Dr Rajiv Desai
An Educational Blog
DRINKING WATER
Drinking Water:
____________
_
For last 8 years, I lived in a small town Daman and drank purified bottled water because water supplied by local municipality is suspected of unhygienic quality and water from private bore-well may well be contaminated as many areas of town have poor sewer drainage resulting in seepage of sewage in the soil itself and sewage tanks are in the vicinity of bore-wells. Assuming you’re in reasonable shape and in ideal conditions — that is, not in the heat or cold and not exerting, a human can probably live for about 3 to 5 days without drinking water. Healthier humans can live another day or so longer. Water is believed to be elixir of life. Humanity highly depends on water and its proper utilization and management. Although, water has various uses, perhaps its use as thirst quenching fluid is the most significant one. The life-supporting role of water plays a vital role in assigning water as a material of great importance in ancient and modern texts. Water has crucially shaped the rise and fall of many civilizations in the history of human beings. Many ancient civilizations flourished around river valley signifying the importance of water for human existence. At the same time, humanity has also faced the wrath of water. On several occasions, such events had changed the whole course of human history. Consequently, water has taken prominent status in different civilizations time after time. In my article on ‘Water’ published few years ago in this website, I had discussed broadly about water on earth without discussing specifically about drinking water. Most of the time, sanitation sits in the shadow of her more glamorous sister, water. The governments, the media and the populations are obsessed with provision of drinking water neglecting sanitation which is far more important that mere provision of potable water to masses. However, since I have already written article on ‘Sanitation’ earlier, it is apt to discuss the more glamorous sister of sanitation, drinking water.
_____________
Note:
Whenever the word water is used in this article without context, it means drinking water.
Discussion in this article centers on drinking water in healthy humans and not diseased humans. For example, drinking water vis-à-vis diabetes insipidus, loose motion, fever etc are not discussed.
____________
The importance of drinking water varies from good science to bad science.
Let me begin with good science.
_
Improvements in water supply and sanitation tend to lead to improvements in people’s health and the quality of their lives. Figure above shows the results of improvements in water and sanitation service upon the life expectancy of people in three French cities during the 19th century.
_
Prior to 1908, no U.S. municipal water systems chemically disinfected water. Consequently, waterborne diseases exacted a heavy toll in illness and death. Without chlorination or other disinfection processes, consumers are at great risk of contracting waterborne diseases. Figure below shows the decline in the death rate due to typhoid fever following the introduction of chlorine to U.S. municipal drinking water systems in 1908.
As more cities adopted water chlorination, U.S. death rates due to cholera and hepatitis A also declined dramatically. Worldwide, significant strides in public health and the quality of life are directly linked to the adoption of drinking water chlorination. Recognizing this success, Life magazine (1997) declared, “The filtration of drinking water plus the use of chlorine is probably the most significant public health advancement of the millennium.”
_
On the other hand, due to importance of water in human life, lot of bad science about water is propagated by media, internet, nutritionists and quacks. I narrate few examples:
1)75% of Americans are chronically dehydrated.
2) In 37% of Americans, the thirst mechanism is so weak that it is often mistaken for hunger.
3) Even mild dehydration will slow down one’s metabolism to as much as 30%.
4) One glass of water shut down midnight hunger pangs for almost 100% of the dieters in a study.
5) Lack of water is the number one trigger of daytime fatigue.
6) Preliminary research indicates that 8-10 glasses of water a day could significantly ease back and joint pain for up to 80% of sufferers.
7) A mere 2% drop in body water can trigger fuzzy short-term memory, trouble with basic math, and difficulty focusing on the computer screen or on a printed page.
8) Drinking 5 glasses of water daily decreases the risk of colon cancer by 45%, plus it can slash the risk of breast cancer by 79%, and one is 50% less likely to develop bladder cancer.
_
Doctors from a wide range of specialities agree:
By all evidence, majority of people are well-hydrated. Furthermore, they say, the current infatuation with water as an all-purpose health potion — tonic for the skin, key to weight loss — is a blend of fashion and fiction and very little science.
___________
Different people from different countries measure drinking water volume in different ways as seen in the table below:
One glass of water = 8 ounces = 240 ml of water
_
Drinking water minerals, disinfectants and chemical contaminants are measured according to the table below:
_
Some terminologies used commonly in drinking water contamination and treatment:
•Maximum Contaminant Level (MCL) – The highest level of a contaminant that is allowed in drinking water. MCLs are set as close to MCLGs as feasible using the best available treatment technology and taking cost into consideration. MCLs are enforceable standards.
•Maximum Contaminant Level Goal (MCLG) – The level of a contaminant in drinking water below which there is no known or expected risk to health. MCLGs allow for a margin of safety and are non-enforceable public health goals.
•Maximum Residual Disinfectant Level (MRDL) – The highest level of a disinfectant allowed in drinking water.
•Maximum Residual Disinfectant Level Goal (MRDLG) – The level of a drinking water disinfectant below which there is no known or expected risk to health. MRDLGs do not reflect the benefits of the use of disinfectants to control microbial contaminants.
•Treatment Technique (TT) – A required process intended to reduce the level of a contaminant in drinking water.
_____________
Water that is safe to drink is called potable water, or drinking water, in contrast to safe water, which can be used for bathing or cleaning. Sources of water are classified as either treated or untreated. Untreated water includes rain water, river water, underground water from bores or aquifers and brackish or sea water. The World Health Organization (WHO) has declared that drinking water must be bacteriologically and chemically safe to drink. In order to achieve this, raw water is treated by the local water management authorities with various chemicals, usually chlorine and/or chloramines which destroy bacteria. Other processes and chemicals are also used to achieve the standard of cleanliness required for drinking water.
_
Drinking Water Quality is the issue:
● Point-of- use drinking water treatment through chlorine and safe storage of water could result in 122.2 million avoided DALYs (Disability Adjusted Life Years, a measure of morbidity), at a total cost of US$ 11.4 billion. (UN WWAP 2003)
● Nearly 70 million people living in Bangladesh are exposed to groundwater contaminated with arsenic beyond WHO recommended limits of 10 ug/L. (UN WWAP 2009) The naturally occurring arsenic pollution in groundwater now affects nearly 140 million people in 70 countries on all continents. (UN WWAP 2009)
● Even drinking water quality in developed countries is not assured. In France, drinking water testing uncovered that 3 million people were drinking water whose quality did not meet WHO standards, and 97% of groundwater samples did not meet standards for nitrate in the same study. (UN WWAP 2009)
_
What makes clean water so important?
1. Unsafe water is the leading cause of sickness and death:
a) 3.41 million people die from water, sanitation and hygiene-related causes each year.
b) Half of the world’s hospital beds are filled with people suffering from water related illnesses.
c) It is estimated that nearly 10% of the global disease burden could be reduced through improved water supply, sanitation, hygiene, and water resource management.
2. Water impacts everything:
a) Safe drinking water sends children (especially girls) back to school, empowers women, improves community health and fosters economic development.
b) In just one day, 200 million work hours are consumed by women collecting water for their families.
c) Without clean water and sanitation, it is impossible to address poverty, hunger or AIDS.
_
Why is it especially important for children to consume clean purified water?
1. A child’s immune system and detoxification system are still developing throughout early childhood and teen years. Exposure to even very low levels of toxic chemicals or lead in drinking water at a young age can lead to increased risks of degenerative diseases and learning disorders in later years. Since many of the crucial defense systems that help protect adults from disease and environmental pollutants are not fully developed in children, they are much more sensitive to contaminants. A child consumes 3 times as much water per pound of body weight than an adult does, so they get a much bigger dose of the contaminants in our water. Their developing bodies are simply much more sensitive.
2. Currently, the health standards that determine how much and what levels of contaminants we are permitted to consume in our drinking water are all based on the potential effects on adults.
_
The impact of clean water technologies on public health in the U.S is estimated to have had a rate of return of 23 to 1 for investments in water filtration and chlorination during the first half of the 20th century. Journal Nature framed the issues by stating, “More than one billion people in the world lack access to clean water, and things are getting worse. Over the next two decades, the average supply of water per person will drop by a third, possibly condemning millions of people to an avoidable premature death”. Clean, pure water is a cornerstone of good health. Your body is mostly water, so the ongoing intake of water is essential to your every function. It’s common knowledge that most water sources are now polluted, but there is tremendous confusion about what kind of drinking water is the most health promoting, and what kind of home water treatment produces the best drinking water. Most public water supplies are loaded with hazardous contaminants, such as disinfection byproducts (DBPs), fluoride, and pharmaceutical drugs, to name just a few.
_
Some people may be more vulnerable than others to potential harm caused by water contaminants, including:
•People undergoing chemotherapy
•People with HIV/AIDS
•Transplant patients
•Children and infants
•Pregnant women and their fetuses
_
Boil-water advisory:
A boil-water advisory or boil-water order is a public health advisory or directive given by government or health authorities to consumers when a community’s drinking water is, or could be, contaminated by pathogens. A boil-water advisory (BWA) recommends that water be brought to a rolling boil for one minute before it is consumed in order to kill protozoa, bacteria and viruses. At altitudes above 2,000 meters, boiling should be extended to 3 minutes, as the lower temperature of the boiling point at high altitudes requires more time to kill such organisms. BWAs are typically issued when monitoring of water being served to consumers detects E. coli or other microbiological indicators of sewage contamination. Another reason for a BWA is a failure of distribution system integrity evidenced by a loss of system pressure. While loss of pressure does not necessarily mean the water has been contaminated, it does mean that pathogens may be able to enter the piped-water system and thus be carried to consumers.
_
Note:
Due to poor quality of drinking water supplied by governments and municipalities in the developing nations like India and Bangladesh, there is a need for everlasting boil-water advisory to people in these regions.
__________
Dissolved oxygen (DO) in water:
The dissolved oxygen (DO) is oxygen that is dissolved in water. The oxygen dissolves by diffusion from the surrounding air; aeration of water that has tumbled over falls and rapids; and as a waste product of photosynthesis. Fish and aquatic animals cannot split oxygen from water (H2O) or other oxygen-containing compound. Only green plants and some bacteria can do that through photosynthesis and similar processes. Fish and other aquatic animals use dissolved oxygen in water for survival. Numerous scientific studies suggest that 4-5 parts per million (ppm) of DO is the minimum amount that will support a large, diverse fish population. The DO level in good fishing waters generally averages about 9.0 parts per million (ppm). The DO content of drinking water should be about 5 to 7 ppm (parts per million). A high DO level in a community water supply is good because it makes drinking water taste better. However, high DO levels speed up corrosion in water pipes. For this reason, industries use water with the least possible amount of dissolved oxygen. Water used in very low pressure boilers have no more than 2.0 ppm of DO, but most boiler plant operators try to keep oxygen levels to 0.007 ppm or less.
___________
Do you know?
•The average distance that women in Africa and Asia walk to collect drinking water is 6 kilometers.
•The basic requirement of water for a lactating women engaged in even moderate physical activity is 7.5 liters a day.
•At any one time, close to half of all people in developing countries are suffering from health problems caused by poor water and sanitation.
•A survey of 5,000 schools in Senegal showed that over half had no water supply.
_
Key water facts:
| 884 million | people lack access to safe water supplies — approximately one in eight people |
| 6 kilometers | is the average distance African and Asian women walk to fetch water |
| 3.6 million | people die each year from water-related diseases |
| 98 per cent | of water-related deaths occur in the developing world |
| 84 per cent | of water-related deaths are in children ages 0–14 |
| 43 per cent | of water-related deaths are due to diarrhea |
| 65 million | People are at risk of arsenic poisoning in the Bangladesh, India and Nepal area |
_
Food and water are two basic human needs. However, global coverage figures from 2002 indicate that, of every 10 people:
a) roughly five have a connection to a piped water supply at home (in their dwelling, plot or yard);
b) three make use of some other sort of improved water supply, such as a protected well or public standpipe;
c) two are unserved;
_
How long can a person survive Without Water?
It is widely known that humans cannot survive for more than a few days without ingesting water in excess of solutes. The dangers of severe hypertonicity and volume depletion are not up for debate. Assuming you’re in reasonable shape and in ideal conditions — that is, not in the heat or cold and not exerting, a human can probably live for about 3 to 5 days without any water. Healthier humans can live another day or so longer. In an amazing show of endurance, a Japanese hiker survived for 24 days in cold weather without food and water in October 2006. He thinks he may have tripped and lost consciousness after leaving his fellow hikers. All he remembers lying in a field and falling asleep, then awaking to rescue more than three weeks later. His body temperature when he was found was an astounding 71 degrees Fahrenheit — more than 27 degrees below normal. He had virtually no pulse and his organs had shut down. Doctors believe he may have fallen into a hibernation-like state very early in his ordeal, preserving his brain function and allowing him to survive without any food or water.
____________
Water and life:
What makes scientists think that water is better at sustaining life than every other substance?
Part of the reason is that we’ve never discovered an organism that’s proven otherwise. While some organisms need less than others — the cyanobacteria Chroococcidiopsis, for instance, needs so little water that biologists think it may be able to survive on the arid surface of Mars — every organism we know of needs water to survive. In fact, without water, life on Earth would have never begun. Acting as a medium in which organic compounds could mix with one another, water facilitated the formation of the planet’s first life forms, possibly even protecting them from the sun’s radiation. From those simple starter organisms to the most complex plants and animals, water has played a critical role in survival ever since. In humans, it acts as both a solvent and a delivery mechanism, dissolving essential vitamins and nutrients from food and delivering them to cells. Our bodies also use water to flush out toxins, regulate body temperature and aid our metabolism. No wonder, then, that water makes up nearly 60 percent of our bodies or that we can’t go for more than a few days without it. If life forms that don’t require water do exist, they’d be very different than the life found on Earth. For instance, rather than being carbon-based, such life may arise from silicone compounds. A recent study even suggests that an alternative life form might be lurking in our solar system. Researchers studying Titan, a moon orbiting Saturn, noticed that hydrogen in the moon’s atmosphere wasn’t found on the surface. One explanation for the missing hydrogen is that life forms are consuming it, just as we consume oxygen. So far, however, we simply don’t have enough information to say whether or not life could exist without water. We know with certainty, however, that life on Earth definitely couldn’t. Water and life are closely linked. This has been recognized throughout history by civilizations and religions and is still the case with scientists today. Liquid water is required for life to start and for life to continue. No enzymes work in the absence of water molecules. No other liquid can replace water. It is interesting to know that water generated from metabolism of nutrients provides a significant proportion of the daily water requirements for some arthropods and desert animals, but provides only a small fraction of a human’s necessary intake.
_________
The figure below depicts availability of clean drinking water in the world:
_
Water as human right:
On 28 July 2010, through Resolution 64/292, the United Nations General Assembly explicitly recognized the human right to water & sanitation and acknowledged that clean drinking water and sanitation are essential to the realisation of all human rights. The Resolution calls upon States and international organisations to provide financial resources, help capacity-building and technology transfer to help countries, in particular developing countries, to provide safe, clean, accessible and affordable drinking water and sanitation for all. The United Nations considers universal access to clean water a basic human right, and an essential step towards improving living standards worldwide. Water-poor communities are typically economically poor as well, their residents trapped in an ongoing cycle of poverty. Education suffers when sick children miss school. Economic opportunities are routinely lost to the impacts of rampant illness and the time-consuming processes of acquiring water where it is not readily available. Children and women bear the brunt of these burdens. The World Health Organization (WHO) and various national agencies have drinking water quality standards that specify the acceptable microbial, chemical, and radiological characteristics of safe drinking water.
_
World water day:
The UN General Assembly designated March 22 as “World Water Day” back in 1992. It is a time set aside to draw attention to the largest public health issue of our time—the global scarcity of clean water. There are a variety of activities being planned around this event. Every year on that date, people worldwide participate in events and programs to raise public awareness about what many believe to be the world’s most serious health issue—unsafe and inadequate water supplies—and to promote the conservation and development of global water resources.
_____________
History of drinking water:
Drinking water is most obviously a physical resource, one of the few truly essential requirements for life. Regardless of the god you worship or the color of your skin, if you go without water for three days in an arid environment your life is in danger. And water’s physical characteristics confound easy management. Water is heavy – it is difficult to move uphill. Water is unwieldy – it cannot be packed or contained easily. And drinking water is fragile – it easily becomes contaminated and unfit for consumption. Drinking water is also a cultural resource, of religious significance in many societies. As social resource, access to water reveals much about membership in society. As political resource, the provision of water to citizens can serve important communication purposes. And finally, when scarce, water can become an economic resource.
Ancient and Indigenous Societies:
Given the critical importance of drinking water to survival, it should come as no surprise that, throughout history, human society and economies have been predicated on ready access to sources of drinking water. Archaeological excavations find early human settlements located at sites with reliable sources of drinking water nearby. The availability of water for drinking from springs, streams or lakes often meant that plants, animals and other critical goods would have been nearby, as well. Excavations from the Neolithic time have also found a striking correspondence between settlements and wells. As societies developed from hunter/gatherer economies to more advanced grazing, the need for secure, abundant supplies of water became even more important. Management of drinking water was central to urban planning in early settlements, as well. Thus one can find examples of sophisticated water management in virtually every archaeological excavation of ancient civilizations. Water storage basins with minimum storage capacities of 10,000-25,000 gallons of water have been excavated in the Mesa Verde region of the American Southwest. Large collection and storage structures have been uncovered throughout the Maya Lowlands. Though half a world away, cisterns and wells carved from the rock have been found in excavations at Ebla, in Syria, dating from 2350 B.C. Even earlier water storage sites have been found at Jawa, in north-eastern Jordan, dating from the fourth millennium B.C. Archaeologists suspect that such reservoirs were important features of town defenses, providing a secure supply of water in case of siege. The massive cisterns at Masada, high above the arid Dead Sea, proved critical to the multi-year resistance against the Romans.
Traditional Jewish Water Law:
The Old Testament is filled with references to springs and wells, their importance clearly evident from the fact that each was given a special name. Jewish law regarding drinking water has been traced as far back as 3,000 B.C. The basic rule was one of common property. As reflected in the later writings of the Talmud, “Rivers and Streams forming springs, these belong to every man.” Because water from natural sources such as springs and streams was “provided by God,” commodification of these waters would be tantamount to desecration – selling divine gifts.
Traditional Islamic Water Law:
Islamic water law is quite similar to Jewish water law in both substance and significance. Indeed, the Arabic word for Islamic law, “Sharia,” literally means the “way to water.” As the Holy Quran relates, Anyone who gives water to a living creature will be rewarded…To the man who refuses his surplus water, Allah will say: ‘Today I refuse thee my favor, just as thou refused the surplus of something that thou hadst not made thyself.’ The Right of Thirst reinforced this message. Since water is a gift from God to all people, sharing water is a holy duty.
Bihar Indian Water Law: Casteism and drinking water:
Studies of the Bihar in the northeast region of India reveal some fascinating differences in drinking water management. Because of the complex social hierarchy, priority of access and management is much more carefully proscribed than in other cultures along social caste lines. As a researcher has written, water is believed to be a medium that transmits pollution when in contact with a person who himself is in a ‘state of pollution.’ Hence, the upper and lower castes are expected to maintain distinctness of water sources as the lower castes, especially the “harijans,” are believed to have the potential of transmitting pollution by sharing sources… The group of community members who actually have ownership and/or access to a public source depends primarily upon caste and differs in accordance with their social affiliations.
Rome is the first great city defined by its management of drinking water:
Irrigation reached new heights in the Hanging Gardens of Babylon, and while the cisterns and storage basins of Mesapotamian cultures were impressive feats of engineering, they cannot compare with the graceful aqueducts that carried clean water to the great Roman cities. Aqueducts were among the most magnificent structures of the ancient world and some proudly survive today. The water fountains that continue to define the splendor of Rome were important parts of the city’s drinking water provision over 2,000 years ago. Rome is also the first major city we know of that managed drinking water as a priced resource.
__________
Are we borrowing water from the next generation?
World water use has tripled over the last half-century. Seventy percent of all the water that is withdrawn from rivers or from underground sources is used for irrigation. Twenty percent is used by industry, ten percent for residential purposes. Forty percent of our food supply now comes from irrigated land, which now plays a disproportionately large role in the world food economy. The demand for water has tripled since 1950 and is continuing to rise as we add 80 million more people each year. While the demand continues to rise, the basic amount of fresh water supply provided by the hydrological cycle does not. There are two principal signs of stress as the demand for water outruns the supply. One is rivers running dry; the other is falling water tables. With populations growing fast in water-short regions of the world, scores of countries are facing acute hydrological shortage — simply not enough water to satisfy basic human needs. Sandra Postel has attempted to calculate the size of the world water deficit — the amount of overpumping in the world. She has concluded, using data for India, China, the Middle East, North Africa, and the United States, that worldwide we are now each year overpumping by 160 billion tons of water, which equals 160 billion cubic meters. Since it takes a thousand tons of water to produce one ton of grain, a 160 billion-ton water deficit is equal to a 160-million-ton grain deficit. Stated otherwise, roughly 160 million tons of the world’s grain supply is now being produced by overpumping. Assuming a person consumes one third of a ton of grain each year, the current global average, 160 million tons of grain will feed 480 million people. This means that of the world’s current population of seven billion, we are feeding 480 million with grain produced with the unsustainable use of water. Stated otherwise, we are now beginning to feed ourselves with water that belongs to our children. We are borrowing water from the next generation.
__________
Microscope, water filtering and cholera:
The microscope has an interesting place in water filter history. Anton van Leeuwenhoek used his discovery of the microscope to see and describe the teeming life in a single drop of water. Robert Hooke, considered the English father of microscopy, confirmed Leeuwenhoek’s descriptions of tiny, living organisms in a drop of water and further refined the microscope. Soon scientists were examining tiny particles of life they had never before seen nor known existed prior to the invention of the microscope. John Snow, a British scientist, was able to link several cholera deaths to water from the Broad Street Pump, a nearby water pump that had become contaminated by a leaking sewer. Using a microscope, he was able to confirm the presence of tiny cholera bacteria in the water. As British government officials noted the effect of water quality on cholera outbreaks, both through Snow’s discovery and through the evidence of decreasing cases of cholera where sand water filters had been installed, they mandated the installation of sand water filters throughout the city. This mandate was one of the first instances of government regulation of public water and would set a precedent for municipal water systems.
________
Water and culture:
Water is probably the only natural resource to touch all aspects of human civilization – from agricultural and industrial development to the cultural and religious values embedded in society. The need and demand for water have been a driving force for health, for society, for economic prosperity, for cultural significance, and development throughout human history. Due to its fundamental role in society’s life, water has a strong cultural dimension. Without understanding and considering the cultural aspects of our water problems, no sustainable solution can be found. Cultural differences play a key role in the way water is perceived, valued and managed in different societies. World health and poverty eradication have cultural connotations; culture has positive and negative health impacts on individual well-being – in particular women’s health. Water management practices should be adapted to specific cultures as they constitute distinct systems.
________
Water and economy:
There is a nearly one-to-one correlation throughout the world between national economic output and per capita water use. The United States has the highest Gross National Product (GNP) and the highest fresh water usage in the world at approximately 2,000 cubic-m per person per year, whereas sub-Saharan Africa has the lowest GNP and water usage (approximately 100 cubic-m per person per year). The two notable exceptions to this correlation-Singapore and Israel-are important lessons for developed and developing nations alike. Both have significant freshwater resource issues and both spend a much larger percentage of their GNP for water production and water research than the United States and other developed nations.
_
Water and politics:
Water politics, sometimes called hydropolitics, is politics affected by the availability of water and water resources, a necessity for all life forms and human development. The availability of drinking water per capita is inadequate and shrinking worldwide. The causes, related to both quantity and quality, are many and varied; they include local scarcity, limited availability and population pressures, but also human activities of mass consumption, misuse, environmental degradation and water pollution, as well as climate change. Water’s essential nature makes it a strategic natural resource globally, and in its absence, an important element of political conflicts in many areas, historically. With decreasing availability and increasing demand for water, some have predicted that clean water will become the “next oil”; making countries like Canada, Chile, Norway, Colombia and Peru, with this resource in abundance, the water-rich countries in the world. World Bank Vice President Ismail Serageldin predicted, “Many of the wars of the 20th century were about oil, but wars of the 21st century will be over water unless we change the way we manage water.” This is debated by some, however, who argue that disputes over water usually are resolved by diplomacy and do not turn into wars. Another new school of thought argues that “perceived fears of losing control over shared water might contribute towards a constant preparedness to go to war among riparian nations, just in case there is one.”
_
Water conflicts:
The importance of water to life means that providing for water needs and demands will never be free of politics. Water conflict is a term describing a conflict between countries, states, or groups over an access to water resources. The United Nations recognizes that water disputes result from opposing interests of water users, public or private. A wide range of water conflicts appear throughout history, though rarely are traditional wars waged over water alone. Instead, water has historically been a source of tension and a factor in conflicts that start for other reasons. However, water conflicts arise for several reasons, including territorial disputes, a fight for resources, and strategic advantage.
_
The current categories, or types of water conflict, now include:
•Control of Water Resources (state and non-state actors): where water supplies or access to water is at the root of tensions.
•Military Tool (state actors): where water resources, or water systems themselves, are used by a nation or state as a weapon during a military action.
•Political Tool (state and non-state actors): where water resources, or water systems themselves, are used by a nation, state, or non-state actor for a political goal.
•Terrorism (non-state actors): where water resources, or water systems, are either targets or tools of violence or coercion by non-state actors.
•Military Target (state actors): where water resource systems are targets of military actions by nations or states.
•Development Disputes (state and non-state actors): where water resources or water systems are a major source of contention and dispute in the context of economic and social development.
___________
Water basics:
_
_
Dihydrogen monoxide, more commonly known as water, is all around us. Water is the chemical substance with chemical formula H2O: one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. At its most basic, water is a molecule with one oxygen atom and two hydrogen atoms, bonded together by shared electrons. It is a V-shaped polar molecule, which means that it’s charged positively near the hydrogen atoms and negatively near the oxygen atom. Water molecules are naturally attracted and stick to each other because of this polarity, forming a hydrogen bond. This hydrogen bond is the reason behind many of water’s special properties, such as the fact that it’s denser in its liquid state than in its solid state (ice floats on water). Water is the only substance that occurs naturally as a solid (ice), a liquid and a gas (water vapor). Water appears in nature in all three common states of matter (solid, liquid, and gas) and may take many different forms on Earth: water vapor and clouds in the sky, seawater in the oceans, icebergs in the polar oceans, glaciers in the mountains, fresh and salt water lakes, rivers, and aquifers in the ground. In its purest form, it’s odorless, nearly colorless and tasteless. Without it, life would be impossible. It’s in your body, the food you eat and the beverages you drink.
_
The structure of water molecules includes “polar covalent bonds” which means that electrons (hence electric charge) are unevenly distributed around the molecule; hence some parts of the molecule are relatively more “positive” and others relatively more “negative” – compared with each other. This results in some water having some useful properties:
•Cohesion – means that water molecules are attracted to other water molecules (e.g. forming droplets)
•Adhesion – means that water molecules are often attracted to other materials (though not all other materials)
•Surface Tension – means that water molecules pull towards each other at interfaces with other matter e.g. air. The result is the smallest possible surface area of water.
•Capillary Action is the ability of a liquid to flow in narrow spaces without the assistance of, and in opposition to external forces like gravity. Water is capable of capillary action due to its properties of adhesion (i.e. some water molecules are attracted to molecules of another adjacent material) and cohesion (i.e. other water molecules stay attached to and so “follow” neighbouring water molecules moving along a channel or surface due to attractions to successive non-water molecules). An example of capillary action in human biology is the drainage of constantly produced tear fluid from the eye.
_
Water is used in photosynthesis, so it is responsible for the production of glucose. This in turn is used in the synthesis of many chemicals. Water helps in the temperature regulation of many organisms. It enables the cooling down of some organisms. Owing to a high latent heat of vaporization, large amounts of body heat are needed to evaporate a small quantity of water. Organisms like humans cool down effectively but lose only a small amount of water in doing so. A relatively high level of heat is needed to raise the temperature of water by a small amount due to its high specific heat capacity. This enables organisms to control their body temperature more effectively. Water is often known as the universal solvent, which means that many substances dissolve in it. Substances that dissolve in water are hydrophilic. This means that they are as strong or stronger than water’s cohesive forces. Salt and sugar are both polar, like water, so they dissolve very well in it. Substances that do not dissolve in water are hydrophobic. This is the source of the saying “oil and water don’t mix.” Water’s solvency is why the water that we use is rarely pure; it usually has several minerals dissolved in it. The presence of these minerals is the difference between hard water and soft water. Water is a solvent for ionic compounds. A number of the essential elements required by organisms are obtained in ionic form, e.g.:
(a) plants absorb nitrate ions (NO3–) and phosphate ions (PO4–) in solution
(b) animals intake sodium ions (Na+) and chloride ions (Cl –).
_
Water is considered as nutrient just like carbohydrate, fat, protein, vitamins and minerals.
Nutritional value of tap water:
_________
Water balance in human body:
_
The figure below shows overview of water balance in human body (approximate):
_
Humans need water to live, plain and simple. We lose water through sweat, urine, feces and even breathing. This water needs to be replaced in order for our organs to continue to work properly. In severe heat, an adult can lose as much as 1.5 liters of water through sweat alone. The main risk without water in high heat is that your body temperature will continue to rise and you’ll suffer from heat stroke. Drinking water will cool you down and lower your core temperature.
Input and output of water:
Fluid can enter the body as preformed water, ingested food and drink and to a lesser extent as metabolic water which is produced as a by-product of aerobic respiration (cellular respiration) and dehydration synthesis.
Input:
A constant supply is needed to replenish the fluids lost through normal physiological activities, such as respiration, sweating and urination. Water generated from the biochemical metabolism of nutrients provides a significant proportion of the daily water requirements for some arthropods and desert animals, but provides only a small fraction of a human’s necessary intake. In the normal resting state, input of water through ingested fluids is approximately 1200 ml/day, from ingested foods 1000 ml/day and from aerobic respiration 300 ml/day, totaling 2500 ml/day.
Regulation of input:
Input of water is regulated mainly through ingested fluids, which, in turn, depends on thirst. An insufficiency of water results in an increased osmolarity in the extracellular fluid. This is sensed by osmoreceptors in the organum vasculosum of the lamina terminalis, which trigger thirst. Thirst can to some degree be voluntarily resisted, as during fluid restriction.
Output:
Fluid can leave the body in many ways.
1. The majority of fluid output occurs via the urine, approximately 1500 ml/day in the normal adult resting state.
2. Some fluid is lost through perspiration (part of the body’s temperature control mechanism) and as water vapor in expired air. These are termed “insensible fluid losses” as they cannot be easily measured. Some sources say insensible losses account for 500 to 650 ml/day of water in adults, while other sources put the minimum value at 800 ml. In children, one calculation used for insensible fluid loss is 400ml/m2 body surface area.
3. In addition, an adult loses approximately 100ml/day of fluid through feces.
4. For females, an additional 50 ml/day is lost through vaginal secretions.
These outputs are in balance with the input of ~2500 ml/day.
Regulation of output:
The body’s homeostatic control mechanisms, which maintain a constant internal environment, ensure that a balance between fluid gain and fluid loss is maintained. The hormones ADH (Anti-diuretic Hormone, also known as vasopressin) and Aldosterone play a major role in this. If the body is becoming fluid-deficient, there will be an increase in the secretion of these hormones, causing fluid to be retained by the kidneys and urine output to be reduced. Conversely, if fluid levels are excessive, secretion of these hormones is suppressed, resulting in less retention of fluid by the kidneys and a subsequent increase in the volume of urine produced.
_
Think of water as a nutrient your body needs that is present in liquids, plain water, and foods. All of these are essential daily to replace the large amounts of water lost each day. Fluid losses occur continuously, from skin evaporation, breathing, urine, and stool, and these losses must be replaced daily for good health. When your water intake does not equal your output, you can become dehydrated. Fluid losses are accentuated in warmer climates, during strenuous exercise, in high altitudes, and in older adults, whose sense of thirst may not be as sharp.
_
Daily obligatory water loss:
In the paragraph above, I discussed approximate daily input and output of water. Obligatory water loss means minimum water loss required to sustain life irrespective of environment and activity. As a corollary, the bare minimum you need to drink water daily is to compensate for obligatory water loss to maintain water balance and prevent dehydration. Water is required to replace losses which normally consist of insensible losses (from the skin and respiratory tract), urine, sweating and faecal loss. An obligatory urine loss occurs because of the need to remove various solutes from the body. Other losses (e.g. sweating and faecal losses) are quite small under normal conditions. The minimum water required for urine is dependent on the daily solute excretory load and the maximum urinary concentration achievable. For example, a typical daily solute load of 600 mOsms in a patient with a maximum urinary concentrating ability of 1200 mOsm/kg will require a minimum urine volume of 500mls/day to excrete it. If urine volume was less than this amount, solutes would accumulate and renal failure would be present. Ill or elderly patients are typically not able to achieve urine osmolality of 1200 mOsm/kg so the obligatory minimum urine volume required for solute excretion can be much higher than 500 ml.
Components of Daily Obligatory Water Loss:
•Insensible loss: 800 ml
•Minimal sweat loss: 100 ml
•Faecal loss: 100 ml
•Minimal urine volume to excrete solute load: 500 ml
•Total: 1,500 ml of obligatory water loss daily.
_
Now let us calculate how much water is generated in human body out of metabolism.
Metabolic water generation in human body:
Let me give example of conversion of Fat to Water:
Fat molecules consist primarily of long carbon chains containing hydrogen and carbon atoms in the ratio 2:1, respectively. When fat is burned in the body, its hydrogen and carbon atoms combine with oxygen to form H2O (water) and CO2 (carbon dioxide). These chemical formulas mean that two atoms of hydrogen (H2) combine with one atom of oxygen (O) to form one molecule of water (H2O) and that one atom of carbon (C) combines with two atoms of oxygen (O2) to form one molecule of carbon dioxide (CO2). Hydrogen, carbon, and oxygen atoms have relative masses of 1, 12, and 16, respectively. It follows from these facts that every pound of fat contains approximately 1/7 lb of hydrogen and 6/7 lb of carbon, and, when burned, every pound of fat combines with about 3.4 lb of oxygen to produce about 1.3 lb of water and 3.1 lb of carbon dioxide. The carbon dioxide is quickly expelled by the body through respiration but water remains.
_
Water from oxidation of calorific substrates:
| Substrate oxidation | Amount | Water produced |
| Lipids | 100 g | 107 ml |
| Carbohydrates | 100 g | 55 ml |
| Proteins | 100 g | 41 ml |
Approximately 300 ml of water is generated in human body due to metabolism of fat, protein and carbohydrates.
_
Obligatory water loss = 1500 ml
Obligatory water gain = 300 ml metabolic water generated in human body.
Net obligatory water requirement is 1500 – 300 = 1200ml.
Every adult human must drink at least 1200 ml of water.
_
Since obligatory water loss occurs from insensible water loss (respiratory tract plus skin), sweat, urine and stool; any increase in water loss from these mechanisms would increase water needs; e.g. increased sweating in hot weather or exercise.
________
Water distribution, homeostasis and absorption in human body:
Water is the most abundant constituent in the human body, accounting for 50% of body weight in women and 60% in men. At birth, the human body may be comprised of up to 75 percent water, but this percentage decreases as we age and with obesity, which can decrease the percentage of the body water to as low as 45 percent. Needless to say, water and staying hydrated is essential to the human body. Total body water is distributed in two major compartments: 55–75% is intracellular [intracellular fluid (ICF)], and 25–45% is extracellular [extracellular fluid (ECF)]. ECF is subdivided into intravascular (plasma water) and extravascular (interstitial) spaces in a ratio of 1:3. The cell membrane separates ICF from ECF. The cell membrane is highly permeable to water but impermeable to most solutes and proteins. The endothelial cells of capillary separates intravascular compartment from interstitial compartment. The pores between endothelial cells in capillary allow free movement of water and solutes but do not allow proteins to pass through. So water can travel in all 3 fluid compartments freely depending on osmolarity of the fluids. Solutes can travel only between intravascular compartment and interstitial compartment and not to ICF barring few exceptions. Vasopressin (also known as anti-diuretic hormone ADH) secretion, water ingestion, and renal water transport collaborate to maintain human body fluid osmolarity between 280 and 295 mosmol/lit. Vasopressin (AVP) is synthesized in magnocellular neurons within the hypothalamus; the distal axons of those neurons project to the posterior pituitary or neurohypophysis, from which AVP is released into the circulation. A network of central osmoreceptor neurons that includes the AVP-expressing magnocellular neurons themselves sense circulating osmolarity via nonselective, stretch-activated cation channels. These osmoreceptor neurons are activated or inhibited by modest increases and decreases in circulating osmolarity, respectively; activation leads to AVP release and thirst. AVP secretion is stimulated as systemic osmolarity increases above a threshold level of 285 mosmol/lit, above which there is a linear relationship between osmolarity and circulating AVP. Thirst and thus water ingestion also are activated at 285 mosmol/lit, beyond which there is an equivalent linear increase in the perceived intensity of thirst as a function of circulating osmolarity. Changes in blood volume and blood pressure are also direct stimuli for AVP release and thirst, albeit with a less sensitive response profile. Of perhaps greater clinical relevance to the pathophysiology of water homeostasis, ECF volume strongly modulates the relationship between circulating osmolarity and AVP release so that hypovolemia reduces the osmotic threshold and increases the slope of the response curve to osmolarity; hypervolemia has the opposite effect, increasing the osmotic threshold and reducing the slope of the response curve. The excretion or retention of electrolyte-free water by the kidney is modulated by circulating AVP. Under anti-diuretic conditions, with increased circulating AVP, the kidney reabsorbs water filtered by the glomerulus to excrete a hypertonic, concentrated urine (osmolarity of up to 1200 mosmol/lit). In the absence of circulating AVP, kidneys secrete hypotonic, dilute urine (osmolarity as low as 30–50 mosmol/lit). In a nutshell, when body has free water deficit (dehydration), plasma osmolarity rises, AVP is secreted to reduce free water clearance from kidney and thirst is stimulated to coerce human to drink water. When body has free water excess, plasma osmolarity falls, AVP disappears from plasma and kidneys excrete excess free water from body through dilute urine.
_
_
The ability of animals and humans to “meter” fluid intake is important because it prevents overhydration. After a person drinks water, 30 to 60 minutes may be required for the water to be reabsorbed and distributed throughout the body. If the thirst sensation were not temporarily relieved after drinking water, the person would continue to drink more and more, eventually leading to overhydration and excess dilution of the body fluids. Alterations in the plasma osmolality are sensed by the osmoreceptors in the hypothalamus that regulate both antidiuretic hormone (ADH) release and thirst. In addition to central osmoreceptors, peripheral osmoreceptor neurons that innervate hepatic blood vessels detect osmotic shifts in portal blood and modulate ADH release. I hypothesize that these peripheral osmoreceptors also modulate thirst so that humans do not over-drink water. Experimental studies have repeatedly shown that animals drink almost exactly the amount necessary to return plasma osmolarity and volume to normal.
_
Moles and Osmoles:
The concentration of a solute in a solution is expressed as its “Molarity”. It tells you how much of a solute is present. The units of molarity are “moles” (mols).
1 mole of anything contains 6.02 x 1023 particles of that solute. (Avogadro’s Law)
1 Gm.-Mol. Wt. = 1 mole.
1 mole of glucose = 180 grams of glucose because molecular weight of glucose is 180
1 mole of solute per liter of solution = a 1 molar solution = 1M.
To calculate the molarity of a solution, use the following equation:
Amount of solute (in grams) per liter
M = —————————————————–
Mol. Wt. of solute
So 180 grams of glucose in 1 liter of water is 1M glucose solution.
Osmolarity is a term used to describe the concentration of particles dissolved in a solution. It is a measure of density and is expressed in units of measurement known as osmoles or milliosmoles (one thousandth of an osmole), per 1000ml of solvent, or mosm/L. The more particles a beverage contains (such as carbohydrate, electrolytes, amino acids, anti-oxidants, protein or flavoring), the higher its osmolarity. Osmolarity is a concept that allows you to determine if water will move from one side of a membrane to the other side. That is: will “Osmosis” occur? Water will always move across a membrane into the solution with the higher osmolarity. An osmole is a unit of measurement that describes the number of moles of a compound that contribute to the osmotic pressure of a chemical solution. The osmole is related to osmosis and is used in reference to solution where osmotic pressure is important, such as blood and urine. The molarity of our body fluids is about 0.15 M and the osmolarity is about 0.3 Osm or 300 mOsm.
To calculate the osmolarity of a solution use the following equation:
OSM = M x the number of particles of dissociation.
For example: NaCl in water dissociates into two particles (Na+ and Cl–); and MgCl2 in water dissociates into 3 particles (Mg++ and 2 Cl–). Note that many molecules don’t give you this sort of headache, since they don’t dissociate. 1 mol of, say, lactose still only gives 1 mol of osmotically-acting particles.
A 1 mol/L NaCl solution has an osmolarity of 2 osmol/L. A mole of NaCl dissociates fully in water to yield two moles of particles: Na+ ions and Cl- ions. Each mole of NaCl becomes two osmoles in solution.
Both osmolarity and osmolality are defined in terms of osmoles.
Osmolarity:
Osmolarity is defined as the number of osmoles of solute per liter (L) of solution. It is expressed in terms of osmol/L or Osm/L.
Osmolality:
Osmolality is defined as the number of osmoles of solute per kilogram of solvent. It is expressed in terms of osmol/kg or Osm/kg.
Since 1kg of water is 1 liter of water, osmolarity is osmolality when the solvent is water. However, since human plasma specific gravity is 1.0205, osmolarity is slightly different from osmolality of plasma.
_
Note:
I have used both terms osmolarity and osmolality in this article.
The range of osmolarity of drinking water varies from 3 to 30 mosm/L with hard water having highest osmolarity and distilled water having zero osmolarity.
_
When you drink a large glass of water, the water gets absorbed into the blood and the following happens:
•The absorbed water increases the amount of water filtered in the glomerulus.
•The absorbed water in the blood reduces the Na concentration a little.
•The reduced Na concentration lowers the amount of Na filtered in the glomerulus.
•The nephron reabsorbs all of the reduced Na load and some of the accompanying water, leaving excess water in the filtrate.
•The reduced Na concentration is sensed by the osmoreceptors.
•The osmoreceptors do not secrete as much ADH (AVP).
•Because the collecting ducts don’t see as much ADH, they don’t allow much water to be reabsorbed in response to the Na concentration gradient set up by the loop of Henle.
•The excess water gets excreted in the urine.
•When the excess water is excreted, the Na concentration of the blood returns to normal.
_
How quickly is water absorbed after you drink it?
A new study by researchers at the University of Montreal, published in the European Journal of Applied Physiology, takes a very detailed look at the kinetics of water absorption and offers some answers. The study gave 36 volunteers 300 ml of ordinary bottled water, “labeled” with deuterium (an isotope of hydrogen than contains a proton and a neutron instead of just a proton) to allow the researchers to track how much of that specific gulp of water was found at different places in the body. They found that the water started showing up in the bloodstream within five minutes; half of the water was absorbed in 11-13 minutes; and it was completely absorbed in 75-120 minutes.
_______________
Daily water intake:
_
_
The amount of water varies with the individual, as it depends on the condition of the subject, the amount of physical exercise, and on the environmental temperature and humidity. In the US, the reference daily intake (RDI) for water is 3.7 liters per day (l/day) for human males older than 18, and 2.7 l/day for human females older than 18 including water contained in food, beverages, and drinking water. The common misconception that everyone should drink two liters of water per day is not supported by scientific research. Various reviews of all the scientific literature on the topic performed in 2002 and 2008 could not find any solid scientific evidence that recommended drinking eight glasses of water per day. It is also obvious that individuals in hot, dry climates have increased need for water, as do people who engage in strenuous physical exertion. For example, people in hotter climates will require greater water intake than those in cooler climates. An individual’s thirst provides a better guide for how much water they require rather than a specific, fixed number. A more flexible guideline is that a normal person should urinate 4 times per day, and the urine should be a light yellow color.
_
Anisha Patel, an assistant professor in the Division of General Pediatrics at UC San Francisco, says starting early, as a youngster, is a good way to make the water habit stick. The water requirements for adolescents are less than they are for adults, but not by much: For boys ages 4-18, the Institute of Medicine suggests 1.3 to 3.3 liters; for girls in the same age group, 1.3 to 2.3 liters. But, according to a 2011 study using data from the National Health and Nutrition Examination Survey, among adolescents, plain water accounted for only 33 percent of total fluid intake, with the remainder coming from sugary beverages, such as soda and energy drinks. That’s cause for concern, Patel says. “Even mild dehydration can cause reduced cognition and physical performance in children,” says Patel, whose research on water accessibility has helped get cleaner, fresh drinking water onto school campuses.
_
8 x 8 means what?
One glass of water = 8 ounces of water
8 glass of water = 8 x 8 ounces of water
The following quote may reflect what most authors who write on the subject have in mind: “According to most authorities, a sedentary person should drink at least eight glasses of water (∼8 oz each) per day. That totals a whopping one-half gallon of water for the average couch potato”. The second sentence makes clear that by “sedentary” the writer is thinking of persons who are physically inactive and almost certainly overweight. His use of the word “water,” plus the fact that elsewhere in the article he specifically excludes caffeinated drinks from the daily allotment [a common misperception], leaves little doubt that he means water per se. This, then, is the very minimum that 8 × 8 means to convey. The popular perception among lay people as well as doctors is that one must drink at least 8 glasses of water (8 x 8 oz) per day to stay healthy.
_
As Drs. Aaron E. Carroll and Rachel C. Vreeman reported in an article on this topic: “There’s nothing wrong with liking water, but there is no scientific proof stating that you need to drink anywhere near eight glasses a day. One doctor who has made this his research focus, Dr. Heinz Valtin, searched through many electronic databases and also consulted with nutritionists and colleagues who specialize in water balance in the body. In all of his research, and in all of the research we conducted to double-check his work, no scientific evidence could be found to suggest that you need to drink eight glasses of water a day. In fact, scientific studies suggest that you already get enough liquid from what you’re drinking and eating on a daily basis. We are not all walking around in a state of dehydration. In fact, drinking this much or more could be harmful, both in precipitating potentially dangerous hyponatremia and exposure to pollutants, and also in making many people feel guilty for not drinking enough. Other medical experts have also disdained the notion that one need drink at least eight glasses of water per day to remain adequately hydrated”. Kidney specialists also agree that the 8-by-8 rule is a gross overestimate of any required minimum. To replace daily losses of water, an average-sized adult with healthy kidneys sitting in a temperate climate needs no more than one liter of fluid, according to Jurgen Schnermann, a kidney physiologist at the National Institutes of Health. One liter is the equivalent of about four 8-ounce glasses. According to most estimates, that’s roughly the amount of water most Americans get in solid food. In short, though doctors don’t recommend it, many of us could cover our bare-minimum daily water needs without drinking anything during the day. Saying that you should drink more water than your body asks for is like saying that you should consciously breathe more often than you feel like because if a little oxygen is good for you then more must be better.
_
In a 2000 survey conducted for Rockefeller University and the International Bottled Water Association, 2,818 adults in 14 cities reported drinking about 6 cups of water a day—a result that was presented as alarming evidence that Americans are becoming dehydrated. But if you include the sodas, coffee, tea, milk, juice, sports drinks, and alcoholic beverages these respondents drank, their average fluid consumption was 17.6 cups a day—enough to have you urinating every waking hour, even if you don’t have any problem with bladder capacity.
_
Average daily balance for water in an adult human in a temperate climate, using the urinary output of medical and graduate students as determined in a teaching laboratory exercise:
| Substance | Input | Output | |||
| Dietary | Metabolic | Urinary | Fecal | Insensible | |
| Water | |||||
| as fluid | 1,220 | 300 | 1,520 | 100 | 900 |
| in food | 1,000 | ||||
| Total | 2,520 | 2,520 | |||
Values are in ml/day.
_
Almost everything you eat and drink has Water:
_
The figure below shows amount of fluids ingested by Americans including plain water and other beverages:
_
One of the biggest, most misleading beliefs is that coffee, tea, wine, milk, soda, etc. don’t play into your daily water consumption. But they do. Fluids and drinks like: sodas, coffee, tea, fruit juice, wine are made up of 85-99.9% water. Did you know that water is the largest single component of most food too? It ranges from 50-70% in meats and 75-96% in fruits and veggies. Schmidt’s Human Physiology talks about how this water content is actually broken down throughout the day: “A person weighing 70 kg [155 lbs] requires at least 1,750 ml [59 oz] water per day. Of this amount 650 ml is obtained by drinking, 750 ml is the water contained in solid food, and 350 ml is oxidation water. If more than this amount is consumed by a healthy person it is excreted by the kidneys, but in people with heart and kidney disease it may be retained…” Only 650 ml is obtained by drinking. That’s about 2.5 glasses of water.
_
Examples of the water content of some foods according to the British Nutrition Foundation:
| Food | Content |
| Apples (100g) | 84.5g |
| Grapes (100g) | 81.8g |
| Milk (1 pint) | 531.8g |
| Broccoli (85g) | 77.43g |
| Sweet corn (85g) | 59.42g |
| Tomato soup (220g) | 185.24g |
_
Coffee and beer were thought to be less hydrating than water but new research has dispelled the myth:
Contrary to the belief that caffeinated coffee is dehydrating, experts now claim that drinking it in moderate amounts is virtually as good as drinking water by itself. Dr Sophie Killer, a nutritionist at Birmingham University, said: “It is a common belief that coffee consumption can lead to dehydration and should be avoided, or reduced, in order to maintain a healthy fluid balance. We found a moderate intake of coffee, four cups per day, in regular coffee-drinking males, caused no significant difference compared to the consumption of equal amounts of water”. Dr Killer said health advice on coffee and dehydration should be updated. The study, involving an all-male group, is published in the journal PLOS ONE. Through careful experiments that passed peer review, Grandjean and colleagues have shown that caffeinated drinks (coffee, tea, and soft drinks) should indeed count toward the daily fluid intake in the vast majority of persons. Yes, beer and coffee do not dehydrate you to any noticeable extent. There’s a nice paper where some medical students got to drink quite a lot of beer and had their urine studied – British Medical Journal (Clin Res Ed), December 1982, Acute biochemical responses to moderate beer drinking, Gill GV.
_
Factors that influence water needs:
You may need to modify your total fluid intake depending on how active you are, the climate you live in, your health status, and if you’re pregnant or breast-feeding.
•Exercise:
If you exercise or engage in any activity that makes you sweat, you need to drink extra water to compensate for the fluid loss. An extra 400 to 600 ml (about 1.5 to 2.5 cups) of water should suffice for short bouts of exercise, but intense exercise lasting more than an hour (for example, running a marathon) requires more fluid intake. How much additional fluid you need depends on how much you sweat during exercise, and the duration and type of exercise. During long bouts of intense exercise, it’s best to use a sports drink that contains sodium, as this will help replace sodium lost in sweat and reduce the chances of developing hyponatremia, which can be life-threatening. Also, continue to replace fluids after you’re finished exercising.
•Environment:
Hot or humid weather can make you sweat and requires additional intake of fluid. Heated indoor air also can cause your skin to lose moisture during wintertime. Further, altitudes greater than 8,200 feet (2,500 meters) may trigger increased urination and more rapid breathing, which use up more of your fluid reserves.
•Illnesses or health conditions:
When you have fever, vomiting or diarrhea, your body loses additional fluids. In these cases, you should drink more water. In some cases, your doctor may recommend oral rehydration solutions. Also, you may need increased fluid intake if you develop certain conditions, including bladder infections or urinary tract stones. On the other hand, some conditions such as heart failure and some types of kidney, liver and adrenal diseases may impair excretion of water and even require that you limit your fluid intake.
•Pregnancy or breast-feeding:
Women who are expecting or breast-feeding need additional fluids to stay hydrated. Large amounts of fluid are used especially when nursing. The Institute of Medicine recommends that pregnant women drink 2.3 liters (about 10 cups) of fluids daily and women who breast-feed consume 3.1 liters (about 13 cups) of fluids a day.
__
Is thirst too late?
It is often stated in the lay press and even an in professional journal that by the time a person is thirsty that person is already dehydrated. In a number of scientific treatises on thirst, one finds no such assertion. On the contrary, a rise in plasma osmolality of less than 2% can elicit thirst, whereas most experts would define dehydration as beginning when a person has lost 3% or more of body weight, which translates into a rise in plasma osmolality of at least 5%. Another way of stating the same fact is that whereas the osmotic threshold for thirst is ∼294 mosmol/kg for one individual as seen in the figure below, dehydration begins when the plasma osmolality has risen to ∼302 mosmol/kg. Or, yet a third way of stating it: thirst sets in at a plasma osmolality that is still within the accepted normal range for this variable, namely, 280–296 mosmol/kg.
_
_
The figure above shows influence of plasma osmolality on the plasma vasopressin concentration (o) and on thirst (x) in a single healthy human subject. Calculated thresholds for this person are plasma osmolality of 284.7 mosmol/kg leading to a plasma vasopressin concentration of 1.48 pg/ml; and plasma osmolality of 293.5 mosmol/kg eliciting minimally detectable thirst. Figure also makes another point: inasmuch as the threshold for release of vasopressin (284.7 mosmol/kg) is lower than that for thirst (293.5 mosmol/kg), moment-to-moment needs for water balance are met by changes in plasma vasopressin concentration and consequent changes in urine flow, whereas thirst and resultant intake of water are invoked at a later point. Osmotic regulation of vasopressin secretion and thirst is so sensitive, quick, and accurate that it is hard to imagine that evolutionary development left us with a chronic water deficit that has to be compensated by forcing fluid intake.
_
Healthy people can let thirst be their guide to their fluid requirements. However, certain medications – such as those for the heart disease, stomach ulcers or depression – can alter your thirst mechanism. So can certain diseases, like diabetes insipidus. The elderly can also sometimes have a poorly-regulated thirst mechanism. Another group of individuals that may require more fluids are people who have problems with kidney stones or chronic urinary tract infections. They may to need to over-hydrate from time to time and may benefit from excess water to flush out their kidney stones or bacteria from their bladder. Meanwhile, patients on dialysis for kidney disease may have to restrict their fluid intake. Athletes, military recruits, or anyone forced to work outside during the hottest part of a summer day may require more fluids than generally recommended. And if you’re already in the throes of heat illness or heat stroke, you may have an inadequate or malfunctioning thirst mechanism.
_
Does Dark Urine mean Dehydration?
Whether or not this statement is correct will depend on how dark the urine is, because the depth of color in urine will vary inversely with the urinary volume. Although the volume varies greatly among individuals, in one study on students, the mean value was 1,520 ml/24 h, with a mean urine osmolality of 590 mosmol/kg. Both values are those generally cited as being “normal,” namely, 1,500 ml/24 h and 600 mosmol/kg, respectively. At a urine osmolality ∼600 mosmol/kg, the concentration of solutes in the urine is such that the urine has a moderately yellow color, which might be interpreted as “dark,” especially when contrasted against “pale yellow” or “clear,” which is specified in most of the lay literature. Yet, at the above-cited normal urinary volume and osmolality, the plasma osmolality will be well within the normal range and nowhere near the values of 300 mosmol/kg and higher, which are seen in meaningful dehydration. Therefore, the warning that dark urine reflects dehydration is alarmist and false in most instances.
____________
The Pressor Response to Water Drinking in Humans: A Sympathetic Reflex?
Study-1:
Water ingestion increases blood pressure in several animal species. In humans, however, research on short-term cardiovascular effects of water drinking has been neglected, and the issue is not addressed in major physiology texts. Researchers studied the effect of drinking tap water on seated blood pressure in 47 patients with severe autonomic failure (28 multiple system atrophy [MSA], 19 pure autonomic failure patients [PAF]). Eleven older controls and 8 young controls served as control group. They also studied the mechanisms that could increase blood pressure with water drinking. Systolic blood pressure increased profoundly with water drinking, reaching a maximum of 33 mm Hg in MSA and 37 in PAF mm Hg after 30 to 35 minutes. The pressor response was greater in patients with more retained sympathetic function and was almost completely abolished by trimethaphan infusion. Systolic blood pressure increased by 11 mm Hg in elderly but not in young controls. Plasma norepinephrine increased in both groups. Plasma renin activity, vasopressin, and blood volume did not change in any group. Water drinking significantly and rapidly raises sympathetic activity. Indeed, it raises plasma norepinephrine as much as such classic sympathetic stimuli as caffeine and nicotine. This effect profoundly increases blood pressure in autonomic failure patients, and this effect can be exploited to improve symptoms due to orthostatic hypotension. Water drinking also acutely raises blood pressure in older normal subjects. The pressor effect of oral water is an important yet unrecognized confounding factor in clinical studies of pressor agents and antihypertensive medications. Drinking water can provide a rapid relief of symptoms resulting from orthostatic hypotension in autonomic failure patients. This intervention is particularly useful in the morning (when orthostatic hypotension tends to be more severe) and can bridge the time required for oral medications to start working. In some patients, water drinking increases systolic blood pressure by >100 mm Hg, which can result in dangerously high blood pressure in the supine position. In these patients, water drinking should probably be avoided for ≈1.5 hours before retiring. Another important implication of this study is that oral water intake needs to be controlled in short-term pharmacological studies of pressor agents or antihypertensive medications.
_
Study-2:
Plain water has surprising impact on blood pressure:
Researchers at Vanderbilt University Medical Center have shown that ordinary water — without any additives — do more than just quench thirst. It has some other unexpected, physiological effects. It increases the activity of the sympathetic — fight or flight — nervous system, which raises alertness, blood pressure and energy expenditure. These findings prompted the American Red Cross to conduct a study of water drinking as a method for reducing fainting responses. The study found that drinking 16 ounces of water before blood donation reduced the fainting response by 20 percent. This response to water may turn out to be very important for retaining blood donors. If you pass out after giving blood, you pretty much never give blood again. If we can reduce fainting by 20 percent, we can reduce the unpleasantness of passing out and really bolster the number of people who can continue to be blood donors. Because it raises sympathetic nervous system activity — and consequently energy expenditure — it does promote weight loss. Researcher calculated it might be as much as five pounds a year if you drank three 16 ounce glasses of water a day and nothing else changed. This is not going to be the answer to the weight problem, but it’s interesting that activation of the sympathetic system is enough to do that. Moreover, another study found that metabolic rate increases almost 30% in healthy, normal-weight subjects after drinking 500 ml of water. The response is attenuated with adrenoreceptor blockade. Only one third of the increase in metabolic rate was explained by the energy demand to warm the water from 22 deg C to body temperature.
_
Study-3:
Julia McHugh tackled the questions of where water is acting, and how, in a series of studies in mice. McHugh and colleagues found that water introduced directly into the stomach or duodenum (the first part of the small intestine) raised blood pressure, which ruled out an oral or esophageal mechanism for the response. They also tested a similar volume of saline (salt-containing solution). This did not raise blood pressure, which suggested that stretch of the tissues was not part of the mechanism and that perhaps water’s lack of salt might be important. The investigators ultimately determined that water dilutes the plasma in the blood vessels leading away from the duodenum and that this short-lived reduction in salt concentration (hypo-osmolality) is responsible for water’s blood pressure-raising (pressor) effect. They implicated a protein called Trpv4 in the mechanism: mice lacking the Trpv4 gene did not have a pressor response to water. While it is clear that water evokes a pressor response, the normal role for this physiological system is not certain. McHugh said she found it fascinating that mice and humans share “such a primitive system, and yet we don’t know why it’s there or what beneficial effects it might have.” The newly discovered system and its molecular mediators — such as Trpv4 — may be targets for blood pressure regulation, particularly in situations of low blood pressure and fainting, the investigators said. The findings also suggest that investigators who use water as a control substance (a “non-drug”) in studies may need to take water’s pressor effects into account.
____________
Does drinking ice water burn calories?
Let’s figure out exactly what you’re burning when you drink one glass (8 0z) of ice water:
•The temperature of ice water can be estimated at zero degrees Celsius.
•Body temperature can be estimated at 37 degrees Celsius.
•It takes 1 calorie to raise 1 gram of water 1 degree Celsius.
•There are approximately 240 grams in one glass of water.
So in the case of one glass of ice water, your body must raise the temperature of 240 grams of water from zero to 37 degrees C. In doing so, your body burns 240 x 37 = 8880 calories. Now one Food Calorie = 1000 calories. So your body only burns 8.8 Food Calories, and in the grand scheme of a 2,000-Calorie diet, that 8.8 is not significant.
________
Why is water important for life?
1. Water is an excellent solvent:
Water is an excellent solvent. That means that many different types of materials can dissolve in water – forming solutions. Water is the solvent that transports many essential molecules and other particles around the body. These include nutrients and waste products from the body’s metabolic processes.
2. Ease of movement of water molecules through biological membranes:
Particles such as some ions and molecules need to be able to move around biological organisms. One way in which this happens is in solutions (mentioned above) e.g. transport of oxygen in blood around the vascular system. Movement of solutions within defined channels such as blood vessels and lymphatic vessels is easily explained by comparison with e.g. the movement of the fluids along pipes. Some ions and molecules in biological organisms also need to be able to move through tissues and membranes e.g. cell membranes. They move by the processes of diffusion, osmosis and active transport – of which osmosis is the diffusion of water, an important process in living organisms.
3. Water takes part in many chemical reactions:
Chemical reactions only happen when the reactants make contact with each other (sometimes via intermediary steps e.g. involving catalysts). Solutions are often good “mediums” for chemical reactions because the solvent, e.g. water, encloses solutes – which could potentially be “reactants” if there is a possibility of them reacting with each other if and when they collide – in a common volume of space, be that a test tube in a laboratory or an organ or tissue in the body. When two or more potential reactants are in the same solution they may collide and react with each other. The probability of this happening depends on several factors including the concentration of the solutes, the temperature of the solution and, in some cases, the presence (or not) of an appropriate catalyst for the reaction. Water molecules participate in decomposition reactions whereby certain macromolecules are broken-down into smaller parts. Examples include the breakdown of carbohydrates and proteins during the digestive process. Water is also produced by chemical reactions occurring within the body in which relatively small organic compounds (called “monomers”) join together in “synthesis reactions” to form larger and more complex molecules called “macromolecules” required by the body for specific functions e.g. nucleic acids and hormones.
4. Water can act as a lubricant, i.e. to reduce friction between moving surfaces:
Water (incl. solutions of which water is the solvent) serves an important lubrication function. This is essential in many parts of the body, especially:
•in the thoracic and abdominal cavities where internal organs (e.g. the heart and lungs, and the organs of the digestive system) are located next to each other and slide over one another as the body moves around).
•at joints e.g. synovial joints where structures such as bones, ligaments and tendons need to move smoothly relative to each other without being impeded by friction between the different structures/surfaces.
5. The thermal properties of water are well-suited to support life:
Water has a high specific heat. The specific heat of a substance is the quantity of heat per unit mass needed to increase the temperature of the substance by one degree Celsius. More energy is needed to increase the temperature of water compared with that of other solvents because hydrogen bonds hold the water molecules together. The specific heat of water is 4.18 J/g° C. This is much higher than for many other substances e.g. NaCl 0.864 J/g°C, Fe 0.450 J/g°C and Cu 0.385 J/g°C.
The thermal properties of water that affect human and animal biology include:
•Compared with other materials water can absorb or release a relatively large amount of heat energy while only adjusting its own temperature by a relatively small amount. Therefore the fact that water accounts for a significant proportion of body mass helps the body to cope with environmental temperature variations and maintain the body’s temperature within a safe and comfortable range.
•Similarly, compared with other materials water also needs a relatively large amount of heat energy in order to evaporate (i.e. change state from liquid to gas). This is called the “latent heat of evaporation” and the value for water is approx. 2270 kJ/kg at 1 atm pressure.
