Dr Rajiv Desai
An Educational Blog
CELL DEATH
______________
CELL DEATH:
____________
_
The image shows webbed hand due to apoptosis abnormalities. It is important that inter-digital cell death happens otherwise a webbed limb will develop rather than a five digit limb.
_
The image shows paw of a mouse embryo that has been stained with a dye that specifically labels cells that have undergone apoptosis. The apoptotic cells appear as bright green dots between the developing digits.
_
As a tadpole changes into a frog, the cells in the tadpole tail are induced to undergo apoptosis; as a consequence, the tail is lost. All the changes that occur during metamorphosis, including the induction of apoptosis in the tail, are stimulated by an increase in thyroid hormone in the blood of a frog.
_
The figure shows light micrograph of normal and apoptotic human leukemia cell illustrating chromatin condensation and nuclear fragmentation.
_________________________________
_________________________________
Prologue:
A vast amount of work has been devoted since the late 19th century to discovering how cells multiply. The study of how and why they die is a relatively recent concern: a rubric entitled “cell death” only appeared in the Index Medicus, an index to medical literature, in 1979. One fertilized human ovum differentiates into 200 different types of cells. The human body contains cells with different life expectancies. Some, like white blood cells and skin cells, are programmed to rapidly die and be replaced by new cells. Others, such as nerve cells in the brain, are programmed to survive the lifetime of the individual and are seldom replaced. The naturally occurring turnover of cells in the body is called programmed cell death (PCD). An average adult human body contains one hundred thousand billion cells. Each second of each day, approximately one million of these cells will be in the process of programmed cell death; and by the end of human life almost 99.9% of all cells once made have undergone this fate. Between 50 and 70 billion cells die each day due to PCD in the average human adult and to maintain homeostasis in the adult human body, the same number of cells is made each day just to balance those dying by PCD. And that number can increase significantly when there is increased PCD during normal development and aging or during disease. Indeed, the mass of cells we lose each year through normal cell death is close to our entire body weight! All cells are endowed with the genetic program for self-destruction in order to balance cell production with cell loss. Within the uterus, we are constantly making and destroying cells, and we generate about three times as many neurons as we eventually end up with when we are born. Cell death also occurs in non-programmed fashion, the accidental cell death due to trauma or disease. The formation of the hand during embryogenesis, the peeling of sunburned skin and the tremor associated with Parkinson’s disease all result from a common process: cell death. How does a cell select a particular type of death? How does a cell switch from a stress-recovery program to cell death? What criteria drive the selection of a cell-death pathway? When is a cell irrevocably committed to death? Answers to these and other questions will ultimately lead to a more profound understanding of cell death, better insight into basic developmental issues and an expanding foundation on which increasingly effective therapeutic interventions may be modeled and introduced into clinical practice. The goal of this article is to provide an overview of the basic principles that govern cell death to students and to enlighten lay people about fascinating aspects of cell death.
__________
The pearl:
_____________
Abbreviations:
FADD, Fas-associated death domain protein;
mTOR, (mammalian) target of rapamycin;
ROS, reactive oxygen species
TNF, tumor necrosis factor;
TRAIL, TNF-related apoptosis-inducing factor;
Apaf-1, apoptotic protease activating factor-1;
AIF, apoptosis inducing factor;
TNFR, tumor necrosis factor receptor;
DED, death-effector domains;
CARD, caspase activation and recruitment domains;
IAPs, inhibitor of apoptosis proteins
ADP, Adenosine diphosphate
ALS, Amyotrophic lateral sclerosis (Lou Gehrig’s disease)
AMP, Adenosine monophosphate
ATF, Activating transcription factor
ATP, Adenosine triphosphate
Bcl-2, B-cell lymphoma 2
BH, Bcl-2 homology
BiP, Binding immunoglobulin protein – glucose-regulated protein (GRP-78); heat shock – protein 5 (HSPA5)
BIR, Baculovirus IAP repeat –domain
Cdk5, Cyclin-dependent kinase 5
CED, Cell death;
CES, Cell death Specification;
CNS, Central nervous system;
ER, Endoplasmic reticulum;
mGluR, Metabotropic glutamate receptor;
mRNA, Messenger RNA;
NMDA, N-methyl-D-aspartic acid;
UPR, Unfolded protein response;
UPS, Ubiquitin proteasome system.
_
The list of abbreviations is not complete and comprehensive. Some abbreviations might have been inadvertently missed.
_
Glossary:
AIF: apoptosis-inducing factor, a flavoprotein normally located in the mitochondrial intermembrane space that can translocate to the nucleus on induction of cell death. Mitochondrial AIF participates in local redox homeostasis, whereas nuclear AIF can contribute to chromatin condensation and degradation.
Akt: a survival kinase (also called protein kinase B or PKB) that, when activated, indirectly enhances glucose metabolism and suppresses autophagy through the mTOR kinase.
Atg: a family of evolutionarily conserved genes, whose products are essential for different steps of the autophagic process. One of these genes encodes Atg6, which is also known as Beclin-1.
Bcl-2: the prototypic anti-apoptotic protein of the Bcl-2 family. The Bcl-2 protein is inserted in the outer mitochondrial membrane and protects mitochondria against MMP.
Bax and Bak: the pro-apoptotic multidomain proteins of the Bcl-2 family. Bax and/or Bak are often required for the apoptosis-specific outer MMP. Whereas Bak is pre-inserted in the outer mitochondrial membrane, Bax has to translocate from the cytosol to mitochondria to mediate MMP.
LMP: lysomal membrane permeabilization, a process leading to leakage of catabolic enzymes from the lysosomal lumen, is induced by ROS, sphingolipids and lysosomotropic agents.
MMP: mitochondrial membrane permeabilization, a process affecting both mitochondrial membranes to a variable extent, leading to disruption of mitochondrial structure and function. Outer MMP leads to leakage of intermembrane proteins from mitochondria. Inner MMP is linked to bioenergetic failure caused by loss of the inner mitochondrial transmembrane potential.
ROS: reactive oxygen species, a side product of normal oxidative phosphorylation that need to be scavenged by the anti-oxidant system of the cell. ROS can be overproduced during deleterious and pathological processes.
NCX: a plasma membrane Na+/Ca2+ exchanger required for maintaining physiological low levels of Ca2+ in cells.
Permeability transition: a process leading to the permeabilizaton of the inner mitochondrial membrane to solutes to up to 1500 Da, causing dissipation of the inner mitochondrial transmembrane potential, colloid osmotic swelling of the mitochondrial matrix and physical dysruption of the outer mitochondrial membrane.
RIP1: a specific kinase that is recruited to the death-inducing signaling complex after occupation of the TNF-R1, and that on activation can mediate various effects including the induction of necrosis.
TNFa: tumor-necrosis factor-a, an inflammatory cytokine that can cause cell death by acting on a specific cell-surface receptor.
_____________________
Up until 1971, the term “necrosis” was used for all types of cell death. When Kerr (1971) et al. first observed a form of nonpathologic cell death in certain tissues, they termed it shrinkage necrosis. As shrinkage necrosis became implicated in the control of organ homeostasis, it was renamed apoptosis (Kerr et al. 1972). Over the last three decades, apoptotic cell death has been well characterized at both the genetic and biochemical levels. These studies have demonstrated that while apoptosis is often initiated in response to toxic insults, the ultimate fate of the injured cell is self-determined. During apoptosis, the injured cell degrades its internal constituents and signals for the clearance of the resulting corpse through phagocytosis.
_
Apoptosis, a form of programmed cell death, is a genetically regulated cell-suicide mechanism that is essential for our well-being. Multicellular organisms protect and even “sculpt” themselves by programmed cell suicide — a process called apoptosis (sometimes spelled aptosis or apotosis, and also known as Programmed Cell Death or PCD). In this process, cells acquire the means of their own destruction in the form of an arsenal of deadly proteins, which they turn upon themselves. Simply put, the principle is that all of a multicellular organism’s cells are prepared to suicide when needed for the benefit of the organism as a whole. They eliminate themselves in a very carefully programmed way so as to minimize damage to the larger organism. Moreover, they don’t do it only when things go wrong! The apoptosis mechanism is a normal and creative aspect of multicellular life. It is required in biological processes such as embryogenesis and homeostasis (maintenance of a stable body). It also destroys cells, which may present a risk to our health, such as cells, which have undergone DNA damage. Its importance is shown by considering the serious consequences of reduced apoptosis. Tumors often form because of cancer cells developing the ability to suppress apoptosis, making them immortal and very dangerous. The other extreme, when too much apoptosis occurs, is thought to play a role in the development of diseases such as Alzheimer’s and in the massive destruction of lymphocytes in AIDS and in the adverse consequences of heart attacks. Scientists are trying to learn how they can modulate apoptosis, so that they can control which cells live and which undergo programmed cell death. Anti-cancer drugs and radiation, for example, work by triggering apoptosis in diseased cells. Understanding how to regulate apoptosis could be the first step to treating these conditions.
_
Apoptosis is over 20 times faster than mitosis. Sightings of dying cells in vivo are therefore rare. Apoptotic cells are engulfed and degraded by neighboring cells without a trace. For cell homeostasis to be maintained, a balance between the increase (by differentiation from precursors and by proliferation) and decrease (by further differentiation and cell death) in the number of a cell population has to be neatly balanced. If mitosis proceeded without cell death, an 80-year-old person would have 2 tons of bone marrow and lymph nodes, and a gut 16 km long.
_
Cell death is currently the subject of considerable research activity. This interest stems, in part, from the potential for understanding oncogenesis and the possibility of exploiting the cell death program for therapeutic purposes. For example, inhibition of cell death might contribute to oncogenesis by promoting cell survival instead of death. Likewise, triggering cell death might provide the means for eliminating unwanted cells (e.g., tumor cells). This might be accomplished by harnessing tumor necrosis factor (TNF), which triggers apoptosis in some target cells. Since all cells have the genetic machinery required to commit suicide, the ability to initiate it in a lineage-specific, non-inflammatory manner would allow for the eradication of specific cancers. Alternatively, inhibition of cell death pathways could rescue valuable but condemned cells, such as HIV infected CD4+ T cells or dopaminergic neurons in Parkinson’s disease. There are now over 200,000 publications listed by the United States Library of Medicine for the topics ‘apoptosis or cell death or programmed cell death’ and a new publication appears every 24 min. One could therefore reasonably conclude that this group of topics would be a major aspect of clinical medicine. The astonishing total number of publications on apoptosis in international arena is now over 35539, including some of the world’s leading scientific journals, such as Nature and Science. Apoptosis is now a growth industry, the clinical implications of which can be applied to chemotherapy, the endocrine treatment of cancer, autoimmune disease and neurodegenerative disease.
_
____________
The cell:
The word cell comes from the Latin cella, meaning “small room”. It was coined by Robert Hooke in his book Micrographia (1665), in which he compared the cork cells he saw through his microscope to the small rooms monks lived in. The cell is the basic structural, functional and biological unit of all known living organisms. Cells are the smallest unit of life that can replicate independently, and are often called the “building blocks of life”. While the number of cells in plants and animals varies from species to species, humans contain about 100 trillion ((1014) cells. Most plant and animal cells are visible only under the microscope, with dimensions between 1 and 100 micrometres. Cells emerged on Earth at least 3.5 billion years ago. The cell was discovered by Robert Hooke in 1665. The cell theory, first developed in 1839 by Matthias Jakob Schleiden and Theodor Schwann, states that all organisms are composed of one or more cells, that all cells come from preexisting cells, that vital functions of an organism occur within cells, and that all cells contain the hereditary information necessary for regulating cell functions and for transmitting information to the next generation of cells. Viruses have been described as “organisms at the edge of life”, since they resemble organisms in that they possess genes and evolve by natural selection, and reproduce by creating multiple copies of themselves through self-assembly. Although they have genes, they do not have a cellular structure, which is often seen as the basic unit of life. Viruses do not have their own metabolism, and require a host cell to make new products. They therefore cannot naturally reproduce outside a host cell. Accepted forms of life use cell division to reproduce, whereas viruses spontaneously assemble within cells. They differ from autonomous growth of crystals as they inherit genetic mutations while being subject to natural selection. Virus self-assembly within host cells has implications for the study of the origin of life, as it lends further credence to the hypothesis that life could have started as self-assembling organic molecules.
_
Cell division, cell death and homeostasis:
Cell division and cell death are the two predominant physiological processes that regulate tissue homeostasis in the adult organism. The importance of dysregulation of these processes in the pathogenesis of major diseases, such as cancer, myocardial infarction, stroke, atherosclerosis, infection, inflammation and neurodegenerative disorders, is becoming increasingly evident. Hence, attempts to find modulators of the cell cycle and cell death programs are being made with the hope of creating novel therapeutic approaches to the treatment of these diseases. It is clear that improved understanding of how cells balance life-and-death processes is crucial for this development. Regulation of the homeostatic balance between cell proliferation and cell death is essential for development and maintenance of multicellular organisms. Physiologic, or programmed cell death is dependent on a genetically encoded and evolutionarily conserved pathway that induces a form of cellular suicide known as apoptosis. In the past decade, it has become clear that the regulatory mechanisms controlling programmed cell death are as fundamental, and as complex, as those regulating cell proliferations. Perturbation of the signaling cascades regulating apoptosis, whether by extracellular triggers, acquired or germline genetic mutations, or viral mimicry of signaling molecules, can result in a wide variety of human diseases. Analysis of these regulatory pathways has led to a better understanding of the etiology and pathogenesis of many human diseases, notably cancers, infectious diseases including AIDS, autoimmune diseases, and neurodegenerative/ neurodevelopmental diseases. Our understanding of the regulation of programmed cell death in health and disease is far from complete, and the challenge of converting that understanding into new therapeutic modalities has only begun to be approached.
___________
Prokaryotes and eukaryotes:
_
Typical prokaryote:
_
The prokaryotes are a group of organisms whose cells lack a membrane-bound nucleus (karyon). The organisms whose cells do have a nucleus are called eukaryotes. Most prokaryotes are unicellular organisms, although a few such as myxobacteria have multicellular stages in their life cycles or create large colonies like cyanobacteria. Prokaryotes do not have a nucleus, mitochondria, or any other membrane-bound organelles. In other words, all their intracellular water-soluble components (proteins, DNA and metabolites) are located together in the same volume enclosed by the cell membrane, rather than in separate cellular compartments. The genome in a prokaryote is held within a DNA/protein complex in the cytosol called the nucleoid, which lacks a nuclear envelope. The complex contains a single, cyclic, double-stranded molecule of stable chromosomal DNA, in contrast to the multiple linear, compact, highly organized chromosomes found in eukaryotic cells. In addition, many important genes of prokaryotes are stored in separate circular DNA structures called plasmids. Prokaryotes lack mitochondria and chloroplasts. Instead, processes such as oxidative phosphorylation and photosynthesis take place across the prokaryotic cell membrane. However, prokaryotes do possess some internal structures, such as prokaryotic cytoskeletons, and the bacterial order Planctomycetes have a membrane around their nucleoid and contain other membrane-bound cellular structures. Both eukaryotes and prokaryotes contain large RNA/protein structures called ribosomes, which produce protein. Prokaryotic cells are usually much smaller than eukaryotic cells. Therefore, prokaryotes have a larger surface-area-to-volume ratio, giving them a higher metabolic rate, a higher growth rate, and as a consequence, a shorter generation time than eukaryotes. Prokaryotes include two major classification domains: the bacteria and the archaea. While prokaryotes are considered to be strictly unicellular, most are capable of forming stable aggregate communities. When such communities are encased in a stabilizing polymer matrix (“slime”), they may be called “biofilms”. Cells in biofilms often show distinct patterns of gene expression (phenotypic differentiation) in time and space. Bacteria and archaea reproduce through asexual reproduction, usually by binary fission. Genetic exchange and recombination still occur, but this a form of horizontal gene transfer and is not a replicative process, simply involving the transference of DNA between two cells, as in bacterial conjugation. The oldest known fossilized prokaryotes were laid down approximately 3.5 billion years ago, only about 1 billion years after the formation of the Earth’s crust. Eukaryotes only appear in the fossil record later, and may have formed from endosymbiosis of multiple prokaryote ancestors. The oldest known fossil eukaryotes are about 1.7 billion years old. However, some genetic evidence suggests eukaryotes appeared as early as 3 billion years ago.
_
One criticism of this classification points out that the word “prokaryote” is based on what these organisms are not (they are not eukaryotic), rather than what they are (either archaea or bacteria). Another difference is that ribosomes in prokaryotes are smaller than in eukaryotes. However, two organelles found in many eukaryotic cells, mitochondria and chloroplasts, contain ribosomes similar in size and makeup to those found in prokaryotes. This is one of many pieces of evidence that mitochondria and chloroplasts are themselves descended from free-living bacteria. This theory holds that early eukaryotic cells took in primitive prokaryotic cells by phagocytosis and adapted themselves to incorporate their structures, leading to the mitochondria we see today.
_
Ancient Invasions: From Endosymbionts to Organelles:
The acquisitions of mitochondria and plastids were important events in the evolution of the eukaryotic cell, supplying it with compartmentalized bioenergetic and biosynthetic factories. Ancient invasions by eubacteria through symbiosis more than a billion years ago initiated these processes. Advances in geochemistry, molecular phylogeny, and cell biology have offered insight into complex molecular events that drove the evolution of endosymbionts into contemporary organelles. In losing their autonomy, endosymbionts lost the bulk of their genomes, necessitating the evolution of elaborate mechanisms for organelle biogenesis and metabolite exchange. In the process, symbionts acquired many host-derived properties, lost much of their eubacterial identity, and were transformed into extraordinarily diverse organelles that reveal complex histories that we are only beginning to decipher.
_
Mitochondria: a link between prokaryotes and eukaryotes:
One of the major features distinguishing prokaryotes from eukaryotes is the presence of mitochondria. Mitochondria contain their own ribosomes and DNA; combined with their double membrane, these features suggest that they might have once been free-living prokaryotes that were engulfed by a larger cell. Complete sequences of numerous mitochondrial, many prokaryotic, and several nuclear genomes confirm that the mitochondrial genome originated from a eubacterial (specifically α-proteobacterial) ancestor. As such, they are able to maintain genomic independence from the nucleus. However, as a consequence of proto-mitochondrial genes integrating into the nuclear genome throughout evolution, most mitochondrial proteins are encoded by nuclear DNA (nDNA) and imported into mitochondria. Although the replication of mitochondrial DNA (mtDNA) is not synchronized with nDNA replication, the overall number of mitochondria per cell remains fairly constant for specific cell types during proliferation, suggesting that the generation of mitochondria is largely influenced by extra-mitochondrial signal transduction events. This conclusion is further supported by the observation that mitochondrial biosynthesis continues even when mtDNA is deleted. Thus, the replication of mitochondria does not require the presence of mtDNA. Each mitochondrion contains 2–10 copies of its genome. Although the number of mitochondria per cell varies with cell type, an individual cell typically contains a fairly constant copy number of mtDNA. During fertilization, the mitochondria are transmitted through the oocyte’s cytoplasm. Thus, the mitochondrial genome is distinct from the nuclear genome in that it follows a strict maternal inheritance pattern. Within a cell, mtDNA replication is semi-autonomous and is not synchronized with the S phase of the cell cycle. However, the two genetic systems (nuclear and mitochondrial) appear to be closely coordinated by yet unknown mechanisms. The overall biogenesis of mitochondria keeps pace with cell proliferation and cell growth.
_
A eukaryote is any organism whose cells contain a nucleus and other structures (organelles) enclosed within membranes. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear envelope, within which the genetic material is carried. Most eukaryotic cells also contain other membrane-bound organelles such as mitochondria or the Golgi apparatus. In addition, plants and algae contain chloroplasts. Many unicellular organisms are eukaryotes, such as protozoa. All multicellular organisms are eukaryotes, including animals, plants and fungi. Cell division in eukaryotes is different from that in organisms without a nucleus (Prokaryote). There are two types of division processes. In mitosis, one cell divides to produce two genetically identical cells. In meiosis, which is required in sexual reproduction, one diploid cell (having two instances of each chromosome, one from each parent) undergoes recombination of each pair of parental chromosomes, and then two stages of cell division, resulting in four haploid cells (gametes). Each gamete has just one complement of chromosomes, each a unique mix of the corresponding pair of parental chromosomes. Eukaryotes represent a tiny minority of all living things; even in a human body there are 10 times more microbes than human cells. However, due to their much larger size, their collective worldwide biomass is estimated at about equal to that of prokaryotes.
_
Typical eukaryote cell:
_
Eukaryotic cells are typically much larger than those of prokaryotes. They have a variety of internal membranes and structures, called organelles, and a cytoskeleton composed of microtubules, microfilaments, and intermediate filaments, which play an important role in defining the cell’s organization and shape. Eukaryotic DNA is divided into several linear bundles called chromosomes, which are separated by a microtubular spindle during nuclear division. Eukaryote cells include a variety of membrane-bound structures, collectively referred to as the endomembrane system. Simple compartments, called vesicles or vacuoles, can form by budding off other membranes. Many cells ingest food and other materials through a process of endocytosis, where the outer membrane invaginates and then pinches off to form a vesicle. It is probable that most other membrane-bound organelles are ultimately derived from such vesicles. The nucleus is surrounded by a double membrane (commonly referred to as a nuclear envelope), with pores that allow material to move in and out. Various tube- and sheet-like extensions of the nuclear membrane form what is called the endoplasmic reticulum or ER, which is involved in protein transport and maturation. It includes the rough ER where ribosomes are attached to synthesize proteins, which enter the interior space or lumen. Subsequently, they generally enter vesicles, which bud off from the smooth ER. In most eukaryotes, these protein-carrying vesicles are released and further modified in stacks of flattened vesicles, called Golgi bodies or dictyosomes. Vesicles may be specialized for various purposes. For instance, lysosomes contain enzymes that break down the contents of food vacuoles, and peroxisomes are used to break down peroxide, which is toxic otherwise. Mitochondria are organelles found in nearly all eukaryotes. They are surrounded by two membranes (each a phospholipid bi-layer), the inner of which is folded into invaginations called cristae, where aerobic respiration takes place. Mitochondria contain their own DNA. They are now generally held to have developed from endosymbiotic prokaryotes, probably proteobacteria. Plants and various groups of algae also have plastids. Again, these have their own DNA and developed from endosymbiotes, in this case cyanobacteria. They usually take the form of chloroplasts, which like cyanobacteria contain chlorophyll and produce organic compounds (such as glucose) through photosynthesis. Others are involved in storing food.
_
Differences among eukaryotic cells:
There are many different types of eukaryotic cells, though animals and plants are the most familiar eukaryotes, and thus provide an excellent starting point for understanding eukaryotic structure. Fungi and many protists have some substantial differences, however. An animal cell is a form of eukaryotic cell that makes up many tissues in animals. The animal cell is distinct from other eukaryotes, most notably plant cells, as they lack cell walls and chloroplasts. They also have smaller vacuoles. Due to the lack of a cell wall, animal cells can adopt a variety of shapes. A phagocytic cell can even engulf other structures. There are many different cell types. For instance, there are approximately 200 distinct cell types in the adult human body.
____________
Unicellular Organism and Multicellular Organism:
The number of cells in the body varies with different organisms. In some lower forms of life (Example: Euglena, Amoeba) the body is formed of a single cell; these organism are described as unicellular. In a unicellular organism, all life activities are carried out by itself with its internal structures. The intracellular structures are called the organelles.
In the vast majority of plants and animals, the body is made up of numerous cells. They are called multicellular organisms. A multicellular organism is actually an aggregation of cells. Here a group of cells functions in a same way to form a tissue or an organ (Example: Xylem vessels, cork cells, cells in the skin). Multicellular organisms have a great capacity to survive than unicellular organisms.
_
Metazoan:
Metazoans are multicellular, mitochondrial eukaryotes having well differentiated tissues. All multicellular animals besides sponges are metazoans. They evolved from the protists approximately 700 million years ago.
_
Programmed cell death is one of the architectural principles of metazoans:
______________
Cell division:
_
_
Cell division is the process by which a parent cell divides into two or more daughter cells. Cell division usually occurs as part of a larger cell cycle. In eukaryotes, there are two distinct type of cell division: a vegetative division, whereby each daughter cell is genetically identical to the parent cell (mitosis), and a reductive cell division, whereby the number of chromosomes in the daughter cells is reduced by half, to produce haploid gametes (meiosis). Both of these cell division cycles are in sexually reproducing organisms at some point in their life cycle, and both are believed to be present in the last eukaryotic common ancestor. Prokaryotes also undergo a vegetative cell division known as binary fission, where their genetic material is segregated equally into two daughter cells. All cell divisions, regardless of organism, are preceded by a single round of DNA replication. For simple unicellular organisms such as the amoeba, one cell division is equivalent to reproduction – an entire new organism is created. On a larger scale, mitotic cell division can create progeny from multicellular organisms, such as plants that grow from cuttings. Cell division also enables sexually reproducing organisms to develop from the one-celled zygote, which itself was produced by cell division from gametes. And after growth, cell division allows for continual construction and repair of the organism. A human being’s body experiences about 10,000 trillion cell divisions in a lifetime. The primary concern of cell division is the maintenance of the original cell’s genome. Before division can occur, the genomic information that is stored in chromosomes must be replicated, and the duplicated genome must be separated cleanly between cells. A great deal of cellular infrastructure is involved in keeping genomic information consistent between “generations”.
_
Normal cell versus cancer cell:
Normal cells can undergo the cell cycle for about 50 times and then they die. Cancer cells can enter the cell cycle repeatedly, and in this way, they are potentially immortal. They are everlasting. Ordinarily, cells with damaged DNA undergo apoptosis, a series of enzymatic reaction that lead to the death of the cell. Cancer cells fail to undergo apoptosis. Normal cells anchor themselves to a substratum and adhere to their neighbors. They exhibit contact inhibition and that is when they come in contact with a neighbor, they stop dividing. Cancer cells have lost all restraint and they pile on top of one another to grow in multiple layers and that’s why they grow. Normal cells do not grow and divide unless they are stimulated to do so by a growth factor. Cancer cells have a reduced need for growth factor. Cancer cells no longer respond to inhibitory growth factors such as transforming growth factors beta from their neighbors. The cancer cell must make their way across a basement membrane and into a blood vessel or lymphatic vessel. They induce angiogenesis and cause nearby blood vessels to form a capillary network that services the tumor. Cancer cells require a series of mutations. Each of the cells propel towards the development of a tumor, which is an accumulation of cancer cells that no longer function properly. Even though tumor cells may show commons characteristics, each type of cancer may have its own particular sequence of mutation. This results in the uncontrolled growth. Cancer cells characteristics distinguish them from normal cells. They have abnormal nuclei with many chromosomal irregularities. Cancer cells can be recognized from normal cells in many different ways. The best distinguishing difference between the two types is their growth in soft agar. Soft agar is a semisolid medium that encourages the division of cancer cells but not most normal cells. Cancer cells survive better in soft agar because they are less dependent than normal cells on attaching to a substratum. Normal cells secrete fewer proteases than cancer cells and have a more organized cytoskeleton. Normal cells grown in culture are mortal, usually succumbing after 25 to 50 divisions. Cancer cells, by definition, are immortal and can divide continuously in culture as long as they are given a continuous supply of nutrients. Finally, cancer cells, but not normal cells, can cause tumors when injected into the appropriate animal host. Normal cells can be converted to cancer cells through a variety of mechanisms. Radiation such as ultraviolet light and X rays can cause mutations in DNA that in turn can cause unregulated cell division. Viruses can cause a variety of human cancers by introducing their DNA into the human genome and altering its function. Chemical carcinogens in the environment can cause cancer by binding to bases. Spontaneous mutations can occur and if this alteration is not corrected by one of the myriad DNA correction enzymes, it could lead to altered genetic activity and cancer. No matter how a normal cell is converted to a cancer cell, however, some stable type of gene alteration must occur. One of these changes is the conversion of a protooncogene, a gene in normal cells that functions during cell growth and division, to an active oncogene, a gene whose activity results in cell transformation from the normal phenotype to a cancer cell. A variety of oncogenes have been identified.
_
Cell population:
In adult tissues, the size of a cell population is determined by the rates of cell differentiation, proliferation, and cell deaths. This dynamic state occurs in tissues such as the bone marrow, skin and gastro-intestinal tract epithelia, in which there is equilibrium between these various processes. The equilibrium may be disrupted by increased or decreased cell proliferation, or by agents such as irradiation that may induce cell death and inhibit stem cell differentiation. Tissues in which there is constitutive cell proliferation are often called labile tissues, to contrast them with stable tissues, such as the liver, pancreas, kidney and endothelial cells, in which cell proliferation is normally very low. However, cells from stable tissues can readily enter the cell cycle and replicate in response to certain stimuli. The most dramatic example of this type of response is liver regeneration after partial hepatectomy. The third type of tissue, called permanent or non-dividing tissues, contain cells that have left the cell cycle permanently and are not capable of proliferation. This category includes the brain, and cardiac and skeletal muscle. Nevertheless, skeletal muscle contains stem-like cells called satellite cells that have the capacity to differentiate and regenerate muscle fibers. Recently, stem cells have been identified in at least two areas of mammalian brain. It is not known if these cells may contribute to brain remodeling and regeneration.
_______________
Senescence:
Everyone gets old and everyone dies. Aging is the changes that occur in an organism (or a part of an organism) between maturity and death. Characteristically there is deterioration in various functions as cells become less efficient in maintaining and replacing vital cell components. In animals this results in a decline in physical ability and, in humans, there is also often a reduction in mental ability. Not all part of the body necessarily become senescent at the same time or age at the same rate. Aging and death seems somehow inherent to human cells, or at least those cells within our environment. Normal human cells die out after dividing a number of times, even when kept alive in ideal laboratory nutrient conditions. But some cancer cells and virus-infected cells can be “immortal” and divide indefinitely. There is much circumstantial evidence that aging is well-defined and even programmatic. Humans age in similar ways and on a similar schedule.
_
_
Aging is an essential, inevitable physiological phenomenon characterized by a progressive accumulation of deleterious molecular damages in cells and tissues during the post-maturational deterioration, which decreases the ability to survive and increases risk of death. The aging process has many facets and multiple causes. The primary molecular phenotype of aging is the stochastic occurrence and accumulation of molecular damage leading to a progressive increase in molecular heterogeneity and functional impairment. Cellular senescence is the state where cells have irreversibly lost their proliferation ability, and they exhibit deficiencies in maintaining their homeostatic processes. The term cellular senescence therefore denotes a stable and long-term loss of proliferative capacity, despite continued viability and metabolic activity. The number of senescent cells increases in tissues with aging. Many theories about the causes of aging have been proposed, and could be divided into two broad categories: the stochastic (statistical) theories and the developmental-genetic theories.
_
Mechanism of cellular aging:
_
Classification of Cellular Senescence:
A. Replicative cellular senescence due to shortening of telomeres (vide infra)
B. Premature cellular senescence
Senescence can also be induced in the absence of any detectable telomere loss or dysfunction, by a variety of conditions.
This type of senescence has been termed premature, since it arises prior to the stage at which it is induced by telomere shortening. In recent years, evidence for the existence of premature senescence in vivo has been accumulating rapidly and points to a critical role in tumor suppression. There are 3 types of premature cellular senescence.
1. Stress‐induced premature senescence (SIPS)
2. Oncogene‐induced senescence
3. Tumor suppressor loss‐induced senescence
_
The most commonly used marker for senescence is senescence-associated β-galactosidase, which is detected by a colorimetric assay using 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-Gal) as a substrate at a pH of 6.0. This assay is limited in its application, however, because molecular mechanisms that define this activity are not understood. Accumulation of heterochromatic foci that are concentrated spots of transcriptionally silenced DNA have also been seen in senescent cells. Staining of these foci and their microscopic determination is another detection method for senescence.
_
Theories of Aging:
There are numerous different theories in the literature on aging research. They can largely be categorized into the following types of theories:
- Statistical theories: cumulative random cell damage over time
- Programmed cell death theories (called “apoptosis”)
- High-level program control theories
_
Cumulative Random Cell Damage Aging Theories:
There are several aging theories that are statistical or stochastic. The idea is that there is no built-in aging program, and that aging occurs as a statistical process. If we were just lucky enough to avoid the bad things in the world, we would live much longer. The basis of these theories is that all humans are subjected to numerous cellular incidents that damage the cells. These accumulate over a lifetime and gradually overwhelm the body, causing aging and the cellular decline that eventually leads to death. Some of the possible theories of random cell damage include free radicals, DNA mutations, or the gradual build-up of the body’s own waste poisons.
_
The DNA damage theory of aging proposes that aging is a consequence of unrepaired DNA damage accumulation:
Damage in this context is a DNA alteration that has an abnormal structure. Although both mitochondrial and nuclear DNA damage can contribute to aging, nuclear DNA is the main subject of this analysis. Nuclear DNA damage can contribute to aging either indirectly (by increasing apoptosis or cellular senescence) or directly (by increasing cell dysfunction). In humans, DNA damage occurs frequently and DNA repair processes have evolved to compensate. On average, approximately 800 DNA lesions occur per hour in each cell. A number of factors can accelerate and/or trigger cell senescence, one of which is oxidative stress. In addition to O2, other sources of oxidative damage, such as H2O2 and tert-butylhydroperoxide, and other stressors–e.g., ethanol, ionizing radiations, and mitomycin C–can induce stress induced premature senescence (SIPS) in many types of proliferative cells such as lung and skin fibroblasts, endothelial cells, melanocytes, and retinal pigment epithelial cells (reviewed in Toussaint et al., 2002b; Dierick et al., 2003). The list of stressors that can cause SIPS is constantly growing. Instead of chronic stress, SIPS can be induced based on a single or repeated short exposure(s) to stressors. Oncogenes such as ras can also induce senescence (Serrano et al., 1997). Because organisms and cells are constantly being exposed to stressors, senescent cells in vivo may derive not only from cell divisions but from cells being exposed to stress.
_
Programmed Cell Death Aging Theories:
All cells have a feature known as “programmed cell death” or “apoptosis”. It is really a form of cell suicide. In fact, it is a very ordered shutdown of the cell through gradual shrinkage and eventual dispersion. It is much less messy than the abrupt death of a cell through cellular injury. The steps involved in apoptosis have been immensely studied in recent years and seem very regular. Theories of aging that use apoptosis seem to imply that as people age, more of their cells start to decide to suicide. One of the main theories uses the Hayflick phenomenon. According to this theory, cells divide until they cannot further, whereupon this is recognized and triggers the apoptosis sequence.
_
High-Level Control Aging Theories:
This type of theory has the idea of a kind of master control or master clock. Realistically, there are two main ways that this theory of aging could work:
- Cells act independently: each cell somehow knows the age of the person, and starts running the later stages of its DNA program. Thus a cell ages on its own.
- System control: the entire body somehow keeps its own clock, and as we age, starting off the end-stage sequences using whatever major cells, organs or enzymes are required. In this theory, a cell does not age unless it gets the aging signals from the body’s master program.
Both variants of the overall control theory of aging share a common element: the program that controls when various aging features are activated are stored in genes in the DNA code. Whether it is each cell making age-related genetic decisions independently, or the whole organism making decisions centrally somehow, both theories find the code in the DNA. Therefore, the key to aging is the master genes in the DNA. And with no real surprise, we note that similar master genes are responsible for cell differentiation and timing of gene activation during early growth. Aging is just another stage of life.
_
Hayflick limit:
The Hayflick limit is the number of times a normal human cell population will divide until cell division stops. Empirical evidence shows that the telomeres associated with each cell’s DNA will get slightly shorter with each new cell division until they shorten to a critical length. The concept of the Hayflick limit was advanced by Leonard Hayflick in 1961, at the Wistar Institute in Philadelphia. Hayflick demonstrated that a population of normal human fetal cells in a cell culture will divide between 40 and 60 times. The population will then enter a senescence phase. Each mitosis slightly shortens each of the telomeres on the DNA of the cells. Telomere shortening in humans eventually makes cell division impossible, and this aging of the cell population appears to correlate with the overall physical aging of the human body. This mechanism also appears to prevent genomic instability. Telomere shortening may also prevent the development of cancer in human aged cells by limiting the number of cell divisions. However, shortened telomeres impair immune function that might also increase cancer susceptibility.
_
Telomere and telomerase:
Inside the nucleus of a cell, our genes are located on twisted, double-stranded molecules of DNA called chromosomes. A telomere is a region of repetitive nucleotide sequences at each end of a chromosome. It protects the end of the chromosome from deterioration or from fusion with neighboring chromosomes. Telomeres have been compared with the plastic tips on shoelaces because they prevent chromosome ends from fraying and sticking to each other, which would scramble an organism’s genetic information to cause cancer, other diseases or death. Yet, each time a cell divides, the telomeres get shorter. When they get too short, the cell no longer can divide and becomes inactive or “senescent” or dies. This process is associated with aging, cancer and a higher risk of death. Telomeres act as a molecular clock, reflecting the replicative history of a primary cell. When telomeres reach a critical minimal length, their protective structure is disrupted. This triggers a DNA damage response (DDR). Telomerase is a ribonucleoprotein that is an enzyme which adds DNA sequence repeats (“TTAGGG” in all vertebrates) to the end of DNA strands in the telomere regions. Telomerase is a reverse transcriptase, which is a class of enzyme that creates single-stranded DNA using single-stranded RNA as a template. Telomerase extends the length of a telomere. In young cells, telomerase keeps telomeres from wearing down too much. But as cells divide repeatedly, there is not enough telomerase, so the telomeres grow shorter and the cells age. Telomerase remains active in sperm and eggs, which are passed from one generation to the next. If reproductive cells did not have telomerase to maintain the length of their telomeres, any organism with such cells soon would go extinct.
_
_
Characteristics of telomeres:
- They are made up of a repetitive sequence of DNA
- Shorten with cell divisions and can signal when a cell should die
- If telomeres are missing, the ends of chromosomes tend to get chewed away or they may stick to other chromosomes
- They allow the chromosomes to be replicated in their entirety, avoiding errors
- In cells like germ or stem cells, the action of the enzyme telomerase adds DNA stretches to the telomeres.
__
_
Telomere length:
Telomeres are non-coding regions at the tips of chromosomes. In vertebrates, they are composed of repeated sequences of TTAGGG. The Hayflick limit has been found to correlate with the length of the telomere region at the end of a strand of DNA. During the process of DNA replication, small segments of DNA at each end of the DNA strand (telomeres) are unable to be copied and are lost after each time DNA is duplicated. The telomere region of DNA does not code for any protein; it is simply a repeated code on the end region of DNA that is lost. After many divisions, the telomeres become depleted and the cell begins apoptosis. This is a mechanism that prevents replication error that would cause mutations in DNA. Once the telomeres are depleted, due to the cell dividing many times, it will no longer divide having reached its Hayflick limit. This process does not take place in cancer cells due to an enzyme called telomerase. This enzyme maintains telomere length, which results in the telomeres of cancer cells never shortening. This gives these cells infinite replicative potential. A proposed treatment for cancer is the usage of telomerase inhibitors that would prevent the restoration of the telomere, allowing the cell to die like other body cells. On the other hand, telomerase activators might repair or extend the telomeres of healthy cells, thus extending their Hayflick limit. Telomerase activation might also lengthen the telomeres of immune system cells enough to prevent cancerous cells from developing from cells with very short telomeres.
_
_
Telomeres as modulators of self-renewal:
An important component of self-renewal is the maintenance of telomere length. In many cancers, the ability to proliferate limitlessly is dependent in part on the activation of telomerase, which enables the cell to maintain telomere length and subvert a normal state of senescence. While normal somatic cells do not express telomerase, high telomerase activity has also been associated with a high degree of self renewal in hematopoietic stem cells (HSCs), and telomere length decreases with repeated transplantation of HSCs. Thus, if one mechanism of stem cell exhaustion is the reduction of telomere length, one prediction would be that increased or decreased telomere length may lead to greater or lesser self-renewal activity, respectively. This idea was tested recently by examining the extent of serial transplantation that can be carried out by HSCs from telomerase reverse transcriptase (TERT)-deficient and transgenic mice. While wild-type HSCs can maintain reconstitution ability for four rounds of serial transplantation, TERT-/- HSCs exhaust prematurely after two rounds of serial transplantation, suggesting that telomere length is indeed critical for maintaining the self-renewal ability of HSCs. Whether extending the length of telomeres allows HSCs to extend their natural lifespan was tested by the generation of a transgenic mouse in which telomerase was overexpressed using an H2K promoter. Overexpression of telomerase was found to increase telomere lengths in the transgenic mouse, and transgenic mice clearly maintained longer telomeres with serial transplantation. However, neither wild-type nor transgenic mouse bone marrow were able to contribute to more than four rounds of serial transplantation. This suggests that extending telomere length alone is not sufficient to extend self-renewal capacity of HSCs and that other factors must contribute to the clock that limits the HSC lifespan. Some long-lived species like humans have telomeres that are much shorter than species like mice, which live only a few years. Nobody yet knows why. But it’s evidence that telomeres alone do not dictate lifespan. It is possible oxidative stress, glycation, telomere shortening and chronological age – along with various genes – all work together to cause aging.
_
Besides telomere shortening, there are other factors involved in aging:
_
Role of telomeres in apoptosis: telomere shortening precedes apoptosis:
_
In a nutshell, cellular senescence is a state between cell maturity and cell death where cells have irreversibly lost their proliferation ability despite continued viability & metabolic activity, and exhibit deficiencies in maintaining their homeostatic processes. Both genetic and environmental factors contribute to senescence.
____________
Cell injury:
The term cell injury is used to indicate a state in which the capacity for physiological adaptation is exceeded. This may occur when the stimulus is excessive or when the cell is no longer capable to adapt without suffering some form of damage. The capacity for adaptation and the sensitivity to different types of injury varies according to cell type (i.e. myocardial cells and neurons are highly sensitive to ischemic injury; hepatocytes are more sensitive to chemical than ischemic injury). Cell injury may be reversible (non-lethal damage which generally can be corrected by removal of the stimulus) or irreversible (lethal damage). The most common types of reversible cell injury are manifested by accumulation of fluid (cellular swelling) and of fat (fatty change). Irreversibly injured cells die and have altered morphology. These morphologic patterns are recognized as necrosis or apoptosis. The transition between reversible and irreversible damage, commonly referred to as the “point of no return” is of major importance. Recognition of the point of no return is a key element for devising therapeutic strategies to prevent cell death after injury.
_
Types of cell injury:
_
Cells have intrinsic signaling mechanisms that are capable of sensing various deleterious conditions, both normal and pathological, and respond by mounting a variety of stress responses. Examples of normal signals are the cytokines that induce inflammatory responses in cells. Pathological signals include UV and X-ray irradiation, hydrogen peroxide (H2O2), abrupt anoxia and physicochemical injury through heat or noxious chemicals. A process of wound healing can function to repair the damage caused by such injuries. In many cases, especially if the stress signal is not too severe, the cell can survive and can even become tolerant to further insults. If cells are growing, such sub-lethal insults can either cause the cell to stop growing temporarily to allow adequate time to repair the damage, or the process of cell proliferation can be stopped more permanently and the cell will enter a state of senescence. Another example of an evolutionarily conserved survival mechanism is autophagy (vide infra), which enables cells to cope with periods of starvation. If such stresses become too severe, however, the cell dies, either through a process of necrosis, which is rapid and catastrophic, or through a slower and more controlled process that is carried out by a highly regulated process of programmed cell death known as apoptosis.
_
Main cellular mechanisms of cell injury
- ATP depletion
- Loss of calcium homeostasis
- Oxidative stress (excess Reactive Oxygen Species)
- Damage to mitochondria, and increased permeability of membranes
_
_
Free radicals and oxidative stress:
_
_
Oxidation is the loss of electrons, whilst reduction is a gain in electrons. Adenosine triphosphate (ATP) is produced as an energy source during the photosynthesis & cellular respiration and is consumed by many enzymes and many cellular processes. During its generation, electrons are transferred from reduced coenzymes to oxygen. Between 1 and 5% of this production escapes and results in the production of free radicals. During transport to the inner mitochondrial membrane, oxygen molecules gain four electrons, leading to the creation of superoxide radicals. Some of these are converted to hydrogen peroxide, H2O2 which diffuses through cell walls and generates more free radicals, in particular the hydroxyl radical, HO. Oxidation produces free radicals, which are molecules missing an electron in their outer shell. Highly unstable and reactive, these molecules “attack” other molecules attempting to “steal” electrons from their outer shells in order to gain stability. Free radicals damage other cells and DNA, creating more free radicals in the process and a chain reaction of oxidative damage. In cells they react with proteins, nucleic acids and (particularly) lipids, damaging the cell and its membrane. Animal experiments have shown that reducing free radicals increases longevity. This has sparked a whole diet and health industry. Oxidative stress is an increased level of free radicals and comes from an imbalance between the production of reactive oxygen and the body’s ability to detoxify the reactive intermediaries or repair resulting damage. Oxygen-based free radicals plus non-radical oxidants (such as hydrogen peroxide) are called reactive oxygen species (ROS). These can also be caused by environmental pollution and radiation, as well as internal cell effects. Nitric oxide and peroxynitrate are reactive nitrogen species (RNS). RNS reacts with amino acids, thus disrupting function. Cells have defense mechanisms to combat oxidative damage, with antioxidants including vitamins A and E, glutathione (GSH), superoxide dismutase (SOD), catalyse and glutathione peroxidase. These primary antioxidants remove free radicals, scavenging ROS and RNS, binding to metals ions or converting free radicals to less harmful forms. Secondary antioxidants are enzymes that repair damage done by free radicals.
_
_
Oxidative stress and cell death will be discussed later on.
___________
Cellular adaptations:
_
_
Cells constantly adapt to physiological demands to maintain a homeostatic steady state. Cells adapt by performing excess work, replicating, decreasing functions, changing its differentiated properties etc. The main adaptations to a persistent stimulus may involve cellular hypertrophy, hyperplasia and metaplasia. Atrophy occurs whenever certain normal stimuli (workload, blood supply, etc) are decreased or lost. Depending on the specific condition, adaptive reactions can produce organ damage. Through these adaptations cells maintain their viability.
_
Inflammatory responses:
The innate immune system is our first line of defense against invading pathogens. Not only does it initiate a vigorous and rapid inflammatory response to attack foreign pathogens, but it also plays a key role in activating the more slowly developing adaptive immune response. The latter results in the activation of specific B and T cells to extend the host defense system further. The initial line of defense during the innate response is carried out by a complex series of cellular interactions, which is known collectively as the inflammatory response. Such responses are particularly evident during wound healing. The major cellular players are the blood platelets, macrophages, mast cells, neutrophils and endothelial cells.
_
The inflammasome is a multiprotein oligomer consisting of caspase 1, PYCARD, NALP and sometimes caspase 5. It is expressed in myeloid cells and is a component of the innate immune system. The exact composition of an inflammasome depends on the activator which initiates inflammasome assembly, e.g. dsRNA will trigger one inflammasome composition whereas asbestos will assemble a different variant. The inflammasome promotes the maturation of inflammatory cytokines Interleukin 1β (IL-1β) and Interleukin 18 (IL-18). The inflammasome is responsible for activation of inflammatory processes, and has been shown to induce cell pyroptosis, a process of programmed cell death distinct from apoptosis.
_
Cell stress response:
During tissue homeostasis there is equilibrium between the net growth rate and the net rate of cell death. Upon exposure to cellular stress this physiological homeostasis is in danger. Cells can respond to stress in various ways ranging from the activation of survival pathways to the initiation of cell death that eventually eliminates damaged cells. Whether cells mount a protective or destructive stress response depends to a large extent on the nature and duration of the stress as well as the cell type. Also, there is often the interplay between these responses that ultimately determines the fate of the stressed cell. The mechanism by which a cell dies (i.e., apoptosis, necrosis, pyroptosis, or autophagic cell death) depends on various exogenous factors as well as the cell’s ability to handle the stress to which it is exposed. The implications of cellular stress responses to human physiology and diseases are manifold and are connected to some major world health issues such as diabetes, Parkinson’s disease, myocardial infarction, and cancer.
_
Cell stress, cell adaptation, cell injury and cell death:
_
_
In response to cell stress and/or injury, body launches prosurvival response:
Prosurvival signaling and responses:
1. The Heat Shock Response:
One of the main prosurvival activities of cells, the heat shock response, was originally described as the biochemical response of cells to mild heat stress (i.e., elevations in temperature of C above normal). It has since been recognized that many stimuli can activate this response, including oxidative stress and heavy metals. One of the main cellular consequences of these stresses is protein damage leading to the aggregation of unfolded proteins. In order to counteract this, cells increase the expression of chaperone proteins that help in the refolding of misfolded proteins and alleviate protein aggregation. This confers a transient protection, leading to a state that is known as thermotolerance, whereby cells become more resistant to various toxic insults, including otherwise lethal temperature elevations, oxidative stress, various anticancer drugs, and trophic factor withdrawal.
2. The Unfolded Protein Response (UPR):
Secretory and membrane proteins undergo posttranslational processing, including glycosylation, disulfide bond formation, correct folding, and oligomerization, in the ER. In order to effectively produce and secrete mature proteins, cellular mechanisms for monitoring the ER environment are essential. Exposure of cells to conditions such as glucose starvation, inhibition of protein glycosylation, disturbance of Ca2+ homeostasis and oxygen deprivation causes accumulation of unfolded proteins in the ER (ER stress) and results in the activation of a well orchestrated set of pathways during a phenomenon known as the unfolded protein response.
3. The DNA Damage Response:
Upon cellular stress conditions that are caused by exposure to chemotherapeutic agents, irradiation, or environmental genotoxic agents such as polycyclic hydrocarbons or ultraviolet (UV) light, damage to DNA is a common initial event. DNA double strand breaks (DSBs) and single strand breaks (SSBs) are considered as key lesions that initiate the activation of the DNA damage response. Since the DNA duplex is more vulnerable to chemical attack or nucleases when it is separated into two single-stranded DNA strands, for example, during DNA replication and transcription, SSBs are preferentially generated under these conditions. Defined SSBs are also generated during distinct pathways of DNA repair, for example, in the course of nucleotide excision repair (NER). DSBs are produced directly or indirectly by many anticancer drugs, including DNA intercalating, alkylating or crosslinking agents, topoisomerase inhibitors, and nucleotide analogs. Once DSBs are generated, ataxia telangiectasia mutated (ATM) is recruited by the MRE-11-Rad50-NBS1 (MRN) complex to sites of broken DNA and phosphorylates downstream substrates such as checkpoint kinase 2 (Chk2) and p53. p53 induces transcriptional activation of different functional programs, for example, cell cycle regulatory proteins such as p21 and pro-apoptotic factors such as CD95, PUMA, and BAX. In addition, recent studies have also defined a nontranscriptional pro-apoptotic activity of p53 that regulates the intrinsic mitochondria-mediated pathway of apoptosis. Damage to DNA engages DNA repair processes to ensure the cell’s survival in the case of sublethal damage. Alternatively, if the damage is too severe to be repaired—the DNA-damaging insult is transmitted by the cellular stress response to the activation of effector systems to mediate cell death. In the latter case, various stress-inducible molecules, including NF-κB, p53, JNK, or MAPK/ERK, have been implicated in propagating and modulating the cell death signal.
4. The Response to Oxidative Stress:
Cell survival requires appropriate proportions of molecular oxygen and various antioxidants. Reactive products of oxygen are amongst the most potent and omnipresent threats faced by cells. These include ROS such as superoxide anion, hydrogen peroxide (H2O2), singlet oxygen, hydroxyl radical (OH-), peroxy radical, as well as the second messenger nitric oxide (NO) which can react with to form peroxynitrite (ONOO−). Normally in cells there exists equilibrium between pro-oxidant species and antioxidant defense mechanisms such as ROS-metabolizing enzymes including catalase, glutathione peroxidase, and superoxide dismutases (SODs) and other antioxidant proteins such as glutathione (GSH) as seen in the figure below. Oxidative stress occurs when there is a disturbance in this pro-oxidant: antioxidant balance and it has been implicated in several biological and pathological processes. Although most oxidative insults can be overcome by the cell’s natural defenses, sustained perturbation of this balance may result in either apoptotic or necrotic cell death.
_____________
The figure below shows that apoptosis is held in check by prosurvival signals received by cell surface receptors:
_
Cell survival signaling:
_
_
The figure above shows the PI 3-kinase pathway of cell survival. Survival factors such as NGF activate receptor protein-tyrosine kinases, leading to activation of PI 3-kinase and formation of PIP3. PIP3 recruits the protein kinase Akt to the plasma membrane where it is activated as a result of phosphorylation by PDK. Akt then appears to phosphorylate a number of proteins that contribute to cell survival. The targets of Akt that have been implicated in suppression of apoptosis include the Bcl-2 family member Bad, caspase-9, several transcription factors, and the protein kinase GSK-3, which affects cell metabolism and protein synthesis. Cell survival is mediated not only by PI 3-kinase/Akt signaling, but also by other signaling pathways including the Ras/Raf/MAP kinase pathway. One mechanism through which this pathway inhibits apoptosis involves the phosphorylation and activation of a protein kinase called RSK by ERK. Like Akt, RSK phosphorylates the Bcl-2 family member Bad, so Bad serves as a site of convergence for the PI 3-kinase/Akt and MAP kinase pathways in signaling cell survival. In addition, ERK and RSK may act by phosphorylating transcription factors that affect the expression of genes that regulate apoptosis.
_
Switch from Prosurvival Signaling to Cell Death Signaling:
While conditions of stress stimulate cells to mount protective responses to counteract the effect of the stress on cellular processes, if the stress remains unresolved, eventual death of the cell ensues. This raises key questions about the molecular mechanisms involved in this switch from prosurvival signaling to prodeath signaling. For example, is there a particular molecule that acts as a molecular switch? How do the duration and severity of the stress contribute to activation of this switch? As described above, in the face of exposure to cell stress, the cell mounts protective responses such as the heat shock response, or the unfolded protein response, in order to relieve the stress and promote survival. However, it is known that if the stress is very severe or if it is prolonged, the cell will die in spite of the activation of prosurvival signaling.
_
What triggers cellular apoptosis?
No sooner has a Metazoan (i.e., multicellular organism) begun to organize itself, then it is subject to the slings and arrows of fate. Constituent cells can become infected with viruses or attacked by bacterial predators, their DNA can be damaged by replication errors, radiation or mutagenic chemicals. Or cells can lose their differentiation and thereby become neoplastic and eventually cancerous. If a cell is infected it may, in turn, infect its neighbors. DNA replication errors or mutations will be passed on to the cell’s progeny. Cancer cells, of course, grow rapidly without bound and disrupt vital bodily functions. Metazoan cells have evolved mechanisms for detecting internal errors or external assaults that might threaten the whole organism. When such threats are detected, the cells react by killing themselves. For many types of cell, loss of contact to the extracellular matrix (ECM) triggers apoptosis. That is, if the cell somehow becomes detached from its proper place in the body, it self-destructs. The apoptosis mechanism removes the cell with a minimum of risk or damage to nearby cells. Apoptosis shrinks the cell, degrades the DNA in its nucleus, breaks down the mitochondria, and then breaks the cell into small, neat, membrane-wrapped, fragments. Finally, nearby phagocytic cells engulf the cell fragments. The phagocytic cells also secrete cytokines that inhibit inflammation that would otherwise be a danger to the surrounding cells. Apoptosis must remove cells in such a careful and well controlled manner because removing cells is just as important to the health of the multicellular organism as growing new cells.
________________
Cell Death Definition:
Cell death is any biological process that results in permanent cessation of all vital functions of a cell. A cell should be considered dead when any one of the following molecular or morphological criteria is met:
(1) The cell has lost the integrity of its plasma membrane as defined by the incorporation of vital dyes (e.g., PI) in vitro;
(2) The cell, including its nucleus, has undergone complete fragmentation into discrete bodies (frequently referred to as “apoptotic bodies”); and/or
(3) Its corpse (or its fragments) has been engulfed by an adjacent cell in vivo.
___________
Cell death itself is a complex phenomenon that forms the basis for most disease processes. Until a few years ago the term necrosis was used as a synonym to cell death. It is now known that there are at least two distinct types of cell death: apoptosis (also known as programmed cell death) and necrosis. The major importance of this distinction between types of cell death is that while necrosis is always a pathological process, apoptosis may take place as a physiological phenomenon that is essential for life. Moreover, necrosis generally elicits an inflammatory reaction while apoptosis is not accompanied by inflammation.
_
Cell death by injury (necrosis)
-Mechanical damage
-Exposure to toxic chemicals
Cell death by suicide (apoptosis)
-Internal signals
-External signals
_____________
_______________
When is cell dead?
Dying cells are engaged in a process that is reversible until a first irreversible phase or ‘point-of-no-return’ is trespassed as seen in the table below.
_____________
Nomenclature of cell death:
Cell death can be classified according to its morphological appearance (which may be apoptotic, necrotic, autophagic or associated with mitosis), enzymological criteria (with and without the involvement of nucleases or of distinct classes of proteases, such as caspases, calpains, cathepsins and transglutaminases), functional aspects (programmed or accidental, physiological or pathological) or immunological characteristics (immunogenic or non-immunogenic).
_
Different types of cell death are often defined by morphological criteria, without a clear reference to precise biochemical mechanisms. The Nomenclature Committee on Cell Death (NCCD 2009) proposes unified criteria for the definition of cell death and of its different morphologies, while formulating several caveats against the misuse of words and concepts that slow down progress in the area of cell death research.
_
Typical cell death modalities:
_
_
Apoptosis:
The expression ‘apoptosis’ has been coined by Kerr et al. to describe a specific morphological aspect of cell death as seen in the table above. Apoptosis is accompanied by rounding-up of the cell, retraction of pseudopodes, reduction of cellular volume (pyknosis), chromatin condensation, nuclear fragmentation (karyorrhexis), classically little or no ultrastructural modifications of cytoplasmic organelles, plasma membrane blebbing (but maintenance of its integrity until the final stages of the process) and engulfment by resident phagocytes (in vivo). Hence, the term ‘apoptosis’ should be applied exclusively to cell death events that occur while manifesting several among these morphological features. It is worth noting that it is not correct to assume that ‘programmed cell death’ (PCD) and ‘apoptosis’ are synonyms because cell death, as it occurs during physiological development, can manifest non-apoptotic features.
_
Autophagy:
Autophagic cell death is morphologically defined (especially by transmission electron microscopy) as a type of cell death that occurs in the absence of chromatin condensation but accompanied by massive autophagic vacuolization of the cytoplasm. In contrast to apoptotic cells (whose clearance is ensured by engulfment and lysosomal degradation), cells that die with an autophagic morphology have little or no association with phagocytes. Although the expression ‘autophagic cell death’ is a linguistic invitation to believe that cell death is executed by autophagy, the term simply describes cell death with autophagy.
_
Necrosis:
‘Necrotic cell death’ or ‘necrosis’ is morphologically characterized by a gain in cell volume (oncosis), swelling of organelles, plasma membrane rupture and subsequent loss of intracellular contents. For a long time, necrosis has been considered merely as an accidental uncontrolled form of cell death, but evidence is accumulating that the execution of necrotic cell death may be finely regulated by a set of signal transduction pathways and catabolic mechanisms.
_
Cornification:
Cornification is a very specific form of PCD (programmed cell death) that occurs in the epidermis, morphologically and biochemically distinct from apoptosis. It leads to the formation of corneocytes, that is dead keratinocytes containing an amalgam of specific proteins (e.g., keratin, loricrin, SPR and involucrin) and lipids (e.g., fatty acids and ceramides), which are necessary for the function of the cornified skin layer (mechanical resistance, elasticity, water repellence and structural stability). Cornification is less often referred to as ‘keratinization’ or ‘cornified envelope formation’, and it is generally considered as a terminal differentiation program similar to those leading to other anucleated tissues (such as the lens epithelium and mature red blood cells). This is mainly due to the fact that these processes display the (often limited) activation of the molecular machinery for cell death, in particular of caspases. In contrast with corneocytes, however, both mature red blood and lens epithelial cells retain the ability to undergo stress-induced death, and hence only cornification should be regarded as a bona fide cell death program.
_
Tentative Definitions of Atypical Cell Death Modalities:
Mitotic catastrophe:
Mitotic catastrophe is a cell death mode occurring either during or shortly after a dysregulated/ failed mitosis and can be accompanied by morphological alterations including micronucleation (which often results from chromosomes and/or chromosome fragments that have not been distributed evenly between daughter nuclei) and multinucleation (the presence of two or more nuclei with similar or heterogeneous sizes, deriving from a deficient separation during cytokinesis). However, there is no broad consensus on the use of this term; mitotic catastrophe can lead either to apoptotic morphology or to necrosis. As a result, the NCCD recommends the use of expressions such as ‘cell death preceded by multinucleation’ or ‘cell death occurring during metaphase’, which are more precise and more informative.
_
Anoikis:
Apoptosis induced by the loss of the attachment to the substrate or to other cells is called anoikis. Besides its specific form of induction, the molecular mechanisms of anoikis-associated cell death match those activated during classical apoptosis. The NCCD acknowledges the use of this term for historical reasons, as it is already quite diffuse in the literature. However, it will be necessary to determine whether under certain circumstances other modalities of cell death occur in vivo following detachment, that is, whether there are forms of anoikis refractory to caspase inhibitors and/or others that manifest necrotic features.
_
Excitotoxicity:
This is a form of cell death occurring in neurons challenged with excitatory amino acids, such as glutamate, that leads to the opening of the N-methyl-Daspartate Ca2þ-permeable channel, followed by cytosolic Ca2þ overload and activation of lethal signaling pathways. Excitotoxicity seemingly overlaps with other types of death such as apoptosis and necrosis (depending on the intensity of the initiating stimulus), and involves MMP as a critical event. For these reasons, and for the presence of common regulators such as nitric oxide itself, excitotoxicity cannot be considered as a separate cell death modality.
_
Wallerian degeneration:
Additional less-characterized forms of cellular catabolism take place in the nervous system, such as Wallerian degeneration, in which part of a neuron or axon degenerates without affecting the main cell body. This term does not describe a type of cell death sensu stricto, because neurons affected by Wallerian degeneration remain alive.
_
Paraptosis:
This term was originally introduced to describe a form of PCD morphologically and biochemically distinct from apoptosis. In multiple cell types, paraptosis was triggered by the expression of the insulin-like growth factor receptor I, and it was associated with extensive cytoplasmic vacuolization and mitochondrial swelling, but without any other morphological hallmark of apoptosis. The manifestations of paraptosis could not be prevented by caspase inhibitors, nor by the overexpression of antiapoptotic Bcl-2-like proteins, and seemingly resulted from a signaling cascade involving specific members of the mitogen-activated protein kinase family. At present, it is still unclear whether paraptosis represents a route of cell death that is truly distinct from all others.
