Dr Rajiv Desai
An Educational Blog
SELF MONITORING (MEASUREMENT) OF BLOOD GLUCOSE (SMBG)
_______
SELF MONITORING (MEASUREMENT) OF BLOOD GLUCOSE (SMBG):
_______
_______
Prologue:
Way back in 1991, on a Sunday afternoon, a young Parsi lady from Mumbai who was holidaying in a nearby village came to me with sudden breathlessness at my nursing home at Vapi, 160 km north of Mumbai. Clinical examination was normal except severe breathlessness. I suspected diabetic ketoacidosis and asked about history of diabetes. Patient and her relatives flatly denied any history of diabetes and told me that she was investigated in Mumbai for weakness recently and there is no diabetes. In those days, glucometer was not available in India. We used to blood sugar by Folin-Wu method. Being Sunday, laboratory was closed, so I could not do blood and urine sugar. I believed the story of relatives, ignored my gut feeling of diabetes, did ECG and X-ray chest which were normal, gave some primary care and sent patient back to Mumbai in their car. She got admitted in Mumbai same night and they repeated the same story of not having diabetes. However, blood tests in Mumbai revealed diabetic ketoacidosis and she died at Mumbai. Till today I have a guilty feeling of missing diabetes diagnosis. If I had glucometer available, I could have clinched diagnosis, could have given insulin and saved her life. I hope her relatives forgive me. Diabetes was originally identified by the presence of glucose in the urine. Indian physicians around 3500 years ago identified diabetes and classified it as madhumeha or honey urine noting that the urine would attract ants. In the 18th and 19th centuries the sweet taste of urine was used for diagnosis before chemical methods became available to detect sugars in the urine. Tests to measure glucose in the blood were developed over 100 years ago, and hyperglycemia subsequently became the sole criterion recommended for the diagnosis of diabetes. Self-monitoring of blood glucose (SMBG) was described as one of the most important advancements in diabetes management since the invention of insulin in 1920. Since approximately 1980, a primary goal of the management of type 1 and type 2 diabetes mellitus has been achieving closer-to-normal levels of glucose in the blood for as much of the time as possible, guided by SMBG several times a day. The benefits include a reduction in the occurrence rate and severity of long-term complications from hyperglycemia as well as a reduction in the short-term, potentially life-threatening complications of hypoglycemia. Unlike some other diseases that rely primarily on professional medical treatment, diabetes treatment requires active participation by the person who has it. Monitoring your blood glucose level on a regular basis and analyzing the results is believed by many to be a crucial part of the treatment equation. Worldwide, the glucose monitoring devices market is expected to be more than $16 billion by the end of this year. World Diabetes Day is celebrated every year on November 14 and this article will promote awareness about diabetes via SMBG.
________
Abbreviations and synonyms:
DM = diabetes mellitus = diabetes (and not diabetes insipidus)
T1DM = type 1 DM
T2DM = type 2 DM
BG = blood glucose
FPG = fasting plasma glucose (venous)
PPG = post-prandial plasma glucose (venous, 2 hours after meal)
FBS = fasting blood sugar (venous)
PPBS = post prandial blood sugar (venous, 2 hours after meal)
Pre-prandial = before meal
Post-prandial = after meal (usually 2 hour)
Fasting = no calorie intake for 8 hours
Random = anytime other than fasting (test taken from a non-fasting subject)
Glycated Hemoglobin = Hemoglobin A1c = HbA1c = Hb1c = HbA1c = A1c = A1C
SMBG = self monitoring (measurement) of blood glucose = capillary whole blood/plasma glucose
SMUG = self monitoring (measurement) of urine glucose
CGM = continuous glucose monitoring
Hyperglycemia = high blood glucose
Hypoglycemia = low blood glucose
When you ask FBS/PPBS from lab, they usually do FPG/PPG.
ADA = American Diabetes Association
IDF = International Diabetes Federation
__________
Terminology of Blood samples:
Blood is pumped around the body by the heart. The major vessels that take blood away from the heart are called arteries. The major vessels that take blood back to the heart are called veins. Between the two networks are many tiny blood vessels called capillaries. The composition of the blood in the three types of vessel varies slightly. When a blood sample is taken by the doctor or nurse, it is taken from a vein and called a venous sample. At the laboratory, the blood may be analysed as it is, in which case it is a ‘whole blood’ measurement. Often the clear liquid part of the blood may be separated from the red blood cells. This yields either serum or plasma (depending on whether or not the blood sample in the tube is treated with a special reagent called an anticoagulant). A serum or plasma measurement of glucose will give a result which is 10 – 15 % higher than a whole blood measurement. When a home blood glucose test is performed, blood is usually taken from a finger-prick sample which gives capillary whole blood glucose. Now there is a move towards all glucometers giving plasma-calibrated results so that readings made at home and at the laboratory can be more easily compared.
_
Sugar vs. glucose:
Sugar is the generalized name for sweet, short-chain, soluble carbohydrates, many of which are used in food. They are carbohydrates, composed of carbon, hydrogen, and oxygen. Simple sugars are called monosaccharides and include glucose (also known as dextrose), fructose and galactose. The table or granulated sugar most customarily used as food is sucrose, a disaccharide. (In the body, sucrose hydrolyses into fructose and glucose.) Other disaccharides include maltose and lactose. In the physiological context, the term sugar is a misnomer because it refers to glucose, yet other sugars besides glucose are always present. Food contains several different types [e.g., fructose (largely from fruits/table sugar/industrial sweeteners), galactose (milk and dairy products), as well as several food additives such as sorbitol, xylose, maltose, etc.]. But because these other sugars are largely inert with regard to the metabolic control system (i.e., that controlled by insulin secretion), and since glucose is the dominant controlling signal for metabolic regulation, the term has gained currency, and is used by medical staff and lay folk alike. In this article, sugar means glucose.
_
Units of glucose measurement in blood/plasma:
A blood glucose measurement will provide the concentration of glucose that is in your bloodstream; the result is given as the amount of glucose per unit volume (of whole blood/plasma/serum). The measurement unit used for indicating the concentration of blood or plasma glucose can either have a weight dimension (mg/dl) or a molarity (mmol/l). In some countries, they use millimoles to measure the amount, and take a ‘unit volume’ of blood to be one liter. ‘Millimoles per liter’ is written mmol/l. In the US, and some other countries, milligrams are used to measure the amount and a deciliter is taken as ‘unit volume’. ‘Milligrams per deciliter’ is written mg/dl. Deciliter means 100 ml. Since the molecular weight of glucose C6H12O6 is 180, for the measurement of glucose, the difference between the two scales is a factor of 18, so that 1 mmol/L of glucose is equivalent to 18 mg/dL. To convert from mmol/l to mg/dl, simply multiply the figure by a factor of 18.
________
Introduction to SMBG:
An important goal in the treatment of diabetes is to achieve and maintain blood glucose levels as close to normal as possible. That is why it is essential to train patients in how to effectively self-manage their diabetes, not only to improve their treatment but also to improve their quality of life. The development in the late 1970s of methods to self-monitor blood glucose levels was an indispensable prerequisite for this. Only through regular self-monitoring of blood glucose levels (SMBG) it has become possible to coordinate drug therapy as well as food intake and exercise so that a good metabolic control can be achieved. Furthermore, it has become easier to identify asymptomatic hypo- and hyperglycemias and blood glucose fluctuations.
_
Blood glucose monitoring is a way of testing the concentration of glucose in the blood (glycemia). Particularly important in the care of diabetes mellitus, a blood glucose test is performed by piercing the skin (typically, on the finger) to draw blood, then applying the blood to a chemically active disposable ‘test-strip’. Different manufacturers use different technology, but most systems measure an electrical characteristic, and use this to determine the glucose level in the blood. The test is usually referred to as capillary blood glucose. Blood glucose monitoring reveals individual patterns of blood glucose changes, and helps in the planning of meals, activities, and at what time of day to take medications. Also, testing allows for quick response to high blood sugar (hyperglycemia) or low blood sugar (hypoglycemia). This might include diet adjustments, exercise, and insulin (as instructed by the health care provider).
_
Self-monitoring of blood glucose (SMBG) has been accepted as an important instrument that empowers people with diabetes to achieve and maintain therapeutic goals. Nevertheless, it is underprescribed and underused by patients. On the other hand, determination of HbA1c is accepted as the gold standard for assessing glycemic control, but its limitations are not sufficiently appreciated. Patients with normal or near-normal HbA1c levels may still display postprandial hyperglycemia, putting them at risk for long-term adverse outcomes. In addition, frequent unrecognized hypoglycemia may lead to falsely low HbA1c levels, and HbA1c does not allow any estimate of glycemic variability. Determination of immediate blood glucose control is best assessed by SMBG because this provides timely information of hyperglycemia and hypoglycemia. Thus, SMBG is a prerequisite for implementing strategies to optimally treat, as well as to avoid, out-of-range glucose values. Healthcare professionals must be capable of making evidence-based clinical decisions in regard to the use of SMBG and balance issues, such as patient abilities, costs, and clinical outcomes. As a base of diabetes treatment, blood glucose monitoring contributes to clinically determining the level of carbohydrate metabolism, formulating therapeutic measures, evaluating effects, and realizing optimal blood glucose control. Intensive blood glucose monitoring and strict blood glucose control significantly eliminate or postpone occurrence or development of chronic diabetic complications. It is important to monitor accurate blood glucose concentrations which may obviously fluctuate from time to time due to various factors such as daily activity, mental status, diet component, environmental change. Blood glucose monitoring is also a necessary method adopted by many food nutrition experts to investigate the carbohydrate-induced glycemic reaction in addition to its clinic applications to diabetes patients.
______
Blood glucose basics:
Glucose is the most important carbohydrate fuel in the body. In the fed state, the majority of circulating glucose comes from the diet; in the fasting state, gluconeogenesis and glycogenolysis maintain glucose concentrations. Very little glucose is found in the diet as glucose; most is found in more complex carbohydrates that are broken down to monosaccharides though the digestive process. About half of the total carbohydrates in the diet are in the form of polysaccharides and the remainder as simpler sugars. About two-thirds of the sugar in the diet is sucrose, which is a disaccharide of glucose and fructose. Glucose is classified as a monosaccharide because it cannot be broken down further by hydrolysis. It is further classified as a hexose because of its six-carbon skeleton and as an aldose, because of the presence of an aldehyde group on carbon 1. The aldehyde group condenses with a hydroxyl group so that glucose exists as a hemiacetal ring structure. This ring structure explains many of the reactions of glucose. Ordinarily the concentration of glucose in the blood is maintained at a relatively stable concentration from 80 to 120 mg/dl. The strong reducing properties of glucose made it relatively easy to measure and thus the clinical estimation of circulating glucose was one of the earliest tests available to the clinician. The recent introduction of microglucose oxidase technology has now made it possible for the patient to measure his or her own blood glucose concentration and undoubtedly makes the estimation of blood glucose the most widely used test of blood chemistry.
_
Natural blood glucose regulation:
Glucose is a simple sugar which is a permanent and immediate primary source of energy to all of the cells in our body. Glucose C6H12O6 is a carbohydrate whose most important function is to act as a source of energy for the human body, by being the essential precursor in the synthesis of ATP (adenosine triphosphate). The energy stored in ATP can then be used to drive processes requiring energy, including biosynthesis, and locomotion or transportation of molecules across cell membranes. According to cellular requirements, glucose can also be used in the creation of proteins, glycogen, and lipids. The blood glucose concentration is very tightly regulated. Human body has two hormones released by pancreas that have opposite effects: insulin and glucagon. Insulin is produced by beta cells of the pancreas while glucagon is produced by alpha cells. The release of insulin is triggered when high levels of glucose are found in the bloodstream, and glucagon is released with low levels of glucose in the blood.
This blood glucose regulation process can be explained in the following steps:
1. After the glucose has been absorbed from the food eaten, it gets released in the bloodstream. High blood glucose levels triggers the pancreas to produce insulin. Insulin enables the muscle cells to take glucose as their source of energy and to form a type of molecule called glycogen that works as secondary energy storage in the case of low levels of glucose. In the liver cells, insulin instigates the conversion of glucose into glycogen and fat. In the fat cells of the adipose tissue, insulin also promotes the conversion of glucose into more fat and the uptake of glucose.
2. The pancreas will continue to release insulin and liver and fat cells continue to use glucose till the drop of concentration of glucose is below a threshold; in that case, glucagon will be released instead of insulin.
3. When glucagon reaches the liver cells, it initiates the conversion of glycogen into glucose, and fat into fatty acids, which many body cells can use as energy after the glucagon enables them to. The cells will continue to burn fat from the adipose tissue as an energy source, and follow with the protein of the muscles, until the levels of glucose increase again by the digestion of food, and that terminates the cycle.
_
Homeostasis of glucose:
_
Essentially blood glucose levels determine the time of secretion of these hormones. The glucose in blood is obtained from the food that you eat. This glucose gets absorbed by intestines and distributed to all of the cells in body through bloodstream and breaks it down for energy. Body tries to maintain a constant supply of glucose for your cells by maintaining a constant blood glucose concentration. The concentration of glucose in blood, expressed in mg/dl, is defined by the term glycemia.
_
Glucose metabolism in normal person:
_
The blood sugar concentration or blood glucose level is the amount of glucose (sugar) present in the blood of a human or animal. The body naturally tightly regulates blood glucose levels as a part of metabolic homeostasis. With some exceptions, glucose is the primary source of energy for the body’s cells, and blood lipids (in the form of fats and oils) are primarily a compact energy store. Glucose is transported from the intestines or liver to body cells via the bloodstream, and is made available for cell absorption via the hormone insulin, produced by the body primarily in the pancreas. Glucose levels are usually lowest in the morning, before the first meal of the day (termed “the fasting level”), and rise after meals for an hour or two by a few millimoles. Blood sugar levels outside the normal range may be an indicator of a medical condition. A persistently high level is referred to as hyperglycemia; low levels are referred to as hypoglycemia. Diabetes mellitus is characterized by persistent hyperglycemia from any of several causes, and is the most prominent disease related to failure of blood sugar regulation. Intake of alcohol causes an initial surge in blood sugar, and later tends to cause levels to fall. Also, certain drugs can increase or decrease glucose levels.
_
The figure below shows fluctuation of blood sugar (red) and the sugar-lowering hormone insulin (blue) in humans during the course of a day with three meals. One of the effects of a sugar-rich vs. a starch-rich meal is highlighted.
_
Normal values in humans:
Normal value ranges may vary slightly among different laboratories. Many factors affect a person’s blood sugar level. A body’s homeostatic mechanism, when operating normally, restores the blood sugar level to a narrow range of about 4.4 to 6.1 mmol/L (79.2 to 110 mg/dL) (as measured by a fasting blood glucose test). The normal blood glucose level (tested while fasting) for non-diabetics, should be between 70 and 100 milligrams per deciliter (mg/dL). The mean normal blood glucose level in humans is about 5.5 mM (5.5 mmol/L or 100 mg/dL; however, this level fluctuates throughout the day. Blood sugar levels for those without diabetes and who are not fasting should be below 125 mg/dL. Despite widely variable intervals between meals or the occasional consumption of meals with a substantial carbohydrate load, human blood glucose levels tend to remain within the normal range. However, shortly after eating, the blood glucose level may rise, in non-diabetics, temporarily up to 7.8 mmol/L (140 mg/dL) or slightly more. The actual amount of glucose in the blood and body fluids is very small. In a healthy adult male of 75 kg with a blood volume of 5 liters, a blood glucose level of 5.5 mmol/L (100 mg/dL) amounts to 5 grams, slightly less than two typical American restaurant sugar packets for coffee or tea. Part of the reason why this amount is so small is that, to maintain an influx of glucose into cells, enzymes modify glucose by adding phosphate or other groups to it.
________
Diabetes Mellitus:
Diabetes mellitus (DM) refers to a group of common metabolic disorders that share the phenotype of hyperglycemia. Several distinct types of DM are caused by a complex interaction of genetics and environmental factors. Depending on the etiology of the DM, factors contributing to hyperglycemia include reduced insulin secretion, decreased glucose utilization, and increased glucose production. The metabolic dysregulation associated with DM causes secondary pathophysiologic changes in multiple organ systems that impose a tremendous burden on the individual with diabetes and on the health care system.
_
Diagnosis of DM:
_
Classification of DM:
_
The figure above shows spectrum of glucose homeostasis and diabetes mellitus (DM). The spectrum from normal glucose tolerance to diabetes in type 1 DM, type 2 DM, other specific types of diabetes (type 3 DM), and gestational DM (type 4 DM) is shown from left to right. In most types of DM, the individual traverses from normal glucose tolerance to impaired glucose tolerance to overt diabetes (these should be viewed not as abrupt categories but as a spectrum). Arrows indicate that changes in glucose tolerance may be bidirectional in some types of diabetes. For example, individuals with type 2 DM may return to the impaired glucose tolerance category with weight loss; in gestational DM, diabetes may revert to impaired glucose tolerance or even normal glucose tolerance after delivery. The fasting plasma glucose (FPG), the 2-h plasma glucose (PG) after a glucose challenge, and the A1c for the different categories of glucose tolerance are shown at the lower part of the figure. These values do not apply to the diagnosis of gestational DM. The World Health Organization uses an FPG of 110–125 mg/dL for the prediabetes category. Some types of DM may or may not require insulin for survival. *Some use the term “increased risk for diabetes” (ADA) or “intermediate hyperglycemia” (WHO) rather than “prediabetes.”
_
DM is classified on the basis of the pathogenic process that leads to hyperglycemia, as opposed to earlier criteria such as age of onset or type of therapy. The two broad categories of DM are designated type 1 (T1DM) and type 2 (T2DM). Both types of diabetes are preceded by a phase of abnormal glucose homeostasis as the pathogenic processes progress. Type 1 DM is the result of complete or near-total insulin deficiency.
Glucose metabolism in T1DM:
_
Type 2 DM is a heterogeneous group of disorders characterized by variable degrees of insulin resistance, impaired insulin secretion, and increased glucose production. Distinct genetic and metabolic defects in insulin action and/or secretion give rise to the common phenotype of hyperglycemia in type 2 DM and have important potential therapeutic implications now that pharmacologic agents are available to target specific metabolic derangements. Type 2 DM is preceded by a period of abnormal glucose homeostasis classified as impaired fasting glucose (IFG) or impaired glucose tolerance (IGT).
Glucose metabolism in T2DM:
_
Two features of the current classification of DM diverge from previous classifications. First, the terms insulin-dependent diabetes mellitus (IDDM) and non-insulin-dependent diabetes mellitus (NIDDM) are obsolete. Since many individuals with type 2 DM eventually require insulin treatment for control of glycemia, the use of the term NIDDM generated considerable confusion. A second difference is that age is not a criterion in the classification system. Although type 1 DM most commonly develops before the age of 30, an autoimmune beta cell destructive process can develop at any age. It is estimated that between 5 and 10% of individuals who develop DM after age 30 years have type 1 DM. Although type 2 DM more typically develops with increasing age, it is now being diagnosed more frequently in children and young adults, particularly in obese adolescents.
_
Comparison of variation of plasma glucose between non-diabetic and diabetic individuals:
_
Other Types of DM (type 3 DM):
Other etiologies for DM include specific genetic defects in insulin secretion or action, metabolic abnormalities that impair insulin secretion, mitochondrial abnormalities, and a host of conditions that impair glucose tolerance . Maturity-onset diabetes of the young (MODY) is a subtype of DM characterized by autosomal dominant inheritance, early onset of hyperglycemia (usually <25 years), and impairment in insulin secretion. Mutations in the insulin receptor cause a group of rare disorders characterized by severe insulin resistance. DM can result from pancreatic exocrine disease when the majority of pancreatic islets are destroyed. Cystic fibrosis-related DM is an important consideration in this patient population. Hormones that antagonize insulin action can also lead to DM. Thus, DM is often a feature of endocrinopathies such as acromegaly and Cushing’s disease. Viral infections have been implicated in pancreatic islet destruction but are an extremely rare cause of DM. A form of acute onset of type 1 diabetes, termed fulminant diabetes, has been noted in Japan and may be related to viral infection of islets.
_
Gestational Diabetes Mellitus (GDM) (type 4 DM):
Glucose intolerance developing during pregnancy is classified as gestational diabetes. Insulin resistance is related to the metabolic changes of late pregnancy, and the increased insulin requirements may lead to IGT or diabetes. GDM occurs in 7% (range 2–10%) of pregnancies in the United States; most women revert to normal glucose tolerance postpartum but have a substantial risk (35–60%) of developing DM in the next 10–20 years. The International Diabetes and Pregnancy Study Groups now recommend that diabetes diagnosed at the initial prenatal visit should be classified as “overt” diabetes rather than gestational diabetes.
_
Note:
This article is written on SMBG and not DM and therefore detailed discussion on DM is inappropriate.
_
Hyperglycemia vs. DM:
For diagnosis of DM, persistent hyperglycemia is must but occasionally you may have transient hyperglycemia without DM. Stress hyperglycemia is a medical term referring to transient elevation of the blood glucose due to the stress of illness. Transient hyperglycemia occurs as a part of stress response in acute illnesses and is brought about by elevated levels of counter regulatory hormones. It usually resolves spontaneously. Stress hyperglycemia is especially common in patients with hypertonic dehydration and those with elevated catecholamine levels (e.g., after emergency department treatment of acute asthma with epinephrine). Steroid diabetes is a specific and prolonged form of stress hyperglycemia. In some people, stress hyperglycemia may indicate a reduced insulin secretory capacity or a reduced sensitivity, and is sometimes the first clue to incipient diabetes. Because of this, it is occasionally appropriate to perform diabetes screening tests after recovery from an illness in which significant stress hyperglycemia occurred. Even fear of needles or pain during blood collection may provoke transient hyperglycemia. Blood glucose is also amplified by drugs or intravenous glucose.
_
Why do diabetics develop complications?
Chronic elevation of blood glucose level leads to damage of blood vessels (angiopathy). The endothelial cells lining the blood vessels take in more glucose than normal, since they do not depend on insulin. They then form more surface glycoproteins than normal, and cause the basement membrane to grow thicker and weaker. In diabetes, the resulting problems are grouped under “microvascular disease” (due to damage to small blood vessels) and “macrovascular disease” (due to damage to the arteries). The risk of chronic complications increases as a function of the duration and degree of hyperglycemia; they usually do not become apparent until the second decade of hyperglycemia. Since type 2 DM often has a long asymptomatic period of hyperglycemia, many individuals with type 2 DM have complications at the time of diagnosis. The microvascular complications of both type 1 and type 2 DM result from chronic hyperglycemia. Large, randomized clinical trials of individuals with type 1 or type 2 DM have conclusively demonstrated that a reduction in chronic hyperglycemia prevents or delays retinopathy, neuropathy, and nephropathy. Other incompletely defined factors may modulate the development of complications. For example, despite long-standing DM, some individuals never develop nephropathy or retinopathy. The fact that 40% of diabetics who carefully control their blood sugar nevertheless develop neuropathy, and that some of those with good blood sugar control still develop nephropathy, requires explanation. Many of these patients have glycemic control that is indistinguishable from those who develop microvascular complications, suggesting that there is a genetic susceptibility for developing particular complications. The familial clustering of the degree and type of diabetic complications indicates that genetics may also play a role in causing complications such as diabetic retinopathy and nephropathy. Non-diabetic offspring of type 2 diabetics have been found to have increased arterial stiffness and neuropathy despite normal blood glucose levels, and elevated enzyme levels associated with diabetic renal disease have been found in non-diabetic first-degree relatives of diabetics. Evidence implicating a causative role for chronic hyperglycemia in the development of macrovascular complications is less conclusive. However, coronary heart disease events and mortality rate are two to four times greater in patients with type 2 DM. These events correlate with fasting and postprandial plasma glucose levels as well as with the A1c. Other factors (dyslipidemia and hypertension) also play important roles in macrovascular complications.
Mechanisms of Complications:
Although chronic hyperglycemia is an important etiologic factor leading to complications of DM, the mechanism(s) by which it leads to such diverse cellular and organ dysfunction is unknown. At least four prominent theories, which are not mutually exclusive, have been proposed to explain how hyperglycemia might lead to the chronic complications of DM. An emerging hypothesis is that hyperglycemia leads to epigenetic changes in the affected cells. One theory is that increased intracellular glucose leads to the formation of advanced glycosylation end products (AGEs), which bind to a cell surface receptor, via the nonenzymatic glycosylation of intra- and extracellular proteins. Nonenzymatic glycosylation results from the interaction of glucose with amino groups on proteins. AGEs have been shown to cross-link proteins (e.g., collagen, extracellular matrix proteins), accelerate atherosclerosis, promote glomerular dysfunction, reduce nitric oxide synthesis, induce endothelial dysfunction, and alter extracellular matrix composition and structure. The serum level of AGEs correlates with the level of glycemia, and these products accumulate as the glomerular filtration rate (GFR) declines. A second theory is based on the observation that hyperglycemia increases glucose metabolism via the sorbitol pathway. Intracellular glucose is predominantly metabolized by phosphorylation and subsequent glycolysis, but when increased, some glucose is converted to sorbitol by the enzyme aldose reductase. Increased sorbitol concentration alters redox potential, increases cellular osmolality, generates reactive oxygen species, and likely leads to other types of cellular dysfunction. However, testing of this theory in humans, using aldose reductase inhibitors, has not demonstrated significant beneficial effects on clinical endpoints of retinopathy, neuropathy, or nephropathy. A third theory proposes that hyperglycemia increases the formation of diacylglycerol leading to activation of protein kinase C (PKC). Among other actions, PKC alters the transcription of genes for fibronectin, type IV collagen, contractile proteins, and extracellular matrix proteins in endothelial cells and neurons. Inhibitors of PKC are being studied in clinical trials. A fourth theory proposes that hyperglycemia increases the flux through the hexosamine pathway, which generates fructose-6-phosphate, a substrate for O-linked glycosylation and proteoglycan production. The hexosamine pathway may alter function by glycosylation of proteins such as endothelial nitric oxide synthase or by changes in gene expression of transforming growth factor (TGF-) or plasminogen activator inhibitor-1 (PAI-1). Growth factors appear to play an important role in some DM-related complications, and their production is increased by most of these proposed pathways. Vascular endothelial growth factor A (VEGF-A) is increased locally in diabetic proliferative retinopathy and decreases after laser photocoagulation. TGF- is increased in diabetic nephropathy and stimulates basement membrane production of collagen and fibronectin by mesangial cells. Other growth factors, such as platelet-derived growth factor, epidermal growth factor, insulin-like growth factor I, growth hormone, basic fibroblast growth factor, and even insulin, have been suggested to play a role in DM-related complications. A possible unifying mechanism is that hyperglycemia leads to increased production of reactive oxygen species or superoxide in the mitochondria; these compounds may activate all four of the pathways described above. Although hyperglycemia serves as the initial trigger for complications of diabetes, it is still unknown whether the same pathophysiologic processes are operative in all complications or whether some pathways predominate in certain organs.
___
Blood glucose tests:
- fasting blood sugar (i.e., glucose) test (FBS)—it means fasting plasma glucose (FPG)
- two-hr postprandial blood sugar test (2-h PPBS)—it means postprandial plasma glucose (PPG)
- oral glucose tolerance test (OGTT)
- intravenous glucose tolerance test (IVGTT)
- glycated hemoglobin (HbA1C)
- self-monitoring of blood glucose (SMBG) level via patient testing
- Random blood sugar (RBS)
- Average blood glucose (eAG = estimated average glucose) may be estimated by measuring HbA1c
_
What are the Target Ranges?
Blood glucose targets are individualized based on:
- duration of diabetes
- age/life expectancy
- comorbid conditions
- known CVD or advanced microvascular complications
- hypoglycemia unawareness
- individual patient considerations.
____
_____
Blood glucose profile:
No matter how mild your diabetes may be, it is very unlikely that any physician can tell you how to normalize your blood sugars throughout the day without knowing what your blood glucose values are around the clock. Don’t believe anyone who tells you otherwise. The only way to know what your around the clock levels are is to monitor them yourself. A table of blood sugar levels, with associated events (meals, exercise, and so on), measured at least 4 times daily over a number of days, is the key element in what is called a blood glucose profile. This profile gives you and your physician or diabetes educator a glimpse of how your medication, lifestyle, and diet converge, and how they affect your blood sugars. Without this information, it is impossible to come up with a treatment plan that will normalize blood sugars. If your treatment includes insulin injections before each meal, your diabetes is probably severe enough to render it impossible for your body to automatically correct small deviations from a target blood glucose range. To achieve blood sugar normalization, it therefore may be necessary for you to record blood glucose profiles every day for the rest of your life, so that you can fine tune any out of range values. If you are not treated with insulin, or if you have a very mild form of insulin treated diabetes, it may only be necessary to prepare blood glucose profiles when needed for readjustment of your diet or medication. Typically, this might be for one to two weeks prior to every routine follow up visit to your physician, and for a few weeks while your treatment plan is being fine tuned for the first time. After all, your physician or diabetes educator cannot tell if a new regimen is working properly without seeing your blood glucose profiles. It is wise, however, that you also do a blood glucose profile for 1 day at least every other week, so you will be assured that things are continuing as planned.
_______
Hypo and hyperglycemia:
Levels which are significantly above or below normal range are problematic and can in some cases be dangerous. A level of <3.8 mmol/L (<70 mg/dL) is usually described as a hypoglycemic attack (low blood sugar). Most diabetics know when they are going to “go hypo” and usually are able to eat some food or drink something sweet to raise levels. A patient who is hyperglycemic (high blood glucose) can also become temporarily hypoglycemic, under certain conditions (e.g. not eating regularly, or after strenuous exercise, followed by fatigue). Intensive efforts to achieve blood sugar levels close to normal have been shown to triple the risk of the most severe form of hypoglycemia, in which the patient requires assistance from by-standers in order to treat the episode. There were annually 48,500 hospitalizations for diabetic hypoglycemia and 13,100 for diabetic hypoglycemia resulting in coma in the period 1989 to 1991 in the U.S., before intensive blood sugar control was as widely recommended as today. One study found that hospital admissions for diabetic hypoglycemia increased by 50% from 1990-1993 to 1997-2000, as strict blood sugar control efforts became more common. Among intensively controlled type 1 diabetics, 55% of episodes of severe hypoglycemia occur during sleep, and 6% of all deaths in diabetics under the age of 40 are from nocturnal hypoglycemia in the so-called ‘dead-in-bed syndrome,’ while National Institute of Health statistics show that 2% to 4% of all deaths in diabetics are from hypoglycemia. In children and adolescents following intensive blood sugar control, 21% of hypoglycemic episodes occurred without explanation. In addition to the deaths caused by diabetic hypoglycemia, periods of severe low blood sugar can also cause permanent brain damage. Interestingly, although diabetic nerve disease is usually associated with hyperglycemia, hypoglycemia as well can initiate or worsen neuropathy in diabetics intensively struggling to reduce their hyperglycemia. Levels greater than 13-15 mmol/L (230–270 mg/dL) are considered high, and should be monitored closely to ensure that they reduce rather than continue to remain high. The patient is advised to seek urgent medical attention as soon as possible if blood sugar levels continue to rise after 2-3 tests. High blood sugar levels are known as hyperglycemia, which is not as easy to detect as hypoglycemia and usually happens over a period of days rather than hours or minutes. If left untreated, this can result in diabetic coma and death. Prolonged and elevated levels of glucose in the blood, which is left unchecked and untreated, will, over time, result in serious diabetic complications in those susceptible and sometimes even death. There is currently no way of testing for susceptibility to complications. Diabetics are therefore recommended to check their blood sugar levels either daily or every few days. There is also diabetes management software available from blood testing manufacturers which can display results and trends over time. Type 1 diabetics normally check more often, due to insulin therapy. A history of blood sugar level results is especially useful for the diabetic to present to their doctor or physician in the monitoring and control of the disease. Failure to maintain a strict regimen of testing can accelerate symptoms of the condition, and it is therefore imperative that any diabetic patient strictly monitor their glucose levels regularly.
_
Hypoglycemia is most commonly caused by drugs used to treat diabetes mellitus or by exposure to other drugs, including alcohol. However, a number of other disorders, including critical organ failure, sepsis and inanition, hormone deficiencies, non–beta-cell tumors, insulinoma, and prior gastric surgery, may cause hypoglycemia. Hypoglycemia is most convincingly documented by Whipple’s triad: (1) symptoms consistent with hypoglycemia, (2) a low plasma glucose concentration measured with a precise method, and (3) relief of those symptoms after the plasma glucose level is raised. The lower limit of the fasting plasma glucose concentration is normally approximately 70 mg/dL (3.9 mmol/L), but substantially lower venous glucose levels occur normally, late after a meal. Glucose levels <55 mg/dL (3.0 mmol/L) with symptoms that are relieved promptly after the glucose level is raised document hypoglycemia. Hypoglycemia can cause serious morbidity; if severe and prolonged, it can be fatal. It should be considered in any patient with episodes of confusion, an altered level of consciousness, or a seizure.
______
Glycemic control:
Glycemic control is a medical term referring to the typical levels of blood sugar (glucose) in a person with diabetes mellitus. Much evidence suggests that many of the long-term complications of diabetes, especially the microvascular complications, result from many years of hyperglycemia (elevated levels of glucose in the blood). Good glycemic control, in the sense of a “target” for treatment, has become an important goal of diabetes care, although recent research suggests that the complications of diabetes may be caused by genetic factors or, in type 1 diabetics, by the continuing effects of the autoimmune disease which first caused the pancreas to lose its insulin-producing ability. Because blood sugar levels fluctuate throughout the day and glucose records are imperfect indicators of these changes, the percentage of hemoglobin which is glycosylated is used as a proxy measure of long-term glycemic control in research trials and clinical care of people with diabetes. In nondiabetic persons with normal glucose metabolism the glycosylated hemoglobin is usually 4-6% by the most common methods (normal ranges may vary by method). Measurement of glycated hemoglobin is the standard method for assessing long-term glycemic control. When plasma glucose is consistently elevated, there is an increase in nonenzymatic glycation of hemoglobin; this alteration reflects the glycemic history over the previous 2–3 months, since erythrocytes have an average life span of 120 days (glycemic level in the preceding month contributes about 50% to the A1C value). In patients achieving their glycemic goal, the ADA recommends measurement of the A1C at least twice per year. More frequent testing (every 3 months) is warranted when glycemic control is inadequate or when therapy has changed. The degree of glycation of other proteins, such as albumin, can be used as an alternative indicator of glycemic control when the A1C is inaccurate (hemolytic anemia, hemoglobinopathies). The fructosamine assay (measuring glycated albumin) reflects the glycemic status over the prior 2 weeks. Alternative assays of glycemic control should not be routinely used since studies demonstrating that it accurately predicts the complications of DM are lacking. “Perfect glycemic control” would mean that glucose levels were always normal (70–130 mg/dl, or 3.9-7.2 mmol/L) and indistinguishable from a person without diabetes. In reality, because of the imperfections of treatment measures, even “good glycemic control” describes blood glucose levels that average somewhat higher than normal much of the time. In addition, one survey of type 2 diabetics found that they rated the harm to their quality of life from intensive interventions to control their blood sugar to be just as severe as the harm resulting from intermediate levels of diabetic complications. Accepted “target levels” of glucose and glycosylated hemoglobin that are considered good control have been lowered over the last 25 years, because of improvements in the tools of diabetes care, because of increasing evidence of the value of glycemic control in avoiding complications, and by the expectations of both patients and physicians. What is considered “good control” also varies by age and susceptibility of the patient to hypoglycemia. In the 1990s the American Diabetes Association conducted a publicity campaign to persuade patients and physicians to strive for average glucose and hemoglobin A1c values below 200 mg/dl (11 mmol/l) and 8%. Currently many patients and physicians attempt to do better than that. Poor glycemic control refers to persistently elevated blood glucose and glycosylated hemoglobin levels, which may range from 200–500 mg/dl (11-28 mmol/L) and 9-15% or higher over months and years before severe complications occur. Meta-analysis of large studies done on the effects of tight vs. conventional, or more relaxed, glycemic control in type 2 diabetics have failed to demonstrate a difference in all-cause cardiovascular death, non-fatal stroke, or limb amputation, but decreased the risk of nonfatal heart attack by 15%. Additionally, tight glucose control decreased the risk of progression of retinopathy and nephropathy, and decreased the incidence peripheral neuropathy, but increased the risk of hypoglycemia 2.4 times.
__
_______
DM prevalence, awareness, morbidity and mortality:
_
In 2006, the General Assembly of the United Nations unanimously adopted a resolution (61/225) which recognizes that diabetes is a global pandemic posing a serious threat to global health, acknowledging it to be a chronic, debilitating, and costly disease associated with major complications. Diabetes reduces the quality of life, can generate multi-system morbidities and premature death, and consequently increases healthcare costs. Currently, in many countries, people with diabetes have a significantly decreased life expectancy.
_
The figure below shows rising worldwide DM prevalence:
_
In our modern world, diabetes prevalence is on the rise. In 2010, statistics showed that over 25 million people in the United States, including children, have diabetes mellitus. Of that population, seven million people who have diabetes are undiagnosed. In addition, diabetes prevalence increases with age. Between 2005 and 2008, statistics showed 26.9% of people with type 1 or type 2 diabetes mellitus were over the age of 65 years, while 17.4% comprised those between 20 and 64 years of age. At this rate, the number of people diagnosed with diabetes in the world is expected to increase by 114% from the year 2000 to 2030. As a result, effective diabetes management will continue to be an important consideration for patients and is key to reducing the risk of complications such as heart disease, blindness, renal disease, and unnecessary amputations.
_
WHO on diabetes 2013:
Key facts:
1. 347 million people worldwide have diabetes.
2. In 2004, an estimated 3.4 million people died from consequences of high fasting blood sugar.
3. More than 80% of diabetes deaths occur in low- and middle-income countries.
4. WHO projects that diabetes will be the 7th leading cause of death in 2030.
5. Healthy diet, regular physical activity, maintaining a normal body weight and avoiding tobacco use can prevent or delay the onset of type 2 diabetes.
_
World health statistics 2012 reports data on people with raised blood glucose levels. One in 10 adults has diabetes. While the global average prevalence is around 10%, up to one third of populations in some Pacific Island countries have this condition. Left untreated, diabetes can lead to cardiovascular disease, blindness and kidney failure. Already, diabetes extracts a high cost in health care dollars, economies’ financial stability, lost productivity, and it destroys lives and families.
_
The International Diabetes Federation (IDF) — the umbrella organization for 200 diabetes associations in more than 160 countries — just released its 2013 Diabetes Atlas. It cites current statistics and the rise of diabetes worldwide. If you’ve been following the trend in diabetes, it will not surprise you to know diabetes continues to rise, unabated, around the world. Type 2 diabetes, which many consider an epidemic currently, is increasing worldwide predominantly due to poor diet, sedentary lifestyle and the fact that we are living longer. The research, published by the American Heart Association’s journal Circulation, found that eating fast food two or more times a week increases the risk of developing Type 2 diabetes by 27 percent.
_
New wealth and development in the Middle East has already led to one in 10 adults having the disease. The greatest number of people with diabetes worldwide is between the ages of 40 and 59. Every six seconds someone dies from diabetes. Diabetes imposes unacceptably high human, social and economic costs on countries at all income levels. In Africa, three quarters of diabetes deaths are in people under 60 years old, handicapping Africa’s ability for development. In 2013, the world spent $548 billion (US) on diabetes health care — 11 percent of the total spent for health care worldwide. 175 million people are currently undiagnosed and progressing toward complications unaware. The number of people with diabetes globally will increase by 55 percent by 2035.
_
Diabetic death rates:
Diabetes causes 4.6 million deaths and costs over 465 billion US dollars in global healthcare expenditure every year. Diabetes is already the world’s most costly epidemic. By 2020, in countries such as the US, Malaysia and Indonesia over 10% of the population will be diabetic and there will be over 300m diabetics worldwide. Up to 5% of GDP and over 25% of many public healthcare budgets globally will be typically being spent on dealing with the consequences of diabetes.
_
Diabetic awareness:
46 % of diabetics are unaware that they have diabetes.
_
The incidence of both type 1 and type 2 diabetes mellitus is increasing; the former has been attributed to an increase in environmental factors, whereas the latter is strongly associated with increasing rates of obesity. Alarmingly, during the past 10 years, type 2 diabetes has been diagnosed more frequently in patients younger than 44 years. In this context, physicians face a dual challenge: not only are there more patients with diabetes, but also the disease is being increasingly diagnosed in younger patients who will require lifelong management. Adding to this burden is the increasing complexity of caring for patients with type 1 diabetes and the expanding armamentarium of medications for patients with type 2 diabetes. The chronic hyperglycemia of diabetes is associated with both micro- and macrovascular complications, which result in significant increases in morbidity and mortality. Improving glycemic control in diabetic patients has been shown to reduce these complications. The main goal of treatment is to keep blood sugar levels in the normal or near-normal range. Checking one’s blood sugar is one of the best ways to know how well the diabetes treatment plan is working.
_
Diabetes mellitus is a condition characterized biochemically by increased blood glucose concentrations and associated with both small blood vessel complications in the eyes (retinopathy), kidneys (nephropathy), and peripheral nerves (neuropathy) and large blood vessel complications of the heart (causing heart attacks), head and neck (causing strokes), and legs (leading to gangrene and amputations). Diabetic retinopathy is the leading cause of blindness in industrialized countries in people between the ages of 20 and 74 years. Diabetic nephropathy is the leading cause of people requiring dialysis for kidney failure. Diabetic neuropathy underlies most cases of lower extremity amputations, much more so than the large vessel complication in the legs. There is overwhelming evidence that keeping blood glucose near normal will have a marked beneficial effect of limiting (and possibly preventing) the small vessel complications. Although one recent article showed that lowering blood glucose concentrations in type 1 diabetic patients had a beneficial effect on coronary artery disease (CAD) many years later, five previous articles in type 2 diabetic patients did not.
___
What is the DCCT?
The Diabetes Control and Complications Trial (DCCT) was a major clinical study conducted from 1983 to 1993 and funded by the National Institute of Diabetes and Digestive and Kidney Diseases. The study showed that keeping blood glucose levels as close to normal as possible slows the onset and progression of the eye, kidney, and nerve damage caused by diabetes. In fact, it demonstrated that any sustained lowering of blood glucose, also called blood sugar, helps, even if the person has a history of poor control. The DCCT involved 1,441 volunteers, ages 13 to 39, with type 1 diabetes and 29 medical centers in the United States and Canada. Volunteers had to have had diabetes for at least 1 year but no longer than 15 years. They also were required to have no, or only early signs of, diabetic eye disease. The study compared the effects of standard control of blood glucose versus intensive control on the complications of diabetes. Intensive control meant keeping hemoglobin A1C levels as close as possible to the normal value of 6 percent or less. The A1C blood test reflects a person’s average blood glucose over the last 2 to 3 months. Volunteers were randomly assigned to each treatment group.
What is the EDIC?
When the DCCT ended in 1993, researchers continued to study more than 90 percent of participants. The follow-up study, called Epidemiology of Diabetes Interventions and Complications (EDIC), is assessing the incidence and predictors of cardiovascular disease events such as heart attack, stroke, or needed heart surgery, as well as diabetic complications related to the eye, kidney, and nerves. The EDIC study is also examining the impact of intensive control versus standard control on quality of life. Another objective is to look at the cost-effectiveness of intensive control.
_
DCCT Study Findings:
Intensive blood glucose control reduces risk of
- eye disease
76% reduced risk - kidney disease
50% reduced risk - nerve disease
60% reduced risk
EDIC Study Findings:
Intensive blood glucose control reduces risk of
- any cardiovascular disease event
42% reduced risk - nonfatal heart attack, stroke, or death from cardiovascular causes
57% reduced risk
_
Large, long-term, randomized controlled trials in both type 1 diabetes (T1DM) and T2DM have shown that aggressive treatment of hyperglycemia significantly reduces the development and progression of microvascular complications. A weaker relationship is observed in most studies between hyperglycemia and the development/ progression of macrovascular disease. However, in a systematic review with meta-analysis including 6 randomized controlled trials involving 27,654 patients, tight blood glucose control reduces the risk for some macrovascular and microvascular events, without effect on all-cause mortality and cardiovascular mortality. Recent RCTs have not shown a benefit of tight glucose control on macrovascular disease in people with T2DM of long duration and high cardiovascular risk. In the earlier studies, the benefits of tight control on macrovascular outcomes were seen only many years after the initial trial had ended and when levels of glycemic control in the intervention and control arms had converged. This so called ‘metabolic memory’ or ‘legacy effect’ suggests that, while the short-term benefits of tight glycemic control for macrovascular disease have not been shown in RCTs, the longer-term benefits may be substantive particularly when good HbA1c levels are achieved and maintained early in the course of the disease. The longer-term findings suggest that greater benefits (clinical and economic) are obtained when simultaneous control of glycemia, blood pressure and lipid levels has been achieved. Diabetes is a significant and growing worldwide concern with potentially devastating consequences. Numerous studies have demonstrated that optimal management of glycemia and other cardiovascular risk factors can reduce the risk of development and progression of both microvascular and macrovascular complications.
_
Proper glycemic control, including self-monitoring of blood glucose (SMBG) is key to managing diabetes. It has been shown that microvascular complications, such as neuropathy, nephropathy, and retinopathy, are reduced 40% for every percentage reduction in hemoglobin A1c values. Furthermore, a survey of 1,895 diabetic patients suggested that decreased blood glucose monitoring compliance was observed in patients who had more than two hospitalizations in a 2-year period. Yet, despite current evidence of the importance of daily SMBG, many patients who have diabetes do not regularly check their blood glucose at home. For example, up to 67% of patients do not check their blood glucose regularly for reasons such as sore fingers, inconvenience, and the fear of needles. Hence, some patients choose to avoid these unpleasant aspects by simply not checking blood sugars on a regular basis, especially since hyperglycemia is often asymptomatic in the early stages of diabetes mellitus. In addition to the difficulties posed by SMBG, maintaining proper glycemic control can be a challenge, especially for patients who are on insulin therapy. Patients who use short-acting insulin to help control blood glucose during a meal must constantly estimate their insulin doses by counting the carbohydrate content of the meal. Since most of us do not eat the same meal every day for breakfast, lunch, and dinner, counting carbohydrates can become a cumbersome process. Furthermore, improperly estimating an insulin dose can potentially result in undertreatment or overtreatment, which may have grave consequences. Based on discharge data of Californian hospitals, hypoglycemia was found to be responsible for approximately 1.7% of hospitalized diabetic patients. In today’s society, even with better understanding of the importance of glycemic control, only 41% of people with diabetes have the ability to calculate an insulin dose based on carbohydrate intake and blood glucose levels. Controlling blood sugar with fast-acting insulin is difficult because it poses the risk of hypoglycemia or hyperglycemia if insulin is not administered in a correct manner. It is difficult to estimate the amount of insulin required with varying portion sizes and fluctuating sugar levels throughout the day.
_
Screening for DM:
Widespread use of the FPG or the A1c as a screening test for type 2 DM is recommended by experts because (1) a large number of individuals who meet the current criteria for DM are asymptomatic and unaware that they have the disorder, (2) epidemiologic studies suggest that type 2 DM may be present for up to a decade before diagnosis, (3) some individuals with type 2 DM have one or more diabetes-specific complications at the time of their diagnosis, and (4) treatment of type 2 DM may favorably alter the natural history of DM. The ADA recommends screening all individuals >45 years every 3 years and screening individuals at an earlier age if they are overweight [body mass index (BMI) >25 kg/m2] and have one additional risk factor for diabetes. In contrast to type 2 DM, a long asymptomatic period of hyperglycemia is rare prior to the diagnosis of type 1 DM. A number of immunologic markers for type 1 DM are becoming available, but their routine use is discouraged pending the identification of clinically beneficial interventions for individuals at high risk for developing type 1 DM.
_
Note:
I have seen many patients who have normal FPG but higher PPG and these patients ultimately develop frank type 2 DM. I therefore recommend only PPG as a screening test for T2DM (vide infra).
_________
History of SMBG:
In 1957, Kohn showed that Clinistix could also give approximate results for blood glucose. In 1965 an Ames research team under Ernie Adams went on to develop the first blood glucose test strip, the Dextrostix, a paper reagent strip which used the glucose oxidase/peroxidise reaction but with an outer semipermeable membrane which trapped red blood cells but allowed soluble glucose to pass through to react with the dry reagents. A large drop of blood (approximately 50–100 μL) was applied to the reagent pad, and after one minute the surface blood was gently washed away and the pad colour visually assessed against a colour chart to give a semiquantitative blood glucose value. However, the colours were difficult to visualise as the colour blocks were affected by ambient lighting conditions, and variation in individual visual acuity made it difficult to obtain accurate and precise readings. Although the Dextrostix was designed for use in doctors’ offices, the concept of diabetic patients undertaking the measurements had not been considered. Around the same time, the German company Boehringer Mannheim developed a competitive blood glucose strip, the Chemstrip bG. This was easier to use because the drop of blood was wiped off using a cotton wool ball, and, as it had a dual colour pad (one beige, the other blue), it was easier to visualise the colour. The visually monitored blood glucose test strips, Dextrostix (Ames) and Chemstrip bG (Boehringer Mannheim), were widely used in clinics, surgeries and hospital wards, notably intensive care units, for adults and neonates. However, colours were prone to fade and it was realised that there were highly significant visual variations in the assessment of colours across the range of glucose concentrations using Dextrostix. These limitations became the trigger to develop an automatic, electronic glucose test strip reader to improve precision and give more quantitative blood glucose results. The development in the 1950s of the oxygen electrode by Clarke for the measurement of pO2 was the forerunner in the development of the first biosensor electrode. The first description of a biosensor, an amperometric enzyme method for glucose measurement, was made by Clarke and Lyons in 1962. This concept was incorporated in the measurement of blood glucose in the Yellow Spring 24AM ‘desktop’ analyser, which became commercially available in the mid-1970s. The first blood glucose biosensor system, the ExacTech, was launched in 1987 by MediSense. It used an enzyme electrode strip developed in the UK at Cranford and Oxford universities. The strip contained glucose oxidase and an electron transfer mediator, ferrocene, which replaced oxygen in the original glucose oxidase reaction; the reduced mediator was reoxidised at the electrode to generate a current detected by an amperometric sensor. The meter was available in two highly original forms, a slim pen or a thin card the size of a credit card. Evaluation reports showed that accuracy, precision and error grid analysis were satisfactory. The use of electrode technology thus heralded what became designated the third-generation BGMS. In 1987, with the increased use of SMBG systems, the American Diabetic Association (ADA) lowered the preferred glucose meter deviation compared to laboratory reference methods to 15%. A useful evaluation statistical tool, error grid analysis, was developed by Clarke et al. and applied by Kochinsky et al., which gave an improved measure of accuracy related to clinical significance and decision making.