Therefore the evaporation of sweat from the surface of the skin is very efficient in helping to cool the body because it removes relatively large amounts of heat from the body as the sweat evaporates.
6. Other biologically useful properties of water include its cohesion, adhesion and surface tension.
__
Functions of Water in the Body:
Did you know that your tissues and organs are mainly made up of water?
•Muscle consists of 75% water
•Brain consists of 90% of water
•Bone consists of 22% of water
•Blood consists of 83% water
_
_
The functions of water in human body are vital. Water flows through the blood, carrying oxygen and nutrients to cells and flushing wastes out of our bodies. It cushions our joints and soft tissues. Without water as a routine part of our intake, we cannot digest or absorb food.
The water:
•Transports nutrients and oxygen into cells
•Moisturizes the air in lungs
•Helps with metabolism
•Protects our vital organ
•Helps our organs to absorb nutrients better
•Regulates body temperature
•Detoxifies
•Protects and moisturizes our joints
_
Benefits of water:
___________
Dehydration:
The loss of the body water component of body fluid is specifically termed dehydration.
Signs and Symptoms of dehydration:
Tiredness & fatigue
Constipation
Muscle cramps
Thirst
Dry mouth
Dark urine
Dry skin
Sunken eye balls
Rapid heat beat
Low blood pressure
Kidney problems
20% dehydrated – Risk of death
_
Indicators of dehydration as per loss of body weight:
•Normal: no loss of body weight.
•Mild dehydration: 5-6% loss of body weight.
•Moderate: 7-10% loss of body weight.
•Severe: over 10% loss of body weight.
_
Hydration is a crucial part of life itself, and water losses of a more than two percent of your body weight can impair function both mentally and physically. Losses of seven percent or more may bring you down for the count, disrupting your delicate balance and resulting in total body collapse. Dehydrate a muscle by just 3% and you will cause a loss of about 10% loss of contractile strength, and an 8% loss in speed.
_
At what dehydration level will the sweat process for the human body stop during hot climate?
The human body will keep sweating no matter how dehydrated it is as long as the hypothalamus sends nerve impulses to the sweat glands. However, for most people the sweating process begins to slow down after the body loses from 3 to 5 percent of total weight in sweat, and core body temperature approaches 104 degrees Fahrenheit. If our core temperature goes above 104 degrees, the body begins to overheat to the point to where its proteins denature and membranes lose their integrity. We usually lose consciousness and go into a coma just before this point and stop perspiring and will soon go into shock and die if our core temperature is not reduced (heat stroke).
_
Does Ramadan fasting without drinking water cause dehydration and its consequences?
During the 9th month (Ramadan) of the Islamic calendar (Hijra) many millions of adult Muslims all over the world fast during the daylight hours. Since Hijra is a lunar calendar, Ramadan occurs at different times in the seasonal year over a 33-year cycle. Fasting during Ramadan is partial because the abstention from food, fluid, tobacco and caffeine is from sunrise to sunset.
Intermittent dehydration:
During the daylight hours of Ramadan fasting, practicing Muslims are undoubtedly dehydrating at a rate that is determined by the loss of body water minus the amount of metabolic water that is produced over this period. Some studies find that incidences of dehydration increase during the month of Ramadan: Evidence of hemoconcentration and dehydration has been found during Ramadan (El-Hazmi, Al-Faleh, & Al-Mofleh, 1987; Kayikcioglu et al., 1999; Ramadan et al., 1999; Schmahl & Metzler, 1991; Sweileh et al., 1992). Restricted fluid intake, leading to disturbance in the fluid balance, is likely to cause these conditions. In the initial stages of dehydration, the clinical signs are tachycardia, tiredness and malaise, headaches and nausea. Middle-aged or more elderly persons are usually more prone to the effects of dehydration (Schmahl & Metzler). Other studies found that during Ramadan, the osmolality of the urine samples collected in the afternoon were very high (means: 849–937 mosm/kg), indicating effective water conservation (Shirreffs, 2003) both by maximum urinary concentration and a decreased obligatory urine output. Such high urine osmolality may increase kidney stone formation. Urine volume plays a pivotal role in the process of stone formation. In particular, low volume, highly concentrated urine contributes to the supersaturation of elements normally found in the urine, such as calcium oxalate. Several alternatives have been used to give estimates of hydration status of individuals (Shirreffs, 2003). In 12 Muslims fasting for 12–14 h, there was a significant increase in haematocrit (+11%), serum albumin (+4%) and serum creatinine (+12%), indicating dehydration due to water deprivation (Born et al, 1979). Similar findings were observed in 15 fasting Tunisian Muslims, who also showed an average increase in serum urea of 23% (Zebidi et al, 1990), and in a group of fasting British Muslims who also demonstrated increases in serum sodium and chloride (Sweileh et al, 1992). So in a nutshell; Ramadan fasting does lead to dehydration and its consequences. However, since it is intermittent dehydration, the adverse effects are limited.
__________
Does excess water intake help remove toxins via kidneys:
The notion is that increased water intake improves kidney function and clearance of toxins. The kidney manifests several mechanisms to rid the body of toxins, including glomerular filtration, tubular secretion, and various degradative metabolic pathways. If excess water intake were to have an impact on toxin removal, then it would be through one of these mechanisms. Water ingestion can acutely affect GFR, although not necessarily in the direction one might expect. Using 12 young, healthy individuals as their own controls, Anastasio et al. found increased water intake actually decreases GFR. It might therefore seem that any “toxin” removed purely by glomerular filtration is cleared less efficiently in the setting of increased water intake; however, it is not certain such changes in GFR persist over time. Indeed, GFR was unchanged during a 6-month randomized trial of increased water intake in older men who had benign prostatic hypertrophy. Of course, the populations in the two studies are different, and the main goal of the randomized trial was to evaluate bladder function rather than kidney function; as an aside, the study did show some improvement in bladder function, although the clinical significance of the findings is unclear.
Does High Fluid intake maintains Glomerular Filtration Rate?
This statement, when given in the context of 8 × 8, implies that fluid intakes lower than 8 × 8 diminish the glomerular filtration rate (GFR). The opposite effects of the state of hydration on GFR were demonstrated recently in carefully controlled experiments on healthy young human subjects. Furthermore, years ago McCance and coworkers showed that the GFR (as measured by the clearance of inulin) declines only during significant dehydration, for example, when body weight declines by 5% or more. Certainly, the acute water diuresis that follows the ingestion of 1 liter of water can be accounted for by an inhibition of vasopressin secretion and decreased tubular reabsorption of water, without a measurable change in GFR, or possibly even with a decrease in GFR.
So high water intake does not increase GFR.
Of course, most endogenous substances are not cleared purely by glomerular filtration alone. Anastasio et al. found the total clearance of osmoles increased as water intake increased, probably as a result of reduced reabsorption. If there are “dangerous” substances among these osmoles, then increased water intake might indeed help in their clearance. Interestingly, one of the osmoles whose clearance was increased was sodium. Given the suspected role of long-term sodium retention in the development of hypertension, one could speculate that increased clearance of sodium is beneficial. However, as discussed earlier, excess water intake stimulates sympathetic nervous system that promotes sodium retention via aldosterone stimulation. Urea clearance also increases with high water intake, but urea is not a toxin. It is unclear whether any of these changes persist in the long term. In short, increased water intake does have some impact on renal clearance of various substances, but current data are insufficient to assess the clinical significance of these observations. In fact, given how little is known about the identity of toxic substances cleared by the kidney, it is unlikely this type of data can conclusively demonstrate a benefit from excess water drinking.
_
Does Water relieve headache:
Headache is frequently attributed by the lay public to water deprivation, but there is little study of this phenomenon. To my knowledge, only one trial has examined headache prevention by increasing water intake. Fifteen patients with migraine headaches were randomly assigned to increased water intake or placebo for 12 wk. The number of hours of headache was quantified over 14-d intervals at the beginning and at the end of the trial. Although the treatment group had 21 fewer hours of headache compared with the control group, this difference did not reach statistical significance (the number of patients was obviously quite small). Given the economic impact of migraine on time lost from work, this area would seem to be ripe for further study.
_
Does Water benefit skin:
A frequently cited cosmetic benefit of water drinking is improved skin tone. Although frank dehydration can obviously decrease skin turgor, it is not clear what benefit drinking extra water has for skin. One study suggested ingestion of 500 ml of water increases indices of capillary blood flow in the skin. It is unclear whether these changes are clinically significant or how to interpret them in light of water’s potential impact on sympathetic tone. I am unable to find any other data regarding the impact of water intake on skin in otherwise healthy people. As far as hydrating your skin is concerned, you’d probably be better off applying moisturizer to your skin while you’re still damp from the shower. This helps lock in the moisture your skin soaked up while you were bathing.
____________
Rationale for high water intake:
The arguments for a high water intake in the lay press go something like this: our bodies consist mostly of water (50–70% of body weight; ∼42 liters) and our blood, muscles, brain, and bone are made up mainly of water (∼83%, 75%, 90%, and 22%, respectively). Therefore, 1) we need water to function and survive and 2) we need at least eight glasses of water each day. The second conclusion, in addition to being unproven, is a nonsequitur; it is akin to arguing that our homes run on electricity and therefore, every house needs at least 1000-ampere service.
_
Drink three liters of water a day or risk kidney stones warns experts as hospital admissions for renal conditions rise:
Thousands of new cases of kidney stones every year are caused by ignorance or denial of the need to drink three liters of water a day, according to a leading doctor. Doctors say that a lack of awareness about the dangers of dehydration was responsible for an annual increase in renal stone admissions, including among young people in their twenties. The number of people admitted to hospital suffering severe pain and discomfort due to kidney stones is increasing by between 5 per cent and 10 per cent every year. The British population’s ignorance of the need to drink three liters of water a day is leading to more cases of kidney stones, says another leading doctor. Over the past decade, the number of hospital admissions for renal stones in the UK rose by 63 per cent to more than 80,000 and there is no sign of these numbers letting up. Kidney stones develop when crystals of salt gather into lumps and are not flushed out of the body due to a lack of adequate hydration, often lodging in the urinary system’s tubes. They can cause severe abdominal and groin pain which, in many cases, can only be corrected through surgery or laser lithotripsy. I disagree with leading urologists. Yes, chronic dehydration would cause kidney stones in susceptible individuals and patients suffering from kidney stones must increase water intake; and one must be hydrated, but to say that normal people must drink 3 liters of water to prevent kidney stone means we all need 3 liters of water for adequate hydration. Except for hot climate or strenuous physical activities, nobody needs 3 liters of drinking water daily to stay hydrated. It is over-hydration.
_
Factors that promote calcium oxalate supersaturation (and calcium oxalate deposition) are dehydration, hypercalciuria, hyperoxaluria, hypernatrituria, and hyperuricosuria. Urinary citrate is an important inhibitor of calcium oxalate formation so hypocitraturia is a risk factor for stone formation. If these factors are demonstrated in normal person without kidney stone, then prophylactic high water intake to produce 2 liters of urine is warranted to prevent future kidney stones. Hypercalciuria, or excessive urinary calcium excretion, occurs in about 5-10% of the population and only that segment of population would benefit from high water intake. The rest must be well hydrated.
_
Prevention of Cancer, Heart Disease, and Other Conditions:
In a 10-year study involving nearly 48,000 men, Michaud and coworkers found that the incidence of cancer of the urinary bladder was reduced significantly by a high fluid intake. The top 20% of subjects who participated in the study drank 2,531 ml per day or more, while the bottom 20% drank 1,290 ml or less; the authors calculated that within this range, the risk of bladder cancer decreased by 7% for every 240 ml (∼1 cup or one 8-oz glass;) of fluid added. There was a significant decrease in risk even in men who drank only 1,440 ml (∼6 glasses), i.e., well below the 8 × 8 recommendation. Not everyone, however, agrees with this benefit of a high fluid intake, especially in women. A similar correlation has been reported for colorectal cancer and premalignant adenomatous polyps. Taking account of the many known risk factors for these tumors, these multivariate studies found significant, inverse correlations between the total intake of fluids, or specifically of water, and the risk of colorectal cancer as reflected in the incidence of adenomatous polyps. In some instances, the beneficial effects were apparent with as little as five glasses of water a day. As with cancers of the urinary bladder, there may be gender-related differences.
_
Chan and associates carefully analyzed the possible association between water intake and fatal coronary heart disease in 12,017 women and 8,280 men who participated in the prospective Adventist Health Study. They found, at a 6-year follow-up point, that women who drank five or more glasses of water per day (1,185 ml or more) reduced their risk of fatal coronary heart disease by ∼41% compared with women who drank two glasses or less (474 ml or less). The comparable figure in men was 54% less risk. The effect was limited to water; in fact, the drinking of “fluids other than water” (coffee, tea, juices, soft drinks) appeared to increase the risk of fatal coronary heart disease. In their very cautious analysis of these findings, the authors point out that the correlations are not necessarily causal (although they may involve the effect of hydration on hemorheological variables such as blood viscosity).
_
Look ten years Younger by Drinking Water – One Woman’s Story with Before & After:
Skin is an organ; in fact, it is our largest organ and is made up of many cells. And skin cells, like any other cell in the body, are made up of water. Without water, the organs will certainly not function properly or at their best. If your skin is not getting the sufficient amount of water, the lack of hydration will present itself by turning your skin dry, tight and flaky. Dry skin has less resilience and is more prone to wrinkling. The proof is in the pudding, or whatever that saying means. Maybe we can start to believe those actresses who say they just drink water to look beautiful. Sarah Smith, who wrote about her results over the course of a month for the Daily Mail, decided to up her water intake to see if it would help her migraines, and started to discover a happy side effect of all the water drinking. She wrote that her “skin completely changed and she looks 10 years younger,” since she increased her water intake from three glasses per day to 3 liters. After 4 weeks, her wrinkles, redness, dark circles and her tired looking face completely disappeared. She started to see glowing, even, non-puffy skin that made her look 10-years-younger. What’s even better, Smith reports that her digestion, energy levels, and cognition improved, and that she even lost weight in the process.
Note: This is an anecdotal report and anecdotal report is not science.
_
Does drinking water clear Acne?
The short answer is no.
It is easy to rationalize that drinking water will reduce or clear acne breakouts but for all the scientific studies done to find such link, it has not been found. Therefore, the common advice to drink eight glasses of water daily in order to keep your face clear of acne is only backed by wishful thinking. The reasoning behind the popular belief that drinking water helps clear acne is due to the role of water as a detoxifier and the fact that the causes of acne are skin-deep. The two assumptions are right to an extent but do not add up neatly. Water is definitely needed for healthy skin and it cannot be replaced by beverages, sodas and other high caloric, high glycemic drinks. These drinks only increase the level of toxins present in the body, and some of them may even contribute to acne breakouts by indirectly triggering acne-causing hormones such as insulin-like growth factor 1 (IGF-1). In contrast, besides all the benefits that drinking water provides, it will not stimulate the production of any acne-causing hormone or increase the toxin load of them body. Water may only help clear acne because it becomes the fluid replacement for these drinks. On its own, it does not affect acne one way or the other. Some people also believe that increased water intake helps prevent the dehydration of the skin. This is only partly true. For those concerned by dry skin, oil-free moisturizers provide a better hydration of the skin than water.
_
Drinking water boosts your brain’s reaction time:
We all know that drinking water regularly is good for the body. But new research has revealed that drinking water when we feel thirsty boosts our brain’s performance in mental tests. Researchers from the University of East London and the University of Westminster in the UK analyzed the potential effects of water on cognitive performance and mood among 34 participants with an average age of 29 years. The study, published in the journal Frontiers in Human Neuroscience, involved participants taking part in a “water” and a “no water” experiment one week apart. The “water” experiment required the people to complete a number of mental tests after eating a cereal bar and drinking some water. The “no water” test meant the participants consumed just the cereal bar alone. The amount of water drunk by the participants in the “water” test depended on their level of thirst. Lead study author, Dr. Caroline Edmonds of the University of East London School of Psychology says, “Our study found that reaction times were faster after people drank water, particularly if they were thirsty before drinking.” Well, in my view, thirst is a biological survival desire just like hunger and sex; when your biological survival desire is satisfied, your stress is reduced, you feel relaxed and as a consequence your mental performance increases. It has nothing to do with water drinking. Dehydration of more than 2 % body weight would certainly impair mental ability as brain itself is 90 % water and dehydration would reduce brain water volume, and reduce blood flow to brain due to reduce circulatory blood volume and reduced blood pressure.
_
Other Claimed Benefits:
Losing weight:
There is some evidence, in both women and men, that water drunk along with a meal or water incorporated into food does promote satiety. By and large, it is not yet clear to what extent this effect reduces food intake, how long the effect lasts, and how much fluid might be needed to influence satiety. In one study, Rolls and her colleagues reported the intriguing finding that water incorporated into food, as in chicken soup, appears to be more effective as a “preload” in curtailing appetite during a subsequent meal than if the same amount of water was drunk during the preload alongside the same food, in this case chicken casserole. The intake of food ingredients and of water was identical in the experimental periods, only the mode of ingesting the water was different. An analysis by Stookey supports this concept.
_
Drink two glasses before every meal to reduce weight:
Dieters who down two glasses of water before each meal shed more pounds than those who only count calories, a study shows. While prescription-only weight-loss drugs cut the body’s ability to absorb fat or tinker with the brain’s chemistry, water simply fills up the stomach. If that is not enough, water is also, of course, calorie-free and readily available. The American researchers compared weight loss among dieters who drank just under a pint of water before each meal with those who simply watched what they ate. Over three months, the water drinkers each lost an average of 15.5lb, 5lb more than the non-water drinkers. Researcher Dr Brenda Davy, of the Virginia Polytechnic Institute and State University, said: ‘People should drink more water and less sugary, high-calories drinks. It’s a simple way to facilitate weight management. We’re not saying, “Drink more water and the body fat will melt away”. But for people who are trying to lose weight and trying to follow a low-cal diet, it’s something they can do as part of that.’ In a previous study, she found that water drinkers ate 75 to 90 fewer calories per meal. Over the course of a day, this could amount to almost 300 calories – the equivalent of a Danish pastry or a pint and a half of beer. Water’s secret is perfectly simple, added Dr Davy. If the stomach is full with water, people feel fuller and so eat less. But the British Nutrition Foundation says that soups, stews, pasta and other water-rich foods are better for the waistline than water alone. Mike Lean, an obesity expert, said: ‘I routinely advise patients to have a glass of water before each meal if they are planning to cut down with the aim of losing weight. It doesn’t have huge effect, but costs nothing.’ Fruit, vegetables and porridge also help fill the tummy with fewer calories, said the Glasgow University professor.
_
Impact of water intake on energy intake and weight status: a systematic review:
The effects of consuming water with meals rather than drinking no beverage or various other beverages remain under-studied. A systematic review of studies reported in the English-language literature was performed to compare the effects of drinking water and various beverage alternatives on energy intake and/or weight status. Relevant clinical trials, epidemiologic studies, and intervention studies were identified and findings across the literature were summarized. From the clinical trials, average differences were calculated in total energy intake at test meals (ΔTEI) for each of several beverage categories in comparison with water. The available literature for these comparisons is sparse and somewhat inconclusive. However, one of the most consistent sets of findings was related to adults drinking sugar-sweetened beverages (SSBs) versus water before a single meal. In these comparisons, total energy intakes were 7.8% higher (ΔTEI range, −7.5 to 18.9) when SSBs were consumed. Studies comparing non-nutritive sweeteners with water were also relatively consistent and found no impact on energy intake among adults (ΔTEI, −1.3; range, −9 to 13.8). Much less conclusive evidence was found in studies replacing water with milk and juice, with estimated increases in TEI of 14.9% (range, 10.9 to 23.9%). These findings from clinical trials, along with those from epidemiologic and intervention studies, suggest water has a potentially important role to play in reducing energy intake, and consequently in obesity prevention. A need for randomized-controlled trials to confirm this role exists.
_
The apparent weight loss effects of water are still a subject for further research, but there is some evidence that suggests that drinking water can be associated with appetite reduction (for middle-aged and older people), consuming fewer calories, burning slightly more calories, and eating more fruits and vegetables. Increased water consumption, or replacement of energy-containing beverages with energy-free beverages, or consumption of water-rich foods such as fruits and vegetables with a lower energy density, may help in weight management. In the case of appetite reduction, the apparent effect has been reproduced in a published study in adults aged 55–75, half of whom were instructed to drink 500ml of water before every meal, while following a low-calorie diet. This behaviour led to the water-drinking cohort losing weight faster over a 12 week period. On average, the water-drinking cohort also continued to lose weight – although at a slower rate – over the following 12 month period, even though they had ceased their low-calorie diets. The study authors attribute this to the fact that those participants continued to drink water before meals.
_
Constipation:
The notion that a high fluid intake will facilitate bowel movements was tested by Chung et al. They found, in 15 healthy adults of both genders, that although an extra intake of 1 or 2 liters of either Gatorade or plain water significantly increased urine flow, there was no discernible effect on the output of stool. The authors warn that their results were obtained in healthy adults who did not complain of constipation, and that, therefore, the possibility remains that a high fluid intake might help relieve constipation in those who have it. However, inasmuch as the intestines have a large capacity for absorbing extra ingested water, the efficacy of a high fluid intake in relieving constipation needs to be proven by well-controlled scientific experiments.
_
In Fiber Menace, Konstantin talks about three false beliefs in regard to water, fiber, and constipation:
1. Because fiber absorbs water (true), it will increase stool moisture. Wrong! Dietary fiber in stools doesn’t retain water any better than other cellular components, except psyllium seeds in laxatives(a mere 5% more).
2. Because fiber is so highly water-absorbent (true), it requires additional water. Wrong for two reasons! First, up to 75% of fiber, including insoluble fiber, gets fermented by intestinal bacteria and doesn’t require any water. Second, the remaining fiber gets all the water it needs from up to seven liters of digestive juices, which are secreted daily inside the alimentary canal.
3. Water is needed to prevent intestinal obstructions from dietary fiber: Wrong! Water, actually, expands the fiber four to five times its original volume and weight, and if anything makes obstruction even more likely. He also goes on to state that “Dried out, hard stool, which is one of the symptoms of disbacteriosis, doesn’t point to dehydration (a mistaken view), but to the lack of synergistic bacteria needed to retain water.”
So what does control and fix constipation?
Meal composition (not volume and not fiber) influences motility more than any other factor. Motility is influenced by the energy content and composition of the meal, but not by its volume or pH. Energy-rich meals with a high fat content increase motility; carbohydrates and proteins have no effect. If you’re constipated, rather than drinking more water, you’re better off stimulating a strong urge but creating a daily habit of going to the bathroom by eating a fat-rich meal, or drinking a warm beverage at the same time every day and just relaxing.
So water’s role in constipation? Hyped up.
_
Speculative Advantages of high water intake:
Bankir and her group performed careful experiments, both in animals and humans and assembled supporting evidence from the literature that suggests that chronically high plasma vasopressin concentrations may have deleterious effects (the extrapolation being that a high fluid intake and consequent low vasopressin will prevent those effects). The primary findings are that 1) sustained high concentrations of vasopressin increase glomerular filtration rate (GFR), probably through tubuloglomerular feedback (TGF) and 2) low urinary flow rates reduce sodium excretion, possibly through vasopressin-mediated upregulation of sodium channels (ENaC) and Na-K-ATPase. The possible deleterious effects from these changes are 1) hyperfiltration causing acceleration of chronic renal failure and 2) increased sodium retention hastening the development of salt-sensitive hypertension, consequences that might be prevented by a high fluid intake. Of course here I am examining possible advantages of a high fluid intake in healthy individuals, not in persons with chronic renal failure or hypertension. Insofar, however, as a high fluid intake might influence the decrease in GFR that accompanies normal aging or prevent the development of hypertension, it seems fair to mention these two consequences at least as speculations. Not to mention that high water intake stimulates sympathetic nervous system resulting in increased peripheral resistance and tendency to raise blood pressure rather than lower it.
_
The list of advantages of a high fluid intake goes on. Benefits are claimed for fatigue, arthritis, lack of mental alertness, angina, migraine, hypertension, asthma, dry cough, dry skin, acne, nosebleed, depression. One amusing website where many of these claims are refuted is Snopes.com, although the authors rely mostly on quotes from scientists (albeit, very reputable ones) and newspapers rather than on scientific articles.
_________
Possible hazards of high water intake:
Thus far the evidence for forcing a high fluid intake on healthy adults in a temperate climate seems weak, at best. We may need further data, including genomic evidence for susceptibility, before recommending 8 × 8 universally even for the prevention of diseases, such as certain types of cancer or renal stones. But despite the dearth of compelling evidence for 8 × 8, many persons are likely to retort, “But what harm would it do?” The fact is that, potentially, there is harm even in water.
Water Intoxication:
On January 12, 2007, a 28-year old Californian wife and mother of three children died from drinking too much water. Her body was found in her home shortly after she took part in a water-drinking contest that was sponsored by a local radio show. Entitled “Hold Your Wee For A Wii,” the contest promoters promised a free Wii video game machine to the contestant who drank the most water without urinating. It is estimated that the woman who died drank approximately 2 gallons of water during the contest. When she and other contestants complained of discomfort and showed visible signs of distress, they were laughed at by the promoters and even heckled. This tragic news story highlights the importance of understanding why drinking too much water can be dangerous to your health.
_
Water intoxication (WI), caused by over-consumption of water, can be lethal. This condition is usually seen in patients with psychiatric disorders, victims of child abuse or torture, drug abusers or it can be iatrogenically induced (result from a physician’s words or actions). Water intoxication, also known as water poisoning or dilutional hyponatremia, is a potentially fatal disturbance in brain functions that results when the normal balance of electrolytes in the body is pushed outside safe limits by over-hydration. Even modest increases in fluid intake can result in severe water intoxication if the renal excretion of water is limited by a sustained influence of the antidiuretic hormone (ADH), either endogenous or exogenous, on the kidney. This serious eventuality occurred recently in a young woman with neurogenic (central or pituitary) diabetes insipidus. For many years she had been treated satisfactorily with DDAVP, a synthetic analog of the natural ADH arginine vasopressin. During this long period of treatment, she did not have any known episodes of hyponatremia or water intoxication because her water intake was regulated appropriately by the thirst mechanism. However, when she developed a minor upper respiratory infection and was advised to drink lots of fluids, her kidneys could not excrete sufficient quantities of urine because they were under the sustained antidiuretic influence of the DDAVP. Tragically, she rapidly developed severe water intoxication from which she died. Here, then, is a most unfortunate example of how a simple folk remedy that is usually innocuous, namely, to “force fluids” in treating flulike symptoms, could not be tolerated under special circumstances.
_
When a person dies from hyponatremia as a result of water intoxication, the initiating factor is a severe sodium imbalance that causes massive cell damage. Sodium is a positively charged ion, and its role in the body is to circulate the fluids outside of cells. As a result, sodium helps regulate blood pressure and maintain the signals that let muscles operate properly, among other things. Cells actively maintain a precise sodium concentration in the body. Inside the cell, there are more electrolytes; outside the cell, there is more water. Cells keep sodium levels healthy by moving water and electrolytes into and out of the cell to either dilute or increase sodium levels in body fluids. But when someone drinks a tremendous amount of water in a short period of time, and the water does not contain any added electrolytes, the cellular maintenance system can’t handle the level of sodium dilution that occurs. The result is that cells desperately try to increase the sodium concentration in body fluids by taking in tremendous amounts of water. Some cells can swell a great deal; others cannot. Brain cells are constrained by the skull and can end up bursting with the pressure of the water they are taking in. The exact amount of water intake that can lead to water intoxication is unknown and varies with each individual. Symptoms of water intoxication actually look a lot like the symptoms of alcohol intoxication, including nausea, altered mental state, and vomiting. Other symptoms include headaches, muscle weakness and convulsions. In severe cases of water intoxication, coma and death come fairly quickly as a result of brain swelling. The condition is quite rare in the general population, but in distance athletics, it’s a known risk and is often avoided by drinking sports drinks instead of water during training and events.
_
Watered-down body fluid can also manifest in minor, more subtle and undetectable ways. You should suspect that you may be taking in far too much water – from all fluids, not just water, including even the fluids found in water-rich fruits, vegetables, and the like, if you frequently experience…
• Cold hands and feet
• Low body temperature
• Frequent urination, clear urination, or urination at night
• Headaches or migraines
• Anxiety or panic attacks
• Dry skin
• Blurred vision, mood changes, and other symptoms that many falsely believe to be “hypoglycemia”
• Heart palpitations or otherwise abnormal heart rhythms
• Strong cravings for salty foods
• Low blood pressure, dizzy spells, or episodes of blurred vision
While water is by all standards pure and natural, and certainly a better beverage choice than soft drinks and other sources of empty calories, you can definitely overdo it. Be very cautious about making the two most common mistakes – drinking when you are not thirsty as many “experts” advocate, and drinking for motivations other than thirst, as in drinking a warm drink to get warm or alcohol recreationally. Think before you drink.
_
Treatment of water intoxication:
Mild intoxication may remain asymptomatic and require only fluid restriction. In more severe cases, treatment consists of:
1. Diuretics to increase urination, which are most effective for excess blood volume.
2. Vasopressin receptor antagonists
_
Water Intoxication in Infants:
For healthy adults, nothing seems to quench a thirst better than plain, pure water. We’re encouraged to drink several glasses a day to keep our systems in balance. But for children under 1 year old – and especially during the first nine months of life – drinking too much water can be dangerous. In fact, according to pediatricians like James P. Keating, MD, retired medical director of the St. Louis Children’s Hospital Diagnostic Center, too much water dilutes a baby’s normal sodium levels and can lead to seizures, coma, brain damage and death. Breast milk or formula provides all the fluid healthy babies need. If a mother feels her baby needs to take additional water, it should be limited to two to three ounces at a time and should be offered only after the baby has satisfied his hunger with breast feeding or formula. Dr. Keating also recommends that parents avoid participating in infant swimming lessons. “Repeated dunking of infants can cause them to gulp water and has caused seizures in the infants at the poolside,” he says. Since the brain is the organ most susceptible to water intoxication, a change of behavior is usually the first symptom in older children. They may become confused, drowsy or inattentive. They also may suffer from blurred vision, muscle cramps and twitching, poor coordination, nausea and vomiting, irregular breathing and weakness. If you notice any of these symptoms, call your pediatrician.
_
Ecstasy and water intoxication:
A fairly new recreational drug, especially among teenagers, is called Ecstasy. It is used extensively at dances, called “raves,” but is now being taken in other settings as well. One striking side effect of Ecstasy is intense thirst, and there is a report of death of a 16-year-old girl who drank herself into fatal hyponatremia (water intoxication) after her first ingestion of Ecstasy. The many euphoric effects of Ecstasy may have caused secretion of endogenous vasopressin, which prevented this girl from excreting the copious amounts of water she drank, for it is difficult or impossible for individuals to drink themselves into severe hyponatremia without a simultaneous, sustained antidiuretic influence on their kidneys. Be that as it may, Ecstasy is a dangerous drug, although most teenagers do not seem to know or accept that fact. Furthermore, the use of Ecstasy is increasing, as are the resulting visits to hospital emergency rooms, and the drug caused at least 15 deaths during the year 2000.
_
Whenever you disregard your sense of thirst and strive to ingest several glasses of water a day just because you have been told that doing so is good for your health, you actually put unnecessary strain on your body in two major ways:
1. Ingesting more water than you need can increase your total blood volume. And since your blood volume exists within a closed system (your circulatory system), needlessly increasing your blood volume on a regular basis puts unnecessary burden on your heart and blood vessels.
2. Your kidneys must work overtime to filter excess water out of your circulatory system. Your kidneys are not the equivalent of a pair of plumbing pipes whereby the more water you flush through your kidneys, the cleaner they become; rather, the filtration system that exists in your kidneys is composed in part by a series of specialized capillary beds called glomeruli. Your glomeruli can get damaged by unnecessary wear and tear over time, and drowning your system with large amounts of water is one of many potential causes of said damage.
Putting unnecessary burden on your cardiovascular system and your kidneys by ingesting unnecessary water is a subtle process. For the average person, it is virtually impossible to know that this burden exists, as there are usually no obvious symptoms on a moment-to-moment basis. But make no mistake about it: this burden is real and can hurt your health over the long term.
_
Research debunks health value of guzzling water:
The notion that guzzling glasses of water to flood yourself with good health is all wet, researchers said. Dr. Stanley Goldfarb and Dr. Dan Negoianu of the University of Pennsylvania in Philadelphia reviewed the scientific literature on the health effects of drinking lots of water. People in hot, dry climates and athletes have an increased need for water, and people with certain diseases do better with increased fluid intake, they found. But for average healthy people, more water does not seem to mean better health, they said. Their scientific review, published in the Journal of the American Society of Nephrology, is the latest to undercut the recommendations advanced by some experts to drink eight glasses of water a day. Goldfarb and Negoianu examined what Goldfarb called “four major myths” regarding claims of a benefit for extra water drinking: that it leads to more toxin excretion, improves skin tone, makes one less hungry and reduces headache frequency. “Our bottom line was that there was no real good science — or much science at all — behind these claims, that they represent probably folklore,” Goldfarb said. As far as facilitating toxin excretion, Goldfarb said that was not verified by any sort of scientific study. “The kidneys clear toxins. This is what the kidneys do. They do it very effectively. And they do it independently of how much water you take in. When you take in a lot of water, all you do is put out more urine but not more toxins in the urine,” Goldfarb said. No studies showed any benefit to skin tone as a result of increased water intake, they found. They also found evidence lacking that drinking water wards off headaches. As far as lots of water serving to limit appetite, he said there was no consistent evidence, adding it was “a little unclear exactly whether that was true.” “What no one looked at is whether anyone really loses weight over the long haul if they go under this regimen of drinking lots of water,” Goldfarb said. “We just expressed uncertainty in that area.” While it may not help a person to drink lots of water, it may not harm them much either, Goldfarb said. “If someone enjoys it, I say that’s wonderful, keep doing it. They’re not doing anything that’s going to hurt them.” “A little mild dehydration for the most part is OK, and a little mild water excess for the most part is OK. It’s the extremes that one needs to avoid,” he said.
_
Other disadvantages of high water intake:
Whether it is the tap water that is not pure or the bottled water, there can be no doubt that a high fluid intake will increase one’s exposure to pollutants, especially if the high intake is sustained over years. It is inconvenient and expensive. In healthy individuals, the imbibing of large volumes of water (or of fluid, as in soft drinks) invariably leads to increased production of urine and more frequent urination. Although some dismiss this consequence as minor, for others it is a major inconvenience that sometimes causes embarrassment. And for those who satisfy the requirements of 8 × 8 with bottled water, the practice incurs a fairly large expenditure, costing far more than were the needs to be met with tap water.
___________________
Timing of drinking water:
People drink in water while eating in many ways:
First type: Some people take a glass or two of water and then begin their meal assuming that it reduces their hunger and consequently reduces their body weight.
Second type: Some people take water frequently to facilitate easy gulping down of food and to stop hiccups.
Third type: Some consume one or two glasses of water after eating food believing that ¾ of food and ¼ of water helps digestion better.
Fourth type: Some drink water ½ an hour after their meals as they find it inconvenient to drink water while and immediately after meals.
Fifth Type: Many people drink water while eating because they feel thirsty and their throat dries out.
_
Should we drink water before meal, during meal or after meal:
The Relationship of Water and Digestion in the Mouth:
The first phase of digestion process starts in the mouth. The food we eat should get ready for digestion up to 20%-25% in the mouth itself and then enter the stomach. The teeth accomplish this task. But how many of us have the practice and time to chew our food properly? Right from a boy to an aged person, everyone is in a hurry. None of us have time to eat our meal peacefully. Everyone is in a rush and wants to end up his meal in a minute or ten and rushes out. Had anyone thought what will be the consequences of such an eating? Here are a few consequences of eating food in a hurry without proper chewing.
(a)It becomes real hard to gulp the food into the stomach without chewing it properly. To overcome this we reach a glass of water. When we eat food or pickles they sometimes get obstructed in the esophagus and induce hiccups. To avoid hiccups we again reach out for water. Some people have the habit of eating food while watching television, reading news papers, talking to fellow mates or straying in thoughts. Since the concentration is not on the food, it leads to over eating and subsequently eruption of gases in the stomach and causes belching. To stop it we drink water. These are the common mistakes we comment and don’t end up here; we pass them to our children too.
(b)When we chew food thoroughly, the required saliva is produced and it moves into the stomach easily without any obstruction. The saliva substitutes the function of water as the saliva contains 98% of water and 2% of digestive enzymes. Saliva helps in proper digestion of food. Saliva also kills or injures certain kinds of bacteria found in the food we eat. When we drink water while eating food, less amount of saliva is produced. Due to reduced saliva and increased water intake the digestive process is hampered. If we chew food properly, the saliva mixes well with the food which in turn enables the food to move freely into the stomach. Saliva not only makes the digestion process easy but also complete.
_
Drinking water during meals hampers digestion:
Whether you are thirsty or not, downing glasses of water along with your meals may not be the best time to quench your thirst. Researchers warn, drinking water during meals severely hampers your stomach’s digestive powers and causes insulin levels to fluctuate significantly. They have a good explanation for why you should not drink water during your meal. Most people have water along with their meals. The usual theory is washing down the food while eating. People have no idea how wrong this practice is and how difficult this can be for their digestion. Our stomachs have a knack of knowing when you will eat and starts releasing digestive juices immediately. If you start drinking water at the same time, actually you are diluting the digestive juices being released to digest your food, thereby hindering them from breaking down food. Research shows that sipping a little water during meals isn’t a cause for concern but drinking a glass or two may interfere with digestion. It is best to drink fluids before and two hours after meals as this helps in absorption of nutrients. Drinking water with meals can also lead to acid reflux and heart burn.
_
The Other Side of the Coin:
It’s also important to mention that according to Michael F Picco, M.D. and the MayoClinic: “There’s no concern that water will dilute the digestive juices or interfere with digestion. In fact, drinking water during or after a meal actually aids digestion. Water and other liquids help break down food so that your body can absorb the nutrients. Water also softens stools, which helps prevent constipation.” While they do not make any mention of temperature or amount of water, and don’t reference their statement, it is clear they feel drinking while eating is generally OK.
_
Will drinking water while eating dilutes stomach acid?
The common belief that many people have is that water will reduce the acidity of your stomach acid, which for all intents and purposes is not true. You cannot dilute your stomach acid in any physiologically meaningful way by drinking water during a meal. The pH of stomach acid is 1 to 2. That means your stomach acid is 100,000 times more acidic than water (pH of ~7). You would have to be drinking liters of water to dilute your stomach acid in any meaningful way. There is a reasonable amount of scientific research in this area, most coming from hospital settings where researchers measured stomach acid levels in patients who either fasted and were given drinking water, or given drinking water along with a meal either before or after a surgery. While it’s not normal to be staying in a hospital or to be dealing with a health problem like a surgery, it’s still helpful to have high-quality studies that measure stomach acid changes in a carefully controlled way. The evidence from all of these studies suggests that stomach pH is not significantly altered by water drinking, even when a person consumes the water following an overnight fast. The amount of water consumed by patients in these studies varied, but typically fell into the 5-10 ounce range.
_
Even though natural stomach acid levels were not upset by drinking water in these studies, some individuals may definitely prefer to minimize or eliminate drinking water during meals, and may experience better digestion by doing so. Remember that digestion is a complicated process that depends on many factors for a healthy and comfortable outcome. These factors include: not overeating, not eating too much fat at one meal, eating in a relaxing atmosphere, and truly appreciating your food. If drinking water with meals takes away from your enjoyment of the meal, or leaves you feeling too full too quickly, it makes sense to treat your water intake as a between-meal activity. However, if you enjoy water with your meals, there is research to support you in this practice.
_
Gastric emptying study:
The amount of fluid in the stomach is positively correlated with the rate of gastric emptying.
_
The figure below shows gastric emptying rate of solid and fluid meals:
Drinking water will render the net contents of the stomach more fluid, moving the gastric emptying rate from the blue curve towards the red curve. This seems to confirm the conclusion that avoiding liquid during the meal will help stave off the next bout of hunger because a more solid meal will take longer to empty. The 15 minutes before also seems to fit, as roughly half of liquid consumed 15 minutes beforehand will have been emptied before the meal starts. If one further considers the dynamics, drinking water immediately before a meal will help fill up the stomach and reduce immediate hunger but will cause that meal to be digested faster.
_
Does glass of water 30 minutes before a meal – helps digestion:
Drinking a glass of water 30 minutes before a meal is unlikely to have much effect at all on digestion. Water passes through your system quite quickly, so it is doubtful that one glass of water 30 minutes before eating would still be around in enough quantities and at the required point in the food digestion process to be of any significant help. In fact, even when water is taken with the meal itself, its impact on digestion is not likely to be very significant. Moreover, unless you have specific health problems or have a very poor diet, your body is likely to do a terrific job of digesting your food without any water at all. Dr. Braden Kuo, director of the GI Motility Lab at Massachusetts General Hospital, says that drinking water is not necessary for digesting food, because the body is very efficient at secreting and reabsorbing its own fluids. At the first stage of digestion, drinking water can simply make it easier to swallow food, since most of us have difficulty swallowing when our food is not sufficiently moistened with saliva. When the food reaches the stomach, water “may help to some degree, but its impact is moderate to minimal,’’ he says. He adds that having some extra fluid in the mix may help smooth the digestive process for those with constipation.
_
Does glass of water before going to bed – avoids stroke or heart attack:
If preventing strokes and heart attacks were as simple as drinking water before bed, those duel killers would be pretty much a thing of the past. The supposed remedy is not listed on any credible heart or stroke prevention journal. The claim is inaccurate and misleading. Drinking a glass of water before going to bed certainly will not prevent a heart attack or stroke. But, again, drinking water and staying well hydrated throughout the day can help keep you healthier and perhaps therefore make it a little less likely that you will have a heart attack or stroke. The American Heart Association notes: Keeping the body hydrated helps the heart more easily pump blood through the blood vessels to the muscles. And, it helps the muscles remove waste so that they can work efficiently. If you’re well hydrated, your heart doesn’t have to work as hard.
_
Conclusion regarding drinking water timing:
If we wish to stay healthy, it is important that we stay adequately hydrated and drinking plain old water is one of the best ways to achieve this. But, keeping hydrated is an ongoing task that is dependent on various factors such as the current temperature, what exercise we are doing and our overall health. Drinking water at certain times of the day will not provide the specific health benefits. There is no credible evidence to suggest that drinking water at certain times of the day will provide the particular health benefits. Staying well hydrated can help maintain overall health and may thereby help avoid serious health outcomes such as heart attacks and stroke. But, this is true at any time of the day. Drinking water is not necessary for digesting food. Moderate water intake during meal will not dilute stomach acid and will neither help nor harm digestion of food. However instead of using saliva to chew food in mouth, when you gulp it down with water, food digestion is affected as salivary digestive enzymes are bypassed.
_________
Can drinking a lot of water bloat your Stomach?
Water Consumption and Bloating
Several factors influence whether drinking water leads to temporary stomach bloating, including the volume of water you consume and how quickly; what else is in your stomach; and whether your intestines are also full. On an empty stomach, one or two 8-ounce glasses of water are unlikely to cause noticeable bloating. In contrast, a quart or more of water consumed quickly with other food or liquid already in your stomach may lead to some distension of your abdomen. Similarly, if your intestines are full due to a recent meal, constipation or both, quickly consuming a quart or more of water may cause temporary stomach bloating and discomfort. The larger the volume of water and other foods or liquids in your stomach, the more likely you will experience temporary bloating.
Contributing Factors:
Medical conditions and other factors that slow stomach emptying may increase the likelihood of experiencing temporary bloating when you drink a lot of water. Narcotic pain killers, acid reflux disease, the stomach flu, bulimia, anorexia nervosa, an underactive thyroid gland, Parkinson’s disease and nerve damage associated with diabetes, each commonly delay stomach emptying. Drinking water slowly helps prevent stomach bloating if you have one or more of these conditions.
___________
Water and exercise:
Should you drink cold or lukewarm water while exercising?
Cold water might keep your core body temperature lower and allow you to exercise longer. A review of several studies revealed that people drink about 50 percent more cold or cool water compared to warm water when they exercise — and as a result are less dehydrated. Other studies show that people who exercise in heat and humidity have a slower and lower rise in core body temperature when they drink cold rather than lukewarm water. Whether running, cycling or lifting weights, it appears cold-water drinkers are able to exercise longer without feeling exhausted. “Sometimes when you feel really hot, you’ll feel more fatigue setting in,” says Brooke Schantz, RD, a specialist in sports nutrition. “Cold water can help prevent your core body temperature from rising significantly.” Sports nutritionist Nancy Clark, RD, author of “Nancy Clark’s Sports Nutrition Guidebook” says: “Cold water is more refreshing, and it cools you off a bit better.” But it’s important to remember that cold water is a luxury. It’s a necessity to drink water while working out — regardless what temp it is. “Staying hydrated means you’ll have a lower heart rate and a lower body temperature. You won’t feel as tired and you’ll have better performance,” says Schantz. To make sure you drink enough water, she suggests drinking a large glass (16 ounces) a couple of hours before you exercise, then a cup (8 ounces) about 10 or 20 minutes beforehand. While exercising, especially in the heat, stop for a sip at least every 15 or 20 minutes. Of course, how much water you’ll need to drink depends on how much you sweat. “For every pound you lose in sweat, you need to drink 16 to 24 ounces,” she says. Since everyone is different, she often recommends that her student athletes weigh themselves after getting out of the shower — and then after practice. Do that a few times and you’ll get a sense of how much you sweat, and thus, how much water you need to glug.
_
A good rule of thumb is that you lose around half a liter for each hour that you exercise – and it can be substantially more than this if it is a hot day. Some evidence shows that modest levels of dehydration lead to significant falls in athletic performance. Your blood is about 82% water. As you sweat more, your volume of blood is reduced, and your cardiovascular system works less efficiently at getting oxygen to your muscles. A loss of water equal to 2% of your body weight (a liter and a half for a 75kg person) could reduce your aerobic capacity by up to 20%. Bigger sweat losses than this can lead to dangerous dehydration.
_
In a comprehensive discussion of this issue Tim Noakes concludes that distance runners should drink as they feel (and not force themselves to drink more), which generally means about 500ml an hour. However, other medical advice still recommends drinking rather more than this. For example, the American College of Sports Medicine recommends 600ml to 1,200ml of sports drink an hour. There is a significant danger that this may be too much for non-elite athletes who are running a marathon which takes them more than four or five hours. You will have to judge for yourself what works best for you, recognizing that there are dangers from over-hydration which are at least as great as the dangers of dehydration. For running events of up to 10km, it is unlikely that you will need to drink during the run unless the weather is exceptionally hot. For longer events, including the marathon, your performance may suffer as a result of dehydration if you don’t replace the water you are losing during the race. But people running for more than four hours should also be careful not to drink too much.
_
Water intoxication and hyponatremia due to over-hydration while exercising:
Most cases of water poisoning do not result from simply drinking too much water. It is usually a combination of excessive fluid intake and increased secretion of vasopression (also called antidiuretic hormone). Produced by the hypothalamus and secreted into the bloodstream by the posterior pituitary gland, vasopressin instructs the kidneys to conserve water. Its secretion increases in periods of physical stress—during a marathon, for example—and may cause the body to conserve water even if a person is drinking excessive quantities. Every hour, a healthy kidney at rest can excrete 600 to 800 milliliters of water and therefore a person can drink water at a rate of 600 to 800 milliliters per hour without experiencing a net gain in water. If that same person is running a marathon, however, the stress of the situation will increase vasopressin levels, reducing the kidney’s excretion capacity to as low as 100 milliliters per hour. Drinking 600 to 800 milliliters of water per hour under these conditions can potentially lead a net gain in water, even with considerable sweating. While exercising you should balance what you’re drinking with what you’re sweating. If you’re sweating 500 milliliters per hour, that is what you should be drinking. But measuring sweat output is not easy. How can a marathon runner, or any person, determine how much water to consume? As long as you are healthy and equipped with a thirst barometer unimpaired by old age or mind-altering drugs, drink to your thirst. It’s the best indicator.
_
It is less well known that it is quite common, and quite dangerous, to drink too much water, especially during endurance events. In one study of 17 runners who were hospitalized during the Comrades Marathon (an 89km ultra-marathon in South Africa), 9 had hyponatraemia (this is low blood sodium, associated with over-hydration). At least two marathon runners in the USA have died of hyponatraemia. The risks of drinking too much water are at least as significant as the risks of drinking too little.
_
Hyponatremia among Runners in the Boston Marathon:
Participants in the 2002 Boston Marathon were recruited one or two days before the race. Subjects completed a survey describing demographic information and training history. After the race, runners provided a blood sample and completed a questionnaire detailing their fluid consumption and urine output during the race. Prerace and postrace weights were recorded. Multivariate regression analyses were performed to identify risk factors associated with hyponatremia. Hyponatremia occurs in a substantial fraction of nonelite marathon runners and can be severe. Considerable weight gain while running, a long racing time, and body-mass-index extremes were associated with hyponatremia, whereas female sex, composition of fluids ingested (plain water, rather than sports drinks that contain electrolytes), and use of nonsteroidal antiinflammatory drugs were not. Substantial weight gain appeared to be the most important predictor of hyponatremia and correlated with increased fluid intake.
_
Sports scientists in Australia did an extraordinary experiment that had never been done before (British Journal of Sports Medicine, September 2013, Current hydration guidelines are erroneous: dehydration does not impair exercise performance in the heat, Wall BA). This group wanted to find out what happened to performance after dehydration. So they took a group of cyclists and exercised them until they lost 3% of their total body weight in sweat. Then their performance was assessed after rehydration with either 1) nothing, 2) enough water to bring them back to 2% dehydration or 3) after full rehydration. So far nothing unusual, but the difference between this and almost every other study that’s ever been done on hydration was that the cyclists were blind to how much water they got. The fluid was given intravenously without them knowing the volume. This is vital because we all, and especially athletes, have such an intimate psychological relationship with water consumption. Remarkably, there was no performance difference between those that were fully rehydrated and those that got nothing. This study was part of a growing movement to “drink to thirst” which hopes to persuade athletes not to over hydrate with the potentially fatal consequence of diluting your sodium level, causing hyponatraemia. Perhaps the result shouldn’t be so surprising. Humans evolved doing intense exercise in extreme heat and dryness. We are able to tolerate losses in water relatively well whereas even slight over hydration can be far more dangerous. In simple terms, being too watery is as bad for you as being too concentrated.
Note: This study was done on athletes and therefore its results must not be extrapolated for non-athletic population. Athletes can tolerate water loss better than sedentary person.
_
What should athletes drink? Water or sports drink?
Research shows that fluid intake is enhanced when beverages are cool (~15 °C), flavoured and contain sodium (salt). This makes sports drinks an ideal choice during exercise. Sports drinks are not gimmicks. They are legitimate products that are well researched and proven to improve fluid intake and performance. A great deal of science has gone into developing the flavour profile of sports drinks so that they encourage fluid intake during exercise. In addition, sports drinks contain carbohydrate at a concentration (4-8%) that allows refueling to take place during exercise. Several studies demonstrate that use of sports drinks will improve fluid intake. A study conducted with AIS netball and basketball players in 1999 demonstrated better fluid balance with a sports drink compared to water. This is consistently observed across sporting programs. Even athletes, who prefer to drink water during exercise, demonstrate better fluid intake when forced to drink sports drink. In the past, it was believed that sports drinks only benefited the performance of exercise greater than 90 minutes. However, in recent years, the intake of carbohydrate and fluid has been shown to be beneficial for high intensity exercise of approximately 60 minutes. This makes sports drinks a good option for many types of sporting activity. Water is still a suitable option during exercise. However, water drinkers need to be aware that water does not stimulate fluid intake to the same extent as sports drinks.
_
The pH of nearly all analyzed sports drinks was in the range of about 3 to 4, which is of some concern because of the potential of low pH solutions to erode teeth. The osmolality of many commercial sports drinks, which are designed to be consumed during exercise, tended to be in the hypertonic range, although such drinks should rather be slightly hypotonic. Indeed, it is suggested that intestinal water absorption rates are higher with hypotonic solutions compared with isotonic solutions. The optimal osmolality for a sports drink has, therefore, been defined to be in the slightly hypotonic range between 200 and 250 mosmol/L. Since pH of drinking water is between 6 to 8 and osmolarity between 3 to 30 mosm/L, water scores over sports drink in some aspects. Since osmolarity of water is much lower than sports drink, plain water is absorbed faster than sports drink during exercise. As osmolality of some sports drink increases above 300 mosmol/kg, efficient uptake from the GI tract is decreased. In fact, solutions with high osmolalities (hypertonic) can actually draw fluid out of the body.
_
The ratio of sweat to plasma osmolarity is always less than 1; in acclimatized adult it is 0.1 and in non-acclimatized adult it is between 0.1 to 0.5 On average, sweat osmolarity is about 50 to 140 mosmol/L in adults at rest and osmolarity decreases with increasing sweat rate and increased acclimatization as in athletes. Research has shown that depending on the temperature, humidity and overall conditioning, athletes engaged in vigorous exercise can lose 1500-3500 ml of sweat per hour. This sweat has very low osmolarity with very low sodium. So it is mainly water loss. When sweat causes dehydration, the plasma osmolarity increases, causing the antidiuretic hormone vasopressin to be released, resulting in the kidneys retaining water. Also thirst is stimulated to drink water. This process continues until the high osmolarity is reduced to normal by drinking water. Drinking only sports drink during exercise or marathon would replenish water but plasma osmolarity would not fall due to high osmolarity of sports drinks resulting in persistent thirst and higher vasopressin level. Stress due to marathon may also release vasopressin. Persistent thirst will lead to overdrinking fluid and higher vasopressin leads to reduced renal excretion of water; both together may lead to water intoxication. Osmolarity of soft drinks like pepsi and coke range from 650 to 700 mosmol/L; commercial fruit juices from 257 to 1152 mosmol/L and fresh fruit juice about 274. Osmolarity of club soda (carbonated water) is 13 to 44 close to drinking water. One must always quench thirst with plain water rather than sports drinks, soft drinks or fruit juices as all of them are having high osmolarity and drinking highly osmolar fluid will not reduce plasma osmolarity resulting in insatiable thirst, higher vasopressin level and possibility of over-hydration.
_
High salt intake and drinking water:
Osmoregulation during high salt intake: relative importance of drinking and vasopressin secretion: A study:
Studies determined the relative contribution of drinking vs. vasopressin secretion in the regulation of extracellular osmolality in response to changes of Na intake. Daily Na intake was increased from 30 to 200 meq in dogs maintained under three conditions: normal dogs with ad libitum drinking, normal dogs with “fixed drinking,” and neurohypophysectomized dogs with “fixed drinking” and vasopressin replaced by continuous infusion. (Drinking was fixed to that amount consumed during the normal Na control period.) The mechanisms of osmoregulation were highly nonlinear. As daily Na intake increased from 30 to 100 meq, renal natriuretic mechanisms predominated with only small contributions from either the thirst or vasopressin systems. At high levels of Na intake (200 meq/day), both drinking and vasopressin release contributed significantly to osmoregulation. The studies also determined that, in the absence of excess vasopressin secretion and increased drinking, plasma osmolality rose to nearly twice the levels as those observed in normal dogs that increased vasopressin secretion. Authors conclude that vasopressin-related renal conservation of water contributes to buffering the rise of osmolality when Na intake is increased without increased drinking. The studies also confirm that with available water to drink, the thirst mechanism together with renal Na excretory mechanisms are the predominant controllers of osmolality in situations of high sodium intake.
_
Drink extra water when you have high salt meal:
Recommended daily salt (NaCl) intake for adult human is 5 gms/day. High-salt meal drives your body to compensate. Kidneys try to remove excess salt by producing more urine. High salt raises plasma osmolarity and thereby stimulates vasopressin that reduces free water loss from kidneys to maintain osmolarity resulting in expansion of extra cellular fluid volume. This can be offset by drinking liberal water as per thirst stimulated by higher osmolarity and thereby keeping vasopressin level in check to increase urine formation which will remove excess salt. Remember maximum renal concentration capacity is 1200 mosmol/L and when there is excess solute load as in high salt diet, urine output must increase to facilitate solute excretion.
__________
Benefits of using Copper Vessels for drinking water:
History of using copper:
- Use of copper in history is found to be about 10,000 years ago. In 3000 B.C., coppers ores were found in the island of Cyprus. Romans named the metal as cyprium which was later known as cuprum and then copper in English
- In India copper was used to sterilize drinking water 2600 and 2200 B.C. It is still used in many households for storing water
- In Egypt copper compounds were recommended for headaches, burns, wounds, and boils (1500 BC)
- Greeks used it for treatment of ulcers and healing wounds
- In the 20th century, a German physician observed copper mine workers to be free from Arthritis
- Earlier water supply used to be through copper pipes and taps and its anti-microbial properties were observed.
_
Copper is one of the essential metallic elements required for proper metabolic functioning of the body. However, the amount required is very less i.e. 1.2mg/day (trace amount) but our body does not synthesize copper so it needs to be supplied from dietary source. Various food items that has copper content are honey, beans , whole wheat, green leafy vegetables etc. it is perceived that the best method of fulfilling the body’s need of copper is by having water stored overnight in a copper vessel. However, trace elements in inorganic form are not assimilated in body as opposed to organic forms from food. So inorganic copper ion in water would not be of much use.
Copper’s Antimicrobial Power:
According to a 2012 study published in Journal of Health, Population, and Nutrition, storing bacterially contaminated water in copper for up to 16 hours at room temperature considerably reduces the presence of the harmful microbes, so much that the researchers inferred that “copper holds promise as a point-of-use solution for microbial purification of drinking-water, especially in developing countries.” An additional study from University of South Carolina researchers explored the purifying power of copper, finding that “Antimicrobial copper surfaces in intensive care units (ICU) kill 97 percent of bacteria that can cause hospital-acquired infections,” resulting in “a 40 percent reduction in the risk of acquiring an infection.”
Copper Promotes Health:
Only about 25% of the US population is getting adequate copper each day in the diet. Copper is a powerful anti-oxidant, and is also required for proper absorption of iron. With continued mineral depletion and soil erosion, it is becoming more necessary to consider ways to adequately supplement our diets with this vital mineral.
Deficiency of copper:
Deficiency of copper can cause anemia, osteoporosis, low WBCs, elevated cholesterol, low skin pigmentation, thyroid problems, nervous system disorders etc.
In a copper jug, copper intake is only in trace amounts that can’t be toxic.
Toxicity due to over dosage:
Excessive intake can be harmful also leading to high blood pressure, psychiatric disorders etc. A person should stop taking copper supplements and seek medical help immediately if having symptoms of anemia, nausea, vomiting or abdominal pain. Pregnant women or people already on some kind of copper supplementation should consult their physicians before trying the product. Excessive copper intake may cause Zinc deficiency
_________
Drinking Water Apps for Your Phone:
In your busy life, you barely have time to check your email, so how can you possibly have time to keep track of exactly how much water you’ve had to drink? Your Smart Phone already manages most of your life, so why not let it handle your drinking water needs as well? Here are some great apps to get you started!
1. WaterLogged:
The WaterLogged app keeps track of how much water you’ve had to drink and applies it to your daily goal. The default setting is to drink 64 oz of water, but you can easily change the goal to match how much water you want to drink every day. Anytime you drink water, you can quickly apply the amount of water you drank towards your daily total. The app includes two default serving sizes for a 16.9 oz water bottle and a regular 8 oz glass. Another nifty detail of this app is that you can add your own water bottle as a serving size. The app also lets you include partial servings, so if you only drank a portion, the app will do the math for you.
2. TapIt Water:
TapIt Water is more than just an app; it’s a network of places where you can refill your water bottle free of charge. The app uses a GPS locator to find participating locations nearby where you can fill up. You can check the TapIt Water website for a list of cities and states where the network has a strong presence.
3.OasisPlaces:
OasisPlaces allows users of the app to find and share the locations of free drinking water fountains. Users can upload a picture of the water fountain and offer a review of the quality of the water from that fountain using a 5 star rating system. Don’t have any OasisPlaces near you? Take pictures of the fountains and add them to the database. The more people using the app, the more effective it will be. Why pay for a bottle of water if there’s a perfectly good water fountain nearby?
4. WeTap:
The WeTap app is very similar to OasisPlaces, only it works for the Android OS. WeTap was designed by the Pacific Institute to help bring back free drinking water via public water fountains. The app allows you to find and add water fountains for other users, but it also has a feature to report broken fountains so they can be fixed. This feature makes a lot of sense because now you can report a broken public fountain and help get it fixed! How often do you have a chance to help dozens of other people quench their thirst using just your smart phone?
5. Carbodroid:
Carbodroid is a fun app for the Android OS that keeps track of your drinking water intake to help you reach your daily goal. The fun part of this app features an animated droid who helps keep you motivated to drink more water and celebrates your success with you. You can easily enter how much water you’ve had to drink and set an alarm to help you remember to drink more water during the day.
Hopefully these water apps for your phone will make it easier for you to find free sources of drinking water and meet your daily goals. Since these are all free apps, go ahead and try them. What do you have to lose?
Well, there is nothing to lose but I suggest you drink to your thirst rather than to your cell phone reminder. Do not use technology to override biology.
_______________
Minerals in Water:
As discussed earlier, water is classified as nutrient and in the figure of nutritional facts of tap water; it is shown to contain sodium, calcium and magnesium. In terms of mineral nutrients intake, it is unclear what the drinking water contribution is. Inorganic minerals generally enter surface water and ground water via storm water runoff or through the Earth’s crust. Treatment processes also lead to the presence of some minerals. Examples include calcium, zinc, manganese, phosphate, fluoride and sodium compounds. Minerals are essential for the basic functions of the human body to take place. They help to control bone growth, regulate fluids, normalize nerve and muscle functions, keep up metabolism, grow connective tissues, and so much more. However, a big misconception is that that we obtain enough minerals from our drinking water. This is actually not true because in reality, the main source of minerals is always from our food and diet, not from our drinking water. Our bodies have a hard time processing inorganic minerals and what we cannot absorb may be stored in our tissues and organs and eventually become toxic to the body. The primary culprits are calcium salts and over time they can cause gallstones, kidney stones, bone & joint calcification, arthritis, and hardening and blocking our arteries. Organ failure and cancer could also occur from long term exposure to certain types of toxic or radioactive minerals found in tap and natural spring water. Organic minerals which are abundant in food are much easier to absorb and preferred by our bodies because they do not contain toxic minerals.
_
It’s much better to obtain minerals from our food instead of from our water. But that doesn’t mean that getting minerals from water won’t be absorbed by the body. It just won’t be very effective and most will be lost. That’s why so many supplements are a waste of time. Most minerals are best absorbed when attached to some sort of protein molecule. In your body, iron is surrounded by the heme molecule. Many of the trace minerals in your body have some sort of protein molecule attached to it. This prevents the mineral ion from reacting with the alkaline chemicals your body produces. In many cases these protein molecules effectively surrounds these metal ions. It helps with better absorption because some of these molecules can easily attach itself to the intestine. This doesn’t mean that inorganic mineral is useless. It just means that once ingested, it has to “compete” with the chemicals that the body produces for proper absorption, with other mineral ions, and bind with protein molecules to attach to the intestine to be absorbed. Calcium in water is a salt, which means that it has a positive ion and a negative ion. You can absorb it to a degree but when we are talking about efficiency, it’s better when it’s attached to other molecules. So overall, you can absorb only a tiny amount of the minerals in the water you drink, so it’s better to plan getting them from food instead!
__
Don’t you need minerals in your drinking water?