_
Pyroptosis:
Pyroptosis has first been described in macrophages infected with Salmonella typhimurium. It involves the apical activation of caspase-1 (but not of caspase-3), a protease that is mostly known as interleukin- 1b (IL-1b)-converting enzyme. Caspase-1 activation induced by S. typhimurium (and by other pathogens such as Pseudomonas aeruginosa and Shigella flexneri) occurs through Ipaf, an Apaf-1-related NLR protein. In contrast, pyroptosis induced by Bacillus anthracis lethal toxin does not require Ipaf and rather involves another NLR protein, that is Nalp1. In addition, lipopolysaccharidetreated macrophages (either in the presence or in the absence of ATP) undergo pyroptosis mediated by the adaptor protein ASC, which together with caspase-1 forms a supramolecular cytoplasmic complex also known as ‘pyroptosome’. Thus, distinct routes to caspase-1 activation induce pyroptosis. As this form of cell death leads to the release of IL-1b (which is one of the major feverinducing cytokines or pyrogens) and of IL-18, it may play a relevant role in both local and systemic inflammatory reactions. As it stands, macrophages undergoing pyroptosis not only exhibit morphological features that are typical of apoptosis, but also display some traits associated with necrosis.
_
Pyronecrosis:
Nalp3 and ASC are involved in the necrotic cell death of macrophages infected by S. flexneri at high bacteria/ macrophage ratios and associated with the release of HMGB-1, caspase-1 and IL-1b, which is called pyronecrosis. Pyronecrosis and pyroptosis are distinguished based on the fact that the latter (but not the former) requires caspase-1. It remains to be determined whether RIP1 is implicated in pyronecrosis, as well as whether pyroptosis and pyronecrosis play any role outside of the innate immune system.
_
Entosis:
Entosis, originally described as a form of ‘cellular cannibalism’ in lymphoblasts from patients with Huntington’s disease, has been reported as a new cell death modality in which one cell engulfs one of its live neighbors, which then dies within the phagosome. Intriguingly, the most efficient cells in performing entosis are MCF-7 breast cancer cells, which lack both caspase-3 and beclin-1 and hence are (relatively) apoptosis- and autophagy incompetent. This points to the possibility, which remains to be explored, that entosis is a default pathway that is unmasked (and hence can be observed) exclusively when other catabolic reactions are suppressed. Entosis is not inhibited by Bcl-2 or Z-VAD-fmk, and internalized cells appear virtually normal. Later they disappear, presumably through lysosomal degradation. In rare cases, however, internalized cells are able to divide within the engulfing cell or are released. Hence, it is difficult to know whether the entosis truly represents a novel cell death modality.
____________
The figure below shows electron microscopic appearance of various modes of cell deaths:
____________
From morphological (2009) to molecular definitions (2012) of cell death modalities: Nomenclature updated in 2012:
Molecular definitions of cell death subroutines:
Recommendations of the Nomenclature Committee on Cell Death 2012:
In 2009, the Nomenclature Committee on Cell Death (NCCD) proposed a set of recommendations for the definition of distinct cell death morphologies and for the appropriate use of cell death-related terminology, including ‘apoptosis’, ‘necrosis’ and ‘mitotic catastrophe’. In view of the substantial progress in the biochemical and genetic exploration of cell death, time has come to switch from morphological to molecular definitions of cell death modalities. Here NCCD proposes a functional classification of cell death subroutines that applies to both in vitro and in vivo settings and includes extrinsic apoptosis, caspase-dependent or -independent intrinsic apoptosis, regulated necrosis, autophagic cell death and mitotic catastrophe. On the basis of the new, revised NCCD classification, cell death subroutines are defined by a series of precise, measurable biochemical features as discussed below.
_
Definition of Extrinsic Apoptosis:
The term ‘extrinsic apoptosis’ has been extensively used to indicate instances of apoptotic cell death that are induced by extracellular stress signals that are sensed and propagated by specific transmembrane receptors. Extrinsic apoptosis can be initiated by the binding of lethal ligands, such as FAS/CD95 ligand (FASL/CD95L), tumor necrosis factor α (TNFα) and TNF (ligand) superfamily, member 10 (TNFSF10, best known as TNF-related apoptosis inducing ligand, TRAIL), to various death receptors (i.e., FAS/CD95, TNFα receptor 1 (TNFR1) and TRAIL receptor (TRAILR)1–2, respectively). Alternatively, an extrinsic pro-apoptotic signal can be dispatched by the so-called ‘dependence receptors’, including netrin receptors (e.g., UNC5A-D and deleted in colorectal carcinoma, DCC), which only exert lethal functions when the concentration of their specific ligands falls below a critical threshold level.
_
Definition of Intrinsic apoptosis:
In response to multiple intracellular stress conditions (e.g., DNA damage, cytosolic Ca2+ overload), pro-survival and pro-death signals are generated and converge to a mitochondrion-centered control mechanism. When lethal signals prevail, mitochondrial outer membrane permeabilization (MOMP) occurs and leads to mitochondrial transmembrane potential (Δψm) dissipation, arrest of mitochondrial ATP synthesis and Δψm-dependent transport activities. Moreover, the respiratory chains gets uncoupled, leading to reactive oxygen species (ROS) overgeneration, and proteins that are normally confined within the mitochondrial intermembrane space (IMS) are released into the cytosol. Among these, cytochrome c (CYTC) drives – together with the cytoplasmic adaptor protein APAF1 and dATP – the assembly of the so-called apoptosome, a multiprotein complex that triggers the caspase-9 caspase-3 proteolytic cascade. Direct IAP-binding protein with low pI (DIABLO, also known as second mitochondria-derived activator of caspases, SMAC) and high temperature requirement protein A2 (HTRA2) facilitate caspase activation by sequestering and/or degrading several members of the inhibitor of apoptosis protein (IAP) family. On the contrary, apoptosis-inducing factor (AIF) and endonuclease G (ENDOG) function in a caspase-independent manner by relocating to the nucleus and mediating large-scale DNA fragmentation. Of note, the serine protease HTRA2 also contributes to caspase-independent apoptosis by cleaving a wide array of cellular substrates (including cytoskeletal proteins).
_
Definition of ‘Regulated Necrosis’:
For a long time, necrosis has been considered as a merely accidental cell death mechanism and was defined by the absence of morphological traits of apoptosis or autophagy. Owing to the work of several laboratories, it is now clear that necrosis can occur in a regulated manner, and that necrotic cell death has a prominent role in multiple physiological and pathological settings. Several triggers can induce regulated necrosis, including alkylating DNA damage, excitotoxins and the ligation of death receptors, at least under selected circumstances. Indeed, when caspases (and in particular caspase-8) are inhibited by genetic manipulations (e.g., by gene knockout or RNA interference, RNAi) or blocked by pharmacological agents (e.g., chemical caspase inhibitors), RIP1 and its homolog RIP3 are not degraded and rather engage in physical and functional interactions that ultimately activate the execution of necrotic cell death. Regulated necrosis can be further characterized with regard to its dependence on specific signaling modules, and should be named consequently. For instance, cases of regulated necrosis that exhibit RIP1 activation (which can be measured by enzymatic assays or by monitoring RIP1 phosphorylation on S161) and that can be suppressed by RIP1 inhibitors including necrostatin-110, 86, 87 should be labeled ‘RIP1-dependent regulated necroses. Of note, RIP3-dependent, but RIP1-independent instances of regulated necrosis have recently been identified, and these are insensitive to necrostatins. The term ‘necroptosis’ has recently been used as a synonym of regulated necrosis, but it was originally introduced to indicate a specific case or regulated necrosis, which is ignited by TNFR1 ligation and can be inhibited by the RIP1-targeting chemical necrostatin-1. However, ‘necroptosis’ can be used to indicate RIP1- and/or RIP3-dependent regulated necrosis, provided that this expression is explicitly defined at its first appearance and used consistently thereafter.
_
Definition of ‘Autophagic Cell Death’:
On the basis of morphological features, the term ‘autophagic cell death’ has widely been used to indicate instances of cell death that are accompanied by a massive cytoplasmic vacuolization, which often (although not always) indicates increased autophagic flux. Although originally this expression did not imply any functional consideration, scientists quickly adopted the term ‘autophagic cell death’ and used it to imply that autophagy would actually execute the cell demise. This applies to at least two very distinct settings. First, autophagy has been shown to mediate physiological cell death in vivo, during the developmental program of D. melanogaster. Second, autophagy appears to be responsible for the death of some cancer cells (especially when they lack essential apoptotic modulators like BAX and BAK or caspases) that respond to a selected panel of chemotherapeutic agents in vitro. Nonetheless, in most known cases, autophagy constitutes a cytoprotective response activated by dying cells in the attempt to cope with stress, and its inhibition accelerates, rather than prevents, cell death.
_
Definition of ‘Mitotic Catastrophe’:
During the past decade, several attempts have been made to delineate the molecular pathways leading to mitotic catastrophe. Occasionally, researchers restrictively employ the term ‘mitotic catastrophe’ for cell death occurring in mitosis. More frequently, mitotic catastrophe refers to cases of cell death that are triggered by aberrant mitosis and executed either during mitosis or in the subsequent interphase. Recently, it has been proposed that mitotic catastrophe might not even constitute a bona fide cell death executioner mechanism, but an oncosuppressive pathway that precedes and is distinct from, yet operates through, cell death or senescence. After aberrant mitosis, cells frequently exhibit gross nuclear alterations (e.g., micro- and multinucleation), which have been used as morphological markers for the detection of mitotic catastrophe. However, apoptotic and necrotic traits have also been detected in such cells, either concomitant with or following multinucleation. Thus, end-point techniques are intrinsically unsuitable for assessing mitotic catastrophe, as they cannot reconstruct the sequence of events that have lead to cell death. To circumvent this issue, novel methods relying on high-throughput video microscopy or time-lapse fluorescence microscopy are under development. Several processes were originally associated with and were then shown to be dispensable for (at least some instances of) mitotic catastrophe. These include cell-cycle-specific kinases (such as the cyclin B1-dependent kinase Cdk1, polo-like kinases and Aurora kinases), cell-cycle checkpoint proteins, survivin, the activation of the DNA damage-responsive protease caspase-2, members of Bcl-2family, the tumor suppressor TP53 and other members of the TP53 family, including the TP73 variant TAp73.
_
Definition of Parthanatos:
Coined after Thanatos, the personification of death in Greek mythology, the term ‘parthanatos’ has been introduced to indicate a particular cell death mode involving the DNA damage-responsive enzymes poly(ADP-ribose) polymerases (PARPs), and in particular PARP1, which alone accounts for more than 90% cellular PARP activity. In physiological conditions, PARP1 cooperates with the DNA repair machinery to ensure genomic homeostasis upon mild DNA damage. Conversely, PARP1 overactivation has several toxic consequences, including NAD+ and ATP depletion, as well as the accumulation of mitochondriotoxic PAR, which favors Δψm dissipation and AIF release. Of note, AIF has recently been shown to possess a high-affinity PAR-binding site, and the physical interaction between PAR and AIF appears to be required for parthanatos, both in vitro and in vivo. Parthanatos have a role in multiple experimental and physiopathological scenarios, including stroke, diabetes, inflammation and neurodegeneration. In line with the original definition of parthanatos, cell death instances should be considered as parthanatic when they depend on early PARP1 activation (i.e., they can be blocked by its chemical and/or genetic inhibition), and exhibit NAD+ plus ATP depletion paralleled by AIF-mediated chromatinolysis. Parthanatos constitutes a caspase-independent cell death pathway.
_______________
Functional classification of regulated cell death modes as recommended by NCCD 2012:
| Main biochemical features | Caspase dependence | Examples of inhibitory interventions | |
| Anoikis | Downregulation of EGFR Inhibition of ERK1 signaling Lack of β1-integrin engagement Overexpression of BIM Caspase-3 (-6,-7) activation |
++ | BCL-2 overexpression Z-VAD-fmk administration |
| Autophagic cell death | MAP1LC3 lipidation SQSTM1 degradation |
−− | VPS34 inhibitors AMBRA1, ATG5, ATG7, ATG12 or BCN1 genetic inhibition |
| Caspase-dependent intrinsic apoptosis Caspase-independent intrinsic apoptosis | MOMP Irreversible Δψm dissipation Release of IMS proteins Respiratory chain inhibition |
++ −− | BCL-2 overexpression Z-VAD-fmk administration BCL-2 overexpression |
| Cornification | Activation of transglutaminases Caspase-14 activation |
+ | Genetic inhibition of TG1, TG3 or TG5 Genetic inhibition of caspase-14 |
| Entosis | RHO activation ROCK1 activation |
−− | Genetic inhibition of metallothionein 2A Lysosomal inhibitors |
| Extrinsic apoptosis by death receptors | Death receptor signaling Caspase-8 (-10) activation BID cleavage and MOMP (in type II cells) Caspase-3 (-6,-7) activation |
++ | CrmA expression Genetic inhibition of caspases (8 and 3) Z-VAD-fmk administration |
| Extrinsic apoptosis by dependence receptors | Dependence receptor signaling PP2A activation DAPK1 activation Caspase-9 activation Caspase-3 (-6,-7) activation |
++ | Genetic inhibition of caspases (9 and 3) Genetic inhibition of PP2A Z-VAD-fmk administration |
| Mitotic catastrophe | Caspase-2 activation (in some instances) TP53 or TP73 activation (in some instances) Mitotic arrest |
−− | Genetic inhibition of TP53 (in some instances) Pharmacological or genetic inhibition of caspase-2 (in some instances) |
| Necroptosis | Death receptor signaling Caspase inhibition RIP1 and/or RIP3 activation |
−− | Administration of necrostatin(s) Genetic inhibition of RIP1/RIP3 |
| Netosis | Caspase inhibition NADPH oxidase activation NET release (in some instances) |
−− | Autophagy inhibition NADPH oxidase inhibition Genetic inhibition of PAD4 |
| Parthanatos | PARP1-mediated PAR accumulation Irreversible Δψm dissipation ATP and NADH depletion PAR binding to AIF and AIF nuclear translocation |
−− | Genetic inhibition of AIF Pharmacological or genetic inhibition of PARP1 |
| Pyroptosis | Caspase-1 activation Caspase-7 activation Secretion of IL-1β and IL-18 |
++ | Administration of Z-YVAD-fmk Genetic inhibition of caspase-1 |
Abbreviations:
ATG, autophagy; BCN1, beclin 1; Δψm, mitochondrial transmembrane potential; CrmA, cytokine response modifier A; DAPK1, death-associated protein kinase 1; EGFR, epidermal growth factor receptor; ERK1, extracellular-regulated kinase 1; IL, interleukin; MAP1LC3, microtubule-associated protein 1 light chain 3; MOMP, mitochondrial outer membrane permeabilization; NET, neutrophil extracellular trap; PAD4, peptidylarginine deiminase 4; PAR, poly(ADP-ribose); PARP1, poly(ADP-ribose) polymerase 1; PP2A, protein phosphatase 2A; ROCK1, RHO-associated, coiled-coil containing protein kinase 1; SQSTM1, sequestosome 1; TG, transglutaminase; Z-VAD-fmk, N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone; Z-YVAD-fmk, N-benzyloxycarbonyl-Tyr-Val-Ala-DL-Asp-fluoromethylketone.
Note:
For classification purposes, pharmacological and genetic interventions should be considered inhibitory when they truly reduce the incidence of cell death, but not when they only provoke a shift between different cell death modalities or when they change the morphology of cell death.
________
Confusing nomenclature of cell death:
The nomenclature pertaining to cell death is confusing, and no single classification scheme encompasses all variant forms of cell death reported in the literature. Many authors use the terms apoptosis and programmed cell death synonymously, while others consider programmed cell death a more general term embracing different morphologies and biochemical processes. Cell death often proceeds by activating biochemical pathways that culminate in the activation of effector enzymes known as caspases. This type of cell death is known commonly as apoptosis and is being referred to also as type 1 cell death (Schweichel and Merker, 1973). Two major pathways participate in this type of cell death. They are not isolated from each other but cross-talk exists between them. The mitochondria-independent apoptosis pathway (also known as extrinsic apoptosis) is elicited by external ligands binding to their receptors. The intrinsic apoptosis pathway, also known as mitochondrial apoptosis (mitochondria-dependent apoptosis) involves the release of proteins from mitochondria. Type 2 cell death (Schweichel and Merker, 1973) involves a physiological process known as autophagy, which may be cytoprotective or result in apoptosis (Rami and Kõgel, 2008). The term type 3 cell death (Schweichel and Merker, 1973) refers to necrosis, as characterized by general disintegration and deletion of organelles. Clarke (1990) distinguishes between type 3A cell death, which does not involve lysosomal disintegration, and type 3B cell death, which is cytoplasmic cell death. A nomenclature committee of the Society of Toxicologic Pathologists has suggested that the term necrosis should be reserved for characterizing dead cells regardless of the pathway by which the cells died (Levin et al, 1999). As such, necrosis is not a form of cell death but a descriptive term for secondary processes taking place after a cell is dead (Majno and Joris, 1995; Wyllie and Golstein, 2001). Note also that any kind of protein implicated specifically in different kinds of cell death may have unrelated vital functions (Galluzzi et al, 2008).
_
The most widely used classification of mammalian cell death recognizes two types: apoptosis and necrosis. Autophagy, which has been proposed as a third mode of cell death, is a process in which cells generate energy and metabolites by digesting their own organelles and macromolecules. Autophagy allows a starving cell, or a cell that is deprived of growth factors, to survive. However, cells that do not receive nutrients for extended periods ultimately digest all available substrates and die (autophagy-associated cell death). Distinctions between apoptosis, necrosis, and autophagy entail differences in the mode of death and morphologic, biochemical, and molecular attributes.
_
1. Apoptosis (PCD Type 1) is the best-characterized type of programmed cell death because of its importance in development and homeostasis, and in the pathogenesis of different diseases such as cancer. Apoptotic cells die in a controlled fashion in response to a variety of extrinsic or intrinsic signals (e.g., activation of TNF receptors, DNA damage, and mitochondrial pathways). The hallmarks of apoptotic cell death include exposure of phosphatidylserine on the extracellular face of the plasma membrane, activation of caspases, disruption of mitochondrial membrane potential, cell shrinkage, DNA fragmentation and DNA condensation.
2. Autophagy (PCD Type 2) is selective degradation of intracellular targets, such as misfolded proteins and damaged organelles, and is an important homeostatic function. Autophagy performs in concert with the Ubiquitin-Proteasome System (UPS) to degrade aggregated/misfolded proteins that are ubiquitinated, targeting them for degradation by autophagy. The ubiquitinated cargo is carried to the phagophore and surrounds its cargo forming a double membrane vesicle, the autophagosome. The lyososome fuses to the autophagosome and the cargo is degraded inside the autolysosome.
3. Necrosis (PCD Type 3) is an uncontrolled cell death characterized by cell swelling, as well as destruction of the plasma membrane and subcellular organelles, without nuclear fragmentation and condensation. Necrotic cell death is considered a heterogeneous phenomenon including both programmed and accidental cell death. Necrosis is often defined in a negative manner, as a type of cell death that involves rupture of the plasma membrane without the hallmarks of apoptosis and without massive autophagic vacuolization.
____________
Programmed cell death (PCD):
Programmed cell-death (or PCD) is death of a cell in any form, mediated by an intracellular program. PCD is carried out in a regulated process, which usually confers advantage during an organism’s life-cycle. For example, the differentiation of fingers and toes in a developing human embryo occurs because cells between the fingers apoptose; the result is that the digits are separate. PCD serves fundamental functions during both plant and metazoa tissue development. Mutated cells incapable of performing their normal functions self-destruct in service of the multicellular organism as a whole. Apoptotic cell death occurs with certain mutational events, DNA damage, oxidative stress and withdrawal of some growth factors particularly within the immune system. Non-apoptotic programmed cell death includes: programmed necrosis, para-apoptosis, autophagic cell death, nutrient withdrawal, and subtypes associated with mis-folded protein response, and PARP mediated cell death. While apoptotic cell death follows a recognized cascade of caspase mediated enzymatic events, non-apoptotic cell death occurs in the absence of caspase activation. In vertebrates it has been called apoptosis and in invertebrates, cell deletion. Apoptosis and autophagy are both forms of programmed cell death, but necrosis is a non-physiological process that occurs as a result of infection or injury. Factors which directly influence the type of cell death include numerous intrinsic factors, cell type, organelles and their cross-talk.
_
Programmed cell death is an important concept. Cell death is “programmed” if it is genetically controlled. The recognition that cell death can occur by genetically controlled processes has enabled advances in unraveling the mechanisms of many diseases, and this new knowledge has facilitated the development of pharmacologic agents that initiate or inhibit programmed cell death. Moreover, there is now evidence that necrosis, traditionally considered an accidental form of cell death, can in certain instances be initiated or modulated by programmed control mechanisms. Senescence is an essential process of aging and occurs following a gene-directed program involving the erosion of telomeres and the activation of tumor suppression signaling pathways.
_
Programmed cell death plays an important role in vertebrate ontogeny (embryological development) and teratogenesis (the production of malformations), as well as in the spectacular metamorphoses that affect tadpoles or caterpillars. Such programmed events are essential if the organism as a whole is to develop its normal final form. Waves of genetically driven cell death are critical to the proper modeling of organs and systems. The inflections (curvatures) of the developing mammalian brain and spinal cord, for instance, or the achievement of a proper numerical balance between functionally related cell groups, cannot be understood without an appreciation of how the death of some (or many) cells is necessary for others to reach maturity. Localized cell death, occurring at precise moments during normal ontogeny, explains phenomena as varied as the fashioning of the digits or the involution of phylogenetic vestiges. Several congenital abnormalities can be attributed to disorders of programmed cell death. Cell death occurs spontaneously in normally involuting tissues such as the thymus. It can be initiated or inhibited by a variety of environmental stimuli, both physiological and pathological. Cell death even occurs in some of the cells of untreated malignant tumours, and it is seen during tumour regression induced by X rays or cytotoxic agents. Programmed cell death may also play a part in the process of aging, cells being designed to die after a certain number of mitotic divisions. Groups of cells responsible for the colour of human hair, for instance, may cease to function years before the hair itself loses the capacity to grow: the result is the “uncoloured” white hair of old age.
_
The term programmed cell death refers to controlled or regulated forms of death associated with a series of biochemical and morphological changes. The realization that some forms of cell death were biologically controlled or programmed has led to exploitation of these processes and has made profound impact in various fields of biology and medicine. The term apoptosis was first used to describe a particular morphology of cell death common to the vast majority of physiological cell deaths. This morphology includes shrinkage and blebbing of cells, rounding and fragmentation of nuclei with condensation, and margination of chromatin, shrinkage, and phagocytosis of cell fragments without accompanying inflammatory responses (in most cases). The morphology of cells undergoing apoptosis appeared dissimilar and distinct from the morphology associated with necrosis. Necrosis, a term commonly used by pathologists, refers to any deaths associated with the loss of control of ionic balance, uptake of water, swelling, and cellular lysis. This lysis releases many intracellular constituents, attracting immune cells and provoking an inflammatory response.
_
What makes a cell decide to commit suicide?
1. Withdrawal of positive signals:
Examples:
Growth factors for neurons
Interleukin-2 (IL-2)
2. Receipt of negative signals
Examples:
Increased levels of oxidants within the cell
Damage to DNA by oxidants
Death activators:
a) Tumor necrosis factor alpha (TNF-a)
b) Lymphotoxin (TNF-β)
c) Fas ligand (FasL)
_
Why are cells that die by programmed cell death generated?
Why do these cells die instead of surviving?
The answer to the first of these questions depends on the cell being considered. For example, some cells are generated in excess and only those that become properly functional survive (as happens in parts of the nervous system). In some cases, the mechanism that generates cells that are needed also fortuitously generates unneeded ones as well (as happens in the immune system). And some cells that die are needed, but only transiently. More generally, building a complex organism like a human being is like creating an intricate sculpture. Cell division forms the clay, whereas cell death sculpts the clay into the desired form. Consider human hands, which start out as paddle-like structures. Fingers develop in the paddles, but then the cells in the tissue between the fingers must die for a proper hand to form. There are cells that are probably evolutionary relics useful in the past, but no longer serve any valuable function. For example consider the cells in the tail of the tadpole, which become unnecessary when the animal develops into a frog. Cells die either because they are harmful or because it takes less energy to kill them than to maintain them. One of the most fascinating reasons for cell death is to get rid of dangerous cells, those that could be harmful to the rest of the organism. One might say that the cells kill themselves for the greater good. They could be mutants that would become cancerous–apoptosis is therefore very important in the formation (or nonformation) of cancer. Also, positive and negative selection occurs among the cells of the immune system. Cells that recognize ‘self’ (that is, ones that would attack the organism’s own cells) are instructed to die during this process. Finally, cells that are infected by a virus can sometimes recognize the infection and kill themselves before the virus has time to replicate and spread to other cells.
_
Evolutionary origin of PCD:
Biologists had long suspected that mitochondria originated from bacteria that had been incorporated as endosymbionts (“living together inside”) of larger eukaryotic cells. It was Lynn Margulis who from 1967 on championed this theory, which has since become widely accepted. The most convincing evidence for this theory is the fact that mitochondria possess their own DNA and are equipped with genes and replication apparatus. This evolutionary step would have been risky for the primitive eukaryotic cells, which began to engulf the energy-producing bacteria, as well as a perilous step for the ancestors of mitochondria, which began to invade their proto-eukaryotic hosts. This process is still evident today, between human white blood cells and bacteria. Most of the times invading bacteria are destroyed by the white blood cells; however, it is not uncommon for the chemical warfare waged by prokaryotes to succeed, with the consequence known as infection and its resulting damage. One of these rare evolutionary events, about two billion years before the present, made it possible for certain eukaryotes and energy-producing prokaryotes to coexist and mutually benefit from their symbiosis. Mitochondriate eukaryotic cells live poised between life and death, because mitochondria still retain their repertoire of molecules that can trigger cell suicide. This process has now been evolved to happen only when programmed. Given certain signals to cells (such as feedback from neighbors, stress or DNA damage), mitochondria release caspase activators that trigger the cell-death-inducing biochemical cascade. As such, the cell suicide mechanism is now crucial to all of our lives.
_
The figure below shows evolutionary conservation of apoptotic pathways:
______________
Pseudo-apoptosis:
Pseudo-apoptosis has been used to define a cellular state closely resembling the initial stages of apoptosis, but asserts a readily reversible state of which a cell can resume normal cellular function. Chemical and morphological changes morphology- a cell may undergo associated with pseudo-apoptosis include blebbing, plasma membrane lipid asymmetry cell membrane, cytoskeleton alterations cytoskeleton, mitochondrial function mitochondria, and increased concentration of cytosolic calcium. Regardless of these cellular alterations, pseudo-apoptotic cells reverse these changes to resume normal cellular process. Bleomycin (BLM) is a cytotoxic, anticancerous drug that catalyzes double-stranded breaks (DSB) and single-stranded breaks (SSB) along DNA molecules. At appropriate dosages, BLM generates morphological changes resembling typical apoptotic events, such as membrane blebbing and altered mitochondrial functioning. Degradation of DNA is also induced without the presence or assistance of specific endonuclease or protease that are involved under classic apoptotic conditions, which defines the usage of this form of pseudo-apoptosis. The relative dose administered determines the extent to which DNA fragmentation occurs. In the presence of large BLM concentrations, pseudo-apoptosis is observed as rapid DNA fragmentation occurs, resulting in cell death in the absence of typical apoptotic components such as specific endonucleases and proteases. Experimental evidence has suggested that every BLM molecule induces an average of 8 to 10 DNA strand breaks. An average ratio of 6 single-stranded breaks is generated for every double-stranded break. These numbers are dependent upon the form of BLM taken into consideration as deglyco-bleomycin has been found to be 100 times less toxic than wild-type BLM. Other forms of BLM forming complexes with various metals have suggested other variability when inducing pseudoapoptosis.
_
Slow cell death:
Though the term apoptosis was originated in pathology and developmental biology as an alternative to necrosis, the tissue necrosis with inflammation is irrelevant to cell culture conditions where apoptosis is mostly studied. Furthermore, no one single morphological feature is either necessary or sufficient to define apoptosis. The emerging biochemical definition, a cell death with caspase activation, allows the distinction of alternative forms of cell death. Thus, inhibition of caspases delays but does not prevent cell death. Slow cell death without caspase activation may nevertheless be associated with DNA fragmentation. Oncogenic Ras, Raf, and mitogen-activated kinases inhibit apoptosis by affecting the cytochrome C/caspase-9 pathway but may arrest growth and cause slow cell death with delayed DNA fragmentation. Such ‘slow’ cell death without caspase activation is often caused by chemotherapeutic drugs. Whether a cell will undergo apoptosis or slow death depends not only on a chemotherapeutic agent but also on the readiness of cellular caspases. Therefore, one can distinguish apoptosis-prone (e.g. leukemia) vs. apoptosis-resistant cells. Cell susceptibilities to spontaneous, starvation-induced and drug-induced apoptosis are correlated and characterize an apoptosis-prone phenotype. Finally, distinction of slow cell death allows rephrasing of a question regarding the goal of cancer therapy: apoptosis vs. slow cell death, or cancer cell-selectivity regardless of the mode of cell death.
_____________
How do you measure cell death?
LDH Cytotoxicity Detection Kit- Easy: Colorimetric Test for Cell Death:
The easiest way to measure cell death is by measuring lactate dehydrogenase (LDH), a stable cytoplasmic enzyme which is present in all cells but only released when the plasma membrane is damaged. The LDH Cytotoxicity Detection Kit provides a simple and precise colorimetric assay for LDH activity: a two-step enzymatic reaction creates a formazan dye that is easily measured by A492.
_
Common methods for measuring apoptotic cell death by flow cytometry:
Flow cytometry can be applied in basic research and in the clinic to identify and measure apoptotic cells. The choice of a particular flow cytometry method depends on several variables (cell system, type of flow cytometer, type of apoptosis, type of apoptosis inducer, type of information required). Regardless of the flow cytometry technique used to measure apoptosis, in most situations the type of cell death should be confirmed by direct microscope inspection. Apoptotic cells display a very specific pattern of morphological changes at the light, electron and fluorescence microscope and this should be the deciding factor when ambiguity arises regarding the mechanism of cell death. It should also be stressed that apoptosis is a sequential process of variable (1-8 h) duration, depending on inducer and cell type. Any method has an optimal ‘time window’ for analyzing the apoptotic process and it is possible that the percentage of apoptotic cells in the same population may not be identical when two methods are utilized (i.e. Annexin-V and TdT-assay are more sensitive than DNA-content analysis in the early phases of apoptosis). The type of information that is being sought is also critical. For example, if specificity of apoptosis with respect to cell-cycle phase is investigated, TdT-assay with PI counterstaining is the method of choice.
_
Membrane integrity essay:
Possibly the simplest assay for cell death is measurement of plasma membrane integrity. This can be assessed in two ways: The ability of a cell to prevent a fluorescent dye from entering it and the ability of a cell to retain a fluorescent dye within it.
_
The figure below shows flow-chart for measurement of apoptotic cell death:
_____________
Apoptosis:
Already since the mid-nineteenth century, many observations have indicated that cell death plays a considerable role during physiological processes of multicellular organisms, particularly during embryogenesis and metamorphosis [Gluecksmann, 1951; Lockshin, 2001]. The term programmed cell death was introduced in 1964, proposing that cell death during development is not of accidential nature but follows a sequence of controlled steps leading to locally and temporally defined self-destruction [Lockshin, 1964]. Eventually, the term apoptosis had been coined in order to describe the morphological processes leading to controlled cellular self-destruction and was first introduced in a publication by Kerr, Wyllie and Currie [Kerr, 1972]. Apoptosis is of Greek origin, having the meaning “falling off or dropping off”, in analogy to leaves falling off trees or petals dropping off flowers. This analogy emphasizes that the death of living matter is an integral and necessary part of the life cycle of organisms. The apoptotic mode of cell death is an active and defined process which plays an important role in the development of multicellular organisms and in the regulation and maintenance of the cell populations in tissues upon physiological and pathological conditions. It should be stressed that apoptosis is a well-defined and possibly the most frequent form of programmed cell death, but that other, non-apoptotic types of cell death also might be of biological significance [Leist, 2001a].
_
Definition of apoptosis (Greek: apo – from, ptosis – falling):
In Greek, apoptosis translates to the “dropping off” of petals or leaves from plants or trees:
A type of cell death in which the cell uses specialized cellular machinery to kill itself; a cell suicide mechanism that enables metazoans to control cell number and eliminate cells that threaten the animal’s survival. Apoptosis a form of cell death in which a controlled sequence of events (or program) leads to the elimination of cells without releasing harmful substances into the surrounding area. Many types of cell damage can trigger apoptosis, and it also occurs normally during development of the nervous system and other parts of the body. Strictly speaking, the term apoptosis refers only to the structural changes cells go through, and programmed cell death (PCD) refers to the complete underlying process, but the terms are often used synonymously. However, PCD includes other modes of cell deaths besides apoptosis but apoptosis remains the prototype of PCD. Apoptosis is a form of programmed cell death involving a biochemical cascade including such proteins as Bcl-2, Bax, Apaf-1, caspases such as caspase-9, caspase-3, and caspase-7, as well as proteins involved in digestion of proteins, degradation of DNA, and phagocytosis. Apoptosis is one of the main types of programmed cell death which involves a series of biochemical events leading to specific cell morphology characteristics and ultimately death of cells. Characteristic cell morphology of cells undergoing apoptosis include blebbing, changes to the cell membrane such as loss of membrane asymmetry and attachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation. During apoptosis, the cell membrane loses its asymmetry, and phosphatidylserine (PS) becomes exposed on the cell surface. The simplest way to observe this phenomenon in vitro is to use a cell permeate DNA-staining fluorescent dye such as Hoechst 33342, which allows a striking visualization of the chromatin condensation. Apoptosis differentiates from necrosis as the processes associated with apoptosis in disposal of cellular debris do not damage the organism in apoptosis. Unlike necrosis, apoptosis produces cell fragments called apoptotic bodies that phagocytic cells are able to engulf and quickly remove before the contents of the cell can spill out onto surrounding cells and cause damage
_
Apoptosis versus mitosis:
It is now realised that the processes of apoptosis and mitosis are really ‘two sides of the same coin’. Mitosis, as all biology students are taught, is cell multiplication, leading to tissue growth. Apoptosis is cell deletion, leading to tissue shrinkage locally. The intentional rhyming of apoptosis with mitosis shows that there is normally a balance between cell death and cell birth respectively.
_
Apoptosis occurs normally during development, aging and as a homeostatic mechanism to maintain cell populations in tissues. Apoptosis also occurs as a defense mechanism such as in immune reactions or when cells are damaged by disease or noxious agents (Norbury and Hickson, 2001). Although there are a wide variety of stimuli and conditions, both physiological and pathological, that can trigger apoptosis, not all cells will necessarily die in response to the same stimulus. Irradiation or drugs used for cancer chemotherapy results in DNA damage in some cells, which can lead to apoptotic death through a p53-dependent pathway. Some hormones, such as corticosteroids, may lead to apoptotic death in some cells (e.g., thymocytes) although other cells are unaffected or even stimulated. Some cells express Fas or TNF receptors that can lead to apoptosis via ligand binding and protein cross-linking. Other cells have a default death pathway that must be blocked by a survival factor such as a hormone or growth factor. There is also the issue of distinguishing apoptosis from necrosis, two processes that can occur independently, sequentially, as well as simultaneously (Hirsch, 1997; Zeiss, 2003). In some cases it’s the type of stimuli and/or the degree of stimuli that determines if cells die by apoptosis or necrosis. At low doses, a variety of injurious stimuli such as heat, radiation, hypoxia and cytotoxic anticancer drugs can induce apoptosis but these same stimuli can result in necrosis at higher doses. Finally, apoptosis is a coordinated and often energy-dependent process that involves the activation of a group of cysteine proteases called “caspases” and a complex cascade of events that link the initiating stimuli to the final demise of the cell. Persuasive evidence that these proteases are involved in most examples of apoptotic cell death has come from the ability of specific caspase inhibitors to block cell death, as well as the demonstration that knockout mice lacking caspase 3, 8 and 9 fail to complete normal embryonic development.
_
Morphological features of apoptosis:
Apoptotic cells can be recognized by stereotypical morphological changes: the cell shrinks, shows deformation and looses contact to its neighbouring cells. Its chromatin condenses and marginates at the nuclear membrane, the plasma membrane is blebbing or budding, and finally the cell is fragmented into compact membrane-enclosed structures, called ‘apoptotic bodies’ which contain cytosol, the condensed chromatin, and organelles. The apoptotic bodies are engulfed by macrophages and thus are removed from the tissue without causing an inflammatory response. Mitochondria remain unchanged morphologically. This type of cell death is often hard to observe in vivo because the dying cells are rapidly phagocytosed by tissue macrophages, and this phagocytosis is clearly different from that seen in inflammation, when activated macrophages are recruited from outside the immediate area of death. Those morphological changes are a consequence of characteristic molecular and biochemical events occurring in an apoptotic cell, most notably the activation of proteolytic enzymes which eventually mediate the cleavage of DNA into oligonucleosomal fragments as well as the cleavage of a multitude of specific protein substrates which usually determine the integrity and shape of the cytoplasm or organelles [Saraste, 2000]. It is in contrast to the necrotic mode of cell-death in which case the cells suffer a major insult, resulting in a loss of membrane integrity, swelling and rupture of the cells. During necrosis, the cellular contents are released uncontrolled into the cell’s environment which results in damage of surrounding cells and a strong inflammatory response in the corresponding tissue [Leist, 2001a].
_
_
_
Physiological and pathological apoptosis:
Apoptosis can be physiological or pathological and often results in the elimination of abnormal or “unwanted” cells (it is as if these cells commit suicide by eliminating themselves through the activation of the apoptotic machinery).
Physiologic Apoptosis:
The role of apoptosis in normal physiology is as significant as that of its counterpart, mitosis. It demonstrates a complementary but opposite role to mitosis and cell proliferation in the regulation of various cell populations. It is estimated that to maintain homeostasis in the adult human body, around 50 billion cells are made each day just to balance those dying by apoptosis. And that number can increase significantly when there is increased apoptosis during normal development and aging or during disease. Apoptosis is critically important during various developmental processes. As examples, both the nervous system and the immune system arise through overproduction of cells. This initial overproduction is then followed by the death of those cells that fail to establish functional synaptic connections or productive antigen specificities, respectively (Nijhawan et al., 2000; Opferman and Korsmeyer, 2003). Apoptosis is also necessary to rid the body of pathogen-invaded cells and is a vital component of wound healing in that it is involved in the removal of inflammatory cells and the evolution of granulation tissue into scar tissue (Greenhalgh, 1998). Dysregulation of apoptosis during wound healing can lead to pathologic forms of healing such as excessive scarring and fibrosis. Apoptosis is also needed to eliminate activated or auto-aggressive immune cells either during maturation in the central lymphoid organs (bone marrow and thymus) or in peripheral tissues (Osborne, 1996). Additionally, apoptosis is central to remodeling in the adult, such as the follicular atresia of the postovulatory follicle and post-weaning mammary gland involution, to name a couple of examples (Tilly, 1991; Lund et al., 1996). Furthermore, as organisms grow older, some cells begin to deteriorate at a faster rate and are eliminated via apoptosis. One theory is that oxidative stress plays a primary role in the pathophysiology of age-induced apoptosis via accumulated free-radical damage to mitochondrial DNA (Harman, 1992; Ozawa, 1995). It is clear that apoptosis has to be tightly regulated since too little or too much cell death may lead to pathology, including developmental defects, autoimmune diseases, neurodegeneration, or cancer.
Physiological apoptosis occurs in:
Destruction of cells during embryonic development
Balance between cell death/proliferation in normal tissues
Regulation of cellular populations in hormonally sensitive tissues
_
Pathologic Apoptosis:
Abnormalities in cell death regulation can be a significant component of diseases such as cancer, autoimmune lymphoproliferative syndrome, AIDS, ischemia, and neurodegenerative diseases such as Parkinson’s disease, Alzheimer ’s disease, Huntington ’s disease, and Amyotrophic Lateral Sclerosis. Some conditions feature insufficient apoptosis whereas others feature excessive apoptosis.
Pathological apoptosis occurs in:
Cell death after DNA damage caused by radiation, cancer treatment drugs
Cell death caused by cytotoxic T cells; death of B and T lymphocytes
Cell death caused by many viruses
Cell death in tumors, in growing tumor but particularly during tumor regression
Cell death in reperfusion injury
_
Why should a cell commit suicide?
Apoptosis is needed for proper development:
Examples:
The resorption of the tadpole tail
The formation of the fingers and toes of the fetus
The sloughing off of the inner lining of the uterus
The formation of the proper connections between neurons in the brain
Apoptosis is needed to destroy cells:
Examples:
Cells infected with viruses
Cells of the immune system
Cells with DNA damage
Cancer cells
_
Functions of apoptosis:
The functionality of cell death is different during development and adult life. During development apoptosis serves three major functions: deleting vestigial structures, i.e. phylogenetic cell death; controlling cell numbers, i.e. histogenetic cell death, and remodeling structures, i.e. morphogenetic cell death. In adult life, apoptosis mainly serves to maintain homeostasis by counterbalancing mitosis and deleting cells, which are potentially autoimmunoreactive, malignant, or virus infected. The development and maintenance of multicellular biological systems depends on a sophisticated interplay between the cells forming the organism, it sometimes even seems to involve an altruistic behaviour of individual cells in favour of the organism as a whole. During development many cells are produced in excess which eventually undergo programmed cell death and thereby contribute to sculpturing many organs and tissues
_
_
A particularly instructive example for the implication of programmed cell death in animal development is the formation of free and independent digits by massive cell death in the interdigital mesenchymal tissue [Zuzarte-Luis, 2002]. Other examples are the development of the brain, during which half of the neurons that are initially created will die in later stages when the adult brain is formed [Hutchins, 1998] and the development of the reproductive organs [Meier, 2000]. Also cells of an adult organism constantly undergo physiological cell death which must be balanced with proliferation in order to maintain homeostasis in terms of constant cell numbers. The majority of the developing lymphocytes die either during genetic rearrangement events in the formation of the antigen receptor, during negative selection or in the periphery, thereby tightly controlling the pool of highly efficient and functional but not self-reactive immune cells and at the same time keeping lymphocyte numbers relatively constant [Rathmell, 2002]. Taken together, apoptotic processes are of widespread biological significance, being involved in e.g. development, differentiation, proliferation/ homoeostasis, regulation and function of the immune system and in the removal of defect and therefore harmful cells. Thus, dysfunction or dysregulation of the apoptotic program is implicated in a variety of pathological conditions. Defects in apoptosis can result in cancer, autoimmune diseases and spreading of viral infections, while neurodegenerative disorders, AIDS and ischaemic diseases are caused or enhanced by excessive apoptosis [Fadeel, 1999a].
_________________
The nematode Caenorhabditis elegans (C. elegans):
_
C. elegans:
The nematode Caenorhabditis elegans is a spool-shaped approximately 1 millimeter long worm with 959 cells that eats bacteria. Sydney Brenner, Robert Horvitz and John Sulston’s discoveries concerning the genetic regulation of organ development and programmed cell death have truly opened new avenues for biological and medical research and got them Nobel Prize for medicine in 2002. Using the nematode C. elegans Nobel Laureates have demonstrated how organ development and programmed cell death are genetically regulated. They have identified key genes regulating programmed cell death and demonstrated that corresponding genes exist also in higher animals, including man. In C. elegans, all cell divisions and differentiations are invariant, i.e. identical from individual to individual, which made it possible to construct a cell lineage for all cell divisions.
_
Our understanding of the mechanisms involved in the process of apoptosis in mammalian cells transpired from the investigation of programmed cell death that occurs during the development of the nematode Caenorhabditis elegans (Horvitz, 1999). In this organism 1090 somatic cells are generated in the formation of the adult worm, of which 131 of these cells undergo apoptosis or “programmed cell death.” This results in an adult nematode (the hermaphrodite), composed of 959 somatic cells. These 131 cells die at particular points during the development process, which is essentially invariant between worms, demonstrating the remarkable accuracy and control in this system. Apoptosis has since been recognized and accepted as a distinctive and important mode of “programmed” cell death, which involves the genetically determined elimination of cells. However, it is important to note that other forms of programmed cell death have been described and other forms of programmed cell death may yet be discovered (Formigli et al., 2000; Sperandio et al., 2000; Debnath et al., 2005). Many of the genes that control the killing and engulfment processes of programmed cell death have been identified, and the molecular mechanisms underlying these processes have proven to be evolutionarily conserved (Metzstein et al., 1998). Until recently, apoptosis has traditionally been considered an irreversible process with caspase activation committing a cell to death and the engulfment genes serving the purpose of dead cell removal. However, the uptake and clearance of apoptotic cells by macrophages may involve more than just the removal of cell debris. Hoeppner et al. have shown that blocking engulfment genes in C. elegans embryos enhances cell survival when cells are subjected to weak pro-apoptotic signals (Hoeppner et al., 2001). Reddien et al. demonstrated that, in C. elegans, mutations that cause partial loss of function of killer genes allow the survival of some cells that are programmed to die via apoptosis, and mutations in engulfment genes enhance the frequency of this cell survival. Moreover, mutations in engulfment genes alone allowed the survival and differentiation of some cells that were otherwise destined to die via apoptosis (Reddien et al., 2001). These findings suggest that genes that mediate corpse removal can also function to actively kill cells. In other words, the engulfing cells may act to ensure that cells triggered to undergo apoptosis will die rather than recover after the initial stages of death.
_
The C. elegans cell-death gene ced-3 encodes a protein similar to mammalian interleukin-1beta-converting enzyme (ICE), a cysteine protease [caspase-1] implicated in mammalian apoptosis. The proximal cause of apoptosis in C. elegans is the activation of the cysteine protease ced-3, which is mediated by its oligomerization at the activator protein ced-4. Activity of the ced-3/ced-4 complex is regulated by the apoptosis inhibitor ced-9 and the apoptosis inducer egl-1. Subsequent studies in mammals and in the fly, D. melanogaster, have identified counterparts for these C. elegans genes, demonstrating that the core components of the cell death machinery are conserved through evolution [Richardson, 2002]. Accordingly, ced-3 is the single C. elegans member of a family of cysteine proteases, the caspases, whereas ced-4 corresponds to the mammalian apoptotic protease activating factor 1, Apaf-1, which is the core of a caspase-activating signaling complex, the apoptosome. Egl-1 and ced-9 are members of the Bcl-2 family of pro- or antiapoptotic proteins, respectively, which play an important role in the mediation and regulation of apoptotic signaling pathways.
_
_
Genetic regulation of apoptosis:
For the nematode Caenorhabditis elegans, the complete genome has been sequenced, a complete cellular fate map has been established and genetic mutants are easily obtained. C. elegans hermaphrodites have 1090 somatic cells, 131 of which commit suicide by apoptosis. 959 cells live and develop into defined tissues. 116 of the 131 dying cells are cells of the nervous system and other ectoderm. Two ‘‘cell death abnormal’’ genes, ced-3 and ced-4 are required for PCD of all 131 somatic cells. The product of the ced-9 gene inhibits the products of ced-3 and ced-4. The action of ces-1, ces-3, ces-4 and egl-1 determines whether a cell will die or survive. A range of genes is involved in engulfment of dead cells (e.g., ced-1 and ced-6). Finally, nuc-1 (a nuclease) is needed for DNA degradation to occur. The mammalian homologues of the C. elegans death genes have been identified. Examples of the protein homologues are: (1) ced-9—anti-apoptotic Bcl-2 family proteins, e.g., Bcl-2/BclxL, (2) ced-4—Apaf-1 and related proteins, (3) ced-3—the caspases, and (4) egl-1—BH3-only proteins, e.g., Bik/Bad/Bim.
_
The figure below shows regulation of PCD by genes:
_______________
Mechanisms of Apoptosis:
The mechanisms of apoptosis are highly complex and sophisticated, involving an energy-dependent cascade of molecular events. To date, research indicates that there are two main apoptotic pathways: the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway. However, there is now evidence that the two pathways are linked and that molecules in one pathway can influence the other (Igney and Krammer, 2002). Chemotherapeutic agents, UV irradiation, and other agents induce caspase activation mainly via the mitochondrial pathway, which involves mitochondrial integration of apoptotic signals and subsequent release of cytochrome c into the cytosol. The liberated cytochrome c, along with adenine nucleotides, initiates formation of an apoptosome consisting of Apaf-1 3 oligomers. This apoptosome recruits and activates caspase-9, which in turn activates the executioner caspases, caspase-3 and caspase-7. Thus, the activated caspases kill the cells by proteolysis of key cellular substrates. There is an additional pathway that involves T-cell mediated cytotoxicity and perforin-granzyme-dependent killing of the cell. The perforin/granzyme pathway can induce apoptosis via either granzyme B or granzyme A. The extrinsic, intrinsic, and granzyme B pathways converge on the same terminal, or execution pathway. This pathway is initiated by the cleavage of caspase-3 and results in DNA fragmentation, degradation of cytoskeletal and nuclear proteins, cross-linking of proteins, formation of apoptotic bodies, expression of ligands for phagocytic cell receptors and finally uptake by phagocytic cells. The granzyme A pathway activates a parallel, caspase-independent cell death pathway via single stranded DNA damage (Martinvalet et al., 2005).
_
_
The figures below shows overview of apoptotic mechanisms:
_
_
Intrinsic pathway:
_
The figure below shows both extrinsic and intrinsic factors that trigger intrinsic apoptotic pathway:
_
There are two main pathways leading to the activation of caspases. The intrinsic pathway depends upon the participation of mitochondria (receptor-independent) and the extrinsic pathway involves the interaction of a death receptor with its ligand. Pro- and anti-apoptotic members of the Bcl-2 family regulate the mitochondrial pathway. Intracellular stress signals, such as growth factor withdrawal, DNA damage, oxidative stress or oncogene activation, lead to permeabilization of the mitochondrial outer membrane. Cellular stress induces pro-apoptotic Bcl-2 family members to translocate from the cytosol to the mitochondria, where they induce the release of cytochrome c, while the anti-apoptotic Bcl-2 proteins work to prevent cytochrome c release from mitochondria, and thereby preserve cell survival. The multidomain proapoptotic Bax and Bak are essential, since mitochondria deficient for Bax and Bak fail to release cytochrome c. Bax and Bak are thought to induce permeabilization by forming pores upon oligomerization. The pro-apoptotic BH3-only family members (such as Bid, Bim, Bad, Noxa and Puma) activate Bax and/or Bak. Two models have been proposed for this activation; several peptide studies suggest that they do so through binding of anti-apoptotic Bcl-2 proteins (such as Bcl-2, Bcl-xL, Bcl-w and Mcl-1), thereby relieving the inhibitory function of these anti-apoptotic proteins. Others propose that a subset of BH3-only proteins can directly bind and activate Bax and/or Bak. The consequent release of cytochrome c leads to the formation of a complex – the apoptosome – which contains cytochrome c, Apaf-1 and initiator caspase-9. This aggregation requires energy from ATP. Caspase-9 is auto-activated by induced proximity in the apoptosome. Active caspase-9 cleaves and thereby activates the executioner caspases. Depending on the agent and the cell type, apoptosis is highly dependent on mitochondrial damage. Particularly important is the loss of mitochondrial membrane permeability, creating a “high-conductance channel” that causes the release of cytochrome c into the cytoplasm.
_
_
Extrinsic pathway:
In receptor-mediated apoptosis, the process is initiated by the binding of the ligand to its receptor in the cell membrane. TNF binds to its type 1 receptor (TNFR1) while the Fas ligand binds to Fas. Both receptors have sequences called “death effector protein domains” that serve as docking sites for binding adapter proteins such as FADD (Fas-associated protein with death domain) and TRADD (TNFR adapter protein with death domain). An example of this process is when cytotoxic T cells bind to a target cell. The T cells produce more FasL that binds to the Fas receptor on the target cell leading to apoptosis of the target cell. When extracellular ligands such as Fas ligand, TNFa or TRAIL (TNF-related apoptosis-inducing ligand) bind to their receptors, the intracellular death domains of these receptors recruit adaptor proteins (such as FADD and TRADD) and initiator caspase-8 and -10. Together these comprise the death-inducing signaling complex (DISC). Caspase- 8 and -10 are activated at the DISC, due to induced proximity of the caspases. This activation is controlled by c-FLIP (cellular FLICE inhibitory proteins). Interaction of TNF-α with TNFαR may activate the NF-κB pathway via NIK/IKK. The activation of NF-κB induces the expression of pro-survival genes including Bcl-2 and FLIP. Both the short (c-FLIPS) and long (c-FLIPL) forms prevent caspase activation, although c-FLIPL is proposed to facilitate caspase binding and activation when lowly expressed. Once caspase-8 is active, it propagates apoptosis via direct cleavage of executioner caspases. However, the extracellular and intracellular apoptotic pathways cross at the level of the mitochondria since caspase-8 can also cleave the protein Bid into its active form tBid. Being a pro-apoptotic member of the Bcl-2 family of proteins, tBid induces Bax/Bak-dependent permeabilization of the outer mitochondrial membrane and release of cytochrome c. Both apoptotic pathways lead to activation of the executioner caspases, caspase-3, -6 and -7, which are the main proteases that degrade the cell. The end result of either pathway is caspase activation and the cleavage of specific cellular substrates, resulting in the morphological and biochemical changes associated with the apoptotic phenotype. Their activity is, at least to some extent, kept in check by IAPs (Inhibitor of Apoptosis Proteins). IAPs themselves are inhibited by the proteins SMAC/DIABLO30, and the serine protease HtrA2/Omi.32 These proteins are also released from the mitochondria, possibly simultaneously with cytochrome c to alleviate the inhibitory signal and to enhance the apoptotic signal. The early steps in apoptosis are reversible — at least in C. elegans. In some cases, final destruction of the cell is guaranteed only with its engulfment by a phagocyte. In the absence of caspase activation, stimulation of death receptors can lead to the activation of an alternative programmed cell death pathway termed necroptosis by forming complex IIb.
_
____________
Regulation of apoptosis:
Bcl-2 gene family: Bcl-2 protein family:
Apoptosis occurs on a cell-by-cell basis. For each affected cell, two primary phases are observed: one of initiation and a second of execution. The resulting cell remnants are processed for reuse. Both phases are complex and require exquisite organization of multiple cellular systems, including interactions between proteins and cellular membranes. The initiation phase, or “death decision,” became of significant interest following the description of a group of proteins in mammals known as the Bcl-2 protein family. This protein family, which provides the framework for controlling apoptosis, takes its name from a type of cancer called B-cell lymphoma. Bcl-2, the first family member, forms the molecular basis for sustaining the lymphoma cancer cells. The Bcl-2 protein itself is an anti-apoptotic protein that can suppress apoptosis by protecting mitochondrial permeability, and in some cells, by binding to a pro-apoptotic protein Apaf-1 (apoptotic protease activating factor). The Bcl-2 gene was first identified by its overexpression in more than 80% of patients with follicular lymphomas. They carry a 14:18 translocation which brings together the IgH locus on chromosome 14 and the Bcl-2 gene in chromosome 18. B cells transfected with Bcl-2 were shown to be rendered resistant towards apoptosis induced by IL-3 withdrawal: for the first time it was shown that the pathway toward tumorigenesis depends not only on the ability to escape growth control but also depends on the ability to prevent apoptosis. When homologues of Bcl-2 had been identified, it became apparent that a Bcl-2 family of proteins can be defined by the presence of conserved sequence motifs known as Bcl-2 homology domains (BH1 to BH4). The Bcl-2 family of proteins has at least 25 members. Most of these are known as BH-3-only proteins. BH-3-only proteins function as activators or sensitizers of apoptosis and monitor important cell processes for dysfunction. They also control the function of two death-initiating, or pro-apoptotic, proteins (Bax and Bak) and a large number of death-preventing, or anti-apoptotic, proteins, which include Bcl-XL and Bcl-2. In mammals this control occurs primarily on the membranes of mitochondria, where the mortality decision for each cell is constantly reviewed under the supervision of the these contesting factions of the Bcl-2 protein family. A second family of proteins, the caspase proteolytic enzymes, contributes to both regulation by the Bcl-2 family and execution of apoptosis after the death decision is confirmed. Caspases function in large part by the activation of other enzymes that dismantle the cellular cytoskeleton and cellular organelles and that degrade the nuclear DNA (deoxyribonucleic acid), from which there is no possibility of recovery. The cellular destruction occurs in place (in situ), and the cellular membrane system is reorganized to package the degraded components, including the digested genetic material, in membrane compartments. This packaging system prevents the materials from being released generally within tissues. Macrophages and other scavenger cells then engulf the membrane packets and process them for reuse as the most basic cellular components. In some instances, neighbouring cells may also engulf the degraded components. Employing the intrinsic pathway, cancer cells, cells that are infected with bacteria or virus particles, and mutant cells can be assigned to apoptosis. The extrinsic pathway is commonly associated with cellular death receptors.
_
How Bcl-2 protein family control apoptosis?
There has been quite some debate about how the Bcl-2 family controls apoptosis: one model proposes that Bcl-2 members might directly control caspase activation [Strasser, 2000], whereas another model claims that they mainly act by guarding mitochondrial integrity [Wang, 2001]. In support of the first model, the worm Bcl-2 orthologue ced-9 binds to the Apaf-1-like adaptor protein ced-4 and prevents it from activating the caspase ced-3 unless the BH3-only protein Egl-1 displaces ced-4.[Conradt, 1998]. In contrast, the mammalian ced-4 homologue Apaf-1 obviously does not interact with Bcl-2-like proteins [Moriishi, 1999] but is activated by cytosolic cytochrome c, and it is the release of cytochrome c from the mitochondria that can be controlled by Bcl-2 [Kluck, 1997; Yang, 1997]. Therefore it appears likely, that the central function of mammalian Bcl-2 family members is to guard mitochondrial integrity and to control the release of mitochondrial proteins into the cytoplasm [Cory, 2002]. How then is mitochondrial integrity affected by proapoptotic Bcl-2 family members? Central to this question are Bax and Bak, even though inactivation of the Bax gene alone affected apoptosis only slightly and disruption of Bak alone did not show any effect. However, the double knockout of Bax and Bak resulted in dramatic impairment of apoptosis during development in many tissues with superfluous cells accumulating in the hematopoietic system and in the brain. Additionally, cells derived from those Bax -/- Bak -/- mice are insensitive to treatment with e.g. etoposide or irradiation [Lindsten, 2000; Wei, 2001]. Bax is a cytosolic monomer in viable cells but during apoptosis changes its conformation, integrates into the outer mitochondrial membrane and oligomerizes [Nechushtan, 2001]. Although the mechanism is controversial, Bax and Bak oligomers are believed to provoke or contribute to the permeabilization of the outer mitochondrial membrane (PT), either by forming channels by themselves [Antonsson, 2000] or by interacting with components of the PT pore such as VDAC [Tsujimoto, 2000]. In contrast, antiapoptotic Bcl-2 members sequester proapoptotic Bcl-2 members by bindig to their BH3 domains and thereby ultimately prevent Bax or Bak activation/ oligomerization and consequently inhibit mitochondrial proapoptotic events: overexpression of Bcl-2 or Bcl-XL potently inhibits apoptosis in response to many cytotoxic insults, among others by suppressing the generation of ROS, stabilizing the mitochondrial inner transmembrane potential (Dy), preventing PT and consequently blocking the release of e.g. cytochrome c [Reed, 1998]. Besides eliciting its antiapoptotic effects on the mitochondrial level by indirectly controlling the activation of the apoptosome, Bcl-2 also appears to inhibit apoptotic pathways that are independent of Apaf-1/caspase-9 and which might depend on caspase-7 as a central effector [Marsden, 2002]. In this context one might even expect the existence of another but up to now unidentified Apaf-1 homologue that can be directly controlled by Bcl-2/Bcl-XL [Puthalakath, 2002]. Whereas Bax and Bak represent the central core of a proapoptotic Bcl-2 death machinery that is held in check by the pro-survival members Bcl-2 and Bcl-XL, members of the BH3-only subfamily are required for the activation of proapoptotic Bax/Bak function [Bouillet, 2002]. On the other hand, the killing effect of BH3-only members depends on Bax/Bak, since cells double-deficient for Bax and Bak do not die upon overexpression of BH3-only proteins as it would be the case in wild type cells, indicating that BH3-only members function upstream of Bax and Bak [Lindsten, 2000; Wei, 2001]. Importantly, just as the proapoptotic activity of multidomain proteins Bax and Bak is controlled by their interaction with the antiapoptotic guardians Bcl-2/Bcl-XL, also most BH3-only members display a strong binding preference to antiapoptotic Bcl-2/Bcl-XL and in this way are kept under control [Scorrano, 2003]. Individual BH3-only proteins are believed to transduce specific death signals since they can be activated for apoptosis signalling by sensing cell stress, such as DNA damage (Noxa and Puma are p53-inducible genes), growth factor deprivation (Hrk and Bim mRNA expression is increased), or anoikis (Bmf is activated by subcellular relocalization) [Borner, 2003]. As another example, cytoplasmic Bid is processed by caspase-8 to its truncated form tBid, which after myristoylation translocates to the mitochondria where it triggers cytochrome c release by affecting Bax/Bak oligomerization and/or by mobilizing cytochrome c stores in cristae [Cory, 2002; Scorrano, 2002]. In general, BH3-only proteins are thought to interfere with the fine-tuned balance of homo- or heterooligomerization between proapoptotic multidomain members Bax/Bak and antiapoptotic members Bcl-2/Bcl-XL. It has been proposed that Bid and Bim possess BH3 domains (Bid-like BH3 domain) which can directly mediate Bax/Bak oligomerization, whereas Bad and Bik possess Bad-like BH3 domains which do not directly act on Bax/Bak but preferentially interact with antiapoptotic Bcl-2/Bcl-XL. As a consequence, activated Bad/Bik might be able to displace Bid/Bim from the binding pocket of antiapoptotic Bcl-2/Bcl-XL, and – in this way released – Bid/Bim might provoke Bax/Bak oligomerization and cytochrome c release even at subliminal levels [Letai, 2002].