_
Four generations of glucometer:
The figure above shows four generations of blood glucose meter. Sample sizes vary from 30 to 0.3 μl. Test times vary from 5 seconds to 2 minutes (modern meters typically provide results in 5 seconds).
_________
Body fluid sample for glucose measurement:
_
The figure below shows overview of body fluid glucose measurement by various techniques and from various sites:
_
Three of the major factors that influence glucose test results are the type of chemical analysis used for the test, the type of sample analyzed (whole blood verses plasma), and the source of the blood (venous, capillary, or arterial).
_________
Whole blood vs. plasma glucose:
Glucose is measured in whole blood, plasma or serum. Historically, blood glucose values were given in terms of whole blood, but most laboratories now measure and report plasma or serum glucose levels. Because red blood cells (erythrocytes) have a higher concentration of protein (e.g., hemoglobin) than serum/plasma, serum/plasma has higher water content and consequently more dissolved glucose than does whole blood. To convert from whole-blood glucose, multiplication by 1.15 has been shown to generally give the serum/plasma level. Under usual circumstances, the concentration of glucose in whole blood is about 15% lower than in plasma or serum, but the difference will be less in patients with low hematocrits. Collection of blood in clot tubes for serum chemistry analysis permits the metabolism of glucose in the sample by blood cells until separated by centrifugation. Red blood cells, for instance, do not require insulin to intake glucose from the blood. Higher than normal amounts of white or red blood cell counts can lead to excessive glycolysis in the sample, with substantial reduction of glucose level if the sample is not processed quickly. Ambient temperature at which the blood sample is kept prior to centrifuging and separation of plasma/serum also affects glucose levels. At refrigerator temperatures, glucose remains relatively stable for several hours in a blood sample. Loss of glucose can be prevented by using Fluoride tubes (i.e., gray-top) since fluoride inhibits glycolysis. However, these should only be used when blood will be transported from one hospital laboratory to another for glucose measurement. Red-top serum separator tubes also preserve glucose in samples after being centrifuged isolating the serum from cells. If you have so far been using a blood glucose monitoring system calibrated for whole blood and are now switching to one calibrated for plasma or vice versa, you may need new target values. You will have to re-adjust though when interpreting the results: since glucose concentration in plasma is approx. 10-15 per cent higher than in whole blood, the levels indicated by meters with plasma-calibrated test strips are approx. 10-15 per cent higher. All the manufacturers will probably switch to plasma in future and in many European countries it has already taken place. Diabetics will find the information about how their meter has been calibrated on the leaflet accompanying the test strips or also in the operating instructions for the meter.
_
Is plasma glucose measurement best?
Conversion of glucose concentrations determined in different sample systems by use of factors is an oversimplification and probably leads to unpredictable rates of discordant disease classifications. These problems are becoming more relevant with the widespread use of point-of-care testing instruments, including blood gas analyzers with integrated glucose sensors that measure glucose in the plasma water fraction. The only solution for this dilemma is to use only one sample system. The experimental data clearly indicate that the use of plasma should be preferred to diagnose glucose intolerance, including diabetes. The logistic disadvantages are the centrifugation step and the prevention of glycolysis. Chan showed that delays in processing blood specimens in hospital practice may lead to misclassification in up to 7% of GTTs. Stahl proposed storage on ice for not more than 1 h until centrifugation. However, this recommendation may not be acceptable for many hospitals. The use of capillary hemolysate together with a reduced decision limit thus may be a second choice for the detection of diabetes.
__________
IV fluid and blood glucose measurement:
To prevent contamination of the sample with intravenous fluids, particular care should be given to drawing blood samples from the arm opposite the one in which an intravenous line is inserted. Alternatively, blood can be drawn from the same arm with an IV line after the IV has been turned off for at least 5 minutes, and the arm has been elevated to drain infused fluids away from the vein. Inattention can lead to large errors, since as little as 10% contamination with a 5% glucose solution (D5W) will elevate glucose in a sample by 500 mg/dL or more.
_
60 % of body weight in men and 50 % of body weight in women is water. Total body water is distributed between 3 fluid compartments in body. Intracellular water (intracellular fluid-ICF) makes up about two- thirds of total body water, with remaining one-third, the extracellular water (extracellular fluid- ECF) being distributed between intravascular (25 %) and interstitial (75 %) compartment. The pores between endothelial cells in capillary allow free movement of water and solutes but do not allow proteins to pass through. So glucose readily passes through intravascular compartment to interstitial compartment. When any glucose-containing IV drip is given, each pint (500ml) of D5W/D5NS contains 25 gm glucose. If you give each pint slowly in 8 hours, body gets 52 mg of glucose every minute. This 52 mg glucose is distributed in 13.8 liter of extracellular water in a 70 kg man (blood water plus interstitial water). So blood glucose value will rise by 0.38 mg/dL every minute if there is no insulin. So if you have given glucose containing IV drip to a diabetic who has near zero insulin secretion, blood glucose will rise by 0.38mg/dL every minute when drip duration is 8 hour. If the same drip is given in 4 hour, the rate of glucose rise is 0.76mg/dL every minute. However, if the same drip is given to a normal non-diabetic person, slight increase in blood glucose will stimulate insulin secretion and therefore blood glucose will be reasonably maintained provided drip rate is 4 to 8 hours per each pint of fluid. The corollary is that if you have collected blood from the arm opposite the one in which an intravenous line is inserted, and if you are getting high glucose level, do not blame IV drip but patient may be diabetic.
_
Every day average person eats 900 to 1300 Kcal of carbohydrates in 2000 Kcal diet. If you divide it in three meals, breakfast, lunch and dinner equally, you eat approximately 300 to 430 Kcal of carbohydrate in each meal. Even if half of carbohydrate is converted into glucose, every meal contains 150 to 215 Kcal from glucose [the other being fructose/galactose]. In other words, every meal generates minimum 37 to 53 gm of glucose to be assimilated in body. Yet in normal non-diabetic person, insulin secretion does not allow the blood sugar to rise much and maximum blood sugar 2 hr after meal is < 140 mg/dL. If normal person can dispose off 50 gm glucose in 2 hours with blood sugar < 140 mg/dL; sure it can dispose off IV D5W/D5NS 500 ml containing 25 gm glucose without rise in blood sugar provided drip rate is not very fast. So drip rate of 125ml/hr to 60 ml/hr would not cause hyperglycemia in normal non-diabetic person.
_
_
Why have I not taken renal glucose loss into consideration in the above formula?
First let me discuss normal non-diabetic person. His GFR is 125 ml/min and blood glucose 100 mg/dL. His kidneys would filter 180 gms of glucose in 24 hrs and more than 99.9 % of filtered glucose is reabsorbed resulting in urine glucose of < 130 mg/24 hr. Now let me discuss uncontrolled diabetic with GFR 125 ml/min and blood glucose 450 mg/dL. He will filter 810 gms of glucose in 24 hrs. Since his blood sugar is far higher than renal threshold, he would have massive renal glycosuria with osmotic diuresis and polyuria. I have gone through various studies on osmotic diuresis in diabetes. For blood glucose of 450mg/dL, when urine output is 5 liter/24 hr, urine glucose concentration is about 8 gm/liter. Further increase in urine output reduces urine glucose concentration, so urine output of 12 liter/24 hour has urine glucose concentration 5 gm/liter. In other words, maximum urine glucose loss in uncontrolled diabetes is 40 to 60 gm glucose in 24 hours. So approximately 6 % of blood glucose is lost in urine and 94 % of filtered glucose is still reabsorbed in uncontrolled diabetes. Therefore, rate of rise of blood glucose after IV D5W/D5NS even in uncontrolled diabetes will change slightly due to renal glucose loss.
_
Note:
Excess water infused through routine IV drip would be excreted by kidneys and would not dilute blood glucose concentration.
_________
Artery vs. capillary vs. vein vis-à-vis blood glucose:
_
Artery to capillary to vein:
_
Home glucose monitoring has traditionally relied on a drop of capillary blood from the finger. Blood glucose is generally measured as the venous plasma level. There is a 3–5 mg/dL difference between arterial and venous levels, with higher differences in the postprandial state. Levels are higher in the arterial blood because some of the glucose diffuses from the plasma to interstitial fluid (IF) as blood circulates through the capillary system. Arterial blood glucose and capillary blood glucose have been shown to be almost identical in concentration, even though the distribution of the glucose to the systemic capillaries does not occur instantaneously. The finger-prick blood sampling is to collect blood in peripheral capillaries and the blood glucose concentration approximates to the level of arterial blood glucose (Rasaiah, 1985). Despite few differences between fasting capillary blood glucose and fasting venous blood glucose, postprandial venous blood glucose is lower than postprandial capillary blood glucose by 7 % because glucose absorbed by the human tissues and remaining glucose returns to veins. Accordingly, the level of arterial blood glucose or postprandial capillary blood glucose is higher than that of postprandial venous blood glucose.
______
Glucose test values may not match with different blood samples because glucose is being consumed by the body:
Glucose diffuses through the capillaries and is consumed by the cells, so arterial glucose concentration (the capillaries’ source) should be higher than venous glucose concentration (the capillaries’ drain) unless capillary diffusion or muscle glucose consumption has been stopped. It has been shown that in fasting subjects the glucose levels in arterial, capillary, and venous samples are practically the same (venous glucose is generally 2-5 mg/dL lower than fingerstick capillary or arterial blood glucose). It is only after meals, when glucose uptake in the periphery is rapid, that glucose levels in fingerstick capillary blood samples can exceed those in concurrently drawn venous samples. A typically quoted value is up to 80 mg/dL difference between venous and fingerstick capillary blood glucose values one hour after ingestion of 100 grams of glucose. Current literature has attempted to determine exactly how glucose levels in venous, arterial and fingerstick capillary blood vary so comparisons can be made. Venous blood is usually employed for laboratory analysis and is preferable in diabetes testing. However, because of the widespread use of SMBG instruments, fingerstick capillary blood samples have also become a standard. Fingerstick capillary blood has been shown to be predominantly arterial and so approximates the concentration of arterial blood. Somogyi compared the glucose content of blood samples simultaneously drawn from the femoral artery and the fingertip of non-diabetics one-hour after ingestion of 50 grams of glucose. The ingested glucose would produce a substantial difference between the arterial and venous glucose levels, and so should indicate whether fingerstick capillary blood was predominantly arterial, venous, or a combination of the two. The discrepancies between arterial and fingerstick capillary blood were less than 1 mg/dL for all three subjects studied and seemed to justify the substitution of fingerstick capillary for arterial blood glucose. Somogyi also studied the difference between fingerstick capillary and venous glucose levels during the fasting state on 100 healthy individuals (fasting for 10-14 hours). The average fasting glucose value in fingerstick capillary blood samples was 89 mg/dL (78 – 97 mg/dL) with the average venous blood glucose value 5 mg/dL lower (84 mg/dL). In the same study, both venous and fingerstick capillary blood glucose values were followed for a period of 4 hours in 44 healthy individuals that had ingested a 100-gram glucose load (see figure below). A substantial increase in the fingerstick capillary to venous blood glucose difference were measured after an oral administration of glucose, and this difference remained consistently higher than the initial fasting level until the blood glucose returned to the fasting blood glucose level. The paper also found that the larger the glucose load ingested, the higher the glucose peaks, and the greater the maximal difference between the fingerstick capillary (paper assumed this to be arterial) blood glucose level and the venous blood glucose level.
_
The difference between capillary and venous blood in the postprandial state is due to muscles removing more glucose from the blood than the liver in the presence of adequate insulin action. It has been shown that a lack of insulin (in the de-pancreatized animal) shows an arterial to venous glucose difference that is extremely small and that injection of insulin produces an increase in this difference. As such, glucose uptake by the tissue is dependent on the sensitivity of the tissue to insulin, the circulating insulin level and the local blood flow. Diabetics may have various degrees of peripheral insulin resistance or various blood insulin levels or both, so a single patient’s nonfasting difference may not be seen in other patients. The nonfasting difference will depend on meal size, meal content, time of sample collection, and individual patient variability. In summary, glucose levels in arterial and fingerstick capillary blood have been so closely correlated that most studies refer to arterial glucose measurements even if they measure fingerstick capillary samples. When studies are performed with the patient under fasting conditions, glucose levels in fingerstick capillary blood gives reliable quantitative estimates of the venous glucose concentration as determined in the laboratory for most patients. However, when the patients are under a glucose load the venous and fingerstick capillary glucose levels diverge in a similar but unpredictable manner where the venous value may be anywhere from 2% lower during fasting to 26% lower within one hour after a glucose load. Unfortunately, empirical conversion factors have been applied to generate equivalent glucose values for different blood sample compartments without adequate data to show equivalence. One such conversion is that fingerstick capillary blood has a glucose concentration that is 7-8% higher than the concurrently drawn venous concentration. Others have presented charts showing the equivalence of venous and capillary glucose levels that differ between 0% to 13% depending on the glucose level. The validity of these conversion factors has been called into question since individual differences between capillary and venous blood glucose values are too great to allow for a meaningful transformation to be applied. It can be reasonably concluded that there is no simple conversion factor available to explain differences between glucose values in the various blood compartments.
_
Glucose test values may not match because the body is consuming oxygen:
A study by Liu measured arterial, fingerstick capillary, and venous blood samples from six healthy males for oxygen saturation and glucose. Each subject’s right hand was placed in a warm air box at 55-60 degrees C to determine if warm air would arterialize the venous blood obtained from a cannula inserted into the dorsal right-hand vein. The oxygen saturation measured in the arterial blood was 97%. The oxygen saturation measured in venous blood on a nonheated hand was 80%. The oxygen saturation measured in the heated ‘arterialized’ venous blood was 94% or approximately 3% below the average arterial value. Glucose levels also showed equilibration between the two blood compartments with heating. The difference between fasting arterial glucose levels and venous glucose levels with no heating of the hand ranged between 4-9 mg/dL (6% – 9%), and this glucose difference significantly correlated with the differences in oxygen saturation between the two blood supplies. The difference between the arterial glucose levels and ‘arterialized’ venous glucose levels obtained by heating the hand averaged less than 2 mg/dL difference, and this glucose difference had a low correlation with the differences in oxygen saturation between the two blood supplies.
_
Many analytical procedures are used to measure blood glucose but the most common techniques are enzymatic. Enzymes commonly used in commercial test strips are glucose oxidase, glucose dehydrogenase, or hexokinase combined with glucose-6-phosphate dehydrogenase. Glucose oxidase has historically been the preferred enzyme because of its excellent specificity for glucose, good room temperature stability, and relatively low cost. However, the reaction requires an adequate oxygen supply, and this leads to an oxygen dependence problem in certain measurement systems. Electrochemical measurement combined with glucose oxidase involves a mediator to transfer electrons between the electrodes. The mediator attempts to replace oxygen in the reaction sequence. This makes oxygen in the blood sample a competitor in the reaction and produces varying results with varying oxygen concentrations (oxygen dependence). Electrochemical test strips that are calibrated using fingerstick capillary blood can read up to 30% higher when tested with venous blood because of its 50-60% lower pO2 values. A similar situation exists with some optical reflectance methods. Generally, atmospheric oxygen is sufficient to meet the glucose oxidase reaction requirements, but different test strip design can block the diffusion of oxygen to the reaction site. Commercial analyzers attempt to circumvent oxygen effects by pre-dilution of the sample into an oxygenated buffer. Glucose dehydrogenase can be made oxygen independent when it is combined with a cofactor called pyrroloquiniline quinone (PQQ). Using this enzyme combination effectively eliminates oxygen competition and enables the use of venous or arterial samples where extremes of pO2 may occur. The trade-off is reduced specificity for glucose in that it also detects maltose, galactose, and metabolites of maltodextrins. There is also reduced operational stability when compared to glucose oxidase. Hexokinase combined with glucose-6-phosphate dehydrogenase also avoids oxygen dependence, but the test strip is inherently more sensitive to heat and moisture, and therefore special attention is paid to packaging. Glucose comparison studies between arterial, capillary, and venous blood must consider the significant differences in oxygen tension between the blood compartments when using analytical systems that are oxygen dependent. Ideally, the effect of pO2 needs to be examined by monitoring oxygen concentrations and determining if a correlation exists for glucose.
_
Glucose test values may not match because of low blood flow in the forearm:
Glucose consumption and oxygen variation concern physiological parameters that would lead to a bias between glucose test results taken simultaneously from two different blood compartments during either fasting or the meal cycle. A third physiological parameter that would cause glucose in one blood compartment to lag or lead another is flow or circulation problems in a capillary bed. Many medical and physical conditions can affect capillary blood flow with the problem being either systemic or localized. Localized variations in blood flow associated with the capillary beds would be a major contributing factor to erroneous comparison data between two capillary blood supplies such as within the finger and forearm. A localized variation in blood flow would also be a contributing factor in glucose differences measured within capillary, arterial, and venous blood. Lower flow in the capillaries will lead to greater exchange of nutrients and metabolites. Simplistically, a drop of blood moving slowly will have more time to lose glucose to the consuming tissue compared to a drop of blood moving quickly. In tissues like the heart, all capillaries are normally open to perfusion, but in skeletal muscle and intestine only 20% – 30% of capillaries are normally open. As an example, it is possible that only 70% of the forearm capillaries are flowing normally at any one time, and 30% have slower-moving blood that is being depleted of glucose and oxygen by diffusion into the cellular space. Lancing into such a location would produce glucose readings lower than both arterial and venous blood glucose since more glucose consumption would occur in areas with no flow. If the measurement technique were oxygen sensitive, then the measurement would also be lower because of oxygen consumption by the surrounding tissue. Ideally, blood collection from sites such as the forearm and thigh should target a highly perfused capillary bed, and either compensate for or be independent of temporal changes in blood flow.
_
Simultaneous measurements of arterial and venous blood samples should produce different glucose values in healthy people due to glucose utilization by peripheral tissues. Unfortunately, the magnitude of this glucose difference cannot be predicted due to the large number of variables that affect it. Since capillary blood has been expanded to refer to blood collected from the finger, forearm, ear, heel, calf, and stomach, questions have arisen if each of these is predominantly arterial or venous. Published studies have justified equating arterial and fingerstick capillary glucose levels under most conditions but no other capillary blood source has been equally studied. Local, rhythmic changes of blood flux within capillary beds play a larger role in the variation of forearm capillary blood glucose vs. fingerstick capillary blood glucose than the differences between arterial and venous values. It is not to say that forearm capillary is more like venous, but that the independent temporal changes in select capillary beds affect the venous value because it is upstream.
_
A Comparison between Venous and Finger-Prick Blood Sampling on Values of Blood Glucose: a 2012 study:
The purpose of this study is to investigate changes in fasting and postprandial blood glucose values of 12 healthy voluntary subjects, who were asked to take 50g glucose solution, during 2 hours and compare correlations and differences based on two types of blood samplings, venous blood sampling and finger-prick blood sampling. It can be seen from experimental results that (1) there is no significant difference between the fasting venous blood glucose value (87.4±0.4 mg/dL) and the fasting capillary blood glucose (91.6±4.4 mg/dL) (0 min); (2) there is significant difference between the postprandial venous blood glucose concentration and the postprandial capillary blood glucose concentration, both of which reach the maximum levels at 30 min (postprandial venous blood glucose value=122.0±1.2 mg/dL; postprandial capillary blood glucose value=163.8±1.3 mg/dL), with glucose solution ingested by subjects; (3) the mean capillary blood glucose concentration is higher than the mean venous blood glucose concentration by 35%; (4) the correlation coefficient r=0.875(p<0.001) suggests statistical discrepancy and positive correlation between two groups of blood glucose concentrations which imply the venous blood glucose concentration is a better indicator to clinically test blood glucose due to higher stability and fewer interference factors. The blood glucose is defined as venous plasma glucose according to the criteria of WHO to diagnose diabetes but the whole blood glucose on the peripheral capillary basis is available in a glucose meter. As one simple and convenient tool, the glucose meter is applicable to self-monitoring of blood glucose values which are accurate enough but proportional to venous plasma glucose values by a stable factor of 1.12 due to a different numerical benchmark rather than an error.
_
Comparability of Blood Glucose Concentrations Measured in Different Sample Systems for Detecting Glucose Intolerance: A study:
The interconversion of glucose values for venous and capillary blood is further complicated by the arteriovenous difference. In the fasting state, the glucose concentrations in arterial, capillary, and (forearm) venous blood are supposed to be almost indistinguishable. In contrast, arterial blood glucose values may differ by 20% or as much as 70% in the postprandial state. The mean arteriovenous differences are largest in lean nondiabetic individuals, smallest in diabetic individuals, and larger in deep veins than in superficial vessels. Other factors can influence the differences in glucose concentrations among the various samples. Thus, the conversion of concentration values from one system (or sample type) to another is subject to unpredictable errors. Several authors have already rejected the practice of converting glucose concentrations and have recommended that plasma be used for all glucose determinations. In a recent editorial, glucose measurement in whole blood was considered anachronistic, but only whole blood is used by home monitoring and near-patient monitoring devices. Many laboratories measure the glucose concentration in whole blood, especially in capillary whole blood, for therapeutic monitoring and for diagnosing hypo-, normo-, and hyperglycemia. However, the applicability of whole blood for determining glucose intolerance is still a matter of debate. Many practitioners tend to use capillary blood (CB) for diagnostic purposes. The decision limits usually applied for whole blood are those recommended by WHO and the American Diabetes Association, which are based on epidemiologic studies with venous plasma (VP). In practice, either measured values or decision limits are converted from one sample system to another.
________
Blood glucose vs. Interstitial fluid (IF) glucose:
_
Interstitial fluid (IF):
IF constitutes approximately 45% of the volume fraction of human skin, with blood vessels contributing to the 5% of the skin volume. IF is a relatively passive medium that has one-third of the total protein concentration as compared to plasma with an average albumin/globulin ratio of 1.85 The total body volume of the interstitial space is three times that of plasma; however, IF compartments around the cells are microscopic. IF bathes the cells and feeds them with nutrients, including glucose, by providing a corridor between the capillaries and the cell. There is less IF in the subcutaneous tissue than in the dermis. Adipose tissue, just below the dermis, is richly vascularized with capillary walls that are relatively thinner (0.03 μm vs. 0.1 μm) than the capillaries of the dermis. The basal membranes of the capillaries are in direct contact with the adipose cell cytoplasmic membrane. The size of adipocytes might affect the amount of IF in the subcutaneous tissue, suggesting that adiposity might have an effect on IF glucose concentrations.
_
The Relationship between Plasma Glucose and Interstitial Glucose:
Plasma and IF have different characteristics and should be considered as separate glucose compartments. Glucose is transferred from the capillary endothelium to the IF by simple diffusion across a concentration gradient without the need of an active transporter. Blood flow to the area dictates the amount of glucose delivered. Interstitial glucose values are determined by the rate of glucose diffusion from plasma to the IF and the rate of glucose uptake by subcutaneous tissue cells. Thus, the metabolic rate of the adjacent cells and other factors, like insulin, affecting glucose uptake by cells, the glucose supply from the blood vessel, blood flow to the area, and the permeability of the capillary that can be altered by many factors, including nerve stimulation, influence the interstitial glucose levels. The time required for glucose to diffuse from the capillary to the tissue plays an important role in the lag time between changes in plasma and interstitial glucose levels, but the lag during rapid changes of blood glucose is likely due to the magnitude of concentration differences in various tissues at a time of rapid change. A major confounding factor in evaluating the dynamics of changes in IF glucose concentrations has been the complexity of direct sampling methods, including insertion of wicks, blister formation, lymph sampling, and ultrafiltration. Microdialysis is an indirect method of estimating IF glucose values. Lönnroth et al. was the first to use this method to show that IF glucose was almost identical to venous plasma glucose in healthy individuals during steady state. Jannson et al. demonstrated an increase in lag time between IF and plasma glucose when there is a rapid rise in the plasma glucose level. The data of Jensen et al. revealed lower IF glucose levels than plasma glucose during clamp experiments extracting IF by suction blister technique. There are relatively limited data on dermal IF glucose levels. Bantle and Thomas demonstrated no significant difference between the dermal and plasma pre- and postprandial glucose levels in subjects with type 1 diabetes. A plasma and dermal interstitial glucose concentration lag time of 10–20 min was reported by Stout et al. In general, IF and plasma glucose variations were evaluated in two different conditions: steady state and non–steady state. Under steady-state conditions, IF glucose generally correlated with the blood glucose with a lag time reported to be between 0 and 45 min and an average lag of 8–10 min. Increasing the blood flow to the interstitial glucose sampling site by applying controlled pressure has been shown to decrease the lag time between the blood and interstitial glucose at times of increasing plasma glucose levels. The reported gradient between interstitial and plasma glucose concentrations has varied between 20% to 110%. During the time of decreasing glucose, interstitial glucose may fall in advance of plasma glucose and reach nadir values that are lower than corresponding venous glucose levels. Interstitial glucose levels have been shown to remain below plasma glucose concentrations for prolonged periods of time after correction of insulin-induced hypoglycemia. These findings could be explained by the push–pull phenomenon during which the glucose is pushed from the blood to the interstitial space at times of increased blood glucose, and later on glucose being pulled from the IF to the surrounding cells during decreasing blood glucose levels. This phenomenon has been a matter of debate for some time, in light of data failing to support the push–pull phenomenon and instead reporting compensation of enhanced uptake of glucose in the IF by increased plasma glucose delivery and lack of glucose removal effect of insulin in the adipose IF. Current continuous glucose monitoring systems have the advantage of direct insertion of electrochemical sensors into the IF space rather than transporting the sampled fluid outside the body to detect glucose concentrations. Software programs have been designed to accommodate the lag in IF glucose readings. Recent advances in glucose sensor technology for measuring interstitial glucose concentrations have challenged the dominance of glucose meters in diabetes management, while raising questions about the relationships between interstitial and blood glucose levels.
_
For glucose meter measurements, a skin-pricking device is used to access the dermal capillary plexus. Human skin consists of two layers—epidermis and dermis—residing above the adipose and muscle tissue. Epidermis is an avascular epithelial membrane. It has enzymes with glucose metabolizing effect. Moreover, glucose is formed from the breakdown of ceramide at the stratum granulosum–corneum interface. Dermis comprises many arterioles, venules, and capillaries, including a deep vascular plexus interfacing dermis and the subcutaneous tissue as seen in the figure below. Another vascular plexus located 0.3–0.6 mm from the skin surface is formed by the feeding vessels arising from the deep vascular plexus. It supplies the blood flow to the dermis and epidermis with the help of small capillary loops branching from the superficial plexus. The blood sampled from the skin prick comes from the capillaries of dermis with a small amount of blood from cut arterioles and venules providing a mix concentration. Blood flow to the skin is controlled by many factors, including autonomic nervous system, temperature, hormonal changes during menstrual cycle for females, and chemical inputs.
_
The figure above shows skin layers with the magnified IF space. (a) Vasculature in different skin layers with the CGM inserted into the subcutaneous tissue. (b) Diffusion of glucose from plasma to IF is in proportion to the concentration in each compartment. IF glucose is cleared by the surrounding cell uptake. Insulin may increase cellular glucose uptake after binding its membrane receptor.
________
Methods of monitoring glucose:
Intermittent vs. continuous glucose monitoring:
The difference between an intermittent and a continuous monitor for monitoring blood glucose is similar to that between a regular camera and a continuous security camera for monitoring an important situation. A regular camera takes discrete, accurate snapshots; its pictures do not predict the future; it produces a small set of pictures that can all be carefully studied; and effort is required to take each picture. A continuous security camera, on the other hand, takes multiple, poorly focused frames; displays a sequential array of frames whose trend predicts the future; produces too much information for each frame to be studied carefully; and operates automatically after it is turned on. The two types of blood glucose monitors differ in much the same way: 1) an intermittent blood glucose monitor measures discrete glucose levels extremely accurately, whereas a continuous monitor provides multiple glucose levels of fair accuracy; 2) with an intermittent monitor, current blood glucose levels do not predict future glucose levels, but with a continuous monitor, trends in glucose levels do have this predictive capability; 3) with an intermittent monitor, it is easy to study every measured blood glucose value over most time periods, but with a continuous monitor, too many data are generated to study all data points; and 4) an intermittent blood glucose monitor requires effort to operate, whereas a continuous monitor does not. Returning to the camera analogy, just as the best tool for closely monitoring a situation when the outcome is important often may be a continuous security camera rather than a regular camera, likewise the best way to monitor diabetes often may be a continuous glucose monitor (CGM) rather than an intermittent monitor.
_
Various methods of glucose monitoring are available, including HbA1c measurement, blood glucose monitoring and urine glucose testing.
_
Basic approaches for blood glucose measurement:
There are three basic approaches to the laboratory measurement of blood glucose concentration: reducing methods, condensation methods, and enzymatic methods. Reducing methods are the oldest and take advantage of the reducing properties of glucose to change the state of a metal ion while glucose is being oxidized. Reducing methods are nonspecific, and any strong reducing agent can cross react to yield spuriously elevated values. While steps can be added to remove most cross-reacting reducing agents, this approach has largely been abandoned in the clinical laboratory. The aldehyde group of glucose can undergo condensation with aromatic compounds to yield a colored product. In the most commonly used condensation reaction, o-toluidine reacts with glucose to form a glucosamine that has an intense green color. The color is then measured spectrophotometrically to estimate the glucose concentration. The reaction is rapid, and the intense color allows a high degree of sensitivity. Other aldoses can cross react, but only mannose and galactose give a highly colored product. These sugars are not found in great concentrations in the blood and their cross reactivity is ordinarily not significant. o-Toluidirie has the drawback of being highly corrosive and toxic. For this reason, this method is rapidly being phased out of the clinical laboratory. More precise blood glucose measurements are performed in a medical laboratory, using hexokinase, glucose oxidase, or glucose dehydrogenase enzymes.
_
The enzyme glucose oxidase reacts with glucose, water, and oxygen to form gluconic acid and hydrogen peroxide. The hydrogen peroxide can then be used to oxidize a chromogen or the consumption of oxygen measured to estimate the amount of glucose present. Glucose oxidase is specific for β-d-glucose, so cross reaction with other sugars is not a problem. In aqueous solution, approximately 66% of d-glucose is in the β state and 34% exists as α- d-glucose. The rate of interconversion is pH and temperature dependent. Some methods add a glucomutarostase to the reagents to speed up the conversion to the beta anomere, but this does not seem to alter the clinical results. The measurement of generated hydrogen peroxide is not as specific as the first glucose oxidase reaction. Numerous reducing substances can potentially inhibit the oxidation of the chromogen. Although uric acid and creatinine, even in uremic patients, seem to have little effect on the results, ascorbic acid will yield spuriously low blood glucose measurements. The high concentration of uric acid found in urine will affect the result and so glucose oxidase methods are not directly applicable to urine samples. The measurement of oxygen consumption using an oxygen-specific electrode avoids the problem of interfering reducing agents. In general, the glucose oxidase method is relatively inexpensive and specific. Many glucose meters employ the oxidation of glucose to gluconolactone catalyzed by glucose oxidase (sometimes known as GOx). Others use a similar reaction catalysed instead by another enzyme, glucose dehydrogenase (GDH). This has the advantage of sensitivity over glucose oxidase but is more susceptible to interfering reactions with other substances.
_
Enzymatic methods to measure blood glucose:
_
Amperometric and photometric techniques for measurement of blood glucose using enzymatic methods:
__
Timing of the test:
Blood sugar is measured at various points of time to give an idea about the body’s blood glucose regulation system. The primary test is the fasting blood glucose (FBG). This is measured after overnight fasting. Blood glucose normally is lowest early in the morning after 6 to 8 hours of fasting overnight. FBG is also called fasting blood sugar (FBS). However, the correct terminology is venous fasting plasma glucose (FPG) as whole blood sugar is obsolete in most laboratories. Two hours post prandial plasma glucose or PPG is the next common test. After a carbohydrate rich, full meal, two hours are allowed to elapse before blood is taken again for estimation of glucose. This test gives an estimation of glucose handling by the body. Other tests include oral glucose tolerance test (OGTT) and intravenous glucose tolerance test (IVGTT) wherein a fixed amount of glucose is administered orally or intravenously respectively and repeated blood sugar tests are performed to check on the body’s glucose handling. Another important test is the glycosylated haemoglobin (HbA1C). This test gives an idea about fluctuations of glucose in blood over a period of last three months.
_
These glucose measurements are useful for different reasons:
Fasting plasma glucose levels, taken in the morning before eating, should fall in a normal range. Normal value for FPG is 70 to 100 mg/dL. Measurement of glucose in plasma of fasting subjects is widely accepted as a diagnostic criterion for diabetes. Advantages include inexpensive assays on automated instruments that are available in most laboratories worldwide. Nevertheless, FPG is subject to some limitations. One report that analyzed repeated measurements from 685 fasting participants without diagnosed diabetes from the Third National Health and Nutrition Examination Survey (NHANES III) revealed that only 70.4% of people with FPG <126 mg/dL on the first test had FPG <126 mg/dL when analysis was repeated ~2 weeks later. Numerous factors may contribute to this lack of reproducibility.
Two hours after eating:
Blood sugar rises and then falls to a baseline level. By sampling blood sugar levels two hours after eating, you find out if glucose is being removed from your blood in a reasonable time. The sugar level peaks in 30-60 minutes and the falls back to a baseline level. The timing and height of the peak level will vary with the composition of the meal and activity levels. Normal PPG value is 100 to 140 mg/dL.
OGTT:
The OGTT evaluates the efficiency of the body to metabolize glucose and for many years has been used as the “gold standard” for diagnosis of diabetes. An increase in postprandial glucose concentration usually occurs before fasting glucose increases. Therefore, postprandial glucose is a sensitive indicator of the risk for developing diabetes and an early marker of impaired glucose homeostasis. Published evidence suggests that increased 2-h plasma glucose during an OGTT is a better predictor of both all-cause mortality and cardiovascular mortality or morbidity than the FPG. The OGTT is accepted as a diagnostic modality by the ADA, WHO/International Diabetes Federation (IDF), and other organizations. However, extensive patient preparation is necessary to perform an OGTT. Important conditions include, among others, ingestion of at least 150 g of dietary carbohydrate per day for 3 days prior to the test, a 10- to 16-h fast, and commencement of the test between 7:00 A.M. and 9:00 A.M. In addition, numerous conditions other than diabetes can influence the OGTT. Consistent with this, published evidence reveals a high degree of intraindividual variability in the OGTT, with a CV of 16.7%, which is considerably greater than the variability for FPG. These factors result in poor reproducibility of the OGTT, which has been documented in multiple studies. The lack of reproducibility, inconvenience, and cost of the OGTT led the ADA to recommend that FPG should be the preferred glucose-based diagnostic test. Note that glucose measurement in the OGTT is also subject to all the limitations described for FPG. An abbreviated screening glucose tolerance test is recommended for all women between their 24th and 28th week of pregnancy. The test consists of 50 g of oral glucose and the measurement of venous plasma glucose 1 hour later. The test may be administered at any time of day and non-fasting. A 1 hour plasma glucose of 140 mg/dl or greater indicates the need for a full-scale glucose tolerance test as described above.
Checking symptomatic episodes:
You measure blood sugar when you are not feeling well to find out how your symptoms correlate with the blood sugar level. High levels are associated with an intoxicated feeling – drowsy, hard to concentrate, judgment impaired. Levels above 17 mmol or 300 mg are dangerously high – you are likely to want to sleep at this level but the most effective way to reduce the sugar levels is to exercise as vigorously as you can. Levels below 4.5 mmol capillary may be associated with hypoglycemic symptoms – you feel strange, anxious, irritable; a tremor develops if the blood sugar value falls lower and you become desperate to eat something. If you can take a quick sugar hit – a glass of orange juice will do and measure your sugar immediately you can determine how low the value dropped; as you feel better do another blood sugar check to find the value that feels normal.
___________
Diabetes and urine glucose monitoring:
The glucose urine test measures the amount of sugar (glucose) in a urine sample. The presence of glucose in the urine is called glycosuria or glucosuria. After you provide a urine sample, it is tested right away. The health care provider uses a dipstick made with a color-sensitive pad. The color the dipstick changes to tell the provider the level of glucose in your urine. The proximal tubules reabsorb more than 99.9 % of glucose filtered by glomerulus in a normal person. When the blood glucose level exceeds about 160 – 180 mg/dl, the proximal tubule becomes overwhelmed and begins to excrete glucose in the urine. This point is called the renal threshold of glucose. If renal threshold is so low that even normal blood glucose levels produce glycosuria, it is referred to as renal glycosuria. Although used in the past to self-monitor diabetes control, urine glucose testing has been largely replaced by self blood glucose monitoring using a small, personal meter. The reason for this is the greater accuracy with which blood glucose monitoring reflects your blood glucose level. However, if you have difficulty obtaining a drop of blood, or you have some other difficulty performing blood glucose monitoring, your doctor or diabetes educator may suggest that urine glucose monitoring is suitable for you. A urine glucose test determines whether or not glucose (sugar) is present in the urine. Glucose will overflow into the urine only when the blood glucose level is high, that is, too high for the kidneys to stop it spilling over into the urine. In most people, blood glucose levels above 10 mmol of glucose per liter of plasma will cause glucose to appear in the urine. This level is called the ‘renal threshold’ for glucose. However, the renal threshold for glucose can be lower in some people who are otherwise healthy, during pregnancy, and in people who have a kidney disorder. In these people, glucose may be present in the urine despite the blood glucose being normal. This can sometimes make urine glucose tests difficult to interpret.
To perform the test:
1. Collect a small amount of urine;
2. Apply this to the test strip, usually by dipping the strip in the urine sample;
3. Read the test result at the specified time, by comparing the colour change on the test strip with the standard colour range for your brand of test strip. The reference colour chart is usually printed on the container.
_
Advantages of urine glucose monitoring
1. Urine glucose testing is easy to do: just dip the test strip in the urine and read the result at the allocated time.
2. It is less painful than blood glucose monitoring — no finger pricks to collect blood!
3. Urine test strips are less costly than buying a blood glucose monitor and its test strips.
_
Limitations of urine glucose monitoring:
If monitoring your diabetes control by testing your urine glucose, it’s important to understand the limitations of this method.
1. A urine glucose test does not reflect your blood glucose level at the time of testing; instead, it gives an indication of your blood glucose level over the past several hours. For example, some of the urine present in your bladder may be 2 hours old, and may show glucose even though your blood glucose may have normalised since then. Compare this to a blood glucose test which gives you a reading of your current blood glucose level.
2. A urine glucose test does not give you any information about low blood glucose levels, as glucose is only found in the urine when the blood glucose level is above 10 mmol/L. That is, a negative urine glucose test may be the result of a normal blood glucose level or a dangerously low blood glucose level, with the urine glucose test unable to differentiate between the 2 situations.
3. The results of a urine glucose test are influenced by the volume and concentration of urine that you pass, which will vary with the amount of fluid you consume and your fluid loss due to such things as heavy sweating or vomiting.
4. Urine glucose tests designed for home use rely on interpreting a colour change to define the result. Subtle colour differences may be difficult to interpret.
5. If a urine glucose test is not read at the specified time after applying the urine to the test strip, then the result is prone to error.
6. Some medications may interfere with the results of urine glucose testing.
_
Urine ketone testing:
People with type 1 diabetes should perform urine testing for ketones if the blood sugar level is above 240 mg/dL (13.3 mmol/L), during periods of illness or stress, or if you have symptoms of ketoacidosis, such as nausea, vomiting, and abdominal pain. Ketones are acids that are formed when the body does not have enough insulin to get glucose into the cells, causing the body to break down fat for energy. Ketones can also develop during illness, if an inadequate amount of glucose is available (due to skipped meals or vomiting). Ketoacidosis occurs when high levels of ketones are present and can lead to serious complications such as diabetic coma. Urine ketone testing is done with a dipstick, available in pharmacies without a prescription. If you have moderate to large ketones, you should call your healthcare provider immediately to determine the best treatment. You may need to take an additional dose of insulin, or your provider may instruct you to go to the nearest emergency room.
_
Urine protein testing:
Urine is tested for microalbuminuria in diabetes. Microalbuminuria is defined as levels of albumin ranging from 30 to 300 mg in a 24-h urine collection. Overt albuminuria, macroalbuminuria, or proteinuria is defined as a urinary albumin excretion of ≥300 mg/24 h. The presence of albuminuria is a powerful predictor of renal and cardiovascular risk in patients with type 2 diabetes and hypertension. In addition, multiple studies have shown that decreasing the level of albuminuria reduces the risk of adverse renal and cardiovascular outcomes. The pathophysiology is not definitively known, but is hypothesized to be related to endothelial dysfunction, inflammation, or possibly abnormalities in the renin-angiotensin-aldosterone system.
______
Glycosylated Hemoglobin: Glycated Hemoglobin:
Glycated hemoglobin (hemoglobin A1c, HbA1c, A1C, Hb1c or HbA1c) is a form of hemoglobin that is measured primarily to identify the average plasma glucose concentration over prolonged periods of time. It is formed in a non-enzymatic glycation pathway by hemoglobin’s exposure to plasma glucose. When hemolysates of red cells are chromatographed, three or more small peaks named hemoglobin A1a, A1b, and A1c are eluted before the main hemoglobin A peak. These “fast” hemoglobins are formed by the irreversible attachment of glucose to the hemoglobin in a two-step reaction. The percentage of hemoglobin glycosylated depends on the average glucose concentration the red cell is exposed to over time. Since the average life of the red cell is 120 days, the percentage of glycosylated hemoglobin gives a good indication of the degree of blood sugar control over the preceding weeks. Hemoglobin A1c is quantifiably the largest peak so that most laboratories measure it selectively, although some laboratories measure all the “fast” hemoglobins. Normal levels of glucose produce a normal amount of glycated hemoglobin. As the average amount of plasma glucose increases, the fraction of glycated hemoglobin increases in a predictable way. This serves as a marker for average blood glucose levels over the previous months prior to the measurement. Glycation of proteins is a frequent occurrence, but in the case of hemoglobin, a nonenzymatic reaction occurs between glucose and the N-end of the beta chain. This forms a Schiff base which is itself converted to 1-deoxyfructose. This rearrangement is known as Amadori rearrangement. When blood glucose levels are high, glucose molecules attach to the hemoglobin in red blood cells. The longer hyperglycemia occurs in blood, the more glucose binds to hemoglobin in the red blood cells and the higher the glycated hemoglobin. Once a hemoglobin molecule is glycated, it remains that way. A buildup of glycated hemoglobin within the red cell, therefore, reflects the average level of glucose to which the cell has been exposed during its life-cycle. Measuring glycated hemoglobin assesses the effectiveness of therapy by monitoring long-term serum glucose regulation. At any moment, the glucose attached to the hemoglobin A protein reflects the level of the blood sugar over the last two to three months. The A1c test measures how much glucose is actually stuck to hemoglobin A, or more specifically, what percent of hemoglobin proteins are glycated. Thus, having a 7% A1c means that 7% of the hemoglobin proteins are glycated. A person’s A1c level will not change significantly over the course of a few days, but it will shift in response to a change in overall glucose control. It is estimated that the past month will account for about 50% of an A1c value, so that value can change within just a few weeks. Some researchers state that the major proportion of its value is weighted toward the most recent 2 to 4 weeks. This is also supported by the data from actual practice showing that HbA1c level improved significantly already after 20 days since glucose-lowering treatment intensification. It has also been noted that at any given time a blood sample contains erythrocytes of varying ages, with different levels of exposure to hyperglycemia. Although older erythrocytes are likely to have more exposure to hyperglycemia, younger erythrocytes are more numerous. Consequently, BG levels from the preceding 30 days have been shown to contribute approximately 50% to HbA1c, whereas those from the period 30–90 days and 90–120 days earlier contribute approximately 40% and 10%, respectively.
_
Glycated hemoglobin measurement is not appropriate where there has been a change in diet or treatment within 6 weeks. Likewise, the test assumes a normal red blood cell aging process and mix of hemoglobin subtypes (predominantly HbA in normal adults). Hence, people with recent blood loss, hemolytic anemia, or genetic differences in the hemoglobin molecule (hemoglobinopathy) such as sickle-cell disease and other conditions, as well as those that have donated blood recently, are not suitable for this test. Concentrations of hemoglobin A1 (HbA1) are increased, both in diabetic patients and in patients with renal failure, when measured by ion-exchange chromatography. The thiobarbituric acid method (a chemical method specific for the detection of glycation) shows that patients with renal failure have values for glycated hemoglobin similar to those observed in normal subjects, suggesting that the high values in these patients are a result of binding of something other than glucose to hemoglobin. In autoimmune hemolytic anemia, concentrations of hemoglobin A1 (HbA1) is undetectable. Administration of prednisolone will allow the HbA1 to be detected. The alternative fructosamine test may be used in these circumstances and it also reflects an average of blood glucose levels over the preceding 2 to 3 weeks.
_
A number of techniques are used to measure A1c:
•High-performance liquid chromatography (HPLC): The HbA1c result is calculated as a ratio to total hemoglobin by using a chromatogram.
•Immunoassay
•Enzymatic
•Capillary electrophoresis
•Boronate affinity chromatography
Point of care (e.g., doctor’s office) devices use:
•Immunoassay
•Boronate affinity chromatography
_
Indications and use:
Glycated hemoglobin testing is recommended for both (a) checking the blood sugar control in people who might be pre-diabetic and (b) monitoring blood sugar control in patients with more elevated levels, termed diabetes mellitus. There is a significant proportion of people who are unaware of their elevated HbA1c level before they have blood lab work. For a single blood sample, it provides far more revealing information on glycemic behavior than a fasting blood sugar value. However, fasting blood sugar tests are crucial in making treatment decisions. The American Diabetes Association guidelines are similar to others in advising that the glycated hemoglobin test be performed at least two times a year in patients with diabetes that are meeting treatment goals (and that have stable glycemic control) and quarterly in patients with diabetes whose therapy has changed or that are not meeting glycemic goals. In diabetes mellitus, higher amounts of glycated hemoglobin, indicating poorer control of blood glucose levels, have been associated with cardiovascular disease, nephropathy, and retinopathy. Monitoring HbA1c in type 1 diabetic patients may improve outcomes.
_
The HbA1c level measures glycemic control during the preceding 2 to 3 months, but it does not provide information about day-to-day glucose levels, nor does it provide immediate feedback to patients about medication or lifestyle choices. For these reasons, self-monitoring of blood glucose (SMBG) levels is considered an important adjunct to HbA1c measurements for achieving and maintaining glycemic control and consequently for reducing diabetes-related complications. Self-monitoring of blood glucose represents an important adjunct to HbA1c because it can distinguish among fasting, preprandial, and postprandial hyperglycemia; detect glycemic excursions; identify hypoglycemia; and provide immediate feedback to patients about the effect of food choices, activity, and medication on glycemic control.
_
HbA1c as a measure of glycemic control:
Numerous studies have shown that elevated HbA1c is associated with an increased risk of complications in patients with type 1 and type 2 diabetes mellitus and that lowering HbA1c reduces such risk. In the DCCT, reductions in HbA1c were accompanied by proportional reductions in the risk of complications, with clinically meaningful risk reductions observed even when HbA1c was reduced toward the normal range of less than 6%. Similar findings were observed in patients with newly diagnosed type 2 diabetes in the United Kingdom Prospective Diabetes Study, in which intensive blood glucose control yielded a 25% reduction in the risk of microvascular complications (P=0.0099) and a 16% risk reduction for myocardial infarction (P=0.05) compared to conventional therapy. Analysis of these data in terms of HbA1c levels revealed a continuous relationship between HbA1c and the risk of complications, with each 1% decrease in HbA1c resulting in statistically significant reductions of 37% for microvascular complications and 14% for myocardial infarction (P<0.0001). These findings are similar to those derived from the DCCT and indicate that the much larger number of patients with type 2 diabetes benefit from glucose lowering to the same degree as those with type 1 diabetes. Thus, HbA1c serves as a surrogate for the risk of microvascular and macrovascular complications, and these results firmly establish HbA1c as a useful measure of long-term glycemic control.
_
For someone who doesn’t have diabetes, a normal A1c level can range from 4.5 to 6 percent. Someone who’s had uncontrolled diabetes for a long time might have an A1c level above 8 percent. When the A1c test is used to diagnose diabetes, an A1c level of 6.5 percent or higher on two separate tests indicates you have diabetes. A result between 5.7 and 6.4 percent is considered prediabetes, which indicates a high risk of developing diabetes. For most people who have previously diagnosed diabetes, an A1c level of 7 percent or less is a common treatment target. Higher targets may be chosen in some individuals. If your A1c level is above your target, your doctor may recommend a change in your diabetes treatment plan. Remember, the higher your A1c level, the higher your risk of diabetes complications. Also keep in mind that the normal range for A1c results may vary somewhat among labs. If you consult a new doctor or use a different lab, it’s important to consider this possible variation when interpreting your A1c test results.