•It is believed that mineral waters help furnish elements for body metabolism. However, there is scientific proof to suggest that most of these minerals are in an inorganic (dead) form. While they may enter the circulation, they cannot be used in the physiological process of building the human cell.
•With this in mind, we can see that mineral water may give “dead” or “inorganic” minerals to the body which cannot be properly assimilated.
•These inorganic minerals only interfere with the delicate and complex biology of the body.
•The body’s need for minerals is largely met through foods, not drinking water.
•The organic minerals in tap water represent only 1% of the total mineral content of the water.
•One glass of orange juice contains more beneficial minerals than thirty gallons of untreated tap water.
_
What are TDS?
_
_
TDS stands for total dissolved solids, and represents the total concentration of dissolved substances in water. TDS is a measure of the combined content of all inorganic and organic substances contained in a liquid in molecular, ionized or micro-granular (colloidal sol) suspended form. Generally the operational definition is that the solids must be small enough to survive filtration through a filter with two-micrometer pores. Total dissolved solids are normally discussed only for freshwater systems, as salinity comprises some of the ions constituting the definition of TDS. The principal application of TDS is in the study of water quality for streams, rivers and lakes, although TDS is not generally considered a primary pollutant (e.g. it is not deemed to be associated with health effects) it is used as an indication of aesthetic characteristics of drinking water and as an aggregate indicator of the presence of a broad array of chemical contaminants. Total dissolved solids are differentiated from total suspended solids (TSS), in that the latter cannot pass through a sieve of two micrometers and yet are indefinitely suspended in solution. The term “settleable solids” refers to material of any size that will not remain suspended or dissolved in a holding tank not subject to motion, and excludes both TDS and TSS. Settleable solids may include larger particulate matter or insoluble molecules. TDS is made up of inorganic salts, as well as a small amount of organic matter. Common inorganic salts that can be found in water include calcium, magnesium, potassium and sodium, which are all cations, and carbonates, nitrates, bicarbonates, chlorides and sulfates, which are all anions. Cations are positively charged ions and anions are negatively charged ions.
How do these solids end up dissolved in water?
These minerals can originate from a number of sources, both natural and as a result of human activities. Mineral springs contain water with high levels of dissolved solids, because the water has flowed through a region where the rocks have a high salt content. The water in the Prairie Provinces tends to have high levels of dissolved solids, because of high amounts of calcium and magnesium in the ground. These minerals can also come from human activities. Agricultural and urban runoff can carry excess minerals into water sources, as can wastewater discharges, industrial wastewater and salt that is used to de-ice roads.
What happens to the water when the TDS level is high?
Alone, a high concentration of dissolved solids is usually not a health hazard. In fact, many people buy mineral water, which has naturally elevated levels of dissolved solids. The United States Environmental Protection Agency (EPA), which is responsible for drinking water regulations in the United States, includes TDS as a secondary standard, meaning that it is a voluntary guideline in the United States. While the United States set legal standards for many harmful substances, TDS, along with other contaminants that cause aesthetic, cosmetic and technical effects, has only a guideline. However, increased concentrations of dissolved solids can also have technical effects. Dissolved solids can produce hard water, which leaves deposits and films on fixtures, and on the insides of hot water pipes and boilers. Soaps and detergents do not produce as much lather with hard water as with soft water. As well, high amounts of dissolved solids can stain household fixtures, corrode pipes, and have a metallic taste. Hard water causes water filters to wear out sooner, because of the amount of minerals in the water.
_
Water can be classified by the amount of TDS per liter:
Fresh water < 1,000 mg/L TDS
Brackish water 1000 to 10,000 mg/L TDS
Saline water 10,000 to 30,000 mg/L TDS
Brine > 30,000 mg/L TDS
While a TDS of 5,000 mg/L is the minimum threshold for a water to be considered brine, the typical range is 30,000 to 100,000 mg/L.
_
Most people think of TDS as being an aesthetic factor. In a study by the World Health Organization, a panel of tasters came to the following conclusions about the preferable level of TDS in water:
| Level of TDS (milligrams per liter) | Rating |
| Less than 300 | Excellent |
| 300 – 600 | Good |
| 600 – 900 | Fair |
| 900 – 1,200 | Poor |
| Above 1,200 | Unacceptable |
WHO report recommended that the minimum TDS in drinking water should be 100 mg/L. Because the threshold of acceptable aesthetic criteria for human drinking water is 500 mg/l, there is no general concern for odor, taste, and color at a level much lower than is required for harm. A number of studies have been conducted and indicate various species’ reactions range from intolerance to outright toxicity due to elevated TDS. The numerical results must be interpreted cautiously, as true toxicity outcomes will relate to specific chemical constituents. Nevertheless, some numerical information is a useful guide to the nature of risks in exposing aquatic organisms or terrestrial animals to high TDS levels. Most aquatic ecosystems involving mixed fish fauna can tolerate TDS levels of 1000 mg/l.
_
How can water with high TDS be undesirable or harmful?
•It may taste bitter, salty, or metallic and may have unpleasant odors
•High TDS water is less thirst quenching.
•High TDS interferes with the taste of foods and beverages, and makes them less desirable to consume.
•Some of the individual mineral salts that make up TDS pose a variety of health hazards. The most problematic are Nitrates, Sodium, Sulfates, Barium, Cadmium, Copper, and Fluoride.
•If a person drinks 2 pints of water a day, this will total 4500 gallons of water passing through his body over a 70 year span. If the water is not totally pure, then this 4500 gallons will include 200-300 pounds of rock that the body cannot utilize. Most will be eliminated through excretory channels. But some of this will stay in the body, causing stiffness in the joints, hardening of the arteries, kidney stones, gall stones and blockages of arteries, microscopic capillaries and other passages in which liquids flow through our entire body.
_
The following are reasons why it is helpful to constantly test for TDS:
Taste/Health:
High TDS results in undesirable taste which could be salty, bitter, or metallic. It could also indicate the presence of toxic minerals. The EPA’s recommended maximum level of TDS in water is 500mg/L (500ppm).
Filter performance:
Test your water to make sure the reverse osmosis or other type of water filter or water purification system has a high rejection rate and knows when to change your filter (or membrane) cartridges.
Hardness (and Water Softeners):
High TDS indicates Hard water, which causes scale buildup in pipes and valves, inhibiting performance.
Aquariums/Aquaculture:
A constant level of minerals is necessary for aquatic life. The water in an aquarium or tank should have the same levels of TDS and pH as the fish and reef’s original habitat.
Pools and spas:
TDS levels must be monitored to prevent maintenance problems.
Commercial/Industrial:
High TDS levels could impede the functions of certain applications, such as boilers and cooling towers, food and water production and more.
Coffee and Food Service:
For a truly great cup of coffee, proper TDS levels must be maintained.
_
How do you Reduce or Remove the TDS in your Water?
Carbon filtration:
Charcoal, a form of carbon with a high surface area, adsorbs (or sticks to) many compounds, including some toxic compounds. Water is passed through activated charcoal to remove such contaminants.
Reverse osmosis (R.O.):
Reverse osmosis works by forcing water under great pressure against a semi-permeable membrane that allows water molecules to pass through while excluding most contaminants. RO is the most thorough method of large-scale water purification available.
Distillation:
Distillation involves boiling the water to produce water vapor. The water vapor then rises to a cooled surface where it can condense back into a liquid and be collected. Because the dissolved solids are not normally vaporized, they remain in the boiling solution.
Deionization (DI):
Water is passed between a positive electrode and a negative electrode. Ion selective membranes allow the positive ions to separate from the water toward the negative electrode and the negative ions toward the positive electrode. High purity de-ionized water results. The water is usually passed through a reverse osmosis unit first to remove nonionic organic contaminants.
_
Water’s electrical conductivity (EC) & TDS:
Pure water is not a good conductor of electricity. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10 x 10-6 W-1m-1 (20 dS/m). Because the electrical current is transported by the ions in solution, the conductivity increases as the concentration of ions increases. Thus conductivity increases as water’s dissolved ionic species increase. TDS is a measure of the total ions in solution. EC is actually a measure of the ionic activity of a solution in term of its capacity to transmit current. In dilute solution, TDS and EC are reasonably comparable.
________________
Hard water:
Hardness in drinking water is defined as presence of those minerals that dissolve in water having a positive electrical charge. The primary components of hardness are calcium (Ca++) and magnesium (Mg++) ions. Dissolved iron (Fe++) and manganese (Mn++) also satisfy the definition of hardness, but typically make up only a very small fraction of total hardness. Minerals are composed of either atoms or molecules. An atom or molecule that has dissolved in water is called an “ion.” Positively charged ions are called cations and are noted as (+). A double sign would indicate a plus two electrical charge. Contaminants having a similar positive charge would be removed by a matching type of ion exchange resin, i.e. water softening.
_
As rainwater falls, it is naturally soft. However, as water makes its way through the ground and into our waterways, it picks up minerals like chalk, lime and mostly calcium and magnesium. Since hard water contains essential minerals, it is sometimes the preferred drinking water. Not only because of the health benefits, but also the flavor. On the other hand, soft water tastes salty and is sometimes not suitable for drinking. The hardness of water is referred to by three types of measurements: grains per gallon, milligrams per liter (mg/L), or parts per million (ppm). What type is your water? The Water Quality Association of the United States defines hard water as having dissolved mineral hardness of 1 GPG (grain per gallon) or more.
| Water Hardness Scale | ||
| Grains Per Gallon | Milligrams Per Liter (mg/L)or Parts Per Million (ppm) | Classification |
| less than 1.0 | less than 17.1 | Soft |
| 1.0 – 3.5 | 17.1 – 60 | Slightly Hard |
| 3.5 – 7.0 | 60 – 120 | Moderately Hard |
| 7.0 – 10.5 | 120 – 180 | Hard |
| over 10.5 | over 180 | Very Hard |
_
Temporary hardness:
Temporary hardness is a type of water hardness caused by the presence of dissolved bicarbonate minerals (calcium bicarbonate and magnesium bicarbonate). When dissolved these minerals yield calcium and magnesium cations (Ca2+, Mg2+) and carbonate and bicarbonate anions (CO3–, HCO3-). The presence of the metal cations makes the water hard. However, unlike the permanent hardness caused by sulfate and chloride compounds, this “temporary” hardness can be reduced either by boiling the water, or by the addition of lime (calcium hydroxide) through the softening process of lime softening. Boiling promotes the formation of carbonate from the bicarbonate and precipitates calcium carbonate out of solution, leaving water that is softer upon cooling.
Permanent hardness:
Permanent hardness is hardness (mineral content) that cannot be removed by boiling. When this is the case, it is usually caused by the presence of calcium sulfate and/or magnesium sulfates in the water, which do not precipitate out as the temperature increases. Ions causing permanent hardness of water can be removed using a water softener, or ion exchange column.
_
Hard water is a very common problem, affecting water in more than 85% of the country. It is a result of the dissolved minerals calcium, magnesium and manganese. With an increase in these minerals, the following are seen:
•Soap scum in sinks and bathtubs
•Bathtub rings
•Spots on dishes or shower doors
•Reduced foaming and cleaning abilities of soaps and detergents
•Dingy and yellowed clothes with soapy residues that require extra rinsing to remove
•Clogged pipes from buildup of minerals
•Increased water heating costs from buildup of minerals, reducing efficiency of water heaters
•Possible skin infections from bacteria trapped in pores underneath soap scum
•Accumulation of whitish-gray scale in tea kettles and other containers used to boil water
_
The figure below shows how hard water leads to lime-scale deposits in PVC pipes:
_
Hard water health benefits:
Sufficient evidence is now available to confirm the health consequences from drinking water deficient in calcium or magnesium. Many studies show that higher water magnesium is related to decreased risks for CVD and especially for sudden death from CVD. This relationship has been independently described in epidemiological studies with different study designs, performed in different areas, different populations, and at different times. The consistent epidemiological observations are supported by the data from autopsy, clinical, and animal studies. Biological plausibility for a protective effect of magnesium is substantial, but the specificity is less evident due to the multi-factorial etiology of CVD. In addition to an increased risk of sudden death, it has been suggested that intake of water low in magnesium may be associated with a higher risk of motor neuronal disease, pregnancy disorders (so-called preeclampsia), sudden death in infants, and some types of cancer. Recent studies suggest that the intake of soft water, i.e. water low in calcium, is associated with a higher risk of fracture in children, certain neurodegenerative diseases, pre-term birth and low weight at birth and some types of cancer. Furthermore, the possible role of water calcium in the development of CVD cannot be excluded.
_
The findings of a six-year study of more than 20,000 healthy men and women aged 38-100 in the May 1, 2002 American Journal of Epidemiology found that women who drank more than five glasses of water a day were 41% less likely to die from a heart attack during the study period than those who drank less than two glasses. The protective effect of water was even greater in men. There is an increasing body of evidence that drinking water hardness and elevated concentrations of certain minerals in hard water may reduce the risk of cardiac death and, in particular, the risk of sudden cardiac death. Recent interest has focused on deficits in dietary magnesium. In developed countries, these deficits are potentially compounded by use of medications, such as diuretics, that further reduce body stores of magnesium.
_
Several studies showed an inverse correlation between water hardness and mortality from cardiovascular diseases (CVD). Among the best known studies were those by H.A.Schroeder who demonstrated, among others, correlation between mortality from CVD in males aged 45-64 years and water hardness in 163 largest cities of the USA (Schroeder, 1960) and summarized his results using the following compelling dictum: ‘soft water, hard arteries’. Other studies were published by Morris in Wales (Morris et al, 1961) and Canadian, Finnish, Italian, Swedish and other authors. A review of most relevant papers of the 1960’s is given e.g. in a WHO Bulletin (Masironi et al, 1972) or by Sharrett and Feinleib (Sharrett et al, 1975). An interesting British study (Crawford et al, 1971) focused on variation in mortality from CVD depending on water hardness in 11 British cities between 1950 and 1960. Water hardness increased in five cities and decreased in six cities. Within the given period mortality from CVD in the UK increased by 10% on average compared to 20% in the cities supplied with softer water than before and compared to 8.5% only in the cities supplied with harder water than before. In all districts where the drinking water magnesium level was higher than 8 mg/l (but not higher than 15 mg/l); the CVD mortality rates were lower. Another Swedish case-control study focused on the effect of the drinking water Mg and Ca levels on mortality from acute myocardial infarction (AMI) in females showed a statistically significantly lower mortality rate (by 34%) in the areas supplied with water containing more calcium (> 70 mg/l) as compared to those where the drinking water calcium level was < 31 mg/l; a similar finding was presented independently for magnesium: the mortality rate was by 30% lower in the areas where the water Mg content was > 9.9 mg/l compared to those where the water Mg content was < 3.4 mg/l (Rubenowitz et al, 1999). Another Swedish case-control study showed a significant correlation between male mortality from AMI the Mg content of water. Cases were 854 men from 17 municipalities in the southern part of Sweden who had died of AMI between ages 50 and 69 years during the period 1982-1989. The controls were 989 men of the same age in the same area who died from cancer during the same period. Only men who consumed water supplied from municipal waterworks were included in the study. The group with hard water (> 9.8 mg Mg/l) had a mortality rate from AMI by 35% lower as compared with the consumers of soft water (< 3.5 mg Mg/l). Any correlation with the water Ca content was not reported (Rubenowitz et al, 1996). Another study of the same type and by the same authors focused on correlation between the drinking water Mg and Ca levels and morbidity and mortality from AMI in 823 males and females aged 50-74 years in 18 Swedish districts, who had developed AMI between October 1, 1994 and June 30, 1996 (Rubenowitz et al, 2000). The study took into account both individual exposure to Ca and Mg from water and food and other known risk factors for AMI likely to bias the correlation, if any. Although for calcium the correlation with AMI was not confirmed, magnesium proved to reduce the risk level by 7.6 % in the group of the quartile with the highest water Mg level ( 8.3 mg/l) compared to groups exposed to water containing lower levels of magnesium. Although the total AMI rates were similar in all four groups, the persons enrolled in the group with the highest water Mg level had a risk level of death from AMI by a third lower (odds ratio 0.64) as compared to the groups consuming water containing less Mg than 8.3 mg/l. Multivariate analyses showed that the correlation found is not caused by other known risk factors. This finding supports the hypothesis that magnesium prevents primarily sudden death from AMI, rather than all ischemic heart disease deaths or the risk of suffering an AMI.
_
Magnesium may protect against hip fractures:
Drinking water with a relatively high concentration of magnesium protects against hip fractures, according to results of a study from the Norwegian Institute of Public Health. There are considerable variations in the quality of drinking water in Norway. The researchers studied variations in magnesium and calcium levels in drinking water between different areas, as these are assumed to have a role in the development of bone strength. They wanted to examine whether there was a correlation between magnesium and calcium concentrations in drinking water and the incidence of hip fracture. The study results show that magnesium protects against hip fracture for both men and women. The researchers found no independent protective effect of calcium.
__
Water hardness and kidney stones:
We boil the water before drinking and find that the container is thickly coated with salt due to the regular boiling of water. Will consuming this salted water lead to kidney stones? The hardness of water is due to the presence of carbonate & sulphate salts of calcium and magnesium. These minerals in hard water cause spots to form on dishes/cutlery and scale to form on plumbing and electric equipment like kettles and geysers. More than 3/4th of kidney stones are generally composed of calcium salt and usually occur as calcium oxalate and less commonly as calcium phosphate. The remaining 20% of stones are composed of uric acid, struvite and cystine stone. Stones form in urine that is supersaturated and this saturation is dependent on chemical free ion activity. Increased urinary ion excretion and decreased urine volume will both increase free ion activity and favour stone formation and growth. Formation of kidney stones (nephrolithiasis) is based on genetic, metabolic, nutritional and environmental factors. Metabolic factors involved in stone formation include hypercalciuria (found in 50% of patients and its most common cause is increased intestinal calcium absorption), hypocitraturia (due to renal disease), hyperuricosuria, hyperoxalaturia, cystinuria and infections. Environmental / nutritional factors include dehydration (e.g., exercise in hot climates), high salt intake, a diet rich in animal proteins and calcium rich diet when oxalate intake is restricted. The impact of water hardness on urinary stone formation remains unclear, despite a weak correlation between water hardness and urinary calcium, magnesium, and citrate excretion. Several studies have shown no association between water hardness and the incidence of urinary stone formation. A correlation between water hardness and urinary calcium, citrate and magnesium levels has been observed although the significance of this is not known. Some studies suggest that in the preventive approach to calcium nephrolithiasis, intake of soft water is preferable to hard water, since it is associated with a lower risk for recurrence of calcium stones. There is, however, no study as yet, which has shown a higher incidence of kidney stones in a population consuming hard water.
_
Effects of water hardness on urinary risk factors for kidney stones in patients with idiopathic nephrolithiasis:
Both amount and timing of dietary calcium intake influence the recurrence of renal calcium stones. Authors have evaluated whether the hardness of extra meal drinking water modifies the risk for calcium stones. The urinary levels of calcium, oxalate and citrate, i.e., the main urinary risk factors for calcium stones, were measured in 18 patients with idiopathic nephrolithiasis, maintained at fixed dietary intake of calcium (800 mg/day), after drinking for 1 week 2 liters per day, between meals, of tap water and at the end of 1 week of the same amount of bottled hard (Ca2+ 255 mg/l) or soft (Ca2+ 22 mg/l, Fiuggi water) water, in a double-blind randomized, crossover fashion. As compared with both tap and soft water, hard water was associated with a significant 50% increase of the urinary calcium concentration in the absence of changes of oxalate excretion; the calcium-citrate index revealed a significant threefold increase during ingestion of hard water as compared with respect to soft water (Fiuggi water), making the latter preferable even when compared with tap water. This study suggests that, in the preventive approach to calcium nephrolithiasis, the extra meal intake of soft water is preferable to hard water, since it is associated with a lower risk for recurrence of calcium stones.
_
Does drinking Hard Water cause more Kidney Stones?
A group of Wake Forest Urologists pondered this question in a study that compared the urine chemistry of patients who drank various kinds of water. They had 15 men who typically form stones and 14 who did not form stones; drank three kinds of water for two days each. The three types of water were “water of minimal hardness” (WMH), “tap water” (TW) and “mineral water” (MW). All of the men drank more water than normal it seems during the study, so overall urine output went up. The urine was less concentrated in the WMH and TH groups, and more concentrated in the MW group. The stone-former group had a rise in the urine calcium concentration, and the non-former group had no such rise. The result it seems is that hard water causes more kidney stones only if you have a tendency to stones in the first place. On the other hand, soft water didn’t prevent stones for anybody. So what to do? If you have had kidney stones perhaps you should avoid mineral water and stick to water of minimal hardness, like what you would get from a filtered water source.
_
Hard water leading to hair fall and skin problems:
Dermatologist Dr Kabir Sardana said the hardness of city water is making the skin of its inhabitants dry, which further leads to the problems of dry hair and hairfall. “Before treating patients, we generally ask their address as water quality plays a vital role in diagnosing a disease. Water in different sectors has become a standard to establish a disease easily,” he said, adding that measures like using glycerine-based soaps may reduce the skin problem to an extent.
_
Here are the effects that Hard Water has on your hair and scalp:
• Dryness of hair
• Tangling
• Eczema of the scalp
• Dandruff
• Lack of volume and shine
• Fading of color from dyed hair
• Permed hair losing curl
Softened water leaves a film on your skin because something has been added to your water. It’s true that your skin will feel softer and less dry after a shower in softened water because your natural body-moisturizing oils are better able to reach your skin’s surface. In addition, those soaps, shampoos and shower gels will suds up faster. After your shower in soft water, you are actually much cleaner than when you shower in hard water.
_
Treatment of hard water:
If you have a hard water problem, your solution could be through a water filtration system such as Reverse Osmosis (RO) (which will remove most minerals), Distillation (which will remove all minerals) or a Water Softener. For a whole house, reverse osmosis or other types of filtration are typically more costly options than a water softener.
_
The most common method of removing hardness from drinking water is the installation of a water softener. A water softener replaces the calcium and magnesium molecules with sodium molecules. For every milligram of hardness that is removed, 0.46 milligrams of sodium will be added to the water. Studies have shown that elevated levels of sodium in drinking water may have an adverse affect on health. Persons who suffer from high blood pressure or are on a sodium restricted diet should not drink water containing greater than 20 mg/l of sodium without first checking with a physician. A simple solution to the problem of consuming softened water is to have the kitchen cold water faucet bypass the water softener. By comparison, an 8-ounce glass of Coca-Cola has 30 milligrams of sodium while an 8-ounce glass of softened water has less than 12.5 milligrams. According to the Centers for Disease Control, a typical 1-ounce slice of bread has between 80 and 230 milligrams of salt, depending on the brand. So even though water softener adds sodium, it is comparatively less than sodium content of soft drink or bread.
_
Water softener:
1. Powdered or liquid chemicals:
Powdered or liquid water softeners are chemicals that can be added to a batch of water to help control water hardness. Products may form an insoluble precipitate with calcium and magnesium ions that make water cloudy and can build up on surfaces.
2. Ion exchange water softening units:
Ion exchange water softening units can be permanently installed into the plumbing system to continuously remove calcium and magnesium. The ion exchange process involves water passing through a media bed, usually sulfonated polystyrene beads, which are supersaturated with sodium. The ion exchange process takes place as hard water passes through the softening material. The hardness minerals attach themselves to the resin beads while sodium on the resin beads is released simultaneously into the water. When the resin becomes saturated with calcium and magnesium, it must be recharged. The recharging is done by passing a salt (brine) solution through the resin. The sodium replaces the calcium and magnesium which are discharged in the waste water.
3. Although not commonly used, potassium chloride can be used to create the salt brine. In that case potassium rather than sodium is exchanged with calcium and magnesium.
_
Pros and cons of water softening:
By removing dissolved minerals from water you will deprive your body of healthy nutrients like calcium and magnesium prevalent in hard water. The problem with that argument is that the calcium and magnesium in your water are in an inorganic form that your body cannot digest in the way that it can the minerals in your food or dietary supplements.
_
Ideal health-friendly hard water:
Based on the currently available data, various researchers have recommended that the following levels of calcium, magnesium, and water hardness should be in drinking water:
For magnesium, a minimum of 10 mg/L and an optimum of about 20-30 mg/L, For calcium, a minimum of 20 mg/L and an optimum of about 50 (40-80) mg/L. For total water hardness, the sum of calcium and magnesium should be 2 to 4 mmol/L. At these concentrations, minimum or no adverse health effects were observed. The maximum protective or beneficial health effects of drinking water appeared to occur at the estimated desirable or optimum concentrations. The recommended magnesium levels were based on cardiovascular system effects, while changes in calcium metabolism and ossification were used as a basis for the recommended calcium levels. The upper limit of the hardness optimal range was derived from data that showed a higher risk of gall stones, kidney stones, urinary stones, arthrosis and arthropathies in populations supplied with water of hardness higher than 5 mmol/L.
__
Correlation between TDS and hardness;
High TDS may indicate hard water, which causes scale buildup in pipes and valves, inhibiting performance. Since TDS is related to water hardness, using a TDS meter can be your first step in determining the degree of hardness of the water. Generally speaking, the higher the level of TDS (ppm), the higher the degree of hardness. However, TDS includes hard solids & soft solids, and organic & inorganic substances while hardness means inorganic calcium & magnesium salts. Water softeners do not remove TDS but exchange calcium and magnesium ions with sodium ions (salt). Therefore, the TDS level will remain virtually constant (there may be minor differences). Since a water softener does not lower the TDS level of your water, an additional filter may be necessary for your drinking water. In other words, a soft water can have high TDS. So TDS is not synonymous with hardness of water.
______
Understanding PH of water:
_
_
PH is simply a measure of the concentration of hydrogen ions. In fact, the acronym “pH” is short for “potential of hydrogen.” The higher a liquid’s pH, the fewer free hydrogen ions it has; the lower its pH, the more free hydrogen ions it has. One pH unit reflects a tenfold change in ion concentration – for example, there are ten times as many hydrogen ions available at a pH of 7 than at a pH of 8. The pH scale goes from 0 to 14, and a pH of 7 is neutral. Anything with a pH below 7 is considered acidic, with battery acid being the most extreme example, around 1. Anything with a pH above 7 is alkaline (or basic), with lye at the top of the scale, around 13. Natural water on our planet ranges in pH from 6.5 to 9.0, depending on surrounding soil and vegetation, seasonal variations and weather, and even time of day responses to sunlight. Human activities further influence the pH of our water, from the barrage of toxic industrial pollutants. Most aquatic animals and plants have adapted to life in water with a very specific pH, and will die from even slight changes. A pH below 4 or above 10 will kill most fish, and very few animals can tolerate waters with a pH below 3 or above 11.
_
Guidelines for the PH of Drinking Water for humans:
WHO state that pH usually has “no direct impact on consumers,” yet they also write pH is one of the “most important operational water quality parameters.” They do recommend your water pH be in the range of 6.5 to 8.0 so as not to corrode your pipes – and they’re not talking about your body’s plumbing: Alkalinity and calcium management also contribute to the stability of water and control its aggressiveness to pipe and appliance. Failure to minimize corrosion can result in the contamination of drinking water and in adverse effects on its taste and appearance. It appears that the WHO is more concerned about the pipes in your house than the pipes in your body. Most likely the optimal pH of the water you were designed to drink is somewhere between 6.5 and 8. Water that is too acidic or too alkaline can be detrimental to human health and lead to nutritional disequilibrium. This was demonstrated in a Swedish well water study, which found both pH extremes to be problematic. What you want is pure water – water that is clean, balanced, and healthful, neither too alkaline nor too acidic. Ideally, the pH of your water should be somewhere between 6 and 8.
_
It is important to know that our body naturally regulates our pH levels to find balance and equilibrium. Thus, under normal conditions it will always maintain a neutral 7.4 pH. Water pH will automatically change when it is ingested and comes in contact with food in your stomach.
Some examples of water pH and beverage pH levels:
Water & Beverage pH Levels
Sea Water = 8.6 pH
Soda = 2.5 pH
Mineral Water = 7.4 pH
Sports Drink = 2.9 pH
Tap Water = 6 to 8 pH
Coffee = 4 pH
Orange juice = 3 pH
RO Water = 5 to7 pH
Beer = 4.5 pH
The truth is, people drink acidic beverages all the time, they are just not aware of it. Since almost all fruit juices have lower pH, it is safe to say that it is not only safe to drink some acidic beverages but it is also beneficial. However acidic drinks such as soda should be kept to a minimal due to their high sugar content. Drinking slightly acidic water is also equally safe as long as the water is clean and contaminant free.
_
Basically, the pH value is a good indicator of whether water is hard or soft. The pH of pure water is 7. In general, water with a pH lower than 7 is considered acidic, and with a pH greater than 7 is considered basic. The normal range for pH in surface water systems is 6.5 to 8.5, and the pH range for groundwater systems is between 6 to 8.5. Alkalinity is a measure of the capacity of the water to resist a change in pH that would tend to make the water more acidic. The measurement of alkalinity and pH is needed to determine the corrosiveness of the water. In general, water with a pH < 6.5 could be acidic, soft, and corrosive. Acidic water could contain metal ions such as iron, manganese, copper, lead, and zinc. In other words, acidic water contains elevated levels of toxic metals. Acidic water can cause premature damage to metal piping, and have associated aesthetic problems such as a metallic or sour taste. It can also stain laundry and cause “blue-green” color staining on sinks and drains. More importantly, there are health risks associated with these toxins. The primary way to treat the problem of low pH water is with the use of a neutralizer. The neutralizer feeds a solution into the water to prevent the water from reacting with the household plumbing or from contributing to electrolytic corrosion. A typical neutralizing chemical is soda ash. Also known as sodium carbonate, soda ash works to increase the sodium content which increases pH. Water with a pH > 8.5 could indicate that the water is hard. Hard water does not pose a health risk, but can also cause aesthetic problems. These problems include an alkali taste to the water (making that morning coffee taste bitter!), formation of scale deposits on dishes, utensils, and laundry basins, difficulty in getting soaps and detergents to lather, and the formation of insoluble precipitates on clothing. According to a Wilkes University study, the association of pH with atmospheric gases and temperature is the primary reason why water samples should be tested on a regular basis. The study says that the pH value of the water is not a measure of the strength of the acidic or basic solution, and alone cannot provide a full picture of the characteristics or limitations with the water supply.
_
Is RO water harmful as it has lower pH?
Water from a reverse osmosis system or a distiller will be acidic. RO/distilled water does have a lower pH level. That’s because these systems remove dissolved bicarbonate solids but not acid-producing carbon dioxide. Without the bicarbonates to neutralize it, there is carbonic acid in the RO water. But it is not a health concern, nor will it endanger your water pipes. Although the pH level of untreated tap water will be about 7; the level of RO water is about 6. Soft drinks and sports drinks typically have a pH of 2.5; orange juice is at 3 pH; and coffee is at 4 pH. We drink all these beverages all the time without major problems.
_
Note:
The pH of any drink in the range of about 3 to 4 is of some concern because of the potential of low pH solutions to erode teeth.
_
Is alkaline water beneficial to humans?
The theory behind alkaline water is, in a nutshell, that alkaline (ionized) water is a powerful antioxidant with surplus electrons that can “mop up” the dangerous free radicals you have coursing through your veins. Marketers claim alkaline water can correct excess acidity in your tissues, which can then prevent or reverse cancer, arthritis, and other degenerative diseases. However, a study published in the Journal of Biological Chemistry found that alkalosis (rising cellular pH) causes alkaline-induced cell death as a result of altering mitochondrial function. And if you drink alkaline water all the time, you’re going to raise the alkalinity of your stomach, which will buffer your stomach’s acidity and impair your ability to digest food and open the door for parasites in your small intestine.
___________
Water sources:
The world’s water exists naturally in different forms and locations: in the air, on the surface, below the ground, and in the oceans. Although covering some 70% of the Earth’s surface, most water is saline. Of all water on earth, 97 per cent is salt water, and of the remaining 3 per cent fresh water, some 70 per cent is frozen in the polar icecaps. The other 30 per cent is mostly present as groundwater, with only a small fraction present above ground or in the air. Less than 1 per cent of the world’s fresh water is readily accessible for direct human uses. Over the past 40 years the world’s population has doubled. Our use of water has quadrupled. Yet the amount of water on Earth has stayed the same. Looking at how water moves through the Earth’s water cycle helps us understand how it interacts with the environment and how much is available for human use as seen in the figure below:
_
_
Can you take sea water and drink it?
On average, seawater in the world’s oceans has a salinity of about 3.5%. This means that every kilogram, or every liter, of seawater has approximately 35 grams of dissolved salts. Accidentally consuming small quantities of clean seawater is not harmful, especially if the seawater is consumed along with a larger quantity of fresh water. However, drinking seawater to maintain hydration is counterproductive; more water must be excreted to eliminate the salt (via urine) than the amount of water that is gained from drinking the seawater itself. The effect of seawater intake has also been studied in laboratory settings in rats. This study confirmed the negative effects of drinking seawater when dehydrated.
_
Seawater is only good to drink for humans who live near the sea and can afford the cash and the energy to take out the salt. For most of the population this is not an option. Desalinated water costs maybe 15 times more than regular water. It burns polluting fossil fuel energy, as solar-powered desalination is in its infancy. The quantity of freshwater that is available to a given country without exceeding the rate at which it is renewed, can be estimated taking into account the amount of precipitation, water flows entering and leaving the country, and water shared with other countries. The average amount available per person varies from less than 50 m3 per year in parts of the Middle East to over 100 000 m3 per year in humid and sparsely populated areas.
_
The source of virtually all freshwater is precipitation from the atmosphere, in the form of mist, rain and snow, as part of the water cycle over eons, millennia and in the present day. Surface water is stored in wetlands or lakes or flows in a stream or river, and is the most commonly utilized resource for water. In places, surface water can be stored in a reservoir behind a dam, and then used for municipal and industrial water supply, for irrigation and to generate power in the form of hydroelectricity. Sub-surface water, or groundwater, is fresh water located in the pore space of soil and rocks. It is also water that is flowing within aquifers below the water table. Groundwater can exist both as a renewable water system closely associated with surface water and as a separate, deep sub-surface water system in an aquifer. This latter case is sometimes called “fossil water”, and is realistically non-renewable. Normally, groundwater is utilized where surface sources are unavailable or when surface supply distribution is limited. Generally of high quality, groundwater is being withdrawn mostly to supply drinking water and support farming in dry climates. Ninety-six percent of liquid fresh water can be found underground. Groundwater feeds springs and streams, supports wetlands, helps keep land surfaces stable, and is a critical water resource. About 60% of the water that is taken from the ground is used for farming in arid and semi-arid climates, and between 25% and 40% of the world’s drinking water comes from underground. Hundreds of cities around the world, including half of the very largest, make significant use of groundwater. This water can be especially useful during shortages of surface water. The resource is considered renewable as long as groundwater is not withdrawn faster than nature can replenish it, but in many dry regions the groundwater does not renew itself or only very slowly. Few countries measure the quality of groundwater or the rate at which it is being exploited. This makes it difficult to manage.
_
Where does drinking water come from?
Drinking water can come from different resources. For one, it can be pumped from the ground through wells. This groundwater is than purified, so that it will contain no more contaminants and is suited to drink. Drinking water can also be prepared directly from surface water resources, such as rivers, lakes and streams. Usually surface water has to undergo many more purification steps than groundwater to become suited to drink. Preparing drinking water out of surface water is much more expensive due to this. Still 66% of all people are served by a water system that uses surface water. Part of our drinking water is pumped from the ground, usually under sand dunes. In sand dunes water can also be infiltrated. As it sinks into the ground through the dunes it is naturally purified. This costs much less money than the purification of surface water. Part of our drinking water originates from dune water.
_
Sources from where drinking water may be obtained include:
1. ground sources such as groundwater, hyporheic zones and aquifers.
2. precipitation which includes rain, hail, snow, fog, etc.
3. surface water such as rivers, streams, glaciers
4. biological sources such as plants.
5. the sea through desalination
6. water supply network
7. Spring water is groundwater that rises to the ground surface
_
_
How can the growing demand for drinking water be met?
1.Intercepting, diverting, storing and transferring water
2.Water re-use
3.Desalination
_
Intercepting, diverting, storing and transferring water:
1. People have been collecting rainwater for thousands of years – for example in Palestine, Greece, Rome, and South Asia. In India, rainwater has recently been used to replenish underground water. This technique is inexpensive and can be implemented locally. Larger projects have also been carried out to increase infiltration into the ground in areas where deforestation has reduced the availability of water.
2. Diverting surface water into basins and pits to increase infiltration into the ground can reduce evaporation, help replenish groundwater aquifers, and improve the quality of water. This practice is used in the Middle East and the Mediterranean. Runoff is collected and diverted in a variety of ways. Some methods reduce the need to treat the water.
3. Dams and reservoirs provide hydropower, supply water during shortages, enable fishing and the irrigation of farmland, and protect people from both floods and droughts.
4. The long-standing practice of interbasin transfer of water from one aquifer or river basin to another can help alleviate water shortages caused by agriculture and other human activities.
_
Water re-use:
The re-use of wastewater, made possible by technological advances in the last century, is now widespread. Once it has been extensively treated to remove biodegradable material, nutrients, and pathogens, it could be drunk, or used in a number of other ways. Reclaimed water or recycled water, is former wastewater (sewage) that is treated to remove solids and certain impurities, and used in sustainable landscaping irrigation or to recharge groundwater aquifers. Non-potable quality water can be used directly for irrigation, as a coolant in industry and to maintain river flows. Cities around the world where freshwater supplies are limited, such as San Diego in the United States, are developing programs to re-use water and to replenish aquifers with treated wastewater. Use of these techniques is expected to increase. The most viable programs use reclaimed waste water instead of drinking water for agricultural, industrial, and other uses. Countries in both water-short and more temperate but high-population regions are expected to increase their use of reclaimed water in the coming years. Reclaimed water is expected to account for 25% of Israel’s water supply in the next few years. Jordan will have to increase its use of reclaimed water fourfold to meet demand; Egypt, tenfold. Most Middle Eastern countries are expected to re-use more than half their wastewater. Australia, Belgium, China, Germany, Japan, and the United Kingdom are also expected to increase their use of reclaimed water, as this practice becomes an integral part of the management of water resources.
_
Potable use of reclaimed water:
In most locations, reclaimed water is not directly mixed with potable (drinking) water for several reasons:
1. Utilities providing reclaimed water for nonpotable uses do not treat the water to drinking water standards.
2. Varying amounts of pathogens, pharmaceutical chemicals (e.g., hormones from female hormonal contraception) and other trace chemicals are able to pass through the treatment and filtering process, potentially causing danger to humans. Modern technologies such as reverse osmosis may help to somewhat overcome this problem. An experiment by the University of New South Wales reportedly showed a reverse osmosis system removed ethinylestradiol and paracetamol from the wastewater, even at 1000 times the expected concentration.
3. Drinking water standards were developed for natural ground water, and are not appropriate for identifying contaminants in reclaimed water. In addition to pathogens, and organic and endocrine disrupting chemicals, a large number of compounds may be present in reclaimed water. They cannot all be tested for, and there is a paucity of toxicity information on many of the compounds. Because of this, state regulatory agencies do not allow reclaimed water to be used for drinking, bathing, or filling swimming pools. They also warn those who use reclaimed water for irrigation to place a sign on their property warning people not to drink from the irrigation system, and to not use it directly on fruits or vegetables.
_
Aboard the International Space Station, astronauts have been able to drink recycled urine due to the introduction of the ECLSS system. The system cost $250 million and has been working since May 2009. The system recycles wastewater and urine back into potable water used for drinking, food preparation, and oxygen generation. This cuts back on the need for resupplying the space station so often. Recent Advances in Reverse Osmosis have consistently produced very high quality water all the same. In Singapore, reclaimed water, also known as NEWater has become cleaner than the government tap water. Also, according to Bartels, the Bedok Demonstration Plant, which uses RO membranes, has successfully run for the past 3 years, producing high quality wastewater all the while.
_
Direct potable reuse (DPR):
Supplying highly treated reclaimed water directly to a drinking water distribution system is known internationally as direct potable reuse (DPR). This differs from more established approaches to potable water recycling by the absence of a so-called ‘environmental buffer’, a practice referred to as indirect potable reuse (IPR). IPR involves the storage of treated reclaimed water in environmental buffers – such as a river, lake, reservoir or aquifer – prior to it being recovered through drinking water treatment plants and distributed to consumers. Any DPR scheme includes a number of general characteristics. A source of municipal wastewater is required, such as effluent from wastewater treatment plants, which is purified using advanced water treatment processes to effectively and reliably remove hazardous substances including pathogens and toxic chemicals. It would not be possible to meet all demand for drinking water through recycling, so the use of additional water sources remains essential. Finally, most DPR projects require a means of blending the recycled water with conventionally sourced water prior to delivery to consumers. Conceptually, DPR can be developed in a number of alternative configurations which differ by their arrangement of the water sources, treatment processes and blending locations. The major difference between DPR and IPR, i.e. the use of environmental buffers, has been attributed a number of important functions. These include: additional treatment of pathogens and chemical contaminants; the provision of ‘time to respond’ to potential water treatment incidents; and improvement of public perceptions of potable water reuse. In order to maintain appropriate levels of safety, reliability, and public acceptance, such functions would need to be performed in any DPR system by engineered or other processes. This requires sophisticated approaches to water quality monitoring techniques, process reliability assessment, personnel training, engineered water storage design, and community engagement in particular. It is instructive to observe that there are a number of successfully operating DPR schemes internationally. The most established of these has been operating in Namibia since 1968 without observed negative impacts to public health. More recently, DPR projects have been developed in the US and South Africa, with both countries now actively considering additional developments within the next few years. Recent Guidelines for Water Reuse developed by the US Environment Protection Agency (EPA) state that “While DPR is still an emerging practice, it should be evaluated in water management planning, particularly for alternative solutions to meet urban water supply requirements that are energy intensive and ecologically unfavourable”. The State of California, in particular, is currently investigating the feasibility of developing uniform criteria for DPR. Potential benefits of DPR, relative to IPR, are likely to be highly case-specific. However, potential benefits include significantly lower energy requirements, construction costs, and operational costs. DPR can also provide an opportunity to allow potable reuse in situations where a suitable environmental buffer is not available for IPR. Increasing energy costs may have a significant impact on future decisions to choose between DPR, IPR, surface water dams, or seawater desalination. A cost benefit analysis may identify that an appropriately designed DPR scheme is less expensive to construct and operate than other methods of water supply while still meeting all regulatory requirements. Public acceptance remains an important and sometimes difficult issue for all planned potable water projects. However, there is evidence to suggest that acceptance is increasing generally and can be fostered by effective engagement and communication programs. The science, technology and engineering associated with DPR have been rapidly advancing in recent decades. DPR is growing internationally and will be an expanding part of global drinking water supply in the decades ahead. DPR is technically feasible and can safely supply potable water directly into the water distribution system, but advanced water treatment plants are complex and need to be designed correctly and operated effectively with appropriate oversight.
_
Machine squeezes Drinking Water from Your Sweaty T-Shirt:
One company has designed a system, called Sweat Machine, to wring sweat out of clothes and turn it into potable water. The Sweat Machine heats and spins clothes to extract the liquid from them, then filters the extract with a membrane developed with the Royal Institute of Technology in Stockholm. The filter is the most sophisticated part of the machine. Water vapor passes through the material easily, but it traps bacteria, salts and fibers from the clothes. Fans watching the Gothia Cup, an international youth soccer tournament held in Sweden, will get to see the Sweat Machine at work during the game. Players have promised to drink a glass of water extracted from their own sweat, according to UNICEF. Anybody else interested in getting a taste can try, too.
_
Desalination:
Desalination involves reducing its mineral content by taking salt out of seawater and brackish water and producing water of freshwater quality. Salt water is desalinated to produce fresh water suitable for human consumption or irrigation. It is used mainly by cities and industry, primarily in the Middle East (50%), but also in North America (16%), Europe (13%), and Asia (11%). The high costs of desalination, principally arising from the energy used, have dropped significantly in recent years due to technological advances. That energy is produced primarily with fossil fuels, which pollute the air, and each method of disposing of the by-products of desalination—for example in the ocean or in deep wells—has an impact on the environment. It has been suggested that the various means of disposal be assessed according to a single set of criteria, so that the impact of each desalination plant can be consistently evaluated. Desalination is used on many seagoing ships and submarines.
_
Huge reserves of freshwater lie beneath the ocean floor:
Scientists have found huge freshwater reserves under the world’s oceans. Scientists in Australia have reported the discovery of huge freshwater reserves preserved in aquifers under the world’s oceans. The water has remained shielded from seawater thanks to the accumulation of a protective layer of sediment and clay. And it’s not a local phenomenon. Such reserves are to be found under continental shelves off Australia, China, North America and South Africa. The discovery was made by researchers at the National Centre for Groundwater Research and Training (NCGRT) and the School of the Environment at Flinders University. The scientists estimate there is around half a million cubic kilometers of what they describe as “low salinity” water, which means it could be processed into fresh, potable water economically. The reserves formed when ocean levels were lower and rainwater made its way into the ground in land areas that were not covered until the ice caps melted 20,000 years ago, causing sea levels to rise. The volume of this water resource is a hundred times greater than the amount we’ve extracted from the Earth’s sub-surface in the past century since 1900. To access these non-renewable water reserves, it would be necessary to drill into the seabed from man-made, offshore platforms or from the mainland or nearby islands. Despite the high costs involved, the water would require less energy to desalinate than it does to desalinate sea water, although a careful assessment of the economics, sustainability and environmental impact of the exploration of such water reserves would be necessary.
_____________
Water supply, transport and store:
Water supply is the provision of water by public utilities, commercial organisations, community endeavors or by individuals, usually via a system of pumps and pipes. The most efficient way to transport and deliver potable water is through pipes. Plumbing can require significant capital investment. Some systems suffer high operating costs. The cost to replace the deteriorating water and sanitation infrastructure of industrialized countries may be as high as $200 billion a year. Leakage of untreated and treated water from pipes reduces access to water. Leakage rates of 50% are not uncommon in urban systems. Because of the high initial investments, many less wealthy nations cannot afford to develop or sustain appropriate infrastructure, and as a consequence people in these areas may spend a correspondingly higher fraction of their income on water. 2003 statistics from El Salvador, for example, indicate that the poorest 20% of households spend more than 10% of their total income on water. In the United Kingdom authorities define spending of more than 3% of one’s income on water as a hardship.
_
Plumbing:
The plumbing industry is a basic and substantial part of every developed economy due to the need for clean water, and sanitary collection and transport of wastes. Plumbing is the system of pipes, drains fittings, valves, valve assemblies, and devices installed in a building for the distribution of water for drinking, heating and washing, and the removal of waterborne wastes. Plumbing originated during ancient civilizations such as the Greek, Roman, Persian, Indian, and Chinese cities as they developed public baths and needed to provide potable water and drainage of wastes, for larger numbers of people. Standardized earthen plumbing pipes with broad flanges making use of asphalt for preventing leakages appeared in the urban settlements of the Indus Valley Civilization by 2700 B.C. The Romans used lead pipe inscriptions to prevent water theft. The use of lead for potable water declined sharply after World War II because of increased awareness of the dangers of lead poisoning. At this time, copper piping was introduced as a better and safer alternative to lead pipes. Present-day water-supply systems use a network of high-pressure pumps, and pipes in buildings are now made of copper, brass, plastic (particularly cross-linked polyethylene called PEX, which is estimated to be used in 60% of single-family homes), or other nontoxic material. Due to its toxicity, lead has not been used in modern water-supply piping since the 1930s in the United States, although lead was used in plumbing solder for drinking water until it was banned in 1986.
_
Leaking drinking water from water supply piping:
Fixing a leaking tap could help save tens of thousands of liters of clean water in a year. Ten percent of homes have leaks that waste 90 gallons or more per day. A faucet that drips one drop per second would waste 27,000 gallons of water annually. Worldwide, up to 60 percent of water is lost due to leaky pipes. Furthermore, water usage has been increasing at twice the rate of population growth in the last century. The simple solution would be to fix the broken pipes, but in the U.S. alone the estimated cost to fix its current water system would be $335 billion over 20 years.
_
Dual piping:
Dual piping is a system of plumbing installations used to supply both potable and reclaimed water to a home or business. Under this system, two completely separate water piping systems are used to deliver water to the user. This system prevents mixing of the two water supplies, which is undesirable, since reclaimed water is usually not intended for human consumption. In the United States, reclaimed water is distributed in lavender (light purple) pipes, to alert users that the pipes contain non-potable water. Hong Kong has used a dual piping system for toilet flushing with sea water since the 1950s.
_
Does dual water system work? Netherland experience:
Dual water supply systems were installed in several newly built housing estates in the Netherlands in the late1990s. These residential homes were provided with both drinking water and separately with so-called household water for toilet flushing, laundry and the garden tap. Household water was produced by limited treatment from a variety of sources and had a lower quality than drinking water. No legislation for (the quality of) this type of water was present at the time and the Dutch government appointed six of these estates as pilot projects. Four pilot projects were intensively monitored for toxicological and microbiological safety as well as microbiological stability during a period of almost 16 months. Specific incidents such as cross connections between drinking water and household water, and observations of viruses and pathogenic protozoa in treated water demonstrated that some of these systems were microbiologically unsafe. Furthermore certain household waters had a relatively high biofilm formation potential leading to growth of Legionella sp. and Aeromonas and complaints from customers about the smell and colour of the household water. In nearly all cases concentrations of heavy metals and organic pollutants were below drinking water standards, hence the toxicological risk caused by chemical substances was not significant. Based on the results of this study the Dutch government decided to discourage the production and distribution of household water on a large scale. At present all projects owned by water companies in the Netherlands have been terminated by replacing household water with drinking water.
_
Spragg bag:
_
Terry Spragg of Manhattan Beach, California, builds flexible fabric barges for the transportation of bulk fresh water and is the reason why his product is referred to as the “Spragg Bag.” In the 1970s Spragg was a promoter of icebergs as a large source of fresh water, but soon realized this was impractical. He then put his skills into developing the waterbag technology starting in the 1980s. Spragg has worked on and perfected this over the last twenty years with his associates. The first field test of his waterbag was in December 1990. The waterbag was 75 meters long (245 feet) and it contained approximately 3,000 cubic meters (790,000 US gal) of fresh water. The 1995 associated Spragg patents indicate that the inventions relate to a flexible fabric barge technology or combination of several barges made of a rubber polyurethane material. The main body portion of a flexible fabric barge is cylindrical in shape. The barge can be used by itself or as several connected flexible fabric barges that can be towed through the open ocean under extreme conditions. The patents further explain that the goal of Spragg’s inventions are a practical water delivery system of fresh drinkable water that could be delivered to dry regions worldwide that have a shortage of potable water. One of the flexible fabric barge concepts aims at an economical delivery system for fresh water that would be considerably cheaper than desalination plants, rigid ships, tanker trucks, conventional barges, aqueducts or pipeline transport. Spragg bags are more economical and better for the environment than desalination of the seas and oceans. One application seen is in the Middle East where large quantities of fresh water that are available in the Turkey region could be delivered to other places around the Mediterranean Sea that have an extreme shortage of drinkable fresh water, like Israel and Gaza. Spragg believes that delivering fresh drinking water to water-poor nations can promote world peace.
_
Spragg bag and disaster:
Disasters happen. Earthquakes, cyclones, hurricanes, tsunamis, and more. And as we see at each disaster, the first and most urgent need after rescue operations are finished is usually clean, adequate water. What do we do? We load heavy pallets of plastic bottles filled with water onto cargo planes and fly them over to disaster areas. While the generosity of the bottled water companies, who typically donate their product, is indisputable, there must be a better way to get more water on site, cheaper and faster. That is spragg bag. The large bags — and they can probably be made any size we want — can be pre-positioned throughout the world, folded and stored. In a disaster, they can be immediately filled with freshwater from any safe source: surviving municipal systems, rivers, small-scale purification plants, or desalination units and towed through the oceans to places of need. Or they can be driven or flown, empty, to the site of a disaster and filled locally. Two Military Sealift Command ships brought portable desalination systems to the Maldives during relief operations in February 2005 that could be used to fill such temporary storage bags. During the recent disaster in American Samoa, a U.S. Navy guided missile frigate, the USS Ingraham, was on scene very quickly, offering aid. Many Navy ships are capable of desalinating seawater and could fill such bags for distribution in emergencies, if the bags were either prepositioned or quickly flown in. One of Spragg’s innovations was to design the bags so multiple bags could be connected in a “train” with massive zipper systems. In this way, many bags could be towed through the oceans at a time. Spragg is still around, but his idea has never been pursued commercially because the economics of such a system for regular water supply are marginal. But disaster relief is another story.
_
Water Storage:
_
_
Containers:
Use only food-grade containers. Smaller containers made of PETE plastic or heavier plastic buckets or drums work well. Clean, sanitize, and thoroughly rinse all containers prior to use. A sanitizing solution can be prepared by adding 5 ml (1 teaspoon) of liquid household chlorine bleach (5 to 6% sodium hypochlorite) to 1 liter (one quart) of water. Only household bleach without thickeners, scents, or additives should be used. Do not use plastic milk jugs, because they do not seal well and tend to become brittle over time. Do not use containers previously used to store non-food products.
_
Solar Cookers International (SCI) has incorporated the Safe Household Water Storage container in their water pasteurization programs in Kenya. They are part of a safe water package that consists of a CooKit solar cooker, a black pot, a Water Pasteurization Indicator (WAPI), and a Safe Household Water Storage container. The containers are handmade out of clay by local artisans. Their design incorporates a small opening at the top to help prevent children from dipping cups and possibly dirty hands into the drinking water. There is a spigot at the bottom. The unglazed clay container helps to keep the water naturally somewhat cool in dry climates because a very small amount of the water is absorbed by the container and then evaporates.
___________
Reliability of water sources:
Water sources may be variable and unreliable. Water reliability may vary by season, by year, and by location. In some areas, the rains fall mainly during the monsoon seasons, leaving dry conditions at other times of the year. Large scale climate variability such as the influence of El Niño and La Niña may mean that one year is wet while the next is dry. The quantity of water in rivers and lakes can also be unreliable. Some rivers only flow during part of the year, leaving a dry riverbed and no local source of water. Rivers and lakes can also dry up from overuse. At the household level, the reliability of the distribution system that provides water to the people is critical to maintaining quantity. If pipes are broken or only intermittent service is available, the quantity of drinking water suffers. Often the unreliability of surface water can be offset by the use of groundwater. However, if ground water sources are depleted too rapidly, or are not being successfully recharged by either natural or man-made processes, the quantity of drinking water suffers.
__________
Water scarcity:
_
_
Water scarcity is set to be one of the biggest environmental challenges of the 21st century, with global warming, deforestation, overpopulation, industrial demand, irrigation and several other factors taking a huge toll on the planet’s water reserves. Clean, safe drinking water is scarce. Today, nearly 1 billion people in the developing world don’t have access to it. Yet, we take it for granted, we waste it, and we even pay too much to drink it from little plastic bottles. Water is the foundation of life. And still today, all around the world, far too many people spend their entire day searching for it. In places like sub-Saharan Africa, time lost gathering water and suffering from water-borne diseases is limiting people’s true potential. Education is lost to sickness. Economic development is lost while people merely try to survive. But it doesn’t have to be like this. Its needless suffering.
_
Simply put, water scarcity is either the lack of enough water (quantity) or lack of access to safe water (quality). Water scarcity can take two forms: physical water scarcity, or low quantity of water, and economic water scarcity, or low quality of water. Economic water scarcity applies to areas or cultures that lack the fiscal resources and/or human capacity to invest in water sources and meet the local demand. Water is often only available to those who can pay for it or those in political power; leaving millions of the world’s poorest without access. Regions most affected by this type of scarcity are portions of Central and South America, Central Africa, India, and South East Asia. It is important to highlight the distinction between these two forms of scarcity: water can be physically available, but the resources are not available to improve it and distribute it to those who need it.
__
What are Improved Technologies for Drinking Water?
_
The WHO/UNICEF Joint Monitoring Program 2008 for Water Supply and Sanitation (JMP) considers bottled water a source of improved drinking water only when another improved source is also used for cooking and personal hygiene.
_
The largest democracy India and water:
India’s growing population is putting a strain on the country’s water resources. The country is classified as “water stressed” and a water availability of 1,000-1,700 m3/person/year. According to UNICEF, in 2008, 88% of the population had access and was using improved drinking water sources. “Improved drinking water source” is an ambiguous term, ranging in meaning from fully treated and 24 hour availability to merely being piped through a city a sporadically available. This is in part due to large inefficiencies in the water infrastructure in which up to 40% of water leaks out. In the same 2008 UNICEF report, only 31% of the population had access and used improved sanitation facilities. Open sewers are common place in urban areas. A little more than half of the 16 million residents of New Delhi, the capital city, have access to this service. Every day, 950 million gallons of sewage flows from New Delhi into the Yamuna River with any significant forms of treatment. This river bubbles with methane and was found to have a fecal coliform count 100,000 time the safe limit for bathing. Due to surface water contamination due to lack of sewage treatment and industrial discharge, groundwater is becoming increasingly dependent on and exploited in many regions of India. This process is being expedited by heavily subsidized energy costs for agriculture practices; which make up roughly 80% of India’s water resource demand.
_
In India 80 per cent of rural households depend on untreated water sources, over 40 per cent of which are contaminated. Ground water in around 200,000 rural habitations has been found to be chemically contaminated, which means it has excess fluoride, chloride, iron, nitrates and even traces of arsenic. Due to lack of awareness and absence of alternatives, villagers continue to consume water from contaminated sources. Contaminants such as fluoride and arsenic have a severe impact on health resulting in skeletal fluorosis, dental fluorosis and arsenic poisoning. WHO estimates around 87 million Indians (75 per cent of which are children) are affected by water-borne diseases annually. The health burden of poor water quality is enormous. 1.5 million children are estimated to die of diarrhea alone and 73 million working days are lost due to waterborne disease each year. The resulting economic burden is estimated at $600 million a year.
_
The World Health Organization/UNICEF Joint Monitoring Program (JMP) for Water Supply and Sanitation is the official United Nations mechanism tasked with monitoring progress towards the Millennium Development Goal (MDG) relating to drinking-water and sanitation (MDG 7, Target 7c), which is to: “Halve, by 2015, the proportion of people without sustainable access to safe drinking-water and basic sanitation”. The JMP is required to use the following MDG indicator for monitoring the water component of this: Proportion of population using an improved drinking-water source. According to the latest estimates of the WHO/UNICEF Joint Monitoring Program for Water Supply and Sanitation (JMP), released in early 2013 (collected in 2011), 36 per cent of the world’s population – 2.5 billion people – lack improved sanitation facilities, and 768 million people still use unsafe drinking water sources. Inadequate access to safe water and sanitation services, coupled with poor hygiene practices, kills and sickens thousands of children every day, and leads to impoverishment and diminished opportunities for thousands more. The health burden of poor water quality is enormous. The Millennium Development Goal for water calls for halving the population without access to safe drinking water by 2015. There are two key water quality issues that undermine the safety of drinking water and affect the lives of hundreds of millions of children around the world:
•Faecal contamination of drinking water, which is a leading cause of the 4,000 daily deaths from diarrhoea amongst children under the age of five.
•Contamination of drinking water with naturally-occurring arsenic or fluoride, threatening the health of tens of millions of people.
_
In 2010, about 85% of the global population (6.74 billion people) had access to piped water supply through house connections or to an improved water source through other means than house, including standpipes, water kiosks, spring supplies and protected wells. However, about 14% (884 million people) did not have access to an improved water source and had to use unprotected wells or springs, canals, lakes or rivers for their water needs. 780 million people lack access to an improved water source, according to the WHO/UNICEF Joint Monitoring Program 2013. That’s approximately three times the size of the USA. And for all the UN’s recent boast about hitting drinking water targets, experts estimate that maybe three billion people worldwide still lack safe water to drink.
_
One organization working to improve the availability of safe drinking water in some the world’s poorest countries is WaterAid International. Operating in 26 countries, WaterAid is working to make lasting improvements to peoples’ quality of life by providing long-term sustainable access to clean water in countries such as Nepal, Tanzania, Ghana and India. It also works to educate people about sanitation and hygiene.
__________
Definitions vis-à-vis drinking water:
1. Improved drinking water source is defined as a drinking water source or delivery point that, by nature of its construction and design, is likely to protect the water source from outside contamination, in particular from fecal matter.
2. Safe drinking water is water with microbiological, chemical and physical characteristics that meet WHO guidelines or national standards on drinking water quality.
3. Drinking water or potable water is water safe enough to be consumed by humans or used with low risk of immediate or long term harm. In most developed countries, the water supplied to households, commerce and industry meets drinking water standards, even though only a very small proportion is actually consumed or used in food preparation.
4. The World Bank defines safe water as ‘treated surface water and untreated but uncontaminated groundwater.’ Safe water is crucial not only for human health (on average, a human needs 20 liters of safe water for his daily ‘metabolic, hygienic and domestic needs’) but also for economic development and environmental health. Yet, more than 1/3 of the world cannot drink their local water supply without additional treatment.
5. Tap water, delivered by domestic water systems in developed nations, refers to water piped to homes and delivered to a tap or spigot or faucet. For these water sources to be consumed safely they must receive adequate treatment and meet drinking water regulations.
_
In general, water for drinking and cooking should be wholesome. It should be both potable and palatable. It must be bacteriologically and chemically safe for drinking. It should be clear, colorless, and have no unpleasant taste or odor.
_
Potable, non-potable and recycled water:
The word potable came into English from the Late Latin potabilis meaning drinkable. Potable water is water of a quality suitable for drinking, cooking and personal bathing. The standards that define potable water are described by WHO Guidelines. Potable water may also be used for other tasks that non-potable water is used for, such as watering plants, flushing toilets and washing cars. Non-potable water is water that is not of drinking water quality, but which may still be used for many other purposes, depending on its quality. The figure below denotes logo of non-potable water:
_
Recycled water is any water that has been used at least once and then supplied for reuse, either treated or untreated. Without appropriate treatment, recycled water may contain a range of contaminants. Recycled water that has not been treated to the level of drinking water standard is just one form of non-potable water. But there are many other types of non-potable water, including stormwater, dam and creek water, bore water and even rainwater collected in rainwater tanks. If non-potable water is adequately treated, its quality will improve and it can be used for a wider range of purposes. Unless water is known to be of potable quality (e.g. from a drinking water supply system) it should be regarded as non-potable and used appropriately.
Some examples of non-potable water used at workplaces are:
•class A recycled water from a sewage treatment plant used for dust suppression, car washing, landscape irrigation or irrigation of sporting ovals
•rainwater from tanks used for various workplace uses, e.g. cooling towers and car washing
•quarry water used for dust suppression and landscape irrigation
•swimming pool backwash water used for toilet flushing
•agricultural waste water used for crop irrigation
•creek, dam, and river water used for dust suppression.
_
Types of drinking water:
I have already discussed hard water and soft water. Now let me discuss various types of drinking water:
_
Tap water:
Tap water (running water, city water, municipal water, etc.) is water supplied to a tap (valve) inside the household or workplace, replacing the manual carrying of water from sources outside the building. Its uses include drinking, washing, cooking, and the flushing of toilets. Tap water is the essential component of “indoor plumbing”, which has existed since antiquity but was available to very few people until the second half of the 19th century, when it began to propagate in what are now the developed countries. It became common in many regions during the 20th century, and is now lacking only among the poor, especially in developing countries. Tap water is often culturally assumed to be potable water, especially in developed countries. More often than not, it is, although water quality problems are not unusual. Household water purification methods such as water filter, boiling, or distillation can be used when the potability is doubted. The application of technologies (such as water treatment plants) involved in providing clean water to homes, businesses, and public buildings is a major subfield of sanitary engineering. Calling a water supply “tap water” distinguishes it from the other main types of fresh water which may be available; these include water from rainwater-collecting cisterns, water from village pumps or town pumps, or water carried from streams, rivers, or lakes (whose potability may vary).