_
The figure blow show how apoptosis is regulated by balancing pro-apoptotic and anti-apoptotic proteins:
_
_
Damage to the Bcl-2 gene has been identified as a cause of a number of cancers, including melanoma, breast, prostate, chronic lymphocytic leukemia, and lung cancer, and a possible cause of schizophrenia and autoimmunity. It is also a cause of resistance to cancer treatments. Cancer occurs as the result of a disturbance in the homeostatic balance between cell growth and cell death. Over-expression of anti-apoptotic genes, and under-expression of pro-apoptotic genes, can result in the lack of cell death that is characteristic of cancer. An example can be seen in lymphomas. The over-expression of the anti-apoptotic Bcl-2 protein in lymphocytes alone does not cause cancer. But simultaneous over-expression of Bcl-2 and the proto-oncogene myc may produce aggressive B-cell malignancies including lymphoma. In follicular lymphoma, a chromosomal translocation commonly occurs between the fourteenth and the eighteenth chromosomes—t(14;18) — which places the Bcl-2 gene next to the immunoglobulin heavy chain locus. This fusion gene is deregulated, leading to the transcription of excessively high levels of Bcl-2. This decreases the propensity of these cells for undergoing apoptosis. Bcl proved to block programmed cell death rather than promote proliferation. Transgenic mice that overexpress Bcl in the B cell lineage demonstrate extended cell survival and progress to high-grade lymphomas. Thus, Bcl initiated a new category of oncogenes, regulators of cell death. Bcl-deficient mice demonstrate fulminant apoptosis of lymphocytes, profound renal cell death and loss of melanocytes. Bcl protein duels with its counteracting twin, a partner known as Bax. When Bax is in excess, cells execute a death command; but, when Bcl dominates, the program is inhibited and cells survive. Bax-deficient mice display cellular hyperplasia, confirming its role as a proapoptotic molecule. The pro- and antiapoptotic Bcl family members represent central regulators in an evolutionarily conserved pathway of cell death. Aberrations in the Bcl-2 family result in disordered homeostasis, a pathogenic event in diseases, including cancer.
_
Regulation of apoptosis by p 53:
_
_
p53 (also known as protein 53, tumor protein 53, tumor antigen p53, and as several other synonyms), is a tumor suppressor protein that in humans is encoded by the TP53 gene. p 53 is crucial in multicellular organisms, where it regulates the cell cycle and, thus, functions as a tumor suppressor that is involved in preventing cancer. It is a proapoptotic mediator. If there is any DNA damage, then p53 restrict the replication and gives sufficient time for repairing of the cell. If cell repair can’t be done, apoptosis will be induced preventing multiplication of the damaged cell. Cell arrest is quite impossible in neoplastic cells in which p53 activity is mutated. p 53 gene is an important cell growth regulator. Decrease in p53 in a cell also makes it resistant for radiation and chemotherapy and inhibiting cancer treatment. As such, p53 has been described as “the guardian of the genome” because of its role in conserving stability by preventing genome mutation. The name p53 is in reference to its apparent molecular mass: It runs as a 53-kilodalton (kDa) protein. But, based on calculations from its amino acid residues, p53’s mass is actually only 43.7 kDa. This difference is due to the high number of proline residues in the protein, which slows its migration, thus making it appear heavier than it actually is. This effect is observed with p53 from a variety of species, including humans, rodents, frogs, and fish. In humans, p53 is encoded by the TP53 gene located on the short arm of chromosome 17 (17p13.1). In humans, a common polymorphism involves the substitution of an arginine for a proline at codon position 72. Many studies have investigated a genetic link between this variation and cancer susceptibility; however, the results have been controversial. For instance, a meta-analysis from 2009 failed to show a link for cervical cancer. A 2011 study found that the TP53 proline mutation did have a profound effect on pancreatic cancer risk among males. A study of Arab women found that proline homozygosity at TP53 codon 72 is associated with a decreased risk for breast cancer. One study suggested that TP53 codon 72 polymorphisms, MDM2 SNP309, and A2164G may collectively be associated with non-oropharyngeal cancer susceptibility and that MDM2 SNP309 in combination with TP53 codon 72 may accelerate the development of non-oropharyngeal cancer in women. A 2011 study found that TP53 codon 72 polymorphism was associated with an increased risk of lung cancer. Meta-analyses from 2011 found no significant associations between TP53 codon 72 polymorphisms and both colorectal cancer risk and endometrial cancer risk. A 2011 study of a Brazilian birth cohort found an association between the non mutant arginine TP53 and individuals without a family history of cancer. Another 2011 study found that the p53 homozygous (Pro/Pro) genotype was associated with a significantly increased risk for renal cell carcinoma.
_
_
PUMA:
The p53 upregulated modulator of apoptosis (PUMA) also known as Bcl-binding component 3 (BBC3), is a pro-apoptotic protein, member of the Bcl protein family. In humans, the Bcl-binding component 3 protein is encoded by the BBC3 gene. The expression of PUMA is regulated by the tumor suppressor p53. PUMA is involved in p53-dependent and -independent apoptosis induced by a variety of signals, and is regulated by transcription factors, not by post-translational modifications. After activation, PUMA interacts with antiapoptotic Bcl family members, thus freeing Bax and/or Bak which are then able to signal apoptosis to the mitochondria. Following mitochondrial dysfunction, the caspase cascade is activated ultimately leading to cell death.
_
In a nutshell, Bcl-2 and p 53 are important genes, which control apoptosis. The dysfunction and mutation of these genes cause 50% of human cancers and are associated with resistance to treatment.
_
Expression levels of antiapoptotic proteins such as Bcl-2, Bcl-XL, and A1 were reported to be upregulated by the transcription factor NF-kB which besides being a central regulator of the innate and adaptive immune response is commonly described as an antiapoptotic transcription factor [Heckman, 2002; Karin, 2002], although under certain circumstances NF-kB also might positively contribute to apoptosis induction [Grimm, 1996b]. Besides inducing the expression of pro-survival Bcl-2 members, NF-kB additionally transactivates a number of other antiapoptotic genes, such as the IAPs (inhibitors of apoptosis proteins).
_
Regulation of apoptosis by IAPs:
The inhibitor of apoptosis proteins (IAPs):
The inhibitor of apoptosis proteins (IAPs) plays a central role in repressing caspase-mediated cell death. IAPs are BIR-domain-containing protein members that suppress apoptosis induction. The antiapoptotic activity of several IAPs has been attributed especially to their ability to inhibit caspases. IAPs are a family of antiapoptotic proteins whose prototype originally was described in baculovirus with many homologues found to be conserved across several species. So far, eight human IAP homologues have been identified, among others NAIP, c-IAP1, c-IAP2, XIAP and survivin. The XIAP is a prototype IAP family member that directly binds and inhibits several effector caspases. All IAPs contain baculovirus IAP repeat (BIR) domains, 70 amino acid motifs, which are essential for the antiapoptotic properties of IAPs [Takahashi, 1998] because it is the interaction between the BIR domains and caspases that is believed to confer most of the antiapoptotic activity of IAPs. Indeed, XIAP, c-IAP1 and c-IAP2 are thought to directly inhibit caspases-3, -7, and –9 [Salvesen, 2002a]. In case of XIAP, it is the BIR3 domain that directly binds to the small subunit of caspase-9, whereas it is the BIR2 domain that interacts with the active-site substrate binding pocket of caspases-3 and –7 [Huang, 2001; Srinivasula, 2001]. In addition to the BIR domains, c-IAP1, c-IAP2, and XIAP contain a highly conserved RING domain at their C-terminal end which possesses E3 ubiquitin ligase activity. Via this RING domain, IAPs are able to catalyze their own ubiquitination, thereby targeting themselves for degradation by the proteasome [Yang, 2000], but they also might target other proteins such as caspase-3 and –7 for ubiquitination and degradation [Huang, 2000; Suzuki, 2001]. Direct inhibition of caspase activity by c-IAPs is certainly a very important means of regulation when considered that signaling cascades mediated by proteolytic enzymes such as caspases is irreversible once activated and therefore must be precisely regulated in order to prevent locally and temporally inappropriate demise of cells. IAPs are themselves regulated by proteins that block their antiapoptotic activity. In Drosophila, Reaper (RPR), Hid, Grim, and, more recently, Sickle are reported to be proapoptotic proteins. They activate caspase-dependent cell death pathways by suppressing the ability of IAPs to inhibit caspases. It has been shown that they can physically interact with the fly members of IAP family, including D-IAP1 and D-IAP2, and antagonize the binding of the IAP proteins to caspases. Smac/DIABLO performs a similar function to these proteins in mammals. The only sequence homology among insect Reaper, Hid, Grim, Sickle, and human Smac is in the four NH2-terminal residues of the active proteins. This short peptide fits into a hydrophobic pocket on the surface of the BIR domain of IAPs and is essential for binding IAPs and blocking their caspase-inhibitory activity.
______________
The executioner of apoptosis: Caspases:
Caspase (cysteine-dependent aspartate-specific proteases):
Caspases, or cysteine-aspartic proteases or cysteine-dependent aspartate-directed proteases are a family of cysteine proteases that play essential roles in apoptosis (programmed cell death), necrosis, and inflammation. Caspases are essential in cells for apoptosis, or programmed cell death, in development and most other stages of adult life, and have been termed “executioner” proteins for their roles in the cell. Some caspases are also required in the immune system for the maturation of lymphocytes. Caspases play an essential role during apoptotic cell death. The discrete and highly limited subset of cellular polypeptides that are cleaved by these proteases is sufficient to account for the majority of cellular and morphological events that occur during cell death. In some cases, caspases also play a contributory role in escalating the propensity for apoptosis, and in doing so may exacerbate disease pathogenesis.
_
Caspase activation:
The adapter proteins bound to death-domain receptor, or the release of proaptotic molecules such as cytochrome c through the mitochondria pathway, trigger the activation of caspases. There are many different caspases, which are present in the cell in inactive, precursor forms (pro-caspases). Some of these caspases (caspases 8 and 9) initiate the process (initiator caspases) whiles others, such as (caspase 3), deliver the final blow, and are known as executioner caspases.
_
_
The caspases, cysteine proteases homologous to C. elegans ced-3, are of central importance in the apoptotic signaling network which is activated in most cases of apoptotic cell death [Bratton, 2000]. Actually, strictly defined, cell death only can be classified to follow a classical apoptotic mode if execution of cell death is dependent on caspase activity [Leist, 2001a]. The term caspases is derived from cysteine-dependent aspartate-specific proteases: their catalytical activity depends on a critical cysteine-residue within a highly conserved active-site pentapeptide QACRG, and the caspases specifically cleave their substrates after Asp residues. So far, 7 different caspases have been identified in Drosophila, and 14 different members of the caspase-family have been described in mammals, with caspase-11 and caspase–12 only identified in the mouse [Denault, 2002; Richardson, 2002]. According to a unified nomenclature, the caspases are referred to in the order of their publication: caspase-1 is ICE (Interleukin-1ß-Converting Enzyme), the first mammalian caspase described to be a homologue of Ced-3 [Creagh, 2001; Miura, 1993]. Caspase-1 as well as caspases-4, -5, -11, and –12 appear to be mainly involved in the proteolytic maturation of pro-inflammatory cytokines such as pro-IL-1ß and pro-IL-18 and their contribution to the execution of apoptosis remains questionable [Denault, 2002]. Indeed, mice deficient for caspase-1 or caspase-11 develop normally and cells from those knockout mice remain sensitive to various death stimuli [Li, 1995; Wang, 1998]. In contrast, gene knockout experiments targeting caspase-3 and caspase-9 resulted in perinatal mortality as a result of severe defects in brain development [Kuida, 1998; Kuida, 1996], whereas caspase-8 deficient embryos died after day 12 [Varfolomeev, 1998]. This and the observation that cell lines derived from those knockout experiments are resistant to distinct apoptosis stimuli underlines the importance of caspases as proapoptotic mediators. Indeed, caspase-3, caspase-9, caspase-8, and additionally caspases-2, -6, -7, and –10 have been recognized to play an important role in the apoptotic signaling machinery [Earnshaw, 1999].
_
The figure below shows human caspase gene family:
_
In the cell, caspases are synthesized as inactive zymogens, the so called procaspases, which at their N-terminus carry a prodomain followed by a large and a small subunit which sometimes are separated by a linker peptide. Upon maturation, the procaspases are proteolytically processed between the large and small subunit, resulting in a small and a large subunit. The prodomain is also frequently but not necessarily removed during the activation process. A heterotetramer consisting of each two small and two large subunits then forms an active caspase.
_
_
The proapoptotic caspases can be divided into the group of initiator caspases including procaspases-2, -8, -9 and –10, and into the group of executioner caspases including procaspases-3, -6, and –7. Whereas the executioner caspases possess only short prodomains, the initiator caspases possess long prodomains, containing death effector domains (DED) in the case of procaspases-8 and –10 or caspase recruitment domains (CARD) as in the case of procaspase-9 and procaspase-2. Via their prodomains, the initiator caspases are recruited to and activated at death inducing signaling complexes either in response to the ligation of cell surface death receptors (extrinsic apoptosis pathways) or in response to signals originating from inside the cell (intrinsic apoptosis pathways).
_
Caspase cascade leads to cell death:
_
The figure below shows how IAPs inhibit caspases:
___________
Calcium and cell death:
Research during the past several decades has provided convincing evidence for a crucial role of the Ca2+ ion in cell signaling. Hence, intracellular Ca2+ transients have been implicated in most aspects of cell physiology, including gene transcription, cell cycle regulation and cell proliferation. Further, the Ca2+ ion has been found to also play an important role in cell death regulation. Thus, necrotic cell death was early associated with intracellular Ca2+ overload, and multiple functions in the apoptotic process have subsequently been found to be governed by Ca2+ signaling. More recently, other modes of cell death, notably anoikis and autophagic cell death, have been demonstrated to also be modulated by Ca2+ transients.
_
_
____________
Mitochondria as central regulators of apoptosis pathways:
_
_
The mitochondria are essential to multicellular life. Without them, a cell ceases to respire aerobically and quickly dies. This fact forms the basis for some apoptotic pathways. Apoptotic proteins that target mitochondria affect them in different ways. They may cause mitochondrial swelling through the formation of membrane pores, or they may increase the permeability of the mitochondrial membrane and cause apoptotic effectors to leak out. These are very closely related to intrinsic pathway, and tumors arise more frequently through intrinsic pathway than the extrinsic pathway because of sensitivity. There is also a growing body of evidence indicating that nitric oxide is able to induce apoptosis by helping to dissipate the membrane potential of mitochondria and therefore make it more permeable. Nitric oxide has been implicated in initiating and inhibiting apoptosis through its possible action as a signal molecule of subsequent pathways that activate apoptosis. Besides amplifying and mediating extrinsic apoptotic pathways, mitochondria also play a central role in the integration and propagation of death signals originating from inside the cell such as DNA damage, oxidative stress, starvation, as well as those induced by chemotherapeutic drugs [Kaufmann, 2000; Wang, 2001]. Most apoptosis-inducing conditions involve the disruption of the mitochondrial inner transmembrane potential (Dy) as well as the so called permeability transition (PT), a sudden increase of the inner mitochondrial membrane permeability to solutes with a molecular mass below approximately 1.5 kDa. Concomitantly, osmotic mitochondrial swelling has been observed by influx of water into the matrix with eventual rupture of the outer mitochondrial membrane, resulting in the release of proapoptotic proteins from the mitochondrial intermembrane space into the cytoplasm [Bernardi, 1999; Loeffler, 2000]. Released proteins include cytochrome c, which activates the apoptosome and therefore the caspase cascade, but also other factors such as the apoptosis-inducing factor AIF [Susin, 1999], the endonuclease endoG [Li, 2001], Smac/Diablo [Verhagen, 2000], and Htr/Omi [Verhagen, 2002]. Interestingly, PT is always followed by Dy, but Dy is not always caused by PT, and cytochrome c release has been observed even in absence of Dy [Bernardi, 1999; Kroemer, 2000]. In addition to the release of mitochondrial factors, the dissipation of Dy and PT also cause a loss of the biochemical homeostasis of the cell: ATP synthesis is stopped, redox molecules such as NADH, NADPH, and glutathione are oxidized, and reactive oxygen species (ROS) are increasingly generated [Kroemer, 2000; Kroemer, 1997]. Increased levels of ROS directly cause the oxidation of lipids, proteins, and nucleic acids, thereby enhancing the disruption of Dy as part of a positive feedback [Marchetti, 1997]. Several possible mechanisms for PT have been proposed, but there appears to exist consent that a so-called permeability transition pore (PTP) is formed consisting of the adenin nucleotide translocator (ANT) and the voltage-dependent anion channel (VDAC) as its core components. ANT is the most abundant protein of the inner mitochondrial membrane and as a transmembrane channel is responsible for the export of ATP in exchange with ADP (antiport). Overexpression of ANT-1 in human cancer cell lines dominantly induces apoptosis with all its characteristic features whereas its closely conserved homologue ANT-2 does not, indicating a specific mechanistic role of ANT-1 in mitochondrial apoptosis events [Bauer, 1999]. VDAC, also called porin, is the most abundant protein of the outer mitochondrial membrane and forms a non-selective pore through the outer membrane. Indicated by direct protein-protein interactions, VDAC-ANT complexes presumably connect inner and outer mitochondrial membrane to so-called ‘contact sites’, corresponding to a close association of the two membranes and thereby possibly constituting the PT pore [Beutner, 1998]. Since PT, loss of Dy, and release of mitochondrial proteins are of central importance in mediating and enhancing apoptotic pathways, those mitochondrial events must be kept under strict control of regulatory mechanisms which are in many ways dependent on members of the Bcl-2 family. Mitochondrial proteins known as SMACs (small mitochondria-derived activator of caspases) are released into the cytosol following an increase in permeability. SMAC binds to inhibitor of apoptosis proteins (IAPs) and deactivates them, preventing the IAPs from arresting the apoptotic process and therefore allowing apoptosis to proceed. IAP normally suppresses the activity of caspases, which carry out the degradation of the cell, therefore the actual degradation enzymes can be seen to be indirectly regulated by mitochondrial permeability. Cytochrome c is also released from mitochondria due to formation of a channel, the mitochondrial apoptosis-induced channel (MAC), in the outer mitochondrial membrane, and serves a regulatory function as it precedes morphological change associated with apoptosis. The Bcl-2 family proteins regulate apoptosis by controlling the formation of MAC. Depending on cell type and apoptotic inducer, Bax and/or Bak are structural component(s) of MAC. Overexpression of the antiapoptotic protein Bcl-2 eliminates MAC activity. Once cytochrome c is released it binds with Apoptotic protease activating factor – 1 (Apaf-1) and ATP, which then bind to pro-caspase-9 to create a protein complex known as an apoptosome. The apoptosome cleaves the pro-caspase to its active form of caspase-9, which in turn activates the effector caspase-3.
_
__
The Mitochondrial Permeability Transition, or MPT, is defined as an increase in the permeability of the mitochondrial membranes to molecules of less than 1500 Daltons in molecular weight. MPT results from the opening of a mitochondrial permeability transition pore, also known as the MPT pore or MPTP. The MPT pore is a protein pore that is formed in the inner membrane of the mitochondria under certain pathological conditions such as traumatic brain injury and stroke. Induction of the permeability transition pore can lead to mitochondrial swelling and cell death through apoptosis or necrosis depending on the particular biological setting.
_____
Apoptosome:
The apoptosome is a large quaternary protein structure formed in the process of apoptosis. Its formation is triggered by the release of cytochrome c from the mitochondria in response to an internal (intrinsic) or external (extrinsic) cell death stimulus. Stimuli can vary from DNA damage and viral infection to developmental cues such as those leading to the degradation of a tadpole’s tail. In mammalian cells, once cytochrome c is released, it binds to the cytosolic protein Apaf-1 to facilitate the formation of apoptosome. An early biochemical study suggests a two-to-one ratio of cytochrome c to apaf-1 for apoptosome formation. However, recent structural studies suggest the cytochrome c to apaf-1 ratio is one-to-one.
_____
Lysosomes and other organelle-specific initiation of cell death pathways:
Most of the early studies on the connections of organelles with cell death focused on mitochondria. Initially, lysosomes were mostly linked with necrotic cell death following major cell damage, although they were not generally considered as its primary cause. Nuclear DNA damage and ligation of plasma-membrane death receptors have long been recognized as initial triggers of apoptosis that induce mitochondrial membrane permeabilization (MMP) and/or the direct activation of caspases. Accumulating evidence suggests that other organelles, including the endoplasmic reticulum (ER), lysosomes and the Golgi apparatus, are also major points of integration of pro-apoptotic signalling or damage sensing. Each organelle possesses sensors that detect specific alterations, locally activates signal transduction pathways and emits signals that ensure inter-organellar cross-talk. The ER senses local stress through chaperones, Ca2+-binding proteins and Ca2+ release channels, which might transmit ER Ca2+ responses to mitochondria. The ER also contains several Bcl-2-binding proteins, and Bcl-2 has been reported to exert part of its cytoprotective effect within the ER. Upon membrane destabilization, lysosomes release cathepsins that are endowed with the capacity of triggering MMP. The Golgi apparatus constitutes a privileged site for the generation of the pro-apoptotic mediator ganglioside GD3, facilitates local caspase-2 activation and might serve as a storage organelle for latent death receptors. Intriguingly, most organelle-specific death responses finally lead to either MMP or caspase activation, both of which might function as central integrators of the death pathway, thereby streamlining lysosome-, Golgi- or ER-elicited responses into a common pathway. In addition, caspase-independent cell death mechanisms have been identified. It is clear that the boundary between autophagic and apoptotic cell death is not completely clear. This may reflect a high degree of flexibility to the cell’s response to environmental changes under physiological or pathological conditions. At the level of lysosomes, the magnitude of lysosomal rupture and the release of hydrolytic enzymes into the cytosol may thus have different consequences ranging from reparable sub lethal damage, to apoptosis, necrosis or autophagy.
____
Execution Pathway:
The extrinsic and intrinsic pathways both end at the point of the execution phase, considered the final pathway of apoptosis. It is the activation of the execution caspases that begins this phase of apoptosis. Execution caspases activate cytoplasmic endonuclease, which degrades nuclear material, and proteases that degrade the nuclear and cytoskeletal proteins. Caspase-3, caspase-6, and caspase-7 function as effector or “executioner” caspases, cleaving various substrates including cytokeratins, PARP, the plasma membrane cytoskeletal protein alpha fodrin, the nuclear protein NuMA and others, that ultimately cause the morphological and biochemical changes seen in apoptotic cells (Slee et al., 2001).
_
Apoptotic DNA fragmentation:
Apoptotic DNA fragmentation is a key feature of apoptosis, a type of programmed cell death. Apoptosis is characterized by the activation of endogenous endonucleases with subsequent cleavage of chromatin DNA into internucleosomal fragments of roughly 180 base pairs (bp) and multiples thereof (360, 540 etc.). The enzyme responsible for apoptotic DNA fragmentation is the Caspase-Activated Dnase (CAD). CAD is normally inhibited by another protein, the Inhibitor of Caspase Activated Dnase (ICAD). During apoptosis, the apoptotic effector caspase, caspase 3, cleaves ICAD and thus causes CAD to become activated. CAD cleaves the DNA at the internucleosomal linker sites between the nucleosomes, protein-containing structures that occur in chromatin at ~180-bp intervals. This is because the DNA is normally tightly wrapped around histones, the core proteins of the nucleosomes. The linker sites are the only parts of the DNA strand that are exposed and thus accessible to CAD. Degradation of nuclear DNA into nucleosomal units is one of the hallmarks of apoptotic cell death. It occurs in response to various apoptotic stimuli in a wide variety of cell types.
_________
Efferocytosis (phagocytosis of apoptotic cell):
_
_
Because the phagocytosis of apoptotic cells has distinctive morphologic features and unique downstream consequences, deCathelineau and Henson and Gardai et al coined the unique term efferocytosis (taken from the Latin effero, meaning to take to the grave or to bury). During efferocytosis, large membrane ruffles sprout out from the phagocyte surface and engulf apoptotic cells into large, fluid-filled phagosomes, or “efferosomes”. Unlike complement or Fcγ receptor-mediated phagocytosis, efferocytosis and macropinocytosis are morphologically similar, and both are inhibited by amiloride. While efferocytosis was originally thought to be limited to “professional” phagocytes, such as macrophages and dendritic cells, we now know that most cell types in the body are capable of ingesting apoptotic cells, including epithelial cells and fibroblasts.
_
“Eat-Me” Signals:
In order for apoptotic cells to be recognized and ingested, fundamental membrane alterations must occur in a way that differentiates them from viable cells. In short, apoptotic cells must express clear-cut “eat-me” signals. The changes in the apoptotic cell which trigger phagocytosis by non-activated macrophages have been investigated by several groups. Macrophages appear to recognize apoptotic cells via several different recognition systems, which seem to be used preferentially by different macrophage subpopulations. There is good evidence that apoptotic cells lose the normal phospholipid asymmetry in their plasma membrane, as manifested by the exposure of normally inward-facing phosphatidyl serine on the external face of the bilayer. Macrophages can recognize this exposed lipid headgroup via an unknown receptor, triggering phagocytosis. Surprisingly, little is known about these alterations, but it has become clear that the externalization of phosphatidylserine (PS) is an essential element. During apoptosis, PS (which is normally sequestered to the inner membrane leaflet) redistributes to the outer membrane leaflet in a process that is fueled by the inhibition of aminophospholipid translocases and the activation of phospholipid flip-flop. This exposure of PS by apoptotic cells is of particular interest, because it induces membrane ruffling and efferocytosis, and it has been implicated in many of the immunomodulatory effects associated with efferocytosis. Calreticulin has also been identified as another eat-me signal on apoptotic cells. During apoptosis, calreticulin increases on the cell surface and redistributes into PS-rich patches. These calreticulin/PS-rich patches distribute away from regions rich in CD47 (a newly described “don’t-eat-me” signal on viable cells), ultimately resulting in enhanced efferocytosis. Finally, CD31 (also known as platelet-endothelial cell adhesion molecule-1) is a fascinating molecule with dual roles, functioning as both a don’t-eat-me (detachment) signal on viable leukocytes and as an eat-me facilitator on apoptotic leukocytes. In an elegant series of studies, Brown and colleagues demonstrated that homophilic ligation of CD31 on viable leukocytes causes them to detach from macrophages. In contrast, the homophilic ligation of CD31 on apoptotic leukocytes enhances their attachment (tethering) to macrophages, the engagement of eat-me signals (e.g., PS), and ingestion. It therefore appears that the apoptotic cell membrane is actively reconfigured in a way that enhances recognition, tethering, and removal by the phagocyte.
_
The Phagocytic Synapse:
Because the interaction between an apoptotic cell and a phagocyte involves an array of receptors and bridging molecules, it has been termed a phagocytic synapse. Receptors known to be associated with efferocytosis include the low-density lipoprotein receptor-related protein (LRP [or CD91]), Mer receptor tyrosine kinase, αvβ3- and αvβ5-integrins, scavenger receptors, CD44, CD14, and complement receptors 3 and 4, Adenosine triphosphate-binding cassette proteins including ABC-A1, ABC-A7 (A.W. Jehle; personal communication; March 2006), and cystic fibrosis transmembrane regulator (CFTR) also contribute to efferocytosis, although perhaps not as true receptors. Some efferocytosis receptors appear to perform primarily a tethering function by holding the apoptotic cell and phagocyte close together (e.g., CD14 or CD31), while other receptors are primarily involved with transducing the engulfment signal (e.g., LRP and PS receptors [PSRs]), hence the so-called tether-and-tickle hypothesis. This, however, does not preclude the possibility that some receptors may ultimately be shown to perform both tethering and tickling (signal transduction) functions.
_
Consequences of efferocytosis:
1. Burying the Bodies:
The most immediate consequence of efferocytosis is the physical removal of apoptotic cells before membrane permeability sets in, thus preventing the release of potentially toxic intracellular contents. This process may be particularly important in the case of neutrophils, because of their large numbers during acute inflammation, their short lifespan, and their internal stores of proteases, inflammatory mediators, and oxidants. This concern seems to be substantiated by in vitro studies showing the proinflammatory effect of lysed neutrophils in an elastase-dependent manner. It may be, however, that the expeditious removal of all types of apoptotic cells is critical, because caspases 2, 3, and 7 have been shown to be present and active on the surface of apoptotic smooth muscle cells and to exhibit extensive elastolytic activity. Therefore, apoptotic cells of all types may be capable of remodeling tissues if not removed swiftly.
2. Innate Immunity:
The interaction of apoptotic cells and phagocytes suppresses the innate immune response and promotes its resolution by actively suppressing inflammatory mediator production through the action of transforming growth factor (TGF)-β1, prostanoids, peroxidase proliferator-activated receptor (PPAR)-γ (C. Freire de Lima; personal communication; March 2006), and in some cases interleukin (IL)-10. Antiinflammatory mediators, such as TGF-β1, that are released from the phagocyte act in an autocrine/paracrine manner to suppress the production of inflammatory cytokines (e.g., tumor necrosis factor [TNF]-α, IL-6, and IL-1β), chemokines (e.g., IL-8 and macrophage inhibitory protein-2), and lipid mediators (e.g., thromboxane A2). The antiinflammatory effect of efferocytosis can also be seen in vivo where the instillation of apoptotic cells suppresses endotoxin-induced lung inflammation in a TGF-β1-dependent manner. In contrast, the phagocytosis of lysed neutrophils is much less effective at inducing TGF-β1 production and inhibiting inflammatory mediator production. In fact, the ingestion of lysed neutrophils does not suppress the release of macrophage inhibitory protein-2 or IL-8, both of which are neutrophil chemotactic factors. Efferocytosis also increases the production of secretory leukoprotease inhibitor, which is an antiprotease with activity against elastase and cathepsin G. This may be especially important in suppressing protease/antiprotease imbalance during acute inflammation.
3. Adaptive Immunity:
The strongest evidence linking efferocytosis with the regulation of adaptive immunity is the association of human and murine autoimmune diseases with the impaired removal of apoptotic cells. The suppressive effect of efferocytosis on the adaptive immune system appears to be largely PS-dependent, to include both TGF-β1-dependent and TGF-β1-independent mechanisms, and to result in decreased antigen-specific CD4 T cells, antigen-specific IgG, dendritic cell maturation, and IL-12 production. In light of this, it is provocative that the infusion of donor apoptotic lymphocytes in a rat heart transplantation model induced allograft tolerance and was shown to be dependent on intact efferocytosis.
4. Proliferation:
The maintenance of alveolar integrity may be critically dependent on intact efferocytosis mechanisms, because it results in the release of growth factors and the activation of signaling molecules involved with the maintenance of the epithelium and endothelium. For example, apoptotic cell ingestion induces the production of hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), and causes the activation of β-catenin. In this paradigm, efferocytosis may be a key homeostatic regulator by locally inducing proliferation as structural cells die. Activation of the β-catenin signaling cascade regulates post-injury epithelial repair. This key regulator of cellular integrity controls the transcriptional expression of downstream cell cycle regulators, as well as the interactions between the actin cytoskeleton and cadherin within the adherens junctions. In a sub-lethal oxidant lung injury model, the pharmacologic inhibition of caspase-dependent apoptosis amplified necrotic injury, reduced β-catenin expression, and impaired post-injury repair, suggesting a pivotal role for post-injury apoptotic clearance in initiating epithelial repair. Efferocytosis also induces the secretion of VEGF by both epithelial cells and macrophages, which enhances the proliferation of pulmonary microvascular endothelial cells, and protects both endothelial and epithelial cells against ultraviolet-induced apoptosis. Similarly, apoptotic cell ingestion in vitro and in vivo increases HGF release from both alveolar macrophages and airway epithelial cells. The importance of this response is suggested by the well-known ability of HGF to enhance epithelial cell proliferation and to facilitate lung regeneration.
____
Inhibition of Apoptosis:
There are many pathological conditions that feature excessive apoptosis (neurodegenerative diseases, AIDS, ischemia, etc.) and thus may benefit from artificially inhibiting apoptosis. As our understanding of the field evolves, the identification and exploitation of new targets remains a considerable focus of attention (Nicholson, 2000). A short list of potential methods of anti-apoptotic therapy includes stimulation of the IAP (inhibitors of apoptosis proteins) family of proteins, caspase inhibition, PARP (poly [ADP-ribose] polymerase) inhibition, stimulation of the PKB/Akt (protein kinase B) pathway, and inhibition of Bcl-2 proteins.
_________
Caspase independent cell death:
_
_
Caspase independent cell death could be apoptotic, necrotic or other modes of cell death.
_
_
There is a cross-talk between cellular organelles during cell death. Upon a lethal stimulus, a cell has access to different death programs that can be executed via caspases (apoptosis) or independent of caspases. Mitochondria, lysosomes and the ER can be involved in various pathways but may play a more prominent role in certain types of PCD. As depicted in the figure above, the signals from the different organelles are linked and may act both upstream and downstream of each other. It has therefore been postulated that the dominant cell death phenotype is determined by the relative speed of the available death programs, and only the fastest and most effective pathway is usually evident.
_
Patterns of cell death have been divided into apoptosis, which is actively executed by specific proteases, the caspases, and accidental necrosis. However, there is now accumulating evidence indicating that cell death can occur in a programmed fashion but in complete absence and independent of caspase activation. Alternative models of programmed cell death (PCD) have therefore been proposed, including autophagy, paraptosis, mitotic catastrophe, and the descriptive model of apoptosis-like and necrosis-like PCD. Caspase-independent cell death pathways are important safeguard mechanisms to protect the organism against unwanted and potential harmful cells when caspase-mediated routes fail but can also be triggered in response to cytotoxic agents or other death stimuli. As in apoptosis, the mitochondrion can play a key role but also other organelles such as lysosomes and the endoplasmic reticulum have an important function in the release and activation of death factors such as cathepsins, calpains, and other proteases.
_
_
Apoptosis inducing factor (AIF):
Apoptosis inducing factor is involved in initiating a caspase-independent pathway of apoptosis (positive intrinsic regulator of apoptosis) by causing DNA fragmentation and chromatin condensation. It also acts as an NADH oxidase. Another AIF function is to regulate the permeability of the mitochondrial membrane upon apoptosis. Neurons, and perhaps other cells, have another way to self-destruct that does not use caspases. Apoptosis-inducing factor (AIF) is a protein that is normally located in the intermembrane space of mitochondria. When the cell receives a signal telling it that it is time to die, AIF
- is released from the mitochondria (like the release of cytochrome c in the intrinsic pathway);
- migrates into the nucleus;
- binds to DNA, which triggers the destruction of the DNA and cell death.
Normally it is found behind the outer membrane of the mitochondria and is therefore secluded from the nucleus. However, when the mitochondrion is damaged, it moves to the cytosol and to the nucleus. Inactivation of AIF leads to resistance of embryonic stem cells to death following the withdrawal of growth factors indicating that it is involved in apoptosis.
_______
Inflammatory Caspases: Inflammation and Pyroptosis:
Several caspases function as critical mediators of innate immune responses rather than proapoptotic factors. Caspase-1, -4, -5, and -12 comprise the inflammatory subset in humans, whereas caspase-1, -11, and -12 serve the same function in mice. Interestingly, the genes encoding inflammatory caspases are located in close proximity on human chromosome 11 and murine chromosome 9, suggesting that they may have arisen from gene duplication events. At the protein level, inflammatory caspases, like their proapoptotic counterparts, are produced as inactive procaspases in resting cells. Only after cellular stimulation via engagement of pattern-recognition receptors are inflammatory caspases activated through the formation of a cytosolic complex termed the inflammasome (Martinon et al. 2002).
Inflammasome Formation (vide supra):
Inflammasome formation resembles apoptosome formation and has been best studied for the nucleotide-binding domain, leucine-rich repeat-containing (NLR) proteins, which are a family of pattern-recognition receptors (PRRs), and other proteins. In a resting cell, NLR monomers are held in an inactive conformation until an external or internal stimulus promotes their assembly (similar to the assembly of APAF1 monomers in the apoptosome). NLR monomers interact through their NACHT domains and bind to the adapter protein apoptosis-associated speck-like protein containing a CARD (ASC/PYCARD) (Ting et al. 2008b). The presence of ASC permits the recruitment of an inactive inflammatory procaspase, typically procaspase-1, to the inflammasome, followed by cleavage and activation of caspase-1 through induced proximity autocatalysis (Davis et al. 2011). Activated caspase-1 in turn cleaves pro-IL-1β and pro-IL-18, which facilitates the secretion of these proinflammatory cytokines (Ting et al. 2008b). Although caspase-1 activation most often contributes to inflammation, excessive caspase-1 activity can cause pyroptosis, a nonapoptotic type of programmed cell death that is characterized by plasma membrane rupture and the release of proinflammatory intracellular contents (Cookson and Brennan 2001; Fink and Cookson 2006). Pyroptosis does not involve classical apoptotic caspases like caspase-3 and -8. Instead, activated caspase-1 activates caspase-7 and an unidentified nuclease that induces DNA cleavage and nuclear condensation without compromising nuclear integrity (Molofsky et al. 2006; Bergsbaken and Cookson 2007). Because caspase-1 activation is required for cell death in a variety of experimental settings, including in the immune system (Shi et al. 1996), the cardiovascular system (Kolodgie et al. 2000; Frantz et al. 2003), and the central nervous system (Liu et al. 1999; Yang et al. 1999; Zhang et al. 2003), pyroptosis has been thought to have an important physiological role.
_________
Apoptosis and regulation of Immune System:
Apoptosis also plays a very active role in regulating the immune system. When it is functional, it can cause immune unresponsiveness to self-antigens via both central and peripheral tolerance. In the case of defective apoptosis, it may contribute to etiological aspects of autoimmune diseases. The autoimmune disease, type 1 diabetes can be caused by defective apoptosis, which leads to aberrant T cell AICD and defective peripheral tolerance. Due to the fact that dendritic cells are the most important antigen presenting cells of the immune system, their activity must be tightly regulated by such mechanisms as apoptosis. Researchers have found that mice containing dendritic cells that are Bim -/-, thus unable to induce effective apoptosis, obtain autoimmune diseases more so than those that have normal dendritic cells. Other studies have shown that the lifespan of dendritic cells may be partly controlled by a timer dependent on anti-apoptotic Bcl-2.
_
The figure below shows anti-tumour immune response mediated through apoptosis:
Induction of tumor cell death leads to recognition of cell death by DCs. Apoptotic bodies are engulfed by APC, and tumor-derived antigens are processed and presented along with CMH and costimulatory molecules, after DC maturation, to naive T lymphocytes. This process can be amplified at different steps. Selection of immunogenic inducers of cell death allows enhancing of recognition and phagocytosis of apoptotic debris, maturation of DCs, processing and presentation of tumor-derived antigens. This leads to the induction of a cytotoxic immune response, involving CD4+ and CD8+ T lymphocytes, resulting in complete eradication of tumor cells. Additional immunotherapies targeting DCs and T lymphocytes can further enhance this immune response.
_
The figure below shows how killer T lymphocyte activates caspase mediated apoptosis in target cell:
_
One of the most obvious examples of the overlap between apoptosis and the immune response is the processing of cytokines by caspases. Mammalian caspase-1, which may not be involved in apoptosis, is clearly required for the processing and secretion of interleukin-1 and interleukin-18, both of which participate in inflammatory responses to infection. Similarly, caspase-3, the prime apoptotic executioner caspase, may be involved in the processing and secretion of interleukin-16, which acts as a chemotactic factor for T lymphocyte recruitment. This function of caspases extends to insects; the caspase DREDD is required for the anti-bacterial response in Drosophila, and appears to play a fundamental role in the processing and activation of Relish, 16 a member of the Nuclear Factor-kappaB (NF-kappaB) family of transcription factors.
_
Interferons and cell death:
Interferons(IFNs) were discovered over a half century ago as anti-viral factors. The role IFNs has been studied in the pathogenesis of both acute and chronic microbial infections. Deregulated IFN production results in a damaging cascade of cell death, inflammation, and immunological host responses that can lead to tissue injury and disease progression. Recently IFNs were recognized as crucial regulators of non-canonical NLRP3 inflammasome activation and pyroptosis. Although the majority of literature indicates a positive role for inflammasomes in anti-microbial host defense, recent reports indicate detrimental effects due to excessive cell death, inflammation, and collateral tissue damage in vital organs (Lupfer and Kanneganti,2012). A paradox exists, where inflammasomes intended to defend against cytoplasmic invaders by pyroptosis, which is predominantly protective in vitro, however in vivo, if exceeds a certain level, can lead to cell and tissue damage and organ failure resulting in negative outcomes. The paradox for IFNs is that their ability to inhibit microbial spread by inducing cell death is counteracted by apoptotic depletion of immune cells and inhibiting anti-microbial immune responses leading to immune suppression.
_
The NLR (nucleotide-binding domain, leucine-rich repeat containing) family of proteins at the intersection of cell death and immunity:
Inflammation is a crucial element of the host response to cellular insult. Pathogen induced inflammation includes a molecular pathway which proceeds through activation of the protease caspase‑1 to the release of the inflammatory cytokines interleukin‑1 (IL‑1) and IL‑18. Importantly, pathogens may also induce forms of cell death that have inherently pro‑inflammatory features. Recent evidence demonstrate that NLR (nucleotide-binding domain, leucine-rich repeat containing) family proteins serve as a common component of both caspase‑1-activated apoptotic pathways and caspase independent necrotic pathways. Parallels are drawn between NLR protein function and the activity of structurally similar proteins involved in cell death: the apoptotic mediator APAF1 (apoptotic-protease-activating factor 1) and the plant disease resistance NBS-LRR (nucleotide-binding site leucine-rich repeats) proteins.
_________
Stem cell and apoptosis:
The figure below shows stem cell pathways that lead to their apoptosis, renewal and differentiation:
__________
Assays for Apoptosis:
Since apoptosis occurs via a complex signaling cascade that is tightly regulated at multiple points, there are many opportunities to evaluate the activity of the proteins involved. As the activators, effectors and regulators of this cascade continue to be elucidated, a large number of apoptosis assays are devised to detect and count apoptotic cells. However, many features of apoptosis and necrosis can overlap, and it is therefore crucial to employ two or more distinct assays to confirm that cell death is occurring via apoptosis. Apoptosis assays, based on methodology, can be classified into six major groups and a subset of the available assays in each group is indicated and briefly discussed:
- Cytomorphological alterations
- DNA fragmentation
- Detection of caspases, cleaved substrates, regulators and inhibitors
- Membrane alterations
- Detection of apoptosis in whole mounts
- Mitochondrial assays.
____________________
Other Forms of Programmed Cell Death besides apoptosis:
In their seminal article published in 1972, Kerr, Wyllie, and Currie described two major types of cell death: apoptosis, the genetically controlled PCD, and necrosis, the nonprogramed and accidental type of cell death (Kerr et al., 1972). In the following three decades, the term ‘‘apoptosis’’ was used as a general term to describe PCD and an impressive amount of information has accumulated regarding the molecular mechanisms governing this phenomenon. Because the apoptosis versus necrosis concept of cell death was so dominant, early observations about the existence of alternative, nonapoptotic PCD types were ignored by the majority of the scientific community (Schweichel and Merker, 1973). There is evidence of other forms of non-apoptotic programmed cell death that should also be considered since they may lead to new insights into cell death programs and reveal their potentially unique roles in development, homeostasis, neoplasia and degeneration. It has become increasingly apparent that cell death mechanisms include a highly diverse array of phenotypes and molecular mechanisms. In recent years, however, increasing interest in alternative PCD types emerged once diVerent tools to study these other genetically controlled systems at the molecular level became available. One type, autophagic cell death, recently received considerable momentum in light of the identification of the mammalian orthologues of the yeast autophagic genes. As a consequence, autophagic cell death is currently recognized as one of the major alternative or complementary cell death pathways to apoptosis in several experimental systems. And there is evidence that modulation of one form of cell death may lead to another. Because other types of cell death may require gene activation and function in an energy dependent manner, they are also considered to be forms of “programmed cell death.” Therefore, there is some resistance to the exclusive use of the term “programmed cell death” to specifically describe apoptosis.
_
Cells respond to changes in their environment and intracellular milieu by altering their anabolic and catabolic pathways. Two major pathways are involved in the catabolism of cellular material: the multi-enzyme proteasome system and autophagy, which culminates in lysosomal degradation. Both proteasome and autophagy are able to degrade proteins, but only autophagy can also degrade other macromolecules and even entire organelles. The ubiquitin-proteasome pathway predominantly degrades short-lived nuclear and cytosolic proteins. The lysosomal-vacuolar pathway degrades larger substrates such as protein complexes and organelles.
___________
Autophagy:
There is speculation that “autophagy” represents another mechanism for programmed cell death and, similar to apoptosis, has important roles in developmental processes, human diseases and cellular responses to nutrient deprivation (Schwartz et al., 1993; Gozuacik and Kimchi, 2004; Debnath et al., 2005). Other terms used synonymously are “macroautophagy” and “autophagic type II cell death” (Klionsky and Emr, 2000). Autophagic cell death is characterized by the sequestration of cytoplasm and organelles in double or multimembrane vesicles and delivery to the cells own lysosomes for subsequent degradation (Noda et al., 2002). In a sense, the cell “cannibalizes” itself. The mechanisms and morphology of autophagy are evolutionarily conserved with strong similarities between organisms as diverse as animals, plants and yeast. The process of autophagy depends on both continuous protein synthesis and the continuous presence of ATP. The molecular mechanisms have been extensively studied in yeast and mammalian orthologues continue to be elucidated (Ohsumi, 2001; Huang and Klionsky, 2002). The distinction of a autophagic programmed cell death was made because it was determined that some cells would undergo caspase-independent gene-activated cell death but would display few of the ultrastructural features characteristic of apoptosis and would not exhibit DNA laddering (Cohen, 1991). However these cells do require de novo gene expression with an increase in expression of the polyubiquitin gene, similar to apoptosis (Ohsumi, 2001; Gozuacik and Kimchi, 2004). Specifically, autophagy occurs in all eukaryotic cells and involves the dynamic rearrangement of subcellular membranes to sequester cytoplasm and organelles for delivery to the lysosome or vacuole where degradation occurs. This is considered to be the major inducible pathway for the general turnover of cytoplasmic components. A unique ubiquitin-like protein conjugation system and a protein complex that directs membrane docking and fusion at the lysosome or vacuole are important components of autophagy. In general, the process of autophagy can be divided into 4 steps: (1) induction, (2) formation of the autophagosome, (3) fusion with the lysosome or vacuole, and (4) autophagic body breakdown and recycling. Although the molecular details are still being elucidated, the regulation of this process occurs through various kinases, phosphatases and guanosine triphosphatases (GTPases).
_
Definition of autophagy:
The word “autophagy,” which is derived from the Greek “to eat” (“phagy”) oneself (“auto”), was first used for structures that were observed on electron microscopy and that consisted of single-or double-membrane lysosomal-derived vesicles containing cytoplasmic particles, including organelles, in various stages of disintegration. We now understand that autophagy is the process by which cells recycle their own nonessential, redundant, or damaged organelles and macromolecular components. It is an adaptive response to sublethal stress, such as nutrient deprivation, that supplies the cell with metabolites it can use for fuel. Autophagy also has a role in the suppression of tumor growth, deletion of toxic misfolded proteins, elimination of intracellular microorganisms, and antigen presentation.
_
_
Autophagy is characterized by sequestration of bulk cytoplasm and organelles in double or multimembrane
autophagic vesicles, and their delivery to and subsequent degradation by the cell’s own lysosomal system. Autophagy has multiple physiological functions in multicellular organisms, including protein degradation and organelle turnover. Genes and proteins that constitute the basic machinery of the autophagic process were first identified in the yeast system and some of their mammalian orthologues have been characterized as well. Increasing lines of evidence indicate that these molecular mechanisms may be recruited by an alternative, caspase-independent form of programmed cell death, named autophagic type II cell death. In some settings, autophagy and apoptosis seem to be interconnected positively or negatively, introducing the concept of ‘molecular switches’ between them. Additionally, mitochondria may be central organelles integrating the two types of cell death. Malignant transformation is frequently associated with suppression of autophagy. The recent implication of tumor suppressors like Beclin 1, DAP-kinase and PTEN in autophagic pathways indicates a causative role for autophagy deficiencies in cancer formation. Autophagic cell death induction by some anticancer agents underlines the potential utility of its induction as a new cancer treatment modality.
_
Three forms of autophagy have been defined on the basis of howlysosomes receive material for degradation. In macroautophagy,a double-membrane structure (the autophagosome) envelops thecargo and then fuses with lysosomes. In microautophagy, an invaginationof the lysosomal membrane engulfs the cargo. In chaperone-mediatedautophagy, heat-shock cognate proteins deliver substrates tolysosomes. Macro-autophagy is an evolutionary mechanism conserved in eukaryotic cells that starts with the formation of an autophagosome, enclosed within a double membrane that engulfs part of the cytoplasm. In mammalian cells, autophagosomes undergo a maturation process by fusing with endocytic compartments and lysosomes. Autophagy is active at a basal level in most of the cells in the body, and this probably reflects its role in regulating the turnover of long-lived proteins, and getting rid of damaged structures. This activity is probably responsible for the anti-aging role of autophagy. However, autophagy can be stimulated by various stress situations, including nutrient depletion. During periods of nutrient shortage, autophagy provides the constituents required to maintain the metabolism essential for survival. Autophagy is also implicated in various diseases, including cancer, neurodegenerative diseases, pathogen invasion, and muscle and liver disorders.
_
Macro-autophagy pathways:
_
Autophagy is activated when nutrients are deficient:
When cells lack essential nutrients, autophagy is activated to supply the missing components. A classical example is the mammalian liver, in which autophagy is switched on during starvation to produce amino acids which, after conversion into glucose, are used to meet the energy requirements of the brain and erythrocytes. Whereas starvation-induced autophagy is a mechanism tightly controlled by amino acids and hormones, it appears that greater complexity of the signaling pathways, which overlap with those of apoptotic signaling, is observed during cell death.
_
Dual role of autophagy:
Autophagic cell death is a type of cell death occurring together with (but not necessarily caused by) autophagic vacuolization. Autophagy, or autophagocytosis, is a catabolic process involving the degradation of a cell’s own components through the lysosomal machinery. It is a tightly-regulated process that plays a normal part in cell growth, development, and homeostasis, helping to maintain a balance between the synthesis, degradation, and subsequent recycling of cellular products. It is a major mechanism by which a starving cell reallocates nutrients from unnecessary processes to more-essential processes. A causative relationship between autophagy and cell death has not been established. Recently it has been argued that autophagy might actually be a survival mechanism on behalf of the cell. In most situations, autophagy has both beneficial and harmful effects. One aspect of this complexity probably reflects the dual role of autophagy, which is both cell-protective and -destructive. Autophagy also plays a housekeeping role in removing misfolded or aggregated proteins, clearing damaged organelles, such as mitochondria, endoplasmic reticulum and peroxisomes, as well as eliminating intracellular pathogens. Thus, autophagy is generally thought of as a survival mechanism, although its deregulation has been linked to non-apoptotic cell death. In addition to elimination of intracellular aggregates and damaged organelles, autophagy prevents cellular senescence and cell surface antigen presentation, protects against genome instability and prevents necrosis, giving it a key role in preventing diseases such as cancer, neurodegeneration, cardiomyopathy, diabetes, liver disease, autoimmune diseases and infections. The role of autophagy during cell death has been discussed in recent reviews. During apoptosis, the stimulation of autophagy can be either a protective mechanism or a process that contributes to cell death. In the absence of apoptosis, autophagy can trigger a form of cell death known as autophagic cell death or Type II programmed cell death, which is distinct from Type I programmed cell death(apoptosis).
_
Autophagy genes:
Autophagy is a multistep process that is characterized by the vesicular sequestration and degradation of long-lived cytoplasmic proteins and organelles, for example, mitochondria. The resulting double-membrane vesicle is termed an autophagosome. The discovery of autophagy-related (atg) genes [atg = AuTophaGy-related], first in yeast and subsequently in humans, has greatly enhanced the molecular understanding of the mechanisms that are involved in the control of autophagy. The protein product of the tumor suppressor gene Beclin 1 is the mammalian homolog of Atg6 and forms a multiprotein complex together with Vps34, a class III phosphatidylinositol 3-kinase, UVRAG (UV irradiation resistance-associated tumor suppressor gene), and a myristylated kinase (Vps15, or p150 in humans). This complex is required for the initiation of the formation of the autophagosome. Once this complex forms, Vps34 becomes activated and catalyzes the generation of phosphatidylinositol-3-phosphate, which is required for vesicle nucleation.
_
Autophagy mechanism:
_
_
Two major protein conjugation systems exist that are required for autophagosome formation, that is, the Atg12–Atg5 conjugation and Atg8-phosphatidylethanolamine conjugation systems. Mechanistically, both conjugation systems function in a manner that is closely related to ubiquitin conjugation to proteins, with corresponding conjugation-assisting enzymes that resemble the E1 and E2 enzymes in ubiquitin conjugation. In the Atg12–Atg5 conjugation pathway, Atg12 is covalently conjugated to Atg5 with the help of the E1-like enzyme Atg7 and the E2-like enzyme Atg10. In the other conjugation pathway, phosphatidylethanolamine (PE) is conjugated to LC3, one of the mammalian homologues of Atg8. This process involves the sequential action of the protease Atg4, the E1-like enzyme Atg7 and the E2-like enzyme Atg3. Subsequently, lipid conjugation results in the conversion of the soluble form of LC3, that is, LC3-I, to the autophagic-vesicle-associated form that is termed LC3-II. Thus, LC3 is soluble under unstressed conditions and undergoes association with peripheral membranes of autophagosomes during the induction of autophagy. Via the fusion with lysosomes, the content of autophagosomes is degraded by the action of acid-dependent enzymes. Autophagy is typically observed in cells that are exposed to a variety of metabolic and therapeutic stresses, including growth factor deprivation, inhibition of the receptor tyrosine kinase/Akt/ mammalian target of rapamycin (mTOR) signaling, shortage of nutrients, ischemia/reperfusion, inhibition of proteasomal degradation, the accumulation of intracellular calcium, and endoplasmic reticulum (ER) stress. Reactive oxygen species (ROS) may provide a common link between cellular stress signals and the initiation of autophagy, as ROS accumulation has been reported to result in inactivation of the cysteine protease ATG4, which in turn causes accumulation of the ATG8-phosphoethanolamine precursor that is required for the initiation of autophagosome formation. The functional relationship between autophagy and cell death is complex in the sense that, under most cellular settings, autophagy functions as a stress adaptation that prevents cell death, whereas in some circumstances, it constitutes an alternative route to cell death. This complex interrelationship between autophagy and cell death implies that these responses are somewhat linked at the molecular level. However, the key molecular events that eventually determine whether autophagy is protective or destructive are still poorly understood.
_
Autophagy, mitochondria and oxidative stress: cross-talk and redox signaling:
Reactive oxygen and nitrogen species change cellular responses through diverse mechanisms that are now being defined. At low levels, they are signaling molecules, and at high levels, they damage organelles, particularly the mitochondria. Oxidative damage and the associated mitochondrial dysfunction may result in energy depletion, accumulation of cytotoxic mediators and cell death. Understanding the interface between stress adaptation and cell death then is important for understanding redox biology and disease pathogenesis. Recent studies have found that one major sensor of redox signaling at this switch in cellular responses is autophagy. Autophagic activities are mediated by complex molecular machinery including more than 30 Atg proteins and 50 lysosomal hydrolases. Autophagosomes form membrane structures, sequester damaged, oxidized or dysfunctional intracellular components and organelles, and direct them to the lysosomes for degradation. This autophagic process is the sole known mechanism for mitochondrial turnover. It has been speculated that dysfunction of autophagy may result in abnormal mitochondrial function and oxidative or nitrative stress. Emerging investigations have provided new understanding of how autophagy of mitochondria (also known as mitophagy) is controlled, and the impact of autophagic dysfunction on cellular oxidative stress. This is particularly relevant to degenerative diseases in which oxidative stress occurs over time, and dysfunction in both the mitochondrial and autophagic pathways play a role. Impaired mitochondrial function, oxidative stress, accumulation of protein aggregates and autophagic stress are common in many pathologies, including neurodegenerative diseases. Oxidative stress can lead to the non-specific post-translational modifications of proteins and contributes to protein aggregation. Since the brain uses 20% of the inspired oxygen and 90% of the consumed oxygen to produce energy during oxidative phosphorylation, it is not surprising that neuronal cells are particularly sensitive to oxidative stress. During oxidative phosphorylation, neurons in the brain are vulnerable to oxidative damage because of their high metabolic activity, low antioxidant capacity and non-replicative nature. Oxidative stress is inseparably linked to mitochondrial dysfunction, as mitochondria are both generators of and targets for reactive species. Mitochondrial turnover is dependent on autophagy, which declines with age and is frequently dysfunctional in neurodegenerative diseases. The cross-talk between autophagy, redox signaling and mitochondrial dysfunction is not well understood. Some possibilities include the accumulation of toxic proteins and the decrease in mitochondrial function, leading to further oxidative stress when the autophagic process is disrupted. Dysregulated redox signaling or mitochondrial dysfunction can also influence autophagic activities.
_
Functions of autophagy:
_
_
Nutrient starvation:
Autophagy has important roles in various cellular functions, one particular example is in yeasts, where the nutrient starvation induces a high level of autophagy, which allows unneeded proteins to be degraded and the amino acids recycled for the synthesis of proteins that are essential for survival. In higher eukaryotes, autophagy is also induced in response to the nutrient depletion that occurs in animals at birth after severing of the trans-placental food supply, as well as that of nutrient starved cultured cells and tissues. Mutant yeast cells that have a reduced autophagic capability rapidly perish in nutrition-deficient conditions. Studies on the apg mutants suggest that autophagy via autophagic bodies is indispensable for protein degradation in the vacuoles under starvation conditions, and that at least 15 atg genes are involved in autophagy in yeast. A gene known as Atg7 has been implicated in nutrient-mediated autophagy, as mice studies have shown that starvation-induced autophagy was impaired in Atg7-deficient mice.
Infection:
Autophagy has been recognized as an immune mechanism. It plays a role in the destruction of intracellular pathogens, in a process of degradation of dysfunctional intracellular organelles. Intracellular pathogens such as Mycobacterium tuberculosis is the bacterium which is responsible for tuberculosis and it can survive within the cells by blocking the maturation of its phagosome into a degradative organelle called a phagolysosome. Stimulation of autophagy in infected cells helps overcome the block and aids the cell to eliminate the pathogens. Vesicular stomatitis virus is believed to be taken up by the autophagosome from the cytosol and translocated to the endosomes where detection takes place by a member of the PRRs called toll-like receptor 7, detecting single stranded RNA. Following activation of the toll-like receptor, intracellular signaling cascades are initiated, leading to induction of interferon and other antiviral cytokines. A subset of viruses and bacteria subvert the autophagic pathway to promote their own replication. Galectin-8 has recently been identified as an intracellular “danger receptor”, able to initiate autophagy against intracellular pathogens. When galectin-8 binds to a damaged vacuole, it recruits autophagy adaptor such as NDP52 leading to the formation of an autophagosome and bacterial degradation.
Repair mechanism:
Autophagy degrades damaged organelles, cell membranes and proteins, and the failure of autophagy is thought to be one of the main reasons for the accumulation of cell damage and aging.
Programmed cell death:
Apoptosis (cell suicide) is not the only form of programmed cell death (PCD). Recent studies have provided evidence that there is another mechanism of PCD which is associated with the appearance of autophagosomes and depends on autophagy proteins. This form of cell death most likely corresponds to a process that has been morphologically defined as autophagic PCD. This hypothesis as well as previous reports that cells with autophagic features often exist in regions where PCD is occurring seem to support the existence of autophagic PCD. One question that constantly arises, however, is whether autophagic activity in dying cells is the cause of death or is actually an attempt to prevent it. Morphological and histochemical studies cannot prove a causative relationship between the autophagic process and cell death. In fact, there have recently been strong arguments that autophagic activity in dying cells might actually be a survival mechanism. Studies of the metamorphosis of insects have shown cells undergoing a form of PCD that appears distinct from other forms; these have been proposed as examples of autophagic cell death.
_
Is autophagy a cell death imposter?
Although strictly speaking, the term autophagy merely means ‘self-eating’, many presume that this cellular self-eating is inevitably a form of cellular self-destruction. Indeed, within the cell death research field, autophagy has also long been defined as a form of non-apoptotic, or type II, programmed cell death. However, as revealed in an issue of Cell Death and Differentiation, which contains nine review papers on the topic of Autophagy in Aging, Disease and Death and one article outlining the recommendations of the Nomenclature Committee on Cell Death 2009, a consensus is emerging that autophagy is largely a cell death impostor which, in reality, functions primarily to promote cellular and organismal health. The delivery function of autophagy – either to the lysosome for degradation or to other cellular compartments for immune activation – is important in protection against aging, neurodegenerative diseases, α-1-antitrypsin deficiency, and infectious diseases and indeed, clinical trials with autophagy-inducing agents are now being planned for the prevention of aging, and treatment of certain neurodegenerative and infectious diseases. In the case of lysosomal delivery, ‘self-eating’ (as well as xenophagy, the digestion of microbes) certainly does occur. However, it is not part of a program of cellular self-destruction that leads to cell death. Rather, it is a mechanism by which the cell rids itself of potentially harmful constituents, and thereby helps to maintain its normal functioning. Thus, after years of being (mis)interpreted as a cell death process, the study summarized in the review papers in this issue shed important light on the true identity of autophagy – which is, in part, an adaptive cellular housekeeping mechanism. It is not yet clear how to reconcile the few examples of ‘cell death by autophagy’ with the larger number of studies in which autophagy suppression by genetic knockout/knockdown of essential autophagy genes increases cell death (reviewed in Scarlatti et al., Kourtis and Tavernakis and Maiuri et al.), indicating a prosurvival function of autophagy. Further, as noted by Scarlatti et al., the evidence for cell death by autophagy remains to be demonstrated in mammals; in fact, embryonic mice lacking autophagy genes, including ambra1, beclin 1, and atg5, have been shown to have increased, not decreased, numbers of apoptotic cells (reviewed in Cecconi and Levine). Thus, although it is certainly possible that autophagy is not always a cell death impostor; the majority of studies discussed in this issue of Cell Death and Differentiation (and recently reviewed elsewhere) suggest that the primary identity of autophagy lies elsewhere, namely, in cytoprotection. It will be important to further unravel the mysteries of the complex interplay between autophagy and cell death, but arguably, it may be even more important to further unravel the relationships between autophagy, aging, disease, and cell survival. The review papers in this series provide an excellent point of departure for meeting both of these scientific challenges.
_
Hormesis and autophagy:
Frequently, low doses of toxins and other stressors not only are harmless but also activate an adaptive stress response that raises the resistance of the organism against high doses of the same agent. This phenomenon, which is known as “hormesis”, is best represented by ischemic preconditioning, the situation in which short ischemic episodes protect the brain and the heart against prolonged shortage of oxygen and nutrients. Many molecules that cause cell death also elicit autophagy, a cytoprotective mechanism relying on the digestion of potentially harmful intracellular structures, notably mitochondria. When high doses of these agents are employed, cells undergo mitochondrial outer membrane permeabilization and die as seen in the figure below. In contrast, low doses of such cytotoxic agents can activate hormesis in several paradigms, and this may explain the lifespan-prolonging potential of autophagy inducers including resveratrol and caloric restriction.