_
Convert A1c to estimated average glucose (eAG):
The A1c-Derived Average Glucose (ADAG) Study is an international study sponsored by the American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD), and International Diabetes Federation (IDF). The objective of the ADAG Study was to define the mathematical relationship between A1c and estimated average glucose (eAG) and determine if A1c could be reliably reported as eAG, which would be in the same units as daily self-monitoring. The ADAG Study establishes what has long been assumed but never demonstrated… that A1c does represent average glucose over time. With that relationship demonstrated and defined, health care providers can now report A1c results to patients in the same units that they are using for self-monitoring (i.e., mg/dl) which should benefit clinical care. Reporting glucose control as ‘average glucose’ will assist health care providers and their patients in being able to better interpret the A1C value in units similar to what patients see regularly through their self-monitoring.
_
_
New formula to convert your A1c to estimated average blood sugar in either mg/dl or mmol/L:
This new formula is based on CGMS data and is believed to be more accurate than either the DCCT formula or the Nathan Formula.
Estimated Average Blood Glucose in mmol/L = [(1.583 X A1c) – 2.52]
The conversion factor used to convert mmol/L to mg/dl is 18.
_
Many patients who practice SMBG already see an “average glucose” on their blood glucose meters.
Is eAG the same thing?
No, an eAG value is unlikely to match the average glucose level shown on a person’s meter. Because people with diabetes are more likely to test more often when their blood glucose levels are low—first thing in the morning, and before meals—the average of the readings on their meter is likely to be lower than their eAG, which represents an average of their glucose levels 24 hours a day, including post-meal periods of higher blood glucose when people are less likely to test. One advantage of using eAG as a measure of glucose control is that it will help patients more directly see the difference between their individual meter readings and how they are doing with their glucose management overall. A range of factors has been postulated to influence the relationship between HbA1c and BG. In particular, the time of BG measurement (fasting, postprandial, etc.) and the frequency and timing of BG measurement appear to have significant impact on this relationship. Analysis of data from one clinical study found that among individual time points, the afternoon and evening prandial glucose readings showed higher correlations with HbA1c than the morning time points.
_
Estimating A1c from SMBG:
Accuracy and Robustness of Dynamical Tracking of Average Glycemia (A1c) to Provide Real-Time Estimation of Hemoglobin A1c Using Routine Self-Monitored Blood Glucose Data: 2013 study:
Laboratory hemoglobin A1c (HbA1c) assays are typically done only every few months. However, self-monitored blood glucose (SMBG) readings offer the possibility for real-time estimation of HbA1c. Researchers present a new dynamical method tracking changes in average glycemia to provide real-time estimation of A1c (eA1c). In diabetes, the struggle for tight glycemic control results in large BG fluctuations over time. These fluctuations are the measurable result of the action of a complex dynamical system, influenced by many internal and external factors, including the timing and amount of insulin and other drug therapies, food eaten, physical activity, etc. The macro (human)-level optimization of this system depends on self-treatment behavior. Such an optimization has to be based on feedback utilizing readily available data, such as SMBG. Although HbA1c is the gold standard marker for average glycemia, HbA1c assays typically require a laboratory and are routinely done only every few months. Thus, a method to track changes in average glycemia in between laboratory assessments is needed. SMBG offers this possibility, provided that appropriate algorithms are used to retrieve SMBG data. This report describes a method for tracking changes in average glycemia, based on a conceptually new approach to the retrieval of SMBG data. The principal premise of this approach is the understanding of HbA1c fluctuation as the measurable effect of the action of an underlying dynamical system. SMBG provides occasional glimpses at the state of this system, and, using these measurements, the hidden underlying system trajectory can be reconstructed for each individual. Using compartmental modeling, researchers constructed a new two-step algorithm that includes real-time eA1c from fasting glucose readings, updated with any new incoming fasting SMBG data point, and initialization and calibration of the estimated HbA1c trace with daily SMBG profiles taken approximately every month. A conceptually new, clinically viable procedure has been developed for real-time tracking of average glycemia from self-monitoring data. The average glucose tracing is then converted into running estimates of A1c, which can be presented to the patient daily. This technique allows for simple parameterization of the dynamics of average glycemia and thereby HbA1c, has a robust estimation procedure capable of working on sparse readings of fasting BG and occasional seven-point SMBG profiles, and has an inherent capability for calibration of the algorithm using SMBG profiles and/or reference HbA1c readings. It should be emphasized, however, that this procedure is not intended as a substitute for laboratory assessments of HbA1c—it should be viewed as a surrogate measure that allows convenient tracing of average glucose, readily implementable in a point-of-care SMBG device.
_
Potential Alternatives to HbA1C:
Two other analytes, fructosamine and 1,5-anhydroglucitol (1,5-AG), have been evaluated as intermediate markers of glycemia. The fructosamine assay measures glycation of serum proteins, principally albumin, that have a shorter half-life than hemoglobin. Thus, fructosamine provides an index of glycemia over a shorter period (approximately 2 weeks) compared to HbA1c measurements. Each 75 µmol change equals a change of approximately 60 mg/dl blood sugar or 2% HbA1c. Unlike A1c, fructosamine is not affected by the varying length of red blood cell lifespans in different individuals. Fructosamine is especially useful in people who are anemic, or during pregnancy, when hormonal changes cause greater short-term fluctuations in blood glucose levels. The accuracy and clinical utility of fructosamine have been questioned because of interference from various substances. The 1,5-AG assay measures serum levels of a compound that competes with glucose for reabsorption at the renal tubule and was recently approved by the US Food and Drug Administration. Used in Japan for more than a decade, 1,5-AG levels appear to be less sensitive to small changes in glycemic control at high HbA1c levels. It cannot identify hypoglycemia, and results are influenced by impaired renal function. Future studies may support the use of 1,5-AG as a means to detect postprandial glycemic excursions.
_________
Advantages and disadvantages of FPG, OGTT and HbA1c vis-à-vis diagnosis of diabetes:
_
FPG for the diagnosis of diabetes:
Advantages:
•Glucose assay easily automated
•Widely available
•Inexpensive
•Single sample
Disadvantages:
•Patient must fast 8 hr
•Large biological variability
•Diurnal variation
•Sample not stable
•Numerous factors alter glucose concentrations, e.g., stress, acute illness
•No harmonization of glucose testing
•Concentration varies with source of the sample (venous, capillary, or arterial blood)
•Concentration in whole blood is different from that in plasma
•Guidelines recommend plasma, but many laboratories measure serum glucose
•FPG less tightly linked to diabetes complications (than A1c)
•Reflects glucose homeostasis at a single point in time
_
OGTT for the diagnosis of diabetes:
Advantages
•Sensitive indicator of risk of developing diabetes
•Early marker of impaired glucose homeostasis
Disadvantages:
•Lacks reproducibility
•Extensive patient preparation
•Time-consuming and inconvenient for patients
•Unpalatable
•Expensive
•Influenced by numerous medications
•Subject to the same limitations as FPG, namely, sample not stable, needs to be performed in the morning, etc.
_
A1c for the diagnosis of diabetes:
Advantages
•Subject need not be fasting
•Samples may be obtained any time of the day
•Very little biological variability
•Sample stable
•Not altered by acute factors, e.g., stress, exercise
•Reflects long-term blood glucose concentration
•Assay standardized across instruments
•Accuracy of the test is monitored
•Single sample, namely whole blood
•Concentration predicts the development of microvascular complications of diabetes
•Used to guide treatment
Disadvantages:
•May be altered by factors other than glucose, e.g., change in erythrocyte life span, ethnicity
•Some conditions interfere with measurement, e.g., selected hemoglobinopathies
•May not be available in some laboratories/areas of the world
•Cost
____________
Self monitoring (measurement) of blood sugar (SMBG) definition:
SMBG is checking the level of glucose in their blood regularly by patients themselves with diabetes mellitus. SMBG can be performed at home, work, or elsewhere — the process involves pricking a fingertip to collect a drop of blood, absorbing the blood with a test strip, and inserting the test strip into an electronic glucose monitor (glucometer) which then displays a number on its screen. Glucose meters are widely used in hospitals, outpatient clinics, emergency rooms, ambulatory medical care (ambulances, helicopters, cruise ships) besides home self-monitoring. Glucose meters provide fast analysis of blood glucose levels and allow management of both hypoglycemic and hyperglycemic disorders with the goal of adjusting glucose to a near-normal range, depending on the patient group. As SMBG is invasive and painful, non-invasive techniques are developed to determine glucose level in body fluids. Non-invasive techniques can be used by patients themselves or medical personnel. So if I have to define SMBG today, I would call it measurement of glucose in blood either directly (invasive) or indirectly through other body fluids (non-invasive) by patients themselves or their care givers or medical personnel outside laboratory. So blood glucose measured by nurses by glucometer or non-invasive technique would also fall under umbrella of SMBG as it is not done in a laboratory.
__________
SMBG technique:
__
First drop vs. second drop of blood for SMBG:
Many insulin-treated patients have to perform SMBG for a lifetime—some of them every day. Discarding the first drop of blood and refraining from squeezing the finger makes measurements more complex and necessitates deeper and more painful punctures. International guidelines and studies about SMBG (e.g., the American Diabetes Association [ADA] and the Diabetes UK guidelines) recommend using the first drop of blood after washing the hands. Some also allow squeezing or milking the finger. The manufacturer’s instructions of the meter used in the study include washing hands with warm water and soap and drying the hands. The first drop of blood can be used after gently squeezing the finger. In daily practice, patients cannot or do not always wash their hands before performing SMBG. In international guidelines, these situations are not discussed.
_
The Use of the First or the Second Drop of Blood: squeezing or not: a study:
There is no general agreement regarding the use of the first or second drop of blood for glucose monitoring. This study investigated whether capillary glucose concentrations, as measured in the first and second drops of blood, differed ≥10% compared with a control glucose concentration in different situations. Capillary glucose concentrations were measured in two consecutive drops of blood in the following circumstances in 123 patients with diabetes: without washing hands, after exposing the hands to fruit, after washing the fruit-exposed hands, and during application of different amounts of external pressure around the finger. The results were compared with control measurements. Not washing hands led to a difference in glucose concentration of ≥10% in the first and in the second drops of blood in 11% and 4% of the participants, respectively. In fruit-exposed fingers, these differences were found in 88% and 11% of the participants, respectively. Different external pressures led to ≥10% differences in glucose concentrations in 5–13% of the participants. Authors recommend washing the hands with soap and water, drying them, and using the first drop of blood for self-monitoring of blood glucose. It does not matter which finger is used for glucose measurements. If washing hands is not possible, and they are not visibly soiled or exposed to a sugar-containing product, it is acceptable to use the second drop of blood after wiping away the first drop. External pressure may lead to unreliable readings. Firm squeezing of the finger should be avoided.
_
Other two studies investigated the differences between glucose concentrations in the first and the second drops of blood. Both of these studies, however, involved volunteers without diabetes. In one study of 53 volunteers, no differences were found in the readings when the hands were clean. Glucose readings for 25 volunteers in the other study were shown to be greatly affected when the fingers were exposed to glucose (i.e., fruit). Even the third drop of blood cannot be used in these cases. Fruhstorfer and Quarder also investigated the influence of milking the finger in 10 volunteers without diabetes and concluded that milking the finger gives correct glucose values. In another study, authors used two pressures to explore whether there would be any influence on the capillary glucose concentration. Venous stasis is achieved with a pressure of 40 mmHg. A pressure of 240 mmHg is above the systolic pressure of the participants. This study shows more deviation between the glucose concentrations with the higher pressure.
___
Lancet: Finger-pricking (lancing) device:
A finger-pricking device (called a lancet) is used to get the drop of blood. The lancet can often be set at different depths for different people. The adjustable lancets are particularly good for young children who have tender skin and may not need much lancing depth. Remember to change the lancet every day. A sharp lancet helps prevent injury and infection.
_
_
How often do you recommend changing lancets?
In the early days of blood glucose self-monitoring, pricking the finger to get a “hanging drop” of blood often hurt and left a scar. This was because the procedure created a laceration, rather than a puncture. We’ve come a long way since then, with improved spring-loaded devices, strips that require less blood and lancets that are sharper and usually coated with a lubricant. Lancets are now much more comfortable to use and less likely to cause a scar. Today’s lancets are so good that they are commonly reused. The reasons to reuse lancets are obvious: It’s cheaper and quicker not to have to change them each time; it’s easier not to carry extra lancets around; and, for some users, the lancets actually seem more comfortable after being “broken in.” Since the lancet goes into the subcutaneous space and is not being used intravenously, and since blood is flowing out of the body, sterility is generally not an issue. The rate of infections and injury from lancets is extremely low. Many people, however, are not able to reuse lancets because they feel discomfort or they experience scarring if the lancet is not in optimal condition. Once a lancet has been used, its surface is rougher, the lubricant wears off and the point is duller. Any handling of the lancet, such as cleaning with alcohol, tends to worsen it. For these individuals, using a new lancet each time is well worthwhile.
_
Fingertip puncture pain:
Although the fingertip possesses well-developed capillaries to provide enough blood for the test, pain receptors concentrated on the fingertip induce significant pain when the skin is punctured. As a result, some patients avoid the self test, which could lead to failure of glucose control. In a survey by Park et al., 55% of the diabetes patients responded to the survey questions, and only 35% performed the self-test. These survey results show that only 20% of the patients may perform the routine self-test to control their blood glucose levels. SMBG using capillary blood sampled from the finger is a standard technique for the management of diabetes. However, it induces pain and may force the patient to avoid the test, thereby leading to a failure in maintaining the appropriate glucose levels. Therefore, the pain experienced during sampling is considered to be a significant problem, and a few alternative methods have been suggested. Noninvasive bloodless glucose measurement techniques have been evaluated; however, their accuracy and consistency have not been proven in clinical application, and the higher costs of commercialization of these techniques may also have to be considered. Capillary blood sampling from an alternative site, such as the forearm, could minimize pain, and perhaps be a practical solution to this problem. While blood sampling from the forearm induces significantly less pain than that from the finger, only a small amount of blood, usually less than a few microliters, can be obtained due to the low degree of capillary distribution in the forearm. This small volume of blood is not sufficient for traditional glucometers. Fortunately, modern high-end but inexpensive glucometers can provide accurate glucose measurements within 5 sec by using less than 1 micro-liter of blood. Therefore, to minimize the pain during glucose self-testing, blood sampling from the forearm is a feasible and practical option.
_
Take the pain out of blood sugar checks:
Pricking fingers is a vital part of daily diabetes management. In a recent study, up to 35% of the participants stated that pain is the main reason people with diabetes refrain from regular blood glucose testing. One factor contributing to greater pain sensation when pricking the finger is wrong handling of the lancing device. You can test more comfortably with these 7 easy steps:
1. Ensure hands are clean and dry.
2. Lance on the side of the fingertip rather than the pad.
3. Keep the skin taut by pressing the lancing device firmly against the skin.
4. Select a penetration depth as shallow as possible but still produces blood.
5. Alternate fingers daily and take the necessary steps to ensure good blood circulation.
6. Consider testing beyond the fingertip. If you and your healthcare professional agree that checking from other sites is right for you, you may experience less pain after a blood sugar test if you use your palm, forearm or upper arm instead of your sensitive fingertips.
7. Use a fresh lancet. Today’s lancets are so tiny that just a single use can bend or dull the tips. As a result, they can hurt more if you try to reuse them.
_
Laser lancet:
It is a laser lancing device that uses a laser beam to draw a drop of blood rather than using a steel lancet.
How does it work?
The fingertip is placed over the disposable lens cover where the laser beam comes out of. Water in the skin absorbs the energy from the laser beam, instantly vaporizing tissue which draws blood.
_______
Why alternate site?
During the past decade, several studies have clearly established the importance of frequent daily self-monitoring of blood glucose to control one’s glycemic condition and thereby reduce the onset of complications caused by diabetes. Pain associated with finger lancing is one of the major barriers to frequent daily testing. Consequently, it has been argued that skin lancing at less sensitive parts of the body would increase testing compliance. Suzuki was the first to perform such alternate-site testing. In response to the need for less painful testing, several manufacturers have now released products that are specifically designed to be used at body sites other than the fingertip.
_
Finger tip vs. alternate site:
Capillary blood glucose levels at the fingertip have been shown to correlate well with systemic arterial blood glucose levels. During times of blood glucose stability, identical glucose levels were demonstrated from alternate sites (e.g., forearm) as compared with finger tip samples. However, at times of rapid change, mainly due to blood flow variability, levels from alternate sites differ considerably. Capillary blood glucose measured from the forearm is lower than fingertip values at times of rapid increases (>2 mg/dL/min) in systemic blood and higher during rapid decreases. Samples from the dorsal forearm have been shown to correspond better to fingertip values when compared with volar forearm samples. The only exception for the alternate site testing is the palm. The skin type of the palm is in the same skin category, hairless or glabrous skin, as the fingertip, and they share the same amount of blood flow, which is considerably more (five to 20 times) than the blood flow to most alternate sites like the forearm. In that respect, blood flow to forearm and abdomen upper dermal region has been reported to be comparable.
_
Alternate sites:
Many children prick sites other than the fingers or toes because they may not hurt as much. The most common alternate site is the forearm. Other places to get blood include the fleshy part of the hand, upper arm, thigh, and back of the calf. The lancet must be dialed to the maximum depth to get enough blood from these sites. You would need a meter that works for these testing sites. The main problem with not using the fingertips is that the blood flow through the arm is slower than through the fingers. The slower blood flow means the blood sugar value from the arm is 10 minutes behind the fingertip.
_
Alternate site testing:
Several blood glucose meters are now available that use sites other than the finger to obtain blood samples in an effort to reduce the discomfort involved with finger sticks. Monitoring at alternate sites, such as the forearm, palm of the hand or thigh, may give slightly lower results than those taken at the fingertips, since they may sample venous blood rather than capillary blood. While this should not be a problem if the patient uses one or the other site exclusively, the between-test variability will increase if numerous sites (such as fingertips and forearm sites) are used. In addition, during times when the blood glucose concentration is either rising rapidly (such as immediately after food ingestion) or falling rapidly (in response to rapidly acting insulin or exercise), blood glucose results from alternate sites may give significantly delayed results compared with finger stick readings. In comparison, blood samples taken from the palm near the base of the thumb (thenar area), demonstrate a closer correlation to fingertip samples at all times of day, and during periods of rapid change in BG levels.
_
Alternate site testing should only be used when blood sugar is stable:
•Immediately before a meal
•When fasting
•Near bedtime
_
Always check from your fingertip, however, when blood sugar may be changing:
1. Following a meal, when blood sugar is rising quickly
2. After exercise
3. Whenever you think your blood sugar might be low or falling
4. You have just taken insulin
5. The results do not agree with the way you feel
6. You are ill
7. You are under stress
Also, you should never use results from an alternative sampling site to calibrate a continuous glucose monitor (CGM), or in insulin dosing calculations.
_
Forearm meter:
_
Whole-Blood Glucose Testing at Alternate Sites: 2001 study:
Glucose values and hematocrit of capillary blood drawn from fingertip and forearm:
In this cross-sectional study of 50 nonfasting subjects whose blood glucose concentration changed to various degrees during the experiment, no significant glucose difference was observed between the capillary beds of the forearm and fingertip, regardless of whether glucose was assayed with HemoCue or the Sof-Tact Blood Glucose System. On the other hand, Hb concentration and Hct were found to be significantly higher in the capillary blood of the forearm. No explanation has yet been given for the occurrence of Hb concentration differences across the integument.
_
Forearm blood glucose testing in diabetes mellitus: 2002 study:
Self monitoring of blood glucose plays a vital role in the treatment plan of children with diabetes mellitus. Regular self blood glucose monitoring enables the appropriate changes to be made in the treatment and management of the child’s diabetes to meet individual goals and needs. Barriers to frequent self monitoring include the pain and trauma associated with the finger prick necessary to obtain blood for the test. Non-compliance with blood glucose monitoring is common, especially in adolescents. Although modern blood glucose meters only require a small sample of blood, monitoring remains a problem. Using an alternate site for sampling, namely the forearm, may be beneficial to the patient and reduce the level of pain they experience. The main objective of the study was to assess the accuracy of a forearm testing device (SoftSense) in a paediatric population, in comparison to a standard reference laboratory method. Blood glucose measurements from samples taken from the forearm and the finger were compared in an outpatient setting from 52 children and adolescents with diabetes mellitus aged 6–17 years. Opinions on forearm sampling were collected by questionnaire. Blood glucose results obtained from forearm sampling correlated well with results from the finger measured by the Yellow Springs Instrument analyser. Error grid analysis showed that 100% of measurements were clinically acceptable; 61% of children reported that forearm testing was painless and 19% that it was less painful than finger prick testing.
Conclusion: Forearm testing is an acceptable alternative to finger prick testing for blood glucose measurement in children and adolescents.
_________
What if I can’t get a drop of blood?
If you don’t get blood from your fingertip, try washing your hands in hot water to get the blood flowing. Then dangle your hand below your heart for a minute. Prick your finger quickly and then put your hand back down below your heart. You might also try slowly squeezing the finger from the base to the tip. If lancing device has dial-a-depth, increase setting by 1 level. Use a new lancet every time you check blood glucose.
_
Your fingertips may get sore from frequent pricking for blood sugar testing. To help prevent sore fingertips:
1. Always prick the side of your finger. Do not prick the tip of your finger. This increases the pain, and you may not get enough blood to do the test accurately. Also, do not prick your toes to get a blood sample. This can increase your risk of getting an infection in your foot.
2. Don’t squeeze the tip of your finger. If you have trouble getting a drop of blood large enough to cover the test area of the strip, hang your hand down below your waist and count to 5. Then squeeze your finger, beginning close to your hand and moving outward toward the tip of your finger.
3. Use a different finger each time. Keep track of which finger you stick so that you don’t use some fingers more than others. If a finger becomes sore, avoid using it to test your blood sugar for a few days.
4. Use a different device. If you are having trouble with sore fingers, you may want to try a meter that obtains a blood sample from sites other than the fingers, such as the palm of the hand or the forearm.
_
What supplies are needed for SMBG?
Doing a blood test requires a method of pricking the skin to get a drop of blood as well as a method of reading the results. Results are read using test strips that are put in a blood glucose meter.
______
HOW TO PERFORM BLOOD SUGAR TESTING:
To test your blood sugar level, collect your blood glucose meter, a test strip and lancing device. The following steps include general guidelines for testing blood sugar levels; you should get specific details for your blood glucose monitors from the package insert or your healthcare provider. Never share blood glucose monitoring equipment or fingerstick lancing devices. Sharing of this equipment could result in transmission of infection, such as hepatitis B.
1. Wash hands with soap and warm water. Dry hands.
2. Prepare the lancing device by inserting a fresh lancet. Lancets that are used more than once are not as sharp as a new lancet, and can cause more pain and injury to the skin.
3. Prepare the blood glucose meter and test strip (instructions for this depend upon the type of glucose meter used).
4. Choose your spot—don’t check from the same finger all the time.
5. Use the lancing device to obtain a small drop of blood from your fingertip or alternate site (like the skin of the forearm). Alternate sites are often less painful than the fingertip. However, results from alternate sites are not as accurate as fingertip samples when the blood glucose is rising or falling rapidly. If you have difficulty getting a good drop of blood from the fingertip, try rinsing your fingers with warm water, shaking the hand below the waist, or squeezing (“milking”) the fingertip.
6. Apply the blood drop to the test strip in the blood glucose meter. The results will be displayed on the meter after several seconds.
7. View your test result and take the proper steps if your blood sugar is too high or low, based on your healthcare professionals’ recommendations.
8. Dispose of the used lancet in a puncture-resistant sharps container (not in household trash).
9. Record the results in a logbook, hold them in the meter’s memory or download to a computer so you can review and analyze them later.
______
What affects the Test:
Reasons you may not be able to have the test or why the results may not be helpful include:
1. Alcohol in the drop of blood. If you clean your skin with rubbing alcohol, let the area dry completely before sticking it with the lancet.
2. Water or soap on your finger.
3. Squeezing your fingertip.
4. A drop of blood that is either too large or too small.
5. Very low (below 40 mg/dL or 2.2 mmol/L) or very high (above 400 mg/dL or 22.2 mmol/L) blood sugar levels.
6. Humidity or a wet test strip. Do not store your test strips in the washroom. When you remove a strip from the bottle, promptly secure the lid back on the bottle to prevent humidity from damaging the unused strips.
__________
Glucometer overview:
_
_
A glucose meter (or glucometer) is a medical device for determining the approximate concentration of glucose in the blood. It can also be a strip of glucose paper dipped into a substance and measured to the glucose chart. It is a key element of home blood glucose monitoring by people with diabetes mellitus or hypoglycemia. A small drop of blood, obtained by pricking the skin with a lancet, is placed on a disposable test strip that the meter reads and uses to calculate the blood glucose level. The meter then displays the level in mg/dl or mmol/l. Blood glucose meters today are small, portable, and easy to use. The mark of a good meter is one that the patient will use regularly and that returns accurate and precise results. Over the past few years the trend with blood glucose meters has been to maximize patient comfort and convenience by reducing the volume of the blood sample required. The blood sample size is now small enough that alternate-site testing is possible. This eliminates the need to obtain blood from the fingers and greatly reduces the pain associated with daily testing. Accurate and precise results have been increased by using better test strips, electronics, and advanced measurement algorithms. Other conveniences include speedy results, edge fill strips, and illuminated test strip ports, to name just a few. Although the cost of using blood glucose meters seems high, it is believed to be a cost benefit relative to the avoided medical costs of the complications of diabetes.
_
Benefits of glucometer:
There are many benefits of using a glucometer for diabetics:
1. It allows diabetics to take care of themselves sans any need to visit doctors and labs regularly.
2. It works towards promoting well-being of the patient.
3. It helps to detect and confirm hypoglycemia.
4. These meters ensure better understanding of medications.
5. The meters help in altering medications.
6. It also helps in detecting infections. Since high blood sugars may be a sign of infection or illness, timely assistance can save many health problems.
_
Disadvantages of home blood glucose testing:
The disadvantages are mainly seen when either the patient lacks motivation to test or does not have sufficient education on how to interpret the results to make sufficient use of home testing equipment. Where this is the case, the following disadvantages may outweigh the potential benefits:
1. Anxiety about one’s blood sugar control and state of health
2. The physical pain of finger pricking
3. Costly affair
_
There are several key characteristics of glucose meters which may differ from model to model:
•Size: The average size is now approximately the size of the palm of the hand. They are battery-powered.
•Test strips: A consumable element containing chemicals that react with glucose in the drop of blood is used for each measurement. For some models this element is a plastic test strip with a small spot impregnated with glucose oxidase and other components. Each strip is used once and then discarded. Instead of strips, some models use discs, drums, or cartridges that contain the consumable material for multiple tests.
•Coding: Since test strips may vary from batch to batch, some models require the user to manually enter in a code found on the vial of test strips or on a chip that comes with the test strip. By entering the coding or chip into the glucose meter, the meter will be calibrated to that batch of test strips. However, if this process is carried out incorrectly, the meter reading can be up to 4 mmol/L (72 mg/dL) inaccurate. The implications of an incorrectly coded meter can be serious for patients actively managing their diabetes. This may place patients at increased risk of hypoglycemia. Alternatively, some test strips contain the code information in the strip; others have a microchip in the vial of strips that can be inserted into the meter. These last two methods reduce the possibility of user error. One manufacturer has standardized their test strips around a single code number, so that, once set, there is no need to further change the code in their older meters, and in some of their newer meters, there is no way to change the code.
•Volume of blood sample: The size of the drop of blood needed by different models varies from 0.3 to 1 μl. (Older models required larger blood samples, usually defined as a “hanging drop” from the fingertip.) Smaller volume requirements reduce the frequency of unproductive pricks.
•Alternative site testing: Smaller drop volumes have enabled “alternate site testing” — pricking the forearms or other less sensitive areas instead of the fingertips. Although less uncomfortable, readings obtained from forearm blood lag behind fingertip blood in reflecting rapidly changing glucose levels in the rest of the body.
•Testing times: The times it takes to read a test strip may range from 3 to 60 seconds for different models.
•Display: The glucose value in mg/dl or mmol/l is displayed on a digital display. The preferred measurement unit varies by country: mg/dl are preferred in the U.S., France, Japan, Israel, and India. mmol/l are used in Canada, Australia, China and the UK. Germany is the only country where medical professionals routinely operate in both units of measure. (To convert mmol/l to mg/dl, multiply by 18. To convert mg/dl to mmol/l, divide by 18.) Many meters can display either unit of measure; there have been a couple of published instances in which someone with diabetes has been misled into the wrong action by assuming that a reading in mmol/l was really a very low reading in mg/dl, or the converse. In general, if a value is presented with a decimal point, it is in mmol/l, without a decimal it is most likely mg/dl.
•Clock/memory: All meters now include a clock that is set by the user for date and time and a memory for past test results. The memory is an important aspect of diabetes care, as it enables the person with diabetes to keep a record of management and look for trends and patterns in blood glucose levels over days and weeks. Most memory chips can display an average of recent glucose readings. A known deficiency of all current meters is that the clock is often not set to the correct time (i.e. – due to time changes, static electricity, etc…) and therefore has the potential to misrepresent the time of the past test results making pattern management difficult.
•Data transfer: Many meters now have more sophisticated data handling capabilities. Many can be downloaded by a cable or infrared to a computer that has diabetes management software to display the test results. Some meters allow entry of additional data throughout the day, such as insulin dose, amounts of carbohydrates eaten, or exercise. A number of meters have been combined with other devices, such as insulin injection devices, PDAs, cellular transmitters and Game Boys. A radio link to an insulin pump allows automatic transfer of glucose readings to a calculator that assists the wearer in deciding on an appropriate insulin dose.
_
Blood glucose vs. plasma glucose:
Glucose levels in plasma (one of the components of blood) are generally 10%–15% higher than glucose measurements in whole blood (and even more after eating). This is important because home blood glucose meters measure the glucose in whole blood while most lab tests measure the glucose in plasma. Currently, there are many meters on the market that give results as “plasma equivalent,” even though they are measuring whole blood glucose. The plasma equivalent is calculated from the whole blood glucose reading using an equation built into the glucose meter. This allows patients to easily compare their glucose measurements in a lab test and at home. It is important for patients and their health care providers to know whether the meter gives its results as “whole blood equivalent” or “plasma equivalent.”
_
Glucose meters vary in their method of analysis. Some meters take a fixed volume of patient whole blood, lyse the cells, and analyze the amount of glucose in that volume of lysate. Other meters utilize a series of absorbent pads to separate the cellular portion of a sample from the serum/plasma portion. This allows only serum/plasma to react with the enzymatic reagents. In order to harmonize glucose results, consensus recommends reporting serum/plasma-based results from glucose meters such that the value will most closely match that of a laboratory method using a serum/plasma sample. Glucose meter whole blood lysate results must therefore be corrected to serum/plasma by either applying a fixed mathematical offset to obtain a “plasma-corrected result” (assuming a normal hematocrit) or correcting the whole blood lysate result using the patient’s actual hematocrit. There are meters on the market that use both types of correction. However, it is more common for manufacturers whose meters separate the cellular portion of the sample to set the calibration of the meter against a laboratory method in order to report a “plasma-calibrated” result. The differences between these various calibration and correction functions are one source of variability among the many glucose meter models when analyzing the same specimen.
_
Measurement of glucose content in plasma from capillary blood in diagnosis of diabetes mellitus: a 2003 study:
Overall, there is good correlation between glucose values obtained from ear capillary blood and those from peripheral venous plasma, but there are considerable individual differences. Results obtained with these two methods are generally not interchangeable and the converted values should not be used in the diagnosis of diabetes mellitus, because of the risk of misclassification. The aim of this study was to investigate whether these differences might be less significant if measurements were taken at the plasma phase of capillary blood and expressed directly as capillary plasma results and if finger capillary blood were used instead of ear capillary blood. The Hitachi 717 instrument was used for measurements of glucose concentrations in venous plasma, the Cobas Mira S in capillary whole blood and the Accu-Chek Inform from Roche in capillary plasma. The conclusions drawn were (1) capillary ear blood glucose concentration correlates well with capillary finger blood concentration and the two sites can be used interchangeably, yielding similar results in the individual patient; (2) sampling variation is almost the same (approx. 0.16 mmol/L) on capillary plasma and capillary whole blood from finger and ear. Sampling variation for venous plasma measured on the Hitachi instrument was 0.13 mmol/L; not significantly better; (3) the analytical imprecision of glucose measurements on capillary plasma (Accu-Chek Inform) and capillary whole blood (haemolysate method) is almost the same (approx. 2.0%). The analytical imprecision of glucose measurements on venous plasma is 0.9% using a laboratory method and almost twice as high using Accu-Chek Inform (2.1%); (4) determination of capillary plasma values in the finger did not improve the correlation with venous plasma values. Even though average values were in better concordance, individual differences did not change. For some persons, both ear- and finger capillary blood measurements deviate significantly from results on venous plasma, such that they cannot be used for diagnosis of diabetes mellitus; (5) the main factor for good correlation is the sampling site. Results obtained on plasma and whole blood from the same puncture correlate well; (6) neither capillary blood nor capillary plasma correlates with the venous plasma method recommended by the American Diabetes Association. It is concluded that physiologic differences in glucose content in capillary- and venous blood prohibit the random use of these two materials in the diagnosis of diabetes.
_
Meter Types:
There are continuous and discrete (single-test) meters on the market today, and implantable and noninvasive meters are in development. Continuous meters are by prescription only and use a subcutaneous electrochemical sensor to measure at a programmed interval.
_
Electrochemical meter:
Single-test meters use electrochemical or optical reflectometry to measure the glucose level in units of mg/dL or mmol/L. The majority of blood glucose meters are electrochemical. Electrochemical test strips have electrodes where a precise bias voltage is applied with a digital-to-analog converter (DAC), and a current proportional to the glucose in the blood is measured as a result of the electrochemical reaction on the test strip. There can be one or more channels, and the current is usually converted to a voltage by a transimpedance amplifier (TIA) for measurement with an analog-to-digital converter (ADC). The full-scale current measurement of the test strip is in the range of 10µA to 50µA with a resolution of less than 10nA. Ambient temperature needs to be measured because the test strips are temperature dependent.
_
Optical-reflectometry meter:
Optical-reflectometry test strips use color to determine the glucose concentration in the blood. Typically, a calibrated current passes through two light-emitting diodes (LEDs) that alternately flash onto the colored test strip. A photodiode senses the reflected light intensity, which is dependent on the color of the test strip, which, in turn, is dependent on the amount of glucose in the blood. The photodiode current is usually converted to a voltage by a TIA for measurement with an ADC. The full-scale current from the photodiode ranges from 1µA to 5µA with a resolution of less than 5nA. Ambient temperature is required for this method.
_
Meter use for hypoglycemia:
Although the apparent value of immediate measurement of blood glucose might seem to be higher for hypoglycemia than hyperglycemia, meters have been less useful. The primary problems are precision and ratio of false positive and negative results. An imprecision of ±15% is less of a problem for high glucose levels than low. There is little difference in the management of a glucose of 200 mg/dl compared with 260 (i.e., a “true” glucose of 230±15%), but a ±15% error margin at a low glucose concentration brings greater ambiguity with regards to glucose management. The imprecision is compounded by the relative likelihoods of false positives and negatives in populations with diabetes and those without. People with type 1 diabetes usually have glucose levels above normal, often ranging from 40 to 500 mg/dl (2.2 to 28 mmol/l), and when a meter reading of 50 or 70 (2.8 or 3.9 mmol/l) is accompanied by their usual hypoglycemic symptoms, there is little uncertainty about the reading representing a “true positive” and little harm done if it is a “false positive.” However, the incidence of hypoglycemia unawareness, hypoglycemia-associated autonomic failure (HAAF) and faulty counterregulatory response to hypoglycemia make the need for greater reliability at low levels particularly urgent in patients with type 1 diabetes mellitus, while this is seldom an issue in the more common form of the disease, type 2 diabetes mellitus. In contrast, people who do not have diabetes may periodically have hypoglycemic symptoms but may also have a much higher rate of false positives to true, and a meter is not accurate enough to base a diagnosis of hypoglycemia upon. A meter can occasionally be useful in the monitoring of severe types of hypoglycemia (e.g., congenital hyperinsulinism) to ensure that the average glucose when fasting remains above 70 mg/dl (3.9 mmol/l).
_
False results:
Probably the greatest concern when using glucose meters is false results. All users should be educated about factors contributing to false results. The Office of In Vitro Diagnostics (OIVD), a service of the FDA, evaluates glucose meters. They evaluate long term safety and effectiveness of the analysers and how devices are used. OIVD, in consultation with manufacturers and users, have produced a table of common problems encountered when using glucose meters as seen in the table below. Causes of false results may be patient/sample based or user/device based. Probably the most important advice for any user of a blood glucose meter is to question any result not consistent with the clinical picture. This needs to be investigated and, at a minimum, the test repeated.
Common problems with glucose meter results.
| Results | Problem | Recommendation |
| Falsely low results | Sensor strips not fully inserted into meter | Always be sure strip is fully inserted in meter |
| Not enough blood applied to strip | Repeat test with a new sample | |
| Patient in shock | Treat appropriately. Venous sample should be sent immediately to a laboratory | |
| Squeezing fingertip too hard because blood is not flowing | Repeat test with a new sample from a new stick | |
| Polycythaemia/increased haematocrit | Venous sample should be sent to a laboratory | |
| Falsely high results | Patient sample site (for example the fingertip) is contaminated with sugar | Always clean test site before sampling |
| Patient is dehydrated | Treat appropriately. Venous sample should be sent immediately to a laboratory | |
| Anemia/decreased haematocrit | Venous sample should be sent to a laboratory | |
| Variable results | Test strips/controls stored at temperature extremes | Store kit according to directions |
| Sites other than fingertips | Results from alternative sites may not match finger stick results | |
| Test strips/controls damaged | Always inspect package for cracks, leaks, etc. | |
| Dirty meter | Even small amounts of blood, grease, or dirt on a meter’s lens can alter the reading | |
| Error codes | Batteries low on power | Change batteries and repeat sample collection |
| Test will not complete | Check package details, calibration code, and expiry dates are all compatible |
_
Variables:
We make decisions based on the results of our blood glucose measurements – it is therefore important to us that the readings we obtain are true. What then, other than glucose, may affect the outcome of the test?
•System variables:
– Batch-to-batch or strip-to-strip variation of strips
– Meter-to-meter variability
•Testing variables:
– Environment – temperature, humidity, altitude
– User – technique (note hands should be dry and clean before finger-pricking), timing
•Patient variables:
– Blood sample – capillary or venous, red blood cell count
– Dehydration of the patient
Extreme hypo- or hyperglycemia (if the blood glucose level falls outside the working range of the meter a ‘LO’ or ‘HI’ message will usually be displayed)
_
Calibration:
Each pack of test strips usually comes with a special ‘calibration code’. This is a correction factor for the meter which is derived by comparing meter response with a standardised laboratory assay or ‘reference method’. For accurate readings it is essential that the meter is recalibrated between batches of strips. If you choose to check the accuracy of your meter using a ‘quality control’ solution then you must use one specially formulated by the meter manufacturer. It may be easy enough to make up a standard solution with known concentration of glucose; unfortunately though, such home-made standards, usually water-based, do not behave the same way as blood on the test strip.
_
Considerations for Glucose Meter selection:
| Feature | Clinical advantages |
| Smaller sample size requirement | Less painful, permits alternate site testing |
| Alternate site testing | Less discomfort for patients who use fingertips regularly (e.g., for typing) |
| Results in less than 15 seconds | Increased convenience |
_
_
How do you choose a Glucose Meter?
There are many different types of meters available for purchase that differs in several ways, including:
- accuracy
- amount of blood needed for each test
- how easy it is to use
- pain associated with using the product
- testing speed
- overall size
- ability to store test results in memory
- likelihood of interferences
- ability to transmit data to a computer
- cost of the meter
- cost of the test strips used
- doctor’s recommendation
- technical support provided by the manufacturer
- special features such as automatic timing, error codes, large display screen, or spoken instructions or results
Talk to your health care provider about the right glucose meter for you, and how to use it.
_
How can you check your meter’s performance? There are three ways to make sure your meter works properly:
1. Use liquid control solutions:
–every time you open a new container of test strips
–occasionally as you use the container of test strips
–if you drop the meter
–whenever you get unusual results
To test a liquid control solution, you test a drop of these solutions just like you test a drop of your blood. The value you get should match the value written on the test strip vial label.
2. Use electronic checks:
Every time you turn on your meter, it does an electronic check. If it detects a problem it will give you an error code. Look in your meter’s manual to see what the error codes mean and how to fix the problem. If you are unsure if your meter is working properly, call the toll-free number in your meter’s manual, or contact your health care provider.
3. Compare your meter with a blood glucose test performed in a laboratory:
Take your meter with you to your next appointment with your health care provider. Ask your provider to watch your testing technique to make sure you are using the meter correctly. Ask your health care provider to have your blood tested with a laboratory method. If the values you obtain on your glucose meter match the laboratory values, then your meter is working well and you are using good technique.
What should you do if your meter malfunctions?
If your meter malfunctions, you should tell your health care provider and contact the company that made your meter and strips.
_
What other supplies do you need?
All meters require test strips to operate – a small chemically treated strip that slides into the meter. After insertion, a drop of blood is placed on the opposite end of the strip that protrudes from the meter, and the meter reads the glucose level and displays the number on the screen. Some monitors use test strip drums or discs, which are self-enclosed packages of strips that automatically load without user intervention or handling. Small children and adults who have difficulties with their fine motor skills may find this type of monitor easier to use. You’ll also need a lancet (a small, fine needle) to get a blood sample for testing. Lancets are inserted into a lancet device – a spring-loaded mechanism about the size and shape of a pen. A dial allows the user to adjust the depth of the lancet stick. Typically there is a button that you push to release the lancet into a fingertip or other site to draw a blood sample. Lancets come in different gauges; the higher the gauge, the finer (i.e., thinner) the needle. Higher gauge needles are less painful, but they also may create a smaller blood sample. Your blood glucose monitor may also come with control solution (for calibrating the monitor per manufacturer’s directions for use) and a carrying case.
______
Glucometer sensor (test strips):
_
_
The sensor used has an electroenzymatic approach, which means that it takes advantage of glucose oxidation with a glucose oxidase enzyme. The presence of glucose oxidase catalyzes the chemical reaction of glucose with oxygen, which causes an increase in pH, decrease in the partial pressure of oxygen, and increase of hydrogen peroxide because of the oxidation of glucose to gluconic acid: The test strip measures changes in one or several of these components to determine the concentration of glucose. A negative voltage of –0.4 V is applied at the reference electrode. When blood or a glucose solution is placed in the strip, a chemical reaction occurs inside it, generating a small electrical current proportional to the glucose concentration. This current is constantly monitored while the strip is in place, allowing the device to monitor when blood is placed. After the chemical reaction stabilizes, 5 s, the voltage is read by the ADC and compared using a look-up table to obtain the proportional glucose value in mg/dL. This value is sent to the host computer to inform the glucose value. When choosing test strips, make sure they work in the meter you are using. Look for strips that need only a small drop of blood and can draw the blood into the strip (capillary action).
_
Caring for Strips:
It is important to care for your strips so that you get an accurate reading. To do this, refer to the manufacturer’s instructions. It will include recommendations like:
•Storing them in a dry place
•Replacing the cap immediately after use
•Checking the expiry date is valid.
__
_
Avoiding problems with meter usage:
Blood sugar meters need to be used and maintained properly. Follow these tips to ensure proper usage:
•Follow the instructions in the user manual for your device, as procedures may vary from one device to another.
•Use a blood sample size as directed in the manual because different meters require different sample sizes.
•Change batteries as recommended by the manufacturer.
•Use only test strips designed for your meter because not all devices and strips are compatible.
•Store test strips as directed.
•Don’t use expired test strips.
•Clean the device regularly as directed.
•Run quality control tests as directed.
•Check the manual for additional troubleshooting tips.
•Bring the meter with you to doctor appointments to address any questions and to demonstrate how you use your meter.
____
Recent advances in glucometer:
Recent advances include:
1. ‘Alternate site testing’, the use of blood drops from places other than the finger, usually the palm or forearm. This alternate site testing uses the same test strips and meter, is practically pain free, and gives the real estate on the finger tips a needed break if they become sore. The disadvantage of this technique is that there is usually less blood flow to alternate sites, which prevents the reading from being accurate when the blood sugar level is changing.
2. ‘No coding’ systems. Older systems required ‘coding’ of the strips to the meter. This carried a risk of ‘miscoding’, which can lead to inaccurate results. Two approaches have resulted in systems that no longer require coding. Some systems are ‘autocoded’, where technology is used to code each strip to the meter. And some are manufactured to a ‘single code’, thereby avoiding the risk of miscoding.
3. ‘Multi-test’ systems. Some systems use a cartridge or a disc containing multiple test strips. This has the advantage that the user doesn’t have to load individual strips each time, which is convenient and can enable quicker testing.
4. ‘Downloadable’ meters. Most newer systems come with software that allows the user to download meter results to a computer. This information can then be used, together with health care professional guidance, to enhance and improve diabetes management. The meters usually require a connection cable, unless they are designed to work wirelessly with an insulin pump, or are designed to plug directly into the computer.
_______
Specialized glucometer:
Hospital glucose meters:
Special glucose meters for multi-patient hospital use are now used. These provide more elaborate quality control records. Their data handling capabilities are designed to transfer glucose results into electronic medical records and the laboratory computer systems for billing purposes.
_
ACCU-CHEK® Aviva Expert, the first and only stand-alone blood glucose meter system with a built-in insulin calculator:
Roche announced that Roche’s ACCU-CHEK® Aviva Expert system, the first and only blood glucose meter system with a built-in insulin calculator to be approved by the U.S. Food and Drug Administration (FDA), is now available by prescription. The device represents a significant advancement in blood glucose meter technology for people with diabetes who take multiple daily insulin injections. The meter’s integrated bolus calculator provides easy-to-use and reliable dose recommendations based on automated calculations, eliminating the need for manual dosing calculations and estimations. A survey of ACCU-CHEK Aviva Expert users found that 79 percent reported increased confidence with insulin dose calculation, and 52 percent reported a reduced fear of hypoglycemia. In the United States, approximately 6 million people take insulin to help manage their diabetes. Many people also take multiple daily injections of insulin to help manage their disease, which requires them to calculate proper insulin dosage amounts based on their food intake and blood glucose readings. These calculations are complex, and constant precision is critical to determine the proper insulin dose. A multicenter study found that 63 percent of manually calculated insulin doses were incorrect. As an incorrect insulin dose can lead to serious health complications, including hypoglycemia, accurate calculations are required. Researchers from the U.S. Centers for Disease Control and Prevention (CDC) reported that there were nearly 100,000 emergency room (ER) visits each year between 2007 and 2011 that were attributed to insulin-related hypoglycemia and other errors, and that these visits accounted for roughly 9 percent of all ER visits due to drug reactions during this timeframe. The availability of the ACCU-CHEK Aviva Expert system marks an important, game-changing milestone in diabetes self-management by making the process of calculating insulin dosage easier and less susceptible to error. One of the biggest barriers to optimal self-management is the ability to calculate bolus doses. It is hoped is that the device will become the standard of care for patients on multiple daily insulin injection therapy due to the simplicity of the built-in bolus calculator.
_
Glucometer for blind:
If you’ve been certified as legally blind, it’s likely you’ll meet the requirements of most insurers to obtain a blood glucose monitor with speech capability, also called a talking blood glucose monitor. Be aware that talking meters fall into 2 categories – those with partial speech and those with full speech. Those with partial speech may only announce your blood glucose result while meters with full speech not only announce your result but also the results in memory, low battery warning, and audible steps to set the time and other monitor features. I wonder how a blind person would use lancet to pierce finger tip and put drop of blood on test strip.
_
Meter to determine glucose plus ketones in blood:
__________
Integrated Self-Monitoring of Blood Glucose System: Handling Step Analysis: 2012:
Self-monitoring of blood glucose (SMBG) implicates a number of handling steps with the meter and the lancing device. Numerous user errors can occur during SMBG, and each step adds to the complexity of use. This report compares the required steps to perform SMBG of one fully integrated (the second generation of the Accu-Chek® Mobile), three partly integrated (Accu-Chek Compact Plus, Ascensia® Breeze®2, and Accu-Chek Aviva), and six conventional (Bayer Contour®, Bayer Contour USB, BGStar™, FreeStyle Lite®, OneTouch® Ultra® 2, and OneTouch Verio™Pro) systems. The results show that the fully integrated system reduces the number of steps to perform SMBG. The mean decrease is approximately 70% compared with the other systems. Authors assume that a reduction of handling steps also reduces the risk of potential user errors and improves the user-friendliness of the system.
_
Individuals achieve more accurate results with Meters that are Codeless and employ Dynamic Electrochemistry: a study:
Four blood glucose monitoring systems (with or without dynamic electrochemistry algorithms, codeless or requiring coding prior to testing) were evaluated and compared with respect to their accuracy. Altogether, 108 blood glucose values were obtained for each system from 54 study participants and compared with the reference values. Analytical performance of these blood glucose meters differed significantly depending on their technologic features. Meters that utilized dynamic electrochemistry and did not require coding were more accurate than meters that used static electrochemistry or required coding.