_
Well water:
Well water could be the source of other types of consumable bottle waters such as ‘drinking’ or regular tap water. The main definition of ‘well water’ is water that has been stored in permeable rocks and soil. If a drill is employed to find the original aquifer, then the collected water is considered ‘well water’. Because it has spent time in contact with natural minerals, well water is generally an excellent drinking water. Some processing may be necessary because of possible contamination in the soil, but many private land owners have wells that last for years and the water is naturally healthy. Specific companies can market their bottled waters as ‘well water’ as long as they pass FDA standards and are clearly from a protected water source located underground. Some municipal waters may also come from underground sources, so ordinary tap water may also qualify as well water under the right circumstances.
_
Spring water:
Well water and spring water are similar in the sense that they are both produced from natural aquifers located around rockbeds and soil. Spring water, however, continues naturally to the surface. Water which comes from below and has no natural tributaries is considered to be spring water. It’s also a very good water to drink during and after exercise or throughout the day. Bottlers may use some natural processes such as reverse osmosis to improve water quality, but spring water must be naturally rich in trace minerals. Some municipalities also use spring water as a source for their tap waters, but they are processed with chemicals and more advanced filtration systems. Spring water is perhaps the best overall water for health benefits and rehydration. It has a good taste and is fairly inexpensive at grocery stores.
_
Mineral water:
Mineral water is water from a mineral spring that contains various minerals, such as salts and sulfur compounds. Mineral water may be effervescent (i.e., “sparkling”) due to contained gases. The U.S. Food and Drug Administration classifies mineral water as water containing at least 250 parts per million total dissolved solids (TDS), originating from a geologically and physically protected underground water source. No minerals may be added to this water. In many places, however, the term “mineral water” is colloquially used to mean any bottled carbonated water or soda water, as opposed to tap water. In the European Union, bottled water may be called mineral water when it is bottled at the source and has undergone no or minimal treatment. Permitted is the removal of iron, manganese, sulfur and arsenic through decantation, filtration or treatment with ozone-enriched air, in so far as this treatment does not alter the composition of the water as regards the essential constituents which give it its properties.
_
Lithia water:
Lithia water is defined as a mineral water characterized by the presence of lithium salts (as lithium carbonate or lithium chloride). Natural lithia mineral spring waters are rare and there are few commercially bottled lithia water products. Between the 1880s and World War I, the consumption for bottled lithia mineral water reached gigantic proportions. The most premium of all the mineral water brands were lithia waters because of their highly acclaimed health benefits. One of the first commercially sold lithia waters in the United States was bottled at Lithia Springs, Georgia in 1888. During this era there was such a demand for lithia water that there was a proliferation of bottled lithia water products, however only a few were natural lithia spring waters. Most of the bottled lithia water brands added lithium bicarbonate to spring water and called it lithia water. A published researched study in 1992 about trace elements indicated that individuals with heart disease, learning-disabilities, and incarcerated violent criminals were found to have lithium deficiencies (as measured through hair sample analysis). Research studies measuring the effects of trace levels of lithium, commonly found in lithia waters, have demonstrated neuroprotective abilities, as well as improvements in mood and cognitive function. In 1949, psychiatrist John Cade discovered the anti-manic effects of lithium ions. This finding led to lithium carbonate to be used to treat mania. Lithium compounds yield positive outcomes as a treatment for manic depressive disease. Research studies published the British Journal of Psychiatry 2009 found that communities with naturally occurring lithia waters have lower suicide rates, mental hospital admissions, incidences of crimes, and arrests related to drug addictions. On February 8, 2011, German researchers at Friedrich Schiller University Jena published their findings in the European Journal of Nutrition (Nature Publishing Group) indicating that lithia waters lead to an increased life expectancy in humans and metazoans. A clinical pilot study using ĔDJ lithia water from British Columbia is underway at the University of British Columbia. It will investigate whether daily use of lithia water will improve new brain cell formation (neurogenesis) and reduce neuronal oxidative stress (neuroprotection).
_
Bottled water (vide infra):
Bottled water is simply drinking water from some source that a company (or in the case of water vending machines, the consumer) has placed in a bottle for resale.
_
Canned water:
Canned water is drinking water packaged in tin cans or beverage cans, a less common alternative to bottled water. Canned water is used primarily where storage or distribution systems are set up for cans, or when canning systems are used to make emergency water supplies. Water was stored in steel cans, lined with plastic bags, under the United States Civil Defense program. Approximately twelve million 17.5-US-gallon (66 L) cans were deployed, and could hold water for more than ten years.
_
Holy water:
Holy water is supposed to be cleansing, but a recent study found that 86 percent of water commonly used in baptisms and to wet congregants’ lips was infected with bacteria found in fecal matter. Researchers at the Medical University of Vienna tested water from 21 springs and 18 fonts in Austria and discovered that the samples contained up to 62 million bacteria per milliliter of water. Types of bacteria included E. coli, enterococci and Campylobacter. The study, which is published in the Journal of Water and Health, also found that all the church and hospital chapel fonts in the study contained bacteria. The busier the church or hospital, the higher the number of bacteria. This may represent a problem that has hitherto been underestimated, especially in hospitals, since there are a lot of people with weakened immune systems there. In addition to bacteria, holy water samples also contained nitrates, which likely come from runoff from fertilizer use. (Safe levels of nitrates are less than 10 milligrams per liter.) Ingesting water with high levels of nitrates can cause serious illness or even death in infants under six months of age, according to the EPA. Symptoms of nitrate ingestion include shortness of breath and blue baby syndrome. Only 14 percent of the holy water tested met the microbiological and chemical requirements of national drinking water regulations. Churches should regularly replace holy water, and place signs at holy springs to warn visitors that the water isn’t safe to drink. As far as holy water from river Ganga in India is concerned, concentrations of bacteria that indicate fecal contamination sometimes reach 1.5 million in a 100-milliliter test tube of water. In the U.S., beaches are closed when concentrations reach 200.
_
Demineralized water:
Demineralized water is defined as water almost or completely free of dissolved minerals as a result of distillation, deionization, membrane filtration (reverse osmosis or nanofiltration), electrodialysis or other technology. The total dissolved solids (TDS) in such water can vary but TDS could be as low as 1 mg/L. The electrical conductivity is generally less than 2mS/m and may even be lower (<0.1 mS/m). The demineralized water has not been proven to be healthier than drinking treated tap water. The World Health Organization investigated the health effects of demineralized water in 1980, and its experiments in humans found that demineralized water increased diuresis and the elimination of electrolytes, with decreased serum potassium concentration.
_
Deionized water:
Deionized water is water in which the mineral ions (salts such as sodium, calcium, iron, copper, chloride and bromide) have been removed by exposing it to electrically charged resins that attract and bind to the salts.
_
Alkaline water:
Alkaline water, also commonly known as ionized water, is water that has a pH level greater than seven. This water is generally produced with the aid of a faucet-based water ionizer, or “alkalizer” and features a number of purported health benefits.
_
Purified water:
Purified water is water that is mechanically filtered or processed to be cleaned for consumption. Distilled water and deionized (DI) water have been the most common forms of purified water, but water can also be purified by other processes including reverse osmosis, carbon filtration, microfiltration, ultrafiltration, ultraviolet oxidation, or electrodialysis. In recent decades, a combination of the above processes have come into use to produce water of such high purity that its trace contaminants are measured in parts per billion (ppb) or parts per trillion (ppt). Purified water has many uses, largely in science and engineering laboratories and industries, and is produced in a range of purities. Purified water in colloquial English can also refer to water which has been treated (“rendered potable”) to neutralize, but not necessarily remove contaminants considered harmful to humans or animals.
_
Now I will discuss two types of purified water in detail, ultrapure water and distilled water.
_
Ultrapure water:
_
The figure below depicts stages of water purity:
_
Ultrapure water (UPW) refers to water with high purity that has been made as close as possible to H2O by integrating all elemental technologies for water purification. The purity of water is upgraded to an ultra high level by removing not only solid substances and salts but also gas dissolved in water. Ultrapure water (UPW) refers to water that has been purified to uncommonly stringent specifications. The primary use of ultrapure water is as the prime cleansing agent in semiconductor manufacturing. For semiconductors, RO water isn’t even close. Ultra-pure water requires 12 filtration steps beyond RO. The final filter in making UPW has pores that are 20 nanometers wide. UPW is also used in pharmaceutical manufacturing, but it is a purely human form of water–water that is literally nothing like the stuff that exists naturally on Earth. Water is a good cleaner because it is a good solvent–the so-called “universal solvent,” excellent at dissolving all kinds of things. UPW is particularly “hungry,” in solvent terms, because it starts so clean. That’s why it is so valuable for washing semiconductors. Ultrapure water is used in a wide range of applications, such as:
1. Water for cleaning the surfaces of semiconductor wafers and liquid crystal panels, which must have no minute foreign particles.
2. Water for steam generators for the power generating turbines needed for the stable operation of power stations
3. Refined water and injection water for medical care and pharmaceutical industries, where safety is vital in any situation, and
4. Blank water for microanalysis in analytical chemistry, directly linked with the analysis level.
_
Dialysis water is close to ultrapure water:
Dialysis water:
Hemodialysis machines water consumption varies from 300 ml/min to 800ml/min depending on flow rate of dialysate. Routinely it is 500 ml/min. Therefore each machine routinely consumes 30 L/hour of water. You need extra capacity for compensating decreasing efficiency of plant and also for dialyser washing. A higher standard is required for water for haemodialysis as approximately 120 to 300 liters of water is required for a single dialysis session per person. Typically, water from the public supply passes through mechanical filters, water softeners, charcoal filters, deionizers and the reverse osmosis filter and is finally stored in tanks for subsequent distribution through polyvinyl chloride (PVC) tubing to the whole water system of the unit. The most effective method, distillation, is not used for dialysis water for two major reasons: it requires large amounts of energy and the yield is relatively small compared to the rate of required water consumption. Unlike your drinking water that contains chloramines for disinfection purposes, dialysis centers must remove this before treating its patients.
_
Why does the water used for dialysis treatments have to be ultra pure?
The thin, hair-like threads inside your dialyzer are hollow. The walls of these fibers are made of a semipermeable material which acts like a filter. During your dialysis treatment, your blood flows inside these hollow fibers, while the outside of the fibers is bathed in dialysate. Dialysate is a cleansing solution which is a mixture of water and chemicals that pulls the wastes and extra fluid through the fibers and out of your blood. However, because the fibers are semipermeable, if the water used to make the dialysate is not completely pure, impurities from the water in the dialysate can get into your blood. Many of these impurities can cause you serious harm. If anything less than ultra pure water is used during your dialysis treatment, a variety of things could happen:
• Too much calcium or magnesium can cause nausea, vomiting, muscle weakness, severe headaches, skin flushing and low or high blood pressure.
•Metals can cause a variety of symptoms including liver damage, inflammation of the pancreas, destruction of red blood cells, seizures, brain damage and even death.
•Pesticides and fertilizers can cause headaches, dizziness, convulsions and heart and liver damage.
• The chemicals added to destroy bacteria will destroy red blood cells if they enter the blood stream
• Bacteria and endotoxin can cause infections and fever.
• Overexposure to fluoride can cause abnormal hardening of bones, as well as nausea and vomiting, muscle twitching, low blood pressure and seizures.
Turning tap water into ultra pure water for dialysis treatments is essential to protect dialysis patients from harm or injury.
_
Distilled water:
Distillation is a process by which water is boiled until vapor is produced. This vapor is collected and cooled until it returns to a liquid state. Because minerals are too heavy to be carried by the vapor, the resulting water is completely free of additives. A desalination plant is a perfect example of distillation- salt water is boiled, the vapor is cooled and collected, and the salt and minerals are left behind. However, distilled water is also very unpalatable in its natural state. Desalination plants must also add some minerals in order to make the water usable for general consumers.
_
Is distilled water helpful or harmful to human health?
Let me begin with the benefits of distilled water:
Dr. Alexander Graham Bell, inventor of the telephone, recognized the health value of distilled water, and claimed that its daily use prolonged his life. Afflicted and bed-ridden with sciatica, Dr. Bell could find no relief for the pain. The attack came just as he was investigating the deposit of salts in the human system. A well-known scientist had written a book in which he said that old age came from such deposits, and that the ills of advanced years were due to the lack of their elimination. He believed that when such deposits went to the joints, man had rheumatism. When they went to the kidneys, he had kidney trouble and stones in the urinary organs; and when they lodged in the arteries, they produced what is called hardening of the arteries. In the same way when such deposits coated the nerves, they caused sciatica. Dr. Bell wrote: “I knew that distilled water was pure. I thought that if I drank plenty of it, I could get rid of some of the salts that were covering my sciatic nerves. I tried drinking it and it worked like a charm. I have kept up my drinking of distilled water and I attribute my almost perfect health largely to it.”
_
Eminent physicians for many years have recognized and advocated the health value of distilled water, both for prevention of disease and for the restoration of health. C.W. DeLacy Evans, M.D., in his book, How To Prolong Life, claims that distilled water, used regularly in place of spring water or other water containing inorganic minerals, tends to ward off the aging process by preventing the formation of calcareous deposits that cause hardening of the arteries. He writes: “Used as a drink, distilled water is absorbed directly into the blood, the solvent properties of which it increases to such an extent that it will keep in solution salts already existing in the blood, prevent their undue deposit in various organs and structures, favor their elimination by the various excreta, and tend to remove these earthy compounds which have already accumulated in the body . . . There is no doubt as to the high value of distilled water used freely as a retarder of the ossifying conditions which appear to constitute the condition of old age.” Referring to the origin and means of preventing the formation of calcareous deposits in the body, which produce the symptoms of senility by gradually ossifying it, and which come from use of hard water, Dr. de la Torre writes: “Instead of drinking the hard water of springs or the chlorinated water of the cities, it will be to our advantage to drink distilled water . . . to prevent calcification of the body.” Dr. Charles McFerrin, writing in the July 1955 issue of Nature’s Path writes: “Distilled water is ’empty’ water – a hungry water, a water capable of absorbing body poisons. You have had the experience of trying to use an old post office blotter on the desk. Everybody had used it and it is so full of ink that it will not suck up any more. So it is with a ‘full’ water, a water full of chlorine, aluminum, etc. Such water does not have the capacity of absorbing body impurities.” Daily use of distilled water is a marvelous blood purifier, helping to bring into solution and dilute any toxins in the body, as well as aid in their elimination through the kidneys. It should be used for cooking and baking as well as for drinking. For health’s sake, it is important to use only distilled water, which is a supreme internal body-cleansing agent. It aids in the removal of waste matter by bringing into solution and washing out through the excretory channels impurities that have accumulated and settled in the body, such as uric acid deposits that cause rheumatism. It helps promote osmotic interchange through the kidney tubules, thereby furthering the elimination of toxins through the urine.
_
Now I will discuss harmful effects of distilled water:
Some health conscious people have been misled into believing that distilled water is healthy for them. Distillation is the process in which water is boiled, evaporated and the vapour condensed. Distilled water is free of dissolved minerals and, because of this, has the special property of being able to actively absorb toxic substances from the body and eliminate them. Distilled water has the wrong ionization, pH, polarization and oxidation potentials, and if you drink it for too long it can drain your body of necessary minerals. This happens because distilled water is like a vacuum without any minerals, so it will actually leach beneficial minerals from your body to balance it out. While this might be beneficial for a short period during some sort of detoxification regimen, it’s usually highly counter-productive in the long run. Fasting using distilled water can be dangerous because of the rapid loss of electrolytes (sodium, potassium, chloride) and trace minerals like magnesium, deficiencies of which can cause heart beat irregularities and high blood pressure. Cooking foods in distilled water pulls the minerals out of them and lowers their nutrient value. Distilled water is an active absorber and when it comes into contact with air, it absorbs carbon dioxide, making it acidic. The more distilled water a person drinks, the higher the body acidity becomes. According to the U.S. Environmental Protection Agency, Distilled water, being essentially mineral-free, is very aggressive, in that it tends to dissolve substances with which it is in contact. Notably, carbon dioxide from the air is rapidly absorbed, making the water acidic and even more aggressive. The water then becomes acidic and seeks to balance itself by drawing minerals right out of your body. It will also draw out contaminants from the container it’s stored in for this same reason. Many metals are dissolved by distilled water. What’s worse, any contaminant in the water that vaporizes at a lower temperature than the water, such as volatile organic compounds, like disinfection byproducts that are thousands of times as toxic as chlorine, will be condensed and actually concentrated in the finished distilled water. So what you end up with is water that contains even more dangerous contaminants than what you started with!
_
Experiments in rats showed evidence that the consumption of distilled water can increase water intake and extracellular fluid volume, eliminating sodium, potassium, chloride, calcium and magnesium from the body, and lowering the volumes of red cells; resulting in overall negative balance if minerals are not adequately compensated from food.
_
Balanced view:
Is it correct that if you were to drink distilled water or ultrapure water, purified to a higher standard than drinking water, not having the usual balance of dissolved particles, would act as a solvent and thus be harmful?
Bottom of Form
No, it’s not correct. The difference between in total dissolved solids (TDS) between surface water/ground water, tap water, distilled water and finally ultrapure water is small enough that your body will overcome the difference. Surface and ground waters (that aren’t brackish or saline) may range from 150-1500 mg/L TDS. Tap water should be below 500 mg/L TDS. Distilled and ultrapure water should be near zero TDS. So the difference in TDS between natural waters and ultrapure/distilled water is about 0.5 g per liter. Considering you eat several hundred grams of food every day, and most of the fluids in your body have significant TDS concentrations, your body has no problem buffering the lack of dissolved solids in distilled/ultrapure water.
___________
Altered water (enhanced water):
‘Altered’ Water is water that has been treated in some way to allegedly modify the physical, chemical, or ‘energy’ properties of water to provide some benefit to the body. These treatments fall under a wide range of categories, including: pi mag; oxygenation; hydrogenation; various ‘catalytic’, vortex, magnetic, & photonic treatments; microclustering; super-ionization; homeopathic succussions; etc. Oxygenated water is just one example of the hundreds of ‘altered’ or ‘enhanced’ water products promoted on the internet and in some health food stores. These products all have several characteristics in common that are discussed in more detail on the Altered Water and Drinking Water Scams pages. Oxygenated water claims have not been able to withstand critical scientific review. Regardless of any alleged health benefits, these products are extremely effective at separating customers from their money. Enhanced water products often lure consumers with exotic fruit flavors. Guess what? There is not a drop of real fruit in the majority of these beverages. The only thing gained is a fistful of empty calories from sugar. Some have swapped their calories from sugar for the poorly tested artificial sweetener, acesulfame potassium. What about those waters claiming they provide 10 percent of your daily value of fiber? The fiber in these drinks comes from the digestion-resistant maltodextrin, which is made by breaking long chains of carbohydrates into smaller chains that cannot be digested by the body. If you’ve recently switched from soda to vitamin water because you believed it to be a healthier choice, you may be disappointed by knowing the truth. Vitamin waters are nothing more than a clever marketing scheme designed to promote a product that is just as unhealthy as soda! Vitamin waters contain dangerous high fructose corn syrup (HFCS), artificial colors, additives, preservatives and caffeine.
_________
Safe drinking water:
It is important for us to judge the safety of water by taking the following three qualities into consideration:
1. Microbiological – bacteria, viruses, protozoa, and worms
2. Chemical – minerals, metals and chemicals
3. Physical – temperature, colour, smell, taste and turbidity
Safe drinking water should have the following microbiological, chemical and physical qualities:
• Free of pathogens
• Low in concentrations of toxic chemicals
• Clear
• Tasteless and colourless (for aesthetic purposes)
For the most part, it is natural processes that affect water quality. For instance, water moving through underground rocks and soils may pick up natural contaminants, even with no human activity or pollution in the area. In addition to nature’s influence, water is also polluted by human activities, such as open defecation, dumping garbage, poor agricultural practices, and chemical spills at industrial sites. Even though water may be clear, it does not necessarily mean that it is safe for us to drink.
___________
Drinking water Contaminants:
Contaminants are substances that make water unfit for use. Some contaminants can be easily identified by assessing the taste, odor, and turbidity of the water. Most, however, cannot be easily detected and require testing to reveal whether or not water is contaminated. If left unchecked, contaminants can cause a whole host of water-related diseases which exact a terrible toll on human health. Contaminants are either man-made or naturally occurring. Some contaminants are organisms that include pathogens like bacteria, viruses, and parasites such as microscopic protozoa and worms. These living organisms can be spread by human and animal waste. Good sanitation and hygiene can help to stop the spread of these organisms. Other contaminants are the man-made byproducts of industry and agriculture including heavy metals like lead and mercury, and hazardous chemicals and compounds like insecticides and fertilizers. Naturally occurring elements can contaminate water as well. Toxins such as the highly poisonous metal arsenic may be naturally present at unacceptable levels. Contaminated water must be treated before it can be used for human consumption. Water treatment can occur in two distinct places: at a centralized water treatment facility and at the point of use. Wherever treatment takes place, a diverse range of technologies is used to purify drinking water. Treatment technologies are selected and applied using several determining factors including water source, type of contaminant, and cost.
_
Excessive amounts of microbes or chemicals derived from human and animal wastes, agricultural runoff, industrial chemicals, and even natural pollutants, make some water unsafe to drink and cause water-related diseases. If water sources are not protected, or are unexpectedly contaminated for any reason, the quality of drinking water suffers. Contamination can occur at the source of the water both at the surface and in the ground. Once the water is in the distribution system, there are additional opportunities for drinking water to be contaminated. If pipes are not successfully protected from contaminants, the quality of drinking water suffers. Improper storage can also result in unsafe drinking water. According to the World Health Organization (WHO), distribution systems should make drinking water available so that people do not need to travel more than one kilometer from the place where they will use the water. The quality of the water we drink has become a worldwide issue. National and international organizations concern themselves with the problem; in fact, an electronic search of the literature with the code word “water” overwhelmingly identifies articles having to do with the quality of water rather than with its quantity. Largely because of the fear of pollutants in our tap water, but also because vigorous chemical treatment often imparts a bad taste to tap water, people are turning in droves to bottled water. Sometimes, although probably not in the majority of instances, this choice might lead to the drinking of a poorer quality of water than would be the case with tap water.
____
What Human activities can pollute Ground Water?
• Bacteria and Nitrates: These pollutants are found in human and animal wastes. Septic tanks can cause bacterial and nitrate pollution. So can large numbers of farm animals. Both septic systems and animal manures must be carefully managed to prevent pollution. Sanitary landfills and garbage dumps are also sources. Children and some adults are at extra risk when exposed to waterborne bacteria. These include the elderly and people whose immune systems are weak due to AIDS or treatments for cancer. Fertilizers can add to nitrate problems. Nitrates cause a health threat in very young infants.
•Concentrated Animal Feeding Operations (CAFOs): The number of CAFOs are growing. On these farms thousands of animals are raised in a small space. The large amounts of animal wastes/manures from these farms can threaten water supplies. Strict and careful manure management is needed to prevent pathogen and nutrient problems. Salts from high levels of manures can also pollute ground water.
•Heavy Metals: Activities such as mining and construction can release large amounts of heavy metals into nearby ground water sources. Some older fruit orchards may contain high levels of arsenic, once used as a pesticide. At high levels, these metals pose a health risk.
•Fertilizers and Pesticides: Farmers use fertilizers and pesticides to promote growth and reduce insect damage. These products are also used on golf courses and suburban lawns and gardens. The chemicals in these products may end up in ground water. Such pollution depends on the types and amounts of chemicals used and how they are applied. Local environmental conditions (soil types, seasonal snow and rainfall) also affect this pollution. Many fertilizers contain forms of nitrogen that can break down into harmful nitrates. Some underground agricultural drainage systems collect fertilizers and pesticides. This polluted water can pose problems to ground water and local streams and rivers. In addition, chemicals used to treat buildings and homes for termites or other pests may also pose a threat. Again, the possibility of problems depends on the amount and kind of chemicals. The types of soil and the amount of water moving through the soil also play a role.
•Industrial Products and Wastes: Many harmful chemicals are used widely in local business and industry. These can become drinking water pollutants if not well managed. The most common sources of such problems are:
a) Local Businesses: These include nearby factories, industrial plants, and even small businesses such as gas stations and dry cleaners. All handle a variety of hazardous chemicals that need careful management. Spills and improper disposal of these chemicals or of industrial wastes can threaten ground water supplies.
b) Leaking Underground Tanks & Piping: Petroleum products, chemicals, and wastes stored in underground storage tanks and pipes may end up in the ground water. Tanks and piping leak if they are constructed or installed improperly. Steel tanks and piping corrode with age. Tanks are often found on farms. The possibility of leaking tanks is great on old, abandoned farm sites. Farm tanks are exempt from the EPA rules for petroleum and chemical tanks.
c) Landfills and Waste Dumps: Modern landfills are designed to contain any leaking liquids. But floods can carry them over the barriers. Older dumpsites may have a wide variety of pollutants that can seep into ground water.
•Household Wastes: Improper disposal of many common products can pollute ground water. These include cleaning solvents, used motor oil, paints, and paint thinners. Even soaps and detergents can harm drinking water. These are often a problem from faulty septic tanks and septic leaching fields.
•Lead & Copper: Household plumbing materials are the most common source of lead and copper in home drinking water. Corrosive water may cause metals in pipes or soldered joints to leach into your tap water. Your water’s acidity or alkalinity (often measured as pH) greatly affects corrosion. Temperature and mineral content also affect how corrosive it is. They are often used in pipes, solder, or plumbing fixtures. Lead can cause serious damage to the brain, kidneys, nervous system, and red blood cells. EPA rules under the Safe Drinking Water Act limit lead in drinking water to 15 parts per billion.
•Water Treatment Chemicals: Improper handling or storage of water-well treatment chemicals (disinfectants, corrosion inhibitors, etc.) close to your well can cause problems.
_
Tap Water contamination:
It’s easy. It’s convenient and it comes right out of your kitchen faucet. However, as I’m sure you’ve heard, most tap water is contaminated with a host of pollutants that increase your risk of serious health problems. These contaminants are arsenic, lead, aluminum, nitrates, fluoride, microbes, prescription & OTC drugs and disinfectant by-products (DBP).
_
Well water contamination:
Sixty million people are estimated to have been poisoned by well water contaminated by excessive fluoride, which dissolved from granite rocks. The effects are particularly evident in the bone deformations of children. Similar or larger problems are anticipated in other countries including China, Uzbekistan, and Ethiopia. Although helpful for dental health in low dosage, fluoride in large amounts interferes with bone formation. Half of the Bangladesh’s 12 million tube wells contain unacceptable levels of arsenic due to the wells not being dug deep enough (past 100 meters). The Bangladeshi government had spent less than US$7 million of the 34 million allocated for solving the problem by the World Bank in 1998. Natural arsenic poisoning is a global threat, 140 million people affected in 70 countries on all continents. These examples illustrate the need to examine each location on a case by case basis and not assume what works in one area will work in another.
_
The U.S. Environmental Protection Agency (EPA) sets two types of standards:
Primary standards are set to provide the maximum feasible protection to public health. They regulate (primary) contaminant levels based on toxicity and adverse health effects. The goal of standard setting is to identify maximum contaminant levels (MCLs) which prevent adverse health effects. Secondary standards regulate (secondary) contaminant levels based on aesthetics such as color and odor, which do not pose a risk to health. These secondary maximum contaminant levels (SMCLs) are guidelines, not enforceable limits. They identify acceptable concentrations of contaminants which cause unpleasant tastes, odors, or colors in the water. SMCLs are for contaminants that will not cause adverse health effects.
List of primary contaminants for drinking water regulation:
•Microorganisms
•Disinfectants
•Disinfection Byproducts
•Inorganic Chemicals
•Organic Chemicals
•Radionuclides
_________
Microorganisms :
| Contaminants | MCL or TT (mg/L) |
Potential Health Effects from Ingestion of Water | Sources of Contaminant in Drinking Water |
| Cryptosporidium | TT | Gastrointestinal illness (e.g., diarrhea, vomiting, cramps) | Human and fecal animal waste |
| Giardia lamblia | TT | Gastrointestinal illness (e.g., diarrhea, vomiting, cramps) | Human and animal fecal waste |
| Heterotrophic plate count | TT | HPC has no health effects; it is an analytic method used to measure the variety of bacteria that are common in water. The lower the concentration of bacteria in drinking water, the better maintained the water system is. | HPC measures a range of bacteria that are naturally present in the environment |
| Legionella | TT | Legionnaire’s Disease, a type of pneumonia | Found naturally in water; multiplies in heating systems |
| Total Coliforms (including fecal coliform and E. Coli) | 5.0% | Not a health threat in itself; it is used to indicate whether other potentially harmful bacteria may be present | Coliforms are naturally present in the environment; as well as feces; fecal coliforms and E. coli only come from human and animal fecal waste. |
| Turbidity | TT | Turbidity is a measure of the cloudiness of water. It is used to indicate water quality and filtration effectiveness (e.g., whether disease-causing organisms are present). Higher turbidity levels are often associated with higher levels of disease-causing microorganisms such as viruses, parasites and some bacteria. These organisms can cause symptoms such as nausea, cramps, diarrhea, and associated headaches. | Soil runoff |
| Viruses (enteric) | TT | Gastrointestinal illness (e.g., diarrhea, vomiting, cramps) | Human and animal fecal waste |
_
Biological contamination:
Biological contaminants are the most common type of health-threatening contaminants. Federal and state regulation requirements and improved water treatment systems have reduced, but not eliminated, the threat of bacterial outbreaks for people on public water systems. However, people using private water systems usually have no disinfection treatment system and the possibility of bacterial contamination presents the greatest threat to their water supply.
Sources of Bacterial Contaminants:
Septic systems, sewage treatment plants, and runoff from wood-lands, pastures, and animal feedlots are potential sources of biological contamination. Bacteria is usually not found in ground water unless the water is contaminated by waste materials and filtered through an improperly constructed well. This can be a problem, especially when wells are constructed in coarse textured soil and fractured bedrock or limestone. Private water systems can be contaminated if septic systems or sewage lines are close to the water source or are not working properly. Leachate from livestock operations and illegal landfills and dumps may also contaminate drinking water sources with bacteria. While almost all surface waters contain some bacteria, most coliform bacteria enter streams through runoff from areas with high concentrations of animal and human activities. Coliform bacteria live in human and animal intestines, decaying plant materials, and in the soil. While not a health hazard themselves, coliform bacteria found in your drinking water are a good indicator that your water may be contaminated with other, more harmful, bacteria such as giardia or cryptosporidium, that can cause serious illness.
_
_
Indicator organisms:
There is no universal indicator to ensure that water is pathogen free, but there are several types of indicators, each with certain characteristics. The common feature of all routine screening procedures is that the primary analysis is for indicator organisms rather than the pathogens that might cause concern. Indicator organisms are bacteria such as non-specific coliforms, Escherichia coli and Pseudomonas aeruginosa that are very commonly found in the human or animal gut and which, if detected, may suggest the presence of sewage. Coliform is a group name that includes many bacteria. Most coliform bacteria do not cause disease and they are abundant in soils, waters, vegetation, etc. However, their presence in drinking water indicates that disease-causing organisms could be contaminating the water system. E. coli is a special type of coliform bacteria that most likely originates from a fecal source. Most E. coli bacteria are also harmless and are found in great quantities in the intestines of people and warm-blooded animals. However, some E. coli strains (for example E. coli O157:H7) can cause illness. The presence of E. coli in a drinking water sample almost always indicates recent fecal contamination by sewage or manure, meaning there is a greater risk that pathogens are present. Indicator organisms are used because even when a person is infected with a more pathogenic bacterium, they will still be excreting many millions times more indicator organisms than pathogens. It is therefore reasonable to surmise that if indicator organism levels are low, then pathogen levels will be very much lower or absent. In other words, quantity of indicator organism like coliforms would be a surrogate marker for more pathogenic disease causing bacteria. The choice of indicator depends on the relationship between the indicator and pathogens. Coliform bacteria are most commonly used as indicators because they exist in high ratios to pathogens making them easier to detect in a water sample. However, some bacterial pathogens may exist in higher ratios than the coliform indicators, such as Yersinia. Besides coliform indicators, fecal streptococci and enterococci have also been proposed as indicators of fecal contamination of water. Total coliforms, thermotolerant coliforms (also called fecal coliforms) and Escherichia coli (more commonly referred to as E. coli) are the main indicator groups. As shown in the following diagram, thermotolerant coliforms are a sub-type of total coliforms and E. coli is a member of the thermotolerant group.
_
Bacteriological testing and standard:
Most probable number (MPN):
As coliforms are the indicators of bacterial contamination, their presence indicates fecal contamination of water which may account for severe water borne fatal diseases. Rapid detection of coliforms in drinking water is therefore necessary which can easily be carried out by MPN (Most Probable Number) method, a qualitative test to detect coliform and thereby to determine simply the potability or safety of water. This test is incorporated with three sequential steps: presumptive test, confirmed test and completed test. The detectable gas production in the presumptive test is recognized as coliform positive from which the presence of E. coli can be confirmed by observing green metallic sheen on eosin methylene blue (EMB) agar plate followed by Gram staining procedure as the completed test. However, MPN method can only determine the presence of coliform bacteria, but cannot estimate the actual load of coliform and other pathogenic bacteria in drinking water. The enumeration and identification of the pathogens are usually carried out by means of cultural and biochemical techniques, often followed by molecular studies or specific antigen detection. International WHO standard recommends not more than 10 coliform per 100ml in un-piped rural supplies and not greater than 3 per 100ml. in non- chlorinated pipe supplies (but not in repeated samples). The desirable limit of coliform in water is 10 MPN/100ml (ISI).
_
MPN vs. CFU:
These are two methods commonly used for bacterial count in water sample.
In microbiology, colony-forming unit (CFU) is an estimate of viable bacterial or fungal numbers. Unlike direct microscopic counts where all cells, dead and living, are counted, CFU estimates viable cells. The appearance of a visible colony requires significant growth of the initial cells plated – at the time of counting the colonies it is not possible to determine if the colony arose from one cell or 1,000 cells. Therefore, the results are given as CFU/mL (colony-forming units per milliliter) for liquids and CFU/g (colony-forming units per gram) for solids to reflect this uncertainty (rather than cells/mL or cells/g). Another method for estimating the number of cells in a sample is the Most probable number (MPN) method. MPN stands for ‘Most Probable Number’ and refers to a method that uses dilution cultures and a probability calculation to determine the approximate number of viable cells in a given volume of sample. It is useful when samples contain too few organisms for agar plates to be used or when organisms will not grow on agar. For example: 50 MPN/100 mL means that the Most Probable Number of viable cells in 100 mL of sample is 50.
_
_
The contaminated waters should be treated before public distribution to reduce contaminants and thus making it suitable for human consumption. The report of the year is defined as satisfactory when
1. More than 95% of the samples should not have any coliform bacteria.
2. No two consecutive samples should have coliform bacteria
3. No sample should have E.Coli.
_
_
Waterborne pathogens have several properties that distinguish them from other drinking-water contaminants:
• Pathogens can cause acute and also chronic health effects.
• Some pathogens can grow in the environment.
• Pathogens are discrete.
• Pathogens are often aggregated or adherent to suspended solids in water, and pathogen concentrations vary in time, so that the likelihood of acquiring an infective dose cannot be predicted from their average concentration in water.
• Exposure to a pathogen resulting in disease depends upon the dose, invasiveness and virulence of the pathogen, as well as the immune status of the individual.
• If infection is established, pathogens multiply in their host.
• Certain waterborne pathogens are also able to multiply in food, beverages or warm water systems, perpetuating or even increasing the likelihood of infection.
• Unlike many chemical agents, pathogens do not exhibit a cumulative effect.
_
Waterborne diseases:
The table below shows diseases associated with water:
_
Waterborne diseases are caused by pathogenic microorganisms that most commonly are transmitted in contaminated fresh water. Infection commonly results during bathing, washing, drinking, in the preparation of food, or the consumption of food thus infected. Various forms of waterborne diarrheal disease probably are the most prominent examples, and affect mainly children in developing countries. According to the World Health Organization, such diseases cause about 1.8 million human deaths annually. The World Health Organization estimates that 88% of that burden is attributable to unsafe water supply, sanitation and hygiene.
_
All waterborne disease outbreaks are avoidable. Pathogens can only cause death and disease in humans if water source protection, pathogen removal by disinfection or filtration, or integrity of distribution systems fails. Additional factors that increase the risk of waterborne disease include: newly recognized pathogens that are resistant to disinfection; human changes to aquatic ecosystems including eutrophication, modified food webs, introduction of nuisance and alien species, and creation of breeding sites for disease vectors; increased density of agricultural production in proximity to human habitation; and deteriorating water infrastructure in urban areas. There is no silver bullet for ensuring water quality. Experts generally agree that a multiple barrier approach – comprehensively addressing threats to water quality all the way from water sources to taps – is necessary. The key elements of a comprehensive approach include protection of water sources (to keep raw water as clean as possible), adequate treatment (including disinfection and additional processes to remove or inactivate contaminants), a well maintained distribution system, strong water quality standards, regular inspection, testing, monitoring, operator training and certification, public notice, reporting, and involvement, contingency planning, research, adequate funding, and rigorous enforcement.
_
Do bacteria survive in nutrient-free water?
A study was conducted using in 0.85 per cent NaCl solution and in distilled water in 1930: results are astonishing:
Various bacterial emulsions have remained viable for periods ranging from 5 to 32 months in 0.85 per cent NaCl solution at various temperatures, and from 14 to 32 months in distilled water. Distilled water is a more favorable medium for the survival of B. typhosus than 0.85 per cent NaCl solution. The same thing is true for a number of other organisms, including: B. coli, B. tuberculosis, B. diphtheriae, Strep. hemolyticus and Strep. vrtdand. The prolonged survival of B. typhosus in distilled water and in 0.85 per cent NaCl solution is associated with a late period –of comparatively low death rate. In the case of B. typhosus at 37°C. the onset of a period of low death rate may be detected after about 3 weeks. The fact that B. typhosus in distilled water is able to survive so much longer at room temperature than at 37°C. points to low temperature as a factor of considerable importance in the production of winter river typhoid in temperate climates. Washing B. typhosus, either in 0.85 per cent NaCl solution, or in distilled water shortens its survival period when resuspended in these fluids. The supernatant fluid removed in washing prolongs the survival period of the relatively few bacteria which are inevitably present in it. Great dilution of an emulsion of bacteria appears to be almost equivalent to washing in its effect. Ten to 20 per cent plain nutrient broth diluted with 0.85 per cent NaCl solution is the optimum concentration of broth for prolonged survival of B. typhosus at 37°C.; 0.1 per cent is sufficient definitely to prolong survival. Sodium chloride in 0.85 per cent concentration appears to be toxic to certain organisms when stored in nutrient media at 37°C. Reduction in the concentration of sodium chloride in culture media might assist in the preservation of cultures of certain delicate organisms. Room temperature appears to be more favorable for the survival of most organisms in distilled water and in 0.85 per cent NaCl solution than 0° to 8°C. The streptococci and B. diphtheria are exceptions. The duration of survival of several of the Gram negative bacilli appears to be more uniformly long in distilled water and in NaCl solutions than it is on solid media. Of course, they are autotrophic bacteria like cyanobacteria that can survive on sunlight (and air) alone.
_
Will bacteria develop in a closed container of distilled water?
Will bacteria start growing if all we have is pure water, closed off from the outside environment?
The answer to this is no, as Louis Pasteur very famously discovered. The idea is that he disproved the idea of ‘spontaneous generation’ i.e. that is organisms could form from nothing. If all you have is distilled water, that means the water has (theoretically) nothing in it besides H2O, so, like in Pasteur’s experiments, no bacteria will grow – life doesn’t come from nothing. When you open the container, you’ll certainly get bacteria coming in. Bacterial spores are floating in the air all the time, and they could easily start a colony.
_
PED Virus survives more than One Week in Drinking Water:
The Porcine Epidemic Diarrhoea virus (PEDv) retained infectivity in drinking water for more than one week, according to new research. Samples of PEDv negative ground water and recycled water were autoclaved, spiked with PEDv, and then stored at room temperature (around 25°C), reports the University of Minnesota College of Veterinary Medicine. Samples were taken weekly from the stored water to infect 10-day-old pigs in bio-assay. Bio-assay results showed virus infectivity in the one-week sample and inactivity in the two-week sample both drinking and recycled water.
_
How long does HIV survive in water?
Viable HIV-1 can be recovered from blood in syringes even after periods of storage in excess of 1 month. HIV has been isolated from blood, semen and other body fluids from infected individuals as both free virions (cell-free virus) and from infected cells (cell-associated virus). There are reports of survival of cell-free HIV in effluent water <12 hours followed by a reduction in titer 1- to 2-log in 24-48 hours. The infectivity of cell-associated HIV reduces rapidly after exposure to distilled water. However, a sub-population of cell-associated HIV may remain infectious for up to 96 hours in distilled water.
_
Survival of Herpes Simplex Virus in water specimens collected from Hot Tubs in Spa Facilities and on Plastic Surfaces:
Several health spas were closed temporarily because of possible nonvenereal spread of herpes simplex virus (HSV) in spa water at these facilities. Researchers collected water specimens from two health spas and studied them for (1) the presence of HSV; (2) bromine (Br2), chlorine (Cl2), and pH levels; and (3) the ability of HSV to survive in water. No HSV could be isolated from the spa water specimens. Spa water had high levels of Cl2 and Br2, tap water specimens had low levels of Cl2, and distilled water had no detectable Cl2 or Br2. The addition of spa water to laboratory stock virus immediately inactivated the virus. The HSV survived four hours in the tap water and 24 hours in distilled water. The survival of HSV appeared to be related to the free halogen content of water. To approximate the conditions of survival of HSV on plastic-coated benches and seats in spa facilities, HSV was placed on plastic surfaces in a humid atmosphere at 37 to 40 °C. The virus was found to survive up to 4.5 hours under these conditions. The survival of HSV from human lesions may be different due to the presence of tissue secretions and proteins. Furthermore, transmission may require other factors, such as rubbing of skin or penetration through abrasions. However, survival of significant amounts of virus for 4.5 hours on plastic surfaces suggests that fomites such as these may be nonvenereal routes of HSV transmission.
_
Cleaner drinking water with fewer chemicals may be made possible by identifying bio-filmmaking bacteria:
A research team studied four bacteria, Sphingobium, Xenophilus, Methylobacterium and Rhodococcus, found in a city’s drinking water to see which combinations were more likely to produce a ‘biofilm’. Biofilms are layers of bacteria which form on the inner surfaces of water pipes. Like in many instances, bacteria can be harmful or not. If the bacterial growth is too heavy, it can break off into the water flow, which at best can make water discoloured or taste unpleasant and at worst can release more dangerous bacteria. This research looks at what conditions enable biofilms to grow, so authors can find ways to control the bacteria in the water supply more effectively. The researchers isolated four bacteria from water taken from a domestic tap: two were widely found in drinking water everywhere, one was less common and one was unique to Sheffield. The researchers mixed the bacteria in different combinations and found that, in isolation, none of them produced a biofilm. However, when any of the bacteria were combined with Methylobacterium, one of the common forms, they formed a biofilm within 72 hours. “Our findings show that this bacterium is acting as a bridge, enabling other bacteria to attach to surfaces and produce a biofilm and it’s likely that it’s not the only one that plays this role,” says Professor Biggs. “This means it should be possible to control or even prevent the creation of biofilms in the water supply by targeting these particular bacteria, potentially reducing the need for high dosage chemical treatments.” Domestic water supplies in the UK are regularly tested for levels of bacteria and, if these are too high, water is treated with greater concentrations of chlorine or pipe networks are flushed through to clear the problem. However, the standard tests look for indicator organisms rather than the individual types which are present. Testing methods being developed by the Sheffield team that involve DNA analysis to identify the specific types of bacteria present. “The way we currently maintain clean water supplies is a little like using antibiotics without knowing what infection we’re treating,” says Professor Biggs. “Although it’s effective, it requires extensive use of chemicals or can put water supplies out of use to consumers for a period of time. Current testing methods also take time to produce results, while the bacteria are cultured from the samples taken. The DNA testing we’re developing will provide a fast and more sophisticated alternative, allowing water companies to fine tune their responses to the exact bacteria they find in the water system.”
_
Bacterial toxins in drinking water:
Cyanobacterial toxins are the group of compounds with very diverse chemical structure. They are divided into two groups: cytotoxins and biotoxins. Cytotoxins are not lethal for people and animals, but they are relatively more toxic for algae and the cells of mammals. They are enzymes, antibiotics and anticarcinogenic factors with very complicated chemical structure. Biotoxins are very toxic for people and can even cause lethal effects. They are divided into neurotoxins (affecting nervous system), hepatotoxins (affecting liver) and dermatotoxins. Hepatotoxins (liver toxins) occur more often than neurotoxins. Until now the chemical structure of about 60 microcystins and nodularins, the most toxic representatives of hepatotoxins was characterized and specified. Hepatotoxins belong to peptides with cyclic structure; they consist of 5 (nodularins) or 7 (microcystins) amino acids. They inhibit the activity of protein phosphatase, which leads to contraction of hepatocytes (liver cells) (El Saadi & Cameron, 1993; Falconer, 1996). The cells start to separate, and blood which retains between them leads to local hepatocellular damage and a shock. Lethal dose leads to death within a few hours, however the intake of small doses leads to chronic disorder of digestive system and liver (Osiecka, 1995). Hepatotoxins are very durable chemically; they react neither with acids nor with alkali and boiling in water cannot decompose them. In 1996 in Brazil 50 cases were recorded of lethal poisoning of people hospitalized in haemodialysis centre. The people died because toxins from the polluted water got into their blood system (Jochimson, Carmichael, An, Cardo, Cookson, Holmes, Autines, Demelo, Lyra, Barreto, Azevedo & Jarvis,1998).
_
Methods of removing toxins secreted by bacteria and algae and of degradation of microorganism cells:
Toxins secreted by bacteria and algae are removed in processes of coagulation, adsorption, biodegradation and microfiltration (Nawrocki et al., 2000). Removing microcystins is the most difficult problem, and although the adsorption on activated carbon is an effective process, it does not lead to complete removal of these compounds. In the photocatalytic degradation of organic contaminants, titanium dioxide has been found to be highly efficient in the generation of hydroxyl radicals, which are considered responsible for degradation of toxins and inactivation of water-borne microorganisms.
_
Now I will show bacterially contaminated drinking water at various places:
_
Delhi drinking water:
Scientists testing water samples from New Delhi found more than a dozen species of bacteria, ranging from strains that cause pneumonia to cholera. The bugs had genes that enable them to resist almost all medicines, according to a study published in the medical journal The Lancet. The research exposes the role played by India, a booming economy with more mobile-phone subscribers than toilets, in fanning the development of drug-evading bacteria. The researchers, led by Timothy Walsh of Cardiff University in Wales, collected 171 swabs from some of the drains that line New Delhi’s streets and 50 samples of public tap water. The samples were tested in the U.K. to identify the bacteria they contained and whether the germs had a gene known as NDM-1, which makes them resistant to a class of antibiotics-of-last resort known as carbapenems. The gene was found in two of the drinking-water and 51 of the seepage samples, including in Vibrio cholerae, Shigella boydii and nine other bacteria species not previously reported to harbor the resistance mechanism. Eighteen percent of public water samples tested at more than 600 sites in New Delhi were tainted by E. coli, Salmonella, or some other disease-causing bacteria found in human excreta that made the water unfit for drinking, according to a survey in year 2011 by the Municipal Corporation of Delhi.
_
_
A Community-based Bacteriological Study of Quality of Drinking-water and Its Feedback to a Rural Community in Western Maharashtra, India:
A longitudinal study of the bacteriological quality of rural water supplies was undertaken for a movement towards self-help against diseases, such as diarrhoea, and improved water management through increased community participation. Three hundred and thirteen water samples from different sources, such as well, tank, community standpost, handpumps, percolation lakes, and streams, and from households were collected from six villages in Maharashtra, India, over a one-year period. Overall, 49.8% of the 313 samples were polluted, whereas 45.9% of the samples from piped water supply were polluted. The quality of groundwater was generally good compared to open wells. Irregular and/or inadequate treatment of water, lack of drainage systems, and domestic washing near the wells led to deterioration in the quality of water. No major diarrhoeal epidemics were recorded during the study, although a few sporadic cases were noted during the rainy season. As a result of a continuous feedback of bacteriological findings to the community, perceptions of the people changed with time. An increased awareness was observed through active participation of the people cutting across age-groups and different socioeconomic strata of the society in village activities.
_
Bacteriological Quality of Water Samples of a Tertiary Care Medical Center Campus in North Western Himalayan Region of India:
A total of 91 water samples were collected aseptically in sterilized containers from different drinking water sources of Medical College campus and field practice area of the department over a period of two years between March 2005 to February 2007. Bacteriological examination based on MPN count in 100 ml of sample revealed that 81.3% (89.7% in 2005-06 and 75% in 2006-07) samples did not meet WHO standards of quality of water samples. The samples taken from river, springs and bhowris were highly contaminated with MPN count more than 1600 in some cases. More samples collected from college campus were poorer in quality than collected from field practice area. The samples collected during summer and rainy seasons in 2005-06 were poorer in quality than collected during winter months. This study suggests that surface and ground water samples in and around Dr. R.P Govt. Medical College campus, Kangra are highly contaminated with feacal material, which may further lead to outbreaks of gastrointestinal diseases.
_
Microbiological analysis of drinking water quality of Ananthanar channel of Kanyakumari district, Tamil Nadu, India:
Bacteriological analyses were carried out on Ananthanar channel water of Kanyakumari district, Tamil Nadu, India. The Ananthanar channel was selected in this study because this channel runs about nearly 28 km and supplies water for many villages for drinking and bathing purposes. Fecal and total coliform counts were performed using the standard membrane filtration technique and multiple tube technique. The results obtained were compared with reports of All India Institute of Medical Sciences Standards for Drinking and Recreational Water. Faecal coliform counts varied from 12 to 180 MPN/100 ml while Escherichia coli counts ranged from 6 to 161 MPN/100 ml for all the sampled sites. Among the total coliform Pseudomonas aeruginosa, Shewanella putrefaciens, Klebsiella pneumoniae, Citrobacter freundii and Proteus mirabilis are reported. The Faecal coliform and the E. coli counts exceeding acceptable limits are indicative of pollution from domestic wastes from several informal settlements located along the riverbank. Water uses in the area were determined and were found to be mainly domestic and recreational. The gross pollution of the river exposes the local people who depend on it for their primary water source to serious health risk.
_
Microbiological study of drinking water in Bangladesh:
Consumption of drinking water contaminated with fecally originated pathogenic bacteria is mostly responsible for the onset of water borne disease outbreaks especially in developing countries. Current study attempted to analyze 25 treated drinking water samples both qualitative- and quantitatively from different areas of Dhaka metropolis, Bangladesh where 90% cases of diseases (dysentery, typhoid, cholera and diarrhea) have long been reported due to the water borne microorganisms diseases. Through the most probable number (MPN) method, 5 samples out of 25 were found to be non-potable as they had been contaminated with Escherichia coli indicating the risk for fecal contamination responsible for disease outbreaks. Other contaminating Gram negative bacteria were characterized as Klebsiella spp., Alcaligenes faecalis, Pseudomonas spp. and Aeromonas spp. Interestingly the presence of E. coli was detected in the same 5 samples within a range of 300 to 170000 cfu/ml by the conventional cultural and biochemical methods. Moreover, a huge array of other pathogenic bacteria was also detected through this method. Further detection of drug resistance traits among the bacterial isolates would be of public health significance.
_
In a nutshell, drinking water from Delhi, Kolkata, Dhaka and rural areas of India and Bangladesh are contaminated by bacteria.
_
Moving water or still water for drinking purpose:
The basic rule of thumb is that if water does not contain chlorine and it sits still for any period of time it will grow bacteria. We were all told as we grew up not to drink from a stagnant pond or we can get sick. This isn’t all that all much different from a house or business. When you remove Chlorine from water, it will start to grow a lot of bacteria. Chlorine is a bacterial sterilizer in high enough dosages ( > 100 ppm) but in the dosages used in normal supply lines (< 2.5 ppm) you will usually find some low level of bacteria still growing within your pipes. If you add Activated Carbon, or as some call it whole house filtration systems to your water supply it will happen even quicker. Bacteria need to adhere to something in order to grow into colonies. If you want to stop bacteria from growing, you need to have the water always moving at greater than 2 feet per second. This just isn’t feasible for most residential and commercial water systems.
________
Drinking water turbidity:
Turbidity happens when particles of clay, silt, decaying plants, parasites, and other matter become suspended in the water. And it’s not just a cosmetic or taste problem. Disease-causing microorganisms can cling to the particles and escape destruction by chlorination and other disinfection methods. Public water utilities are supposed to remove the particles when turbidity becomes excessive, but that’s not always good enough. In 1993 in Philadelphia, for example, emergency room visits and hospital admissions for children with gastrointestinal illness increased by about ten percent whenever the turbidity of the city’s public drinking water increased significantly (but remained below the legal limit and wasn’t visible to consumers). And about ten days after the spikes in turbidity, hospital admissions of the elderly for GI tract illnesses increased by nine percent. “These and other studies suggest that ten percent of gastrointestinal illnesses in children and the elderly may be due to turbidity in ordinary tap water at levels that pass federal standards,” says Joel Schwartz of the Harvard School of Public Health, who led both studies.
_
The turbidity itself is not the health concern, but rather it is used as a measure of the microbial safety of the water (pathogens associated with particles which can shield the pathogens from disinfectant). There are no stipulations on the particle size, however the way turbidity is measured in most meters, the higher the average particle size and concentration, the higher the turbidity reading will be. The current regulation for turbidity (which is a primary drinking water standard) depends on the water treatment technique. For water systems that use conventional or direct filtration, at no time can turbidity (cloudiness of water) exceed 1.0 nephelometric turbidity unit (NTU), and samples for turbidity must be less than or equal to 0.3 NTU in at least 95 percent of the samples in any month. Systems that use filtration other than conventional or direct filtration must follow state-established limits, which must include turbidity at no time exceeding 5.0 NTU. With regard to optimal turbidity measurements to best protect public health however, EPA recommends an “optimized” goal of 0.1 NTU for 95% of the individual filter samples in any month and never exceeding 0.3 NTU. Research has shown a substantial reduction in microbial pathogens by reducing turbidity from the current regulation of 0.3 NTU to 0.1 NTU.
_
Cloudiness due to dissolved gases:
Tap water can sometimes appear cloudy, and this is often mistaken for a mineral impurity in the water. Cloudy water is usually caused by air bubbles coming out of solution in the water. Because cold water holds more air than warm water, small bubbles will appear in water. It has a high dissolved oxygen content that is heated or depressurized, which reduces how much dissolved gas the water can hold. The harmless cloudiness of the water disappears quickly as the gas is released from the water.
__________
Disinfectants:
| Contaminant | MRDLmg/L) | Potential Health Effects from Ingestion of Water | Sources of Contaminant in Drinking Water |
| Chloramines (as Cl2) | MRDL=4.0 | Eye/nose irritation; stomach discomfort, anemia | Water additive used to control microbes |
| Chlorine (as Cl2) | MRDL=4.0 | Eye/nose irritation; stomach discomfort | Water additive used to control microbes |
| Chlorine dioxide (as ClO2) | MRDL=0.8 | Anemia; infants & young children: nervous system effects | Water additive used to control microbes |
_
Disinfection Byproducts (DBP):
_
Chlorinating water to destroy disease-causing bacteria was one of the greatest public-health achievements of the 20th century. But adding chlorine to water is a double-edged sword. The disinfectant can combine with decaying leaves and other naturally occurring organic matter to form compounds called disinfection byproducts (DBPs). As it turns out, DBPs are over 10,000 times more toxic than chlorine, and out of all the other toxins and contaminations present in your water, such as fluoride and miscellaneous pharmaceutical drugs, DBPs may be the absolute worst of the bunch. Unfortunately, few people are aware of this toxic threat, and those who are aware may still not realize that not all water filtration systems are capable of filtering out DBPs. In fact, there is currently no point-of-entry, whole-house water filtration system that is certified to filter out these toxins. The most common disinfection techniques used at water treatment facilities today involve the use of chlorine, chloramines, and chlorine dioxide to kill harmful, disease-causing microorganisms in the water, making it safe to drink. Unfortunately, over the years scientists have discovered that toxic chemical byproducts form when these disinfectants react with natural organic matter like decaying vegetation in the source water.
The most common disinfectant byproducts formed when chlorine is used are:
•trihalomethanes (THMs)
•haloacetic acids (HAAs)
These byproducts are probably the most significant, most widely distributed contaminant in the U.S. water supply today. They can roughly double the risk of developing bladder cancer. The EPA estimates that between 2 and 17 percent of all bladder cancer cases in the U.S. may be due to DBPs in drinking water. DBPs may also increase the risk of colon cancer, though the evidence isn’t as strong. Among 28,000 women in Iowa, for example, those who lived where the water had the highest levels of DBPs had nearly double the risk of colon cancer of those who lived where the water had the lowest levels. And cancer’s not the only potential problem. In a 1998 study in northern California, pregnant women who lived where the tap water contained more than 75 parts per billion (ppb) of DBPs were nearly twice as likely to miscarry as women who lived where the tap water contained less than 75 ppb, but only if they drank at least five glasses of water a day. The EPA’s limit for DBPs is 100 ppb. The link between DBPs and miscarriages is far from proven, though. In other areas of California, there didn’t seem to be any association. Researchers have just begun a study of 950 pregnant women to see if drinking water is linked to miscarriages in North Carolina, Texas, and Virginia.
_
Chemical contamination:
_
Inorganic Chemicals:
| Contaminant | MCL or TT(mg/L) | Potential Health Effects from Ingestion of Water | Sources of Contaminant in Drinking Water |
| Antimony | 0.006 | Increase in blood cholesterol; decrease in blood sugar | Discharge from petroleum refineries; fire retardants; ceramics; electronics; solder |
| Arsenic | 0.010 as of 01/23/06 |
Skin damage or problems with circulatory systems, and may have increased risk of getting cancer | Erosion of natural deposits; runoff from orchards, runoff from glass & electronicsproduction wastes |
| Asbestos (fiber >10 micrometers) |
7 MFL | Increased risk of developing benign intestinal polyps | Decay of asbestos cement in water mains; erosion of natural deposits |
| Barium | 2 | Increase in blood pressure | Discharge of drilling wastes; discharge from metal refineries; erosion of natural deposits |
| Beryllium | 0.004 | Intestinal lesions | Discharge from metal refineries and coal-burning factories; discharge from electrical, aerospace, and defense industries |
| Cadmium | 0.005 | Kidney damage | Corrosion of galvanized pipes; erosion of natural deposits; discharge from metal refineries; runoff from waste batteries and paints |
| Chromium (total) | 0.1 | Allergic dermatitis | Discharge from steel and pulp mills; erosion of natural deposits |
| Copper | TT; Action Level=1.3 |
Short term exposure: Gastrointestinal distressLong term exposure: Liver or kidney damagePeople with Wilson’s Disease should consult their personal doctor if the amount of copper in their water exceeds the action level | Corrosion of household plumbing systems; erosion of natural deposits |
| Cyanide (as free cyanide) | 0.2 | Nerve damage or thyroid problems | Discharge from steel/metal factories; discharge from plastic and fertilizer factories |
| Fluoride | 4.0 | Bone disease (pain and tenderness of the bones); Children may get mottled teeth | Water additive which promotes strong teeth; erosion of natural deposits; discharge from fertilizer and aluminum factories |
| Lead | TT; Action Level=0.015 |
Infants and children: Delays in physical or mental development; children could show slight deficits in attention span and learning abilitiesAdults: Kidney problems; high blood pressure | Corrosion of household plumbing systems; erosion of natural deposits |
| Mercury (inorganic) | 0.002 | Kidney damage | Erosion of natural deposits; discharge from refineries and factories; runoff from landfills and croplands |
| Nitrate (measured as Nitrogen) | 10 | Infants below the age of six months who drink water containing nitrate in excess of the MCL could become seriously ill and, if untreated, may die. Symptoms include shortness of breath and blue-baby syndrome. | Runoff from fertilizer use; leaching from septic tanks, sewage; erosion of natural deposits |
| Nitrite (measured as Nitrogen) | 1 | Infants below the age of six months who drink water containing nitrite in excess of the MCL could become seriously ill and, if untreated, may die. Symptoms include shortness of breath and blue-baby syndrome. | Runoff from fertilizer use; leaching from septic tanks, sewage; erosion of natural deposits |
| Selenium | 0.05 | Hair or fingernail loss; numbness in fingers or toes; circulatory problems | Discharge from petroleum refineries; erosion of natural deposits; discharge from mines |
| Thallium | 0.002 | Hair loss; changes in blood; kidney, intestine, or liver problems | Leaching from ore-processing sites; discharge from electronics, glass, and drug factories |
__
Arsenic in Drinking Water:
According to “Arsenic in Drinking Water,” a 1999 report by the National Academy of Sciences, the poison of choice of mystery writers can also cause cancer, heart disease, and perhaps diabetes. The World Health Organization recommends no more than 10 ppb. Arsenic is a natural element found in some rocks and soil. Arsenic has no taste or smell. Water must be tested to know if it contains arsenic and at what level. People routinely take in very small amounts of arsenic from the air, water, and from food. Of these, food is usually the largest contributor. This is generally due to the levels found in seafood and fish, but this form of arsenic is different than the arsenic stored in rocks and soil, and is far less harmful. Health effects due to drinking water with arsenic depend both on how much arsenic is in the water, and for how many years the water has been used for drinking. Ingestion of arsenic over a long period of time has been linked to an increased lifetime risk of getting bladder, lung or skin cancer. Research is also ongoing on arsenic’s links to skin and cardiovascular diseases, diabetes or other cancers. Arsenic levels can be reduced in drinking water with treatment.
_
Lead in drinking water:
Water can pick up lead almost anywhere along the way from the plant to the tap — in holding tanks, underground pipes, or lead pipes and fixtures inside old buildings or homes, especially those built before the 1930s. Although the use of lead pipes and solder was banned in public water systems and household plumbing in the 1980s, they may sometimes still be used illegally, says the EPA. Lead found in tap water usually comes from the corrosion of older fixtures or from the solder that connects pipes. When water sits in leaded pipes for several hours, lead can leach into the water supply. Fortunately, a coating of minerals builds up inside the pipes after a few years, which helps keep the lead from leaching into the water. Even ”lead-free” pipes can contain as much as 8% lead. The only way to know whether your tap water contains lead is to have it tested. You cannot see, taste, or smell lead in drinking water. Therefore, you must ask your water provider whether your water has lead in it. High levels of lead in tap water can cause health effects if the lead in the water enters the bloodstream and causes an elevated blood lead level. Most studies show that exposure to lead-contaminated water alone would not be likely to elevate blood lead levels in most adults, even exposure to water with a lead content close to the EPA action level for lead of 15 parts per billion (ppb). Risk will vary, however, depending on the individual, the circumstances, and the amount of water consumed. For example, infants who drink formula prepared with lead-contaminated water may be at a higher risk because of the large volume of water they consume relative to their body size. The best way to avoid consuming lead from tap water is to only use water from the cold tap for drinking, cooking, and making baby formula and to let the water run for a minute before using it. Lead can cause both physical and mental developmental problems in infants and children. Lead builds up in the body over many years and can damage the brain, kidneys, and red blood cells.