_________________
Necrosis:
Definition of necrosis:
Necrosis (from the Greek “dead”) is the premature death of cells and living tissue. Necrosis is a form of cell injury that results in the premature death of cells in living tissue by autolysis. Necrosis has been defined as a type of cell death that lacks the features of apoptosis and autophagy, and is usually considered to be uncontrolled. Recent research suggests, however, that its occurrence and course might be tightly regulated. Necrosis is caused by factors external to the cell or tissue, such as infection, toxins, or trauma, that result in the unregulated digestion of cell components. In contrast, apoptosis is a naturally occurring programmed and targeted cause of cellular death. While apoptosis often provides beneficial effects to the organism, necrosis is almost always detrimental and can be fatal. Cells that die due to necrosis do not follow the apoptotic signal transduction pathway but rather various receptors are activated that result in the loss of cell membrane integrity and an uncontrolled release of products of cell death into the intracellular space. This initiates in the surrounding tissue an inflammatory response which prevents nearby phagocytes from locating and eliminating the dead cells by phagocytosis. For this reason, it is often necessary to remove necrotic tissue surgically, a procedure known as debridement. Untreated necrosis results in a build-up of decomposing dead tissue and cell debris at or near the site of the cell death. A classic example is gangrene. Necrosis is usually considered an accidental (i.e., nonprogrammed) form of cell death that occurs in response to acute hypoxic or ischemic injury, such as myocardial infarction and stroke. It occurs spontaneously in neoplasms when cell proliferation outpaces angiogenesis. The exposure of cells to supraphysiologic conditions (e.g., mechanical force, heat, cold, and membrane-permeabilizing toxins) also precipitates necrosis. Necrosis, which can also be initiated by bacterial or viral infection, is believed to help the body’s immune system detect threats. When dying cells release their contents during necrosis, it serves as a warning signal for your body that there is a virus there and recruits macrophages and other immune cells to the area.
_
Necrosis is best defined by light or electron microscopic detection of cell and organelle swelling or rupture of surface membranes with spillage of intracellular contents. The term “oncosis” (Greek for swelling) is preferred by some investigators, and “oncotic necrosis” has also been used. The compromise of organellar membranes allows proteolytic enzymes to escape from lysosomes, enter the cytosol, and cause cell demolition. Necrosis usually results from metabolic failure that has coincided with rapid depletion of ATP; it classically occurs in ischemia. Necrosis is a type of irreversible and pathological cell death. It is mainly caused by early plasma membrane rupture, mitochondrial dysfunction, cell injury, infarction, inflammation and lysosomal rupture. There are several patterns of necrosis classified based on morphological criteria, including coagulative necrosis, liquefactive necrosis, caseous necrosis, fibrinoid necrosis and fat necrosis. Unlike apoptosis, necrosis is considered as uncontrolled, however current research suggests that this perhaps may not be the not the case.
_
Necrosis is characterized by early swelling of the cytoplasm and of the mitochondria (energy-releasing organelles) within it. Later changes include the appearance of localized densities, possibly related to calcium deposition, in the matrix (ground substance) of the mitochondria. This is followed by the dissolution of other cytoplasmic organelles and the separation of affected cells from their neighbours through shearing of intercellular junctions. Nuclear alterations occur late and are relatively unremarkable. The nucleus swells, becomes darker (pyknosis), and ruptures (karyolysis) at about the same time as does the plasma membrane, the outer envelope of the cell. The basic mechanism of necrosis is thought to be a loss of control over cell volume, related to changes in the permeability of the cell membrane. These changes form the basis of several of the tests used to diagnose a necrotic cell in the laboratory. The affected membrane rapidly loses its ion-pumping capacity, and there are dramatic increases in the intracellular concentrations of sodium and calcium ions. This is followed by osmotic shock and the development of intracellular acidosis. The early injury to the mitochondria has profound repercussions on intracellular oxidative metabolism. The point of no return is reached with irreversible damage to mitochondrial structure and function. Later still, the lysosomes rupture, releasing their acid enzymes into the cytoplasm of the cell. All this produces an ionic milieu unsuitable to the survival of the nucleus. Loss of the cell’s capacity to synthesize protein is the ultimate proof that it is functionally dead.
_
Mediators of necrosis:
Reactive oxygen species (ROS), calcium ions, poly–ADP–ribose polymerase (PARP), calcium-activated nonlysosomal proteases (calpains), and cathepsins mediate necrosis. PARP is a DNA-repair enzyme that can deplete cellular ATP stores when it catalyzes the repair of multiple DNA strand breaks that occur in cell injury. In apoptosis, PARP undergoes rapid cleavage and inactivation (detection of cleaved PARP is a diagnostic test for apoptosis), so stores of ATP are preserved. ATP is necessary for numerous effector processes in apoptosis, whereas exhaustion of ATP shifts the cell from apoptosis to necrosis. PARP inhibition mitigates necrosis in ischemia–reperfusion injury and other types of damage. Increased intracellular calcium ions, a central feature of necrosis, activate proteases that degrade critical proteins. Intriguingly, the source and amount of increased calcium ions may induce different types of cell death: the influx of calcium ions across the plasma membrane triggers necrosis, whereas the release of calcium ions from the endoplasmic reticulum more readily induces apoptosis. In addition, the inhibition of specific proteins involved in regulating apoptosis or autophagy can change the type of cell death to necrosis. Because necrosis is prominent in ischemia, trauma and possibly some forms of neurodegeneration, further biochemical comprehension and molecular definition of this process could have important clinical implications.
_
Mechanism of necrosis:
Necrosis has been considered as an accidental mode of cell death for many years, implying that within a multicellular organism it is an unregulated process. However, there is now mounting evidence that the execution of necrotic cell death is also regulated by a set of signaling pathways. For instance, death domain receptors, for example, TNFR1, and Toll-like receptors have been reported to trigger necrosis, in particular in the presence of caspase inhibitors. In addition, necrotic cell death has been reported in response to cellular stress stimuli, including ischemia or glutamate excitotoxicity in neurons or cancer cells exposed to alkylating DNA damaging agents. Morphologically, necrosis is characterized by a gain in cell volume, swelling of organelles and plasma membrane rupture, which results in the loss of intracellular contents. Several signal transduction cascades have been described that are involved in the propagation of necrotic cell death. There is mounting evidence that the serine/threonine kinase RIP1 is one of the key mediators of necrotic cell death, at least in the case of death receptors or Toll-like receptors. Studies in RIP1-deficient leukemia cells revealed that RIP1 is required for death receptor-induced necrosis. Furthermore, RIP1 has been described to be required for lipopolysaccharide-induced cell death of macrophages. In line with a central role of RIP1 in necrotic cell death, small molecule inhibitors of RIP1 kinase were reported to protect against ischaemic brain injury in an in vivo model of necrosis. In addition to RIP1, there is very recent evidence that RIP3 is also critical for necrotic cell death. To this end, RIP3 was identified in an RNA interference screen to be essential for necrosis in response to TNF stimulation and during virus infection. RIP3 interacts with RIP1 and regulates RIP1 phosphorylation and the generation of ROS. Moreover, ROS and calcium constitute important mediators that are involved in the propagation of the necrotic signal in various forms of necrosis, for example, upon stimulation with TNF or exposure to double-stranded DNA. ROS may be generated intracellularly by mitochondria and glycolysis. While the ER is the main intracellular calcium store, mitochondrial calcium has been described to stimulate oxidative phosphorylation, thereby promoting ROS generation. Both ROS and calcium can cause damage to organelles and macromolecules, which contributes to the loss of cell integrity. In addition calcium-mediated activation of calpain can lead to cleavage and inactivation of caspases, whereas the ROS can target the active site of caspases and render them inactive. Many stimuli that drive necrosis can inhibit the apoptotic machinery.
_
A cell may undergo two different processes leading to necrosis.
- Primary necrosis: characterized by external chemical and physical damage to the cell.
- Secondary necrosis: occurs in late apoptotic cells which fail to be engulfed by macrophages. Cells are not phagocytosed and thus lose membrane integrity, cease to be metabolically active and release their cytoplasmic content out into the extracellular matrix, or if in vitro, into the culture medium.
Primary necrosis is triggered through unregulated processes of membrane and cytosolic destruction under extreme conditions. These extreme conditions/stresses arise from various bacterial toxins and viruses, inadequate secretions of cytokines, nitric oxide and reactive oxygen species and calcium cytotoxicity. The mechanisms of necrosis include:
1. Mitochondrial Dysfunction and Reactive Oxygen Species (ROS):
- Mitochondrial dysfunction is one of the major causes of necrosis due to ATP depletion. The mitochondria is a site of cellular oxygen consumption and responsible for generating ATP by oxidative phosphorylation. Insufficient oxygen supply to the cell causes the uncoupling of oxidative phosphorylation and the mitochondria cannot generate ATP.
- As a consequence of energy depletion, the maintenance of the cell membrane potential is neglected. Early depolarization leads to excitotoxicity through excessive upregulation of glutamate and an increase in intracellular calcium concentration. At the early stage of oxygen deprivation, there is a formation of protrusions on the plasma membrane called blebs causing the cell to swell. Once the plasma membrane with blebs disrupts, the cell necrosis is irreversible.
- In the case of ischaemia, the lack of oxygen causes an increase Ca2+. In prolonged ischaemia, the Ca2+ overloaded mitochondria produce free radicals such as superoxide, singlet oxygen and hydroxyl radicals. These react with proteins and lipids inside the cell and form oxidized protein and peroxidised lipids which then disrupt the membrane structure. Recovery from ischaemia by reintroduction of oxygen will not prevent the formation of OFR but will cause an excess production of OFR leading to a worse condition (reperfusion injury).
2. Control of intracellular calcium:
The endoplasmic reticulum is the major Ca2+ store for the cell and its regulated release of calcium is important for cellular signaling. Ca2+ is a stimulator of oxidative phosphorylation in the mitochondria. Mitochondrial calcium overload will cause an elevated flow of electrons to the electron transport chain resulting in increased levels of ROS. If the electron transport chain is not functioning well, there will be a decrease in the energy levels of the cell.
This depletion is further enhanced during oxygen deprivation in ischaemia.
- The cell begins to use its glycogen stores anaerobically when energy levels are too low.
- This causes acidosis and a further increase in Ca2+ influx into the cell.
- Elevated increase in calcium will overstimulate oxidative phosphorylation and increase ROS.
- ROS will disrupt the mitochondrial inner membrane and result in further loss of energy.
- Transmembrane ion transport will slow down and cause increase cell membrane permeability and thus a loss in the homeostasis of K+, Na+ and Ca2+ ions.
- Now that the cell is severely deprived of ATP, the mitochondria and other organelles will swell and burst causing cell lysis; a characteristic feature of necrosis.
3. Proteases and necrosis:
Proteases are considered the “executioners” of cell death because once a proteolytic cascade is set in motion, death is the only outcome. There are a variety of proteases that are involved in necrosis.
- a) Cytosolic cysteine proteases (Caspases and Calpains)
- b) Lysosomal proteases (Cathepsins)
a) Cytosolic Cysteine Proteases:
- Caspases :
Caspases are cysteine proteases that are produced as inactive proteases called “zymogens” and are activated both during necrotic and apoptotic cell death. Mitochondrial damage due to excessive intracellular calcium and the release of cytochrome c and free radicals can lead to the activation of caspases. Once activated, they proteolytically cleave a large range of cellular targets leading to cell death. In necrosis, caspases mediate the degradation of calpastatin and thus activate calpain proteases.
- Calpains:
Calpains are calcium dependent cysteine proteases that perform only a limited amount of proteolytic cleavage of cellular substrates. There are two main isoforms of calpains, Calpain 1 and Calpain 2 that have different sensitivity to calcium concentrations. An acute increase in the intracellular calcium concentration will trigger calpain activation leading to cleavage of cytoskeletal proteins. During an event of extreme intracellular calcium, Calpains localize to the lysosomal membrane and cathepsins spill out into the cytoplasm catastrophically cleaving regulatory and structural proteins. Calpains then cleave the Na+/Ca2+ exchanger which functions to extrude Ca2+ from the cell further increasing calcium levels and thus overactivating calpains. Calpain activation is prominently seen in neuronal cell necrosis.
b) Lysosomal proteases:
- Cathepsins:
Cathepsins are lysosomal proteolytic enzymes that are also synthesized as inactive zymogens called “procathepsins”. Cathepsins are localized in the lysosomal compartments of the cell traveling from the Golgi apparatus. With the right signaling, cathepsins are activated and released from these lysosomal compartments to begin their role of degrading extracellular matrix proteins such as collagen, laminin and elastin. Cathepsins can be divided into
- Aspartic Cathepsins (Cathepsin D and E)
- Cysteine Cathepsins (Cathepsin B, C, H, L, and S)
- Serine Cathepsins (Cathepsin A and G)
Cathepsin activation and release is also highly dependent on intracellular Ca2+ concentrations. Elevated Ca2+ levels cause an overactivation of calpains which in turn cause lysosomal disruption. Lysosomal disruption will cause the release of cathepsins, particularly Cahtepsin B and Cathepsin L which degrade structural proteins in the cell.
_
Necrosis cascade:
_
The figure below shows how calcium influx promotes necrosis:
_
Necrosis: Programmed or Regulated?
Accumulating evidence indicates that necrosis is more ordered than was originally thought. When cells die from necrosis, damage-associated molecular-pattern (DAMP) molecules, such as high-mobility group box 1 (HMGB1) protein, enter the circulation and activate innate immune cells. Thus, the first cells that die from trauma or infection may function as sentinels, alerting the host to the need for defensive or reparative responses. In addition, necrosis can be initiated by the activation of selected cell-surface receptors. For example, high concentrations of TNF induce hepatocyte necrosis. The identification of an intracellular serpin (protease inhibitor) that prevents necrosis caused by multiple noxious stimuli indicates that necrosis can be regulated, programmed, and driven by a peptidase stress-response pathway.
_
Viral infection can induce or suppress Programmed Necrosis of host cell:
_
Necroptosis:
A regulated form of necrosis, that is, necroptosis, has recently been identified that proceeds in a programmed and controlled manner. Necroptosis refers to RIP1- and/or RIP3-dependent regulated necrosis. A better understanding of the molecular mechanisms that regulate necroptosis signal transduction may open new perspectives for targeted modulation and therapeutic exploitation of necroptosis signaling. While necroptosis has for long been viewed as an accidental mode of cell death triggered by physical or chemical damage, it has become clear over the last years that necroptosis can also represent a programmed form of cell death in mammalian cells. Key discoveries in the field of cell death research, including the identification of critical components of the necroptotic machinery, led to a revised concept of cell death signaling programs. Several regulatory check and balances are in place in order to ensure that necroptosis is tightly controlled according to environmental cues and cellular needs. This network of regulatory mechanisms includes metabolic pathways, especially those linked to mitochondrial signaling events. A better understanding of these signal transduction mechanisms will likely contribute to open new avenues to exploit our knowledge on the regulation of necroptosis signaling for therapeutic application in the treatment of human diseases.
______________
Apoptosis versus Necrosis:
Apoptosis is under genetic control, requires ATP and is regulated by a variety of cellular signaling pathways. In apoptosis, the cell shrinks and becomes denser. Karyohexis, or breakdown of the nucleus, takes place. The DNA degenerates and chromosomal DNA is cleaved into internucleosomal fragments. The apoptotic cell divides into many parts by “budding”, forming what are known as ‘apoptotic bodies’ surrounded by an intact plasma membrane, containing cell organelles and nuclear materials. These bodies are phagocytosed by neighbouring cells. Necrosis is not a programmed cell death and is considered to be uncontrolled. It can be initiated accidentally by ischaemia, trauma or ATP depletion. No apoptotic bodies are formed. The plasma membrane integrity is not maintained in necrosis; the necrotic cell explodes and releases its cellular contents, including lysosomal enzymes, into the extracellular space. This cellular leakage triggers an inflammatory response. Necrosis is also known as caspase-independent cell death – caspases are not involved in this type of cell death and also no ATP is required. However, recent research shows that even necrosis can be regulated and caspases are also involved in necrosis.
_
| Apoptosis-cell death | Death that utilizes energy | No inflammation |
| Necrosis-cell death | Energy depletion causing death | Produce inflammation |
_
_
Difference between Immunomodulatory Effects of Dying Cells: apoptosis vs. necrosis:
The effect of dying cells on immunity is an exciting area of investigation. Apoptotic cells induce anergy or an immunosuppressive phenotype, whereas necrotic cells augment inflammation, in part by binding the receptor C-type lectin domain family 9 (CLEC9A) on dendritic cells. The administration of apoptotic cells to mice before challenge with parasites greatly increased the number of blood parasites, as compared with control mice, whereas in mice given necrotic cells, there was greatly decreased parasitemia. Similarly, mice that received apoptotic cells before the induction of peritonitis had a higher death rate than control mice, whereas mice that received necrotic cells had improved survival.
________
Apoptosis versus autophagy:
_________
Inter-connection and overlap between various cell death modes:
Cell death can occur through 3 mechanisms: apoptosis, autophagy, and necrosis. Apoptosis, or programmed cell death, results in controlled cell shrinkage and nuclear fragmentation via the action of caspases, as well as an anti-inflammatory cytokine release. In contrast, necrosis signals via RIPK1 (RIP1), leading to cell swelling, lysis, and a pro-inflammatory cytokine release. Autophagy destroys the cell’s damaged proteins and organelles via an intracellular catabolic process in the lysosome. Multiple physiological processes require the removal of specific cells by a controlled cell-death program. For example, tissue remodeling activates apoptosis, whereas energy metabolism and growth regulation responses rely on autophagy. Developmental processes often activate apoptosis, while bodily injuries or infection more commonly induce necrosis. The molecular mechanisms behind these cell death pathways overlap, and can be co-activated during some cellular functions. Apoptosis and necrosis both signal through the death domain receptors FAS, TNFRSF1A (TNFR1), and TNFRSF10A (TRAIL-R), while autophagy and apoptosis share BCL2 family members as key players.
_
_
There are necrotic-like phenotypes that require gene activation and protein synthesis so they are, strictly speaking, forms of programmed cell death (Proskuryakov et al., 2003). These forms of cell death that have certain morphological features of both necrosis and apoptosis have been given the term “aponecrosis” (Formigli et al., 2000). By affecting the mitochondrial respiratory chain with antimycin A, Formigli and coworkers induced a type of cell death that shared dynamic, molecular, and morphological features with both apoptosis and necrosis. Caspase-independent mechanisms of neuronal cell death have also been identified. This specific type of programmed cell death may involve specific mitochondrial factors. In experimental models, apoptosis-inducing factor (AIF) and endonuclease G promote this type of cell death; however, Smac/DIABLO and HtrA2/Omi may also contribute (Ravagnan et al., 2002; van Loo et al., 2002b). Oppenheim and coworkers (2001) have shown that programmed cell death occurs in developing mammalian neurons, even after the genetic deletion of caspases. Other research has shown that inhibition of the caspase execution machinery may only temporarily rescue damaged neurons and that classical apoptotic features can still appear in caspase-inhibited neurons (Volbracht et al., 2001). It appears that caspase-dependent and caspase-independent mechanisms of neuronal cell death may depend on brain region, cell type, and age. Sperandio and coworkers (2000) have described a form of programmed cell death that is morphologically and biochemically distinct from apoptosis, dubbed “paraptosis.” Although this form of cell death does not respond to caspase inhibitors or BCL-XL, it is driven by an alternative form of caspase-9 activity that is Apaf-1 independent. This alternative form of programmed cell death is reported to occur during development and in transgenic models of Huntington’s disease and human amyotrophic lateral sclerosis (Dal Canto and Gurney, 1994; Turmaine et al., 2000). There are some settings where autophagy and apoptosis seem to be interconnected and the idea of “molecular switches” between the two processes has been introduced (Piacentini et al., 2003). This is similar to the concept of the necrosis-apoptosis “continuum” or “paradox” which suggests that both apoptosis and necrosis represent morphologic expressions of a shared biochemical network in which the route of cell death depends on a variety of factors such as the physiologic milieu, developmental stage, tissue type, and the nature of the cell death signal (Hirsch et al., 1997; Zeiss, 2003). However, the core apoptotic pathway can be diverted to induce a necrotic phenotype by alteration of the availability of intracellular ATP and the availability of caspases. A similar relationship may occur between apoptosis and autophagy. It has been suggested that mitochondria may be central organelles that integrate both apoptosis and autophagy (Elmore et al., 2001). Moreover, some of the same signals that are involved in apoptosis may also be involved in autophagy. For example, in both apoptosis and autophagy, there is the coordinated regulation of Akt (protein kinase B) and p70S6 kinase. Other proteins that may be part of the network connecting the two types of cell death include DAPk, Beclin 1, BNIP3, HSpin1, or protymosin-α (Klionsky and Emr, 2000). Malignant transformation is another link between autophagy and apoptosis (Gozuacik and Kimchi, 2004). The role of genetic alterations in the pathways that control cellular proliferation/apoptosis in cancer development has been well established. Similarly, a correlation between reduced autophagy and cancer has also been documented. Studies have indicated that during malignant transformation several proteins and pathways that are related to autophagy signaling are deregulated resulting in reduced autophagocytic activity. This suggests that, in some circumstances, autophagy may function as a safeguard mechanism that restricts uncontrolled cell growth. Autophagy has also been considered a protective mechanism against apoptosis. Lemasters and coworkers (1998) observed that depolarized mitochondria, a feature of apoptosis, are rapidly eliminated by autophagy in primary hepatocytes. Eliminating damaged mitochondria prevents the release of proapoptotic substances from the mitochondria, thus preventing apoptosis. Autophagy is considered the major cellular mechanism for disposing of long-lived proteins and cytoplasmic organelles; however, the concept of autophagic cell death has been a matter of debate within the scientific community. Since there is a distinct advantage of increased autophagy in various physiological and stress conditions, it has been suggested that autophagy represents an important adaptive mechanism that attempts to rescue cells from death. In other words, the presence of autophagic vesicles in dying cells may reflect an adaptive response to maintain cell survival under stress conditions rather than a reflection of “autophagic cell death.” Alternatively, are apoptosis and autophagic cell death mutually exclusive or can both apply in a situation similar to the apoptosis-necrosis continuum? It may be that the type of cell death depends on the severity of the response, the influences of cellular constituents and/or the influences of other signaling pathways. There are reports that apoptosis and autophagic programmed cell death are not mutually exclusive and the diverse morphologies are attributed, in part, to distinct biochemical and molecular events (Gozuacik and Kimchi, 2004). In certain cells and under certain conditions the morphological features of autophagy may occur prior to apoptotic cell death, representing an early phase of apoptosis. The controversy still remains, however interest in the field of autophagic cell death is constantly increasing with the emergence of new assays and markers for elucidating the molecular basis of autophagy and its possible implications in programmed cell death and malignant cell transformation.
_
The figure below shows connections between apoptosis and autophagy:
_
The figure above shows the relationship between necrosis, autophagy, and apoptosis. Metabolic and therapeutic stresses lead to acute NAD+ and ATP depletion accompanied by increased intracellular calcium and ROS. Cells that do not adapt to these changes undergo necrotic cell death. The activation of stress regulators, such as AMPK, allows cells to acutely survive these changes. AMPK-dependent phosphorylation results in the inhibition of mammalian target of rapamycin, which inhibits autophagy. AMPK-dependent phosphorylation also activates p53, which can lead to autophagy or apoptosis, through the activation of Bax and Bak, the cytoplasmic release of cytochrome c (Cyt. C), and the activation of caspases. Unlike apoptosis or necrosis, stress-induced autophagy can lead to autophagic cell death or to cell survival.
_
The figure below shows how p 53 regulates various cell death modes:
_
p53 localization is a key regulator of p53-induced apoptosis and autophagy. Nuclear p53 induces cellular autophagy, apoptosis and repair through its transcriptional activity while cytosolic p53 inhibits autophagy and induces apoptosis.
_
Cross-Talk between Cell-Death Mechanisms:
The type and intensity of noxious signals, ATP concentration, cell type, and other factors determine how cell death occurs. Acute myocardial ischemia (which precipitates rapid and profound decreases in ATP) induces necrosis, whereas chronic congestive heart failure (with more modest yet chronic decreases in ATP) induces apoptosis. The blockade of a particular pathway of cell death may not prevent the destruction of the cell but may instead recruit an alternative path: antiapoptotic caspase inhibitors cause hyperacute necrosis of hepatocytes and kidney tubular cells induced by TNF. The overexpression of antiapoptotic proteins may allow injured cells to survive, and autophagy may assist by providing critical metabolites. However, if death stimuli persist, antiapoptotic pathways and autophagy are unlikely to continue, and necrosis ensues. Furthermore, cells may be more susceptible to apoptosis if autophagy is inhibited. Nuclear factor B, ATG5, ATP, and PARP probably function as molecular switches that determine whether a cell undergoes apoptosis, necrosis, or autophagy. Protein p53 also modulates autophagy and other responses to cell stress. Recent work indicates that basal p53 activity suppresses autophagy, whereas the activation of p53 by certain stimuli induces autophagy and the activation of p53 by different stimuli results in the engagement of apoptosis, mediated by PUMA and NOXA.
______________
Apoptosis in the pathogenesis and treatment of disease:
In multicellular organisms, homeostasis is maintained through a balance between cell proliferation and cell death. Although much is known about the control of cell proliferation, less is known about the control of cell death. Physiologic cell death occurs primarily through an evolutionarily conserved form of cell suicide termed apoptosis. The decision of a cell to undergo apoptosis can be influenced by a wide variety of regulatory stimuli. Recent evidence suggests that alterations in cell survival contribute to the pathogenesis of a number of human diseases, including cancer, viral infections, autoimmune diseases, neurodegenerative disorders, and AIDS (acquired immunodeficiency syndrome). Treatments designed to specifically alter the apoptotic threshold may have the potential to change the natural progression of some of these diseases.
__
_
Disease as a consequence of dysregulated apoptosis:
In the adult human body several hundred thousand cells are produced every second by mitosis and a similar number die by apoptosis for the maintenance of homeostasis and for specific tasks such as the regulation of immune cell selection and activity [Fadeel, 1999b]. Dysregulation of apoptotic signaling can play a primary or secondary role in various diseases with insufficient apoptosis leading to e.g. cancer (cell accumulation, resistance to therapy, defective tumor surveillance by the immune system), autoimmunity (failure to eliminate autoreactive lymphocytes), persistent infections (failure to eradicate infected cells), whereas excessive apoptosis contributes to e.g. neurodegeneration (Alzheimers’ disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis), autoimmunity (uncontrolled apoptosis induction in specific organs), AIDS (depletion of T lymphocytes), and ischaemia (stroke, myocardial infarction) [Reed, 2002]. Malfunction of the death machinery results from the mutation of genes that code for factors directly or indirectly involved in the initiation, mediation, or execution of apoptosis, and several mutations in apoptosis genes have been identified as a causing or contributing factor in human diseases [Mullauer, 2001].
_
_
Pharmacological manipulation of cell death: clinical applications:
Apoptosis, the major form of cellular suicide, is central to various physiological processes and the maintenance of homeostasis in multicellular organisms. Presumably, even more important is a causative or contributing role of apoptosis to various human diseases. These include situations with unwanted cell accumulation (cancer) and failure to eradicate aberrant cells (autoimmune diseases) or disorders with an inappropriate loss of cells (heart failure, stroke, AIDS, neurodegenerative diseases, and liver injury). The past decade has witnessed a tremendous progress in the knowledge of the molecular mechanisms that regulate apoptosis and the mediators that either prevent or trigger cell death. Consequently, apoptosis regulators have emerged as key targets for the design of therapeutic strategies aimed at modulating cellular life-and-death decisions. Numerous novel approaches are currently being followed employing gene therapy and antisense strategies, recombinant biologics, or classical organic and combinatorial chemistry to target specific apoptotic regulators. Convincing proof-of-principle evidence obtained in several animal models confirms the validity of strategies targeting apoptosis and revealed an enormous potential for therapeutic intervention in a variety of illnesses. Although numerous apoptotic drugs are currently being developed, several therapeutics have progressed to clinical testing or are already approved and marketed.
_
The prevention of cell death is more technically challenging than the induction of cell death. Different forms of cell death can occur simultaneously because of the coordinated release of multiple death-inducing stimuli. In ischemia–reperfusion injury, reactive oxygen species, calcium-ion overload, and destructive protease activation may induce cell death independently. Thus, it may be necessary to target multiple death pathways or identify and block common funnel points of cell-death signaling to enable survival. Despite these challenges, meaningful clinical advances are emerging. The inhibition of calpains and cathepsins, potent proteases that are responsible for necrosis, mitigates disease in animal models. Nicorandil, a cardioprotective drug that acts on mitochondrial ATP-sensitive potassium channels, lowers levels of serum troponin T in patients undergoing cardiac bypass surgery. Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis are current investigational targets of antiapoptotic and autophagy-enhancing drugs. The therapeutic induction of metabolic arrest may also prove valuable. Although the means by which cells enter a “hibernating” state remain unknown, important mediators of this low-energy state are now better understood; 5′-AMP is one such mediator that allows nonhibernating animals to safely enter a hypothermic condition.
_
Signal transduction pathways controlling cell death and the molecular core machinery responsible for cellular self-destruction have been elucidated with unprecedented celerity during the last decade, leading to the design of novel strategies for blocking pathological cell loss or for killing unwanted cells. Thus, an increasing number of compounds targeting a diverse range of apoptosis-related molecules are being explored at the preclinical and clinical levels. Beyond the agents that are already FDA approved, a range of molecules targeting apoptosis-regulatory transcription factors, regulators of mitochondrial membrane permeabilization, and inhibitors or activators of cell death–related proteases are under close scrutiny for drug development. The Review by Amaravadi and Thompson considers this problem from the perspective of the signaling pathways, in particular the kinases, that control cell survival. This includes not only regulation of apoptosis, per se, but also the signals that control metabolic demand and, in turn, control life and death. Other survival signals, notably those controlled by NF-κB, are reviewed by Luo et al. and considered with respect to cancer therapy. In addition, cellular survival can be preserved by the functions of members of the heat shock protein family, an active area of research that is reviewed by Beere. In all of these cases, important targets have been identified, and agents to modulate them are in different stages of development and testing for use in human disease. The mitochondria sit at the center of a major pathway for cell death controlled by multiple inputs, and this can result in apoptosis or other forms of cell death. Bouchier-Hayes and colleagues consider the mitochondria as a target for pharmacological intervention, especially with respect to control of the Bcl-2 family proteins. Strategies for elaborating specific apoptosis modulators that mimic Bcl-2 family proteins or act on such proteins are discussed by Letai. Another organelle that appears to control cell life and death is the ER, which coordinates complex signaling events around calcium storage and the unfolded protein response as well as other forms of ER stress. Xu et al. consider the signal transduction pathways that center on the ER with respect to cell death and its control. The regulation of caspase activation is, of course, highly relevant to the manipulation of apoptosis, and this subject is reviewed by Lavrik and colleagues. Similarly, the IAPs have for some time been thought of predominantly as caspase inhibitors. Recent discoveries have shown that these also play roles distinct from the control of caspases, acting as ubiquitin ligases for other proteins, passenger molecules, and signal transduction proteins. Wright and Duckett consider IAPs as pharmacological targets and how these affect various outcomes in the cell and organism. Finally, the process of autophagy, distinct from that of apoptosis, accompanies cell death in many cases, and it is possible that this “self-eating” may result in death, or, alternatively, may represent a mechanism for cell survival. Levine and Yuan discuss this process and consider pharmacological approaches to its manipulation.
_
Many drug targets elucidated in studies of cell death have led to approved pharmacological agents that are currently in use, while many more are in clinical trial. These are promising, of course, but we are only beginning to understand these processes and their potential for clinical benefit. The application of our understanding is now in sight, but the potential extends beyond our vision.
_
Inhibition of cell death at the preclinical and clinical levels:
The death of postmitotic cells in the CNS and myocardium, be it the result of an acute or a chronic degenerative process, is a pathogenic event. Similarly, septic shock, ischemia, and intoxication can cause massive apoptosis in multiple organs. Infections can cause an elevated apoptotic turnover of specific cell types, for instance, lymphocytes in AIDS or gastric mucosa cells in Helicobacter pylori infection. These diseases are therefore candidates for therapeutic cell death suppression. A cornucopia of putative drugs and targets for cytoprotection are being explored at the preclinical and clinical levels. MOMP inhibitors have attracted interest, with encouraging results in animal models. Similarly, a variety of protease inhibitors are being designed to prevent cell death. Among this class of agents, caspase inhibitors have been extensively explored. This exploration has been driven by the often-erroneous belief that caspases would generally dictate the “point of no return” of cell death. Although some preclinical results are promising, many of the specific caspase inhibitors (such as zVAD-fmk and zDEVD-fmk) can actually inhibit calpains and cathepsins. Calpains and cathepsin can also be involved in pathogenic cell death, as indicated by pharmacological studies in rodents and genetic validation in mice. Genetic methods to inhibit caspases, for instance by overexpressing the baculovirus protein p35, have not validated the role of caspases in cell death, for instance in kainate-induced neurotoxicity. Moreover, a proof-of-principle experiment illustrating that the conditional or tissue-specific knockout of 1 or several caspase genes can confer true cytoprotection is still elusive. In this sense, it is not surprising that clinical studies of caspase inhibitors assess the treatment of inflammatory diseases such as hepatitis C, in which the inhibition of inflammatory caspases (caspase-1 and perhaps caspase-4 and -5) may be the therapeutic goal.
Therapeutic targets for cell death inhibition at the preclinical stage are depicted in the table below:
_
Pharmacological inhibitors of apoptosis that are FDA approved or in clinical development are depicted in the table below:
_
Induction of cell death:
Although there are many ways to stimulate apoptotic responses by compounds inducing organelle-specific damage, thus far no agents specifically acting on cytoplasmic organelles has made it into clinical trials for the treatment of neoplasia. However, ligands of so-called death receptors (such as CD95/Fas, TNFR, and the 2 receptors of TRAIL) are being evaluated as potential anticancer agents that could bypass the resistance against conventional chemotherapy.
Pharmacological inducers of apoptosis that are FDA approved or in clinical development are depicted in the table below:
_
Apoptosis can be manipulated either by mutations or external agents as seen in the table below:
_________
Cell death and infection:
One of the most intrinsic immune defense mechanisms of multicellular organisms is to sacrifice infected cells for the benefit of the remaining cells. The major cell death modalities, including apoptotic cell death, necrotic cell death, and pyroptotic cell death, are critical defense mechanisms against microbial infection (Bergsbaken et al., 2009; Lamkanfi and Dixit, 2010; Zitvogel et al., 2010). For instance, in response to bacterial infection, programmed cell death, such as apoptosis, is induced as a host innate immune response. This cell death plays two defensive roles in infection. One is to eliminate pathogens at the early stage of infection without emitting alarm signals. The other role is to induce dendritic cells (DCs) to engulf apoptotic bodies containing infected microbes, which allows extracellular antigens to access MHC I molecules and subsequently induce a protective immune response (Elliott and Ravichandran, 2010). Cell death can also benefit pathogens: one prominent strategy of many bacterial pathogens is to induce the demise of infected host cells, which allows the bacteria to efficiently exit the host cell, spread to neighboring cells, evade immune cells, and/or gain nutrients. Meanwhile, many bacterial pathogens, especially those capable of invading and multiplying within host cells, use multiple mechanisms to manipulate host cell death and survival pathways in order to maintain their replicative compartment (Behar et al., 2010; Kim et al., 2010; Lamkanfi and Dixit, 2010).
_
Bacterial infection elicits a diverse array of host protective and stress responses, including the cell death and proliferative responses, inflammatory response, and innate immune response. The host cell death response critically influences the fate of bacterial infection, the integrity of the host innate defense barrier, innate and acquired immunity, and disease outcome. Bacteria-triggered cell death results in various modes of cell death that greatly vary with the host cell type, stage of infection, infectious dosage, physiological condition of the cell, bacterial factors, and experimental setting. Host cell death is an intrinsic immune defense mechanism in response to microbial infection. However, bacterial pathogens use many strategies to manipulate the host cell death and survival pathways to enhance their replication and survival. This manipulation is quite intricate, with pathogens often suppressing cell death to allow replication and then promoting it for dissemination. Frequently, these effects are exerted through modulation of the mitochondrial pro-death, NF-κB–dependent pro-survival, and inflammasome-dependent host cell death pathways during infection. Understanding the molecular details by which bacterial pathogens manipulate cell death pathways will provide insight into new therapeutic approaches to control infection. In addition, our understanding of the dynamic interplay between pathogens and host innate defense responses in vitro and in vivo are occasionally contradictory. Nevertheless, as has been described here and in other literature (Lamkanfi and Dixit, 2010; Rudel et al., 2010), future studies on the cell death response during host–pathogen interactions will provide further insight into the mechanisms by which cells undergo death as an innate defense against microbial infection. Furthermore, a better understanding of the molecular details by which bacterial pathogens manipulate cell death pathways will undoubtedly provide insight into new therapeutic approaches that can be used to control bacterial infection and inflammatory disease progression.
_
Intracellular bacteria can trigger NF-kappaB-mediated gene activation through interaction with another CARD-containing protein, NOD1 (also called CARD4), which appears to be required for the intracellular response (with NF-kappaB activation) to bacterial lipopolysaccharides or Shigella infection. Recently, mutations in a related molecule, NOD2, were implicated in susceptibility to Crohn’s disease, an inflammatory bowel disease. Most strikingly, the domain structure of the NOD proteins, which in addition to a CARD contain a nucleotide binding domain and a leucine rich repeat domain, is homologous to a family of proteins in plants that are involved in host defense. The plant hypersensitivity response involves the death of infected cells in response to infection, which has the effect of blocking systemic dissemination of the pathogen. A caspase related protein, metacaspase, has also been identified in plants. Another scenario linking infection with the evolution of apoptosis, is that infectious organisms will develop mechanisms to disable the apoptotic machinery of their host cell as a means of countering this defensive response. This phenomenon has been well described for infections with viruses, bacteria, fungi or protozoa. While in many cases the mechanism for resistance to apoptosis is unknown, in others there appear to be a variety of ways in which the parasite ensures cellular survival. These include the production of molecules that resemble and mimic Bcl-2, the expression of inhibitors of death receptor signaling, the generation of IAPs and other caspase inhibitors and interference with NF-kappaB pathways.
_
Manipulation of host cell death pathways during microbial infections:
Viral and microbial infections often elicit programmed cell death as part of the host defense system or as a component of the survival strategy of the pathogen. It is thus not surprising that pathogens have evolved an array of toxins and virulence factors to modulate host cell death pathways. Apoptosis, necrosis, and pyroptosis represent the three major programmed cell death modes during infection, and the choice of death mode depends on a variety of factors, including the nature of the pathogen, pathogen load, and the site of infection. Indeed, various host cell types are likely to react differently to an infectious agent. For example, Shigella flexneri inhibits apoptosis in epithelial cells (Clark and Maurelli, 2007) but triggers apoptotic and pyroptotic cell death of infected macrophages (Hilbi et al., 1998; Suzuki et al., 2005). A major difference in the biological outcome of inducing apoptosis, necrosis, or pyroptosis is the ensuing inflammatory response. Apoptosis is generally immunologically silent (reviewed in Savill et al., 2002), while necrosis and pyroptosis are accompanied by secretion of proinflammatory cytokines and the extracellular release of the cytosolic content (Berghe et al., 2010; Lamkanfi and Dixit, 2009a).
_
The figure below shows how caspase-1 activation in inflammasome induces pyroptosis:
Do mitochondria trigger the intracellular response to infection?
There is another consequence of the scenario linking infection to apoptosis that leads to some intriguing questions. If the process of active cell death in response to infection is ancestral to the emergence of the eukaryotes, then the invasion by proto-mitochondria (as well, perhaps, as proto-chloroplasts and apicoplasts) into primitive eukaryotes should be capable of triggering the death of the cell. Clearly, at some point, this did not happen and symbioses arose. Did mitochondria contribute the Bcl-2 family proteins to the apoptotic machinery of the eukaryotic cell as a mechanism for evasion of the infection-induced death response? Did the cell incorporate the regulation of infection signals from mitochondria into the regulated cell death apparatus of the cell? And did, therefore, infection (and the monitoring of host relatedness that is implied by host specificity) drive the evolution of apoptosis in unicellular organisms, providing the route to social control of cell life and death that permitted these organisms to eventually become multicellular? We are searching answers.
_
Viral infection:
Viral induction of apoptosis occurs when one or several cells of a living organism are infected with a virus leading to cell death. Viruses can trigger apoptosis of infected cells via a range of mechanisms including:
1. Receptor binding.
2. Activation of protein kinase R (PKR).
3. Interaction with p53.
4.Expression of viral proteins coupled to MHC proteins on the surface of the infected cell, allowing recognition by cells of the immune system (such as Natural Killer and cytotoxic T cells) that then induce the infected cell to undergo apoptosis.
_
Canine distemper virus (CDV) is known to cause apoptosis in central nervous system and lymphoid tissue of infected dogs in vivo and in vitro. Apoptosis caused by CDV is typically induced via the extrinsic pathway which activates caspases that disrupt cellular function and eventually leads to the cells death. In normal cells CDV activates caspase-8 first which works as the initiator protein followed by the executioner protein caspase-3. However apoptosis induced by CDV in HeLa cells does not involve the initiator protein caspase-8. HeLa cell apoptosis caused by CDV follows a different mechanism than in vero cell lines. This change in the caspase cascade suggests CDV induces apoptosis via the intrinsic pathway, excluding the need for the initiator caspase-8. The executioner protein is instead activated by the internal stimuli caused by viral infection not a caspase cascade. The Oropouche virus (OROV) is found in the family Bunyaviridae. The study of apoptosis brought on by Bunyaviridae was initiated in 1996 when it was observed that apoptosis was induced by the La Crosse virus into the kidney cells of baby hamsters and into the brains of baby mice. Many viruses encode proteins that can inhibit apoptosis. Several viruses encode viral homologs of Bcl-2. These homologs can inhibit proapoptotic proteins such as BAX and BAK, which are essential for the activation of apoptosis. Examples of viral Bcl-2 proteins include the Epstein-Barr virus BHRF1 protein and the adenovirus E1B 19K protein. Some viruses express caspase inhibitors that inhibit caspase activity and an example is the CrmA protein of cowpox viruses. Whilst a number of viruses can block the effects of TNF and Fas. For example the M-T2 protein of myxoma viruses can bind TNF preventing it from binding the TNF receptor and inducing a response. Furthermore, many viruses express p53 inhibitors that can bind p53 and inhibit its transcriptional transactivation activity. Consequently p53 cannot induce apoptosis since it cannot induce the expression of proapoptotic proteins. The adenovirus E1B-55K protein and the hepatitis B virus HBx protein are examples of viral proteins that can perform such a function. p53 signals growth arrest of the cell at a checkpoint to allow for DNA damage repair or can cause the cell to undergo apoptosis if the damage cannot be repaired. This system can also be inactivated by a number of mechanisms including somatic genetic/epigenetic alterations and expression of oncogenic viral proteins such as the HPV, leading to tumorigenesis. Viruses can remain intact from apoptosis particularly in the latter stages of infection. They can be exported in the apoptotic bodies that pinch off from the surface of the dying cell and the fact that they are engulfed by phagocytes prevents the initiation of a host response. This favours the spread of the virus.
_
Programmed cell death activates latent herpesviruses:
Researchers have found that apoptosis, a natural process of programmed cell death, can reactivate latent herpesviruses in the dying cell. The results of the research, which could have broad clinical significance since many cancer chemotherapies cause apoptosis, was published in the Journal of Virology. Human herpesviruses (HHV) are linked to a range of childhood and adult diseases, including chickenpox, mononucleosis, cold sores, and genital sores, and are of a particular concern for patients who are immunosuppressed due cancer or AIDS. Some HHV types are so common they are nearly universal in humans. A key feature of these viruses is their ability to remain latent for long periods of time, and then reactivate after the latent phase. Previously, reactivation was thought to be primarily due to waning immunity, immunosuppression, or exposure to certain inducing agents. This study began when principal investigator Steven Zeichner of Children’s National Medical Center and George Washington University in Washington, DC, followed up earlier findings that high concentrations of the antibiotic doxycycline can induce apoptosis, and can also activate replication by the Kaposi’s Sarcoma-associated Herpesvirus (KSHV), and a study by his former mentor, Bernard Roizman of the University of Chicago, which showed that apoptosis also triggers replication of herpes simplex virus-1, which causes cold sores in the mouth. They decided to test… several additional human herpesviruses that cause notable diseases and which have good latent infection cell line models, including human herpesviruses (HHV)-6A, 6B, and 7, and Epstein-Bar virus (EBV). That all of these herpesviruses were activated by apoptosis suggested that this mechanism might apply to all herpesviruses. The clinical implications could be staggering. Some important cytotoxic cancer chemotherapeutic drugs, including doxorubicin, vincristine, and prednisone act in part by inducing apoptosis, according to the study. Additionally, treatment with glucocorticoids has been known to worsen Kaposi’s Sarcoma. The investigators also note that herpesvirus activation has been associated with poor outcomes following bone marrow transplantation. Activation of herpesviruses in these states and disorders has previously been variably attributed to general immune suppression, suppression of specific arms of the immune system, and increased concentrations of inflammatory and activating cytokines. If this activation occurs in potentially damaging ways, then perhaps patients at risk for herpesvirus activation should be treated with antiviral medications in addition to antineoplastic cytotoxic chemotherapy. Almost all humans are infected with HHV-6, and many are infected with the other aforementioned herpesviruses, as well as cytomegalovirus, oral and genital herpes, and Varicella zoster, the virus that causes chicken pox and shingles.
_
Reovirus activates a Caspase-Independent Cell Death Pathway:
Virus-induced apoptosis is thought to be the primary mechanism of cell death following reovirus infection. Induction of cell death following reovirus infection is initiated by the incoming viral capsid proteins during cell entry and occurs via NF-κB-dependent activation of classical apoptotic pathways. Prototype reovirus strain T3D displays a higher cell-killing potential than strain T1L. To investigate how signaling pathways initiated by T3D and T1L differ, researchers methodically analyzed cell death pathways activated by these two viruses in L929 cells. They found that T3D activates NF-κB, initiator caspases, and effector caspases to a significantly greater extent than T1L. Surprisingly, blockade of NF-κB or caspases did not affect T3D-induced cell death. Cell death following T3D infection resulted in a reduction in cellular ATP levels and was sensitive to inhibition of the kinase activity of receptor interacting protein 1 (RIP1). Furthermore, membranes of T3D-infected cells were compromised. Based on the dispensability of caspases, a requirement for RIP1 kinase function, and the physiological status of infected cells, they conclude that reovirus can also induce an alternate, necrotic form of cell death described as necroptosis. They also found that induction of necroptosis requires synthesis of viral RNA or proteins, a step distinct from that necessary for the induction of apoptosis. Thus, our studies reveal that two different events in the reovirus replication cycle can injure host cells by distinct mechanisms.
_
Viral Bcl-2 homologs and their role in virus replication and associated diseases:
Cellular Bcl-2 family proteins regulate a critical step in the mammalian programmed cell death pathway by modulating mitochondrial permeability and function. Bcl-2 family proteins are also encoded by several large DNA viruses, including all known gamma herpesviruses, adenoviruses, and several other unrelated viruses. Viral Bcl-2 proteins can prevent cell death but often escape cellular regulatory mechanisms that govern their cellular counterparts. By evading the ‘‘altruistic’’ suicide of infected cells, viruses can ensure replication and propagation in the infected host, but sometimes in surprising ways. Many human cancers and other disorders are associated with viruses that encode Bcl-2 homologs.
_
Viral Bcl-2 and associated diseases:
_
The figure above shows the role of viral Bcl-2 in the virus life cycle. E1B 19K promotes acute replication by inhibiting apoptosis and preventing premature death of the infected cell. gHV68 Bcl-2 is suggested to promote reactivation from latency and persistent replication but has not been shown to play a role in acute replication or the establishment of latency. Further research is needed to elucidate the role of persistent replication in the gamma herpesvirus life cycle and associated disease states. KSBcl-2 and BHRF1 are speculated to participate in the tumorigenesis associated with their respective viruses by analogy to the function of Bcl-2 in follicular lymphomas and its conservation in clinical isolates, but because of the lack of good animal models this hypothesis has yet to be tested. Although E1B 19K can cooperate with adenovirus E1A in cell transformation, adenovirus infections are not associated with human cancers.
_
The role of host cell death in Salmonella infections:
Salmonella enterica is an important enteric pathogen of humans and a variety of domestic and wild animals. Infection is initiated in the intestinal tract, and severe disease produces widespread destruction of the intestinal mucosa. Salmonella strains can also disseminate from the intestine and produce serious, sometimes fatal infections with considerable cytopathology in a number of systemic organs. A combination of bacterial genetic and cell biology studies have shown that Salmonella uses specific virulence mechanisms to induce host cell death during infection. Salmonella produces one set of virulence proteins to promote invasion of the intestine and a different set to mediate systemic disease. Significantly, each set of virulence factors mediates a distinct mechanism of host cell death. The Salmonella pathogenicity island-1 (SPI-1) locus encodes a type III protein secretion system (TTSS) that delivers effector proteins required for intestinal invasion and the production of enteritis. The SPI-1 effector SipB activates caspase-1 in macrophages, releasing IL-1beta and IL-18 and inducing rapid cell death by a mechanism that has features of both apoptosis and necrosis. Caspase-1 is required for Salmonella to infect Peyer’s patches and disseminate to systemic tissues in mice. Progressive Salmonella infection in mice requires the SPI-2 TTSS and associated effector proteins as well as the SpvB cytotoxin. Apoptosis of macrophages in the liver is found during systemic infection. In cell culture, Salmonella strains induce delayed apoptosis dependent on SPI-2 function in macrophages from a variety of sources. This delayed apoptosis also requires activation of TLR4 on macrophages by the bacterial LPS. Downstream activation of kinase pathways leads to balanced pro- and antiapoptotic regulatory factors in the cell. NF-kappaB and p38 mitogen-activated protein kinase (MAPK) are particularly important for the induction of antiapoptotic factors, whereas the kinase PKR is required for bacterial-induced apoptosis. The Salmonella SPI-2 TTSS is essential for altering the balance in favor of apoptosis during intracellular infection, but the effectors involved remain poorly characterized. The SpvB cytotoxin has been shown to play a role in apoptosis in human macrophages by depolymerizing the actin cytoskeleton. In the intestine, the Salmonella SPI-1 TTSS and SipB mediate macrophage death by caspase-1 activation, which also releases IL-1beta and IL-18, promoting inflammation and subsequent phagocytosis by incoming macrophages and leading to dissemination to systemic tissues. Intracellular secretion of virulence effector proteins by the SPI-2 TTSS facilitates growth of Salmonella in these macrophages and the delayed onset of apoptosis in extraintestinal tissues. These infected, apoptotic cells are targeted for engulfment by incoming macrophages, thus perpetuating the cycle of cell-to-cell spread that is the hallmark of systemic Salmonella infection.
_
Sepsis:
Sepsis is perhaps the most remarkable clinical setting in which apoptosis occurs. Massive apoptosis of immune effector cells and gastrointestinal epithelial cells develops in patients with sepsis. The profound loss of immune effector cells in sepsis inhibits the ability of the immune system to eradicate the primary infection and renders the patient susceptible to nosocomial infections. Numerous studies in animals have highlighted the role of apoptosis in aggravating sepsis. They have shown that the prevention of sepsis-induced apoptosis improves survival.
__________
Cell death, HIV and AIDS:
HIV progression:
The progression of the human immunodeficiency virus infection into AIDS is primarily due to the depletion of CD4+ T-helper lymphocytes in a manner that is too rapid for the body’s bone marrow to replenish the cells, leading to a compromised immune system. One of the mechanisms by which T-helper cells are depleted is apoptosis, which results from a series of biochemical pathways:
1. HIV enzymes deactivate anti-apoptotic Bcl-2. This does not directly cause cell death, but primes the cell for apoptosis should the appropriate signal be received. In parallel, these enzymes activate proapoptotic procaspase-8, which does directly activate the mitochondrial events of apoptosis.
2. HIV may increase the level of cellular proteins which prompt Fas-mediated apoptosis.
3. HIV proteins decrease the amount of CD4 glycoprotein marker present on the cell membrane.
4. Released viral particles and proteins present in extracellular fluid are able to induce apoptosis in nearby “bystander” T helper cells.
5. HIV decreases the production of molecules involved in marking the cell for apoptosis, giving the virus time to replicate and continue releasing apoptotic agents and virions into the surrounding tissue.
6. The infected CD4+ cell may also receive the death signal from a cytotoxic T cell.
Cells may also die as a direct consequence of viral infection. HIV-1 expression induces tubular cell G2/M arrest and apoptosis. The progression from HIV to AIDS is not immediate or even necessarily rapid; HIV’s cytotoxic activity towards CD4+ lymphocytes is classified as AIDS once a given patient’s CD4+ cell count falls below 200.
_
Mitochondrion-mediated apoptosis in HIV-1 infection:
Acquired immunodeficiency syndrome (AIDS), which is caused by human immunodeficiency virus (HIV-1), involves the apoptotic destruction of lymphocytes and, in the context of AIDS-associated pathologies, of neurons and myocytes. Several proteins encoded by HIV-1 trigger apoptosis by inducing permeabilization of the mitochondrial membrane. Several nucleoside analogs used clinically in the treatment of HIV-1 inhibit the replication of mitochondrial DNA (mtDNA) and/or increase the frequency of mtDNA mutations. These cause severe mitochondriopathy and might contribute to lipodystrophy, the complication associated with HIV-1 therapy. HIV-1 protease inhibitors can inhibit apoptosis at the mitochondrial level, which might help to alleviate lymphopenia. Thus, it appears that the pathogenesis of AIDS, and the pharmacological interventions and complications associated with this disease, affect the mitochondrial regulation of apoptosis, which, therefore, largely determines the outcome of HIV-1 infection.
_
Scientists discover how the body’s own immune response to HIV causes CD4 T cell death:
Two separate journal articles, published simultaneously in Nature and Science, detail the research from the laboratory of Warner C. Greene, MD, PhD, who directs virology and immunology research at Gladstone, an independent biomedical-research nonprofit. His lab’s Science paper reveals how, during an HIV infection, a protein known as IFI16 senses fragments of HIV DNA in abortively infected immune cells. This triggers the activation of the human enzyme caspase-1 and leads to pyroptosis, a fiery and highly inflammatory form of cell death. As revealed in the Nature paper, this repetitive cycle of abortive infection, cell death, inflammation and recruitment of additional CD4 T cells to the infection “hot zone” ultimately destroys the immune system and causes AIDS. Researchers have made two important discoveries, first by showing how the body’s own immune response to HIV causes CD4 T cell death via a pathway triggering inflammation, and secondly by identifying the host DNA sensor that detects the viral DNA and triggers this death response. Once the scientists discovered this key process, as described in Nature, they began to investigate how the body senses the fragments of HIV’s DNA in the first place, before alerting the enzyme caspase-1 to launch an immune response in the CD4 T cells. To identify the so-called DNA sensor, the scientists found a way to genetically manipulate CD4 T cells in spleen and tonsil tissue. In doing so, they discovered that reducing the activity of a protein known as IFI16 inhibited pyroptosis. This identified IFI16 as the DNA sensor, which then sends signals to caspase-1 and triggers pyroptosis. The Phase 2 trial—which will test an existing anti-inflammatory’s ability to block inflammation and pyroptosis in HIV-infected people—promises to validate a variety of expected advantages to this therapy. For example, by targeting the human body, or host, instead of the virus, the drug is likely to avoid the rapid emergence of drug resistance that often plagues the use of ARVs. The anti-inflammatory may also provide a bridge therapy for the millions without access to ARVs, while also reducing persistent inflammation in HIV-infected people already on ARVs. Many suspect this inflammation drives the early onset of aging-related conditions such as dementia and cardiovascular disease. By reducing inflammation, the drug might also prevent expansion of a reservoir of latent virus that hides in the body where it thwarts a cure for HIV/AIDS.
____________
Cell death and Cancer:
Cancer is an example where the normal mechanisms of cell cycle regulation are dysfunctional, with either an overproliferation of cells and/or decreased removal of cells (King and Cidlowski, 1998). In fact, suppression of apoptosis during carcinogenesis is thought to play a central role in the development and progression of some cancers (Kerr et al., 1994). There are a variety of molecular mechanisms that tumor cells use to suppress apoptosis. Tumor cells can acquire resistance to apoptosis by the expression of anti-apoptotic proteins such as Bcl-2 or by the down-regulation or mutation of pro-apoptotic proteins such as Bax. The expression of both Bcl-2 and Bax is regulated by the p53 tumor suppressor gene (Miyashita, 1994). Certain forms of human B cell lymphoma have overexpression of Bcl-2, and this is one of the first and strongest lines of evidence that failure of cell death contributes to cancer (Vaux et al., 1988). Another method of apoptosis suppression in cancer involves evasion of immune surveillance (Smyth et al., 2001). Certain immune cells (T cells and natural killer cells) normally destroy tumor cells via the perforin/granzyme B pathway or the death-receptor pathway. In order to evade immune destruction, some tumor cells will diminish the response of the death receptor pathway to FasL produced by T cells. This has been shown to occur in a variety of ways including down-regulation of the Fas receptor on tumor cells. Other mechanisms include expression of nonfunctioning Fas receptor, secretion of high levels of a soluble form of the Fas receptor that will sequester the Fas ligand or expression of Fas ligand on the surface of tumor cells (Cheng et al., 1994; Cefai et al., 2001; Elnemr et al., 2001). In fact, some tumor cells are capable of a Fas ligand-mediated “counterattack” that results in apoptotic depletion of activated tumor infiltrating lymphocytes (Koyama et al., 2001). Other cell signaling pathways can also be involved in tumor development. For example, upregulation of the phosphatidylinositol 3-kinase/AKT pathway in tumor cells renders them independent of survival signals. In addition to regulation of apoptosis, this pathway regulates other cellular processes, such as proliferation, growth, and cytoskeletal rearrangement (Vivanco and Sawyers, 2002).
_
The figure below shows tumorigenic stress and the response of organism namely cell cycle arrest and cell death to prevent cancer.
_
The essential role of evasion from cell death in cancer:
The link between evasion of apoptosis and the development of cellular hyperplasia and ultimately cancer is implicitly clear if one considers how many cells are produced each day and, hence, how many cells must die to make room for the new ones (reviewed in Raff, 1996). Furthermore, cells are frequently experiencing noxious stimuli that can cause lesions in their DNA and faults in DNA replication can occur during cellular proliferation. Such DNA damage needs to be repaired efficiently or cells with irreparable damage must be killed to prevent subsequent division of aberrant cells that may fuel tumorigenesis (reviewed in Weinberg, 2007). The detection of genetic lesions in human cancers that activate prosurvival genes or disable proapoptotic genes have provided the first evidence that defects in programmed cell death can cause cancer (Tagawa et al., 2005; Tsujimoto et al., 1984; Vaux, Cory, and Adams, 1988) and this concept was proven by studies with genetically modified mice (Egle et al., 2004b; Strasser et al., 1990a). It is therefore now widely accepted that evasion of apoptosis is a requirement for both neoplastic transformation and sustained growth of cancer cells (reviewed in Cory and Adams, 2002; Hanahan and Weinberg, 2000; Weinberg, 2007). Importantly, apoptosis is also a major contributor to anticancer therapy-induced killing of tumor cells (reviewed in Cory and Adams, 2002; Cragg et al., 2009). Consequently, a detailed understanding of apoptotic cell death will help to better comprehend the complexities of tumorigenesis and should assist with the development of improved targeted therapies for cancer based on the direct activation of the apoptotic machinery (reviewed in Lessene, Czabotar, and Colman, 2008).
_
The figure below shows generation of cancer from dysregulation and dysfunction of various cell death modes:
_
_
Tumour resistance to apoptosis:
Cancer cells that avoid apoptosis continue to proliferate uncontrollably, which results in an increased tumor mass. Resisting apoptosis is a key process in cancer development and progression. Cells resist apoptosis if they have an overexpression of anti-apoptotic proteins, such as Bcl-2, Bcl-XL, or IAP proteins. When these anti-apoptotic proteins are overexpressed, the cell’s ability to resist apoptosis is increased, and the cells will continue to proliferate, regardless of pro-apoptotic cues. Virtually all cancer cells are prone to resisting apoptosis. One of the hallmarks of human cancers is the intrinsic or acquired resistance to apoptosis. Evasion of apoptosis may contribute to carcinogenesis, tumor progression and also to treatment resistance, since most current anticancer therapies including chemotherapy, radio- and immunotherapy primarily act by activating cell death pathways including apoptosis in cancer cells. Hence, a better understanding of the molecular mechanisms underlying tumor resistance to apoptotic cell death is expected to provide the basis for a rational approach to develop molecular targeted therapies.
_
Inhibition of apoptosis in human laryngeal cancer cells by E6 and E7 oncoproteins of human papillomavirus 16:
The carcinogenesis of human papillomaviruses type 16 (HPV-16) is mainly due to its two oncoproteins, E6 and E7. Their carcinogenic features in term of their relationship with Bcl-2 family are still unclear. Researchers thus aimed to analyze the expression of Bcl-2 family members, Bcl-2, Bax, and Bak in laryngeal cancer cells transfected with the E6 or E7 and to determine the sensitivity of these cells to apoptotic stimuli. They employed two human laryngeal cancer cell lines, UMSCC12 and UMSCC11A in this study. These two cell lines were stably transfected with HPV16 E6, E7 or empty vector, pcDNA3.1. they found that E6 and E7 inhibited apoptosis induced by TNF-α/CHX in both UMSCC11A and UMSCC12 cells, enhanced the stability of Bcl-2 protein and increased the degradation of Bak protein. Furthermore, it was found that HPV-16 E7 statistically enhanced the expression of Bcl-2 in laryngeal cancer. The alteration of Bak by E6 and E7 was not through the influence on the Bak promoter, as the luciferase assay showed that neither E6 nor E7 changed the Bak promoter activity. Researchers conclude that the evasion of apoptosis mediated by HPV-16 E6 and E7 is associated with increased Bcl-2 and decreased Bak in laryngeal carcinogenesis and that the decreased level of Bak by E6 and E7 is not caused by the regulation of the Bak promoter but by reducing its protein stability.
_
More than 50% of neoplasms have defects in the apoptotic machinery. Among the best characterized of these abnormalities are the increased expression of prosurvival BCL2 family proteins and mutations in the tumor-suppressor gene TP53, which encodes tumor protein p53. This gene, called the “guardian of the genome,” initiates apoptosis in response to DNA damage induced by radiation, chemical agents, oxidative stress, and other agents by transcriptional induction of many proapoptotic proteins, including PUMA, NOXA, and BAX. Inherited defects in TP53 (e.g., the Li–Fraumeni syndrome) result in numerous neoplasms, including gliomas and sarcomas. Most chemotherapeutic agents induce apoptosis in tumor cells. The tyrosine kinase inhibitor imatinib (Gleevec) kills chronic myeloid leukemia cells by up-regulating the proapoptotic BCL2 family members BIM and BAD. ABT-737, a small-molecule mimic of the BH3-only proteins that bind to antiapoptotic BCL2 and BCL-XL, kills certain tumor cells on its own and greatly enhances the efficacy of other anticancer drugs. An orally active analogue of ABT-737 (ABT-263) has entered clinical trials in hematologic cancers and as adjuvant therapy in solid-organ tumors. Other proapoptotic chemotherapeutic agents that are in clinical trials target survivin and X-linked inhibitor of apoptosis (XIAP), which are endogenous inhibitors of proapoptotic caspases.