_____
Heel-stick SMBG in neonate:
Heel stick is a minimally invasive and easily accessible way of obtaining capillary blood samples for SMBG in newborn. The development of newer, more effective and less painful lancing devices may increase the relative utility of heel stick. Heel stick sampling can also help preserve venous access for future intravenous (IV) lines in neonates. The normal range of blood glucose is around 1.5–6 mmol/l in the first days of life, depending on the age of the baby, type of feed, assay method used, and possibly the mode of delivery. Up to 14% of healthy term babies may have blood glucose less than 2.6 mmol/l in the first three days of life. The normal blood glucose level in full-term babies is 40 mg/dL to 150 mg/dL. In premature infants, it is 30 mg/dL to 150 mg/dL. The healthy, term infant experiences a brief, self-limited period of relatively low blood glucose during the first two hours of life. Infants are normally asymptomatic during this time. As this transient drop is physiologic, routine glucose screening is not recommended. Lowest concentrations are more likely on day 1. There is no reason to routinely measure blood glucose in appropriately grown term babies who are otherwise well. Screening should be directed towards those infants at risk for pathologic hypoglycemia. Glucose screening is recommended for infants in the following categories who are at increased risk for pathological hypoglycemia:
- Born to mothers with gestational diabetes or diabetes mellitus
- Large for gestational age (LGA) ( >3969g)
- Small for gestational age (SGA) (<2608g)
- Premature (<37 weeks gestation)
- Low birth weight (<2500g)
- Smaller twin when sizes are discordant
- Polycythemia (hct >70%)
- Hypothermia
- Low Apgar scores (<5 at one minute, <6 at five minutes)
- Stress (sepsis, respiratory distress, etc)
________
Standardization of glucometer:
There are two ways to assess the accuracy of glucose measurement techniques: technical or clinical. Technical accuracy assesses the agreement between the measured and reference glucose values. Clinical accuracy judges how the differences in the measurements impact clinical decision processes. Both have clinical implications. A review by Krouwer and Cembrowski details the standards and statistical methods used to characterize accuracy of SMBG Devices and highlight the different criteria acceptable for accuracy between standard organizations and professional societies. In 1987, an American Diabetes Association (ADA) consensus statement recommended that the acceptable error for SMBG DEVICEs from all sources (user, analytical, etc.) should be less than 10% for glucoses ranging from 30 to 400 mg/dl at all times. This ADA consensus statement also recommended that glucose measurements should not differ more than 15% from values obtained by a laboratory reference method. The ADA decreased the maximum allowable analytical error to <5% in 1996. International Organization for Standardization (ISO) 15197 provided different recommendations in 2003. These state that 95% of the individual glucose measurements compared to the reference measurements are required to be in the range ±15 mg/dl for values less than or equal to 75 mg/dl and ±20% for glucose values greater than 75 mg/dl. This is the standard that the FDA normally uses as the goal for approval of SMBG DEVICEs. The standards set by the ADA (ADA 1987/1996), requiring all glucose measurements with SMBG DEVICEs to be within 5% of CLD values, were deemed technically unachievable by the International Federation of Clinical Chemistry and Laboratory Medicine.
_
Differing glucometer standards:
Although there is no universal standard for accuracy of glucose meters, several groups have defined acceptable ranges. The U.S. Food and Drug Administration (FDA) requires glucose meters to produce self-monitoring results within 20 percent of a reference measurement but recommends results within 15 percent; the FDA has stated that future meters should achieve results within 10 percent of reference at serum glucose concentrations of 30 to 400 mg per dL (1.7 to 22.2 mmol per L). The American Diabetes Association (ADA) recommends that meters produce readings within 5 percent of laboratory values. All meters currently on the market are considered to be clinically accurate in that they at least meet the FDA standard, although it is important to remember that they are not as accurate as a standard laboratory test. Given this broad range of possible error, making treatment decisions based solely on self-monitoring of blood glucose (SMBG) is not advised. Glucose meters are most accurate when used properly. Thus, educating patients on proper use and what to do with the results is vital. Although the exact procedure for using a meter varies by product, potential pitfalls are similar. Common errors include poor maintenance (e.g., soiled meter), using expired test strips, obtaining an inadequate sample size, and failing to calibrate the meter.
_
International Organization for Standardization of SMBG devices:
In a recent study in 2012 of 43 glucose meters, only 34 systems met ISO standards. ISO 15197:2013 specifies requirements for in vitro glucose monitoring systems that measure glucose concentrations in capillary blood samples, for specific design verification procedures and for the validation of performance by the intended users. These systems are intended for self-measurement by lay persons for management of diabetes mellitus. ISO 15197:2013 is applicable to manufacturers of such systems and those other organizations (e.g. regulatory authorities and conformity assessment bodies) having the responsibility for assessing the performance of these systems. Based on ISO 15197:2013, the blood-glucose monitoring system shall meet both the following minimum criteria for acceptable system accuracy.
1. 95% of the measured glucose values shall fall within the standard:
2. 99% of individual glucose measured values shall fall within zones A and B of the Clarke Error Grid (CEG) for type 1 diabetes. The Clarke Error Grid Analysis (EGA) was developed in 1987 to quantify clinical accuracy of patient estimates of their current blood glucose as compared to the blood glucose value obtained in their meter. The grid breaks down a scatter plot of a reference glucometer and evaluated glucometer into 5 regions; A, B, C, D, and E.
____________
Why SMBG is done?
_
_
Self-monitoring is an integral part of diabetes management because it puts you in charge. Regardless of how you manage your diabetes — through diet and exercise alone or combined with oral medicines or insulin — regular blood glucose monitoring provides immediate feedback on how your program is working. Checking your blood glucose gives you the freedom to make choices without worry, the confidence to learn from your actions, and the motivation to keep striving to do better. Monitoring tells you that what you’re doing either is working or isn’t, and it serves as motivation to keep up actions that are working or to make changes. The important thing is to know how to interpret the numbers and take the necessary action. For example, if you take insulin and your blood glucose is high, you may need to bolus, or take more rapid-acting insulin, to bring your levels down into range. If you manage your Type 2 diabetes with diet and exercise, you might treat high blood glucose with a walk around the block. People who use insulin and certain oral diabetes drugs are also at risk of developing low blood glucose, or hypoglycemia, which needs to be treated promptly when it occurs. Regular monitoring may enable you to catch and treat it early, and any symptoms of hypoglycemia should be checked with a meter reading. Over time, blood glucose monitoring records can be analyzed for patterns of highs or lows that may suggest that a change is needed in the treatment regimen. Regular monitoring is especially helpful for showing the positive effects of exercise. Say your readings have regularly been around 140 mg/dl, but you start taking a walk every day and you start getting more readings around 120 mg/dl. That will definitely boost your motivation.
_
Self-monitoring blood glucose — provides useful information for diabetes management.
It can help you to:
•Judge how well you’re reaching overall treatment goals
•Understand how diet, stress and exercise affect blood sugar levels
•Understand how as illness affect blood sugar levels
•Monitor the effect of diabetes medications on blood sugar levels
•Identify blood sugar levels that are dangerously high or low
•Help prevent low blood sugar at night
•Reduce the risk of eye, kidney and nerve complications
•Help you make informed decisions about the amount and type of insulin to use
•Help you manage illness at home and alert you if you need to do a ketone test
_
Based upon the results of randomized trials, self-monitoring of blood glucose (SMBG) is recommended for in patients who take medications that can cause hypoglycemia and that need to be adjusted based on ambient glucose levels. For example, in order to avoid hypoglycemia and achieve target glucose levels, patients with type 1 diabetes who take mealtime insulin should usually test before meals to adjust doses, based on meal size and content, anticipated activity levels, and glucose levels. Similar guidelines apply to insulin-treated type 2 diabetes, although their glucose levels are characteristically more stable, and they may require less frequent monitoring. Patients treated with sulfonylureas or meglitinides, which can also cause hypoglycemia, should be tested once to twice per day during titration of their doses, but after a stable dose and target glycemic targets are achieved, may only need to test several times per week, usually in the morning or before dinner. All insulin and sulfonylurea patients need to test more frequently before and during long car rides, during sick days, and when there are changes in diet and exercise patterns.
_
Clinical utility of SMBG:
Uses of SMBG data include identifying and treating hyper- and hypoglycemia; making decisions about food intake or medication adjustment when exercising; determining the effect of ingested food on blood glucose; and managing glucose fluctuations resulting from illness. Although the data are somewhat conflicting, larger, better-designed trials have shown that SMBG improves glycemic control when the results are used to adjust therapy. However, the data for reducing long-term complications are more conclusive for patients on insulin therapy. In most hands, the glucose oxidase strip method is accurate and reliable. Since whole blood is used, the results tend to be slightly lower than simultaneous venous samples, but this is balanced by the fact that capillary blood has a higher glucose concentration than venous blood. Most patients can visually estimate the correct value, but a few patients consistently misread the visual charts and must use a reflectance meter. This may be due to an unexpectedly high prevalence of disturbances of color perception in diabetics. Most patients feel more comfortable with the digital readout of the reflectance meter, although it is not necessarily more accurate. The major sources of error are in failing to put a large enough drop of blood on the strip and inaccurate timing. For patients who use reflectance meters, another source of error is failure to keep the machine clean and calibrated. Once the color is developed, it is relatively stable, so patients can be instructed to bring developed strips to the physician’s office so that the accuracy can be checked.
_
Optimal use of SMBG:
Establishing a Glucose Profile:
To take full advantage of the benefits of SMBG, patients must collect data at appropriate times during the day, recognize readings that are outside their target range, and take action to improve their glycemic control. This is most easily accomplished by having patients compile a periodic glucose profile by taking a series of blood glucose measurements throughout the day, capturing information from the fasting, postprandial, and postabsorptive (or late postprandial) periods. Conversely, by staggering SMBG measurements at different times on different days, patients can generate an accurate portrait of day-to-day glycemic excursions while avoiding the need to test many times in a single day. Regardless of the testing regimen, patients should be encouraged to collect data on glucose levels relative to meals. Studies that have used meal-based SMBG testing have demonstrated improvements in HbA1c. The ability to download memory meters with a date and time stamp greatly facilitates this process. Some meters have event markers for meal times, insulin doses, exercise, and hypoglycemia, substantially adding to the power of the analysis.
_
Pattern Recognition:
Regardless of the monitoring regimen, a key to effective use of SMBG in clinical practice is “pattern management,” a systematic approach to recognizing the glycemic patterns within SMBG data and then taking action based on those results. This approach consists of several key steps: (1) establish both premeal and postmeal blood glucose targets; (2) gather data on blood glucose levels, carbohydrate in-take, insulin dose (when applicable), activity levels, schedule, and physical and emotional stress; (3) analyze data to determine whether any patterns emerge; (4) assess any influencing factors; (5) take action; and (6) regularly monitor blood glucose levels to evaluate the impact of actions taken. By using the data gathered during a specified period, the clinician and patient can review patterns of glycemic excursions and then make adjustments to meals, activities, and medications to better control glucose levels, minimize glycemic excursions, and limit hypoglycemia.
_
Inconsistent Highs & Lows:
Sometimes you may get a lower or higher blood glucose reading than usual and you may not be able to figure out the reason. When you are sick with a virus or flu, your blood glucose levels will nearly always go up and you may need to contact your doctor. There are a number of other common causes for blood glucose levels to increase or decrease. These include:
•Food – time eaten, type and amount of carbohydrate for example: bread, pasta, cereals, vegetables, fruit and milk
•Exercise or physical activity
•Illness and pain
•Diabetes medication
•Alcohol
•Emotional stress
•Other medications
•Testing techniques.
Contact your doctor or Credentialed Diabetes Educator if you notice that your blood glucose patters change or are consistently higher or lower than usual.
_
A change in attitude:
For many people with diabetes, striving for tight control is a full-time job, and numbers outside the parameters of your goals can make you crazy. Dale, the diabetes educator from the University of Michigan, suggests a shift in perception that can help avoid knee-jerk reactions to high or low numbers: Instead of “testing” your blood glucose, “monitor” it. “When you ‘test,’” she says, “the results can be interpreted to mean that you’ve ‘passed’ or ‘failed.’ It’s emotionally charged. When you ‘monitor’ instead, you gather information and make adjustments as necessary. You just need to ask, ‘What can I learn from this? Was my serving of pasta too large? Do I need to lower my insulin dose before exercise? What can I do better to prevent this from happening in the future?’ That’s how it should be for everyone.”
___
Barriers to SMBG:
Barriers to optimal use of SMBG include limited knowledge, both by clinicians and patients, as well as from perceived inconvenience or discomfort with the measurement. Motivational/behavioral issues, particularly in the adolescent subgroup, may also be a barrier. These issues, however, should never distract from the fact that failure to achieve glycemic control with SMBG is often the result of a failure to properly educate patients how to monitor blood glucose levels and the importance of accuracy in doing so. Thus, clinicians must be aware of these potential barriers and be prepared to address them with individual patients and other caregivers, such as families or guardians.
_
Barriers and facilitators to SMBG by people with type 2 diabetes using insulin
_
_______
Conceptual framework of factors influencing the use of SMBG:
_
_______
Rate of daily SMBG among Adults with Diabetes aged 18 Years and Older, 1997-2006: CDC Data & Statistics:
_
_
From 1997 to 2006, rates of SMBG increased overall, in all age groups examined, and in the majority of states examined. Health insurance policy changes and improvements in monitoring devices during this period might have influenced the rate increases. The Balanced Budget Act of 1997 provided Medicare coverage for blood-glucose monitors and testing strips for persons with insulin-treated or non–insulin-treated diabetes. This change in Medicare coverage and its possible influence on the policies of private insurers might have contributed to the increases in SMBG rates. The improvement in monitoring technology makes the monitoring practice more convenient, which might also contribute to the upward trends. Consistent with findings from other studies, lower rates of SMBG were correlated with being male, having less than a high school education, having no health insurance coverage, taking no medication or oral medication only, making two or fewer doctor visits annually, and not having taken a diabetes-education course. The negative associations between SBMG and lower education or lack of health insurance coverage suggest that socioeconomic barriers might impede the practice of SMBG. The cost of blood glucose–monitoring supplies might be a barrier for patients with limited economic resources. Positive associations were observed between SMBG and number of doctor visits, insulin use, or having ever taken a diabetes-education course, which indicates that SMBG might be associated with better disease management or more intensive medical care. Access to health care is an important factor associated with SMBG. Health insurance coverage of monitoring devices and supplies is integral in encouraging self-monitoring and self-management practices. Collaborations to ensure adequate insurance coverage for blood-glucose monitors, test strips, and lancets are essential for increasing the rates and benefits of SMBG. Recommendations from health professionals and the provision of diabetes education can influence the self-management practices of patients. Diabetes-education programs might increase the benefits of self-monitoring by teaching patients the optimal timing and frequency of self-monitoring, how to interpret the results correctly, and how to make appropriate diet, exercise, and pharmacologic-therapy adjustments in response to SMBG readings. Continued surveillance will be important for monitoring future trends in SMBG and the effectiveness of intervention strategies.
_________
When to do SMBG?
The more often you measure your blood sugar level, the more information you and your diabetes care provider will have for making the right decisions about your diabetes management. The most common times to do a blood sugar test include:
1. Before breakfast: This test reflects the blood sugar values during the night and is probably the most important time to test. The rapid-acting insulin dose can be adjusted based, in part, by the value of this test. The dose of Lantus or Levemir insulin is also based on this test.
2. Before lunch: This helps you decide if the morning Humalog/NovoLog/Apidra and/or Regular insulin dosage was correct.
3. Before dinner: This test reflects how well the dose of morning NPH or lunchtime rapid-acting insulin worked. It may also reflect the effect of afternoon sports activities and an afternoon snack. A test should not be done unless it has been at least 2 hours since food was eaten. If it is time for dinner and your child had an afternoon snack 1 hour earlier, it is best to wait and do a test before the bedtime snack. If this is a common occurrence, change to doing a blood sugar test before the afternoon snack.
4. Before the bedtime snack: This test lets you know if the rapid-acting insulin dose given at dinner was correct. This test is important for people who tend to have reactions during the night, children who play outside after dinner, and anyone who did not eat well at dinner. If the bedtime values are low, an extra snack should be given in addition to the usual solid protein and carbohydrate so your child’s blood sugar does not drop too low during the night. Recheck the value 15 or more minutes after the snack to make sure that it has come back up.
5. Testing after meals: Doing a blood sugar test 2 hours after eating a meal is becoming a more common practice. You should check blood sugar values 2 hours after each meal once or twice weekly. The blood sugar value goals are the same for 2 hours after a meal as they are before a meal. Testing after meals is a useful testing time for people who count carbohydrates and inject insulin just before eating based on how many carbohydrates they plan to eat.
6. Testing at night: Occasionally, you may need to do a blood test in the middle of the night to make sure the value is not getting too low. A nighttime blood sugar test is important for people who tend to have low blood sugars during the night. More than half of the severe low sugars occur during the night. It is important to test on nights when there has been extra physical activity (for example, a basketball game in the evening or playing hard outside on a nice summer evening). The best time to do a check varies with each person. For some, between midnight and 2 AM is the best. For others, the early morning hours are better
________
Dawn phenomenon and Somogyi effect:
If your fasting readings are consistently higher than these goals, it may be because of the dawn phenomenon or a result of the Somogyi effect. In the dawn phenomenon, hormones released in the very early morning cause increased insulin resistance, resulting in higher blood glucose levels. This occurs in everyone, with diabetes or without. However, in people who don’t have diabetes, extra insulin is secreted, so the rise in blood glucose level is minimal. Common preventive treatments for high morning blood glucose caused by the dawn phenomenon include getting daily exercise, eating a carbohydrate-containing bedtime snack, or adding the drug metformin to the diabetes control regimen.
_
The Somogyi effect, which is more likely to occur in people who use insulin, is a phenomenon in which low blood glucose during the night causes the body to release hormones that raise blood glucose levels, resulting in high morning levels. While a person’s first instinct for treating high morning readings may be to increase nighttime insulin, in fact, taking less insulin and going to bed with a higher blood glucose reading may be more effective at preventing the low that leads to the morning rise in glucose. People who are experiencing high morning blood glucose levels are often encouraged to wake up at 3 AM on several occasions to check their blood glucose. High blood glucose at this time may point to the dawn phenomenon as the cause of the high morning readings, while low blood glucose at 3 AM may suggest the Somogyi effect.
________
What do my blood sugar levels tell me?
| Time of Test | Can be used to adjust meal/medicine |
| Fasting blood sugar (FBG) and Nighttime (3-4 a.m.) | Adjust medicine or long-acting insulin |
| Before a meal | Modify meal or medicine |
| 1-2 hours after a meal | Learn how food affects sugar values (often the highest blood sugars of the day*) |
| At bedtime | Adjust diet or medicine (last chance for the next 8 hours) |
*Depends on the size of the meal and the amount of insulin in your medicine
____
Frequency of SMBG:
_
_
Although the optimal frequency of monitoring is unknown, the ADA recommends SMBG three or more times a day for patients with type 1 diabetes. Patients with type 2 diabetes still benefit from at least periodic monitoring. Ultimately, the frequency and timing of SMBG should be determined by how the data will be used. SMBG can assist the patient and physician with adjusting diet and medications and maintaining appropriate glucose control. More frequent monitoring is beneficial during insulin dose adjustments. Postprandial monitoring is important to identify the effect of various foods on glucose levels and to monitor the effects of preprandial medications. Other factors, such as desire for tight control and current degree of control, will influence frequency of monitoring.
_
A major obstacle to increased SMBG utilization is the lack of clear guidelines for testing frequency. A global consensus conference was convened in 2004 to address this issue. The results of that conference were published as a supplement in the American Journal of Medicine. Table below shows a summary of the recommendations presented.
_
When you should test your blood glucose levels and how often you should test varies depending on each individual, the type of diabetes and the tablets and/or insulin being used. Your doctor or Credentialed Diabetes Educator will help you decide how many tests are needed and the levels to aim for.
Possible times to test are:
•Before breakfast (fasting)
•Before lunch/dinner
•Two hours after a meal
•Before bed
•Before rigorous exercise
•When you are feeling unwell
You may need to record all your tests. Even though your meter may have a memory, it is important to keep a record of your readings in a diary and to take this with you to all appointments with your diabetes team. Testing four times a day is usually recommended for people with type 1 diabetes. People using an insulin pump may need to test more often.
_
SMBG more often:
There will be times when you need to test more often, however you should first discuss this with your doctor or Credentialed Diabetes Educator. Example of these times include when you are:
•Being more physically active or less physically active
•Sick or stressed
•Experiencing changes in routine or eating habits, e.g. travelling
•Changing or adjusting your insulin or medication
•Experiencing symptoms of hypoglycemia
•Experiencing symptoms of hyperglycemia
•Experiencing night sweats or morning headaches
•A female planning pregnancy or are pregnant.
•Pre/post minor surgical day procedures
•Post dental procedures
Your Credentialed Diabetes Educator can help you work out a testing plan especially for you.
___
SMBG vis-à-vis diabetes table:
| Basic SMBG requirements (must be met): The person with diabetes (or a family member/caregiver) must have the knowledge and skills to use a home blood glucose monitor and to record the results in an organized fashion. The person with diabetes and/or members of the healthcare team must be willing to review and act upon the SMBG results in addition to the A1C results. __ A. REGULAR SMBG is required if the person with diabetes is: |
|
| SITUATION | SMBG RECOMMENDATION |
| Using multiple daily injections of insulin (≥4 times per day) Using an insulin pump |
SMBG ≥4 times per day |
| Using insulin <4 times per day | SMBG at least as often as insulin is being given |
| Pregnant (or planning a pregnancy), whether using insulin or not Hospitalized or acutely ill |
SMBG individualized and may involve SMBG ≥4 times per day |
| Starting a new medication known to cause hyperglycemia (e.g. steroids) Experiencing an illness known to cause hyperglycemia (e.g. infection) |
SMBG individualized and may involve SMBG ≥2 times per day |
| B. INCREASED FREQUENCY OF SMBG may be required if the person with diabetes is: | |
| SITUATION | SMBG RECOMMENDATION |
| Using drugs known to cause hypoglycemia (e.g. sulfonylureas, meglitinides) |
SMBG at times when symptoms of hypoglycemia occur or at times when hypoglycemia has previously occurred |
| Has an occupation that requires strict avoidance of hypoglycemia | SMBG as often as is required by employer |
| Not meeting glycemic targets | SMBG ≥2 times per day, to assist in lifestyle and/or medication changes until such time as glycemic targets are met |
| Newly diagnosed with diabetes (<6 months) | SMBG ≥1 time per day (at different times of day) to learn the effects of various meals, exercise and/or medications on blood glucose |
| Treated with lifestyle and oral agents and is meeting glycemic targets | Some people with diabetes might benefit from very infrequent checking (SMBG once or twice per week) to ensure that glycemic targets are being met between A1C tests |
| C. DAILY SMBG is not USUALLY required if the person with diabetes: | |
| Screen for diabetes complications annually or as indicated | |
| Is treated only with lifestyle and is meeting glycemic targets | |
| Has pre-diabetes | |
_
_
SMBG vis-à-vis insulin table:
| Suggested SMBG Patterns for Patients Using Insulin: Basal Insulin Only – NPH or long-acting insulin analog, typically given at bedtime. SMBG at least as often as insulin is being given. Optional, less frequent SMBG can be done at other times of day to ensure glycemic stability throughout the day. |
||||||||
| BREAKFAST | LUNCH | SUPPER | BED- TIME |
NIGHT | ||||
| before | after | before | after | before | after | |||
| Insulin | NPH/ long (basal) |
|||||||
| SMBG pattern |
SMBG test |
|||||||
| Adjustment | Basal insulin ↑ if BG high ↓ if BG low |
|||||||
| Premixed – typically given pre-breakfast and pre-supper. SMBG at least as often as insulin is being given. SMBG QID until glycemic targets are met; SMBG BID (alternating times) is usually sufficient once glycemic targets are met. | ||||||||
| BREAKFAST | LUNCH | SUPPER | BED- TIME |
NIGHT | ||||
| before | after | before | after | before | after | |||
| Insulin | pre- test |
pre- test |
||||||
| SMBG pattern 1: Starting |
SMBG test |
SMBG test |
SMBG test |
SMBG test |
||||
| SMBG pattern 2: Stable |
SMBG test |
SMBG test |
||||||
| Alternating daily | SMBG test |
SMBG test |
||||||
| Adjustment | Pre-supper insulin ↑if BG high ↓if BG low |
Pre-breakfast insulin ↑if BG high ↓if BG low |
Pre-breakfast insulin ↑if BG high ↓if BG low |
Pre-supper insulin ↑if BG high ↓if BG low |
||||
| QID (basal-bolus/MDI) – typically given as a rapid-acting analog or regular insulin (bolus) before each meal and NPH or long-acting analog (basal) typically given at bedtime. SMBG should be QID, pre-meal and bedtime, in order to assess previous dose and to adjust next dose. Some patients find that post-prandial checking can also be helpful. | ||||||||
| BREAKFAST | LUNCH | SUPPER | BED- TIME |
NIGHT | ||||
| before | after | before | after | before | after | |||
| Insulin | rapid regular bolus |
rapid regular bolus |
rapid regular bolus |
NPH/ long (basal) |
||||
| SMBG pattern 1: Starting or Stable |
SMBG test |
SMBG test |
SMBG test |
SMBG test |
||||
| SMBG pattern 2: Stable, Focus on post-meal BG |
SMBG test |
SMBG test |
SMBG test |
SMBG test |
||||
| SMBG pattern 3: Intensive management |
SMBG test |
SMBG test |
SMBG test |
SMBG test |
SMBG test |
SMBG test |
SMBG test |
SMBG test |
| Adjustment | Basal insulin ↑if BG high ↓if BG low |
Pre-breakfast insulin ↑if BG high ↓if BG low |
Pre-lunch insulin ↑if BG high ↓if BG low |
Pre-supper insulin ↑if BG high ↓if BG low |
Basal insulin ↓if BG low |
|||
| MDI = multiple daily injections No funding sources were used by the CDA for the development or launch of this document on SMBG. |
||||||||
_____
SMBG in Type 1 diabetes:
•Self-monitoring of blood glucose levels should be used as part of an integrated package that includes appropriate insulin regimens and education to help choice and achievement of optimal diabetes outcomes.
•Self-monitoring skills should be taught close to the time of diagnosis and initiation of insulin therapy.
•Self-monitoring results should be interpreted in the light of clinically significant life events.
•Self-monitoring should be performed using meters and strips chosen by adults with diabetes to suit their needs, and usually with low blood requirements, fast analysis times and integral memories.
•Structured assessment of self-monitoring skills, the quality and use made of the results obtained and the equipment used should be made annually. Self-monitoring skills should be reviewed as part of annual review, or more frequently according to need, and reinforced where appropriate.
•Adults with type 1 diabetes should be advised that the optimal frequency of self-monitoring will depend on:
-The characteristics of an individual’s blood glucose control.
-The insulin treatment regimen.
-Personal preference in using the results to achieve the desired lifestyle.
_
SMBG in Type 2 diabetes:
National Institute for Health and Care Excellence (NICE) recommendations for patients with type 2 diabetes:
•Offer self-monitoring of plasma glucose to a person newly diagnosed with type 2 diabetes only as an integral part of his or her self-management education.
•Discuss its purpose and agree how it should be interpreted and acted upon.
•Self-monitoring of plasma glucose should be available:
-To those on insulin treatment.
-To those on oral glucose-lowering medications to provide information on hypoglycemia.
-To assess changes in glucose control resulting from medications and lifestyle changes.
-To monitor changes during intercurrent illness.
-To ensure safety during activities, including driving.
•Assess at least annually and in a structured way:
-Self-monitoring skills.
-The quality and appropriate frequency of testing.
-The use made of the results obtained.
-The impact on quality of life.
-The continued benefit.
-The equipment used.
•If self-monitoring is appropriate but blood glucose monitoring is unacceptable to the individual, discuss the use of urine glucose monitoring.
_
At least some studies have found that the more often people monitor their blood glucose with a conventional blood glucose meter, the better their glycosylated hemoglobin (HbA1c) levels. (The HbA1c test is a measure of blood glucose control over the previous two to three months.) Other studies have reported similar benefits for continuous monitoring, in which a sensor worn under the skin transmits glucose measurements every few minutes to a receiver. The GuardControl Trial, for example, found that participants with Type 1 diabetes who used a continuous glucose monitor for three months experienced a 1-percentage-point drop in their HbA1c levels. In a perfect world, people with Type 1 diabetes should monitor six or seven times a day. However, that’s often impractical because of time and resources. A person whose Type 1 diabetes is in stable control should monitor a minimum of four times a day. For people whose Type 2 diabetes in good control, they should monitor twice a day.
_
SMBG is currently recommended for all type 1 and type 2 diabetes diabetic patients being treated with insulin. SMBG should be determined individually, and be part of a total treatment regimen that includes diet, exercise, weight loss, and insulin or oral medications when indicated. The optimal frequency and timing of SMBG depends on many variables, including diabetes type, level of glycemic control, management strategy, and individual patient factors. Healthcare professionals will also need to modify SMBG regimens to accommodate changes in therapy and lifestyle. For people with type 1 diabetes, SMBG is an essential component of daily diabetes management and it has been shown that testing 3 or more times a day was associated with a statistically and clinically significant 1.0% reduction in A1C levels. Furthermore, blood glucose measurements taken post-lunch, post-dinner and at bedtime have demonstrated the highest correlation to A1C. Frequent SMGB pre-and post meals several times a day will provide useful information for adjusting insulin and carbohydrate intake. In addition, patients with hypoglycemia unawareness may need to test more frequently, particularly prior to driving or operating any machinery, watching small children, and other activities where compromise of cognitive function may be dangerous. The results of multiple testing each day provide information that is better correlated to A1C than fasting results alone.
_
SMBG for people with type 2 diabetes who are not using insulin:
Although self-monitoring of blood glucose has been found to be effective for patients with type 1 diabetes and for patients with type 2 diabetes using insulin, evidence suggests that self-monitoring of blood glucose is of limited clinical effectiveness in improving glycemic control in people with type 2 diabetes on oral agents or diet alone. A Cochrane review found that the overall effect of self-monitoring of blood glucose on glycemic control in patients with type 2 diabetes who are not using insulin is small up to six months after initiation and subsides after 12 months. There was no evidence that self-monitoring of blood glucose affected patient satisfaction, general well-being or general health-related quality of life.
_
_
Accumulating Evidence for Improved Glycemic Control in Type 2 Diabetes:
The use of SMBG to detect hypoglycemia and hyperglycemia and then adjusting therapy to minimize glycemic excursions is generally considered standard practice in type 1 diabetes. In patients with type 2 diabetes, especially those not taking insulin, SMBG use has been more controversial. The observational Fremantle Diabetes Study, which reported cross-sectional and longitudinal data, found no significant benefit associated with SMBG. Likewise, a small study by Davidson et al found no statistically significant improvements in HbA1c for patients randomized to SMBG vs. controls. Cross-sectional SMBG studies are incapable of demonstrating a cause-and-effect relationship between SMBG and HbA1c because the data do not evaluate changes in HbA1c over time in the presence of an intervention. In the longitudinal arm of the Fremantle Diabetes Study, the mean SMBG testing frequency of less than 1 test per day in patients treated with diet or oral agents or less than 2 tests per day in insulin-treated patients may have been suboptimal for providing actionable feedback to patients. Additionally, the study did not clearly indicate how SMBG was integrated into the diabetes management plan or whether patients were taught how to respond to out-of-target blood glucose readings. Similarly, the study by Davidson et al did not clearly report how SMBG results were used by patients or their health care professionals. The sample size, wide 95% confidence interval, less than 40% adherence to recommended SMBG frequency, and poorly educated study population may have contributed to the failure to achieve a significant difference. Recently, however, 2 meta-analyses demonstrated that including SMBG as part of a multicomponent management strategy results in a statistically significant decrease in HbA1c of approximately 0.40% in patients with type 2 diabetes who are not taking insulin. When extrapolated to findings from the United Kingdom Prospective Diabetes Study, this decrease would be expected to reduce the risk of microvascular complications by approximately 14%. A 4-year longitudinal study that differentiated new users of SMBG from experienced users found a proportional relationship between SMBG frequency and HbA1c reduction regardless of therapy for new users and a similar association among pharmacologically treated experienced users. Finally, a large epidemiological study of patients with type 2 diabetes that spanned 6.5 years showed that SMBG was associated with lower diabetes-related morbidity and all-cause mortality, even among patients not receiving insulin. Hence, a growing body of evidence suggests that daily SMBG has clinical value in type 2 and type 1 diabetes.
_______
Synopsis of SMBG vis-à-vis methods of glycemic control:
_
In a nutshell, frequent SMBG is indicated all diabetics who take daily insulin and who take oral agents which can cause severe hypoglycemia.
_______
For those with unstable diabetes:
For those suffering from brittle diabetes (unstable diabetes) along with Type 1 diabetes, should use the CGMS (Continuous Glucose Monitoring System) of monitoring their blood glucose. Unlike traditional meters that provide a one-time snapshot of one’s blood glucose levels, continuous glucose monitors (CGMSs) measure one’s glucose levels every few minutes. This system is essential for people suffering from this kind of diabetes since they need to keep a tab on their blood glucose levels at all times.
_
Should I keep written records?
Keeping good records to look for patterns in blood sugars is essential. It is wise to keep written records even if your meter is able to store results (in case the meter breaks). Write down the time of the test, the date, how your child feels, and the blood sugar value. You may also want to note times of heavy exercise, illness, or stress. It may be helpful to record what was eaten for the bedtime snack or any evening exercise to see if these are related to morning blood sugars. Also, keep a record of when your child has low blood sugar reactions and possible causes. Bring these results to your appointments. Good record keeping and bringing the results to clinic visits allow the family and diabetes team to work together most effectively to achieve good diabetes management.
_
SMBG: Accuracy of Self-Reported Data and Adherence to Recommended Regimen: a1988 study:
Reflectance meters containing memory chips were used in a study that addressed several questions concerning routine use of self-monitoring of blood glucose (SMBG), including accuracy of patient blood glucose (BG) diaries, reliability of self-reported frequency of SMBG, and adherence to recommended SMBG regimen. Thirty adults with insulin-dependent diabetes used memory meters and recorded test results in diaries for 2 wk while performing their normal SMBG regimen. Analysis of glucose diaries showed that only 23% of the subjects had no diary errors and 47% had clinically accurate diaries (>10% error rate). The most common types of errors were omissions of values contained in meter memory and additions of values not contained in meter memory, with significantly more omissions than additions. Alterations of test values (e.g., changing a 300-mg/dl reading to 200 mg/dl) were extremely rare. There was no difference in the rate of errors that resulted in a more positive clinical profile (omitting unacceptable values and adding acceptable values) or a more negative clinical profile (omitting acceptable values and adding unacceptable values). Examination of the actual frequency of SMBG showed that most subjects (56.6%) measured their BG an average of two to three times each day. Self-report of SMBG frequency correlated with both actual frequency and HbA1. Although actual frequency of SMBG was not related to physicians’ recommendations, the majority (64%) of subjects were self-testing as often or more often than they had been instructed.
___
Structured SMBG:
Self-monitoring should be assessed at least annually and in a structured way:
_
If self-monitoring is appropriate but blood glucose monitoring is unacceptable to the individual, discuss the use of urine glucose monitoring.
_
SMBG education:
SMBG should be part of an educational program including the patient and close relatives (family members) where necessary. When prescribing the SMBG device, it is essential to explain the issues to the patient and to organise this self-monitoring with the patient, including the frequency, scheduling, blood glucose targets and treatment adjustments to be made by the patient or doctor based on the results. In all cases, the patient should maintain an appropriate diet and physical exercise. Glucose meters are most accurate when used properly. Thus, educating patients on proper use and what to do with the results is vital. Also, lower rates of SMBG are correlated with having less than a high school education; and in my view, uneducated people are unlikely to learn right way to use glucometer.
_
The figure below shows impacts of SMBG as a component of education/treatment program:
____
____
Successful SMBG:
_______________
SMBG Special patient groups:
While the overall effect of self-monitoring seems modest, there is a paucity of data on special groups, including heavy goods vehicle drivers for whom hypoglycemia may pose an unacceptable occupational risk to themselves and the public. Also, people starting or changing their oral diabetes medication may benefit from self-monitoring.
_
Barriers to self-monitoring of blood glucose among adults with diabetes in an HMO: A cross sectional study: 2003:
In addition to logistic barriers to SMBG, some recent evidence suggests that adult diabetes patients who may be at greatest risk for poor outcomes (e.g., minorities, elderly, lower SES) may be least likely to self-monitor. In a study of more than 44,000 managed care patients with type 1 (2,818) and type 2 (41,363) diabetes, Karter et al identified older age, male gender, non-white race, lower socioeconomic status, English language difficulty, higher out of pocket test strip costs, intensity of insulin therapy, greater alcohol consumption, and smoking as independent predictors of less frequent self-monitoring in diabetes patients. This study was the first to move beyond simple reporting of descriptive statistics in order to assess predictors of SMBG in managed care settings. Unfortunately, the validity of the study findings is limited by the reliance on self-reports of self-monitoring, an unreliable measure of actual behavior. The purpose of the current study was to examine the relationship between patient characteristics and SMBG in a large health maintenance organization (HMO) using objective measures of self-monitoring practice. Specifically, we tested the hypothesis that, controlling for type of drug therapy and severity of illness, diabetes patients at greatest risk for poor health outcomes (e.g., older age, multiple chronic conditions, non-white race, lower neighborhood SES) are less likely to practice SMBG. The study population included more than 4,500 adult managed care patients using insulin, oral, or a combination of the two drug therapies. Our use of objective measures of SMBG distinguishes this study from previous attempts to identify predictors of SMBG in managed care. This paper represents the first phase of a larger study to evaluate the effect of distributing free home glucose monitors to diabetes patients at this New England HMO. In multivariate analyses, lower neighborhood socioeconomic status, older age, fewer HbA1c tests, and fewer physician visits were associated with lower rates of self-monitoring. Obesity and fewer comorbidities were also associated with lower rates of self-monitoring among insulin-managed patients, while black race and high glycemic level (HbA1c>10) were associated with less frequent monitoring. For patients taking oral sulfonylureas, higher dose of diabetes medications was associated with initiation of self-monitoring and HbA1c lab testing was associated with more frequent testing. Managed care organizations may face the greatest challenges in changing the self-monitoring behavior of patients at greatest risk for poor health outcomes (i.e., the elderly, minorities, and people living in low socioeconomic status neighborhoods).
_____
Self-monitoring of blood glucose during pregnancy: indications and limitations:
Approximately 5 percent of all pregnancies are complicated by gestational diabetes mellitus, which increases both maternal and perinatal morbidity. In treating women with this condition, many have advocated minimizing fluctuations in blood glucose concentrations to avert maternal hyperglycemia and thus decrease the risk of fetal hyperglycemia and its consequences, fetal hyperinsulinemia and excess fetal growth. Perinatal morbidity and mortality rates, often affected by maternal diabetes, have dramatically been reduced since the discovery of insulin and its therapeutic implementation. In addition to increased availability of insulin, many important technological advances have been developed over the preceding decades. These advances culminated in a larger array of diagnostic and therapeutic capabilities that contributed to improved outcomes in high-risk pregnancies. The availability of glucose meters has represented an important positive impact in the treatment of pregnant women with any type of diabetes. Data frequently show patients who perform self-monitoring of blood glucose (SMBG) more strictly adhere to treatment programs due to increased comprehension regarding treatment and participation in the prescribed treatment regimen. Treating hyperglycemia during pregnancy reduces adverse pregnancy outcomes. The first step towards a tight glucose control in pregnancy is patient adherence to SMBG.
_
Indications for self-monitoring of blood glucose during pregnancy complicated by diabetes:
SMBG is an integral part of standard diabetes care. It allows pregnant women and their healthcare providers to determine the most effective therapeutic modality (e.g. diet, physical activity, or insulin) to control glucose levels and reduce risks of diabetes-related complications. The number of daily tests required to adequately monitor blood glucose levels is specific to the patient and based on the recommendation of the practitioner. Several characteristics, unique to each pregnant woman should be considered. For example, the type of treatment (diet and/or insulin), frequency and intensity of physical activity, and the risk of hypoglycemia. Additionally, SMBG makes patients feel more secure and comfortable using insulin since it allows early recognition of symptoms of hypoglycemia. The indications for, and frequency of SMBG in pregnant women that are not under insulin treatment must be tailored to the individual. Patients must be trained to adjust the amount of food intake with the frequency, intensity, and timing of physical exercise. It is unclear whether SMBG alone leads to improved glycemic control in non-insulin treated subjects with type 2 diabetes. Additionally, there is no data in women with gestational diabetes mellitus (GDM). Measured glucose values need to be frequently checked to ensure both accuracy and the patient’s understanding of any alterations to prescribed treatment. For the vast majority of patients using insulin, SMBG is recommended three or more times per day. A more intensive SMBG regimen is indicated for women with pre-gestational type 1 or 2 diabetes. The aim is to reach adequate HbA1c levels safely without inducing hypoglycemia.
_
When to monitor:
Strict monitoring of postprandial glucose levels is paramount during pregnancy. Many studies have shown that postprandial hyperglycemia beyond the 16th week of pregnancy is the main predictor for fetal macrosomia. Peak plasma glucose levels during pregnancy occur between 60 and 90 minutes after eating. It is recommended to perform SMBG one hour after food intake to evaluate potential adjustments in meal composition and/or in the prandial insulin dose. In special circumstances, like women with slowed gastric emptying, a high-fat meal, or women who use regular insulin for a prandial bolus, it might be more appropriate to perform SMBG two hours after meals instead of one. SMBG performed before eating is the most useful parameter to identify optimal basal insulin doses. Evaluating glycemic levels during the night is recommended to diagnose and prevent nocturnal hypoglycemia. One randomized study of 66 women with GDM observed better neonatal outcomes by aiming for 1-hour postprandial glucose levels less than 140 mg/dL as opposed to a preprandial target of 59 to 106 mg/dL. In another study, 61 women with type 1 diabetes were randomly assigned into two groups at 16 weeks gestation. Women either monitored blood glucose levels preprandially or postprandially. Postprandial capillary blood glucose monitoring significantly reduced the incidence of preeclampsia and neonatal triceps skinfold thickness compared to preprandial monitoring. These studies have been criticized for not using comparable target blood glucose levels for pre- and post-prandial monitoring. Regardless, most specialists prefer postprandial testing at least partly, for the physiologic changes discussed earlier.
_
Barriers to self-monitoring of blood glucose during pregnancy:
The first step towards a successful SMBG during pregnancy is patient education and an understanding of the importance of SMBG to reducing complications during and after pregnancy. The patient must be properly educated on all aspects of meter use. It is important she be aware of how to properly code her meter, wash her hands prior to the test, and to apply the correct amount of blood to the test strip. It is also critical to educate patients on how glucose from food can affect the test results, to use test strips before the expiration date and not longer than 90 days after the vial was opened. Lastly, it is crucial to educate patients on proper storage of strips and disposal of strips if they are subjected to extreme humidity or temperature. Other common barriers to SMBG include costs of the meters and strips, lower socio-economic status, fewer HbA1c tests, obesity and other comorbidities, poor glycemic control, stigmas of testing in public places, pain, and inconvenience.
_
An association between diabetes in pregnancy and fetal overgrowth has long been recognized. Fetal overgrowth is associated with a number of adverse outcomes for both the mother and her baby, such as a higher rate of difficult delivery. To reduce the rate of fetal overgrowth and its associated complications, women with diabetes in pregnancy undergo a number of interventions. Among these interventions is glucose monitoring. The utility of a hemoglobin A1c value appears to be greatest when performed periconceptionally to estimate the risk of congenital anomalies, but it does not appear to confer substantial benefit for estimating the risk of fetal overgrowth or other adverse pregnancy outcomes. Recent studies suggest that CGMS may be beneficial for certain women with diabetes treated with insulin, particularly in women with diabetes that is difficult to control. However, these data require further evaluation and do not yet support the incorporation of CGMS into routine practice.
_
Postprandial versus Preprandial Blood Glucose Monitoring in Women with Gestational Diabetes Mellitus requiring Insulin Therapy: a 1995 study:
In the management of gestational diabetes, various methods of glucose monitoring have been proposed, including the measurement of fasting, preprandial, postprandial, and mean 24-hour blood glucose concentrations. In a retrospective pilot study comparing the outcomes of pregnancy among women with gestational diabetes who were followed with preprandial or postprandial glucose measurements, authors found that there was less macrosomia (defined as a birth weight greater than 4000 g) among their infants when treatment was based on the results of postprandial measurements.
_________
Variability of blood glucose:
Biological Variation:
Fasting glucose concentrations vary considerably both in a single person from day to day and also between different subjects. Intraindividual variation in a healthy person is reported to be 5.7% to 8.3%, whereas interindividual variation of up to 12.5% has been observed. Based on a CV (coefficient of variation) of 5.7%, FPG can range from 112 to 140 mg/dL in an individual with an FPG of 126 mg/dL. (It is important to realize that these values encompass the 95% confidence interval, and 5% of values will be outside this range.)
_
The concentration of glucose is highest in the arterial circulation. Laboratory determinations are usually done on venous samples. If the venous circulation is delayed, such as by leaving a tourniquet on for a prolonged period of time, the concentration falls even further. Thus, samples should be obtained after releasing the tourniquet. Studies have shown that blood glucose concentration may fall as much as 25 mg/dl when a tourniquet has been left in place for 6 minutes. The concentration of glucose in capillary samples is intermediate between venous and arterial. Warming the extremity increases the capillary flow and “arterializes” the sample, while cooling or a tourniquet decreases the flow and lowers the concentration of glucose.
_
Glycolysis:
Both red cells and leukocytes contain glycolytic enzymes. Therefore glucose will be consumed and the concentration of glucose in a sample of whole blood will decline with time. The rate of loss is generally said to be approximately 5% per hour, but may be as rapid as 40% in 3 hours. Consumption of glucose in whole blood samples can be prevented by adding sodium fluoride to the specimen to inhibit the glycolytic enzymes. This approach is the generally applied method in the clinical laboratory. It is effective except in situations where the system is overwhelmed, such as in specimens from patients with leukemia, which contain large numbers of leukocytes. Sodium fluoride has a major disadvantage in that its use makes the sample unacceptable for other determinations such as sodium and uric acid. However, while fluoride does attenuate in vitro glycolysis, it has no effect on the rate of decline in glucose concentrations in the first 1 to 2 h after blood is collected, and glycolysis continues for up to 4 h in samples containing fluoride. The delay in the glucose stabilizing effect of fluoride is most likely the result of glucose metabolism proximal to the fluoride target enolase. After 4 h, fluoride maintains a stable glucose concentration for 72 h at room temperature. A recent publication showed that acidification of the blood sample inhibits glycolysis in the first 2 h after phlebotomy, but the collection tubes used in that study are not commercially available. Placing tubes in ice water immediately after collection may be the best method to stabilize glucose initially, but this is not a practical solution in most clinical situations. Separating cells from plasma within minutes is also effective, but impractical. Rapid separation of the sample or cooling will also prevent glycolysis and will allow the sample to be used for other determinations. Unhemolyzed samples that have been separated within 30 minutes of drawing are generally considered adequate. Rapid cooling of the sample followed by centrifugation is even more effective in preventing glycolysis. Blood glucose cannot be determined accurately on postmortem specimens because both glycogenolysis and glycolysis continue after death. A reasonable estimate of the antemortem blood glucose concentration can be obtained by measuring the glucose concentration of the vitreous of the eye, which does not contain glycolytic enzymes.
_
Variability of capillary blood glucose monitoring measured on home glucose monitoring devices:
Self monitoring of blood glucose helps achieve glycemic goals. Glucometers must be accurate. Many variables affect blood glucose levels. Factors are analytical variables (intrinsic to glucometer and glucose strips) and pre analytical related to patients. Analytical variables depend on factors like shelf life, amount of blood and enzymatic reactions. Preanalytical variables include pH of blood, hypoxia, hypotension, hematocrit etc. CGMS has the potential to revolutionise diabetes care but accuracy needs to be proven beyond doubt before replacing current glucometer devices.
_______
Factors that alter blood glucose results by SMBG:
Table above outlines preanalytical, analytical, and postanalytical factors that can alter the glucose result when a SMBG device is used. The FDA accumulated over 400 medical device reports on blood glucose monitors used in hospitals over 2 years. The 4 most frequent errors reported included 2 preanalytical errors (inadequate instrument cleaning; incorrect quality control or proficiency testing procedures) and 2 analytical errors (improper technique; an incorrect match between the glucose monitor for calibration and test strip calibration when required by the manufacturer).
_______
Preanalytical Variation:
Numerous factors that occur before a sample is measured can influence results of blood tests. Examples include medications, venous stasis, posture, and sample handling. The concentration of glucose in the blood can be altered by food ingestion, prolonged fasting, or exercise. It is also important that measurements are performed in subjects in the absence of intercurrent illness, which frequently produces transient hyperglycemia. Similarly, acute stress (e.g., not being able to find parking or having to wait) can alter blood glucose concentrations. Samples for fasting glucose analysis should be drawn after an overnight fast (no caloric ingestion for at least 8 h), during which time the subject may consume water ad lib. The requirement that the subject be fasting is a considerable practical problem as patients are usually not fasting when they visit the doctor, and it is often inconvenient to return for phlebotomy. For example, at an HMO affiliated with an academic medical center, 69% (5,752 of 8,286) of eligible participants were screened for diabetes. However, FPG was performed on only 3% (152) of these individuals. Ninety-five percent (5,452) of participants were screened by random plasma glucose measurements, a technique not consistent with ADA recommendations. In addition, blood drawn in the morning as FPG has a diurnal variation. Analysis of 12,882 participants aged 20 years or older in NHANES III who had no previously diagnosed diabetes revealed that mean FPG in the morning was considerably higher than in the afternoon. Prevalence of diabetes (FPG > 126 mg/dL) in afternoon-examined patients was half that of participants examined in the morning. Other patient-related factors that can influence the results include food ingestion when supposed to be fasting and hypocaloric diet for a week or more prior to testing.
_
Pre-analytical variables:
Choosing the correct blood sample:
There are several aspects concerning the blood sample that needs attention. Although there are different recommendations, the first choice is to wash the hands with soap and water, dry them, and use the first drop of blood for assessment. Erroneous blood glucose levels (pseudo hyperglycemia) have been recorded when patients did not wash their hands with water after peeling fruits and such false readings were still noted when hand washing was substituted with the use of an alcohol swab. If washing hands is not possible, and they are not visibly soiled or exposed to a sugar-containing product, it is acceptable to use the second drop of blood after wiping away the first drop. Firm squeezing of the finger should be avoided.