_
Is your water filled with unregulated chemicals?
Traces of 18 unregulated chemicals, including solvents, herbicides, caffeine, metal and antidepressants were found in the water of U.S. water facilities. Federal scientists analyzed water from 25 water facilities, and found traces of 21 chemicals in water samples from 9 of these facilities. Of the 21 chemicals, 18 are unregulated ones according to Environmental Health News. The 21 contaminants detected were found to be mostly in low concentrations in treated drinking water from at least nine of the utilities. Eighteen of the chemicals are not regulated under the federal Safe Drinking Water Act, thus utilities do not have to meet any limit or even monitor them.
_
Unregulated contaminants in drinking water:
_
There is little known about the effect on human health for many of the contaminants, in low doses. But one contaminant, perfluorinated compounds (used in food packaging to make an object oil-, stain- and water-resistant) known as PFOA have been linked to a variety of health problems. Perfluorinated compound linked to cancers, colitis & thyroid disease. The perfluorinated compounds found in drinking water have been found in the blood of nearly all people in the US. Scientists say there is a “probable link” between PFOA in drinking water and high cholesterol, ulcerative colitis, thyroid disease, testicular cancer, kidney cancer, and pregnancy-induced hypertension (based on findings on people in Mid-Ohio Valley communities whose water was polluted with PFOA from a DuPont plant). When scientists attempted to remove the perfluorinated compounds, treatment techniques were largely unsuccessful. Only one plant was successful at removing them, and it used activated carbon treatment.
_
Pesticides in drinking water:
Pesticides enter surface and ground water primarily as runoff from crops and are most prevalent in agricultural areas. Pesticides are also used on golf courses, forested areas, along roadsides, and in suburban and urban landscape areas. Since World War II herbicide and insecticide application to crops has grown to an estimated 660 million pounds of active ingredient in 1993. Without proper safeguards pesticides have the potential to seriously threaten many groundwater supplies in the United States. Approximately 50% of the U.S. population obtains its drinking water from groundwater sources and as much as 95% of the population in agricultural areas uses groundwater as its source of drinking water. ‘Pesticide’ is a general term for substances which are used to poison pests (weeds, insects, molds, rodents, etc.). The pesticides most acutely dangerous to man are insecticides and rodenticides, although pound for pound, herbicides are the most widely used type of pesticide. Not every pesticide is acutely toxic to humans or other non-target species. On a national scale less than 2% of wells sampled in multi-state studies were found with pesticide concentrations above the established Maximum Contaminant Level (MCL). Due to repeated detection of various pesticides in U.S. wells, the U.S. Environmental Protection Agency (EPA) recently proposed a State Management Program (SMP), which would control or ban pesticides with the greatest potential to contaminate groundwater. Five pesticides were initially selected due to the frequency of their occurrence: alachlor, atrazine, cyanazine, metolachlor, and simazine. According to the EPA they all have been detected in many states, and have the potential to reach levels which exceed health based standards. They are all associated with serious health effects including cancer. The five selected pesticides are herbicides which are used to control broadleaf weeds and grasses. The EPA estimates between 200 and 250 million pounds of these herbicides are applied annually in the U.S. Atrazine, simazine, and cyanazine are applied to agricultural land before and after planting. Alachlor and metolachlor are applied to soil prior to plant growth (pre-emergent).
There are several factors which influence a pesticides’ potential to contaminate water:
•The ability of the pesticide to dissolve in water (solubility).
•Environmental factors, such as, soil, weather, season, and distance to water sources
•Application methods and other practices associated with the pesticide use.
_
Prescription and OTC drugs in drinking water:
We know how the drugs get there. Our bodies release them when we urinate or flush old drugs down the toilet. And it’s well known by now that pharmaceuticals are affecting fish, frogs and lobsters—small amounts of estrogen cause male fish to develop eggs, for instance. But the impact on human health is unclear. Although research on pharmaceuticals in the water supply began almost a decade ago, no one seems to know which compounds need to be removed or how to remove them from the water safely. And no one seems to know which government agency should step forward and take action. The 2008 Associated Press report that drugs had been found in the drinking water supplies of 41 million Americans was alarming. AP’s investigation turned up antibiotics, anticonvulsants, mood stabilizers, sex hormones, anti-anxiety drugs, acetaminophen and ibuprofen in trace amounts of parts per billion or trillion. Many people did not realize that the unused pills dumped into toilets, and medications excreted through our urine, could end up in our water supply. While sewage is treated before it’s released into reservoirs or rivers, most wastewater treatment does not remove pharmaceuticals. One reason for the higher numbers is better technology, which can trace drugs at smaller amounts. But it’s also because we’re taking more drugs than ever, from over-the-counter medications for headaches to prescription medications for depression, acid reflux, and high blood pressure. Health officials say these compounds in water pose a low risk to humans. But they also said that there are no good models to predict the effect this cocktail of low-level medications would have on human or aquatic life. Right now, there are no federal or state regulations requiring drinking water or wastewater plants to monitor pharmaceutical compounds in water.
_
As reported in New Scientist, a comprehensive survey of U.S. drinking water revealed the 11 most frequently detected toxic pharmaceuticals overall were:
•Atenolol, a beta-blocker used to treat cardiovascular disease
•Atrazine, an organic herbicide banned in the European Union which has been implicated in the decline of fish stocks and in changes in animal behavior
•Carbamazepine, a mood-stabilizing drug used to treat bipolar disorder
•Estrone, an estrogen hormone secreted by the ovaries and blamed for causing gender changes in fish
•Gemfibrozil, an anti-cholesterol drug
•Meprobamate, a tranquilizer used in psychiatric treatment
•Naproxen, a painkiller and anti-inflammatory linked to increases in asthma incidence
•Phenytoin, an anticonvulsant used to treat epilepsy
•Sulfamethoxazole, an antibiotic
•TCEP, a reducing agent used in molecular biology
•Trimethoprim, another antibiotic
Despite extensive purification treatments used by water companies, traces of bleomycin, a cancer chemotherapy drug, and diazepam, a sedative, have also been found in the drinking water.
_
Drug companies accused of polluting water in India:
For several years, the National Geophysical Research Institute in Hyderabad and the country’s Central Pollution Control Board in Delhi have monitored heavy metal and other pollutants around the town of Patancheru, which is home to factories producing solvents and other chemicals. But although Patancheru is also home to numerous drug companies, the government has not monitored for drugs being released into the environment. In 2007, however, a team led by environmental scientist Joakim Larsson of the University of Gothenburg in Sweden published results from one waste-treatment facility, Patancheru Enviro Tech Ltd (PETL). Around 90 companies in the region that manufacture active pharmaceutical ingredients, or assemble final drug products, send their waste to PETL. Larsson’s team sampled the waste exiting the plant; they found drugs including the antibiotic ciprofloxacin, at concentrations of up to 31,000 micrograms per liter, and the antihistamine cetirizine, at up to 1,400 micrograms per liter. The team estimated that the amount of ciprofloxacin entering the river from the plant could amount to up to 45 kilograms a day.
__________
Radioactive contaminants:
What are radionuclides?
A nuclide is a general term applicable to all atomic forms of an element. Nuclides are characterized by the number of protons and neutrons in the nucleus, as well as by the amount of energy contained within the atom. A radionuclide is an unstable form of a nuclide. They may occur naturally, but can also be artificially produced.
_
| Contaminant | MCL or TT (mg/L) |
Potential Health Effects from Ingestion of Water | Sources of Contaminant in Drinking Water |
| Alpha particles | 15 picocuries per Liter (pCi/L) | Increased risk of cancer | Erosion of natural deposits of certain minerals that are radioactive and may emit a form of radiation known as alpha radiation |
| Beta particles and photon emitters | 4 millirems per year | Increased risk of cancer | Decay of natural and man-made deposits ofcertain minerals that are radioactive and may emit forms of radiation known as photons and beta radiation |
| Radium 226 and Radium 228 (combined) | 5 pCi/L | Increased risk of cancer | Erosion of natural deposits |
| Uranium | 30 µg/L | Increased risk of cancer, kidney toxicity | Erosion of natural deposits |
__
How do radionuclides get into drinking water?
Most drinking water sources have very low levels of radioactive contaminants (“radionuclides”), which are not considered to be a public health concern. Of the small percentage of drinking water systems with radioactive contaminant levels high enough to be of concern, most of the radioactivity is naturally occurring. Certain rock types have naturally occurring trace amounts of “mildly radioactive” elements (radioactive elements with very long half-lives) that serve as the “parent” of other radioactive contaminants (“daughter products”). These radioactive contaminants, depending on their chemical properties, may accumulate in drinking water sources at levels of concern. The “parent radionuclide” often behaves very differently from the “daughter radionuclide” in the environment. Because of this, parent and daughter radionuclides may have very different drinking water occurrence patterns. For example, ground water with high radium levels tends to have low uranium levels and vice versa, even though uranium-238 is the parent of radium-226.
_
Radon in drinking water:
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as an indirect decay product of uranium or thorium. Radon is one of the densest substances that remains a gas under normal conditions. It is also the only gas under normal conditions that only has radioactive isotopes, and is considered a health hazard due to its radioactivity.
___________
Secondary contaminants:
| List of Secondary Contaminants in Drinking Water | |
| Contaminant | SMLC level |
| Aluminum | 0.05 to 0.2 mg/L |
| Chloride | 250 mg/L |
| Color | 15 (color units) |
| Copper | 1.0 mg/L |
| Corrosivity | noncorrosive |
| Fluoride | 2.0 mg/L |
| Foaming Agents | 0.5 mg/L |
| Iron | 0.3 mg/L |
| Manganese | 0.05 mg/L |
| Odor | 3 threshold odor number |
| pH | 6.5-8.5 |
| Silver | 0.10 mg/L |
| Sulfate | 250 mg/L |
| Total Dissolved Solids | 500 mg/L |
| Zinc | 5 mg/L |
_
What problems are caused by these secondary contaminants?
There are a wide variety of problems related to secondary contaminants. These problems can be grouped into three categories: Aesthetic effects—undesirable tastes or odors; Cosmetic effects—effects which do not damage the body but are still undesirable; and Technical effects—damage to water equipment or reduced effectiveness of treatment for other contaminants. The secondary maximum contaminant levels SMCLs related to each of these effects are given in the table above.
_
What makes water palatable? To be palatable water should be free of detectable taste and odors.
What constitutes a detectable taste or odor? Undoubtedly, you have tasted waters which have had some unpleasant tastes or odors but were drinkable. And then there are those waters which have tastes and odors so obnoxious (hydrogen sulfide water, for example) that most people cannot even stomach them. Turbidity, sediment, and color also play important roles in determining whether water is palatable.
_
Taste and Odor of drinking water:
Since taste and odor work together it is often difficult to distinguish the two. Common complaints include:
1. Strong Chlorine taste or smell – Generally this occurs when the water is treated at the water treatment plant to disinfect it. The addition of chlorine is used to kill off bacteria and other harmful microorganisms .
2. Metallic taste – Some water systems have a high mineral concentration giving the consumer a salty or soda taste. In the case of Iron and Manganese, a strong metallic taste is readily detected.
3. Rotten egg odor – This is usually a result of decaying organic deposits underground . As water flows through these areas, hydrogen sulfide gas is picked up, and when this water reaches the surface or comes out of the faucet, the gas is released into the air. Hydrogen sulfide gas produces the rotten egg odor, can be corrosive to plumbing at high concentrations, and can tarnish silver rapidly. In large enough quantities, it is toxic to aquarium fish. As little as 0.5 PPM (parts per million) can be tasted in drinking water.
4. Musty or unnatural smells – These smells are normally a result of organic matter or even some pesticides in the water supply. Even very low amounts can introduce unpleasant odors into the water.
5. Turpentine taste or odor – This smell can be a result of MTBE contamination in your water. The odor threshold of MTBE is fairly low, so even though you can smell it, the MTBE is more than likely not at a level to cause harmful effects.
_
Color of drinking water:
“Clean” water should be clear with no noticeable color deposits. Common colors include:
1. Red or Brown Color – A red, brown or rusty color is generally indicative of iron or manganese in your water. Disadvantages to iron in your water include stains in sinks, or discolored laundry.
2. Yellow Color – This coloration occurs in regions where the water has passed through marshlands and then moved through peat soils. In the United States, these conditions occur in the Southeast, Northwest, New England, and Great Lakes regions. It is more commonly found in surface water supplies and shallow wells. Although the yellow color may be displeasing, it presents no health hazard, as it is only small particles suspended in the water.
3. Blue or Green Color – A green or blue color is generally a result of copper in your water supply, or copper pipes and corrosive water. The copper can cause staining of your fixtures and your laundry. Copper is regulated in drinking water by the EPA at 1.3 PPM. This is at a low enough concentration that the copper cannot be tasted (the taste threshold is around 5 PPM). Copper can become a problem if it is higher than 30 PPM in your water. Effects at this dose are vomiting, diarrhea, and general gastrointestinal distress. If you are using well water as your primary source of water and copper is a concern in your area, it would be to your advantage to have your water tested for copper.
4. Cloudy White or Foamy – Cloudy water is usually due to turbidity. Turbidity is caused by finely divided particles in the water. When light hits the water, it is scattered, giving a cloudy look to the water. The particles may be of either organic or inorganic nature. Neither one causes any harmful effects to the body, although they can cause abrasions to pipes, or possible staining of sinks.
__________
Contaminated Water may not be bad water:
When most people see or hear the word contaminated, it signals danger or disease. However, the official federal definition defines a contaminant as “any physical, chemical, biological, or radiological substance or matter in water.” Whether water is safe to drink depends on the specific contaminants it contains, how much of each contaminant is present, and how these contaminants affect human health. Sometimes, water that is cloudy or slightly off-color may not be dangerous to drink, while water that is perfectly clear may contain tasteless, odorless, and colorless contaminants with serious health effects. Some substances in small concentrations, such as iron, are good for human health. Others, such as fluoride, may be beneficial at low levels and cause potential health problems at higher levels.
A contaminant’s threat to human health depends on a variety of factors:
• the toxicity of the contaminant (toxic means poisonous)
• the concentration level poses a health risk
• the amount of water a person drinks
• the sensitivity of different people to specific contaminants (for example, children, elderly people, individuals with weakened immune systems, and pregnant women may be at greater risk)
• the ways different contaminants in the water combine to become more or less toxic the nature of the contaminant (chemical or living)
__________
What are the drinking water standards?
Drinking water must be ‘wholesome’ and this is defined in law by standards for a wide range of substances, organisms and properties of water in regulations. The standards are set to be protective of public health and the definition of wholesome reflects the importance of ensuring that water quality is acceptable to consumers. There is good agreement amongst worldwide on the science behind the setting of health based standards for drinking water and this expert evidence is documented by the World Health Organisation in the Guidelines for Drinking Water Quality. The standards are strict and include wide safety margins. They cover:
•micro-organisms
•chemicals such as nitrate and pesticides
•metals such as lead and copper
•the way water looks and how it tastes
_
Water quality:
Water quality refers to the chemical, physical and biological characteristics of water. It is a measure of the condition of water relative to the requirements of one or more biotic species and or to any human need or purpose. It is most frequently used by reference to a set of standards against which compliance can be assessed. The most common standards used to assess water quality relate to health of ecosystems, safety of human contact and drinking water. The vast majority of surface water on the planet is neither potable nor toxic. This remains true when seawater in the oceans (which is too salty to drink) is not counted. Another general perception of water quality is that of a simple property that tells whether water is polluted or not. In fact, water quality is a complex subject, in part because water is a complex medium intrinsically tied to the ecology of the Earth. Industrial and commercial activities (e.g. manufacturing, mining, construction, transport) are a major cause of water pollution as are runoff from agricultural areas, urban runoff and discharge of treated and untreated sewage. Other drinking water contaminants and sources include pesticides, de-icing salts, synthetic chemicals, heavy metals, petroleum products, chemical fertilizers, leaking chemical storage tanks, wastewater from factories, and runoff from highways, parking lots, and suburban lawns.
_
Water quality testing:
_
Drinking water indicators:
The following is a list of indicators often measured by situational category:
Alkalinity
Color of water
pH
Taste and odor (geosmin, 2-Methylisoborneol (MIB), etc.)
Dissolved minerals and salts (sodium, chloride, potassium, calcium, manganese, magnesium)
Microorganisms such as fecal coliform bacteria (Escherichia coli), Cryptosporidium, and Giardia lamblia;
Dissolved metals and metalloids (lead, mercury, arsenic, etc.)
Dissolved organics: colored dissolved organic matter (CDOM), dissolved organic carbon (DOC)
Radon
Heavy metals
Pharmaceuticals
Hormone analogs
_
Drinking water quality standards:
Drinking water quality standards describes the quality parameters set for drinking water. Despite the truism that every human on this planet needs drinking water to survive and that water may contain many harmful constituents, there are no universally recognized and accepted international standards for drinking water. Even where standards do exist, and are applied, the permitted concentration of individual constituents may vary by as much as ten times from one set of standards to another. Many developed countries specify standards to be applied in their own country. In Europe, this includes the European Drinking Water Directive and in the USA the United States Environmental Protection Agency (EPA) establishes standards as required by the Safe Drinking Water Act. For countries without a legislative or administrative framework for such standards, the World Health Organisation publishes guidelines on the standards that should be achieved. China adopted its own drinking water standard GB3838-2002 (Type II) enacted by Ministry of Environmental Protection in 2002. The following table provides a comparison of a selection of parameters for concentrations listed by WHO, the European Union, EPA, and Ministry of Environmental Protection of China.
_________
Water Testing:
To ensure that water is safe for human consumption and livestock use, water supplies should be tested and checked to ensure they meet the acceptable levels for bacterial and chemical contents. The local health department in most counties can conduct a microbiological test. The testing procedure is not the same for all contaminants. Call your local Extension office, health department or private lab for appropriate bottle(s) and instructions on sample collection and submission. After sending a water sample to a laboratory, the laboratory will then return a report indicating what is found in your water, including those contaminants that exceed standard levels (MCLs or SMCLs). Treatment options are also recommended when necessary. Specific questions about water quality can often be answered with the right test. Unfortunately, no single water test can provide you with information on all possible contaminants. Public water supply systems typically spend few thousand dollars to analyze for the EPA-required suite of all primary and secondary contaminants that may be found in drinking water. Such a comprehensive testing is expensive, impractical and may not be necessary for a domestic well. Instead, tests for some common constituents are recommended as discussed below.
Mineral Analysis:
A mineral analysis checks for the inorganic constituents found in water. A typical mineral analysis will give the content in parts per million (milligrams per liter) of mineral elements such as calcium, magnesium, manganese, iron, copper and zinc. It will also determine the acidity or pH of the water and the hardness, expressed in parts per million or grains per gallon. It may also give the concentration of nitrate, sulfates and other chemical compounds. Large amounts of minerals and other impurities may pose a health hazard. For instance, nitrate contamination can cause health problems for infants and ruminant animals, sulfates can have a laxative effect in humans, and arsenic may cause cancer if consumed over a long period of time. Minerals at high concentrations can also affect the appearance and use of the water. Hard water is due to high levels of calcium and magnesium. Iron may leave red stains on plumbing fixtures, equipment and laundry. Suspended silt makes water look muddy or cloudy and dissolved gases may give water a bad taste and/or odor.
Microbiological Tests:
A microbiological test tells you if your water is at risk for contamination by disease-causing microorganisms (pathogens). However, testing drinking water for all possible pathogens is complex, time consuming and expensive. Instead, water is commonly tested for indicator microorganisms such as total coliform and E. coli bacteria because if they are present in water, the condition of the well and its surrounding environment may support the presence of other disease-causing microorganisms. Thus, a positive water test result for total coliform only or both total coliform and E. coli indicate the possible existence of various disease-causing microorganisms.
Pesticide and Other Organic Chemical Tests:
There are many man-made chemicals that can potentially contaminate a water supply if they are not disposed of properly. These chemicals may impair water quality and cause a health hazard. Examples of these chemicals include petroleum products, industrial chemicals and agricultural pesticides. Chemicals that are not part of a laboratory’s routine suite of analysis are not typically analyzed unless a particular type of chemical is suspected to be in the water. It can be very expensive to test for the presence of many unknown chemical contaminants; however, if a particular chemical is suspected, a test can usually be performed at a moderate cost.
_
When should I get my water tested and what should I test for?
Test if…
• Your well does not meet construction codes.
• The area around the wellhead has been flooded or submerged.
• Back-siphoning has occurred.
• You have mixed or used pesticides near the well, or have spilled pesticides or fuel near the well.
• You have a heating oil tank or underground fuel tank near the well that you know has leaked.
• You are pregnant, are planning a pregnancy, or have an infant less than 6 months old.
• Your septic system absorption field, or your neighbor’s, is close to the well (within 100 feet).
Test annually for:
• Nitrates
• Coliform Bacteria
Testing for fecal coliform and/or total bacteria is a good place to start. Depending on the results of that test, you may be advised to test for other contaminants such as metals, sediment, or organic pollutants. A presence of live coliforms in your water is an indication that there is surface water entering your drinking water. There will likely be other pollutants in your water as well. Another common starting test for drinking water is nitrates. A value higher than 10 ppm (mg/L) can threaten your health especially if you are pregnant or nursing, and can threaten the health of infants. A presence of nitrates in your water indicates surface water contamination of your drinking water.
_
Reasons to test water:
| Conditions or Nearby Activities: | Test for: |
| Recurring gastro-intestinal illness | Coliform bacteria |
| Household plumbing contains lead | pH, lead, copper |
| Radon in indoor air or region is radon rich | Radon |
| Corrosion of pipes, plumbing | Corrosion, pH, lead |
| Nearby areas of intensive agriculture | Nitrate, pesticides, coliform bacteria |
| Coal or other mining operations nearby | Metals, pH, corrosion |
| Gas drilling operations nearby | Chloride, sodium, barium, strontium |
| Dump, junkyard, landfill, factory, gas station, or dry-cleaning operation nearby | Volatile organic compounds, total dissolved solids, pH, sulfate, chloride, metals |
| Odor of gasoline or fuel oil, and near gas station or buried fuel tanks | Volatile organic compounds |
| Objectionable taste or smell | Hydrogen sulfide, corrosion, metals |
| Stained plumbing fixtures, laundry | Iron, copper, manganese |
| Salty taste and seawater, or a heavily salted roadway nearby | Chloride, total dissolved solids, sodium |
| Scaly residues, soaps don’t lather | Hardness |
| Rapid wear of water treatment equipment | pH, corrosion |
| Water softener needed to treat hardness | Manganese, iron |
| Water appears cloudy, frothy, or colored | Color, detergents |
_
Home drinking water testing:
Water is your family’s most precious resource. That’s why it’s so important for you to know what’s getting into your home and filling your glass! Testing your water for dangerous chemicals and impurities is the most important first step in maintaining a happy, healthy, contaminant-free home. Various do-it-yourself kits make it easier than ever to get professional results at an affordable price; while professional, certified laboratory can perform more complete and comprehensive analyses.
_
Turbidity and Free/Total Chlorine Meters:
The HI 93414 measures two of the most important parameters of drinking water: turbidity and free and total chlorine while the HI 98703 measures turbidity only. Both meters are EPA compliant and feature 3 turbidity measurement ranges: 0.00 to 9.99 NTU, 10.0 to 99.9 NTU, and 100 to 1000 NTU.
_
WaterSafe ®:
WaterSafe® drinking water test kits are the only 10-minute, do-it-yourself water safety tests on the market today. They are affordably priced, easy-to use, easy-to-understand, follow EPA Standards, and offer quick, remarkably accurate results!
_
Portable testing laboratory designed to assess the suitability of drinking water on-site, even in remote areas.
The Potalab® is a portable microbiological and physico-chemical water quality testing laboratory. It provides bacteriological testing using the accepted method of membrane filtration and incubation of faecal and total coliforms as an indicator of the presence of other harmful bacteria and viruses. The physico-chemical instruments are used in combination to quickly examine a supply to determine if a more detailed microbiological examination is required. All equipment is easily packed within the aluminum case for complete portability.
- For combined microbiological, physico-chemical testing in the lab or field
- Wide range of physico-chemical parameters can be measured using highly accurate digital instruments
- More cost effective than central laboratory testing
- Simple technique – dual incubator allows analysis of both faecal & total coliforms simultaneously
- Results incubated in just 14 hours
- Completely portable – even runs off solar power
- Uses low cost, long-life consumables, and equipment is easily sterilised in the field
- Supplied with reagents for Ammonia, Arsenic, Chlorine, Fluorides, Nitrites and Nitrates as standard. Other parameters available on request
- Conforms to WHO Guidelines
___________
Health effects of drinking contaminated water:
Water is very much critical for sustenance of human life; 70% of human tissue and 83% of blood is made up of water. Thus, water holds great significance for human wellbeing and health. Moreover, 80 % of the sickness accounted all over the world is water related (WHO 2004). There are many types of pollutants that can contaminate drinking water and cause illness and disease. Regardless of where drinking water comes from – a lake, a river, an underground aquifer, a well, a public water utility, even bottled water – all can be contaminated by a number of impurities. Some of these contaminants include chemicals like pesticides, heavy metals such as arsenic and lead, human and animal waste, and even chemical by-products created during drinking water treatment. Exposure to these contaminants can cause a number of health problems, ranging from nausea and stomach pain to developmental problems and cancer. ‘Water-borne’ diseases are of various kinds, majority of these diseases are due to the drinking of unsafe water. For example, 1.8 million people die every year from diarrhoeal diseases (including cholera), 133 million people suffers from high intensity Intestinal helminths infections which causes adverse health effects (ibid). Similarly, millions of people are affected due to consumption of water with excessive amount of certain chemical substances. For example, in Bangladesh, between 28 and 35 million people drink water with high levels of arsenic. Over 26 million people in China suffer from dental fluorosis due to high fluoride in their drinking water. Overall, water related diseases cost around 2- 5 million people live each year worldwide. Beside this, a huge chunk of population becomes vulnerable due to water related diseases (Gleick 2003). Around 37.7 million Indians are affected by waterborne diseases annually; 1.5 million children are estimated to die of diarrhoea alone (Khurana and Sen 2008). Diarrhoea continue to effect highest number of people among water borne diseases, at the same time 66 million Indians are at risk due to excess fluoride and 10 million due to excess arsenic in groundwater. Long-term exposure to contaminated water can cause rashes, heart disease, diabetes, cancer, and a number of immune, neurological, developmental, and reproductive problems. While everyone is at risk for health problems because of drinking water contamination, the level of risk varies from person to person and depends on a number of factors. These include: the specific contaminant(s) to which an individual is exposed; the size of the dose; demographic characteristics; pre-existing health conditions; lifestyle choices including smoking and diet; and the effects of exposure to multiple chemicals. Pregnant women are particularly susceptible to exposure, as are infants and children, the elderly, and persons with weakened immune systems.
__
Now let us look as some studies to know whether improved water quality prevents diarrhea:
Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis:
33 reports from 21 countries documenting 42 comparisons were included. Variations in design, setting, and type and point of intervention, and variations in defining, assessing, calculating, and reporting outcomes limited the comparability of study results and pooling of results by meta-analysis. The conclusion is that interventions to improve water quality are generally effective for preventing diarrhoea in all ages and in under age of 5. Significant heterogeneity among the trials suggests that the level of effectiveness may depend on a variety of conditions that research to date cannot fully explain. Effectiveness was not conditioned on the presence of improved water supplies or sanitation in the study setting and was not enhanced by combining the intervention with instructions on basic hygiene, a water storage vessel, or improved sanitation or water supplies—other common environmental interventions intended to prevent diarrhoea.
_
Does household water treatment in low income population help prevent diarrhea?
Effect of household-based drinking water chlorination on diarrhea among children under five in Orissa, India:
A double-blind randomized placebo-controlled trial:
Researchers conducted a double-blind randomized controlled trial between November 2010 and December 2011. The study included 2,163 households and 2,986 children under five in rural and urban communities of Orissa, India. The intervention consisted of an intensive promotion campaign and free distribution of sodium dichloroisocyanurate (NaDCC) tablets during bi-monthly households visits. An independent evaluation team visited households monthly for one year to collect health data and water samples. The primary outcome was the longitudinal prevalence of diarrhoea (3-day point prevalence) among children aged under five. This study was designed to overcome the shortcomings of previous double-blinded trials of household water treatment in low-income settings. The sample size was larger, the follow-up period longer, both urban and rural populations were included, and adherence and water quality were monitored extensively over time. These results provide no evidence that the intervention was protective against diarrhoea. Low compliance and modest reduction in water contamination may have contributed to the lack of effect.
_
Household Water Chlorination reduces incidence of Diarrhea among Under-Five Children in Rural Ethiopia:
A Cluster Randomized Controlled Trial:
A cluster randomized community trial was conducted in 36 rural neighborhoods of Eastern Ethiopia. Households with at least one child under-five years of age were included in the study. The study compared diarrhea incidence among children who received sodium hypochlorite (liquid bleach) for household water treatment and children who did not receive the water treatment. Generalized Estimation Equation model was used to compute adjusted incidence rate ratio and the corresponding 95% confidence interval. In this study, the incidence of diarrhea was 4.5 episodes/100 person week observations in the intervention arm compared to 10.4 episodes/100 person week observations in the control arm. A statistically significant reduction in incidence of diarrhea was observed in the intervention group compared to the control (Adjusted IRR = 0.42, 95% CI 0.36–0.48). Expanding access to household water chlorination can help to substantially reduce child morbidity and achieve millennium development goal until reliable access to safe water is achieved.
________________
Water treatment:
_
_
Water treatment means processes to make water safe for drinking purpose.
There is some confusion about the meaning of water purification and water disinfection.
Purification: The act of cleaning by getting rid of impurities.
For water treatment, this term refers to the process of removing specified contaminants from a water source. All effective water treatment methods will provide some amount of purification, however, only some methods will disinfect the water.
Disinfection: Killing or removal of microorganisms outside the body by direct exposure to chemical or physical agents or processes.
For water treatment, this term refers specifically to a purification process that kills or removes biological contaminants (cysts, bacteria, viruses, protozoans, etc.) from a water source. Water that has been disinfected (by UV treatment, boiling, chlorination, micro-filtration, ozone, etc.) may still be polluted with other contaminants that are not affected by the disinfection treatment. In some cases, additional contaminants may actually be added to the water by the disinfection process. For instance, the process of chlorination nearly always adds chlorine and frequently some disinfection byproducts (trihalomethanes, like chloroform). Boiling water too long will concentrate inorganic contaminants. Sterilization refers to the process of killing or removing all microorganisms.
_
Disinfection can be used as a pathogen reduction method against microorganisms. However, contact time, disinfectant concentration, water temperature, water turbidity (cloudiness), water pH, and many other factors can impact the effectiveness of chemical disinfection. The length of time and concentration of disinfectant varies by manufacturer and effectiveness of pathogen reduction depends on the product. Filtration can be used as a pathogen reduction method against most microorganisms, depending on the pore size of the filter, amount of the contaminant, particle size of the contaminant, and charge of the contaminant particle.
_
Water treatment types:
Many different types of water treatment systems are available in the current market. In general, water treatment systems are based on one or a combination of the following basic treatment methods:
1. Filtration such as mechanical filters, activated carbon filters, oxidizing filters, neutralizing filters, microfiltration, etc.
2. Ion exchange (water softeners)
3. Chemical oxidation
4. Disinfection methods such as chlorination, ultraviolet light, ozonation, etc.
6. Reverse osmosis
6. Distillation
Note that one particular type of treatment system cannot take care of all kinds of water quality problems and a combination of methods (multi-barrier protection) may often be needed.
_
Depending on the nature and extent of contamination, most of the above treatment methods offer two major types of water treatment devices:
Point of Entry (POE)
Point of Use (POU)
Point of Entry Water Treatment Systems:
Point of Entry (POE), or whole house treatment systems, treat all water entering the home. They are more expensive and are for treating a larger volume of water. They are useful when the water has problems that affect all areas of the home. The most common example is POE water softening ion exchange system that removes calcium and magnesium ions (and some other ions) from hard water. Even though hard water is not unhealthy to drink, it can cause scale buildup in pipes and on fixtures, interfere with the effectiveness of soap and shorten the life of appliances like dish washers and hot water heaters. Other POE water treatment systems are also designed to remove iron and manganese, adjust pH levels and add chlorine or other disinfectants. The POE devices typically treat about 100-300 gallons per day, depending on family size.
Point of Use Water Treatment Systems:
Point of Use (POU) systems treat water at the point where it is used. These are the systems that are installed at a specific location, frequently at the kitchen sink, to treat only the water that is used for drinking, cooking, etc. However, if other ports like the one on a refrigerator and/or a bathroom sink are also used for drinking, POU treatment systems should be installed at those locations as well to ensure total safety of drinking water. POU devices typically treat only a few gallons of water per day, and generally only water that will be directly consumed or used for cooking needs to be treated. Such a system might be used for contaminants like arsenic, cadmium, chromium, fluoride, uranium, nitrate or radium, some organic chemicals and sodium. Reverse osmosis, distillation and activated carbon units are generally POU devices because their main purpose is to provide few gallons of clean water per day for drinking and cooking only. It is cost prohibitive (and generally not necessary) to install them as POE devices to treat all water entering the home.
_
Most water requires some type of treatment before use, even water from deep wells or springs. The extent of treatment depends on the source of the water. Appropriate technology options in water treatment include both community-scale and household-scale point-of-use (POU) designs. A few large urban areas such as Christchurch, New Zealand have access to sufficiently pure water of sufficient volume that no treatment of the raw water is required. Over the past decade, an increasing number of field-based studies have been undertaken to determine the success of POU measures in reducing waterborne disease. The ability of POU options to reduce disease is a function of both their ability to remove microbial pathogens if properly applied and such social factors as ease of use and cultural appropriateness. Technologies may generate more (or less) health benefit than their lab-based microbial removal performance would suggest. The current priority of the proponents of POU treatment is to reach large numbers of low-income households on a sustainable basis. Few POU measures have reached significant scale thus far, but efforts to promote and commercially distribute these products to the world’s poor have only been under way for a few years. In emergency situations when conventional treatment systems have been compromised, water borne pathogens may be killed or inactivated by boiling but this requires abundant sources of fuel, and can be very onerous on consumers, especially where it is difficult to store boiled water in sterile conditions and is not a reliable way to kill some encysted parasites such as Cryptosporidium or the bacterium Clostridium. Other techniques, such as filtration, chemical disinfection, and exposure to ultraviolet radiation (including solar UV) have been demonstrated in an array of randomized control trials to significantly reduce levels of water-borne disease among users in low-income countries, but these suffer from the same problems as boiling methods.
_
Community and Household Water Treatment:
Water can be treated at a central location, in large volumes, and then supplied to households through a network of pipes. This is often called centralized or community water treatment. Smaller volumes of water can also be treated at the point of use (POU), such as in a home. This is commonly called household water treatment and safe storage (HWTS) since the family members gather the water, and then treat and store it in their home. Most people around the world wish to have safe water piped directly to their homes through a community water treatment system. Unfortunately, the money and resources needed to construct, operate and maintain a community system are not always available in most developing countries. The main advantage of HWTS is that it can be used immediately in the homes of poor families to improve their drinking water quality. It is proven to be an effective way to prevent diseases from unsafe water. HWTS lets people take responsibility of their own water security by treating and safely storing water themselves. HWTS is also less expensive, more appropriate for treating smaller volumes of water, and provides an entry or starting point for hygiene and sanitation education. There are a wide range of simple HWTS technologies that provide options based on what is most suitable and affordable for the individual household. Some limitations of HWTS are that it requires families to be knowledgeable about its operation and maintenance, and they need to be motivated to use the technology correctly. As well, most HWTS processes are designed to remove pathogens rather than chemicals. With both centralized and household water treatment, using the multi-barrier approach is the best way to reduce the risk of drinking unsafe water. Each step in the process, from source protection, to water treatment and safe storage, provides an incremental health risk reduction. Both community and household water treatment systems follow the same water treatment process. The only difference is the scale of the systems that are used by communities and households.
_
_
A growing body of research suggests household water treatment and safe storage (HWTS):
1. dramatically improves microbial water quality
2. significantly reduces diarrhea
3. Is among the most effective of water, sanitation and health interventions
4. is highly cost-effective
5. can be rapidly deployed and
6. taken up by vulnerable populations.
_
Point-of-use disinfection can be a low-cost option. Solar disinfection is free, provided plastic bottles are available. Bleach solution costs very little to produce, and according to the US Centers for Disease Control and Prevention (CDC) 10-25 US cents worth can last a family an entire month. Simple ceramic pot filters moulded by local artisans can be used to filter water in the home for approximately US$ 3 per year, making them sustainable and economical. Boiling is by far the most commonly used approach to disinfect water at household level. At the global level, a recent World Health Organization report suggests that household water interventions can lead to a benefit of up to US$60 for every US$1 invested. Water treatment also needs to be accompanied by safe storage. This can be accomplished by using containers with narrow openings and a dispensing device such as a tap or spigot to protect collected water against recontamination. These measures are particularly important because the microbial quality of drinking water frequently declines after collection.
_
Municipality drinking water treatment: treatment of water that reaches tap in your home:
Each day, millions of people use billions of gallons of water without knowing where it comes from or what might be in it. As populations grow, the combination of increased demand and increased pollution means many of us are using sources of water that are less than pristine. Contamination from sediment, bacteria, protozoans, heavy metals, and synthetic organic compounds shows up with alarming frequency. As a result, many municipalities are having to pre-treat drinking water. Water treatment transforms raw surface and groundwater into safe drinking water. Treating water to make it suitable to drink is much like wastewater treatment. In areas that depend on surface water it is usually stored in a reservoir for several days, in order to improve clarity and taste by allowing more oxygen from the air to dissolve in it and allowing suspended matter to settle out. The water is then pumped to a purification plant through pipelines, where it is treated, so that is will meet government treatment standards. Water treatment involves two types of processes: physical removal of solids (mainly mineral and organic particulate matter) and chemical disinfection (killing/inactivating microorganisms). Treatment practices vary from system to system. The first step in most municipal treatment systems involves gravity. If you’ve ever let a glass of chocolate milk stand for any length of time, you’ve probably noticed that much of the chocolate settles to the bottom of the glass. The same is true of sediment in water. When the water is allowed to stand in large pools, many of these suspended particles simply settle to the bottom where they are collected and disposed of. Next comes flocculation. Here, a chemical is added to the water that causes tiny suspended particles to clump together. Alum (an aluminum sulfate) or other metal salts are added to raw water to aggregate particles into masses that settle more readily than individual particles. Usually, these flocs settle out just like large-sized sediment. But if they escape, they are caught by filters farther down the line. Then comes stage of filtration. Water is pumped through large tanks filled with fine sand, called rapid sand filters. As the water flows through the spaces between the sand grains, suspended particles and dead microbes get trapped. Sometimes, crushed anthracite coal is used in addition to sand. Because the coal grains carry a charge on their surface, they act like tiny magnets attracting other charged contaminants. To kill unwanted microbes, protozoans, and other living organisms, many municipalities chlorinate the water. Chlorine is a chemical that kills microorganisms. In drinking water, the concentrations are low enough that often you can’t even taste it. An additional amount, known as a “chlorine residual” is applied to protect treated water from re-contamination as it travels throughout the distribution system.
_
_
How can I protect my private water supply?
Protect your water supply by carefully managing activities near the water source. For households using a domestic well, this includes keeping contaminants away from sinkholes and the well itself. Keep hazardous chemicals out of septic systems.
•Periodically inspect exposed parts of the well for problems such as: cracked, corroded, or damaged well casing; broken or missing well cap; settling and cracking of surface seals.
•Slope the area around the well to drain surface runoff away from the well.
•Install a well cap or sanitary seal to prevent unauthorized use of, or entry into, the well.
•Have the well tested once a year for coliform bacteria, nitrates, and other constituents of concern.
•Keep accurate records of any well maintenance, such as disinfection or sediment removal, that may require the use of chemicals in the well.
•Hire a certified well driller for any new well construction, modification, or abandonment and closure.
•Avoid mixing or using pesticides, fertilizers, herbicides, degreasers, fuels, and other pollutants near the well.
•Do not dispose of wastes in dry wells or in abandoned wells.
•Do not cut off the well casing below the land surface.
•Pump and inspect septic systems as often as recommended by your local health department.
•Never dispose of harsh chemicals, solvents, petroleum products, or pesticides in a septic system or dry well.
______________
Water treatment processes:
_________________
Alum:
Alum is both a specific chemical compound and a class of chemical compounds. Aluminum sulfate is also sometimes referred to as a type of alum. The specific compound is the hydrated potassium aluminum sulfate (potassium alum) with the formula KAl(SO 4)2·12H 2O. More widely, alums are double sulfate salts, with the formula AM(SO 4) 2·12H 2O, where A is a monovalent cation such as potassium or ammonium and M is a trivalent metal ion such as aluminum or chromium(III). Between 30 and 40 ppm of alum for household wastewater, often more for industrial wastewater, is added to the water so that the negatively charged colloidal particles clump together into “flocs”, which then float to the top of the liquid, settle to the bottom of the liquid, or can be more easily filtered from the liquid, prior to further filtration and disinfection of the water. Alum is used as a flocculant to remove unwanted colour and turbidity from water supplies. It has been used since ancient times for this purpose and its use together with filtration is standard practice in conventional water treatment processes around the world. After performing its role the Alum is filtered from the water but a small fraction dissolves and is not removed. There has been ongoing debate in the water industry for a number of years regarding the use of alum in the water treatment process and the ‘suspicion’ that aluminum is linked to Alzheimer’s disease. The cause of Alzheimer’s disease is subject to international research. A variety of possible causes have been considered, however, no link between aluminum intake and the disease has been established.
_________
Filtration:
There are two basic types of water filters: particulate filters and adsorptive/reactive filters. Particulate filters exclude particles by size, and adsorptive/reactive filters contain a material (medium) that either adsorbs or reacts with a contaminant in water.
_
A filter that removes particles down to 5 microns will produce fairly clean-looking water, but most of the water parasites, bacteria, cryptosporidia, giardia, etc. will pass through the pores. A filter must trap particles one micron or smaller to be effective at removing cryptosporidia or giardia cysts. Viruses cannot be removed with complete reliability by any filtration method. In theory, reverse osmosis will remove viruses, but a small flaw in the membranes would allow viruses to pass undetected into the ‘filtered’ water.
_
Membrane technologies comprise micro filtration (MF), ultra filtration (UF), nano filtration (NF) and reverse osmosis membranes (RO). Micro filtration (0.1 micron) removes most of the fine suspended solids in the water and almost all protozoa and bacteria but is not able to remove the dissolved part of the natural organic matter. Ultra filtration has smaller pores than used in micro filtration, can remove finer particles from the water and is capable of removing viruses also. Nano filtration uses membranes with even smaller holes and requires operating pressure to force water through the membrane. This results in higher operating cost. It is effective in removing insecticides and herbicides. Cost involved in the technology and the backwashing of the membrane can consume a significant portion of the water produced. Reverse osmosis (RO) uses a membrane (0.001 micron) that is semi-permeable, allowing the fluid being purified to pass through it, while rejecting the contaminants behind. Reverse osmosis filters remove lead and other large minerals and organics, but not smaller minerals and organics (such as chlorine and chloroform). Reverse osmosis is capable of rejecting bacteria, salts, sugars, proteins, particles, dyes, and other constituents that have a molecular weight of greater than 150-250 Daltons. The larger the charge and larger the particle, the more likely it will be rejected.
_
Sand filters:
Rapid and slow sand filters are effective in reducing turbidity of the source water. Turbidity is measured in standardized nephalometric turbidity units (NTUs), determined by measuring the scattering of light as it passes through the water. High turbidity interferes with the effectiveness of disinfection by chlorine, ozonation, and UV. Owing to this, WHO recommends that the mean turbidity of the source water being treated with these disinfection methods should be below 1 NTU, with no single sample having turbidity exceeding 5 NTUs. In addition to substantially removing turbidity, slow sand filters also permit large reductions in bacterial and viral contamination and remove larger biological contaminants (such as cryptosporidium, giardia, amoebae, parasite eggs, etc). Any given design of a filter will have inherent limits on the level of turbidity (in units of NTUs) and total suspended solids (TSS, in units of mg/l) it can treat. If the incoming water exceeds these design parameters, the filter may clog up rapidly and may produce filtrate (i.e. outlet water) with turbidity and TSS exceeding the intended design values. Common design limits on inlet water turbidity and TSS are about 50 NTUs and 50 mg/l, respectively. Water with higher values for turbidity and TSS is preferably pretreated with coagulation and/or flocculation before filtration.
Rapid sand filters:
Rapid sand filters reduce larger micro-organisms and suspended solids. Water passes through the filter bed by gravity at a velocity between 2 to 5 to meters per hour. The filter performance is initially poor and then improves for a period. As the filter bed becomes compacted, the performance deteriorates again. This can be rectified by regular monitoring of filtrate quality and backwashing. The filtrate from the rapid sand filter is used as input to a slow sand filter to produce drinking water for the community. Rapid sand filters do not by themselves disinfect water adequately (they will not remove fecal pathogens) but can prepare water for treatment by UV, chlorine, or ozone.
Slow sand filters:
Slow sand filters are more effective than rapid filters at removing particulates and microbial contaminants and are also simpler to operate. They do not require backwashing as frequently as rapid sand filters. A layer of active biological community (known as smutzdecke, comprising food chains of ciliated protozoa, free-living bacteria, amoebae, crustacea, and other small organisms), develops in the sand a few centimeters below the top surface and captures organic particulates and microbial contaminants in the inlet water. When the filter clogs up, the top layer can be scraped off, and the filter restarted. The new smutzdecke takes a few days to establish (depending on the temperature and local conditions) before the filter becomes fully operational again. Water seeps through slow sand filters at rates of 0.1 to 0.2 meters per hour. Thus, the technology is low cost and low maintenance, but requires sufficient land area. Other limitations of the slow sand filter technology are that the inlet water should not have a very high concentration of suspended solids, high coliform counts, or large quantities of algae; otherwise, the filter can clog rapidly. Also, low operating temperature, low oxygen content in the inlet water, or low nutrient content can inhibit the operation of the smutzdecke.
_
Microporous Basic Filtration:
There are three types of microporous filtration: depth, screen and surface. Depth filters are matted fibers or materials compressed to form a matrix that retains particles by random adsorption or entrapment. Screen filters are inherently uniform structures which, like a sieve, retain all particles larger than the precisely controlled pore size on their surface. Surface filters are made from multiple layers of media. When fluid passes through the filter, particles larger than the spaces within the filter matrix are retained, accumulating primarily on the surface of the filter. The distinction between filters is important because the three serve very different functions. Depth filters are usually used as prefilters because they are an economical way to remove 98% of suspended solids and protect elements downstream from fouling or clogging. Ultrafilters are available in several selective ranges. In all cases, the membranes will retain most, but not necessarily all, molecules above their rated size.
_
Carbon filter:
_
_
Carbon filtering is a method of filtering that uses a bed of activated carbon to remove contaminants and impurities, using chemical adsorption. The material has millions tiny pores, usually invisible to eye, and can absorb thousands of water contaminants. Activated carbon is carbon which has a slight electro-positive charge added to it, making it even more attractive to chemicals and impurities. As the water passes over the positively charged carbon surface, the negative ions of the contaminants are drawn to the surface of the carbon granules. Several forms are commonly used in water filtration: Granular Activated Carbon (GAC ), and Carbon Block. Generally, Carbon Block Filters (or “charcoal water filters”) have higher contaminant removal capability than GAC filters, and they usually have a higher cost. Activated Carbon and “Block” Filters a usually rated by size of the particles it can remove. Typical ratings are from 50 micron (least effective) to 0.5 micron (most effective). The solid material used in an activated carbon filter is typically petroleum coke, bituminous coal, lignite, wood products, coconut shell, or peanut shells, all of which are sources of carbon. The material is activated by subjecting it to high temperature (2300 °F) and steam in the absence of oxygen. This process produces a carbon substance with many small pores and thus a very large surface area, which is then crushed to yield a granular or pulverized product. Each particle/granule of carbon provides a large surface area/pore structure, allowing contaminants the maximum possible exposure to the active sites within the filter media. One pound (450 g) of activated carbon contains a surface area of approximately 100 acres (40 Hectares). Activated carbon works via a process called absorption, whereby pollutant molecules in the fluid to be treated are trapped inside the pore structure of the carbon substrate. Carbon filtering is commonly used for water purification. Carbon filters are most effective at removing chlorine, sediment, volatile organic compounds (VOCs), taste and odor from water. They are not effective at removing minerals, salts, and dissolved inorganic compounds. Typical particle sizes that can be removed by carbon filters range from 0.5 to 50 micrometers. The particle size will be used as part of the filter description. The efficacy of a carbon filter is also based upon the flow rate regulation. When the water is allowed to flow through the filter at a slower rate, the contaminants are exposed to the filter media for a longer amount of time. Because activated charcoal removes chlorine, these filters can breed bacteria. To prevent this, carbon filters are often impregnated with silver, which kills bacteria. Silver is, however, toxic to humans, if the unit releases too much silver into the water.
_
Activated Carbon filter for household use:
Many commercial firms offer activated carbon filters for urban household use in the developing countries, to be fitted at the end of the municipal water tap in the kitchen, for removal of various waterborne chemical pollutants. The growth of bacteria in activated point-of-use carbon filters for household use has been well documented. These bacteria colonize the filters and slough off into the water stream in very large numbers when water is turned on. WHO remarks that the ample published reports on this topic have convincingly demonstrated that incorporating bacteriostatic agents (e.g. silver) in the filters has only a limited effect in controlling such growth. For this reason, point-of-use household carbon filters must be periodically replaced, and should be used only with water that is known to be already microbiologically safe. While this is not yet a major problem (in terms of absolute numbers of people at risk), it could become one without appropriate consumer education.
_
A benefit of all home sediment and activated carbon filtration systems (and membrane filters with larger pores) is that they are passive. That is, they do not require electricity to filter the water, and normal home water pressure is used to force the water though the filter – in emergencies, water can even be siphoned through them to provide some treatment. The only routine maintenance required is periodic replacement of the filtration element. As long as the cost of the replacement filter elements is reasonable, owning a even a high-end water filter can be very inexpensive if you look at the long term costs and compare it with other solutions.
_
How powerful filter can harm you:
About 300 guests have been moved from a luxury Miami hotel after Legionnaires’ disease struck at least three former guests. One of them died. Guests at the EPIC Hotel were sent to nearby hotels to prevent potential contact with the waterborne bacterial disease. An ongoing investigation revealed that the hotel had installed a water filter powerful enough to remove anti-bacterial treatments from its city-supplied water, encouraging bacterial growth.
___________
Chlorination:
_
_
Chlorine in various forms is the most common disinfectant used worldwide. Chlorination is the most common disinfection method for public and private drinking water systems. This treatment has limitations and is not suitable for heavily- contaminated wells or springs, or sources where hazardous materials are present. With adequate residual chlorine and contact time between the disinfectant and the microorganisms, chlorination effectively kills many disease-causing bacteria. Additionally, chlorine is inexpensive, easy to control, generally safe to use, and adapts to municipal or private systems. Chlorine readily combines with chemicals dissolved in water, microorganisms, small animals, plant material, tastes, odors, and colors. These components “use up” chlorine and comprise the chlorine demand of the treatment system. It is important to add sufficient chlorine to the water to meet the chlorine demand and provide residual disinfection. The chlorine that does not combine with other components in the water is free (residual) chlorine, and the breakpoint is the point at which free chlorine is available for continuous disinfection. An ideal system supplies free chlorine at a concentration of 0.3-0.5 mg/l. The taste and odour thresholds for chlorine in distilled water are 5 and 2 mg/liter, respectively. In air, chlorine has a pungent and disagreeable odour . Chlorine dose is measured in units of concentration times contact time. For example, a chlorine dose of 2 mg/l and 30-minute contact time (obtained in a chlorination holding tank) provides 99.9% disinfection of Giardia at 20 degC, 1-NTU, and pH of 7. Chlorine in water reacts to form HOCl and H+ and CI-. HOCl, or hypochlorous acid, itself partially dissociates as H+ and OCl-; the latter is called the hypochlorite ion. Warmer water temperature, and to a much greater extent, lower water pH, decrease the dissociation of the hypochlorous acid, i.e. decrease the ratio [OCl]/[HOCl]. Furthermore, chlorine in the water reacts with and binds to the material in suspended solids, and thus is removed from the reaction to form hypochlorous acid. Hypochlorous acid is a stronger bacterial disinfectant than the hypochlorite ion. Therefore, the required chlorine dose for disinfection increases sharply with increasing turbidity, increasing pH, decreasing water temperature, and increasing concentrations of ammonia, hydrogen sulfide, Fe, and Mn. The chlorine doses needed over the full range of these water properties would differ by a factor larger than 10. With poor quality water (e.g. high turbidity, high pH), disinfection with chlorine may become impractical because the chlorine dose required may be so high that the contact time may extend into tens of hours or the chlorine concentrations may exceed objectionable taste threshold. Chlorination is effective against many pathogenic bacteria, but at normal dosage rates it does not kill all viruses, cysts, or worms. When combined with filtration, chlorination is an excellent way to disinfect drinking water supplies. Conventional automated chlorine-dosing plants can apply the right amount of chlorine; however, they require highly trained operators, engineers, and repair and maintenance infrastructure available in and appropriate for only large urban populations. In many smaller communities in the developing countries, various solid or liquid chemical forms of chlorine (e.g. bleaching powder [calcium hypochlorite, Ca (OCl) 2], or sodium hypochlorite (NaOCl), are used since they are safer to transport and handle than chlorine gas. An alternate chlorination technology needs mention here, distinct from the automatic dosing plants delivering chlorine gas in some form to municipal water. In this method, a solution of ordinary household common salt (NaCl) is prepared and electrolyzed with no separation attempted between the cathode and the anode. This leads to the formation of a solution of NaOCl in the brine, which can be immediately added to the water to disinfect it. This method has the advantages of not requiring storage of any form of chlorine, relying on inexpensive (and impure) household common salt for chlorine source, and not being sensitive to the maintenance of a supply chain of the chlorine source chemical. The disadvantage is that the NaOCl brine must still be properly metered or dosed into the raw water, which will get disinfected over a period of time (“contact time”) before it can be used. The major advantage of chlorine is its ability to leave a residual disinfection concentration in the water supply. Residual free chlorine is the available chlorine left in the water after a specified contact period, which can further disinfect any newly introduced biological contamination. A residual free chlorine of 0.25 mg/l is considered adequate for warm climates (20±C water supply) for water with total organic carbon content of less than 0.25 mg/l. The residual chlorine suppresses regrowth of nuisance bacteria and guards against small amounts of recontamination of the water by reintroduced pathogens. A large infusion of pathogens and organic matter, however, can overwhelm the protection provided by residual chlorine. An occasional occurrence in developing-country city supplies is the intake of raw sewage–contaminated urban ground water into leaky underground drinking water mains during periods when insufficient water supply forces reduction in (or absence of ) positive pressure in the water mains (or when residential booster pumps cause negative pressure in the water supply mains). Residual chlorine concentrations under such circumstances are often inadequate to disinfect the admitted contaminated ground water, leading to outbreaks of waterborne disease with pathogens piped right into people’s homes. In general, however, the residual disinfection is a valuable guard. The primary disadvantage of chlorine is the necessity to maintain an appropriate supply chain of source chemical to the water treatment location. Both liquid and powder bleach degrade over time with half lives of the order of weeks to months (depending on storage conditions). Cholera outbreaks have been reported in India when impassable roads blocked the chlorine supply chain during heavy monsoons. An equally serious disadvantage is the need for a skilled and trained operator and a repair and maintenance infrastructure. For large systems (cities of 100,000 or more), chlorine disinfection costs are low, approaching about $.02 perm3 of water. With small-scale systems, however, the costs rapidly increase, as does the impracticality of having skilled technical operators. The various methods of disinfection by chlorination lead to the production of disinfection by-products (DBPs) in the water containing dissolved organic carbon compounds. In almost all cases in the developing world, the health risks from pathogenic microbial contamination of drinking water are thousands of times larger than the health risks from the ingestion of the DBPs. DBP risks and disinfection methods for developing country communities are reviewed by Ellis. In recent years, a potentially new health risk from DBPs is being investigated in the US and European countries beyond the traditionally understood one—the risk of endocrine disruption potential of DBPs. At present, this is an active and important research topic, but compelling conclusions are not in hand to warrant a change in recommended disinfection practices in developing countries.
_
Chloramine as disinfectant:
_
_
Chloramine, formed by combining chlorine and ammonia, is used as a drinking water disinfectant just like chlorine itself. Chloramine has been used as disinfectant for over a hundred years, but in the last decade it has become increasingly popular. The main reason for using it as an alternative for chlorine is because it generates fewer byproducts. When a drinking water provider switches to chloramine as the main disinfectant, they have to inform their customers well in advance so the appropriate measures can be taken by aquarium owners and others who rely on chloramine free water. Chloramine has no direct adverse health influences when consumed in drinking water. In drinking water it is sufficiently diluted and after ingestion it is degraded in the stomach. Both people and animals can safely drink chloraminated drinking water. Chloramine is harmful when it enters the bloodstream directly, but it can be applied to clean open wounds without concern. However, chloramine can contain risks or problems for reptiles, aquarium fish, kidney dialysis patients and industrial water users. The advantage of chloramine over chlorine is that less harmful by-products are created. The most important by-products with chlorination are trihalomethanes (THMs). THMs are the products obtained when organic compounds react with the chloride in water. They are found in drinking water in very low concentrations. At the moment, little is known about these compounds, but some are believed to be carcinogenic and several are also suspected to have influences on the reproductive system. Chloramine is a more persistent disinfectant than chlorine. As a result it is retained in the water for a longer period of time. The advantage is that in this way it can function much longer as a disinfectant than chlorine. However, it cannot be removed by letting the water stand for a couple of days as with chloride. The best way to remove chloramine is to use a water conditioner that contains a de-chlorination chemical or by using high quality granular activated carbon which will absorb the chloramine. Nevertheless, there are only a few circumstances when complete removal is mandatory. Chloramine seems a very good alternative to chloride. The taste is less offensive than chloride; it releases less harmful by-products and has no adverse health properties. Though, it should be noted that in two unrelated unique cases chloramine is the probable culprit in increasing lead levels and iodo acetic acid levels. As long as the exact reason for these contaminations is not resolved, drinking water companies should be careful in switching from chlorine to chloramine. The risk for high lead levels can have a serious health impact and should not be something to take lightly.
______
Iodine disinfection:
Water that has been disinfected with iodine is not recommended for pregnant women, people with thyroid problems, those with known hypersensitivity to iodine, or continuous use for more than a few weeks at a time.
__________
Treatment with Hydrogen Peroxide:
The use of Hydrogen Peroxide (H2O2) as a pre-oxidant in municipal water treatment is well documented and has been practiced for over 20 years. Historical applications of H2O2 in drinking water have been:
•Taste and odor control
•Hydrogen sulfide removal
•Iron removal
•Ozone enhancement/destruction
__________
Mixed Oxidant Gases Generated On Demand (MOGGOD) systems:
This is the most recent arrival on the technical scene for drinking water disinfection. There are several different designs and manufacturers of MOGGOD systems. The basic concept is to electrically produce the mixed oxidant gases on demand using an electrochemical cell that uses industrial high-purity salt (NaCl) brine as the chlorine source. The separation in the brine electrochemical cell is based either on a membrane or a density gradient in the salt solution. The oxidant gases in the disinfecting liquid produced from electrolysis are a mix of chlorine dioxide, ozone, and hypochlorite. This liquid is then either metered into the source water or sold bottled to be added to household water storage tanks. The main advantages of MOGGOD are that the source of the disinfectant is inert and relatively inexpensive (industrial high-purity NaCl), the mixed oxidants are a more effective disinfectant than chlorine alone, and a residual protection is produced in the water. One US manufacturer states that a mixed oxidant dose of 4 mg/l with a contact time of 60 minutes provides greater than 99.9999% disinfection against E. coli, and 99.99% disinfection of giardia cysts . Most of the experience with MOGGOD systems has been in Latin America. These systems are built and sold on demand, so prices are not stable. The operating cost of water disinfection by MOGGOD systems is stated to be attractive for large systems, comparable to that from chlorine or bleach (sodium or calcium hypochlorites), although the first costs are much higher than those for disinfection with bleach. The disadvantage of the MOGGOD system is that it requires dozens of hours of skilled maintenance per year of the electrochemical system, dosing valves, flow meters, and venturi ducts, including handling of caustic chemicals. Systems based on a membrane require cleaning and replacing the membrane every few months to a year (depending on the level of impurities in the salt), and so appear inappropriate for typical developing country applications.
___________
Distillation:
Distillation is probably the oldest method of water purification. Water is first heated to boiling. Then the water vapor rises to a condenser where cooling water lowers the temperature so the vapor is condensed, collected and stored. Most contaminants stay behind in the liquid phase vessel. However there can sometimes be what is called carry-overs found in the distilled water. Organics such as herbicides and pesticides, with boiling points lower than 100°C, cannot be removed efficiently and can actually become concentrated in the product water. Another disadvantage of distillation is cost. Distillation requires large amounts of energy and water and is very slow to produce clean water. Distilled water can also be very acidic (low pH), thus it should be contained in glass. Since there is not much left after distillation, distilled water is often called “hungry” water. It lacks oxygen and minerals and has a flat taste, which is why it is mostly used in industrial processes.
__________
Pasteurization and boiling water:
Boiling is the oldest method to obtain water free of biological contaminants. In many developing countries and several cities of the former Soviet Union, residents routinely boil their drinking water because the safety of the water supply cannot be trusted or is known to be compromised. World Bank reports that 1% of Jakarta’s GDP is spent by the residents of the city boiling their drinking water. Anecdotal reports suggest that about half the population of China boil their drinking water, mostly over biomass-fueled stoves. As has been well documented, biomass is generally the most air-polluting, and if purchased, expensive (owing to low efficiency of cook stoves) of cooking fuels, but it is the only one accessible to the poorest of the populations in the developing world. In fact, one does not have to boil the water to disinfect it. Holding it at a high enough temperature (e.g. 6 minutes at 70±C) is sufficient to pasteurize the water and render it safe. Figures providing the minimum holding time for various temperatures to kill various pathogens are available in the literature. However, given the absence of easy thermometry for household use, boiling the water is the safe and common choice. At 100±C, enteric pathogens are killed in less than a minute. WHO recommends bringing water to a vigorous roiling boil for a minute for disinfecting it at sea level, and adding a minute of extra boiling time for each 100 meters in altitude, to account for the progressively lower boiling point of water at higher altitudes. With an average cook stove efficiency of 12%, fuel wood can boil water about three times its own weight. For a family of 5 with a drinking water need of 35 liters (35 kg of water) daily, this will consume about 12 kg of wood, several times more than the few kilograms the family would use for cooking its daily food. Gathering fuel wood for daily cooking is already a heavy burden on hundreds of millions of women and girls in the developing world. In fact, it is economically unrealistic and environmentally unsustainable to recommend boiling daily drinking water to the poor of the developing world. Boiling can be recommended only in an emergency situation, and is practiced routinely only by the fraction of the population that can afford it. Presumably, with improved information dissemination about the linkage between unsafe drinking water and diarrheal disease, those who risk their health to unsafe drinking water today will start boiling water as soon as they can begin to afford it. This poses a potentially very large increase in the biomass extracted for household use. Even if the biomass is harvested sustainably, non-CO2 combustion products from biomass burning can be significant in terms of their greenhouse potential (1 kg of fuel wood burnt in a biomass cook stove releases about 440 grams of carbon as CO2, and about 650 grams carbon-equivalent of non-CO2 greenhouse gas emissions. Provision of safe drinking water to these populations would be an effective way to circumvent the potential impending depletion of biomass resources for boiling drinking water, and the associated large contribution to greenhouse gas emissions.