_
The figure below shows capabilities of cancer cells:
_
Let’s use the example of a simple mathematical equation. Every child would recognize the principles of the following formula:
Tumor mass = growth rate – death rate
This simple equation represents the principle of modern cancer biology. Where cancer researchers went wrong was that they mistakenly posited that the only way a tumor mass could increase was through an increase in the growth rate. However, as any child will tell you, a negative of a negative is a positive. That is, at a given growth rate, the tumor mass can also increase if you reduce the death rate. Thus, the “growth” so obvious to earlier investigators did not reflect an increase in proliferation but instead a decrease in cell attrition. Cancer didn’t grow too much it died too little, but the end result was exactly the same. It should now be abundantly clear exactly why chemotherapy drugs, designed to stop cells from growing, didn’t work. Yes, the drugs stopped cells from growing, and yes any population of “growing cells” would suffer the effect. But they didn’t cure cancers because the cancers weren’t growing particularly fast. Indeed, the fact that chemotherapy works at all is almost an accident. Contrary to our long held belief that we were inhibiting cell proliferation, chemotherapy drugs designed to damage DNA and disrupt mitosis, were actually working (when they did at all) by forcing the cells to take inventory and decide whether they could continue to survive. If the injury were too extreme, the cells would commit suicide through the process of cell death. If the cells were not severely damaged or could repair the damage, then they carried on to fight another day. None of this, however, had anything to do with cell growth. Following the description of apoptosis in the British Journal of Cancer in 1972, scientists around the world incorporated the concept of programmed cell death into their cancer research. With the recognition of programmed cell death as a principal factor in carcinogenesis and cancer response to therapy, there has been a growing belief that the measurement of apoptosis alone will provide the insights needed in cancer biology. This oversimplification underestimates the complexity of cell biology and suggests that cancer cells have but one mechanisms of response to injury. It has previously been shown that cancer cells that suffer lethal injury and initiate the process of apoptosis can be treated with caspase inhibitors to prevent caspase-mediated apoptosis. Of interest, these cells are not rescued from death. Instead, these cells committed to death, undergo a form of non-apoptotic programmed cell death more consistent with necrosis. Thus, commitment to death overrides mechanism of death. Labs that focus on measurements of caspase activation can only measure apoptotic cell death. While apoptotic cell death is of importance in hematologic cancers and some solid tumors, it does not represent the mechanism of cell death in all tumors. This is why researchers measure all cell death events by characterizing metabolic viability at the level of cell membrane integrity, ATP content, or mitochondrial function. While caspase activation is of interest, comparably easy to measure and useful in many leukemias and lymphomas, it does not represent cancer cell death in all circumstances and can be an unreliable parameter in many solid tumors.
_
Chemotherapy of cancer:
_
Chemotherapy of cancer targeting cell death pathways:
__
Some viruses associated with cancers use tricks to prevent apoptosis of the cells they have transformed.
- Several human papilloma viruses (HPV) have been implicated in causing cervical cancer. One of them produces a protein (E6) that binds and inactivates the apoptosis promoter p53.
- Epstein-Barr Virus (EBV), the cause of mononucleosis and associated with some lymphomas
- produces a protein similar to Bcl-2
- produces another protein that causes the cell to increase its own production of Bcl-2. Both these actions make the cell more resistant to apoptosis (thus enabling a cancer cell to continue to proliferate).
Even cancer cells produced without the participation of viruses may have tricks to avoid apoptosis.
- Some B-cell leukemias and lymphomas express high levels of Bcl-2, thus blocking apoptotic signals they may receive. The high levels result from a translocation of the BCL-2 gene into an enhancer region for antibody production.
- Melanoma (the most dangerous type of skin cancer) cells avoid apoptosis by inhibiting the expression of the gene encoding Apaf-1.
- Some cancer cells, especially lung and colon cancer cells, secrete elevated levels of a soluble “decoy” molecule that binds to FasL, plugging it up so it cannot bind Fas. Thus, cytotoxic T cells (CTL) cannot kill the cancer cells by the mechanism shown above.
- Other cancer cells express high levels of FasL, and can kill any cytotoxic T cells (CTL) that try to kill them because CTL also express Fas (but are protected from their own FasL).
_
Of special interest is the involvement of defective apoptosis pathways in tumor formation, progression, and metastasis as well as the occurrence of multidrug resistance during cancer therapy [Johnstone, 2002]. During the last years it became more and more evident that tumorigenesis is not merely the result of excessive proliferation due to the activation of oncogenes but to the same extent depends on the – frequently concurrent – impairment of apoptosis checkpoints [Hanahan, 2000; Wang, 1999]. Intriguingly, many of the alterations that induce malignant transformation, such as oncogene-driven deregulated proliferation and invasion, actually sensitize a cell to apoptosis, and therefore only those oncogenic transformed cells will survive and become malignant which additionally acquire defects in apoptosis pathways and therefore are protected against cell death induction [Vousden, 2002]. A transformed cell can achieve protection against apoptosis by inappropriate activation or expression of antiapoptotic proteins (which usually act as oncogenes), or by the inactivation of proapoptotic factors (which usually are tumor-suppressors). As an example and as already mentioned, Bcl-2 was the first apoptosis-related gene that was recognized to play a role in tumorigenesis, and indeed, Bcl-2 is overexpressed in a variety of cancers, contributing to cancer cell survival through direct inhibition of apoptosis [Hockenbery, 1990; Reed, 1999]. Conversely, mutated or downregulated Bax and Bak are observed in certain cancers [Kondo, 2000; Rampino, 1997] and disruption of those genes promotes tumorigenesis in mice [Yin, 1997]. Further, proapoptotic Bad and procaspase-9 are negatively regulated by the oncogenic Akt/PKB kinase, which on the other hand is frequently constitutively active or amplified in many types of human cancer [Nicholson, 2002], and its antagonist, the phosphatase PTEN, is one of the most commonly mutated tumor-suppressors [Yamada, 2001]. Even more underlining its potential as an oncogene, Akt/PKB is also stimulating the NF-kB survival pathway by phosphorylation of IkB kinase alpha (IKK alpha) and it is suppressing p53 proapoptotic signaling by phosphorylation of the oncogene Mdm2 which thereby is activated for inhibition of p53 [Mayo, 2002]. Both, NF-kB and Mdm2, are themselves inappropriately activated or overexpressed in the process of transformation [Chene, 2003; Orlowski, 2002]. A paradigm for the central importance of apoptosis checkpoints in the defense against malignant transformation presents the tumor suppressor p53, which is presumably the most intensely studied apoptosis factor contributing to cancer because it is inactivated in presumably more than 50% of all human cancers [Hainaut, 2000]. p53 is a tumor suppressor protein which is activated as a transcription factor in response to e.g. oncogene activation, hypoxia and especially DNA damage, resulting in growth arrest and/or apoptosis by stimulating the expression of various p53 target genes such as p21, Bax, Puma, Noxa, Apaf-1, Fas, and DR5 [Vousden, 2002] or by repressing the expression of antiapoptotic proteins, e.g. Bcl-2, Bcl-XL or survivin [Hoffman, 2002; Wu, 2001]. Recent evidence suggests transcription-independent p53 apoptosis pathways in which p53 translocates to the mitochondria, interacts with Bcl-XL, induces PT and the release of cytochrome c [Mihara, 2003]. In non-stressed, undamaged cells p53 therefore must be kept under stringent control: it is present only at low cellular concentrations, it is retained in the cytosol and prevented to enter the nucleus, and its transactivation domain is inactivated [Chene, 2003]. Central to p53 regulation is the oncogene Mdm2 which binds to and thereby inhibits p53. Mdm2 is a ubiquitin-ligase which mediates ubiquitination of p53, thereby targeting it for degradation by the proteasome. In this way, p53 levels are kept low in normal cells [Kubbutat, 1997]. The importance of Mdm2 in the control of p53 is demonstrated by mdm2 gene knockout mice which die early during development but are rescued from death by additional deletion of the p53 gene [Montes de Oca Luna, 1995]. In response to cellular stress (such as DNA damage), p53 is phosphorylated at specific serine/threonine residues which prevents the Mdm2-p53 interaction, and thus p53 is stabilized and activated [Schon, 2002]. Moreover, p53 is central to oncogene-induced cell death because it is induced by oncogenes such as c-myc, adenovirus E1A, and ras as well as by loss of the retinoblastoma tumor suppressor pRb [Henriksson, 2001]. All those oncogenes activate the transcription factor E2F-1 which not only can promote cell cycle progression and proliferation but at the same time directly triggers expression of the tumor suppressor ARF which leads to stabilization and activation of p53 [Ginsberg, 2002]. This explains in part why oncogene activation not always leads to uncontrolled cell proliferation but under certain circumstances to the stabilization of p53 and activation of cell death, provided that p53 signaling pathways are intact [Eischen, 1999].
_
Tumor Suppressor p53:
The tumor suppressor protein p53 is an important integrator of cellular stresses that can either trigger cell cycle arrest or apoptosis. The p53 tumor suppressor gene is a transcription factor that regulates the cell cycle and is the most widely mutated gene in human tumorigenesis (Wang and Harris, 1997). The critical role of p53 is evident by the fact that it is mutated in over 50% of all human cancers. p53 can activate DNA repair proteins when DNA has sustained damage, can hold the cell cycle at the G1/S regulation point on DNA damage recognition, and can initiate apoptosis if the DNA damage proves to be irreparable (Pientenpol and Stewart, 2002). Tumorigenesis can occur if this system goes awry. If the p53 gene is damaged, then tumor suppression is severely reduced. The p53 gene can be damaged by radiation, various chemicals, and viruses such as the Human papillomavirus (HPV). People who inherit only one functional copy of this gene will most likely develop Li–Fraumeni syndrome, which is characterized by the development of tumors in early adulthood (Varley et al., 1997; Gu et al., 2001). Due to the importance of p53 in apoptosis induction following DNA damage, different strategies have been undertaken to target the tumor suppressor as seen in the table below. A promising approach is the restoration of normal functions of mutant p53. For instance, the radioprotector amifostine can restore the transcriptional activity of some p53 mutants in yeast functional assays, provided that they are conformationally flexible (Maurici et al., 2001). Similarly, introduction of small synthetic molecules, which stabilize p53 in a transcriptionally active state by allosteric modulation of its conformation, is thought to especially target tumor cells with a high accumulation of mutant p53 (Foster et al., 1999). Some reports demonstrated that the transcriptional activity of mutant p53 can be rescued by application of synthetic peptides derived from the C terminus of p53. These peptides interact with the core domain of mutant p53 and restore growth-suppressing functions of p53 (Selivanova et al., 1997, 1999; Kim et al., 1999).
_
Reagents targeting p 53:
_
The p53 network – survival and cell death regulation:
In many instances, an oncogenic insult only results in increased proliferation and eventually malignant transformation if activators of p53 (such as ARF, Chk2, or ATM), p53 itself (e.g. by Mdm2, the adenovirus E1B, papillomavirus E6, or SV40 large T antigen), or p53 downstream signaling components (p53 target genes) are inactivated. On the other hand, p53-mediated apoptosis pathways can be suppressed by survival signals, such as growth factors binding to their cognate growth factor receptors what eventually results in activation of the Akt kinase [Datta, 1999]. Akt kinase is known to mediate a number of antiapoptotic mechanisms, such as the direct phosphorylation and inactivation of Bad and caspase-9, the activation of NF-kB antiapoptotic signaling via phosphorylation of IkB, but also phoshorylation and activation of Mdm2 as an inhibitor of p53 [Mayo, 2002]. Besides phosphorylation, ubiquitination and protein-protein interactions, p53 is also regulated by acetylation what affects its transcriptional activity, as well as by sumoylation [Melchior, 2003; Appella, 2001].
_
Abrogation of p53-induced apoptosis by the hepatitis B virus X gene:
The p53 tumor suppressor gene product is a transcriptional transactivator and a potent apoptotic inducer. The fact that many of the DNA tumor virus oncoproteins bind to p53 and affect these p53 functions indicates that this interaction is an important step in oncogenic transformation. Researchers have recently demonstrated that the hepatitis B virus oncoprotein, HBx, can form a complex with p53 and inhibit its DNA consensus sequence binding and transcriptional transactivator activity. Using a microinjection technique, they report here that HBx efficiently blocks p53-mediated apoptosis and describe the results of studies exploring two possible mechanisms of HBx action. First, inhibition of apoptosis may be a consequence of the failure of p53, in the presence of HBx, to upregulate genes, such as p21WAF1, Bax, or Fas, that are involved in the apoptotic pathway. Data consistent with this hypothesis include HBx reduction of p53-mediated p21WAF1 expression. Alternatively, HBx could affect p53 binding to the TFIIH transcription-nucleotide excision repair complex as HBx binds to the COOH terminus of p53 and inhibits its binding to XPB or XPD. Binding of p53 to these constituents of the core TFIIH is a process that may be involved in apoptosis. Because the HBx gene is frequently integrated into the genome of hepatocellular carcinoma cells, inhibition of p53-mediated apoptosis by HBx may provide a clonal selective advantage for hepatocytes expressing this integrated viral gene during the early stages of human liver carcinogenesis.
_
Bcl-2 and cancer:
Normal cells tightly control apoptosis in order to maintain homeostasis. Resisting apoptosis is one of the hallmarks of cancer and allows for uncontrolled proliferation and the formation of a tumor. The Bcl-2 superfamily is made up of both pro-apoptotic and anti-apoptotic proteins that are involved in apoptosis. Two important anti-apoptotic proteins, Bcl-2 and Bcl-XL, inhibit cellular apoptosis and are overexpressed in many cancers.
Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells:
A common feature of follicular lymphoma, the most prevalent haematological malignancy in humans, is a chromosome translocation (t (14;18)) that has coupled the immunoglobulin heavy chain locus to a chromosome 18 gene denoted bcl-2. By analogy with the translocated c-myc oncogene in other B-lymphoid tumours, bcl-2 is a candidate oncogene, but no biological effects of bcl-2 have yet been reported. To test whether bcl-2 influences the growth of haematopoietic cells, either alone or together with a deregulated c-myc gene, researchers have introduced a human bcl-2 complementary DNA using a retroviral vector into bone marrow cells from either normal or Eµ-myc transgenic mice, in which B-lineage cells constitutively express the c-myc gene8–10. Bcl-2 cooperated with c-myc to promote proliferation of B-cell precursors, some of which became tumorigenic. To determine how bcl-2 expression impinges on growth factor requirements, the gene was introduced into a lymphoid and a myeloid cell line that require interleukin 3 (IL-3). In the absence of IL-3, bcl-2 promoted the survival of the infected cells but they persisted in a G0 state, rather than proliferating. These results argue that bcl-2 provided a distinct survival signal to the cell and may contribute to neoplasia by allowing a clone to persist until other oncogenes, such as c-myc, become activated.
_
Restoring the cell’s ability to respond to apoptotic stimuli may combat tumorigenesis. Inhibiting anti-apoptotic proteins like Bcl-2 and Bcl-XL may lead to advances in cancer therapy.
_
AbbVie leukemia drug (Bcl-2 blocker) impresses in early-stage trial:
An experimental AbbVie Inc drug for leukemia controlled or eliminated signs of the disease in more than 80 percent of patients who had failed to benefit from previous treatments, an unprecedented finding that could spur use of the medicine for other cancers, researchers said. The AbbVie drug, ABT-199, works by blocking a protein called BCL-2 that allows cancer cells to overcome a natural mechanism called programmed cell death, in which the body kills off defective or cancerous cells. The favorable data was seen in a phase I, or early-stage, trial involving 67 patients with chronic lymphocytic leukemia who had not been helped by chemotherapy or relapsed from such treatment. The slowly progressing blood cancer, which mainly affects people age 65 and older, occurs when white blood cells build up in the blood, bone marrow and lymph nodes, causing them to enlarge. About 15,000 Americans are diagnosed with the disease each year. Some 84 percent of patients who took ABT-199 strongly responded to the drug, with at least a 50 percent reduction in signs of the disease. And 23 percent had complete remissions, meaning all signs of the disease vanished.
_
Atm and Bid:
Cellular DNA is under constant assault – from normal metabolism and from environmental factors like chemicals, UV light and radiation. To maintain genomic integrity and resist tumor-causing mutations, cells sense DNA damage and activate DNA repair processes or programmed cell death. Loss of the DNA damage sensor Atm in mice results in T-cell leukemia. Atm phosphorylates Bid, a protein that promotes cell death. To explore Bid’s role in vivo and probe its Atm-independent functions, Sandra Zinkel, M.D., Ph.D., assistant professor of Medicine, and colleagues generated mice missing both Bid and Atm. They were surprised to find that T-cell leukemia developed more slowly in these mice, compared to mice missing only Atm. Mice missing Atm and Bid have impaired cell cycle checkpoint control and increased cell death of developing T cells, which prevents the propagation of deleterious mutations. The findings, reported in the journal Cell Death and Differentiation, support the important roles of Bid and related Bcl-2 family members in regulating cell death and suggest their actions depend on cell-type and context.
_
APG350 effectively induces Apoptosis of Tumor Cells via TRAIL Pathway Independent of Fc-gamma Cross-Linking:
Apogenix, a clinical stage biopharmaceutical company published in the journal of Molecular Cancer Therapeutics the effective antitumor activity of the company’s drug candidate APG350, an activator for TRAIL receptors. The publication shows that APG350’s novel molecular structure allows for potent induction of apoptosis of tumor cells independent of the innate immune system, thus overcoming the limitations of other TRAIL receptor agonists. “Due to the potential role of the TRAIL pathway in the treatment of cancer, there have been several attempts to develop substances for therapeutic use that utilize this pathway,” said Harald Fricke, M.D., Chief Medical Officer and Chief Operating Officer of Apogenix. “However, to date, none of these approaches have been successful in clinical trials. APG350 was designed to optimize both the activation of TRAIL receptors on tumor cells as well as the pharmacokinetic properties of the substance. Its unique molecular structure ensures effectiveness without the need for cross-linking via Fc-gamma receptors on immune cells. Since such cross-linking cannot be effectively achieved in the human body, previous TRAIL receptor agonists have ultimately failed to show antitumor efficacy in clinical studies.” “In the different colon carcinoma animal models that we examined, treatment with APG350 resulted in efficient induction of tumor-specific apoptosis and consequently the complete remission of the tumors. Because activation of the TRAIL signaling pathway is possible in many tumor types, APG350 has the potential for wide use in oncology. We are convinced that this novel drug design concept will be successful in clinical applications,” Harald Fricke concluded.
_
PKM2 protein controls mitosis, saving cancer cells from death and promoting brain tumor growth:
Researchers have caught a protein they previously implicated in a variety of cancer-promoting roles performing a vital function in cell division, survival and development of brain tumors. In a paper published in Molecular Cell, Zhimin Lu, Ph.D., professor of Neuro-Oncology at The University of Texas MD Anderson Cancer Center and colleagues report how a tumor-specific protein flips a crucial switch in an irregular mechanism for mitosis that allows cancer cells to safely divide. “Our research shows that tumor cells rely heavily on a distinct mechanism for orderly cell division that’s driven by oncogene-induced pyruvate kinase M2,” Lu said. After a cell begins division by replicating all of its chromosomes, mitosis separates them into two identical sets of chromosomes for both cells. After mitosis, cytokinesis completes cell divison. “Without PKM2 regulating a checkpoint in mitosis, the tumor cell would not successfully divide,” Lu said. “Depleting PKM2 led to an uneven distribution of DNA to the two new cells, triggering programmed cell death, or apoptosis, of those cells after division.” “This new, additional role for PKM2 in cancer development and survival may provide a molecular basis for diagnosing and treating tumors with upregulated PKM2,” Lu said. He and his colleagues have now identified four specific mechanisms by which PKM2 promotes cancer development. The key relationship between PKM2 activity and mitosis uncovered by the researchers led to rapid brain tumor growth when activated in mice, while blocking it reduced tumor volume by 83 percent and more than doubled survival from about 20 days to beyond 40 days. Analysis of 50 human glioblastoma multiforme tumors and 50 lung cancer tumors confirmed the relationship in human cancer and indicated an effect on survival for patients with glioblastoma, the most common and lethal form of brain tumor. PKM2 can act as a protein kinase, which gives orders to other proteins by attaching phosphate groups to them. While it plays a normal role in sugar metabolism, PKM2 also actively promotes cell growth during infancy when such growth is desired. Usually, Lu said, it eventually turns off, but tumor cells reactivate PKM2, and it is famously overexpressed in solid tumors. This tumor-specific PKM2 is activated by the epidermal growth factor receptor (EGFR), which is overactive in a variety of cancers. Deplete PKM2 in mitosis, tumor cells abnormally divide in multiple cancer types A series of experiments in glioblastoma cell lines revealed that PKM2 phosphorylates a protein called Bub3, activating it to interact with others in a protein complex that assures orderly and equal chromosome separation. Depleting PKM2 blocked Bub3 activation, leading to an increase in cells with abnormal numbers of chromosomes and programmed cell death. The team confirmed its findings in human breast, prostate, lung, pancreatic and colon cancer cell lines. PKM2-induced Bub3 activation was essential for development of brain tumors in mice.
_
Antibody-drug-conjugate (ADC):
Antibody-drug conjugates (ADCs) offer a potentially new way to treat cancer by combining the proven antigen-specific selectivity and antitumor activity of monoclonal antibodies with the potency of cytotoxic molecules. ADCs have the potential to optimize the best features of both components by
- Increasing the cell-killing potential of monoclonal antibodies
- Conferring higher tumor selectivity and, therefore, increased tolerability while
_
The figure below shows ADC:
_
How are ADCs designed to work?
ADCs are designed to target antigens that are exclusively or preferentially expressed on the surface of cancer cells. ADCs have multiple proposed mechanisms of action, including antibody-mediated anticancer activities and targeted intracellular delivery of a potent cytotoxic. By combining the antigen-targeting precision and antitumor activity of monoclonal antibodies with the cell-killing potency of cytotoxic molecules, ADCs are designed to deliver potent anticancer agents to tumors in a targeted manner to limit systemic exposure. The monoclonal antibody component of the ADC binds to the target antigen on the cell surface.
The monoclonal antibody component of an ADC may possess its own anticancer activities, which may include
- Prevention of signaling
- Antibody-dependent cell-mediated cytotoxicity (ADCC)
- Induction of apoptosis
_
SNS01-T Selectively kills Cancer Cells and not Healthy Cells in Disease Models:
SNS01-T is a novel, gene regulatory approach to cancer therapy that is designed to selectively trigger apoptosis in B-cell cancers such as multiple myeloma, and, mantle cell and diffuse large B-cell lymphomas. Laboratory studies have already shown that, due to its design, SNS01-T does not produce significant levels of the message that makes the death-inducing protein in normal tissues including heart and liver. SNS01-T, which is the subject of an on-going Phase 1b/2a clinical study in B-cell cancers, induces cell death in cancer cells by reducing the levels of a protective protein and replacing it with a protein that induces cell death. The drug candidate was taken up more efficiently by malignant B-cells than normal human B-cells in non-clinical studies. Although 50% of normal B-cells took up SNS01-T, it did not cause cell death in the healthy cells, whereas cell death was readily seen in human myeloma cells. The new discoveries provide evidence suggesting SNS01-T may not affect normal human B-cells, which are an important protective component of the body’s immune system. This is in contrast to existing B-cell cancer therapies like rituximab, which is widely used in mantle cell and diffuse large cell lymphomas.
_
Light triggers death switch in cancer cells:
Researchers at Cardiff University have created a peptide (a small piece of protein), linked to a light-responsive dye, capable of switching ‘on’ death pathways in cancer cells. The peptide remains inactive until exposed to external light pulses which convert it into a cell death signal. Complex mechanisms in healthy cells normally protect us from developing cancer. However, when the finely balanced networks of interactions between proteins that control such mechanisms are disturbed, uncontrolled cell growth can occur. The Cardiff team has developed a peptide-switch to alter critical interactions in B-cell lymphoma cancer cells in a ‘smart’ and controlled way. This new pathway activation technology, called transient photoactivation, may enable scientists to identify cells normally resistant to chemotherapy leading to the development of more effective treatment strategies. Professor Rudolf Allemann from Cardiff University’s School of Chemistry, who led the research, said: “Whilst killing cancer cells is a goal in itself, this is also proof of a wider principle. Directing therapeutic peptides to the precise location where they are required can be difficult, but activating peptides with light will allow us to precisely define the area where we wish a peptide to act. “Our research demonstrates that we can control cellular processes with light, which has implications for research in biology and medicine, as our tools can be used to understand the inner workings of cells and to work out how to correct misfiring pathways that lead to disease. “This work may eventually lead to photo-controlled drugs and tools to probe molecular interactions in intact cells and whole organisms with enormous consequences for biomedical research.”
_
‘Cold Plasma’ Researchers See Success in New Leukemia Cancer Treatment Method:
In a new study published in a high-impact biotechnology and bioengineering journal Wiley, Thiyagarajan explains that his research team has developed a way to make this happen without using the harmful heat and he says when he directs that ray of light called “cold plasma” at cancer cells, what happens is incredible. “Within 24 hours, we began to see the destruction of over 90 percent of the cancer cells,” said Thiyagarajan. “Cold plasma induces the cancer cells to self-destruct, but it can leave the healthy cells unharmed.” Thiyagarajan says the key reason healthy cells may not be affected during this process is due to the reactive species produced by the cold plasma also being a natural byproduct of a cell’s own metabolic processes. “The cold plasma forces those cancer cells to self-destruct in a rage of apoptosis,” said Thiyagarajan. “In contrast, the healthy cells are unharmed due to their lower levels of reactive species to begin with.”
____________
Vitamin C and cell death in cancer:
Autoschizis is a term derived from the Greek roots “auto” meaning self, and “skhizein” to split. It was introduced in 1998 to describe a novel form of cancer cell death characterized by a reduction in cell size that occurs due to the loss of cytoplasm through self-excision (the cell splits open) without the loss of cell organelles, morphologic degradation of the cell’s nucleus and nucleolus without the formation of apoptotic bodies and destruction of the cell membrane. The cell death results from karyorrhexis and karyolysis. Autoschizis can be initiated via in vivo treatment with Vitamin C (VC), synthetic Vitamin K (VK3) or, better, a combination of both. The treatment has been tested on various types of cancer cells in vitro and in vivo with positive results.
_
Fatty acids stops growth and incite cell death in cancer:
Animal studies have found that fish oils rich in omega-3 fatty acids suppress the formation and growth of some types of cancer however; studies in human subjects have shown conflicting results. Scientists at Queen Mary, University of London, had been examining squamous cell carcinoma (SCC). Squamous cell carcinoma is an uncontrolled growth of abnormal cells arising in the squamous cells, which compose most of the skin’s upper layers. Squamous cell carcinoma (SCC) is a common skin cancer in humans. About 700,000 new cases of this skin cancer are diagnosed in the United States each year. However, squamous cells also occur in the lining of the digestive tract, lungs, and other areas of the body. Oral squamous cell carcinomas (OSCC) are the sixth most common cancer worldwide and are difficult and very expensive to treat. In experiments the scientists grew cell cultures in the lab from several different cells lines that included both malignant oral and skin SCCs, along with pre-malignant cells and normal skin and oral cells to which they added fatty acids. Their results showed that, in part, EPA specifically inhibits SCC growth and development by creating a sustained signaling imbalance to amplify the EGFR/ERK/p90RSK pathway in neoplastic keratinocytes to a supra-optimal level, supporting the chemo preventative potential of EPA, whose toxicity to normal cells might be reduced further by blocking its metabolism to DHA. As the doses needed to kill the cancer cells do not affect normal cells, especially with one particular fatty acid we used called Eicosapentaenoic acid (EPA), there is potential for using omega-3 fatty acids in the prevention and treatment of skin and oral cancers. It may be that those at an increased risk of such cancers — or their recurrence — could benefit from increased omega-3 fatty acids. Moreover, as the skin and oral cancers are often easily accessible, there is the potential to deliver targeted doses locally via aerosols or gels. However further research is needed to define the appropriate therapeutic doses.
_
B2G2 component of grape seed extract induces apoptosis in prostate cancer cells:
A University of Colorado Cancer Center study published online ahead of print in the journal Nutrition and Cancer describes the laboratory synthesis of the most active component of grape seed extract, B2G2, and shows this synthesized compound induces the cell death known as apoptosis in prostate cancer cells while leaving healthy cells unharmed. “We’ve shown similar anti-cancer activity in the past with grape seed extract (GSE), but now we know B2G2 is its most biologically active ingredient which can be synthesized in quantities that will allow us to study the detailed death mechanism in cancer cells,” says Alpna Tyagi, PhD, of the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences. Tyagi works in the lab of CU Cancer Center investigator and Skaggs School of Pharmacy faculty member, Chapla Agarwal, PhD.
_
Herb Triggers Cancer Cell Death:
A substance found in the popular Chinese herb huang qin triggers the death of tumor cells, while having virtually no effect on healthy cells. Many researchers have looked to the capability of controlled cell suicide as a means of fighting cancer. But it is a risky approach, because it involves the danger of damaging healthy tissue, too. Scientists have been searching for substances that induce cell death selectively in tumor cells. Now scientists of the German Cancer Research Centre have discovered that wogonin, a component of huang qin, causes apoptosis (cellular suicide) in cultured leukemia cells without affecting healthy blood cells. Wogonin has already been shown to reduce cancer growth in mice. Dr. Min Li-Weber of the Division of Immunogenetics has found that wogonin works by radically increasing the formation of hydrogen peroxide in tumor cells compared to healthy cells. The peroxide, in turn, produces a calcium response, which triggers the apoptosis reaction.
_
Researchers use magnets to cause programmed cancer cell deaths:
A team of researchers in South Korea has developed a method to cause cell death in both living fish and lab bowel cancer cells (in vivo and in vitro) using a magnetic field. The application of the magnetic field, as described in their paper published in the journal Nature Materials, triggers a “death signal” that leads to programmed cell death. A major problem with treating cancer is how to effectively and efficiently apply a therapy, one that discriminates between cancer and healthy cells: killing the bad while retaining the good. Numerous methods have been tried over the years with varying degrees of success. In this new research, the team has been experimenting with the introduction of iron oxide nanoparticles, which attach to antibodies, into the system. The antibodies, in turn, bind to tumor cell receptors. When a magnetic field is introduced, the nanoparticles bunch up or cluster, which triggers a natural response called a death signal. When that happens, apoptosis (programmed cell death) occurs, causing destruction of the tumor. The work is based on apoptosis, a process that continually occurs in living organisms. In the present study, researchers took advantage of this process by causing chemicals to be sent to tumor cells. The researchers applied zinc-doped iron oxide nanoparticles to colon cancer cells, which naturally bind to antibodies. Those antibodies then bind very strongly to what is known as the death receptor 4(DR4) which exists on DLD-1 colon cancer cells. When a magnetic field is applied, the death receptor sends out a signal telling the system to attack the cell. Chemicals are then sent, killing the tumor. In their experiment, the team found that more than half of the tumor cells exposed to the treatment were killed, while none of the untreated cells died. Unfortunately, other experiments with zebra fish resulted in the growth of abnormal tails. The team notes that their research is still in its infancy and that far more research will need to be done to see if the process can be refined and eventually tested in human trials.
__________
Oxidative stress and cancer cell death:
Due to oncogenic stimulation and high metabolic rates, cancer cells exhibit high levels of reactive oxygen species (ROS) that stimulate cell proliferation and promote genetic instability. Such a biochemical difference between transformed and non-transformed cells represents a redox vulnerability of malignant cells that can be targeted by chemotherapeutic intervention using redox modulators. Since cancer cells usually lack antioxidant enzymes, researchers hypothesize that a selective exposure of cancer cells to oxidants will render these cells more vulnerable to an oxidative stress. To this end, they used an H2O2-generating system, namely the association between ascorbate and redox-active quinones. Ascorbate plays a key role in this system because it is preferentially taken up by cancer cells, thus favouring the in situ formation of ROS. Researchers have shown that the association of ascorbate and menadione (Asc/Men) inhibits glycolysis leading to an ATP depletion and cancer cell death. Since cancer cells are highly dependent on glycolysis, the impairment of this pathway will be critical for their survival. In addition, oxidative stress affects the activity of two major chaperones, the cytosolic heat shock protein 90 (Hsp90) and the glucose regulated protein 94 (GRP94) in the endoplasmic reticulum. Since the stability of several proteins (like Bcr-Abl, Akt, RIP…) that are essential for malignant transformation, is made possible by Hsp90, its inhibition represents an interesting target for cancer therapies. On the other hand, they have shown that an increase in the expression of GRP94 is tightly linked to an enhancement in cell proliferation and migration capacities. Since they showed that GRP94 expression was accrued in recurrent human breast cancers compared to their paired primary neoplasias, it appears that GRP94 overexpression may be a hallmark of aggressiveness and recurrence in breast cancers. An interesting molecular link between cancer cell metabolism, resistance acquisition and oxidative stress is the activation of Nrf2, a transcription factor associated to pro-survival pathways. Preliminary results about redox modulation in this field are strongly exciting and it anticipates that our approach may have potential clinical applications.
_________
Viral Death Proteins Apoptin and E4orf4:
Promising candidates for tumor therapy are the viral proteins apoptin (derived from the chicken anemia virus) and the adenoviral protein E4orf4 . Sharing the same preference as TRAIL, they specifically kill tumor cells while leaving normal cells unharmed (Kleinberger, 2000; Rohn and Noteborn, 2004). The reason for this tumor selectivity is still unclear. In the case of apoptin, it seems to depend on its subcellular localization, as the protein is localized in the cytoplasm of normal cells, but migrates into the nucleus of transformed cells. This is at least in part a consequence of apoptin phosphorylation at Thr-108, which seems to take place only in transformed but not in normal cells. An interesting feature of apoptin is that it triggers cell death very efficiently in tumor cells that are resistant to chemotherapy due to Bcl-2 overexpression, p53 mutations, or expression of the BCR-ABL fusion oncoprotein. Both viral proteins are still in preclinical studies. Delivery with gene therapy vectors or fusion with the protein transduction domain from HIV-Tat, which enable fused proteins to transfer cell membranes, are currently being investigated (Lavoie et al., 1998; van der Eb et al., 2002; Guelen et al., 2004). Preclinical studies showed no adverse effects of apoptin delivered by an adenoviral vector, and an antitumor effect was observed in nude mice challenged with human hepatoma (Pietersen and Noteborn, 2000).
_
The figure below shows viral proapoptotic strategies:
__________
Apoptosis to non-apoptosis cell death in cancer:
The process by which a normal cell develops into a malignant cell with the capacity to form a tumor requires several cellular alterations. Evasion of apoptotic cell death is one of the proposed alterations. Importantly, evasion of apoptosis is also recognized to result in resistance to anti-cancer therapies. Much research has therefore focused on finding ways to circumvent this resistance to apoptosis in order to improve the treatment of cancer patients. However, the contribution of apoptosis resistance to treatment success remains a matter of debate, especially in solid tumors. Increasing attention is being directed towards other types of cell death, such as mitotic catastrophe, autophagy and necrosis. These alternative types of cell death may compensate for the resistance to apoptosis. Understanding the regulation of apoptosis and non-apoptotic death pathways will help us to better evaluate their impact on tumor development and treatment response in vivo. Moreover, detailed knowledge regarding the molecular events that contribute to treatment success will facilitate a more rational approach of anti-cancer treatments.
_
Protein that pushes cancer cells those are resistant to apoptosis into necrosis instead:
ALKBH7 belongs to a family of proteins first discovered in E. coli about a dozen years ago as part of a DNA-repair mechanism. In humans, there are nine different ALKBH proteins, which Samson’s lab has been studying for several years. Most of the mammalian ALKBH proteins appear to be involved in DNA repair, similar to the original E. coli version. In particular, they respond to DNA damage caused by alkylating agents. These agents can be found in pollutants such as fuel exhaust and tobacco smoke, and are also used to treat cancer. In the new paper, Samson, a professor of biology and biological engineering, and her colleagues found that ALKBH7 has an unexpected effect. When the researchers lowered ALKBH7 levels in human cells grown in the lab, those cells were much more likely to survive DNA damage than cells with normal ALKBH7 levels. This suggests that ALKBH7 actually promotes cell death. “That was a surprising finding, because previously all of these ALKBH proteins were shown to be helping the cell survive when exposed to damage,” says Fu, who is now a visiting research fellow at the University of Zurich. Upon further investigation, the researchers found that when healthy cells suffer massive DNA damage from alkylating agents, they enter the programmed necrosis pathway. The findings suggest that when DNA is so badly harmed that cells can’t repair it, the programmed necrosis pathway kicks in to prevent cells with major genetic damage from potentially become cancerous. Other researchers have shown that some types of cancer cells have much lower ALKBH7 levels than normal cells. This suggests that the cancer cells have gained the ability to evade programmed necrosis, helping them to survive, Fu says. The necrosis pathway appears to be initiated by an enzyme called PARP, which becomes hyperactive following DNA damage and shuts down the cell’s production of two molecules that carry energy, ATP and NAD. The MIT team found that ALKBH7 prevents ATP and NAD levels from returning to normal by disrupting the function of mitochondria — the cell structures that generate energy for a cell. Without an adequate supply of those critical energy-carrying molecules, the cell cannot survive and undergoes necrosis. In cells that lack ALKBH7, ATP and NAD levels rebound, and the cells survive, carrying a heavy burden of DNA damage. The researchers are now investigating the molecular details of the programmed necrosis pathway in hopes of identifying ways to activate it in cancer cells. “The observations reported in this paper open up the possibility that novel treatments could be developed to treat tumors that are relatively resistant to killing via the apoptotic pathway,” says Ashok Bhagwat, a professor of chemistry at Wayne State University who was not part of the research team.
_
Scientists find necrosis to kill Cancer Cells:
This finding shows, for the first time, that cancer cells are unusually sensitive to dying by necrosis, when their ability to metabolize glucose is blocked,” said Craig Thompson, MD, Principal Investigator of the study and Scientific Director of the Abramson Family Cancer Research Institute (AFCRI). “Up until now, research has focused on finding ways to program cancer cells to die through apoptosis – a very regulated, orderly form of cell death that does not trigger an immune response. Now, we know that cancer cells can be forced to die, suddenly, through necrosis. If we can harness this method, which does trigger an immune response, then, the door will be opened to a whole new and less toxic way to treat cancer.” To study this issue, the researchers created mouse cells that were unable to die by apoptosis. The cells were engineered to be deficient in either the tumor suppressor gene p53, the most commonly mutated gene in human cancer, or two key proteins essential for the execution of apoptotic cell death, Bax and Bak. The researchers then determined whether any standard chemotherapeutic drugs could kill these cells. They discovered that some commonly used chemotherapeutic drugs – alkylating agents such as mechlorethamine hydrochloride (nitrogen mustard) – retained the ability to kill the cells engineered to be resistant to apoptosis. When exposed to alkylating agents, the cancer cells died by necrosis, a form of cell death that results from energy depletion. Of equal importance, the researchers found that the induced necrotic cell death was specific to proliferating cancer cells. The rapid energy depletion was triggered by activation of a DNA repair protein, called PARP. Its activation leads to an inhibition of the cancer cell’s ability to break down glucose to produce the cellular fuel ATP, a process termed glycolysis. In contrast, non-proliferating or non-cancerous cells did not exhibit energy depletion, as they produce most of their ATP by metabolizing a mixture of fats, proteins, and carbohydrates in a process termed oxidative phosphorylation. This explains why necrotic cell death, induced by the chemotherapeutic agents, was specific to cancer cells and did not affect healthy, non-proliferating cells. When PARP activation was blocked, necrotic cell death failed to occur despite exposure to the chemotherapeutic agents. Chemotherapeutic drugs activate PARP by damaging DNA. While this is effective at killing tumor cells, it comes at the price of damaging many normal cells, creating mutations that might lead to new cancers. In contrast, the new work suggests that drugs directly activating PARP might prove effective at treating cancer without many of the serious side effects of existing chemotherapy.
_____________
Target mitochondria in cancer:
It is known that mtDNA is far more vulnerable to mutations than nuclear DNA due to its lack of histone protection, limited repair capacity, and close proximity to the electron transport chain, which constantly generates superoxide radicals. Considering mtDNA lacks introns, most mutations occur in the coding sequences and are thus, likely to be of biological consequence. Mutations in mtDNA have been identified in various types of human cancer. Given the critical role of mitochondria in apoptosis, it is conceivable that mutations in mtDNA in cancer cells could significantly affect the cellular apoptotic response to anticancer agents.
_
_
Targeting the mitochondria as a novel strategy for cancer therapy:
Though targeting the inherent adaptive anti-apoptotic strategies enhance sensitization of cancer cells to therapeutic regimes, the rise of resistance in cancer cells poses a challenge for scientists and physicians. In this regard, numerous anticancer agents target pathways that lie upstream of the mitochondria, which then converge onto the intrinsic death pathway. However, the deregulation of several master switch proteins such as HIF1, HKII, c-Myc and P53, leads to resistance in these cells. Thus, directly targeting the mitochondria may allow us to overcome the problem of resistance that arises from engaging the upstream pathways. The mitochondria are the cellular powerhouses and hence the primary source of energy for the cells. Since cancer cells generally display a higher energy demand, targeting the cellular energy source seems to be an attractive solution. ATP is the energy currency of cells and a high ratio of ATP/ADP is produced primarily by the oxidation of glucose. Oxidation of glucose is a two stage process namely, glycolysis, which is the first stage, that occurs at the cytosol and oxidative phosphorylation, the second stage that occurs at the mitochondria, where most of the ATP is produced. In addition to the pertinent role in cell survival, the mitochondria are critical regulators of the intrinsic pathway of apoptosis. They control several death effector cascades through the release of pro-apoptotic proteins such as Apaf-1, cyt c and Smac/DIABLO, which normally reside in the inter-membranous space. Thus, the convergence of these life and death regulatory roles at the mitochondria has made it a highly favorable target for therapeutic manipulation.
_
Differences in mitochondria between normal and cancer cells as molecular cancer target for natural chemotherapy:
There is compelling evidence that mitochondrial dysfunction coincides with many cancers. Consequently, cancer prevention therapies have recently focusing on mitochondrial targeting strategies. The induction of the programmed suicide cell death (apoptosis) in cancer cells with least adverse effects on normal cells is particularly desirable. Researchers demonstrated that a matched pair of isogenetic normal and transformed counterpart cells (WI38/WI38VA) showed a selective induction of the mitochondrial-mediated apoptotic pathway only in tumor but not in normal cells in response to resveratrol analogs, black tea-derived theaflavins, and oxidative stress as demonstrated by membrane permeability transition, cell blebbing, cytochrome c release, Bax/Bcl-2 ratio increase, caspase activation, and DNA laddering. Noteworthy, a mitochondrial clustering around the nucleus (perinuclear clustering) along microtubule tracks occurred as very early event only in cancer cells. Further analysis demonstrated a significant difference in mitochondrial morphology which correlated to their capacity for apoptosis. Tumor cells bearing a bubble-like mitochondrial network (e.g. WI38VA, HeLa) showed intrinsic apoptosis in response to various inducers. In contrast, normal counterpart cells (WI38) or tumor cells (C6 glioma, MG63 osteosarcoma, Caco-2 colorectal adenocarcinoma) which are resistant to apoptosis are composed of a web-like mitochondrial reticulum. Interestingly, researchers observed strong anti-cancer effects of hyperthermia towards resistant cancers (e.g. glioma, colorectal, prostate). The results indicate a correlation between mitochondrial morphology and the capacity for induction of apoptosis. Knowledge about mitochondrial morphology or cellular components impacting mitochondria (e.g. microtubule) thus offers a unique molecular site against which selective natural-derived bioactives and/or physico-chemical inducers might be targeted.
_
Apoptosis-resistant mitochondria and cancer:
_
Targeting the Mitochondria activates two Independent Cell Death Pathways in Ovarian Cancer Stem Cells:
Cancer stem cells are responsible for tumor initiation and chemoresistance. In ovarian cancer, the CD44+/MyD88+ ovarian cancer stem cells are also able to repair the tumor and serve as tumor vascular progenitors. Targeting these cells is therefore necessary to improve treatment outcome and patient survival. The previous demonstration that the ovarian cancer stem cells are resistant to apoptotic cell death induced by conventional chemotherapy agents suggests that other forms of targeted therapy should be explored. Researchers show in this study that targeting mitochondrial bioenergetics is a potent stimulus to induce caspase-independent cell death in a panel of ovarian cancer stem cells. Treatment of these cells with the novel isoflavone derivative, NV-128, significantly depressed mitochondrial function exhibited by decrease in ATP, Cox-I, and Cox-IV levels, and by increase in mitochondrial superoxide and hydrogen peroxide. This promotes a state of cellular starvation that activates two independent pathways: (i) AMPKα1 pathway leading to mTOR inhibition; and (ii) mitochondrial MAP/ERK kinase/extracellular signal-regulated kinase pathway leading to loss of mitochondrial membrane potential. The demonstration that a compound can specifically target the mitochondria to induce cell death in this otherwise chemoresistant cell population opens a new venue for treating ovarian cancer patients.
_
Cisplatin and mitochondria:
Cisplatin, which contains the metal platinum, was approved to treat ovarian and testicular tumors in 1978 and is now used for many other cancers, including lung and bladder. The drug forms crosslinks in DNA, creating blockages that interfere with a cell’s ability to read or replicate its genome. If enough of these blockages form, the cell undergoes a type of programmed cell suicide called apoptosis. Cisplatin is a chemotherapy drug given to more than half of all cancer patients. The drug kills cells very effectively by damaging nuclear DNA, but if tumors become resistant to cisplatin they often grow back. A new study from the Massachusetts Institute of Technology (MIT) and the University of Toronto offer a possible way to overcome that resistance. The researchers found that when cisplatin was delivered to cellular structures called mitochondria; DNA in this organelle was damaged, leading to cancer cell death. Moreover, the mitochondrial-targeted drug could overcome cisplatin resistance. “These results suggest that the mitochondria can be an important target for platinum-based drugs,” said Robert Radford. Mitochondria-targeting cisplatin might also be effective at lower doses than regular cisplatin, helping to avoid some of the severe side effects often seen with the drug, according to the researchers.
Because mitochondria are involved in apoptosis, the researchers wanted to see whether they could induce cell death by targeting mitochondrial DNA, particularly in cells that are already resistant to regular cisplatin. To do that, they developed a new way to tag the drug with a protein fragment developed in Kelley’s lab that can enter the cell and accumulate in mitochondria. The mitochondrial-targeted version of the drug killed cancer cells and cisplatin-resistant cells with the same success rate. With regular cisplatin, killing resistant cells requires about 10 times the amount of drug needed to kill the same number of nonresistant cells. However, by targeting the platinum-based drug to mitochondria, at a given dose, the researchers showed they could kill equal numbers of resistant and nonresistant cells. The drug was even more effective in cells with an impaired ability to repair mitochondrial DNA. This result was one of several pieces of evidence the researchers obtained proving that the new mitochondria-targeted, platinum-based compound was working by targeting mitochondrial DNA. The researchers also showed that the cells were dying through apoptosis, and not some less-controlled form of cell death.
_
Drugs acting on mitochondrial membrane permeabilization (MMP):
An important apoptotic alteration is an event characterized as mitochondrial membrane permeabilization (MMP). Several cytotoxic drugs induce MMP by a direct action on mitochondria and might therefore trigger death in cells in which upstream apoptotic signals have been disabled (Debatin et al., 2002). Components acting directly on mitochondria can be either proteins of the permeability transition pore complex, including the peripheral benzodiazepine receptor (PBR) and the adenine nucleotide translocase (ANT), or can be small molecules that exert a cytotoxic effect by virtue of their physicochemical properties as seen in the table below. Interestingly, certain compounds acting directly on mitochondria can overcome the antiapoptotic effect of Bcl-2 proteins, suggesting that their action is independent of the Bcl-2 pathway. Some of these agents are already used in anticancer chemotherapy, and new drugs specifically designed to target mitochondria are being developed.
______________
Metastasis:
Anoikis and metastasis:
Anoikis is a form of programmed cell death which is induced by anchorage-dependent cells detaching from the surrounding extracellular matrix (ECM). Usually cells stay close to the tissue to which they belong since the communication between proximal cells as well as between cells and ECM provide essential signals for growth or survival. When cells are detached from the ECM, i.e. there is a loss of normal cell–matrix interactions, they may undergo anoikis. However, metastatic tumor cells may escape from anoikis and invade other organs. The mechanism by which invading tumor cells survive the anoikis process remains largely unknown. Recent findings suggest that the protein TrkB, best known for its role in the nervous system, might be involved together with its ligand, brain-derived neurotrophic factor (BDNF). It seems that TrkB could make tumor cells resistant to anoikis by activating phosphatidylinositol 3-kinase (PI3K) signaling cascade. In squamous cell carcinoma, researchers have found that anoikis resistance can be induced through hepatocyte growth factor (HGF) activating both extracellular signaling-receptor kinase (ERK) and PI3K. Using a novel high-throughput screening assay, Mawji et al. showed that anisomycin can sensitize metastatic epithelial cells to anoikis and reduce circulating tumor cell implantation in vivo. Anisomycin achieved this anti-metastatic activity in part by decreasing the abundance of the death receptor inhibiting protein FLIP. In related work, Schimmer’s team showed that FLIP levels are higher in metastatic cells than non-metastatic cells, and that reducing FLIP levels using RNAi (RNA Interference) or other small molecule inhibitors of FLIP can sensitize metastatic cells to anoikis. Given that FLIP is an inhibitor of anoikis, and that reducing FLIP can sensitize metastatic cells to anoikis, Mawji et al. hypothesize that FLIP reduction may be a viable therapeutic strategy against cancer metastasis.
_
Targeting anti-oxidant enzyme to kill metastatic cancer cells:
Metastasis, the spread of cancer from one organ to other parts of the body, relies on cancer cells ability to evade a cell death process called anoikis. Metastasizing cancer cells are able to survive anoikis, which normally results from detachment from the extracellular matrix. A paper reveals that cancer cells that are detached from their normal environment, as they would be during metastasis, rely on the activity of antioxidant enzymes to facilitate their survival. This class of enzymes is critical for neutralizing oxidative stress and function much like the compounds that are present in a variety of foods. The paper describes a prominent role for antioxidant enzymes in facilitating the survival of breast cancer cells after detachment from the extracellular matrix. Conversely, the researchers report, silencing antioxidant enzyme expression reduced tumor formation. The results in this paper suggest that targeting antioxidant enzymes with novel therapeutics may selectively kill off metastasizing cancer cells. The paper appears in the journal Cancer Research, which is the most frequently cited cancer journal in the world.
_
Blocking antioxidants in cancer cells reduces tumor growth in mice:
Many cancers have adapted to cope with high levels of immune system-produced free radicals, also referred to as reactive oxygen species, by overproducing antioxidant proteins. One of these proteins, superoxide dismutase 1 (SOD1), is overproduced in lung adenocarcinomas and has been implicated as a target for chemotherapy. In the issue of the Journal of Clinical Investigation, Navdeep Chandel and colleagues from Northwestern University report the effects of a SOD1 pharmacological inhibitor on non-small-cell lung cancer (NSCLC) cells. The inhibitor, called ATN-224, stunted the growth of human NSCLC cells in culture and induced their death. The researchers also found that ATN-224 inhibited other antioxidant proteins, which caused high levels of hydrogen peroxide inside the cells. The ability of cancer cells to produce hydrogen peroxide was required for ATN-224-dependent effects, because hydrogen peroxide activated cell death pathways. Furthermore, ATN-224 induced cancer cell death and reduced tumor sizes in a mouse model of lung adenocarcinoma. ATN-224 dependent effects in animals were improved when the inhibitor was used in combination with another drug that activates programmed cell death. This study suggests inhibition of antioxidants may be a viable chemotherapeutic option.
_____________
The Immune System and apoptosis:
Abnormalities in apoptosis can increase susceptibility to autoimmune diseases. During development, clones of B and T cells that express autoreactive antigen receptors are deleted from the immune repertoire. The deletion relies on the proapoptotic BH3-only protein BIM. Moreover, killing of mature, antigen-activated B and T cells during shutdown of immune responses is mediated by both BIM and the death receptor FAS. Apoptosis of intestinal epithelial cells and basal keratinocytes in graft-versus-host disease is a functionally related phenomenon. In type 1 diabetes, the loss of beta cells from the pancreatic islets is probably mediated by the FAS death receptor; CD8 T cells expressing FAS ligand interact with FAS receptors on the insulin-secreting cells to induce death.
_
Autoimmune lymphoproliferative syndrome (ALPS):
The immune response to a foreign invader involves the proliferation of lymphocytes — T and/or B cells. When their job is done, they must be removed leaving only a small population of memory cells. This is done by apoptosis. Very rarely humans are encountered with genetic defects in apoptosis. The most common one is a mutation in the gene for Fas, but mutations in the gene for FasL or even one of the caspases are occasionally seen. In all cases, the genetic problem produces autoimmune lymphoproliferative syndrome or ALPS. The autoimmune lymphoproliferative syndrome (ALPS), which is typified by massive lymphadenopathy, hypersplenism, and autoimmune cytopenias, develops in patients with a defect in the FAS ligand or receptor. ALPS occur when there is insufficient apoptosis of auto-aggressive T cells, resulting in multiple autoimmune diseases. An overproliferation of B cells occurs as well, resulting in excess immunoglobulin production, leading to autoimmunity. Some of the common diseases of ALPS include hemolytic anemia, immune-mediated thrombocytopenia, and autoimmune neutropenia. The different types of this condition are caused by different mutations. Type 1A results from a mutation in the death domain of the Fas receptor, Type 1B results from a mutation in Fas ligand and Type 2 results from a mutation in caspase-10, reducing its activity.
_
T cells and apoptosis:
Immune cells, and especially T cells, can’t work properly if they die too quickly – you want them to live and survive for long periods of time to be completely functional, in order to provide protection against whatever your immune system is reacting. Part of what we do is to understand how proteins that are expressed on the surface of T cells affect the cell’s ability to live, to survive, and not to undergo apoptosis, which is the process of cell death. Apoptosis in one form is programmed cell death that is a natural part of the cell’s lifecycle and is often triggered once the cell has performed its primary function, for example in protecting the body from a threat. Apoptosis can also be a regulated activity that is promoted by protein receptors on cells that directly induce death of a cell containing pathogens or a cancer cell. T cells normally have a short lifespan. There are processes in place that are required to limit the lifespan of a T cell — negative suppressive influences as well as positive influences. It would be unfavorable for these cells to be stimulated to respond to something and never die. The key lies in the balance between cell death and cell lifespan, and both are controlled by different proteins expressed on the surface of the cell. The molecules that signal a T cells’ availability to respond also can determine how long the cell should live and whether it will die. In cancer, the logical goal is for the T cell to live longer, and in greater numbers than normal, so it can attack the tumor cell. In autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, or in asthma, where the cells create an inappropriate or overactive immune response, the ideal is for the cell to respond more slowly or not at all. In infectious disease, understanding apoptosis is key in the creation of vaccines that target viruses and bacteria, from Dengue virus to Cytomegalovirus (CMV), while leaving the body’s immune cells to flourish. The implications for understanding apoptosis and cell death may even lead to cures for AIDS and Type 1 Diabetes.
_
Integration of interferon-α/β signaling to p53 responses in tumour suppression and antiviral defense:
Swift elimination of undesirable cells is an important feature in tumour suppression and immunity. The tumour suppressor p53 and interferon-α and -β (IFN-α/β) are essential for the induction of apoptosis in cancerous cells and in antiviral immune responses, respectively, but little is known about their interrelationship. Researchers show that transcription of the p53 gene is induced by IFN-α/β, accompanied by an increase in p53 protein level. IFN-α/β signaling itself does not activate p53; rather, it contributes to boosting p53 responses to stress signals. They show examples in which p53 gene induction by IFN-α/β contributes to tumour suppression. Furthermore, they show that p53 is activated in virally infected cells to evoke an apoptotic response and that p53 is critical for antiviral defense of the host. Their study reveals a hitherto unrecognized link between p53 and IFN-α/β in tumour suppression and antiviral immunity, which may have therapeutic implications. The regulation of the tumour suppressor p53 has been extensively studied at the protein level; however, little is known about the transcriptional regulation of the p53 gene. In this study, researchers have shown that the p53 gene is transcriptionally induced by IFN-α/β through ISGF3 activation, demonstrating p53 gene induction by a cytokine. Whereas IFN-α/β induces p53 mRNA and increases its protein level, p53-mediated responses such as cell cycle arrest or apoptosis were not observed in cells treated with IFN-α/β alone. Although seemingly futile, this induction could therefore be viewed as a regulated ‘trade-off’ to provide the cell with a greater dynamic range in its response to a variety of stress signals. This notion is supported by the present findings that cells treated with IFN-β evoke more robust p53 responses than do untreated cells. Notably, induction of the p53 mRNA by IFN-β is relatively weak compared with that of the mRNAs of conventional IFN-inducible genes. It is likely that the low affinity of the p53-ISREs to IFN-activated ISGF3 accounts for the weak induction. In view of the fact that p53 is an extremely efficient inhibitor of cell growth, the modest induction of p53 by IFNs may help to avoid adverse p53 actions in normal growing cells. There are numerous reports on the usefulness of IFN-α/β for the treatment of some types of human cancer, including HPV-associated cervical cancer and hepatic cancer. However, the molecular basis of IFN action in cancer treatment still remains largely elusive. This study suggests that one mechanism of the anti-tumour action of IFN may involve p53 induction. In this regard, it is interesting that IFNAR1-/- MEFs undergo spontaneous transformation in vitro and that the mutant mice develop papilloma of the skin at a high incidence (about eightfold relative to wild-type mice) when treated with chemical carcinogens (A.T. and N. Tanaka). In addition, this study suggests the possible usefulness of treating human cancers with IFN-α/β in combination with chemotherapeutic drugs that activate p53. Combined therapy with IFN-α/β and chemotherapeutic drugs such as 5-FU may permit the use of lower doses of chemotherapeutic agents, which would otherwise exert toxic side effects by p53-independent mechanisms. This will be a clinically interesting issue to address further. Their results reveal an important role of p53 in the complex innate antiviral host defences. Prompt induction of apoptosis of virus-infected cells via p53 activation will be beneficial to the host in limiting virus replication: virus production would be suppressed if the infected cells undergo rapid apoptosis before the onset of cellular lysis. Apoptotic cells would be swiftly engulfed by phagocytic cells in vivo and this event would also contribute to inhibiting the spread of virus. This p53 response is not absolutely dependent on but could be enhanced by IFN-α/β. Interestingly, in the absence of p53, IFN-treated cells are more resistant to apoptosis on infection with VSV or encephalomyocarditis virus (EMCV), and the total VSV virus yield becomes higher in p53-/- MEFs than in wild-type MEFs when these cells were treated by IFN-β followed by the infection. On the basis of these findings, one may infer the following events: at the early phase of virus infection, virus-infected cells produce IFN-α/β and eventually undergo p53-dependent apoptosis. On the other hand, virus-induced IFN-α/β may act on the surrounding, uninfected cells to help antiviral defenses by inducing cellular genes that inhibit virus replication and, in addition, by inducing p53 to prime cells for enhanced apoptosis. This study reveals a hitherto unrecognized cooperation between p53 and IFN-α/β, providing a new link between tumour suppression and antiviral host defense.
_
Apoptosis and Organ Transplants:
For many years it has been known that certain parts of the body such as
- the anterior chamber of the eye
- the testes
are “immunologically privileged sites”. Antigens within these sites fail to elicit an immune response. It turns out that cells in these sites differ from the other cells of the body in that they express high levels of FasL at all times. Thus antigen-reactive T cells, which express Fas, would be killed when they enter these sites. [Exactly opposite of how killer T lymphocyte activates caspase mediated apoptosis in target cell]
This finding raises the possibility of a new way of preventing graft rejection. If at least some of the cells on a transplanted kidney, liver, heart, etc. could be made to express high levels of FasL, that might protect the graft from attack by the T cells of the host’s cell-mediated immune system. If so, then the present need for treatment with immunosuppressive drugs for the rest of the transplant recipient’s life would be reduced or eliminated. So far, the results in animal experiments have been mixed. Allografts engineered to express FasL have shown increased survival for kidneys but not for hearts or islets of Langerhans.
____________
Apoptosis and liver:
Alcoholic liver disease:
Heavy alcohol consumption over long periods of time can result in severe liver damage, including death of liver cells (i.e., hepatocytes). Two mechanisms—apoptosis and necrosis—can contribute to hepatocyte death. In apoptosis, the affected cell actively participates in the cell death process, whereas in necrosis the cell death occurs in response to adverse conditions in the cell’s environment. Numerous factors that may contribute to the initiation of hepatocyte apoptosis are affected by alcohol consumption. These factors include the enzyme cytochrome P450 2E1 (i.e., CYP2E1), small molecules (i.e., cytokines) involved in cell communication, oxidative stress, and changes in iron metabolism. Similarly, alcohol consumption can influence several factors believed to be involved in hepatocyte necrosis, including depletion of the energy-storing molecule adenosine-triphosphate, reduced oxygen levels (i.e., hypoxia) in the liver, oxidativestress, and bacterial molecules called endotoxins.
_
Hepatitis:
Hepatocytes are particularly prone to apoptosis in response to various types of stress, including infections. A trial of a potent caspase inhibitor (IDN-6556) in patients with chronic hepatitis C showed that the drug caused a highly significant lowering of serum alanine aminotransferase and aspartate aminotransferase levels in patients with chronic hepatitis C. IDN-6556 is also being evaluated for its ability to reduce ischemia–reperfusion injury after liver transplantation.
_
Liver Cancer due to Chronic Inflammation:
Liver cancer (Hepatocellular Carcinoma, HCC) usually arises as the result of a chronic, inflammatory liver disease. The most common causes here are excessive alcohol consumption as well as a high-fat diet and also chronic infection with the hepatitis viruses B and C. In the course of the inflammatory process, the liver cells (hepatocytes) die more frequently due to programmed cell death. The result is increased cell growth, also referred to as compensatory proliferation, which can lead to tumour development.
