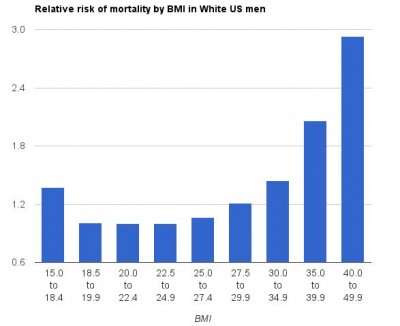
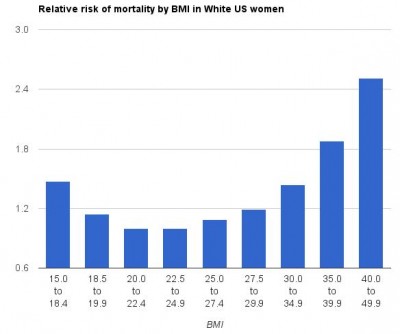
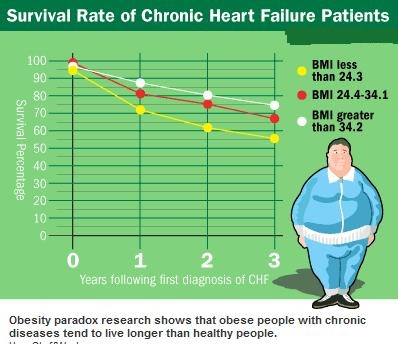
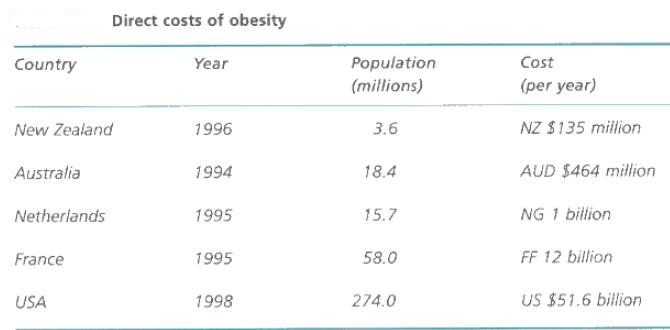
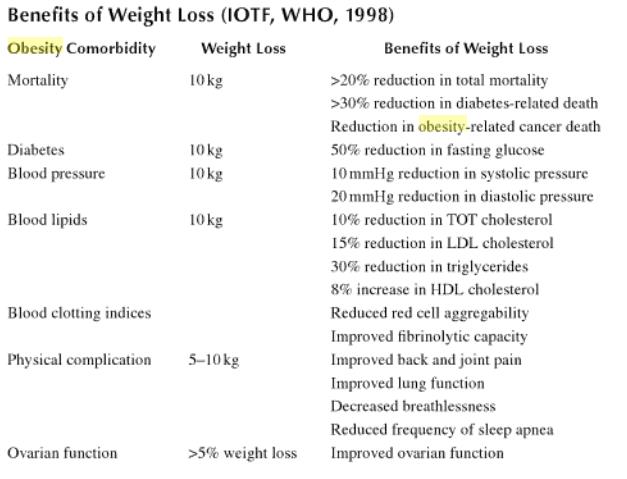

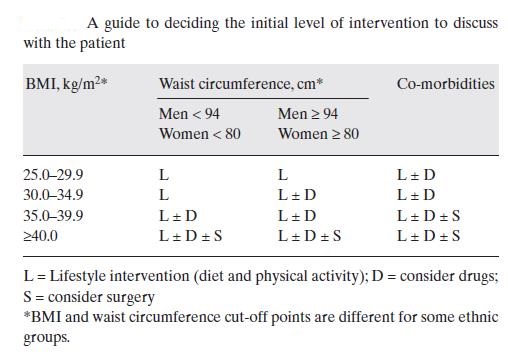
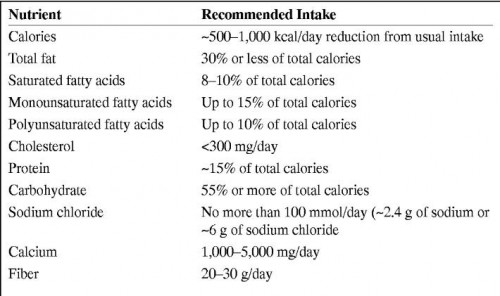
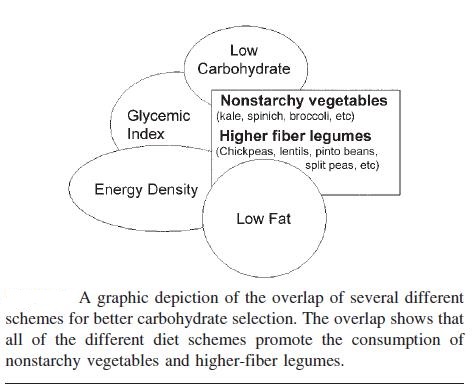
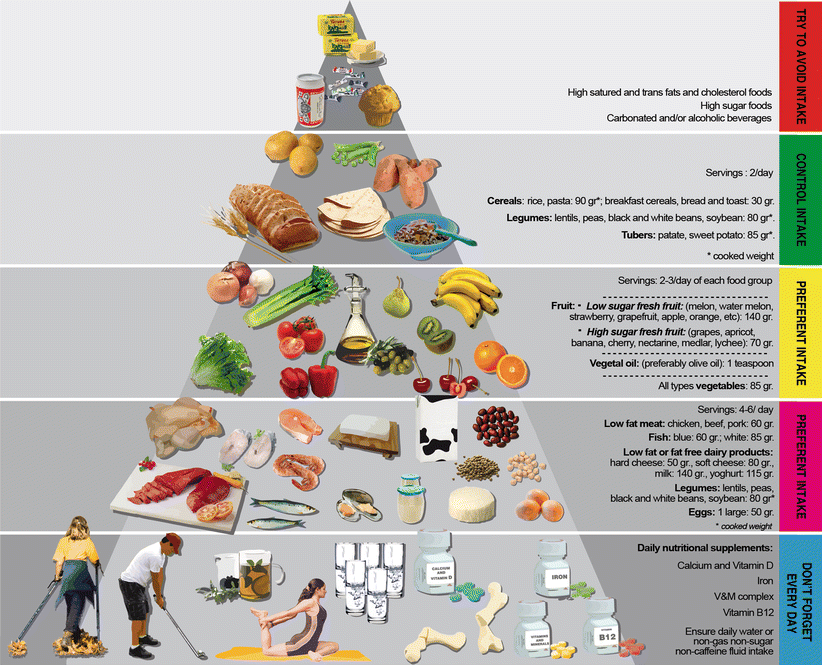

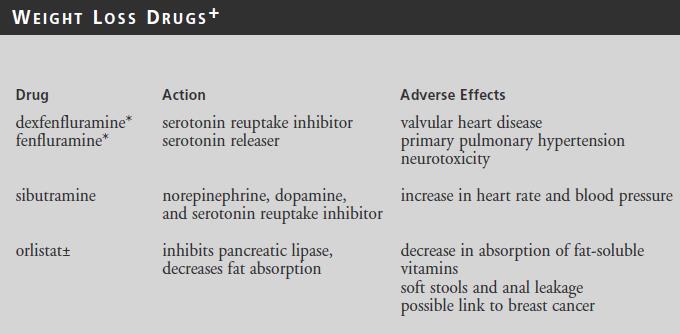

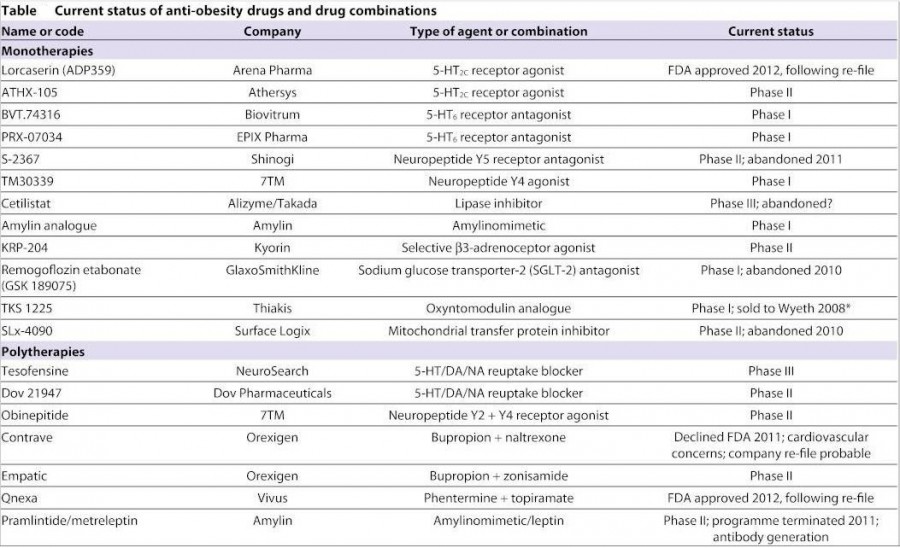
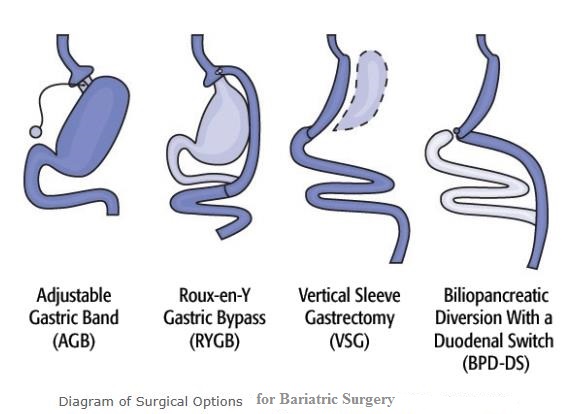
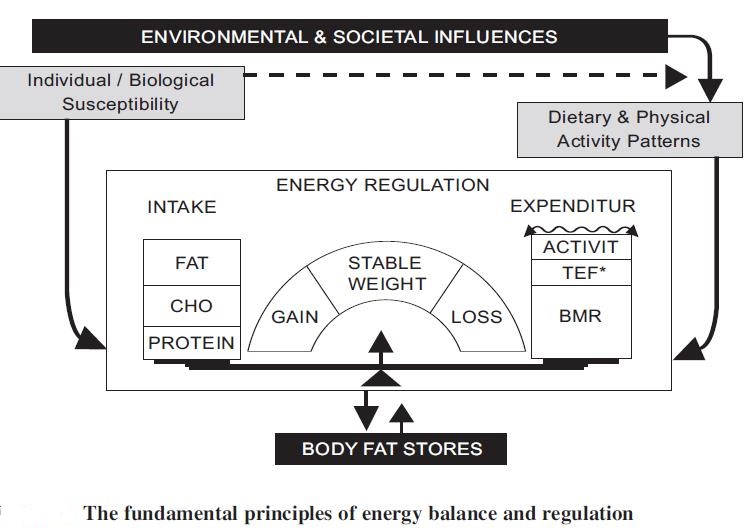
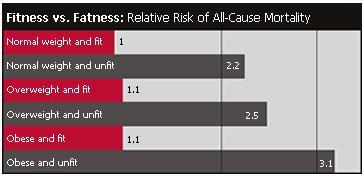
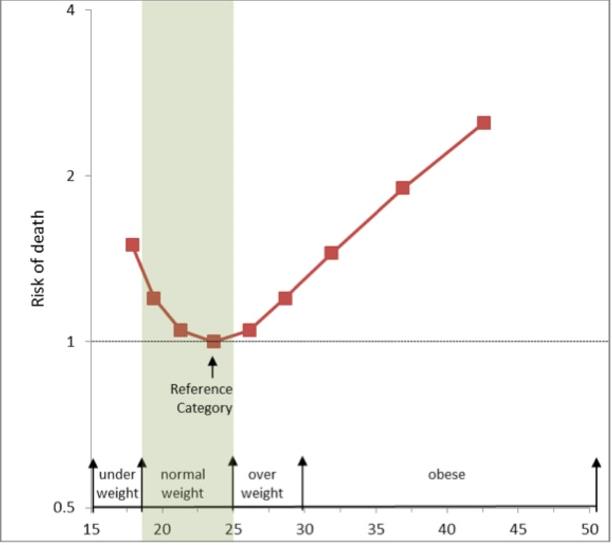
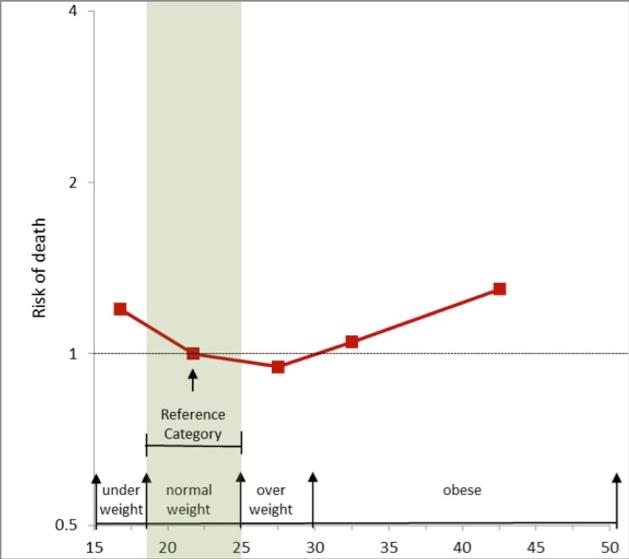