_
Operator error:
The technique of the user or operator of the glucometer usually is responsible for more inaccuracy than the glucometer itself. Applying insufficient blood to the strip, using strips that are out of date or exposed to excess moisture or humidity, and failure to enter the proper code, can compromise accuracy. Several important technologic advances that decrease operator error have been made in the last few years. These include “no wipe” strips, automatic commencement of timing when both the sample and the strip are in the meter, smaller sample volume requirements, an error signal if sample volume is inadequate, “lock out” if controls are not assayed, barcode readers, and the ability to store up to several hundred results that can subsequently be downloaded for analysis. Together these improvements have produced superior performance by newer meters. Patient education can have significant influence on the accuracy of the readings shown on the glucometer. Operator error such as differences as much as 14.5 mg/dl between lots of test strips has been reported. Most of the glucometers require coding to be done prior to use. A study by Raine et al. have suggested that up to 16% of patients in endocrine practice miscode their glucometers. This can lead to -37% to + 29% errors in clinical practice. The probability of giving additional 3 units of insulin dose when meters are miscoded was as high as 22.5%.
_
Hematocrit:
Variation in the patient hematocrit can result in inaccuracies in the blood glucose reading. Abnormal hematocrit concentrations can result in falsely low (hematocrit >50%) or high (hematocrit <40%) glucose levels. In one study, hematocrit effect was studied by adjusting the hematocrit of donor sodium heparin blood at glucose concentrations of 54, 247, and 483 mg/dl. At low glucose concentrations (54 mg/dl), the mean glucose difference changed by more than 10 mg/dl. At higher glucose concentrations, meters demonstrated more than 10% change in the mean glucose percentage difference between the lowest and highest hematocrit values. Hematocrit variations may occur frequently in daily routine (e.g., due to dehydration/exercise, nicotin and alcohol abuse, pregnancy etc.). The uses of meters with stable performance are recommended under these conditions.
_
Whole blood vs. plasma vs. interstitial fluid:
The estimation of whole blood glucose levels are usually 10-15% lower than plasma glucose alone. The glucose concentration in the water that makes up plasma is equal to that of erythrocytes. Plasma has greater water content than erythrocytes and, therefore, exhibits higher glucose levels than whole blood. The World health Organization (WHO) has devised a conversion factor of 1.12, which has been mathematically, derived assuming a hematocrit of 45% and red- cell to plasma ratio of ~ 0.8. In a critical care setting, multiple variables affecting the blood glucose may be present at one time. Hypotension, hypoxia, pH of blood, temperature are amongst the many variables affecting the blood glucose measurement. Glucose is measured in the interstitial fluid by an electrochemical glucose oxidase method. Each measurement cycle requires 20 minutes to complete. The measured interstitial glucose value lags behind the serum glucose concentration by about 18 minutes, secondary to the time required for a change in serum glucose to equilibrate with the interstitial fluid. Sweat on the skin will dilute the collection fluid. Sweat and/or elevated body temperature will initiate a skipped measurement cycle.
_
Hypotension:
Hypotension results in decrease in perfusion and increase in glucose utilization resulting in false results in capillary blood glucose. Atkin et al. assessed the validity of the finger stick glucose measurements in the hypotensive critically-ill patients. They found that the fingerstick glucose values were significantly lower than the values obtained by venous reagent strips or laboratory glucose measurements. Fingerstick glucose values in the hypotensive group were 67.5% of laboratory glucose values and were significantly lower than the values obtained in the normotensive group (91.8%, P less than 0.001). Juneja et al. aimed to compare the accuracy of capillary bedside glucometry with arterial samples in critically-ill patients with shock through a prospective case-control study. They studied 100 patients on vasopressor support, and the control group had 100 normotensive patients. Mean arterial and capillary sugars (mg/dl) in study and control groups were 164.7 ± 70 and 157.4 ± 68.9 and 167.1 ± 62.2 and 167.5 ± 61, respectively. They concluded that arterial blood glucose is better measurement compared to capillary blood glucose in hypotensive patients. Venous blood glucose values are also stated to be significantly better than capillary blood glucose measurement and correlate better with the laboratory measurements in a critical care setting. Also, the prandial state accentuated the difference between various measurements in hospital setting. Capillary blood glucose levels were 20-25% higher than venous plasma glucose level in prandial state, whereas it was only 2- 5 mg/ dl higher in fasting state.
_
PH:
Like any other enzymatic reaction, change in pH is likely to affect the enzymatic reaction. However, in the range of pH 6.89 to 7.4, it is found to not have much effect on the blood glucose levels measured.
_
Alternate site:
Bina et al. studied the differences in the measurement of blood glucose at various site (forearm, palm, and thigh) with respect to the finger tip capillary blood glucose. Also, the effect of prandial state and moderate exercise at the blood glucose levels on the different sites were studied. Significant differences in BG at alternative sites were found 60 min post-meal (P < 0.0003) and post-exercise (P < 0.037). However, no significant differences were observed between sites in either the fasting state or at 90 and 120 min post-meal. It has been observed that there is a considerable time lag in measurement at alternate sites. It can be particularly dangerous in hypoglycemia situations, and hence clinicians must be aware of this. The effect of oxygen concentration in the sample has already been discussed.
_
Drugs:
There are various drugs affecting the capillary glucose readings. Of particular importance were the acetaminophen, dopamine, mannitol, and the ascorbic acid. Glucose meters use enzyme-based amperometric biosensors to measure glucose concentrations. Glucose oxidase oxidizes glucose to gluconolactone while reducing oxygen to H2O2. Other mediators like ascorbic acid, uric acid, acetaminophen, and salicylic acid can falsify the results by nonspecifically oxidizing H2O2. Acetaminophen increased glucose readings with GDH meters but decreased readings with some, but not all, GO-based meters at therapeutic drug levels. Dopamine increased glucose values on GDH-based meters, primarily at high drug concentrations. Mannitol increased GO-based meter readings, possibly through detection by the analyzer or by a non-specific osmotic effect. At high doses, ascorbic acid increased GDH-based meter readings but decreased those that used glucose oxidase.
_
Other interfering substances:
Some naturally occurring substances in the body tend to interfere in the blood glucose readings. High triglyceride levels cause falsely low blood glucose values as they tend to take up volume reducing the glucose levels. Also, bilirubin has also noted to cause pseudohypoglycemia .
_
Implications:
With such vast data regarding the various variables affecting the blood glucose reading in glucometer, the clinician must be alert while interpreting the values while treating a patient. Also, the patients need to be educated regarding their glucometers, which can prevent false readings and inadvertent admission of excess insulin resulting in severe hypoglycemia.
_________
Analytical Variation:
Glucose is measured in central laboratories almost exclusively using enzymatic methods, predominantly with glucose oxidase or hexokinase. The following terms are important for understanding measurement: accuracy indicates how close a single measurement is to the “true value” and precision (or repeatability) refers to the closeness of agreement of repeated measurements under the same conditions. Precision is usually expressed as CV (coefficient of variation); methods with low CV have high precision. Numerous improvements in glucose measurement have produced low within-laboratory imprecision (CV <2.5%). Thus, the analytical variability is considerably less than the biological variability, which is up to 8.3%. Nevertheless, accuracy of measurement remains a problem. There is no program to standardize results among different instruments and different laboratories. Bias (deviation of the result from the true value) and variation among different lots of calibrators can reduce the accuracy of glucose results. (A calibrator is a material of known concentration that is used to adjust a measurement procedure.) A comparison of serum glucose measurements (target value 98.5 mg/dL) was performed among ~6,000 laboratories using 32 different instruments. Analysis revealed statistically significant differences in bias among clinical laboratory instruments, with biases ranging from -6 to +7 mg/dL (-6 to +7%) at a glucose concentration of 100 mg/dL. These considerable differences among laboratories can result in the potential misclassification of >12% of patients. Similarly, inspection of a College of American Pathologists (CAP) survey comprising >5,000 laboratories revealed that one-third of the time the results among instruments for an individual measurement could range between 141 and 162 mg/dL. This variation of 6.9% above or below the mean reveals that one-third of the time the glucose results on a single patient sample measured in two different laboratories could differ by 14%.
_
Analytical variables:
Detection Method:
Glucometers use predominantly 2 principles: Electrochemical (ameperometry) and reflectance photometry. In glucometers, the enzyme used (glucose oxidase) induces an electric current through the strip, which is proportionate to the amount of glucose. In the reflectance glucometers, the strip changes color according to the amount of glucose in the sample. These glucometers quantify the color change by reflectance photometry. If the drop of blood does not cover the entire testing area of reflectance, glucometers can give falsely low value. Also, they are either automatic (non-wiping) or manual (wiping). The ambient temperature has shown to affect the glucose readings in the reflectance meters. In one study, it was demonstrated that the manual reflectance glucometer overestimated glucose concentrations by 14% at 44°C and underestimated by 12.7% at 25°C.
_
Enzymatic reactions:
Glucose meters contain strips that contain two enzymes; glucose oxidase (GO) and glucose dehydrogenase (GDH) or hexokinase. Glucose oxidase meters require oxygen and water for their reaction and hence are susceptible to extremes of hydration or oxygenation. GO-mediated reactions result in generation of gluconic acid and hydrogen peroxide. Capillary blood glucose was measured in mountain climbers at 13500 ft by various glucometers. GO glucometers overestimated blood glucose by 6-15%, whereas the GDH meters were all within 5%. This is because the glucose oxidase biosensor strips are sensitive to the oxygen concentration. But, a recent study shows no such difference. Maltose, galactose and xylose will be misinterpreted as glucose by GDH based methods. So patients on icodextrin peritoneal dialysis using GDH meters will result in falsely high values as it can be metabolized to maltose cross-reacting as glucose.
_
Glucostrips:
Glucostrips are another potential source of variability of blood glucose levels. Glucostrips have a finite life; it is usually for 2 years in ideal storage conditions. Exposure of strips to light causes discoloration of test area resulting in falsely elevated glucose levels. Exposure of strips to humidity and temperature by open cap vials decreases their stability by day 14 due to exposure to heat and humidity.
_____________
Accuracy of glucometer:
Accuracy can be defined as the variation from the reference value. When assessing laboratory values for glucose, the testing method is accurate if the measurement is within acceptable error compared to the reference method. Within the range of hypoglycemia, if the values reported by the SMBG device are inaccurate (e.g., reported higher than actual values), this inaccuracy could lead to failure to recognize and treat life-threatening values or even more worrisome result in a different treatment (e.g., increasing insulin infusions) that could pose a serious patient safety risk. The importance of accuracy for clinical treatment assesses whether the measurement value is within a range close enough to the actual value that the clinical approach to therapy remains the same. The current ADA device recommendations for SMBG with SMBG devices include the following: (a) achieve and maintain glycemic control, (b) prevent and detect hypoglycemia, (c) avoid severe hyperglycemia, and (d) facilitate diabetes therapy adjustment to lifestyle changes (activity, diet changes, etc.). The accuracy requirements set by the professional organizations are still rarely met by SMBG devices. With outpatients and other hospitalized noncritically ill patients, most clinicians appear satisfied with SMBG device accuracy when glucose values avoid the extremes of hypoglycemia and hyperglycemia. This is because, in the range of normal glucose, the accuracy in this range is typically acceptable for clinical decision-making. For the care of critically ill patients, accuracy becomes more important as some of the early signs present with hypoglycemia and hyperglycemia may be difficult to detect in this patient population due to decreased mental status, sedatives, and other patient conditions. For optimal glucose control in high-demand states in critically ill patients, SMBG device technology has yet to provide a high enough degree of accuracy and reliability that leads to appropriate clinical decision-making. Continuous glucose monitoring devices based on invasive, minimal invasive, or noninvasive methodology are being developed to improve blood glucose monitoring.
_
Establishing glucose meter accuracy is challenging. Glucose meters only accept whole blood, but existing standards are serum based. Glucose as an analyte is unstable in whole blood, and the process of stabilizing glucose through glycolysis inhibitors can interfere with some glucose meters. Technical accuracy for glucose meters is defined by comparing meter results against clinical laboratory methods that use plasma/serum-based samples. There is no consensus among standards organizations and professional societies, however, for acceptable performance criteria. While technical accuracy defines meter performance, clinical accuracy establishes how treatment decisions agree between meter results and laboratory glucose results. Glucose meters should be evaluated before use, and the specific meter model selected should be based on technical and clinical performance in the intended patient population.
_
Accuracy of glucose meters is a common topic of clinical concern. Blood glucose meters must meet accuracy standards set by the International Organization for Standardization (ISO). According to ISO 15197 Blood glucose meters must provide results that are within 20% of a laboratory standard 95% of the time (for concentrations about 75 mg/dL, absolute levels are used for lower concentrations). However, a variety of factors can affect the accuracy of a test. Factors affecting accuracy of various meters include calibration of meter, ambient temperature, pressure use to wipe off strip (if applicable), size and quality of blood sample, high levels of certain substances (such as ascorbic acid) in blood, hematocrit, dirt on meter, humidity, and aging of test strips. Models vary in their susceptibility to these factors and in their ability to prevent or warn of inaccurate results with error messages. The Clarke Error Grid has been a common way of analyzing and displaying accuracy of readings related to management consequences. More recently an improved version of the Clarke Error Grid has come into use: It is known as the Consensus Error Grid. Older blood glucose meters often need to be “coded” with the lot of test strips used, otherwise, the accuracy of the blood glucose meter may be compromised due to lack of calibration.
_
Independent accuracy testing is expensive, complicated, and rare. Diabetes Forecast, for example, doesn’t test or recommend products because the American Diabetes Association is a nonprofit organization without a laboratory or expertise in lab comparisons of products. Where the data do exist, in the form of manufacturers’ tests, accuracy is reported in different ways. Some companies report accuracy as a “regression line,” involving correlation coefficients, slopes, and Y-axes. Others report in a friendlier table format using percentages. Those measures of accuracy are apples and oranges. It’s not possible [for a consumer] to do a direct comparison of how accurate one meter is to another. We’ve seen cases where cheaper meters don’t necessarily have all the bells and whistles, but have better accuracy. So users have to evaluate all of the features that are important to them.
_
Factors that affect Blood Glucose Meter Accuracy:
Expired test strips:
Always check the expiry date of test strips before performing a test as expired test strips could produce a false reading.
Testing in very warm or very cold temperatures:
Accuracy of blood glucose meters can be affected by temperature. Blood glucose meters are designed to be at their most accurate at room temperature. If you need to test in very warm or very cold temperatures, refer to your meter’s user guide to see what temperature range the meter is most accurate at.
Testing in very humid conditions:
Humid conditions can also affect meter accuracy. Always remember to keep the test strip pot closed after a test has been performed and, where possible, avoid testing in very humid environments.
Coding mistakes:
Some blood glucose meters require test strips which need a code to be entered into the meter before testing with a new batch of test strips. If the wrong code is entered, this can affect accuracy of the result. A number of blood glucose meters these days do not need coding.
Too little blood applied:
If too little applied is applied to the strip, this can cause a false reading. A number of blood glucose monitors won’t give a reading if this happens and will instead alert that too little blood has been applied or give an error message.
Contamination on skin:
Dirty or wet hands can make readings very inaccurate in some cases. You should ensure your hands have been cleaned, with soap and water, and dried then before performing a blood test.
____
Temperature and SMBG:
In principle, SMBG values are measured with blood glucose meters that use an enzyme reaction. A temperature sensor is built into the main device as a control mechanism for adjusting the enzyme reaction rate to match the ambient temperature, allowing accurate values to be obtained. Differences in SMBG values due to temperature, within the range of usual ambient temperatures, are reported to be negligible to the extent that clinical decisions are not affected. Conversely, one study comparing SMBG values for ground temperatures between 25°C and 8°C reported that the meters can either underestimate or overestimate BG values. In addition, when patient skin temperature is cool (15.5°C), lower SMBG values have been reported compared with warm skin temperature (35°C). Particularly during the winter, the SMBG values are higher than the PG values.
_______
Common User’s Errors:
A tool such as SMBG can contribute substantially to improved glycemic control if reasonably accurate and used appropriately. What if, however, the information is incorrect either because of technical inaccuracies or user error? Confounding issues related to blood glucose testing in the inpatient setting have been well elucidated. In the outpatient setting, common errors in SMBG have been documented in observational studies. I have already discussed technical inaccuracies but SMBG data can be rendered inaccurate by several user errors, including:
• failure to store glucose strips properly;
• failure to set glucose meter codes to match strip codes;
• failure to apply sufficient blood on the meter’s strip;
• failure to use control solutions;
• use of date-expired control solutions;
• use of date-expired strips; and
• failure to wash hands properly.
The frequency of user error relating to meter codes has been reported at approximately 16%. In one study, exactly half of the patients were elderly. As these patients are often challenged by cognitive and dexterity limitations and frequently have long-standing diabetes requiring insulin, therapeutic interventions based on such erroneous data can be destructive.
_
The American Diabetes Association (ADA) assumed in a consensus report published in 1990 that up to 50% of the self-monitored blood glucose readings have more than 20% deviation from the true values. However, more recent studies found the percentage of deviation to be less. Alto and colleagues conducted a study of 111 patients in two family practice settings to determine the technical skill and accuracy of SMBG in an outpatient population. The patients were observed using a 13-point checklist of critical steps in the calibration and operation of their glucose monitor. Overall, 53% of patient glucose values were within 10% of the control value, 84% were within 20% of the control value and 16% varied 20% or more from the control value. In short, the study showed that despite multiple technical errors when using SMBG, most patients obtained clinically useful values. A study reported by Bergenstal and colleagues found that 19% percent of patients had inaccuracy rates of more than 15% in blood glucose monitoring. Some of the most common causes of inaccurate readings included: lack of periodic meter technique evaluation, difficulty using wipe meters, incorrect use of control solutions, lack of hand washing (even when under clinical observation), and using unclean meters. These studies demonstrate the need for healthcare providers to monitor patient use of SMBG to help improve the accuracy of test results.
_
Error categories:
For the evaluation, an error classification system was developed containing a weighting and interpretation of the errors and the resulting consequences. Errors were classified in the categories F1 to F5:
_
Community pharmacy-based intervention to improve self-monitoring of blood glucose in type 2 diabetic patients: a 2006 study:
The objective of this study was to record and assess the errors patients make in preparing, performing, and processing self-monitoring of blood glucose (SMBG). This study shows that the majority of individuals with type 2 diabetes (83%) make at least one mistake in carrying out the measurement of blood glucose levels with their own device. The study revealed two kinds of errors that were quite frequent: errors that falsify the measurement reading as well as errors that can have a negative effect on patient compliance. In the reference literature there is a consensus that individuals with diabetes make numerous mistakes in the self-monitoring of their blood glucose levels and that remedial training sessions are required. The kinds of mistakes observed in the studies, however, were very similar. In other studies, too, the main mistakes were in cleaning of the hands, making adjustments to the settings of the meter and problems with coding. But there is little data on how much impact to expect from these remedial training sessions for type 2 diabetic patients. One problem is that there is no standard method for remedying the errors, so that the kind of error assessment is variable at the time of our study. A validated documentation sheet was not available. The documentation sheets used in various studies are designed to record 13 to 45 sources of error. Thus, no comparability exists among the various studies. The main difference in the evaluations consisted in whether the components of the SMBG were summarized or recorded in a very detailed way. Common to all of them, however, is that it could be shown in principle that diabetes management education sessions instructing how to carry out blood glucose self-testing are both necessary and effective, even if no general statement could be made about the extent of the success. The study presented here was able to show that a one-time, standardized intervention in community pharmacies specialized in diabetes care is able to more than triple the number of patients who carried out the self-monitoring without making any errors: initially 17% compared to 59% at the end of the study. However, a selection bias in the patient population of the study cannot be fully excluded, since such offers are probably accepted more frequently by motivated patients rather than by unmotivated patients. Altogether, the pharmacy setting is suited for carrying out such evaluations along with giving corresponding instructions on how to correctly perform SMBG. Such an intervention comprised verbal instructions as well as practical exercises and took on average about 20 to 30 minutes, including the study documentation.
_
Synopsis of SMBG variation and error:
___________
Efficacy of SMBG:
_
Why SMBG is helpful for Patients with Diabetes:
SMBG is helpful to patients with diabetes in four distinct ways.
1. First, it allows patients and clinicians to detect high or low blood glucose levels, thereby facilitating therapeutic adjustments to achieve long-term A1c goals.
2. Second, SMBG helps protect patients by allowing them to immediately confirm acute hypoglycemia or hyperglycemia.
3. Third, the technology facilitates patient education about diabetes and its management by giving patients more self-care responsibilities.
4. Fourth, SMBG helps motivate people toward healthier behavior.
_
SMBG facilitates improved A1c:
Many published studies have demonstrated that regular and frequent SMBG improves glycemic control in T1DM and T2DM patients on insulin treatment. There is also very strong evidence that SMBG improves control in T2DM patients who are not on insulin therapy. Davidson and colleagues showed that there is an inverse correlation between frequency of SMBG and A1c values in T1DM patients. Patients using SMBG have lower A1c than those who do not. The authors found that the more times per day that people check their blood glucose levels, the lower their A1c. However, after reaching a frequency of 6-7 tests per day, the improvement levels off. Strowig and colleagues showed similar results, reporting a 0.25% decrease in A1c for each blood glucose test per day. Again, there was a point of diminishing returns; improvements in A1c leveled off at approximately 8 tests per day. Studies of pediatric T1DM patients have demonstrated similar findings. In a retrospective study of more than 24,000 patients, Karter and colleagues found that increased frequency of SMBG correlated strongly with improved A1c regardless of the type of diabetes or therapy used.
_
Non-Insulin-Treated T2DM patients:
There has been much debate on the impact of SMBG on A1c in T2DM patients who are not treated with insulin. Skeptics of the benefits of SMBG use in this patient group often cite small or poorly designed studies that demonstrate no A1c benefit. This perspective often overlooks the fact that many T2DM patients are not adequately trained to interpret and respond to their test results. Utilization of SMBG involves more than simply documenting test results in a logbook; patients must understand and be able to make appropriate changes in therapy or activity based upon those results. SMBG testing in T2DM patients has also been hampered by a lack of consensus on the timing and frequency with which testing should be performed. Most patients who do perform blood glucose monitoring seldom test postprandial glucose. Other factors that inhibit testing frequency include the cost, pain, and inconvenience. All of these factors work against seeing a benefit in T2DM patients. Despite these factors, there is strong evidence that SMBG is, in fact, an effective method for lowering A1c in this patient group. A meta-analysis by Sarol and colleagues found an overall A1c improvement of 0.4% in non-insulin-treated T2DM patients who use SMBG compared with those who do not monitor. To counter potential criticism of their report, the authors critiqued the studies included in their meta-analysis and found no publication bias in their selection. A second meta-analysis conducted by Welschen and colleagues found similar results: an overall 0.39% improvement in A1c in type 2 patients not on insulin. The authors concluded that SMBG lowers A1c levels. Another review of the literature by Saudek in 2006 yielded similar findings.
_
SMBG and clinical outcome:
In a recent epidemiologic, non-randomized retrospective study, Martin and colleagues looked at disease-related fatal and non-fatal events in approximately 3,200 T2DM patients. Unlike the meta-analyses cited above, this study directly assessed clinical outcomes relative to SMBG utilization. Fewer patients who used SMBG experienced fatal or non-fatal events than patients who did not monitor their glucose (7.2 versus 10.4%, p=0.002). The authors concluded that SMBG may be associated with a healthier lifestyle and/or better disease management. Significantly, this study did not simply show that SMBG correlates with improved A1c; it demonstrated that SMBG is actually linked to better clinical outcomes. Furthermore, a recent study showed that patients described as being “Uncontrolled Diabetics” (defined in this study by HbA1C levels >8%) showed a statistically significant decrease in the HbA1C levels after a 90-day period of seven-point Self-Monitoring of Blood Glucose (SMBG) with a Relative Risk Reduction (RRR) of 0.18% (95% CI, 0.86-2.64%, p<.001).
________
Pros and cons of SMBG:
In contrast to the average blood glucose concentration reflected in an A1c level, the measurement of blood glucose itself, termed self monitoring of blood glucose (SMBG), only reflects what is going on at that moment. This has both advantages and disadvantages. One advantage is that, at least theoretically, interventions can be carried out by the patient at that moment to counter the high (or low) blood glucose concentration. Furthermore, when adjusting insulin doses, it is important to know the pattern of blood glucose values, i.e., when during the day the levels are high, in range, or low, since different parts of the insulin prescription affect glucose concentrations at various times after injection. The disadvantage is that the value only reflects one instance in time and glucose concentrations fluctuate throughout the day and night. Therefore, one value does not accurately portray what the overall levels of glucose are. It is certainly not unheard for patients to manipulate their behavior to ‘look good’ (i.e., have a glucose concentration near normal) when seen by their doctor by restricting their diets several days before, omitting food for 18–24 hours before the visit, taking extra insulin, etc. In that vein, it has been amply demonstrated that up to a quarter of patients will falsify their SMBG values when writing the results in their log books. In that case, they usually conveniently forget to bring in the meter, most of which contain a memory chip. Discrepancies between A1C levels and proffered SMBG values usually flush out this misguided behavior.
_
Most people would agree that treatments, especially those that have invasive components and/or are expensive, should result in improved clinical outcomes. SMBG, as part of a treatment plan, is both expensive and invasive but does have the potential to improve outcomes by helping to lower glucose concentrations and thereby decrease the small vessel complications of diabetes. In patients taking insulin, performing SMBG offers the opportunity to correct high measured values at that moment by injecting additional rapid-acting insulin. More importantly, the pattern of results over longer periods of time enables the physician (or selected patients) to make insulin dose adjustments to counteract blood glucose concentrations exceeding the desired range. Therefore, it is not surprising that in at least eight studies, A1c levels were inversely related to the frequency of SMBG measurements in insulin-requiring patients, i.e., the more frequently that patients tested, the lower their A1C levels. However, simply measuring blood glucose is ineffective. In one of the studies, increased frequency of SMBG resulted in lower A1C levels only in those who self-adjusted their insulin doses, not in the insulin-requiring patients who did not. This strongly suggests that acting on the measured values is necessary.
_
At least 19 studies have been carried out to evaluate the effect of SMBG on A1c levels in diabetic patients not receiving insulin. Only five have been positive, i.e., showing that performing SMBG in these patients was associated with statistically significant lower A1c levels than in control groups that did not carry out SMBG. However, in each one of them, factors other than SMBG were probably responsible for the positive results. These include greater attention to education and decision making in the group performing SMBG compared with the control group, self-selection or a preferential drop-out rate. In the first case, those in the SMBG group either received more intensive nutritional counseling or decisions on changing therapy were made more frequently than in their matched control group. In the second case, patients were given the choice of performing SMBG or not. Those who chose to also had better self care practices and healthy lifestyle behaviors documented by a questionnaire, thus invalidating the conclusion that SMBG per se is what led to the lower A1c levels. Finally, in one study, nearly 50% failed to finish it. If the nearly half of the SMBG group that failed to complete the study were enriched in those who were showing the least response, these results could also be due to self selection.
_
The gold standard for carrying out clinical studies is randomization and blinding. This means that the subjects are randomly chosen to be placed in the control or intervention group (which avoids self selection) and the person(s) carrying out the study are blinded so that they do not know whether the subject is in the control or intervention group (which avoids preferential treatment of one group). A nurse-directed diabetes disease management program afforded the author the opportunity to carry out such a study evaluating SMBG in type 2 diabetic patients who were taking pills but no insulin. In this program, a nurse (under the supervision of a physician) followed detailed treatment algorithms. Patients on pills were randomized to perform SMBG or not. Both groups were seen by a dietitian who taught the selected patients SMBG and provided nutritional counseling to both groups five times during a six-month period. The dietitian utilized the SMBG values (recommended before and after one meal every day but Sunday and carried out 45% of the time) in his nutritional counseling. Neither the nurse nor the physician when consulted by the nurse knew which patients were performing SMBG. Although A1c levels fell significantly in both groups, the decrease was not statistically significantly different between the groups. In other words, SMBG had no beneficial effect when patients not taking insulin received good diabetes care. There are at least three possible explanations for the lack of an effect of SMBG in patients not taking insulin. First, patients receive little or no feedback on their results. This was not the case in the randomized blinded study described above. Second, related to the first, they are not taught the self-management skills to use to lower the measured glucose values. However, there are a limited number of behaviors possible for patients not receiving insulin to counter a high SMBG value. If the measurement was taken before a meal, options include delaying that meal, eating less (especially carbohydrates), exercising at that point, or increasing the dose of a pill before that meal that rapidly increases insulin secretion. (That medication, however, is used by only a very small minority of patients.) Even if taught, given patients’ usual lifestyles, these self management activities are not very likely to occur. Third, in the author’s experience, the vast majority of patients measure their glucose level before meals, rather than after meals. This limits the two potential benefits of SMBG in patients not taking insulin – motivation and education. Fasting values serve neither to educate (there is no information on the effect of the meal composition or size) nor to motivate well (postprandial values are much higher). Except for early type 2 diabetes, in which the before-meal glucose values are near normal, the most important determinant of after-meal glucose concentrations is the before-meal value. Therefore, in the author’s view, if SMBG is to be recommended in patients not receiving insulin, it should be carried out before and one to two hours after a meal to maximize the educational value of how the size and composition of the meal contributes to the rise of glucose concentrations after eating (from the difference between the two SMBG values) and the motivational aspect by showing the patient how high the glucose level rises.
_
In addition to its drawbacks of invasiveness and lack of efficacy, SMBG is expensive. In the Kaiser Permanente Northern California Region, the cost for strips alone in 1998 was the fourth largest out-patient pharmacy expenditure, accounting for 2% of the entire budget. Some of these costs would, of course, be attributed to patients receiving insulin. Although it is not possible to completely isolate SMBG costs for diabetic patients not taking insulin, the Medicare B fee-for-service program run by the government affords a fairly accurate estimate of this cost. The ICD-9 code, 250.00 (type 2 diabetes, uncomplicated, not uncontrolled), is the one most often used for diabetic patients on either diet alone or taking oral antidiabetes medications. The total cost in 2002 for reagent strips, lancets, lancing devices, meters, batteries, calibration solutions or calibration chips was US$465,503,576, which represented 58.8% of the total outlay of the Medicare B program for the ICD-9 code of 250.00 (personal communication, staff, Center for Medicare & Medicaid Services).To the extent that type 2 diabetic patients receiving insulin were given this ICD- 9 code, this cost would be an overestimate. On the other hand, to the extent that type 2 diabetic patients not taking insulin were given another ICD-9 code, this cost would be an underestimate. However, since this cost does not include the 10% of Medicare beneficiaries enrolled in health maintenance organiztion (HMO) Managed Medicare, this figure is certainly an underestimate of the total cost for SMBG in type 2 diabetic Medicare patients not taking insulin. Given that this nearly half a billion dollars is only for Medicare patients, the total cost for SMBG for all type 2 diabetic patients not taking insulin is obviously much, much higher.
______
SMBG clinical trials:
Self-monitoring blood glucose (SMBG) improves glycaemic control in patients with type 1 diabetes and possibly also in insulin-treated type 2 diabetes (T2D), especially when treated with multiple insulin injections per day. However, the value and frequency of SMBG in non-insulin-treated patients with T2D is a matter of controversy. A consensus opinion among a group of experts from the UK suggested that patients with T2D using oral antidiabetic drugs (OAD) should monitor their blood glucose at least once daily, varying the time of testing between fasting, preprandial and postprandial levels during the day. A global consensus conference on SMBG recommended eleven measurements a week in these patients and another recent consensus conference noted that patents with T2D on OAD may use SMBG but specific recommendations with respect to frequency were not made. A cross-sectional and longitudinal study of patients with T2D in Australia showed that HbA1c was not significantly different between SMBG users and nonusers, either overall or within diabetes treatment groups such as diet, OAD or insulin, with or without OAD. Although such observational data can be useful in determining the effect of an intervention, conclusive evidence of this assumption is not available from randomized controlled trials. A recent study reported on the effect of a more and less intensive diabetes education combined with recommendations on the frequency of SMBG in patients with T2D. They found that a more intensive education did not result in an improved HbA1c (%) compared to standard information and care.
______
Summary of key observational studies on Self-Monitoring of Blood Glucose in Non-Insulin Treated Type 2 Diabetes:
| Study | Description of purpose | Findings/comments |
| Fremantle Diabetes Study | Assessed whether SMBG is an independent predictor of improved outcome in a community-based cohort of T2DM patients. Used longitudinal data from 1,280 T2DM participants (70% ongoing SMBG users at baseline) and a subset of 531 individuals who attended annual assessments over a 5-year period | SMBG was associated with a 48% decreased risk of cardiovascular mortality in insulin-treated patients, but a 79% increased risk in non-insulin-treated patients. Time-dependent SMBG was independently associated with a 48% reduced risk of retinopathy in the 5-year cohort.‘Inconsistent findings relating to the association of SMBG with cardiac death and retinopathy may be due to confounding, incomplete covariate adjustment or chance’ |
| Kaiser Permanente | Assessed longitudinal association between SMBG and glycemic control in diabetic patients from an integrated health plan. Followed 16,091 new SMBG users and 15,347 ongoing users over a 4-year period | Greater SMBG frequency among new users was associated with a graded decrease in HbA1c (relative to non-users) regardless of diabetes therapy. Longitudinal changes in SMBG frequency were related to significant changes in glycemic control |
| QuED | Assessed impact of SMBG on metabolic control in non-insulin-treated T2DM subjects (41% ongoing SMBG users at baseline). Followed 1,896 patients over a 3-year period | Performance and frequency of SMBG did not predict better metabolic control over 3 years. Investigators could not identify any specific subgroups for whom SMBG practice was associated with lower HbA1c levels during the study |
| ROSSO | Investigated relationship of SMBG with disease-related morbidity and mortality. Followed 3,268 patients from diagnosis of T2DM between 1995 and 1999 until end of 2003 (mean follow-up 6.5 years) retrospectively from medical records | SMBG was associated with decreased diabetes-related severe morbidity and all-cause mortality. This association was also seen in subgroup of non-insulin-treated patients. Medical records contained data on some biochemical parameters, retinopathy and neuropathy for only a small proportion of patients |
| King-Drew Medical Center Trial | Randomized, single-blind study designed to determine whether SMBG improves HbA1c in non-insulin-treated T2DM patients. Clinical management decisions were blinded to SMBG data and use 89 non-insulin-treated T2DM patients were followed for 6 months | At 6 months, differences in decrease in HbA1c levels were not statistically significant. The rapid upgrading of medication every two weeks if goals were not met may have obscured the potential of SMBG for supporting self-management |
| ESMON | Prospective randomized controlled trial assessed the effect of SMBG vs. no monitoring on glycemic control and psychological indices in patients with newly diagnosed T2DM. Evaluated 184 non-insulin-treated patients with no previous use of SMBG over 12 months | There were no significant differences in HbA1c between groups at any time point. SMBG was associated with a 6% higher score on the depression subscale of the well-being questionnaire. The major improvement of mean HbA1c levels in the control group, from 8.6 to 6.9% indicates a dominant role of medication in disease management |
| DINAMIC | Multicentre, randomized, parallel-group trial was designed to determine if therapeutic management programs for T2DM that included SMBG result in greater reductions in HbA1c compared with programs without SMBG in non-insulin-treated patients.Followed 610 T2DM patients with early or mild diabetes receiving an identical oral anti-diabetic therapy regimen with gliclazide for 27 weeks | There was a major decrease of HbA1c which was significantly larger in the SMBG group than the control group. The incidence of symptomatic hypoglycemia was lower in the SMBG group. The major improvement of HbA1c levels in the control group from 8.1 to 7.2% indicates a dominant role of medication in disease management |
| German-Austrian | Prospective, multicenter, randomized controlled study Investigated the effect of meal-related SMBG on glycemic control and well-being in non-insulin-treated T2DM subjects. Followed 250 non-insulin-treated T2DM patients for 6 months | In per-protocol analysis (n=223) use of SMBG significantly reduced HbA1c levels. SMBG use resulted in a marked improvement of general well-being with significant improvements in the subject’s depression and lack of well-being. The benefit of intense patient care is evident but the contribution of intense vs. SMBG cannot be assessed |
| DiGEM | Three-arm, open, parallel group randomized trial designed to determine whether SMBG alone, or with instruction in incorporating results into self-care, is more effective than standardized usual care in improving glycaemic control in non-insulin-treated T2DM patients. Followed 453 patients with a mean HbA1c level of 7.5% for a median duration of 1 year. | At 12 months the differences in HbA1c level between the three groups were not statistically significant. Investigators concluded that evidence is not convincing of an effect of SMBG, with or without instruction in incorporating findings into self-care, compared with usual care in reasonably well controlled non-insulin-treated patients with type 2 diabetes. |
_______
_______
Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin:
A 2012 study by Cochrane Collaboration:
Self-monitoring of blood glucose (SMBG) has been found to be effective for patients with type 1 diabetes and for patients with type 2 diabetes using insulin. There is much debate on the effectiveness of SMBG as a tool in the self-management for patients with type 2 diabetes who are not using insulin. The Objective of this study was to assess the effects of SMBG in patients with type 2 diabetes mellitus who are not using insulin. Twelve randomised controlled trials were included and evaluated outcomes in 3259 randomised patients. Intervention duration ranged from 6 months (26 weeks) to 12 months (52 weeks). Nine trials compared SMBG with usual care without monitoring, one study compared SMBG with SMUG, one study was a three-armed trial comparing SMBG and SMUG with usual care and one study was a three-armed trial comparing less intensive SMBG and more intensive SMBG with a control group. Seven out of 11 studies had a low risk of bias for most indicators. From this review, authors conclude that when diabetes duration is over one year, the overall effect of self-monitoring of blood glucose on glycemic control in patients with type 2 diabetes who are not using insulin is small up to six months after initiation and subsides after 12 months. Furthermore, based on a best-evidence synthesis, there is no evidence that SMBG affects patient satisfaction, general well-being or general health-related quality of life. More research is needed to explore the psychological impact of SMBG and its impact on diabetes specific quality of life and well-being, as well as the impact of SMBG on hypoglycemia and diabetic complications. The aim of this systematic review was to assess the effects of SMBG in patients with type 2 diabetes who are not using insulin. Six randomised controlled trials were added to the six trials included in the original review (Welschen 2005a). In non-insulin treated type 2 diabetes patients with a diabetes duration of at least one year the overall effect of SMBG compared to control groups and a follow-up of six months showed a statistically significant 0.3% HbA1c decrease. In contrast, authors saw a non-significant decrease of 0.1% in HbA1c in patients in SMBG groups compared to control groups over a 12 months follow-up period. Secondly, the overall effect of SMBG compared to SMUG over a follow-up of six months showed a statistically non-significant decrease of 0.2% HbA1c. Thirdly, it was not possible to estimate an overall effect of SMBG over a follow-up of six months for newly diagnosed non-insulin treated type 2 diabetes patients, due to substantial inconsistency in the direction of the effect. However, the overall effect of SMBG with a follow-up of 12 months demonstrated a statistically significant decrease of 0.5% in HbA1c compared to control groups (two trials). Concerning health-related quality of life, well-being and patient satisfaction outcomes, based on a best-evidence synthesis authors conclude that there was no significant evidence available that SMBG had an effect on patient satisfaction (4 out of 4 trials), general well-being (4 out of 4 trials) or general health-related quality of life (3 out of 3 trials). Regarding levels of depression (WBQ-22, sub scale), inconsistent findings were observed (2 out of 2 trials). Lastly, regarding the secondary outcomes authors conclude that based on a best-evidence synthesis periods of both asymptomatic and symptomatic hypoglycaemia are more frequent in patients performing SMBG (3 out of 4 trials); and secondly, there is no statistically significant difference in fasting plasma glucose levels between SMBG and control intervention groups (3 out of 3 trials).
_
Results from studies of SMBG use in non-insulin-treated T2DM have been mixed, due to differences in study design, populations, outcome indicators, and inherent limitations of the traditional RCT models used. However, current evidence suggests that using SMBG in this population has the potential to improve glycemic control, especially when incorporated into a comprehensive and ongoing education program that promotes management adjustments according to the ensuing blood glucose values. SMBG use should be based on shared decision making between people with diabetes and their healthcare providers and linked to a clear set of instructions on actions to be taken based upon SMBG results. SMBG prescription is discouraged in the absence of relevant education and/or ability to modify behaviour or therapy modalities. In summary, the appropriate use of SMBG by people with non-insulin-treated diabetes has the potential to optimize diabetes management through timely treatment adjustments based on SMBG results and improve both clinical outcomes and quality of life. However, the value and utility of SMBG may evolve within a preventive care model that is based on ongoing monitoring and the ability to adjust management as the diabetes progresses over time. In the meantime, more effective patient and provider training around the use of SMBG is needed. Because skilled healthcare professionals are needed now and in the future to address the growing diabetes epidemic, it is hoped that this report will encourage the development and systematic introduction of more effective diabetes self-management education/training and the value-based models of clinical decision making and care delivery.
_
A systematic review of self blood glucose monitoring (SMBG) in type 2 patients not taking insulin concluded: “The overall effect of SMBG was a statistically significant decrease of 0.39% in glycated hemoglobin (HbA1c) compared with the control groups. This is considered clinically relevant. Based on the UK Prospective Diabetes Study, a decrease of 0.39% in HbA1c is expected to reduce risk of microvascular complications by 14%. Davidson, on the other hand, in a counterpoint to this study, reviewed several trials and concluded that SMBG fails to reduce HbA1c in type 2 patients not taking insulin and is therefore a waste of money.
_
HBA1c as a function of SMBG measurements per day:
The figure above conclusively proves that SMBG improves A1c in all types of DM and consequently reduce diabetic complications and improve clinical outcomes.
________
Continuous glucose monitoring (CGM):
_
_
A continuous glucose monitoring system (CGMS) measures blood glucose on a continuous basis (every few minutes). Two types of devices are available: newer systems that display “real time” glucose results directly on the monitor system, and earlier “non-real time” devices that do not have this result display capability and results are only available for retrospective viewing and analysis when data are downloaded to computer.
A typical “real-time” system consists of:
- a disposable glucose sensor placed just under the skin, which is worn for a few days until replacement,
- a link from the sensor to a non-implanted transmitter which communicates to a radio receiver,
- an electronic receiver worn like a pager (or insulin pump) that displays blood glucose levels on a practically continuous manner, as well as monitors rising and falling trends in glycemic excursions.
Continuous monitoring allows examination of how the blood glucose level reacts to insulin, exercise, food, and other factors. The additional data can be useful for setting correct insulin dosing ratios for food intake and correction of hyperglycemia. Monitoring during periods when blood glucose levels are not typically checked (i.e. overnight) can help to identify problems in insulin dosing (such as basal levels for insulin pump users or long-acting insulin levels for patients taking injections). Monitors may also be equipped with alarms to alert patients of hyperglycemia or hypoglycemia so that a patient can take corrective action(s) (after finger stick testing, if necessary) even in cases where they do not feel symptoms of either condition. While the technology has its limitations, studies have demonstrated that patients with real-time continuous sensors experience less hyperglycemia, hyperglycemia, nocturnal hypoglycemia and even improvement in A1C levels. CGMS do not directly measure glucose levels in the blood, but measure the glucose level of interstitial fluid. This results in two disadvantages as compared to traditional blood glucose monitoring.
1. Using current technology, continuous systems must be calibrated with a traditional blood glucose measurement and therefore do not yet fully replace “finger stick” measurements.
2. Glucose levels in interstitial fluid temporally lag behind blood glucose values. The lag time has been reported to be 5 minutes in general. This lag time is insignificant when blood sugar levels are relatively consistent. However, blood sugar levels, when changing rapidly (rising such as after a meal, or dropping in case of hypoglycemia), may read in the normal range on a CGMS while in reality the patient is already experiencing symptoms of an out-of-range blood glucose value and may require treatment. For these and other reasons related to this first generation technology, patients using CGMS are typically advised to take traditional finger stick measurements at least twice a day (for calibration), to verify that the sensor readings are accurate, and whenever they wish to self-treat their diabetes. Currently available CGMS are relatively expensive. While changing a subcutaneous cannula every 3–7 days would be less invasive than 3 or more finger sticks per day, the technology is still invasive. Coincidentally, the standard finger stick method must be used alongside the CGM technology to guarantee its functionality and accuracy. CGM technology is a major step in the advancement of diabetes care and has been proven effective. The ultimate goal of CGM technology is to use it in combination with subcutaneous insulin pumps, and in effect create an external “artificial pancreas” thereby providing better overall health and improved HbA1C tests.
_
The currently available CGMs measure blood glucose either with minimal invasiveness through continuous measurement of interstitial fluid (IF) or with the noninvasive method of applying electromagnetic radiation through the skin to blood vessels in the body. The technologies for bringing a sensor into contact with IF include inserting an indwelling sensor subcutaneously (into the abdominal wall or arm) to measure IF in situ or harvesting this fluid by various mechanisms that compromise the skin barrier and delivering the fluid to an external sensor. These IF measurement technologies are defined as minimally invasive because they compromise the skin barrier but do not puncture any blood vessels. After a warm-up period of up to 2 h and a device-specific calibration process, each device’s sensor will provide a blood glucose reading every 1–10 min for up to 72 h with the minimally invasive technology and up to 3 months with the noninvasive technology. Results are available to the patient in real time or retrospectively. Every manufacturer of a CGM produces at least one model that sounds an alarm if the glucose level falls outside of a preset euglycemic range. Invasive indwelling intravascular sensors that measure blood glucose directly are also under development for monitoring hospitalized patients. Prolonged use of such devices might cause vascular damage or infection. No articles have been published on their performance.
_
Noninvasive CGM Sensor:
A novel noninvasive method introduced by Glucon in 2007 is a CGM sensor called Aprise. This technology uses the photo acoustic properties of blood to measure glucose levels. The technique involves a sensor placed on a blood vessel that emits sound and pressure waves generated with short laser pulses absorbable by human tissue. The absorption causes a rise in temperature, and this creates an acoustic pressure impulse on the tissue surface. This impulse carries information about the properties of the structure underlying the skin. Glucose is known to affect the optical properties of blood, and thus the ultrasonic image changes of the tissue can estimate the blood glucose concentration. A study of 62 inpatients showed similar results with the Aprise CGM system compared with the more invasive CGM systems. Results showed accuracy in measuring glucose in 51%, 67%, and 86% of samples amongst low (<150 mg/dL), mid (151–200 mg/dL), and high (>251 mg/dL) glucose ranges respectively. They were able to directly measure the blood instead of using the interstitial fluid compartment and inferring the systemic glucose levels. The results from this noninvasive CGM trial were promising; however, the Aprise is yet to be marketed to the public.
_
According to a recent report the CGM technology is not as reliable as initially anticipated. In a clinical trial following 11 patients with either Type I or Type II diabetes, Mazze and colleagues found that the 2 leading CGM systems, DexCom (San Deigo, CA) and MiniMed Guardian RT (Northhridge, CA), are less accurate than expected due to a delay in the time it takes to monitor glucose in interstitial fluid and display it to the patient. There were lag times of 21 ± 5 min for the Guardian system and 7 ± 7 min for Dexcom. The CGM devices were also found to be less reliable than the traditional finger stick method. While both the Guardian and the Dexcom monitors should display 282 readings per day, the Dexcom averaged 204 ± 37 and the Guardian 229 ± 29. The inaccuracies did not induce episodes of acute crisis, such as diabetic coma, nor did they stop the technology from being marketed. As a result it is recommended that finger sticks continue to be performed to confirm high and low glucose readings before relying solely on CGM. The potential for somewhat continuous detection of glycemic abnormalities and maintaining overall glycemic control was achieved, and the technology was appoved for sale. There is no clear consensus about the clinical indications for CGM in actual clinical practice. A Cochrane review found that there is limited evidence for the effectiveness of real-time CGM use in children, adults and patients with poorly controlled diabetes. However there were indications that higher compliance of wearing the CGM device improved glycosylated HbA1c level to a larger extent.
_
Clinical indications of CGM:
Situations that require detailed information about blood glucose fluctuations that only continuous monitoring can provide include when adjusting therapy, quantifying the response in a trial of a diabetes therapy, assessing the impact of lifestyle modifications on glycemic control, monitoring conditions where tighter control without hypoglycemia is sought (e.g., gestational diabetes, pediatric diabetes, in the intensive care unit), diagnosing and then preventing hypoglycemia (e.g., during sleep, with hypoglycemia unawareness), and diagnosing and preventing postprandial hypoglycemia. The most important use of continuous blood glucose monitoring is to facilitate adjustments in therapy to improve control. Specific therapeutic adjustments include changing from regular to a synthetic ultrashort-acting insulin analog at mealtime, changing from NPH to a synthetic ultralong-acting insulin once or twice per day, increasing or decreasing the mealtime insulin bolus dosage, increasing or decreasing the basal insulin rate, altering the treatment of intermittent hypoglycemia or hyperglycemia, changing the insulin-to-glucose correction algorithm for premeal hyperglycemia, changing the insulin-to-carbohydrate ratio at mealtime, changing the method for counting carbohydrates, changing the carbohydrate composition of the diet, changing the discount in short-acting insulin dosage for exercise, changing the nighttime regimen because of the dawn phenomenon, changing the target preprandial or postprandial blood glucose values, or before referring a patient for psychological counseling to improve adherence to the treatment regimen. The most frequent therapy adjustment by Sabbah et al. (out of eight adjustments) was to increase the mealtime bolus dosage. The most frequent therapy adjustment by Kaufman et al. (out of nine adjustments) was to modify the type of basal long-acting insulin.