_______________
Cardiovascular Diseases and apoptosis:
Programmed necrosis of cardiac myocytes is critical in the pathogenesis of myocardial infarction resulting from ischemia/reperfusion and may also play a role in heart failure. Accordingly, there is a strong rationale for seeking pharmacological approaches to inhibit necrosis in heart disease. Researchers consider the merits of various strategies to inhibit necrosis and discuss some currently available drugs. A key issue for any anti–cell death strategy is whether to target proximal versus distal pathways. An advantage of distal inhibition is that it may avoid the redundancy resulting from multiple proximal inputs. This advantage comes at a price, however, in that distal blockade may not rescue key organelles. For example, caspase inhibitors often fail to preserve mitochondrial function in apoptotic cells. In the case of necrosis, the organelles that need protection are the mitochondria, which sustain considerably more damage than in apoptosis, and the various cell membranes (plasma, ER, and lysosomal). Thus, they suspect that inhibition of proximal pathways will be needed to preserve mitochondrial and membrane function, both of which are critical for cell viability.
Several types of small molecules inhibit proximal events:
(1) nec-1, which antagonizes the kinase activity of RIP1, blocks the death receptor necrosis pathway;
(2) PARP inhibitors; and
(3) cyclosporine A (and related compounds), which binds cyclophilin D and inhibits its interactions or function, thereby decreasing the likelihood of MPTP opening.
Nec-1 appears to reduce infarct size markedly, although these data come from a single study with a small number of animals. In contrast, substantial data support the notion that cyclosporine A can reduce infarct size in various animal models and perhaps humans. The reported overlap between the effects of nec-1 and cyclophilin D absence on infarct size reduction suggest that there would be little benefit to combining nec-1 with cyclosporine A. However, the effects of combinations of these drugs have been studied in relatively few animals and at only a single time point. There are likely to be some actions of nec-1 that do not overlap with those of cyclosporine A. For these reasons, it will be important to re-evaluate necrosis inhibition by nec-1 and cyclosporine A, alone and in combination. Even if proximal inhibition proves most critical, the addition of distal inhibitors may be advantageous. Examples of compounds that may work through distal inhibition include calpain antagonists and polymers that seal plasma membranes.
_
Necrosis predominates in ischemic injury, but often there are apoptotic cells in the hypoxic penumbra in myocardial infarction and stroke and in globally hypoxic zones after reperfusion injury. When there is hypoxia-induced premature activation of the apoptotic program, the inhibition of apoptosis (e.g., by caspase inhibitors) might prevent cell loss. Cyclosporine, which inhibits apoptosis by blocking mitochondrial permeability-transition pores, can decrease the infarct size in patients with acute myocardial infarction. In a pilot trial, 58 patients with acute myocardial infarction received an intravenous bolus of either cyclosporine or saline immediately before undergoing percutaneous coronary intervention. On day 5, the absolute mass of the area of infarcted tissue on magnetic resonance imaging was significantly reduced in the cyclosporine group, as compared with the control group. Patients with acute stroke who were treated within 24 hours with minocycline, an antiapoptotic compound with multiple actions, had superior neurologic outcomes, as compared with patients who were given placebo.
_
The Switch: Apoptosis or Oncosis (Necrosis)?
After prolonged myocardial ischemia, a critical depletion of energy levels leads to the loss of ionic homeostasis, with cell swelling, rupture and necrotic death. As programmed cell death (PCD) or apoptosis is energy-dependent, the switch in the decision between oncosis and apoptosis depends on ATP concentrations. Severe ischemia results in significant reduction and final depletion of adenine nucleotides accompanied by cellular injury. ATP loss of 70% as usually present in myocardial infarction is incompatible with apoptosis and causes oncosis (necrosis). In several models of myocardial ischemia and reperfusion, necrosis and apoptosis can coexist, though it is still unclear exactly what factors determine the ultimate fate of a cardiomyocyte. It has been proposed that myocardial segments exposed to the most severe areas of ischemia and reperfusion undergo necrosis, whereas cells on the periphery undergo apoptosis (Gottlieb et al., 1994; Fliss and Gattinger, 1996). This is consistent with the notion that cells exposed to severe stimuli undergo necrosis, whereas cells exposed to less hypoxia become apoptotic and sustain partial recovery.
_
Although necrosis does occur, overexpression of BAX has been detected in ischemic myocardial tissue and therapy aimed at reducing apoptosis has shown some success in reducing the degree of tissue damage (Hochhauser et al., 2003). One hypothesis is that the damage produced by ischemia is capable of initiating apoptosis but if ischemia is prolonged, necrosis occurs. If energy production is restored, as with reperfusion, the apoptotic cascade that was initiated by ischemia may proceed (Freude et al., 2000). Although the extent to which apoptosis is involved in myocardial ischemia remains to be clarified, there is clear evidence that supports a role for this mode of cell death.
_
Apoptosis: Mitochondria and Cardiomyocytes:
More recently, mitochondria have been shown to be involved with apoptosis and mitochondria have been implicated in the generation of reactive oxygen species (ROS) during ischemia and apoptosis. Due to the fact that cardiomyocytes (CM) contain the highest volume density of all mammalian cells, (25% in human cells and 37% in mice) these quantitative differences significantly influence the role that mitochondria play in CM cell death (Elsasser et al., 2000). Moreover, ATP production is needed to fuel PCD and apoptosis, and the beating heart has high energy requirements than other organs. Thus the sensitivity of apoptosis in CM may be higher in CM than other cells due to these cells having the highest mitochondrial volume (Jugdutt et al., 2005). As mentioned, cardiomyocytes showed DNA fragmentation as early as 12 hours, whereas non-myocyte fibroblasts did not show fragmentation of DNA even up to 72 hours with Fas stimulation. (Tanaka et al., 1994). Also, as demonstrated in an in vivo study, cardiac cells have a significantly lower sensitivity to apoptotic stimulation than liver cells of adult rat hearts, although the rate of clearance of cardiomyocytes was similar to that of apoptotic hepatocytes. (Hayakawa et al., 2003).
___________
Neurologic Diseases and apoptosis:
There is growing evidence that neuronal apoptosis plays a key role in neonatal brain disorders. Developing neurons are particularly susceptible to apoptosis in response to noxious stimuli during the period of synaptogenesis. In neonatal hypoxic brain injury, the cell-death phenotype changes over time from early necrosis to apoptosis, an evolution that has been termed the “necrosis–apoptosis” continuum. There is evidence that apoptosis is a more important mechanism of neonatal brain injury than necrosis. The fetal alcohol syndrome is due to apoptotic neurodegeneration that results from ethanol-induced blockade of the N-methyl-d-aspartate (NMDA) receptor and activation of the -aminobutyric acid (GABA) receptor. General anesthetics also modulate NMDA and GABA receptors, and studies in animals showing that general anesthetics induce extensive neuronal apoptosis in neonates have raised considerable concern that the use of general anesthetics in neonates might cause long-term cognitive defects.
_
Neurodegeneration:
- A neurodegenerative disease is one in which the cells of the brain and spinal cord are lost
- The functions of these cells include decision making and control of movements
- These cells are not easily regenerated, so the effects of diseases can be devastating
- Neurodegenerative diseases include Alzheimer’s (AD), Parkinson’s disease (PD) and Huntington’s disease (chorea) (HD).
When a virus hijacks a brain cell it leads to a build-up of viral proteins. Cells respond by shutting down nearly all protein production in order to halt the virus’s spread. Similarly many neurodegenerative diseases involve the production of faulty or “misfolded” proteins. These activate the same defenses, but with more severe consequences. The misfolded proteins linger and the brain cells shut down protein production for so long that they eventually starve themselves to death. This process, repeated in neurons throughout the brain, can destroy movement or memory or even kill, depending on the disease. This process is thought to take place in many forms of neurodegeneration, so safely disrupting it could treat a wide range of diseases. The researchers used a compound which prevented those defense mechanisms kicking in and in turn halted neurodegeneration.
_
The death of brain cells caused by diseases like Alzheimer’s is the result of a natural defense mechanism called the unfolded protein response, or UPR. Alzheimer’s disease is a neurodegenerative condition that is thought to be caused by mutations in certain proteins such as APP (amyloid precursor protein) and presenilins. Presenilins are thought to be involved in the processing of APP to amyloid β. This condition is associated with the deposition of amyloid β in extracellular deposits known as plaques and amyloid β is thought to be neurotoxic when found in aggregated plaque form. Amyloid β is thought to induce apoptosis by causing oxidative stress or by triggering increased Fas ligand expressions in neurons and glia. It may also activate microglia, which would result in TNFα secretion and activation of the TNF-R1, leading to apoptosis (Ethell and Buhler, 2003). Having recognized the build-up of rogue, unfolded proteins in the brain – a first stage of many such neurodegenerative illnesses – the organ shuts down protein production to stop the problem from spreading. However, the UPR can only halt all production of proteins, including those necessary over time to keep neurons alive and synapses functioning. The researchers found that the drug, made by British firm GlaxoSmithKline and labeled GSK2606414, was able to keep protein production functioning in the brain by inhibiting a key enzyme that facilitates this process. In humans, however, inhibiting the PERK enzyme can lead to side-effects including weight loss and diabetes. It is also important to note that this study was carried out on mice with prion disease and so it is not clear how applicable it is to humans with diseases such as Alzheimer’s.
_
Targeting programmed cell death in neurodegenerative disease:
Neuronal loss is a relatively late event in neurodegenerative diseases, following neuronal dysfunction, synapse loss and, often, somal atrophy. But targeting PCD has been successful in at least some model systems of neurodegeneration, and it is possible that targeting multiple PCD pathways will be even more effective. Caspase inhibition in vivo retarded the degeneration in transgenic mouse models of both ALS and HD, despite the fact that the morphological description of neuronal cell death in these diseases is not compatible with apoptosis. Bcl-2 expression in a transgenic model of ALS delayed symptom onset and increased lifespan, but did not alter the disease duration. Minocycline, a second-generation tetracycline that inhibits mitochondrial cytochrome c release, effected neuroprotection in mouse models of HD, PD and ALS. Minocycline is orally bioavailable, penetrates the blood–brain barrier and has been proved safe for use in humans. It is currently being evaluated in clinical trials in patients with HD and ALS. Rasagiline, which has been approved for the treatment of PD, has also been proposed to target apoptosis, but because it is a potent, selective, irreversible inhibitor of monoamine oxidase type B, its therapeutic effect on PD may have nothing to do with effects on apoptosis. Trophic factors have multiple effects, including the inhibition of apoptosis, the stimulation of neural precursors and the stimulation of neurite outgrowth. The literature is rife with examples of trophic-factor therapy for various neurological conditions, and although many have been unsuccessful, the delivery of the correct factor(s) to the right target in the correct concentration for a particular disease still holds great promise (although hyperactivation of at least some trophic-factor receptors may induce PCD). A recent study of fibroblast growth factor 2 (FGF2) in a mouse model of HD prolonged survival, improved motor performance and reduced polyglutamine aggregates. A number of approaches have been taken to deliver nerve growth factor (NGF) to patients with AD, the most recent being by means of genetically engineered fibroblasts in a phase I trial. In this study, improvements in cognition and positron-emission tomography (used to monitor fluorodeoxyglucose uptake) were documented. Glial-derived neurotrophic factor (GDNF) has shown promise in the treatment of PD. HD may represent one of the best possibilities for trophic-factor therapy: presymptomatic diagnosis is readily available, and although a number of different trophic factors have shown promise in animal models (for example, FGF2, NGF and ciliary neurotrophic factor), brain-derived neurotrophic factor (BDNF) is both reduced in the disease and therapeutic in models. Furthermore, cysteamine has been shown to increase BDNF levels in brain.
_
Mutated gene causes nerve cell death:
Researchers identify new mechanism in the onset of incurable nerve disease Stephen Hawking, a British astrophysicist, is likely to be the world’s most famous person living with amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease. ALS is a progressive disease affecting motor neurons, nerve cells that control muscle function, and nearly always leads to death. Researchers at the Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA) in Vienna have now identified a completely new mechanism in the onset of motor neuron diseases. Their findings could be the basis for future treatments for these presently incurable diseases. The IMBA scientists, working with an international team of researchers under the leadership of Josef Penninger and Javier Martinez, discovered a completely new fundamental mechanism that triggers the death of motor neurons. Motor neurons are nerve cells responsible for stimulating muscles. The loss of these motor neurons in mice with a genetic mutation in a gene named CLP1 leads to severe and progressive muscular paralysis and, in some cases, to death. They have developed model mice in which the function of CLP1 was genetically inactivated. To their utter surprise they discovered that deactivating CLP1 increases the sensitivity of cell to die when exposed to oxidative stress. That leads to enhanced activity of the p53 protein and then to the permanent destruction of motor neurons. By deactivating CLP1, they have discovered a previously unknown new species of RNA. The accumulation of this RNA is a consequence of increased oxidative stress in the cell. Researchers see this as one of the triggers for the loss of motor neurons that occurs in ALS and other neuromuscular diseases. Nearly all genetic mutations found in ALS patients affect either RNA metabolism or oxidative stress, suggesting a possibly unifying principle for these diseases. Removing p53 saves mice with CLP1 mutations from certain death. If scientists are successful in applying these findings to people, the researchers may have discovered a treatment approach to cure ALS and similar diseases.
___________
Apoptosis in physiological and pathological skin: implications for therapy:
Acanthosis, the hallmark of psoriatic skin, is an example for diminished epidermal apoptosis. Defects in termination of inflammatory reactions occur in atopic dermatitis. Lupus erythematosus may arise due to disturbed apoptosis on several check points of the apoptosis cascade. Experimental evidence suggests a role for Bcl-2 and CD95L in the inhibition of programmed cell death in UV-induced skin cancer or malignant melanoma cells. Thus, it leads to survival of malignant cell clones. The slow growth of basal cell carcinomas is due to an increased apoptosis to mitosis ratio. Spontaneous regression of tumors is associated with increased apoptotic rates. Malignant melanoma cells characteristically show different anti-apoptotic strategies which underscore its aggressive behavior and its refractory towards classic therapeutic regimens. Additionally, induction of apoptosis in tumor infiltrating immune cells seems to be a strategy by which the tumor escapes from an immunological attack (tumor counter-attack). Since apoptosis is either absent or altered under pathological conditions therapeutic procedures should correct this. Established therapies like dithranol, vitanin-D3 analogs, low-dose methotrexate, induce apoptosis. Future treatment regimens like vaccine and gene therapy are designed to selectively induce apoptosis. Therefore, pharmacological agents and therapeutic strategies interfering with disrupted apoptosis regulation could improve the therapeutic arsenal in the future.
_
Programmed Cell Death in Retinal Degeneration: Targeting Apoptosis in Photoreceptors as Potential Therapy for Retinal Degeneration:
Retinal degenerations are the major cause of incurable blindness characterized by loss of retinal photoreceptor cells. Several genes causing these genetic diseases have been identified, however the molecular characterization of a high percentage of patients affected by retinitis pigmentosa (RP), a common form of retinal degeneration, is still unknown. The high genetic heterogeneity of these diseases hampers the comprehension of the pathogenetic mechanism causing photoreceptor cell death. Therapies are not available yet and for this reason there is a lot of interest in understanding the etiology and the pathogenesis of these disorders at a cellular and molecular level. Some common features have been identified in different forms of RP. Apoptosis was reported to be the final outcome in all RP animal models and patients analyzed so far. Researchers recently identified two apoptotic pathways co-activated in photoreceptors undergoing cell death in the retinal degeneration (rd1) mouse model of autosomal recessive RP. Their studies opened new perspectives together with many questions that require deeper analyses in order to take advantage of this knowledge and develop new therapeutic approaches. They believe that minimizing cell demise may represent a promising curing strategy that needs to be exploited for retinal degeneration.
_
Diabetes: Cell Death and KIRAs (Kinase Inhibitory RNAse Attenuators):
Type 1 and type 2 diabetes are characterized by progressive β-cell failure. Apoptosis is probably the main form of β-cell death in both forms of the disease. It has been suggested that the mechanisms leading to nutrient- and cytokine-induced β-cell death in type 2 and type 1 diabetes, respectively, share the activation of a final common pathway involving interleukin (IL)-1β, nuclear factor (NF)-κB, and Fas. When insulin-secreting beta cells of the pancreas die, the resultant loss of control over blood sugar wreaks havoc if left untreated. However, why these cells die is perhaps the biggest unanswered question in the field of diabetes, according to UCSF researcher Feroz Papa, MD, PhD. Papa, winner of a New Innovator Award from the National Institutes of Health, investigates whether beta cells die when they become stressed in trying to make large amounts of insulin to meet the body’s increasing demands. This particular type of stress is called ER stress. In the journal Cell, Papa reports that a specialized form of a type of drug known as a kinase inhibitor may prolong survival among cells experiencing ER stress. It may one day become possible to use a member of this class of drugs to forestall cell death in diseases such as type 2 diabetes, he says. Papa calls the newly identified class of drugs KIRAs. No diabetes drugs to date have had a direct impact on cell survival. “This paper describes how a cellular protein called IRE1 controls a life-or-death decision in cells experiencing ER stress,” Papa says. He describes IRE1 as a switch. “IRE1 helps cells survive under ER stress, but only up to a point,” Papa says. “If the stress continues, IRE1 flips into executioner mode and polishes off the cell.” To try to stop cell death due to ER stress, it makes sense to target IRE1. “We identified a class of drugs that targets IRE1,” Papa says. “We call these drugs KIRAs, for Kinase Inhibitory RNAse Attenuators. KIRAs may protect cells by reducing death signals emanating from IRE1. We hope that it may be possible someday to develop KIRAs for use in humans in order to slow progression of ER stress-related diseases such as diabetes.” “Diabetes is a growing health problem in the world and we urgently need new medicines to reverse the disease process,” said acting NIH director Raynard Kington, MD, PhD. “Dr. Papa’s discovery opens up promising new approaches for saving crucial insulin-producing cells. This is exactly the type of research that the New Innovator Program was designed to foster.” The ER, or endoplasmic reticulum, acts within cells as a protein-folding factory. Like other proteins, insulin needs to fold into its proper shape within the ER. When the amount of protein that needs folding becomes excessive, protein molecules tend to fold incorrectly, causing ER stress. Beta cells are especially prone to ER stress because the normal level of insulin traffic going through the protein-folding factory is high. Becoming insulin-resistant causes even more insulin traffic, increasing the risk of ER stress and cell death. The protective side of IRE1’s response to ER stress was understood previously. IRE1 increases the capacity of the ER’s protein-folding factory by making another protein, called XBP1. By producing XBP1, the ER has a better chance to eliminate the backlog of unfolded proteins, so cells can recover and once again perform their protein-folding jobs accurately. If the cellular strategy of producing XBP1 fails to restore cells to their pre-stressed state, then IRE1 switches tactics, and instead helps the dysfunctional cells commit suicide. Working with insulin-secreting beta cells, Papa determined that IRE1 destroys the messenger RNAs that produce insulin and enzymes needed to fold insulin into its proper shape in the ER. This “kill-the-messenger” strategy, which was first discovered in fly cells by UCSF Professor Jonathan Weissman, PhD, is conserved in mammalian cells and ensures that highly stressed cells cannot recover. “IRE-mediated, messenger RNA decay appears to be strictly required for IRE1’s ability to induce cell death,” Papa says. “Mammals appear to have evolved a strategy to discard ER-stressed cells by forcing them to commit suicide. This is a very stringent, but ultimately a harsh form of quality control. It is conceivable that if the process gets out of hand, it could lead to disease.” Papa discovered that IRE1’s protective and destructive impulses were both triggered by the same enzymatic part of the protein, called an RNAse, acting in two different ways. Using KIRAs, Papa reports that he was able to force the RNAse to activate the protective response while turning off the suicidal response. Papa hopes that such an approach can eventually be used to keep insulin-secreting cells alive in individuals who are insulin-resistant, and help keep their blood sugar under control.
_
Endothelial Cell Aging and Apoptosis in Prevention and Disease: E-Selectin Expression and Modulation as a Model:
Endothelial cell senescence and apoptosis are features of numerous human pathologies including atherosclerosis, allograft vasculopathy, heart failure, diabetic retinopathy and scleroderma. In contrast, endothelial cell activation and replication associated with vessel proliferation and angiogenesis are now therapeutic targets in other diseases such as cancer and macular dystrophy. Finally, preventive medicine, in particular cardiovascular and cancer chemoprevention, commonly involve the endothelium. Here researchers discuss several aspects of the interplay between endothelial cell aging, apoptosis and senescence. Further, they show novel microarray data on endothelial cells “aged” in culture, and note that many genes regulated by the aging process are also modulated by a chemopreventive anti-angiogenic and anti-apoptotic drug, N-acetyl-cysteine (NAC). Focusing on one of these genes, the leukocyte adhesion protein E-selectin, they show that E-selectin is down-modulated with time in culture and upon treatment with NAC at mRNA and protein levels. This correlates with reduced adhesion of breast cancer cells and NF-kB activation in NAC treated endothelial cells. These data underscore the effects of a chemoprevention agent in modulating parameters associated with endothelial cell aging.
_
Researchers find way to make unlimited amounts of red blood cells from iPS cells:
Japanese scientists have developed a technique using induced pluripotent stem (iPS) cells to engineer red blood cells in sufficient amounts to make shortages of blood for transfusions a thing of the past. Researchers from Kyoto University’s Center for iPS Cell Research and Application (CiRA) and other institutions transduced c-MYC and BCL-XL genes, which are involved in cell proliferation and help prevent cell death or apoptosis, respectively, into iPS cells. The iPS cells then turned into erythrocyte progenitor cells. The team led by Koji Eto, a stem cell biology professor at CiRA, was able to inhibit specific functions in the two genes, which turned the progenitor cells into mature erythrocytes–or red blood cells. The erythrocyte progenitor cells have an almost unlimited ability to replicate in vitro, allowing researchers to produce almost infinite red blood cells from limited amounts of blood samples. According to the researchers, the new technology produces 20 times the number of red blood cells compared with an existing method using the same amount of culture fluid.
_
Oxidative Stress, Cell Death, and Other Damage to Alveolar Epithelial Cells Induced by Cigarette Smoke:
Cigarette smoking is a major risk factor in the development of various lung diseases, including pulmonary emphysema, pulmonary fibrosis, and lung cancer. The mechanisms of these diseases include alterations in alveolar epithelial cells, which are essential in the maintenance of normal alveolar architecture and function. Following cigarette smoking, alterations in alveolar epithelial cells induce an increase in epithelial permeability, a decrease in surfactant production, the inappropriate production of inflammatory cytokines and growth factors, and an increased risk of lung cancer. However, the most deleterious effect of cigarette smoke on alveolar epithelial cells is cell death, i.e., either apoptosis or necrosis depending on the magnitude of cigarette smoke exposure. Cell death induced by cigarette smoke exposure can largely be accounted for by an enhancement in oxidative stress. In fact, cigarette smoke contains and generates many reactive oxygen species that damage alveolar epithelial cells. Whether apoptosis and/or necrosis in alveolar epithelial cells is enhanced in healthy cigarette smokers is presently unclear. However, recent evidence indicates that the apoptosis of alveolar epithelial cells and alveolar endothelial cells is involved in the pathogenesis of pulmonary emphysema, an important cigarette smoke-induced lung disease characterized by the loss of alveolar structures.
_____________
Clinical Implications of autophagy:
_
The figure below shows connections between autophagy and various diseases:
_
Although autophagy is a survival mechanism that provides the cell with alternative sources of substrates when nutrients are limited, it could also protect the cell by eliminating damaged mitochondria (which can trigger apoptosis by generating excess reactive oxygen species) or toxic misfolded proteins, including those believed to induce neurodegeneration. Drugs that activate autophagy can clear toxic protein aggregates, such as mutant huntingtin protein and mutant tau in models of neurologic disease. Rapamycin analogues (which induce autophagy by inhibiting mTOR) decrease polyglutamine proteins and are effective in animal models of Huntington’s disease; they are also being examined in acute brain injury.
_
_
Autophagy and Cancer:
Autophagy has a complex role in cancer. Although current data are only associative, autophagy presumably functions as a suppressor of neoplasia. Many oncogenes (including PI3K and AKT family members, BCL2, and MTOR) suppress autophagy, whereas tumor suppressors (PTEN, TSC2, and HIF1A) promote autophagy. In addition, the loss of individual autophagy-related genes (especially Beclin1, UVRAG, and BIF1 [ZBTB24]) results in lymphomas and gastrointestinal tumors in mouse models, and these same genes are frequently mutated in human cancers, including bowel and hepatocellular carcinomas. Thus, it is seemingly paradoxical that hydroxychloroquine, an antimalarial drug that blocks autophagy by raising intralysosomal pH, is under evaluation in cancer trials. In patients undergoing chemotherapy, autophagy can promote resistance to cell death, especially to DNA-damaging agents, and hydroxychloroquine blocks this cellular adaptive response, which results in increased tumor killing. Mutations in oncogenes and oncosuppressor genes dysregulate autophagy. Up-regulated autophagy may confer chemo- and radio-resistance to cancer cells, and also a pro-survival advantage in cancer cells experiencing oxygen and nutrient shortage. This fact is the rationale for using autophagy inhibitors along with anti-neoplastic therapies. Yet, aberrant hyper-induction of autophagy can lead to cell death, and this phenomenon could also be exploited for cancer therapy.
_
Autophagy, immunity and infections:
Over the past several years, accumulating evidence has suggested that autophagy can function as an intracellular innate defense pathway in response to infection with a variety of bacteria and viruses. Autophagy plays a role as a specialized immunologic effector and regulates innate immunity to exert antimicrobial defense mechanisms. Numerous bacterial pathogens have developed the ability to invade host cells or to subvert host autophagy to establish a persistent infection.
_
The figure above shows the role of autophagy in control of innate immunity and the survival mechanisms of intracellular bacteria. Various intracellular bacteria are internalized by phagocytes; these survive in the cytosol or phagosome through various strategies. First, some bacteria are able to escape from the phagosome by secreting factors and replicating in the host cytoplasm (left panel). When autophagy is induced, Listeria monocytogenes and Rickettsia conorii present in the cytoplasm are captured by the autophagosomal membrane and degraded by the autolysosome; however, Shigella flexneri has the ability to avoid import into the autophagosome. Second, some bacteria reside in the phagosomal compartment (right panel). Upon autophagy activation, phagosomes containing Mycobacterium tuberculosis are caught by the autophagic vacuole and then degraded by fusion with the lysosome. In contrast, Brucella abortus andLegionella pneumophila can inhibit autophagosome maturation through inhibition of lysosome fusion, allowing the pathogen to survive and replicate.
_
Subversion of Cellular Autophagosomal Machinery by RNA Viruses:
Infection of human cells with poliovirus induces the proliferation of double-membraned cytoplasmic vesicles whose surfaces are used as the sites of viral RNA replication and whose origin is unknown. Researchers show that several hallmarks of cellular autophagosomes can be identified in poliovirus-induced vesicles, including colocalization of LAMP1 and LC3, the human homolog of Saccharomyces cerevisiae Atg8p, and staining with the fluorophore monodansylcadaverine followed by fixation. Colocalization of LC3 and LAMP1 was observed early in the poliovirus replicative cycle, in cells infected with rhinoviruses 2 and 14, and in cells that express poliovirus proteins 2BC and 3A, known to be sufficient to induce double-membraned vesicles. Stimulation of autophagy increased poliovirus yield, and inhibition of the autophagosomal pathway by 3-methyladenine or by RNA interference against mRNAs that encode two different proteins known to be required for autophagy decreased poliovirus yield. Researchers propose that, for poliovirus and rhinovirus, components of the cellular machinery of autophagosome formation are subverted to promote viral replication. Although autophagy can serve in the innate immune response to microorganisms, their findings are inconsistent with a role for the induced autophagosome-like structures in clearance of poliovirus. Instead, they argue that these double-membraned structures provide membranous supports for viral RNA replication complexes, possibly enabling the nonlytic release of cytoplasmic contents, including progeny virions, from infected cells.
_
Cell death and autophagy in prion diseases (transmissible spongiform encephalopathies):
Neuronal autophagy, like apoptosis, is one of the mechanisms of programmed cell death. In a review, authors summarize current information about autophagy in naturally occurring and experimentally induced scrapie, Creutzfeldt-Jakob disease and Gerstmann-Sträussler-Scheinker syndrome against the broad background of neural degenerations in transmissible spongiform encephalopathies (TSEs). Typically a sequence of events is observed: from a part of the neuronal cytoplasm sequestrated by concentric arrays of double membranes (phagophores); through the enclosure of the cytoplasm and membrane proliferation; to a final transformation of the large area of the cytoplasm into a collection of autophagic vacuoles of different sizes. These autophagic vacuoles form not only in neuronal perikarya but also in neurites and synapses. On the basis of ultrastructural studies, authors suggest that autophagy may play a major role in transmissible spongiform encephalopathies and may even participate in the formation of spongiform change.
_
Autophagy regulates TNFα-mediated joint destruction in experimental arthritis:
Autophagy was activated in osteoclasts of human rheumatoid arthritis (RA) showing increased expression of Beclin1 and Atg7. TNFα potently induced the expression of autophagy-related genes and activated autophagy in vitro and in vivo. Activation of autophagy by overexpression of Beclin1-induced osteoclastogenesis and enhanced the resorptive capacity of cultured osteoclasts, whereas pharmacologic or genetic inactivation of autophagy prevented osteoclast differentiation. Arthritic hTNFα tg mice transplanted with Atg7fl/fl×LysMCre+ bone marrow cells (BMC) showed reduced numbers of osteoclasts and were protected from TNFα-induced bone erosion, proteoglycan loss and chondrocyte death. These findings demonstrate that autophagy is activated in RA in a TNFα-dependent manner and regulates osteoclast differentiation and bone resorption. Researchers provide evidence for a central role of autophagy in joint destruction in RA.
_
Autophagy in Idiopathic Pulmonary Fibrosis:
Lung tissues from IPF patients demonstrate evidence of decreased autophagic activity as assessed by LC3, p62 protein expression and immunofluorescence, and numbers of autophagosomes. TGF-β1 inhibits autophagy in fibroblasts in vitro at least in part via activation of mTORC1; expression of TIGAR is also increased in response to TGF-β1. In the bleomycin model of pulmonary fibrosis, rapamycin treatment is antifibrotic, and rapamycin also decreases expression of á-smooth muscle actin and fibronectin by fibroblasts in vitro. Inhibition of key regulators of autophagy, LC3 and beclin-1, leads to the opposite effect on fibroblast expression of á-smooth muscle actin and fibronectin. Autophagy is not induced in pulmonary fibrosis despite activation of pathways known to promote autophagy. Impairment of autophagy by TGF-β1 may represent a mechanism for the promotion of fibrogenesis in IPF.
_
Autophagy and schizophrenia:
Prof. Illana Gozes – the Lily and Avraham Gildor Chair for the Investigation of Growth Factors, the director of the Adams Super Center for Brain Studies at the Sackler Faculty of Medicine, and a member of the Sagol School of Neuroscience at Tel Aviv University – has discovered that an important cell-maintenance process called autophagy is reduced in the brains of schizophrenic patients. The findings, published in Nature’s Molecular Psychiatry, advance the understanding of schizophrenia and could enable the development of new diagnostic tests and drug treatments for the disease. They found RNA evidence of decreased levels of the protein beclin 1 in the hippocampus of schizophrenia patients, a brain region central to learning and memory. Beclin 1 is central to initiating autophagy – its deficit suggests that the process is indeed blocked in schizophrenia patients. Developing drugs to boost beclin 1 levels and restart autophagy could offer a new way to treat schizophrenia, the researchers say. Next, the researchers looked at protein levels in the blood of schizophrenia patients. They found no difference in beclin 1 levels, suggesting that the deficit is limited to the hippocampus. But the researchers also found increased levels of another protein, activity-dependent neuroprotective protein (ADNP), discovered by Prof. Gozes and shown to be essential for brain formation and function, in the patients’ white blood cells. Previous studies have shown that ADNP is also deregulated in the brains of schizophrenia patients. The researchers think the body may boost ADNP levels to protect the brain when beclin 1 levels fall and autophagy is derailed. ADNP, then, could potentially serve as a biomarker, allowing schizophrenia to be diagnosed with a simple blood test.
___________
Senescence and PCD:
_
_
Replicative senescence was first described in the context of normal human cells explanted in culture that failed to divide beyond a finite number of population doublings. One mechanism involved in the activation of replicative senescence is the DNA damage response activated by the shortening of telomeres. Telomeres are repetitive DNA sequences that protect the ends of chromosomes. After each cell division, telomeres in human cells progressively shorten because of the erosion of the telomeric repeats. Most human adult cells lack sufficient amounts of the enzyme telomerase that adds the telomeric repeats to chromosome ends. When the telomere shortens beyond a certain limit, a DNA damage response is triggered that results in cell cycle arrest. The DNA damage response triggered by other cellular stresses, like exposure to chemotherapeutic agents or oncogenic or mitogenic signals, also can induce senescence, often termed premature senescence, both in vitro and in vivo. Therefore, it has been proposed that defects in senescence signaling contribute to tumorigenesis. Indeed, the p53 and retinoblastoma (Rb) tumor suppressor genes have been identified as two principal regulators of senescence. Different classes of chemotherapeutic agents and IR induce senescence in human cancer cell lines in vitro and in mouse tumor xenografts. The molecular signals that play critical roles in mediating DNA damage–induced senescence include p53, Rb, p16INK4A, p21, and Bcl-2. Knockout of p53 or p16 eliminates treatment-induced senescence in the Eμ-myc lymphoma mouse model. Senescent cells in culture are large in size, appear flattened, and are often vacuolated.
_
The relationship between apoptosis and aging:
Apoptosis is involved in aging and age-related disease, with respect to aging, apoptosis acting in a cell type- specific manner. The rate of apoptosis is elevated in- most types of aging cell populations and organs. In stable cells and certain continuously dividing cells, apoptosis serves to eliminate presumably dysfunctional cells that show homeostatic failure due to oxidative stress, glycation, and DNA damage, thereby maintaining homeostasis in the body. What’s more, apoptosis, at least in part, plays some important role in the regulation of aging process and anti-tumorigenesis in mammals. Age-enhanced apoptosis may be an innate protective mechanism against age-associated tumorigenesis. Age-related degeneration can be a consequence of a genetic program or it may be an entropic process. Ultimately, disorders in housekeeping ability jeopardize homeostasis and expose cells to apoptotic forms of cell death. An increased incidence of apoptosis has been reported for several tissues during aging, even in the absence of overt aging-related disease. For example, aging-associated atrophy of muscle, called sarcopenia in mammals, is observed in organisms ranging from invertebrates to humans. Detailed analysis of sarcopenia in rodents indicates an apoptotic-like mechanism, characterized by mitochondrial changes and caspase-independence (Marzetti et al., 2009). During aging in Drosophila, apoptotic-like events are observed in both muscle and fat tissue, as indicated by DNA fragmentation assay and caspase activation (Zheng et al., 2005). On the other hand, there is clear evidence in some tissues that senescent cells are remarkably resistant to apoptosis and therefore prone to tumorigenesis.
_
When Lockshin and Zakeri discussed the relevance of apoptosis to aging, the common view was that apoptosis had primarily a negative impact on aging by destroying essential and often irreplaceable cells. That view has now changed to one that acknowledges that there are two general ways in which apoptosis can play a role in aging: (1) elimination of damaged and presumably dysfunctional cells (e.g., fibroblasts, hepatocytes) which can then be replaced by cell proliferation, thereby maintaining homeostasis and (2) elimination of essential postmitotic cells (e.g., neurons) which cannot be replaced, thereby leading to pathology. Evidence exists in two systems (fibroblasts and thymocytes/ lymphocytes) that there are age-related decreases in the potential for apoptosis, although the molecular bases for these decreases appear to differ. Fibroblasts (and neurons?) lose the ability to downregulate bcl-2 in response to an apoptotic signal; thus, apoptosis is blocked even though an initiating signal has been received. In contrast, thymocytes/ lymphocytes lack the ability to initiate the signal due to downregulation of the cell surface receptor Fas. There is limited information available for other tissue types, and nothing is known about why and how these age-related changes occur. An interesting observation, but not necessarily a critical one, is that the frequency of upregulation of the bcl-2 gene due to chromosome translocation increases with age. The role of apoptosis in regulating cell number is also a promising area of research. The studies on liver damage and neoplastic lesions suggest an extremely important role for apoptosis in controlling cancer. This may be particularly important in the prostate, where hypertrophy and cancer are a virtual certainty with ever-increasing age. It is not known whether the ability to undergo apoptosis declines in the prostate with increasing age, but it appears likely that it does. Apoptosis appears to be either “on” or “off” in cells, while the basic cell-killing machinery may often be present, but in an inactive form. The results currently available do suggest that apoptosis is a process which may be important in aging, at least in some tissues, and the mechanism of its regulation needs to be understood. Although a variety of tumor suppressor gene and oncogene products are known to be involved in signal transduction associated with apoptosis, it remains to be shown which of these, if any, are actually involved in “on-off” switches for apoptosis and which might regulate the intrinsic rate of apoptosis. As Driscoll has already pointed out: “regulation and execution of cell death is an absolutely critical process that interfaces with nearly every aspect of life. Future investigation of the links of cell death to cellular aging and the aging of organisms should be an exciting enterprise.”
_
Role of Programmed Cell Death in Life Span Regulation:
Because normally regulated PCD is required for tissue homeostasis, it seems likely this process will be required for optimal life span, particularly in species like mammals with abundant adult cell turnover. In contrast, the ectopic and counterproductive apoptosis associated with tissues that incur damage during aging, such as in sarcopenia, might be expected to limit life span. In C. elegans, where adult somatic cells are all postmitotic, mutation of key conserved apoptosis regulators such as the caspase gene ced-3 did not affect life span (Garigan et al., 2002). Similarly, in Drosophila, over-expression of powerful caspase inhibitors such as baculovirus p35 and DIAP1 in the adult animal had no detectable effect on life span (Shen et al., 2009), suggesting that, at least in the invertebrates, life span is not limited by a canonical caspase-dependent apoptosis pathway. However, it remains possible that life span might be limited by caspase-independent PCD mechanisms, perhaps including the apoptotic-like events associated with muscle atrophy, and this will be an interesting area for future research. Even the cell death observed in aging yeast cells has several characteristics indicative of PCD (Rockenfeller and Madeo, 2008). In mammals, investigating the role of PCD pathway genes in life span is complicated by the normal requirement for PCD in development and adult tissue homeostasis, but in the future, the targeting of PCD inhibition to specific tissues in the adult may yield answers to the question of whether one or more PCD pathways function in regulating mammalian life span.
_
Programmed cell death as a target to interrupt the aging program:
There are two opposite points of view on aging of organisms. The traditional concept assumes that aging is a stochastic process consisting in age-dependent accumulation of random injuries in living systems. However, many pieces of evidence are recently obtained in favor of an alternative scheme suggesting that aging is genetically programmed being the final step of ontogenesis. The latter concept predicts (i) the existence of non-aging species which have lost the aging program and (ii) that the program in question can be experimentally interrupted by manipulations with corresponding genes or by small molecules operating as inhibitors of the execution of aging program. In a paper authors summarize observations which are consistent with these two predictions. In both cases, interruption of the aging program is based upon inhibition of programmed cell death (apoptosis) mediated by mitochondrial reactive oxygen species (ROS). Researchers argue that the main difference between young and old multicellular organisms consists in the cellularity, i.e. in number of functional cells in organs or tissues rather than in quality of these cells. The cellularity decreases due to domination of apoptosis over proliferation in aging organisms. This means that apoptosis appears to be the basis of aging program. A pharmacological approach to switch off the aging program is considered, and this approach involves mitochondria-targeted antioxidants and uncouplers. Such compounds prevent mitochondrial oxidative stress which increases with age and stimulates the age-dependent apoptosis.
_
Aging, cancer and apoptosis:
Cancer rates increase markedly with age. This is a major clue that there is some connection between aging and the regulation of cell division. While childhood cancers are rare, cancers among the elderly are common. The few treatments that have been found to slow aging, such as caloric restriction and dampened insulin/insulin-like growth factor 1 signaling, also delay the onset of cancer. On the other hand, senescence protects against tumorigenesis in some tissues. Aging mammalian cells can stop dividing and enter senescence if they are damaged or have defective telomeres. Senescence protects against tumor formation, and tumor suppressor genes include some that regulate cell division and lead to senescence. Two pathways – one involving the tumor suppressor gene p53, the other involving the tumor suppressor gene RB – lead to a division arrest followed by either apoptosis (cell suicide) or senescence (the stopping of cell division). Studies of mutant p53 genes have supported the idea that increased tumor protection by p53 can shorten life spans of mice.
_
_
p53 is a key player in Programmed Cell Death and Life Span Regulation:
p53 functions to suppress cancer through at least two mechanisms: in response to cellular damage and/or abnormal signaling, p53 causes cells to enter either apoptosis or senescence, depending upon the signal and the cellular context as seen in the figure above. Either way, further cell division is halted, thereby preventing that cell from becoming cancerous. p53 has also been found to regulate life span in several species. For example, in mouse, abnormal p53 activity can cause a premature aging-like phenotype, associated with the accumulation of abnormal cells (Hinkal et al., 2009). In both Drosophila and C. elegans, p53 function appears to limit life span, as mutation of the endogenous p53 gene or expression of dominant-negative forms of p53 produces long-lived animals (Arum and Johnson, 2007; Donehower 2009; Shen et al., 2009; Waskar et al., 2009). In C. elegans the increased life span of p53 mutants and several other long-lived mutants appears to be dependent upon increased autophagy (Tavernarakis et al., 2008). Intriguingly, in Drosophila, p53 has been found to have both positive and negative effects on adult life span, depending upon the particular tissue and whether the animal is male or female (Shen and Tower, 2009; Waskar et al., 2009), supporting a link between sexual differentiation, PCD, and aging (Tower, 2006). Because p53 is able to regulate PCD, cell senescence, autophagy, mitochondrial metabolism, and other critical cell processes (Green and Kroemer, 2009), determining how p53 and autophagy are affecting life span will be an important area for future research.
_
The figure below shows how senescence pathways are switched off during the development of cancer:
_
What role do telomeres play in cancer?
As a cell begins to become cancerous, it divides more often, and its telomeres become very short. If its telomeres get too short, the cell may die. It can escape this fate by becoming a cancer cell and activating an enzyme called telomerase, which prevents the telomeres from getting even shorter. Studies have found shortened telomeres in many cancers, including pancreatic, bone, prostate, bladder, lung, kidney, and head and neck. Measuring telomerase may be a new way to detect cancer. If scientists can learn how to stop telomerase, they might be able to fight cancer by making cancer cells age and die. In one experiment, researchers blocked telomerase activity in human breast and prostate cancer cells growing in the laboratory, prompting the tumor cells to die. But there are risks. Blocking telomerase could impair fertility, wound healing, and production of blood cells and immune system cells.
_
Scientists believe genetic tweaks could significantly extend our lifespan:
Californian scientists tweaked two genetic pathways in the worm Caenorhabditis elegans to amplify its lifespan. The new research, reported in the journal Cell Reports, involved blocking key molecules that affect the action of insulin and a nutrient signaling pathway called Target of Rapamycin (TOR). Single mutations in the TOR pathway were known to extend the lifespan of C. elegans by 30 per cent, while insulin-signaling mutations could double the amount of time they lived. Adding the two together might have been expected to extend longevity by 130 per cent, but the combined impact turned out to be much greater.
_
Autophagy and Aging: The Importance of Maintaining “Clean” Cells:
A decrease in the turnover of cellular components and the intracellular accumulation of altered macromolecules and organelles are features common to all aged cells. Diminished autophagic activity plays a major role in these age-related manifestations. Dietary caloric restriction and antilipolytic agents have been proven to efficiently stimulate autophagy in old rodents.
_
Anti-aging effects of caloric restriction:
_
_
Anti-aging caloric restrictions prevent the accumulation of altered proteins and the age-related alteration of autophagic proteolysis. Prolonged calorie restriction has been shown to extend both the median and maximal lifespan in a variety of lower species such as yeast, worms, fish, rats, and mice and to improve the health conditions of primates, depending on level and duration. In addition, caloric restriction has also been shown to improve recovery from toxic challenge in lower animals. Restriction of food intake 40% below food consumption of ad libitum fed rats, or every other day feeding, makes animals spend a large part of their time in the state of fasting, with lower glucose and insulin plasma levels and consequently, favoring activation of the two inducible forms of autophagy, macroautophagy and CMA. Ten years ago it was proposed that autophagy, as a highly regulated cell repair mechanism, could mediate in part the anti-ageing effects of calorie restriction. Extensive evidence may now support this hypothesis, showing that caloric restriction prevents the accumulation of altered proteins in cytosol and membranes, the increase in liver tissue dolichol, and the accumulation of altered mtDNA. Caloric restriction preserves the juvenile function and regulation of macroautophagy. Functioning of macroautophagic proteolysis in caloric restricted rats was studied in vivo by the injection of an antilipolytic drug to older rats fasted overnight, which had been on food restriction since early in life. Results showed that caloric restriction prevented the age-related changes in regulatory plasma nutrients and hormones, and in the proteolysis of resident liver proteins. In vitro incubated hepatocytes from these rats, dietary anti-aging intervention preserved the juvenile amino acid and hormone regulation of autophagy. Further support for the hypothesis came from the observation that the protection of rat liver autophagic proteolysis from the age-related decline covaried with the duration and level of anti-ageing food restriction in agreement with the known effects of the dietary intervention on longevity. Probably, by maintaining plasma insulin at a markedly low level during long periods of fasting throughout life, caloric restriction is in effect increasing autophagic proteolysis. Treatment with insulin was shown to reverse at least some beneficial anti-aging effects of caloric restriction. The finding that daily stimulation of autophagic proteolysis by fasting may prevent the age-dependent deregulation of the process in life-long caloric restricted rats, invited studies on the effects of chronic pharmacological stimulation of macroautophagy on the age-dependent decline in autophagic proteolysis. Stimulation of macroautophagy was performed by the administration to fasted rats of antilipolytic drugs like 3,5-dimethylpyrazole or ACIPIMOX, which is available on the market for human use. Treatment causes a sudden decrease in the availability of lipid fuel and induces a compensatory increase in protein degradation. The chronic weekly administration of the drug, starting from age six months, restored autophagic proteolysis and its hormonal control in 24-month old rats and prevented the accumulation of the biomarker of aging, dolichol in liver tissue. In principle, it can be predicted that the long-lasting administration of caloric restriction mimetics and stimulators of macroautophagy via mTOR blockage, like rapamycin, should have similar effects (while GH, Insulin, IGF-1 and anabolic steroids should have opposite effects, and cause aging and the onset of age-associated diseases). Although experimental evidence in mammals is still lacking, studies in C. elegans have recently revealed that TOR deficiency doubles its natural lifespan.
_
_
Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy:
Caloric restriction and autophagy-inducing pharmacological agents can prolong lifespan in model organisms including mice, flies, and nematodes. In this study, researchers show that transgenic expression of Sirtuin-1 induces autophagy in human cells in vitro and in Caenorhabditis elegans in vivo. The knockdown or knockout of Sirtuin-1 prevented the induction of autophagy by resveratrol and by nutrient deprivation in human cells as well as by dietary restriction in C. elegans. Conversely, Sirtuin-1 was not required for the induction of autophagy by rapamycin or p53 inhibition, neither in human cells nor in C. elegans. The knockdown or pharmacological inhibition of Sirtuin-1 enhanced the vulnerability of human cells to metabolic stress, unless they were stimulated to undergo autophagy by treatment with rapamycin or p53 inhibition. Along similar lines, resveratrol and dietary restriction only prolonged the lifespan of autophagy-proficient nematodes, whereas these beneficial effects on longevity were abolished by the knockdown of the essential autophagic modulator Beclin-1. Researchers conclude that autophagy is universally required for the lifespan-prolonging effects of caloric restriction and pharmacological Sirtuin-1 activators.
_
Intermittent Fasting in humans, autophagy and health:
The repertoire of routine housekeeping functions performed by autophagy includes the elimination of defective proteins and organelles, the prevention of abnormal protein aggregate accumulation, and the removal of intracellular pathogens. Such functions are likely critical for autophagy-mediated protection against aging, cancer, neurodegenerative diseases, and infection. Fasting has been an integral part of health and healing practices throughout the recorded history of mankind. This ancient tradition may be partially rooted in a cellular process we are now beginning to understand in modern scientific terms. One of the most evolutionarily conserved cellular responses to organismal fasting is the activation of the lysosomal degradation pathway of autophagy, a process in which the cell self-digests its own components. This self-digestion not only provides nutrients to maintain vital cellular functions during fasting but also can rid the cell of superfluous or damaged organelles, misfolded proteins, and invading micro-organisms. Interestingly, self-digestion by autophagy—a process that is potently triggered by fasting—is now emerging as a central biological pathway that functions to promote health and longevity. Animal studies show a dramatic decrease in cancer after only 16 weeks of intermittent fasting; largely by allowing the body to perform autophagic functions. In human studies, intermittent fasting showed increased insulin sensitivity and increased HDL levels.
_
Eating stimulates the body to go into building phase where we are anabolic in nature and store both nutrients and toxins. This phase is essential for building new cells and tissues and store nutrients for times of scarcity. This building phase of physiology is predominately led by the hormone insulin. Fasting for more than 6 hours begins the cleansing phase. The cleansing phase is catabolic in nature in that it tears down old damaged cells. This process turns on body autophagy, or “self-eating,” in where the cells recycle waste material, regulate waste products and repair. These genetic repair mechanisms are turned on through the release of human growth hormone (HGH). Intermittent fasting is one of the most powerful modalities for reducing inflammation; boosting immunity and enhancing tissue healing. This is one of the reasons why many people feel nauseated when they have infections. This innate mechanism is the body’s way of influencing us to fast so it can produce the right environment to boost natural immunity. In nature, when animals get sick they stop eating and instead focus on resting. This is a primal instinct to reduce stress on their internal system so there body can fight off infection. This natural mechanism allows the animal to concentrate all their internal energy systems towards immunity. Fasting also stimulates ketone bodies that are neuroprotective, but the basis of this effect is still poorly understood. Recent studies demonstrate that ketone bodies increase neuronal levels of hypoxiainducible factor-1α (HIF-1α), possibly owing to succinate-mediated inhibition of prolyl hydroxylase activity. Another recent study has observed reduced activity of mTORC1 in the cortex of rats fed a ketogenic diet. These effects could be expected to collaborate in the induction of neuronal autophagy. Considerable evidence points to moderate up-regulation of neuronal autophagy as a rational strategy for prevention of neurodegenerative disorders; hence, autophagy may mediate some of the neuroprotective benefits of ketogenic diets.
_
Resources seem to suggest that intermittent fasting is a feasible way to increase autophagy, but by no means suggest that this is the best (most effective or healthiest) method. Many sources suggest that mere caloric restrictions, not outright fasting, are the key.
_
The benefits of Intermittent Fasting inferred from the effects of autophagy include:
- Increasing lifespan.
- Increasing insulin sensitivity, which has many health benefits in and of itself. Of interest, the resulting increase in insulin signaling in the brain is thought to be how fasting/calorie restriction works to increase lifespan.
- Lowering blood lipids, triglycerides and other markers of metabolic syndrome.
- Fighting/preventing cancer. There is also some evidence that fasting before chemotherapy treatments can help reduce the negative side effects.
- Increasing growth hormone secretion (which builds muscle and burns fat).
- Normalizing expression of the hunger hormone ghrelin, thereby reducing appetite.
- Promoting brain and peripheral nervous system health by increasing neuronal plasticity and promoting neurogenesis, which has a large variety of effects such as boosting mood, memory, and mental clarity.
- Increasing dopamine production, thereby boosting mood and increasing anticipation and response to rewards (meaning we get more enjoyment from less food).
- Increasing energy through regulating metabolic hormones.
_
The figure below shows how increased autophagy (intermittent fasting) can promote longevity:
_
Mitochondria and the Autophagy–Inflammation–Cell Death Axis in Organismal Aging:
Alterations of mitochondrial functions are linked to multiple degenerative or acute diseases. As mitochondria age in our cells, they become progressively inefficient and potentially toxic, and acute damage can trigger the permeabilization of mitochondrial membranes to initiate apoptosis or necrosis. Moreover, mitochondria have an important role in pro-inflammatory signaling. Autophagic turnover of cellular constituents, be it general or specific for mitochondria (mitophagy), eliminates dysfunctional or damaged mitochondria, thus counteracting degeneration, dampening inflammation, and preventing unwarranted cell loss. Decreased expression of genes that regulate autophagy or mitophagy can cause degenerative diseases in which deficient quality control results in inflammation and the death of cell populations. Thus, a combination of mitochondrial dysfunction and insufficient autophagy may contribute to multiple aging-associated pathologies.
_____________
PCD in non-metazoans:
Due to its importance in such various biological processes, programmed cell death is a widespread phenomenon, occuring in all kinds of metazoans [Tittel, 2000] such as in mammals, insects [Richardson, 2002], nematodes [Liu, 1999b], and cnidaria [Cikala, 1999]. Moreover, programmed cell death also might play a role in plant biology [Solomon, 1999], and apoptosis-like cell death mechanisms even have been observed and used as a model system in yeast [Frohlich, 2000; Skulachev, 2002]. Fascinating insights into the origin and evolution of programmed cell death might possibly be given by the fact, that programmed cell death is also an integral part of the life cycle of other unicellular eukaryotes (such as the kinetoplastid parasite Trypanosoma brucei brucei, the ciliate Tetrahymena thermophila, and the slime mold Dictyostelium discoideum) and that even prokaryotes (such as Bacillus subtilis, Streptomyces and Myxobacteria) sometimes undergo regulated cell death [Ameisen, 2002].
_
Cell death in plants:
Researchers have noted a number of similarities between apoptosis in animals and PCD in plants (Greenberg, 1996), and comparing the functions of key components involved in these processes between kingdoms may serve fruitful for understanding the regulation of PCD in both. For example, cytochrome c release from mitochondria is a key event in apoptosis in animal cells and has also been shown to be an early event in PCD in plants. There is clear evidence that cell death during plant development and interactions with the environment involves PCD. PCD in plants can influence the outcome of yield and quality of crops through its important role in seed germination and the defense process against pathogens. Cell death is common among plants, especially among the higher plants. The xylem in trees, through which water rises to the leaves, consists of spaces left by dead cells. Cell death occurs very visibly when deciduous trees drop their leaves in the fall. (Incidentally, this is where the name ‘apoptosis’ comes from: it is the Greek word for the falling of leaves from trees, as well as the losing of hair from balding men–which incidentally is also thought to involve apoptosis!) Plant cells cannot move, so plants use a slash-and-burn technique to cope with infection: all the cells in an infected area may kill themselves to halt the spread of the disease.
_
In plants, selective cell death is necessary for growth and survival and can occur on a local or large scale (Barlow,
1982). As shown in the figure below, cell death causes the deletion of the suspensor and aleurone cells, which serve temporary functions during development (Yeung and Meinke, 1993), and can also eliminate stamen primordia cells in female flowers of unisexual species (Dellaporta and Calderon-Urrea, 1994). Cell death may also cause the formation of certain leaf lobes and perforations (Greenberg, 1996), and cell death in xylem tracheary elements (TEs) and in some kinds of trichomes is essentia1 for the specialization of these cells. Senescence involves cell death on a large scale.
_
Yeast programmed cell death and aging:
Similarly to metazoans, yeast cells can exhibit several characteristics of apoptosis, including chromatin condensation, DNA breakage, flipping of phosphatidylserine to the outer leaflet of the plasma membrane, accumulation of reactive oxygen species (ROS), and release of pro-death factors such as cytochrome c or Endonuclease G from mitochondria. Yeast programmed cell death has been shown to occur in response to a variety of stimuli, such as oxidative stress, exposure to acetic acid, and expression of mammalian pro-apoptotic proteins. This program is also inherent to the yeast life cycle, as aged mother cells and cells exposed to pheromone also display an apoptotic and necrotic phenotype. Yeast therefore comprises a conserved core programmed cell death process that shares several regulators with mammalian cells, which play major roles in the pathogenesis of human diseases. At the same time, it lacks many of the cell death regulators that have evolved in higher eukaryotes, probably due to the invention of multicellularity. The simplicity of the yeast model allows elucidating the basic molecular pathways of programmed cell death without interference from multifaceted regulation, due to various protein isoforms or cellular specificity often observed in studies using mammalian systems. In addition, yeast heterologous expression systems offer the opportunity to exploit the individual functional and mechanistic properties of mammalian apoptotic regulators.
_
Apoptosis-like programmed cell death (PCD) has recently been described in multiple taxa of unicellular protists, including the protozoan parasites Plasmodium, Trypanosoma and Leishmania. Apoptosis-like PCD in protozoan parasites shares a number of morphological features with programmed cell death in multicellular organisms. However, both the evolutionary explanations and mechanisms involved in parasite PCD are poorly understood. Explaining why unicellular organisms appear to undergo ‘suicide’ is a challenge for evolutionary biology and uncovering death executors and pathways is a challenge for molecular and cell biology. Bioinformatics has the potential to integrate these approaches by revealing homologies in the PCD machinery of diverse taxa and evaluating their evolutionary trajectories.
_
PCD in prokaryotes:
PCD in bacteria:
PCD is a genetically encoded process that leads to cell death. It is an essential mechanism in multicellular organisms for proper development and homeostasis. Although PCD has classically been studied in the context of multicellular organisms, it is now widely recognized that bacteria and other single-cell organisms also exhibit PCD in response to environmental stimuli. Over the past two decades, PCD has been identified in various bacterial species and found to play critical roles in their survival and pathogenesis. In particular, recent studies discovered that cell death in Escherichia coli also exhibits typical biochemical markers of apoptosis when induced with bactericidal antibiotics. This phenomenon has been termed ‘apoptosis-like death’. The origin and physiological implications of PCD are still the subjects of active debate: for instance, is it a trait that is selected for during evolution or a maladaptive byproduct of another trait? A common mechanism to realize PCD in bacteria is through toxin–antitoxin (TA) modules, which are present in almost all free-living bacteria species that have been sequenced. A TA module typically consists of genes encoding a protein toxin and an antitoxin (a protein or an RNA) that neutralizes the toxin by either directly binding to the toxin or inhibiting translation of the toxin. The antitoxin is often less stable than the toxin and has to be constantly produced to inhibit the toxin. Under certain stressful conditions, antitoxins are quickly degraded, thus freeing toxins to exert their poisoning effects. The targets of toxins are diverse: they include DNA replication, translation, cell division, and cell wall synthesis. One of the most studied TA modules is the mazEF system, which was first found on chromosomes of E. coli and later in other bacteria. mazF codes for a toxin MazF, an endoribonuclease that cleaves mRNAs; mazE codes for an antitoxin MazE, which can be degraded quickly by the ClpPA serine protease. The mazEF system is activated under various stressful conditions, including amino acid starvation, antibiotic treatment, DNA damage, and oxidative stress. Once induced, it causes PCD in most of the population by increasing the synthesis of ‘death proteins’, while allowing survival of a small subpopulation by increasing the synthesis of ‘survival proteins’. Moreover, mazEFmediated death is regulated by a small peptide called extracellular death factor that is secreted by cells, suggesting fine tuning of PCD through population density. PCD can also occur by activation of a prophage, a bacteriophage DNA that is inserted into a bacterial host genome as a result of the lysogenic life cycle of bacteriophage. Normally dormant, the prophage can be activated by environmental stresses that damage host DNA, which in turn results in host lysis. Because prophage- coded genes play critical roles in bacterial evolution and prophages reside in bacterial chromosomes, authors consider prophage activation as PCD. Also, this notion is consistent with the general definition of PCD. Genomic analyses have identified prophages in many sequenced bacteria; in many cases, a bacterium contains multiple prophages in its genome, although many of them have become defective in producing phage particles. A notable example is the food pathogen Shiga toxin-producing E. coli (STEC) O157:H7 strain Sakai, which contains 18 prophage elements.
_
_
PCD as a target for antibacterial therapy:
Since the introduction of antibiotics in the 1940s, antibiotics have become the largest component in our arsenal against bacterial infections. However, decades of overuse of antibiotics are now undermining their own effectiveness. Bacteria have been developing resistance to existing antibiotics at an alarming rate, and there is an urgent need for developing new antibiotics and new treatment strategies for better use of existing antibiotics. The presence of PCD has profound implications for both aspects. On the one hand, if antibiotic-mediated killing of pathogens leads to the release of enzymes that degrade the antibiotic (realizing the basic logic of altruistic PCD), the bacterial growth can exhibit non-monotonic responses to the antibiotic doses, whereby the bacteria grow better at a higher dose of antibiotic. On the other hand, elements of PCD can serve as potential drug targets to achieve effective inhibition of bacterial survival. For example, PCD can be artificially induced to promote greater cell death. This strategy has been proposed for TA systems, where the induction can be achieved by disruption or prevention of TA complex formation or enhanced proteolytic degradation of antitoxin. This strategy is analogous to cancer treatment by chemically inducing or promoting apoptosis. While intuitively appealing, however, this strategy could lead to counterintuitive consequences when PCD is coupled with release of public goods. Specifically, induction of PCD will result in faster public good generation in the short term, which can lead to a greater fitness benefit for the pathogen in the long term. As a result, triggering death in some cells could, in principle, lead to apparently better growth in the overall population, although it remains to be seen whether the same effect takes place in vivo systems.
_
Antibiotic-induced Bacterial Cell Death exhibits Physiological and Biochemical Hallmarks of Apoptosis:
Programmed cell death is a gene-directed process involved in the development and homeostasis of multicellular organisms. The most common mode of programmed cell death is apoptosis, which is characterized by a stereotypical set of biochemical and morphological hallmarks. Researchers report that Escherichia coli also exhibit characteristic markers of apoptosis—including phosphatidylserine exposure, chromosome condensation, and DNA fragmentation— when faced with cell death-triggering stress, namely bactericidal antibiotic treatment. Notably, researchers also provide proteomic and genetic evidence for the ability of multifunctional RecA to bind peptide sequences that serve as substrates for eukaryotic caspases, and regulation of this phenotype by the protease, ClpXP, under conditions of cell death. Their findings illustrate that prokaryotic organisms possess mechanisms to dismantle and mark dying cells in response to diverse noxious stimuli and suggest that elaborate, multilayered proteolytic regulation of these features may have evolved in eukaryotes to harness and exploit their deadly potential.
_____________
Not to oversimplify cell death:
Cell death is an essential phenomenon in normal development and homeostasis, but also plays a crucial role in various pathologies. Our understanding of the molecular mechanisms involved has increased exponentially, although it is still far from complete. The morphological features of a cell dying either by apoptosis or by necrosis are remarkably conserved for quite different cell types derived from lower or higher organisms. At the molecular level, several gene products play a similar, crucial role in a major cell death pathway in a worm and in man. However, one should not oversimplify. It is now evident that there are multiple pathways leading to cell death, and some cells may have the required components for one pathway, but not for another, or contain endogenous inhibitors which preclude a particular pathway. Furthermore, different pathways can co-exist in the same cell and are switched on by specific stimuli. Apoptotic cell death, reported to be non-inflammatory, and necrotic cell death, which may be inflammatory, are two extremes, while the real situation is usually more complex. Researchers review the distinguishing features of the various cell death pathways: caspases (cysteine proteases cleaving after particular aspartate residues), mitochondria and/or reactive oxygen species are often, but not always, key components. As these various caspase-dependent and caspase-independent cell death pathways are becoming better characterized, we may learn to differentiate them, fill in the many gaps in our understanding, and perhaps exploit the knowledge acquired for clinical benefit.