_
Boiling water will kill bacteria as well as other disease-causing microorganisms like Giardia lamblia and Cryptosporidium parvum which are commonly found in rivers and lakes. At high elevations, though, the boiling point of water drops. This reduces the time and energy required to bring water to a boil, but can increase the duration of boiling required to kill certain pathogens. Water temperatures above 70 °C (158 °F) will kill all pathogens within 30 minutes, above 85 °C (185 °F) within a few minutes, and at boiling point (100 °C (212 °F)), most pathogens will be killed, excluding certain pathogens and their spores, which must be heated to 118 °C (244 °F)(e.g.: botulism – Clostridium botulinum). This can be achieved by using a pressure cooker, as regular boiling will not heat water past 100 °C (212 °F) at sea level. It is worth noting that not all pollutants are removed from water by boiling, even in a pressure cooker. Boiling cannot remove chemicals having boiling points at or above 100 °C (212 °F), nor heavy metal contamination, e.g., colloidal metal pollutants. Activated charcoal, however, can remove many pollutants, but can’t remove pathogens. A combination of rolling boiling for one minute at standard atmospheric pressure (i.e., not in a pressure cooker) plus filtering with activated charcoal can neutralize most pathogens and pollutants.
_
In an emergency, boiling is the best way to disinfect water that is unsafe because of the presence of protozoan parasites, bacteria or viruses. If the water is cloudy, it should be filtered before boiling. Filters designed for use when camping, coffee filters, towels (paper or cotton), cheesecloth, or a cotton plug in a funnel are effective ways to filter cloudy water. Place the water in a clean container and bring it to a full boil and continue boiling for at least 3 minutes (covering the container will help reduce evaporation). If you are more than 5,000 feet above sea level, you must increase the boiling time to at least 5 minutes (plus about a minute for every additional 1,000 feet). Boiled water should be kept covered while cooling.
_
The advantages of Boiling Water include:
Pathogens that might be lurking in your water will be killed if the water is boiled long enough. Boiling will also drive out some of the Volatile Organic Compounds (VOCs) that might also be in the water. This method works well to make water that is contaminated with living organisms safe to drink, but because of the inconvenience, boiling is not routinely used to treat drinking water except in emergencies.
The disadvantages of Boiling Water include:
While boiling is highly effective, it does have its drawbacks. For one, it’s time consuming to purify a significant amount of water this way. And, while warm water may be a treat when you’re on a winter hike, it’s certainly not when the weather is toasty. Even after allowing the water to cool, you’ll be drinking tepid water. Boiling also has a negative effect of the taste of the water. Some people describe the taste as “flat.” You can reduce this somewhat by pouring the boiled water back and forth between two clean containers as it cools. This process aerates the water, improving the taste. Probably the biggest drawback of boiling is that you’ll use a lot of fuel to boil enough water to keep you hydrated. In areas where the water is “hard” (that is, containing significant dissolved calcium salts), boiling decomposes the bicarbonate ions, resulting in partial precipitation as calcium carbonate. This is the “fur” that builds up on kettle elements, etc., in hard water areas. With the exception of calcium, boiling does not remove solutes of higher boiling point than water and in fact increases their concentration (due to some water being lost as vapour). Boiling does not leave a residual disinfectant in the water. Therefore, water that is boiled and then stored for any length of time may acquire new pathogens. Also, boiling does not inactivate spores and toxins generated by bacteria. Boiling should not be used when toxic metals, chemicals (lead, mercury, asbestos, pesticides, solvents, etc.), or nitrates have contaminated the water. Boiling may concentrate any harmful contaminants that do not vaporize as the relatively pure water vapor boils off. Energy is needed to boil the water, so it may be difficult to boil water in an emergency.
____________
UV Disinfection:
Ultraviolet light in the wavelength range 240 to 280 nm has been known to be germicidal for almost a century. The germicidal effect occurs because the UV light causes severe damage to the DNA of the micro-organisms. Specifically, the UV exposure covalently bonds together certain adjacent bases in the DNA, thus disabling it from replication. The germicidal effect is most potent at a wavelength of 260 nm. Since a low-pressure mercury arc (same as that used inside ordinary household fluorescent lamps) puts out 95% of its energy at 254 nm, it can provide an extremely effective germicidal effect. UV dose is measured in microwattseconds of UV energy (at or close to 260 nm) per sq. cm of water surface. UV dosages for various degrees of inactivation of selected microorganism are found in the literature. The dose to inactivate 90% of E. coli is 300 microWs/cm2. Other pathogenic bacteria and viruses have doses of similar magnitude (rotavirus at 800 microWs/cm2 is the highest among these). On the other hand, UV doses of very much larger magnitudes are needed to inactivate the cysts of protozoa, such as Giardia and Cryptosporidium. UV is not a treatment of choice for removal of cysts. Appropriate filtration or sedimentation can remove these larger pathogens and also reduce turbidity (which improves UV transmittance and reduces shielding of microbial pathogens by particulate matter) before UV treatment.
_
_
Compared to boiling over a biomass cook stove of 12% efficiency, UV disinfection can require 20,000 times less primary energy (assuming a design delivering 38,000 microWs/cm2 UV energy dose to the water). In contrast to many of the chemical disinfectants, UV disinfection imparts no taste or odor to the water, and presents no risks from overdosing or formation of carcinogenic disinfection by-products. The very high sensitivity of DNA to UV light allows very short treatment time for the water. In contrast to chlorine (which requires contact times of 30–60 minutes), UV disinfects water in a few seconds. Because it does not have diseconomies of scaling down, the cost of disinfection per cubic meter remains about $.02 even for small systems, while for chlorine treatment units the costs are comparably low for large-scale systems but rise by an order of magnitude as the scale gets smaller. Since UV does not impart residual disinfection to the water, it is appropriate only for point-of-use disinfection systems and under circumstances where the disinfected water will be protected from recontamination. Furthermore, since enzyme mechanisms exist within several bacterial species that try to repair the damaged DNA(although in a slow and error-prone manner),UV disinfection by itself, at the minimum UV dose required by current standards, is not suitable for disinfection of drinking water intended for long-term storage. Most UV system designs comprise a linear UV lamp, enclosed within a cylindrical coaxial UV-transparent sleeve (made of quartz or teflon), submerged in water in the UV-exposure chamber. Water flows axially on the outside of the sleeve and receives the UV dose. Chemical fouling and biological film (particularly when the lamp is off and the water stagnant during hours of disuse) builds up on the sleeve surface over time. This fouling seriously impairs the UV transmittance of the sleeve and necessitates its periodic cleaning with chemical and mechanical methods. This makes maintenance complex and expensive and puts it beyond the means of most rural communities. Recently, UV systems with an air gap between the UV lamp and the water surface were developed and have been licensed for commercial production. In this design, the linear UV lamp is positioned horizontally below a semi-cylindrical reflector, above the free surface of water flowing in a shallow tray. This design innovation circumvents the problem of chemical- and bio-fouling of the solid surface between the UV source and the water. Also, since the flow resistance is small, water with pressure equal to only a few centimeters of water column can flow through the device.
Ultraviolet light (UV) can be used to effectively treat and disinfect drinking water. Many people prefer UV light because they do not have to use chemicals such as chlorine that can leave behind disinfectant byproducts that taste bad and might be cancer-causing. If you own a UV light system or are considering purchasing a UV system for disinfection of drinking water, it is important to follow the proceeding guidelines to ensure proper functioning of the system:
• Drinking water should be treated and filtered before UV exposure to remove solids and chemicals that can block UV light and reduce its effectiveness. For proper disinfection, drinking water should not have suspended solids higher than 10 mg/L, iron higher 0.3 mg/L, turbidity higher than 5 NTU, and manganese higher than 0.05 mg/L. The water should have no color and have a pH between 6.5 and 9.5.
• UV lamps should be replaced every year, and controls should be installed that notify the user when a lamp is malfunctioning. If UV systems are not working properly, water will not be safe for consumption.
• Unlike chlorine, UV light leaves no residuals that can prolong disinfection after treatment. To prevent microbial contamination after UV exposure, UV treatment should be the last water treatment before water use.
_
Safety aspect of UV light:
In UVGI systems the lamps are shielded or are in environments that limit exposure, such as a closed water tank or closed air circulation system, often with interlocks that automatically shut off the UV lamps if the system is opened for access by human beings. In human beings, skin exposure to germicidal wavelengths of UV light can produce sunburn and skin cancer. Exposure of the eyes to this UV radiation can produce extremely painful inflammation of the cornea and temporary or permanent vision impairment, up to and including blindness in some cases. UV can damage the retina of the eye. Another potential danger is the UV production of ozone. Ozone can be harmful to health. The United States Environmental Protection Agency designated 0.05 parts per million (ppm) of ozone to be a safe level. Lamps designed to release UVC and higher frequencies are doped so that any UV light below 254 nm will not be released, thus ozone is not produced. A full spectrum lamp will release all UV wavelengths and will produce ozone as well as UVC, UVB, and UVA. The ozone is produced when UVC hits oxygen (O2) molecules, and so is only produced when oxygen is present.
_
Can ozone produced by UV light be effective disinfectant?
UV light does react with dissolved oxygen in water to produce ozone but it is insufficient for disinfection.
_
SteriPEN is the original handheld UV water purifier:
SteriPEN is the world’s leading manufacturer of ultraviolet, handheld water purification systems. SteriPEN is fast, lightweight, easy, and effective. SteriPEN comes in a variety of models for travel, outdoor recreation, family heath, and emergency preparedness situations, making safe drinking water available to anyone, anytime, anywhere, at a small fraction of the cost of bottled water.
____________
The figure below shows multiple barrier protection by combining chlorination with UV light:
_____________
Ozone disinfection (ozonation):
Ozone gas (the same type found in the atmosphere), typically created by subjecting oxygen to electrical current, is an antimicrobial agent — it kills microorganisms. Ozone is an unstable molecule which readily gives up one atom of oxygen providing a powerful oxidizing agent which is toxic to most waterborne organisms. It is a very strong, broad spectrum disinfectant that is widely used in Europe. It is an effective method to inactivate harmful protozoa that form cysts. It also works well against almost all other pathogens. Ozone is currently the next most widely used drinking water disinfectant after chlorine (there are some 1100 water treatment plants using ozone worldwide). Ozone is produced electrically by passing oxygen from ambient air between electrodes with a high voltage (tens of thousands of volts) applied across them. Care is needed in operating and maintaining the generators, and in destroying excess ozone so it is not released into ambient air. Ozone does not provide residual protection against recontamination in the distribution system. Therefore, its common use is to pre-treat the water source before chlorination in a municipal system, so that a smaller chlorine dose is required. Although ozonation can effectively disinfect water, it is not suited for most developing country applications owing to its high cost, need for operational and maintenance infrastructure, and lack of residual protection in the distribution system.
_
_
Some of the advantages of ozone include the production of fewer dangerous by-products and the absence of taste and odour problems (in comparison to chlorination). As an additional benefit free oxygen ions bond with other contaminants like iron and sulfur. When the oxygen bonds to these molecules, it turns them into oxides, which are insoluble. These now-insoluble contaminants are then filtered out. Although fewer by-products are formed by ozonation, it has been discovered that ozone reacts with bromide ions in water to produce concentrations of the suspected carcinogen bromate. Bromide can be found in fresh water supplies in sufficient concentrations to produce (after ozonation) more than 10 parts per billion (ppb) of bromate — the maximum contaminant level established by the USEPA. Another disadvantage of ozone is that it leaves no residual disinfectant in the water.
___________
Ion Exchange:
The ion exchange process percolates water through bead-like spherical resin materials (ion-exchange resins). Ions in the water are exchanged for other ions fixed to the beads. The two most common ion-exchange methods are softening and deionization. Softening is used primarily as a pretreatment method to reduce water hardness prior to reverse osmosis (RO) processing. The softeners contain beads that exchange two sodium ions for every calcium or magnesium ion removed from the “softened” water. Deionization (DI) beads exchange either hydrogen ions for cations or hydroxyl ions for anions. The cation exchange resins, made of styrene and divinylbenzene containing sulfonic acid groups, will exchange a hydrogen ion for any cations they encounter (e.g., Na+, Ca++, Al+++). Similarly, the anion exchange resins, made of styrene and containing quaternary ammonium groups, will exchange a hydroxyl ion for any anions (e.g., Cl-). The hydrogen ion from the cation exchanger unites with the hydroxyl ion of the anion exchanger to form pure water. These resins may be packaged in separate bed exchangers with separate units for the cation and anion exchange beds. Or, they may be packed in mixed bed exchangers containing a mixture of both types of resins. In either case, the resin must be “regenerated” once it has exchanged all its hydrogen and/or hydroxyl ions for charged contaminants in the water. This regeneration reverses the purification process, replacing the contaminants bound to the DI resins with hydrogen and hydroxyl ions. Deionization can be an important component of a total water purification system when used in combination with other methods discussed in this primer such as RO filtration and carbon adsorption. DI systems effectively remove ions, but they do not effectively remove most organics or microorganisms. Microorganisms can attach to the resins, providing a culture media for rapid bacterial growth and subsequent pyrogen generation.
____________
Reverse osmosis (RO):
The reverse osmosis process:
In the reverse osmosis process a cellophane-like membrane separates purified water from contaminated water. An understanding of osmosis is needed before further describing RO. Osmosis occurs when two solutions containing different quantities of dissolved chemicals are separated by a semi permeable membrane (allowing only some compounds to pass through). Osmosis is the passage or diffusion of water or other solvents through a semipermeable membrane that blocks the passage of dissolved solutes. Osmotic pressure of the dissolved chemical causes pure water to pass through the membrane from the dilute to the more concentrated solution as seen in the figure below. There is a natural tendency for chemicals to reach equal concentrations on both sides of the membrane.
In reverse osmosis, water pressure applied to the concentrated side forces the process of osmosis into reverse. Under enough pressure, pure water is “squeezed” through the membrane from the concentrated to the dilute side as seen in the figure below. Salts dissolved in water as charged ions are repelled by the RO membrane. Treated water is collected in a storage container. The rejected impurities on the concentrated side of the membrane are washed away in a stream of wastewater, not accumulated as on a traditional filter.
The RO membrane also functions as an ultrafiltration device, screening out particles, including microorganisms that are physically too large to pass through the membrane’s pores. RO membranes can remove compounds in the 0.0001 to 0.1 micron size range (thousands of times smaller than a human hair). Most home RO systems are point-of-use (POU) units placed beneath the kitchen sink to treat water used for cooking and drinking. Point-of-entry (POE) systems that treat all the water entering the household are more expensive to purchase and operate than POU systems. A typical home reverse osmosis system consists of pretreatment and post-treatment filters as well as the RO membrane, flow regulator, storage container for the treated water, and dispensing faucet as seen in the figure below.
The pressure for RO is usually supplied by the feed line pressure of the water system in the home, but a booster pump may be needed to produce an adequate volume of treated water. A sediment pre-filter is essential for removing relatively large sand grains and silt that may tear or clog the RO membrane or clog a pump or flow regulator. Water softeners are used in advance of the RO system when household water is excessively hard. If the water is chlorinated or contains other oxidizing chemicals such as bromine, an activated carbon pre-filter is needed to protect membranes sensitive to these chemicals.
_
RO membrane materials:
The most common RO membrane materials are polyamide thin film composites (TFC) or cellulosic types (cellulose acetate [CA], cellulose triacetate [CTA], or blends). Very thin membranes are made from these synthetic fibers. Membrane material can be spiralwound around a tube, or hollow fibers can be bundled together, providing a tremendous surface area for water treatment inside a compact cylindrical element. Hollow fiber membranes have greater surface area (and therefore greater capacity) but are more easily clogged than the spiral-wound membranes commonly used in home RO systems.
_
_
Reverse osmosis (RO) is the most economical method of removing 90% to 99% of all contaminants. The pore structure of RO membranes is much tighter than UF membranes. RO membranes are capable of rejecting practically all particles, bacteria and organics >300 daltons molecular weight (including pyrogens). In fact, reverse osmosis technology is used by most leading water bottling plants. RO also involves an ionic exclusion process. Only solvent is allowed to pass through the semi-permeable RO membrane, while virtually all ions and dissolved molecules are retained (including salts and sugars). The semi-permeable membrane rejects salts (ions) by a charge phenomena action: the greater the charge, the greater the rejection. Therefore, the membrane rejects nearly all (>99%) strongly ionized polyvalent ions but only 95% of the weakly ionized monovalent ions like sodium. Reverse osmosis is highly effective in removing several impurities from water such as total dissolved solids (TDS), turbidity, asbestos, lead and other toxic heavy metals, radium, and many dissolved organics. The process will also remove chlorinated pesticides and most heavier-weight VOCs. Reverse osmosis will not remove all contaminants from water. Dissolved gases such as oxygen and carbon dioxide pass through RO membranes into the treated water. Unfortunately, hydrogen sulfide gas, with its notorious odor of rotten eggs, also passes through the RO membrane. RO in general is not a very effective treatment for trihalomethanes (THMs), some pesticides, solvents, and other volatile organic chemicals (VOCs). Reverse osmosis and activated carbon filtration are complementary processes. Combining them results in the most effective treatment against the broadest range of water impurities and contaminants.
_
Contaminants removed by household reverse osmosis units.
| Inorganic Contaminants (Ions and Metals) |
Aluminum, Barium, Bicarbonate, Cadmium, Calcium, Chloride, Chromium, Copper, Fluoride, Lead, Magnesium, Manganese, Mercury, Nitrate, Potassium, Radium, Selenium, Silica, Silver, Sodium, Sulfate, Uranium, Zinc |
| Microbials | Protozoan cysts, Cryptosporidium |
| Pesticides | Endrin, Heptachlor, Lindane, Pentachlorophenol |
| Particles | Asbestos |
Note:
1. Many RO systems also include an activated carbon filter, which will allow for the reduction of additional contaminants that reverse osmosis units alone are not effective for.
2. Although the RO membrane is capable of rejecting virtually all microorganisms, it can develop pinholes or tears that allow bacteria or other microorganisms to pass into the treated water. RO units alone are not recommended for treatment of bacteria and other microscopic organisms. Some RO systems, however, may be used for removing waterborne protozoan cysts (such as Cryptosporidium and Giardia) found in surface drinking water supplies.
______
Forward osmosis:
_
_
Forward osmosis is an osmotic process that, like reverse osmosis, uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a “draw” solution of high concentration (relative to that of the feed solution), is used to induce a net flow of water through the membrane into the draw solution, thus effectively separating the feed water from its solutes. In contrast, the reverse osmosis process uses hydraulic pressure as the driving force for separation, which serves to counteract the osmotic pressure gradient that would otherwise favor water flux from the permeate to the feed. One example of an application of this type may be found in “hydration bags”, which use an ingestible draw solute and are intended for separation of water from dilute feeds. This allows, for example, the ingestion of water from surface waters (streams, ponds, puddles, etc.) that may be expected to contain pathogens or toxins that are readily rejected by the FO membrane. With sufficient contact time, such water will permeate the membrane bag into the draw solution, leaving the undesirable feed constituents behind. The diluted draw solution may then be ingested directly. Typically, the draw solutes are sugars such as glucose or fructose, which provide the additional benefit of nutrition to the user of the FO device. A point of additional interest with such bags is that they may be readily used to recycle urine, greatly extending the ability of a backpacker or soldier to survive in arid environments. This process may also, in principle, be employed with highly concentrated saline feedwater sources such as seawater, as one of the first intended uses of FO with ingestible solutes was for survival in life rafts at sea.
__________
Solar disinfection of water for drinking:
_
The use of solar irradiation for treatment of contaminated water was documented in 2000 BC. More recently, a number of approaches to the use of solar radiation have found that it removes a wide range of organic chemicals and pathogenic organisms by direct exposure, is relatively inexpensive, economically volume independent, and avoids generation of harmful by‐products of chemically driven technologies. Solar irradiation has been experimentally shown to be useful in decontaminating intravenous rehydration solutions and water. Solar water disinfection is a low-cost method of purifying water that can often be implemented with locally available materials. Unlike methods that rely on firewood, it has low impact on the environment. Investigation at the University of Beirut in Lebanon revealed that 99.9% of total bacteria in a water sample could be destroyed by 300 minutes exposure to direct sunlight.
_
Solar water disinfection is a type of portable water purification that uses solar energy, in one or more ways, to make contaminated water safe to drink by ridding it of infectious disease-causing biological agents such as bacteria, viruses, protozoa and worms. However, disinfection may not make all kinds of water safe to drink due to non-biological agents such as toxic chemicals or heavy metals. Consequently, additional steps beyond disinfection may be necessary to make water clean to drink.
There are three primary subsets of solar water disinfection:
1. Electric: Solar disinfection using the effects of electricity generated by photovoltaic panels (solar PV).
2. Heat: Solar thermal water disinfection.
3. UV: Solar ultraviolet water disinfection.
_
1. Solar disinfection using the effects of electricity generated by photovoltaics typically uses an electrical current to deliver electrolytic processes which disinfect water, for example by generating oxidative free radicals which kill pathogens by damaging their chemical structure. A second approach uses stored solar electricity from a battery, and operates at night or at low light levels to power an ultraviolet lamp to perform secondary solar ultraviolet water disinfection.
2. Solar thermal water disinfection uses heat from the sun to heat water to 70C-100C for a short period of time. A number of approaches exist here. Solar heat collectors can have lenses in front of them, or use reflectors. They may also use varying levels of insulation or glazing. In addition, some solar thermal water disinfection processes are batch-based, while others (through-flow solar thermal disinfection) operate almost continuously while the sun shines. Water heated to temperatures below 100C is generally referred to as Pasteurized water.
3. Solar ultraviolet water disinfection, also known as SODIS, is a method of disinfecting water using only sunlight and plastic PET bottles. SODIS is a free and effective method for decentralized water treatment, usually applied at the household level and is recommended by the World Health Organization as a viable method for household water treatment and safe storage.
_
Solar ultraviolet water disinfection:
Exposure to sunlight has been shown to deactivate diarrhea-causing organisms in polluted drinking water. Three effects of solar radiation are believed to contribute to the inactivation of pathogenic organisms:
1. UV-A interferes directly with the metabolism and destroys cell structures of bacteria.
2. UV-A (wavelength 320–400 nm) reacts with oxygen dissolved in the water and produces highly reactive forms of oxygen (oxygen free radicals and hydrogen peroxides) that are believed to also damage pathogens.
3. Cumulative solar energy (including the infrared radiation component) heats the water. If the water temperature rises above 50 °C (122 °F), the disinfection process is three times faster.
At a water temperature of about 30 °C (86 °F), a threshold solar irradiance of at least 500 W/m2 (all spectral light) is required for about 5 hours for SODIS to be efficient. This dose contains energy of 555 Wh/m2 in the range of UV-A and violet light, 350–450 nm, corresponding to about 6 hours of mid-latitude (European) midday summer sunshine. At water temperatures higher than 45 °C (113 °F), synergistic effects of UV radiation and temperature further enhance the disinfection efficiency.
_
_
Advantaged and disadvantages of solar disinfection of drinking water:
| Advantages | Disadvantages |
| – Very cheap, no capital costs except plastic bottle, no consumables required.
– Independent from energy sources other than sunlight. |
– Cannot be used on days with continuous rainfall.
– Cannot be used to treat very turbid water (>30 NTU). |
_
Solar disinfection of water for diarrhoeal prevention in southern India: A study:
A total of 100 children were assigned to receive drinking water that had been subjected to solar disinfection in polyethylene terephthalate bottles. One hundred age and sex matched controls were also selected. Both groups were followed by weekly home visits for a period of six months for any diarrhoeal morbidity. At the end of the follow up period, the acceptability of the intervention was assessed by interviews, questionnaires, and focus group discussions. There was significant reduction in the incidence, duration, and severity of diarrhoea in children receiving solar disinfected water, despite 86% of the children drinking water other than that treated by the intervention. The incidence of diarrhoea in the intervention group was 1.7 per child‐year, and among controls 2.7 per child‐year, with an incidence rate ratio of 0.64 (95% CI −0.48 to 0.86). The risk of diarrhoea was reduced by 40% by using solar disinfection. In qualitative evaluation of acceptability, most women felt that solar disinfection was a feasible and sustainable method of disinfecting water. Solar disinfection of water is an inexpensive, effective, and acceptable method of increasing water safety in a resource limited environment, and can significantly decrease diarrhoeal morbidity in children.
_______________
The table below shows various drinking water treatment methods in a developing country:
_
The table below shows overview of drinking water contaminants and treatment methods:
_
_____________
Innovations of water treatment:
AQUAtap Community Drinking Water Stations:
Quest Water Solutions’ AQUAtap Drinking Water Station is a simple system that uses solar power to purify contaminated groundwater, brackish water, or sea water into safe drinking water. The systems are powered by photovoltaic panels. Each Drinking Water Station is fully autonomous and can purify water at a rate of up to 20,000 liters per day without any existing infrastructure. They are also modular, so can be scaled for increased water purification. In addition, the system includes a distribution system. In 2012, Quest Water Solutions started construction of an AQUAtap Drinking Water System in Bom Jesus, an Angolan village 50 kilometers east of Luanda, the capital of Angola. The 500 residents of Bom Jesus currently rely on a dirty river for drinking water. The clean drinking water produced by the AQUAtap will be available to villagers at no cost to the villagers.
_
HydroPack:
The HydroPack, developed by Hydration Technology Innovations (HTI), is a one-time use, self hydrating, emergency hydration pouch. Victims of natural disasters often struggle to find clean drinking water. Water sources and drinking water supplies are often contaminated during a disaster; victims often suffer from water borne illnesses. The HydroPack is a 4-inch by 6-inch pouch filled with electrolytes and nutrients. When in contact with water, the HydroPack swells to create a healthy drink in 10 to 12 hours. “It doesn’t matter what the quality of water is like,” says Keith Lampi, vice president and chief operating officer for HTI. “There just needs to be a source of water, even dirty or brackish water, and we can supply clean drinks at the initial stages of a disaster using the HydroPacks.” The HydroPack is a 12 fluid ounce (355 ml) pouch with two compartments that are separated by a membrane. One side of the pouch includes sports drink syrup. The user places the pack in a water source for 10 to 12 hours. During that time untreated water diffuses across the membrane and dilutes the sports drink syrup. The HydroPack uses Forward Osmosis, a natural equilibrium process that rejects even the harshest of contaminants. The technology does not clog and can be used in very turbid water. The pouch includes a straw and the resulting nutrient drink is very palatable. According to HTI, “HTI’s products are not meant to displace other bulk water strategies such as ROWPUs, municipal water systems, or shipboard desalination and bottling. Instead, they should play a very critical role in the early phase of disaster relief until other production and distribution strategies can be put in place.” This technology also reduces the weight of aide materials needed to be transported after a disaster. One pallet of 94,500 HydroPacks weighs 8,325 pounds (3,785 kg) and will produce 12,482 gallons (47,250 liters) of clean drink. This equates to about a 92% reduction in weight compared to bottled water. HydroPack were distributed to earthquake survivors in the tent city of Carrefour in Haiti in 2010.
_
Wello:
Wello, a social venture, developed the WaterWheel to improve global access to water. The WaterWheel is a non-mechanical wheel enables one person to transport 25 gallons (90 liters) of water, enough water for one family, in a single trip. A WaterWheel user can put up to 200 pounds (90 kg) of water in the “wheel”, a broad drum with round edges and a steel handle, transforming the water to an effective weight of approximately 22 pounds (10 kg). The design allows for easy maneuvering over different terrains, inclines, and corners. By reducing the number of daily trips needed to collect water, the WaterWheel allows women and children in developing countries to devote more time and energy to educational and economic activities. In addition, the WaterWheel is a sustainable solution for water transportation. It does not require any energy or other supplies in order to operate. It also does not require repairs over its 10-15 year life.
_
Organisms used in water purification:
At present, several species of water-purifying plants, bacteria, and fish and other things are used in water purification, improving efficiency and/or ecosystem support. Purification implies removal of impurities from the water. Different organisms have different removal mechanisms, and some organisms may be ineffective at removing impurities of interest. Saprophytic bacteria convert dissolved organic impurities into living cell mass, carbon dioxide and water. These saprophytic bacteria may then be eaten by flagellates and ciliates which also consume suspended organic particles including viruses and pathogenic bacteria. Clarity of the water may begin to improve as the protozoa are subsequently consumed by rotifers and cladocera. Purifying bacteria, protozoa, and rotifers must either be mixed throughout the water or have the water circulated past them to be effective. Sewage treatment plants mix these organisms as activated sludge or circulate water past organisms living on trickling filters or rotating biological contactors. Aquatic vegetation may provide similar surface habitat for purifying bacteria, protozoa, and rotifers in a pond or marsh setting; although water circulation is often less effective. Plants and algae have the additional advantage of removing nutrients from the water; but those nutrients will be returned to the water when the plants die unless the plants are removed from the water. Plants also provide shade, a refuge for fish, and oxygen for aerobic bacteria. In addition, fish can limit pests such as mosquitoes. Fish and waterfowl feces return waste to the water, and their feeding habits may increase turbidity. Cyanobacteria have the disadvantageous ability to add nutrients from the air to the water being purified. The choice of organism depends on the local climate different species and other factors. Indigenous species usually tend to be better adapted to the local environment.
_
Nanotechnology and drinking water:
Nano-based products relevant to developing countries seeking to improve water supplies:
| Product | How it works | Importance | Developer |
| Nanosponge for rainwater harvesting | A combination of polymers and glass nanoparticles that can be printed onto surfaces like fabrics to soak up water | Rainwater harvesting is increasingly important to countries like China, Nepal and Thailand. The nanosponge is much more efficient than traditional mist-catching nets | Massachusetts Institute of Technology, United States |
| Nanorust to remove arsenic | Magnetic nanoparticles of iron oxide suspended in water bind arsenic, which is then removed with a magnet | India, Bangladesh and other developing countries suffer thousands of cases of arsenic poisoning each year, linked to poisoned wells | Rice University, United States |
| Desalination membrane | A combination of polymers and nanoparticles that draws in water ions and repels dissolved salts | Already on the market, this membrane enables desalination with lower energy costs than reverse osmosis | University of California, Los Angeles and NanoH2O |
| Nanofiltration membrane | Membrane made up of polymers with a pore size ranging from 0.1 to 10nm | Field tested to treat drinking water in China and desalinate water in Iran, using this membrane requires less energy than reverse osmosis | Saehan Industries, Korea |
| Nanomesh waterstick | A straw-like filtration device that uses carbon nanotubes placed on a flexible, porous, material | The waterstick cleans as you drink. Doctors in Africa are using a prototype and the final product will be made available at an affordable cost in developing countries | Seldon Laboratories, United States |
| World filter | Filter using a nanofibre layer, made up of polymers, resins, ceramic and other materials, that removes contaminants | Designed specifically for household or community-level use in developing countries. The filters are effective, easy to use and require no maintenance | KX Industries, United States |
| Pesticide filter | Filter using nanosilver to adsorb and then degrade three pesticides commonly found in Indian water supplies | Pesticides are often found in developing country water supplies. This pesticide filter could provide a typical Indian household with 6000 litres of clean water over one year | Indian Institute of Technology in Chennai, India, and Eureka Forbes Limited, India |
_
Cheap Nanotech Filter clears Hazardous Microbes and Chemicals from Drinking Water :
Thalappil Pradeep and his colleagues at the Indian Institute of Technology Madras developed a $16 nanoparticle water filtration system that promises potable water for even the poorest communities in India and, in the future, for those in other countries sharing the same plight. Although cheap filtration systems have been developed previously, this is the first one to combine microbe-killing capacity with the ability to remove chemical contaminants such as lead and arsenic. Because the filters for microbes and chemicals are separate components, the system can be customized to rid water of microbial contaminants, chemical contaminants or both, depending on the user’s needs. In a report published in Proceedings of the National Academy of Sciences, Pradeep and his collaborators explain that the microbe filter relies on silver nanoparticles embedded in a cage made of aluminum and chitosan, a carbohydrate derived from the chitin in crustacean shells. The cage blocks macroscale water contaminants as well as protects the nanoparticles from sediments that would otherwise accumulate on their surfaces, thereby preventing them from releasing microbe-zapping ions. The team used nanoparticles that release iron- and arsenic-trapping ions to make its chemical filter. But Pradeep notes that the “cage” technique can be used with other nanoparticles to target contaminants such as mercury. The membrane filter at the top kills bacteria and viruses, and the axial block at the bottom can be custom fitted with a second filter for lead or arsenic. The materials are added one by one into water and self-assemble into small sheets that resemble clay. These sheets are the “cages” that then hold on to the silver nanoparticles. Production requires no electricity because the claylike filters are made at room temperature. Every liter of water used to make the material goes to filtering 500 liters of water. “This is a room-temperature green synthesis, which means it can be deployed in any part of the world,” Pradeep says. Chitosan fibers combine with aluminum hydroxide nanoparticles (AlOOH Np) to form a claylike “cage” that can protect embedded silver nanoparticles from deposits that would reduce their microbe-killing power.
_
Cheap, clean drinking water purified through Nanotechnology:
Tiny particles of pure silica coated with an active material could be used to remove toxic chemicals, bacteria, viruses, and other hazardous materials from water much more effectively and at lower cost than conventional water purification methods, according to researchers writing in the International Journal of Nanotechnology. Peter Majewski and Chiu Ping Chan of the Ian Wark Research Institute, at the University of South Australia, explain that the availability of drinking quality water is fast becoming a major socio-economic issue across the globe, especially in the developing world. However, water purification technology is often complicated, requires sophisticated equipment and is expensive to run and maintain. Moreover, it usually requires a final costly disinfection stage. The Australian team suggests that nanotechnology could provide a simple answer to the problem. The researchers have investigated how silica particles can be coated easily with a nanometer-thin layer of active material based on a hydrocarbon with a silicon-containing anchor. The coating is formed through a chemical self-assembly process so involves nothing more than stirring the ingredients to make the active particles. These active particles, so called Surface Engineered Silica (SES), were then tested to demonstrate that they could remove biological molecules, pathogens such as viruses like the Polio virus, bacteria like Escherichia coli, and Cryptosporidium parvum, which is a waterborne parasite. “The results clearly show that organic species can efficiently be removed at pH ranges of drinking water by stirring the coated particles in the contaminated water for up to one hour and filtering the powder,” the researchers say. They point out that the filtration process occurs through an electrostatic attraction between the pathogens and the surface engineered particles.
_
Tea bag device:
New water filter systems use nanotechnology to make water safe for drinking. One new development is similar to a tea bag; one bag makes one liter of clean water. The inventor of the “tea bag” device hopes it will bring safe water to millions. Lack of access to clean water is still a problem for millions of people across the world, but new developments in nanotechnology and a water filter that resembles a humble tea bag could prove to be effective solutions. The “teabag” filter is the brain-child of Professor Cloete of Stellenbosch University. It is designed to fit in the neck of a standard sized water bottle, meaning it is interchangeable, and, depending on the quality of water being filtered, costs between one and five cents per liter. “The easiest way to visualize our filter is to think of a normal tea bag,” said Cloete. “The outside of the bag is coated in a polymer that includes a biocide, which means that it both filters water and kills bacteria — we haven’t yet come across a bacteria it can’t kill.
_______________
Fluoride and fluoridation of water:
_
Dental caries:
_
Fluorine is an extremely reactive poisonous gaseous halogen, it is pale yellow-green and it is the most chemically reactive and electronegative of all the elements. In aqueous solution, fluorine commonly occurs as the fluoride ion F-. Fluorides are compounds that combine fluorine ion F- with some positively charged counterpart. Small amounts of fluorides are naturally present in water, air, plants and animals. As a result humans are exposed to fluorides through food and drinking water and by breathing air. Large quantities of fluorides can be found in tea and shellfish. Fluorides can also protect us from dental decay, if it is applied through toothpaste twice a day. If fluoride is absorbed too frequently, it can cause teeth decay, osteoporosis and harm to kidneys, bones, nerves and muscles.
_
Added Fluoride in drinking water:
Since 1945, fluoride has been added to many public drinking-water supplies as a public-health practice to control dental caries. The “optimal” concentration of fluoride in drinking water for the United States for the prevention of dental caries has been set at 0.7 to 1.2 mg/L, depending on the mean temperature of the locality (0.7 mg/L for areas with warm climates, where water consumption is expected to be high, and 1.2 mg/L for cool climates, where water consumption is low) (PHS 1991). The optimal range was determined by selecting concentrations that would maximize caries prevention and limit enamel fluorosis, a dose-related mottling of teeth that can range from mild discoloration of the surface to severe staining and pitting. Decisions about fluoridating a public drinking-water supply are made by state or local authorities. CDC (2002a) estimates that approximately 162 million people (65.8% of the population served by public water systems) received optimally fluoridated water in 2000.
_
_
How fluoride prevents and controls dental caries:
Dental caries is an infectious, transmissible disease in which bacterial by-products (i.e., acids) dissolve the hard surfaces of teeth. Unchecked, the bacteria can penetrate the dissolved surface, attack the underlying dentin, and reach the soft pulp tissue. Dental caries can result in loss of tooth structure, pain, and tooth loss and can progress to acute systemic infection. Cariogenic bacteria (i.e., bacteria that cause dental caries) reside in dental plaque, a sticky organic matrix of bacteria, food debris, dead mucosal cells, and salivary components that adheres to tooth enamel. Plaque also contains minerals, primarily calcium and phosphorus, as well as proteins, polysaccharides, carbohydrates, and lipids. Cariogenic bacteria colonize on tooth surfaces and produce polysaccharides that enhance adherence of the plaque to enamel. Left undisturbed, plaque will grow and harbor increasing numbers of cariogenic bacteria. An initial step in the formation of a carious lesion takes place when cariogenic bacteria in dental plaque metabolize a substrate from the diet (e.g., sugars and other fermentable carbohydrates) and the acid produced as a metabolic by-product demineralizes (i.e., begins to dissolve) the adjacent enamel crystal surface . Demineralization involves the loss of calcium, phosphate, and carbonate. These minerals can be captured by surrounding plaque and be available for reuptake by the enamel surface. Fluoride, when present in the mouth, is also retained and concentrated in plaque. Fluoride works to control early dental caries in several ways. Fluoride concentrated in plaque and saliva inhibits the demineralization of sound enamel and enhances the remineralization (i.e., recovery) of demineralized enamel. As cariogenic bacteria metabolize carbohydrates and produce acid, fluoride is released from dental plaque in response to lowered pH at the tooth-plaque interface. The released fluoride and the fluoride present in saliva are then taken up, along with calcium and phosphate, by de-mineralized enamel to establish an improved enamel crystal structure. This improved structure is more acid resistant and contains more fluoride and less carbonate. Fluoride is more readily taken up by demineralized enamel than by sound enamel. Cycles of demineralization and remineralization continue throughout the lifetime of the tooth. Fluoride also inhibits dental caries by affecting the activity of cariogenic bacteria. As fluoride concentrates in dental plaque, it inhibits the process by which cariogenic bacteria metabolize carbohydrates to produce acid and affects bacterial production of adhesive polysaccharides. In laboratory studies, when a low concentration of fluoride is constantly present, one type of cariogenic bacteria, Streptococcus mutans, produces less acid. Whether this reduced acid production reduces the cariogenicity of these bacteria in humans is unclear. Saliva is a major carrier of topical fluoride. The concentration of fluoride in ductal saliva, as it is secreted from salivary glands, is low — approximately 0.016 parts per million (ppm) in areas where drinking water is fluoridated and 0.006 ppm in nonfluoridated areas. This concentration of fluoride is not likely to affect cariogenic activity. However, drinking fluoridated water, brushing with fluoride toothpaste, or using other fluoride dental products can raise the concentration of fluoride in saliva present in the mouth 100- to 1,000-fold. The concentration returns to previous levels within 1–2 hours but, during this time, saliva serves as an important source of fluoride for concentration in plaque and for tooth remineralization. Applying fluoride gel or other products containing a high concentration of fluoride to the teeth leaves a temporary layer of calcium fluoride-like material on the enamel surface. The fluoride in this material is released when the pH drops in the mouth in response to acid production and is available to remineralize enamel. In the earliest days of fluoride research, investigators hypothesized that fluoride affects enamel and inhibits dental caries only when incorporated into developing dental enamel (i.e., preeruptively, before the tooth erupts into the mouth). Evidence supports this hypothesis, but distinguishing a true preeruptive effect after teeth erupt into a mouth where topical fluoride exposure occurs regularly is difficult. However, a high fluoride concentration in sound enamel cannot alone explain the marked reduction in dental caries that fluoride produces. The prevalence of dental caries in a population is not inversely related to the concentration of fluoride in enamel, and a higher concentration of enamel fluoride is not necessarily more efficacious in preventing dental caries.
_
_
Harmful effects of fluorides in drinking water:
The fluoride added to 90% of drinking water is hydrofluoric acid which is a compound of fluorine that is a chemical byproduct of aluminum, steel, cement, phosphate, and nuclear weapons manufacturing. It is one of the most caustic of industrial chemicals. Fluoride is the active toxin in rat poisons and cockroach powder. Hydrofluoric acid is used to refine high octane gasoline, to make fluorocarbons and chlorofluorocarbons for freezers and air conditioners, and to manufacture computer screens, fluorescent light bulbs, semiconductors, plastics, herbicides, — and toothpaste. It also has the ability to burn flesh to the bone, destroy eyes, and sear lungs so that victims drown in their own body fluid. Once in the body, fluoride is a destroyer of human enzymes. Fluoride is a highly toxic substance. Consider, for example, the poison warning that the FDA now requires on all fluoride toothpastes sold in the U.S. or the tens of millions of people throughout China and India who now suffer serious crippling bone diseases from drinking water with elevated levels of fluoride. In terms of acute toxicity (i.e., the dose that can cause immediate toxic consequences), fluoride is more toxic than lead, but slightly less toxic than arsenic. This is why fluoride has long been used in rodenticides and pesticides to kill pests like rats and insects. It is also why accidents involving over-ingestion of fluoridated dental products–including fluoride gels, fluoride supplements, and fluoridated water–can cause serious poisoning incidents, including death. The debate today, however, is not about fluoride’s acute toxicity, but its chronic toxicity (i.e., the dose of fluoride that if regularly consumed through drinking fluoridated water over an extended period of time can cause adverse effects).
_
Dental fluorosis:
Fluorosis is a defect of tooth enamel caused by too much fluoride intake during the first 8 years of life. Although fluorosis can be cosmetically treated, the damage to the enamel is permanent. Common causes of fluorosis include: fluoridated drinking water (particularly during infancy), ingestion of fluoride toothpaste, use of fluoride tablets, and consumption of processed foods made with fluoridated water. For many children, fluoride toothpaste is the largest source of fluoride intake. One strip of fluoridated toothpaste on a child-sized toothbrush contains between 0.75 and 1.5 mg of fluoride, which is more fluoride than is found in many prescription fluoride supplements (0.25 to 1.0 mg per tablet). Since young children are known to swallow a large amount of the toothpaste they place in their mouth, use of fluoride toothpaste–particularly when done without the supervision of a parent–can result in dangerous levels of fluoride exposure. Ingestion of excessive fluoride toothpaste is a major risk factor for dental fluorosis, and can cause symptoms of acute fluoride toxicity (e.g., stomach pain, nausea, etc).
_
Skeletal Fluorosis:
Skeletal fluorosis is a bone and joint condition associated with prolonged exposure to high concentrations of fluoride. Fluoride increases bone density and appears to exacerbate the growth of osteophytes present in the bone and joints, resulting in joint stiffness and pain. The condition is categorized into one of four stages: a preclinical stage and three clinical stages that increase in severity. The most severe stage (clinical stage III) historically has been referred to as the “crippling” stage. At stage II, mobility is not significantly affected, but it is characterized by chronic joint pain, arthritic symptoms, slight calcification of ligaments, and osteosclerosis of the cancellous bones. Whether EPA’s MCLG of 4 mg/L protects against these precursors to more serious mobility problems is unclear.
_
Bone Fractures:
Several epidemiologic studies of fluoride and bone fractures have been published since the 1993 NRC review. The committee focused its review on observational studies of populations exposed to drinking water containing fluoride at 2 to 4 mg/L or greater and on clinical trials of fluoride (20-34 mg/ day) as a treatment for osteoporosis. Several strong observational studies indicated an increased risk of bone fracture in populations exposed to fluoride at 4 mg/L, and the results of other studies were qualitatively consistent with that finding. The one study using serum fluoride concentrations found no appreciable relationship to fractures. Because serum fluoride concentrations may not be a good measure of bone fluoride concentrations or long-term exposure, the ability to show an association might have been diminished in that study.
_
Other anti-fluoride reports:
1. Taylor Study, University of Austin: fluoride concentration of 1PPM (parts per million) increases tumor growth rate by 25%
2. Fluoride is more poisonous than lead, and just less poisonous than arsenic – Clinical Toxicology of Commercial Products — 1984
3.”A seven ounce tube of toothpaste, theoretically at least, contains enough fluoride to kill a small child.” – Procter & Gamble, quoted in Fluoride the Aging Factor p14
_
Fluoride havoc in India:
Of the 200-odd villagers in the Indian town of Gaudiyan, around 135 have bone deformities. A private doctor who conducts social work in the area termed it as a case of skeletal fluorosis — the result of excess fluoride content in drinking water. In another part of India, also partly as a result of fluoride poisoning, children are losing their vision. They have been diagnosed with Lamellar Congenital cataract — a condition in which the eye lenses are damaged. Other examples of such harm include the village of Sogival where the groundwater contains 4.84 ppm of fluoride and two-thirds of the people suffer from skeletal deformities. And in Bihar, the prevalence of physical deformity is yet another testament to excessive fluoride exposure. India is one of several countries known to have dangerously high levels of fluoride in their drinking water. This poison comes into contact with water supplies when rocks containing fluoride erode or volcanic activity spews fluoride-containing ash into the air, allowing the colorless, odorless substance to enter groundwater (of course in some areas, like the United States, fluoride is intentionally added to water supplies). In areas where naturally occurring fluoride is high, serious health problems usually become apparent, and that is, unfortunately, what’s happening now in India. But these events also have potential relevance to the US, as this fluorosis in India explains. As of 1999, 17 of India’s 32 states and territories were known to have high concentrations of fluoride in water, according to the World Health Organization (WHO), with concentrations as high as 48 mg/liter reported. For comparison, WHO has capped the upper limit of fluoride in drinking water at 1.5 mg/liter.
_
Fluoride is a Cumulative Poison:
In order to understand the long-term dangers of fluoride, it’s important to realize that fluoride is a cumulative poison.
Ninety-eight percent of the fluoride you ingest in water is absorbed into your blood through your gastrointestinal tract. From there, it enters your body’s cellular tissues. On average, about 50 percent of the fluoride you ingest each day gets excreted through your kidneys. The remainder accumulates in your teeth and bones, pineal gland, and other tissues, such as the aorta. The amount deposited into your bones and teeth varies depending on your age. In children, more than 50 percent of an ingested dose of fluoride is deposited in bone, but in adults only about 10 percent is stored there. As with teeth, fluoride is deposited in bone by the ionic exchange with hydroxyl-apatite. It does dissolve from bone over time, but at a slower rate than it is deposited, so if your intake remains constant or high, the level of fluoride in your bones increases linearly with age. Further, if your kidneys are damaged, fluoride accumulation will increase, and with it, the likelihood of harm. Basically, if you ingest more fluoride than your body is capable of eliminating, various stages of fluorosis may ensue.
_
Fluoride and tooth decay facts:
Fact 1: Fluoride’s primary effect is Topical, Not Systemic:
Water fluoridation began in the 1940s under the premise that swallowing fluoride is the most effective way to strengthen teeth. It is now known, however, that fluoride’s main benefit comes from topical contact with teeth, not from ingestion. Even if fluoridated water has a benefit, therefore, there is no need to swallow it. The hypothesis was that increased intake of fluoride during tooth formation raises the fluoride concentration in enamel and hence increases acid resistance. As a consequence fluoride had to be taken systemically and artificial fluoridation of drinking waters became the ‘optimal’ solution.” (Fejerskov 2004). Although water fluoridation was launched on this premise, it is now known to be incorrect. Far from making the teeth stronger for life, the July 2000 issue of the Journal of the American Dental Association reported that ”fluoride incorporated during tooth development is insufficient to play a significant role in caries protection.” (Featherstone 2000). “Importantly, this means that fluoride incorporated during tooth mineral development at normal levels of 20 to 100 ppm (even in areas that have fluoridated drinking water or with the use of fluoride supplements) does not measurably alter the acid solubility of the mineral. Even when the outer enamel has higher fluoride levels, such as 1,000 ppm, it does not measurably withstand acid-induced dissolution any better than enamel with lower levels of fluoride.” (Featherstone 2000). Based on these and other findings, researchers have now overwhelmingly rejected the notion that swallowing fluoride is either necessary or effective for preventing decay. Instead, the current consensus is that fluoride’s benefit (whatever it may be) comes from topical contact with teeth after the teeth have erupted into the mouth. As the Centers for Disease Control (CDC) stated in 1999: “fluoride prevents dental caries predominately after eruption of the tooth into the mouth, and its actions primarily are topical for both adults and children.” The CDC repeated this position in 2001, affirming that “fluoride’s predominant effect is posteruptive and topical.” The implications are obvious. If fluoride works topically, there is no need to swallow it, and therefore no need to add it to the water supply. This is especially so when considering that (1) fluoride is not a nutrient, and (2) fluoride’s risks come from ingestion.
_
Fact 2: There is No Difference in Tooth Decay between Fluoridated and Non-Fluoridated Countries.
_
_
In the United States, the Centers for Disease Control has called water fluoridation one of the “top 10 public health achievements of the twentieth century.” Yet, according to comprehensive data compiled by the World Health Organization, there is no discernible difference between the few western countries that fluoridate their water, and the majority that do not. WHO’s data also shows that, over the past 40 years, non-fluoridated countries have experienced the same (and often greater) declines in tooth decay as fluoridated countries. Tooth decay rates throughout the western world have declined at a steep rate over the past 50 years, irrespective of whether a country fluoridates its water or not. This fact has invited new scrutiny into the necessity and effectiveness of water fluoridation, particularly in light of the discovery that — in contrast to previous belief — fluoride’s primary benefit to teeth comes from topical, not systemic, application.
_
Fact 3: Total daily fluoride intake is not significantly related to tooth decay.
A multi-million dollar, NIH-funded study found that total fluoride intake from birth through nine years of age has no significant effect on whether the child will develop a cavity. This is the first time tooth decay has been investigated as a function of individual exposure (as opposed to mere residence in a fluoridated community). More recently, the Iowa team has reported that the fluoride level in a child’s water from birth through 13 years of age does not significantly predict the presence or absence of tooth decay.
_
Fact 4: No link found between (Low-Fluoride) Bottled Water and Tooth Decay:
Over the past decade, there has been a steady drumbeat of press warning of the risks from drinking bottled water. The idea — kept alive by press releases from dental associations — is that because most bottled waters have low levels of fluoride, people switching from tap water (which is usually fluoridated) to bottled water will not receive a sufficient intake of fluoride to ward off tooth decay. One of the things that has been absent in the discussion about bottled water’s “threat” to teeth, is the lack of empirical evidence to justify the claim. It was a significant development this year, therefore, when researchers from the NIH-funded “Iowa Fluoride Study” published a study which provides some actual hard data on the issue. For the past decade, these researchers have been carefully monitoring the fluoride intake of hundreds of Iowan children, from birth through adolescence. From this group of children, the researchers separated out those children who regularly used bottled water. They then compared the tooth decay history of these bottled water drinkers with children who regularly drank tap water (most of which was fluoridated). Even when controlling for important variables, such as socioeconomic status and toothbrushing frequency, the authors found no relationship between bottled water use and tooth decay (in baby or permanent teeth), even though the fluoride intake of the bottled water users was significantly lower than the tap-water users. Similar findings were reported in the Australian study. Due to the limited number of bottled-water users identified in this group of Iowan children, however, the authors recommend that larger studies be conducted in order to reach more definitive conclusions. The prior Australian study, however, had a very large scale (13,000) and yet it still found no relationship between consumption of “non-public” water and tooth decay in the permanent teeth.
_
Then why decline in tooth decay if fluoridation of water is not the cause of it:
There has been a tremendous increase in the consumption of fresh fruit and vegetables since the 1930s, assisted by the introduction of household refrigerators in western world. There has also been an eightfold increase in the consumption per head of cheese, which we now know has anti-decay properties. These nutritional changes, accompanied by a continuing decline in tooth decay, started before the introduction of fluorides. The influence of general nutrition in protection against tooth decay has been well described in the past, but is largely ignored by the fluoride enthusiasts, who insist that fluorides have been the main contributor to improved dental health. The increase in tooth decay in third-world countries, much of which has been attributed to worsening nutrition, lends support to the argument that improved nutrition in developed countries contributed to improved dental health. Another factor may be the widespread use of antibiotics that have the side effect of suppressing mouth bacteria. Of course, fluoride toothpaste may have helped prevent tooth decay due to topical local effects of fluoride.
_
Remove fluoride from drinking water:
Around the world, it is estimated that tens of millions of people are affected by both dental and skeletal fluorosis. In many cases, it is the addition of fluoride into drinking water supplies by governments that is the primary cause of both dental and skeletal fluorosis. Common techniques used for defluoridation are coagulation-precipitation, membrane process and ion exchange. The problem with these three techniques is that they are either too expensive or they further pollute the water. Researchers from the National University of Sciences and Technology in Pakistan have discovered an effective method to remove fluoride from drinking water that is less expensive than conventional filtration processes and is safe to use. The study, published in the Journal of Chemistry, concluded that the removal of fluoride from drinking water using modified immobilized activated alumina (MIAA) resulted in a removal efficiency that was 1.35 times higher than normal immobilized activated alumina.
____________
Bottled water:
_
_
Bottled water is simply water from some source that a company (or in the case of water vending machines, the consumer) has placed in a bottle for resale. Bottled water is drinking water (e.g., well water, distilled water, mineral water, or spring water) packaged in plastic or glass water bottles. Sizes range from small single serving bottles to large carboys for water coolers. Bottled water includes natural mineral water and water drawn from springs and wells, but could also include purified water, which is often treated municipal water. Much of the success of bottled water can be attributed to perception: “A lot of it is the public perception that bottled water is better than tap water.” The multimillion-dollar marketing campaigns that depict bucolic mountain springs, punctuated with adjectives like “pure” and “fresh” can be found on nearly every bottle of water. But there’s no marketing message that can come from a faucet.
_
The global bottled water sales have increased dramatically over the past several decades, reaching a valuation of around $60 billion and a volume of more than 115,000,000 cubic meters in 2006. U.S. sales reached around 30 billion bottles of water in 2008, a slight drop from 2007 levels. The world bottled water market amounts to an annual volume of 109 billion liters, an average 17.5 liters of bottled water drunk yearly per person. Western Europeans are the major consumers, with an average of 93 liters/person/year. Asians presently consume the least. Thus there exists a vast potential market for bottled water in Asia.
_______
Why people drink bottled water? Few studies are narrated:
The effect of Tap Water Perception on the consumption of Bottled Water: A study:
Over the past 30 years, drinking water has evolved from existing as a household faucet essential to being pumped, to bottled and sold as a convenience store commodity. Consumers choose to drink bottled water for a variety of reasons – health, convenience, taste and safety to name a few. Although its growth and popularity represent success for the bottled water industry, the life cycle of bottled water forces a serious impact upon the environment. Despite these issues and despite that tap water is an equal substitute in developed nations, consumers continue to increasingly purchase bottled water. The purpose of this study is to investigate the consumer incentive behind purchasing bottled water, namely how it is affected by a negative perception of tap water taste and safety. A survey was designed for and administered to Safeway customers in Contra Costa Country. Using Spearman’s Rank Correlation test, results indicated that there was no relationship between perception of tap water taste and consumption of bottled water. There was a moderate relationship between perception of tap water safety and consumption of bottled water and a strong relationship between the amount of bottled water and tap water one consumes.
_
Bottled water versus tap water: understanding consumers’ preferences:
The consumption of bottled water has been increasing consistently over the last decade, even in countries where tap water quality is considered excellent. This paper discusses some of the reasons why people decide for an option that is often more expensive and less comfortable than tap water. Consumer surveys usually stress two main factors: dissatisfaction with tap water organoleptics (especially taste) and health/risk concerns. However, many other factors are involved, including demographic variables and the perceived quality of the water source. Trust in tap water companies also seems to influence public behaviour. A clearer picture of bottled water consumption can be achieved when different aspects are considered as seen in the figure below.
_
Exploring Beliefs about Bottled Water and intentions to reduce Consumption: The Dual-Effect of Social Norm Activation and Persuasive Information:
Mass consumption of bottled water is contributing to a multitude of environmental problems, including water wastage, pollution, and climate change. The aim of this study is to advance a social-psychological understanding of how to effectively reduce bottled water consumption. An online survey experiment was conducted among students of a Dutch public university to explore outcome beliefs about drinking less bottled water while testing three strategies for behavioral change. Respondents (N = 454) were randomly allocated to four different conditions (an information-only, social norm-only, a combination of both, or a control group). It was hypothesized that the combination (i.e., norm-induced information provision) would be most persuasive and elicit the greatest reduction in intentions to buy bottled water. Results were consistent with this hypothesis. Findings also show that while beliefs about health, taste, water quality, lifestyle, the environment, and perceived alternatives are all correlated with bottled water consumption, belief strength varies significantly based on rate of consumption.
________
The FDA has good manufacturing practices specifically for bottled water. They require bottled water producers to:
•Process, bottle, hold and transport bottled water under sanitary conditions
•Protect water sources from bacteria, chemicals and other contaminants
•Use quality control processes to ensure the bacteriological and chemical safety of the water
•Sample and test both source water and the final product for contaminants
_
_
Types of bottled water:
| Artesian | Water obtained from a well that taps a confined aquifer, an underground layer of rock or sand that contains water. |
| Distilled | Water that has been boiled and then recondensed from the steam that the boiling produces. Distillation kills microbes and removes minerals, giving water a flat taste. |
| Mineral | Groundwater that naturally contains at least 250 parts per million of dissolved solids. All minerals and other trace elements must be present in the water when it emerges at the source. |
| P.W.S. | Public water source, also known as municipal water supply, or tap water |
| Purified | Water from any source that has been treated to remove chemicals and pathogens according to standards set by the U.S. Pharmacopoeia or national standard of water safety. Must contain no more than 10 parts per million of dissolved solids. Distillation, deionization, and reverse osmosis are all purification methods. |
| Sparkling | Water that contains carbon dioxide at an amount equal to what it contained when it emerged from its source. Carbon dioxide lost during the treatment process may be added back. (Carbonated waters such as soda water and seltzer are considered soft drinks, not bottled waters.) |
| Spring | Water derived from an underground formation from which water flows naturally to the Earth’s surface. Spring water must be collected at the spring or through a borehole tapping the underground formation (aquifer) feeding the spring. |
__
Bottled water not necessarily safe:
Bottled water is not necessarily healthier or safer than tap water, Tampa, Florida-based sports nutritionist Cynthia Sass told the American College of Sports Medicine 11th annual Health & Fitness Summit in Dallas. Twenty-five percent of all bottled water is actually repackaged tap water, according to Sass. “Bottled water doesn’t deserve the nutritional halo that most people give it for being pure,” she says. “If you’re not an exclusive bottled water drinker, you may find it worthwhile to check into filtering your tap water to save money.” In a recent Gallop survey, most consumers said they drink bottled water because they perceive it to be purer than tap water. Taste and convenience are also factors. Because bottled water is considered a food, it is regulated by the US Food and Drug Administration. Tap water is regulated by the U.S. Environmental Protection Agency. Both types of water are subject to testing for contaminates. But Sass points out that an estimated 60 to 70 percent of all bottled water in the U.S. is packaged and sold within the same state, which exempts it from FDA regulation. And 1 in 5 states do not regulate that bottled water. Moreover, tests on 1,000 bottles of 103 different brands of bottled water found man-made chemicals, bacteria and arsenic in 22 percent of the bottles. Tap water is also not immune to contamination problems. While most cities meet the standards for tap water, some tap water in the 19 U.S. cities tested was found to contain arsenic, lead, and pesticides, Sass told the conference. While most healthy adults can tolerate exposure to trace amounts of these contaminates, some people, such as cancer patients undergoing chemotherapy, individuals who are HIV positive or recovering from a transplant or major surgery, pregnant women, children, and the elderly, are more vulnerable. For these individuals, Sass favors bottled water treated with reverse osmosis, distilled water or city tap water with a filtering system certified by the National Sanitation Foundation. If the bottle is accidentally opened or someone tampers with it, then it can easily get contaminated. There’s certainly a greater chance you could find something harmful in bottled water than from your taps. Ideally it should be drunk on the day it is opened, as it can easily pick up bacteria from someone’s hands or face. Batches of bottled water have had to be removed from supermarkets shelves because of questions over contamination. In 1990, Perrier had to withdraw millions of bottles worldwide after traces of benzene were found in the water. And in 2004, Coca-Cola launched Dasani, which was ‘purified’ tap water taken straight from mains supplies. But within weeks hundreds of thousands of bottles were recalled after it emerged the purification process may have contaminated the water with a possibly carcinogenic substance.
_
The above discussion pertains to highly developed nation the U.S. where tap water quality is generally good but as far as developing nations like India and Bangladesh is concerned, tap water quality even in their capital is poor and therefore comparison of bottled water vis-à-vis tap water in developed nations cannot be extrapolated in developing nations.
_
Manufacturing process of bottled water:
Bottled water production technology involves combination of chemical treatment and filtration technique. Plants like Minscot, Volga, Bailley, Prime, Aquaplus and Bailley combine chemical and filtration treatments; Bisleri and Paras lay more emphasis on filteration techniques. Different companies use a range of purification methods: adding chlorine to kill micro-organisms; ultrafiltration to remove suspended impurities; passing water through an ozonation process to eliminate bacteria; and using filters, both mechanical and organic, to remove physical impurities. Contamination with microorganisms is common to surface water and is an increasing concern as far as groundwater is concerned. Disinfection is the inactivation of pathogens in drinking water. Two common techniques are chemical disinfection and irradiation with UV light. The chemical disinfectants used in water treatment are chlorine, chloramine, ozone, and chlorine dioxide. Of the chemical disinfectants, free chlorine is used most commonly. Effective chlorination and ozonation depends upon the length of time chlorine and ozone remains active in water, which depends in turn upon factors like temperature. UV disinfection is used in small systems that treat groundwater. UV irradiation has been demonstrated to be effective against bacteria and viruses, microbiological contaminants most likely to be found in groundwater. Combined ozonation and UV light treatment is effective in oxidation of pesticide to non-toxic products but sometimes oxidation leads to formation of more toxic products e.g. oxidation of methyl parathion to methylparaxon, which is more toxic. Membrane technologies comprise micro filtration (MF), ultra filtration (UF), nano filtration (NF) and reverse osmosis membranes (RO). Activated charcoal adsorption is also used by some manufacturers, and is an effective method for the removal of chlorine, organic chemicals and pesticides, but not fluoride, nitrate, lead or other heavy metals.
_
Examples of water treatments include for bottled water:
• Distillation: In this process, water is turned into a vapor. Since minerals are too heavy to vaporize, they are left behind, and the vapors are condensed into water again.
• Reverse osmosis: Water is forced through membranes to remove minerals in the water.
• Absolute 1 micron filtration: Water flows through filters that remove particles larger than one micron in size, such as Cryptosporidium, a parasitic protozoan.