_
Accuracy of CGM:
A real-time CGM can be programmed to sound an alarm for readings below or above a target range. The most important use of an alarm is to detect unsuspected hypoglycemia (such as during sleep) so that glucose can be administered to prevent brain damage. There is a trade-off between an alarm’s sensitivity and specificity. In general, if the alarm is set to sound at a lower level than the hypoglycemic threshold, then the specificity will be good but the sensitivity may be poor. If the alarm is set to sound at a glucose level higher than the hypoglycemic threshold, then the sensitivity will be good but the specificity may be poor. The greater accuracy a continuous monitor can provide, the less of a trade-off is necessary.
_
The Diabetes Research in Children Network (DirecNet) is a U.S. network of five clinical centers and a coordinating center dedicated to researching glucose monitoring technology in children with type 1 diabetes. The network’s investigators, the DirecNet Study Group, assessed the accuracy of the first- and second-generation CGMS and the GW2B in children with type 1 diabetes in concurrently published studies. The second-generation CGMS Gold, compared with the first-generation CGMS, had a lower median relative absolute difference (RAD) between CGMS glucose and reference serum glucose paired values (11 and 19%, respectively). For the GW2B, the median RAD between GW2B glucose and reference serum glucose paired values was 16%. Similar RAD values of 21% have been reported for the first-generation CGMS by Kubiak et al. RAD values of 12.8% and 12.8–15.7% have been reported for the second-generation CGMS Gold system by Goldberg et al. and Guerci et al, respectively. Bode et al. evaluated the performance of the Guardian Continuous Monitoring System (Medtronic MiniMed) and whether using real-time alarms reduced hypo- and hyperglycemic excursions in a type 1 diabetic population. The mean absolute relative error between home blood glucose meter readings and sensor values was 21.3% (median 7.3%); further, on average the Guardian read 12.8 mg/dl below the concurrent home blood glucose meter readings. The hypoglycemia alert was able to distinguish glucose values ≤70 mg/dl with 67% sensitivity, 90% specificity, and 47% false alerts. The hyperglycemia alert showed a similar ability, detecting values ≥250 mg/dl with 63% sensitivity, 97% specificity, and 19% false alerts. The alerts resulted in a significant (P = 0.03) reduction in the duration of hypoglycemic excursions and a marginally significant (P = 0.07) increase in the frequency of hyperglycemic excursions. The International Organization for Standardization (ISO) standards for accuracy of point blood glucose tests require that a sensor blood glucose value be within 15 mg/dl of reference for a reference value ≤75 mg/dl and within 20% of reference for a reference value >75 mg/dl. Sensor accuracy by this definition is expressed as the percentage of data pairs meeting these requirement. The DirecNet group found that for hypoglycemic blood glucose levels (determined by a reference blood glucose monitor, the OneTouch Ultra), the CGMS Gold met the ISO standards in only 48% of readings and the GW2B met these standards in only 32% of readings. The percentage of data points attaining ISO accuracy standards climbed as the blood glucose level rose, topping out for the highest segment of reference blood glucose levels (i.e., blood glucose values ≥240 mg/dl). In this glycemic category, the CGMS Gold and GW2B, respectively, met ISO accuracy for 81 and 67% of data points. In a separate series of 15 healthy nondiabetic children undergoing continuous glucose monitoring over 24 h, the DirecNet Group reported that the median absolute difference in concentrations for the GW2B was 13 mg/dl and for the CGMS was 17 mg/dl. Furthermore, 30% of the values from the GW2B and 42% of the values from the CGMS deviated by >20 mg/dl from the reference value.
_
Continuous glucose monitoring offers advantages over intermittent glucose monitoring when glycemic patterns are poorly understood. The information about direction, magnitude, duration, frequency, and causes of fluctuations in blood glucose levels that can be obtained by continuous glucose monitoring is simply not available with intermittent blood glucose monitoring. When retrospective patterns are needed to adjust therapy or document the state of physiology, CGMs are useful. When real-time recognition of both the absolute magnitude of glycemia as well as trend patterns are needed, then a real-time CGM provides a wealth of information. Technologies for continuous glucose monitoring require patient education for proper use. During hypoglycemia or periods of rapid fluctuation, values provided by CGMs may be inaccurate. Clinical outcome studies suggest that measures of mean glycemia and hypoglycemic burden both improve with the use of continuous glucose monitoring, but more studies are needed to convince payers to reimburse for this technology. In this data-hungry world, it appears likely that CGMs will eventually become a routine part of diabetes management, initially for patients with difficult-to-control diabetes and eventually for most patients with diabetes. Retrospective reporting will eventually give way to real-time readings, and adjunctive use requiring a confirmatory finger-stick blood test will eventually give way to primary use without the requirement of such confirmation. As methods for minimally invasive and noninvasive continuous monitoring advance, diabetic patients will use this technology more routinely. Data printouts from CGMs will increasingly provide a roadmap for effective diabetes management in the 21st century.
__
Continuous glucose monitoring systems for type 1 diabetes mellitus: Cochrane review 2012:
Type 1 diabetes is a disease in which the pancreas has lost its ability to make insulin. A deficit in insulin leads to increases in blood glucose levels, these elevated blood glucose levels can lead to complications which may affect the eyes, kidneys, nerves and the heart and blood vessels. Since there is no cure for type 1 diabetes, patients need to check their blood glucose levels often by fingerprick and use these blood glucose values to decide on their insulin dosages. Fingerpricks are often regarded as cumbersome and uncomfortable by patients. In addition, fingerprick measurements only provide information about a single point in time, so it is difficult to discern trends in decline of rises in blood glucose levels. Continuous glucose monitoring systems (CGM) measure blood glucose levels semi-continuously. In this review 22 studies were included. These studies randomised 2883 patients with type 1 diabetes to receive a form of CGM or to use self measurement of blood glucose (SMBG) using fingerprick. The duration of follow-up varied between 3 and 18 months; most studies reported results for six months of CGM use. This review shows that CGM helps in lowering the glycosylated haemoglobin A1c (HbA1c) value (a measure of glycemic control). In most studies the HbA1c value decreased (denoting improvement of glycemic control) in both the CGM and the SMBG users, but more in the CGM group. The difference in change in HbA1c levels between the groups was on average 0.7% for patients starting on an insulin pump with integrated CGM and 0.2% for patients starting with CGM alone. The most important adverse events, severe hypoglycemia and ketoacidosis did not occur frequently in the studies, and absolute numbers were low (9% of the patients, measured over six months). Diabetes complications, death from any cause and costs were not measured. There are no data on pregnant women with diabetes type 1 and patients with diabetes who are not aware of hypoglycemia. There is limited evidence for the effectiveness of real-time continuous glucose monitoring (CGM) use in children, adults and patients with poorly controlled diabetes. The largest improvements in glycaemic control were seen for sensor-augmented insulin pump therapy in patients with poorly controlled diabetes who had not used an insulin pump before. The risk of severe hypoglycemia or ketoacidosis was not significantly increased for CGM users, but as these events occurred infrequent these results have to be interpreted cautiously. There are indications that higher compliance of wearing the CGM device improves glycosylated haemoglobin A1c level (HbA1c) to a larger extent.
_
Glucose sensing bio-implants:
A significant improvement of diabetes therapy might be achieved with an implantable sensor that would continuously monitor blood sugar levels within the body and transmit the measured data outside. The burden of regular blood testing would be taken from the patient, who would instead follow the course of their glucose levels on an intelligent device like a laptop or a smart phone. Glucose concentrations do not necessarily have to be measured in blood vessels, but may also be determined in the interstitial fluid, where the same levels prevail – with a time lag of a few minutes – due to its connection with the capillary system. However, the enzymatic glucose detection scheme used in single-use test strips is not directly suitable for implants. One main problem is caused by the varying supply of oxygen, by which glucose is converted to glucono lactone and H2O2 by glucose oxidase. Since the implantation of a sensor into the body is accompanied by growth of encapsulation tissue as seen in the figure below, the diffusion of oxygen to the reaction zone is continuously diminished. This decreasing oxygen availability causes the sensor reading to drift, requiring frequent re-calibration using finger-sticks and test strips.
_
_
Other approaches replace the troublesome glucose oxidase reaction with a reversible sensing reaction, known as an affinity assay. This scheme was originally put forward by Schultz & Sims in 1978. A number of different affinity assays have been investigated, with fluorescent assays proving most common. MEMS technology has recently allowed for smaller and more convenient alternatives to fluorescent detection, via measurement of viscosity. Investigation of affinity-based sensors has shown that encapsulation by body tissue does not cause a drift of the sensor signal, but only a time lag of the signal compared to the direct measurement in blood. Longer term solutions to continuous monitoring, not yet available but under development, use a long-lasting bio-implant. These systems promise to ease the burden of blood glucose monitoring for their users, but at the trade off of a minor surgical implantation of the sensor that lasts from one year to more than five years depending on the product selected. Products under development include: The Senseonics Continuous Glucose Monitoring System, Implanted Glucose Bio-sensor, The Dexcom LTS (long term system) and The Animas Glucose Sensor.
_
Function of an Implanted Tissue Glucose Sensor for more than 1 Year in Animals:
An implantable sensor capable of long-term monitoring of tissue glucose concentrations by wireless telemetry has been developed for eventual application in people with diabetes. The sensor telemetry system functioned continuously while implanted in subcutaneous tissues of two pigs for a total of 222 and 520 days, respectively, with each animal in both nondiabetic and diabetic states. The sensor detects glucose via an enzyme electrode that is based on differential electrochemical oxygen detection, which reduces the sensitivity of the sensor to encapsulation by the body, variations in local microvascular perfusion, limited availability of tissue oxygen, and inactivation of the enzymes. After an initial 2-week stabilization period, the implanted sensors maintained stability of calibration for extended periods. The lag between blood and tissue glucose concentrations was 11.8 ± 5.7 and 6.5 ± 13.3 minutes (mean ± standard deviation), respectively, for rising and falling blood glucose challenges. The lag resulted mainly from glucose mass transfer in the tissues, rather than the intrinsic response of the sensor, and showed no systematic change over implant test periods. These results represent a milestone in the translation of the sensor system to human applications.
__________
Artificial pancreas:
To overcome the limitations of current insulin therapy, researchers have long sought to link glucose monitoring and insulin delivery by developing an artificial pancreas. An artificial pancreas is a system that will mimic, as closely as possible, the way a healthy pancreas detects changes in blood glucose levels and responds automatically to secrete appropriate amounts of insulin. Although not a cure, an artificial pancreas has the potential to significantly improve diabetes care and management and to reduce the burden of monitoring and managing blood glucose.
An artificial pancreas based on mechanical devices requires at least three components:
- a CGM system
- an insulin delivery system
- a computer program that “closes the loop” by adjusting insulin delivery based on changes in glucose levels
With recent technological advances, the first steps have been taken toward closing the loop. The first pairing of a CGM system with an insulin pump—the MiniMed Paradigm REAL-Time System—is not an artificial pancreas, but it does represent the first step in joining glucose monitoring and insulin delivery systems using the most advanced technology available.
_
A Breakthrough in better Glucose Control: MiniMed 530G with Enlite:
As an integrated insulin pump and CGM system, MiniMed 530G with Enlite offers better control than multiple daily injections or conventional insulin pumps. It increases confidence to achieve better control. Bayer’s Contour Next Link allows seamless integration as a part of the MiniMed 530G system, transmitting exceptionally accurate blood glucose results wirelessly to the insulin pump. This makes it easier for you and your doctor to make better therapy adjustments than with logbooks alone. CareLink is a convenient online tool that collects and organizes information from your system into personalized reports.
_
Bionic pancreas:
The device represents a solution that is as close to a cure as some feel possible for now and may be available to the commercial market by 2017. Developed by a collaborative biomedical team between BU and Massachusetts General Hospital, the device is worn externally and consists of two hormone pumps (insulin and glucagon), an iPhone and a continuous glucose monitor that measures blood sugar levels every five minutes. It is a marriage of biology and technology, with a powerful algorithm capable of recommending and delivering instantaneous hormone that balances blood sugar better than any diabetic can replicate on his or her own. It is self-correcting, constantly making little adjustments to keep the blood sugars in the optimal range and learning from the patient’s own history how much insulin or glucagon to provide. All of the participants in the study had blood sugars that would greatly reduce, if not eliminate, the long-term risks of diabetes.
__________
ICU and glucose monitoring:
Accuracy of different methods for blood glucose measurement in critically ill patients:
Measurement of blood glucose concentration in ICUs is currently performed almost entirely intermittently, with analysis using either point-of-care glucose meters or blood gas analyzers. Although accurate data are not available, most measurements are probably made on glucose meters and the majority of samples are capillary blood obtained by finger pricks. The use of glucose meters and sampling capillary blood both have the potential to introduce errors into the measurement of blood glucose concentration. Severe sepsis and septic shock are the main causes of death in intensive care units. More than 750,000 cases of severe sepsis occur annually in the United States, amounting to 215,000 deaths/year in that country. Impaired microcirculation plays a leading role in this setting and, unless corrected, it can evolve to multiple organ dysfunction and death. Glucose homeostasis becomes modified in these patients, thereby resulting in insulin resistance, hyperinsulinemia and consequent hyperglycemia. This set of conditions is named stress diabetes, and it is a physiological response that ensures glucose supply to non-insulin-dependent tissues such as hepatocytes, nerve cells and alveolar, endothelial and immune system cells. Hyperglycemia is an independent predictor of adverse outcomes in cases of cardiovascular disease, neurological disorders, respiratory, liver and gastrointestinal disease, malignancy, sepsis and surgical patients. Normoglycemia is related to lower morbidity and mortality because of improvements in systemic inflammatory processes and in immune, endothelial and mitochondrial dysfunctions. Normoglycemic patients are less susceptible to bloodstream infection, renal failure, anemia and transfusion, polyneuropathy, hyperbilirubinemia and prolonged dependence on both mechanical ventilation and intensive care therapy. Additionally, glucose control is cost-effective. Thus, although glucose control is a priority in treating critically ill patients, glucose monitoring can be quite challenging. Considering that many intensive care patients are unable to express signs and symptoms of hypoglycemia, frequent and accurate measurements are pivotal. Given the low cost, easy sampling and prompt results of glucometers, capillary blood glucose levels are often determined using this method, although it has not been validated for intensive care patients. Critically ill patients have multiple relevant conditions that can interfere with measurements such as pH, partial pressure of oxygen, hematocrit, low blood pressure levels and tissue hypoperfusion. Measurement mistakes may lead to unnecessary procedures regarding insulin doses and increase the risk of severe or prolonged hypoglycemia and its complications such as seizures, coma, arrhythmia and irreversible cerebral damage. So fingerstick capillary blood glucose method usually overestimates the true glucose levels and gives rise to management errors in ICU patients. The use of glucometer in intensive care must also be avoided, particularly if noradrenalin is being used. Predominantly, this method overestimates blood glucose levels, which implies procedural errors and exposes patients to more frequent and prolonged hypoglycemic events. There is an increasing volume of evidence that maintaining as close to normal glucose as possible in hospitalized patients, especially those in intensive, surgical, and critical care units, can significantly improve outcomes, decreasing both morbidity and mortality.
_
Alternatives to the use of glucose meters are measurement in the hospital’s central laboratory or using a blood gas analyzer in the ICU. Although central laboratory measurement is much more accurate, the time delay in sending samples to the laboratory makes this an impractical solution for the ICUs in most hospitals. The frequency with which the blood glucose concentration is measured in the ICU makes venipuncture impractical, and viable alternatives are to sample from indwelling arterial or venous catheters. Sampling from indwelling vascular catheters may increase the risk of catheter-related bloodstream infection but this risk has not been quantified. Obviously, when sampling from indwelling catheters it is essential to avoid contamination from infusions of glucose-containing fluids. A more practical solution, but one that may have considerable cost implications, is to measure the blood glucose concentration in a blood gas analyzer because the majority of ICUs in the developed world will have such an analyzer in the ICU. Measurements from a properly maintained blood gas analyzer will have similar accuracy to central laboratory measurements. An additional consideration is that the blood glucose concentration varies in different vascular beds and the site from which blood is sampled can introduce further errors. The blood glucose concentration in radial arterial blood will be approximately 0.2 mmol/l higher than that in blood sampled from a peripheral vein, and 0.3 to 0.4 mmol/l higher than that in blood sampled from the superior vena cava. Sampling capillary blood in ICU patients, particularly in those who are hemodynamically unstable and being treated with vasopressors, can introduce large errors when compared with a reference method in which glucose is measured in central venous or arterial samples.
_
CGM in ICU:
Numerous techniques are available for continuous glucose monitoring in the ICU, including microdialysis and optical methods such as absorption spectroscopy, optical scattering and fluorescence. The blood glucose concentration can be measured in vitro by sensors that sit in the vascular or interstitial space or ex vivo by drawing blood samples or a dialysate to a sensor from an indwelling vascular catheter or dialysis membrane. Systems that intermittently draw blood to an externally based sensor may be described as automated intermittent monitors rather than continuous glucose monitors. Potential advantages of continuous glucose monitors include the ability to observe trends in blood glucose concentration and to intervene before the blood glucose concentration enters an unacceptable range, and removal of operator error both in the timing of blood glucose measurements and in the sampling and analysis of blood. Almost all monitoring of the blood glucose concentration in critically ill patients is by intermittent measurement. Although intermittent measurement is current standard practice, there is no agreed metric for reporting glycemic control and many of the metrics currently reported are affected by the frequency of measurement. Current systems for continuous or automated intermittent monitoring may measure the blood glucose concentration at a frequency varying from every minute to every 15 minutes. Such monitors will not only increase the number of measurements, but will also standardize the frequency of measurements amongst patients monitored with each device. This may allow for a better reporting of glucose control metrics, and if sufficiently accurate may offer a better understanding of the association between those metrics and outcomes.
__________
Limitations of Conventional Methods of Self-Monitoring of Blood Glucose: a 2001study:
Children with type 1 diabetes are usually asked to perform self-monitoring of blood glucose (SMBG) before meals and at bedtime, and it is assumed that if results are in target range, along with HbA1c measurements, then overall glycemic control is adequate. However, the brief glimpses in the 24-h glucose profile provided by SMBG may miss marked glycemic excursions. The MiniMed Continuous Glucose Monitoring System (CGMS) has provided a new method to obtain continuous glucose profiles and opportunities to examine limitations of conventional monitoring. A total of 56 children with type 1 diabetes (age 2–18 years) wore the CGMS for 3 days. Patients entered four fingerstick blood samples into the monitor for calibration and kept records of food intake, exercise, and hypoglycemic symptoms. Data were downloaded, and glycemic patterns were identified. Despite satisfactory HbA1c levels (7.7 ± 1.4%) and premeal glucose levels near the target range, the CGMS revealed profound postprandial hyperglycemia. Almost 90% of the peak postprandial glucose levels after every meal were >180 mg/dl (above target), and almost 50% were >300 mg/dl. Additionally, the CGMS revealed frequent and prolonged asymptomatic hypoglycemia (glucose <60 mg/dl) in almost 70% of the children. Despite excellent HbA1c levels and target preprandial glucose levels, children often experience nocturnal hypoglycemia and postprandial hyperglycemia that are not evident with routine monitoring. These observations have important clinical implications, because recent evidence suggests that postprandial hyperglycemia plays a particularly important role in the development of vascular complications of diabetes. These data also illustrate the potential usefulness of monitoring postprandial as well as preprandial glucose levels in youth with type 1 diabetes. The sensor also detected many more hypoglycemic events during the day than were appreciated clinically. Repeated use of the CGMS may provide a means to optimize basal and bolus insulin replacement in patients with type 1 diabetes.
__________
Home vs. Hospital Testing:
Most home meters measure glucose in so-called “whole blood” (blood as it comes out of our body). Whole blood consists of a liquid, called plasma, and cells, mainly red cells. The percentage of red cells is called the hematocrit. The standard reference lab test measures glucose in plasma (about half to two thirds of the volume of blood). Home meters are calibrated to give results as though they are measuring glucose in plasma only (called “plasma-equivalent” results). That said, to some degree we’re already on two different playing fields. Second, laboratory tests eliminate virtually all variation, except for manufacturing variation, from their testing. What that means is that hospital standards are much more exacting than testing at home because in hospitals you have: trained technicians, a controlled environment for temperature and humidity, constant maintenance of the machine that performs the test, with checking and refining of the machine’s calibration several times a day, and a much larger sample of blood (5 ml) that’s analyzed for 60 seconds or more, and at much greater expense. Lab tests generally come within about plus/minus 4% of a perfect reading.
_______
SMBG vs. HbA1c:
Hemoglobin A1c outperforms Fasting Glucose for Risk Prediction:
Measurements of hemoglobin A1c (HbA1c) more accurately identify persons at risk for clinical outcomes than the commonly used measurement of fasting glucose, according to a study by researchers at the Johns Hopkins Bloomberg School of Public Health. HbA1c levels accurately predict future diabetes, and they better predict stroke, heart disease and all-cause mortality as well. The study appeared in the March 4, 2010, issue of New England Journal of Medicine. As a diagnostic, “HbA1c has significant advantages over fasting glucose,” said Elizabeth Selvin, PhD, MPH, the study’s lead author. The A1c test has low variability from day to day, levels are not as affected by stress and illness, it has greater stability and the patient is not required to fast before the test is performed. In the study, people with HbA1c levels between 5.0 to 5.5 percent were identified as being within “normal” range. The majority of the U.S. adult population is within this range. With each incremental HbA1c increase, the study found, the incidence of diabetes increased as well; those at a level of 6.5 percent or greater are considered diabetic, and those between 6.0 and 6.5 percent are considered at a “very high risk” (9 times greater than those at the “normal” range) for developing diabetes. The revised ADA guidelines classify people with HbA1c levels in the range of 5.7 to 6.4 percent as “at very high risk” for developing diabetes over 5 years. The range of 5.5 to 6 percent, according to the ADA guidelines, is the appropriate level to initiate preventive measures. The study measured HbA1c in blood samples from more than 11,000 people, black and white adults, who had no history
__
Limitations of monitoring glycemic control using only HbA1c:
HbA1c does not provide information about Glycemic Variability:
As an integrated measure of fasting, preprandial, and postprandial glucose levels, HbA1c may not completely represent the risks that patients with diabetes are exposed to on a daily basis. Although it provides a quantitative measure of mean glucose exposure over an extended period, HbA1c alone does not indicate the degree of glycemic variability (the frequency and magnitude of glucose excursions) that a patient may experience during a given day. The potential importance of glycemic variability is suggested by findings from the DCCT. Even at equivalent HbA1c levels, patients receiving intensive therapy (involving more frequent preprandial insulin injections) had a reduction in the risk of progression of retinopathy over time compared with patients receiving conventional treatment. The DCCT research group speculated that complications might be highly dependent on the extent of postprandial glycemic excursions and that conventionally treated patients were more likely to be exposed to greater glycemic excursions than those in the intensive treatment group.
_
The figure below proves that HbA1c misses glycemic excursions:
_
The figure below shows that HbA1c misses many lows of glucose:
_
HbA1C does not differentiate among Fasting, Preprandial, and Postprandial Glycemia:
Optimal diabetes management involves control of fasting, preprandial, and postprandial glucose levels. However, because HbA1c represents mean glycemic exposure over time, it cannot be used to identify whether a given patient’s abnormal glycemic levels are primarily due to high fasting plasma glucose levels or high postprandial plasma glucose (PPG) levels. Simply put, an elevated HbA1c measurement signals a need for a change in therapy, but it cannot indicate what type of change is necessary. In fact, the relative contributions of fasting plasma glucose and PPG to HbA1c vary according to HbA1c level, with PPG becoming increasingly important as HbA1c decreases toward target levels.
_
A considerable body of evidence points to the clinical importance of postprandial hyperglycemia, typically evaluated in clinical studies as postchallenge glucose levels in an oral glucose tolerance test. Although the terms postchallenge and postprandial are not synonymous because of the inherent differences between ingesting a pure glucose solution and eating a mixed meal, postchallenge hyperglycemia is often regarded as a surrogate for postprandial hyperglycemia. Even when HbA1c and fasting glucose levels are within the normal range, postchallenge hyperglycemia has been associated with a 2-fold increase in the risk of death from cardiovascular disease. In the Funagata Diabetes Study, impaired glucose tolerance (IGT), defined as a 2-hour postchallenge plasma glucose level between 140 and 198 mg/dL (7.8-11.0 mmol/L), as opposed to impaired fasting glucose (defined in that study as 110-125 mg/dL [6.1-7.0 mmol/L]) was shown to double the risk of death from cardiovascular disease.
_
The increased risk of macrovascular disease among patients with IGT but not impaired fasting glucose, combined with the greater contribution of postprandial hyperglycemia to overall glycemia when HbA1c levels are lower, may help to explain why even newly diagnosed patients have an increased cardiovascular risk. When IGT progresses to diabetes, the patient has been exposed to postprandial glycemic excursions for many years. In the Diabetes Intervention Study, elevated PPG levels after a normal breakfast were associated with significantly higher mortality during an 11-year follow-up of patients with newly diagnosed type 2 diabetes (P<.01). In support of these findings, prospective interventions that control PPG have been shown to improve endothelial function and reduce carotid atherosclerosis in patients with type 2 diabetes. Postprandial hyperglycemia was recently linked to microvascular complications as well. In a study of 151 Japanese patients, PPG levels correlated better than HbA1c measurements with the risk of retinopathy progression.
_
Inaccuracies in HbA1c Test Results:
More than 30 different HbA1c assays are currently available. Differences among these assays as well as variations between and within laboratories can affect HbA1c results. In 1996, the National Glycohemoglobin Standardization Program (NGSP) was established in the United States to certify assay methods as traceable to DCCT reference values. Certification from the NGSP requires an assay method’s reference interval to be within 5% of the normal HbA1c level of 4% to 6%, with variations limited to less than 3% within a laboratory and less than 5% between laboratories.6 The ADA now recommends that laboratories use only NGSP-certified assays. Some medical conditions may cause inaccurate HbA1c test results. Conditions or factors that shorten red blood cell life span, such as acute blood loss, hemolytic anemia, and some medications used for human immunodeficiency virus-positive patients, will yield falsely low HbA1c values regardless of assay method. Hemoglobin variants, hemoglobinopathies, conditions that result in increased erythrocyte turnover, and blood transfusions can increase or decrease HbA1c levels depending on the condition and the HbA1c assay method used. Iron deficiency anemia, hypertriglyceridemia, hyperbilirubinemia, uremia, and high doses of acetylsalicylic acid can produce falsely high HbA1c measurements. Dietary supplements and opiate or alcohol abuse can also affect HbA1c results. Vitamins C and E may lower test results by inhibiting glycation of hemoglobin, and vitamin C has also been reported to increase HbA1c values, depending on the assay used.
_
_
Why is hemoglobin A1c unreliable?
While this sounds good in theory, the reality is not so black and white. The main problem is that there is actually a wide variation in how long red blood cells survive in different people. A study shows that red blood cells live longer than average at normal blood sugars. Researchers found that the lifetime of hemoglobin cells of diabetics turned over in as few as 81 days, while they lived as long as 146 days in non-diabetics. This proves that the assumption that everyone’s red blood cells live for three months is false, and that hemoglobin A1c can’t be relied upon as a blood sugar marker. In a person with normal blood sugar, hemoglobin will be around for a lot longer, which means it will accumulate more sugar. This will drive up the A1c test result – but it doesn’t mean that person had too much sugar in their blood. It just means their hemoglobin lived longer and thus accumulated more sugar. The result is that people with normal blood sugar often test with unexpectedly high A1c levels. So people with completely normal fasting and post-meal blood sugars have A1c levels of >5.4%. In fact this is not abnormal, when we understand that people with normal blood sugar often have longer-lived red blood cells – which gives those cells time to accumulate more sugar. On the other hand, if someone is diabetic, their red blood cells live shorter lives than non-diabetics. This means diabetics and those with high blood sugar will test with falsely low A1c levels. And we already know that fasting blood glucose is the least sensitive marker for predicting future diabetes and heart disease. This is a serious problem, because fasting blood glucose and hemoglobin A1c are almost always the only tests doctors run to screen for diabetes and blood sugar issues. Another condition that affects hemoglobin A1c levels is anemia. People who are anemic have short-lived red blood cells, so like diabetics, they will test with falsely low A1c levels. In my practice, about 30-40% of my patients have some degree of anemia, so this is not an uncommon problem.
_________
Using SMBG to Complement HbA1c:
Self-monitoring of blood glucose provides a real-time measure of blood glucose levels and consequently represents a valuable adjunct to the periodic determination of HbA1c values. Accordingly, SMBG provides patients with instant feedback about the effects of food choices, exercise, stress, and medications on their glycemic levels. Although the optimal frequency and timing of SMBG depend on many variables including diabetes type, level of glycemic control, management strategy, and individual patient factors, SMBG allows clinicians to fine-tune therapy and thus more effectively manage their patients’ glucose levels. When used properly, most modern glucose meters demonstrate a high degree of clinical accuracy compared with laboratory instruments, and average SMBG readings generally correlate well with HbA1c values. Without regular self-testing to provide day-to-day insights, an A1c result can be misleading. Because it gives a long-term view, a person with frequent highs and lows could have an average A1c that looks quite healthy. The only way to get a complete picture of your blood sugar control is by reviewing your day-to-day self-checks along with your regular A1c tests, and working closely with your healthcare team to interpret the results.
_
SMBG can identify Hypoglycemic Episodes:
Fear of hypoglycemia often leads to a less intensive management approach by clinicians and patients, resulting in suboptimal glycemic control. Hypoglycemia is a concern to patients with type 1 diabetes and those with type 2 diabetes managed with insulin or oral agents. In a study using CGM in elderly patients with type 2 diabetes, no patients reported symptoms of hypoglycemia, yet 80% had glucose values lower than 50 mg/dL (2.8 mmol/L) on at least 1 occasion. Self-monitoring of blood glucose provides a means of identifying daily hypoglycemic events, allowing immediate treatment and modification of therapeutic regimens to allow tighter glycemic control while minimizing future hypoglycemic risk.
_
SMBG detects Glycemic Excursions:
Continuous glucose monitoring studies show that daily blood glucose values range widely in both hypoglycemic and hyperglycemic ranges. Although real-time CGM devices that measure glucose concentrations in interstitial fluid have recently entered the market, they are indicated as adjuncts to standard SMBG, and all therapy adjustments should be based on measurements obtained from a blood glucose meter. Both SMBG and CGM have the ability to identify glycemic excursions. In a study of 600 insulin-treated patients, regular SMBG—an average of 3 times per day for 3 months—revealed a wide range of daily glucose values. When mean minimal and maximal values were determined, blood glucose ranged from 40 to 449 mg/dL in patients with type 1 diabetes and 63 to 382 mg/dL in patients with type 2. The wide variation in glucose levels, particularly the dangerously low values observed in the patients with diabetes, would not have been detected by HbA1c alone. For patients treated with insulin, an occasional SMBG reading in the middle of the night can help detect nocturnal hypoglycemia. It is important to recognize that postprandial glucose excursions may still be present in patients who have achieved their HbA1c target. A study of patients with type 2 diabetes who performed SMBG before and 2 hours after meals showed that many with HbA1c levels lower than 7.0% had postprandial glucose levels in excess of 160 mg/dL and glucose excursions of more than 40 mg/dL. In a cross-sectional analysis of the Third National Health and Nutrition Examination Survey cohort, postchallenge hyperglycemia was identified in 39% of patients with type 2 diabetes who were not using insulin and had an HbA1c level lower than 7.0%. Diabetes management software, with data uploaded from blood glucose meters, can be used to calculate the SDs of blood glucose values. Analyzing SDs is perhaps the simplest method to identify the degree of glycemic fluctuations.
_
Management of glycemia in diabetes is crucially important to the prevention of both acute and long-term complications. The two fundamental approaches to assessment, SMBG and HbA1c, provide fundamentally different but complementary information. Regular SMBG is to be encouraged, particularly in patients using insulin, although the frequency can vary widely dependent particularly on the glycemic stability of the patient and the need to follow treatment changes. HbA1c, the criterion standard measure of chronic glycemic control and complication risk, should be measured every 3 to 6 months to assess the success of the treatment regimen. Changes in both approaches are ongoing but with proper control of
glycemia, diabetes can be successfully managed.
________
What blood sugar marker is most reliable? FPG, PPG or A1c?
Testing accurately for blood sugar is like putting pieces of a puzzle together. Fasting blood glucose, A1c and post-meal blood sugar are all pieces of the puzzle. But post-meal blood glucose testing is by far the most reliable and accurate way to determine what’s happening with blood sugar, and the most sensitive way of predicting future diabetic complications and heart disease.
_
Why post-meal blood sugar is a superior marker than fasting BG and A1c:
Fasting blood sugar:
According to continuous glucose monitoring studies of healthy people, a normal fasting blood sugar is 83 mg/dL or less. Many normal people have fasting blood sugar in the mid-to-high 70s. While most doctors will tell you that anything under 100 mg/dL is normal, it may not be. In a study, people with FBG levels above 95 had more than 3x the risk of developing future diabetes than people with FBG levels below 90. This study showed progressively increasing risk of heart disease in men with FBG levels above 85 mg/dL, as compared to those with FBG levels of 81 mg/dL or lower. What’s even more important to understand about FBG is that it’s the least sensitive marker for predicting future diabetes and heart disease. Several studies show that a “normal” FBG level in the mid-90s predicts diabetes diagnosed a decade later. Far more important than a single fasting blood glucose reading is the number of hours a day our blood sugar spends elevated over the level known to cause complications, which is roughly 140 mg/dl (7.7 mmol/L). One caveat here is that very low-carb diets will produce elevated fasting blood glucose levels. Why? Because low-carb diets induce insulin resistance. Restricting carbohydrates produces a natural drop in insulin levels, which in turn activates hormone sensitive lipase. Fat tissue is then broken down, and non-esterified fatty acids are released into the bloodstream. These FFA are taken up by the muscles, which use them as fuel. And since the muscle’s needs for fuel has been met, it decreases sensitivity to insulin. So, if you eat a low-carb diet and have borderline high FBG (i.e. 90-105), it may not be cause for concern. Your post-meal blood sugars and A1c levels are more important.
_
Hemoglobin A1c:
In spite of what the American Diabetes Association (ADA) tells us, a truly normal A1c is between 4.6% and 5.3%. But while A1c is a good way to measure blood sugar in large population studies, it’s not as accurate for individuals. An A1c of 5.1% maps to an average blood sugar of about 100 mg/dL. But some people’s A1c results are always a little higher than their FBG and OGTT numbers would predict, and other people’s are always a little lower. This is probably due to the fact that several factors can influence red blood cells (vide supra). A number of studies show that A1c levels below the diabetic range are associated with cardiovascular disease. A study showed that A1c levels lower than 5% had the lowest rates of cardiovascular disease (CVD) and that a 1% increase (to 6%) significantly increased CVD risk. Another study showed an even tighter correlation between A1c and CVD, indicating a linear increase in CVD as A1c rose above 4.6% – a level that corresponds to a fasting blood glucose of just 86 mg/dL. Also earlier study showed that the risk of heart disease in people without diabetes doubles for every percentage point increase above 4.6%. Studies also consistently show that A1c levels considered “normal” by the ADA fail to predict future diabetes. This study found that using the ADA criteria of an A1c of 6% as normal missed 70% of individuals with diabetes, 71-84% with dysglycemia, and 82-94% with pre-diabetes. How’s that for accuracy? What we’ve learned so far, then, is that the fasting blood glucose and A1c levels recommended by the ADA are not reliable cut-offs for predicting or preventing future diabetes and heart disease. This is problematic, to say the least, because the A1c and FBG are the only glucose tests the vast majority of people get from their doctors.
_
OGTT / post-meal blood sugars:
The more realistic and convenient way to achieve PPG rather than conventional OGTT is simply using a glucometer to test your blood sugar one and two hours after you eat a meal. This is called post-prandial (post-meal) blood sugar testing. The ADA considers OGTT of between 140 – 199 two hours after the challenge to be pre-diabetic, and levels above 200 to be diabetic. But once again, continuous glucose monitoring studies suggest that the ADA levels are far too high. Most people’s blood sugar drops below 120 mg/dL two hours after a meal, and many healthy people drop below 100 mg/dL or return to baseline. This study showed that even after a high-carb meal, normal people’s blood sugar rises to about 125 mg/dL for a brief period, with the peak blood sugar being measured at 45 minutes after eating, and then drops back under 100 mg/dL by the two hour mark. Another continuous glucose monitoring study confirmed these results. Sensor glucose concentrations were between 71 – 120 mg/dL for 91% of the day. Sensor values were less than or equal to 60 or 140 mg/dL for only 0.2% and 0.4% of the day, respectively. On the other hand, some studies suggest that even healthy people with no known blood sugar problems can experience post-meal spikes above 140 mg/dL at one hour. If post-meal blood sugars do rise above 140 mg/dL and stay there for a significant period of time, the consequences are severe. Prolonged exposure to blood sugars above 140 mg/dL causes irreversible beta cell loss (the beta cells produce insulin) and nerve damage. One in two “pre-diabetics” gets retinopathy, a serious diabetic complication. Cancer rates increase as post-meal blood sugars rise above 160 mg/dL. This study showed stroke risk increased by 25% for every 18 mg/dL rise in post-meal blood sugars. Finally, 1-hour OGTT readings above 155 mg/dL correlate strongly with increased CVD risk.
_
Does targeting postprandial hyperglycemia improve overall glycemic control?
In a study of patients with type 2 diabetes with secondary failure of sulfonylurea therapy, Feinglos et al. showed that improvement of postprandial hyperglycemia, using insulin lispro (Humalog) at mealtime in combination with a sulfonylurea, not only reduced 2-h postprandial glucose excursions, but also reduced both fasting glucose and A1C levels from 9.0% to 7.1% (P < 0.0001). Subjects in the lispro group also benefited from significantly decreased total cholesterol levels and improved HDL cholesterol concentrations. Improvements in A1C levels were also reported in a study by Bastyr et al., which showed that therapy focused on lowering postprandial glucose versus fasting glucose may be better for lowering glycated hemoglobin levels. Further, in a study of patients with gestational diabetes, De Veciana et al. demonstrated that targeting treatment to 1-h postprandial glucose levels rather than fasting glucose reduces glycated hemoglobin levels and improves neonatal outcomes. Regardless of whether postprandial glucose is a better predictor of A1C than fasting/preprandial glucose, most researchers agree that the best predictor of A1C is mean blood glucose, which is a composite of both fasting/preprandial and postprandial glucose. Therefore, it is reasonable to conclude that achieving near-normal postprandial glucose levels is essential to achieving overall glycemic control.
_
Is postprandial glucose control an independent contributor to diabetes outcomes?
Numerous epidemiological studies have shown elevated postprandial/post-challenge glucose to be independent and significant risk factors for macrovascular complications and increased mortality risk. The Honolulu Heart Study found a strong correlation between postchallenge glucose levels and the incidence of cardiovascular mortality. The Diabetes Intervention Study, which followed newly diagnosed patients with type 2 diabetes, found moderate postprandial hyperglycemia to be more indicative of artherosclerosis than was fasting glucose, and found postprandial but not fasting glucose to be an independent risk factor for cardiovascular mortality. The DECODE Study, which followed more than 25,000 subjects for a mean period of 7.3 years, showed that increased mortality risk was much more closely associated with 2-h post–glucose load plasma levels than with fasting plasma glucose. Similar to these findings, de Vegt et al. found that the degree of risk conferred by the 2-h postprandial glucose concentration was nearly twice that conferred by A1C level. Further, recent studies have demonstrated that even moderate postprandial hyperglycemia (148–199 mg/dl) is not only more indicative of artherosclerosis than is fasting glucose, but also may have direct adverse effects on the endothelium.
_
What is 4 point SMBG?
Fasting, before lunch, before dinner and bedtime SMBG is 4 point SMBG.
What is 7 point SMBG?
Fasting (before breakfast), after breakfast, before & after lunch, before and after dinner, and bedtime is 7 point SMBG.
7 point SMBG takes into consideration post-meal blood glucose and since post-meal blood glucose correlates well with vascular diabetic complications, 7 point SMBG not only improves glycemic control better than 4 point SMBG, but also greater reduction in diabetic vascular complications.
_
So in a nutshell, I would like to have all three: fasting blood glucose, A1c and post-meal glucose. But if I had to choose one, it would definitely be post-meal glucose.
______
SMBG vs. CGM:
Glucose monitoring is an important component of type 1 diabetes treatment. Careful consideration of the advantages and disadvantages of SMBG and CGM can help providers identify the approach that best fits with patient’s lifestyle and treatment goals.
_
The advantages of SMBG are that it is relatively inexpensive, easy to train patient to complete, provides an accurate measure of capillary glucose concentrations, and available glucose meters can offer features including memory, downloading software, no coding strips, and small blood sample requirements. Disadvantages are the impact of user error on test accuracy, the need for multiple finger-stick blood samples each day, and the limited data available (e.g., SMBG provides a single snap shot of glucose concentrations, not trending data). Efficacy studies support the use of SMBG in diabetes management and suggest that it likely to remain the most common form of glucose monitoring practiced by patient today.
_
The main advantage of CGM is that it can provide a near-continuous read-out of interstitial glucose concentration, which adequately reflects blood glucose concentration and can help to identify trends and patterns in glucose control with only a single needle stick to place the sensor. In addition, in the case of real-time CGM, monitors can be programmed to alarm for either high or low glucose values, thus allowing parents and youth to treat for these abnormal values and potentially reducing fear related to hypo or hyperglycemia. Disadvantages include the cost of CGM, lack of universal insurance coverage for this technology, limited FDA approval for CGM devices, and cosmetic (e.g., additional infusion site/monitor) and psychological concerns (e.g., frustration, helplessness if glucose control is not perceived as adequate). There is also limited evidence supporting use of CGM with type 1 diabetes as a means of improving long-term glycemic control. One barrier to CGM use appears to be patient’s willingness to accept and use this technology for diabetes management, a problem which likely will need to be addressed before it is possible to adequately examine for the efficacy of CGM use on glycemic control.
_______
Cost effectiveness of SMBG:
The worldwide epidemic of diabetes is producing unacceptable human suffering. This in turn produces economic losses from direct costs and lost production. Therapeutic endeavors must be directed to attenuation of this effect. A cure is not on the horizon; the best tools available to doctors are those that reduce risks and delay or prevent disease progression. In type 2 patients, therapeutic approaches must be progressive, reflecting the gradual loss of b-cell function. SMBG is the singular, immediate, accurate measure available to the patient allowing therapy adjustment. With appropriate education, the patient and healthcare team can adjust therapy to approach glycemic goals. The value of testing, not simply the cost, must be appreciated by patients, doctors, and the healthcare system. Prevention or delay of complications and improvement in daily symptoms and quality of life are priceless. The cost of home blood glucose monitoring is substantial due to the cost of the test strips. In 2006, the consumer cost of each glucose strip ranged from about $0.35 to $1.00. Manufacturers often provide meters at no cost to induce use of the profitable test strips. Type 1 diabetics may test as often as 4 to 10 times a day due to the dynamics of insulin adjustment, whereas type 2 typically test less frequently, especially when insulin is not part of treatment. As described earlier, frequent SMBG results in a statistically and clinically significant improvement in A1c which can range up to reductions of 2.5-4.0%. To determine whether this reduction results in economic benefits, Neeser and colleagues performed a cost-effectiveness analysis of SMBG using a Markov state model of diabetes to assess the clinical impact and related costs when SMBG is provided to patients not on insulin therapy. They assumed an improvement in A1c of 0.39%. The results of the analysis showed a slight increase in life expectancy and a reduced cost of complications, 70% of which was attributable to reductions in microvascular events. The cost per life-year gained was approximately $39,650, which is considered to be an acceptable cost-effective intervention from a health insurance perspective.
_
An analysis by Simon and colleagues assessed the cost-effectiveness of SMBG in type 2 subjects who participated in the DiGEM study. The average annual cost of intervention was £89 (€113; $179) for standardized usual care, £181 for less intensive self-monitoring, and £173 for more intensive self-monitoring, showing an additional cost per patient of £92 (95% confidence interval £80 to £103) in the less intensive group and £84 (£73 to £96) in the more intensive group. Given that there were no significant differences in clinical outcomes (change in HbA1c), the authors concluded that SMBG is unlikely to be cost-effective when additional to standardized usual care.
_
A 2008 BMJ study found that self monitoring of blood glucose with or without additional training in incorporating the results into self care was associated with higher costs and lower quality of life in patients with non-insulin treated type 2 diabetes. In light of this, and no clinically significant differences in other outcomes, self monitoring of blood glucose is unlikely to be cost effective in addition to standardised usual care. Canadian study in 2010 found that routine use of SMBG (one or more test strips per day) in patients with non–insulin-treated type 2 diabetes is associated with an incremental cost of $113,643 per QALY gained, relative to no SMBG. A reduction in the price of blood glucose test strips would improve the cost-effectiveness of SMBG. For patients with insulin-treated type 2 diabetes, SMBG testing frequencies beyond 21 test strips per week require unrealistically large A1C estimates of effect to achieve favourable incremental cost per QALY estimates.
_
Self-monitoring of Blood Glucose in Type 2 Diabetes: Cost-effectiveness in the United States: 2008:
Compared with no SMBG, quality adjusted life expectancy increased with SMBG frequency. Increases were 0.103 and 0.327 quality-adjusted life-years (QALYs) for SMBG at 1 and 3 times per day, respectively. Corresponding incremental cost-effective ratios (ICERs) were $7856 and $6601 per QALY gained. Results indicate that SMBG at both 1 and 3 times per day in this cohort of patients with T2DM taking OADs would represent good value for money in the United States, with ICERs being most sensitive to the time horizon. Longer time horizons generally led to greater SMBG cost-effectiveness. The ICER for SMBG 3 times per day was $518 per QALY over a 10-year time horizon, indicating very good value.
__
Self-monitoring of blood glucose (SMBG) in patients with type 2 diabetes on oral anti-diabetes drugs: cost-effectiveness in France, Germany, Italy, and Spain: 2010:
With cost assumptions reflecting current reimbursement levels in France, Germany, Italy, and Spain, SMBG was found to be cost-effective across a 40-year time horizon, with all base case ICERs <16,000/QALY. This study adds to the literature on the country-specific, long-term value of SMBG for type 2 diabetes patients treated with OADs. Under current model assumptions, variations in cost-effectiveness results stemmed primarily from payer reimbursement practices for SMBG within each country
________
Counterfeit SMBG: Bogus Diabetes Test Strips Traced to Chinese Distributor:
Batches of counterfeit test strips for some meters have been identified, which have been shown to produce inaccurate results. A global hunt started by Johnson & Johnson has tracked to China some counterfeit versions of the test strips used by 10 million Americans to measure their blood sugar levels. Potentially dangerous copies of the OneTouch Test Strip sold by the company’s LifeScan unit surfaced in American and Canadian pharmacies last year. J.& J., one of the world’s largest makers of consumer health products, learned of the bogus test strips from patients’ complaints. Tipped off by the company, the Food and Drug Administration issued a consumer alert without disclosing the link to China. No injuries were reported, but inaccurate test readings may lead a person with diabetes to inject the wrong amount of insulin, causing harm or death. The investigation found that a distributor in China was the source of about a million fake test strips that have turned up in at least 35 states and eight countries. The trail, initiated by calls to a LifeScan hot line, led detectives to 700 pharmacies where the products were sold, then to eight American wholesalers, then to two importers. One importer was in the United States and was found in a Las Vegas hotel room. Records seized from the importers showed that the counterfeit strips were bought from Henry Fu and his company, Halson Pharmaceuticals, which is based in Shanghai. Mr. Fu was arrested by Chinese authorities and remains in prison in China.
_________
Research and newer technology in SMBG:
_____
Dual-Analyte Detection:
Various clinical situations require the simultaneous monitoring of glucose and of other clinically important analytes, such as lactate or insulin. Such coupling of two sensing elements requires both analytes to be monitored independently at different levels and without cross talk. Wang and Zhang developed a needle-type sensor for the simultaneous continuous monitoring of glucose and insulin. The integrated microsensor consisted of dual electrocatalytic (RuOx) and biocatalytic (GOx) modified carbon electrodes inserted into a needle and responded independently to nanomolar and millimolar concentrations of insulin and glucose, respectively.
_
__________
Less Invasive Technology to Monitor Blood Glucose Levels in Patients with Diabetes:
The current standard for self monitoring blood glucose levels is through a lancet device that pricks the finger or forearm. The droplet of blood is placed on an optical disposable strip that is then placed into a glucose meter, or glucometer, giving a reading of blood glucose in milligrams per deciliter. High or low glucose levels can then be corrected using insulin or glucose depending on whether the patient is hyper- or hypoglycemic, respectively. This gold standard of diabetes self testing has been improved upon in recent years and many monitors require less blood and allow more sites than just the fingertip and forearm to be tested. There have also been attempts to make the standard glucometers less noticeable and easier to carry. One example is a cellular phone which can double as a glucose monitor, thus making it something that could be discretely taken anywhere. However, the invasiveness of the procedure persists as there is still a requirement to puncture skin to produce a blood droplet. The gold standard for self monitoring currently involves a small device measuring the blood glucose level in a droplet of blood taken from the fingertip or forearm of a patient. This lancet approach is done on average 3–4 times a day and can be uncomfortable and inconvenient. It is known that monitoring blood glucose more frequently leads to better control and maintaining overall health. However, invasiveness of the finger stick method has caused patients to ignore the need to monitor their blood glucose levels and leave themselves at risk for future complications.