1. Apoptosis and necrosis can occur in the same cell:
As mentioned already, TNF induces typical apoptotic death in many cell types (KYM, MCF-7, PC60), while in L929 and other fibrosarcoma cells TNF causes typical necrosis. The simplest explanation for these different TNF-activated pathways would be that in the latter cell types a critical component of the apoptotic pathway, e.g. FADD or caspase-8, is missing. But when this hypothesis was tested, came a first surprise. L929.hCD95 is a transfected L929 derivative in which the human CD95 gene was expressed constitutively; upon treatment with monoclonal anti-CD95, these cells died rapidly by typical apoptosis (Vercammen et al., 1998b) (Figure 1). Hence, all the machinery necessary for the apoptotic pathway is present in the cells. The introduction of the human CD95 gene did not affect at all the necrotic response to TNF. When the L929.hCD95 cells were treated with an agonistic anti-CD95 antibody and in addition a broad-spectrum caspase inhibitor such as benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD-fmk) was added, as expected, the apoptotic pathway was blocked. But remarkably, the cells now died by necrosis; this is an active process, involving again enhanced ROS production, which could be inhibited by BHA (in the presence of both zVAD-fmk and BHA, the cells no longer proliferated and slowly disappeared). So researchers can conclude that CD95 triggering can lead to a vigorous apoptotic response, but when the latter is blocked, at least in some cell types, an alternative, slower process leading to necrosis becomes apparent (Vercammen et al., 1998b). Kawahara et al. (1998) recently described another Jurkat cell-derived system in which apoptotic death could be replaced by necrosis. They made a construct coding for a fusion protein consisting of an inducible oligomerization domain followed by a FADD domain. As expected, upon addition of an oligomerizing agent, typical apoptosis was observed. Remarkably, however, when the caspase-dependent pathway was blocked, either by using a caspase-8-negative Jurkat subline or by addition of a potent caspase inhibitor, induced oligomerization of FADD still led to cell death. But characteristic apoptotic features were not observed (no internucleosomal DNA cleavage or lamin breakdown, etc.); rather, the cells seemed to die by necrosis! In fact, treatment of Jurkat cells or peripheral blood lymphocytes with CD95L results in increased ROS production and slightly enhanced DeltaPsim, even in the presence of the broad-spectrum caspase inhibitor zVAD-fmk (Banki et al., 1999). These events are reminiscent of those occurring in L929 cells treated with TNF, and suggest a similar necrotic pathway. In fact, a number of cell systems have been reported in the literature, where in the presence of a broad-spectrum caspase inhibitor, necrotic cell death features were observed (reviewed by Kitanaka and Kuchino, 1999). A typical example was a transfected glioma cell where induction of an introduced activated H-ras gene led to necrotic death characterized by extensive autophagy (Kitanaka and Kuchino, 1999). It is of interest that in several of these caspase-independent systems the normally apoptogenic Bax protein was implicated. Furthermore, induced expression of mammalian Bax in yeast cells leads to cell death due to mitochondria-derived ROS (Madeo et al., 1999). Similar remarkable findings have even been described for an in vivo system. Death and removal of interdigital cells in the mouse embryo is a prototype example of developmental PCD. Studying this system, Chautan et al. (1999) found that blocking of apoptosis, either by addition of a caspase inhibitor or by using apaf-1-/- mice, did not prevent interdigital cell death or removal. In the absence of caspase activity, these cells revealed features strongly suggesting ongoing necrosis. Even under normal conditions, a fraction of interdigital cells apparently died by necrosis rather than by apoptosis. These results are in agreement with earlier studies on PCD in embryogenesis, where necrotic cell death has often been observed (Schweichel and Merker, 1973; Kitanaka and Kuchino, 1999). It seems likely, therefore, that in many cell types both an apoptotic and a necrotic pathway are latently present. When upon stimulation, the caspase-dependent pathway is non-functional or blocked, an underlying, slower pathway becomes in evidence, which leads to the cell’s demise by necrosis. However, one should not equate caspase-independent cell death with necrosis. There are many examples of apoptotic cell death occurring even in the presence of broad-spectrum caspase inhibitors such as zVAD-fmk (Borner and Monney, 1999). For example, one mechanism may involve an apoptosis-inducing factor (AIF). Various triggers of cell death cause release of AIF from the mitochondrial intermembrane space; AIF then moves to the nucleus, where it causes apoptotic features (Lorenzo et al., 1999).
2, Apoptosis vs. necrosis: not the two sides of a coin:
The neat features of apoptotic cell death have been extolled in numerous reviews. The cells die rapidly, without any spilling of their content, and the corpses are removed without delay. In short, the perfect suicide/murder. This may be ideal for development, for tissue remodeling, or for termination of a vigorous immune response. On the other hand, when a cell is attacked by a pathogen or sustains chemical or physical insults, it is of advantage to the organism that signals are sent out, such that proper specific and non-specific defenses are set up, in short an inflammatory response. This is best achieved by necrotic cell death. Although in necrosis, by definition, the cell content is released, this does not necessarily mean that the patient or organism mounts an auto-immune response. In reality, the aforementioned alternative pathways of apoptotic and necrotic cell death corresponding to non-inflammatory and inflammatory outcomes, respectively, are extreme examples of a wide spectrum of cellular responses. CD95L is the best known inducer of bona fide apoptosis. Yet, tumour cells expressing a stable form of CD95L on their surface, when injected into mice, elicited a vigorous inflammatory response, which was mediated by IL-1 (Miwa et al., 1998). Another example is ischemia-reperfusion injury, which is characterized not only by extensive apoptotic cell death, but also by damaging inflammation; blocking apoptosis also alleviated the inflammation (Daemen et al., 1999). Cells which are recognized as non-self, are killed by cytotoxic T-cells in a typical apoptotic process initiated either by CD95 clustering or granzyme B/perforin action. Yet, for many years immunologists have been following the cytotoxic T-cell reactivity by release into the medium of 51Cr from the target cells! In fact, more detailed studies have revealed that when a cell dies by a typical apoptotic process, usually there occurs a late-phase necrosis, characterized by spillage of cell content (Dive et al., 1992; Geier et al., 1995; Slater et al., 1995). In the studies of Chautan et al. (1999) on the removal of interdigital cells in the developing mouse embryo referred to above, when apoptosis was prevented, the cells died by necrosis, and even under normal conditions a certain percentage of the cells are removed by necrosis; yet, in neither condition was there evidence for an inflammatory response. In Jurkat and in HeLa cells it has been observed that when intracellular ATP levels drop below a critical value, CD95 triggering no longer elicits apoptotic cell death, but a pathway leading to necrosis becomes in evidence (Eguchi et al., 1997; Leist et al., 1997). It is tempting to believe that this observation is related to the known requirement for dATP, in direct equilibrium with ATP, for the Apaf-1-mediated activation of procaspase-9. It is not known whether the necrotic pathway observed under low ATP conditions is caspase-independent, as in the studies of Kawahara et al. (1998) and the other examples referred to above. In those cells where both pathways can occur, apoptosis fails when ATP levels drop below 15 – 25% of control cells (Lieberthal et al., 1998; Lelli et al., 1998). But, as mentioned above, in fibrosarcoma cells, TNF treatment results in necrosis without a decrease of the ATP level (G Denecker, 1999 unpublished results). AKR-2B fibroblasts die upon withdrawal of serum; the intracellular ATP content remained high, yet detailed analysis revealed features both of necrosis (rapid membrane leakage, no oligonucleosomal DNA fragmentation) as well as of apoptosis (membrane blebbing, strong nuclear condensation) (Simm et al., 1997). Although dATP/ATP is required for apoptosis, absence or blockage of the latter pathway apparently reveals a slower, necrotic process, independent of the ATP level. Other conditions, such as relative abundance of Bax and Bcl-xL, may also determine whether a cell will die by apoptosis or by necrosis.
___________
A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants:
As an animal interacts with its environment, it invariably encounters stressful conditions such as extreme temperatures, drought, UV exposure and harmful xenobiotics. Since the ability to respond appropriately to stressful stimuli is paramount to survival, organisms have developed sophisticated stress response programs. Some stressful conditions cause damaged cells to commit suicide (undergo apoptosis), whereas others cause the entire organism to develop mechanisms to resist environmental stress. Studying the small roundworm C. elegans, researchers find that these two responses are somehow linked: perturbing the mechanisms that allow cells to undergo apoptosis changes the whole animal’s response to environmental stress. In fact, perturbing the apoptosis machinery in any way—through mutations that prevent apoptosis altogether, or through mutations that either slow or accelerate the clearance of dying cells—causes the animal to become more stress resistant. Together these findings raise the possibility that the animal may have a way of detecting defects in the normal programmed cell death pathway, and that in response it induces a new program that protects itself from a harsh environment. Researchers find that C. elegans mutations blocking the normal course of programmed cell death and clearance confer animal-wide resistance to a specific set of environmental stressors; namely, ER, heat and osmotic stress. Remarkably, this pattern of stress resistance is induced by mutations that affect cell death in different ways, including ced-3 (cell death defective) mutations, which block programmed cell death, ced-1 and ced-2 mutations, which prevent the engulfment of dying cells, and progranulin (pgrn-1) mutations, which accelerate the clearance of apoptotic cells. Stress resistance conferred by ced and pgrn-1 mutations is not additive and these mutants share altered patterns of gene expression, suggesting that they may act within the same pathway to achieve stress resistance. Together, these findings demonstrate that programmed cell death effectors influence the degree to which C. elegans tolerates environmental stress. While the mechanism is not entirely clear, it is intriguing that animals lacking the ability to efficiently and correctly remove dying cells should switch to a more global animal-wide system of stress resistance. In summary, researchers’ findings indicate that programmed cell death effectors not only kill and remove individual cells, but also influence environmental stress resistance at the level of the whole animal. This is the first time that cell-death effectors like ced-3, pgrn-1 and ced-1 have been implicated in organismal stress resistance, and these findings raise many interesting new questions about both mechanism and evolution.
_
In other words, blocking programmed cell death increases stress resistance of cells:
We know that cancer cell have reduced apoptosis and they survive harsh conditions as compared to normal cells. Many cancer cells have adapted distinct metabolism and can grow under harsh conditions in terms of substrate availability. Survival of the fittest might be the best way to explain the genetic and molecular machinery behind cancer metastasis. Researchers believe that overexpression of some genes in melanoma and other cancers allows some cells to survive the very harsh conditions that occur as they leave a primary tumor, travel to a distant site, and establish a new location for malignancy. It is a similar theme to Darwin with natural selection, although it works out in a microenvironment.
_
A study showing differential stress response between cancer cells and non-cancer cells of breast:
Breast cancer cells can survive and proliferate under harsh conditions of nutrient deprivation, including limited oxygen and glucose availability. Researchers hypothesized that such environments trigger metabolic adaptations of mitochondria, which promote tumor progression. So they mimicked aglycemia and hypoxia in vitro and compared the mitochondrial and cellular bioenergetic adaptations of human breast cancer (HTB-126) and non-cancer (HTB-125) cells that originate from breast tissue. Using high-resolution respirometry and western blot analyses, they demonstrated that 4 days of glucose deprivation elevated oxidative phosphorylation five-fold, increased the spread of the mitochondrial network without changing its shape, and decreased the apparent affinity of oxygen in cancer cells (increase in C50), whereas it remained unchanged in control cells. The substrate control ratios also remained constant following adaptation. They also observed the Crabtree effect, specifically in HTB-126 cells. Likewise, sustained hypoxia (1% oxygen during 6 days) improved cell respiration in non-cancer cells grown in glucose or glucose-deprived medium (+ 32% and +38%, respectively). Conversely, under these conditions of limited oxygen or a combination of oxygen and glucose deprivation for 6 days, routine respiration was strongly reduced in cancer cells (-36% in glucose medium, -24% in glucose-deprived medium). The data demonstrate that cancer cells behave differently than normal cells when adapting their bioenergetics to microenvironmental conditions.
_
Programmed cell death (PCD) is a beneficial trait for organism. However, when PCD is blocked, another inbuilt program exists that increases stress resistance. Reduced PCD is the basis of cancer. This reduced PCD has provoked increased stress resistance and survival in harsh conditions by cancer cells. Of course, treatment of cancer include inducing PCD in cancer cells but if we can map out that another inbuilt program related to survival in harsh conditions at the DNA level, we can kill cancer cells by blocking that program. I call this novel program as “Programmed Cell Resistance” [PCR]. So all cells contain genes for PCD as well as PCR. In normal cell, there is a fine balance between PCD and PCR. In cancer, PCD is reduced and consequently PCR is increased. In neurodegenerative disorders, PCD is enhanced and consequently PCR is reduced. Since chemotherapy targets PCD alone, it fails. Targeting both PCD and PCR would certainly cure cancer.
_____________
Moral of the story:
_