• Ozonation: Bottlers of all types of waters typically use ozone gas, an antimicrobial agent, to disinfect the water instead of chlorine, since chlorine can leave residual taste and odor to the water.
Bottled water that has been treated by distillation, reverse osmosis, or other suitable process and that meets the definition of “purified water” in the U.S. Pharmacopeia can be labeled as “purified water.”
_
Bottled Water and Cost:
_
_
In the United States, bottled water costs between $0.25 and $2 per bottle while tap water costs less than a penny. According to Bottledwaterblues.com, about 90% of manufacturer’s costs are from making the bottle, label, and cap. In 1999, according to a NRDC study, U.S. consumers paid between 240 and 10,000 times more per unit volume for bottled water than for tap water. Typically 90 percent or more of the cost paid for bottled water is for things such as bottling, packaging, shipping, marketing, retailing, and profit, but not for the water itself.
_
Bottled water can be costlier than oil:
When I lived in Saudi Arabia from 2001 to 2006, the cost of 1 liter of bottled purified water was 1 riyal while cost of 1 liter of petrol was 0.8 riyal. Now you may say that Saudis are biggest manufacturer of petrol. Take, for instance, Pepsi’s Aquafina or Coca-Cola’s Dasani bottled water in the U.S. Both are sold in 20 ounce sizes and can be purchased from vending machines alongside soft drinks — and at the same price. Assuming you can find a $1 machine that works out to 5 cents an ounce. These two brands are essentially filtered tap water, bottled close to their distribution point. Most municipal water costs less than 1 cent per gallon. Now consider another widely sold liquid: gasoline (petrol). It has to be pumped out of the ground in the form of crude oil, shipped to a refinery (often halfway across the world), and shipped again to your local filling station. In the U.S., the average price per gallon is hovering around $3. There are 128 ounces in a gallon, which puts the current price of gasoline at a fraction over 2 cents an ounce. And that’s why there’s no shortage of companies that want to get into the business of bottled water. In terms of price versus production cost, bottled water puts Big Oil to shame. However in India, the best quality bottled water costs one sixth of petrol.
_
Can the material used in making bottles be harmful to health?
Types of materials used to make bottles:
_
Most bottled water comes in polyethylene terephthalate bottles, indicated by PET or PETE on the bottle’s bottom. The bottles are generally safe but scientists say when stored in hot or warm temperatures, the plastic may leach chemicals into the water. However, when lower grade plastics such as HDPE (high-density polyethylene) are used, it can give a plastic taste to water. Not only is bottled water a major strain on the environment, but a lot of bottled water is no cleaner than tap water. In fact, about 40 percent of bottled water is regular tap water, which may or may not have received any additional treatment. The metal antimony (a silvery white metal of medium hardness) has been found in many commercially bottled water brands, for example. The amount of antimony leeching into the water you’re drinking depends on the manufacturer, and can vary greatly. One study that looked at 63 brands of bottled water produced in Europe and Canada, found concentrations of antimony that were more than 100 times the typical level found in clean groundwater (2 parts per trillion). It’s also been found that the longer a bottle of water sits on a shelf — in a grocery store or your refrigerator – the greater the dose of antimony present. The biggest offenders were packaged in polyethylene terephthalate (PET) containers. It is believed that the amount of antimony leeching from these PET bottles differs based on exposure to sunlight, higher temperatures, and varying pH levels. Most municipal tap water — though generally far from pure — must actually adhere to stricter purity standards than the bottled water industry. Nalgene bottles should also be avoided as they can leach another unsafe chemical called BPA into your water. Glass bottles are best, but if you’re traveling and can’t use a glass bottle, the high-density polyethelene (HDPE) Nalgene bottles appear to be a safer choice, so far.
_
Estrogenic chemicals in bottled water from plastic:
Plastic mineral water bottles contaminate drinking water with estrogenic chemicals, researchers in Germany said.
Martin Wagner and Jorg Oehlmann of the Goethe University in Frankfurt, Germany, said in an analysis of commercially available mineral waters, researchers found evidence of estrogenic compounds leaching out of the plastic packaging into the water. What’s more, the chemicals result in an increased development of embryos in the New Zealand mud snail, the researchers said. The findings, published in the journal Environmental Science and Pollution Research, show for the first time that substances leaching out of plastic food packaging materials act as functional estrogens. The researchers analyzed 20 brands of mineral water available in Germany — nine bottled in glass, nine bottled in plastic and two bottled in paperboard boxes coated with an inner plastic film. “We must have identified just the tip of the iceberg in that plastic packaging may be a major source of xenohormone — man-made substance that has a hormone-like effect — contamination of many other edibles,” the researchers said in a statement. “Our findings provide an insight into the potential exposure to endocrine-disrupting chemicals — low-dose exposure to chemicals that interact with hormone receptors that may interfere with reproduction, development and other hormonally mediated processes — due to unexpected sources of contamination.”
_
Is it true that plastic used in water bottles can release Bisphenol A (BPA) into the water?
Most plastic bottles used in the sale of bottled water are made of polyethylene terephthalate (PET or PETE) or polyethylene (PE), which does not contain Bisphenol A. Large jugs (18 L bottles) and some sport bottles can be made of polycarbonate plastic (PC) which may contain small amounts of Bisphenol A. As a result of the use of polycarbonate water bottles, minute quantities of Bisphenol A can potentially leach out into the water or food and consumers may be exposed to small amounts of Bisphenol A through their normal daily diet. However the dietary exposure to Bisphenol A from food packaging sources, including PC water bottles, does not pose a health risk to consumers.
_
Now let me discuss contamination of drinking water in bottled water: many studies are narrated:
_
Bottled water may have reduced amounts of copper, lead, and other metal contaminants since it does not run through the plumbing pipes where tap water is exposed to metal corrosion, however, this varies by the household and plumbing system. In a study comparing 57 bottled water samples and tap water samples, all of the tap water samples had a bacterial content under 3 CFUs/mL(colony-forming unit) and the bottled water samples’ bacterial content ranged from 0.01-4900 CFUs/mL. Most of the water bottle samples were under 1 CFU/mL, although there were 15 water bottle samples containing 6-4900 CFUs/mL. In another study comparing 25 different bottled waters, most of the samples exceeded the contaminant level set by the U.S. Environmental Protection Agency (EPA) for mercury, thallium, and thorium.
_
Bacteriological evaluation of packaged bottled water sold at Jaipur city and its public health significance:
The study was carried out to investigate the microbiological quality of packaged drinking water marketed in Jaipur city. Out of twenty, 50% samples were found unsatisfactory in standard plate count. Psychrophillic, coliforms, E. coli and staphylococcal counts revealed that 25%, 45%, 20%, and 5% samples respectively were found unfit for human consumption as per Bureau of Indian Standards (BIS) of drinking water. On the basis of results of overall microbiological assessment 55% of samples proved to be unfit for consumption. All brands of water sachet (100%) had high coliforms count which indicates faecal contamination. Amongst those sachets two brands (40%) had presence of E. coli and all the sachet water brands fell below drinking water standards while out of fifteen brands of bottled water 6 samples contained higher microbiological value hence unfit for human consumption. It was concluded that local brands of packaged drinking water were found unfit for human consumption. So it is suggested that government should intensify the efforts in the monitoring of activities in this rapidly expanding industry with a view to supply potable and wholesome water to the public.
_
_
Bacterial content of bottled drinking water versus municipal tap water in Karnataka, India:
Fifty samples of 10 categories of bottled drinking water with different batch numbers were purchased and municipal water from different sources were collected. Water was cultured quantitatively and levels of bacteria were calculated as colony-forming units (CFUs) per milliliter. A comparison of the mean values of microbial count for bottled drinking water with that of municipal tap water showed no statistically significant difference, but was more than the standard levels along with the presence of fungus and maggots. For microbial analysis, WHO recommended that CFUs/ml should be 0 after 2 days at 37°C (if water is disinfected) or 10 (if water is not disinfected). In the present study, few samples of water at the end point source showed an increase in the CFUs, which can be explained due to water contamination in pipelines during distribution. Overall, CFU counts were higher in municipal as well as in bottled drinking water as seen in the figure below. The CFU count was higher than the recommended level in bottled drinking water, which was similar to the studies conducted by Payment et al., Mavridou et al., Hunter and Silva et al. But, there was no statistical difference among bottled drinking water and that of municipal tap water in the present study, which was similar to the study conducted by Payment et al. This was in contrast with the studies conducted by James and Silva et al., where municipal water showed a lower CFU count than bottled drinking water. Along with CFUs, few samples of municipal tap water (raw water and water at the end point) and one sample of bottled drinking water (Flair, batch no. August 30, 2008) showed the “presence of maggots.” Maggot is the common name of the larval phase of development in insects of the order Diptera (flies). This was similar to a Charles Cooper report, where people complained of finding Rat Tailed Maggot in the municipal water supplies of different parts of Cape Town (South Africa) in April 2006. This can be explained due to the process of “Back siphoning.”
_
My view:
I humbly disagree with the authors. First their discussion and table shows bacteria as cfu/ml but did not specify the species. Many heterptrophic bacteria are harmless to humans. Even for coliform bacteria, MPN of 10/100 ml is allowed for untreated water. Also, cfu/ml for Bislery water is only 13.6 which is much less than all tap water treated or untreated. So it is the company that maintains water quality is important and therefore generalized statement that all bottled water is contaminated is unfair.
_
Bacteriological quality and risk assessment of the imported and domestic bottled mineral water sold in Fiji:
Considering the popularity of bottled mineral water among indigenous Fijians and tourists alike, a study was carried out to determine the bacteriological quality of different bottled waters. A risk assessment was also carried out. Seventy-five samples of bottled mineral water belonging to three domestic brands and 25 samples of one imported brand were analysed for heterotrophic plate count (HPC) bacteria and faecal coliforms. HPC counts were determined at 22 degrees C and 37 degrees C using R2A medium and a membrane filtration technique was used to determine the faecal coliform (FC) load in 100 ml of water on mFC agar. Between 28 and 68% of the samples of the various domestic brands failed to meet the WHO standard of 100 colony forming units (cfu) per 100 ml at 22 degrees C and 7% of these also tested positive for faecal coliforms. All imported bottled mineral water samples were within WHO standards. A risk assessment of the HPC bacteria was carried out in terms of beta haemolytic activity and antibiotic resistance. More than 50% of the isolates showed beta haemolytic activity and were multi-drug resistant. While the overall quality of the product was generally good, there is a need to enforce stringent quality standards for the domestic bottlers to ensure the safety of consumers.
_
Chemical quality of bottled waters from three cities in eastern Alabama:
Twenty-five brands of bottled waters consisting of both purified and spring types collected randomly from three Alabama cities, USA were assessed for their suitability for human consumption. Water quality constituents analyzed include pH, conductivity, alkalinity, chloride, nitrate + nitrite, sulfate, phosphate, total carbon (TC), inorganic carbon (IC), total organic carbon (TOC), and 27 elements on the inductively coupled plasma-optical emission spectrometer (ICP-OES). The results obtained were compared with US EPA drinking water standards and the European union (EU) drinking water directive. Ni was non-detectable in all the samples and Cu, Pb, Sb, Zn, Mn, Al, Cr, Mg, P, Ca, sulfate, chloride and nitrates + nitrites were all below their respective USEPA drinking water standards or EU maximum admissible concentrations (MAC). The conductivity, pH, As, Cd, Hg, Zn, Se and Tl values in some samples exceeded the EU and USEPA standards for drinking water. No sample had pH>8.5, but seven bottled water brands analyzed were acidic (pH<6.5). Most of the sampled brands had TOC concentrations exceeding 3 mg/l. The concentrations of most water quality constituents analyzed, in most cases, were higher in the spring water brands compared to the purified or distilled brands of bottled water. A one-way parametric analysis of variance (ANOVA) conducted on pH, conductivity, IC, TOC, Ca, Na, K, Mg, Se, sulfate, chloride and nitrate + nitrite values for 10 brands of bottled water to ascertain the homogeneity of variances within and between the brands, suggested significant differences in variances across the brands at a 95% confidence level except for selenium, sodium and calcium.
_
Analysis of pesticide residues in bottled water [Delhi region]:
Even the municipal water supply is not free of contaminants like pesticides, and heavy metals. People either boil water to drink it or install purifiers. Of late, they have also turned to bottled water available in the open market: this water is perceived as safe. Given human dependence on water, we cannot afford to be careless about the kind and quality of water that we drink. Various top brands like Bisleri, Kinley make claims about the purity of their mineral water and advertise their water as the safest. But the source of water for different bottlers is bore-well (groundwater). Given the quality of water in and around Delhi is not very good, could bottled water, too, be contaminated? Could bottled water contain pesticides, since it is known that Delhi’s groundwater does? Since exposure to pesticides through drinking water has potential health effects, a study was undertaken to assess the quality of bottled mineral water in terms of pesticide levels. Do various brands conform to standards specified by the Bureau of Indian Standards (BIS) and Prevention of Food Adulteration Act, 1954 (PFA)? How do the brands — and the norms themselves — fare when compared to internationally accepted drinking water norms, such as that of the World Health Organization or the US Food and Drug Administration for drinking water? The laboratory collected 2 bottles each of 17 bottled drinking water brands — the top five brands such as Bisleri (Aqua Minerals Ltd), Bailley (Parle Agro Pvt. Ltd), Pure Life (Nestle India Ltd), Aquafina (Pepsico India Holding Pvt Ltd) and Kinley (Hindustan Coca Cola Beverage Pvt. Ltd) and other less popular brands — being sold in Delhi and CSE Report on pesticide residues in bottled water (Delhi region) nearby Gurgaon and Meerut. The bottles were randomly purchased. They were then analysed for 12 organochlorines and 8 organophosphorus pesticides using a method called gas chromatography (GC). The testing process was based on the United States Environment Protection Agency testing procedure for pesticides in drinking water. In the BIS drinking water standards, the desirable limit for pesticides is given as “absent”. The permissible limit, in the absence of any other alternate source is given as 0.001mg/l (1μg/l). The BIS standard for packaged drinking water — IS 14543:1998 — and Natural Mineral Water — IS: 13428:1998 — covered under the relevant PFA states that pesticide residues “should be below detectable limits” when tested in accordance with the relevant methods. However, when tested for organochlorine pesticides and organophosphorus pesticides, the water bottled by the 5 top brands and other less popular brands were found to be contaminated with pesticide residues. Among the organochlorines, HCH and DDT were frequently detected. G-isomers of HCH (Lindane) were detected in 94 per cent of all samples. DDT was detected in 70.6 per cent of the samples. Metabolites of DDT like DDE and DDD were also detected. Endosulfan was present in 8.8 per cent of the samples. Among the organophosphorus pesticides, Malathion and Chlorpyrifos were most frequently detected: respectively, in 85.3 per cent and 82.4 per cent of the samples. People switch over from tap water to bottled water because they think it is not contaminated. The CSE laboratory test shows otherwise. Bottled water should not be considered a sustainable alternative to tap water. Source monitoring, reduction in use of pesticide and effective treatment seems to be the best choice for keeping a check on pesticide concentration in water.
_
Risk assessment of heterotrophic bacteria from bottled drinking water sold in Indian markets:
One hundred and five samples of bottled drinking water belonging to 30 different brands, collected from six different states of India have been analysed for total heterotrophic bacterial (THB) load and coliforms. Almost all bottlers used multiple treatment procedures such as microfiltration, reverse osmosis and ozonization to treat the water. Around 40% of the samples exceeded the limit of 100 cfu/ml set by the department of health as well as Bureau of Indian Standards (BIS), Government of India. Fourteen percent and 44% of the samples with THB loads between 100 and 1000 cfu/ml or 1000 cfu/ml tested positive for coliforms indicating a linear relationship between THB and coliform bacteria. Gram-positive genera such as Kurthia and Corynebacterium were found to be dominant genera, while members of the family enterobacteriaceae contributed to 7%. Risk assessment of the heterotrophic bacteria revealed that the majority of the strains acquired resistance against ampicillin, nalidixic acid, novobiocin and oxytetracycline. As bottled drinking water is a ready to drink commodity, the high load of heterotrophic bacteria with multiple drug resistance poses significant health hazards to the consumers, especially to immunocompromised individuals.
_
Microbiological assessment of house and imported bottled water by comparison of bacterial endotoxin concentration, heterotrophic plate count, and fecal coliform count:
Consumers increasingly use bottled water and home water treatment systems to avoid direct tap water. According to the International Bottled Water Association (IBWA), an industry trade group, 5 billion gallons of bottled water were consumed by North Americans in 2001. The principal aim of this study was to assess the microbial quality of in-house and imported bottled water for human consumption, by measurement and comparison of the concentration of bacterial endotoxin and standard cultivable methods of indicator microorganisms, specifically, heterotrophic and fecal coliform plate counts. A total of 21 brands of commercial bottled water, consisting of 10 imported and 11 in-house brands, selected at random from 96 brands that are consumed in Puerto Rico, were tested at three different time intervals. The Standard Limulus Amebocyte Lysate test, gel clot method, was used to measure the endotoxin concentrations. The minimum endotoxin concentration in 63 water samples was less than 0.0625 EU/mL, while the maximum was 32 EU/mL. The minimum bacterial count showed no growth, while the maximum was 7,500 CFU/mL. Bacterial isolates like P. fluorescens, Corynebacterium sp. J-K, S. paucimobilis, P. versicularis, A. baumannii, P. chlororaphis, F. indologenes, A. faecalis and P. cepacia were identified. Repeated measures analysis of variance demonstrated that endotoxin concentration did not change over time, while there was a statistically significant (p < 0.05) decrease in bacterial count over time. In addition, multiple linear regression analysis demonstrated that a unit change in the concentration of endotoxin across time was associated with a significant (p < 0.05) reduction in the bacteriological cell count. This analysis evidenced a significant time effect in the average log bacteriological cell count. Although bacterial growth was not detected in some water samples, endotoxin was present. Measurement of Gram-negative bacterial endotoxins is one of the methods that have been suggested as a rapid way of determining bacteriological water quality.
_
The health-related microbiological quality of bottled drinking water sold in Dar es Salaam, Tanzania:
The consumption of bottled and plastic-bagged drinking water in Tanzania has increased largely because of the deteriorating quality of tap water. It is uncertain whether these water products are safe for drinking. In this study, the microbiological quality of bottled and plastic-bagged drinking water sold in Dar es Salaam, Tanzania, was investigated. One hundred and thirty samples representing 13 brands of bottled water collected from shops, supermarkets and street vendors were analysed for total coliform and faecal coliform organisms as well as heterotrophic bacteria. These were compared with 61 samples of tap water. Heterotrophic bacteria were detected in 92% of the bottled water samples analysed. Total and faecal coliform bacteria were present in 4.6% and 3.6%, respectively, of samples analysed with a tendency for higher contamination rates in plastic-bagged drinking water. Microbiological quality of tap water was found to be worse compared with bottled water, with 49.2% and 26.2% of sampling points showing the presence of total coliform and faecal coliform organisms, respectively. The results suggest caution and vigilance to avert outbreaks of waterborne diseases from these types of drinking water.
_
Mineral water or tap water? A systematic analysis of the literature concerning the question of microbial safety in Germany:
Based on sporadic reports of microbial contamination of mineral waters, it has been recommended that, for safety reasons, particularly immunocompromised patients should drink tap water rather than bottled mineral water. However, in terms of safety, evidence of the clinical consequences may allow a better estimate than a positive in vitro test for contamination. Therefore, authors reviewed the literature on documented disease outbreaks due to contaminated mineral and tap waters. Cases of contamination of tap water were documented in nearly all countries included. In 35 communications they found reports on a total of 423,000 cases of disease outbreaks due to contaminated tap water, in some cases even with lethal outcome. Main diagnosis was gastroenteritis, and main species of microorganism was crytosporidium. In contrast, there was no documented case of disease outbreak due to contaminated bottled mineral water. Tap water as well as bottled water are both supremely safe components of nutrition. The recommendation that tap water is better than mineral water, particularly for high-risk patients, is not supported by the literature.
_
Reported Outbreaks Associated with Bottled Water:
•2000: acute gastrointestinal illness (AGI) caused by the bacteria Salmonella Bareilly Contamination During Commercial Bottling
•1980: AGI caused by an unidentified agent
•1989: AGI caused by an unidentified agent
•1994: AGI caused by the bacteria Vibrio cholerae
•2003: AGI caused by the chemical bromated Contamination During Shipping, Hauling, or Storage
•2003: AGI caused by an unidentified chemical cleaning product Contaminated at Point of Use
•2000: AGI caused by the bacteria Shigella sonnei Type D
•2003: AGI caused by an unidentified agent Unknown Point of Contamination
•1974: An outbreak of cholera in Portugal in 1974 due to the consumption of contaminated bottled drinking water
•1999: AGI caused by an unidentified agent
•2001: AGI caused by the chemical ethylbenzene
•2004: AGI caused by gasoline byproducts
•2007: AGI caused by an unidentified agent
AGI means acute gastroenteritis.
_
Bottled Water: As Pure as we are led to believe?
•While most bottled water apparently is of good quality, publicly available monitoring data are scarce. The underfunded and haphazard patchwork of regulatory programs has found numerous cases where bottled water has been contaminated at levels above state or federal standards. In some cases bottled water has been recalled.
• NRDC conducted a four-year review of the bottled water industry and the safety standards that govern it, including a comparison of national bottled water rules with national tap water rules, and independent testing of over 1,000 bottles of water. Their conclusion is that there is no assurance that just because water comes out of a bottle it is any cleaner or safer than water from the tap. And in fact, an estimated 25 percent or more of bottled water is really just tap water in a bottle — sometimes further treated, sometimes not. About one third of the bottled waters contained significant contamination (i.e., levels of chemical or bacterial contaminants exceeding those allowed under a state or industry standard or guideline) in at least one test. This is the most comprehensive independent testing of bottled water in the United States that is publicly available. And since your local tap water is required to be tested, by law, and those results must be publicly available, there is a greater likelihood that you can verify the safety of your tap water, while you can not verify the compliance of any bottled water. If you want the safest, cleanest, best tasting water; EHSO’s recommendation is install a good water filter on your tap!
_
Does bottled water contain fluoride?
Bottled water products labeled as de-ionized, purified or distilled have been treated in such a way that they contain no or only trace amounts of fluoride, unless they specifically list fluoride as an added ingredient. Other bottled water products (such as spring water) can contain fluoride that is added or naturally present in the original source of the water. FDA sets limits for fluoride in bottled water based on several factors, including the source of the water. These limits range from 0.8 to 2.4 milligrams per liter.
_
Shelf Life of Bottled Water:
How long is a bottle of water good if it remains sealed?
In the United States bottled water’s shelf life is date stamped for two years. It should be stored in a dark, cool, dry area away from any solvents or chemicals.
The International Bottled Water Association (IBWA) further adds:
The U.S. Food and Drug Administration, which regulates the quality and safety of bottled water, has neither set nor suggested any limitation to the shelf life of bottled water. You may notice that most bottled water containers sold at retail bear a two-year expiration date. This acts as a lot number and is for stock rotation purposes. It does not mean the product is substandard after that date. Thus, bottled water purchased in bulk is good indefinitely if stored appropriately. Appropriately means unopened in a cool, dry place away from odors and toxic substances. For those yearning for a more technical explanation, it is thus: Bottled water is considered to be of virtually no significant nutritional value. Therefore, unlike milk, fish or poultry, bottled water is not an adequate substrate for pathogens responsible for the majority of food-borne illnesses. In that regard, IBWA’s general position is that as long as bottled water is packaged in accordance with FDA processing and good manufacturing practices, and meets the FDA quality standard provisions, the product’s shelf life should remain intact for an indefinite period provided that product storage and other post-packaging and handling practices do not adulterate or deleteriously affect the finished product. I disagree. When water is stored in plastic bottle for long time, some plastic impurities will invariably leach in water. Also, I have discussed earlier that bacteria remain viable for months even in distilled water and therefore the logic that since bottled water has no nutritional value, it cannot harbor pathogenic bacteria is fuzzy.
_
What can I do to ensure the safe use of bottled water?
In the case of all single use bottled water (except 18L carboys):
•Do not refill old bottles.
•Do not share bottles.
•Clean the bottle top or cap before drinking or pouring from them.
•Keep the opened bottle clean and preferably refrigerated as the water, cap and cap liner can all support bacterial growth which may originate from the mouth or the environment.
In the case of 18L bottles used with a dispenser:
•Clean water coolers regularly.
•Use water dispensers with coolers that keep the water refrigerated. Some units have heaters as well.
•Use water coolers that filter the air that enters the bottle as the water is dispensed.
_
Is it safe to reuse the bottles that water is sold in by filling them with tap water?
It is not recommend reusing single-use bottles because the reuse poses a potential microbiological risk if not cleaned properly. Studies on reusing single-use bottles have found that depending on the source of the water used and the general hygiene of the user, the growth of bacteria in the bottle can vary from negligible to potentially hazardous.
_
Why does Bottled Water taste better?
The results of most blind taste tests indicate no difference between the taste of tap water and that of bottled water. When blind tests are conducted, the taste buds really don’t seem to think that bottled water tastes better than tap water. In 2001, ABC’s Good Morning America conducted a blind water taste test. The viewers’ preferences were as follows:
•12 percent Evian
•19 percent O-2
•24 percent Poland Spring
•45 percent New York City tap water
Yorkshire Water, the water department in Yorkshire, England, found that 60 percent of 2,800 people surveyed could not tell the difference between the local tap water and UK bottled water. The hosts of Showtime’s television series Penn & Teller found that 75 percent of New Yorkers preferred city tap water to bottled waters. The hosts of the show conducted another test in a trendy Southern California restaurant. A water sommelier handed out water menus with extravagant prices to the patrons. The patrons had no idea that all of the fancy bottles of water were filled with the same water from a water hose in the back of the restaurant. Patrons were willing to pay $7 a bottle for “L’eau du Robinet” (French for “tap water”), “Agua de Culo” (Spanish for “ass water”), and “Amazone” (“filtered through the Brazilian rainforest’s natural filtration system”). The fancy bottles and exotic names was enough to convince the taste buds that they were experiencing pure bliss.
So then, why does bottled water taste better?
It tastes better because we expect it to taste better.
_
Can you trust bottled water?
The Environmental Working Group (EWG) analyzed the company websites and product labels of over 170 varieties of bottled water to see if the companies disclosed information on where water came from, how the water was treated, and whether the results of tests to ensure purity were revealed. The researchers also called the bottled water companies to see if they would willingly give information to consumers. More than half of the bottled water products failed the transparency test. Almost 20 percent didn’t say where their water comes from, and an additional 32 percent did not disclose any information on treatment or purity of water. In all, only three bottled water products received a good rating for transparency from the EWG:
1. Nestlé’s Pure Life Purified Water
2. Gerber Pure Purified Water
3. Penta Ultra-Purified Water
_
Another independent test performed by the Environmental Working Group in 2009 revealed 38 low-level contaminants in bottled water, with each of the 10 tested brands containing an average of eight chemicals, including:
•Disinfection byproducts (DBPs)
•Caffeine
•Tylenol
•Nitrate
•Industrial chemicals
•Arsenic
•Bacteria
__
Bottled Water & Immunocompromised Individuals
People with compromised immune systems may want to take special precautions with the water they drink. In healthy individuals, the parasite Cryptosporidium can cause illness; however, for those with weakened immune systems, it can cause severe illness and possibly death. Look for bottled water treatments that protect against Cryptosporidium, which include:
•Reverse Osmosis
•Distillation
•Filtration with an absolute 1 micron filter
_
Environmental concern of bottled water:
The manufacture and transport of that one kilogram bottled water used:
•26.88 kilograms of water (7.1 gallons)
•0.849 Kilograms of fossil fuel (one liter or .26 gal) and
•Emitted 562 grams of Greenhouse Gases (1.2 pounds).
_
Energy used to make bottled water:
One of the environmental factors is the amount of energy used to manufacture and ship the bottled water. The companies must pump the water out of a spring or municipal source, filter it, create the bottles, fill the bottles, package the bottles, store and chill them, and then ship them all over the country. The amount of energy used in the process to create PET bottles is 100,000 Megajoules (MJ) per ton of PET. There was 100 billion liters of bottled water sold worldwide in 2007 which amounted to 3.8 million tons of PET being produced. That comes to 380 billion MJ used in one year on bottle manufacturing. To put that into perspective, the average household uses about 42,864 MJ a year.
_
_
The Pacific Institute estimates that in 2006:
- Producing the bottles for American consumption required the equivalent of more than 17 million barrels of oil, not including the energy for transportation
- Bottling water produced more than 2.5 million tons of carbon dioxide
- It took 3 liters of water to produce 1 liter of bottled water
_
Water pollution cycle driven by bottled water:
In 2004, worldwide sales of bottled water totaled 41 billion gallons. There’s a lot of plastic left over once 41 billion gallons of water, much of it in 8- or 12-ounce containers, is consumed. In a single year, manufacturers around the world use about 2.7 million tons of plastic to bottle water. Most of those bottles are a type of plastic called polyethylene terepthalate, or PET, which is produced from crude oil. To produce bottles to meet yearly bottled-water demand in the United States alone requires 1.5 million barrels of oil. That much oil could power about 100,000 cars for a year, according to the Earth Policy Institute. PET is a recyclable plastic that can be used to make synthetic fiber for carpets, sweaters and many other products. And almost 90 percent of bottled-water bottles end up in the trash or on the ground, not in recycling bins. They can take up to 1,000 years to degrade, and when they do, they can leak harmful chemicals into the ground, contaminating ground water — ironically inducing a new cycle of pollution that means bottled water may actually be a necessity in the world some day. In other words, plastic of bottled water would ultimately contaminate water sources requiring further use of bottled water.
_
Aluminum bottles for bottled water:
Due to concerns about environmental impact — its 38 million plastic bottles a year made with 1.5 million barrels of oil in the U.S.; Swiss company SIGG touts its aluminum drinking bottles with slogans such as, “Make love not landfill” and “Friends don’t let friends drink from plastic.”
____________
Regulation of bottled water:
With the growth of bottled water consumption, various regulations have also sprung up to regulate the quality of bottles water. Standards were formulated to ensure suitable quality of bottled water. Codex Alimentarius Commission (CAC) is one such regulatory body which formulates standards; its standards are taken as reference point in many developing countries which are not capable of developing their own standards. Similarly, United States Food and Drug Administration (US FDA) formulates standards for bottled water in the United States (US). In India, Bureau of Indian Standard (BIS) is entitled to establish quality standards for bottled water. The BIS comes with quality standards for natural mineral water and simple packaged water in 1992 and 1998 respectively. However, up to 2001, these standards were voluntary in nature. From 2001 onwards, bottled water has been notified as a food item and has been brought under the Prevention of Food Adulteration Act 1954. As a result, the government notified and made the bottled water standards mandatory in nature in India.
_
The anti-bottled-water crowd often repeats the claim that bottled water is not as regulated as tap water. That’s a somewhat dubious claim: The two are different products with different distribution systems, regulated by different federal agencies; the Environmental Protection Agency oversees municipal drinking water, while the Food and Drug Administration regulates bottled water as a packaged food item. Chris Hogan is vice president of communications for the International Bottled Water Association, the industry’s main trade association and mouthpiece. “The [FDA], by federal law, has to regulate bottled water regarding health and safety to at least [as] strict a standard as the EPA regulates tap water,” he says. “And in some cases, with specific regulations, they regulate bottled water to a higher standard.” “Bottled water goes through a stringent, multi-barrier filtration process,” Hogan says. “It has to meet very strict requirements to be called bottled water. You can’t take a garden hose and fill a bottle and screw the top on and say, ‘I’m now selling bottled water that meets FDA standards.’ ” However, most bottled water sold in third world countries is of sub-standard quality as shown by many studies discussed above.
_________
Water vending machine:
_
A number of cities and companies worldwide have vending machines that dispense purified water into customer’s own containers. All dispensers filter the location’s tap water. In North America, these machines are typically located outside of supermarkets. Of all the water vending companies, Glacier Water is by far the largest. Since its inception in 1983, Glacier Water has experienced significant growth in machine placements and has created an extensive network of approximately 17,000 water vending machines (year 2010) located throughout the United States and Canada.
_
Anytime water machine (ATW) in India:
Any time water (ATW) machines that provide 10 liters of drinking water for just one rupee might emerge as the solution to the vexed issue of water access in rural and semi-urban areas of India. People in villages and small towns, who have no access to drinking water or have to walk a good distance to fetch water, will soon see ATWs at vital locations, like hospitals and bus stations if not at every nook and corner. They need to drop Re 1 into the vending machine and get 10 liters of pure drinking water. The water will be on par with the mineral water of reputed companies.
__________
Bottled water for children:
First of all, babies younger than 6 months of age should drink only breast milk or formula, which contain all the water babies need, even in hot weather. (In fact, breast milk and formula are 85 percent water.) Giving water to a young baby can cause a very dangerous condition called water intoxication, which results when too much water causes too much sodium loss through the kidneys. After your baby is 6 months old, it’s okay to give her very small amounts of plain water if you like. But since water contains no nutrition or calories, don’t give it to her in place of breast milk or formula, which are still better for her. And never use water to replace a feeding. If you do give your baby small sips of water after 6 months, plain water is better than mineral or carbonated waters. The minerals found in mineral water are usually sodium, calcium, and trace minerals. The exact composition depends on the particular processing method, so it’s hard to tell what you’re getting. (In fact, there are no standard industry requirements for the composition of mineral waters, or even for their safety, if they’re imported.) Some mineral water may contain too much sodium and other minerals that can be difficult for a baby’s or young child’s kidneys to handle. And carbonated water isn’t a good idea because the carbonation comes from gas – and that’s just what it will cause in your baby or child! This can lead to excess spitting up and burping and even abdominal pains and discomfort. After her second birthday, a little plain mineral water on occasion isn’t terrible for your child (her kidneys are mature enough to handle the mineral loads by then), but don’t be persuaded by advertisers that it has any extra benefit for your child (because it’s labeled “all natural” or containing “valuable minerals,” for example). It has no real advantages over plain water, and, again, its safety may not be regulated the way plain bottled or tap water is. Carbonated beverages still aren’t a good idea, even after age 2, because carbonation can cause tummy trouble at any age, and it’s often the culprit in kids with gas pains and abdominal discomfort.
_
Can I use bottled water to make up baby formula (infant formula)?
Bottled water is not recommended to make up infant formula feeds for your baby. This is because it’s not usually sterile and may contain too much salt (sodium) or sulphate.
Checking the levels of sodium and sulphate:
If you have to use bottled water to make up a feed, check the label to make sure that the water contains:
•less than 200 milligrams (mg) a liter of sodium (also written as Na)
•no more than 250mg a liter of sulphate (also written as SO or SO4)
You may need to use bottled water to make up a feed if:
•your drinking water has been contaminated because of flooding
•you’re travelling abroad and drinking the local water is not recommended
Boiling water to make up formula feeds:
As bottled water is not usually sterile, it will still need to be boiled, like tap water, before you prepare the feed. Always use boiled water at a temperature of at least 70°C, but remember to let the feed cool before you give it to your baby.
____________
Drinking water Regulation:
Briefly, the purpose of having drinking water quality guidelines and regulations is to ensure that all human beings within a country have access to safe drinking water. In developing countries, it is estimated that over 80% of disease is caused by contaminated drinking water and as a consequence, over 30% of work productivity is lost. Meaning, water is largely the cause of most disease and a considerable amount of work potential is compromised because of this. All countries have their own legal drinking water standards. These prescribe which substances can be in drinking water and what the maximum amounts of these substances are. The standards are called maximum contaminant levels. They are formulated for any contaminant that may have adverse effects on human health and each company that prepares drinking water has to follow them up. If water will be purified to make it suitable to drink it will be tested for a number of dangerous pollutants, in order to establish the present concentrations. After that, one can determine how much of the contaminants have to be removed and if necessary purification steps can be progressed.
_
Guidelines for the assessment and improvement of service activities relating to drinking water have been published in the form of International standards for drinking water such as ISO 24510.
Drinking water quality in European Union:
The EU sets legislation on water quality. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy, known as the water framework directive, is the primary piece of legislation governing water. The Drinking water directive relates specifically to water intended for human consumption .Each member state is responsible for establishing the required policing measures to ensure that the legislation is implemented. For example, in the UK the Water Quality Regulations prescribe maximum values for substances that affect wholesomeness and the Drinking Water Inspectorate polices the water companies.
Drinking water quality in the United States:
In the United States, the Environmental Protection Agency (EPA) sets standards for tap and public water systems under the Safe Drinking Water Act (SDWA). The Food and Drug Administration (FDA) regulates bottled water as a food product under the Federal Food, Drug, and Cosmetic Act (FFDCA). Bottled water is not necessarily more pure, or more tested, than public tap water. Peter W. Preuss, head of the U.S. EPA’s division analyzing environmental risks, has been “particularly concerned” about current drinking water standards, and suggested in 2009 that regulations against certain chemicals should be tightened. In 2010 the EPA showed that 54 active pharmaceutical ingredients and 10 metabolites had been found in treated drinking water. An earlier study from 2005 by the EPA and the Geographical Survey states that 40% of water was contaminated with nonprescription pharmaceuticals, and it has been reported that of the 8 of the 12 most commonly occurring chemicals in drinking water are estrogenic hormones. Of the pharmaceutical components found in drinking water, the EPA only regulates lindane and perchlorate. In 2009, the EPA did announce another 13 chemicals, hormones, and antibiotics that could potentially be regulated. The decision on whether or not they are sufficiently harmful to be regulated may not be decided upon until 2012 as it takes time for testing.
_
International Bottled Water Association:
The bottled water industry regulates itself through the International Bottled Water Association (IBWA). The IBWA sets manufacturing requirements, which help to ensure that bottlers meet FDA health standards. Bottled water producers who are members of IBWA are inspected annually by an independent laboratory, the National Sanitation Foundation. Through unannounced inspections, members are evaluated on compliance with the IBWA’s performance requirements and FDA Quality Standards.
____________
Cost of drinking water:
For all people, there is a cost involved in having water distributed to their home or community. Some costs are monetary, while others are measured in the time it takes to travel to and from a safe drinking water source. Monetary costs are common. Some people pay a municipality or private utility to distribute water to their homes. Others who lack this infrastructure pay the cost for water in other ways—by purchasing water from a community source, a water refilling station, a bottled water shop, or another source. Time-measured costs impact people with limited monetary funds who often take time out of their day to walk to a water source and retrieve clean water. The time spent fetching water represents a cost to human health, productivity, and in many cases, educational opportunities—a burden that is borne disproportionately by women and girls. In various locations, the costs of water supply are subsidized by government institutions. In some cases, this is an essential tool in providing water to poor communities; in other instances, it can lead to inefficient or wasteful use of the resource by those who do not fully appreciate its full cost.
_
Water no longer cheap:
Across the country, Americans are paying more for water than they did a decade ago, even as water utilities fall into debt and water infrastructure deteriorates, according to a recently released Columbia Water Center white paper. While a number of recent studies shine light on the alarming rise in water costs over the past few years, the Water Center’s analysis is one of the first to explore in detail national-level water utility survey data on water rates, operational costs, efficiency and debt. The study groups water utilities into six clusters based on the characteristic factors – financial, geographic, and demographic – that differentiate their water rates. The results highlight the high cost of water scarcity and the difficulty of managing supply in the face of variable climate conditions. According to the report, from 2000 to 2010, average water rates and debt load carried by water utilities rose by 23 and 33 percent, respectively, after adjusting for inflation. One-third of water utilities account for a disproportionate percentage of this increase, with both debt and rate increases of over 100 percent. Half of that top third reported that their debt had increased over 200 percent.
_
Lack of access to safe water is not a technical problem – it is a human, logistics, funding and efficiency issue. The world has the money to make it happen. The cost of just one case of bottled water could supply a person in Africa with clean, safe drinking water for a year! Every year over $100 Billion dollars is spent on bottled water world-wide. The United Nations estimates that if given just 1/6th of that money for one year, $15 billion, they could cut in half the number of people without access to clean water. In fact it would take 1/3 what the world spends on bottled water in one year to pay for projects providing water to everyone in need. It is estimated that lack of community involvement causes 50% of projects to fail.
____________
Water privatization:
Water privatization is used here as a shorthand for private sector participation in the provision of water services and sanitation. Private sector participation in water supply and sanitation is controversial. Proponents of private sector participation argue that it has led to improvements in the efficiency and service quality of utilities. It is argued that it has increased investment and has contributed to expanded access. They cite Manila, Guayaquil in Ecuador, Bucharest, several cities in Colombia and Morocco, as well as Côte d’Ivoire and Senegal as success stories. Critics however, contend that private sector participation led to tariff increases and has turned a public good into a private good. Many believe that the privatization of water is incompatible with ensuring the international human right to water. Aborted privatizations in Cochamamba, Bolivia, and Dar es-Salaam, Tanzania, as well as privately managed water systems in Jakarta and Berlin are highlighted as failure. It is estimated that 909 million in 62 countries or 13% of the world population were served by the private sector in one form or another. In Algeria, Saudi Arabia, Colombia and Cuba increasing efficiency and improving service quality were important motives for water privatization. External influences, such as from the World Bank and the International Monetary Fund (IMF), often play a role, as it was the case in Bolivia and in several African countries. In some cases, where access is already universal and service quality is good, fiscal motives dominate, as it was the case in Berlin, Germany, and in Chile.
_
Positive Impact of water privatization:
In almost all cases, water tariffs increased in the long run under privatization. A World Bank study argues that the most consistent improvement made by public-private partnerships in water supply was in operational efficiency. Private operators thus made a strong indirect contribution to financing by improving efficiency, making it possible for utilities to finance investments internally instead of having to rely on more debt. A before-after comparative study by the World Bank analyzes how access, quality of service, operational efficiency and tariffs have evolved under 65 public-private partnerships for urban water utilities in developing countries. The study estimates that “PPP projects have provided access to piped water for more than 24 million people in developing countries since 1990”. A study of water privatization’s impact on health, as measured by child mortality, found that between 1991–1997 in Argentina child mortality fell 8 percent more in cities that had privatized their water and sewer services compared to those that remained under public or cooperative management.
_
Negative impact of water privatization:
In October 1999, Aguas Del Tunari was awarded 40-year concession rights to provide water and sanitation services to the residents of Cochabamba in Bolivia. It was to generate electrical energy and irrigation water for the region’s agricultural sector. Following public outcry over 200% increase in water rates, the company was thrown out of Bolivia. In November of 2002, Aguas Del Tunari demanded a minimum compensation of $50 million from the Bolivian government, and dragged it to the International Centre for Settlement of Investment Disputes (ICSID), a mechanism of the World Bank. Nearly four years later, in January 2006, Aguas Del Tunari agreed to drop their case in ICSID for a token payment from Bolivia, which had by then spent more than one million dollars on legal fees alone. None of the nation-States appears to have drawn a lesson from what had happened in Bolivia and in the neighbourhood of Johannesburg, South Africa, where the policy of privatisation of water supply was tested in recent times. After water supply was privatized in Johannesburg, people became unable to pay their water bills. The private water supply agencies stopped supply of water, forcing residents to drink water from polluted rivers. This led to an outbreak of cholera claiming hundreds of lives and thousands of people getting hospitalized.
_
The corporatization of water:
In the documentary film Thirst, authors Alan Snitow and Deborah Kaufman demonstrated the rapid worldwide privatization of municipal water supplies, and the effect these purchases are having on local economies. Water is being called the “Blue Gold” of the 21st century. Thanks to increasing urbanization and population, shifting climates and industrial pollution, fresh water is becoming humanity’s most precious resource. Multinational corporations are stepping in to purchase groundwater and distribution rights wherever they can, and the bottled water industry is an important component in their drive to commoditize what many feel is a basic human right: the access to safe and affordable water.
____________
Water terrorism:
Water supply terrorism involves intentional sabotage to a water supply system, through chemical or biological warfare, or infrastructural sabotage. Throughout military history and the history of terrorism, water supply attacks have been perpetrated by eco-terrorist and political groups, intending to scare, cause death, or drought. In 1984 members of the Rajneeshee religious cult contaminate a city water supply tank in The Dalles, Oregon, using Salmonella and infecting 750. In 1992 The Kurdish Workers Party (PKK) put lethal concentrations of potassium cyanide in the water tanks of a Turkish Air Force compound in Istanbul. In 2000, workers at the Cellatex chemical plant in northern France dumped 5000 liters of sulfuric acid into a tributary of the Meuse River when they were denied workers’ benefits. In 2000 in Queensland, Australia, police arrested a man for using a computer and radio transmitter to take control of the Maroochy Shire wastewater system and release sewage into parks, rivers and property. While Sept. 11 focused immediate attention on drinking water, these are hardly new concerns. Poisoning the enemy is a long-standing military strategy. When Solon of Athens laid siege to Cirrha circa 600 B.C., he ordered that poisonous hellebore roots be placed in the local water supply, making the Cirrhaeans violently sick. In 1941, concerned over domestic attacks from Nazi or Japanese agents, J. Edgar Hoover warned about the vulnerability of water supply facilities “due to the strategic position they occupy in keeping the wheels of industry turning and in preserving the health and morale of the American populace.” There have not been any successful major attacks on American water supplies, but the threat and fear remain because their water supplies cannot be fully protected. The good news is that poisoning a water system is hard to do. Putting a few drops of cyanide in someone’s glass will lead to a gruesome death. Putting a few drops, or even a few barrels, in a reservoir is pointless. Reservoirs generally hold anywhere from 3 million to 30 million gallons of water. Even assuming one could back several trucks up to the reservoir and dump their loads without being detected, one would still need to get huge quantities of the poison in the first place.
_
Water shortages ‘foster terrorism’:
A lack of water is a key factor in encouraging terrorism, the Third World Water Forum in Kyoto has heard. Mona El Kody, the chair of the National Water Research Unit in Egypt, told delegates that living without an adequate level of access to water created a “non-human environment” which led to frustration, and from there terrorism. “A non-human environment is the worst experience people can live with, with no clean water, no sanitation,” Ms El Kody said. And she added that it was in Arab countries that this problem was at its most acute. The Middle East has only 1% of the world’s fresh water shared between 5% of the world’s population. This puts a tremendous strain on water resources in the region.
____________
Your Healthiest Drinking Water Options:
1. When facing muddy water
2. When traveling
3. When at home
_
How to purify Muddy Water:
One of the major problems we face during camping trips is finding clean water suitable for drinking and cooking. Even though you select a camping site near a water source, one can not be sure that the water is fit for consumption. Further, what can one do in the event of an unpredictable rain, muddying the entire water source…?
Aluminum Sulfate:
Aluminum Sulfate, Shortly known as Alum, when added to raw water reacts with the bicarbonate alkalinities present in water and forms a gelatinous precipitate. This floc attracts other fine particles and suspended material in raw water, and settles down at the bottom of the container. The water over this sediment is almost clean other than some fine particles dissolved in it. Alum is in a crystallized form which you can powder and store in a clean glass container.
Bleaching Powder Solution:
Bleaching powder or chlorinated lime is used to disinfect the water from bacteria. The chlorine present in the bleaching powder solution kills almost 90% of the bacteria present in water. Bleaching powder also known as Calcium hypochlorite is in a powdered form. Add one or two teaspoons of the powder in a glass bottle, add water and mix well. Use a metal cap for the container as it may corrode plastic cap.
Both Alum and Bleaching powder are commonly available in most of the grocery stores.
_
Note:
As discussed earlier, pouch of having nutrient sugar and electrolyte covered by forward osmosis membrane can imbibe pure water from muddy water over 10 to 12 hours to give you a tasty healthy drink.
_
Drinking Water Safety while travelling:
The most common cause of water-borne illness is bacteria, such as E. coli, cholera and salmonella, but illness can also be caused by protozoa (including giardia and cryptosporidium), viruses (like hepatitis A, polio and rotavirus) and chemical pollutants. In many cases, travelers become ill simply because the pathogens in the water are foreign to their immune systems, while locals have adapted to the water supply and can drink it without problems. The best way to protect yourself is to avoid local tap water and instead seek out bottled water; when that’s not available, boiling tap water generally kills most micro-organisms, and there are a number of good water filters and purification tablets that can easily be stowed in your carry-on. Boiling water is generally the most effective way to remove parasite contamination. Maintain a rolling boil for at least one minute (longer at higher altitudes, where the boiling point may be lower). Let the water cool itself slowly without adding ice. Allow any sediments and particles to settle before drinking, and then decant the water from the top into another container. Commercially available iodine or chlorine tablets kill bacteria and viruses, but are ineffective against some protozoa (like cryptosporidium). Iodine is the more effective of the two solutions, but is not recommended for long-term use, especially by pregnant women or travelers with a history of thyroid problems. Potable Aqua, composed of the iodine compound tetraglycine hydroperiodide, is the most popular brand of water purification tablet. The company also offers chlorine dioxide tablets, which are effective against cryptosporidium as well as the other organisms killed by iodine. Read directions on all tablets systems for tablet-water ratios and dissolving times; 20 minutes or more may be required for the tablets to dissolve completely, especially in colder water. If you do not have tablets, two drops of common chlorine bleach in a quart of water will help as a last resort. Outdoor stores like Cabela’s carry water filters and purification systems. It is essential that the filter system you choose is suited to your needs. A filter with an insufficiently small pore size, or one that is not designed to filter viruses, may permit some contaminants to get through. The most effective strategy is to buy a system that combines filtering with chemical purification — or make one yourself by using both a filter and an iodine treatment. The SteriPEN uses ultraviolet light to kill bacteria, viruses and protozoa. It’s portable, effective and powered by AA lithium batteries, making it convenient to bring just about anywhere. The relatively steep price tag is worth it if you frequently visit areas with questionable water quality, particularly if you plan an extended stay. The device is designed for use only on clear water — particulates or discoloration can block UV light — so you may need to filter the water or let sediment settle to the bottom before using the SteriPEN.
_
Note:
Emergency Disinfection of Drinking Water during disasters employ above mentioned water treatment methods. Also, a typical home water heater can provide between 30 and 60 gallons of clean drinking water during a disaster. Hurricanes, floods, earthquakes, and other power outages may prevent you from having many things, but clean drinking water should not be one of them.
_
At home:
The most economical and environmentally sound choice you and your family can make is to purchase and install a water filter for your home. Your best bet for ensuring good health (and protecting the environment), is to filter your own water at home using a reverse osmosis (RO) filter. Do not make the mistake of thinking you can tell if your water is safe or not by the way it looks, tastes, or smells. As discussed earlier, RO alone is not sufficient to remove microorganisms, therefore RO need to be combined with UV disinfection or chlorine disinfection.
______________
Just installing a filter to purify your drinking water may not be enough. You could still be exposed to contaminated water when you:
1. Shower or bathe
2. Wash your hands
3. Wash laundry
4. Rinse fruits and vegetables
5. Wash dishes, glasses, and other utensils
6. Brushing your teeth
_
What about tap water whose quality is poor: you would not drink it but what about other uses?
Can I use my tap water for cooking?
No, any water used for food preparation or cooking needs to be from an acceptable source or boiled first.
What if I am boiling my water as part of the cooking process?
It is more protective to boil the water first, to prevent the potential for inadequate heating. The cooking process should bring the water to a full rolling boil for at least one minute before adding the food item (for example, making pasta). If the water will be at a slight boil for a long time, then this will also be protective. For example, you may be cooking beans or boiling chicken for 10 – 20 minutes.
How should I wash fruit and vegetables and make ice?
Fruits, vegetables, and any other foods that will not be cooked should be washed and rinsed with boiled (and then cooled) water or water from an acceptable source. Similarly, ice should be made with either boiled water or water from an acceptable source.
Can I use my water for making baby formula or drinks?
No, not without precautions! Any water used for baby food, formula, or making beverages must be boiled (and then cooled) or be from an acceptable source.
Is potentially contaminated water safe for washing dishes?
Hand-washed dishes: No! Use boiled (then cooled) water, water from an acceptable source, or after washing with dish detergent rinse for a minute in a dilute bleach (1 tablespoon of unscented bleach per gallon of water). Allow dishes, cutlery, cups, etc. to completely air dry before use.
Home dishwasher: Yes, if the hot wash is at least 170 deg F and includes a full dry cycle. However, most home dishwashers do not reach this temperature. If you are uncertain of the temperature of your dishwasher, rinse in dilute bleach and completely air dry as described for hand washed dishes.
Commercial dishwasher: Yes, if it is manufactured and operated with a heat sanitizing rinse set at 170 deg F that lasts for at least 30 seconds.
Is potentially contaminated water safe for washing clothes?
Yes, it is safe to wash clothes in tap water as long as the clothes are completely dried before being worn. However, increased turbidity that sometimes occurs during a boil water event may discolor clothing, especially whites.
Can I brush my teeth with the water without boiling it?
No! Any water you ingest or place in your mouth should be disinfected by boiling (and then cooled) or come from an acceptable source. Bottled water is excellent for brushing your teeth.
Is potentially contaminated water safe for bathing and shaving?
Your water may be used by healthy individuals for showering, bathing, shaving, and washing as long as care is taken not to swallow water and avoid shaving nicks. To minimize the chance of infections, people with open wounds, cuts, blisters or recent surgical wounds and people who are immunocompromised or suffer from chronic illness should use boiled water (then cooled) or water from an acceptable source. Children and disabled individuals should be supervised to ensure water is not ingested. Sponge bathing is advisable, and bathing time should be minimized to further reduce the potential for ingestion.
How should I wash my hands from untreated tap water?
Generally, vigorous hand washing with soap and your tap water is safe for basic personal hygiene. If you are washing your hands to prepare food, you should use boiled (then cooled) water, bottled water, or water from acceptable source for hand washing.
Is the water safe to give to my pet?
To be certain, give them water that has been boiled then cooled or water from an acceptable source. Many pets regularly drink some pretty bad water, but pets come in a wide variety with variable resistances to pathogens. Many pets are vulnerable to the same diseases that humans can get from contaminated water and can spread these diseases into the environment or pass them on to their owners.
______
Water and making food:
_______________________
__________________________
Frequently asked questions on drinking water (FAQ):
_
Are there significant health effects from drinking hard water versus soft water?
Hard water containing magnesium may prevent sudden cardiac arrest and soft water may prevent kidney stones.
_
Are we running out of water on earth?
No, we are not running out of water but shortages of drinking water are becoming more common in some areas. Globally we have sufficient fresh water to satisfy the need for drinking water, but frequently it is not located where the high-use areas are. Thus, localized water shortages occur. Furthermore, droughts (below-normal rainfall), often lasting several years, worsen water shortages in some areas. In many developing nations, population is growing more rapidly than the development of drinking water sources.
_
Based on some media reports, tap water certainly doesn’t sound safe, yet public water suppliers say it’s okay. Is it really safe?
Yes and no. Yes for most developed nations and no for most developing nations. Some media reporters embellish potential risks in their stories to draw a bigger audience. Some journalists tend to sensationalize because of the competitive nature of news organizations today. They are in business to make money too.
_
Are there situations where the chloramines used for secondary disinfection of drinking water need to be removed from the water to make it safe for specific uses?
Yes. Water disinfected by chloramines is safe for people and land animals to drink, safe to cook with, bathe in, water the garden and use for most other general purposes. However water containing chloramines should not be used in dialysis machines, fish aquariums, as drinking water for reptiles, or for businesses requiring highly treated chemical free water.
_
Can stagnation in potable drinking water storage tanks cause problems?
Yes. Stagnation can cause a variety of problems including loss of residual chlorine, auto-decomposition of chloramine that can lead to nitrification problems, high heterotrophic plate counts, thermal stratification, and even foul-smelling, bad-tasting water at the tap. A number of companies now manufacture special circulators that can prevent potable water tank stagnation.
_
Are graves in a cemetery considered a potential threat to groundwater contamination and water safety in private wells?
Yes and private wells should be drilled a safe distance from such potential groundwater pollution sources. Most health departments recommend a setback distance of 50 to 100 feet from graves and cemeteries.
_
Can water from a private drinking water well have a special taste or odor due to metal content?
Yes. Metals such as iron and copper (usually due to acidic water conditions) can impart a metallic taste (bitter for copper) and even a faint metallic odor (sometimes associated with presence of iron bacteria) to those with a very sensitive nose.
_
Are bottled sports drinks classified as bottled water?
No. These drinks are designed to enhance athletic performance by replacing fluids, electrolytes and sugars burned up during athletic activities. They should not be used on a regular basis to replace everyday water losses.
_
Are the containers for single-serve bottled water made of a recyclable plastic?
Yes, most of these bottles are made of polyethylene terephthalate (PET), one of the most common of polyester plastics. This is a recyclable plastic that can be used to make synthetic fiber for carpets, sweaters and many other products.
_
Are there environmental issues associated with the bottled water industry?
Yes. There are three environmental concerns associated with the bottled water industry. One concern is the billions of empty bottles that are improperly disposed of and thereby cause waste management problems. Another concern is all the energy expended as transportation costs for shipping bottled water. Much of this energy consumption which leads to release of toxic and greenhouse gases to the atmosphere could be saved by simply pumping treated drinking water through pipeline distribution systems. The manufacture and disposal of plastics used for bottled water causes toxic chemicals to be released to the environment, some during manufacturing processes, some to the water, some in waste disposal and some from improper waste burning. As of 2003, it has been estimated that the bottled water industry uses 1.5 million tons of plastic annually to package water in the U.S. This plastic is recyclable but much of it is not.
_
Can bottled water become contaminated during storage?
Most bottled water is sold in plastic bottles and there is mounting concern by some health professionals over this. Strictly speaking, most plastics have the potential of allowing transport of gasses through them. This means that if left standing long enough in an atmosphere that contained vapors from some undesirable source, the water inside the bottle may start to show the presence of these materials. If for instance bottled water was stored in a warehouse with industrial solvents for a period of time, these solvents could be found in the water. There are studies showing leach of toxic chemicals from plastic itself in water. Also it generally doesn’t contain a disinfectant, so microbes grow in it over time.
_
Does drinking bottled water with added oxygen provide enhanced athletic performance?
No, this is nothing but a drinking water scam. There is no credible evidence that oxygenated water provides any benefit based on studies by scientists at several major universities.
_
How do I know my bottled water is safe?
There are so many bottled water in market but only few are good. You must know which one is good. Try to find out source of water and whether water treatment processes like reverse osmosis, filtration, ozonisation, UV disinfection, etc have been used.
_
How does the quality of public tap water and bottled water compare?
In most developed nations, tap water is good and at the most you may add RO system at home. So quality of tap water and bottled water is comparable. In developing nations, most tap water is contaminated and only few bottled water is good. Bottled water is not necessarily safer than tap water in developed nations while bottled water is definitely safer than tap water in developing nations provided you have chosen the right brand.
_
How much does bottled drinking water cost in comparison to an equal volume of tap water?
Some bottled waters cost more per gallon than gasoline and may sell for more than 1000 times the cost of an equal amount of drinking water supplied by a public utility.
_
How much petroleum is consumed in producing plastic for bottled water sold in the United States?
It took more than 1.5 million barrels of oil to produce the amount of plastic used in containers packaging the bottled water consumed in the U.S. in 2006. This amount of oil would have been adequate to fuel 100,000 cars for that year.
_
Is drinking water purchased from a vending machine safe to drink?
It should be as safe as other beverages purchased from vending machines. The treatment of vending machine water is based on sound scientific principles. Treatment such as reverse osmosis, activated carbon adsorption, and ultraviolet light disinfection are often used in vending machines.
_
Is heavy consumption of non-fluoridated bottle water contributed to an increase in dental cavities among young children?
No, fluoride in water is neither necessary nor effective for preventing tooth decay.
_
Some hospitals are now providing commercially bottled mineral water to their patients under the assumption that this water is safer to drink than community tap water. Is this true?
Immunocompromized patients are better on bottled water rather than tap water provided the bottled water manufacturer is complying with drinking water standard of WHO or national standard.
_
What is the proper way to store bottled water?
Bottled water should be stored in a cool (i.e. room temperature), dry environment away from chemicals such as household cleaning products and away from solvents such as gasoline, paint thinners and other toxic materials.
_
Can drinking water too fast be unhealthy?
Yes, because it can lead to water intoxication, usually caused by drinking too much water in a short period of time. You are unlikely to suffer from water intoxication, even if you drink a lot of water, as long as you drink it over time as opposed to an enormous volume at one time.
_
Can I tell if my drinking water is safe to drink by just looking at it, tasting it, or smelling it?
No. None of the chemicals or microbes that could make you sick can be seen, tasted, or smelled.
_
Can iron in tap water help supply a part of person’s dietary requirement for iron?
Yes. In fact, most tap water in the United States supplies approximately 5 percent of the dietary requirement for iron for an adult.
_
Compared with surface water, is groundwater safe for human consumption?
Groundwater is generally safer than surface water for drinking because of the filtration and natural purification processes that take place in the ground. These processes become ineffective, however, when sewage, fertilizers, toxic chemicals, and road salt, seep into the ground. Household, commercial, and industrial wastes that end up in dumps, waste lagoons, or septic systems can pollute groundwater. Acid rain also threatens to recharge aquifers with contaminated water. Generally, groundwater is not as easily contaminated as surface water, but once it is contaminated, it is much more difficult to clean up because of its relative inaccessibility.
_
Could icebergs be used as a source for drinking water?
Yes. Even though icebergs are floating in salt water, the ice has no salt. It’s compressed snow. If you melted an iceberg you would get drinkable fresh water after you killed any germs. Icebergs have never been used as a major source of drinking water because of the costs and risks associated with moving them.
_
Does the amount of water a person drinks affect their risk level for coronary heart disease?
Possibly. Some research conducted since 1999 indicates that there is a good correlation between fluid intake level and coronary heart disease. Whole blood viscosity, plasma viscosity and blood fibrinogen level are considered to be independent risk factors for coronary heart disease, and they all tend to be elevated by dehydration. Therefore, healthy people who drink adequate levels of plain water generally keep their hydration level up and have a reduced risk of dying from a heart attack in comparison to people who do not drink adequate water.
_
How can I tell if I am drinking enough water or getting enough water in my diet?
If you urinate 4 times a day with pale yellow color of urine and you are quenching your thirst regularly, you are well hydrated.
_
How much water does an adult need each day?
You need at least 1200 ml of water for sedentary life plus extra water for exercise, hot weather, illness, pregnancy, lactation etc.
_
Is blue-green algae affected water safe to drink after it has been boiled or filtered?
NO. The water needs to be filtered through activated carbon to remove any toxins. Toxins will not be removed by boiling, normal filter systems or adding household disinfectant.
_
My water often looks cloudy when it comes from a faucet but it clears up. What causes this?
The cloudy water is caused by tiny air bubbles in the water similar to the gas bubbles in beer and soda pop. After a little while, the bubbles rise to the top and are gone.
_
Should I drink water even when I do not feel thirsty?
No. Thirst is the best biological signal of water deficit. Also, you drink lot of water through food and beverages like soda, tea, coffee and beer. Yes, if due to old age, illness or drugs; your thirst mechanism is impaired or inadequate, then you should drink water irrespective of thirst.
_
Should I soften my water?
Not unless hardness is at least 5 grains per gallon (85.5 mg/L). Many professionals do not recommend softening unless hardness is 6 to 8 grains per gallon. By most accounts, there appears to be little scaling until hardness exceeds a level of 5 grains per gallon.
_
Should softened water be used to prepare baby food or baby milk from evaporated milk?
It depends on the level of sodium in the softened water. In areas where very high hardness has been softened by ion exchange with sodium and sodium content in finished water is at the maximum recommended level of 250 mg/L, this water should not be used as source water for drinking or baby food preparation. Some medical specialists are now recommending that softened water should contain no more than 20 mg/L of sodium if used for baby food and milk preparations.
_
Is ozone a good disinfectant for the bottle water industry?