_
Non-invasive glucose monitoring:
Non-invasive glucose monitoring techniques can be grouped as subcutaneous, dermal, epidermal and combined dermal and epidermal glucose measurements. Matrices other than blood under investigation include interstitial fluid, ocular fluids and sweat. Test sites being explored include finger tips, cuticle, finger web, forearm and ear lobe. Subcutaneous measurements include microdialysis, wick extraction, and implanted electrochemical or competitive fluorescence sensors. Microdialysis is also an investigational dermal and epidermal glucose measurement technique. Epidermal measurements can be obtained via infrared spectroscopy, as well. Combined dermal and epidermal fluid glucose measurements include extraction fluid techniques (iontophoresis, skin suction and suction effusion techniques) and optical techniques. The optical techniques include near infrared spectroscopy, infrared spectroscopy, raman spectroscopy, photoacoustic spectroscopy, scatter and polarization changes
_
The Biosensor for Blood Glucose Concentration:
There are many methods available for glucose determination, with the majority based on enzymatic reactions. In order of accurateness, the most common are directly measuring glucose in blood (invasive), measuring glucose in the interstitial fluid (minimally invasive), and estimating glucose using other corporal fluids like oral mucosa, aqueous humor of the eye, sweat, urine, saliva, tears, and so forth (noninvasive). The technologies employed could be polarimetry, electromagnetism, ultrasound, Raman spectroscopy, reverse iontophoresis, impedance spectroscopy, and so forth. Why noninvasive measurement is important is evident; the pain caused by finger pricking or invasive sensors is the main reason. It is very common that minimally invasive glucose sensors cause irritation, infections, or even bruising. These sensors have to be renewed every 5 or 6 days, and, at worst, may require that the sensor be recalibrated at frequent intervals with a fingerstick meter. Noninvasive monitoring avoids all these disadvantages but is not as accurate as the invasive technologies. The ideal glucose sensor should be selective for glucose with a fast, predictable response to changing glucose concentrations. It should depend on a reversible and reproducible signal to provide results, and sensor fabrication must be reproducible and cheap on a large scale. It should have a long operational lifetime under physiological conditions, but most of all must be acceptable to the patient. Therefore, it should be noninvasive, should not require user calibration, and would ideally provide real-time continuous information regarding glucose.
_
Current continuous glucose monitoring systems have the advantage of direct insertion of electrochemical sensors into the IF space rather than transporting the sampled fluid outside the body to detect glucose concentrations. Software programs have been designed to accommodate the lag in IF glucose readings. Despite the advances in the making of sensors with new and improved designs and materials, sensor insertion causes trauma to the insertion site. It can disrupt the tissue structure, provoking an inflammatory reaction that can consume glucose followed by a repair process. The interaction of the sensor with the traumatized microenvironment warrants the need for a waiting period for the sensor signal to stabilize, and that period varies depending on the sensor type.
_
A variety of noninvasive blood glucose monitoring techniques are currently under evaluation as seen in the table below. However, none of them are commercially available at this time. Noninvasive methods will permit real-time bedside glucose monitoring without the requirement of an indwelling intravenous catheter with a glucose sensor located on its tip. The availability of a device for rapidly assessing glucose concentration without skin puncture by retinal or corneal glucose measurement or skin transillumination will revolutionize monitoring of glucose at home and in the hospital. Products under development include: Fovioptics retinal glucose analyzer, Inlight Solutions, NIR glucose sensor, NIR Diagnostics, NIR glucose sensor, Sinsys Medical GTS, Sontra Ultrasonic Symphony Diabetes management system, Solianis Monitoring AG etc.
__
What is intriguing about these initiatives is that, in their final form, they may create a flow of useful diagnostic data reported to clinical laboratories in real time. This would create the opportunity for pathologists and lab scientists to consult with the patients’ physicians, while archiving this test result data in the laboratory information system (LIS). These glucose monitoring methods would also ensure that a complete longitudinal record of patient tests results is available to all the physicians practicing in an accountable care organization (ACO), medical home, or hospital.
________
Overview of Non-Invasive Optical Glucose Monitoring Techniques:
Non-invasive optical measurement of glucose is performed by focusing a beam of light onto the body. The light is modified by the tissue after transmission through the target area. An optical signature or fingerprint of the tissue content is produced by the diffuse light that escapes the tissue it has penetrated. The absorbance of light by the skin is due to its chemical components (i.e., water, hemoglobin, melanin, fat and glucose). The transmission of light at each wavelength is a function of thickness, color and structure of the skin, bone, blood and other material through which the light passes. The glucose concentration can be determined by analyzing the optical signal changes in wavelength, polarization or intensity of light. The sample volume measured by these methods depends on the measurement site. The correlation with blood glucose is based on the percent of fluid sample that is interstitial, intracellular or capillary blood. Drs. Roe and Smoller have devised the following example. The fluid viewed through the limb is 63% intracellular and 37% extracellular, of which 27% is interstitial and 10% plasma. A blood glucose value of 100mg/dl is equivalent to a tissue sample glucose average of 38mg/dl of which 26% is due to blood, 58% is due to interstitial fluid and 16% is due to intracellular fluid. What the tissue sample glucose means clinically in respect, to therapy is still under investigation. Not only is the optical measurement dependent on concentration changes in all body compartments measured, but changes in the ratio of tissue fluids (as altered by activity level, diet or hormone fluctuations) and this, in turn, effects the glucose measurement. Problems also occur due to changes in the tissue after the original calibration and the lack of transferability of calibration from one part of the body to another. Tissue changes include: body fluid source of the blood supply for the body fluid being measured, medications that affect the ratio of tissue fluids, day-to-day changes in the vasculature, the aging process, diseases and the person‘s metabolic activity.
_
| Dermal and Epidermal Fluid Glucose Measurement Techniques | |
| Technique | Definition |
| Near Infrared Spectroscopy (NIR) | Absorption or emission data in the 0.7 to 2.5 µm region of the spectrum are compared to known data for glucose. |
| Raman Spectroscopy | Laser light is used to induce emission from transitions near the level excited. |
| Photoacoustic Spectroscopy | Laser excitation of fluids is used to generate an acoustic response and a spectrum as the laser is tuned. |
| Scatter Changes | The scattering of light can be used to indicate a change in the material being examined. |
| Polarization Changes | The presence of glucose in a fluid is known to cause a polarization preference in the light transmitted. |
| Mid-Infrared Spectroscopy | Absorption or emission data in the 2.5 µm – 25 µm region are examined and used to quantify glucose in a fluid. |
The market introduction of noninvasive blood glucose measurement by spectroscopic measurement methods, in the field of near-infrared (NIR), by extracorporal measuring devices, failed so far because at this time, the devices measure tissue sugar in body tissues and not the blood sugar in blood fluid. To determine blood glucose, the measuring beam of infrared light, for example, has to penetrate the tissue for measurement of blood glucose.
_
Optical Coherence Tomography:
Optical coherence tomography (OCT) technology is similar to that of pulsatile microcirculation, but it uses infrared light and penetrates deeper into biological tissues. Optical coherence tomography monitors a cylindrical layer of skin in 20μm increments from the skin surface to the subcutaneous tissue. The sensor, trademarked as the GlucoLight Sentris 100 Optical Continuous Glucose Monitor, detects changes in the protein confirmation of collagen and myosin that occur secondary to glucose concentration changes. In a feasibility trial, 33 patients had baseline blood glucose levels sampled via OCT and via capillary derived glucose with subsequent checks every 10–15 min for 2 hours after a 50-g carbohydrate load. The range of blood glucose detected by OCT was 98–442mg/dL. Eighty-three percent of results were within zones A and B of Clark error grid analysis with <1% of values in the clinically unacceptable zones of C and D. Similarly, 83% of the readings were within 20% of reference values obtained with capillary testing. Overall the results of the clinical trial showed that the GlucoLight was safe and effective, and that its reported glucose readings correlate with capillary blood glucose results. However, the trial did not demonstrate the new technology’s ability to accurately monitor blood glucose in the hypoglycemic range and no readings ≤75mg/dL were recorded.
_
At Israel’s Bar-Ilan University, a research team led by Zeev Zallevsky, Ph.D., has developed a non-invasive glucose measuring device that is worn like a wristwatch. This device consists of a laser that generates a wavefront of light to illuminate a patch of skin on the wrist near an artery, and a camera that measures changes over time in the light backscattered off the skin. Unlike other chemicals present in the blood, glucose exhibits a “Faraday effect,”. In the presence of an external magnetic field generated by the attached magnet, the glucose molecule alters the polarization of the wavefront and thus influences the resulting speckle patterns. These changing patterns provide a direct measurement of the glucose concentration.
_
Pulsatile Microcirculation:
Measuring blood glucose using an optical signal rather than blood from the fingertip has recently been developed. In a 15 patient study, each patient placed their finger into a slot of the newly developed meter, the TangTest (TG), and waited 30 sec for results. The meter works by using a weak light source shone onto the tested finger. Variations in intensity of the transmitted light are measured and algorithms used to quantify the amount of glucose present. The times measured were fasting, 20 min, and 40 min after a meal. The results of the study showed a linear relationship between glucose measurements using the TG system and a standard glucometer, with a correlation coefficient of r=0.81. Correcting for finger position and pulsatile components of the TG signals, 100% of results fell within zones A and B in a Clark error grid. There were many factors that might potentially limit this technology’s commercial application. Since blood flow must be unimpeded for testing, the ambient temperature must be within a defined range. As diabetics often have circulatory problems, the test results for this technology might be affected. In addition, the body must be relaxed physically and psychologically when testing; therefore tested patients had to be warm and relaxed for 20 min before performing the study. This is especially challenging in real life when a patient is hypoglycemic and a test result is needed as soon as possible. To obtain optimal results the test finger also has to be perfectly positioned in the meter; an odd angle or improper finger position can affect the transmission of light yielding inaccurate results. The TG meter also takes 30 sec to give a test result, in contrast to 5 seconds for standard glucometers.
_
Pulse glucometry:
A new approach for noninvasive blood glucose measurement using instantaneous differential near-infrared spectrophotometry:
Authors describe a new optical method for noninvasive blood glucose (BGL) measurement. Optical methods are confounded by basal optical properties of tissues, especially water and other biochemical species, and by the very small glucose signal. They address these problems by using fast spectrophotometric analysis in a finger, deriving 100 transmittance spectra per second, to resolve optical spectra (900to1700nm) of blood volume pulsations throughout the cardiac cycle. Difference spectra are calculated from the pulsatile signals, thereby eliminating the effects of bone, other tissues, and nonpulsatile blood. A partial least squares (PLS) model is used with the measured spectral data to predict BGL levels. Using glucose tolerance tests in 27 healthy volunteers, periodic optical measurements were made simultaneously with collection of blood samples for in vitro glucose analysis. Altogether, 603 paired data sets were obtained in all subjects and two-thirds of the data or of the subjects randomly selected were used for the PLS calibration model and the rest for the prediction. Bland-Altman and error-grid analyses of the predicted and measured BGL levels indicated clinically acceptable accuracy. Authors conclude that the new method, named pulse glucometry, has adequate performance for safe, noninvasive estimation of BGL.
_
Vital signs including blood glucose monitoring from arterial pulse:
UFIT, which uses a noninvasive, Web-enabled device that straps around a patient’s wrist, responds to the need for an easy-to-use self-monitoring system that reliably and simultaneously captures key data on heart and blood, including heart rate, blood pressure, blood oxygen and blood glucose. The system is intended to optimize the management of chronic diseases such as high blood pressure, heart disease and diabetes. The study’s findings were presented at the Institute of Electrical and Electronics Engineers (IEEE) International Workshop on Medical Measurements and Applications (MeMeA). This Biosign-sponsored study assumed that the arterial pulse, a rich source of clinically relevant information (e.g., rate, rhythm, pattern, pressure and oxygen), could also provide information on blood glucose. The study gathered glucose measurements from 120 participants with blood glucose levels ranging between 3.5 and 27.4 mmol/L. The results show a tight statistical correlation (0.998, Pearson substantial equivalence) between UFIT and laboratory analysis of blood glucose, with a low (1.63 percent) average of the mean percent difference between the UFIT measurements and the laboratory analysis. The correlation was obtained post-hoc by comparing a feature extracted from the radial artery pulse with laboratory blood glucose data. The methodology resembles that used to correlate HbA1C with the direct measurements of glucose in drawn blood.
_
Pulse gluco-oxymeter: OrSense’s NBM-200G:
This new, noninvasive continuous blood glucose monitoring system, which has already been approved in Europe, measures oxygen saturation, hemoglobin, and blood glucose with very high sensitivity. NBM-200G utilizes occlusion spectroscopy technology that correlates blood glucose levels with light-absorption and scattering measurements. The device is “operated by placing a ring-shaped probe around the patient’s finger, which applies a gentle pressure to the finger, similar to that applied during noninvasive blood-pressure measurement, and temporarily occludes the blood flow. During the occlusion, optical elements in the sensor perform a sensitive measurement of the light transmitted through the finger. In a recent trial of the NBM-200G, 130,000 glucose-paired readings were taken from 450 patients to determine the accuracy of the device compared to invasive products. There was a strong correlation between measurements derived from the NBM-200G and those from invasive measurements. This method is painless as well as accurate in comparison to invasive devices. Therefore, it is likely to improve compliance in patients who tend to avoid fingersticks and are unable to control their diabetes with invasive products. According to OrSense, the NBM-200G is Conformité Européenne (CE) approved for noninvasive continuous monitoring in patients with demanding need for glycemic control, such as those with brittle diabetes, nocturnal hypoglycemia, and gestational diabetes.
_
Azurite: Attempting to develop a Noninvasive Continuous Glucose Monitor using electrical properties of glucose:
Azurite’s approach is apparently quite original. Andrews and Zebrowski intend to measure blood glucose directly through an electromagnetic (EM) sensing system. Current continuous glucose monitors are invasive and rely on the measurement of some secondary characteristic. For example, Google’s contact lens, which is still in the research stage and not yet available on the market, measures the glucose content of tears, not blood. Continuous monitors currently on the market are invasive, requiring a device that attaches to the body with adhesive and a needle that must be replaced every several days. These monitors measure base blood glucose figures on glucose values in interstitial fluid, the liquid that surrounds our cells and tissues. Azurite’s idea is based on the fact that an electromagnetic signal, depending on its wavelength, can bounce off a surface and return to its source with a particular pattern reflective of the surface it encountered. Glucose molecules, like any material, reflect a unique electromagnetic signal based on their inherent electrical properties. So Azurite hopes to bounce an electromagnetic signal off the glucose in your blood, which would then return to a device carrying information about how much glucose it encountered along the journey. Various research groups have successfully ascertained blood glucose levels by observing the electrical properties of glucose in the blood. In an article published in 2011, researchers at the University of Mississippi demonstrated that a microstrip patch antenna could be used to determine the glucose concentration within a sample of blood by measuring its electrical properties. Drawing from this research and the work of other research groups that are examining at the electrical properties of glucose, Azurite has modeled a novel approach that they hope will lead to a device that uses EM technology to measure those electrical properties remotely. Azurite is determined to move beyond the theoretical and make a direct impact on the lives of people with diabetes. Researchers are hopeful that this technology will lead to a product that combines the rich data of continuous sensing and the convenience and ease of a noninvasive meter.
___
Iontophoresis Based Monitoring: GlucoWatch:
The GlucoWatch automatic glucose biographer from Cygnus (San Francisco, CA) has been introduced as a means of measuring blood glucose based on iontophoresis. This technology uses electrical polarity to cross the skin into deeper tissues of the body and is frequently used for drug delivery. In the context of blood glucose monitoring, interstitial fluid is brought to the skin surface and glucose levels are measured with an electrochemical enzymatic sensor worn on the skin. The wearable GlucoWatch device (available from Animas Technologies Inc.) contains both the extraction and the sensing functions along with the operating and data-storage circuitry. It provides up to three glucose readings per hour for up to 12 h (i.e., 36 readings within a 12 h period). The system has been shown to be capable of measuring the electroosmotically extracted glucose with a clinically acceptable level of accuracy. An alarm capability is included to alert the individual of very low or high glucose levels. However, the unit requires a long warm up and calibration against fingerstick blood measurement and is subject to difficulties due to skin rash with irritation under the device, long warm up times, sweating, or change in the skin temperature. A similar device has been developed in Korea called the RIGMD. It uses the same technology as that of the GlucoWatch but the enzymatic sensor measures glucose every 5 min instead of every 10 min.
_
The GlucoWatch didn’t quite live up to doctors’ and consumers’ expectations. Promises of a new, revolutionary way to continuously monitor blood sugar levels fell short. Some patients found this process very uncomfortable, even painful. Many reported skin irritation. A randomized study from researchers at the University College of London pointed out further shortcomings with the GlucoWatch device. Their results were published in the May 2009 edition of the journal Diabetic Medicine. Though only 6 percent were unable to tolerate wearing the device, participants noted inaccuracies in their readings on the GlucoWatch G2 Biographer. Another clinical study from the Stanford School of Medicine found that the GlucoWatch frequently triggered false alarms, erroneously telling users their blood sugar was too high. Out of 20 alarms sounded, only 10 cases actually correctly assessed a too-high reading, the other 10 were false positives. Now GlucoWatch has vanished from the diabetes care scene and its manufacturer has stopped any further development.
_
Exhaled Gases to Measure Blood Glucose:
It has been previously shown that people experiencing hyperglycemia exhale gases such as acetone and ethanol in different amounts than people who are normoglycemic. The concept of analyzing exhaled gases to measure glucose has been pursued by looking at methyl nitrate production. In a research study 18 experiments were conducted among 10 Type I diabetic children. Glucose from plasma and exhaled gases were monitored during euglycemia (normal glucose levels) and during inducement of hyperglycemia and its correction. The study was able to show that methyl nitrate concentrations were around 11 ± 3 parts per trillion by volume (pptv) during euglycemia and increased to 27 ± 6 pptv during hyperglycemia. Methyl nitrate concentrations also normalized and came back to 15 ± 2 pptv after the correction of a hyperglycemic event. The study showed that methyl nitrate concentration correlates with blood glucose fluctuations. Although methyl nitrate was found to be the most significant chemical in glucose fluctuation, there were greater than 50 gases that were detectable. This study shows the potential for the use of exhaled compounds as diagnostic markers for glycemic levels; however, it is far from being ready for commercial use. The authors employed gas chromatography and mass selective detection to monitor the fluctuations in exhalation levels. This is not a method that is feasible for routine patient use in disease monitoring, and the technology must be harnessed into a device that could be marketed and easily used by diabetics in their daily lives. The study also had weaknesses as it only looked at children with Type I diabetes and only looked at hyperglycemia. Finally, algorithms to relate the levels of exhaled gas to actual glucose levels in standard units are undeveloped. Further testing will need to be done on a larger randomized population of people across all different levels of blood glucose measurements. All negative aspects aside, the authors offer a promising avenue to pursue in the methods of noninvasive blood glucose testing.
_
Breathalyzer’s Nanosensor detects glucose in exhaled breath:
Glucose Breathalyzer uses Nano-films and Acetone-Sensitive Polymers:
Western New England University (WNE) researchers also announced another breathalyzer that uses nanotechnology to noninvasively detect blood-glucose levels in the breath of diabetics. The researchers unveiled this technology at the 2013 American Association of Pharmaceutical Scientists (AAPS) Annual Meeting and Exposition in San Antonio, Texas. Ronnie Priefer, Ph.D., a Professor of Medical Chemistry at WNE in Springfield, Massachusetts, created the multilayer technology using nanometer-thick films consisting of two polymers that react with acetone. This film crosslinks the polymers and alters the physicochemical nature of the film to provide quantification of acetone, and thus glucose levels, noted the AAPS press release.
________
Glucose Sensing via the Eye:
An exciting potential avenue for less invasive glucose measurement involves ocular testing. One group created a wearable contact lens that has been analyzed in clinical trial. The lens showed promise as it measures increases in glucose levels by a colorimetric response to systemic glucose fluctuations. Increased glucose levels cause a Rhodopsin fluorescent dye to emit from the lens and recordings were obtained with a hand-held photofluorometer. The color change of the lens was only slightly visible to the naked eye and thus would remain aesthetically acceptable to the wearer. A more recent study introduced a wearable amperometoric glucose sensor to measure tear glucose levels. The sensor, connected to an external measurement system displaying results, was able to detect an increasing dose of glucose as it was manually administered to a rabbit. However, the biosensor showed a limited increase in glucose levels from 0.16 to 0.46 mmol/L in comparison to a commercial glucometer, which showed an increase from 3.7 to 7.6 mmol/L, a clear difference in sensitivity. In addition, there was a measurement delay in the sensor, in the order of tens of minutes, which is not desirable in situations of hypo- or hyperglycemia.
_
Google develops contact lens glucose monitor:
_
Google unveiled a contact lens that monitors glucose levels in tears, a potential reprieve for millions of diabetics who have to jab their fingers to draw their own blood as many as 10 times a day. The prototype, which Google says will take at least five years to reach consumers, is one of several medical devices being designed by companies to make glucose monitoring for diabetic patients more convenient and less invasive than the traditional finger pricks. The lenses use a minuscule glucose sensor and a wireless transmitter to help those among the world’s 382 million diabetics who need insulin keep a close watch on their blood sugar and adjust their dose. The device looked like a typical contact lens when it is held one on index finger. On closer examination, sandwiched in the lens are two twinkling glitter-specks loaded with tens of thousands of miniaturized transistors. It’s ringed with a hair-thin antenna. The Google team built the wireless chips in clean rooms, and used advanced engineering to get integrated circuits and a glucose sensor into such a small space. Researchers also had to build in a system to pull energy from incoming radio frequency waves to power the device enough to collect and transmit one glucose reading per second. The embedded electronics in the lens don’t obscure vision because they lie outside the eye’s pupil and iris. According to Google, the sensor can take about one reading per second, and it is working on adding tiny LED lights to the lens to warn users when their glucose levels cross certain thresholds. The sensors are so small that they “look like bits of glitter.” Google says it is working with the FDA to turn these prototypes into real products and that it is working with experts to bring this technology to market. These partners, the company says, “will use our technology for a smart contact lens and develop apps that would make the measurements available to the wearer and their doctor.”
_
Non-invasive measurement of blood glucose using retinal imaging:
An apparatus carries out measurements of blood glucose in a repeatable, non-invasive manner by measurement of the rate of regeneration of retinal visual pigments, such as cone visual pigments. The rate of regeneration of visual pigments is dependent upon the blood glucose concentration, and by measuring the visual pigment regeneration rate, blood glucose concentration can be accurately determined. This apparatus exposes the retina to light of selected wavelengths in selected distributions and subsequently analyzes the reflection (as color or darkness) from a selected portion of the exposed region of the retina, preferably from the fovea.
_
EyeSense:
EyeSense is a noninvasive technology currently in development that measures blood glucose concentrations simply by placement of the measurement device near the eye. This innovative, noninvasive technology utilizes a novel biochemical sensor that is inserted below the conjunctiva in a simple and painless procedure by the ophthalmologist on an annual basis. The technology would replace conventional fingersticking and would probably increase blood glucose monitoring compliance. The methodology hinges on a biochemical sensor that is embedded on a small, hydrogel disk. The chemical in the disk reacts with blood glucose in the interstitial fluid below the conjunctiva of the eye and emits fluorescent light that is quantified by the photometer device. The photo-meter can be placed in front of the eye to obtain the blood glucose results in less than 20 seconds. The advantage of noninvasive technology is that patients have the ability to measure their blood glucose as frequently as they want without having to lance their fingers. The implanted disk is invisible to the naked eye. Additionally, it is generally well tolerated and does not feel like a foreign body in the eye of the user. EyeSense is still in the advanced stages of development and its approval appears promising.
_ _______
Dario: Turning Your Smartphone into a Glucose Meter:
It’s an integrated unit about the size of a cigarette lighter that includes a basic adapter that connects into a smartphone’s audio jack. As soon as you connect the device, your smartphone switches to BG-monitoring mode. You then click open the self-contained lancing device that has disposable lancets inside and an integrated cartridge of 25 propriety test strips, allowing you to poke your finger just like any other meter. The reading you get is transmitted directly to the smartphone through an app that’ll be available for free for both iPhones and Andriod systems. The app will allow patients not only to immediately see and automatically upload BG results, but also to add food information into a database — along with easy access to carb estimations, an insulin calculator, and other features like data-sharing online. Not to mention the array of alerts and reminders that patients could program in at their choosing. Dario uses ultra-thin lancets, and you would buy the 25-strip cartridges with propriety strips from the company (or from a supply provider who will eventually stock them). Dario is unique in that users will be able to analyze data directly on the phone app, send data to caregivers and doctors, and even have their data examined in clinical research and epidemiology studies about the distribution and patterns of diabetes management. The hope is to have Dario become compatible with electronic health records (EHRs) and other services that would offer interoperability with insulin pumps and CGMS (continuous glucose monitors), and possibly even Pharma-interaction on the app in terms of learning about or ordering prescriptions if users so desire.
_
iBG star:
As the “i” in iBGStar suggests, the glucose monitor is specifically made for the Apple iPhone or iPod touch. iBGStar is the first device that has been cleared by the FDA for use on an Apple device, and it is currently available in some European countries. The iBGStar uses its Diabetes Manager App for the iPhone to help users keep track of blood glucose levels on a daily basis, while the application allows patients to send selected data to their physicians to aid in monitoring their progress. The new monitor uses a novel patented technology called dynamic electrochemistry. Dynamic electrochemistry is a technology that uses complex mathematical methods to calculate and adjust for interference that may be caused by changes in temperature, humidity, and hematocrit levels. The device sends out signals of different frequencies and voltage in order to compensate for the interference that may cause inconsistent blood glucose readings. In patients who have abnormal hematocrit levels, which may be due to a disease state, a low hematocrit level may artificially overestimate actual blood glucose levels. This may pose a safety concern to the patient because it may lead him or her to use a higher insulin dose than required, possibly resulting in hypoglycemia and even hospitalization. Therefore, it is important to use a device that can measure blood glucose levels precisely in various conditions. In order to establish the accuracy of the iBGStar, a comparison study evaluated the BGStar, a device using the same dynamic electrochemistry method, against 12 other glucose monitoring systems from various manufacturers. The study specifically observed the consistency of blood glucose readings with varied hematocrit concentrations. The results showed that only four of the 13 devices—the BGStar, OneTouch Verio, Glucocard G+, and Contour—actually met the study criteria for having less than 10% maximal mean percentage deviation (MMPD) from control glucose readings. In addition, another study has supported the accuracy of the device by showing that the iBGStar has 99.5% accuracy. The iBGStar is an excellent device that will provide consistent blood glucose readings and is easier to use than conventional monitors due to portability and compatibility with smartphones, but it still requires the use of needles, which may hinder compliance to glucose monitoring for some patients.
_
Apple’s HealthKit:
HealthKit, which is still under development, is the center of a new healthcare system by Apple. Regulated medical devices, such as glucose monitors with accompanying iPhone apps, can send information to HealthKit. With a patient’s consent, Apple’s service gathers data from various health apps so that it can be viewed by doctors in one place. Stanford University Hospital doctors said they are working with Apple to let physicians track blood sugar levels for children with diabetes. In the first Stanford trial, young patients with Type 1 diabetes will be sent home with an iPod touch to monitor blood sugar levels between doctor’s visits. HealthKit makes a critical link between measuring devices, including those used at home by patients, and medical information services relied on by doctors, such as Epic Systems Corp, a partner already announced by Apple. Medical device makers are taking part in the Stanford and Duke trials. DexCom Inc, which makes blood sugar monitoring equipment, is in talks with Apple, Stanford, and the US Food and Drug Administration about integrating with HealthKit. DexCom’s device measures glucose levels through a tiny sensor inserted under the skin of the abdomen. That data is transmitted every five minutes to a hand-held receiver, which works with a blood glucose meter. The glucose measuring system then sends the information to DexCom’s mobile app, on an iPhone, for instance. Under the new system, HealthKit can scoop up the data from DexCom, as well as other app and device makers. Data can be uploaded from HealthKit into Epic’s “MyChart” application, where it can be viewed by clinicians in Epic’s electronic health record.
_
Glooko’s new device Bluetooth-enables popular glucose meters:
Glooko, which makes a cable that syncs popular glucose meters to a companion app on smartphones, has always said it plans eventually to replace that cable with a wireless Bluetooth connection. Recently company announced that they’ve finally released that product, the Bluetooth MeterSync Blue. The small box will plug into a patient’s glucose meter and send the information wireless, via Bluetooth, to Android or Apple phones.
__
Telcare Glucose Meter:
Recent advances in cellular data communications technology have enabled the development of glucose meters that directly integrate cellular data transmission capability, enabling the user to both transmit glucose data to the medical caregiver and receive direct guidance from the caregiver on the screen of the glucose meter. The first such device, from Telcare, Inc., was exhibited at the 2010 CTIA International Wireless Expo, where it won an E-Tech award. This device is currently undergoing clinical testing in the US and internationally. The Telcare Blood Glucose Meter (BGM) aims to change that, with a color screen and cellular connectivity that automatically uploads your blood sugars to the cloud. While the Telcare device itself might be more on par stylistically with the Blackberry generation, the Telcare BGM serves as an essential transition step for glucose meters by adding cloud storage and analytics while retaining a familiarity for users of all ages and tech-suaveness. With its own cellular 3G antenna, the Telcare automatically uploads blood glucose recordings to its central cloud server using the Verizon network. At this point, users can access their data through any web browser, or use the partner app Diabetes Pal for Android or iPhone. A key advantage of the Telcare is that the MyTelcare portal allows users to grant read-only access to others (family members, caregivers), and to grant full access to health care providers. The full access permitted for health care providers allows them to adjust feedback messages, change target ranges, and target number of readings per day.
_____
Non-invasive glucose meter (glucometer):
Noninvasive glucose refers to the measurement of blood glucose levels (required by people with diabetes to prevent both chronic and acute complications from the disease) without drawing blood, puncturing the skin, or causing pain or trauma. The search for a successful technique began about 1975 and has continued to the present without a clinically or commercially viable product. A non-invasive glucose meter is a relatively new piece of technology that takes glucose measurements without any finger pricking or skin pricking. The thought of pricking one’s finger several times a day, or even once, makes many diabetics jittery, so a non-invasive glucose meter can be highly desirable. A 2012 study reviewed ten technologies: bioimpedance spectroscopy, electromagnetic sensing, fluorescence technology, mid-infrared spectroscopy, near infrared spectroscopy, optical coherence tomography, optical polarimetry, raman spectroscopy, reverse iontophoresis, and ultrasound technology, concluding with the observation that none of these had produced a commercially available, clinically reliable device and that therefore, much work remained to be done. As of 2014, only two noninvasive glucose meters [GlucoTrack & Orsense] which have obtained CE mark approval are being marketed in a number of countries.
_
The GlucoTrack:
GlucoTrack uses ultrasonic, electromagnetic and thermal technologies to non-invasively measure glucose levels in the blood: In a perfect world, blood sugar testing would be quick and painless. The finger-prick, the blood and the coated strips can be messy, complicated to use and painful—and these issues can contribute to patient noncompliance. A goal of the medical device community has been to develop a blood glucose monitoring device that is noninvasive but still highly effective, and thereby remove what are believed to be among the two most significant barriers to frequent monitoring of blood glucose by diabetes patients: pain and cost. To meet this need, Integrity Applications, based in Ashkelon, Israel, has developed the GlucoTrack® model DF-F non-invasive blood glucose measurement device, which represents a key advance in this area. It is designed to help people with diabetes obtain blood glucose level measurements without the pain, inconvenience, incremental cost and difficulty of conventional (invasive) spot finger stick devices. The GlucoTrack device takes advantage of the natural physiology of the ear lobe and uses an ear lobe clip to deliver blood glucose readings in about a minute, thanks to a trio of technologies: ultrasonic, electromagnetic and thermal.
_
GlucoTrack is battery-operated and includes a Main Unit (MU), which contains display and control features, as well as transmitter, receiver and processor, and a Personal Ear Clip (PEC), which is clipped to the earlobe and contains sensors and calibration electronics. The device is small, light and easy to use and handle. The Main Unit can be shared by up to three users (in model DF-F), although each user requires his/her own (individually calibrated) PEC. The device includes a USB port for data downloading (enables off-line analysis), as well as battery recharging. As a noninvasive device, GlucoTrack does not measure blood glucose levels directly; instead, it harnesses three independent technologies to measure physiological phenomena that correlate with the user’s glucose level. These measurements—which are transmitted from the PEC to the Main Unit—are subsequently analyzed using an algorithm that translates them into blood glucose level readings. Significantly, GlucoTrack does not use optical technology, which, based on others’ experience, was found to be impractical for use in noninvasive glucose monitoring. GlucoTrack performs three independent measurements simultaneously, using thermal, ultrasound, and electromagnetic technologies. The results are weighted, using a patented, unique algorithm to provide a reading which is displayed on a color touch screen of the device by large, clear digits. The result is announced verbally as well, allowing visually impaired users using the device as easy.
_
Why the earlobe and not another anatomical location?
The earlobe is a very convenient place on the body to measure one’s blood sugar levels, since doing so doesn’t interfere with one’s activities. From a physiological standpoint, there are also specific benefits to using the earlobe. For example, the earlobe contains a great number of capillary vessels, and blood within it flows relatively slowly. It also contains a relatively small amount of fat and nerves, as well as no bones. All of these facts help to ensure a better reading. In addition, the earlobe is relatively stable in size in adults, which similarly helps to maintain the calibration valid for relatively long period of time. The device also cuts down on costs for the user, as the Personal Ear Clip only needs to be replaced every six months.
_
Calibration:
Calibration is required to be performed prior to glucose measurements so that the influence of individual quasi-stable factors, such as tissue structure, can be minimized. The process consists of correlating invasive basal and postprandial BG data, taken from finger capillary blood, with six sequential measurements with the GluocTrack instrument, generating a calibration curve that is exclusive to each individual. Six invasive pre and post-prandial measurements generate individual calibration as seen in the figure below. The first measurement pair is taken in the fasting state. The calibration procedure is easy, lasts about 1.5 hours and more importantly, is valid for a month (a longer period is forecast in the future).
_
The figure below shows that non-invasive glucometer saves money as compared to invasive glucometer:
______
Parents hack child’s glucometer:
To deal with childhood diabetes and keep on top of a disease that could turn deadly at a moment’s notice, parents have resorted to hacking medical devices. By creating solutions that help them manage their children’s disease, these innovative parents could push the medical device world in a new direction. Jason Adams, whose eight year old daughter has Type 1 diabetes, was concerned about monitoring her blood sugar at night. Without the ability to monitor her condition, he was forced to keep her home, which prevented her from attending sleepovers with friends. Jason’s daughter Ella uses a Dexcom Inc. glucose monitor, a device that takes blood sugar readings every five minutes, according the WSJ. Unfortunately, however, the monitor has no provision for sharing data over a network. A little internet searching revealed to Jason a system called “NightScout,” a remote-monitoring software developed by other parents of diabetic children. The developers of NightScout, who happen to be software engineers, were frustrated with the limited capabilities of current diabetes monitoring technology. According to the WSJ, the open-source software enables parents to hack the Dexcom glucose monitor and upload its information to the Internet. Two weeks after getting the software setup at home, Ella was able to attend her first sleepover. Other notable successes have occurred as well, according to the WSJ. Kristin Derichsweiler, a nurse and single mother of four, downloaded the software and started using it to help her 15 year old son manage his diabetes. While at work, she noticed his blood sugar dropping to dangerously low levels. When he failed to answer the phone, she rushed home to find he had become unresponsive and needed juice to restore proper sugar levels. Despite the successes, there is justified concern from the FDA. Coming to rely on an untested technology could lead to a potentially deadly false sense of security. Questions that are raised by the FDA related to NightScout center around how users may get support if they run into problems, and how to keep data confidential on the Internet. While questions are raised, the agency is making efforts to facilitate the new software and the parents who are using it.
_
The First Remote Mobile Communications Device Used for Continuous Glucose Monitoring (CGM):
Dexcom, Inc., a leader in continuous glucose monitoring (CGM) for patients with diabetes, announced recently that it has received U.S. Food and Drug Administration (FDA) approval for its CGM remote mobile communications device: Dexcom SHARE. Dexcom SHARE, an accessory to the Dexcom G4® PLATINUM Continuous Glucose Monitoring System, uses a secure wireless connection to transmit the glucose levels of a person with diabetes to the smartphones of up to five designated recipients, or “followers.” These followers can remotely monitor a patient’s glucose information and receive alert notifications from almost anywhere via their Apple® iPhone® or iPod® touch. With Dexcom SHARE, parents and personal caregivers can monitor a child’s or loved one’s glucose data from a remote location, giving them peace of mind and reassurance when they are apart. Now critical glucose data from the Dexcom G4® PLATINUM Continuous Glucose Monitoring System can be remotely monitored using a mobile device. So parents need not hack their child’s glucometer as discussed in previous paragraph.
___________
___________
My logic on Diabetes Mellitus:
Since it has been shown that microvascular diabetes complications are due to chronic hyperglycemia per se, I am surprised to know that most experts including ADA, IDF and WHO recommend venous plasma glucose level for diagnosis of diabetes. It is because all the tissues bearing the brunt of diabetic microvascular complications (e.g. kidneys, nerves, and retina) are perfused by high blood/plasma glucose from their arterial blood supply. Venous blood/plasma glucose does not enter in any tissue except liver through portal vein. Remember, it is the portal vein that brings glucose from food along with insulin from pancreas to liver for metabolism. Remember, both glucose from food and insulin from pancreas enter systemic circulation after first-pass metabolism in liver. However, pancreas secret insulin in response to arterial blood glucose and not venous blood glucose including portal vein glucose. So it is the arterial blood glucose that secretes insulin and it is the high arterial blood glucose that causes diabetic complications when insulin secretion and/or action is reduced. Then why rely on venous blood glucose for diagnosis of diabetes? Since it is difficult to puncture artery every time for blood glucose test and since arterial blood glucose is almost same as capillary blood glucose, why not capillary blood glucose for diagnosis of diabetes mellitus? Of course we have to change cutoff values for diabetes diagnosis as compared to venous blood but it would be more rational, more physiological and correlate well with diabetic complications. Of course there is marked difference between capillary blood glucose and venous blood glucose in non-diabetic individual post prandial, but since capillary (surrogate arterial) blood glucose determines diabetic complications, cutoff value for diagnosis of diabetes, both fasting and post prandial, must be based on capillary blood glucose measurement (SMBG) rather than venous glucose. I would also recommend HbA1c measurement from capillary blood rather than venous blood for the same reason.
___________
___________
The moral of the story:
1. Diabetes mellitus (DM) is defined as a metabolic disorder characterized by hyperglycemia due to reduced insulin secretion and/or reduced insulin action and/or increased glucose production. Persistent hyperglycemia is a hallmark of diabetes but transient hyperglycemia can occur a part of stress response in acute illnesses and is brought about by elevated levels of counter regulatory hormones.
_
2. One in 10 adults has diabetes. 382 millions have diabetes in 2013 worldwide. Every six seconds someone dies from diabetes.
_
3. About half of diabetic population worldwide does not know that they have diabetes.
_
4. Diabetes is not merely a health issue, but also a political issue, one which requires whole society approach. Type 2 diabetes, which many consider an epidemic currently, is increasing worldwide predominantly due to poor diet, sedentary lifestyle, new wealth and the fact that we are living longer. Eating fast food two or more times a week increases the risk of developing Type 2 diabetes by 27 percent.
_
5. Chronic hyperglycemia per se causes chronic diabetic complications by various mechanisms although there is a genetic susceptibility for developing particular complications. There is no way to check genetic susceptibility to diabetic complications.
_
6. Numerous studies have demonstrated that optimal management of glycemia along with other cardiovascular risk factors can reduce risk of development and progression of both microvascular and macrovascular complications. It has been shown that microvascular complications, such as neuropathy, nephropathy, and retinopathy are reduced by 40% for every percentage reduction in hemoglobin A1c (HbA1c or A1c) values.
_
7. Tight glucose control decreased the risk of progression of retinopathy, nephropathy, and neuropathy but increased the risk of hypoglycemia 2.4 times. Intensive efforts to achieve blood sugar levels close to normal have been shown to triple the risk of the most severe form of hypoglycemia, in which the patient requires assistance from by-standers in order to treat the episode. Among intensively controlled type 1 diabetics, 55% of episodes of severe hypoglycemia occur during sleep, and 6% of all deaths in diabetics under the age of 40 are from nocturnal hypoglycemia.
_
8. Since hyperglycemia per se causes diabetic complications, since control of hyperglycemia leads to reduction of diabetic complications and since tight control of blood glucose invariably leads to hypoglycemia and its complications; diabetics are therefore recommended to check their blood glucose levels frequently to prevent and/or treat hyperglycemia and hypoglycemia.
_
9. Researchers found that the lifetime of hemoglobin cells (RBC) of diabetics turned over in as few as 81 days, while they lived as long as 146 days in non-diabetics. In a person with normal blood sugar, hemoglobin will be around for a lot longer, which means it will accumulate more sugar. This will drive up the A1c test result – but it doesn’t mean that person had too much sugar in their blood. It just means their hemoglobin lived longer and thus accumulated more sugar. So normal people with normal fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) can have falsely elevated A1c levels. On the other hand, if someone is diabetic, their red blood cells live shorter lives than non-diabetics. That means diabetics will have falsely low A1c levels. So can we rely on A1c alone?
_
10. It is a fact that A1c is a measure of glycemic control and rise/fall of A1c correlates well with rise/fall of chronic diabetic complications. Nonetheless it does not provide information about day-to-day glucose levels, nor does it provide immediate feedback to patients about medication or lifestyle choices. The biggest limitation of A1c is that it misses wide glycemic excursions and also misses many asymptomatic hypoglycemias. Frequent unrecognized hypoglycemia may lead to falsely low HbA1c levels. Postprandial hyperglycemia was identified in 39% of patients with type 2 diabetes who were not using insulin and had an HbA1c level lower than 7.0%. There is evidence to show that wide glycemic excursions lead to vascular complications despite reasonable A1c. Self-monitoring of blood glucose (SMBG) complements A1c because it can distinguish among fasting, preprandial, and postprandial hyperglycemia; detect wide glycemic excursions; identify hypoglycemia; and provide immediate feedback to patients about the effect of food choices, activity, and medication on glycemic control.
_
11. SMBG is measurement of glucose in blood (or plasma) either directly or indirectly through other body fluids by patients themselves or their care givers or medical personnel by any technique without using laboratory.
_
12. Nobody should do urine glucose test as a surrogate marker for blood glucose test. However, I have diagnosed diabetes in some patients by seeing urine glucose test positive incidentally when urine examination was done for evaluation of fever, jaundice or hematuria and not for diabetes. Also urine ketone test is very helpful to patient to judge severity of illness at home when SMBG level is above 240 mg/dL.
_
13. Collection of blood sample must be avoided from the same arm or the same vein where IV drip D5W/D5NS is infused because only10% contamination with D5W/D5NS will elevate glucose in a sample by 500 mg/dL or more. However, when blood sample is drawn from the arm opposite the one in which an intravenous line is inserted, blood glucose will rise by 0.38mg/dL every minute in a 70 kg diabetic man with little or no insulin secretion when drip duration is 8 hour for 500 ml. And if the same drip is given to a normal non-diabetic person, slight increase in blood glucose will stimulate insulin secretion and therefore blood glucose will be reasonably maintained. The corollary is that if you have collected blood from the arm opposite the one in which an intravenous line is inserted, if the drip rate is average (4 to 8 hour for each pint), and if you are getting high blood glucose level, do not blame IV drip (D5W or D5NS) as patient may indeed be diabetic. This is very important because I have seen in emergency situation as well as routine hospital admission; IV drip is started even before blood is collected for investigation although SMBG by nurse must be done before starting IV drip. Also, diabetics on IV drip for various reasons ought to be monitored for glucose levels.
_
14. Whole blood glucose is 15 % lower than plasma glucose because plasma has higher water content and consequently more dissolved glucose than whole blood. The conversion of concentration values from one system (or sample type) to another is subject to unpredictable errors. Several authors have already rejected the practice of converting glucose concentrations and have recommended that plasma be used for all glucose determinations. The blood glucose is defined as venous plasma glucose according to the criteria of WHO to diagnose diabetes. Venous blood is usually employed for laboratory analysis and is preferable in diabetes testing. Laboratory plasma glucose measurement is far more accurate than plasma equivalent of whole blood capillary glucose measurement by glucometer (SMBG) because laboratory tests virtually eliminate all variations except for manufacturing variation from their testing. A standard lab glucose value is within about plus/minus 4% of a perfect reading while SMBG is within plus/minus 15% of the lab test (current ISO standard). However, because of the widespread use of glucometers, fingerstick capillary whole blood glucose tests have also become a standard. Also, some glucometers can measure capillary plasma glucose directly by a series of absorbent pads to separate the cellular portion of a sample from the plasma portion.
_
15. Plasma glucose is a biological variable and possesses intra-individual variability of 5.7% to 8.3% and inter-individual variability of up to 12.5%. An individual having FPG of 126 mg/dL can show FPG value from 112 to 140 mg/dL based on coefficient of variation (CV) of 5.7%. The analytical variability is considerably less than the biological variability, still one-third of the time, the glucose results on a single patient sample measured in two different laboratories could differ by 14%.
_
16. In the fasting state, the glucose concentrations in arterial, capillary (SMBG), and (forearm) venous blood are supposed to be almost indistinguishable. Fasting venous glucose is generally 2-5 mg/dL lower than fasting arterial blood glucose. Arterial blood glucose and capillary blood glucose (SMBG) have been shown to be almost identical in concentration in fasting as well as post meal time. However, post prandial (after glucose load) venous glucose could be 7 to 35 % lower than arterial glucose (equivalent SMBG) due to muscles removing more glucose from the blood than the liver in the presence of adequate insulin action. It has been shown that a lack of insulin (in the de-pancreatized animal) shows an arteriovenous glucose difference that is extremely small and that injection of insulin produces an increase in this difference. The mean arteriovenous differences are largest in lean nondiabetic individuals and smallest in diabetic individuals. So in a non-diabetic individual, SMBG post prandial can overdiagnose diabetes because SMBG would be markedly higher than venous blood glucose. So if you were a non-diabetic before and now you want to know whether you have developed diabetes, please test fasting and post prandial blood (plasma) glucose in a laboratory. For established diabetes, SMBG is useful for monitoring fasting blood sugar (FBS) and postprandial blood sugar (PPBS). Lower post prandial venous blood glucose proves insulin action in non-diabetic as well as in type 2 diabetics (T2DM). Therefore in T2DM, concurrent post prandial SMBG and lab venous blood glucose can show arteriovenous blood glucose difference and higher the difference, greater the residual insulin action. In other words, large arteriovenous postprandial blood glucose difference in T2DM suggests that pancreas is secreting some residual insulin and that insulin is acting.
_
17. I recommend 2 hour postprandial plasma glucose (PPG) by laboratory as a screening test for T2DM. An increase in postprandial glucose concentration usually occurs before fasting glucose increases. Therefore, postprandial glucose is a sensitive indicator of the risk for developing diabetes and an early marker of impaired glucose homeostasis. Also, PPG is a better predictor of both all-cause mortality and cardiovascular mortality or morbidity than the FPG. Even when HbA1c and fasting glucose levels are within the normal range, post prandial hyperglycemia has been associated with a 2-fold increase in the risk of death from cardiovascular disease. Elevated postprandial glucose is independent and significant risk factors for macrovascular complications and increased mortality risk. Prospective interventions that control PPG have been shown to improve endothelial function and reduce carotid atherosclerosis in patients with type 2 diabetes. PPG levels correlated better than HbA1c measurements with the risk of retinopathy progression. We have to unlearn that FPG and A1c levels are reliable cut-offs for predicting or preventing future diabetes and its complications. PPG scores over FPG and A1c as the best marker for predicting future diabetes, preventing future diabetes, controlling present diabetes and preventing chronic diabetic complications. Many studies have shown that postprandial hyperglycemia beyond the 16th week of pregnancy is the main predictor for fetal macrosomia and postprandial capillary blood glucose monitoring significantly reduced the incidence of preeclampsia. In order to avoid hypoglycemia and achieve target glucose levels, patients with diabetes who take mealtime insulin are advised to test SMBG before meals to adjust doses, based on meal size and content, anticipated activity levels, and glucose levels. The biggest limitation of SMBG is that despite excellent A1c levels and target preprandial glucose levels, patients often experience nocturnal hypoglycemia and postprandial hyperglycemia that are not evident with routine SMBG. Since recent evidence suggests that postprandial hyperglycemia plays a particularly important role in the development of vascular complications of diabetes, it is imperative to monitor postprandial blood glucose by SMBG for better clinical outcome.
_
18. Pain associated with finger lancing is one of the major barriers to SMBG. In a recent study, up to 35% of the participants stated that pain is the main reason people with diabetes refrain from regular blood glucose testing. Everybody nowadays uses computer, internet and cell phones; all need finger tip use; painful finger tips can affect handling of all these devices. To reduce lancing pain, forearm testing is an acceptable alternative to finger prick testing for blood glucose measurement provided blood sugar is stable and not rapidly changing.
_
19. Do not share glucometer or fingerstick lancing devices. Sharing of this equipment could result in transmission of infection such as hepatitis B. However, the rate of infections from lancets is extremely low because the lancet goes into the subcutaneous space and is not being used intravenously, and the blood is flowing out of the body.
_
20. If fingers are not soiled by sugar containing products (biscuits, fruits etc); the first drop of blood can be used after gentle squeezing the finger for SMBG.
_
21. Up to 16% of patients miscode their glucometers. This can lead to -37% to + 29% errors in clinical practice. So no coding strips are recommended.
_
22. Factors affecting accuracy of various glucometers include calibration of meter, ambient temperature, pressure use to wipe off strip (if applicable), size and quality of blood sample, high levels of certain substances (such as ascorbic acid) in blood, hematocrit, dirt on meter, humidity, and aging of test strips. However despite multiple technical errors while using SMBG, most patients obtain clinically useful values. Various studies have shown the clinical accuracy of current glucose meters and concluded that the meters are sufficiently reliable for clinical decision making.