I appreciate you sharing this post.Thanks Again. Awesome.
Hey there this is somewhat of off topic but I was wondering if blogs use WYSIWYG editors or if you have to manually code with HTML. I’m starting a blog soon but have no coding know-how so I wanted to get advice from someone with experience. Any help would be greatly appreciated!
Very informative article post.Really thank you! Great.
I think this is a real great article.Really thank you! Keep writing.
Hi there,
We are just wondering if you would be interested in our Twitter growth service.
We have a network of over 3.4 million followers, and have worked in this industry since 2009.
If you would like more information and would like to see some of our previous work, just reply back and we can discuss further.
Kind Regards,
Lucy
Really informative post.Thanks Again. Much obliged.
I am going to go ahead and bookmark this article for my brother for a study project for class. This is a attractive web page by the way. Where do you pick up the design for this web page?
I love how you make it simple. Very helpful!
I’m not trying to argue.. but are you sure about this? It maybe kinda reactionary and I’m concerned for you :/
Great piece of content.
I actually like what you’ve acquired here, really like what you arestating and the way in which you say it.
I asked you to believe in impossible things. #TheOA needs your help. #SaveTheOA
Your article normally possess much of really up to date info. Where do you come up with this? Just declaring you are very resourceful. Thanks again
That is an extremely amazing powerful resource that you’re offering and you simply provide it away cost-free!! I that can match discovering websites that view the particular property value supplying you with a wonderful learning resource for zero cost. We truly dearly loved examining this page. Enjoy it!
You ought to take part in a contest for one of the greatest websites on the web. I most certainly will recommend this web site!|
I too think therefore, perfectly indited post!
Thanks for sharing your thoughts about pulsa. Regards