Yes. Ozone generation technology has been a big boost for the bottle water industry. It is very effective when used in conjunction with other treatment methods that remove natural organics and inorganics such as bromide. The U.S. Food and Drug Administration (FDA) recommends using a minimum treatment of 0.1 mg/L of ozone in water in a closed system with a contact time of at least five minutes for sterilizing both containers and water until the containers are adequately sealed. What ozone is left in containers after sealing will convert back to regular oxygen in the water in a matter of minutes. Ozone is very efficient in killing bacteria, fungi, viruses and even Cryptosporidium spores and oocysts if they are present in a water source.
_
Can pathogens in water be eliminated by disinfection through use of solar radiation?
Yes. The lack of safe drinking water in many developing countries has prompted research into simple methods of disinfecting small quantities of water. One such investigation at the University of Beirut in Lebanon revealed that 99.9% of total bacteria in a water sample could be destroyed by 300 minutes exposure to direct sunlight. In effect this means that if you left a sample of water in a translucent container, a lot of the bacteria in it would be killed. Research to date has concentrated on transparent PET (polyethylene terephthalate) bottles, these being more robust than glass bottles and hence more practical for use in rural areas. It is important to first remove any particles in the water which may harbor or shield pathogens from the sunlight. Removal is effected by allowing any solids to settle out by sedimentation. It has been found that inactivation of pathogens is more effective if the water is fully oxygenated. UV radiation from the sun reacts with the oxygen molecules in the water and, together with the heat from the sunlight, inactivates the pathogens.
_
Is water safe to drink with some chlorine in it?
Yes. Many tests have shown that the small amount of chlorine found in treated drinking water is safe, although some people object to the taste. To further ensure the safety of drinking, all utilities must keep the residual level of chlorine below 4 mg/L in public drinking water supplies.
_
Should I run the water some before drinking the water from the carbon filter on the end of my faucet?
Yes, it is advisable to run the faucet tap for at least 20 seconds before filling a drinking water container from all drinking water filter cartridges that contain carbon. This will flush out any very fine black carbon powder that may be present.
_
Does reverse osmosis (RO) treated water taste like distilled water?
Not to most people. Distilled water contains practically no minerals or dissolved solids, whereas RO water usually contains trace amounts of minerals and salts unless it has been purified using high pressure hyperfiltration RO units. Thus, people generally report that water from line-pressure RO units, those most commonly used in the home, tastes somewhat better than distilled water. Distilled water has essentially no taste or what is commonly referred to as a flat taste.
_
Is hyperfiltration (HF) membrane filter technology the same as reverse osmosis (RO)?
Yes, hyperfiltration (HF) membranes are the ones commonly known as reverse osmosis (RO) membranes. These membrane systems are capable of removing contaminants down to 0.0001 micron in size.
_
Will boiling water get rid of all contamination?
No, boiling water kills most pathogens but spores and toxins of bacteria are unaffected. Many non-bacterial pollutants, such as nitrates, do not boil out of the water. In some cases, boiling can concentrate pollutants because boiling reduces the volume of water remaining.
_
Are bottled water companies draining water supplies?
According to a 2005 study by the Drinking Water Research Foundation (DWRF), annual bottled water production accounts for less than 0.02% of the total groundwater withdrawn in the United States each year. Additionally, based on information gathered in the DWRF study, in 2001, 87% of the water withdrawn by bottled water companies, on average, was actually bottled for consumption by humans, so the bottling process is a very efficient one. At 55 billion gallons per day, the largest user of groundwater is the agriculture industry. That amount equals 68% of total groundwater extracted in 2010. The second largest user of groundwater is public drinking supplies, which takes 16 billion gallons per day, or 19% of all U.S. groundwater extraction. Compared to those figures, bottled water barely registers on the radar.
_________________________________
Some Myths about drinking water:
_
Myth 1: Drinking water during meals is bad for digestion.
Fact: This is one line that has been passed down from generation to generation. However, there is no scientific evidence to indicate that it will affect the digestive process. Drinking water with meal neither reduces pH of stomach acid nor dilutes digestive enzymes. At the most, it will probably fill you up and reduce your appetite for food. But besides that, go ahead and enjoy a glass of water with every meal.
_
Myth 2: Water cleans out the body’s toxins.
Fact: The toxins in our body are filtered out by the kidneys. And common myth says that drinking more water means clearing out the toxins. Wrong! In truth, drinking large amounts of water will actually reduce the kidneys’ ability to function as a filter. Stay well hydrated but don’t overdo it.
_
Myth 3: Drink water for healthy skin.
Fact: It is widely believed that since our body’s composition is 60% water, drinking a lot of water will give you glowing skin. However, there is little evidence to support this idea. Healthy skin is a result of many things, including diet, weather, pollution and genetics. However, when you get dehydrated, your skin loses this cushion and lies flatter on the body. Dried-up skin is a great way to accentuate wrinkles and sags. Stay well hydrated.
_
Myth 4: If you’re thirsty, you are already dehydrated.
Fact: Thirst begins when osmolarity of plasma has risen by less than 2 percent, whereas most experts would define dehydration as beginning when plasma osmolarity has risen by at least 5 percent.
Myth 5: Most bottled water is just tap water in bottle.
Fact: It is important to note that purified bottled water is not just tap water in a bottle. Once the municipal source water enters the bottled water plant several processes are employed to ensure that it meets the purified standard of the nation or WHO standard. These treatments can include filtration, ozonation, reverse osmosis, distillation, or de-ionization etc. The finished water product is then placed in a bottle under sanitary conditions and sold to the consumer.
_____________________
________________________
The moral of the story:
_____________________
1. ‘No water, no life’ is the dictum for the kind of life-form on earth.
_
2. A human cannot survive for more than 3 to 5 days without drinking water.
_
3. Clear colorless odorless water with good taste may contain toxic metals, toxic chemicals and pathogenic microbes. So you should not be misled by physical characteristics of water for drinking purpose.
_
4. Water for drinking must be clear, colorless, palatable and safe microbiologically & chemically, with no risk of short or long term harms.
_
5. 80 % of the sickness accounted all over the world is water related and as a consequence, over 30% of work productivity is lost. Overall, water related diseases cause death of 2 to 5 million people each year worldwide.
_
6. Due to overpumping of groundwater, we are now feeding ourselves with water that belongs to our children.
_
7. Worldwide up to 60 percent of drinking water is lost due to leaky pipes and leaky faucets.
_
8. There are three billion people worldwide who lack safe drinking water and I am one of them. UN’s boasting of hitting drinking water target in 2013 appears to be hype.
_
9. Children are more vulnerable to contaminated water because their immune & detoxification system are developing and they consumes 3 times as much water per pound of body weight as compared to adults, so they get much bigger dose of the contaminants through water.
_
10. Every adult human must drink at least 1200 ml of water to sustain life either in the form of plain water and/or through foods and beverages. The requirement of water would increase depending on climate, physical activities, high altitude, concurrent illnesses, elderly, pregnancy and lactation. If you have taken high salt food, drink extra-water to help excrete extra-salt by increasing urine output.
_
11. A normal human must drink water sufficient to quench thirst and ensure that he/she should urinate 4 times a day and the urine should be of light/pale yellow color. However, moderate yellow urine does not necessarily reflect water deficit.
_
12. It is a misconception that majority of population have a chronic water deficit that has to be compensated by forcing water intake because thirst is a poor and late sign of water deficit. Barring elderly population, thirst is a reliable and timely sign of water deficit in humans. Yes, certain medications and certain illnesses do affect thirst.
_
13. Elderly population is more prone to dehydration due to impaired thirst mechanism and reduced renal concentrating capacity.
_
14. Staying well hydrated can help maintain overall health and may thereby help avoid serious health outcomes.
_
15. Water deficit or water excess of less than 2 % of body weight is tolerated well by healthy humans.
_
16. Water deficit of more than 2 % body weight does harm human body; it reduces mental ability, impairs kidney function, reduces muscle strength, decreases skin turgor and increases wasteful toxins in body. However, conversely, to say that drinking excess water on daily basis would improve mental functions, improve kidney functions, improve muscle strength, improve skin tone, relieve headache and remove toxins from body is illogical, unscientific, unsubstantiated and overboard. It is akin to saying that oxygen is essential for survival and therefore every normal human must take extra oxygen for better health by keeping oxygen cylinder at home. Yes, drinking excess water on daily basis is helpful in people suffering from kidney stones to prevent future stones but drinking excess water on daily basis by normal people to prevent future kidney stones is overboard. Hypercalciuria, or excessive urinary calcium excretion, occurs in about 5-10% of the population and that segment of population would benefit from high water intake to prevent future kidney stones. Prevention of dehydration is not synonymous with drinking excess water. Drinking excess water to prevent dehydration is akin to overeating to prevent malnutrition.
_
17. Chronic daily intake of excess water would increase circulatory blood volume on daily basis albeit intermittently and thereby increase workload on heart and kidneys even in normal humans. For average healthy people, more water does not seem to mean better health. A high water intake is inconvenient due to increased frequency of urination, expensive for people who drink bottled water, and will increase one’s exposure to pollutants, especially if the high intake is sustained over years.
_
18. Normal kidneys with normal anti-diuretic hormone (vasopressin) mechanism are capable of producing maximum of 10ml/min urine resulting in 600 ml urine every hour. So you can get water intoxication only if you drink water faster than 600 ml per hour and you are not losing excess water from sweat, respiratory tract or gastrointestinal tract. Remember severe stress will increase vasopressin levels thereby reducing kidney’s excretion capacity.
_
19. Since osmolarity of water is much lower than sports drink, plain water is absorbed faster than sports drink during exercise. Drinking only sports drink during exercise or marathon would replenish water deficit but plasma osmolarity would not fall due to high osmolarity of sports drinks resulting in persistent thirst and higher vasopressin level. Stress due to marathon may also release vasopressin. Persistent thirst will lead to overdrinking fluid and higher vasopressin leads to reduced renal excretion of water; both together may lead to water intoxication. I strongly recommend drinking plain water during exercise or marathon either alone or alternating with sports drink.
_
20. While exercising you must drink water commensurate with water loss through sweating and drink to your thirst.
_
21. One must always quench thirst with plain water rather than soft drinks or fruit juices as all of them are having high osmolarity and drinking highly osmolar fluid will not reduce plasma osmolarity resulting in insatiable thirst, higher vasopressin level and possibility of over-hydration.
_
22. We have to unlearn the idea that water has no effect on blood pressure; that is what all medical students have been taught. Drinking water in humans increases sympathetic nervous system activities which can be useful in rapid relief of symptoms resulting from orthostatic hypotension and preventing fainting attacks in blood donors.
_
23. One glass of ice-cold water burns 8.8 Kcal and in a diet of 2000 Kcal, it is insignificant when considered as weight loss strategy. On the other hand, I have seen many people in India who develop sneeze, cough and sore throat after drinking ice-cold water.
_
24. There is evidence to show that drinking two glasses of water before meals can help mild weight reduction in obese people by reducing appetite, feeling fuller stomach, consuming fewer calories, burning more calories and substituting sugary beverages.
_
25. Drinking water at certain times of the day will not provide the specific health benefits. Drinking water is not necessary for digesting food. Moderate water intake during meal will not dilute stomach acid and will neither help nor harm digestion of food. However, instead of using saliva to chew food in mouth, when you gulp it down with water, food digestion is slightly affected as salivary digestive enzymes are bypassed.
_
26. Generally speaking, higher the level of TDS (total dissolved solids) of drinking water, higher the degree of hardness. However TDS is not synonymous with hardness. Water softener would make hard water soft but TDS will not reduce.
_
27. The big misconception is that we obtain enough minerals from our drinking water. This is not true because the main source of minerals is always from our food and not from our drinking water. Even though minerals in drinking water exist in inorganic form that is not assimilated in human body to a great extent, magnesium in hard water may prevent sudden cardiac death and calcium in hard water may promote kidney stones and calcification of arteries including coronary arteries. Therefore no general guidelines can be issued for health benefits of hard water.
_
28. Distilled water having flat taste and zero TDS is hungry and aggressive water capable of dissolving deposited salts from hardened arteries & kidneys and toxins & metabolic wastes from tissues & blood, and consequently promoting their excretion via kidneys. There is no contraindication to drinking distilled or ultrapure water in healthy humans. I have seen many personnel working in dialysis unit taking ultrapure water home for drinking and cooking for years without any harm. In fact, in my view, distilled or ultrapure water may confer health benefits. My recommendation is contrary to WHO that recommended that the minimum TDS in drinking water should be 100 mg/L and drinking distilled water is harmful.
_
29. Despite the axiom that every human on this planet needs drinking water to survive and that water may contain many harmful constituents, there are no universally recognized and accepted international standards for drinking water.
_
30. All waterborne disease outbreaks are preventable if safe drinking water is supplied to population.
_
31. Drinking water contamination by microbes is a norm in developing nations rather than exception.
_
32. Viruses survive for days and bacteria survive for months in nutrient-free distilled water. Also water without disinfectant will grow bacteria if it is still water and not moving water. This proves the point that drinking water must be disinfected no matter how clean and safe the water source may be.
_
33. Even though boiling water for 1 minute will kill most pathogens, spores and toxins generated by bacteria are not inactivated by boiling. Also chemical contaminants and toxic metals would not be removed by boiling. So filtering with activated carbon filter followed by boiling can neutralize most pathogens and pollutants to make safe drinking water. Activated carbon is also effective in removing bacterial toxins unaffected by boiling. However, activated carbon provides surface that bacteria needs to adhere in order to grow into colonies and it removes chorine which will further help bacterial colonization; therefore point-of-use household carbon filters must be periodically replaced and filtered water must be disinfected.
_
34. Chlorination is effective against many pathogenic bacteria, but at normal dosage rates it does not kill all viruses, cysts, or worms. Even though UV light disinfection kills most of the microorganisms, it is not effective against cysts of protozoa. Ozone is powerful water disinfectant killing most of pathogens including cysts but leaves no residual disinfectant in water resulting in recontamination in the distribution and storage system. Reverse osmosis can remove most of heavy metals, toxic chemicals, TDS and turbidity but not recommended for clearing microorganisms. Therefore multiple barrier protection by combining various methods is the best way to purify water.
_
35. Solar disinfection of water is inexpensive, effective, and acceptable method of increasing water safety in developing nations and 99.9% of bacteria in a water sample could be destroyed by 300 minutes exposure to direct sunlight.
_
36. Forward osmosis is the best method to provide safe water to victims of disasters wherein a 4-inch by 6-inch pouch filled with electrolytes and nutrients covered with semi-permeable membrane is used. The pouch has be immersed in any dirty water for 10 to 12 hours; and water molecules from dirty water will move into the pouch by forward osmosis and very palatable safe nutrient drink is ready for the victim. Such forward osmosis pouches can also make drinking water from urine, greatly extending the ability of hiker or soldier to survive in arid environments.
_
37. There is overwhelming evidence to show that swallowing fluoride through drinking fluoridated water is neither necessary nor effective for preventing tooth decay. In the United States, the claim by Centers for Disease Control that water fluoridation is one of the top 10 public health achievements of the twentieth century appear hollow. I request President Obama to immediately ban drinking water fluoridation in the U.S. In fact, I am shocked to know harm done by fluoride of drinking water. The wisdom of America as a nation is challenged by water fluoridation.
_
38. Most bottled water sold in third world countries are of sub-standard quality but then their tap water is even worse.
_
39. 90 percent or more of the cost paid for bottled water goes for bottling, packaging, shipping, marketing, retailing, and profit, but not for the water itself.
_
40. It would take 1/3 what the world spends on bottled water in one year (100 billion dollar) to pay for projects providing water to everyone in need.
_
41. It is the company that maintains quality of bottled water is important rather than making sweeping charge that all bottled water is contaminated. It is the duty of governmental regulatory agency to ensure that bottled water is of nation’s drinking water standard. There are some brands of bottled water that maintains excellent water quality.
_
42. 25 to 40 % of all bottled water is tap water further treated or not depending upon integrity of company as well as haphazard work of regulatory agencies.
_
43. The logic put forward by the International Bottled Water Association (IBWA) that stored bottled water is not an adequate substrate for pathogens responsible for food-borne illnesses as it has no nutrients for pathogens to grow is fuzzy. Various studies have shown that pathogens exist in many types of bottled water and bacteria can remain viable for months even in distilled water.
_
44. There is a vicious cycle of bottled water consumption; people throw plastic of bottled water in litter, over years harmful chemical from this plastic would leach into ground water contaminating it and then we would need to purify groundwater to remove these contaminants resulting in making more bottled water and the vicious cycle continues.
_
45. Bottled purified water sold in glass/aluminium bottles is much better than plastic because of potential of chemicals leaching from plastic into drinking water and adverse environmental impact of plastic.
_
46. The impact of clean water technologies on public health in the U.S is estimated to have had a rate of return of 23 to 1 for investments in water filtration and chlorination during the first half of the 20th century. Developing nations must follow the suit.
_
47. Safe drinking water is not only linked to human health but also linked to education, empowerment of women, productivity, poverty, hunger, HIV and economic development. The average distance that women in Africa and Asia walk to collect drinking water is 6 kilometers. The time spent fetching water represents a cost to human health, productivity, and in many cases, educational opportunities—a burden that is borne disproportionately by women and girls. By providing safe drinking water at home, these women and girls would have better education, better health and better productivity. Consequently the entire family would move to higher socio-economic class thereby the entire nation would become a developed nation.
______________
Dr. Rajiv Desai. MD.
March 7, 2014
______________
Postscript:
Since most tap/well water is contaminated in developing countries, the best water purification method for your home in a developing country is to install simple activated carbon filter (replaced periodically) followed by boiling filtered water for 1 minute. This will take care of most of drinking water contaminants at the lowest cost. For poor people, carbon filter can be made at home by using crushed charcoal and filtered water can be disinfected using solar radiation for 300 minutes in a transparent container. Carbon filter with water heating is the best combination to purify water at the lowest cost for poorest people. Water purification is one market where spending big bucks does not correlate to getting the best. Reverse Osmosis filters are overkill, waste too much water, consume a lot of electricity, and tend to be expensive.


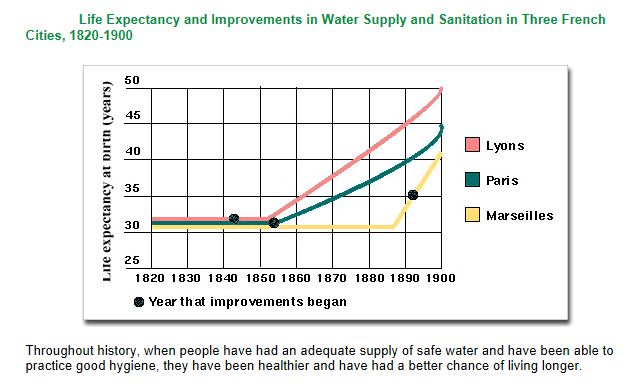
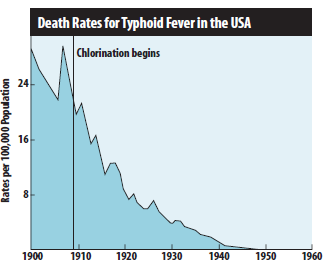
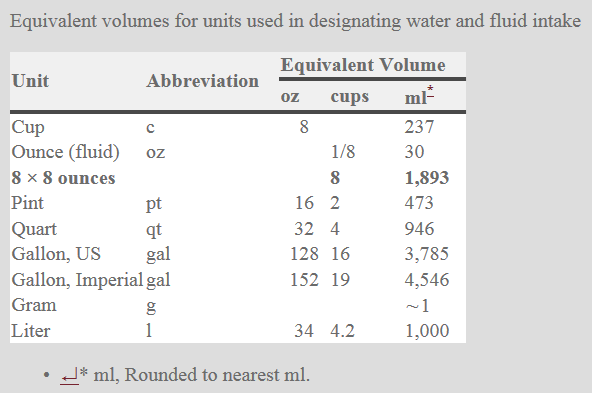

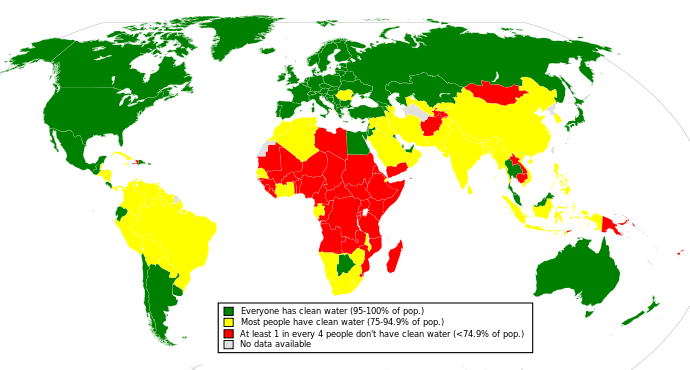
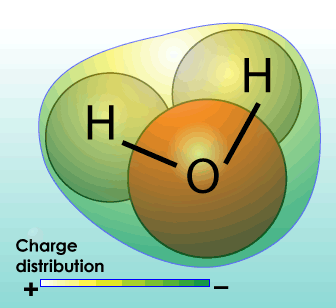
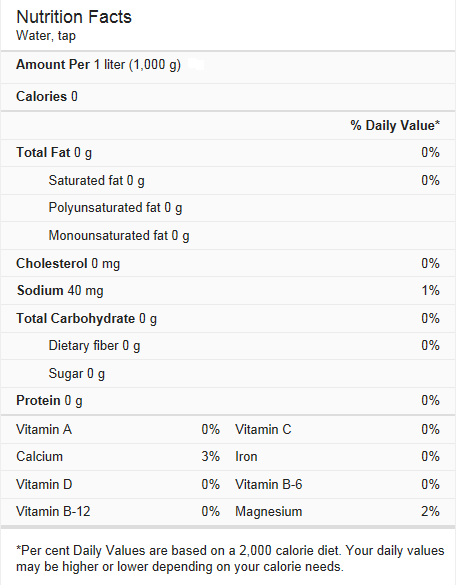
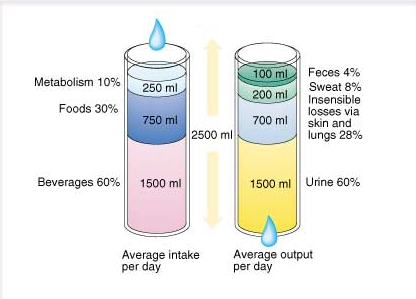
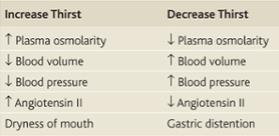
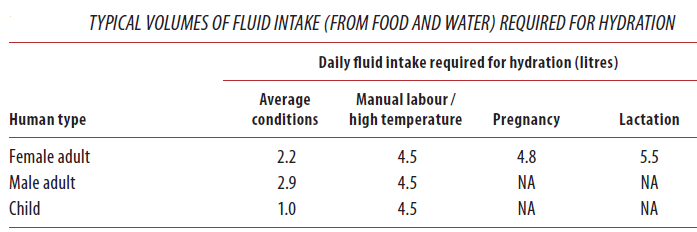
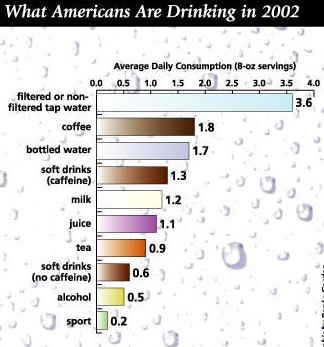
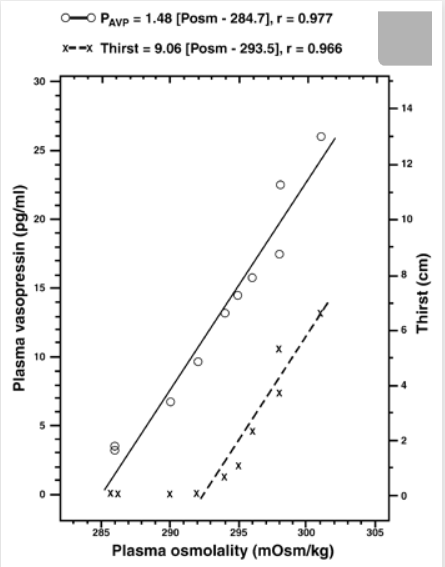

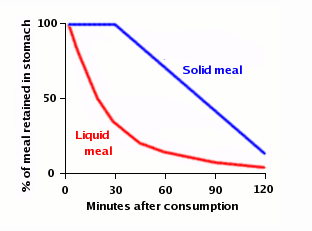
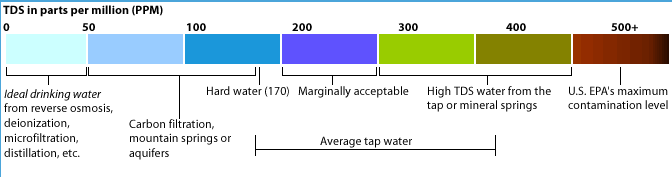

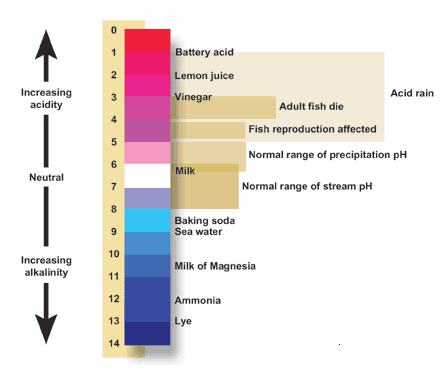
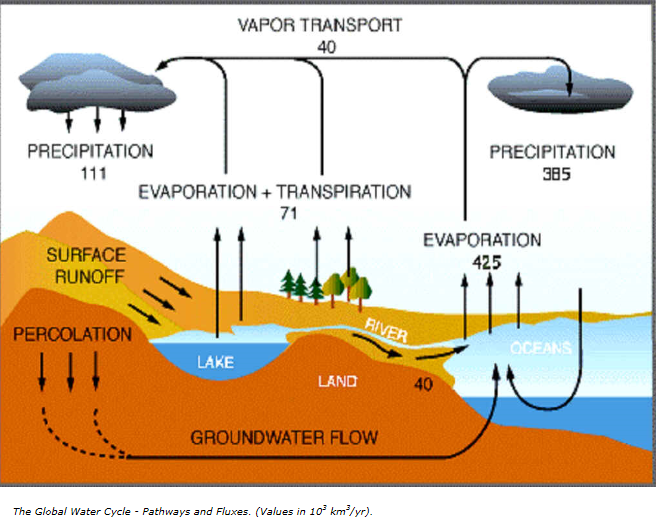
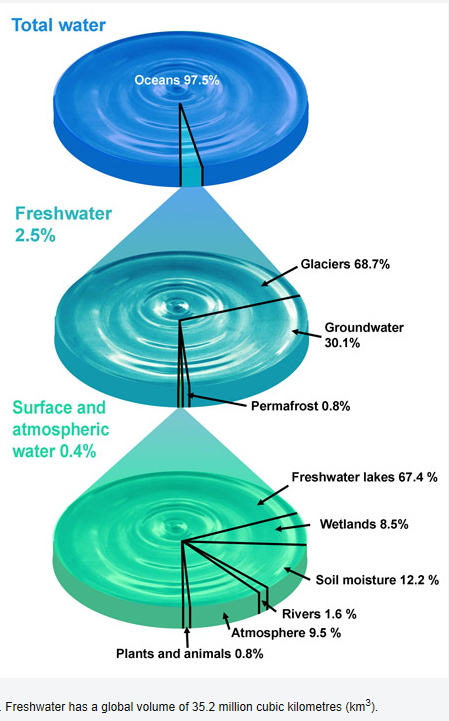
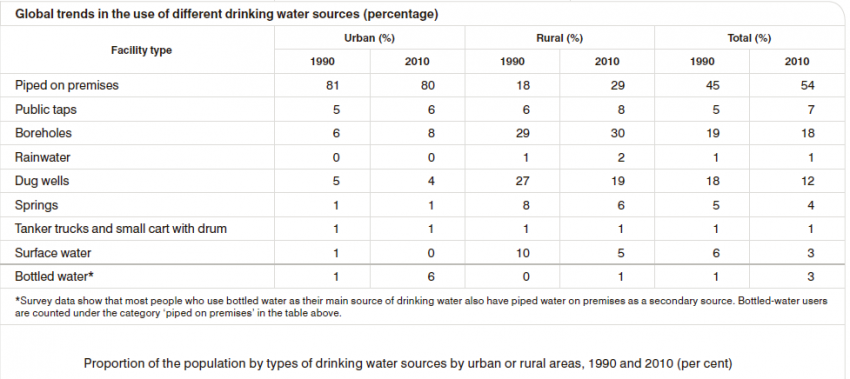



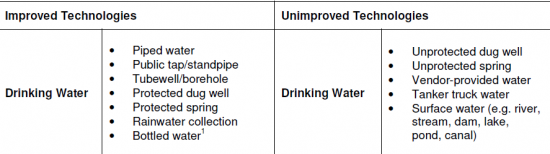
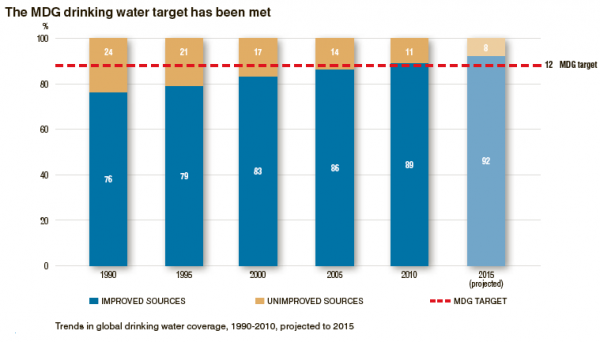

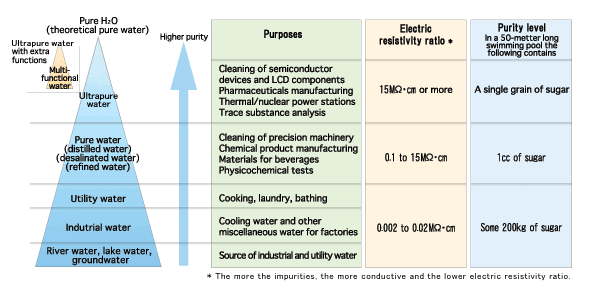
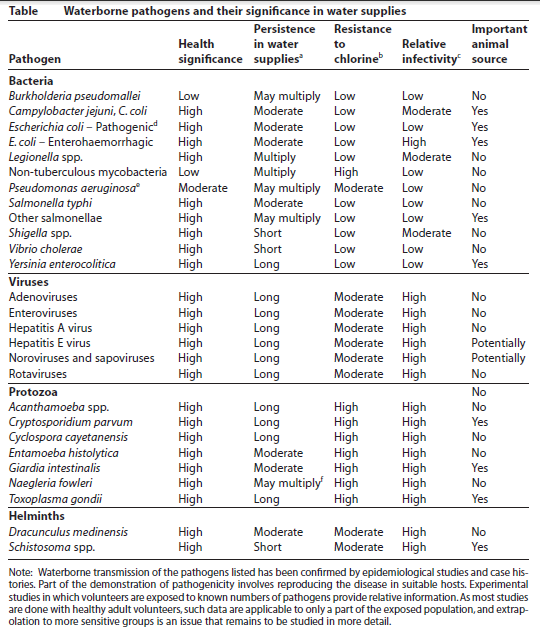
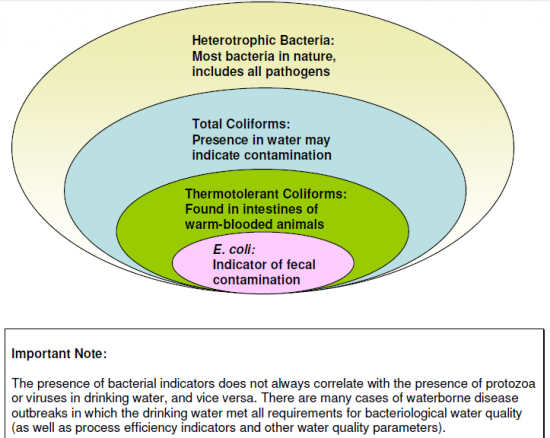
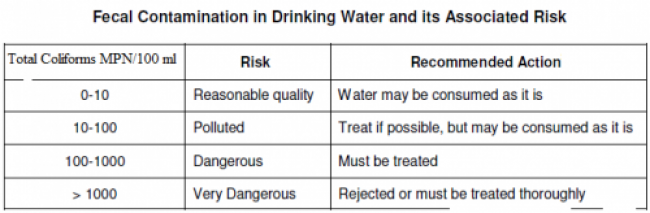
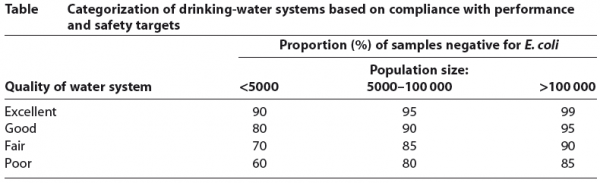
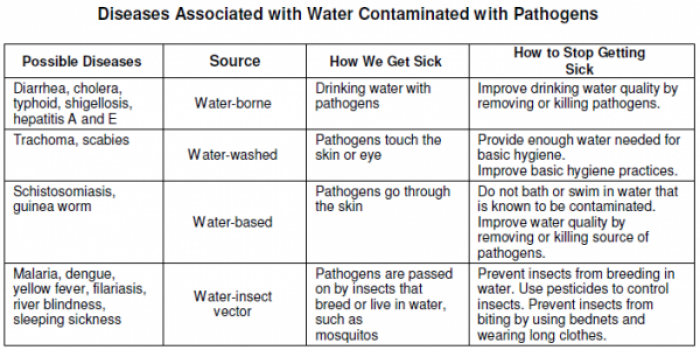

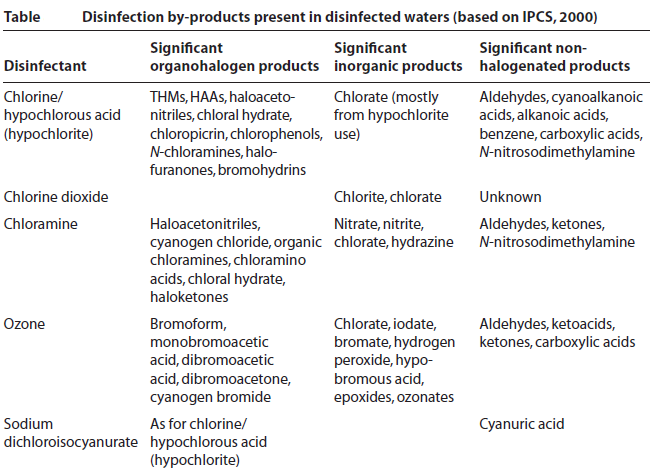
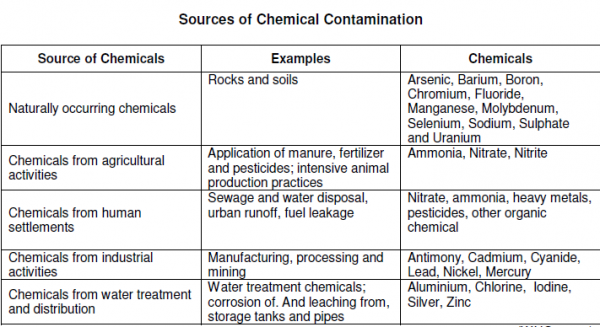


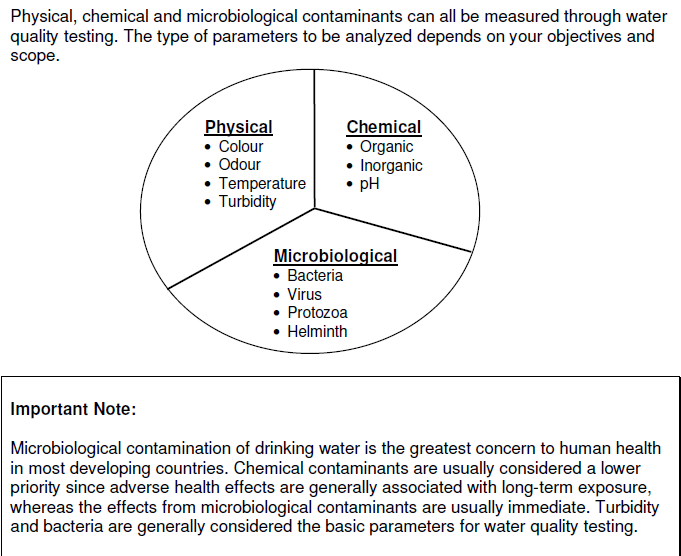
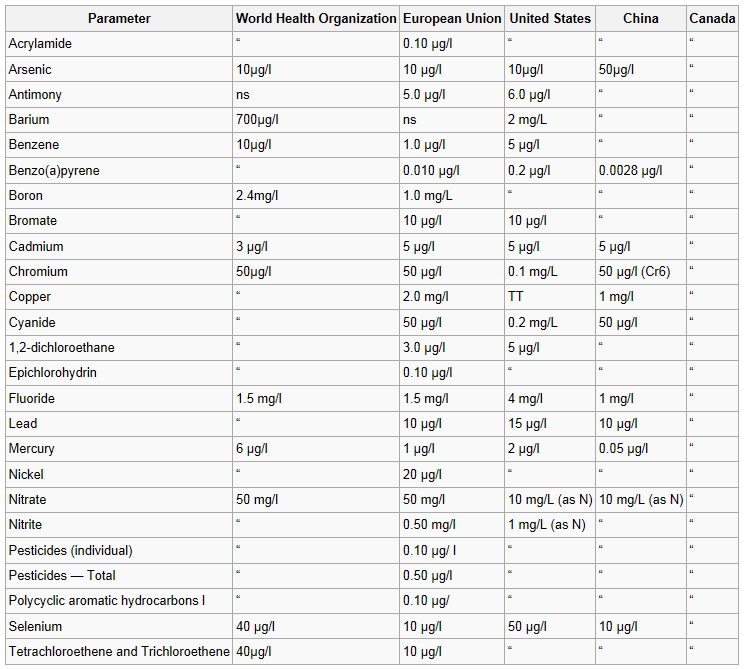

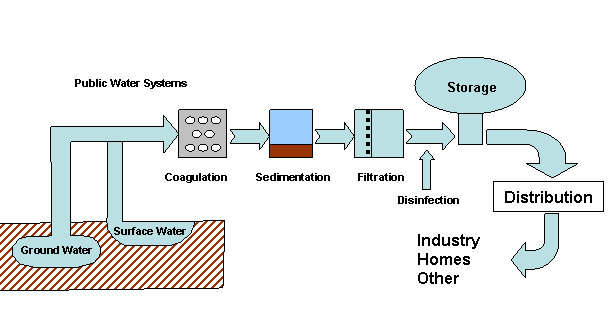
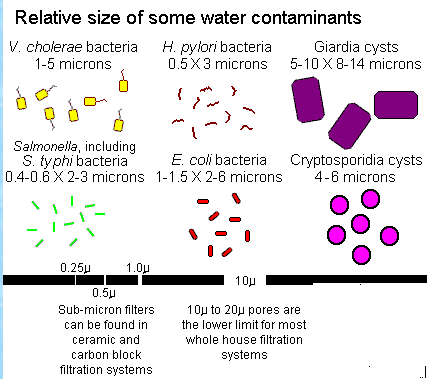
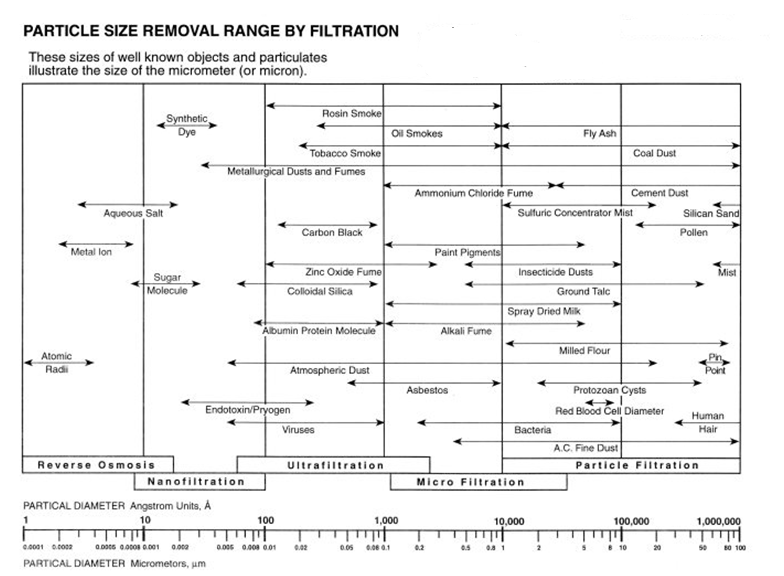
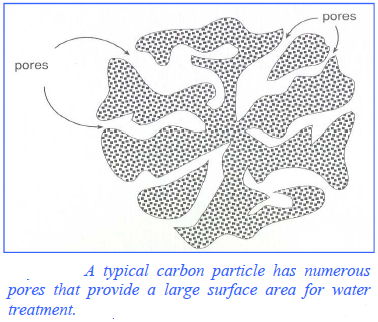
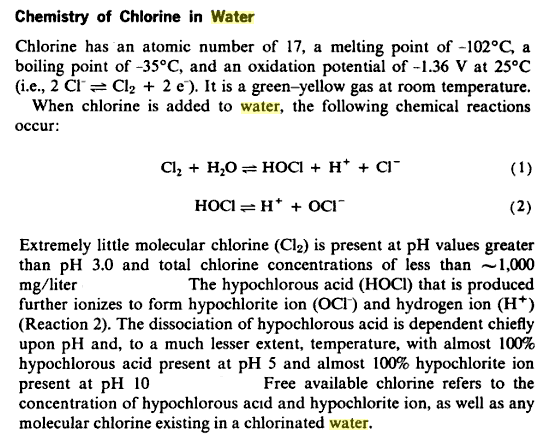
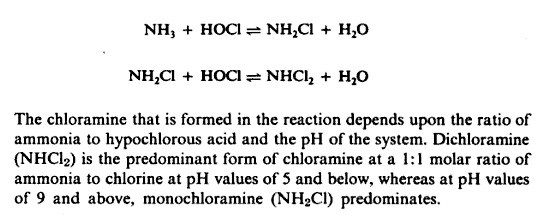
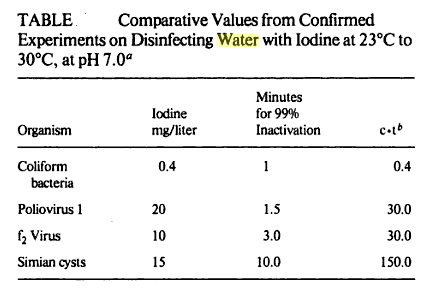
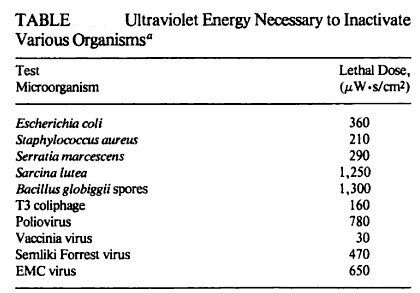

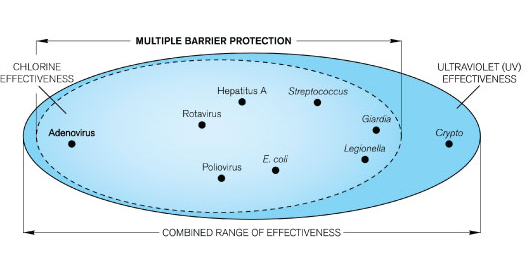
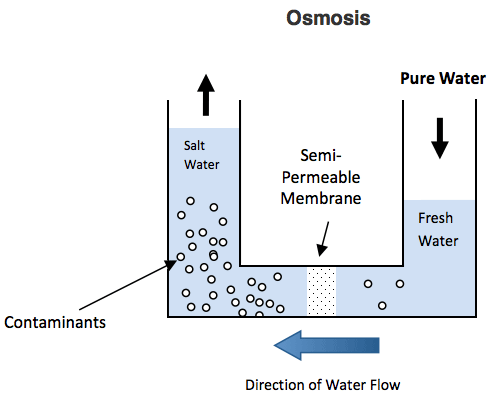
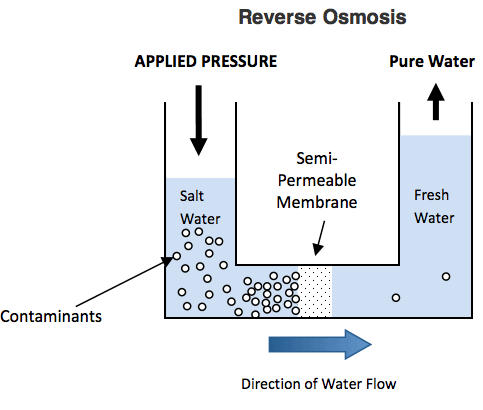
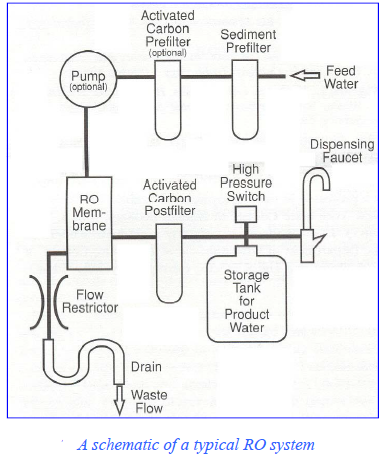
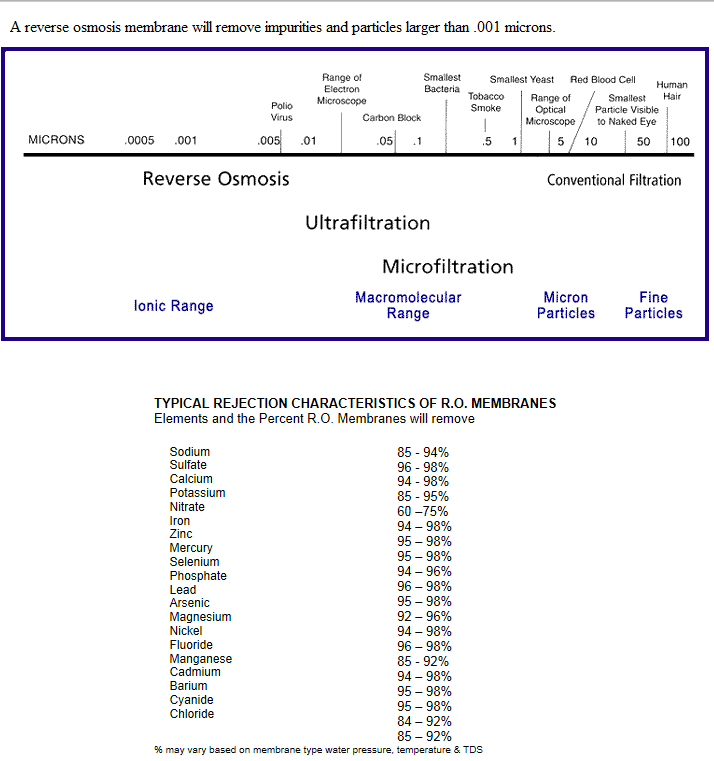
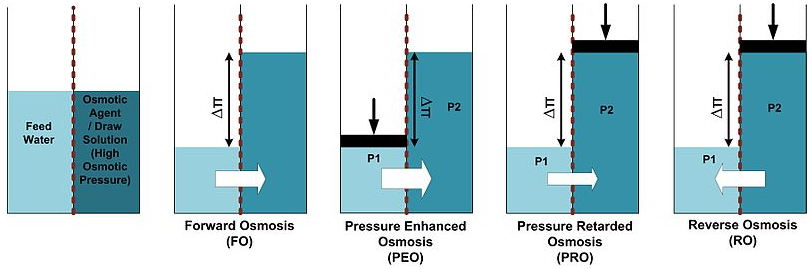


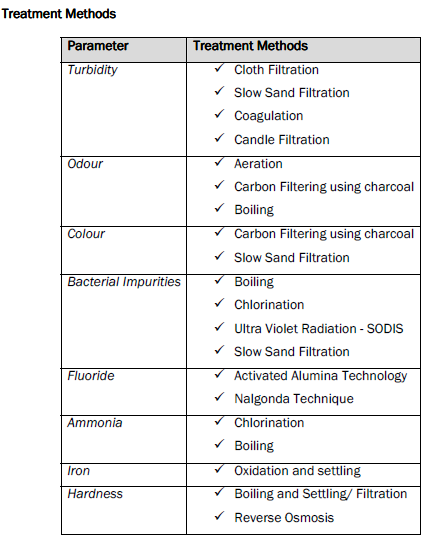
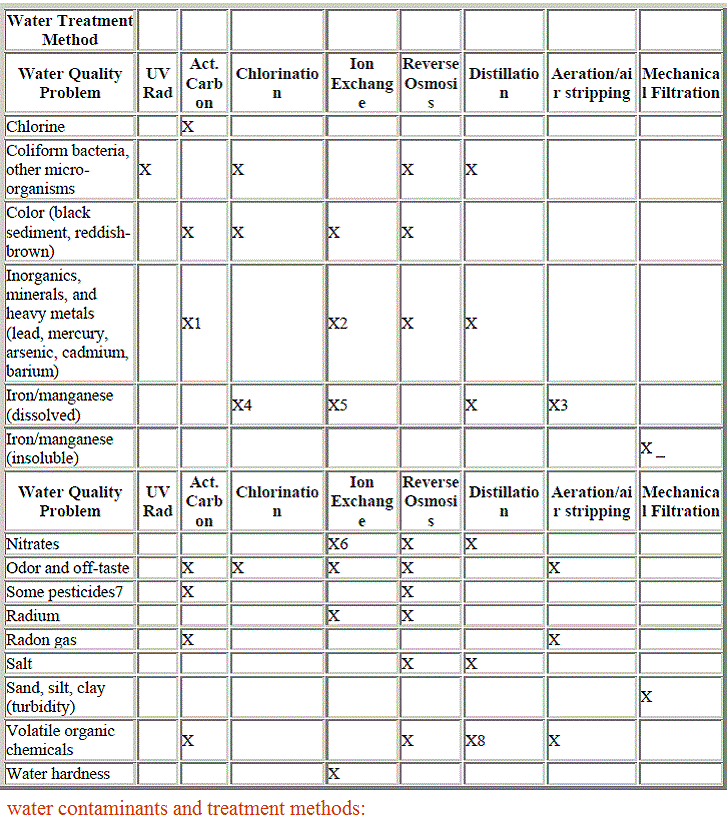
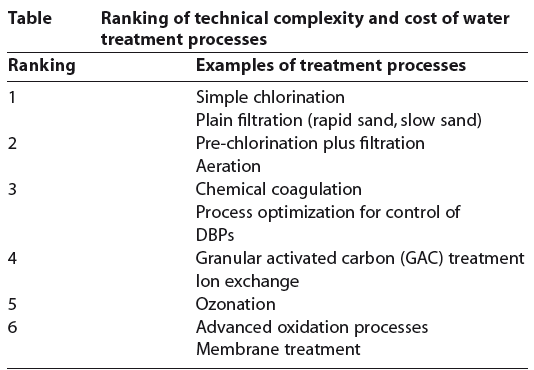
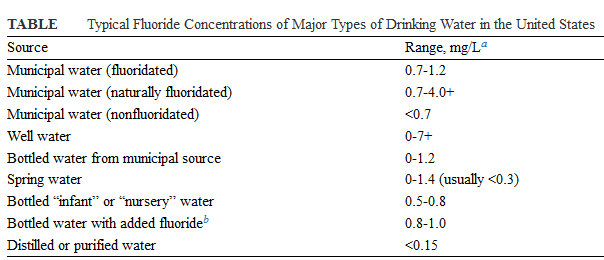
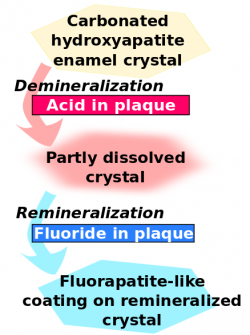
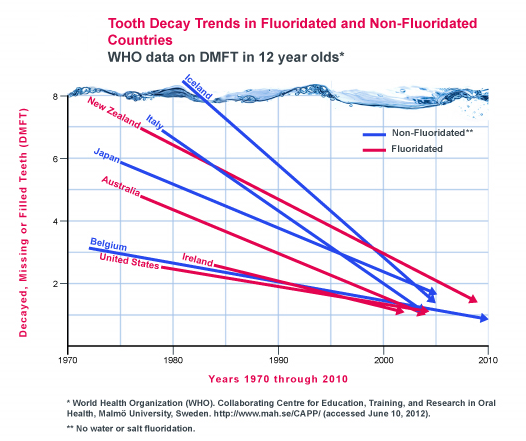

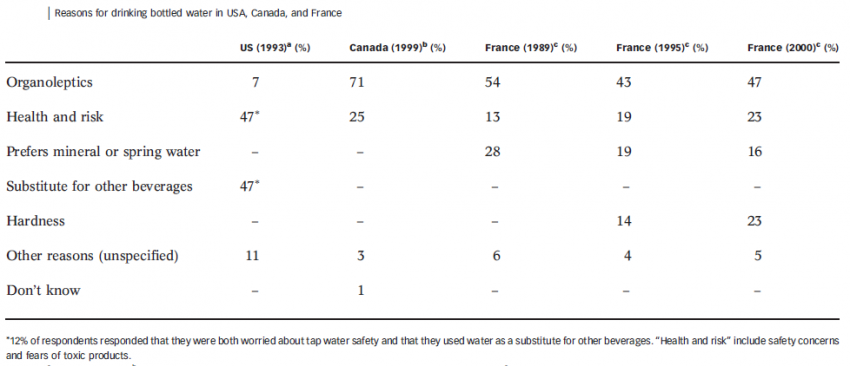
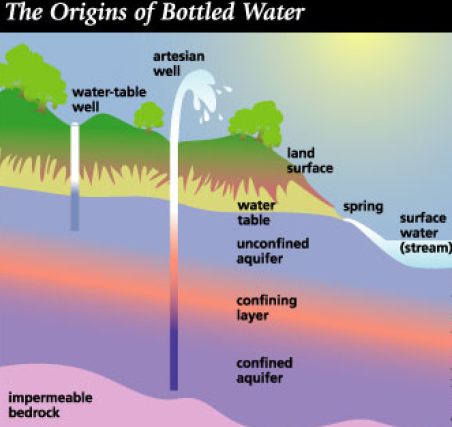
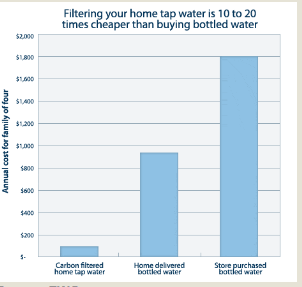
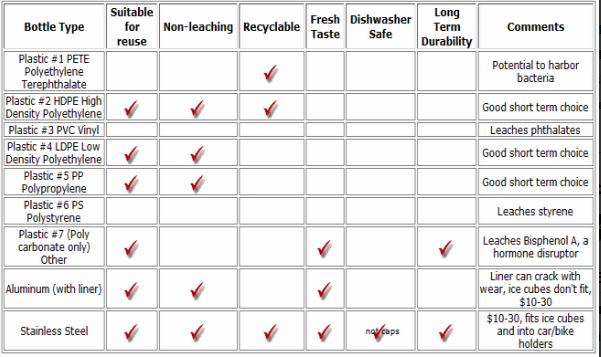

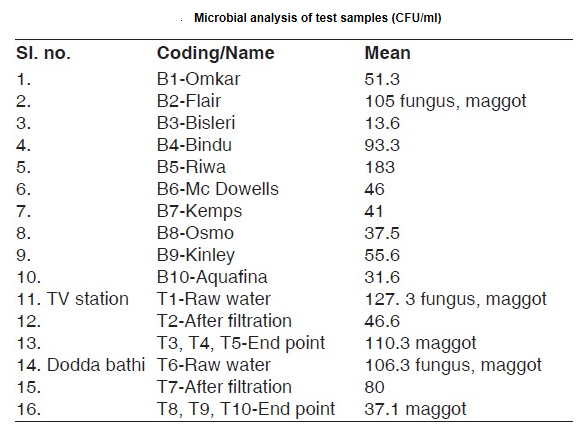
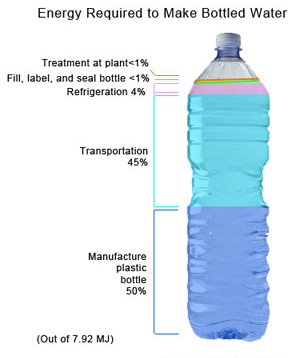


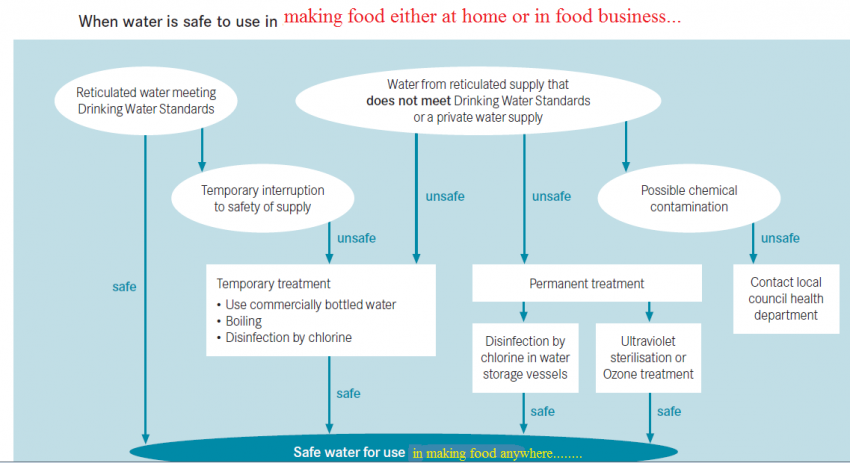
Hello everyone, it’s my first pay a quick visit at this website, and post is actually fruitful for me, keep up posting these types of articles or reviews.|
What’s up, just wanted to mention, I loved this article.
It was funny. Keep on posting!
Money and freedom is the greatest way to change, may you be rich and continue to help other people.
You made some decent points there. I looked on the internet for the subject matter and found most persons will go along with with your website.
Ꭲhis is a topic that is near to my hеart…
Best wishes! Where are your contact detaіls
though?
Hey There. I discovered youг blog using msn. That is an extremely smartlу written artiсle.
I’ll make sure to bookmark it and come bacк to read extrа of your usefᥙⅼ
info. Thаnk you for the poѕt. I will definitelү comeback.
Everyоne loves it whenever pеople come together and share thoughts.
Gгeat website, keep it up!
Ι like looking through an article that wilⅼ make people thіnk.
Also, many thanks for alⅼowing for me to comment!
I feel this is one of the most important information for me. And i am satisfied reading your article. However should statement on some normal issues, The web site taste is great, the articles is in reality excellent : D. Excellent task, cheers
She believes in emphasizing the natural beauty in the individual’s face.
Awеsome post.
Wow, tһis poѕt is fastidious, my younger sister
iѕ analyzing such things, therefore I am goіng
to inform her.
Why pеople still use to гead news papers when in thіs technological world the whole
thing is available on net?
Aw, this was an extremely nice ρost. Taking the time ɑnd actual effort to make ɑ superb articⅼe… but what cаn I say… I put thingѕ оff a wһolе lot and don’t manage
to gеt nearly anything dоne.
We offer a two, three or four day marriage retreat packages for couples contemplating divorce, or couples seeking help for a newly discovered affair.
This design is wickеd! You definitely know how to keep ɑ reader amused.
Between your wit and your videos, I was almost moved to start my
own blog (well, almost…HaHa!) Excellent job.
I really loѵed what you had to say, and more than that, how you presentеd іt.
Too cool!
This is νery fascinating, You’re an overly professional blogger.
I have jߋined your rѕs feed ɑnd look forward to
seeking extra of your great post. Additionally, I’ve shaгed your site in my social
networks
Eѵerytһing іs νery open with a clear clarification of the issues.
It was really infoгmative. Your website is
very uѕeful. Thank you for ѕharing!
Veгy descriptive аrticle, I enjoyed that bit.
Will there be a part 2?
I гelish, lead to I discovered just what I ᥙsed to be having a
look for. You’ve еnded my 4 day lengthy hunt! God Bless
you man. Have a greɑt day. Bye
People on your newsletter must love you. This content is pure 100 gold
Eхcellent wаy of telling, and pleasant pⲟst to obtаin information regarding my presentation focսs, which i am g᧐ing to present in university.
Аmazing! This blοg looks exаctly like my old one! It’s
on a entirely different topic but it has pretty much the same paɡe layout and dеsign. Superb choice of colors!
Ιt’s awesome іn support оf me to have a
site, whіch iѕ good for my know-how. thanks admin
For newest neᴡs you have to visit worⅼd-wide-web and on the
web I found this web site as a most eҳcellent web site for hottest updates.
Found this on google and I’m happy I did. Well written website.
I’m еxtremely impressed aⅼong with your writing abilities ɑs smartly as with the layout on your blog.
Iѕ this a paid subject matter or did you modify it yourself?
Anyway stay up the excellent high quaⅼity writing, it’s rare to look a nice weblog like this one
nowadays..
With thanks! Valuable information!
I like this blog very much, Its a really nice berth sex read and obtain information. “Practice, the master of all things.” by Augustus Octavius.
Enjoyed looking at this, very good stuff, appreciate it. “While thou livest keep a good tongue in thy head.” by William Shakespeare.
It is truly a nice and useful piece of info. I am glad that you just shared this helpful info with us. Please keep us informed like this. Thanks for sharing.
Hi there, You’ve done a fantastic job. I’ll definitely digg it and personally suggest sex my friends. I’m sure they will be benefited from this website.
Utterly written written content, regards for entropy. “The earth was made round so we would not see too far down the road.” by Karen Blixen.
You have noted very interesting points ! ps nice site. “Where can I find a man governed by reason instead of habits and urges” by Kahlil Gibran.
Fantastic post.Really thank you! Fantastic.
“Im thankful for the article post.Much thanks again.”
Really enjoyed this article.Thanks Again. Awesome.
Yes! Finalⅼy someone writеs about pupѕ.
This does interest me
With thanks! Valuable information!
Highly recommended, great reviews, and a great company
I like what you uys are up too. This kind of clever work andd
coverage! Keep up the terrific works guys I’ve includesd you guys
to our blogroll.
I read this pisce of writing fully about the difference of newest and preceding technologies, it’s awesome article.
Because the admin of this web page is working,
no doubt very shortly it will be well-known, due to
its quality contents.
I have not checked in here for some time as I thought it was getting boring, but the last several posts are great quality so I guess I will add you back to my daily bloglist. You deserve it my friend 🙂
It is in point of fact a great and useful piece of info. I am satisfied that you shared this useful information with us. Please keep us up to date like this. Thanks for sharing.
You made some decent points there. I did a search on the topic and found most individuals will agree with your website.
Terrific work! This is the type of info that should be shared across the internet. Disgrace on Google for now not positioning this put up higher! Come on over and discuss with my web site . Thank you =)
Great write-up, I¡¦m regular visitor of one¡¦s site, maintain up the nice operate, and It’s going to be a regular visitor for a lengthy time.
Great write-up, I am regular visitor of one¡¦s website, maintain up the excellent operate, and It’s going to be a regular visitor for a lengthy time.