_
23. It has been amply demonstrated that up to a quarter of patients will falsify their SMBG values when writing the results in their log books to manipulate their behavior to ‘look good’.
_
24. For people with type 1 diabetes and type 2 diabetes taking daily insulin, SMBG is an essential component of daily diabetes management and it has been shown that testing 3 or more times a day was associated with a statistically and clinically significant 1.0% reduction in A1c levels. Furthermore, blood glucose measurements taken post-lunch, post-dinner and at bedtime have demonstrated the highest correlation to A1c. Two meta-analyses demonstrated that SMBG results in a statistically significant decrease in A1c of approximately 0.40% in patients with type 2 diabetes who are not taking insulin. SMBG allows patients to adjust food intake, physical activity, or pharmacologic therapy in response to their blood-glucose readings and to assess whether their blood-glucose levels are under control and thereby reduce diabetic complications.
_
25. Even though SMBG is invasive and expensive, regular and frequent SMBG not only improves glycemic control in all diabetics by improving A1c but also linked to better clinical outcomes; by necessarily acting on the measured values. In other words, mere doing SMBG without acting upon is futile. SMBG prescription is discouraged in the absence of relevant education and/or ability to modify behaviour or therapy modalities. Out of all SMBGs, only 41% of people with diabetes have the ability to calculate an insulin dose based on carbohydrate intake and blood glucose levels. Also glucometers are most accurate when used properly. Thus, educating patients on proper use and what to do with the results is vital.
_
26. Lower rates of SMBG are correlated with having less than a high school education, having no health insurance coverage (poor people), taking no medication or oral medication only, making two or fewer doctor visits annually, and not having taken a diabetes-education course.
_
27. Patients at greatest risk for diabetes complications (i.e. elderly, minorities, low socioeconomic status, obese, alcoholics and smokers) are least likely to self-monitor blood glucose.
_
28. Glucometers and test strips are an acceptable cost-effective intervention in all diabetics from a health insurance perspective.
_
29. Fingerstick blood glucose measurement by glucometer is affected by pH, partial pressure of oxygen, hematocrit, hypotension & tissue hypoperfusion and noradrenalin (norepinephrine) drip in the intensive care unit (ICU) resulting in wrong therapy. Glucometer should not be used in ICU. A blood gas analyzer can measure blood glucose level in the ICU for critically ill patients.
_
30. Continuous glucose monitor (CGM) has two distinct disadvantages over SMBG due to measurement of interstitial fluid glucose and not blood glucose; continuous systems must be calibrated with SMBG and therefore do not yet fully replace “finger stick” SMBG measurements and glucose levels in interstitial fluid temporally lag behind blood glucose values by 5 to 20 minutes. CGM by subcutaneous sensor is classified as minimal invasive technique. Detection of nocturnal hypoglycemia is the main advantage of CGM over SMBG. CGM technology is neither reliable nor accurate as initially anticipated and has limited evidence for the effectiveness. The most important use of continuous blood glucose monitoring is to facilitate adjustments in intensive insulin therapy to improve control. The ultimate goal of CGM technology is to use it in combination with subcutaneous insulin pumps, and in effect create an external “artificial pancreas” thereby providing better overall health and improved HbA1c tests.
_
31. The two most significant barriers to frequent fingerstick SMBG by diabetes patients, the pain and the cost, are overcome by non-invasive blood glucose meter like GlucoTrack and Orsense NBM-200G.
_
32. The arterial pulse provides clinically relevant information like rate, rhythm, pressure and oxygen saturation; could also provide information on blood glucose by non-invasive blood glucose measurement using optical properties of glucose. This is pulse gluco-oximetry.
_
33. Technological advances have enabled glucometer connectivity to smart-phones resulting in transmission of blood glucose data to smart-phones so that users will be able to analyze data directly on the phone app and send data to caregivers and doctors. Recent advances in cellular data communications technology have enabled the development of glucometer that directly integrate cellular data transmission capability, enabling the user to both transmit glucose data to the medical caregiver and receive direct guidance from the caregiver on the screen of the glucometer. In a nutshell, glucometer data can be transmitted to smart phone, smart phone can function as glucometer and glucometer can function as smart phone. I would name such device as Glucophone, a device that measures and sends blood glucose data to caregivers & doctors, and receive guidance from the caregiver & doctors. I hope that ADA and IDF accept this new terminology.
_
34. My logic on Diabetes Mellitus:
Since it has been shown that microvascular diabetes complications are due to chronic hyperglycemia per se, I am surprised to know that most experts including ADA, IDF and WHO recommend venous plasma glucose level for diagnosis of diabetes. It is because all the tissues bearing the brunt of diabetic microvascular complications (e.g. kidneys, nerves, and retina) are perfused by high blood/plasma glucose from their arterial blood supply. Venous blood/plasma glucose does not enter in any tissue except liver through portal vein. Remember, it is the portal vein that brings glucose from food along with insulin from pancreas to liver for metabolism. Remember, both glucose from food and insulin from pancreas enter systemic circulation after first-pass metabolism in liver. However, pancreas secret insulin in response to arterial blood glucose and not venous blood glucose including portal vein glucose. So it is the arterial blood glucose that secretes insulin and it is the high arterial blood glucose that causes diabetic complications when insulin secretion and/or action is reduced. Then why rely on venous blood glucose for diagnosis of diabetes? Since it is difficult to puncture artery every time for blood glucose test and since arterial blood glucose is almost same as capillary blood glucose, why not capillary blood glucose for diagnosis of diabetes mellitus? Of course we have to change cutoff values for diabetes diagnosis as compared to venous blood but it would be more rational, more physiological and correlate well with diabetic complications. Of course there is marked difference between capillary blood glucose and venous blood glucose in non-diabetic individual post prandial, but since capillary (surrogate arterial) blood glucose determines diabetic complications, cutoff value for diagnosis of diabetes, both fasting and post prandial, must be based on capillary blood glucose measurement (SMBG) rather than venous glucose. I would also recommend HbA1c measurement from capillary blood rather than venous blood for the same reason. I hope that ADA, IDF and WHO accept my logic on diabetes mellitus. In my view, capillary whole blood/plasma glucose, fasting and post prandial, by glucometer or laboratory method must be used for diagnosis of diabetes mellitus.
_____________
_____________
Dr. Rajiv Desai. MD.
November 1, 2014
_____________
Postscript:
Many diabetics in developing countries are poor people and cannot afford SMBG. Out of all affording diabetics, 67% of patients do not check their blood glucose regularly for reasons such as sore fingers, inconvenience, and the fear of needles. Additionally uneducated people are unlikely to learn right way to use glucometer and unlikely to act upon SMBG results. So SMBG is not a panacea for diabetes control.
_
Footnote:
When a normal non-diabetic individual goes for diabetes screening, post prandial SMBG overdiagnose diabetes as post prandial SMBG is far higher than concurrent venous blood glucose. So if you were a non-diabetic before and now you want to know whether you have developed diabetes, please test fasting and post prandial blood (plasma) glucose in a laboratory. However, according to my logic on diabetes, capillary blood glucose determines diabetic complications and not venous blood glucose; so you must go for fasting and post prandial SMBG for diagnosis of diabetes. Of course you would need newer cutoff points.
__________


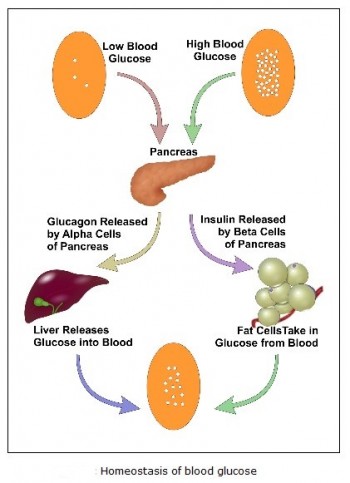
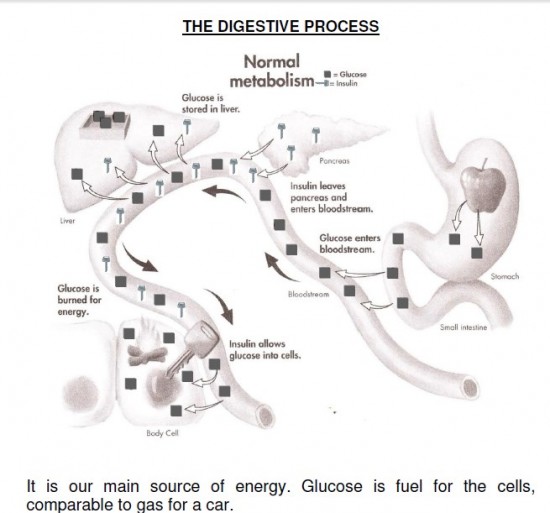
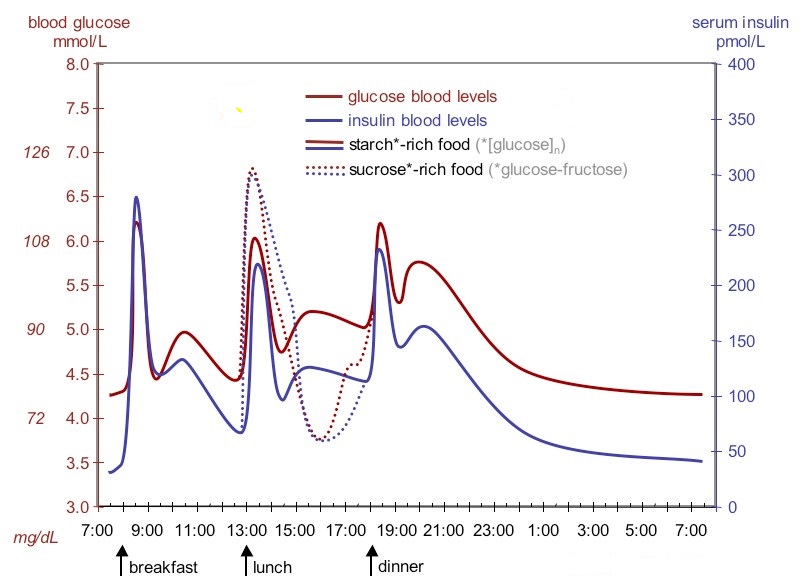
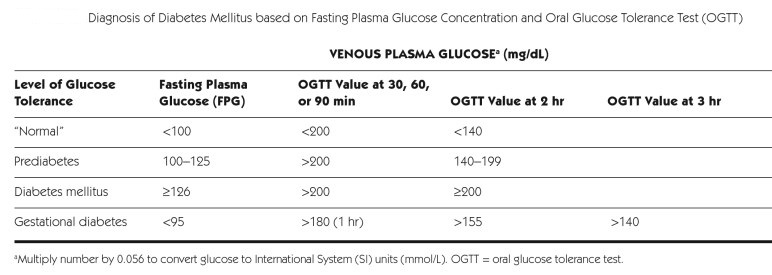
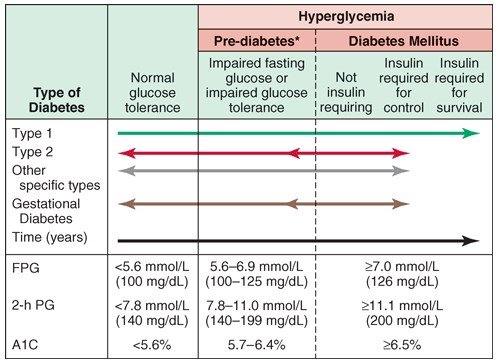
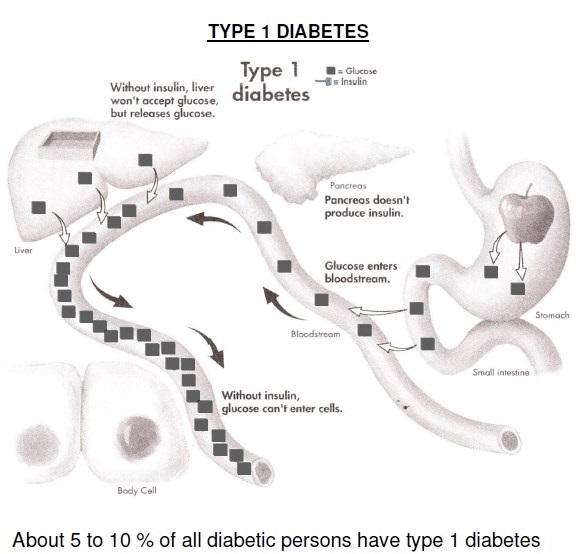
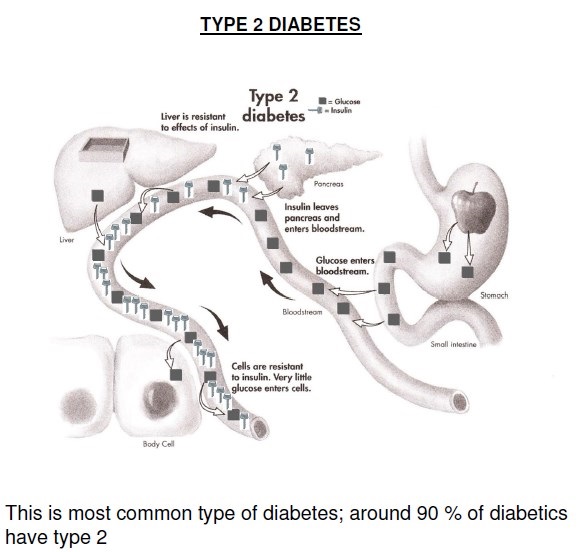
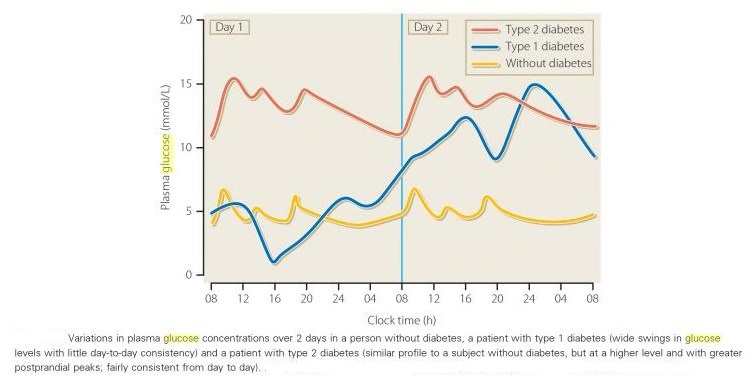
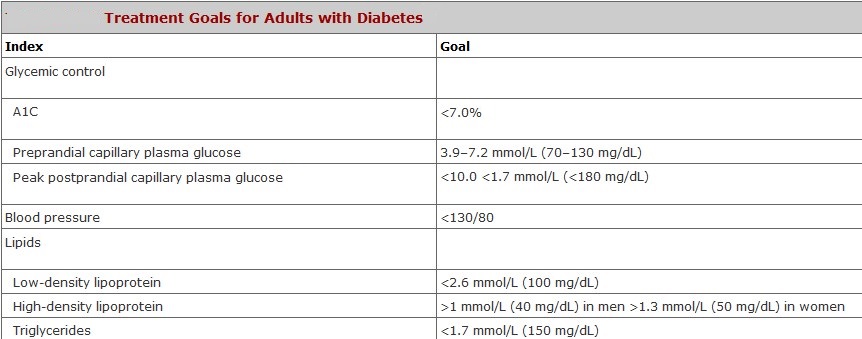
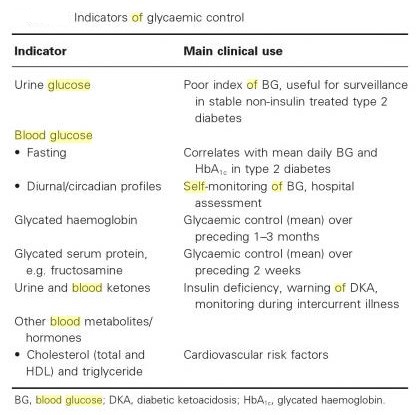
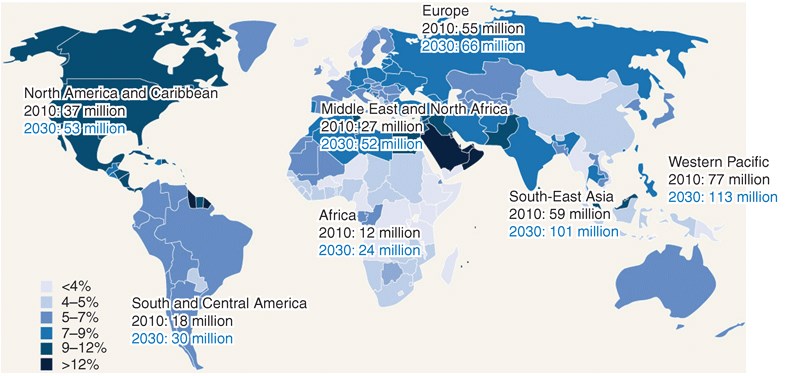
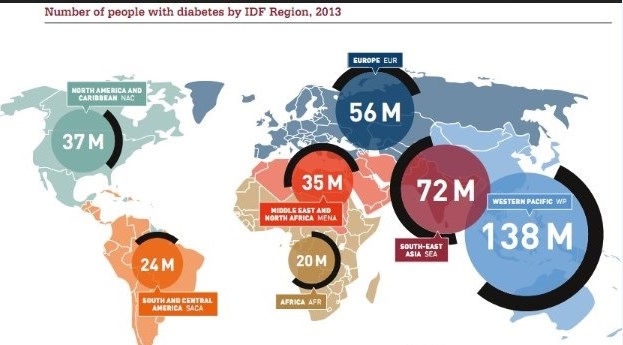
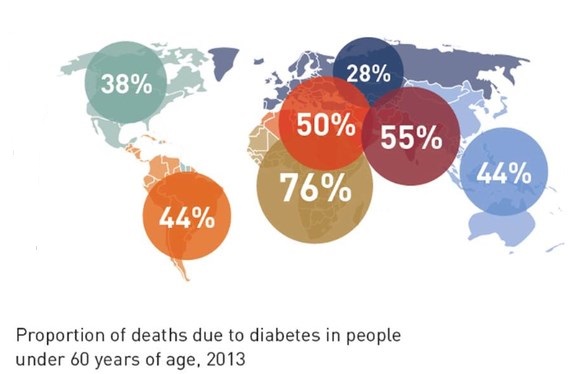
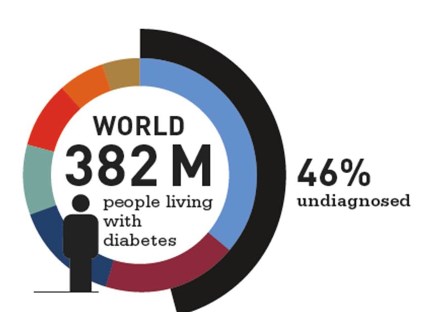
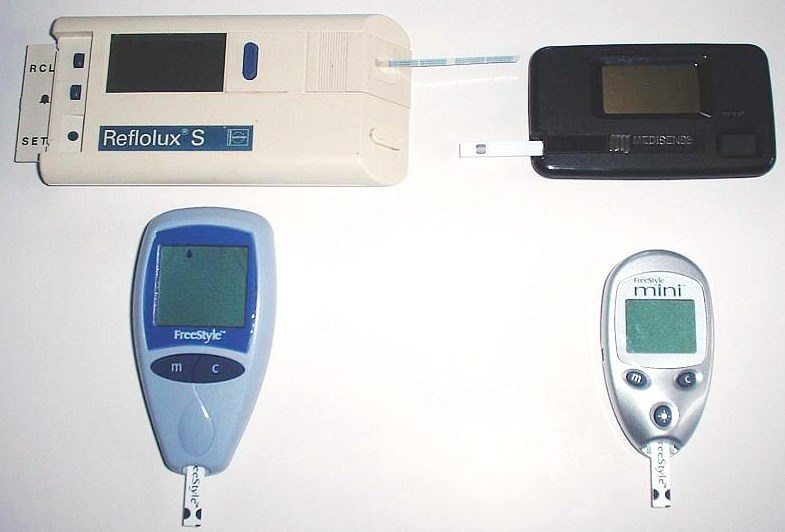
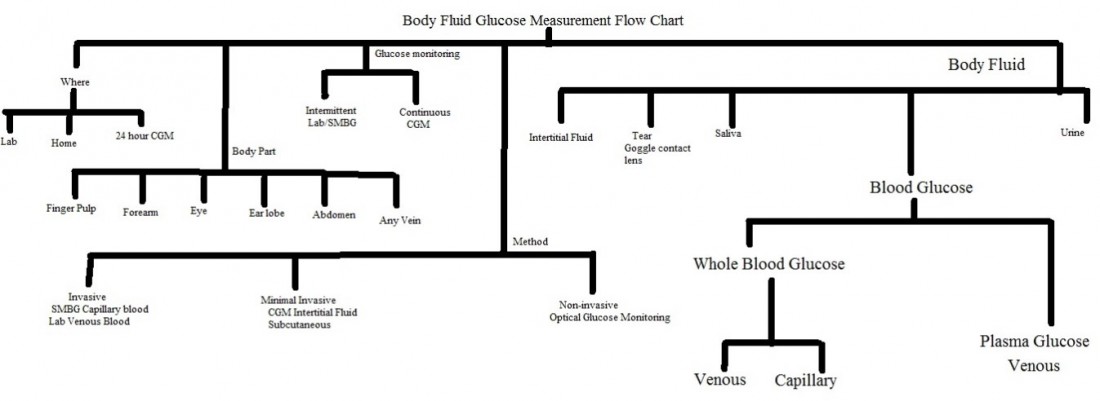

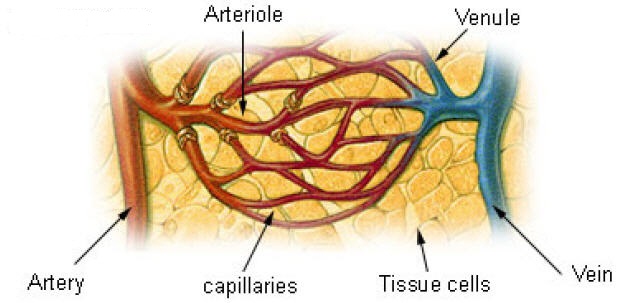
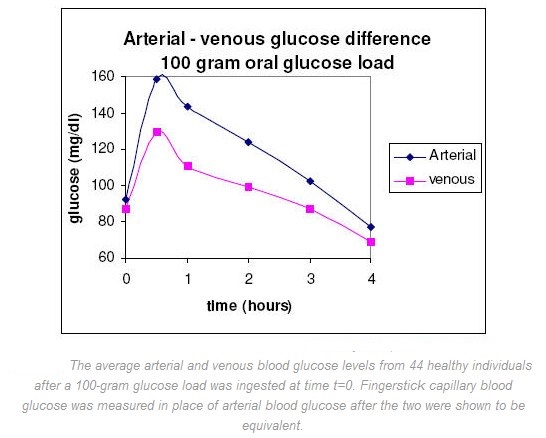
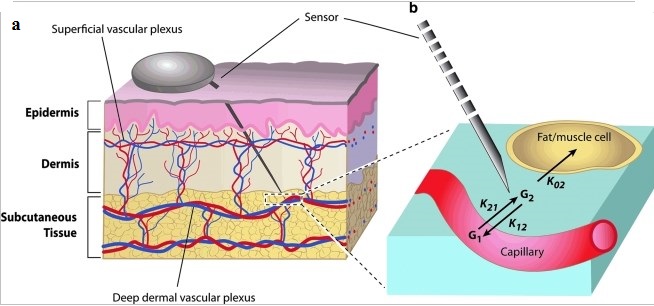
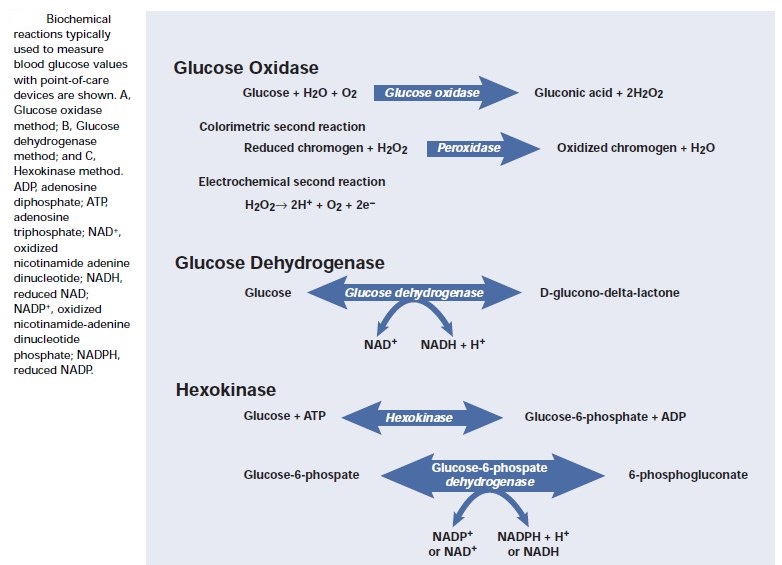
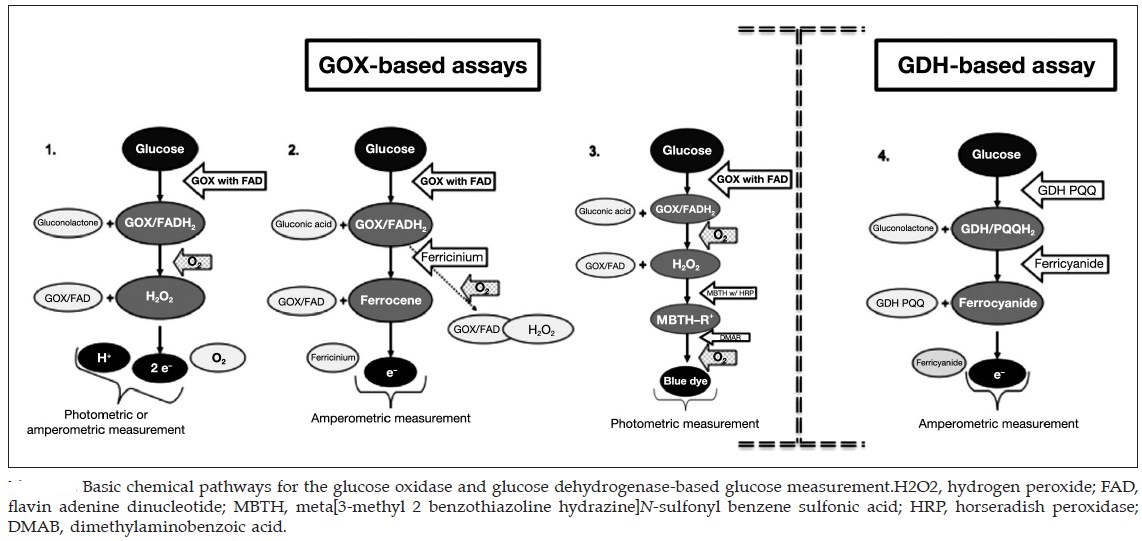
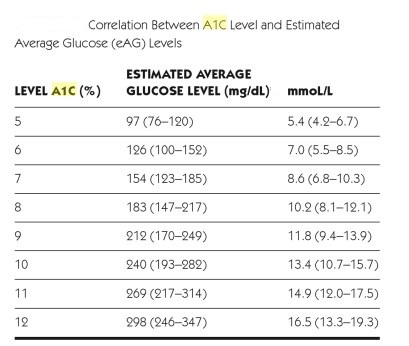

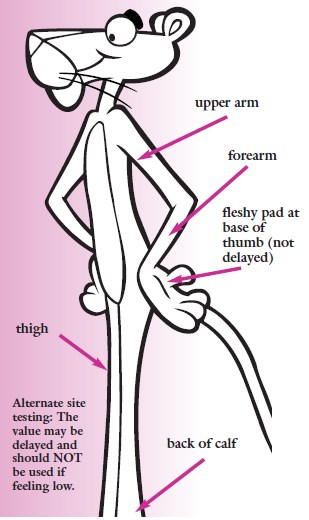
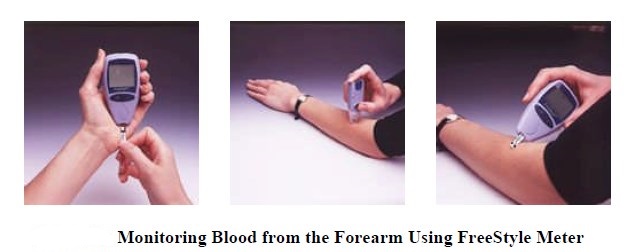

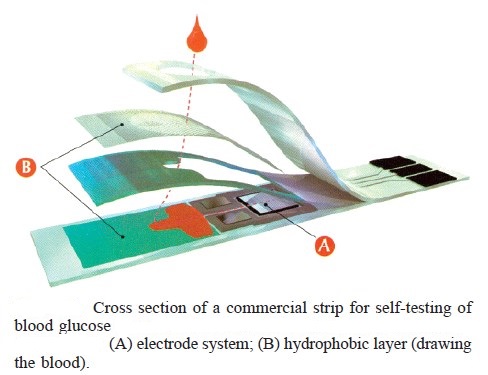

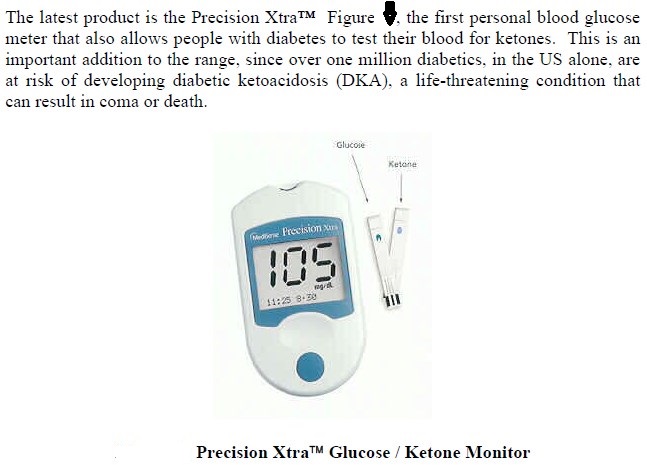
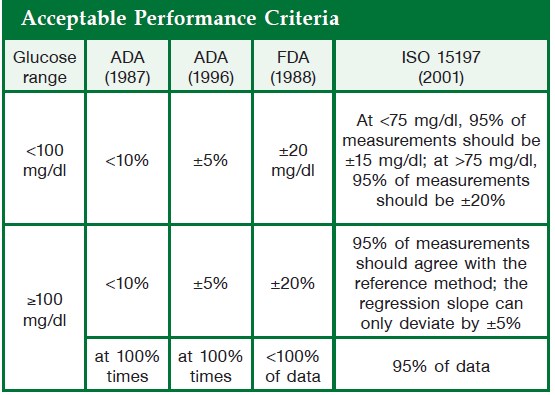


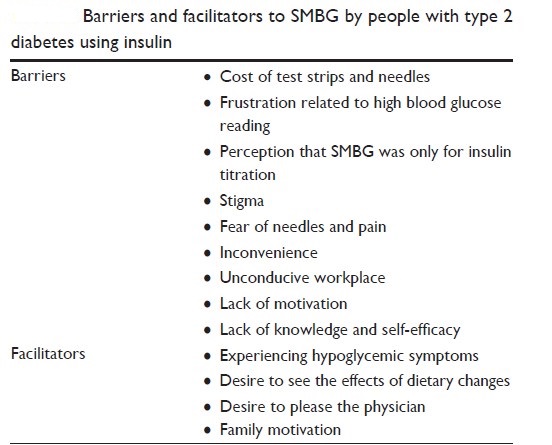
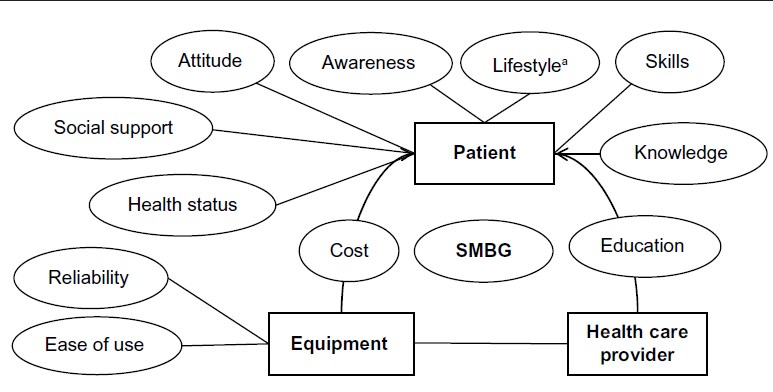
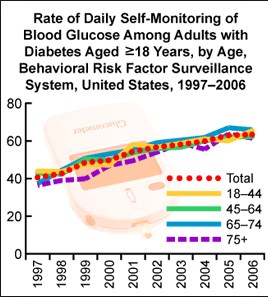


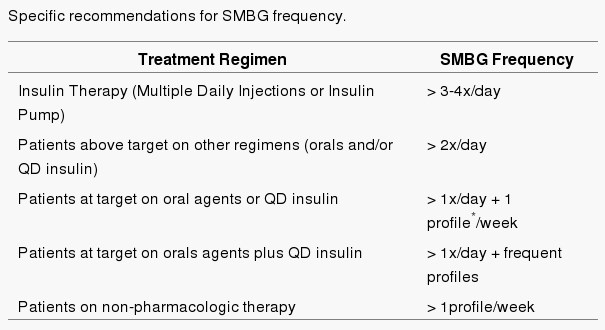

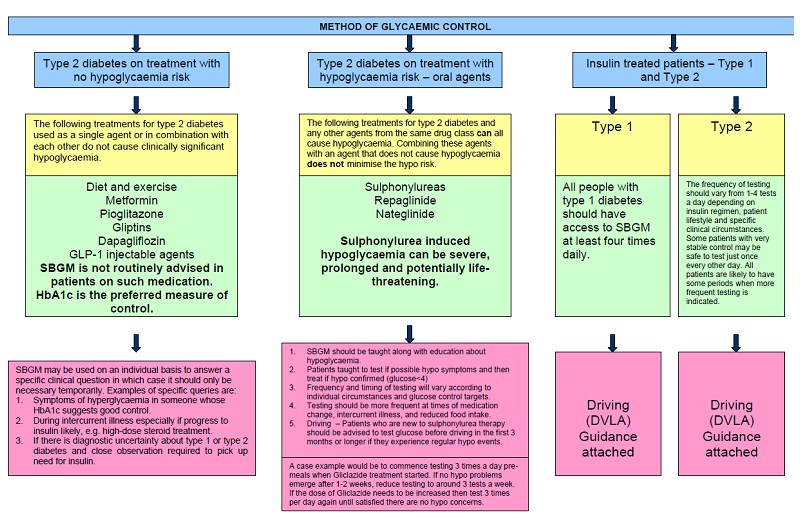

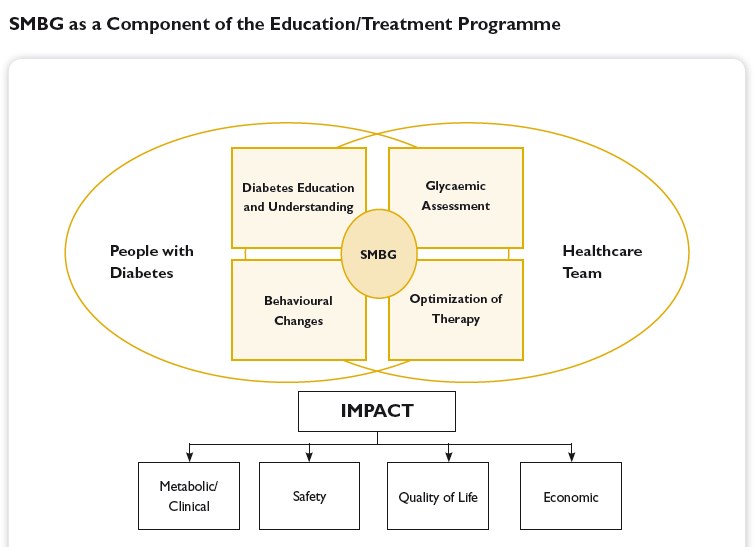

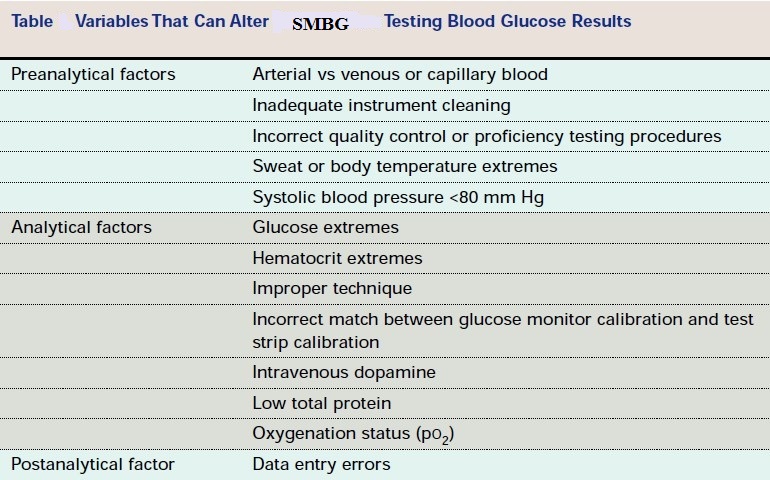
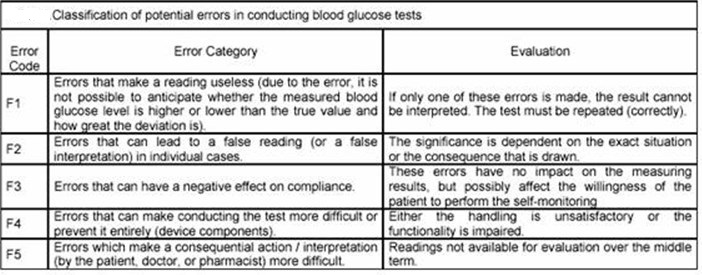
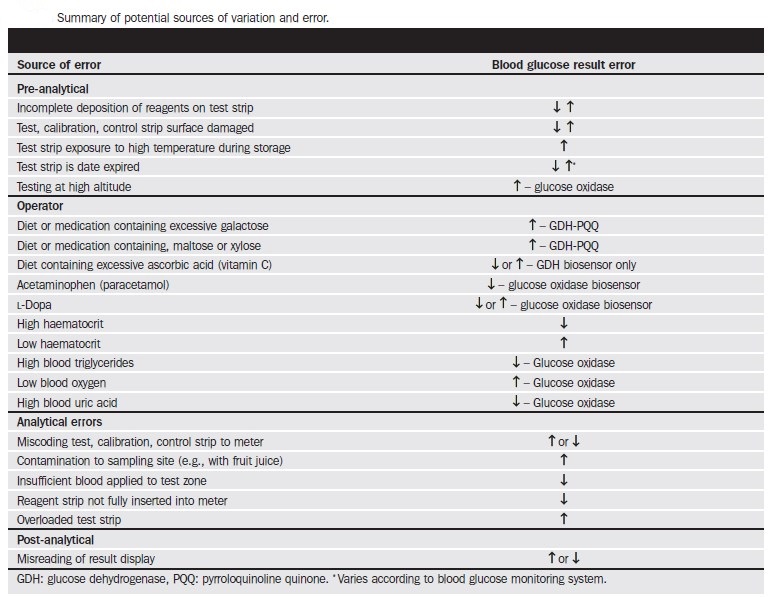
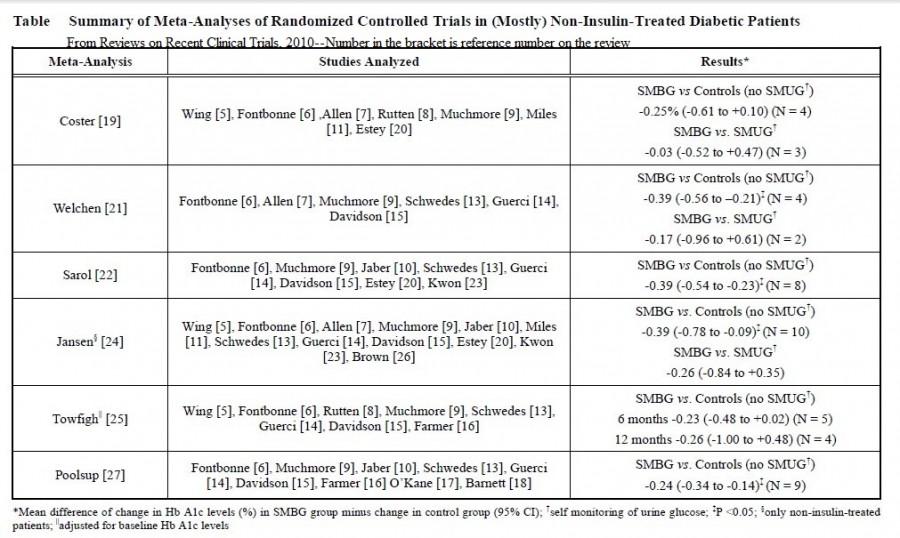
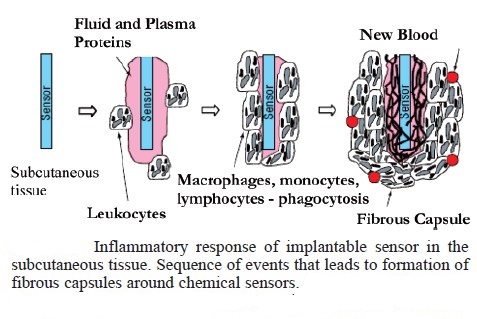
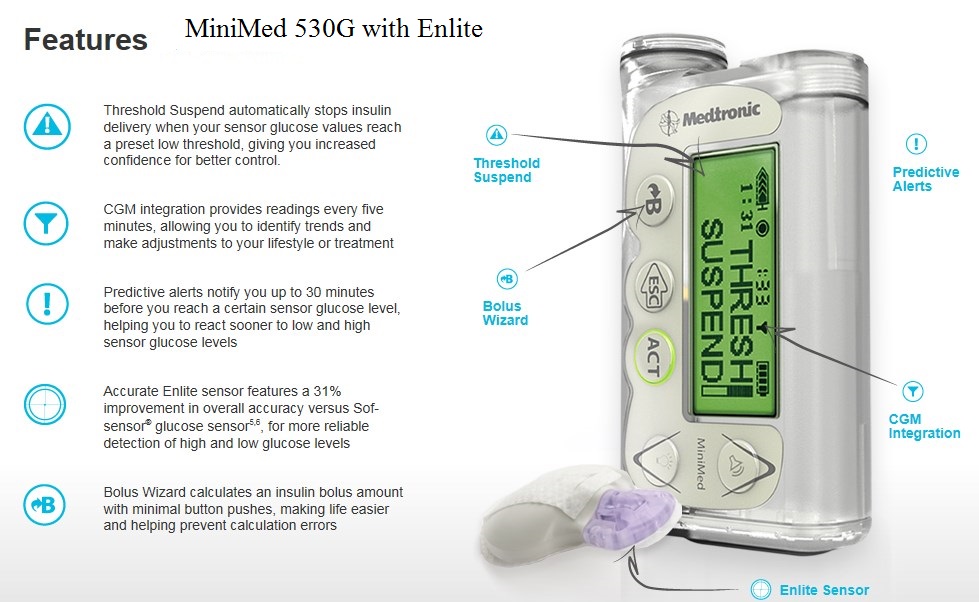
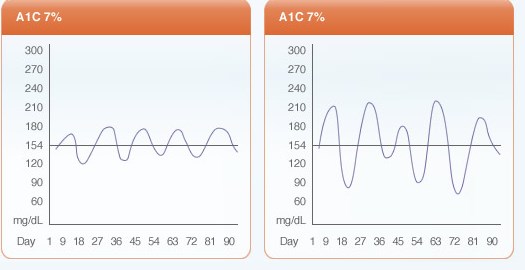
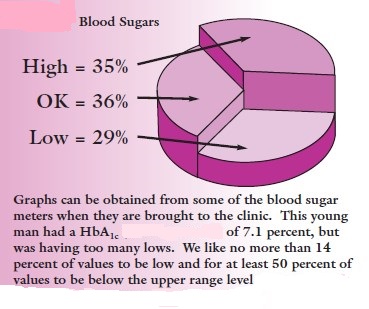
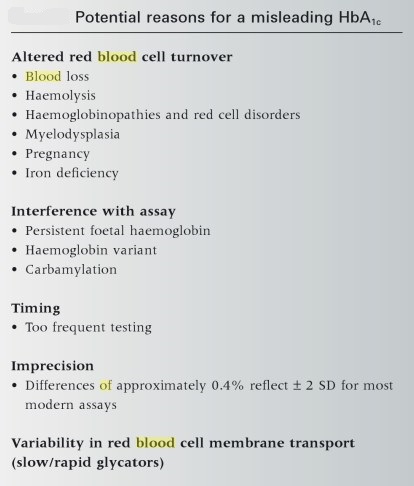
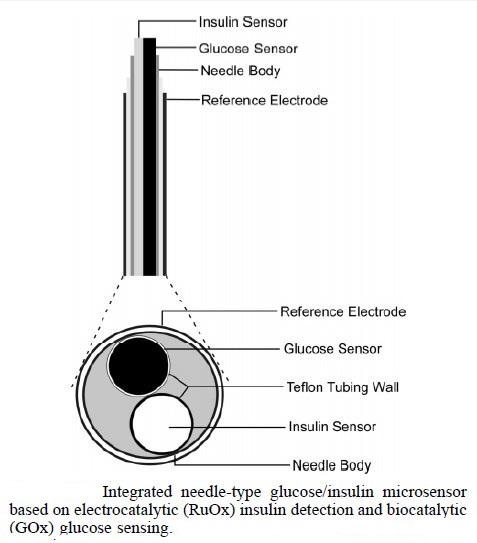
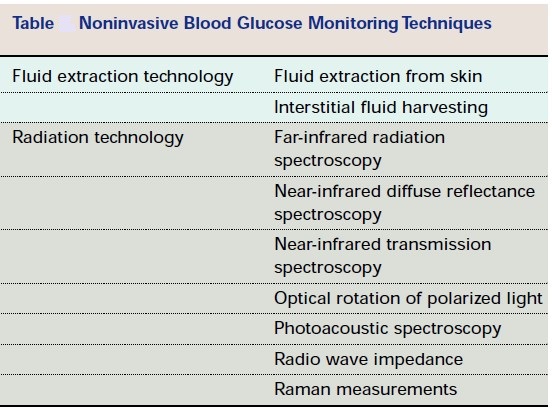
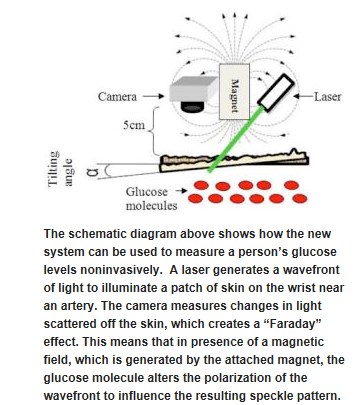


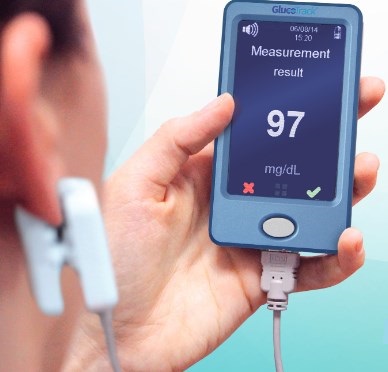
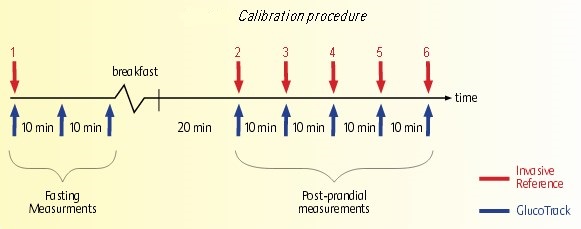
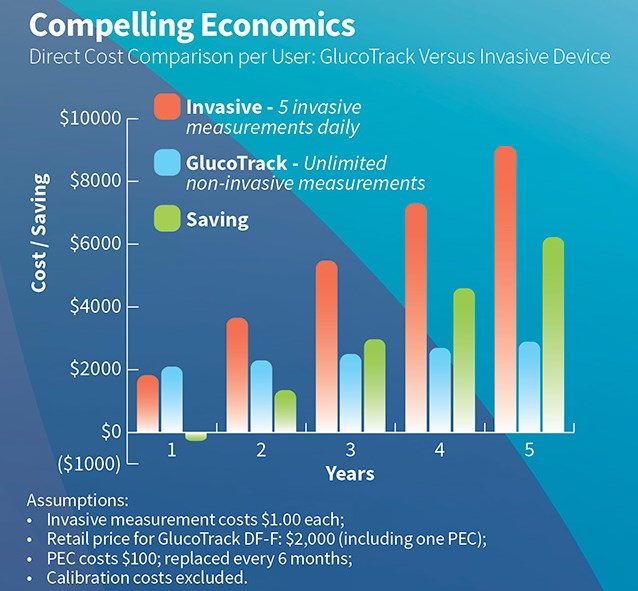
Great article, totally what I needed.|
Everyone loves it when people come together and share thoughts. Great blog, keep it up!
Very good information. Lucky me I ran across your site by accident (stumbleupon). I have saved it for later!
Fantastic items from you, man. I’ve have in mind your stuff previous
to and you’re simply too wonderful. I actually like what you’ve
received here, really like what you’re stating and the
best way during which you assert it. You are making it entertaining and you continue to care for to keep it sensible.
I cant wait to read much more from you. This is actually
a wonderful website.
Just checking in to register my support. Your post is well written Great job!