1. Adult human body contains about one hundred thousand billion cells differentiated into 200 different cell types. Each second, something on the order of one million cells die in our body and by the end of human life almost 99.9% of all cells once made have undergone this fate. Without cell death, an 80-year-old person would have 2 tons of bone marrow & lymph nodes, and a gut 16 km long.
_
2. Programmed cell-death (PCD) is death of a cell by any mode, mediated by an intracellular program under genetic control. PCD is indispensable and perform many functions, right from ontogenesis to metamorphoses to involution of phylogenetic vestiges to modeling of organs & systems including immune system to homeostasis to controlling cell number to process of aging to prevent cancer to fight infection.
_
3. During tissue homeostasis there is equilibrium between the net growth rate and the net rate of cell death. Upon exposure to cellular stress this physiological homeostasis is in danger. Normal cell undergoes adaptation in response to stress. When stress is severe or adaptation fails, cell gets injured. The injured cell mounts pro-survival response. When injury is severe or pro-survival mechanism fails, cell generates cell death response.
_
4. Cellular senescence is a state between cell maturity and cell death where cells have irreversibly lost their proliferation ability despite continued viability & metabolic activity, and exhibit deficiencies in maintaining their homeostatic processes. Both genetic and environmental factors contribute to senescence.
_
5. The nomenclature pertaining to cell death is confusing, and no single classification scheme encompasses all variant forms of cell death reported in the literature. Different morphological, biochemical, molecular, functional, immunological and enzymatic pathways delineate different modes of cell death, and there is overlap between different cell death modalities.
_
6. Apoptosis a prototype of programmed cell death in which a controlled sequence of events leads to the elimination of cells without releasing harmful substances into the surrounding area, enabling metazoans to control cell number and eliminate cells that threaten the animal’s survival. Characteristic cell morphology of cells undergoing apoptosis include blebbing, loss of cell membrane asymmetry and attachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and eventually fragmentation of entire cell into membrane bound apoptotic bodies, triggering phagocytosis by non-activated macrophages and other cells in the vicinity.
_
7. There is substantial evidence to show that autophagy is a survival mechanism rather than programmed cell death mechanism and only when the stressor or injury is severe, the survival mechanism fails resulting in so called autophagic cell death (PCD type 2), the characteristic cell morphology being a massive cytoplasmic vacuolization and absence of chromatin condensation.
_
8. Necrosis is the premature death of cells in living tissue by autolysis due to cell injury, characterized metabolically by rapid depletion of ATP and morphologically by gain in cell volume and swelling of organelles due to loss of ion-pumping capacity of plasma membrane followed by rupture of plasma membrane and loss of intracellular contents resulting in inflammatory response. It was initially considered non-programmed (uncontrolled) but now considered somewhat regulated.
_
9. There is considerable overlap, interconnection and cross-talk between various cell death modes at molecular level. The blockade of a particular pathway of cell death may not prevent the destruction of the cell but may instead recruit an alternative path. So commitment to death overrides mechanism of death. The survival of a multicellular organism as a whole is important rather than the survival of individual cells.
_
10. Apoptotic cell death, reported to be non-inflammatory, and necrotic cell death, which may be inflammatory, are two extremes, while there is lot of overlap between them. When a cell dies by a typical apoptotic process, usually there occurs a late-phase necrosis, characterized by spillage of cell content. Although lower intracellular ATP level favors necrosis over apoptosis as apoptosis needs energy, regulated necrosis can occur with high intracellular ATP levels.
_
11. Apoptosis is involved in the pathogenesis of cancer, infections, autoimmune diseases, neurodegenerative disorders, ischemic disorders, HIV/AIDS, diabetes mellitus, and COPD/ILD etc. Consequently, apoptosis regulators have emerged as key targets for the design of therapeutic strategies aimed at modulating cellular life-and-death decisions.
_
12. Autophagy is involved in disorders of nervous system, muscles, liver, cancer and aging.
_
13. Both apoptosis and necrosis are responsible for liver cell death due to alcohol and alveolar epithelial cell death due to smoking.
_
14. More than 50% of cancers have defects in the apoptotic machinery due to increased expression of pro-survival Bcl-2 family proteins and mutations in the tumor-suppressor gene TP53, which encodes tumor suppressor protein p53. Many human cancers are caused by viruses that encode Bcl-2 homologs and viral protein that inactivate p 53. On the other hand, virus-infected cells produce IFN-α/β and undergo p53-dependent apoptosis. Integration and cooperation between p53 and IFN-α/β, provides a new link between tumour suppression and antiviral host defense. This is useful for treating human cancers with IFN-α/β in combination with chemotherapeutic drugs that activate p53.
_
15. Induction of programmed cell death in tumour cells is the basis of most chemotherapeutic agents for treatment of cancer.
_
16. Evolutionary biologically, mitochondria represent prokaryote (e.g. energy generating bacteria) in eukaryote (e.g. human) cell having symbiosis, therefore mitochondria plays a central role in cell deaths, cancer and neurodegenerative disorders. That is why targeting mitochondria is the best way to kill cancer cells.
_
17. Control of cell death mechanisms may lead to production of unlimited amount of RBC in laboratory and protection of graft from attack by T cells of host immunity; resulting in solving problem of scarcity of blood in blood bank and prevent graft rejection in organ transplant.
_
18. Slowly we are learning that sometimes it is body’s own immune response to infections that cause death of a patient rather than the harm done by infective agent. Repetitive cycle of abortive infection, pyroptosis (PCD) of CD4 T cell, inflammation and recruitment of additional CD4 T cells destroy most of CD4 T cell in HIV infection, and immune response to second dengue infection releases cytokines that cause endothelial tissue to become permeable resulting in fluid loss from the blood vessels causing intravascular volume depletion and shock; are the two classical examples of body’s own immune response to microorganisms causing death of patients. All the time we were targeting the infective agent but now we need a paradigm shift by targeting PCD and/or immune system to save human lives.
_
19. As cells age i.e. number of cell divisions increase, its sensitivity to stressor increases. Depending on cell type, in response to stress or injury, aging cell would increase PCD or decrease PCD resulting in neurodegenerative disorders or cancer respectively. That is why neurodegenerative disorders and cancers are common in aging human population. However, once cell reaches senescence, it stops dividing and cannot become cancerous.
_
20. Aging of an individual means reduced cellularity (number of functional cells) of organs and reduced functions of cells in multicellular organism. So there is gradual decline in the qualitative and quantitative functions of cells. Cellularity can be increased by inhibiting apoptosis and functions can be improved by stimulating autophagy. Therefore, anti-aging measures would include anti-apoptotic and pro-autophagic interventions. However, anti-apoptotic measures would induce tumorigenesis.
_
21. Intermittent fasting stimulates autophagy which protect against aging, cancer, neurodegenerative diseases, and infection. There in some truth in the health benefits of religious fasting. Intermittent fasting promotes health and longevity. Mere caloric restriction, not outright fasting is the key.
_
22. Experiments on C. elegans have shown that perturbing the apoptosis machinery in any way—through mutations that prevent apoptosis altogether, or through mutations that either slow or accelerate the clearance of dying cells—causes the animal to become more stress resistant. This proves that the animal has a way of detecting defects in the normal programmed cell death pathway, and that in response it induces a new program that protects itself from a harsh environment. So even though PCD is evolutionary biologically inherited genetic program to benefit the organism, there is another inbuilt program that detect defect in PCD and consequently raise organism’s stress resistance. Reduced PCD is the basis of cancer. This reduced PCD has provoked increased stress resistance and survival in harsh conditions by cancer cells. Of course, treatment of cancer include inducing PCD in cancer cells but if we can map out that another inbuilt program related to survival in harsh conditions at the DNA level, we can kill cancer cells by blocking that program. I call this novel program as “Programmed Cell Resistance” [PCR]. So all cells contain genes for PCD as well as PCR. In normal cell, there is a fine balance between PCD and PCR. In cancer, PCD is reduced and consequently PCR is increased. In neurodegenerative disorders, PCD is enhanced and consequently PCR is reduced. Since chemotherapy targets PCD alone, it fails. Targeting both PCD and PCR would certainly cure cancer.
_________________
Dr. Rajiv Desai. MD.
January 1, 2014
_________________
Postscript:
I request cell death researchers and cancer researchers to read this article. Their suggestions and criticisms may be conveyed to me at my email ID [email protected]
__________________

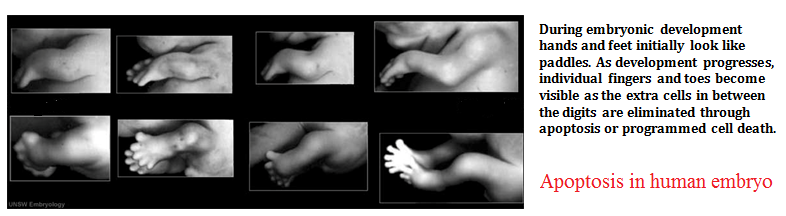
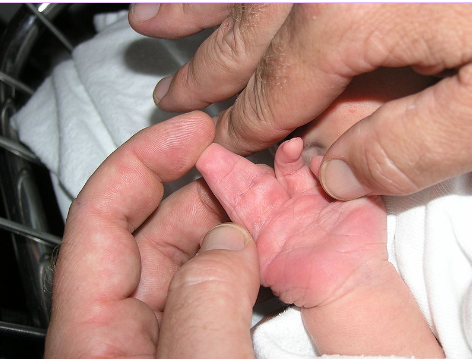
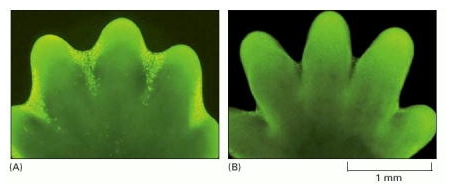
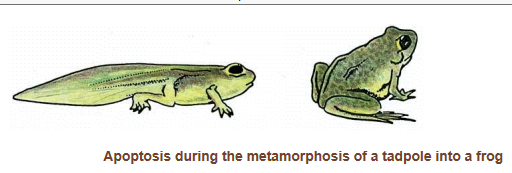
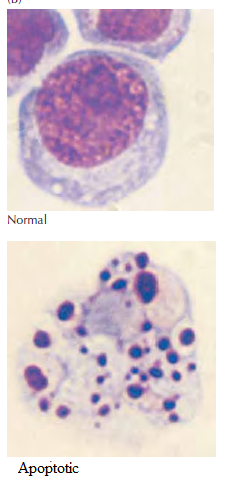

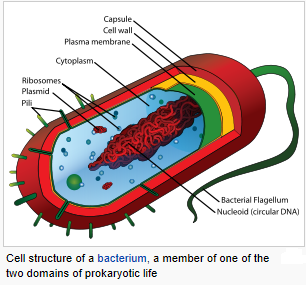
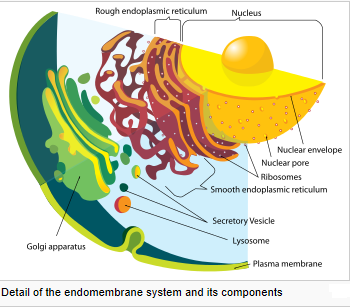
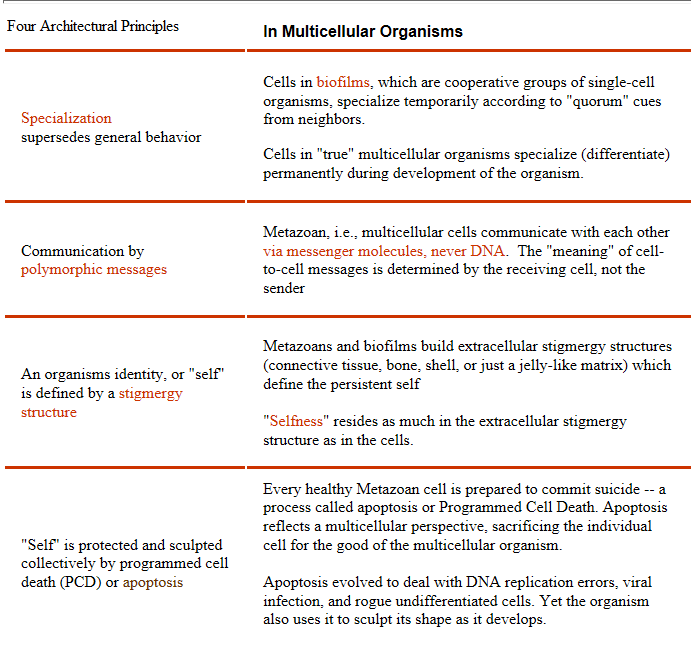
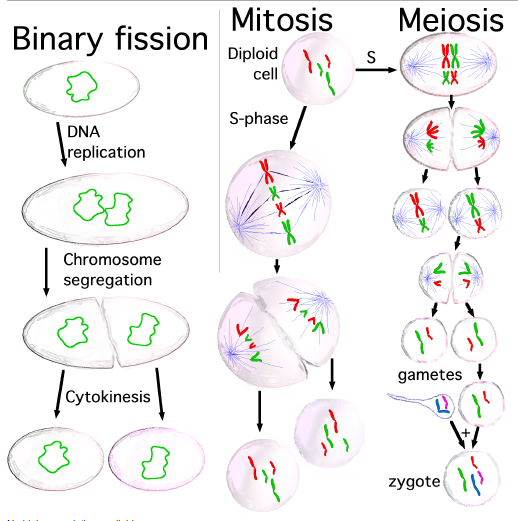
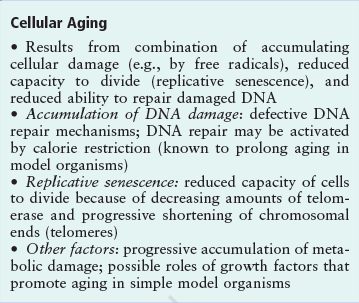
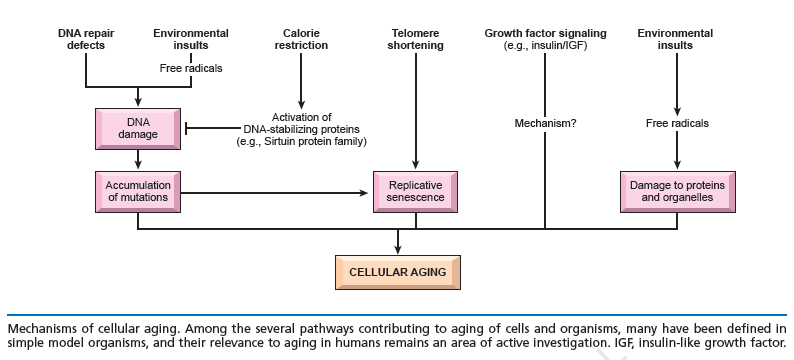
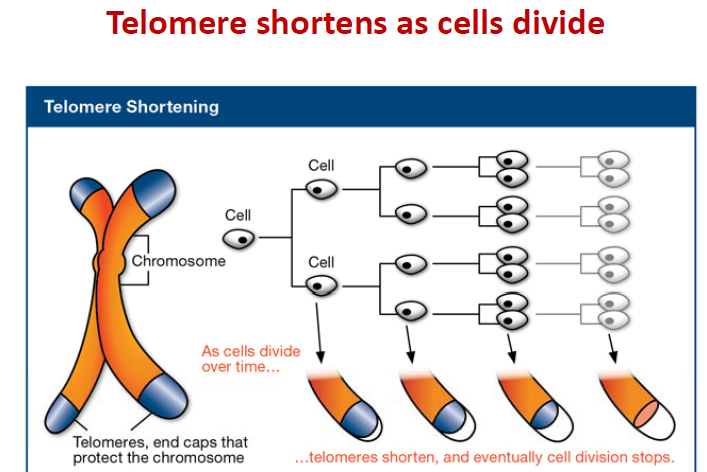
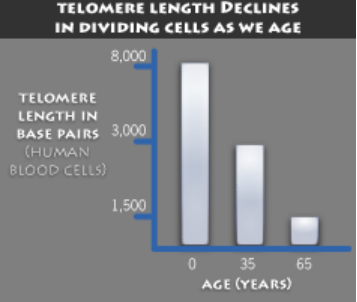
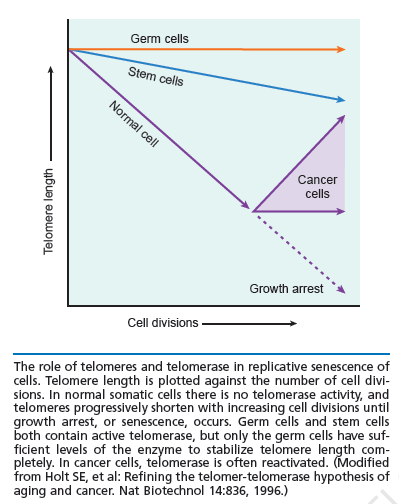
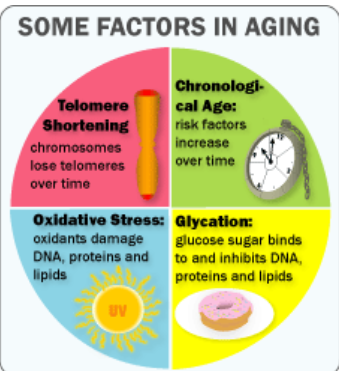
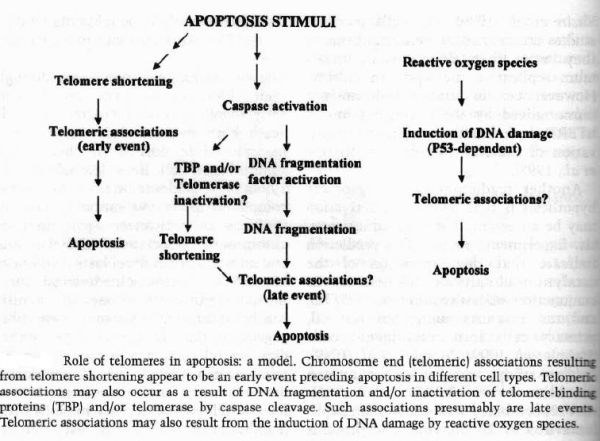
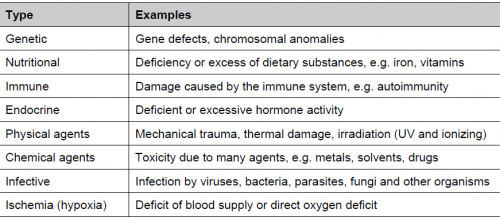
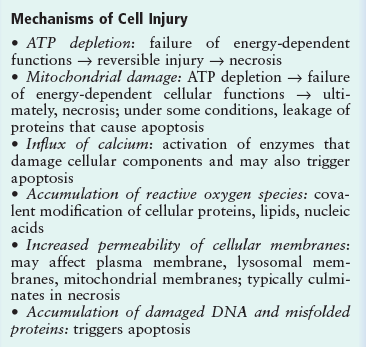
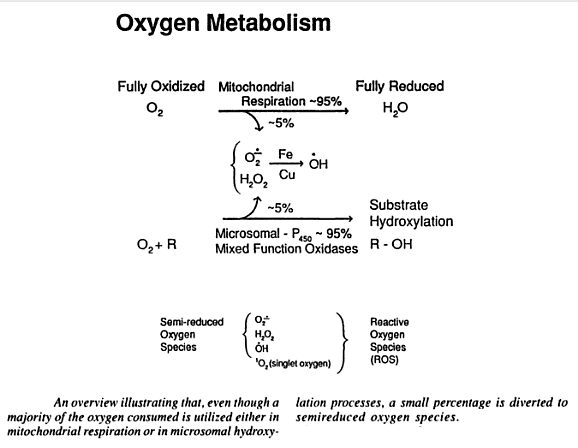
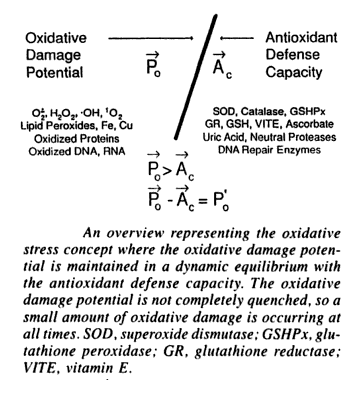
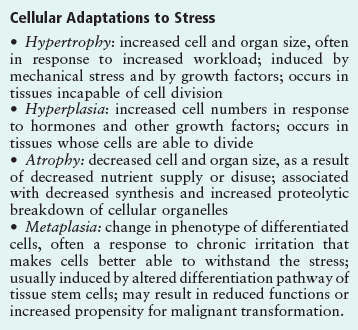
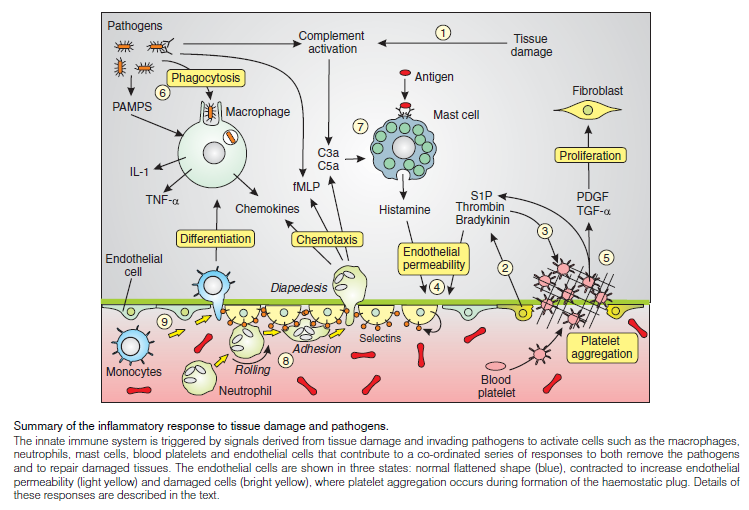
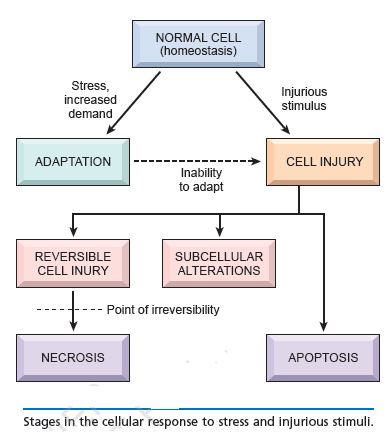
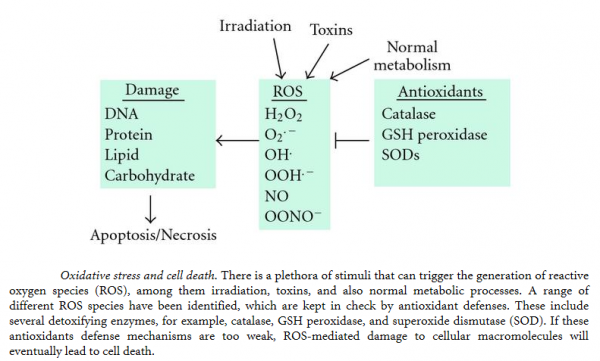
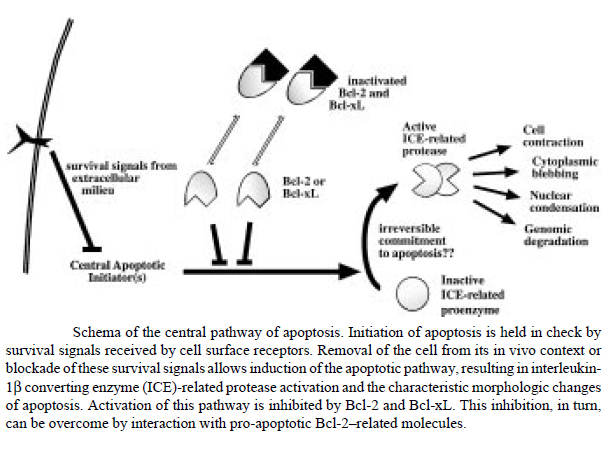
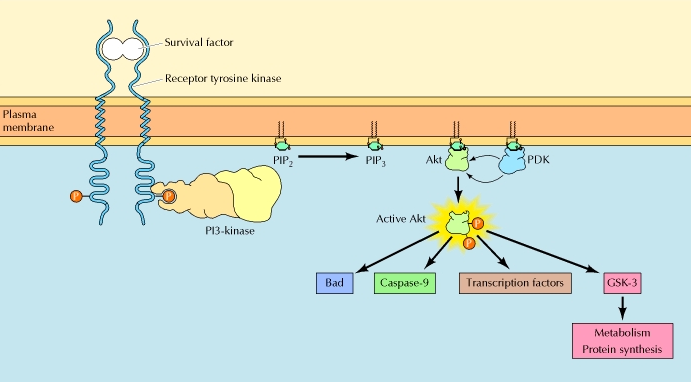
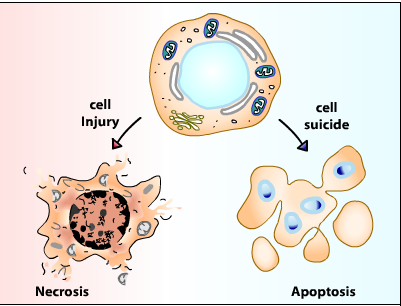

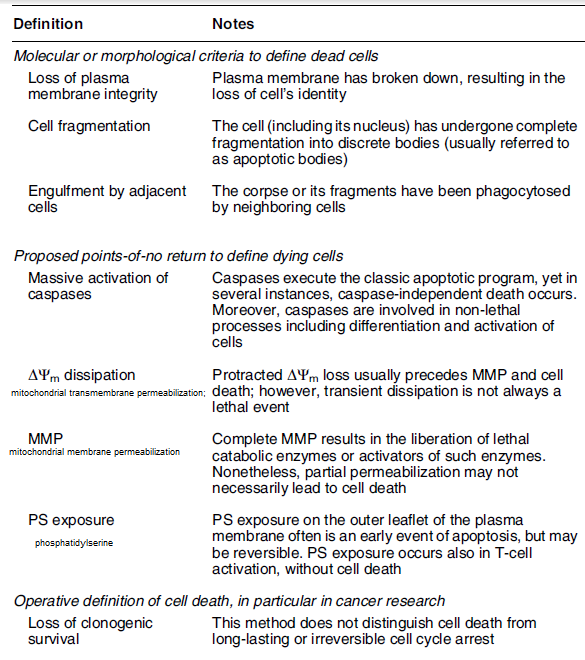
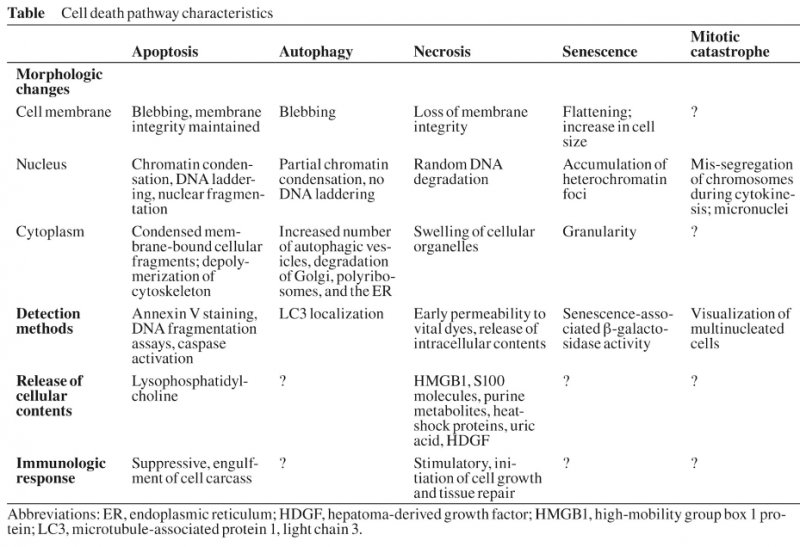
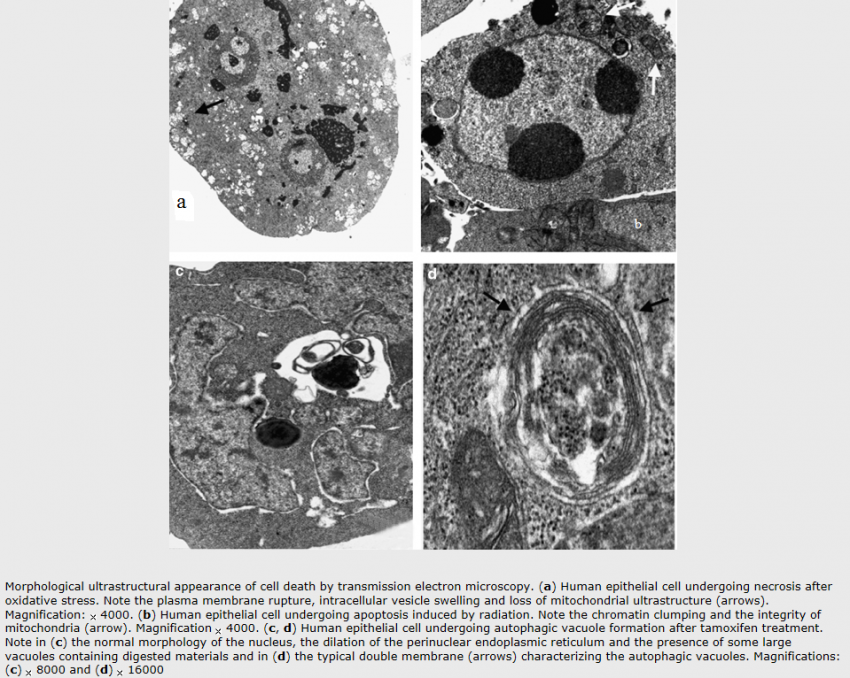
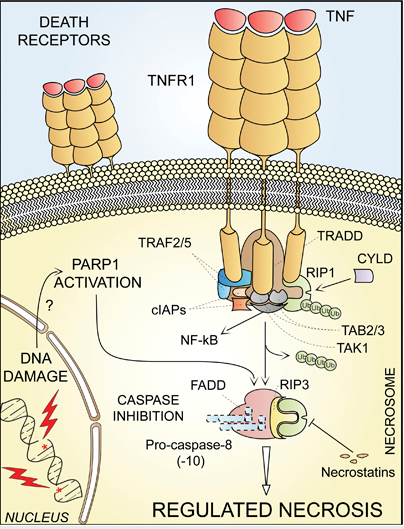
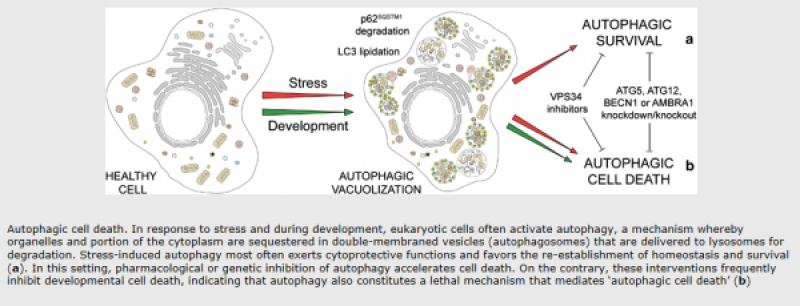
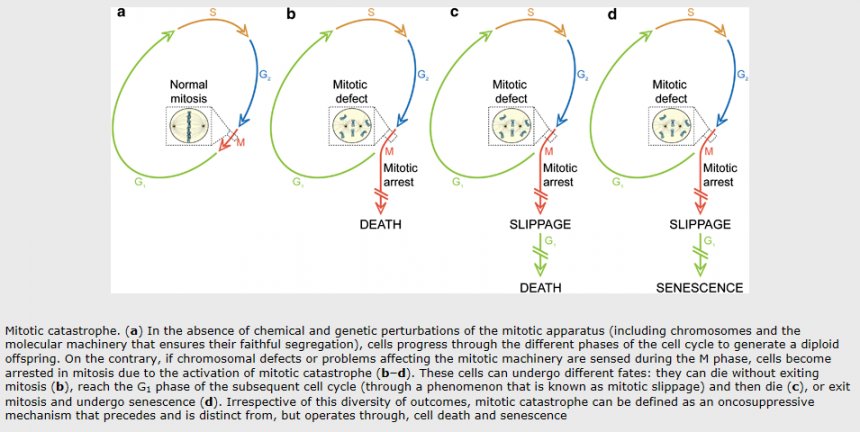
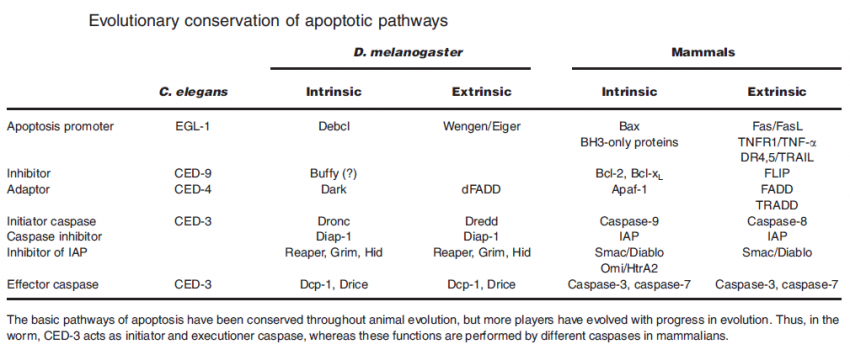
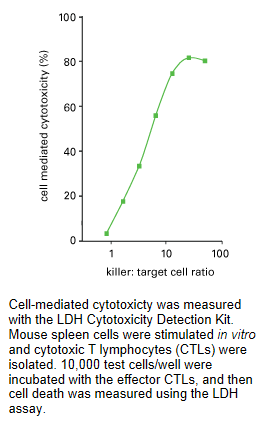
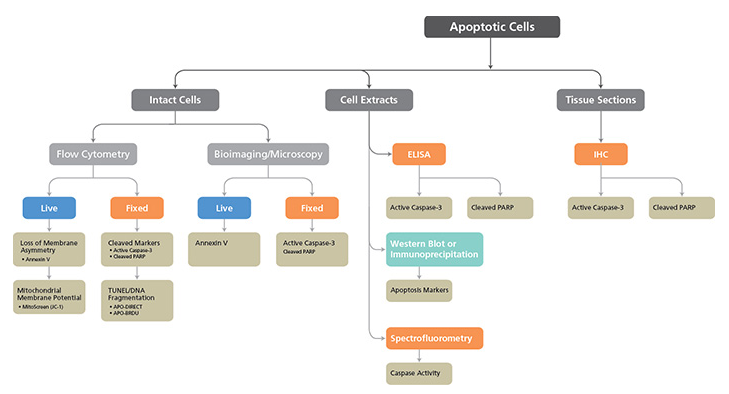
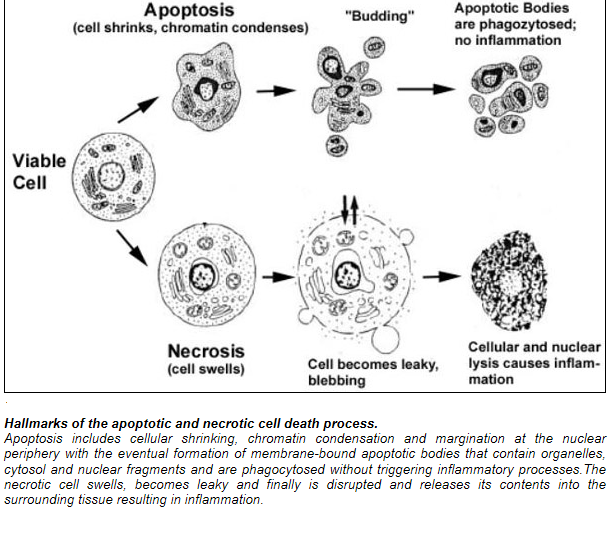
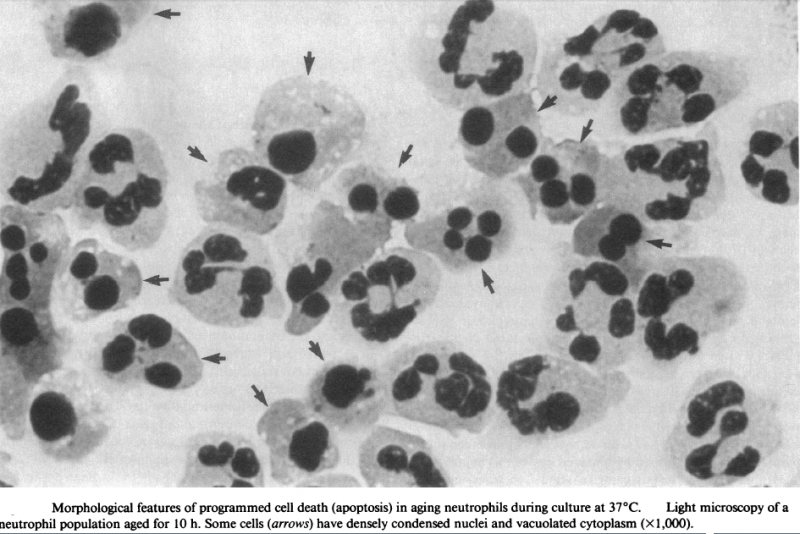
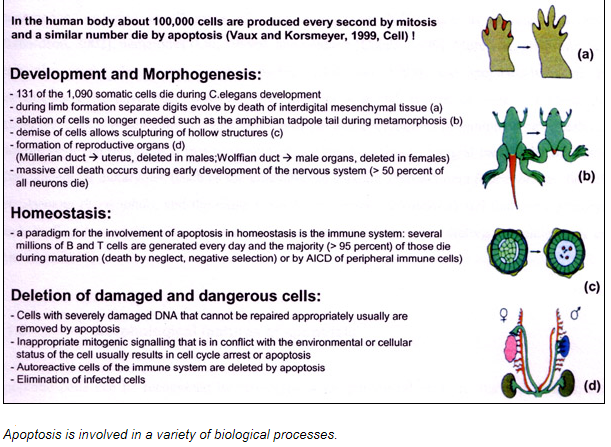
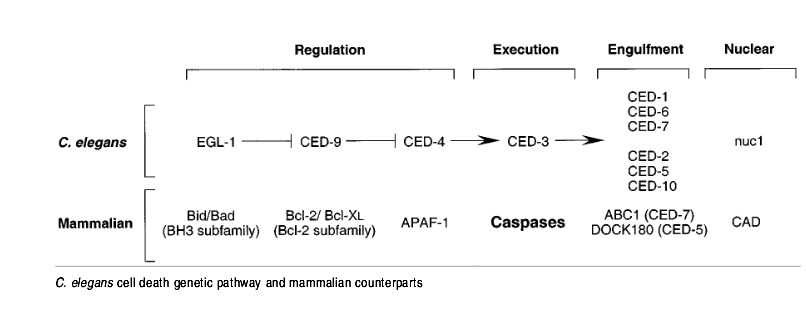
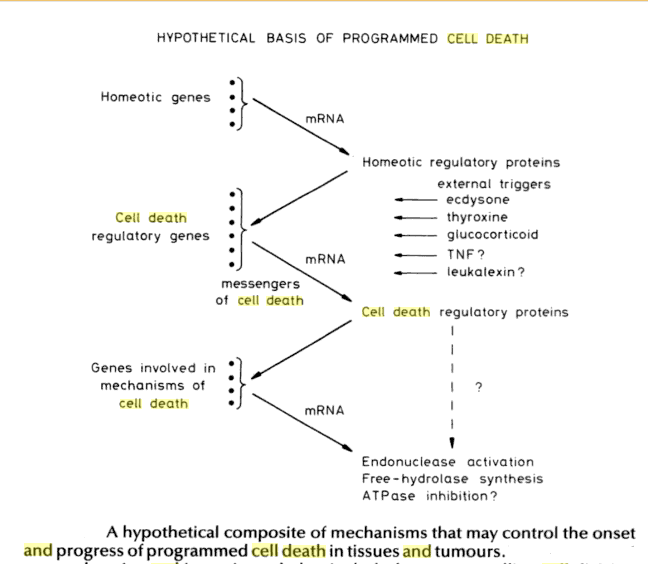
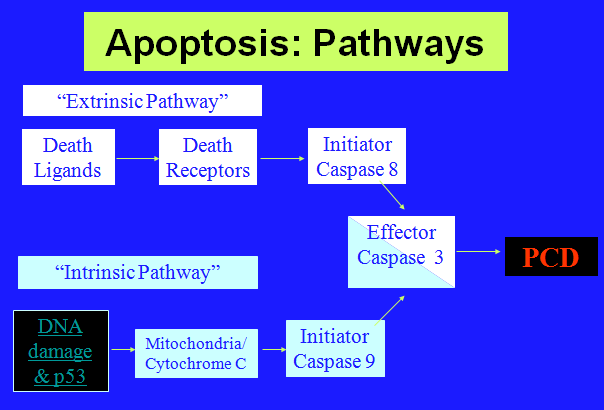
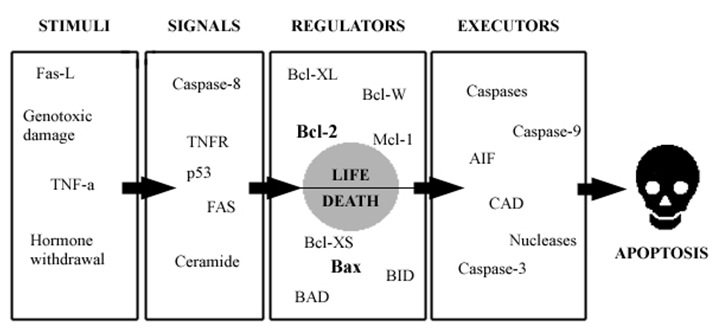
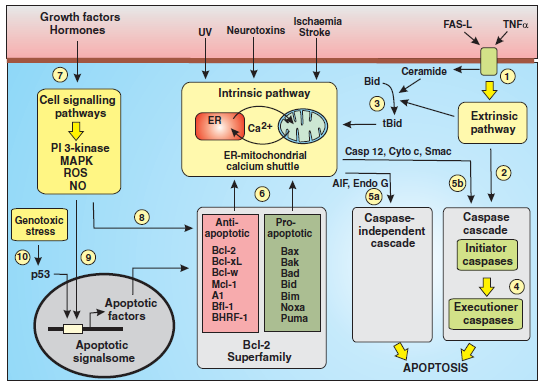
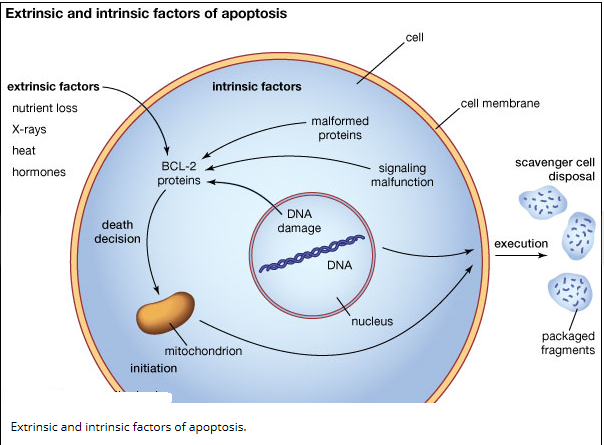
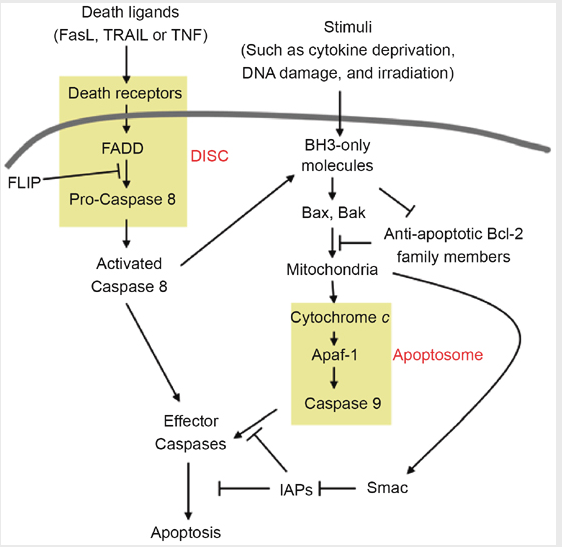
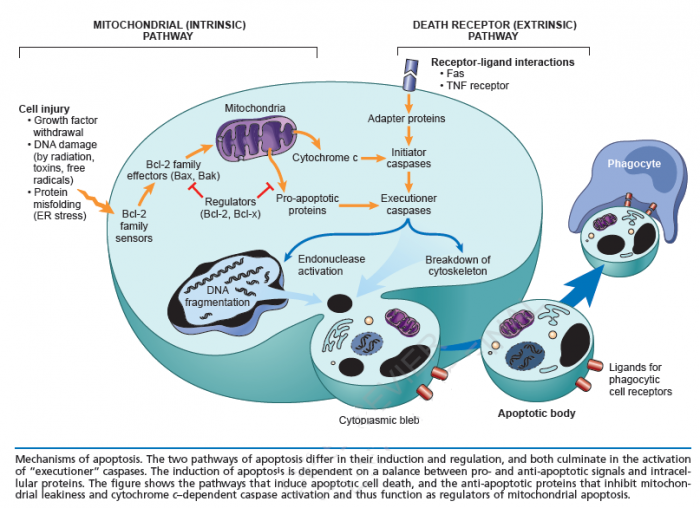
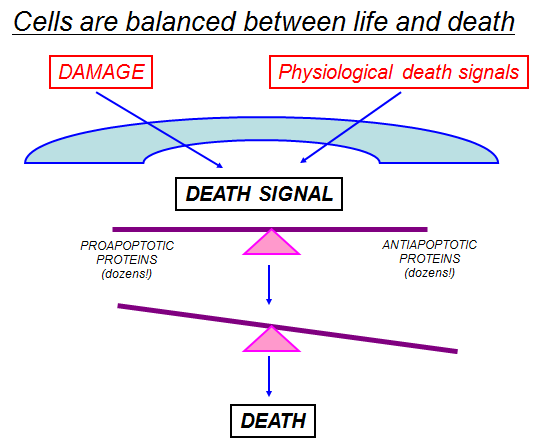
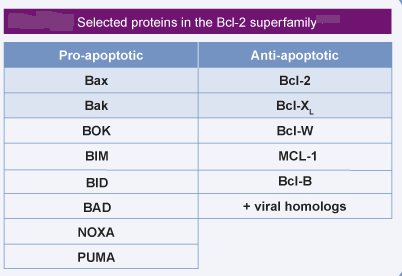
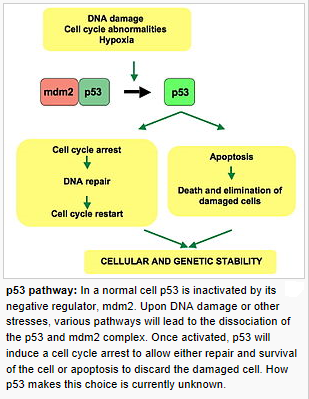
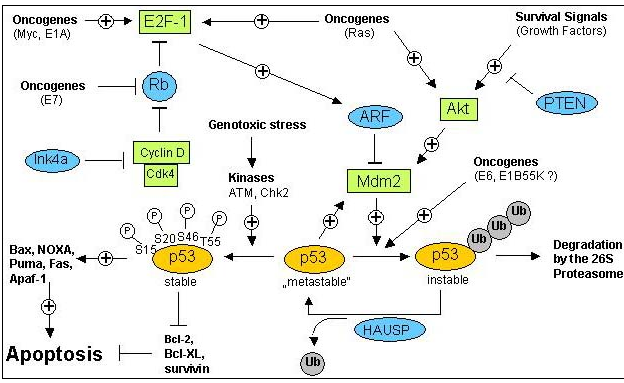
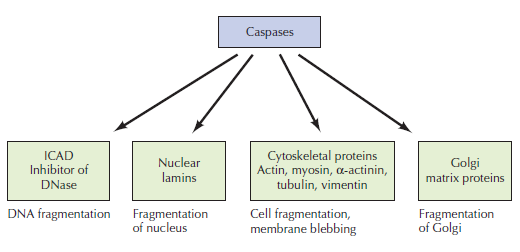
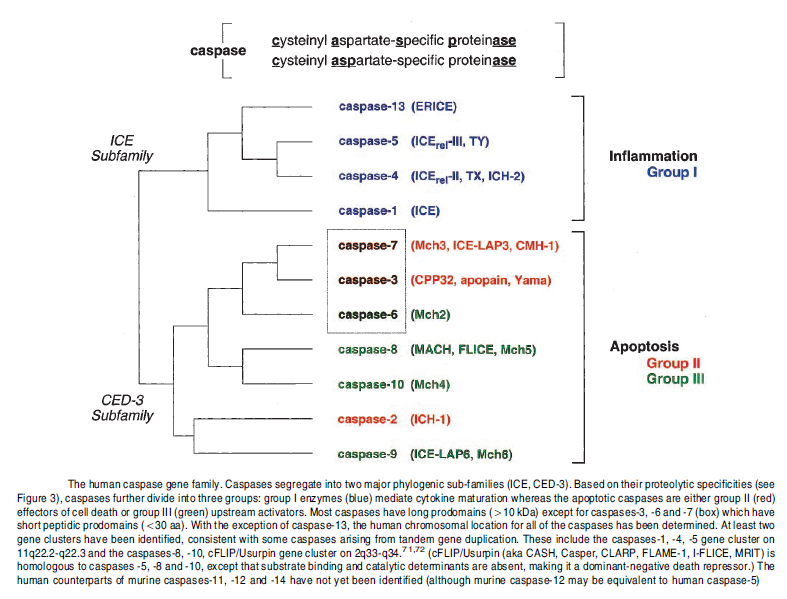
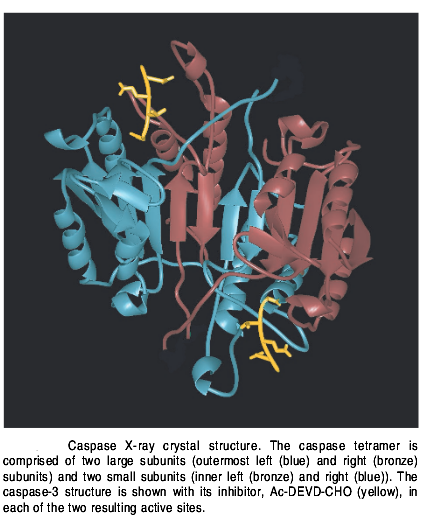
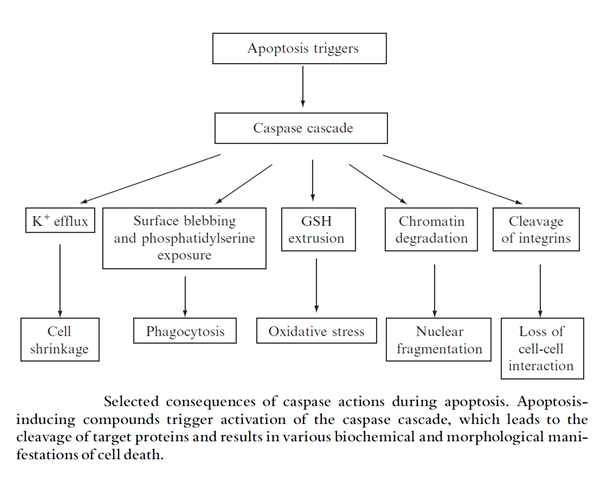
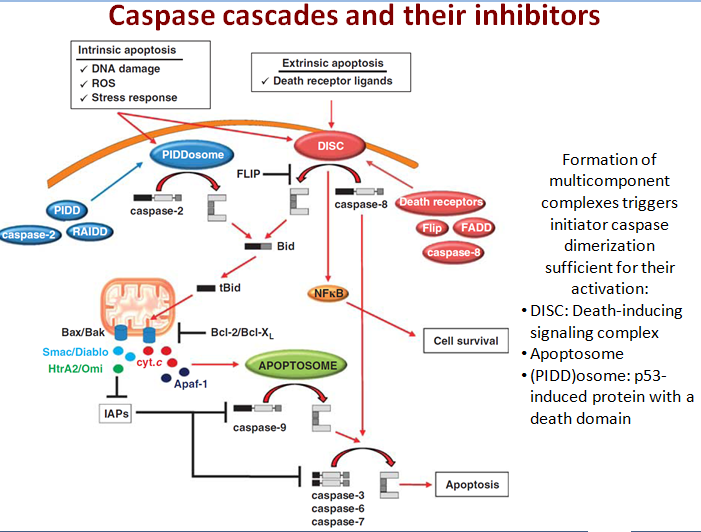
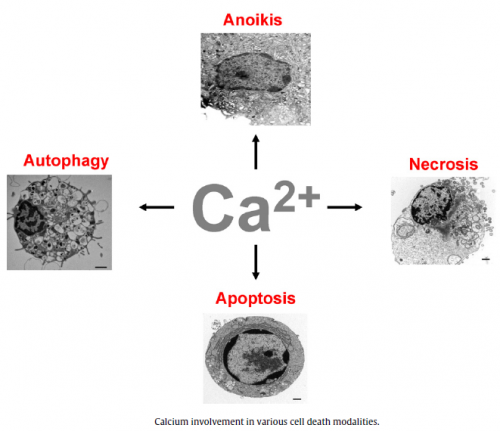
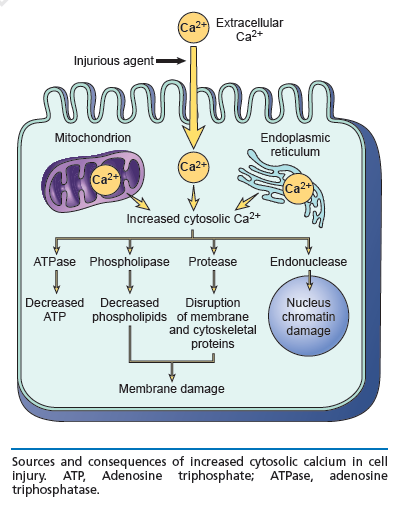
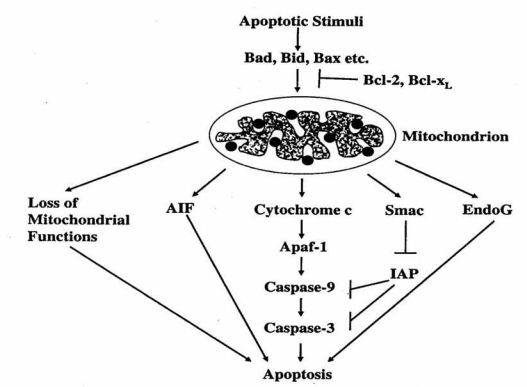
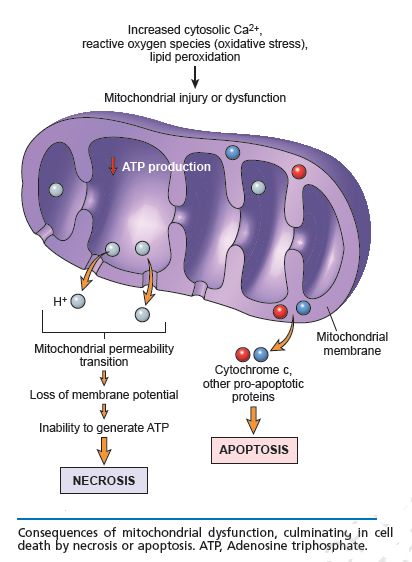
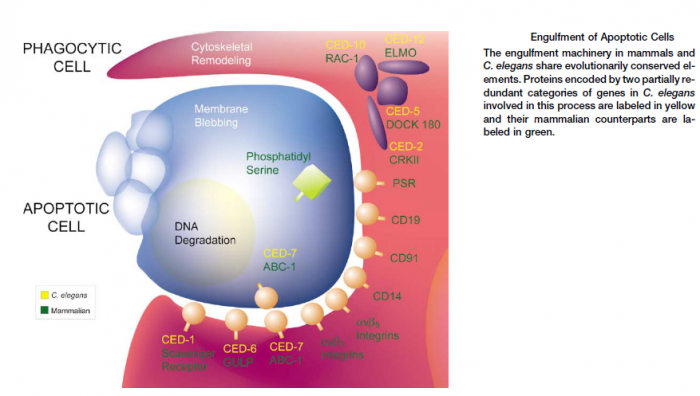
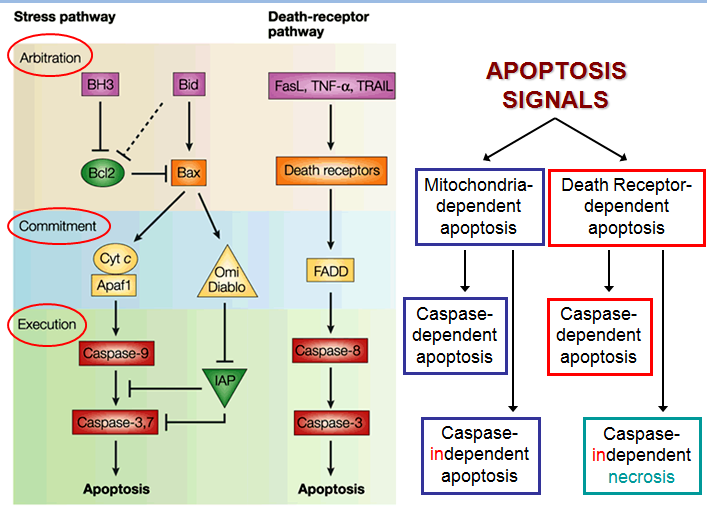
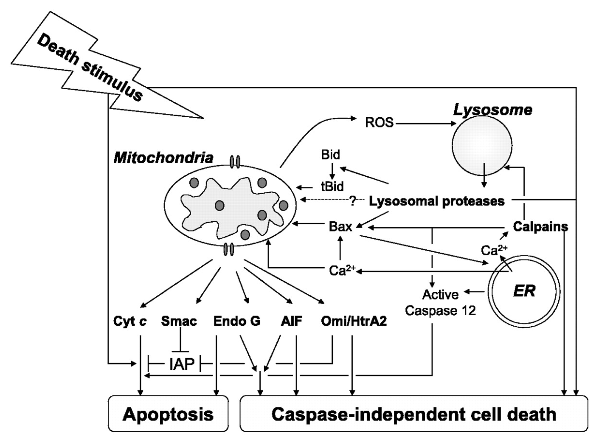
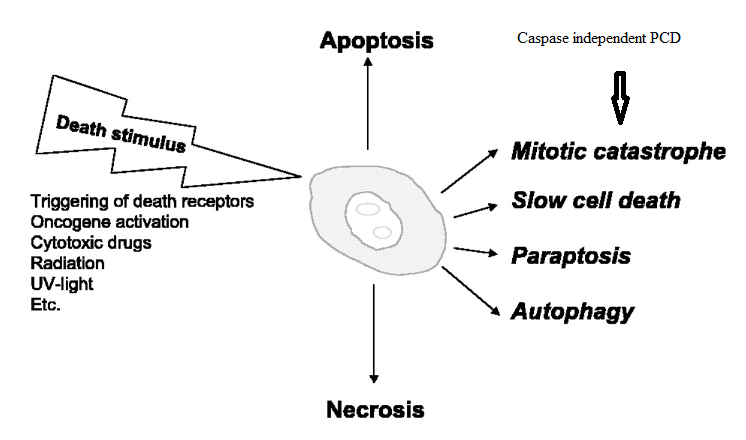
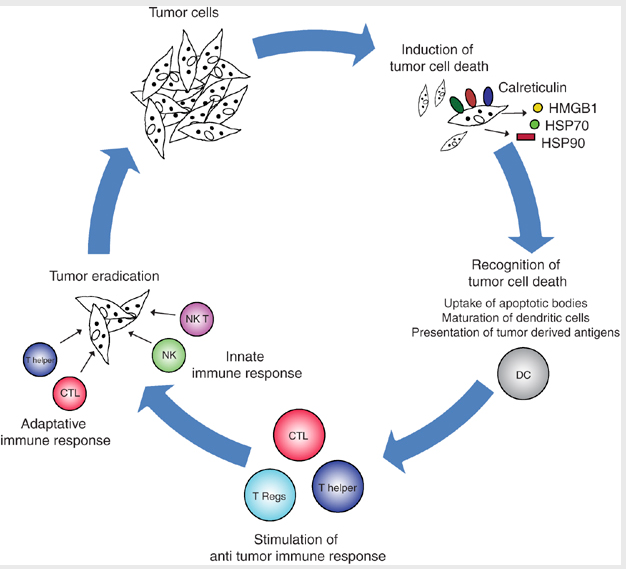
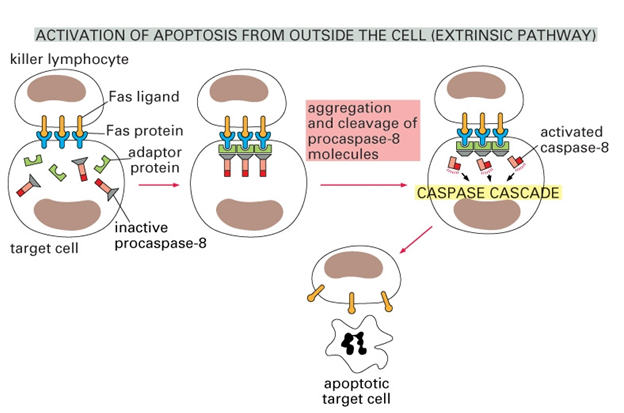
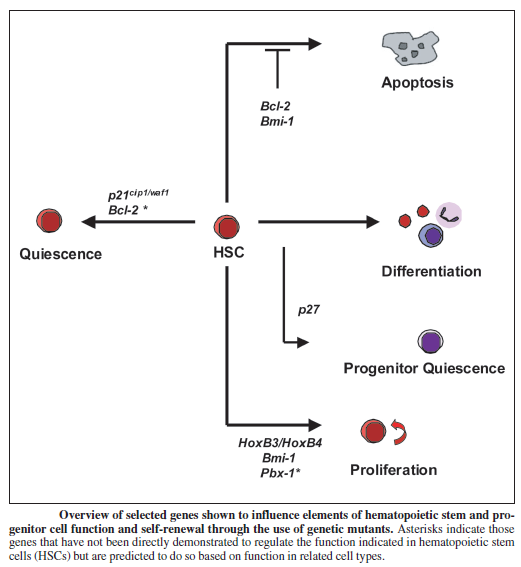
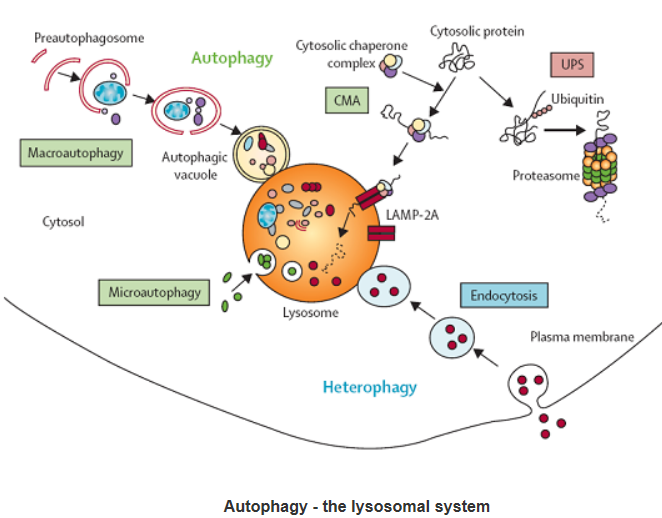
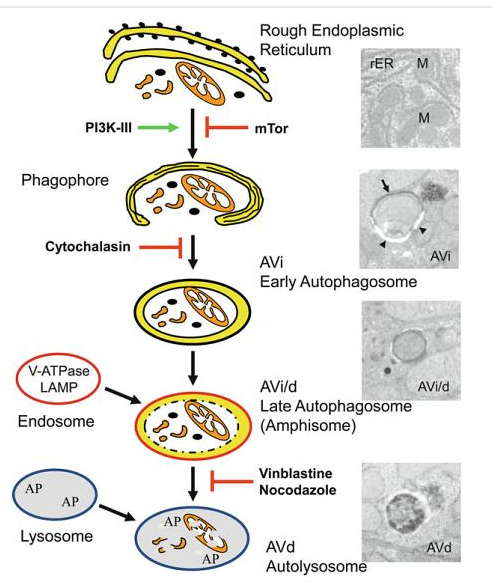
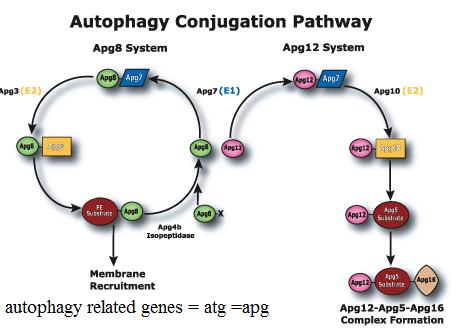
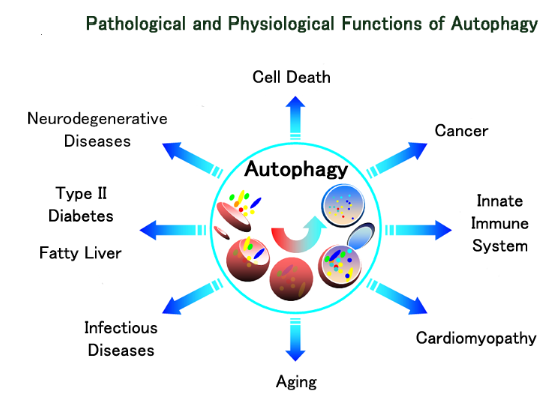
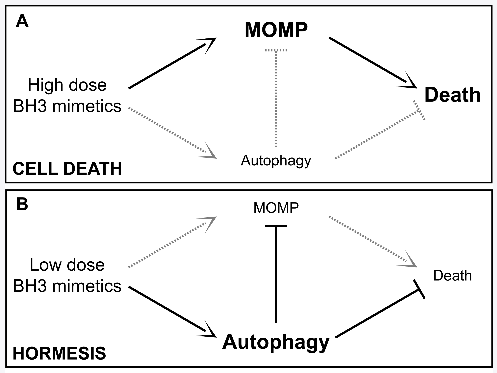
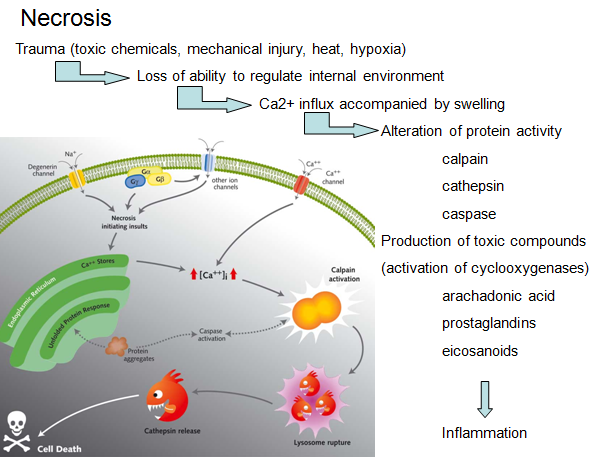
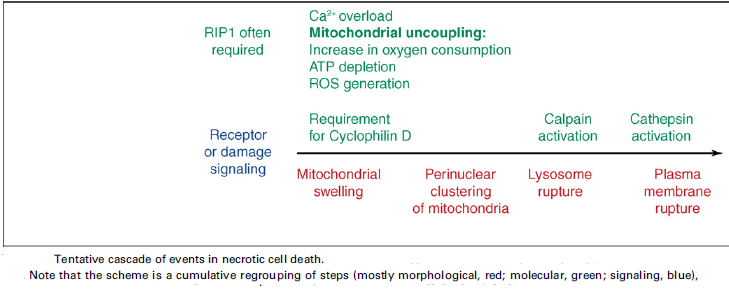
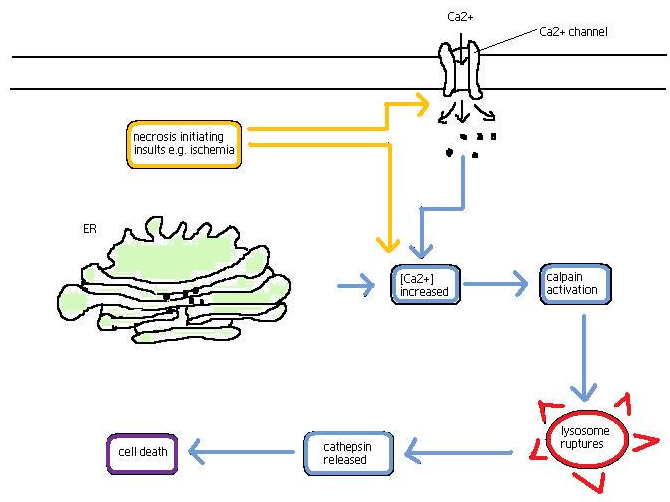
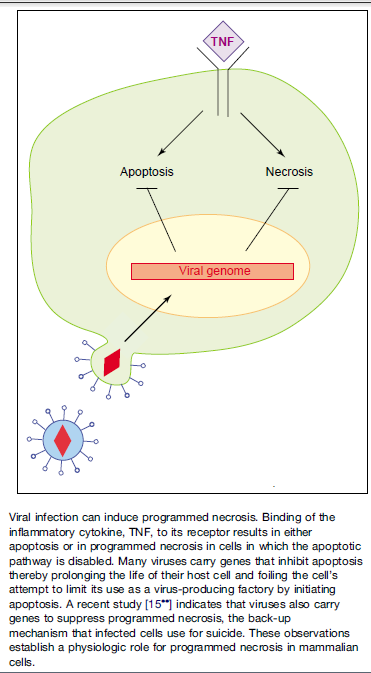
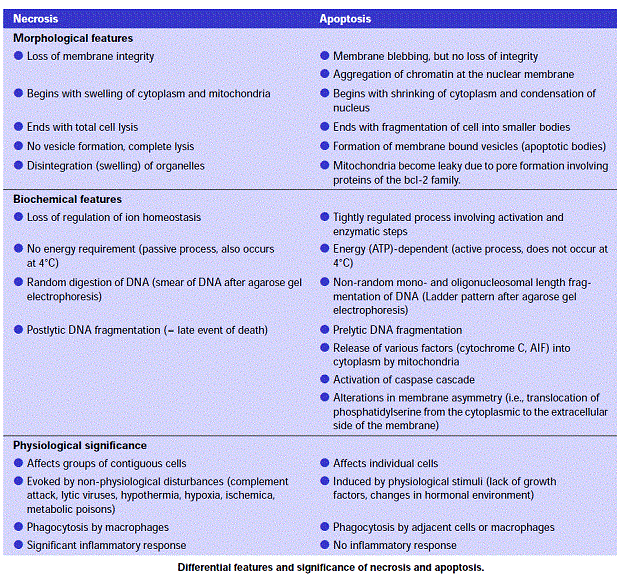
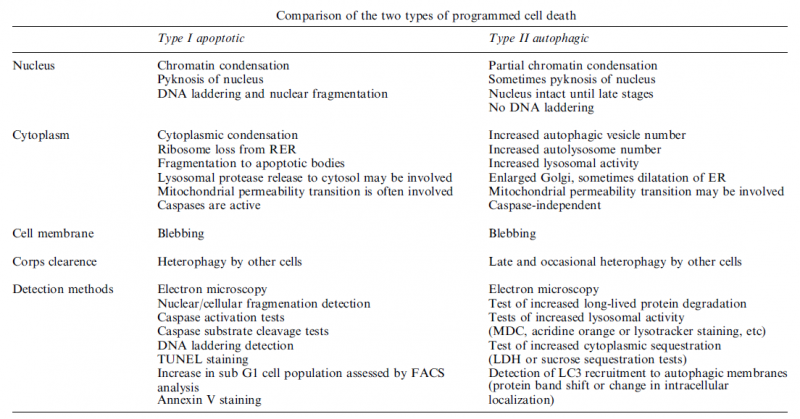
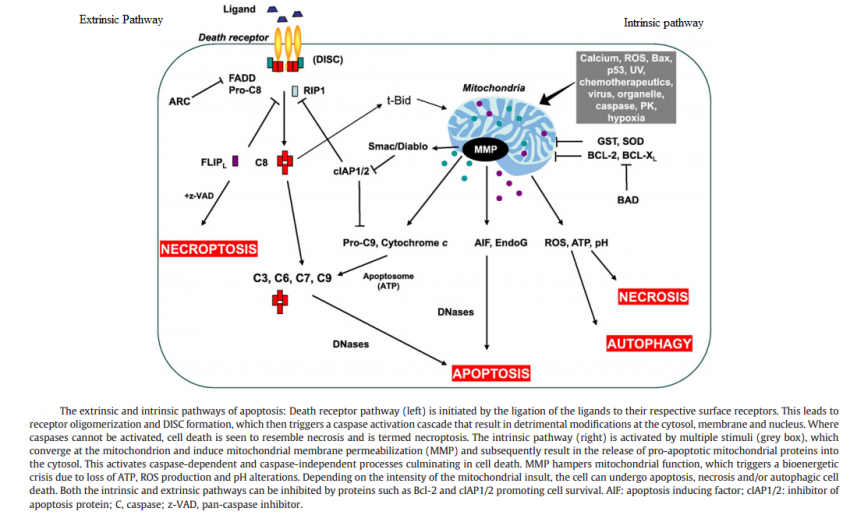
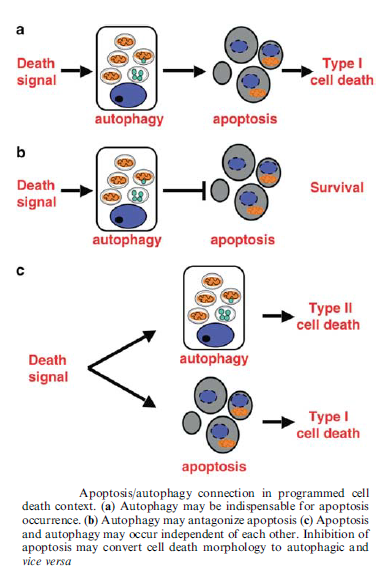
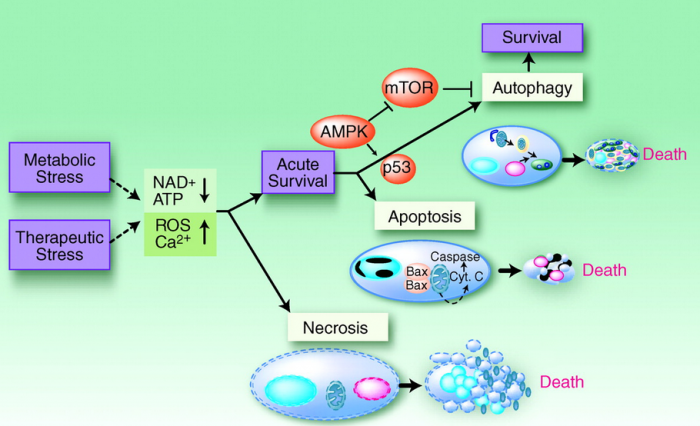
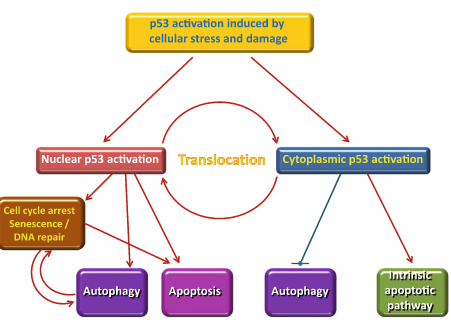
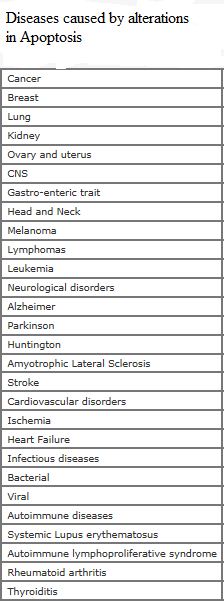
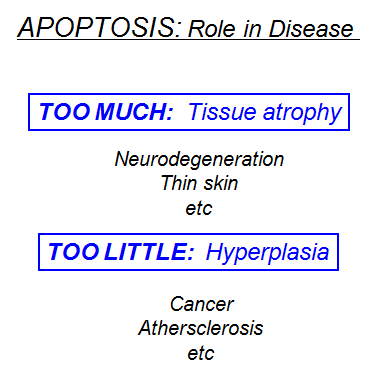
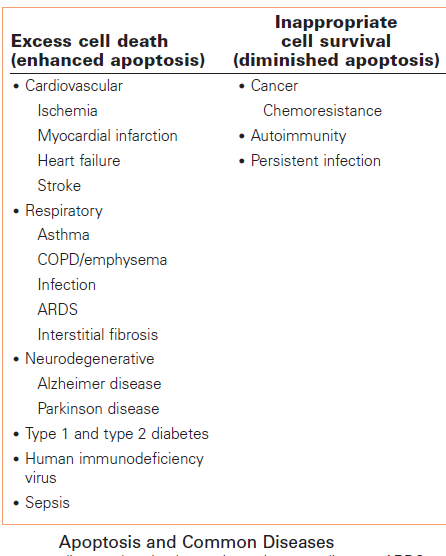
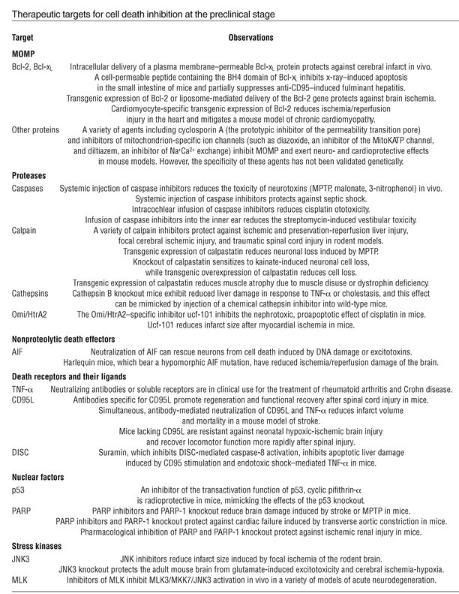
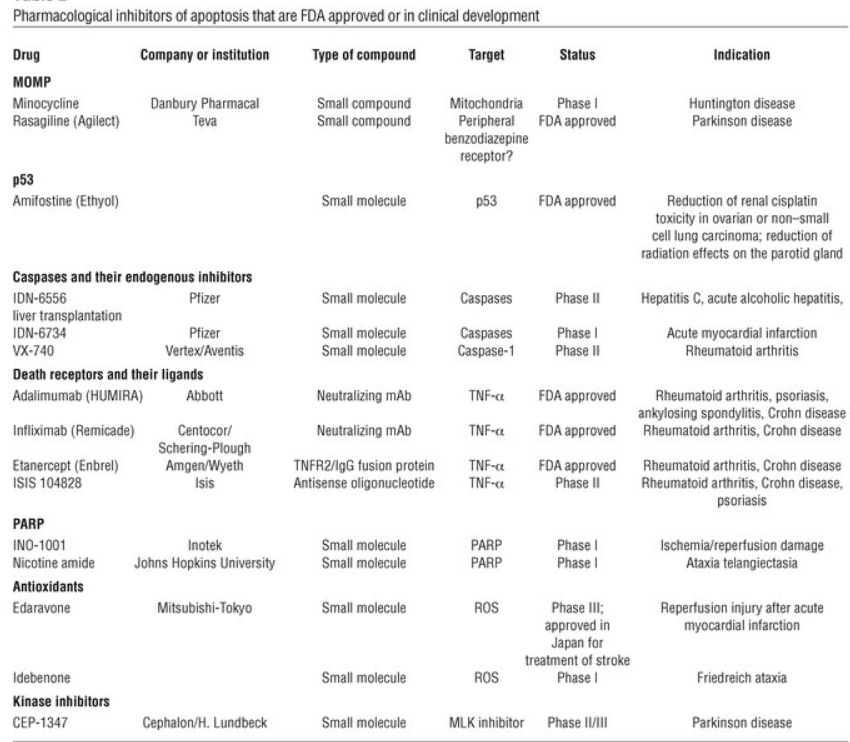
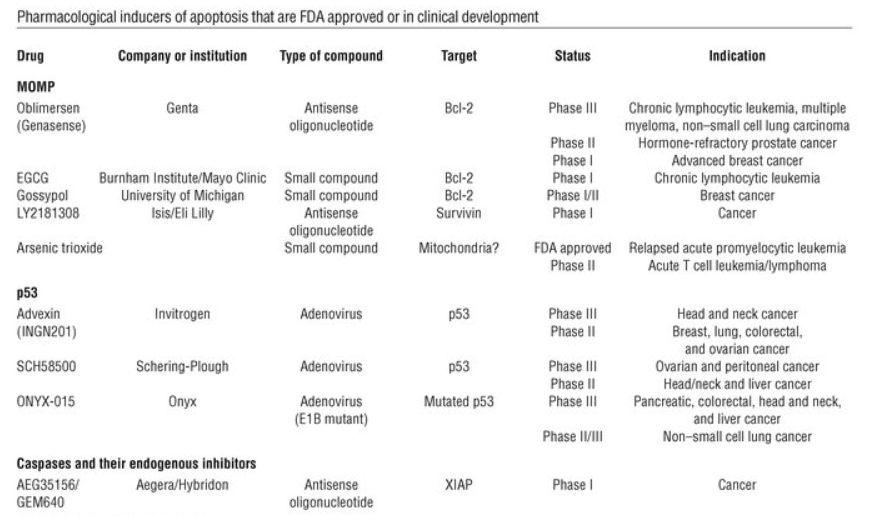
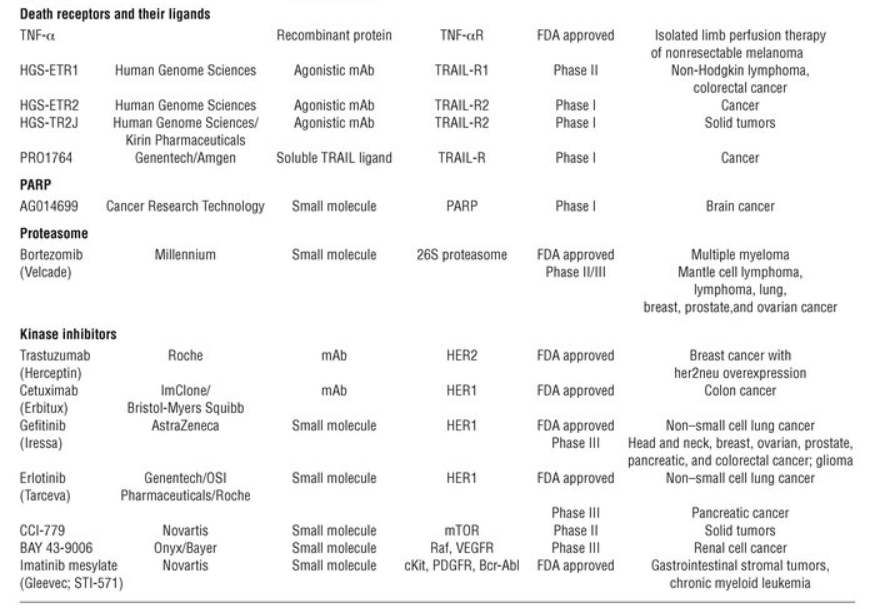
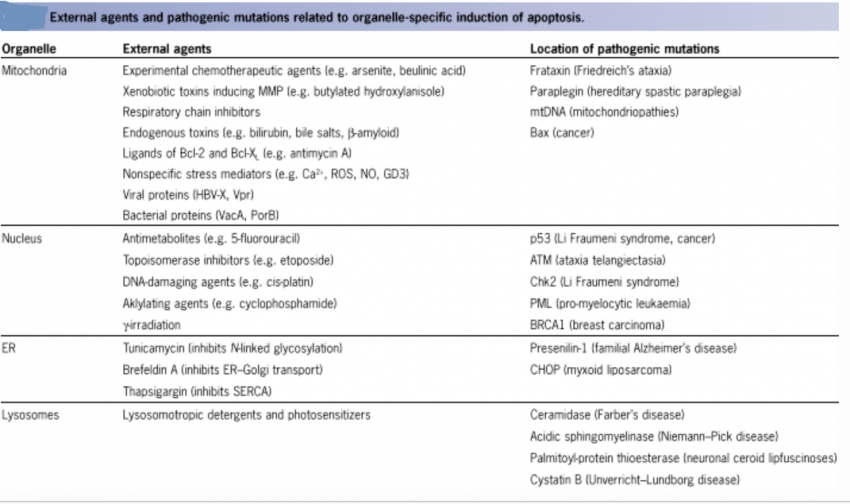
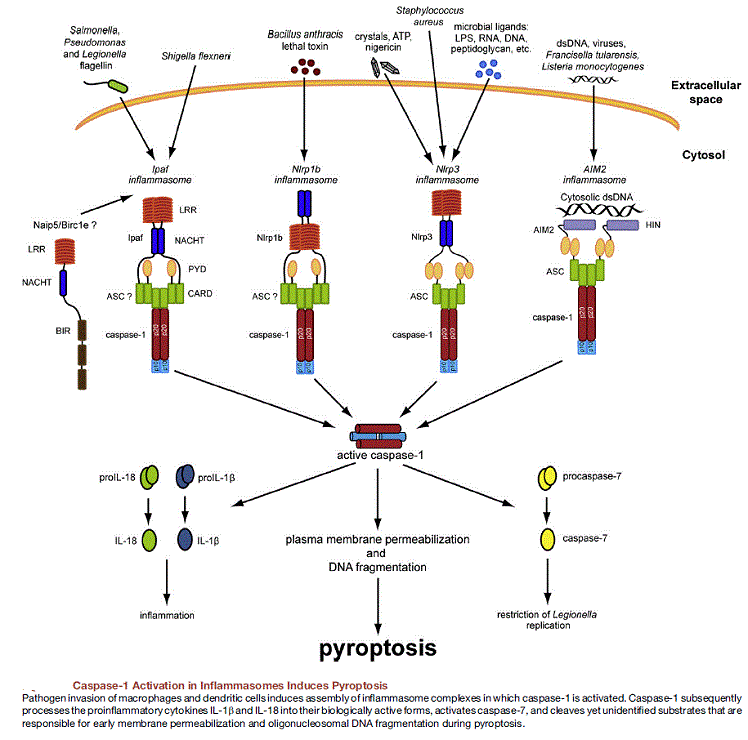
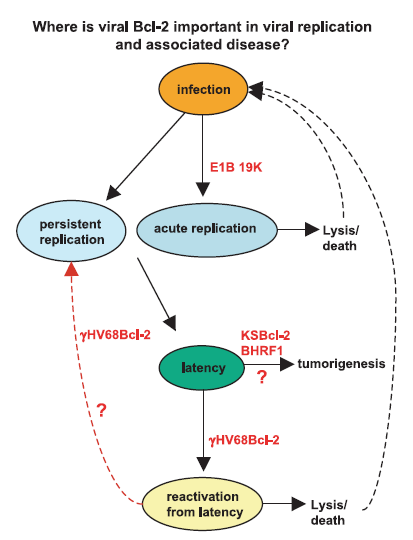
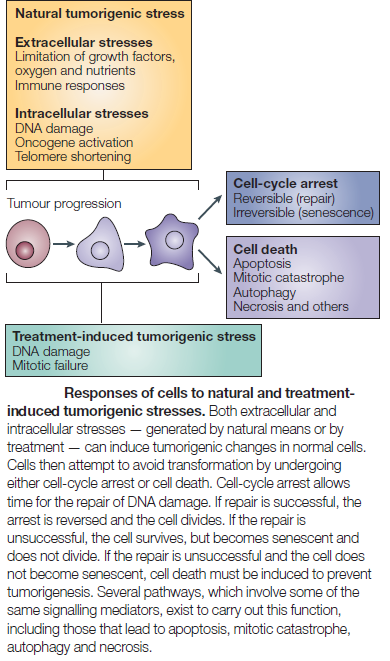
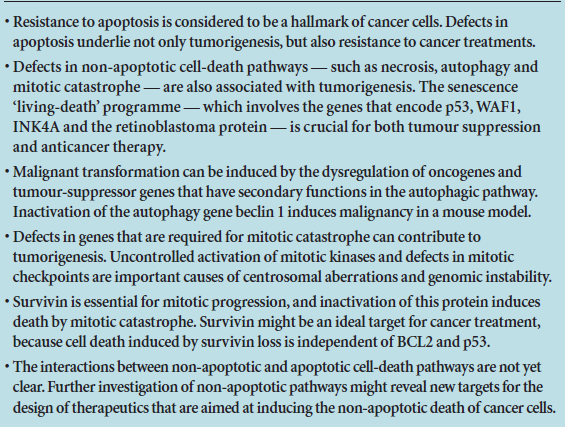
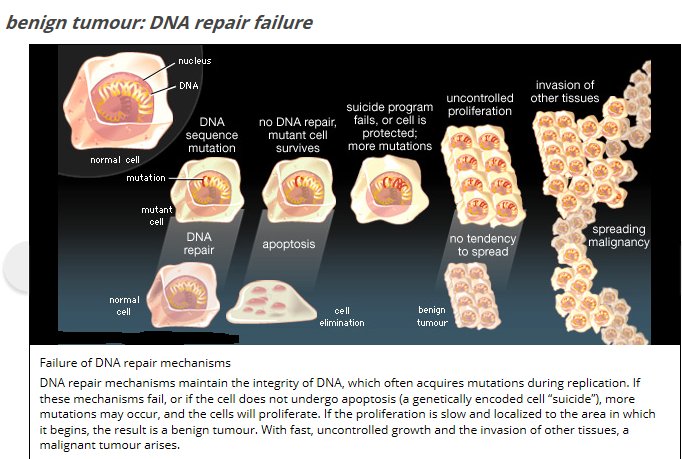
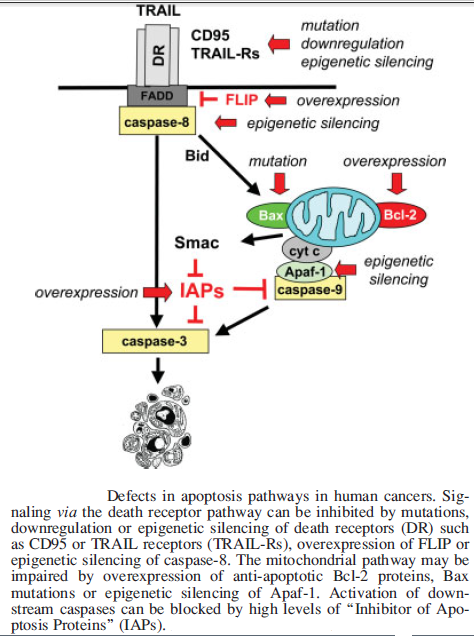
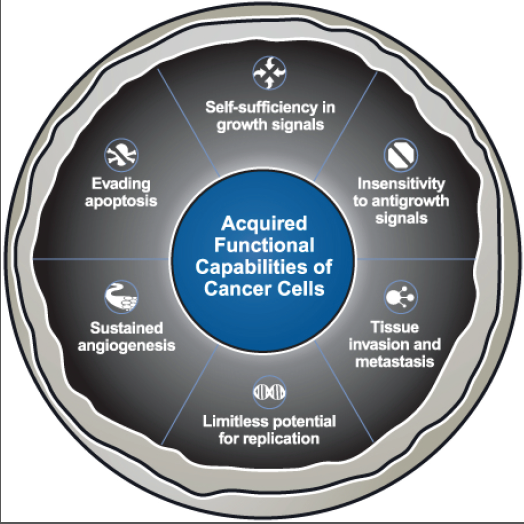
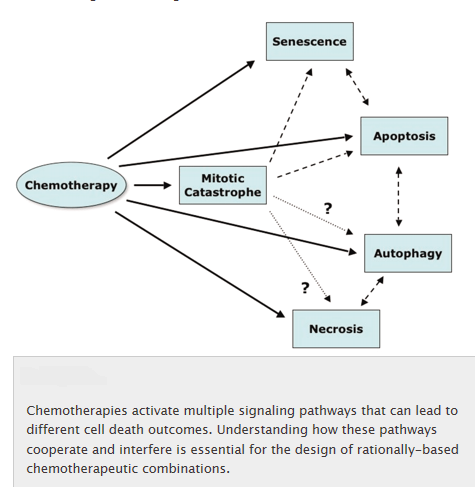
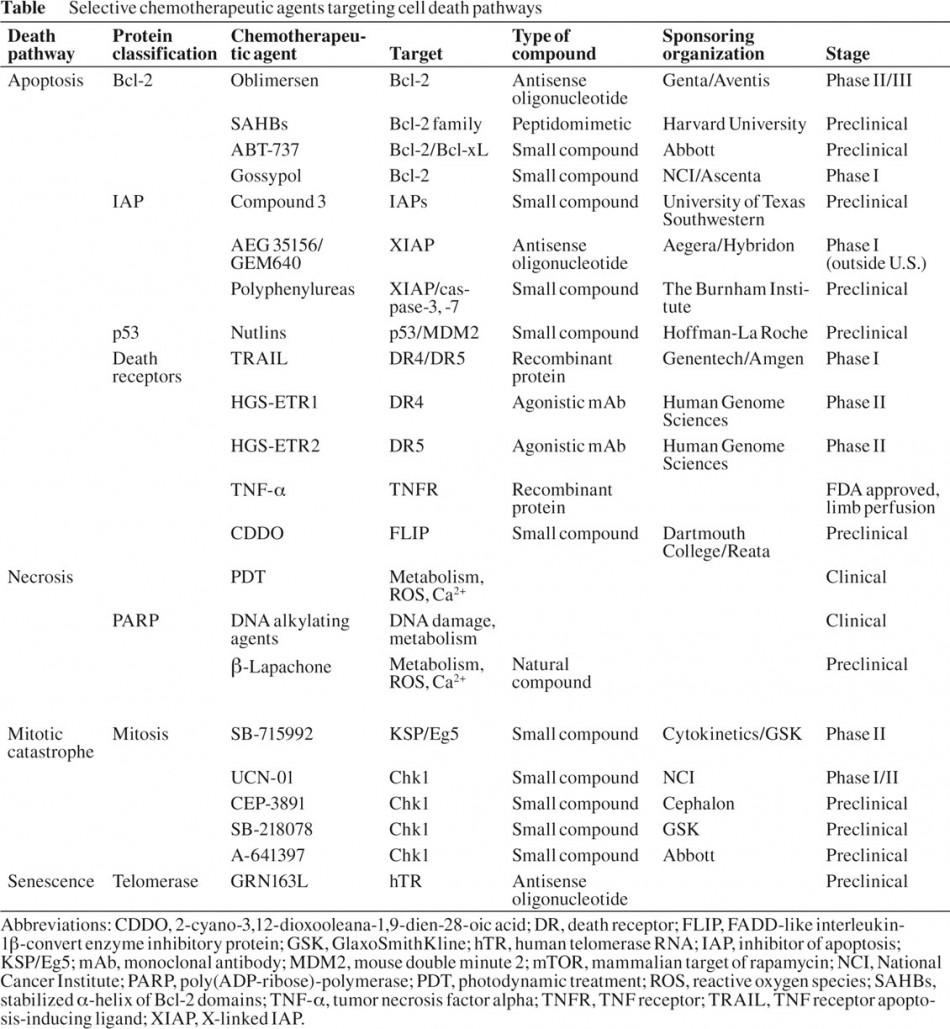
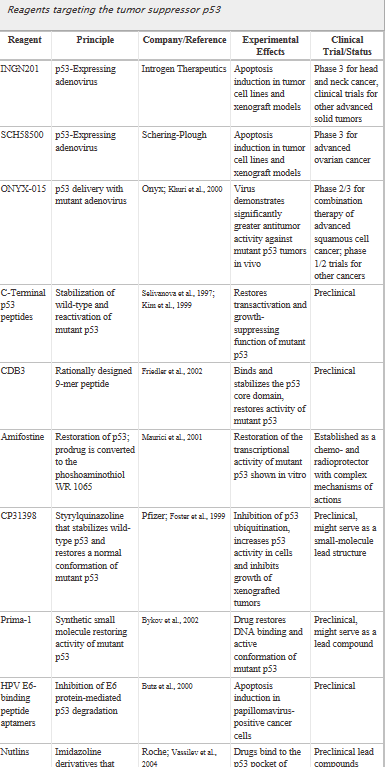
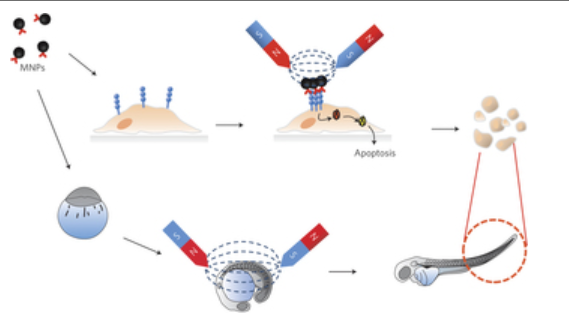
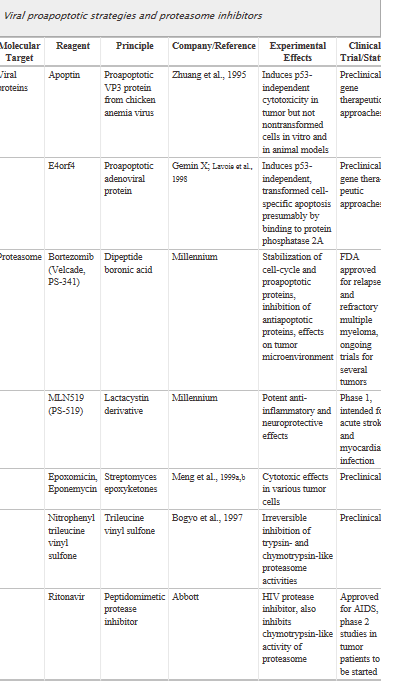
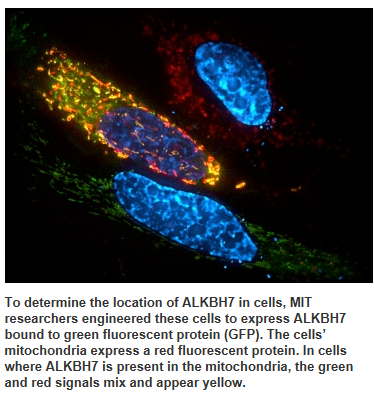
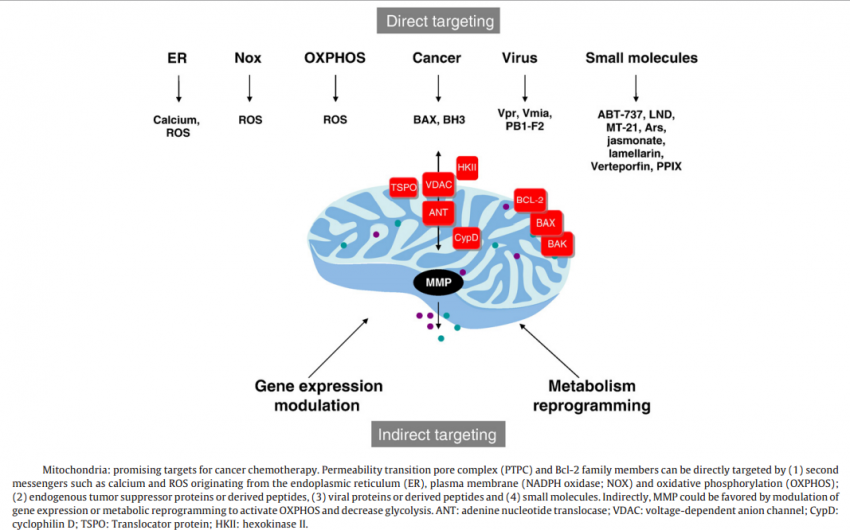
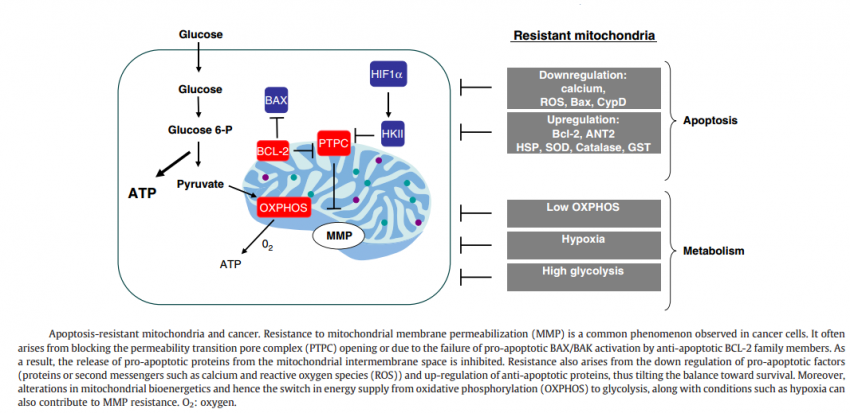
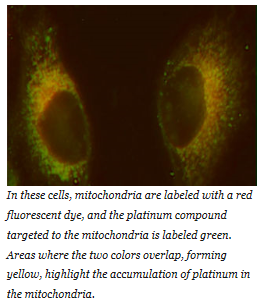
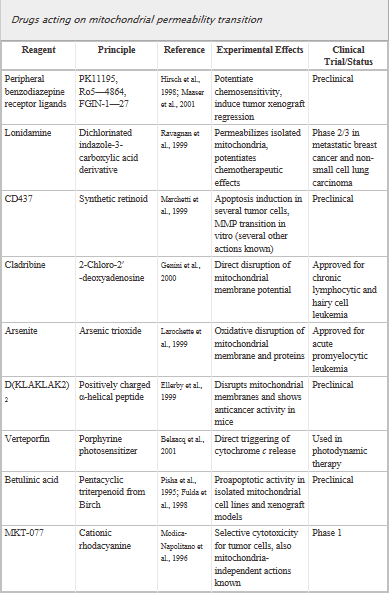
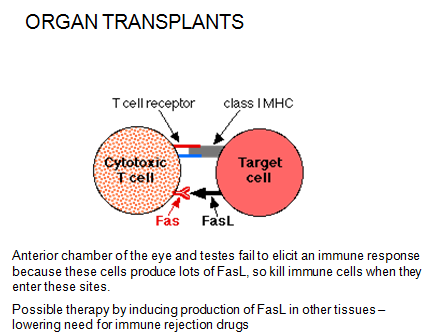
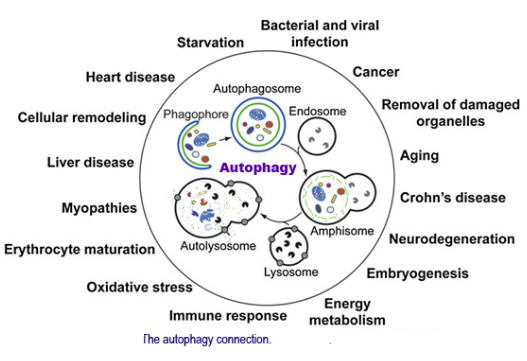
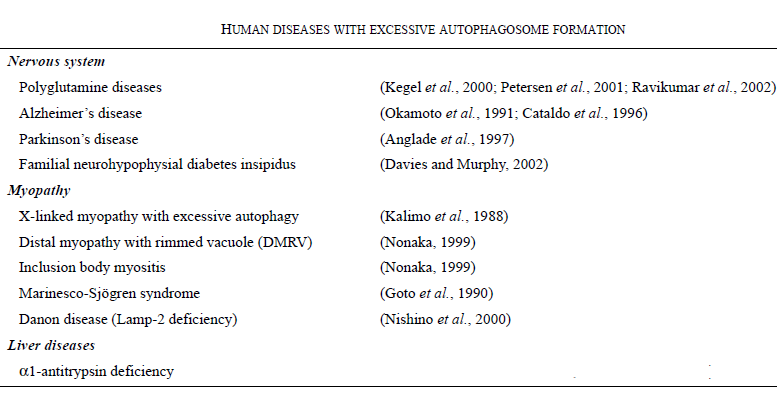
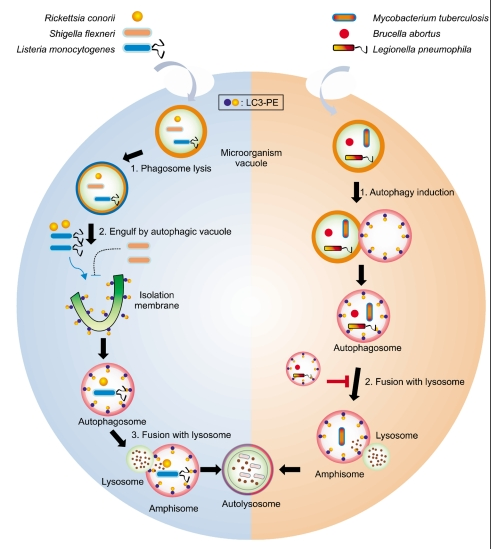
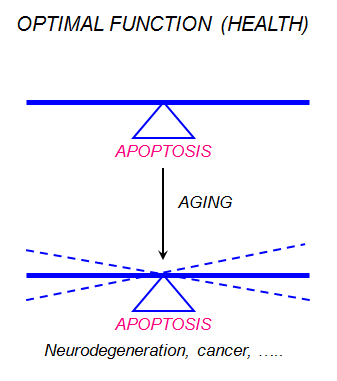
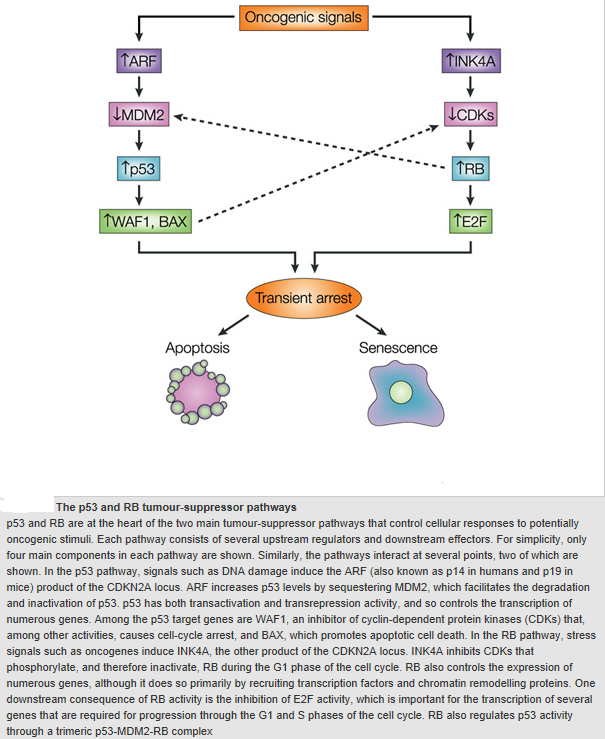
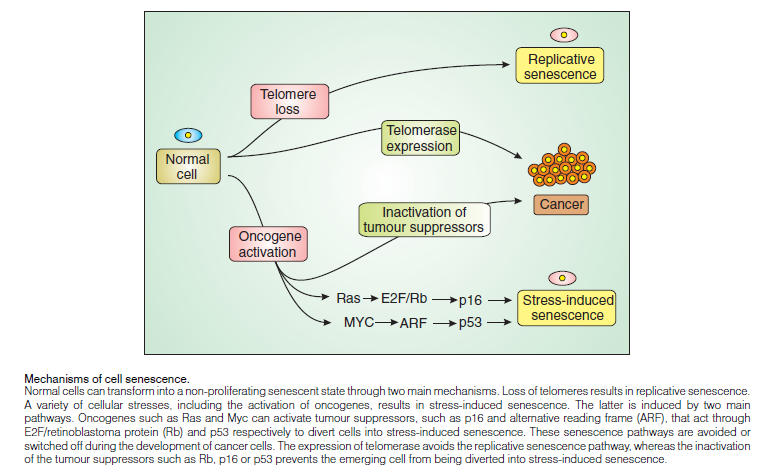

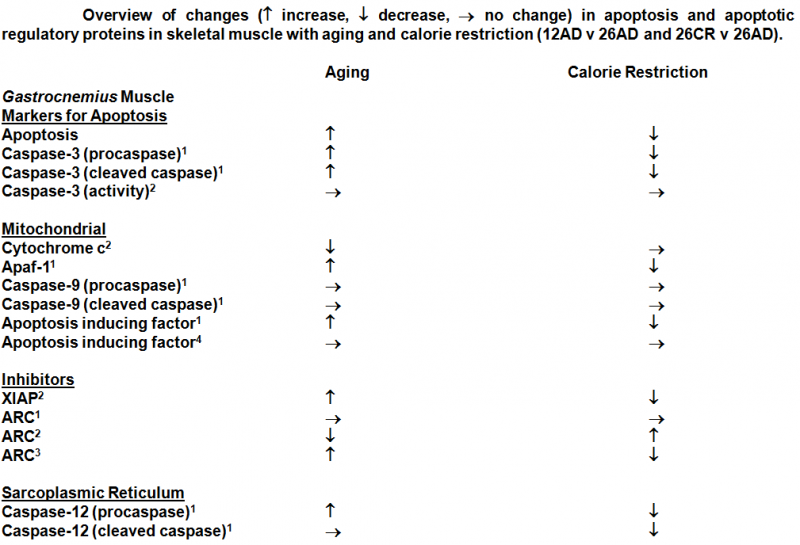
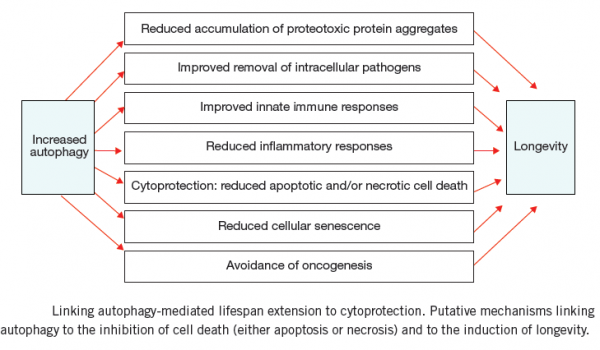
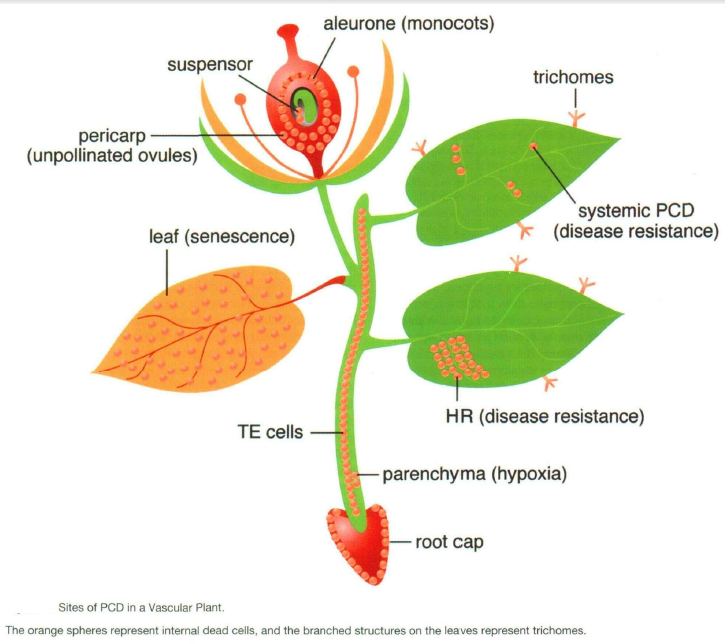
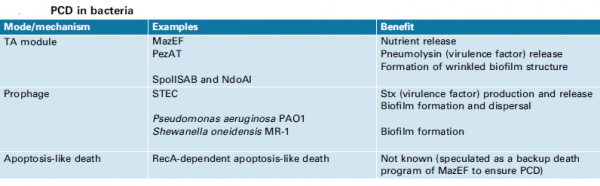
I really like and appreciate your article.Really thank you! Want more.
Terrific article! That is the kind of info that are meant to be shared across the internet. Shame on Google for no longer positioning this post higher! Come on over and seek advice from my site . Thank you =)|
Yes! Finally someone writes about keyword1.|
I think this is a real great blog article.Much thanks again. Really Great.
Excellent post. I used to be checking constantlythis blog and I am impressed! Very useful info specially the last phase 🙂 I handle such info much.I was seeking this particular info for a very lengthy time.Thank you and good luck.